
- Proceedings
Information
Clinical Medicine Research

- Processing Charges

http://www.clinicalmed.org
Time to first decision
First decision to acceptance
Acceptance to publication
- Open Access
Submission Guidelines
We're committed to making your publishing experience as easy and efficient as we can. Our submission guidelines will offer you the essential resources and guidance for a successful process of submitting and publishing your article.
Editorial Board
Clinical Medicine Research benefits from a distinguished editorial board, who actively engage in the peer review process, providing direction for ongoing development to ensure the journal remains dynamic in the field.
Reviewer Guidelines
We encourage you to explore our reviewer guidelines, where you'll discover valuable insights and practical tips to enhance your role as a peer reviewer, promoting a constructive and efficient peer review experience.
Special issues are collections of articles centered around a subject of special interest, which are organized and led by subject experts who take on the role of the guest editor. Authors should be aware that articles included in special issues are subject to the same criteria of quality, originality, and significance as regular articles.
New Development in Optometry
Lead Guest Editor: Gaurav Dubey
Submission deadline: 30 August 2024
Combination Therapy in Bladder Cancer
Lead Guest Editor: Weigang Ren
Submission deadline: 9 August 2024
- Propose a Special Issue
By proposing a special issue, you have the opportunity to undertake the role of lead guest editor and curate a collection of articles focused on a subject of particular interest. This allows you to showcase and explore the chosen topic in-depth.

Benefits of the Lead Guest Editor
Serving as a lead guest editor can bring a variety of career benefits, such as the following:
Thank you to all the peer reviewers who generously donated their time and expertise to Clinical Medicine Research publications. Your contributions are invaluable in maintaining the high-quality standards of our publications.
- Assoc. Prof. Zhang Haiyu Department of Transfusion Blood, Sichuan Cancer Hospital, Chengdu, China
- Assoc. Prof. Neha Hajira Department of Prosthodontics, Rural Dental College, Pravara Institute of Medical Sciences (Deemed University), Ahmednagar, India
How to be an effective Peer Reviewer?
Our comprehensive guide on becoming a peer reviewer provides you with all the essential information to embark on this role successfully. It covers the following key aspects:
Why should you consider becoming a Peer Reviewer?
Engaging in the peer review process not only significantly contributes to the scientific community but also brings considerable benefits for your own research and career development. At SciencePG, we always appreciate and welcome professionals who are interested in becoming part of our dedicated reviewer team.
Clinical Medicine Research maintains an Editorial Board of practicing researchers from around the world, to ensure manuscripts are handled by editors who are experts in the field of study.
- Wahab Owolawi Department of Audiology, University of Medical Sciences, Ondo, Nigeria
- Prof. Carolina Mahuad, PhD Department of Hematology, Hospital Alemán, Buenos Aires, Argentina
- Join the Editorial Board
Would you like to make a valuable contribution to the scientific community by joining the editorial board of this journal? Or perhaps you're a current editorial board member who wishes to recommend a colleague for this role? Your interest and suggestions are highly appreciated and warmly welcomed.
Benefits for Editorial Board Members
Joining the Editorial Board is an opportunity to be recognized as an expert in your field and to contribute to the peer review process of cutting-edge research. By acting as editor, you will have the opportunity to shape the future of research in your field and be part of a community of like-minded researchers.
As an editorial board member, You will Benefit from:
AcademicEvents is an academic event planning platform initiated by Science Publishing Group (SciencePG). AcademicEvents aims to fostering collaboration and facilitating the dissemination of innovative ideas. Whether you're an esteemed professor, a dedicated researcher, or a curious student, our platform provides comprehensive support for hosting academic events in diverse disciplines.

Webinars are typically executed as online events, following the initial planning.
You have the flexibility to organize workshops either in-person or virtually, based on your preference.

Conferences
Conferences are traditionally conducted face-to-face, with in-person attendance being the norm.
AcademicEvents strives to provide you with a seamless experience. By organizing academic events under our service, you will connect with like-minded individuals and expand your professional network. Besides, our user-friendly interface allows you stay informed about the latest conferences in your field of interest.
We also provide publishing services for conference content (past or upcoming conference). The ideas generated during the conference can be developed into articles or abstracts for publication.

Organize Your Event
Find the customized support to host a successful event

Publishing Service
Free you from the burden of managing conference outputs

Crossed Cerebellar Diaschisis and Cerebral Infarction After Cerebral Hyperperfusion Syndrome Following Carotid Artery Stenting: A Case Report
Authors: Wei-Qin Ning, ... Shui-Sheng Zhong
Pages: 13-16 Published Online: 21 February 2024
DOI: 10.11648/j.cmr.20241301.13
Combining Multiple Tumor Markers to Construct a Clinical Prediction Model for Breast Cancer
Authors: Zebin Liu, ... Liying Qiu
Pages: 6-12 Published Online: 8 January 2024
DOI: 10.11648/j.cmr.20241301.12
Therapeutic Effect of Hydroalcoholic Extract for Withania Coagulans for Diuretic Activity in Rodents
Authors: Rajesh Kumar Sharma, ... Sanjana Soni
Pages: 1-5 Published Online: 8 January 2024
DOI: 10.11648/j.cmr.20241301.11
Development and Evaluation of a Questionnaire on the Acceptability of Advance Care Planning for the Families of End-Stage Patients with Chronic Diseases
Authors: Shen Yongqing, ... Li Dongli
Pages: 103-109 Published Online: 29 November 2023
DOI: 10.11648/j.cmr.20231206.11
Mechanisms of Iron Deficiency in Obese Children
Authors: Xin-yan Yan
Pages: 95-102 Published Online: 14 October 2023
DOI: 10.11648/j.cmr.20231205.12
Analysis of Clinical Characteristics of Stress Response in the Digestive System Caused by Altitude Sickness
Authors: Liu Yanxi, ... Liu Chao
Pages: 91-94 Published Online: 1 September 2023
DOI: 10.11648/j.cmr.20231205.11
Science Publishing Group (SciencePG) is an Open Access publisher, with more than 300 online, peer-reviewed journals covering a wide range of academic disciplines.
Learn More About SciencePG

- Special Issues
- AcademicEvents
- ScholarProfiles
- For Authors
- For Reviewers
- For Editors
- For Conference Organizers
- For Librarians
- Article Processing Charges
- Special Issues Guidelines
- Editorial Process
- Peer Review at SciencePG
- Ethical Guidelines
Important Link
- Manuscript Submission
- Become a Reviewer

- © 2018

Clinical Medicine Research
- Mieczyslaw Pokorski 0
Opole Medical School, Opole, Poland
You can also search for this editor in PubMed Google Scholar
- Advancing new ideas and knowledge
- Innovative and insightful clinical research
- Dissemination of best clinical practice
Part of the book series: Advances in Experimental Medicine and Biology (AEMB, volume 1116)
Part of the book sub series: Clinical and Experimental Biomedicine (CLEXBI)
11k Accesses
74 Citations
79 Altmetric
- Table of contents
About this book
Editors and affiliations, about the editor, bibliographic information.
- Publish with us
Buying options
- Available as EPUB and PDF
- Read on any device
- Instant download
- Own it forever
- Durable hardcover edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Other ways to access
This is a preview of subscription content, log in via an institution to check for access.
Table of contents (12 chapters)
Front matter, bioprogressive paradigm in physiotherapeutic and antiaging strategies: a review.
- Mieczyslaw Pokorski, Giovanni Barassi, Rosa G. Bellomo, Loris Prosperi, Matteo Crudeli, Raoul Saggini
Influence of Proprioceptive Neuromuscular Facilitation on Lung Function in Patients After Coronary Artery Bypass Graft Surgery
- Małgorzata Bujar-Misztal, Andrzej Chciałowski
Remote Ischemic Preconditioning in Renal Protection During Elective Percutaneous Coronary Intervention
- Małgorzata Wojciechowska, Maciej Zarębiński, Piotr Pawluczuk, Dagmara Gralak-Łachowska, Leszek Pawłowski, Wioletta Loska et al.
Prognostic Impact of Extracapsular Lymph Node Invasion on Survival in Non-small-Cell Lung Cancer: A Systematic Review and Meta-analysis
- Seyed Vahid Tabatabaei, Christoph Nitche, Maximilian Michel, Kurt Rasche, Khosro Hekmat
Influence of Transurethral Resection of Bladder Cancer on Sexual Function, Anxiety, and Depression
- Wojciech Krajewski, Urszula Halska, Sławomir Poletajew, Radosław Piszczek, Bartosz Bieżyński, Mateusz Matyjasek et al.
Cognitive Predictors of Cortical Thickness in Healthy Aging
- Patrycja Naumczyk, Angelika K. Sawicka, Beata Brzeska, Agnieszka Sabisz, Krzysztof Jodzio, Marek Radkowski et al.
Osteoprotegerin, Receptor Activator of Nuclear Factor Kappa B Ligand, and Growth Hormone/Insulin-Like Growth Factor-1 Axis in Children with Growth Hormone Deficiency
- Ewelina Witkowska-Sędek, Małgorzata Rumińska, Anna Stelmaszczyk-Emmel, Maria Sobol, Urszula Demkow, Beata Pyrżak
Inhibition of Cross-Reactive Carbohydrate Determinants in Allergy Diagnostics
- Maciej Grzywnowicz, Emilia Majsiak, Józef Gaweł, Karolina Miśkiewicz, Zbigniew Doniec, Ryszard Kurzawa
Effects of Glutathione on Hydrolytic Enzyme Activity in the Mouse Hepatocytes
- Iwona Stanisławska, Bożena Witek, Marek Łyp, Danuta Rochon-Szmejchel, Adam Wróbel, Wojciech Fronczyk et al.
Adaptation to Occupational Exposure to Moderate Endotoxin Concentrations: A Study in Sewage Treatment Plants in Germany
- M. A. Rieger, V. Liebers, M. Nübling, T. Brüning, B. Brendel, F. Hoffmeyer et al.
Effects of Low-Level Laser Therapy in Autism Spectrum Disorder
- Gerry Leisman, Calixto Machado, Yanin Machado, Mauricio Chinchilla-Acosta
Epidemiology of Granulomatosis with Polyangiitis in Poland, 2011–2015
- Krzysztof Kanecki, Aneta Nitsch-Osuch, Paweł Gorynski, Patryk Tarka, Magdalena Bogdan, Piotr Tyszko
This book presents an update on new trends and developments in broadly defined medical disciplines. The whole range of multidisciplinary topics are tackled, regarded as being important for advancing the understanding of disease pathogenicity, diagnostic methods, and patient management. The topics include a holistic approach to physiotherapy, with proprioceptive neuromuscular facilitation at the core of it, potential ways to protect kidneys during ischemic coronary interventions, and psychosocial aspects in cancer survivors. Other topics deal with growth hormone deficiency in short children and responses of molecular markers of bone metabolism to growth hormone replacement therapy and with the modern use of transcranial laser-induced photobiomodulation showing surprising benefits in autism disorder. The expert contributions take on the challenges presented to medical professionals by ever growing medical knowledge and various individual and contextual issues that require a multidisciplinary approach in patient management. The authors present a bench-to-bed clinical research to make useful additions to the knowledge on contemporary diagnostic procedures, therapy, and quality of life of patients. The book aims to provide stimulus for new research ideas and to give new perspectives on practical clinical issues. The book is intended for primary care clinicians, family physicians, medical scholars, and other clinicians who treat and manage patients.
- Bioprogressive treatment
- Cancer research
- Cognitive function
- Coronary disease
- Diagnostic markers
- Growth hormone
- Health care
- Occupational endotoxins
- Physiotherapy
Mieczyslaw Pokorski
Book Title : Clinical Medicine Research
Editors : Mieczyslaw Pokorski
Series Title : Advances in Experimental Medicine and Biology
DOI : https://doi.org/10.1007/978-3-030-04837-2
Publisher : Springer Cham
eBook Packages : Biomedical and Life Sciences , Biomedical and Life Sciences (R0)
Copyright Information : The Editor(s) (if applicable) and The Author(s), under exclusive license to Springer Nature Switzerland AG 2018
Hardcover ISBN : 978-3-030-04836-5 Published: 04 December 2018
eBook ISBN : 978-3-030-04837-2 Published: 23 November 2018
Series ISSN : 0065-2598
Series E-ISSN : 2214-8019
Edition Number : 1
Number of Pages : VI, 138
Number of Illustrations : 13 b/w illustrations, 6 illustrations in colour
Topics : Neurochemistry , Cancer Research , Physiotherapy , Cytokines and Growth Factors
Policies and ethics
- Find a journal
- Track your research
Study record managers: refer to the Data Element Definitions if submitting registration or results information.
Search for terms

- Advanced Search
- See Studies by Topic
- See Studies on Map
- How to Search
- How to Use Search Results
- How to Find Results of Studies
- How to Read a Study Record

- Learn About Studies
- Other Sites About Studies
- Glossary of Common Site Terms

- Submit Studies to ClinicalTrials.gov PRS
- Why Should I Register and Submit Results?
- FDAAA 801 and the Final Rule
- How to Apply for a PRS Account
- How to Register Your Study
- How to Edit Your Study Record
- How to Submit Your Results
- Frequently Asked Questions
- Support Materials
- Training Materials

- Selected Publications
- Clinical Alerts and Advisories
- Trends, Charts, and Maps
- Downloading Content for Analysis

- ClinicalTrials.gov Background
- About the Results Database
- History, Policies, and Laws
- ClinicalTrials.gov Modernization
- Media/Press Resources
- Linking to This Site
- Terms and Conditions
ClinicalTrials.gov is a database of privately and publicly funded clinical studies conducted around the world.
Explore 491,719 research studies in all 50 states and in 223 countries..
ClinicalTrials.gov is a resource provided by the U.S. National Library of Medicine.
IMPORTANT : Listing a study does not mean it has been evaluated by the U.S. Federal Government. Read our disclaimer for details.
Before participating in a study, talk to your health care provider and learn about the risks and potential benefits .
- Studies by Topic
- Studies on Map
Patients and Families
Researchers, study record managers.
- Customer Support
- Accessibility
- Viewers and Players
- Freedom of Information Act
- HHS Vulnerability Disclosure
- U.S. National Library of Medicine
- U.S. National Institutes of Health
- U.S. Department of Health and Human Services
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Trending Articles
- ITPRIPL1 binds CD3ε to impede T cell activation and enable tumor immune evasion. Deng S, et al. Cell. 2024. PMID: 38614099
- Small extracellular vesicles from young plasma reverse age-related functional declines by improving mitochondrial energy metabolism. Chen X, et al. Nat Aging. 2024. PMID: 38627524
- Dual-role transcription factors stabilize intermediate expression levels. He J, et al. Cell. 2024. PMID: 38631355
- A pan-cancer analysis of the microbiome in metastatic cancer. Battaglia TW, et al. Cell. 2024. PMID: 38599211
- Time-series reconstruction of the molecular architecture of human centriole assembly. Laporte MH, et al. Cell. 2024. PMID: 38604175
Latest Literature
- Cancer Res (8)
- J Clin Endocrinol Metab (5)
- Nature (42)
- PLoS One (229)
- Pediatrics (1)
- Proc Natl Acad Sci U S A (15)
- Science (26)
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Journals | Policy | Permission Journal of Clinical Medicine Research
INFORMATION
- For Authors
- For Reviewers
- For Editors
- For Readers
- Admin-login
(For the last 6-month published articles)

Original Article Review Case Report Short Communication Letter to the Editor Editorial

All content of World Journal of Oncology is permanently archived and preserved in Portico .

- Online-First
Journal of Clinical Medicine Research
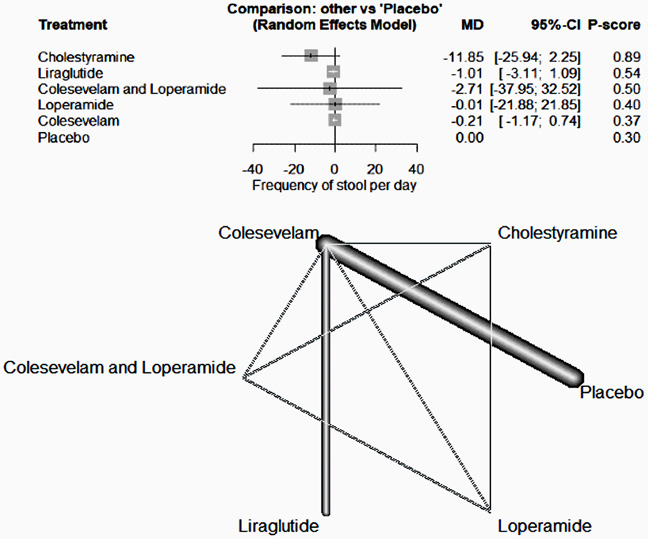
Bile acid malabsorption (BAM) is characterized by chronic watery diarrhea resulting from excessive bile acids in the feces. BAM is often an overlooked cause of chronic diarrhea, with its prevalence not being sufficiently researched.
The field of kidney transplantation is being revolutionized by the integration of artificial intelligence (AI) and machine learning (ML) techniques. AI equips machines with human-like cognitive abilities, while ML enables computers to learn from data.
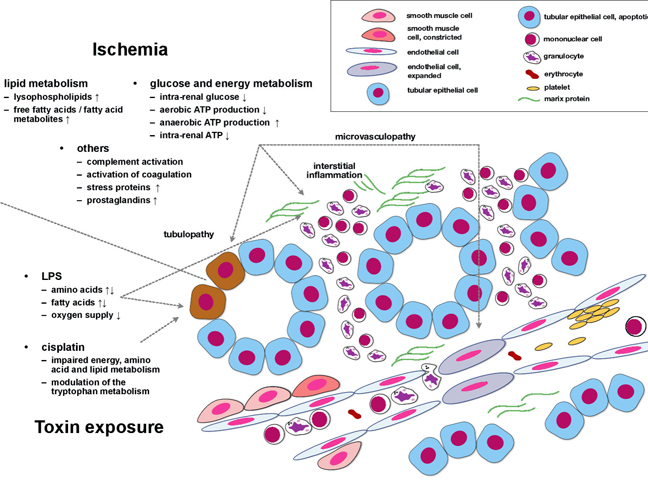
Acute kidney injury (AKI) affects increasing numbers of in-hospital patients in Central Europe and the USA, the prognosis remains poor. Although substantial progress has been achieved in the identification of molecular/cellular processes that induce and perpetuate AKI, more integrated pathophysiological perspectives are missing.

Subjects with mild cognitive impairment (MCI) can progress to dementia. Studies have shown that neuropsychological tests, biological or radiological markers individually or in combination have helped to determine the risk of conversion from MCI to dementia.
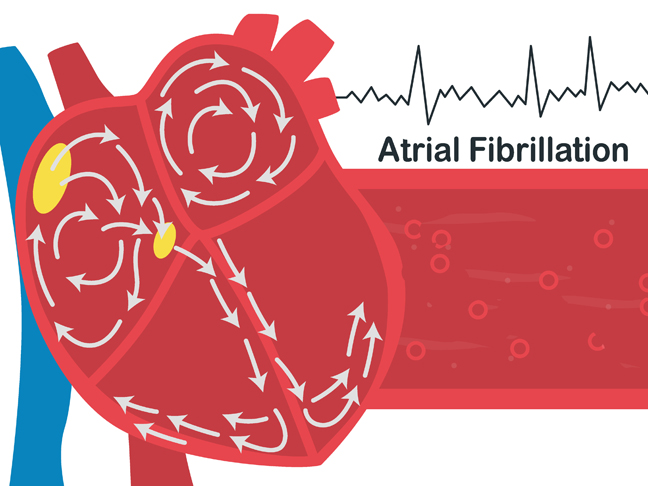
Atrial fibrillation (AF) is the most common arrhythmia with a growing prevalence worldwide, especially in the elderly population. Patients with AF are at higher risk of serious life-threatening events and complications that may lead to long-term sequelae and reduce quality of life.
Vol. 16, No. 2-3, Mar 2024
Table of contents, original article, short communication.
- Special Issues
- Editorial Board
- Article Processing Charges
- Login/Regeister
Clinical Research: An Overview of Study Types, Designs, and Their Implications in the Public Health Perspective
Venkataramana Kandi 1, and Sabitha Vadakedath 2
1 Department of Microbiology, Prathima Institute of Medical Sciences, Karimnagar, Telangana, India
2 Department of Biochemistry, Prathima Institute of Medical Sciences, Karimnagar, Telangana, India
Cite this paper
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 11 April 2024
Why diversity is needed at every level of clinical trials, from participants to leaders
- Khadijah Breathett ORCID: orcid.org/0000-0001-5397-6419 1
Nature Medicine ( 2024 ) Cite this article
140 Accesses
1 Altmetric
Metrics details
- Clinical trials
This article has been updated
Diversity in clinical trials must be accompanied by justice and equity, including benefits for underrepresented participants, in order to boost population health.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Change history
15 april 2024.
The version of this article initially published was an uncorrected proof; it has now been replaced by the final version.
US Department of Health and Human Services. The Belmont Report https://go.nature.com/3mj33xy (2010).
Baptiste, D.-L. et al. Nursing Open 9 , 2236 (2022).
Article PubMed PubMed Central Google Scholar
Pacheco, C. M. et al. Am. J. Public Health 103 , 2152–2159 (2013).
Ndugga, N., Pillai, D. & Artiga, S. Racial and Ethnic Disparities in Access to Medical Advancements and Technologies . https://go.nature.com/4arFv7b (2024).
Breathett, K. & Manning, K. D. Circ. Cardiovasc. Qual. Outcomes 17 , e010009 (2024).
Article PubMed Google Scholar
Cullen, M. R. et al. Contemporary Clinical Trials 129 , 107184 (2023).
Download references
Author information
Authors and affiliations.
Division of Cardiovascular Medicine, Krannert Cardiovascular Research Center, Indiana University, Indianapolis, IN, USA
Khadijah Breathett
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Khadijah Breathett .
Ethics declarations
Competing interests.
The author declares no conflicts of interest.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Breathett, K. Why diversity is needed at every level of clinical trials, from participants to leaders. Nat Med (2024). https://doi.org/10.1038/s41591-024-02914-x
Download citation
Published : 11 April 2024
DOI : https://doi.org/10.1038/s41591-024-02914-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Institute of Medicine (US) Forum on Drug Discovery, Development, and Translation. Transforming Clinical Research in the United States: Challenges and Opportunities: Workshop Summary. Washington (DC): National Academies Press (US); 2010.

Transforming Clinical Research in the United States: Challenges and Opportunities: Workshop Summary.
- Hardcopy Version at National Academies Press
2 The State of Clinical Research in the United States: An Overview
The Institute of Medicine (IOM) reports To Err Is Human: Building a Safer Health System ( IOM, 2000 ) and Crossing the Quality Chasm: A New Health System for the 21st Century ( IOM, 2001a ), focused the nation’s attention on concerns about the quality of health care in the United States. Since those reports were published, efforts have accelerated to develop a health care system that systematically measures and improves the quality of care delivered. Essential to such a system is a systematic approach for assessing which clinical approaches do and do not work and then ensuring that this knowledge is utilized in clinical decision making. This approach is what is often referred to as a learning health care system.
Many different kinds of evidence can inform the policies and practices of a health care system. Clinical trials, a type of clinical research, are one of the most robust sources of this knowledge. A number of workshop speakers from many backgrounds—clinical investigators, research sponsors, practitioners, and patients—expressed the view that the current clinical research enterprise 1 in the United States is unable to produce the high-quality, timely, and actionable evidence needed to support a learning health care system. They identified numerous obstacles to producing this evidence, including the length of time and high financial cost involved in conducting clinical trials, delays associated with navigating the many regulatory and ethical requirements of studies involving human subjects (e.g., Institutional Review Board [IRB] approval), difficulties in recruiting and retaining the appropriate patient population, and the generally fragmented way clinical research is prioritized and undertaken to advance medical care in the United States.
As noted in Chapter 1 , the workshop focused on the randomized controlled trial (RCT), the gold standard in clinical research. Many consider the RCT to be unsustainable as an approach to addressing the large number of research questions that need to be answered because of the time and expense involved. Yet alternative approaches have limitations with respect to producing high-quality data. Christopher Cannon, senior investigator in the Thrombolysis in Myocardial Infarction (TIMI) Study Group, for example, discussed the use of registries, which are large databases that provide extensive observational data on current clinical practice. He commented that while registry data are of good quality and less expensive to obtain compared with data from RCTs, confounding (i.e., why an individual received one therapy versus another) is a significant problem. Because it is difficult to attribute trends in registry data to particular therapies, registries do not provide the conclusive evidence necessary to change clinical practice. Instead, registries generate hypotheses that can then be tested in an RCT. Therefore, while patient registries and other research tools exist, the workshop focused primarily on RCTs.
Results of thousands of RCTs are published each year, yet clinical decision making frequently is not based on the evidence created by these results. A key issue informing the workshop discussions, then, was how RCTs can be conducted in an efficient, timely manner to answer all of the questions and meet all of the needs of a learning health care system. A logical first step in addressing this issue is to examine the clinical research enterprise as it operates in the United States today.
This chapter describes various aspects of clinical research in the United States, beginning with clinical research networks (CRNs). Research commissioned for the workshop from Ronald Krall, former Chief Medical Officer, GlaxoSmithKline, is then presented, addressing tools available for assessing clinical research in the United States; volume and type of clinical trials conducted; the clinical investigator workforce; and the overall capacity of the clinical research enterprise.
- CLINICAL RESEARCH NETWORKS
CRNs have been developed to pool resources and expertise in conducting clinical research. They include clinical sites and investigators usually organized around a specific disease area and can be accessed by many different research stakeholders for the conduct of clinical research.
The National Institutes of Health’s (NIH’s) Roadmap for Medical Research points specifically to CRNs and their ability to rapidly conduct high-quality studies as a way to improve the efficiency and productivity of the clinical research enterprise. In this vein, NIH’s National Center for Research Resources (NCRR) manages the Inventory and Evaluation of Clinical Research Networks (IECRN) project to survey active networks and characterize best practices that could potentially be implemented in other networks or clinical trial settings. Although the exact structures vary, the NIH project defines a CRN as an organization of clinical sites and investigators that conducts or intends to conduct multiple collaborative research protocols. CRNs can carry out a number of different types of studies, including clinical trials, and the organization of sites and investigators can be formal or informal as long as the collaborative accomplishments of the group are clear. For instance, a group of researchers that conducts a single trial and subsequently disbands is not considered to be a network ( NCRR, 2006 ).
By pooling the resources of multiple entities, CRNs can realize efficiencies in implementing and conducting clinical trials. They create a supportive infrastructure for investigators and can facilitate the rapid conduct of trials to answer important research questions. For instance, CRNs organized around a particular disease often have access to patients with that disease who can serve as study participants. The in-house scientific leadership of CRNs can also streamline the protocol development process and create uniformity in clinical trials across the network or disease area. When clinical trials from a particular network generate consistent results, this can also accelerate the drug development pipeline for the disease studied.
TOOLS FOR ASSESSING CLINICAL RESEARCH IN THE UNITED STATES 2
Krall obtained information on the current state of clinical trials in the United States from various public and private sources. A key source was data on submissions to clinicaltrials.gov, a federally sponsored, publicly available registry of clinical trials. Information was also obtained from the Tufts Center for the Study of Drug Development, KMR Group, Citeline, and individual pharmaceutical companies. The Tufts Center and KMR collect data from pharmaceutical companies for the purpose of providing benchmarking data and proprietary analyses. Citeline is a proprietary data source that draws from a number of resources (literature, advertising, and clinicaltrials.gov) to create a comprehensive database of clinical research and the global investigator workforce.
Clinicaltrials.gov
The Food and Drug Administration Modernization Act (FDAMA) of 1997 mandated the creation of the clinicaltrials.gov registry for efficacy trials in serious and life-threatening conditions and interventions regulated by the FDA. Developed by NIH’s National Library of Medicine (NLM) in 2000, it allows interested parties to find information on both completed and ongoing clinical trials. The database includes federally and privately supported clinical trials, and study sponsors are responsible for submitting timely and accurate information about their studies.
The database registered a modest number of clinical trials in its initial years ( Figure 2-1 ). A dramatic increase in trial registration came in 2005 in response to the newly introduced International Committee of Medical Journal Editors’ (ICMJE’s) requirement that studies published in their journals be registered in clinicaltrials.gov or other equivalent publicly available registries. The Food and Drug Administration Amendments Act (FDAAA) of 2007 created a legal requirement for the registration of trials of drugs, biologics, and devices, generating a modest increase in the registration of trials over what had been seen in 2005. Given the increasing number of trials registered on clinicaltrials.gov over time, the database encompasses a broad spectrum of research organized by study sponsor (industry, government, and nonprofit), disease and treatment being studied, and trial design.
Timeline reflecting the number of clinical trials registered on clinicaltrials.gov and regulatory changes affecting the database registration from 2001 to 2009. SOURCE: Krall, 2009. Reprinted with permission from Ronald Krall 2009.
Data Limitations
The information gathered by Krall to inform the workshop discussions of the state of the U.S. clinical research enterprise was not intended to provide an exhaustive analysis of the impact of every role and action of the broad range of research stakeholders involved. Rather, the goal was to highlight the productivity of one aspect of the clinical research enterprise—clinical trials. The data gathered reflect not the “effectiveness” of trials in terms of how well they answer the study questions, but how efficiently they are conducted. The commissioned research was designed to meet the needs of the workshop, however, the topics covered and issues raised by Krall’s analysis could be informative for other areas of the clinical research enterprise as well.
The data collected have some limitations. With respect to certain industry information, individual pharmaceutical company data can vary significantly depending on how the various elements and costs of clinical trials are measured. Also, although NLM reviews information submitted to the clinicaltrials.gov database, neither the accuracy of the data nor the scientific relevance of the study is guaranteed. Thus, while the information gathered on the number and type of clinical trials being conducted today is revealing, it would be incorrect to assume that it reflects the quality or relevance of those trials. Krall also noted that some types of clinical trials do not need to be reported to the database, and that there are concerns about the timeliness and accuracy of the data that are submitted. Variability in the reporting and classification of certain data elements in clinicaltrials.gov (e.g., drugs vs. biologics, phases of research, reporting no funding source, and currency of investigator site information) is another concern. Yet while clinicaltrials.gov is not without limitations, Krall suggested that its creation is undoubtedly a positive step toward developing a clearer picture of the state of clinical research in the United States.
- VOLUME AND TYPE OF CLINICAL TRIALS CONDUCTED
In RCTs, investigators control which participants receive the study treatment by assigning them at random to a particular experimental study group. Observational, non-experimental studies occur in natural settings and involve no manipulation of the interventions or treatments study participants receive. Because RCTs were the focus of the workshop, observational studies were excluded from Krall’s analysis.
Krall reported that as of August 16, 2009, there were 10,974 ongoing, interventional clinical trials with at least one U.S. center. The 10,974 ongoing trials collectively are seeking to enroll 2.8 million subjects. As Figure 2-2 indicates, the majority of trials (59 percent) are testing drugs. A distant second and third to drug interventions are behavioral trials (10 percent) and those testing biologics (9 percent), respectively.
Percentage of the 10,974 ongoing clinical trials and 2.8 million study subjects being sought by intervention being tested. SOURCE: Krall, 2009. Reprinted with permission from Ronald Krall 2009.
Clinical Trials by Phase of Research
The phase of clinical trials (i.e., phases 0-IV; see Chapter 1 ) is considered by some to be a marker of innovation, reported Krall. An analysis of clinical research by phase of experimental clinical trials can indicate the degree to which innovative new therapies are being developed and tested. It takes 10–15 years for a typical drug to be developed successfully from discovery to registration with the FDA. In the earlier phases of research, the chance of a drug reaching patients is small—approximately 1 in 10. In phase III research, however, the odds of registering a new product improve. About two-thirds of drugs that reach pivotal phase III trials will make it to the market ( IOM, 2009c . 85).
To characterize trials by phase more precisely, Krall narrowed the focus of his research to trials for FDA-regulated interventions (drugs, biologics, devices, and dietary supplements). In these FDA-regulated categories, there are 8,386 trials recruiting 1.9 million subjects. As shown in Figure 2-3 , among clinical trials for FDA-regulated products, phase II research is the largest category, followed closely by phase IV. Also referring to Figure 2-3 , although there are larger numbers of phase II and III trials, phase III trials by design involve the largest number of participants; thus it makes sense that 52 percent of all subjects are enrolled in these pivotal trials.
Number of the 8,386 clinical trials involving FDA-regulated products and 1.9 million study subjects being sought for these trials by phase of research. SOURCE: Krall, 2009. Reprinted with permission from Ronald Krall 2009.
Clinical Trials by Disease
Krall described ongoing clinical trials in the four disease areas of focus at the workshop—cardiovascular disease, depression, cancer, and diabetes. Figure 2-4 indicates that approximately half of the 10,974 trials being conducted today are in cancer; however, each such trial involves a relatively small number of participants. Figure 2-4 also reveals that cardiovascular disease trials are seeking more than 300,000 participants—10 percent of all clinical trial participants being recruited and far more than the number of participants sought for cancer, diabetes, or depression trials. Recruiting a large number of subjects per trial is a trademark of cardiovascular disease studies: on average, 275 patients are sought per cardiovascular trial, as compared with 20 patients per cancer trial, 70 patients per depression trial, and 100 per diabetes trial.
Number of the 10,974 ongoing clinical trials and 2.8 million study subjects being sought by disease being studied. NOTE: CV Disease = cardiovascular disease. SOURCE: Krall, 2009. Reprinted with permission from Ronald Krall 2009.
- THE CLINICAL INVESTIGATOR WORKFORCE
Annual surveys from the Tufts Center for the Study of Drug Development indicate a consistently high turnover rate in the clinical investigator community. Investigators conducting a clinical trial to support a New Drug Application (NDA) or a change in labeling are required to complete FDA’s Form 1527. In 2007, 26,000 investigators registered this form with the FDA, 85 percent of whom participated in only one clinical trial. The issues facing clinical investigators were discussed throughout the workshop, and many participants echoed the theme of the Tufts data—it is difficult to conduct clinical trials in the United States and establish a career as a clinical investigator. While opportunities in clinical investigation can vary depending on whether or not an investigator is working in private practice or academia, for example, the challenges to successfully conducting a clinical trial in the United States are substantial. Making clinical investigation an attractive career option for academics and professionals was mentioned by a number of participants as an important component of any approach to improving the capacity of the clinical trials enterprise in the United States.
Globalization
In addition to high turnover, the U.S. clinical investigator workforce is subject to an absolute decrease in its ranks. While there has been an annual decline of 3.5 percent in U.S.-based investigators since 2001, there has been an increase in investigators outside the United States. Figure 2-5 reveals that investigators from the rest of the world increased steadily between 1997 and 2007, making up for the decline in North American investigators over the same period. As of 2007, U.S. investigators constituted 57 percent of the global investigator workforce, a decrease from approximately 85 percent in 1997. According to the Tufts data, there are an estimated 14,000 U.S. investigators, compared with an estimated 12,000 investigators outside the United States. Currently, 8.5 percent of investigators are from Central and Eastern Europe, 5.5 percent from Asia, and 5.5 percent from Latin America.
The proportion of clinical investigators from North America has decreased since 1997, while the proportion of investigators from Western Europe and the rest of the world has increased. SOURCE: Tufts Center for the Study of Drug Development. 2009. Impact (more...)
Finally, Krall noted the difference between the role of a clinical investigator (i.e., the person who establishes the hypotheses to test, designs the trial, analyses and reports the results) and that of the individual who finds patients to participate in a trial and collects information about them. The latter role is essential to the ability to carry out research and should be recognized, rewarded, and developed to a greater degree, according to Krall. Workshop presenters and participants echoed Krall’s sentiment later in the day by discussing the many different levels of staff, in addition to the principal investigator, that ultimately make a clinical trial successful.
- CAPACITY OF THE CLINICAL RESEARCH ENTERPRISE
KMR data from 2006 for the 15 largest pharmaceutical companies show that the majority of patient visits associated with an industry-sponsored clinical trial occur outside the United States. According to Krall, this statistic speaks to the costs and difficulty associated with conducting clinical research in the United States. In terms of cost-effectiveness, 860 patient visits occur in the United States per $1 million spent on clinical operations, whereas for the same cost, 902 patient visits occur outside of the United States. Thus, by the measure of cost per patient visit, U.S.-based clinical trials are not as cost-effective as those in the rest of the world. Krall urged caution in interpreting these data, however, given the high degree of variability among pharmaceutical companies in patient visit and cost measures.
U.S. investigators enroll two-thirds as many subjects into clinical trials as investigators in the rest of the world. Among U.S. investigators participating in a clinical trial, 27 percent fail to enroll any subjects, compared with 19 percent of investigators elsewhere. Investigator performance in the United States and the rest of the world is similar in that 75 percent of investigators fail to enroll the target number of subjects; also, 90 percent of all clinical trials worldwide fail to enroll patients within the target amount of time and must extend their enrollment period. Krall commented that these data on patient enrollment are from one pharmaceutical company but that, based on his industry experience and conversations with colleagues from other companies, he believes the data are generally consistent with the pharmaceutical industry as a whole.
According to clinicaltrials.gov data, clinical trials today call for the enrollment of 1 in every 200 Americans as study participants. Because this is such a remarkable undertaking, Krall questioned whether this high level of human participation is being put to the best use possible—that is, are the right questions being asked through the thousands of clinical trials being conducted today?
The clinical research enterprise is a broad term that encompasses the full spectrum of clinical research and its applications. It includes early-stage, laboratory research and the processes, institutions, and individuals that eventually apply research to patient care ( IOM, 2002 ).
The remainder of this chapter is based on the presentation of Dr. Krall.
- Cite this Page Institute of Medicine (US) Forum on Drug Discovery, Development, and Translation. Transforming Clinical Research in the United States: Challenges and Opportunities: Workshop Summary. Washington (DC): National Academies Press (US); 2010. 2, The State of Clinical Research in the United States: An Overview.
- PDF version of this title (1.9M)
In this Page
- TOOLS FOR ASSESSING CLINICAL RESEARCH IN THE UNITED STATES
Other titles in this collection
- The National Academies Collection: Reports funded by National Institutes of Health
Recent Activity
- The State of Clinical Research in the United States: An Overview - Transforming ... The State of Clinical Research in the United States: An Overview - Transforming Clinical Research in the United States
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
- Download PDF
- Share X Facebook Email LinkedIn
- Permissions
Clinical Reasoning of a Generative Artificial Intelligence Model Compared With Physicians
- 1 Department of Medicine, Beth Israel Deaconess Medical Center, Boston, Massachusetts
- 2 Department of Medicine, Massachusetts General Hospital, Boston
- 3 Department of Pulmonary and Critical Care, Brigham and Women’s Hospital, Boston, Massachusetts
Large language models (LLMs) have shown promise in clinical reasoning, but their ability to synthesize clinical encounter data into problem representations remains unexplored. 1 - 3 We compared an LLM’s reasoning abilities against human performance using standards developed for physicians.
Read More About
Cabral S , Restrepo D , Kanjee Z, et al. Clinical Reasoning of a Generative Artificial Intelligence Model Compared With Physicians. JAMA Intern Med. Published online April 01, 2024. doi:10.1001/jamainternmed.2024.0295
Manage citations:
© 2024
Artificial Intelligence Resource Center
Best of JAMA Network 2022
Browse and subscribe to JAMA Network podcasts!
Others Also Liked
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
- History, Facts & Figures
- YSM Dean & Deputy Deans
- YSM Administration
- Department Chairs
- YSM Executive Group
- YSM Board of Permanent Officers
- FAC Documents
- Current FAC Members
- Appointments & Promotions Committees
- Ad Hoc Committees and Working Groups
- Chair Searches
- Leadership Searches
- Organization Charts
- Faculty Demographic Data
- Professionalism Reporting Data
- 2022 Diversity Engagement Survey
- State of the School Archive
- Faculty Climate Survey: YSM Results
- Strategic Planning
- Mission Statement & Process
- Beyond Sterling Hall
- COVID-19 Series Workshops
- Previous Workshops
- Departments & Centers
- Find People
- Biomedical Data Science
- Health Equity
- Inflammation
- Neuroscience
- Global Health
- Diabetes and Metabolism
- Policies & Procedures
- Media Relations
- A to Z YSM Lab Websites
- A-Z Faculty List
- A-Z Staff List
- A to Z Abbreviations
- Dept. Diversity Vice Chairs & Champions
- Dean’s Advisory Council on Lesbian, Gay, Bisexual, Transgender, Queer and Intersex Affairs Website
- Minority Organization for Retention and Expansion Website
- Office for Women in Medicine and Science
- Committee on the Status of Women in Medicine Website
- Director of Scientist Diversity and Inclusion
- Diversity Supplements
- Frequently Asked Questions
- Recruitment
- By Department & Program
- News & Events
- Executive Committee
- Aperture: Women in Medicine
- Self-Reflection
- Portraits of Strength
- Mindful: Mental Health Through Art
- Event Photo Galleries
- Additional Support
- MD-PhD Program
- PA Online Program
- Joint MD Programs
- How to Apply
- Advanced Health Sciences Research
- Clinical Informatics & Data Science
- Clinical Investigation
- Medical Education
- Visiting Student Programs
- Special Programs & Student Opportunities
- Residency & Fellowship Programs
- Center for Med Ed
- Organizational Chart
- Committee Procedural Info (Login Required)
- Faculty Affairs Department Teams
- Recent Appointments & Promotions
- Academic Clinician Track
- Clinician Educator-Scholar Track
- Clinican-Scientist Track
- Investigator Track
- Traditional Track
- Research Ranks
- Instructor/Lecturer
- Social Work Ranks
- Voluntary Ranks
- Adjunct Ranks
- Other Appt Types
- Appointments
- Reappointments
- Transfer of Track
- Term Extensions
- Timeline for A&P Processes
- Interfolio Faculty Search
- Interfolio A&P Processes
- Yale CV Part 1 (CV1)
- Yale CV Part 2 (CV2)
- Samples of Scholarship
- Teaching Evaluations
- Letters of Evaluation
- Dept A&P Narrative
- A&P Voting
- Faculty Affairs Staff Pages
- OAPD Faculty Workshops
- Leadership & Development Seminars
- List of Faculty Mentors
- Incoming Faculty Orientation
- Faculty Onboarding
- Past YSM Award Recipients
- Past PA Award Recipients
- Past YM Award Recipients
- International Award Recipients
- Nominations Calendar
- OAPD Newsletter
- Fostering a Shared Vision of Professionalism
- Academic Integrity
- Addressing Professionalism Concerns
- Consultation Support for Chairs & Section Chiefs
- Policies & Codes of Conduct
- Health & Well-being
- First Fridays
- Fund for Physician-Scientist Mentorship
- Grant Library
- Grant Writing Course
- Mock Study Section
- Research Paper Writing
- Funding Opportunities
- Join Our Voluntary Faculty
- Faculty Resources
- Research by Keyword
- Research by Department
- Research by Global Location
- Translational Research
- Research Cores & Services
- Program for the Promotion of Interdisciplinary Team Science (POINTS)
- CEnR Steering Committee
- Experiential Learning Subcommittee
- Goals & Objectives
- Issues List
- Print Magazine PDFs
- Print Newsletter PDFs
- YSM Events Newsletter
- Social Media
- Patient Care
INFORMATION FOR
- Residents & Fellows
- Researchers
Precision Medicine for Parkinson’s Disease Is Focus of New Yale Center
Clemens Scherzer, MD , is on a mission to revolutionize the treatment of Parkinson’s disease through the use of genomics and artificial intelligence (AI) to create tailored therapeutics. In January, Scherzer joined Yale School of Medicine (YSM) and stepped into his new role as director of the Stephen & Denise Adams Center for Parkinson’s Disease Research. The center’s unique approach to developing the future of precision medicine for Parkinson’s disease is the first of its kind, he says.
“Right now, we wait for Parkinson’s disease to progress and cause debilitating symptoms that drive the patient to the clinic, where we scramble to catch up with treating them,” says Scherzer. “Through our new center, we want to learn to catch the disease early, be able to predict what the future will hold for each patient, and then intervene to prevent debilitating progression from ever occurring.”
Researchers will identify targets for new Parkinson’s disease medications
Scherzer envisions a future in which a person with Parkinson’s disease can walk into a clinic and provide a few drops of blood that a computer program can analyze to identify the patient’s genome and biomolecules. Clinicians would be able to use this information in addition to the patient’s electronic health data to determine the exact disease driver and to recommend precision therapeutics based on tailored biomarkers.

“Our work is similar to how a search engine targets advertisements to a user based on massive search histories,” he explains. “The goal is to precisely match the right drug to the right person at the right time, based on a search of the entire disease biology.”
To make this vision a reality, Scherzer and his team are building a multi-modal human atlas of Parkinson’s disease by cataloging molecular and clinical data from thousands of patients. Scherzer began this work while at Harvard Medical School, where he was professor of neurology, director of the American Parkinson’s Disease Association (APDA) Center for Advanced Parkinson Research, and director of the Precision Neurology Program at Brigham and Women’s Hospital. He will be advancing these efforts at YSM.
So far, Scherzer’s team has already sequenced the RNA programs of one million human brain cells spanning the entirety of disease progression—from healthy brains, to those in the earliest stages of Parkinson’s, to those in the most advanced manifestations of the disease.
Other ongoing work that also will be expanding at the Yale center includes the Yale Harvard Biomarkers Study, which involves mapping the genetic variants that control the course of Parkinson’s and using multi-omics technology to catalog molecules in patients’ biofluids. The biobank already has hundreds of thousands patient samples of DNA, RNA, plasma, and more—stored at the Yale Adams Center and with collaborators at Mass General Brigham—that he and his program have extensively characterized over several years as patients’ disease advanced. “This is a treasure trove for discovery of genes, therapeutics, and biomarkers,” says Scherzer.
The goal is to precisely match the right drug to the right person at the right time, based on a search of the entire disease biology. Clemens Scherzer, MD
For example, approximately 10% of patients with sporadic Parkinson’s disease [in which the patient has no clear familial history] have a mutation in a gene known as GBA . Researchers have discovered four different types of genetic variants of this gene that regulate the speed of progression of the disease. Those with the most severe mutation suffer a very rapid progression of their condition. After identifying this disease driver, a research team Scherzer led at Harvard collaborated with a pharmaceutical company to target this gene with precision medicine. The collaboration contributed to the first Phase 2 clinical trial focused on a genetic form of Parkinson’s and provided a tool kit for precision trials targeting GBA .
Scherzer hopes to use the power of genomics and AI to turn data into medicine. The center is using computational neuroscience and machine learning to accelerate research. “With powerful sequencing and computational technologies, we can look at 30,000 genes in a million brain cells in parallel and let biology tell us what is truly important to work on.”
Paving the way to precision medicine for Parkinson’s disease
Now, Scherzer’s ambitions include identifying other disease drivers and learning how to target them. “We’re on a quest to decode the RNA software of brain cells and figure out how to develop tailored drugs that correct any glitches,” he says. “Then, our goal is to launch early-stage clinical trials based on our newly identified drug targets.”
To fast-track drug development, the researchers are utilizing electronic patient data from entire populations and from their Yale Harvard Biomarkers Study to find old drugs that could be repurposed for Parkinson’s patients. In collaboration with the University of Bergen in Norway, Clemens’ team is using computer models to compare health outcomes recorded over a decade in thousands of individuals with Parkinson’s disease on a medication compared to millions of individuals with Parkinson’s not on the medication. “We are searching for old drugs that can be taught new tricks to help patients with Parkinson’s disease,” says Scherzer.
This search is identifying drugs for possible repurposing, including medications commonly used for asthma known as beta2 agonists. In the lab, the researchers observed that in neurons grown in a dish, the asthma drugs lowered the activity of the alpha-synuclein gene and improved the cells’ health. “This was intriguing because the brains of Parkinson’s patients are full of Lewy bodies, which are piles of alpha-synuclein,” says Scherzer. “Dialing down alpha-synuclein levels would be ideal to correct this disease driver.”
Several beta2 agonists are currently in clinical trials. Scherzer and colleagues hope that their repurposing platform will spur even more clinical trials.
A central goal of the Yale center is to develop new and more effective Parkinson’s disease medications that slow or block disease progression and prevent disabling symptoms from ever occurring. “We already have dopamine replacement medicines that treat patients very well for several years, but then debilitating complications develop,” says Scherzer. “If we can identify drugs that extend this therapeutic window for 10 years or more, many patients will never suffer these complications.”
His longer-term vision is to one day build a precision neurology clinic where those living with Parkinson’s disease receive personalized treatments. “We are going to change patients’ lives,” says Scherzer.
Featured in this article
- Clemens Scherzer, MD Director of the Stephen & Denise Adams Center for Parkinson’s Disease Research
- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- Alternative routes...
Alternative routes into clinical research: a guide for early career doctors
- Related content
- Peer review
- Phillip LR Nicolson , consultant haematologist and associate professor of cardiovascular science 1 2 3 ,
- Martha Belete , registrar in anaesthetics 4 5 ,
- Rebecca Hawes , clinical fellow in anaesthetics 5 6 ,
- Nicole Fowler , haematology clinical research fellow 7 ,
- Cheng Hock Toh , professor of haematology and consultant haematologist 8 9
- 1 Institute of Cardiovascular Sciences, University of Birmingham, UK
- 2 Department of Haemostasis, Liaison Haematology and Transfusion, University Hospitals Birmingham NHS Foundation Trust, Birmingham
- 3 HaemSTAR, UK
- 4 Department of Anaesthesia, Plymouth Hospitals NHS Trust, Plymouth, UK
- 5 Research and Audit Federation of Trainees, UK
- 6 Department of Anaesthesia, The Rotherham NHS Foundation Trust, Rotherham Hospital, Rotherham
- 7 Department of Haematology, Royal Cornwall Hospitals NHS Trust, Treliske, Truro
- 8 Liverpool University Hospitals NHS Foundation Trust, Prescott Street, Liverpool
- 9 Institute of Infection, Veterinary and Ecological Sciences, University of Liverpool
- Correspondence to P Nicolson, C H Toh p.nicolson{at}bham.ac.uk ; c.h.toh{at}liverpool.ac.uk
Working in clinical research alongside clinical practice can make for a rewarding and worthwhile career. 1 2 3 Building research into a clinical career starts with research training for early and mid-career doctors. Traditional research training typically involves a dedicated period within an integrated clinical academic training programme or as part of an externally funded MD or PhD degree. Informal training opportunities, such as journal clubs and principal investigator (PI)-mentorship are available ( box 1 ), but in recent years several other initiatives have launched in the UK, meaning there are more ways to obtain research experience and embark on a career in clinical research.
Examples of in-person and online research training opportunities
These are available either informally or formally, free of charge or paid, and via local employing hospital trusts, allied health organisations, royal colleges, or universities
Acute medicine
No national trainee research network
Anaesthesia
Research and Audit Federation of Trainees (RAFT). www.raftrainees.org
Cardiothoracic surgery
No national trainee-specific research network. National research network does exist: Cardiothoracic Interdisciplinary Research Network (CIRN). www.scts.org/professionals/research/cirn.aspx
Emergency medicine
Trainee Emergency Medicine Research Network (TERN). www.ternresearch.co.uk
Ear, nose, and throat
UK ENT Trainee Research Network (INTEGRATE). www.entintegrate.co.uk
Gastroenterology
No national trainee research network. Many regional trainee research networks
General practice
No national trainee-specific research network, although national research networks exist: Society for Academic Primary Care (SAPC) and Primary Care Academic Collaborative (PACT). www.sapc.ac.uk ; www.gppact.org
General surgery
Student Audit and Research in Surgery (STARSurg). www.starsurg.org . Many regional trainee research networks
Geriatric Medicine Research Collaborative (GeMRC). www.gemresearchuk.com
Haematology (non-malignant)
Haematology Specialty Training Audit and Research (HaemSTAR). www.haemstar.org

Haematology (malignant)
Trainee Collaborative for Research and Audit in Hepatology UK (ToRcH-UK). www.twitter.com/uk_torch
Histopathology
Pathsoc Research Trainee Initiative (PARTI). www.pathsoc.org/parti.aspx
Intensive care medicine
Trainee Research in Intensive Care Network (TRIC). www.tricnetwork.co.uk
Internal medicine
No trainee-led research network. www.rcp.ac.uk/trainee-research-collaboratives
Interventional radiology
UK National Interventional Radiology Trainee Research (UNITE) Collaborative. https://www.unitecollaborative.com
Maxillofacial surgery
Maxillofacial Trainee Research Collaborative (MTReC). www.maxfaxtrainee.co.uk/
UK & Ireland Renal Trainee Network (NEPHwork). www.ukkidney.org/audit-research/projects/nephwork
Neurosurgery
British Neurosurgical Trainee Research Collaborative (BNTRC). www.bntrc.org.uk
Obstetrics and gynaecology
UK Audit and Research Collaborative in Obstetrics and Gynaecology (UKAROG). www.ukarcog.org
The National Oncology Trainee Collaborative for Healthcare Research (NOTCH). www.uknotch.com
Breast Cancer Trainee Research Collaborative Group (BCTRCG). https://bctrcguk.wixsite.com/bctrcg
Ophthalmology
The Ophthalmology Clinical Trials Network (OCTN). www.ophthalmologytrials.net
Paediatrics
RCPCH Trainee Research Network. www.rcpch.ac.uk/resources/rcpch-trainee-research-network
Paediatric anaesthesia
Paediatric Anaesthesia Trainee Research Network (PATRN). www.apagbi.org.uk/education-and-training/trainee-information/research-network-patrn
Paediatric haematology
Paediatric Haematology Trainee Research Network (PHTN). No website
Paediatric surgery
Paediatric Surgical Trainees Research Network (PSTRN). www.pstrnuk.org
Pain medicine
Network of Pain Trainees Interested in Research & Audit (PAIN-TRAIN). www.paintrainuk.com
Palliative care
UK Palliative Care Trainee Research Collaborative (UKPRC). www.twitter.com/uk_prc
Plastic surgery
Reconstructive Surgery Trials Network (RSTN). www.reconstructivesurgerytrials.net/trainees/
Pre-hospital medicine
Pre-Hospital Trainee Operated Research Network (PHOTON). www.facebook.com/PHOTONPHEM
Information from Royal College of Psychiatrists. www.rcpsych.ac.uk/members/your-faculties/academic-psychiatry/research
Radiology Academic Network for Trainees (RADIANT). www.radiantuk.com
Respiratory
Integrated Respiratory Research collaborative (INSPIRE). www.inspirerespiratory.co.uk
British Urology Researchers in Surgical Training (BURST). www.bursturology.com
Vascular surgery
Vascular & Endovascular Research Network (VERN). www.vascular-research.net
This article outlines these formal but “non-traditional” routes available to early and mid-career doctors that can successfully increase research involvement and enable research-active careers.
Trainee research networks
Trainee research networks are a recent phenomenon within most medical specialties. They are formalised regional or national groups led by early and mid-career doctors who work together to perform clinical research and create research training opportunities. The first of these groups started in the early 2010s within anaesthetics but now represent nearly every specialty ( box 2 ). 4 Trainee research networks provide research training with the aim of increasing doctors’ future research involvement. 5
A non-exhaustive list of UK national trainee led research networks*
Research training opportunities.
Mentorship by PIs at local hospital
Taking on formal role as sub-investigator
Journal clubs
Trainee representation on regional/national NIHR specialty group
API Scheme: https://www.nihr.ac.uk/health-and-care-professionals/training/associate-principal-investigator-scheme.htm .
eLearning courses available at https://learn.nihr.ac.uk (free): Good clinical practice, fundamentals of clinical research delivery, informed consent, leadership, future of health, central portfolio management system.
eLearning courses available from the Royal College of Physicians. Research in Practice programme (free). www.rcplondon.ac.uk
eLearning courses available from the Medical Research Council (free). https://bygsystems.net/mrcrsc-lms/
eLearning courses available from Nature (both free and for variable cost via employing institution): many and varied including research integrity and publication ethics, persuasive grant writing, publishing a research paper. https://masterclasses.nature.com
University courses. Examples include novel clinical trial design in translational medicine from the University of Cambridge ( https://advanceonline.cam.ac.uk/courses/ ) or introduction to randomised controlled trials in healthcare from the University of Birmingham ( https://www.birmingham.ac.uk/university/colleges/mds/cpd/ )
*limited to those with formal websites and/or active twitter accounts. Correct as of 5 January 2024. For regional trainee-led specialty research networks, see www.rcp.ac.uk/trainee-research-collaboratives for medical specialties, www.asit.org/resources/trainee-research-collaboratives/national-trainee-research-collaboratives/res1137 for surgical specialties, and www.rcoa.ac.uk/research/research-bodies/trainee-research-networks for anaesthetics.
Networks vary widely in structure and function. Most have senior mentorship to guide personal development and career trajectory. Projects are usually highly collaborative and include doctors and allied healthcare professionals working together.
Observational studies and large scale audits are common projects as their feasibility makes them deliverable rapidly with minimal funding. Some networks do, however, carry out interventional research. The benefits of increasing interventional research studies are self-evident, but observational projects are also important as they provide data useful for hypothesis generation and defining clinical equipoise and incidence/event rates, all of which are necessary steps in the development of randomised controlled studies.
These networks offer a supportive learning environment and research experience, and can match experience with expectations and responsibilities. Early and mid-career doctors are given opportunities to be involved and receive training in research at every phase from inception to publication. This develops experience in research methodology such as statistics, scientific writing, and peer review. As well as research skills training, an important reward for involvement in a study is manuscript authorship. Many groups give “citable collaborator” status to all project contributors, whatever their input. 6 7 This recognises the essential role everyone plays in the delivery of whole projects, counts towards publication metrics, and is important for future job applications.
Case study—Pip Nicolson (HaemSTAR)
Haematology Specialist Training, Audit and Research (HaemSTAR) is a trainee research network founded because of a lack of principal investigator training and clinical trial activity in non-malignant haematology. It has led and supported national audits and research projects in various subspecialty areas such as immune thrombocytopenia, thrombotic thrombocytopenic purpura, venous thrombosis, and transfusion. 8 9 10 Through involvement in this network as a registrar, I have acted as a sub-investigator and supported the principal investigator on observational and interventional portfolio-adopted studies by the National Institute for Health and Care Research. These experiences gave me valuable insight into the national and local processes involved in research delivery. I was introduced to national leaders in non-malignant haematology who not only provided mentorship and advice on career development, but also gave me opportunities to lead national audits and become involved in HaemSTAR’s committee. 10 11 These experiences in leadership have increased my confidence in management situations as I have transitioned to being a consultant, and have given me skills in balancing clinical and academic roles. Importantly, I have also developed long term friendships with peers across the country as a result of my involvement in HaemSTAR.
Associate Principal Investigator scheme
The Associate Principal Investigator (API) scheme is a training programme run by NIHR to develop research skills and contribute to clinical study delivery at a local level. It is available throughout England, Scotland, Wales, and Northern Ireland for NIHR portfolio-adopted studies. The programme runs for six months and, upon completion, APIs receive formal recognition endorsed by the NIHR and a large number of royal colleges. The scheme is free and open to medical and allied healthcare professionals at all career grades. It is designed to allow those who would not normally take part in clinical research to do so under the mentorship of a local PI. Currently there are more than 1500 accredited APIs and over 600 affiliated studies across 28 specialties. 12 It is a good way to show evidence of training and involvement in research and get more involved in research conduct. APIs have been shown to increase patient recruitment and most people completing the scheme continue to be involved in research. 12 13
Case study—Rebecca Hawes
I completed the API scheme as a senior house officer in 2021. A local PI introduced me to the Quality of Recovery after Obstetric Anaesthesia NIHR portfolio study, 14 which I saw as a training opportunity and useful experience ahead of specialist training applications. It was easy to apply for and straightforward to navigate. I was guided through the six month process in a step-by-step manner and completed eLearning modules and video based training on fundamental aspects of running research projects. All this training was evidenced on the online API platform and I had monthly supervision meetings with the PI and wider research team. As well as the experience of patient recruitment and data collection, other important aspects of training were study set-up and sponsor communications. Key to my successful API scheme was having a supportive and enthusiastic PI and developing good organisational skills. I really enjoyed the experience, and I have since done more research and have become a committee member on a national trainee research network in anaesthesia called RAFT (Research and Audit Federation of Trainees). I’ve seen great enthusiasm among anaesthetists to take part in the API scheme, with over 150 signing up to the most recent RAFT national research project.
Clinical research posts
Dedicated clinical research posts (sometimes termed “clinical research fellow” posts) allow clinicians to explore and develop research skills without committing to a formal academic pathway. They can be undertaken at any stage during a medical career but are generally performed between training posts, or during them by receiving permission from local training committees to temporarily go “out of programme.” These positions are extremely varied in how they are advertised, funded, and the balance between research and clinical time. Look out for opportunities with royal colleges, local and national research networks, and on the NHS Jobs website. Research fellowships are a good way to broaden skills that will have long term impact across one’s clinical career.
Case study—Nicole Fowler
After completing the Foundation Programme, I took up a 12 month clinical trials fellow position. This gave me early career exposure to clinical research and allowed me to act as a sub-investigator in a range of clinical trials. I received practical experience in all stages of clinical research while retaining a patient facing role, which included obtaining consent and reviewing patients at all subsequent visits until study completion. Many of the skills I developed in this post, such as good organisation and effective teamwork, are transferable to all areas of medicine. I have thoroughly enjoyed the experience and it is something I hope to talk about at interview as it is an effective way of showing commitment to a specialty. Furthermore, having a dedicated research doctor has been beneficial to my department in increasing patient involvement in research.
Acknowledgments
We would like to thank Holly Speight and Clare Shaw from the NIHR for information on the API scheme.
*These authors contributed equally to this work
Patient and public involvement: No patients were directly involved in the creation of this article.
PLRN, MB, and CHT conceived the article and are guarantors. All authors wrote and edited the manuscript.
Competing interests: PLRN was the chair of HaemSTAR from 2017 to 2023. MB is the current chair of the Research and Audit Federation of Trainees (RAFT). RH is the current secretary of RAFT. CHT conceived HaemSTAR.
Provenance and peer review: Commissioned; externally peer reviewed.
- Downing A ,
- Morris EJ ,
- Corrigan N ,
- Bracewell M ,
- Medical Academic Staff Committee of the British Medical Association
- ↵ RAFT. The start of RAFT. https://www.raftrainees.org/about
- Jamjoom AAB ,
- Hutchinson PJ ,
- Bradbury CA ,
- McCulloch R ,
- Nicolson PLR ,
- HaemSTAR Collaborators
- Collaborators H ,
- ↵ National Institute for Health and Care Research. Associate Principal Investigator (PI) Scheme. 2023. https://www.nihr.ac.uk/health-and-care-professionals/career-development/associate-principal-investigator-scheme.htm
- Fairhurst C ,
- Torgerson D
- O’Carroll JE ,
- Warwick E ,
- ObsQoR Collaborators
What Is Long COVID? Understanding the Pandemic’s Mysterious Fallout
BY BROOKS LEITNER April 15, 2024

Just weeks after the first cases of COVID-19 hit U.S. shores, an op-ed appeared in The New York Times titled “We Need to Talk About What Coronavirus Recoveries Look Like: They're a lot more complicated than most people realize.” The author, Fiona Lowenstein, is a writer and yoga teacher living in New York City, who wrote about her own illness and the symptoms she was left with once she was released from the hospital. “In the weeks since I was hospitalized for the coronavirus , the same question has flooded my email inbox, texts and direct messages: Are you better yet? I don’t yet know how to answer.”
She was better, she wrote on April 13, 2020 , but she wasn’t well. And others she was in touch with were having the same issue. Unlike most diseases, Long COVID was first described not by doctors, but by the patients themselves. Even the term “Long COVID” was coined by a patient. Dr. Elisa Perego, an honorary research fellow at University College in London, came up with the hashtag #LongCOVID when tweeting about her own experience with the post-COVID syndrome. The term went viral and suddenly social media, and then the media itself, was full of these stories.
Complaints like "I can't seem to concentrate anymore" or "I'm constantly fatigued throughout the day" became increasingly common, seemingly appearing out of nowhere. With nothing abnormal turning up from their many thorough lab tests, patients and their physicians were left feeling helpless and frustrated.
The World Health Organization (WHO) has defined Long COVID as the "continuation or development of new symptoms three months after the initial SARS-CoV-2 infection, with these symptoms lasting for at least two months with no other explanation." This deliberately broad definition reflects the complex nature of this syndrome. We now understand that these symptoms are wide-ranging, including heart palpitations, cough, nausea, fatigue, cognitive impairment (commonly referred to as "brain fog"), and more. Also, many who experience Long COVID following an acute infection face an elevated risk of such medical complications as blood clots and (type 2) diabetes.
As of March 2024 , it’s estimated that about 17% of patients who get COVID-19 will go on to develop post-acute COVID-19 syndrome, the medical term for Long COVID. Data from the Centers for Disease Control and Prevention (CDC) suggest that Long COVID disproportionately affects women, and individuals between the ages of 40 and 49 have the highest reported rates of developing this post-acute infection syndrome.
Long COVID represents a new clinical challenge
Ebony Dix, MD , a Yale School of Medicine (YSM) assistant professor of psychiatry and the medical director of the geriatric psychiatry inpatient unit at Yale New Haven Hospital, said: "Unfortunately, it is not easy to say who is going to get Long COVID and who isn't." She also emphasized that "it can be overlooked and attributed to a preexisting condition. Sometimes the only thing that patients have in their history is a positive COVID test."
Dr. Dix recalled a patient she treated in the COVID psychiatry unit whose unanticipated clinical decline began with increasing fatigue during physical therapy sessions, ultimately necessitating more care over several weeks. Dr. Dix noted, "Long COVID requires time for things to settle down. It might take several months to get back to baseline." Making timely changes to a patient’s treatment plans was essential to helping her patients get back to good health, she said.
A major challenge with Long COVID is how difficult it can be to diagnose. Determining whether new-onset symptoms, such as fatigue or weakness, are related to an underlying condition or entirely attributed to a prior COVID infection is the greatest challenge for those who care for these patients. This is, in large part, due to the lack of research surrounding the topic.
Defining a basis for Long COVID with clinical research
Inderjit Singh, MBChB , a YSM assistant professor specializing in pulmonary, critical care, and sleep medicine, and director of the Pulmonary Vascular Program, is actively engaged in clinical trials aimed at uncovering the fundamental underpinnings of Long COVID. In one research study, patients suffering from unexplained fatigue and shortness of breath undergo exhaustive exercise testing. In order to be enrolled in this study, patients need to have already completed a substantial work-up, including an echocardiogram, pulmonary function testing, chest CT scans, and more, all which result in no alternative diagnosis.
Through this work, a significant revelation emerged. They observed that patients grappling with Long COVID and facing exercise difficulties were unable to efficiently extract oxygen from their bloodstream during physical exertion. This discovery identifies a specific cause underlying the biological underpinnings of Long COVID.
Recognizing the impracticality of conducting comprehensive exercise tests for every Long COVID patient, Dr. Singh, along with other researchers, is focused on the identification of blood-based markers to assess the severity of Long COVID. For example, a research group, led by Akiko Iwasaki, PhD , Sterling Professor of Immunobiology and Molecular, Cellular, and Developmental Biology, and director of the Center for Infection & Immunity at YSM, most recently created a new method to classify Long COVID severity with circulating immune markers.
Further investigations conducted by Dr. Singh's team identified distinctive protein signatures in the blood of Long COVID patients, which correlated with the degree of Long COVID severity. Researchers identified two major and distinct blood profiles among the patients. Some of them exhibited blood profiles indicating that excessive inflammation played a prominent role in their condition, while others displayed profiles indicative of impaired metabolism. Dr. Singh raises a pressing question: "Do we prioritize treating the inflammation or addressing the metabolic defects?"
Although his research findings and those of his peers are progressively unraveling the mysteries of Long COVID, he acknowledges that "significant challenges persist in defining this syndrome.”
Why does Long COVID happen?
The symptoms of Long COVID can vary significantly from one patient to another. Some individuals may be so fatigued that they find it difficult to get out of bed each morning. Others experience heart palpitations, lightheadedness, nausea, vomiting, diarrhea, or brain fog. This broad spectrum of symptoms—more than 200 documented—has led to various hypotheses about the underlying mechanisms at play.
Researchers currently believe that the impairment of a spectrum of key bodily functions may contribute to these diverse symptoms. These potential mechanisms include compromised immune system function, damage to blood vessels, and direct harm to the brain and nervous system. Importantly, it's likely that most patients experience symptoms arising from multiple underlying causes, which complicates both the diagnosis and treatment of Long COVID.
How can Long COVID be treated?
While the diagnosis and treatment of Long COVID remain challenging, the landscape of treatment options is evolving. At Yale’s Multidisciplinary Long COVID Care Center, a team, including respiratory therapists, physical therapists, and clinical social workers, along with an internist, work together to provide a comprehensive evaluation of each patient. Treatment approaches can vary widely and may encompass medications, supplements, physical therapy, or other interventions. Each regimen is designed to meet the specific presentation of Long COVID in each patient.
Dr. Singh’s apt summarization of the situation? "I don't think there's a magic bullet for it." Effective management of Long COVID necessitates a multidisciplinary approach that harnesses the expertise of a wide variety of specialists, working together to provide tailored care and support for these patients.
Brooks Leitner is an MD/PhD candidate at Yale School of Medicine.
The last word from Lisa Sanders, MD:
I’m the internist who sees patients at Yale New Haven Health’s Multidisciplinary Long COVID Care Center. In our clinic, patients are examined by a variety of specialists to determine the best next steps for these complex patients. Sometimes that entails more testing. Often patients have had extensive testing even before they arrive, and far too often—when all the tests are normal—both doctors and patients worry that their symptoms are “all in their head.”
One of our first tasks is to reassure patients that many parts of Long COVID don’t show up on tests. We don’t know enough about the cause of many of these symptoms to create a test for them. The problem is not with the patient with the symptoms, but of the science surrounding them.
If any good can be said to come out of this pandemic, it will be a better understanding of Long COVID and many of the other post-acute infection syndromes that have existed as long as the infections themselves.
If you’d like to share your experience with Long COVID for possible use in a future post (under a pseudonym), write to us at: LongCovid [email protected]
Information provided in Yale Medicine content is for general informational purposes only. It should never be used as a substitute for medical advice from your doctor or other qualified clinician. Always seek the individual advice of your health care provider for any questions you have regarding a medical condition.
More news from Yale Medicine
Clinical Medicine & Research
Skip to main page content
- CURRENT ISSUE
- RAPID RELEASE
- SUBSCRIPTIONS
Information for Authors
To submit a manuscript to Clinical Medicine & Research, please use the web-based manuscript submission system, Editorial Manager .
Clinical Medicine & Research is a peer-reviewed medical research journal presenting relevant, credible information that addresses topics of interest to a multi-specialty audience in medicine, preventive medicine, translational medicine, epidemiology, research, and basic science. Clinical Medicine & Research will consider original manuscripts that are relevant, well-documented, and that appeal to a multi-specialty audience.
Clinical Medicine & Research serves as a source for rural health research, interventions, education, and safety issues, and as a forum on nonclinical aspects of science, medicine, and public health, covering political, philosophical, ethical, environmental, economic, historical, and cultural issues.
OBJECTIVES Clinical Medicine & Research is committed to providing its readers with relevant, rigorously peer reviewed, original scientific medical research that substantially improves human health and well-being. Clinical Medicine & Research is available in both print and electronic formats. Full text articles and abstracts are available on this web site, as well as from the PubMed Central electronic archive.
Clinical Medicine & Research is indexed by Index Medicus /MEDLINE/PubMed, EMBASE, Scopus, EBSCOhost EJS, CINAHL, CAS, Index Copernicus, and is digitally archived by PubMed Central and PubMed Central International, and is listed in the Directory of Open Access Journals.
ETHICS STATEMENT Clinical Medicine & Research follows the International Committee of Medical Journal Editors' (ICMJE) Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical Journals , the Council of Science Editors' (CSE) White Paper on Promoting Integrity in Scientific Journal Publications , and the Committee on Publication Ethics' (COPE) and the Committee on Publication Ethics' (COPE) Core Practices for Promoting Integrity in Research and Its Publication guidance for editors, reviewers, and authors.
Journal-specific Policies for Authors, Reviewers, and Editors:
Authorship Clinical Medicine & Research follows the International Committee of Medical Journal Editors (ICMJE) recommendations on authorship which states all authors should have participated sufficiently in the work to take public responsibility for the content, either all of the work or an important part of it, and must be able to defend the content (all or an important part) and conclusions of the article if publicly challenged. Sufficient participation means that substantial contributions have been made in each of the following areas:
1. Conception and design of the work; or acquisition, analysis, or interpretation of the data for the work; and
2. Drafting the work or revising it critically for important intellectual content; and
3. Approval of the version to be published; and
4. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
All those designated as authors should meet all four criteria for authorship, and all who meet the four criteria should be identified as authors. Those who do not meet all four criteria should be listed in the acknowledgments section. The ICMJE authorship criteria are intended to grant authorship status to those who deserve credit and who can take responsibility for the work. The authorship criteria should not be used as a means to withhold authorship from a contributor who otherwise meets authorship criteria by denying them the opportunity to draft/revise the manuscript and approve its final version. Therefore, all individuals who meet the first criterion should have the opportunity to participate in drafting, reviewing and final approval of the manuscript. For more information about the ICMJE authorship criteria, please go to www.icmje.org .
Each manuscript should have one author designated as the corresponding author to receive all communications about the submission, and, if it is accepted for publication, the published article. The corresponding author assumes primary responsibility for communication with the journal during the manuscript submission, peer review, and publication process, and is responsible for ensuring all the journal's administrative requirements are properly completed.
Authors may not be added or removed from a manuscript after submission and prior to acceptance without a written explanation and statement of agreement for the requested change signed by all listed authors and from the author to be removed or added. Requests for authorship changes will be reviewed by the editor and allowed as deemed appropriate. Authors may not be added or removed after the manuscript has been accepted for publication.
Group authorship : Please follow the current AMA Manual of Style, A Guide for Authors and Editors.
Acknowledgments : Individuals who contribute to a manuscript but do not qualify for authorship should be listed (with their written permission) in an Acknowledgments section with a description of their individual contributions. When a medical writer or editing service was used, their activities and the funding source for these services should be noted.
Statement of Authorship: For transparency, we require authors to complete and sign a Statement of Authorship and Disclosure as part of the copyright transfer agreement (Section 1: Statement of Authorship and Disclosure). This statement collects each author’s contribution to Concept/Design, Data Collection and Analysis, Writing, Fund Procurement, Study Subjects/Facilities/Equipment, Project Administrative Support, Authorship credit or Acknowledgement. The information provided will help the editor determine if authorship has been appropriately granted.
Conflict of Interest Authors: Every author is required to complete their own ICMJE Disclosure Form for disclosing potential Conflicts of Interest. This form is available from http://www.icmje.org/disclosure-of-interest/ and should be completed by each author. All completed forms uploaded at submission by the corresponding author. Authors should also include all conflict of interest disclosures and any financial support of the research on the title page of the manuscript at submission.
Reviewers: Reviewers are required to disclose (on the reviewer score sheet) whether they have any financial or professional affiliations that may be perceived as a conflict of interest in reviewing a specific manuscript. Reviewers should decline to review any manuscript with which they have any conflict of interest issues regarding the topic, authors, or related affiliations that may hinder providing a fair and unbiased review. If unsure, reviewers should contact the Editorial Office for clarification (email: clinmedres{at}marshfieldresearch.org ).
Editors and Journal Staff: Editors who make final decisions about manuscripts should recuse themselves from editorial decisions if they have conflicts of interest or relationships that pose potential conflicts related to articles under consideration. Other editorial staff members who participate in editorial decisions must provide editors with a current description of their financial interests or other conflicts (as they might relate to editorial judgments) and recuse themselves from any decisions in which a conflict of interest exists. Editors and editorial staff must not use information gained through working with manuscripts for private gain.
Disclosure of Support Authors must disclose any financial and/or material support (including grant information) received for the conduct of the research and/or preparation of the article. The authors, also, should briefly describe the role of the sponsor(s), if any, in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. If the funding source(s) had no such involvement, this should be stated. This information should be entered in the online manuscript submission system and included on the title page of the manuscript during submission.
Data Access, Responsibility, and Analysis For all reports containing original data (regardless of funding source), authors are asked to identify who had full access to all the data in the study and who takes responsibility for the integrity of the data and the accuracy of the data analysis. A statement identifying these authors or contributors should be included in the Acknowledgment section at the end of the manuscript. The names and affiliations of all authors and contributors who conducted and are responsible for the data access and data analysis must be indicated on the title page of the manuscript at submission.
Data Sharing Authors are asked to state if original study data will be shared and provide an explanation if data cannot be shared. If authors choose to share data, it will be published as an online supplement linked to the published article. Authors are asked to indicate if any restrictions on use of the data or types of analyses that are permitted. This information should be included on the title page of the manuscript at submission.
Data Privacy The Clinical Medicine & Research data privacy policy for authors and reviewers is available to view/download here: Data Privacy Policy .
Human and Animal Research All human studies must contain a statement within the methods section indicating that the study has been approved by an institutional review board and that participants have signed written informed consent or that the institutional review board has waived the need for informed consent. If animals were used in the research, approval from the institutional animal care and use board must be provided.
Notice of Fundamental Errors (Corrections/Errata/Retractions) If an author discovers a significant error or inaccuracy in their published work, it is the author’s obligation to promptly notify the journal editor and work with the editor to correct or retract the paper if deemed necessary by the editor. If the editor learns from a third party that a published work contains an error, the author is obliged to cooperate with the editor, including providing evidence to the editor when requested. Clinical Medicine & Research will follow the current guidance of the International Committee of Medical Journal Editors (ICMJE) and the Committee on Publication Ethics (COPE) regarding handling of errors in published manuscripts.
EDITORIAL FREEDOM Clinical Medicine & Research will openly publish both sides of controversial issues.
Clinical Medicine & Research will not publish unethical studies, whether they are controversial or not, and whether they were performed in a quality manner or not.
Authors submitting manuscripts understand that the editor and editorial staff will edit manuscripts for clarity and conformity to the style of Clinical Medicine & Research . Revisions of this type will not require input from the author for clarification. Authors will receive a final galley proof for their approval. Changes will not be made once the final galley proof is approved.
The Clinical Medicine & Research Editorial Board will, at all time, retain total editorial control of the content, structure, presentation of articles and editorials, and all decisions relating to the publication and publication schedules.
The Clinical Medicine & Research Editorial Board, staff, and its publisher, Marshfield Clinic Health System, do not endorse companies, products, or services displayed in advertisements or within the content of published articles.
Clinical Medicine & Research will not accept advertisements that advertisers or advertising agencies specify must appear at the same time as an article describing the product or service in the advertisement.
MANUSCRIPT SUBMISSION INFORMATION
Information for Authors , click here to view/download this document
Manuscript Preparation Information , click here to view/download this document.
Copyright Transfer Agreement , click here to view/download this document.
Peer Review Process information, click here to view/download this document.
Call for Papers document, click here to view/download this document.
Current Issue
- March 2024, 22 (1)
Issue Highlights
- A National Survey of Neonatologists’ Perspectives on Probiotics Use in Neonatal Intensive Care Units in the U.S.A.
- Clinical Presentation of Blastomycosis is Associated with Infecting Species, Not Host Genotype
- Mycoplasmoides genitalium Macrolide Resistance Detection is Needed in University Settings
- Alert me to new issues of Clinical Medicine & Research
- Instructions to authors
- Subscriptions
- About the journal
- Editorial Board
- Advertising Information
- Email alerts
Marshfield Clinic publishes Clinical Medicine & Research.
Stanford University Libraries' HighWire Press ® assists in the publication of CM&R Online
Copyright © 2024 by the Marshfield Clinic
- Print ISSN: 1539-4182
- Online ISSN: 1554-6179
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
April 16, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
African-led clinical research essential to tackling the continent's health care challenges, says expert
by SciDev.Net
Africa is experiencing unprecedented growth in terms of both population and economic transformation.
As witnessed during the pandemic, there is an increasing critical mass of expertise and ability for health innovation contributing to the global health agenda.
These factors, and the rising burden of both neglected tropical diseases and noncommunicable diseases, present an opportunity to strengthen and expand clinical research and trials throughout Africa.
"Pharma must prioritize uninterrupted health care access by investing in sustainable local capacity, collaboration, and alignment with Africa's public health needs," said Caxton Murira, of the Science for Africa Foundation.
According to Clinicaltrials.gov , Africa accounts for around 4% of global clinical studies. Although reports are sometimes classified as "African" in scope, randomized clinical trials are rarely continent-wide, and many nations are not represented in even one study.
Drug discovery is also inconsistently matched with public health needs, which limits African-led health innovation.
If vaccines, drugs, and medical devices are to work better, they need to be tested on groups of people who have the same epidemiological and demographic traits as the people who will be receiving the treatment.
In recent years, Africa has increased its efforts to be conducive for vaccine development. However, more work is needed to increase Africa's part of vaccine development from its present worldwide proportion of 0.1%.
The inequity can be attributed to several interrelated challenges. They include unpredictable regulatory environments, duplication of efforts because of poor stakeholder coordination, and ecosystem challenges like limited infrastructure, unharmonized regulations, poor market access, a limited policy landscape, insufficient funding.
The Pan African Clinical Trials Registry and the Clinical Trials Community have extensively documented these characteristics.
Why capacity development is critical
Africa-led health research and sustained capacity building in clinical research and trials are essential to tackling Africa's specific health care challenges.
Building a critical mass of expertise through training, infrastructure development, access to technological, and long-term funding, are key components for increasing participation in clinical research and trials. This contributes significantly to the discovery and development of effective and customized vaccines, diagnostics, and therapeutics.
Existing strengths and capacities should be leveraged through equitable partnerships to ensure adequate resource allocation and utilization for positive impact.
COVID-19 underscored the need for a continent-wide, multisectoral, multi-stakeholder approach to achieve desired results. This served as a long-term example of how to cooperate during emergencies. Many lives were saved through initiatives that utilized industry cooperation as part of a coordinated response.
Among Africa's multisectoral initiatives is the Partnerships for African Vaccine Manufacturing (PAVM) initiative, which collaborates with pharmaceutical companies to advance vaccine development and manufacturing through technology transfer, intellectual property support, trade, and policy advocacy.
There is also The H3D Foundation, which partnered with the International Federation of Pharmaceutical Manufacturers & Associations (IFPMA) to advance drug research and development in Africa, as well as the African Pharmaceutical Technology Foundation, which strengthens bilateral initiatives to manufacture pharmaceuticals locally, including a partnership between BioNTech and Senegal's Pasteur Institute to produce COVID-19 vaccines.
The role of pharmaceuticals
The pharmaceutical industry has learned that considerable progress requires increasing collaboration and the establishment of well-defined priorities in Africa and other underrepresented regions.
The pharmaceutical business models revolve around the creation of profitable new drugs to prevent and treat existing and emerging diseases to boost company value. This may not always align with patients' desire to receive uninterrupted and affordable access to health care.
Africa as a continent remains an important market for medicines and medical equipment from major pharmaceutical companies and device manufacturers in the higher income countries. Businesses are starting to facilitate the transfer of funds, knowledge, and technology to establish long-term local capability.
Africa's pharmaceutical sector has more than tripled, from US$19 billion in 2012 to $66 billion by 2022, making it the world's fastest-growing market, according to a UN report . With projected growth in the industry, there is the opportunity to create millions of employment and improve the lives of African residents and their families.
Institutions such as the Africa Centers for Disease Control and the African Medicines Agency have been mandated to coordinate with industry players to create an equitable framework to drive the delivery of new products for the African patient. This will require strong commitment from key stakeholders, regulatory oversight, and stakeholder cooperation. It will also require a strong coordination framework.
The Science for Africa Foundation recently announced a new project through its Clinical Research and Trials Community program. The initiative will promote collaboration for clinical research capacity in Africa. It also encourages active industry participation through initiatives such as the Clinical Trials Community Africa Network to enable visibility of clinical trial capacity in Africa and access to clinical trial networks and epidemiological data.
The growth of the pharmaceutical industry in Africa has shown pharma's ability to drive innovation and global health through collaboration. Pharma must prioritize uninterrupted health care access by investing in sustainable local capacity, collaboration, and alignment with Africa's public health needs.
Explore further
Feedback to editors

Researchers discover new therapeutic target for non-small cell lung cancer
7 hours ago

Immune cells carry a long-lasting 'memory' of early-life pain
8 hours ago

Cannabis legalization and rising sales have not contributed to increase in substance abuse, study finds

No negative impact from prolonged eye patching on child's development or family stress levels

COVID-19 booster immunity lasts much longer than primary series alone, study shows

Study finds that human neuron signals flow in one direction
9 hours ago

A common pathway in the brain that enables addictive drugs to hijack natural reward processing identified
10 hours ago

Scientists identify airway cells that sense aspirated water and acid reflux

Environment may influence metacognitive abilities more than genetics

Contracting RSV before age two can cause long-term lung changes and impairment
Related stories.

BioNTech hails key step in building Rwanda mRNA vaccine hub
Dec 18, 2023

Three pillars to strengthening health systems in African countries
Jan 19, 2022
Germany's BioNTech to build Africa vaccine plant from 2022
Oct 26, 2021
What will it take to boost Africa's vaccine production?
Mar 1, 2023
Rwanda breaks ground on first mRNA vaccine plant
Jun 23, 2022
North Africa disconnect 'blocks health collaboration,' say health experts
Mar 24, 2023
Recommended for you

Study links lack of diversity in Alzheimer's disease clinical trials to differences in amyloid levels
Apr 17, 2024

Researchers find glucose levels of nondiabetic people vary more than thought

Mouse study shows how a father's diet can shape the health of his offspring

Study on rats shows a junk food diet can cause long-term damage to adolescent brains
Apr 16, 2024

Mouse study finds small extracellular vesicles from young blood extend lifespan and restore physiological functions
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter

IMAGES
VIDEO
COMMENTS
A National Survey of Neonatologists' Perspectives on Probiotics Use in Neonatal Intensive Care Units in the U.S.A. Clinical Presentation of Blastomycosis is Associated with Infecting Species, Not Host Genotype. Mycoplasmoides genitalium Macrolide Resistance Detection is Needed in University Settings.
Nutrition in Medicine — A New Review Article Series. C.D. Lee and OthersN Engl J Med 2024;390:1324-1325. The editors announce a new series focusing on fundamental and emerging concepts in ...
Clinical Medicine Research is a peer-reviewed journal that publishes original articles on various aspects of clinical medicine. It offers submission guidelines, editorial board, reviewer guidelines, special issues, and academic events for researchers and professionals in the field.
The New England Journal of Medicine (NEJM) is a weekly general medical journal that publishes new medical research and review articles, and editorial opinion on a wide variety of topics of ...
Clinical Medicine is a peer-reviewed journal that publishes original research, reviews and CME articles on general and specialist medicine. It aims to improve clinical practice by providing evidence-supported and relevant learning for physicians across the specialties.
In traditional clinical research, when progress takes the form of a new drug for a definable condition, the standards for testing and accepting the drug as an advance are well established ...
Clinical Medicine & Research is a peer reviewed publication of original scientific medical research that is relevant to a broad audience of medical researchers and healthcare professionals.Clinical Medicine & Research publishes collections of articles in quarterly issues of the journal and, upon acceptance, author manuscripts are published electronically in the RAPID RELEASE section of this ...
Clinical research. Nature Communications is interested in publishing high-quality clinical research in all areas of clinical medicine. In this collection, which is curated by the Life Sciences ...
This book presents an update on new trends and developments in broadly defined medical disciplines, such as physiotherapy, coronary disease, cancer, and autism. The book is intended for clinicians, scholars, and other professionals who treat and manage patients.
Clinical research is an alternative terminology used to describe medical research. Clinical research involves people, and it is generally carried out to evaluate the efficacy of a therapeutic drug, a medical/surgical procedure, or a device as a part of treatment and patient management. Moreover, any research that evaluates the aspects of a ...
Medical and clinical research can be classified in many different ways. Probably, most people are familiar with basic (laboratory) research, clinical research, healthcare (services) research, health systems (policy) research, and educational research. Clinical research in this review refers to scientific research related to clinical practices.
Medical research can be broadly categorised into primary and secondary research. ... The validity of clinical research findings depends on a variety of factors, such as study design, sampling techniques and statistical analysis. Choosing an appropriate study design requires detailed knowledge of the types of clinical study, the situations where ...
Explore 491,535 research studies in all 50 states and in 223 countries. See listed clinical studies related to the coronavirus disease (COVID-19) ClinicalTrials.gov is a resource provided by the U.S. National Library of Medicine.
Clinical Queries Single Citation Matcher Download. E-utilities API FTP Batch Citation Matcher Explore. MeSH Database Journals Trending Articles ... National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894. Web Policies FOIA HHS Vulnerability Disclosure.
Clinical research is the comprehensive study of the safety and effectiveness of the most promising advances in patient care. Clinical research is different than laboratory research. It involves people who volunteer to help us better understand medicine and health. Lab research generally does not involve people — although it helps us learn ...
Journal of Clinical Medicine Research, monthly, ISSN 1918-3003 (print), 1918-3011 (online), published by Elmer Press Inc. The content of this site is intended for health care professionals. This is an open-access journal distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License, which permits ...
Journal of Clinical Medicine publishes peer-reviewed articles on various topics of clinical medicine, indexed in Scopus, Web of Science, PubMed and other databases. Browse the latest articles on metabolic syndrome, atrial fibrillation, prostate cancer, ADPKD and more.
Venkataramana Kandi and Sabitha Vadakedath. Clinical Research: An Overview of Study Types, Designs, and Their Implications in the Public Health Perspective. American Journal of Clinical Medicine Research. 2021; 9(2):36-42. doi: 10.12691/AJCMR-9-2-1. Abstract Human health is plagued by several challenges throughout life.
Clinical research and care in the USA has a sinister history of exploitation and injustice 1,2,3.This includes (but is not limited to) the failure to treat Black men with syphilis in the Tuskegee ...
Clinical Medicine & Research is an open-access, peer-reviewed, academic journal of clinical medicine published by the Marshfield Clinic Research Foundation. The journal is currently edited by Adedayo A. Onitilo (Marshfield Clinic). Abstracting and indexing. The journal is abstracted and indexed in the following bibliographic databases:
In a world where chronic non-communicable diseases increasingly dominate public health concerns, the concept of "food as medicine" is undergoing a renaissance. Emerging scientific research now ...
The Institute of Medicine (IOM) reports To Err Is Human: Building a Safer Health System (IOM, 2000) and Crossing the Quality Chasm: A New Health System for the 21st Century (IOM, 2001a), focused the nation's attention on concerns about the quality of health care in the United States. ... An analysis of clinical research by phase of ...
Limitations For clinical cases chosen…there seemed to be lack of compensation for inter rater variability for the 5 EDITORS chosen? For survey, lack of demographical data for residents and attendees besides pgy1 since most internal medical residents are pgy1…pgy2 they are more specialized ..ie ophthalmology
The collaboration contributed to the first Phase 2 clinical trial focused on a genetic form of Parkinson's and provided a tool kit for precision trials targeting GBA. Scherzer hopes to use the power of genomics and AI to turn data into medicine. The center is using computational neuroscience and machine learning to accelerate research.
Working in clinical research alongside clinical practice can make for a rewarding and worthwhile career.123 Building research into a clinical career starts with research training for early and mid-career doctors. Traditional research training typically involves a dedicated period within an integrated clinical academic training programme or as part of an externally funded MD or PhD degree.
Inderjit Singh, MBChB, a YSM assistant professor specializing in pulmonary, critical care, and sleep medicine, and director of the Pulmonary Vascular Program, is actively engaged in clinical trials aimed at uncovering the fundamental underpinnings of Long COVID.In one research study, patients suffering from unexplained fatigue and shortness of breath undergo exhaustive exercise testing.
Clinical Medicine & Research is committed to providing its readers with relevant, rigorously peer reviewed, original scientific medical research that substantially improves human health and well-being. Clinical Medicine & Research is available in both print and electronic formats. Full text articles and abstracts are available on this web site ...
Why capacity development is critical. Africa-led health research and sustained capacity building in clinical research and trials are essential to tackling Africa's specific health care challenges.