Anti freeze proteins (Afp): Properties, sources and applications - A review
Affiliations.
- 1 Centre for Drug Discovery and Development, Sathyabama Institute of Science and Technology, Chennai 600 119, Tamil Nadu, India.
- 2 Centre for Drug Discovery and Development, Sathyabama Institute of Science and Technology, Chennai 600 119, Tamil Nadu, India. Electronic address: [email protected].
- 3 National Centre for Polar and Ocean Research, Ministry of Earth Sciences, Vasco-da-Gama 403804, Goa, India.
- PMID: 34419548
- DOI: 10.1016/j.ijbiomac.2021.08.105
Extreme cold marine and freshwater temperatures (below 4 °C) induce massive deterioration to the cell membranes of organisms resulting in the formation of ice crystals, consequently causing organelle damage or cell death. One of the adaptive mechanisms organisms have evolved to thrive in cold environments is the production of antifreeze proteins with the functional capabilities to withstand frigid temperatures. Antifreeze proteins are extensively identified in different cold-tolerant species and they facilitate the persistence of cold-adapted organisms by decreasing the freezing point of their body fluids. Various structurally diverse types of antifreeze proteins detected possess the ability to modify ice crystal growth by thermal hysteresis and ice recrystallization inhibition. The unique properties of antifreeze proteins have made them a promising resource in industry, biomedicine, food storage and cryobiology. This review collates the findings of the various studies carried out in the past and the recent developments observed in the properties, functional mechanisms, classification, distinct sources and the ever-increasing applications of antifreeze proteins. This review also summarizes the possibilities of the way forward to identify new avenues of research on anti-freeze proteins.
Keywords: Antifreeze proteins; Applications; Ice recrystallization inhibition; Thermal hysteresis.
Copyright © 2021 Elsevier B.V. All rights reserved.

Publication types
- Antifreeze Proteins / chemistry*
- Cryoprotective Agents / pharmacology
- Crystallization
- Food Preservation
- Antifreeze Proteins
- Cryoprotective Agents
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- In Your Element
- Published: 24 October 2022
Antifreeze proteins solve cold problems
- Anna Ampaw 1 na1
Nature Chemistry volume 14 , page 1336 ( 2022 ) Cite this article
2004 Accesses
3 Citations
6 Altmetric
Metrics details
- Biomaterials – proteins
Anna Ampaw reflects on the effects of antifreeze proteins, from Arctic fish to fruity ice creams.
This is a preview of subscription content, access via your institution
Relevant articles
Open Access articles citing this article.
The role of antifreeze genes in the tolerance of cold stress in the Nile tilapia (Oreochromis niloticus)
- Abdel-Fattah M. El-Sayed
- , Asmaa A. Khaled
- … Ahmed A. Saleh
BMC Genomics Open Access 23 August 2023
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
251,40 € per year
only 20,95 € per issue
Buy this article
Purchase on Springer Link
Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Gordon, M. S., Amdur, B. H. & Scholander, P. F. Biol. Bull. 122 , 52–62 (1962).
Article Google Scholar
DeVries, A. L. & Wohlschlag, D. E. Science 163 , 1073–1075 (1969).
Article CAS PubMed Google Scholar
Duman, J. G. & Olsen, T. M. Cryobiology 30 , 322–328 (1993).
Chao, H., Davies, P. L. & Carpenter, J. F. J. Exp. Biol. 199 , 2071–2076 (1996).
Wang, T., Zhu, Q., Yang, X., Layne, J. R. & DeVries, A. L. Cryobiology 31 , 185–192 (1994).
Raymond, J. A. & DeVries, A. L. Proc. Natl Acad. Sci. USA 74 , 2589–2593 (1977).
Article CAS PubMed PubMed Central Google Scholar
Sreter, J. A., Foxall, T. L. & Varga, K. Biomolecules 12 , 669 (2022).
Moskin, J. Creamy, Healthier Ice Cream? What’s the Catch? The New York Times (July 2006).
Download references
Author information
Twitter: @anna_thechemist
Authors and Affiliations
University of Toronto Mississauga, Mississauga, ON, Canada
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Anna Ampaw .
Ethics declarations
Competing interests.
The author declares no competing interests.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Ampaw, A. Antifreeze proteins solve cold problems. Nat. Chem. 14 , 1336 (2022). https://doi.org/10.1038/s41557-022-01075-z
Download citation
Published : 24 October 2022
Issue Date : November 2022
DOI : https://doi.org/10.1038/s41557-022-01075-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
- Asmaa A. Khaled
- Ahmed A. Saleh
BMC Genomics (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Molecular dynamics-guided insight into the adsorption–inhibition mechanism for controlling ice growth/melt of antifreeze protein type IV mutant from longhorn sculpin fish
- Original Paper
- Published: 01 April 2024
Cite this article
- Azadeh Eskandari ORCID: orcid.org/0000-0003-0143-425X 1 , 2 ,
- Thean Chor Leow 1 , 3 , 5 ,
- Mohd Basyaruddin Abdul Rahman 4 &
- Siti Nurbaya Oslan 1 , 2 , 5
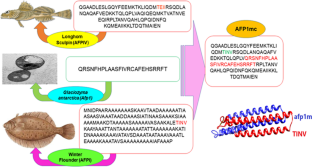
Antifreeze proteins (AFPs) represent a distinctive class of proteins that exist in organisms thriving in sub-zero conditions and act as an inhibitor of ice growth by binding to the ice interfaces. The melting or growing inhibition characterization can explain the adsorption–inhibition mechanism. This mechanism occurred within the thermal hysteresis activity of AFPs and is not amenable to measure experimentally. AFPIV is a newly discovered type of AFPs that exhibit remarkably low activity in inhibiting ice recrystallization. Herein, the novel mutation of AFPIV has been developed through the incorporation of afp1m peptide fused to the AFPIV’s third helix with a newly designed linker. The bioinformatics tools were employed for verification purposes to evaluate and analyze the model. The main focus of the present study pertains to the ice growth inhibition and Kelvin effect associated with the AFPIV mutant (AFP1mc) in comparison with AFPIV at different temperatures. The investigation revealed that in AFP1mc the rate of ice growth in the surrounding area experiences a significant reduction regarding the ice depression point as dictated by the Gibbs–Thomson effect. Moreover, it can be deduced that above the equilibrium melting point, ice melting is inhibited by the formation of the concave ice/water while, below that temperature, the ice growth inhibition observed through the ice water convex formation; however, this mechanism exhibits greater strength in AFP1mc owing to its superior affinity toward ice interaction. These findings provide evidence that the activity of AFP1mc is much higher than the original AFPIV, rendering it competent for additional experimental investigations and practical deployment in AFP contexts.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Data availability
The analysis was exclusively carried out using publicly available data, as specified in the text. Code and the datasets used are made freely available.
Aho N, Buslaev P, Jansen A, Bauer P, Groenhof G (2022) Scalable constant pH molecular dynamics in GROMACS. J Chem Theory Comput 18:6148–6160. https://doi.org/10.1021/acs.jctc.2c00516
Article CAS PubMed PubMed Central Google Scholar
Alaidarous M (2020) In silico structural homology modeling and characterization of multiple N-Terminal domains of selected bacterial Tcps. Peer J 8:e11043. https://doi.org/10.7717/peerj.10143.eCollection2020
Article Google Scholar
Barrett J (2001) Thermal hysteresis proteins. Int J Biochem Cell Biol 33:105–117. https://doi.org/10.1016/s1357-2725(00)00083-2
Article CAS PubMed Google Scholar
Baskaran A, Kaari M, Venugopal G, Manikkam R, Joseph J, Bhaskar P (2021) Antifreeze proteins (Afp) properties, sources and applications. Int J Biol Macromol 189:292–305. https://doi.org/10.1016/j.ijbiomac.2021.08.105
Benkert P, Kunzli M, Schwede T (2009) QMEAN server for protein model quality estimation. Nucleic Acids Res 37:W510–W514. https://doi.org/10.1093/nar/gkp322
Benkert P, Tosatto SCE, Schomburg D (2008) QMEAN: a comprehensive scoring function for model quality assessment. Proteins Struct Funct Bioinform 71:261–277. https://doi.org/10.1002/prot.21715
Article CAS Google Scholar
Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE (2000) The protein data bank. Nucleic Acids Res 28:235–242. https://doi.org/10.1093/nar/28.1.235
Bhattachary M, Hota A, Kar M, Chini DS, Malick RC (2018) In-silico Structural and Functional modeling of Antifreeze protein (AFP) sequences of Ocean pout (Zoarces americanus, Bloch & Schneider 1801). J Genet Eng Biotechnol 16:721–730. https://doi.org/10.1016/j.jgeb.2018.08.004
Bjelkmar P, Larsson P, Cuendet MA, Hess B (2010) Implementation of the CHARMM force field in GROMACS: analysis of protein stability effects from correction maps, virtual interaction sites, and water models. Chem Theory Comput. https://doi.org/10.1021/ct900549r
Combet C, Blanchet C, Geourjon C, Deléage G (2000) NPS@: network protein sequence analysis. Trends Biochem Sci 25:147–150. https://doi.org/10.1016/s0968-0004(99)01540-6
Darden T, York D, Pedersen L (1993) Particle mesh Ewald: an N-log(N) method for Ewald sums in large systems. J Chem Phys 98:10089–10092. https://doi.org/10.1063/1.464397
Davies PL, Baardsnes J, Kuiper MJ, Walker VK (2002) Structure and function of antifreeze proteins. Philos Trans R Soc Lond B 357:927–935. https://doi.org/10.1098/rstb.2002.1081
Deng G, Laursen RA (1998) Isolation and characterization of an antifreeze protein from the longhorn sculpin. Myoxocephalus Octodecimspinosis Biochimica Et Biophysica Acta 1388:305–314. https://doi.org/10.1016/s0167-4838(98)00180-0
DePristo MA, DeBakker PI, Blundell TL (2004) Heterogeneity and inaccuracy in protein structures solved by x-ray. Crystallography J Struc 12:831–838. https://doi.org/10.1016/j.str.2004.02.031
Ebbinghaus S, Meister K, Prigozhin MB, DeVries AL, Havenith M, Dzubiella J, Gruebele M (2012) Functional importance of short-range binding and long-range solvent interactions in helical antifreeze peptides. Biophys J 103(7):L20–L22
Ekpo MD, Xie J, Hu Y, Liu X, Liu F (2022) Antifreeze proteins: Novel applications and navigation towards their clinical application in cryobanking. Int J Mol Sci 23:2639. https://doi.org/10.3390/ijms23052639
Ellis CR, Shen J (2015) pH-dependent population shift regulates BACE1 activity and inhibition. J Am Chem Soc 137:9543–9546. https://doi.org/10.1021/jacs.5b05891
Eskandari A, Leow TC, Rahman MBA, Oslan SN (2020) Antifreeze proteins and their practical utilizations in industry, medicine, and agriculture. Biomol J 10:1649. https://doi.org/10.3390/biom10121649
Fernandez RG, Abascal JLF, Vega C (2006) The melting point of ice ih for common water models calculated from direct coexistence of the solid-liquid interface. J Chem Phys 124:144506. https://doi.org/10.1063/1.2183308
Frenkel D, Smit B (1996) Understanding molecular simulation. Academic Press, San Diego
Google Scholar
Gasteiger, E, Hoogland C, Gattiker A, Duvaud S, Wilkins M, Appel A, Bairoch A (2005) Protein identification and analysis tools on the ExPASy server 112: 531–52. https://doi.org/10.1385/1-59259-584-7:531
Gerhauser J, Gauker V (2021) Detailed analysis of the ice surface after binding of an insect antifreeze protein and correlation with Gibbs-Thomson equation. Langmuir 37:11716–11725. https://doi.org/10.1021/acs.langmuir.1c01620
Ghalamara S, Silva S, Brazinha C, Pintado M (2022) Structural diversity of marine anti-freezing proteins, properties and potential applications. Bioresour Bioprocess. https://doi.org/10.1186/s40643-022-00494-7
Gharib G, Saeidiharzand SH, Sadaghian A, Kosar A (2022) Antifreeze proteins: a tale of evolution from origin to energy application. Front Bioeng Biotechnol 9:770588. https://doi.org/10.3389/fbioe.2021.770588
Article PubMed PubMed Central Google Scholar
Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJ (2015) The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc 10:845–858. https://doi.org/10.1038/nprot.2015.053
Kim HJ, Lee JH, Hur YB (2017) Marine antifreeze protein: structure and application to cryopreservation as a potential cryoprotectant. Mar Drugs 15:27. https://doi.org/10.3390/md15020027
Knight CA, Wierzbicki A (2001) Adsorption of biomolecules to ice and their effects upon ice growth. 2. A discussion of the basic mechanism of “antifreeze” phenomena. Cryst Growth Des 1:439–446. https://doi.org/10.1021/cg015532l
Koyama Y, Tanaka H, Gao G, Zeng XC (2004) Melting points and thermal expansivities of proton-disordered hexagonal ice with several model potentials. J Chem Phys 121:7926. https://doi.org/10.1063/1.1801272
Kristiansen E, Zachariassen KE (2005) The mechanism by which fish antifreeze proteins cause thermal hysteresis. Cryobiology 51:262–280. https://doi.org/10.1016/j.cryobiol.2005.07.007
Kuiper M, Morton CM, Abraham M, Weale A (2015) The biological function of an insect antifreeze protein simulated by molecular dynamics. Struct Biol Mol Biophys 7:4e05142. https://doi.org/10.7554/eLife.05142
Kulieva V, Zamora W, Martinez E, Tirado L (2022) Effect of antifreeze proteins on the freeze-thaw cycle of foods: fundamentals, mechanism of action, current challenges and recommendations for future work. Heliyon 8:e10973. https://doi.org/10.1016/j.heliyon.2022.e10973
Kumari S, Muttachikavil AV, Tiwari JK, PunnathanaM SN (2020) Computational study of differences between antifreeze activity of Type III antifreeze protein from ocean pout and its mutant. Langmuir 36:2439–2448. https://doi.org/10.1021/acs.langmuir.0c00065
Kurniawan J, Ishida T (2022) Protein model quality estimation using molecular dynamics simulation. ACS Omega 7:24274–24281. https://doi.org/10.1021/acsomega.2c01475
Lovell SC, Davis IW, Arendall WB, De Bakker PI et al (2003) Structure validation by C-alpha geometry: phi, psi and C-beta deviation. Proteins J 50:437–450. https://doi.org/10.1002/prot.10286
Lee H (2018) Structures, dynamics, and hydrogen-bond interactions of antifreeze proteins in TIP4P/Ice water and their dependence on force fields. PLoS ONE 13:e0198887. https://doi.org/10.1371/journal.pone.0198887
Maddah M, Shahrabi M, Peyvandi K (2021) How does DcAFP , a plant antifreeze protein, control ice inhibition through the Kelvin effect? Ind Eng Chem Res 60:18230–18242. https://doi.org/10.1021/acs.iecr.1c02559
Madura JD, Wierzbicki A, Harrington JP, Maughon RH, Raymond JA, Sikes CS (1994) Interactions of the D and L forms of winter flounder antifreeze peptide with the 201 planes of ice. J Am Chem Soc 116:417–418. https://doi.org/10.1021/ja00080a066
Marilene SR, Chirle F, Joao G, Edna RA (2019) Effect of the antifreeze protein on microstructure of strawberries. Food Technol 22:9. https://doi.org/10.1590/1981-6723.21818
Melo F, Feytmans E (1998) Assessing protein structures with a non-local atomic interaction energy. J Mol Biol 277:1141–1152. https://doi.org/10.1006/jmbi.1998.1665
Midya US, Bandyopadhyay S (2018) Operation of Kelvin effect in the activities of an antifreeze protein: a molecular dynamics simulation. J Phys Chem B 1:1–31. https://doi.org/10.1021/acs.jpcb.8b00846
Mofiz Uddin Khan NM, Atari T, Tsuda S, Kondo H (2021) Characterization of microbial antifreeze protein with intermediate activity suggests that a bound-water network is essential for hyperactivity. Sci Rep 11:5971. https://doi.org/10.1038/s41598-021-85559-x
Naing A, Kim CH (2019) A brief review of applications of antifreeze proteins in cryopreservation and metabolic genetic engineering. 3 Biotech J 9:329. https://doi.org/10.1007/s13205-019-1861-y
Nguyen H, Le L (2017) Investigation of changes in structure and thermodynamic of spruce budworm antifreeze protein under subfreezing temperature. Sci Rep 7:40032. https://doi.org/10.1038/srep40032
Nguyen H, Le L, Ho TB (2014) Computational study on ice growth inhibition of Antarctic bacterium antifreeze protein using coarse grained simulation. J Chem Phys 140:225101. https://doi.org/10.1063/1.4881895
Pakhrin SC, Sherstha B, Adhikari B, Dukka B (2021) Deep learning-based advances protein structure prediction. Int J Mol Sci 22:5553. https://doi.org/10.3390/ijms22115553
Pal P, Aich R, Chakraborty S, Jana B (2022) Molecular factors of ice growth inhibition for hyperactive and globular antifreeze proteins: insight from molecular dynamics simulation. Langmuir 38:15132–15144. https://doi.org/10.1021/acs.langmuir.2c02149
Pettersen EF, Goddard TD et al (2004) UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem 25:1605–1612. https://doi.org/10.1002/jcc.20084
Raymond JA, DeVries AL (1977) Adsorption inhibition as a mechanism of freezing resistance in polar fishes. Proc Natl Acad Sci USA 74:2589–2593. https://doi.org/10.1073/pnas.74.6.2589
Rifai EA, Dijk MN, Vermeulen PE, Yanuar A, Geerke DP (2019) A comparative linear interaction energy and MM/PBSA study on SIRT1–ligand binding free energy calculation. JCIM 59:4018–4033. https://doi.org/10.1021/acs.jcim.9b00609
Robles V, Valcarce DG, Riesco MF (2019) The use of antifreezeproteins in cryopreservation of gametes and embryos. Biomolecules 9:181. https://doi.org/10.3390/biom9050181
Schauperl M, Podewitz M, Ortner T, Waibl F (2017) Balance between hydration enthalpy and entropy is important for ice binding surfaces in antifreeze protein. Scientific Rep 7:11901. https://doi.org/10.1038/s41598-017-11982-8
Schwede T (2003) Swiss-model: an automated protein homology-modeling server. Nucleic Acids Res 31:3381–3385. https://doi.org/10.1093/nar/gkg520
Shah SH, Rajv K, Azren A, Asmavi A et al (2012) Solution structure, Dynamic and ice growth inhibitory activity of peptide fragments Derived from an Antarctic yeast protein. PLoS ONE 7:e49788. https://doi.org/10.1371/journal.pone.0049788
Sheikh M, Tsuda S (2018) Application of antifreeze proteins: practical use of the quality products from Japanese fishes. Adv Exp Med Biol 1081:321–337. https://doi.org/10.1007/978-981-13-1244-1_17
Steinhardt PJ, Nelson DR, Ronchetti M (1983) Bond-orientational order in liquids and glasses. Phys Rev B 28:784–805. https://doi.org/10.1103/PhysRevB.28.784
Suros-Valls R, Votes IK (2019) Peptidic antifreeze materials: prospects and challenges. Int J Mol Sci 20:5149. https://doi.org/10.3390/ijms20205149
Thomson SW (1871) On the equilibrium of vapor at a curved surface of liquid. Phil Mag 4:448–452
Vega C, Sanz E, Abascal JLF (2005) The melting temperature of the most common models of water. J Chem Phys 122:114507. https://doi.org/10.1063/1.1862245
Wang C, Pakhomova S, Newcomer ME, Christner BC, Luo BH (2017) Structural basis of antifreeze protein activity of a bacterial multi-domain antifreeze protein. PLoS ONE 12:1–19. https://doi.org/10.1371/journal.pone.0187169
Wang J, Yoo S, Bai J, Morris JR, Zeng XC (2005) Melting temperature of ice ih calculated from coexisting solid-liquid phases. J Chem Phys 123:36101. https://doi.org/10.1063/1.1950647
Waterhouse A, Bertoni M, Bienert S, Studer G, Tauriello G et al (2018) SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res 46:768. https://doi.org/10.1093/nar/gky427
Wathen B, Kuiper M, Walker V, Jia Z (2003) A new model for simulating 3-D crystal growth and its application to the study of antifreeze proteins. J Am Chem Soc 125:729–737. https://doi.org/10.1021/ja0267932
Wen D, Laursen RAA (1992) A model for binding of an antifreeze polypeptide to ice. Biophys J 63:1659–1662. https://doi.org/10.1016/S0006-3495(92)81750-2
Wilson P (1993) Explaining thermal hysteresis by the Kelvin effect. Cryo-Lett 14:31–36
Wu X, Yao F, Zhang H, Li J (2021) Antifreeze proteins and their biomimetic for cell cryopreservation, mechanism, function and application. Int J Biol Macromol 192:1276–1291. https://doi.org/10.1016/j.ijbiomac.2021.09.211
Zandiyeh S, Ebrahimi F, Sabbaghian M (2018) Application of antifreeze protein on sperm cryopreservation. Crimson Publ 1:22–34. https://doi.org/10.31031/PRM.2018.01.000520
Download references
Acknowledgements
The authors are thankful to the members of the Enzyme and Microbial Technology Research Center (EMTech) for the constructive comments and help in the completion of this manuscript.
This project was supported by the Prototype Research Grant Scheme (PRGS) from the Ministry of Higher Education (MoHE) Malaysia PRGS/1/2021/STG02/UPM/02/2 which was awarded to the last author (SNO).
Author information
Authors and affiliations.
Enzyme and Microbial Technology Research Centre, Universiti Putra Malaysia (UPM), 43400, Serdang, Selangor, Malaysia
Azadeh Eskandari, Thean Chor Leow & Siti Nurbaya Oslan
Department of Biochemistry, Faculty of Biotechnology and Biomolecular Sciences, Universiti Putra Malaysia (UPM), 43400, Serdang, Selangor, Malaysia
Azadeh Eskandari & Siti Nurbaya Oslan
Department of Cell and Molecular Biology, Faculty of Biotechnology and Biomolecular Sciences, Universiti Putra Malaysia (UPM), 43400, Serdang, Selangor, Malaysia
Thean Chor Leow
Department of Chemistry, Faculty of Science, Universiti Putra Malaysia (UPM), 43400, Serdang, Selangor, Malaysia
Mohd Basyaruddin Abdul Rahman
Enzyme Technology and X-Ray Crystallography Laboratory, VacBio 5, Institute of Bioscience, Universiti Putra Malaysia (UPM), 43400, Serdang, Selangor, Malaysia
Thean Chor Leow & Siti Nurbaya Oslan
You can also search for this author in PubMed Google Scholar
Contributions
EA, TCL, MBAR, and SNO wrote original preparation, EA, TLC, MBAR and SNO wrote abstract, prepared tables, and all figures, and EA and SNO edited the manuscript. All authors have reviewed and agreed to the published version of the manuscript.
Corresponding author
Correspondence to Siti Nurbaya Oslan .
Ethics declarations
Conflict of interest.
The authors declare no conflict of interest.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.

Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Eskandari, A., Leow, T.C., Rahman, M.B.A. et al. Molecular dynamics-guided insight into the adsorption–inhibition mechanism for controlling ice growth/melt of antifreeze protein type IV mutant from longhorn sculpin fish. Chem. Pap. (2024). https://doi.org/10.1007/s11696-024-03407-4
Download citation
Received : 08 November 2023
Accepted : 07 March 2024
Published : 01 April 2024
DOI : https://doi.org/10.1007/s11696-024-03407-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Novel mutant
- Ice growth rate inhibition
- Kelvin effect
- MD simulation
- Find a journal
- Publish with us
- Track your research
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.8(10); 2022 Oct

Effect of antifreeze proteins on the freeze-thaw cycle of foods: fundamentals, mechanisms of action, current challenges and recommendations for future work ☆
Vicente amirpasha tirado-kulieva.
a Facultad de Ingeniería de Industrias Alimentarias y Biotecnología, Universidad Nacional de Frontera, Peru
William Rolando Miranda-Zamora
Ernesto hernández-martínez.
b Facultad de Ingeniería, Universidad Nacional de Jaén, Peru
Lucia Ruth Pantoja-Tirado
c Carrera Profesional de Ingeniería en Industrias Alimentarias, Universidad Nacional Autónoma de Tayacaja Daniel Hernández Morillo, Peru
Delicia Liliana Bazán-Tantaleán
Ever william camacho-orbegoso.
d Facultad de Ingeniería y Gestión, Universidad Nacional Tecnológica de Lima Sur, Peru
Associated Data
No data was used for the research described in the article.
Freezing is widely used in food preservation, but if not carried out properly, ice crystals can multiply (nucleation) or grow (recrystallization) rapidly. This also affects thawing, causing structural damage and affecting overall quality. The objective of this review is to comprehensively study the cryoprotective effect of antifreeze proteins (AFPs), highlighting their role in the freeze-thaw process of food. The properties of AFPs are based on their thermal hysteresis capacity (THC), on the modification of crystal morphology and on the inhibition of ice recrystallization. The mechanism of action of AFPs is based on the adsorption-inhibition theory, but the specific role of hydrogen and hydrophobic bonds/residues and structural characteristics is also detailed. Because of the properties of AFPs, they have been successfully used to preserve the quality of a wide variety of refrigerated and frozen foods. Among the limitations of the use of AFPs, the high cost of production stands out, but currently there are solutions such as the use the production of recombinant proteins, cloning and chemical synthesis. Although in vitro, in vivo and human studies have shown that AFPs are non-toxic, their safety remains a matter of debate. Further studies are recommended to expand knowledge about AFPs, to reduce costs in their large-scale production, to understand their interaction with other food compounds and their possible effects on the consumer.
AFP; ISP; IBP; Food cryopreservation; Freeze-thaw; Recrystallization; Thermal hysteresis.
1. Introduction
Freezing is one of the most widely used processes in food preservation. Despite the advantages of this technique, it can generate large ice crystals due to poor operating conditions ( Chen et al., 2021a ). This causes changes in food texture, induces lipid oxidation, protein denaturation and enzyme activation ( Nian et al., 2020 ). It also causes cell wall rupture, structural damage and, finally, cell lysis ( Dalvi-Isfahan et al., 2019 ). Zhang et al. (2022) evaluated the effect of different freezing conditions (−6 to −30 °C for 10–30 days) on the quality of wheat gluten protein in unfermented doughs. As the temperature and storage time increased, the solubility, foamability and water holding capacity of the protein decreased. Damage to the gluten structure was proportional to the number and size of ice crystals; this affected sensory, physical and nutritional characteristics of the products during thawing ( Song et al., 2019 ; Zhang et al., 2020 ). During storage of star fruit at −20 °C for 90 days, Provesi et al. (2019) noted that there was considerable drip loss due to the fact that during thawing, some of the water in the food escaped to the outside. Freeze-thaw (F-T) cycles resulted in severe cooking losses, centrifugal losses, reduced amino acid composition, and structural damage in fish Trachurus murphyi . The impact was proportional to the size of the ice crystals formed ( Hu and Xie, 2021 ).
To solve this problem, it is necessary to know the F-T process and to investigate the application of cryoprotective additives ( Chen et al., 2021a ). The most commonly used are glycerol, salt, sorbitol, trehalose and sucrose ( Eskandari et al., 2020 ). However, the sweetness of sugars is undesirable for many foods and for people who are intolerant or have diabetes. In addition, these cryoprotectants are used in high concentrations ( Rosa et al., 2019 ). The need arises to use a viable agent such as antifreeze proteins (AFPs), which are healthier, provide a better cryoprotective effect and in lower concentrations ( Provesi et al., 2019 ). AFPs have been studied in the medical, pharmaceutical, biotechnological and, mainly, food industries ( Eskandari et al., 2020 ; Ustun and Turhan, 2020 ). Specifically, AFPs are widely used to protect the integrity of frozen foods by slowing the development of ice crystals at sub-zero temperatures ( Gün et al., 2020 ). The use of AFPs also encompasses refrigerated foods ( Tian et al., 2020 ).
It is still a challenge to establish a technique to control the size and shape of crystals produced during freezing and their stabilization during thawing. The existing literature on AFPs is scarce and there are many gaps on their application in food products. In this regard, this study will highlight the most significant applications of AFPs in frozen and refrigerated foods. First, the F-T process will be described and the fundamentals/characteristics of the AFPs will be detailed in order to better understand the subject. To fill another knowledge gap, emphasis will be placed on the different mechanisms of action. The specific damages of the F-T process in different foods will also be described. Finally, some limitations of the technique, alternative solutions and recommendations for future work will be mentioned.
2. Freeze-thaw process: water as a key element
Water, as the main compound of food, directly influences its quality depending on the processes to which it is exposed. In this context, water plays a fundamental role in the F-T process and if not properly managed, it could be lethal for the food ( Białkowska et al., 2020 ).
Knowing the interaction of water (activity, mobility and distribution) in refrigerated and frozen foods is relevant to understand the basis of their quality and stability ( Kontogiorgos et al., 2008 ; Ding et al., 2015 ). During freezing, water is mobilized, molecules are organized and form more crystals (nucleation) ( Chen et al., 2021a ). This produces the internal expansion of the food, causing the rupture of its membranes and cell walls ( Provesi and Amante, 2015 ). In addition to nucleation, recrystallization of ice can also occur; it consists of increasing the amount of free water and inducing larger crystals by melting the smaller crystals (because they are thermodynamically unstable). This causes changes in the morphology, shape, orientation and distribution of the crystals ( Kong et al., 2016 ; Eskandari et al., 2020 ). Recrystallization occurs due to temperature variation, and its speed increases at temperatures below the freezing point (FP) ( Chen et al., 2021b ).
Fennema et al. (1973) classified recrystallization according to the mechanisms that induce it: accretion, migration, surface isomass, irruption and pressure induction. Regarding frozen foods, the authors mention that the first three mechanisms are those that induce recrystallization. According to Zhu et al. (2019) , accretion is the melting of small crystals in adjacent zones; in migration, due to temperature fluctuation, the liquid generated by the melted crystals flows into the large crystals; isomass refers to the fact that, in order to be thermodynamically stable, the crystal surface becomes sharper and smoother.
To avoid the formation of large crystals and the impact on thawing, where undesirable physical, chemical and microbiological changes also occur, the process should be carried out at very low temperatures (<−20 °C) ( Calderara et al., 2016 ). Throughout the food chain, conditions such as relative humidity and temperature must be controlled. In addition, the use of safe and effective cryoprotectants is recommended.
Another factor related to recrystallization is the glass transition temperature (Tg); temperature at which the thermodynamic pseudotransition occurs irreversibly. It is suggested that, during storage, the temperature should be lower than the Tg of the water because at a higher temperature, water has greater mobility, acquiring greater speed in increasing the number and size of crystals ( Ustun and Turhan, 2020 ).
3. Fundamentals of antifreeze proteins
3.1. origin and history.
Antifreeze activity (AFA), later determined to be a product of AFPs, was first observed in Antarctic fish by Scholander et al., in 1957, and in the hemolymph from Tenebrio molitor in 1964 by Ramsay ( Ramløv and Johnsen, 2014 ). Since then, AFPs have also been discovered (but structurally different in most cases) in nematodes, amphibians, algae ( Eskandari et al., 2020 ), plants, insects, spiders ( Kristiansen, 2020 ), bacteria, fungi, ciliates, diatoms ( Baskaran et al., 2021 ), and lichens ( Crevel et al., 2007 ). These organisms are usually exposed to cold environments and therefore, they acquired the necessary tolerance for their survival, avoiding the usual damages that occur in sub-zero environments ( Xiang et al., 2020 ). Among other sources of AFPs, promising cases of recombinant AFPs expressed in Escherichia coli , Lactococcus Lactis and Pichia pastoris were also reported ( Liu et al., 2018a ).
AFPs are also known as ice structuring proteins (ISPs), ice-binding proteins (IBPs) or thermal hysteresis proteins (THPs) ( Liu et al., 2018b ; Ding et al., 2020 ; Eskandari et al., 2020 ). They are peculiar compounds that adhere to ice, specifically at the water-ice interface ( Chen et al., 2021a ). In this way, AFPs interfere with the speed of crystal growth, shape and orientation, decreasing the damage to the structure of the organism.
3.2. Types of antifreeze proteins
From the structural point of view and according to their composition, there are AFPs of type I, II, III and IV, antifreeze glycoproteins (AFGPs) and hyperactive AFPs ( Boonsupthip and Lee, 2003 ; Tejo et al., 2020 ). The first five types are referred to as moderately active AFPs ( Cao et al., 2021 ). These have an AFA or difference between the melting point (MP) and the hysteresis freezing point (HFP) of 1–2 °C in fish ( Ramløv and Johnsen, 2014 ; Damodaran and Wang, 2017 ), 0.2–0.4 °C in plants ( Ding et al., 2020 ), 0.2–0.5 °C in insects ( Ustun and Turhan, 2020 ), and 0.1–0.35 °C in bacteria ( Griffith and Ewart, 1995 ). Hyperactive AFPs present in insects have different AFA values, from 8 °C ( Ramløv and Johnsen, 2014 ), ≤6 °C at low concentrations ( Cao et al., 2021 ), 10 to 100 times more AFA than active AFPs ( Gün et al., 2020 ). AFPs from insects have the greatest potential as an antifreeze agent; plants have low AFA, but have higher ice recrystallization inhibition (IRI) activity ( Gruneberg et al., 2021 ). The IRI activity of the plants is so high that a 100 to 500 times lower concentration is required to exert the effect, compared to the concentration required for AFA ( Ding et al., 2020 ). It was reported that a solution with AFPs from fish could cause negative impacts when added to food because the crystals take the form of needles or spicules, which is sensorily undesirable. In contrast, crystals generated in a solution with AFPs from insects have a more rounded and pleasing shape ( Ramløv and Johnsen, 2014 ). This morphological irregularity is due to the fact that AFPs from fish are used in high concentrations due to their low AFA potential ( Griffith and Ewart, 1995 ).
Another difference between moderately active and hyperactive AFPs is with respect to growth of ice crystals. First, to understand the morphology of ice crystals, they have three symmetrical a -axis and a perpendicular c -axis, called prismatic and basal plane, respectively ( Provesi and Amante, 2015 ). With respect to moderate AFPs, crystal growth is parallel to the c -axis. They do not cover the basal planes, morphologically achieving a crystal with the shape of a hexagonal bipyramid ( Kong et al., 2016 ). In contrast, hyperactive AFPs also cover the basal planes, providing complete protection ( Cao et al., 2016 ). A graphical representation is shown in Figure 1 . Khan et al. (2021) characterized AFPs with different activity from the fungus Typhula ishikariensis . When evaluating the crystal structure, all AFPs presented a bound-water network on the surface of the ice-binding site. However, the water network of moderately active AFPs was not as wide, which prevented their expansion in all planes of the ice crystal, unlike hyperactive AFPs.

Difference between the effect of the integration of moderate and hyperactive AFPs on the morphological modification of ice crystals.
3.3. Properties
AFPs have mainly THC, IRI and morphological modification of ice crystals, and they form the biological antifreeze mechanism ( Liu et al., 2018b ; Białkowska et al., 2020 ; Ustun and Turhan, 2020 ). The action as a whole is detailed later, but to clarify the terms, TH is the difference between MP and FP and is used to measure the activity of the AFPs ( Tian et al., 2020 ). HFP is the point at which ice growth begins spontaneously; the difference between HFP and MP is known as hysteresis activity ( Zhan et al., 2018 ).
AFPs kinetically depress the growth temperature of ice crystals, reducing the freezing temperature, but not the melting temperature ( Nian et al., 2020 ). This state is known as the supercooling phase, in which water maintains its liquid state in a specific temperature range ( Calderara et al., 2016 ). The decrease in PF is due to the saturation of the non-colligative activity of AFPs which is 300–500 times higher than compounds with colligative property such as ethylene glycol ( Das et al., 2018 ) and without the need to increase the concentration.
3.4. Mechanisms of action
All AFPs share the same adsorption-inhibition mechanism, whose freezing point suppression is explained by the Kelvin effect ( Meister et al., 2019 ). Thus, as AFPs adsorb on the crystal, the curvature of the crystal surface and vapor pressure increase, while the droplet radius decreases until equilibrium is reached at the water-ice interface ( Kontogiorgos et al., 2008 ). There is evidence that AFPs also provide additional protection to membranes by preventing thermotropic phase changes and preventing leakage by blocking ion channels ( Chattopadhyay, 2007 ).
In general, in the absence of AFPs ( Figure 2 a), nucleation and/or recrystallization is inevitable at 0 °C. In the presence of AFPs ( Figure 2 b), their interaction with ice crystals occurs as follows: a) adhesion of the AFPs on the crystals; b) decrease of FP; c) prevention of the nucleation and recrystallization (even at sub-zero temperatures). This mechanism of action is considered as the adsorption-inhibition theory ( Tian et al., 2020 ). However, this process is not permanent since, even if AFPs are present in the solution (or matrix), if the temperature decreases to a certain point, crystal growth will inevitably and violently occur ( Ramløv and Johnsen, 2014 ).

Nucleation and recrystallization in a) absence of AFPs, at T = 0 °C and, b) presence of AFPs, even at T < 0 °C.
Specifically, different mechanisms of action of AFPs have been proposed. Lately, the influence of hydrophobic residues and hydrogen bonding groups has been hypothesized. Precisely, according to Tian et al. (2020) , the ice-binding surface of AFPs possesses TxT repeat domains, whose methyl (hydrophobic) and hydroxyl groups play a key role in ice binding. This paradox is called affinity-specificity ( Kumari et al., 2020 ). It was reported that, the binding between AFPs-I and the ice surface is due to hydrogen bonding ( Kumari et al., 2020 ). This hypothesis was ruled out in the study by Zhang and Laursen (1998) , who, despite substituting serine (capable of forming hydrogen bonds) by threonine residues of AFP-I from winter flounder, AFA decreased. On the other hand, the substitution of threonine residues by valine did not modify the AFA of AFP-I from winter flounder by retaining the methyl (hydrophobic) groups ( Chao et al., 1997 ). Similarly, replacing larger hydrophobic groups with smaller hydrophobic groups decreased the AFA of recombinant AFP-III ( Baardsnes and Davies, 2002 ). This is still under investigation. Hudait et al. (2019) carried out molecular simulations to elucidate the role of hydrophilic and hydrophobic AFPs groups of Tenebrio molitor . The methyl group hardens the ice binding site, slows the water mobility and stabilizes the clathrate-like water in the anchored clathrate motif that binds AFPs to ice. Hydrogen bonds act on the anchor the clathrate-like water, slowing down water dynamics. Mochizuki and Molinero (2018) evaluated the binding mechanism between AFPs and ice. By molecular simulation, it was determined that the key role in ice binding and inhibition is played by the segregation of hydrophobic and hydrophilic groups of the PPII helix of AFPs. The methyl groups and disaccharides of the AFPs also act in the aforementioned mechanism, but to a lesser extent.
In a simulation study, Kumari et al. (2020) evaluated the mechanism of AFA of AFP-III expressed in Zoarces americanus . AFPs bound at the water-ice interface due to hydrophobic hydration caused by the formation of clathrate structures of water molecules near the ice-binding surface. After adsorption, it was determined that, any surface of AFPs can bind to ice by hydrogen bonds. Similar results were shown experimentally and by simulation in the study by Gandini et al. (2020) . They determined that the affinity between AFPs and the ice surface was mainly for hydrophobic residues.
Although hydrophobic interaction significantly influences the adsorption of AFPs at the water-ice interface, hydrogen bonding interactions are also necessary. Lee (2019) simulated ice binding of AFPs-I from Tenebrio molitor rich in threonine, which showed affinity only to the primary plane. Threonine was replaced by alanine, achieving binding to the secondary plane. The more hydrophobic residues there were, the higher the binding affinity to ice. However, to some extent, the more the threonine (hydrogen bond former) was substituted, the affinity decreased. These findings indicated that hydrophobic residues play the main role in AFP-ice binding, but hydrophilic residues are also necessary.
The role of other factors in the antifreeze mechanism of AFPs has also been evaluated. Surís-Valls and Voets (2019) elucidated the influence of structural characteristics on AFPs activity. The following were mentioned: a) α-helical AFPs (expressed in several fish species); b) β-strand AFPs (insects, bacteria and plants); c) AFGPs (blood serum of Antarctic notothenioids and northern cod); d) polyproline II containing AFPs (insect AFPs without α/β-helices, such as snow flea AFPs).
4. Antifreeze proteins as cryoprotective agents for foods
The proper management of crystal formation and growth conditions in different different frozen foods is a challenge that persists to this day. If the F-T process has been optimal, food spoilage can also occur in storage, due to biochemical reactions such as lipid oxidation, denaturation, oxidation and aggregation of proteins, degradation of vitamins and pigments ( Nian et al., 2020 ; Chen et al., 2021a ; Zhu et al., 2021b ). This affect the acceptance of the food in the market ( Tan et al., 2018 ; Cheng et al., 2019 ); causing significant economic losses.
AFPs are a natural, safe and effective alternative as a food additive ( Kashyap et al., 2020 ). Their use in preservation during refrigeration, freezing, storage, transport and thawing of the product is emphasized ( Tejo et al., 2020 ). By delaying recrystallization, AFPs help to avoid loss of texture (more noticeable in products consumed raw) and loss of volatile components ( Kashyap et al., 2020 ). They prevent or reduce protein deterioration, cell damage, dripping (on thawing), and water holding capacity ( Cai et al., 2020 ). AFPs also maintain organoleptic characteristics ( Xiang et al., 2020 ), demonstrating a high potential for extending the shelf life of refrigerated and frozen products.
It is worth mentioning that the integration of AFPs into food matrices can be by direct physical processes or by genetic transfer ( Ustun and Turhan, 2020 ). If AFPs are transmitted genetically, the offspring of the organism will also be resistant to low temperatures ( Chen et al., 2021b ). This would be promising; however, for the success of the process, a number of hurdles must be overcome.
AFPs are used in concentrations below approximately 100 μg/L ( Provesi and Amante, 2015 ). Their application in food provides benefits in physical and chemical characteristics and also presents antimicrobial properties ( Griffith and Ewart, 1995 ; Lee et al., 2015 ). Promising results were obtained for fruits and vegetables ( Crevel et al., 2007 ), frozen dough, meats, fish ( Nian et al., 2020 ), cereals ( Tian et al., 2020 ), powdered sugar, milk ( Ustun and Turhan, 2020 ), ice cream, yogurt ( Zhan et al., 2018 ), sorbets, frozen custard, fruit purees ( Das et al., 2018 ), gelatin, noodles, boiled eggs, and tofu ( Eskandari et al., 2020 ).
AFPs (2%) were added to quick-frozen pork patties to evaluate their effect on myofibrillar proteins. Surface roughness decreased by 9.7% at 180 days of freezing, carbonyl content decreased and free amino acids increased by 32 and 14.99%, respectively. In addition, the authors indicated that the structural stability of the myofibrillar proteins in the patties was maintained ( Li et al., 2021 ). Similarly, the carbonyl content in mirror carp subjected to F-T was 16.5% lower with the addition of AFPs (2 g/L). After five F-T cycles, the structural deterioration was ≈13.64% lower ( Du et al., 2021 ). AFPs (0.015, 0.2 and 0.3%) from cold-acclimated wheatgrass juice were added to solutions of pure water, sugar, salt, lipids and commercial Italian pasta sauces. The freezing time of all samples was reduced by 20%, showing the same effect on thawing, especially in the tomato sauce ( Calderara et al., 2016 ). Class IV chitinase as an AFPs (0.1 mg/mL in 9% (w/v) saline solution) from Hippophae rhamnoides were applied to green beans. Reduced electrolytic leakage and drip loss, aiding in structural cryopreservation of green beans, as well as maintaining their volatile components after thawing ( Kashyap et al., 2020 ). Kashyap and Kumar (2022) immersed fresh green peas in a 9% (w/v) saline solution with 0.1 mg/mL of extract of Hordeum vulgare rich in AFPs. Treated peas had 5% less drip loss after thawing; loss of vitamin B6 was reduced from 32 to 30%, loss of vitamin B2 from 20 to 10% and loss of vitamin C from 16 to 5%. Impregnation of Patinopecten yessoensis adductor muscles in AFPs solution showed higher moisture retention, lower loss by centrifugation and by cooking during F-T cycles. Improved springiness, chewiness, hardness and shear force were also observed. When the morphology of the ice crystals was evaluated, they were more rounded in the experimental group ( Shi et al., 2022 ).
AFPs have also been used in confectionery. AFPs-III were applied to truffles, which after thawing maintained their consistency by inhibiting recrystallization ( Derossi et al., 2015 ). The products with the highest application of AFPs in the last decade are dough, fruits and vegetables ( Table 1 ), and to a lesser extent, meat, and fish.
Table 1
Some studies on the use of AFPs to maintain the quality of various refrigerated and frozen foods.
Note: In the case of doughs and related products, the percentage of AFPs is with respect to the overall formulation of the product and not on the basis of the amount of flour, which is usually considered. N.S.: Not specified.
4.1. Meat and fish
The F-T cycle causes severe damage to refrigerated and frozen meat and fish due to large ice crystals affecting their tissues and cell walls ( Kong et al., 2016 ). Ice crystals can cause water loss and/or leakage of reactive species; causing protein oxidation, degradation and aggregation, and lipid oxidation resulting in texture alteration, undesirable changes in taste, color and odor, and loss of nutrients ( Egelandsdal et al., 2019 ; Zhu et al., 2021b ). Lan et al. (2020) subjected Litopenaeus vannamei to eight F-T cycles and evaluated textural properties and sensory acceptability. From cycle 0 to cycle 8 of F-T, hardness/g decreased from 625.13 to 303.27, springiness decreased from 0.861 to 0.623, chewiness decreased from 265.32 to 84.93, and the sensory score was drastically reduced from 30.00 to 9.92. In addition, abrupt temperature fluctuations and the resulting structural damage to muscle cells affect the taste and odor of meat and fish. This is due to the formation of malondialdehyde, carbonyl compounds and the release of pro-oxidative factors ( Wang et al., 2021a ). For example, due to the F-T process subjected to pork, Wu et al. (2021) indicate that heterocyclic aromatic amines and malonaldehyde were produced.
The property of inhibiting recrystallization by AFPs in products influences the post-mortem stage during refrigeration, freezing, thawing or storage ( Ding et al., 2015 ) and also the pre-mortem stage ( Feeney and Yeh, 1998 ). Wang et al., 2021a , Wang et al., 2021b incorporated AFPs (0.2%) from winter wheat into frozen pork patties. After five F-T cycles, the treated patties had 3.84% more hardness and 10.61% more springiness than the untreated samples. Structural damage and moisture migration were significantly lower, resulting in a 43.64% reduction in thawing loss. Regarding oxidation, the treated patties had 25% less thiobarbituric acid reactive substance (TBARS) and 32% less carbonyl concentration. According to Tan et al. (2021) , large ice crystals cause discoloration of meat and fish. In the study by Jiang et al. (2019) , as the number of F-T cycles increased, greater discoloration was observed in salted tuna meat.
In the first reported study on the application of AFPs in meat and/or fish, AFGPs (0.1 mg/mL) from Dissostichus mawsoni were added to chilled and frozen bovine muscle for 3 days. There was a significant reduction in crystal size in all samples, but only under refrigerated conditions ( Payne et al., 1994 ). The same source and proportion of AFPs were used in lambs 24 h before slaughter. Muscles were extracted, frozen and stored. During thawing, crystal size and drip loss were reduced ( Payne and Young, 1995 ). A reduction in drip loss after thawing and an increase in the sensory evaluation score with respect to the level of juiciness in frozen meat was achieved after applying a 0.045 g/mL of recombinant AFPs-I produced by Lactobacillus Lactis ( Yeh et al., 2009 ). Lee et al. (2015) focused on other evaluations of Korean beef with the application of recombinant AFPs (5 ng/mL) from Glaciozyma sp . The results indicated that there was a delay in lipid peroxidation and microbial growth rate during storage prior to freezing. In addition, it increased glutathione activity and the activity of glutathione S-transferase, glutathione peroxidase, catalase and superoxide dismutase.
Regarding the use in fish, the addition of AFPs (0.1%) from Clupea harengus in sea bream improved viscoelasticity of the myofibrillar protein and the stability of its structure. Protein denaturation and F-T cycle damage were also reduced ( Cai et al., 2020 ). Micropterus salmoides was immersed in a saline solution of sodium chloride (9% w/v), trehalose (0.1 M) and AFPs-II (0.1%) from Clupea harengus . By preventing recrystallization, mechanical damage and oxidation are avoided ( Nian et al., 2020 ). The effect of AFPs (0.2%) on Cyprinus carpio during F-T cycles was also evaluated. After three F-T cycles, by preventing moisture migration, weight loss by centrifugation, thawing and cooking was reduced by 43.3%, 57.3% and 27.6%, respectively. In addition, oxidative damage was reduced by reducing the carbonyl content by 16.7% and the TBARS concentration by 21.5% ( Du et al., 2020 ). In Cyprinus carpio , AFPs (0.2%) decreased 17.5% of the ice crystal diameter in the third F-T cycle and 16.5% of the carbonyl in the fifth cycle. The content of free amines was also reduced, preventing oxidative deterioration and keeping the structure of the fish ( Du et al., 2021 ).
The application of AFPs-III (50 g/L) in the actomyosin gel of tilapia hybrids helped preserve Ca 2+ ATPase activity during freezing, remaining after 3 days of storage ( Boonsupthip and Lee, 2003 ). Similar results were shown when AFPs from collagen were applied to frozen surimi ( Chen et al., 2021b ).
4.2. Fruits and vegetables
Refrigeration and freezing are widely used to preserve the quality of perishable foods such as fruits and vegetables, such as strawberries, cherries ( Kong et al., 2017 ), raspberries, blueberries, cucumbers and zucchini ( Tian et al., 2020 ); however, these foods are sensitive. The formation of intracellular and/or extracellular ice crystals (depending on their location, number, size and morphology) can cause a mechanical rupture of the protoplasmic structure in fruits and vegetables, generating drip losses and consequent cellular dehydration; preventing them from returning to their natural state. It can also increase salt concentration and decrease water activity, in addition to leakage of intracellular compounds, cell contraction, stretching, and tissue deformation ( Liu et al., 2020 ). After thawing, accelerated oxidation reactions and increased enzyme activity occur ( Arai et al., 2021 ). This damage causes the loss of nutrients, in addition to the alteration of the sensory characteristics of fruits and vegetables. It was reported that after the F-T cycle, fruits and vegetables experience undesirable dehydration and damage to their overall quality ( Chen et al., 2021b ). Zhu et al. (2021a) determined the effect of the F-T process on the quality of blueberry juice. Antioxidant activity, total anthocyanin content and the concentration of volatile compounds decreased. The original flavor of the fruit was also lost due to the increase in ethanol concentration.
At extremely low temperatures (−196 to −60 °C) and at high speed, the formation of large crystals can be avoided, but this requires high energy levels ( Ustun and Turhan, 2020 ). Even if the operating conditions are relatively correct, deterioration will still occur, so treatment with AFPs is ideal. Efficacy of adding AFPs-I (0.01 mg/mL) from winter flounder on Nasturtium officinale was demonstrated. A more defined cell wall, smaller and rounder crystals, higher mechanical strength and turgidity were obtained. In addition, the thawed samples showed higher turgidity and a more uniform green color ( Cruz et al., 2009 ). In a peculiar experiment, frozen carrots were immersed in a saline solution (0.9%, w/v) with 0.1 mg/mL of DCR26 and DCR39 peptide analogues designed from AFPs from Dendroides canadensis , and AFPs replicated from AFPs-I from winter flounder. The shape of the ice crystals was positively modified and the drip loss on thawing was reduced, preserving the structure, texture, color and volatile compounds of the carrots ( Kong et al., 2016 ).
4.3. Frozen dough
Since the 1960s, the dough has been frozen to solve the problem with its short shelf life ( Gün et al., 2020 ), increasing its demand ever since ( Ding et al., 2015 ). However, due to the formation of large crystals, and their interaction with the water and gluten proteins in the dough, freezing generates disadvantages in the product. Freezing can damage the structure of gluten network. The yeast can suffer damage to its cell membrane, which would cause its dehydration and subsequent death and/or reduced viability. During thawing, yeast cells can undergo oxidative stress, decreasing the CO 2 production capacity. This affects fermentation time, CO 2 retention capacity, specific volume, firmness, color, flavor and causing loss of nutrients in the bread (or other related product) ( Panadero et al., 2005 ; Luo et al., 2018 ; Chen et al., 2021b ). To avoid all this, hydrocolloids ( Zhang et al., 2015 ), wheat flour or low-temperature tolerant yeasts are used, but these increase costs and modify product characteristics ( Ustun and Turhan, 2020 ). Moreover, they are not as efficient as AFPs, which, due to their IRI and TH properties, prevent Ostwald ripening and other negative effects during and after freezing.
In one of the first experiments, recombinant AFPs-I from Myoxocephalus aenaeus were used in a wild laboratory strain and in the commercial yeast Saccharomyces cerevisiae . Yeasts showed higher viability during freezing and thawing (at different times). There was also less loss in gas production when inoculated in a liquid mass model ( Panadero et al., 2005 ). 0.35% carrot concentrated protein (18.3% AFPs) was added to frozen dough. Retention capacity was increased and yeast mortality was reduced. In addition, the bread made with the carrot concentrated protein had the same sensory quality as the control sample ( Zhang et al., 2007 ). Adding the same protein (but with 15.4% AFPs) in the crumb and bread dough improved texture (less hardness) during frozen storage and improved aroma due to reduced loss of volatile compounds ( Zhang et al., 2008 ). The addition of muscle hydrolysate (14%) from Hypophthalmichthys molitrix rich in AFPs was evaluated in Saccharomyces cerevisiae . Yeast survival rate increased by 82–91% after 1 to 2 F-T cycles ( Wang et al., 2021b ).
By using AFPs (0.3 and 0.6%) from winter wheat, it was possible to increase the water holding capacity, the specific volume of the bread and reduce the leavening time during the F-T cycles, unlike the frozen dough without AFPs ( Xu et al., 2009 ). Regarding recombinant AFPs, the incorporation of a solution of AFPs-I (0.045 g/mL) from frozen Lactobacillus lactis in frozen dough improved its fermentation, without affecting consumer acceptability when evaluating breads made with the dough after a F-T cycle ( Yeh et al., 2009 ). AFPs-I (0.5%) of barley increased the apparent specific heat of fresh dough, FP and Tg, in addition to decreasing the enthalpy of fusion and freezable water content. AFPs also influenced gelation property, melting performance, and water mobility and distribution in the frozen dough after F-T cycles ( Ding et al., 2015 ). AFPs from carrot expressed in Pichia Pastoris reduced the freezable water content of frozen dough during F-T cycles; in addition to maintaining the fermentation capacity and microstructure of the bread, improving its texture and specific volumen ( Liu et al., 2018c ).
In particular, the use of AFPs (0.1%) from cold-acclimated Triticum aestivum in a mixture of flour and water (37% w/w), caused the embedding of starch granules in the gluten network after F-T cycle due to the increase in water mobility and consequent recrystallization. The product had a total deterioration of mechanical properties during 30 days of storage under fixed freezing conditions and fluctuating temperatures under refrigeration ( Kontogiorgos et al., 2008 ). In this case, according to electron microscopy analysis, the presence of AFPs did not influence ice formation and recrystallization.
4.4. Current challenges and recommendations for future work
Regardless of the use of AFPs, the main challenge is their high cost due to their low yield after extraction, isolation and purification ( Yeh et al., 2009 ). For example, 1 g of AFGPs from winter flounder costs $500 and 1 mg of AFPs-III from teleost fish costs approximately $10, depending on their purity ( Ustun and Turhan, 2020 ). To meet demand, microorganisms are a more cost-effective source of AFPs than plants, insects and fish. However, microbes produce many types of proteins and purification of AFPs remains a challenge ( Han et al., 2020 ). The expression of AFPs in microbial hosts is presented as a promising alternative. The production of recombinant AFPs with yields up to 300 mg/L has been reported, with potential for large-scale application ( Tab et al., 2017 ). Tab et al. (2017) carried out a batch fermentation to express recombinant AFPs-I from Glaciozyma antarctica in Pichia pastoris , obtaining a high yield of 39.5 mg/L and at a pH value of 5. Liu et al. (2018c) expressed AFPs from carrot in P . pastoris GS115 with a yield of 379.47 mg/L and TH of 1.96 °C.
Other sources of AFPs should be exploited, such as chemical synthesis and the production of analogues. These alternatives are very profitable and offer the opportunity to improve the characteristics of the AFPs through a correct design. Kun and Mastai (2007) synthesized short AFPs-I from winter flounder with three times less amino acids, significantly reducing the cost of production. Han et al. (2020) expressed AFPs-II from Clupea harangues and AFPs-III from Anarhichas minor in E . coli . The preparation of longer peptides improved the ability to inhibit hydrate crystals with ice-like clathrate structure. In another study, hyperactive AFPs from Rhagium inquisitor with shorter motifs showed the highest IRI activity ( Kong et al., 2019 ). Synthetic AFPs of intermediate length showed better antifreeze capacity than those of short and long length ( Rojas et al., 2022 ). It is recommended to optimize the peptide length to achieve an adequate cost/efficiency ratio. On the other hand, Liu et al. (2021b) suggest the use of BL21 (DE3), a cell with high efficiency in protein expression. The authors used BL21 (DE3) to express the AFPs gene from Marinomonas primoryensis ; producing high purity proteins with high IRI activity and at low cost.
It is also possible to take advantage of advances in biotechnological advances to clone AFPs. AFPs were successfully cloned from Solanum dulcamara ( Huang and Duman, 2002 ), Dendroctonus armandi ( Fu et al., 2022 ), Brassica rapa ( Liu et al., 2019 ; Dong et al., 2020 ), Zoarces americanus (type III, Hobbs et al., 2020 ), Gadus macrocephalus (type IV, Mao et al., 2018 ), Hypogastrura harveyi ( Chen et al., 2022 ), Lycodichthys dearborni (type III, Huang et al., 2019 ), Cottoidea (type I, Yamazaki et al., 2019 ), Apis cerana cerana ( Xu et al., 2018 ), Ammopiptanthus nanus ( Zhang et al., 2021 ), Larimichthys Crocea ( Qian, 2021 ), Glaciozyma antarctica ( Firdaus-raih et al., 2018 ), Chloromonas sp. KNF0032 (type II, Cho et al., 2019 ), Brachypodium distachyon ( Bredow et al., 2018 ).
Another limitation is that purification of the plant-derived AFPs extract is mandatory because if applied directly, the endogenous enzymes can cause undesirable reactions in the treated food, causing substantial damage to its quality. One solution is to treat the extract by heat treatment, but this would result in denaturation of the AFPs and reduction of their AFA ( Ustun and Turhan, 2020 ). The use of non-thermal methods such as ultrasound, cold plasma, high hydrostatic pressures and pulsed electric field can be evaluated ( Tirado-Kulieva et al., 2021 ).
One food safety issue that has been raised is the potential allergic reaction and toxicity that AFPs may induce in consumers ( Panadero et al., 2005 ). There is still no evidence of toxicity on the use of AFPs in frozen foods; however, there is general information that may be of interest. It was reported that 0.63 and 2 mg/mL of AFPs present toxicity to embryonic kidney cells and human embryonic liver, respectively ( Liu et al., 2021a ). At 72 h, AFPs (500 mg/L) from Tenebrio molitor and Dendroides canadensis showed no toxicity in MA-10 and RAW264.7 cell lines, but decreased the viability of C18-4 and HUH7 cell lines by 20 and 40%, respectively. At 1000 mg/L, AFPs from both sources induced inflammatory reactions in the RAW264.7 cell line ( Tran-Guzman et al., 2022 ). This is a matter of debate because other authors indicate that AFPs are safe because they contain common dietary compounds such as amino acids ( Kong et al., 2016 ; Song et al., 2019 ). Their (relatively) safe use as a food additive has also been proven by several studies. Healthy people consumed AFPs-III for 2 months and after an analysis of 5 days per week, it was shown that there was no impact in terms of allergenicity ( Crevel et al., 2007 ). The null cytotoxicity in human cells was also demonstrated by an in vitro assay in HUVEC (human umbilical vein endothelial cells), HDFA (human dermal fibroblasts) and HEK-293 (human embryonic kidney cells) ( Kong et al., 2017 ). Recombinant AFPs-III from Saccharomyces cerevisiae showed no genotoxicity in bacteria, mammalian cells or mouse bone marrow. In addition, administration in rats for 3 months did not cause subchronic toxicity ( Hall-Manning et al., 2004 ). Despite promising results, techniques are needed to reduce this potential risk. Chen et al. (2021a) suggest preparing AFPs by microbial fermentation; they are converted into peptides with equal or higher activity, easily absorbable during human digestion and with lower allergenic risk. The authors indicate that the AFPs have a better flavor than if they are treated with other methods such as enzymatic hydrolysis. Another option to avoid toxicity is the use of artificially synthesized AFPs or the combination of AFPs with other cryoprotectants. Promising results have been obtained; the quality of frozen Pagrosomus major was maintained after thawing by immersing in a solution of AFPs (0.1%) from herring plus chitosan magnetic nanoparticles (CMN, 0.01%) ( Cai et al., 2019 ) or carboxymethyl CMN (0.01%) ( Cai et al., 2020 ).
To evaluate the properties of AFPs and their possible negative effects, further research is needed. In silico studies (in addition to in vitro and in vivo studies) are recommended to perform experiments quickly, with a significant reduction in costs and time, in addition to being able to perform modeling and predictions. Eslami et al. (2018) used a computational method based on machine learning to rapidly identify AFPs in a sample with more than 400 AFPs and 9000 non-AFPs. Zhu et al. (2022) determined in silico that two AFPs from shrimp by-products have high potential. AFPs (0.1–3%) preserved shrimp muscle quality after up to 6 F-T cycles. This is useful for, predicting the possible toxicity of AFPs and even the chemical reactions that may occur due to their interaction with other compounds in the food. Li et al. (2018) also suggest to investigate in depth the damage caused by the F-T cycle due to its complexity and, mainly, due to the complex composition of different food products.
5. Conclusions
AFPs are an ideal additive whose popularity has increased in recent years due to their potential to preserve the quality of refrigerated and frozen foods, and during their thawing. Currently, it is essential to take advantage of the particularities of AFPs, since limitations such as cost, performance and stability are being solved thanks to advances in biotechnology. Furthermore, although the mechanism of action is relatively clear and possible toxic effects of AFPs have been ruled out, further research is needed. In silico studies are suggested to rapidly evaluate them and predict their possible negative effects on food and, consequently, on the consumer. This will help to better understand the technique and to be able to classify it as safe and promising in the food sector.
Declarations
Author contribution statement.
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Declaration of interest’s statement.
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
☆ This article is a part of the “Structure and function of food proteins and peptides” Special issue.
- Arai N., Fujiwara A., Wakuda M., Fujimoto T., Nambu Y., Ishii T., et al. Anti-freeze effect of Enoki mushroom extract on the quality preservation of frozen whipped cream. J. Food Eng. 2021; 291 [ Google Scholar ]
- Baardsnes J., Davies P.L. Contribution of hydrophobic residues to ice binding by fish type III antifreeze protein. Biochim. Biophys. Acta, Proteins Proteomics. 2002; 1601 :49–54. [ PubMed ] [ Google Scholar ]
- Baskaran A., Kaari M., Venugopal G., Manikkam R., Joseph J., Bhaskar P.V. Anti freeze proteins (Afp): properties, sources and applications – a review. Int. J. Biol. Macromol. 2021; 189 :292–305. [ PubMed ] [ Google Scholar ]
- Białkowska A., Majewska E., Olczak A., Twarda-clapa A. Ice binding proteins: diverse biological roles and applications in different types of industry. Biomolecules. 2020; 10 :274. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Boonsupthip W., Lee T.C. Application of antifreeze protein for food preservation: effect of Type III antifreeze protein for preservation of gel-forming of frozen and chilled actomyosin. J. Food Sci. 2003; 68 :1804–1809. [ Google Scholar ]
- Bredow M., Tomalty H.E., Smith L., Walker V.K. Ice and anti-nucleating activities of an ice-binding protein from the annual grass, Brachypodium distachyon. Plant Cell Environ. 2018; 41 :983–992. [ PubMed ] [ Google Scholar ]
- Cai L., Nian L., Cao A., Zhang Y., Li X. Effect of carboxymethyl chitosan magnetic nanoparticles plus herring antifreeze protein on conformation and oxidation of myofibrillar protein from red sea bream (Pagrosomus major) after freeze-thaw treatment. Food Bioprocess Technol. 2020; 13 :355–366. [ Google Scholar ]
- Cai L., Nian L., Zhao G., Zhang Y., Sha L., Li J. Effect of herring antifreeze protein combined with chitosan magnetic nanoparticles on quality attributes in red sea bream (Pagrosomus major) Food Bioprocess Technol. 2019; 12 :409–421. [ Google Scholar ]
- Calderara M., Deorsola F.A., Bensaid S., Fino D., Russo N., Geobaldo F. Role of ice structuring proteins on freezing–thawing cycles of pasta sauces. J. Food Sci. Technol. 2016; 53 :4216–4223. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Cao C., Xiao Z., Ge C., Wu Y. Animal by-products collagen and derived peptide, as important components of innovative sustainable food systems—a comprehensive review. Crit. Rev. Food Sci. Nutr. 2021 [ PubMed ] [ Google Scholar ]
- Cao H., Zhao Y., Zhu Y.B., Xu F., Yu J.S., Yuan M. Antifreeze and cryoprotective activities of ice-binding collagen peptides from pig skin. Food Chem. 2016; 194 :1245–1253. [ PubMed ] [ Google Scholar ]
- Chao H., Houston M.E., Hodges R.S., Kay C.M., Sykes B.D., Loewen M.C., et al. A diminished role for hydrogen bonds in antifreeze protein binding to ice. Biochemistry. 1997; 36 :14652–14660. [ PubMed ] [ Google Scholar ]
- Chattopadhyay M.K. Antifreeze proteins of bacteria. Resonance. 2007; 12 :25–30. [ Google Scholar ]
- Chen X., Shi X., Cai X., Yang F., Li L., Wu J., et al. Ice-binding proteins: a remarkable ice crystal regulator for frozen foods. Crit. Rev. Food Sci. Nutr. 2021; 61 :3436–3449. [ PubMed ] [ Google Scholar ]
- Chen X., Wu J., Cai X., Wang S. Production, structure–function relationships, mechanisms, and applications of antifreeze peptides. Compr. Rev. Food Sci. Food Saf. 2021; 20 :542–562. [ PubMed ] [ Google Scholar ]
- Chen X., Wu J. hong, Li L., Wang S. yun. The cryoprotective effects of antifreeze peptides from pigskin collagen on texture properties and water mobility of frozen dough subjected to freeze–thaw cycles. Eur. Food Res. Technol. 2017; 243 :1149–1156. [ Google Scholar ]
- Chen X., Wu J., Yang F., Zhou M., Wang R., Huang J., et al. New insight into the mechanism by which antifreeze peptides regulate the physiological function of Streptococcus thermophilus subjected to freezing stress. J. Adv. Res. 2022 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Cheng S., Wang X., Li R., Yang H., Wang H., Wang H., et al. Influence of multiple freeze-thaw cycles on quality characteristics of beef semimembranous muscle: with emphasis on water status and distribution by LF-NMR and MRI. Meat Sci. 2019; 147 :44–52. [ PubMed ] [ Google Scholar ]
- Cho S.M., Kim S., Cho H., Lee H., Lee J.H., Lee H., et al. Type II ice-binding proteins isolated from an arctic microalga are similar to adhesin-like proteins and increase freezing tolerance in transgenic plants. Plant Cell Physiol. 2019; 60 :2744–2757. [ PubMed ] [ Google Scholar ]
- Crevel R.W.R., Cooper K.J., Poulsen L.K., Hummelshoj L., Bindslev-Jensen C., Burks A.W., et al. Lack of immunogenicity of ice structuring protein type III HPLC12 preparation administered by the oral route to human volunteers. Food Chem. Toxicol. 2007; 45 :79–87. [ PubMed ] [ Google Scholar ]
- Cruz R.M.S., Vieira M.C., Silva C.L.M. The response of watercress (Nasturtium officinale) to vacuum impregnation: effect of an antifreeze protein type I. J. Food Eng. 2009; 95 :339–345. [ Google Scholar ]
- Cui M., Liu H., Liu Y., Yu J., Li X., Huang Y., et al. Cryoprotective effects of silver carp muscle hydrolysate on frozen dough subjected to multiple freeze–thaw cycles and their underlying mechanisms. J. Food Meas. Charact. 2021; 15 :5507–5514. [ Google Scholar ]
- Dalvi-Isfahan M., Jha P.K., Tavakoli J., Daraei-Garmakhany A., Xanthakis E., Le-Bail A. Review on identification, underlying mechanisms and evaluation of freezing damage. J. Food Eng. 2019; 255 :50–60. [ Google Scholar ]
- Damodaran S., Wang S.Y. Ice crystal growth inhibition by peptides from fish gelatin hydrolysate. Food Hydrocolloids. 2017; 70 :46–56. [ Google Scholar ]
- Das A., Chauhan G., Satyaprakash K., Tomar S. Application of antifreeze proteins in foods of animal origin- A review. Int. J. Livest. Res. 2018; 8 :70. [ Google Scholar ]
- Derossi A., Iliceto A., De Pilli T., Severini C. Application of vacuum impregnation with anti-freezing proteins to improve the quality of truffles. J. Food Sci. Technol. 2015; 52 :7200–7208. [ Google Scholar ]
- Ding X., Li T., Zhang H., Guan C., Qian J., Zhou X. Effect of barley antifreeze protein on dough and bread during freezing and freeze-thaw cycles. Foods. 2020; 9 :1698. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ding X., Zhang H., Liu W., Wang L., Qian H., Qi X. Extraction of carrot (daucus carota) antifreeze proteins and evaluation of their effects on frozen white salted noodles. Food Bioprocess Technol. 2014; 7 :842–852. [ Google Scholar ]
- Ding X., Zhang H., Wang L., Qian H., Qi X., Xiao J. Effect of barley antifreeze protein on thermal properties and water state of dough during freezing and freeze-thaw cycles. Food Hydrocolloids. 2015; 47 :32–40. [ Google Scholar ]
- Dong X., Liu Z., Mi W., Xu C., Xu M., Zhou Y., et al. Plant Physiology and Biochemistry Overexpression of BrAFP1 gene from winter rapeseed (Brassica rapa) confers cold tolerance in Arabidopsis. Plant Physiol. Biochem. 2020; 155 :338–345. [ PubMed ] [ Google Scholar ]
- Du X., Chang P., Tian J., Kong B., Sun F., Xia X. Effect of ice structuring protein on the quality, thermal stability and oxidation of mirror carp (Cyprinus carpio L.) induced by freeze-thaw cycles. LWT--Food Sci. Technol. 2020; 124 [ Google Scholar ]
- Du X., Li H., Dong C., Ren Y., Pan N., Kong B., et al. Effect of ice structuring protein on the microstructure and myofibrillar protein structure of mirror carp (Cyprinus carpio L.) induced by freeze-thaw processes. LWT--Food Sci. Technol. 2021; 139 [ Google Scholar ]
- Egelandsdal B., Abie S.M., Bjarnadottir S., Zhu H., Kolstad H., Bjerke F., et al. Detectability of the degree of freeze damage in meat depends on analytic-tool selection. Meat Sci. 2019; 152 :8–19. [ PubMed ] [ Google Scholar ]
- Eskandari A., Leow T.C., Rahman M.B.A., Oslan S.N. Antifreeze proteins and their practical utilization in industry, medicine, and agriculture. Biomolecules. 2020; 10 :1–18. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Eslami M., Shirali R., Zade H., Takalloo Z., Mahdevar G., Emamjomeh A., et al. afpCOOL : a tool for antifreeze protein prediction. Heliyon. 2018; 4 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Feeney R.E., Yeh Y. Antifreeze proteins: current status and possible food uses. Trends Food Sci. Technol. 1998; 9 :102–106. [ Google Scholar ]
- Fennema O.R., Powrie W.D., Marth E.M. Food Science. Taylor & Francis; 1973. Low temperature preservation of foods and living matter; pp. 467–472. [ Google Scholar ]
- Firdaus-raih M., Haza N., Hashim F., Bharudin I., Abu M.F., Huang K.K., et al. The Glaciozyma Antarctica genome reveals an array of systems that provide sustained responses towards temperature variations in a persistently cold habitat. PLoS One. 2018; 13 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Fu D., Sun Y., Gao H., Liu B., Kang X., Chen H. Identification and functional characterization of antifreeze protein and its mutants in Dendroctonus armandi (Coleoptera: Curculionidae: scolytinae) larvae under cold stress. Environ. Entomol. 2022; 51 :167–181. [ PubMed ] [ Google Scholar ]
- Gandini E., Sironi M., Pieraccini S. Modelling of short synthetic antifreeze peptides: insights into ice-pinning mechanism. J. Mol. Graph. Model. 2020; 100 [ PubMed ] [ Google Scholar ]
- Griffith M., Ewart K.V. Antifreeze proteins and their potential use in frozen foods. Biotechnol. Adv. 1995; 13 :375–402. [ PubMed ] [ Google Scholar ]
- Gruneberg A.K., Graham L.A., Eves R., Agrawal P., Oleschuk R.D., Davies P.L. Ice recrystallization inhibition activity varies with ice-binding protein type and does not correlate with thermal hysteresis. Cryobiology. 2021; 99 :28–39. [ PubMed ] [ Google Scholar ]
- Gün İ., Aslı A., Asuman G. Turkish journal of agriculture - food science and technology antifreeze proteins : an inovative agent for the prevention of foods antifriz proteinler : gıdaların korunmasında i?novatif bir ajan. Turkish J. Agric. - Food Sci. Technol. 2020; 8 :1433–1439. [ Google Scholar ]
- Hall-Manning T., Spurgeon M., Wolfreys A.M., Baldrick A.P. Safety evaluation of ice-structuring protein (ISP) type III HPLC 12 preparation . Lack of genotoxicity and subchronic toxicity. Food Chem. Toxicol. 2004; 42 :321–333. [ PubMed ] [ Google Scholar ]
- Han S., Kannan M., Lee W., Ho S., Kang S. Efficacy of antifreeze proteins from Clupea harangues and Anarhichas minor on gas hydrate inhibition via cell surface display. Chem. Eng. Sci. 2020; 215 [ Google Scholar ]
- Hobbs R.S., Hall J.R., Graham L.A., Id P.L.D., Fletcher L. Antifreeze protein dispersion in eelpouts and related fishes reveals migration and climate alteration within the last 20 Ma. PLoS One. 2020; 15 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hu C., Xie J. The effect of multiple freeze–thaw cycles on the microstructure and quality of trachurus murphyi. Foods. 2021; 10 :1–13. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Huang Q., Hu R., Peng C., Chen L. The cold resistant activities of multi-domain type III antifreeze proteins from Antarctic eelpout Lycodichths dearborni revealed by transgenic tobaccos. Aquac. Fish. 2019 0–1. [ Google Scholar ]
- Huang T., Duman J.G. Cloning and characterization of a thermal hysteresis (antifreeze) protein with DNA-binding activity from winter bittersweet nightshade , Solanum dulcamara. Plant Mol. Biol. 2002; 48 :339–350. [ PubMed ] [ Google Scholar ]
- Hudait A., Qiu Y., Odendahl N., Molinero V. Hydrogen-bonding and hydrophobic groups contribute equally to the binding of hyperactive antifreeze and ice-nucleating proteins to ice. J. Am. Chem. Soc. 2019; 141 :7887–7898. [ PubMed ] [ Google Scholar ]
- Jia C., Huang W., Wu C., Zhong J., Rayas-Duarte P., Guo C. Frozen bread dough properties modified by thermostable ice structuring proteins extract from Chinese privet (Ligustrum vulgare) leaves. Cereal Chem. 2012; 89 :162–167. [ Google Scholar ]
- Jiang Q., Nakazawa N., Hu Y., Osako K., Okazaki E. Changes in quality properties and tissue histology of lightly salted tuna meat subjected to multiple freeze-thaw cycles. Food Chem. 2019; 293 :178–186. [ PubMed ] [ Google Scholar ]
- Kashyap P., Kumar S. Ice structuring protein extract of Hordeum vulgare var. dolma grain reduces drip loss and loss of soluble vitamin content in peas during frozen storage. Cryobiology. 2022; 104 :1–7. [ PubMed ] [ Google Scholar ]
- Kashyap P., Kumar S., Singh D. Performance of antifreeze protein HrCHI4 from Hippophae rhamnoides in improving the structure and freshness of green beans upon cryopreservation. Food Chem. 2020; 320 [ PubMed ] [ Google Scholar ]
- Khan N.M.M.U., Arai T., Tsuda S., Kondo H. Characterization of microbial antifreeze protein with intermediate activity suggests that a bound-water network is essential for hyperactivity. Sci. Rep. 2021; 11 :1–15. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kong C.H.Z., Hamid N., Liu T., Sarojini V. Effect of antifreeze peptide pretreatment on ice crystal size, drip loss, texture, and volatile compounds of frozen carrots. J. Agric. Food Chem. 2016; 64 :4327–4335. [ PubMed ] [ Google Scholar ]
- Kong C.H.Z., Hamid N., Ma Q., Lu J., Wang B.G., Sarojini V. Antifreeze peptide pretreatment minimizes freeze-thaw damage to cherries: an in-depth investigation. LWT--Food Sci. Technol. 2017; 84 :441–448. [ Google Scholar ]
- Kong L.F., Al-khdhairawi A.A.Q., Tejo B.A. Rational design of short antifreeze peptides derived from Rhagium inquisitor antifreeze protein. Biocatal. Agric. Biotechnol. 2019 [ Google Scholar ]
- Kontogiorgos V., Goff H.D., Kasapis S. Effect of aging and ice-structuring proteins on the physical properties of frozen flour-water mixtures. Food Hydrocolloids. 2008; 22 :1135–1147. [ Google Scholar ]
- Kristiansen E. In: Characteristics of Antifreeze Proteins. first ed. Ramløv H., Friis D.S., editors. Springer; Switzerland: 2020. [ Google Scholar ]
- Kumari S., Muthachikavil A.V., Tiwari J.K., Punnathanam S.N. Computational study of differences between antifreeze activity of type-III antifreeze protein from ocean pout and its mutant. Langmuir. 2020; 36 :2439–2448. [ PubMed ] [ Google Scholar ]
- Kun H., Mastai Y. Activity of short segments of Type I antifreeze protein. Biopolymers. 2007; 88 :807–814. [ PubMed ] [ Google Scholar ]
- Lan W., Hu X., Sun X., Zhang X., Xie J. Effect of the number of freeze-thaw cycles number on the quality of Pacific white shrimp (Litopenaeus vannamei): an emphasis on moisture migration and microstructure by LF-NMR and SEM. Aquac. Fish. 2020; 5 :193–200. [ Google Scholar ]
- Lee H. Effects of hydrophobic and hydrogen-bond interactions on the binding affinity of antifreeze proteins to specific ice planes. J. Mol. Graph. Model. 2019; 87 :48–55. [ PubMed ] [ Google Scholar ]
- Lee S.J., Kim H.J., Cheong S.H., Kim Y.S., Kim S.E., Hwang J.W., et al. Antioxidative effect of recombinant ice-binding protein (rLeIBP) from Arctic yeast Glaciozyma sp. on lipid peroxidation of Korean beef. Process Biochem. 2015; 50 :2099–2104. [ Google Scholar ]
- Li D., Zhu Z., Sun D.W. Effects of freezing on cell structure of fresh cellular food materials: a review. Trends Food Sci. Technol. 2018; 75 :46–55. [ Google Scholar ]
- Li F., Du X., Ren Y., Kong B., Wang B., Xia X., et al. Impact of ice structuring protein on myofibrillar protein aggregation behaviour and structural property of quick-frozen patty during frozen storage. Int. J. Biol. Macromol. 2021; 178 :136–142. [ PubMed ] [ Google Scholar ]
- Liu D.K., Xu C.C., Guo C.X., Zhang X.X. Sub-zero temperature preservation of fruits and vegetables: a review. J. Food Eng. 2020; 275 [ Google Scholar ]
- Liu M., Liang Y., Wang Y., Zhang H., Wu G., Wang L., et al. Effects of recombinant carrot antifreeze protein from Pichia pastoris GS115 on the physicochemical properties of hydrated gluten during freeze-thawed cycles. J. Cereal. Sci. 2018; 83 :245–251. [ Google Scholar ]
- Liu M., Liang Y., Zhang H., Wu G., Wang L., Qian H., et al. Comparative study on the cryoprotective effects of three recombinant antifreeze proteins from Pichia pastoris GS115 on hydrated gluten proteins during freezing. J. Agric. Food Chem. 2018; 66 :6151–6161. [ PubMed ] [ Google Scholar ]
- Liu M., Liang Y., Zhang H., Wu G., Wang L., Qian H., et al. Production of a recombinant carrot antifreeze protein by Pichia pastoris GS115 and its cryoprotective effects on frozen dough properties and bread quality. LWT--Food Sci. Technol. 2018 [ Google Scholar ]
- Liu X., Pan Y., Liu F., He Y., Zhu Q., Liu Z., et al. A review of the material characteristics, antifreeze mechanisms, and applications of cryoprotectants (CPAs) J. Nanomater. 2021; 2021 [ Google Scholar ]
- Liu X., Peng H., Xie J., Hu Y., Liu F., Wang X., et al. Methods in biosynthesis and characterization of the antifreeze protein (AFP) for potential blood cryopreservation. J. Nanomater. 2021; 2021 [ Google Scholar ]
- Liu Z., Dong X., Ma L., Sun W., Yang G., Fang Y. Separation and identification of Brassica rapa BrAFP and its gene cloning and expression under freezing stress. Plant Breed. 2019; 138 :193–201. [ Google Scholar ]
- Luo W., Sun D.W., Zhu Z., Wang Q.J. Improving freeze tolerance of yeast and dough properties for enhancing frozen dough quality - a review of effective methods. Trends Food Sci. Technol. 2018; 72 :25–33. [ Google Scholar ]
- Mao M., Chen Y., Liu R., Lü H., Gu J., Jiang Z. Comparative Biochemistry and Physiology - Part D Transcriptome from Paci fi c cod liver reveals types of apolipoproteins and expression analysis of AFP-IV , structural analogue with mammalian ApoA-I. Comp. Biochem. Physiol. - Part D. 2018; 28 :204–212. [ PubMed ] [ Google Scholar ]
- Meister K., Moll C.J., Chakraborty S., Jana B., DeVries A.L., Ramløv H., et al. Molecular structure of a hyperactive antifreeze protein adsorbed to ice. J. Chem. Phys. 2019; 150 [ PubMed ] [ Google Scholar ]
- Mochizuki K., Molinero V. Antifreeze glycoproteins bind reversibly to ice via hydrophobic groups. J. Am. Chem. Soc. 2018; 140 :4803–4811. [ PubMed ] [ Google Scholar ]
- Muñoz P.A., Márquez S.L., González-Nilo F.D., Márquez-Miranda V., Blamey J.M. Structure and application of antifreeze proteins from Antarctic bacteria. Microb. Cell Factories. 2017; 16 :1–13. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Nian L., Cao A., Cai L. Investigation of the antifreeze mechanism and effect on quality characteristics of largemouth bass (Micropterus salmoides) during F-T cycles by hAFP. Food Chem. 2020; 325 [ PubMed ] [ Google Scholar ]
- Panadero J., Randez-Gil F., Prieto J.A. Heterologous expression of type I antifreeze peptide GS-5 in baker’s yeast increases freeze tolerance and provides enhanced gas production in frozen dough. J. Agric. Food Chem. 2005; 53 :9966–9970. [ PubMed ] [ Google Scholar ]
- Payne S.R., Sandford D., Harris A., Young O.A. The effects of antifreeze proteins on chilled and frozen meat. Meat Sci. 1994; 37 :429–438. [ PubMed ] [ Google Scholar ]
- Payne S.R., Young O.A. Effects of pre-slaughter administration of antifreeze proteins on frozen meat quality. Meat Sci. 1995; 41 :147–155. [ PubMed ] [ Google Scholar ]
- Provesi J.G., Amante E.R. Revisão: proteínas anticongelantes - Uma tecnologia emergente para o congelamento de alimentos. Braz. J. Food Technol. 2015; 18 :2–13. [ Google Scholar ]
- Provesi J.G., Valentim Neto P.A., Arisi A.C.M., Amante E.R. Extraction of antifreeze proteins from cold acclimated leaves of Drimys angustifolia and their application to star fruit (Averrhoa carambola) freezing. Food Chem. 2019; 289 :65–73. [ PubMed ] [ Google Scholar ]
- Qian B. 2021. Molecular Characterization and mRNA Expression of ISP2 and ISP4 in the Large Yellow Croaker (Larimichthys Crocea) under Acute Cold Stress. Prepr. (Version 1) available Res. Sq. [ Google Scholar ]
- Ramløv H., Johnsen J.L. second ed. Elsevier; 2014. Controlling the Freezing Process with Antifreeze Proteins. [ Google Scholar ]
- Rojas R., Aróstica M., Carvajal-rondanelli P., Albericio F., Guzmán F., Cárdenas C. Relationship between type II polyproline helix secondary structure and thermal hysteresis activity of short homopeptides. Electron. J. Biotechnol. 2022; 59 :62–73. [ Google Scholar ]
- Rosa M.S., Ferreira C., Provesi J.G., Amante E.R. Effect of the antifreeze protein on the microstructure of strawberries (Fragaria ananassa Duch) Braz. J. Food Technol. 2019; 22 [ Google Scholar ]
- Shi Y., Wang H., Zheng Y., Qiu Z., Wang X. Effects of ultrasound-assisted vacuum impregnation antifreeze protein on the water-holding capacity and texture properties of the yesso scallop adductor muscle during freeze – thaw cycles. Foods. 2022; 11 :320. https://www.mdpi.com/2304-8158/11/3/320 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Song D.H., Kim M., Jin E.S., Sim D.W., Won H.S., Kim E.K., et al. Cryoprotective effect of an antifreeze protein purified from Tenebrio molitor larvae on vegetables. Food Hydrocolloids. 2019; 94 :585–591. [ Google Scholar ]
- Surís-Valls R., Voets I.K. Peptidic antifreeze materials: prospects and challenges. Int. J. Mol. Sci. 2019; 20 :5149. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Tab M.M., Hashim N.H.F., Najimudin N., Mahadi N.M., Bakar F.D.A., Murad A.M.A. Large-scale production of Glaciozyma Antarctica antifreeze protein 1 (Afp1) by fed-batch fermentation of Pichia pastoris. Food Res. Int. 2017; 43 :133–141. [ Google Scholar ]
- Tan M., Lin Z., Zu Y., Zhu B., Cheng S. Effect of multiple freeze-thaw cycles on the quality of instant sea cucumber: emphatically on water status of by LF-NMR and MRI. Food Res. Int. 2018; 109 :65–71. [ PubMed ] [ Google Scholar ]
- Tan M., Mei J., Xie J. The formation and control of ice crystal and its impact on the quality of frozen aquatic products: a review. Crystals. 2021; 11 :1–17. [ Google Scholar ]
- Tejo B.A., Asmawi A.A., Rahman M.B.A. Antifreeze proteins: characteristics and potential applications. Makara J. Sci. 2020; 24 :8. [ Google Scholar ]
- Tian Y., Zhu Z., Sun D.W. Naturally sourced biosubstances for regulating freezing points in food researches: fundamentals, current applications and future trends. Trends Food Sci. Technol. 2020; 95 :131–140. [ Google Scholar ]
- Tirado-Kulieva V., Miranda Zamora W.R., Leyva Povis N.L. Análisis crítico del potential del plasma frío como tecnología no destructiva en el procesamiento alimentario: situación actual y tendencias futuras. Rev. la Univ. del Zulia. 2021; 12 :284–316. [ Google Scholar ]
- Tran-Guzman A., Moradian R., Walker C., Cui H., Corpuz M., Gonzalez I., et al. Toxicity profiles and protective effects of antifreeze proteins from insect in mammalian models. Toxicol. Lett. 2022; 368 :9–23. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ustun N.S., Turhan S. In: Antifreeze Proteins. Ramløv H., Friis D.S., editors. Springer; Switzerland: 2020. Antifreeze Proteins in Foods; pp. 231–260. [ Google Scholar ]
- Velickova E., Tylewicz U., Dalla Rosa M., Winkelhausen E., Kuzmanova S., Gómez Galindo F. Effect of vacuum infused cryoprotectants on the freezing tolerance of strawberry tissues. LWT--Food Sci. Technol. 2013; 52 :146–150. [ Google Scholar ]
- Wang B., Li F., Pan N., Kong B., Xia X. Effect of ice structuring protein on the quality of quick-frozen patties subjected to multiple freeze-thaw cycles. Meat Sci. 2021; 172 [ PubMed ] [ Google Scholar ]
- Wang F., Cui M., Liu H., Li X., Yu J., Huang Y., et al. Characterization and identification of a fraction from silver carp (Hypophthalmichthys molitrix) muscle hydrolysates with cryoprotective effects on yeast. LWT--Food Sci. Technol. 2021; 137 [ Google Scholar ]
- Wu X., Zhang Z., He Z., Wang Z., Qin F., Zeng M., et al. Effect of freeze-thaw cycles on the oxidation of protein and fat and its relationship with the formation of heterocyclic aromatic amines and advanced glycation end products in raw meat. Molecules. 2021; 26 :1264. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Xiang H., Yang X., Ke L., Hu Y. The properties, biotechnologies, and applications of antifreeze proteins. Int. J. Biol. Macromol. 2020; 153 :661–675. [ PubMed ] [ Google Scholar ]
- Xu H.N., Huang W., Jia C., Kim Y., Liu H. Evaluation of water holding capacity and breadmaking properties for frozen dough containing ice structuring proteins from winter wheat. J. Cereal. Sci. 2009; 49 :250–253. [ Google Scholar ]
- Xu K., Niu Q., Zhao H., Du Y., Guo L., Jiang Y. Sequencing and expression characterization of antifreeze protein maxi-like in Apis cerana cerana. J. Insect Sci. 2018; 18 :1–11. [ Google Scholar ]
- Yamazaki A., Nishimiya Y., Tsuda S., Togashi K. Freeze tolerance in sculpins (pisces ; Cottoidea) inhabiting north pacific and arctic oceans : antifreeze activity and gene sequences of the antifreeze protein. Biomolecules. 2019; 9 :139. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Yeh C.M., Kao B.Y., Peng H.J. Production of a recombinant Type 1 antifreeze protein analogue by L. lactis and its applications on frozen meat ar frozen dough. J. Agric. Food Chem. 2009; 57 :6216–6223. [ PubMed ] [ Google Scholar ]
- Zhan X., Sun D.W., Zhu Z., Wang Q.J. Improving the quality and safety of frozen muscle foods by emerging freezing technologies: a review. Crit. Rev. Food Sci. Nutr. 2018; 58 :2925–2938. [ PubMed ] [ Google Scholar ]
- Zhang C., Zhang H., Wang L. Effect of carrot (Daucus carota) antifreeze proteins on the fermentation capacity of frozen dough. Food Res. Int. 2007; 40 :763–769. [ Google Scholar ]
- Zhang C., Zhang H., Wang L., Guo X. Effect of carrot (Daucus carota) antifreeze proteins on texture properties of frozen dough and volatile compounds of crumb. LWT--Food Sci. Technol. 2008; 41 :1029–1036. [ Google Scholar ]
- Zhang L., Zeng J., Gao H., Zhang K., Wang M. Effects of different frozen storage conditions on the functional properties of wheat gluten protein in nonfermented dough. Food Sci. Technol. 2022; 42 [ Google Scholar ]
- Zhang W., Laursen R.A. Structure-function relationships in a type I antifreeze polypeptide. The role of threonine methyl and hydroxyl groups in antifreeze activity. J. Biol. Chem. 1998; 273 :34806–34812. [ PubMed ] [ Google Scholar ]
- Zhang Y., Cao Y., Zheng H., Feng W., Qu J., Fu F., et al. Ectopic expression of antifreeze protein gene from Ammopiptanthus nanus confers chilling tolerance in maize. Crop J. 2021; 9 :924–933. [ Google Scholar ]
- Zhang Y., Zhang H., Wang L., Qian H., Qi X. Extraction of oat (avena sativa L.) antifreeze proteins and evaluation of their effects on frozen dough and steamed bread. Food Bioprocess Technol. 2015; 8 :2066–2075. [ Google Scholar ]
- Zhang Y., Zhang Y., Ai Z., Zhang H. Thermal, rheological properties and microstructure of hydrated gluten as influenced by antifreeze protein from oat (Avena sativa L.) J. Cereal. Sci. 2020; 93 [ Google Scholar ]
- Zhu K., Zheng Z., Dai Z. Identification of antifreeze peptides in shrimp byproducts autolysate using peptidomics and bioinformatics. Food Chem. 2022; 383 [ PubMed ] [ Google Scholar ]
- Zhu L., Liang X., Lu Y., Tian S., Chen J., Lin F., et al. Effect of freeze-thaw cycles on juice properties, volatile compounds and hot-air drying kinetics of blueberry. Foods. 2021; 10 :2362. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Zhu S., Yu J., Chen X., Zhang Q., Cai X., Ding Y., et al. Dual cryoprotective strategies for ice-binding and stabilizing of frozen seafood: a review. Trends Food Sci. Technol. 2021; 111 :223–232. [ Google Scholar ]
- Zhu Z., Zhou Q., Sun D.W. Measuring and controlling ice crystallization in frozen foods: a review of recent developments. Trends Food Sci. Technol. 2019; 90 :13–25. [ Google Scholar ]

IMAGES
VIDEO
COMMENTS
Antifreeze proteins (AFPs) or thermal hysteresis (TH) proteins are biomolecular gifts of nature to sustain life in extremely cold environments. This family of peptides, glycopeptides and proteins produced by diverse organisms including bacteria, yeast, insects and fish act by non-colligatively depressing the freezing temperature of the water ...
Antifreeze proteins (AFPs) are specific proteins, glycopeptides, and peptides made by different organisms to allow cells to survive in sub-zero conditions. AFPs function by reducing the water's freezing point and avoiding ice crystals' growth in the frozen stage. ... Research shows that AFPs can be applied for agriculture and aquaculture ...
Antifreeze proteins are a class of proteins that widely exist in organisms living in cold conditions, such as fish, insects, plants and microorganisms in Antarctica and other cold regions. Antifreeze proteins can reduce the freezing point of water, inhibit recrystallisation effects, modify ice crystal morphology and effectively suppress the ...
1. Introduction. Antarctic and Arctic regions have extremely low temperatures and are almost completely covered by ice. Therefore, the organisms have developed several survival strategies therein, including the production of antifreeze peptides, exopolysaccharides and compatible solutes [1, 2].Antifreeze proteins (AFPs) were initially discovered in Antarctic fish and defined as cryoprotectants ...
Antifreeze glycoproteins or glycosylated proteins consist of repeated alanine-alanine-threonine peptides with each threonine residue bound by a disaccharide. The antifreeze activity of AFGPs depends on the number of repeating units; therefore AFGPs are classified into 8 types (AFGP1-8) based on their repeating units.
Abstract. Antifreeze proteins (AFPs) are proteins that protect cellular fluids and body fluids from freezing by inhibiting the nucleation and growth of ice crystals and preventing ice recrystallization, thereby contributing to the maintenance of life in living organisms. They exist in fish, insects, microorganisms, and fungi.
The unique properties of antifreeze proteins have made them a promising resource in industry, biomedicine, food storage and cryobiology. This review collates the findings of the various studies carried out in the past and the recent developments observed in the properties, functional mechanisms, classification, distinct sources and the ever ...
Antifreeze proteins (AFPs) act both by lowering the freezing point of water and by inhibiting ice crystals, and prevent recrystallization during storage. Because of their advantageous characteristics in recent years, numerous studies have been made on the use of AFPs in improving the properties of frozen foods.
They offer distinct advantages over conventional commercial antifreeze agents and natural antifreeze proteins. This review provides an overview of the current state of research on antifreeze agents, elucidates their characteristics and mechanisms, and examines their applications in aquatic products. ... Feature papers represent the most ...
Antifreeze Proteins: Characteristics, Function, Mechanism of Action, Sources and Application to Foods. Antifreeze proteins (AFPs) are synthesized by various organisms to enable their cells to survive subzero environments. AFPs exert their effects by lowering the freezing point of water as well as….
Antifreeze proteins (AFPs) or thermal hysteresis (TH) proteins are biomolecular gifts of nature to sustain life in extremely cold environments. This family of peptides, glycopeptides and proteins produced by diverse organisms including bacteria, yeast, insects and fish act by non-colligatively depressing the freezing temperature of the water below its melting point in a process termed thermal ...
Antifreeze proteins (AFPs) confer the ability to survive at subzero temperatures and are found in many different organisms, including fish, plants, and insects. They prevent the formation of ice crystals by non-colligative adsorption to the ice surface and are essential for the survival of organisms in cold environments. These proteins are also widely used for cryopreservation, food technology ...
Antifreeze proteins solve cold problems. Nature Chemistry 14 , 1336 ( 2022) Cite this article. Anna Ampaw reflects on the effects of antifreeze proteins, from Arctic fish to fruity ice creams ...
Antifreeze proteins (AFPs) represent a distinctive class of proteins that exist in organisms thriving in sub-zero conditions and act as an inhibitor of ice growth by binding to the ice interfaces. The melting or growing inhibition characterization can explain the adsorption-inhibition mechanism. This mechanism occurred within the thermal hysteresis activity of AFPs and is not amenable to ...
The Origin of Antifreeze Proteins and Their Diversity. A notable diversity within various species of AFP families and their arbitrary placement in the respective phylogeny revealed their evolutionary history back to almost 10-30 million years ago when the sea level began to freeze in Antarctica; however; the degree of expression of ice-binding proteins differs in various species (Seifert, 2003).
The Altmetric Attention Score is a quantitative measure of the attention that a research article has received online. ... the asterisk indicates the name of the author to whom inquiries about the paper should be addressed. ... Reversible Binding of the HPLC6 Isoform of Type I Antifreeze Proteins to Ice Surfaces and the Antifreeze Mechanism ...
Research paper : Efforts toward commercialization of antifreeze proteins (ISHII H. et al.) 竏・1竏・BR>Synthesiology - English editionVol.12 No.2 (2021) this paper, was in charge of verification of effect on foods, effort in product realization of the reagents, and writing of this paper. INOUEToshifumi.
Elucidating the role of key structural motifs in antifreeze glycoproteins. The simulations reveal the importance of steric bulk, intra-molecular carbohydrate-protein H-bonds and conformational preferences in controlling the spatial segregation of the hydrophilic and hydrophobic regions of AFGPs. Expand.
1. The present state of knowledge of antifreeze protein structure, composition, bioactivity, biosynthesis and the application of these substances is reviewed. Studies on insect antifreeze proteins have focused on several species, so far over twenty antifreeze proteins (AFPs) had been isolated. This paper reviews the present state of knowledge ...
Antifreeze proteins have been isolated from many different organs, such as the liver, stomach, heart, seeds, stems, bark, leaves, ... AFP type and concentration, and cryopreservation protocol. Extensive research has focused on the development of AFP-overexpressing transgenic animals or plants to improve their survival in extreme cold conditions ...
1. Introduction. Antifreeze proteins (AFPs) and antifreeze glycoproteins (AFGPs) protect organisms living in subzero environments from freezing injury, and even death [1-10].The mechanism relies on inhibiting the growth of seed ice crystals, and the recrystallization of ice [].Type I AFPs have α-helical secondary structures with molecular weights in the range of 3.3-4.5 kDa [4,12-14].
The recent progress of research made at home and abroad on low-temperature-induced proteins and antifreeze proteins (AFPs) with thermal hysteresis (THA) and the identification and expression regulation of low- temperature-induced genes are reviewed. Over the past several years, the proteins and genes associated with freezing resistance and molecular genetic improvement in freezing resistance ...
Antifreeze proteins as cryoprotective agents for foods. ... To evaluate the properties of AFPs and their possible negative effects, further research is needed. In silico studies (in addition to in vitro and in vivo studies) are recommended to perform experiments quickly, with a significant reduction in costs and time, in addition to being able ...
The feature,activity,application,biochemical characteristic of antifreeze proteins,and their expression in bacteria,their role in plant antifreeze physiology and their genetic engineering are systematically reviewed from the view of application.The present situation and current progress in study of antifreeze proteins are discussed in a pithy ...