Top five research articles of 2020
Despite the significant challenges this year has posed, The Pharmaceutical Journal has continued to publish high-quality peer-reviewed research.
Our researchers have made a range of investigations — from evaluating pharmacist interventions using the Simpler tool in Malaysia , to a pharmacist-led virtual thiopurine clinic to support people with inflammatory bowel disease and auto-immune hepatitis, here in the UK.
We have some exciting research coming up in 2021, but in case you missed them the first time around, here are the top five most popular research articles of 2020:

5. Misuse of prescription and over-the-counter drugs to obtain illicit highs: how pharmacists can prevent abuse
Use of prescription and over-the-counter drugs for recreational purposes is increasing, and this perspective article collates the existing literature to provide an in-depth overview of the misuse and diversion of a range of drugs with psychoactive potential, including gabapentinoids, antihistamine drugs and loperamide.
4. Effective detection and management of hypertension through community pharmacy in England
Community pharmacists can play a big role in managing hypertension — from the identification of medication-related problems, to providing lifestyle advice. Despite this, they are not routinely involved in structured hypertension management or screening programmes. So, this review summarises the evidence to recommend the roll-out of a community pharmacy-led hypertension management service.
3. Recent advances in the oral delivery of biologics
Oral administration of medicines is often preferred by patients for its convenience, but, for biologics, the gastrointestinal tract poses challenges for administering in this way. This review discusses the advantages and limitations of several novel drug delivery strategies, and highlights the work to be done to put this technology into clinical practice.
2. Immuno-oncology agents for cancer therapy
Immuno-oncology is a novel treatment that works by conditioning the body’s immune cells to recognise and kill cancer cells — combining this treatment with conventional therapies has led to promising improvements in patient outcomes. This review looks at the range of immuno-oncology agents, and how problems such as their toxicity and high cost can be overcome.
1. Investigational treatments for COVID-19
The emergence of COVID-19 resulted in a global research effort to find effective treatment options to relieve healthcare burdens and, ultimately, save lives. In June 2020, this rapid review summarised the clinical trials and treatment evidence at the time.
Check out The Pharmaceutical Journal’ s ‘Everything you should know about the coronavirus outbreak’ for the latest on this continually evolving situation.
Find the full catalogue of articles in our research section .
Call for submissions
In 2021, The Pharmaceutical Journal will keep adding to the evidence base with review, perspective and research articles. If you have undertaken research into innovations and initiatives that can improve pharmacy services and administration, the pharmacological management of disease, or advances in drug development, please submit your article for consideration by email to: [email protected]
You may also be interested in
The importance of diverse clinical imagery within health education.

Entrustable professional activities: a new approach to supervising trainee pharmacists on clinical placements

Roz Gittins: ‘The longer fitness-to-practise processes take, the longer registrants are left in purgatory’

Pharmaceutical Research
An Official Journal of the American Association of Pharmaceutical Scientists
- Publishes papers on small drug molecules, biotechnology products including genes, peptides, proteins, vaccines, and genetically engineered cells.
- Known for excellent author service with 97% of authors expressing interest to publish again.
- Publishes twelve issues a year.
Societies and partnerships
Latest issue
Volume 41, Issue 3
Latest articles
A refined thin-film model for drug dissolution considering radial diffusion – simulating powder dissolution.
- Karthik Salish
Development and Validation of Matrix of Chemistry, Manufacturing, and Control ( MoCMC ) System for Intramammary Drug Products (IMM)
- Nada A. Helal
- Marilyn N. Martinez
- Mansoor A. Khan

Antibiotic Development: Lessons from the Past and Future Opportunities
- Michael S. Kinch
- Zachary Kraft
- Tyler Schwartz

Combating Alcohol Adduct Impurity in Immunosuppressant Drug Product Manufacturing: A Scientific Investigation for Enhanced Process Control
- Vasanthakumar Sekar
- Devarajan Vedhachalam
- Jean-Marie M. Geoffroy

The Use of Global Sensitivity Analysis to Assess the Oral Absorption of Weakly Basic Compounds: A Case Example of Dipyridamole
- Siddharth S. Kesharwani
- Guillaume Louit
- Fady Ibrahim

Journal updates
The aaps journals make use of portable peer review.
If your manuscript has been declined, and you are planning to submit your article to another of the four AAPS journals ( AAPS Open, The AAPS Journal, AAPSPharmSciTech , or Pharmaceutical Research ), we can help make the peer review process faster and smoother by extending the use of previous peer reviewer comments! Portable Peer Review, an opt-into service available for any author of these journals, allows us to share, between the portfolio, previous peer review comments and reviewer names, subject to approval of all parties. This can help speed up the assessment process, should the editor choose to make a decision on the basis of the comments and revision. Interested? Email us with the name of the journal to which you are going to submit. Note: Portable peer review is opt-in only, available at the authors’ preference, and the reviewer identities and comments will only be shared between offices, not to the author.
AAPS Pharmaceutical Research Meritorious Manuscript Award
AAPS Pharmaceutical Research Meritorious Manuscript Award Recognizing outstanding achievement in the pharmaceutical sciences as demonstrated by the author(s) in the quality and originality of a manuscript published in Pharmaceutical Research. Watch the video here .
Awardee/Corresponding Author: Dennis Douroumis, Ph.D., University of Greenwich Co-Authors: Nicolaos Scoutaris, Ph.D. and Steven A. Ross, Ph.D.
Featured article
Repurposing of kinase inhibitors for treatment of COVID-19 There are three major needs that have yet to be met for effective management of COVID19 disease: 1) anti-viral therapies that limit viral transmission, cell entry, and replication, 2) therapies that attenuate the non-productive immune response and thus decrease end-organ damage, and 3) therapies that have an anti-fibrotic effect in patients with ARDS and thus decrease long-term sequelae of disease. Read the article above for the full review and proposal.
Announcing new Editor-in-Chief of AAPS Open: Dr. Andrea Allmendinger
AAPS Open is proud to announce new Editor-in-Chief Dr. Andrea Allmendinger. Dr. Allmendinger obtained her PhD in Pharmaceutical Technology from the University of Basel and joined the Pharmaceutical Development department of Roche/Genentech in Basel in 2013, where she is responsible for formulation and process development for clinical Phase I-III projects and commercial transfer activities for both large molecules and for synthetic drugs.
Journal information
- Biological Abstracts
- CAB Abstracts
- Chemical Abstracts Service (CAS)
- Current Contents/Life Sciences
- Google Scholar
- Japanese Science and Technology Agency (JST)
- OCLC WorldCat Discovery Service
- Pathway Studio
- Science Citation Index Expanded (SCIE)
- TD Net Discovery Service
- UGC-CARE List (India)
Rights and permissions
Springer policies
© Springer Science+Business Media, LLC, part of Springer Nature
- Find a journal
- Publish with us
- Track your research
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
Pharmacology articles from across Nature Portfolio
Pharmacology is a branch of biomedical science, encompassing clinical pharmacology, that is concerned with the effects of drugs/pharmaceuticals and other xenobiotics on living systems, as well as their development and chemical properties.
Related Subjects
- Clinical pharmacology
- Pharmacodynamics
- Pharmacogenetics
- Pharmacokinetics
- Receptor pharmacology
Latest Research and Reviews
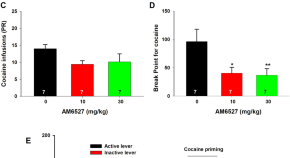
AM6527, a neutral CB1 receptor antagonist, suppresses opioid taking and seeking, as well as cocaine seeking in rodents without aversive effects
- Omar Soler-Cedeño
- Hannah Alton
- Zheng-Xiong Xi
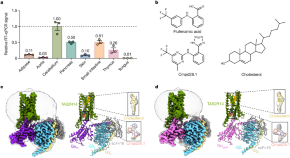
Bitter taste receptor activation by cholesterol and an intracellular tastant
Cryo-electron microscopy structures of the type 2 taste receptor TAS2R14 in complex with Ggust and Gi1 identify cholesterol as an orthosteric agonist and the bitter tastant cmpd28.1 as a positive allosteric modulator and agonist.
- Yoojoong Kim
- Ryan H. Gumpper
- Bryan L. Roth
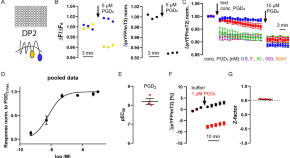
DP2 receptor activity sensor suited for antagonist screening and measurement of receptor dynamics in real-time
- Michael Kurz
- Michaela Ulrich
- Moritz Bünemann
Tumor-selective activity of RAS-GTP inhibition in pancreatic cancer
- Urszula N. Wasko
- Jingjing Jiang
- Kenneth P. Olive
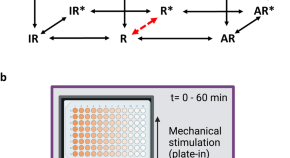
Mechano-sensitivity of β2-adrenoceptors enhances constitutive activation of cAMP generation that is inhibited by inverse agonists
Use of a cAMP biosensor to study real-time mechano-sensitive enhancement of β2-adrenoceptor constitutive activity and its inhibition by β2-inverse agonists in HEK 293 cells.
- Sean A. Cullum
- Simon Platt
- Stephen J. Hill
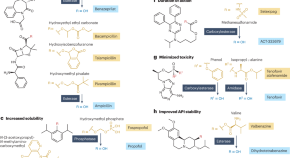
The landscape of small-molecule prodrugs
The development of prodrugs — derivatives of active pharmaceutical ingredients (APIs) with little or no biological activity themselves that are converted into the API after administration — can address issues with properties of the API such as poor bioavailability. This article provides a holistic analysis of approved prodrugs and discusses trends in prodrug design, their indications, mechanisms of API release and the chemistry of promoieties added to APIs to form prodrugs.
- Zachary Fralish
- Ashley Chen
- Daniel Reker
News and Comment
Biosimilar ranibizumab in india- overview of phase 3 clinical trial designs.
- Ashish Sharma
- Nilesh Kumar
- Baruch D. Kuppermann
Given the fraught history of fluorine, Michelle Francl wonders what made medicinal chemists consider fluorine derivatives?
- Michelle Francl
Comment on: “History of testosterone therapy through the ages”
- Diederik F. Janssen
Aflibercept biosimilars – update on the development progress
- Anat Loewenstein
Comment on: Effects of selective dopamine D3 receptor partial agonist/antagonists on oxycodone self-administration and antinociception in monkeys
- Samantha Chong
- Sandra D. Comer
Rapid and novel treatments in psychiatry: the future is now
- Carolyn I. Rodriguez
- Charles F. Zorumski
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
April 10, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
Pharmacy researchers examine trends in rising cost of medicine
by Laurie Fickman, University of Houston

Newly published research from the University of Houston College of Pharmacy reveals an alarming trend in diabetic medication expenditures. While pharmaceutical spending in the U.S. has long been recognized as higher than in other affluent nations, diabetic medications, including insulin, are now at the forefront of this surge in prescription drug costs.
From 2011 to 2020, total annual prescription medication expenditures rose from $341.49 to $473.12 billion per year with metabolic agents being the costliest category. Among the metabolic agents, antidiabetic agents were the most expensive therapeutic area, with an increasing trend observed from $27.15 to $89.17 billion over the same period.
"Despite observed trends in medication expenditures, very little effort has been made to understand how those trends vary by therapeutic class," reports Tyler Varisco, assistant professor of Pharmaceutical Health Outcomes and Policy and assistant director of the Prescription Drug Misuse Education and Research Center in the journal Research in Social and Administrative Pharmacy .
Varisco and Whanhui Chi, a second-year doctoral student in Pharmaceutical Outcomes and Policy, conducted a cross-sectional analysis to identify determinants of increasing medicine expenditures in the U.S. between 2011 and 2020.
Varisco used prescription medication expenditures from the Medical Expenditures Panel Survey to calculate total annual medication expenditures by payer categories (Out-of-pocket, Medicare, Medicaid, TRICARE/Veterans Administration/CHAMPVA (TVAC), Other Government Sources, Private Insurance, and Other Sources).
Spending by Medicare ($38.23 billion) and Medicaid ($8.68 billion) accounted for almost half of all U.S. spending on metabolic agents in 2020.
Varisco also found the growth in prescription drug expenditures in the U.S. can be ascribed to several different factors, such as population demographics, changes in technology, and health care practice. For example, total Medicare enrollment was found to be 48,892,758 in 2011, which later reached 62,840,267 in 2020.
"In light of these developments, research is needed to substantiate concerns that trends in the cost of care are outpacing patients' ability to pay," Varisco says. "Continuing analysis is needed to help policymakers and other key stakeholders understand how changes in practice, policy, and drug marketing converge to impact total market expenditures."
Explore further
Feedback to editors

Case study of 4-year-old with Down syndrome and sleep apnea suggests procedure can be effective at young ages


Study finds esketamine injection just after childbirth reduces depression in new mothers
7 hours ago

A new screening protocol can detect aggressive prostate cancers more selectively
8 hours ago
How a new drug prototype regenerates lung tissue
9 hours ago

Why some people with rheumatoid arthritis have pain without inflammation
10 hours ago

Researchers show chemical found naturally in cannabis may reduce anxiety-inducing effects of THC
'Virtual biopsy' lets clinicians analyze skin noninvasively
11 hours ago
Research team discovers new way to generate human cartilage

Filling in genomic blanks for disease studies works better for some groups than others

Researchers find new origin of deep brain waves
Related stories.

Investigating racial and ethnic differences in Medicare costs for older adults with dementia
Mar 20, 2024

Study finds that Medicare Part D plans increased restrictions on drug coverage
Mar 4, 2024

New report finds COVID-19 pandemic causes dramatic shifts in prescription drug spending
Apr 21, 2021

National health spending expected to increase through 2028
Mar 25, 2020

National health expenditures set to increase through 2031
Jun 18, 2023

Health expenditures considerable for asthma, COPD in U.S. workers
Jul 6, 2020
Recommended for you

Industry gifts may influence which cardiac device is used in common lifesaving procedure
14 hours ago
Morphine tolerance found to result from Tiam1-mediated maladaptive plasticity in spinal neurons
Apr 9, 2024

Popular diabetes drugs do not increase thyroid cancer risk, study suggests

Job insecurity in early adulthood linked to heightened risk of serious alcohol-related illness in later life

Study finds that efforts to help low-income Americans by buying up their medical debt aren't going as planned
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter

Challenges and Opportunities for Pharmacists in Clinical Research
Although the number of clinical trials has quadrupled, the number of FDA approvals remains stable over a 10-year period, highlighting workforce challenges in clinical research.
FDA approvals for drugs, devices, and biologics have remained relatively steady for the past decade, explained Jennifer Espiritu, PharmD, BCOP, CCRP, clinical research pharmacy specialist, Dana-Farber Cancer Institute, during a presentation at the Hematology/Oncology Pharmacy Association (HOPA) Annual Conference 2024 in Tampa, Florida. However, Espiritu noted that when the number of FDA-approved therapies is compared to the amount of open clinical trials, a pattern emerges.
“The number of clinical trials has more than quadrupled in this amount of time, while the number of approvals has remained steady,” said Espiritu during the HOPA session. “There are many reasons why this might be happening, including advances in technology that allow us to identify potential drug targets faster, as well as drug label expansions, and using drugs in different settings.”
However, as the number of clinical trials increases, so does the number of resources needed to conduct them, explained Espiritu. This includes an additional need for resources from institutions that are enrolling and treating patients.
“To illustrate the tremendous effort that goes into a clinical trial, Tufts University conducted a study looking at the complexity of the different clinical trial protocols. They found that the average phase 3 clinical trial collects 3.6 million data points. That's 3.6 million data points for one clinical trial. And this number has tripled over the past decade,” Espiritu said during the session. “When you consider all the kinds of information that can be collected, multiply it by the amount of patients that are enrolled on a clinical trial, and then further multiply it by the time the patients come in for these assessments, it's easy to see how 3.6 million starts to add up.”
Furthermore, as the number of clinical trials increases and they become more complex, clinical research teams have become more strained, according to Espiritu. As a result, there has been a workforce crisis that has developed within institutions.

Image Credit: © Gorodenkoff - stock.adobe.com

“This has become a real threat to successful clinical trial completion and, consequently, therapeutic innovation,” Espiritu said during the session. “At the heart of the problem is the fact that the clinical research workforce suffers from a lack of professional identity. Aside from physicians, other research professionals are often overlooked as key stakeholders in the research ecosystem. This is reflected in the fact that clinical research professional is not a recognized profession by the US Bureau of Labor Statistics.”
This crisis is then further exacerbated by a scarcity of qualified personnel. Espiritu explained that for every 1 experienced clinical research coordinator, there are 7 jobs available. Additionally, for every 1 clinical research nurse, there are 10 jobs available, and for every 1 regulatory affairs professional, there are 35 jobs available.
“This lack of sufficient workforce has been traced back to minimal intentional pursuit,” Espiritu said during the session. “Most professionals working in clinical research have found their way there by accident—including myself.”
Espiritu noted that the clinical research professional pathway is also rarely discussed as a career path in undergraduate programs for nursing and related health sciences. Additionally, what has worsened the supply issue for institutions are issues around skillset requirements in relation to salary discrepancies.
“Clinical research professionals require significant intellectual abilities and skills, but most institution-based staff are leaving to pursue roles in contract research organizations or pharmaceutical companies that offer better pay, benefits, and work-life balance,” Espiritu said during the session. “The academic skillset and salary discrepancy has been described as champagne tastes on a beer budget, which is an accurate analogy for what's going on at most academic medical centers.”
Additionally, the COVID-19 pandemic pushed health care to its limits, and the great health care resignation started, explained Espiritu. High turnover rates further increased the pressure on the remaining staff, which contributed to increased stress and burnout, further perpetuating the attrition cycle.
“Ever increasing trial complexity also contributes to the stress, burnout, and attrition of staff. The more complicated the trial, the more data points they're likely going to require you collect. The more data points there are, the more likely you're going to miss some, and you'll have to file deviations with either the sponsor or your local internal review board,” Espiritu said during the session. “But what if pharmacist practitioners can be incorporated into the clinical team to help alleviate the strain.”
There are certain clinical areas where pharmacists’ skills are particularly valuable when applied to clinical research, according to Espiritu. For example, pharmacists can support critical areas such as patient counseling, medication adherence, and reviewing patients for trial eligibility.
“Most clinical trials have a minimum or maximum amount of lines of therapy a patient has to receive before enrolling. While physicians are no doubt experts in this, the reality is that staff that are doing the preliminary detailed eligibility review are not physicians,” Espiritu said. “For example, in our institution, we have a nurse navigator that actually helps evaluate patients against eligibility. [For these professionals,] prior line requirements can be confusing when clinical trials have different rules as to what counts as one line of therapy.”
For example, Espiritu explained that some trials may not count adjuvant chemotherapy as a line, and so may not count maintenance therapies. Such terms are familiar to pharmacists, but may not be to other researchers that may be quickly skimming a chart, according to Espiritu.
“Most clinical trials will also have a predetermined amount of time that needs to pass from a patient's last dose of chemotherapy to starting treatment. These washout periods are typically defined in definitive timeframes, like 4 weeks from the last chemotherapy, or it can be based on the half-life of the previous chemotherapy given, whichever one ends up being shorter,” Espiritu said during the session. “Instances where a patient’s last therapy has a short half-life, pharmacists can actually identify that it's shorter than the predetermined 4 weeks, for example, and the patient can actually begin treatment a lot sooner.”
Espiritu J. Collaborative Practice in Clinical Trials: Reinforcements for the Crisis in Clinical Research. Presented at: Hematology/Oncology Pharmacy Association Annual Conference 2024; Tampa, Florida; April 3-6, 2024.

Public Health Matters: Seacrest Studios’ Impact on Children’s Hospitals and Healing Through Broadcasting

Digital Therapeutics Might Address Gaps in Care for Mental Health Patients

Pharmacy Focus: Limited Series - Celebrity Endorsements in Public Health

Pharmacists Improve Quality of Depression Care at Patient, Community Levels

More Research Needed for Treatment of Clozapine-Resistant Schizophrenia
2 Commerce Drive Cranbury, NJ 08512
609-716-7777

INNOVATIONS in pharmacy
Vol. 15 No. 1 (2024)
Pharmacy Practice & Practice-Based Research
- Original Research
Copyright (c) 2024 Mohammed AL Qahtani, Ahmed Al-Jedai, Albert Wertheimer

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License .
Copyright of content published in INNOVATIONS in pharmacy belongs to the author(s).
Factors that Influence Healthcare Professionals' Intentions towards Biosimilars
Mohammed AL Qahtani
Security Forces Hospital - Dammam, Saudi Arabia
Ahmed Al-Jedai
Colleges of Medicine and Pharmacy, AL Faisal University, Riyadh, Saudi Arabia
Albert Wertheimer
College of Pharmacy, Department of Sociobehavioral and Administrative Pharmacy, Nova Southeastern University, USA
DOI: https://doi.org/10.24926/iip.v15i1.5922
Keywords: biosimilars, intention, Theory of Planned Behavior
Background : Physicians often prescribe original biologic products to patients who have not used them before and are reluctant to switch to biosimilars. Biosimilars are highly similar versions of already-approved biologics, but healthcare professionals typically hesitate to transition patients from the original products to biosimilars. This study aims to investigate the factors that influence U.S. healthcare professionals' intentions to use biosimilars.
Methods : A cross-sectional study was conducted. 510 participants were eligible healthcare professionals (279 physicians and 231 pharmacists). The theory of planned behavior (TPB) is used to identify which factors affect healthcare professionals' intentions. Descriptive statistics, chi-square, and the logistic regression model tested the TPB constructs as predictors of intentions toward biosimilars.
Results : Among 279 physicians, most were aged 61 and above, with high (n = 142) and low (n = 137) intentions. Male physicians constituted 71% of the population. Attending physicians (66.3%) showed consistent perceptions towards biosimilars, primarily in the private sector (76.3%). Pharmacists (n = 231), a higher percentage of females demonstrated higher intentions compared to males (35.5% vs. 28.1%); the majority were community pharmacists. Associations between years of practice and intentions were significant. Positive correlations existed between beliefs and intentions, except for normative beliefs.
Conclusions : This study revealed diverse attitudes among healthcare professionals towards biosimilars in the USA. Pharmacists and physicians, especially those with limited experience, require ongoing education on biosimilar manufacturing pathways. This education supports the appropriate use of biosimilars and helps standardize federal and state legislation.
Author Biographies
Mohammed al qahtani, security forces hospital - dammam, saudi arabia.
Dr. Mohammad Abdullah N. Al-Qahtani is a healthcare professional with a bachelor’s degree in pharmacy, a master’s in science in Pharmaceutical Economics and Policy, and a Ph.D. in social and administrative pharmacy. Through his positions, he acquired many skills and a comprehensive understanding of the pharmaceutical field. Dr. Al-Qahtani contributed to this field through his work at the Security Forces Hospital in Dammam, Kingdom of Saudi Arabia.
Ahmed Al-Jedai, Colleges of Medicine and Pharmacy, AL Faisal University, Riyadh, Saudi Arabia
Professor at Alfaisal University, Colleges of Medicine and Pharmacy, Riyadh, Saudi Arabia Assistant Deputy for Support Medical Services, Saudi MoH. Consultant Clinical Pharmacist, Solid Organ Transplant at King Faisal Specialist Hospital and Research Center, Riyadh, Saudi Arabia.
Albert Wertheimer , College of Pharmacy, Department of Sociobehavioral and Administrative Pharmacy, Nova Southeastern University, USA
Dr. Albert Wertheimer has been active in sociobehavioral and administrative pharmacy research and education for more than 30 years. He earned a pharmacy degree at the University of Buffalo, an M.B.A. from the State University of New York at Buffalo and his Ph.D. at Purdue University. In addition, he completed a post-doctoral research fellowship in the department of social medicine at St. Thomas” Hospital Medical School of the University of London. He has directed nearly 100 Ph.D. students and more than that number of master’s students. He is the author or editor of 40 books, numerous book chapters and about 430 journal articles. He has lectured or consulted in about 75 countries, and he holds visiting professor appointments at universities in Mexico, Taiwan, Turkey, Slovenia and China. He performed pioneering work in the area of social and behavioral sciences in pharmacy and in more recent years has conducted research in pharmaco-economics, outcomes research, and health policy analysis. Prior to his appointment at NSU, he has been a professor at Temple University, dean of the Philadelphia College of Pharmacy, a researcher at Merck and Company, a vice president of First Health, Inc., a Pharmacy Benefit Manager and a professor and director of graduate studies at the University of Minnesota. Dr. Wertheimer has received many awards and recognitions including fellowships from the American Association of Pharmaceutical Scientists and the International Pharmacy Federation (FIP). He is Scheele Laureate and an honorary member of several professional societies. He has been a study section member/grant reviewer for the US NIH and for AHRQ. Current research interests include pharmaceutical counterfeiting, off label prescribing and cost:benefit studies.

Contact Publishing Services | Acceptable Use of IT Resources
The copyright of these individual works published by the University of Minnesota Libraries Publishing remains with the original creator or editorial team. For uses beyond those covered by law or the Creative Commons license, permission to reuse should be sought directly from the copyright owner listed on each article.
- Introduction
- Conclusions
- Article Information
RCT indicates randomized clinical trial.
Bold copy emphasizes the random-effects model and common effects model. Square size indicates the weight of the study; diamonds, the total weight. ADE indicates adverse drug effect; RR, risk ratio.
a High-dose psilocybin.
b Moderate-dose psilocybin.
eFigure 1. Sensitivity Analysis
eFigure 2. Funnel Plots
Data Sharing Statement
See More About
Sign up for emails based on your interests, select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Get the latest research based on your areas of interest.
Others also liked.
- Download PDF
- X Facebook More LinkedIn
Yerubandi A , Thomas JE , Bhuiya NMMA , Harrington C , Villa Zapata L , Caballero J. Acute Adverse Effects of Therapeutic Doses of Psilocybin : A Systematic Review and Meta-Analysis . JAMA Netw Open. 2024;7(4):e245960. doi:10.1001/jamanetworkopen.2024.5960
Manage citations:
© 2024
- Permissions
Acute Adverse Effects of Therapeutic Doses of Psilocybin : A Systematic Review and Meta-Analysis
- 1 Department of Clinical and Administrative Pharmacy, College of Pharmacy, University of Georgia, Athens
- 2 Department of Clinical and Administrative Sciences, Larkin University, Miami, Florida
- 3 Lloyd L. Gregory School of Pharmacy, Palm Beach Atlantic University, West Palm Beach, Florida
Question What are the notable acute adverse effects for therapeutic doses of psilocybin in the treatment of depression and anxiety?
Findings In this meta-analysis of 6 randomized, double-blind clinical trials with 528 patients, headaches, nausea, anxiety, dizziness, and fluctuations in blood pressure occurred significantly more frequently with psilocybin vs comparators. Psilocybin use was not associated with risk of paranoia and transient thought disorder.
Meaning The findings of this study suggest a tolerable acute adverse effect profile for therapeutic doses of psilocybin, but rare and long-term adverse effects need to be further elucidated.
Importance Psilocybin has been studied in the treatment of depression and anxiety disorders. Clinical studies have mainly focused on efficacy, with systematic reviews showing favorable efficacy; however, none have primarily focused on psilocybin safety.
Objective To evaluate the acute adverse effects of psilocybin at therapeutic doses in the treatment of depression and anxiety.
Data Sources MEDLINE via PubMed, Web of Science, and ClinicalTrials.gov were searched for publications available between 1966 and November 30, 2023.
Study Selection Randomized, double-blind clinical trials that reported adverse effects of psilocybin in patients treated for depression and anxiety were screened.
Data Extraction and Synthesis Data were independently extracted by 2 authors and verified by 2 additional authors following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guideline. The inverse variance method with the Hartung-Knapp adjustment for the random-effects model was used, with a continuity correction of 0.5 for studies with 0 cell frequencies. Sensitivity analysis was conducted by sequentially removing 1 study at a time to assess the robustness of the results.
Main Outcomes and Measures The primary outcome was considered as the adverse effects of psilocybin at high and moderate (ie, therapeutic) dose regimens and compared with placebo, low-dose psilocybin, or other comparator in the treatment of depression and/or anxiety.
Results Six studies met the inclusion criteria with a total sample of 528 participants (approximately 51% female; median age 39.8 years; IQR, 39.8-41.2). Seven adverse effects were reported in multiple studies and included in the analysis. Among these, headache (relative risk [RR], 1.99; 95% CI 1.06-3.74), nausea (RR, 8.85; 95% CI, 5.68-13.79), anxiety (RR, 2.27; 95% CI, 1.11-4.64), dizziness (RR, 5.81; 95% CI, 1.02-33.03), and elevated blood pressure (RR, 2.29; 95% CI, 1.15- 4.53) were statistically significant. Psilocybin use was not associated with risk of paranoia and transient thought disorder.
Conclusions and Relevance In this meta-analysis, the acute adverse effect profile of therapeutic single-dose psilocybin appeared to be tolerable and resolved within 48 hours. However, future studies need to more actively evaluate the appropriate management of adverse effects.
Psilocybin is classified as a serotonergic psychedelic and a prodrug of psilocin (4-hydroxy-dimethyltryptamine), which converts to the active form once ingested. 1 The theoretical mechanism of action involves binding to serotonin 2A (5-HT 2a ) predominantly in the amygdala, thalamus, and prefrontal cortex. The psychopharmacologic profile of psilocybin was examined in the 1960s. It was proposed that oral administration of approximately 10 mg was needed to induce psychological effects, with more potent effects developing with increasing doses. 2 - 4 Psilocybin’s psychological effects were comparable to those of lysergic acid diethylamide, but thought to be more vividly visual, less emotionally intense, more euphoric, and less likely to cause panic attacks or paranoia. 4 Clinical studies suggest psilocybin produces an antidepressant benefit in patients with treatment-resistant depression. 5 This impact is believed to be connected to its affinity for the serotonergic pathway in the brain, which is essential in controlling mood. 5
In recent years, there has been renewed interest in the therapeutic potential of psilocybin in the treatment of mental health (eg, depression, anxiety) disorders. 6 - 8 Psilocybin-assisted therapy typically involves 1 or 2 dosing sessions with individuals encouraged to explore their thoughts and emotions with the support of a therapist. Clinical studies have focused on psilocybin efficacy, resulting in studies pooling and presenting aggregate results. 6 , 7 One recent meta-analysis investigated psilocybin adverse effects as a secondary aim, using a dose-dependent approach focused on select adverse effects. 8 However, these studies have not primarily focused on or explored the adverse effect profile of psilocybin in depth. 6 - 8 Therefore, the purpose of this study was to summarize and examine the relative risk (RR) of acute adverse effects of therapeutic doses of psilocybin in patients with depression and anxiety.
A systematic review of the literature following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses ( PRISMA ) reporting guideline 9 was conducted to identify studies involving participants receiving psilocybin in the treatment of major depressive disorder or depression associated with other related disorders (eg, cancer-related anxiety and depression). Studies included randomized clinical trials comparing psilocybin with either placebo or another comparator (eg, niacin, escitalopram, low-dose psilocybin). Doses were grouped into low (1-3 mg), moderate (10-20 mg), and high (20-30 mg) categories. These dosing ranges were based on previous clinical data. 10 , 11 All studies were evaluated for adverse effects of psilocybin in the treatment of depression and anxiety in study participants. When selecting adverse event profile rates, the shortest time period available was selected and analyzed (eg, day 1 instead of day 30) since the half-life of psilocin is 3 ± 1.1 hours when taken orally and the duration of action can range between 3 to 12 hours. 12 , 13 Therefore, it is expected that psilocin concentrations would be minimal by 24 hours.
Published studies were identified by conducting a search of MEDLINE via PubMed, Web of Science, and ClinicalTrials.gov for publications available between 1966 and November 30, 2023. Search terms included psilocybin , side effects , depression , anxiety , adverse effects , and adverse side effects . Only randomized, double-blind clinical trials published in the English language were included in the systematic review. Studies were included in the analysis if they reported the adverse effects of psilocybin. Studies were excluded if they reported the same adverse events from previous studies or follow-up studies in which adverse event data were already described (eg, deferring adverse event profile to most recent exposure). When appropriate and based on our expertise, adverse effects having different terminology but similar definitions were grouped. These included nausea and transient nausea, headache and transient headache, anxiety and transient anxiety, paranoia and paranoid ideation, and transient thought disorder and abnormal thinking.
Data were independently extracted by 2 of us (A.Y. and N.M.M.A.B.) and verified by an additional 2 of us (J.E.T. and J.C.). The primary outcome was considered the adverse effects of psilocybin at high- and moderate-dose regimens (ie, therapeutic doses) and compared with placebo, low-dose psilocybin, or another comparator in the treatment of depression and anxiety with other related disorders.
The meta-analysis was conducted using R statistical software, version 4.3.1 (R Foundation for Statistical Computing). We used the inverse variance method with the Hartung-Knapp adjustment for the random-effects model and used a continuity correction of 0.5 for studies with 0 cell frequencies. Sensitivity analysis was conducted by sequentially removing 1 study at a time to assess the robustness of our results. For outcomes with 4 or less studies, a common effect model was considered. Heterogeneity among the studies was quantified using the I 2 measure. Additionally, for each study, a funnel plot was created to evaluate publication bias. Study quality was assessed by the risk-of-bias tool for randomized trials (RoB2). 14 Two of us (A.Y. and N.M.M.A.B) determined initial RoB2 scores for the included studies. An additional 2 of us (J.C. and J.E.T.) then independently assessed and verified initial scores. Any discrepancies were discussed and rescored as needed. With 2-sided analysis, the significance threshold was P ≤ .05.
Overall, of 70 published studies identified through PubMed, Web of Science, and ClinicalTrials.gov, 64 studies were excluded ( Figure 1 ). Therefore, 6 studies (total sample of 528 participants; approximately 51% female; 49% male; median age, 39.8 [IQR, 39.8-41.2] years) met our inclusion criteria ( Table ). 11 , 15 - 19 In general, the population was middle-aged adults and more than 90% of the participants were White. The RoB2 assessment tool showed an overall low risk of bias for all included studies. Several adverse effects were identified throughout the studies. Comparators identified in these studies included placebo, niacin, escitalopram, and low-dose psilocybin (1-3 mg). In general, participants experienced adverse effects immediately or within 24 hours after administration of various doses of psilocybin.
Safety data ( Figure 2 and Figure 3 ) from the randomized clinical trials to assess risk ratio via meta-analysis were available for the following 7 adverse effects: headache, nausea, anxiety, dizziness, paranoia, transient thought disorder, and elevated blood pressure. Overall, psilocybin was associated with a greater risk of adverse effects of headache (RR, 1.99; 95% CI, 1.06-3.74; P = .04), nausea (RR, 8.85; 95% CI, 5.68-13.79; P < .001), anxiety (RR, 2.27; 95% CI, 1.11-4.64; P = .02), dizziness (RR, 5.81; 95% CI, 1.02-33.03; P = .047), and elevated blood pressure (RR, 2.29; 95% CI, 1.15-4.53; P = .02) compared with control. Psilocybin use was not associated with risk of paranoia and transient thought disorder. Overall, 2 adverse effects appeared in all 6 studies, including headache, with an incidence ranging from 2% to 66% and nausea with an occurrence varying from 4% to 48%. 11 , 15 - 19 Anxiety was documented in 3 studies, with an incidence ranging from 4% to 26%. 11 , 16 , 17 All adverse effects had an estimated I 2 value of less than 50%, except elevated blood pressure ( I 2 = 78%), suggesting most results were not affected by heterogeneity. In the sensitivity analysis, minor adjustments in RR were observed for headache and nausea, while anxiety, dizziness, paranoia, and transient thought disorder showed unchanged RR values. This consistency, despite limited studies for some conditions, highlights the robustness of our findings. Sensitivity and funnel plot analysis are located in eFigure 1 and eFigure 2 in Supplement 1 .
Additionally, there were 6 acute adverse effects that appeared in greater than 5% of the population, including elevated heart rate (76%), visual perceptual effects (44%), physical discomfort (21%), fatigue (approximately 6%), euphoric mood (approximately 5%), and mood alteration (approximately 5%). 11 , 15 - 17 However, these adverse effects were identified in only 1 study and therefore not included in the meta-analysis. The studies reported none of the adverse events listed were considered serious. A summary of the studies is presented in the Table .
A summary of the acute adverse effects of psilocybin in treating depression and anxiety is needed for health care professionals to identify expected adverse effects and provide effective patient counseling. This study focused on therapeutic doses to clarify the expected adverse effects in potential future practice. The results overall suggest a statistically significant incidence of headache, nausea, anxiety, dizziness, and elevated blood pressure. However, caution is advised in interpreting elevated blood pressure due to its heterogeneity ( I 2 = 78%), indicating potential variability. Given the psilocybin mechanism of action, these adverse effects are expected as they are similar among serotonergic antidepressants. 1 The adverse effects were also anticipated based on previous survey data from adult participants who ingested active doses of psilocybin mushrooms. 20 Additionally, data on adverse effect severity appear to align with documented psychedelic adverse effects over the past 60 years or more. 21 - 23
All 6 studies identified headache as a statistically significant adverse effect of psilocybin, with an incidence ranging from 2% to 66%. 11 , 15 - 19 Headaches were typically mild to moderate in severity, and none required medications for relief. Literature reports corroborate headaches as a known adverse effect of psilocybin. A recent study found a significant dose-response relationship, with the RR of headaches/migraines increasing by 1.42% for each unit increase in psilocybin dose. 8 Moreover, a small double-blind study in healthy individuals indicated a dose-related response to psilocybin with respect to headache occurrence, duration, and intensity. 24 These headaches subsided within 24 hours of psilocybin administration.
All 6 studies identified nausea as an adverse effect. 11 , 15 - 19 Incidence varied between 4% and 48%. 15 , 18 One study reported nausea at 22% with a high dose, but the rate decreased with lower doses (eg, 7% with moderate dose, 1% with low dose). 11 An additional study reported 15% of participants experienced nausea at a high dose and none with a low dose. 16 Recent data support a dose-response association with a relative risk of 1.25% ( P < .001). 8 Five studies stated nausea was not severe and resolved within 60 minutes. 11 , 16 - 19 While the severity of nausea was not discussed in the studies, none reported using any pharmacologic agent to assist with nausea. Additionally, medications to alleviate any severe nausea or vomiting, if needed, were not identified in the study protocols. There are anecdotal reports suggesting eating 1 to 2 hours before taking psilocybin or taking with a small snack, using lemon juice and/or ginger, and hydrating well may be helpful. 25 , 26 Others advise patients can concentrate on themselves in an act of surrendering to the psychedelic experience. 27 , 28 However, there are no clinical studies to support these suggestions or allopathic treatments and caution is warranted. For example, ginger may potentiate the effects of psilocybin due to its ability to increase serotonin and produce negative consequences. 29 The impact a therapist had in assisting patients in managing nausea is also unknown. For example, anecdotal evidence suggests a patient fully immerses themselves into their experience through the guide of a therapist to alleviate nausea dates to the late 1950s-1960s but has not been validated. 27 , 28
Three studies identified anxiety as an adverse effect. 11 , 16 , 17 According to 1 study, anxiety was reported in 4% of participants administered high-dose psilocybin, 8% with a moderate dose, and none with a low dose. 11 However, another study stated 26% of participants with high-dose psilocybin and 15% with low-dose psilocybin experienced an anxiety episode. 16 All 3 studies identifying anxiety stated that, similar to nausea, anxiety resolved between 24 and 48 hours. While the severity of anxiety was not thoroughly discussed in any of the studies, the studies mentioned that anxiety was not serious. In the data set reviewed, 1 case was identified in which a patient received a pharmacologic intervention (ie, lorazepam, 2 mg) after taking high-dose psilocybin and experiencing acute anxiety. 11 In general, the study protocols discussed using medications to treat anxiety not resolving after nonpharmacologic interventions (eg, guidance from therapist). For example, Goodwin et al 11 noted benzodiazepine anxiolytics, such as lorazepam or alprazolam, given orally may be preferred due to rapid onset, short duration of action, and the possibility that another route (eg, intravenous injection) may exacerbate anxiety. Two additional studies listed oral diazepam, 5 to 10 mg, and oral olanzapine, 5 to 10 mg, as rescue medications for severe adverse psychological distress or severe anxiety in the protocols. 17 , 19 Another study mentioned diazepam and risperidone as rescue medications for anxiety or psychosis, with no dosing range. 15 Carhart-Harris et al 18 listed lorazepam (oral or injectable) as a rescue medication for treating events of severe panic that would place people at risk after not responding to psychological intervention. Perhaps having the therapist present may assist in decreasing or managing anxiety through simple arm-holding for patients experiencing anxiety 18 ; however, given the increase in anxiety, there may be a need to provide such pharmacologic transparency on protocols designed to manage anxiety not controlled by the therapist.
Although a recent meta-analysis focused on dose-dependent response did not report dizziness as an adverse effect of psilocybin, 8 our analysis found it statistically significant. Two studies identified dizziness as an adverse effect. 11 , 19 One study reported 6% dizziness with high-dose psilocybin, 1% with moderate dose and none with low dose. 11 The other study reported 8% of participants with dizziness after administration of psilocybin. 19 Both studies stated dizziness resolved between 24 and 48 hours. Neither study used any medication to treat dizziness, and both reported it was a nonserious adverse effect. None of the study protocols defined any medications to treat severe dizziness. Similar to their interventions with other adverse effects, the role a therapist has in managing dizziness (eg, asking patients to lie down, encouraging them to trust the experience) is also unknown. Some protocols state that a patient should lie down with eye coverings, 11 , 16 , 17 , 19 and therefore such measures may be enough to decrease the severity of the dizziness and avoid requiring any medications.
Two studies reported elevated blood pressure and heart rate. 16 , 17 In one trial, 76% of participants experienced elevated blood pressure at a therapeutic dose of 21 mg. 17 In another study, 34% of participants had elevated systolic blood pressure (>160 mm Hg) and 13% had elevated diastolic blood pressure (>100 mm Hg) with high-dose psilocybin. 16 However, patients taking low-dose psilocybin (in control group) also had elevated systolic (17%) and diastolic (2%) blood pressure. There is a possibility the blood pressure increases in the low-dose psilocybin (part of control group) in one study 16 but absent in the other 17 (using niacin as the control) may have contributed to the high heterogeneity. The observed high I 2 statistic points to heterogeneity among the studies because only 2 studies contributed to this result. The limited number of studies and difference in control groups may inflate the heterogeneity measure; therefore, the findings should be interpreted within the context of this limitation. However, recent findings suggest an increased blood pressure dose-related response with a relative risk of 1.04% ( P = .04) appear to support our results. 8 Peak heart rate was 71 beats/min at 300 minutes postdosing in one study, while peak heart rate was 84 beats/min postdosing using high-dose psilocybin in another. 16 , 17 Elevated heart rate in both studies was not considered serious and resolved within 24 hours. Additionally, 1 of the studies noted elevated blood pressure with the moderate dose, which was reported as nonsignificantly different from placebo. 19 However, the sample size of this adverse effect was not described in the study or supplemental materials and therefore not included in the meta-analysis. Also, 4 of the 6 studies 11 , 15 - 17 excluded patients with uncontrolled hypertension or elevated blood pressure at baseline and, therefore, the effects of psilocybin on blood pressure need to be further explored. At this time, we are not aware of any medications used to treat this effect, even though elevated heart rate has been described in the literature. 17 Despite limited data on listing medications to treat increased blood pressure or heart rate, based on its mechanism of action and pharmacokinetic profile, clonidine may be an option for psychedelic-induced increased blood pressure and/or heart rate but has only been studied in mice. 30 Protocols and guidelines note oral nifedipine, 10 mg, or intravenous labetalol as rescue medications for hypertension. 19 , 23 Data suggest both clonidine and nifedipine are equally effective to treat urgent hypertension in general. 31 While dosing per se has not been studied in this target population, based on studies and the pharmacokinetic profile of psilocybin, clonidine, 0.1 to 0.2 mg, orally or nifedipine, 10 to 20 mg, orally per dose appears reasonable and recommended for short-term resolution. 31 , 32
Other adverse effects analyzed in the meta-analysis but not showing a significant difference included paranoia and transient thought disorder. Three studies reported a total of 3 cases of paranoia with high-dose psilocybin across 128 patients. 15 - 17 Additionally, 5 patients in 2 studies totaling 103 patients experienced transient thought disorder with psilocybin (1 with high dose, 4 with moderate dose). 11 , 17 One study listed risperidone, while another reported olanzapine and diazepam as agents to treat psychological distress. 11 , 17 Risperidone can also be a potential option in treating acute psychological distress. 11 While the incidence of both paranoia and transient thought disorder appears to be low, this may be an adverse effect worth monitoring in the future and supported by a recent study suggesting a dose-response relationship. 8 All 6 studies used a therapist/facilitator to assist patients during treatment. The use of these therapists may have played a role in supporting these cases and preventing increased severity or complications. Published guidelines provide recommendations for study personnel to assist participants during their psychedelic experience. 23 Additionally, there are a number of certificate programs designed to provide psychedelic psychotherapy training. 33 None of the studies stated whether any of the therapists had any specific psychedelic certifications. Therefore, the utility of such certifications also merits further study in managing patient response to adverse effects.
Single studies reported other acute adverse effects, including visual perceptive effects (44%), physical discomfort (21%), fatigue (approximately 6%), euphoric mood (approximately 5%), and mood alteration (approximately 5%). 11 , 15 , 16 While these adverse effects were only identified in single studies, it is unknown whether they were specifically evaluated across other studies, and it is difficult to speculate any dose-related response. Regardless, all the adverse effects identified in single trials were not considered serious and resolved within 24 hours with no pharmacologic treatment needed. Visual perceptual effects, an expected adverse effect of psilocybin, was only identified in 1 study. 15 While the incidence occurred in 44% of the participants, 6% continued to experience symptoms after the dosing day and resolved by day 9.
Overall, the strengths of this study include the ability to evaluate the adverse event profile of therapeutic doses of psilocybin using a meta-analysis approach when possible given the currently small number of studies with limited sample sizes. However, these studies had a low risk of bias. There are several limitations to our study results. First, our meta-analysis is based on 6 randomized controlled studies published only in English, which have less sample sizes for analysis to conclude the potential adverse effects caused by psilocybin. Additionally, the studies focus more on acute adverse effects, usually concentrating on the first 48 hours and appear to be less stringent with time. There are some adverse effects that are mentioned in only 1 study and cannot be further analyzed. A bias within the studies for focusing on certain adverse effects and not others may be possible. Selection bias may also be a limitation. Participants in these studies have been predominantly White adults without comorbidities that may be exacerbated (eg, hypertension) with psilocybin use. Also, since psilocybin appears to act as an antidepressant, future studies need to evaluate suicidality that, although rare, may pose a risk in younger adults and is a boxed warning on all antidepressants. This is of particular interest since 5 of the 6 studies appeared to exclude patients with potential suicide risk. 11 , 15 , 16 , 18 , 19 Additionally, there is a lack of recent research data discussing the treatment of psychedelic adverse effects. One study describes safety guidelines for hallucinogen research, and while rescue medications are briefly mentioned, the guidelines primarily focus on participant selection and preparation, study personnel and appropriate conduct, physical environment, and postsession procedures. It is also unknown whether future studies exploring higher doses or more frequent use of psilocybin may carry additional adverse effects or increase the severity of symptoms. Furthermore, it is important to assess the impact a therapist may have in mitigating any of these adverse effects.
In this systematic review and meta-analysis, therapeutic doses of psilocybin appeared to produce tolerable acute adverse effects that typically resolved within 24 to 48 hours. However, less common adverse effects, such as paranoia and prolonged visual perceptual effects, warrant attention. Larger trials are necessary to fully assess these adverse effects, particularly in populations with comorbid health conditions. Recommendations for solicited acute adverse effects should, at a minimum, include headache, nausea, anxiety, dizziness, paranoia, blood pressure and/or heart rate changes, visual perceptual effects, physical discomfort, and mood changes. Although infrequent, the possibility of suicidality, prolonged paranoia, and persistent visual perceptual effects should be monitored over the long term. The effectiveness of medications and alternative treatments in managing these symptoms requires further investigation. Additionally, the role of licensed therapists in managing adverse effects presents an avenue for future research.
Accepted for Publication: February 12, 2024.
Published: April 10, 2024. doi:10.1001/jamanetworkopen.2024.5960
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2024 Yerubandi A et al. JAMA Network Open .
Corresponding Author: Joshua Caballero, PharmD, BCPP, FCCP, University of Georgia, 250 W Green St, Athens, GA 30602 ( [email protected] ).
Author Contributions: Drs Joshua Caballero and Akhila Yerubandi had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Thomas, Harrington, Caballero.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Yerubandi, Thomas, Bhuiya, Caballero.
Critical review of the manuscript for important intellectual content: All authors.
Statistical analysis: Harrington, Villa Zapata.
Administrative, technical, or material support: Yerubandi, Harrington, Villa Zapata, Caballero.
Supervision: Villa Zapata, Caballero.
Conflict of Interest Disclosures: None reported.
Data Sharing Statement: See Supplement 2 .
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
- UB Directory
- School of Pharmacy and Pharmaceutical Sciences >
- News and Events >
- School News >
UB Pharmacy School continues sustained leadership with Top 20 U.S. News & World Report ranking
Published April 9, 2024

BUFFALO, N.Y. — The University at Buffalo School of Pharmacy and Pharmaceutical Sciences (UB SPPS) was again ranked a Top 20 pharmacy school in the recently released 2024 U.S. News and World Report Doctor of Pharmacy (PharmD) school rankings. The report ranked UB SPPS No. 19 nationally and No. 1 in New York State, further solidifying its reputation as an academic and research leader, both regionally and across the country.
“This achievement is a testament to our relentless pursuit of groundbreaking research, transformative discoveries, and unwavering commitment to the success of our students,” says Dean Gary Pollack, PhD. “It is also a reaffirmation of our steadfast commitment to cultivating the next generation of pharmacy and pharmaceutical science leaders who will push the boundaries of scientific inquiry, drive innovation in patient care, and elevate the standards of pharmaceutical research and pharmacy practice on a global scale."
The USNWR Top 20 ranking recognizes school-wide efforts in the expansion of graduate programs and strategic enhancements to the PharmD curriculum, along with the rollout of high impact research work in drug discovery, drug development and translational/experimental therapeutics. These areas have provided the foundation for a strategic faculty hiring initiative, securing top-notch research scientists, clinical practitioners and educators to ensure student success and outcomes.
The PharmD schools and programs ranking is part of U.S. News & World Report’s ranking of “Best Graduate Schools.” A number of other graduate programs at the University at Buffalo were ranked as well.
For over 135 years, the University at Buffalo School of Pharmacy and Pharmaceutical Sciences has continually been a leader in the education of pharmacists and pharmaceutical scientists, renowned for innovation in clinical practice and research. The school is accredited by the American Council of Pharmaceutical Education and is the No. 1 ranked school of pharmacy in New York State and No. 19 in the United States by U.S. News & World Report.
Media Contact Information
Rebecca Brierley Assistant Dean, Communications and Alumni Relations University at Buffalo School of Pharmacy and Pharmaceutical Sciences Tel: 716-645-6965 [email protected]
- School of Pharmacy and Pharmaceutical Sciences
- UB Directory
- TPRC Home >
- News and Announcements >
- UB schools among the best in U.S. News & World Report’s Best Graduate Schools rankings
UB schools among the best in U.S. News & World Report’s Best Graduate Schools rankings

Photo: Douglas Levere
By David J. Hill
Release Date: April 9, 2024

BUFFALO, N.Y. – Several schools within the University at Buffalo, New York’s flagship, are ranked among the top nationwide in U.S. News & World Report’s Best Graduate Schools rankings , released this morning.
The School of Pharmacy and Pharmaceutical Sciences is among the top 20 in the country, coming in at No. 19 overall and 18th among public universities.
“This achievement is a testament to our relentless pursuit of groundbreaking research, transformative discoveries, and unwavering commitment to the success of our students,” said Gary Pollack, PhD, dean of the School of Pharmacy and Pharmaceutical Sciences. “Our top 20 ranking reaffirms our steadfast commitment to cultivating the next generation of pharmacy and pharmaceutical science leaders who will push the boundaries of scientific inquiry, drive innovation in patient care, and elevate the standards of pharmaceutical research on a global scale.”
The School of Social Work is once again in the top 25, coming in at 24th nationally and No. 13 among public universities.
The School of Nursing’s Doctor of Nursing Practice (DNP) program moved up 11 spots to 34th in the country and No. 24 among public universities. In addition, the nursing anesthesia program was ranked 22nd.
“We are immensely proud to rank among the best DNP programs in the United States,” says School of Nursing Dean Annette Wysocki, PhD. “Our commitment to exceptional nursing education cultivates discipline of the mind to educate highly discerning nurse practitioners, who are indispensable in addressing the nation’s critical shortage of primary care providers. Quality nursing education remains paramount in safeguarding the health and wellness of our community.”
UB’s Graduate School of Education moved up four spots to No. 54 in Best Education Schools. Among public universities, the Graduate School of Education ranks 42nd. “Our steady climb in the rankings over the years is a testament to the hard work and high-quality research of GSE faculty,” says dean Suzanne Rosenblith, PhD.
The School of Public Health and Health Professions moved up four spots, to No. 46 nationally and No. 29 among public universities. Its programs in occupational therapy (41) and physical therapy (74) were also ranked, as was biostatistics (22).
Meanwhile, the School of Law moved up 17 places to No. 108 among Best Law Schools nationwide and 56th among public universities.
The School of Management’s MBA program came in at No. 74 in Best Business Schools and 38th among public universities, making it the highest rated business school within SUNY.
In addition, UB’s graduate programs in audiology (22) and speech language pathology (32) were also ranked this year.
Media Contact Information
David J. Hill Director of Media Relations Public Health, Architecture, Urban and Regional Planning, Sustainability Tel: 716-645-4651 [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Can J Hosp Pharm
- v.64(2); Mar-Apr 2011

What is Pharmacy Research?
The question of what constitutes pharmacy research emerged at a recent meeting to discuss a pharmacy resident’s project. The pharmacists around the table were attempting to clearly define this seemingly straightforward term in the context of a residency project surveying hospital pharmacists about “pharmacy research”. Surprisingly, this “obvious” term could not be consistently defined by any of the members present, most of whom were well versed in this type of research. This event triggered a lengthy search to define the enigma called “pharmacy research”.
One of our first stops was the Merriam-Webster dictionary. As expected, we did not find the specific term “pharmacy research” in the dictionary. However, a subsequent search for the individual words revealed the following 1 :
Pharmacy: the art, practice, or profession of preparing, preserving, compounding, and dispensing medical drugs Research: studious inquiry or examination; especially: investigation or experimentation aimed at the discovery and interpretation of facts, revision of accepted theories or laws in the light of new facts, or practical application of such new or revised theories or laws
While combining these definitions yields a broad definition, it is limited by the traditional definition of “pharmacy” that the dictionary provides. However, some may consider “pharmacy research”, as defined by this combination of definitions, to be synonymous with the more modern term “pharmacy practice research”. The Canadian Pharmacists Association (CPhA) defines pharmacy practice research as a component of health services research that focuses on the assessment and evaluation of pharmacy practice. 2 While this definition is clearly unique to the profession, not all research in which pharmacists are involved reflects their practice, nor can it solely reflect the practice of pharmacists, especially in the era of collaborative practice teams. Additionally, research done by pharmacists may address important questions that facilitate improved patient care or service delivery, without specifically advancing pharmacy practice, but still contributing to the scientific literature as a whole. The Canadian Institutes of Health Research (CIHR) lists health services research, which includes the efficiency and effectiveness of health professionals, as 1 of its 4 pillars of health research (the others being biomedical; clinical; and social, cultural, environmental, and population health). 3 However, limiting “pharmacy research” to pharmacy practice research limits the impact and relevance of pharmacists’ work to the other CIHR pillars of research deemed to be important to Canadians.
To further refine our definition, we looked to the literature and leading Canadian and US pharmacy organizations for additional insight. An Internet and literature search provided little help with our dilemma. While generally advocating and supporting research, none of these organizations—specifically the CPhA, the Canadian Society of Hospital Pharmacists (CSHP), the American College of Clinical Pharmacy (ACCP), and the American Society of Health-System Pharmacists—have clearly defined pharmacy research. However, both the CSHP and the ACCP have attempted, at least in part, to define “research”.
CSHP has published 2 papers discussing institutional pharmacy research, a statement (published in 1995) 4 and a set of guidelines (published in 1997) 5 . The 1995 statement 4 loosely states that “any unknown in the practice field is a potential research idea” and includes the following as research topics for institutional pharmacists:
- basic pharmaceutical sciences, including the development and testing of new dosage forms or medication-administration modalities
- clinical research concerning the efficacy, safety, and pharmacokinetics of drugs
- pharmacy practice research addressing various issues such as the evaluation of new and existing services, workload measurement, pharmacoeconomics, and quality management
The 1997 guidelines 5 are just as vague and incomplete. They state that “the term ‘research’ can be used to describe many endeavours in institutional pharmacy practice” which may include literature reviews, descriptive studies, and hypothesis-driven research. Neither of the CSHP documents provides the reader with a clear definition of pharmacy research, even though this term is used in the title of both documents. These documents are currently being revised, and we hope that these ambiguities will be addressed.
The ACCP has defined clinical pharmacy as “that area of pharmacy concerned with the science and practice of rational medication use”. 6 This definition offers a more contemporary perspective of pharmacy. The ACCP further defines clinical research as “studies of human subjects, including surveys, cross-sectional studies, case-series, case–control studies, cohort studies, first-in-human studies, proof-of-principle projects and all phases of clinical trials”. 7 By marrying these definitions (which do not appear in the same document), we can create a broad definition of clinical pharmacy research. However, it is likely intentional that these 2 terms were not presented in a single document, as the ACCP is advocating for increased pharmacist participation in all types of clinical research, not just practice-based research. 8
It is clear that while we continue to use the term “pharmacy research”, it carries no universally accepted meaning. As our group discovered, there is far too much ambiguity related to this term. Perhaps we should consider abandoning it.
Perhaps we also need to re-examine our approach to research as a profession. Instead of undertaking so-called ”pharmacy research”, we should follow the lead of our US counterparts and develop a system of pharmacist-researchers and scientists and describe research as any research activity done by pharmacists, regardless of the topic. As health care professionals, pharmacists represent only one aspect of the complex and interdependent health care system. Focusing our energies and resources solely on studying the practice of pharmacy may or may not help in developing our practice, but it will likely add little to the entire health care system. Pharmacists must be involved in all aspects of health research, from basic laboratory investigations to population-based studies. Our unique set of skills and our focus will ensure that we have distinctive research topics. Limiting our contributions to the pillars of health services and clinical research represents a disservice to the advancement of pharmacy and to Canadians.

IMAGES
VIDEO
COMMENTS
JAMA Network Open. Research. April 5, 2024. This cross-sectional study examines the proportion of supply chain issue reports associated with drug shortages overall and during the COVID-19 pandemic. Health Policy Pharmacy and Clinical Pharmacology Coronavirus (COVID-19) Regulatory Agencies Pharmacoeconomics.
Call for submissions. In 2021, The Pharmaceutical Journal will keep adding to the evidence base with review, perspective and research articles. If you have undertaken research into innovations and initiatives that can improve pharmacy services and administration, the pharmacological management of disease, or advances in drug development, please submit your article for consideration by email to ...
The Journal of Pharmacy Practice (JPP) is a peer-reviewed journal that was established in 1988 and is published 6 times per year. The journal is read and cited both nationally and internationally. ... Research article. First published Feb 17, 2021. Insulin Dosage Adjustments After Initiation of GLP-1 Receptor Agonists in Patients With Type 2 ...
JACCP accepts original research and review articles on all aspects of clinical pharmacy practice and clinical pharmacy education and training. JACCP will also publish editorials, ... Those interested in becoming a reviewer for JACCP should send a curriculum vitae and their areas of practice and research expertise to Keri Sims ([email protected]).
Journal of Pharmacy and Pharmacology ( JPP ), founded in 1870, is one of the leading pharmaceutical sciences journals publishing original research papers, critical reviews and rapid communications keeping you up-to-date with the latest developments in the pharmaceutical sciences. Find out more. Scutellarein: a review of chemistry and pharmacology.
Various classifications for research designs and methods used in pharmacy practice have been used in the literature. The following are some of the approaches for the classification of research designs: 1. Classification based on time orientation: Retrospective vs. prospective designs. a.
1. The Emergence of Pharmacy. Throughout the 19th century, pharmacy emerged as an identifiable profession emanating from a nebulous background in which various actors delved in medicinal science and other aspects of healthcare [1,2,3] At the beginning of the 19th century in the United Kingdom, parliament passed an act (1815) stating "There were four degrees in the medical profession ...
Pharmaceutics articles from across Nature Portfolio. Pharmaceutics is the scientific discipline concerned with the process of creating the dosage form (such as a pill for oral administration or a ...
Journal of Research in Pharmacy Practice. 12(1):29-31, Jan-Mar 2023. Abstract. Favorite; PDF; Permissions Open. Letter to the Editor Keep Away from Children's Reach: A Missing Link of Pharmacy Practice Research. Kanan, Mohammed. Journal of Research in Pharmacy Practice. 12(1):32, Jan-Mar 2023. ...
Restricted access Other First published January 18, 2024 pp. 522-525. xml GET ACCESS. Table of contents for Journal of Pharmacy Practice, 37, 2, Apr 01, 2024.
Journal of Pharmacy Research. Articles & Issues. Menu. Articles & Issues. Latest issue; All issues; Submit search. About the journal. Title transferred to JPR Solutions as of 2014; ... Research article Full text access. Synthesis and in-vitro anticancer activity of 3-cyano-6,9-dimethyl-4-imino 2-methylthio 4H-pyrimido [2,1-b] ...
Pharmaceutical Research is an official journal of the American Association of Pharmaceutical Scientists, covering innovative research in drug discovery, development, evaluation, and regulatory approval.. Current emphasis of the journal includes: preformulation; drug delivery and targeting; formulation design, engineering, and processing; pharmacokinetics, pharmacodynamics, and pharmacogenomics ...
Pharmacology articles from across Nature Portfolio. Pharmacology is a branch of biomedical science, encompassing clinical pharmacology, that is concerned with the effects of drugs/pharmaceuticals ...
The objectives of this article are to describe recent health care trends, evidence and policies and to identify opportunities for the profession of pharmacy. ... Data from the Ontario Pharmacy Evidence Network (OPEN) research group and others demonstrate that uptake of community pharmacist-delivered medication reviews and vaccinations in ...
The Journal of the American Pharmacists Association (JAPhA) is a peer-reviewed forum to improve medication use and health outcomes, inform health care policies, and advance pharmacist-provided services. Editor in Chief: Pamela Heaton, University of Cincinnati. Deputy Editor: Spencer Harpe, Midwestern University Chicago.
Hospital Pharmacy is an independent, peer-reviewed journal. For 50 years it has been practitioner-focused and dedicated to the promotion of best practices and medication safety. Turn to HPX for essential information on medication errors, adverse reaction reporting, formulary drug reviews, original research, current FDA-related drug information, off-label drug uses, new technology, and more.
Hot Topics in Pharmaceutical Research. In this virtual issue, we highlight some of the most impactful recent articles in the journal as reflected by citations in 2022. Highly cited articles provide insight into which research topics are attracting the most attention and reflect innovative new discoveries, or timely reviews and perspectives on ...
Pharmacy is an international, scientific, peer-reviewed, open access journal dealing with pharmacy education and practice and is published bimonthly online by MDPI.. Open Access — free for readers, with article processing charges (APC) paid by authors or their institutions.; High Visibility: indexed within ESCI (Web of Science), PubMed, PMC, Embase, and other databases.
From January 1, 2023, Pharmacological Research will become a full gold open access journal freely available for everyone to access and read. All articles submitted after October 14, 2022, are subject to an article publishing charge (APC) after peer review and acceptance.Learn more about hybrid journals moving to open access. $3680
Pharmacy Times offers the latest news and insights for the pharmacy professional and solutions that impact the everyday practice of pharmacy. News. All News. Press Releases. ... highlighting workforce challenges in clinical research. Read More. FDA Approves Fam-Trastuzumab Deruxtecan-nxki for Unresectable or Metastatic HER2-Positive Solid Tumors.
Newly published research from the University of Houston College of Pharmacy reveals an alarming trend in diabetic medication expenditures. While pharmaceutical spending in the U.S. has long been ...
Challenges and Opportunities for Pharmacists in Clinical Research. April 6, 2024. Alana Hippensteele, Managing Editor. Feature. Article. Although the number of clinical trials has quadrupled, the number of FDA approvals remains stable over a 10-year period, highlighting workforce challenges in clinical research. FDA approvals for drugs, devices ...
The main focus of JRPP will be on evidence-based drug-related human research. However, a wide range of closely related issues will also be covered in a new approach to have a holistic look at Pharmacy Practice. These will include Pharmaceutical Care, Pharmacoepidemiology, Social Pharmacy, Pharmacy Education, Process and Outcome Research ...
Inspiring Inquiry and Improvement in Pharmacy Practice, Education, and Policy A quarterly publication featuring case studies, clinical experiences, commentaries, idea papers, original research, and review articles that focus on the leading edge, novel ideas for improving, modernizing, and advancing pharmacy practice, education, and policy.
Clinical Pharmacy and Pharmacology; Complementary and Alternative Medicine; Consensus Statements; Coronavirus (COVID-19) ... there is a lack of recent research data discussing the treatment of psychedelic adverse effects. One study describes safety guidelines for hallucinogen research, and while rescue medications are briefly mentioned, the ...
BUFFALO, N.Y. — The University at Buffalo School of Pharmacy and Pharmaceutical Sciences (UB SPPS) was again ranked a Top 20 pharmacy school in the recently released 2024 U.S. News and World Report Doctor of Pharmacy (PharmD) school rankings. The report ranked UB SPPS No. 19 nationally and No. 1 in New York State, further solidifying its reputation as an academic and research leader ...
The application of rigorous qualitative research in pharmacy education holds great potential in addressing complex and evolving healthcare problems. This work provides empirical evidence of the ongoing anecdotal dialogue that has long existed in pharmacy education concerning why some researchers are hesitant to conduct qualitative research, the ...
The School of Pharmacy and Pharmaceutical Sciences is among the top 20 in the country, coming in at No. 19 overall and 18th among public universities. "This achievement is a testament to our relentless pursuit of groundbreaking research, transformative discoveries, and unwavering commitment to the success of our students," said Gary Pollack ...
Pharmacy: the art, practice, or profession of preparing, preserving, compounding, and dispensing medical drugs. Research: studious inquiry or examination; especially: investigation or experimentation aimed at the discovery and interpretation of facts, revision of accepted theories or laws in the light of new facts, or practical application of ...