More Practical Problem Solving in HPLC
Kromidas, Stavros

1. Edition November 2004 XV, 294 Pages, Softcover 97 Pictures 31 tables Practical Approach Book
ISBN: 978-3-527-31113-2 Wiley-VCH, Weinheim
Wiley Online Library

Short Description
A unique approach to solving HPLC problems. Everyone who bought "Problem Solving in HPLC" by Stavros Kromidas will equally benefit from nearly 100 new practical examples for optimization, trouble-shooting, and instrument performance given in this sequel. In each case the problem, the solution and the conclusions are presented over a maximum of 4 pages, and in addition the book contains manufacturers' addresses, references, data tables and checklists.
Price: 92,90 €
Price incl. VAT, excl. Shipping
- Out of print -
Further versions
- Description
- Author information
A unique approach to solving HPLC problems. Everyone who bought "Problem Solving in HPLC" by Stavros Kromidas will equally benefit from nearly 100 new practical examples for optimization, trouble-shooting, and instrument performance given in this sequel. The author provides - guidance for selecting and evaluating methods, intstruments and columns, - practical help with everyday trouble-shooting, - advice for optimizing separations, always explaining the reason why. In each case the problem, the solution and the conclusions are presented over a maximum of 4 pages, and in addition the book contains manufacturers' addresses, references, data tables and checklists.
S. Kromidas, Consultant, Saarbruecken, Germany
©2024 Wiley-VCH GmbH - Provider - www.wiley-vch.de - [email protected] - Data Policy - Cookie Preferences Copyright © 2000-2024 by John Wiley & Sons, Inc., or related companies. All rights reserved.

- Data Integrity 2024: A Virtual Symposium

- Publications
- Conferences
The LCGC Blog: Back to School—Solving Practical HPLC Problems with Basic Theory
I’m very much a “big picture” type of thinker. By that, I mean unless I can understand all of the working parts of a problem and understand how they interact, I find it difficult to decide the best approach to figuring out how to solve the issue. While this is certainly a personality trait, I often wonder how chromatographers with some thirty-plus years less experience than myself begin to fathom the complexities of separation science and so begin to develop their own approach to practical problem solving.
The Purnell Equation shown in Figure 1 does an excellent job of describing the change in chromatographic Resolution (R) obtained when altering each of the principle chromatographic variables, namely efficiency (N), selectivity (a) or retention factor (k). When I’m teaching chromatography, it’s at the point we begin to roll out the governing equations that I see that glazed look come over many students, as the old guy “tries to tie everything back to theory,” “when was the last time I ever used an equation in the laboratory,” and “when was the last time he ever did any real chromatography.” Most times I can retrieve this situation by answering, “yesterday,” and by relating the mathematical relationships to the last time they stood in front of their HPLC equipment scratching their head for inspiration! Let’s do the same thing here and see how this particular equation can help with practical problems.

Figure 2: Separation of neutral test probes (LogP(1) – 2.0, LogP(2) – 2.4, LogP(3) – 3.0), other conditions shown in the figure
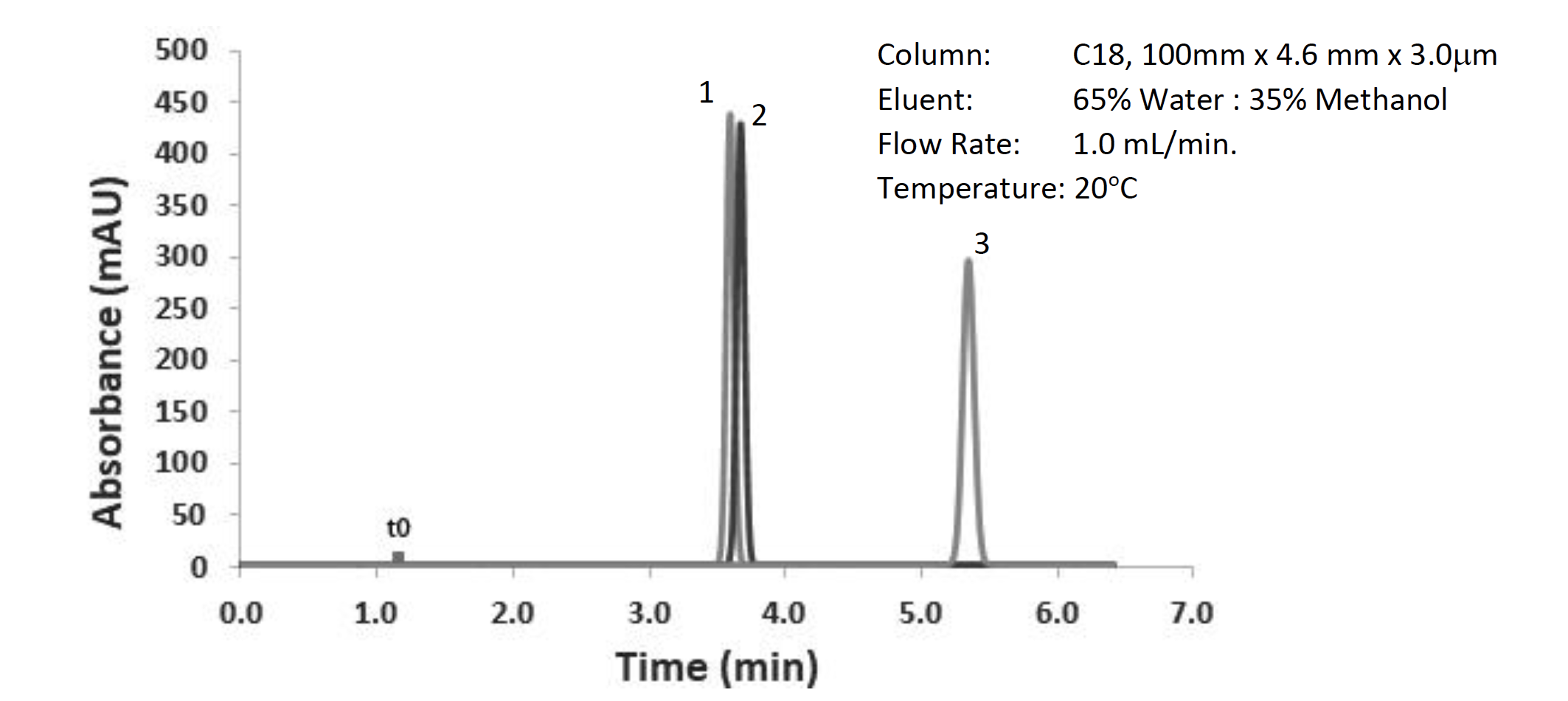
Clearly, we have a separation issue between the first two peaks within the chromatogram and need to think about how to improve the separation. We have seen that changing the selectivity of the separation makes a big impact on resolution, so how can that be done in this case? Well, my own personal golden rules of selectivity optimization follow a “lazy chromatographers” pathway:
- Optimize the % organic modifier
- Optimize temperature
- Optimize eluent pH (for ionizable analytes)
- Change the stationary phase
Why do I put such a big-hitting selectivity variable, such as the stationary-phase chemistry, last? Well, I’m lazy, and I’d like to see if I can get the separation done with the variables at hand, without having the change the column. And it’s not just about choosing a different phase, equilibrating the new column, and performing an injection. What if the new phase doesn’t work? Remember there are around 200 C18 stationary phases on the market to choose from, and they will all (or close to all), give a different selectivity. You might get lucky. You might not!

Figure 3 : Optimization of the separation from Figure 2 using eluent isotropic strength (top) and temperature (bottom).
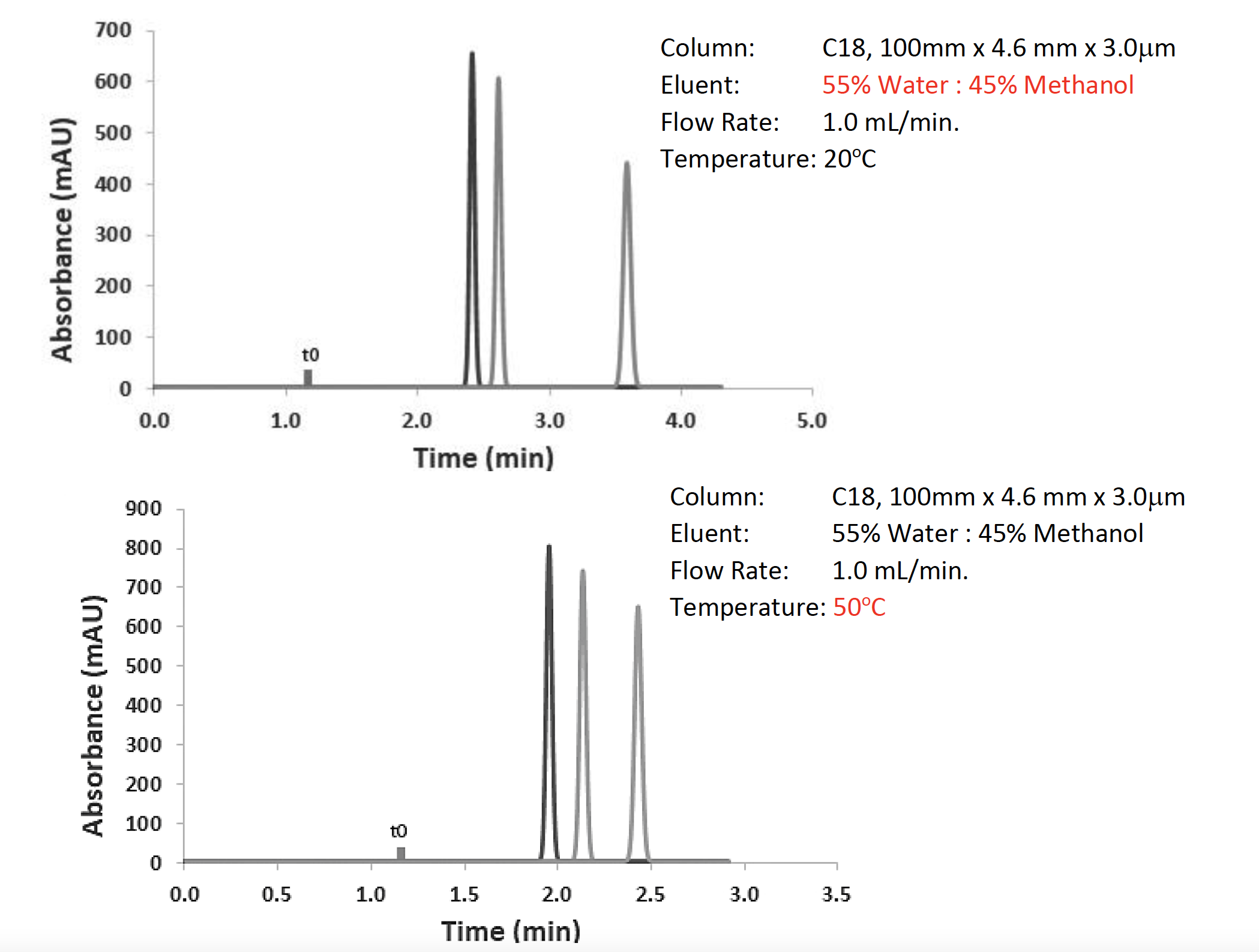
As can be seen from Figure 3, by following my rules of thumb, I managed to affect a good resolution for the separation in a very reasonable analysis time. I increased the % methanol as a first intent, because lowering it just gives me longer analysis time, and if I can get away with a faster analysis, then so much the better. The same is true with temperature. Higher temperatures give faster separations, although I should note that I got a little lucky with this separation, and temperature doesn’t always have such a profound effect on the separation of non-ionogenic analytes. All of this was achieved simply by changing settings in the data system. I didn’t touch the HPLC at any time during this optimization, only the data system.
Of course, if I was dealing with ionizable analytes, I could have gone on to optimize the eluent pH. But I wasn’t, primarily because I wanted to keep this article a little shorter! One could argue that optimizing eluent pH and changing the stationary phase are both complex and can be iterative processes if optimization software isn’t used. And that would be right, but I bet these very processes are going around in laboratories all over the world, right now. For pH optimization I would urge the reader to select one of the analytes in the mixture, find out its pKa value, and then adjust eluent pH so that the analyte is fully non-ionized (eluent pH two units above the pka for bases and two units below the pKa for acids). This will be a good starting point for the optimization. For stationary-phase selection, use a website such as www.HPLCcolumns.org to better understand what type of stationary phase is orthogonal to your current phase. You can choose the same chemistry (such as another C18) or try a different chemistry. I would recommend the use of polar-embedded phases for lower Log P analytes, Phenyl Hexyl for aromatic analytes, and Penta Fluoro Phenyl (PFP) for analytes that are closely structurally related.
Of course, one could try to change the efficiency using a different column diameter or smaller particle size or even optimizing the eluent flow rate, but as we saw in Figure 1, it’s better to start with the big-hitting variables in order to quickly get to the result you want. On that latter point of changing flow rate, remember that capacity factor in isocratic separations does not appreciably change with flow rate as can be seen from Equation 1 for calculating k:

Figure 4 (a) shows a gradient separation of 4 neutral test compounds of various LogP values, which isn’t quite working out as we had hoped. Using Equation 3, we first doubled the flow rate and, as can be seen, the average capacity factor (k*) of the peaks increased by a factor of two. The same effect can be seen when halving the gradient change in %B (gradient range) or doubling the gradient time.
What should be obvious is that in each case the resolution of the critical pair of peaks has improved! Figure 4 (c) is somewhat of a compromise separation in that most of the peaks are eluting in the “isocratic” phase at the end of the gradient. In practical terms, the conditions set out Figure 4(d) may be the most promising in terms of a basis for further optimization, given that most of the peaks elute within the gradient range. Of course, one might suspect that isocratic mode would have been more successful for this separation, but that will need to be reserved for a future technical tip.
I should state that this is quite a simple separation, and I did get a little lucky on this particular example, however, the fact remains that by doing not very much "practical" work, it has been possible to make some informed decisions, based on basic chromatographic relationships, which presented possible solutions for the optimization of the separation.
I realize that many of us working in HPLC don’t plan or even optimize separations based on equations. Many of us develop a “feel” for what is required through many years of experience. However, I do strongly believe that this “feel” can be very usefully backed up and even better informed when one understands and can use some of these simple relationships to understand the cause-and-effect nature of our work.

Tony Taylor

Tony Taylor is the Chief Scientific Officer of Arch Sciences Group and the Technical Director of CHROMacademy. His background is in pharmaceutical R&D and polymer chemistry, but he has spent the past 20 years in training and consulting, working with Crawford Scientific Group clients to ensure they attain the very best analytical science possible. He has trained and consulted with thousands of analytical chemists globally and is passionate about professional development in separation science, developing CHROMacademy as a means to provide high-quality online education to analytical chemists. His current research interests include HPLC column selectivity codification, advanced automated sample preparation, and LC–MS and GC–MS for materials characterization, especially in the field of extractables and leachables analysis.

Natural Deep Eutectic Solvents’ Potential in HPLC Mobile Phases
Scientists from Auburn University recently investigated how natural deep eutectic solvents (NADES) can be incorporated into high-performance liquid chromatography (HPLC) mobile phases.

HPLC-MS/MS for Extracting Favipiravir from Plasma Samples
Scientists recently tested a new system for detecting and extracting favipiravir from plasma samples.

Best of the Week: What’s New in MS, 2024 Young Chemist Award Winner
This week, LCGC International published a variety of articles on the hottest topics in chromatography and beyond. Below, we’ve highlighted some of the most popular articles, according to our readers. Happy reading!

HPLC–MS/MS For Screening Neuraminidase Inhibitors
A recent study examined using high performance liquid chromatography coupled to mass spectrometry (HPLC–MS/MS) to optimize this key class of drugs that are used for the treatment of the common cold and flu.

The LCGC Blog: Charged Stationary Phases in Reversed Phase HPLC
In this LCGC Blog, the author explores stationary phases that incorporate permanent or induced charges within, or alongside, hydrophobic alkyl silica type bonded ligands.
2 Commerce Drive Cranbury, NJ 08512
609-716-7777


- Medical Books

Download the free Kindle app and start reading Kindle books instantly on your smartphone, tablet, or computer - no Kindle device required .
Read instantly on your browser with Kindle for Web.
Using your mobile phone camera - scan the code below and download the Kindle app.

Image Unavailable

- To view this video download Flash Player
Follow the author

More Practical Problem Solving in HPLC 1st Edition
- ISBN-10 9783527311132
- ISBN-13 978-3527311132
- Edition 1st
- Publisher Wiley-VCH
- Publication date January 24, 2005
- Language English
- Dimensions 8 x 1.3 x 10 inches
- Print length 309 pages
- See all details

Editorial Reviews
From the inside flap, from the back cover, about the author, product details.
- ASIN : 3527311130
- Publisher : Wiley-VCH; 1st edition (January 24, 2005)
- Language : English
- Paperback : 309 pages
- ISBN-10 : 9783527311132
- ISBN-13 : 978-3527311132
- Item Weight : 1.31 pounds
- Dimensions : 8 x 1.3 x 10 inches
- #1,603 in Analytic Chemistry (Books)
- #4,058 in Molecular Biology (Books)
- #4,211 in Pathology Clinical Chemistry (Books)
About the author
Stavros kromidas.
Discover more of the author’s books, see similar authors, read author blogs and more
Customer reviews
Customer Reviews, including Product Star Ratings help customers to learn more about the product and decide whether it is the right product for them.
To calculate the overall star rating and percentage breakdown by star, we don’t use a simple average. Instead, our system considers things like how recent a review is and if the reviewer bought the item on Amazon. It also analyzed reviews to verify trustworthiness.
- Sort reviews by Top reviews Most recent Top reviews
Top reviews from the United States
There was a problem filtering reviews right now. please try again later..

Practical problem solving in HPLC
By stavros kromidas.
- 0 Want to read
- 0 Currently reading
- 0 Have read

My Reading Lists:
Use this Work
Create a new list
My book notes.
My private notes about this edition:
Download Options
Check nearby libraries
- Library.link
Buy this book
This edition doesn't have a description yet. Can you add one ?
Previews available in: English
Showing 3 featured editions. View all 3 editions?
Add another edition?
Book Details
Published in.
Weinheim, New York
Edition Notes
Includes bibliographical references and index.

Classifications
The physical object, community reviews (0).
- Created September 29, 2008
- 9 revisions
Wikipedia citation
Copy and paste this code into your Wikipedia page. Need help ?
High-Performance Liquid Chromatography (HPLC)
Cite this chapter.

- Veronika R. Meyer 3
2688 Accesses
- Cardiac Glycoside
- Pulse Amperometric Detection
- Gradient Separation
- Column Switching
- HPLC Instrument
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Durable hardcover edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Unable to display preview. Download preview PDF.
Cataldi TRI, Campa C, De Benedetto GE (2000) Carbohydrate analysis by high-performance anion-exchange chromatography with pulsed amperometric detection: the potential is still growing. Fresenius J. Anal. Chem. 368:739–758
Article CAS PubMed Google Scholar
Dolan JW, Snyder LR (2000) Essential guides to method development in liquid chromatography. In: Wilson ID (ed) Encyclopedia of Separation Science. Academic Press, London, p 4626–4636
Google Scholar
Erickson BE (2000) Electrochemical detectors for liquid chromatography. Anal. Chem. 72:353A–357A
CAS PubMed Google Scholar
Ermer J, Vogel M (2000) Applications of hyphenated LC-MS techniques in pharmaceutical analysis. Biomed. Chromatogr. 14:373–383
Hanai T (ed) (1991) Liquid Chromatography in Biomedical Analysis. Elsevier, Amsterdam
Meyer VR (2004) Practical High-Performance Liquid Chromatography. John Wiley & Sons, Chichester, 4th ed.
Papadoyannis IN (1990) HPLC in Clinical Chemistry. Marcel Dekker, New York
Parvez H, Bastart-Malsot M, Parvez S, Nagatsu T, Carpentier G (eds) (1987) Electrochemical Detection in Medicine and Chemistry. VNU Science Press, Utrecht
Patel D (1997) Liquid Chromatography Essential Data. John Wiley & Sons, Chichester
Snyder LR, Kirkland JJ, Glajch JL (1997) Practical HPLC Method Development. Wiley-Interscience, New York, 2nd ed.
Wolfender JL, Ndjoko K, Hostettmann K (2003) Liquid chromatography with ultraviolet absorbance — mass spectrometry: a powerful combination for the on-line strutural investigation of plant metabolites. J. Chromatogr. A 1000:437–455
Sample Preparation
Cortes HJ, Rothman D (1990) Multidimensional high-performance liquid chromatography. In: Cortes HJ (ed) Multidimensional Chromatography, Techniques and Applications. Marcel Dekker, New York, p 219–250
Hennion MC (1999) Solid-phase extraction: Method development, sorbents, and coupling with liquid chromatography. J. Chromatogr. A 856:3–54
Moldoveanu SC, David V (2002) Sample Preparation in Chromatography. Elsevier, Amsterdam
Moldoveanu SC (2004) Solutions and challenges in sample preparations for chromatography. J Chromatogr. Sci. 42:1–14
Simpson NJK (ed) (2000) Solid-Phase Extraction, Principles, Techniques and Applications. Marcel Dekker, New York
Wells MJM (2000) Essential guides to method development in solid-phase extraction. In: Wilson ID (ed) Encyclopedia of Separation Science. Academic Press, London, p 4636–4643
Catecholamines
Boos KS, Wilmers B, Sauerbrey R, Schlimme E (1987) Development and performance of an automated HPLC-analyzer for catecholamines. Chromatographia 24:363–370
CAS Google Scholar
Hansen AM, Kristiansen J, Nielsen JL, Byrialsen K, Christensen JM (1999) Validation of a high performance liquid chromatography analysis for the determination of noradrenaline and adrenaline in human urine with an on-line sample purification. Talanta 50:367–379
Article CAS Google Scholar
Holmes C, Eisenhofer G, Goldstein DS (1994) Improved assay for plasma dihydroxyphenylacetic acid and other catechols using high-performance liquid chromatography with electrochemical detection. J. Chromatogr. B 653:131–138
Krstuloviæ AM (ed) (1986) Quantitative Analysis of Catecholamines and Related Compounds. Ellis Horwood, Chichester
Musso NR, Vergassola C, Pende A, Lotti G (1990) HPLC with electrochemical detection of catecholamines in human plasma. A mini-review. J. Liquid Chromatogr. 13:1075–1090
Nikolajsen RPH, Hansen AM (2001) Analytical methods for determining urinary catecholamines in healthy subjects. Anal. Chim. Acta 449:1–15
Raggi MA, Sabbioni C, Nicoletta G, Mandrioli R, Gerra G (2003) Analysis of plasma catecholamines by liquid cromatography with amperometric detection using a novel SPE ion-exchange procedure. J. Sep. Sci. 26: 1141–1146
Cardiac Glycosides
Guan F, Ishii A, Seno H, Watanabe-Suzuki K, Kumazawa T, Suzuki O (1999) Identification and quantification of cardiac glycosides in blood and urine samples by HPLC/MS/MS. Anal. Chem. 71:4034–4043
Kelly KL, Kimball BA, Johnston JJ (1995) Quantitation of digitoxin, digoxin, and their metabolites by high-performance liquid chromatography using pulsed amperometric detection. J. Chromatogr. A 711:289–295
Vetticaden SJ, Chandrasekaran A (1990) Chromatography of cardiac glycosides. J. Chromatogr. 531:215–234
Cunico RL, Gooding KM, Wehr T (1998) Basic HPLC and CE of Biomolecules. Bay Bioanalytical Laboratory, Richmond
Jäger D, Jungblut PR, Müller-Werdan U (2002) Separation and identification of human heart proteins. J. Chromatogr. B 771:131–153
Kastner M (ed) (2000) Protein Liquid Chromatography. Elsevier, Amsterdam
Troubleshooting
Dolan JW, Snyder LR (1989) Troubleshooting LC Systems. Aster, Chester
Kromidas S (2000) Practical Problem Solving in HPLC. Wiley-VCH, Weinheim
Meyer VR (1997) Pitfalls and Errors of HPLC in Pictures. Hüthig, Heidelberg
Sadek PC (2000) Troubleshooting HPLC Systems, A Bench Manual. Wiley-Interscience, New York
Download references
Author information
Authors and affiliations.
Institut für Physiologie II, Rheinische-Friedrich-Wilhelms- Universität Bonn, Wilhelmstraße 31, 53111, Bonn, Germany
Veronika R. Meyer
You can also search for this author in PubMed Google Scholar
Editor information
Editors and affiliations.
Klinik für Herzchirurgie, Universität Leipzig, Herzzentrum, Strümpellstraße 39, 04289, Leipzig, Germany
Stefan Dhein & Friedrich Wilhelm Mohr &
Department of Pharmacology, SUNY Upstate Medical University, 766 Irving Avenue, Syracuse, NY, 13210, USA
Mario Delmar MD PhD
Rights and permissions
Reprints and permissions
Copyright information
© 2005 Springer-Verlag Berlin Heidelberg
About this chapter
Meyer, V.R. (2005). High-Performance Liquid Chromatography (HPLC). In: Dhein, S., Mohr, F.W., Delmar, M. (eds) Practical Methods in Cardiovascular Research. Springer, Berlin, Heidelberg. https://doi.org/10.1007/3-540-26574-0_35
Download citation
DOI : https://doi.org/10.1007/3-540-26574-0_35
Publisher Name : Springer, Berlin, Heidelberg
Print ISBN : 978-3-540-40763-8
Online ISBN : 978-3-540-26574-0
eBook Packages : Medicine Medicine (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research

VIDEO
COMMENTS
Back to School—Solving Practical HPLC Problems with Basic Theory. September 10, 2021. Tony Taylor. Publication. Article. Column September 2021. Volume 17. Issue 9. Pages: 26-30.
Quick and easy solutions to HPLC problems! For 45 typical questions of HPLC users, the answer and general conclusions are presented on no more than 4 pages each. The book covers - simple equipment tests and selection criteria for columns, buffers etc. - specific problems and how to solve them - options for optimizing separations. The book also features a special chapter on the retention of ...
From the Inside Flap. Stavros Kromidas Practical Problem Solving in HPLC Quick and easy solutions to HPLC problems! For 45 typical questions of HPLC users, the answer and general conclusions are presented on no more than 4 pages each. The book covers. simple equipment tests and selection criteria for columns, buffers etc.
Let's do the same thing here and see how this particular equation can help with practical problems. Figure 1 should immediately tell us that when trying to optimise resolution, the selectivity gives us by far the biggest return on our investment. It also seems to be the gift that keeps on giving with no real 'plateau' in resolution as the ...
A unique approach to solving HPLC problems. Everyone who bought "Problem Solving in HPLC" by Stavros Kromidas will equally benefit from nearly 100 new practical examples for optimization, trouble-shooting, and instrument performance given in this sequel. The author provides - guidance for selecting and evaluating methods, intstruments and columns, - practical help with everyday trouble ...
A unique approach to solving HPLC problems. Everyone who bought Problem Solving in HPLC by Stavros Kromidas will equally benefit from nearly 100 new practical examples for optimization, trouble-shooting, and instrument performance given in this sequel. The author provides - guidance for selecting and evaluating methods, intstruments and columns, - practical help with everyday trouble-shooting ...
48-Hour online access $10.00. Details. Online-only access $18.00. Details. Single Chapter PDF Download $42.00. Details. Check out.
Troublesome small peaks. Lowering the detection limit by optimizing the injection. Setting the parameters of an HPLC instrument. The right wavelength - old hat to some, a revelation to others. Characteristics of refraction, fluorescence and conductivity detectors.
HPLC is one of the most frequently used techniques for separation and analysis of compounds, used extensively in food, pharmaceutical, biochemical, and environmental analysis. This highly practical guide shows how to solve real-world problems when using HPLC, offering numerous tips and examples.
The book covers. - simple equipment tests and selection criteria for columns, buffers etc. - specific problems and how to solve them. - options for optimizing separations. The book also features a special chapter on the retention of ionizable components in RP-HPLC, references, data tables and check lists. It's a first-aid kit for every HPLC user.
A unique approach to solving HPLC problems.Everyone who bought "Problem Solving in HPLC" by Stavros Kromidas will equally benefit from nearly 100 new practical examples for optimization, trouble-shooting, and instrument performance given in this sequel.The author provides- guidance for selecting and evaluating methods, intstruments and columns,- practical help with everyday trouble-shooting ...
A unique approach to solving HPLC problems. Everyone who bought Problem Solving in HPLC by Stavros Kromidas will equally benefit from nearly 100 new practical examples for optimization, trouble-shooting, and instrument performance given in this sequel. The author provides - guidance for selecting and evaluating methods, intstruments and columns, - practical help with everyday trouble-shooting ...
The book covers. - simple equipment tests and selection criteria for columns, buffers etc. - specific problems and how to solve them. - options for optimizing separations. The book also features a special chapter on the retention of ionizable components in RP-HPLC, references, data tables and check lists. It's a first-aid kit for every HPLC user.
A unique approach to solving HPLC problems. Everyone who bought "Problem Solving in HPLC" by Stavros Kromidas will equally benefit from nearly 100 new practical examples for optimization, trouble-shooting, and instrument performance given in this sequel. The author provides. - guidance for selecting and evaluating methods, intstruments and columns,
A unique approach to solving HPLC problems. Everyone who bought "Problem Solving in HPLC" by Stavros Kromidas will equally benefit from nearly 100 new practical examples for optimization, trouble-shooting, and instrument performance given in this sequel. The author provides - guidance for selecting and evaluating methods, intstruments and columns,
A unique approach to solving HPLC problems. Everyone who bought "Problem Solving in HPLC" by Stavros Kromidas will equally benefit from nearly 100 new practical examples for optimization, trouble-shooting, and instrument performance given in this sequel. The author provides - guidance for selecting and evaluating methods, intstruments and columns,
Tailing in RP HPLC - Part 1: Fast troubleshooting. Tailing in RP HPLC - Part 2: Further causes and time-served cures. Peak deformation and a shift in retention time due to an unsuitable sample solvent.
Practical Problem Solving in HPLC. 2000, ISBN 3-527-29842-8. P.C. Sadek. The HPLC Solvent Guide. 2002, ISBN -471-41138-8. P.C. Sadek. Troubleshooting HPLC Systems. A Bench Manual. 1999, ISBN -471-17834-9. Stavros Kromidas. More Practical Problem Solving in HPLC. with Contributions by
While this is certainly a personality trait, I often wonder how chromatographers with some thirty-plus years less experience than myself begin to fathom the complexities of separation science and so begin to develop their own approach to practical problem solving. The Purnell Equation shown in Figure 1 does an excellent job of describing the ...
Paperback. $88.57 3 Used from $85.08. A unique approach to solving HPLC problems. Everyone who bought "Problem Solving in HPLC" by Stavros Kromidas will equally benefit from nearly 100 new practical examples for optimization, trouble-shooting, and instrument performance given in this sequel. The author provides.
Stavros Kromidas Practical Problem Solving in HPLC Quick and easy solutions to HPLC problems! For 45 typical questions of HPLC users, the answer and general conclusions are presented on no more than 4 pages each. The book covers. simple equipment tests and selection criteria for columns, buffers etc. specific problems and how to solve them
July 6, 2019. Edited by MARC Bot. import existing book. September 29, 2008. Created by ImportBot. Imported from Oregon Libraries MARC record . Practical problem solving in HPLC by Stavros Kromidas, 2000, Wiley-VCH edition, in English.
Kromidas S (2000) Practical Problem Solving in HPLC. Wiley-VCH, Weinheim. Google Scholar Meyer VR (1997) Pitfalls and Errors of HPLC in Pictures. Hüthig, Heidelberg. Google Scholar Sadek PC (2000) Troubleshooting HPLC Systems, A Bench Manual. Wiley-Interscience, New York. Google Scholar