Gastroesophageal reflux disease: the case for improving patient education in primary care
Affiliation.
- 1 Advanced Gastroenterology Associates, St. Alexius Medical Center, Hoffman Estates, IL, USA. Email: [email protected].
- PMID: 24340333
Purpose: Gastroesophageal reflux disease (GERD) affects up to 25% of the western population, and the annual expenditure for managing GERD is estimated to be more than $14 billion. Most GERD patients do not consult a specialist, but rather rely on their primary care physician for symptom management. Research has shown that many patients--regardless of diagnosis--do not fully understand what their doctors tell them and remain uncertain as to what they are supposed to do to take care of themselves. To determine if patients are adequately educated in the management of GERD, we conducted a survey.
Method: We administered a survey to patients with GERD in an outpatient setting and explored their knowledge of such management practices as modification of behavior and diet and use of medication.
Results: Of 333 patients enrolled, 66% reported having an in-depth discussion with their primary care physician. Among patients taking a proton pump inhibitor, 85% of those who’d had an in-depth discussion were aware of the best time to take their medication, compared with only 18% of those who did not have an in-depth discussion. In addition, patients who’d had in-depth conversations were significantly more likely than those who didn’t to know some of the behavior modification measures that might improve their symptoms.
Conclusion: Our study underscores the need for primary care providers to fully discuss GERD with their patients to improve overall management of the disease.
- Aged, 80 and over
- Gastroesophageal Reflux / psychology
- Gastroesophageal Reflux / therapy*
- Health Care Surveys
- Health Knowledge, Attitudes, Practice*
- Middle Aged
- Patient Compliance*
- Patient Education as Topic*
- Physician-Patient Relations*
- Primary Health Care*
- Proton Pump Inhibitors / therapeutic use
- Risk Reduction Behavior
- Proton Pump Inhibitors


Introduction
Disclosure statement, clinical presentation of gastroesophageal reflux disease: a prospective study on symptom diversity and modification of questionnaire application.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Get Permissions
- Cite Icon Cite
- Search Site
Ryan Broderick , Karl-Hermann Fuchs , Wolfram Breithaupt , Gabor Varga , Thomas Schulz , Benjamin Babic , Arielle Lee , Frauke Musial , Santiago Horgan; Clinical Presentation of Gastroesophageal Reflux Disease: A Prospective Study on Symptom Diversity and Modification of Questionnaire Application. Dig Dis 13 May 2020; 38 (3): 188–195. https://doi.org/10.1159/000502796
Download citation file:
- Ris (Zotero)
- Reference Manager
Introduction: Symptoms occurring in gastroesophageal reflux disease (GERD) such as heartburn, regurgitation, thoracic pain, epigastric pain, respiratory symptoms, and others can show a broad overlap with symptoms from other foregut disorders. The goal of this study is the accurate assessment of symptom presentation in GERD. Methods: Patients with foregut symptoms were investigated for symptoms as well as endoscopy and gastrointestinal-functional studies for presence of GERD and symptom evaluation by standardized questionnaire. Questionnaire included a graded evaluation of foregut symptoms documenting severity and frequency of each symptom. The three types of questionnaires include study nurse solicitated, self-reported, and free-form self-reported by the patient. Results: For this analysis, 1,031 GERD patients (572 males and 459 females) were enrolled. Heartburn was the most frequently reported chief complaint, seen in 61% of patients. Heartburn and regurgitation are the most common (82.4/58.8%, respectively) in overall symptom prevalence. With regard to modification in questionnaire technique, if patients fill in responses without prompting, there is a trend toward more frequent documentation of respiratory symptoms (up to 54.5% [ p < 0.01]), fullness (up to 93.9%), and gas-related symptoms ( p < 0.001). Self-reported symptoms are more diverse (e.g., throat-burning [12%], mouth-burning [9%], globus [6%], dyspnea [9%], and fatigue [7%]). Conclusions: GERD symptoms are commonly heartburn and regurgitation, but overall symptom profile for patients may change depending on the type of questionnaire.
Since gastroesophageal reflux disease (GERD) has a prevalence of 20% in industrialized countries, symptoms associated with the disease are common in these populations [ 1, 2 ]. In order to define GERD, the authors of the Montreal classification relied heavily on symptoms and their effect on patients: “GERD is a condition which develops when the reflux of stomach contents causes troublesome symptoms and/or complications” [ 1 ]. These symptoms can reduce patient’s well-being and have a negative influence on the quality of life [ 3, 4 ].
In many studies, GERD symptoms are used to define the study populations [ 5‒13 ]. Other studies, however, have some evidence that symptoms are not always reliable as a guide to the diagnosis of GERD [ 14‒17 ]. GERD symptoms such as heartburn, regurgitation, thoracic pain, epigastric pain, respiratory symptoms, globus, and others show a broad overlap with symptoms from other esophageal and gastric disorders such as dyspepsia, esophageal motility disorders, functional heartburn, hypersensitive esophagus, irritable stomach and bowel, and somatoform disorders [ 1 , 14‒17 ]. The wide array of symptoms and potential diagnoses make one consider if there is a specific questioning technique or symptom profile that is more highly suggestive of GERD. Klauser et al. [ 18 ] have stated that heartburn and regurgitation are the most typical symptoms characterizing GERD, but in clinical practice, a large variety of esophageal and extra-esophageal symptoms can be reported.
Over the last 3 decades, our team had documented symptoms of GERD patients in a large data bank. Initially, the evaluations were standardized and leaned heavily on the early DeMeester symptom score and Gastrointestinal Quality of Life Index [ 19‒22 ]. Several years later, these questions were validated within the project of creating a symptom questionnaire featuring 53 items to determine somatoform tendencies [ 17 ]. With the exception of respiratory symptoms, all items in this current questionnaire differentiated significantly between healthy volunteers and patients with foregut symptoms [ 17 ].
The goals of this study are to determine the diversity and most common symptoms of GERD in large patient populations over time. Additionally, we aim to determine if the method of questioning is significant in altering the symptom profile of GERD patients.
Study Design
Over the course of more than 2 decades, our working group had the opportunity to investigate a large population of patients with GERD in a specialized center for benign esophageal and gastric disorders. All patients with foregut symptoms referred for further exploration of esophageal and/or gastric disease underwent a history and physical examination. The symptoms of the patients were evaluated by a standardized questionnaire over the complete time period from 1995 to 2017. Only the method of application for the questionnaires was changed over time, as described in detail below. All patients received an upper gastrointestinal endoscopy and esophageal manometry. In more recent years, a high-resolution manometry was performed [ 23 ]. The presence of pathologic reflux was evaluated by 24-h pH monitoring and later by impedance-pH monitoring.
Varying methods of questionnaire administration were used over the years in different time segments to evaluate the patient’s symptoms, as indicated below:
Group 1: (Study period 1995–1999) The study nurse used the standard questionnaire to ask the patients for the symptoms and marked the answers of the patients regarding presence and severity of the symptoms herself.
Group 2: (Study period 2005–2009) The study nurse handed the questionnaire over to the patients and the patients were left alone to fill in the presence and the severity of the symptoms. The patients could ask for assistance to the nurse, if needed.
Group 3a: (Study period 2015–2017) The study nurse handed the questionnaire over to the patients and the patients were left alone to fill in the presence and the severity of the symptoms in the document.
Group 3b: (Study period 2015–2017) Patients (same patients of Group 3a) were asked to document in a free-text version the 3 most important symptoms that limit or reduce the patient’s quality of life. Patients were instructed by the study nurse to document their most relevant symptoms as precisely as possible. Additionally, the study nurse also handed the standard questionnaire over to the patients and the patients were left alone to fill in the presence and the severity of the symptoms. It is important to notice that the free formulated description of the symptoms by the patients themselves was always conducted before the patients filled in the standardized questionnaire. This order was kept with the aim to avoid influences of the standard questionnaire to the patient formulated free text.
The groups were chosen for different time periods, in which changes of the symptom evaluation was established (solicited, self-reported, and free-form self-reported). The standard symptom questionnaire remained the same over the study duration.
Patient Selection and Inclusion/Exclusion Criteria
The patients were recruited in a tertiary referral center for foregut disorders and its diagnostic functional laboratory and surgery unit. The management of the patients was performed by the same team (same study nurse) over the complete period 1995–2017. The patients were asked to give informed consent to the study evaluation and the diagnostic work-up. The study was approved by our Institutional Review Board.
The data were reviewed in a prospectively maintained databank. Inclusion criteria for this analysis were patients with documented GERD, which required either the presence of esophagitis (esophagitis grading according to Savary-Miller 1–4), pathologic esophageal acid exposure on pH testing, and/or a hiatal hernia with heartburn and/or regurgitation. The hiatal hernia was documented during endoscopy by measuring the vertical extent of the distance between the cardia (beginning of the gastric folds) and the waist of the crurae, best assessed during inspiration (distance >1 cm). Care was taken to measure this length in the beginning of the endoscopy without major air insufflation of the stomach to avoid hernia reduction.
This analysis was not performed in some time periods (2000–2004 and 2010–2014), during which the documentation of symptoms was not rigorously followed due to shortage in personnel for administering the questionnaire. In addition, other exclusion criteria were if patients had other diseases such as cancer, inflammatory bowel disease, esophageal spasm, achalasia, or if they had prior operations for GERD.
The Questionnaire
For symptom evaluation, a standardized questionnaire was established and used over 25 years. The questionnaire included a graded evaluation of foregut symptoms: heartburn, regurgitation, retrosternal/thoracic pain, respiratory symptoms (cough/hoarseness), dysphagia, epigastric pain (pain/cramps/burning), nausea/vomiting, fullness (unpleasant fullness, early satiety), and gas-related symptoms (belching/bloating/flatulence). Patients had to document the severity and frequency of each symptom by grading according to the following system: 0 = no symptoms; 1 = symptom occurring rarely; 2 = symptom occurring occasionally; 3 = symptom occurring monthly and/or with mild intensity; 4 = symptom occurring weekly and/or with moderate intensity; 5 = symptoms occurring daily and/or with severe intensity.
Statistical Methods
Symptom results were analyzed according to their documented overall presence in these patients, independent of their severity, as well as by the most frequently reported significant/chief complaints. The mean intensity of the presented symptoms was analyzed. Statistical comparison with a t test for unpaired samples was used for the comparison of data from the different samples. A chi-square test was used for comparison of group data.
From 1995 to 2017, over 2,000 patients with symptoms indicative of GERD were seen by our team. Patients with other gastrointestinal diseases that could influence foregut symptoms were excluded from this study. In total, 1,031 met all inclusion criteria as GERD patients and were enrolled from 3 different time segments. Group 1 (1995–1999) included 481 patients, Group 2 (2005–2009) had 333 patients, and Group 3a/3b (2015–2017) had 217 patients. There were 572 males and 459 females. Table 1 demonstrates the characteristics of patients in the different groups. Presence of esophagitis, evidence of lower esophageal sphincter incompetence, esophageal acid exposure, and the level of quality of life showed severity of GERD among the patients in different groups over the years.
Patients’ characteristics for each group

Frequency of Chief Complaints and Overall Presence of Symptoms
Heartburn (retrosternal burning rising from the epigastrium to the chest) was the most frequent chief symptom (intensity: 5), independent of exam technique (Table 2 : Group 1: 60%; Group 2: 61%; Group 3a: 61.6%; Group 3b: 48.5%). Table 2 shows the frequency of chief complaints in the different groups. When the questionnaire is filled in by the study nurse (Group 1), the most common symptoms are heartburn and regurgitation (60%, 17%). Additionally in Group 1, other symptoms such as epigastric pain, dysphagia, or gas-related symptoms such as bloating, belching, and flatulence are not often experienced as the primary symptom (frequencies <15%). When comparing between groups, there are significant differences between the reported symptoms (Group 1 vs. Group 2/Group 3a). More often patients self-report respiratory symptoms (1.6% vs. 21.3%/20.2%; p < 0.001), epigastric pain (13.1% vs. 24.7%/12.1%), and gas-related problems (2.6% vs. 27.2%/22.0%; p < 0.01).
Overview on the percentage of documented symptoms with intensity 5 (chief complaint) differentiated for each group

Table 3 provides an overview on the overall presence of symptoms as evaluated in the various time periods. Heartburn and regurgitation are most frequent in Group 1 (82.4 and 58.8%, respectively). If patients fill in the questionnaire themselves, there are significant differences between groups in the presence of documentation of respiratory symptoms (Group 1: 11.8%; Group 2: 24.9%; Group 3a: 54.5%; p < 0.01), fullness (1: 11%; 2: 72.7%; 3: 93.9%; p < 0.001), and gas-related symptoms (1: 34%; 2: 72.7%; 3: 93.9%). These differences are even more pronounced in recent years.
Overview on the percentage of overall presence of documented symptoms differentiated for each group

Administration of Free-Text Form of Symptom Evaluation
When patients report their symptoms in their own words prior to completing the standard questionnaire (Group 3b), the documented variety of symptoms increases compared to the structured questionnaire alone (Table 4 ). In Group 3b, heartburn remains the most frequently reported symptom both as chief complaint (31%) and in the overall presence (48.5%). Reported symptoms are much more diversified: burning in the throat (12%), burning in the mouth (9%), globus (6%), headache (1%), dyspnea (9%), and fatigue (7%; Table 4 ).
Overview on percentage of symptoms in a free-text version self-assessed symptoms versus documentation in a self-assessed structured questionnaire

Intensity of Symptoms and Their Relation to Objective Functional Data
Data on the intensity of symptoms are summarized in Table 5 . The intensity of heartburn is highest in all groups (Group 1: 3.61; Group 2: 3.88; Group 3a: 3.39). The nurse documented the intensity of the symptoms such as regurgitation, retrosternal pain, epigastric pain, and respiratory symptoms higher (Group 1) than the patients themselves (Groups 2 and 3).
Overview on the mean intensity of symptoms differentiated for each group

The relationship between symptom intensity and the esophageal functional status show only for heartburn a significant rise in intensity for patients with and without lower esophageal sphincter-incompetence. These differences were for Group 1: 3.1 vs. 3.9; for Group 2: 3.2 vs. 3.9; for Group 3: 1.8 vs. 3.4 (all p < 0.005). The differences in symptom intensity are also significant for some comparisons with regurgitation; however, all other symptoms have no remarkable differences detected for changes in objective functional status.
We show that despite altering modality of questioning and symptom assessment in GERD patients, heartburn is the most frequently reported symptom. The severity and intensity of heartburn were documented to be the highest among all other symptoms through all years of investigation. The reported intensity of heartburn is significantly increased when the functional status of the antireflux barrier deteriorates. On the other hand, the presence/absence and intensity of other symptoms (e.g., regurgitation, respiratory symptoms, bloating) can depend on the concept and details of questioning. Allowing the patients to report free-form selection of symptoms shows a larger variety of documented chief complaints and other gas-related symptoms that may not be appreciated on standardized questionnaire.
Similar to our study, literature review shows that heartburn is reported to be present in patients with pathologic esophageal acid exposure in 72–99% [ 1 , 3 , 14 , 17 , 18 , 24‒28 ]. Regurgitation is another important symptom in GERD, with a prevalence of 33–86% [ 1, 14, 17, 29, 30 ]. According to some studies, epigastric pain is present in patients with foregut symptoms in 70% and in those with documented pathologic acid reflux in 12–67% [ 1, 3, 14, 17 ]. Our study confirms the importance of heartburn as the classic symptom with the highest intensity and the highest frequency as a chief complaint throughout the study. In Group 3b (free-text format), the symptom of heartburn was further delineated as “burning in the throat” or “burning in the mouth” in up to 14%.
Results of the present study show that the documented presence of symptoms can depend on the method of questioning (e.g., whether the symptoms are asked by a study nurse or if the patients are documenting without solicitation). The more the patient is free in her/his answering the questionnaire, symptom variability increases, especially with increased incidence of gas-related and atypical symptoms. The overall presence of heartburn remains independent of questionnaire administration around 80%. Notably, a statistically significant finding of respiratory symptom presence increases from 11 to 50% and the gas-related symptoms from 30 to 90% depending on questionnaire modality of application. All other symptoms have a much lower incidence in our GERD patients, and therefore, functional investigations are helpful to confirm the disease if esophagitis is absent.
There has been a controversial discussion about symptoms as a diagnostic tool for the presence of GERD, initiated by the Montreal definition [ 1 , 14 , 18‒20 ]. Our study confirms that there is a significant diversity of foregut symptoms present in GERD patients, as well as numerous extra-esophageal complaints such as cough, hoarseness, burning sensation in pharynx, mouth, and tongue in patients [ 1 , 14‒17 ]. Extra-esophageal symptoms can be respiratory symptoms such as chronic cough, hoarseness, and shortness of breath [ 31‒38 ]. There may also be symptoms at the level of the head and neck such as globus or burning in the mouth or throat. Recent studies show limitations of measuring acid reflux in the pharynx with current technology [ 37, 39, 40 ]. It remains difficult to correlate these symptoms with reflux episodes, even with objective testing.
We show that our validated questionnaire provides adequate assessment of patient symptoms. Allowing free-form reporting of symptoms in addition to a structured questionnaire may provide a more robust symptom profile in reflux disease. There is evidence in literature that structured questionnaires are very helpful and effective for symptom evaluation, and this is confirmed by our study [ 41‒46 ]. Several instruments have been published, validated, and successfully used in clinical practice [ 41‒46 ]. Various questionnaires published include the Patient Assessment of Upper Gastrointestinal Symptom Severity Index, the Gastrointestinal Rating Scale, the Chinese GERD Questionnaire, the GERD-Health Related Quality of Life Instrument, the Esophageal Symptoms Questionnaire, and the Reflux Disease Questionnaire [ 41‒43 , 47‒50 ]. A systematic review of all the available questionnaires for the assessment of GERD showed that many differ in design, validation, and translation [ 43 ]. One should be aware of the strength and shortcomings of each before selecting one for use [ 43 ]. All instruments have a self-assessment or self-administered mode of application, usually evaluating severity and/or frequency of GERD symptoms with a median of 15 items (6–30 items) [ 41‒43 , 47‒50 ]. The most useful instruments allowed for self-assessment by the patients [ 43 ]. However, none of these surveys allow for a free-text version of symptom documentation such as the one tested in this study.
When using the questionnaire over the years we noticed that many patients added remarks in the margin, indicating a possible lack of options or inadequate description. The unprompted free-form clarification of symptoms stimulated the impetus for providing patients more space to document symptoms in this way. None of the available validated questionnaires leaves room for the patient’s free text. Variations in patient symptoms such as burning in the mouth, tongue, and throat may be important features to document. In the past, one could only speculate that these symptoms were superficially classified as heartburn or odynophagia. Most of the available structured and validated questionnaires focus on heartburn, epigastric pain, fullness, bloating, regurgitation, and dysphagia. Therefore, it may be reasonable to add a free-text section to GERD questionnaires for detection of rare but important symptoms restricting the patient’s quality of life.
While expanding structured questionnaires to integrate all possible symptoms would be able to register all symptom variations, the more items to be answered lengthen and complicate the questionnaire process, potentially reducing applicability. Recently developed technologies allow patients to record symptoms in an electronic diary using a mobile electronic device. These technologies may be able to integrate self-administered and free text from evaluations to receive a more realistic and clinically valuable assessment.
Limitations of this study include the retrospective character of the analysis and the long duration of data sampling. Additionally, there were periods of time during the study period where documentation was not able to be rigorously completed due to shortage of nurses (2000–2004, 2010–2014), so data from these periods were excluded and sample size reduced as a result. Overall, the size of the patient data sampling performed by one team and one study nurse provides a dependable performance of data sampling and robust data for comparison of the changing techniques of administrating the assessment of GERD symptoms.
GERD remains a disease with a wide variety of symptoms experienced by patients. While heartburn and regurgitation remain mainstays of symptom reporting, there may be a range of symptoms and intensities of symptoms that go unreported if not elicited in a free-text format. The variety of symptoms experienced also shows the importance of a full correlating objective workup with esophago-gastro-duodenoscopy, high-resolution manometry, and impedance-pH testing to assist with accurate diagnosis of patients who may need surgical correction of their disease.
GERD symptoms are commonly heartburn, regurgitation, fullness, respiratory, and gas/bloat-related. The most important and frequent symptom is heartburn and its intensity parallels objective functional parameters of the esophagus. The overall symptom profile of patients may vary depending on the modality of questioning: practitioner directed, patient questionnaire, or free-form patient reporting of symptoms. Objective studies should be a key component in determining treatment for GERD due to the wide disparity in presenting symptoms.
The authors have no conflicts of interest to declare.
Email alerts
Citing articles via, suggested reading.
- Online ISSN 1421-9875
- Print ISSN 0257-2753
INFORMATION
- Contact & Support
- Information & Downloads
- Rights & Permissions
- Terms & Conditions
- Catalogue & Pricing
- Policies & Information
- People & Organization
- Stay Up-to-Date
- Regional Offices
- Community Voice
SERVICES FOR
- Researchers
- Healthcare Professionals
- Patients & Supporters
- Health Sciences Industry
- Medical Societies
- Agents & Booksellers
Karger International
- S. Karger AG
- P.O Box, CH-4009 Basel (Switzerland)
- Allschwilerstrasse 10, CH-4055 Basel
- Tel: +41 61 306 11 11
- Fax: +41 61 306 12 34
- Contact: Front Office
- Experience Blog
- Privacy Policy
- Terms of Use
This Feature Is Available To Subscribers Only
Sign In or Create an Account
We have a new app!
Take the Access library with you wherever you go—easy access to books, videos, images, podcasts, personalized features, and more.
Download the Access App here: iOS and Android . Learn more here!
- Remote Access
- Save figures into PowerPoint
- Download tables as PDFs

Chapter 34: Gastroesophageal Reflux Disease: A Burning Question Level II
Brian A. Hemstreet
- Download Chapter PDF
Disclaimer: These citations have been automatically generated based on the information we have and it may not be 100% accurate. Please consult the latest official manual style if you have any questions regarding the format accuracy.
Download citation file:
- Search Book
Jump to a Section
Learning objectives, patient presentation.
- CLINICAL PEARL
- Full Chapter
- Supplementary Content
Instructors can request access to the Casebook Instructor's Guide on AccessPharmacy. Email User Services ( [email protected] ) for more information.
After completing this case study, the reader should be able to:
Describe the clinical presentation of gastroesophageal reflux disease (GERD), including typical, atypical, and alarm symptoms.
Discuss appropriate diagnostic approaches for GERD, including when patients should be referred for further diagnostic evaluation.
Recommend appropriate nonpharmacologic and pharmacologic measures for treating GERD.
Develop a treatment plan for a patient with GERD, including both nonpharmacologic and pharmacologic measures and monitoring for efficacy and toxicity of selected drug regimens.
Outline a patient education plan for proper use of drug therapy for GERD.
Chief Complaint
“I’m having a lot of heartburn. These pills I have been using have helped a little but it’s still keeping me up at night.”
Janet Swigel is a 68-year-old woman who presents to the GI clinic with complaints of heartburn four to five times a week over the past 5 months. She also reports some regurgitation after meals that is often accompanied by an acidic taste in her mouth. She states that her symptoms are worse at night, particularly when she goes to bed. She finds that her heartburn worsens and she coughs a lot at night, which keeps her awake. She has had difficulty sleeping over this time period and feels fatigued during the day. She reports no difficulty swallowing food or liquids. She has tried OTC Prevacid 24HR once daily for the past 3 weeks. This has reduced the frequency of her symptoms to 3–4 days per week, but they are still bothering her.
Atrial fibrillation × 12 years
Asthma × 10 years
Type 2 DM × 5 years
HTN × 10 years
Patient is married with three children. She is a retired school bus driver. She drinks one to two glasses of wine 4–5 days per week. She does not use tobacco. She has commercial prescription drug insurance.
Father died of pneumonia at age 75; mother died at age 68 of gastric cancer
Diltiazem CD 120 mg PO once daily
Hydrochlorothiazide 25 mg PO once daily
Metformin 500 mg PO twice daily
Aspirin 81 mg PO daily
Fluticasone/salmeterol DPI 100 mcg/50 mcg one inhalation twice daily
Peanuts (hives)
Sign in or create a free Access profile below to access even more exclusive content.
With an Access profile, you can save and manage favorites from your personal dashboard, complete case quizzes, review Q&A, and take these feature on the go with our Access app.
Pop-up div Successfully Displayed
This div only appears when the trigger link is hovered over. Otherwise it is hidden from view.
Please Wait

- Nutrition counsellor
- Food Allergy
- gastroenterology
- Heptologist In Delhi
- Liver transplant
- Case Study on Gastroesophageal Reflux in Middle-Aged Woman
Patient: Female, 52
Final diagnosis: Gastroesophageal Reflux
Gastroesophageal Reflux signs and Symptoms: burning pain in the chest that usually occurs after eating and worsens when lying down.
Speciality: Gastroenterology
Causes, symptoms, and treatment of Gastroesophageal Reflux
Gastroesophageal reflux disease (GERD) affects as many middle-aged women as males. It might manifest as heartburn, regurgitation, dysphagia, or chest discomfort. We examined the severity of symptoms in women is significantly more than in men and may contribute to earlier disease recognition and different disease management.
Gastroesophageal Reflux Case Study
A 52-year-old woman was referred to gastroenterology practice for a history of gastroesophageal reflux disease. The patient claims to have had heartburn symptoms for at least five years. Her symptoms responded to over-the-counter medications such as antacid tablets and liquids, but they grew so frequent that she sought medical attention from her primary care physician.
Later, she reported minor acid reflux at least twice a week. She does not have any other chronic medical issues and does not use any other drugs. Her social background includes severe alcohol usage for 20 years, which she discontinued after being diagnosed with liver illness four years ago. There is no family history of gastrointestinal cancer in her family.
Gastroesophageal Reflux Causes
Gastroesophageal reflux disease, often known as GERD, is a digestive illness that affects the muscular ring between your oesophagus and stomach. The lower esophageal sphincter is the term given to this ring (LES). You may have heartburn or acid indigestion if you have it. Doctors believe that some people develop it as a result of a disease known as hiatal hernia. In most situations, GERD symptoms can be alleviated via dietary and lifestyle modifications. However, some people may require taking medication or going under surgery.
Dysphagia, nausea or vomiting, blood in her stool, or accidental weight loss were all symptoms she encountered. Most people can control their GERD symptoms with simple lifestyle modifications and over-the-counter medicines. However, she may require more potent medication or surgery to cure her problems. Because the patient has a history of alcoholism, she is particularly sensitive to this condition.
Gastroesophageal Reflux Age Range
We observed that the usual age group with GERD symptoms is primarily middle-aged women, i.e. (age range 36 to 55).
Gastroesophageal Reflux symptoms
Heartburn is the most common symptom of GERD. (GERD) occurs when stomach acid regularly rushes back into the tube that links your mouth and stomach (oesophagus). Acid reflux (backwash) can irritate the esophageal lining. Many people experience acid reflux on a regular basis.
Common signs and symptoms of GERD include:
- A burning sensation in your chest (heartburn), usually after eating, which could be worse during the night
- Difficulty swallowing
- Regurgitation of food or sour liquid
- Throat lump sensation
Night-time acid reflux, one might also experience:
- Chronic cough
- New or worsening asthma
- Disrupted sleep
Gastroesophageal reflux risk factors include:
- Bulging the top of the stomach up into the diaphragm
- Connective tissue disorders, such as scleroderma
- Delayed stomach emptying
Gastroesophageal Reflux Symptoms and Treatment
Lying down is one of the home treatments for GERD. According to most researchers, the optimal height of bed head elevation is at least 6-8 inches (15-20 centimetres). This height has been shown in studies to reduce acid reflux when lying down. In reality, the higher the height, the better.
Gastroesophageal reflux management
GERD therapy aims to reduce the quantity of reflux or decrease the damage to the oesophagus lining affected by the refluxed materials.
Over the counter or prescription, medicine recommendations work to address Gastroesophageal reflux causes and symptoms.
- Antacids: These medications can help neutralize the acid in the oesophagus and stomach, therefore alleviating heartburn. Non-prescription antacids give brief or partial relief for many people. Some people benefit from an antacid coupled with a foamy agent. These chemicals, according to researchers, form a foam barrier on top of the stomach, preventing acid reflux.
- H2 blockers: For persistent reflux and heartburn, the doctor may prescribe medicines to decrease stomach acid. H2 blockers, which assist in inhibiting acid production in the stomach, are among these medications. Cimetidine (Tagamet), famotidine (Pepcid), and nizatidine are models of H2 blockers.
- Proton pump inhibitors (PPIs): Often known as acid pumps, medications that reduce stomach acid production by blocking a protein. Dexlansoprazole (Dexilant), esomeprazole (Nexium), lansoprazole (Prevacid), omeprazole (Prilosec), omeprazole/sodium bicarbonate (Zegerid), pantoprazole (Protonix), and rabeprazole are all proton pump inhibitors (Aciphex).
- Prokinetics: In rare circumstances, these medicines assist your stomach empty faster, resulting in less acid being left behind. They may also aid in the treatment of symptoms such as bloating, nausea, and vomiting. Domperidone and metoclopramide are two samples of prokinetics (Clopra, Maxolon, Metozolv, Reglan). Many individuals are unable to take them, and those who can only do so for a short time.
In this case, she was initially given an H2 blocker, which proved ineffective, so she was put on proton pump inhibitor treatment for a while. She presently takes 20mg of omeprazole daily, which she finds beneficial, although she does have heartburn if she skips a dosage. The patient can now comfortably swallow meals and liquids and shows no indications of vomiting or nausea. The patient is constantly monitored and encouraged to make certain dietary and lifestyle modifications. She is recommended to abstain totally from alcohol and cigarettes.
Routine check-ups and proper treatments can help patients with Gastroesophageal Reflux cure. Suppose you are experiencing any symptoms mentioned above. In that case, you can follow the Gastroesophageal reflux care plan by getting in touch with the online gastroenterologist doctor . You can talk to the gastroenterologist and consult gastroenterologist online free. Services are available in different cities, consult her as a gastroenterologist, the best doctor in Patna for the stomach , best female gynaecologist in Jhansi , gastro surgeon in Delhi , liver cirrhosis specialist doctor in India, NCR gastro liver clinic Gurgaon, max hospital liver specialist, the gastro & liver clinic Patna Bihar and best physician in Jammu city .
1. Can GERD affect my heart?
GERD and the associated heartburn have nothing to do with heart or heart disease even though the burning chest in pain seems like a pain in the heart.
2. Are there symptoms other than heartburn for GERD?
Other symptoms include regurgitation of acid up in the throat, bitter taste in the mouth, persistent dry cough, and wheezing among others.
3. Why doesn’t the acid harm the stomach?
The stomach has a thick lining that protects it from damage by acid. The oesophagus does not have this lining.
Privacy Preference Center
Privacy preferences, nutrition counselling.
Nutrition counseling is the assessment of an individual’s dietary intake after which, they are helped set achievable goals and taught various ways of maintaining these goals. The nutrition counselor provides information, educational materials, support and follow-up care to help an individual make and maintain the needed dietary changes for problems like obesity.
Obesity/ Food allergy
I assist people dealing with weight-related health problems by evaluating the health risks and help in obesity management. I also help patients manage various food allergies.
As a hepatologist, I specialize in the treatment of liver disorders, pancreas, gallbladder, hepatitis C, jaundice and the biliary tree. I also see patients suffering from pancreatitis, liver cancers alcoholic cirrhosis and drug induced liver disease(DILI), which has affected the liver.
Gastroenterology
As a gastroenterologist, my primary focus is the overall health of the digestive system. I treat everything from acid reflux to ulcers, IBS, IBD: Crohns disease and ulcerative colitis, and colon cancer.
Endoscopy is a nonsurgical procedure to examine a person’s digestive tract. It is carried out with an endoscope, a flexible tube with a light and camera attached to it so that the doctor can see pictures of the digestive tract on a color TV monitor.
Medical Gastroenterology
Gastroenterology is a specialty that evaluates the entire alimentary tract from the mouth to anus and involves studying the diseases of the pancreas.
Liver Transplant Services
A liver transplant is a surgical procedure that removes a patient’s non-functioning liver and replaces it with a healthy liver from a deceased donor or a portion of healthy liver from a living donor. It is reserved as a treatment option for people who have significant complications due to end-stage chronic liver disease or in case of sudden failure of a previously healthy liver.
- Case Report
- Open access
- Published: 13 April 2016
A case of advanced systemic sclerosis with severe GERD successfully treated with acotiamide
- Ryo Kato 1 ,
- Kiyokazu Nakajima 1 , 2 ,
- Tsuyoshi Takahashi 1 ,
- Yasuhiro Miyazaki 1 ,
- Tomoki Makino 1 ,
- Yukinori Kurokawa 1 ,
- Makoto Yamasaki 1 ,
- Shuji Takiguchi 1 ,
- Masaki Mori 1 &
- Yuichiro Doki 1
Surgical Case Reports volume 2 , Article number: 36 ( 2016 ) Cite this article
4017 Accesses
5 Citations
1 Altmetric
Metrics details
The majority of systemic sclerosis (SSc) patients have gastrointestinal tract involvement, but therapies of prokinetic agents are usually unsatisfactory. Patients are often compromised by the use of steroid; therefore, a surgical indication including fundoplication has been controversial. There is no report that advanced SSc with severe gastroesophageal reflux disease (GERD) is successfully treated with acotiamide, which is the acetylcholinesterase (AChE) inhibitor designed for functional dyspepsia (FD). We report a 44-year-old woman of SSc with severe GERD successfully treated with acotiamide. She had received medical treatment in our hospital since 2003. She had been aware of the significant gastroesophageal reflux symptoms (e.g., heartburn, chest pain, and dysphagia) due to the development of esophageal hardening associated with SSc since 2014. As a result of upper gastrointestinal series, upper gastrointestinal endoscopy, and 24-h pH monitoring and frequency scale for the symptoms of the GERD (FSSG) scoring, she has been diagnosed with GERD associated with SSc. First of all, she started to take prokinetic agents Rikkunshito and mosapride and proton pump inhibitor; there was no change in reflux symptoms. So, we started to prescribe her the acotiamide.
After oral administration started, reflux symptoms have been improved. Five months after oral administration, FSSG score, a questionnaire for evaluation of the symptoms of GERD, was improved. Since its introduction of acotiamide, the patient has kept free from symptoms for 6 months.
Systemic sclerosis (SSc) is a multisystem and chronic disease characterized by abnormalities of small blood vessels and fibrosis of the skin and internal organs. SSc, when advanced, is often compromised with severe gastroesophageal reflux disease (GERD), which may be lethal in a worst-case scenario. A wide variety of medication has been used [ 1 – 5 ]; however, none of them are promising for patients with SSc. In addition, patients with SSc are often compromised by the use of steroid; therefore, a surgical indication including fundoplication has been controversial.
In this short communication, we describe our recent case of advanced SSc patients with severe GERD, who was successfully treated with a new drug originally designed for functional dyspepsia (FD).
Case presentation
A 44-year-old woman of SSc had received medical treatment in our hospital since 2003. She had been aware of the significant gastroesophageal reflux symptoms and esophagus stasis due to the development of esophageal hardening associated with SSc since 2014. On physical examination, cachexia, a “mouse face” appearance and ulceration in the distal phalanges were identified. The abnormal build-up of fibrous tissue in the skin can cause the skin to tighten so severely that her fingers curl and lose their mobility (Fig. 1 ). Because she had been aware of the worsening of gastroesophageal reflux symptoms, she received a medical examination from this department. Upper gastrointestinal series revealed no expansion and meandering esophagus, and reflux into the esophagus in the Trendelenburg position (Fig. 2 ). The upper gastrointestinal endoscopy showed reflux esophagitis of Los Angeles classification grade C and esophagus residue (Fig. 3 ).
The abnormal build-up of fibrous tissue in the skin can cause the skin to tighten so severely that fingers curl and lose their mobility in SSc
Upper gastrointestinal series revealed no expansion and meandering esophagus and reflux into the esophagus in the Trendelenburg position
The upper gastrointestinal endoscopy showed reflux esophagitis of Los Angeles classification grade C and esophagus residue
A 24-h esophageal pH monitoring revealed significant acid reflux: the number of refluxes was 81 times, pH was below 4.0 for 32.1 %, mean pH was 4.55, and DeMeester score (normal <14.75) was 117.5 (Fig. 4 ). Symptoms of gastroesophageal reflux disease (frequency scale for the symptoms of the GERD (FSSG)) score, a questionnaire evaluating the symptoms of GERD, was 34 points [ 6 ] (maximum 48 points). As a result of these tests, she has been diagnosed with GERD associated with SSc. Treatment with Rikkunshito and mosapride, which are prokinetic agents, and proton pump inhibitor was started. However, her symptoms were not improved. Therefore, we started the acotiamide on June 2015, which was a new drug originally designed for FD. Since then, her symptoms which were heartburn, burp, and nausea after a meal were improved. Five months after acotiamide was started, the FSSG score was reduced to 21 points (Fig. 5 ). However, the results of 24-h esophageal pH monitoring showed worsening acid reflux: the number of refluxes was 152 times, pH was below 4.0 for 60.5 %, mean pH was 3.73, and DeMeester score (normal <14.75) was 211.6 (Fig. 4 ). The upper gastrointestinal series and upper gastrointestinal endoscopy did not change.
A 24-h pH monitoring was performed before and after acotiamide oral administration. These four items showed worsening before and after the administration
After oral administration of acotiamide, FSSG score was improved from 34 points to 21 points
Since the introduction of acotiamide, the patient has been free from symptoms to date.
In 1994, Sjogren proposed a progression of SSc with gastrointestinal involvement, vascular damage, neurogenic impairment, and myogenic dysfunction with the replacement of normal smooth muscle by collagens fibrosis and atrophy [ 7 ]. It is distinguished in diffuse cutaneous SSc (dcSSc) or limited cutaneous SSc (lcSSc) whether skin hardening exceeds an elbow or a knee [ 8 ]. Next to the skin, the gastrointestinal tract is the second most common site of SSc organ damage that can affect patients with lcSSc and dcSSc [ 9 ]. It affects the gastrointestinal tract in more than 80 % of patients. Reflux esophagitis is found in 50–90 % of SSc patients [ 7 , 10 ].
The common characteristics of GERD seen in SSc patients are as follows. The upper GI series often shows peristaltic decrease and expansion of the lower esophagus. The upper gastrointestinal endoscopy shows linear redness and erosion of the lower esophagus caused by the reflux, which often merges with the Barett esophagus. In addition, the merger frequency of esophageal cancer is often in SSc. Esophageal dysmotility leads to impaired acid clearance, and 24-h monitoring shows prolongation of esophageal exposure time to gastric acid [ 11 ].
Its treatment, either medical or surgical, has been still challenging. The major medical treatment option includes use of histamine-2 receptor antagonist (H2RA) and/or proton pomp inhibitor (PPI) for the purpose of controlling stimulation and inflammation of the esophageal mucosa by the gastric acid reflux [ 12 – 14 ]. Surgery, e.g., Nissen fundoplication, can be considered for drug-resistant reflux disease [ 15 ]. These medical/surgical treatments have been shown not as promising as those for reflux patients without SSc [ 16 ].
The last option for SSc patients with severe GERD is a group of prokinetic drugs. A wide variety of prokinetic agents have been used, such as mosapride citrate, metoclopramide, domperidone, erythromycin, octreotide, and dinoprost [ 1 – 5 ]. However, therapies of traditional prokinetic agents are usually unsatisfactory for severe GERD patients.
Acotiamide is the novel prokinetic agent basically designed for FD; it is the acetylcholinesterase (AChE) inhibitor. FD is a chronic disorder of sensation and movement (peristalsis) in the upper gastrointestinal tract. The acetylcholine (Ach) is released from cholinergic nerve terminals and lets the gastrointestinal tract shrink by binding to the muscarinic receptor of the gastrointestinal smooth muscle. It is thought that the Ach is broken down immediately by AChE and enterokinesis is regulated by this reaction. Acotiamide inhibits AChE and regulates the resolution of Ach. As a result, it increases the quantity of ACh available in the synaptic cleft and therefore improves enterokinesis [ 17 ].
We have prescribed a variety of traditional prokinetic agents, without obtaining even temporary relief of her symptoms. Therefore, we prescribed acotiamide. We performed upper gastrointestinal series, gastrointestinal endoscopy, 24-h pH monitoring, and the quality of life (QOL) scores for the assessment of gastroesophageal reflux before and after oral administration of acotiamide on this patient. No changes were observed before and after treatment in the upper GI series and upper gastrointestinal endoscopy. Acotiamide does not show the emission promoting effect on the normal gastric emptying in rats. However, prior research reports that acotiamide does improve the gastric emptying during restraint stress. Acotiamide might have led to symptom improvement due to the suppressed response to stress by decreasing the expression of NmU, a stress-related gene in the hypothalamus, via the vagus nerve [ 18 ].We think this may be a partial reason why our patient showed improvement of her GI symptoms.
In the 24-h pH monitoring, DeMeester score even showed a worsening from 117 to 211. However, FSSG, which is one of the established QOL scoring system, showed improvement from total 34 to 21 points. FSSG is a questionnaire composed of 12 questions and classified into two groups, which are five items related to “dysmotility” symptoms and seven items to “acid reflux” symptoms. In our case, the score related to both symptoms were improved from 17 to 10 points and 17 points to 11 points, respectively. As we describe later, these improvements can be explained by the pharmacological effects of acotiamide on both the gastric and the esophageal functions.
In fact, this patient was clearly aware of the improvement of clinical symptoms, and QOL has been improved.
In our case, traditional prokinetic agents attempted prior to acotiamide were not effective. Only acotiamide showed substantial relief of the patient’s symptom. We speculated this might be because of these two factors: (1) pharmacologically, acotiamide may not only affect the gastric emptying but also improve fundic accommodation of adaptive relaxation, and (2) acotiamide may directly act on the esophageal body and improve esophageal peristalsis. The authors have reached the above speculation based on the results of FSSG score: improvements of early satiety and chest discomfort. The question score “Do you feel full while eating meals?” was improved from 4 points to 2 points and “Do some things get stuck when you swallow?” was improved from 3 points to 2 points, respectively.
We just experienced one case; therefore, further accumulation of similar cases is definitely required. In our case, no objective improvement was observed on classical 24-h pH monitoring. The authors believe this examination might not be appropriate as an evaluation tool of GERD in patients with severe esophageal motor dysfunction like advanced SSc patients. Novais et al. reported that the 24-h abnormal pH tracings were classified into three types: (i) 24-h abnormal pH, with a true GERD pattern, i.e., sharp sudden pH drops, reaching values below 3 and then returning to usual esophageal pH (pH 6–7) (Fig. 6 a); (ii) 24-h abnormal pH with a pattern suggesting esophageal fermentation due to retained food, i.e., steady drop of pH not reaching values below 3.0 (Fig. 6 b); and (iii) negative 24-h pH, i.e., presence of physiological reflux (reflux episodes occurring in less than 4.5 % of total examining time) or zero reflux (absence of any episode of pH lower than 4.0) [ 19 ]. In a 24-h pH monitoring after acotiamide was started, there were many frequent waveforms of (ii) than those of (i). The esophageal food fermentation may have affected the results of this case. The QOL score, including FSSG is likely to accurately reflect the symptoms of patients than 24-h pH monitoring. Future tasks are to perform a detailed study by using a new method of measuring such as high resolution manometry.
A 24-h pH tracing of this patient. True gastroesophageal reflux ( a ) and fermentation ( b )
Conclusions
We have experienced a case of advanced SSc with severe GERD successfully treated with acotiamide. Acotiamide might become a help of advanced SSc with severe GERD patient whose surgical indication has been controversial.
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Abbreviations
acetylcholine
acetylcholinesterase
functional dyspepsia
frequency scale for the symptoms of the GERD
gastroesophageal reflux disease
- systemic sclerosis
Johnson DA, Drane WE, Curran J, Benjamin SB, Chobanian SJ, et al. Metoclopramide response in patients with progressive systemic sclerosis. Effect on esophageal and gastric motility abnormalities. Arch Intern Med. 1987;147:1597–1601.1.
Article CAS PubMed Google Scholar
Smout AJ, Bogaard JW, Grade AC, ten Thije OJ, Akkermans LM, et al. Effects of cisapride, a new gastrointestinal prokinetic substance, on interdigestive and postprandial motor activity of the distal oesophagus in man. Gut. 1985;26:246–51.
Article CAS PubMed PubMed Central Google Scholar
Soudah HC, Hasler WL, Owyang C, et al. Effect of octreotide on intestinal motility and bacterial overgrowth in scleroderma. N Engl J Med. 1991;325:1461–7.
Dull JS, Raufman JP, Zakai MD, Strashun A, Straus EW, et al. Successful treatment of gastroparesis with erythromycin in a patient with progressive systemic sclerosis. Am J Med. 1990;89:528–30.
Tomomasa T, Kuroume T, Arai H, Wakabayashi K, Itoh Z, et al. Erythromycin induces migrating motor complex in human gastrointestinal tract. Dig Dis Sci. 1986;31:157–61.
Kusano M, Shimoyama Y, Sugimoto S, et al. Development and evaluation of FSSG: frequency scale for the symptoms of GERD. J Gastroenterol. 2004;39:888–91.
Article PubMed Google Scholar
Sjögren RW. Gastrointestinal motility disorders in scleroderma. Arthritis Rheum. 1994;37:1265–82.
LeRoy EC, Black C, Fleischmajer R. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988;15(2):202–5.
CAS PubMed Google Scholar
Clements PJ, Becvar R, Drosos AA, Ghattas L, Gabrielli A. Assessment of gastrointestinal involvement. Clin Exp Rheumatol. 2003;21:S15–8.
Young MA, Rose S, Reynolds JC. Gastrointestinal manifestations of scleroderma. Rheum Dis Clin North Am. 1996;22(4):797–823.
Tasleem A, Qazi M, Jaswinder S, et al. Assessment of esophageal involvement in systemic sclerosis and morphea (localized scleroderma) by clinical, endoscopic, manometric and pH metric features: a prospective comparative hospital based study. BMC Gastroenterol. 2015;15:24.
Petrokubi RJ, Jeffries GH. Cimtidine versus an acid in scleroderma with reflux esophagitis: a randomized double-blind controlled study. Gastroenterology. 1979;77:691–5.
Hendel L, Aggestrup S, Stentoft P. Long-term ranitidine in progressive systemic sclerosis (scleroderma) with gastroesophageal reflux. Scan J Gastroenterol. 1986;21:799–805.
Article CAS Google Scholar
Wigley FM, Sule SD. Novel therapy in the treatment of scleroderma. Expert Opin Investig Drugs. 2001;10:31–48.
Cicala M, Emerenziani S, Guarino MP, Ribolsi M. Proton pump inhibitor resistance, the real challenge in gastro-esophageal reflux disease. World J Gastroenterol. 2013;19(39):6529–35.
Article PubMed PubMed Central Google Scholar
Carlson DA, Hinchcliff M, Pandolfino JE. Advances in the evaluation and management of esophageal disease of systemic sclerosis. Curr Rheumatol Rep. 2015;17(1):475.
Kawachi M, Matsunaga Y, Tanaka T. Acotiamide hydrochloride (Z-338) enhances gastric motility and emptying by inhibiting acetylcholinesterase activity in rats. Eur J Pharmacol. 2011;666:218–25.
Seto K et al. Acotiamide, hydrocholoride (Z-338), a novel prokinetics agent, restores delayed gastric emptying and feeding inhibition induced by restraint stress in rats. Neurogastroenterol Motil. 2008;20(9):1051–9.
Novais PA, Lemme EMO. 24-h pH monitoring patterns and clinical response after achalasia treatment with pneumatic dilation or laparoscopic Heller myotomy. Aliment Pharmacol Ther. 2010;32:1257–65.
Download references
Author information
Authors and affiliations.
Department of Gastroenterological Surgery, Graduate School of Medicine, Osaka University, 2-2, E-2, Yamadaoka, Suita, Osaka, 565-0871, Japan
Ryo Kato, Kiyokazu Nakajima, Tsuyoshi Takahashi, Yasuhiro Miyazaki, Tomoki Makino, Yukinori Kurokawa, Makoto Yamasaki, Shuji Takiguchi, Masaki Mori & Yuichiro Doki
Division of Next Generation Endoscopic Intervention (Project ENGINE), Global Center for Medical Engineering and Informatics, Center of Medical Innovation and Translational Research, Osaka University, 2-2, Yamadaoka, Suita, Osaka, 565-0871, Japan
Kiyokazu Nakajima
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Kiyokazu Nakajima .
Additional information
Competing interests.
The authors declare that they have no competing interests.
Authors’ contributions
All authors participated in the management of the patient in this case report. KN is a chief surgeon of our hospital and supervised the case and also supervised the writing of the manuscript. YD is a chairperson of our department and supervised the entire process. All authors read and approved the final manuscript.
Ryo Kato is the first author.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Reprints and permissions
About this article
Cite this article.
Kato, R., Nakajima, K., Takahashi, T. et al. A case of advanced systemic sclerosis with severe GERD successfully treated with acotiamide. surg case rep 2 , 36 (2016). https://doi.org/10.1186/s40792-016-0162-5
Download citation
Received : 14 January 2016
Accepted : 06 April 2016
Published : 13 April 2016
DOI : https://doi.org/10.1186/s40792-016-0162-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- gastroesophageal reflux disease (GERD)
- functional dyspepsia (FD)
- frequency scale for the symptoms of the GERD (FSSG)
- Cell Biology
Case Study: Gastroesophageal Reflux Disease

Related documents

Add this document to collection(s)
You can add this document to your study collection(s)
Add this document to saved
You can add this document to your saved list
Suggest us how to improve StudyLib
(For complaints, use another form )
Input it if you want to receive answer
Gerd Case Study and Pharmacotherapy
This document presents a case study of a 60-year-old male patient admitted to the hospital with abdominal discomfort for 10 days and a history of bronchial asthma and GERD. Examination findings and investigation reports are provided. The patient is assessed and diagnosed with bronchial asthma and GERD. A drug chart outlines the treatment plan and discharge summary is presented advising the patient to continue medications and make lifestyle modifications. The case study concludes with a discussion of monitoring parameters, pharmacist interventions, and patient counseling on drug therapy and disease and lifestyle management. Read less

Recommended
More related content, what's hot, what's hot ( 20 ), similar to gerd case study and pharmacotherapy, similar to gerd case study and pharmacotherapy ( 20 ), recently uploaded, recently uploaded ( 20 ).
- 1. PHARMACOTHERAPEUTICS-III CASE PRESENTATION NANDHA COLLEGE OF PHARMACY RAJNANDINI SINGHA IV Pharm D
- 2. CASE STUDY ON GERD
- 3. SUBJECTIVE A 60 year old Male patient was admitted in Perundarai medical college hospital on 25/1/2018 with the complaints of abdominal discomfort for 10 days.
- 4. HISTORY OF PRESENT ILLNESS H/O Abdominal discomfort H/O Difficulty in breathing H/O skin allergy H/O cough with expectoration
- 5. PAST HISTORY K/C bronchial asthma before 3 days
- 6. PERSONAL HISTORY Diet: Mixed.
- 7. GENERAL EXAMINATION Patient conscious, oriented BP :160/70 mmHg PR :79 bpm
- 8. SYSTEMIC EXAMINATION CVS – S1S2 Heard RS - B/L AE+,B/L WHEEZ+ CNS – NFND P/A - Soft
- 9. OBJECTIVE INVESTIGATION CHART NAME OF INVESTIGATION OBSERVED VALUE NORMAL VALUE WBC 14.0x109/L 4.5-10.5×109/L RBC 4.26x1012/L 3.8-5.9×1012/L HAEMOGLOBIN 10.5g/dl 12-14g/dl PLATELETS 173.0109/L 130-400109/L L/M/G 2.5/1.5/11.0109/L MCV 92.9 FL 80-100FL HCT 40.2% 35-50% MCH 27.6pg 27- 34pg
- 10. MCHC 29.7g/dl 32-36g/dl ESR 32mm/hr 0-20mm/hr BIOCHEMISTRY RBS 67 mg/dl Up to 140 mg/dl BLOOD UREA 30 mg/dl 10-40 mg/dl SERUM CREATININE 1.0mg/dl 0.6-1.3 mg/dl SERUM PHOSPHATE 3.5 mg/dl 2.5-4.5 mg/dl URINE ANALYSIS COLOUR PALE YELLOW REACTION Acidic ALBUMIN NIL
- 11. PROGRESS CHART DATE TEMP (°F) B.P P.R R.R 25/1/18 98.4 110/80 80 20 26/1/18 98.4 110/80 75 22 27/1/18 98.8 100/80 84 20 28/1/18 98.6 140/60 82 20 29/1/18 98.4 110/80 78 22 30/1/18 99 140/80 82 20 31/1/18 98.5 120/80 80 20 1/1/18 98.7 140/80 85 22 2/1/18 98.4 120/80 82 22
- 12. OTHER INVESTIGATION X-RAY –Increased BVM (bronchovascular marking)
- 14. ASSESMENT FINAL DIAGNOSIS: Bronchial Asthma GERD( Gastroesophageal reflux disease)
- 15. DRUG CHART DRUG GENERIC NAME DOSE ROUTE FREQ 3 4 5 6 7 Inj.genta Gentamycin 1mg IV 1-0-1 √ √ √ √ √ INJ .RANTAC Ranitidine 2ml IM 1-0-0 √ √ √ √ √ T.CPM Chlorphenaramin e 10mg PO 1-0-1 √ √ √ √ √ T. Dolo Paracetamol 650mg PO 1-1-1 √ √ √ √ √ Inj. Deri Theophylline+Eto phylline 2ml IV 1-0-1 √ √ √ √ √ T.celin Vitamin C 40mg PO 1-0-0 √ √ √ √ √ SYRUP. Rantac ranitidine 15mg PO 1-0-1 √ √ √ √ √ Liquid paraffin Liquid paraffin 10ml topical 0-0-1 √ √ √ √ √
- 16. DRUG GENERIC NAME DOSE ROUTE FREQ 3 4 5 6 7 BVM OINTMENT Betamethasone valarate 0.1% 1-1-1 topical √ √ √ √ √ SYRUP. REXCOF Dextromethophan and chlorpheniramine maleate 100ml 1-0-1 PO √ √ SALBUTAMOL NEB SUBUTAMOL 1-1-1 NASAL √ √ √ √ √
- 17. DISCHARGE SUMMARY The patient was discharged on 7/02/18 DISCHARGE ADVICE T.PARACETAMOL(1-1-1) 650mg T.CPM(1-0-1)10mg SYRUP.RANTAC(1-1-1)10ml T.CELIN(VITAMIN C) (1-0-0)500mg Liquid paraffin(1-0-1) BVM OINTMENT(1-0-1)
- 19. PATIENT COUNSELLING: DRUG RELATED DISEASE RELATED LIFE STYLE MODIFICATION •Take SYRUP Rantac before 30mins of food •LIQUIID PARAFFIN and BVM OINTMENT should be apply on dry skin. • Advice the patient not to skip meals. •Avoid stress and NSAIDS • Elevate the head on the bed. COPD: On coughing mouth should be closed. Avoid dust, allergen, alcohol, smoking. •Avoid choclate, coffee, soda, alcoholic drinks. • Avoid Citrus fruits and juices of tomato. •Advice the patient to eat boil food for 2-3 weeks. •Green vegetables should be taken to regenerate the lining of stomach.
- 20. S.N O ASSESMENT PLAN 1. Chlorpheniramine + food Alcohol can increase the CNS Side effect such as drowsiness, dizziness .. MANAGEMENT: To avoid consumption of alcohol , hazardous activities require complete alterness ,mental alertness. Syrup Ranitidine should administered 30mins before food. 3. T.CPM AND SYRUP .Rexcof Side effect - sleepiness Do not prescribe in the morning
- 21. MONITORING PARAMTER: pH Monitoring for GERD Increased acid exposure time.
- 22. PHARMACIST INTERVENTION: Antacid should be recommended in the prescription. Suggesting to Reduce the adminstration of PARACETAMOL to reduce toxicity. Proton pump inhibitor is recommended to be add in the prescription.
- 23. THANK YOU

Case Studies
CR, a 44-year-old man, comes to the pharmacy looking for a remedy for his heartburn. He reports that his heartburn has been bothering him for the past few weeks, and he complains of an acidic taste in his mouth and a burning feeling in his throat about twice a week. CR does not complain of any other related symptoms, such as pain when swallowing. CR has a box of omeprazole (Prilosec) in his hand. He asks if it would be the best product to help alleviate his symptoms.
As the pharmacist, how would you respond?
EF is a 30-year-old woman who comes to the pharmacy with dry, demarcated lesions in linear streaks, with some vesicles, on her hands, arms, and face. She says she was gardening yesterday for a few hours and must have touched poison ivy. EF says she tried to hide it with makeup to go to work this morning, but it only made it worse. She exclaims, “I cannot stand the itching anymore.” Upon questioning, you find out that she has had similar lesions before, but they were less extensive and not as bothersome. EF asks if there is pharmacy product that could help. She has no significant medical history and is not taking any prescription or OTC medications.
As the pharmacist, what would you recommend?
Case 1: Based on his reported symptoms, CR likely suffers from mild/ episodic gastroesophageal reflux disease (GERD), so he is a candidate for self-treatment. OTC proton pump inhibitors (PPIs) such as omeprazole, lansoprazole, and esomeprazole are appropriate for self-treatment of GERD for up to 14 days. However, before you recommend these products, you should educate CR that OTC omeprazole, lansoprazole, and esomeprazole are not intended for immediate relief of heartburn. These drugs have a slow onset but a long duration of action, and CR may have to take one of these drugs for 1 to 4 days before he feels better. CR should be cautioned to speak to his doctor if his symptoms do not resolve after 2 weeks or his heartburn worsens.
Alternatively, CR could try a histamine2 (H2)-receptor antagonist such as ranitidine, cimetidine, famotidine, or nizatidine. H2-receptor antagonists have a different mechanism of action than PPIs and provide relief of heartburn more quickly than PPIs. H2-receptor antagonists can be taken prophylactically before meals to prevent GERD.
CR might also consider taking an antacid, including calcium carbonate, sodium bicarbonate, magnesium hydroxide/aluminum hydroxide, or bismuth subsalicylate. These agents have the fastest onset of action, but they provide only symptomatic relief of heartburn and have the shortest duration of action.
Case 2: Allergic contact dermatitis is an inflammatory skin reaction to a foreign substance, such as urushiol in the sap of the poison ivy plant. Sensitized patients can develop clinical symptoms such as erythema, intense itching, and formation of plaques and vesicles within 4 to 96 hours after exposure to an allergen.
EF appears to have severe contact dermatitis. She is not a candidate for self-treatment because of the facial involvement of her dermatitis and the presence of vesicles and intense itching. If left untreated, allergic contact dermatitis resolves within 1 to 3 weeks; however, it can cause significant discomfort. EF should be referred to her primary care provider to obtain a prescription for an oral corticosteroid, such as prednisone to decrease itching, and perhaps a high-potency topical corticosteroid such as clobetasol propionate 0.05% cream, which is generally not applied to the face. A 21-day course of oral prednisone (starting at 1 mg/kg/day and tapered over 3 weeks) is appropriate and can significantly reduce symptoms, including itching.
EF should be told to keep the area clean and to avoid scratching and using makeup, as they can irritate the skin. In addition, nonpharmacologic treatments, including the application of cold compresses, can be recommended. EF might try using astringents such as aluminum acetate (Burrow’s solution) or calamine to reduce inflammation and promote drying, and healing of the lesions.
Read the answers
function showAnswer() {document.getElementById("answer").style.display = 'block';document.getElementById("link").style.display = 'none';}
Dr. Coleman is professor of pharmacy practice, as well as codirector and methods chief at Hartford Hospital Evidence-Based Practice Center, at the University of Connecticut School of Pharmacy.

Condition Watch: Asthma
HSSP Model Can Reduce Financial Toxicity of Oral Oncology Treatment
SGLT2 Inhibitors Show Considerable Efficacy for Diabetes Management
Case Study: Advise Patients on Eligibility, Efficacy of Zoster Vaccine

Counsel Patients About Self-Care Measures, OTC Treatments for Seasonal Allergies

The Fludarabine Shortage and Its Ripple Effects: Navigating the Crisis
2 Commerce Drive Cranbury, NJ 08512
609-716-7777

An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Prev Nutr Food Sci
- v.26(4); 2021 Dec 31
Dietary Intake in Relation to the Risk of Reflux Disease: A Systematic Review
Neda heidarzadeh-esfahani.
1 Student Research Committee, School of Nutritional Sciences and Food Technology, Kermanshah University of Medical Sciences, Kermanshah 6719851552, Iran
Davood Soleimani
2 Research Center of Oils and Fats, Kermanshah University of Medical Sciences, Kermanshah 6719851552, Iran
3 Nutritional Sciences Department, School of Nutrition Sciences and Food Technology, Kermanshah University of Medical Sciences, Kermanshah 6719851552, Iran
Salimeh Hajiahmadi
4 Department of Nutrition, School of Public Health, Shahid Sadoughi University of Medical Sciences, Yazd 8916188635, Iran
Shima Moradi
5 Department of Nutritional Sciences, Research Center for Environmental Determinants of Health (RCEDH), Kermanshah University of Medical Sciences, Kermanshah 6719851552, Iran
Nafiseh Heidarzadeh
6 Depertment of Genetics, Faculty of Basic Sciences, Shahrekord University, Shahrekord 881863414, Iran
Seyyed Mostafa Nachvak
Gastroesophageal reflux disease (GERD) is a chronic condition which has a high global prevalence. Dietary intake is considered to be a contributing factor for GERD. However, scientific evidence about the effect of diet on the risk of GERD is controversial. This systematic review was conducted to address this issue. A comprehensive structured search was performed using the MEDLINE, Scopus, and Web of Science databases up to August 2020, in accordance with the PRISMA statement. No restrictions were set in terms of language, time of publication, or study location. Study selection and data abstraction was conducted independently by two authors, and risk of bias was assessed using a modified Quality in Prognosis Studies Tool. Eligible studies evaluating the impact of food and dietary pattern on GERD were included in qualitative data synthesis. After excluding duplicate, irrelevant, and low quality studies, 25 studies were identified for inclusion: 5 case-control studies, 14 cross-sectional studies, and 6 prospective studies. This review indicates that high-fat diets, carbonated beverages, citrus products, and spicy, salty, and fried foods are associated with risk of GERD.
INTRODUCTION
Gastroesophageal reflux disease (GERD) is a common disorder that affects quality of life. GERD develops when reflux of stomach contents causes troublesome symptoms and long-term complications ( Rajaie et al., 2020 ). The major symptoms of GERD include heartburn and regurgitation ( Kahrilas, 2003 ), however, GERD can also manifest with atypical symptoms including epigastric pain, dyspepsia, nausea, bloating, and belching ( Badillo and Francis, 2014 ). GERD pathogenesis involves esophagitis, hemorrhage, stricture, Barrett’s esophagus, and adenocarcinoma ( Rajaie et al., 2020 ). Moreover, GERD is independently associated with increased risk of cardiovascular diseases, including acute myocardial infarction ( Lei et al., 2017 ). It is a global disease, with an estimated highest incidence in North America (18.1%∼27.8%), followed by the Middle-East America (8.7%∼33.1%), Europe (8.8%∼25.9%), and East Asia (2.5%∼7.8%) ( Seremet et al., 2015 ).
GERD is a multifactorial disease influenced by both genetic predisposition and environmental factors. Diet (an environmental factor) has important roles in gastrointestinal and cardio metabolic disorders ( Argyrou et al., 2018 ; Heshmati et al., 2019 ; Surdea-Blaga et al., 2019 ; Heshmati et al., 2020 ), and modifiable risk factors included long meal-to-sleep intervals, speed of eating, and scale and temperature of foods ( Esmaillzadeh et al., 2013 ; Yuan et al., 2017 ). Intake of alcohol, chocolate, and high-fat meals reduces esophageal sphincter pressure and increases esophageal exposure to gastric juices ( Kaltenbach et al., 2006 ). Some studies have reported that both the quality and quantity of carbohydrates in diet may be associated with GERD ( Keshteli et al., 2017 ; Wu et al., 2018 ). However, current data are contradictory ( Kim et al., 2014 ).
Emerging data indicates that appropriate eating behaviors, i.e., healthy diets involving high intakes of fruits and whole grains ( Wu et al., 2013 ), such as the Mediterranean diet ( Mone et al., 2016 ), improves GERD symptoms. Therefore, improving diets can decrease the occurrence of GERD and should be considered a cost-effective strategy instead of pharmacotherapy.
Review studies have investigated predictors of GERD risk in terms of food related factors such as probiotics ( Cheng and Ouwehand, 2020 ) and food components ( Surdea-Blaga et al., 2019 ). However, to our knowledge, no systematic reviews have been conducted to assess the impact of diet on risk of reflux disease. We conducted a systematical review of articles investigating the association between food and dietary patterns with GERD.
MATERIALS AND METHODS
Search strategy.
The literature search was conducted by two independent researchers using electronic databases, including the Web of Sciences, PubMed/MEDLINE, and Scopus, to identify relevant publications up to August 2020. This study was given ethical approval by the Ethics Committee of Research Council of Kermanshah University of Medical Sciences (Ethics Code: IR.KUMS.REC.1399.941).
We performed the systematic search using Medical Subject Headings (MeSH) along with non-MeSH keywords in the title and abstract as follows: “Diet” OR “Food” OR “Dietary Pattern” OR “Food Pattern” AND “Gastroesophageal Reflux” OR “Gastric Acid Reflux” OR “Gastroesophageal Reflux Disease” OR “GERD” OR “Esophageal Reflux” OR “Pyrosis” OR “Pyroses” OR “Heartburn” OR “Barrett’s Esophagus”. We did not consider any restrictions in terms of language, time of publication, and study location.
Inclusion and exclusion criteria
Eligible studies were performed on adults and evaluated all components of the dietary patterns and risk of reflux disease. In addition, we considered all observational studies, including cross-sectional, case-control, prospective, and retrospective studies. Interventional studies were not included in this study since the duration of exposure was short. Overall, 991 articles were identified during the initial search and duplicate studies (n=26) were removed. The remaining studies were screened based on topic and 833 irrelevant studies were excluded. Thereafter, 119 studies were reviewed in more detail, and 24 classed as irrelevant (including 17 that did not assess dietary pattern) and 52 that did not evaluate our outcome (“reflux diseases”) were excluded. In addition, the qualification of 26 articles were evaluated and one study was excluded because of low quality. In total, 25 articles were eligible for inclusion in this review study ( Fig. 1 ).

Flow chart of the search and publication selection.
Quality assessment
Using all the data extracted, we scored the risk of bias of the selected studies on a six-point scale using a modified version of the Quality in Prognosis Studies ( Hayden et al., 2006 ). Using this system, we assessed the quality of individual studies using the following criteria (one point per criterion): (I) study participation (the study sample represents the key characteristics of the population of interest sufficiently well to limit potential bias to the results); (II) study attrition (loss to follow-up is not associated with key characteristics); (III) prognostic factor measurement (prognostic factors of interest are measured in study participants in such a way that potential bias is limited); (IV) confounding measurement and account (outcomes of interest are measured in study participants in such a way that potential bias is limited); (V) outcome measurement (important potential confounders are appropriately accounted for, limiting potential bias with respect to the prognostic factor of interest); and (VI) analysis (the statistical analysis is appropriate for the design of the study, and limits the potential for invalid results). Studies with a score between 0 and 3 points were considered to be of low quality, while studies with a score >3 to 6 were considered to be of high quality.
Data extraction
Data extraction was performed independently by two researchers using a data collection checklist. Any disagreement has been discussed and resolved accordingly. For each article, the first author’s name, publication year, study design (trial/prospective/cross-sectional/case control), sample size, study population demographics [age, sex, body mass index (BMI), and country], dietary assessment tools, dietary components, and outcomes (all reported data on association between GERD and dietary components) were extracted. All data are presented in the Results.
Study characteristics
In this literature review, we identified 25 eligible studies with quality scores from 3.5 to 6.0. As shown in Table 1 , these studies included 5 case-control studies ( Nandurkar et al., 2004 ; Murphy et al., 2010 ; Wu et al., 2013 ; Asl et al., 2015 ; Ebrahimi-Mameghani et al., 2017 ), 14 cross-sectional studies ( El-Serag et al., 2005a ; El-Serag et al., 2005b ; Shapiro et al., 2007 ; Friedenberg et al., 2010 ; Kubo et al., 2014 ; Khodarahmi et al., 2016 ; Mone et al., 2016 ; Alkhathami et al., 2017 ; Eslami et al., 2017 ; Keshteli et al., 2017 ; Atta et al., 2019 ; Kim et al., 2019 ; Kariri et al., 2020 ; Rajaie et al., 2020 ), and 6 prospective studies ( Ruhl and Everhart, 1999 ; Gutschow et al., 2005 ; Austin et al., 2006 ; Bhatia et al., 2011 ; López-Colombo et al., 2017 ; Wu et al., 2018 ). There were a total of 8 to 12,349 subjects per study. Studies were conducted in America ( Ruhl and Everhart, 1999 ; Nandurkar et al., 2004 ; El-Serag et al., 2005a ; El-Serag et al., 2005b ; Austin et al., 2006 ; Shapiro et al., 2007 ; Friedenberg et al., 2010 ; Kubo et al., 2014 ; López-Colombo et al., 2017 ), Asia ( Bhatia et al., 2011 ; Wu et al., 2013 ; Asl et al., 2015 ; Khodarahmi et al., 2016 ; Alkhathami et al., 2017 ; Ebrahimi-Mameghani et al., 2017 ; Eslami et al., 2017 ; Keshteli et al., 2017 ; Wu et al., 2018 ; Atta et al., 2019 ; Kim et al., 2019 ; Kariri et al., 2020 ; Rajaie et al., 2020 ), and Europe ( Gutschow et al., 2005 ; Murphy et al., 2010 ; Mone et al., 2016 ). All studies included participates aged ≥18 years of age, and most assessed dietary intake using the food frequency questionnaire.
Important characteristics of the included studies
Values are presented as mean±SD or number (%).
BMI, body mass index; GERD, gastroesophageal reflux disease; FFQ, food frequency questionnaire; RDQ, reflux diagnostic questionnaire; GSAS-ds, gastroesophageal reflux disease symptom assessment scale-distress subscale; -, not available.
Study outcomes
A total of nine studies examined the negative effects of refluxtriggering foods, including high-fat, spicy, fried, and citrus foods, carbonated beverages, and tea ( Shapiro et al., 2007 ; Kubo et al., 2014 ; Asl et al., 2015 ; Alkhathami et al., 2017 ; Eslami et al., 2017 ; López-Colombo et al., 2017 ; Atta et al., 2019 ; Kim et al., 2019 ; Kariri et al., 2020 ). Other food components, such as saturated fatty acids (SFA), monounsaturated fatty acids, polyunsaturated fatty acids, cholesterol, smoky foods, salty foods, coffee, alcohol, chocolate, and dairies, did not contribute to risk of GERD ( Nandurkar et al., 2004 ; El-Serag et al., 2005a ; El-Serag et al., 2005b ; Shapiro et al., 2007 ; Friedenberg et al., 2010 ; Murphy et al., 2010 ; Bhatia et al., 2011 ; Wu et al., 2013 ; Kubo et al., 2014 ; Asl et al., 2015 ; Alkhathami et al., 2017 ; Eslami et al., 2017 ; López-Colombo et al., 2017 ; Atta et al., 2019 ). The results of studies examining the role of dietary components in reflux disease are shown in Table 2 .
Summary of findings on the relationship of each dietary component with gastroesophageal reflux disease
GERD, gastroesophageal reflux disease; OR, odds ratio; CI, confidence interval; SFA, saturated fattyacids; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids.
Shapiro et al. (2007) , Kubo et al. (2014) , Asl et al. (2015) , and Kim et al. (2019) observed that high-fat diets contributed to the risk of reflux disease. However, Ruhl and Everhart (1999) and Wu et al. (2013) did not observe any association between high-fat diets and reflux disease. On the other hand, Ruhl and Everhart (1999) reported that cholesterol may increase the risk of reflux disease, and El-Serag et al. (2005a ; 2005b) found the relationship between dietary fat and reflux disease was non-significant after adjusting for BMI, energy intake, and demographic characteristics. Moreover, daily intakes of total fat, SFA, cholesterol, energy from dietary fat, and fat were significantly higher in subjects without GERD symptoms than those with GERD symptoms.
Fast food is a possible causal risk factor for reflux disease ( Alkhathami et al., 2017 ; Kariri et al., 2020 ). Indeed, Kubo et al. (2014) and Atta et al. (2019) found a significant relationship between fried foods and symptoms of GERD.
Keshteli et al. (2017) showed that foods with high-glycemic indexes increase the risk of uninvestigated heartburn [odd ratio (OR=1.75; 95% confidence interval (CI): 1.03, 2.97; P -value=0.04)] and uninvestigated chronic dyspepsia (OR=2.14; 95% CI: 1.04, 4.37; P -value=0.04) in men but not in women, even after adjusting for potential confounders, such as age, marital status, medications, education, sleeping, eating rate, and intake of spicy foods, cocoa, sugar-sweetened beverages, tea, coffee, energy, fat, fructose, and fiber. However, Kubo et al. (2014) , Asl et al. (2015) , Alkhathami et al. (2017) , Atta et al. (2019) , and Kariri et al. (2020) found a significant relationship between reflux disease and carbonated beverages, including soda, aerated, and soft drinks.
Wu et al. (2013) observed a negative relationship between intake of fruits and reflux disease, whereas other studies did not observe any significant association ( Friedenberg et al., 2010 ; El-Serag et al., 2005a ; El-Serag et al., 2005b ). Kubo et al. (2014) , Alkhathami et al. (2017) , Eslami et al. (2017) , and López-Colombo et al. (2017) found that citrus was associated with risk of GERD. Moreover, consumption of non-vegetarian foods was an independent predictor of GERD ( Bhatia et al., 2011 ). However, El-Serag et al. (2005a ; 2005b) and Wu et al. (2013) did not observe any association between vegetables and GERD.
Two studies reported that fast foods, including sausage, fried chicken, pizza, hamburgers, french fries, and doughnuts, were not significantly associated with reflux disease ( Friedenberg et al., 2010 ; Eslami et al., 2017 ). Alkhathami et al. (2017) reported that the prevalence of GERD was higher in those who did not consume dietary fibers regularly.
To the best of our knowledge, this is the first systematic review to investigate the relationship between different foods and dietary patterns with the occurrence of GERD. Unlike previous review studies, the present study reviewed all previous observational studies. The results of this study showed a significant association between adherences to high-fat diets and increased the risk of GERD.
Consumption of large high-fat meals appears to accelerate development of GERD ( Surdea-Blaga et al., 2019 ) by reducing lower esophageal sphincter (LES) pressure ( Kumar and Katz, 2013 ; Kubo et al., 2014 ; Asl et al., 2015 ). Furthermore, large high-fat meals are correlated with increased acid exposure time in patients compared with low fat meals ( Kahrilas et al., 2008 ; Kubo et al., 2010 ; Castillo et al., 2015 ; Ireland et al., 2016 ; Sethi and Richter, 2017 ). However, previous review studies in 2000 and 2009 investigating the pathogenic relationship between eating habits and occurrence of GERD were unable support the effect of dietary fat on incidence of GERD ( Meining and Classen, 2000 ; Festi et al., 2009 ). In contrast to the results of the present study, Eslick and Talley (2009) emphasized the relationship between high cholesterol consumption and increased risk of GERD. The authors concluded that confounding factors such as BMI, energy and demographic variables were responsible for this result.
Decreased LES pressure is involved in progression of GERD in overweight individuals consuming a high-cholesterol diet. However, further studies are needed to investigate the independent association of dietary fat as a risk factor for GERD. Several studies reported that consuming a high-fat diet and food rich in cholesterol and SFA ( El-Serag et al., 2005a ; El-Serag et al., 2005b ; Shapiro et al., 2007 ; Wu et al., 2013 ), and unsaturated fatty acids ( Shapiro et al., 2007 ) was not associated with increased risk of GERD. Similarly, review studies investigating the pathogenic relationship between eating habits and occurrence of GERD in 2000 ( Meining and Classen, 2000 ) and 2009 ( Festi et al., 2009 ) did not support an effect of dietary fat on the incidence of GERD.
Consuming high-salt foods ( Asl et al., 2015 ; Alkhathami et al., 2017 ), spicy foods ( Bhatia et al., 2011 ; Eslami et al., 2017 ; López-Colombo et al., 2017 ), smoky foods ( Asl et al., 2015 ), and fast foods ( Friedenberg et al., 2010 ; Eslami et al., 2017 ) does not significantly increase the risk of GERD. However, several studies have emphasized the relationship between consumption of high-salt foods ( Wu et al., 2013 ) and high-spice foods ( Asl et al., 2015 ; Alkhathami et al., 2017 ; Kariri et al., 2020 ) in accelerating development of GERD. Furthermore, Wu et al. (2013) showed that increasing salt intake is only effective in reducing LES pressure, and alone cannot increase the risk of GERD. In addition, in Asian populations, consuming high-spice foods followed by the habit of lying down after eating increases the risk of GERD. Lying down after eating may be a major cause of this disorder since it reduces LES pressure and affects the reflux of gastric contents ( Asl et al., 2015 ).
Vegetables (cooked vegetables and salad) ( El-Serag et al., 2005a ; El-Serag et al., 2005b ; Friedenberg et al., 2010 ; Wu et al., 2013 ), dietary fiber ( Nandurkar et al., 2004 ; El-Serag et al., 2005a ; El-Serag et al., 2005b ; Wu et al., 2013 ), dairy products ( El-Serag et al., 2005a ; El-Serag et al., 2005b ; Wu et al., 2013 ), and antioxidants ( Murphy et al., 2010 ) were also not significantly associated with increased incidence of GERD. A previous review study investigating the relationship between diet and GERD showed that adherence to the Mediterranean diet (rich in vegetables, fiber, and antioxidants) ( Winberg et al., 2012 ), could play a preventive role in GERD, especially among patients with underlying diseases such as diabetes, heart disease, and cancer ( Badillo and Francis, 2014 ; Newberry and Lynch, 2017 ). However, these results are inconsistent with those of the present study. Further studies are needed to assess the effectiveness of these food groups in preventing GERD in all individuals.
Consumption of high-caffeine products such as tea ( Asl et al., 2015 ; Eslami et al., 2017 ), coffee ( Nandurkar et al., 2004 ; Friedenberg et al., 2010 ; Kubo et al., 2014 ; Asl et al., 2015 ; Eslami et al., 2017 ; López-Colombo et al., 2017 ), and chocolate ( Eslami et al., 2017 ; López-Colombo et al., 2017 ) are also not significantly associated with risk of developing GERD. Review studies investigating the relationship between lifestyle and GERD also did not support a role of high-caffeine sources in increasing the incidence of GERD ( Meining and Classen, 2000 ; Kaltenbach et al., 2006 ; Vemulapalli, 2008 ). However, in 2014, a study suggested that tea consumption was associated with increased risk of GERD. These contradictory results may be due to differences between the types of tea consumed by the subjects in the study ( Kubo et al., 2014 ). In addition, some studies have shown that coffee relaxes the LES, and increases percentage reflux time in the fasting state ( Akbar and Howden, 2016 ).
Similarly to our results, other studies have not shown a significant relationship between alcohol consumption and increased risk of GERD ( Festi et al., 2009 ; Kubo et al., 2010 ; Esmaillzadeh et al., 2013 ; Ireland et al., 2016 ). A further review study was conducted in parallel with our own, which showed that consumption of carbonated beverages increases the risk of GERD ( Newberry and Lynch, 2017 ). Carbonated beverages may increase the likelihood of dysphagia reflexes by altering the acidity of the gastrointestinal tract, especially the stomach, and affecting digestion (intragastric residence time and inducing poor digestion) ( Asl et al., 2015 ). Furthermore, these beverages contain high levels of acidity, added sugars and artificial sweeteners, and caffeine, which alter LES pressures and intraesophageal pH ( Newberry and Lynch, 2017 ).
The results of the present study show that daily consumption of citrus increases risk of GERD ( López-Colombo et al., 2017 ), which is consistent with the results of prior review studies ( Meining and Classen, 2000 ; Kaltenbach et al., 2006 ; Sethi and Richter, 2017 ). These fruits increase the risk of GERD by reducing LES pressure or delayed gastric emptying ( Eslami et al., 2017 ). A previous study conducted on adherence to dietary recommendations in GERD ( Kubo et al., 2014 ) showed that frequent consumption of citrus fruits and juices of adjusted pH plays a preventive role in acid-sensitive individuals. Other compounds in citrus fruits in addition to acidity are also likely to play a preventive role. However, this study attributed infrequent citrus fruit intake to the strong recommendations on limiting these fruits in people predisposed to dysphagia, and did not provide any documented data linking this group of fruits to GERD ( Kubo et al., 2014 ).
In the present study, a diet rich in fruits did not significantly effect on impact the risk of developing GERD. Nevertheless, results from previous review studies support the protective effect of fruits in reducing the incidence of reflux disorders ( Wu et al., 2013 ; Badillo and Francis, 2014 ). Indeed, these studies attribute this positive effect to the high-fiber content in fruits ( Badillo and Francis, 2014 ). Moreover, consumption of fruit juices increases pressure the gradient from the abdomen to the chest, resulting in GERD symptoms by increasing abdominal pressure or decreasing LES pressure ( Fallah et al., 2020 ). Further studies are needed to investigate the relationship between different compounds in fruits and GERD.
In parallel with the current study, a review study was conducted in 2017 that showed whole grain consumption is significantly correlated with reduced incidence of GERD in people with underlying diseases such as diabetes, heart disease, and cancer ( Badillo and Francis, 2014 ). Further studies are needed to evaluate the effectiveness of grain consumption in reducing the incidence of this disorder in all individuals. The inconsistencies between studies may arise from differences in the sociodemographic statuses of the study populations, sample sizes, and criteria used to diagnose reflux disease.
Review studies have several inherent limitations that should be considered. First, although the present study investigated the relationship between different food groups and GERD in adults of different races and in different geographical conditions by extracting data from observational studies, it was not possible to investigate different age groups due to data limitations. Hence, we recommend other researchers conduct systematic review of observational and clinical trial studies investigating the effect of diet on GERD in children. Second, there were significant heterogeneity between studies due to varying regimens, doses, duration, center settings, and populations. Hence, we could not determine the fundamental factors to explain the observed heterogeneity due to the limited number of studies and lack of information for pooling. Third, it is crucial to investigate grey literature as an important resource in systematic review in order to reduce publication bias, which was unfortunately neglected from the current study.
In this study, we concluded that diets rich in vegetables, fiber, antioxidants, and caffeine were not significantly associated with increased risk of dysphagia. However, consumption of citrus fruits, carbonate beverages, spicy, and fried food increases the risk of developing this disorder. Furthermore, we did not identify a specific diet that plays an effective role in GERD. Other large-scale studies with robust study designs are needed to investigate the effect of different diets associated with this disorder in all age groups.
ACKNOWLEDGEMENTS
We thank gratefully acknowledge the Research Council of Kermanshah University of Medical Sciences.
AVAILABILITY OF DATA AND MATERIALS
The datasets used and analyzed during the present study are available from the corresponding author on reasonable request.
This research was funded by Research Council of Kermanshah University of Medical Sciences, Kermanshah, Iran (Grant No: 3010879).
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
- Akbar A, Howden CW. Lifestyle modifications in GERD. In: Vaezi MF, editor. Diagnosis and Treatment of Gastroesophageal Reflux Disease. Springer; Cham, Switzerland: 2016. pp. 59–70. [ CrossRef ] [ Google Scholar ]
- Alkhathami AM, Alzahrani AA, Alzhrani MA, Alsuwat OB, Mahfouz MEM. Risk factors for gastroesophageal reflux disease in Saudi Arabia. Gastroenterology Res. 2017; 10 :294–300. doi: 10.14740/gr906w. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Argyrou A, Legaki E, Koutserimpas C, Gazouli M, Papaconstantinou I, Gkiokas G, et al. Risk factors for gastroesophageal reflux disease and analysis of genetic contributors. World J Clin Cases. 2018; 6 :176–182. doi: 10.12998/wjcc.v6.i8.176. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Asl SF, Mansour-Ghanaei F, Samadi H, Joukar F. Evaluations of life style factors and the severity of gastroesophageal reflux disease; a case-control study. Int J Mol Epidemiol Genet. 2015; 6 :27–32. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Atta MM, Sayed MH, Zayed MA, Alsulami SA, Al-Maghrabi AT, Kelantan AY. Gastro-oesophageal reflux disease symptoms and associated risk factors among medical students, Saudi Arabia. Int J Gen Med. 2019; 12 :293–298. doi: 10.2147/IJGM.S206995. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Austin GL, Thiny MT, Westman EC, Yancy WS, Jr, Shaheen NJ. A very low-carbohydrate diet improves gastroesophageal reflux and its symptoms. Dig Dis Sci. 2006; 51 :1307–1312. doi: 10.1007/s10620-005-9027-7. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Badillo R, Francis D. Diagnosis and treatment of gastroesophageal reflux disease. World J Gastrointest Pharmacol Ther. 2014; 5 :105–112. doi: 10.4292/wjgpt.v5.i3.105. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bhatia SJ, Reddy DN, Ghoshal UC, Jayanthi V, Abraham P, Choudhuri G, et al. Epidemiology and symptom profile of gastroesophageal reflux in the Indian population: report of the Indian Society of Gastroenterology Task Force. Indian J Gastroenterol. 2011; 30 :118–127. doi: 10.1007/s12664-011-0112-x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Castillo R, Otero W, Trespalacios A. Evidence based review of the impact of treatments of gastroesophageal reflux disease. Rev Col Gastroenterol. 2015; 30 :427–442. [ Google Scholar ]
- Cheng J, Ouwehand AC. Gastroesophageal reflux disease and probiotics: a systematic review. Nutrients. 2020; 12 :132. doi: 10.3390/nu12010132. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ebrahimi-Mameghani M, Sabour S, Khoshbaten M, Arefhosseini SR, Saghafi-Asl M. Total diet, individual meals, and their association with gastroesophageal reflux disease. Health Promot Perspect. 2017; 7 :155–162. doi: 10.15171/hpp.2017.28. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- El-Serag HB, Graham DY, Satia JA, Rabeneck L. Obesity is an independent risk factor for GERD symptoms and erosive esophagitis. Am J Gastroenterol. 2005a; 100 :1243–1250. doi: 10.1111/j.1572-0241.2005.41703.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- El-Serag HB, Satia JA, Rabeneck L. Dietary intake and the risk of gastro-oesophageal reflux disease: a cross sectional study in volunteers. Gut. 2005b; 54 :11–17. doi: 10.1136/gut.2004.040337. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Eslami O, Shahraki M, Bahari A, Shahraki T. Dietary habits and obesity indices in patients with gastro-esophageal reflux disease: a comparative cross-sectional study. BMC Gastroenterol. 2017; 17 :132. doi: 10.1186/s12876-017-0699-1. https://doi.org/10.1186/s12876-017-0699-1 . [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Eslick GD, Talley NJ. Gastroesophageal reflux disease (GERD): risk factors, and impact on quality of life-a population-based study. J Clin Gastroenterol. 2009; 43 :111–117. doi: 10.1097/MCG.0b013e31815ea27b. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Esmaillzadeh A, Keshteli AH, Feizi A, Zaribaf F, Feinle-Bisset C, Adibi P. Patterns of diet-related practices and prevalence of gastro-esophageal reflux disease. Neurogastroenterol Motil. 2013; 25 :831–e638. doi: 10.1111/nmo.12192. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fallah Z, Ferns GA, Ghayour-Mobarhan M. Fluid intake and functional gastrointestinal disease: a narrative review. Crit Comments Biomed. 2020; 1 :e10020. https://doi.org/10.18502/ccb.v1i1.2872 . [ Google Scholar ]
- Festi D, Scaioli E, Baldi F, Vestito A, Pasqui F, Di Biase AR, et al. Body weight, lifestyle, dietary habits and gastroesophageal reflux disease. World J Gastroenterol. 2009; 15 :1690–1701. doi: 10.3748/wjg.15.1690. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Friedenberg FK, Rai J, Vanar V, Bongiorno C, Nelson DB, Parepally M, et al. Prevalence and risk factors for gastroesophageal reflux disease in an impoverished minority population. Obes Res Clin Pract. 2010; 4 :e261–e269. doi: 10.1016/j.orcp.2010.06.001. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gutschow CA, Bollschweiler E, Schröder W, Collet P, Collard JM, Hölscher AH. Effect of "white diet" during bile monitoring with Bilitec 2000 on esophageal pH-metry in patients with gastroesophageal reflux disease. J Gastrointest Surg. 2005; 9 :508–513. doi: 10.1016/j.gassur.2004.09.035. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hayden JA, Côté P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med. 2006; 144 :427–437. doi: 10.7326/0003-4819-144-6-200603210-00010. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Heshmati J, Golab F, Morvaridzadeh M, Potter E, Akbari-Fakhrabadi M, Farsi F, et al. The effects of curcumin supplementation on oxidative stress, Sirtuin-1 and peroxisome proliferator activated receptor γ coactivator 1α gene expression in polycystic ovarian syndrome (PCOS) patients: a randomized placebo-controlled clinical trial. Diabetes Metab Syndr. 2020; 14 :77–82. doi: 10.1016/j.dsx.2020.01.002. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Heshmati J, Sepidarkish M, Namazi N, Shokri F, Yavari M, Fazelian S, et al. Impact of dietary calcium supplement on circulating lipoprotein concentrations and atherogenic indices in overweight and obese individuals: a systematic review. J Diet Suppl. 2019; 16 :357–367. doi: 10.1080/19390211.2018.1440685. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ireland CJ, Thompson SK, Laws TA, Esterman A. Risk factors for Barrett's esophagus: a scoping review. Cancer Causes Control. 2016; 27 :301–323. doi: 10.1007/s10552-015-0710-5. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kahrilas PJ, Shaheen NJ, Vaezi MF American Gastroenterological Association Institute; Clinical Practice and Quality Management Committee, author. American Gastroenterological Association Institute technical review on the management of gastroesophageal reflux disease. Gastroenterology. 2008; 135 :1392–1413. 1413.e1–1413.e5. doi: 10.1053/j.gastro.2008.08.044. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kahrilas PJ. GERD pathogenesis, pathophysiology, and clinical manifestations. Cleve Clin J Med. 2003; 70 Suppl 5 :S4–S19. doi: 10.3949/ccjm.70.Suppl_5.S4. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kaltenbach T, Crockett S, Gerson LB. Are lifestyle measures effective in patients with gastroesophageal reflux disease? An evidence-based approach. Arch Intern Med. 2006; 166 :965–971. doi: 10.1001/archinte.166.9.965. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kariri AM, Darraj MA, Wassly A, Arishi HA, Lughbi M, Kariri A, et al. Prevalence and risk factors of gastroesophageal reflux dis-ease in Southwestern Saudi Arabia. Cureus. 2020; 12 :e6626. doi: 10.7759/cureus.6626. https://doi.org/10.7759/cureus.6626 . [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Keshteli AH, Haghighatdoost F, Azadbakht L, Daghaghzadeh H, Feinle-Bisset C, Afshar H, et al. Dietary glycaemic index and glycaemic load and upper gastrointestinal disorders: results from the SEPAHAN study. J Hum Nutr Diet. 2017; 30 :714–723. doi: 10.1111/jhn.12480. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Khodarahmi M, Azadbakht L, Daghaghzadeh H, Feinle-Bisset C, Keshteli AH, Afshar H, et al. Evaluation of the relationship between major dietary patterns and uninvestigated reflux among Iranian adults. Nutrition. 2016; 32 :573–583. doi: 10.1016/j.nut.2015.11.012. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kim J, Oh SW, Myung SK, Kwon H, Lee C, Yun JM, et al. Korean Meta-analysis (KORMA) Study Group, author. Association between coffee intake and gastroesophageal reflux disease: a meta-analysis. Dis Esophagus. 2014; 27 :311–317. doi: 10.1111/dote.12099. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kim YM, Kim JH, Baik SJ, Jung DH, Park JJ, Youn YH, et al. Association between skeletal muscle attenuation and gastroesophageal reflux disease: a health check-up cohort study. Sci Rep. 2019; 9 :20102. doi: 10.1038/s41598-019-56702-6. https://doi.org/10.1038/s41598-019-56702-6 . [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kubo A, Block G, Quesenberry CP, Jr, Buffler P, Corley DA. Dietary guideline adherence for gastroesophageal reflux disease. BMC Gastroenterol. 2014; 14 :144. doi: 10.1186/1471-230X-14-144. https://doi.org/10.1186/1471-230X-14-144 . [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kubo A, Corley DA, Jensen CD, Kaur R. Dietary factors and the risks of oesophageal adenocarcinoma and Barrett's oesophagus. Nutr Res Rev. 2010; 23 :230–246. doi: 10.1017/S0954422410000132. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kumar AR, Katz PO. Functional esophageal disorders: a review of diagnosis and management. Expert Rev Gastroenterol Hepatol. 2013; 7 :453–461. doi: 10.1586/17474124.2013.811028. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lei WY, Wang JH, Wen SH, Yi CH, Hung JS, Liu TT, et al. Risk of acute myocardial infarction in patients with gastroesophageal reflux disease: a nationwide population-based study. PLoS One. 2017; 12 :e0173899. doi: 10.1371/journal.pone.0173899. https://doi.org/10.1371/journal.pone.0173899 . [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- López-Colombo A, Pacio-Quiterio MS, Jesús-Mejenes LY, Rodrí-guez-Aguilar JE, López-Guevara M, Montiel-Jarquín AJ, et al. Risk factors associated with gastroesophageal reflux disease relapse in primary care patients successfully treated with a proton pump inhibitor. Rev Gastroenterol Mex. 2017; 82 :106–114. doi: 10.1016/j.rgmxen.2017.03.002. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Meining A, Classen M. The role of diet and lifestyle measures in the pathogenesis and treatment of gastroesophageal reflux disease. Am J Gastroenterol. 2000; 95 :2692–2697. doi: 10.1111/j.1572-0241.2000.03175.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mone I, Kraja B, Bregu A, Duraj V, Sadiku E, Hyska J, et al. Adherence to a predominantly Mediterranean diet decreases the risk of gastroesophageal reflux disease: a cross-sectional study in a South Eastern European population. Dis Esophagus. 2016; 29 :794–800. doi: 10.1111/dote.12384. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Murphy SJ, Anderson LA, Ferguson HR, Johnston BT, Watson PR, McGuigan J, et al. Dietary antioxidant and mineral intake in humans is associated with reduced risk of esophageal adenocarcinoma but not reflux esophagitis or Barrett's esophagus. J Nutr. 2010; 140 :1757–1763. doi: 10.3945/jn.110.124362. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nandurkar S, Locke GR, 3rd, Fett S, Zinsmeister AR, Cameron AJ, Talley NJ. Relationship between body mass index, diet, exercise and gastrooesophageal reflux symptoms in a community. Aliment Pharmacol Ther. 2004; 20 :497–505. doi: 10.1111/j.1365-2036.2004.02156.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Newberry C, Lynch K. Can we use diet to effectively treat esopha-geal disease? A review of the current literature. Curr Gastroenterol Rep. 2017; 19 :38. doi: 10.1007/s11894-017-0578-5. https://doi.org/10.1007/s11894-017-0578-5 . [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rajaie S, Ebrahimpour-Koujan S, Hassanzadeh Keshteli A, Esmaillzadeh A, Saneei P, Daghaghzadeh H, et al. Spicy food consumption and risk of uninvestigated heartburn in Isfahani adults. Dig Dis. 2020; 38 :178–187. doi: 10.1159/000502542. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ruhl CE, Everhart JE. Overweight, but not high dietary fat intake, increases risk of gastroesophageal reflux disease hospitalization: the NHANES I epidemiologic followup study. First National Health and Nutrition Examination Survey. Ann Epidemiol. 1999; 9 :424–435. doi: 10.1016/S1047-2797(99)00020-4. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Seremet N, Karaagaoglu N, Kaner G, Tel K. Gastroesophageal reflux symptoms and nutritional preferences. Stud Ethno-Med. 2015; 9 :305–318. doi: 10.1080/09735070.2015.11905448. [ CrossRef ] [ Google Scholar ]
- Sethi S, Richter JE. Diet and gastroesophageal reflux disease: role in pathogenesis and management. Curr Opin Gastroenterol. 2017; 33 :107–111. doi: 10.1097/MOG.0000000000000337. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Shapiro M, Green C, Bautista JM, Dekel R, Risner-Adler S, Whitacre R, et al. Assessment of dietary nutrients that influence perception of intra-oesophageal acid reflux events in patients with gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2007; 25 :93–101. doi: 10.1111/j.1365-2036.2006.03170.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Surdea-Blaga T, Negrutiu DE, Palage M, Dumitrascu DL. Food and gastroesophageal reflux disease. Curr Med Chem. 2019; 26 :3497–3511. doi: 10.2174/0929867324666170515123807. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Vemulapalli R. Diet and lifestyle modifications in the management of gastroesophageal reflux disease. Nutr Clin Pract. 2008; 23 :293–298. doi: 10.1177/0884533608318106. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Winberg H, Lindblad M, Lagergren J, Dahlstrand H. Risk factors and chemoprevention in Barrett's esophagus-an update. Scand J Gastroenterol. 2012; 47 :397–406. doi: 10.3109/00365521.2012.667145. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wu KL, Kuo CM, Yao CC, Tai WC, Chuah SK, Lim CS, et al. The effect of dietary carbohydrate on gastroesophageal reflux disease. J Formos Med Assoc. 2018; 117 :973–978. doi: 10.1016/j.jfma.2017.11.001. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wu P, Zhao XH, Ai ZS, Sun HH, Chen Y, Jiang YX, et al. Dietary intake and risk for reflux esophagitis: a case-control study. Gastroenterol Res Pract. 2013; 2013 :691026. doi: 10.1155/2013/691026. https://doi.org/10.1155/2013/691026 . [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Yuan L, Tang D, Peng J, Qu N, Yue C, Wang F. Study on lifestyle in patients with gastroesophageal reflux disease. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2017; 42 :558–564. doi: 10.11817/j.issn.1672-7347.2017.05.013. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Open access
- Published: 22 April 2024

Clinicopathologic and endoscopic characteristics of ten patients with gastric hamartomatous inverted polyp: a single center case series
- Ningning Dong ORCID: orcid.org/0000-0002-3497-727X 1 ,
- Fandong Meng 1 ,
- Bing Yue 2 &
- Junzhen Hou ORCID: orcid.org/0000-0002-9874-9938 3
BMC Gastroenterology volume 24 , Article number: 139 ( 2024 ) Cite this article
Metrics details
Gastric hamartomatous inverted polyps (GHIPs) are not well characterized and remain diagnostically challenging due to rarity. Therefore, this study aims to investigate the clinicopathologic and endoscopic characteristics of patients with GHIP.
We retrospectively reviewed clinicopathologic and endoscopic features of ten patients with GHIP who were admitted to Beijing Friendship Hospital from March 2013 to July 2022. All patients were treated successfully by endoscopic resection.
GHIPs were usually asymptomatic and found incidentally during gastroscopic examination. They may be sessile or pedunculated, with diffuse or local surface redness or erosion. On endoscopic ultrasonography, the sessile submucosal tumor-type GHIP demonstrated a heterogeneous lesion with cystic areas in the third layer of the gastric wall. Histologically, GHIPs were characterized by a submucosal inverted proliferation of cystically dilated hyperplastic gastric glands accompanied by a branching proliferation of smooth muscle bundles. Inflammatory cells infiltration was observed in the stroma, whereas only one patient was complicated with glandular low-grade dysplasia. Assessment of the surrounding mucosa demonstrated that six patients (60%) had atrophic gastritis or Helicobacter pylori –associated gastritis, and four patients (40%) had non-specific gastritis. Endoscopic resection was safe and effective.
Conclusions
GHIPs often arise from the background of abnormal mucosa, such as atrophic or H.pylori -associated gastritis. We make the hypothesis that acquired inflammation might lead to the development of GHIPs. We recommend to make a full assessment of the background mucosa and H. pylori infection status for evaluation of underlying gastric mucosal abnormalities, which may be the preneoplastic condition of the stomach.
Peer Review reports
Gastric hamartomatous inverted polyps (GHIPs), characterized by the downward growth of hyperplastic mucosal component into the submucosal layer [ 1 ], account for fewer than 1% of all gastric polyps [ 2 ]. They have also been called gastric inverted hyperplastic polyps(GIHPs) [ 3 , 4 , 5 , 6 ], because of the similarity to their colonic counterpart [ 7 ]. Collectively, lesions exhibiting inverted growth are referred to as “gastric inverted polyps (GIPs)” [ 2 ]. Kim et al. [ 8 ] divided gastric inverted polyps (GIPs) into three subtypes based on their communication with the mucosal surface, smooth muscle boundary, and tissue organization. Type 1 has a central mucosal communicating structure and a recognizable smooth muscle boundary, and has a typical round vase shape when viewed under low magnification. Half of type 1 may be accompanied by simultaneous cancer transformation. Type 2 is similar to type 1 but with no central communicating structure. Type 3 is characterized by distorted lobular tissue organization composed of cystic or hyperplastic glands and smooth muscle, without a mucosal communicating structure or smooth muscle boundary.
Because of their rarity, GHIPs are not well characterized and remain diagnostically challenging based on endoscopic findings [ 1 ]. Moreover, the pathogenesis and precancerous potential of GHIPs are still uncertain, meanwhile their association with various forms of gastritis has not been well documented in the literature. Herein we retrospectively reviewed clinical, endoscopic, and histological data of ten patients with GHIPs, all of which were resected successfully by endoscopy.
This retrospective study was approved by the Ethic Committee of Beijing Friendship Hospital, Capital Medical University (Beijing, China). Written informed consent was obtained from all patients. A total of ten patients with GHIP were included in the study from March 2013 to July 2022. None of the patients had prior gastric surgery or a family history of gastric cancer or gastrointestinal polyposis syndromes. The demographics, clinical manifestations, endoscopic and histopathological features were obtained from the patients’ medical records. Endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD) was performed for GHIPs. Helicobacter pylori ( H. pylori ) infection status was assessed by 13 C-urea breath test (UBT) (Shenzhen China National Nuclear Corporation Heidewe Biotechnology, China), past history of prior successful H. pylori eradication therapy, microscopic observations of biopsied/resected specimens, serum H. pylori antibody test, endoscopic manifestations [ 9 ], or a combination of these methods. 13 C-UBT was performed in the morning after a at least of 6 h fasting, with no close (within the past 4 weeks) or concomitant medical history of proton pump inhibitors, antibiotics and bismuth, with a dosage of Urea of 75 mg, and the cut-off value to distinguish whether the 13 C-UBT is positive or negative was defined as 4‰. Based on the results of these tests, we divided the patients into two groups according to the H. pylori infection status: Hp group (consisting of patients with current or past H. pylori infection) and uninfected group (consisting of H. pylori -uninfected patients).
The histopathological findings were analyzed by hematoxylin and eosin (HE) staining and immunohistochemical staining (for Mucin 6, Mucin-5AC, Mucin 2, Pepsinogen I and Desmin), including the glandular components (foveolar, fundic, cardiac/pyloric/mucous-neck, and intestinal type), the presence of epithelial dysplasia or not, and the characteristics of stroma, muscularis mucosae and background gastric mucosa. The exact sample size was a total of ten lesions from ten patients. All the submitted specimens were fixed with 10% neutral formaldehyde solution, followed by routine dehydration, paraffin embedding, tissue sectioning at a thickness of 4 µm and HE staining. An En-Vision two-step method was used for immunohistochemical labelling. The pepsinogen-I antibody was purchased from Abcam Company in the United States. Other antibodies (including Mucin 6, Mucin-5AC, Mucin 2 and Desmin) were purchased from Fuzhou Maixin Medical Technology Co., Ltd. Negative and positive controls were established for the above markers.
Subsequently, we investigated the atrophy status of the gastric mucosa surrounding/overlying each GHIP endoscopically (according to “Kimura and Takemoto's endoscopic-atrophic border scale [ 10 , 11 ]”) and pathologically. In biopsy/resected specimens, mucosa with glandular atrophy or metaplasia (including focal or extensive intestinal/pseudo-pyloric metaplasia) was determined to be atrophic gastritis.
We compared the main clinical and endoscopic characteristics of GHIPs between patients with and without H. pylori infection. The statistical analysis was performed using R language Statistical Software (R 4.3.2). Fisher's precision probability test was used to compare categorical variables, and the independent-sample t test for quantitative variables.
Clinical and endoscopic findings
A summary of the clinical and endoscopic findings in patients with GHIP was shown in Table 1 . GHIPs typically presented in late adulthood (median age of diagnosis, 59.5 years old; range, 42–79 years old), with a modest male predominance (7/10, 70%). They located in the proximal stomach (four in the middle-upper body, four in the fornix and two in the cardia), with the maximum diameter ranging from 6 to 20 mm (median size, 13.5 mm). Most patients were asymptomatic and diagnosed incidentally during endoscopic examination, however, a minority (3/10, 30%) presented with non-specific symptoms, including heartburn, acid regurgitation and epigastric distension. Endoscopically, GHIPs were solitary, and could be classified into pedunculated polyp-type (Fig. 1 ) (7/10, 70%), which were all completely resected by EMR, and sessile submucosal tumor (SMT)-type (Fig. 2 ) (3/10, 30%), which were all completely resected by ESD. All of the ten GHIPs exhibited diffuse (3/10, 30%) or local (7/10, 70%) surface redness or erosion. On endoscopic ultrasonography (EUS), all of the three sessile SMT-type GHIPs demonstrated a heterogeneous lesion with anechoic cystic areas in the third layer of the gastric wall (Fig. 3 ). H. pylori -infection of the gastric mucosa was confirmed in four cases (4/10, 40%), including three patients with current infection (Fig. 4 a and b) and one patient with past infection. All patients were discharged without any significant complications after the endoscopic resection.
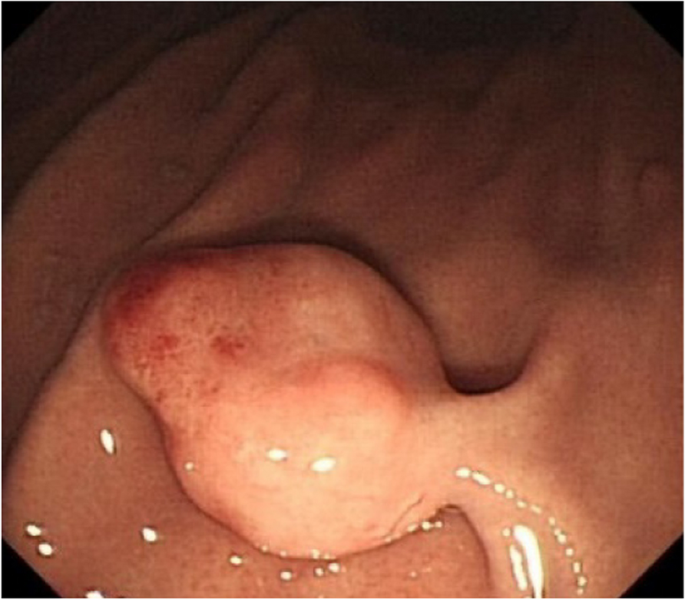
Image of endoscopy of a pedunculated polyp-type GHIP in the gastric body without mucosal diffuse redness or atrophy
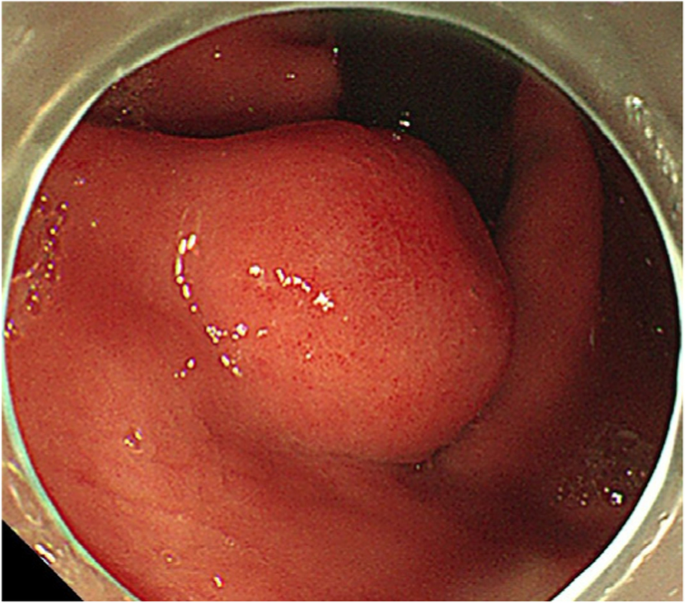
Image of endoscopy of a sessile submucosal tumor (SMT)-type GHIP in the gastric cardia
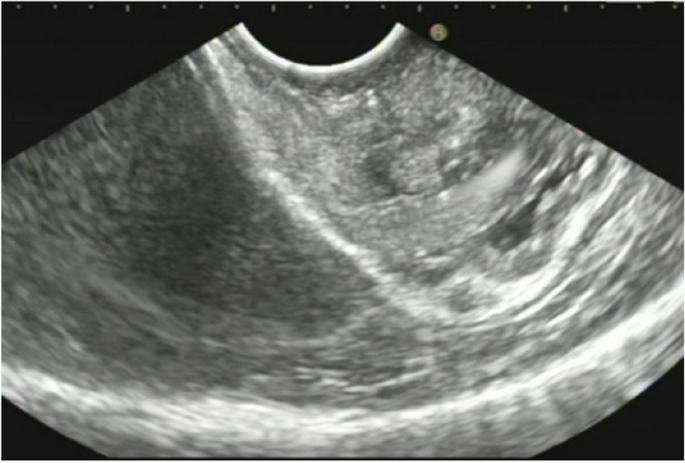
Endoscopic ultrasound (radial scan, 10 MHz) revealed the GHIP as a heterogeneous tumor with multiple small hypoechoic or anechoic areas in the third layer of the gastric wall
Images of endoscopy of a GHIP in the gastric fundus with mucosal diffuse redness, which indicated H. pylori currently infection ( a white light image. b narrow-band image)
In order to investigate the correlation to Helicobacter pylori infection status, we compared the age, gender, morphology, maximum diameter and location of GHIPs between patients with and without H. pylori infection. The differences were not statistically significant ( P > 0.05), as shown in Table 2 .
Histopathological findings
Histopathological findings in the ten GHIPs were summarized in Table 3 . The histopathological examination of the ten GHIPs revealed well-circumscribed and lobulated submucosal proliferation of cystically dilated hyperplastic glands and smooth muscle bundles, partly including fibroblast cells and calcification (Fig. 5 a and b). Within the GHIPs, the glandular structures mainly consisted of foveolar type (Fig. 6 ), cardiac/pyloric/mucous-neck type epithelium (Fig. 7 ), meanwhile a small quantity of fundic type or intestinal metaplasia cells were found in four cases. The continuity between the submucosal glands or cystic elements and the overlying gastric mucosa through a defect of the muscularis mucosa was observed in six GHIPs (6/10, 60%) (Fig. 8 ). Inflammatory cell infiltration was observed in the submucosal stroma within all the ten GHIPs. Only one GHIP (1/10, 10%) complicated with submucosal glandular low-grade dysplasia, but none was accompanied by adenocarcinoma. Assessment of the surrounding mucosa demonstrated that six patients (60%) had H. pylori –associated gastritis or atrophic gastritis with intestinal metaplasia (one of them was diagnosed as autoimmune gastritis), and four patients (40%) had non-specific gastritis.
a Low-power view of HE staining illustrated the inverted growth lesion, which consisted of dilated glands in various sizes and shapes in the submucosa. b Medium-power magnification demonstrated foveolar and mucous-neck glands without cytological atypia and partial cystic dilation
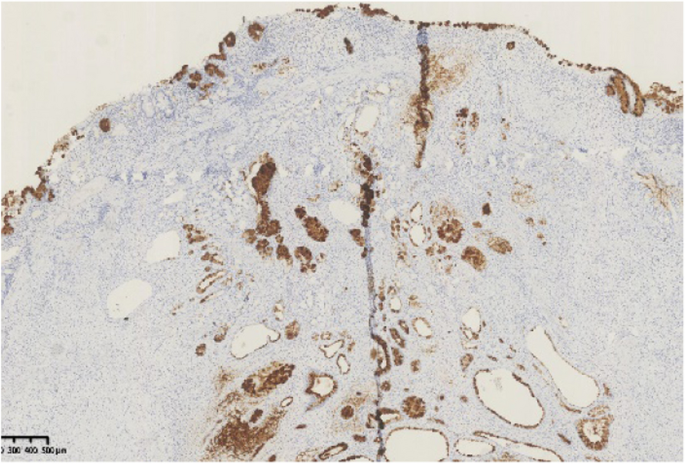
The foveolar epithelium of the overlying mucosa and foveolar type glands in the submucosal lesion were positive for the mucin-5AC immunohistochemical stain
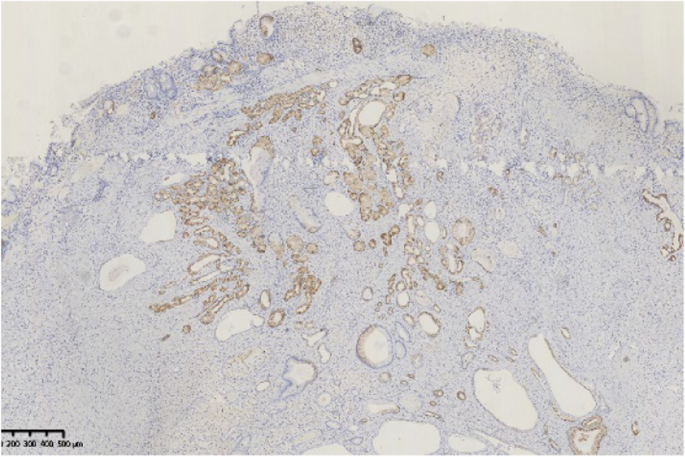
Immunohistochemical stain for mucin 6 showed positive glands in both the overlying mucosa and the submucosal lesion
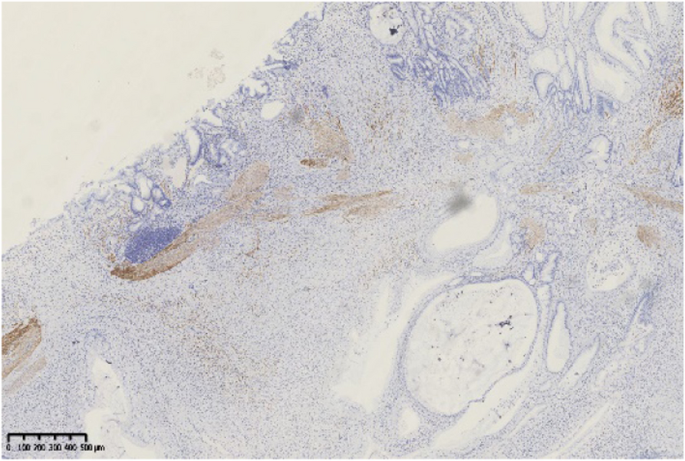
Immunohistochemical stain for Desmin showed the submucosal glands or cystic elements were connected with the overlying gastric mucosa through defects of the muscularis mucosa
In the present study, we reported the clinicopathologic and endoscopic features of ten patients with GHIPs (the second largest case study of GIPs up to now). Furthermore, a literature review was conducted on previously reported cases of GIPs in PubMed using various keywords such as 'gastric inverted polyp', 'gastric inverted hyperplastic polyp', or 'gastric hamartomatous inverted polyp' between January 1978 and May 2023. To our knowledge, only 45 cases of GHIPs have been reported in English [ 1 , 3 , 5 , 6 , 7 , 8 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 ]. Table 4 summarizes the clinicopathological and endoscopic characteristics of these patients. Our review of the previously reported patients, as well as the ten present patients (totally 55 patients and 56 lesions), revealed a slight male predominance (33 males and 22 females) and a median age at diagnosis of 58 years (range: 23–81 years). Most of the patients were asymptomatic and found incidentally. However, some patients (13/55, 23.6%) presented with non-specific symptoms, including epigastric pain/discomfort, dyspepsia or heartburn and acid regurgitation. Furthermore, few patients presented with anemia secondary to chronic hemorrhage [ 3 , 5 , 14 ] and gastrointestinal obstruction [ 28 ] due to the lesion. The vast majority of GHIPs (54/56, 96.4%) located in the proximal stomach and the most common location was in the body (40/56, 71.4%), followed by the fundus (11/56, 19.6%), cardia (3/56, 5.4%) and antrum (2/56, 3.6%). The median diameter was 17 mm (range, 5–45 mm). The vast majority of the patients (54/55, 98.2%) had only one GHIP in the stomach, except for one patient [ 5 ] (1/55, 1.8%) who had 2 GHIPs.
The endoscopic manifestations of GHIPs were diverse. Aoki et al. [ 17 ] classified the appearances of GHIPs into sessile SMT-type and pedunculated polyp-type on endoscopy. According to this classification, sessile SMT-type was more frequently noted in GHIPs (38/56, 67.9%). Typically, the surface of GHIPs was covered with almost intact gastric mucosa, and an erosive redness or depression was frequently noted, which would indicate the relationship between GHIPs and mucosal inflammation, meanwhile a central orifice or dell with or without milky mucus outflow was occasionally observed, which would indicate the communication between submucosal lesion and gastric lumen. On EUS, the majority demonstrated a heterogeneous lesion with multiple anechoic cystic areas in the third layer of the gastric wall (23/29, 79.3%), however, a minority demonstrated a hypoechoic lesion (6/29, 20.7%). It is difficult to distinguish a GHIP with or without adenocarcinoma based on the EUS manifestation s.
Furthermore, the assessment of the surrounding mucosa in the 55 patients revealed the presence of atrophic gastritis/intestinal metaplasia in 21 patients (among them 1 patient was diagnosed as autoimmune gastritis), H.pylori -associated gastritis in 10 patients, non-specific chronic gastritis in 11 patients, and data not available in 19 patients. The continuity between the submucosal glands or cystic elements and the overlying gastric mucosa through a defect of the muscularis mucosa or direct communication with the gastric mucosa was observed in 24 GIPs, suggesting that the polyp may have been formed by the heterotopic inverted downgrowth of mucosal glands into the submucosa. In addition, infiltration of chronic inflammatory cells was observed in the submucosal stroma within all GHIPs. According to all these findings, although the pathogenesis of GIPs is unknown, the heterotopic inverted downgrowth of mucosal components in GIPs is thought to develop as a result of infiltration of the mucosa through the muscularis, mucosal crevices or defects caused by repeated erosion due to various types of chronic gastritis [ 32 ]. Smooth muscle proliferated bundles would be induced by the regenerative process of both the mucosa and muscularis mucosae caused by repeated erosion [ 7 ], supporting the view of GIPs as regenerative lesions as well.
According to the classification of Kim et al. [ 8 ], the present study consisted of type 2 and type 3 GHIPs, and within the lesions no carcinomatous component was observed. However, although the exact association between gastric adenocarcinoma and GHIP is still controversial, a few studies reported GHIP coexisted with adenocarcinoma within the lesion [ 8 , 24 , 27 , 33 ], or outside the lesion presented as synchronous or metachronous gastric adenocarcinoma [ 5 , 6 , 7 , 8 , 25 ]. The surrounding mucosa was assessed in 9 out of the 14 patients accompanied by adenocarcinoma, revealing H. pylori –associated gastritis or atrophic gastritis with intestinal metaplasia in eight patients (8/9, 88.9%), and non-specific chronic gastritis in only one patient. All the six GHIPs coexisted with adenocarcinoma within the lesion were classified into type 1 [ 8 ], characterized by a central mucosal communicating structure, which may be the reason for neoplasia because it allowed a continuous exposure to luminal carcinogen and mechanical stress [ 8 ]. In four out of the six patients, a hyperplasia–dysplasia–carcinoma sequence was noted within the lesion [ 8 , 24 , 33 ], which indicated that adenocarcinoma might originate from a benign GHIP. Moreover, Ohtsu et al. [ 14 ] reported three patients with GHIP whose gene analysis demonstrated no significant mutation. It seems that GHIPs may not be premalignant lesions, but the gastric mucosa with H. pylori –associated gastritis or atrophic gastritis with intestinal metaplasia in or outside the polyp is more likely to harbor an adenocarcinoma. Therefore, one important implication of GHIPs appears to be a marker for an abnormal gastric mucosal background that is associated with the development of gastric cancer.
Moreover, as is known to all, gastrin plays a key role in gastric physiology, including various cellular processes such as proliferation, differentiation, angiogenesis, and apoptosis [ 34 , 35 , 36 ]. Gastric mucosal inflammation and hypergastrinemia, especially due to atrophic gastritis in oxyntic mucosa and H.pylori infection may play a major role in the development and neoplasia of GIPs as a result of repair, regeneration and proliferation. Additional clinicopathological studies are needed to further clarify the pathogenesis of GIPs and the association between the development of GHIPs and precancerous potential with various forms of gastritis.
In terms of treatment, endoscopic diagnosis of a GIP and neoplastic potential within a GIP may be difficult, and biopsy often faces incomplete pathological sampling of the remaining masses, therefore, complete resection may be required for subsequent pathological examination. GHIPs need to be differentiated from ectopic pancreas, gastritis cystica profunda (GCP), gastrointestinal stromal tumor (GIST) and neuroendocrine tumor (NET) [ 1 , 17 , 31 ], mainly through pathological characteristics. The key points of differentiation are as follows: (1) Ectopic pancreas: Microscopically pancreatic acini and ducts can help to distinguish from GHIP, (2) GCPs usually locate at the anastomotic site of the gastrointestinal tract, and the submucosal glands are composed of simple glands without obvious proliferative changes. (3) GIST and NET, as common submucosal tumors in the stomach, can be distinguished from GHIP through immune phenotypes (including immunohistochemical staining for CD34、CD117、DOG1 and Chromogranin A).The polyp-type GHIPs can be resected endoscopically by EMR, but for SMT-type especially larger than 20 mm in diameter, ESD is practical for en bloc resection currently [ 1 , 3 , 6 , 13 , 14 , 16 , 18 , 19 , 21 , 24 , 25 , 26 , 28 , 29 , 37 ], which is consistent with our present 10 cases. However, it has also been reported that laparoscopic resection may be suitable for SMT-type GHIPs with a diameter more than 20 mm [ 17 , 23 , 27 , 33 ].
In summary, we present a comprehensive clinicopathologic analysis of 10 GHIPs with a literature review. GHIPs often arise from the background of abnormal mucosa, such as atrophic or H.pylori -associated gastritis. We make the hypothesis that acquired inflammation might lead to the development of GHIPs, and the gastric mucosa with H. pylori–associated gastritis or atrophic gastritis with intestinal metaplasia, whether inside or outside the polyp, is more likely to harbor an adenocarcinoma. We recommend to make a full assessment of the background mucosa and H. pylori infection status, so as to evaluate the underlying gastric mucosal abnormalities, which may be the preneoplastic condition of the stomach. Nonetheless, considering the limited number of cases and the nature of retrospective analysis in this study, further studies are needed.
Availability of data and materials
The authors confirm that the data supporting the findings of this study are available within the article.
Abbreviations
- Gastric hamartomatous inverted polyp
Gastric inverted hyperplastic polyp
Gastric inverted polyp
Endoscopic mucosal resection
Endoscopic submucosal dissection
Helicobacter pylori
Urea breath test
Hematoxylin and eosin
Submucosal tumor
Endoscopic ultrasonography
Low grade intraepithelial neoplasia
Autoimmune gastritis
Atrophic gastritis
Intestinal metaplasia
H.pylori–associated gastritis
Non-specific gastritis
Gastritis cystica profunda
Gastrointestinal stromal tumor
Neuroendocrine tumor
Mori H, Kobara H, Tsushimi T, Fujihara S, Nishiyama N, Matsunaga T, et al. Two rare gastric hamartomatous inverted polyp cases suggest the pathogenesis of growth. World J Gastroenterol. 2014;20:5918–23.
Article PubMed PubMed Central Google Scholar
Sipponen P, Siurala M. Cystic, “hamartomatous” epithelial polyps of the stomach. Acta Hepatogastroenterol (Stuttg). 1978;25:380–3.
CAS PubMed Google Scholar
Yun JT, Lee SW, Kim DP, Choi SH, Kim SH, Park JK, et al. Gastric inverted hyperplastic polyp: A rare cause of iron deficiency anemia. World J Gastroenterol. 2016;22:4066–70.
Lee YH, Joo MK, Lee BJ, Lee JA, Kim T, Yoon JG, et al. Inverted Hyperplastic Polyp in Stomach: A Case Report and Literature Review. Korean J Gastroenterol. 2016;67:98–102.
Article PubMed Google Scholar
Yamashita M, Hirokawa M, Nakasono M, Kiyoku H, Sano N, Fujii M, et al. Gastric inverted hyperplastic polyp Report of four cases and relation to gastritis cystica profunda. APMIS. 2002;110:717–23.
Lee SJ, Park JK, Seo HI, Han KH, Kim YD, Jeong WJ, et al. A case of gastric inverted hyperplastic polyp found with gastritis cystica profunda and early gastric cancer. Clin Endosc. 2013;46:568–71.
Kamata Y, Kurotaki H, Onodera T, Nishida N. An unusual heterotopia of pyloric glands of the stomach with inverted downgrowth. Acta Pathol Jpn. 1993;43:192–7.
Kim JY, Ahn S, Kin KM, Chang SH, Kim HS, Lee JH, et al. Gastric inverted polyps-distinctive Subepithelial lesions of the Stomach: Clinicopathologic analysis of 12 cases: with an emphasis on neoplastic potential. Am J Surg Pathol. 2021;45:680–9.
Kamada T, Haruma K, Inoue K, et al. Helicobacter pylori infection and endoscopic gastritis -Kyoto classification of gastritis[J]. Nihon Shokakibyo Gakkai Zasshi. 2015;112(6):982–93.
PubMed Google Scholar
Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy. 1969;3:87–97.
Article Google Scholar
Sakaki N, Arakawa T, Katou H, et al. Relationship between progression of gastric mucosal atrophy and Helicobacter pylori infection: retrospective long-term endoscopic follow-up study. J Gastroenterol. 1997;32:19–23.
Article CAS PubMed Google Scholar
Ma Q, Gao L, Sun N, Chen Y, Liu L, Liu L, et al. Gastric inverted hyperplastic polyp: A case report. Exp Ther Med. 2023;25:6.
Han YP, Min CC, Li YB, Chen YQ, Liu H, Tian ZB, et al. Diagnosis and treatment of gastric hamartomatous inverted polyp (GHIP) by endoscopic submucosal dissection: A case report. Medicine (Baltimore). 2023;102:e33443.
Ohtsu T, Takahashi Y, Tokuhara M, Tahara T, Ishida M, Miyasaka C, et al. Gastric hamartomatous inverted polyp: Report of three cases with a review of the endoscopic and clinicopathological features. DEN Open. 2023;3:e198.
Miyamoto Y, Muguruma N, Okamura S, Okada Y, Kitamura S, Okamoto K, et al. A pedunculated submucosal lesion in the stomach with inverted downgrowth. Intern Med. 2014;53:1625–8.
Hashimoto R, Okuzono T, Alahira J. An Uncommon Gastric Submucosal Tumor With Mucosal Erosion. Clin Gastroenterol Hepatol. 2018;16:e57–8.
Aoki M, Yoshida M, Saikawa Y, Otani Y, Kubota T, Kumai K, et al. Diagnosis and treatment of a gastric hamartomatous inverted polyp: report of a case. Surg Today. 2004;34:532–6.
Jung M, Min KW, Ryu YJ. Gastric inverted hyperplasic polyp composed only of pyloric glands: a rare case report and review of the literature. Int J Surg Pathol. 2015;23:313–6.
Matsui S, Kashida H, Kudo M. Gastric Inverted Hyperplastic Polyp Mimicking a Papilla. Am J Gastroenterol. 2018;113:462.
Koh J, Lee KL, Lee MS, Ahn HS, Chang MS. Gastric inverted hyperplastic polyp with inflammatory myofibroblastic tumor-like stroma, mimicking GI stromal tumor. Gastrointest Endosc. 2019;89:433–5.
Ng HI, Li ZQ, Zhang YM, Hu CF, Wang GQ. Gastric inverted hyperplastic polyp, an exceptional case diagnosed after endoscopic submucosal dissection. Clin Res Hepatol Gastroenterol. 2022;46:101890.
Noh BJ, Min JW, Sung JY, Park YK, Lee J, Kim YW. Inflammatory myofibroblastic tumor-like stromal proliferation within gastric inverted hyperplastic polyp. Pathol Int. 2016;66:180–2.
Hayase S, Sakuma M, Chida S, Saito M, Ami H, Koyama Y, et al. Diagnosis and treatment of gastric hamartomatous inverted polyp (GHIP) using a modified combination of laparoscopic and endoscopic approaches to neoplasia with a non-exposure technique (modified CLEAN-NET): a case report. Surg Case Rep. 2020;6:200.
Kim HS, Hwang EJ, Jang JY, Lee J, Kim YW. Multifocal Adenocarcinomas Arising within a Gastric Inverted Hyperplastic Polyp. Korean J Pathol. 2012;46:387–91.
Ono S, Kamoshida T, Hiroshima Y, Okawara A, Matsuo T, Kakinoki N, et al. A case of early gastric cancer accompanied by a hamartomatous inverted polyp and successfully managed with endoscopic submucosal dissection. Endoscopy. 2007;39:E202.
Dohi M, Gen Y, Yoshioka M. A case of gastric hamartomatous inverted polyp resected endoscopically. Endosc Int Open. 2016;4:E608–9.
Okamura T, Iwaya Y, Nagaya T, Muranaka F, Ota H, Umemura T. Gastric adenocarcinoma arising from hamartomatous inverted polyp during 8-year follow-up. DEN open. 2022;2:e16.
Sudo G, Tanuma T, Nakase H. Gastric Hamartomatous Inverted Polyp Causing Ball Valve Syndrome. Clin Gastroenterol Hepatol. 2019;17:e119–20.
Odashima M, Otaka M, Nanjo H, Jin M, Horikawa Y, Matsuhashi T, et al. Hamartomatous inverted polyp successfully treated by endoscopic submucosal dissection. Intern Med. 2008;47:259–62.
Carfagna G, Pilato FP, Bordi C, Barsotti P, Riva C. Solitary polypoid hamartoma of the oxyntic mucosa of the stomach. Pathol Res Pract. 1987;182:326–30.
Itoh K, Tsuchigame T, Matsukawa T, Takahashi M, Honma K, Ishimaru Y. Unusual gastric polyp showing submucosal proliferation of glands: case report and literature review. J Gastroenterol. 1998;33:720–3.
Yamagiwa H, Matsuzaki O, Ishihara A, Yoshimura H. Heterotopic gastric glands in the submucosa of the stomach. Acta Pathol Jpn. 1979;29:347–50.
Kono T, Imai Y, Ichihara T, Miyagawa K, Kanemitsu K, Ajiki T, et al. Adenocarcinoma arising in gastric inverted hyperplastic polyp: a case report and review of the literature. Pathol Res Pract. 2007;203:53–6.
Dockray GJ. Topical review Gastrin and gastric epithelial physiology. J Physiol. 1999;518:315–24.
Article CAS PubMed PubMed Central Google Scholar
Peach HG, Barnett NE. Determinants of basal plasma gastrin levels in the general population. J Gastroenterol Hepatol. 2000;15:1267–71.
Wroblewski LE, Pritchard DM, Carter S, Varro A. Gastrin-stimulated gastric epithelial cell invasion: the role and mechanism of increased matrix metalloproteinase 9 expression. Biochem J. 2002;365:873–9.
Yang TC, Hou MC, Chen PH, Liao WC, Li AF. Gastric hamartomatous inverted polyp mimicking ectopic pancreas on endoscopy and endosonography. Endoscopy. 2014;46:E119–20.
Download references
Acknowledgements
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and affiliations.
Department of Gastroenterology, Beijing Friendship Hospital, Capital Medical University, State Key Laboratory for Digestive Health, National Clinical Research Center for Digestive Diseases, Beijing Digestive Disease Center, Beijing Key Laboratory for Precancerous Lesion of Digestive Diseases, Beijing, 100050, China
Ningning Dong & Fandong Meng
Department of Pathology, Beijing Friendship Hospital, Capital Medical University, Beijing, 100050, China
Department of Gastroenterology, Shijingshan Teaching Hospital of Capital Medical University, Beijing Shijingshan Hospital, 24 Shi-Jing-Shan Road Shi-Jing-Shan District, Beijing, 100040, China
Junzhen Hou
You can also search for this author in PubMed Google Scholar
Contributions
N.D. conceived of and performed the study, wrote the manuscript, and gave final approval of the version to be published. F.M.participated in the conception and data collection. B.Y. was responsible for the pathological analysis. J.H. conceived of and performed the study, participated in critically revising the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Junzhen Hou .
Ethics declarations
Ethics approval and consent to participate.
This study was approved by the Ethic Committee of Beijing Friendship Hospital, Capital Medical University (Beijing, China). All the ten patients provided informed consent for treatment and participation.
Consent for publication
Not applicable. All details of participants’ images or information have been de-identified so that the identities of the patients may not be ascertained in any way.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Dong, N., Meng, F., Yue, B. et al. Clinicopathologic and endoscopic characteristics of ten patients with gastric hamartomatous inverted polyp: a single center case series. BMC Gastroenterol 24 , 139 (2024). https://doi.org/10.1186/s12876-024-03233-8
Download citation
Received : 09 September 2023
Accepted : 16 April 2024
Published : 22 April 2024
DOI : https://doi.org/10.1186/s12876-024-03233-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Clinicopathologic characteristic
- Gastric atrophy
BMC Gastroenterology
ISSN: 1471-230X
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Case report
- Open access
- Published: 23 April 2024
Genetic exploration of Dravet syndrome: two case report
- Agung Triono 1 ,
- Elisabeth Siti Herini ORCID: orcid.org/0000-0003-2571-8310 1 &
Journal of Medical Case Reports volume 18 , Article number: 215 ( 2024 ) Cite this article
Metrics details
Dravet syndrome is an infantile-onset developmental and epileptic encephalopathy (DEE) characterized by drug resistance, intractable seizures, and developmental comorbidities. This article focuses on manifestations in two Indonesian children with Javanese ethnicity who experienced Dravet syndrome with an SCN1A gene mutation, presenting genetic analysis findings using next-generation sequencing.
Case presentation
We present a case series involving two Indonesian children with Javanese ethnicity whom had their first febrile seizure at the age of 3 months, triggered after immunization. Both patients had global developmental delay and intractable seizures. We observed distinct genetic findings in both our cases. The first patient revealed heterozygous deletion mutation in three genes ( TTC21B , SCN1A , and SCN9A ). In our second patient, previously unreported mutation was discovered at canonical splice site upstream of exon 24 of the SCN1A gene. Our patient’s outcomes improved after therapeutic evaluation based on mutation findings When comparing clinical manifestations in our first and second patients, we found that the more severe the genetic mutation discovered, the more severe the patient’s clinical manifestations.
These findings emphasize the importance of comprehensive genetic testing beyond SCN1A , providing valuable insights for personalized management and tailored therapeutic interventions in patients with Dravet syndrome. Our study underscores the potential of next-generation sequencing in advancing genotype–phenotype correlations and enhancing diagnostic precision for effective disease management.
Peer Review reports
Dravet syndrome (DS), previously known as severe myoclonic epilepsy of infancy (SMEI), is an infantile-onset developmental and epileptic encephalopathy (DEE) characterized by drug resistance, intractable seizures, and comorbidities including intellectual disability, behavioral problems, sleep disturbances, gait disturbances, and an increased risk of sudden unexpected death in epilepsy [ 1 , 2 ]. The incidence of DS is approximately 1 in every 15,700 births [ 3 ]. The first symptom of DS is seizures in the first year of life, followed by developmental delay [ 1 ]. This first seizure is either generalized tonic–clonic or focal (occasionally hemiclonic) clonic, and in more than half of the cases, it is a febrile seizure, making it difficult to distinguish from a self-limiting febrile seizure. Infection, hot environment, exhaust, sunlight, or exercise can initiate an attack of DS [ 4 , 5 ]. Approximately 80% of patients with DS carry a pathogenic variant of the sodium channel alpha 1 subunit ( SCN1A ) gene resulting in haploinsufficiency Nav1.1, the alpha-1 subunit of the sodium channel. PCDH19, SCN2A, SCN8A, SCN1B, GABRA1, GABRB3, GABRG2, KCNA2, CHD2, CPLX1, HCN1A, and STXBP1 variants may also be involved in DS or DS-like phenotypes. Accordingly, genetic testing is required to identify other genes that play a role in the DS phenotype and to expand genotype-DS phenotype correlations to enhance the future management of this disease [ 6 ]. In the last decade, next-generation sequencing (NGS) technology has been able to analyze a set of genes (targeted panel sequencing), exome [(whole exome sequencing (WES)], or genome [whole genome sequencing (WGS)] in a single sequencing process, making it possible to diagnose rare diseases such as early childhood epilepsy [ 7 ]. Identification of the genetic basis of DS can provide additional information regarding pathophysiology, prognosis, and individual drug therapy options according to the patient’s condition.
We present a case series involving two children, one aged 11 years and 2 months, and the other aged 1 year and 4 months. Both children were diagnosed with DS, exhibiting symptoms of intractable seizures, global developmental delay, and seizures triggered by postimmunization fever. Despite displaying similar symptoms, the two individuals possess different genetic variants of the SCN1A gene and also possible novel mutation in DS. We also discuss the main clinical characteristics, treatment course, and management of DS at tertiary referral hospitals in Indonesia.
A boy with Javanese ethnicity aged 11 years and 2 months with uncontrollable seizures regularly visits our hospital. The patient had his first seizure at the age of 3 months with a duration of 15 min, and it was triggered after receiving diphtheria–pertussis–tetanus (DPT) immunization, which was accompanied by fever. The patient has about six to seven seizures per day for 1 min in the form of generalized tonic–clonic and absence seizures. He was the first child of nonconsanguineous healthy parents with normal prenatal and birth history. He has a younger sister with normal development. There is no history of family members with febrile seizure. The patient was born at 40 weeks of gestation, with a birth weight of 4000 g, length of 52 cm, and head circumference of 33 cm. The patient is currently experiencing global developmental delay and is still in kindergarten. He had learning difficulties and was unable to speak words at an age-appropriate level. He had delayed motor development and was unable to perform age-appropriate motor activities. Head circumference was 46.5 cm (microcephaly). There were no signs of meningeal irritation nor Babinski response. The motor examination revealed no increased tone in the upper and lower limb. Other systemic examinations revealed no abnormalities. Interictal electroencephalography (EEG) showed diffuse epileptiform irritative abnormality on a normal background (Fig. 1 ). Magnetic resonance imaging (MRI) of the brain showed cerebral atrophy, bilateral frontal subarachnoid enlargement, bilateral occipital lobe and polymicrogyria, and a neuroglial cyst in the right temporal lobe (Fig. 2 ). He was recommended to get genetic testing done since he was suspected of having DS.
Electroencephalography (EEG) shows diffuse epileptiform irritative abnormality on a normal background
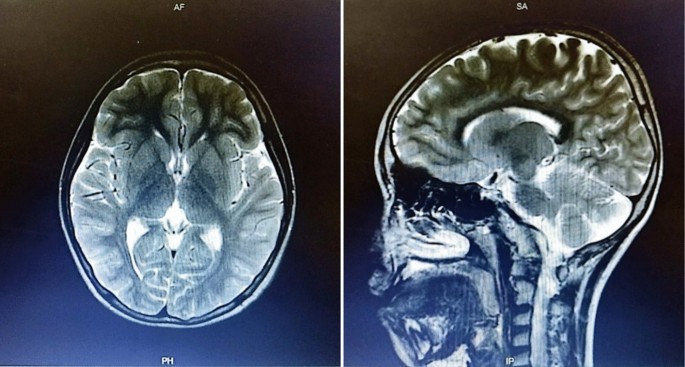
Axial brain magnetic resonance imagery shows cerebral atrophy, bilateral frontal subarachnoid enlargement, bilateral occipital lobe polymicrogyria, and a neuroglial cyst in the right temporal lobe
Whole genome sequencing (WGS), whole exome sequencing (WES), and Sanger sequencing were performed at 3Billion (Seoul, Korea). The WGS and WES procedures were conducted according to the protocols of Richards et al . [ 8 ] and Seo et al . [ 9 ], respectively. Both WES and WGS are comprised of four main parts: (1) high-quality sequencing; (2) sequencing data analysis including alignment to the genome reference consortium human 37 (GRCh37)/hg19 for WES, also alignment to the genome reference consortium human 38 (GRCh38) and revised Cambridge reference sequence (rCRS) of the mitochondrial genome for WGS; (3) variant annotation and prioritization by EVIDENCE [a software that was developed in house to prioritize variants based on the American College of Medical Genetics and Genomics (ACMG) guidelines [ 10 ]]; and (4) variant interpretation in the context of the patient’s symptoms and reporting of disease-causing variants. Once EVIDENCE prioritizes the top candidate variants/genes, 3Billion’s highly-trained clinical/medical geneticists manually curate each variant to identify the disease-causing variant for reporting.
In our initial examination, we performed WES on patient 1 and subsequently identified a copy number variant (CNV), prompting us to proceed with WGS. The WGS analysis revealed a heterozygous pathogenic 552.9 Kb deletion variant in 2q24.3. The heterozygous deletion NC_000002.12:g.165811316_166364199delinsTGTACACTA at 2q24.3 spans across three genes ( TTC21B , SCN1A , and SCN9A ). The variant is not observed in the gnomAD SVs v2.1.1 dataset. SCN1A is subject to haploinsufficiency. Other pathogenic variants have been reported in this region. There are multiple similarly affected individuals reported with similar likely pathogenic copy–number–loss overlapping this region [ 11 , 12 ]. Therefore, this variant was classified as pathogenic. Due to region-spanning mutation in SCN1A, which suitable with clinical manifestation, the patient was diagnosed with DS (OMIM 607208: since we were unable to perform a Sanger sequencing study on both of the parents, the pattern of inheritance is still uncertain.
The arents were counseled about their child’s condition and agreed to undergo multipronged therapy. Before the patient was diagnosed with DS, he had received valproic acid (30 mg/kg per day), phenobarbital (2.5 mg/kg per day), and oxcarbazepine (5 mg/kg per day), also physio, occupation, and speech therapy but had not shown significant improvement. He was seizure-free for 3 months after oxcarbazepine was changed to levetiracetam (27 mg/kg per day). However, the patient then had another episodes of less than 5 minutes general tonic–clonic seizure (GTCS)-induced by fever. Interictal EEG was performed to evaluate his condition, and we found that the diffuse epileptiform irritative abnormality persisted.
A 1 year and 4 month-old-girl with Javanese ethnicity was referred to our hospital due experiencing myoclonic seizure followed by 20 minute GTCS at 3 months, after fever following DPT immunization. She then continued to experience generalized tonic–clonic seizures one to two times per day for 10–15 seconds. At 9 months of age, the patient received a second DPT immunization, and on the same day, she had another generalized tonic–clonic seizure that lasted > 30 minutes, resulting in her admission to the pediatric intensive care unit. Before the first seizure, the patient could lift her head, grasp a toy and make eye contact, but after that, she could neither lift her head nor grasp an object. The patient has no previous history of trauma.
She had a normal head circumference increased physiological reflexes in all extremities. Other systemic examinations revealed no abnormalities. Computed tomography (CT) scan examination of the head showed a subdural hygroma in the right and left frontoparietal region, without any other abnormalities (Fig. 3 ). Electroencephalography (EEG) at the beginning of the seizure did not show any abnormalities, but the EEG follow-up 7 months after the onset of the seizure showed an abnormal epileptiform (spike wave) with a normal background (Fig. 4 ). Thus, she was suspected of having DS and was recommended to undergo genetic examination.
Axial brain computed tomography scan shows a subdural hygroma in the right and left frontoparietal region, without any other abnormalities
Electroencephalography shows abnormal irritative epileptiform with a normal background
Whole exome sequencing (WES) showed a likely pathogenic variant identified as a heterozygous mutation of the SCN1A gene with genomic position 2-166859265-T-C (GRCh37), [NM_001165963.4:C.4003-2A > G [NP_001159435.1:p.?]. The variant is located in the canonical splice site upstream of exon 24 of SCN1A gene (NM_001165963.4 transcript). Since this variant is an essential splicing variant, the protein consequence is uncertain and therefore represented as (p.?). In this patient’s genetic mutation, the canonical junction site occurs which is expected to alter the junction and result in loss or disruption of normal protein function. However, using an in silico predictor, spliceAI ( https://spliceailookup.broadinstitute.org/ ), the variant is predicted to result in a loss of 22 base pairs at end of exon 24. This loss is expected to create a frameshift at the Gly1342 position. Sanger sequencing confirmed the patient’s genotype (Fig. 5 A), but the mother’s Sanger analysis was negative (Fig. 5 B). Due to familial issues, Sanger sequencing was not performed on the father, leaving the inheritance pattern unresolved.
A Sanger sequencing result of patient 2 showed a heterozygous mutation of the SCN1A gene with the genomic position 2-166859265-T-C (GRCh37), [NM_001165963.4:C.4003-2A >G [NP_001159435.1:p.?] (red arrow); and B Sanger sequencing result of patient 2’s mother showed normal sequence
The parents were counseled about their child’s condition and agreed to undergo multipronged therapy. Before patient was diagnosed with DS, she received clonazepam (0.01 mg/kg per day), valproic acid (29 mg/kg per day), and phenytoin (5 mg/kg per day), but seizure persisted. When phenytoin was stopped, with valproic acid (30 mg/kg per day) and clonazepam (0.04 mg/kg per day) adjusted, seizures were greatly decreased. Later, patient only experienced one seizure per year. The patient routinely received physio, speech, and occupational therapy.
When comparing the clinical features and outcomes of the two patients (Table 1 ), we found that our first patient, who had three medications, was still having a generalized seizure induced by fever with duration less than 5 minutes after they had been seizure-free for 3 months (at the age 11 years and 8 months. Our second patient, however, only experienced one seizure annually after receiving two medications (at the age 1 year and 10 months). This difference implies that the clinical state of the first patient was worse than that of the second.
Research on the identification of DS genetic mutations using NGS has never been done in Indonesia. In 2010, we conducted a study to identify pathogenic variants of the SCN1A gene using the Sanger sequencing method and successfully reported cases of novel SCN1A mutations in Indonesia in patients with severe myoclonic epilepsy in infancy (SMEI) and borderline SMEI (SMEB). The first boy identified with SMEI experienced a variety of seizures, including his first febrile seizure and general tonic–clonic seizure at 7 months of age, and later suffered from myoclonic seizures, left-sided hemiconvulsions, also focal convulsions without fever, along with delayed speech development. The second patient with SMEB had his first febrile seizures with GTCS after immunization at 3 months old, then later on experienced status epilepticus, GTCS, and atonic convulsions without fever [ 13 ]. We also conducted another research on the spectrum of generalized epilepsy with febrile seizure plus (GEFS+) focusing on clinical manifestations and SCN1A gene mutations. That study analyzed a total of 34 patients who suffered from SMEI (7 patients), SMEB (7 patients), febrile seizure plus (FS+) and absence/myoclonic/atonic/partial seizures (11 patients), and FS+ (9 patients) [ 14 ].
However, the research that we have done uses the Sanger sequencing genetic examination, which is expensive and takes considerable time. Additionally, it is unable to find any other gene besides SCN1A in patients with DS. A study by Djémié et al . in Belgium reported the discovery of 28 pathogenic variants of the SCN1A gene using the NGS method which were previously missed or undiagnosed using Sanger sequencing [ 7 ]. To link DS cases more effectively, we are attempting to conduct NGS genetic tests, specifically WES and WGS.
Dravet syndrome (DS) was infrequently reported in Indonesia due to its difficulty in diagnosis, misdiagnosis as febrile seizures or other epilepsy syndromes, or lack of follow-up and genetic testing in our country. According to the to the International League Against Epilepsy (ILAE) [ 15 ], the diagnostic criteria for this condition should consist of a number of the following symptoms: (1) a family history of epilepsy or febrile seizures; (2) normal development before seizures onset; (3) seizure before 1 year of age; (4) EEG with generalized spike and polyspike waves; (5) pleomorphic epilepsy (myoclonic, focal, clonic, absence, and generalized seizures); (6) focal abnormalities or early photosensitivity; (7) psychomotor retardation after 24 months; (8) exacerbation of seizures with increased body temperature; and (9) the appearance of subsequent ataxia, pyramidal signs or interictal myoclonus after the beginning of psychomotor slowing. Both of our patients had seizures beginning with increased body temperature and regression of development after seizure onset, which were resistant to the majority of anticonvulsant medications. The seizures began as generalized tonic–clonic seizures, followed by absence seizures. Both of our patients also experienced subsequent ataxia and pyramidal signs. Thus, they were suspected of having DS and were advised to undergo genetic testing.
Infants with DS have normal physical and psychomotor development at the time of their first seizure, which typically occurs between the ages of 5 and 8 months. In our case series, both of our patients experienced their first seizure at the age of 3 months [ 16 , 17 ]. In the first year of life, the most common form of seizure is febrile tonic–clonic. Some patients may experience myoclonic and dyscognitive seizures infrequently. Frequently, protracted seizures result in status epilepticus. In the first year of life, seizures are precipitated by fever/illness, immunization, and cleansing [ 16 ]. As the infant develops, he or she will experience a variety of seizure types, as well as fever and emotional stress, flashes of light, and overexertion being seizure triggers. The child with DS will develop hypotonia, ataxia, incoordination, and pyramidal signs, dysautonomia events, cognitive impairment, and behavioral disturbances such as attention deficit, hyperactivity, or autistic characteristics [ 15 ]. Some of the conditions above are very consistent with what happened to our patients.
The EEG performed during the early phases of the disease is normal. However, as the child grows, generalized spike waves with isolated or brief discharges of fast polyspike waves may be present [ 15 , 18 ]. In the first case, we found diffuse epileptiform irritative abnormality with a normal background, whereas in the second case, initially it was found normal, then a few months later it became abnormal irritative epileptiform with a normal background.
Genetic testing is developing rapidly and playing a significant role in the specific diagnosis and management of epilepsy [ 19 , 20 ]. Several genes with pathogenic mutations produce DS or DS-like phenotypes, which inevitably require different drug therapy approaches. Genes that cause DS can be grouped based on how they work: specifically, three sodium channel-related genes ( SCN2A, SCN8A , and SCN1B ), one potassium channel-related gene ( KCNA2 ), three gamma-aminobutyric acid receptors ( GABAR ) genes ( GABRA2, GABRB3 , and GABRG2 ), a cyclic nucleotide gated cation channel gene ( HCN1 ), and other functional genes including CHD2, CPLX1 , and STXBP1 . Approximately 80% of patients with DS have a pathogenic variant of the SCN1A gene, from which the majority of SCN1A variants are de novo, but 10% of people inherit the SCNA1 mutation from one or both parents [ 6 ]. Both of our patients had a mutation in the SCN1A gene, which is the most common mutation seen in DS.
Furthermore, TTC21B and SCN9A mutations were also found in our first patient. A study conducted by Suls et al . also reported a four generation Bulgarian family with epilepsy, revealing a heterozygous 400 kb deletion on chromosome 2q24 that included the SCN1A and TTC21B genes [ 21 ]. The patients exhibited variable phenotypes, but all experienced generalized tonic–clonic seizures around the first year of life, with some presenting myoclonic or absence seizures. Febrile seizures occurred in three of the four patients during infancy. Notably, one patient had mild mental retardation, another had psychomotor slowing, and a third had mental retardation from early infancy; all showed reduced seizures on medication. The findings in that study parallel the situation observed in our initial patient case. Meanwhile, a study by Singh et al . identified a heterozygous mutation in the SCN9A gene in two patients diagnosed with DS [ 22 ]. One of these patients also exhibited a mutation in the SCN1A gene. The study provided evidence suggesting that the SCN9A gene on chromosome 2q24 could potentially serve as a modifier for DS. Among 109 patients with DS, 8% were found to have an SCN9A mutation. This included six patients with double heterozygosity for SCN9A and SCN1A mutations and three patients with only heterozygous SCN9A mutations, supporting the notion of a multifactorial inheritance pattern [ 22 ]. The previous research confirmed the severity of clinical symptoms in our first patient, whom we identified mutations in the SCN1A, SCN9A , and TTC21B genes.
In the last decade, there has been a very rapid development of neurogenetic science and diagnostic technology. NGS is the latest method of genetic examination that allows for the discovery of causal mutations, including de novo, novel, and familial mutations related to epilepsy syndromes that have variable phenotypic features [ 23 ]. The first generation of DNA sequencing using the Sanger method could only examine one gene at a time and had limitations especially when examining large genomic regions, so the NGS method is more widely used today [ 7 , 23 ]. A study conducted by Kim et al . in Seoul reported an increase in diagnostic yield using WES after targeted panel sequencing with negative results in infantile onset epilepsy by 8%. This result suggests that WES assays increase the opportunity to search for new epilepsy genes and uncover less well-known epileptic phenotypes from known neurological diseases [ 24 ]. The WES examination also allows for the discovery of de novo or inherited mutations if the patient and both parents are examined [ 25 ].
According to the recommendations of the North American consensus panel, clobazam and valproic acid are the first-line therapies for antiepileptic drugs, followed by stiripentol, topiramate and levetiracetam. Patients with a suboptimal response to clobazam and valproic acid have been advised to consider the ketogenic diet as a second-line treatment [ 17 ]. SCN1A is a gene that codes for sodium channel channels, so drugs that work as sodium channel blockers, such as lamotrigine, phenytoin, carbamazepine, oxcarbazepine, lacosamide, and rufinamide, are contraindicated in patients with DS because they can increase the frequency of seizures [ 4 ]. After the failure of first- and second-line therapy, surgical therapies, such as vagus nerve stimulation (VNS), were moderately agreed upon and should be considered [ 17 ]. Besides medication, controlling infections and body temperature variations also showed to decrease the frequency of seizures and severity of the disease [ 18 ]. Initially, the first patient received oxcarbazepine and the second patient got phenytoin, which had been contraindicated to patients with DS. Futhermore, after eliminating medications that were contraindicated, both patients’ outcome improved.
In this study, we discovered unique mutations that have never been documented before, particularly in Indonesia, where NGS analysis of DS genetic variants has never been done. However, the limitation of this study, is that the information comes from two cases only. Further research is needed to explore more cases from Indonesia population.
In summary, our case series utilizing next-generation sequencing (NGS) unveils the intricate genetic landscape of Dravet syndrome (DS) in two Indonesian pediatric cases. By using WGS and WES, we identified distinct mutations in the SCN1A gene, as well as contributions from genes, such as TTC21B and SCN9A . The power of WGS lies in its ability to uncover rare pathogenic variants, including a 552.9 Kb deletion in the 2q24.3 region. These findings emphasize the importance of comprehensive genetic testing beyond SCN1A , providing valuable insights for personalized management and tailored therapeutic interventions in patients with DS. Our study underscores the potential of NGS in advancing genotype–phenotype correlations and enhancing diagnostic precision for effective disease management. Furthermore, we found that the clinical condition of the first patient was worse than that experienced by the second patient. This difference suggests that the more severe the genetic mutation detected, the more severe the clinical manifestations of the patient.
Availability of data and materials
The dataset used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
American College Of Medical Genetics
Copy number variant
Computed tomography
- Dravet syndrome
Developmental and epileptic encephalopathy
Electroencephalography
Febrile seizure plus
Generalized epilepsy with febrile seizure plus
Genome reference consortium human 37
Genome reference consortium human 38
General tonic clonic seizure
International league against epilepsy
Magnetic resonance imaging
- Next-generation sequencing
Revised Cambridge reference sequence
Sodium channel alpha 1 subunit
Severe myoclonic epilepsy of infancy-borderline
Severe myoclonic epilepsy in infancy
Vagus nerve stimulation
Whole-exome sequencing
Whole-genome sequencing
Zuberi SM, Wirrell E, Yozawitz E, Wilmshurst JM, Specchio N, Riney K, et al . ILAE classification and definition of epilepsy syndromes with onset in neonates and infants: position statement by the ILAE Task Force on Nosology and Definitions. Epilepsia. 2022;63(6):1349–97.
Article PubMed Google Scholar
Wirrell EC, Hood V, Knupp KG, Meskis MA, Nabbout R, Scheffer IE, et al . International consensus on diagnosis and management of Dravet syndrome. Epilepsia. 2022;63(7):1761–77.
Article CAS PubMed PubMed Central Google Scholar
Isom LL, Knupp KG. Dravet syndrome: novel approaches for the most common genetic epilepsy. Neurotherapeutics. 2021;18(3):1524–34.
Cardenal-Muñoz E, Auvin S, Villanueva V, Cross JH, Zuberi SM, Lagae L, et al . Guidance on Dravet syndrome from infant to adult care: road map for treatment planning in Europe. Epilepsia Open. 2022;7(1):11–26.
Chen C, Fang F, Wang X, Lv J, Wang X, Jin H. Phenotypic and genotypic characteristics of SCN1A associated seizure diseases. Front Mol Neurosci. 2022;28(15): 821012.
Article Google Scholar
Ding J, Wang L, Jin Z, Qiang Y, Li W, Wang Y, et al . Do all roads lead to Rome? Genes causing Dravet syndrome and Dravet syndrome-like phenotypes. Front Neurol. 2022;11(13): 832380.
Djémié T, Weckhuysen S, Von Spiczak S, Carvill GL, Jaehn J, Anttonen A, et al . Pitfalls in genetic testing: the story of missed SCN1A mutations. Mol Genet Genomic Med. 2016;4(4):457–64.
Article PubMed PubMed Central Google Scholar
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al . Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–24.
Seo GH, Kim T, Choi IH, Park J, Lee J, Kim S, et al . Diagnostic yield and clinical utility of whole exome sequencing using an automated variant prioritization system, EVIDENCE . Clin Genet. 2020;98(6):562–70.
Riggs ER, Andersen EF, Cherry AM, Kantarci S, Kearney H, Patel A, et al . Technical standards for the interpretation and reporting of constitutional copy-number variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics (ACMG) and the Clinical Genome Resource (ClinGen). Genet Med. 2020;22(2):245–57.
Lim BC, Hwang H, Kim H, Chae JH, Choi J, Kim KJ, et al . Epilepsy phenotype associated with a chromosome 2q243 deletion involving SCN1A: migrating partial seizures of infancy or atypical Dravet syndrome? Epilepsy Res. 2015;109:34–9.
Article CAS PubMed Google Scholar
Fry AE, Rees E, Thompson R, Mantripragada K, Blake P, Jones G, et al . Pathogenic copy number variants and SCN1A mutations in patients with intellectual disability and childhood-onset epilepsy. BMC Med Genet. 2016;17(1):34.
Herini ES, Gunadi, Van Kempen MJA, Yusoff S, Sutaryo, Sunartini, et al. Novel SCN1A mutations in Indonesian patients with severe myoclonic epilepsy in infancy. Pediatr Int. 2010;52(2):234–9.
Herini ES, Gunadi, Harahap ISK, Yusoff S, Morikawa S, Patria SY, et al. Generalized epilepsy with febrile seizures plus (GEFS+) spectrum: clinical manifestations and SCN1A mutations in Indonesian patients. Epilepsy Res. 2010;90(1–2):132–9.
Anwar A, Saleem S, Patel UK, Arumaithurai K, Malik P. Dravet syndrome: an overview. Cureus. 2019. https://www.cureus.com/articles/20900-dravet-syndrome-an-overview . Accessed 20 Nov 2023.
Brunklaus A, Dorris L, Ellis R, Reavey E, Lee E, Forbes G, et al . The clinical utility of an SCN1A genetic diagnosis in infantile-onset epilepsy. Dev Med Child Neurol. 2013;55(2):154–61.
Wirrell EC, Laux L, Donner E, Jette N, Knupp K, Meskis MA, et al . Optimizing the diagnosis and management of Dravet syndrome: recommendations from a North American Consensus Panel. Pediatr Neurol. 2017;68:18-34.e3.
Yadav R, Shah S, Bhandari B, Marasini K, Mandal P, Murarka H, et al . Patient with Dravet syndrome: a case report. Clin Case Rep. 2022;10(5): e05840.
Møller RS, Dahl HA, Helbig I. The contribution of next generation sequencing to epilepsy genetics. Expert Rev Mol Diagn. 2015;15(12):1531–8.
Yozawitz E, Moshé SL. The influence of genetics on epilepsy syndromes in infancy and childhood. Acta Epileptol. 2022;4(1):41.
Suls A, Velizarova R, Yordanova I, Deprez L, Van Dyck T, Wauters J, et al . Four generations of epilepsy caused by an inherited microdeletion of the SCN1A gene. Am Acad Neurol. 2010;75(72):72–6.
CAS Google Scholar
Singh NA, Pappas C, Dahle EJ, Claes LRF, Pruess TH, De Jonghe P, et al . A role of SCN9A in human epilepsies, as a cause of febrile seizures and as a potential modifier of Dravet syndrome. PLoS Genet. 2009;5(9): e1000649.
Dunn P, Albury CL, Maksemous N, Benton MC, Sutherland HG, Smith RA, et al . Next generation sequencing methods for diagnosis of epilepsy syndromes. Front Genet. 2018;7(9):20.
Kim SY, Jang SS, Kim H, Hwang H, Choi JE, Chae J, et al . Genetic diagnosis of infantile-onset epilepsy in the clinic: application of whole-exome sequencing following epilepsy gene panel testing. Clin Genet. 2021;99(3):418–24.
Poduri A, Sheidley BR, Shostak S, Ottman R. Genetic testing in the epilepsies—developments and dilemmas. Nat Rev Neurol. 2014;10(5):293–9.
Download references
Acknowledgements
The authors express their gratitude to the patient and their families for their cooperation, as well as to all the staff and nurses who provided care for the patient. We are also thankful for the Faculty of Medicine, Public Health, and Nursing, Universitas Gadjah Mada for funding this research and providing English editing services for assistance in the editing and proofreading process. Additionally, we appreciate the assistance of Kristy Iskandar, Marissa Leviani Hadiyanto and Khansadhia Hasmaradana Mooiindie during the data collection and editing phases.
This study was supported by the Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada, (Dana Masyarakat to ESH). The funding body did not influence the study design, data analysis, data interpretation, nor manuscript writing.
Author information
Authors and affiliations.
Department of Child Health, Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada, Dr. Sardjito Hospital, Jl. Kesehatan No. 1, Yogyakarta, 55281, Indonesia
Agung Triono & Elisabeth Siti Herini
Pediatric Surgery Division, Department of Surgery/Genetics Working Group/Translational Research Unit, Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada, Dr. Sardjito Hospital, Yogyakarta, 55281, Indonesia
You can also search for this author in PubMed Google Scholar
Contributions
ESH, AG, and G made substantial contributions to the conception and design of the work. AG contributed to data acquisition. ESH, AG, and G performed the data analyses and the interpretation of the data. ESH and AG drafted the text and prepared the figures. ESH, AG, and G revised, read, and approved the final manuscript. All authors approve the present version for publication, and are accountable for all aspects related to the study.
Corresponding author
Correspondence to Elisabeth Siti Herini .
Ethics declarations
Ethics approval and consent to participate.
The Medical and Health Research Ethics Committee of the Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada, Yogyakarta, Indonesia approved all recruitment and research protocols. Eligible patients signed an informed consent form (ethical clearance number KE-FK-0455-EC-2023; dated March 2023). Patients older than 12 years old and/or their parents or guardian (for patients < 12 years old) signed a written informed consent form to be included in this study.
Consent for publication
Written informed consent was obtained from the patients’ legal guardians for publication of this case report and any accompanying images.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Triono, A., Herini, E.S. & Gunadi Genetic exploration of Dravet syndrome: two case report. J Med Case Reports 18 , 215 (2024). https://doi.org/10.1186/s13256-024-04514-2
Download citation
Received : 31 January 2024
Accepted : 18 March 2024
Published : 23 April 2024
DOI : https://doi.org/10.1186/s13256-024-04514-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Case series
Journal of Medical Case Reports
ISSN: 1752-1947
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Laparoscopic totally extraperitoneal hernia repair in patients with a history of previous abdominopelvic surgery
- Original Article
- Open access
- Published: 23 April 2024
Cite this article
You have full access to this open access article

- Romilly Hayward 1 na1 ,
- Jacob J. Smith 1 na1 ,
- Christos Kontovounisios 2 , 3 ,
- Shengyang Qiu ORCID: orcid.org/0000-0001-8699-4621 2 , 3 &
- Oliver J. Warren 2 , 3
A retrospective cohort study of patients undergoing laparoscopic inguinal hernia repair compared short- and long-term outcomes between individuals with or without history of previous abdominopelvic surgery, aiming to determine the feasibility of totally extraperitoneal (TEP) repair within this population. All patients who underwent elective TEP inguinal hernia repair by one consultant surgeon across three London hospitals from January 2017 to May 2023 were retrospectively analysed to assess perioperative outcomes. Two hundred sixty-two patients were identified, of whom two hundred forty-three (93%) underwent laparoscopic TEP repair. The most frequent complications were haematoma (6.2%) and seroma (4.1%). Recurrence occurred in four cases (1.6% of operations, 1.1% of hernias). One hundred eighty-four patients (76%) underwent day-case surgery. There were no mesh infections or explanations, vascular or visceral injuries, port-site hernias, damage to testicle, or persisting numbness. There were no requirements for blood transfusion, returns to theatre, or readmissions within 30 days. There was one conversion to open and one death within 60 days of surgery. Eighty-three (34%) had a history of previous AP surgery. There was no significant difference in perioperative outcomes between the AP and non-AP arms. This finding carried true for subgroup analysis of 44 patients whose AP surgical history did not include previous inguinal hernia repair and for those undergoing repair of recurrent hernia. In expert hands, laparoscopic TEP repair is associated with excellent outcomes and low rates of long-term complications, and thus should be considered as standard for patients regardless of a history of AP surgery.
Avoid common mistakes on your manuscript.
Inguinal hernia repairs are amongst the most frequent abdominal surgeries in the UK with over 60,000 performed within NHS England each year [ 1 ]. Despite extensive collective experience and knowledge within the field, there is not yet consensus on the optimum surgical approach.
Laparoscopic repair is increasingly preferred over open repair, associated with reduced recovery time and lower rates of chronic pain and numbness [ 2 ]. There are two standardised laparoscopic approaches, transabdominal pre-peritoneal (TAPP) and totally extraperitoneal (TEP) [ 3 ]. TEP repair is minimally invasive and unlike TAPP, the repair does not require violation of the peritoneal cavity, reducing the risk of injury to intra-peritoneal organs [ 4 ]. However, both TEP and TAPP are associated with steep learning curves and considered more technically challenging than open procedures [ 2 ]. Moreover, many surgeons view certain patient factors such as a history of abdominopelvic (AP) surgery as relative contraindications to TEP repair; hence, the approach is not always considered feasible [ 5 , 6 ]. Previous AP surgery is widely considered a relative contraindication to laparoscopic repair owing to the expectation that this approach would prove more complex in the presence of intra-abdominal adhesions [ 7 ].
A retrospective cohort study of patients undergoing laparoscopic inguinal hernia repair was conducted to compare short- and long-term outcomes between individuals with or without history of previous AP surgery, aiming to determine the feasibility of TEP repair within this population.
All patients who underwent elective TEP inguinal hernia repair by one consultant surgeon across three London hospitals from January 2017 to May 2023 were retrospectively analysed to assess perioperative outcomes.
Surgical technique
A standard three-port multi-trocar TEP repair was performed under general anaesthesia. Blunt dissection using a balloon trocar and subsequent carbon dioxide insufflation using a Hasson’s cannula through a 1-cm subumbilical incision was performed to create the initial working space. Reduction of the hernia sac was achieved through two midline working ports, one 2–3 cm above the pubic symphysis and one in the midway between the other two ports. Polypropylene mesh (Bard 3D Max™) was introduced through the infraumbilical port, placed over the defect and secured without fixation.
Data collection
Data were retrieved from preoperative clinic notes, operative notes and letters to General Practitioners. Standard follow-up occurred at 2 and 6 weeks. All patient identifiable data were removed, and data were collated in a dedicated electronic database.
Outcome measures included conversion rates, early complications (<30 days) (need for reintervention, haematoma, seroma, superficial or mesh infection, vascular or visceral injury, damage to testicle, port-site hernia) and late complications (>30 days) (persisting numbness (>2 months), chronic pain (>2 months), recurrence).
Groups were categorised for comparison based on history of previous AP surgery with subgroup analysis excluding previous inguinal hernia repair. Further subcategorisation was conducted based on hernia characteristics (primary versus recurrent; unilateral versus bilateral).
Statistical analysis
Data were analysed using IBM SPSS Statistics programme version 28.0.0.0 (190). Continuous parametric data are reported as mean (standard deviation), continuous non-parametric data as median (interquartile range) and categorical data as frequency (percentage). Groups were compared using unpaired two-tailed t tests (continuous parametric), Mann–Whitney rank sum tests (continuous non-parametric) and Chi-square tests (categorical). P values <0.05 were considered significant.
Two hundred sixty-two patients underwent elective inguinal hernia repair between January 2017 and May 2023. Laparoscopic TEP repair with Bard 3D Max™ mesh was offered as standard, and was used in 243 cases (93%). Nineteen patients (7%) underwent Lichtenstein open repair with prolene mesh, the most common indications for open repair being previous laparoscopic repair, previous lower midline major surgery, inguinoscrotal hernia, and patient preference.
Outcomes in laparoscopic totally extraperitoneal repair
One hundred fifty TEP repairs (62%) were unilateral and ninety-three (32%) were bilateral; thus, a total of three hundred fifty-six hernias were repaired laparoscopically. Median (IQR) length of follow-up was 2.18 (1.07–4.01) years. Two hundred seven patients were male (85%) with a median age of 60 years (IQR: 47–71). Eighty-three patients (34%) had a history of previous AP surgery and twenty-one (9%) presented for repair of a recurrent hernia.
Complication rates are summarised in Table 1 . The most frequent complications were haematoma (6.2%) and seroma (4.1%), all cases of which resolved spontaneously without the need for further reintervention. Recurrence occurred in four cases (1.6% of operations, 1.1% of hernias). One hundred eighty-four patients (76%) underwent day-case surgery.
There were no mesh infections or explanations, vascular or visceral injuries, port-site hernias, damage to testicle or persisting numbness. There were no requirements for blood transfusion, returns to theatre or readmissions within 30 days. There was one conversion to open and one death within 60 days of surgery.
TEP repair outcomes for patients with and without history of abdominopelvic surgery
Of the 243 patients who underwent laparoscopic TEP repair, 83 (34%) had a history of previous AP surgery.
Patient demographics and hernia characteristics were similar between groups (Table 2 ).
There was no significant difference in perioperative outcomes between the AP and non-AP arms (Table 3 ).
AP subgroup analysis—excluding previous inguinal repair
Thirty-nine patients whose AP surgical history included previous inguinal hernia repair were excluded from subgroup analysis. The AP arm, thus, consisted of 44 patients, and the non-AP arm of 160.
Thirteen patients had a history of multiple previous AP surgeries. The most frequent types of previous AP surgery included: appendectomy ( n = 21), urological procedures ( n = 13, of which the most common was prostatectomy, n = 4), gynaecological procedures ( n = 10, of which the most common was caesarean section, n = 6), and bowel resection ( n = 4).
There was a greater proportion of male patients in the AP arm. Other patient demographics and hernia characteristics were similar between groups (Table 4 ).
There was no significant difference in perioperative outcomes between the AP and non-AP arms (Table 5 ).
Outcomes in bilateral versus unilateral TEP repair
One hundred fifty TEP repairs (59.5%) were unilateral and ninety-three (40.5%) were bilateral. Length of follow-up was greater in the bilateral arm ( P = 0.018) (Table 6 ).
There was no significant difference in overall early complication rates or any late complication rates for unilateral versus bilateral repair. Patients who underwent bilateral repair had higher incidence of seroma (7.5% versus 2%, P = 0.035) and overnight stay (31.2% versus 20%, P = 0.048) (Table 7 ).
Outcomes following TEP repair of primary versus recurrent hernias
Two hundred twenty-two TEP repairs (91%) were for primary hernias and twenty-one (9%) were for recurrent hernias. Patient demographics and hernia characteristics were similar between groups (Table 8 ).
There was no significant difference in perioperative outcomes between the primary and recurrent arms (Table 9 ).
This dataset provides real-world insight into outcomes after inguinal hernia repair performed by a surgeon experienced in laparoscopic techniques. Excellent outcomes are reported following TEP repair, with low complication and recurrence rates. These findings carry true for patients with a history of previous AP surgery, suggesting that for experienced surgeons, such history should not be a contraindication to laparoscopic repair. Overall short- and long-term complication rates were similar for bilateral versus unilateral repair, lending favour to the opportunistic repair of incidental asymptomatic hernias discovered during the primary repair.
TEP repair for patients with previous abdominopelvic surgery history
Patient characteristics and medical history are key considerations when deciding on surgical approach, especially history of previous pelvic or lower abdominal surgery. Previous AP surgery is widely considered a relative contraindication to laparoscopic repair owing to the expectation that this approach would prove more complex in the presence of intra-abdominal adhesions [ 7 ]. This study found no increased risk of complications for patients with a history of AP surgery or for those presenting with a recurrent hernia following laparoscopic repair, with no conversions to open repair in either subgroup. The European Hernia Society recommends recurrent inguinal hernias should be repaired using the opposite approach to the previous surgery [ 8 ]. For example, after previous open repair via an anterior approach, laparoscopic repair (TEP/TAPP) via the posterior route is preferred. Although the Lichtenstein technique is recommended following prior laparoscopic repair, European Association of Endoscopic Surgery guidelines consider laparoscopic repair following previous TEP/TAPP, a complex situation that ought to be elected by surgeons with experience in minimally invasive surgery [ 9 , 10 ]. To fully assess the feasibility of laparoscopic TEP repair in the hands of an experienced surgeon, subgroup analysis was performed for patients with a history of AP surgery that excluded previous repair of inguinal hernia. This analysis showed no significant difference in post-operative outcomes between the AP and non-AP arms, further indicating the safety and feasibility of laparoscopic TEP repair these patients.
Whilst this data is from a single surgeon, findings nonetheless demonstrate that, regardless of past surgical history, laparoscopic TEP repair is an excellent option for most patients when performed by an experienced surgeon. This is supported by previous research demonstrating similar outcomes following TEP repair for patients with or without history of previous lower abdominal surgery [ 6 , 7 ]. Patients should, therefore, be informed and consented with consideration of their surgeon’s preferred approach and relevant expertise. Moreover, given the strong correlation between a surgeon’s experience and improved post-operative outcomes [ 11 , 12 , 13 ], alongside reduced recovery time and long-term complication rates observed for laparoscopic versus open repair [ 14 , 15 ], increased emphasis on training surgeons in laparoscopic techniques could hold benefit in improving patient outcomes and reducing costs and healthcare burden.
Although incidence of seroma and overnight stay were greater following bilateral repair, overall short-term complications were similar between groups and there was no significant difference in any long-term complications. Overnight stay was often a decision based on convenience, taking into account the time of surgery and distance travelled by patients to return home, in particular for those who were elderly, comorbid, or who lived alone. Subsequently, the finding of increased overnight stay in the bilateral group may have been confounded by these factors. Prevalence of asymptomatic contralateral inguinal hernia in those with unilateral inguinal hernia is around 22%, with 30% eventually becoming symptomatic [ 16 , 17 ]. With no increased risk of long-term complications or recurrence observed for patients undergoing bilateral versus unilateral repair, this dataset lends favour to the practise of identification and opportunistic repair of contralateral asymptomatic hernias during repair of the primary to reduce future morbidity.
Determining optimal surgical approach
Different surgical approaches for inguinal hernia repair have been extensively reviewed and compared to determine the optimal method, with outcomes shown to vary depending on numerous factors. Guidelines determined by the HerniaSurge group in 2018 reflected this, recommending that the choice of surgical approach be tailored to ‘the surgeon’s expertise, patient- and hernia-related characteristics, and local/national resources’, with further emphasis on the idea that ‘one single standard technique for all hernias does not exist’ [ 8 ].
The recommendation to consider the operating surgeon’s preference, training, and capabilities when deciding on surgical approach was strongly supported by the results of this study where, in the hands of a surgeon experienced in laparoscopic techniques, this approach was associated with excellent outcomes. In the literature, complication rates for TEP range from 1.3 to 50.3% (median 12.5%). A review of these complications with regard to the Clavien–Dindo classification determined 22% to be clinically relevant in the long term (Grade ≥ III) [ 18 ]. This study reports 19% of complications as long term, giving an overall long-term complication rate of just 2.5% with recurrence rate 1.6%.
As surgeons gain experience with each respective approach, outcomes tend to improve [ 8 , 11 , 13 ]. Prospectively, low-volume surgeons (25–30 surgeries per year) have markedly higher recurrence rates compared to high-volume surgeons [ 12 ]. The trend holds true for those experienced in a single approach, with retrospective analysis of 2410 TEP repairs conducted by the Mayo Clinic demonstrating a correlation between higher annual surgeon volume and decreased perioperative complications, overnight stays and recurrence rates [ 19 ]. It is, therefore, recommended that inguinal hernia surgery be conducted by an experienced high-volume surgeon specialising in their preferred technique [ 20 ].
This has led to difficulty in designing valid randomised controlled trials to compare surgical approaches; hence, alternative methods of evaluating clinical outcomes are required. Moreover, increasing utilisation of robotic surgery and the emergence of robotic TAPP repair have necessitated the creation of a framework to quickly assess clinical outcomes and learning curves associated with novel surgical techniques. This study demonstrates the routine evaluation of a surgeon’s practise is a suitable, low-cost and highly feasible approach to outcome analysis, in high-volume centres. This approach provides up-to-date, real-world clinical data, allowing surgeons to track their progress and respective learning curves over time, and providing patients with accurate and applicable information to facilitate informed consent.
Limitations
The findings of this study were restricted to the practise of one experienced London-based surgeon, reducing the generalisability of results. It would be important to apply this model to other high-volume hernia surgeons and centres to test its validity. Furthermore, the retrospective design meant certain demographic data including BMI, smoking status and activity levels were incomplete, which may have confounded results. Future studies should include detailed demographic data, alongside more structured follow-up to screen for recurrence and long-term complications. The association between history of laparoscopic and open repair in patients presenting with recurrence may also be considered to further analyse this correlation. This will be well supported by the launch of the British Hernia Society Registry similar to the Danish Hernia Database which has proved indispensable in clinical research and improvement of patient outcomes [ 21 , 22 ].
In expert hands, laparoscopic TEP repair is associated with excellent outcomes and low rates of long-term complications, and thus should be considered as standard for patients regardless of a history of AP surgery or hernia recurrence. Given no increased risk of long-term complications or recurrence following bilateral repair, surgeons may consent patients undergoing unilateral repair for potential concomitant repair of incidental contralateral hernia to reduce future morbidity.
Data availability
All data underlying the results are included in this article or are available from the corresponding author on reasonable request.
Pawlak M, Tulloh B, de Beaux A (2020) Current trends in hernia surgery in NHS England. Ann R Coll Surg Engl 102(1):25–27. https://doi.org/10.1308/rcsann.2019.0118
Article CAS PubMed Google Scholar
McCormack K, Scott N, Go PMNYH, Ross SJ, Grant A (2010) Laparoscopic techniques versus open techniques for inguinal hernia repair. Cochrane Database Syst Rev 1:2003. https://doi.org/10.1002/14651858.CD001785
Article Google Scholar
Wake BL, McCormack K, Fraser C, Vale L, Perez J, Grant A (2010) Transabdominal pre-peritoneal (TAPP) vs. totally extraperitoneal (TEP) laparoscopic techniques for inguinal hernia repair. Cochrane Database Syst Rev 1:2005. https://doi.org/10.1002/14651858.CD004703.pub2
Ferzli G, Iskandar M (2019) Laparoscopic totally extra-peritoneal (TEP) inguinal hernia repair. Ann Laparosc Endosc Surg 4:35–35. https://doi.org/10.21037/ales.2019.03.03
Dulucq JL, Wintringer P, Mahajna A (2006) Totally extraperitoneal (TEP) hernia repair after radical prostatectomy or previous lower abdominal surgery: is it safe? A prospective study. Surg Endosc 20(3):473–476. https://doi.org/10.1007/S00464-006-3027-3
Article PubMed Google Scholar
Prassas D et al (2019) Effect of previous lower abdominal surgery on outcomes following totally extraperitoneal (TEP) inguinal hernia repair. Surg Laparosc Endosc Percutan Tech 29(4):267–270. https://doi.org/10.1097/SLE.0000000000000633
Claus CMP et al (2014) Laparoscopic inguinal hernioplasty after radical prostatectomy: is it safe? Prospective clinical trial. Hernia 18(2):255–259. https://doi.org/10.1007/s10029-013-1204-6
HerniaSurge Group (2018) International guidelines for groin hernia management. Hernia 22(1):1–165. https://doi.org/10.1007/s10029-017-1668-x
Poelman MM et al (2013) EAES consensus development conference on endoscopic repair of groin hernias. Surg Endosc 27(10):3505–3519. https://doi.org/10.1007/S00464-013-3001-9
Köckerling F, Schug-Pass C (2014) Tailored approach in inguinal hernia repair—decision tree based on the guidelines. Front Surg 1:89631. https://doi.org/10.3389/FSURG.2014.00020/BIBTEX
Neumayer LA et al (2005) Proficiency of surgeons in inguinal hernia repair: effect of experience and age. Ann Surg 242(3):344–8; discussion 348–352. doi: https://doi.org/10.1097/01.sla.0000179644.02187.ea
Köckerling F, Bittner R, Kraft B, Hukauf M, Kuthe A, Schug-Pass C (2017) Does surgeon volume matter in the outcome of endoscopic inguinal hernia repair? Surg Endosc 31(2):573–585. https://doi.org/10.1007/s00464-016-5001-z
Feliu-Palà X, Martín-Gómez M, Morales-Conde S, Fernández-Sallent E (2001) The impact of the surgeon’s experience on the results oflaparoscopic hernia repair. Surg Endosc 15(12):1467–1470. https://doi.org/10.1007/s00464-001-9017-6
Schmedt CG, Sauerland S, Bittner R (2005) Comparison of endoscopic procedures vs. Lichtenstein and other open mesh techniques for inguinal hernia repair: a meta-analysis of randomized controlled trials. Surg Endosc 19(2):188–199. https://doi.org/10.1007/S00464-004-9126-0
Karthikesalingam A, Markar SR, Holt PJE, Praseedom RK (2010) Meta-analysis of randomized controlled trials comparing laparoscopic with open mesh repair of recurrent inguinal hernia. Br J Surg 97(1):4–11. https://doi.org/10.1002/BJS.6902
van den Heuvel B, Beudeker N, van den Broek J, Bogte A, Dwars BJ (2013) The incidence and natural course of occult inguinal hernias during TAPP repair. Surg Endosc 27(11):4142–4146. https://doi.org/10.1007/s00464-013-3008-2
Park JB, Chong DC, Reid JL, Edwards S, Maddern GJ (2022) Should asymptomatic contralateral inguinal hernia be laparoscopically repaired in the adult population as benefits greatly outweigh risks? A systematic review and meta-analysis. Hernia 26(4):999–1007. https://doi.org/10.1007/s10029-022-02611-z
Article PubMed PubMed Central Google Scholar
Weyhe D, Tabriz N, Sahlmann B, Uslar V-N (2017) Risk factors for perioperative complications in inguinal hernia repair—a systematic review. Innov Surg Sci 2(2):47–52. https://doi.org/10.1515/iss-2017-0008
Aljamal YN et al (2016) Annual surgeon volume and patient outcomes following laparoscopic totally extraperitoneal inguinal hernia repairs. J Laparoendosc Adv Surg Tech A 26(2):92–98. https://doi.org/10.1089/LAP.2015.0368
McCormack K et al (2005) Laparoscopic surgery for inguinal hernia repair: systematic review of effectiveness and economic evaluation. Health Technol Assess 9(14):1–203, iii–iv. doi: https://doi.org/10.3310/hta9140
Kyle-Leinhase I et al (2018) Comparison of hernia registries: the CORE project. Hernia 22(4):561–575. https://doi.org/10.1007/s10029-017-1724-6
Article CAS PubMed PubMed Central Google Scholar
Scrimgeour DSG et al (2022) A modified Delphi process to establish research priorities in hernia surgery. Hernia 26(3):751–759. https://doi.org/10.1007/S10029-021-02519-0/TABLES/2
Download references
No funds, grants, or other support were received.
Author information
Romilly Hayward and Jacob J. Smith have contributed equally to this work and should be recognised as co-first authors.
Authors and Affiliations
Imperial College London School of Medicine, London, UK
Romilly Hayward & Jacob J. Smith
Chelsea and Westminster Hospital NHS Foundation Trust, 369 Fulham Road, London, SW10 9NH, UK
Christos Kontovounisios, Shengyang Qiu & Oliver J. Warren
Department of Surgery and Cancer, Imperial College London, London, UK
You can also search for this author in PubMed Google Scholar
Contributions
RH (data curation, formal analysis, investigation, methodology, project administration, writing—original draft). JJS (data curation, formal analysis, investigation, methodology, project administration, writing—original draft). CK (conceptualization, methodology, project administration, supervision, writing—review and editing). SQ (conceptualization, methodology, project administration, supervision, writing—review and editing). OJW (conceptualization, methodology, project administration, supervision, writing—review and editing).
Corresponding author
Correspondence to Shengyang Qiu .
Ethics declarations
Conflict of interest.
The authors declare that there is no conflict of interest in this article.
Ethics approval
For this type of study, ethical approval is not required.
Human and animal rights
This article does not contain any studies with human participants performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Hayward, R., Smith, J.J., Kontovounisios, C. et al. Laparoscopic totally extraperitoneal hernia repair in patients with a history of previous abdominopelvic surgery. Updates Surg (2024). https://doi.org/10.1007/s13304-024-01810-w
Download citation
Received : 19 November 2023
Accepted : 04 March 2024
Published : 23 April 2024
DOI : https://doi.org/10.1007/s13304-024-01810-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Laparoscopic
- Total extraperitoneal
- Previous surgery
- Abdominopelvic
- Find a journal
- Publish with us
- Track your research

IMAGES
VIDEO
COMMENTS
Several prospective studies have demonstrated that a substantial proportion of incident BE occurs in persons without typical reflux symptoms (Table 1). 3, 31-35 Thus, while patients with symptomatic heartburn may have a slightly increased incidence of BE, screening on the basis on GERD alone may miss more subjects with BE than it finds.
The patient had a history of exercise-induced asthma, gastroesophageal reflux disease, vitiligo, and genital warts. Two years before presentation, she had had negative screening tests for ...
The type of practitioner may also impact a GERD patient's perception. A study comparing the satisfaction of patients with GERD who saw gastroenterologists to those who saw a family physician indicated that the ... Qaz B, Davis N, Shapiro AB, Kavin H. Gastroesophageal reflux disease: the case for improving patient education in primary care. J ...
Gastroesophageal reflux disease (GERD) is mainly a clinical diagnosis based on typical symptoms of heartburn and acid regurgitation. Current guidelines indicate that patients with typical symptoms should first try a proton pump inhibitor (PPI). If reflux symptoms persist after 8 weeks on a PPI, endoscopy of the esophagus is recommended, with biopsies taken to rule out eosinophilic esophagitis ...
Introduction. Gastroesophageal reflux disease (GERD) affects 10% to 20% of people in the Western world. 1 It is the most common gastrointestinal disorder, leading to an overall spending of 15 to $20 billion yearly. 2 The disease is defined by the presence of recurrent reflux of stomach contents causing troublesome symptoms. Most patients present with repeated episodes of heartburn and/or ...
A 33-year-old pregnant woman with ulcerative colitis presented at 10 weeks of gestation with fever, nausea, vomiting, abdominal pain, and headache. On hospital day 3, the systolic blood pressure de...
In a small, randomized trial involving patients with GERD and refractory heartburn, the percentage of patients who had a successful outcome (≥50% improvement in scores on GERD-related quality-of ...
Network. Symptoms and risks associated with GERD, benefits of treatment and continued monitoring are key in patient communication for treatment and management of GERD in this case-based educational activity.
Purpose: Gastroesophageal reflux disease (GERD) affects up to 25% of the western population, and the annual expenditure for managing GERD is estimated to be more than $14 billion. Most GERD patients do not consult a specialist, but rather rely on their primary care physician for symptom management. Research has shown that many patients--regardless of diagnosis--do not fully understand what ...
Patient Background: Patient is a 79 year old female who was admitted for weakness and lethargy after taking antibiotics for 2 days for her UTI. She had a history of atrial fibrillation, bladder spasms, moderate left hydronephrosis, and gastroesophageal reflux disease.
A 2010 systematic review of four studies found conflicting evidence regarding iron absorption with prolonged (greater than 1 year) PPI use. 40 Adding to the controversy, a study of seven patients with hereditary hemochromatosis who took PPIs for 1 year found a significant reduction in the volume of blood phlebotomized to maintain appropriate ...
Introduction: Symptoms occurring in gastroesophageal reflux disease (GERD) such as heartburn, regurgitation, thoracic pain, epigastric pain, respiratory symptoms, and others can show a broad overlap with symptoms from other foregut disorders. The goal of this study is the accurate assessment of symptom presentation in GERD. Methods: Patients with foregut symptoms were investigated for symptoms ...
After completing this case study, the reader should be able to: + + Describe the clinical presentation of gastroesophageal reflux disease (GERD), including typical, atypical, and alarm symptoms. ... Develop a treatment plan for a patient with GERD, including both nonpharmacologic and pharmacologic measures and monitoring for efficacy and ...
Routine check-ups and proper treatments can help patients with Gastroesophageal Reflux cure. Suppose you are experiencing any symptoms mentioned above. In that case, you can follow the Gastroesophageal reflux care plan by getting in touch with the online gastroenterologist doctor. You can talk to the gastroenterologist and consult ...
The majority of systemic sclerosis (SSc) patients have gastrointestinal tract involvement, but therapies of prokinetic agents are usually unsatisfactory. Patients are often compromised by the use of steroid; therefore, a surgical indication including fundoplication has been controversial. There is no report that advanced SSc with severe gastroesophageal reflux disease (GERD) is successfully ...
Clinical Presentation. Heartburn and acid regurgitation are the classic symptoms of GERD. Patients generally report a burning feeling in the retrosternal area, raising into the chest and radiating toward the neck, throat and occasionally the back.() It occurs post-prandially—particularly after large fatty meals or the ingestion of spicy foods, citrus products, fats, chocolates, or alcohol.
CASE STUDY - CHAPTER 41 UPPER GI PROBLEMS. Gastroesophageal Reflux Disease. Patient Profile C. is a 49-year-old male who goes to the health care provider because he is experiencing heartburn more frequently and it is keeping him awake at night. He had asthma as a child. He is currently taking Mylanta as needed for heartburn.
Case Study #1: Case 7 (GERD) Answer the following questions: 1. How is acid produced and controlled within the gastrointestinal tract? Gastric acid secretion contains three phases: the cephalic phase, the gastric phase, and the intestinal phase. The cephalic phase occurs when there is the anticipation. of eating food thus sending signals to the ...
GASTROESOPHAGEAL REFLUX DISEASE (GERD): A CASE STUDY ABDULAZIZ M ALBAKER ABSTRACT Gastroesophageal Reflux Disease (GERD) has different signs and symptom depending on the sever - ... detected gastroesophageal reflux in patients with cerebral palsy. Turkish Journal of Medical Sciences 2013; 43: 6. Pakistan Oral & Dental Journal Vol 36, No. 2 ...
This document presents a case study of a 60-year-old male patient admitted to the hospital with abdominal discomfort for 10 days and a history of bronchial asthma and GERD. Examination findings and investigation reports are provided. The patient is assessed and diagnosed with bronchial asthma and GERD. A drug chart outlines the treatment plan ...
Case 1: Based on his reported symptoms, CR likely suffers from mild/ episodic gastroesophageal reflux disease (GERD), so he is a candidate for self-treatment. OTC proton pump inhibitors (PPIs) such as omeprazole, lansoprazole, and esomeprazole are appropriate for self-treatment of GERD for up to 14 days. ... Sensitized patients can develop ...
A previous review study investigating the relationship between diet and GERD showed that adherence to the Mediterranean diet (rich in vegetables, fiber, and antioxidants) (Winberg et al., 2012), could play a preventive role in GERD, especially among patients with underlying diseases such as diabetes, heart disease, and cancer (Badillo and ...
Lifestyle strategies to reduce body weight without aggravating GERD symptoms. Study with Quizlet and memorize flashcards containing terms like 1. Symptoms similar to both heart disease and GERD, 2. Why a hiatal hernia can contribute to an increased risk of GERD, 3. How GERD may contribute to an arrhythmia (irregular heartbeat) and more.
A total of ten patients with GHIP were included in the study from March 2013 to July 2022. None of the patients had prior gastric surgery or a family history of gastric cancer or gastrointestinal polyposis syndromes. ... 30%) presented with non-specific symptoms, including heartburn, acid regurgitation and epigastric distension. Endoscopically ...
The findings in that study parallel the situation observed in our initial patient case. Meanwhile, a study by Singh et al. identified a heterozygous mutation in the SCN9A gene in two patients diagnosed with DS . One of these patients also exhibited a mutation in the SCN1A gene.
A retrospective cohort study of patients undergoing laparoscopic inguinal hernia repair compared short- and long-term outcomes between individuals with or without history of previous abdominopelvic surgery, aiming to determine the feasibility of totally extraperitoneal (TEP) repair within this population. All patients who underwent elective TEP inguinal hernia repair by one consultant surgeon ...