- Today's news
- Reviews and deals
- Climate change
- 2024 election
- Fall allergies
- Health news
- Mental health
- Sexual health
- Family health
- So mini ways
- Unapologetically
- Buying guides

Entertainment
- How to Watch
- My Portfolio
- Stock Market
- Biden Economy
- EV Deep Dive
- Stocks: Most Actives
- Stocks: Gainers
- Stocks: Losers
- Trending Tickers
- World Indices
- US Treasury Bonds
- Top Mutual Funds
- Highest Open Interest
- Highest Implied Volatility
- Stock Comparison
- Advanced Charts
- Currency Converter
- Investment Ideas
- Research Reports
- Basic Materials
- Communication Services
- Consumer Cyclical
- Consumer Defensive
- Financial Services
- Industrials
- Real Estate
- Mutual Funds
- Credit Cards
- Balance transfer cards
- Cash-back cards
- Rewards cards
- Travel cards
- Personal Loans
- Student Loans
- Car Insurance
- Morning Brief
- Market Domination
- Market Domination Overtime
- Opening Bid
- Stocks in Translation
- Lead This Way
- Good Buy or Goodbye?
- Fantasy football
- Pro Pick 'Em
- College Pick 'Em
- Fantasy baseball
- Fantasy hockey
- Fantasy basketball
- Download the app
- Daily fantasy
- Scores and schedules
- GameChannel
- World Baseball Classic
- Premier League
- CONCACAF League
- Champions League
- Motorsports
- Horse racing
- Newsletters
New on Yahoo
- Privacy Dashboard
Yahoo Finance
Curevac and md anderson enter strategic collaboration to develop novel cancer vaccines.
Agreement creates strong synergies between CureVac's unique end-to-end mRNA capabilities and MD Anderson's translational and clinical research expertise
Collaboration aims to develop novel, off-the-shelf, mRNA-based cancer vaccines in selected hematological and solid cancers with high unmet medical need
MD Anderson responsible for leading initial Phase 1/2 studies; CureVac retains worldwide exclusive rights to late-stage development, commercialization, or partnering of cancer vaccine candidates
TÜBINGEN, GERMANY and HOUSTON, TX / ACCESSWIRE / April 16, 2024 / CureVac N.V. (Nasdaq:CVAC) ("CureVac"), a global biopharmaceutical company developing a new class of transformative medicines based on messenger ribonucleic acid ("mRNA"), and The University of Texas MD Anderson Cancer Center today announced a co-development and licensing agreement to develop novel mRNA-based cancer vaccines.
The collaboration creates strong synergies between CureVac's unique end-to-end capabilities for cancer antigen discovery, mRNA design, and manufacturing and MD Anderson's expertise in cancer antigen discovery and validation, translational drug development, and clinical research. The collaboration will focus on the development of differentiated cancer vaccine candidates in selected hematological and solid tumor indications with high unmet medical need.
"We look forward to collaborating with the team at MD Anderson to push the boundaries of mRNA technology and develop impactful therapeutic options for patients in need," said Dr. Alexander Zehnder, Chief Executive Officer of CureVac. "In combining our respective expertise, we believe we can go further and faster to develop novel, off-the-shelf, mRNA-based cancer vaccines that have the potential to significantly improve patient outcomes."
Both parties will contribute to the identification of differentiated cancer antigens based on whole genome sequencing, combined with long- and short-read RNA sequencing and cutting-edge bioinformatics. Joint preclinical validation of the highest-quality cancer antigens will be supported by Sachet Shukla, Ph.D., Assistant Professor of Hematopoietic Biology & Malignancy and director of the department's cancer vaccine program, and by MD Anderson's ECLIPSE (Evolution of Cancer, Leukemia, and Immunity Post Stem cEll transplant) platform, part of the institution's Therapeutics Discovery division.
"We are excited for cancer vaccines to potentially emerge as an essential therapeutic tool in the future," Shukla said. "This collaboration with CureVac is an important milestone in our efforts and brings together complementary strengths toward our goal of developing transformative vaccines for cancer."
Following selection of the most promising validated vaccine candidates and completion of Investigational New Drug (IND) approvals, MD Anderson will be responsible for conducting initial Phase 1/2 studies in appropriate clinical indications.
"Our ECLIPSE team uses proprietary high-throughput technology to identify and validate immune targets, and we are driven to advance impactful immunotherapies with the potential to transform the lives of patients with cancer," said Jeffrey Molldrem, M.D., chair of Hematopoietic Biology and Malignancy and leader of the ECLIPSE platform at MD Anderson. "Together with CureVac, we hope to embrace this exciting area of drug discovery and development in pursuit of mRNA vaccines that will address significant unmet medical need."
Under the terms of the collaboration agreement, CureVac and MD Anderson will jointly contribute to and support development of those programs designated to move forward. CureVac has worldwide exclusive rights to late-stage development, commercialization, or partnering of the cancer vaccine candidates. MD Anderson is eligible for certain downstream payments based on potential future commercialization.
About CureVac
CureVac (Nasdaq:CVAC) is a global biopharmaceutical company in the field of messenger RNA (mRNA) technology, with more than 20 years of expertise in developing, optimizing, and manufacturing this versatile biological molecule for medical purposes. The principle of CureVac's proprietary technology is the use of optimized mRNA as a data carrier to instruct the human body to produce its own proteins capable of fighting a broad range of diseases. In July 2020, CureVac entered in a collaboration with GSK to jointly develop new products in prophylactic vaccines for infectious diseases based on CureVac's second-generation mRNA technology. This collaboration was later extended to the development of second-generation COVID-19 vaccine candidates, and modified mRNA vaccine technologies. Based on its proprietary technology, CureVac has built a deep clinical pipeline across the areas of prophylactic vaccines, cancer therapies, antibody therapies, and the treatment of rare diseases. CureVac N.V. has its headquarters in Tübingen, Germany, and has more than 1,100 employees across its sites in Germany, the Netherlands, Belgium, Switzerland, and the U.S. Further information can be found at www.curevac.com .
CureVac Media Contact
Patrick Perez, Junior Manager Public Relations CureVac, Tübingen, Germany T: +49 7071 9883-1831 [email protected]
CureVac Investor Relations Contact
Dr. Sarah Fakih, Vice President Corporate Communications and Investor Relations CureVac, Tübingen, Germany T: +49 7071 9883-1298 M: +49 160 90 496949 [email protected]
About MD Anderson
The University of Texas MD Anderson Cancer Center in Houston ranks as one of the world's most respected centers focused on cancer patient care, research, education and prevention. The institution's sole mission is to end cancer for patients and their families around the world, and, in 1971, it became one of the nation's first National Cancer Institute (NCI)-designated comprehensive cancer centers. MD Anderson is No. 1 for cancer in U.S. News & World Report's "Best Hospitals" rankings and has been named one of the nation's top two hospitals for cancer since the rankings began in 1990. MD Anderson receives a cancer center support grant from the NCI of the National Institutes of Health (P30 CA016672).
MD Anderson Media Contact
Clayton Boldt, Ph.D. Public Relations [email protected] +1-713-792-9518
Forward-Looking Statements CureVac
This press release contains statements that constitute "forward looking statements" as that term is defined in the United States Private Securities Litigation Reform Act of 1995, including statements that express the opinions, expectations, beliefs, plans, objectives, assumptions or projections of CureVac N.V. and/or its wholly owned subsidiaries CureVac SE, CureVac Manufacturing GmbH, CureVac Inc., CureVac Swiss AG, CureVac Corporate Services GmbH, CureVac RNA Printer GmbH, CureVac Belgium SA and CureVac Netherlands B.V. (the "company") regarding future events or future results, in contrast with statements that reflect historical facts. Examples include discussion of the potential efficacy of the company's vaccine and treatment candidates and the company's strategies, financing plans, growth opportunities and market growth. In some cases, you can identify such forward-looking statements by terminology such as "anticipate," "intend," "believe," "estimate," "plan," "seek," "project," or "expect," "may," "will," "would," "could," "potential," "intend," or "should," the negative of these terms or similar expressions. Forward-looking statements are based on management's current beliefs and assumptions and on information currently available to the company. However, these forward-looking statements are not a guarantee of the company's performance, and you should not place undue reliance on such statements. Forward-looking statements are subject to many risks, uncertainties and other variable circumstances, including negative worldwide economic conditions and ongoing instability and volatility in the worldwide financial markets, ability to obtain funding, ability to conduct current and future preclinical studies and clinical trials, the timing, expense and uncertainty of regulatory approval, reliance on third parties and collaboration partners, ability to commercialize products, ability to manufacture any products, possible changes in current and proposed legislation, regulations and governmental policies, pressures from increasing competition and consolidation in the company's industry, the effects of the COVID-19 pandemic on the company's business and results of operations, ability to manage growth, reliance on key personnel, reliance on intellectual property protection, ability to provide for patient safety, fluctuations of operating results due to the effect of exchange rates, delays in litigation proceedings, different judicial outcomes or other factors. Such risks and uncertainties may cause the statements to be inaccurate and readers are cautioned not to place undue reliance on such statements. Many of these risks are outside of the company's control and could cause its actual results to differ materially from those it thought would occur. The forward-looking statements included in this press release are made only as of the date hereof. The company does not undertake, and specifically declines, any obligation to update any such statements or to publicly announce the results of any revisions to any such statements to reflect future events or developments, except as required by law.
For further information, please reference the company's reports and documents filed with the U.S. Securities and Exchange Commission (SEC). You may get these documents by visiting EDGAR on the SEC website at www.sec.gov .
SOURCE: CureVac
View the original press release on accesswire.com
Tissue Procurement Specialist - Institutional Tissue Bank - 9am-6pm
- Requisition #: 163118
- Department: Translational Molecular Path
- Location: Houston, TX
- Posted Date: 4/24/2024
- Requisition ID: 163118
- Employment Status: Full-Time
- Employee Status: Regular
- Work Week: Days
- Minimum Salary: US Dollar (USD) 36,500
- Midpoint Salary: US Dollar (USD) 45,500
- Maximum Salary : US Dollar (USD) 54,500
- FLSA: non-exempt and eligible for overtime pay
- Fund Type: Soft
- Work Location: Onsite
- Pivotal Position: No
- Referral Bonus Available?: No
- Relocation Assistance Available?: No
- Science Jobs: No
Be more at MD Anderson
- Applicant Rights & Notices
- EEO / Accessibility
- mdanderson.org
© 2024 The University of Texas MD Anderson Cancer Center

MD Anderson at Cooper Achieves Accreditation for Excellence in Radiation Oncology Services

“Receiving APEx accreditation from ASTRO, the largest radiation oncology society in the world, is an outstanding achievement by our Radiation Oncology team,” said Anthony Dragun, MD, chairman and chief of the Department of Radiation Oncology at MD Anderson at Cooper. “Our team was invested in evaluating our processes to meet ASTRO’s high standards for safety and quality. By achieving this accreditation, we demonstrate our unwavering commitment to deliver consistently safe patient-centered cancer care.”
“Seeking and achieving this voluntary APEx accreditation affirms our commitment to excellence in patient care and safety,” said Generosa Grana, MD, FACP, director of MD Anderson at Cooper. “This accreditation is a direct reflection of our entire team’s dedication and steadfast focus to provide the highest level of radiation oncology services at all of our locations.”
APEx, the fastest-growing radiation oncology practice accreditation program in the United States, is a voluntary, objective and rigorous multi-step process during which a radiation oncology practice is evaluated using consensus-based standards. The practice must demonstrate its safety and quality processes and show that it adheres to patient-centered care by promoting effective communication, coordinated treatments and strong patient engagement.
“ASTRO commends MD Anderson at Cooper for achieving APEx accreditation,” said Jeff M. Michalski, MD, MBA, FASTRO, chair of the ASTRO Board of Directors. “By undergoing this comprehensive review, the facility demonstrated their strong commitment to delivering safe, high-quality radiation oncology services to their patients.”
APEx is the only radiation oncology accreditation program that includes a self-assessment, which allows practices to internally assess compliance with quality improvement standards. The practice then has a facility review by an external surveyor team that includes a radiation oncologist and a medical physicist. The program reflects the recommendations endorsed in the ASTRO publication Safety is No Accident: A Framework for Quality Radiation Oncology and Care .To date, more than 300 U.S. facilities have earned APEx accreditation.
About MD Anderson Cancer Center at Cooper
MD Anderson Cancer Center at Cooper is South Jersey’s leading cancer center. Through our partnership with MD Anderson Cancer Center – a world-renowned cancer center – our patients have access to advanced cancer treatments. Our multidisciplinary, patient-centered approach to cancer care combines the expertise of our highly skilled cancer specialists with innovative diagnostic and treatment technologies and groundbreaking clinical trials.
MD Anderson at Cooper takes a disease-site-specific approach to cancer care. Experts from every area of cancer medicine work together to provide each patient with outstanding care from diagnosis to treatment, recovery, and beyond. Patients also have access to our full range of supportive care services throughout their cancer journey. Learn more at MDAndersonCooper.org .
Leave a Reply Cancel reply
DO NOT USE THIS FORM FOR APPOINTMENTS. Using this form will only delay your ability to get an appointment. Please use the contact information in the article or visit appointments.cooperhealth.org .

Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
Latest science news, discoveries and analysis

Bird flu in US cows: is the milk supply safe?

Future of Humanity Institute shuts: what's next for ‘deep future’ research?

Judge dismisses superconductivity physicist’s lawsuit against university

NIH pay raise for postdocs and PhD students could have US ripple effect
Hello puffins, goodbye belugas: changing arctic fjord hints at our climate future, china's moon atlas is the most detailed ever made, ‘shut up and calculate’: how einstein lost the battle to explain quantum reality, rat neurons repair mouse brains — and restore sense of smell, ecologists: don’t lose touch with the joy of fieldwork chris mantegna.

Should the Maldives be creating new land?

Lethal AI weapons are here: how can we control them?

Algorithm ranks peer reviewers by reputation — but critics warn of bias

How gliding marsupials got their ‘wings’
Audio long read: why loneliness is bad for your health, nato is boosting ai and climate research as scientific diplomacy remains on ice, plastic pollution: three numbers that support a crackdown, the maldives is racing to create new land. why are so many people concerned.
Retractions are part of science, but misconduct isn’t — lessons from a superconductivity lab

Any plan to make smoking obsolete is the right step


Citizenship privilege harms science
European ruling linking climate change to human rights could be a game changer — here’s how charlotte e. blattner, will ai accelerate or delay the race to net-zero emissions, current issue.

Surprise hybrid origins of a butterfly species
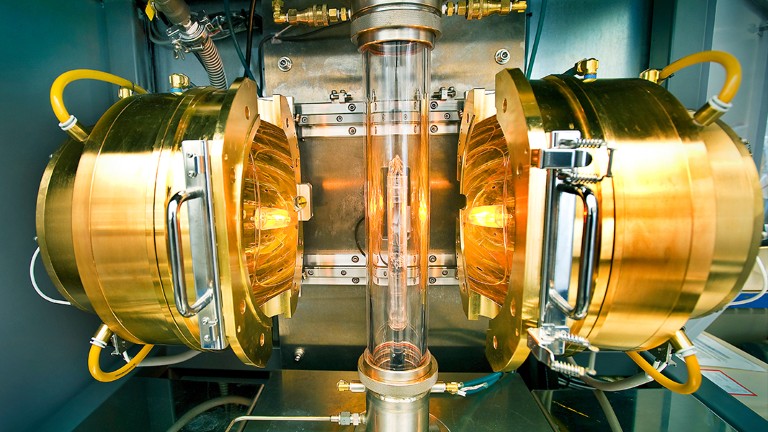
Stripped-envelope supernova light curves argue for central engine activity, optical clocks at sea, research analysis.

Ancient DNA traces family lines and political shifts in the Avar empire
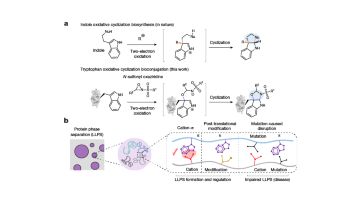
A chemical method for selective labelling of the key amino acid tryptophan
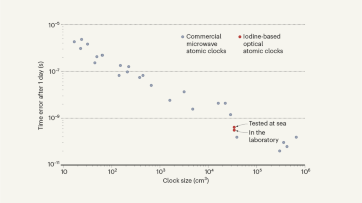
Robust optical clocks promise stable timing in a portable package
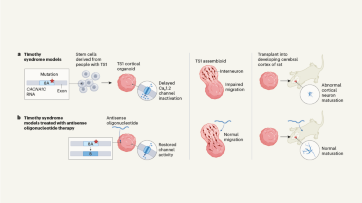
Targeting RNA opens therapeutic avenues for Timothy syndrome
Bioengineered ‘mini-colons’ shed light on cancer progression, galaxy found napping in the primordial universe, tumours form without genetic mutations, marsupial genomes reveal how a skin membrane for gliding evolved.

Scientists urged to collect royalties from the ‘magic money tree’

Breaking ice, and helicopter drops: winning photos of working scientists

Shrouded in secrecy: how science is harmed by the bullying and harassment rumour mill
How ground glass might save crops from drought on a caribbean island, londoners see what a scientist looks like up close in 50 photographs, books & culture.

How volcanoes shaped our planet — and why we need to be ready for the next big eruption

Dogwhistles, drilling and the roots of Western civilization: Books in brief

Cosmic rentals
Las borinqueñas remembers the forgotten puerto rican women who tested the first pill, dad always mows on summer saturday mornings, nature podcast.

Latest videos
Nature briefing.
An essential round-up of science news, opinion and analysis, delivered to your inbox every weekday.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Search all jobs
Current Employee

- All Job Categories
( While navigating through the site, please be sure to disable your pop-up blocker . )
Clinical research scientist, gi med oncology - research, 🔍 united states, texas, houston, houston (tx med ctr).
Required: PhD or Medical degree.
EXPERIENCE:
Required: None.
Preferred: Prior Clinical Research
It is the policy of The University of Texas MD Anderson Cancer Center to provide equal employment opportunity without regard to race, color, religion, age, national origin, sex, gender, sexual orientation, gender identity/expression, disability, protected veteran status, genetic information, or any other basis protected by institutional policy or by federal, state or local laws unless such distinction is required by law. http://www.mdanderson.org/about-us/legal-and-policy/legal-statements/eeo-affirmative-action.html
- Requisition ID: 166877
- Employment Status: Full-Time
- Employee Status: Regular
- Work Week: Days
- Minimum Salary: US Dollar (USD) 66,500
- Midpoint Salary: US Dollar (USD) 83,000
- Maximum Salary : US Dollar (USD) 99,500
- FLSA: exempt and not eligible for overtime pay
- Fund Type: Soft
- Work Location: Remote
- Pivotal Position: Yes
- Referral Bonus Available?: Yes
- Relocation Assistance Available?: Yes
- Science Jobs: Yes
My Submissions
Track your opportunities.
Similar Listings
Lymphoma - Myeloma 900193
United States, Texas, Houston, Houston (TX Med Ctr)
📁 Research
Requisition #: 167082
Hematopoietic Biology&Malignan 710597
Requisition #: 167270
Genomic Med Rsch Department 710562
Requisition #: 167083

- Patients & Family
- Prevention & Screening
- Donors & Volunteers
- For Physicians
- Education & Research
- Knowledge Center
- About MD Anderson
- Publications
- For Employees
- Finding Your Way
- Get In Touch
- Call: 1-877-632-6789
Study record managers: refer to the Data Element Definitions if submitting registration or results information.
Search for terms

- Advanced Search
- See Studies by Topic
- See Studies on Map
- How to Search
- How to Use Search Results
- How to Find Results of Studies
- How to Read a Study Record

- Learn About Studies
- Other Sites About Studies
- Glossary of Common Site Terms

- Submit Studies to ClinicalTrials.gov PRS
- Why Should I Register and Submit Results?
- FDAAA 801 and the Final Rule
- How to Apply for a PRS Account
- How to Register Your Study
- How to Edit Your Study Record
- How to Submit Your Results
- Frequently Asked Questions
- Support Materials
- Training Materials

- Selected Publications
- Clinical Alerts and Advisories
- Trends, Charts, and Maps
- Downloading Content for Analysis

- ClinicalTrials.gov Background
- About the Results Database
- History, Policies, and Laws
- ClinicalTrials.gov Modernization
- Media/Press Resources
- Linking to This Site
- Terms and Conditions
- Search Results
- Study Record Detail

Heart Failure Patients Registry (Priority-HF)
- Study Details
- Tabular View
- No Results Posted

This study is a multicenter non-interventional observational prospective registry. This non-interventional study (NIS) does not imply any intervention into a routine clinical practice, including choice of treatment modality or special methods of investigation. The study will include only those patients who sign the informed consent form (ICF) after explanation of the study objectives and methods by the study physician.
Planned study population consists of 20 000 adult outpatients with HF. All patients with HF who signed an ICF will be included to this study. Planned number of study sites is 150 outpatient centers in about 50 regions (in order to describe characteristics of outpatients with HF in different regions in the most comprehensive way).
Expected inclusion period duration - 24 months OR reaching 20 000 patients, if this takes less than 24 months. Planned follow-up period duration for 1 patient is about 52 weeks (12 months), which includes 3 visits (visit 1 - inclusion; visit 2 - approximately 6 months after inclusion; visit 3 - approximately 12 months after inclusion)

- Functional Class I - ≤3 points;
- Functional Class II - from 4 to 6 points;
- Functional Class III - from 7 to 9 points;
- Functional Class IV - more than 9 points;
At baseline of this study.
- <90 mm Hg;
- 90-99 mm Hg;
- 100-109 mm Hg;
- 110-119 mm Hg;
- 120-139 mm Hg;
- 140-159 mm Hg;
- ≥160 mm Hg;
At baseline of this study
- ≥90 mm Hg;
eGFR = 141 x min(SCr/κ, 1)^α x max(SCr/κ, 1)^-1.209 x 0.993^Age x 1.018 [if female] x 1.159 [if Black], where eGFR (estimated glomerular filtration rate) = mL/min/1.73 m^2, SCr (standardized serum creatinine) = mg/dL, κ = 0.7 (females) or 0.9 (males), α = -0.329 (females) or -0.411 (males), min = indicates the minimum of SCr/κ or 1, max = indicates the maximum of SCr/κ or 1, age = years.
- <30 mg/g;
- 30-300 mg/g;
- >300 mg/g;
- Sinus rhythm;
- Pacemaker rhythm;
- <130 ms;
- 130-150 ms;
- >150 ms;
- ACE inhibitors (duration, dosage, proportion of patients receiving: <50% of target dose, 50-100% of target dose, ≥100% of target dose);
- B-blockers (duration, dosage, proportion of patients receiving: <50% of target dose, 50-100% of target dose, ≥100% of target dose);
- ARBs (duration, dosage, proportion of patients receiving: <50% of target dose, 50 100% of target dose, ≥100% of target dose);
- MRAs (duration, dosage, proportion of patients receiving: <50% of target dose, 50 100% of target dose, ≥100% of target dose);
- ARNI (duration, dosage, proportion of patients receiving: <50% of target dose, 50 100% of target dose, ≥100% of target dose);
- Implantable cardioverter-defibrillator (ICD) (recommended; implanted);
- Cardiac resynchronisation therapy (CRT) (recommended; implanted);
- CRT with defibrillation (CRT-D) (recommended; implanted);
- Left ventricular assist device (LVAD);
- Revascularisation with PCI;
- Revascularisation with CABG;
- Valvular surgery;
- Heart transplantation;
- Modification of diet and lifestyle only;
- Sulfonylureas;
- Inhibitors of dipeptidyl peptidase-4 (iDPP4);
- Glucagon-like peptide-1 receptor agonists (GLP1 RA);
Inclusion Criteria:
- Age ≥ 18 years at the time of inclusion;
- Signed and dated written informed consent in accordance with ICH GCP and local law prior to inclusion in the study;
- Documented diagnosis of HF (according to Clinical Guidelines "Chronic Heart Failure", 2020, approved by MoH of RF) with typical symptoms/signs of HF consistent with I-IV functional classes of HF according to NYHA classification.
Exclusion Criteria:
- The absence of signed ICF;
- The participation in any randomised controlled trial within 3 months before the inclusion in this study or during the participation in this study.

- For Patients and Families
- For Researchers
- For Study Record Managers
- Customer Support
- Accessibility
- Viewers and Players
- Freedom of Information Act
- HHS Vulnerability Disclosure
- U.S. National Library of Medicine
- U.S. National Institutes of Health
- U.S. Department of Health and Human Services

IMAGES
VIDEO
COMMENTS
Requisition #: 166877. Department: GI Med Onc Rsch Department. Location: Houston, TX. Posted Date: 4/10/2024. The primary purpose of the Clinical Research Scientist is to assist in the interrogation of large datasets, development and design of clinical and translational research goals and objectives. Reviews, evaluates and correlates research data.
Thanks for your interest in the Clinical Research Scientist - Investigational Cancer Therapeutics position. ... It is the policy of The University of Texas MD Anderson Cancer Center to provide equal employment opportunity without regard to race, color, religion, age, national origin, sex, gender, sexual orientation, gender identity/expression ...
Research Assistant I - Neurosurgery - Research. 🔍 United States, Texas, Houston, Houston (TX Med Ctr) The mission of The University of Texas MD Anderson Cancer Center is to eliminate cancer in Texas, the nation, and the world through outstanding... 167200. 📅 18 minutes ago.
TRANSCEND Cancer Initiative. Resources for investigators designing, instituting and analyzing samples from translational research studies. MD Anderson's programs & centers focus on a variety of issues ranging from bone disease research to intervention programs. Learn more today.
MD Anderson is one of the world's most respected centres focused on cancer patient care, research, education and prevention. Its physicians treated more than 170,000 patients in 2021, and it has ...
The University of Texas MD Anderson Cancer Center in Houston is one of the world's most respected centers focused on cancer patient care, research, education and prevention. ... The primary purpose of the Research Scientist is to plan, organize, coordinate, direct and participate in scientific research projects. Assigns and reviews work of ...
Clinical Research Scientist ... It is the policy of The University of Texas MD Anderson Cancer Center to provide equal employment opportunity without regard to race, color, religion, age, national origin, sex, gender, sexual orientation, gender identity/expression, disability, protected veteran status, genetic information, or any other basis ...
The MD Anderson Lung Cancer Interception Program is a pioneering initiative dedicated to preventing lung cancer before it takes hold. We leverage cutting-edge research and a multidisciplinary team to identify and intercept pre-cancerous lesions, ultimately aiming to transform lung cancer care.
Preferred experience: Research in machine learning and data science. It is the policy of The University of Texas MD Anderson Cancer Center to provide equal employment opportunity without regard to race, color, religion, age, national origin, sex, gender, sexual orientation, gender identity/expression, disability, protected veteran status ...
The University of Texas MD Anderson Cancer Center's Research Highlights showcases the latest breakthroughs in cancer care, research and prevention. These advances are made possible through seamless collaboration between MD Anderson's world-leading clinicians and scientists, bringing discoveries from the lab to the clinic and back. Recent developments at MD Anderson offer insights into a ...
Research Scientist - Imaging Physics ... It is the policy of The University of Texas MD Anderson Cancer Center to provide equal employment opportunity without regard to race, color, religion, age, national origin, sex, gender, sexual orientation, gender identity/expression, disability, protected veteran status, genetic information, or any other ...
Agreement creates strong synergies between CureVac's unique end-to-end mRNA capabilities and MD Anderson's translational and clinical research expertise Collaboration aims to develop novel, off ...
That Data Scientist provides analysis and develops and maintains software tools for clinical and scientific problems in the Bioinformatics and Computational Biology department. This position involves application of complex statistical models to patient cohort data, revolving around a software tool suite called LFSPRO, and communicating findings ...
Impact patient care through clinical and research procurement of human tissue. ... With preferred degree, one year of required experience. It is the policy of The University of Texas MD Anderson Cancer Center to provide equal employment opportunity without regard to race, color, religion, age, national origin, sex, gender, sexual orientation ...
This is a phase 3, multicenter, double-blind, randomized, placebo-controlled, parallel-group study to evaluate the efficacy and safety of CSL112 on reducing the risk of major adverse CV events [MACE - cardiovascular (CV) death, myocardial infarction (MI), and stroke] in subjects with acute coronary syndrome (ACS) diagnosed with either ST-segment elevation myocardial infarction (STEMI) or non ...
The primary purpose of the Clinical Research Scientist is to assist in the interrogation of large datasets, development and design of clinical and translational research goals and objectives. ... It is the policy of The University of Texas MD Anderson Cancer Center to provide equal employment opportunity without regard to race, color, religion ...
(CAMDEN, NJ) —MD Anderson Cancer Center at Cooper successfully attained accreditation at its Camden, Voorhees, and Mount Laurel locations from the American Society for Radiation Oncology (ASTRO) APEx - Accreditation Program for Excellence®. APEx provides external validation that a radiation oncology facility is delivering high-quality patient care. "Receiving APEx accreditation from ...
Find breaking science news and analysis from the world's leading research journal.
The primary purpose of the Clinical Research Scientist is to assist in the interrogation of large datasets, development and design of clinical and translational research goals and objectives. ... It is the policy of The University of Texas MD Anderson Cancer Center to provide equal employment opportunity without regard to race, color, religion ...
Choosing to participate in a study is an important personal decision. Talk with your doctor and family members or friends about deciding to join a study. To learn more about this study, you or your doctor may contact the study research staff using the contacts provided below. For general information, Learn About Clinical Studies.