- Locations and Hours
- UCLA Library
- Research Guides
- Biomedical Library Guides

Systematic Reviews
- Types of Literature Reviews
What Makes a Systematic Review Different from Other Types of Reviews?
- Planning Your Systematic Review
- Database Searching
- Creating the Search
- Search Filters & Hedges
- Grey Literature
- Managing & Appraising Results
- Further Resources
Reproduced from Grant, M. J. and Booth, A. (2009), A typology of reviews: an analysis of 14 review types and associated methodologies. Health Information & Libraries Journal, 26: 91–108. doi:10.1111/j.1471-1842.2009.00848.x
- << Previous: Home
- Next: Planning Your Systematic Review >>
- Last Updated: Apr 10, 2024 11:08 AM
- URL: https://guides.library.ucla.edu/systematicreviews
Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, generate accurate citations for free.
- Knowledge Base
Methodology
- Systematic Review | Definition, Example, & Guide
Systematic Review | Definition, Example & Guide
Published on June 15, 2022 by Shaun Turney . Revised on November 20, 2023.
A systematic review is a type of review that uses repeatable methods to find, select, and synthesize all available evidence. It answers a clearly formulated research question and explicitly states the methods used to arrive at the answer.
They answered the question “What is the effectiveness of probiotics in reducing eczema symptoms and improving quality of life in patients with eczema?”
In this context, a probiotic is a health product that contains live microorganisms and is taken by mouth. Eczema is a common skin condition that causes red, itchy skin.
Table of contents
What is a systematic review, systematic review vs. meta-analysis, systematic review vs. literature review, systematic review vs. scoping review, when to conduct a systematic review, pros and cons of systematic reviews, step-by-step example of a systematic review, other interesting articles, frequently asked questions about systematic reviews.
A review is an overview of the research that’s already been completed on a topic.
What makes a systematic review different from other types of reviews is that the research methods are designed to reduce bias . The methods are repeatable, and the approach is formal and systematic:
- Formulate a research question
- Develop a protocol
- Search for all relevant studies
- Apply the selection criteria
- Extract the data
- Synthesize the data
- Write and publish a report
Although multiple sets of guidelines exist, the Cochrane Handbook for Systematic Reviews is among the most widely used. It provides detailed guidelines on how to complete each step of the systematic review process.
Systematic reviews are most commonly used in medical and public health research, but they can also be found in other disciplines.
Systematic reviews typically answer their research question by synthesizing all available evidence and evaluating the quality of the evidence. Synthesizing means bringing together different information to tell a single, cohesive story. The synthesis can be narrative ( qualitative ), quantitative , or both.
Receive feedback on language, structure, and formatting
Professional editors proofread and edit your paper by focusing on:
- Academic style
- Vague sentences
- Style consistency
See an example

Systematic reviews often quantitatively synthesize the evidence using a meta-analysis . A meta-analysis is a statistical analysis, not a type of review.
A meta-analysis is a technique to synthesize results from multiple studies. It’s a statistical analysis that combines the results of two or more studies, usually to estimate an effect size .
A literature review is a type of review that uses a less systematic and formal approach than a systematic review. Typically, an expert in a topic will qualitatively summarize and evaluate previous work, without using a formal, explicit method.
Although literature reviews are often less time-consuming and can be insightful or helpful, they have a higher risk of bias and are less transparent than systematic reviews.
Similar to a systematic review, a scoping review is a type of review that tries to minimize bias by using transparent and repeatable methods.
However, a scoping review isn’t a type of systematic review. The most important difference is the goal: rather than answering a specific question, a scoping review explores a topic. The researcher tries to identify the main concepts, theories, and evidence, as well as gaps in the current research.
Sometimes scoping reviews are an exploratory preparation step for a systematic review, and sometimes they are a standalone project.
A systematic review is a good choice of review if you want to answer a question about the effectiveness of an intervention , such as a medical treatment.
To conduct a systematic review, you’ll need the following:
- A precise question , usually about the effectiveness of an intervention. The question needs to be about a topic that’s previously been studied by multiple researchers. If there’s no previous research, there’s nothing to review.
- If you’re doing a systematic review on your own (e.g., for a research paper or thesis ), you should take appropriate measures to ensure the validity and reliability of your research.
- Access to databases and journal archives. Often, your educational institution provides you with access.
- Time. A professional systematic review is a time-consuming process: it will take the lead author about six months of full-time work. If you’re a student, you should narrow the scope of your systematic review and stick to a tight schedule.
- Bibliographic, word-processing, spreadsheet, and statistical software . For example, you could use EndNote, Microsoft Word, Excel, and SPSS.
A systematic review has many pros .
- They minimize research bias by considering all available evidence and evaluating each study for bias.
- Their methods are transparent , so they can be scrutinized by others.
- They’re thorough : they summarize all available evidence.
- They can be replicated and updated by others.
Systematic reviews also have a few cons .
- They’re time-consuming .
- They’re narrow in scope : they only answer the precise research question.
The 7 steps for conducting a systematic review are explained with an example.
Step 1: Formulate a research question
Formulating the research question is probably the most important step of a systematic review. A clear research question will:
- Allow you to more effectively communicate your research to other researchers and practitioners
- Guide your decisions as you plan and conduct your systematic review
A good research question for a systematic review has four components, which you can remember with the acronym PICO :
- Population(s) or problem(s)
- Intervention(s)
- Comparison(s)
You can rearrange these four components to write your research question:
- What is the effectiveness of I versus C for O in P ?
Sometimes, you may want to include a fifth component, the type of study design . In this case, the acronym is PICOT .
- Type of study design(s)
- The population of patients with eczema
- The intervention of probiotics
- In comparison to no treatment, placebo , or non-probiotic treatment
- The outcome of changes in participant-, parent-, and doctor-rated symptoms of eczema and quality of life
- Randomized control trials, a type of study design
Their research question was:
- What is the effectiveness of probiotics versus no treatment, a placebo, or a non-probiotic treatment for reducing eczema symptoms and improving quality of life in patients with eczema?
Step 2: Develop a protocol
A protocol is a document that contains your research plan for the systematic review. This is an important step because having a plan allows you to work more efficiently and reduces bias.
Your protocol should include the following components:
- Background information : Provide the context of the research question, including why it’s important.
- Research objective (s) : Rephrase your research question as an objective.
- Selection criteria: State how you’ll decide which studies to include or exclude from your review.
- Search strategy: Discuss your plan for finding studies.
- Analysis: Explain what information you’ll collect from the studies and how you’ll synthesize the data.
If you’re a professional seeking to publish your review, it’s a good idea to bring together an advisory committee . This is a group of about six people who have experience in the topic you’re researching. They can help you make decisions about your protocol.
It’s highly recommended to register your protocol. Registering your protocol means submitting it to a database such as PROSPERO or ClinicalTrials.gov .
Step 3: Search for all relevant studies
Searching for relevant studies is the most time-consuming step of a systematic review.
To reduce bias, it’s important to search for relevant studies very thoroughly. Your strategy will depend on your field and your research question, but sources generally fall into these four categories:
- Databases: Search multiple databases of peer-reviewed literature, such as PubMed or Scopus . Think carefully about how to phrase your search terms and include multiple synonyms of each word. Use Boolean operators if relevant.
- Handsearching: In addition to searching the primary sources using databases, you’ll also need to search manually. One strategy is to scan relevant journals or conference proceedings. Another strategy is to scan the reference lists of relevant studies.
- Gray literature: Gray literature includes documents produced by governments, universities, and other institutions that aren’t published by traditional publishers. Graduate student theses are an important type of gray literature, which you can search using the Networked Digital Library of Theses and Dissertations (NDLTD) . In medicine, clinical trial registries are another important type of gray literature.
- Experts: Contact experts in the field to ask if they have unpublished studies that should be included in your review.
At this stage of your review, you won’t read the articles yet. Simply save any potentially relevant citations using bibliographic software, such as Scribbr’s APA or MLA Generator .
- Databases: EMBASE, PsycINFO, AMED, LILACS, and ISI Web of Science
- Handsearch: Conference proceedings and reference lists of articles
- Gray literature: The Cochrane Library, the metaRegister of Controlled Trials, and the Ongoing Skin Trials Register
- Experts: Authors of unpublished registered trials, pharmaceutical companies, and manufacturers of probiotics
Step 4: Apply the selection criteria
Applying the selection criteria is a three-person job. Two of you will independently read the studies and decide which to include in your review based on the selection criteria you established in your protocol . The third person’s job is to break any ties.
To increase inter-rater reliability , ensure that everyone thoroughly understands the selection criteria before you begin.
If you’re writing a systematic review as a student for an assignment, you might not have a team. In this case, you’ll have to apply the selection criteria on your own; you can mention this as a limitation in your paper’s discussion.
You should apply the selection criteria in two phases:
- Based on the titles and abstracts : Decide whether each article potentially meets the selection criteria based on the information provided in the abstracts.
- Based on the full texts: Download the articles that weren’t excluded during the first phase. If an article isn’t available online or through your library, you may need to contact the authors to ask for a copy. Read the articles and decide which articles meet the selection criteria.
It’s very important to keep a meticulous record of why you included or excluded each article. When the selection process is complete, you can summarize what you did using a PRISMA flow diagram .
Next, Boyle and colleagues found the full texts for each of the remaining studies. Boyle and Tang read through the articles to decide if any more studies needed to be excluded based on the selection criteria.
When Boyle and Tang disagreed about whether a study should be excluded, they discussed it with Varigos until the three researchers came to an agreement.
Step 5: Extract the data
Extracting the data means collecting information from the selected studies in a systematic way. There are two types of information you need to collect from each study:
- Information about the study’s methods and results . The exact information will depend on your research question, but it might include the year, study design , sample size, context, research findings , and conclusions. If any data are missing, you’ll need to contact the study’s authors.
- Your judgment of the quality of the evidence, including risk of bias .
You should collect this information using forms. You can find sample forms in The Registry of Methods and Tools for Evidence-Informed Decision Making and the Grading of Recommendations, Assessment, Development and Evaluations Working Group .
Extracting the data is also a three-person job. Two people should do this step independently, and the third person will resolve any disagreements.
They also collected data about possible sources of bias, such as how the study participants were randomized into the control and treatment groups.
Step 6: Synthesize the data
Synthesizing the data means bringing together the information you collected into a single, cohesive story. There are two main approaches to synthesizing the data:
- Narrative ( qualitative ): Summarize the information in words. You’ll need to discuss the studies and assess their overall quality.
- Quantitative : Use statistical methods to summarize and compare data from different studies. The most common quantitative approach is a meta-analysis , which allows you to combine results from multiple studies into a summary result.
Generally, you should use both approaches together whenever possible. If you don’t have enough data, or the data from different studies aren’t comparable, then you can take just a narrative approach. However, you should justify why a quantitative approach wasn’t possible.
Boyle and colleagues also divided the studies into subgroups, such as studies about babies, children, and adults, and analyzed the effect sizes within each group.
Step 7: Write and publish a report
The purpose of writing a systematic review article is to share the answer to your research question and explain how you arrived at this answer.
Your article should include the following sections:
- Abstract : A summary of the review
- Introduction : Including the rationale and objectives
- Methods : Including the selection criteria, search method, data extraction method, and synthesis method
- Results : Including results of the search and selection process, study characteristics, risk of bias in the studies, and synthesis results
- Discussion : Including interpretation of the results and limitations of the review
- Conclusion : The answer to your research question and implications for practice, policy, or research
To verify that your report includes everything it needs, you can use the PRISMA checklist .
Once your report is written, you can publish it in a systematic review database, such as the Cochrane Database of Systematic Reviews , and/or in a peer-reviewed journal.
In their report, Boyle and colleagues concluded that probiotics cannot be recommended for reducing eczema symptoms or improving quality of life in patients with eczema. Note Generative AI tools like ChatGPT can be useful at various stages of the writing and research process and can help you to write your systematic review. However, we strongly advise against trying to pass AI-generated text off as your own work.
If you want to know more about statistics , methodology , or research bias , make sure to check out some of our other articles with explanations and examples.
- Student’s t -distribution
- Normal distribution
- Null and Alternative Hypotheses
- Chi square tests
- Confidence interval
- Quartiles & Quantiles
- Cluster sampling
- Stratified sampling
- Data cleansing
- Reproducibility vs Replicability
- Peer review
- Prospective cohort study
Research bias
- Implicit bias
- Cognitive bias
- Placebo effect
- Hawthorne effect
- Hindsight bias
- Affect heuristic
- Social desirability bias
A literature review is a survey of scholarly sources (such as books, journal articles, and theses) related to a specific topic or research question .
It is often written as part of a thesis, dissertation , or research paper , in order to situate your work in relation to existing knowledge.
A literature review is a survey of credible sources on a topic, often used in dissertations , theses, and research papers . Literature reviews give an overview of knowledge on a subject, helping you identify relevant theories and methods, as well as gaps in existing research. Literature reviews are set up similarly to other academic texts , with an introduction , a main body, and a conclusion .
An annotated bibliography is a list of source references that has a short description (called an annotation ) for each of the sources. It is often assigned as part of the research process for a paper .
A systematic review is secondary research because it uses existing research. You don’t collect new data yourself.
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the “Cite this Scribbr article” button to automatically add the citation to our free Citation Generator.
Turney, S. (2023, November 20). Systematic Review | Definition, Example & Guide. Scribbr. Retrieved April 9, 2024, from https://www.scribbr.com/methodology/systematic-review/
Is this article helpful?

Shaun Turney
Other students also liked, how to write a literature review | guide, examples, & templates, how to write a research proposal | examples & templates, what is critical thinking | definition & examples, what is your plagiarism score.

Systematic Reviews
- Introduction to Systematic Reviews
Traditional Systematic Reviews
Meta-analyses, scoping reviews, rapid reviews, umbrella reviews, selecting a review type.
- Reading Systematic Reviews
- Resources for Conducting Systematic Reviews
- Getting Help with Systematic Reviews from the Library
- History of Systematic Reviews
- Acknowledgements
Systematic Reviews are a family of review types that include:
This page provides information about the most common types of systematic reviews, important resources and references for conducting them, and some tools for choosing the best type for your research question .
Additional Information
- A typology of reviews: an analysis of 14 review types and associated methodologies This classic article is a valuable reference point for those commissioning, conducting, supporting or interpreting reviews.
- Traditional Systematic Reviews follow a rigorous and well-defined methodology to identify, select, and critically appraise relevant research articles on a specific topic and within a specified population of subjects
- The primary goal of this type of study is to comprehensively find the empirical data available on a topic, identify relevant articles, synthesize their findings and draw evidence-based conclusions to answer a clinical question
- Cochrane Handbook for Systematic Reviews of Interventions The Cochrane Handbook for Systematic Reviews of Interventions provides direction on the standard methods involved in conducting a systematic review. It is the official guide to the process involved in preparing and maintaining Cochrane systematic reviews on the effects of healthcare interventions.
- JBI Manual for Evidence Synthesis The JBI Manual for Evidence Synthesis is designed to provide authors with a comprehensive guide to conducting JBI systematic reviews. It describes in detail the process of planning, undertaking and writing up a systematic review using JBI methods. The JBI Manual for Evidence Synthesis should be used in conjunction with the support and tutorials offered at the JBI SUMARI Knowledge Base.
These are some places where protocols for systematic reviews might be published.
- PROSPERO: International prospective register of systematic reviews PROSPERO is an international database of prospectively registered systematic reviews in health and social care, welfare, public health, education, crime, justice, and international development, where there is a health related outcome. Key features from the review protocol are recorded and maintained as a permanent record. PROSPERO aims to provide a comprehensive listing of systematic reviews registered at inception to help avoid duplication and reduce opportunity for reporting bias by enabling comparison of the completed review with what was planned in the protocol.
- Guidance Notes for Registering A Systematic Review Protocol with PROSPERO
- OSF Registries Open Science Framework (OSF) Registries is an open network of study registgrations and pre-registrations. It can be used to pre-register a systematic review protocol. Note that OSF pre-registrations are not reviewed.
- OSF Preregistration Initiative This page explains the motivation behind preregistrations and best practices for doing so.
- Protocols.io A secure platform for developing and sharing reproducible methods, including protocols for systematic reviews.
- PRISMA 2020 Statement The PRISMA 2020 Statement was published in 2021. It consists of a checklist and a flow diagram, and is intended to be accompanied by the PRISMA 2020 Explanation and Elaboration document.
- Meta-analysis is a statistical method that can be applied during a systematic review to extract and combine the results from multiple studies
- This pooling of data from compatible studies increases the statistical power and precision of the conclusions made by the systematic review
- Systematic reviews can be done without doing a meta-analysis, but a meta-analysis must be done in connection with a systematic review
- Scoping reviews identify the existing literature available on a topic to help identify key concepts, the type and amount of evidence available on a subject, and what research gaps exist in a specific area of study
- They are particularly useful when a research question is broad and the goal is to provide an understanding of the available evidence on a topic rather than providing a focused synthesis on a narrow question
- JBI Manual Chapter 11: Scoping Reviews
- Updated methodological guidance for the conduct of scoping reviews The objective of this paper is to describe the updated methodological guidance for conducting a JBI scoping review, with a focus on new updates to the approach and development of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews (the PRISMA-ScR).
- Steps for Conducting a Scoping Review This article in the Journal of Graduate Medical Education provides a comprehensive yet brief overview of the scoping review process.
Note: Protocols for scoping reviews can be published in all the same places as traditional systematic reviews except PROSPERO.
- Best practice guidance and reporting items for the development of scoping review protocols The purpose of this article is to clearly describe how to develop a robust and detailed scoping review protocol, which is the first stage of the scoping review process. This paper provides detailed guidance and a checklist for prospective authors to ensure that their protocols adequately inform both the conduct of the ensuing review and their readership.
- PRISMA for Scoping Reviews (PRISMA-ScR) The PRISMA extension for scoping reviews was published in 2018. The checklist contains 20 essential reporting items and 2 optional items to include when completing a scoping review. Scoping reviews serve to synthesize evidence and assess the scope of literature on a topic. Among other objectives, scoping reviews help determine whether a systematic review of the literature is warranted.
- Touro College: What is a Scoping Review? This page describes scoping reviews, including their limitations, alternate names, and how they differ from traditional systematic reviews.
- What are scoping reviews? Providing a formal definition of scoping reviews as a type of evidence synthesis This article from JBI Evidence Synthesis provides a thorough definition of what scoping reviews are and what they are for.
- The role of scoping reviews in reducing research waste This article from the Journal of Clinical Epidemiology looks at how scoping reviews can reduce research waste.
- Rapid reviews streamline the systematic review process by omitting certain steps or accelerating the timeline
- They are useful when there is a need for timely evidence synthesis, such as in response to questions concerning an urgent policy or clinical situation such as the COVID-19 pandemic
- Rapid Review Guidebook This document provides guidance on the process of conducting rapid reviews to use evidence to inform policy and program decision making.
- Rapid reviews to strengthen health policy and systems: a practical guide This guide from the World Health Organization offers guidance on how to plan, conduct, and promote the use of rapid reviews to strengthen health policy and systems decisions. The Guide explores different approaches and methods for expedited synthesis of health policy and systems research, and highlights key challenges for this emerging field, including its application in low- and middle-income countries. It touches on the utility of rapid reviews of health systems evidence, and gives insights into applied methods to swiftly conduct knowledge syntheses and foster their use in policy and practice.
- Cochrane Rapid Reviews Methods Group offers evidence-informed guidance to conduct rapid reviews The Cochrane Rapid Reviews Methods Group offers new, interim guidance to support the conduct of Rapid Reviews.
- Touro College: What is a Rapid Review? This page describes rapid reviews, including their limitations, alternate names, and how they differ from traditional systematic reviews.
- Umbrella reviews synthesize evidence from multiple systematic reviews and meta-analyses on a specific topic
- They provide a next-generation level of evidence synthesis, analyzing evidence taken from multiple systematic reviews to offer a broader perspective on a given subject
- JBI Manual Chapter 10: Umbrella reviews
- Preferred Reporting Items for Overviews of Reviews (PRIOR) Overviews of reviews (i.e., overviews) compile information from multiple systematic reviews to provide a single synthesis of relevant evidence for healthcare decision-making. Despite their increasing popularity, there are currently no systematically developed reporting guidelines for overviews. This is problematic because the reporting of published overviews varies considerably and is often substandard. Our objective is to use explicit, systematic, and transparent methods to develop an evidence-based and agreement-based reporting guideline for overviews of reviews of healthcare interventions (PRIOR, Preferred Reporting Items for Overviews of Reviews).
- Touro College: What is an Overview of Reviews? This page describes umbrella reviews, including their limitations, alternate names, and how they differ from traditional systematic reviews.
- Cornell University Systematic Review Decision Tree This decision tree is designed to assist researchers in choosing a review type.
- Right Review This tool is designed to provide guidance and supporting material to reviewers on methods for the conduct and reporting of knowledge synthesis.
- << Previous: Introduction to Systematic Reviews
- Next: Reading Systematic Reviews >>
- Last Updated: Mar 27, 2024 4:35 PM
- URL: https://libguides.ohsu.edu/systematic-reviews
Systematic Reviews: Types of literature review, methods, & resources
- Types of literature review, methods, & resources
- Protocol and registration
- Search strategy
- Medical Literature Databases to search
- Study selection and appraisal
- Data Extraction/Coding/Study characteristics/Results
- Reporting the quality/risk of bias
- Manage citations using RefWorks This link opens in a new window
- GW Box file storage for PDF's This link opens in a new window
Analytical reviews
GUIDELINES FOR HOW TO CARRY OUT AN ANALYTICAL REVIEW OF QUANTITATIVE RESEARCH
Enhancing the QUAlity and Transparency Of health Research (EQUATOR) network. (Tracking and listing over 550 reporting guidelines for various different study types including Randomised trials, Systematic reviews, Study protocols, Diagnostic/prognostic studies, Case reports, Clinical practice guidelines, Animal pre-clinical studies, etc). http://www.equator-network.org/resource-centre/library-of-health-research-reporting/
When comparing therapies :
PRISMA (Guideline on how to perform and write-up a systematic review and/or meta-analysis of the outcomes reported in multiple clinical trials of therapeutic interventions. PRISMA replaces the previous QUORUM statement guidelines ): Liberati, A,, Altman, D,, Moher, D, et al. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Plos Medicine, 6 (7):e1000100. doi:10.1371/journal.pmed.1000100
When comparing diagnostic methods :
Checklist for Artificial Intelligence in Medical Imaging (CLAIM). CLAIM is modeled after the STARD guideline and has been extended to address applications of AI in medical imaging that include classification, image reconstruction, text analysis, and workflow optimization. The elements described here should be viewed as a “best practice” to guide authors in presenting their research. Reported in Mongan, J., Moy, L., & Kahn, C. E., Jr (2020). Checklist for Artificial Intelligence in Medical Imaging (CLAIM): A Guide for Authors and Reviewers. Radiology. Artificial intelligence , 2 (2), e200029. https://doi.org/10.1148/ryai.2020200029
STAndards for the Reporting of Diagnostic accuracy studies (STARD) Statement. (Reporting guidelines for writing up a study comparing the accuracy of competing diagnostic methods) http://www.stard-statement.org/
When evaluating clinical practice guidelines :
AGREE Research Trust (ART) (2013). Appraisal of Guidelines for Research & Evaluation (AGREE-II) . (A 23-item instrument for as sessing th e quality of Clinical Practice Guidelines. Used internationally for evaluating or deciding which guidelines could be recommended for use in practice or to inform health policy decisions.)
National Guideline Clearinghouse Extent of Adherence to Trustworthy Standards (NEATS) Instrument (2019). (A 15-item instrument using scales of 1-5 to evaluate a guideline's adherence to the Institute of Medicine's standard for trustworthy guidelines. It has good external validity among guideline developers and good interrater reliability across trained reviewers.)
When reviewing genetics studies
Human genetics review reporting guidelines. Little J, Higgins JPT (eds.). The HuGENet™ HuGE Review Handbook, version 1.0 .
When you need to re-analyze individual participant data
If you wish to collect, check, and re-analyze individual participant data (IPD) from clinical trials addressing a particular research question, you should follow the PRISMA-IPD guidelines as reported in Stewart, L.A., Clarke, M., Rovers, M., et al. (2015). Preferred Reporting Items for a Systematic Review and Meta-analysis of Individual Participant Data: The PRISMA-IPD Statement. JAMA, 313(16):1657-1665. doi:10.1001/jama.2015.3656 .
When comparing Randomized studies involving animals, livestock, or food:
O’Connor AM, et al. (2010). The REFLECT statement: methods and processes of creating reporting guidelines for randomized controlled trials for livestock and food safety by modifying the CONSORT statement. Zoonoses Public Health. 57(2):95-104. Epub 2010/01/15. doi: 10.1111/j.1863-2378.2009.01311.x. PubMed PMID: 20070653.
Sargeant JM, et al. (2010). The REFLECT Statement: Reporting Guidelines for Randomized Controlled Trials in Livestock and Food Safety: Explanation and Elaboration. Zoonoses Public Health. 57(2):105-36. Epub 2010/01/15. doi: JVB1312 [pii] 10.1111/j.1863-2378.2009.01312.x. PubMed PMID: 20070652.
GUIDELINES FOR HOW TO WRITE UP FOR PUBLICATION THE RESULTS OF ONE QUANTITATIVE CLINICAL TRIAL
When reporting the results of a Randomized Controlled Trial :
Consolidated Standards of Reporting Trials (CONSORT) Statement. (2010 reporting guideline for writing up a Randomized Controlled Clinical Trial). http://www.consort-statement.org . Since updated in 2022, see Butcher, M. A., et al. (2022). Guidelines for Reporting Outcomes in Trial Reports: The CONSORT-Outcomes 2022 Extension . JAMA : the Journal of the American Medical Association, 328(22), 2252–2264. https://doi.org/10.1001/jama.2022.21022
Kilkenny, C., Browne, W. J., Cuthill, I. C., Emerson, M., & Altman, D. G. (2010). Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biology, 8(6), e1000412–e1000412. https://doi.org/10.1371/journal.pbio.1000412 (A 20-item checklist, following the CONSORT approach, listing the information that published articles reporting research using animals should include, such as the number and specific characteristics of animals used; details of housing and husbandry; and the experimental, statistical, and analytical methods used to reduce bias.)
Narrative reviews
GUIDELINES FOR HOW TO CARRY OUT A NARRATIVE REVIEW / QUALITATIVE RESEARCH / OBSERVATIONAL STUDIES
Campbell, M. (2020). Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ, 368. doi: https://doi.org/10.1136/bmj.l6890 (guideline on how to analyse evidence for a narrative review, to provide a recommendation based on heterogenous study types).
Community Preventive Services Task Force (2021). The Methods Manual for Community Guide Systematic Reviews . (Public Health Prevention systematic review guidelines)
Enhancing the QUAlity and Transparency Of health Research (EQUATOR) network. (Tracking and listing over 550 reporting guidelines for various different study types including Observational studies, Qualitative research, Quality improvement studies, and Economic evaluations). http://www.equator-network.org/resource-centre/library-of-health-research-reporting/
Cochrane Qualitative & Implementation Methods Group. (2019). Training resources. Retrieved from https://methods.cochrane.org/qi/training-resources . (Training materials for how to do a meta-synthesis, or qualitative evidence synthesis).
Cornell University Library (2019). Planning worksheet for structured literature reviews. Retrieved 4/8/22 from https://osf.io/tnfm7/ (offers a framework for a narrative literature review).
Green, B. N., Johnson, C. D., & Adams, A. (2006). Writing narrative literature reviews for peer-reviewed journals: secrets of the trade . Journal of Chiropractic Medicine, 5(3): 101-117. DOI: 10.1016/ S0899-3467 (07)60142-6. This is a very good article about what to take into consideration when writing any type of narrative review.
When reviewing observational studies/qualitative research :
STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement. (Reporting guidelines for various types of health sciences observational studies). http://www.strobe-statement.org
Meta-analysis of Observational Studies in Epidemiology (MOOSE) http://jama.jamanetwork.com/article.aspx?articleid=192614
RATS Qualitative research systematic review guidelines. https://www.equator-network.org/reporting-guidelines/qualitative-research-review-guidelines-rats/
Methods/Guidance
Right Review , this decision support website provides an algorithm to help reviewers choose a review methodology from among 41 knowledge synthesis methods.
The Systematic Review Toolbox , an online catalogue of tools that support various tasks within the systematic review and wider evidence synthesis process. Maintained by the UK University of York Health Economics Consortium, Newcastle University NIHR Innovation Observatory, and University of Sheffield School of Health and Related Research.
Institute of Medicine. (2011). Finding What Works in Health Care: Standards for Systematic Reviews . Washington, DC: National Academies (Systematic review guidelines from the Health and Medicine Division (HMD) of the U.S. National Academies of Sciences, Engineering, and Medicine (formerly called the Institute of Medicine)).
International Committee of Medical Journal Editors (2022). Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly work in Medical Journals . Guidance on how to prepare a manuscript for submission to a Medical journal.
Cochrane Handbook of Systematic Reviews of Interventions (International Cochrane Collaboration systematic review guidelines). The various Cochrane review groups comporise around 30,000 physicians around the world working in the disciplines on reviews of interventions with very detailed methods for verifying the validity of the research methods and analysis performed in screened-in Randmized Controlled Clinical Trials. Typically published Cochrane Reviews are the most exhaustive review of the evidence of effectiveness of a particular drug or intervention, and include a statistical meta-analysis. Similar to practice guidelines, Cochrane reviews are periodically revised and updated.
Joanna Briggs Institute (JBI) Manual of Evidence Synthesis . (International systematic review guidelines). Based at the University of Adelaide, South Australia, and collaborating with around 80 academic and medical entities around the world. Unlike Cochrane Reviews that strictly focus on efficacy of interventions, JBI offers a broader, inclusive approach to evidence, to accommodate a range of diverse questions and study designs. The JBI manual provides guidance on how to analyse and include both quantitative and qualitative research.
Cochrane Methods Support Unit, webinar recordings on methodological support questions
Cochrane Qualitative & Implementation Methods Group. (2019). Training resources. Retrieved from https://methods.cochrane.org/qi/training-resources . (How to do a meta-synthesis, or qualitative evidence synthesis).
Center for Reviews and Dissemination (University of York, England) (2009). Systematic Reviews: CRD's guidance for undertaking systematic reviews in health care . (British systematic review guidelines).
Agency for Health Research & Quality (AHRQ) (2013). Methods guide for effectiveness and comparative effectiveness reviews . (U.S. comparative effectiveness review guidelines)
Hunter, K. E., et al. (2022). Searching clinical trials registers: guide for systematic reviewers. BMJ (Clinical research ed.) , 377 , e068791. https://doi.org/10.1136/bmj-2021-068791
Patient-Centered Outcomes Research Institute (PCORI). The PCORI Methodology Report . (A 47-item methodology checklist for U.S. patient-centered outcomes research. Established under the Patient Protection and Affordable Care Act, PCORI funds the development of guidance on the comparative effectivess of clinical healthcare, similar to the UK National Institute for Clinical Evidence but without reporting cost-effectiveness QALY metrics).
Canadian Agency for Drugs and Technologies in Health (CADTH) (2019). Grey Matters: a practical tool for searching health-related grey literature. Retrieved from https://www.cadth.ca/resources/finding-evidence/grey-matters . A checklist of N American & international online databases and websites you can use to search for unpublished reports, posters, and policy briefs, on topics including general medicine and nursing, public and mental health, health technology assessment, drug and device regulatory, approvals, warnings, and advisories.
Hempel, S., Xenakis, L., & Danz, M. (2016). Systematic Reviews for Occupational Safety and Health Questions: Resources for Evidence Synthesis. Retrieved 8/15/16 from http://www.rand.org/pubs/research_reports/RR1463.html . NIOSH guidelines for how to carry out a systematic review in the occupational safety and health domain.
A good source for reporting guidelines is the NLM's Research Reporting Guidelines and Initiatives .
Grading of Recommendations Assessment, Development and Evaluation (GRADE). (An international group of academics/clinicians working to promote a common approach to grading the quality of evidence and strength of recommendations.)
Phillips, B., Ball, C., Sackett, D., et al. (2009). Oxford Centre for Evidence Based Medicine: Levels of Evidence. Retrieved 3/20/17 from https://www.cebm.net/wp-content/uploads/2014/06/CEBM-Levels-of-Evidence-2.1.pdf . (Another commonly used criteria for grading the quality of evidence and strength of recommendations, developed in part by EBM guru David Sackett.)
Systematic Reviews for Animals & Food (guidelines including the REFLECT statement for carrying out a systematic review on animal health, animal welfare, food safety, livestock, and agriculture)
Grant, M. J., & Booth, A. (2009). A typology of reviews: an analysis of 14 review types and associated methodologies . Health Information & Libraries Journal, 26(2), 91-108. doi:10.1111/j.1471-1842.2009.00848.x. (Describes 14 different types of literature and systematic review, useful for thinking at the outset about what sort of literature review you want to do.)
Sutton, A., Clowes, M., Preston, L., & Booth, A. (2019). Meeting the review family: exploring review types and associated information retrieval requirements . Health information and libraries journal, 36(3), 202–222. doi:10.1111/hir.12276 (An updated look at different types of literature review, expands on the Grant & Booth 2009 article listed above).
Garrard, J. (2007). Health Sciences Literature Review Made Easy: The Matrix Method (2nd Ed.). Sudbury, MA: Jones & Bartlett Publishers. (Textbook of health sciences literature search methods).
Zilberberg, M. (2012). Between the lines: Finding the truth in medical literature . Goshen, MA: Evimed Research Press. (Concise book on foundational concepts of evidence-based medicine).
Lang, T. (2009). The Value of Systematic Reviews as Research Activities in Medical Education . In: Lang, T. How to write, publish, & present in the health sciences : a guide for clinicians & laboratory researchers. Philadelphia : American College of Physicians. (This book chapter has a helpful bibliography on systematic review and meta-analysis methods)
Brown, S., Martin, E., Garcia, T., Winter, M., García, A., Brown, A., Cuevas H., & Sumlin, L. (2013). Managing complex research datasets using electronic tools: a meta-analysis exemplar . Computers, Informatics, Nursing: CIN, 31(6), 257-265. doi:10.1097/NXN.0b013e318295e69c. (This article advocates for the programming of electronic fillable forms in Adobe Acrobat Pro to feed data into Excel or SPSS for analysis, and to use cloud based file sharing systems such as Blackboard, RefWorks, or EverNote to facilitate sharing knowledge about the decision-making process and keep data secure. Of particular note are the flowchart describing this process, and their example screening form used for the initial screening of abstracts).
Brown, S., Upchurch, S., & Acton, G. (2003). A framework for developing a coding scheme for meta-analysis . Western Journal Of Nursing Research, 25(2), 205-222. (This article describes the process of how to design a coded data extraction form and codebook, Table 1 is an example of a coded data extraction form that can then be used to program a fillable form in Adobe Acrobat or Microsoft Access).
Elamin, M. B., Flynn, D. N., Bassler, D., Briel, M., Alonso-Coello, P., Karanicolas, P., & ... Montori, V. M. (2009). Choice of data extraction tools for systematic reviews depends on resources and review complexity . Journal Of Clinical Epidemiology , 62 (5), 506-510. doi:10.1016/j.jclinepi.2008.10.016 (This article offers advice on how to decide what tools to use to extract data for analytical systematic reviews).
Riegelman R. Studying a Study and Testing a Test: Reading Evidence-based Health Research , 6th Edition. Lippincott Williams & Wilkins, 2012. (Textbook of quantitative statistical methods used in health sciences research).
Rathbone, J., Hoffmann, T., & Glasziou, P. (2015). Faster title and abstract screening? Evaluating Abstrackr, a semi-automated online screening program for systematic reviewers. Systematic Reviews, 480. doi:10.1186/s13643-015-0067-6
Guyatt, G., Rennie, D., Meade, M., & Cook, D. (2015). Users' guides to the medical literature (3rd ed.). New York: McGraw-Hill Education Medical. (This is a foundational textbook on evidence-based medicine and of particular use to the reviewer who wants to learn about the different types of published research article e.g. "what is a case report?" and to understand what types of study design best answer what types of clinical question).
Glanville, J., Duffy, S., Mccool, R., & Varley, D. (2014). Searching ClinicalTrials.gov and the International Clinical Trials Registry Platform to inform systematic reviews: what are the optimal search approaches? Journal of the Medical Library Association : JMLA, 102(3), 177–183. https://doi.org/10.3163/1536-5050.102.3.007
Ouzzani, M., Hammady, H., Fedorowicz, Z., & Elmagarmid, A. (2016). Rayyan a web and mobile app for systematic reviews. Systematic Reviews, 5 : 210, DOI: 10.1186/s13643-016-0384-4. http://rdcu.be/nzDM
Kwon Y, Lemieux M, McTavish J, Wathen N. (2015). Identifying and removing duplicate records from systematic review searches. J Med Libr Assoc. 103 (4): 184-8. doi: 10.3163/1536-5050.103.4.004. https://www.ncbi.nlm.nih.gov/pubmed/26512216
Bramer WM, Giustini D, de Jonge GB, Holland L, Bekhuis T. (2016). De-duplication of database search results for systematic reviews in EndNote. J Med Libr Assoc. 104 (3):240-3. doi: 10.3163/1536-5050.104.3.014. Erratum in: J Med Libr Assoc. 2017 Jan;105(1):111. https://www.ncbi.nlm.nih.gov/pubmed/27366130
McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS Peer Review of Electronic Search Strategies: 2015 Guideline Statement. J Clin Epidemiol. 2016;75:40–46. doi: 10.1016/j.jclinepi.2016.01.021 . PRESS is a guideline with a checklist for librarians to critically appraise the search strategy for a systematic review literature search.
Clark, JM, Sanders, S, Carter, M, Honeyman, D, Cleo, G, Auld, Y, Booth, D, Condron, P, Dalais, C, Bateup, S, Linthwaite, B, May, N, Munn, J, Ramsay, L, Rickett, K, Rutter, C, Smith, A, Sondergeld, P, Wallin, M, Jones, M & Beller, E 2020, 'Improving the translation of search strategies using the Polyglot Search Translator: a randomized controlled trial', Journal of the Medical Library Association , vol. 108, no. 2, pp. 195-207.
Journal articles describing systematic review methods can be searched for in PubMed using this search string in the PubMed search box: sysrev_methods [sb] .
Software tools for systematic reviews
- Covidence GW in 2019 has bought a subscription to this Cloud based tool for facilitating screening decisions, used by the Cochrane Collaboration. Register for an account.
- NVIVO for analysis of qualitative research NVIVO is used for coding interview data to identify common themes emerging from interviews with several participants. GW faculty, staff, and students may download NVIVO software.
- RedCAP RedCAP is software that can be used to create survey forms for research or data collection or data extraction. It has very detailed functionality to enable data exchange with Electronic Health Record Systems, and to integrate with study workflow such as scheduling follow up reminders for study participants.
- SRDR tool from AHRQ Free, web-based and has a training environment, tutorials, and example templates of systematic review data extraction forms
- RevMan 5 RevMan 5 is the desktop version of the software used by Cochrane systematic review teams. RevMan 5 is free for academic use and can be downloaded and configured to run as stand alone software that does not connect with the Cochrane server if you follow the instructions at https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman/revman-5-download/non-cochrane-reviews
- Rayyan Free, web-based tool for collecting and screening citations. It has options to screen with multiple people, masking each other.
- GradePro Free, web application to create, manage and share summaries of research evidence (called Evidence Profiles and Summary of Findings Tables) for reviews or guidelines, uses the GRADE criteria to evaluate each paper under review.
- DistillerSR Needs subscription. Create coded data extraction forms from templates.
- EPPI Reviewer Needs subscription. Like DistillerSR, tool for text mining, data clustering, classification and term extraction
- SUMARI Needs subscription. Qualitative data analysis.
- Dedoose Needs subscription. Qualitative data analysis, similar to NVIVO in that it can be used to code interview transcripts, identify word co-occurence, cloud based.
- Meta-analysis software for statistical analysis of data for quantitative reviews SPSS, SAS, and STATA are popular analytical statistical software that include macros for carrying out meta-analysis. Himmelfarb has SPSS on some 3rd floor computers, and GW affiliates may download SAS to your own laptop from the Division of IT website. To perform mathematical analysis of big data sets there are statistical analysis software libraries in the R programming language available through GitHub and RStudio, but this requires advanced knowledge of the R and Python computer languages and data wrangling/cleaning.
- PRISMA 2020 flow diagram generator The PRISMA Statement website has a page listing example flow diagram templates and a link to software for creating PRISMA 2020 flow diagrams using R software.
GW researchers may want to consider using Refworks to manage citations, and GW Box to store the full text PDF's of review articles. You can also use online survey forms such as Qualtrics, RedCAP, or Survey Monkey, to design and create your own coded fillable forms, and export the data to Excel or one of the qualitative analytical software tools listed above.
Forest Plot Generators
- RevMan 5 the desktop version of the software used by Cochrane systematic review teams. RevMan 5 is free for academic use and can be downloaded and configured to run as stand alone software that does not connect with the Cochrane server if you follow the instructions at https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman/revman-5-download/non-cochrane-reviews.
- Meta-Essentials a free set of workbooks designed for Microsoft Excel that, based on your input, automatically produce meta-analyses including Forest Plots. Produced for Erasmus University Rotterdam joint research institute.
- Neyeloff, Fuchs & Moreira Another set of Excel worksheets and instructions to generate a Forest Plot. Published as Neyeloff, J.L., Fuchs, S.C. & Moreira, L.B. Meta-analyses and Forest plots using a microsoft excel spreadsheet: step-by-step guide focusing on descriptive data analysis. BMC Res Notes 5, 52 (2012). https://doi-org.proxygw.wrlc.org/10.1186/1756-0500-5-52
- For R programmers instructions are at https://cran.r-project.org/web/packages/forestplot/vignettes/forestplot.html and you can download the R code package from https://github.com/gforge/forestplot
- << Previous: Home
- Next: Protocol and registration >>

- Last Updated: Mar 21, 2024 11:08 AM
- URL: https://guides.himmelfarb.gwu.edu/systematic_review

- Himmelfarb Intranet
- Privacy Notice
- Terms of Use
- GW is committed to digital accessibility. If you experience a barrier that affects your ability to access content on this page, let us know via the Accessibility Feedback Form .
- Himmelfarb Health Sciences Library
- 2300 Eye St., NW, Washington, DC 20037
- Phone: (202) 994-2850
- [email protected]
- https://himmelfarb.gwu.edu

- Duke NetID Login
- 919.660.1100
- Duke Health Badge: 24-hour access
- Accounts & Access
- Databases, Journals & Books
- Request & Reserve
- Training & Consulting
- Request Articles & Books
- Renew Online
- Reserve Spaces
- Reserve a Locker
- Study & Meeting Rooms
- Course Reserves
- Digital Health Device Collection
- Pay Fines/Fees
- Recommend a Purchase
- Access From Off Campus
- Building Access
- Computers & Equipment
- Wifi Access
- My Accounts
- Mobile Apps
- Known Access Issues
- Report an Access Issue
- All Databases
- Article Databases
- Basic Sciences
- Clinical Sciences
- Dissertations & Theses
- Drugs, Chemicals & Toxicology
- Grants & Funding
- Interprofessional Education
- Non-Medical Databases
- Search for E-Journals
- Search for Print & E-Journals
- Search for E-Books
- Search for Print & E-Books
- E-Book Collections
- Biostatistics
- Global Health
- MBS Program
- Medical Students
- MMCi Program
- Occupational Therapy
- Path Asst Program
- Physical Therapy
- Researchers
- Community Partners
Conducting Research
- Archival & Historical Research
- Black History at Duke Health
- Data Analytics & Viz Software
- Data: Find and Share
- Evidence-Based Practice
- NIH Public Access Policy Compliance
- Publication Metrics
- Qualitative Research
- Searching Animal Alternatives
Systematic Reviews
- Test Instruments
Using Databases
- JCR Impact Factors
- Web of Science
Finding & Accessing
- COVID-19: Core Clinical Resources
- Health Literacy
- Health Statistics & Data
- Library Orientation
Writing & Citing
- Creating Links
- Getting Published
- Reference Mgmt
- Scientific Writing
Meet a Librarian
- Request a Consultation
- Find Your Liaisons
- Register for a Class
- Request a Class
- Self-Paced Learning
Search Services
- Literature Search
- Systematic Review
- Animal Alternatives (IACUC)
- Research Impact
Citation Mgmt
- Other Software
Scholarly Communications
- About Scholarly Communications
- Publish Your Work
- Measure Your Research Impact
- Engage in Open Science
- Libraries and Publishers
- Directions & Maps
- Floor Plans
Library Updates
- Annual Snapshot
- Conference Presentations
- Contact Information
- Gifts & Donations
- What is a Systematic Review?
Types of Reviews
- Manuals and Reporting Guidelines
- Our Service
- 1. Assemble Your Team
- 2. Develop a Research Question
- 3. Write and Register a Protocol
- 4. Search the Evidence
- 5. Screen Results
- 6. Assess for Quality and Bias
- 7. Extract the Data
- 8. Write the Review
- Additional Resources
- Finding Full-Text Articles
Review Typologies
There are many types of evidence synthesis projects, including systematic reviews as well as others. The selection of review type is wholly dependent on the research question. Not all research questions are well-suited for systematic reviews.
- Review Typologies (from LITR-EX) This site explores different review methodologies such as, systematic, scoping, realist, narrative, state of the art, meta-ethnography, critical, and integrative reviews. The LITR-EX site has a health professions education focus, but the advice and information is widely applicable.
Review the table to peruse review types and associated methodologies. Librarians can also help your team determine which review type might be appropriate for your project.
Reproduced from Grant, M. J. and Booth, A. (2009), A typology of reviews: an analysis of 14 review types and associated methodologies. Health Information & Libraries Journal, 26: 91-108. doi:10.1111/j.1471-1842.2009.00848.x
- << Previous: What is a Systematic Review?
- Next: Manuals and Reporting Guidelines >>
- Last Updated: Mar 20, 2024 2:21 PM
- URL: https://guides.mclibrary.duke.edu/sysreview
- Duke Health
- Duke University
- Duke Libraries
- Medical Center Archives
- Duke Directory
- Seeley G. Mudd Building
- 10 Searle Drive
- [email protected]

Library Services
UCL LIBRARY SERVICES
- Guides and databases
- Library skills
- Systematic reviews
Types of systematic reviews
- What are systematic reviews?
- Formulating a research question
- Identifying studies
- Searching databases
- Describing and appraising studies
- Synthesis and systematic maps
- Software for systematic reviews
- Online training and support
- Live and face to face training
- Individual support
- Further help

There are many types of systematic reviews. They may ask different kinds of questions and use a variety of methods, just like primary research. As with primary research, they vary in terms of perspective, purpose, approach, methods, and the time and resources used to conduct them.
Some reviews may ask broad questions (over a wide topic area) and examine them in little detail whilst others may ask narrow questions that are examined in great detail. Some reviews ask such broad questions that these are split into sub-questions that are addressed by different sub-components in the review each with different review methods considering different types of primary studies (multi-component reviews).
Dimensions of difference in systematic reviews
- EPPI Centre: Dimensions of Difference in Systematic Reviews This video (22 mins) describes the Dimensions of Difference in Systematic Reviews by David Gough of the UCL IOE EPPI-Centre.
Further reading
- Clarifying differences between review designs and methods / Gough et al. 2012 This paper clarifies differences between review designs and methods.
- Approaches to evidence synthesis in international development: a research agenda / Oliver et al. 2018 International development is an area where systematic review methods have been applied to complex situations for a variety of purposes. This paper details some of these different approaches and synthesis methods,
- Perspectives on the methods of a large systematic mapping of maternal health interventions / Chersich et al. 2016 One large systematic map describes maternal health interventions in 2292 full text articles. The perspectives of 15 researchers from the review team provide insights into some of the challenges in systematically identifying and describing studies, and in being part of a large review team.
Demystifying literature reviews
- Demystifying Literature Reviews: What I Have Learned From an Expert? This paper illustrates some differences between three broad groups of reviews, provides some practical insights into searching and other stages in reviewing, and gives further readings. Please note: this paper briefly groups scoping reviews and mapping reviews together, but these two types of reviews can be very different from each other.
- Systematic evidence maps Find out more about how scoping reviews and mapping reviews differ.
- << Previous: What are systematic reviews?
- Next: Stages in a systematic review >>
- Last Updated: Apr 4, 2024 10:09 AM
- URL: https://library-guides.ucl.ac.uk/systematic-reviews

- University of Texas Libraries
- UT Libraries
Systematic Reviews & Evidence Synthesis Methods
Types of reviews.
- Formulate Question
- Find Existing Reviews & Protocols
- Register a Protocol
- Searching Systematically
- Supplementary Searching
- Managing Results
- Deduplication
- Critical Appraisal
- Glossary of terms
- Librarian Support
- Video tutorials This link opens in a new window
- Systematic Review & Evidence Synthesis Boot Camp
Not sure what type of review you want to conduct?
There are many types of reviews --- narrative reviews , scoping reviews , systematic reviews, integrative reviews, umbrella reviews, rapid reviews and others --- and it's not always straightforward to choose which type of review to conduct. These Review Navigator tools (see below) ask a series of questions to guide you through the various kinds of reviews and to help you determine the best choice for your research needs.
- Which review is right for you? (Univ. of Manitoba)
- What type of review is right for you? (Cornell)
- Review Ready Reckoner - Assessment Tool (RRRsAT)
- A typology of reviews: an analysis of 14 review types and associated methodologies. by Grant & Booth
- Meeting the review family: exploring review types and associated information retrieval requirements | Health Info Libr J, 2019
Reproduced from Grant MJ, Booth A. A typology of reviews: an analysis of 14 review types and associated methodologies . Health Info Libr J. 2009 Jun;26(2):91-108. doi: 10.1111/j.1471-1842.2009.00848.x
- Last Updated: Apr 9, 2024 8:57 PM
- URL: https://guides.lib.utexas.edu/systematicreviews


Systematic Review Process: Types of Reviews
- Definitions of a Systematic Review
Types of Reviews
- Systematic Review Planning Process
- Resources Needed to Conduct a Review
- Reporting Guidelines
- Where to Search
- How to Search
- Screening and Study Selection
- Data Extraction
- Appraisal and Analysis
- Citation Management
- Additional Resources: Guides and Books
- Using Covidence for Your Systematic Review
- Librarian Collaboration
Narrative vs. Systematic Reviews
People often confuse systematic and literature (narrative) reviews. They both are used to provide a summary of the existing literature or research on a specific topic.
A narrative or traditional literature review is a comprehensive, critical, and objective analysis of the current knowledge on a topic. They are an essential part of the research process and help to establish a theoretical framework and focus or context for your research. A literature review will help you to identify patterns and trends in the literature so that you can identify gaps or inconsistencies in a body of knowledge. This should lead you to a sufficiently focused research question that justifies your research.
A systematic review is comprehensive and has minimal bias. It is based on a specific question and uses eligibility criteria and a pre-planned protocol. This type of study evaluates the quality of evidence.
A systematic review can be either quantitative or qualitative:
- If quantitative, the review will include studies that have numerical data.
- If qualitative, the review derives data from observation, interviews, or verbal interactions and focuses on the meanings and interpretations of the participants. It will include focus groups, interviews, observations and diaries.
Narrative reviews in comparison provide a perspective on topic (like a textbook chapter), may have no specified search strategy, might have significant bias issues, and may not evaluate quality of evidence.
This table provides a detailed comparison of systematic and literature (narrative) reviews.
Tools to Help You Choose a Review Type
There are other comprehensive literature reviews of similar methodology to the systematic review. These tools can help you determine which type of review you may want to conduct.
- The Review Ready Reckoner - Assessment Tool (RRRsAT) is a chart created as an adaptation of Andrew Booth's article on review typology. The chart that describes the features of multiple review types listing characteristics that distinguish each type and including sample of each type of review.
- The What Review is Right for You tool asks five short questions to help you identify the most appropriate method for a review.
Use this chart to determine the type of review you are interested in writing and to learn the differences in the stages and processes of various reviews compared to systematic reviews.
Source: Yale University
The type of review you conduct will depend on the purpose of the review, your question, your resources, expertise, and type of data.
Here are two suggested articles to consult if you want to know more about review types:
Grant, M. J., & Booth, A. (2009). A typology of reviews: an analysis of 14 review types and associated methodologies. Health information & libraries journal , 26 (2), 91-108. This article defines 14 types of reviews. There is a helpful summary table on pp.94-95
Sutton A, Clowes M, Preston L, Booth A. Meeting the review family: exploring review types and associated information retrieval requirements. Health information & libraries journal . 2019;36(3):202–222. doi:10.1111/hir.12276
This Comparison table is derived from a guide which is licensed under Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International license , and was originally included in a workbook by Amanda Wanner at Plymouth University for Systematic Reviews and Scoping Reviews. Stephanie Roth at Temple University remixed the original version. Many thanks and much appreciation to Amanda Wanner and Stephanie Roth for allowing me to create a derivative of their work.

Funaro, M., Nyhan, K., & Brackett, A. (n.d.). What type of review could you write? Yale Harvey Cushing/John Hay Whitney Medical Library.
- << Previous: Definitions of a Systematic Review
- Next: Systematic Review Planning Process >>
- Last Updated: Dec 27, 2023 12:48 PM
- URL: https://library.aah.org/guides/systematicreview
- Correspondence
- Open access
- Published: 10 January 2018
What kind of systematic review should I conduct? A proposed typology and guidance for systematic reviewers in the medical and health sciences
- Zachary Munn ORCID: orcid.org/0000-0002-7091-5842 1 ,
- Cindy Stern 1 ,
- Edoardo Aromataris 1 ,
- Craig Lockwood 1 &
- Zoe Jordan 1
BMC Medical Research Methodology volume 18 , Article number: 5 ( 2018 ) Cite this article
120k Accesses
2019 Citations
406 Altmetric
Metrics details
Systematic reviews have been considered as the pillar on which evidence-based healthcare rests. Systematic review methodology has evolved and been modified over the years to accommodate the range of questions that may arise in the health and medical sciences. This paper explores a concept still rarely considered by novice authors and in the literature: determining the type of systematic review to undertake based on a research question or priority.
Within the framework of the evidence-based healthcare paradigm, defining the question and type of systematic review to conduct is a pivotal first step that will guide the rest of the process and has the potential to impact on other aspects of the evidence-based healthcare cycle (evidence generation, transfer and implementation). It is something that novice reviewers (and others not familiar with the range of review types available) need to take account of but frequently overlook. Our aim is to provide a typology of review types and describe key elements that need to be addressed during question development for each type.
Conclusions
In this paper a typology is proposed of various systematic review methodologies. The review types are defined and situated with regard to establishing corresponding questions and inclusion criteria. The ultimate objective is to provide clarified guidance for both novice and experienced reviewers and a unified typology with respect to review types.
Peer Review reports
Introduction
Systematic reviews are the gold standard to search for, collate, critique and summarize the best available evidence regarding a clinical question [ 1 , 2 ]. The results of systematic reviews provide the most valid evidence base to inform the development of trustworthy clinical guidelines (and their recommendations) and clinical decision making [ 2 ]. They follow a structured research process that requires rigorous methods to ensure that the results are both reliable and meaningful to end users. Systematic reviews are therefore seen as the pillar of evidence-based healthcare [ 3 , 4 , 5 , 6 ]. However, systematic review methodology and the language used to express that methodology, has progressed significantly since their appearance in healthcare in the 1970’s and 80’s [ 7 , 8 ]. The diachronic nature of this evolution has caused, and continues to cause, great confusion for both novice and experienced researchers seeking to synthesise various forms of evidence. Indeed, it has already been argued that the current proliferation of review types is creating challenges for the terminology for describing such reviews [ 9 ]. These fundamental issues primarily relate to a) the types of questions being asked and b) the types of evidence used to answer those questions.
Traditionally, systematic reviews have been predominantly conducted to assess the effectiveness of health interventions by critically examining and summarizing the results of randomized controlled trials (RCTs) (using meta-analysis where feasible) [ 4 , 10 ]. However, health professionals are concerned with questions other than whether an intervention or therapy is effective, and this is reflected in the wide range of research approaches utilized in the health field to generate knowledge for practice. As such, Pearson and colleagues have argued for a pluralistic approach when considering what counts as evidence in health care; suggesting that not all questions can be answered from studies measuring effectiveness alone [ 4 , 11 ]. As the methods to conduct systematic reviews have evolved and advanced, so too has the thinking around the types of questions we want and need to answer in order to provide the best possible, evidence-based care [ 4 , 11 ].
Even though most systematic reviews conducted today still focus on questions relating to the effectiveness of medical interventions, many other review types which adhere to the principles and nomenclature of a systematic review have emerged to address the diverse information needs of healthcare professionals and policy makers. This increasing array of systematic review options may be confusing for the novice systematic reviewer, and in our experience as educators, peer reviewers and editors we find that many beginner reviewers struggle to achieve conceptual clarity when planning for a systematic review on an issue other than effectiveness. For example, reviewers regularly try to force their question into the PICO format (population, intervention, comparator and outcome), even though their question may be an issue of diagnostic test accuracy or prognosis; attempting to define all the elements of PICO can confound the remainder of the review process. The aim of this article is to propose a typology of systematic review types aligned to review questions to assist and guide the novice systematic reviewer and editors, peer-reviewers and policy makers. To our knowledge, this is the first classification of types of systematic reviews foci conducted in the medical and health sciences into one central typology.
Review typology
For the purpose of this typology a systematic review is defined as a robust, reproducible, structured critical synthesis of existing research. While other approaches to the synthesis of evidence exist (including but not limited to literature reviews, evidence maps, rapid reviews, integrative reviews, scoping and umbrella reviews), this paper seeks only to include approaches that subscribe to the above definition. As such, ten different types of systematic review foci are listed below and in Table 1 . In this proposed typology, we provide the key elements for formulating a question for each of the 10 review types.
Effectiveness reviews [ 12 ]
Experiential (Qualitative) reviews [ 13 ]
Costs/Economic Evaluation reviews [ 14 ]
Prevalence and/or Incidence reviews [ 15 ]
Diagnostic Test Accuracy reviews [ 16 ]
Etiology and/or Risk reviews [ 17 ]
Expert opinion/policy reviews [ 18 ]
Psychometric reviews [ 19 ]
Prognostic reviews [ 20 ]
Methodological systematic reviews [ 21 , 22 ]
Effectiveness reviews
Systematic reviews assessing the effectiveness of an intervention or therapy are by far the most common. Essentially effectiveness is the extent to which an intervention, when used appropriately, achieves the intended effect [ 11 ]. The PICO approach (see Table 1 ) to question development is well known [ 23 ] and comprehensive guidance for these types of reviews is available [ 24 ]. Characteristics regarding the population (e.g. demographic and socioeconomic factors and setting), intervention (e.g. variations in dosage/intensity, delivery mode, and frequency/duration/timing of delivery), comparator (active or passive) and outcomes (primary and secondary including benefits and harms, how outcomes will be measured including the timing of measurement) need to be carefully considered and appropriately justified.
Experiential (qualitative) reviews
Experiential (qualitative) reviews focus on analyzing human experiences and cultural and social phenomena. Reviews including qualitative evidence may focus on the engagement between the participant and the intervention, as such a qualitative review may describe an intervention, but its question focuses on the perspective of the individuals experiencing it as part of a larger phenomenon. They can be important in exploring and explaining why interventions are or are not effective from a person-centered perspective. Similarly, this type of review can explain and explore why an intervention is not adopted in spite of evidence of its effectiveness [ 4 , 13 , 25 ]. They are important in providing information on the patient’s experience, which can enable the health professional to better understand and interact with patients. The mnemonic PICo can be used to guide question development (see Table 1 ). With qualitative evidence there is no outcome or comparator to be considered. A phenomenon of interest is the experience, event or process occurring that is under study, such as response to pain or coping with breast cancer; it differs from an intervention in its focus. Context will vary depending on the objective of the review; it may include consideration of cultural factors such as geographic location, specific racial or gender based interests, and details about the setting such as acute care, primary healthcare, or the community [ 4 , 13 , 25 ]. Reviews assessing the experience of a phenomenon may opt to use a mixed methods approach and also include quantitative data, such as that from surveys. There are reporting guidelines available for qualitative reviews, including the ‘Enhancing transparency in reporting the synthesis of qualitative research’ (ENTREQ) statement [ 26 ] and the newly proposed meta-ethnography reporting guidelines (eMERGe) [ 27 ].
Costs/economic evaluation reviews
Costs/Economics reviews assess the costs of a certain intervention, process, or procedure. In any society, resources available (including dollars) have alternative uses. In order to make the best decisions about alternative courses of action evidence is needed on the health benefits and also on the types and amount of resources needed for these courses of action. Health economic evaluations are particularly useful to inform health policy decisions attempting to achieve equality in healthcare provision to all members of society and are commonly used to justify the existence and development of health services, new health technologies and also, clinical guideline development [ 14 ]. Issues of cost and resource use may be standalone reviews or components of effectiveness reviews [ 28 ]. Cost/Economic evaluations are examples of a quantitative review and as such can follow the PICO mnemonic (see Table 1 ). Consideration should be given to whether the entire world/international population is to be considered or only a population (or sub-population) of a particular country. Details of the intervention and comparator should include the nature of services/care delivered, time period of delivery, dosage/intensity, co-interventions, and personnel undertaking delivery. Consider if outcomes will only focus on resource usage and costs of the intervention and its comparator(s) or additionally on cost-effectiveness. Context (including perspective) can also be considered in these types of questions e.g. health setting(s).
Prevalence and/or incidence reviews
Essentially prevalence or incidence reviews measure disease burden (whether at a local, national or global level). Prevalence refers to the proportion of a population who have a certain disease whereas incidence relates to how often a disease occurs. These types of reviews enable governments, policy makers, health professionals and the general population to inform the development and delivery of health services and evaluate changes and trends in diseases over time [ 15 , 29 ]. Prevalence or incidence reviews are important in the description of geographical distribution of a variable and the variation between subgroups (such as gender or socioeconomic status), and for informing health care planning and resource allocation. The CoCoPop framework can be used for reviews addressing a question relevant to prevalence or incidence (see Table 1 ). Condition refers to the variable of interest and can be a health condition, disease, symptom, event of factor. Information regarding how the condition will be measured, diagnosed or confirmed should be provided. Environmental factors can have a substantial impact on the prevalence or incidence of a condition so it is important that authors define the context or specific setting relevant to their review question [ 15 , 29 ]. The population or study subjects should be clearly defined and described in detail.
Diagnostic test accuracy reviews
Systematic reviews assessing diagnostic test accuracy provide a summary of test performance and are important for clinicians and other healthcare practitioners in order to determine the accuracy of the diagnostic tests they use or are considering using [ 16 ]. Diagnostic tests are used by clinicians to identify the presence or absence of a condition in a patient for the purpose of developing an appropriate treatment plan. Often there are several tests available for diagnosis. The mnemonic PIRD is recommended for question development for these types of systematic reviews (see Table 1 ). The population is all participants who will undergo the diagnostic test while the index test(s) is the diagnostic test whose accuracy is being investigated in the review. Consider if multiple iterations of a test exist and who carries out or interprets the test, the conditions the test is conducted under and specific details regarding how the test will be conducted. The reference test is the ‘gold standard’ test to which the results of the index test will be compared. It should be the best test currently available for the diagnosis of the condition of interest. Diagnosis of interest relates to what diagnosis is being investigated in the systematic review. This may be a disease, injury, disability or any other pathological condition [ 16 ].
Etiology and/or risk reviews
Systematic reviews of etiology and risk are important for informing healthcare planning and resource allocation, and are particularly valuable for decision makers when making decisions regarding health policy and prevention of adverse health outcomes. The common objective of many of these types of reviews is to determine whether and to what degree a relationship exists between an exposure and a health outcome. Use of the PEO mnemonic is recommended (see Table 1 ). The review question should outline the exposure, disease, symptom or health condition of interest, the population or groups at risk, as well as the context/location, the time period and the length of time where relevant [ 17 ]. The exposure of interest refers to a particular risk factor or several risk factors associated with a disease/condition of interest in a population, group or cohort who have been exposed to them. It should be clearly reported what the exposure or risk factor is, and how it may be measured/identified including the dose and nature of exposure and the duration of exposure, if relevant. Important outcomes of interest relevant to the health issue and important to key stakeholders (e.g. knowledge users, consumers, policy makers, payers etc.) must be specified. Guidance now exists for conducting these types of reviews [ 17 ]. As these reviews rely heavily on observational studies, the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) [ 30 ] reporting guidelines should be referred to in addition to the PRISMA guidelines.
Expert opinion/policy reviews
Expert opinion and policy analysis systematic reviews focus on the synthesis of narrative text and/or policy. Expert opinion has a role to play in evidence-based healthcare, as it can be used to either complement empirical evidence or, in the absence of research studies, stand alone as the best available evidence. The synthesis of findings from expert opinion within the systematic review process is not well recognized in mainstream evidence-based practice. However, in the absence of research studies, the use of a transparent systematic process to identify the best available evidence drawn from text and opinion can provide practical guidance to practitioners and policy makers [ 18 ]. While a number of mnemonics have been discussed previously that can be used for opinion and text, not all elements necessarily apply to every text or opinion-based review, and use of mnemonics should be considered a guide rather than a policy. Broadly PICo can be used where I can refer to either the intervention or a phenomena of interest (see Table 1 ). Reviewers will need to describe the population, giving attention to whether specific characteristics of interest, such as age, gender, level of education or professional qualification are important to the question. As with other types of reviews, interventions may be broad areas of practice management, or specific, singular interventions. However, reviews of text or opinion may also reflect an interest in opinions around power, politics or other aspects of health care other than direct interventions, in which case, these should be described in detail. The use of a comparator and specific outcome statement is not necessarily required for a review of text and opinion based literature. In circumstances where they are considered appropriate, the nature and characteristics of the comparator and outcomes should be described [ 18 ].
Psychometric reviews
Psychometric systematic reviews (or systematic reviews of measurement properties) are conducted to assess the quality/characteristics of health measurement instruments to determine the best tool for use (in terms of its validity, reliability, responsiveness etc.) in practice for a certain condition or factor [ 31 , 32 , 33 ]. A psychometric systematic review may be undertaken on a) the measurement properties of one measurement instrument, b) the measurement properties of the most commonly utilized measurement instruments measuring a specific construct, c) the measurement properties of all available measurement instruments to measure a specific construct in a specific population or d) the measurement properties of all available measurement instruments in a specific population that does not specify the construct to be measured. The COnsensus-based Standards for the selection of health Measurement Instruments (COSMIN) group have developed guidance for conducting these types of reviews [ 19 , 31 ]. They recommend firstly defining the type of review to be conducted as well as the construct or the name(s) of the outcome measurement instrument(s) of interest, the target population, the type of measurement instrument of interest (e.g. questionnaires, imaging tests) and the measurement properties on which the review investigates (see Table 1 ).
Prognostic reviews
Prognostic research is of high value as it provides clinicians and patients with information regarding the course of a disease and potential outcomes, in addition to potentially providing useful information to deliver targeted therapy relating to specific prognostic factors [ 20 , 34 , 35 ]. Prognostic reviews are complex and methodology for these types of reviews is still under development, although a Cochrane methods group exists to support this approach [ 20 ]. Potential systematic reviewers wishing to conduct a prognostic review may be interested in determining the overall prognosis for a condition, the link between specific prognostic factors and an outcome and/or prognostic/prediction models and prognostic tests [ 20 , 34 , 35 , 36 , 37 ]. Currently there is little information available to guide the development of a well-defined review question however the Quality in Prognosis Studies (QUIPS) tool [ 34 ] and the Checklist for critical appraisal and data extraction for systematic reviews of prediction modelling studies (CHARMS Checklist) [ 38 ] have been developed to assist in this process (see Table 1 ).
Methodology systematic reviews
Systematic reviews can be conducted for methodological purposes [ 39 ], and examples of these reviews are available in the Cochrane Database [ 40 , 41 ] and elsewhere [ 21 ]. These reviews can be performed to examine any methodological issues relating to the design, conduct and review of research studies and also evidence syntheses. There is limited guidance for conducting these reviews, although there does exist an appendix in the Cochrane Handbook focusing specifically on methodological reviews [ 39 ]. They suggest following the SDMO approach where the types of studies should define all eligible study designs as well as any thresholds for inclusion (e.g. RCTS and quasi-RCTs). Types of data should detail the raw material for the methodology studies (e.g. original research submitted to biomedical journals) and the comparisons of interest should be described under types of methods (e.g. blinded peer review versus unblinded peer review) (see Table 1 ). Lastly both primary and secondary outcome measures should be listed (e.g. quality of published report) [ 39 ].
The need to establish a specific, focussed question that can be utilized to define search terms, inclusion and exclusion criteria and interpretation of data within a systematic review is an ongoing issue [ 42 ]. This paper provides an up-to-date typology for systematic reviews which reflects the current state of systematic review conduct. It is now possible that almost any question can be subjected to the process of systematic review. However, it can be daunting and difficult for the novice researcher to determine what type of review they require and how they should conceptualize and phrase their review question, inclusion criteria and the appropriate methods for analysis and synthesis [ 23 ]. Ensuring that the review question is well formed is of the utmost importance as question design has the most significant impact on the conduct of a systematic review as the subsequent inclusion criteria are drawn from the question and provide the operational framework for the review [ 23 ]. In this proposed typology, we provide the key elements for formulating a question for each of the 10 review types.
When structuring a systematic review question some of these key elements are universally agreed (such as PICO for effectiveness reviews) whilst others are more novel. For example, the use of PIRD for diagnostic reviews contrasts with other mnemonics, such as PITR [ 43 ], PPP-ICP-TR [ 44 ] or PIRATE [ 45 ]. Qualitative reviews have sometimes been guided by the mnemonic SPIDER, however this has been recommended against for guiding searching due to it not identifying papers that are relevant [ 46 ]. Variations on our guidance exist, with the additional question elements of ‘time’ (PICOT) and study types (PICOS) also existing. Reviewers are advised to consider these elements when crafting their question to determine if they are relevant for their topic. We believe that based on the guidance included in this typology, constructing a well-built question for a systematic review is a skill that can be mastered even for the novice reviewer.
Related to this discussion of a typology for systematic reviews is the issue of how to distinguish a systematic review from a literature review. When searching the literature, you may come across papers referred to as ‘systematic reviews,’ however, in reality they do not necessarily fit this description [ 21 ]. This is of significant concern given the common acceptance of systematic reviews as ‘level 1’ evidence and the best study design to inform practice. However, many of these reviews are simply literature reviews masquerading as the ideal product. It is therefore important to have a critical eye when assessing publications identified as systematic reviews. Today, the methodology of systematic reviews continues to evolve. However, there is general acceptance of certain steps being required in a systematic review of any evidence type [ 2 ] and these should be used to distinguish between a literature review and a systematic review. The following can be viewed as the defining features of a systematic review and its conduct [ 1 , 2 ]:
Clearly articulated objectives and questions to be addressed
Inclusion and exclusion criteria, stipulated a priori (in a protocol), that determine the eligibility of studies
A comprehensive search to identify all relevant studies, both published and unpublished
A process of study screening and selection
Appraisal of the quality of included studies/ papers (risk of bias) and assessment of the validity of their results/findings/ conclusions
Analysis of data extracted from the included research
Presentation and synthesis of the results/ findings extracted
Interpret the results, potentially establishing the certainty of the results and making and implications for practice and research
Transparent reporting of the methodology and methods used to conduct the review
Prior to deciding what type of review to conduct, the reviewer should be clear that a systematic review is the best approach. A systematic review may be undertaken to confirm whether current practice is based on evidence (or not) and to address any uncertainty or variation in practice that may be occurring. Conducting a systematic review also identifies where evidence is not available and can help categorize future research in the area. Most importantly, they are used to produce statements to guide decision-making. Indications for systematic reviews:
uncover the international evidence
confirm current practice/ address any variation
identify areas for future research
investigate conflicting results
produce statements to guide decision-making
The popularity of systematic reviews has resulted in the creation of various evidence review processes over the last 30 years. These include integrative reviews, scoping reviews [ 47 ], evidence maps [ 48 ], realist syntheses [ 49 ], rapid reviews [ 50 ], umbrella reviews (systematic reviews of reviews) [ 51 ], mixed methods reviews [ 52 ], concept analyses [ 53 ] and others. Useful typologies of these diverse review types can be used as reference for researchers, policy makers and funders when discussing a review approach [ 54 , 55 ]. It was not the purpose of this article to describe and define each of these diverse evidence synthesis methods as our focus was purely on systematic review questions. Depending on the researcher, their question/s and their resources at hand, one of these approaches may be the best fit for answering a particular question.
Gough and colleagues [ 9 ] provided clarification between different review designs and methods but stopped short of providing a taxonomy of review types. The rationale for this was that in the field of evidence synthesis ‘the rate of development of new approaches to reviewing is too fast and the overlap of approaches too great for that to be helpful.’ [ 9 ] They instead provide a useful description of how reviews may differ and more importantly why this may be the case. It is also our view that evidence synthesis methodology is a rapidly developing field, and that even within the review types classified here (such as effectiveness [ 56 ] or experiential [qualitative [ 57 ]]) there may be many different subsets and complexities that need to be addressed. Essentially, the classifications listed above may be just the initial level of a much larger family tree. We believe that this typology will provide a useful contribution to efforts to sort and classify evidence review approaches and understand the need for this to be updated over time. A useful next step might be the development of a comprehensive taxonomy to further guide reviewers in making a determination about the most appropriate evidence synthesis product to undertake for a particular purpose or question.
Systematic reviews of animal studies (or preclinical systematic reviews) have not been common practice in the past (when comparing to clinical research) although this is changing [ 58 , 59 , 60 , 61 ]. Systematic reviews of these types of studies can be useful to inform the design of future experiments (both preclinical and clinical) [ 59 ] and address an important gap in translation science [ 5 , 60 ]. Guidance for these types of reviews is now emerging [ 58 , 60 , 62 , 63 , 64 ]. These review types, which are often hypothesis generating, were excluded from our typology as they are only very rarely used to answer a clinical question.
Systematic reviews are clearly an indispensable component in the chain of scientific enquiry in a much broader sense than simply to inform policy and practice and therefore ensuring that they are designed in a rigorous manner, addressing appropriate questions driven by clinical and policy needs is essential. With the ever-increasing global investment in health research it is imperative that the needs of health service providers and end users are met. It has been suggested that one way to ensure this occurs is to precede any research investment with a systematic review of existing research [ 65 ]. However, the only way that such a strategy would be effective would be if all reviews conducted are done so with due rigour.
It has been argued recently that there is mass production of reviews that are often unnecessary, misleading and conflicted with most having weak or insufficient evidence to inform decision making [ 66 ]. Indeed, asking has been identified as a core functional competency associated with obtaining and applying the best available evidence [ 67 ]. Fundamental to the tenets of evidence-based healthcare and, in particular evidence implementation, is the ability to formulate a question that is amenable to obtaining evidence and “structured thinking” around question development is critical to its success [ 67 ]. The application of evidence can be significantly hampered when existing evidence does not correspond to the situations that practitioners (or guideline developers) are faced with. Hence, determination of appropriate review types that respond to relevant clinical and policy questions is essential.
The revised JBI Model of Evidence-Based Healthcare clarifies the conceptual integration of evidence generation, synthesis, transfer and implementation, “linking how these occur with the necessarily challenging dynamics that contribute to whether translation of evidence into policy and practice is successful” [ 68 ]. Fundamental to this approach is the recognition that the process of evidence-based healthcare is not prescriptive or linear, but bi-directional, with each component having the potential to affect what occurs on either side of it. Thus, a systematic review can impact upon the types of primary research that are generated as a result of recommendations produced in the review (evidence generation) but also on the success of their uptake in policy and practice (evidence implementation). It is therefore critical for those undertaking systematic reviews to have a solid understanding of the type of review required to respond to their question.
For novice reviewers, or those unfamiliar with the broad range of review types now available, access to a typology to inform their question development is timely. The typology described above provides a framework that indicates the antecedents and determinants of undertaking a systematic review. There are several factors that may lead an author to conduct a review and these may or may not start with a clearly articulated clinical or policy question. Having a better understanding of the review types available and the questions that these reviews types lend themselves to answering is critical to the success or otherwise of a review. Given the significant resource required to undertake a review this first step is critical as it will impact upon what occurs in both evidence generation and evidence implementation. Thus, enabling novice and experienced reviewers to ensure that they are undertaking the “right” review to respond to a clinical or policy question appropriately has strategic implications from a broader evidence-based healthcare perspective.
Systematic reviews are the ideal method to rigorously collate, examine and synthesize a body of literature. Systematic review methods now exist for most questions that may arise in healthcare. This article provides a typology for systematic reviewers when deciding on their approach in addition to guidance on structuring their review question. This proposed typology provides the first known attempt to sort and classify systematic review types and their question development frameworks and therefore it can be a useful tool for researchers, policy makers and funders when deciding on an appropriate approach.
Abbreviations
CHecklist for critical Appraisal and data extraction for systematic Reviews of prediction Modelling Studies
Condition, Context, Population
COnsensus-based Standards for the selection of health Measurement Instruments
- Evidence-based healthcare
Meta-ethnography reporting guidelines
Enhancing transparency in reporting the synthesis of qualitative research
Joanna Briggs Institute
Meta-analysis Of Observational Studies in Epidemiology
Population, Exposure, Outcome
Population, Prognostic Factors (or models of interest), Outcome
Population, Intervention, Comparator, Outcome
Population, Phenomena of Interest, Context
Population, Intervention, Comparator/s, Outcomes, Context
Population, Index Test, Reference Test, Diagnosis of Interest
Quality in Prognosis Studies
Randomised controlled trial
Studies, Data, Methods, Outcomes
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (Clinical research ed). 2009;339:b2700.
Article Google Scholar
Aromataris E, Pearson A. The systematic review: an overview. AJN. Am J Nurs. 2014;114(3):53–8.
Article PubMed Google Scholar
Munn Z, Porritt K, Lockwood C, Aromataris E, Pearson A. Establishing confidence in the output of qualitative research synthesis: the ConQual approach. BMC Med Res Methodol. 2014;14:108.
Article PubMed PubMed Central Google Scholar
Pearson A. Balancing the evidence: incorporating the synthesis of qualitative data into systematic reviews. JBI Reports. 2004;2:45–64.
Pearson A, Jordan Z, Munn Z. Translational science and evidence-based healthcare: a clarification and reconceptualization of how knowledge is generated and used in healthcare. Nursing research and practice. 2012;2012:792519.
Steinberg E, Greenfield S, Mancher M, Wolman DM, Graham R. Clinical practice guidelines we can trust: National Academies Press 2011.
Bastian H, Glasziou P, Chalmers I. Seventy-five trials and eleven systematic reviews a day: how will we ever keep up? PLoS Med. 2010;7(9):e1000326.
Chalmers I, Hedges LV, Cooper HA. Brief history of research synthesis. Eval Health Prof. 2002;25(1):12–37.
Gough D, Thomas J, Oliver S. Clarifying differences between review designs and methods. Systematic Reviews. 2012;1:28.
Munn Z, Tufanaru C, Aromataris EJBI. S systematic reviews: data extraction and synthesis. Am J Nurs. 2014;114(7):49–54.
Pearson A, Wiechula R, Court A, Lockwood C. The JBI model of evidence-based healthcare. International Journal of Evidence-Based Healthcare. 2005;3(8):207–15.
PubMed Google Scholar
Tufanaru C, Munn Z, Stephenson M, Aromataris E. Fixed or random effects meta-analysis? Common methodological issues in systematic reviews of effectiveness. Int J Evid Based Healthc. 2015;13(3):196–207.
Lockwood C, Munn Z, Porritt K. Qualitative research synthesis: methodological guidance for systematic reviewers utilizing meta-aggregation. Int J Evid Based Healthc. 2015;13(3):179–87.
Gomersall JS, Jadotte YT, Xue Y, Lockwood S, Riddle D, Preda A. Conducting systematic reviews of economic evaluations. Int J Evid Based Healthc. 2015;13(3):170–8.
Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc. 2015;13(3):147–53.
Campbell JM, Klugar M, Ding S, et al. Diagnostic test accuracy: methods for systematic review and meta-analysis. Int J Evid Based Healthc. 2015;13(3):154–62.
Moola S, Munn Z, Sears K, et al. Conducting systematic reviews of association (etiology): the Joanna Briggs Institute's approach. Int J Evid Based Healthc. 2015;13(3):163–9.
McArthur A, Klugarova J, Yan H, Florescu S. Innovations in the systematic review of text and opinion. Int J Evid Based Healthc. 2015;13(3):188–95.
Mokkink LB, Terwee CB, Patrick DL, et al. The COSMIN checklist for assessing the methodological quality of studies on measurement properties of health status measurement instruments: an international Delphi study. Qual Life Res. 2010;19(4):539–49.
Dretzke J, Ensor J, Bayliss S, et al. Methodological issues and recommendations for systematic reviews of prognostic studies: an example from cardiovascular disease. Systematic reviews. 2014;3(1):1.
Campbell JM, Kavanagh S, Kurmis R, Munn Z. Systematic Reviews in Burns Care: Poor Quality and Getting Worse. Journal of Burn Care & Research. 9000;Publish Ahead of Print.
France EF, Ring N, Thomas R, Noyes J, Maxwell M, Jepson RA. Methodological systematic review of what’s wrong with meta-ethnography reporting. BMC Med Res Methodol. 2014;14(1):1.
Stern C, Jordan Z, McArthur A. Developing the review question and inclusion criteria. Am J Nurs. 2014;114(4):53–6.
Higgins J, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. ed: The Cochrane Collaboration 2011.
Hannes K, Lockwood C, Pearson AA. Comparative analysis of three online appraisal instruments' ability to assess validity in qualitative research. Qual Health Res. 2010;20(12):1736–43.
Tong A, Flemming K, McInnes E, Oliver S, Craig J. Enhancing transparency in reporting the synthesis of qualitative research: ENTREQ. BMC Med Res Methodol. 2012;12:181.
France EF, Ring N, Noyes J, et al. Protocol-developing meta-ethnography reporting guidelines (eMERGe). BMC Med Res Methodol. 2015;15:103.
Article CAS PubMed PubMed Central Google Scholar
Shemilt I, Mugford M, Byford S, et al. In: JPT H, Green S, editors. Chapter 15: incorporating economics evidence. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration: In; 2011.
Google Scholar
Munn Z, Moola S, Riitano D, Lisy K. The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. Int J Health Policy Manag. 2014;3(3):123–8.
Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–12.
Article CAS PubMed Google Scholar
COSMIN: COnsensus-based Standards for the selection of health Measurement INstruments. Systematic reviews of measurement properties. [cited 8th December 2016]; Available from: http://www.cosmin.nl/Systematic%20reviews%20of%20measurement%20properties.html
Terwee CB, HCWd V, CAC P, Mokkink LB. Protocol for systematic reviews of measurement properties. COSMIN: Knowledgecenter Measurement Instruments; 2011.
Mokkink LB, Terwee CB, Stratford PW, et al. Evaluation of the methodological quality of systematic reviews of health status measurement instruments. Qual Life Res. 2009;18(3):313–33.
Hayden JA, van der Windt DA, Cartwright JL, CÃ P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158(4):280–6.
The Cochrane Collaboration. Cochrane Methods Prognosis. 2016 [cited 7th December 2016]; Available from: http://methods.cochrane.org/prognosis/scope-our-work .
Rector TS, Taylor BC, Wilt TJ. Chapter 12: systematic review of prognostic tests. J Gen Intern Med. 2012;27(Suppl 1):S94–101.
Peters S, Johnston V, Hines S, Ross M, Coppieters M. Prognostic factors for return-to-work following surgery for carpal tunnel syndrome: a systematic review. JBI Database of Systematic Reviews and Implementation Reports. 2016;14(9):135–216.
Moons KG, de Groot JA, Bouwmeester W, et al. Critical appraisal and data extraction for systematic reviews of prediction modelling studies: the CHARMS checklist. PLoS Med. 2014;11(10):e1001744.
Clarke M, Oxman AD, Paulsen E, Higgins JP, Green S, Appendix A: Guide to the contents of a Cochrane Methodology protocol and review. In: Higgins JP, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 ed: The Cochrane Collaboration 2011.
Jefferson T, Rudin M, Brodney Folse S, Davidoff F. Editorial peer review for improving the quality of reports of biomedical studies. Cochrane Database Syst Rev. 2007;2:MR000016.
Djulbegovic B, Kumar A, Glasziou PP, et al. New treatments compared to established treatments in randomized trials. Cochrane Database Syst Rev. 2012;10:MR000024.
PubMed PubMed Central Google Scholar
Thoma A, Eaves FF 3rd. What is wrong with systematic reviews and meta-analyses: if you want the right answer, ask the right question! Aesthet Surg J. 2016;36(10):1198–201.
Deeks JJ, Wisniewski S, Davenport C. In: Deeks JJ, Bossuyt PM, Gatsonis C, editors. Chapter 4: guide to the contents of a Cochrane diagnostic test accuracy protocol. Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy The Cochrane Collaboration: In; 2013.
Bae J-M. An overview of systematic reviews of diagnostic tests accuracy. Epidemiology and Health. 2014;36:e2014016.
White S, Schultz T. Enuameh YAK. Lippincott Wiliams & Wilkins: Synthesizing evidence of diagnostic accuracy; 2011.
Methley AM, Campbell S, Chew-Graham C, McNally R, Cheraghi-Sohi SPICO. PICOS and SPIDER: a comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv Res. 2014;14:579.
Peters MD, Godfrey CM, Khalil H, McInerney P, Parker D, Soares CB. Guidance for conducting systematic scoping reviews. International journal of evidence-based healthcare. 2015;13(3):141–6.
Hetrick SE, Parker AG, Callahan P, Purcell R. Evidence mapping: illustrating an emerging methodology to improve evidence-based practice in youth mental health. J Eval Clin Pract. 2010;16(6):1025–30.
Wong G, Greenhalgh T, Westhorp G, Pawson R. Development of methodological guidance, publication standards and training materials for realist and meta-narrative reviews: the RAMESES (Realist And Meta-narrative Evidence Syntheses - Evolving Standards) project. Southampton UK: Queen's Printer and Controller of HMSO 2014. This work was produced by Wong et al. under the terms of a commissioning contract issued by the secretary of state for health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR journals library, National Institute for Health Research, evaluation, trials and studies coordinating Centre, alpha house, University of Southampton Science Park, Southampton SO16 7NS, UK. 2014.
Munn Z, Lockwood C, Moola S. The development and use of evidence summaries for point of care information systems: a streamlined rapid review approach. Worldviews Evid-Based Nurs. 2015;12(3):131–8.
Aromataris E, Fernandez R, Godfrey CM, Holly C, Khalil H, Tungpunkom P. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid Based Healthc. 2015;13(3):132–40.
Pearson A, White H, Bath-Hextall F, Salmond S, Apostolo J, Kirkpatrick PA. Mixed-methods approach to systematic reviews. Int J Evid Based Healthc. 2015;13(3):121–31.
Draper PA. Critique of concept analysis. J Adv Nurs. 2014;70(6):1207–8.
Grant MJ, Booth A. A Typology of reviews: an analysis of 14 review types and associated methodologies. Health Inf Libr J. 2009;26(2):91–108.
Tricco AC, Tetzlaff J, Moher D. The art and science of knowledge synthesis. J Clin Epidemiol. 2011;64(1):11–20.
Bender R. A practical taxonomy proposal for systematic reviews of therapeutic interventions. 21st Cochrane Colloquium Quebec, Canada 2013.
Kastner M, Tricco AC, Soobiah C, et al. What is the most appropriate knowledge synthesis method to conduct a review? Protocol for a scoping review. BMC Med Res Methodol. 2012;12:114.
Leenaars M, Hooijmans CR, van Veggel N, et al. A step-by-step guide to systematically identify all relevant animal studies. Lab Anim. 2012;46(1):24–31.
de Vries RB, Wever KE, Avey MT, Stephens ML, Sena ES, Leenaars M. The usefulness of systematic reviews of animal experiments for the design of preclinical and clinical studies. ILAR J. 2014;55(3):427–37.
Hooijmans CR, Ritskes-Hoitinga M. Progress in using systematic reviews of animal studies to improve translational research. PLoS Med. 2013;10(7):e1001482.
Mignini LE, Khan KS. Methodological quality of systematic reviews of animal studies: a survey of reviews of basic research. BMC Med Res Methodol. 2006;6:10.
van Luijk J, Bakker B, Rovers MM, Ritskes-Hoitinga M, de Vries RB, Leenaars M. Systematic reviews of animal studies; missing link in translational research? PLoS One. 2014;9(3):e89981.
Vesterinen HM, Sena ES, Egan KJ, et al. Meta-analysis of data from animal studies: a practical guide. J Neurosci Methods. 2014;221:92–102.
CAMARADES. Collaborative Approach to Meta-Analysis and Review of Animal Data from Experimental Studies. 2014 [cited 8th December 2016]; Available from: http://www.dcn.ed.ac.uk/camarades/default.htm#about
Moher D, Glasziou P, Chalmers I, et al. Increasing value and reducing waste in biomedical research: who's listening? Lancet. 2016;387(10027):1573–86.
Ioannidis J. The mass production of redundant, misleading, and conflicted systematic reviews and meta-analyses. The Milbank Quarterly. 2016;94(3):485–514.
Rousseau DM, Gunia BC. Evidence-based practice: the psychology of EBP implementation. Annu Rev Psychol. 2016;67:667–92.
Jordan Z, Lockwood C, Aromataris E. Munn Z. The Joanna Briggs Institute: The updated JBI model for evidence-based healthcare; 2016.
Cooney GM, Dwan K, Greig CA, et al. Exercise for depression. Cochrane Database Syst Rev. 2013;9:CD004366.
Munn Z, Jordan Z. The patient experience of high technology medical imaging: a systematic review of the qualitative evidence. JBI Libr. Syst Rev. 2011;9(19):631–78.
de Verteuil R, Tan WS. Self-monitoring of blood glucose in type 2 diabetes mellitus: systematic review of economic evidence. JBI Libr. Syst Rev. 2010;8(7):302–42.
Munn Z, Moola S, Lisy K, Riitano D, Murphy F. Claustrophobia in magnetic resonance imaging: a systematic review and meta-analysis. Radiography. 2015;21(2):e59–63.
Hakonsen SJ, Pedersen PU, Bath-Hextall F, Kirkpatrick P. Diagnostic test accuracy of nutritional tools used to identify undernutrition in patients with colorectal cancer: a systematic review. JBI Database System Rev Implement Rep. 2015;13(4):141–87.
Australia C. Risk factors for lung cancer: a systematic review. NSW: Surry Hills; 2014.
McArthur A, Lockwood C. Maternal mortality in Cambodia, Thailand, Malaysia and Sri Lanka: a systematic review of local and national policy and practice initiatives. JBI Libr Syst Rev. 2010;8(16 Suppl):1–10.
Peek K. Muscle strength in adults with spinal cord injury: a systematic review of manual muscle testing, isokinetic and hand held dynamometry clinimetrics. JBI Database of Systematic Reviews and Implementation Reports. 2014;12(5):349–429.
Hayden JA, Tougas ME, Riley R, Iles R, Pincus T. Individual recovery expectations and prognosis of outcomes in non-specific low back pain: prognostic factor exemplar review. Cochrane Libr. 2014. http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD011284/full .
Download references
Acknowledgements
No funding was provided for this paper.
Availability of data and materials
Not applicable
Author information
Authors and affiliations.
The Joanna Briggs Institute, The University of Adelaide, 55 King William Road, North Adelaide, Soueth Australia, 5005, Australia
Zachary Munn, Cindy Stern, Edoardo Aromataris, Craig Lockwood & Zoe Jordan
You can also search for this author in PubMed Google Scholar
Contributions
ZM: Led the development of this paper and conceptualised the idea for a systematic review typology. Provided final approval for submission. CS: Contributed conceptually to the paper and wrote sections of the paper. Provided final approval for submission. EA: Contributed conceptually to the paper and reviewed and provided feedback on all drafts. Provided final approval for submission. CL: Contributed conceptually to the paper and reviewed and provided feedback on all drafts. Provided final approval for submission. ZJ: Contributed conceptually to the paper and reviewed and provided feedback on all drafts. Provided approval and encouragement for the work to proceed. Provided final approval for submission.
Corresponding author
Correspondence to Zachary Munn .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
All the authors are members of the Joanna Briggs Institute, an evidence-based healthcare research institute which provides formal guidance regarding evidence synthesis, transfer and implementation.
The authors have no other competing interests to declare.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Munn, Z., Stern, C., Aromataris, E. et al. What kind of systematic review should I conduct? A proposed typology and guidance for systematic reviewers in the medical and health sciences. BMC Med Res Methodol 18 , 5 (2018). https://doi.org/10.1186/s12874-017-0468-4
Download citation
Received : 29 May 2017
Accepted : 28 December 2017
Published : 10 January 2018
DOI : https://doi.org/10.1186/s12874-017-0468-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Systematic reviews
- Question development
BMC Medical Research Methodology
ISSN: 1471-2288
- General enquiries: [email protected]
- Resources Home 🏠
- Try SciSpace Copilot
- Search research papers
- Add Copilot Extension
- Try AI Detector
- Try Paraphraser
- Try Citation Generator
- April Papers
- June Papers
- July Papers

Types of Literature Review — A Guide for Researchers

Table of Contents
Researchers often face challenges when choosing the appropriate type of literature review for their study. Regardless of the type of research design and the topic of a research problem , they encounter numerous queries, including:
What is the right type of literature review my study demands?
- How do we gather the data?
- How to conduct one?
- How reliable are the review findings?
- How do we employ them in our research? And the list goes on.
If you’re also dealing with such a hefty questionnaire, this article is of help. Read through this piece of guide to get an exhaustive understanding of the different types of literature reviews and their step-by-step methodologies along with a dash of pros and cons discussed.
Heading from scratch!
What is a Literature Review?
A literature review provides a comprehensive overview of existing knowledge on a particular topic, which is quintessential to any research project. Researchers employ various literature reviews based on their research goals and methodologies. The review process involves assembling, critically evaluating, and synthesizing existing scientific publications relevant to the research question at hand. It serves multiple purposes, including identifying gaps in existing literature, providing theoretical background, and supporting the rationale for a research study.
What is the importance of a Literature review in research?
Literature review in research serves several key purposes, including:
- Background of the study: Provides proper context for the research. It helps researchers understand the historical development, theoretical perspectives, and key debates related to their research topic.
- Identification of research gaps: By reviewing existing literature, researchers can identify gaps or inconsistencies in knowledge, paving the way for new research questions and hypotheses relevant to their study.
- Theoretical framework development: Facilitates the development of theoretical frameworks by cultivating diverse perspectives and empirical findings. It helps researchers refine their conceptualizations and theoretical models.
- Methodological guidance: Offers methodological guidance by highlighting the documented research methods and techniques used in previous studies. It assists researchers in selecting appropriate research designs, data collection methods, and analytical tools.
- Quality assurance and upholding academic integrity: Conducting a thorough literature review demonstrates the rigor and scholarly integrity of the research. It ensures that researchers are aware of relevant studies and can accurately attribute ideas and findings to their original sources.
Types of Literature Review
Literature review plays a crucial role in guiding the research process , from providing the background of the study to research dissemination and contributing to the synthesis of the latest theoretical literature review findings in academia.
However, not all types of literature reviews are the same; they vary in terms of methodology, approach, and purpose. Let's have a look at the various types of literature reviews to gain a deeper understanding of their applications.
1. Narrative Literature Review
A narrative literature review, also known as a traditional literature review, involves analyzing and summarizing existing literature without adhering to a structured methodology. It typically provides a descriptive overview of key concepts, theories, and relevant findings of the research topic.
Unlike other types of literature reviews, narrative reviews reinforce a more traditional approach, emphasizing the interpretation and discussion of the research findings rather than strict adherence to methodological review criteria. It helps researchers explore diverse perspectives and insights based on the research topic and acts as preliminary work for further investigation.
Steps to Conduct a Narrative Literature Review

Source:- https://www.researchgate.net/figure/Steps-of-writing-a-narrative-review_fig1_354466408
Define the research question or topic:
The first step in conducting a narrative literature review is to clearly define the research question or topic of interest. Defining the scope and purpose of the review includes — What specific aspect of the topic do you want to explore? What are the main objectives of the research? Refine your research question based on the specific area you want to explore.
Conduct a thorough literature search
Once the research question is defined, you can conduct a comprehensive literature search. Explore and use relevant databases and search engines like SciSpace Discover to identify credible and pertinent, scholarly articles and publications.
Select relevant studies
Before choosing the right set of studies, it’s vital to determine inclusion (studies that should possess the required factors) and exclusion criteria for the literature and then carefully select papers. For example — Which studies or sources will be included based on relevance, quality, and publication date?
*Important (applies to all the reviews): Inclusion criteria are the factors a study must include (For example: Include only peer-reviewed articles published between 2022-2023, etc.). Exclusion criteria are the factors that wouldn’t be required for your search strategy (Example: exclude irrelevant papers, preprints, written in non-English, etc.)
Critically analyze the literature
Once the relevant studies are shortlisted, evaluate the methodology, findings, and limitations of each source and jot down key themes, patterns, and contradictions. You can use efficient AI tools to conduct a thorough literature review and analyze all the required information.
Synthesize and integrate the findings
Now, you can weave together the reviewed studies, underscoring significant findings such that new frameworks, contrasting viewpoints, and identifying knowledge gaps.
Discussion and conclusion
This is an important step before crafting a narrative review — summarize the main findings of the review and discuss their implications in the relevant field. For example — What are the practical implications for practitioners? What are the directions for future research for them?
Write a cohesive narrative review
Organize the review into coherent sections and structure your review logically, guiding the reader through the research landscape and offering valuable insights. Use clear and concise language to convey key points effectively.
Structure of Narrative Literature Review
A well-structured, narrative analysis or literature review typically includes the following components:
- Introduction: Provides an overview of the topic, objectives of the study, and rationale for the review.
- Background: Highlights relevant background information and establish the context for the review.
- Main Body: Indexes the literature into thematic sections or categories, discussing key findings, methodologies, and theoretical frameworks.
- Discussion: Analyze and synthesize the findings of the reviewed studies, stressing similarities, differences, and any gaps in the literature.
- Conclusion: Summarizes the main findings of the review, identifies implications for future research, and offers concluding remarks.
Pros and Cons of Narrative Literature Review
- Flexibility in methodology and doesn’t necessarily rely on structured methodologies
- Follows traditional approach and provides valuable and contextualized insights
- Suitable for exploring complex or interdisciplinary topics. For example — Climate change and human health, Cybersecurity and privacy in the digital age, and more
- Subjectivity in data selection and interpretation
- Potential for bias in the review process
- Lack of rigor compared to systematic reviews
Example of Well-Executed Narrative Literature Reviews
Paper title: Examining Moral Injury in Clinical Practice: A Narrative Literature Review

Source: SciSpace
While narrative reviews offer flexibility, academic integrity remains paramount. So, ensure proper citation of all sources and maintain a transparent and factual approach throughout your critical narrative review, itself.
2. Systematic Review
A systematic literature review is one of the comprehensive types of literature review that follows a structured approach to assembling, analyzing, and synthesizing existing research relevant to a particular topic or question. It involves clearly defined criteria for exploring and choosing studies, as well as rigorous methods for evaluating the quality of relevant studies.
It plays a prominent role in evidence-based practice and decision-making across various domains, including healthcare, social sciences, education, health sciences, and more. By systematically investigating available literature, researchers can identify gaps in knowledge, evaluate the strength of evidence, and report future research directions.
Steps to Conduct Systematic Reviews

Source:- https://www.researchgate.net/figure/Steps-of-Systematic-Literature-Review_fig1_321422320
Here are the key steps involved in conducting a systematic literature review
Formulate a clear and focused research question
Clearly define the research question or objective of the review. It helps to centralize the literature search strategy and determine inclusion criteria for relevant studies.
Develop a thorough literature search strategy
Design a comprehensive search strategy to identify relevant studies. It involves scrutinizing scientific databases and all relevant articles in journals. Plus, seek suggestions from domain experts and review reference lists of relevant review articles.
Screening and selecting studies
Employ predefined inclusion and exclusion criteria to systematically screen the identified studies. This screening process also typically involves multiple reviewers independently assessing the eligibility of each study.
Data extraction
Extract key information from selected studies using standardized forms or protocols. It includes study characteristics, methods, results, and conclusions.
Critical appraisal
Evaluate the methodological quality and potential biases of included studies. Various tools (BMC medical research methodology) and criteria can be implemented for critical evaluation depending on the study design and research quetions .
Data synthesis
Analyze and synthesize review findings from individual studies to draw encompassing conclusions or identify overarching patterns and explore heterogeneity among studies.
Interpretation and conclusion
Interpret the findings about the research question, considering the strengths and limitations of the research evidence. Draw conclusions and implications for further research.
The final step — Report writing
Craft a detailed report of the systematic literature review adhering to the established guidelines of PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses). This ensures transparency and reproducibility of the review process.
By following these steps, a systematic literature review aims to provide a comprehensive and unbiased summary of existing evidence, help make informed decisions, and advance knowledge in the respective domain or field.
Structure of a systematic literature review
A well-structured systematic literature review typically consists of the following sections:
- Introduction: Provides background information on the research topic, outlines the review objectives, and enunciates the scope of the study.
- Methodology: Describes the literature search strategy, selection criteria, data extraction process, and other methods used for data synthesis, extraction, or other data analysis..
- Results: Presents the review findings, including a summary of the incorporated studies and their key findings.
- Discussion: Interprets the findings in light of the review objectives, discusses their implications, and identifies limitations or promising areas for future research.
- Conclusion: Summarizes the main review findings and provides suggestions based on the evidence presented in depth meta analysis.
*Important (applies to all the reviews): Remember, the specific structure of your literature review may vary depending on your topic, research question, and intended audience. However, adhering to a clear and logical hierarchy ensures your review effectively analyses and synthesizes knowledge and contributes valuable insights for readers.
Pros and Cons of Systematic Literature Review
- Adopts rigorous and transparent methodology
- Minimizes bias and enhances the reliability of the study
- Provides evidence-based insights
- Time and resource-intensive
- High dependency on the quality of available literature (literature research strategy should be accurate)
- Potential for publication bias
Example of Well-Executed Systematic Literature Review
Paper title: Systematic Reviews: Understanding the Best Evidence For Clinical Decision-making in Health Care: Pros and Cons.

Read this detailed article on how to use AI tools to conduct a systematic review for your research!
3. Scoping Literature Review
A scoping literature review is a methodological review type of literature review that adopts an iterative approach to systematically map the existing literature on a particular topic or research area. It involves identifying, selecting, and synthesizing relevant papers to provide an overview of the size and scope of available evidence. Scoping reviews are broader in scope and include a diverse range of study designs and methodologies especially focused on health services research.
The main purpose of a scoping literature review is to examine the extent, range, and nature of existing studies on a topic, thereby identifying gaps in research, inconsistencies, and areas for further investigation. Additionally, scoping reviews can help researchers identify suitable methodologies and formulate clinical recommendations. They also act as the frameworks for future systematic reviews or primary research studies.
Scoping reviews are primarily focused on —
- Emerging or evolving topics — where the research landscape is still growing or budding. Example — Whole Systems Approaches to Diet and Healthy Weight: A Scoping Review of Reviews .
- Broad and complex topics : With a vast amount of existing literature.
- Scenarios where a systematic review is not feasible: Due to limited resources or time constraints.
Steps to Conduct a Scoping Literature Review
While Scoping reviews are not as rigorous as systematic reviews, however, they still follow a structured approach. Here are the steps:
Identify the research question: Define the broad topic you want to explore.
Identify Relevant Studies: Conduct a comprehensive search of relevant literature using appropriate databases, keywords, and search strategies.
Select studies to be included in the review: Based on the inclusion and exclusion criteria, determine the appropriate studies to be included in the review.
Data extraction and charting : Extract relevant information from selected studies, such as year, author, main results, study characteristics, key findings, and methodological approaches. However, it varies depending on the research question.
Collate, summarize, and report the results: Analyze and summarize the extracted data to identify key themes and trends. Then, present the findings of the scoping review in a clear and structured manner, following established guidelines and frameworks .
Structure of a Scoping Literature Review
A scoping literature review typically follows a structured format similar to a systematic review. It includes the following sections:
- Introduction: Introduce the research topic and objectives of the review, providing the historical context, and rationale for the study.
- Methods : Describe the methods used to conduct the review, including search strategies, study selection criteria, and data extraction procedures.
- Results: Present the findings of the review, including key themes, concepts, and patterns identified in the literature review.
- Discussion: Examine the implications of the findings, including strengths, limitations, and areas for further examination.
- Conclusion: Recapitulate the main findings of the review and their implications for future research, policy, or practice.
Pros and Cons of Scoping Literature Review
- Provides a comprehensive overview of existing literature
- Helps to identify gaps and areas for further research
- Suitable for exploring broad or complex research questions
- Doesn’t provide the depth of analysis offered by systematic reviews
- Subject to researcher bias in study selection and data extraction
- Requires careful consideration of literature search strategies and inclusion criteria to ensure comprehensiveness and validity.
In short, a scoping review helps map the literature on developing or emerging topics and identifying gaps. It might be considered as a step before conducting another type of review, such as a systematic review. Basically, acts as a precursor for other literature reviews.
Example of a Well-Executed Scoping Literature Review
Paper title: Health Chatbots in Africa Literature: A Scoping Review

Check out the key differences between Systematic and Scoping reviews — Evaluating literature review: systematic vs. scoping reviews
4. Integrative Literature Review
Integrative Literature Review (ILR) is a type of literature review that proposes a distinctive way to analyze and synthesize existing literature on a specific topic, providing a thorough understanding of research and identifying potential gaps for future research.
Unlike a systematic review, which emphasizes quantitative studies and follows strict inclusion criteria, an ILR embraces a more pliable approach. It works beyond simply summarizing findings — it critically analyzes, integrates, and interprets research from various methodologies (qualitative, quantitative, mixed methods) to provide a deeper understanding of the research landscape. ILRs provide a holistic and systematic overview of existing research, integrating findings from various methodologies. ILRs are ideal for exploring intricate research issues, examining manifold perspectives, and developing new research questions.

Steps to Conduct an Integrative Literature Review
- Identify the research question: Clearly define the research question or topic of interest as formulating a clear and focused research question is critical to leading the entire review process.
- Literature search strategy: Employ systematic search techniques to locate relevant literature across various databases and sources.
- Evaluate the quality of the included studies : Critically assess the methodology, rigor, and validity of each study by applying inclusion and exclusion criteria to filter and select studies aligned with the research objectives.
- Data Extraction: Extract relevant data from selected studies using a structured approach.
- Synthesize the findings : Thoroughly analyze the selected literature, identify key themes, and synthesize findings to derive noteworthy insights.
- Critical appraisal: Critically evaluate the quality and validity of qualitative research and included studies by using BMC medical research methodology.
- Interpret and present your findings: Discuss the purpose and implications of your analysis, spotlighting key insights and limitations. Organize and present the findings coherently and systematically.
Structure of an Integrative Literature Review
- Introduction : Provide an overview of the research topic and the purpose of the integrative review.
- Methods: Describe the opted literature search strategy, selection criteria, and data extraction process.
- Results: Present the synthesized findings, including key themes, patterns, and contradictions.
- Discussion: Interpret the findings about the research question, emphasizing implications for theory, practice, and prospective research.
- Conclusion: Summarize the main findings, limitations, and contributions of the integrative review.
Pros and Cons of Integrative Literature Review
- Informs evidence-based practice and policy to the relevant stakeholders of the research.
- Contributes to theory development and methodological advancement, especially in the healthcare arena.
- Integrates diverse perspectives and findings
- Time-consuming process due to the extensive literature search and synthesis
- Requires advanced analytical and critical thinking skills
- Potential for bias in study selection and interpretation
- The quality of included studies may vary, affecting the validity of the review
Example of Integrative Literature Reviews
Paper Title: An Integrative Literature Review: The Dual Impact of Technological Tools on Health and Technostress Among Older Workers

5. Rapid Literature Review
A Rapid Literature Review (RLR) is the fastest type of literature review which makes use of a streamlined approach for synthesizing literature summaries, offering a quicker and more focused alternative to traditional systematic reviews. Despite employing identical research methods, it often simplifies or omits specific steps to expedite the process. It allows researchers to gain valuable insights into current research trends and identify key findings within a shorter timeframe, often ranging from a few days to a few weeks — unlike traditional literature reviews, which may take months or even years to complete.
When to Consider a Rapid Literature Review?
- When time impediments demand a swift summary of existing research
- For emerging topics where the latest literature requires quick evaluation
- To report pilot studies or preliminary research before embarking on a comprehensive systematic review
Steps to Conduct a Rapid Literature Review
- Define the research question or topic of interest. A well-defined question guides the search process and helps researchers focus on relevant studies.
- Determine key databases and sources of relevant literature to ensure comprehensive coverage.
- Develop literature search strategies using appropriate keywords and filters to fetch a pool of potential scientific articles.
- Screen search results based on predefined inclusion and exclusion criteria.
- Extract and summarize relevant information from the above-preferred studies.
- Synthesize findings to identify key themes, patterns, or gaps in the literature.
- Prepare a concise report or a summary of the RLR findings.
Structure of a Rapid Literature Review
An effective structure of an RLR typically includes the following sections:
- Introduction: Briefly introduce the research topic and objectives of the RLR.
- Methodology: Describe the search strategy, inclusion and exclusion criteria, and data extraction process.
- Results: Present a summary of the findings, including key themes or patterns identified.
- Discussion: Interpret the findings, discuss implications, and highlight any limitations or areas for further research
- Conclusion: Summarize the key findings and their implications for practice or future research
Pros and Cons of Rapid Literature Review
- RLRs can be completed quickly, authorizing timely decision-making
- RLRs are a cost-effective approach since they require fewer resources compared to traditional literature reviews
- Offers great accessibility as RLRs provide prompt access to synthesized evidence for stakeholders
- RLRs are flexible as they can be easily adapted for various research contexts and objectives
- RLR reports are limited and restricted, not as in-depth as systematic reviews, and do not provide comprehensive coverage of the literature compared to traditional reviews.
- Susceptible to bias because of the expedited nature of RLRs. It would increase the chance of overlooking relevant studies or biases in the selection process.
- Due to time constraints, RLR findings might not be robust enough as compared to systematic reviews.
Example of a Well-Executed Rapid Literature Review
Paper Title: What Is the Impact of ChatGPT on Education? A Rapid Review of the Literature

A Summary of Literature Review Types
Tools and resources for conducting different types of literature reviews, online scientific databases.
Platforms such as SciSpace , PubMed , Scopus , Elsevier , and Web of Science provide access to a vast array of scholarly literature, facilitating the search and data retrieval process.
Reference management software
Tools like SciSpace Citation Generator , EndNote, Zotero , and Mendeley assist researchers in organizing, annotating, and citing relevant literature, streamlining the review process altogether.
Automate Literature Review with AI tools
Automate the literature review process by using tools like SciSpace literature review which helps you compare and contrast multiple papers all on one screen in an easy-to-read matrix format. You can effortlessly analyze and interpret the review findings tailored to your study. It also supports the review in 75+ languages, making it more manageable even for non-English speakers.

Goes without saying — literature review plays a pivotal role in academic research to identify the current trends and provide insights to pave the way for future research endeavors. Different types of literature review has their own strengths and limitations, making them suitable for different research designs and contexts. Whether conducting a narrative review, systematic review, scoping review, integrative review, or rapid literature review, researchers must cautiously consider the objectives, resources, and the nature of the research topic.
If you’re currently working on a literature review and still adopting a manual and traditional approach, switch to the automated AI literature review workspace and transform your traditional literature review into a rapid one by extracting all the latest and relevant data for your research!
There you go!
Frequently Asked Questions
Narrative reviews give a general overview of a topic based on the author's knowledge. They may lack clear criteria and can be biased. On the other hand, systematic reviews aim to answer specific research questions by following strict methods. They're thorough but time-consuming.
A systematic review collects and analyzes existing research to provide an overview of a topic, while a meta-analysis statistically combines data from multiple studies to draw conclusions about the overall effect of an intervention or relationship between variables.
A systematic review thoroughly analyzes existing research on a specific topic using strict methods. In contrast, a scoping review offers a broader overview of the literature without evaluating individual studies in depth.
A systematic review thoroughly examines existing research using a rigorous process, while a rapid review provides a quicker summary of evidence, often by simplifying some of the systematic review steps to meet shorter timelines.
A systematic review carefully examines many studies on a single topic using specific guidelines. Conversely, an integrative review blends various types of research to provide a more comprehensive understanding of the topic.
You might also like

AI for Meta Analysis — A Comprehensive Guide

How To Write An Argumentative Essay

Beyond Google Scholar: Why SciSpace is the best alternative

Literature Review: Types of literature reviews
- Traditional or narrative literature reviews
- Scoping Reviews
- Systematic literature reviews
- Annotated bibliography
- Keeping up to date with literature
- Finding a thesis
- Evaluating sources and critical appraisal of literature
- Managing and analysing your literature
- Further reading and resources
Types of literature reviews

The type of literature review you write will depend on your discipline and whether you are a researcher writing your PhD, publishing a study in a journal or completing an assessment task in your undergraduate study.
A literature review for a subject in an undergraduate degree will not be as comprehensive as the literature review required for a PhD thesis.
An undergraduate literature review may be in the form of an annotated bibliography or a narrative review of a small selection of literature, for example ten relevant articles. If you are asked to write a literature review, and you are an undergraduate student, be guided by your subject coordinator or lecturer.
The common types of literature reviews will be explained in the pages of this section.
- Narrative or traditional literature reviews
- Critically Appraised Topic (CAT)
- Scoping reviews
- Annotated bibliographies
These are not the only types of reviews of literature that can be conducted. Often the term "review" and "literature" can be confusing and used in the wrong context. Grant and Booth (2009) attempt to clear up this confusion by discussing 14 review types and the associated methodology, and advantages and disadvantages associated with each review.
Grant, M. J. and Booth, A. (2009), A typology of reviews: an analysis of 14 review types and associated methodologies . Health Information & Libraries Journal, 26 , 91–108. doi:10.1111/j.1471-1842.2009.00848.x
What's the difference between reviews?
Researchers, academics, and librarians all use various terms to describe different types of literature reviews, and there is often inconsistency in the ways the types are discussed. Here are a couple of simple explanations.
- The image below describes common review types in terms of speed, detail, risk of bias, and comprehensiveness:

"Schematic of the main differences between the types of literature review" by Brennan, M. L., Arlt, S. P., Belshaw, Z., Buckley, L., Corah, L., Doit, H., Fajt, V. R., Grindlay, D., Moberly, H. K., Morrow, L. D., Stavisky, J., & White, C. (2020). Critically Appraised Topics (CATs) in veterinary medicine: Applying evidence in clinical practice. Frontiers in Veterinary Science, 7 , 314. https://doi.org/10.3389/fvets.2020.00314 is licensed under CC BY 3.0
- The table below lists four of the most common types of review , as adapted from a widely used typology of fourteen types of reviews (Grant & Booth, 2009).
Grant, M.J. & Booth, A. (2009). A typology of reviews: An analysis of 14 review types and associated methodologies. Health Information & Libraries Journal, 26 (2), 91-108. https://doi.org/10.1111/j.1471-1842.2009.00848.x
See also the Library's Literature Review guide.
Critical Appraised Topic (CAT)
For information on conducting a Critically Appraised Topic or CAT
Callander, J., Anstey, A. V., Ingram, J. R., Limpens, J., Flohr, C., & Spuls, P. I. (2017). How to write a Critically Appraised Topic: evidence to underpin routine clinical practice. British Journal of Dermatology (1951), 177(4), 1007-1013. https://doi.org/10.1111/bjd.15873
Books on Literature Reviews
- << Previous: Home
- Next: Traditional or narrative literature reviews >>
- Last Updated: Apr 10, 2024 5:05 PM
- URL: https://libguides.csu.edu.au/review

Charles Sturt University is an Australian University, TEQSA Provider Identification: PRV12018. CRICOS Provider: 00005F.

- About Covidence and systematic reviews
What are the different types of review?
Systematic literature reviews (slrs).
SLR’s attempt to collate all empirical evidence that fit pre-specified eligibility criteria in order to answer a specific clearly-formulated research question. A SLR uses explicit and reproducible systematic methods that are selected with a view to minimizing bias, thus providing more reliable findings from which conclusions can be drawn and decisions made.
The process starts with a research question and a protocol or research plan. A review team searches for studies to answer the question using a highly sensitive search strategy. The retrieved studies are then screened for eligibility using pre-specified inclusion and exclusion criteria (this is done by at least two people working independently). Next, the reviewers extract the relevant data and assess the quality of the included studies. Finally, the review team synthesizes the extracted study data and presents the results.
A SLR may contain meta-analyses (statistical analysis). A SLR which is continually updated, incorporating relevant new evidence as it becomes available is often known as a living SLR.
Rapid reviews
Rapid reviews aim to produce a rigorous synthesis quickly (due to time constraints/urgency), based on a pre-defined research question. The review process for rapid reviews is the same as for a more traditional systematic review: the emphasis is on a replicable pre-specified search, and screening methods that minimize the risk of bias, although potentially isn’t as stringent as a formal systematic review.
The process operates within pre-specified limits (for example, by restricting searches to articles published during a specific timeframe) and is usually run by a multidisciplinary team with expertise in systematic review methods.
Umbrella reviews or Overview of reviews
An umbrella review is a review of multiple systematic reviews. The process uses explicit and systematic methods to search for, and identify, systematic reviews on related research questions in the same topic area. The purpose of an umbrella review is to synthesize the results of the systematic reviews across important outcomes.
Scoping reviews
Scoping reviews are exploratory and they typically address a broad question, compared to a systematic review that typically has a more targeted question.
Researchers conduct scoping reviews to assess the extent of the available evidence, to organize it into groups and to highlight gaps. If a scoping review finds no studies, this might help researchers to decide that a systematic review is likely to be of limited value and that resources could be better directed elsewhere.
Literature reviews or narrative reviews
Literature, or narrative, reviews provide an overview of what is known about a particular topic. They evaluate the material, rather than simply restating it, but the methods used to do this are not usually prespecified and they are not described in detail in the review. The search might be comprehensive but it does not aim to be exhaustive. Literature reviews are often topic based and can take the form of a discussion. Literature reviews lack precision and replicability and can present their findings in the context of what has come before. Often, this sort of synthesis does not attempt to control for the author’s own bias. The results or conclusion of a literature review is likely to be presented in a narrative format rather than statistical methods.
Take a look at the articles about the different types of review on the Covidence blog:
- Systematic review types: meet the family
- The difference between a systematic review and a literature review
- The difference between a systematic review and a meta-analysis

Help us improve our Library guides with this 5 minute survey . We appreciate your feedback!
- UOW Library
- Key guides for researchers
Systematic Review
- What is a systematic review?
- Five other types of systematic review
- How is a literature review different?
- Search tips for systematic reviews
- Controlled vocabularies
- Grey literature
- Transferring your search
- Documenting your results
- Support & contact
Frameworks for systematic reviews
Using a framework to structure your research question will assist you to structure the entire process - determine the scope of your review, provide a focus while searching for literature, help identify key concepts and guide your selection of papers for inclusion.
PICO framework
Use a framework like PICO when developing a good clinical research question:
PICO examples from the Cochrane Library
- Log into the Cochrane Library
- Enter your UOW username and password
- From the home page click “Advanced Search”
- Click on the “PICO search” tab and search for your topic
- Click on “Run search”
- Choose a review and click on the “ShowPICOs” drop-down menu
- You will then see the PICO attached to the systematic review.
PICO searching on Medline
- Advanced searching in Medline.
- Understanding Focus and Explode.
- Conduct a search strategy using the PICO framework.
- Searching using Medical Subject Headings.
- PICO Searches and Systematic Reviews (Academic skills and study support)
- Describes PICO Searches and systematic literature reviews.
PRISMA is an evidence-based minimum set of items for reporting in systematic reviews and meta-analyses.
PRISMA Checklist The 27 checklist items relate to the content of a systematic review and meta-analysis, which includes:
A PRISMA extension for scoping reviews, PRISMA-ScR , has been created to provide reporting guidance for this specific type of review. This extension is also intended to apply to evidence maps, as these share similarities with scoping reviews and involve a systematic search of a body of literature to identify knowledge gaps.
The PRISMA extension for scoping reviews contains 20 essential reporting items and 2 optional items to include when completing a scoping review. Scoping reviews serve to synthesize evidence and assess the scope of literature on a topic. Among other objectives, scoping reviews help determine whether a systematic review of the literature is warranted.
The SPIDER question format was adapted from the PICO tool to search for qualitative and mixed-methods research. Questions based on this format identify the following concepts:
- P henomenon of I nterest
- E valuation
- R esearch type.
Defining your question for finding qualitative research: SPIDER tool
Example: What are young parents’ experiences of attending antenatal education?
Search for ( S AND P of I AND ( D OR E ) AND R ) (Cooke, Smith, & Booth, 2012).
"Beyond PICO: the SPIDER tool for qualitative evidence synthesis" (Cooke, Smith, & Booth, 2012)
- Previous: Tools
- Next: Support & contact
- Last Updated: Feb 22, 2024 12:34 PM
- URL: https://uow.libguides.com/systematic-review
Insert research help text here
LIBRARY RESOURCES
Library homepage
Library SEARCH
A-Z Databases
STUDY SUPPORT
Academic Skills Centre
Referencing and citing
Digital Skills Hub
MORE UOW SERVICES
UOW homepage
Student support and wellbeing
IT Services

On the lands that we study, we walk, and we live, we acknowledge and respect the traditional custodians and cultural knowledge holders of these lands.

Copyright & disclaimer | Privacy & cookie usage
University of Tasmania, Australia
Literature reviews.
- Introduction: who will benefit from this guide?
- Getting started: what is a literature review?
- How to develop a researchable question
- How to find the literature
- How to manage the reading and take notes that make sense
- How to bring it all together: examples, templates, links, guides
Who will benefit from this guide?
This guide is written for undergraduates and postgraduate, course work students who are doing their first literature review.
Higher degree research candidates and academic researchers, please also refer to the Resources for Researchers library guides for more detailed information on writing theses and systematic reviews.
What is a literature review?
A literature review is an examination of research in a particular field.
- It gathers, critically analyses, evaluates, and synthesises current research literature in a discipline,
- indicates where there may be strengths, gaps, weaknesses, and agreements in the current research.
It considers:
- what has been done,
- the current thinking,
- research trends,
- principal debates,
- dominant ideas,
- methods used in researching the topic
- gaps and flaws in the research.
http://libguides.lib.msu.edu/c.php?g=96146&p=904793
Different Types of reviews
You may be asked to complete a literature review that is done in a systematic way, that is like a systematic review.
Mostly, the literature review you will be asked to do will be integrative – that is, conclusions are drawn from the literature in order to create something new, such as a new hypothesis to address a question, a solution to a complex problem, a new workplace procedure or training program.
Some elements of what you are asked to do may be like a systematic review, particularly in health fields.
Systematic approach does not mean a systematic review.
A true systematic review is a complex research project:
- conducted in a scientific manner,
- usually with more than one person involved,
- they take a long time to complete
- are generally a project in themselves.
For more information have a look at the Systematic Review library guide .
If you would like to know more about different types of reviews, have a look at the document below:

At the core of a literature review is a synthesis of the research.
While both analysis and synthesis are involved, s ynthesis goes beyond analysis and is a higher order thinking.(Bloom's taxonomy).
Looking at the diagram below, it is evident that synthesis goes well beyond just analysis.

- Analysis asks you to break something down into its parts and compare and contrast with other research findings.
- where they agree and disagree
- the major themes, arguments, ideas in a field
- the questions raised and those yet to be answered.
- This will show the relationships between different aspects of the research findings in the literature.
- It is not a summary, but rather is organised around concepts and themes, where there is a combining of elements to form something new.
Watch this short clip from Utah State University which defines how to go about achieving synthesis.
Synthesis: True or False.
Quick Quiz: check your understanding of synthesis from the video by deciding which of these statements are true or false .
- Next: How to develop a researchable question >>
- Last Updated: Apr 10, 2024 11:56 AM
- URL: https://utas.libguides.com/literaturereviews

- Open access
- Published: 11 April 2024
Anatomical variants of the intercostobrachial nerve and its preservation during surgery, a systematic review and meta-analysis
- Roberto Cirocchi 1 ,
- Matteo Matteucci 2 ,
- Justus Randolph 3 ,
- Francesca Duro 1 ,
- Luca Properzi 1 ,
- Stefano Avenia 1 ,
- Bruno Amato 4 ,
- Ruggiero Iandoli 5 ,
- Giovanni Tebala 6 ,
- Carlo Boselli 1 ,
- Piero Covarelli 1 &
- Paolo Sapienza 7
World Journal of Surgical Oncology volume 22 , Article number: 92 ( 2024 ) Cite this article
27 Accesses
Metrics details
The anatomic variants of the intercostobrachial nerve (ICBN) represent a potential risk of injuries during surgical procedure such as axillary lymph node dissection and sentinel lymph node biopsy in breast cancer and melanoma patients. The aim of this systematic review and meta-analysis was to investigate the different origins and branching patterns of the intercostobrachial nerve also providing an analysis of the prevalence, through the analysis of the literature available up to September 2023.
Materials and methods
The protocol for this study was registered on PROSPERO (ID: CRD42023447932), an international prospective database for reviews. The PRISMA guideline was respected throughout the meta-analysis. A systematic literature search was performed using PubMed, Scopus and Web of Science. A search was performed in grey literature through google.
We included a total of 23 articles (1,883 patients). The prevalence of the ICBN in the axillae was 98.94%. No significant differences in prevalence were observed during the analysis of geographic subgroups or by study type (cadaveric dissections and in intraoperative dissections). Only five studies of the 23 studies reported prevalence of less than 100%. Overall, the PPE was 99.2% with 95% Cis of 98.5% and 99.7%. As expected from the near constant variance estimates, the heterogeneity was low, I 2 = 44.3% (95% CI 8.9%−65.9%), Q = 39.48, p = .012. When disaggregated by evaluation type, the difference in PPEs between evaluation types was negligible. For cadaveric dissection, the PPE was 99.7% (95% CI 99.1%–100.0%) compared to 99.0% (95% CI 98.1%–99.7%).
Conclusions
The prevalence of ICBN variants was very high. The dissection of the ICBN during axillary lymph-node harvesting, increases the risk of sensory disturbance. The preservation of the ICBN does not modify the oncological radicality in axillary dissection for patients with cutaneous metastatic melanoma or breast cancer. Therefore, we recommend to operate on these patients in high volume center to reduce post-procedural pain and paresthesia associated with a lack of ICBN variants recognition.
Introduction
The Intercostobrachial nerve (ICBN) emerges from the second intercostal space and it traverses the axilla horizontally [ 1 , 2 ]. Then it perforates the deep fascia of the arm, providing the sensory supply to the upper medial region of the arm [ 3 ]. The anatomy of ICBN represents a potential risk of unintentional injuries during routine surgical procedures such as axillary lymph node dissection (ALND) and sentinel lymph node biopsy (SLNB) [ 4 ].
Breast cancer stands as the most prevalent malignant in women throughout the world, affecting almost 12% of women in Western countries [ 5 ]. The ICBN seems to be frequently damaged during mastectomy procedures and other techniques such as ALND (Axillary Lymph Node Dissection) and SLNB (Sentinel Lymph Node Biopsy); some studies suggest that injuring ICBN could play a role in Persistent Pain After Breast Cancer Treatment (PPBCT) and reduction of sensory function in the affected area [ 1 , 3 ]. Additionally, the possibility of injury of the ICBN should also be considered in axillary surgery for melanoma [ 6 ].
Researchers have demonstrated that ICBN preservation significantly reduce post-procedural paresthesia and improve quality of life after the treatment for breast cancer and axillary surgery for melanoma. In fact, damaging ICBN or one of its primary branches could represents a cause of dysesthesia, paresthesia and chronic pain in these patients. Surgeons need to be accurate during the exploration of the axillary region, as the initial divisions and the connections between the ICBN and the brachial plexus may be damaged [ 3 ]. The configuration of the ICBN has an important variability, showing multiple points of origin, patterns of division, and connections with other branches. If surgeons could know the real frequency, the characteristics of these division and the origin models of ICBN they could reduce the ICBN lesions and the post operatory morbility [ 3 , 4 ].
The aims of this systematic review and meta-analysis are to assess the prevalence, the different origins and branching patterns of the intercostobrachial nerve, analyzing the literature available up to the present day.
Search strategy
The protocol for this study was registered on PROSPERO (ID: CRD42023447932), an international prospective database for reviews.
The PRISMA (preferred reporting items for systematic reviews and meta-analyses) guideline was respected throughout the meta-analysis [ 7 ]. The identification of articles to be included in the meta-analysis was carried out with searches up to September 2023 in the following databases: PubMed, Scopus and Web of Science. The search strategy performed in PubMed is presented as follows: nerve and Intercostobrachial or nervus intercostobrachialis. No date or language restrictions were applied. Identification of additional studies eligible for the meta-analysis was performed by searching the references of all included articles.
Evaluation of inclusion criteria
Eligibility for inclusion in this systematic review and meta-analysiss was performed by M.M. and R.C. All intraoperative or cadaveric studies reporting extractable prevalence data on the origin or branching of ICBN were included. Exclusion criteria included: case reports, case series, letters to the editor, or conference abstracts.
Data extraction
Data from the included studies were extracted by R.C. and M.M. For each article, the following information have been extracted: first author and year of publication, nation of study, sample size (number of patients and number of axillae analyzed), time of enrollment, type of study, type of evaluation (cadaveric or surgical), type of surgical treatment, prevalence of ICBN, origin of ICBN, mode of branching of ICBN. All selected full text articles were evaluated by quality assessment and analysis of the risk of bias using the Anatomical Quality Assurance (AQUA) [Henry, B.M. et al. 2017 [ 8 ]].
Study identification
The initial literature search identified 497 articles. Following the removal of duplicates and primary screening, 43 articles were assessed as full text for eligibility in the meta-analysis records. Finally, we included a total of 23 articles [ 2 , 3 , 4 , 6 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 ] selected by eliminating articles with incomplete information and articles that used different classification (Fig. 1 ).
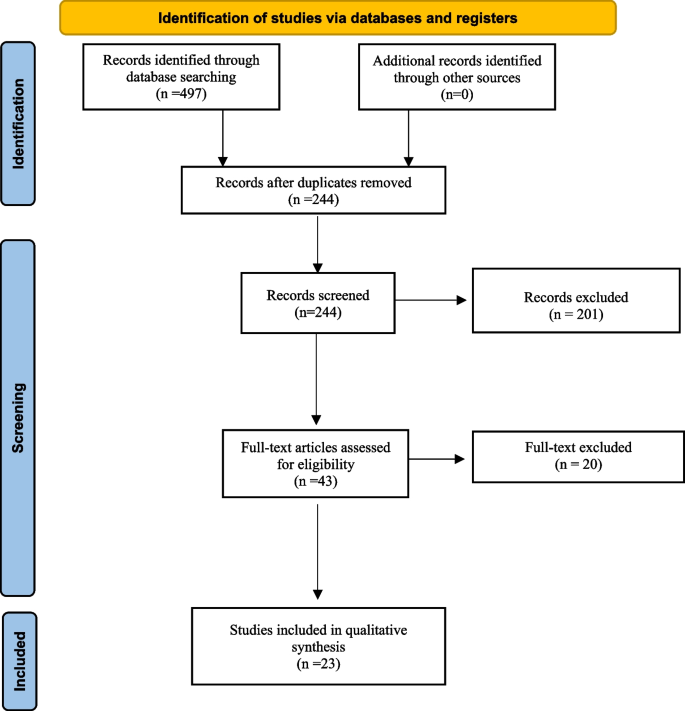
Prisma flow diagram of studies included
Characteristics of the included studies
The systematic review and meta-analysis include twenty-three studies published between 1999 and 2023. Six studies were conducted on cadaveric samples [ 9 , 15 , 16 , 23 , 27 , 28 ], and seventeen were performed intraoperatively [ 2 , 3 , 4 , 6 , 10 , 11 , 12 , 13 , 14 , 17 , 18 , 19 , 20 , 21 , 22 , 24 , 25 , 26 ]. The studies exhibit a broad geographic distribution, with 10 studies from Asia [ 3 , 4 , 9 , 10 , 11 , 12 , 16 , 18 , 21 , 28 ], 6 from Europe [ 6 , 14 , 19 , 20 , 25 , 26 ], 4 from South America [ 2 , 15 , 22 , 24 ], and one each from North America [ 23 ], Africa [ 17 ], and Australia [ 27 ]. Among the twenty-three studies, 1,636 patients were included, and 1,883 axillae were evaluated (494 from cadaveric dissections and 1,389 from intraoperative dissections): 765 from Asians, 570 from Europeans, 318 from South Americans, 200 from North Americans, 30 from Africans, and 28 from Australians (Table 1 ).
Quality valutation of the studies included
The AQUA tool probes for potential risk of bias in 5 studies domains (objectives and subject characteristics, study design, methodology characterization, descriptive anatomy and reporting of results) (Henry, B.M. et al. 2017 [ 8 ]). The risk of bias within each domain is normally categorized as “Low”, “High” or “Unclear”. Twenty-two of the studies included showed low risk in domain one (Objectives and Subject characteristics), ten studies showed high risk of bias in domain three (Methodology characterization), mainly because there is an important reduction of possibility of studying anatomy during an intervention. A summary of the assessment of quality and risk of bias by the AQUA tool is displayed in the Fig. 2 .
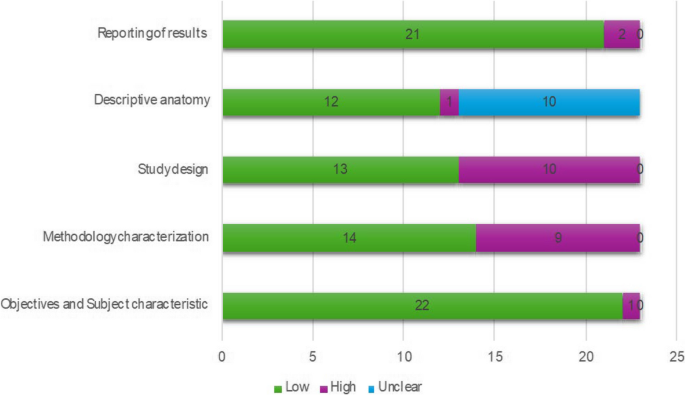
Assessment of quality and risk of bias by the AQUA tool
Statistical methods
For the primary outcome—prevalence of the ICBN—pooled prevalence estimates (PPES) and their 95% confidence intervals are reported using MetaXL software (V. 5.3). We used a DerSimonian and Larid random effects model with a double arcsin transformation, normalized prevalence, and a 0.5 continuity correction. Heterogeneity was investigated through the I 2 statistics, Cochrane’s Q statistic, and a visual analysis of forest plots and funnel plots. In addition, we examined evaluation type (cadaveric dissection or intraoperative dissection) and geographic region of the first author’s affiliation (Africa, Americas, Asia, Oceania, or Europe) as factors. A leave-one-out sensitivity analysis was conducted to examine the effect of outlying studies. Funnel plots and Doi plots were used to investigate possible sources of bias—including publication bias. The leave-one-out sensitively analyses yielded PPEs from 99.3% with the Andersen 2014 [ 19 ] study excluded to 99.1% with the Loukas et al. 2016 [ 23 ] study excluded.
There was great variety in the outcome categories that authors used in the secondary outcomes: this is the reason why a single multicategory pooled prevalence estimate was not possible for any secondary outcomes. Furthermore, it was not feasible to report on the PPEs for tens of secondary outcomes if each of the outcome categories were reported as individual binary outcomes. Therefore, we descriptively report the secondary outcomes using only raw, marginal proportions.
Primary outcome
Prevalence of the icbn.
All studies reported data on the prevalence of the ICBN (1,883 axillae). The overall total prevalence of the ICBN in the axillae was 98.94% (1,863 ICBN). No significant differences in prevalence were observed during the analysis of geographic subgroups [99.35% in Asians (760 ICBN), 97.54% in Europeans (556 ICBN), 99.68% in South Americans (317 IBCN), 100% in North Americans (200 IBCN), 100% in Africans (30 ICBN), and 100% in Australians (28 IBCN)] or by study type [99.8% (93.75–100%) in cadaveric dissections (493 ICBN) and 98.63% (94.69–100%) in intraoperative dissections (1,370 ICBN)] (Fig. 3 ).
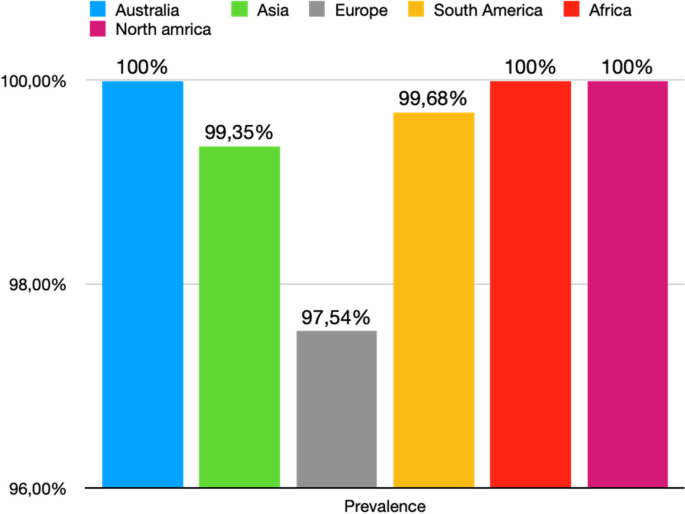
Prevalence of ICBN for continent
Figure 4 is a forest plot shows that the prevalence estimates for the ICBN had a near constant variance of 100%. Only five studies of the 23 studies reported prevalences of less than 100%. Overall, the PPE was 99.2% with 95% CIs of 98.5% and 99.7%. As expected from the near constant variance estimates, the heterogeneity was low, I 2 = 44.3% (95% CI 8.9%–65.9%), Q = 39.48, p = .012. The Doi plot showed minor asymmetry (Fig. 5 ) and the funnel plot (Fig. 6 ) as indicative of an outcome with near constant variance. Rather than a random display of data points within a funnel pattern, there was a line of studies with a prevalence of 100% and the other data points to the left of that line. When disaggregated by evaluation type, the difference in PPEs between evaluation types was negligible (Fig. 7 ). For cadaveric dissection, the PPE was 99.7% (95% CI 99.1%–100.0%) compared to 99.0% (95% CI 98.1%–99.7%). There were differences in PPEs between subgroups was also negligible for geographic region (Fig. 8 ).: Africa = 99.2% (95% CI 94.3%–100.0%), Americas = 99.7% (95% CI 99.0%–100.0%), Asia = 99.3% (95% CI 98.6%–99.9%), Oceania = 99.2% (95% CI 93.9%–100.0%), and Europe = 98.4% (95% CI 95.9%–100.0%).
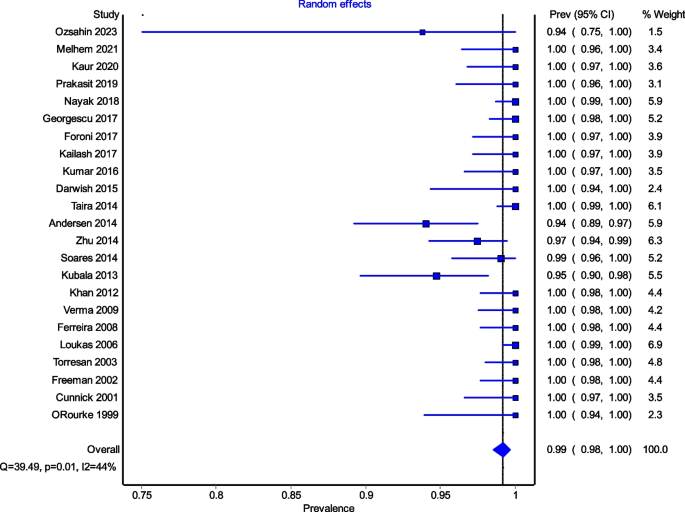
Prevalence estimates for the ICBN
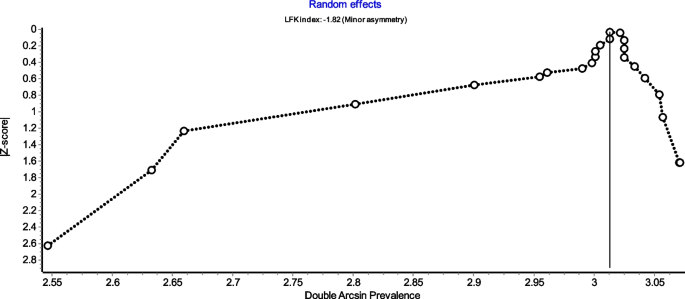
Funnel plot
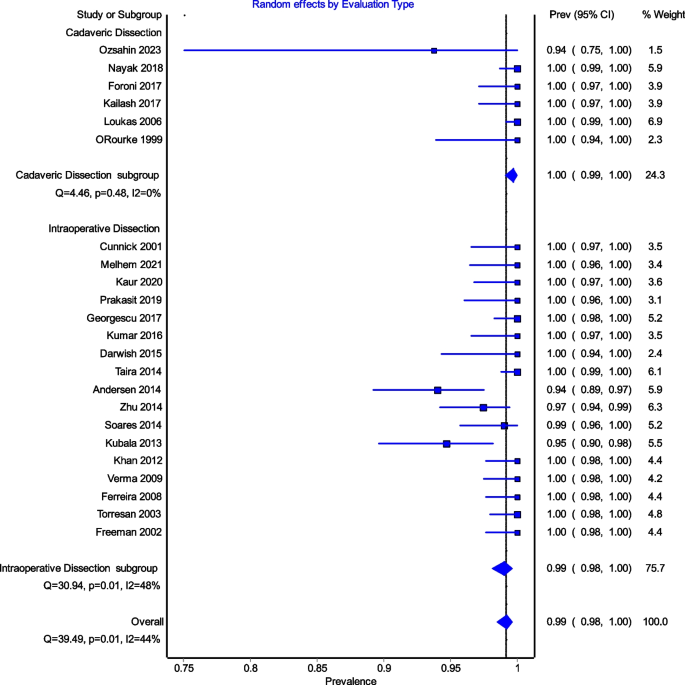
Random effects by Evaluation type
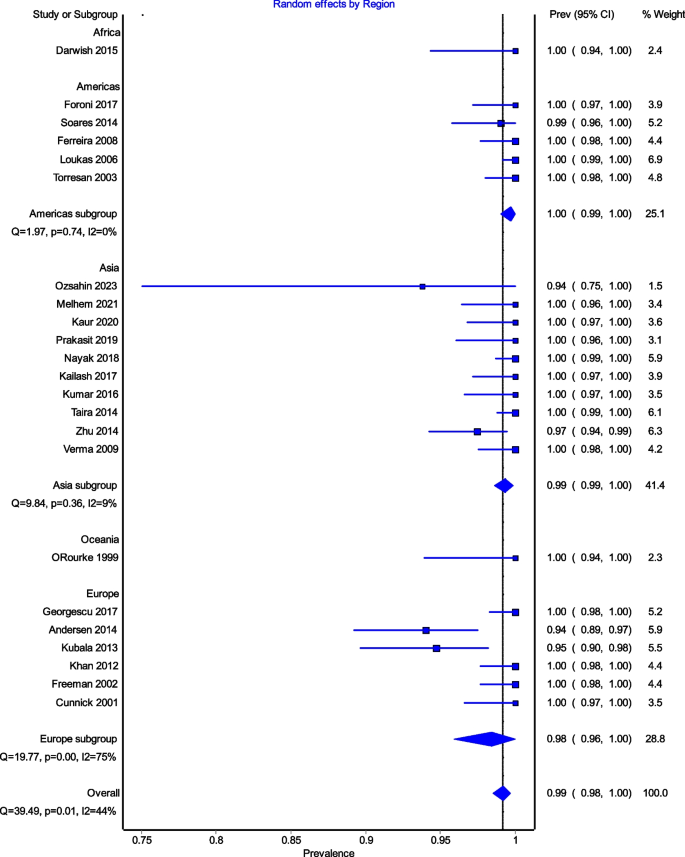
Random effects by Region
Secondary outcomes
Origin of the intercostobrachial nerve.
Eight studies (747 ICBN) reported data on the origin of the ICBN. The most common origin was at the T2 vertebral level, accounting for 81.79% of cases (611 ICBN), followed by T2-T3 at 8.17% (61 ICBN), T3 at 4.55% (34 ICBN), and T1-T2 at 2.9% (24 ICBN); much rarer origins were T1 at 1.07% (8 ICBN) and T1, T2, T3 at 1.2% (9 ICBN). To further clarify, combined origins were indicated when two separate roots were observed to merge into a common ICBN (Table 2 ). A total of 12 studies (1,060 ICBNs) reported data on the ICBN branching pattern. Table 3 provides detailed information on the ramifications of the ICBN. The ICBN most appeared as a single trunk in 51.6% of cases (547 ICBN), followed by the bifurcation pattern at 29.71% (315 IBCN), and the multiple branch pattern at 14.81% (157 IBCN). Subgroup analysis did not reveal significant differences.
Surgical analysis
Intraoperative preservation of the intercostobrachial nerve.
Sixteen studies (1,311 ICBN) reported data on the preservation of the ICBN during axillary dissection for breast cancer (15 studies) and melanoma (1 study). It was found that the ICBN was completely preserved in 63.39% (34-95.65%) (831 ICBN) and partially preserved in 4.27% (15–29%) (56 ICBN). Only three studies reported the reasons for ICBN division (52 ICBN): accidental injury (53.85%, 28 ICBN), necessity dissection due to nerve involvement in lymph node clusters (30.77%, 16 ICBN), necessity dissection due to the nerve hindering proper access to the axillary cavity (15.38%, 8 ICBN).
Sensorial analysis
Pain in patients with intercostobrachial nerve section.
Postoperative pain was assessed at the time of discharge (7 studies, 209 ICBN) and after 3 months (4 studies, 92 ICBN). Postoperative pain was present in 38.75% at discharge and 46.74% after 3 months from the procedure (Fig. 9 ).
Hypoesthesia in patients with intercostobrachial nerve section
The decrease in postoperative sensitivity was evaluated at the time of discharge (5 studies, 169 ICBN) and after 3 months (4 studies, 105 ICBN). Postoperative hypoesthesia was present in 62.13% at the time of discharge and 51.42% after 3 months from the procedure (Fig. 9 ).
Paresthesia in patients with intercostobrachial nerve section
The presence of postoperative paresthesia was assessed at the time of discharge (9 studies, 283 ICBN) and after 3 months (2 studies, 42 ICBN). Postoperative paresthesia was present in 40.99% at the time of discharge and 10.04% after 3 months from the procedure (Fig. 9 ).
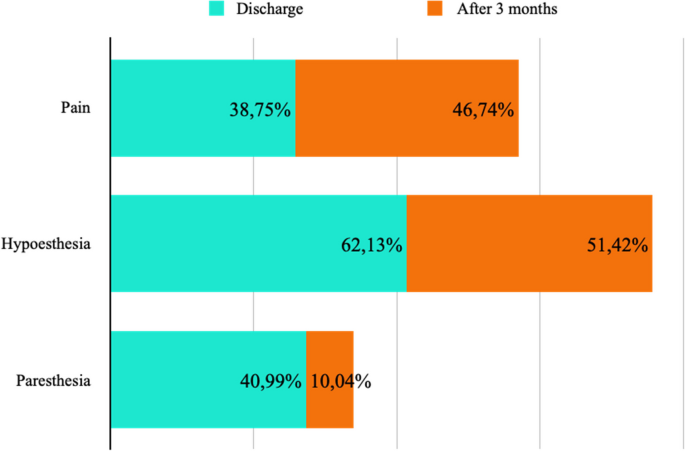
Patients with ICBN section
Pain in patients with intact intercostobrachial nerve
Postoperative pain was assessed at the time of discharge (7 studies, 288 ICBN) and after 3 months (4 studies, 103 ICBN). Postoperative pain was present in 31.59% at discharge and 12.62% after 3 months (Fig. 10 ).
Hypoesthesia in patients with intact intercostobrachial nerve
The decrease in postoperative sensitivity was evaluated at the time of discharge (5 studies, 228 ICBN) and after 3 months (4 studies, 123 ICBN). Postoperative hypoesthesia was present in 45.17% at discharge and 18.70% after 3 months (Fig. 10 ).
Paresthesia in patients with intact intercostobrachial nerve
The presence of postoperative paresthesia was assessed at the time of discharge (8 studies, 321 ICBN) and after 3 months (3 studies, 57 ICBN). Postoperative paresthesia was present in 29.6% at discharge and 22.8% after 3 months (Fig. 10 ).
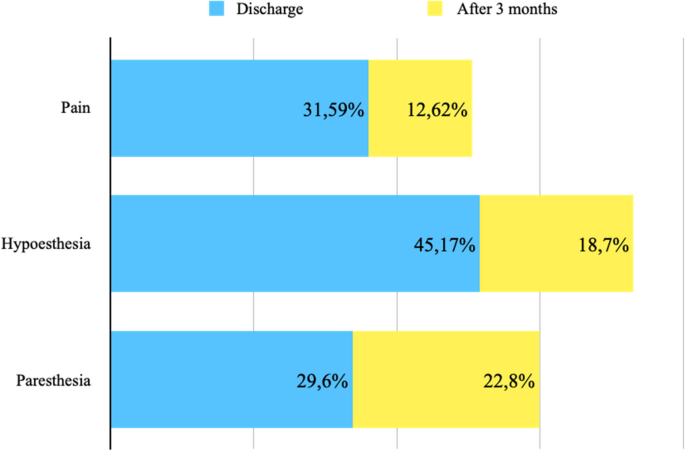
Patients with ICBN preservation
The intercostobrachial nerve (ICBN) is an anatomical structure with a high variability in origin and branching pattern. The ICBN is a branch of the second intercostal nerve: therefore, special attention should be paid to the area of the second intercostal space, where the origin of the ICBN is most likely (90.6%). However, the ICBN could present occasional contribution also from the third intercostal nerve and it can be identified in an anterior position during the exposure of the thoracic and thoracodorsal long nerves. The ICBN is at high risk of injury during operative procedures into the axilla. ICBN fiber’s injury has been associated with post-procedural pain, paresthesia and with a reduction of the quality of life.
The evaluation of prevalence of ICBN might have an impact on surgical plan and patient outcomes: in fact, lymphedema, motor and/or sensor alterations of the arm have an impact on the quality of life of patients who underwent to operative procedure into the axillary region. Moderate or severe post-operative pain is experienced by 50% of patients after breast surgery (Andersen; Besic).
The ICBN should always be preserved. However, current guidelines do not provide specific recommendations regarding the preservation or sacrifice of the ICBN during axillary lymph-node dissection or sentinel lymph-node biopsy. A study conducted by Henry et al. in 2017 [ 29 ] showed that the ICBN is a variable structure at risk for injury during operative procedures of the axilla and due to the postoperative pain and paresthesia experienced by patients following injury, surgeons need to exercise caution and need to preserve the ICBN. Another study, conducted by Warrier et al. in 2014 [ 30 ] showed that the incidence of sensory disorder was lower in case of preservation of ICBN compared with the division of the ICBN.
On the other hand, in three different studies, conducted respectively by Abdullah et al. 1998 [ 31 ], Salmon et al. 1998 [ 32 ] and Torresan et al. 2003 [ 24 ], showed that patients who presented sensory distur in the immediate post-operative period may have a resolution of the symptoms and patients who may not notice any sensory disturbance initially may develop it at a later stage.
The ICBN can be damaged for a variety of reasons, from traction to transection. In addition, damaged nerves can develop neuromas that can further complicate the patient’ symptoms.
Our systematic review and meta-analysis showed that the overall prevalence of ICBN was 98.94% and that more frequently it exists as a single trunk (51.6%) originating from the T2 vertebral level (81.79%). The branching subgroups are differently based on the type of the study (cadaveric or operational). In fact, we noticed that the ICBNs examined in cadavers (99.8%) had a bifurcation rate more than double than those evaluated during operation (98.63%).
From these differences we deduced two different hypotheses. Firstly, the limited intraoperative field of view and the inability to freely dissect tissue without consequences may limit the in vivo identification of the nerve branches. These differences may help to explain the reason why post-operative complications might be present even if nerves are successfully identified and protected. Kumar et al. 2016 [ 3 ] noted that, six months after surgery, 20% of patients who had successfully preserved ICBN still had numbness and paresthesia. The ICBN may also be damaged due to stretching by retractors or other intraoperative stresses on nerve fibers. Secondly, ICBN might have a propensity to bifurcate unequally when analyzing cadaveric or intraoperative studies (63.4% unequal bifurcation versus 36.6% equal bifurcation). Therefore, surgeons can easily identify the larger trunk, but a failure to identify the smaller branch puts it at high risk of operative injury.
Our meta-analysis was limited by several factors related to inconsistent reporting and small sample sizes. Some studies presented the origins of the first or third intercostal nerve as separate data. It was unclear whether these origins were duplications or simply contributing fibers and therefore were excluded from the present analysis. In addition, we observed a high heterogeneity which might have the result of an inherent variable nature of the ICBN course. Another limitation of our study is the lack of data regarding the preservation of the intercostobrachial nerve during SLNB. However, larger studies are needed to demonstrate this observation.
Furthermore, in our analysis, cadaveric dissection rather than intraoperative dissection was more specific in the demonstration of multiple branching pattern. In fact, anatomical data retrieved from operative field were less accurate. Probably this rate discrepancy was associated to reporting errors.
In general, the studies did not provide information on factors such as sex and laterality, which could allow further subgroup analyses. Most studies have been conducted on women; however, Loukas et al. 2006 [ 23 ] found no gender differences. Limited data from regions such as Africa, North America, Oceania, and South America precluded analysis of other regions besides Europe and Asia. Many studies have a lack of detailed data on the branching site or symmetry of post-division branches.
Future studies need to be carried out to further elucidate the behavior of the terminal branching of the ICBN and its anastomoses with the brachial plexus. There is also the possibility of studying the use of landmarks or relationships with adjacent structures to be able to intraoperatively identify the nerves. Structures such as the lateral thoracic vein discussed in O’Rourke et al. 1999 [ 27 ] could be a potential candidate. Integration with future research will provide valuable information for surgeons and, ideally, lead to more positive outcomes for patients undergoing surgeries in the axillary region. In fact, ICBN neuralgia and post-operative pain syndrome can be successfully managed with loco-regional anesthesia techniques, but the primary goal should be the overall reduction of their incidence.
An accurate knowledge of the anatomy of axillary region is crucial to reduce nerve’ injuries. Another possible strategy for minimizing nerve’ injuries during surgery could be the ultrasound guided identification of ICBN before surgery. In fact, in a study conducted by Feigl et al. the ICBN blocks with ultrasound guided anterior approach to intercostal nerves was evaluated to supplement the axillary block. The sonographic identification of ICBN and its possible anatomical variations may be useful to reduce ICBN’s injuries [ 33 ].
Furthermore, the group with intact ICBN have a higher percentage of paresthesia (32.8%) after three months than the group with ICBN section. We were unable to explain this result, but we think that this pain might be similar to groin pain after inguinal hernia repair [ 34 ]. For this reason, some surgeons suggest to perform the inguinal nerve neurectomy in all patient underwent open mesh hernia repair [ 35 ].
The prevalence of ICBN is very high, for this reason during axillary dissection a small operative theatre is associated at the inability to freely dissect tissue without a reduction of post-procedural pain and paresthesia. The division of the ICBN during axillary lymph node dissection, increases the risk of sensory disturbance such as hyposensitivity of the arm. The preservation of the ICBN do not modify the oncological radicality in axillary dissection for patients with cutaneous metastatic melanoma or breast cancer.
Availability of data and materials
The data of the article can be accessed by requesting the corresponding author.
Mewa Kinoo S, Singh B. Complex regional pain syndrome of the breast and chest wall. Breast J. 2016;22(3):366–8.
Article PubMed Google Scholar
Soares EW. Anatomical variations of the axilla. Springerplus. 2014;3:306.
Article PubMed PubMed Central Google Scholar
Kumar P, Meena RN, Sheikh BH, Belliappa V, Pais AV, et al. Intercostobrachial nerve - anatomical considerations and its importance in carcinoma breast of female patients. Ann Surg Perioper Care. 2016;1(2):1013.
Google Scholar
Zhu JJ, Liu XF, Zhang PL, Yang JZ, Wang J, Qin Y, Zhang GL, et al. Anatomical information for intercostobrachial nerve preservation in axillary lymph node dissection for breast cancer. Genet Mol Res. 2014;13(4):9315–23.
Article CAS PubMed Google Scholar
DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Goding Sauer A, et al. Breast cancer statistics, 2013. CA Cancer J Clin. 2014;64(1):52–62.
Kubala O, Prokop J, Jelínek P, Ostruszka P, Tošenovský J, Ihnát P, et al. Anatomicko-chirurgická studie průběhu interkostobrachiálních nervů (ICBN) v axile při exenteraci I. a II. etáže axily u karcinomu prsu a maligního melanomu [Anatomic-surgical study of intercostobrachial nerve (ICBN) course in axilla during I. and II. Level of axilla clearance in breast cancer and malignant melanoma]. Rozhl Chir. 2013;92(6):320–9. Czech.
CAS PubMed Google Scholar
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Henry BM, Tomaszewski KA, Ramakrishnan PK, et al. Development of the Anatomical Quality Assessment (AQUA) Tool for the quality assessment of anatomical studies included in meta-analyses and systematic reviews. Clin Anat. 2017;30:6–13.
Özşahin MK, Kaynak G, Afacan MY, et al. Anatomical variations of intercostobrachial nerve: a potential candidate for neurotization after traumatic median nerve injury? Ulus Travma Acil Cerrahi Derg. 2023;29:22–9.
PubMed Central Google Scholar
Melhem J, Amarin M, Odeh G, Al-Bustami N, Al-Lauzy H, Ayoub R. Intercostobrachial nerve (ICBN) preservation versus sacrifice in axillary dissection: randomized controlled trial. Am J Clin Oncol. 2021;44(5):206–9.
Kaur N, Kumar R, Jain A, et al. Sensory changes and postmastectomy pain following preservation of intercostobrachial nerve in breast cancer surgery: a prospective randomized study. Indian J Surg Oncol. 2021;12(1):108–13.
Chirappapha P, Kongdan Y, Vassanasiri W, Ratchaworapong K, Sukarayothin T, Supsamutchai C, et al. Evaluation the effect of preserving intercostobrachial nerve in axillary dissection for breast cancer patient. Gland Surg. 2019;8(6):599–608.
Soubhagya Ranjan Nayak, Smita Singh Banerjee. Anatomic variations of the extrathoracic course of the intercostobrachial nerve and its clinical significance. Gland Surg. 2019;8(6):599–608.
Orsolya HB, Coros MF, Stolnicu S, Georgescu R, et al. Does the surgical management of the intercostobrachial nerve influence the Postoperatory Paresthesia of the upper limb and life quality in breast cancer patients? Chirurgia (Bucur). 2017;112(4):436–42.
Foroni L, Siqueira MG, Martins RS, Oliveira GP. The intercostobrachial nerve as a sensory donor for hand reinnervation in brachial plexus reconstruction is a feasible technique and may be useful for restoring sensation. Arq Neuropsiquiatr. 2017;75(7):439–45.
Kailash D, Balkund S, Priyadharshini. A study on variations of intercostobrachial nerve. MedPulse – Int J Anat 2017;4(2):14–6.
Article Google Scholar
Darwish A, Mlees A, El-Gendy M, Elghazeery M. Preservation of lateral thoracic vein and intercostobrachial nerve in breast conservative surgery. Ain Shams J Surg. 2015;8(1):53–60.
Taira N, Shimozuma K, Ohsumi S, et al. Impact of preservation of the intercostobrachial nerve during axillary dissection on sensory change and health-related quality of life 2 years after breast cancer surgery. Breast Cancer. 2014;21(2):183–90.
Andersen KG, Aasvang EK, Kroman N, Kehlet H. Intercostobrachial nerve handling and pain after axillary lymph node dissection for breast cancer. Acta Anaesthesiol Scand. 2014;58(10):1240–8.
Khan A, Chakravorty A, Gui GP. In vivo study of the surgical anatomy of the axilla. Br J Surg. 2012;99(6):871–7.
Verma S, Kala R, Bhargava G, Yadav R, Singh R, Maurya P, et al. Evaluation of the role of preservation of the intercostobrachial nerve on the post-mastectomy pain syndrome in breast cancer patients of North India. Internet J Surg 23. 2009;23:2.
Ferreira BP, Soares. Morbidade cirúrgica pós-biópsia de linfonodo sentinela e esvaziamento axilar: estudo comparativo em mulheres com e sem preservação do nervo intercostobraquial. Rev Assoc Med Bras. 2008;54(6):517–21.
Loukas M, Hullett J, Louis RG Jr, Holdman S, Holdman D. The gross anatomy of the extrathoracic course of the intercostobrachial nerve. Clin Anat. 2006;19(2):106–11.
Torresan RZ, Cabello C, Conde DM, Brenelli HB. Impact of the preservation of the intercostobrachial nerve in axillary lymphadenectomy due to breast cancer. Breast J. 2003;9(5):389–92.
Freeman SR, Washington SJ, Pritchard T, Barr L, Baildam AD, Bundred NJ. Long term results of a randomised prospective study of preservation of the intercostobrachial nerve. Eur J Surg Oncol. 2003;29(3):213–5.
Cunnick GH, Upponi S, Wishart GC. Anatomical variants of the intercostobrachial nerve encountered during axillary dissection. Breast. 2001;10(2):160–2.
O’Rourke MG, Tang TS, Allison SI, Wood W. The anatomy of the extrathoracic intercostobrachial nerve. Aust N Z J Surg. 1999;69(12):860–4.
Nayak SR, Banerjee SS. Anatomic variations of the extrathoracic course of the intercostobrachial nerve and its clinical significance. Asian J Med Sci. 2018;9(5):77–80.
Henry BM, Graves MJ, Pękala JR, Sanna B, Hsieh WC, Tubbs RS, et al. Origin, branching, and communications of the intercostobrachial nerve: a meta-analysis with implications for Mastectomy and axillary lymph node dissection in breast cancer. Cureus. 2017;9(3):e1101.
PubMed PubMed Central Google Scholar
Warrier S, Hwang S, Koh CE, Shepherd H, Mak C, Carmalt H, et al. Preservation or division of the intercostobrachial nerve in axillary dissection for breast cancer: meta-analysis of randomised controlled trials. Breast. 2014;23(4):310–6.
Abdullah TI, Iddon J, Barr L, Baildam AD, Bundred NJ. Prospective randomized controlled trial of preservation of the intercostobrachial nerve during axillary node clearance for breast cancer. Br J Surg. 1998;85(10):1443–5.
Salmon RJ, Ansquer Y, Asselain B. Preservation versus section of intercostal-brachial nerve (IBN) in axillary dissection for breast cancer–a prospective randomized trial. Eur J Surg Oncol. 1998;24(3):158–61.
Feigl GC, Litz RJ, Marhofer P. Anatomy of the brachial plexus and its implications for daily clinical practice: regional anesthesia is applied anatomy. Reg Anesth Pain Med. 2020;45(8):620–7.
Charalambous MP, Charalambous CP. Incidence of chronic groin pain following open mesh inguinal hernia repair, and effect of elective division of the ilioinguinal nerve: meta-analysis of randomized controlled trials. Hernia. 2018;22(3):401–9.
Cirocchi R, Sutera M, Fedeli P, Anania G, Covarelli P, Suadoni F, Boselli C, Carlini L, Trastulli S, D’Andrea V, Bruzzone P. Ilioinguinal nerve neurectomy is better than preservation in Lichtenstein Hernia repair: a systematic literature review and meta-analysis. World J Surg. 2021;45(6):1750–60.
Download references
Acknowledgements
We would like to thank all the participants who contributed to the study.
We do not have any funding.
Author information
Authors and affiliations.
Department of Medicine and Surgery, University of Perugia, Perugia, 06132, Italy
Roberto Cirocchi, Francesca Duro, Luca Properzi, Stefano Avenia, Carlo Boselli & Piero Covarelli
Department of Medicine and Surgery, University of Milan, Milan, 20122, Italy
Matteo Matteucci
Georgia Baptist College of Nursing, Mercer University, Atlanta, GA, 30341, USA
Justus Randolph
Department of Public Health, University of Naples “Federico II”, Naples, 80131, Italy
Bruno Amato
Department of General Surgery, P.O Frangipane Ariano Irpino, Avellino, 83031, Italy
Ruggiero Iandoli
Department of Digestive and Emergency Surgery, AOSP of Terni, Terni, 05100, Italy
Giovanni Tebala
Department of Surgery, “Sapienza“ University of Rome, Roma, 00161, Italy
Paolo Sapienza
You can also search for this author in PubMed Google Scholar
Contributions
R.C., M.M. and P.S. contributed to the design of study, conducted study and drafted the initial manuscript. G.T. and L.P., B.A., F.D revised manuscript. R.A. contributed to data collection. J.R. assisted in analyzing data and interpreting the data. P.C. and C.B. contributed to perform surgery and supervision. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Roberto Cirocchi .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Cirocchi, R., Matteucci, M., Randolph, J. et al. Anatomical variants of the intercostobrachial nerve and its preservation during surgery, a systematic review and meta-analysis. World J Surg Onc 22 , 92 (2024). https://doi.org/10.1186/s12957-024-03374-w
Download citation
Received : 16 November 2023
Accepted : 28 March 2024
Published : 11 April 2024
DOI : https://doi.org/10.1186/s12957-024-03374-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
World Journal of Surgical Oncology
ISSN: 1477-7819
- General enquiries: [email protected]
- Open access
- Published: 05 April 2024
Concern about the risk of aerosol contamination from ultrasonic scaler: a systematic review and meta-analysis
- Priscilla Gonçalves Lomardo 1 ,
- Mariana Campello Nunes 1 ,
- Patrícia Arriaga 1 ,
- Lívia Azeredo Antunes 2 ,
- Aldir Machado 1 ,
- Valquiria Quinelato 3 ,
- Telma Regina da Silva Aguiar 1 &
- Priscila Ladeira Casado 1
BMC Oral Health volume 24 , Article number: 417 ( 2024 ) Cite this article
162 Accesses
Metrics details
Many instruments used in dentistry are rotary, such as handpieces, water syringes, and ultrasonic scalers that produce aerosols. The spray created by these instruments can carry, in addition to water, droplets of saliva, blood, and microorganisms, which can pose a risk of infections for healthcare professionals and patients. Due to the COVID-19 pandemic, this gained attention.
The aim was to carry out a systematic review of the evidence of the scope of the aerosol produced by ultrasonic scaler in environmental contamination and the influence of the use of intraoral suction reduction devices.
Scientific literature was searched until June 19, 2021 in 6 databases: Pubmed, EMBASE, Web of science, Scopus, Virtual Health Library and Cochrane Library, without restrictions on language or publication date. Studies that evaluated the range of the aerosol produced by ultrasonic scaler during scaling/prophylaxis and the control of environmental contamination generated by it with the use of low (LVE) and high (HVE) volume evacuation systems were included.
Of the 1893 potentially relevant articles, 5 of which were randomized controlled trials (RCTs). The meta-analysis of 3 RCTs showed that, even at different distances from the patient’s oral cavity, there was a significant increase in airborne bacteria in the dental environment with the use of ultrasonic scaler. In contrast, when meta-analysis compared the use of HVE with LVE, there was no significant difference ( P = 0.40/CI -0.71[-2.37, 0.95]) for aerosol produced in the environment.
Conclusions
There is an increase in the concentration of bioaerosol in the dental environment during the use of ultrasonic scaler in scaling/prophylaxis, reaching up to 2 m away from the patient’s mouth and the use of LVE, HVE or a combination of different devices, can be effective in reducing air contamination in the dental environment, with no important difference between different types of suction devices.
Peer Review reports
Every aspect of life has been influenced by an outbreak of the new coronavirus disease (COVID-19) in China [ 1 ] which greatly changed the routine in dental clinics. Due to the COVID-19 pandemic, the Centers for Disease Control and Prevention (CDC) recommended in principle, avoiding aerosol-generating procedures in the dental environment whenever possible, not using equipment that produces aerosols, and prioritizing the use of hand instruments only [ 2 ].
However, many of the instruments used in dentistry are rotary, such as handpieces, water syringes, and ultrasonic scalers. The spray created by these instruments can carry, in addition to water, droplets of saliva, blood, and microorganisms, which can pose a risk of infections for healthcare professionals and patients [ 3 ].
Particles formed by liquids and solids dispersed and suspended in the air are aerosols, which become bioaerosols when microorganisms excreted by the body dissolve with the aerosols through the act of coughing, breathing vigorously, sneezing, or speaking loudly [ 4 , 5 ].
Micik et al. used the terms “aerosol” and “splatter” in 1969, in which they were defined as particles smaller and larger than 50 micrometers (µm) in diameter, respectively. The first term refers to small particles that remain in the air for a period of time before being deposited on surfaces or entering the airways. The second are particles or droplets that are forcibly ejected from the operating site, reaching a trajectory similar to that of a bullet until they come into contact with a surface or fall to the ground [ 6 ].
With a simulation using computational fluid dynamics to quantify the transport of large droplets and aerosols in dental clinic environments, we better understand the risks associated with a common dental procedure such as ultrasonic scaling. Aerosols below 15 μm remain in the air for up to 7.13 min on average and can travel up to 25.45 m on average from their source, potentially contaminating entire clinics [ 3 ].
The water spray droplets produced during ultrasonic scaling are extremely light in weight and release large numbers of microorganisms into the air [ 7 ]. The bacterial challenge appears to be considerable and it is likely that viruses and bacteria can be spread in this way [ 8 ]. It should also be taken into account that particles ranging from 0.3 to 5 µm increase significantly after instrumentation with an ultrasonic scaler [ 9 ] and the variation in ultrasonic frequency causes an increase in surface contamination, as well as the type of suction used, influences the degree of contamination [ 10 ].
Due to the fact that ultrasonic scaler is one of the equipment that produces the most aerosol and can be responsible for spreading the SARS-CoV-2 virus during dental care, which is a major concern among dentists, especially periodontists, this work aimed to carry out a systematic review of the evidence of the reach of the aerosol, produced by ultrasonic scaler during scaling and prophylaxis, in the contamination of the dental environment and the influence of the use of intraoral suction devices in the reduction of this contamination.
Materials and methods
The present systematic review was registered in the PROSPERO (International Prospective Register of Systematic Reviews) [ 11 ] under the number #CRD42020191209 and conducted in accordance with the recommendations of the “Cochrane Handbook for Systematic Reviews of Interventions” [ 12 ] and following the guidelines of the PRISMA checklist [ 13 ]. Clinical questions were organized using the “PECO” (Population, Exposition, Comparison and Outcome) strategy.
The objective of this study was to carry out a systematic review of the evidence of the reach of the aerosol in distance traveled, produced by ultrasonic scaler during scaling and prophylaxis, in the contamination of the dental environment and the influence of the use of intraoral suction devices in the reduction of this contamination.
Focus question
What is the evidence of the reach of the aerosol in distance traveled produced by scaling with ultrasonic scaler in the contamination of the dental environment and the influence of intraoral suction devices in the reduction of this contamination?
Search strategy
Scientific literature was searched in six electronic databases until June 19, 2021 through Pubmed ( https://pubmed.ncbi.nlm.nih.gov ), EMBASE ( https://www.embase.com ), Web of Science ( www.webofscience.com ), Scopus ( www.scopus.com ), Virtual Health Library (VHL - in the LILACS, BBO, and IBECS databases) (bvsalud.org), and Cochrane Library ( www.cochranelibrary.com ). No restrictions on language or publication date were imposed. In addition to the electronic search, a manual search was performed using the reference lists of the selected articles. In addition, information was searched in the OpenGrey open access database [ 14 ] for unpublished studies (grey literature) using the same terms.
The following MeSH terms (Medical Subjects Headings) [ 15 ] were used for the search: “dental care”, “dental prophylaxis”, “ultrasonic therapy”, “dental scaling” and “aerosols”. In addition, other synonyms of DeCS (Health Sciences Descriptors) [ 16 ] and free terms were applied in the search, they are: “delivery of dental care”, “dental treatment”, “ultrasonic instrumentation”, “ultrasonic dental scale”, “ultrasonic scaling”, “dental cleaning”, “subgingival scaling”, “supragingival scaling”, “splatter”, “aerosol contamination”, “bioaerosol”, “bio-aerosol”, “airborne”, “dental aerosols”. All descriptors were connected through the Boolean operators “AND” and “OR”. The search strategy is described in Tables 1 and 2 . The Endnote web software was used to organize the studies [ 17 ].
Selection criteria
The eligibility requirements were outlined according to the PECOS strategy:
P (Population of interest): patients undergoing dental scaling treatment with ultrasonic scaler were included;
E (Exposure): Aerosol produced by ultrasonic scaler;
C (Comparison): comparison of contamination reduction with the use of different intraoral suction devices;
O (Outcome): contamination of the environment caused by aerosol from ultrasonic scaler;
S (Study design): randomized controlled trials (RCT) were included.
Exclusion criteria were studies that use manual scaling; studies that included prior use of mouthwashes and studies that used external air decontamination systems.
The eligibility requirements considered for studies to be included in this review were: human studies; studies that evaluated the range of the aerosol produced by ultrasonic during scaling procedures; studies that evaluated the contamination of the environment by the aerosol produced by dental ultrasonic and studies that used intraoral suction reduction devices to control the aerosol.
Screening process
At first, two reviewers (PGL and MCN) independently selected titles and abstracts. Disagreements were resolved through discussion with a third reviewer (TRSA). Studies that appeared to meet the inclusion criteria or that did not have sufficient information in their titles and abstracts were selected for evaluation of the full article at a later stage. The same reviewers independently assessed full texts to determine whether studies were eligible. Data extraction and risk of bias were performed in studies that met the inclusion criteria.
Data extraction
All data were extracted individually by two reviewers (PGL and MCN) and discrepancies were discussed by a third reviewer (TRSA). Reviewers were calibrated in applying the inclusion and exclusion criteria applied to a sample of 20% of the studies to determine inter-rater agreement (Kappa = 0.80). All necessary data were found in the studies, and it is not necessary to contact the authors for clarification.
The synthesis of the extracted data was organized in table with the following variables: first author, year of publication, country of origin, type of clinic, patient involved, type of suction device, collected distances, type of incubation, outcome measure and results.
Outcome measures
The outcome measure was the count of bacterial colony forming units (CFU) present in the oral aerosol, produced by ultrasonic scaler during scaling and prophylaxis, collected through plates with culture media positioned at different distances around the patient and/or the clinic.
Assessment of the risk of bias and quality
The quality assessment of the studies was performed by the same reviewers (PGL and MCN) independently and any disagreement between them was resolved through consultation with a third party (TRSA).
The Cochrane Collaboration Tool was used to assess the risk of bias using the updated Risk of Bias 2 (RoB 2) tool [ 18 ].
This tool evaluates five domains that can be classified as: low risk of bias, some concerns or high risk of bias. The domains are:
D1: Randomization process;
D2: Deviations from intended interventions;
D3: Missing result data;
D4: Measurement of the result; and.
D5: Selection of reported result.
This tool also allows for ranking the overall risk of bias, which receives the least favorable ranking among the assessed risks for the domains. The judgment about the risk of bias resulting from each domain is proposed by an algorithm, based on signaling questions, which help the reviewer to assess the important factors for the evaluation of each domain.
Statistical analysis
Meta-analyses were performed using the Review Manager software, Version 5.4.1 (Nordic Cochrane Center, Cochrane Collaboration) [ 19 ]. A meta-analysis of the reach of the aerosol in the contamination of the environment and a meta-analysis of the reduction of the contamination of the environment were performed, comparing the use of high-volume evacuation (HVE) and low-volume evacuation (LVE), both expressed in mean and standard deviation of CFU/m³. The inverse variance statistical method was used, with a random effects analysis model. Forest plots were calculated for 95% confidence intervals (CI) and P values. Heterogeneity between study results and quantification of inconsistency was assessed using the I 2 test. Results were expressed as standardized mean difference. Subgroups were established according to the distance of the aerosol reach in relation to the patient’s oral cavity.
Analysis of certainty of evidence
The quality of evidence (certainty in effect estimates) was analyzed by two reviewers (PGL and PA) using the assessment, development and assessment of recommendations (GRADE) approach [ 20 ]. The domains evaluated in clinical studies were: risk of bias, inconsistency, indirectness, imprecision and publication bias.
GRADE defines the quality of scientific evidence in a clearer and more objective way, and can be classified as high, moderate, low or very low.
Selection of studies
A total of 1893 relevant records were found: 298 references from Pubmed, 191 from Web of Science, 502 from Scopus, 413 from EMBASE, 385 from VHL, 103 from Cochrane Library and 1 from Opengrey. 619 duplicate references were removed; 1274 studies were analyzed by title and abstract; 1236 were excluded after this selection; and 38 studies were selected for full-text analysis. Among the 38 selected studies, 33 studies were excluded. Figure 1 outlines the search process and reasons for exclusions. Five randomized controlled trials (RCTs) were included. The synthesis of the extracted data was organized in the Table 3 .
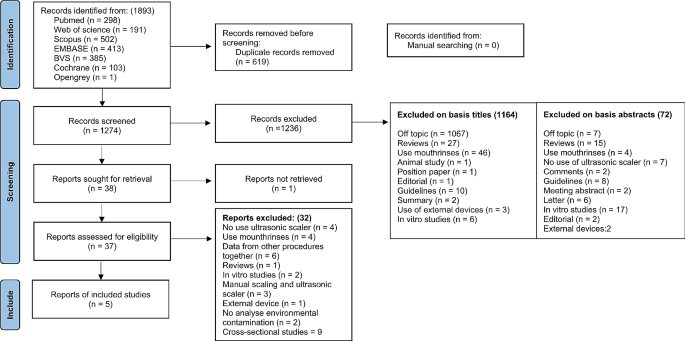
PRISMA 2020 flow diagram (13) of the screening and selection process
Study characteristics
The studies originated from 3 different countries: 1 from India [ 21 ], 3 from the United States [ 22 , 23 , 24 ] and 1 from Netherlands [ 25 ]. All selected articles were written in English. All included studies used ultrasonic scaler during treatments, but not all specified the type used, whether piezoelectric, magnetostrictive or sonic. In addition, they used high-volume and/or low-volume suction devices and made comparisons between two types of suction. Four studies were carried out in a dental environment with a single and only chair [ 21 , 22 , 23 , 25 ], and one study used a multi-chair environment [ 24 ].
Two studies had as sample patients diagnosed with periodontal disease [ 21 , 25 ]. The other studies were considered as if they had evaluated periodontally healthy patients [ 22 , 23 , 24 ].
All the studies measured aerosol contamination using colony forming units (CFU). The culture medium varied between studies. Some studies used culture media for aerobic and anaerobic bacteria [ 25 ], others only for aerobic bacteria culture [ 21 , 23 , 24 ], and one study only used anaerobic culture [ 22 ].
Assessment of risk of bias and quality
The quality of randomized controlled trials is shown in Fig. 2 . None of the randomized controlled trials scored the highest in the quality analysis. The five studies did not describe the allocation sequence and two [ 21 , 25 ] did not analyze the data according to a pre-specified analysis plan. Thus, they were characterized as some concerns.
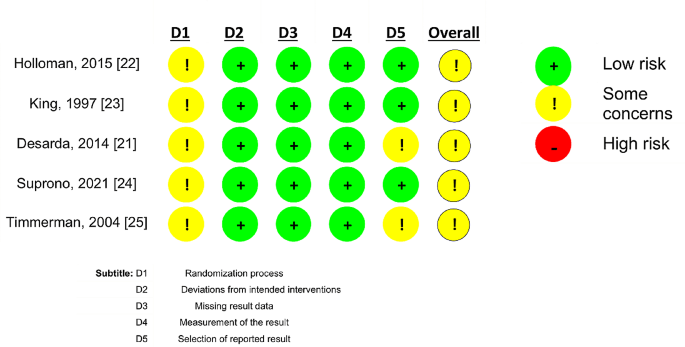
Risk of bias in randomized controlled trials analyzed using the RoB 2 tool
Meta-analysis
Two meta-analyses of RCTs were performed. The first in relation to contamination of the environment before and during the use of ultrasonic scaler and the second, referring to the reduction of contamination when comparing the use of high-volume suction versus low-volume suction. The analyzes carried out, taking into account the primary outcome of contamination of the dental environment, are shown in Fig. 3 , and the secondary outcome related to the reduction of contamination of the environment, comparing the use of HVE and LVE, is shown in Fig. 4 .
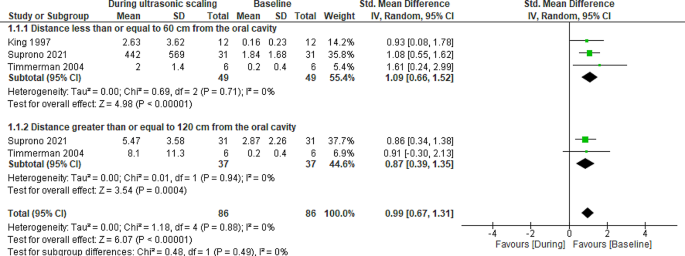
Analysis 1 – Contamination of the dental environment by aerosol produced during scaling with ultrasonic, in randomized controlled studies. Primary outcome: contamination of the dental environment. Subtitle: SD: Standard deviation, CI: Confidence interval
Analysis 2 - Reduction in the level of aerosol contamination by comparing the use of high (HVE) and low (LVE) volume suction in randomized controlled trials. Secondary outcome: reduction in the level of contamination. Subtitle: SD: Standard deviation, CI: Confidence interval
Only three randomized controlled studies [ 23 , 24 , 25 ] were included in the meta-analysis of data on environmental contamination and were divided into two subgroups: one considering data at a distance of less than or equal to 60 cm and another at a distance greater than or equal to 120 cm. For the contamination reduction analysis comparing different types of suction, data from three RCTs [ 21 , 23 , 25 ] were also included.
In the meta-analysis of the RCTs [ 23 , 24 , 25 ] (Fig. 3 ), the studies were homogeneous and indicated that, both at a distance less than or equal to 60 cm and at a distance greater than or equal to 120 cm from the patient’s oral cavity, there is disclosure of a smaller amount of bacteria in the dental environment before the ultrasonic procedure, even though high-volume suction was used, showing a significant increase in bacteria in the air in the dental environment with the use of ultrasonic scaler ( P < 0.00001/CI 0.99, [0.67, 1.31]), quantifying the magnitude of the effect, according to the Cohen scale [ 26 ], in large.
On the other hand, when a meta-analysis was performed comparing the use of HVE with LVE (Fig. 4 ), there was no significant difference ( P = 0.40/CI -0.71[-2.47, 0.95]) in reducing the amount of aerosol produced in the environment, quantifying the magnitude of the effect, according to the Cohen scale [ 26 ], in medium.
Certainty of evidence
The certainty of the evidence is represented in Tables 3 and 4 .
In the subgroup analysis for distances less than or equal to 60 cm and greater than or equal to 120 cm from the oral cavity, contamination of the dental environment with the use of ultrasonic scaler was greater than without the use of ultrasonic scaler and the certainty of the evidence was considered moderate for both distances (Table 4 ).
When comparing the use of high-volume suction with the use of low-volume suction in reducing levels of contamination in the dental environment, the certainty of the evidence was considered low (Table 5 ), with no significant differences between these devices. Serious problems with inconsistency and imprecision were detected in the studies included in the meta-analyses.
Aerosols and splashes are the main sources of environmental contamination during dental procedures [27]. This fact has become one of the biggest concerns among dentists during the COVID-19 pandemic. In order to review the evidence related to air contamination generated by the reach of the aerosol produced during the use of ultrasonic scaler for scaling and prophylaxis, a detailed search was carried out in six databases and five randomized controlled trials who met the inclusion criteria were found.
The high bacterial counts (log10 5.0 CFUs/mL) indicate that there is a worrying contamination of the air after the use of ultrasonic scaler, even when using a high volume suction combined with another device [ 22 ]. This contamination was shown in the first meta-analysis (Fig. 3 ) carried out on the results of randomized controlled studies [ 23 , 24 , 25 ], in which even with the use of high-volume suction, there was a significant difference in the increase of bacteria in the air ( P < 0.00001/CI 0.99, [0.67, 1.31]).
Of the five randomized controlled trials included, only two [ 23 , 24 ] found a statistically significant reduction in the mean CFUs ( p < 0.001) collected during the use of ultrasonic scaler when using two different suction methods, where one used the high-pressure suction cannula volume attached directly to the ultrasonic pen [ 23 ], and the other the high volume suction combination added plus a high volume suction hose [ 24 ]. The other RCTs [ 21 , 22 ] did not find significant differences ( p > 0.05) between the two suction methods studied. However, the number of bacteria in the air tends to be higher when conventional suction devices are used, that is, low volume ones [ 25 ]. Furthermore, the high-volume suction device used separately, without any modification, does not appear to be as effective in reducing the amount of aerosol formed [ 21 ]. Despite this, when a meta-analysis (Fig. 4) was performed comparing the use of high and low volume suction devices in three RCTs [ 21 , 23 , 25 ], there was no significant difference in the amount of aerosol formed during the use of ultrasonic scaler.
As limitations of the study, a difference was observed in the methodologies used by the studies, which makes a more accurate comparison difficult, as the distance at which the agar plates are placed to collect the samples, the plate exposure time and the different dental environments, can influence the comparison of results. Two RCTs [ 22 , 23 ] placed the sample collection plates six inches (15.24 cm) from the patient’s mouth and the others used different distances such as 40 and 150 cm [ 25 ]; 12 and 20 inches (approximately 30 and 50 cm) [ 21 ] and at three different distances between 2 and 4 feet (approximately 60–120 cm) (24). Regarding the exposure time of the plaque during the use of ultrasound, there was a variation between 5 min [ 23 , 25 ] and 20 min [ 24 ]. Therefore, the shorter the plate exposure time and the greater the distance, the lower the chance of CFU collection. And regarding the dental environments just one study used a multi-chair environment [ 24 ].
Another limitation of the study refers to the fact that it was not possible to assess publication bias as only five studies were included for meta-analysis, with low power to detect possible bias.
There is an increase in the concentration of bioaerosol in the dental environment during the use of ultrasonic scaler in scaling/prophylaxis, reaching up to 2 m away from the patient’s mouth.
The use of good suction, whether low volume, high volume or a combination of different devices, can be effective in reducing air contamination in the dental environment, with no important difference between different types of suction devices.
Final considerations: To minimize the risk of infection for the operator, it is recommended to use adequate precautions, such as the use of adapted masks. And to minimize the risk of cross-infection, especially between patients, and contamination of surfaces, it is recommended to space appointments by at least 30 min and always use suction devices respectively.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
Colony forming units
Confidence intervals
Coronavirus disease 2019
Centers for Disease Control and Prevention
High Volume Suction
Low Volume Suction
Population, Exposition, and Outcome
Randomized controlled trials
Risk of Bias 2
Severe acute respiratory syndrome coronavirus 2
Gralinski LE, Menachery VD. Return of the coronavirus: 2019-nCoV. Viruses. 2020;12:135.
Article PubMed PubMed Central Google Scholar
Centers for Disease Control and Prevention (CDC). COVID-19 Overview and Infection Prevention and Control Priorities in non-U.S. 2021.
Komperda J, Peyvan A, Li D, Kashir B, Yarin AL, Megaridis CM, et al. Computer simulation of the SARS-CoV-2 contamination risk in a large dental clinic. Phys Fluids. 2021;33:033328.
Article CAS Google Scholar
Adhikari U, Chabrelie A, Weir M, Boehnke K, McKenzie E, Ikner L, et al. A Case Study evaluating the risk of infection from Middle Eastern Respiratory Syndrome Coronavirus (MERS-CoV) in a hospital setting through Bioaerosols. Risk Anal. 2019;39:2608–24.
Yu ITS, Li Y, Wong TW, Tam W, Chan AT, Lee JHW, et al. Evidence of Airborne Transmission of the severe Acute Respiratory Syndrome Virus. N Engl J Med. 2004;350:1731–9.
Article CAS PubMed Google Scholar
Micik RE, Miller RL, Mazzarella MA, Ryge G. Studies on Dental Aerobiology: I. Bacterial Aerosols Generated during Dental procedures. J Dent Res. 1969;48:49–56.
Larato DC, Ruskin PF, Martin A. Effect of an Ultrasonic Scaler on Bacterial counts in Air. J Periodontol. 1967;38:550–4. 6 Part I:.
Holbrook WP, Muir KF, MacPhee IT, Ross PW. Bacteriological investigation of the aerosol from ultrasonic scalers. Br Dent J. 1978;144:245–7.
Graziani F, Izzetti R, Lardani L, Totaro M, Baggiani A. Experimental evaluation of Aerosol Production after Dental Ultrasonic Instrumentation: an analysis on fine particulate matter perturbation. IJERPH. 2021;18:3357.
Balcoș CB, Săveanu I, Bobu L, Bosînceanu D, Bolat M, Grădinaru I, et al. The risk of contamination through ultrasonic scaling. Romanian J Oral Rehabilitation. 2019;11:140–7.
Google Scholar
International prospective register of systematic reviews. 2020.
Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.3. 2022.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;:n71.
OpenGrey open access database. 2021.
Medical S. Headings. 2021.
Health S. Descriptors. 2021.
Endnote. web software. 2021.
Higgins JPT, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343(oct18 2):d5928–8.
Review Manager (RevMan). [Computer program]. Version 5.4.1, The Cochrane Collaboration. 2020.
Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso-Coello P, et al. GRADE guidelines: 9. Rating up the quality of evidence. J Clin Epidemiol. 2011;64:1311–6.
Article PubMed Google Scholar
Desarda H, Gurav A, Dharmadhikari C, Shete A, Gaikwad S. Efficacy of high-volume Evacuator in Aerosol reduction: truth or myth? A clinical and microbiological study. J Dent Res. 2014;Dental Clinics:Dental Prospects; eISSN 20082118.
Holloman JL, Mauriello SM, Pimenta L, Arnold RR. Comparison of suction device with saliva ejector for aerosol and spatter reduction during ultrasonic scaling. J Am Dent Association. 2015;146:27–33.
Article Google Scholar
King TB, Muzzin KB, Berry CW, Anders LM. The effectiveness of an Aerosol reduction device for Ultrasonic Sealers. J Periodontol. 1997;68:45–9.
Suprono MS, Won J, Savignano R, Zhong Z, Ahmed A, Roque-Torres G, et al. A clinical investigation of dental evacuation systems in reducing aerosols. J Am Dent Association. 2021;152:455–62.
Timmerman MF, Menso L, Steinfort J, Van Winkelhoff AJ, Van Der Weijden GA. Atmospheric contamination during ultrasonic scaling. J Clin Periodontol. 2004;31:458–62.
Cohen J Statistical power analysis for the behavioral sciences. Academic press., Harrel SK, Barnes JB, Rivera-Hidalgo F. Aerosol and splatter contamination from the operative site during ultrasonic scaling. The Journal of the American Dental Association. 1998;129:1241–9.
Download references
Author information
Authors and affiliations.
Department of Periodontology, School of Dentistry, Fluminense Federal University, Rua Mário Santos Braga, nº 28, Centro, Niterói, Rio de Janeiro, CEP 24040-110, Brazil
Priscilla Gonçalves Lomardo, Mariana Campello Nunes, Patrícia Arriaga, Aldir Machado, Telma Regina da Silva Aguiar & Priscila Ladeira Casado
Department of Specific Information, School of Dentistry, Fluminense Federal University, Dr. Silvio Henrique Braune Street, 22 – Centro, Nova Friburgo, RJ, 28625- 650, Brazil
Lívia Azeredo Antunes
National Institute of Traumatology and Orthopedics, Research department, Rio de Janeiro, Brazil
Valquiria Quinelato
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualisation, Methodology, Investigation, Formal analysis, Writing - Original Draft (Priscilla Lomardo, Mariana Campello Nunes, Patrícia Arriaga); Formal analysis, Data Curation, Writing - Review & Editing, Visualisation (Valquiria Quinelato, Aldir Machado); Conceptualisation, Methodology, Writing - Review & Editing, Visualisation, Project administration (Priscila Ladeira Casado, Lívia Azeredo Antunes, Telma Regina da Silva Aguiar).
Corresponding author
Correspondence to Valquiria Quinelato .
Ethics declarations
Ethical approval and informed consent.
Not applicable.
Consent for publication
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Gonçalves Lomardo, P., Nunes, M.C., Arriaga, P. et al. Concern about the risk of aerosol contamination from ultrasonic scaler: a systematic review and meta-analysis. BMC Oral Health 24 , 417 (2024). https://doi.org/10.1186/s12903-024-03996-2
Download citation
Received : 16 October 2023
Accepted : 07 February 2024
Published : 05 April 2024
DOI : https://doi.org/10.1186/s12903-024-03996-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Ultrasonic scaler
- Environmental contamination
- SARS-CoV-2 virus
BMC Oral Health
ISSN: 1472-6831
- Submission enquiries: [email protected]
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.8(11); 2016 Nov

How to Conduct a Systematic Review: A Narrative Literature Review
Nusrat jahan.
1 Psychiatry, Mount Sinai Chicago
Sadiq Naveed
2 Psychiatry, KVC Prairie Ridge Hospital
Muhammad Zeshan
3 Department of Psychiatry, Bronx Lebanon Hospital Icahn School of Medicine at Mount Sinai, Bronx, NY
Muhammad A Tahir
4 Psychiatry, Suny Upstate Medical University, Syracuse, NY
Systematic reviews are ranked very high in research and are considered the most valid form of medical evidence. They provide a complete summary of the current literature relevant to a research question and can be of immense use to medical professionals. Our goal with this paper is to conduct a narrative review of the literature about systematic reviews and outline the essential elements of a systematic review along with the limitations of such a review.
Introduction and background
A literature review provides an important insight into a particular scholarly topic. It compiles published research on a topic, surveys different sources of research, and critically examines these sources [ 1 ]. A literature review may be argumentative, integrative, historical, methodological, systematic, or theoretical, and these approaches may be adopted depending upon the types of analysis in a particular study [ 2 ].
Our topic of interest in this article is to understand the different steps of conducting a systematic review. Systematic reviews, according to Wright, et al., are defined as a “review of the evidence on a clearly formulated question that uses systematic and explicit methods to identify, select and critically appraise relevant primary research, and to extract and analyze data from the studies that are included in the review” [ 3 ]. A systematic review provides an unbiased assessment of these studies [ 4 ]. Such reviews emerged in the 1970s in the field of social sciences. Systematic reviews, as well as the meta-analyses of the appropriate studies, can be the best form of evidence available to clinicians [ 3 ]. The unsystematic narrative review is more likely to include only research selected by the authors, which introduces bias and, therefore, frequently lags behind and contradicts the available evidence [ 5 ].
Epidemiologist Archie Cochrane played a vital role in formulating the methodology of the systematic review [ 6 ]. Dr. Cochrane loved to study patterns of disease and how these related to the environment. In the early 1970s, he found that many decisions in health care were made without reliable, up-to-date evidence about the treatments used [ 6 ].
A systematic review may or may not include meta-analysis, depending on whether results from different studies can be combined to provide a meaningful conclusion. David Sackett defined meta-analysis as a “specific statistical strategy for assembling the results of several studies into a single estimate” [ 7 - 8 ].
While the systematic review has several advantages, it has several limitations which can affect the conclusion. Inadequate literature searches and heterogeneous studies can lead to false conclusions. Similarly, the quality of assessment is an important step in systematic reviews, and it can lead to adverse consequences if not done properly.
The purpose of this article is to understand the important steps involved in conducting a systematic review of all kinds of clinical studies. We conducted a narrative review of the literature about systematic reviews with a special focus on articles that discuss conducting reviews of randomized controlled trials. We discuss key guidelines and important terminologies and present the advantages and limitations of systematic reviews.
Narrative reviews are a discussion of important topics on a theoretical point of view, and they are considered an important educational tool in continuing medical education [ 9 ]. Narrative reviews take a less formal approach than systematic reviews in that narrative reviews do not require the presentation of the more rigorous aspects characteristic of a systematic review such as reporting methodology, search terms, databases used, and inclusion and exclusion criteria [ 9 ]. With this in mind, our narrative review will give a detailed explanation of the important steps of a systematic review.
Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) checklist
Systematic reviews are conducted based on predefined criteria and protocol. The PRISMA-P checklist, developed by Moher, et al., contains 17 items (26 including sub-items) comprising the important steps of a systematic review, including information about authors, co-authors, their mailing and email addresses, affiliations, and any new or updated version of a previous systematic review [ 9 ]. It also identifies a plan for documenting important protocol amendments, registry names, registration numbers, financial disclosures, and other support services [ 10 ]. Moher, et al. also state that methods of systematic reviews involve developing eligibility criteria and describing information sources, search strategies, study selection processes, outcomes, assessment of bias in individual studies, and data synthesis [ 10 ].
Research question
Writing a research question is the first step in conducting a systematic review and is of paramount importance as it outlines both the need and validity of systematic reviews (Nguyen, et al., unpublished data). It also increases the efficiency of the review by limiting the time and cost of identifying and obtaining relevant literature [ 11 ]. The research question should summarize the main objective of a systematic review.
An example research question might read, “How does attention-deficit/hyperactivity disorder (ADHD) affect the academic performance of middle school children in North America?” The question focuses on the type of data, analysis, and topic to be discussed (i.e., ADHD among North American middle school students). Try to avoid research questions that are too narrow or broad—they can lead to the selection of only a few studies and the ability to generalize results to any other populations may be limited. An example of a research question that is too narrow would be, “What is the prevalence of ADHD in children and adolescents in Chicago, IL?” Alternately, if the research question is too broad, it can be difficult to reach a conclusion due to poor methodology. An example of a research question that is too broad in scope would be, “What are the effects of ADHD on the functioning of children and adolescents in North America?”
Different tools that can be used to help devise a research question, depending on the type of question, are: population, intervention, comparator, and outcomes (PICO); sample, phenomenon of interest, design, evaluation, and research type (SPIDER); setting, perspective, intervention, comparison, and evaluation (SPICE); and expectation, client group, location, impact, professionals, and service (ECLIPSE).
The PICO approach is mostly used to compare different interventions with each other. It helps to formulate a research question related to prognosis, diagnosis, and therapies [ 12 ].
Scenario: A 50-year-old white woman visited her psychiatrist with a diagnosis of major depressive disorder. She was prescribed fluoxetine, which she feels has been helpful. However, she experienced some unpleasant side effects of nausea and abdominal discomfort. She has recently been told by a friend about the use of St. John’s wort in treating depression and would like to try this in treating her current depression. (Formulating research questions, unpublished data).
In the above-mentioned scenario, the sample population is a 50-year-old female with major depressive disorder; the intervention is St. John’s wort; the comparison is fluoxetine; and the outcome would be efficacy and safety. In order to see the outcome of both efficacy and safety, we will compare the efficacy and safety of both St. John’s wort and fluoxetine in a sample population for treating depression. This scenario represents an example where we can apply the PICO approach to compare two interventions.
In contrast, the SPIDER approach is focused more on study design and samples rather than populations [ 13 ]. The SPIDER approach can be used in this research question: “What is the experience of psychiatry residents attending a transgender education?” The sample is psychiatry residents; the phenomenon of interest is transgender education; the design is a survey; the evaluation looks at the experience; and the research type is qualitative.
The SPICE approach can be used to evaluate the outcome of a service, intervention, or project [ 14 ]. The SPICE approach applies to the following research question: “In psychiatry clinics, does the combined use of selective serotonin reuptake inhibitor (SSRI) and psychotherapy reduce depression in an outpatient clinic versus SSRI therapy alone?” The setting is the psychiatry clinic; the perspective/population is the outpatient; the intervention is combined psychotherapy and SSRI; the comparison is SSRI alone; and the evaluation is reduced depression.
The ECLIPSE approach is useful for evaluating the outcome of a policy or service (Nguyen, et al., unpublished data). ECLIPSE can apply in the following research question: “How can a resident get access to medical records of patients admitted to inpatient from other hospitals?” The expectation is: “What are you looking to improve/change to increase access to medical records for patients admitted to inpatient?” The client group is the residents; the location is the inpatient setting; the impact would be the residents having easy access to medical records from other hospitals; and the professionals in this scenario would be those involved in improving the service experiences such as hospital administrators and IT staff.
Inclusion and exclusion criteria
Establishing inclusion and exclusion criteria come after formulating research questions. The concept of inclusion and exclusion of data in a systematic review provides a basis on which the reviewer draws valid and reliable conclusions regarding the effect of the intervention for the disorder under consideration [ 11 ]. Inclusions and exclusion are based on preset criteria for specific systematic review. It should be done before starting the literature search in order to minimize the possibility of bias.
Eligibility criteria provide the boundaries of the systematic review [ 15 ]. Participants, interventions, and comparison of a research question provide the basis for eligibility criteria [ 15 ]. The inclusion criteria should be able to identify the studies of interest and, if the inclusion criteria are too broad or too narrow, it can lead to an ineffective screening process.
Protocol registration
Developing and registering research protocol is another important step of conducting a systematic review. The research protocol ensures that a systematic review is carefully planned and explicitly documented before the review starts, thus promoting consistency in conduct for the review team and supporting the accountability, research integrity, and transparency of the eventually completed review [ 10 ]. PROSPERO and the Cochrane Database of Systematic Reviews are utilized for registering research protocols and research questions, and they check for prior existing duplicate protocols or research questions. PROSPERO is an international database of prospectively registered systematic reviews related to health care and social sciences (PRISMA, 2016). It is funded by the National Institute for Health Research. The Cochrane Collaboration concentrates on producing systematic reviews of interventions and diagnostic test accuracy but does not currently produce reviews on questions of prognosis or etiology [ 16 ].
A detailed and extensive search strategy is important for the systematic review since it minimizes bias in the review process [ 17 ].
Selecting and searching appropriate electronic databases is determined by the topic of interest. Important databases are: MEDLARS Online (MEDLINE), which is the online counterpart to the Medical Literature Analysis and Retrieval System (MEDLARS); Excerpta Medica Database (EMBASE); and Google Scholar. There are multiple electronic databases available based on the area of interest. Other important databases include: PsycINFO for psychology and psychiatry; Allied and Complementary Medicine Database (AMED) for complementary medicine; Manual, Alternative, and Natural Therapy Index System (MANTIS) for alternative medical literature; and Cumulative Index to Nursing and Allied Health Literature (CINAHL) for nursing and allied health [ 15 ].
Additional studies relevant for the review may be found by looking at the references of studies identified by different databases [ 15 ]. Non-indexed articles may be found by searching the content of journals, conferences proceedings, and abstracts. It will also help with letters and commentaries which may not get indexed [ 15 ]. Reviewing clinical trial registries can provide information about any ongoing trials or unpublished research [ 15 ]. A gray literature search can access unpublished papers, reports, and conference reports, and it generally covers studies that are published in an informal fashion, rather than in an indexed journal [ 15 ]. Further search can be performed by selecting important key articles and going through in-text citations [ 15 ].
Using Boolean operators, truncation, and wildcards
Boolean operators use the relationship between different search words to help with the search strategy. These are simple words (i.e., AND, OR, and NOT) which can help with more focused and productive results (poster, Jahan, et al.: How to conduct a systematic review. APPNA 39th Summer Convention. Washington, DC. 2016). The Boolean operator AND finds articles with all the search words. The use of OR broadens the focus of the search, and it will include articles with at least one search term. The researchers can also ignore certain results from the records by using NOT in the search strategy.
An example of AND would be using “depression” AND “children” in the search strategy with the goal of studying depression in children. This search strategy will include all the articles about both depression and children. The researchers may use OR if the emphasis of the study is mood disorders or affective disorders in adolescents. In that case, the search strategy will be “mood disorders” OR “affective disorders” AND “adolescents.” This search will find all the articles about mood disorders or affective disorders in adolescents. The researchers can use NOT if they only want to study depression in children and want to ignore bipolar disorder from the search. An example search in this scenario would be “depression” NOT “bipolar disorder” AND “children.” This will help ignore studies related to bipolar disorder in children.
Truncation and wildcards are other tools to make search strategy more comprehensive and focused. While the researchers search a database for certain articles, they frequently face terminologies that have the same initial root of a word but different endings. An example would be "autism," "autistic," and "autism spectrum disorder." These words have a similar initial root derived from “autis” but they end differently in each case. The truncation symbol (*) retrieves articles that contain words beginning with “autis” plus any additional characters. Wildcards are used for words with the same meanings but different spellings due to various reasons. For the words with spelling variations of a single letter, wildcard symbols can be used. When the researcher inputs “M+N” in the search bar, this returns results containing both “man” or “men” as the wildcard accounts for the spelling variations between the letters M and N.
Study selection
Study selection should be performed in a systematic manner, so reviewers deal with fewer errors and a lower risk of bias (online course, Li T, Dickersin K: Introduction to systematic review and meta-analysis. 2016. https://www.coursera.org/learn/systematic-review #). Study selection should involve two independent reviewers who select studies using inclusion and exclusion criteria. Any disagreements during this process should be resolved by discussion or by a third reviewer [ 10 ]. Specific study types can be selected depending on the research question. For example, questions on incidence and prevalence can be answered by surveys and cohort studies. Clinical trials can provide answers to questions related to therapy and screening. Queries regarding diagnostic accuracy can be answered by clinical trials and cross-sectional studies (online course, Li T, Dickersin K: Introduction to systematic review and meta-analysis. 2016. https://www.coursera.org/learn/systematic-review #). Prognosis and harm-related questions should use cohort studies and clinical trials, and etiology questions should use case-control and cohort studies (online course, Li T, Dickersin K: Introduction to systematic review and meta-analysis. 2016. https://www.coursera.org/learn/systematic-review #).
Data screening and data extractions are two of the major steps in conducting a systematic review [ 18 ]. Data screening involves searching for relevant articles in different databases using keywords. The next step of data screening is manuscript selection by reviewing each manuscript in the search results to compare that manuscript against the inclusion criteria [ 18 ]. The researchers should also review the references of the papers selected before selecting the final paper, which is the last step of data screening [ 18 ].
The next stage is extracting and appraising the data of the included articles [ 18 ]. A data extraction form should be used to help reduce the number of errors, and more than one person should record the data [ 17 ]. Data should be collected on specific points like population type, study authors, agency, study design, humanitarian crisis, target age groups, research strengths from the literature, setting, study country, type(s) of public health intervention, and health outcome(s) addressed by the public health intervention. All this information should then be put into an electronic database [ 18 ].
Assessing bias
Bias is a systematic error (or deviation from the truth) in results or inferences. Biases can change the results of any study and lead to an underestimation or overestimation of the true intervention effect [ 19 ]. Biases can impact any aspect of a review, including selecting studies, collecting and extracting data, and making a conclusion. Biases can vary in magnitude; some are small, with negligible effect, but some are substantial to a degree where an apparent finding may be entirely due to bias [ 19 ]. There are different types of bias, including, but not limited to, selection, detection, attrition, reporting, and performance.
Selection bias occurs when a sample selected is not representative of the whole general population. If randomization of the sample is done correctly, then chances of selection bias can be minimized [ 20 ].
Detection bias refers to systematic differences between groups in how outcomes are determined. This type of bias is based on knowledge of the intervention provided and its outcome [ 19 ].
Attrition bias refers to systematic differences between groups in withdrawals from a study [ 19 ]. The data will be considered incomplete if some subjects are withdrawn or have irregular visits during data collection.
Reporting bias refers to systematic differences between reported and unreported findings, and it is commonly seen during article reviews. Reporting bias is based on reviewer judgment about the outcome of selected articles [ 20 ].
Performance bias develops due to the knowledge of the allocated interventions by participants and personnel during the study [ 20 ]. Using a double-blind study design helps prevent performance bias, where neither the experimenter nor the subjects know which group contains controls and which group contains the test article [ 14 ].
Last step of systematic review: discussion
The discussion of a systematic review is where a summary of the available evidence for different outcomes is written and discussed [ 10 ]. The limitations of a systematic review are also discussed in detail. Finally, a conclusion is drawn after evaluating the results and considering limitations [ 10 ].
Discussion of the current article
Systematic reviews with or without a meta-analysis are currently ranked to be the best available evidence in the hierarchy of evidence-based practice [ 21 ]. We have discussed the methodology of a systematic review. A systematic review is classified in the category of filtered information because it appraises the quality of the study and its application in the field of medicine [ 21 ]. However, there are some limitations of the systematic review, as we mentioned earlier in our article. A large randomized controlled trial may provide a better conclusion than a systematic review of many smaller trials due to their larger sample sizes [ 22 ], which help the researchers generalize their conclusions for a bigger population. Other important factors to consider include higher dropout rates in large studies, co-interventions, and heterogeneity among studies included in the review.
As we discussed the limitations of the systematic review and its effect on quality of evidence, there are several tools to rate the evidence, such as the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system [ 22 ]. GRADE provides a structured approach to evaluating the risk of bias, serious inconsistency between studies, indirectness, imprecision of the results, and publication bias [ 22 ]. Another approach used to rate the quality of evidence is a measurement tool to assess systematic reviews (AMSTAR) [ 23 ]. It is also available in several languages [ 23 ].
Conclusions
Despite its limitations, a systematic review can add to the knowledge of the scientific community especially when there are gaps in the existing knowledge. However, conducting a systematic review requires different steps that involve different tools and strategies. It can be difficult at times to access and utilize these resources. A researcher can understand and strategize a systematic review following the different steps outlined in this literature review. However, conducting a systematic review requires a thorough understanding of all the concepts and tools involved, which is an extensive endeavor to be summed up in one article.
The Cochrane Handbook for Systematic Reviews of Interventions and the Center for Reviews and Dissemination (CRD) provide excellent guidance through their insightful and detailed guidelines. We recommend consulting these resources for further guidance.
Given that our article is a narrative review of the scholarly literature, it contains the same limitations as noted for any narrative review. We hope that our review of the means and methods for conducting a systematic review will be helpful in providing basic knowledge to utilize the resources available to the scientific community.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.

IMAGES
VIDEO
COMMENTS
What Makes a Systematic Review Different from Other Types of Reviews? Reproduced from Grant, M. J. and Booth, A. (2009), A typology of reviews: an analysis of 14 review types and associated methodologies. ... Refers to any combination of methods where one significant component is a literature review (usually systematic). Within a review context ...
As such, ten different types of systematic review foci are listed below and in Table ... Systematic reviews are the ideal method to rigorously collate, examine and synthesize a body of literature. Systematic review methods now exist for most questions that may arise in healthcare. This article provides a typology for systematic reviewers when ...
Scoping reviews provide an understanding of the size and scope of the available literature and can inform whether a full systematic review should be undertaken. If you're not sure you should conduct a systematic review or a scoping review, this article outlines the differences between these review types and could help your decision making. 2.
What makes a systematic review different from other types of reviews is that the research methods are designed to reduce bias. The methods are repeatable, and the approach is formal and systematic: ... Systematic review vs. literature review. A literature review is a type of review that uses a less systematic and formal approach than a ...
Introduction. A systematic review collects secondary data, and is a synthesis of all available, relevant evidence which brings together all existing primary studies for review (Cochrane 2016).A systematic review differs from other types of literature review in several major ways.
This page provides information about the most common types of systematic reviews, ... and assess the scope of literature on a topic. Among other objectives, scoping reviews help determine whether a systematic review of the literature is warranted. Additional Information ... The Guide explores different approaches and methods for expedited ...
(Describes 14 different types of literature and systematic review, useful for thinking at the outset about what sort of literature review you want to do.) ... Health information and libraries journal, 36(3), 202-222. doi:10.1111/hir.12276 (An updated look at different types of literature review, expands on the Grant & Booth 2009 article ...
A systematic review collects secondary data, and is a synthesis of all available, relevant evidence which brings together all existing primary studies for review (Cochrane 2016).A systematic review differs from other types of literature review in several major ways.
Literature reviews establish the foundation of academic inquires. However, in the planning field, we lack rigorous systematic reviews. In this article, through a systematic search on the methodology of literature review, we categorize a typology of literature reviews, discuss steps in conducting a systematic literature review, and provide suggestions on how to enhance rigor in literature ...
Included SRs evaluated 24 unique methodological approaches used for defining the review scope and eligibility, literature search, screening, data extraction, and quality appraisal in the SR process. Limited evidence supports the following (a) searching multiple resources (electronic databases, handsearching, and reference lists) to identify ...
This site explores different review methodologies such as, systematic, scoping, realist, narrative, state of the art, meta-ethnography, critical, and integrative reviews. The LITR-EX site has a health professions education focus, but the advice and information is widely applicable. Types of Reviews. Review the table to peruse review types and ...
There are many types of systematic reviews. They may ask different kinds of questions and use a variety of methods, just like primary research. As with primary research, they vary in terms of perspective, purpose, approach, methods, and the time and resources used to conduct them.
There are many types of reviews --- narrative reviews, scoping reviews, systematic reviews, integrative reviews, umbrella reviews, rapid reviews and others --- and it's not always straightforward to choose which type of review to conduct.These Review Navigator tools (see below) ask a series of questions to guide you through the various kinds of reviews and to help you determine the best choice ...
A literature review will help you to identify patterns and trends in the literature so that you can identify gaps or inconsistencies in a body of knowledge. This should lead you to a sufficiently focused research question that justifies your research. A systematic review is comprehensive and has minimal bias. It is based on a specific question ...
Method details Overview. A Systematic Literature Review (SLR) is a research methodology to collect, identify, and critically analyze the available research studies (e.g., articles, conference proceedings, books, dissertations) through a systematic procedure [12].An SLR updates the reader with current literature about a subject [6].The goal is to review critical points of current knowledge on a ...
Systematic reviews have been considered as the pillar on which evidence-based healthcare rests. Systematic review methodology has evolved and been modified over the years to accommodate the range of questions that may arise in the health and medical sciences. This paper explores a concept still rarely considered by novice authors and in the literature: determining the type of systematic review ...
1. Narrative Literature Review. A narrative literature review, also known as a traditional literature review, involves analyzing and summarizing existing literature without adhering to a structured methodology. It typically provides a descriptive overview of key concepts, theories, and relevant findings of the research topic.
Systematic reviews have historically focused on the benefits and harms of interventions; over time, various types of systematic reviews have emerged to address the diverse information needs of clinicians, patients, and policy makers Systematic reviews with traditional components have become defined by the different topics they assess (Table 2.1 ...
The common types of literature reviews will be explained in the pages of this section. Narrative or traditional literature reviews. Critically Appraised Topic (CAT) Scoping reviews. Systematic literature reviews. Annotated bibliographies. These are not the only types of reviews of literature that can be conducted.
An umbrella review is a review of multiple systematic reviews. The process uses explicit and systematic methods to search for, and identify, systematic reviews on related research questions in the same topic area. The purpose of an umbrella review is to synthesize the results of the systematic reviews across important outcomes.
A PRISMA extension for scoping reviews, PRISMA-ScR, has been created to provide reporting guidance for this specific type of review. This extension is also intended to apply to evidence maps, as these share similarities with scoping reviews and involve a systematic search of a body of literature to identify knowledge gaps.
Different Types of reviews. You may be asked to complete a literature review that is done in a systematic way, that is like a systematic review. Mostly, the literature review you will be asked to do will be integrative - that is, conclusions are drawn from the literature in order to create something new, such as a new hypothesis to address a ...
The anatomic variants of the intercostobrachial nerve (ICBN) represent a potential risk of injuries during surgical procedure such as axillary lymph node dissection and sentinel lymph node biopsy in breast cancer and melanoma patients. The aim of this systematic review and meta-analysis was to investigate the different origins and branching patterns of the intercostobrachial nerve also ...
A systematic review collects all possible studies related to a given topic and design, and reviews and analyzes their results [ 1 ]. During the systematic review process, the quality of studies is evaluated, and a statistical meta-analysis of the study results is conducted on the basis of their quality. A meta-analysis is a valid, objective ...
Background: Physical activity (PA) and/or exercise improves postprandial cardiometabolic risk markers; however, the optimal exercise intensity, frequency, and dose remain unclear. We aimed to (1) compare the acute metabolic effects of interrupted prolonged sitting with PA bouts of different frequencies and durations on blood glucose, insulin, and triacylglycerol responses, and (2) compare the ...
The aim was to carry out a systematic review of the evidence of the scope of the aerosol produced by ultrasonic scaler in environmental contamination and the influence of the use of intraoral suction reduction devices. Scientific literature was searched until June 19, 2021 in 6 databases: Pubmed, EMBASE, Web of science, Scopus, Virtual Health ...
Introduction and background. A literature review provides an important insight into a particular scholarly topic. It compiles published research on a topic, surveys different sources of research, and critically examines these sources [].A literature review may be argumentative, integrative, historical, methodological, systematic, or theoretical, and these approaches may be adopted depending ...