
Chemistry Steps


General Chemistry
Stoichiometry.
This is a comprehensive, end-of-chapter set of practice problems on stoichiometry that covers balancing chemical equations, mole-ratio calculations, limiting reactants, and percent yield concepts.
The links to the corresponding topics are given below.
- The Mole and Molar Mass
- Molar Calculations
- Percent Composition and Empirical Formula
- Stoichiometry of Chemical Reactions
Limiting Reactant
- Reaction/Percent Yield
- Stoichiometry Practice Problems
Balance the following chemical equations:
a) HCl + O 2 → H 2 O + Cl 2
b) Al(NO 3 ) 3 + NaOH → Al(OH) 3 + NaNO 3
c) H 2 + N 2 → NH 3
d) PCl 5 + H 2 O → H 3 PO 4 + HCl
e) Fe + H 2 SO 4 → Fe 2 (SO 4 ) 3 + H 2
f) CaCl 2 + HNO 3 → Ca(NO 3 ) 2 + HCl
g) KO 2 + H 2 O → KOH + O 2 + H 2 O 2
h) Al + H 2 O → Al 2 O 3 + H 2
i) Fe + Br 2 → FeBr 3
j) Cu + HNO 3 → Cu(NO 3 ) 2 + NO 2 + H 2 O
k) Al(OH) 3 → Al 2 O 3 + H 2 O
l) NH 3 + O 2 → NO + H 2 O
m) Ca(AlO 2 ) 2 + HCl → AlCl 3 + CaCl 2 + H 2 O
n) C 5 H 12 + O 2 → CO 2 + H 2 O
o) P 4 O 10 + H 2 O → H 3 PO 4
p) Na 2 CrO 4 + Pb(NO 3 ) 2 → PbCrO 4 + NaNO 3
q) MgCl 2 + AgNO 3 → AgCl + Mg(NO 3 ) 2
r) KClO 3 → KClO 4 + KCl
s) Ca(OH) 2 + H 3 PO 4 → Ca 3 (PO 4 ) 2 + H 2 O
Consider the balanced equation:
C 5 H 12 + 8 O 2 → 5CO 2 + 6H 2 O
Complete the table showing the appropriate number of moles of reactants and products.
How many grams of CO 2 and H 2 O are produced from the combustion of 220. g of propane (C 3 H 8 )?
C 3 H 8 (g) + 5O 2 (g) → 3CO 2 (g) + 4H 2 O(g)
How many grams of CaCl 2 can be produced from 65.0 g of Ca(OH) 2 according to the following reaction,
Ca(OH) 2 + 2HCl → CaCl 2 + 2H 2 O
How many moles of oxygen are formed when 75.0 g of Cu(NO 3 ) 2 decomposes according to the following reaction?
2Cu(NO 3 ) 2 → 2CuO + 4NO 2 + O 2
How many grams of MnCl 2 can be prepared from 52.1 grams of MnO 2 ?
MnO 2 + 4HCl → MnCl 2 + Cl 2 + 2H 2 O
Determine the mass of oxygen that is formed when an 18.3-g sample of potassium chlorate is decomposed according to the following equation:
2KClO 3 (s) → 2KCl(s) + 3O 2 (g).
How many grams of H 2 O will be formed when 48.0 grams H 2 are mixed with excess hydrogen gas?
2H 2 + O 2 → 2H 2 O
Consider the chlorination reaction of methane (CH4):
CH 4 (g) + 4Cl 2 (g) → CCl 4 (g) + 4HCl(g)
How many moles of CH 4 were used in the reaction if 51.9 g of CCl4 were obtained?
How many grams of Ba(NO 3 ) 2 can be produced by reacting 16.5 g of HNO 3 with an excess of Ba(OH) 2 ?
Ethanol can be obtained by fermentation – a complex chemical process breaking down glucose to ethanol and carbon dioxide.
C 6 H 12 O 6 → 2C 2 H 5 OH + 2CO 2 glucose ethanol
How many mL of ethanol (d =0.789 g/mL) can be obtained by this process starting with 286 g of glucose?
36.0 g of butane (C 4 H 10 ) was burned in an excess of oxygen and the resulting carbon dioxide (CO 2 ) was collected in a sealed vessel.
2C 4 H 10 + 13O 2 → 8CO 2 + 10H 2 O
How many grams of LiOH will be necessary to consume all the CO 2 from the first reaction?
2LiOH + CO 2 → Li 2 CO 3 + H 2 O
13. Which statement about limiting reactant is correct?
a) The limiting reactant is the one in a smaller quantity.
b) The limiting reactant is the one in greater quantity.
c) The limiting reactant is the one producing less product.
d) The limiting reactant is the one producing more product.
Find the limiting reactant for each initial amount of reactants.
4NH 3 + 5O 2 → 4NO + 6H 2 O
a) 2 mol of NH 3 and 2 mol of O 2
b) 2 mol of NH 3 and 3 mol of O 2
c) 3 mol of NH 3 and 3 mol of O 2
d) 3 mol of NH 3 and 2 mol of O 2
Note: This is not a multiple-choice question. Each row represents a separate question where you need to determine the limiting reactant.
How many g of hydrogen are left over in producing ammonia when 14.0 g of nitrogen is reacted with 8.0 g of hydrogen?
N 2 (g) + 3 H 2 (g) → 2 NH 3 (g)
How many grams of PCl 3 will be produced if 130.5 g Cl 2 is reacted with 56.4 g P 4 according to the following equation?
6Cl 2 (g) + P 4 (s) → 4PCl 3 (l)
How many grams of sulfur can be obtained if 12.6 g H 2 S is reacted with 14.6 g SO 2 according to the following equation?
2H 2 S(g) + SO 2 (g) → 3S(s) + 2H 2 O(g)
The following equation represents the combustion of octane, C 8 H 18 , a component of gasoline:
2C 8 H 18 (g) + 25O 2 (g) → 16CO 2 (g) + 18H 2 O(g)
Will 356 g of oxygen be enough for the complete combustion of 954 g of octane?
When 140.0 g of AgNO 3 was added to an aqueous solution of NaCl, 86.0 g of AgCl was collected as a white precipitate. Which salt was the limiting reactant in this reaction? How many grams of NaCl were present in the solution when AgNO 3 was added?
AgNO 3 (aq) + NaCl(aq) → AgCl(s) + NaNO 3 (aq)
Consider the reaction between MnO 2 and HCl:
MnO 2 + 4HCl → MnCl 2 + Cl 2 + 2H 2 O
What is the theoretical yield of MnCl 2 in grams when 165 g of MnO 2 is added to a solution containing 94.2 g of HCl?
Percent Yield
21. In a chemistry experiment, a student obtained 5.68 g of a product. What is the percent yield of the product if the theoretical yield was 7.12 g?
When 38.45 g CCl 4 is reacted with an excess of HF, 21.3 g CCl 2 F 2 is obtained. Calculate the theoretical and percent yields of this reaction.
CCl 4 + 2HF → CCl 2 F 2 + 2HCl
Iron(III) oxide reacts with carbon monoxide according to the equation:
Fe 2 O 3 ( s ) + 3CO( g ) → 2Fe( s ) + 3CO 2 ( g )
What is the percent yield of this reaction if 623 g of iron oxide produces 341 g of iron?
Determine the percent yield of the reaction if 77.0 g of CO 2 are formed from burning 2.00 moles of C 5 H 12 in 4.00 moles of O 2 .
C 5 H 12 + 8 O 2 → 5CO 2 + 6H 2 O
The percent yield for the following reaction was determined to be 84%:
N 2 ( g ) + 2H 2 ( g ) → N 2 H 4 ( l )
How many grams of hydrazine (N 2 H 4 ) can be produced when 38.36 g of nitrogen reacts with 6.68 g of hydrogen?
Silver metal can be prepared by reducing its nitrate, AgNO 3 with copper according to the following equation:
Cu( s ) + 2AgNO 3 ( aq ) → Cu(NO 3 ) 2 ( aq ) + 2Ag( s )
What is the percent yield of the reaction if 71.5 grams of Ag was obtained from 132.5 grams of AgNO 3 ?
Industrially, nitric acid is produced from ammonia by the Ostwald process in a series of reactions:
4NH 3 ( g ) + 5O 2 ( g ) → 4NO( g ) + 6H 2 O( l )
2NO( g ) + O 2 ( g ) → 2NO 2 ( g )
2NO 2 ( g ) + H 2 O( l ) → HNO 3 ( aq ) + HNO 2 ( aq )
Considering that each reaction has an 85% percent yield, how many grams of NH 3 must be used to produce 25.0 kg of HNO 3 by the above procedure?
Aspirin (acetylsalicylic acid) is widely used to treat pain, fever, and inflammation. It is produced from the reaction of salicylic acid with acetic anhydride. The chemical equation for aspirin synthesis is shown below:
In one container, 10.00 kg of salicylic acid is mixed with 10.00 kg of acetic anhydride.
a) Which reactant is limiting? Which is in excess? b) What mass of excess reactant is left over? c) What mass of aspirin is formed assuming 100% yield (Theoretical yield)? d) What mass of aspirin is formed if the reaction yield is 70.0% ? e) If the actual yield of aspirin is 11.2 kg, what is the percent yield? f) How many kg of salicylic acid is needed to produce 20.0 kg of aspirin if the reaction yield is 85.0% ?
3 thoughts on “Stoichiometry Practice Problems”
You forgot the subscript 3 for O in the molecular formula for acetic anhydride and the reaction is not balanced as written. For part F) it’s 18.1 kg and not1.81 kg as written in the final line of the solution.
Thanks for letting me know! Fixed.
You’re welcome!
Leave a Comment Cancel reply
Notify me of followup comments via e-mail. You can also subscribe without commenting.
Pathways to Chemistry
Chemistry for college, middle and high school, homeschooled students, teachers and parents, stoichiometry part 2 answer key.
Back to Stoichiometry Part 2 Worksheet Back to Worksheets Back to Stoichiometry, Limiting Reactants, and Percent Yield Study Guide
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
The temperature of 1 gram of burning wood is approximately the same for both a match and a bonfire. This is an intensive property and depends on the material (wood). However, the overall amount of produced heat depends on the amount of material; this is an extensive property. The amount of wood in a bonfire is much greater than that in a match; the total amount of produced heat is also much greater, which is why we can sit around a bonfire to stay warm, but a match would not provide enough heat to keep us from getting cold.
Heat capacity refers to the heat required to raise the temperature of the mass of the substance 1 degree; specific heat refers to the heat required to raise the temperature of 1 gram of the substance 1 degree. Thus, heat capacity is an extensive property, and specific heat is an intensive one.
(a) 47.6 J/°C; 11.38 cal °C −1 ; (b) 407 J/°C; 97.3 cal °C −1
1310 J; 313 cal
(a) 0.390 J/g °C; (b) Copper is a likely candidate.
We assume that the density of water is 1.0 g/cm 3 (1 g/mL) and that it takes as much energy to keep the water at 85 °F as to heat it from 72 °F to 85 °F. We also assume that only the water is going to be heated. Energy required = 7.47 kWh
lesser; more heat would be lost to the coffee cup and the environment and so Δ T for the water would be lesser and the calculated q would be lesser
greater, since taking the calorimeter’s heat capacity into account will compensate for the thermal energy transferred to the solution from the calorimeter; this approach includes the calorimeter itself, along with the solution, as “surroundings”: q rxn = −( q solution + q calorimeter ); since both q solution and q calorimeter are negative, including the latter term ( q rxn ) will yield a greater value for the heat of the dissolution
The temperature of the coffee will drop 1 degree.
5.7 × × 10 2 kJ
−2.2 kJ; The heat produced shows that the reaction is exothermic.
22.6. Since the mass and the heat capacity of the solution is approximately equal to that of the water, the two-fold increase in the amount of water leads to a two-fold decrease of the temperature change.
1.4 × × 10 2 Calories
The enthalpy change of the indicated reaction is for exactly 1 mol HCL and 1 mol NaOH; the heat in the example is produced by 0.0500 mol HCl and 0.0500 mol NaOH.
25 kJ mol −1
81 kJ mol −1
1.83 × × 10 −2 mol
–802 kJ mol −1
−494 kJ/mol
44.01 kJ/mol
90.3 kJ/mol
(a) −1615.0 kJ mol −1 ; (b) −484.3 kJ mol −1 ; (c) 164.2 kJ; (d) −232.1 kJ
−54.04 kJ mol −1
−2660 kJ mol −1
On the assumption that the best rocket fuel is the one that gives off the most heat, B 2 H 6 is the prime candidate.
(a) C 3 H 8 ( g ) + 5 O 2 ( g ) ⟶ 3 CO 2 ( g ) + 4 H 2 O ( l ) ; C 3 H 8 ( g ) + 5 O 2 ( g ) ⟶ 3 CO 2 ( g ) + 4 H 2 O ( l ) ; (b) 330 L air; (c) −104.5 kJ mol −1 ; (d) 75.4 °C
As an Amazon Associate we earn from qualifying purchases.
This book may not be used in the training of large language models or otherwise be ingested into large language models or generative AI offerings without OpenStax's permission.
Want to cite, share, or modify this book? This book uses the Creative Commons Attribution License and you must attribute OpenStax.
Access for free at https://openstax.org/books/chemistry-2e/pages/1-introduction
- Authors: Paul Flowers, Klaus Theopold, Richard Langley, William R. Robinson, PhD
- Publisher/website: OpenStax
- Book title: Chemistry 2e
- Publication date: Feb 14, 2019
- Location: Houston, Texas
- Book URL: https://openstax.org/books/chemistry-2e/pages/1-introduction
- Section URL: https://openstax.org/books/chemistry-2e/pages/chapter-5
© Jan 8, 2024 OpenStax. Textbook content produced by OpenStax is licensed under a Creative Commons Attribution License . The OpenStax name, OpenStax logo, OpenStax book covers, OpenStax CNX name, and OpenStax CNX logo are not subject to the Creative Commons license and may not be reproduced without the prior and express written consent of Rice University.
Just added to your cart

Stoichiometry: Limiting & Excess Reactants
Adding product to your cart
- Choosing a selection results in a full page refresh.
- Press the space key then arrow keys to make a selection.
Stoichiometry Unit Review Bundle & Answer Keys

Products in this Bundle (6)
showing 1 - 5 of 6 products
Also included in

Description
This bundle includes five review documents and the "5 Steps to Live By" 1/2 sheet that encourages students to review balancing equations using correct formulas, the use of coefficients to complete a mole to mole ratio, completing stoichiometry problems using the "5 Steps to Live By" and solving for percent yield (based on an actual yield provided in the problem). The answer keys are provided.
This bundle will work optimally after a student has completed the Stoichiometry Unit Notes Bundle, Guided Practice questions, and Labs.
(This document was made with narrow margins set and Century Gothic font)

Questions & Answers
Becky youngkent.
- We're hiring
- Help & FAQ
- Privacy policy
- Student privacy
- Terms of service
- Tell us what you think

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

3.E: Stoichiometry (Exercises)
- Last updated
- Save as PDF
- Page ID 43470
These are homework exercises to accompany the Textmap created for "Chemistry: The Central Science" by Brown et al. Complementary General Chemistry question banks can be found for other Textmaps and can be accessed here . In addition to these publicly available questions, access to private problems bank for use in exams and homework is available to faculty only on an individual basis; please contact Delmar Larsen for an account with access permission.
3.1: Chemical Equations
Conceptual problems.
- How does a balanced chemical equation agree with the law of definite proportions?
- What is the difference between S 8 and 8S? Use this example to explain why subscripts in a formula must not be changed.
- What factors determine whether a chemical equation is balanced?
- What information can be obtained from a balanced chemical equation? Does a balanced chemical equation give information about the rate of a reaction?
Numerical Problems
1. Balance each chemical equation.
a. KI(aq) + Br 2 (l) → KBr(aq) + I 2 (s)
b. MnO 2 (s) + HCl(aq) → MnCl 2 (aq) + Cl 2 (g) + H 2 O(l)
c. Na 2 O(s) + H 2 O(l) → NaOH(aq)
d. Cu(s) + AgNO 3 (aq) → Cu(NO 3 ) 2 (aq) + Ag(s)
e. SO 2 (g) + H 2 O(l) → H 2 SO 3 (aq)
f. S 2 Cl 2 (l) + NH 3 (l) → S 4 N 4 (s) + S 8 (s) + NH 4 Cl(s)
2. Balance each chemical equation.
a. Be(s) + O 2 (g) → BeO(s)
b. N 2 O 3 (g) + H 2 O(l) → HNO 2 (aq)
c. Na(s) + H 2 O(l) → NaOH(aq) + H 2 (g)
d. CaO(s) + HCl(aq) → CaCl 2 (aq) + H 2 O(l)
e. CH 3 NH 2 (g) + O 2 (g) → H 2 O(g) + CO 2 (g) + N 2 (g)
f. Fe(s) + H 2 SO 4 (aq) → FeSO 4 (aq) + H 2 (g)
3. Balance each chemical equation.
a. N 2 O 5 (g) → NO 2 (g) + O 2 (g)
b. NaNO 3 (s) → NaNO 2 (s) + O 2 (g)
c. Al(s) + NH 4 NO 3 (s) → N 2 (g) + H 2 O(l) + Al 2 O 3 (s)
d. C 3 H 5 N 3 O 9 (l) → CO 2 (g) + N 2 (g) + H 2 O(g) + O 2 (g)
e. reaction of butane with excess oxygen
f. IO 2 F(s) + BrF 3 (l) → IF 5 (l) + Br 2 (l) + O 2 (g)
4. Balance each chemical equation.
a. H 2 S(g) + O 2 (g) → H 2 O(l) + S 8 (s)
b. KCl(aq) + HNO 3 (aq) + O 2 (g) → KNO 3 (aq) + Cl 2 (g) + H 2 O(l)
c. NH 3 (g) + O 2 (g) → NO(g) + H 2 O(g)
d. CH 4 (g) + O 2 (g) → CO(g) + H 2 (g)
e. NaF(aq) + Th(NO 3 ) 4 (aq) → NaNO 3 (aq) + ThF 4 (s)
f. Ca 5 (PO 4 ) 3 F(s) + H 2 SO 4 (aq) + H 2 O(l) → H 3 PO 4 (aq) + CaSO 4 •2H 2 O(s) + HF(aq)
5. Balance each chemical equation.
a. NaCl(aq) + H 2 SO 4 (aq) → Na 2 SO 4 (aq) + HCl(g)
b. K(s) + H 2 O(l) → KOH(aq) + H 2 (g)
c. reaction of octane with excess oxygen
d. S 8 (s) + Cl 2 (g) → S 2 Cl 2 (l)
e. CH 3 OH(l) + I 2 (s) + P 4 (s) → CH 3 I(l) + H 3 PO 4 (aq) + H 2 O(l)
f. (CH 3 ) 3 Al(s) + H 2 O(l) → CH 4 (g) + Al(OH) 3 (s)
6. Write a balanced chemical equation for each reaction.
a. Aluminum reacts with bromine.
b. Sodium reacts with chlorine.
c. Aluminum hydroxide and acetic acid react to produce aluminum acetate and water.
d. Ammonia and oxygen react to produce nitrogen monoxide and water.
e. Nitrogen and hydrogen react at elevated temperature and pressure to produce ammonia.
f. An aqueous solution of barium chloride reacts with a solution of sodium sulfate.
7. Write a balanced chemical equation for each reaction.
a. Magnesium burns in oxygen.
b. Carbon dioxide and sodium oxide react to produce sodium carbonate.
c. Aluminum reacts with hydrochloric acid.
d. An aqueous solution of silver nitrate reacts with a solution of potassium chloride.
e. Methane burns in oxygen.
f. Sodium nitrate and sulfuric acid react to produce sodium sulfate and nitric acid.
3.2: Some Simple Patterns of Chemical Reactivity
3.3: formula masses.
- What is the relationship between an empirical formula and a molecular formula
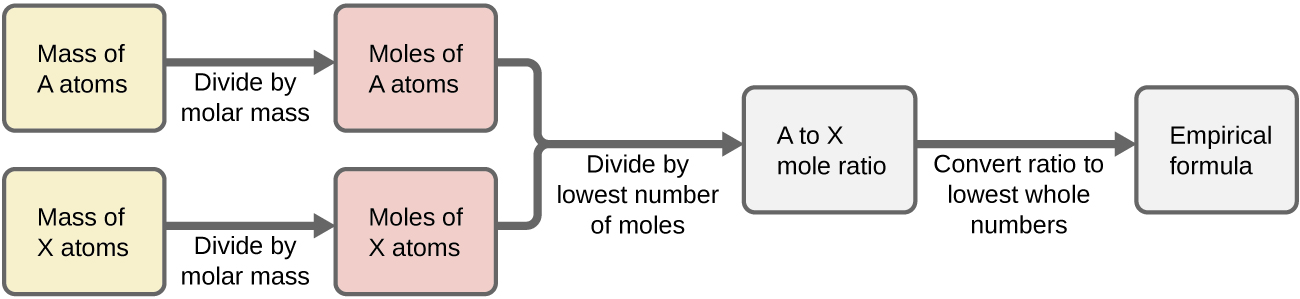
- Construct a flowchart showing how you would determine the empirical formula of a compound from its percent composition.
Please be sure you are familiar with the topics discussed in Essential Skills 2 ( Section 3.7 "Essential Skills 2" ) before proceeding to the Numerical Problems.
a. What is the formula mass of each species?
a. ammonium chloride
b. sodium cyanide
c. magnesium hydroxide
d. calcium phosphate
e. lithium carbonate
f. hydrogen sulfite ion
b. What is the molecular or formula mass of each compound?
a. potassium permanganate
b. sodium sulfate
c. hydrogen cyanide
d. potassium thiocyanate
e. ammonium oxalate
f. lithium acetate
1. What is the mass percentage of water in each hydrate?
a. H 3 AsO 4 ·5H 2 O
b. NH 4 NiCl 3 ·6H 2 O
c. Al(NO 3 ) 3 ·9H 2 O
2. What is the mass percentage of water in each hydrate?
a. CaSO 4 ·2H 2 O
b. Fe(NO 3 ) 3 ·9H 2 O
c. (NH 4 ) 3 ZrOH(CO 3 ) 3 ·2H 2 O
3. Which of the following has the greatest mass percentage of oxygen—KMnO 4 , K 2 Cr 2 O 7 , or Fe 2 O 3 ?
4. Which of the following has the greatest mass percentage of oxygen—ThOCl 2 , MgCO 3 , or NO 2 Cl?
5. Calculate the percent composition of the element shown in bold in each compound.
b. As 2 I 4
c. Al P O 4
d. C 6 H 10 O
6. Calculate the percent composition of the element shown in bold in each compound.
a. H Br O 3
c. C 3 H 8 O
d. Fe S O 4
7. A sample of a chromium compound has a molar mass of 151.99 g/mol. Elemental analysis of the compound shows that it contains 68.43% chromium and 31.57% oxygen. What is the identity of the compound?
8. The percentages of iron and oxygen in the three most common binary compounds of iron and oxygen are given in the following table. Write the empirical formulas of these three compounds.
9. What is the mass percentage of water in each hydrate?
a. LiCl·H 2 O
b. MgSO 4 ·7H 2 O
c. Sr(NO 3 ) 2 ·4H 2 O
10. What is the mass percentage of water in each hydrate?
a. CaHPO 4 ·2H 2 O
b. FeCl 2 ·4H 2 O
c. Mg(NO 3 ) 2 ·4H 2 O
11. Two hydrates were weighed, heated to drive off the waters of hydration, and then cooled. The residues were then reweighed. Based on the following results, what are the formulas of the hydrates?
12. Which contains the greatest mass percentage of sulfur—FeS 2 , Na 2 S 2 O 4 , or Na 2 S?
13. Given equal masses of each, which contains the greatest mass percentage of sulfur—NaHSO 4 or K 2 SO 4 ?
14. Calculate the mass percentage of oxygen in each polyatomic ion.
a. bicarbonate
b. chromate
15. Calculate the mass percentage of oxygen in each polyatomic ion.
c. dihydrogen phosphate
d. thiocyanate
16. The empirical formula of garnet, a gemstone, is Fe 3 Al 2 Si 3 O 12 . An analysis of a sample of garnet gave a value of 13.8% for the mass percentage of silicon. Is this consistent with the empirical formula?
17. A compound has the empirical formula C 2 H 4 O, and its formula mass is 88 g. What is its molecular formula?
18. Mirex is an insecticide that contains 22.01% carbon and 77.99% chlorine. It has a molecular mass of 545.59 g. What is its empirical formula? What is its molecular formula?
19. How many moles of CO 2 and H 2 O will be produced by combustion analysis of 0.010 mol of styrene?
20. How many moles of CO 2 , H 2 O, and N 2 will be produced by combustion analysis of 0.0080 mol of aniline?
21. How many moles of CO 2 , H 2 O, and N 2 will be produced by combustion analysis of 0.0074 mol of aspartame?
22. How many moles of CO 2 , H 2 O, N 2 , and SO 2 will be produced by combustion analysis of 0.0060 mol of penicillin G?
23. Combustion of a 34.8 mg sample of benzaldehyde, which contains only carbon, hydrogen, and oxygen, produced 101 mg of CO 2 and 17.7 mg of H 2 O.
a. What was the mass of carbon and hydrogen in the sample?
b. Assuming that the original sample contained only carbon, hydrogen, and oxygen, what was the mass of oxygen in the sample?
c. What was the mass percentage of oxygen in the sample?
d. What is the empirical formula of benzaldehyde?
e. The molar mass of benzaldehyde is 106.12 g/mol. What is its molecular formula?
24. Salicylic acid is used to make aspirin. It contains only carbon, oxygen, and hydrogen. Combustion of a 43.5 mg sample of this compound produced 97.1 mg of CO 2 and 17.0 mg of H 2 O.
a. What is the mass of oxygen in the sample?
b. What is the mass percentage of oxygen in the sample?
c. What is the empirical formula of salicylic acid?
d. The molar mass of salicylic acid is 138.12 g/mol. What is its molecular formula?
25. Given equal masses of the following acids, which contains the greatest amount of hydrogen that can dissociate to form H + —nitric acid, hydroiodic acid, hydrocyanic acid, or chloric acid?
26. Calculate the formula mass or the molecular mass of each compound.
a. heptanoic acid (a seven-carbon carboxylic acid)
b. 2-propanol (a three-carbon alcohol)
d. tetraethyllead
e. sulfurous acid
f. ethylbenzene (an eight-carbon aromatic hydrocarbon)
27. Calculate the formula mass or the molecular mass of each compound.
c. bromobenzene
d. cyclohexene
e. phosphoric acid
f. ethylamine
28. Given equal masses of butane, cyclobutane, and propene, which contains the greatest mass of carbon?
29. Given equal masses of urea [(NH 2 ) 2 CO] and ammonium sulfate, which contains the most nitrogen for use as a fertilizer?
Conceptual Answers
1) What is the relationship between an empirical formula and a molecular formula
- An empirical formula refers to the simplest ratio of elements that is obtained from a chemical formula while a molecular formula is calculated to show the actual formula of a molecular compound.
2) Construct a flowchart showing how you would determine the empirical formula of a compound from its percent composition.
Numerical Answers
a. 53.49146 amu
b. 49.0072 amu
c. 58.3197 amu
d. 310.177 amu
e. 73.891 amu
f. 81.07 amu
a.158.034 amu
b. 142.04 amu
c. 27.0253 amu
d. 97.181 amu
e. 124.1 amu
f. 65.99 amu
1. To two decimal places, the percentages are:
2. To two decimal places, the percentages of water are:
3. % oxygen: KMnO 4 , 40.50%; K 2 Cr 2 O 7 , 38.07%; Fe 2 O 3 , 30.06%
4. % oxygen: ThOCl2, 5.02%; MgCO3, 56.93%; NO2Cl, 39.28%
5. To two decimal places, the percentages are:
a. 66.32% Br
b. 22.79% As
c. 25.40% P
d. 73.43% C
a. 61.98% Br
b. 34.69% Cs
c. 59.96% C
d. 21.11% S
7. Cr 2 O 3 .
8. Empirical Formulas
9. To two decimal places, the percentages are:
11. NiSO 4 · 6H 2 O and CoCl 2 · 6H 2 O
13. NaHSO 4
No, the calculated mass percentage of silicon in garnet is 16.93%
17. C 4 H 8 O 2
Empirical Formula: C 10 Cl 12
Molecular Formula: C 10 Cl 12
Moles of CO2: 0.08 mol CO 2
Moles of H2O: 0.04 mol H 2 O
Mole of CO2: 0.048 mol CO 2
Mole of H2O: 0.028 mol H 2 O
Mole of N2: 0.004 mol N 2
Mole of CO2: 0.104 mol CO 2
Mole of H2O: 0.666 mol H 2 O
Mole of N2: 0.0074 mol N 2
Mole of CO2: 0.096 mol CO 2
Mole of H2O: 0.054 mol H 2 O
Mole of N2: 0.060 mol N 2
Mole of SO2: 0.060 mol SO 2
a. 27.6 mg C and 1.98 mg H
b. 5.2 mg O
d. C 7 H 6 O
e. C 7 H 6 O
C 7 H 6 O 3
25. hydrocyanic acid, HCN
a. 130.1849 amu
b. 60.1 amu
c. 158.034 amu
d. 323.4 amu
e. 82.07 amu
f. 106.17 amu
27. To two decimal places, the values are:
a. 273.23 amu
b. 69.62 amu
c. 157.01 amu
d. 82.14 amu
e. 98.00 amu
f. 45.08 amu
28. Cyclobutene
3.4: Avogadro's Number and the Mole
Please be sure you are familiar with the topics discussed in Essential Skills 2 before proceeding to the Conceptual Problems.
- Describe the relationship between an atomic mass unit and a gram.
- Is it correct to say that ethanol has a formula mass of 46? Why or why not?
- If 2 mol of sodium reacts completely with 1 mol of chlorine to produce sodium chloride, does this mean that 2 g of sodium reacts completely with 1 g of chlorine to give the same product? Explain your answer.
- Construct a flowchart to show how you would calculate the number of moles of silicon in a 37.0 g sample of orthoclase (KAlSi 3 O 8 ), a mineral used in the manufacture of porcelain.
- Construct a flowchart to show how you would calculate the number of moles of nitrogen in a 22.4 g sample of nitroglycerin that contains 18.5% nitrogen by mass.
Please be sure you are familiar with the topics discussed in Essential Skills 2 before proceeding to the Numerical Problems.
1. Derive an expression that relates the number of molecules in a sample of a substance to its mass and molecular mass.
2. Calculate the molecular mass or formula mass of each compound.
- KCl (potassium chloride)
- NaCN (sodium cyanide)
- H 2 S (hydrogen sulfide)
- NaN 3 (sodium azide)
- H 2 CO 3 (carbonic acid)
- K 2 O (potassium oxide)
- Al(NO 3 ) 3 (aluminum nitrate)
- Cu(ClO 4 ) 2 [copper(II) perchlorate]
3. Calculate the molecular mass or formula mass of each compound.
- V 2 O 4 (vanadium(IV) oxide)
- CaSiO 3 (calcium silicate)
- BiOCl (bismuth oxychloride)
- CH 3 COOH (acetic acid)
- Ag 2 SO 4 (silver sulfate)
- Na 2 CO 3 (sodium carbonate)
- (CH 3 ) 2 CHOH (isopropyl alcohol)
4. Calculate the molar mass of each compound.
5. Calculate the molar mass of each compound.
6. For each compound, write the condensed formula, name the compound, and give its molar mass.
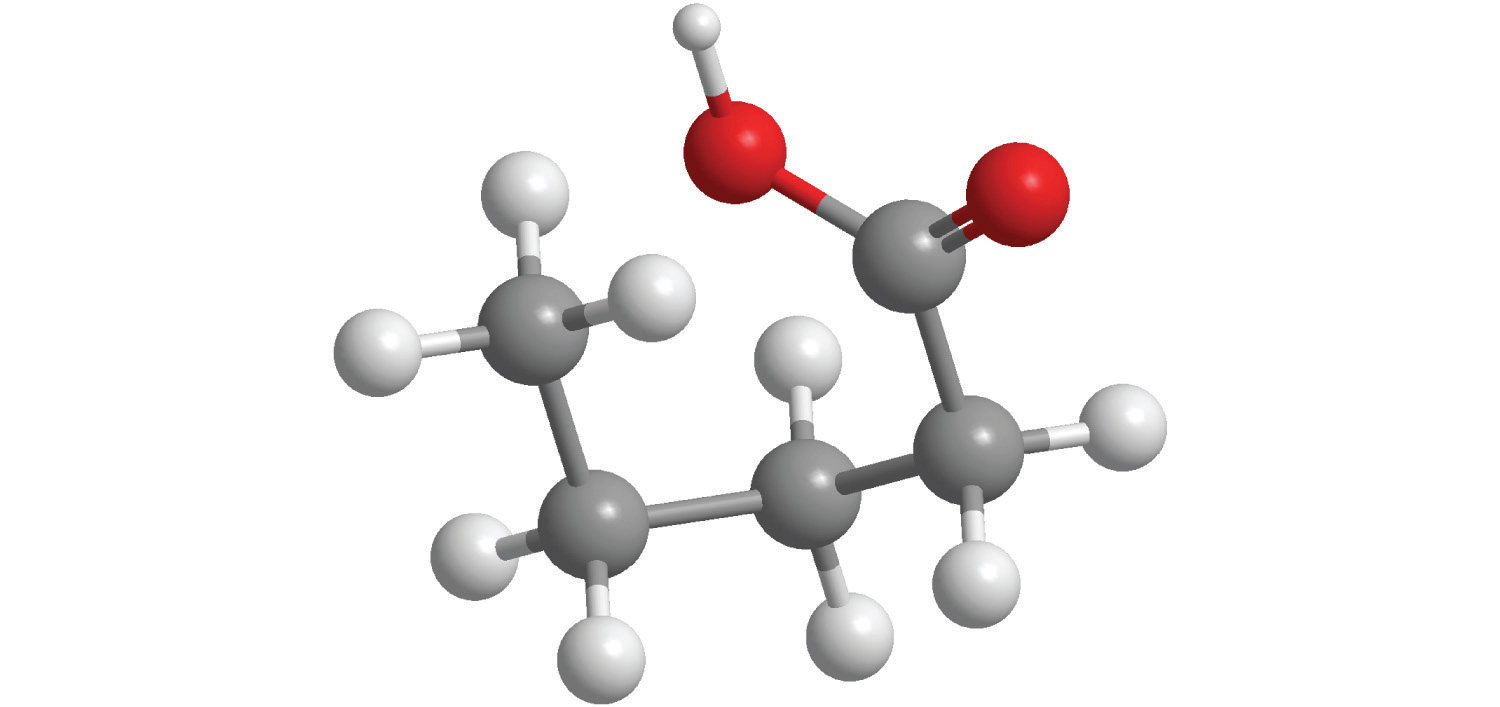
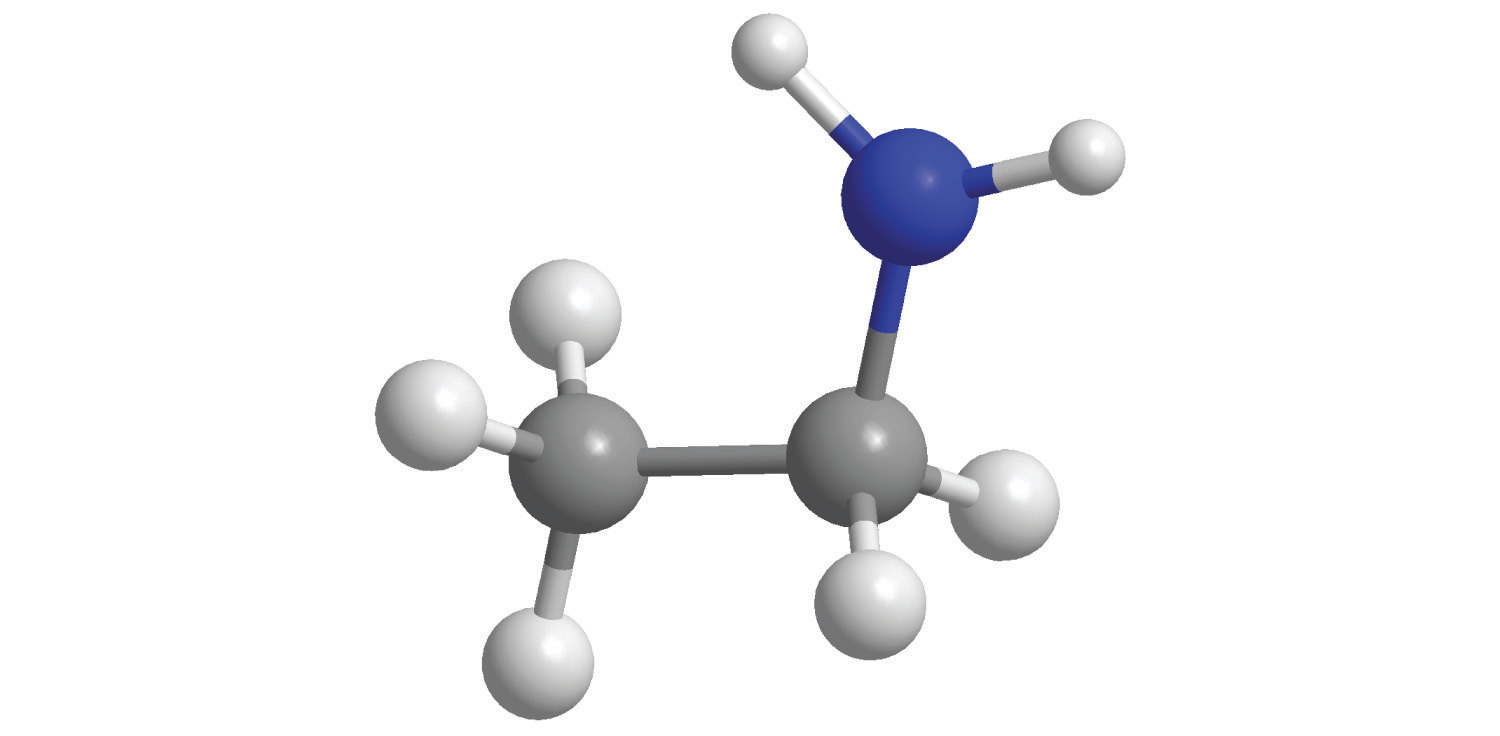
7. For each compound, write the condensed formula, name the compound, and give its molar mass.
8. Calculate the number of moles in 5.00 × 10 2 g of each substance. How many molecules or formula units are present in each sample?
a. CaO (lime)
b. CaCO 3 (chalk)
c. C 12 H 22 O 11 [sucrose (cane sugar)]
d. NaOCl (bleach)
e. CO 2 (dry ice)
9. Calculate the mass in grams of each sample.
a. 0.520 mol of N 2 O 4
b. 1.63 mol of C 6 H 4 Br 2
c. 4.62 mol of (NH 4 ) 2 SO 3
10. Give the number of molecules or formula units in each sample.
a. 1.30 × 10 −2 mol of SCl 2
b. 1.03 mol of N 2 O 5
c. 0.265 mol of Ag 2 Cr 2 O 7
11. Give the number of moles in each sample.
a. 9.58 × 10 26 molecules of Cl 2
b. 3.62 × 10 27 formula units of KCl
c. 6.94 × 10 28 formula units of Fe(OH) 2
12. Solutions of iodine are used as antiseptics and disinfectants. How many iodine atoms correspond to 11.0 g of molecular iodine (I 2 )?
13. What is the total number of atoms in each sample?
a. 0.431 mol of Li
b. 2.783 mol of methanol (CH 3 OH)
c. 0.0361 mol of CoCO 3
d. 1.002 mol of SeBr 2 O
14. What is the total number of atoms in each sample?
a. 0.980 mol of Na
b. 2.35 mol of O 2
c. 1.83 mol of Ag 2 S
d. 1.23 mol of propane (C 3 H 8 )
15. What is the total number of atoms in each sample?
a. 2.48 g of HBr
b. 4.77 g of CS 2
c. 1.89 g of NaOH
d. 1.46 g of SrC 2 O 4
16. Decide whether each statement is true or false and explain your reasoning.
- There are more molecules in 0.5 mol of Cl 2 than in 0.5 mol of H 2 .
- One mole of H 2 has 6.022 × 10 23 hydrogen atoms.
- The molecular mass of H 2 O is 18.0 amu.
- The formula mass of benzene is 78 amu.
17. Complete the following table.
18. Give the formula mass or the molecular mass of each substance.
- \(Cu_2P_2O_7\)
- \(BiONO_3\)
- \(Tl_2SeO_4\)
19. Give the formula mass or the molecular mass of each substance.
- \(UO_2CO_3\)
- \(NH_4UO_2AsO_4\)
- While both are units of mass, a gram is Avogadro’s number of atomic mass units so you would multiply the number of amu by 6.022x10^23 to find total number of grams
- The correct way to state formula mass of ethanol is to show the units of mass which is amu.
- No because moles and weight operate on different set of standards meaning that they’re not equal to each other. This means that moles of different compounds contain different weights. For example, 2 moles of Na = 2 x 22.989 g = 45.98g while 1 mole of Cl = 1 x 35.453 g = 35.453 g Cl. This makes the sodium react completely with chlorine. 2g of sodium would react with = (35.453/45.978) x 2 = 1.542 g Cl
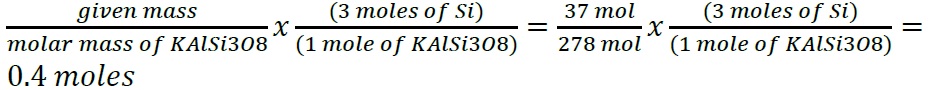
- The information required to determine the mass of the solute would be the molarity of the solution because once that is achieved, volume of the solution and molar mass of the solute can be used to calculate the total mass. A derivatization that achieves this goes as: Molarity = moles of solute / volume of solution in liter -> Moles = molarity x volume in liter -> Mass= moles x molar mass.
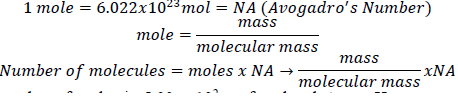
a. 153.82 g/mol
b. 80.06 g/mol
c. 92.01 g/mol
d. 70.13 g/mol
e. 74.12 g/mol
a. 92.45 g/mol
b. 135.04 g/mol
c. 44.01 g/mol
d. 40.06 g/mol
a. C 5 H 10 O 2 , Valeric Acid, 102.13 g/mol
b. H 3 PO 3, Phosphorous acid, 82 g/mol
a. C 2 H 5 NH 2, Ethylamine, 45.08 g/mol
b. HIO 3, Iodic acid, 175.91 g/mol
a. 5.37 × 10 24 mol
b. 3.01 × 10 24 mol
c. 8.80 × 10 23 mol
d. 4.04 × 10 24 mol
e. 6.84 × 10 24 mol
a. 47.85 grams
b. 384.52 grams
c. 536.57 grams
a. 7.83x10 21 molecules
b. 6.20x10 23 molecules
c. 1.60x10 23 molecules
a. 1590.8 moles
b. 6011.3 moles
c. 115244.1 moles
2.61 x10 22 molecules
a. 2.60x10 23 atoms
b. 1.01x10 25 atoms
c. 1.09x10 23 atoms
d. 2.41x10 24 atoms
a. 5.9x10 23 atoms
b. 2.8x10 24 atoms
c. 3.31x10 24 atoms
d. 8.15x10 24 atoms
a. 3.69x10 22 atoms
b. 1.13x10 23 atoms
c. 8.54x10 22 atoms
d. 3.50x10 23 atoms
a. False, the number of molecules in 0.5 mol Cl2 are the same amount of molecules in H2
b. False, the number of molecules in H2 is 2 x (6.022 x10^23) H atoms
c. True, 2 H (1.01 amu) + 1 O (16.01) = 18.0 amu
d. True, C6H6 -> 12(6) + 1(6) = 78 amu
17. Complete the following table
b. 2.36x10^23
c. 7.08x10^23
e. 1.71x10^24
f. 8.55x10^24
i. 5.36x10^26
n. 7.77x10^23
o. 5.44x10^24
q. 8.27x10^22
r. 1.65x10^24
u. 1.23x10^2
3.6: Empirical Formulas from Analysis
3.6: quantitative information from balanced equations.
- What information is required to determine the mass of solute in a solution if you know the molar concentration of the solution?
- Refer to the Breathalyzer test described in Example 8. How much ethanol must be present in 89.5 mL of a person’s breath to consume all the potassium dichromate in a Breathalyzer ampul containing 3.0 mL of a 0.40 mg/mL solution of potassium dichromate?
- Phosphoric acid and magnesium hydroxide react to produce magnesium phosphate and water. If 45.00 mL of 1.50 M phosphoric acid are used in the reaction, how many grams of magnesium hydroxide are needed for the reaction to go to completion?
- Barium chloride and sodium sulfate react to produce sodium chloride and barium sulfate. If 50.00 mL of 2.55 M barium chloride is used in the reaction, how many grams of sodium sulfate are needed for the reaction to go to completion?
- How many grams of sodium phosphate are obtained in solution from the reaction of 75.00 mL of 2.80 M sodium carbonate with a stoichiometric amount of phosphoric acid? The second product is water; what is the third product? How many grams of the third product are obtained?
- How many grams of ammonium bromide are produced from the reaction of 50.00 mL of 2.08 M iron(II) bromide with a stoichiometric amount of ammonium sulfide? What is the second product? How many grams of the second product are produced?
- The information required to determine the mass of the solution with a known molar concentration are the molar mass of the solute and the volume of the solute.
- 5.905 g Mg(OH) 2
- 18.105 g Na 2 SO 4
- Third Product: CO 2, Grams of Third Product: 9.24 g, Grams of Sodium Phosphate: 22.26g
- Grams Produced: 20.37 grams NH 4 Br 2 , Second Product: Iron (II) Sulfide, 9.15 grams FeS
3.7: LIMITING REACTANTS
- Engineers use conservation of mass, called a “mass balance,” to determine the amount of product that can be obtained from a chemical reaction. Mass balance assumes that the total mass of reactants is equal to the total mass of products. Is this a chemically valid practice? Explain your answer.
- Given the equation \[2H_{2\;(g)} + O_{2\;(g)} \rightarrow 2H+2O_{(g)},\] is it correct to say that 10 g of hydrogen will react with 10 g of oxygen to produce 20 g of water vapor?
- What does it mean to say that a reaction is stoichiometric?
- When sulfur is burned in air to produce sulfur dioxide, what is the limiting reactant? Explain your answer.
- Is it possible for the percent yield to be greater than the theoretical yield? Justify your answer.
Please be sure you are familiar with the topics discussed in Essential Skills 2 () before proceeding to the Numerical Problems.
12. Write a balanced chemical equation for each reaction and then determine which reactant is in excess.
a. 2.46 g barium(s) plus 3.89 g bromine(l) in water to give barium bromide
b. 1.44 g bromine(l) plus 2.42 g potassium iodide(s) in water to give potassium bromide and iodine
c. 1.852 g of Zn metal plus 3.62 g of sulfuric acid in water to give zinc sulfate and hydrogen gas
d. 0.147 g of iron metal reacts with 0.924 g of silver acetate in water to give iron(II) acetate and silver metal
e. 3.142 g of ammonium phosphate reacts with 1.648 g of barium hydroxide in water to give ammonium hydroxide and barium phosphate
13. Under the proper conditions, ammonia and oxygen will react to form dinitrogen monoxide (nitrous oxide, also called laughing gas) and water. Write a balanced chemical equation for this reaction. Determine which reactant is in excess for each combination of reactants.
a. 24.6 g of ammonia and 21.4 g of oxygen
b. 3.8 mol of ammonia and 84.2 g of oxygen
c. 3.6 × 10 24 molecules of ammonia and 318 g of oxygen
d. 2.1 mol of ammonia and 36.4 g of oxygen
14. When a piece of zinc metal is placed in aqueous hydrochloric acid, zinc chloride is produced, and hydrogen gas is evolved. Write a balanced chemical equation for this reaction. Determine which reactant is in excess for each combination of reactants.
a. 12.5 g of HCl and 7.3 g of Zn
b. 6.2 mol of HCl and 100 g of Zn
c. 2.1 × 10 23 molecules of Zn and 26.0 g of HCl
d. 3.1 mol of Zn and 97.4 g of HCl
15. Determine the mass of each reactant needed to give the indicated amount of product. Be sure that the chemical equations are balanced.
a. NaI(aq) + Cl 2 (g) → NaCl(aq) + I 2 (s); 1.0 mol of NaCl
b. NaCl(aq) + H 2 SO 4 (aq) → HCl(g) + Na 2 SO 4 (aq); 0.50 mol of HCl
c. NO 2 (g) + H 2 O(l) → HNO 2 (aq) + HNO 3 (aq); 1.5 mol of HNO 3
16. Determine the mass of each reactant needed to give the indicated amount of product. Be sure that the chemical equations are balanced.
a. AgNO 3 (aq) + CaCl 2 (s) → AgCl(s) + Ca(NO 3 ) 2 (aq); 1.25 mol of AgCl
b. Pb(s) + PbO 2 (s) + H 2 SO 4 (aq) → PbSO 4 (s) + H 2 O(l); 3.8 g of PbSO 4
c. H 3 PO 4 (aq) + MgCO 3 (s) → Mg 3 (PO 4 ) 2 (s) + CO 2 (g) + H 2 O(l); 6.41 g of Mg 3 (PO 4 ) 2
17. Determine the percent yield of each reaction. Be sure that the chemical equations are balanced. Assume that any reactants for which amounts are not given are in excess. (The symbol Δ indicates that the reactants are heated.)
a. KClO 3 (s) \(\underrightarrow {\Delta} \) KCl(s)+O 2 (g);2.14 g of KClO 3 produces 0.67 g of O 2
b. Cu(s) + H 2 SO 4 (aq) → CuSO 4 (aq) + SO 2 (g) + H 2 O(l); 4.00 g of copper gives 1.2 g of sulfur dioxide
c. AgC 2 H 3 O 2 (aq) + Na 3 PO 4 (aq) → Ag 3 PO 4 (s) + NaC 2 H 3 O 2 (aq); 5.298 g of silver acetate produces 1.583 g of silver phosphate
18. Each step of a four-step reaction has a yield of 95%. What is the percent yield for the overall reaction?
19. A three-step reaction yields of 87% for the first step, 94% for the second, and 55% for the third. What is the percent yield of the overall reaction?
20. Give a general expression relating the theoretical yield (in grams) of product that can be obtained from x grams of B, assuming neither A nor B is limiting.
A + 3B → 2C
21. Under certain conditions, the reaction of hydrogen with carbon monoxide can produce methanol.
a. Write a balanced chemical equation for this reaction.
b. Calculate the percent yield if exactly 200 g of methanol is produced from exactly 300 g of carbon monoxide.
22. Chlorine dioxide is a bleaching agent used in the paper industry. It can be prepared by the following reaction:
NaClO 2 (s) + Cl 2 (g) → ClO 2 (aq) + NaCl(aq)
a. What mass of chlorine is needed for the complete reaction of 30.5 g of NaClO 2 ?
b. Give a general equation for the conversion of x grams of sodium chlorite to chlorine dioxide.
23. The reaction of propane gas (CH 3 CH 2 CH 3 ) with chlorine gas (Cl 2 ) produces two monochloride products: CH 3 CH 2 CH 2 Cl and CH 3 CHClCH 3 . The first is obtained in a 43% yield and the second in a 57% yield.
a. If you use 2.78 g of propane gas, how much chlorine gas would you need for the reaction to go to completion?
b. How many grams of each product could theoretically be obtained from the reaction starting with 2.78 g of propane?
c. Use the actual percent yield to calculate how many grams of each product would actually be obtained.
24. Protactinium (Pa), a highly toxic metal, is one of the rarest and most expensive elements. The following reaction is one method for preparing protactinium metal under relatively extreme conditions:
\[ \ce{2PaI_5 (s) ->[\Delta] 2Pa (s) + 5I_2 (s)} \nonumber \]
a. Given 15.8 mg of reactant, how many milligrams of protactinium could be synthesized?
b. If 3.4 mg of Pa was obtained, what was the percent yield of this reaction?
c. If you obtained 3.4 mg of Pa and the percent yield was 78.6%, how many grams of PaI5 were used in the preparation?
25. Aniline (C 6 H 5 NH 2 ) can be produced from chlorobenzene (C 6 H 5 Cl) via the following reaction:
C 6 H 5 Cl(l) + 2NH 3 (g) → C 6 H 5 NH 2 (l) + NH 4 Cl(s)
Assume that 20.0 g of chlorobenzene at 92% purity is mixed with 8.30 g of ammonia.
a. Which is the limiting reactant?
b. Which reactant is present in excess?
c. What is the theoretical yield of ammonium chloride in grams?
d. If 4.78 g of NH 4 Cl was recovered, what was the percent yield?
e. Derive a general expression for the theoretical yield of ammonium chloride in terms of grams of chlorobenzene reactant, if ammonia is present in excess.
26. A stoichiometric quantity of chlorine gas is added to an aqueous solution of NaBr to produce an aqueous solution of sodium chloride and liquid bromine. Write the chemical equation for this reaction. Then assume an 89% yield and calculate the mass of chlorine given the following:
a. 9.36 × 10 24 formula units of NaCl
b. 8.5 × 10 4 mol of Br 2
c. 3.7 × 10 8 g of NaCl
- It is not a chemically valid practice because the law of conservation of mass applies to closed/isolated systems only, so in this case of an open system converting mass into energy and allowing it to escape the system wouldn’t be valid.
- No it is not correct because in order to examine the molecular weight you need to take log of both molecules to see what there true weight is. In this case, log of O2 = 10g/(32 g/mol) = 0.3125 g/mol while log of H2 = 10g/(2 g/mol) = 5 g/mol. With that when hydrogen and oxygen react against each other ((2 x (0.3125 g/L)) x (18 g/mol H2O)) = 11.25 g of water vapor
- When a reaction is stoichiometric it means that the none of the reactants remain after being introduced leaving no excess or total leaving quantity.
- From a reaction of S + O 2 -> SO 2 we’re able to determine that the limiting reactant would be sulfur because the amount of O 2 in the air is higher than the amount of sulfur that is burned in the reaction.
- Yes it is possible, given the equation of: percent yield = (actual yield)/(Theoretical Yield) its entirely possible to have the percent yield become greater than the theoretical yield when the product of the reaction has impurities that increase the mass compared if the product was pure.
a. Ba(s) + Br 2 (l) = BaBr 2 (aq)
Reactant in excess: Br 2
b. Br 2 (l) + 2KI(s) = 2KBr + I 2
Reactant in Excess: Br 2
c. Zn + H 2 SO 4 = ZnSO 4 + H 2
Reactant in excess: H 2 SO 4
d. Fe + 2 AgC 2 H 3 O 2 = Fe(C 2 H 3 O 2 ) 2 + 2 Ag
Reactant in excess: AgC 2 H 3 O 2
e. 2 (NH 4 ) 3 PO 4 + 3 Ba(OH) 2 = 6 NH 4 OH + Ba 3 (PO 4 ) 2
Reactant in excess: Ba(OH) 2
13. The balanced chemical equation for this reaction is
2NH 3 + 2O 2 → N 2 O + 3H 2 O
14. a. Excess: HCl
b. Excess: HCl
c. Excess: HCl
d. Excess: Zn
a. 150 g NaI and 35 g Cl 2
b. 29 g NaCl and 25 g H 2 SO 4
c. 140 g NO 2 and 27 g H 2 O
a. 2 AgNO 3 (aq) + CaCl 2 (s) = 2 AgCl(s) + Ca(NO 3 ) 2 (aq); Mass of Reactant: 212.34g AgNO3 and 69.4 g CaCl2
b. Pb(s) + PbO 2 (s) + 2 H 2 SO 4 (aq) = 2 PbSO 4 (s) + 2 H 2 O(l); Mass of Reactant: 1.3g Pb, 1.5g PbO 2 , 1.2g H 2 SO 4
c. 2 H 3 PO 4 (aq) + 3 MgCO 3 (s) = Mg 3 (PO 4 ) 2 (s) + 3 CO 2 (g) + 3 H 2 O(l); Mass of Reactant: 6.17 g of MgCO 3 , 4.78g of H 3 PO 4
20. (x/molecular mass of B) x (2/3) x (molecular mass of C)
a. CO + 2H 2 → CH 3 OH
22. 2 NaClO 2 (s) + Cl 2 (g) → ClO 2 (aq) + NaCl(aq)
a. 11.9 g Cl 2
b. (0.7458g= Grams of ClO2 = (x/(90.44 g/mol)) x (2/2) x (67.45 g ClO2/ 1 mol ClO2
a. 2.24 g Cl 2
c. 2.13 g CH 3 CH 2 CH 2 Cl plus 2.82 g CH 3 CHClCH 3
24. a. 4.14mg Pa
c. 0.0162 g PaI5
a. chlorobenzene
c. 8.74 g ammonium chloride.
e. \(Theoretical \, yield \, (NH_4Cl) = {mass \, of \, chlorobenzene \, (g) \times 0.92 \times \times 53.49 \, g/mol \over 112.55 \, g/mol} \)
a. 6619.19g Cl 2
b. 6.77 x 10 6 g Cl 2
c. 2.52 x 10 8 g Cl 2
Conceptual Problems II
Numerical problems ii.
\[ 2PaI_5 (s) \underrightarrow {\Delta} 2Pa (s) + 5I_2 (s) \]
Numerical Answers II

IMAGES
VIDEO
COMMENTS
Answers: 1. 116 g AgCl 5. 1.40 x 1019 molecules CS 2 9. 4.63 x 10 24 molecules I 2 13. 3.97 x 10 ... Chemistry: Stoichiometry - Problem Sheet 2 KEY 9) 2 24 2 2 23 2 2 2 2 4.63 x 10molecules I 1 mol I 6.02 x 10 moleculesI 1 mol Cl 1mol 71 g Cl Cl x 546 g Cl
KEY Chemistry: Stoichiometry ... 2 mol Al O x g O 3.75 mol Al Answers: 1A. 30 mol Ag 1C. 20 mol H 2 O 2A. 38 mol N 2 H 4 2C. 76 mol H 2 O 1B. 30 mol AgNO 3 1D. 10 mol NO 2B. 19 mol N 2 O 4 3. 191 g Al 2 O 3. 4. At a very high temperature, manganese is isolated from its ore, manganomanganic oxide, via the following
Stoichiometry Practice Problems. This is a comprehensive, end-of-chapter set of practice problems on stoichiometry that covers balancing chemical equations, mole-ratio calculations, limiting reactants, and percent yield concepts. The links to the corresponding topics are given below. The Mole and Molar Mass.
2 O 3 is used, how many kilograms of iron can be produced? The reaction is: Fe 2 O 3 + 3 C ---> 2 Fe + 3 CO 9. The average human requires 120.0 grams of glucose (C 6 H 12 O 6) per day. How many grams of CO 2 (in the photosynthesis reaction) are required for this amount of glucose? The photosynthetic reaction is: 6 CO 2 + 6 H 2 O ---> C 6 H 12 O ...
By Chemistry Corner. Everything you need for a successful Chemical Reactions Unit! Six complete lessons: each lesson comes with student notes, detailed teacher notes, check for understanding exit tickets, and homework. Also included in this Mega Unit Bundle is two. Subjects: Chemistry, Physical Science.
PROBLEM 5.2.1.1 5.2.1. 1. Write the balanced equation and determine the information requested. Don't worry about state symbols in these reactions. The number of moles and the mass (in grams) of chlorine, Cl 2, required to react with 10.0 g of sodium metal, Na, to produce sodium chloride, NaCl. The number of moles and the mass (in milligrams) of ...
Answer. The empirical formula is BH 3. The molecular formula is B 2 H 6. This page titled 4.E: Stoichiometry of Chemical Reactions- Homework is shared under a CC BY license and was authored, remixed, and/or curated by Scott Van Bramer. End of chapter homework problems for Chapter \ (\PageIndex {1}\).
Q4. Given the following reaction: H2SO4 + Na2CO3 → Na2SO4 +H2O + CO2 H 2 S O 4 + N a 2 C O 3 → N a 2 S O 4 + H 2 O + C O 2. Calculate the molarity of the H2SO4 H 2 S O 4 solution if it takes 40.0 mL of H2SO4 H 2 S O 4 to neutralize 46.7 mL of a 0.364 M Na2CO3 N a 2 C O 3 solution.
(2) Graphic Organizers: The Mole, and Stoichiometry Conversions (2) Homework/Practice assignments w/KEY—all problems completely worked out; Quiz w/KEY; Check for Understanding (exit ticket) w/KEY ; Teacher Notes pages (3 pages) Slide-by-slide background notes for the PowerPoint. Note on the PowerPoint: The PowerPoint included in this product ...
Stoichiometry Part 2 Answer Key. View in Full Screen. Back to Stoichiometry Part 2 Worksheet. Back to Worksheets. Back to Stoichiometry, Limiting Reactants, and Percent Yield Study Guide. *.
The lesson addresses conversions involving mass, number of particles, and liters @ STP. Objectives: Define stoichiometry. Describe the function of the mole to mole conversion factor. Write mole to mole conversion factors for a chemical equation. Calculate the amount of product or reactant in moles, mass, or liters from an amount of a different ...
The Stoichiometry practice is a 4-page worksheet that allows students to feel confident in their ability to determine how much product can be produced from a given amount of reactant. The answer key has detailed answers for every problem that includes each step in finding the solution.It is scaffold...
Find step-by-step solutions and answers to Pearson Chemistry - 9780132525763, as well as thousands of textbooks so you can move forward with confidence. ... Section 1.2: Chemistry and You. Section 1.3: Thinking Like a Scientist. Section 1.4: Problem Solving in Chemistry. Page 28: ... Stoichiometry. Section 12.1: The Arithmetic of Equations ...
www.njctl.org Chemistry Stoichiometry Homework Set 4: 1. No one likes cockroaches. Since the 1970's exterminators have used a chemical called diazinon, ... www.njctl.org Chemistry Stoichiometry ANSWER KEY!!! Classwork Set 1: 1) 2C 2 H 6 + 7O 2--> 4CO 2 + 6H 2 O a) How many moles of O 2 are required to react with 24 moles of C 2 H 6? 84 moles
A stoichiometric quantity of chlorine gas is added to an aqueous solution of NaBr to produce an aqueous solution of sodium chloride and liquid bromine. Write the chemical equation for this reaction. Then assume an 89% yield and calculate the mass of chlorine given the following: a. 9.36 × 10 24 formula units of NaCl.
Why was 2 mol CaO/2mol SO 2 included in the second example if it did not affect the final number? Answers. Gases are at STP. Units will cancel and you will be left with only the desired units for the problem. It did affect the units. If it were missing the SO 2 values would not have cancelled and the CaO would have cancelled. 12 Limiting Reactant
Student Answer Sheets (2)- one for basic stoichiometry cards, and one for cards with limiting and excess reactants/percent yield cards) Complete Key for both sets of task cards with problems completely worked out. Teacher Notes, tips, and strategies for setting up this activity. (2 pages) You will want to save this lesson and use it for years ...
You will also have access to all of the support materials that you will need. Curriculum Guide, Pacing Guide, Unit Plans, NGSS & TEKs Correlations, Mid-Term and Finals Exams. BONUS: You will have access to the Chemistry Corner Community. Meet and collaborate with chemistry teachers from around the world in our robust community!
Our mission is to improve educational access and learning for everyone. OpenStax is part of Rice University, which is a 501 (c) (3) nonprofit. Give today and help us reach more students. This free textbook is an OpenStax resource written to increase student access to high-quality, peer-reviewed learning materials.
Objectives: • Define limiting and excess reactants. • Use stoichiometric calculations to determine the limiting and excess reactant. • Determine the amount of excess reactant remaining after reaction has run. • Using the limiting reactant, use stoichiometric calculations to determine the amount of product formed.
View Stoichiometry Worksheet 2.docx from CHEM 1411 at Lone Star College System, North Harris. CHEM 1411 Lone Star College - Kingwood STOICHIOMETRY WORKSHEET #2 INSTRUCTIONS Please show all work on a
This substantial bundle contains everything you would need for a complete advanced chemistry course: at least two sets of PowerPoints, the Guided Practice Questions & answer key to engage students during instruction, an average of 4-6 review documents (& answer keys), a list of key terms, an. 244. Products. $475.00 $521.00 Save $46.00.
A stoichiometric quantity of chlorine gas is added to an aqueous solution of NaBr to produce an aqueous solution of sodium chloride and liquid bromine. Write the chemical equation for this reaction. Then assume an 89% yield and calculate the mass of chlorine given the following: a. 9.36 × 10 24 formula units of NaCl.