Clinical Trials
Type 1 diabetes.
Displaying 71 studies
The purpose of this study is to demonstrate that a morning injection of Toujeo compared to Lantus will provide better glycemic control, as shown by Continuous Glucose Monitoring (CGM), in adult patients with type 1 diabetes mellitus.
The purpose of this study is to identify risk factors for ICI associated diabetes mellitus and to assess the severity and natural course of this immune related adverse effect.
The purpose of this study is to collect blood samples for biomarker assessment in type 1 diabetes prior to and at specific time points during closed loop control.
Hypothesis: Increased contact with the diabetes care team throughout pregnancy will lead to improved glucose control during pregnancy.
The purpose of this study is to serve as a comparator group to a group of patients that will be managed with AP for varying periods of time during pregnancy.
The purpose of this study is to evaluate glucose variability in patients with type 1 diabetes (T1D) and insulin antibodies, to evaluate the clinical significance of insulin antibodies, and to establish an in vitro assay that would detect antibodies to insulin and insulin analogs.
This clinical trial will identify exercise-related and emotional stress related effects on glycemic control in patients with type 1 diabetes using sensor-augmented pump (SAP) therapy.
This study will test the efficacy of BKR-017 (colon-targeted 500 mg butyrate tablets) on insulin sensitivity, glucose control and triglycerides in type-1 diabetes subjects.
Our goal in this pilot study is to test and develop a novel method that will accurately measure, in vivo, glucagon kinetics in healthy humans and generate preliminary data in type 1 diabetes (T1DM) subjects under overnight fasted conditions.
The purpose of this research is to create a single registry for type 1 diabetes at Mayo Rochester and affiliated Mayo sites.
The purpose of this study is to assess a novel informatics approach that incorporates the use of patient’s diabetes self-care data into the design and delivery of individualized education interventions to improve diabetes control.
The purpose of this study is to assess the glycemic variability in patients with complex diabetes admitted in the hospital using a glycemic sensor.
The multi-purpose of this study is to examine the effectiveness of “InsulisiteGuider” in patients with type 1 diabetes (T1D) through a two-group randomized controlled trial, to characterize the RNA biomarkers in skin epithelial cells isolated from the continuous subcutaneous insulin infusion (CSII) cannulas from T1D patients, and to characterize RNA biomarkers in the blood and saliva of TID patients.
The purpose of this research is to test the safety and effectiveness of the interoperable Artificial Pancreas System Smartphone App (iAPS) in managing blood sugars in pregnant patients with type 1 diabetes.
The objective of this study is to evaluate the EWIS in patients with type 1 diabetes on insulin pump therapy.
This study is a multi-center, non-randomized, prospective single arm study with type 1 patients with diabetes on insulin pump therapy with Continuous Glucose Monitoring (CGM).
A total of up to 300 subjects will be enrolled at up to 20 investigational centers in the US in order to have 240 subjects meeting eligibility criteria. Each subject will wear their own MiniMed™ 670G insulin system. Each subject will be given 12 infusion sets to wear (each infusion set for at least 174 hours, or ...
The purpose of this study is to use the USS Virginia Closed-Loop system for overnight insulin delivery in adults with Type 1 Diabetes (T1DM) in an outpatient setting to evaluate the system's ability to significantly improve blood glucose levels. This protocol will test the feasibility of "bedside" closed-loop control - an approach comprised of standard sensor-augmented pump therapy during the day using off-the-shelf devices and overnight closed-loop control using experimental devices in an outpatient setting. The rationale for this study is as follows: we anticipate that closed-loop control may ultimately be adopted by patients with T1DM in a selective manner. ...
The overall objective of this study is to perform baseline and repeat assessments over time of the metabolic and immunologic status of individuals at risk for type 1 diabetes (T1D) to:
- characterize their risk for developing T1D and identify subjects eligible for prevention trials;
- describe the pathogenic evolution of T1D; and
- increase the understanding of the pathogenic factors involved in the development of T1D.
The study purpose is to understand patients’ with the diagnosis of Diabetes Mellitus type 1 or 2 perception of the care they receive in the Diabetes clinic or Diabetes technology clinic at Mayo Clinic and to explore and to identify the healthcare system components patients consider important to be part of the comprehensive regenerative care in the clinical setting.
However, before we can implement structural changes or design interventions to promote comprehensive regenerative care in clinical practice, we first need to characterize those regenerative practices occurring today, patients expectations, perceptions and experiences about comprehensive regenerative care and determine the ...
This study is being done to determine the roles that several molecules play in the repair of injured cells that line your blood vessels.
This purpose of this study is to determine if activation of a person's immune system in the small intestine could be a contributing cause of Type 1 Diabetes.
The purpose of this project is to collect data over the first year of clinical use of the FDA approved 670G closed loop insulin delivery system among patients with type 1 diabetes. The goal is to evaluate how this newly approved system impacts both clinical and patient-reported outcomes.
The objective of the study is to assess efficacy and safety of a closed loop system (t:slim X2 with Control-IQ Technology) in a large randomized controlled trial.
Can QBSAfe be implemented in a clinical practice setting and improve quality of life, reduce treatment burden and hypoglycemia among older, complex patients with type 2 diabetes?
Questionnaire administered to diabetic patients in primary care practice (La Crosse Mayo Family Medicine Residency /Family Health Clinic) to assess patient’s diabetic knowledge. Retrospective chart review will also be done to assess objective diabetic control based on most recent hemoglobin A1c.
The primary goal of this study protocol is to determine the candidate ratio of pramlintide and insulin co-infusion in individuals with type 1 diabetes (T1DM) to enable stable glucose control during the overnight post-absorptive and in the postprandial periods.
The purpose of this trial is to assess the performance of an Artificial Pancreas (AP) device using the Portable Artificial Pancreas System (pAPS) platform for subjects with type 1 diabetes using an insulin pump and rapid acting insulin. This proposed study is designed to compare closed-loop control with or without optimization of initialization parameters related to basal insulin infusion rates and insulin to carbohydrate (I:C) ratios for meals and snacks. The study consists of an evaluation of the Artificial Pancreas device system during two 24-27.5-hour closed-loop phases in an outpatient/hotel environment. Prior to the closed-loop phases, each subject will undergo ...
The study is being done to find out if low blood sugar (hypoglycemia) can be reduced in people with type 1 diabetes (T1D) 65 years and older with use of automated insulin delivery (AID) system.
The device systems used in this study are approved by the Food and Drug Administration (FDA) for diabetes management. We will be collecting data about how they are used, how well they work, and how safe they are.
This study aims to identify an early stage biomarker for type 1 diabetes. In vitro evidence identified a significant enrichment of the chemokine CXCL10 in β-cell derived EXO upon exposure to diabetogenic pro-inflammatory cytokines. The study also aims to test protocols for efficient isolation of plasma-derived EXO from small volumes of sample, develop an assay for the sensitive detection of CXCL10 in plasma-derived EXO, and characterization of plasma-derived EXO through assessment of concentration, size, and content (proteomics).
The study is designed to understand the confidence and competence level of patients with type 1 diabetes mellitus in their ability to make changes to their insulin pump.
The objective for thisstudy is to characterize the impact of glycemic excursions on cognition in Type 1 Diabetes (T1D) and determine mediators and moderators of this relationship. This study will allow us to determine how glycemic excursions impact cognition, as well as to identify mediators and moderators of this relationship that could lead to novel interventions.
The purpose of this study is to gather preliminary data to better understand acute effects of exercise on glucose metabolism. We will address if subjects with Type 1 Diabetes (T1D) are more insulin sensitive during and following a short bout of exercise compared to healthy controls. We will also determine insulin dependent and insulin independent effects on exercise in people with and without type 1 diabetes.
The purpose of this study is to retrospectively and prospectively compare maternal and fetal/newborn clinical outcomes in age-matched pregnant patients with T1D and healthy controls and to assess the relationship between glycemic variability and pregnancy outcomes in the current era.
The purpose of this study is to compare the effectiveness and safety of an automated insulin delivery (AID) system using a model predictive control (MPC) algorithm versus Sensor-Augmented Pump/Predictive Low Glucose Suspend (SAP/PLGS) therapy with different stress assessments over a 4-week period.
This research study is being done to develop educational materials that will help patients and clinicians talk about diabetes treatment and management options.
The purpose of this study is to evaluate whether or not a 6 month supply (1 meal//day) of healthy food choices readily available in the patient's home and self management training including understanding of how foods impact diabetes, improved food choices and how to prepare those foods, improve glucose control. In addition, it will evaluate whether or not there will be lasting behavior change modification after the program.
The purpose of this study is to measure and characterize specific immune cell abnormalities found in patients who have type 1 diabetes and may or may not be on the waiting list for either a pancreas alone or a pancreas and kidney transplant.
What are the effects of transient insulin deprivation on brain structure, blood flow, mitochondrial function, and cognitive function in T1DM patients? What are the effects of transient insulin deprivation on circulating exosomes and metabolites in T1DM patients?
The primary objective of this study is to determine if continuous glucose monitoring (CGM) can reduce hypoglycemia and improve quality of life in older adults with type 1 diabetes (T1D).
The purpose of this study is to identify novel genetic variants that predispose to Type 1 Diabetes.
The purpose of this study is to demonstrate the safety and effectiveness of the Hybrid Closed Loop system (HCL) in adult and pediatric patients with type 1 diabetes in the home setting. A diverse population of patients with type 1 diabetes will be studied. The study population will have a large range for duration of diabetes and glycemic control, as measured by glycosylated hemoglobin (A1C). They will be enrolled in the study regardless of their prior diabetes regimen, including using Multiple Daily Injections (MDI), Continuous Subcutaneous Insulin Infusion (CSII) or Sensor-Augmented Pump therapy (SAP)
The purpose of this study is to evaluate the safety of utilizing insulin lispro-aabc in the MiniMed™ 780G System to support product and system labeling.
The purpose of this study is to evaluate the effects of improving glycemic control, and/or reducing glycemic variability on gastric emptying, intestinal barrier function, autonomic nerve functions, and epigenetic changes in subjects with type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM) who are switched to intensive insulin therapy as part of clinical practice.
This study is designed to compare an intensive lifestyle and activity coaching program ("Sessions") to usual care for diabetic patients who are sedentary. The question to be answered is whether the Sessions program improves clinical or patient centric outcomes. Recruitment is through invitiation only.
The purpose of this 3-month extension study (DCLP3 Extension) following a primary trial (DCLP3 or NCT03563313) to assess effectiveness and safety of a closed loop system (t:slim X2 with Control-IQ Technology) in a large randomized controlled trial.
The goal of this work is to identify an early stage biomarker for type 1 diabetes. In vitro evidence using rodent models has identified a significant enrichment of the chemokine CXCL10 in β-cell derived sEV upon exposure to diabetogenic pro-inflammatory cytokines. The aims of this project will focus on 1) testing protocols for efficient isolation of plasma-derived sEV from small volumes of sample, 2) development of an assay for the sensitive detection of CXCL10 in plasma-derived sEV, and 3) characterization of plasma-derived sEV through assessment of concentration, size, and content (proteomics). The study plans to include children that ...
This is a study to evaluate a new Point of Care test for blood glucose monitoring.
The objective of the study is to assess the efficacy and safety of home use of a Control-to-Range (CTR) closed-loop (CL) system.
The purpose of this study is assess the feasibility, effectiveness, and acceptability of Diabetes-REM (Rescue, Engagement, and Management), a comprehensive community paramedic (CP) program to improve diabetes self-management among adults in Southeast Minnesota (SEMN) treated for servere hypoglycemia by the Mayo Clinic Ambulance Services (MCAS).
Diabetics are at risk for invasive pneumococcal infections and are more likely to have severe outcomes with infection compared to the general population. The pneumococcal (PPSV23) vaccination is recommended for all people with type 1 diabetes, but whether the vaccine is beneficial for this population has not been established. The purpose of this study is to determine if children with type 1 diabetes have adequate immune response to the PPSV23 vaccination and to assess factors affecting immune response through a pre and post vaccination blood sample.
The purpose of this study is to develop a better blood test to diagnose early kidney injury in type 1 diabetes.
The purpose of this study is to evaluate the effectiveness and safety of brolucizumab vs. aflibercept in the treatment of patients with visual impairment due to diabetic macular edema (DME).
Although vitreous hemorrhage (VH) from proliferative diabetic retinopathy (PDR) can cause acute and dramatic vision loss for patients with diabetes, there is no current, evidence-based clinical guidance as to what treatment method is most likely to provide the best visual outcomes once intervention is desired. Intravitreous anti-vascular endothelial growth factor (anti-VEGF) therapy alone or vitrectomy combined with intraoperative PRP each provide the opportunity to stabilize or regress retinal neovascularization. However, clinical trials are lacking to elucidate the relative time frame of visual recovery or final visual outcome in prompt vitrectomy compared with initial anti-VEGF treatment. The Diabetic Retinopathy Clinical Research ...
The purpose of this study is to demonstrate feasibility of dynamic 11C-ER176 PET imaging to identify macrophage-driven immune dysregulation in gastric muscle of patients with DG. Non-invasive quantitative assessment with PET can significantly add to our diagnostic armamentarium for patients with diabetic gastroenteropathy.
The purpose of this study is to collect device data to assist in the development of a Personalized Closed Loop (PCL) system.
The purpose of this study is to evaluate the effects of multiple dose regimens of RM-131 on vomiting episodes, stomach emptying and stomach paralysis symptoms in patients with Type 1 and Type 2 diabetes and gastroparesis.
The purpose of this study is to use multiple devices to measure blood sugar changes and the reasons for these changes in healthy and diabetic children.
The objectives of this study are to evaluate the safety of IW-9179 in patients with diabetic gastroparesis (DGP) and the effect of treatment on the cardinal symptoms of DGP.
The purpose of this study is to understand why patients with indigestion, with or without diabetes, have gastrointestinal symptoms and, in particular, to understand where the symptoms are related to increased sensitivity to nutrients.Subsequently, look at the effects of Ondansetron on these patients' symptoms.
The purpose of this study is to evaluate the safety, tolerability, pharmacokinetics, and exploratory effectiveness of nimacimab in patients with diabetic gastroparesis.
The purpose of this study is gain the adolescent perspective on living with type 1 diabetes.
The purpose of this study is to demonstrate the performance of the Guardian™ Sensor (3) with an advanced algorithm in subjects age 2 - 80 years, for the span of 170 hours (7 days).
The primary purpose of this study is to prospectively assess symptoms of bloating (severity, prevalence) in patients with diabetic gastroparesis.
The purpose of this study is to track the treatment burden experienced by patients living with Type 2 Diabetes Mellitus (T2DM) experience as they work to manage their illness in the context of social distancing measures.
To promote social distancing during the COVID-19 pandemic, health care institutions around the world have rapidly expanded their use of telemedicine to replace in-office appointments where possible.1 For patients with diabetes, who spend considerable time and energy engaging with various components of the health care system,2,3 this unexpected and abrupt transition to virtual health care may signal significant changes to ...
The purpose of this study is to evaluate the ability of appropriately-trained family physicians to screen for and identify Diabetic Retinopathy using retinal camera and, secondarily, to describe patients’ perception of the convenience and cost-effectiveness of retinal imaging.
Hypothesis: We hypothesize that patients from the Family Medicine Department at Mayo Clinic Florida who participate in RPM will have significantly reduced emergency room visits, hospitalizations, and hospital contacts.
Aims, purpose, or objectives: In this study, we will compare the RPM group to a control group that does not receive RPM. The primary objective is to determine if there are significant group differences in emergency room visits, hospitalizations, outpatient primary care visits, outpatient specialty care visits, and hospital contacts (inbound patient portal messages and phone calls). The secondary objective is to determine if there are ...
The purpose of this research is to determine if CGM (continuous glucose monitors) used in the hospital in patients with COVID-19 and diabetes treated with insulin will be as accurate as POC (point of care) glucose monitors. Also if found to be accurate, CGM reading data will be used together with POC glucometers to dose insulin therapy.
The purpose of this study is to evaluate the effect of fenofibrate compared with placebo for prevention of diabetic retinopathy (DR) worsening or center-involved diabetic macular edema (CI-DME) with vision loss through 4 years of follow-up in participants with mild to moderately severe non-proliferative DR (NPDR) and no CI-DME at baseline.
The purpose of this study is to see if there is a connection between bad experiences in the patient's childhood, either by the patient or the parent, and poor blood sugar control, obesity, poor blood lipid levels, and depression in patients with type 1 diabetes.
The purpose of this study is to assess painful diabetic peripheral neuropathy after high-frequency spinal cord stimulation.
The purpose of this study is to evaluate the effietiveness of remdesivir (RDV) in reducing the rate of of all-cause medically attended visits (MAVs; medical visits attended in person by the participant and a health care professional) or death in non-hospitalized participants with early stage coronavirus disease 2019 (COVID-19) and to evaluate the safety of RDV administered in an outpatient setting.
This study (SE2030) will establish a platform of data to build the perfect stress echo test, suitable for all patients, anywhere, anytime, also quantitative and operator independent.

Mayo Clinic Footer
- Request Appointment
- About Mayo Clinic
- About This Site
Legal Conditions and Terms
- Terms and Conditions
- Privacy Policy
- Notice of Privacy Practices
- Notice of Nondiscrimination
- Manage Cookies
Advertising
Mayo Clinic is a nonprofit organization and proceeds from Web advertising help support our mission. Mayo Clinic does not endorse any of the third party products and services advertised.
- Advertising and sponsorship policy
- Advertising and sponsorship opportunities
Reprint Permissions
A single copy of these materials may be reprinted for noncommercial personal use only. "Mayo," "Mayo Clinic," "MayoClinic.org," "Mayo Clinic Healthy Living," and the triple-shield Mayo Clinic logo are trademarks of Mayo Foundation for Medical Education and Research.
Advertisement
Discoveries from the study of longstanding type 1 diabetes
- Published: 04 March 2021
- Volume 64 , pages 1189–1200, ( 2021 )
Cite this article
- Bruce A. Perkins ORCID: orcid.org/0000-0002-5885-0046 1 , 2 ,
- Leif Erik Lovblom ORCID: orcid.org/0000-0002-6774-8924 1 , 2 ,
- Sebastien O. Lanctôt 1 , 2 ,
- Krista Lamb 1 &
- David Z. I. Cherney ORCID: orcid.org/0000-0003-4164-0429 3
5039 Accesses
11 Citations
14 Altmetric
Explore all metrics
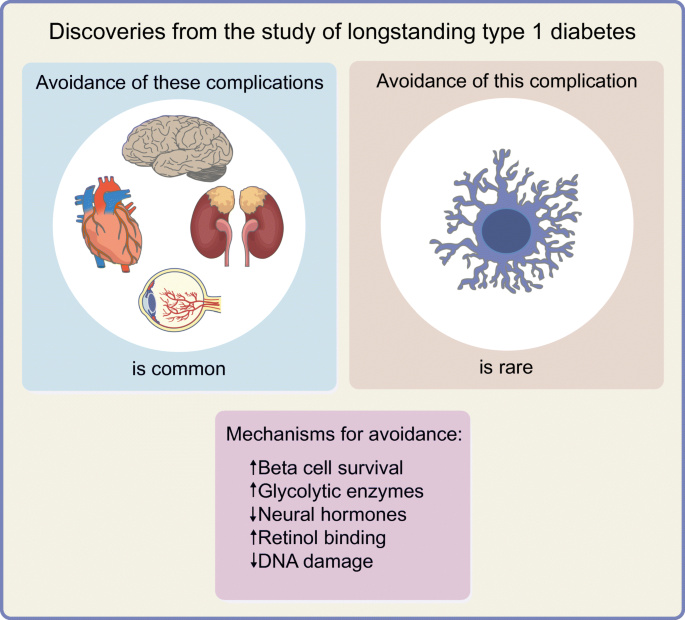
Award programmes that acknowledge the remarkable accomplishments of long-term survivors with type 1 diabetes have naturally evolved into research programmes to determine the factors associated with survivorship and resistance to chronic complications. In this review, we present an overview of the methodological sources of selection bias inherent in survivorship research (selection of those with early-onset diabetes, incidence–prevalence bias and bias from losses to follow-up in cohort studies) and the breadth and depth of literature focusing on this special study population. We focus on the learnings from the study of longstanding type 1 diabetes on discoveries about the natural history of insulin production loss and microvascular complications, and mechanisms associated with them that may in future offer therapeutic targets. We detail descriptive findings about the prevalence of preserved insulin production and resistance to complications, and the putative mechanisms associated with such resistance. To date, findings imply that the following mechanisms exist: strategies to maintain or recover beta cells and their function; activation of specific glycolytic enzymes such as pyruvate kinase M2; modification of AGE production and processing; novel mechanisms for modification of renin–angiotensin–aldosterone system activation, in particular those that may normalise afferent rather than efferent renal arteriolar resistance; and activation and modification of processes such as retinol binding and DNA damage checkpoint proteins. Among the many clinical and public health insights, research into this special study population has identified putative mechanisms that may in future serve as therapeutic targets, knowledge that likely could not have been gained without studying long-term survivors.
Graphical abstract
Similar content being viewed by others

Novel Treatments and the Future of Diabetic Nephropathy: What Is on the Horizon?
Disease-modifying therapies and features linked to treatment response in type 1 diabetes prevention: a systematic review
Jamie L. Felton, Kurt J. Griffin, … ADA/EASD PMDI
Diagnosis and treatment of type 1 diabetes at the dawn of the personalized medicine era
Ammira Al-Shabeeb Akil, Esraa Yassin, … Khalid A. Fakhro
Avoid common mistakes on your manuscript.
Introduction
Although the discovery of insulin in 1921 converted type 1 diabetes from a universally fatal to a chronic condition, in the middle of that century there was approximately 50% mortality risk, primarily from end-stage renal failure, in people with type 1 diabetes who had survived to their 40s [ 1 ]. It was in this context that the Joslin Diabetes Center in Boston acknowledged that surviving decades was a self-management accomplishment worthy of immense pride and accolade [ 2 , 3 ]. Thus began in 1948 the Joslin Medalist Program awarding 25 year medals and, in later years, the 50 and 75 year medals to honour these duration milestones. Rather than assuming such success is purely behavioural, in 1997 Joslin Diabetes Center investigators began a formal research programme to create cross-sectional and cohort study analyses with the goal of better understanding the factors (genetic, physiological, environmental) associated with protection from complications. Such initiatives were not unique. Many international groups have acknowledged long-term survivors and contributed descriptive and causal inference research into complications susceptibility. Interestingly, mortality rates have appeared to decline in the past decades [ 4 ], and it is likely that life expectancy for many people with type 1 diabetes may approach or even exceed that of the age-, sex- and race-standardised general population [ 5 ]. Table 1 provides a non-exhaustive list of the investigational centres that have focused on long-term survivors, either through the direct recruitment of participants into traditional cohort studies or through analyses nested within larger longitudinal cohorts and registry designs. Analyses most frequently align with the Joslin Medalist cohort’s initial operating definition of long-duration diabetes exceeding 50 years, yet many of these have examined, for different purposes, cohorts and subgroups of perfectly valid shorter or longer extreme durations (shown in Table 1 ).
For this review, our main objective was to present the rationale for the study of such a specialised population, present the key methodological issues that inform analytical design, and highlight key research findings that may not have been possible through the study of only participants with diabetes of shorter duration.
Key methodological considerations in the study of longstanding diabetes: Incidence–prevalence bias
The core objective in the study of longstanding diabetes is to better understand an ‘extreme phenotype’ such as an individual who, despite more than 50 years, for example, of diabetes duration has survived without substantial burden of complications. This survival occurred, in part, through the era that included lack of physiological insulins, lack of capacity for self-monitoring of glucose, or even the knowledge of interventions such as intensive insulin therapy, access to measures of mean glucose exposure (e.g. HbA 1c ), or limitations in the screening tests for early complications [ 2 , 3 ]. It stands to reason, then, that such an individual is very likely to have a major protective behaviour or environment such as regular exercise or a supportive spouse or social network [ 6 , 7 ], or perhaps biological resistance to a pathophysiological process, or existence of a protective gene profile. On the other hand, though complications can occur at any age and duration of diabetes beyond 5 years, after 50 years of diabetes there is greater certainty that an individual at risk of an outcome will have declared this risk or not [ 8 ]. The extreme phenotype of an individual with 50 years of diabetes without complications could be compared, for example, to an individual with short duration of diabetes who has developed extensive diabetes complications. A less-extreme comparison frequently used is with individuals in whom complications are present (rather than absent) after longstanding diabetes.
These research approaches, however, are susceptible to forms of selection bias. First, they favour selection of childhood-onset type 1 diabetes. Second, incidence–prevalence bias (frequently referred to as Neyman’s bias or survivor bias) occurs when the risk of a complication is estimated on the basis of data collected at a given time point in a series of survivors rather than data gathered during a certain time period in a study population of incident diabetes [ 9 , 10 ]. Imagine a hypothetical population cohort of 2000 individuals followed from birth wherein eight individuals develop type 1 diabetes, four of whom have greater disease severity such that they die within a decade and the other four have more indolent disease such that their survivorship is similar to that of the general population. If at a point in time a group of investigators seek to recruit all individuals with type 1 diabetes from this implicit, but unknown, underlying cohort, they are most likely to select those with more indolent diabetes as they are more likely to be alive at the time of screening (see Fig. 1 ). Furthermore, if they seek individuals with diabetes of 50 years’ duration, those with greater disease severity have not survived for the opportunity to be accrued. However, creating an incident type 1 diabetes cohort does not fully overcome this bias as selection bias may be induced by censorship, either through losses to follow-up or death from competing risks. In summary, selection bias occurs from the focus on childhood-onset type 1 diabetes, the existence of incidence–prevalence bias, and selection from losses to follow-up in cohort studies. Rather than focus on measures such as cumulative incidence, researchers have focused efforts on the less-biased objectives of determining factors associated with the extreme phenotypes, through inventive adaptations of case series and cross-sectional, case–control and cohort study designs.
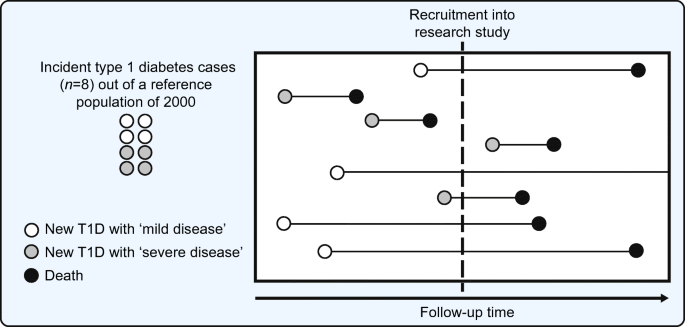
Schematic representation of incidence–prevalence bias. In a hypothetical reference population of 2000 general population members, eight have new onset diabetes. Of these eight index cases, four have ‘mild disease’ such that they survive longer, while four have ‘severe disease’ (perhaps owing to adverse genetic, environmental, behavioural or physiological factors) such that survival is shorter. At the time of recruitment into a research study of type 1 diabetes (indicated by the dashed vertical line in the figure), available cases for recruitment are imbalanced in favour of those with greater survival. Rather than selection of all eight individuals, the four with ‘mild disease’ are available for recruitment but only one with ‘severe disease’ is available. In the process of selection of prevalent type 1 diabetes cases, the probability of selecting individuals with longer survival is greater than if all new-onset cases had been included on their index (diagnosis date). This phenomenon is referred to as incidence–prevalence bias (Neyman’s bias or survivor bias). Incidence–prevalence bias, preferential selection for childhood-onset diabetes and selection based on losses to follow-up (from competing events such as death or outcomes like admission to long-term care facilities) make up the three fundamental sources of selection bias in the traditional cohort studies and registry-based cohort studies for longstanding type 1 diabetes. T1D, type 1 diabetes. Adapted from Tripepi et al. [ 9 ]. This figure is available as part of a downloadable slideset
Residual beta cell function in longstanding diabetes
The study of people with longstanding diabetes has drastically changed the traditionally accepted model of type 1 diabetes, which states that endogenous insulin production declines within 5 years of diabetes onset and that there is a complete loss of beta cells and insulin production [ 11 ]. While the study of long-term survivors might be expected to overestimate the proportion with endogenous insulin production if it is indeed associated with survival (owing to the issues of selection bias just discussed), we must accept that the finding of even a small proportion of long-term survivors would challenge the traditionally accepted model. Such preservation could protect against hypoglycaemia and extreme glycaemic exposure and therefore may be a key protective factor for the development of glucose-dependent long-term complications such as retinopathy [ 12 , 13 , 14 ]. Leading advances in this line of research, the Joslin Medalist investigators examined endogenous insulin production through multiple physiological stimuli including random and mixed-meal stimulated C-peptide production, and a hyperglycaemic i.v. clamp with arginine infusion for stimulation of insulin and thus C-peptide production [ 15 ]. C-peptide, proinsulin’s cleaved ‘connecting peptide’ between the α- and β-chains that make up the functioning insulin molecule, serves as a dependable measure of endogenous insulin production as it is not present in pharmaceutical insulin. These research methods indicated that a large proportion (one-third to one-half) of the individuals surviving for 50 years had some form of detectable C-peptide production and that levels of production fluctuated over a subsequent follow-up period of 4 years. Furthermore, examination of 68 post-mortem pancreases (from an innovative collaboration with the National Disease Research Interchange and its affiliated organ procurement networks) revealed detectable beta cells in all individuals, either atypically as single cells or within morphologically distinct islets. Although the forces of selection may have led to an overestimate of preserved function, studies of shorter-duration diabetes in which C-peptide expression is substantial suggest that selection cannot fully explain these findings, and detectable beta cells by post-mortem histological examination was ubiquitous [ 13 , 16 , 17 , 18 ]. Large-scale registry data from the Scottish Diabetes Research Network Type 1 Bioresource (SDRNT1BIO) study has revealed that persistent C-peptide secretion may have a particular association with aspects of the type 1 diabetes phenotype, including variants in HLA gene regions that are different from the specific regions associated with early-onset type 1 diabetes [ 19 ]. A focus on determining the relevance of these HLA regions and how they impact maintenance of insulin secretion may in future help determine mechanisms of islet cell autoimmunity and may inform novel pathways that could be targeted to limit, or ideally reverse, the loss of insulin production in type 1 diabetes [ 19 ]. Taken together, these findings raise the possibility that hypoglycaemia-related outcomes, long-term glycaemic control, and even retinopathy risk may be better in those who maintain some insulin production [ 14 , 20 , 21 ], and that interventions that counteract autoimmunity or that have trophic effects on beta cells could have putative impact beyond the time surrounding the diagnosis of type 1 diabetes.
It is necessary to acknowledge that these study populations may include individuals with a classification of diabetes other than type 1. For example, monogenic diabetes variants (especially the more common HNF1α and HNF4α [also known as HNF1A and HNF4A , respectively] may be confused with a diagnosis of type 1 diabetes [ 22 , 23 ]. This was systematically studied in the Joslin Medalist cohort in which 7.9% of participants were found to have likely pathogenic variants in genetic studies [ 15 ]. However, the relevance of these variants as the sole cause of the diabetes is quite unlikely. Specifically, such monogenic forms of diabetes would most likely be associated with levels of C-peptide much higher than the levels observed in these longevity studies [ 24 ]. For example, in a study of 77 individuals with fairly long-duration HNF1α diabetes, the lowest C-peptide level was 0.36 nmol/l, which far exceeded the highest level observed in the Joslin Medalist cohort. Moreover, 0.2 nmol/l has recently been proposed as a threshold above which to prompt monogenic diabetes screening, again exceeding the highest levels in the Joslin Medalist cohort [ 22 ]. However, future work should focus on the determination of genetic factors, including the polygenic risk score for type 1 diabetes and targeted genetic screening.
Diabetic kidney disease and renin–angiotensin–aldosterone system activation
Even at the end of the last century it was evident that individuals with longstanding diabetes maintained risk for chronic kidney disease. Research from the Newcastle group demonstrated that albuminuria was present in approximately 27% of individuals with 30 or more years of type 1 diabetes [ 25 ] and that this was independently associated with greatly augmented 7 year mortality risk [ 26 , 27 ]. These prevalence estimates were consistent with Swedish National Registry data showing that chronic kidney disease, primarily defined by albuminuria, was present in nearly half of those with type 1 diabetes of 50 or more years’ duration [ 28 ]. Cumulative incidence from these earlier reports stands in contrast to cohorts accrued more recently in which prevalence of albuminuria and chronic kidney dysfunction appears to be much lower, likely in view of secular trends in the use of intensive insulin and renoprotective therapies, as well as the successful strategies in those earlier reports of employing long-term cohort and registry data to reduce selection bias [ 29 , 30 ]. Though undoubtedly underestimating lifetime diabetic kidney disease (DKD) risk, these studies can nevertheless serve the important purpose of identifying specific pathways and protective factors.
Through examination of post-mortem renal glomerular specimens from 50 year Medalists, the Joslin investigators were able to determine that those protected from clinical nephropathy displayed exaggerated glycolytic flux. This appears to be associated with decreasing accumulation of toxic glucose metabolites which in turn improves mitochondrial function, podocyte survival and other morphological markers of glomerular pathology. Extending this finding to rodent models, activation of the key glycolytic enzyme pyruvate kinase M2 (PKM2) resulted in normalisation of renal haemodynamic and mitochondrial dysfunction, and subsequent glomerular morphological changes [ 31 , 32 , 33 ]. While renal handling of dynamic glycolytic flux appeared to be salutary, there are also data to suggest that the renal handling of AGEs may play a role. Higher exposure of kidneys to circulating levels of AGEs would generally be predicted to be associated with chronic kidney disease, such as exposure to higher N ε -(1-carboxyethyl)lysine (CEL) and pentosidine level. However, it was observed that lower levels of certain AGEs ( N ε -carboxymethyl-lysine [CML] and fructosyl lysine) were associated with nephropathy [ 30 ], perhaps through increased renal processing of these toxic metabolites leading to their increased clearance but resulting in greater renal tubule-interstitial exposure and damage [ 34 ]. Similar lower levels of other markers of protein glycation damage were observed for retinopathy and other complications in Joslin Medalists [ 30 ]. This concept that hyperglycaemia may initiate renal damage by altering both haemodynamics and metabolism to cause dysfunction of renal vascular, glomerular and tubulo-interstitial cells in parallel is plausible since therapeutics that only affect haemodynamics, such as traditional inhibition of the renin–angiotensin–aldosterone system (RAAS), have not appeared to substantially counteract the burden of kidney disease in diabetes populations [ 31 , 35 ].
In our own work in the Canadian Study for Longevity in Type 1 Diabetes, we committed to a primary outcome of determining the significance of RAAS activation on DKD. If indeed RAAS activation is the fundamental process of renal injury in type 1 diabetes, certainly this should be evident among individuals with a lifetime of diabetes in that they will have declared with certainty their risk of chronic kidney disease onset. By way of background, it has been established that a subset of individuals (even those with short-duration type 1 diabetes) exhibit hyperglycaemia-induced RAAS activation, in turn leading to increased renal vascular resistance (RVR) and consequent increased glomerular pressure and renal hyperfiltration as compared with during euglycaemia [ 36 ]. In those of intermediate age and diabetes duration, evidence of this activation appears to be amplified such that RVR is further increased compared with age- and sex-matched control individuals, and it is associated with the development of indicators of clinical chronic kidney disease [ 37 , 38 ]. To probe the level of endogenous intrarenal RAAS activity, we applied an infusion of angiotensin-II (ANG-II) to people with 50 years or more of diabetes and a second group of generally age- and sex-matched non-diabetes control individuals to induce angiotensin 1 (AT1) receptors. AT1 receptors are primarily located on the efferent arteriole and their stimulation causes constriction of the efferent arteriole and raises intraglomerular pressure. The physiological role of this mechanism is to maintain glomerular filtration on renal blood flow in the setting of hypovolaemia or hypotension. Abnormal neurohormonal activation induced by hyperglycaemia in diabetes, rather than serving the purpose to maintain glomerular filtration, raises intraglomerular pressure to levels that can induce renal injury. Study of renal haemodynamics has established the degree of change in RVR as the reference measure of endogenous intrarenal RAAS activation. In simple terms, an individual who responds less to exogenous stimulation of the RAAS using ANG-II infusion has developed resistance to its effect because of chronic, maximal stimulation by endogenous ANG-II. An exaggerated RVR response to ANG-II is indicative of lower, nearer normal, endogenous RAAS activation [ 39 , 40 , 41 , 42 , 43 ]. We found that even nephropathy resistors (long-term survivors of type 1 diabetes without evidence of chronic kidney disease) did indeed have evidence of a substantial degree of endogenous RAAS activation compared with non-diabetic control individuals. This effect was most pronounced on the traditional target of this neurohormonal activation, the efferent arteriole (Fig. 2 ). Individuals with nephropathy had an even greater degree of RAAS activation; however, rather than the predominant effect being constriction of the efferent arteriole, there was greater dilatation of the afferent arteriole, with an amplified effect of increasing intraglomerular pressure (Fig. 2 ).
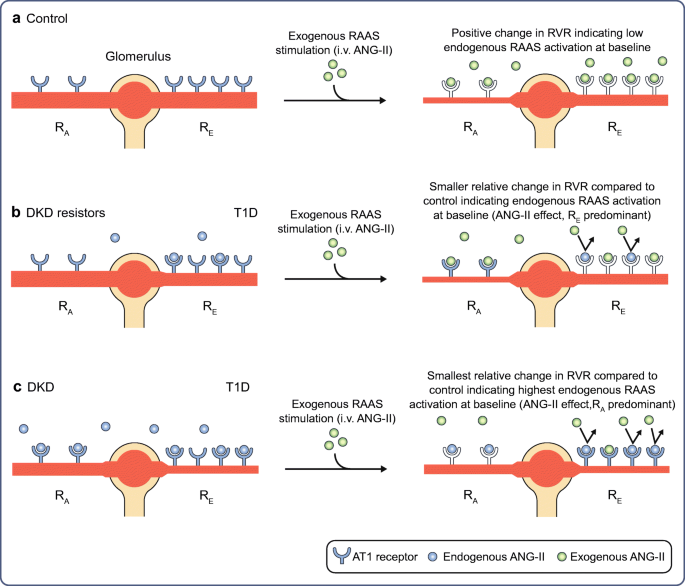
RAAS activation in longstanding type 1 diabetes. In individuals with longstanding type 1 diabetes, when compared with age- and sex-matched control individuals, abnormal afferent arteriolar dilatation, rather than classical efferent constriction, is associated with DKD. AT1 receptors are predominantly expressed at the renal efferent arteriole with less relative expression at the afferent arteriole. In non-diabetic control individuals ( a ), upon exogenous RAAS stimulation with i.v. infusion of ANG-II, ANG-II freely interacts with available AT1 receptors at the afferent and efferent arterioles, initiating vasoconstrictive responses. In individuals with type 1 diabetes without DKD (DKD resistors) ( b ), locally within the kidney there is more endogenous intrarenal ANG-II at baseline occupying AT1 receptors (relative to non-diabetes controls), predominantly at the efferent arterioles compared with the afferent arterioles. In contrast, in individuals with DKD ( c ), there is exaggerated expression of endogenous ANG-II both at the afferent and efferent arterioles at baseline, such that upon exogenous RAAS stimulation, there are fewer AT1 receptors available for ANG-II binding and therefore fewer vasoconstrictive changes relative to DKD resistors and controls predominantly at the afferent arteriole. This finding indicates that abnormal dilatation of the afferent arteriole is a key feature of individuals with DKD after longstanding type 1 diabetes, a process not amenable to pharmacological inhibition from angiotensin converting enzyme inhibitors or aldosterone receptor blockers. R A , renal afferent arteriole; R E , renal efferent arteriole; T1D, type 1 diabetes. Republished with permission of the American Society for Clinical Investigation from Lovshin et al. [ 29 ]; permission conveyed through Copyright Clearance Center, Inc. This figure is available as part of a downloadable slideset
This was an important finding from a pathophysiological perspective, simply because RAAS inhibitors, the mainstay of management of DKD, are expected to exert protective effect through inhibition of efferent arteriolar AT1 receptors, leading to relative dilatation and reduction in renovascular resistance. However, given that the dominant finding was abnormal afferent arteriolar dilatation leading to greater intraglomerular pressure in individuals with DKD, this could explain the incomplete renoprotection observed with RAAS inhibitors [ 35 ]. In our study of renal haemodynamic abnormalities, we found that higher-than-normal uric acid was associated with afferent arteriolar dysfunction [ 44 , 45 ]. Nevertheless, a recent definitive clinical trial of uric-acid-lowering therapy in people with type 1 diabetes did not protect against renal function loss over 3 years [ 46 ]. The second putative strategy would be the normalisation of afferent arteriolar tone induced by sodium–glucose cotransporter (SGLT) inhibition, which has a proven dramatic cardio-renal protective effect in those with type 2 diabetes [ 47 , 48 ], and substantial supportive physiological evidence in type 1 diabetes [ 49 , 50 ]. The findings from the study of longstanding diabetes, in addition to key findings related to endogenous mechanisms of protection from cardiovascular [ 6 , 28 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 ] and renal disease, such as maintenance of circulating progenitor cells [ 63 ], strongly support further research into the potential major effect of pharmacotherapies such as SGLT inhibition on cardio-renal protection in those with type 1 diabetes, especially on the progression of DKD and heart failure.
Peripheral and central nervous system disease
Though people with diabetes are susceptible to a number of types of peripheral nerve damage, by far the most common is the symmetrical, length-dependent clinical presentation of diabetic distal symmetric polyneuropathy [ 64 ]. When compared with other complications, such neuropathy has received less focus in previous studies of longevity cohorts, likely owing to the complexities in objective tests for its identification and quantification of severity. Using self-reported outcome scales or physical examination scales, it has been determined that 40–60% of individuals with longstanding diabetes may be resistant to the development of clinical neuropathy [ 30 , 65 , 66 , 67 ]. However, in our Canadian Study for Longevity in Type 1 Diabetes research programme, we focused on objective measures of neuropathy examining peripheral nerve function. When subjected to objective testing, we found that the presence of at least one neuropathic symptom or sign corroborated by abnormal peripheral nerve function by the gold standard nerve conduction study testing in those with 50 or more years of diabetes was nearly ubiquitous (approximately 90% of participants studied) [ 68 ]. Neuropathy was associated with accentuated diabetes-related distress and depressive symptoms (even independent of the presence of neuropathic pain symptoms), greater than the level of distress associated with any other diabetes complication [ 69 ]. Furthermore, we found sex differences such that women appeared to have a greater burden of neuropathic pain than men even with lower severity of objective peripheral nerve dysfunction [ 68 ]. These findings have implications for treatment of people with neuropathy, in which strategies to reduce emotional distress and sex-specific strategies for the management of neuropathic pain should be considered.
Several research groups have examined aspects of cognitive function and dementia as central nervous system complications of diabetes [ 70 ]. The University of California at San Francisco group have contributed systematically through use of administrative databases (in which at least approximately 5% of those with 50 or more years of type 1 diabetes carry a diagnosis of dementia) and cross-sectional (and future cohort) methods through the Study Of Longevity In Diabetes (SOLID). In SOLID, though the researchers did not define the proportion of participants with dementia, they found a number of factors to be associated with cognition: one’s self-determination for diabetes management (‘locus of control’); glycaemic exposure; history of severe hypoglycaemia; history of diabetic ketoacidosis; history of traumatic brain injury; and sleep quality [ 71 , 72 , 73 , 74 , 75 , 76 ]. Of great historical concern was the potential causal association between hypoglycaemia and subsequent cognitive impairment. This association was not observed in shorter duration of diabetes [ 77 ]. However, in SOLID, focusing on long-duration diabetes, recent severe hypoglycaemia was associated with an OR of 3.22 (95% CI 1.30, 7.90) for impaired global cognition and 3.15 (95% CI 1.19, 8.29) for cognitive impairment on the language domain, each defined by standardised z scores of less than 1.5 SD below the population mean from cognitive assessment tests. The Joslin Medalist Study similarly evaluated factors associated with cognitive functioning and found influence of concomitant CVD and retinopathy [ 70 ]. There is currently a major research effort examining cognitive function and neuro-imaging in the study population from the Diabetes Control and Complications Trial and Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC), now followed for over 30 years, as it includes a greater proportion of older adults reaching 50 years duration [ 78 ]. Additionally, compared with the studies discussed above, this analysis will permit evaluation of causality between hypoglycaemia and diabetic ketoacidosis with the risk of long-term cognitive impairment. The burden of cognitive dysfunction and dementia in those with longstanding diabetes and the identification of interventions for prevention of decline represent an urgent research need.
Retinopathy and macular oedema
Although still susceptible to secular trends and the same forces of selection bias described previously (childhood-onset diabetes, incidence–prevalence bias, death and competing risks), cohort studies have suggested that the cumulative incidence of diabetic retinopathy likely exceeds 90% in people with type 1 diabetes, and that of macular oedema is likely substantially lower [ 79 , 80 , 81 ]. However, selected cohorts with longstanding type 1 diabetes demonstrate larger proportions of individuals free of retinopathy and macular oedema even after 50 or more years of diabetes duration (e.g. 35% of the Joslin Medalists and 16% of participants in the Canadian Study of Longevity in Type 1 Diabetes) [ 30 , 82 ]. The role of long-term excess glycaemic exposure (and the formation of AGEs) has been clear from multiple experimental sources [ 83 ], including the Joslin Medalist Study that found higher levels of plasma carboxyethyl lysine and pentosidine but not traditional markers of glycaemic exposure (discussed above in the context of renal injury) also associated with the presence of retinopathy [ 30 ]. In that same study, individuals free of retinopathy appeared to be at minimal risk of progression [ 30 ]; the forces of selection may explain this so it is important that clinicians consider the appropriate risk factor modification for prevention of incidence and progression of complications at any age or duration. However, a number of mechanisms have been identified to potentially explain resistance to retinopathy and other complications. Higher concentrations of retinol binding protein 3 (RBP3), a transport protein secreted mainly by the retinal photoreceptors, were observed in those resistant to the development of retinopathy [ 84 , 85 ]. This fundamental discovery was of great importance as RBP3 overexpression in resistors could affect multiple known pathological pathways including inhibition of the tyrosine phosphorylation of vascular endothelial growth factor receptors, decreasing glucose uptake via GLUT1 into retinal endothelial cells and Müller cells, thus acting as a countermeasure to the downstream hyperglycaemia-induced injurious pathways including local cytokine production [ 86 ]. Additionally, impaired growth, reprogramming and differentiation of circulating inducible pluripotent stem cells were observed in those with any complication (but prevalence and severity of retinopathy was the greatest), and genomic and proteomic analysis revealed association with DNA damage checkpoint proteins, specifically the miT-200 microRNA transcriptional regulator [ 87 ]. Differential exaggerated responses to RAAS activation in the peripheral vasculature of those with proliferative retinopathy were observed in Canadian participants such that even in the absence of kidney disease, neurohormonal abnormalities are a fundamental pathway in those with retinopathy and longstanding type 1 diabetes duration as they have been demonstrated to have particular effect on novel aspects of renal vascular (afferent arteriole) and peripheral vascular dysfunction [ 52 ].
Taken together as an extensive literature, the study of retinopathy in longstanding diabetes has identified that greater glycaemic exposure and its downstream injurious mechanisms can putatively be modified by overexpression of a retinol binding protein pathway and that modification of the DNA damage checkpoint pathways in circulating stem cells and novel pharmacological mechanisms for modifying RAAS activation may serve as potential therapeutic targets for diabetes complications.
Concluding considerations
In this review, we have focused on the learnings from the study of longstanding type 1 diabetes on discoveries about the natural history of insulin production loss and microvascular complications and the mechanisms associated with them that may in future offer therapeutic targets. Such fundamental findings likely could not have occurred without studying the extraordinary people who have survived longstanding diabetes. To date, prominent among these potentially beneficial approaches are strategies to maintain or recover existing beta cells and their function, activation of specific glycolytic enzymes (e.g. PKM2), modification of AGE production and processing, novel mechanisms for modification of RAAS activation (in particular, those that may normalise afferent rather than efferent renal arteriolar resistances), and activation and modification of processes such as retinol binding and DNA damage checkpoint proteins. Highlighting these particular discoveries in no way is meant to overshadow the wealth of descriptive data from longevity studies, implications on process of care and public health policies [ 66 ], or the need to better implement and emphasise standard clinical practices in the management of this unique patient group [ 51 ]. Additionally, we must further the knowledge on specific findings such as poorer bone health despite maintenance of adequate levels of bone density [ 88 , 89 , 90 ], the dramatically high risk of troublesome cheiroarthropathy [ 91 ], a better understanding of heart failure and atherosclerotic CVD, and the wealth of research around mental health, diabetes-specific emotional distress, and the factors associated with the resilience shown by so many outstanding people with a lifetime of type 1 diabetes.
Abbreviations
Angiotensin-II
Angiotensin 1
Diabetic kidney disease
Pyruvate kinase M2
Renin–angiotensin–aldosterone system
Retinol binding protein 3
Renal vascular resistance
Sodium–glucose cotransporter
Study Of Longevity In Diabetes
Krolewski AS, Warram JH, Christlieb AR, Busick EJ, Kahn CR (1985) The changing natural history of nephropathy in type I diabetes. Am J Med 78:785–794. https://doi.org/10.1016/0002-9343(85)90284-0
Article CAS PubMed Google Scholar
Clarke SF, Foster JR (2012) A history of blood glucose meters and their role in self-monitoring of diabetes mellitus. Br J Biomed Sci 69:83–93
Article CAS Google Scholar
Bliss M (1993) The history of insulin. Diabetes Care 16(Suppl 3):4–7
Article Google Scholar
Rawshani A, Rawshani A, Franzen S et al (2017) Mortality and Cardiovascular Disease in Type 1 and Type 2 Diabetes. N Engl J Med 376:1407–1418. https://doi.org/10.1056/NEJMoa1608664
Article PubMed Google Scholar
The Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Study Research Group (2016) Mortality in Type 1 Diabetes in the DCCT/EDIC Versus the General Population. Diabetes Care 39:1378–1383. https://doi.org/10.2337/dc15-2399
He ZH, D’Eon SA, Tinsley LJ et al (2015) Cardiovascular disease protection in long-duration type 1 diabetes and sex differences. Diabetes Care 38:e73–e74. https://doi.org/10.2337/dc15-0017
Article PubMed PubMed Central Google Scholar
Altman JJ, Vincent-Cassy C, Feldman-Billard S (2009) Improvements in the lifestyle of patients who have had type 1 diabetes for 50 years: an optimistic message. Diabetologia 52:364–366. https://doi.org/10.1007/s00125-008-1216-4
The Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group et al (2015) Effect of intensive diabetes therapy on the progression of diabetic retinopathy in patients with type 1 diabetes: 18 years of follow-up in the DCCT/EDIC. Diabetes 64:631–642
Tripepi G, Jager KJ, Dekker FW, Wanner C, Zoccali C (2008) Bias in clinical research. Kidney Int 73:148–153. https://doi.org/10.1038/sj.ki.5002648
Hernan MA, Hernandez-Diaz S, Robins JM (2004) A structural approach to selection bias. Epidemiology 15:615–625. https://doi.org/10.1097/01.ede.0000135174.63482.43
DiMeglio LA, Evans-Molina C, Oram RA (2018) Type 1 diabetes. Lancet 391:2449–2462
Roep BO, Stobbe I, Duinkerken G et al (1999) Auto- and alloimmune reactivity to human islet allografts transplanted into type 1 diabetic patients. Diabetes 48:484–490
Greenbaum CJ, Anderson AM, Dolan LM et al (2009) Preservation of beta-cell function in autoantibody-positive youth with diabetes. Diabetes Care 32:1839–1844. https://doi.org/10.2337/dc08-2326
Article CAS PubMed PubMed Central Google Scholar
Keenan HA, Sun JK, Levine J et al (2010) Residual insulin production and pancreatic β-cell turnover after 50 years of diabetes: Joslin Medalist Study. Diabetes 59:2846–2853. https://doi.org/10.2337/db10-0676
Yu MG, Keenan HA, Shah HS et al (2019) Residual beta cell function and monogenic variants in long-duration type 1 diabetes patients. J Clin Invest 129:3252–3263
McGee P, Steffes M, Nowicki M et al (2014) Insulin secretion measured by stimulated C-peptide in long-established Type 1 diabetes in the Diabetes Control and Complications Trial (DCCT)/ Epidemiology of Diabetes Interventions and Complications (EDIC) cohort: a pilot study. Diabet Med 31:1264–1268
Davis AK, DuBose SN, Haller MJ et al (2015) Prevalence of detectable C-Peptide according to age at diagnosis and duration of type 1 diabetes. Diabetes Care 38:476–481. https://doi.org/10.2337/dc14-1952
Oram RA, Jones AG, Besser RE et al (2014) The majority of patients with long-duration type 1 diabetes are insulin microsecretors and have functioning beta cells. Diabetologia 57:187–191. https://doi.org/10.1007/s00125-013-3067-x
McKeigue PM, Spiliopoulou A, McGurnaghan S et al (2019) Persistent C-peptide secretion in Type 1 diabetes and its relationship to the genetic architecture of diabetes. BMC Med 17:165
Lovblom L, Cardinez N, Orszag A et al (2019) 126 - Prevalence of Detectable C-peptide in Longstanding Type 1 Diabetes (T1D). Can J Diabetes 43:S43. https://doi.org/10.1016/j.jcjd.2019.07.135
Rother KI, Harlan DM (2010) Comment on: Keenan et al. (2010) residual insulin production and pancreatic ss-Cell turnover after 50 years of diabetes: Joslin Medalist Study. Diabetes 2010;59:2846–2853. Diabetes 59:e26 author reply e27
Broome DT, Pantalone KM, Kashyap SR, Philipson LH (2020) Approach to the Patient with MODY-Monogenic Diabetes. J Clin Endocrinol Metab 106:237–250
Thirumalai A, Holing E, Brown Z, Gilliam LK (2013) A case of hepatocyte nuclear factor-1b (TFC2) maturity onset diabetes of the young misdiagnosed as type 1 diabetes and treated unnecessarily with insulin. J Diabetes 5:462–464
Szopa M, Klupa T, Kapusta M et al (2019) A decision algorithm to identify patients with high probability of monogenic diabetes due to HNF1A mutations. Endocrine 64:75–81. https://doi.org/10.1007/s12020-019-01863-7
Mackin P, Macleod JM, New JP, Marshall SM (1996) Renal function in long-duration type I diabetes. Diabetes Care 19:249–251. https://doi.org/10.2337/diacare.19.3.249
Arun CS, Stoddart J, Mackin P, MacLeod JM, New JP, Marshall SM (2003) Significance of microalbuminuria in long-duration type 1 diabetes. Diabetes Care 26:2144–2149. https://doi.org/10.2337/diacare.26.7.2144
Stadler M, Auinger M, Anderwald C et al (2006) Long-term mortality and incidence of renal dialysis and transplantation in type 1 diabetes mellitus. J Clin Endocrinol Metab 91:3814–3820. https://doi.org/10.1210/jc.2006-1058
Adamsson Eryd S, Svensson AM, Franzen S, Eliasson B, Nilsson PM, Gudbjornsdottir S (2017) Risk of future microvascular and macrovascular disease in people with Type 1 diabetes of very long duration: a national study with 10-year follow-up. Diabet Med 34:411–418. https://doi.org/10.1111/dme.13266
Lovshin JA, Boulet G, Lytvyn Y et al (2018) Renin-angiotensin-aldosterone system activation in long-standing type 1 diabetes. JCI Insight 3:e96968
Sun JK, Keenan HA, Cavallerano JD et al (2011) Protection from retinopathy and other complications in patients with type 1 diabetes of extreme duration: the joslin 50-year medalist study. Diabetes Care 34:968–974. https://doi.org/10.2337/dc10-1675
Gordin D, Shah H, Shinjo T et al (2019) Characterization of Glycolytic Enzymes and Pyruvate Kinase M2 in Type 1 and 2 Diabetic Nephropathy. Diabetes Care 42:1263–1273. https://doi.org/10.2337/dc18-2585
Qi W, Li Q, Gordin D, King GL (2018) Preservation of renal function in chronic diabetes by enhancing glomerular glucose metabolism. J Mol Med (Berl) 96:373–381. https://doi.org/10.1007/s00109-018-1630-0
Qi W, Keenan HA, Li Q et al (2017) Pyruvate kinase M2 activation may protect against the progression of diabetic glomerular pathology and mitochondrial dysfunction. Nat Med 23:753–762. https://doi.org/10.1038/nm.4328
Perkins BA, Rabbani N, Weston A et al (2012) Serum levels of advanced glycation endproducts and other markers of protein damage in early diabetic nephropathy in type 1 diabetes. PLoS One 7:e35655. https://doi.org/10.1371/journal.pone.0035655
Jones CA, Krolewski AS, Rogus J, Xue JL, Collins A, Warram JH (2005) Epidemic of end-stage renal disease in people with diabetes in the United States population: do we know the cause? Kidney Int 67:1684–1691. https://doi.org/10.1111/j.1523-1755.2005.00265.x
Miller JA (1999) Impact of hyperglycemia on the renin angiotensin system in early human type 1 diabetes mellitus. J Am Soc Nephrol 10:1778–1785
Nosadini R, Velussi M, Brocco E et al (2006) Increased renal arterial resistance predicts the course of renal function in type 2 diabetes with microalbuminuria. Diabetes 55:234–239
Vervoort G, Wetzels JF, Lutterman JA, van Doorn LG, Berden JH, Smits P (1999) Elevated skeletal muscle blood flow in noncomplicated type 1 diabetes mellitus: role of nitric oxide and sympathetic tone. Hypertension 34:1080–1085. https://doi.org/10.1161/01.HYP.34.5.1080
Matsumoto N, Ishimura E, Taniwaki H et al (2000) Diabetes mellitus worsens intrarenal hemodynamic abnormalities in nondialyzed patients with chronic renal failure. Nephron 86:44–51. https://doi.org/10.1159/000045711
Ronnback M, Fagerudd J, Forsblom C, Pettersson-Fernholm K, Reunanen A, Groop PH (2004) Altered age-related blood pressure pattern in type 1 diabetes. Circulation 110:1076–1082. https://doi.org/10.1161/01.CIR.0000139903.29522.8D
Herbert KE, Mistry Y, Hastings R, Poolman T, Niklason L, Williams B (2008) Angiotensin II-mediated oxidative DNA damage accelerates cellular senescence in cultured human vascular smooth muscle cells via telomere-dependent and independent pathways. Circ Res 102:201–208. https://doi.org/10.1161/CIRCRESAHA.107.158626
Kunieda T, Minamino T, Nishi J et al (2006) Angiotensin II induces premature senescence of vascular smooth muscle cells and accelerates the development of atherosclerosis via a p21-dependent pathway. Circulation 114:953–960. https://doi.org/10.1161/CIRCULATIONAHA.106.626606
Cherney DZ, Reich HN, Miller JA et al (2010) Age is a determinant of acute hemodynamic responses to hyperglycemia and angiotensin II in humans with uncomplicated type 1 diabetes mellitus. Am J Physiol Regul Integr Comp Physiol 299:R206–R214
Lytvyn Y, Har R, Locke A et al (2017) Renal and Vascular Effects of Uric Acid Lowering in Normouricemic Patients With Uncomplicated Type 1 Diabetes. Diabetes 66:1939–1949
Lytvyn Y, Bjornstad P, Lovshin JA et al (2019) Association between uric acid, renal haemodynamics and arterial stiffness over the natural history of type 1 diabetes. Diabetes Obes Metab 21:1388–1398. https://doi.org/10.1111/dom.13665
Doria A, Galecki AT, Spino C et al (2020) Serum Urate Lowering with Allopurinol and Kidney Function in Type 1 Diabetes. N Engl J Med 382:2493–2503. https://doi.org/10.1056/NEJMoa1916624
Zelniker TA, Wiviott SD, Raz I et al (2019) SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet 393:31–39. https://doi.org/10.1016/S0140-6736(18)32590-X
Perkovic V, Jardine MJ, Neal B et al (2019) Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med 380:2295–2306. https://doi.org/10.1056/NEJMoa1811744
Cherney DZ, Perkins BA, Soleymanlou N et al (2014) Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 129:587–597. https://doi.org/10.1161/CIRCULATIONAHA.113.005081
Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ (2016) Sodium Glucose Cotransporter 2 Inhibitors in the Treatment of Diabetes Mellitus: Cardiovascular and Kidney Effects, Potential Mechanisms, and Clinical Applications. Circulation 134:752–772. https://doi.org/10.1161/CIRCULATIONAHA.116.021887
Bai JW, Boulet G, Halpern EM et al (2016) Cardiovascular disease guideline adherence and self-reported statin use in longstanding type 1 diabetes: results from the Canadian study of longevity in diabetes cohort. Cardiovasc Diabetol 15:14
Lovshin JA, Bjornstad P, Lovblom LE et al (2018) Atherosclerosis and Microvascular Complications: Results From the Canadian Study of Longevity in Type 1 Diabetes. Diabetes Care 41:2570–2578. https://doi.org/10.2337/dc18-1236
Theilade S, Rossing P, Jensen JS, Jensen MT (2018) Arterial-ventricular coupling in type 1 diabetes: arterial stiffness is associated with impaired global longitudinal strain in type 1 diabetes patients-the Thousand & 1 Study. Acta Diabetol 55:21–29. https://doi.org/10.1007/s00592-017-1062-2
Hjortebjerg R, Tarnow L, Jorsal A et al (2015) IGFBP-4 Fragments as Markers of Cardiovascular Mortality in Type 1 Diabetes Patients With and Without Nephropathy. J Clin Endocrinol Metab 100:3032–3040
Grauslund J, Jørgensen TM, Nybo M, Green A, Rasmussen LM, Sjølie AK (2010) Risk factors for mortality and ischemic heart disease in patients with long-term type 1 diabetes. J Diabetes Complicat 24:223–228
Conway BN, Aroda VR, Maynard JD et al (2012) Skin intrinsic fluorescence is associated with coronary artery disease in individuals with long duration of type 1 diabetes. Diabetes Care 35:2331–2336
Holte KB, Svanteson M, Hanssen KF, Haig Y, Solheim S, Berg TJ (2019) Undiagnosed coronary artery disease in long-term type 1 diabetes. The Dialong study. J Diabetes Complicat 33:383–389. https://doi.org/10.1016/j.jdiacomp.2019.01.006
Larsen J, Brekke M, Sandvik L, Arnesen H, Hanssen KF, Dahl-Jorgensen K (2002) Silent coronary atheromatosis in type 1 diabetic patients and its relation to long-term glycemic control. Diabetes 51:2637–2641. https://doi.org/10.2337/diabetes.51.8.2637
Orchard TJ, Olson JC, Erbey JR et al (2003) Insulin resistance-related factors, but not glycemia, predict coronary artery disease in type 1 diabetes: 10-year follow-up data from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Care 26:1374–1379. https://doi.org/10.2337/diacare.26.5.1374
Pongrac Barlovic D, Harjutsalo V, Gordin D et al (2018) The Association of Severe Diabetic Retinopathy With Cardiovascular Outcomes in Long-standing Type 1 Diabetes: A Longitudinal Follow-up. Diabetes Care 41:2487–2494. https://doi.org/10.2337/dc18-0476
Turek SJ, Hastings SM, Sun JK, King GL, Keenan HA (2013) Sexual dysfunction as a marker of cardiovascular disease in males with 50 or more years of type 1 diabetes. Diabetes Care 36:3222–3226. https://doi.org/10.2337/dc13-0294
Vaisar T, Kanter JE, Wimberger J et al (2020) High Concentration of Medium-Sized HDL Particles and Enrichment in HDL Paraoxonase 1 Associate With Protection From Vascular Complications in People With Long-standing Type 1 Diabetes. Diabetes Care 43:178–186
Hernandez SL, Gong JH, Chen L et al (2014) Characterization of circulating and endothelial progenitor cells in patients with extreme-duration type 1 diabetes. Diabetes Care 37:2193–2201
Pop-Busui R, Boulton AJ, Feldman EL et al (2017) Diabetic Neuropathy: A Position Statement by the American Diabetes Association. Diabetes Care 40:136–154
Weisman A, Rovinski R, Farooqi MA et al (2016) Commonly Measured Clinical Variables Are Not Associated With Burden of Complications in Long-standing Type 1 Diabetes: Results From the Canadian Study of Longevity in Diabetes. Diabetes Care 39:e67–e68
Weisman A, Lovblom LE, Keenan HA et al (2018) Diabetes Care Disparities in Long-standing Type 1 Diabetes in Canada and the U.S.: A Cross-sectional Comparison. Diabetes Care 41:88–95
Cardinez N, Lovblom LE, Bai JW et al (2018) Sex differences in neuropathic pain in longstanding diabetes: Results from the Canadian Study of Longevity in Type 1 Diabetes. J Diabetes Complicat 32:660–664. https://doi.org/10.1016/j.jdiacomp.2018.05.001
Cardinez N, Lovblom LE, Orszag A, Bril V, Cherney DZ, Perkins BA (2019) Sex differences in neuropathy & neuropathic pain: A brief report from the Phase 2 Canadian Study of Longevity in Type 1 Diabetes. J Diabetes Complicat 33:107397. https://doi.org/10.1016/j.jdiacomp.2019.06.002
Bai JW, Lovblom LE, Cardinez M et al (2017) Neuropathy and presence of emotional distress and depression in longstanding diabetes: Results from the Canadian study of longevity in type 1 diabetes. J Diabetes Complicat 31:1318–1324. https://doi.org/10.1016/j.jdiacomp.2017.05.002
Musen G, Tinsley LJ, Marcinkowski KA et al (2018) Cognitive Function Deficits Associated With Long-Duration Type 1 Diabetes and Vascular Complications. Diabetes Care 41:1749–1756. https://doi.org/10.2337/dc17-1955
Gilsanz P, Albers K, Beeri MS, Karter AJ, Quesenberry CP Jr, Whitmer RA (2018) Traumatic brain injury associated with dementia risk among people with type 1 diabetes. Neurology 91:e1611–e1618
Lacy ME, Gilsanz P, Eng CW, Beeri MS, Karter AJ, Whitmer RA (2020) Recurrent diabetic ketoacidosis and cognitive function among older adults with type 1 diabetes: findings from the Study of Longevity in Diabetes. BMJ Open Diabetes Res Care 8. https://doi.org/10.1016/j.ajo.2020.12.001
Lacy ME, Gilsanz P, Karter AJ, Quesenberry CP, Pletcher MJ, Whitmer RA (2018) Long-term Glycemic Control and Dementia Risk in Type 1 Diabetes. Diabetes Care 41:2339–2345. https://doi.org/10.2337/dc18-0073
Lacy ME, Gilsanz P, Eng C, Beeri MS, Karter AJ, Whitmer RA (2020) Severe Hypoglycemia and Cognitive Function in Older Adults With Type 1 Diabetes: The Study of Longevity in Diabetes (SOLID). Diabetes Care 43:541–548. https://doi.org/10.2337/dc19-0906
Gilsanz P, Lacy ME, Beeri MS, Karter AJ, Eng CW, Whitmer RA (2020) Sleep Quality and Cognitive Function in Type 1 Diabetes: Findings From the Study of Longevity in Diabetes (SOLID). Alzheimer Dis Assoc Disord 34:18–24
Eng CW, Gilsanz P, Lacy ME, Beeri MS, Whitmer RA (2020) Locus of Control and Cognition in Older Adults With Type 1 Diabetes: Evidence For Sex Differences From the Study of Longevity in Diabetes (SOLID). Alzheimer Dis Assoc Disord 34:25–30
The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group et al (2007) Long-term effect of diabetes and its treatment on cognitive function. N Engl J Med 356:1842–1852. https://doi.org/10.1056/NEJMoa066397
Jacobson AM, Ryan CM, Cleary PA et al (2011) Biomedical risk factors for decreased cognitive functioning in type 1 diabetes: an 18 year follow-up of the Diabetes Control and Complications Trial (DCCT) cohort. Diabetologia 54:245–255. https://doi.org/10.1007/s00125-010-1883-9
Schreur V, van Asten F, Ng H et al (2018) Risk factors for development and progression of diabetic retinopathy in Dutch patients with type 1 diabetes mellitus. Acta Ophthalmol 96:459–464. https://doi.org/10.1111/aos.13815
Lee R, Wong TY, Sabanayagam C (2015) Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis (Lond) 2:17
Klein R, Knudtson MD, Lee KE, Gangnon R, Klein BE (2009) The Wisconsin Epidemiologic Study of Diabetic Retinopathy XXIII: the twenty-five-year incidence of macular edema in persons with type 1 diabetes. Ophthalmology 116:497–503. https://doi.org/10.1016/j.ophtha.2008.10.016
Lovshin JA, Lytvyn Y, Lovblom LE et al (2019) Retinopathy and RAAS Activation: Results From the Canadian Study of Longevity in Type 1 Diabetes. Diabetes Care 42:273–280. https://doi.org/10.2337/dc18-1809
Klein BEK, Horak KL, Maynard JD, Lee KE, Klein R (2017) Association of Skin Intrinsic Fluorescence with Retinal Microvascular Complications of Long Term Type 1 Diabetes in the Wisconsin Epidemiologic Study of Diabetic Retinopathy. Ophthalmic Epidemiol 24:211–216. https://doi.org/10.1080/09286586.2016.1269934
Yokomizo H, Maeda Y, Park K et al (2019) Retinol binding protein 3 is increased in the retina of patients with diabetes resistant to diabetic retinopathy. Sci Transl Med 11:eaau6627
Fickweiler W, Aiello LP, Sun JK, King GL (2019) Retinol binding protein 3 as biomarker for diabetic retinopathy. Ann Transl Med 7:706. https://doi.org/10.21037/atm.2019.10.95
Marsh S, Nakhoul FM, Skorecki K et al (2000) Hypoxic induction of vascular endothelial growth factor is markedly decreased in diabetic individuals who do not develop retinopathy. Diabetes Care 23:1375–1380. https://doi.org/10.2337/diacare.23.9.1375
Bhatt S, Gupta MK, Khamaisi M et al (2015) Preserved DNA Damage Checkpoint Pathway Protects against Complications in Long-Standing Type 1 Diabetes. Cell Metab 22:239–252. https://doi.org/10.1016/j.cmet.2015.07.015
Alhuzaim ON, Lewis EJH, Lovblom LE et al (2019) Bone mineral density in patients with longstanding type 1 diabetes: Results from the Canadian Study of Longevity in Type 1 Diabetes. J Diabetes Complicat 33:107324
Maddaloni E, D’Eon S, Hastings S et al (2017) Bone health in subjects with type 1 diabetes for more than 50 years. Acta Diabetol 54:479–488. https://doi.org/10.1007/s00592-017-0973-2
Biragova MS, Gracheva SA, Glazunova AM et al (2016) The role of mineral and bone disorders in the development and progression of cardiac and renal pathology in patients with type 1 diabetes mellitus of long duration. Diabetes Res Clin Pract 118:29–37. https://doi.org/10.1016/j.diabres.2016.04.049
Holte KB, Juel NG, Brox JI et al (2017) Hand, shoulder and back stiffness in long-term type 1 diabetes; cross-sectional association with skin collagen advanced glycation end-products. The Dialong study. J Diabetes Complicat 31:1408–1414
Keenan HA, Costacou T, Sun JK et al (2007) Clinical factors associated with resistance to microvascular complications in diabetic patients of extreme disease duration: the 50-year medalist study. Diabetes Care 30:1995–1997. https://doi.org/10.2337/dc06-2222
Bain SC, Gill GV, Dyer PH et al (2003) Characteristics of Type 1 diabetes of over 50 years duration (the Golden Years Cohort). Diabet Med 20:808–811. https://doi.org/10.1046/j.1464-5491.2003.01029.x
Prior SL, Tang TS, Gill GV, Bain SC, Stephens JW (2011) Adiponectin, total antioxidant status, and urine albumin excretion in the low-risk “Golden Years” type 1 diabetes mellitus cohort. Metab Clin Exp 60:173–179. https://doi.org/10.1016/j.metabol.2009.12.008
Gordin D, Harjutsalo V, Tinsley L et al (2018) Differential Association of Microvascular Attributions With Cardiovascular Disease in Patients With Long Duration of Type 1 Diabetes. Diabetes Care 41:815–822. https://doi.org/10.2337/dc17-2250
Juel NG, Brox JI, Brunborg C, Holte KB, Berg TJ (2017) Very High Prevalence of Frozen Shoulder in Patients With Type 1 Diabetes of ≥45 Years’ Duration: The Dialong Shoulder Study. Arch Phys Med Rehabil 98:1551–1559. https://doi.org/10.1016/j.apmr.2017.01.020
Guo J, Naimi AI, Brooks MM, Muldoon MF, Orchard TJ, Costacou T (2020) Mediation analysis for estimating cardioprotection of longitudinal RAS inhibition beyond lowering blood pressure and albuminuria in type 1 diabetes. Ann Epidemiol 41:7–13.e11. https://doi.org/10.1016/j.annepidem.2019.12.004
Braffett BH, Dagogo-Jack S, Bebu I et al (2019) Association of Insulin Dose, Cardiometabolic Risk Factors, and Cardiovascular Disease in Type 1 Diabetes During 30 Years of Follow-up in the DCCT/EDIC Study. Diabetes Care 42:657–664. https://doi.org/10.2337/dc18-1574
Otani T, Kasahara T, Miura J, Uchigata Y, Babazono T (2019) Clinical background of Japanese patients with type 1 diabetes mellitus who have received insulin therapy for 50 years or longer. Diabetol Int 10:288–294. https://doi.org/10.1007/s13340-019-00393-x
Bjornstad P, Lovshin JA, Lytvyn Y et al (2018) Adiposity Impacts Intrarenal Hemodynamic Function in Adults With Long-standing Type 1 Diabetes With and Without Diabetic Nephropathy: Results From the Canadian Study of Longevity in Type 1 Diabetes. Diabetes Care 41:831–839. https://doi.org/10.2337/dc17-2475
Bjornstad P, Singh SK, Snell-Bergeon JK et al (2019) The relationships between markers of tubular injury and intrarenal haemodynamic function in adults with and without type 1 diabetes: Results from the Canadian Study of Longevity in Type 1 Diabetes. Diabetes Obes Metab 21:575–583. https://doi.org/10.1111/dom.13556
Boulet G, Halpern EM, Lovblom LE et al (2016) Prevalence of Insulin Pump Therapy and Its Association with Measures of Glycemic Control: Results from the Canadian Study of Longevity in Type 1 Diabetes. Diabetes Technol Ther 18:298–307. https://doi.org/10.1089/dia.2015.0216
Distiller LA, Joffe BI, Melville V, Welman T, Distiller GB (2006) Carotid artery intima-media complex thickening in patients with relatively long-surviving type 1 diabetes mellitus. J Diabetes Complicat 20:280–284. https://doi.org/10.1016/j.jdiacomp.2005.07.012
Mäkimattila S, Ylitalo K, Schlenzka A et al (2002) Family histories of Type II diabetes and hypertension predict intima-media thickness in patients with Type I diabetes. Diabetologia 45:711–718. https://doi.org/10.1007/s00125-002-0817-6
Download references
Acknowledgements
We wish to thank the many inspiring participants who have taken part in studies that focus on longstanding type 1 diabetes survivorship. For the Canadian Study of Longevity in Type 1 Diabetes project, we thank our key study co-investigators: G. Boulet (L’Université de Laval); J. Lovshin, M. H. Brent, N. Paul and V. Bril (all at the University Health Network); and H. A. Keenan (Joslin Diabetes Center). We thank E. M. Halpern, D. Eldelekli (Lunenfeld-Tanenbaum Research Institute) and G. Boulet (L’Université de Laval) for their valuable recruitment work, including translation for our French-speaking participants.
Authors’ relationships and activities
BAP has received speaker honoraria from Abbott, Medtronic, Insulet and Novo-Nordisk, research support to his research institute from Boehringer Ingelheim and the Bank of Montreal (BMO), and has served as a consultant to Boehringer Ingelheim, Abbott and Novo-Nordisk. LEL receives support from a CIHR Canada Graduate Scholarship Doctoral Award. DZIC is supported by a Department of Medicine, University of Toronto Merit Award and receives support from the CIHR, Diabetes Canada and the Heart and Stroke Richard Lewar Centre of Excellence. All other authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
We acknowledge the support from Diabetes Canada and JDRF Canada (grant no. 17-2013-312) and its Canadian Clinical Trial Network, BMO (the Bank of Montreal), and Boehringer Ingelheim, as well as Randy and Jenny Frisch and The Harvey and Annice Frisch Family Fund, the contributions of David and Jill Wright, and the Menkes Family Fund. BAP is grateful for funding from the Sam and Judy Pencer Family Chair in Diabetes Clinical Research.
Author information
Authors and affiliations.
Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, ON, Canada
Bruce A. Perkins, Leif Erik Lovblom, Sebastien O. Lanctôt & Krista Lamb
Division of Endocrinology and Metabolism, Department of Medicine, University of Toronto, Toronto, ON, Canada
Bruce A. Perkins, Leif Erik Lovblom & Sebastien O. Lanctôt
Division of Nephrology, Department of Medicine, University of Toronto, Toronto, ON, Canada
David Z. I. Cherney
You can also search for this author in PubMed Google Scholar
Contributions
All authors were responsible for drafting the article and revising it critically for important intellectual content. All authors approved the version to be published.
Corresponding author
Correspondence to Bruce A. Perkins .
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Slideset of figures.
(PPTX 273 kb)
Rights and permissions
Reprints and permissions
About this article
Perkins, B.A., Lovblom, L.E., Lanctôt, S.O. et al. Discoveries from the study of longstanding type 1 diabetes. Diabetologia 64 , 1189–1200 (2021). https://doi.org/10.1007/s00125-021-05403-9
Download citation
Received : 28 July 2020
Accepted : 07 December 2020
Published : 04 March 2021
Issue Date : June 2021
DOI : https://doi.org/10.1007/s00125-021-05403-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Cardiovascular disease
- Cognitive function
- Long duration
- Nephropathy
- Retinopathy
- Type 1 diabetes
- Find a journal
- Publish with us
- Track your research

‘Need for fast-acting insulin disappeared’: UB professor discovers new way to treat Type 1 diabetes

AMHERST, N.Y. — Dr. Paresh Dandona at the University at Buffalo Jacobs School of Medicine may have discovered a medication that could change the way Type 1 diabetes is treated.
“What I’m talking about right now is far ahead of anyone else in the world in this area today,” Dandona said. “Had I been at Harvard, this would have made massive noise.”
Dandona is the SUNY distinguished professor at the University at Buffalo Jacobs School of Medicine.

His studies have found that treating Type 1 diabetics (who still meet a certain threshold of insulin production) may have a way to reduce their insulin injection amount.
Dandona found that treating these Type 1 diabetics with semaglutide, the drug in products like Ozempic, may drastically reduce or even eliminate their need for injected insulin.

“With all of them, within 6 months, the need for fast acting insulin disappeared,” Dandona said.
There are several types of diabetes:
According to the CDC, the most common and preventable form of diabetes, Type 2, is when your body has insulin, but doesn’t use it well enough to keep blood sugar at normal levels.
Type 1 diabetes is a reaction that stops your body from making insulin altogether, with no prevention or cure.
One of Dr. Dandona’s patients, Ginny Bullock shared her struggles with her Type 1 diagnosis.
A year and half ago, Bullock was diagnosed with Type 1 diabetes. She lives in Colorado and has never been to Buffalo, but her search for help led her to UB.
“The insulin thing is just really complicated,” Bullock said. “I emailed him, I really didn’t think he’d get back to me. Within 10 mins he got back to me, and I spoke on the phone with him, and he got the process rolling.”

Ever since she started using the semaglutide medication, she no longer needs injections every meal.
The treatment reduced her weekly injections by over 90%, to one dose of the medication and one dose of long-acting insulin.
“I’m extremely grateful, I love him,” Bullock said.
“One of the joys of being a physician is to see your patients doing well,” Dandona said.
He is still researching these effects and encourages other diabetics to reach out to him by emailing him at [email protected] .
A Voice For Everyone
We want to hear what’s going on in your community. Share your voice and hear from your neighbors.
Sign up for the Headlines Newsletter and receive up to date information.
Now signed up to receive the headlines newsletter..

7 News I Team
WATCH: I-Team obtains secret recording of Buffalo dentist

As type 1 diabetes expands nationwide and in CT, Congress approves more money for research

A federal program researching type 1 diabetes was recently allocated more funding by Congress to study how to prevent and cure the condition.
Congress approved an additional $10 million for the Special Diabetes Program , a special program administered by the National Institutes of Health. The additional money, which was approved Friday, drives up the program’s annual funding to $160 million.
The allocation comes as rates of type 1 diabetes are rising nationally and in Connecticut.
People with type 1 diabetes are unable to make insulin, or make very little insulin, which prevents blood sugar from entering cells. High blood sugar is damaging to the body and can cause severe, life-threatening complications.
Little is known about what causes type 1 diabetes, but endocrinologists believe it has a genetic component.
“We know it's an autoimmune disease that physically destroys the cells in the pancreas that produce insulin, [but] what triggers the autoimmunity at the individual level is not clear at all,” Dr. Francesco Celi, chair of the Department of Medicine at UConn Health, says.
Type 1 diabetes rates are going up, Celi says, and the demographic is expanding to older adults .
“It is what was previously known as juvenile diabetes except that it’s in individuals who are 30, 40, 50 years old,” he says.
The condition is also expanding to new demographics, Celi says, including Black and Hispanic populations.
As rates go up and populations impacted by type 1 diabetes expand, access to care remains a barrier, despite Medicaid expansion in Connecticut, which improved access to medication and insulin pumps.
Certain demographic groups still have trouble accessing the full spectrum of care they would need to manage the condition, Celi says, including diabetes educators, dieticians, and support groups.
Congress created the Special Diabetes Program in the late 90s to fund research on type 1 diabetes and to narrow the disproportionate burden of type 2 diabetes on American Indians and Alaska Natives.
In a statement, the head of JDRF, a nonprofit that funds type 1 diabetes research, praised Congress’ recent funding boost for the program.
“JDRF is thrilled the Special Diabetes Program has been renewed until December 2024 — and with a much-needed increase in funding — ensuring that critical type 1 diabetes research continues,” Aaron Kowalski, CEO of JDRF, says.
Across America, more than 38 million people have diabetes. American Indians and Alaska Natives are almost three times more likely to be diagnosed with diabetes than white adults, according to the U.S. Centers for Disease Control and Prevention (CDC).
More than 1.7 million adults and 304,000 children and teenagers have type 1 diabetes, according to the CDC. According to the T1D Index , a data simulation tool that provides the a global picture of type 1 diabetes, the condition is growing 2.9% each year in the U.S.

Stand up for civility
This news story is funded in large part by Connecticut Public’s Members — listeners, viewers, and readers like you who value fact-based journalism and trustworthy information.
We hope their support inspires you to donate so that we can continue telling stories that inform, educate, and inspire you and your neighbors. As a community-supported public media service, Connecticut Public has relied on donor support for more than 50 years.
Your donation today will allow us to continue this work on your behalf. Give today at any amount and join the 50,000 members who are building a better—and more civil—Connecticut to live, work, and play.

An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Entire Site
- Research & Funding
- Health Information
- About NIDDK
- Diabetes Overview
- What Is Diabetes?
Type 1 Diabetes
- Español
On this page:
What is type 1 diabetes?
Who is more likely to develop type 1 diabetes, what are the symptoms of type 1 diabetes, what causes type 1 diabetes, how do health care professionals diagnose type 1 diabetes, what medicines do i need to treat my type 1 diabetes, how else can i manage type 1 diabetes, do i have other treatment options for my type 1 diabetes, what health problems can people with type 1 diabetes develop, can i lower my chance of developing type 1 diabetes.
Diabetes occurs when your blood glucose, also called blood sugar, is too high. Blood glucose is your main source of energy and comes mainly from the food you eat. Insulin , a hormone made by the pancreas , helps the glucose in your blood get into your cells to be used for energy. Another hormone, glucagon , works with insulin to control blood glucose levels.
In most people with type 1 diabetes, the body’s immune system , which normally fights infection, attacks and destroys the cells in the pancreas that make insulin. As a result, your pancreas stops making insulin. Without insulin, glucose can’t get into your cells and your blood glucose rises above normal. People with type 1 diabetes need to take insulin every day to stay alive.

Type 1 diabetes typically occurs in children and young adults, although it can appear at any age. Having a parent or sibling with the disease may increase your chance of developing type 1 diabetes. In the United States, about 5 percent of people with diabetes have type 1. 1
Symptoms of type 1 diabetes are serious and usually happen quickly, over a few days to weeks. Symptoms can include
- increased thirst and urination
- increased hunger
- blurred vision
- unexplained weight loss
Sometimes the first symptoms of type 1 diabetes are signs of a life-threatening condition called diabetic ketoacidosis (DKA) . Some symptoms of DKA include
- breath that smells fruity
- dry or flushed skin
- nausea or vomiting
- stomach pain
- trouble breathing
- trouble paying attention or feeling confused
DKA is serious and dangerous. If you or your child have symptoms of DKA, contact your health care professional right away, or go to the nearest hospital emergency room.
Experts think type 1 diabetes is caused by genes and factors in the environment, such as viruses , that might trigger the disease. Researchers are working to pinpoint the causes of type 1 diabetes through studies such as TrialNet .
Health care professionals usually test people for type 1 diabetes if they have clear-cut diabetes symptoms. Health care professionals most often use the random plasma glucose (RPG) test to diagnose type 1 diabetes. This blood test measures your blood glucose level at a single point in time. Sometimes health professionals also use the A1C blood test to find out how long someone has had high blood glucose.
Even though these tests can confirm that you have diabetes, they can’t identify what type you have. Treatment depends on the type of diabetes, so knowing whether you have type 1 or type 2 is important.
To find out if your diabetes is type 1, your health care professional may test your blood for certain autoantibodies. Autoantibodies are antibodies that attack your healthy tissues and cells by mistake. The presence of certain types of autoantibodies is common in type 1 but not in type 2 diabetes.
Because type 1 diabetes can run in families, your health care professional can test your family members for autoantibodies. Type 1 diabetes TrialNet, an international research network, also offers autoantibody testing to family members of people diagnosed with the disease. The presence of autoantibodies, even without diabetes symptoms, means the family member is more likely to develop type 1 diabetes. If you have a brother or sister, child, or parent with type 1 diabetes, you may want to get an autoantibody test. People age 20 or younger who have a cousin, aunt, uncle, niece, nephew, grandparent, or half-sibling with type 1 diabetes also may want to get tested.
If you have type 1 diabetes, you must take insulin because your body no longer makes this hormone. Different types of insulin start to work at different speeds, and the effects of each last a different length of time. You may need to use more than one type. You can take insulin a number of ways . Common options include a needle and syringe , insulin pen , or insulin pump .
Some people who have trouble reaching their blood glucose targets with insulin alone also might need to take another type of diabetes medicine that works with insulin, such as pramlintide . Pramlintide, given by injection, helps keep blood glucose levels from going too high after eating. Few people with type 1 diabetes take pramlintide, however. The NIH has recently funded a large research study to test use of pramlintide along with insulin and glucagon in people with type 1 diabetes. Another diabetes medicine, metformin, may help decrease the amount of insulin you need to take, but more studies are needed to confirm this. Reseachers are also studying other diabetes pills that people with type 1 diabetes might take along with insulin.
Hypoglycemia, or low blood sugar, can occur if you take insulin but don’t match your dose with your food or physical activity. Severe hypoglycemia can be dangerous and needs to be treated right away. Learn more about hypoglycemia and how to prevent or treat it.
Along with insulin and any other medicines you use, you can manage your diabetes by taking care of yourself each day. Following your diabetes meal plan, being physically active, and checking your blood glucose often are some of the ways you can take care of yourself. Work with your health care team to come up with a diabetes care plan that works for you. If you are planning a pregnancy with diabetes , try to get your blood glucose levels in your target range before you get pregnant.
The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) has played an important role in developing “artificial pancreas” technology. An artificial pancreas replaces manual blood glucose testing and the use of insulin shots. A single system monitors blood glucose levels around the clock and provides insulin or a combination of insulin and glucagon automatically. The system can also be monitored remotely, for example by parents or medical staff.
In 2016, the U.S. Food and Drug Administration approved a type of artificial pancreas system called a hybrid closed-loop system. This system tests your glucose level every 5 minutes throughout the day and night through a continuous glucose monitor , and automatically gives you the right amount of basal insulin , a long-acting insulin, through a separate insulin pump. You still need to manually adjust the amount of insulin the pump delivers at mealtimes and when you need a correction dose. You also will need to test your blood with a glucose meter several times a day. Talk with your health care provider about whether this system might be right for you.
The illustration below shows the parts of a type of artificial pancreas system.

The continuous glucose monitor sends information through a software program called a control algorithm. Based on your glucose level, the algorithm tells the insulin pump how much insulin to deliver. The software program could be installed on the pump or another device such as a cell phone or computer.
Starting in late 2016 and early 2017, the NIDDK has funded several important studies on different types of artificial pancreas devices to better help people with type 1 diabetes manage their disease. The devices may also help people with type 2 diabetes and gestational diabetes.
NIDDK is also supporting research into pancreatic islet transplantation —an experimental treatment for hard-to-control type 1 diabetes. Pancreatic islets are clusters of cells in the pancreas that make insulin. Type 1 diabetes attacks these cells. A pancreatic islet transplant replaces destroyed islets with new ones that make and release insulin. This procedure takes islets from the pancreas of an organ donor and transfers them to a person with type 1 diabetes. Because researchers are still studying pancreatic islet transplantation, the procedure is only available to people enrolled in a study. Learn more about islet transplantation studies .
Over time, high blood glucose leads to problems such as
- heart disease
- kidney disease
- eye problems
- dental disease
- nerve damage
- foot problems
- sleep apnea
If you have type 1 diabetes, you can help prevent or delay the health problems of diabetes by managing your blood glucose, blood pressure, and cholesterol, and following your self-care plan.
At this time, type 1 diabetes can’t be prevented. However, through studies such as TrialNet, researchers are working to identify possible ways to prevent or slow down the disease.
This content is provided as a service of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), part of the National Institutes of Health. NIDDK translates and disseminates research findings to increase knowledge and understanding about health and disease among patients, health professionals, and the public. Content produced by NIDDK is carefully reviewed by NIDDK scientists and other experts.
The NIDDK would like to thank: Rita Basu, M.D., Mayo Clinic
- Patient Care & Health Information
- Diseases & Conditions
- Type 1 diabetes
Diagnostic tests include:
- Glycated hemoglobin (A1C) test. This blood test shows your average blood sugar level for the past 2 to 3 months. It measures the amount of blood sugar attached to the oxygen-carrying protein in red blood cells (hemoglobin). The higher the blood sugar levels, the more hemoglobin you'll have with sugar attached. An A1C level of 6.5% or higher on two separate tests means you have diabetes.
If the A1C test isn't available, or if you have certain conditions that can make the A1C test inaccurate — such as pregnancy or an uncommon form of hemoglobin (hemoglobin variant) — your provider may use these tests:
- Random blood sugar test. A blood sample will be taken at a random time and may be confirmed by additional tests. Blood sugar values are expressed in milligrams per deciliter (mg/dL) or millimoles per liter (mmol/L). No matter when you last ate, a random blood sugar level of 200 mg/dL (11.1 mmol/L) or higher suggests diabetes.
- Fasting blood sugar test. A blood sample will be taken after you don't eat (fast) overnight. A fasting blood sugar level less than 100 mg/dL (5.6 mmol/L) is healthy. A fasting blood sugar level from 100 to 125 mg/dL (5.6 to 6.9 mmol/L) is considered prediabetes. If it's 126 mg/dL (7 mmol/L) or higher on two separate tests, you have diabetes.
If you're diagnosed with diabetes, your provider may also run blood tests. These will check for autoantibodies that are common in type 1 diabetes. The tests help your provider decide between type 1 and type 2 diabetes when the diagnosis isn't certain. The presence of ketones — byproducts from the breakdown of fat — in your urine also suggests type 1 diabetes, rather than type 2.
After the diagnosis
You'll regularly visit your provider to talk about managing your diabetes. During these visits, the provider will check your A1C levels. Your target A1C goal may vary depending on your age and various other factors. The American Diabetes Association generally recommends that A1C levels be below 7%, or an average glucose level of about 154 mg/dL (8.5 mmol/L).
A1C testing shows how well the diabetes treatment plan is working better than daily blood sugar tests. A high A1C level may mean you need to change the insulin amount, meal plan or both.
Your provider will also take blood and urine samples. They will use these samples to check cholesterol levels, as well as thyroid, liver and kidney function. Your provider will also take your blood pressure and check the sites where you test your blood sugar and deliver insulin.
More Information
- Blood pressure test
Treatment for type 1 diabetes includes:
- Taking insulin
- Counting carbohydrates, fats and protein
- Monitoring blood sugar often
- Eating healthy foods
- Exercising regularly and keeping a healthy weight
The goal is to keep the blood sugar level as close to normal as possible to delay or prevent complications. Generally, the goal is to keep the daytime blood sugar levels before meals between 80 and 130 mg/dL (4.44 to 7.2 mmol/L). After-meal numbers should be no higher than 180 mg/dL (10 mmol/L) two hours after eating.
Insulin and other medications
Anyone who has type 1 diabetes needs insulin therapy throughout their life.
There are many types of insulin, including:
- Short-acting insulin. Sometimes called regular insulin, this type starts working around 30 minutes after injection. It reaches peak effect at 90 to 120 minutes and lasts about 4 to 6 hours. Examples are Humulin R, Novolin R and Afrezza.
- Rapid-acting insulin. This type of insulin starts working within 15 minutes. It reaches peak effect at 60 minutes and lasts about 4 hours. This type is often used 15 to 20 minutes before meals. Examples are glulisine (Apidra), lispro (Humalog, Admelog and Lyumjev) and aspart (Novolog and FiAsp).
- Intermediate-acting insulin. Also called NPH insulin, this type of insulin starts working in about 1 to 3 hours. It reaches peak effect at 6 to 8 hours and lasts 12 to 24 hours. Examples are insulin NPH (Novolin N, Humulin N).
- Long- and ultra-long-acting insulin. This type of insulin may provide coverage for as long as 14 to 40 hours. Examples are glargine (Lantus, Toujeo Solostar, Basaglar), detemir (Levemir) and degludec (Tresiba).
You'll probably need several daily injections that include a combination of a long-acting insulin and a rapid-acting insulin. These injections act more like the body's normal use of insulin than do older insulin regimens that only required one or two shots a day. A combination of three or more insulin injections a day has been shown to improve blood sugar levels.
- Insulin pump
An insulin pump is a device about the size of a cellphone that's worn on the outside of your body. A tube connects the reservoir of insulin to a catheter that's inserted under the skin of your abdomen. Insulin pumps are programmed to dispense specific amounts of insulin automatically and when you eat.
Insulin delivery options
Insulin can't be taken by mouth to lower blood sugar because stomach enzymes will break down the insulin, preventing it from working. You'll need to either get shots (injections) or use an insulin pump.
Injections. You can use a fine needle and syringe or an insulin pen to inject insulin under the skin. Insulin pens look like ink pens and are available in disposable or refillable varieties.
If you choose shots (injections), you'll probably need a mixture of insulin types to use during the day and night.
An insulin pump. This is a small device worn on the outside of your body that you program to deliver specific amounts of insulin throughout the day and when you eat. A tube connects a reservoir of insulin to a catheter that's inserted under the skin of your abdomen.
There's also a tubeless pump option that involves wearing a pod containing the insulin on your body combined with a tiny catheter that's inserted under your skin.
Blood sugar monitoring
Depending on the type of insulin therapy you select or need, you may have to check and record your blood sugar level at least four times a day.
The American Diabetes Association recommends testing blood sugar levels before meals and snacks, before bed, before exercising or driving, and whenever you think you have low blood sugar. Careful monitoring is the only way to make sure that your blood sugar level remains within your target range. More frequent monitoring can lower A1C levels.
Even if you take insulin and eat on a strict schedule, blood sugar levels can change. You'll learn how your blood sugar level changes in response to food, activity, illness, medications, stress, hormonal changes and alcohol.
Continuous glucose monitoring
Continuous glucose monitoring (CGM) monitors blood sugar levels. It may be especially helpful for preventing low blood sugar. These devices have been shown to lower A1C .
Continuous glucose monitors attach to the body using a fine needle just under the skin. They check blood glucose levels every few minutes.
Closed loop system
A closed loop system is a device implanted in the body that links a continuous glucose monitor to an insulin pump. The monitor checks blood sugar levels regularly. The device automatically delivers the right amount of insulin when the monitor shows that it's needed.
The Food and Drug Administration has approved several hybrid closed loop systems for type 1 diabetes. They are called "hybrid" because these systems require some input from the user. For example, you may have to tell the device how many carbohydrates are eaten, or confirm blood sugar levels from time to time.
A closed loop system that doesn't need any user input isn't available yet. But more of these systems currently are in clinical trials.
Other medications
Other medications also may be prescribed for people with type 1 diabetes, such as:
- High blood pressure medications. Your provider may prescribe angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blockers (ARBs) to help keep your kidneys healthy. These medications are recommended for people with diabetes who have blood pressures above 140/90 millimeters of mercury (mm Hg).
- Aspirin. Your provider may recommend you take baby or regular aspirin daily to protect your heart. Your provider may feel that you have an increased risk of a cardiovascular event. Your provider will discuss the risk of bleeding if you take aspirin.
Cholesterol-lowering drugs. Cholesterol guidelines are stricter for people with diabetes because of their higher risk of heart disease.
The American Diabetes Association recommends that low-density lipoprotein (LDL, or "bad") cholesterol be below 100 mg/dL (2.6 mmol/L). High-density lipoprotein (HDL, or "good") cholesterol is recommended to be over 50 mg/dL (1.3 mmol/L) in women and over 40 mg/dL (1 mmol/L) in men. Triglycerides, another type of blood fat, should be less than 150 mg/dL (1.7 mmol/L).
Healthy eating and monitoring carbohydrates
There's no such thing as a diabetes diet. However, it's important to center your diet on nutritious, low-fat, high-fiber foods such as:
- Whole grains
Your registered dietitian will recommend that you eat fewer animal products and refined carbohydrates, such as white bread and sweets. This healthy-eating plan is recommended even for people without diabetes.
You'll need to learn how to count the amount of carbohydrates in the foods you eat. By doing so, you can give yourself enough insulin. This will allow your body to properly use those carbohydrates. A registered dietitian can help you create a meal plan that fits your needs.
Physical activity
Everyone needs regular aerobic exercise, including people who have type 1 diabetes. First, get your provider's OK to exercise. Then choose activities you enjoy, such as walking or swimming, and do them every day when you can. Try for at least 150 minutes of moderate aerobic exercise a week, with no more than two days without any exercise.
Remember that physical activity lowers blood sugar. If you begin a new activity, check your blood sugar level more often than usual until you know how that activity affects your blood sugar levels. You might need to adjust your meal plan or insulin doses because of the increased activity.
Activities of concern
Certain life activities may be of concern for people who have type 1 diabetes.
- Driving. Low blood sugar can occur at any time. It's a good idea to check your blood sugar anytime you're getting behind the wheel. If it's below 70 mg/dL (3.9 mmol/L), have a snack with 15 grams of carbohydrates. Retest again in 15 minutes to make sure it has risen to a safe level before you start driving.
- Working. Type 1 diabetes can pose some challenges in the workplace. For example, if you work in a job that involves driving or operating heavy machinery, low blood sugar could pose a serious risk to you and those around you. You may need to work with your provider and your employer to ensure that certain adjustments are made. You may need additional breaks for blood sugar testing and fast access to food and drink. There are federal and state laws that require employers to provide these adjustments for people with diabetes.
Being pregnant. The risk of complications during pregnancy is higher for people with type 1 diabetes. Experts recommend that you see your provider before you get pregnant. A1C readings should be less than 6.5% before you try to get pregnant.
The risk of diseases present at birth (congenital diseases) is higher for people with type 1 diabetes. The risk is higher when diabetes is poorly controlled during the first 6 to 8 weeks of pregnancy. Careful management of your diabetes during pregnancy can lower your risk of complications.
- Being older or having other conditions. For those who are weak or sick or have difficulty thinking clearly, tight control of blood sugar may not be practical. It could also increase the risk of low blood sugar. For many people with type 1 diabetes, a less strict A1C goal of less than 8% may be appropriate.
Potential future treatments
- Pancreas transplant. With a successful pancreas transplant, you would no longer need insulin. But pancreas transplants aren't always successful — and the procedure poses serious risks. Because these risks can be more dangerous than the diabetes itself, pancreas transplants are generally used for those with very difficult-to-manage diabetes. They can also be used for people who also need a kidney transplant.
- Islet cell transplantation. Researchers are experimenting with islet cell transplantation. This provides new insulin-producing cells from a donor pancreas. This experimental procedure had some problems in the past. But new techniques and better drugs to prevent islet cell rejection may improve its chances of becoming a successful treatment.
Signs of trouble
Despite your best efforts, sometimes problems will happen. Certain short-term complications of type 1 diabetes, such as low blood sugar, require care immediately.

Low blood sugar (hypoglycemia)
Diabetic hypoglycemia occurs when someone with diabetes doesn't have enough sugar (glucose) in the blood. Ask your provider what's considered a low blood sugar level for you. Blood sugar levels can drop for many reasons, such as skipping a meal, eating fewer carbohydrates than called for in your meal plan, getting more physical activity than normal or injecting too much insulin.
Learn the symptoms of hypoglycemia. Test your blood sugar if you think your levels are low. When in doubt, always test your blood sugar. Early symptoms of low blood sugar include:
- Looking pale (pallor)
- Dizziness or lightheadedness
- Hunger or nausea
- An irregular or fast heartbeat
- Difficulty concentrating
- Feeling weak and having no energy (fatigue)
- Irritability or anxiety
- Tingling or numbness of the lips, tongue or cheek
Nighttime hypoglycemia may cause you to wake with sweat-soaked pajamas or a headache. Nighttime hypoglycemia sometimes might cause an unusually high blood sugar reading first thing in the morning.
If diabetic hypoglycemia isn't treated, symptoms of hypoglycemia worsen and can include:
- Confusion, unusual behavior or both, such as the inability to complete routine tasks
- Loss of coordination
- Difficulty speaking or slurred speech
- Blurry or tunnel vision
- Inability to eat or drink
- Muscle weakness
Severe hypoglycemia may cause:
- Convulsions or seizures
- Unconsciousness
- Death, rarely
You can raise your blood sugar quickly by eating or drinking a simple sugar source, such as glucose tablets, hard candy or fruit juice. Tell family and friends what symptoms to look for and what to do if you're not able to treat the condition yourself.
If a blood glucose meter isn't readily available, treat for low blood sugar anyway if you have symptoms of hypoglycemia, and then test as soon as possible.
Inform people you trust about hypoglycemia. If others know what symptoms to look for, they might be able to alert you to early symptoms. It's important that family members and close friends know where you keep glucagon and how to give it so that a potentially serious situation can be easier to safely manage. Glucagon is a hormone that stimulates the release of sugar into the blood.
Here's some emergency information to give to others. If you're with someone who is not responding (loses consciousness) or can't swallow due to low blood sugar:
- Don't inject insulin, as this will cause blood sugar levels to drop even further
- Don't give fluids or food, because these could cause choking
- Give glucagon by injection or a nasal spray
- Call 911 or emergency services in your area for immediate treatment if glucagon isn't on hand, you don't know how to use it or the person isn't responding
Hypoglycemia unawareness
Some people may lose the ability to sense that their blood sugar levels are getting low. This is called hypoglycemia unawareness. The body no longer reacts to a low blood sugar level with symptoms such as lightheadedness or headaches. The more you experience low blood sugar, the more likely you are to develop hypoglycemia unawareness.
If you can avoid having a hypoglycemic episode for several weeks, you may start to become more aware of coming lows. Sometimes increasing the blood sugar target (for example, from 80 to 120 mg/DL to 100 to 140 mg/DL) at least for a short time can also help improve low blood sugar awareness.
High blood sugar (hyperglycemia)
Blood sugar can rise for many reasons. For example, it can rise due to eating too much, eating the wrong types of foods, not taking enough insulin or fighting an illness.
- Frequent urination
- Increased thirst
- Blurred vision
- Irritability
If you think you have hyperglycemia, check your blood sugar. If it is higher than your target range, you'll likely need to administer a "correction." A correction is an additional dose of insulin given to bring your blood sugar back to normal. High blood sugar levels don't come down as quickly as they go up. Ask your provider how long to wait until you recheck. If you use an insulin pump, random high blood sugar readings may mean you need to change the place where you put the pump on your body.
If you have a blood sugar reading above 240 mg/dL (13.3 mmol/L), test for ketones using a urine test stick. Don't exercise if your blood sugar level is above 240 mg/dL or if ketones are present. If only a trace or small amounts of ketones are present, drink extra noncalorie fluids to flush out the ketones.
If your blood sugar is persistently above 300 mg/dL (16.7 mmol/L), or if your urine ketones stays high in spite of taking correction doses of insulin, call your provider or seek emergency care.
Increased ketones in your urine (diabetic ketoacidosis)
If your cells are starved for energy, the body may begin to break down fat. This produces toxic acids known as ketones. Diabetic ketoacidosis is a life-threatening emergency.
Symptoms of this serious condition include:
- Abdominal pain
- A sweet, fruity smell on your breath
- Shortness of breath
If you suspect ketoacidosis, check the urine for excess ketones with an over-the-counter ketones test kit. If you have large amounts of ketones in the urine, call your provider right away or seek emergency care. Also, call your provider if you have vomited more than once and you have ketones in the urine.
- Bone marrow transplant
- Pancreas transplant
- Type 1 diabetes FAQs
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
From Mayo Clinic to your inbox
Sign up for free and stay up to date on research advancements, health tips, current health topics, and expertise on managing health. Click here for an email preview.
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing!
You'll soon start receiving the latest Mayo Clinic health information you requested in your inbox.
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
Clinical trials
Explore Mayo Clinic studies testing new treatments, interventions and tests as a means to prevent, detect, treat or manage this condition.
Lifestyle and home remedies
Careful management of type 1 diabetes can lower your risk of serious — even life-threatening — complications. Consider these tips:
- Make a commitment to manage your diabetes. Take your medications as recommended. Learn all you can about type 1 diabetes. Make healthy eating and physical activity part of your daily routine. Establish a relationship with a diabetes educator. Ask your health care team for help.
- Identify yourself. Wear a tag or bracelet that says you are living with diabetes. Keep a glucagon kit nearby in case of a low blood sugar emergency. Make sure your friends and loved ones know how to use the kit.
- Schedule a yearly physical exam and regular eye exams. Your regular diabetes checkups aren't meant to replace yearly physicals or routine eye exams. During the physical, your provider will look for any diabetes-related complications. Your provider will also look for other medical problems. Your eye care specialist will check for signs of eye complications, such as retina damage, cataracts and glaucoma.
Keep your vaccinations up to date. High blood sugar can weaken the immune system. Get a flu shot every year. Your provider will likely recommend the pneumonia vaccine, too. They may also recommend getting the COVID-19 vaccine.
The Centers for Disease Control and Prevention (CDC) recommends hepatitis B vaccination if you haven't had it before and you're an adult between the ages of 19 and 59 years with type 1 or type 2 diabetes. The CDC recommends vaccination as soon as possible after diagnosis with type 1 or type 2 diabetes. If you are age 60 or older and have diabetes and haven't received the vaccine, talk to your provider about whether it's right for you.
- Pay attention to your feet. Wash your feet daily in lukewarm water. Dry them gently, especially between the toes. Moisturize your feet with lotion. Check your feet every day for blisters, cuts, sores, redness or swelling. Consult your provider if you have a sore or other foot problem that doesn't heal.
- Keep your blood pressure and cholesterol under control. Eating healthy foods and exercising regularly can help control high blood pressure and cholesterol. Medication also may be needed.
- If you smoke or use other forms of tobacco, ask your provider to help you quit. Smoking increases your risk of diabetes complications. These include heart attack, stroke, nerve damage and kidney disease. Talk to your provider about ways to stop smoking or to stop using other types of tobacco.
- If you drink alcohol, do so responsibly. Alcohol can cause either high or low blood sugar. It depends on how much you drink and if you eat at the same time. If you choose to drink, do so only in moderation and always with a meal. Check your blood sugar levels before going to sleep.
- Take stress seriously. The hormones the body produces when you're under long-term stress may prevent insulin from working properly. This can stress and frustrate you even more. Take a step back and set some limits. Prioritize your tasks. Learn ways to relax. Get plenty of sleep.
Coping and support
Diabetes can affect emotions both directly and indirectly. Poorly controlled blood sugar can directly affect emotions by causing behavior changes, such as irritability. There may be times when you resent your diabetes.
People living with diabetes have an increased risk of depression and diabetes-related distress. Many diabetes specialists regularly include a social worker or psychologist as part of their diabetes care team.
You may find that it helps to talk to other people with type 1 diabetes. Online and in-person support groups are available. Group members often know about the latest treatments. They may also share their own experiences or helpful information. For example, they may share where to find carbohydrate counts for your favorite takeout restaurant.
If you're interested in a support group, your provider may be able to recommend one in your area. Or you can visit the websites of the American Diabetes Association (ADA) or the Juvenile Diabetes Research Foundation (JDRF). These sites may list support group information and local activities for people with type 1 diabetes. You can also reach the ADA at 800-DIABETES ( 800-342-2383 ) or JDRF at 800-533-CURE ( 800-533-2873 ).
Preparing for your appointment
If you think that you or your child might have type 1 diabetes, see your provider immediately. A simple blood test can show if you need more evaluation and treatment.
After diagnosis, you'll need close medical follow-up until your blood sugar level is stable. A provider who specializes in hormonal disorders (endocrinologist) usually works with other specialists on diabetes care. Your health care team will likely include:
- Certified diabetes educator
- Registered dietitian
- Social worker or mental health professional
- Health care provider who specializes in eye care (ophthalmologist)
- Health care provider who specializes in foot health (podiatrist)
Once you've learned how to manage type 1 diabetes, your provider likely will recommend checkups every few months. A thorough yearly exam and regular foot and eye exams also are important. This is especially true if you're having a hard time managing your diabetes, if you have high blood pressure or kidney disease, or if you're pregnant.
These tips can help you prepare for your appointments. They can also let you know what to expect from your provider.
What you can do
- Write down any questions you have. Once you begin insulin treatment, the first symptoms of diabetes should go away. However, you may have new issues that you need to address. These include having low blood sugar that happens often or finding ways to control high blood sugar after eating certain foods.
- Write down key personal information, including any major sources of stress or recent changes in your life. Many factors can affect your diabetes control, including stress.
- Make a list of all the medications, vitamins and supplements you're taking.
- For your regular checkups, bring the records of your glucose values or your meter to your appointments.
- Write down questions to ask your provider.
Preparing a list of questions can help you make the most of your time with your provider and the rest of your health care team. Things you want to discuss with your provider, registered dietitian or diabetes educator include:
- When and how often you should monitor your blood glucose
- Insulin therapy — types of insulin used, timing of dosing, amount of dose
- Insulin administration — shots versus a pump
- Low blood sugar — how to recognize and treat
- High blood sugar — how to recognize and treat
- Ketones — testing and treatment
- Nutrition — types of food and their effect on blood sugar
- Carbohydrate counting
- Exercise — adjusting insulin and food intake for activity
- Medical management — how often to visit your provider and other diabetes care team members
- Sick day management
What to expect from your doctor
Your provider is likely to ask you many questions, including:
- How comfortable are you managing your diabetes?
- How frequent are your low blood sugar episodes?
- Do you know when your blood sugar is getting low?
- What's a typical day's diet like?
- Are you exercising? If so, how often?
- On average, how much insulin are you using daily?
What you can do in the meantime
If you're having trouble managing your blood sugar or you have questions, contact your health care team in between appointments.
- Summary of revisions: Standards of medical care in diabetes — 2022. Diabetes Care. 2022; doi:10.2337/dc22-Srev.
- Papadakis MA, et al., eds. Diabetes mellitus. In: Current Medical Diagnosis & Treatment 2022. 61st ed. McGraw Hill; 2022. https://accessmedicine.mhmedical.com. Accessed May 4, 2022.
- What is diabetes? National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/diabetes/overview/what-is-diabetes. Accessed May 4, 2022.
- Levitsky LL, et al. Epidemiology, presentation, and diagnosis of type 1 diabetes mellitus in children and adolescents. https://www.uptodate.com/contents/search. Accessed May 4, 2022.
- Diabetes mellitus (DM). Merck Manual Professional Version. https://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/diabetes-mellitus-and-disorders-of-carbohydrate-metabolism/diabetes-mellitus-dm. Accessed May 4, 2022.
- AskMayoExpert. Type 1 diabetes mellitus. Mayo Clinic; 2021.
- Robertson RP. Pancreas and islet transplantation in diabetes mellitus. https://www.uptodate.com/contents/search. Accessed May 4, 2022.
- Levitsky LL, et al. Management of type 1 diabetes mellitus in children during illness, procedures, school, or travel. https://www.uptodate.com/contents/search. Accessed May 4, 2022.
- Hyperglycemia (high blood glucose). American Diabetes Association. https://www.diabetes.org/healthy-living/medication-treatments/blood-glucose-testing-and-control/hyperglycemia. Accessed May 4, 2022.
- Diabetes and DKA (ketoacidosis). American Diabetes Association. https://www.diabetes.org/diabetes/dka-ketoacidosis-ketones. Accessed May 4, 2022.
- Insulin resistance & prediabetes. National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/diabetes/overview/what-is-diabetes/prediabetes-insulin-resistance. Accessed May 4, 2022.
- Blood sugar and insulin at work. American Diabetes Association. https://www.diabetes.org/tools-support/diabetes-prevention/high-blood-sugar. Accessed May 4, 2022.
- Inzucchi SE, et al. Glycemic control and vascular complications in type 1 diabetes. https://www.uptodate.com/contents/search. Accessed May 4, 2022.
- Diabetes and oral health. American Diabetes Association. https://www.diabetes.org/diabetes/keeping-your-mouth-healthy. Accessed May 4, 2022.
- Drug treatment of diabetes mellitus. Merck Manual Professional Version. https://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/diabetes-mellitus-and-disorders-of-carbohydrate-metabolism/drug-treatment-of-diabetes-mellitus. Accessed May 4, 2022.
- Weinstock DK, et al. Management of blood glucose in adults with type 1 diabetes mellitus. https://www.uptodate.com/contents/search. Accessed May 7, 2022.
- FDA proves first automated insulin delivery device for type 1 diabetes. U.S. Food and Drug Administration. https://www.fda.gov/news-events/press-announcements/fda-approves-first-automated-insulin-delivery-device-type-1-diabetes. Accessed May 4, 2022.
- Boughton CK, et al. Advances in artificial pancreas systems. Science Translational Medicine. 2019; doi:10.1126/scitranslmed.aaw4949.
- Hypoglycemia (low blood sugar). American Diabetes Association. https://www.diabetes.org/healthy-living/medication-treatments/blood-glucose-testing-and-control/hypoglycemia. Accessed May 4, 2022.
- Diabetes in the workplace and the ADA. U.S. Equal Opportunity Employment Commission. https://www.eeoc.gov/laws/guidance/diabetes-workplace-and-ada. Accessed May 4, 2022.
- Cardiovascular disease and risk management: Standards of medical care in diabetes — 2022. Diabetes Care. 2022; doi:10.2337/dc22-S010.
- Diabetes technology. Standards of Medical Care in Diabetes — 2022. 2022; doi:10.2337/dc22-S007.
- FDA authorizes a second artificial pancreas system. JDRF. https://www.jdrf.org/blog/2019/12/13/jdrf-reports-fda-authorizes-second-artificial-pancreas-system/. Accessed May 4, 2022.
- Classification and diagnosis of diabetes: Standards of medical care in diabetes — 2022. Diabetes Care. 2022; doi:10.2337/dc22-S002.
- Retinopathy, neuropathy, and foot care: Standards of medical care in diabetes — 2022. Diabetes Care. 2022; doi:10.2337/dc22-S012.
- Glycemic targets: Standards of medical care in diabetes — 2022. Diabetes Care. 2022; doi:10.2337/dc22-S012.
- Pharmacologic approaches to glycemic treatment: Standards of medical care in diabetes — 2022. Diabetes Care. 2022; doi:10.2337/dc22-S009.
- Facilitating behavior change and well-being to improve health outcomes: Standards of medical care in diabetes — 2022. Diabetes Care. 2022; doi:10.2337/dc22-S005.
- Centers for Disease Control and Prevention. Use of hepatitis B vaccination for adults with diabetes mellitus: Recommendations of the Advisory Committee on Immunization Practices (ACIP). Morbidity and Mortality Weekly Report. 2011;60:1709.
- Management of diabetes in pregnancy: Standards of medical care in diabetes — 2022. Diabetes Care. 2022; doi:10.2337/dc22-S015.
- Older adults: Standards of medical care in diabetes — 2022. Diabetes Care. 2022; doi:10.2337/dc22-S013.
- FDA approves first-of-its-kind automated insulin delivery and monitoring system for use in young pediatric patients. U.S. Food and Drug Administration. https://www.fda.gov/news-events/press-announcements/fda-approves-first-its-kind-automated-insulin-delivery-and-monitoring-system-use-young-pediatric#:~:text=Today, the U.S. Food and,by individuals aged 2 to. Accessed May 8, 2022.
- What you need to know: Getting a COVID-19 vaccine. American Diabetes Association. https://www.diabetes.org/coronavirus-covid-19/vaccination-guide. Accessed June 1, 2022.
- What is type 1 diabetes? A Mayo Clinic expert explains
Associated Procedures
News from mayo clinic.
- Driven by family, fueled by hope: Mayo Clinic researcher fights against Type 1 diabetes Aug. 06, 2023, 11:00 a.m. CDT
Products & Services
- A Book: The Essential Diabetes Book
- Symptoms & causes
- Diagnosis & treatment
- Doctors & departments
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
Your gift holds great power – donate today!
Make your tax-deductible gift and be a part of the cutting-edge research and care that's changing medicine.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- 06 March 2024
Type 1 diabetes: from the dream of automated insulin delivery to a fully artificial pancreas
- Moshe Phillip 0 ,
- Aaron Kowalski 1 &
- Tadej Battelino 2
The Institute for Endocrinology and Diabetes, Schneider Children's Medical Center, Petah Tikva, and Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel.
You can also search for this author in PubMed Google Scholar
JDRF International, New York, NY, USA.
Faculty of Medicine, University of Ljubljana, and Department of Endocrinology, Diabetes and Metabolism, University Children's Hospital, University Medical Centre Ljubljana, Ljubljana, Slovenia.
The journey from pioneering concept to practical clinical application of automated insulin delivery (AID) systems traces back to 1963, when Arnold Kadish described the first wearable system for delivering insulin, calibrated by an in vivo continuous glucose monitoring (CGM) system (Fig. 1). In effect, this was the first automated closed-loop device, also able to infuse glucagon to counter hypoglycemia. Dubbed ‘The Backpack’, it was an impractical proof-of-concept system that ignited the development of genuinely wearable insulin pumps and CGM systems. By 1974, a portable closed-loop artificial pancreas, which used algorithms on a computer consol to deliver intravenous insulin, was used successfully to treat diabetic ketoacidosis and coma in five adult patients 22–89 years of age 1 . By 1977, this bulky ‘Biostator’ was in limited use in hospitals. From that point on, the development of diabetes technology focused separately on insulin delivery and CGM systems, rather than on an integrated artificial pancreas. Commercial, wearable, continuous subcutaneous insulin infusion (CSII) pumps were introduced from 1978 onward, and the first ‘smart insulin pump’ was launched in 2002, with a bolus calculator and missed-meal-bolus alarms.
The technological and clinical development of fully closed-loop automated insulin delivery systems is shown.
A critical milestone in the evolution of AID systems was reached in 1999, when the first commercial CGM system was approved by the US Food and Drug Administration (FDA). This system sampled glucose in the interstitial fluid every 10 seconds and recorded an average glucose value every 5 minutes, for a total of 288 readings per day. The glucose patterns and trends were retrospectively reviewed by a physician to recommend changes to therapy or lifestyle. This led to lower glycated hemoglobin (HbA1c) and fewer episodes of hypoglycemia than with fingerstick blood glucose testing 2 .
In 2003, a group of diabetes clinicians founded The Loop Club 3 , which met for the first time ahead of the 42nd Annual Meeting of the European Society for Paediatric Endocrinology in Ljubljana, Slovenia. This event brought together diabetes clinical professionals and commercial stakeholders from the diabetes technology sector, including from established and start-up companies. The common purpose of this group was to accelerate the development of a wearable AID system for use in daily diabetes care, outside of the hospital setting, by children and adults with insulin-dependent diabetes. Additional momentum was provided by the 2006 Juvenile Diabetes Research Foundation’s Artificial Pancreas Project 4 .
The realization of automated insulin delivery
The efficacy of CSII pumps in lowering HbA1c, reducing hypoglycemia and improving quality of life spurred research and technological innovation on ways to close the diabetes management loop. The goal was for CGM to be used with CSII pumps to provide real-time glucose readings on which AID doses could be adjusted, in a safe and effective system. This heralded the dawn of sensor-augmented pump therapy, in which users are provided CGM devices on which they can base their insulin adjustments. The STAR 3 study of this therapy indicated that relative to results obtained with multiple daily injections, children and adolescents with suboptimal management of type 1 diabetes (T1D) were able to decrease their HbA1c, hyperglycemic excursions and glycemic variability in a short period, in a sustainable and safe manner 5 . This study also emphasized the importance of using the CGM sensor for a considerable proportion of time each day.
The next key advance was the development of CSII pumps able to receive CGM data from compatible systems as part of sensor-augmented pump therapy. Although this did not facilitate full AID functionality, such systems were able to accept CGM data in order to suspend insulin infusion when glucose readings hit a pre-set target, to avoid hypoglycemia. This low-glucose suspend (LGS) combination was safe and effective, with no deterioration in glucose control 2 .
LGS systems, with reactive use of CGM data, were followed by the development of predictive LGS systems that proactively interrupt insulin delivery as CGM sensor glucose readings fall. These systems were shown to be more-effective for reducing hypoglycemia than were sensor-augmented pump systems, and were associated with diminished diabetes distress and improved quality of life for people with T1D 6 .
Closing the loop
A major goal for the care of people with T1D is the closed-loop AID system, an ‘artificial pancreas’ that automatically infuses insulin at a rate and dose that maintains glucose within a defined target range 24 hours a day, including at mealtimes. To achieve this, a CGM sensor feeds glucose data into a control algorithm that determines how much insulin must be delivered via the CSII pump. In achieving this goal, the development of sophisticated algorithms that mimic the reasoning of a diabetes practitioner has been a critical step.
The first studies of fully closed-loop AID systems were undertaken under hospital supervision and showed that these systems improved glucose control and reduced the risk of nocturnal hypoglycemia in children, adolescents and adults with T1D 7 . The next step was to show that closed-loop AID systems could be used successfully and safely outside of the hospital setting. This happened in 2011 with the first randomized crossover trial of AID technology in children and adolescents over two nights at diabetes youth camps in Israel, Slovenia and Germany 8 . The study compared the use of AID against sensor-augmented pump therapy and showed that episodes of nocturnal hypoglycemia below 63 mg/dl were significantly fewer in the group using AID than with sensor-augmented pump therapy, and that fasting plasma glucose on waking was lower as well. Time with glucose in the range of 70–140 mg/dl overnight (‘time in range’) was also significantly greater for users of AID than with sensor-augmented pumps. Adverse events were also fewer with AID.
AID systems in fully closed-loop configurations must be modified to minimize the degree of post-prandial glycemic excursions; these are a consequence of the lag between the automatic insulin response to mealtime carbohydrates and the speed of glucose absorption. Consequently, hybrid closed-loop systems have been developed, with which users must manually announce anticipated mealtime carbohydrates and calculate increased insulin doses before eating. Similarly, periods of planned physical activity and exercise require the AID user to manually adjust insulin delivery rates and timings. Since the first hybrid closed-loop AID system was introduced, studies have demonstrated that people with T1D, including young children and older adults, can improve their time in range and decrease their HbA1c, with no additional risk of hypoglycemia 9 .
Innovation from companies and people with diabetes
Advances in diabetes technology have let to the emergence of new companies and changes in regulatory approach, such as FDA acceptance in 2008 of an in silico computer simulation, rather than animal trials, of a closed-loop control algorithm as part of a regulatory submission when assessing these control systems for inclusion in hybrid CSII–CGM products. A key element driving innovation has been the participation of commercial diabetes stakeholders, who have provided proprietary data and fostered projects with clinical experts, including funding clinical trials. A parallel process of innovation has been generated by people with diabetes, including the development of open-source data architectures and the creation of ‘do-it-yourself’ artificial pancreas systems, ahead of commercially regulated and approved systems.
An ongoing goal for AID systems is to maintain day-to-day glycemic control within recommended glucose target ranges, without intervention by the person with T1D. This is emphasized the most in AID systems that do not require mealtime insulin bolus announcements. Ongoing research includes the use of acoustic mealtime detection, based on the AID system’s responding to chewing and swallowing noises that precede the entry of food into the stomach, which should give the system a 10- to-15-minute warning before post-prandial glucose levels begin to rise. Hand-gesture recognition associated with eating is another avenue of enquiry.
Similar approaches may also provide solutions for fully closed-loop AID systems to understand the physical cues associated with anticipated exercise. Artificial-intelligence-adapted algorithms can model pancreatic beta-cell physiology; this has been successfully tested in fully closed-loop computer simulations against regulatory-body-approved AID systems 10 , and results show they cope better with the real-world challenge of missed or delayed meal announcements. The addition of adjunct therapy can also improve fully closed-loop performance. The inclusion of a sodium–glucose co-transporter-2 inhibitor (SGLT-2i) to closed-loop AID therapy in T1D has been shown to increase time in range and reduce post-prandial excursions relative to AID therapy alone 11 .
Continuous ketone and lactate monitoring
Several daily situations can lead to rapid production of ketone bodies in people with T1D, resulting in diabetic ketoacidosis within a few hours. For example, ketogenesis can occur when insulin infusion from a CSII pump is interrupted, during fasting or when carbohydrate intake is low. It is estimated that as many as 3% of adolescents and adults with T1D on insulin-pump therapy have experienced at least one such episode in the past 3 months. Despite this, ketone testing by people with T1D is uncommon because of lack of awareness or the inconvenience of carrying a separate device for this. A real-time continuous ketone-monitoring sensor has been developed that measures β-hydroxybutyrate, a product of ketosis, in the interstitial fluid of adults on ketogenic diets 12 . Additional studies are needed to assess the performance of such sensors in people with T1D across the range of daily scenarios.
The detection of lactate, due to anaerobic metabolism during physical training and exercise, has clear applications for the future of AID systems. The hybrid closed-loop operation of current AID systems allows people with T1D to manually specify periods of exercise and physical activity during which their insulin needs and insulin sensitivity change. Fully closed-loop AID operation would reduce or eliminate the need for users to announce their exercise. Continuous lactate-monitoring sensors have been under development for many inpatient scenarios related to infection, sepsis and trauma, as well as for monitoring exercise training in athletes. Lactate biosensing in AID systems could enable the control algorithm to make automatic decisions on insulin needs and dosing for people with T1D, to support healthful exercise.
Analogues and bi-hormonal systems
Ultra-rapid-acting insulin analogues further reduce the lag time between subcutaneous insulin infusion and the onset and peak of action. Formulations of insulin aspart and insulin lispro have been approved by the European Medicines Agency and the FDA. Both analogues have been clinically tested in hybrid closed-loop or fully closed-loop AID systems and do decrease post-prandial glucose and mean glucose and improve time in the target glucose range, relative to results obtained with the original formulations 13 , indicative of a faster insulin response to rising glucose. These studies also used existing control algorithms not adapted to the delivery of ultra-rapid analogues; in silico simulations have indicated a greater potential for substantially improved glycemic control when algorithms are optimized for their pharmacokinetics.
A physiological glycemic-control strategy is being developed in bi-hormonal AID systems, which incorporate glucagon to prevent hypoglycemia by counter-regulation of insulin. A 2021 trial of such a device, using a self-learning glucose-control algorithm, was undertaken in 23 adults with T1D who used the system at home for 2 weeks, after a 4-day training period 14 . Participants had more time in range, less time in hypoglycemia and less time in hyperglycemia than with standard insulin pump therapy. In this small study cohort, use of the fully closed-loop bi-hormonal AID system relieved users of the burden of carbohydrate counting, or other behavioral adaptations, in order to achieve good glycemic control.
Use of the amylin analogue pramlintide, to reduce the rate of glucose absorption after meals, has also been investigated in a bi-hormonal AID system with insulin 15 . This system also used a meal-detection algorithm to co-deliver basal and bolus insulin doses and pramlintide in a fixed ratio, mimicking co-formulation. This open-label study was undertaken at a research center, rather than under real-world conditions, but demonstrated non-inferiority to a hybrid closed-loop system that delivered insulin only.
Conclusions
The period that has defined the development of the artificial pancreas spans 60 years, but most achievements have occurred in the past 20 years. This pace of clinical progress has provided people with diabetes on intensive insulin treatment with a powerful tool to achieve a better metabolic control. Future developments in AID systems will further reduce the burden of diabetes and narrow the gap between technology and physiology, to deliver the fully autonomous artificial pancreas envisioned by Arnold Kadish at the start of this journey.
doi: https://doi.org/10.1038/d41591-024-00013-5
Competing interests
M.P. has participated on advisory boards for AstraZeneca, Eli Lilly, Insulet, Mannkind, Medtronic Diabetes, Pfizer, Sanofi and Dompé, has received consulting fees from Eli Lilly, Medtronic Diabetes, Novo Nordisk, Pfizer, Sanofi and Qulab Medical, has received, through his institute, research grants from Dexcom, Eli Lilly, Insulet, Medtronic Diabetes, Novo Nordisk, Pfizer, Roche Diagnostics, Sanofi, DreaMed Diabetes, NG Solutions, Dompe, Lumos, GWAVE and OPKO, and owns stocks in DreaMed-Diabetes and NG Solutions. T.B. has participated on advisory boards for Novo Nordisk, Sanofi, Eli Lilly, Boehringer-Ingelheim, Medtronic and Indigo Diabete, and as a speaker for AstraZeneca, Eli Lilly, Novo Nordisk, Medtronic, Sanofi and Roche, and owns stocks of DreaMed Diabetes, and his institution has received research grant support and travel expenses from Abbott Diabetes Care, Medtronic, Novo Nordisk, Sanofi, Sandoz and Novartis. A.K. declares no competing interests.
Pfeiffer, E., Thum, Ch. & Clemens, A. Horm. Metab. Res. 6 , 339–342 (1974).
Google Scholar
Bergenstal, R.M. et al. N. Engl. J. Med. 369 , 224–232 (2013).
Battelino, T. & Phillip, M. J. Pediatr. Endocrinol. Metab. 17 , 375–376 (2004).
Kowalski, A.J. Diabetes Technol. Ther. 11 S1, S113–S119 (2009).
Bergenstal, R.M. et al. N. Engl. J. Med. 363 , 311–320 (2010).
Battelino, T., Nimri, R., Dovc, K., Phillip, M. & Bratina, N. Diabetes Care 40 , 764–770 (2017).
Hovorka, R. et al. Lancet 375 , 743–751 (2010).
Phillip, M. et al. N. Engl. J. Med. 368 , 824–833 (2013).
Tauschmann, M. et al. Lancet 392 , 1321–1329 (2018).
Armiger, R., Reddy, M., Oliver, N.S., Georgiou, P. & Herrero, P. J. Diabetes Sci. Technol. 16 , 29–39 (2022).
Biester, T. et al. Diabetes Obes. Metab. 23 , 599–608 (2021).
Alva, S., Castorino, K., Cho, H. & Ou, J. J. Diabetes Sci. Technol. 15 , 768–774 (2021).
Nwokolo, M. et al. Diabetes Technol. Ther. 25 , 856–863 (2023).
Blauw, H., Onvlee, A.J., Klaassen, M., van Bon, A.C. & DeVries, J.H. Diabetes Care 44 , 836–838 (2021).
Tsoukas, M.A. et al. Lancet Digital Heal 3, e723–e732 (2021).
Download references
Reprints and permissions
Tenure-Track Assistant Professor to the rank of Associate Professor in computational biology
UNIL is a leading international teaching and research institution, with over 5,000 employees and 15,500 students split between its Dorigny campus, ...
Lausanne, Canton of Vaud (CH)
University of Lausanne (UNIL)
Assistant Scientist/Professor in Rare Disease Research, Sanford Research
Assistant Scientist/Professor in Rare Disease Research, Sanford Research Sanford Research invites applications for full-time faculty at the rank of...
Sioux Falls, South Dakota
Sanford Research
Junior Group Leader
The Cancer Research UK Manchester Institute seeks to appoint an outstanding scientist to a new Junior Group Leader position.
United Kingdom
Research projects in all fields of the humanities, social sciences, and natural sciences are welcome
The aim of fostering future world-class researchers at Kyoto University.
Hakubi Center for Advanced Research, Kyoto University
Staff Scientist (Virology)
Staff Scientist position available at Scripps Research
La Jolla, California
The Scripps Research Institute (TSRI)
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

Inclusive Health: Creating Access to Type 1 Diabetes Screening in Underserved Communities
Editor’s Note: This interview was condensed and edited for clarity.
Thanks to decades of research, it’s possible to identify type 1 diabetes ( T1D ) in its earliest stages and potentially delay the full onset, requiring daily insulin therapy.
Spotting those early stages, though, requires autoantibody screening. Screening looks for the autoantibodies that develop when the immune system attacks those insulin-producing cells. This autoimmune attack can start years before noticeable symptoms develop.
While screening itself is a simple blood test, the biggest challenge is educating people about the importance of getting screened and providing support for individuals who test positive for early-stage type 1 diabetes.
This effort is easier said than done, especially in underserved communities.
“We’re in the community——we don’t just sit here in the hospital,” said Franklin Hickey, PhD, RN, NEA-BC, chief of Staff of University Hospital , when asked about local screening efforts for T1D in Newark, New Jersey.
Research and outreach efforts to screen for T1D have made incredible strides to reduce the risk and severity of DKA (diabetic ketoacidosis) at the time of diagnosis. This outreach includes educating the general community about the symptoms of T1D, the earlier stages of T1D before symptoms develop, FDA-approved medications to delay the full onset of T1D, and ongoing prevention research. In partnership with JDRF’s early detection program , University Hospital in Newark, NJ, worked with JDRF to screen for type 1 diabetes and educate their community members about the signs and symptoms of T1D.
University Hospital plays a critical role in providing care to patients who are uninsured, qualify for Medicaid, and may be facing economic challenges, food insecurity, and housing insecurity. The hospital strives to address the health inequities that complicate a person’s ability to manage chronic conditions like diabetes.
Here’s a closer look at the partnership between University Hospital and JDRF, and their screening efforts.
The Power of Community Outreach
“Since the launch of this program in July of 2022, University Hospital has identified 1,000 individuals to be screened,” said Hickey. “To date, we’ve had 50 community events and screened 883 community members.”
The program offered screening to everyone—with an emphasis on children. Parents and caregivers were notified about the opportunity to have their children screened. Out of all of the patients they screened, 15 tested positive for T1D autoantibodies and two of those patients had two persistent diabetes-related autoantibodies. One child had an elevated hemoglobin A1C.
After a patient has been screened, University Hospital provides follow-up support and additional education based on the results. If a child tests positive for autoantibodies, University Hospital ensures they receive necessary support and guidance while getting connected to specialty care.
“We were able to find a pediatric endocrinologist and get that patient treated,” recalls Hickey. “We’re proud because that kid never had any issues with DKA. We prevented that from happening with our program.”
T1D screening and prevention may require educating individuals who’ve never heard of T1D and may only be familiar with type 2 diabetes (T2D) , which is a metabolic condition.
“You don’t hear a lot about type 1 diabetes. Instead, most people in this community think of diabetes as type 2,” said Hickey. “When we’re calling these families of those kids that we identified with a diagnosis of T1D, we wanted to screen the parents and the siblings. But to do that, we had a script by JDRF explaining why this was important.”
Even if the screening results came back negative, participants were provided resources and education on the stages and symptoms of T1D.
University Hospital staff understand how much representation matters when sending people to educate the community about screening. The majority of the community members are African American and Latinx, and span nationalities including the Caribbean. This ensures they are reaching diverse communities to bring awareness to screening for T1D.
“We train our staff, including those who speak Spanish to spread our message to the Latino community,” said Hickey. “We even put a cultural liaison in our Portuguese community. We need people who understand these communities and cultural norms. That’s part of building trust.”
Addressing Socioeconomic Challenges Facing Community Members
Socioeconomic challenges can keep people from seeking preventative care, or any care at all, in a timely manner. Screening for T1D was no different—as some of the challenges people face include travel access, such as needing to take public transportation to get to the hospital. Understanding those challenges is why Hickey and his team meet people where they are and go directly into communities as much as possible.
Despite challenges, University Hospital maintains multiple touchpoints to ensure patients get test results and reminders for follow-up appointments.
“In addition to calls and emails, we use an electronic health record platform, where they can see their medical records, including test results, and send notifications about returning for another visit,” said Hickey.
Expanding T1D Screening to New Communities & Health Systems
Getting ahead of a potential T1D diagnosis is an all-hands-on-deck effort—it requires having adequate staff on hand and everyone from administrative staff to community healthcare workers, to primary care doctors to understand T1D, as it is a complex condition often conflated with type 2 diabetes.
The staff’s buy-in into the program is just as important as the community members who are at the receiving end. JDRF and University Hospital are learning what staff members need to be successful in interacting with program participants, especially as they consider its long- term future.
“We’re all learning in humility,” said Anastasia Albanese-O’Neill, PhD, APRN, CDCES , the AVP of Community Screening and Clinical Trial Education at JDRF. “We’re learning in partnership with Franklin and his team and incorporating those learnings into the next phase of this work. I think we’ve demonstrated feasibility and acceptability, but we want to know if this program is sustainable over time at University Hospital. We will work on answering those questions in the next phase of the partnership.”
JDRF and University Hospital are looking to replicate the success of this initiative with other healthcare professionals. Currently, they have over 30 pilot clinics across the country, ranging from federally qualified health centers to large health systems.
“We know healthcare professionals can go to a conference and learn about the importance of T1D screening, but they may not implement it when they return to work,” said Albanese-O’Neill.
“This initiative is proximate to care and community education. Trust is critical. That’s why we supported University Hospital to hire a full-time T1D navigator who is working to build relationships in the community. These partnerships are essential in identifying and prioritizing the needs of the community.”
How the T1D Community Can Support Early Detection of T1D
The T1D online community has been a beacon of support for many who are newly diagnosed. People impacted by this chronic condition often use their platforms to educate others about managing diabetes, as well as the signs to look for in others. JDRF has a dedicated community of volunteers who spread awareness about the organization’s initiatives.
By joining forces, University Hospital and JDRF have successfully broadened their ability to reach more diverse communities.
Hickey also emphasized the importance of volunteers helping to spread the word about screening, especially within underserved communities.
Albanese-O’Neill agreed, adding that JDRF volunteers play an important role in spreading the word about this initiative.
“It’s important for our volunteers and the T1D community to know that these screening programs exist. They can be our allies in finding more clinical champions and connecting people back to our work at University Hospital.”
This article was made possible due to the JDRF x Beyond Type 1 Alliance. For more resources on screening for type 1 diabetes, please visit JDRF’s T1Detect website and Beyond Type 1’s resources . To become a JDRF volunteer, please click here .

WRITTEN BY T'ara Smith, MS, Nutrition Education, POSTED 03/08/24, UPDATED 03/11/24
The night my son nearly died for a sprite -, a mother’s cautionary tale -, signs of type 1 diabetes -.

- TERMS OF USE
- 400 CONCAR DR.
- SAN MATEO, CA 94402

- BEYOND TYPE 1 BEYOND TYPE 1 EN ESPAÑOL
- IN ENGLISH EN ESPANOL BEYOND TYPE 1 AUF DEUTSCH BEYOND TYPE 1 IN ITALIANO BEYOND TYPE 1 EN FRANÇAIS BEYOND TYPE 1 IN HET NEDERLANDS BEYOND TYPE 1 PÅ SVENSKA BEYOND TYPE 1 EM PORTUGUÊS BEYOND TYPE 1 بالعربية

- BEYOND TYPE 2 BEYOND TYPE 2 EN ESPAÑOL IN ENGLISH EN ESPANOL BEYOND TYPE 2 AUF DEUTSCH BEYOND TYPE 2 en Français BEYOND TYPE 2 in Italiano BEYOND TYPE 2 Canada (French) BEYOND TYPE 2 Canada (English)
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Advanced Search
- Journal List
- HHS Author Manuscripts

Type 1 diabetes
Linda a dimeglio.
Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN, USA;
Carmella Evans-Molina
Department of Medicine, Indiana University School of Medicine, Indianapolis, IN, USA;
Richard A Oram
Institute of Biomedical and Clinical Science, University of Exeter Medical School, and The Academic Kidney Unit, Royal Devon and Exeter NHS Foundation Trust, Exeter, UK
Contributors
Type 1 diabetes is a chronic autoimmune disease characterised by insulin deficiency and resultant hyperglycaemia. Knowledge of type 1 diabetes has rapidly increased over the past 25 years, resulting in a broad understanding about many aspects of the disease, including its genetics, epidemiology, immune and β-cell phenotypes, and disease burden. Interventions to preserve β cells have been tested, and several methods to improve clinical disease management have been assessed. However, wide gaps still exist in our understanding of type 1 diabetes and our ability to standardise clinical care and decrease disease-associated complications and burden. This Seminar gives an overview of the current understanding of the disease and potential future directions for research and care.
Introduction
At first consideration, type 1 diabetes pathophysiology and management might seem straightforward; however, the more that is learnt about the disease, the less it seems is truly known. Improved understanding of the disease’s pathogenesis has not led to a single unifying Koch’s postulate for all cases. What once seemed like a single autoimmune disorder, with roots in T-cell mediated attack of insulin-producing β cells, is now recognised to result from a complex interplay between environmental factors and microbiome, genome, metabolism, and immune systems that vary between individual cases.
Despite known genetic underpinnings, most people who are diagnosed with type 1 diabetes do not have a relative with the disease or even the highest risk combination of HLA alleles, making attempts at primary disease prevention difficult. Although survival and patient health have improved considerably, particularly in the past 25 years, a cure for type 1 diabetes remains elusive. 1 , 2 Additionally, despite advances in technology, glycaemic control for most people with type 1 diabetes is not optimised, and many cannot access modern therapies because of the high costs of even basic care.
In 1984, George Eisenbarth developed a conceptual model for the pathogenesis of type 1 diabetes that is still used nowadays ( figure 1 ). 3 The model plots β-cell mass against age, highlighting an event sequence starting with predisposing genetic risk, then a precipitating environmental trigger that causes islet-specific auto-immunity, followed by β-cell loss, dysglycaemia, clinical diabetes, and rapid progression to complete β-cell loss. Although useful, this model does not address the increasingly apparent complexity of type 1 diabetes pathogenesis. Additionally, the disease pathogenesis is shown by a single line of disease course over time; however, at all stages of the disease heterogeneity exists that is not well understood.

Key events of the Eisenbarth model 3 over the course of the disease (measured in years) are shown by dotted lines at different time points. Challenges to this model, taking into account the increasing complexity of type 1 diabetes, include the following: precipitating immune events that might occur prenatally (A); large variation in starting β-cell mass and function, defects in one or both could be developmentally programmed (B); initiation of autoimmunity is measured by autoantibodies, but other immunological abnormalities probably precede the presence of detectable pancreatic antibodies (C); the patient’s environment could affect their entire disease course (D); β-cell loss could relapse or remit (E); dysglycaemia occurs before clinical diagnosis (F); decline in β-cell function might not mirror decline in β-cell mass—methods to measure β-cell mass have not been established (G); and residual C-peptide is detectable in many people who have long duration type 1 diabetes (H). Furthermore, progression through stages A–C is heterogeneous, and will be affected by immune, genetic, environment, and key demographic features (ie, age, body-mass index). Adapted from Atkinson et al. 4
This Seminar provides a review of type 1 diabetes and the status of research in the field. We focus on developments from the past 5 years that highlight the heterogeneity and complexity of the disease.
A diagnosis of diabetes is based on a fasting blood glucose concentration above 7·0 mmol/L (126 mg/dL), a random blood glucose concentration above 11·1 mmol/L (200 mg/dL) with symptoms, or an abnormal result from an oral glucose tolerance test. 5 In the absence of symptoms, abnormal glycaemia must be present on two different occasions. A diagnosis of diabetes can also be made on the basis of a glycated haemoglobin (HbA 1c ) concentration above 48 mmol/mol (6·5%). However, since dysglycaemia progression can be rapid in patients with type 1 diabetes, HbA 1c is less sensitive for diagnosis than fasting or stimulated blood glucose measurements. 5
Children with type 1 diabetes commonly present with symptoms of polyuria, polydipsia, and weight loss; approximately a third present with diabetic ketoacidosis. 6 The onset of type 1 diabetes can be more variable in adults, who might not present with the classic symptoms seen in children. Although traditional definitions classified type 1 diabetes as juvenile onset, the disease can occur at any age, with up to 50% of cases occurring in adulthood. 7 As many as 50% of adults with type 1 diabetes might be initially misclassified as having type 2 diabetes. 8 Similarly, in conjunction with the epidemic of childhood obesity, type 2 diabetes is increasingly common in adolescents (particularly in non-white individuals), and monogenic diabetes (eg, maturity diabetes onset of the young) accounts for 1–6% of childhood diabetes cases. 9 – 11
Although low C-peptide concentration as a marker of severe endogenous insulin deficiency is useful to guide both classification and treatment in cases of diabetes assessed over 3 years after clinical diagnosis, 12 no single clinical feature can perfectly distinguish type 1 from non-type 1 diabetes at diagnosis. Classification depends on an appreciation of other risk factors for type 1 versus other subtypes and the integration of clinical features (eg, age of diagnosis and body-mass index) with biomarkers (eg, pancreatic autoantibodies). 13
Over 90% of people with newly diagnosed type 1 diabetes have measurable antibodies against specific β-cell proteins, including insulin, glutamate decarboxylase, islet antigen 2, zinc transporter 8, and tetraspanin-7. 14 Birth cohort studies 15 , 16 of individuals with a high genetic risk for diabetes have shown a peak incidence of first autoantibody development before age 2 years. Most people with a single autoantibody do not progress to type 1 diabetes, but seroconversion to the presence of two or more serum autoantibodies in children is associated with an 84% risk of clinical type 1 diabetes by the age of 18 years ( figure 2A ). 16 The high risk of progression in the presence of multiple autoantibodies has led to a redefining of type 1 diabetes stages. In this new paradigm, a preclinical stage 1 case of type 1 diabetes is defined as the presence of two or more autoantibodies, while stages 2 and 3 are defined as the progression of metabolic abnormalities from abnormal glycaemia to overt diabetes, diagnosed by standard criteria ( figure 2B ). 18 Since the progression from islet autoantibody positivity to clinical diabetes could take months or years, defining multiple auto-antibody positivity as stage 1 allows targeting of immune interventions to a realistic primary outcome and facilitates early life intervention studies. 19

(A) The probability of developing diabetes in childhood stratified by the number of islet antibodies. In a study by Zeigler and colleagues, 16 13 377 children were identified as at risk in the newborn or infant period on the basis of high-risk HLA genotypes or having a relative with type 1 diabetes, or both, and were followed-up regularly. The numbers at risk are the number of children receiving follow-up at ages 0, 5, 10, 15, and 20 years. Adapted from Ziegler et al 16 with permission of the American Medical Association. (B) Type 1 diabetes progression and stages of type 1 diabetes. Stage 1 is the start of type 1 diabetes, marked by individuals having two or more diabetes-related autoantibodies and normal blood sugar concentrations. In stage 2, individuals have dysglycaemia without symptoms. Stage 3 is the time of clinical diagnosis. Reproduced from Greenbaum et al, 17 with permission from the American Diabetes Association. T1D=type 1 diabetes.
Type 1 diabetes is a heritable polygenic disease with identical twin concordance of 30–70%, 20 sibling risk of 6–7%, and a risk of 1–9% for children who have a parent with diabetes. 21 The overall lifetime risk varies greatly by country and geographical region but overall is around one in 250 people. 22 The disease is slightly more common in men and boys than in women and girls. 23 Two HLA class 2 haplotypes involved in anti gen presentation, HLA DRB1*0301-DQA1*0501-DQ* {"type":"entrez-nucleotide","attrs":{"text":"B10201","term_id":"2091320","term_text":"B10201"}} B10201 ( DR3 ) and HLA DRB1*0401-DQA1*0301-DQB1*0301 (DR4-DQ8) , are linked to approximately 50% of disease heritability and are prevalent in white people. 24 Other haplotypes are known to reduce type 1 diabetes risk, including DRB1*1501-DQA1*0102-DQB1–0602 ( DR15-DQ6 ). 24 The mechanisms by which these HLA haplotypes interact and alter risk are not completely understood. Different HLA associations in other racial groups are recognised but remain poorly characterised. 24 Genome-wide association studies have identified over 60 additional non-HLA loci associated with the risk of type 1 diabetes. These variants have been predominantly associated with the immune system and highlight pathways that are important in disease development—eg, insulin gene expression in the thymus, regulation of T-cell activation, and viral responses. 24 These HLA and non-HLA genetic associations could identify potential targets for future disease-modifying therapies or subgroups of patients who could benefit from specific immune interventions.
Historically, people at high risk of type 1 diabetes have been identified for research by HLA risk or familial risk, or both. 25 By contrast, individual non-HLA loci cannot be used to predict type 1 diabetes or discriminate it from other types of diabetes. Combined measurement of HLA and non-HLA loci into genetic risk scores could offer improved prediction of the risk of developing type 1 diabetes and discrimination of type 1 from type 2 diabetes. 26 , 27 Furthermore, the continuing fall of genotyping costs could facilitate future population-level disease prediction by use of genetic risk scores. 19 , 28
Epidemiology
Globally, type 1 diabetes is increasing both in incidence and prevalence, with overall annual increases in incidence of about 2–3% per year. 29 , 30 US data 31 suggest an overall annualised incidence from 2001 to 2015 of about 22·9 cases per 100 000 people among those younger than 65 years; data from other regions suggest similar incidences. 32 The greatest observed increases in incidence of type 1 diabetes are among children younger than 15 years, particularly in those younger than 5 years. 33 These increases cannot be explained by genetic changes, implicating environmental or behavioural factors, or both. Many environmental exposures are associated with type 1 diabetes, including infant and adult diet, vitamin D sufficiency, early-life exposure to viruses associated with islet inflammation (eg, enteroviruses), and decreased gut-microbiome diversity. 34 Obesity is associated with increasing presentation of type 1 diabetes, with β-cell stress potentially providing a mechanistic underpinning. 34 , 35 The large differences in the incidence of type 1 diabetes in genetically similar populations that are separated by socioeconomic borders 36 and the increasing incidence of type 1 diabetes in genetically low-risk individuals 37 highlight the importance of environmental risk factors regardless of genetic background risk. Further work is being done to understand the role of gene–environment interactions in the pathogenesis of type 1 diabetes, the role of different loci and pathways at different stages of the disease, and whether loci that are independent of disease risk could have a role in disease progression after development of autoimmunity. 38 – 40 Some data 31 , 41 suggest that the observed incidence could be declining in adults or potentially even levelling off across all age ranges; worldwide registry data will eventually reveal if this pattern is indeed true. 42
The incidence of type 1 diabetes varies by country and by region within countries. 31 At northern latitudes, people born in the spring are more likely to develop the disease than those born in the other seasons. 43 The peak incidence of diagnosis is seen in children aged 10–14 years. 31 , 32 Although many people present with type 1 diabetes in adulthood, 44 the higher incidence of type 2 diabetes in adulthood compared with type 1 diabetes and the flawed criteria for distinguishing these forms of disease make assessment of the incidence of type 1 diabetes in adults very difficult. 23 , 45 Most people living with type 1 diabetes are adults. 46
The immune phenotype of type 1 diabetes
The pathogenesis of type 1 diabetes results from a complex interaction between the pancreatic β-cell and innate and adaptive immune systems ( figure 3 ). 47 The question of whether a trigger for the immune response against β cells exists or whether the immune response is a random stochastic event has been a subject of considerable speculation and controversy. Several viral infections are associated with type 1 diabetes, with enterovirus being one of the most commonly associated infections. Enteroviral major capsid protein VP1 and RNA have been detected in islets from people with recent-onset type 1 diabetes, 48 along with hyper-expression of the class 1 major histo compatibility complex 49 and other indices of viral infection. One possibility is that some people with type 1 diabetes have an atypical, chronic viral infection of β cells, leading to chronic inflammation and the development of autoimmunity. The viral hypothesis has been difficult to test, although both antiviral therapy and the development of vaccines targeting enteroviruses are being pursued for this purpose.

The development of type 1 diabetes is thought to be initiated by the presentation of β-cell peptides by antigen-presenting cell (APCs). APCs bearing these autoantigens migrate to the pancreatic lymph nodes where they interact with autoreactive CD4+ T lymphocytes, which in turn mediate the activation of autoreactive CD8+T cells (A). These activated CD8+ T cells return to the islet and lyse β cells expressing immunogenic self-antigens on major histocompatibility complex class I surface molecules (B). β-cell destruction is further exacerbated by the release of proinflammatory cytokines and reactive oxygen species from innate immune cells (macrophages, natural killer cells, and neutrophils; C). This entire process is amplified by defects in regulatory T lymphocytes, which do not effectively suppress autoimmunity (D). Activated T cells within the pancreatic lymph node also stimulate B lymphocytes to produce autoantibodies against β-cell proteins. These autoantibodies can be measured in circulation and are considered a defining biomarker of type 1 diabetes (E).
In the field, much effort has been given to the study of the adaptive immune system in type 1 diabetes by use of assays of peripheral lymphocytes selected for autoreactivity to islet antigens. Increased frequency of islet-specific autoreactive CD8+ T lymphocytes and decreased regulatory immune function have been associated with type 1 diabetes. 50 Experiments, such as the transfer of type 1 diabetes following non-T-cell depleted allogeneic bone-marrow transplantation, 51 development of type 1 diabetes in an individual with B-lymphocyte and antibody deficiency, 52 and inherited genetic defects of T-lymphocyte function causing type 1 diabetes 53 highlight the crucial role of T cells in the pathophysiology of type 1 diabetes. 54 Almost all studies of peripheral autoimmunity in people with type 1 diabetes show overlap of phenotypes seen in the general population, and the proportion of islet autoreactive cells present in the periphery is often tiny (only a few cells among millions of non-autoreactive cells). As a result, connecting the population of autoreactive immune cells that is detectable in blood to the disease process in islets has been difficult. A key development has been the isolation of T lymphocytes that are reactive to β-cell antigen peptides from islets of organ donors with type 1 diabetes. 55 – 57
Histopathologically, these processes are observed as insulitis or immune-infiltrated (insulitic) islets. 58 CD8+ T lymphocytes are the most common immune cells within insulitic lesions, with CD4+ T cells present in lower numbers. Distinct patterns of insulitis that stratify with the aggressiveness of β-cell loss and age of diagnosis have been identified in insulitic islets. 59 Although insulitis is common and intense in animal models of type 1 diabetes, it is much rarer and more variable in human beings ( figure 3 ). 60
The β-cell phenotype of type 1 diabetes
At diagnosis, people with type 1 diabetes have reduced β-cell function compared with healthy controls. 61 With amelioration of hyperglycaemia, these β cells can have a partial recovery of insulin secretory function, leading to a so-called honeymoon period after diagnosis with minimal or no exogenous insulin needed. Over time, many of these residual cells are lost. However, analysis of pancreatic sections from individuals with long-term type 1 diabetes show the presence of residual β cells decades after diagnosis. 62 , 63 When sensitive C-peptide measure ments are performed, 30–80% of people with long-term type 1 diabetes are found to be insulin microsecretors. 64 – 67 So, although endogenous β-cell quantity and function decline with longer disease duration, this decline does not progress to a complete loss of all β cells. 64 – 67 This finding is noteworthy because in the Diabetes Control and Complications Trial 68 , 69 persistent C-peptide secretion was associated with reduced development of retinopathy, nephropathy, and hypoglycaemia. Additionally, the persistence of C-peptide secretion in people with long-term type 1 diabetes could improve glucagon responses to hypoglycaemia. 70 Moreover, the presence of residual C-peptide secretion after the diagnosis of disease could also increase the possibility of an improved effect of interventions targeted at rescuing or augmenting the survival of this residual pool of β cells. Analyses of pancreatic specimens from the Network of Pancreatic Organ Donors repository have not found evidence of either increased neogenesis or proliferation in pancreatic cells from donors with type 1 diabetes. 63 Thus, the mechanisms underlying the persistence of residual β cells in people with long-term type 1 diabetes remain unclear. Identifying pathways that have allowed these cells to escape the autoimmune attack could yield insight into new therapeutic approaches.
β-cell abnormalities might also contribute to type 1 diabetes pathogenesis, leading to the notion of so-called β-cell suicide. β-cell HLA class I overexpression is common in pancreatic sections from cadaveric donors with type 1 diabetes. This overexpression serves as a homing signal for cytotoxic T lymphocytes. 49 However, whether this signal is a primary β-cell defect or a response to a stimulus (eg, a viral infection) is not yet known. Additionally, evidence also exists for increased β-cell endoplasmic reticulum stress linked with accelerated β-cell death. 71 , 72 Endoplasmic reticulum stress in β cells has also been associated with alterations in mRNA splicing and errors in protein translation and folding; the resultant protein products have been proposed as potential immunogenic neoantigens. 73
In addition to these defects in the β-cell compartment, alterations in non-endocrine islet cells and the exocrine pancreas have also been described ( figure 4 ). These defects include abnormalities in the islet extracellular matrix 74 , 75 and in islet innervation and vascularity. 76 – 78 Data have also placed a renewed emphasis on the role of exocrine pancreatic pathology in type 1 diabetes. Compared with healthy individuals, people with type 1 diabetes have a decreased pancreatic weight and volume that continues to decrease with disease duration. 79 , 80 This finding could be explained by developmental defects, or pancreatic atrophy in response to loss of the paracrine and pro-growth effects of insulin or chronic inflammation, or even autoimmune-mediated exocrine destruction. These possibilities are all topics of active investigation.

(A) Type 1 diabetes is characterised by a variety of abnormalities that involve both the islet and the exocrine pancreas. The hallmark of type 1 diabetes is loss of insulin-producing β cells and immune infiltration of islets. However, the presence of insulitis, even within an individual pancreas, can be highly variable. (B) Immunofluorescent image of an insulitic islet from a cadaveric donor with long-term type 1 diabetes. Insulin is shown in blue and CD8+ T cells surrounding the islet are shown in yellow. (C) Haematoxylin and eosin staining of an islet from a cadaveric donor that exhibits a classic pattern of insulitis. The islet is circled with a yellow dotted line. The infiltrating immune cells are circled in red and indicated by arrows. (D) Haematoxylin and eosin staining of an islet, circled in yellow dotted line, from a cadaveric donor with long-term type 1 diabetes without any discernible immune infiltrate. By contrast with the islet in (C), this islet has evidence of peri-islet fibrosis as shown circled in red and indicated by arrows. Images B–D courtesy of M Campbell-Thompson, University of Florida, Gainesville, FL, USA.
Management of clinical disease
Methods of managing type 1 diabetes continue to improve, and although progress is generally slow and incremental, occasionally it is punctuated by rapid change. One such moment of change happened in 1993 with the publication of the Diabetes Control and Complication Trial. 81 This trial and the follow-up observational Epidemiology of Diabetes Interventions and Complications trial convincingly showed that achieving and maintaining glucose concentrations as close to those seen in people without diabetes as possible leads to a reduction in microvascular and cardiovascular type 1 diabetes complications. 82
Although insulin remains the mainstay of therapy, new insulin analogues with varying onsets and durations of action are widely available. Optimal glycaemic control requires multiple-dose insulin regimens that mimic physiological insulin release, with basal insulin for overnight and between-meal control, plus bolus doses of rapid-acting insulin analogues to cover ingested carbohydrate loads and treat hyperglycaemia. Insulin can be taken by injection (with an insulin pen if available) or, preferably for many people, with an insulin pump. 83 Ultra-rapid inhaled insulin is also available, but little enthusiasm for this preparation exists because of its fixed dosing (four or eight unit increments only), issues with consistent delivery, cost, and the need for pulmonary function testing. 84 A faster-acting subcutaneously-administered insulin (via injection or infusion) has also recently become available for clinical use. Appropriate insulin use requires frequent dosing adjustments for ingested carbohydrates, physical activity, and illness or stress.
While pramlintide is the only non-insulin medication approved for improved glycaemic control in patients with type 1 diabetes, metformin, glucagon-like peptide-1 receptor agonists, dipeptidyl peptidase-4 inhibitors, and sodium-glucose co-transporter-2 (SGLT2) inhibitors have also been used of-label; however, fewer than 5% of patients use these medications. 85 Metformin, an insulin sensitiser, is the most commonly prescribed drug for people with type 1 diabetes who have insulin resistance but it has not been shown to be effective in people younger than 18 years who are overweight or obese and have type 1 diabetes. 86 Use of SGLT2 inhibitors is restricted in part because of early reports of euglycaemic diabetic ketoacidosis in people with type 1 diabetes treated with these compounds. A 2018 meta-analysis of these inhibitors suggests they are safe, 87 but more data are needed.
Glucagon therapy is also poised to undergo a resurgence in management of type 1 diabetes. Although only an emergency kit has been commercially available up until now for cases of severe hypoglycaemia leading to seizure or loss of consciousness, nasal and stable liquid formulations are being developed. The nasal formulation will be available as a rapid rescue therapy only, 88 whereas the stable liquid formulation could also be used in small doses for exercise and in dual hormone (ie, insulin and glucagon) closed-loop systems. 89 , 90
In the past 13 years, continuous glucose monitoring (CGM) and intermittently viewed CGM devices for at-home patient use with minimally invasive devices have become available, which have similar accuracy to capillary blood glucose monitors. 91 Both CGM and intermittently viewed CGM allow examination of glucose concentration patterns over time and, although CGM devices still need periodic calibration, they obviate the need for frequent capillary blood glucose measurements. CGM is more sophisticated than intermittently viewed CGM because it can give the user a warning on the basis of absolute or projected glucose values. When CGM is incorporated into hybrid closed-loop insulin-pump systems that automatically regulate basal infusion rates, but that require manual delivery of meal boluses by trained wearers to cover estimated carbohydrate intakes, substantial improvements in glucose variability and overall glycaemic control are seen ( figure 5 ). 93 Combined use of automated insulin delivery and CGM offers the prospect of an artificial pancreas with little input from the user. The substantial advances that have been made in pump and sensor technology and the increase in the number of trials to test their efficacy show that partially or fully automated systems could become a reality.

Sensor glucose profiles from 124 people with type 1 diabetes, of which 30 were adolescents (14–21 years; A) and 94 were adults (22–75 years; B), before (during run-in phase) and during the study phase using the Medtronic MiniMed 670 g hybrid closed-loop system (Medtronic, Northridge CA, USA) under clinical trial conditions. Median and IQR of sensor glucose values are given as a green line and band for the run-in phase, and a pink line and band for the study phase, respectively. In the run-in phase, the hybrid closed-loop system was in manual mode, with participants making all treatment decisions except for the pump automatically suspending before senor glucose concentrations became too low. In the study phase, the hybrid closed-loop system was in auto mode. Participants had less variability in their blood glucose concentration during auto mode. Reproduced from Garg et al, 92 with permission from Mary Ann Liebert.
Guidelines from the American Diabetes Association, International Society for Pediatric and Adolescent Diabetes, and Candian Diabetes Association suggest a HbA 1c target of less than 53 mmol/mol (7·0%) for adults and less than 58 mmol/mol (7·5%) in paediatric patients with type 1 diabetes; 94 – 96 however, most individuals do not achieve these targets. Although setting more aggressive targets is associated with achieving lower HbA 1c , 97 these targets should be individualised on the basis of many factors including comorbidities, patient capability and attitude, and available care resources 98 —eg, even lower targets are often prescribed for pregnant women and women anticipating pregnancy than those prescribed to other patients. 99 Higher targets might be appropriate for people with hypoglycaemia unawareness, history of severe hypoglycaemia, advanced complications, and short life expectancy. For optimal outcomes, people with diabetes should be cared for by a multidisciplinary care team, including diabetes educators, nurse practitioners, nurses, nutritionists, physician assistants, exercise physiologists, social workers, and psychologists. To optimise glycaemic control, clinical care with skilled and structured patient education and training sessions should be provided—including information on insulin adjustments, carbohydrate counting, and optimal use of available technology. 100
People with type 1 diabetes also risk developing other autoimmune diseases, sometimes as part of a poly-glandular autoimmune syndrome. A study 101 from the Type 1 Diabetes Exchange clinic registry noted the prevalence of autoimmune disease was 27% in a population of over 25 000 people with type 1 diabetes with a mean age of 23 years. The most common autoimmune disease is autoimmune thyroiditis (ie, Hashimoto thyroid itis and Graves’ disease) followed by coeliac disease. Other associated conditions include collagen-vascular diseases (eg, rheumatoid arthritis and lupus), autoimmune gastritis or pernicious anaemia, vitiligo, and Addison’s disease. Guidelines for the care of people with diabetes include periodic screening for these diseases, particularly thyroid and coeliac diseases. 102
Complications of type 1 diabetes
The discovery of insulin in 1922 transformed type 1 diabetes from a terminal to a treatable disease. Despite the advances in care discussed previously, the disease continues to be associated with substantial medical, psychological, and financial burden. Hypoglycaemia and ketoacidosis are persistent potentially life-threatening complications. Severe hypoglycaemic events requiring treatment assistance from another person occur at rates of 16–20 per 100 person-years; hypoglycaemic events leading to loss of consciousness or seizure occur at a rate of 2–8 per 100 person-years. 103 – 105 Recurrent hypoglycaemia results in an increased likelihood of hypoglycaemia unawareness and subsequent severe hypoglycaemic events, since recurrent hypoglycaemia reduces the glucose concentration that triggers the counter-regulatory responses to return to euglycaemia. 106 Hypoglycaemia unawareness can improve with edu cation, support, and glucose targets that are aimed at avoiding biochemical hypoglycaemia, while maintaining overall metabolic control. 107
Hypoglycaemic events are associated with adverse effects on cognitive function, 108 , 109 and are associated with 4–10% of type 1 diabetes-related deaths. 110 – 112 Observational studies suggest poor diabetes control does not reduce the risk of severe hypoglycaemia. 113 Notably, rates of severe hypoglycaemic events have been decreasing over time 104 and with CGM and other advanced diabetes technologies HbA 1c can be lowered into the target range without increasing the risk of severe hypoglycaemia. 114 Treatment in hospital for diabetic ketoacidosis occurs at a rate of 1–10 per 100 patient-years in paediatric populations with established type 1 diabetes, and accounts for 13–19% of type 1 diabetes-related mortality. 105 , 110 , 111 Incidence of diabetic ketoacidosis is higher among women than among men, and among people with higher HbA 1c levels than other people with type 1 diabetes.
Microvascular complications of the disease manifest primarily as retinopathy, neuropathy, and nephropathy, but also can affect cognitive function, the heart, and other organs. Hyperglycaemia is the primary risk factor for microvascular disease, and reducing HbA 1c through intensive diabetes management, particularly early during disease, is associated with striking (about 70%) reductions in incidence and slower progression of microvascular disease. However, differences in HbA 1c do not fully explain the variation in the incidence of complications and the severity of disease between individuals. Variability in glucose concentrations (both during the day and longer term) and glycosylation rates also probably have a role in interindividual differences. 115 , 116 Type 1 diabetes during puberty also appears to accelerate the development of complications. 117
Macrovascular complications of type 1 diabetes include atherosclerosis and thrombosis in the heart, peripheral arteries, and brain. By contrast with microvascular complications, the risk of cardiovascular complications does not appear to be as attenuated by intensive blood sugar control. Diabetic nephropathy, whether manifesting as microalbuminuria, macroalbuminuria, or a reduced glomerular filtration rate progressively augments the overall risk of macrovascular complications. 118 Cardiovascular disease remains the major cause of premature morbidity and mortality, with data 119 , 120 suggesting an 8–13-year shorter life expectancy for people with type 1 diabetes than for healthy individuals.
People with diabetes might also have both chronic and acute neurocognitive changes that include decline in cognitive function with detrimental effects on psychomotor speed, cognitive flexibility, attention, and visual perception. 121 , 122 Although the pathophysiology of neurocognitive changes is poorly understood, their development has been linked with both microvascular and macrovascular changes and changes in brain structure, neuronal loss, and cerebral atrophy. 123 , 124 Risk factors include developing diabetes early in life, chronic hyperglycaemia, and repeated hypoglycaemia.
In the past 25 years, among people with type 1 diabetes the risks of microvascular and macrovascular compli-cations have substantially decreased and outcomes have improved. 125 , 126 These improvements have been largely driven by better glycaemic control and improved management of associated risk factors—eg, hypertension and hyperlipidaemia. Several studies 127 – 130 have identified additional non-glycaemic risk factors for the development of complications. Genetic studies have not yielded strong associations between specific gene variants and complication status. Low levels of education and income have been associated with high risks of both micro-vascular and macrovascular complications. 127 Sex also appears to modify risk, since women with type 1 diabetes have been shown to have higher rates of all-cause premature mortality and vascular events than do men with type 1 diabetes. 128 In the past 5 years, new technologies have been designed to attempt to better predict future risk and complications by combining risk factors into probability models. Two examples are the QDiabetes 129 and QRISK3 130 web calculators that were developed with a prospective general practice dataset of 803 044 people with diabetes (44 440 with type 1 diabetes). These calculators can be used to predict 10-year risk for microvascular and macrovascular complications. However, continued work is needed in this area to combine prediction models with disease-specific bio- markers and disease-modifying therapies that can prevent sequelae.
An additional noteworthy complication of type 1 diabetes is the patient-reported burden of adverse also their family, friends, and caregivers. 131 Fear of hypoglycaemia is a prevalent issue, particularly for the families of very young children with type 1 diabetes. 132 Furthermore, poor quality of life is predictive of subsequent poor glycaemic control. 133
Disease-modifying therapies
For over 30 years, most efforts to cure type 1 diabetes have focused on altering the immune system’s attack on β cells. This approach began with trials of ciclosporin, an immunosuppressant that was given to inhibit T-cell activation. Although ciclosporin was unable to induce a durable disease remission, insulin requirements of patients decreased during active treatment, generating enthusiasm that immune modulation could treat type 1 diabetes. 134 – 136 Subsequently, other strategies have been tested in both primary and secondary prevention paradigms. Most efforts have focused broadly on tolerance induction by use of antigens or modulation of T-lymphocyte, B-lymphocyte, and cytokine responses. Some primary prevention studies have also used dietary approaches. 137 , 138
Antigen-based trials have used various forms of glutamate decarboxylase (GAD) protein, which have shown mixed but mostly negative results. 139 – 141 The Diabetes Prevention Trial—Type 1, tested whether oral or parental insulin prevented the development of type 1 diabetes in people who were autoantibody positive. Neither approach reduced diabetes development, but subgroup analyses suggested a benefit of oral insulin in individuals with the highest titres of insulin auto-antibodies. 142 , 143 Based on this finding, the Type 1 Diabetes TrialNet Network completed a trial 144 of low-dose oral insulin in a second cohort of individuals who were autoantibody positive with similar insulin autoantibody profiles, but this trial was also negative. Negative results were also observed in another trial investigating intranasal insulin. 145
Personalised strategies for tolerance induction are now also being pursued. One study tested repeated intradermal doses of a specific proinsulin peptide fragment in people with the HLA DRB1*0401 genotype, 146 for whom this peptide was identified to be specifically immunogenic. Clinical trials at diagnosis have also tested approaches aimed at modulating T-cell and B-cell responses. Despite many attempts at immune intervention, only four categories of drugs have shown efficacy in preserving C-peptide secretion in recent onset type 1 diabetes in randomised placebocontrolled trials. These drugs include a monoclonal antibody against the B-cell CD20 receptor (rituximab), 147 monoclonal antibodies against the T-cell CD3 receptor (teplizumab 148 , 149 and otelixizumab 150 ), cytotoxic T-lymphocyte protein 4 (CTLA4)-immunoglobulin-mediated co-stimulatory blockade with abatacept, 151 and alefacept, 152 which is a fusion protein that binds CD2 and targets CD4+ and CD8+ effector memory T cells. Although the phase 2 trials of these drugs met their primary or secondary endpoints, defined as an improvement in the C-peptide area-under-the-curve response during a mixed meal tolerance test, no drug has yet been able to induce insulin independence or progressed to a positive phase 3 trial that was translatable into clinical care. This gap in translating results from trials into clinical practice could highlight the need for alternative strategies. Combinatorial approaches that modulate multiple aspects of the immune response could result in better efficacy. For example, low-dose anti-thymocyte globulin in combination with granulocyte colony-stimulating factor has shown early and sustained efficacy in pilot studies 153 , 154 and is being tested in a phase 2 study () in recent-onset type 1 diabetes. Another approach is to intervene earlier in the disease process, at a time when greater β-cell mass remains. To this end, abatacept () and teplizumab () are being tested in stage 1 and stage 2 type 1 diabetes through the TrialNet Network. Even modest preservation of β-cell function could have long-term benefits, and better glycaemic control early in the disease course could mitigate the likelihood of complications. 155 – 157
One potential future therapy for type 1 diabetes is with replacement of β cells from an external source. Pancreas transplants have been performed for over 50 years and have become a standard-of-care treatment in individuals who have developed end-stage renal failure and require kidney transplantation. 158 Simultaneous kidney and pancreas transplantation in experienced centres can offer an up to 80% chance of insulin independence for over 5 years, but there is substantial surgical risk, and the requirement of immunosuppression. 159 Islet transplantation is a low-risk procedure, with donor islets infused into the liver via the portal vein. Shapiro and colleagues’ landmark work, by use of a steroid free Edmonton Protocol, 160 showed that islet transplantation could achieve insulin independence and offered an example of a successful and low-risk cell-based therapy. However, only a minority of islet transplant recipients achieve durable insulin independence. Moreover, morbidity associated with immunosuppression and limitations in the supply of donor islets restricts the number of people who can benefit from islet transplantation. 161 Currently, islet transplantation is used in a small subset of patients who have extremely severe hypoglycaemic unawareness. Even if insulin independence is not achieved, severe life-threatening hypoglycaemia can be prevented with minimal islet transplant function. 162 , 163
Cell therapy as a potential cure for type 1 diabetes remains a field of great interest. 2 Considerable effort has been focused on protocols to generate functional and glucose-responsive β cells from human embryonic stem cells or induced pluripotent stem cells from living donors. This approach offers the possibility of a limitless source of β cells that could be delivered in a semipermeable device that would permit functional insulin secretion but avoid the need for immuno-suppression. 164 Several small molecules, growth factors, hormones, and nutrients have been shown to promote modest β-cell neogenesis and proliferation. However, most positive results come from animal models and have been difficult to replicate in human studies. While stem-cell-based therapies and neogenesis are a source of hope for potential cures, they are not realistic treatments in the immediate future. 2
Other novel approaches include autologous haemopoietic stem-cell transplantation 165 , 166 and autologous T-regulatory cell administration. 167 – 169 In response to growing evidence highlighting an active role for the β cell in disease pathogenesis, several ongoing trials are testing drugs that have successfully targeted β-cell stress responses in mouse models of diabetes. 170
Conclusions
Over the past 50 years, people with type 1 diabetes and their medical-care providers have been tantalised with optimism and subsequently disappointed at the seemingly unobtainable cure on the horizon. However, this long journey has been punctuated by several pivotal successes, including the discovery of insulin in 1922, the first pancreatic transplantation in 1966, 171 the first insulin-pump studies, the first immunomodulatory trial in 1986, 136 and the first definitive evidence linking glycaemic control with complication status in 1993. 81 The past 25 years has brought an upsurge of technological advances, including designer insulin analogues, smart insulin pumps, continuous glucose sensors, and closed-loop insulin delivery systems.
Clinicians, investigators, and patients have gained a better appreciation of the true complexity of type 1 diabetes, and humility in the face of many unsuccessful trials aimed at inducing a durable disease remission. While scientists continue to untangle the complicated pathogenesis of the disease, patients and health-care providers should focus on advocating for improved access to modern advances in diabetes care, especially for affordable insulin analogues and technologies that can reduce the burden of managing this chronic disease. When insulin was discovered, the University of Toronto freely licensed the right to manufacture the drug; yet, people in resource-limited environments continue to die because they have no access to insulin. 172
Additionally, crucial research must continue into strategies to prevent disease onset and preserve or restore β-cell function. These approaches offer the promise of ameliorating or eliminating disease complications, and greatly improving outcomes for those who have the disease. Continued development of new low-cost, low-burden, and highly effective therapies to improve glycaemic control is also needed. These approaches could include investigation into the effects of different dietary composition on glycaemic outcomes, and the safety and efficacy of open-source patient-designed artificial pancreas innovations. Given observed differences in care, health-care providers must be committed to initiatives for continuous quality improvement, with a focus on increasing uptake and implementation of best standards of care. A greater focus on patient-centred outcomes has been present in trials, and further exploration of these important endpoints is also crucial. If stakeholders in the field concentrate on the areas that are most likely to have a long-term effect, management of type 1 diabetes is poised to undergo further radical transformation.
Search strategy and selection criteria
We searched MEDLINE for publications in English published between Jan 1, 2014, and March 1, 2018, using the term “type 1 diabetes” and MEDLINE subheadings and selected papers on the basis of our opinion of their scientific importance. Research published since the 2014 Lancet Seminar on this topic was given particular attention. We provide an overview of type 1 diabetes focusing on updating the reader on recent advances and controversies.
Acknowledgments
This work was partly supported by grants from the National Institutes of Health, JDRF, the Veteran’s Administration, Diabetes UK, the Leona M and Harry B Helmsley Charitable Trust, the BIRAX Regenerative Medicine Initiative, the Ball Brothers Foundation, George and Francis Ball Foundation, Sigma Beta Sorority, Cryptic Masons Medical Research Foundation, and the Luke Weise Research Fund. We thank W Tamborlane, C Matthews, and J Kushner for their review of a draft of this Seminar. We thank M Campbell-Thompson, F Syed, and T Weinzerl for assistance with figures. And we thank T Lewallen and M Wales for administrative support.
Declaration of interests
LAD reports personal fees from Eli Lilly, and grants from Medtronic, Sanofi, Xeris, Caladrius, Dexcom, and Janssen outside the submitted work. RAO holds a UK Medical Research Council institutional Confidence in Concept grant to develop a 10 SNP biochip type 1 diabetes genetic test in collaboration with Randox. CE-M declares no competing interests.
Contributor Information
Linda A DiMeglio, Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN, USA;
Carmella Evans-Molina, Department of Medicine, Indiana University School of Medicine, Indianapolis, IN, USA;
Richard A Oram, Institute of Biomedical and Clinical Science, University of Exeter Medical School, and The Academic Kidney Unit, Royal Devon and Exeter NHS Foundation Trust, Exeter, UK.

IMAGES
VIDEO
COMMENTS
The study is being done to find out if low blood sugar (hypoglycemia) can be reduced in people with type 1 diabetes (T1D) 65 years and older with use of automated insulin delivery (AID) system. The device systems used in this study are approved by the Food and Drug Administration (FDA) for diabetes management.
RSS Feed. Type 1 diabetes (also known as diabetes mellitus) is an autoimmune disease in which immune cells attack and destroy the insulin-producing cells of the pancreas. The loss of insulin leads ...
The burden of type 1 diabetes remains substantial, and more research is needed to improve the lives of people with type 1 diabetes and to find a cure. To this end, ADA-funded research continues to drive progress by funding research projects topics spanning technology, islet transplantation, immunology, improving transition to self-management ...
Correspondingly, beta cell rescue strategies are being pursued, which include antigen vaccination using, for example, oral insulin or peptides, as well as agents with suggested benefits on beta cell stress, such as verapamil and glucagon-like peptide-1 receptor agonists. Whilst autoimmune-focused prevention approaches are central in type 1 ...
Type 1 Research Highlights. While the Association's priority is to improve the lives of all people affected by diabetes, type 1 diabetes is a critical focus of the organization. In fact, in 2016, 37 percent of our research budget was dedicated to projects relevant to type 1 diabetes.
Liam Drew. Encapsulated stem cell-derived islets could shield β cells from the immune system. Credit: Ref. 8. Insulin has been one of the most transformative discoveries in medicine. The ...
Hummel, S. et al. Children diagnosed with presymptomatic type 1 diabetes through public health screening have milder diabetes at clinical manifestation. Diabetologia 66 , 1633-1642 (2023).
Type 1 diabetes is a chronic disease caused by autoimmune destruction of pancreatic β cells. Individuals with type 1 diabetes are reliant on insulin for survival. Despite enhanced knowledge related to the pathophysiology of the disease, including interactions between genetic, immune, and environmental contributions, and major strides in treatment and management, disease burden remains high.
The trial was done between 2011 and 2018 at sites in the USA, Canada, Germany, and Australia. 76 relatives of patients with type 1 diabetes, identified in the TrialNet Natural History Study, between the ages of 8 and 49 years, who were at high risk for type 1 diabetes (multiple autoantibody positive and dysglycaemic—ie, stage 2), were enrolled.
Clinical trials have the potential to transform the lives of many thousands of people at risk for type 1 diabetes and people who already have the disease. On July 14, diaTribe hosted its second Musings event of the year: " Type 1 Diabetes Research 2021: Science, Hope, and Clinical Reality .". The virtual event drew over 300 participants who ...
The SEARCH for Diabetes in Youth study in the United States found a 1.4% per year increase in T1D incidence from 2002 to 2012, with an unexpected increase in Hispanic youths ( 14 ). This increase in the United States is similar to the gradual worldwide annual increase in T1D incidence over the past 30 years.
2. Type 1 Diabetes Mellitus. Type 1 diabetes mellitus (T1DM) incidence has been increasing by 2-5% worldwide with significant heterogeneity in this diagnosis by regions or continents [5,6].Clinical care has significantly improved, raising quality of life and clinical outcomes for these patients, but more must be done to find a cure.
The burden of type 1 diabetes remains substantial, and more research is needed to improve the lives of people with type 1 diabetes and to find a cure. To this end, ADA-funded research continues to drive progress by funding research projects topics spanning technology, islet transplantation, immunology, improving transition to self-management ...
Beena Akolkar, Ph.D. Clinical research in the prevention and immunopathogenesis of Type 1 Diabetes and the genetics and genomics of Type 1 and Type 2 Diabetes. Guillermo A. Arreaza-Rubín, M.D. Diabetes and endocrine disease bioengineering and glucose sensing. Miranda Broadney, M.D., M.P.H. Pediatrics, Pediatric Endocrinology, Clinical ...
Figure 1: Windows for Prevention of Type 1 Diabetes (T1D): This graphic illustrates how type 1 diabetes progresses. Genetic risk, combined with an unknown environmental trigger (s), is followed by inappropriate activation of the immune system to attack the insulin-producing β cells. The appearance of more than one islet-cell autoantibody in a ...
Type 1 Research Highlights. While the Association's priority is to improve the lives of all people affected by diabetes, type 1 diabetes is a critical focus of the organization. In fact, in 2016, 37 percent of our research budget was dedicated to projects relevant to type 1 diabetes.
At the time of the centennial anniversary of the first clinical use of insulin, the treatment of type 1 diabetes has undergone multiple innovations that have advanced the health, well-being, and ...
The study of people with longstanding diabetes has drastically changed the traditionally accepted model of type 1 diabetes, which states that endogenous insulin production declines within 5 years of diabetes onset and that there is a complete loss of beta cells and insulin production [].While the study of long-term survivors might be expected to overestimate the proportion with endogenous ...
Through the JDRF - Beyond Type 1 Alliance, Beyond Type 1 has partnered with JDRF—the world's biggest nonprofit funder of type 1 diabetes research —to educate our community on the important role research plays in the lives of everyone affected by type 1 diabetes (T1D).It was diabetes research that led to the discovery of insulin in 1921. It was research that led to the creation of the ...
Type 1 diabetes is a reaction that stops your body from making insulin altogether, with no prevention or cure. One of Dr. Dandona's patients, Ginny Bullock shared her struggles with her Type 1 ...
New technologies in type 1 diabetes. Intensive insulin therapy for the management of type 1 diabetes (T1D) was established as the standard of care based on the results of the Diabetes Control and Complication Trial (DCCT), which conclusively demonstrated the benefits of tight glycemic control. 1 However, those who received intensive insulin ...
A federal program researching type 1 diabetes was recently allocated more funding by Congress to study how to prevent and cure the condition. Congress approved an additional $10 million for the Special Diabetes Program, a special program administered by the National Institutes of Health.The additional money, which was approved Friday, drives up the program's annual funding to $160 million.
Sometimes the first symptoms of type 1 diabetes are signs of a life-threatening condition called diabetic ketoacidosis (DKA). Some symptoms of DKA include. breath that smells fruity. dry or flushed skin. nausea or vomiting. stomach pain. trouble breathing. trouble paying attention or feeling confused.
Anyone who has type 1 diabetes needs insulin therapy throughout their life. There are many types of insulin, including: Short-acting insulin. Sometimes called regular insulin, this type starts working around 30 minutes after injection. It reaches peak effect at 90 to 120 minutes and lasts about 4 to 6 hours.
Automated insulin delivery systems have transformed the care of people living with type 1 diabetes and continue to move closer to the ultimate goal of a fully autonomous artificial pancreas.
Editor's Note: This interview was condensed and edited for clarity. Thanks to decades of research, it's possible to identify type 1 diabetes (T1D) in its earliest stages and potentially delay the full onset, requiring daily insulin therapy.Spotting those early stages, though, requires autoantibody screening.
Diagnosis. A diagnosis of diabetes is based on a fasting blood glucose concentration above 7·0 mmol/L (126 mg/dL), a random blood glucose concentration above 11·1 mmol/L (200 mg/dL) with symptoms, or an abnormal result from an oral glucose tolerance test. 5 In the absence of symptoms, abnormal glycaemia must be present on two different occasions. A diagnosis of diabetes can also be made on ...
At a meeting in Florence last week, the Juvenile Diabetes Research Foundation (JDRF) presented draft recommendations on caring for those who test positive. Mass screening is "a totally different way of thinking about type 1 diabetes," says Emily Sims, a pediatric endocrinologist at the Indiana University School of Medicine.
54 likes, 0 comments - danajfnutrition on February 27, 2024: "Let's get to know my favorite organ! The pancreas is an obvious choice when it comes to a favo..."