- Search Menu
- Advance articles
- Editor's Choice
- Supplements
- French Abstracts
- Portuguese Abstracts
- Spanish Abstracts
- Author Guidelines
- Submission Site
- Open Access
- About International Journal for Quality in Health Care
- About the International Society for Quality in Health Care
- Editorial Board
- Advertising and Corporate Services
- Journals Career Network
- Self-Archiving Policy
- Dispatch Dates
- Contact ISQua
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Introduction, results of data synthesis, conclusions, acknowledgements.
- < Previous
How are medication errors defined? A systematic literature review of definitions and characteristics
- Article contents
- Figures & tables
- Supplementary Data
M. Lisby, L.P. Nielsen, B. Brock, J. Mainz, How are medication errors defined? A systematic literature review of definitions and characteristics, International Journal for Quality in Health Care , Volume 22, Issue 6, December 2010, Pages 507–518, https://doi.org/10.1093/intqhc/mzq059
- Permissions Icon Permissions
Multiplicity in terminology has been suggested as a possible explanation for the variation in the prevalence of medication errors. So far, few empirical studies have challenged this assertion. The objective of this review was, therefore, to describe the extent and characteristics of medication error definitions in hospitals and to consider the consequences for measuring the prevalence of medication errors.
Studies were searched for in PubMed, PsychINFO, Embase and CINAHL employing primary search terms such as ‘medication errors’ and ‘adverse drug events’. Peer-reviewed articles containing these terms as primary end-points were included. Study country, year, aim, design, data-collection methods, sample-size, interventions and main results were extracted.
Forty-five of 203 relevant studies provided a generic definition of medication errors including 26 different forms of wordings. The studies conducted in nine countries represented a variety of clinical settings and the approach was mainly descriptive. Of utmost importance is the documented prevalence of medication errors, which ranged from 2 to 75% with no associations found between definitions and prevalence.
Inconsistency in defining medication errors has been confirmed. It appears that definitions and methods of detection rather than being reproducible and reliable methods are subject to the individual researcher's preferences. Thus, application of a clear-cut definition, standardized terminology and reliable methods has the potential to greatly improve the quality and consistency of medication error reporting. Efforts to achieve a common accepted definition that defines the scope and content are therefore needed.
In the Harvard Medical Practice studies of adverse events in hospitals, medication errors were found to be the main contributor constituting around one in five of the events, which were subsequently confirmed in comparable studies and studies of adverse drug events (ADEs) [ 1–4 ]. This has led to an increased focus on epidemiology and prevention of medication error in hospital settings around the world prompting numerous studies [ 5–13 ]. However, this contribution has not provided clarity or consistent findings with respect to medication errors. Quite the contrary, there appears to be a multiplicity of terms involved in defining the clinical range of medication errors and classifying consequences e.g. error, failure, near miss, rule violation, deviation, preventable ADE and potential ADE [ 14–18 ]. Moreover, it has been suggested that this inconsistency has contributed to the substantial variation in the reported occurrences of medication errors [ 19–21 ]. Thus, compared with other epidemiological fields in health care, no single definition is currently being used to determine medication errors although attempts to develop an international definition have been made e.g. National Coordinating Council for Medication Error Reporting and Prevention (NCC MERP) [ 22 ], which is clearly reflected in the referred literature. As an important consequence, this lack of clarity hinders reliable comparison of findings across studies, clinical settings and countries.
Another obstacle to achieving consensus of a common definition is the different approaches towards interpreting and detecting medication errors such as the system-oriented approach using mandatory or voluntary-based reporting systems in contrast to the epidemiological approach using specific research methods. Choice of reliable methods, including denominators, data completeness and systematic data collection, is essential in the epidemiological approach. However, these considerations are likely to be secondary in a system approach where causation is paramount. Unfortunately, identifying error causes without consistent, reliable measures is unlikely to lead to well-targeted prevention strategies. So far, the literature has mainly emphasized the problem of inconsistent use of definitions and data collection methods, whereas few studies have explored medical error subsets, and these have often been in specific clinical settings or particular to specific patient safety organizations [ 5 , 19–21 , 23–25 ]. Most importantly, no studies have to our knowledge systematically provided an overview of the extent of existing definitions and their possible impact on the occurrence of medication errors. Hence, the objective of this study was to investigate definitions of medication errors and, furthermore, to describe characteristics as well as to assess whether or not there were any associations between definitions and observed prevalence in hospitals.
Data sources
A systematic search of studies related to medication errors was performed in the databases on 18 December 2006 in PubMed (1951), Embase (1948), CINAHL (1981) and PsycINFO (1806) using the following key terms: ‘medication errors’, ‘adverse drug events’, ‘adverse drug events and errors’ and ‘medication errors and adverse drug events’ (Fig. 1 ). To capture all possible studies of medication errors in hospitals, the search was not restricted to MeSH terms in PubMed. However, a comparable search using the MeSH term ‘medication errors’ was performed in which all studies from the key term search could be retrieved.
Summary of search and review process.
Study selection
Only studies performed in hospital settings having medication errors and/or ADEs as the main objective were included in the present review, excluding studies performed solely in primary health care and literature reviews of medication errors and ADE (Fig. 1 ). Although there is no reason to believe that the occurrence of medication errors would be significantly different from hospitals, primary health care was excluded in this review due to assumed differences in medication handling and to the limited amount of completed medication error research in primary health care when the literature search was conducted. Finally, the search was limited to peer-reviewed studies with abstracts and studies presented in English.
First, titles and abstracts were examined in accordance with inclusion and exclusion criteria. Secondly, papers that met the inclusion criteria or papers in which inclusion could not be determined directly e.g. whether a setting was representing primary care were obtained. Thirdly, all duplicates between databases, papers that did not meet the inclusion criteria or papers that could not be obtained were excluded.
Data extraction
Definitions of medication errors and ADEs were registered along with included error types and whether the paper focused on ordering, dispensing, administering and monitoring. Moreover, general information regarding journal, author, year, title, aim, setting, participants, design, methods, intervention, results and evidence level were registered in an Access database.
Determination of evidence level was based on modified Oxford Criteria (Table 1 ). Studies in which evidence level could not be determined on behalf of available information, were discussed with a clinical pharmacologist and a professor in Public Health.
Levels of evidence, Oxford Centre for Evidence-based Medicine (2001) and pharmaco-epidemiological study design
In Table 1 , study-designs, in respectively, Oxford Centre for Evidence-based Medicine (therapy/prevention/aetiology/harm) and in the Pharmaco-epidemiological literature are provided along with the matching evidence levels (right column). In the present review, the evidence levels of the included studies were classified in accordance to these study-designs, as appropriate.
Due to the obvious lack of standard methodology and outcome measures, data could not be statistically summarized. However, prevalences of medication errors were reported for studies in which denominators were accessible. In pre–post studies and controlled studies, only prevalences of medication errors at baseline or from a control group were presented, whereas no prevalences could be calculated in studies using data from reporting systems [ 26 ]. Definitions were analysed with regard to similarities in content leading to the following five categories: (i) studies using the term error; (ii) studies using the NCC MERP definition; (iii) studies using failure; (iv) studies using deviation; and, finally (v) other terms. In each category, definitions from included studies were presented along with study characteristics. Finally, possible tendencies towards associations between definitions and prevalences were examined.
The literature search revealed 203 eligible papers (Fig. 1 ) of which 45 (23%) included a generic definition of medication errors. An additional 30 studies included a stage-specific definition; 22 prescribing, 3 in dispensing, 5 in administering and, finally, 4 studies contained a definition of intravenous errors. However, in 124 studies, no definitions were provided.
Overall characteristics
The 45 included studies were published in 26 different peer-reviewed journals in the period from 1984 to 2006 with half of them in the period 2005–06. The majority of studies were conducted in North America, representing 36 studies; 2 were done in Australia, 6 in Western Europe and, finally, 1 in Asia. The studies were conducted in a variety of clinical settings with almost 50% assessing more than one type of setting e.g. medical and surgical departments. Moreover, 20 studies included only adults, 9 studies only children, 9 studies both adults and children and, finally, 8 studies included other types of participants e.g. nurses and pharmacists. In 13 studies an intervention was addressed of which 9 were technologies in the medication process (e.g. computerized order entry (CPOE) either alone or combined with clinical decision support (CDS) systems, dose dispensing systems and infusion pumps with CDS). Descriptive designs were employed in 37 studies, whereas 2 studies were conducted as randomized clinical controlled studies, 1 as a case–control study and 1 as a prospective cohort study, and, finally, 4 studies were conducted using other designs e.g. case reports. Nine out of 10 studies were classified as evidence level IV or V, and, finally, chart review and reporting systems were the most frequently used methods to detect medication errors.
Prevalence of medication errors
In 21 of 45 studies, it was not possible to determine a prevalence of medication errors due to lack of valid denominators. These were in particular studies using reporting systems, interview and questionnaires as data collection method. Overall, a prevalence of 75% was found, with the majority being below <10% (Tables 2–4 ).
Studies using errors in definition of medication errors
a Oxford Centre for Evidence-based Medicine Levels of Evidence. Abbreviations: P: prescription; T: transcription; D: dispensing; A: administration; CPOE: computerized order entry; CDS: clinical decision support; OE: opportunities for errors; MEOS: medication error outcome scale. b Pre-intervention. c Preventable ADE + potential ADE.
Studies using the NCC MERP definition of medication errors
NCC MERP definition: ‘Any preventable event that may cause or lead to inappropriate medication use or patient harm while the medication is in control of the health-care professional, patient or consumer. Such events may be related to professional practice, health-care products, procedures and systems, including prescribing; order communication; product labelling, packaging and nomenclature; compounding; dispensing; distribution; administration; education; monitoring; and use.’ a Oxford Centre for Evidence-based Medicine Levels of Evidence. Abbreviations: P: prescription; T: transcription; D: dispensing; A: administration; CPOE: computerized order entry; CDS: clinical decision support; N/A: not applicable.
Studies using ‘failure, deviation or other’ terms in definition of medication errors
a Oxford Centre for Evidence-based Medicine Levels of Evidence. Abbreviations: AUS: Australia; UK: United Kingdom; P: prescription; T: transcription; D: dispensing; A: administration; CPOE: computerized order entry; CDS: clinical decision support; OE: opportunities for errors; N/A: not applicable; MEOS: medication error outcome scale. b Prevalence from a control group or pre-intervention.
An average of nine error types (min/max: 1/38) were identified in 38 of the 45 studies. In seven studies no error types were included due to study design. The study having one error type, namely, overdose (gentamicin) accounted for the highest prevalence in the review. Unfortunately, it was not possible to retrieve prevalence in the study using the highest number of error types, as data were collected from voluntary reporting. Dosing errors were the most frequent single error type, and in studies including all stages in the medication process, prescribing errors accounted for the highest percentage.
Definitions
Of the 45 definitions, 26 differed in wording and/or content. One definition used harm or potential for harm as a criterion for medication error, whereas one explicitly included intercepted medication errors [ 27–29 ]. Finally, five definitions were limited to deviations between ordered and administered drugs and doses [ 30–34 ]. In all other definitions no restrictions were specified.
A crude categorization of the revealed definitions was performed based on similarities in wording and/or content. Tables 2–4 provide an overview of definitions and characteristics of each study. Table 2 shows 15 definitions using the word ‘error/s’ followed by information about included stages in the medication process. In seven definitions, information regarding injury or intercepted errors is stated. Table 3 reveals characteristics of 17 studies using the definition from NCC MERP [ 22 ]. Finally, Table 4 presents five definitions using failure instead of error; five focusing on deviations between ordered and administered drugs/doses and three using other definitions.
Trends towards association between definition and prevalence
In the first category (Table 2 ), it was possible to ascertain prevalence in all studies ranging from 2 to 75% with the two European studies as the main contributors. In the second category (Table 3 ), which included studies using the definition from NCC MERP, it was possible to retrieve prevalence in 1 out of 17 studies, due to the use of reporting systems. This study revealed a prevalence of 8%. In the third category (Table 4 ) consisting of five studies using the term ‘failure’, it was not possible to provide information about prevalence due to study design and data collection methods. Finally, in the fourth category (Table 4 ), a prevalence of 3–16% was observed in studies focusing on deviations between ordered and administered drugs/doses.
To our knowledge, this is the first study to systematically explore the extent and impact of generic definitions of medication errors in hospital settings. The literature review confirmed an inconsistent use of definitions. However, other aspects have to be considered in order to explain the variation in prevalence of medication errors, as interpretation of the included definitions did not suggest any tendencies.
It is of particular relevance that fewer than half of the studies explicitly defined medication errors either as an overall definition (generic) or a stage/route-specific term. Furthermore, fewer than a quarter presented a generic definition despite that being the main objective of the studies. Thus, the inconsistency in terminology only represents the tip of the iceberg. Additionally, the present review has confirmed that the overall poor understanding of the epidemiology of medication errors can, at least, partly be explained by choice of design, data collection methods and study population, including denominators [ 19–21 , 23 ]. Based on these shortcomings, we have revealed a prevalence of 2–75% in studies that included a generic definition of medication error.
The second important problem is the choice of denominator or study population. It has previously been suggested that to use opportunities for errors rather than number of patients as denominator reduces the risk of case-mix bias [ 26 ]. Here we demonstrated a variety of denominators including drug order, doses, opportunities for errors, patients, nurses, reports and triggers. In addition, the frequent use of a reporting system excluded calculation of valid prevalence in almost half of all the studies thereby increasing the lack of clarity.
Thirdly, the impact of error types should be considered. It could be assumed that increasing the number of error types being measured, would automatically result in higher occurrences of medication errors due to an increased probability of detecting more errors. However, the study with the highest prevalence of errors (75%) in the present review included only one error type, namely, dosing errors, which conflicts with this assumption [ 35 ]. On the other hand, not all error types are mutually exclusive e.g. dosing errors, which inevitably includes all errors resulting in wrong or omitted dose (under-dose, overdose, omission of dose). Thus, the number of error types has to be weighed against type of error and the sensitivity of error detection methods. Unfortunately, the present review did not provide sufficient information on the impact of error types with regard to prevalence.
Finally, choice of data collection method should be considered important. Previously, chart review has been considered as the most appropriate method to detect prescribing errors and direct observation the most sensitive method to detect dispensing and administration errors, as opposed to voluntary reporting, which was found to be the least sensitive method [ 32 , 36 ]. In recent years the availability of computer-generated signals in error detection has increased, which allow an objective detection of all incidents that have been defined as an error in the computer. Thus, it can be assumed that such systems will increase the detection of systematic documented electronic data such as dosing of gentamicin [ 35 ]. In the present review the most frequently applied error detection method was chart review, which might have contributed to an underestimation of the occurrence of medication errors when applied to detection of errors other than prescribing.
Definition and prevalence
Interestingly, definitions, which at first glance appeared to be similar (Table 2 ), turned out to have the widest range in prevalence of medication errors. A closer scrutiny revealed that 10 of 15 studies in this category were affiliated with the same institutions in Boston, USA [ 7 , 15 , 28 , 37–43 ]. In addition, these studies demonstrated the lowest occurrence of medication errors ranging from 2 to 8% regardless of whether intercepted errors were included or not, suggesting consistency in error detection methods. However, prevalence in the two studies from Europe exceeded the American studies by as much as eight times, despite use of virtually identical definitions [ 10 , 35 ]. No obvious circumstances can explain these extreme differences, apart from use of data collection methods, as the study with the highest prevalence used computer-generated signals to detect dosing errors [ 35 ].
The majority of studies used the definition by NCC MERP. Unfortunately, it was only possible to retrieve one valid prevalence of medication errors [ 44 ]. This definition was initially developed for medication error reporting and, therefore, was an obvious choice for studies using reporting systems, which was the case for almost all the studies in this category [ 22 ]. An important drawback to reporting systems is an increased risk of underestimating the occurrence of medication errors due to the reporter's awareness of errors, attitudes towards reporting errors and fear of sanctions [ 45 ]. In addition, reporting systems are by nature denominator free as they do not provide information on the whole population; on the contrary, retrospective fitted denominators, such as time period or admissions, are frequently employed to demonstrate error rates [ 26 ]. Thus, reports of incidence or prevalence in studies using reporting systems should be avoided or interpreted with caution. Unfortunately, this expelled a unique opportunity to compare prevalence in studies using identical definitions.
Surprisingly, only 1 of the 45 definitions restricted medication errors to failures that either result in harm or have the potential to lead to harm [ 27 ]. Contrary to other definitions of medication errors in the present review, this approach relates process and outcome factors within the same definition, which previously has been suggested as minimizing the risk of accepting associations between errors and processes as synonyms for causation [ 46 ]. Moreover, this definition has been tested in an Australian study, in which it proved to be the most robust among other definitions, when evaluated in comparison with different medication error scenarios [ 25 ]. However, due to the design of this study it was not possible to elucidate that a restricted definition would lead to lower occurrences of medication errors compared with more profound definitions [ 15 ].
Finally, definitions that considered a medication error as a deviation between an ordered and administered drug and dose seemed to be more homogeneous with regard to prevalence despite representing different countries and employing different study designs [ 30 , 32–34 , 47 ]. However, these studies predominantly used the same types of denominator (opportunities for errors; doses and orders) as well as the most sensitive and appropriate data collection methods, e.g. direct observation in studies of dispensing and administration errors. A possible explanation for this consistency is the clear-cut limitation to deviations, which might appear simpler and be a less subjective approach in determination of medication errors. However, this approach excludes prescribing errors, as prescriptions serve as the gold standard in these definitions.
Limitations
The aim of this review was to investigate the multiplicity of definitions used in studies having medication errors and/or ADEs as the main objective. Hence, the characteristics and prevalence reported here might not reflect the overall occurrence of medication errors. However, it could be assumed that prevalence ranging from 2 to 75% represents the vast majority of studies in medication errors. Secondly, the literature search was limited to four major databases and restricted to papers in the English language. It is, therefore, possible that studies that would have met the inclusion criteria, were not indexed by these databases or were published in other languages than English. Nevertheless, due to experience from the current literature search, in which studies from a time span of >20 years were included, we assume that studies that might have been unintentionally disregarded in the search strategy would rather have added to the current inconsistency than contributed to clarification of the terminology. Third, the groupings we selected were somewhat arbitrary and this might have affected our chances of seeing an effect.
In the present systematic literature review of 45 studies we have confirmed inconsistency in defining medication errors as well as lack of definitions. Most of the definitions were profound, including minor deviations as well as fatal errors, whereas a single definition was restricted to harmful or potentially harmful errors.
Most importantly, it appears that definitions of medication errors and methods of detection, rather than being reproducible and reliable methods, are subject to individual researcher's preferences. Thus, it is obvious that application of a clear-cut definition, standardized terminology and reliable methods will greatly improve the quality and consistency of medication error findings. Efforts to achieve a commonly accepted definition that defines the scope and content is required in future studies.
We would like to thank, Prof. D.W. Bates, Harvard Medical School and Harvard School of Public Health, Boston, MA, for commenting on the present article.
Google Scholar
- medication errors
Supplementary data
Email alerts, citing articles via.
- Recommend to your Library
Affiliations
- Online ISSN 1464-3677
- Print ISSN 1353-4505
- Copyright © 2024 International Society for Quality in Health Care and Oxford University Press
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, generate accurate citations for free.
- Knowledge Base
Methodology
- Systematic Review | Definition, Example, & Guide
Systematic Review | Definition, Example & Guide
Published on June 15, 2022 by Shaun Turney . Revised on November 20, 2023.
A systematic review is a type of review that uses repeatable methods to find, select, and synthesize all available evidence. It answers a clearly formulated research question and explicitly states the methods used to arrive at the answer.
They answered the question “What is the effectiveness of probiotics in reducing eczema symptoms and improving quality of life in patients with eczema?”
In this context, a probiotic is a health product that contains live microorganisms and is taken by mouth. Eczema is a common skin condition that causes red, itchy skin.
Table of contents
What is a systematic review, systematic review vs. meta-analysis, systematic review vs. literature review, systematic review vs. scoping review, when to conduct a systematic review, pros and cons of systematic reviews, step-by-step example of a systematic review, other interesting articles, frequently asked questions about systematic reviews.
A review is an overview of the research that’s already been completed on a topic.
What makes a systematic review different from other types of reviews is that the research methods are designed to reduce bias . The methods are repeatable, and the approach is formal and systematic:
- Formulate a research question
- Develop a protocol
- Search for all relevant studies
- Apply the selection criteria
- Extract the data
- Synthesize the data
- Write and publish a report
Although multiple sets of guidelines exist, the Cochrane Handbook for Systematic Reviews is among the most widely used. It provides detailed guidelines on how to complete each step of the systematic review process.
Systematic reviews are most commonly used in medical and public health research, but they can also be found in other disciplines.
Systematic reviews typically answer their research question by synthesizing all available evidence and evaluating the quality of the evidence. Synthesizing means bringing together different information to tell a single, cohesive story. The synthesis can be narrative ( qualitative ), quantitative , or both.
Here's why students love Scribbr's proofreading services
Discover proofreading & editing
Systematic reviews often quantitatively synthesize the evidence using a meta-analysis . A meta-analysis is a statistical analysis, not a type of review.
A meta-analysis is a technique to synthesize results from multiple studies. It’s a statistical analysis that combines the results of two or more studies, usually to estimate an effect size .
A literature review is a type of review that uses a less systematic and formal approach than a systematic review. Typically, an expert in a topic will qualitatively summarize and evaluate previous work, without using a formal, explicit method.
Although literature reviews are often less time-consuming and can be insightful or helpful, they have a higher risk of bias and are less transparent than systematic reviews.
Similar to a systematic review, a scoping review is a type of review that tries to minimize bias by using transparent and repeatable methods.
However, a scoping review isn’t a type of systematic review. The most important difference is the goal: rather than answering a specific question, a scoping review explores a topic. The researcher tries to identify the main concepts, theories, and evidence, as well as gaps in the current research.
Sometimes scoping reviews are an exploratory preparation step for a systematic review, and sometimes they are a standalone project.
A systematic review is a good choice of review if you want to answer a question about the effectiveness of an intervention , such as a medical treatment.
To conduct a systematic review, you’ll need the following:
- A precise question , usually about the effectiveness of an intervention. The question needs to be about a topic that’s previously been studied by multiple researchers. If there’s no previous research, there’s nothing to review.
- If you’re doing a systematic review on your own (e.g., for a research paper or thesis ), you should take appropriate measures to ensure the validity and reliability of your research.
- Access to databases and journal archives. Often, your educational institution provides you with access.
- Time. A professional systematic review is a time-consuming process: it will take the lead author about six months of full-time work. If you’re a student, you should narrow the scope of your systematic review and stick to a tight schedule.
- Bibliographic, word-processing, spreadsheet, and statistical software . For example, you could use EndNote, Microsoft Word, Excel, and SPSS.
A systematic review has many pros .
- They minimize research bias by considering all available evidence and evaluating each study for bias.
- Their methods are transparent , so they can be scrutinized by others.
- They’re thorough : they summarize all available evidence.
- They can be replicated and updated by others.
Systematic reviews also have a few cons .
- They’re time-consuming .
- They’re narrow in scope : they only answer the precise research question.
The 7 steps for conducting a systematic review are explained with an example.
Step 1: Formulate a research question
Formulating the research question is probably the most important step of a systematic review. A clear research question will:
- Allow you to more effectively communicate your research to other researchers and practitioners
- Guide your decisions as you plan and conduct your systematic review
A good research question for a systematic review has four components, which you can remember with the acronym PICO :
- Population(s) or problem(s)
- Intervention(s)
- Comparison(s)
You can rearrange these four components to write your research question:
- What is the effectiveness of I versus C for O in P ?
Sometimes, you may want to include a fifth component, the type of study design . In this case, the acronym is PICOT .
- Type of study design(s)
- The population of patients with eczema
- The intervention of probiotics
- In comparison to no treatment, placebo , or non-probiotic treatment
- The outcome of changes in participant-, parent-, and doctor-rated symptoms of eczema and quality of life
- Randomized control trials, a type of study design
Their research question was:
- What is the effectiveness of probiotics versus no treatment, a placebo, or a non-probiotic treatment for reducing eczema symptoms and improving quality of life in patients with eczema?
Step 2: Develop a protocol
A protocol is a document that contains your research plan for the systematic review. This is an important step because having a plan allows you to work more efficiently and reduces bias.
Your protocol should include the following components:
- Background information : Provide the context of the research question, including why it’s important.
- Research objective (s) : Rephrase your research question as an objective.
- Selection criteria: State how you’ll decide which studies to include or exclude from your review.
- Search strategy: Discuss your plan for finding studies.
- Analysis: Explain what information you’ll collect from the studies and how you’ll synthesize the data.
If you’re a professional seeking to publish your review, it’s a good idea to bring together an advisory committee . This is a group of about six people who have experience in the topic you’re researching. They can help you make decisions about your protocol.
It’s highly recommended to register your protocol. Registering your protocol means submitting it to a database such as PROSPERO or ClinicalTrials.gov .
Step 3: Search for all relevant studies
Searching for relevant studies is the most time-consuming step of a systematic review.
To reduce bias, it’s important to search for relevant studies very thoroughly. Your strategy will depend on your field and your research question, but sources generally fall into these four categories:
- Databases: Search multiple databases of peer-reviewed literature, such as PubMed or Scopus . Think carefully about how to phrase your search terms and include multiple synonyms of each word. Use Boolean operators if relevant.
- Handsearching: In addition to searching the primary sources using databases, you’ll also need to search manually. One strategy is to scan relevant journals or conference proceedings. Another strategy is to scan the reference lists of relevant studies.
- Gray literature: Gray literature includes documents produced by governments, universities, and other institutions that aren’t published by traditional publishers. Graduate student theses are an important type of gray literature, which you can search using the Networked Digital Library of Theses and Dissertations (NDLTD) . In medicine, clinical trial registries are another important type of gray literature.
- Experts: Contact experts in the field to ask if they have unpublished studies that should be included in your review.
At this stage of your review, you won’t read the articles yet. Simply save any potentially relevant citations using bibliographic software, such as Scribbr’s APA or MLA Generator .
- Databases: EMBASE, PsycINFO, AMED, LILACS, and ISI Web of Science
- Handsearch: Conference proceedings and reference lists of articles
- Gray literature: The Cochrane Library, the metaRegister of Controlled Trials, and the Ongoing Skin Trials Register
- Experts: Authors of unpublished registered trials, pharmaceutical companies, and manufacturers of probiotics
Step 4: Apply the selection criteria
Applying the selection criteria is a three-person job. Two of you will independently read the studies and decide which to include in your review based on the selection criteria you established in your protocol . The third person’s job is to break any ties.
To increase inter-rater reliability , ensure that everyone thoroughly understands the selection criteria before you begin.
If you’re writing a systematic review as a student for an assignment, you might not have a team. In this case, you’ll have to apply the selection criteria on your own; you can mention this as a limitation in your paper’s discussion.
You should apply the selection criteria in two phases:
- Based on the titles and abstracts : Decide whether each article potentially meets the selection criteria based on the information provided in the abstracts.
- Based on the full texts: Download the articles that weren’t excluded during the first phase. If an article isn’t available online or through your library, you may need to contact the authors to ask for a copy. Read the articles and decide which articles meet the selection criteria.
It’s very important to keep a meticulous record of why you included or excluded each article. When the selection process is complete, you can summarize what you did using a PRISMA flow diagram .
Next, Boyle and colleagues found the full texts for each of the remaining studies. Boyle and Tang read through the articles to decide if any more studies needed to be excluded based on the selection criteria.
When Boyle and Tang disagreed about whether a study should be excluded, they discussed it with Varigos until the three researchers came to an agreement.
Step 5: Extract the data
Extracting the data means collecting information from the selected studies in a systematic way. There are two types of information you need to collect from each study:
- Information about the study’s methods and results . The exact information will depend on your research question, but it might include the year, study design , sample size, context, research findings , and conclusions. If any data are missing, you’ll need to contact the study’s authors.
- Your judgment of the quality of the evidence, including risk of bias .
You should collect this information using forms. You can find sample forms in The Registry of Methods and Tools for Evidence-Informed Decision Making and the Grading of Recommendations, Assessment, Development and Evaluations Working Group .
Extracting the data is also a three-person job. Two people should do this step independently, and the third person will resolve any disagreements.
They also collected data about possible sources of bias, such as how the study participants were randomized into the control and treatment groups.
Step 6: Synthesize the data
Synthesizing the data means bringing together the information you collected into a single, cohesive story. There are two main approaches to synthesizing the data:
- Narrative ( qualitative ): Summarize the information in words. You’ll need to discuss the studies and assess their overall quality.
- Quantitative : Use statistical methods to summarize and compare data from different studies. The most common quantitative approach is a meta-analysis , which allows you to combine results from multiple studies into a summary result.
Generally, you should use both approaches together whenever possible. If you don’t have enough data, or the data from different studies aren’t comparable, then you can take just a narrative approach. However, you should justify why a quantitative approach wasn’t possible.
Boyle and colleagues also divided the studies into subgroups, such as studies about babies, children, and adults, and analyzed the effect sizes within each group.
Step 7: Write and publish a report
The purpose of writing a systematic review article is to share the answer to your research question and explain how you arrived at this answer.
Your article should include the following sections:
- Abstract : A summary of the review
- Introduction : Including the rationale and objectives
- Methods : Including the selection criteria, search method, data extraction method, and synthesis method
- Results : Including results of the search and selection process, study characteristics, risk of bias in the studies, and synthesis results
- Discussion : Including interpretation of the results and limitations of the review
- Conclusion : The answer to your research question and implications for practice, policy, or research
To verify that your report includes everything it needs, you can use the PRISMA checklist .
Once your report is written, you can publish it in a systematic review database, such as the Cochrane Database of Systematic Reviews , and/or in a peer-reviewed journal.
In their report, Boyle and colleagues concluded that probiotics cannot be recommended for reducing eczema symptoms or improving quality of life in patients with eczema. Note Generative AI tools like ChatGPT can be useful at various stages of the writing and research process and can help you to write your systematic review. However, we strongly advise against trying to pass AI-generated text off as your own work.
If you want to know more about statistics , methodology , or research bias , make sure to check out some of our other articles with explanations and examples.
- Student’s t -distribution
- Normal distribution
- Null and Alternative Hypotheses
- Chi square tests
- Confidence interval
- Quartiles & Quantiles
- Cluster sampling
- Stratified sampling
- Data cleansing
- Reproducibility vs Replicability
- Peer review
- Prospective cohort study
Research bias
- Implicit bias
- Cognitive bias
- Placebo effect
- Hawthorne effect
- Hindsight bias
- Affect heuristic
- Social desirability bias
A literature review is a survey of scholarly sources (such as books, journal articles, and theses) related to a specific topic or research question .
It is often written as part of a thesis, dissertation , or research paper , in order to situate your work in relation to existing knowledge.
A literature review is a survey of credible sources on a topic, often used in dissertations , theses, and research papers . Literature reviews give an overview of knowledge on a subject, helping you identify relevant theories and methods, as well as gaps in existing research. Literature reviews are set up similarly to other academic texts , with an introduction , a main body, and a conclusion .
An annotated bibliography is a list of source references that has a short description (called an annotation ) for each of the sources. It is often assigned as part of the research process for a paper .
A systematic review is secondary research because it uses existing research. You don’t collect new data yourself.
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the “Cite this Scribbr article” button to automatically add the citation to our free Citation Generator.
Turney, S. (2023, November 20). Systematic Review | Definition, Example & Guide. Scribbr. Retrieved April 15, 2024, from https://www.scribbr.com/methodology/systematic-review/
Is this article helpful?

Shaun Turney
Other students also liked, how to write a literature review | guide, examples, & templates, how to write a research proposal | examples & templates, what is critical thinking | definition & examples, unlimited academic ai-proofreading.
✔ Document error-free in 5minutes ✔ Unlimited document corrections ✔ Specialized in correcting academic texts
1.2.2 What is a systematic review?
A systematic review attempts to collate all empirical evidence that fits pre-specified eligibility criteria in order to answer a specific research question. It uses explicit, systematic methods that are selected with a view to minimizing bias, thus providing more reliable findings from which conclusions can be drawn and decisions made (Antman 1992, Oxman 1993) . The key characteristics of a systematic review are:
a clearly stated set of objectives with pre-defined eligibility criteria for studies;
an explicit, reproducible methodology;
a systematic search that attempts to identify all studies that would meet the eligibility criteria;
an assessment of the validity of the findings of the included studies, for example through the assessment of risk of bias; and
a systematic presentation, and synthesis, of the characteristics and findings of the included studies.
Many systematic reviews contain meta-analyses. Meta-analysis is the use of statistical methods to summarize the results of independent studies (Glass 1976). By combining information from all relevant studies, meta-analyses can provide more precise estimates of the effects of health care than those derived from the individual studies included within a review (see Chapter 9, Section 9.1.3 ). They also facilitate investigations of the consistency of evidence across studies, and the exploration of differences across studies.

An official website of the Department of Health & Human Services
- Search All AHRQ Sites
- Email Updates

1. Use quotes to search for an exact match of a phrase.
2. Put a minus sign just before words you don't want.
3. Enter any important keywords in any order to find entries where all these terms appear.
- The PSNet Collection
- All Content
- Perspectives
- Current Weekly Issue
- Past Weekly Issues
- Curated Libraries
- Clinical Areas
- Patient Safety 101
- The Fundamentals
- Training and Education
- Continuing Education
- WebM&M: Case Studies
- Training Catalog
- Submit a Case
- Improvement Resources
- Innovations
- Submit an Innovation
- About PSNet
- Editorial Team
- Technical Expert Panel
How are medication errors defined? A systematic literature review of definitions and characteristics.
Lisby M, Nielsen LP, Brock B, et al. How are medication errors defined? A systematic literature review of definitions and characteristics. International Journal for Quality in Health Care. 2010;22(6). doi:10.1093/intqhc/mzq059.
This systematic review found wide variation in how medication errors are defined between studies. This variation has significant implications for determining the prevalence of medication errors. Prior commentaries have noted the need for standardized, universally applicable definitions of adverse drug events.
How should medication errors be defined? Development and test of a definition. May 30, 2012
Errors in the medication process: frequency, type, and potential clinical consequences. March 6, 2005
Identifying high-risk medication: a systematic literature review. August 13, 2014
Case study: getting boards on board at Allen Memorial Hospital, Iowa Health System. April 2, 2008
Association of hospital participation in a regional trauma quality improvement collaborative with patient outcomes. June 20, 2018
Selection of indicators for continuous monitoring of patient safety: recommendations of the project 'safety improvement for patients in Europe.' June 10, 2009
Novel analysis of clinically relevant diagnostic errors in point-of-care devices. October 19, 2011
Measuring patient safety climate: a review of surveys. October 12, 2005
National Patient Safety Foundation agenda for research and development in patient safety. March 27, 2005
Physician assistants and the disclosure of medical error. June 18, 2014
Insulin dosing error in a patient with severe hyperkalemia. January 17, 2018
Effective interventions and implementation strategies to reduce adverse drug events in the Veterans Affairs (VA) system. February 20, 2008
Implementing an error disclosure coaching model: a multicenter case study. February 22, 2017
Building safer systems by ecological design: using restoration science to develop a medication safety intervention. April 12, 2006
Disclosing adverse events in clinical practice: the delicate act of being open. February 2, 2022
Use of a safety climate questionnaire in UK health care: factor structure, reliability and usability. November 22, 2006
Using standardised patients in an objective structured clinical examination as a patient safety tool. March 6, 2005
Time of day effects on the incidence of anesthetic adverse events. August 23, 2006
Discrimination, abuse, harassment, and burnout in surgical residency training. November 20, 2019
Effect of computerized physician order entry and a team intervention on prevention of serious medication errors. March 27, 2005
Relationship between tort claims and patient incident reports in the Veterans Health Administration. April 21, 2005
An intervention to decrease patient identification band errors in a children's hospital. May 12, 2010
"Every error counts": a web-based incident reporting and learning system for general practice. August 20, 2008
Effects of skilled nursing facility structure and process factors on medication errors during nursing home admission. November 5, 2014
Frequency and type of situational awareness errors contributing to death and brain damage: a closed claims analysis. May 24, 2017
A simulation design for research evaluating safety innovations in anaesthesia. January 28, 2009
Teaching teamwork during the Neonatal Resuscitation Program: a randomized trial. June 20, 2007
Implementation of bar-code medication administration to reduce patient harm. February 20, 2019
Design and implementation of a point-of-care computerized system for drug therapy in Stockholm metropolitan health region--bridging the gap between knowledge and practice. August 29, 2007
Analysis of suicides reported as adverse events in psychiatry resulted in nine quality improvement initiatives. July 21, 2021
Nil per os orders for imaging: a teachable moment. September 27, 2017
An observational study of direct oral anticoagulant awareness indicating inadequate recognition with potential for patient harm. April 13, 2016
Clinical pharmacists and inpatient medical care: a systematic review. May 17, 2006
Safety learning among young newly employed workers in three sectors: a challenge to the assumed order of things. November 17, 2021
Competencies for patient safety and quality improvement: a synthesis of recommendations in influential position papers. April 6, 2016
Patterns of potential opioid misuse and subsequent adverse outcomes in Medicare, 2008 to 2012. June 6, 2018
Video capture of clinical care to enhance patient safety. March 1, 2006
Do faculty and resident physicians discuss their medical errors? October 15, 2008
Getting teams to talk: development and pilot implementation of a checklist to promote interprofessional communication in the OR. October 19, 2005
Why psychiatry is different--challenges and difficulties in managing a nosocomial outbreak of coronavirus disease (COVID-19) in hospital care. January 20, 2021
The "Seven Pillars" response to patient safety incidents: effects on medical liability processes and outcomes. September 7, 2016
Regional surveillance of emergency-department visits for outpatient adverse drug events. April 22, 2009
Errors with concentrated epinephrine in otolaryngology. September 3, 2008
Doctors debate safety of their white coats. December 2, 2015
Multicentre study to develop a medication safety package for decreasing inpatient harm from omission of time-critical medications. March 4, 2015
Strengthening leadership as a catalyst for enhanced patient safety culture: a repeated cross-sectional experimental study. June 22, 2016
Errors during the preparation of drug infusions: a randomized controlled trial. August 22, 2012
The impact of a tele-ICU on provider attitudes about teamwork and safety climate. May 26, 2010
Non-intercepted dose errors in prescribing antineoplastic treatment: a prospective, comparative cohort study. March 11, 2015
Polypharmacy in hospitalized older adult cancer patients: experience from a prospective, observational study of an oncology-acute care for elders unit. August 5, 2009
Anaesthetists' management of oxygen pipeline failure: room for improvement. January 31, 2007
Attitudes and barriers to incident reporting: a collaborative hospital study. February 22, 2006
Using simulation to identify sources of medical diagnostic error in child physical abuse. April 27, 2016
An alternative strategy for studying adverse events in medical care. March 27, 2005
A prospective study of patient safety in the operating room. February 22, 2006
Wrong-site sinus surgery in otolaryngology. August 11, 2010
Reducing serious safety events and priority hospital-acquired conditions in a pediatric hospital with the implementation of a patient safety program. June 6, 2018
The SAGES Fundamental Use of Surgical Energy program (FUSE): history, development, and purpose. February 14, 2018
Does a suggested diagnosis in a general practitioners' referral question impact diagnostic reasoning: an experimental study. April 27, 2022
Adverse-event-reporting practices by US hospitals: results of a national survey. January 7, 2009
Involvement of parents in critical incidents in a neonatal-paediatric intensive care unit. December 16, 2009
Using computerized virtual cases to explore diagnostic error in practicing physicians. February 13, 2019
The role of housestaff in implementing medication reconciliation on admission at an academic medical center. June 16, 2010
The Safety Attitudes Questionnaire: psychometric properties, benchmarking data, and emerging research. April 19, 2006
National Partnership for Maternal Safety: Consensus Bundle on Venous Thromboembolism. December 7, 2016
Enhancing psychological safety in mental health services. June 9, 2021
Teamwork behaviours and errors during neonatal resuscitation. March 24, 2010
The use of medical emergency teams in medical and surgical patients: impact of patient, nurse and organisational characteristics. October 29, 2008
Augmenting health care failure modes and effects analysis with simulation. March 5, 2014
Relationship between complaints and quality of care in New Zealand: a descriptive analysis of complainants and non-complainants following adverse events. February 15, 2006
Nurses' perspective on a serious adverse drug event. March 6, 2005
Medication safety program reduces adverse drug events in a community hospital. June 22, 2005
Disclosing large scale adverse events in the US Veterans Health Administration: lessons from media responses. April 13, 2016
The experiences of risk managers in providing emotional support for health care workers after adverse events. May 11, 2016
Risk managers' descriptions of programs to support second victims after adverse events. May 13, 2015
Interprofessional education in team communication: working together to improve patient safety. March 27, 2013
Beyond "see one, do one, teach one": toward a different training paradigm. February 25, 2009
Effect of an in-hospital multifaceted clinical pharmacist intervention on the risk of readmission: a randomized clinical trial. February 7, 2018
Preventable errors in organ transplantation: an emerging patient safety issue? July 11, 2012
Levels of agreement on the grading, analysis and reporting of significant events by general practitioners: a cross-sectional study. October 29, 2008
The impact of trained assistance on error rates in anaesthesia: a simulation-based randomised controlled trial. February 25, 2009
The effect of executive walk rounds on nurse safety climate attitudes: a randomized trial of clinical units. April 27, 2005
Association of surgical resident wellness with medical errors and patient outcomes. May 6, 2020
Relationship between patient complaints and surgical complications. February 15, 2006
Error rating tool to identify and analyse technical errors and events in laparoscopic surgery. September 11, 2013
The influence of standardisation and task load on team coordination patterns during anaesthesia inductions. April 29, 2009
Incident reporting system does not detect adverse drug events: a problem for quality improvement. March 27, 2005
A systematic review of clinical decision support systems for clinical oncology practice. May 15, 2019
Development and validation of a taxonomy of adverse handover events in hospital settings. February 18, 2015
Evaluation of reasons why surgical residents exceeded 2011 duty hour requirements when offered flexibility. June 20, 2018
Error, stress, and teamwork in medicine and aviation: cross sectional surveys. December 21, 2005
Readiness for organisational change among general practice staff. April 28, 2010
Universal surveillance for methicillin-resistant Staphylococcus aureus in 3 affiliated hospitals. April 2, 2008
The costs of adverse drug events in hospitalized patients. March 27, 2005
Medication administration discrepancies persist despite electronic ordering. November 28, 2007
A family-centered rounds checklist, family engagement, and patient safety: a randomized trial. May 31, 2017
Improving team information sharing with a structured call-out in anaesthetic emergencies: a randomized controlled trial. March 12, 2014
Communication failures contributing to patient injury in anaesthesia malpractice claims. September 1, 2021
Are informed policies in place to promote safe and usable EHRs? A cross-industry comparison. March 8, 2017
Evaluation of adverse drug events and medication discrepancies in transitions of care between hospital discharge and primary care follow-up. October 29, 2014
Thematic reviews of patient safety incidents as a tool for systems thinking: a quality improvement report. May 17, 2023
Driving Learning and Improvement After RCA2 Event Reviews. January 26, 2023 - January 26, 2023
HSIB Education. October 19, 2022
Interventions to reduce medication dispensing, administration, and monitoring errors in pediatric professional healthcare settings: a systematic review. September 29, 2021
Critical incidents involving the medical emergency team: a 5-year retrospective assessment for healthcare improvement. April 28, 2021
Suicide as an incident of severe patient harm: a retrospective cohort study of investigations after suicide in Swedish healthcare in a 13-year perspective. March 31, 2021
Learning from incident reporting? Analysis of incidents resulting in patient injuries in a web-based system in Swedish health care. December 9, 2020
How incident reporting systems can stimulate social and participative learning: a mixed-methods study. September 2, 2020
Register-based research of adverse events revealing incomplete records threatening patient safety. August 19, 2020
Identifying no-harm incidents in home healthcare: a cohort study using trigger tool methodology. August 5, 2020
Apparent cause analysis: a safety tool. May 20, 2020
Medical teamwork and the evolution of safety science: a critical review. March 11, 2020
The Field Guide to Human Error Investigations, Third Edition. August 24, 2017
Monitoring the anaesthetist in the operating theatre—professional competence and patient safety. March 1, 2017
Measurement of patient safety: a systematic review of the reliability and validity of adverse event detection with record review. September 28, 2016
Is there evidence for a better health care for cancer patients after a second opinion? A systematic review. September 28, 2016
Healthcare staff wellbeing, burnout, and patient safety: a systematic review. August 24, 2016
Performance of the Global Assessment of Pediatric Patient Safety (GAPPS) tool. June 15, 2016
Vaccination errors in general practice: creation of a preventive checklist based on a multimodal analysis of declared errors. June 15, 2016
Medical error—the third leading cause of death in the US. May 11, 2016
Patients' views of adverse events in primary and ambulatory care: a systematic review to assess methods and the content of what patients consider to be adverse events. February 17, 2016
Aviation and healthcare: a comparative review with implications for patient safety. February 3, 2016
Interorganizational complexity and organizational accident risk: a literature review. November 25, 2015
Interventions to reduce nurses' medication administration errors in inpatient settings: a systematic review and meta-analysis. September 30, 2015
The influence of context on the effectiveness of hospital quality improvement strategies: a review of systematic reviews. September 16, 2015
Ethical issues in patient safety research: a systematic review of the literature. September 9, 2015
"First, know thyself": cognition and error in medicine. June 3, 2015
Insulin pump risks and benefits: a clinical appraisal of pump safety standards, adverse event reporting, and research needs: a joint statement of the European Association for the Study of Diabetes and the American Diabetes Association Diabetes Technology Working Group. May 6, 2015
Hospital organisation, management, and structure for prevention of health-care-associated infection: a systematic review and expert consensus. March 11, 2015
Peer review of medical practices: missed opportunities to learn. January 28, 2015

Connect With Us
Sign up for Email Updates
To sign up for updates or to access your subscriber preferences, please enter your email address below.
Agency for Healthcare Research and Quality
5600 Fishers Lane Rockville, MD 20857 Telephone: (301) 427-1364
- Accessibility
- Disclaimers
- Electronic Policies
- HHS Digital Strategy
- HHS Nondiscrimination Notice
- Inspector General
- Plain Writing Act
- Privacy Policy
- Viewers & Players
- U.S. Department of Health & Human Services
- The White House
- Don't have an account? Sign up to PSNet
Submit Your Innovations
Please select your preferred way to submit an innovation.
Continue as a Guest
Track and save your innovation
in My Innovations
Edit your innovation as a draft
Continue Logged In
Please select your preferred way to submit an innovation. Note that even if you have an account, you can still choose to submit an innovation as a guest.
Continue logged in
New users to the psnet site.
Access to quizzes and start earning
CME, CEU, or Trainee Certification.
Get email alerts when new content
matching your topics of interest
in My Innovations.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Wiley-Blackwell Online Open

An overview of methodological approaches in systematic reviews
Prabhakar veginadu.
1 Department of Rural Clinical Sciences, La Trobe Rural Health School, La Trobe University, Bendigo Victoria, Australia
Hanny Calache
2 Lincoln International Institute for Rural Health, University of Lincoln, Brayford Pool, Lincoln UK
Akshaya Pandian
3 Department of Orthodontics, Saveetha Dental College, Chennai Tamil Nadu, India
Mohd Masood
Associated data.
APPENDIX B: List of excluded studies with detailed reasons for exclusion
APPENDIX C: Quality assessment of included reviews using AMSTAR 2
The aim of this overview is to identify and collate evidence from existing published systematic review (SR) articles evaluating various methodological approaches used at each stage of an SR.
The search was conducted in five electronic databases from inception to November 2020 and updated in February 2022: MEDLINE, Embase, Web of Science Core Collection, Cochrane Database of Systematic Reviews, and APA PsycINFO. Title and abstract screening were performed in two stages by one reviewer, supported by a second reviewer. Full‐text screening, data extraction, and quality appraisal were performed by two reviewers independently. The quality of the included SRs was assessed using the AMSTAR 2 checklist.
The search retrieved 41,556 unique citations, of which 9 SRs were deemed eligible for inclusion in final synthesis. Included SRs evaluated 24 unique methodological approaches used for defining the review scope and eligibility, literature search, screening, data extraction, and quality appraisal in the SR process. Limited evidence supports the following (a) searching multiple resources (electronic databases, handsearching, and reference lists) to identify relevant literature; (b) excluding non‐English, gray, and unpublished literature, and (c) use of text‐mining approaches during title and abstract screening.
The overview identified limited SR‐level evidence on various methodological approaches currently employed during five of the seven fundamental steps in the SR process, as well as some methodological modifications currently used in expedited SRs. Overall, findings of this overview highlight the dearth of published SRs focused on SR methodologies and this warrants future work in this area.
1. INTRODUCTION
Evidence synthesis is a prerequisite for knowledge translation. 1 A well conducted systematic review (SR), often in conjunction with meta‐analyses (MA) when appropriate, is considered the “gold standard” of methods for synthesizing evidence related to a topic of interest. 2 The central strength of an SR is the transparency of the methods used to systematically search, appraise, and synthesize the available evidence. 3 Several guidelines, developed by various organizations, are available for the conduct of an SR; 4 , 5 , 6 , 7 among these, Cochrane is considered a pioneer in developing rigorous and highly structured methodology for the conduct of SRs. 8 The guidelines developed by these organizations outline seven fundamental steps required in SR process: defining the scope of the review and eligibility criteria, literature searching and retrieval, selecting eligible studies, extracting relevant data, assessing risk of bias (RoB) in included studies, synthesizing results, and assessing certainty of evidence (CoE) and presenting findings. 4 , 5 , 6 , 7
The methodological rigor involved in an SR can require a significant amount of time and resource, which may not always be available. 9 As a result, there has been a proliferation of modifications made to the traditional SR process, such as refining, shortening, bypassing, or omitting one or more steps, 10 , 11 for example, limits on the number and type of databases searched, limits on publication date, language, and types of studies included, and limiting to one reviewer for screening and selection of studies, as opposed to two or more reviewers. 10 , 11 These methodological modifications are made to accommodate the needs of and resource constraints of the reviewers and stakeholders (e.g., organizations, policymakers, health care professionals, and other knowledge users). While such modifications are considered time and resource efficient, they may introduce bias in the review process reducing their usefulness. 5
Substantial research has been conducted examining various approaches used in the standardized SR methodology and their impact on the validity of SR results. There are a number of published reviews examining the approaches or modifications corresponding to single 12 , 13 or multiple steps 14 involved in an SR. However, there is yet to be a comprehensive summary of the SR‐level evidence for all the seven fundamental steps in an SR. Such a holistic evidence synthesis will provide an empirical basis to confirm the validity of current accepted practices in the conduct of SRs. Furthermore, sometimes there is a balance that needs to be achieved between the resource availability and the need to synthesize the evidence in the best way possible, given the constraints. This evidence base will also inform the choice of modifications to be made to the SR methods, as well as the potential impact of these modifications on the SR results. An overview is considered the choice of approach for summarizing existing evidence on a broad topic, directing the reader to evidence, or highlighting the gaps in evidence, where the evidence is derived exclusively from SRs. 15 Therefore, for this review, an overview approach was used to (a) identify and collate evidence from existing published SR articles evaluating various methodological approaches employed in each of the seven fundamental steps of an SR and (b) highlight both the gaps in the current research and the potential areas for future research on the methods employed in SRs.
An a priori protocol was developed for this overview but was not registered with the International Prospective Register of Systematic Reviews (PROSPERO), as the review was primarily methodological in nature and did not meet PROSPERO eligibility criteria for registration. The protocol is available from the corresponding author upon reasonable request. This overview was conducted based on the guidelines for the conduct of overviews as outlined in The Cochrane Handbook. 15 Reporting followed the Preferred Reporting Items for Systematic reviews and Meta‐analyses (PRISMA) statement. 3
2.1. Eligibility criteria
Only published SRs, with or without associated MA, were included in this overview. We adopted the defining characteristics of SRs from The Cochrane Handbook. 5 According to The Cochrane Handbook, a review was considered systematic if it satisfied the following criteria: (a) clearly states the objectives and eligibility criteria for study inclusion; (b) provides reproducible methodology; (c) includes a systematic search to identify all eligible studies; (d) reports assessment of validity of findings of included studies (e.g., RoB assessment of the included studies); (e) systematically presents all the characteristics or findings of the included studies. 5 Reviews that did not meet all of the above criteria were not considered a SR for this study and were excluded. MA‐only articles were included if it was mentioned that the MA was based on an SR.
SRs and/or MA of primary studies evaluating methodological approaches used in defining review scope and study eligibility, literature search, study selection, data extraction, RoB assessment, data synthesis, and CoE assessment and reporting were included. The methodological approaches examined in these SRs and/or MA can also be related to the substeps or elements of these steps; for example, applying limits on date or type of publication are the elements of literature search. Included SRs examined or compared various aspects of a method or methods, and the associated factors, including but not limited to: precision or effectiveness; accuracy or reliability; impact on the SR and/or MA results; reproducibility of an SR steps or bias occurred; time and/or resource efficiency. SRs assessing the methodological quality of SRs (e.g., adherence to reporting guidelines), evaluating techniques for building search strategies or the use of specific database filters (e.g., use of Boolean operators or search filters for randomized controlled trials), examining various tools used for RoB or CoE assessment (e.g., ROBINS vs. Cochrane RoB tool), or evaluating statistical techniques used in meta‐analyses were excluded. 14
2.2. Search
The search for published SRs was performed on the following scientific databases initially from inception to third week of November 2020 and updated in the last week of February 2022: MEDLINE (via Ovid), Embase (via Ovid), Web of Science Core Collection, Cochrane Database of Systematic Reviews, and American Psychological Association (APA) PsycINFO. Search was restricted to English language publications. Following the objectives of this study, study design filters within databases were used to restrict the search to SRs and MA, where available. The reference lists of included SRs were also searched for potentially relevant publications.
The search terms included keywords, truncations, and subject headings for the key concepts in the review question: SRs and/or MA, methods, and evaluation. Some of the terms were adopted from the search strategy used in a previous review by Robson et al., which reviewed primary studies on methodological approaches used in study selection, data extraction, and quality appraisal steps of SR process. 14 Individual search strategies were developed for respective databases by combining the search terms using appropriate proximity and Boolean operators, along with the related subject headings in order to identify SRs and/or MA. 16 , 17 A senior librarian was consulted in the design of the search terms and strategy. Appendix A presents the detailed search strategies for all five databases.
2.3. Study selection and data extraction
Title and abstract screening of references were performed in three steps. First, one reviewer (PV) screened all the titles and excluded obviously irrelevant citations, for example, articles on topics not related to SRs, non‐SR publications (such as randomized controlled trials, observational studies, scoping reviews, etc.). Next, from the remaining citations, a random sample of 200 titles and abstracts were screened against the predefined eligibility criteria by two reviewers (PV and MM), independently, in duplicate. Discrepancies were discussed and resolved by consensus. This step ensured that the responses of the two reviewers were calibrated for consistency in the application of the eligibility criteria in the screening process. Finally, all the remaining titles and abstracts were reviewed by a single “calibrated” reviewer (PV) to identify potential full‐text records. Full‐text screening was performed by at least two authors independently (PV screened all the records, and duplicate assessment was conducted by MM, HC, or MG), with discrepancies resolved via discussions or by consulting a third reviewer.
Data related to review characteristics, results, key findings, and conclusions were extracted by at least two reviewers independently (PV performed data extraction for all the reviews and duplicate extraction was performed by AP, HC, or MG).
2.4. Quality assessment of included reviews
The quality assessment of the included SRs was performed using the AMSTAR 2 (A MeaSurement Tool to Assess systematic Reviews). The tool consists of a 16‐item checklist addressing critical and noncritical domains. 18 For the purpose of this study, the domain related to MA was reclassified from critical to noncritical, as SRs with and without MA were included. The other six critical domains were used according to the tool guidelines. 18 Two reviewers (PV and AP) independently responded to each of the 16 items in the checklist with either “yes,” “partial yes,” or “no.” Based on the interpretations of the critical and noncritical domains, the overall quality of the review was rated as high, moderate, low, or critically low. 18 Disagreements were resolved through discussion or by consulting a third reviewer.
2.5. Data synthesis
To provide an understandable summary of existing evidence syntheses, characteristics of the methods evaluated in the included SRs were examined and key findings were categorized and presented based on the corresponding step in the SR process. The categories of key elements within each step were discussed and agreed by the authors. Results of the included reviews were tabulated and summarized descriptively, along with a discussion on any overlap in the primary studies. 15 No quantitative analyses of the data were performed.
From 41,556 unique citations identified through literature search, 50 full‐text records were reviewed, and nine systematic reviews 14 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 were deemed eligible for inclusion. The flow of studies through the screening process is presented in Figure 1 . A list of excluded studies with reasons can be found in Appendix B .

Study selection flowchart
3.1. Characteristics of included reviews
Table 1 summarizes the characteristics of included SRs. The majority of the included reviews (six of nine) were published after 2010. 14 , 22 , 23 , 24 , 25 , 26 Four of the nine included SRs were Cochrane reviews. 20 , 21 , 22 , 23 The number of databases searched in the reviews ranged from 2 to 14, 2 reviews searched gray literature sources, 24 , 25 and 7 reviews included a supplementary search strategy to identify relevant literature. 14 , 19 , 20 , 21 , 22 , 23 , 26 Three of the included SRs (all Cochrane reviews) included an integrated MA. 20 , 21 , 23
Characteristics of included studies
SR = systematic review; MA = meta‐analysis; RCT = randomized controlled trial; CCT = controlled clinical trial; N/R = not reported.
The included SRs evaluated 24 unique methodological approaches (26 in total) used across five steps in the SR process; 8 SRs evaluated 6 approaches, 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 while 1 review evaluated 18 approaches. 14 Exclusion of gray or unpublished literature 21 , 26 and blinding of reviewers for RoB assessment 14 , 23 were evaluated in two reviews each. Included SRs evaluated methods used in five different steps in the SR process, including methods used in defining the scope of review ( n = 3), literature search ( n = 3), study selection ( n = 2), data extraction ( n = 1), and RoB assessment ( n = 2) (Table 2 ).
Summary of findings from review evaluating systematic review methods
There was some overlap in the primary studies evaluated in the included SRs on the same topics: Schmucker et al. 26 and Hopewell et al. 21 ( n = 4), Hopewell et al. 20 and Crumley et al. 19 ( n = 30), and Robson et al. 14 and Morissette et al. 23 ( n = 4). There were no conflicting results between any of the identified SRs on the same topic.
3.2. Methodological quality of included reviews
Overall, the quality of the included reviews was assessed as moderate at best (Table 2 ). The most common critical weakness in the reviews was failure to provide justification for excluding individual studies (four reviews). Detailed quality assessment is provided in Appendix C .
3.3. Evidence on systematic review methods
3.3.1. methods for defining review scope and eligibility.
Two SRs investigated the effect of excluding data obtained from gray or unpublished sources on the pooled effect estimates of MA. 21 , 26 Hopewell et al. 21 reviewed five studies that compared the impact of gray literature on the results of a cohort of MA of RCTs in health care interventions. Gray literature was defined as information published in “print or electronic sources not controlled by commercial or academic publishers.” Findings showed an overall greater treatment effect for published trials than trials reported in gray literature. In a more recent review, Schmucker et al. 26 addressed similar objectives, by investigating gray and unpublished data in medicine. In addition to gray literature, defined similar to the previous review by Hopewell et al., the authors also evaluated unpublished data—defined as “supplemental unpublished data related to published trials, data obtained from the Food and Drug Administration or other regulatory websites or postmarketing analyses hidden from the public.” The review found that in majority of the MA, excluding gray literature had little or no effect on the pooled effect estimates. The evidence was limited to conclude if the data from gray and unpublished literature had an impact on the conclusions of MA. 26
Morrison et al. 24 examined five studies measuring the effect of excluding non‐English language RCTs on the summary treatment effects of SR‐based MA in various fields of conventional medicine. Although none of the included studies reported major difference in the treatment effect estimates between English only and non‐English inclusive MA, the review found inconsistent evidence regarding the methodological and reporting quality of English and non‐English trials. 24 As such, there might be a risk of introducing “language bias” when excluding non‐English language RCTs. The authors also noted that the numbers of non‐English trials vary across medical specialties, as does the impact of these trials on MA results. Based on these findings, Morrison et al. 24 conclude that literature searches must include non‐English studies when resources and time are available to minimize the risk of introducing “language bias.”
3.3.2. Methods for searching studies
Crumley et al. 19 analyzed recall (also referred to as “sensitivity” by some researchers; defined as “percentage of relevant studies identified by the search”) and precision (defined as “percentage of studies identified by the search that were relevant”) when searching a single resource to identify randomized controlled trials and controlled clinical trials, as opposed to searching multiple resources. The studies included in their review frequently compared a MEDLINE only search with the search involving a combination of other resources. The review found low median recall estimates (median values between 24% and 92%) and very low median precisions (median values between 0% and 49%) for most of the electronic databases when searched singularly. 19 A between‐database comparison, based on the type of search strategy used, showed better recall and precision for complex and Cochrane Highly Sensitive search strategies (CHSSS). In conclusion, the authors emphasize that literature searches for trials in SRs must include multiple sources. 19
In an SR comparing handsearching and electronic database searching, Hopewell et al. 20 found that handsearching retrieved more relevant RCTs (retrieval rate of 92%−100%) than searching in a single electronic database (retrieval rates of 67% for PsycINFO/PsycLIT, 55% for MEDLINE, and 49% for Embase). The retrieval rates varied depending on the quality of handsearching, type of electronic search strategy used (e.g., simple, complex or CHSSS), and type of trial reports searched (e.g., full reports, conference abstracts, etc.). The authors concluded that handsearching was particularly important in identifying full trials published in nonindexed journals and in languages other than English, as well as those published as abstracts and letters. 20
The effectiveness of checking reference lists to retrieve additional relevant studies for an SR was investigated by Horsley et al. 22 The review reported that checking reference lists yielded 2.5%–40% more studies depending on the quality and comprehensiveness of the electronic search used. The authors conclude that there is some evidence, although from poor quality studies, to support use of checking reference lists to supplement database searching. 22
3.3.3. Methods for selecting studies
Three approaches relevant to reviewer characteristics, including number, experience, and blinding of reviewers involved in the screening process were highlighted in an SR by Robson et al. 14 Based on the retrieved evidence, the authors recommended that two independent, experienced, and unblinded reviewers be involved in study selection. 14 A modified approach has also been suggested by the review authors, where one reviewer screens and the other reviewer verifies the list of excluded studies, when the resources are limited. It should be noted however this suggestion is likely based on the authors’ opinion, as there was no evidence related to this from the studies included in the review.
Robson et al. 14 also reported two methods describing the use of technology for screening studies: use of Google Translate for translating languages (for example, German language articles to English) to facilitate screening was considered a viable method, while using two computer monitors for screening did not increase the screening efficiency in SR. Title‐first screening was found to be more efficient than simultaneous screening of titles and abstracts, although the gain in time with the former method was lesser than the latter. Therefore, considering that the search results are routinely exported as titles and abstracts, Robson et al. 14 recommend screening titles and abstracts simultaneously. However, the authors note that these conclusions were based on very limited number (in most instances one study per method) of low‐quality studies. 14
3.3.4. Methods for data extraction
Robson et al. 14 examined three approaches for data extraction relevant to reviewer characteristics, including number, experience, and blinding of reviewers (similar to the study selection step). Although based on limited evidence from a small number of studies, the authors recommended use of two experienced and unblinded reviewers for data extraction. The experience of the reviewers was suggested to be especially important when extracting continuous outcomes (or quantitative) data. However, when the resources are limited, data extraction by one reviewer and a verification of the outcomes data by a second reviewer was recommended.
As for the methods involving use of technology, Robson et al. 14 identified limited evidence on the use of two monitors to improve the data extraction efficiency and computer‐assisted programs for graphical data extraction. However, use of Google Translate for data extraction in non‐English articles was not considered to be viable. 14 In the same review, Robson et al. 14 identified evidence supporting contacting authors for obtaining additional relevant data.
3.3.5. Methods for RoB assessment
Two SRs examined the impact of blinding of reviewers for RoB assessments. 14 , 23 Morissette et al. 23 investigated the mean differences between the blinded and unblinded RoB assessment scores and found inconsistent differences among the included studies providing no definitive conclusions. Similar conclusions were drawn in a more recent review by Robson et al., 14 which included four studies on reviewer blinding for RoB assessment that completely overlapped with Morissette et al. 23
Use of experienced reviewers and provision of additional guidance for RoB assessment were examined by Robson et al. 14 The review concluded that providing intensive training and guidance on assessing studies reporting insufficient data to the reviewers improves RoB assessments. 14 Obtaining additional data related to quality assessment by contacting study authors was also found to help the RoB assessments, although based on limited evidence. When assessing the qualitative or mixed method reviews, Robson et al. 14 recommends the use of a structured RoB tool as opposed to an unstructured tool. No SRs were identified on data synthesis and CoE assessment and reporting steps.
4. DISCUSSION
4.1. summary of findings.
Nine SRs examining 24 unique methods used across five steps in the SR process were identified in this overview. The collective evidence supports some current traditional and modified SR practices, while challenging other approaches. However, the quality of the included reviews was assessed to be moderate at best and in the majority of the included SRs, evidence related to the evaluated methods was obtained from very limited numbers of primary studies. As such, the interpretations from these SRs should be made cautiously.
The evidence gathered from the included SRs corroborate a few current SR approaches. 5 For example, it is important to search multiple resources for identifying relevant trials (RCTs and/or CCTs). The resources must include a combination of electronic database searching, handsearching, and reference lists of retrieved articles. 5 However, no SRs have been identified that evaluated the impact of the number of electronic databases searched. A recent study by Halladay et al. 27 found that articles on therapeutic intervention, retrieved by searching databases other than PubMed (including Embase), contributed only a small amount of information to the MA and also had a minimal impact on the MA results. The authors concluded that when the resources are limited and when large number of studies are expected to be retrieved for the SR or MA, PubMed‐only search can yield reliable results. 27
Findings from the included SRs also reiterate some methodological modifications currently employed to “expedite” the SR process. 10 , 11 For example, excluding non‐English language trials and gray/unpublished trials from MA have been shown to have minimal or no impact on the results of MA. 24 , 26 However, the efficiency of these SR methods, in terms of time and the resources used, have not been evaluated in the included SRs. 24 , 26 Of the SRs included, only two have focused on the aspect of efficiency 14 , 25 ; O'Mara‐Eves et al. 25 report some evidence to support the use of text‐mining approaches for title and abstract screening in order to increase the rate of screening. Moreover, only one included SR 14 considered primary studies that evaluated reliability (inter‐ or intra‐reviewer consistency) and accuracy (validity when compared against a “gold standard” method) of the SR methods. This can be attributed to the limited number of primary studies that evaluated these outcomes when evaluating the SR methods. 14 Lack of outcome measures related to reliability, accuracy, and efficiency precludes making definitive recommendations on the use of these methods/modifications. Future research studies must focus on these outcomes.
Some evaluated methods may be relevant to multiple steps; for example, exclusions based on publication status (gray/unpublished literature) and language of publication (non‐English language studies) can be outlined in the a priori eligibility criteria or can be incorporated as search limits in the search strategy. SRs included in this overview focused on the effect of study exclusions on pooled treatment effect estimates or MA conclusions. Excluding studies from the search results, after conducting a comprehensive search, based on different eligibility criteria may yield different results when compared to the results obtained when limiting the search itself. 28 Further studies are required to examine this aspect.
Although we acknowledge the lack of standardized quality assessment tools for methodological study designs, we adhered to the Cochrane criteria for identifying SRs in this overview. This was done to ensure consistency in the quality of the included evidence. As a result, we excluded three reviews that did not provide any form of discussion on the quality of the included studies. The methods investigated in these reviews concern supplementary search, 29 data extraction, 12 and screening. 13 However, methods reported in two of these three reviews, by Mathes et al. 12 and Waffenschmidt et al., 13 have also been examined in the SR by Robson et al., 14 which was included in this overview; in most instances (with the exception of one study included in Mathes et al. 12 and Waffenschmidt et al. 13 each), the studies examined in these excluded reviews overlapped with those in the SR by Robson et al. 14
One of the key gaps in the knowledge observed in this overview was the dearth of SRs on the methods used in the data synthesis component of SR. Narrative and quantitative syntheses are the two most commonly used approaches for synthesizing data in evidence synthesis. 5 There are some published studies on the proposed indications and implications of these two approaches. 30 , 31 These studies found that both data synthesis methods produced comparable results and have their own advantages, suggesting that the choice of the method must be based on the purpose of the review. 31 With increasing number of “expedited” SR approaches (so called “rapid reviews”) avoiding MA, 10 , 11 further research studies are warranted in this area to determine the impact of the type of data synthesis on the results of the SR.
4.2. Implications for future research
The findings of this overview highlight several areas of paucity in primary research and evidence synthesis on SR methods. First, no SRs were identified on methods used in two important components of the SR process, including data synthesis and CoE and reporting. As for the included SRs, a limited number of evaluation studies have been identified for several methods. This indicates that further research is required to corroborate many of the methods recommended in current SR guidelines. 4 , 5 , 6 , 7 Second, some SRs evaluated the impact of methods on the results of quantitative synthesis and MA conclusions. Future research studies must also focus on the interpretations of SR results. 28 , 32 Finally, most of the included SRs were conducted on specific topics related to the field of health care, limiting the generalizability of the findings to other areas. It is important that future research studies evaluating evidence syntheses broaden the objectives and include studies on different topics within the field of health care.
4.3. Strengths and limitations
To our knowledge, this is the first overview summarizing current evidence from SRs and MA on different methodological approaches used in several fundamental steps in SR conduct. The overview methodology followed well established guidelines and strict criteria defined for the inclusion of SRs.
There are several limitations related to the nature of the included reviews. Evidence for most of the methods investigated in the included reviews was derived from a limited number of primary studies. Also, the majority of the included SRs may be considered outdated as they were published (or last updated) more than 5 years ago 33 ; only three of the nine SRs have been published in the last 5 years. 14 , 25 , 26 Therefore, important and recent evidence related to these topics may not have been included. Substantial numbers of included SRs were conducted in the field of health, which may limit the generalizability of the findings. Some method evaluations in the included SRs focused on quantitative analyses components and MA conclusions only. As such, the applicability of these findings to SR more broadly is still unclear. 28 Considering the methodological nature of our overview, limiting the inclusion of SRs according to the Cochrane criteria might have resulted in missing some relevant evidence from those reviews without a quality assessment component. 12 , 13 , 29 Although the included SRs performed some form of quality appraisal of the included studies, most of them did not use a standardized RoB tool, which may impact the confidence in their conclusions. Due to the type of outcome measures used for the method evaluations in the primary studies and the included SRs, some of the identified methods have not been validated against a reference standard.
Some limitations in the overview process must be noted. While our literature search was exhaustive covering five bibliographic databases and supplementary search of reference lists, no gray sources or other evidence resources were searched. Also, the search was primarily conducted in health databases, which might have resulted in missing SRs published in other fields. Moreover, only English language SRs were included for feasibility. As the literature search retrieved large number of citations (i.e., 41,556), the title and abstract screening was performed by a single reviewer, calibrated for consistency in the screening process by another reviewer, owing to time and resource limitations. These might have potentially resulted in some errors when retrieving and selecting relevant SRs. The SR methods were grouped based on key elements of each recommended SR step, as agreed by the authors. This categorization pertains to the identified set of methods and should be considered subjective.
5. CONCLUSIONS
This overview identified limited SR‐level evidence on various methodological approaches currently employed during five of the seven fundamental steps in the SR process. Limited evidence was also identified on some methodological modifications currently used to expedite the SR process. Overall, findings highlight the dearth of SRs on SR methodologies, warranting further work to confirm several current recommendations on conventional and expedited SR processes.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Supporting information
APPENDIX A: Detailed search strategies
ACKNOWLEDGMENTS
The first author is supported by a La Trobe University Full Fee Research Scholarship and a Graduate Research Scholarship.
Open Access Funding provided by La Trobe University.
Veginadu P, Calache H, Gussy M, Pandian A, Masood M. An overview of methodological approaches in systematic reviews . J Evid Based Med . 2022; 15 :39–54. 10.1111/jebm.12468 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
Impulsive suicide attempts: a systematic literature review of definitions, characteristics and risk factors
Affiliations.
- 1 Australian Institute for Suicide Research and Prevention, National Centre of Excellence in Suicide Prevention, World Health Organisation Collaborating Centre for Research and Training in Suicide Prevention, Griffith University, Australia. Electronic address: [email protected].
- 2 Australian Institute for Suicide Research and Prevention, National Centre of Excellence in Suicide Prevention, World Health Organisation Collaborating Centre for Research and Training in Suicide Prevention, Griffith University, Australia; Griffith Health Institute, Australia.
- 3 Australian Institute for Suicide Research and Prevention, National Centre of Excellence in Suicide Prevention, World Health Organisation Collaborating Centre for Research and Training in Suicide Prevention, Griffith University, Australia.
- PMID: 25299440
- DOI: 10.1016/j.jad.2014.08.044
Background: Extensive research on impulsive suicide attempts, but lack of agreement on the use of this term indicates the need for a systematic literature review of the area. The aim of this review was to examine definitions and likely correlates of impulsive attempts.
Methods: A search of Medline, Psychinfo, Scopus, Proquest and Web of Knowledge databases was conducted. Additional articles were identified using the cross-referencing function of Google Scholar.
Results: 179 relevant papers were identified. Four different groups of research criteria used to assess suicide attempt impulsivity emerged: (a) time-related criteria, (b) absence of proximal planning/preparations, (c) presence of suicide plan in lifetime/previous year, and (d) other. Subsequent analysis used these criteria to compare results from different studies on 20 most researched hypotheses. Conclusions regarding the characteristics of impulsive attempts are more consistent than those on the risk factors specific to such attempts. No risk factors were identified that uniformly related to suicide attempt impulsivity across all criteria groups, but relationships emerged between separate criteria and specific characteristics of suicide attempters.
Limitations: Only published articles were included. Large inconsistencies in methods of the studies included in this review prevented comparison of effect sizes.
Conclusions: The vast disparities in findings on risk factors for impulsive suicide attempts among different criteria groups suggest the need to address the methodological issues in defining suicide attempt impulsivity before further research into correlates of such attempts can effectively progress. Specific recommendations are offered for necessary research.
Keywords: Impulsive suicide; Impulsivity; Planned suicide; Planning; Suicide attempts.
Copyright © 2014 Elsevier B.V. All rights reserved.
Publication types
- Research Support, Non-U.S. Gov't
- Systematic Review
- Impulsive Behavior*
- Risk Factors
- Suicide, Attempted / psychology*
- Suicide, Attempted / statistics & numerical data*
Defining the Metaverse: A Systematic Literature Review
Ieee account.
- Change Username/Password
- Update Address
Purchase Details
- Payment Options
- Order History
- View Purchased Documents
Profile Information
- Communications Preferences
- Profession and Education
- Technical Interests
- US & Canada: +1 800 678 4333
- Worldwide: +1 732 981 0060
- Contact & Support
- About IEEE Xplore
- Accessibility
- Terms of Use
- Nondiscrimination Policy
- Privacy & Opting Out of Cookies
A not-for-profit organization, IEEE is the world's largest technical professional organization dedicated to advancing technology for the benefit of humanity. © Copyright 2024 IEEE - All rights reserved. Use of this web site signifies your agreement to the terms and conditions.
Advertisement
Toward a framework for selecting indicators of measuring sustainability and circular economy in the agri-food sector: a systematic literature review
- LIFE CYCLE SUSTAINABILITY ASSESSMENT
- Published: 02 March 2022
Cite this article
- Cecilia Silvestri ORCID: orcid.org/0000-0003-2528-601X 1 ,
- Luca Silvestri ORCID: orcid.org/0000-0002-6754-899X 2 ,
- Michela Piccarozzi ORCID: orcid.org/0000-0001-9717-9462 1 &
- Alessandro Ruggieri 1
2866 Accesses
11 Citations
9 Altmetric
Explore all metrics
A Correction to this article was published on 24 March 2022
This article has been updated
The implementation of sustainability and circular economy (CE) models in agri-food production can promote resource efficiency, reduce environmental burdens, and ensure improved and socially responsible systems. In this context, indicators for the measurement of sustainability play a crucial role. Indicators can measure CE strategies aimed to preserve functions, products, components, materials, or embodied energy. Although there is broad literature describing sustainability and CE indicators, no study offers such a comprehensive framework of indicators for measuring sustainability and CE in the agri-food sector.
Starting from this central research gap, a systematic literature review has been developed to measure the sustainability in the agri-food sector and, based on these findings, to understand how indicators are used and for which specific purposes.
The analysis of the results allowed us to classify the sample of articles in three main clusters (“Assessment-LCA,” “Best practice,” and “Decision-making”) and has shown increasing attention to the three pillars of sustainability (triple bottom line). In this context, an integrated approach of indicators (environmental, social, and economic) offers the best solution to ensure an easier transition to sustainability.
Conclusions
The sample analysis facilitated the identification of new categories of impact that deserve attention, such as the cooperation among stakeholders in the supply chain and eco-innovation.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
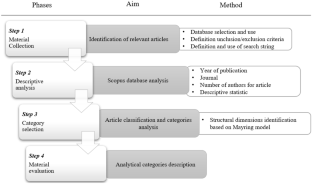
Source: Authors’ elaboration. Notes: The graph shows the temporal distribution of the articles under analysis
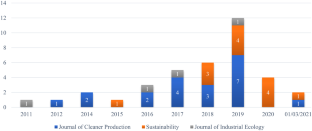
Source: Authors’ elaborations. Notes: The graph shows the time distribution of articles from the three major journals
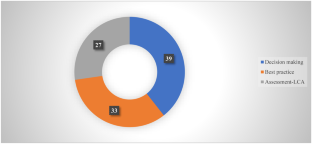
Source: Authors’ elaboration. Notes: The graph shows the composition of the sample according to the three clusters identified by the analysis
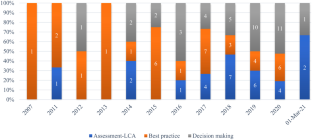
Source: Authors’ elaboration. Notes: The graph shows the distribution of articles over time by cluster
Source: Authors’ elaboration. Notes: The graph shows the network visualization
Source: Authors’ elaboration. Notes: The graph shows the overlay visualization
Source: Authors’ elaboration. Notes: The graph shows the classification of articles by scientific field
Source: Authors’ elaboration. Notes: Article classification based on their cluster to which they belong and scientific field
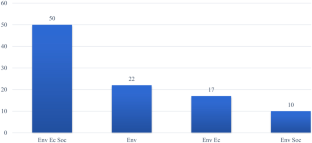
Source: Authors’ elaboration
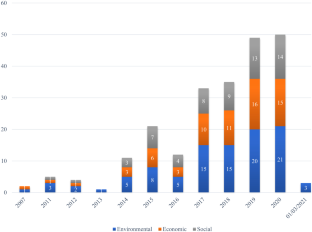
Source: Authors’ elaboration. Notes: The graph shows the distribution of items over time based on TBL
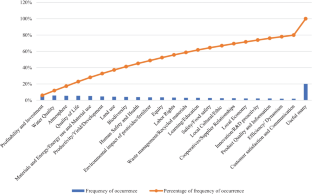
Source: Authors’ elaboration. Notes: The graph shows the Pareto diagram highlighting the most used indicators in literature for measuring sustainability in the agri-food sector
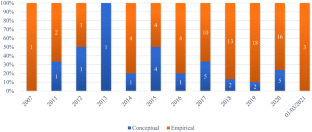
Source: Authors’ elaboration. Notes: The graph shows the distribution over time of articles divided into conceptual and empirical
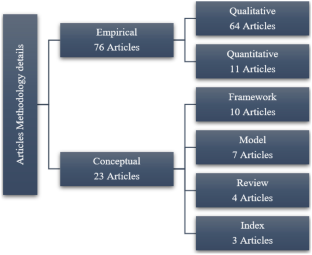
Source: Authors’ elaboration. Notes: The graph shows the classification of articles, divided into conceptual and empirical, in-depth analysis
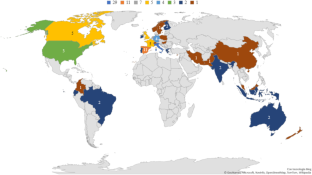
Source: Authors’ elaboration. Notes: The graph shows the geographical distribution of the authors
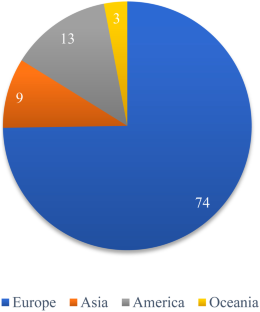
Source: Authors’ elaboration. Notes: The graph shows the distribution of authors according to the continent from which they originate
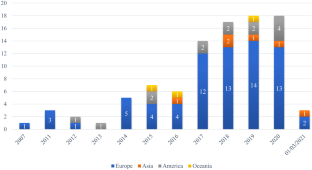
Source: Authors’ elaboration. Notes: The graph shows the time distribution of publication of authors according to the continent from which they originate
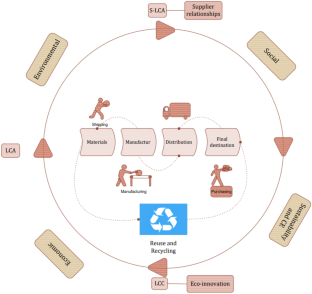
Source: Authors’ elaboration. Notes: Sustainability measurement indicators and impact categories of LCA, S-LCA, and LCC tools should be integrated in order to provide stakeholders with best practices as guidelines and tools to support both decision-making and measurement, according to the circular economy approach
Similar content being viewed by others
The socio-economic performance of agroecology. A review
Ioanna Mouratiadou, Alexander Wezel, … Paolo Bàrberi

The Circular Economy: An Interdisciplinary Exploration of the Concept and Application in a Global Context
Alan Murray, Keith Skene & Kathryn Haynes
Food systems and rural wellbeing: challenges and opportunities
Jim Woodhill, Avinash Kishore, … Saher Hasnain
Change history
24 march 2022.
A Correction to this paper has been published: https://doi.org/10.1007/s11367-022-02038-9
Acero AP, Rodriguez C, Ciroth A (2017) LCIA methods: impact assessment methods in life cycle assessment and their impact categories. Version 1.5.6. Green Delta 1–23
Accorsi R, Versari L, Manzini R (2015) Glass vs. plastic: Life cycle assessment of extra-virgin olive oil bottles across global supply chains. Sustain 7:2818–2840. https://doi.org/10.3390/su7032818
Adjei-Bamfo P, Maloreh-Nyamekye T, Ahenkan A (2019) The role of e-government in sustainable public procurement in developing countries: a systematic literature review. Resour Conserv Recycl 142:189–203. https://doi.org/10.1016/j.resconrec.2018.12.001
Article Google Scholar
Aivazidou E, Tsolakis N, Vlachos D, Iakovou E (2015) Water footprint management policies for agrifood supply chains: a critical taxonomy and a system dynamics modelling approach. Chem Eng Trans 43:115–120. https://doi.org/10.3303/CET1543020
Alhaddi H (2015) Triple bottom line and sustainability: a literature review. Bus Manag Stud 1:6–10
Allaoui H, Guo Y, Sarkis J (2019) Decision support for collaboration planning in sustainable supply chains. J Clean Prod 229:761–774. https://doi.org/10.1016/j.jclepro.2019.04.367
Alshqaqeeq F, Amin Esmaeili M, Overcash M, Twomey J (2020) Quantifying hospital services by carbon footprint: a systematic literature review of patient care alternatives. Resour Conserv Recycl 154:104560. https://doi.org/10.1016/j.resconrec.2019.104560
Anwar F, Chaudhry FN, Nazeer S et al (2016) Causes of ozone layer depletion and its effects on human: review. Atmos Clim Sci 06:129–134. https://doi.org/10.4236/acs.2016.61011
Aquilani B, Silvestri C, Ruggieri A (2016). A Systematic Literature Review on Total Quality Management Critical Success Factors and the Identification of New Avenues of Research. https://doi.org/10.1108/TQM-01-2016-0003
Aramyan L, Hoste R, Van Den Broek W et al (2011) Towards sustainable food production: a scenario study of the European pork sector. J Chain Netw Sci 11:177–189. https://doi.org/10.3920/JCNS2011.Qpork8
Arfini F, Antonioli F, Cozzi E et al (2019) Sustainability, innovation and rural development: the case of Parmigiano-Reggiano PDO. Sustain 11:1–17. https://doi.org/10.3390/su11184978
Assembly UG (2005) Resolution adopted by the general assembly. New York, NY
Avilés-Palacios C, Rodríguez-Olalla A (2021) The sustainability of waste management models in circular economies. Sustain 13:1–19. https://doi.org/10.3390/su13137105
Azevedo SG, Silva ME, Matias JCO, Dias GP (2018) The influence of collaboration initiatives on the sustainability of the cashew supply chain. Sustain 10:1–29. https://doi.org/10.3390/su10062075
Bajaj S, Garg R, Sethi M (2016) Total quality management: a critical literature review using Pareto analysis. Int J Product Perform Manag 67:128–154
Banasik A, Kanellopoulos A, Bloemhof-Ruwaard JM, Claassen GDH (2019) Accounting for uncertainty in eco-efficient agri-food supply chains: a case study for mushroom production planning. J Clean Prod 216:249–256. https://doi.org/10.1016/j.jclepro.2019.01.153
Barth H, Ulvenblad PO, Ulvenblad P (2017) Towards a conceptual framework of sustainable business model innovation in the agri-food sector: a systematic literature review. Sustain 9. https://doi.org/10.3390/su9091620
Bastas A, Liyanage K (2018) Sustainable supply chain quality management: a systematic review
Beckerman W (1992) Economic growth and the environment: whose growth? Whose environment? World Dev 20:481–496. https://doi.org/10.1016/0305-750X(92)90038-W
Belaud JP, Prioux N, Vialle C, Sablayrolles C (2019) Big data for agri-food 4.0: application to sustainability management for by-products supply chain. Comput Ind 111:41–50. https://doi.org/10.1016/j.compind.2019.06.006
Bele B, Norderhaug A, Sickel H (2018) Localized agri-food systems and biodiversity. Agric 8. https://doi.org/10.3390/agriculture8020022
Bilali H El, Calabrese G, Iannetta M et al (2020) Environmental sustainability of typical agro-food products: a scientifically sound and user friendly approach. New Medit 19:69–83. https://doi.org/10.30682/nm2002e
Blanc S, Massaglia S, Brun F et al (2019) Use of bio-based plastics in the fruit supply chain: an integrated approach to assess environmental, economic, and social sustainability. Sustain 11. https://doi.org/10.3390/su11092475
Bloemhof JM, van der Vorst JGAJ, Bastl M, Allaoui H (2015) Sustainability assessment of food chain logistics. Int J Logist Res Appl 18:101–117. https://doi.org/10.1080/13675567.2015.1015508
Bonisoli L, Galdeano-Gómez E, Piedra-Muñoz L (2018) Deconstructing criteria and assessment tools to build agri-sustainability indicators and support farmers’ decision-making process. J Clean Prod 182:1080–1094. https://doi.org/10.1016/j.jclepro.2018.02.055
Bonisoli L, Galdeano-Gómez E, Piedra-Muñoz L, Pérez-Mesa JC (2019) Benchmarking agri-food sustainability certifications: evidences from applying SAFA in the Ecuadorian banana agri-system. J Clean Prod 236. https://doi.org/10.1016/j.jclepro.2019.07.054
Bornmann L, Haunschild R, Hug SE (2018) Visualizing the context of citations referencing papers published by Eugene Garfield: a new type of keyword co-occurrence analysis. Scientometrics 114:427–437. https://doi.org/10.1007/s11192-017-2591-8
Boulding KE (1966) The economics of the coming spaceship earth. New York, 1-17
Bracquené E, Dewulf W, Duflou JR (2020) Measuring the performance of more circular complex product supply chains. Resour Conserv Recycl 154:104608. https://doi.org/10.1016/j.resconrec.2019.104608
Burck J, Hagen U, Bals C et al (2021) Climate Change Performance Index
Calisto Friant M, Vermeulen WJV, Salomone R (2020) A typology of circular economy discourses: navigating the diverse visions of a contested paradigm. Resour Conserv Recycl 161:104917. https://doi.org/10.1016/j.resconrec.2020.104917
Campbell BM, Beare DJ, Bennett EM et al (2017) Agriculture production as a major driver of the earth system exceeding planetary boundaries. Ecol Soc 22. https://doi.org/10.5751/ES-09595-220408
Capitanio F, Coppola A, Pascucci S (2010) Product and process innovation in the Italian food industry. Agribusiness 26:503–518. https://doi.org/10.1002/agr.20239
Caputo P, Zagarella F, Cusenza MA et al (2020) Energy-environmental assessment of the UIA-OpenAgri case study as urban regeneration project through agriculture. Sci Total Environ 729:138819. https://doi.org/10.1016/j.scitotenv.2020.138819
Article CAS Google Scholar
Chabowski BR, Mena JA, Gonzalez-Padron TL (2011) The structure of sustainability research in marketing, 1958–2008: a basis for future research opportunities. J Acad Mark Sci 39:55–70. https://doi.org/10.1007/s11747-010-0212-7
Chadegani AA, Salehi H, Yunus M et al (2017) A comparison between two main academic literature collections : Web of Science and Scopus databases. Asian Soc Sci 9:18–26. https://doi.org/10.5539/ass.v9n5p18
Chams N, Guesmi B, Gil JM (2020) Beyond scientific contribution: assessment of the societal impact of research and innovation to build a sustainable agri-food sector. J Environ Manage 264. https://doi.org/10.1016/j.jenvman.2020.110455
Chandrakumar C, McLaren SJ, Jayamaha NP, Ramilan T (2019) Absolute sustainability-based life cycle assessment (ASLCA): a benchmarking approach to operate agri-food systems within the 2°C global carbon budget. J Ind Ecol 23:906–917. https://doi.org/10.1111/jiec.12830
Chaparro-Africano AM (2019) Toward generating sustainability indicators for agroecological markets. Agroecol Sustain Food Syst 43:40–66. https://doi.org/10.1080/21683565.2019.1566192
Colicchia C, Strozzi F (2012) Supply chain risk management: a new methodology for a systematic literature review
Conca L, Manta F, Morrone D, Toma P (2021) The impact of direct environmental, social, and governance reporting: empirical evidence in European-listed companies in the agri-food sector. Bus Strateg Environ 30:1080–1093. https://doi.org/10.1002/bse.2672
Coppola A, Ianuario S, Romano S, Viccaro M (2020) Corporate social responsibility in agri-food firms: the relationship between CSR actions and firm’s performance. AIMS Environ Sci 7:542–558. https://doi.org/10.3934/environsci.2020034
Corona B, Shen L, Reike D et al (2019) Towards sustainable development through the circular economy—a review and critical assessment on current circularity metrics. Resour Conserv Recycl 151:104498. https://doi.org/10.1016/j.resconrec.2019.104498
Correia MS (2019) Sustainability: An overview of the triple bottom line and sustainability implementation. Int J Strateg Eng 2:29–38. https://doi.org/10.4018/IJoSE.2019010103
Coteur I, Marchand F, Debruyne L, Lauwers L (2019) Structuring the myriad of sustainability assessments in agri-food systems: a case in Flanders. J Clean Prod 209:472–480. https://doi.org/10.1016/j.jclepro.2018.10.066
CREA (2020) L’agricoltura italiana conta 2019
Crenna E, Sala S, Polce C, Collina E (2017) Pollinators in life cycle assessment: towards a framework for impact assessment. J Clean Prod 140:525–536. https://doi.org/10.1016/j.jclepro.2016.02.058
D’Eusanio M, Serreli M, Zamagni A, Petti L (2018) Assessment of social dimension of a jar of honey: a methodological outline. J Clean Prod 199:503–517. https://doi.org/10.1016/j.jclepro.2018.07.157
Dania WAP, Xing K, Amer Y (2018) Collaboration behavioural factors for sustainable agri-food supply chains: a systematic review. J Clean Prod 186:851–864
De Pascale A, Arbolino R, Szopik-Depczyńska K et al (2021) A systematic review for measuring circular economy: the 61 indicators. J Clean Prod 281. https://doi.org/10.1016/j.jclepro.2020.124942
De Schoenmakere M, Gillabel J (2017) Circular by design: products in the circular economy
Del Borghi A, Gallo M, Strazza C, Del Borghi M (2014) An evaluation of environmental sustainability in the food industry through life cycle assessment: the case study of tomato products supply chain. J Clean Prod 78:121–130. https://doi.org/10.1016/j.jclepro.2014.04.083
Del Borghi A, Strazza C, Magrassi F et al (2018) Life cycle assessment for eco-design of product–package systems in the food industry—the case of legumes. Sustain Prod Consum 13:24–36. https://doi.org/10.1016/j.spc.2017.11.001
Denyer D, Tranfield D (2009) Producing a systematic review. In: Buchanan B (ed) The sage handbook of organization research methods. Sage Publications Ltd, Cornwall, pp 671–689
Google Scholar
Dietz T, Grabs J, Chong AE (2019) Mainstreamed voluntary sustainability standards and their effectiveness: evidence from the Honduran coffee sector. Regul Gov. https://doi.org/10.1111/rego.12239
Dixon-Woods M (2011) Using framework-based synthesis for conducting reviews of qualitative studies. BMC Med 9:9–10. https://doi.org/10.1186/1741-7015-9-39
do Canto NR, Bossle MB, Marques L, Dutra M, (2020) Supply chain collaboration for sustainability: a qualitative investigation of food supply chains in Brazil. Manag Environ Qual an Int J. https://doi.org/10.1108/MEQ-12-2019-0275
dos Santos RR, Guarnieri P (2020) Social gains for artisanal agroindustrial producers induced by cooperation and collaboration in agri-food supply chain. Soc Responsib J. https://doi.org/10.1108/SRJ-09-2019-0323
Doukidis GI, Matopoulos A, Vlachopoulou M, Manthou V, Manos B (2007) A conceptual framework for supply chain collaboration: empirical evidence from the agri‐food industry. Supply Chain Manag an Int Journal 12:177–186. https://doi.org/10.1108/13598540710742491
Durach CF, Kembro J, Wieland A (2017) A new paradigm for systematic literature reviews in supply chain management. J Supply Chain Manag 53:67–85. https://doi.org/10.1111/jscm.12145
Durán-Sánchez A, Álvarez-García J, Río-Rama D, De la Cruz M (2018) Sustainable water resources management: a bibliometric overview. Water 10:1–19. https://doi.org/10.3390/w10091191
Duru M, Therond O (2015) Livestock system sustainability and resilience in intensive production zones: which form of ecological modernization? Reg Environ Chang 15:1651–1665. https://doi.org/10.1007/s10113-014-0722-9
Edison Fondazione (2019) Le eccellenze agricole italiane. I primati europei e mondiali dell’Italia nei prodotti vegetali. Milan (IT)
Ehrenfeld JR (2005) The roots of sustainability. MIT Sloan Manag Rev 46(2)46:23–25
Elia V, Gnoni MG, Tornese F (2017) Measuring circular economy strategies through index methods: a critical analysis. J Clean Prod 142:2741–2751. https://doi.org/10.1016/j.jclepro.2016.10.196
Elkington J (1997) Cannibals with forks : the triple bottom line of 21st century business. Capstone, Oxford
Esposito B, Sessa MR, Sica D, Malandrino O (2020) Towards circular economy in the agri-food sector. A systematic literature review. Sustain 12. https://doi.org/10.3390/SU12187401
European Commission (2018) Agri-food trade in 2018
European Commission (2019) Monitoring EU agri-food trade: development until September 2019
Eurostat (2018) Small and large farms in the EU - statistics from the farm structure survey
FAO (2011) Biodiversity for food and agriculture. Italy, Rome
FAO (2012) Energy-smart food at FAO: an overview. Italy, Rome
FAO (2014) Food wastage footprint: fool cost-accounting
FAO (2016) The state of food and agriculture climate change, agriculture and food security. Italy, Rome
FAO (2017) The future of food and agriculture: trends and challenges. Italy, Rome
FAO (2020) The state of food security and nutrition in the world. Transforming Food Systems for Affordable Healthy Diets. Rome, Italy
Fassio F, Tecco N (2019) Circular economy for food: a systemic interpretation of 40 case histories in the food system in their relationships with SDGs. Systems 7:43. https://doi.org/10.3390/systems7030043
Fathollahi A, Coupe SJ (2021) Life cycle assessment (LCA) and life cycle costing (LCC) of road drainage systems for sustainability evaluation: quantifying the contribution of different life cycle phases. Sci Total Environ 776:145937. https://doi.org/10.1016/j.scitotenv.2021.145937
Ferreira VJ, Arnal ÁJ, Royo P et al (2019) Energy and resource efficiency of electroporation-assisted extraction as an emerging technology towards a sustainable bio-economy in the agri-food sector. J Clean Prod 233:1123–1132. https://doi.org/10.1016/j.jclepro.2019.06.030
Fiksel J (2006) A framework for sustainable remediation. JOM 8:15–22. https://doi.org/10.1021/es202595w
Flick U (2014) An introduction to qualitative research
Franciosi C, Voisin A, Miranda S et al (2020) Measuring maintenance impacts on sustainability of manufacturing industries : from a systematic literature review to a framework proposal. J Clean Prod 260:1–19. https://doi.org/10.1016/j.jclepro.2020.121065
Gaitán-Cremaschi D, Meuwissen MPM, Oude AGJML (2017) Total factor productivity: a framework for measuring agri-food supply chain performance towards sustainability. Appl Econ Perspect Policy 39:259–285. https://doi.org/10.1093/aepp/ppw008
Galdeano-Gómez E, Zepeda-Zepeda JA, Piedra-Muñoz L, Vega-López LL (2017) Family farm’s features influencing socio-economic sustainability: an analysis of the agri-food sector in southeast Spain. New Medit 16:50–61
Gallopín G, Herrero LMJ, Rocuts A (2014) Conceptual frameworks and visual interpretations of sustainability. Int J Sustain Dev 17:298–326. https://doi.org/10.1504/IJSD.2014.064183
Gallopín GC (2003) Sostenibilidad y desarrollo sostenible: un enfoque sistémico. Cepal, LATIN AMERICA
Garnett T (2013) Food sustainability: problems, perspectives and solutions. Proc Nutr Soc 72:29–39. https://doi.org/10.1017/S0029665112002947
Garofalo P, D’Andrea L, Tomaiuolo M et al (2017) Environmental sustainability of agri-food supply chains in Italy: the case of the whole-peeled tomato production under life cycle assessment methodology. J Food Eng 200:1–12. https://doi.org/10.1016/j.jfoodeng.2016.12.007
Gava O, Bartolini F, Venturi F et al (2018) A reflection of the use of the life cycle assessment tool for agri-food sustainability. Sustain 11. https://doi.org/10.3390/su11010071
Gazzola P, Querci E (2017) The connection between the quality of life and sustainable ecological development. Eur Sci J 7881:1857–7431
Geissdoerfer M, Savaget P, Bocken N, Hultink EJ (2017) The circular economy – a new sustainability paradigm ? The circular economy – a new sustainability paradigm ? J Clean Prod 143:757–768. https://doi.org/10.1016/j.jclepro.2016.12.048
Georgescu-Roegen N (1971) The entropy low and the economic process. Harward University Press, Cambridge Mass
Book Google Scholar
Gerbens-Leenes PW, Moll HC, Schoot Uiterkamp AJM (2003) Design and development of a measuring method for environmental sustainability in food production systems. Ecol Econ 46:231–248. https://doi.org/10.1016/S0921-8009(03)00140-X
Gésan-Guiziou G, Alaphilippe A, Aubin J et al (2020) Diversity and potentiality of multi-criteria decision analysis methods for agri-food research. Agron Sustain Dev 40. https://doi.org/10.1007/s13593-020-00650-3
Ghisellini P, Cialani C, Ulgiati S (2016) A review on circular economy: the expected transition to a balanced interplay of environmental and economic systems. J Clean Prod 114:11–32. https://doi.org/10.1016/j.jclepro.2015.09.007
Godoy-Durán Á, Galdeano- Gómez E, Pérez-Mesa JC, Piedra-Muñoz L (2017) Assessing eco-efficiency and the determinants of horticultural family-farming in southeast Spain. J Environ Manage 204:594–604. https://doi.org/10.1016/j.jenvman.2017.09.037
Gold S, Kunz N, Reiner G (2017) Sustainable global agrifood supply chains: exploring the barriers. J Ind Ecol 21:249–260. https://doi.org/10.1111/jiec.12440
Goucher L, Bruce R, Cameron DD et al (2017) The environmental impact of fertilizer embodied in a wheat-to-bread supply chain. Nat Plants 3:1–5. https://doi.org/10.1038/nplants.2017.12
Green A, Nemecek T, Chaudhary A, Mathys A (2020) Assessing nutritional, health, and environmental sustainability dimensions of agri-food production. Glob Food Sec 26:100406. https://doi.org/10.1016/j.gfs.2020.100406
Guinée JB, Heijungs R, Huppes G et al (2011) Life cycle assessment: past, present, and future †. Environ Sci Technol 45:90–96. https://doi.org/10.1021/es101316v
Guiomar N, Godinho S, Pinto-Correia T et al (2018) Typology and distribution of small farms in Europe: towards a better picture. Land Use Policy 75:784–798. https://doi.org/10.1016/j.landusepol.2018.04.012
Gunasekaran A, Patel C, McGaughey RE (2004) A framework for supply chain performance measurement. Int J Prod Econ 87:333–347. https://doi.org/10.1016/j.ijpe.2003.08.003
Gunasekaran A, Patel C, Tirtiroglu E (2001) Performance measures and metrics in a supply chain environment. Int J Oper Prod Manag 21:71–87. https://doi.org/10.1108/01443570110358468
Hamam M, Chinnici G, Di Vita G et al (2021) Circular economy models in agro-food systems: a review. Sustain 13
Harun SN, Hanafiah MM, Aziz NIHA (2021) An LCA-based environmental performance of rice production for developing a sustainable agri-food system in Malaysia. Environ Manage 67:146–161. https://doi.org/10.1007/s00267-020-01365-7
Harvey M, Pilgrim S (2011) The new competition for land: food, energy, and climate change. Food Policy 36:S40–S51. https://doi.org/10.1016/j.foodpol.2010.11.009
Hawkes C, Ruel MT (2006) Understanding the links between agriculture and health. DC: International Food Policy Research Institute. Washington, USA
Hellweg S, Milà i Canals L (2014) Emerging approaches, challenges and opportunities in life cycle assessment. Science (80)344:1109LP–1113. https://doi.org/10.1126/science.1248361
Higgins V, Dibden J, Cocklin C (2015) Private agri-food governance and greenhouse gas abatement: constructing a corporate carbon economy. Geoforum 66:75–84. https://doi.org/10.1016/j.geoforum.2015.09.012
Hill T (1995) Manufacturing strategy: text and cases., Macmillan
Hjeresen DD, Gonzales R (2020) Green chemistry promote sustainable agriculture?The rewards are higher yields and less environmental contamination. Environemental Sci Techonology 103–107
Horne R, Grant T, Verghese K (2009) Life cycle assessment: principles, practice, and prospects. Csiro Publishing, Collingwood, Australia
Horton P, Koh L, Guang VS (2016) An integrated theoretical framework to enhance resource efficiency, sustainability and human health in agri-food systems. J Clean Prod 120:164–169. https://doi.org/10.1016/j.jclepro.2015.08.092
Hospido A, Davis J, Berlin J, Sonesson U (2010) A review of methodological issues affecting LCA of novel food products. Int J Life Cycle Assess 15:44–52. https://doi.org/10.1007/s11367-009-0130-4
Huffman T, Liu J, Green M et al (2015) Improving and evaluating the soil cover indicator for agricultural land in Canada. Ecol Indic 48:272–281. https://doi.org/10.1016/j.ecolind.2014.07.008
Ilbery B, Maye D (2005) Food supply chains and sustainability: evidence from specialist food producers in the Scottish/English borders. Land Use Policy 22:331–344. https://doi.org/10.1016/j.landusepol.2004.06.002
Ingrao C, Faccilongo N, Valenti F et al (2019) Tomato puree in the Mediterranean region: an environmental life cycle assessment, based upon data surveyed at the supply chain level. J Clean Prod 233:292–313. https://doi.org/10.1016/j.jclepro.2019.06.056
Iocola I, Angevin F, Bockstaller C et al (2020) An actor-oriented multi-criteria assessment framework to support a transition towards sustainable agricultural systems based on crop diversification. Sustain 12. https://doi.org/10.3390/su12135434
Irabien A, Darton RC (2016) Energy–water–food nexus in the Spanish greenhouse tomato production. Clean Technol Environ Policy 18:1307–1316. https://doi.org/10.1007/s10098-015-1076-9
ISO 14040:2006 (2006) Environmental management — life cycle assessment — principles and framework
ISO 14044:2006 (2006) Environmental management — life cycle assessment — requirements and guidelines
ISO 15392:2008 (2008) Sustainability in building construction–general principles
Istat (2019) Andamento dell’economia agricola
Jaakkola E (2020) Designing conceptual articles : four approaches. AMS Rev 1–9. https://doi.org/10.1007/s13162-020-00161-0
Jin R, Yuan H, Chen Q (2019) Science mapping approach to assisting the review of construction and demolition waste management research published between 2009 and 2018. Resour Conserv Recycl 140:175–188. https://doi.org/10.1016/j.resconrec.2018.09.029
Johnston P, Everard M, Santillo D, Robèrt KH (2007) Reclaiming the definition of sustainability. Environ Sci Pollut Res Int 14:60–66. https://doi.org/10.1065/espr2007.01.375
Jorgensen SE, Burkhard B, Müller F (2013) Twenty volumes of ecological indicators-an accounting short review. Ecol Indic 28:4–9. https://doi.org/10.1016/j.ecolind.2012.12.018
Joshi S, Sharma M, Kler R (2020) Modeling circular economy dimensions in agri-tourism clusters: sustainable performance and future research directions. Int J Math Eng Manag Sci 5:1046–1061. https://doi.org/10.33889/IJMEMS.2020.5.6.080
Kamilaris A, Gao F, Prenafeta-Boldu FX, Ali MI (2017) Agri-IoT: a semantic framework for Internet of Things-enabled smart farming applications. In: 2016 IEEE 3rd World Forum on Internet of Things, WF-IoT 2016. pp 442–447
Karuppusami G, Gandhinathan R (2006) Pareto analysis of critical success factors of total quality management: a literature review and analysis. TQM Mag 18:372–385. https://doi.org/10.1108/09544780610671048
Kates RW, Parris TM, Leiserowitz AA (2005) What is sustainable development? Goals, indicators, values, and practice. Environ Sci Policy Sustain Dev 47:8–21. https://doi.org/10.1080/00139157.2005.10524444
Khounani Z, Hosseinzadeh-Bandbafha H, Moustakas K et al (2021) Environmental life cycle assessment of different biorefinery platforms valorizing olive wastes to biofuel, phosphate salts, natural antioxidant, and an oxygenated fuel additive (triacetin). J Clean Prod 278:123916. https://doi.org/10.1016/j.jclepro.2020.123916
Kitchenham B, Charters S (2007) Guidelines for performing systematic literature reviews in software engineering version 2.3. Engineering 45. https://doi.org/10.1145/1134285.1134500
Korhonen J, Nuur C, Feldmann A, Birkie SE (2018) Circular economy as an essentially contested concept. J Clean Prod 175:544–552. https://doi.org/10.1016/j.jclepro.2017.12.111
Kuisma M, Kahiluoto H (2017) Biotic resource loss beyond food waste: agriculture leaks worst. Resour Conserv Recycl 124:129–140. https://doi.org/10.1016/j.resconrec.2017.04.008
Laso J, Hoehn D, Margallo M et al (2018) Assessing energy and environmental efficiency of the Spanish agri-food system using the LCA/DEA methodology. Energies 11. https://doi.org/10.3390/en11123395
Lee KM (2007) So What is the “triple bottom line”? Int J Divers Organ Communities Nations Annu Rev 6:67–72. https://doi.org/10.18848/1447-9532/cgp/v06i06/39283
Lehmann RJ, Hermansen JE, Fritz M et al (2011) Information services for European pork chains - closing gaps in information infrastructures. Comput Electron Agric 79:125–136. https://doi.org/10.1016/j.compag.2011.09.002
León-Bravo V, Caniato F, Caridi M, Johnsen T (2017) Collaboration for sustainability in the food supply chain: a multi-stage study in Italy. Sustainability 9:1253
Lepage A (2009) The quality of life as attribute of sustainability. TQM J 21:105–115. https://doi.org/10.1108/17542730910938119
Li CZ, Zhao Y, Xiao B et al (2020) Research trend of the application of information technologies in construction and demolition waste management. J Clean Prod 263. https://doi.org/10.1016/j.jclepro.2020.121458
Lo Giudice A, Mbohwa C, Clasadonte MT, Ingrao C (2014) Life cycle assessment interpretation and improvement of the Sicilian artichokes production. Int J Environ Res 8:305–316. https://doi.org/10.22059/ijer.2014.721
Lueddeckens S, Saling P, Guenther E (2020) Temporal issues in life cycle assessment—a systematic review. Int J Life Cycle Assess 25:1385–1401. https://doi.org/10.1007/s11367-020-01757-1
Luo J, Ji C, Qiu C, Jia F (2018) Agri-food supply chain management: bibliometric and content analyses. Sustain 10. https://doi.org/10.3390/su10051573
Lynch J, Donnellan T, Finn JA et al (2019) Potential development of Irish agricultural sustainability indicators for current and future policy evaluation needs. J Environ Manage 230:434–445. https://doi.org/10.1016/j.jenvman.2018.09.070
MacArthur E (2013) Towards the circular economy. J Ind Ecol 2:23–44
MacArthur E (2017) Delivering the circular economy a toolkit for policymakers, The Ellen MacArthur Foundation
MacInnis DJ (2011) A framework for conceptual. J Mark 75:136–154. https://doi.org/10.1509/jmkg.75.4.136
Mangla SK, Luthra S, Rich N et al (2018) Enablers to implement sustainable initiatives in agri-food supply chains. Int J Prod Econ 203:379–393. https://doi.org/10.1016/j.ijpe.2018.07.012
Marotta G, Nazzaro C, Stanco M (2017) How the social responsibility creates value: models of innovation in Italian pasta industry. Int J Glob Small Bus 9:144–167. https://doi.org/10.1504/IJGSB.2017.088923
Martucci O, Arcese G, Montauti C, Acampora A (2019) Social aspects in the wine sector: comparison between social life cycle assessment and VIVA sustainable wine project indicators. Resources 8. https://doi.org/10.3390/resources8020069
Mayring P (2004) Forum : Qualitative social research Sozialforschung 2. History of content analysis. A Companion to Qual Res 1:159–176
McKelvey B (2002) Managing coevolutionary dynamics. In: 18th EGOS Conference. Barcelona, Spain, pp 1–21
McMichael AJ, Butler CD, Folke C (2003) New visions for addressing sustainability. Science (80- ) 302:1191–1920
Mehmood A, Ahmed S, Viza E et al (2021) Drivers and barriers towards circular economy in agri-food supply chain: a review. Bus Strateg Dev 1–17. https://doi.org/10.1002/bsd2.171
Mella P, Gazzola P (2011) Sustainability and quality of life: the development model. In: Kapounek S (ed) Enterprise and competitive environment. Mendel University: Brno, Czechia. 542–551
Merli R, Preziosi M, Acampora A (2018) How do scholars approach the circular economy ? A systematic literature review. J Clean Prod 178:703–722. https://doi.org/10.1016/j.jclepro.2017.12.112
Merli R, Preziosi M, Acampora A et al (2020) Recycled fibers in reinforced concrete: a systematic literature review. J Clean Prod 248:119207. https://doi.org/10.1016/j.jclepro.2019.119207
Miglietta PP, Morrone D (2018) Managing water sustainability: virtual water flows and economic water productivity assessment of the wine trade between Italy and the Balkans. Sustain 10. https://doi.org/10.3390/su10020543
Mitchell MGE, Chan KMA, Newlands NK, Ramankutty N (2020) Spatial correlations don’t predict changes in agricultural ecosystem services: a Canada-wide case study. Front Sustain Food Syst 4:1–17. https://doi.org/10.3389/fsufs.2020.539892
Moraga G, Huysveld S, Mathieux F et al (2019) Circular economy indicators: what do they measure?. Resour Conserv Recycl 146:452–461. https://doi.org/10.1016/j.resconrec.2019.03.045
Morrissey JE, Dunphy NP (2015) Towards sustainable agri-food systems: the role of integrated sustainability and value assessment across the supply-chain. Int J Soc Ecol Sustain Dev 6:41–58. https://doi.org/10.4018/IJSESD.2015070104
Moser G (2009) Quality of life and sustainability: toward person-environment congruity. J Environ Psychol 29:351–357. https://doi.org/10.1016/j.jenvp.2009.02.002
Muijs D (2010) Doing quantitative research in education with SPSS. London
Muller MF, Esmanioto F, Huber N, Loures ER (2019) A systematic literature review of interoperability in the green Building Information Modeling lifecycle. J Clean Prod 223:397–412. https://doi.org/10.1016/j.jclepro.2019.03.114
Muradin M, Joachimiak-Lechman K, Foltynowicz Z (2018) Evaluation of eco-efficiency of two alternative agricultural biogas plants. Appl Sci 8. https://doi.org/10.3390/app8112083
Naseer MA, ur R, Ashfaq M, Hassan S, et al (2019) Critical issues at the upstream level in sustainable supply chain management of agri-food industries: evidence from Pakistan’s citrus industry. Sustain 11:1–19. https://doi.org/10.3390/su11051326
Nattassha R, Handayati Y, Simatupang TM, Siallagan M (2020) Understanding circular economy implementation in the agri-food supply chain: the case of an Indonesian organic fertiliser producer. Agric Food Secur 9:1–16. https://doi.org/10.1186/s40066-020-00264-8
Nazari-Sharabian M, Ahmad S, Karakouzian M (2018) Climate change and eutrophication: a short review. Eng Technol Appl Sci Res 8:3668–3672. https://doi.org/10.5281/zenodo.2532694
Nazir N (2017) Understanding life cycle thinking and its practical application to agri-food system. Int J Adv Sci Eng Inf Technol 7:1861–1870. https://doi.org/10.18517/ijaseit.7.5.3578
Negra C, Remans R, Attwood S et al (2020) Sustainable agri-food investments require multi-sector co-development of decision tools. Ecol Indic 110:105851. https://doi.org/10.1016/j.ecolind.2019.105851
Newsham KK, Robinson SA (2009) Responses of plants in polar regions to UVB exposure: a meta-analysis. Glob Chang Biol 15:2574–2589. https://doi.org/10.1111/j.1365-2486.2009.01944.x
Niemeijer D, de Groot RS (2008) A conceptual framework for selecting environmental indicator sets. Ecol Indic 8:14–25. https://doi.org/10.1016/j.ecolind.2006.11.012
Niero M, Kalbar PP (2019) Coupling material circularity indicators and life cycle based indicators: a proposal to advance the assessment of circular economy strategies at the product level. Resour Conserv Recycl 140:305–312. https://doi.org/10.1016/j.resconrec.2018.10.002
Nikolaou IE, Tsagarakis KP (2021) An introduction to circular economy and sustainability: some existing lessons and future directions. Sustain Prod Consum 28:600–609. https://doi.org/10.1016/j.spc.2021.06.017
Notarnicola B, Hayashi K, Curran MA, Huisingh D (2012) Progress in working towards a more sustainable agri-food industry. J Clean Prod 28:1–8. https://doi.org/10.1016/j.jclepro.2012.02.007
Notarnicola B, Tassielli G, Renzulli PA, Monforti F (2017) Energy flows and greenhouses gases of EU (European Union) national breads using an LCA (life cycle assessment) approach. J Clean Prod 140:455–469. https://doi.org/10.1016/j.jclepro.2016.05.150
Opferkuch K, Caeiro S, Salomone R, Ramos TB (2021) Circular economy in corporate sustainability reporting: a review of organisational approaches. Bus Strateg Environ 1–22. https://doi.org/10.1002/bse.2854
Padilla-Rivera A, do Carmo BBT, Arcese G, Merveille N, (2021) Social circular economy indicators: selection through fuzzy delphi method. Sustain Prod Consum 26:101–110. https://doi.org/10.1016/j.spc.2020.09.015
Pagotto M, Halog A (2016) Towards a circular economy in Australian agri-food industry: an application of input-output oriented approaches for analyzing resource efficiency and competitiveness potential. J Ind Ecol 20:1176–1186. https://doi.org/10.1111/jiec.12373
Parent G, Lavallée S (2011) LCA potentials and limits within a sustainable agri-food statutory framework. Global food insecurity. Springer, Netherlands, Dordrecht, pp 161–171
Chapter Google Scholar
Pattey E, Qiu G (2012) Trends in primary particulate matter emissions from Canadian agriculture. J Air Waste Manag Assoc 62:737–747. https://doi.org/10.1080/10962247.2012.672058
Pauliuk S (2018) Critical appraisal of the circular economy standard BS 8001:2017 and a dashboard of quantitative system indicators for its implementation in organizations. Resour Conserv Recycl 129:81–92. https://doi.org/10.1016/j.resconrec.2017.10.019
Peano C, Migliorini P, Sottile F (2014) A methodology for the sustainability assessment of agri-food systems: an application to the slow food presidia project. Ecol Soc 19. https://doi.org/10.5751/ES-06972-190424
Peano C, Tecco N, Dansero E et al (2015) Evaluating the sustainability in complex agri-food systems: the SAEMETH framework. Sustain 7:6721–6741. https://doi.org/10.3390/su7066721
Pearce DW, Turner RK (1990) Economics of natural resources and the environment. Harvester Wheatsheaf, Hemel Hempstead, Herts
Pelletier N (2018) Social sustainability assessment of Canadian egg production facilities: methods, analysis, and recommendations. Sustain 10:1–17. https://doi.org/10.3390/su10051601
Peña C, Civit B, Gallego-Schmid A et al (2021) Using life cycle assessment to achieve a circular economy. Int J Life Cycle Assess 26:215–220. https://doi.org/10.1007/s11367-020-01856-z
Perez Neira D (2016) Energy sustainability of Ecuadorian cacao export and its contribution to climate change. A case study through product life cycle assessment. J Clean Prod 112:2560–2568. https://doi.org/10.1016/j.jclepro.2015.11.003
Pérez-Neira D, Grollmus-Venegas A (2018) Life-cycle energy assessment and carbon footprint of peri-urban horticulture. A comparative case study of local food systems in Spain. Landsc Urban Plan 172:60–68. https://doi.org/10.1016/j.landurbplan.2018.01.001
Pérez-Pons ME, Plaza-Hernández M, Alonso RS et al (2021) Increasing profitability and monitoring environmental performance: a case study in the agri-food industry through an edge-iot platform. Sustain 13:1–16. https://doi.org/10.3390/su13010283
Petti L, Serreli M, Di Cesare S (2018) Systematic literature review in social life cycle assessment. Int J Life Cycle Assess 23:422–431. https://doi.org/10.1007/s11367-016-1135-4
Pieroni MPP, McAloone TC, Pigosso DCA (2019) Business model innovation for circular economy and sustainability: a review of approaches. J Clean Prod 215:198–216. https://doi.org/10.1016/j.jclepro.2019.01.036
Polit DF, Beck CT (2004) Nursing research: principles and methods. Lippincott Williams & Wilkins, Philadelphia, PA
Porkka M, Gerten D, Schaphoff S et al (2016) Causes and trends of water scarcity in food production. Environ Res Lett 11:015001. https://doi.org/10.1088/1748-9326/11/1/015001
Prajapati H, Kant R, Shankar R (2019) Bequeath life to death: state-of-art review on reverse logistics. J Clean Prod 211:503–520. https://doi.org/10.1016/j.jclepro.2018.11.187
Priyadarshini P, Abhilash PC (2020) Policy recommendations for enabling transition towards sustainable agriculture in India. Land Use Policy 96:104718. https://doi.org/10.1016/j.landusepol.2020.104718
Pronti A, Coccia M (2020) Multicriteria analysis of the sustainability performance between agroecological and conventional coffee farms in the East Region of Minas Gerais (Brazil). Renew Agric Food Syst. https://doi.org/10.1017/S1742170520000332
Rabadán A, González-Moreno A, Sáez-Martínez FJ (2019) Improving firms’ performance and sustainability: the case of eco-innovation in the agri-food industry. Sustain 11. https://doi.org/10.3390/su11205590
Raut RD, Luthra S, Narkhede BE et al (2019) Examining the performance oriented indicators for implementing green management practices in the Indian agro sector. J Clean Prod 215:926–943. https://doi.org/10.1016/j.jclepro.2019.01.139
Recanati F, Marveggio D, Dotelli G (2018) From beans to bar: a life cycle assessment towards sustainable chocolate supply chain. Sci Total Environ 613–614:1013–1023. https://doi.org/10.1016/j.scitotenv.2017.09.187
Redclift M (2005) Sustainable development (1987–2005): an oxymoron comes of age. Sustain Dev 13:212–227. https://doi.org/10.1002/sd.281
Rezaei M, Soheilifard F, Keshvari A (2021) Impact of agrochemical emission models on the environmental assessment of paddy rice production using life cycle assessment approach. Energy Sources. Part A Recover Util Environ Eff 1–16
Rigamonti L, Mancini E (2021) Life cycle assessment and circularity indicators. Int J Life Cycle Assess. https://doi.org/10.1007/s11367-021-01966-2
Risku-Norja H, Mäenpää I (2007) MFA model to assess economic and environmental consequences of food production and consumption. Ecol Econ 60:700–711. https://doi.org/10.1016/j.ecolecon.2006.05.001
Ritzén S, Sandström GÖ (2017) Barriers to the circular economy – integration of perspectives and domains. Procedia CIRP 64:7–12. https://doi.org/10.1016/j.procir.2017.03.005
Rockström J, Steffen W, Noone K et al (2009) A safe operating space for humanity. Nature 461:472–475. https://doi.org/10.1038/461472a
Roos Lindgreen E, Mondello G, Salomone R et al (2021) Exploring the effectiveness of grey literature indicators and life cycle assessment in assessing circular economy at the micro level: a comparative analysis. Int J Life Cycle Assess. https://doi.org/10.1007/s11367-021-01972-4
Roselli L, Casieri A, De Gennaro BC et al (2020) Environmental and economic sustainability of table grape production in Italy. Sustain 12. https://doi.org/10.3390/su12093670
Ross RB, Pandey V, Ross KL (2015) Sustainability and strategy in U.S. agri-food firms: an assessment of current practices. Int Food Agribus Manag Rev 18:17–48
Royo P, Ferreira VJ, López-Sabirón AM, Ferreira G. (2016) Hybrid diagnosis to characterise the energy and environmental enhancement of photovoltaic modules using smart materials. Energy 101:174–189. https://doi.org/10.1016/j.energy.2016.01.101
Ruggerio CA (2021) Sustainability and sustainable development: a review of principles and definitions. Sci Total Environ 786:147481. https://doi.org/10.1016/j.scitotenv.2021.147481
Ruiz-Almeida A, Rivera-Ferre MG (2019) Internationally-based indicators to measure agri-food systems sustainability using food sovereignty as a conceptual framework. Food Secur 11:1321–1337. https://doi.org/10.1007/s12571-019-00964-5
Ryan M, Hennessy T, Buckley C et al (2016) Developing farm-level sustainability indicators for Ireland using the Teagasc National Farm Survey. Irish J Agric Food Res 55:112–125. https://doi.org/10.1515/ijafr-2016-0011
Saade MRM, Yahia A, Amor B (2020) How has LCA been applied to 3D printing ? A systematic literature review and recommendations for future studies. J Clean Prod 244:118803. https://doi.org/10.1016/j.jclepro.2019.118803
Saitone TL, Sexton RJ (2017) Agri-food supply chain: evolution and performance with conflicting consumer and societal demands. Eur Rev Agric Econ 44:634–657. https://doi.org/10.1093/erae/jbx003
Salim N, Ab Rahman MN, Abd Wahab D (2019) A systematic literature review of internal capabilities for enhancing eco-innovation performance of manufacturing firms. J Clean Prod 209:1445–1460. https://doi.org/10.1016/j.jclepro.2018.11.105
Salimi N (2021) Circular economy in agri-food systems BT - strategic decision making for sustainable management of industrial networks. In: International S (ed) Rezaei J. Publishing, Cham, pp 57–70
Salomone R, Ioppolo G (2012) Environmental impacts of olive oil production: a life cycle assessment case study in the province of Messina (Sicily). J Clean Prod 28:88–100. https://doi.org/10.1016/j.jclepro.2011.10.004
Sánchez AD, Río DMDLC, García JÁ (2017) Bibliometric analysis of publications on wine tourism in the databases Scopus and WoS. Eur Res Manag Bus Econ 23:8–15. https://doi.org/10.1016/j.iedeen.2016.02.001
Saputri VHL, Sutopo W, Hisjam M, Ma’aram A (2019) Sustainable agri-food supply chain performance measurement model for GMO and non-GMO using data envelopment analysis method. Appl Sci 9. https://doi.org/10.3390/app9061199
Sassanelli C, Rosa P, Rocca R, Terzi S (2019) Circular economy performance assessment methods : a systematic literature review. J Clean Prod 229:440–453. https://doi.org/10.1016/j.jclepro.2019.05.019
Schiefer S, Gonzalez C, Flanigan S (2015) More than just a factor in transition processes? The role of collaboration in agriculture. In: Sutherland LA, Darnhofer I, Wilson GA, Zagata L (eds) Transition pathways towards sustainability in agriculture: case studies from Europe, CPI Group. Croydon, UK, pp. 83
Seuring S, Muller M (2008) From a literature review to a conceptual framework for sustainable supply chain management. J Clean Prod 16:1699–1710. https://doi.org/10.1016/j.jclepro.2008.04.020
Silvestri C, Silvestri L, Forcina A, et al (2021) Green chemistry contribution towards more equitable global sustainability and greater circular economy: A systematic literature review. J Clean Prod 294. https://doi.org/10.1016/j.jclepro.2021.126137
Smetana S, Schmitt E, Mathys A (2019) Sustainable use of Hermetia illucens insect biomass for feed and food: attributional and consequential life cycle assessment. Resour Conserv Recycl 144:285–296. https://doi.org/10.1016/j.resconrec.2019.01.042
Sonesson U, Berlin J, Ziegler F (2010) Environmental assessment and management in the food industry: life cycle assessment and related approaches. Woodhead Publishing, Cambridge
Soussana JF (2014) Research priorities for sustainable agri-food systems and life cycle assessment. J Clean Prod 73:19–23. https://doi.org/10.1016/j.jclepro.2014.02.061
Soylu A, Oruç C, Turkay M et al (2006) Synergy analysis of collaborative supply chain management in energy systems using multi-period MILP. Eur J Oper Res 174:387–403. https://doi.org/10.1016/j.ejor.2005.02.042
Spaiser V, Ranganathan S, Swain RB, Sumpter DJ (2017) The sustainable development oxymoron: quantifying and modelling the incompatibility of sustainable development goals. Int J Sustain Dev World Ecol 24:457–470. https://doi.org/10.1080/13504509.2016.1235624
Stewart R, Niero M (2018) Circular economy in corporate sustainability strategies: a review of corporate sustainability reports in the fast-moving consumer goods sector. Bus Strateg Environ 27:1005–1022. https://doi.org/10.1002/bse.2048
Stillitano T, Spada E, Iofrida N et al (2021) Sustainable agri-food processes and circular economy pathways in a life cycle perspective: state of the art of applicative research. Sustain 13:1–29. https://doi.org/10.3390/su13052472
Stone J, Rahimifard S (2018) Resilience in agri-food supply chains: a critical analysis of the literature and synthesis of a novel framework. Supply Chain Manag 23:207–238. https://doi.org/10.1108/SCM-06-2017-0201
Strazza C, Del Borghi A, Gallo M, Del Borghi M (2011) Resource productivity enhancement as means for promoting cleaner production: analysis of co-incineration in cement plants through a life cycle approach. J Clean Prod 19:1615–1621. https://doi.org/10.1016/j.jclepro.2011.05.014
Su B, Heshmati A, Geng Y, Yu X (2013) A review of the circular economy in China: moving from rhetoric to implementation. J Clean Prod 42:215–227. https://doi.org/10.1016/j.jclepro.2012.11.020
Suárez-Eiroa B, Fernández E, Méndez-Martínez G, Soto-Oñate D (2019) Operational principles of circular economy for sustainable development: linking theory and practice. J Clean Prod 214:952–961. https://doi.org/10.1016/j.jclepro.2018.12.271
Svensson G, Wagner B (2015) Implementing and managing economic, social and environmental efforts of business sustainability. Manag Environ Qual an Int Journal 26:195–213. https://doi.org/10.1108/MEQ-09-2013-0099
Tasca AL, Nessi S, Rigamonti L (2017) Environmental sustainability of agri-food supply chains: an LCA comparison between two alternative forms of production and distribution of endive in northern Italy. J Clean Prod 140:725–741. https://doi.org/10.1016/j.jclepro.2016.06.170
Tassielli G, Notarnicola B, Renzulli PA, Arcese G (2018) Environmental life cycle assessment of fresh and processed sweet cherries in southern Italy. J Clean Prod 171:184–197. https://doi.org/10.1016/j.jclepro.2017.09.227
Teixeira R, Pax S (2011) A survey of life cycle assessment practitioners with a focus on the agri-food sector. J Ind Ecol 15:817–820. https://doi.org/10.1111/j.1530-9290.2011.00421.x
Tobergte DR, Curtis S (2013) ILCD Handbook. J Chem Info Model. https://doi.org/10.278/33030
Tortorella MM, Di Leo S, Cosmi C et al (2020) A methodological integrated approach to analyse climate change effects in agri-food sector: the TIMES water-energy-food module. Int J Environ Res Public Health 17:1–21. https://doi.org/10.3390/ijerph17217703
Tranfield D, Denyer D, Smart P (2003) Towards a methodology for developing evidenceinformed management knowledge by means of systematic review. Br J Manag 14:207–222
Trivellas P, Malindretos G, Reklitis P (2020) Implications of green logistics management on sustainable business and supply chain performance: evidence from a survey in the greek agri-food sector. Sustain 12:1–29. https://doi.org/10.3390/su122410515
Tsangas M, Gavriel I, Doula M et al (2020) Life cycle analysis in the framework of agricultural strategic development planning in the Balkan region. Sustain 12:1–15. https://doi.org/10.3390/su12051813
Ülgen VS, Björklund M, Simm N (2019) Inter-organizational supply chain interaction for sustainability : a systematic literature review.
UNEP S (2020) Guidelines for social life cycle assessment of products and organizations 2020.
UNEP/SETAC (2009) United Nations Environment Programme-society of Environmental Toxicology and Chemistry. Guidelines for social life cycle assessment of products. France
United Nations (2011) Guiding principles on business and human rights. Implementing the United Nations “protect, respect and remedy” framework
United Nations (2015) Transforming our world: the 2030 agenda for sustainable development. sustainabledevelopment.un.org
Van Asselt ED, Van Bussel LGJ, Van Der Voet H et al (2014) A protocol for evaluating the sustainability of agri-food production systems - a case study on potato production in peri-urban agriculture in the Netherlands. Ecol Indic 43:315–321. https://doi.org/10.1016/j.ecolind.2014.02.027
Van der Ploeg JD (2014) Peasant-driven agricultural growth and food sovereignty. J Peasant Stud 41:999–1030. https://doi.org/10.1080/03066150.2013.876997
van Eck NJ, Waltman L (2010) Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 84:523–538. https://doi.org/10.1007/s11192-009-0146-3
Van Eck NJ, Waltman L (2019) Manual for VOSviwer version 1.6.10. CWTS Meaningful metrics 1–53
Vasa L, Angeloska A, Trendov NM (2017) Comparative analysis of circular agriculture development in selected Western Balkan countries based on sustainable performance indicators. Econ Ann 168:44–47. https://doi.org/10.21003/ea.V168-09
Verdecho MJ, Alarcón-Valero F, Pérez-Perales D et al (2020) A methodology to select suppliers to increase sustainability within supply chains. Cent Eur J Oper Res. https://doi.org/10.1007/s10100-019-00668-3
Vergine P, Salerno C, Libutti A et al (2017) Closing the water cycle in the agro-industrial sector by reusing treated wastewater for irrigation. J Clean Prod 164:587–596. https://doi.org/10.1016/j.jclepro.2017.06.239
WCED (1987) Our common future - call for action
Webster K (2013) What might we say about a circular economy? Some temptations to avoid if possible. World Futures 69:542–554
Wheaton E, Kulshreshtha S (2013) Agriculture and climate change: implications for environmental sustainability indicators. WIT Trans Ecol Environ 175:99–110. https://doi.org/10.2495/ECO130091
Wijewickrama MKCS, Chileshe N, Rameezdeen R, Ochoa JJ (2021) Information sharing in reverse logistics supply chain of demolition waste: a systematic literature review. J Clean Prod 280:124359. https://doi.org/10.1016/j.jclepro.2020.124359
Woodhouse A, Davis J, Pénicaud C, Östergren K (2018) Sustainability checklist in support of the design of food processing. Sustain Prod Consum 16:110–120. https://doi.org/10.1016/j.spc.2018.06.008
Wu R, Yang D, Chen J (2014) Social Life Cycle Assessment Revisited Sustain 6:4200–4226. https://doi.org/10.3390/su6074200
Yadav S, Luthra S, Garg D (2021) Modelling Internet of things (IoT)-driven global sustainability in multi-tier agri-food supply chain under natural epidemic outbreaks. Environ Sci Pollut Res 16633–16654. https://doi.org/10.1007/s11356-020-11676-1
Yee FM, Shaharudin MR, Ma G et al (2021) Green purchasing capabilities and practices towards Firm’s triple bottom line in Malaysia. J Clean Prod 307:127268. https://doi.org/10.1016/j.jclepro.2021.127268
Yigitcanlar T (2010) Rethinking sustainable development: urban management, engineering, and design. IGI Global
Zamagni A, Amerighi O, Buttol P (2011) Strengths or bias in social LCA? Int J Life Cycle Assess 16:596–598. https://doi.org/10.1007/s11367-011-0309-3
Download references
Author information
Authors and affiliations.
Department of Economy, Engineering, Society and Business Organization, University of “Tuscia, ” Via del Paradiso 47, 01100, Viterbo, VT, Italy
Cecilia Silvestri, Michela Piccarozzi & Alessandro Ruggieri
Department of Engineering, University of Rome “Niccolò Cusano, ” Via Don Carlo Gnocchi, 3, 00166, Rome, Italy
Luca Silvestri
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Cecilia Silvestri .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Communicated by Monia Niero
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: a number of ill-placed paragraph headings were removed and the source indication "Authors' elaborations" was added to Tables 1-3.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (DOCX 31 KB)
Rights and permissions.
Reprints and permissions
About this article
Silvestri, C., Silvestri, L., Piccarozzi, M. et al. Toward a framework for selecting indicators of measuring sustainability and circular economy in the agri-food sector: a systematic literature review. Int J Life Cycle Assess (2022). https://doi.org/10.1007/s11367-022-02032-1
Download citation
Received : 15 June 2021
Accepted : 16 February 2022
Published : 02 March 2022
DOI : https://doi.org/10.1007/s11367-022-02032-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Agri-food sector
- Sustainability
- Circular economy
- Triple bottom line
- Life cycle assessment
- Find a journal
- Publish with us
- Track your research
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Public & Patients
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 31, Issue 1
- Definitions of digital biomarkers: a systematic mapping of the biomedical literature
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- Ana Karen Macias Alonso 1 , 2 ,
- http://orcid.org/0000-0001-6589-3936 Julian Hirt 2 , 3 , 4 ,
- Tim Woelfle 2 , 5 ,
- Perrine Janiaud 2 , 3 and
- Lars G Hemkens 2 , 3 , 6 , 7
- 1 Department of Applied Natural Sciences , Technische Hochschule Lübeck , Lübeck , Germany
- 2 Pragmatic Evidence Lab, Research Center for Clinical Neuroimmunology and Neuroscience Basel (RC2NB) , University Hospital Basel and University of Basel , Basel , Switzerland
- 3 Department of Clinical Research , University Hospital Basel and University of Basel , Basel , Switzerland
- 4 Department of Health , Eastern Switzerland University of Applied Sciences , St.Gallen , Switzerland
- 5 Department of Neurology and MS Center , University Hospital Basel and University of Basel , Basel , Switzerland
- 6 Meta-Research Innovation Center at Stanford (METRICS) , Stanford University , Stanford , California , USA
- 7 Meta-Research Innovation Center Berlin (METRIC-B) , Berlin Institute of Health , Berlin , Germany
- Correspondence to Dr Lars G Hemkens; lars.hemkens{at}usb.ch
Background Technological devices such as smartphones, wearables and virtual assistants enable health data collection, serving as digital alternatives to conventional biomarkers. We aimed to provide a systematic overview of emerging literature on ‘digital biomarkers,’ covering definitions, features and citations in biomedical research.
Methods We analysed all articles in PubMed that used ‘digital biomarker(s)’ in title or abstract, considering any study involving humans and any review, editorial, perspective or opinion-based articles up to 8 March 2023. We systematically extracted characteristics of publications and research studies, and any definitions and features of ‘digital biomarkers’ mentioned. We described the most influential literature on digital biomarkers and their definitions using thematic categorisations of definitions considering the Food and Drug Administration Biomarkers, EndpointS and other Tools framework (ie, data type, data collection method, purpose of biomarker), analysing structural similarity of definitions by performing text and citation analyses.
Results We identified 415 articles using ‘digital biomarker’ between 2014 and 2023 (median 2021). The majority (283 articles; 68%) were primary research. Notably, 287 articles (69%) did not provide a definition of digital biomarkers. Among the 128 articles with definitions, there were 127 different ones. Of these, 78 considered data collection, 56 data type, 50 purpose and 23 included all three components. Those 128 articles with a definition had a median of 6 citations, with the top 10 each presenting distinct definitions.
Conclusions The definitions of digital biomarkers vary significantly, indicating a lack of consensus in this emerging field. Our overview highlights key defining characteristics, which could guide the development of a more harmonised accepted definition.
- Medical Informatics
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information.
This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/ .
https://doi.org/10.1136/bmjhci-2023-100914
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Introduction
Biomarkers are defined as a set of characteristics that are objectively measured and used as indicators of normal biological processes, pathogenic processes or biological responses that appear due to exposure or therapeutic interventions. 1 This comprises physiological, molecular, histologic and radiographic measurements. 2 The US Food and Drug Administration (FDA) subclassifies susceptible/risk, diagnostic, monitoring, prognostic, predictive, response and safety biomarkers. 1 They highlight that a full biomarker description must include the source or matrix, the measurable characteristic(s) and the methods used to measure the biomarker. 1 The digitalisation of our world impacting daily living and healthcare broadens the spectrum of the possible source and methods used to measure biomarkers and introduces a novel dimension of measurable characteristics. This allows digital devices used daily, such as smartphones, wearable devices, sensors and smart home devices, to provide a new category of biomarkers, often called ‘digital biomarkers’. In recent years, digital biomarkers became increasingly present in routine care and in research in many areas of medicine, such as cardiology, oncology or COVID-19. For example, smartphone recorded cough sounds have been used as a digital biomarker to detect asthma and respiratory infections in clinical trials, 3 4 or deep learning was applied to data from a three-axis accelerometer to predict sleep/wake patterns. 4 5 Moreover, such digital biomarkers have spread in the field of neurology, which has a large unmet need for non-invasive and objective biomarkers reflecting cognitive and motor functions that are traditionally assessed with specific tests performed by neurologists. 6 Beyond monitoring health and disease status, predicting the occurrence and development of diseases would be promising applications of such novel approaches. 7
Thus, digital biomarkers have the potential to offer valuable insights on the health of patients. They usually have high temporal resolution (up to (quasi-)continuous), are usually objective (and not subject to interobserver variability) and can have high external validity as they may be applied in the patient’s routine environment (as opposed to, eg, the clinic or a research environment). 8
Many everyday digital tools used mainly for entertainment/leisure purposes (eg, fitness trackers) are increasingly considered as a source of helpful information that may be transformed into digital biomarkers. Yet, with all this diversity in application and complex interaction with rapidly evolving technology, it becomes necessary to provide a clear and precise definition of the fundamental underlying concepts to facilitate research and decision-making with and on these novel approaches.
One of the first definitions of this novel type of biomarker was provided by Dorsey et al , who defined digital biomarkers as ‘the use of a biosensor to collect objective data on a biological (eg, blood glucose, serum sodium), anatomical (eg, mole size) or physiological (eg, heart rate, blood pressure) parameter obtained using sensors followed by algorithms to transform these data into interpretable outcome measures, helping to address many of the shortcomings in current measures.’ Furthermore, they stated that these new measures ‘include portable (eg, smartphones), wearable, and implantable devices, and are by their nature largely independent of raters.’ 9 A later definition given in 2020 by the European Medicines Agency (EMA) was based on ‘digital measures’ (‘measured through digital tools’) and did not include the requirement of algorithms as a defining feature: ‘a digital biomarker is an objective, quantifiable measure of physiology and/or behaviour used as an indicator of biological, pathological process or response to an exposure or an intervention that is derived from a digital measure. […]’) 10
Others gave broader definitions including further defining features, for example, defining digital biomarkers as ‘objective, quantifiable, quantitative, physiological and behavioural data that are collected and measured by means of digital devices such as portables, wearables, implantables or digestibles. The data collected are used to explain, influence and/or predict health-related outcomes’. 2 6 11
Overall, such a disagreement between definitions used by regulators and in articles published in high-impact biomedical journals raised concerns that no clear consensus exists among researchers and users of this novel approach and terminology, increasing the risk for miscommunication. There are numerous examples where differences in definitions have been recognised as critical cause of inefficiencies and delay in health research and avoidable controversy, uncertainty and potential harm in clinical care and public health. 12–15 The Biomarkers, EndpointS and other Tools (BEST) framework developed by the FDA and US National Institutes of Health with ‘the goals of improving communication, aligning expectations, and improving scientific understanding’ highlights that ‘unclear definitions and inconsistent use of key terms can hinder the evaluation and interpretation of scientific evidence and may pose significant obstacles to medical product development programmes’. 1 We aimed to provide a systematic overview of the emerging literature on digital biomarkers and characterisation of the definitions of digital biomarkers that are provided in biomedical journal articles by performing a systematic mapping and citation analysis of all articles that prominently used the term ‘digital biomarker’. We sought to determine differences in characteristics of common definitions to provide a foundation for subsequent activities to develop clearer and consistent definitions that ensure improved application of digital biomarkers in research and healthcare decision-making.
We analysed all articles published at any time in PubMed that prominently used the term ‘digital biomarker’, that is, either in title or abstract.
We systematically explored definitions of digital biomarkers that are provided and/or referred to in the biomedical literature, that is, journal articles that are indexed in PubMed, in a mapping review without a formal assessment of included studies. 16 We structured our review report to the ‘Preferred Reporting Items for Systematic Reviews and Meta-Analyses’ guidance, where applicable. 17 We did not use a prespecified protocol.
Eligibility criteria, information source and search strategy
We searched PubMed and included all articles mentioning ‘digital biomarker’ or ‘digital biomarkers’ in their title or abstract (by searching PubMed for ‘digital biomarker*(tiab)’; date of last search: 8 March 2023). We excluded animal research.
Study selection
One reviewer (AKMA) screened titles, abstracts and full texts for eligibility. Confirmation by a second reviewer (JH or LGH) was planned for situations where the reviewer was unsure, but this case never occurred given the clear and objective selection criteria.
Data extraction
We developed a spreadsheet to structure the data extraction process. One reviewer (AKMA) extracted data with confirmation by a second reviewer (JH or LGH) in case of any uncertainty.
We extracted from every article: author(s), publication year, title, journal, corresponding author, and country of correspondence, article type (ie, primary research, review or other type (eg, editorial, comment, opinion-based letter)). Of primary research articles, we additionally extracted definitions of digital biomarkers that are provided and/or referred to (based on a semantic search for indicators of definition such as ‘digital biomarkers are’, ‘… are defined as’, ‘… can be defined’, ‘the definition of … is’), medical context, and whether the article is about the development and/or validation of a digital biomarker. The number of global citations was obtained by using metadata from OpenAlex 18 ; accessed via the Local Citation Network 19 (as of 26 June 2023).
Data analysis and categorisation of definition components
We considered the BEST framework to derive components of definitions for digital biomarkers. 1 We analysed the identified digital biomarker definitions by assessing if they contained descriptions that fall within three key components, that is, the (1) type of data that is measured (eg, whether data were measured objectively, continuously or quantitatively), (2) data collection method (eg, whether sensors, computers, portables, wearables, implantables or digestibles were used to collect data) and (3) purpose of the digital biomarker (eg, whether a biomarker was used as measure of disease progression or to predict health-related outcomes). We defined definitions as duplicates when they used the same sequence of words. We illustrate the frequency of various terminologies used in all provided definitions with a word cloud. 20 We analysed the structural similarity of definitions that were provided without a reference by performing hierarchical clustering on the distance-matrix containing pairwise ‘Indel’-distances, that is, ‘the minimum number of insertions and deletions required to change one (definition) into the other’. 21 Since we aimed at exploring how digital biomarkers are defined in the biomedical literature, we did not critically assess the included articles and studies. For the analysis of citations, we calculated the quotient of number of global citations (retrieved by the Local Citation Network 19 ) and years since publication per article. To create a citation network of citing and cited relationships between the articles, we used the Local Citation Network with the OpenAlex scholarly index. 19 22
We used descriptive statistics by reporting numbers and percentages. For all analyses, we used R (V.4.2.2) or Python (V.3.11.4).
We identified 415 articles that had ‘digital biomarker’ in their title or abstract ( online supplemental S1 ). The first article was published in 2014 (median publication year 2021; figure 1 ; online supplemental S2 ). Most articles described primary studies (n=283; 68%) and were published in digital medicine specialty journals, including Digital Biomarkers (n=35; 8%), Journal of Medical Internet Researc h (n=21; 5%) or npj Digital Medicine (n=19; 4%; table 1 ). Of the 415 articles, 128 (31%) provided at least 1 definition of a digital biomarker.
Supplemental material
- Download figure
- Open in new tab
- Download powerpoint
The annual number of published article types referring to digital biomarkers as of 8 March 2023 (n=415).
- View inline
Characteristics of all 415 articles in PubMed using ‘digital biomarker’ in title or abstract
Characteristics of articles providing a definition of digital biomarker
The 128 articles with a definition of digital biomarker were published between 2015 and 2023 (median: 2021). Of them, 59 articles were primary studies, 50 were reviews and 19 were other types of articles ( table 1 ).
Almost all primary studies described the development of one or more digital biomarkers (53 of 59 articles), and many described a validation process of biomarkers (35 of 59 articles). The most frequent medical field of the primary research articles that described the development of one or more digital biomarkers was neurology (25 of 53), while the spectrum of medical fields was overall very wide ( table 1 ). The most frequent diseases were dementia and related disorders (16 of 53 articles, ie, (mild) cognitive impairment or Alzheimer’s disease), Parkinson’s disease (5 of 53 articles) and diabetes (3 of 53 articles), with numerous other conditions addressed in one or two studies (eg, atrial fibrillation, cervical cancer, depression, heart failure and muscular dystrophy; online supplemental S2 ).
The corresponding authors were mostly from the USA (69 of 128 articles), Switzerland (22 of 128 articles), Germany (16 of 128 articles) and the UK (16 of 128 articles; table 1 ).
The articles were cited a median of 6 times (range 0–517, IQR 2–20, overall 2,705); on average two times per year (range 0–86, IQR 1–5; online supplemental S2 ). We show the citation network (ie, citing and cited relationships within the sample of these 128 articles) online ( https://LocalCitationNetwork.github.io/?fromJSON=Digital-Biomarker -Definitions.json).
Definitions of digital biomarkers
Overall, 128 articles reported between 1 and 7 definitions (median 1, IQR 1–2). In 91 articles, at least 1 reference was provided for these definitions made by the authors (median 1, range 1–13, IQR 1–2, overall 274 references); for 37 articles with 51 definitions, no reference was provided ( online supplemental S2 ).
The mostly used references to support the definitions were Coravos et al 4 (referenced by 51 of 91 articles); Dorsey et al 9 (11 articles); Califf 23 (9 articles); Piau et al 24 (9 articles); Babrak et al 6 (8 articles) and Coravos et al 25 (8 articles). All these articles were among the 415 articles analysed here. The original definitions in these top-cited articles can be found in table 2 . Other references were used by less than five articles.
The top cited definitions of Digital Biomarkers within the 415 articles
In total, the 128 articles reported 202 definitions; 75 of which were duplicates. Hence, we identified 127 unique definitions across the 128 articles.
The 10 most frequently used terms that most of the 127 unique definitions contained were ‘digital’ (125 of 127 definitions; 98%), ‘biomarkers’ (109 of 127 definitions; 85%), ‘data’ (62 of 127 definitions; 48%), ‘collected’ (55 of 127 definitions; 43%), ‘devices’ (50 of 127 definitions; 39%), ‘health’ (42 of 127 definitions; 33%), ‘physiological’ (37 of 127 definitions; 29%), ‘objective’ (37 of 127 definitions; 29%), ‘wearable’ (34 of 127 definitions; 26%) and ‘behavioural’ (33 from 127 definitions; 25%; figure 2 ).
Word cloud with the most frequently used terms in the analysed digital biomarker(s) definitions.
Of the 127 unique definitions, 56 definitions refer to the type of data that are collected, 78 definitions contain information on the data collection method, and 50 definitions provide information on the purpose of the digital biomarker. Only 23 of 127 definitions involve all 3 components and 26 contain none of these components ( table 3 ; online supplemental S3 ; online supplemental S2 ).
Definitions of digital biomarkers that include three key components: type of data, data collection method and purpose of a digital biomarker (n=23)
There were almost no structural similarities between the 51 identified definitions in 37 articles without a reference (for those with a reference, similarities such as paraphrasing are expected; online supplemental S4 ).
We systematically searched and characterised the biomedical literature that used the term digital biomarker and analysed the provided definitions of the concept. We identified 415 articles using ‘digital biomarker’ in title and/or abstract that were published between 2014 and 2023. Of them, 128 articles provided 127 different definitions. By comparing the defining features, we aimed to better understand what those who use this term in the context of biomedical research or healthcare mean by ‘digital biomarker’ and which components are deemed the essence of it. 26
The first definition of a digital biomarker is from 2015. 27 Within 8 years, more than 127 definitions have been used, with none of them clearly being the most widely used; indicating a high heterogeneity of the concept of digital biomarkers. The definitions often cover different aspects of definitional components that are traditionally used to describe more conventional biomarkers. Authors have created their own concepts and gave an identity to this type of biomarker. The variation in these definitions and the fact that only 23 of them provide a full description containing all components of FDA’s BEST framework, shows how broad the current understanding of this fundamental concept is.
Digital biomarkers emerged as a concept in medical and technological domains, although with a diverse terminology across different academic journals. In the medical field, digital biomarkers are often referred to as biomarkers of health or disease obtained through digital health technologies. In the technical field, these biomarkers are viewed as data-driven indicators collected from sensors, wearables and other portable digital technologies that provide an assessment of the health status. These diverse terminologies and definitions reflect the interdisciplinary nature of digital biomarkers with their application in a broad spectrum of biomedicine which underlines the importance of unified concepts to enhance the communications and cross-disciplinary collaborations on this evolving field.
Regulatory perspectives
The EMA has defined digital biomarkers in 2020 in their draft guidance ‘Questions and answers: Qualification of digital technology-based methodologies to support approval of medicinal products’, stating their ‘clinical meaning is established by a reliable relationship to an existing, validated endpoint’. 10 EMA draws a clear line to electronic clinical outcome assessments (eCOA), whose ‘clinical meaning is established de novo’. According to EMA’s terminology, both digital biomarkers and eCOA are derived from ‘digital measures’ and can be used as ‘digital endpoints’. 10
On the other hand, the term ‘digital biomarker’ cannot be found in the FDA draft guidance ‘Digital Health Technologies for Remote Data Acquisition in Clinical Investigations’, which instead features eCOA as examples of digital health technologies. 28 Figure 3 contains our semantic interpretation of the terminology used by EMA and FDA.
Semantic overview of terminology used by EMA and FDA. Digital health technologies obtain digital measures, which include digital biomarkers and electronic clinical outcome assessment (eCOA). Digital biomarkers and eCOAs both can provide digital endpoints. EMA, European Medicines Agency; FDA, Food and Drug Administration.
This distinction can rarely be observed in the medical literature—we found this term in 8 of the 415 articles analysed and a PubMed search for ‘electronic clinical outcome assessment*’ returned also only 8 articles mentioning it in title or abstract (as of 31 August 2023), compared with the 415 for our search term ‘digital biomarker*’. As Vasudevan et al stated in 2022: ‘There are currently multiple definitions of the term digital biomarker reported in the scientific literature, and some seem to conflate established definitions of a biomarker and a clinical outcomes assessment (COA)’. 11
This divergency in the terminology of digital biomarkers between the academic literature and the regulators’ language raises challenges and ambiguity. Consequently, a more cohesive and comprehensive framework within the digital biomarker field is needed to strengthen the clarity and continue growing the potential that this data could bring for health.
The development of a substantive and unified definition of digital biomarkers would be an important step in shaping a conceptual framework for the development, assessment and reporting of digital biomarkers. Our results may inform this process by using the existing understanding of digital biomarkers systematically analysed in this study as a basis. To achieve a common and more unified understanding of what digital biomarkers are—and are not—a Delphi study could be useful. 29 30 Such a study would aim to combine multiple views and expectations on the existing definitions of digital biomarkers and their components until a consensus is reached. Ideally, that would be achieved by an international panel with expert’s representative of all relevant stakeholders covering a range of medical fields (eg, cardiology, neurology), professional backgrounds (eg, clinical care/rehabilitation/nursing, software developers, device manufacturer, editors, guideline developers), and professional perspectives (eg, academia, regulatory, industry/technology, publishing) and involving patients.
Limitations
There are some limitations to our study.
First, we used a limited search only in a single database using the single term of ‘digital biomarker*’, which may have overlooked some other relevant studies. PubMed was chosen as literature database given its outstanding role, reflecting the most impactful journals in biomedicine. 31 We focused on this single term because we assume it to be the most central and widely used term describing the concept of ‘digital biomarker’. It is very unlikely that the definitions would be much more uniform in potentially overlooked studies or would we have included other potential concepts, and it is quite possible that many more different definitions would emerge, especially from digital biomarker developments contained in technical literature databases (such as IEEE Explore or ACM Digital Library). Therefore, we may have even underestimated the large number of different definitions.
Second, the screening and data extraction were performed by a single reviewer only. This may have resulted in some studies that were overlooked and some misclassifications, but it is unlikely that our main interpretation would change. Third, we developed a simple framework with three key elements of definitions based on a well-established framework (BEST), but the categorisation of elements is subjective to some degree. However, we employed a structured analysis that confirmed the observed heterogeneity across definitions.
Conclusions
Clear and unambiguous communication and research reporting is essential for the effective implementation of scientific innovations and developments. This requires clear definitions and consistent use and understanding of key terms and concepts. A lack of clarity and consistency can lead to research waste, delay or even misdirection of promising developments and potential. Digital biomarkers offer the opportunity to collect objective, meaningful, patient-relevant data cost-effectively with unprecedented granularity. An exact understanding of what they are and how they are described in biomedical literature is essential to let them shape the future of clinical research and enable them to provide most useful evidence for research and care. Our study can inform the development of a harmonised and more widely accepted definition, for example, with a Delphi study.
Ethics statements
Patient consent for publication.
Not applicable.
Acknowledgments
We thank Saido Haji Abukar (Medical Bachelor student at ETH Zurich) for her support with the study selection and data extraction.
- FDA-NIH Biomarker Working Group
- Motahari-Nezhad H ,
- Gulácsi L , et al
- Moschovis PP ,
- Sampayo EM ,
- Cook A , et al
- Coravos A ,
- Guerrero R , et al
- Babrak LM ,
- Menetski J ,
- Rebhan M , et al
- Buegler M ,
- Balasa M , et al
- Woelfle T ,
- Bourguignon L ,
- Lorscheider J , et al
- Dorsey ER ,
- Papapetropoulos S ,
- Xiong M , et al
- European Medicines Agency
- Vasudevan S ,
- Tarver ME , et al
- Guyatt GH , et al
- Oliveira ML ,
- Lucchetta RC ,
- Bonetti A de F , et al
- Soriano JB ,
- Marshall JC , et al
- Verghis R ,
- Blackwood B ,
- McDowell C , et al
- McKenzie JE ,
- Bossuyt PM , et al
- Piwowar H ,
- Mattek N , et al
- Goldsack JC ,
- Karlin DR , et al
- Mackereth S
- Gerbelot R ,
- Goyer C , et al
- Food and Drug Administration (US)
- Pawlowski SD
- Khodyakov D ,
- Kroger J , et al
- National Cancer Institute at the National Institutes of Health
- Andrade AQ ,
- Kelly T-L , et al
- Bartolome A ,
- Bijlani N ,
- Nilforooshan R ,
- Choi BK , et al
- Parziale A ,
- Mascalzoni D
- Phillips KA ,
- Douglas MP ,
- Trosman JR , et al
- Wright JM ,
- Dillenseger A ,
- Weidemann ML ,
- Trentzsch K , et al
- Vanden Abeele M-E ,
- De Croon R , et al
- Ferrari A ,
- Meier IB , et al
- de Luca V ,
- Kostikova A , et al
- Katsaros D ,
- Hawthorne J ,
- Patel J , et al
- Mahdi Abid M , et al
- Palanica A ,
- Docktor MJ ,
- Lieberman M , et al
- Petersen CL ,
- Norin O , et al
- Nourhashemi F , et al
- Sahandi Far M ,
- Eickhoff SB ,
- Goni M , et al
- Seyhan AA ,
- Shandhi MMH ,
- Roghanizad AR , et al
- Signorini A , et al
- van den Brink WJ ,
- van den Broek TJ ,
- Palmisano S , et al
- Zetterström A ,
- Hämäläinen MD ,
- Karlberg E , et al

Supplementary materials
Supplementary data.
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
- Data supplement 1
- Data supplement 2
Contributors All authors made substantial contributions to the conception and design of the work; all authors have drafted the work or substantively revised it; all authors have approved the submitted version; all authors have agreed both to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved and the resolution documented in the literature.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests RC2NB (Research Center for Clinical Neuroimmunology and Neuroscience Basel) is supported by Foundation Clinical Neuroimmunology and Neuroscience Basel. One of the main projects of RC2NB is the development and evaluation of a digital biomarkers which is supported by grants from Novartis, Roche and Innosuisse (Swiss Innovation Agency). All authors declare no competing interests.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Read the full text or download the PDF:
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 13 April 2024
Acute spinal cord injury serum biomarkers in human and rat: a scoping systematic review
- Sina Shool ORCID: orcid.org/0000-0002-0280-3187 1 , 2 na1 ,
- Saeed Rahmani 1 na1 ,
- Mohammad Amin Habibi 1 , 2 na1 ,
- Seyed Mohammad Piri 2 ,
- Mahmoud Lotfinia 3 ,
- Delara Jashnani 1 &
- Sina Asaadi ORCID: orcid.org/0000-0003-2953-5992 4
Spinal Cord Series and Cases volume 10 , Article number: 21 ( 2024 ) Cite this article
Metrics details
- Diagnostic markers
- Prognostic markers
Study design
Scoping systematic review.
To summarize the available experimental clinical and animal studies for the identification of all CSF and serum-derived biochemical markers in human and rat SCI models.
Tehran, Iran.
In this scoping article, we systematically reviewed the electronic databases of PubMed, Scopus, WOS, and CENTRAL to retrieve current literature assessing the levels of different biomarkers in human and rat SCI models.
A total of 19,589 articles were retrieved and 6897 duplicated titles were removed. The remaining 12,692 studies were screened by their title/abstract and 12,636 were removed. The remaining 56 were considered for full-text assessment, and 11 papers did not meet the criteria, and finally, 45 studies were included. 26 studies were human observational studies comprising 1630 patients, and 19 articles studied SCI models in rats, including 832 rats. Upon reviewing the literature, we encountered a remarkable heterogeneity in terms of selected biomarkers, timing, and method of measurement, studied models, extent, and mechanism of injury as well as outcome assessment measures.
Conclusions
The specific expression and distribution patterns of biomarkers in relation to spinal cord injury (SCI) phases, and their varied concentrations over time, suggest that cerebrospinal fluid (CSF) and blood biomarkers are effective measures for assessing the severity of SCI.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 1 print issues and online access
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
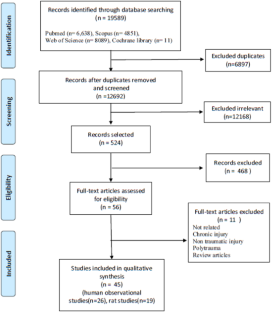
Similar content being viewed by others
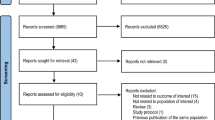
Systematic review of the changes in the microbiome following spinal cord injury: animal and human evidence
Ezra Valido, Alessandro Bertolo, … Jivko Stoyanov
Neurochemical biomarkers in spinal cord injury
Brian K. Kwon, Ona Bloom, … Kevin K. Wang
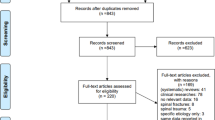
Epidemiology of spinal cord injury in China: A systematic review of the chinese and english literature
Chuandong Chen, Xu Qiao, … Jan D. Reinhardt
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary files.
Tetreault LA, Karadimas S, Wilson JR, Arnold PM, Kurpad S, Dettori JR, et al. The Natural History of Degenerative Cervical Myelopathy and the Rate of Hospitalization Following Spinal Cord Injury: An Updated Systematic Review. Glob Spine J. 2017;7: 28S–34S.
Article Google Scholar
Ahuja CS, Wilson JR, Nori S, Kotter MRN, Druschel C, Curt A, et al. Traumatic spinal cord injury. Nat Rev Dis Prim. 2017;3: 17018.
Article PubMed Google Scholar
Kim YH, Ha KY, Kim SI. Spinal Cord Injury and Related Clinical Trials. Clin Orthopedic Surg. 2017;9: 1–9.
Stein DM, Sheth KN. Management of acute spinal cord injury. Continuum. 2015;21: 159–87.
PubMed Google Scholar
Stahel PF, VanderHeiden T, Finn MA. Management strategies for acute spinal cord injury: current options and future perspectives. Curr Opin Crit Care. 2012;18: 651–60.
Ydens E, Palmers I, Hendrix S, Somers V. The Next Generation of Biomarker Research in Spinal Cord Injury. Mol Neurobiol. 2017;54: 1482–99.
Article CAS PubMed Google Scholar
Mattiassich G, Gollwitzer M, Gaderer F, Blocher M, Osti M, Lill M, et al. Functional Outcomes in Individuals Undergoing Very Early (<5 h) and Early (5-24 h) Surgical Decompression in Traumatic Cervical Spinal Cord Injury: Analysis of Neurological Improvement from the Austrian Spinal Cord Injury Study. J Neurotrauma. 2017;34: 3362–71.
Wutte C, Klein B, Becker J, Mach O, Panzer S, Strowitzki M, et al. Earlier Decompression (<8 h) Results in Better Neurological and Functional Outcome after Traumatic Thoracolumbar Spinal Cord Injury. J Neurotrauma. 2019;36: 2020–7.
White NH, Black NH. National Spinal Cord Injury Statistical Center, Spinal Cord Injury (SCI) 2016 Facts and Figures at a Glance. J Spinal Cord Med. 2016;39:493–4.
Kirshblum S, Waring W 3rd. Updates for the International Standards for Neurological Classification of Spinal Cord Injury. Phys Med Rehabilit Clin North Am. 2014;25: 505–17, vii.
Du W, Li H, Sun J, Xia Y, Zhu R, Zhang X, et al. The Prognostic Value of Serum Neuron Specific Enolase (NSE) and S100B Level in Patients of Acute Spinal Cord Injury. Med Sci Monit. 2018;24: 4510–5.
Article CAS PubMed PubMed Central Google Scholar
Roberts TT, Leonard GR, Cepela DJ. Classifications In Brief: American Spinal Injury Association (ASIA) Impairment Scale. Clin Orthop Relat Res. 2017;475: 1499–504.
Fawcett JW, Curt A, Steeves JD, Coleman WP, Tuszynski MH, Lammertse D, et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord. 2007;45: 190–205.
Rowland JW, Hawryluk GW, Kwon B, Fehlings MG. Current status of acute spinal cord injury pathophysiology and emerging therapies: promise on the horizon. Neurosurg Focus. 2008;25: E2.
Yousefifard M, Sarveazad A, Babahajian A, Baikpour M, Shokraneh F, Vaccaro AR, et al. Potential diagnostic and prognostic value of serum and cerebrospinal fluid biomarkers in traumatic spinal cord injury: A systematic review. J Neurochem. 2019;149: 317–30.
Strimbu K, Tavel JA. What are biomarkers? Curr Opin HIV AIDS. 2010;5: 463–6.
Article PubMed PubMed Central Google Scholar
Badhiwala JH, Wilson JR, Kwon BK, Casha S, Fehlings MG. A Review of Clinical Trials in Spinal Cord Injury Including Biomarkers. J Neurotrauma. 2018;35: 1906–17.
Kwon BK, Casha S, Hurlbert RJ, Yong VW. Inflammatory and structural biomarkers in acute traumatic spinal cord injury. Clin Chem Lab Med. 2011;49: 425–33.
Hulme CH, Brown SJ, Fuller HR, Riddell J, Osman A, Chowdhury J, et al. The developing landscape of diagnostic and prognostic biomarkers for spinal cord injury in cerebrospinal fluid and blood. Spinal Cord. 2017;55: 114–25.
Wu Y, Streijger F, Wang Y, Lin G, Christie S, Mac-Thiong JM, et al. Parallel Metabolomic Profiling of Cerebrospinal Fluid and Serum for Identifying Biomarkers of Injury Severity after Acute Human Spinal Cord Injury. Sci Rep. 2016;6: 38718.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6: e1000097.
Hassannejad Z, Sharif-Alhoseini M, Shakouri-Motlagh A, Vahedi F, Zadegan SA, Mokhatab M, et al. Potential variables affecting the quality of animal studies regarding pathophysiology of traumatic spinal cord injuries. Spinal Cord. 2016;54: 579–83.
Ga W. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. In 3rd Symposium on Systematic Reviews: Beyond the Basics. Oxford, UK; 2000.
Cao F, Yang XF, Liu WG, Hu WW, Li G, Zheng XJ, et al. Elevation of neuron-specific enolase and S-100beta protein level in experimental acute spinal cord injury. J Clin Neurosci. 2008;15: 541–4.
Ding SQ, Chen J, Wang SN, Duan FX, Chen YQ, Shi YJ, et al. Identification of serum exosomal microRNAs in acute spinal cord injured rats. Exp Biol Med. 2019;244: 1149–61.
Article CAS Google Scholar
Guo L, Hou J, Zhong J, Liu J, Sun T, Liu H. Association between injury severity and amyloid β protein levels in serum and cerebrospinal fluid in rats with traumatic spinal cord injury. Mol Med Rep. 2017;15: 2241–6.
Hasturk A, Atalay B, Calisaneller T, Ozdemir O, Oruckaptan H, Altinors N. Analysis of serum pro-inflammatory cytokine levels after rat spinal cord ischemia/reperfusion injury and correlation with tissue damage. Turkish Neurosurg. 2009;19: 353–9.
Google Scholar
Li XH, Wu F, Zhao F, Huang SL. Fractional anisotropy is a marker in early-stage spinal cord injury. Brain Res. 2017;1672: 44–9.
Loy DN, Sroufe AE, Pelt JL, Burke DA, Cao QL, Talbott JF, et al. Serum biomarkers for experimental acute spinal cord injury: rapid elevation of neuron-specific enolase and S-100beta. Neurosurgery. 2005;56: 391–7.
Ma J, Novikov LN, Karlsson K, Kellerth JO, Wiberg M. Plexus avulsion and spinal cord injury increase the serum concentration of S-100 protein: an experimental study in rats. Scand J Plast Reconstruct Surg Hand Surg. 2001;35: 355–9.
Ma Z, Dong Q, Lyu B, Wang J, Quan Y, Gong S. The expression of bradykinin and its receptors in spinal cord ischemia-reperfusion injury rat model. Life Sci. 2019;218: 340–5.
Ur K, Demiroz S, Bengu AS, Ulucan A, Gergin OO, Kizmazoglu C, et al. Serum endocan level and the severity of spinal cord injury. Bratisl Lekarske listy. 2018;119: 298–301.
CAS Google Scholar
Wang CX, Olschowka JA, Wrathall JR. Increase of interleukin-1beta mRNA and protein in the spinal cord following experimental traumatic injury in the rat. Brain Res. 1997;759: 190–6.
Wu F, Ding XY, Li XH, Gong MJ, An JQ, Huang SL. Correlation between elevated inflammatory cytokines of spleen and spleen index in acute spinal cord injury. J Neuroimmunol. 2020;344: 577264.
Zong S, Zeng G, Fang Y, Peng J, Tao Y, Li K, et al. The role of IL-17 promotes spinal cord neuroinflammation via activation of the transcription factor STAT3 after spinal cord injury in the rat. Mediators Inflamm. 2014;2014: 786947.
Caprelli MT, Mothe AJ, Tator CH. Hyperphosphorylated Tau as a Novel Biomarker for Traumatic Axonal Injury in the Spinal Cord. J Neurotrauma. 2018;35: 1929–41.
Hong J, Chang A, Zavvarian MM, Wang J, Liu Y, Fehlings MG. Level-Specific Differences in Systemic Expression of Pro- and Anti-Inflammatory Cytokines and Chemokines after Spinal Cord Injury. Int J Mol Sci. 2018;19: 2167.
Mukhamedshina YO, Akhmetzyanova ER, Martynova EV, Khaiboullina SF, Galieva LR, Rizvanov AA. Systemic and Local Cytokine Profile following Spinal Cord Injury in Rats: A Multiplex Analysis. Front Neurol. 2017;8: 581.
Tanhoffer RA, Yamazaki RK, Nunes EA, Pchevozniki AI, Pchevozniki AM, Nogata C, et al. Glutamine concentration and immune response of spinal cord-injured rats. J Spinal Cord Med. 2007;30: 140–6.
Wang CX, Nuttin B, Heremans H, Dom R, Gybels J. Production of tumor necrosis factor in spinal cord following traumatic injury in rats. J Neuroimmunol. 1996;69: 151–6.
Yang Z, Bramlett HM, Moghieb A, Yu D, Wang P, Lin F, et al. Temporal Profile and Severity Correlation of a Panel of Rat Spinal Cord Injury Protein Biomarkers. Mol Neurobiol. 2018;55: 2174–84.
Shaw G, Yang C, Ellis R, Anderson K, Parker Mickle J, Scheff S, et al. Hyperphosphorylated neurofilament NF-H is a serum biomarker of axonal injury. Biochemical Biophysical Res Commun. 2005;336: 1268–77.
Ahadi R, Khodagholi F, Daneshi A, Vafaei A, Mafi AA, Jorjani M. Diagnostic Value of Serum Levels of GFAP, pNF-H, and NSE Compared With Clinical Findings in Severity Assessment of Human Traumatic Spinal Cord Injury. Spine. 2015;40: E823–30.
Bank M, Stein A, Sison C, Glazer A, Jassal N, McCarthy D, et al. Elevated circulating levels of the pro-inflammatory cytokine macrophage migration inhibitory factor in individuals with acute spinal cord injury. Arch Phys Med Rehabilit. 2015;96: 633–44.
Biglari B, Büchler A, Swing T, Biehl E, Roth HJ, Bruckner T, et al. Increase in soluble CD95L during subacute phases after human spinal cord injury: a potential therapeutic target. Spinal Cord. 2013;51: 183–7.
Biglari B, Büchler A, Swing T, Child C, Biehl E, Reitzel T, et al. Serum sCD95L concentration in patients with spinal cord injury. J Int Med Res. 2015;43: 250–6.
Biglari B, Swing T, Child C, Büchler A, Westhauser F, Bruckner T, et al. A pilot study on temporal changes in IL-1β and TNF-α serum levels after spinal cord injury: the serum level of TNF-α in acute SCI patients as a possible marker for neurological remission. Spinal Cord. 2015;53: 510–4.
Ferbert T, Child C, Graeser V, Swing T, Akbar M, Heller R, et al. Tracking Spinal Cord Injury: Differences in Cytokine Expression of IGF-1, TGF- B1, and sCD95l Can Be Measured in Blood Samples and Correspond to Neurological Remission in a 12-Week Follow-Up. J Neurotrauma. 2017;34: 607–14.
Hassanshahi G, Amin M, Shunmugavel A, Vazirinejad R, Vakilian A, Sanji M, et al. Temporal expression profile of CXC chemokines in serum of patients with spinal cord injury. Neurochemistry Int. 2013;63: 363–7.
Hayakawa K, Okazaki R, Ishii K, Ueno T, Izawa N, Tanaka Y, et al. Phosphorylated neurofilament subunit NF-H as a biomarker for evaluating the severity of spinal cord injury patients, a pilot study. Spinal Cord. 2012;50: 493–6.
Heller RA, Seelig J, Bock T, Haubruck P, Grützner PA, Schomburg L, et al. Relation of selenium status to neuro-regeneration after traumatic spinal cord injury. J Trace Elem Med Biol. 2019;51: 141–9.
Heller RA, Raven TF, Swing T, Kunzmann K, Daniel V, Haubruck P, et al. CCL-2 as a possible early marker for remission after traumatic spinal cord injury. Spinal Cord. 2017;55: 1002–9.
Jin GX, Li L, Cui SQ, Duan JZ, Wang H. Persistent hypoalbuminemia is a predictor of outcome in cervical spinal cord injury. Spine J. 2014;14: 1902–8.
Kuhle J, Gaiottino J, Leppert D, Petzold A, Bestwick JP, Malaspina A, et al. Serum neurofilament light chain is a biomarker of human spinal cord injury severity and outcome. J Neurol Neurosurg Psychiatry. 2015;86: 273–9.
Lee SJ, Kim CW, Lee KJ, Choe JW, Kim SE, Oh JH, et al. Elevated serum S100B levels in acute spinal fracture without head injury. Emerg Med J. 2010;27: 209–12.
Li H, Zhao D, Zhang M, editors. Temporal expression MicroRNA-21 in serum of patients with spinal cord injury. International conference on biomedical and biological engineering. Proceedings of the 2016 International Conference on Biomedical and Biological Engineering. Atlantis Press; 2016. pp. 116–22.
Moghaddam A, Child C, Bruckner T, Gerner HJ, Daniel V, Biglari B. Posttraumatic inflammation as a key to neuroregeneration after traumatic spinal cord injury. Int J Mol Sci. 2015;16: 7900–16.
Moghaddam A, Heller R, Daniel V, Swing T, Akbar M, Gerner HJ, et al. Exploratory study to suggest the possibility of MMP-8 and MMP-9 serum levels as early markers for remission after traumatic spinal cord injury. Spinal Cord. 2017;55: 8–15.
Moghaddam A, Sperl A, Heller R, Gerner HJ, Biglari B. sCD95L in serum after spinal cord injury. Spinal Cord. 2016;54: 957–60.
Paczkowska E, Rogińska D, Pius-Sadowska E, Jurewicz A, Piecyk K, Safranow K, et al. Evidence for proangiogenic cellular and humoral systemic response in patients with acute onset of spinal cord injury. J Spinal Cord Med. 2015;38: 729–44.
Papatheodorou A, Stein A, Bank M, Sison CP, Gibbs K, Davies P, et al. High-Mobility Group Box 1 (HMGB1) Is Elevated Systemically in Persons with Acute or Chronic Traumatic Spinal Cord Injury. J neurotrauma. 2017;34: 746–54.
Singh A, Kumar V, Ali S, Mahdi AA, Srivastava RN. Phosphorylated neurofilament heavy: A potential blood biomarker to evaluate the severity of acute spinal cord injuries in adults. Int J Crit Illn Inj Sci. 2017;7: 212–7.
Wang HC, Lin YT, Hsu SY, Tsai NW, Lai YR, Su BY, et al. Serial plasma DNA levels as predictors of outcome in patients with acute traumatic cervical spinal cord injury. J Transl Med. 2019;17: 329.
Wolf H, Krall C, Pajenda G, Leitgeb J, Bukaty AJ, Hajdu S, et al. Alterations of the biomarker S-100B and NSE in patients with acute vertebral spine fractures. Spine J. 2014;14: 2918–22.
Xu L, Zhang Y, Zhang R, Zhang H, Song P, Ma T, et al. Elevated plasma BDNF levels are correlated with NK cell activation in patients with traumatic spinal cord injury. Int Immunopharmacol. 2019;74: 105722.
Zhao P, Wang S, Zhou Y, Zheng H, Zhao G. MicroRNA-185 regulates spinal cord injuries induced by thoracolumbar spine compression fractures by targeting transforming growth factor-β1. Exp Therapeutic Med. 2017;13: 1127–32.
Bickenbach J, Shakespeare T, von Groote PM, World Health Organization, International Spinal Cord Society. International perspectives on spinal cord injury. World Health Organization; 2013. online source: https://www.who.int/publications/i/item/international-perspectives-on-spinal-cord-injury .
Grassner L, Maier D. Impact of surgery on the outcome after spinal cord injury - current concepts and an outlook into the future. Neural Regen Res. 2016;11: 1928–9.
Fehlings MG, Vaccaro A, Wilson JR, Singh A, Cadotte DW, Harrop JS, et al. Early versus delayed decompression for traumatic cervical spinal cord injury: results of the Surgical Timing in Acute Spinal Cord Injury Study (STASCIS). PloS One. 2012;7: e32037.
Silva NA, Sousa N, Reis RL, Salgado AJ. From basics to clinical: a comprehensive review on spinal cord injury. Prog Neurobiol. 2014;114: 25–57.
Rodrigues LF, Moura-Neto V. Biomarkers in Spinal Cord Injury: from Prognosis to Treatment. Mol Neurobiol. 2018;55: 6436–48. TCLS ES
Anwar MA, Al Shehabi TS, Eid AH. Inflammogenesis of Secondary Spinal Cord Injury. Front Cell Neurosci. 2016;10: 98.
Tator CH, Fehlings MG. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg. 1991;75: 15–26.
Winter B, Pattani H, Temple E. Spinal cord injury. Anaesth Intensive Care Med. 2014;15: 424–7.
Haider T, Höftberger R, Rüger B, Mildner M, Blumer R, Mitterbauer A, et al. The secretome of apoptotic human peripheral blood mononuclear cells attenuates secondary damage following spinal cord injury in rats. Exp Neurol. 2015;267: 230–42.
Denslow N, Michel ME, Temple MD, Hsu CY, Saatman K, Hayes RL. Application of proteomics technology to the field of neurotrauma. J Neurotrauma. 2003;20: 401–7.
Wang KK, Ottens A, Haskins W, Liu MC, Kobeissy F, Denslow N, et al. Proteomics studies of traumatic brain injury. Int Rev Neurobiol. 2004;61: 215–40.
Alemi-Neissi A, Rosselli FB, Zoccolan D. Multifeatural shape processing in rats engaged in invariant visual object recognition. J Neurosci. 2013;33: 5939–56.
Pouw MH, Kwon BK, Verbeek MM, Vos PE, van Kampen A, Fisher CG, et al. Structural biomarkers in the cerebrospinal fluid within 24 h after a traumatic spinal cord injury: a descriptive analysis of 16 subjects. Spinal Cord. 2014;52: 428–33.
Marquardt G, Setzer M, Seifert V. Serum biomarkers for experimental acute spinal cord injury: rapid elevation of neuron-specific enolase and S-100 beta. Neurosurgery. 2006;58: E590.
Dalkilic T, Fallah N, Noonan VK, Salimi Elizei S, Dong K, Belanger L, et al. Predicting Injury Severity and Neurological Recovery after Acute Cervical Spinal Cord Injury: A Comparison of Cerebrospinal Fluid and Magnetic Resonance Imaging Biomarkers. J Neurotrauma. 2018;35: 435–45.
Kwon BK, Stammers AM, Belanger LM, Bernardo A, Chan D, Bishop CM, et al. Cerebrospinal fluid inflammatory cytokines and biomarkers of injury severity in acute human spinal cord injury. J Neurotrauma. 2010;27: 669–82.
Yokobori S, Zhang Z, Moghieb A, Mondello S, Gajavelli S, Dietrich WD, et al. Acute diagnostic biomarkers for spinal cord injury: review of the literature and preliminary research report. World Neurosurg. 2015;83: 867–78.
Leister I, Haider T, Mattiassich G, Kramer JLK, Linde LD, Pajalic A, et al. Biomarkers in Traumatic Spinal Cord Injury-Technical and Clinical Considerations: A Systematic Review. Neurorehabilit Neural Repair. 2020;34: 95–110.
Faridaalee G, Keyghobadi Khajeh F. Serum and Cerebrospinal Fluid Levels of S-100β Is A Biomarker for Spinal Cord Injury; a Systematic Review and Meta-Analysis. Arch Acad Emerg Med. 2019;7: e19.
PubMed PubMed Central Google Scholar
Sroga JM, Jones TB, Kigerl KA, McGaughy VM, Popovich PG. Rats and mice exhibit distinct inflammatory reactions after spinal cord injury. J Comp Neurol. 2003;462: 223–40.
Nakamura M, Houghtling RA, MacArthur L, Bayer BM, Bregman BS. Differences in cytokine gene expression profile between acute and secondary injury in adult rat spinal cord. Exp Neurol. 2003;184: 313–25.
Blight AR, Leroy EC Jr., Heyes MP. Quinolinic acid accumulation in injured spinal cord: time course, distribution, and species differences between rat and guinea pig. J Neurotrauma. 1997;14: 89–98.
Blight AR, Tuszynski MH. Clinical trials in spinal cord injury. J Neurotrauma. 2006;23: 586–93.
Tuszynski MH, Grill R, Jones LL, McKay HM, Blesch A. Spontaneous and augmented growth of axons in the primate spinal cord: effects of local injury and nerve growth factor-secreting cell grafts. J Comp Neurol. 2002;449: 88–101.
Courtine G, Bunge MB, Fawcett JW, Grossman RG, Kaas JH, Lemon R, et al. Can experiments in nonhuman primates expedite the translation of treatments for spinal cord injury in humans? Nat Med. 2007;13: 561–6.
Download references
Acknowledgements
The authors thank Shahid Beheshti University of Medical Science for their help and support.
Author information
These authors contributed equally: Sina Shool, Saeed Rahmani, Mohammad Amin Habibi.
Authors and Affiliations
Department of Neurosurgery, Loghman Hakim Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Sina Shool, Saeed Rahmani, Mohammad Amin Habibi & Delara Jashnani
Sina Trauma and Surgery Research Center, Sina Hospital, Tehran University of Medical Sciences, Hassan-Abad Square, Imam Khomeini Ave, 11365-3876, Tehran, Iran
Sina Shool, Mohammad Amin Habibi & Seyed Mohammad Piri
Resident of Neurosurgery, Department of Neurosurgery, Klinikum Saarbrücken, University of Saarland, Saarbrücken, Germany
Mahmoud Lotfinia
Department of Surgery, Division of Acute Care Surgery, Loma Linda University, Loma Linda, CA, USA
Sina Asaadi
You can also search for this author in PubMed Google Scholar
Contributions
SS was responsible for: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Roles/Writing - original draft; Writing - review & editing. SR was responsible for: Data curation; Roles/Writing - original draft; MAH was responsible for: Conceptualization; Roles/Writing - original draft; Writing - review & editing. SMP was responsible for: Data curation; Roles/Writing - original draft; ML was responsible for: Data curation; Roles/Writing - original draft; DJ was responsible for: Data curation; Roles/Writing - original draft; SA was responsible for: Conceptualization; Project administration; Resources; Supervision; Writing - review & editing.
Corresponding author
Correspondence to Sina Asaadi .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Ethics approval
The present study received the approval of the Ethics Committee of Shahid Beheshti University of Medical Sciences with reference number No: IR.SBMU.RETECH.REC.1399.478.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplemental tables, rights and permissions.
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Shool, S., Rahmani, S., Habibi, M.A. et al. Acute spinal cord injury serum biomarkers in human and rat: a scoping systematic review. Spinal Cord Ser Cases 10 , 21 (2024). https://doi.org/10.1038/s41394-024-00636-3
Download citation
Received : 29 March 2023
Revised : 28 March 2024
Accepted : 08 April 2024
Published : 13 April 2024
DOI : https://doi.org/10.1038/s41394-024-00636-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- Open access
- Published: 05 December 2023
Acute care models for older people living with frailty: a systematic review and taxonomy
- Thomas Knight 1 ,
- Vicky Kamwa 1 ,
- Catherine Atkin 1 ,
- Catherine Green 2 ,
- Janahan Ragunathan 3 ,
- Daniel Lasserson 4 &
- Elizabeth Sapey 1
BMC Geriatrics volume 23 , Article number: 809 ( 2023 ) Cite this article
988 Accesses
1 Altmetric
Metrics details
The need to improve the acute care pathway to meet the care needs of older people living with frailty is a strategic priority for many healthcare systems. The optimal care model for this patient group is unclear.
A systematic review was conducted to derive a taxonomy of acute care models for older people with acute medical illness and describe the outcomes used to assess their effectiveness. Care models providing time-limited episodes of care (up to 14 days) within 48 h of presentation to patients over the age of 65 with acute medical illness were included. Care models based in hospital and community settings were eligible.
Searches were undertaken in Medline, Embase, CINAHL and Cochrane databases. Interventions were described and classified in detail using a modified version of the TIDIeR checklist for complex interventions. Outcomes were described and classified using the Core Outcome Measures in Effectiveness Trials (COMET) taxonomy. Risk of bias was assessed using RoB2 and ROBINS-I.
The inclusion criteria were met by 103 articles. Four classes of acute care model were identified, acute-bed based care, hospital at home, emergency department in-reach and care home models. The field is dominated by small single centre randomised and non-randomised studies. Most studies were judged to be at risk of bias. A range of outcome measures were reported with little consistency between studies. Evidence of effectiveness was limited.
Acute care models for older people living with frailty are heterogenous. The clinical effectiveness of these models cannot be conclusively established from the available evidence.
Trial registration
PROSPERO registration (CRD42021279131).
Peer Review reports
Introduction
Population ageing and the increasing prevalence of long-term health conditions represent a significant challenge to many advanced health care systems [ 1 ]. Older people, particularly those living with frailty and multimorbidity, are at high risk of sudden health crisis necessitating urgent assessment to identify and treat causative conditions. The acute care pathway collectively defines the clinical processes employed to achieve this function. It typically comprises sequential assessment in community and hospital settings and culminates in emergency hospital admission when necessary.
Older people living with frailty are at high risk of adverse outcomes such as mortality [ 2 ] and have longer average lengths of hospital stay when accessing the acute care pathway [ 3 ]. The conversion rate from ED attendance to emergency admission is 3 times higher in people aged over 85 relative to people under 65 [ 4 ]. As older people represent a growing proportion of ED attendances the demand for hospital bed-based care is likely to rise [ 4 ]. This must be reconciled with downward trends in the number of acute hospital beds at the population level [ 5 ]. Improved integration between health and social care may help mitigate the impact of these changes to some degree but will not abrogate the need for hospital assessment and inpatient bed-based care in the context of sudden deterioration or severe illness [ 6 ]. Adaptations to the acute care pathway may improve the quality of care for older people while simultaneously reducing pressure on an increasingly congested acute care system.
These factors have collectively driven a rapid expansion of studies investigating models of care intended to mitigate the risk of hospital admission or avoid bed-based hospital care entirely [ 7 ]. Previous systematic reviews of acute care models for older people have focused on interventions located at specific points along the acute care pathway [ 8 , 9 , 10 ]. There has been a tendency to group interventions with different eligibility criteria and clinical processes. Differentiating models of care able to manage acute illness from those primarily engaged with rehabilitation and the functional consequence of resolving acute illness is not straightforward. This distinction is important as policy makers and commissioners look to maximise the efficiency of acute hospital bed use and find credible alternatives to acute inpatient care in the community.
It is possible that a more granular classification of the interventions may foster a greater understanding of which elements of the model drive effectiveness and highlight areas of best practice.
A systematic review was undertaken to describe and classify the range of acute care models designed to manage acute medical illness in older people with the objective of deriving a taxonomy of care models. The review also aimed to describe and classify the outcome measures used in studies investigating these models. A secondary objective was to determine whether the proposed taxonomy was useful in understanding any differences in observed outcomes between studies. We took the novel approach of including acute care models operating in hospital and community settings.
Study design
The systematic review was conducted using a two-step process. The first step was undertaken to describe and classify acute care models for older people and the outcome measures used to demonstrate their clinical effectiveness within the current literature. This information was used to create a taxonomy of care models accompanied by a narrative summary. No restrictions were placed on study design at this stage of the process.
The second step looked to describe the effectiveness of each model and restricted analysis to randomised controlled trials or observational studies with an experimental design (including non-randomised trials, cohort studies with comparator groups, before and after longitudinal studies). Previous systematic reviews and meta-analyses were not used to inform the taxonomy. Primary studies from relevant systematic reviews were included if they met the inclusion criteria. The systematic review was undertaken in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline. The study protocol was registered with PROSPERO (CRD42021279131).
Eligibility criteria and study selection
Inclusion and exclusion criteria were designed to incorporate interventions operating within the hospital and the community. An age threshold of 65 years was used to define care models for older people (mean age of study participants > 65 years. Mean age as opposed to a strict age threshold was employed to ensure care models accepting younger patients with frailty identified using alternative measures, such as validated frailty scores or multi-morbidity were not excluded.
The intervention needed to target acute medical illness or acute exacerbation of chronic disease. There is no consensus definition of acute care. To ensure a focus on acute care, study participants needed to be recruited within 48 h of presentation and the care model had to provide time limited episodes of care (up to 14 days). The requirement for time limited episodes of care was used as a criterion to exclude care models delivering ongoing chronic disease management after resolution of acute illness which were felt likely to employ different care processes and focus on different clinical outcomes. Recruitment direct from the ED was used as a proxy for recruitment within 48 h in studies where this metric was not reported. Community interventions were only included if they were able to provide a credible alternative to hospital bed-based care. This was defined as the capability to provide face-to-face review alongside access to hospital level treatments (eg intravenous treatments) and hospital level diagnostics (eg blood tests, imaging) at home.
A full list of inclusion and exclusion criteria is provided in Table 1 .
Data sources and searches
The search strategy comprised both MeSH terms and keyword text and was performed on 30 th September 2021 with no date restrictions. The search strategy is provided in Supplementary Table 1 . The search was undertaken in 5 electronic databases (Ovid MEDLINE, Ovid Embase, Cumulative Index to Nursing and Allied Health Literature, Cochrane Database of systematic reviews, Cochrane Central Register of Controlled Trials). Hand reference list screening was carried out of all included articles. Systematic reviews were not included directly. All individual studies meeting the inclusion criteria contained within systematic reviews identified by the search were included.
Titles and abstracts were reviewed by two reviewers. (TK reviewed each and at-least one further review from CA, VK, CG, JR). Full-text records were obtained and reviewed against the eligibility criteria. Disagreements were resolved by a third reviewer (DL). Data extraction was undertaken by 1 reviewer (TK). A bespoke data extraction tool was adapted from the TIDIeR checklist to characterise each intervention [ 11 ]. Outcome measurements were classified using the Core Outcome Measures in Effectiveness Trials (COMET) taxonomy [ 12 ].
Data extraction and quality assessment
Risk of bias was assessed using criteria from the Cochrane Handbook. Randomised controlled trials were assessed using RoB-2 tool [ 13 ] and observational studies were assessed using the ROBINS-I tool [ 14 ]. Risk of bias was assessed by 1 reviewer (TK).
Data synthesis
Finding from included articles were grouped and summarised. Due to clinical heterogeneity between studies meta-analysis was not appropriate. A narrative synthesis of the results was undertaken. Visualisations were created using R statistical software (Version 1.3.1093, Vienna. Austria). The geographical location of included studies was mapped using the ggmap package. Source maps were obtained from © Stamen Design, under a Creative Commons Attribution (CC BY 3.0) license. Outcome areas and domains were plotted using the treemap package.
The initial search returned 13,102 relevant articles. Title and abstract screening identified 340 relevant articles for full text review. A total of 90 articles met the eligibility criteria. Hand searching of references identified 13 further articles. Therefore, 103 articles were included in the analysis (see Fig. 1 ). Identified articles were published between April 1991 and April 2021. This comprised 20 randomised controlled trials reported across 26 articles), 6 study protocols (results for 2 had been reported and were included), 38 observational studies with a comparator group reported across 51 articles, and 20 descriptive studies without a comparator group. The search identified 101 conference abstracts which did not contain sufficient information to adequately describe the model of care delivered. These abstracts were not used to inform the taxonomy.
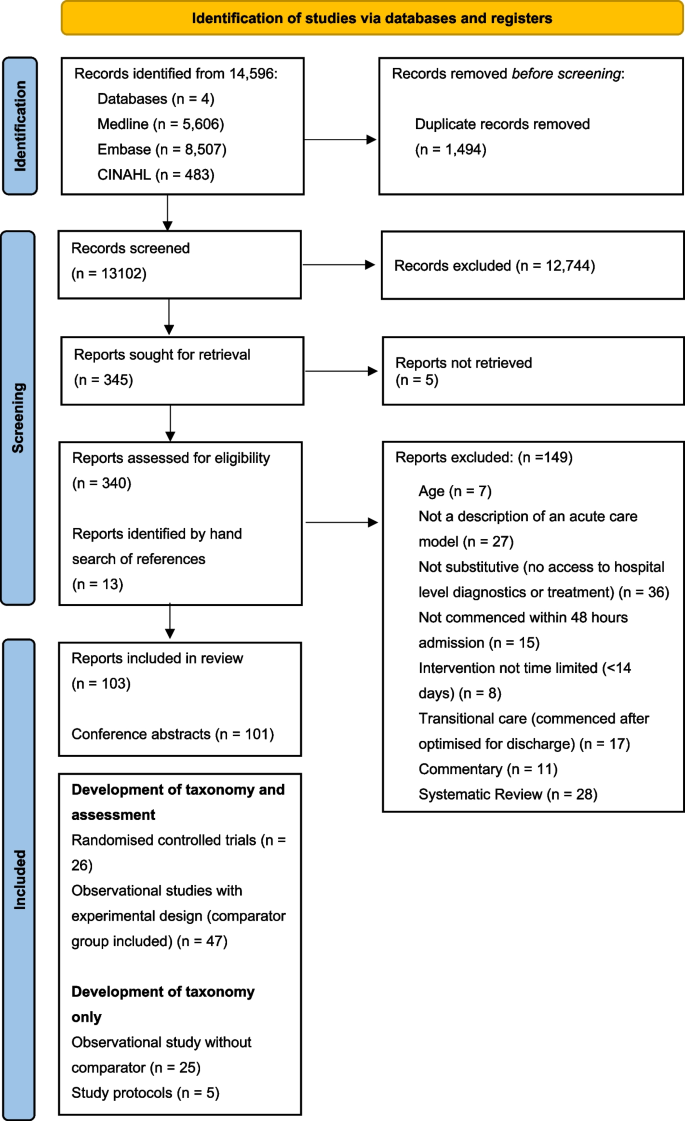
A PRISMA flow diagram for the studies screened and included in the systematic review. Legend: Studies were screened against the inclusion and exclusion criteria described in Table 1 . Reasons for exclusion are provided
The articles could be broadly categorised into four groups based on the model of care they described. These included: bedded acute frailty units (AFU), Hospital at Home models (HaH), ED based in-reach models and acute care home models, see Fig. 2 . A detailed description of the interventions described in each individual study is provided in Supplementary Table 2 . The geographical location of included studies is provided in Fig. 3 .
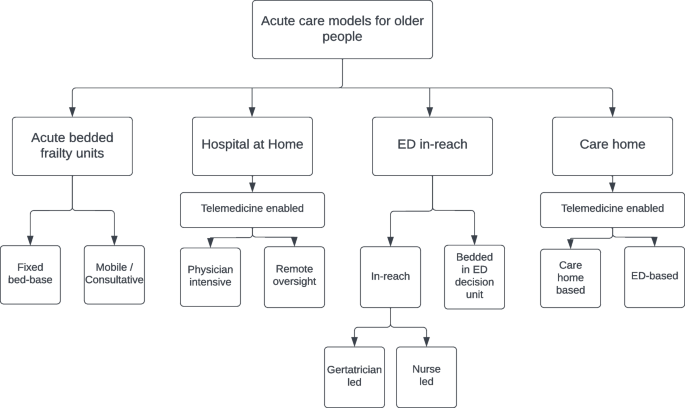
The Proposed taxonomy of acute care models for older people. Legend: The taxonomy was defined using key features of the care models; Care models were initially differentiated based on location. Acute bedded frailty units operated from a fixed bed base or offering consultation to general medical wards. Hospital at home models were differentiated based on their use of telemedicine. Physician intensive models used face to face review at home as standard. Remote oversight models were primarily delivered by specialist nurses with care supported provided remotely by physicians on a selective basis. Emergency Department in reach models could be differentiated by their staffing model. Nurse led care coordination without direct input from a dedicated geriatrician or care delivered by geriatricians within the Emergency Department. Care home models were differentiated by their primary location of activity, either services offered within the care home or adaptations to the care pathway following transfer to the Emergency Department
A map identifying the countries where the included studies were based. Legend. The map shows the location of included studies identifying: Colours to denote the care model type as defined by the taxonomy. Brown dots represent Hospital at Home models, Violet dots represents bedded Acute Frailty Units. Purple dots Emergency Department in-reach models. Green dots care models. Source maps were obtained from © Stamen Design, under a Creative Commons Attribution (CC BY 3.0) license
Bedded acute frailty units models
The provision of tailored bed-based in-patient care for frail adults as a direct alternative to treatment on a general medical ward was described in 32 articles derived from 24 studies. This included 8 articles [ 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 ] reporting results from 6 randomised controlled trials, 1 trial protocol without results [ 23 ], 11 observational studies with a comparator group reported across 15 articles [ 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 ] and 8 descriptive studies without a comparator [ 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 ]. A detailed description of the care models is provided in Supplementary Table 2 A.
The AFU care model has a strong focus on maintaining and restoring function, but in contrast to a rehabilitation ward intervenes prior to full resolution of acute illness. A range of names were used to identify care models with similar underlying approaches, including Acute Frailty units (AFU), Acute Care for Elders (ACE) units and CGA units. Generic descriptions of the model frequently reference four core components, patient centred care, specifically designed environments, review of medical care and early discharge planning as key characteristics of the model. There was considerable variation in how these shared high-level objectives were operationalised within individual care models.
Treatment was delivered within a geographically distinct bedded unit in 20 studies [ 15 , 16 , 17 , 18 , 19 , 21 , 22 , 23 , 24 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 34 , 35 , 38 , 39 , 42 , 44 , 45 , 46 ], of which 7 specifically reported adaptations to optimise the environment for older people [ 15 , 17 , 18 , 23 , 24 , 39 , 41 ]. The mean number of beds in each unit was 18 (SD 8). The number of beds was not reported in 3 studies [ 25 , 41 , 46 ]. A mobile model providing specialist consultations to patients within general medical bed was described in 3 studies [ 20 , 33 , 36 ] (and an integrated service with variable bed capacity operating within an acute medical unit in 1 study [ 45 ].
Eligibility criteria were heterogenous. Age criteria were reported in studies describing 20 care models [ 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 35 , 36 , 37 , 38 , 39 , 41 , 42 , 44 , 45 ]. Descriptions of the process of patient referral and how eligibility criteria were implemented in practice were uncommon. The presence of additional criteria such as functional impairment or specific geriatric conditions were frequently reported, but it was not possible to establish how these criteria were operationalised. The use of validated frailty assessment tools to define eligible patients were reported in 1 study (reported across 5 articles) [ 26 , 28 , 29 , 30 , 31 ]. Patients from residential care homes were excluded in 2 studies [ 18 , 21 ]. Bed availability was cited as a common determinant of receiving treatment on the AFU.
Hospital at home models
Hospital at home (HaH) models describe the provision of acute medical care within a person’s usual place of residence. The care model aims to replicate acute bed-based care and operate under the assumption that care would be delivered in an acute hospital setting if the model were absent. HaH models were described in 37 articles derived from 27 studies. This included 16 articles [ 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 ] reporting results from 12 randomised controlled, 2 protocols (of which 1 had reported results and was included) [ 63 , 64 ], 9 observational studies with a comparator group reported across 15 articles [ 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 ] and 4 descriptive studies without a comparator group [ 79 , 80 , 81 , 82 ]. A detailed description of the care models is provided in Supplementary Table 2 B.
There was significant clinical heterogeneity between included HaH models. The model accommodated patients with unselected acute medical illness in 31 studies and specific disease groups in 7 studies (decompensated heart failure = 3 [ 57 , 58 , 62 ], COPD = 4 [ 47 , 51 , 52 , 70 , 79 ]).
Eligibility criteria to define suitability for HaH care were heterogenous. All included studies made the intention to act as an alternative to hospital bed-based care explicit. Clinical discretion exercised by the HaH team was the arbiter of the appropriateness and safety of HaH care in all the identified studies. No standardised approach to assessment was identified and it was not possible to reliably determine the acuity of included patients from the reported data. The majority of HaH studies specifically targeted adults over the age of 65. In models open to adults of all ages, the mean age of participants was over 65 in all cases. Care home residents were excluded in 9 studies [ 53 , 58 , 59 , 63 , 67 , 73 , 74 , 75 , 80 ].
Care was led by a geriatrician in 6 studies, [ 47 , 59 , 61 , 62 , 73 , 78 ] by a general internal medicine physician in 29 studies and a primary care physician in 2 studies [ 60 , 83 ]. The intensity of physician and nursing involvement varied substantially. Physician involvement ranged from multiple daily physical home visits to remote oversight without direct physical assessment. Specific out-of-hours arrangements were reported in 12 studies reported across 19 articles [ 47 , 53 , 54 , 55 , 61 , 62 , 65 , 67 , 68 , 69 , 71 , 72 , 74 , 75 , 76 , 77 , 81 , 82 , 83 ]. The use of telemedicine was described in 5 studies reported across 11 articles [ 47 , 65 , 66 , 68 , 69 , 71 , 72 , 74 , 75 , 76 , 77 ]. Reporting of the study intervention was often restricted to a description of standardised operating procedure. The frequency of assessment achieved in practice was reported in 6 studies [ 52 , 53 , 58 , 74 , 75 , 81 ] and the proportion of patients receiving specific treatments was reported in 3 studies [ 47 , 53 , 80 ].
ED in-reach models
ED in-reach models aim to optimise processes of care for older people in the ED. The care models typically provide care coordination and elements of CGA to reduce the likelihood of admission to acute-bed based care. ED in-reach models were described in 28 studies describing 27 care models. This included 2 randomised controlled trials, [ 84 , 85 ] 1 randomised controlled trial protocol without results [ 86 ], 12 observational studies with a comparator group [ 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 ] and 13 descriptive studies without a comparator group [ 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 ]. A detailed description of the care models is provided in Supplementary Table 2 C.
Two distinct approaches to the operational design of services were evident. One approach, described in 11 studies, involved the use of bedded areas located within ED clinical decision units (alternatively referred to as ED short stay units) to provide elements of CGA to older patients who required additional assessment and investigation before a decision regarding acute medical admission could be reached [ 87 , 89 , 90 , 91 , 94 , 96 , 104 , 105 , 107 , 109 , 111 ].
An alternative approach, described in 20 studies, involved the provision of elements of CGA directly within the ED. CGA was undertaken by a geriatrician in 10 care models [ 84 , 88 , 97 , 100 , 101 , 102 , 103 , 108 , 110 ] and by specially trained nurses in 7 care models [ 85 , 86 , 92 , 93 , 95 , 98 , 99 , 106 ]. Studies of this care model frequently cited a reduction in the number of avoidable medical admissions as the primary motivation for the service. The distinction between avoidable and unavoidable admissions was poorly defined.
Eligibility criteria were heterogenous. Age criteria were reported in 13 care models [ 84 , 88 , 91 , 93 , 94 , 95 , 98 , 99 , 102 , 103 , 104 , 106 , 108 , 112 ]. The use of validated frailty assessment tools to define eligible patients were reported in 5 care models [ 84 , 86 , 92 , 99 , 106 ]. Care home residents were excluded in 3 studies [ 86 , 91 , 94 ]. Eligibility criteria were not reported in 5 studies [ 87 , 89 , 91 , 109 , 110 ]. A variety of approaches were adopted to identifying potentially eligible patients in the ED. Screening of all patients attending the ED was reported in 3 studies [ 84 , 88 , 93 ]. The service was accessed by a referral from the ED team in 11 care models [ 89 , 90 , 92 , 95 , 98 , 99 , 100 , 101 , 104 , 109 , 110 ]. The process of referral and patient selection were not consistently reported.
Acute care home models
Models targeting care home residents were reported in 5 studies. All 5 studies had an observational design [ 113 , 114 , 115 , 116 , 117 ]. Two categories of intervention were described. The first involved the presence of dedicated staff trained in acute care present with the care home [ 113 , 117 ]. These staff had the ability to deliver acute interventions in the care home. Privileged access was given to the on-call ED physician in both models (augmented by telemedicine in one study) [ 117 ]. The process which triggered assessment by the on-site team were not defined. A detailed description of the care models is provided in Supplementary Table 2 D.
An alternative model involved a hospital-based team providing out-reach to care homes and early assessment of care home resident presenting to ED. Both care models in this category also had the capability to provide ongoing acute care in the care home when required. This was achieved by a geriatrician-led team with the option to provide daily visits in one model [ 116 ] and a specialist ED nursing team in the other [ 114 , 115 ].
Outcome measurements
Outcomes were classified using the COMET taxonomy. Outcomes were reported across 6 core areas and 15 domains. Mortality was reported (in isolation or as part of a composite outcome) in 35 studies, the reporting time horizon ranged from in-hospital mortality to 1 year. Life impact was reported 27 studies, this included measurement of physical function in 21 studies and cognitive function in 6 studies. The tools used to measure physical function and the time horizons of assessment varied.
Resource use was the most reported core outcome measure. Studies frequently described multiple outcome domains related to resource use. The average length of stay was reported in 34 studies and re-admission rate in 39 studies. Readmission rates were reported over a range of time horizons 30 days to 1 year. Care home admission were reported (in isolation of as part of a composite outcome) in 14 studies over a time horizon of 30 days to 6 months. Economic analysis was reported in 19 studies. Adverse events were reported in 22 studies. A detailed summary of the outcome domains, methods of measurement and associated time horizons is provided in Supplementary Table 3 .
The relative frequency with which the outcome domains were reported across all studies is provided in Fig. 4 A and stratified by care model in Fig. 4 B. Outcomes reported by bedded AFU and HaH were broadly similar, although AFU more commonly reported outcomes related to physical function. Economic analysis was less prevalent in studies investigating ED in-reach models. A focus on aspects of care delivery, such as disposition from the ED and analysis of clinical processes relevant to the quality and adequacy of intervention were more common in studies evaluating ED in-reach.
Tree diagrams: A tree diagrams representing the relative proportion of outcomes reported in all studies. B Tree diagrams representing the relative proportion of studies by study group. Legend. * Treemap representing hierarchical outcome data using nested rectangles. Large rectangle represent core outcome areas, smaller rectangular tiles within each core outcome area represent outcome domains. Each rectangle has an area proportional to the frequency reported within included studies. All studies n = 103, Bedded acute frailty unit n = 32, Hospital at Home n = 38, ED in reach models n = 28, Care home n = 5
Effectiveness
Clinical heterogeneity amongst the care models identified and disparity in the outcomes measured used to evaluate the care models precluded meta-analysis. Risk of bias was assessed for each study. Aggregated results of the domain-based risk of bias assessment tools are provided in Fig. 5 and the results of individual study assessments are provided in Supplementary Table 4 .
Summary of bias assessments. A Summary of randomised controlled studies using RoB2 tool. B Summary of non-randomised studies using ROBINS-I tool
The nature of the intervention precluded blinding of participants or personnel to group allocation in all included randomised controlled trials. Partial blinding of outcome assessment was reported in one study investigating the effectiveness of bedded AFUs [ 17 ] and assessment was unblinded in the remainder. Blinding during outcome assessment was reported in 4 randomised controlled trials investigating HaH [ 47 , 52 , 59 , 60 ]. Outcome assessment was unblinded in both randomised controlled trials investigating ED in-reach models [ 84 , 85 ]. All the studies investigating bedded AFUs were undertaken in single sites which may have led to contamination of the control arm. This would be anticipated to favour the null hypothesis [ 15 , 16 , 18 , 19 , 20 , 21 , 22 ]. Contamination of the control arm was less likely in HaH models delivered by distinct clinical teams.
All included observational studies were at serious or critical risk of confounding. The decision to manage patients in the intervention arm is likely to have been selective, based on clinical judgment informed by pre-intervention clinical characteristics. Only 5 studies employed robust statistical techniques to control for confounding [ 65 , 67 , 69 , 78 , 92 ]. Residual confounding from unmeasured prognostic factors posed at risk of bias all included observational studies.
Effectiveness of acute care models
Bedded acute frailty unit models.
No statistical difference in primary outcome was observed in 2 randomised controlled trials (reported across 3 articles) of specialist bed-based care for unselected older medical patients, 1 study measured the composite outcome of death, severe dependence and psychological well-being [ 15 ] and the other physical function at 3 months following discharge [ 19 ]. A planned cost-analysis demonstrated no difference in the total cost of admission between groups [ 16 ]. A single centre randomised controlled trial comparing a specialist unit for acutely unwell patients with cognitive impairment with usual care demonstrated no statistical difference in the composite outcome of days at home [ 17 ]. All included observational studies were judged to be at critical or serious risk of bias.
The largest randomised controlled trial included 1055 participants [ 59 ]. The study was designed to recruit to the HaH intervention at a ratio of 2:1. A significant number of participants moved from the control to the intervention arm due to operational pressures within the hospital. The study found no difference in the primary outcome of living at home at 6 months (the inverse of death or long-term residential care) [ 59 ]. The remaining 11 trials (reported across 15 articles) had smaller sample sizes (mean 81 participants, SD 33). One randomised controlled trial (2 articles) reported a statistically significant reduction in the rate of adverse events [ 50 ] and favourable functional outcomes in the group allocated to HaH care [ 49 ].
HaH care for older people with decompensated heart failure was investigated in 2 randomised controlled trials, 1 reported no difference in mortality or readmission at 6 months [ 62 ] and 1 no difference in mortality or readmission at 12 months [ 57 ]. HaH care for older people with an acute exacerbation of COPD was investigated in 2 randomised controlled trials, 1 reported a statistically significant reduction in readmissions at 6 months and no difference in mortality at 6 months [ 47 ] and 1 reported lower costs at 90 days, driven by shorted length of stay in the HaH group, with no difference in mortality or readmission rate at 90 days [ 52 ]. Economic analysis determined HaH was associated with lower costs in 1 randomised controlled trial of participants with unselected medical-illness [ 53 ]. Nested analysis of patient and carer satisfaction was included in 5 trials [ 47 , 52 , 53 , 59 , 62 ] in 3 trials the findings were reported in separate articles [ 51 , 55 , 66 ]. All showed an increase in measures of patient satisfaction in the HaH intervention group.
One randomised trial compared two contrasting models of HaH. The study arms compared HaH care led by primary care physicians with care led by hospital specialists [ 60 ]. Those in the hospital specialist arm were initially assessed in the ED and discharged within 4 h of assessment with a home-based care plan. The hospital specialist team did not undertake home visits. Those in the primary care physician arm received care exclusively at home. In both arms the plan care was delivered by a dedicated HaH nursing team. The primary care physician model was a associated with a statistically significant reduction in hospital admission at 7 days. A series of articles published as part of a non-randomised controlled trial [ 75 ] reported a reduction in length of admission, [ 75 ] reduced levels of carer stress [ 71 ] and no difference in physical function [ 72 ] in the HaH group.
ED in reach models
No statistical difference in the primary outcome measure was observed in 2 randomised controlled trials investigating ED in-reach models. In one study the provision of geriatrician lead CGA to patients aged over 75 with a clinical frailty scale (CFS) of 4 or above did not affect cumulative length of stay over a 1 year follow up period [ 84 ]. A randomised controlled trial investigating provision of nurse-led care coordination in the ED found no significant effect on the rate of hospital admission [ 85 ]. Uncontrolled before and after studies were a common methodological approach to the assessment of ED in-reach models, employed in 5 studies. All included observational studies were judged to be at serious risk of bias.
This systematic review provides a summary and classification of acute care models for older people living with frailty and an assessment of effectiveness based on current published evidence. The care models identified could be broadly differentiated by the location within the acute care pathway at which they operate. This generic classification provides a degree of structure to a large and complicated field of research, sensitive to the fact that relevant interventions have emerged across hospital and community settings. The spectrum of outcomes reported and differing approaches to measurement suggest consensus on how best to determine the effectiveness of these care models has yet to emerge.
The clinical effectiveness of acute care models for older people was difficult to determine from the available studies. The number of participants within each trial was small. The risk of confounding by indication was pervasive amongst observational studies and statistical techniques to control for cofounding were generally absent or inadequate. These methodological limitations prevented meaningful comparisons of the impact on outcomes between care models. There is a paucity of contemporary data on the effectiveness of acute care models for older people. Some of the most influential studies were conducted over two decades ago. This raises the concern that the clinical processes employed may now be obsolete.
Complex interventions, such as acute care models for older people are often difficult to characterise. The detailed summary of individual interventions provided within this review highlights the contrasting approaches adopted by services under the same umbrella.
Few studies adopted a structured approach to defining the intervention under investigation and the descriptions provided varied in depth and quality. The nature of care provided in the usual care arm of comparative studies was equally difficult to define. The absence of consistent inclusion and exclusion criteria or knowledge of how criteria were operationalised makes it difficult to discern the population targeted by each intervention. Assignment often incorporated a subjective assessment by an individual clinician acting as gatekeeper. Thresholds for admission and discharge are not standardised and risk tolerance may vary at the individual, hospital and system level. This is particularly pertinent to studies investigating the role of HaH and ED in-reach models, predicated on the assumption that care would inevitably require in-patient bed-based care if the intervention was absent. This assumption is inherently difficult to substantiate. All the HaH models included in this systematic review had access to hospital level diagnostics and interventions but the proportion of patients receiving these interventions were inconsistently reported. This obfuscates an objective assessment of acuity and whether hospital admission was warranted.
Comparison with previous literature
Clinical heterogeneity in the studies included in previous systematic reviews and the absence of universally accepted definitions for the care models investigated cloud interpretation of the existing literature. The diverse range of approaches to patient selection, operational design and outcome measurement highlighted in this review suggests caution is warranted when pooling studies in this subject area.
Several systematic reviews investigating acute care models for older people have focused the delivery of comprehensive geriatric assessment (CGA) [ 8 ]. CGA involves multidimensional assessment with particular attention on the functional consequences of illness [ 118 ]. CGA has been shown to increase the likelihood of being alive or returning to home at 3 to 12 months follow up amongst older patients admitted to hospital with acute illness [ 8 ]. Meta-analysis of CGA delivered in bed-based frailty units found a lower risk of functional decline, a higher likelihood of living at home after discharge and no differences in mortality [ 119 ]. CGA delivered in bed-based frailty units may also reduce the incidence of adverse events such as falls, delirium and pressure sores at discharge [ 10 ]. The inclusion of interventions delivered on rehabilitation wards, and patients with surgical and orthopaedic presentations in previous systematic reviews limits generalisation to care models employed at earlier time points in the acute care pathway. The available literature suggests alternatives to usual bed-based care incorporating CGA may be of benefit but offers little to guide how these services should be designed and implemented. When inclusion is limited to interventions employed within 48 h of presentation the evidence of effectiveness is less compelling. This is important given the benefit of CGA is cited as the primary motivation for operational models located upstream in the acute care pathway [ 120 ].
HaH models have also been the subject of systematic review and meta-analysis. A Cochrane review of admission avoidance HaH identified ten randomised controlled trials including 1333 participants of which 850 were included in individual patient level meta-analysis [ 121 ]. The analysis demonstrated a significant reduction in mortality at 6 months (adjusted HR 0.62, 95% CI 0.45–0.87). A more recent systematic review and meta-analysis found patients managed in HaH following discharge from the ED had a lower risk of admission to institutional care (RR 0.16 95% CI 0.03–0.74) and no difference in mortality (RR 0.84 95% CI 0.6–1.2) [ 122 ]. These systematic reviews pooled results from studies investigating HaH in the context of a diverse range of conditions including stroke, cellulitis, fractures and respiratory illness which would be expected to employ very different clinical processes. Applying a more restrictive approach to study inclusion, by only including HaH models with access to hospital level diagnostics and treatments allows greater confidence in the assertion that the HaH models included in the current review offered a true alternative to hospital admission.
Implications for policy and future research
The provision of acute care models for older people are predicated on a logic model rather than empirical evidence of benefit. Further large and rigorously constructed randomised controlled trials may strengthen the evidence base but may not be the most effectual method of influencing local decisions on service provision or the direction of policy.
Research in acute care delivery is complicated by a need to maintain operational performance. Amongst the studies identified, bed availability and restricted operational hours frequently resulted in a large differential between the number of potentially eligible participants and the number of patients ultimately included. Practical considerations aside, the outcomes of interventional studies are likely to be highly dependent on local context and external factors which influence generalisability.
Knowledge in this subject area may be enhanced by developing a consistent approach to outcome reporting and measurement, ideally incorporating the priorities and preferences of patients. Mortality may not be the most appropriate metric of effectiveness given a significant proportion of older people living with frailty requiring acute care for medical illness are entering the last 12 months of life [ 123 ]. Current models of acute care infrequently establish and record individual preferences in relation to location of care in the event of acute medical illness or preferred location of death amongst older people [ 124 ]. A narrow focus on clinical and operational outcomes may simplify study design, facilitate comparisons and provide reassurance around safety but risks ignoring other aspects of care, such as quality of life, which may be more meaningful from the patient perspective.
Given the complexity of the intervention, an understanding of the processes and behaviours which drive successful models may be best approached from a qualitative research paradigm.
Strength and limitations
The primary objective of this systematic review was to describe and categorise acute care models for older people and highlight variation in the outcome measures used to assess them. An extensive search strategy inclusive of the grey literature and indifferent to methodological design was purposefully employed in order to capture a comprehensive representation of the range of models in operation. Every acute hospital encounters older people living with frailty and the potential for variation in approach is vast. Only a small fraction of care models delivered in practice are reported in the literature. The practice of publishing multiple articles from the same original study was relatively common, particularly in literature pertaining to acute bed-based care and HaH models. The account provided is therefore susceptible to both publication and outcome reporting bias.
Availability of data and materials
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
de Meijer C, Wouterse B, Polder J, Koopmanschap M. The effect of population aging on health expenditure growth: a critical review. Eur J Ageing. 2013;10(4):353–61.
Article PubMed PubMed Central Google Scholar
Hao Q, Zhou L, Dong B, Yang M, Dong B, Weil Y. The role of frailty in predicting mortality and readmission in older adults in acute care wards: a prospective study. Sci Rep. 2019;9(1):1207.
Wallis SJ, Wall J, Biram RW, Romero-Ortuno R. Association of the clinical frailty scale with hospital outcomes. QJM. 2015;108(12):943–9.
Article CAS PubMed Google Scholar
Wittenberg R, Sharpin L, McCormick B, Hurst J. The ageing society and emergency hospital admissions. Health Policy. 2017;121(8):923–8.
Article PubMed Google Scholar
Organisation for Economic Co-operation and Development (OECD). "Hospital beds and occupancy", in Health at a Glance 2021: OECD Indicators. Paris: OECD Publishing; 2021. https://doi.org/10.1787/e5a80353-en .
The Kings Fund. Older people and emergency bed use: Exploring variation. 2012.
Google Scholar
Huntley AL, Chalder M, Shaw ARG, Hollingworth W, Metcalfe C, Benger JR, et al. A systematic review to identify and assess the effectiveness of alternatives for people over the age of 65 who are at risk of potentially avoidable hospital admission. BMJ Open. 2017;7(7):e016236.
Ellis G, Gardner M, Tsiachristas A, Burke O, Shepperd S, Langhorne P, et al. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database Syst Rev. 2017;2017(9):CD006211.
PubMed Central Google Scholar
Briggs R, McDonough A, Ellis G, Bennett K, O’Neill D, Robinson D. Comprehensive Geriatric Assessment for community-dwelling, high-risk, frail, older people. Cochrane Database Syst Rev. 2022;5(5):Cd012705.
PubMed Google Scholar
Fox MT, Persaud M, Maimets I, O’Brien K, Brooks D, Tregunno D, et al. Effectiveness of acute geriatric unit care using acute care for elders components: a systematic review and meta-analysis. J Am Geriatr Soc. 2012;60(12):2237–45.
Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:g1687.
Dodd S, Clarke M, Becker L, Mavergames C, Fish R, Williamson PR. A taxonomy has been developed for outcomes in medical research to help improve knowledge discovery. J Clin Epidemiol. 2018;96:84–92.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898.
Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919.
Asplund K, Gustafson Y, Jacobsson C, Bucht G, Wahlin A, Peterson J, et al. Geriatric-based versus general wards for older acute medical patients: a randomized comparison of outcomes and use of resources. J Am Geriatr Soc. 2000;48(11):1381–8.
Covinsky KE, King JT Jr, Quinn LM, Siddique R, Palmer R, Kresevic DM, et al. Do acute care for elders units increase hospital costs? A cost analysis using the hospital perspective. J Am Geriatr Soc. 1997;45(6):729–34.
Goldberg SE, Bradshaw LE, Kearney FC, Russell C, Whittamore KH, Foster PER, et al. Care in specialist medical and mental health unit compared with standard care for older people with cognitive impairment admitted to general hospital: randomised controlled trial (NIHR TEAM trial). BMJ. 2013;347:f4132.
Harris RD, Henschke PJ, Popplewell PY, Radford AJ, Bond MJ, Turnbull RJ, et al. A randomised study of outcomes in a defined group of acutely ill elderly patients managed in a geriatric assessment unit or a general medical unit. Aust N Z J Med. 1991;21(2):230–4.
Landerfeld CS, Palmer RM, Kresevic DM, Fortinsky RH, Kowal J. A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients. N Engl J Med. 1995;332(20):1338–44.
Article Google Scholar
Naughton BJ, Moran MB, Feinglass J, Falconer J, Williams ME. Reducing hospital costs for the geriatric patient admitted from the emergency department: a randomized trial. J Am Geriatr Soc. 1994;42(10):1045–9.
Saltvedt I, Jordhøy M, Opdahl Mo ES, Fayers P, Kaasa S, Sletvold O. Randomised trial of in-hospital geriatric intervention: impact on function and morale. Gerontology. 2006;52(4):223–30.
Saltvedt I, Opdahl E, Fayers P, Kaasa S, Sletvold O. Reduced mortality in treating acutely sick, frail older patients in a geriatric evaluation and management unit. A prospective randomized trial. J Am Geriatr Soc. 2002;50(5):792–8.
Ribbink ME, Van Seben R, Oudejans I, MacNeil-Vroomen JL, Buurman BM. Investigating the effectiveness of care delivery at an acute geriatric community hospital for older adults in the Netherlands: A protocol for a prospective controlled observational study. BMJ Open. 2020;10(3):e033802.
Abdalla A, Adhaduk M, Haddad RA, Alnimer Y, Rios-Bedoya CF, Bachuwa G. Does acute care for the elderly (ACE) unit decrease the incidence of falls? Geriatric nursing (New York, NY). 2018;39(3):292–5.
Abisheganaden J, Ding YY, Chong WF, Heng BH, Lim TK. Effectiveness of acute geriatric units in the real world: The case of short-term mortality among seniors hospitalized for pneumonia. Geriatr Gerontol Int. 2013;13(1):55–62.
Ahlund K, Oberg B, Back M, Ekerstad N. Effects of comprehensive geriatric assessment on physical fitness in an acute medical setting for frail elderly patients. Clin Interv Aging. 2017;12:1929–39.
Chittock DR, McLean N, Wilbur K, Wong RY. Discharge outcomes of older medical in-patients in a specialized acute care for elders unit compared with non-specialized units. Can J Geriatr. 2006;9(3):96–101.
Ekerstad N, Husberg M, Alwin J, Ivanoff SD, Landahl S, Ostberg G, et al. Acute care of severely frail elderly patients in a CGA-unit is associated with less functional decline than conventional acute care. Clin Interv Aging. 2017;12:1239–49.
Ekerstad N, Husberg M, Alwin J, Karlson BW, DahlinIvanoff S, Landahl S, et al. Is the acute care of frail elderly patients in a comprehensive geriatric assessment unit superior to conventional acute medical care? Clin Interv Aging. 2017;12:1–9.
Ekerstad N, Karlson BW, Andersson D, Husberg M, Carlsson P, Heintz E, et al. Short-term resource utilization and cost-effectiveness of comprehensive geriatric assessment in acute hospital care for severely frail elderly patients. J Am Med Dir Assoc. 2018;19(10):871-8.e2.
Ekerstad N, Ostberg G, Johansson M, Karlson BW. Are frail elderly patients treated in a CGA unit more satisfied with their hospital care than those treated in conventional acute medical care? Patient Prefer Adherence. 2018;12:233–40.
Flood KL, MacLennan PA, McGrew D, Green D, Dodd C, Brown CJ. Effects of an acute care for elders unit on costs and 30-day readmissions. JAMA Intern Med. 2013;173(11):981–7.
Hung WW, Ross JS, Farber J, Siu AL. Evaluation of the Mobile Acute Care of the Elderly (MACE) service. JAMA Intern Med. 2013;173(11):990–6.
Jayadevappa R, Chhatre S, Weiner M, Raziano DB. Health resource utilization and medical care cost of acute care elderly unit patients. Value Health. 2006;9(3):186–92.
Salinas R, Zelada MA, Baztan JJ. Reduction of functional deterioration during hospitalization in an acute geriatric unit. Arch Gerontol Geriatr. 2009;48(1):35–9.
Schubert CC, Parks R, Coffing JM, Daggy J, Slaven JE, Weiner M. Lessons and outcomes of mobile acute care for elders consultation in a veterans affairs medical center. J Am Geriatr Soc. 2019;67(4):818–24.
Stewart M, Suchak N, Scheve A, Popat-Thakkar V, David E, Laquinte J, et al. The impact of a geriatrics evaluation and management unit compared to standard care in a community teaching hospital. MD Med J (Baltimore, MD: 1985). 1999;48(2):62–7.
CAS Google Scholar
Wald HL, Glasheen JJ, Guerrasio J, Youngwerth JM, Cumbler EU. Evaluation of a hospitalist-run acute care for the elderly service. J Hosp Med. 2011;6(6):313–21.
Ahmed N, Dyer CB, Taylor K, McDaniel Y. The role of an acute care for the elderly unit in achieving hospital quality indicators while caring for frail hospitalized elders. Popul Health Manag. 2012;15(4):236–40.
Aizen E, Swartzman R, Clarfield AM. Hospitalization of nursing home residents in an acute-care geriatric department: Direct versus emergency room admission. Isr Med Assoc J. 2001;3(10):734–8.
CAS PubMed Google Scholar
Lin M-H, Peng L-N, Chen L-K, Hsu C-C, Yu P-C. Early geriatric evaluation and management services reduced in-hospital mortality risk among frail oldest-old patients. Aging Med Healthc. 2021;12(2):62–7.
Meschi T, Ticinesi A, Prati B, Nouvenne A, Borghi L, Montali A, et al. A novel organizational model to face the challenge of multimorbid elderly patients in an internal medicine setting: a case study from Parma Hospital Italy. Intern Emerg Med. 2016;11(5):667–76.
Nouvenne A, Ticinesi A, Cerundolo N, et al. Implementing a multidisciplinary rapid geriatric observation unit for non-critical older patients referred to hospital: observational study on real-world data. Aging Clin Exp Res. 2022;34:599–609. https://doi.org/10.1007/s40520-021-01967-z .
Shaw M, Acton C, Wilbur K, McMillan M, Breurkens E, Sowden C, et al. An interdisciplinary approach to optimize health services in a specialized acute care for elders unit. Geriatrics Today: J Can Geriatr Soc. 2003;6(3):177–86.
Taylor JK, Murphy S, Fox J, Gaillemin OS, Pearl AJ. Embedding comprehensive geriatric assessment in the emergency assessment unit: The impact of the COPE zone. Clinical Medicine. J R Coll Physicians Lond. 2016;16(1):19–24.
Webb M, Campbell K. Innovating care in the community. Br J Healthc Manag. 2010;16(2):330–2.
AimoninoRicauda N, Scarafiotti C, Marinello R, Zanocchi M, Molaschi M, Leff B, et al. Substitutive “hospital at home” versus inpatient care for elderly patients with exacerbations of chronic obstructive pulmonary disease: A prospective randomized, controlled trial. J Am Geriatr Soc. 2008;56(3):493–500.
Board N, Brennan N, Caplan GA. A randomised controlled trial of the costs of hospital as compared with hospital in the home for acute medical patients. Aust N Z J Public Health. 2000;24(3):305–11.
Caplan GA, Coconis J, Woods J. Effect of hospital in the home treatment on physical and cognitive function: a randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2005;60(8):1035–8.
Caplan GA, Ward JA, Brennan NJ, Coconis J, Board N, Brown A. Hospital in the home: a randomised controlled trial. Med J Aust. 1999;170(4):156–60.
Dismore LL, Van Wersch A, Echevarria C, Bourke S, Gibson J. What are the positive drivers and potential barriers to implementation of hospital at home selected by low-risk DECAF score in the UK: A qualitative study embedded within a randomised controlled trial. BMJ Open. 2019;9(4):e026609.
Echevarria C, Gray J, Hartley T, Steer J, Miller J, Simpson AJ, et al. Home treatment of COPD exacerbation selected by DECAF score: a non-inferiority, randomised controlled trial and economic evaluation. Thorax. 2018;73(8):713–22.
Levine DM, Blanchfield B, Saenz A, Burke K, Paz M, Schnipper JL, et al. Hospital-level care at home for acutely ill adults a randomized controlled trial. Ann Intern Med. 2020;172(2):77–85.
Levine DM, Blanchfield B, Schnipper JL, Ouchi K, Diamond K, Licurse A, et al. Hospital-Level Care at Home for Acutely Ill Adults: a Pilot Randomized Controlled Trial. J Gen Intern Med. 2018:1–8.
Levine DM, Pian J, Mahendrakumar K, Patel A, Saenz A, Schnipper JL. Hospital-level care at home for acutely ill adults: a qualitative evaluation of a randomized controlled trial. J Gen Intern Med. 2021;36(7):1965–73.
Mäkelä P, Stott D, Godfrey M, Ellis G, Schiff R, Shepperd S. The work of older people and their informal caregivers in managing an acute health event in a hospital at home or hospital inpatient setting. Age Ageing. 2020;49(5):856–64.
Mendoza H, Martin MJ, Garcia A, Aros F, Aizpuru F, Regalado De Los Cobos J, et al. ‘Hospital at home’ care model as an effective alternative in the management of decompensated chronic heart failure. Eur J Heart Fail. 2009;11(12):1208–13.
Patel H, Shafazand M, Ekman I, Höjgård S, Swedberg K, Schaufelberger M. Home care as an option in worsening chronic heart failure – a pilot study to evaluate feasibility, quality adjusted life years and cost-effectiveness. Eur J Heart Fail. 2008;10(7):675–81.
Shepperd S, Butler C, Cradduck-Bamford A, Ellis G, Gray A, Hemsley A, et al. Is comprehensive geriatric assessment admission avoidance hospital at home an alternative to hospital admission for older persons? : A randomized trial. Ann Intern Med. 2021;174(7):889–98.
Skjot-Arkil H, Mogensen CB, Lindberg MJ, Ankersen ES, Hansen SL, Solgaard J, et al. Admission rates in a general practitioner-based versus a hospital specialist based, hospital-at-home model: ACCESS, an open-labelled randomised clinical trial of effectiveness. Scand J Trauma Resusc Emerg Med. 2018;26(1):26.
Tibaldi V, Aimonino N, Ponzetto M, Stasi MF, Amati D, Raspo S, et al. A randomized controlled trial of a home hospital intervention for frail elderly demented patients: Behavioral disturbances and caregiver’s stress. Arch Gerontol Geriatr. 2004;38:431–6.
Tibaldi V, Isaia G, Scarafiotti C, Gariglio F, Zanocchi M, Bo M, et al. Hospital at home for elderly patients with acute decompensation of chronic heart failure: a prospective randomized controlled trial. Arch Intern Med. 2009;169(17):1569–75.
Pouw MA, Calf AH, van Munster BC, Ter Maaten JC, Smidt N, de Rooij SE. Hospital at Home care for older patients with cognitive impairment: a protocol for a randomised controlled feasibility trial. BMJ Open. 2018;8(3):e020332.
Shepperd S, Cradduck-Bamford A, Butler C, Ellis G, Godfrey M, Gray A, et al. A multi-centre randomised trial to compare the effectiveness of geriatrician-led admission avoidance hospital at home versus inpatient admission. Trials. 2017;18:1–9.
Augustine MR, Siu AL, Boockvar KS, DeCherrie LV, Leff BA, Federman AD. Outcomes of hospital at home for older adults with and without high levels of social support. Home Healthc Now. 2021;39(5):261–70.
Burton L, Clark R, Steinwachs D, Greenough Iii WB, Guido S, Burton JR, et al. Satisfaction with hospital at home care. J Am Geriatr Soc. 2006;54(9):1355–63.
Cai S, Grubbs A, Makineni R, Kinosian B, Phibbs CS, Intrator O. Evaluation of the Cincinnati veterans affairs medical center hospital-in-home program. J Am Geriatr Soc. 2018;66(7):1392–8.
Clark R, Steinwachs DM, Leff B, Mader SI, Naughton WB, Burl JB, et al. Substitutive hospital at home for older persons: effects on costs. Am J Manag Care. 2009;15(1):49–56.
Federman AD, Soones T, DeCherrie LV, Leff B, Siu AL. Association of a bundled hospital-at-home and 30-day postacute transitional care program with clinical outcomes and patient experiences. JAMA Intern Med. 2018;178(8):1033–41.
Gonzalez Barcala FJ, Alvarez Calderon P, Valdes Cuadrado L, Pose Reino A, De la Fuente CR, Masa Vazquez LA, et al. Hospital at home for acute respiratory patients. Eur J Intern Med. 2006;17(6):402–7.
Greenough Iii WB, Guido S, Leff B, Burton L, Koehn D, Clark R, et al. Comparison of stress experienced by family members of patients treated in hospital at home with that of those receiving traditional acute hospital care. J Am Geriatr Soc. 2008;56(1):117–23.
Greenough Iii WB, Guido S, Leff B, Burton L, Steinwachs D, Mader SL, et al. Comparison of functional outcomes associated with hospital at home care and traditional acute hospital care. J Am Geriatr Soc. 2009;57(2):273–8.
Isaia G, Astengo MA, Tibaldi V, Zanocchi M, Bardelli B, Obialero R, et al. Delirium in elderly home-treated patients: A prospective study with 6-month follow-up. Age. 2009;31(2):109–17.
Leff B, Burton L, Guido S, Greenough WB, Steinwachs D, Burton JR. Home hospital program: a pilot study. J Am Geriatr Soc. 1999;47(6):697–702.
Leff B, Burton L, Mader SL, Naughton B, Burl J, Inouye SK, et al. Hospital at home: Feasibility and outcomes of a program to provide hospital-level care at home for acutely III older patients. Ann Intern Med. 2005;143(11):798–856.
Marsteller JA, Burton L, Steinwachs D, Clark R, Mader SL, Naughton B, et al. Health care provider evaluation of a substitutive model of hospital at home. Med Care. 2009;47(9):979–85.
Saenger P, Lubetsky S, Catalan E, Federman AD, DeCherrie LV, Leff B, et al. Choosing inpatient vs home treatment: why patients accept or decline hospital at home. J Am Geriatr Soc. 2020;68(7):1579–83.
Tsiachristas A, Ellis G, Buchanan S, Langhorne P, Stott DJ, Shepperd S. Should i stay or should i go? A retrospective propensity score-matched analysis using administrative data of hospital-at-home for older people in Scotland. BMJ Open. 2019;9(5):e023350.
Mendoza Ruiz de Zuazu H, Gómez Rodríguez de Mendarozqueta M, Regalado de Los Cobos J, Altuna Basurto E, Marcaide Ruiz de Apodaca MA, Aizpuru Barandiarán F, Cía Ruiz JM. Enfermedad pulmonar obstructiva crónica en hospitalización a domicilio. Estudio de 522 casos [Chronic obstructive pulmonary disease in the setting of hospital at home. Study of 522 episodes]. Rev Clin Esp. 2007;207(7):331-6. https://doi.org/10.1157/13107944 .
Mader SL, Medcraft MC, Joseph C, Jenkins KL, Benton N, Chapman K, et al. Program at home: a Veterans Affairs Healthcare Program to deliver hospital care in the home. J Am Geriatr Soc. 2008;56(12):2317–22.
Montalto M, Lui B, Mullins A, Woodmason K. Medically-managed Hospital in the Home: 7 year study of mortality and unplanned interruption. Aust Health Rev. 2010;34(3):269–75.
Salazar A, Estrada C, Porta R, Lolo M, Tomas S, Alvarez M. Home hospitalization unit: an alternative to standard inpatient hospitalization from the emergency department. Eur J Emerg Med. 2009;16(3):121–3.
Mas MA, Santaeugenia SJ, Tarazona-Santabalbina FJ, Gamez S, Inzitari M. Effectiveness of a hospital-at-home integrated care program as alternative resource for medical crises care in older adults with complex chronic conditions. J Am Med Dir Assoc. 2018;19(10):860–3.
Alakare J, Kemp K, Castren M, Harjola V-P, Strandberg T, et al. Systematic geriatric assessment for older patients with frailty in the emergency department: a randomised controlled trial. BMC Geriatr. 2021;21(1):408.
Basic D, Conforti DA. A prospective, randomised controlled trial of an aged care nurse intervention within the Emergency Department. Aust Health Rev. 2005;29(1):51–9.
Lo AX, Dresden SM, Post LA, Lindquist LA, Kocherginsky M, et al. The impact of Geriatric Emergency Department Innovations (GEDI) on health services use, health related quality of life, and costs: Protocol for a randomized controlled trial. Contemp Clin Trials. 2020;97:106125.
Arendts G, Leyte N, Dumas S, Ahamed S, Khokulan V, Wahbi O, Lomman A, Hughes D, Clayden V, Mandal B. Efficiency gains from a standardised approach to older people presenting to the emergency department after a fall. Aust Health Rev. 2020;44(4):576-81. https://doi.org/10.1071/AH19187 .
Buttery A, O’Neill S, Hopper A, Harari D, Martin FC. The older persons’ assessment and liaison team “OPAL”: Evaluation of comprehensive geriatric assessment in acute medical inpatients. Age Ageing. 2007;36(6):670–5.
Conroy SP, Ansari K, Williams M, Laithwaite E, Teasdale B, Dawson J, et al. A controlled evaluation of comprehensive geriatric assessment in the emergency department: the “Emergency Frailty Unit.” Age Ageing. 2014;43(1):109–14.
Ellis G, Alcorn M, Jamieson CA, Devlin V. An Acute Care for Elders (ACE) unit in the emergency department. Eur Geriatr Med. 2012;3(4):261–3.
Foo CL, Siu VWY, Seow E, Tan TL, Ding YY. Geriatric assessment and intervention in an emergency department observation unit reduced re-attendance and hospitalisation rates. Australas J Ageing. 2012;31(1):40–6.
Hwang U, Dresden SM, Rosenberg MS, Garrido MM, Loo G, Sze J, et al. Geriatric emergency department innovations: transitional care nurses and hospital use. J Am Geriatr Soc. 2018;66(3):459–66.
Kwon N, Carpenter K, Silverman R, Willis H, Liberman T, Cascio K, et al. GAP-ED project: Improving care for frail elderly patients presenting to the emergency department. Acad Emerg Med. 2017;24:S293–4.
Leung TH, Leung SC, Wong CKG. The effectiveness of an emergency physician-led frailty unit for the living-alone elderly: A pilot retrospective cohort study. Hong Kong J Emerg Med. 2020;27(3):162–7.
Marsden E, Taylor A, Wallis M, Craswell A, Broadbent M, Barnett A, et al. Effect of the Geriatric Emergency Department Intervention on outcomes of care for residents of aged care facilities: A non-randomised trial. Emerg Med Australas : EMA. 2020;32(3):422–9.
PuigCampmany M, BlazquezAndion M, Benito Vales S, Ris RJ. Development of a comprehensive, multidisciplinary program of care for frailty in an emergency department. Europ Geriatr Med. 2019;10(1):37–46.
A geriatrician in the emergency department. https://pavilionhealthtoday.com/gm/a-geriatrician-in-the-emergency-department/ .
Wallis M, Marsden E, Taylor A, Craswell A, Broadbent M, Barnett A, et al. The Geriatric Emergency Department Intervention model of care: a pragmatic trial. BMC Geriatr. 2018;18(1):297.
Argento V, Calder G, Ferrigno R, Skudlarska B. Geriatric emergency medicine service: a novel approach to an emerging trend. Conn Med. 2014;78(6):339–43.
Clarfield AM, Bergman H, Beaudet M, Sinoff G. A two-year follow-up of geriatric consults in the emergency department. J Am Geriatr Soc. 1998;46(6):716–20.
Fox J, Pattison T, Wallace J, Pradhan S, Gaillemin O, Feilding E, et al. Geriatricians at the front door: The value of early comprehensive geriatric assessment in the emergency department. Eur Geriatr Med. 2016;7(4):383–5.
Gentric A, Duquesne F, Graziana A, Sivy H, Duges F, Garo B, Boles JM. L'accueil gérontologique médicosocial aux urgences: une alternative à l'hospitalisation des personnes âgées en médecine? [A sociomedical geriatric assessment in the emergency units: an alternative to the hospitalization of aged patients?]. Rev Med Interne. 1998;19(2):85-90. https://doi.org/10.1016/s0248-8663(97)83417-0 .
Jones S, Wallis P. Effectiveness of a geriatrician in the emergency department in facilitating safe admission prevention of older patients. Clinical Medicine. J R Coll Physicians Lond. 2013;13(6):561–4.
Khan SA, Millington H, Miskelly FG. Benefits of an accident and emergency short stay ward in the staged hospital care of elderly patients. J Accid Emerg Med. 1997;14(3):151–2.
Article CAS PubMed PubMed Central Google Scholar
Ngian VJJ, Ong BS, O’Rourke F, Nguyen HV, Chan DKY. Review of a rapid geriatric medical assessment model based in emergency department. Age Ageing. 2008;37(6):696–9.
O’Shaughnessy I, Edge L, Dillon A, Flynn S, Briggs R, Cunningham C, et al. Home FIRsT: interdisciplinary geriatric assessment and disposition outcomes in the Emergency Department. Eur J Intern Med. 2021;85:50–5.
Pareja T, Hornillos M, Rodriguez M, Martinez J, Madrigal M, Mauleon C, et al. Medical short stay unit for geriatric patients in the emergency department: clinical and healthcare benefits. Revista Espanola de Geriatria y Gerontologia. 2009;44(4):175–9.
Roussel-Laudrin S, Paillaud E, Alonso E, Caillet P, Herbaud S, Merlier I, et al. The establishment of geriatric intervention group and geriatric assessment at emergency of Henri-Mondor hospital. Rev Med Interne. 2005;26(6):458–66.
Southerl LT, Nagaraj L, Caterino JM, Gure TR, Vargas AJ. An emergency department observation unit is a feasible setting for multidisciplinary geriatric assessments in compliance with the geriatric emergency department guidelines. Acad Emerg Med. 2018;25(1):76–82.
Tan KM, Lannon R, O’Keeffe L, Barton D, Ryan J, O’Shea D, et al. Geriatric medicine in the emergency department. Ir Med J. 2012;105(8):271–4.
Elias TCN, Bowen J, Hassanzadeh R, Lasserson DS, Pendlebury ST. Factors associated with admission to bed-based care: observational prospective cohort study in a multidisciplinary same day emergency care unit (SDEC). BMC Geriatr. 2021;21(1):8.
Ellis G, Whitehead M, Robinson D, O’Neill D, Langhorne P. Comprehensive geriatric assessment for older adults admitted to hospital: A systematic review. CDSR. 2006;4:CD006211.
Brickman KR, Silvestri JA. The emergency care model: A new paradigm for skilled nursing facilities. Geriatric nursing (New York, NY). 2020;41(3):242–7.
Crilly J, Chaboyer W, Wallis M. A structure and process evaluation of an Australian hospital admission avoidance programme for aged care facility residents. J Adv Nurs. 2012;68(2):322–34.
Crilly J, Chaboyer W, Wallis M, Thalib L, Polit D. An outcomes evaluation of an Australian Hospital in the Nursing Home admission avoidance programme. J Clin Nurs. 2011;20(7–8):1178–87.
Lau L, Chong CP, Lim WK. Hospital treatment in residential care facilities is a viable alternative to hospital admission for selected patients. Geriatr Gerontol Int. 2013;13(2):378–83.
Joseph JW, Kennedy M, Nathanson LA, Wardlow L, Crowley C, Stuck A. Reducing emergency department transfers from skilled nursing facilities through an emergency physician telemedicine service. West J Emerg Med. 2020;21(6):205–9.
Parker SG, McCue P, Phelps K, McCleod A, Arora S, Nockels K, et al. What is Comprehensive Geriatric Assessment (CGA)? An umbrella review. Age Ageing. 2018;47(1):149–55.
Baztan JJ, Suarez-Garcia FM, Lopez-Arrieta J, Rodriguez-Manas L, Rodriguez-Artalejo F. Effectiveness of acute geriatric units on functional decline, living at home, and case fatality among older patients admitted to hospital for acute medical disorders: Meta-analysis. BMJ (Online). 2009;338(7690):334–6.
NHS Improvement NE. Same-day acute frailty services. 2019. Available from: https://www.england.nhs.uk/wp-content/uploads/2021/02/SDEC_guide_frailty_May_2019_update.pdf .
Shepperd S, Goncalves-Bradley DC, Iliffe S, Doll HA, Clarke MJ, Kalra L, et al. Admission avoidance hospital at home. CDSR. 2016;2016(9):CD007491.
Article PubMed Central Google Scholar
Arsenault-Lapierre G, Henein M, Gaid D, Le Berre M, Gore G, Vedel I. Hospital-at-Home Interventions vs In-Hospital Stay for Patients With Chronic Disease Who Present to the Emergency Department: A Systematic Review and Meta-analysis. JAMA Netw Open. 2021;4(6):e2111568. https://doi.org/10.1001/jamanetworkopen.2021.11568 .
Emily M, Rosalia M-A, Lauren S, Alice R, David C, Chris I. Death within 1 year among emergency medical admissions to Scottish hospitals: incident cohort study. BMJ Open. 2018;8(6):e021432.
Knight M, Bergbaum C, Hussein T, Newman N, Bertfield D, Rawle MJ. Comprehensive geriatric assessment at the front-door pilot: Improving older adults care outcomes in Barnet Hospital. Eur Geriatr Med. 2020;11:S176.
Download references
Acknowledgements
Not applicable.
DSL is funded by National Institute for Health Research (NIHR) Applied Research Collaboration (ARC) West Midlands, NIHR Community Healthcare MedTech and IVD Cooperative and NIHR Oxford Biomedical Research Centre (BRC). The views expressed are those of the authors and not necessarily those of the NIHR or Department of Health and Social Care.
Author information
Authors and affiliations.
Acute Care Research Group, Institute of Inflammation and Ageing, University of Birmingham, Birmingham, B15 2TT, UK
Thomas Knight, Vicky Kamwa, Catherine Atkin & Elizabeth Sapey
Department of Geriatric Medicine, Whiston Hospital, Mersey and West Lancashire Teaching Hospital NHS Trust, Prescot, L35 5DR, UK
Catherine Green
Department of Geriatric Medicine, Royal Bolton NHS Foundation Trust, Bolton, BL4 0JR, UK
Janahan Ragunathan
Warwick Medical School, Professor of Acute and Ambulatory Care, University of Warwick, Coventry, CV4 7AL, UK
Daniel Lasserson
You can also search for this author in PubMed Google Scholar
Contributions
TK wrote the manuscript and undertook the primary analysis. CA, CG, JR, VK contributed to abstract screening and review. DSL and ES provided review of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Thomas Knight .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1., additional file 2., additional file 3., additional file 4., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Knight, T., Kamwa, V., Atkin, C. et al. Acute care models for older people living with frailty: a systematic review and taxonomy. BMC Geriatr 23 , 809 (2023). https://doi.org/10.1186/s12877-023-04373-4
Download citation
Received : 11 December 2022
Accepted : 03 October 2023
Published : 05 December 2023
DOI : https://doi.org/10.1186/s12877-023-04373-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Acute frailty
- Care models
BMC Geriatrics
ISSN: 1471-2318
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Loading metrics
Open Access
Peer-reviewed
Research Article
Mathematical models of drug-resistant tuberculosis lack bacterial heterogeneity: A systematic review
Roles Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliations Department of Infectious Disease Epidemiology, Faculty of Epidemiology and Population Health, London School of Hygiene and Tropical Medicine, London, United Kingdom, Centre for Mathematical Modelling of Infectious Diseases, London School of Hygiene and Tropical Medicine, London, United Kingdom, Antimicrobial Resistance Centre, London School of Hygiene and Tropical Medicine, London, United Kingdom, Tuberculosis Centre, London School of Hygiene and Tropical Medicine, London, United Kingdom
Roles Data curation, Validation, Writing – review & editing
Roles Data curation, Writing – review & editing
Roles Conceptualization, Writing – review & editing
Affiliation UCL Centre for Clinical Microbiology, Division of Infection & Immunity, Royal Free Campus, University College London, London, United Kingdom
Roles Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing
- Naomi M. Fuller,
- Christopher F. McQuaid,
- Martin J. Harker,
- Chathika K. Weerasuriya,
- Timothy D. McHugh,
- Gwenan M. Knight

- Published: April 10, 2024
- https://doi.org/10.1371/journal.ppat.1011574
- Peer Review
- Reader Comments
This is an uncorrected proof.
Drug-resistant tuberculosis (DR-TB) threatens progress in the control of TB. Mathematical models are increasingly being used to guide public health decisions on managing both antimicrobial resistance (AMR) and TB. It is important to consider bacterial heterogeneity in models as it can have consequences for predictions of resistance prevalence, which may affect decision-making. We conducted a systematic review of published mathematical models to determine the modelling landscape and to explore methods for including bacterial heterogeneity. Our first objective was to identify and analyse the general characteristics of mathematical models of DR-mycobacteria, including M . tuberculosis . The second objective was to analyse methods of including bacterial heterogeneity in these models. We had different definitions of heterogeneity depending on the model level. For between-host models of mycobacterium, heterogeneity was defined as any model where bacteria of the same resistance level were further differentiated. For bacterial population models, heterogeneity was defined as having multiple distinct resistant populations. The search was conducted following PRISMA guidelines in five databases, with studies included if they were mechanistic or simulation models of DR-mycobacteria. We identified 195 studies modelling DR-mycobacteria, with most being dynamic transmission models of non-treatment intervention impact in M . tuberculosis (n = 58). Studies were set in a limited number of specific countries, and 44% of models (n = 85) included only a single level of “multidrug-resistance (MDR)”. Only 23 models (8 between-host) included any bacterial heterogeneity. Most of these also captured multiple antibiotic-resistant classes (n = 17), but six models included heterogeneity in bacterial populations resistant to a single antibiotic. Heterogeneity was usually represented by different fitness values for bacteria resistant to the same antibiotic (61%, n = 14). A large and growing body of mathematical models of DR-mycobacterium is being used to explore intervention impact to support policy as well as theoretical explorations of resistance dynamics. However, the majority lack bacterial heterogeneity, suggesting that important evolutionary effects may be missed.
Author summary
The emergence of drug-resistant tuberculosis (DR-TB), where the causative bacterium Mycobacterium tuberculosis is resistant to key antibiotics such as rifampicin and isoniazid, poses a significant threat to TB control efforts. To gain a broader understanding of the challenges surrounding DR-TB, mathematical models are increasingly being employed to estimate the impact of interventions, effectiveness of treatment, and to predict the evolution of drug-resistance. However, pragmaticism surrounding model construction often means that important aspects, such as bacterial heterogeneity, are overlooked. We undertook a systematic review of the existing DR-mycobacterium modelling literature, with the specific aim of capturing methods for including bacterial heterogeneity. Our analysis revealed that most models of drug-resistance in mycobacteria primarily focus on intervention strategies and cost-effectiveness analyses, with minimal attention to bacterial heterogeneity. Where heterogeneity is included it mostly consisted of different fitness costs for resistance.
Citation: Fuller NM, McQuaid CF, Harker MJ, Weerasuriya CK, McHugh TD, Knight GM (2024) Mathematical models of drug-resistant tuberculosis lack bacterial heterogeneity: A systematic review. PLoS Pathog 20(4): e1011574. https://doi.org/10.1371/journal.ppat.1011574
Editor: Mark Robert Davies, University of Melbourne, AUSTRALIA
Received: July 25, 2023; Accepted: March 25, 2024; Published: April 10, 2024
Copyright: © 2024 Fuller et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All relevant data are within the manuscript and its Supporting Information files.
Funding: This research and NMF was funded by the Biotechnology and Biological Sciences Research Council through the London Interdisciplinary Doctoral Training Programme (BBSRC LIDO, https://www.lido-dtp.ac.uk ) at the London School of Hygiene and Tropical Medicine (LSHTM) in partnership with University College London (UCL), Grant code - BB/M009513/1. CFM was funded for other work by Bill and Melinda Gates Foundation (TB MAC OPP1135288, INV-059518, https://www.gatesfoundation.org ) and Unitaid (20193–3-ASCENT, https://unitaid.org/calls/#en ). CKW was supported by a grant from the Bill and Melinda Gates Foundation (INV-001754, https://www.gatesfoundation.org ). GMK was supported by Medical Research Council UK, https://www.ukri.org/opportunity/career-development-award/ (MR/ W026643/1). The views expressed are those of the authors and not necessarily those of the BBSRC, LIDO, LSHTM or UCL. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Introduction
Drug-resistant (DR-) strains of Mycobacterium tuberculosis ( M . tuberculosis ) are an urgent threat to the control of tuberculosis disease (TB) globally. For TB, the backbone antibiotics of standard therapy are rifampicin and isoniazid. In 2021, multidrug-resistant (combined rifampicin and isoniazid resistance) or rifampicin-resistant tuberculosis (MDR/RR-TB) caused an estimated 450,000 cases globally [ 1 ].
Routinely collected antimicrobial resistance (AMR) data use microbiological definitions of resistance, which are guided by threshold cut-offs for phenotypic resistance, resulting in discrete categorisations. For TB, these categorisations are further grouped with strains being classified as drug-susceptible (DS-), multidrug- or rifampicin-resistant- (MDR/RR-), pre-extensively-drug (pre-XDR) resistant (MDR plus resistance to a fluoroquinolone) or XDR- resistant (MDR plus resistance to a fluoroquinolone and a Group A drug) [ 1 ]. The MDR/RR grouping is based on the knowledge that isoniazid resistance is commonly acquired prior to rifampicin resistance and the wider prevalence of rifampicin-resistance testing through genotypic testing, making clinical management of RR- and MDR-TB similar [ 2 , 3 ]. These definitions are sufficient for patient care decision-making that does not need to account for the spectrum of phenotypic resistance levels (for example, those below the threshold for successful treatment) or any other bacterial characteristics (such as types of resistance-conferring mutations). However, bacterial populations are often highly diverse with a spectrum of characteristics. Hence, resistance categories will also have a high degree of bacterial heterogeneity, such as variation in transmission fitness between strains with the same phenotypic resistance, which affects the rate at which M . tuberculosis spreads between individuals.
Several important insights into the evolution of DR-TB, its emergence and spread, and the control of resistant bacteria more broadly have been generated by mathematical models. Some examples are the predominance of primary rather than acquired resistance, the effectiveness of TB surveillance for controlling DR-TB, and the potential impact of controlling HIV on reducing TB transmission [ 4 – 7 ]. Most mathematical models of AMR have typically adopted binary ( e . g . resistant versus susceptible) categorisations. When bacterial heterogeneity is included in mathematical models, the predicted public health outcomes can be different from those when bacterial heterogeneity is ignored [ 8 ]. We may lose subtlety in model outputs when modelling antibiotic treatment as a selective pressure if the traits allowing for bacterial heterogeneity are not included. Models may miss key dynamics, such as competition between strains and antibiotic effectiveness against strains with varying resistance levels, and be at risk of incorrectly predicting the effectiveness of a treatment intervention. As Trauer et al. (2018) point out, strain diversity, virulence and fitness costs have implications for the trajectory of drug resistance in TB [ 9 ]. Decisions as to what to include in a model will depend on the questions being asked, the selective pressures modelled, and the time-frame studied. Assessing this balance in model design between detailed and generalised parameters to allow a pragmatic approach for public health interventions can often prove challenging. Hence, assessing the extent to which bacterial heterogeneity has been included in existing models that predict intervention impact for DR-TB control is highly important.
Previous systematic reviews have explored the landscape of mathematical models of AMR [ 7 , 10 ] and TB [ 11 – 14 ], with up to 43 DR-TB transmission and 52 within-host studies being found prior to 2016. To our knowledge, only one expert review from 2009 focused on mathematical models of DR-TB [ 4 ], emphasising the useful insights from modelling but also highlighting important knowledge gaps in the economics, biological impact of mutations and ability to control DR-TB. To date, there is little evidence on how bacterial heterogeneity is incorporated into DR-TB models and little evidence of the effect this would have on model outcomes.
Mycobacteria predominantly develop antibiotic resistance via mutation [ 15 ], resulting in different patterns of resistance dynamics to other bacterial genera. Mycobacterial species other than M . tuberculosis can often be used as experimental or theoretical models for M . tuberculosis and are also responsible for a clinical burden [ 16 – 18 ]. They are often used to understand the resistance dynamics of M . tuberculosis [ 19 , 20 ].
We aimed to support future modelling of interventions against DR-TB by systematically surveying the characteristics of mathematical models of mycobacteria, of which we expect the M . tuberculosis species to dominate due to its substantial clinical burden. Our secondary objective was categorising the amount and type of bacterial heterogeneity included in mathematical models of DR-mycobacteria. We envisaged two broad settings of papers to be included in this review, within-host and between-host transmission models. This was noted by Cohen et al. (2009), a previous review of the DR-TB modelling literature [ 4 ], where “between-host” models refer to models on the human population scale. Since 2009, there has been an increase in models of bacterial populations set in the laboratory. As the populations captured will be similar to within-host models, we combined laboratory models and within-host models and collectively called them “bacterial population” models.
The aims, dynamics and model structure of between-host models differ considerably from bacterial population models, namely by transmission of the pathogen and populations included, making them difficult to compare. Therefore, we defined heterogeneity differently for bacterial populations and between-host models to compare methods within these categories and gain a clearer picture of bacterial heterogeneity modelling. At the between-host level, we were interested in capturing those models that went beyond capturing resistance phenotypes but included any added dimension of bacterial variation, including what may affect survival, such as fitness effects. Models of bacterial populations that captured any resistance variation were included; distinct populations of resistant bacteria needed to be modelled, which differed in their parameter values (e.g. growth rate or mutation rate).
Our review consisted of two stages of selection and data analysis. In Stage 1 of the review, our aim was to identify and analyse the general characteristics of mathematical models pertaining to drug-resistant (DR-) mycobacteria, such as model type and aim. In Stage 2 of the review, our focus was to identify mathematical models of DR-mycobacteria that specifically incorporated the concept of bacterial heterogeneity, as elucidated by the definition in the inclusion and exclusion criteria section.
Search strategy
The systematic review was designed and conducted following the PRISMA reporting protocol to search and review mathematical modelling papers of DR-mycobacteria [ 21 ]. The search terms consisted of those relevant to [ 1 ] “mycobacteria”, [ 2 ] “mathematical modelling”, and [ 3 ] “antibiotic resistance” ( S1 Text ). The search was conducted in five databases (Medline, Embase, Global Health, Web of Science and Scopus) initially on January 22nd, 2021, and then repeated on April 1st, 2022. Duplicates were removed before screening.
Inclusion and exclusion criteria
The screening process of the papers adhered to predefined inclusion and exclusion criteria ( Table 1 ). Initially, the titles and abstracts of the papers were screened to identify mathematical models specifically pertaining to DR-mycobacteria, followed by a full-text screening for inclusion in Stage 1. Finally, another round of full-text screening was carried out on the remaining papers to identify those appropriate for Stage 2 of the study.
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.ppat.1011574.t001
Mathematical models were defined as mechanistic models or simulation models reproducing a mathematically described scenario of DR-mycobacteria or of individuals carrying DR-mycobacteria. We excluded statistical analyses, such as regression models or risk analysis; molecular modelling (those focused on molecular structure of chemical compounds) or those only focused on drug development; models of drug-resistance that only used mycobacteria as an example or discussion point unless results for DR-mycobacteria were specifically included.
We split models into two groupings: “between-host” and “bacterial population” models, with the differences in their model scale, structure, and aims, resulting in a different bacterial heterogeneity definition. A “between-host” model was classed as a heterogenous model when strains infecting a human population resistant to the same drug varied in another characteristic such as fitness, rates of compensatory mutation evolution or associated treatment recovery rates. These characteristics were extracted during the full-text extraction stage. “Bacterial population” models included both within-host and models of bacterial populations capturing dynamics measured in laboratory or experimental conditions. A bacterial population model was classed as a heterogeneous model when there were distinct resistant strains captured which had different parameter values such as fitness, mutation rates and metabolic states. These parameter differences were extracted during the full-text extraction stage.
Selection and extraction: Stage 1
Title and abstract screening were performed for every paper by at least two authors (NMF, GMK, CFM, MJH and CKW) to determine if the paper likely included a mathematical model of DR-mycobacteria. High-level data extraction from these screened papers that continued to match the criteria for Stage 1 upon full-text screening provided a landscape analysis of DR-mycobacteria models. DR-mycobacteria models can address multiple aims with various methods, but they will have a common theme, such as parameter estimation or evaluation of the impact of interventions. We extracted information from the models to categorise and classify them into five categories, focusing on the main theme of the model. 1) model setting (such as geographic location), 2) model aims (7 categories of; non-treatment i nterventions that did not explore antibiotic usage (with and without cost-effectiveness), treatment interventions (with and without cost-effectiveness), parameter estimation, burden estimation or theoretical), 3) model type (7 categories of; bacterial dynamics, decision analytic, PK/PD, state transition (with and without a statistical component) or transmission (with or without an operational or state transition component), 4) mycobacterial species and 5) resistance classifications (such as MDR or XDR) ( S2 Text ). We extracted resistance classifications based on what the authors defined in their papers, as current resistance definitions are continuously updated. A resistance class is defined as a model stratification whereby strains (or the populations including them) are grouped across multiple antibiotic resistances (i.e. MDR could here be a single “resistance class” but represents resistance to multiple antibiotic agents). We only extracted which antibiotics were modelled in papers if their resistance was also considered. This extraction was performed by NMF and GMK, with discussions to resolve any conflicts.
Selection and extraction: Stage 2
For Stage 2, full-text screening of the Stage 1 papers was performed by three authors (NMF, GMK, CFM) to determine the models with bacterial heterogeneity, with subsequent discussions and consensus to resolve any discrepancies. NMF performed full-text extraction and data analysis of the extracted data from these papers ( S2 Table ). Stage 2 extracted data on the methods used to model heterogeneity, types of heterogeneity included, data sources and the effect of resistance inclusion (such as resistance effects on disease progression) ( S3 Text ).
After the removal of duplicates, 3,180 papers were identified ( Fig 1 ). Following a title and abstract screening, 372 papers remained for full-text screening. 195 papers were found to fulfil our Stage 1 criteria having a model of DR-mycobacteria strains ( S1 Table ). Of these papers, only 23 were found to meet the requirements of bacterial heterogeneity in mathematical models of DR-mycobacteria ( S2 Table ).
https://doi.org/10.1371/journal.ppat.1011574.g001
Stage 1 Results: DR-mycobacteria model landscape
Most models of mycobacteria were of M . tuberculosis (190 papers/97%) with HIV (59 papers) and diabetes mellitus (5 papers) often included. There was a rapid increase in the number of papers published on DR-mycobacterium from 2005 onwards ( S1 Fig ).
Settings captured
119 papers aimed to model a specific geographical location, typically at the national level ( Fig 2A and S3 Table ). This reflects the settings with the highest MDR-TB incidence but also highlights some countries that are not being focused on ( Fig 2B ). Of the 117 papers, 82 covered a single national analysis and 35 covered different countries. Other geographical locations included 7 models with a global focus, whilst 6 models covered regions with 4 models of Southeast Asia [ 22 – 24 ], and 1 of Eastern Europe [ 25 ] and 1 of the Asia-Pacific [ 26 ].
(a) Countries captured in models of DR-mycobacteria. Note: some models include outputs for multiple countries, therefore this image represents all countries modelled, not the total number of models. (b) From the WHO Global Tuberculosis Report 2022 [ 1 ], the 10 countries with the highest estimated MDR/RR-TB incidence are given with number of models in brackets. The colours in the table match the corresponding colours of the country in part (a). Map layer made with Natural Earth, free vector and raster map data @ naturalearthdata.com .
https://doi.org/10.1371/journal.ppat.1011574.g002
Model aims and types
Of the seven distinct categories of study aim found ( Fig 3 ), non-treatment interventions without cost-effectiveness considered (n = 45, 23%) was the most common. Transmission models (n = 129, 67%) were the most common model type used for all model aims, except for “treatment interventions with cost-effectiveness”, which mostly used state transition models ( Fig 3 ). As would be expected, PK/PD models were used almost exclusively for “treatment interventions”, with one model being used for parameter estimation. Six models used a combination of methods: transmission and state transition [ 27 , 28 ], transmission and operational [ 29 ]and state transition and statistical [ 30 – 32 ]. “Bacterial dynamics” type models were used for “treatment interventions”, “theoretical” and “parameter estimation” aims only. “Decision analytic” type models were used for all aims other than “theoretical” and “parameter estimation”.
The model type (colours) definitions can be summarised as follows: [ 1 ] Bacterial dynamics: Capture bacterial populations without considering between-host transmission. [ 2 ]; Decision analytic: Track cohorts of human individuals through treatment or diagnostic pathways without ongoing transmission. [ 3 ] Pharmacokinetic/pharmacodynamic (PK/PD): Focus on drug concentrations and their effects in vivo, incorporating parameters related to bacterial populations. [ 4 ] State Transition: Involve individuals or populations transitioning between different disease states, with the force of infection as a static input parameter. [ 5 ] Statistical: inference-based models of collected or population data. [ 6 ] Transmission: Dynamically account for the spread of bacteria between individuals or populations. [ 7 ] Operational models: simulation of patient pathways and treatment or diagnostic procedures. The model aim (x axis) definitions can be summarised as follows: (1) Non-treatment Interventions: Model the impact of interventions not related to changes in antibiotic usage or treatment without considering economic aspects. (2) Non-treatment Interventions + cost-effectiveness: Model the impact of interventions not related to changes in antibiotic usage or treatment while considering their economic impact. (3) Treatment interventions: Model interventions related to changes in antibiotic usage. (4) Treatment interventions + cost-effectiveness: Model interventions related to changes in antibiotic usage while considering their economic impact. (5) Parameter estimation: Estimate parameters by comparing to data, trends, or varying model structures or components. (6) Burden estimation models: Quantify the number of individuals potentially infected with DR-mycobacteria. (7) Theoretical models: Theoretically explore interactions between susceptible and resistant strains. Note: "CE" stands for cost-effectiveness. For full details of aim and model type see S2 Text .
https://doi.org/10.1371/journal.ppat.1011574.g003
Resistance categories
Most models of DR-mycobacteria capture resistance to fewer than three antibiotics. Six models considered all possible combinations of resistance to several antibiotics (‘*’, Fig 4 ). Of 16 models to capture four or more resistances at once, 11 of these models included antibiotic resistance as stepwise accumulation of resistance [ 22 , 30 , 33 – 41 ] and 5 models only included mono-resistance of resistance to multiple antibiotics [ 20 , 42 – 45 ].
Each coloured cell represents a specific combination of resistances included in a model, with the size of the cell representing how many models included this combination of resistances. “Single” and “Multiple” sections refer to the number of antibiotic resistances included in a model, with “Multiple” referring to models that captured resistance to more than one antibiotic. "*" indicates the model included all possible combinations of antibiotic resistance listed. A = INH, RIF, MDR/RR, MOX, PZA, BDQ, PA, RIF + MOX, RIF + PZA, B = INH, RIF, MDR/RR, AMI, MOX, BDQ, RIF + MOX, RIF + AMI, RIF + BDQ, C = INH, RIF, MDR/RR, XDR, MDR + FQ, MDR + SLInject, D = INH, RIF, MDR/RR, XDR, Pre-XDR. Antibiotic abbreviations as follows: AMI = amikacin, BDQ = bedaquiline, CLR = clarithromycin, ETM = ethambutol, FQ = undefined fluoroquinolone, MOX = moxifloxacin, PA = pretomanid, PZA = pyrazinamide, STR = streptomycin, INH = isoniazid, RIF = rifampicin, MDR/RR = multidrug resistant/rifampicin resistant, XDR = extensively drug-resistant, SLInject = second line injectable antibiotic (from WHO guidelines 2014). S1 Fig shows all resistance categories per 195 models.
https://doi.org/10.1371/journal.ppat.1011574.g004
Overall, for stage 1, most models included a resistance class of MDR/RR-TB (129 papers/67%, Fig 4 ) with 85 models that chose to model only a single resistance class of MDR/RR-TB alongside DS-TB ( Fig 4 ). 40/195 models included isoniazid resistance ( Fig 4 ) with 27/40 also including MDR/RR-TB. 21/195 models included rifampicin resistance separate from MDR with 15/21 including isoniazid and rifampicin resistance as mono-resistances that developed into MDR with 6/15 models including the development of XDR-TB. Of 18 models that modelled XDR, 16 included MDR/RR, while two did not [ 46 , 47 ]. Out of the first-line antibiotics used to treat TB, isoniazid (n = 40) and rifampicin (n = 27) resistance were modelled the most, followed by pyrazinamide (n = 8) and then ethambutol (n = 5) resistance. Pyrazinamide resistance was often found to be modelled alongside rifampicin and/or isoniazid resistance with only 3 models including resistance to all 4 first-line antibiotics, 2 with mono-resistances and 1 with a combination of all 4 resistances [ 33 , 37 , 42 ] ( Fig 4 ).
41 theoretical models included resistance to a non-named antibiotic ( S1 Table ). One of these explored differences in drug action (bacteriostatic or bactericidal [ 48 ], and two explored antibiotic persistence [ 49 , 50 ] ( S1 Fig ). There were 38 theoretical modelling studies ( S1 Fig ) capturing “drug resistance”, with four of these models exploring firstly hypothetical and then antibiotic-specific resistance ( S1 Table ).
Stage 2 Results: Heterogeneous models
We found 23 models with bacterial heterogeneity—15 bacterial population and 8 between-host models ( S2 Table ) [ 8 , 20 , 33 , 34 , 37 , 43 – 45 , 48 , 49 , 51 – 63 ]. The distribution of model aims that these papers fall into were different from Stage 1 with 13 “parameter estimation”, 8 “treatment interventions”, 1 “theoretical”, and 1 “non-treatment intervention”. 12 of the 23 models modelled the immune system.
Bacterial population models
The fifteen bacterial population models mostly captured multiple resistance classes (n = 13) ( Fig 5 and S2 Table ). One other considered a single resistance class of isoniazid only in an M . tuberculosis population and explored deterministically the impact of antibiotic exposure on resistance dominance with or without heterogeneity in fitness and mutation distributions [ 52 ]. Including heterogeneity in fitness and mutation distributions was also the most common method for exploring variation in models with multiple resistance classes. This was true both for stochastic and deterministic model structures [ 33 , 43 , 51 , 57 , 59 , 62 ], though one deterministic model only explored differences in mutation rates [ 43 ]. Four models additionally explored the impact of variation in growth rates induced by different metabolic states [ 20 , 34 , 45 , 60 ], with one model including fitness variation too [ 45 ].
https://doi.org/10.1371/journal.ppat.1011574.g005
Different clearance rates were used in 2 models, a PK/PD model and a bacterial dynamics model to differentiate between two resistant bacterial strains with the aim of determining the most effective treatment combination [ 48 , 58 ].
One model did not include AMR as a direct resistance to an antibiotic, but instead as persistence [ 49 ]. This was modelled as non-replicating bacterial populations and antibiotics had little to no effect on these bacterial populations. The model implemented heterogeneity by including fast and slow-growing bacteria.
Between-host models
All eight between-host models were compartmental models. Six of these models explored the impact of including a distribution of fitness costs affecting transmission resulting from resistance-conferring mutations to prevalence of either a single [ 8 , 53 , 55 , 56 ] or multiple resistance classes [ 54 , 63 ]. Four of these six models were deterministic [ 53 , 55 , 56 , 63 ], with Knight et al. (2015) exploring a stochastic version in the supplementary materials [ 8 ]. Blower et al. (2004) explored a stochastic model that included heterogeneity by modelling strains of M . tuberculosis with different fitness rates but also cure, treatment, detection, and resistance mutation rates. The model aimed to estimate MDR-TB prevalence [ 54 ].
Two stochastic models were classified as heterogeneous as they included resistance compartments stratified with different resistant genotypes [ 44 , 61 ]. These papers had different aims: Kendall et al. [ 44 ] explored the impact of high and low levels of moxifloxacin resistance on treatment regimens and drug susceptibility testing. Pecerska et al. [ 61 ] estimated the fitness cost of MDR-TB with and without pyrazinamide resistance from a genetic data set.
Use of data derived from the literature
All Stage 2 papers used at least one parameter sourced from existing literature, so no models were entirely theoretical. Some models used a primary data set that was collected from experiments or a population study [ 20 , 49 , 52 , 58 , 59 , 61 ]. Data types used were experimental (83%), epidemiological (26%), clinical (4%), genetic (4%) and WHO data (30%). All bacterial population models used experimental data, with one paper also including clinical data [ 37 ]. Between-host models used a combination of experimental, epidemiological, and WHO data, with one using only genetic data.
Acquired or primary resistance and discrete resistance
All models with heterogeneity represented resistance as discrete categories, such as MDR/RR-TB, with no models including resistance as a spectrum. 6/8 between-host heterogenous models modelled resistance as both primary and acquired and two models had no primary resistance, with acquired resistance only [ 44 , 63 ].
Resistance effects in models
Resistance affected the ability of M . tuberculosis to transmit in 6/8 between-host heterogenous models, with resistant strains usually having a lower value for the transmission coefficient or fitness parameter than the susceptible strain.
Resistance affected disease progression in all models except Knight et al. (2015) [ 8 ]. For bacterial population models, this was defined as different growth rates. For between-host models, this was included as a separate disease progression parameter for resistant strains [ 54 , 55 , 63 ], different relapse rates for patients with resistant bacteria [ 44 ], different associated mortality rates for each resistant strain [ 61 ], variance in cross-immunity by resistant strain [ 53 ], or different natural history pathways for resistant strains [ 56 ].
13/23 models assumed resistance affected operational parameters. In nine, resistance reduced treatment efficacy [ 8 , 44 , 45 , 53 – 56 , 61 , 63 ], with one also including different diagnostic (GeneXpert rapid nucleic acid amplification test for M . tuberculosis ) sensitivity parameters for each resistant strain [ 44 ]. Four bacterial population models had a different antibiotic kill rate [ 48 , 49 , 58 , 60 ], with one including different clinical conversion factors [ 49 ].
Our review of the mathematical modelling landscape of drug resistance in mycobacteria has revealed a growing body of work mostly using transmission dynamic models to explore intervention impact. We found that a minority (33%) explore resistances other than MDR/RR-TB. Few models account for the known heterogeneity that exists in bacterial populations. Where heterogeneity was captured in both bacterial population and between-host models, it was mostly through a variation in the model-specific fitness parameter (with the definition of fitness varying broadly from being related to transmission, ability to cause disease or speed of bacterial growth).
Our Stage 1 landscape analysis found that several high MDR-TB burden countries (e.g. Pakistan, Nigeria, Ukraine, and Myanmar) are underrepresented in the English DR-TB literature. Increasing modelling of DR-TB in specific countries may aid understanding of epidemiology in the specific country and increase the global understanding of DR-TB, as well as improve estimates of intervention efficacy and hence design of context-specific interventions. This is highly relevant when considering that, as has been found for models of M . tuberculosis in general [ 11 , 64 ], most models aimed to estimate the impact of public health interventions. Transmission models were used more than any other type of model across all categories, except for the category of "treatment interventions + cost-effectiveness”, where state transition models were most used. This indicates that most modellers are interested in modelling M . tuberculosis at a between-human host population scale.
MDR-TB was the most common category of resistance modelled (67% of DR-mycobacterium models)—an expected result linked to the historical importance of this as a clinical treatment threshold and reflected in most data collection [ 1 , 3 ]. Mono-isoniazid resistance was more commonly modelled than explicit mono-rifampicin resistance, with 27 models capturing the pathway from isoniazid resistance developing into MDR-TB. XDR-TB was not considered without MDR-TB other than by two papers by Basu et al. (2008, 2009), who were interested in the burden and interventions specific to XDR-TB [ 46 , 47 ]. XDR-TB was often treated as a final state of resistance in modelling systems, with no further resistance being acquired. This reflects the historic clinical decision-making pathway (susceptible or MDR or XDR) and that XDR-TB is resistant to a large number of anti-TB antibiotics. However, there is a great variation in DR-TB and the pathways that may lead to each level of it. Understanding this variation in DR-TB will drive improvements in treatment success by identifying which antibiotics will be most effective and, therefore improve patient outcomes.
Rifampicin and isoniazid resistance were the most modelled mono-resistances, followed by pyrazinamide and ethambutol, reflecting first-line treatments and prophylaxis for TB and data availability. Testing for pyrazinamide and ethambutol resistance is typically reserved for reference settings, and there is widespread use of GeneXpert (Cepheid 6/10-colour instrument), which tests for rifampicin resistance. Only 21% of models (n = 41) captured resistances beyond these four drugs. This will need to be expanded as we move into a period with many more treatment options–constructing, parameterising, and exploring mathematical models of other antibiotic resistances is vitally needed to optimise future treatment and TB control interventions, as well as to explore evolutionary pathways. For example, we found only two papers which explicitly modelled resistance to bedaquiline [ 44 , 45 ], whilst two new treatment regimens containing bedaquiline were approved by the WHO in 2022 [ 65 ].
Models that capture non-specific DR-TB can be useful in the absence of data or to explore broad trends. We found 45 models in this category and found that these theoretical or non-specific systems were used to understand under what constraints DR-TB would dominate over DS-TB or explored the efficacy of a theoretical intervention.
When designing a model to answer a specific question such as the impact of a public health intervention, a balance needs to be struck between designing a detailed or generalised model to allow for a pragmatic approach. This pragmatism is likely the reason for our stage 2 results that revealed few models including bacterial heterogeneity. This is despite several models showing how heterogeneity in transmission fitness can affect DR-TB prevalence estimates [ 8 , 54 – 56 ]. Or how including multiple levels of resistance to one antibiotic can affect treatment outcomes [ 44 , 61 ]. Authors cannot capture all the subtlety of antibiotics as a selection pressure without including the related resistance dynamics and from this the population diversity it fosters. Mathematically, it can be difficult to include complexities in all aspects, for example, population mixing, and often there is little context-specific data on bacterial heterogeneity to inform models. However, if authors want to understand the risk of antibiotic resistance developing under a new treatment regimen it should follow that those resistances are then included in predictions. Some nuance may be beneficial in results that are only achievable with models that include bacterial heterogeneity, such as in Basu et al. (2008) where their conclusions suggested that a weaker immune response to a DR-TB infection with high fitness levels leads to higher DR-TB prevalence in HIV-positive and -negative populations [ 53 ].
Interestingly, we found that all models included resistance in a small number of discrete compartments, with no near-continuous distributions of resistance. Biologically speaking, resistance exists across a spectrum with strains having a range of minimum inhibitory concentrations, but for therapeutic and diagnostic uses they are classified with discrete values. Modelling resistance at multiple possible sub-levels would enable new research questions to be posed about pathways to evolution and competition due to multiple resistant levels. To our knowledge, such a question has not yet been asked regarding M . tuberculosis .
We found that transmission fitness levels, by contrast to resistance levels, were commonly allowed to vary across a distribution within resistant populations, likely reflecting the available historical data pointing to fitness differences between TB strains [ 66 ]. This contrasts with the lack of data linking resistant strain variation with treatment outcomes such as failure or recovery. Including such fitness effects is a relatively easy single-parameter effect within standard transmission dynamic or bacterial dynamics models and is commonly included in models of drug resistance outside of M . tuberculosis [ 7 ].
In this review, we identified 190 published papers which included drug-resistant strains of M . tuberculosis , a further 5 with a drug-resistant non-tuberculosis mycobacteria species, and 1 including both M . tuberculosis and M . marinum . Our update on the literature shows an increasing trend to model DR-TB.
The limitations of our review included that we conducted the search for English language articles when a substantial burden of DR-TB is found in non-English speaking settings such as Eastern Europe [ 1 ]. We did not capture which antibiotics were explored in the models as our focus was on the resistance captured nor time horizons for each model. Our stage 1 analysis only extracted high-level information as our main interest was the bacterial heterogeneity in stage 2. Future work could use this baseline set of literature to explore how resistance is modelled in the natural history of tuberculosis.
We encourage future modellers to consider if the bacterial component of their research question would benefit from the inclusion of bacterial heterogeneity. By not including it, models miss key features of bacterial populations, such as competition or treatment efficacy differences between strains and may, for example, under or overestimate the degree by which an intervention might increase resistance or prevalence of DR-TB.
We were unable to provide a comprehensive review of how resistance was included in Stage 1 models due to the lack of model information provided in many papers such as parameter tables, model diagrams or equations. Future mathematical models should aim for clear model reporting as suggested by the WHO [ 67 ] and Bennett et al. (2012) for transparency and to enable reproducible research [ 68 ].
In this review, we identified 195 drug-resistant mycobacteria mathematical models, with 190 DR-TB models and 23 models including bacterial heterogeneity. This has provided us with an understanding of how resistant mycobacterial species have been modelled, in terms of geographical settings, model aims and types, resistances modelled and further insights into the inclusion of bacterial heterogeneity. However, we found that bacterial heterogeneity was often ignored despite evidence of its importance at the population level. Balancing pragmaticism with biological reality when building mathematical models is vital within the fundamental evolutionary dynamics of AMR.
Supporting information
S1 text. search strings..
https://doi.org/10.1371/journal.ppat.1011574.s001
S2 Text. Details of extraction table for stage 1.
https://doi.org/10.1371/journal.ppat.1011574.s002
S3 Text. Details of extraction table for stage 2.
https://doi.org/10.1371/journal.ppat.1011574.s003
S1 Fig. Heatmap of all resistance categories in stage 1 models.
Heatmap of resistances included per DR-TB model (n = 195) indicates a lack of diversity in resistances modelled, with MDR/RR-TB featuring in over half of all 195 models. Each coloured line indicates a model (y axis) included in stage 1 (purple) or stage 2 (orange). The graph groups models into specific (captures resistance to a named antibiotic), non-specific (defined resistance that are not specific to an antibiotic) and hypothetical (captures antibiotic resistance not linked to a named drug). Antibiotic acronyms as follows: AMI = amikacin, BDQ = bedaquiline, CLR = clarithromycin, ETM = ethambutol, FQ = undefined fluroquinolone, LZD = linezolid, MOX = moxifloxacin, PA = pretomanid, PZA = pyrazinamide, STR = streptomycin, INH = isoniazid, RIF = rifampicin, MDR/RR = multidrug resistant/ rifampicin resistant, XDR = extensively drug-resistant, SLInject = second line injectable antibiotic (from WHO guidelines 2014), another 1st line = rifampicin, ethambutol, or pyrazinamide. Index links to paper number in S1 Table .
https://doi.org/10.1371/journal.ppat.1011574.s004
S2 Fig. Plot of number of publications over time.
https://doi.org/10.1371/journal.ppat.1011574.s005
S1 Table. Extraction table results from stage 1.
https://doi.org/10.1371/journal.ppat.1011574.s006
S2 Table. Extraction table results from stage 2.
https://doi.org/10.1371/journal.ppat.1011574.s007
S3 Table. Geographic settings in models.
https://doi.org/10.1371/journal.ppat.1011574.s008
Acknowledgments
We would like to thank the support of library staff at the London School of Hygiene and Tropical Medicine. Thank you for the guidance and advice for this work from Quentin Leclerc and Alastair Clements.
- 1. World Health Organisation. Global tuberculosis report 2022 [Internet]. 2022 Oct. Available from: https://www.who.int/publications/i/item/9789240061729
- 2. World Health Organisation. WHO announces updated definitions of extensively drug-resistant tuberculosis [Internet]. 2021 [cited 2021 Jan 27]. Available from: https://www.who.int/news/item/27-01-2021-who-announces-updated-definitions-of-extensively-drug-resistant-tuberculosis
- 3. World Health Organisation. Definitions and reporting framework for tuberculosis–2013 revision: updated December 2014 and January 2020. 2013. Report No.: No. WHO/HTM/TB/2013.2.
- View Article
- PubMed/NCBI
- Google Scholar
- 65. World Health Organisation. WHO consolidated guidelines on tuberculosis. Module 4: treatment—drug-resistant tuberculosis treatment, 2022 update. 2022 Dec.
- 67. World Health Organization. Guidance for country-level TB modelling [Internet]. Geneva: World Health Organization; 2018 [cited 2023 Jun 6]. 37 p. Available from: https://apps.who.int/iris/handle/10665/274279
SYSTEMATIC REVIEW article
Effect of thoracic paravertebral nerve block on delirium in patients after video-assisted thoracoscopic surgery: a systematic review and meta-analysis of randomized controlled trials.

- Department of Anesthesiology, The Second Clinical Medical College, North Sichuan Medical College, Nanchong Central Hospital, Nanchong, China
Background: Nerve blocks are widely used in various surgeries to alleviate postoperative pain and promote recovery. However, the impact of nerve block on delirium remains contentious. This study aims to systematically evaluate the influence of Thoracic Paravertebral Nerve Block (TPVB) on the incidence of delirium in patients post Video-Assisted Thoracoscopic Surgery (VATS).
Methods: We conducted a systematic search of PubMed, Embase, Web of Science, Cochrane Library, and Scopus databases in June 2023. The search strategy combined free-text and Medical Subject Headings (MeSH) terms, including perioperative cognitive dysfunction, delirium, postoperative cognitive dysfunction, paravertebral nerve block, thoracic surgery, lung surgery, pulmonary surgery, and esophageal/esophagus surgery. We utilized a random effects model for the analysis and synthesis of effect sizes.
Results: We included a total of 9 RCTs involving 1,123 participants in our study. In VATS, TPVB significantly reduced the incidence of delirium on postoperative day three (log(OR): −0.62, 95% CI [−1.05, −0.18], p = 0.01, I 2 = 0.00%) and postoperative day seven (log(OR): −0.94, 95% CI [−1.39, −0.49], p < 0.001, I 2 = 0.00%). Additionally, our study indicates the effectiveness of TPVB in postoperative pain relief ( g : −0.82, 95% CI [−1.15, −0.49], p < 0.001, I 2 = 72.60%).
Conclusion: The comprehensive results suggest that in patients undergoing VATS, TPVB significantly reduces the incidence of delirium and notably diminishes pain scores.
Systematic review registration: CRD42023435528. https://www.crd.york.ac.uk/PROSPERO .
1 Introduction
For Lung cancer serves as a prominent contributor to cancer-related fatalities across the globe, accounting for nearly a quarter of all cancer-related deaths ( 1 ). Despite recent advancements in targeted therapies, immunotherapy, and radiation treatment for lung cancer, early-stage surgical resection remains the predominant curative strategy for the vast majority of patients ( 2 ). Video-Assisted Thoracoscopic Surgery (VATS) remains the favored technique for surgical resection in early-stage lung cancer ( 3 ). Nonetheless, the rate of postoperative complications in elderly patients who undergo thoracic surgery varies from 12 to 47%, with delirium frequently observed ( 4 ). Postoperative delirium is defined as delirium occurring within one week after surgery or before the patient’s discharge from the hospital (whichever occurs earlier), meeting the diagnostic criteria for delirium as outlined in the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders ( 5 ). Delirium is categorized into two types based on timing: emergence delirium and postoperative delirium ( 6 , 7 ). Additionally, delirium is divided into three subtypes based on activity levels: hypoactive delirium, hyperactive delirium, and mixed-type delirium ( 8 , 9 ).
The presence of delirium has been definitively associated with higher patient mortality rates, reduced comfort during hospital stays, prolonged discharge times, increased hospital expenses, and additional strain on both patients’ families and the healthcare system ( 10 , 11 ). Recent studies indicate that the implementation of suitable preventive measures can effectively decrease the occurrence of delirium ( 12 ), thereby improving patient comfort and the overall quality of healthcare.
Current studies suggest that delirium arises from a combination of various factors, where neuroinflammation plays a pivotal role in both its onset and progression ( 13 , 14 ). Pain is universally recognized as a leading risk factor for post-surgical delirium ( 15 ). Nerve block is believed to have the potential to decrease postoperative pain ( 16 , 17 ) and diminish the stress and inflammatory responses triggered by surgery ( 18 – 21 ), thus potentially reducing the occurrence of postoperative delirium. However, there is still an ongoing debate regarding whether nerve block can effectively lower the incidence of delirium ( 22 , 23 ). This study aims to investigate the potential impact of Thoracic Paravertebral Nerve Block (TPVB) on postoperative delirium in patients undergoing VATS.
2.1 Study design
This study rigorously adhered to the principles outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) ( 24 ) and Assessing the methodological quality of Systematic Reviews (AMSTAR) ( 25 ) guidelines. All data incorporated into our study were exclusively derived from published literature, obviating the necessity for ethical review. Furthermore, the study is registered in the International Prospective Register of Systematic Reviews (PROSPERO CRD42023435528).
2.2 Data collection
In June 2023, a comprehensive search was carried out across various databases, including PubMed, Embase, Web of Science, Cochrane Library, and Scopus. The retrieval strategy combines a mixture of free-text and MeSH terms, including perioperative neurocognitive disorders, delirium, postoperative cognitive dysfunction, paravertebral nerve block, thoracic surgery, pulmonary surgery, lung surgery, esophageal/esophagus surgery, and others ( Supplementary Appendix 1 ).
2.3 Data selection
Two independent researchers conducted a comprehensive and independent assessment and review of the literature, covering titles, abstracts, and full-text articles, to determine the final inclusion of studies in this research. Any disagreements were resolved through discussion, and in cases where a consensus could not be reached, a third researcher intervened to make the final decision.
Inclusion Criteria.
The following criteria were employed for the inclusion of studies:
1. The literature must comprise a randomized controlled trial.
2. The study subjects must be patients undergoing thoracic or pulmonary surgery.
3. Paravertebral nerve block must be administered during the perioperative period.
4. The literature must evaluate the incidence of delirium.
5. Literature from the control group must also be incorporated into the study.
Exclusion Criteria.
The following criteria were applied for the exclusion of studies:
1. Literature classified as case reports.
2. Observational studies.
3. Retrospective cohort studies.
4. Review articles.
5. Trial protocols.
6. Insufficient or unclear data within the literature.
7. Inability to access the full text or contact the authors.
2.4 Data extraction and integration
We have developed a data extraction table and conducted a preliminary test. Subsequently, two researchers independently conducted data extraction. Any discrepancies in data extraction were thoroughly discussed. In cases where the two independent researchers were unable to reach a consensus, a third researcher was consulted to make the final decision. The data extraction table comprises the following key elements: author names, publication year, study design, participant age, participant count, type of surgery, anesthesia agent types and dosages, Delirium incidence, and postoperative pain scores. These data were primarily sourced from numerical data presented in tables and figures. We employed the online tool WebPlotDigitizer (Version 4.6; WebPlotDigitizer, A. Rohatgi, Pacifica, CA, United States) to extract data presented in graphical form. We employed the equation proposed by Wan et al. to estimate the mean and standard deviation of data described with medians (interquartile range) ( 26 ).
2.5 Bias risk assessment and evidence quality grading
We employed the Cochrane Collaboration’s Risk of Bias 2 tool ( 27 ) to assess potential bias in the included studies. This comprehensive tool evaluates various aspects, including random sequence generation, allocation concealment, blinding of patients, healthcare providers, data collectors, and outcome assessors, completeness of outcome data, selective outcome reporting, and other potential sources of bias. Each article was then categorized into one of three risk levels: “low,” “some concerns,” or “high.” To evaluate the quality of evidence for each outcome, we applied the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) methodology ( 28 ). This systematic approach allowed us to categorize the quality of evidence as either very low, low, moderate, or high.
2.6 Data analysis methods
We carried out data analysis leveraging Stata 17.0 and Review Manager 5.4 software platforms. To gauge the extent of variability among the selected studies, we employed τ 2 (Tau squared) and I 2 (I-squared) statistical metrics. These statistics were used to gauge and measure the degree of heterogeneity within the gathered data, thereby enabling a more rigorous interpretation of the findings ( 29 ). To optimally mitigate potential confounders and to more accurately mirror real-world scenarios, we opted for a random-effects model ( 30 ). Notably, when heterogeneity is notably low, outcomes from the random-effects model align with those of the fixed-effects model ( 30 ). Consequently, this study uniformly applied a random-effects model for calculating and amalgamating log-odds ratios (log(OR)) and their corresponding 95% confidence intervals (CI) in binary data, as well as in determining Hedges’s g ( g ) and their relevant 95% CI in continuous outcomes. This methodology bolsters the accuracy and dependability of our analyses.
In this study, we employed log(OR) and g as statistical measures to assess the effect size differences between the experimental group and the control group. Essentially, log(OR) measures the same relationship as the odds ratio(OR), but it provides a more stable and normally distributed estimate, making it more suitable for studies with small sample sizes. On the other hand, g is a standardized measure of mean difference that accounts for the impact of sample size, allowing it to be compared with results from other studies. Compared to the simple standardized mean difference, g adjusts for estimation bias in studies with small samples, thereby offering a more accurate measure of effect size.
To assess and scrutinize publication bias for each assessed outcome, we utilized funnel plots and conducted Egger’s test as part of our analysis ( 29 ).
3.1 Inclusion of studies
The investigators commenced their study with an initial database search, encompassing PubMed ( n = 170), EMBASE ( n = 217), Cochrane Library ( n = 147), Web of Science ( n = 149), and Scopus ( n = 75), culminating in a total of 758 articles obtained. Subsequently, we removed 258 redundant articles. A pair of researchers screened out 436 papers based on their titles and abstracts. Subsequently, 64 articles underwent full-text assessment by the same duo, resulting in a final selection of 9 articles. For a comprehensive selection process, please consult Figure 1 .
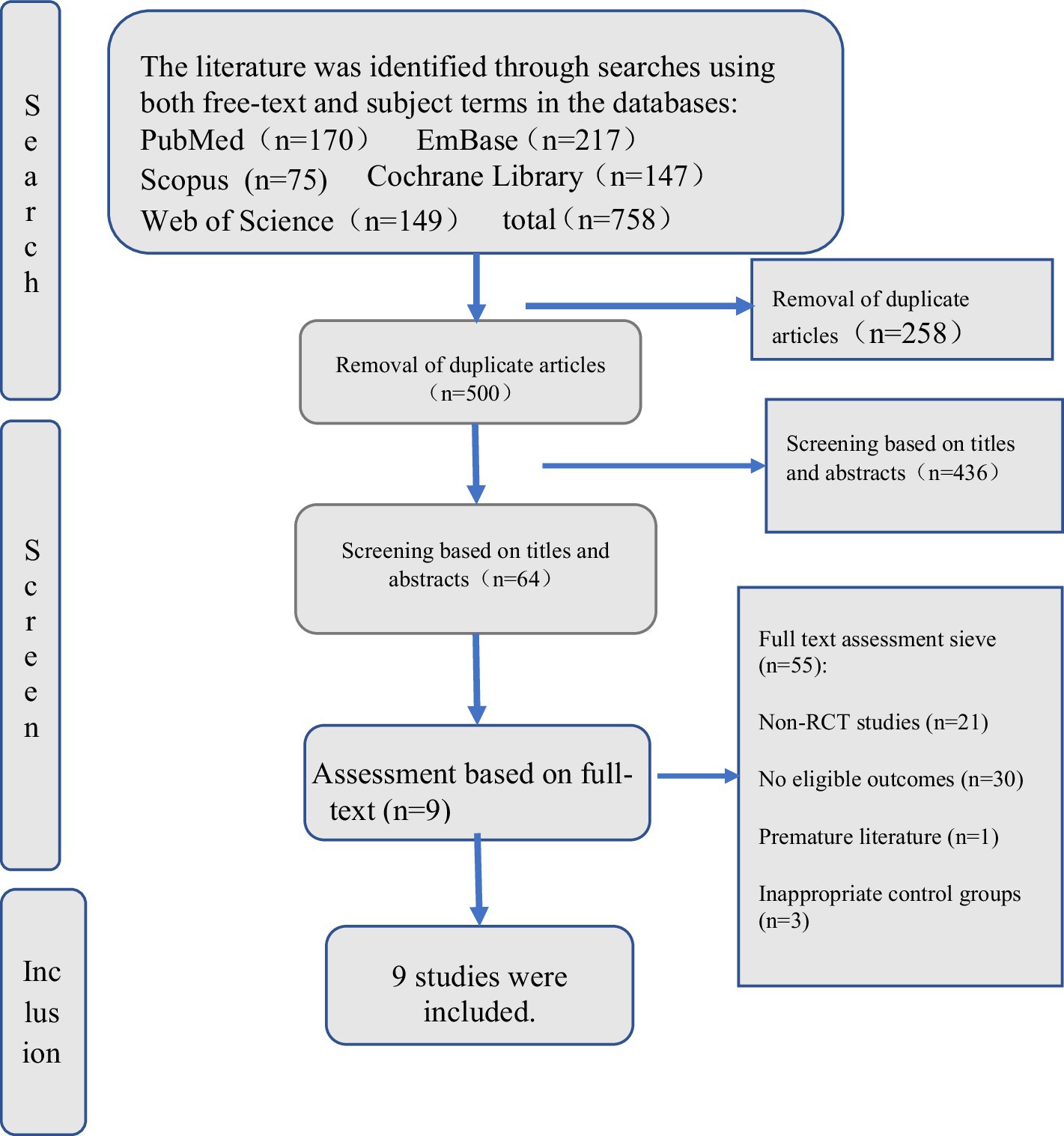
Figure 1 . Flow diagram of study selection.
3.2 Study characteristics
In this study, a total of eight studies conducted thoracoscopic lung lobe resection under general anesthesia combined with TPVB ( 31 – 38 ), while another study performed thoracoscopic surgery under general anesthesia combined with TPVB ( 39 ). In seven of the studies, the postoperative analgesia regimen employed Patient-Controlled Intravenous Analgesia (PCIA). In the remaining two studies, the pain management approach involved either continuous thoracic epidural analgesia or PCIA. Detailed information on each study can be found in Table 1 .
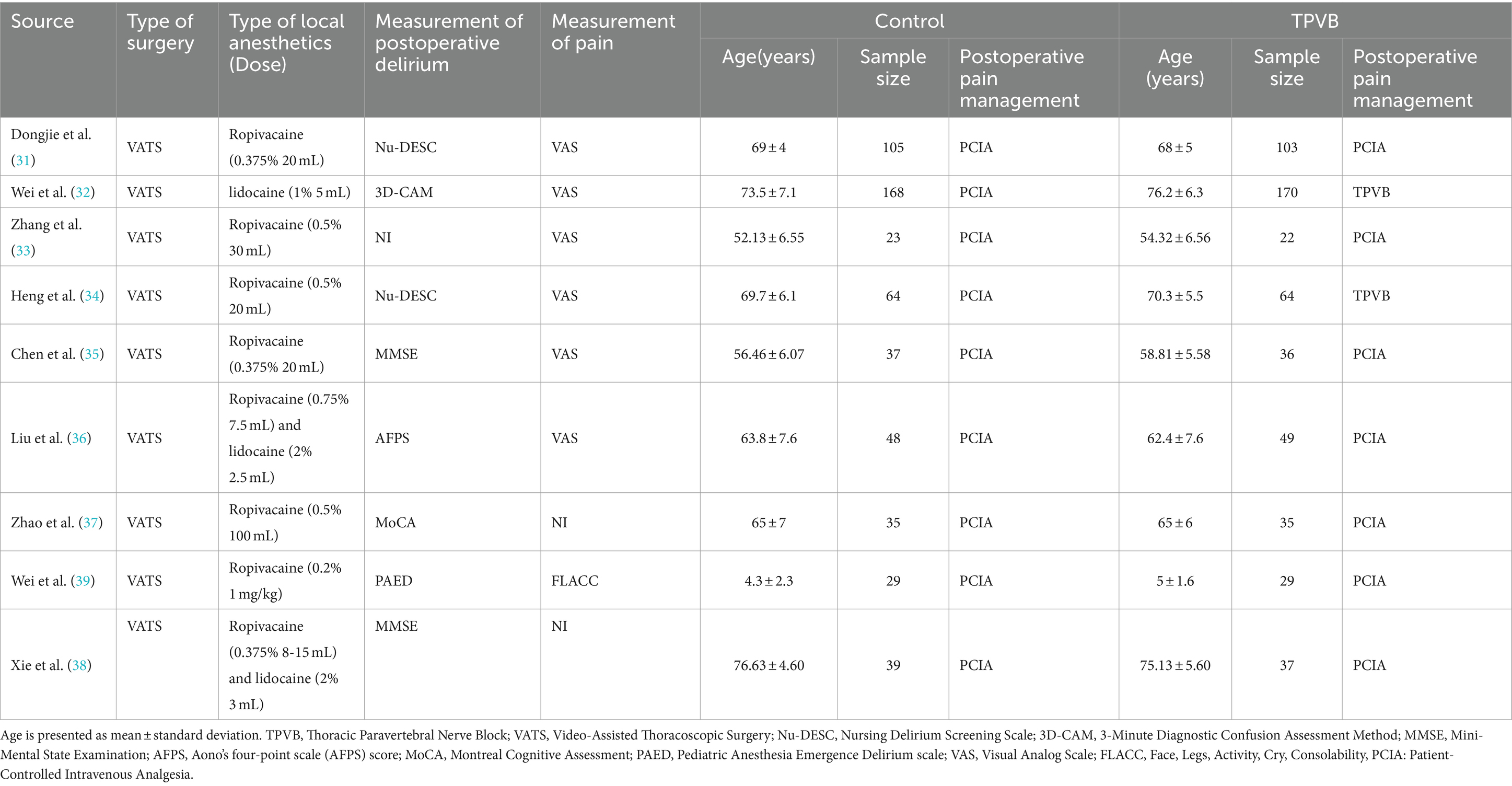
Table 1 . The characteristics of included studies.
3.3 Risk of bias and evidence quality grading
A single study was categorized as posing a low risk of bias ( 35 ), whereas seven studies elicited some level of concern regarding bias risk ( 31 – 33 , 36 – 39 ), and another study was flagged for high bias risk ( 34 ), as depicted in Figure 2 . The quality assessment of the evidence is outlined in Figure 3 .
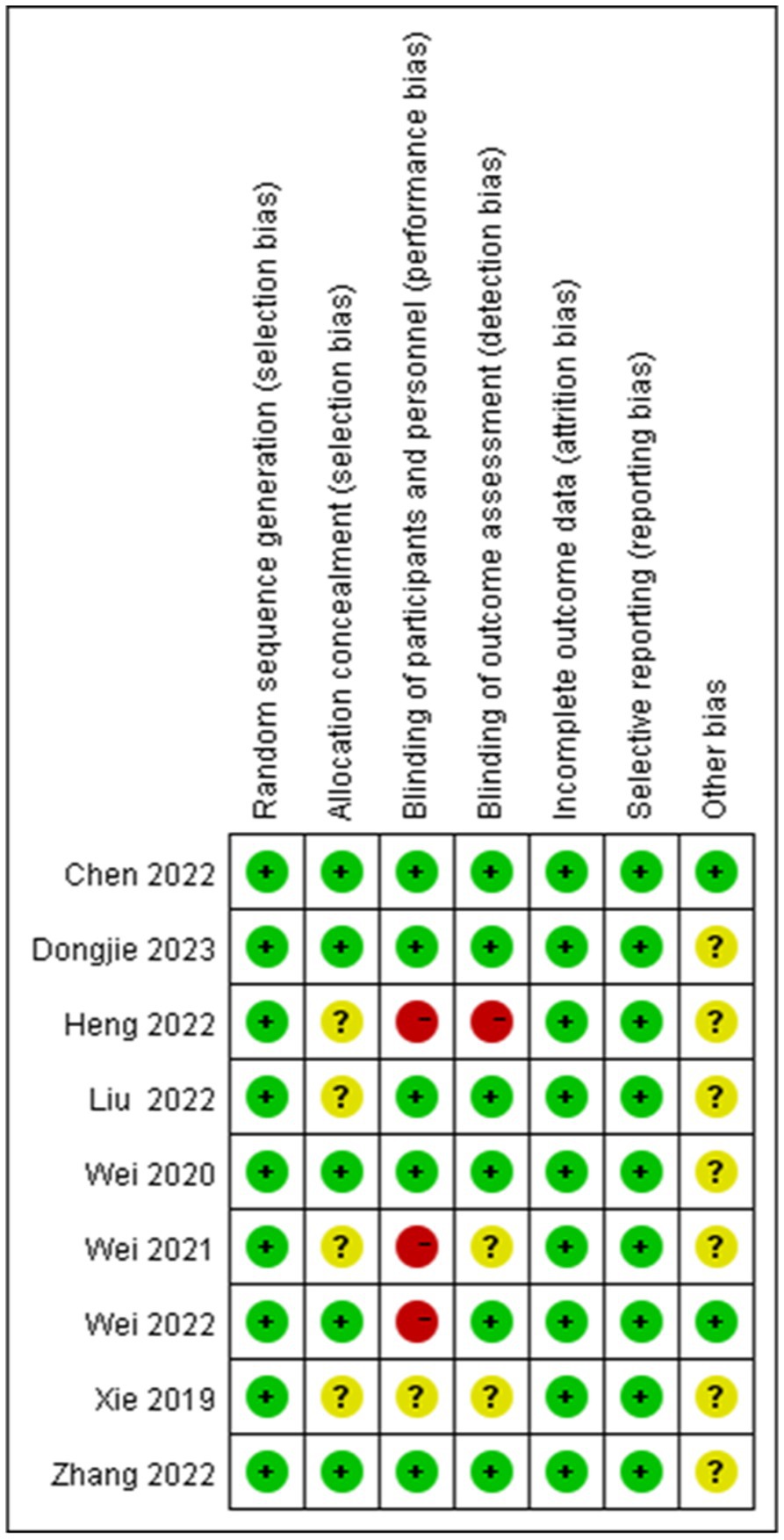
Figure 2 . Risk of bias summary: the author’s assessment of the bias risk factors in various studies.
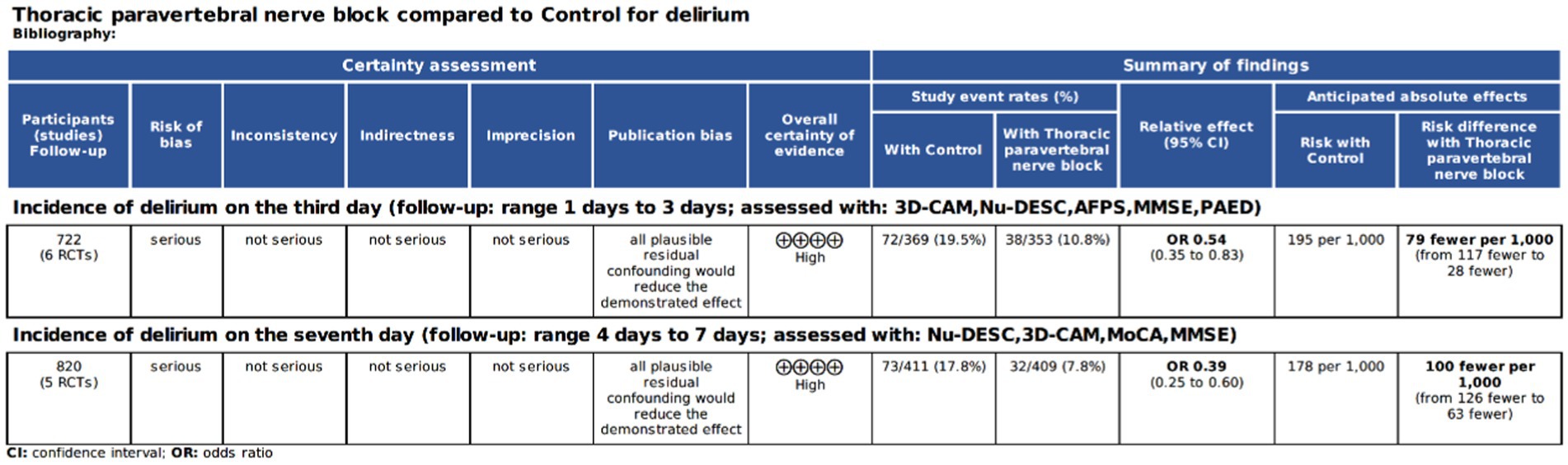
Figure 3 . GRADE evidence summary table.
3.4 The impact of delirium after surgery within three days
Six RCTs studies ( 32 – 36 , 39 ) were included in our postoperative three-day delirium analysis. We incorporated the delirium incidence rate (1–3 days) from each study that was closest to the third day. The TPVB group exhibited a lower delirium incidence rate three days post-surgery compared to the control cohort (log(OR): −0.62, 95% CI [−1.05, −0.18], p = 0.01, I 2 = 0.00%) ( Figure 4 ). No substantial heterogeneity was identified via Galbraith Plot evaluation ( Figure 5 ). We conducted a publication bias funnel plot ( Figure 6 ) and Egger’s test ( p = 0.767; Supplementary Appendix 2 ), both of which did not indicate significant evidence of publication bias.
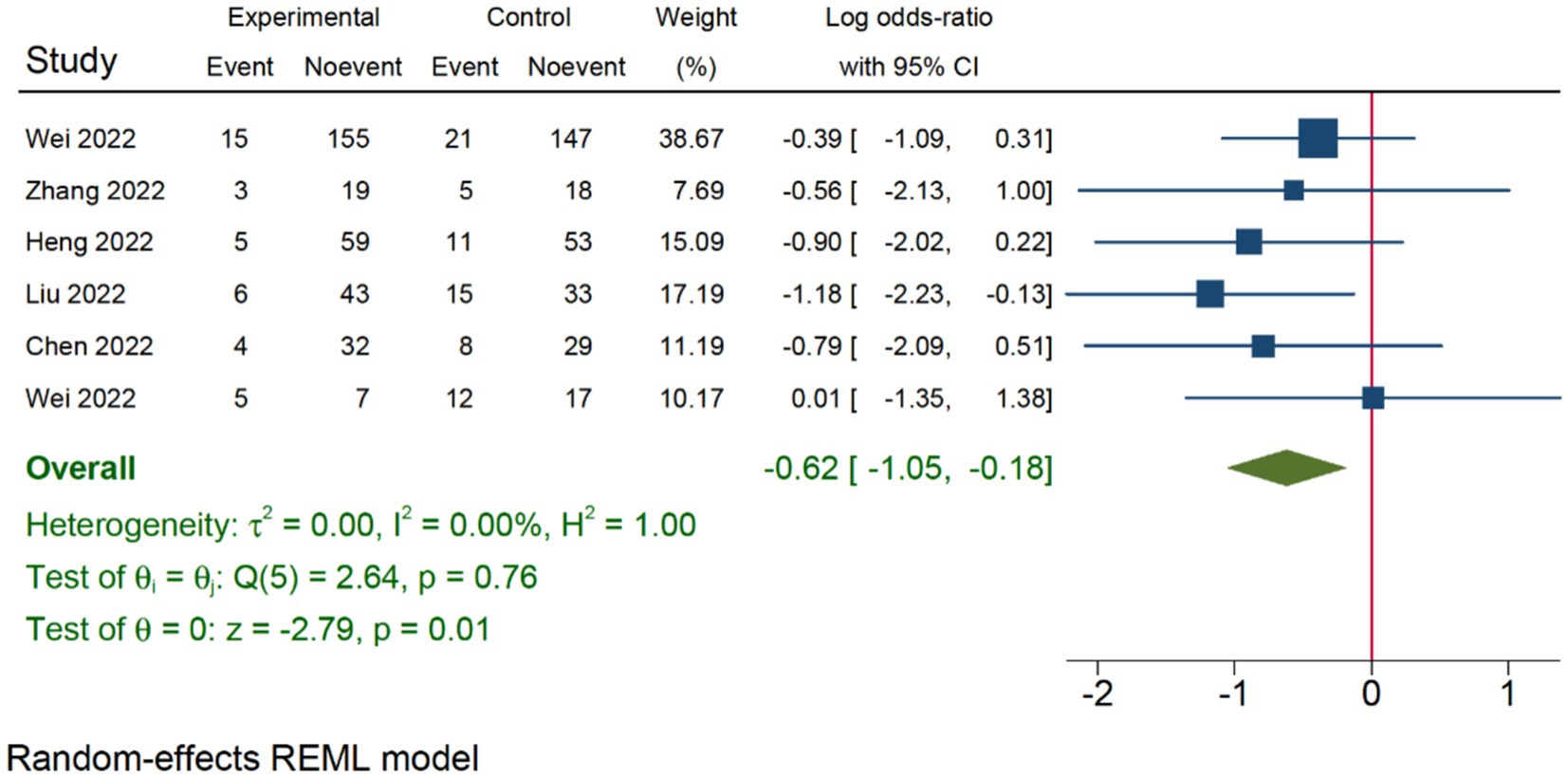
Figure 4 . The impact of TPVB on postoperative delirium three days after surgery.
Figure 5 . Galbraith plot chart at three days post-surgery.
Figure 6 . Publication bias funnel plot at three days post-surgery.
3.5 The impact of delirium after surgery within seven days
Five RCTs studies ( 31 , 32 , 34 , 37 , 38 ) were included in our postoperative seven-day delirium analysis. We included the delirium incidence rate closest to the seventh postoperative day. The delirium incidence rate at seven days postoperatively was lower in the TPVB group compared to the control group (log(OR): –0.94, 95% CI [−1.39, −0.49], p < 0.001, I 2 = 0.00%) ( Figure 7 ). Heterogeneity assessment using the Galbraith Plot indicated low heterogeneity ( Figure 8 ). We conducted a publication bias funnel plot ( Figure 9 ) and Egger’s test ( p = 0.247; Supplementary Appendix 3 ), both of which did not reveal significant evidence of publication bias.
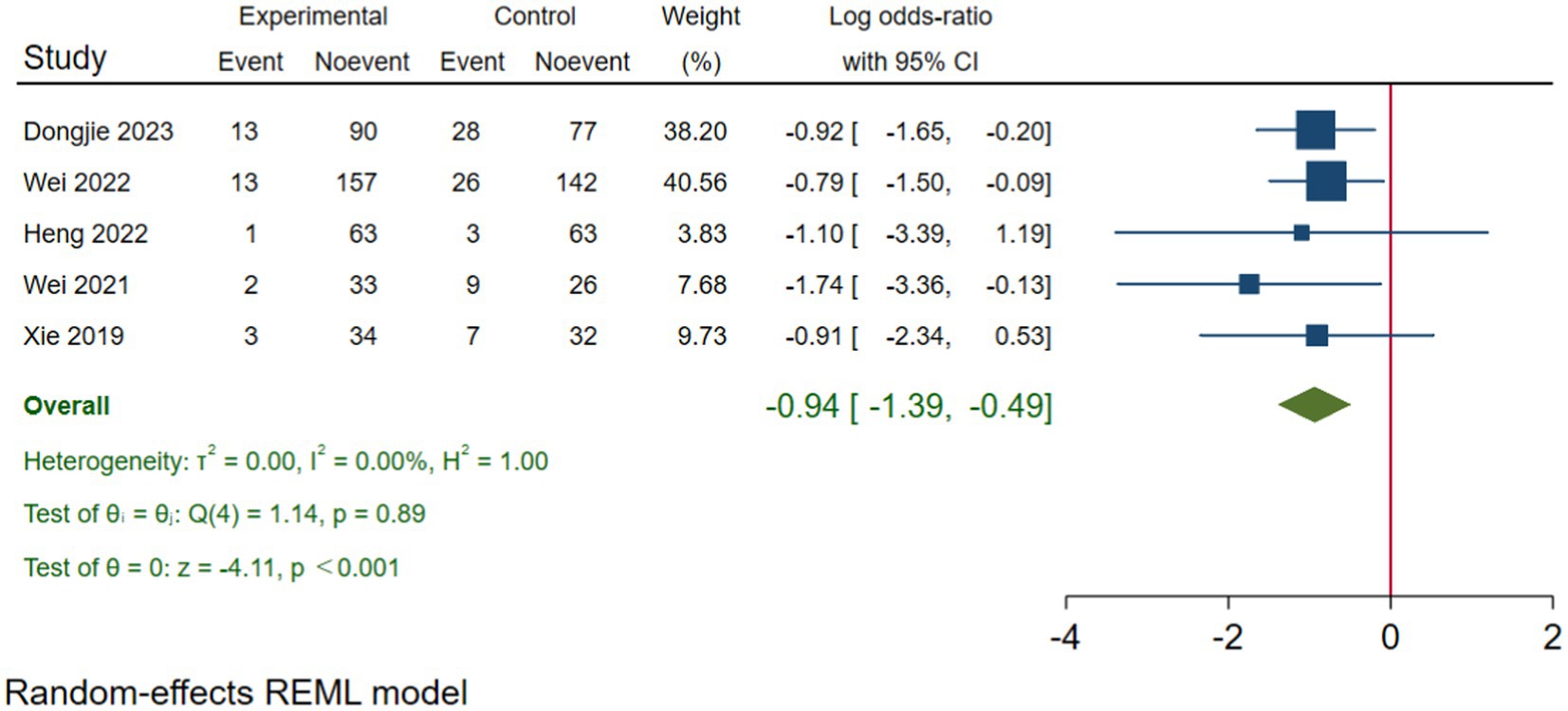
Figure 7 . The impact of TPVB on postoperative delirium seven days after surgery.
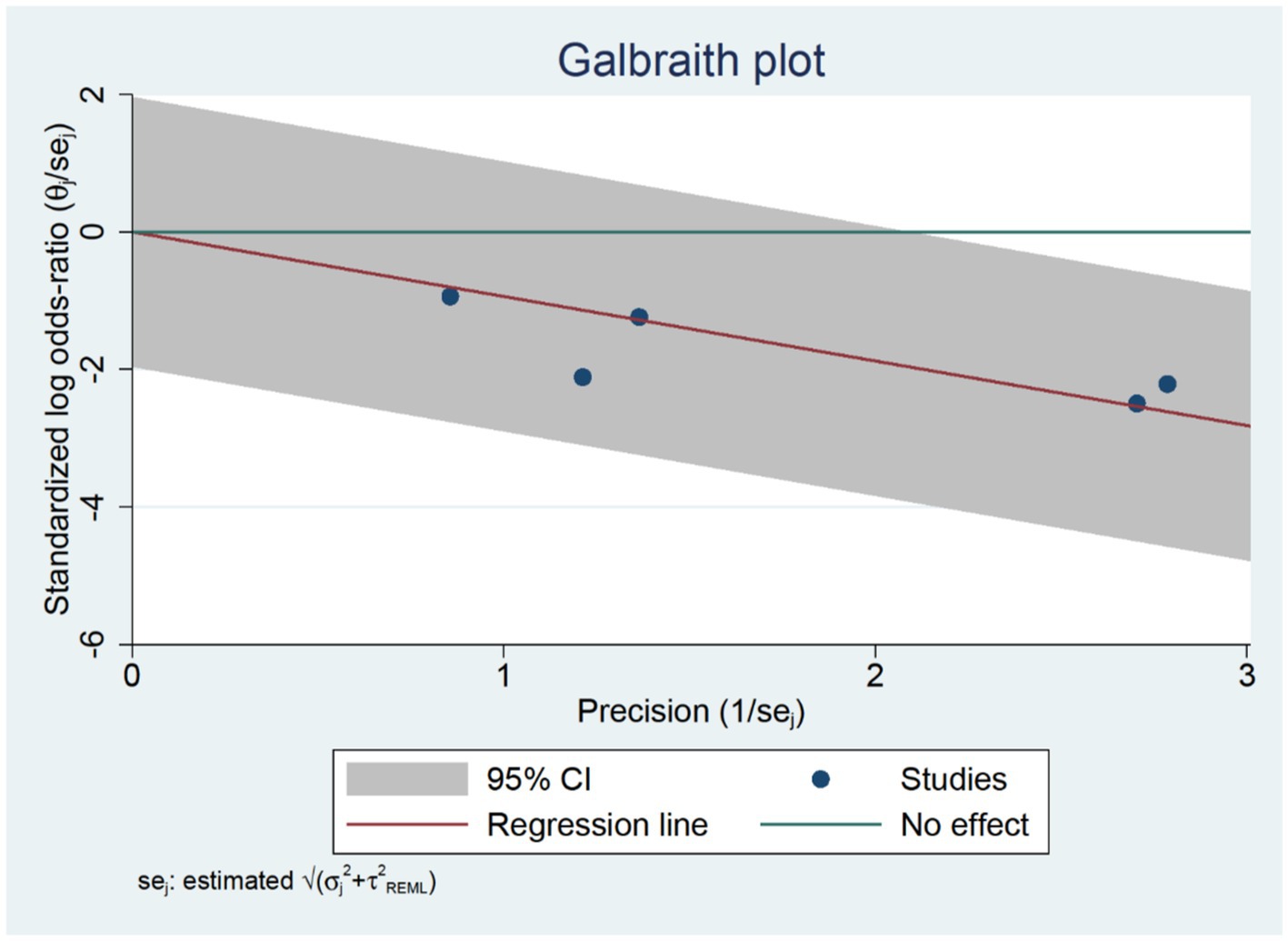
Figure 8 . Galbraith plot chart at seven days post-surgery.
Figure 9 . Publication bias funnel plot at seven days post-surgery.
3.6 The impact of postoperative pain
An analysis of post-surgical first-day pain scores was conducted, omitting a study with a divergent pain assessment approach ( 39 ). Six RCTs were integrated into our evaluation ( 31 – 36 ). When performing a meta-analysis on the remaining seven studies that employed VAS pain scores, we observed that on the first day postoperatively, the TPVB group had lower pain scores compared to the control group ( g : −1.52, 95% CI [−2.87, −0.17], p = 0.03, I 2 = 98.50%) ( Supplementary Appendix 4 ). The documented I 2 value signifies a substantial level of heterogeneity. Heterogeneity analysis was conducted using the Galbraith plot ( Figure 10 ), and the influence of individual studies on the results can be observed in Figure 11 .
Figure 10 . A Galbraith plot related to postoperative pain within a one-day timeframe is presented in the figure.
Figure 11 . The impact of individual studies on the results.
We performed a sensitivity analysis of the pain scores on the first day after surgery. In this process, we excluded the study published by Wei et al. ( 32 ) to enhance the reliability and real-world relevance of our study findings. On the first day postoperatively, the VAS pain scores in the TPVB group were significantly lower than those in the control group, and this result was statistically significant ( g : −0.87, 95% CI [−1.25, −0.48], p < 0.001, I 2 = 77.51%) ( Figure 12 ). This suggests that the application of TPVB effectively reduces postoperative pain in patients, leading to improved comfort, enhanced surgical experience, and a smoother recovery process. Nonetheless, it is important to note that there is a relatively high level of heterogeneity among the included studies. Consequently, we should interpret these results with caution. We conducted a publication bias funnel plot and Egger’s test ( p = 0.662), both of which did not reveal significant evidence of publication bias.
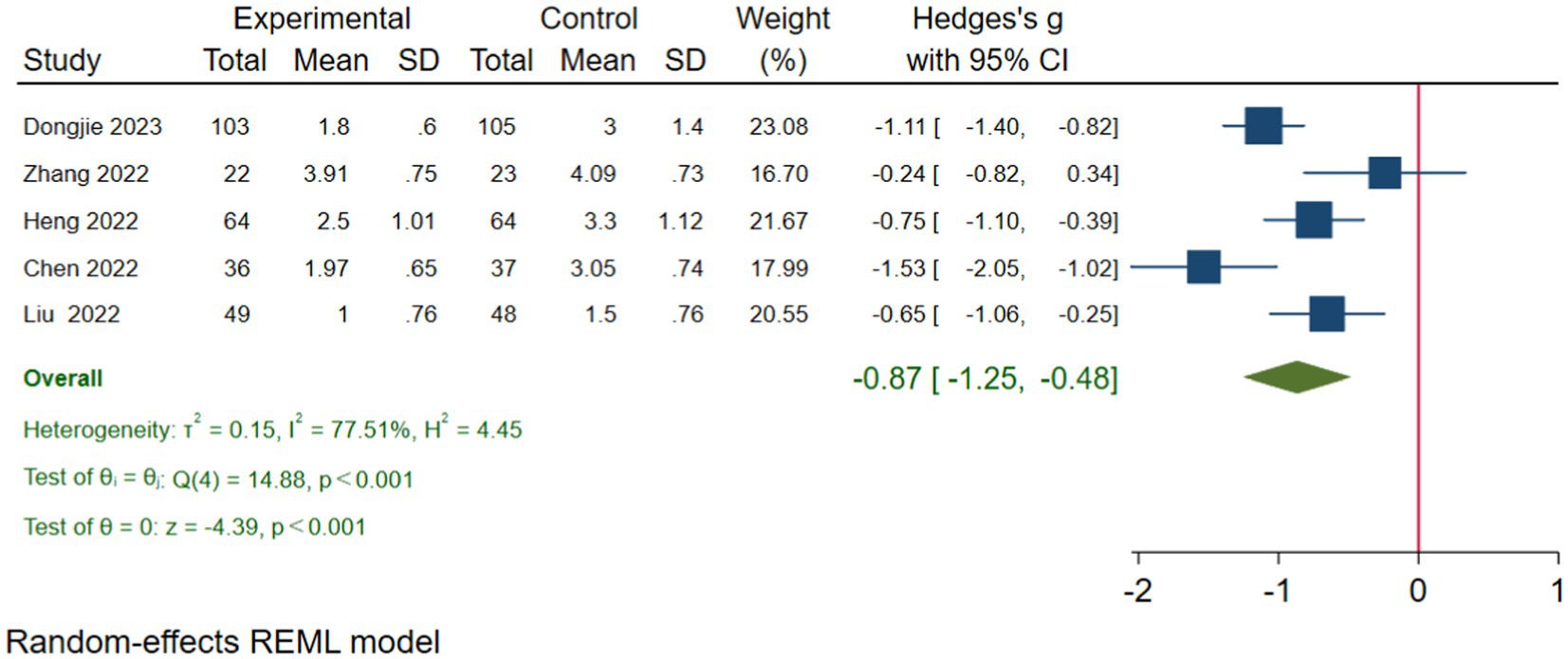
Figure 12 . After one day postoperatively, the forest plot of VAS pain scores.
4 Discussion
Our systematic review and meta-analysis clearly demonstrate that TPVB can effectively reduce the incidence of delirium in patients after VATS. According to the GRADE methodology, the quality of evidence for this conclusion is rated as high. Additionally, patients receiving TPVB also exhibit reduced postoperative pain scores. The importance of this study lies in indicating the effectiveness of TPVB in reducing the rates of delirium and pain intensity in patients undergoing VATS.
Current perioperative neurocognitive disorders (PND) include delirium (occurring within one week post-surgery or until discharge, whichever happens first), delayed neurocognitive recovery (a decline in cognitive abilities occurring within 30 days post-surgery), and postoperative neurocognitive disorder (lasting up to 12 months) ( 5 ). In our investigation, we discerned that TPVB markedly attenuates the incidence of postoperative delirium on the third and seventh days postoperatively, with low heterogeneity. This result suggests that the implementation of TPVB has a short-term impact on the occurrence of PND. However, research on the long-term effects of TPVB on neurocognitive function after the 7th day following VATS is relatively scarce. Therefore, determining the long-term impact of TPVB on neurocognitive function presents a challenge. Further investigation into the effects of TPVB on postoperative neurocognitive function is necessary. These studies should involve larger sample sizes in clinical trials, the use of standardized diagnostic criteria, long-term tracking of neurocognitive function presents after surgery, and comparisons with patients who have not undergone TPVB. Conducting such studies will be instrumental in gaining a more comprehensive understanding of the effects of TPVB on delirium and neurocognitive function. Ultimately, these investigations will furnish more precise recommendations for clinical practice. Through additional research, we can determine whether TPVB can be considered an effective strategy for reducing the occurrence of delirium and improving patients’ neurocognitive function. Furthermore, we can investigate other potential influencing factors to gain a deeper understanding of the mechanisms behind PND and explore preventive measures.
A recent meta-analysis examining the influence of perioperative peripheral nerve blocks on delirium in elderly individuals undergoing hip joint surgery, encompassing 19 randomized controlled trials with a total of 1,977 patients, indicated a decrease in the occurrence of delirium on the third postoperative day (OR: 0.59, 95% CI [0.40–0.87], p = 0.007, I 2 = 35%). ( 40 ). However, it’s worth noting that another meta-analysis yielded contradictory results, indicating no statistically significant difference in the incidence of postoperative delirium between regional anesthesia and general anesthesia ( 41 ). But, this study is subject to considerable heterogeneity influenced by multiple factors. Additionally, there are substantial inconsistencies in both the statistical outcomes and the definitions used. Consequently, drawing a definitive conclusion from this study proves to be challenging.
In our study, we found that TPVB can significantly reduce postoperative pain scores. Postoperative pain is considered one of the significant risk factors for delirium, and this may be associated with the observed decrease in delirium rates on the third postoperative day. At the same time, current research suggests that postoperative opioid use is also one of the risk factors for the occurrence of delirium ( 42 ). Considering that TPVB efficiently diminishes postoperative pain levels, it may contribute to a lower delirium rates by minimizing the need for opioid analgesics ( 43 , 44 ). Nonetheless, it’s worth mentioning that in this study, at the seven-day postoperative time point, patients in the TPVB group also demonstrated a significant decrease in delirium rates. This could be attributed to the ability of TPVB to mitigate perioperative and postoperative stress responses and inflammatory reactions induced by surgery, consequently reducing the incidence of delirium.
In our meta-analysis of pain scores, it is essential to take note that Wei et al. ( 39 ) utilized the FLACC scale for pain evaluation, while other studies employed the Visual Analog Scale (VAS) for pain assessment. As a result, we decided to exclude the study conducted by Wei et al. ( 39 ) from our analysis. Nevertheless, it is noteworthy that the study conducted by Wei et al. ( 39 ) also suggested a tendency towards lower pain scores (OR: 0.55, 95% CI [−0.04, 1.14], p = 0.065). Simultaneously, the heterogeneity analysis of pain scores on postoperative day one, as depicted in the Galbraith plot ( Figure 10 ), and the influence of individual studies on the outcomes ( Figure 11 ), clearly demonstrates the substantial heterogeneity evident in the study by Wei et al. published in 2022 ( 32 ). This heterogeneity has had a notable impact on the experimental results, potentially introducing inherent bias into the study findings. Consequently, during sensitivity analysis, the aforementioned study was excluded.
Our investigation does face certain limitations. Primarily, due to variations in the timing of delirium diagnosis across the included studies, we extracted delirium incidence rates closest to the third and seventh post-surgical days, incorporating them into the analysis. This approach may inevitably introduce some potential bias. Secondly, multiple diagnostic tools are currently at our disposal for diagnosing delirium, including the Confusion Assessment Method (CAM), the Richmond Agitation-Sedation Scale (RASS), the Memorial Delirium Assessment Scale (MDAS), the Nursing Delirium Screening Scale (Nu-DESC), the Mini-Mental State Examination (MMSE), and the Montreal Cognitive Assessment (MOCA), among others ( 45 , 46 ). Nonetheless, there’s currently no uniform standard for diagnosing delirium. The research we incorporated utilized different methods for delirium diagnosis, including Nu-DESC, 3D-CAM, MoCA, MMSE, and one study did not explicitly specify its diagnostic approach. These variations in diagnostic methods may potentially influence the study results. Moreover, concerning the implementation of TPVB, most research favored ropivacaine as the primary agent, with a few opted for a combination of lidocaine. Notably, inconsistencies in dosage, volume, concentration, and injection site among these studies. These disparities could potentially affect the effectiveness of TPVB and create variances in the study outcomes.
In summary, this study indicates that TPVB has a positive short-term impact on reducing the incidence of delirium among patients undergoing VATS, with high-quality GRADE evidence supporting this conclusion. However, contradictions remain, and further research is necessary to fully understand TPVB’s effects on neurocognitive function and to refine postoperative management strategies. Such studies are essential for enhancing recovery support for surgical patients and ensuring a more comprehensive approach to their care.
Data availability statement
The original contributions presented in the study are included in the article/ Supplementary material , further inquiries can be directed to the corresponding author.
Author contributions
XZ: Writing – review & editing, Writing – original draft. WM: Writing – review & editing, Writing – original draft. LZ: Writing – review & editing, Writing – original draft. HZ: Writing – review & editing, Writing – original draft. LC: Writing – review & editing, Writing – original draft. YX: Writing – review & editing, Writing – original draft. LL: Writing – review & editing, Writing – original draft.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors would like to thank all the authors who participated in this study for their contributions to this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1347991/full#supplementary-material
1. Siegel, RL, Miller, KD, Fuchs, HE, and Jemal, A. Cancer statistics, 2021. CA Cancer J Clin . (2021) 71:7–33. doi: 10.3322/caac.21654
Crossref Full Text | Google Scholar
2. Ettinger, DS, Wood, DE, Aisner, DL, Akerley, W, Bauman, JR, Bharat, A, et al. Non-small cell lung Cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw . (2022) 20:497–30. doi: 10.6004/jnccn.2022.0025
3. Sihoe, ADL. Video-assisted thoracoscopic surgery as the gold standard for lung cancer surgery. Respirology . (2020) 25:49–60. doi: 10.1111/resp.13920
4. Revenig, LM, Canter, DJ, Kim, S, Liu, Y, Sweeney, JF, Sarmiento, JM, et al. Report of a simplified frailty score predictive of short-term postoperative morbidity and mortality. J Am Coll Surg . (2015) 220:904–911e1. doi: 10.1016/j.jamcollsurg.2015.01.053
PubMed Abstract | Crossref Full Text | Google Scholar
5. Evered, L, Silbert, B, Knopman, DS, Scott, DA, DeKosky, ST, Rasmussen, LS, et al. Recommendations for the nomenclature of cognitive change associated with anaesthesia and Surgery-2018. Anesthesiology . (2018) 129:872–9. doi: 10.1097/aln.0000000000002334
6. Silverstein Jeffrey, H, Timberger, M, Reich David, L, Uysal, S, and Warltier, DC. Central nervous system dysfunction after noncardiac surgery and Anesthesia in the elderly. Anesthesiology . (2007) 106:622–8. doi: 10.1097/00000542-200703000-00026
7. Zhang, Y, He, ST, Nie, B, Li, XY, and Wang, DX. Emergence delirium is associated with increased postoperative delirium in elderly: a prospective observational study. J Anesth . (2020) 34:675–87. doi: 10.1007/s00540-020-02805-8
8. Lipowski, ZJ. Delirium in the elderly patient. N Engl J Med . (1989) 320:578–82. doi: 10.1056/nejm198903023200907
9. Liptzin, B, and Levkoff, SE. An empirical study of delirium subtypes. Br J Psychiatry . (1992) 161:843–5. doi: 10.1192/bjp.161.6.843
10. Fiest, KM, Soo, A, Hee Lee, C, Niven, DJ, Ely, EW, Doig, CJ, et al. Long-term outcomes in ICU patients with delirium: a population-based cohort study. Am J Respir Crit Care Med . (2021) 204:412–20. doi: 10.1164/rccm.202002-0320OC
11. Hshieh, TT, Yang, T, Gartaganis, SL, Yue, J, and Inouye, SK. Hospital elder life program: systematic review and Meta-analysis of effectiveness. Am J Geriatr Psychiatry . (2018) 26:1015–33. doi: 10.1016/j.jagp.2018.06.007
12. Swarbrick, CJ, and Partridge, JSL. Evidence-based strategies to reduce the incidence of postoperative delirium: a narrative review. Anaesthesia . (2022) 77 Suppl 1:92–01. doi: 10.1111/anae.15607
13. Khachaturian, AS, Hayden, KM, Devlin, JW, Fleisher, LA, Lock, SL, Cunningham, C, et al. International drive to illuminate delirium: a developing public health blueprint for action. Alzheimers Dement . (2020) 16:711–25. doi: 10.1002/alz.12075
14. Davis, DHJ, Skelly, DT, Murray, C, Hennessy, E, Bowen, J, Norton, S, et al. Worsening cognitive impairment and neurodegenerative pathology progressively increase risk for delirium. Am J Geriatr Psychiatry . (2015) 23:403–15. doi: 10.1016/j.jagp.2014.08.005
15. Hayhurst, CJ, Hughes, CG, and Pandharipande, PP. The conundrum of pain, opiate use, and delirium: Analgosedation or analgesia-first approach? Am J Respir Crit Care Med . (2021) 204:502–3. doi: 10.1164/rccm.202104-0968ED
16. Morrison, RS, Dickman, E, Hwang, U, Akhtar, S, Ferguson, T, Huang, J, et al. Regional nerve blocks improve pain and functional outcomes in hip fracture: a randomized controlled trial. J Am Geriatr Soc . (2016) 64:2433–9. doi: 10.1111/jgs.14386
17. Abou-Setta, AM, Beaupre, LA, Rashiq, S, Dryden, DM, Hamm, MP, Sadowski, CA, et al. Comparative effectiveness of pain management interventions for hip fracture: a systematic review. Ann Intern Med . (2011) 155:234–45. doi: 10.7326/0003-4819-155-4-201108160-00346
18. Xu, YJ, Sun, X, Jiang, H, Yin, YH, Weng, ML, Sun, ZR, et al. Randomized clinical trial of continuous transversus abdominis plane block, epidural or patient-controlled analgesia for patients undergoing laparoscopic colorectal cancer surgery. Br J Surg . (2020) 107:e133–41. doi: 10.1002/bjs.11403
19. Liu, R, Qin, H, Wang, M, Li, K, and Zhao, G. Transversus abdominis plane block with general anesthesia blunts the perioperative stress response in patients undergoing radical gastrectomy. BMC Anesthesiol . (2019) 19:205. doi: 10.1186/s12871-019-0861-0
20. Zhang, W, Cong, X, Zhang, L, Sun, M, Li, B, Geng, H, et al. Effects of thoracic nerve block on perioperative lung injury, immune function, and recovery after thoracic surgery. Clin Transl Med . (2020) 10:e38. doi: 10.1002/ctm2.38
21. Bagry, H, de la Cuadra Fontaine, JC, Asenjo, JF, Bracco, D, and Carli, F. Effect of a continuous peripheral nerve block on the inflammatory response in knee arthroplasty. Reg Anesth Pain Med . (2008) 33:17–23. doi: 10.1016/j.rapm.2007.06.398
22. Memtsoudis, SG, Cozowicz, C, Bekeris, J, Bekere, D, Liu, J, Soffin, EM, et al. Peripheral nerve block anesthesia/analgesia for patients undergoing primary hip and knee arthroplasty: recommendations from the international consensus on Anesthesia-related outcomes after surgery (ICAROS) group based on a systematic review and meta-analysis of current literature. Reg Anesth Pain Med . (2021) 46:971–85. doi: 10.1136/rapm-2021-102750
23. Li, T, Jiang, C, and Smith, FG. Regional vs general Anesthesia and incidence of postoperative delirium in older patients undergoing hip fracture surgery-reply. JAMA . (2022) 327:1708–9. doi: 10.1001/jama.2022.3544
24. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ . (2021) 372:n71. doi: 10.1136/bmj.n71
25. Shea, BJ, Reeves, BC, Wells, G, Thuku, M, Hamel, C, Moran, J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ . (2017) 358:j4008. doi: 10.1136/bmj.j4008
26. Wan, X, Wang, W, Liu, J, and Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol . (2014) 14:135. doi: 10.1186/1471-2288-14-135
27. Sterne, JAC, Savović, J, Page, MJ, Elbers, RG, Blencowe, NS, Boutron, I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ . (2019) 28:l4898. doi: 10.1136/bmj.l4898
28. Schünemann, HJ, Oxman, AD, Brozek, J, Glasziou, P, Jaeschke, R, Vist, GE, et al. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ . (2008) 336:1106–10. doi: 10.1136/bmj.39500.677199.AE
29. Cumpston, M, Li, T, Page, MJ, Chandler, J, Welch, VA, Higgins, JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev . (2019) 10:Ed000142. doi: 10.1002/14651858.Ed000142
30. Borenstein, M, Hedges, LV, Higgins, JP, and Rothstein, HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods . (2010) 1:97–11. doi: 10.1002/jrsm.12
31. Dongjie, Q, Longbiao, Z, Peng, L, Li, J, Hongmeng, X, Zhiyan, C, et al. Effects of thoracic paravertebral nerve block on postoperative pain and postoperative delirium in elderly patients undergoing thoracoscopic lobectomy [journal article]. Medicine . (2023) 102:e32907. doi: 10.1097/MD.0000000000032907
32. Wei, W, Zheng, X, Gu, Y, Fu, W, Tang, C, and Yao, Y. Effect of general anesthesia with thoracic paravertebral block on postoperative delirium in elderly patients undergoing thoracoscopic lobectomy: a randomized-controlled trial [journal article]. BMC Anesthesiol . (2022) 22:1. doi: 10.1186/s12871-021-01532-1
33. Zhang, JW, Feng, XY, Yang, J, Wang, ZH, Wang, Z, and Bai, LP. Ultrasound-guided single thoracic paravertebral nerve block and erector spinae plane block for perioperative analgesia in thoracoscopic pulmonary lobectomy: a randomized controlled trial [article]. Insights Imaging . (2022) 13:16. doi: 10.1186/s13244-021-01151-x
34. Heng, L, Wang, M, Wang, M, Li, L, and Zhu, S. Thoracic paravertebral block ameliorates postoperative delirium in geriatric patients [journal article]. Thorac Cardiovasc Surg . (2022) 70:439–44. doi: 10.1055/s-0041-1731788
35. Chen, XD, Liu, QS, and Fan, L. Effects of thoracic paravertebral block combined with s-ketamine on postoperative pain and cognitive function after thoracoscopic surgery [article]. Heliyon . (2022) 8:e12231. doi: 10.1016/j.heliyon.2022.e12231
36. Liu, W, Luo, T, Wang, F, Zhang, D, Liu, T, Huang, J, et al. Effect of preoperative thoracic paravertebral blocks on emergence agitation during tracheal extubation: a randomized controlled trial [article]. Front Med . (2022) 9. doi: 10.3389/fmed.2022.902908
37. Zhao, W, Li, C, Wang, Z, Shen, J, and Jia, H. Effect of paravertebral nerve block combined with general anesthesia on intraoperative regional cerebral oxygen saturation in elderly patients undergoing thoracoscopic lobectomy [article]. Chin J Anesthesiol . (2021) 41:939–42. doi: 10.3760/cma.j.cn131073.20210326.00810
38. Xie, H, Zhou, J, Du, W, Zhang, S, Huang, R, Han, Q, et al. Impact of thoracic paravertebral block combined with general anesthesia on postoperative cognitive function and serum adiponectin levels in elderly patients undergoing lobectomy [journal article]. Wideochirurgia I inne techniki maloinwazyjne . (2019) 14:538–44. doi: 10.5114/wiitm.2019.84742
39. Wei, W, Fan, Y, Liu, W, Zhao, T, Tian, H, Xu, Y, et al. Combined non-intubated anaesthesia and paravertebral nerve block in comparison with intubated anaesthesia in children undergoing video-assisted thoracic surgery [journal article]. Acta Anaesthesiol Scand . (2020) 64:810–8. doi: 10.1111/aas.13572
40. Kim, SY, Jo, HY, Na, HS, Han, SH, Do, SH, and Shin, HJ. The effect of peripheral nerve block on postoperative delirium in older adults undergoing hip surgery: a systematic review and Meta-analysis of randomized controlled trials. J Clin Med . (2023) 12. doi: 10.3390/jcm12072459
41. Bhushan, S, Huang, X, Duan, Y, and Xiao, Z. The impact of regional versus general anesthesia on postoperative neurocognitive outcomes in elderly patients undergoing hip fracture surgery: a systematic review and meta-analysis. Int J Surg . (2022) 105:106854. doi: 10.1016/j.ijsu.2022.106854
42. Duprey, MS, Dijkstra-Kersten, SMA, Zaal, IJ, Briesacher, BA, Saczynski, JS, Griffith, JL, et al. Opioid use increases the risk of delirium in critically ill adults independently of pain. Am J Respir Crit Care Med . (2021) 204:566–72. doi: 10.1164/rccm.202010-3794OC
43. Clegg, A, and Young, JB. Which medications to avoid in people at risk of delirium: a systematic review. Age Ageing . (2011) 40:23–9. doi: 10.1093/ageing/afq140
44. O'Connell, KM, Quistberg, DA, Tessler, R, Robinson, BRH, Cuschieri, J, Maier, RV, et al. Decreased risk of delirium with use of regional analgesia in geriatric trauma patients with multiple rib fractures. Ann Surg . (2018) 268:534–40. doi: 10.1097/sla.0000000000002929
45. Wong, CL, Holroyd-Leduc, J, Simel, DL, and Straus, SE. Does this patient have delirium?: value of bedside instruments. JAMA . (2010) 304:779–86. doi: 10.1001/jama.2010.1182
46. Adamis, D, Sharma, N, Whelan, PJ, and Macdonald, AJ. Delirium scales: a review of current evidence. Aging Ment Health . (2010) 14:543–55. doi: 10.1080/13607860903421011
Keywords: delirium, thoracic paravertebral nerve block, thoracic surgery thoracoscopy, thoracoscope, postoperative analgesia, meta-analysis
Citation: Zhou X, Mao W, Zhao L, Zhu H, Chen L, Xie Y and Li L (2024) Effect of thoracic paravertebral nerve block on delirium in patients after video-assisted thoracoscopic surgery: a systematic review and meta-analysis of randomized controlled trials. Front. Neurol . 15:1347991. doi: 10.3389/fneur.2024.1347991
Received: 15 January 2024; Accepted: 28 March 2024; Published: 10 April 2024.
Reviewed by:
Copyright © 2024 Zhou, Mao, Zhao, Zhu, Chen, Xie and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Linji Li, [email protected]
† These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.

IMAGES
VIDEO
COMMENTS
Literature reviews establish the foundation of academic inquires. However, in the planning field, we lack rigorous systematic reviews. In this article, through a systematic search on the methodology of literature review, we categorize a typology of literature reviews, discuss steps in conducting a systematic literature review, and provide suggestions on how to enhance rigor in literature ...
Inconsistency in defining medication errors has been confirmed. It appears that definitions and methods of detection rather than being reproducible and reliable methods are subject to the individual researcher's preferences. Thus, application of a clear-cut definition, standardized terminology and reliable methods has the potential to greatly ...
In the present systematic literature review of 45 studies we have confirmed inconsistency in defining medication errors as well as lack of definitions. Most of the definitions were profound, including minor deviations as well as fatal errors, whereas a single definition was restricted to harmful or potentially harmful errors.
A systematic review is a type of review that uses repeatable methods to find, select, and synthesize all available evidence. It answers a clearly formulated research question and explicitly states the methods used to arrive at the answer. Example: Systematic review. In 2008, Dr. Robert Boyle and his colleagues published a systematic review in ...
Topic selection and planning. In recent years, there has been an explosion in the number of systematic reviews conducted and published (Chalmers & Fox 2016, Fontelo & Liu 2018, Page et al 2015) - although a systematic review may be an inappropriate or unnecessary research methodology for answering many research questions.Systematic reviews can be inadvisable for a variety of reasons.
CONCLUSION. Siddaway 16 noted that, "The best reviews synthesize studies to draw broad theoretical conclusions about what the literature means, linking theory to evidence and evidence to theory" (p. 747). To that end, high quality systematic reviews are explicit, rigorous, and reproducible. It is these three criteria that should guide authors seeking to write a systematic review or editors ...
a systematic search that attempts to identify all studies that would meet the eligibility criteria; an assessment of the validity of the findings of the included studies, for example through the assessment of risk of bias; and. a systematic presentation, and synthesis, of the characteristics and findings of the included studies. Many systematic ...
Abstract. This article aims to provide an overview of the structure, form and content of systematic reviews. It focuses in particular on the literature searching component, and covers systematic database searching techniques, searching for grey literature and the importance of librarian involvement in the search.
review process as a scientific process in itself, which developed into the SR process (Dixon-Woods, 2010). 2.2 Definition, principles and procedures for systematic reviews SRs are a way of synthesising scientific evidence to answer a particular research question in a way that
This systematic review found wide variation in how medication errors are defined between studies. This variation has significant implications for determining the prevalence of medication errors. Prior commentaries have noted the need for standardized, universally applicable definitions of adverse drug events.
A systematic review collects all possible studies related to a given topic and design, and reviews and analyzes their results [ 1 ]. During the systematic review process, the quality of studies is evaluated, and a statistical meta-analysis of the study results is conducted on the basis of their quality. A meta-analysis is a valid, objective ...
A systematic literature review of definitions and characteristics.}, author={Marianne Lisby and Lars Peter Nielsen and Lars Peter Nielsen and Birgitte Brock and Birgitte Brock and Jan Mainz and Jan Mainz}, journal={International journal for quality in health care : journal of the International Society for Quality in Health Care}, year={2010 ...
A systematic literature review of definitions and classification systems for radiotherapy innovation: A first step towards building a value-based assessment tool for radiation oncology. ... However, despite these recurring characteristics, there is a lack of consensus in terminology. For example, common terms such as 'treatment' or ...
1. INTRODUCTION. Evidence synthesis is a prerequisite for knowledge translation. 1 A well conducted systematic review (SR), often in conjunction with meta‐analyses (MA) when appropriate, is considered the "gold standard" of methods for synthesizing evidence related to a topic of interest. 2 The central strength of an SR is the transparency of the methods used to systematically search ...
3.1. Systematic Literature Review. This section describes the characteristics of the Systematic Literature Review (SLR). A SLR is based on the application of a systematic process to define the research question, identify relevant studies, evaluate their quality and summarise the findings qualitatively or quantitatively [64].Moreover, the tools used for selecting the studies must also be ...
Regarding this point, a systematic review of the literature found 45 generic definitions of medication errors, including 26 different forms of wordings confirming the inconsistency in the ...
Prominent definitions The most referenced PSS definition in the literature review was the definition by Mont from 2002, which was referred to 18 times in the primary literature: “A system of products, services, supporting networks and infrastructure that is designed to be: competitive, satisfy customer needs and have a lower environmental ...
The use of a systematic literature review method complemented by a narrative analysis provided the tools to identify information scattered across different fields of study and analyze their content. ... The characteristics describe the types or "forms" of the gap. ... In this review, there were limited definitions of the gap identified as ...
This paper is a systematic literature review th at attempts to identify and c lassify proposed definitions and measures of IT project complexity. The r esults include a map of the identified ...
Background: Extensive research on impulsive suicide attempts, but lack of agreement on the use of this term indicates the need for a systematic literature review of the area. The aim of this review was to examine definitions and likely correlates of impulsive attempts. Methods: A search of Medline, Psychinfo, Scopus, Proquest and Web of Knowledge databases was conducted.
This paper collects, analyzes, and synthesizes scientific definitions and the accompanying major characteristics of the Metaverse using the methodology of a Systematic Literature Review (SLR). Two revised definitions for the Metaverse are presented, both condensing the key attributes, where the first one is rather simplistic holistic describing ...
4.1 Review methodology. A systematic literature review approach (SLR) was used to answer the research questions. The aim of SLR is "to identify, evaluate, and interpret research relevant to a determined topic area, research question, or phenomenon of interest" (Kitchenham and Charters 2007; Muller et al. 2019, p. 398).
Systematic reviews have specific characteristics that set them apart from traditional literature reviews. A systematic review is "a review of a clearly formulated question that uses systematic and explicit methods to identify, select, and critically appraise relevant research, and to collect and analyze data from the studies that are included ...
Methods We analysed all articles in PubMed that used 'digital biomarker(s)' in title or abstract, considering any study involving humans and any review, editorial, perspective or opinion-based articles up to 8 March 2023. We systematically extracted characteristics of publications and research studies, and any definitions and features of 'digital biomarkers' mentioned.
The current systematic review and meta-analysis synthesized 76 reports that examined the association between unprompted thought and affective well-being. Although our meta-analyses indicated a general negative relationship between the occurrence of unprompted thought and affective well-being, this seemingly robust relationship changes in ...
Scoping systematic review. To summarize the available experimental clinical and animal studies for the identification of all CSF and serum-derived biochemical markers in human and rat SCI models.
The need to improve the acute care pathway to meet the care needs of older people living with frailty is a strategic priority for many healthcare systems. The optimal care model for this patient group is unclear. A systematic review was conducted to derive a taxonomy of acute care models for older people with acute medical illness and describe the outcomes used to assess their effectiveness.
To date, there has been no systematic review of the literature on impulsive suicide attempts. The present paper sought to address this gap. Its purpose was to examine how impulsive suicide attempts have been defined by researchers and to establish, as far as possible, the likely correlates of those attempts described as impulsive. 2. Method
We conducted a systematic review of published mathematical models to determine the modelling landscape and to explore methods for including bacterial heterogeneity. Our first objective was to identify and analyse the general characteristics of mathematical models of DR-mycobacteria, including M. tuberculosis. The second objective was to analyse ...
All data incorporated into our study were exclusively derived from published literature, obviating the necessity for ethical review. Furthermore, the study is registered in the International Prospective Register of Systematic Reviews (PROSPERO CRD42023435528). 2.2 Data collection