- Open access
- Published: 29 September 2020

Medical cannabis use in the United States: a retrospective database study
- V. Kishan Mahabir 1 ,
- Jamil J. Merchant 1 ,
- Christopher Smith 1 &
- Alisha Garibaldi ORCID: orcid.org/0000-0001-6332-7754 1
Journal of Cannabis Research volume 2 , Article number: 32 ( 2020 ) Cite this article
11k Accesses
26 Citations
52 Altmetric
Metrics details
Introduction
Growing interest in the medicinal properties of cannabis has led to an increase in its use to treat medical conditions, and the establishment of state-specific medical cannabis programs. Despite medical cannabis being legal in 33 states and the District of Colombia, there remains a paucity of data characterizing the patients accessing medical cannabis programs.
We retrospectively reviewed a registry with data from 33 medical cannabis evaluation clinics in the United States, owned and operated by CB2 Insights. Data were collected primarily by face-to-face interviews for patients seeking medical cannabis certification between November 18, 2018 and March 18, 2020. Patients were removed from the analysis if they did not have a valid date of birth, were less than 18, or did not have a primary medical condition reported; a total of 61,379 patients were included in the analysis. Data were summarized using descriptive statistics expressed as a mean (standard deviation (SD)) or median (interquartile range (IQR)) as appropriate for continuous variables, and number (percent) for categorical variables. Statistical tests performed across groups included t-tests, chi-squared tests and regression.
The average age of patients was 45.5, 54.8% were male and the majority were Caucasian (87.5%). Female patients were significantly older than males (47.0 compared to 44.6). Most patients reported cannabis experience prior to seeking medical certification (66.9%). The top three mutually exclusive primary medical conditions reported were unspecified chronic pain (38.8%), anxiety (13.5%) and post-traumatic stress disorder (PTSD) (8.4%). The average number of comorbid conditions reported was 2.7, of which anxiety was the most common (28.3%). Females reported significantly more comorbid conditions than males (3.1 compared to 2.3).
This retrospective study highlighted the range and number of conditions for which patients in the US seek medical cannabis. Rigorous clinical trials investigating the use of medical cannabis to treat pain conditions, anxiety, insomnia, depression and PTSD would benefit a large number of patients, many of whom use medical cannabis to treat multiple conditions.
The cannabis plant has been used in traditional medicine for centuries, and within the last few decades it has generated considerable attention among the general population, modern medical community and regulatory bodies for its potential medicinal capabilities (Alsherbiny and Li 2018 ). The effects of cannabis are due to the action of cannabinoids, a diverse group of chemical compounds found in the cannabis plant that act on the human endocannabinoid system, via a series of interactions with cell receptors throughout the human body, and alter neurotransmitter release in the brain affecting various physiological functions (Fraguas-Sanchez and Torres-Suarez 2018 ; Vuckovic et al. 2018 ). While more than 100 cannabinoids have been identified, Δ-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) have undergone the most scientific investigation and are considered to be the greatest contributors to the medicinal effects of cannabis (Pertwee et al. 2010 ; Pertwee 1997 ).
The growing interest in medical cannabis has led to an increase in its use to treat medical conditions or symptoms thereof, such as chronic pain, anxiety, and depression. Individuals do this either through self-medication, accessing the drug via the recreational or illicit markets, or via medical cannabis programs in regions where regulations permit. Other clinical conditions that cannabis is thought to treat include multiple sclerosis, AIDS-associated wasting/cachexia, insomnia, arthritis, epilepsy, post-traumatic stress disorder (PTSD), glaucoma, headaches and migraines, and nausea (Kosiba et al. 2019 ; Lu and Anderson 2017 ; Kaur et al. 2016 ; Zaller et al. 2015 ; Klumpers and Thacker 2019 ; Institute of Medicine (U.S.) 1999 ).
Medical cannabis programs began in the United States (US) in 1996, with California becoming the first state to legalize medical cannabis (Legislatures NC of S 2020 ). Since then, other states have slowly adopted medical cannabis programs, with the programs themselves evolving over time. As of 2000, 8 states had legalized medical cannabis (Yu et al. 2020 ); by 2010, there were 16 and by 2016, there were 29 (Pacula and Smart 2017 ). As of July 2020, medical cannabis is legal in 33 states and the District of Columbia, 12 of which allow adults over the age of 21 to use cannabis recreationally (DISA Global Solutions 2019 ).
Qualifying conditions for medical cannabis vary significantly state-by-state as some states (e.g., California, Massachusetts, Oklahoma, and the District of Columbia) allow physicians to use discretion when recommending patients for certification, while other states only allow certification based on a limited set of qualifying conditions (Legislatures NC of S 2020 ). The allowable THC-percentage component of state-run programs also varies, with certain states only allowing access to high-CBD, low-THC products for medical cannabis patients. Patients seeking medical cannabis in the US in most states are required to obtain a state-specific medical cannabis identification card, allowing them to purchase cannabis products from dispensaries to treat certain medical conditions.
Despite medical cannabis being legal in many states, there remains a paucity of data characterizing the patients accessing it via state-run programs. Two large studies reviewed available state registry data of patients holding medical cannabis licenses; however, these studies came with limitations including voluntary reporting at the state-level, or inability for the authors to access the registry data (Boehnke et al. 2019 ; Fairman 2016 ). One of the studies reviewed the primary conditions for which patients sought medical cannabis, but did not report any other patient characteristics such as age or gender (Boehnke et al. 2019 ). The other reported on the age and gender of patients accessing medical cannabis, but did not report on medical conditions (Fairman 2016 ). While useful, these studies did not adequately characterize medical cannabis use through state programs by age, gender or condition. Other studies that have been published to characterize medical cannabis patients are limited by sample size and selection, include only patient-reported data, or include patients outside of the US (Sexton et al. 2016 ; Eurich et al. 2019 ; Bonn-Miller et al. 2014 ; Reinarman et al. 2011 ).
Given the need for a large data set to contribute to the medical knowledge, inform on policy and identify areas for future research, we designed a retrospective study of a registry database. The primary objective of this study was to thoroughly describe the population of patients seeking treatment with medical cannabis in the US. These data were reviewed at a high-level to answer the following questions:
What are the key demographic characteristics of patients accessing medical cannabis?
Are there differences in characteristics of males and females accessing medical cannabis?
What are the most commonly reported conditions among this sample of patients?
How many conditions do patients seek treatment for, and does this change based on age and gender?
These questions were investigated to assist the medical community in further developing an understanding of patients seeking medical cannabis for treatment of their conditions and symptoms, and to assist others in determining areas of interest for future research. This knowledge may also inform policy makers in states considering medical cannabis legalization or the further development of existing medical cannabis programs in states where medical cannabis has already been legalized.
This was a retrospective database study of patients seeking medical cannabis certification in the US. Data were extracted from the database software utilized in CB2 Insights’ clinical network. CB2 Insights operates one of the largest collections of medical cannabis evaluation clinics in the US, collectively assessing approximately 100,000 patients per year seeking access to medical cannabis, using a single and consistent software that contributes data to a patient registry. These 33 independent clinics are not connected to dispensaries or producers of medical cannabis, and are situated across 12 states (number of clinics): Colorado (6), Connecticut (1), Delaware (2), Illinois (1), Maine (1), Maryland (1), Massachusetts (10), Missouri (1), New Jersey (5), New York (1), Rhode Island (2), and Pennsylvania (2). Patients access these clinics by physician-referral or self-referral through word of mouth, community out-reach and marketing. Over 95% of data were collected via face-to-face interview, with the remaining collected via telemedicine. Patients presenting to any of the clinics are required to complete the same baseline information upon intake, including demographic, medical, and therapeutic information; however, certain characteristics such as race and gender were not made mandatory initially, and are not reported for all patients. Baseline questions include patient-reported tobacco smoking and alcohol use, current or past substance abuse of drugs and/or alcohol, use of illicit (illegal) drugs, medication use and alternative therapies. Medication use is an open-ended question that may be completed by transcribing a medication list into the software, which leaves room for errors and may be a limitation of the data. All patients indicate their primary reason for seeking access to medical cannabis and are asked to report all comorbid conditions for which they are also seeking medical cannabis. Patients are required to provide supporting documentation of their medical histories and relevant conditions for review and verification, in the form of medical records or a letter from another physician. Review of medical documentation, in combination with a medical evaluation by a state-authorized physician or nurse practitioner are used to confirm their qualification for medical cannabis within their respective state. Prior to data export, the protocol was reviewed by the Advarra Institutional Review Board (IRB) and was determined to be exempt from IRB oversight ( Pro00042652 ) as the study had minimal risk, the data exports were void of patient identifiers, and it did not require direct patient contact.
Data were exported for 62,145 patients who were seen for their initial assessment between November 18, 2018 (when the technology and standardized protocol were introduced into the clinics) and March 18, 2020. Data were exported without any patient identifiers to ensure patient anonymity. Eligibility criteria were applied to the data set and the following patients were removed: 1) 77 patients without a valid date of birth; 2) 78 patients younger than 18; and 3) 611 patients without a primary medical condition reported. Overall, 61,379 patients were included in the analysis (Fig. 1 ).
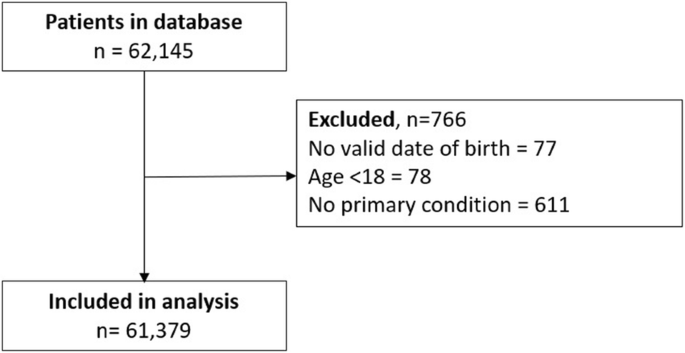
Patient Flow
Data from the database software utilized in CB2 Insights’ clinical network were also merged with US tax data, which provides tabulations of income tax data by ZIP code in order to estimate household income based on individual patients’ ZIP codes. Median household income values from the 2018 dataset purchased from Cubit Planning Inc. were used (US Income Statistics - Current Census Data for Zip Codes 2018 ). Cubit Planning Inc. summarizes the most current income statistics from the US Census Bureau.
When a final dataset was confirmed, data were analyzed using RStudio (Boston, MA). All information was summarized using descriptive statistics expressed as a mean (standard deviation (SD)) or median (interquartile range (IQR)) as appropriate for continuous variables, and number (percent) for categorical variables. Univariate analyses were conducted to inform multivariate analyses including t-tests when comparing means and chi-squared tests when comparing proportions. Regression analyses were conducted to determine if age and gender, specifically, were significant predictors of characteristics of smoking, alcohol consumption, prior cannabis use and medication usage, the number of medications being used, and the number of conditions reported. Logistic regression was used for dichotomous variables and linear regression was used for continuous variables. To analyze whether age and gender were significant predictors of reporting each primary condition, each condition was compared separately to all others using logistic regression. An interaction model with age and gender was included for all regression analyses; if the interaction effect was significant, p -values are reported for the interaction model, otherwise p-values are reported for the model without interaction. All tests were completed with a significance level of 0.05. P -values less than 0.001 are expressed as p < 0.001, and 95% confidence intervals (CI) are provided where appropriate.
The average age of patients in the sample was 45.5 (SD = 15.8) and 54.8% were male (Table 1 ). The average age of females, 47.0 (SD = 15.7), was significantly greater than males, 44.6 (SD = 15.7) ( p < 0.001, difference in means = 2.4, 95% CI: 2.15–2.68) (Table 2 ). Of the patients with race reported, Caucasians represented the largest group of the sample population at 87.5%. The median household income in the ZIP code in which patients resided was available for 56,083 patients. The overwhelming majority of patients lived in a ZIP code where the median household income was above $40,000 (93.7%); the median was $69,481 (IQR $35,807). Most patients (66.9%) reported that they had experience with cannabis prior to seeking medical certification, were non-smokers (81.2%), did not drink (57.5%) and did not have a history of substance abuse (94.4%). Gender was not a significant predictor of reporting prior cannabis experience or history of substance abuse ( p = 0.929 and 0.871, respectively), but was for smoking status and alcohol consumption ( p < 0.001) (Tables 3 and 4 ). Males reported smoking tobacco more than females, whereas females reported the use of alcohol more than males.
Less than half of patients reported prescription medication use (44.2%). Increased age and female gender were significant predictors of reporting at least one medication ( p < 0.001) and a greater number of medications (p < 0.001) (Tables 3 and 4 ). Of patients who reported taking at least one medication ( n = 27,106), the mean number of medications reported was 4.1 (SD = 3.7). Over half the sample (59.4%) reported currently using an alternate form of therapy. Of those who reported using a therapy, the average number reported was 2.5 (SD = 1.7), and the most commonly reported among this group were exercise (70.9%), massage therapy (36.4%), mental health counselling (30.8%) and chiropractor (30.3%). A quarter of the sample (26.1%) did not report any current use of medications or alternate therapy.
Regardless of gender, the top three primary medical conditions were unspecified chronic pain ( n = 23,817, 38.8%), anxiety ( n = 8280, 13.5%) and PTSD ( n = 5143, 8.4%) (Table 5 ). Following the top three were back and neck problems ( n = 3969, 6.5%), arthritis ( n = 2395, 3.9%), insomnia ( n = 2096, 3.4%) and cancer-related pain ( n = 1641, 2.7%). Depression, migraines, muscle spasms, ADD/ADHD, chronic nausea, fibromyalgia, headaches and epilepsy were each reported as the primary medical condition for 2.0% or less of the sample. Of the primary medical conditions, 10.6% of those reported were other medical conditions each representing less than 1.0% of the entire sample. Gender was not a significant predictor of epilepsy, but was a significant predictor for all other conditions (Table 6 ); females were significantly more likely to report anxiety, PTSD, arthritis, cancer related pain, depression, migraines, chronic nausea, fibromyalgia and headaches, whereas males were significantly more likely to report unspecified chronic pain, back & neck problems, insomnia, muscle spasms and ADD/ADHD.
Patients reporting anxiety, PTSD, depression, migraines, ADD/ADHD, chronic nausea, headaches or epilepsy as their primary reason for seeking medical cannabis were significantly more likely to be younger ( p < 0.001), whereas patients seeking medical cannabis primarily for unspecified chronic pain, back and neck problems, arthritis, insomnia, cancer related pain or fibromyalgia were significantly more likely to be older (Table 6 ). Age was not a significant predictor of reporting muscle spasms.
Patients were able to report any number of comorbid medical conditions necessary to describe their reason(s) for seeking medical cannabis (Table 7 ). The average number of comorbid medical conditions reported was 2.7 (SD = 2.6). Anxiety was the most commonly reported comorbid condition ( n = 17,359, 28.3%), followed by back and neck problems ( n = 14,550, 23.7%), insomnia (n = 14,247, 23.2%), depression ( n = 13,413, 21.9%), and unspecified chronic pain ( n = 11,199, 18.2%). Only 17.6% of the sample did not report a comorbid medical condition.
Taking into consideration all medical conditions reported (both primary and comorbid (Table 7 )), over half of the sample reported unspecified chronic pain (57.0%), followed by anxiety (41.8%), back and neck problems (30.2%), insomnia (26.6%) and depression (23.9%). Patients reported an average of 3.7 total medical conditions (SD = 2.6). Younger age and female gender were significant predictors of the number of conditions patients reported ( p = 0.003 and p < 0.001, respectively). Females reported an average of 4.1 (SD = 2.9) total conditions, compared to an average of 3.3 (SD = 2.3) among males.
We conducted an extensive retrospective study with the objective of describing the population of patients seeking treatment with medical cannabis at 33 clinics in the US. Our results indicate that patients seeking medical cannabis in the US most commonly report suffering from unspecified chronic pain (57.0%), regardless of age or gender, which is consistent with similar studies that report 61.2 to 82.6% of patients seeking medical cannabis for chronic pain (Boehnke et al. 2019 ; Sexton et al. 2016 ; Eurich et al. 2019 ; Reinarman et al. 2011 ). Second to unspecified chronic pain, patients were most likely to report anxiety as their primary medical condition, and anxiety was the most commonly reported comorbid condition. This finding is consistent with results from a survey completed by Sexton et al. among self-identifying medical cannabis patients, in which the second and third most common medical conditions that patients reported using medical cannabis for were anxiety (58.1%) and depression (50.3%) (Sexton et al. 2016 ). Gender was a significant predictor for most primary conditions, which is unsurprising as males and females have different risk factors, experiences and perceptions of illness and do not tend to report or be diagnosed with medical conditions in equal proportions (Seeman 1997 ; Buvinić et al. 2006 ; Westergaard et al. 2019 ).
The average number of conditions and comorbidities is not commonly stated, but has been reported at 1.8 and 3.0 in similar studies, both lower than our findings (Reinarman et al. 2011 ; Salazar et al. 2019 ). The average number of conditions reported differed between males and females, with females reporting a higher average number of conditions. This aligns with previous research that has demonstrated that females access health care services more than males and may be more diligent with providing relevant information, which may partially explain why females tend to have higher reported rates of morbidity (Bertakis et al. 2000 ; Verbrugge and Wingard 1987 ; Waldron 1983 ; MacIntyre et al. 1999 ).
Similar to the survey by Salazar et al., this study also demonstrated the wide variety of conditions for which patients access medical cannabis (Salazar et al. 2019 ). Conditions representing less than 1.0% of sample accounted for 10.6% of primary conditions reported and included more than 200 unique conditions (Table 8 ), the majority of which came from states where physicians are able to use their discretion for patients’ qualification (MA, MD, ME, MO). The information on the number and variety of conditions for which patients report seeking medical cannabis treatment is important for medical practitioners for several reasons. Firstly, it highlights the breadth of conditions for which patients are seeking medical cannabis for symptomatic relief. This is important as it identifies patients who may potentially turn to them with questions regarding their suitability for medical cannabis or who may already be seeking medical cannabis without their knowledge, demonstrating the need for practitioners to educate themselves and be prepared to discuss and provide their professional medical opinion. Secondly, these data demonstrate that patients seeking medical cannabis are complex patients who have more than a single ailment. While rigorous clinical trials are still needed to validate the use of medical cannabis for these conditions, real world data are also needed to describe these patients, as complex patients are more likely to be excluded from clinical trials evaluating the effectiveness of a medication (Hanlon et al. 2019 ). Finally, these data highlight the potential utility of medical cannabis and how it is currently utilized for treatment of multiple conditions with which a patient is suffering.
Medication use and average number of medications increased with age; however, patients reported medication use less than the general US population overall. Findings from the National Health and Nutrition Examination Survey reported that 83.6% of adults aged 60 and over used prescription medication in the previous 30 days, compared to only 56.4% of our sample aged 60 and older. For adults aged 40–59, our sample also reported less medication use; 48.3% compared to 59.5% (Martin et al. 2015 ). This difference may be a result of patients under-reporting their medications at the clinic, or medication information not being correctly transcribed into the software from practitioners’ notes. Alternatively, it may show a true difference in characteristics between the general US population and those accessing medical cannabis. The latter may suggest that those accessing medical cannabis may be doing so in lieu of using traditional pharmaceutical medications; however, this theory contradicts results of a study from 2018 that reported that medical cannabis users are more likely to use prescription medications (Caputi and Humphreys 2018 ). Reported medication use was not analyzed in reference to specific primary medical conditions for the purposes of this study, but identified it as an area of interest for future research. It is interesting to note that 26.1% of the sample did not report using medications or alternate therapies at the time of their initial assessment. This may demonstrate a limitation of the data, as there is potential that there is under-reporting at the system level; however, this may also indicate that patients are seeking medical cannabis where other treatments have failed, which would benefit from further investigation.
When comparing our results to a study of 1746 patients attending assessment clinics in California in 2006, who were primarily male (72.9%) and between the ages of 25–54 (69.2%) (Reinarman et al. 2011 ), it suggests that the medical cannabis patient population has evolved over time to include more females , and a wider range of ages; 40% of the sample who reported gender were female, and 31.5% of the patient population in this study were over the age of 54. The increasing age of medical cannabis users and increase in female users were also reported in a review completed by Fairman et al. (Fairman 2016 ). The high representation of Caucasians in this sample is consistent with the literature in which Caucasians represent the majority of medical cannabis users reported in other studies (77.0, 86.5, 61.5%) (Sexton et al. 2016 ; Reinarman et al. 2011 ; Reiman 2007 ). This is substantiated by the fact that Caucasians are the racial majority in the US, particularly in the states where the clinics are located.
The median estimated household income in the ZIP codes where our patient sample resides was higher than the US median, $69,481 compared to $61,937 (Bureau UC 2019 ). When taking into consideration the median household income from just the included states, $58,912 (US Income Statistics - Current Census Data for Zip Codes 2018 ), the median estimated household income from our sample was still higher; however, as the income data for our sample was a surrogate, this may be inflated and potentially inaccurate. Similar studies tend to report that income among medical cannabis users is lower than the average, which highlights the need for additional investigation (Sexton et al. 2016 ; Reiman 2007 ).
Data collected as part of the intake for patients in this retrospective study provide an interesting perspective on cannabis experience prior to seeking medical certification, as almost 70% reported using cannabis prior to their certification. This number is substantially higher than the lifetime cannabis use estimate among Americans from the National Survey on Drug Use and Health, which reported a lifetime cannabis use of 45.3% in 2018 (2018 NSDUH Detailed Tables | CBHSQ Data 2019 ). Unfortunately, it is not known how many of the patients in the dataset had medical certification from a separate clinic or physician elsewhere prior to becoming a patient at a CB2 Insights clinic.
Strengths of this study include the large sample of patients accessing medical cannabis across 12 states. The consistent input of data at the clinics allowed for a comprehensive review of characteristics, and most importantly, provided data on all medical conditions for which patients sought medical cannabis, rather than just one per patient. Additionally, data collection was verified by medical professionals at the time of input. Limitations of this study primarily include missing data (i.e., a large number of patients for whom gender and race were not reported), a lack of ethnicity data, the absence of data on patients who did not qualify for medical cannabis certification and were not included in the registry, and the use of surrogate income data. Another limitation is that the data came from a single network of clinics, and do not represent patients in all states where medical cannabis is legal.
Conclusion and future initiatives
This retrospective study offers insight into the characteristics and commonly reported type and number of medical conditions among patients accessing medical cannabis in the US. It highlighted the conditions that patients are seeking medical cannabis for most often that would benefit from further clinical evidence; mainly pain conditions, anxiety, insomnia, depression and PTSD. This study also demonstrated that patients often use medical cannabis to treat more than one condition, which is important for the medical community to understand and be aware of, as well as the patients who may be turning to cannabis as a treatment option. This finding in particular raises questions that are important to investigate, including why patients use medical cannabis for multiple conditions and whether they use different products to treat their various symptoms. As this study explored demographic and medical characteristics from patients in 12 different states, an in-depth review comparing states with contrasting cannabis regulations would offer further insights into medical cannabis use and access in the US.
Availability of data and materials
The datasets used for this article are not publicly available due to patients’ privacy. Data can be made available upon appropriate request to the authors.
Abbreviations
cannabidiol
confidence interval
interquartile range
institutional review board
post-traumatic stress disorder
standard deviation
Δ-9-tetrahydrocannabinol
United States
2018 NSDUH Detailed Tables | CBHSQ Data. 2019 https://www.samhsa.gov/data/report/2018-nsduh-detailed-tables . Accessed 27 Mar 2020.
Alsherbiny MA, Li CG. Medicinal Cannabis-Potential Drug Interactions. Med (Basel, Switzerland). 2018;6(1). https://doi.org/10.3390/medicines6010003 .
Bertakis K, Azari R, Helms L, Callahan E, Robbins J. Gender differences in the utilization of health care services. J Fam Pr 2000;49(2):147–152. https://pubmed.ncbi.nlm.nih.gov/10718692/ . Accessed 9 June 2020.
Boehnke KF, Gangopadhyay S, Clauw DJ, Haffajee RL. Qualifying conditions of medical cannabis license holders in the United States. Health Aff. 2019;38(2):295–302. https://doi.org/10.1377/hlthaff.2018.05266 .
Article Google Scholar
Bonn-Miller MO, Boden MT, Bucossi MM, Babson KA. Self-reported cannabis use characteristics, patterns and helpfulness among medical cannabis users. Am J Drug Alcohol Abuse. 2014;40(1):23–30. https://doi.org/10.3109/00952990.2013.821477 .
Article PubMed Google Scholar
Bureau UC. U.S. Median Household Income Up in 2018 From 2017. 2019. https://www.census.gov/library/stories/2019/09/us-median-household-income-up-in-2018-from-2017.html . Accessed 26 Sept 2019.
Buvinić M, Medici A, Fernández E, Torres AC. Gender differentials in health. The International Bank for Reconstruction and Development / The World Bank; 2006. http://www.ncbi.nlm.nih.gov/pubmed/21250310 . Accessed 14 Aug 2020.
Caputi TL, Humphreys K. Medical marijuana users are more likely to use prescription drugs medically and nonmedically. J Addict Med. 2018;12(4):295–9. https://doi.org/10.1097/ADM.0000000000000405 .
DISA Global Solutions. Map of marijuana legality by state. Retrieved from URL https://disa.com/map-of-marijuana-legality-by-state . 2019. https://disa.com/map-of-marijuana-legality-by-state .
Eurich DT, Hanlon JG, Boisvenue JJ, Meng H, Dyck JRB. A description of the medical Cannabis use in Ontario, Canada. Cannabis Cannabinoid Res. 2019;4(2):131–5. https://doi.org/10.1089/can.2018.0036 .
Fairman BJ. Trends in registered medical marijuana participation across 13 US states and District of Columbia. Drug Alcohol Depend. 2016;159:72–9. https://doi.org/10.1016/j.drugalcdep.2015.11.015 .
Fraguas-Sanchez AI, Torres-Suarez AI. Medical use of cannabinoids. Drugs. 2018;78(16):1665–703. https://doi.org/10.1007/s40265-018-0996-1 .
Article PubMed CAS Google Scholar
Hanlon P, Hannigan L, Rodriguez-Perez J, et al. Representation of people with comorbidity and multimorbidity in clinical trials of novel drug therapies: an individual-level participant data analysis. BMC Med. 2019;17(1):201. https://doi.org/10.1186/s12916-019-1427-1 .
Article PubMed PubMed Central Google Scholar
Institute of Medicine (U.S.). Marijuana and medicine: assessing the science base. Natl Acad Press https://doi.org/1017226/6376 . 1999. https://doi.org/10.5860/choice.37-2206 .
Kaur R, Ambwani SR, Singh S. Endocannabinoid system: a multi-facet therapeutic target. Curr Clin Pharmacol. 2016;11(2):110–7.
Article CAS Google Scholar
Klumpers LE, Thacker DL. A brief background on Cannabis: from plant to medical indications. J AOAC Int. 2019;102(2):412–20. https://doi.org/10.5740/jaoacint.18-0208 .
Kosiba JD, Maisto SA, Ditre JW. Patient-reported use of medical cannabis for pain, anxiety, and depression symptoms: systematic review and meta-analysis. Soc Sci Med. 2019;233:181–92. https://doi.org/10.1016/j.socscimed.2019.06.005 .
Legislatures NC of S. State Medical Marijuana Laws. https://www.ncsl.org/research/health/state-medical-marijuana-laws.aspx . Published 2020.
Lu Y, Anderson HD. Cannabinoid signaling in health and disease. Can J Physiol Pharmacol. 2017;95(4):311–27. https://doi.org/10.1139/cjpp-2016-0346 .
MacIntyre S, Ford G, Hunt K. Do women “over-report” morbidity? Men’s and women’s responses to structured prompting on a standard question on long standing illness. Soc Sci Med. 1999;48(1):89–98. https://doi.org/10.1016/S0277-9536(98)00292-5 .
Martin CB, Hales CM, Gu Q, Ogden CL. Prescription Drug Use in the United States, 2015–2016 Key Findings Data from the National Health and Nutrition Examination Survey; 2015. https://www.cdc.gov/nchs/data/databriefs/db334_tables-508.pdf#1 . Accessed 3 Apr 2020.
Pacula RL, Smart R. Medical marijuana and marijuana legalization. Annu Rev Clin Psychol. 2017;13:397–419. https://doi.org/10.1146/annurev-clinpsy-032816-045128 .
Pertwee RG. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther. 1997;74(2):129–80. https://doi.org/10.1016/s0163-7258(97)82001-3 .
Pertwee RG, Howlett AC, Abood ME, et al. International union of basic and clinical pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB(1) and CB(2). Pharmacol Rev. 2010;62(4):588–631. https://doi.org/10.1124/pr.110.003004 .
Article PubMed PubMed Central CAS Google Scholar
Reiman A. Medical Cannabis patients: patient profiles and health care utilization patterns. Complement Health Pract Rev. 2007;12(1):31–50. https://doi.org/10.1177/1533210107301834 .
Reinarman C, Nunberg H, Lanthier F, Heddleston T. Who are medical marijuana patients? Population characteristics from nine California assessment clinics. J Psychoactive Drugs. 2011;43(2):128–35. https://doi.org/10.1080/02791072.2011.587700 .
Salazar CA, Tomko RL, Akbar SA, Squeglia LM, Mcclure EA. Medical Cannabis Use among Adults in the Southeastern United States. Cannabis. 2019;2(1):53–65.
Seeman MV. Psychopathology in women and men: focus on female hormones. Am J Psychiatry. 1997;154(12):1641–7. https://doi.org/10.1176/ajp.154.12.1641 .
Sexton M, Cuttler C, Finnell JS, Mischley LK. A cross-sectional survey of medical Cannabis users: patterns of use and perceived efficacy. Cannabis Cannabinoid Res. 2016;1(1):131–8. https://doi.org/10.1089/can.2016.0007 .
US Income Statistics - Current Census Data for Zip Codes. 2018. https://www.incomebyzipcode.com/ . Accessed 27 Mar 2020.
Verbrugge LM, Wingard DL. Sex differentials in health and mortality. Health Matrix. 1987;5(2):3–19. https://doi.org/10.1300/j013v12n02_07 .
Vuckovic S, Srebro D, Vujovic KS, Vucetic C, Prostran M. Cannabinoids and pain: new insights from old molecules. Front Pharmacol. 2018;9:1259. https://doi.org/10.3389/fphar.2018.01259 .
Waldron I. Sex differences in illness incidence, prognosis and mortality: issues and evidence. Soc Sci Med. 1983;17(16):1107–23. https://doi.org/10.1016/0277-9536(83)90004-7 .
Westergaard D, Moseley P, Sørup FKH, Baldi P, Brunak S. Population-wide analysis of differences in disease progression patterns in men and women. Nat Commun. 2019;10(1):1–14. https://doi.org/10.1038/s41467-019-08475-9 .
Yu B, Chen X, Chen X, Yan H. Marijuana legalization and historical trends in marijuana use among US residents aged 12-25: results from the 1979-2016 National Survey on drug use and health. BMC Public Health. 2020;20(1):1–10. https://doi.org/10.1186/s12889-020-8253-4 .
Zaller N, Topletz A, Frater S, Yates G, Lally M. Profiles of medicinal cannabis patients attending compassion centers in Rhode Island. J Psychoactive Drugs. 2015;47(1):18–23. https://doi.org/10.1080/02791072.2014.999901 .
Download references
Acknowledgements
The authors would like to thank Christopher Vannabouathong for assistance with the statistical analysis.
This study was not funded by any external sources. Authors are employees of CB2 Insights and were compensated as per their contracts for the time to analyze the data and prepare the manuscript.
Author information
Authors and affiliations.
CB2 Insights, 5045 Orbitor Dr, Building 11, Suite 300, Mississauga, ON, L4W 4Y4, Canada
V. Kishan Mahabir, Jamil J. Merchant, Christopher Smith & Alisha Garibaldi
You can also search for this author in PubMed Google Scholar
Contributions
CS and KM conceptualized the study question. AG and JM completed the analysis and writing of the initial manuscript. CS and KM were integral in interpreting the data and writing and editing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Christopher Smith .
Ethics declarations
Ethics approval and consent to participate.
This research initiative was submitted for IRB review and determined to be exempt from IRB oversight by the Advarra IRB ( Pro00042652 ) as this study had minimal risk, the data exports were void of patient identifiers, and it did not require direct patient contact.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests aside from being employees of CB2 Insights. All authors contributed to the manuscript as part of an internally guided project. CB2 Insights owns the data, and the authors are all employees of CB2 Insights. No external companies had input on the content of the manuscript, and all data were analyzed in an unbiased fashion. Apart from the authors, CB2 Insights did not play any role in the conduct or analysis of the study, the writing of the manuscript or the decision to publish.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Mahabir, V.K., Merchant, J.J., Smith, C. et al. Medical cannabis use in the United States: a retrospective database study. J Cannabis Res 2 , 32 (2020). https://doi.org/10.1186/s42238-020-00038-w
Download citation
Received : 27 April 2020
Accepted : 09 September 2020
Published : 29 September 2020
DOI : https://doi.org/10.1186/s42238-020-00038-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Medical cannabis
- Chronic pain
- Post-traumatic stress disorder
Journal of Cannabis Research
ISSN: 2522-5782
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
- UCI Center for the Study of Cannabis
- cannabis.uci.edu
Place the widgets in the Top Extra Area.
- What’s New in Cannabis Research
Monthly updates on cannabis and cannabinoid research, by…

June 4, 2021
An original investigation conducted by Arkell et al. assayed the effects of cannabidiol (CBD) compared to ∆9-Tetrahydrocannabidiol (THC) on driving performance. The study investigated the effects of vaporized THC-dominant versus CBD-dominant cannabis o …

March 1, 2021
Regular use of cannabis high in delta-9-tetrahydrocannabinol (THC) is associated with increased risk of psychotic symptoms among young people. This finding has not been explored as deeply in adults who use cannabis-based medications. Velayudhan et al. …

February 12, 2021
Post-Traumatic Stress Disorder (PTSD) is characterized by intrusive memories of life-threatening events accompanied by severe anxiety and sleeplessness that often adversely affect quality of life. PTSD has been reported to have a life-time prevalence o …

January 25, 2021
Cannabinoid receptor activation is known to ameliorate inflammatory bowel disease (IBD) in animal models, but the contributions of other factors in combination with receptor activation remain unstudied. Cannabinoids bind to G protein coupled receptors …

December 10, 2020
This study assesses cannabidiol, which inhibits the reuptake of endocannabinoids, as a treatment for cannabis use disorder. Freeman et al. (2020) conducted a phase 2a, randomized, double-blind, placebo, controlled, clinical trial investigating the heal …

July 20, 2020
Sickle cell disease affects approximately 100,000 Americans. It is a genetic disease characterized by chronic pain and extremely painful episodes, which usually require treatment with large doses of opioids for extended periods of time. Opioids have a …

March 2, 2020
With increasing availability of cannabis products and its decriminalized status in many states, understanding its acute and residual effects on driving is important in promoting public health and in guiding law enforcement with implementations of legal …

January 31, 2020
Cannabis has shown promise in treating several medical conditions but its effects on pregnant women and developing fetuses is unknown. This study aimed to investigate the association of self-reported cannabis use by pregnant women and unfavorable mater …

This review article analyzed nine peer-reviewed studies focused on the effects of cannabinoids on Alzheimer’s disease and dementia. Due to the increasing life expectancy in society aging related Alzheimer’s disease onset has continued to increase since …

December 4, 2019
Cannabidiol’s anxiolytic effects have been demonstrated in several studies. However, the mechanism for this effect remains to be elucidated. The present paper offers additional data that may aid in future research in understanding endocannabinoids’ rol …

October 15, 2019
Benzodiazepines (BDs) like Xanax, are a class of medications used for anxiety, seizures, insomnia, and a variety of other neurological/psychiatric conditions. The adverse side effects of BDs are well documented and include dependence, addiction, withdr …

September 11, 2019
This paper reports that incidences of pediatric cannabis exposure in Massachusetts increased following medical marijuana legalization. The study analyzed cannabis exposure cases amongst individuals aged 0-19 reported to the Regional Center for Poison C …

August 27, 2019
The issue of JAMA published on August 09, 2019 includes an entire section on cannabis curated by Kevin Hill. Topics include cannabis use for medical purposes such as chronic pain and cancer treatment.

August 10, 2019
Currently, there is not enough significant data to conclude that THC or any other cannabinoid is harmful to a developing fetus. This study of 367,403 pregnancies among women in Northern California, reports that the relative rates of daily, weekly, and …

Up to 7.5% of pregnant women in the United States use cannabis during their pregnancy. The concentration of THC in cannabis is increasing as is the number of pregnant women who use cannabis. It is critical that the developmental risk of THC and its mai …

Approximately 35% of Americans suffer from obesity and its related health effects. The activation of cannabinoid 1 receptor [CB1R] has been shown to increase body weight, appetite, insulin resistance, and fat cell production. Thus, overactivity of CB1R …

June 21, 2019
Adolescence is a critical period marked by complex neurological processes and is frequently associated with mood fluctuations and impulsivity. Consequently, understanding the effects of cannabis use during this developmental stage is essential in order …

May 28, 2019
Due to the increasing rates of tobacco and cannabis use co-occurrence among individuals seeking treatment for cannabis use disorder (CUD), appropriate timing of tobacco intervention (TI) initiation is important in maximizing the effects of both the CUD …

February 6, 2019
Cannabis’ increasing popularity is paralleled by growing concerns about its habit-forming propensity along with other important public health concerns including but not limited to driving under the influence and availability to adolescents. This double …

As the cannabis market expands, consumers face a wide variety of products with different methods of consumption such as vaporizing, smoking, edible formulations, etc., Thus, rigorous analyses of pharmacokinetics and pharmacodynamics for each route of c …

Cannabidiol, a non-intoxicating constituent of cannabis, has been implicated as a possible anxiolytic and anti-epileptic with the FDA recently approving a drug to treat epilepsy with cannabidiol as its primary ingredient. The present study aims to exam …

December 16, 2018
Over 12 million births in the US were analyzed to identify pregnant women who use cannabis and to assess obstetric and neonatal outcomes. The results showed that the incidence of cannabis dependence in 2013 is two-times more than in 1999 and that canna …

This review provides insights on three clinical trials that showed cannabidiol’s efficacy in reducing seizure episodes by at least 38%. More importantly, this review highlights the need for further research to fully elucidate CBD’s mechanism of action …

Despite increasing evidence of cannabinoids’ analgesic properties, concerns regarding its potential for abuse limit its utility in pain management. The present study posits a solution to such valid and thoughtful concern. GAT211 is a novel compound tha …

December 14, 2018
With the legalization of cannabis in many states, it is imperative that policies are adopted based on scientific findings. This study, conducted in Canada, where recreational use of cannabis recently became legal, sought to determine how cannabis inhal …

The study used positron emission tomography to show that men may have a greater number of CB1-type cannabinoid receptors than women do. This interesting finding may help in designing future studies regarding the health impact of cannabis in men and wom …

This study examined the anti-anxiety effect of cannabidiol using the Simulated Public Speaking Test to induce anxiety and the Visual Analogue Mood Scale to assess subjective ratings of anxiety. The authors also sought to identify the optimal dose of ca …
- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
U.S. Food and Drug Administration
- Search
- Menu
- News & Events
- Public Health Focus
FDA and Cannabis: Research and Drug Approval Process
On this page:, fda supports sound scientific research.
- Cannabis Study Drugs Controlled Under Schedule I of the CSA
- Cannabis Study Drugs Containing Hemp
Additional Resources
The FDA understands that there is increasing interest in the potential utility of cannabis for a variety of medical conditions, as well as research on the potential adverse health effects from use of cannabis.
To date, the FDA has not approved a marketing application for cannabis for the treatment of any disease or condition. The agency has, however, approved one cannabis-derived drug product: Epidiolex (cannabidiol), and three synthetic cannabis-related drug products: Marinol (dronabinol), Syndros (dronabinol), and Cesamet (nabilone). These approved drug products are only available with a prescription from a licensed healthcare provider. Importantly, the FDA has not approved any other cannabis, cannabis-derived, or cannabidiol (CBD) products currently available on the market.

- Cannabis sativa L. is a plant that contains over 80 different naturally occurring compounds called “cannabinoids”
- Cannabidiol (CBD)
- Tetrahydrocannabinol (THC)
- Plants are grown to produce varying concentrations of cannabinoids – THC or CBD
- These plant variations are called cultivars
Cannabis-derived compounds
- Compounds occurring naturally in the plant – like CBD and THC
- These compounds are extracted directly from the plant
- Can be used to manufacture drug products
- Example: highly-purified CBD extracted from the plant
Cannabis-related compounds
- These synthetic compounds are created in a laboratory
- Can be used to manufacture drug products
- Some synthetic compounds may also occur naturally in the plant and some may not
- Examples: synthetically-derived dronabinol (also naturally occurring) and nabilone (not naturally occurring)
FDA has approved Epidiolex, which contains a purified form of the drug substance cannabidiol (CBD) for the treatment of seizures associated with Lennox-Gastaut syndrome or Dravet syndrome in patients 2 years of age and older. That means FDA has concluded that this particular drug product is safe and effective for its intended use.
The agency also has approved Marinol and Syndros for therapeutic uses in the United States, including for nausea associated with cancer chemotherapy and for the treatment of anorexia associated with weight loss in AIDS patients. Marinol and Syndros include the active ingredient dronabinol, a synthetic delta-9- tetrahydrocannabinol (THC) which is considered the psychoactive intoxicating component of cannabis (i.e., the component responsible for the “high” people may experience from using cannabis). Another FDA-approved drug, Cesamet, contains the active ingredient nabilone, which has a chemical structure similar to THC and is synthetically derived. Cesamet, like dronabinol-containing products, is indicated for nausea associated with cancer chemotherapy.
FDA is aware that unapproved cannabis and/or unapproved cannabis-derived products are being used to treat a number of medical conditions including, AIDS wasting, epilepsy, neuropathic pain, spasticity associated with multiple sclerosis, and cancer and chemotherapy-induced nausea. Caregivers and patients can be confident that FDA-approved drugs have been carefully evaluated for safety, efficacy, and quality, and are monitored by the FDA once they are on the market. However, the use of unapproved cannabis and cannabis-derived products can have unpredictable and unintended consequences, including serious safety risks. Also, there has been no FDA review of data from rigorous clinical trials to support that these unapproved products are safe and efficacious for the various therapeutic uses for which they are being used.
FDA understands the need to develop therapies for patients with unmet medical needs, and does everything it can to facilitate this process. FDA has programs such as Fast Track, Breakthrough Therapy, Accelerated Approval and Priority Review that are designed to facilitate the development of and expedite the approval of drug products. In addition, the FDA’s expanded access (sometimes called “compassionate use”) statutory and regulatory provisions are designed to facilitate the availability of investigational products to patients with serious diseases or conditions when there is no comparable or satisfactory alternative therapy available, either because the patients have exhausted treatment with or are intolerant of approved therapies, or when the patients are not eligible for an ongoing clinical trial. Through these programs and the drug approval process, FDA supports sound, scientifically-based research into the medicinal uses of drug products containing cannabis or cannabis-derived compounds and will continue to work with companies interested in bringing safe, effective, and quality products to market.
↑ Back to top
The FDA has an important role to play in supporting scientific research into the medical uses of cannabis and its constituents in scientifically valid investigations as part of the agency’s drug review and approval process. As a part of this role, the FDA supports those in the medical research community who intend to study cannabis by:
- Providing information on the process needed to conduct clinical research using cannabis.
- Providing information on the specific requirements needed to develop a human drug that is derived from a plant such as cannabis. In December 2016, the FDA updated its Guidance for Industry: Botanical Drug Development , which provides sponsors with guidance on submitting investigational new drug (IND) applications for botanical drug products. The FDA also has issued “ Cannabis and Cannabis-Derived Compounds: Quality Considerations for Clinical Research, Draft Guidance for Industry .”
- Providing specific support for investigators interested in conducting clinical research using cannabis and its constituents as a part of the IND or investigational new animal drug (INAD) process through meetings and regular interactions throughout the drug development process.
- Providing general support to investigators to help them understand and follow the procedures to conduct clinical research through the FDA Center for Drug Evaluation and Research (CDER) Small Business and Industry Assistance group .
To conduct clinical research that can lead to an approved new drug, including research using materials from plants such as cannabis, researchers need to work with the FDA and submit an IND application to CDER. The IND application process gives researchers a path to follow that includes regular interactions with the FDA to support efficient drug development while protecting the patients who are enrolled in the trials. An IND includes protocols describing proposed studies, the qualifications of the investigators who will conduct the clinical studies, and assurances of informed consent and protection of the rights, safety, and welfare of the human subjects. The FDA reviews the IND to ensure that the proposed studies, generally referred to as “clinical trials,” do not place human subjects at an unreasonable risk of harm. The FDA also requires obtaining the informed consent of trial subjects and human subject protection in the conduct of the clinical trials. For research intending to develop an animal drug product, researchers would establish an INAD file with the Center for Veterinary Medicine (CVM) to conduct their research, rather than an IND with CDER.
FDA is committed to encouraging the development of cannabis-related drug products, including CBD. Those interested in cannabis-derived and cannabis-related drug development are encouraged to contact the relevant CDER review division and CDER’s Botanical Review Team (BRT) to answer questions related to their specific drug development program. The BRT serves as an expert resource on botanical issues and has developed the Botanical Drug Development Guidance for Industry to assist those pursuing drug development in this area. FDA encourages researchers to request a Pre-Investigational New Drug application (PIND) meeting to discuss questions related to the development of a specific cannabis-derived and cannabis-related drug product.
Please note that certain cultivars and parts of the Cannabis sativa L. plant are controlled under the Controlled Substances Act (CSA) since 1970 under the drug class "Marihuana" (commonly referred to as "marijuana") [21 U.S.C. 802(16)]. "Marihuana" is listed in Schedule I of the CSA due to its high potential for abuse, which is attributable in large part to the psychoactive intoxicating effects of THC, and the absence of a currently accepted medical use in the United States. From 1970 until December of 2018, the definition of “marihuana” included all types of Cannabis Sativa L. , regardless of THC content. However, in December 2018, the Agriculture Improvement Act of 2018 (also known as the Farm Bill) removed hemp, a type of cannabis that is very low in THC (cannabis or cannabis derivatives containing no more than 0.3% THC on a dry weight basis), from controls under the CSA. This change in the law may result in a more streamlined process for researchers to study cannabis and its derivatives, including CBD, that fall under the definition of hemp, a result which could speed the development of new drugs containing hemp.
Conducting clinical research using cannabis-derived substances that are considered controlled substances under the CSA often involves interactions with several federal agencies. For example:
- Protocols to conduct research with controlled substances listed in Schedule I are required to be conducted under a site-specific DEA investigator registration. For more information, see 21 CFR 1301.18 .
- National Institute on Drug Abuse (NIDA) Drug Supply Program provides research-grade marijuana for scientific study. Through registration issued by DEA, NIDA is responsible for overseeing the cultivation of marijuana for medical research and has contracted with the University of Mississippi to grow marijuana for research at a secure facility. Marijuana of varying potencies and compositions along with marijuana-derived compounds are available. DEA also may allow additional growers to register with the DEA to produce and distribute marijuana for research purposes. DEA that, as the result of a recent amendment to federal law, certain forms of cannabis no longer require DEA registration to grow or manufacture.
- Researchers work with the FDA and submit an IND or INAD application to the appropriate CDER divisions or other center offices depending on the therapeutic indication or population. If the research is intended to support the approval of an animal drug product, an INAD file should be established with CVM. Based on the results obtained in studies conducted at the IND or INAD stage, sponsors may submit a marketing application for formal approval of the drug.
Cannabis Study Drugs Controlled Under Schedule I of the CSA (greater than 0.3% THC on a dry weight basis)
Sponsor obtains pre-IND number through CDER review division to request a pre-IND meeting. For new animal drug research, a sponsor may engage with CVM to establish an INAD file. A pre-IND meeting with CDER is optional, and an opportunity to obtain FDA guidance on sponsor research plans and required content for an IND submission .
The sponsor contacts NIDA or another DEA-registered source of cannabis and/or cannabis-derived substances to obtain information on the specific cultivars available, so that all necessary chemistry, manufacturing, and controls (CMC) and botanical raw material (BRM) information can be included in the IND. Importation of products controlled under the CSA are subject to DEA authorization.
The sponsor may contact DEA to discuss Schedule I drug research plans that may require DEA inspection for an investigator and study site Schedule I license.
Step 4: If the selected BRM or drug substance manufacturer holds a Drug Master File (DMF) , the sponsor must obtain a Letter of Authorization (LOA) to reference CMC and BRM information. Alternatively, an IND submission would need to contain all necessary CMC data characterizing their study drug and ensuring it is safe for use in humans.
The sponsor sends a copy of the IND and clinical protocol, including a LOA (if applicable), to FDA.
FDA reviews the submitted IND. The sponsor must wait 30 calendar days following IND submission before initiating any clinical trials, unless FDA notifies the sponsor that the trials may proceed sooner. During this time, FDA has an opportunity to review the submission for safety to assure that research subjects will not be subjected to unreasonable risk.
If the IND is authorized by FDA as “safe to proceed” the sponsor may then submit their clinical protocol registration application, including referenced IND number, to DEA to obtain the protocol registration. Once this is received, the sponsor contacts NIDA or another DEA-registered source to obtain the cannabis and/or cannabis-derived substances and they can then begin the study.
For nonclinical research, including research conducted under an INAD file submitted established with CVM, there is no requirement of prior authorization of the protocol by FDA before the investigators may proceed with a protocol registration application submitted to DEA. For these nonclinical protocols, investigators may immediately pursue investigator and study site licensure, and protocol registration with DEA, so they may then obtain their Schedule I cannabis-derived study drug from supplier.
Cannabis Study Drugs Containing Hemp (no more than 0.3% THC on a dry weight basis)
Sponsor provides all applicable chemistry, manufacturing, and controls (CMC) and botanical raw material (BRM) information in the IND for review by FDA, including hemp cultivars.
If the selected hemp manufacturer holds a Drug Master File (DMF) , the sponsor must obtain a Letter of Authorization (LOA) to reference CMC and BRM information. Alternatively, an IND submission would need to contain all necessary CMC data characterizing their study drug and ensuring it is safe for use in humans.
FDA’s Role in the Drug Approval Process
The FDA’s role in the regulation of drugs, including cannabis and cannabis-derived products, also includes review of applications to market drugs to determine whether proposed drug products are safe and effective for their intended indications. The FDA’s drug approval process requires that clinical trials be designed and conducted in a way that provides the agency with the necessary scientific data upon which the FDA can make its approval decisions. Without this review, the FDA cannot determine whether a drug product is safe and effective. It also cannot ensure that a drug product meets appropriate quality standards. For certain drugs that have not been approved by the FDA, the lack of FDA approval and oversight means the safety, effectiveness, and quality of the drug – including how potent it is, how pure it is, and whether the labeling is accurate or false – may vary considerably.
- Product-Specific Guidance for Generic Drug Development: Draft Guidance on Cannabidiol Oral Solution (PDF - 42KB)
- Cannabis Clinical Research: Drug Master Files (DMFs) & Quality Considerations Webinar
- Cannabis and Cannabis-Derived Compounds: Quality Considerations for Clinical Research, Draft Guidance for Industry
- FDA Regulation of Cannabis and Cannabis-Derived Products, Including Cannabidiol (CBD): Questions and Answers
- Development & Approval Process (Drugs)
- From an Idea to the Marketplace: The Journey of an Animal Drug through the Approval Process
- FDA Center for Drug Evaluation and Research Small Business and Industry Assistance group
- CVM Small Business Assistance
- National Institutes of Health (NIH): Guidance on Procedures for Provision of Marijuana for Medical Research
- National Institute on Drug Abuse's (NIDA) Role in Providing Marijuana for Research
- Drug Enforcement Administration - Registration of Manufacturers, Distributors, and Dispensers of Controlled Substances
- International Narcotics Control Board: Single Convention on Narcotic Drugs (1961)
- National Institute on Drug Abuse (NIDA): Ordering Guidelines for Marijuana and Marijuana Cigarettes

The background image is Shown is a bottle of hemp oil with a dropper above it, with a blurred background of other medical marijuana products
Welcome to the Pennsylvania-approved Medical Marijuana Academic Clinical Research Center at Penn State
The Penn State Medical Marijuana Academic Clinical Research Center (ACRC) will become an internationally recognized expert in the benefits and dangers of medicinal cannabinoids and medical cannabis.
The goal of the center is to support the development of medical marijuana pre-clinical and clinical research and provide scientific evidence on the utility of medical cannabis. Currently, there are more than 30 researchers engaged in cannabis research within the ACRC; these researchers are divided into two divisions, a basic science division and a clinical science division .
Penn State College of Medicine was recognized as a potential ACRC site with the passage of ACT 16 in 2016, which legalized medical marijuana in Pennsylvania.
A key provision of this act was to “promote high-quality research” on medical marijuana.
In May of 2018, Penn State College of Medicine was one of eight universities approved by Gov. Tom Wolf as a Certified Academic Clinical Research Center. In June 2019, the Penn State College of Medicine ACRC, in a relationship with PA Options for Wellness, was one of the first three centers approved by the Commonwealth of Pennsylvania.
Director: Kent Vrana , PhD, Elliot S. Vesell Professor and Chair, Department of Pharmacology Scientific Director: Wesley M. Raup-Konsavage , PhD, Assistant Professor, Department of Pharmacology
Research areas and current projects
Basic science division.
The basic science division utilizes pre-clinical models, such as in vitro studies and rodent models, to explore the potential therapeutic uses of cannabinoids for a variety of disease.
Current projects are exploring the efficacy of cannabinoids or cannabis to treat cancer, acute and chronic pain, anxiety, high blood pressure and post-traumatic stress disorder (PTSD).
Additional research within this group focuses on the chemistry of cannabinoids including pharmacokinetics, receptor binding and the development of novel cannabinoid-based compounds. These preclinical data will help to steer clinical research projects.
- Basic Science Team
Clinical Science Division
- Introduction
The clinical science division focuses on examining the benefits and potential harm of medical cannabis use for the current state-approved medical conditions.
Currently, there are two teams within this division. The first team is focused on health outcomes in patients currently taking medical marijuana for the treatment of state-approved conditions, with special attention on anxiety, post-traumatic stress disorder and pain. The second team focuses on clinical trials and they will be conducting double blind, placebo-controlled studies on the efficacy of cannabis to treat medical conditions and reduce reliance on opioids for pain.
- Available Clinical Trials
Cannabidiol and Management of Endometriosis Pain
The study team will be looking at the effects of cannabidiol (CBD) in patients with endometriosis. It is believed that CBD will improve both pain and quality of life. The study will last a total of 12 weeks and involve several onsite visits in addition to daily pain assessments.
Contact: Kristin Riley, MD .
- Clinical Science Team
Penn State Medical Marijuana ACRC uses the following facilities to advance research in the field.
Super critical co2 extractor.

Cannabinoid Drug Libraries
Other Reagents
Meet the team

Kent Vrana, PhD
Elliot S. Vesell Professor and Chair Department of Pharmacology

Scientific Director
Wesley M. Raup-Konsavage, PhD
Assistant Professor Department of Pharmacology

Research Technician
Diana Sepulveda
News and Resources
The strength of the Center for Cannabis and Natural Product Pharmaceutics (CCNPP) is directly linked to bringing talented investigators from across the Penn State system. We encourage investigators interested in natural product pharmaceutics research collaborations to apply for the Penn State Center for Cannabis and Natural Product Pharmaceutics membership. Applicants must hold an appointment at Penn State, Penn State Health, a Penn State Medical Marijuana Academic Clinical Research Center (ACRC) partner or an affiliate institution.
Acceptance and/or approval of a membership application is determined by the leadership team.
Benefits of Membership
- Can request supercritical CO 2 extraction of dried plant material
- Access to cannabinoids, natural product library (TimTec), and cannabis extracts
- Assistance with Grant Applications and Letters of Support
- Support for IRB, IND and IACUC protocol submissions
- Assistance developing tools for Mass Spectrometry, Microsome, and PK analysis
- Support for modification of lead compounds and formulation
Expectations and Responsibilities of Membership
- Acknowledgement of the CCNPP in publications
- Collaboration with members of the CCNPP
- Participation in CCNPP meetings
- Attendance at the annual CCNPP retreat
Complete a membership application
- Publications
Support natural product research at Penn State College of Medicine with a donation today
Millions of people currently take herbal supplements, including CBD oil or medical cannabis, that make claims to potential medical benefits. These statements and claims are rarely supported by strong scientific evidence, if any. Importantly, these supplements are not subject to government oversight, potential negative side-effects are not disclosed, and people believe they are safe (and therefore don’t mention to their primary care provider). The goals of the center are to identify novel natural product pharmaceutics, validate reported claims, and assess negative side-effects of natural products that consumers are using. Your generous gift helps to fund this work as well as to our work to educate clinicians on the medicinal value of plants and natural products.
Give hope today – Ways to give
Click here to donate online (credit card) .
Make a gift by check payable to: Penn State University – ACRC Penn State Health Milton S. Hershey Medical Center Penn State College of Medicine Development & Alumni Relations, MC HS20 1249 Cocoa Ave, Suite 115 P.O. Box 852 Hershey, PA 17033-0852
Have a question about giving?
Call 717-531-8497 or email [email protected] .
- Pennsylvania Medical Marijuana Program
- PA Options for Wellness
- Organic Synthesis Core
- Drug-Drug Interactions
CCNPP newsletter
- View past newsletters in Sharepoint (PSU login required)
Sign up for the newsletter
Sign up for the center for cannabis and natural product pharmaceuticals mailing list.
Here’s how you know
- U.S. Department of Health and Human Services
- National Institutes of Health
NIH-Supported Research on Cannabis, Cannabinoids, and Related Compounds

.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Background
The National Institutes of Health (NIH) supports a broad portfolio of research on cannabis and cannabis constituents and related compounds, as well as the endocannabinoid system. Specific topics of interest vary among Institutes, Centers, and Offices, but overall the research portfolio includes studies investigating the whole or parts of the Cannabis sativa plant, cannabis extracts or enriched extracts, cannabinoid compounds extracted and derived from cannabis extracts, non-cannabinoid constituents of cannabis, synthetic cannabinoids, and the components of the endocannabinoid system (the signaling pathways in the body activated by cannabinoids).
There is considerable interest in the possible therapeutic uses of cannabis and its constituent compounds. In 2015, NIH developed three reporting categories to describe and account for the research efforts underway to examine the chemical, physiological, and therapeutic properties of cannabinoids and the physiological systems they affect.
View examples of NIH research grants in each of the categories below.
- Cannabinoid Research – This category reports the total NIH investment in all cannabinoid research including basic research, animal and human preclinical studies, and clinical research. Studies examine cannabis use disorder as well as the societal and/or health impacts of changing cannabis laws and policies; all classes of cannabinoids (purified, synthetic, endocannabinoids, or phytocannabinoids); compounds that modify the activity of consumed cannabis (e.g., cannabinoid receptor allosteric modulators); as well as the physiological systems affected by cannabis (e.g., endocannabinoid system) and modulators thereof (e.g., fatty acid amide hydrolase [FAAH] inhibitors).
- Cannabidiol Research – This subset of the Cannabinoid Research category (above) reports all NIH projects examining basic, preclinical, and therapeutic properties of cannabidiol (CBD).
- Therapeutic Cannabinoid Research – This subset of the Cannabinoid Research category (above) reports all NIH projects examining the therapeutic properties of all classes of cannabinoids (endocannabinoids, phytocannabinoids, and synthetic).
These categories are publicly accessible on the NIH categorical spending website ( NIH RePORTER ) and will be updated annually.
- Skip to main content
- Keyboard shortcuts for audio player
- Your Health
- Treatments & Tests
- Health Inc.
- Public Health
After 50 Years, U.S. Opens The Door To More Cannabis Crops For Scientists

More than 30 states have medical marijuana programs — yet scientists are only allowed to use cannabis plants from one U.S. source for their research. That's set to change, as the federal government begins to add more growers to the mix. Drew Angerer/Getty Images hide caption
More than 30 states have medical marijuana programs — yet scientists are only allowed to use cannabis plants from one U.S. source for their research. That's set to change, as the federal government begins to add more growers to the mix.
After more than 50 years, the federal government is lifting a roadblock to cannabis research that scientists and advocates say has hindered rigorous studies of the plant and possible drug development.
Since 1968, U.S. researchers have been allowed to use cannabis from only one domestic source : a facility based at the University of Mississippi, through a contract with the National Institute on Drug Abuse (NIDA).
That changed earlier this month, when the Drug Enforcement Administration announced it's in the process of registering several additional American companies to produce cannabis for medical and scientific purposes.
It's a move that promises to accelerate understanding of the plant's health effects and possible therapies for treating conditions — chronic pain, the side effects of chemotherapy, multiple sclerosis and mental illness, among many others — that are yet to be well studied .

Shots - Health News
How one boy's fight with epilepsy led to the first marijuana-derived pharmaceutical.
"This is a momentous decision," says Rick Doblin, executive director of the Multidisciplinary Association for Psychedelic Studies (MAPS), which has spearheaded research into other Schedule 1 drugs — the most restrictive class of controlled substance, which the federal government defines as "drugs with no currently accepted medical use."
"This is the last political obstruction of research with Schedule 1 drugs," he says.
About one-third of Americans currently live in a state where recreational marijuana is legal — and more than 30 states have medical marijuana programs . Yet scientists still aren't allowed to simply use the cannabis sold at state-licensed dispensaries for their clinical research because cannabis remains illegal under federal law.

Medical Marijuana's 'Catch-22': Limits On Research Hinder Patient Relief
"It is a big disconnect," says Dr. Igor Grant , a psychiatry professor and director of the Center for Medicinal Cannabis Research at University of California, San Diego.
The new DEA decision doesn't resolve the conflict between federal and state laws, but it does offer researchers a new, federally sanctioned pipeline for more products and strains of cannabis.
"We'll see a decade or more of explosive cannabis research and potential new therapies," says Dr. Steve Groff, founder and chairman of Groff North America , one of three companies that has publicly announced it has preliminary approval from the federal government to cultivate cannabis for research.
A long-running fight to overturn federal "monopoly"
Despite their efforts, scientists have encountered administrative and legal hurdles to growing pharmaceutical-grade cannabis for decades.
In 2001, Dr. Lyle Craker, a prominent plant biologist, first applied for a license to cultivate marijuana for research — only to encounter years of delay that kicked off a prolonged court battle with the DEA, which has to greenlight research into Schedule 1 drugs like cannabis.
"There's thousands of different cannabis varieties that all have unique chemical profiles and produce unique clinical effects, but we didn't have access to that normal diversity," says Dr. Sue Sisley , a cannabis researcher and president of the Scottsdale Research Institute, which also received preliminary DEA approval to produce cannabis for research.
Only in 2016 did the federal government signal a change in policy that would open the door for new growers, but applications to do so languished for years. Craker and others ended up suing the federal government over the delay.

Psychiatrist Explores Possible Benefits Of Treating PTSD With Ecstasy Or Cannabis
Sisley has long taken issue with the supply of cannabis coming from the NIDA facility in Mississippi — in particular, how it's processed. She used cannabis produced there in her recently published clinical trial on treating PTSD in military veterans.
She describes the product as an "anemic" greenish powder.
"It's very difficult to overcome the placebo effect when you have something that diluted," she says.
The 76-person study, which took 10 years to complete, concluded that smoked cannabis was generally well tolerated and did not lead to deleterious effects in this group. But it also did not find any statistically significant difference in abating the symptoms of PTSD when compared to a placebo.
For Grant of UCSD, the problem with the long-standing supply of cannabis isn't so much the quality, but the lack of different products like edibles and oils and of cannabis strains with varying concentrations of CBD and THC, the plant's main psychoactive ingredient.
"We don't have enough research on the kind of marijuana products that people in the real world are using," he says.

As CBD Oils Become More Popular, The FDA Considers Whether To Set New Rules
Because of the limited domestic supply, some researchers have resorted to importing cannabis from outside the U.S. — a legal but wildly counterintuitive arrangement that is "arduous" and prone to hiccups, says Sisley.
The constraints on research cannabis also has impeded the pathway to drug development because the NIDA facility's cannabis could only be used for academic research, not for prescription drug development . A drug studied in phase 3 clinical trials — what's required before submitting for approval from the Food and Drug Administration — must be the same as what's later marketed.
"The NIDA monopoly has primarily been why we have medical marijuana in the states, but we don't have medical marijuana through the FDA," says Doblin of MAPS. "It's a fundamental change that we can now have drug development with domestic supplies."
A few barriers still remain
The few companies that will soon land DEA spots to cultivate cannabis have an eager marketplace of researchers who are "clamoring" for the chance to study the scientific properties and medical potential of the plant, says Groff, whose company is up for DEA approval and who also has an FDA project to study the antimicrobial properties of cannabis for killing dangerous bacteria like MRSA .
By the end of next year, Groff anticipates his company will be producing up to 5,000 pounds of marijuana per year, offering researchers a "full menu of customizable options."
Biopharmaceutical Research Company — a third company that will soon cultivate cannabis with a DEA license — already has dozens of agreements in place with U.S. researchers and is hearing from more academic institutions, drugmakers and biotech companies in the wake of the change in policy, says CEO George Hodgin.
"Now there's a very clear, approved and legal path for them to legally enter the cannabis space in the United States," says Hodgin.

The Coronavirus Crisis
'illegal to essential': how the coronavirus is boosting the legal cannabis industry.
Washington State University's Center for Cannabis Policy, Research and Outreach is one of the places that expects to eventually procure cannabis from Hodgin's business.
"It's definitely a big step in the right direction because the industry is moving much faster than we are in research," says Michael McDonell , an associate professor of medicine and director of the university's cannabis center.
But he also points out that even with more growers coming online, it's still by no means easy to study cannabis, because researchers need a special license when working with a Schedule 1 drug and grants to conduct these studies are hard to come by.
Despite the widespread use of marijuana in the U.S., research into the medical potential of other Schedule 1 drugs like MDMA (ecstasy) is much further along than cannabis .
UCSD's Grant says the biggest leap forward for research would come from moving cannabis out of the Schedule 1 drug classification. "If that were to happen," he says, "that would solve a lot of these problems that we've been talking about."
- medical cannabis
- medical marijuana
Quick Links
- [email protected]
- News/Events Email
Enhancing Cannabis Research
Coordinating and fostering innovative Cannabis research across disciplines

Get Involved

WEBINARS: Medical Properties of Cannabis; Chronic & Acute Effects of High-Potency Cannabis on Cognition
Nephi Stella; Carrie Cuttler
Research Work Groups
Five projects mandated by the Washington State Legislature unite experts to study the impact of Cannabis use.

2nd UW CCR Retreat was a true success!
50 participants, a plenary lecture, 6 presentations by UW and WSU faculty, and cutting-edge research posters.

Meet a Researcher: Denise Walker
Dr. Walker develops and evaluates interventions for cannabis use disorders for adults and adolescents.

Schools & Colleges of UW-CCR Members

funding from the National Institutes of Health
UW departments or divisions
state-funded research projects
Shop legal, local weed.
We use cookies for certain features and to improve your experience. See our Cookie Policy and Privacy Policy to learn more
By accessing this site, you accept the Terms of Use and Privacy Policy .
- Cannabis 101
- Strains & products
- Science & tech
- Leafly Lists
The 5 most intriguing cannabis research studies of 2022
There has been a noticeable shift in the type of cannabis research published in 2022. Reduced government research restrictions and improved global legalization efforts have both paved the way for new and exciting ways to research the plant and its complexities.
While research into the independent effects of THC and CBD are still the main focus of most studies, there is increasing interest in commercially available cannabis products, whole-plant extracts, and the effects of terpenes on brain function. Below are five intriguing cannabis research stories from the last year.
Terpenes, not THC levels, are the best predictors of how much you’ll like a product
Cbd doesn’t necessarily make thc safer, benefits of cannabis on the aging adult brain, thc and cbd don’t tell the whole story of effects, cbd-rich cannabis oil improves core social symptoms in people with autism spectrum disorder.
Many cannabis products are described by their cannabinoid content (e.g., THC or CBD), but the plant also produces hundreds of terpenes , aroma compounds which give cannabis its unique odors and flavors.
However, a recent study led by Arianne Wilson-Poe, revealed that terpenes also provide subjective appeal and determine the desirability of a particular flower or vape cannabis product. Scientists tested the appeal of a cannabis product to an individual with a range of THC potencies (from less than 0.3% to over 30%) in nearly 300 individuals across thousands of consumption sessions.
With rising THC potency in commercially available products, one might predict that THC potency would directly correlate to the overall draw of a product, but that wasn’t the case—there was no relationship between THC potency, total cannabis dose, or total THC dose with subjective appeal. Instead, only the aroma, which comes from the terpenes, directly correlated with individuals’ appeal scores.
Therefore, a product’s smell is a better predictor of enjoyment than its THC content. These findings highlight the importance of terpenes in a product’s quality and propose that you don’t need to be overly stoned to have a pleasurable experience.
Achieving the desired high requires finding the optimal THC dose. Overshoot it and there could be problems: memory becomes impaired, cognitive performance decreases, and all-in-all, it’s just a less pleasurable experience.
Shop highly rated dispensaries near you
- See all dispensaries
One common belief is that CBD can blunt THC’s negative effects. Therefore, products with higher ratios of CBD to THC have been thought to be safer and lead to fewer adverse THC symptoms. Researchers performed a double-blind experiment which tested 46 cannabis users and ultimately found this hypothesis to be inaccurate.
As it turns out, vaporized oils containing ratios of 1:1, 2:1, or 3:1 CBD to 10mg THC did not protect against THC’s effects across a range of measures. CBD level also had no impact on THC’s effects, including feeling stoned, impairments to working and long-term memory, increased pleasurable responses to music and chocolate, or effects on a range of physiological measures, including blood pressure and heart rate.
These findings indicate that the inclusion of CBD to THC products at a common recreational level may not serve any protective benefit against some of THC’s adverse effects. Perhaps even higher CBD:THC ratios may be effective, but to be confident, the safest strategy to avoid potentially adverse effects of THC is to limit the dose instead of masking it with CBD.
The strength of communication across different brain regions changes as people age and contributes to age-related memory impairments and cognitive decline. Scientists in Colorado used functional neuroimaging to assess how regular cannabis use (at least once per week) in adults over 60 years old altered the strength of communication between several brain regions that normally declines with age.
They found that older adults who regularly used cannabis had stronger communication patterns between three brain regions—the hippocampus, the parahippocampal gyrus, and the cerebellum—compared to non-cannabis using older adults. The stronger connectivity among older cannabis users resembled that of much younger non-using adults and suggests that cannabis may protect against some of the age-related declines in brain function.
Although these results are not causal in nature because it wasn’t a randomized, controlled experiment, they provide some of the first human evidence to support observations from rodent studies where small amounts of regular cannabis use protected against age-related brain changes and cognitive decline.
Commercial cannabis products are commonly labeled with THC and CBD content to provide some predictive measure for how they’re going to create effects and impact brain function when consumed. It turns out, that’s not enough information to make an accurate prediction.
For many people, this isn’t surprising. Indicaproducts have historically been thought to be relaxing, whereas sativaproducts were thought to be energizing, but these predictive classifications are less relevant than a strain’s chemical composition, which includes a mix of cannabinoids, terpenes, and other compounds. Nonetheless, THC and CBD content are the primary metrics presented on retail cannabis products.
A recent study found that oral consumption of a commercially available indicaoil reduced the amount of effort animals were willing to exert to achieve a large reward—basically, it made them lazy. However, a sativaoil, even though it had the same THC and CBD content, had no effect.
These findings illustrate that THC and CBD levels, and indica and sativa classifications, are not the only considerations for predicting the effects that oral cannabis consumption will have on brain function—other minor cannabinoids and terpenes matter.
Many parents have been raving about the benefits of cannabis-based treatments for children on the autism spectrum for years. Notably, the goal is not to cure autism, but to facilitate better engagement and promote improved living skills so they can eventually be more independent.
For several years, Israeli scientists have been running clinical trials that reveal promising results for a 20:1 CBD to THC cannabis oil on many secondary symptoms of autism spectrum disorder, such as improved sleep, reduced anxiety, fewer rage attacks, and reductions in self-injury behaviors.
Results from these clinical trials show that cannabis also improved core social communication skills and boosted daily-living skills, such as getting dressed, eating food, and cleaning up, in children and adolescents. While these benefits didn’t extend to other core symptoms like restricted and repetitive behaviors, this study highlights the exciting potential of cannabis to improve the quality of life for those with autism spectrum disorder, and improve the opportunity for them to live a more independent life.
The latest in Science & tech
By providing us with your email address, you agree to Leafly's Terms of Service and Privacy Policy .
Toxic metal particles can be present in cannabis vapes even before the first use, study finds
Vapes have often been heralded as a "safer" way to consume either nicotine or cannabis, where legal to do so. But the devices present their own suite of risks that are slowly being revealed as they undergo increasing research and regulation. Now, researchers have discovered that nano-sized toxic metal particles may be present in cannabis vaping liquids even before the vaping device is heated, and the effect is worse in unregulated products.
The researchers will present their results today at the spring meeting of the American Chemical Society (ACS). ACS Spring 2024 is a hybrid meeting being held virtually and in person March 17-21; it features nearly 12,000 presentations on a range of science topics.
While cannabis regulation and legalization are still growing in the U.S., it was made federally legal in Canada under its Cannabis Act in 2018. "Cannabis vapes are newly regulated products in Canada, so we don't yet have much scientific data about them," says Andrew Waye, who will present the work at the meeting. "This is an opportunity for us to look at some of the questions concerning the risks and unknowns of cannabis vapes." Waye manages the research program at the Office of Cannabis Science and Surveillance at Health Canada.
Unlike smoking, vaping does not involve a combustion reaction, which produces harmful byproducts. Instead, a vaping device heats a liquid until it evaporates into an inhalable vapor. As a result, it is often seen as a safer method to consume cannabis or nicotine. But research on nicotine vapes has shown that the metal components that heat the vape liquid may release harmful elemental metals, including nickel, chromium and lead, which can then be transported into the aerosol and deposited into the user's body.
Waye's team wanted to investigate whether this was also true for cannabis vapes. To do so, the group collaborated with Zuzana Gajdosechova, who is a scientist at the Metrology Research Centre of the National Research Council of Canada, which has been involved in cannabis testing and standardization for several years.
The team gathered 41 samples of cannabis vape liquids -- 20 legal, regulated samples from the Ontario Cannabis Store and 21 samples from the illicit market provided by the Ontario Provincial Police. The liquids were analyzed by mass spectrometry to look for the presence of 12 metals. Regulated cannabis products are routinely tested for some of the analyzed metals, as well as other contaminants.
To verify the team's findings, Gajdosechova collaborated with imaging experts and used techniques such as scanning electron microscopy to provide a visual confirmation of the metal particles. While some metals, such as arsenic, mercury and cadmium, were within the generally accepted tolerance limits for cannabis products, others were detected in concentrations considered to be very high. The most striking example proved to be lead: Some unregulated samples contained 100 times more lead than the regulated samples, far exceeding the generally accepted tolerance limit.
Importantly, this metal contamination was found in the liquid of cannabis vapes that had never been used and were less than six months old. "The evidence strongly suggests that metal contamination can come from the device when it's produced, and not from the heating of the coils," explains Gajdosechova. "But depending on the quality of the device, the contamination may be increased by that heating."
Additionally, the team found that vapes belonging to the same production lot could contain different levels of metal contamination, demonstrating a high level of variability between samples. This could have implications for testing procedures, as Canadian regulations require samples to be representative of the whole lot or batch and that testing be done at or after the last step where contamination can occur. "If contamination is happening when the device is assembled, you should be testing at that stage rather than earlier," says Waye.
Next, the team wanted to investigate the size of the metal particles to understand their potential health risks. Using single particle inductively coupled plasma mass spectrometry, the researchers found many particles that were of nanoscale size. "Some nano-sized metal particles are highly reactive and potentially harmful," says Gajdosechova.
Moving forward, the team wants to determine how many of these particles are transmitted into the vape aerosol when a device is used. This is when the metals could get into users' lungs, which will be important to determine the public health implications of these findings. The effect has been demonstrated in nicotine vapes, and the researchers expect that cannabis vapes could show the same.
"Different types of cannabis products present different risks. Our research doesn't answer whether vaping is riskier than smoking, it just underlines that the risks may be different. Previously uncharacterized risks with cannabis vaping are still being identified," concludes Waye. So, while there isn't necessarily one way to "safely" consume these products, this research demonstrates that regulation can help create safer cannabis products overall.
This research was funded by Health Canada.
- Controlled Substances
- Personalized Medicine
- Alzheimer's Research
- Inorganic Chemistry
- Nature of Water
- Air Pollution
- Mushroom poisoning
- Nuclear fission
- Vegetarianism
- Subatomic particle
Story Source:
Materials provided by American Chemical Society . Note: Content may be edited for style and length.
Cite This Page :
Explore More
- Next-Gen Solar Cells: Perovskite Semiconductors
- When Faces Appear Distorted: Rare Condition
- End of Planet Formation
- Enormous Ice Loss from Greenland Glacier
- Signs of Life Detectable in Single Ice Grain
- Tudor Era Horse Cemetery
- When Do Babies Become Conscious?
- Secrets of the Van Allen Belt Revealed
- Robotic Prostheses, Exoskeletons
- Bronze Age Families: Clothing, Recipes, Pets
Trending Topics
Strange & offbeat.
- Open access
- Published: 20 March 2024
Benzodiazepine use in medical cannabis authorization adult patients from 2013 to 2021: Alberta, Canada
- Cerina Dubois 1 ,
- Heidi Fernandes 1 ,
- Karen J. B. Martins 3 ,
- Jason R. B. Dyck 4 ,
- Scott W. Klarenbach 3 ,
- Lawrence Richer 3 ,
- Ed Jess 5 ,
- John G. Hanlon 6 , 7 ,
- Elaine Hyshka 1 &
- Dean T. Eurich 1
BMC Public Health volume 24 , Article number: 859 ( 2024 ) Cite this article
193 Accesses
Metrics details
Benzodiazepines are a class of medications that are being frequently prescribed in Canada but carry significant risk of harm. There has been increasing clinical interest on the potential “sparing effects” of medical cannabis as one strategy to reduce benzodiazepine use. The objective of this study as to examine the association of medical cannabis authorization with benzodiazepine usage between 2013 and 2021 in Alberta, Canada.
A propensity score matched cohort study with patients on regular benzodiazepine treatment authorized to use medical cannabis compared to controls who do not have authorization for medical cannabis. A total of 9690 medically authorized cannabis patients were matched to controls. To assess the effect of medical cannabis use on daily average diazepam equivalence (DDE), interrupted time series (ITS) analysis was used to assess the change in the trend of DDE in the 12 months before and 12 months after the authorization of medical cannabis.
Over the follow-up period after medical cannabis authorization, there was no overall change in the DDE use in authorized medical cannabis patients compared to matched controls (− 0.08 DDE, 95% CI: − 0.41 to 0.24). Likewise, the sensitivity analysis showed that, among patients consuming ≤5 mg baseline DDE, there was no change immediately after medical cannabis authorization compared to controls (level change, − 0.04 DDE, 95% CI: − 0.12 to 0.03) per patient as well as in the month-to-month trend change (0.002 DDE, 95% CI: − 0.009 to 0.12) per patient was noted.
Conclusions
This short-term study found that medical cannabis authorization had minimal effects on benzodiazepine use. Our findings may contribute ongoing evidence for clinicians regarding the potential impact of medical cannabis to reduce benzodiazepine use.
• Medical cannabis authorization had little to no effect on benzodiazepine usage among patients prescribed regular benzodiazepine treatment in Alberta, Canada.
• Further clinical research is needed to investigate the potential impact of medical cannabis as an alternative to benzodiazepine medication.
Peer Review reports
Benzodiazepines are anxiolytic medications which are known for their sedative properties in the treatment of anxiety [ 1 ]. When first introduced on the market, clinicians enthusiastically prescribed benzodiazepines, quickly becoming one of the most widely prescribed medications [ 2 ]. Benzodiazepines have a relaxing or calming effect, which is beneficial in the treatment of anxiety. Benzodiazepines can also relieve severe emotional distress, such as panic attacks. Alongside its benefits, also comes a list of side effects associated with the medication [ 3 ]. The most reported side effect is drowsiness and hence they are often used as sleep aids. Other side effects include impaired coordination, slurred speech, confusion, disorientation, dizziness, decreased blood pressure and respiratory rate, and memory problems [ 4 ]. Long-term use of the medication, particularly in the elderly population, has been associated with cognitive impairment and increased risk of falls [ 5 ].
To date, an increasing number of benzodiazepines are being misused by patients and prescription percentages have aligned with patients’ increased risk of dependence on this medication [ 6 ]. While over 1 million benzodiazepine prescriptions were dispensed in Alberta in 2021 alone [ 7 ]. Prescribing are seemingly decreasing (4% of Albertans received a benzodiazepine or Z-drug [zopiclone, zaleplon, and zolpidem] prescription in 2021 compared to 9.1% in 2016 – a drop of almost one fifth), in which medical cannabis use may be playing a role in circumventing benzodiazepine usage [ 7 ]. Nevertheless, benzodiazepine prescribing continues to be high in Alberta.
Among the top reasons for medical cannabis use has been an increasing clinical interest in its “sparing effect” on reducing the use of other medications, specifically benzodiazepines (as well as opioids) [ 8 ]. Literature that supports the use of medical cannabis as a substitution for benzodiazepines is currently low; and studies assessing medical cannabis’ association with anxiety report inconclusive or mixed results [ 9 , 10 , 11 ]. One small randomized controlled trial (RCT) investigating 24 patients with generalized social anxiety disorder showed a reduction in public speaking anxiety with the use of cannabidiol (CBD), a therapeutic component of cannabis [ 12 ]. Conversely, there are concerns cannabis can actually increase anxiety levels. One study reported cannabis use increased the risk for more severe anxiety symptoms [ 13 ], however, a meta-analysis showed that cannabis was only a minor risk factor for increased anxiety [ 14 ]. This lack of consensus of cannabis use for anxiety may also be attributed to the inconsistent nature of the current available literature. Previous studies either tend to strongly focus on the negative effects of cannabis and anxiety (rather than searching for how cannabis may improve anxiety) [ 15 , 16 , 17 , 18 ], consist of age-specific and small cohort sizes [ 13 , 19 , 20 , 21 , 22 , 23 ], did not differentiate cannabis from other illicit substances [ 24 , 25 ], or cannot accurately distinguish cannabis for medical purposes versus recreational where non-medical use which often occur in the context of other drug use [ 26 , 27 ]. Consequently, the current clinical practice guidelines for medical cannabis for Canadian physicians do not support its use for anxiety and other mental health conditions [ 28 ].
The legalization of recreational cannabis in October 2018 has led to an increased interest in medical cannabis use, including its use for anxiety and its “sparing effect” for benzodiazepines (and opioids) [ 29 ]. Concurrent with the high prevalence of benzodiazepine prescribing, it is imperative to fully investigate the harms and benefits of medical cannabis in order for clinicians and patients to make the best-informed decisions. Thus, the purpose of this study was to examine the association of medical cannabis authorization on benzodiazepine usage in the Alberta population. We hypothesize that medical cannabis authorization would be associated with a reduction in chronic benzodiazepine use (i..e, reduction in daily dose).
Study design
A matched cohort study with patients on regular benzodiazepine treatment authorized to use medical cannabis and controls who do not have authorization for medical cannabis.
Data source
The College of Physicians and Surgeons of Alberta provided the medical cannabis patient identifiers as they are the regulatory entity for cannabis authorization in the province. Through the use of unique lifetime personnel health numbers, all patients were linked to the administrative databases of Alberta Health which captures all healthcare utilization for all patients in the province of Alberta as part of the universal health insurance plan for residents. These databases included provincial health care registry, vital statistics, all inpatient hospitalizations, ambulatory emergency department visits, all community pharmacy drug dispensations, and physician claims data, providing at least one-year of longitudinal follow-up data following the index date for both patients authorized to access medical cannabis and high dimensional propensity score matched controls as outlined below. All data was de-identified prior to its released to the researchers.
Inclusion criteria
The exposed group were all patients prescribed regular benzodiazepine treatment and authorized for medical cannabis in Alberta between March 31, 2013 and March 31, 2021. Medical cannabis authorization in Canada is defined as a patient being granted authorization by their healthcare provider to access cannabis for medical purpose (i.e., patients self-treating with cannabis for medicinal purposes were not included). Participants were of 18 years of age and older, any sex, ethnicity, and socioeconomic status who received authorization for medical cannabis for any indication (acute and chronic). The index date for each patient was the first recorded date of medical cannabis authorization at the clinics. Regular benzodiazepine treatment was defined as those who had:
Benzodiazepine dispensation within 30 days prior to the first date of medical cannabis authorization (index date); and
A total of 120 or more cumulative calendar days of benzodiazepines prescriptions based on days supply; or 10 or more dispensations in the year prior index date [ 29 ].
The unexposed control group met the same above criteria as the exposed group, with the exception of not having medical cannabis authorization. The index dates of the unexposed group were randomly assigned based on the distribution of exposed group’s index dates.
Exclusion criteria
All patients who were not eligible to receive health benefits in Alberta were excluded (e.g., out of province patients). In addition, to ensure sufficient follow-up time to assess the effects of medical cannabis authorization on regular benzodiazepine use, all patients had to have at least 1 year follow-up time, maintain eligibility for Alberta Health benefits (i.e., did not move out of province) and did not die within 1 year after the index date. Any patients not meeting these criteria were excluded.

Propensity score matched controls
To construct the propensity score, high-dimensional propensity score (HDPS) was used as the approach is known to balance the potential confounders (baseline covariates) and thus, significantly reduce bias by confounding. HDPS can also reduce confounding by some unmeasured characteristics depending on the underlying correlations with known variables. All patients authorized for medical cannabis ( n = 9690) were matched with one unique unexposed control group patient using the high dimensional propensity score (HDPS) matching. We selected the matched control for each cannabis user using the nearest neighbour approach with 1: 1 ratio and a caliper set at 0.2. Based on this algorithm, we were successful in identifying 1 control for each cannabis users. Balance in confounders was fully assessed, and no imbalances were noted (all standardized mean differences < 0.1 as recommended). Variables incorporated into the HDPS matching method included: sex, age, year of index date (categorical), baseline benzodiazepine duration, social deprivation index, age group, CNS medications, living area (rural/urban), comorbidities associated with cannabis use, and all healthcare resource utilization variables (all within the year prior to the index date). This includes healthcare utilization (all hospitalizations with up to 10 CCI (Canadian Classification of Health Interventions) procedure codes and 25 diagnostic ICD-10 codes, emergency visit with up to 10 ICD-10 diagnostic code, physician claims with CCI procedure code and with up to 3 ICD-9 diagnostic codes) and all prescription drugs dispensed to a patient at baseline. Notably, the entire healthcare dataset reported greater than 1000 different variables and categories which were included in the HDPS. The HDPS matching technique used the SAS packages proposed by Rassen et al. [ 30 ] and Schneeweiss et al. [ 31 ].
All benzodiazepine doses were converted into a defined daily dose (DDD) based on the drug’s day supply, dispensation amount, and strength. Using DDD allowed us to estimate the average maintenance dose per day for the different types of benzodiazepines. To standardized the DDD, as benzodiazepines can be short- or long-acting, the strength of each benzodiazepine was then converted to a diazepam equivalence using known pharmacologic conversion factors [ 1 , 32 ]. The primary outcome was the difference in the daily average diazepam equivalence (DDE) between the medically authorized patients and the matched control group in the 12 months prior to index and 12 months following medical cannabis authorization (or equivalent index date for controls).
Ethics approval
This study was approved by the University of Alberta Health Research Ethics Board (PRO 00084689). As the study relied on secondary use of de-identified health data (i.e. administrative data), a waiver for informed consent was provided by the Ethics Board.
Statistical analysis
All data were expressed descriptively using means (standard deviations [SD]) or count (proportions [%]), as appropriate. To assess the effect of medical cannabis use on DDE, interrupted time series (ITS) analysis was used to assess the change in the trend of diazepam equivalence in the 12 months before and 12 months after the authorization of medical cannabis (or pseudo index for matched controls). ITS is a quasi-experimental design that allows comparison of trends in an outcome before and after an intervention [ 33 , 34 ]. This specific analysis was chosen for its effectiveness in clear differentiation between population-level health pre-intervention and post-intervention periods. The controlled ITS employed in this study included an additional control series to account for temporal changes that may have occurred within the population. Controlled ITS has been shown to provide similar results as those observed in RCTs [ 35 , 36 ], which highlights the validity of the approach [ 37 , 38 ].
DDE was assessed in 30-day windows for each patient (i.e., month-to-month average diazepam equivalence). The absolute effect of medical cannabis authorization on average monthly DDE was calculated, which summarizes both the immediate level change (i.e. within the first month following the index date) and change in trend over the 1 year follow-up period with the multivariate delta method used to construct 95% confidence intervals around the estimate [ 39 ].
Sensitivity and stratification analysis
Further stratification was conducted on both authorized medical cannabis patients ( n = 9690) and matched controls in 4 subgroups according to baseline DDE as any change in DDE would be expected to be affected by the initial starting DDE:
DDE between 5 and 10 mg
DDE between 10 and 15 mg
DDE > 15 mg
In total, 9690 medically authorized cannabis patients and 123,899 eligible controls were identified (Fig. 1 ). All 9690 patients were matched to one control, and following HDPS matching, all covariates were well balanced after matching between the groups (standardized difference < 10%; a threshold recommended for declaring imbalance in pharmacoepidemiologic research) [ 40 ] (Table 1 ).
Selection of study population
Over the 1-year follow up period after medical cannabis authorization, there was no overall change in the DDE use in authorized medical cannabis patients compared to matched controls (− 0.08 DDE, 95% CI: − 0.41 to 0.24). Additionally, there was no effect in the month-to-month change in average DDE after cannabis authorization (0.04 DDE, 95% CI: − 0.01 to 0.09). When both the initial change and the longer month to month change were combined, no overall effect was observed in the absolute difference in the total monthly DDE (0.32, 95% CI: − 0.23 to 0.87) per patient between cases and controls (Table 2 ; Fig. 2 ). Assessments on the type of benzodiazepines dispensed also indicated there was no change in the most frequently used benzodiazepines used before and after cannabis authorization ( p > 0.05).
Difference in average daily diazepam equivalents per patient medically authorized cannabis users ( n = 9690) vs controls ( n = 9690)
Sensitivity analyses results
Among patients consuming ≤5 mg baseline DDE, there was no change immediately after medical cannabis authorization compared to controls (level change, − 0.04 DDE, 95% CI: − 0.12 to 0.03) per patient as well as in the month-to-month trend change (0.002 DDE, 95% CI: − 0.009 to 0.12) per patient was noted. Overall, there was no change in the absolute difference in the total monthly DDE among medically authorized patients after accounting for both immediate and overall trend during follow-up (− 0.02 DDE, 95% CI: − 0.16 to 0.11) compared to controls (Table 3 ). Results observed among patients consuming 5–10 DDE, 10–15 mg DDE, and > 15 mg DDE were similar with no immediate change, month-to-month trend change, nor overall absolute difference in the total monthly DDE per patient (Table 3 ).
In this retrospective, observational, population-based study, short-term analysis demonstrated that medical cannabis authorization had little to no effect on benzodiazepine usage among patients prescribed regular benzodiazepine treatment in Alberta, Canada. There were no differences observed across a wide range of initial DDE at baseline. Notably, there were no statistically significant differences when comparing different categories of benzodiazepine DDE groups and any small increases or decreases are likely clinically inconsequential. Collectively, these results may suggest that for the majority of patients on regular benzodiazepine treatment, medical cannabis use is unlikely to alter future benzodiazepine use.
Regarding the literature on benzodiazepine use, more studies are now supporting the role of medical cannabis as an adjunct to benzodiazepine tapering and cessation. Also known as a “sparing effect treatment,” recent studies are reporting that concurrent use with medical cannabis can reduce future benzodiazepine use [ 41 ]. Particularly, Purcell et. al’s work found that 45.2% patients successfully discontinued their pre-existing benzodiazepine therapy over 2 months of medical cannabis therapy [ 42 ]. However, it is important to note that in this study, the sample size was much smaller and had more frequent follow-ups with physicians compared to the larger sample in our study. Additionally, another study [ 43 ] on perioperative cannabis use on surgical patients showed that concurrent use decreased overall benzodiazepine and opioid use. Although our study showed no effect, subgroup effects may still exist where certain patients on benzodiazepine treatment were able to successfully decrease their benzodiazepine dosage through medical cannabis use.
A significant strength of this study is that it is currently, to our knowledge, the largest and longest population-based study of medical cannabis users in Canada. It uses robust measures to track medical cannabis authorization and current benzodiazepine treatment. However, our study is not without limitations. It is an observational study, which can be prone to potential spectrum bias as our cohort of patients were those who individually sought medical cannabis authorization. Secondly, there is a lack of insight into the patient’s adherence to their authorized medical cannabis treatment. The cannabis may have been taken differently than as indicated and/or alternative therapies may have been used to circumvent benzodiazepine usage. Notably, we do not know whether patients were using cannabis prior to authorization. Further, we do not know whether legalization of cannabis during this period impacted the number of patients seeking medical cannabis authorization. In this case, we may expect non-differential misclassification of the control group due to increased widespread cannabis legalization after 2018. For example, some controls may report not having been medically authorized for cannabis, but after legalization of cannabis, were accessing recreational cannabis via storefront for other reasons. The extent of this occurrence is unknown but notably the majority of patients in our study were derived prior to the legalization change in 2018 (i.e., 2013–2018). Furthermore, given the wide variability of medical cannabis products available in Canada, we could not analyze specific strains, modes of consumption, or dosing regimens in this study. All cannabis products were treated as equals, despite there being known clinical difference between products and regimens. Our study is therefore limited by the lack of these clinical details of medical cannabis, in addition to the lack of concomitant use with other non-prescription benzodiazepines agents.
This study found that medical cannabis authorization had minimal effects on benzodiazepine use. Although the clinical importance of medical cannabis as a benzodiazepine sparing treatment is unclear, our findings contribute ongoing evidence for clinicians regarding the association between medical cannabis on benzodiazepine use.
Availability of data and materials
The data that support the findings of this study are available from College of Physicians & Surgeons of Alberta (CPSA) and the Alberta SPOR SUPPORT Unit ( https://absporu.ca ) but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. We had full permission to use this data, however, specific restrictions do apply to the public availability of these data, which are under data access agreements for the current study.
Howard P, Twycross R, Shuster J, Mihalyo M, Wilcock A. Benzodiazepines. J Pain Symptom Manag. 2014;47(5):955–64. https://doi.org/10.1016/j.jpainsymman.2014.03.001 .
Article Google Scholar
Wick JY. The history of benzodiazepines. Consult Pharm. 2013;28(9):538–48. https://doi.org/10.4140/TCP.n.2013.538 .
Article PubMed Google Scholar
Votaw VR, Geyer R, Rieselbach MM, McHugh RK. The epidemiology of benzodiazepine misuse: a systematic review. Drug Alcohol Depend. 2019;200:95–114. https://doi.org/10.1016/j.drugalcdep.2019.02.033 .
Article PubMed PubMed Central Google Scholar
Edinoff AN, Nix CA, Hollier J, et al. Benzodiazepines: Uses, Dangers, and Clinical Considerations. Neurol Int. 2021;13(4):594–607. https://doi.org/10.3390/neurolint13040059 .
Article CAS PubMed PubMed Central Google Scholar
Gray SL, Lai KV, Larson EB. Drug-induced cognition disorders in the elderly: incidence, prevention and management. Drug Saf. 1999;21(2):101–22. https://doi.org/10.2165/00002018-199921020-00004 .
Article CAS PubMed Google Scholar
Panes A, Verdoux H, Fourrier-Reglat A, Berdai D, Pariente A, Tournier M. Misuse of benzodiazepines: prevalence and impact in an inpatient population with psychiatric disorders. Br J Clin Pharmacol. 2020;86(3):601–10. https://doi.org/10.1111/bcp.14165 .
Alberta T. Hot off the press: The 2021 Opioid & Benzodiazepine/Z-drug and Antibiotic Prescription Atlases. . 2021. Accessed February 13, 2023. https://cpsa.ca/news/hot-off-the-press-the-2021-opioid-benzodiazepine-z-drug-and-antibiotic-prescription-atlases/#:~:text=Over%20one%20million%20benzodiazepine%2FZ ,drop%20of%20almost%20one%20fifth.
Clarke H, Fitzcharles M. The evolving culture of medical cannabis in Canada for the management of chronic pain. Front Pharmacol. 2023;14:1153584. https://doi.org/10.3389/fphar.2023.1153584 .
Sharpe L, Sinclair J, Kramer A, de Manincor M, Sarris J. Cannabis, a cause for anxiety? A critical appraisal of the anxiogenic and anxiolytic properties. J Transl Med. 2020;18(1):374. https://doi.org/10.1186/s12967-020-02518-2 .
Van Ameringen M, Zhang J, Patterson B, Turna J. The role of cannabis in treating anxiety: an update. Curr Opin Psychiatry. 2020;33(1):1–7. https://doi.org/10.1097/YCO.0000000000000566 .
Berger M, Amminger GP, McGregor IS. Medicinal cannabis for the treatment of anxiety disorders. Aust J Gen Pract. 2022;51(8):586–92. https://doi.org/10.31128/AJGP-04-21-5936 .
Bergamaschi MM, Queiroz RH, Chagas MH, et al. Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naive social phobia patients. Neuropsychopharmacoly. 2011;36(6):1219–26. https://doi.org/10.1038/npp.2011.6 .
Article CAS Google Scholar
Gobbi G, Atkin T, Zytynski T, et al. Association of Cannabis Use in Adolescence and Risk of Depression, Anxiety, and Suicidality in Young Adulthood: A Systematic Review and Meta-analysis. JAMA Psychiatry. 2019;76(4):426–34. https://doi.org/10.1001/jamapsychiatry.2018.4500 .
Twomey CD. Association of cannabis use with the development of elevated anxiety symptoms in the general population: a meta-analysis. J Epidemiol Community Health. 2017;71(8):811–6. https://doi.org/10.1136/jech-2016-208145 .
Hill KP. Cannabis Use and Risk for Substance Use Disorders and Mood or Anxiety Disorders. JAMA. 2017;317(10):1070–1. https://doi.org/10.1001/jama.2016.19706 .
Mammen G, Rueda S, Roerecke M, Bonato S, Lev-Ran S, Rehm J. Association of Cannabis With Long-Term Clinical Symptoms in Anxiety and Mood Disorders: A Systematic Review of Prospective Studies. J Clin Psychiatry. 2018;79(4) https://doi.org/10.4088/JCP.17r11839 .
Moore TH, Zammit S, Lingford-Hughes A, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370(9584):319–28. https://doi.org/10.1016/S0140-6736(07)61162-3 .
Di Blasi M, Cavani P, Pavia L, et al. Mediating effects of global negative effect expectancies on the association between problematic Cannabis use and social anxiety. Front Psychiatry. 2017;8:249. https://doi.org/10.3389/fpsyt.2017.00249 .
Volkow ND, Baler RD, Compton WM, Weiss SR. Adverse health effects of marijuana use. N Engl J Med. 2014;370(23):2219–27. https://doi.org/10.1056/NEJMra1402309 .
Lum HD, Arora K, Croker JA, et al. Patterns of marijuana use and health impact: a survey among older Coloradans. Gerontol Geriatr Med. 2019;5:2333721419843707. https://doi.org/10.1177/2333721419843707 .
Pedersen ER, Miles JN, Osilla KC, Ewing BA, Hunter SB, D'Amico EJ. The effects of mental health symptoms and marijuana expectancies on marijuana use and consequences among at-risk adolescents. J Drug Issues. 2015;45(2):151–65. https://doi.org/10.1177/0022042614559843 .
Esmaeelzadeh S, Moraros J, Thorpe L, Bird Y. The association between depression, anxiety and substance use among Canadian post-secondary students. Neuropsychiatr Dis Treat. 2018;14:3241–51. https://doi.org/10.2147/NDT.S187419 .
Butler A, Patte KA, Ferro MA, Leatherdale ST. Interrelationships among depression, anxiety, flourishing, and cannabis use in youth. Addict Behav. 2019;89:206–15. https://doi.org/10.1016/j.addbeh.2018.10.007 .
Villarosa-Hurlocker MC, Bravo AJ, Pearson MR. Protective strategies study T. The relationship between social anxiety and alcohol and marijuana use outcomes among concurrent users: a motivational model of substance use. Alcohol Clin Exp Res. 2019;43(4):732–40. https://doi.org/10.1111/acer.13966 .
Degenhardt L, Chiu WT, Sampson N, et al. Toward a global view of alcohol, tobacco, cannabis, and cocaine use: findings from the WHO world mental health surveys. PLoS Med. 2008;5(7):e141. https://doi.org/10.1371/journal.pmed.0050141 .
Wall MM, Liu J, Hasin DS, Blanco C, Olfson M. Use of marijuana exclusively for medical purposes. Drug Alcohol Depend. 2019;195:13–5. https://doi.org/10.1016/j.drugalcdep.2018.11.009 .
Smith JM, Mader J, Szeto ACH, Arria AM, Winters KC, Wilkes TCR. Cannabis use for medicinal purposes among Canadian University students. Can J Psychiatr. 2019;64(5):351–5. https://doi.org/10.1177/0706743718818420 .
Allan GM, Ramji J, Perry D, et al. Simplified guideline for prescribing medical cannabinoids in primary care. Can Fam Phys. 2018;64(2):111–20.
Google Scholar
Lee C, Lin M, Martins KJB, et al. Opioid use in medical cannabis authorization adult patients from 2013 to 2018: Alberta Canada. BMC Public Health. 2021;21(1):843. https://doi.org/10.1186/s12889-021-10867-w .
Rassen JA, Glynn RJ, Brookhart MA, Schneeweiss S. Covariate selection in high-dimensional propensity score analyses of treatment effects in small samples. Am J Epidemiol. 2011;173(12):1404–13. https://doi.org/10.1093/aje/kwr001 .
Schneeweiss S, Rassen JA, Glynn RJ, Avorn J, Mogun H, Brookhart MA. High-dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiol. 2009;20(4):512–22. https://doi.org/10.1097/EDE.0b013e3181a663cc .
Ashton HC. Benzodiazepines: How They Work and How to Withdraw. 2013. https://benzo.org.uk/manual/ . Accessed 12 Mar 2024.
Bernal JL, Cummins S, Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol. 2017;46(1):348–55. https://doi.org/10.1093/ije/dyw098 .
Hamilton I, Lloyd C, Hewitt C, Godfrey C. Effect of reclassification of cannabis on hospital admissions for cannabis psychosis: a time series analysis. Int J Drug Policy. 2014;25(1):151–6. https://doi.org/10.1016/j.drugpo.2013.05.016 .
St. Clair T, Hallberg K, Cook TD. The validity and precision of the comparative interrupted time-series design: three within-study comparisons. J Educ Behav Stat. 2016;41(3):269–99.
St. Clair T, Cook TD, Hallberg K. Examining the internal validity and statistical precision of the comparative interrupted time series design by comparison with a randomized experiment. Am J Eval. 2014;35(3):311–27. https://doi.org/10.1177/1098214014527337 .
Fretheim A, Zhang F, Ross-Degnan D, et al. A reanalysis of cluster randomized trials showed interrupted time-series studies were valuable in health system evaluation. J Clin Epidemiol. 2015;68(3):324–33. https://doi.org/10.1016/j.jclinepi.2014.10.003 .
Fretheim A, Soumerai SB, Zhang F, Oxman AD, Ross-Degnan D. Interrupted time-series analysis yielded an effect estimate concordant with the cluster-randomized controlled trial result. J Clin Epidemiol. 2013;66(8):883–7. https://doi.org/10.1016/j.jclinepi.2013.03.016 .
Zhang F, Wagner AK, Soumerai SB, Ross-Degnan D. Methods for estimating confidence intervals in interrupted time series analyses of health interventions. J Clin Epidemiol. 2009;62(2):143–8. https://doi.org/10.1016/j.jclinepi.2008.08.007 .
Stuart EA, Lee BK, Leacy FP. Prognostic score-based balance measures can be a useful diagnostic for propensity score methods in comparative effectiveness research. J Clin Epidemiol. 2013;66(8 Suppl):S84–S90 e1. https://doi.org/10.1016/j.jclinepi.2013.01.013 .
Pottie K, Thompson W, Davies S, et al. Deprescribing benzodiazepine receptor agonists: evidence-based clinical practice guideline. Can Fam Phys. 2018;64(5):339–51.
Purcell C, Davis A, Moolman N, Taylor SM. Reduction of benzodiazepine use in patients prescribed medical Cannabis. Cannabis Cannabinoid Res. 2019;4(3):214–8. https://doi.org/10.1089/can.2018.0020 .
Stewart C, Fong Y. Perioperative Cannabis as a Potential Solution for Reducing Opioid and Benzodiazepine Dependence. JAMA Surg. 2021;156(2):181–90. https://doi.org/10.1001/jamasurg.2020.5545 .
Download references
Acknowledgements
This study is based in part on data provided by Alberta Health, Alberta Health Services and by the College of Physicians & Surgeons of Alberta (CPSA). The interpretation and conclusions contained herein are those of the researchers and do not necessarily represent the views of the Government of Alberta, Alberta Health Services or CPSA. Neither the Government of Alberta, Alberta Health, Alberta health Services nor CPSA expresses any opinion in relation to this study.
Patient and public involvement
Patients were not involved in the design, conduct and reporting of this research project as it was not applicable to this project. The College of Physicians & Surgeons of Alberta (CPSA) is a public body that was involved in the project in terms of our access to the data. As mentioned in our “Acknowledgements,” although the data was provided by the CPSA, neither the Government of Alberta, Alberta Health, Alberta Health Services, nor CPSA expresses any opinion in relation to this study. Further, the CPSA was not involved in the analysis or reporting of the research project’s outcomes.
Transparency declaration
DTE affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and if relevant) have been explained.
Production of this study has been made possible through a CIHR Catalyst Grant for Cannabis Research in Urgent Priority Areas, funded by the Canadian Centre on Substance Use and Addiction using Health Canada Cannabis Research Initiative funds (CCSA 163022). The views expressed herein do not necessarily represent the views of CIHR or CCSA or its funders. The funders did not participate in the design of the study, collection, analysis, interpretation of the data, and in writing the manuscript.
Author information
Authors and affiliations.
School of Public Health, University of Alberta, 3-300 Edmonton Clinic Health Academy 11405 - 87 Ave Edmonton, AB, T6G 1C9 2E, Edmonton, AB, Canada
Cerina Dubois, Heidi Fernandes, Elaine Hyshka & Dean T. Eurich
SPOR (Strategy for Patient Oriented Research) Data Platform, Alberta Health Services, Edmonton, Alberta, Canada
Faculty of Medicine & Dentistry, University of Alberta, Edmonton, Alberta, Canada
Karen J. B. Martins, Scott W. Klarenbach & Lawrence Richer
Cardiovascular Research Centre, Department of Pediatrics, Faculty of Medicine and Dentistry, University of Alberta, Edmonton, Alberta, Canada
Jason R. B. Dyck
College of Physicians & Surgeons of Alberta, Edmonton, Alberta, Canada
St. Michael’s Hospital Department of Anesthesia, University of Toronto, Ontario, Canada
John G. Hanlon
Department of Anaesthesiology and Pain Medicine, University of Toronto, Ontario, Canada
You can also search for this author in PubMed Google Scholar
Contributions
DTE, JRBD, JGH, EH, SWK, and LR designed the study and DTE, JRBD, KJBM acquired the data. ML was the primary analyst for the data. CD, DTE, and HF drafted the manuscript. All other authors revised it critically for important intellectual content and approved the final version to be published. All authors are accountable for the work and integrity of the work. The corresponding author and guarantor accepts full responsibility of the work and/or conduct of the study, had access to the data and controlled the decision to publish. DTE attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Corresponding author
Correspondence to Dean T. Eurich .
Ethics declarations
Ethics approval and consent to participate, informed consent.
The data that support the findings of this study are available from the College of Physicians & Surgeons of Alberta (CPSA) and the Alberta SPOR SUPPORT Unit ( https://absporu.ca ). We had full permission to use this data, however, but restrictions apply to the public availability of these data, which are under data access agreements for the current study. Patients and the public were not involved in the design, conduct and reporting of this research project as it was not applicable to this project.
Consent for publication
Not applicable.
Competing interests
JRBD was a former member of the board of directors for Aurora Cannabis Inc., which is a for-profit, company licensed for the cultivation and sale of medical cannabis. JRBD currently has no financial interest in Aurora Cannabis Inc. In the past, JGH has worked as a paid advisor and speaker for Canadian Cannabis Clinics- however, currently has no clinical or financial ties. DTE and JRBD held a Mitacs Grant with Aurora as a partner. Mitacs is a national, not-for-profit organization that works with universities, private companies, and both federal and provincial governments, to build partnerships and administer research funding that supports industrial and social innovation in Canada. DTE does not have any past or present financial interest in the companies involved. KJBM, SWK, and LR are members of the Alberta Real World Evidence Consortium, an academic entity that conducts research including investigator-initiated industry-funded studies. All other authors have no conflicts of interest to declare. Moreover, the research funders and companies listed were not involved in any aspect of the design or write-up of the study and all analysis was performed independent from the funders and companies.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Dubois, C., Fernandes, H., Lin, M. et al. Benzodiazepine use in medical cannabis authorization adult patients from 2013 to 2021: Alberta, Canada. BMC Public Health 24 , 859 (2024). https://doi.org/10.1186/s12889-024-18356-6
Download citation
Received : 27 September 2023
Accepted : 14 March 2024
Published : 20 March 2024
DOI : https://doi.org/10.1186/s12889-024-18356-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Medical cannabis
- Benzodiazepine
- Diazepam equivalence
- Epidemiology
- Cohort study
BMC Public Health
ISSN: 1471-2458
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Premium Content

- MIND, BODY, WONDER
Do you smoke weed recreationally? Here's what experts want you to know.
Today’s cannabis strains are not your grandma’s weed—and they may be impacting your mental health, heart health, and more.
Some 23 states and the District of Columbia have legalized recreational cannabis in recent years, and others, including Florida, will vote to do so in November. This changing landscape has led to a dramatic rise in consumption, with some 62 million Americans using cannabis in 2023. But legalization of cannabis doesn’t mean that regular consumption is completely safe.
A growing body of evidence has documented an array of health concerns beyond just dry mouth and fatigue and includes both mental and physical illnesses. One recent study even links cannabis consumption to heart disease .
“People think about Bob Marley when they think about cannabis. They think it’s natural, it’s Mother Nature, that it’s not going to do any harm,” says Marco Solmi, a psychiatrist at the University of Ottawa. Yet his review of the substance published in the BMJ found numerous potential problems .
Cannabis isn’t dangerous in the same way opioids are, says Deborah Hasin, an epidemiologist at Columbia University who has researched cannabis use and abuse. “People don’t die from cannabis overdose,” she says. “But it can have a lot of other consequences to both physical and psychological health.”
Stronger strains abound
Some of the problems can be attributed to the stronger strains now available . As Maria Rahmandar, medical director of the substance use and prevention program at Chicago’s Lurie Children’s Hospital, put it at a recent discussion of cannabis at the National Academies of Sciences, Engineering, and Medicine, today’s products are “not your grandmother’s weed.”
“These products are much more potent and come in so many different formulations, that it’s very different from those in the sixties and even the nineties,” Rahmandar says.
The way people consume cannabis today increases the amount of the active ingredient, tetrahydrocannabinol (THC), they ingest. Vaping and edibles generally deliver higher quantities than rolling and smoking joints does, Rahmandar says.
Psychological distress a significant problem
One of the lesser-known but troublesome risks of regular cannabis use is substance-abuse psychosis, where a person has delusions or paranoia, hears voices, and otherwise temporarily loses touch with reality. The psychosis generally resolves within a few days, but in some cases requires hospitalization.
This condition can occur with any psychologically altering substances, but the risk from cannabis is higher even than from cocaine, Solmi says.
“You’re more likely to develop substance-abuse psychosis if you use cannabis daily, but I cannot tell you there’s a safe amount that would prevent this,” he says. Young adults and males are the most prone.
Especially worrisome, up to a third of people who experience substance-abuse psychosis go on to develop the more permanent condition of schizophrenia, Solmi says.
( Schizophrenia in women is widely misunderstood—and misdiagnosed )
Observational studies also connect other mental-health conditions to frequent cannabis use. Solmi’s review found that depression increases, as does violence among dating couples. And since cannabis causes cognitive impairment—as well as visual impairment—car accidents have risen among users who drive while under the influence.
Experts especially worry about the mental health impacts for teenagers. Some 17 percent of tenth graders report using cannabis, even though no state has legalized the drug for anyone under 21.
Adolescents are 37 percent more likely to develop depression by young adulthood if they regularly use cannabis compared to non-users. Rates of suicide are also higher.
You May Also Like

Do you really need 10,000 steps a day? Here’s what the science says.

There’s a better way to wake up. Here’s what experts advise.

Melanoma is overdiagnosed at ‘alarming’ rates. Here’s what to know.
“Teenage brains are going through a time of maturity and pruning, so when substances are put in there, they have more of an influence than they do on adult brains,” Rahmandar says.
Cannabis harms the heart
Regular use of cannabis can also lead to significant physical problems.
People who use the drug regularly have a higher risk for heart attack, stroke, and other heart disease , according to a large population-based study published in the Journal of the American Heart Association in February. Heart attack rates rose 25 percent while stroke increased 42 percent in this group, the researchers found.
This likely occurs because THC affects blood flow in the arteries and because receptors for cannabinoids exist throughout the cardiovascular system, the authors state. People who smoke their weed also boost their heart disease risk from the particulate matter they inhale alongside the THC.
Other studies have linked cannabis with improving nausea and vomiting after chemotherapy, but the BMJ review found regular users can actually suffer from an extended vomiting condition known as hyperemesis. “This is rare, but it’s increasing as more people use the drug,” Hasin says.
Pregnant women who use cannabis regularly are more likely to have preterm births and dangerously small babies. More research is needed to determine whether this results from the drug itself or from other lifestyle factors among those who choose to use cannabis while they are pregnant, Solmi says.
Cannabis addiction is a concern
Many people perceive cannabis to be safer than alcohol, but one in five cannabis users develop an addiction to the drug. Symptoms of cannabis use disorder are like those for other substances.
“If people experience cravings, feel they need more and more to get the same effects, they’ve had unsuccessful attempts to quit or cut down,” or have any of several other symptoms “that’s a warning,” Hasin says.
As with alcohol, cannabis addiction can lead to personal, financial, legal, and health problems .
Certain groups are at particularly heightened risk for this addiction. Rates in veterans have increased substantially since 2005, Hasin found in her research. She attributes this to a combination of increased potency and greater acceptance of the drug from its legal status, as well as the likely use of cannabis to self-medicate chronic pain and psychiatric disorders. “The VA has done a good job of reducing unnecessary prescribing of opioids in veterans, so some of them might be turning to cannabis,” she says.
( Is pain relief from cannabis all in your head? )
Young people are also at risk for developing this disorder. Youth who begin using the drug at earlier ages or who have a family history of addiction especially heighten their odds for trouble .
“People younger than 25 should avoid cannabis altogether,” Solmi says. “They have no idea how they will react to cannabis. You’re gambling with your brain and your health.”
For everyone else, moderation is key.
“This isn’t a benign substance that has no risk,” Rahmandar says. “Most users will be fine, but we can’t predict who will develop problems.”
Related Topics
- MENTAL HEALTH

Multiple COVID infections can lead to chronic health issues. Here’s what to know.

No food or medicine can do what olive oil can do. Here's why.

What is white lung syndrome? Here's what to know about pneumonia

Ozempic is a serious drug with serious risks. Here’s what to know.

Ozempic was tested on monkeys IUCN listed as endangered. Here’s what we know
- Paid Content
- Environment
History & Culture
- History Magazine
- Women of Impact
- History & Culture
- Mind, Body, Wonder
- Destination Guide
- Adventures Everywhere
- Terms of Use
- Privacy Policy
- Your US State Privacy Rights
- Children's Online Privacy Policy
- Interest-Based Ads
- About Nielsen Measurement
- Do Not Sell or Share My Personal Information
- Nat Geo Home
- Attend a Live Event
- Book a Trip
- Inspire Your Kids
- Shop Nat Geo
- Visit the D.C. Museum
- Learn About Our Impact
- Support Our Mission
- Advertise With Us
- Customer Service
- Renew Subscription
- Manage Your Subscription
- Work at Nat Geo
- Sign Up for Our Newsletters
- Contribute to Protect the Planet
Copyright © 1996-2015 National Geographic Society Copyright © 2015-2024 National Geographic Partners, LLC. All rights reserved

Switzerland Launches Cannabis Study With 7.5k Participants & More Pot Updates In Europe
O ver the last several years, Europe has become more open-minded about medical and recreational marijuana. Here are some of the latest cannabis developments from Switzerland, Germany and the Czech Republic.
Switzerland: The Country's Largest Cannabis Study To Date Launches In Zurich
The Canton of Zurich is launching Switzerland's largest marijuana study to date , which is being financed by donations. The research budget is close to $1.69 million, reported SwissInfo.
The University of Zurich and the Federal Technology Institute ETH Zurich are taking part in the research on cannabis use that will involve 7,500 participants. The study is expected to run for five years, during which time marijuana will be allowed for recreational use.
The goal is to examine cannabis regulations by Paul-Lukas Good , president of the association Swiss Cannabis Research, which is responsible for the project.
Trending: Former Trump Aide Says If Letitia James Seizes Ex-President's Properties It Would Be 'Very Hard On His Eg
Must Read: Trump Vs. Biden Gets More Exciting As Both Secure Key Wins In Ohio, Illinois, Kansas, Florida, And Arizona Primaries
Andreas Beerli , head of research from the KOF Swiss Economic Institute at ETH Zurich, added that the research will concentrate on social and economic consequences for the test subjects, for example, to review whether legal marijuana can have a positive effect on health and education, or if it has negative consequences such as an increase in consumption.
Germany: Health Minister Fights Against Possible Cannabis Legalization Delays
Germany’s Health Minister Karl Lauterbach is working hard to prevent possible delays of cannabis legalization law, reported Euractiv.
At the beginning of March, following Germany's recent partial cannabis legalization , Justice Ministers from various states joined together to push for postponing its enactment from April 1 to October.
On Monday, Lauterbach said he would be lobbying all week to prevent the bill from being referred to the mediation committee in the Bundesrat this Friday. "Then we would lose the unique opportunity to reform the failed cannabis policy here. In my view, that would be a triumph for the black market," Lauterbach said.
The Czech Republic: Government To Weigh In On Two Cannabis Bills
The Czech Republic's anti-drug coordinator, Jindřich Vobořil prepared two separate cannabis bills – one that would fully regulate the commercial market and one without, reported Business of Cannabis. This measure will be submitted to the government in the upcoming weeks.
Photo: Courtesy of Kittyfly via Shutterstock
© 2024 Benzinga.com. Benzinga does not provide investment advice. All rights reserved.
This article Switzerland Launches Cannabis Study With 7.5k Participants & More Pot Updates In Europe originally appeared on Benzinga.com .

- News U.S. News Life Politics Business Science/Health Good News World Tech Entertainment Sports
- Shows Schedule Show List
- Documentaries In Real Life Next Level Bellingcat All Docs
- Investigations
- About Team Viewer Spotlight Hotline Support
FDA recommends reclassifying marijuana; says it has medicinal purpose

As a Schedule I drug, marijuana is currently in the same category as some of the hardest drugs, like heroin and methamphetamine.
The U.S. Food and Drug Administration released a report saying that marijuana does have a legitimate use for medical purposes and recommended the Drug Enforcement Agency change its classification from Schedule I to Schedule III.
“The definition of a Schedule I drug says it has no health benefits to it, and, so, obviously, there’s been plenty of research that has documented the multitudes of ways that cannabis can be helpful,” said David Berger, MD, with Wholistic ReLeaf.
Marijuana use could make you a nicer person, research finds
Researchers said their positive findings are important since weed has generally been associated with negative mental health impacts.
Though not all in the medical community agree, many people swear by the medicinal effects of marijuana to help treat symptoms of cancer, anxiety, post traumatic stress disorder and epilepsy.
“It’s no longer appropriate to say that there’s no medical benefit when there are hundreds if not thousands of medical studies that show the opposite,” explained Dr. Berger.
As a Schedule I drug, marijuana is in the same category as some of the hardest drugs like heroin, LSD and methamphetamine, which means it’s classified as being more dangerous than even fentanyl — a Schedule II narcotic.
Are cannabis edibles safer than smoking? Here's what some experts say
Inhaling cannabis smoke or ingesting edible cannabis products can have different effects on the body in terms of safety and intensity.
“What happens after this is the federal government has more decisions to make as to what they’re going to do next,” said Dr. Berger.
The Department of Health and Human Services formally recommended that the DEA classify marijuana as Schedule III in August of last year after the Biden administration asked it to review how the drug is classified under federal law.
Earlier this year, Senate Democrats urged President Biden to deschedule the drug entirely, meaning you would not need a doctor’s authorization to use marijuana.
This story was originally published by Anthony Hill at Scripps News Tampa.
Latest in U.S.
Opposing bills on lgbtq+ representation in schools advance in 2 states.
Two very different opinions on the inclusion of LGBTQ+ subject matter in the classroom are playing out in state houses, marking a divide in the U.S.
State Farm discontinues 72,000 home policies in California
The announcement this week comes nine months after the Illinois-based company said it would not issue new home policies in the state.
Runway near misses and addictive THC | Scripps News Investigates
Scripps News explores the troubling trend in near-miss encounters on U.S. runways and explains the dangers of potent new THC products.
Top Stories
Doctors are less likely to respond to black patients, study finds.
Researchers found patients belonging to minority groups were less likely to hear back from doctors on their patient portals.
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on the Health Effects of Marijuana: An Evidence Review and Research Agenda. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington (DC): National Academies Press (US); 2017 Jan 12.

The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research.
- Hardcopy Version at National Academies Press
15 Challenges and Barriers in Conducting Cannabis Research
Several states have legalized cannabis for medical or recreational use since the release of the 1999 Institute of Medicine (IOM) 1 report Marijuana and Medicine: Assessing the Science Base ( IOM, 1999 ). As of October 2016, 25 states and the District of Columbia had legalized the medical use of cannabis, while 4 states and the District of Columbia had also legalized recreational cannabis use ( NCSL, 2016 ; NORML, 2016a ). 2 In November 2016, voters in California, Maine, Massachusetts, and Nevada approved ballot initiatives to legalize recreational cannabis, while voters in Arkansas, Florida, Montana, and North Dakota approved ballot initiatives to permit or expand the use of cannabis for medical purposes ( NORML, 2016b ).
Policy changes are associated with marked changes in patterns of cannabis use. In recent years, the number of U.S. adolescents and adults ages 12 and older who reported using cannabis increased by 35.0 percent and 20.0 percent for use in the past month and in the past year, respectively ( Azofeifa et al., 2016 ). Revenue from the sale and taxation of cannabis can serve as a proxy measure for cannabis use and suggests that the scope of cannabis use in the United States is considerable. For example, the total estimated value of legal cannabis sales in the United States was $5.7 billion in 2015 and $7.1 billion in 2016 ( Arcview Market Research and New Frontier Data, 2016 ). At the state level, the Colorado Department of Revenue reported that sales and excise taxes on recreational and medical cannabis sales totaled $88,239,323 in fiscal year 2015 ( CDOR, 2016a, p. 29 ), 3 and in Washington, state and local sales taxes and state business and occupation taxes on recreational and medical cannabis totaled $53,410,661 in fiscal year 2016 ( WDOR, 2016a , b ). 4
Despite these changes in state policy and the increasing prevalence of cannabis use and its implications for population health, the federal government has not legalized cannabis and continues to enforce restrictive policies and regulations on research into the health harms or benefits of cannabis products that are available to consumers in a majority of states. As a result, research on the health effects of cannabis and cannabinoids has been limited in the United States, leaving patients, health care professionals, and policy makers without the evidence they need to make sound decisions regarding the use of cannabis and cannabinoids. This lack of evidence-based information on the health effects of cannabis and cannabinoids poses a public health risk.
In order to promote research on cannabis and cannabinoids, the barriers to such research must first be identified and addressed. The committee identified several barriers to conducting basic, clinical, and population health research on cannabis and cannabinoids, including regulations and policies that restrict access to the cannabis products that are used by an increasing number of consumers and patients in state-regulated markets, funding limitations, and numerous methodological challenges. The following sections discuss these barriers in detail.
- REGULATORY AND SUPPLY BARRIERS
Regulatory Barriers
Investigators seeking to conduct research on cannabis or cannabinoids must navigate a series of review processes that may involve the National Institute on Drug Abuse (NIDA), the U.S. Food and Drug Administration (FDA), the U.S. Drug Enforcement Administration (DEA), institutional review boards, offices or departments in state government, state boards of medical examiners, the researcher's home institution, and potential funders. A brief overview of some of these review processes is discussed.
Researchers conducting clinical research on biological products such as cannabis must submit an investigational new drug (IND) application to the FDA. As a next step, the investigator may contact NIDA, an important source of research-grade cannabis, to obtain an administrative letter of authorization (LOA). An LOA describes the manufacturer's facilities, as well as the availability and pertinent characteristics of the desired cannabis product (e.g., strains, quality, strength, pharmacology, toxicology). To safeguard against the acquisition of cannabis or cannabinoids for non-research purposes, investigators must also apply for a DEA registration and site licensure before conducting studies involving cannabis or any of its cannabinoid constituents, irrespective of their pharmacologic activity. 5 The investigator must submit the IND and LOA to the FDA and the DEA for review ( FDA, 2015 ).
After submitting an IND application, researchers must wait at least 30 days before initiating research, during which period the FDA reviews the application to ensure that research participants will not be exposed to unreasonable risk ( FDA, 2016a ). If the FDA determines that the proposed research would expose study participants to unreasonable risk or that the IND application is in some other way deficient, a clinical hold postponing the research may be imposed. This hold is not lifted until and unless the sponsoring researchers have resolved the deficiencies ( FDA, 2016b ).
It is important to note that the Controlled Substances Act of 1970 classified cannabis as a Schedule I substance, the highest level of drug restriction. 6 As defined by the Act, Schedule I substances are those that (1) have a high potential for abuse; (2) have no currently accepted medical use in treatment in the United States; and (3) have a lack of accepted safety for their use under medical supervision. 7 Other substances classified in Schedule I include heroin, LSD, mescaline, hallucinogenic amphetamine derivatives, fentanyl derivatives (synthetic opioid analgesics), and gammahydroxybutyrate (GHB). 8 By contrast, Schedule II substances—though they also have a high potential for abuse and may lead to severe psychological or physical dependence—are defined as having a currently accepted medical use and can be prescribed with a controlled substance prescription ( DEA, 2006 ). 9
In some states, researchers conducting clinical research on cannabis or cannabinoid products must also apply for and receive a controlled substance certificate from a state board of medical examiners or a controlled substance registration from a department of the state government in order to conduct clinical trials or any other activity involving Schedule I substances ( Alabama Board of Medical Examiners, 2013 ; MDHSS, n.d .). Some state governments require additional approvals. For example, California requires that all trials involving Schedule I or II controlled substances be registered with and approved by the Research Advisory Panel of California ( CADOJ/OAG, 2016 ). When the necessary approvals are secured, only then can the investigator apply for a DEA registration and site licensure to conduct research on a Schedule I controlled substance (see Box 15-1 for examples of research barriers).
Illustrative Examples of the Current Research Barriers to Colorado Researchers.
Researchers conducting trials of Schedule I substances must additionally submit a research protocol to the DEA that includes details regarding the security provisions for storing and dispensing the substance. 10 Previously, nonfederally funded studies on cannabis were also required to undergo an additional review process conducted by the Public Health Service. This review process was determined to unnecessarily duplicate the FDA's IND application process in several ways and, as of June 2015, is no longer required. 11
To ensure that controlled substances obtained for research purposes will be stored and accessed in accordance with DEA security requirements, local DEA officials may perform a preregistration inspection of the facility where the proposed research will take place ( University of Colorado, 2016 ). DEA security requirements include storing cannabis in a safe, a steel cabinet, or a vault, and limiting access to the storage facility to “an absolute minimum number of specifically authorized employees. 12 The extent of the security measures required by DEA varies with the amount of cannabis being stored, 13 and among local DEA jurisdictions ( Woodworth, 2011 ). Funders must bear the costs of meeting the necessary security requirements.
Additionally, as with any human clinical trial, approval from an institutional review board must be sought. 14 Obtaining this approval confirms that an appropriate plan to protect the rights and welfare of human research subjects has been outlined in the proposed research efforts. If a study is being conducted in a clinical research center, a separate review may be required by this entity's medical or research advisory committee.
In summary, basic and clinical researchers seeking to obtain cannabis or cannabinoids from NIDA for research purposes—including efforts to determine the value of cannabis or cannabinoids for treating a medical condition or achieving a therapeutic end need—must obtain a number of approvals from a range of federal, state, or local agencies, institutions, or organizations. This process can be a daunting experience for researchers. The substantial layers of bureaucracy that emerge from cannabis's Schedule I categorization is reported to have discouraged a number of cannabis researchers from applying for grant funding or pursuing additional research efforts ( Nutt et al., 2013 ). Given the many gaps in the research of the health effects of cannabis and cannabinoids, there is a need to address these regulatory barriers so that researchers will be better able to address key public health questions about the therapeutic and adverse effects of cannabis and cannabinoid use.
CONCLUSION 15-1 There are specific regulatory barriers, including the classification of cannabis as a Schedule I substance, that impede the advancement of cannabis and cannabinoid research. 15
Barriers to Cannabis Supply
In the United States, cannabis for research purposes is available only through the NIDA Drug Supply Program ( NIDA, 2016a ). The mission of NIDA is to “advance science on the causes and consequences of drug use and addiction and to apply that knowledge to improve individual and public health,” rather than to pursue or support research into the potential therapeutic uses of cannabis or any other drugs ( NIDA, 2016b ). As a result of this emphasis, less than one-fifth of cannabinoid research funded by NIDA in fiscal year 2015 concerns the therapeutic properties of cannabinoids ( NIDA, 2016c ). 16 Because NIDA funded the majority of all the National Institutes of Health (NIH)-sponsored cannabinoid research in fiscal year 2015 ( NIDA, 2016c ), 17 its focus on the consequences of drug use and addiction constitutes an impediment to research on the potential beneficial health effects of cannabis and cannabinoids.
All of the cannabis that NIDA provides to investigators is sourced from the University of Mississippi, which is currently the sole cultivator of the plant material and has been since 1968 ( NIDA, 1998 , 2016a ). 18 In the past, the varieties of cannabis that were available to investigators through NIDA were limited in scope and were not of comparable potency to what patients could obtain at their dispensaries ( Stith and Vigil, 2016 ). Because of restrictions on production and vicissitudes in supply and demand, federally produced cannabis may have been harvested years earlier, is stored in a freezer (a process that may affect the quality of the product) ( Taschwer and Schmid, 2015 ; Thomas and Pollard, 2016 ), and often has a lower potency than cannabis sold in state-regulated markets ( Reardon, 2015 ; Stith and Vigil, 2016 ). In addition, many products available in state-regulated markets (e.g., edibles, concentrates, oils, wax, topicals) are not commonly available through federal sources ( NIDA, 2016d ). Since the products available through the federal system do not sufficiently reflect the variety of products used by consumers, research conducted using cannabis provided by NIDA may lack external validity. In July 2016, NIDA posted a formal request for information on the varieties of cannabis and cannabis products of interest to researchers ( NIDA, 2016e ). Reflecting the perceived shortcomings of cannabis and cannabis products currently provided by NIDA, a summary of the comments received in response to this request states that “the most consistent recommendation was to provide marijuana strains and products that reflect the diversity of products available in state dispensaries” ( NIDA, 2016e ).
Naturally, it is difficult for a single facility at the University of Mississippi to replicate the array and potency of products available in dispensaries across the country. It is worth noting, however, that NIDA has been increasingly responsive to the needs of clinical investigators. For example, NIDA has contracted with the University of Mississippi to produce cannabis strains with varying concentrations of Δ 9 -tetrahydrocannabinol (THC) and cannabidiol (CBD) ( NIDA, 2016d ), and NIDA has previously authorized development of cannabis extracts, tinctures, and other dosage formulations for research purposes ( Thomas and Pollard, 2016 ). As mentioned above, NIDA has sought public comment on the needs of cannabis researchers in order to inform efforts to “expand access to diverse marijuana strains and products for research purposes” ( NIDA, 2016e ). In addition, cannabis is made available to research investigators funded by NIH at no cost. 19 Finally, the DEA has adopted a new policy that increases the number of entities that may be registered under the Controlled Substances Act (CSA) to grow (manufacture) marijuana to supply legitimate researchers in the United States. 20 Under this new policy, the DEA will facilitate cannabis research by increasing the number of private entities allowed to cultivate and distribute research-grade cannabis. As of December 2016, the University of Mississippi remains the sole cultivator of cannabis provided to researchers by NIDA ( NIDA, 2016a ).
Although new plans are being made to provide a wider array of more clinically relevant cannabis products for research, at present this issue is still a significant barrier for conducting comprehensive research on the health effects of cannabis use. How the proposed changes will affect cannabis research in the future remains to be seen.
CONCLUSION 15-2 It is often difficult for researchers to gain access to the quantity, quality, and type of cannabis product necessary to address specific research questions on the health effects of cannabis use.
Funding Limitations
Funding for research is another key barrier; without adequate financial support, cannabis research will be unable to inform health care or public health practice or to keep pace with changes in cannabis policy and patterns of cannabis use. NIH is responsible for funding research across a number of health domains. In 2015, NIH spending on all cannabinoid research totaled $111,275,219 ( NIDA, 2016c ). NIDA, a member institute of NIH, has as its mission to study factors related to substance abuse and dependence and conducts research on the negative health effects and behavioral consequences associated with the abuse of cannabis and other drugs ( NIDA, 2016b ). Because cannabis was historically perceived to have only negative effects, the majority of cannabis research has been conducted under the auspices of NIDA.
In fiscal year 2015, studies supported by NIDA accounted for 59.3 percent ($66,078,314) of all NIH spending on cannabinoid research; however, only 16.5 percent ($10,923,472) of NIDA's spending on cannabinoid research supported studies investigating therapeutic properties of cannabinoids ( NIDA, 2016c ). 21 , 22 As demonstrated in Chapter 4 of this report, a growing body of evidence suggests that cannabis and cannabinoids also have therapeutic health effects. In light of these findings, a comprehensive research agenda that investigates both the potential adverse and the potential therapeutic health effects of cannabis use is needed.
However, it may be unrealistic to expect NIDA to have the resources or interest to fund this broader research agenda, which could involve investigating the health effects of cannabis use on a diverse range of conditions (e.g., metabolic syndrome, cardiovascular disease, cancer, obesity and sedentary behavior, Alzheimer's disease) that are targeted by other institutes and centers of NIH. While it is not clear how these studies might be funded, almost assuredly the changing norms and the changing legal status of cannabis will have an impact on conditions that are targeted by institutes other than NIDA, and it will become increasingly important to have a funding mechanism to better understand the comprehensive health effects of cannabis so that consumers and policy makers can respond to changing trends accordingly.
CONCLUSION 15-3 A diverse network of funders is needed to support cannabis and cannabinoid research that explores the harmful and beneficial health effects of cannabis use.
- METHODOLOGICAL CHALLENGES
Drug Delivery Challenges
Another challenge in investigating the potential health effects of cannabis and cannabinoids is the identification of a method of administering the drug that is accepted by study participants, that can be performed at most research sites, and that ensures standardized dosing. Smoking as a route of administration is particularly challenging, as some study participants may not view it as an acceptable method of drug administration, and academic medical centers or other locations where cannabis or cannabinoid research takes place may lack facilities where study participants can smoke under controlled conditions. Furthermore, variations among individuals in terms of their cannabis smoking techniques make it difficult to ensure that study participants reliably receive the targeted dose of the drug. Devices for providing a metered dose of cannabis via inhalation exist ( Eisenberg et al., 2014 ), but the FDA has not approved such devices for use. Standardized smoking techniques have also been developed ( Foltin et al., 1988 ) but can be difficult to perform correctly. These difficulties are due, in part, to differences among individuals in their tolerance of the potential psychoactive effects of the drug ( D'Souza et al., 2008 ; Ramaekers et al., 2009 ), which may prevent the receipt of equal doses by all study participants.
Researchers have also explored vaporization as a method for administering cannabis ( Abrams et al., 2007 ). Cannabinoids vaporize at lower temperatures than the temperature at which pyrolytic toxic compounds are created through combustion; as a result, levels of some carcinogenic compounds are lower in cannabis vapor than in cannabis smoke ( Eisenberg et al., 2014 ). However, there is a paucity of research on the effectiveness of these devices as a mode of drug administration. For example, data on the plasma concentrations of cannabinoids achieved through use of vaporizers exists, but they are limited ( Abrams et al., 2007 ; Zuurman et al., 2008 ). In addition, even less is known about the long-term pulmonary effects of inhaling a vaporized liquid than about the effect of inhaling plant material. As vaporizing devices proliferate and evolve, researchers may benefit from advances in their portability and usability, but they will also have to account for clinically relevant differences in the functioning and the effectiveness of an increasingly wide range of models.
To circumvent the practical and methodological challenges involved in administration of cannabis through smoking or vaporization, investigators may choose to study the health effects of orally administered dronabinol or nabilone, which offer a more controlled method of drug delivery. However, the effects generated by these isolated cannabinoids might, at least in part, be different from those produced by the use of the whole cannabis plant, which also contains CBD and other cannabinoids, as well as terpenoids and flavonoids. As a result, extrapolating from the observed health effects associated with use of an isolated cannabinoid such as dronabinol or nabilone in order to predict the health effects associated with the use of cannabis may lead to erroneous conclusions.
The Placebo Issue
The gold standard of drug development is the prospective, randomized, double-blind, placebo-controlled clinical trial. Placebo cannabis produced by solvent extraction is available from NIDA and has a potency of 0.002 percent THC by weight and 0.001 percent CBD by weight ( NIDA, 2016d ). 23 The extraction process seems to retain the terpenoids and flavonoids so that the combusted placebo material smells similar to the true cannabis, thus helping to preserve the blinding to some extent. However, the psychoactive and vasoactive effects of cannabis pose a considerable challenge for effective blinding, since study participants who feel such effects will surmise that they are receiving cannabis or cannabinoids, and not a placebo.
Strategies to promote the effectiveness of blinding exist. For example, if the cannabis being studied has a very low THC content, study participants—especially those who, through regular use of more potent cannabis strains, are inured to the psychoactive effects of cannabis with low THC content—may not notice the psychoactive effects of the cannabis and therefore be unable to reliably determine whether they are using cannabis or a placebo. There is also a possibility that cannabis products with a lower ratio of the concentration of THC to the concentration of CBD may have less psychoactivity than products with a comparatively higher ratio of the concentration of THC to the concentration of CBD ( Hindocha et al., 2015 ; Jacobs et al., 2016 ). Using these strains with diminished psychoactive effects could promote more effective blinding. Researchers may also try treating both study arms in a placebo-controlled cannabis trial with a mildly psychoactive or sedating drug, the effects of which may help to ensure that study participants are unable to determine whether they are receiving a placebo or cannabis. However, by introducing another active agent, the investigators risk obfuscating the results of their study.
A potential method for assessing the effectiveness of blinding in a cannabis trial is to ask study participants to guess whether they are receiving true cannabis or a placebo. If most or all of the participants correctly guess their assignment, it can be inferred that the blinding was ineffective. Whether or not such methods are employed, investigators risk undermining their study results. On the one hand, conducting the test carries the risk of discovering that attempts at blinding were ineffective, thereby rendering the study results invalid. On the other hand, not conducting the test may lead journal reviewers aware of the challenges of blinding in cannabis trials to assume that blinding was ineffective and to discount the study results accordingly. Thus, research to address the challenge of achieving reliably effective blinding in a cannabis trial is of marked importance.
Exposure Assessment
In order to arrive at valid and meaningful results, population studies on the health effects of cannabis require as detailed an ascertainment of exposure to cannabis as possible. However, obtaining such a detailed exposure history can be difficult. This is especially true for recreational cannabis use due to the lack of a standardized dose and the existence of diverse routes of administration, including multiple modes of inhalation ( Schauer et al., 2016 ). In addition, known pharmacological biomarkers of cannabis use may be unreliable in some circumstances, while population studies to identify novel pharmacological biomarkers of cannabis exposure are limited ( Hartman et al., 2016 ; Schwope et al., 2011 ). Furthermore, the wide variety of different cannabis strains developed through a long and ongoing process of cultivation and the associated variation in the concentration of active substances in cannabis further complicate the characterization of cannabis exposure ( ElSohly and Gul, 2014 ; Elsohly et al., 2016 ; Mehmedic et al., 2010 ). Finally, recreational cannabis may contain chemical contaminants or adulterants ( Busse et al., 2008 ). Cannabis users may be unaware of the presence of these chemicals, making it unlikely that such chemicals would be identified through toxicological evaluation unless the user became involved in a forensic investigation.
Most observational studies, particularly case-control and cohort studies, depend on self-report in order to assess cannabis exposure. These reports may be incomplete, inaccurate, or imprecise due to failure on the part of investigators to ask cannabis users detailed questions about their cannabis exposure history, including the source of their cannabis exposure (e.g., smoking, edibles, vaping), or because users themselves may have limited knowledge of some aspects of their exposure or may be resistant to reporting some information. Personal recall of substance use may also be affected by other factors. For example, memory problems have been identified as a cause of inaccuracies in reporting drug use ( Johnson and Fendrich, 2005 ; Pedersen, 1990 ). In other cases, study participants may not report illicit substance use in an attempt to conform to perceived social norms ( Johnson and Fendrich, 2005 ). Similarly, individuals with substance dependency syndromes may have psychiatric comorbidity that affects the accuracy of reporting.
Finally, important information often missing from cannabis exposure histories is the extent of other substance use. As noted in Chapter 14 , there is limited evidence that cannabis use is associated with the use of other licit or illicit substances. Despite this association and the confounding effect of polysubstance use on evaluations of the health effects of cannabis use, surveys used to characterize cannabis exposure histories do not always assess for the presence of other substance use. Since secondhand exposure to cannabis smoke can have minor health effects, there may also be value in assessing for such exposure as part of larger assessments of cannabis exposure ( Herrmann et al., 2015 ).
Cannabis-Related Study Designs
In researching the health outcomes of cannabis use, the committee identified a number of studies, particularly cohort studies, of general health outcomes such as all-cause mortality or important chronic illnesses such as cancers or cardiovascular diseases. For both cohort and case-control studies, a better assessment of cannabis use would offer more valuable information, such as years of use and age at first use. Particularly for cohort studies, this would offer better ascertainment of the duration and net burden of use as well as more insight into period and age effects. As discussed in the proceeding health outcomes chapters of the report, in many of the existing cohort studies cannabis use was often queried only at baseline, and thus there was little information on interval use over time or on the variation or cessation in that use. There was also very limited information on interval health events as the cohorts progressed, impeding a summarization of long-term use and the consequent health effects. Attention to these issues will likely improve the precision of study findings.
CONCLUSION 15-4 To develop conclusive evidence for the effects of cannabis use on short- and long-term health outcomes, improvements and standardization in research methodology (including those used in controlled trials and observational studies) are needed.
The methodological challenges and the regulatory, financial, and access barriers described above markedly affect the ability to conduct comprehensive basic, clinical, and public health research on the health effects of cannabis use, with further consequences for the many potential beneficiaries of such research. In the absence of an appropriately funded and supported cannabis research agenda, patients may be unaware of viable treatment options, providers may be unable to prescribe effective treatments, policy makers may be hindered from developing evidence-based policies, and health care organizations and insurance providers lack a basis on which to revise their care and coverage policies. In short, such barriers represent a public health problem. See Box 15-2 for a summary of the chapter conclusions.
Summary of Chapter Conclusions .
- Abrams DI, Vizoso HP, Shade SB, Jay C, Kelly ME, Benowitz NL. Vaporization as a smokeless cannabis delivery system: A pilot study. Clinical Pharmacology & Therapeutics. 2007; 82 (5):572–578. [ PubMed : 17429350 ]
- Alabama Board of Medical Examiners. Alabama Board of Medical Examiners Administrative Code. 2013. [December 29, 2016]. Chapter 540-X-4: Controlled Substances Certificate. http://www .alabamaadministrativecode .state .al.us/docs/mexam/540-X-4.pdf .
- Arcview Market Research and New Frontier Data. The State of Legal Marijuana Markets, 4th Edition: Executive Summary. San Francisco, CA: The Arcview Group; 2016. [December 8, 2016]. http://mjardin .com/wp-content /uploads/2016 /05/Executive-Summary-State-of-Legal-Marijuana-Markets-4th-Edition-1.pdf .
- Azofeifa A, Mattson ME, Schauer G, McAfee T, Grant A, Lyerla R. National estimates of marijuana use and related indicators—National Survey on Drug Use and Health, United States, 2002-2014. The Morbidity and Mortality Weekly Report Surveillance Summaries. 2016; 65 (11):1–28. [ PubMed : 27584586 ]
- Busse F, Omidi L, Timper K, Leichtle A, Windgassen M, Kluge E, Stumvoll M. Lead poisoning due to adulterated marijuana. New England Journal of Medicine. 2008; 358 (15):1641–1642. [ PubMed : 18403778 ]
- CADOJ/OAG (State of California Department of Justice/Office of the Attorney General). Research Advisory Panel: Guidelines. 2016. [November 3, 2016]. https://oag .ca.gov/research/guide .
- CDOR (Colorado Department of Revenue). Annual Report 2015. Denver: Colorado Department of Revenue; 2016a. [December 8, 2016]. https://www .colorado .gov/pacific/sites/default /files/2015%20Annual%20Report_1 .pdf .
- CDOR. MED 2015 Annual Update. Denver, CO: Colorado Department of Revenue; 2016b. [December 7, 2016]. https://www .colorado .gov/pacific/sites/default /files/2015%20Annual %20Update%20FINAL%2009262016_1.pdf .
- DEA (U.S. Drug Enforcement Administration). Practitioner's Manual: An Informational Outline of the Controlled Substances Act. Washington, DC: Drug Enforcement Administration; 2006. [December 28, 2016]. Section V: Valid Prescription Requirements; pp. 18–22. https://www .deadiversion .usdoj.gov/pubs/manuals /pract/pract_manual012508.pdf .
- D'Souza DC, Ranganathan M, Braley G, Gueorguieva R, Zimolo Z, Cooper T, Perry E, Krystal J. Blunted psychotomimetic and amnestic effects of delta-9-tetrahydrocannabinol in frequent users of cannabis. Neuropsychopharmacology. 2008; 33 (10):2505–2516. [ PMC free article : PMC3799954 ] [ PubMed : 18185500 ]
- Eisenberg E, Ogintz M, Almog S. The pharmacokinetics, efficacy, safety, and ease of use of a novel portable metered-dose cannabis inhaler in patients with chronic neuropathic pain: A phase 1a study. Journal of Pain & Palliative Care Pharmacotherapy. 2014; 28 (3):216–225. [ PubMed : 25118789 ]
- ElSohly M, Gul W. Chapter 5: The Chemical Phenotypes (Chemotypes) of Cannabis. In: Pertwee R, editor. Handbook of Cannabis. New York: Oxford University Press; 2014. pp. 89–110.
- ElSohly MA, Mehmedic Z, Foster S, Gon C, Chandra S, Church JC. Changes in cannabis potency over the last 2 decades (1995-2014): Analysis of current data in the United States. Biological Psychiatry. 2016; 79 (7):613–619. [ PMC free article : PMC4987131 ] [ PubMed : 26903403 ]
- FDA (U.S. Food and Drug Administration). Marijuana research with human subjects. 2015. [January 3, 2017]. http://www .fda.gov/NewsEvents /PublicHealthFocus/ucm421173 .htm .
- FDA. Investigational New Drug (IND) Application: Introduction. 2016a. [December 8, 2016]. http://www .fda.gov/drugs /developmentapprovalprocess /howdrugsaredevelopedandapproved /approvalapplications /investigationalnewdrugindapplication/default.htm .
- FDA. IND application procedures: Clinical hold. 2016b. [December 8, 2016]. http://www .fda.gov/Drugs /DevelopmentApprovalProcess /HowDrugsareDevelopedandApproved /ApprovalApplications /InvestigationalNewDrugINDApplication/ucm362971.htm .
- Foltin R, Fischman M, Byrne M. Effects of smoked marijuana on food intake and body weight of humans living in a residential laboratory. Appetite. 1988; 25 :577–582. [ PubMed : 3228283 ]
- Hartman RL, Brown TL, Milavetz G, Spurgin A, Gorelick DA, Gaffney GR, Huestis MA. Effect of blood collection time on measured delta9-tetrahydrocannabinol concentrations: Implications for driving interpretation and drug policy. Clinical Chemistry. 2016; 62 (2):367–377. [ PubMed : 26823611 ]
- Herrmann ES, Cone EJ, Mitchell JM, Bigelow GE, LoDico C, Flegel R, Vandrey R. Non-smoker exposure to secondhand cannabis smoke II: Effect of room ventilation on the physiological, subjective, and behavioral/cognitive effects. Drug and Alcohol Dependence. 2015; 151 :194–202. [ PMC free article : PMC4747424 ] [ PubMed : 25957157 ]
- Hindocha C, Freeman TP, Schafer G, Gardener C, Das RK, Morgan CJ, Curran HV. Acute effects of delta-9-tetrahydrocannabinol, cannabidiol and their combination on facial emotion recognition: A randomised, double-blind, placebo-controlled study in cannabis users. European Neuropsychopharmacology. 2015; 25 (3):325–334. [ PMC free article : PMC4398332 ] [ PubMed : 25534187 ]
- IOM (Institute of Medicine). Marijuana and medicine: Assessing the science base. Washington, DC: National Academy Press; 1999. [ PubMed : 25101425 ]
- Jacobs DS, Kohut SJ, Jiang S, Nikas SP, Makriyannis A, Bergman J. Acute and chronic effects of cannabidiol on delta(9)-tetrahydrocannabinol (delta(9)-THC)induced disruption in stop signal task performance. Experimental and Clinical Psychopharmacology. 2016; 24 (5):320–330. [ PMC free article : PMC5119678 ] [ PubMed : 27690502 ]
- Johnson T, Fendrich M. Modeling sources of self-report bias in a survey of drug use epidemiology. Annals of Epidemiology. 2005; 15 (5):381–389. [ PubMed : 15840552 ]
- Marijuana Business Daily Staff. Marijuana Business Daily. Jun 13, 2016. [December 29, 2016]. Chart of the Week: Sales of Marijuana Concentrates, Edibles Surging in Colorado. http://mjbizdaily .com /chart-of-the-week-sales-of-marijuana-concentrates-edibles-surging-in-colorado .
- Mehmedic Z, Chandra S, Slade D, Denham H, Foster S, Patel AS, Ross SA, Khan IA, ElSohly MA. Potency trends of delta9-THC and other cannabinoids in confiscated cannabis preparations from 1993 to 2008. Journal of Forensic Science. 2010; 55 (5):1209–1217. [ PubMed : 20487147 ]
- MDHSS (Missouri Department of Health and Senior Services). Frequently Asked Questions. n.d. [December 29, 2016]. http://health .mo.gov/safety/bndd/faqs .php .
- NCSL (National Conference of State Legislatures). State Medical Marijuana Laws. 2016. [December 22, 2016]. http://www .ncsl.org/research /health/state-medical-marijuana-laws.aspx .
- NIDA (National Institute on Drug Abuse). Provision of Marijuana and Other Compounds for Scientific Research—Recommendations of the National Institute on Drug Abuse National Advisory Council. 1998. [December 29, 2016]. https://archives .drugabuse .gov/about/organization /nacda/MarijuanaStatement.html .
- NIDA. Information on Marijuana Farm Contract. 2015. [December 29, 2016]. https://www .drugabuse .gov/drugs-abuse/marijuana /nidas-role-in-providing-marijuana-research /informationmarijuana-farm-contract .
- NIDA. NIDA's Role in Providing Marijuana for Research. 2016a. [December 8, 2016]. https://www .drugabuse .gov/drugs-abuse/marijuana /nidas-role-in-providing-marijuana-research .
- NIDA. National Institute on Drug Abuse (NIDA): Mission. 2016b. [December 9, 2016]. https://www .nih.gov/about-nih /what-we-do /nih-almanac/national-institute-drug-abuse-nida .
- NIDA. NIH Research on Marijuana and Cannabinoids. 2016c. [December 29, 2016]. https://www .drugabuse .gov/drugs-abuse/marijuana /nih-research-marijuana-cannabinoids .
- NIDA. Marijuana plant material available from the NIDA drug supply program. 2016d. [November 3, 2016]. https://www .drugabuse .gov/researchers/research-resources /nida-drug-supplyprogram-dsp /marijuana-plant-material-available-nida-drug-supply-program .
- NIDA. Summary of Request for Information (RFI) Regarding Varieties of Marijuana and Marijuana Products for Research. 2016e. [November 3, 2016]. https://www .drugabuse .gov/drugsabuse/marijuana /nidas-role-in-providing-marijuana-research /summary-requestinformation-rfi-regarding-varieties-marijuana-marijuana-products-research .
- NIH (National Institutes of Health). Estimates of Funding for Various Research, Condition, and Disease Categories (RCDC). 2016. [December 29, 2016]. https://report .nih.gov /categorical_spending.aspx#legend1 .
- NORML (National Organization for the Reform of Marijuana Laws). About Marijuana. 2016a. [December 22, 2016]. http://norml .org/marijuana .
- NORML. Election 2016—Marijuana Ballot Results. 2016b. [December 22, 2016]. http://norml .org/election-2016 .
- Nutt DJ, King LA, Nichols DE. Effects of Schedule I drug laws on neuroscience research and treatment innovation. Nature Reviews Neuroscience. 2013; 14 (8):577–585. [ PubMed : 23756634 ]
- Pedersen W. Reliability of drug use responses in a longitudinal study. Scandinavian Journal of Psychology. 1990; 31 (1):28–33. [ PubMed : 2333484 ]
- Ramaekers JG, Kauert G, Theunissen EL, Toennes SW, Moeller MR. Neurocognitive performance during acute thc intoxication in heavy and occasional cannabis users. Journal of Psychopharmacology. 2009; 23 (3):266–277. [ PubMed : 18719045 ]
- Reardon S. Marijuana gears up for production high in U.S. labs. Nature. 2015; 519 (7543):269–270. [ PubMed : 25788072 ]
- Schauer GL, King BA, Bunnell RE, Promoff G, McAfee TA. Toking, vaping, and eating for health or fun: Marijuana use patterns in adults, U.S., 2014. American Journal of Preventative Medicine. 2016; 50 (1):1–8. [ PubMed : 26277652 ]
- Schwope DM, Karschner EL, Gorelick DA, Huestis MA. Identification of recent cannabis use: Whole-blood and plasma free and glucuronidated cannabinoid pharmacokinetics following controlled smoked cannabis administration. Clinical Chemistry. 2011; 57 (10):1406–1414. [ PMC free article : PMC3717336 ] [ PubMed : 21836075 ]
- Stith SS, Vigil JM. Federal barriers to cannabis research. Science. 2016; 352 (6290):1182. [ PubMed : 27257247 ]
- Taschwer M, Schmid MG. Determination of the relative percentage distribution of THCA and D(9)-THC in herbal cannabis seized in Austria—Impact of different storage temperatures on stability. Forensic Science International. 2015; 254 :167–171. [ PubMed : 26247127 ]
- Thomas BF, Pollard GT. Preparation and distribution of cannabis and cannabis-derived dosage formulations for investigational and therapeutic use in the United States. Frontiers in Pharmacology. 2016; 7 :285. [ PMC free article : PMC5006560 ] [ PubMed : 27630566 ]
- University of Colorado. Drug Enforcement Administration (DEA) Controlled Substances. 2016. [December 22, 2016]. http://www .ucdenver.edu /research/EHS/hazmat/Pages/DEA.aspx .
- WDOR (Washington Department of Revenue). Medical Marijuana Taxes. 2016a. [December 8, 2016]. http://dor .wa.gov/Docs /Reports/2014/MMJTax.xlsx .
- WDOR. Recreational Marijuana Taxes. 2016b. [December 8, 2016]. http://dor .wa.gov/Docs /Reports/2014/RMJTax.xlsx .
- Woodworth TW. How will DEA affect your clinical study? Journal of Clinical Research Best Practices. 2011; 7 (12) [December 8, 2016]; https: //firstclinical .com/journal/2011/1112_DEA.pdf .
- Zuurman L, Roy C, Schoemaker RC, Hazekamp A, den Hartigh J, Bender JC, Verpoorte R, Pinquier JL, Cohen AF, van Gerven JM. Effect of intrapulmonary tetrahydrocannabinol administration in humans. Journal of Psychopharmacology. 2008; 22 (7):707–716. [ PubMed : 18515447 ]
As of March 2016, the Health and Medicine Division continues the task of producing consensus studies and convening activities previously undertaken by the Institute of Medicine (IOM).
The count of states where cannabis is legalized for medical use includes Ohio and Pennsylvania, where medical cannabis laws were not operational as of October 2016 ( NCSL, 2016 ).
$22,225,750 (Marijuana Sales Tax [2.9%]) + $42,017,798 (Retail Marijuana Sales Tax [10%]) + $23,995,775 (Retail Marijuana Excise Tax [15%]) = $88,239,323.
Medical Cannabis: $5,236,536 (State Retail Sales Tax) + $792,906 (State Business and Occupation Tax) + $ 2,084,323 (Local Retail Sales Tax) = $8,113,765. Recreational Cannabis: $30,017,823 (State Retail Sales Tax) + $4,050,212 (State Business & Occupation Tax) + $11,228,861 (Local Retail Sales Tax) = $45,296,896. $8,113,765 (Total Medical Cannabis Taxes) + $45,296,896 (Total Recreational Cannabis Taxes) = $53,410,661.
Code of Federal Regulations, Registration of Manufacturers, Distributors, and Dispensers of Controlled Substances, Title 21, § 1301.11 and Code of Federal Regulations, Schedules of Controlled Substances, Title 21, § 1308.11.
Code of Federal Regulations, Schedules of Controlled Substances, Title 21, § 1308.11; United States Code, Schedules of Controlled Substances, Title 21, § 812.
United States Code, Schedules of Controlled Substances, Title 21, § 812(b)(1).
Code of Federal Regulations, Schedules of Controlled Substances, Title 21, § 1308.11.
United States Code, Schedules of Controlled Substances, Title 21, § 812(b)(2).
Code of Federal Regulations, Registration of Manufacturers, Distributors, and Dispensers of Controlled Substances, Title 21, § 1301.18.
Office of the Secretary, Office of the Assistant Secretary for Health, U.S. Department of Health and Human Services. Notice. “Announcement of Revision to the Department of Health and Human Services Guidance on Procedures for the Provision of Marijuana for Medical Research as Published on May 21, 1999,” Federal Register , 80, no. 120 (June 23, 2015): 35960, https://www .gpo.gov/fdsys /pkg/FR-2015-06-23/pdf/2015-15479 .pdf (accessed November 25, 2016).
Code of Federal Regulations, Registration of Manufacturers, Distributors, and Dispensers of Controlled Substances, Title 21, § 1301.72 (a) and (d).
Code of Federal Regulations, Registration of Manufacturers, Distributors, and Dispensers of Controlled Substances, Title 21, § 1301.71 (c).
Code of Federal Regulations, Institutional Review Boards, Title 21, § 56.103.
The committee was specifically directed in its statement of task not to comment on cannabis policy issues, such as regulatory options for legalization, taxation, or distribution. While the committee has identified the Schedule 1 classification of cannabis as posing a significant barrier to the conduct of scientific research on the health effects of cannabis, the committee is aware that any decision on the regulation of cannabis involves many factors far outside the committee's remit and expertise. Specifically, the committee did not comment on the abuse or dependency liability or accepted medical use of cannabis compared to other scheduled drugs.
In fiscal year 2015, NIDA's investment in cannabinoid research totaled $66,078,314, of which $10,923,472 was allocated for therapeutic cannabinoid research ( NIDA, 2016c ).
In fiscal year 2015, NIH's investment in cannabinoid research totaled $ $111,275,219, of which $66,078,314 was allocated to NIDA ( NIDA, 2016c ).
NIDA contracts with the University of Mississippi through an open solicitation process. Although the University of Mississippi is currently NIDA's only supplier of research-grade cannabis, other groups can compete for the contract ( NIDA, 2015 , 2016a ).
In December 2016, cannabis provided by NIDA was generally free for NIH-sponsored research. For research not funded by the federal government, the cost of non-placebo cannabis was $10.96 per cigarette and $1,133 per pound ($2,497 per kilogram) ( NIDA, 2016d ).
DEA, U.S. Department of Justice. Policy Statement. “Applications to Become Registered Under the Controlled Substances Act to Manufacture Marijuana to Supply Researchers in the United States,” Federal Register , 81, no. 156 (August 12, 2016): 53846, https://www .gpo.gov/fdsys /pkg/FR-2016-08-12/pdf/2016-17955 .pdf (accessed January 7, 2017).
$66,078,314 (Total NIDA spending on cannabinoid research in fiscal year 2015)/$111,275,219 (Total NIH spending on cannabinoid research in fiscal year 2015) = 0.593. $10,923,472 (Total NIDA spending on therapeutic cannabinoid research in fiscal year 2015)/$66,078,314 (Total NIDA spending on cannabinoid research in fiscal year 2015) = 0.165.
By contrast, NIH spending on tobacco research totaled $300 million in 2015, and spending on research related to the harms and benefits of alcohol use totaled $473 million in 2015 ( NIH, 2016 ).
In December 2016, placebo cannabis provided by NIDA was generally free for NIH-sponsored research. For research not funded by the federal government, the cost of placebo cannabis was $13.94 per cigarette and $1,133 per pound ($2,497 per kilogram) ( NIDA, 2016d ).
- Cite this Page National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on the Health Effects of Marijuana: An Evidence Review and Research Agenda. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington (DC): National Academies Press (US); 2017 Jan 12. 15, Challenges and Barriers in Conducting Cannabis Research.
- PDF version of this title (3.3M)
In this Page
Other titles in this collection.
- The National Academies Collection: Reports funded by National Institutes of Health
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Recent Activity
- Challenges and Barriers in Conducting Cannabis Research - The Health Effects of ... Challenges and Barriers in Conducting Cannabis Research - The Health Effects of Cannabis and Cannabinoids
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
- About the Hub
- Announcements
- Faculty Experts Guide
- Subscribe to the newsletter
Explore by Topic
- Arts+Culture
- Politics+Society
- Science+Technology
- Student Life
- University News
- Voices+Opinion
- About Hub at Work
- Gazette Archive
- Benefits+Perks
- Health+Well-Being
- Current Issue
- About the Magazine
- Past Issues
- Support Johns Hopkins Magazine
- Subscribe to the Magazine
You are using an outdated browser. Please upgrade your browser to improve your experience.
Healthy volunteers needed for cannabis and alcohol study
By Hub staff report
Healthy adults age 21-55 are needed to take part in a Johns Hopkins research study. The study will require nine total visits to the lab, located at the Bayview Medical Center.
Participants will first complete an in-person screening session (occurs over two days) to determine study eligibility. Participants who are eligible and agree to be in the study will complete seven drug administration sessions, each lasting about 10 hours. In these sessions, participants will eat a brownie that contains cannabis and consume alcohol and then complete various tests to measure different aspects of performance. Those who complete the study can earn up to $2,660.
Call 410-550-0096 for details. You can also click here to see if you may be eligible.
Principal Investigator: Tory Spindle, PhD IRB00290015
News Network
- Johns Hopkins Magazine
- Get Email Updates
- Submit an Announcement
- Submit an Event
- Privacy Statement
- Accessibility
Discover JHU
- About the University
- Schools & Divisions
- Academic Programs
- Plan a Visit
- my.JohnsHopkins.edu
- © 2024 Johns Hopkins University . All rights reserved.
- University Communications
- 3910 Keswick Rd., Suite N2600, Baltimore, MD
- X Facebook LinkedIn YouTube Instagram
Cannabis (Marijuana) DrugFacts
What is marijuana.

Marijuana refers to the dried leaves, flowers, stems, and seeds from the Cannabis sativa or Cannabis indica plant. The plant contains the mind-altering chemical THC and other similar compounds. Extracts can also be made from the cannabis plant (see " Marijuana Extracts ").
According to the National Survey on Drug Use and Health , cannabis (marijuana) is one of the most used drugs in the United States, and its use is widespread among young people. In 2021, 35.4% of young adults aged 18 to 25 (11.8 million people) reported using marijuana in the past year. 1 According to the Monitoring the Future survey , rates of past year marijuana use among middle and high school students have remained relatively steady since the late 1990s. In 2022, 30.7% of 12th graders reported using marijuana in the past year and 6.3% reported using marijuana daily. In addition, many young people also use vaping devices to consume cannabis products. In 2022, nearly 20.6% of 12th graders reported that they vaped marijuana in the past year and 2.1% reported that they did so daily. 2
Legalization of marijuana for medical use or adult recreational use in a growing number of states may affect these views. Read more about marijuana as medicine in our DrugFacts: Marijuana as Medicine .

How do people use marijuana?
People smoke marijuana in hand-rolled cigarettes (joints) or in pipes or water pipes (bongs). They also smoke it in blunts—emptied cigars that have been partly or completely refilled with marijuana. To avoid inhaling smoke, some people are using vaporizers. These devices pull the active ingredients (including THC) from the marijuana and collect their vapor in a storage unit. A person then inhales the vapor, not the smoke. Some vaporizers use a liquid marijuana extract.
People can mix marijuana in food ( edibles ), such as brownies, cookies, or candy, or brew it as a tea. A newly popular method of use is smoking or eating different forms of THC-rich resins (see " Marijuana Extracts ").
Marijuana Extracts
Smoking THC-rich resins extracted from the marijuana plant is on the rise. People call this practice dabbing . These extracts come in various forms, such as:
- hash oil or honey oil —a gooey liquid
- wax or budder —a soft solid with a texture like lip balm
- shatter —a hard, amber-colored solid
These extracts can deliver extremely large amounts of THC to the body, and their use has sent some people to the emergency room. Another danger is in preparing these extracts, which usually involves butane (lighter fluid). A number of people have caused fires and explosions and have been seriously burned from using butane to make extracts at home. 3,4
How does marijuana affect the brain?
Marijuana has both short-and long-term effects on the brain.
Short-Term Effects
When a person smokes marijuana, THC quickly passes from the lungs into the bloodstream. The blood carries the chemical to the brain and other organs throughout the body. The body absorbs THC more slowly when the person eats or drinks it. In that case, they generally feel the effects after 30 minutes to 1 hour.
THC acts on specific brain cell receptors that ordinarily react to natural THC-like chemicals. These natural chemicals play a role in normal brain development and function.
Marijuana over activates parts of the brain that contain the highest number of these receptors. This causes the "high" that people feel. Other effects include:
- altered senses (for example, seeing brighter colors)
- altered sense of time
- changes in mood
- impaired body movement
- difficulty with thinking and problem-solving
- impaired memory
- hallucinations (when taken in high doses)
- delusions (when taken in high doses)
- psychosis (risk is highest with regular use of high potency marijuana)
Long-Term Effects
Marijuana also affects brain development. When people begin using marijuana as teenagers, the drug may impair thinking, memory, and learning functions and affect how the brain builds connections between the areas necessary for these functions. Researchers are still studying how long marijuana's effects last and whether some changes may be permanent.
For example, a study from New Zealand conducted in part by researchers at Duke University showed that people who started smoking marijuana heavily in their teens and had an ongoing marijuana use disorder lost an average of 8 IQ points between ages 13 and 38. The lost mental abilities didn't fully return in those who quit marijuana as adults. Those who started smoking marijuana as adults didn't show notable IQ declines. 5
In another recent study on twins, those who used marijuana showed a significant decline in general knowledge and in verbal ability (equivalent to 4 IQ points) between the preteen years and early adulthood, but no predictable difference was found between twins when one used marijuana and the other didn't. This suggests that the IQ decline in marijuana users may be caused by something other than marijuana, such as shared familial factors (e.g., genetics, family environment). 6 NIDA’s Adolescent Brain Cognitive Development (ABCD) study, a major longitudinal study, is tracking a large sample of young Americans from late childhood to early adulthood to help clarify how and to what extent marijuana and other substances, alone and in combination, affect adolescent brain development. Read more about the ABCD study on our Longitudinal Study of Adolescent Brain and Cognitive Development (ABCD Study) webpage.
A Rise in Marijuana’s THC Levels
The amount of THC in marijuana has been increasing steadily over the past few decades. 7 For a person who's new to marijuana use, this may mean exposure to higher THC levels with a greater chance of a harmful reaction. Higher THC levels may explain the rise in emergency room visits involving marijuana use.
The popularity of edibles also increases the chance of harmful reactions. Edibles take longer to digest and produce a high. Therefore, people may consume more to feel the effects faster, leading to dangerous results.
Higher THC levels may also mean a greater risk for addiction if people are regularly exposing themselves to high doses.
What are the other health effects of marijuana?
Marijuana use may have a wide range of effects, both physical and mental.
Physical Effects
- Breathing problems. Marijuana smoke irritates the lungs, and people who smoke marijuana frequently can have the same breathing problems as those who smoke tobacco. These problems include daily cough and phlegm, more frequent lung illness, and a higher risk of lung infections. Researchers so far haven't found a higher risk for lung cancer in people who smoke marijuana. 8
- Increased heart rate. Marijuana raises heart rate for up to 3 hours after smoking. This effect may increase the chance of heart attack. Older people and those with heart problems may be at higher risk.
- Problems with child development during and after pregnancy. One study found that about 20% of pregnant women 24-years-old and younger screened positive for marijuana. However, this study also found that women were about twice as likely to screen positive for marijuana use via a drug test than they state in self-reported measures. 9 This suggests that self-reported rates of marijuana use in pregnant females is not an accurate measure of marijuana use and may be underreporting their use. Additionally, in one study of dispensaries, nonmedical personnel at marijuana dispensaries were recommending marijuana to pregnant women for nausea, but medical experts warn against it. This concerns medical experts because marijuana use during pregnancy is linked to lower birth weight 10 and increased risk of both brain and behavioral problems in babies. If a pregnant woman uses marijuana, the drug may affect certain developing parts of the fetus's brain. Children exposed to marijuana in the womb have an increased risk of problems with attention, 11 memory, and problem-solving compared to unexposed children. 12 Some research also suggests that moderate amounts of THC are excreted into the breast milk of nursing mothers. 13 With regular use, THC can reach amounts in breast milk that could affect the baby's developing brain. Other recent research suggests an increased risk of preterm births. 27 More research is needed. Read our Marijuana Research Report for more information about marijuana and pregnancy.
- Intense nausea and vomiting. Regular, long-term marijuana use can lead to some people to develop Cannabinoid Hyperemesis Syndrome. This causes users to experience regular cycles of severe nausea, vomiting, and dehydration, sometimes requiring emergency medical attention. 14
Reports of Deaths Related to Vaping
The Food and Drug Administration has alerted the public to hundreds of reports of serious lung illnesses associated with vaping, including several deaths. They are working with the Centers for Disease Control and Prevention (CDC) to investigate the cause of these illnesses. Many of the suspect products tested by the states or federal health officials have been identified as vaping products containing THC, the main psychotropic ingredient in marijuana. Some of the patients reported a mixture of THC and nicotine; and some reported vaping nicotine alone. No one substance has been identified in all of the samples tested, and it is unclear if the illnesses are related to one single compound. Until more details are known, FDA officials have warned people not to use any vaping products bought on the street, and they warn against modifying any products purchased in stores. They are also asking people and health professionals to report any adverse effects. The CDC has posted an information page for consumers.

Mental Effects
Long-term marijuana use has been linked to mental illness in some people, such as:
- temporary hallucinations
- temporary paranoia
- worsening symptoms in patients with schizophrenia —a severe mental disorder with symptoms such as hallucinations, paranoia, and disorganized thinking
Marijuana use has also been linked to other mental health problems, such as depression, anxiety, and suicidal thoughts among teens. However, study findings have been mixed.
Are there effects of inhaling secondhand marijuana smoke?
Failing a drug test.
While it's possible to fail a drug test after inhaling secondhand marijuana smoke, it's unlikely. Studies show that very little THC is released in the air when a person exhales. Research findings suggest that, unless people are in an enclosed room, breathing in lots of smoke for hours at close range, they aren't likely to fail a drug test. 15,16 Even if some THC was found in the blood, it wouldn't be enough to fail a test.
Getting High from Passive Exposure?
Similarly, it's unlikely that secondhand marijuana smoke would give nonsmoking people in a confined space a high from passive exposure. Studies have shown that people who don't use marijuana report only mild effects of the drug from a nearby smoker, under extreme conditions (breathing in lots of marijuana smoke for hours in an enclosed room). 17
Other Health Effects?
More research is needed to know if secondhand marijuana smoke has similar health risks as secondhand tobacco smoke. A recent study on rats suggests that secondhand marijuana smoke can do as much damage to the heart and blood vessels as secondhand tobacco smoke. 20 But researchers haven't fully explored the effect of secondhand marijuana smoke on humans. What they do know is that the toxins and tar found in marijuana smoke could affect vulnerable people, such as children or people with asthma.
How Does Marijuana Affect a Person's Life?
Compared to those who don't use marijuana, those who frequently use large amounts report the following:
- lower life satisfaction
- poorer mental health
- poorer physical health
- more relationship problems
People also report less academic and career success. For example, marijuana use is linked to a higher likelihood of dropping out of school. 18 It's also linked to more job absences, accidents, and injuries. 19
Is marijuana a gateway drug?
Use of alcohol, tobacco, and marijuana are likely to come before use of other drugs. 21,22 Animal studies have shown that early exposure to addictive substances, including THC, may change how the brain responds to other drugs. For example, when rodents are repeatedly exposed to THC when they're young, they later show an enhanced response to other addictive substances—such as morphine or nicotine—in the areas of the brain that control reward, and they're more likely to show addiction-like behaviors. 23,24
Although these findings support the idea of marijuana as a "gateway drug," the majority of people who use marijuana don't go on to use other "harder" drugs. It's also important to note that other factors besides biological mechanisms, such as a person’s social environment, are also critical in a person’s risk for drug use and addiction. Read more about marijuana as a gateway drug in our Marijuana Research Report .
Can a person overdose on marijuana?
An overdose occurs when a person uses enough of the drug to produce life-threatening symptoms or death. There are no reports of teens or adults dying from marijuana alone. However, some people who use marijuana can feel some very uncomfortable side effects, especially when using marijuana products with high THC levels. People have reported symptoms such as anxiety and paranoia, and in rare cases, an extreme psychotic reaction (which can include delusions and hallucinations) that can lead them to seek treatment in an emergency room.
While a psychotic reaction can occur following any method of use, emergency room responders have seen an increasing number of cases involving marijuana edibles. Some people (especially preteens and teens) who know very little about edibles don't realize that it takes longer for the body to feel marijuana’s effects when eaten rather than smoked. So they consume more of the edible, trying to get high faster or thinking they haven't taken enough. In addition, some babies and toddlers have been seriously ill after ingesting marijuana or marijuana edibles left around the house.
Is marijuana addictive?
Marijuana use can lead to the development of a substance use disorder, a medical illness in which the person is unable to stop using even though it's causing health and social problems in their life. Severe substance use disorders are also known as addiction. Research suggests that between 9 and 30 percent of those who use marijuana may develop some degree of marijuana use disorder. 25 People who begin using marijuana before age 18 are four to seven times more likely than adults to develop a marijuana use disorder. 26
Many people who use marijuana long term and are trying to quit report mild withdrawal symptoms that make quitting difficult. These include:
- grouchiness
- sleeplessness
- decreased appetite
What treatments are available for marijuana use disorder?
No medications are currently available to treat marijuana use disorder, but behavioral support has been shown to be effective. Examples include therapy and motivational incentives (providing rewards to patients who remain drug-free). Continuing research may lead to new medications that help ease withdrawal symptoms, block the effects of marijuana, and prevent relapse.
Points to Remember
- Marijuana refers to the dried leaves, flowers, stems, and seeds from the Cannabis sativa or Cannabis indica plant .
- The plant contains the mind-altering chemical THC and other related compounds.
- People use marijuana by smoking, eating, drinking, or inhaling it.
- Smoking and vaping THC-rich extracts from the marijuana plant (a practice called dabbing ) is on the rise.
- altered senses
- impaired memory and learning
- hallucinations and paranoia
- breathing problems
- possible harm to a fetus's brain in pregnant women
- The amount of THC in marijuana has been increasing steadily in recent decades, creating more harmful effects in some people.
- It's unlikely that a person will fail a drug test or get high from passive exposure by inhaling secondhand marijuana smoke.
- There aren’t any reports of teens and adults dying from using marijuana alone, but marijuana use can cause some very uncomfortable side effects, such as anxiety and paranoia and, in rare cases, extreme psychotic reactions.
- Marijuana use can lead to a substance use disorder, which can develop into an addiction in severe cases.
- No medications are currently available to treat marijuana use disorder, but behavioral support can be effective.
For more information about marijuana and marijuana use, visit our:
- Marijuana webpage
- Drugged Driving DrugFacts
- Substance Abuse Center for Behavioral Health Statistics and Quality. Results from the 2018 National Survey on Drug Use and Health: Detailed Tables. SAMHSA. https://www.samhsa.gov/data/report/2018-nsduh-detailed-tables . Accessed December 2019.
- Miech, R. A., Johnston, L. D., Patrick, M. E., O’Malley, P. M., Bachman, J. G., & Schulenberg J. E. (2023). Monitoring the Future National Survey Results on Drug Use, 1975-2022 . Monitoring the Future Monograph Series. Ann Arbor: Institute for Social Research, The University of Michigan.
- Bell C, Slim J, Flaten HK, Lindberg G, Arek W, Monte AA. Butane Hash Oil Burns Associated with Marijuana Liberalization in Colorado. J Med Toxicol Off J Am Coll Med Toxicol. 2015;11(4):422-425. doi:10.1007/s13181-015-0501-0.
- Romanowski KS, Barsun A, Kwan P, et al. Butane Hash Oil Burns: A 7-Year Perspective on a Growing Problem. J Burn Care Res Off Publ Am Burn Assoc. 2017;38(1):e165-e171. doi:10.1097/BCR.0000000000000334.
- Meier MH, Caspi A, Ambler A, et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci U S A. 2012;109(40):E2657-E2664. doi:10.1073/pnas.1206820109.
- Jackson NJ, Isen JD, Khoddam R, et al. Impact of adolescent marijuana use on intelligence: Results from two longitudinal twin studies. Proc Natl Acad Sci U S A. 2016;113(5):E500-E508. doi:10.1073/pnas.1516648113.
- Mehmedic Z, Chandra S, Slade D, et al. Potency trends of Δ9-THC and other cannabinoids in confiscated cannabis preparations from 1993 to 2008. J Forensic Sci. 2010;55(5):1209-1217. doi:10.1111/j.1556-4029.2010.01441.x.
- National Academies of Sciences, Engineering, and Medicine. The Health Effects of Cannabis and Cannabinoids: Current State of Evidence and Recommendations for Research. Washington, DC: The National Academies Press; 2017.
- Young-Wolff KC, Tucker L-Y, Alexeeff S, et al. Trends in Self-reported and Biochemically Tested Marijuana Use Among Pregnant Females in California From 2009-2016. JAMA. 2017;318(24):2490. doi:10.1001/jama.2017.17225
- The National Academies of Sciences, Engineering, and Medicine, Health and Medicine Division, Board on Population Health and Public Health Practice, Committee on the Health Effects of Marijuana: An Evidence Review and Research Agenda. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. http://nationalacademies.org/hmd/Reports/2017/health-effects-of-cannabis-and-cannabinoids.aspx . Accessed January 19, 2017.
- Goldschmidt L, Day NL, Richardson GA. Effects of prenatal marijuana exposure on child behavior problems at age 10. Neurotoxicol Teratol. 2000;22(3):325-336.
- Richardson GA, Ryan C, Willford J, Day NL, Goldschmidt L. Prenatal alcohol and marijuana exposure: effects on neuropsychological outcomes at 10 years. Neurotoxicol Teratol. 2002;24(3):309-320.
- Perez-Reyes M, Wall ME. Presence of delta9-tetrahydrocannabinol in human milk. N Engl J Med. 1982;307(13):819-820. doi:10.1056/NEJM198209233071311.
- Galli JA, Sawaya RA, Friedenberg FK. Cannabinoid Hyperemesis Syndrome. Curr Drug Abuse Rev . 2011;4(4):241-249.
- Röhrich J, Schimmel I, Zörntlein S, et al. Concentrations of delta9-tetrahydrocannabinol and 11-nor-9-carboxytetrahydrocannabinol in blood and urine after passive exposure to Cannabis smoke in a coffee shop. J Anal Toxicol. 2010;34(4):196-203.
- Cone EJ, Bigelow GE, Herrmann ES, et al. Non-smoker exposure to secondhand cannabis smoke. I. Urine screening and confirmation results. J Anal Toxicol. 2015;39(1):1-12. doi:10.1093/jat/bku116.
- Herrmann ES, Cone EJ, Mitchell JM, et al. Non-smoker exposure to secondhand cannabis smoke II: Effect of room ventilation on the physiological, subjective, and behavioral/cognitive effects. Drug Alcohol Depend. 2015;151:194-202. doi:10.1016/j.drugalcdep.2015.03.019.
- McCaffrey DF, Pacula RL, Han B, Ellickson P. Marijuana Use and High School Dropout: The Influence of Unobservables. Health Econ. 2010;19(11):1281-1299. doi:10.1002/hec.1561.
- Zwerling C, Ryan J, Orav EJ. The efficacy of preemployment drug screening for marijuana and cocaine in predicting employment outcome. JAMA. 1990;264(20):2639-2643.
- Wang X, Derakhshandeh R, Liu J, et al. One Minute of Marijuana Secondhand Smoke Exposure Substantially Impairs Vascular Endothelial Function. J Am Heart Assoc. 2016;5(8). doi:10.1161/JAHA.116.003858.
- Secades-Villa R, Garcia-Rodríguez O, Jin CJ, Wang S, Blanco C. Probability and predictors of the cannabis gateway effect: a national study. Int J Drug Policy. 2015;26(2):135-142. doi:10.1016/j.drugpo.2014.07.011.
- Levine A, Huang Y, Drisaldi B, et al. Molecular mechanism for a gateway drug: epigenetic changes initiated by nicotine prime gene expression by cocaine. Sci Transl Med. 2011;3(107):107ra109. doi:10.1126/scitranslmed.3003062.
- Panlilio LV, Zanettini C, Barnes C, Solinas M, Goldberg SR. Prior exposure to THC increases the addictive effects of nicotine in rats. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2013;38(7):1198-1208. doi:10.1038/npp.2013.16.
- Cadoni C, Pisanu A, Solinas M, Acquas E, Di Chiara G. Behavioural sensitization after repeated exposure to Delta 9-tetrahydrocannabinol and cross-sensitization with morphine. Psychopharmacology (Berl). 2001;158(3):259-266. doi:10.1007/s002130100875.
- Hasin DS, Saha TD, Kerridge BT, et al. Prevalence of Marijuana Use Disorders in the United States Between 2001-2002 and 2012-2013. JAMA Psychiatry. 2015;72(12):1235-1242. doi:10.1001/jamapsychiatry.2015.1858.
- Winters KC, Lee C-YS. Likelihood of developing an alcohol and cannabis use disorder during youth: association with recent use and age. Drug Alcohol Depend. 2008;92(1-3):239-247. doi:10.1016/j.drugalcdep.2007.08.005.
- Corsi DJ, Walsh L, Weiss D, et al. Association Between Self-reported Prenatal Cannabis Use and Maternal, Perinatal, and Neonatal Outcomes. JAMA . Published online June 18, 2019322(2):145–152. doi:10.1001/jama.2019.8734
This publication is available for your use and may be reproduced in its entirety without permission from NIDA. Citation of the source is appreciated, using the following language: Source: National Institute on Drug Abuse; National Institutes of Health; U.S. Department of Health and Human Services.

Study Aims To Determine Role of Shrooms in Battling Alcoholism
- Psychedelics
Organs from Cannabis Consumers Don’t Pose Risks of Infection
State attorneys general ask congress to regulate intoxicating hemp products, socal city, costa mesa, officials consider sweeping new rules for dispensaries.
- California News
- Dispensaries
Study Finds Natural Mushroom Extract Has Better Therapeutic Effects Than Synthesized Psilocybin
A new study shows that natural mushroom extracts may be more therapeutically effective than synthesized psilocybin, which is widely used in research investigating the medicinal potential of the psychedelic drug. The findings suggest that natural mushroom extracts may offer more potential applications for the treatment of serious mental health conditions such as depression, post-traumatic stress disorder (PTSD) and schizophrenia.
The study , which was conducted by an interdisciplinary team of researchers from the Hebrew University of Jerusalem’s Hadassah Medical Center BrainLabs Center for Psychedelic Research, compared the effects of the natural and synthesized versions of psilocybin, the compound primarily responsible for the psychoactive effects of magic mushrooms.
“My colleagues and I are very interested in the potential of psychedelics to treat serious, treatment resistant psychiatric disorders such as depression, PTSD, OCD and even schizophrenia,” study author Bernard Lerer, a professor of psychiatry and director of the Hadassah BrainLabs Center for Psychedelic Research at Hebrew University, told PsyPost .
“There are many anecdotal and clinical reports which suggest that extract of psilocybin-containing mushrooms may have unique effects that are qualitatively and quantitatively different from chemical psilocybin, and also some preclinical studies,” Lerer continued. “This observation has important clinical implications and we wanted to test it empirically in a laboratory study.”
Entourage Effect May Boost The Therapeutic Effects Of Psilocybin
Mushrooms that contain psilocybin also produce many other psychoactive and non-psychoactive compounds that may work together to provide enhanced therapeutic effects through a phenomenon known as the entourage effect. However, most clinical research into psilocybin is conducted with synthesized forms of the drug that do not contain these additional potentially therapeutic compounds.
“In Western medicine, there has historically been a preference for isolating active compounds rather than utilizing extracts, primarily for the sake of gaining better control over dosages and anticipating known effects during treatment,” the researchers said in an emailed statement about the study. “The challenge with working with extracts lay in the inability, in the past, to consistently produce the exact product with a consistent compound profile.”
“Contrastingly, ancient medicinal practices, particularly those attributing therapeutic benefits to psychedelic medicine, embraced the use of extracts or entire products, such as consuming the entire mushroom,” they continued. “Although Western medicine has long recognized the ‘entourage’ effect associated with whole extracts, the significance of this approach has gained recent prominence.”
To conduct the study, the researchers compared the effects of a natural mushroom extract with those of synthesized psilocybin in laboratory mice. The mice were divided into three groups that received either the extract, synthesized psilocybin or a saline solution control. Both forms of psilocybin were given in amounts determined to be therapeutically relevant based on equivalent dosing models between humans and laboratory mice.
The researchers assessed the behavioral effects and potential neuroplasticity induced by psilocybin using the head twitch response assay, a commonly employed method of studying the effects of psychedelics in mice. They also compared the metabolic changes in the frontal cortex following treatment and analyzed the expression of synaptic proteins in the brain that can be used as indicators of neuroplasticity.
The research showed that the mushroom extract demonstrated a stronger and more prolonged impact on synaptic plasticity, which could indicate the extract offers unique therapeutic benefits. Additionally, the metabolic analyses showed distinct metabolic profiles between synthesized psilocybin and the extract, suggesting that the mushroom extract may have a “unique influence on oxidative stress and energy production pathways,” according to a report from Neuroscience News.
While the research showed that the mushroom extract and synthesized psilocybin had different metabolic and neuroplasticity effects, both induced the head twitch response. The findings suggest that the acute effects of both compounds are similar at the basic behavioral level.
“We were surprised by the fact that there were no differences in the acute effect on the head twitch response between chemical psilocybin and psilocybin-containing mushroom extract while the differences emerged in terms of longer term effects on synaptic proteins and metabolomics,” Lerer said. “This has important potential clinical relevance.”
The researchers noted that while the mushroom extract showed potentially enhanced therapeutic effects, creating them in consistent formulations can be a challenge, making synthesized versions of the compound a common alternative for therapeutic research. However, they noted that with careful cultivation and processing, it is possible to make extracts in consistent formulations.
“A major challenge with natural extracts lies in achieving a consistently stable compound profile, especially with plants; however, mushrooms present a unique case,” the researchers wrote. “Mushroom compounds are highly influenced by their growing environment, encompassing factors such as substrate composition, CO2/O2 ratio, light exposure, temperature, and microbial surroundings. Despite these influences, controlled cultivation allows for the taming of mushrooms, enabling the production of a replicable extract.”
The researchers recommended more research, noting that there could be clinical advantages to using a natural mushroom extract instead of synthesized psilocybin.
“Our findings need to be confirmed in human studies but they do suggest that there may be therapeutic advantages to psilocybin-containing mushroom extract over chemically synthesized psilocybin, when both are administered at the same psilocybin dose,” said Lerer.
- Entourage Effect
- magic mushrooms
- mental health
- synthesized psilocybin

Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Sign Up for Our Newsletters
Related posts, first black woman-owned dispensary opens in manhattan, reported thc potency for cali weed drops after new rules take effect, report reveals europe’s cannabis and cocaine capitals, dutch cities reign supreme, study underscores value of spiritual health practitioners in psychedelic-assisted therapies.

COMMENTS
The Journal of Cannabis Research is an international, fully open access, peer-reviewed journal covering all topics pertaining to cannabis, including original research, perspectives, commentaries and protocols. Our goal is to provide an accessible outlet for expert interdisciplinary discourse on cannabis research. Read Aims & Scope.
Adverse effects were reported in most reviews comparing cannabis with placebo (49/59, 83%) and in 20/24 (83%) of the reviews comparing cannabis to active drugs. Minor adverse effects (e.g., drowsiness, dizziness) were common and reported in over half of the reviews. Serious harms were not as common, but were reported in 21/59 (36%) reviews that ...
Donate Now. With nearly a century of research restrictions and limited funding leading to a lack of scientific knowledge about cannabis, particularly in regards to its potential therapeutic use, we need your support now more than ever. Join the UCLA Center for Cannabis and Cannabinoids as we conduct innovative, groundbreaking research designed ...
What kinds of marijuana research does NIDA fund? As part of its mandate to study drug abuse and addiction and other health effects of both legal and illegal drugs, NIDA funds a wide range of research on marijuana (cannabis); its main psychotropic ingredient, delta-9-tetrahydrocannabinol (THC); and chemicals related to THC (cannabinoids), including:
The cannabis plant has been used in traditional medicine for centuries, and within the last few decades it has generated considerable attention among the general population, modern medical community and regulatory bodies for its potential medicinal capabilities (Alsherbiny and Li 2018).The effects of cannabis are due to the action of cannabinoids, a diverse group of chemical compounds found in ...
The NIH supports a broad portfolio of research on cannabinoids and the endocannabinoid system. This research portfolio includes some studies utilizing the whole marijuana plant (Cannabis sativa), but most studies focus on individual cannabinoid compounds.Individual cannabinoid chemicals may be isolated and purified from the marijuana plant or synthesized in the laboratory, or they may be ...
Medical Cannabis Real World Evidence [ 44 - 46] A Canadian, prospective, non-interventional, observational study led by the University Health Network in Toronto. It aims to explore the benefits of medical cannabis in an observational setting for adults with conditions such as chronic pain, anxiety or depression.
Medical Marijuana Laws and Prescription Opioid Use Outcomes. A new study underscores the need for additional research on the effect of medical marijuana laws on opioid overdose deaths and cautions against drawing a causal connection between the two. Early research suggested that there may be a relationship between the availability of medical ...
December 10, 2020. This study assesses cannabidiol, which inhibits the reuptake of endocannabinoids, as a treatment for cannabis use disorder. Freeman et al. (2020) conducted a phase 2a, randomized, double-blind, placebo, controlled, clinical trial investigating the heal …. Read More.
Cannabis sativa has a long history as a medicinal plant, likely dating back more than two millennia (Russo et al., 2007). It was available as a licensed medicine in the United States for about a century before the American Medical Association removed it from the 12th edition of the U.S. Pharmacopeia (IOM, 1999). In 1985, pharmaceutical companies received approval to begin developing Δ9 ...
The FDA has an important role to play in supporting scientific research into the medical uses of cannabis and its constituents in scientifically valid investigations as part of the agency's drug ...
The 7 most important cannabis research studies of 2023. Nick Jikomes, PhD Published on December 19, 2023. (Mitch/AdobeStock) 2023 saw tons of new research come out related to cannabis. Below is a ...
A key provision of this act was to "promote high-quality research" on medical marijuana. In May of 2018, Penn State College of Medicine was one of eight universities approved by Gov. Tom Wolf as a Certified Academic Clinical Research Center. In June 2019, the Penn State College of Medicine ACRC, in a relationship with PA Options for ...
The National Institutes of Health (NIH) supports a broad portfolio of research on cannabis and cannabis constituents and related compounds, as well as the endocannabinoid system. Specific topics of interest vary among Institutes, Centers, and Offices, but overall the research portfolio includes studies investigating the whole or parts of the ...
After more than 50 years, the federal government is lifting a roadblock to cannabis research that scientists and advocates say has hindered rigorous studies of the plant and possible drug development.
open to eligible people ages 18 years and up. This study will address whether cannabis affects antiretroviral therapy (ART) drug concentrations, mood, and thinking. The project will have two phases. Phase 1 is an observational study, in which 120 people will be assessed to evaluate the effects…. San Diego, California.
Cannabis research slowed down in 2020 due to COVID-19, but some great studies still came out showing off the amazing benefits of the plant. Leafly Shop legal, local weed.
Enhancing Cannabis Research. Coordinating and fostering innovative Cannabis research across disciplines. ... Event details Research Work Groups. Five projects mandated by the Washington State Legislature unite experts to study the impact of Cannabis use. Work Groups 2nd UW CCR Retreat was a true success! 50 participants, a plenary lecture, 6 ...
Much of what is known about the adverse effects of medicinal cannabis comes from studies of recreational users of marijuana. 43 Short-term use of cannabis has led to impaired short-term ... other limitations in cannabis research also exist. For example, the Center for Medicinal Cannabis Research at the University of California-San Diego had ...
Imaging studies of marijuana's impact on brain structure in humans have shown conflicting results. Some studies suggest regular marijuana use in adolescence is associated with altered connectivity and reduced volume of specific brain regions involved in a broad range of executive functions such as memory, learning, and impulse control ...
For several years, Israeli scientists have been running clinical trials that reveal promising results for a 20:1 CBD to THC cannabis oil on many secondary symptoms of autism spectrum disorder ...
Waye manages the research program at the Office of Cannabis Science and Surveillance at Health Canada. ... 2019 — An Australian study has demonstrated that cannabis-based medication helps tackle ...
Benzodiazepines are a class of medications that are being frequently prescribed in Canada but carry significant risk of harm. There has been increasing clinical interest on the potential "sparing effects" of medical cannabis as one strategy to reduce benzodiazepine use. The objective of this study as to examine the association of medical cannabis authorization with benzodiazepine usage ...
Observational studies also connect other mental-health conditions to frequent cannabis use. Solmi's review found that depression increases, as does violence among dating couples.
The Canton of Zurich is launching Switzerland's largest marijuana study to date, which is being financed by donations. The research budget is close to $1.69 million, reported SwissInfo.
Though not all in the medical community agree, many people swear by the medicinal effects of marijuana to help treat symptoms of cancer, anxiety, post traumatic stress disorder and epilepsy. "It's no longer appropriate to say that there's no medical benefit when there are hundreds if not thousands of medical studies that show the opposite," explained Dr. Berger.
Researchers conducting trials of Schedule I substances must additionally submit a research protocol to the DEA that includes details regarding the security provisions for storing and dispensing the substance. 10 Previously, nonfederally funded studies on cannabis were also required to undergo an additional review process conducted by the Public Health Service.
Healthy adults age 21-55 are needed to take part in a Johns Hopkins research study. The study will require nine total visits to the lab, located at the Bayview Medical Center. ... In these sessions, participants will eat a brownie that contains cannabis and consume alcohol and then complete various tests to measure different aspects of ...
According to the National Survey on Drug Use and Health, cannabis (marijuana) is one of the most used drugs in the United States, and its use is widespread among young people. In 2021, 35.4% of young adults aged 18 to 25 (11.8 million people) reported using marijuana in the past year. 1 According to the Monitoring the Future survey, rates of ...
A new study shows that natural mushroom extracts may be more therapeutically effective than synthesized psilocybin, which is widely used in research investigating the medicinal potential of the ...