
- Previous Article
- Next Article

Presentation
Clinical pearls, case study: complicated gestational diabetes results in emergency delivery.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Cite Icon Cite
- Get Permissions
Ginny Lewis; Case Study: Complicated Gestational Diabetes Results in Emergency Delivery. Clin Diabetes 1 January 2001; 19 (1): 25–26. https://doi.org/10.2337/diaclin.19.1.25
Download citation file:
- Ris (Zotero)
- Reference Manager
A.R. is a 33-year-old caucasian woman initially diagnosed with diabetes during a recent pregnancy. The routine glucose challenge test performed between 28 and 29 weeks gestation was elevated at 662 mg/dl. A random glucose completed 1–2 days later was also elevated at 500 mg/dl. A follow-up HbA 1c was elevated at 11.6%. Additional symptoms included a 23-lb weight loss over the past 3–4 weeks with ongoing “flu-like” symptoms, including fatigue, nausea, polyuria, and polydypsia.
A.R. had contacted her obstetrician’s office when her symptoms first appeared and was told to contact her primary care provider for the “flu” symptoms. She had called a nurse triage line several times over the previous 2–3 weeks with ongoing symptoms and was told to rest and take fluids.
She presented to her primary care provider 3 days after the HbA 1c was drawn for ongoing evaluation of hyperglycemia. At that time, she was symptomatic for diabetic ketoacidosis. She was hospitalized and started on an insulin drip.
A.R.’s hospitalization was further complicated with gram-negative sepsis, adult respiratory distress syndrome, and Crohn’s disease with a new rectovaginal fistula. She was intubated as her respiratory status continued to decline and was transferred to a tertiary medical center for ongoing management. She required an emergency Caesarian section at 30 1/7 weeks gestation due to increased fetal distress.
A.R. had no family history of diabetes with the exception of one sister who had been diagnosed with gestational diabetes. Her medical history was significant for Crohn’s disease diagnosed in 1998 with no reoccurrence until this hospitalization. Her pre-pregnancy weight was 114–120 lb. She had gained 25 lb during her pregnancy and lost 23 lb just before diagnosis.
A.R.’s blood glucose levels improved postpartum, and the insulin drip was gradually discontinued. She was discharged on no medications.
At her 2-week postpartum visit, home blood glucose monitoring indicated that values were ranging from 72 to 328 mg/dl, with the majority of values in the 200–300 mg/dl range. A repeat HbA 1c was 8.7%. She was restarted on insulin.
1. What is the differential diagnosis of gestational diabetes versus type 1 diabetes?
2. At what point during pregnancy should insulin therapy be instituted for blood glucose control?
3. How can communication systems be changed to provide for integration of information between multiple providers?
Gestational diabetes is defined as “any degree of carbohydrate intolerance with onset first recognized during pregnancy. This definition applies whether insulin ... is used for treatment and whether or not the condition persists after pregnancy.” 1 Risk assessment is done early in the pregnancy, with average-risk women being tested at 24–28 weeks’ gestation and low-risk women requiring no additional testing. 1 , 2 A.R. met the criteria for average risk based on age and a first-degree family member with a history of gestational diabetes.
Screening criteria for diagnosing diabetes include 1 ) symptoms of diabetes plus casual plasma glucose >200 mg/dl (11.1 mmol/l), or 2 ) fasting plasma glucose >126 mg/dl (7.0 mmol/l), or 3 ) 2-h plasma glucose >200 mg/dl (11.1 mmol/l) during an oral glucose tolerance test (OGTT). 3 For women who do not meet the first two criteria, a glucose challenge test (GCT) measuring a 1-h plasma glucose following a 50-g oral glucose load is acceptable. For those women who fail the initial screen, practitioners can then proceed with the OGTT. 1
In A.R.’s case, she most likely would have met the first criterion if a casual blood glucose had been measured. She had classic symptoms with weight loss, fatigue, polyuria, and polydypsia. Her 1-h plasma glucose following the glucose challenge was >600 mg/dl, which suggests that her casual glucose would also have been quite high.
Medical nutrition therapy (MNT) is certainly a major part of diabetes management. However, with this degree of hyperglycemia, MNT would not be adequate as monotherapy. Treatment for gestational diabetes includes the use of insulin if fasting blood glucose levels are >95 mg/dl (5.3 mmol/l) or 2-h postprandial values are >120 mg/dl (6.7 mmol/l). 1
Several days passed from the time of A.R.’s initial elevated blood glucose value and the initiation of insulin therapy. While HbA 1c values cannot be used for diagnostic purposes, in this case they further confirmed the significant degree of hyperglycemia.
Plasma blood glucose values initially improved in the immediate postpartum period. A.R. was sent home without medications but instructed to continue home glucose monitoring.
At her 2-week postpartum visit, whole blood glucose values were again indicating progressive hyperglycemia, and insulin was restarted. A.R.’s postpartum weight was 104 lb—well below her usual pre-pregnancy weight of 114–120 lb. Based on her ethnic background, weight loss, abrupt presentation with classic diabetes symptoms, and limited family history, she was reclassified as having type 1 diabetes.
In immune-mediated, or type 1, diabetes, b-cell destruction can be variable, with a slower destruction sometimes seen in adults. 3 Presentation of type 1 diabetes can also vary with modest fasting hyperglycemia that can quickly change to severe hyperglycemia and/or ketoacidosis in the presence of infection or other stress. 3 A.R. may have had mild hyperglycemia pre-pregnancy that increased in severity as the pregnancy progressed.
The final issue is communication among multiple health care providers. A.R. was part of a system that uses primary care providers, specialists, and triage nurses. She accessed all of these providers as instructed. However, the information did not seem to be clearly communicated among these different types of providers. A.R. called triage nurses several times with her concerns of increased fatigue, nausea, and weight loss. The specialist performed her glucose challenge with follow-up through the primary care office. It seems that if all of these providers had the full information about this case, the diagnosis could have been made more easily, and insulin could have been initiated more quickly.
1. Hyperglycemia diagnosed during pregnancy is considered to be gestational diabetes until it is reclassified in the postpartum period. Immune-mediated diabetes can cause mild hyperglycemia that is intensified with the increased counterregulatory hormone response during pregnancy.
2. Insulin therapy needs to be instituted quickly for cases in which MNT alone is inadequate.
3. The GCT is an appropriate screening test for an average-risk woman with no symptoms of diabetes. In the face of classic symptoms of diabetes, a casual plasma glucose test can eliminate the need for the glucose challenge.
4. As part of the health care industry, we need to continue to work on information systems to track patient data and share data among multiple providers. Patients can become lost in an ever-expanding system that relies on “protocols” and does not always allow for individual differences or for cases with unusual presentation.
Ginny Lewis, ARNP, FNP, CDE, is a nurse practitioner at the Diabetes Care Center of the University of Washington School of Medicine in Seattle.
Email alerts
- Online ISSN 1945-4953
- Print ISSN 0891-8929
- Diabetes Care
- Clinical Diabetes
- Diabetes Spectrum
- Standards of Medical Care in Diabetes
- Scientific Sessions Abstracts
- BMJ Open Diabetes Research & Care
- ShopDiabetes.org
- ADA Professional Books
Clinical Compendia
- Clinical Compendia Home
- Latest News
- DiabetesPro SmartBrief
- Special Collections
- DiabetesPro®
- Diabetes Food Hub™
- Insulin Affordability
- Know Diabetes By Heart™
- About the ADA
- Journal Policies
- For Reviewers
- Advertising in ADA Journals
- Reprints and Permission for Reuse
- Copyright Notice/Public Access Policy
- ADA Professional Membership
- ADA Member Directory
- Diabetes.org
- X (Twitter)
- Cookie Policy
- Accessibility
- Terms & Conditions
- Get Adobe Acrobat Reader
- © Copyright American Diabetes Association
This Feature Is Available To Subscribers Only
Sign In or Create an Account
- Practice Test
- Fundamentals of Nursing
- Anatomy and Physiology
- Medical and Surgical Nursing
- Perioperative Nursing
- Psychiatric Mental Health Nursing
- Maternal & Child Nursing
- Community Health Nursing
- Pathophysiology
- Nursing Research
- Study Guide and Strategies
- Nursing Videos
- Work for Us!
- Privacy Policy

Gestational Diabetes Mellitus Case Study

Gestational diabetes mellitus (GDM), also known as type III diabetes mellitus, is one of the most common types of diabetes mellitus and considered the most common complication of pregnancy. This health problem is like pregnancy-induced hypertension (PIH) that develops during pregnancy and disappears after the delivery of the fetus, or as the maternal body returns to its pre-pregnant state. Gestational diabetes mellitus may or may not with co-existing maternal diabetes. It heightens the level of diabetes (if with previous diabetes) by a notch in response to the rise in fetal carbohydrate demand. 40% of pregnant mothers who develop GDM will eventually develop non-insulin-dependent diabetes mellitus (NIDDM or type II DM) within 5 years.
FACTS ABOUT INSULIN
Knowing the facts about insulin facilitates the understanding of gestational diabetes mellitus. Or any form of diabetes for that matter. This creates/develops ideas on how and why such health problems occur.
- The insulin is a normal body hormone that is produced by the beta cells of the Islets of Langerhans in the pancreas.
- The release of insulin is regulated by negative feedback in response to high glucose levels. The high glucose level may come from excessive glucagon action or high carbohydrate intake.
- The insulin secretion of the pancreas and its action on the liver makes it maintain a normal value of 80-120 mg/dL.
- Carbohydrates— utilization of glucose by the cells
- Proteins— conversion of amino acids to replace muscle tissues
- Fats— conversion of excess glucose to fatty acids and store them to adipose tissues
- Endothelial and nerve cells are the only cells/tissues that can use glucose even without insulin.
- Low insulin level causes the rise in plasma glucose concentration and glycosuria.
- Diabetes mellitus develops as the body secretes a low amount or as body cells reject its utilization.
ANATOMY AND PHYSIOLOGY
A normal body uses insulin as a channel for glucose to enter the cells for utilization. This process is also applicable to the fetus (during pregnancy) for growth and development. As the fetus grows, the maternal body executes an automatic response by doubling the level of glucose level through lowering insulin secretion and with the aid of some gestational hormones that antagonize the effects of insulin, a process known as a protective mechanism. Along with this, this mechanism causes the rise of placental lactogen, estrogen, and progesterone to cause the following effects: 1. antagonizes the effects of insulin, 2. prolong the elevation of stress hormones (cortisol, epinephrine, and glucagon), and 3. degradation of insulin by the placenta. The total effect of these mechanisms raises the maternal glucose level for fetal usage. Hyperglycemia normally occurs with a protective mechanism that predisposes a pregnant mother in the triggering of her pre-diabetic state or heightens an existing diabetes mellitus.
The effects of pregnancy on diabetes mellitus are summarized as:
- The first trimester— glucose level is relatively stable or may decrease
- The second trimester— there is a rapid increase in glucose level
- The third trimester— there is a rapid decrease in glucose level and return to its pre-pregnant state.
CAUSES AND INCIDENCE
The primary cause is almost the same as the other types of diabetes . The inability of the body to produce or synthesize a sufficient amount of insulin in response to glucose level (as in type I DM), or the body’s rejection of insulin (as in type II DM) shows a significant relationship on the development of any form of diabetes. The existence of either of these problems, plus, the interaction of the protective mechanisms in pregnancy doubles the occurrence of GDM.
The incidence of gestational diabetes mellitus is almost 3% in all pregnancies and 2% in all women with diabetes before pregnancy.
GDM causes a high incidence of fetal morbidity and unwanted complications such as polyhydramnios and macrosomia in fetus.
RISK FACTORS
For some clear and unclear pathological reasons, the following are considered the risk factors in the occurrence/development of GDM:
- Family history of DM
- Age of 45 or older (when got pregnant)
- Previous delivery of a baby weighing 9 lbs or more
- History of any autoimmune disease
- Belonging to/with ethnic background from African Americans, Latino, and Native Americans
- History of previous GDM
- With any level of hypertension
- With elevated high-density lipoprotein
SIGNS AND SYMPTOMS
The clinical manifestations of gestational diabetes mellitus coincide with the signs and symptoms of the other types of diabetes mellitus. These are popularly known as the “3 P’s” or polydipsia (excessive thirst), polyphagia (excessive hunger), and polyuria (frequent urination). Aside from these manifestations, there are also other signs and symptoms that are general manifestations and pregnancy-specific manifestations.
PATHOPHYSIOLOGY
COMPLICATIONS
The chronic effects or the uncontrolled glucose level during pregnancy would lead to the development of the following complications:
- Urinary tract infection (UTI)
- Infertility
- Preterm labor and delivery
- Pregnancy-induced hypertension (PIH)- pre-eclampsia and eclampsia
- Congenital anomalies
- Spontaneous abortion
Also, a woman who developed or experienced gestational diabetes mellitus is expected to have type II diabetes mellitus within 5 years for the rest of her life.
The prognosis or the chance of the mother and/or fetus for survival depends on the maternal ability to tolerate and adjust to high glucose levels, medical management, and obedience to the treatment regimen. This means that the more cooperative and responsive the mother to the treatment regimen is, the better chances of both maternal and fetal well being are.
The performance of the following diagnostic tests aims to determine the level of diabetes present in the pregnant mother and determine its extent of damage or impending effects. This serves as the basis for the plan of care for the mother and the fetus.
- Blood glucose monitoring— this can either be done through fasting blood sugar (FBS) or randomly. This reveals the glucose level and indicates the plan of care needed.
- Glucose tolerance test (GTT)— to evaluate the response of insulin to loading glucose.
- Glycated hemoglobin (Glycohemoglobin)— measures glycemic control by evaluating the attachment of glucose to freely permeable erythrocytes during their whole life cycle.
- C-peptide Assay (connecting peptide assay)— useful when the presence of insulin antibodies interferes with direct insulin assay.
- Fructosamine assay— is much more useful than glycosylated hemoglobin tests in cases of hemoglobin variants.
- Urine glucose and ketone monitoring— may be performed in cases where blood glucose monitoring is not available, but, is not as accurate as of the former.
- Amniocentesis
- Non-stress test
NURSING DIAGNOSES
- Altered nutrition, more or less than body requirements related to weight gain.
- High-risk pregnancy: high risk for infection, ketosis, fetal demise, cephalopelvic disproportion, polyhydramnios, congenital anomalies, preterm labor.
- Knowledge deficit related to disease and insulin use and interaction.
The overall goal of management for gestational diabetes mellitus is the control of the maternal glucose level and keep it on a normal or near-normal level to prevent the development of complications that might compromise both the mother and the fetus. The most significant of these managements is the use of insulin. This is the most potent, yet, requires accuracy and monitoring of its unwanted effect (hypoglycemia) that brings immediate danger to both the mother and the fetus. Proper timing, dosage, and knowledge on counteractions of its over-reaction are vital concepts to be incorporated in health education.
Along with this, health promotion and disease prevention activities like diet, exercise, and fetal monitoring are of great importance.
NURSING MANAGEMENTS
History taking on:
- First presentation of the manifestations of diabetes (3 P’s)
- First diagnosis of DM
- Family members with DM
Review of systems:
- Weight gain, increasing fatigue/weakness/tiredness
- Skin lesions, infections, hydration, signs of poor wound healing
- Changes in vision—floaters, halos, blurred vision, dry/burning eyes, cataract, glaucoma
- Gingivitis, periodontal disease
- Orthostatic hypotension, cold extremities, weak pedal pulses
- Diarrhea, constipation, early satiety, bloating, flatulence, hunger and thirst
- Frequent urination, nocturia, vaginal discharge
- Numbness and tingling of the extremities, decrease pain and temperature sensation
Intervention
1. Nutrition
- Assess the timing and content of meals
- Instruct on importance of a well-balanced diet
- Explain the importance of exercise
- Plan for a weight reduction course
2. Insulin use
- Encourage verbalization of feelings
- Demonstrate and explain insulin therapy
- Allow the client to do self-administration
- Review mastery of the whole process
3. Injury from hypoglycemia
- Monitor maternal blood glucose level
- Instruct on insulin-activity-diet interaction
- Teach on the signs and symptoms of hypoglycemia
- Teach/present list of things/foods that need to be available at all times (in cases of hypoglycaemic attacks)
- Have an identification band indicating the health condition (DM) for fainting instances
4. Activity tolerance
- Plan for regular exercise
- Increase carbohydrate intake before exercise
- Instruct to avoid exercise if blood glucose level exceeds 250 mg/dL and urine ketones are present
- Advise to use abdomen for insulin injection if arms and legs are used for exercise
5. Skin integrity
- Avoid alcohol use, instead, lotion
- Teach on proper foot care
- Advice to stop smoking and alcohol use
6. Fetal well-being
- Continuous monitoring of fetal activities and fetal heart tone
- Monitor fetal activities during maternal activities
- Monitor early signs of labor
- Advice to report of any discharge coming from the vagina
- Monitor daily weight and advice to report on rapid weight gain
7. Educative
- Teach on lifestyle modifications
- Advice to see psychologists with other family members for therapy on the possibilities of fetal abnormalities
- Advice to call emergency response team in cases of emergency
- Advise to religiously follow health instructions
- Bodyweight is within the normal range for the age of gestation.
- Demonstrates proper technique in self-administration of insulin
- No episodes of hypoglycemia as claimed by the client
- No skin problems/lesions
- Verbalizes readiness on the possible fetal defects.
- Stable fetal heart rate
RELATED ARTICLES MORE FROM AUTHOR
Anaphylactic shock case study -the impending doom, ectopic pregnancy case study, chronic suppurative otitis media case study, pregnacy induced hypertension (pih) case study, leave a reply cancel reply.
Save my name, email, and website in this browser for the next time I comment.
11 Gestational Diabetes Mellitus Nursing Care Plans

Gestational Diabetes Mellitus (GDM) is glucose intolerance with onset during pregnancy. In true GDM, glucose usually returns to normal by six weeks postpartum , although women with GDM have an increased risk of developing type 2 diabetes mellitus later in life. The primary concern for any woman with this disorder is controlling the balance between insulin and blood glucose levels to prevent hyperglycemia or hypoglycemia. Women with gestational diabetes are at an increased risk of complications during pregnancy and delivery.
Table of Contents
Nursing problem priorities, nursing assessment, nursing diagnosis, nursing goals, 1. managing unstable blood glucose levels, 2. promoting adequate nutrition, 3. promoting safety and preventing injury, 4. preventing macrosomia and fetal injury, 5. initiating infection control measures and preventing infections, 6. enhancing fluid balance and preventing diabetic ketoacidosis, 7. promoting adherence to health management, 8. initiating patient education and health teachings, 9. administer medications and provide pharmacologic support, 10. monitoring results of diagnostic and laboratory procedures, 11. assessing and monitoring for potential complications, recommended resources, references and sources, nursing care plans and management.
The nursing care plan for gestational diabetes mellitus involves providing the client or couple with information regarding the disease condition, teaching insulin administration, achieving and maintaining normoglycemia, and evaluating the present client or fetal well-being.
The following are the nursing priorities for patients with gestational diabetes mellitus (GDM):
- Monitor and manage blood glucose levels.
- Provide dietary guidance and develop a personalized meal plan to maintain stable blood sugar levels.
- Educate patients on self-monitoring of blood glucose and proper technique for glucose testing.
- Collaborate with healthcare professionals to adjust medication, such as insulin, if necessary.
- Monitor fetal growth and development through regular ultrasounds and other tests.
- Offer support and counseling to address emotional and psychological concerns related to GDM.
- Promote physical activity and exercise, as recommended by healthcare providers.
- Educate patients on the potential risks of GDM to both the mother and baby.
- Schedule regular prenatal visits to monitor maternal and fetal health.
Assess for the following subjective and objective data:
- See nursing assessment cues under Nursing Interventions and Actions.
Following a thorough assessment, a nursing diagnosis is formulated to specifically address the challenges associated with gestational diabetes mellitus (GDM) based on the nurse ’s clinical judgment and understanding of the patient’s unique health condition. While nursing diagnoses serve as a framework for organizing care, their usefulness may vary in different clinical situations. In real-life clinical settings, it is important to note that the use of specific nursing diagnostic labels may not be as prominent or commonly utilized as other components of the care plan. It is ultimately the nurse’s clinical expertise and judgment that shape the care plan to meet the unique needs of each patient, prioritizing their health concerns and priorities.
Goals and expected outcomes may include:
- Within 4 hours of nursing intervention, the patient will verbalize understanding of the individual treatment regimen and the need for regular glucose self-monitoring.
- Within 8 hours of nursing action, the patient will maintain fasting serum blood glucose levels between 60-100 mg/dl and 1-hour postprandial of no higher than 140 mg/dl and will be free of signs and symptoms of diabetic ketoacidosis (fruity-scented breath, excessive thirst, frequent urination, weakness , confusion ).
- The client will gain at least 25-30 lbs prenatally or as appropriate for pre-pregnancy weight.
- The client follows the prescribed dietary intake appropriately.
- The client is free from signs of hypoglycemia or hyperglycemia.
- The client identifies the proper food and fluid choices for her diet.
- The client will be free of signs and symptoms of diabetic ketoacidosis (fruity-scented breath, excessive thirst, frequent urination, weakness , and confusion ).
- The client will maintain vital signs within the normal range.
- The client will maintain blood glucose levels within the normal range.
- The client will deliver the infant vaginally and without complications.
- The fetus will display reactive normal stress tests and negative OCT and CST.
- The fetus will be delivered full-term and vaginally without complications.
- The fetus will display weight within the normal range.
- The fetus will have a normal blood glucose level upon birth.
- The client will verbalize understanding of the procedures, laboratory tests, and activities involved in controlling diabetes.
- The client will understand the importance of careful attention to nutrition , exercise, and home monitoring of glucose levels during pregnancy.
- The client will participate in the management of diabetes during pregnancy.
- The client will describe the appropriate nutrition and exercise program.
- The client will demonstrate proficiency in self-monitoring and insulin administration.
- The client will be free of infection.
- The client will identify interventions to prevent or reduce the risk of infection.
- The client will participate and adhere to the treatment plan against infection.
Nursing Interventions and Actions
Therapeutic interventions and nursing actions for patients with gestational diabetes mellitus (GDM) may include:
If a woman’s insulin production is insufficient, glucose cannot be used by the body cells. The cells register the need for glucose, and the liver quickly converts stored glycogen to glucose to increase the serum glucose level. However, because insulin is unavailable, the body cells still cannot use the glucose, so the serum glucose levels rise.
Perform a prenatal screening test to identify gestational diabetes mellitus. Suppose the woman does not have preexisting diabetes mellitus. In that case, a prenatal screening test is routinely performed between 24 and 28 weeks gestation, but it may be done earlier if risk factors are present. The woman drinks 50g of an oral glucose solution, and a blood sample is taken 1 hour later and analyzed for glucose. If the blood glucose level is 130 to 140 mg/dL or higher, a more complex, 3-hour glucose tolerance test is done.
Note signs of hyperglycemia (confusion, increased thirst, frequent urination, changes in visual acuity) or hypoglycemia (dizziness; tremors; lethargy; excessive sweating, pale, cool, moist skin). Observing these signs may alert the nurse to developing hyperglycemia or hypoglycemia. If the woman cannot increase her insulin production, she will have periods of hyperglycemia as glucose accumulates in the blood. Because the fetus continuously draws glucose from the mother, maternal hypoglycemia can occur between meals and night.
Monitor the client’s vital signs, uterine contractions, and fetal heart rate (FHR). Determine the client’s progress through monitoring and physical exam and inform her of signs of beginning labor . Increased FHR is a sign of possible fetal distress. Uterine contractions could mark the beginning of preterm labor .
Assess understanding of the effect of stress on diabetes. Teach the client about stress management and relaxation measures. Hormones released during stress conditions (stress hormones) are counter-regulatory in glucose metabolism because they can induce hyperglycemia. During stress situations, insulin sensitivity is generally reduced, mainly due to signaling defects downstream of the insulin receptor that reduce glucose transport in insulin-sensitive tissues such as the liver, muscle , and fats. In contrast, glucose production is higher due to increased hepatic gluconeogenesis. (Marcovecchio & Chiarelli, 2012).
Teach and demonstrate to the client how to monitor blood glucose levels using a finger-stick method. The pregnant diabetic woman may monitor her blood glucose levels several times a day as directed by the healthcare provider. The client can test their own blood glucose level in their homes. The client not only must be skilled in the techniques but also understand the results and how to incorporate them into the daily regimen. This means involving the entire healthcare team in ongoing supervision, demonstrations, and support. To ensure a successful pregnancy, the client must keep her blood glucose levels as close to normal as possible.
Provide information regarding any required changes in diabetic management, e.g., use of human insulin only, changing from oral diabetic drugs to insulin, and self-monitoring of serum blood glucose levels at least twice a day (e.g., before breakfast and before dinner). Metabolism and maternal/fetal needs fluctuate during gestation, requiring close monitoring and adaptation. The dose and frequency of insulin injections are tailored to a woman’s individual needs. Insulin is often administered on a sliding scale, in which the woman varies her dose of insulin based on each blood glucose level. Two-thirds of daily insulin needs are given before breakfast and one-third before dinner. The client should eat immediately after injecting insulin to avoid hypoglycemia.
Provide information regarding the signs, symptoms, and differences between hyperglycemia and hypoglycemia. The client who takes insulin may experience episodes of hypoglycemia or hyperglycemia. Therefore, she should be taught how to recognize and respond to each condition, and family members are also included in the teaching. Symptoms and interventions include:
Hypoglycemic episodes occur most frequently in the first trimester, owing to continuous fetal drain on serum glucose and amino acids, and too low levels of human placental lactogen (HPL). The blood glucose level that indicates hypoglycemia is usually <60 mg/dL. The client may feel excessive hunger; trembling; weakness; faintness; lethargy; headache; irritability; sweating, pale, cool, moist skin; and even loss of consciousness.
Hyperglycemia results from inadequate insulin, reduced activity, excessive food intake, and infection during pregnancy. A blood glucose level of >120 mg/dL indicates hyperglycemia. Signs of symptoms of hyperglycemia include fatigue; flushed, hot skin; dry mouth ; excessive thirst; dehydration ; frequent urination; nausea and vomiting; rapid, deep respirations; acetone odor of the breath (which indicates ketoacidosis); and depressed reflexes. To correct a hyperglycemic episode, teach the client to evaluate her food intake and emphasize the importance of honesty regarding her food intake to avoid inappropriately adjusting her insulin dose.
Instruct the client on how to treat symptomatic hypoglycemia. During hypoglycemic periods, the client may drink an 8oz glass of milk or juice or eat a piece of fruit or two crackers to relieve the hypoglycemic episode. She may then repeat in 15 minutes if serum glucose levels remain below 70 mg/dl.Using plenty of simple carbohydrates to treat hypoglycemia causes serum glucose values to elevate. A combination of complex carbohydrates and protein maintains normoglycemia longer and helps maintain the stability of serum glucose throughout the day.
Discuss the type of insulin, dosage, and schedule . Division of insulin dosage considers basal maternal needs and mealtime insulin-to-food ratio and allows more freedom in meal-scheduling. The total daily dosage is based on gestational, current maternal body weight, and serum glucose levels. Typically, insulin dosage may be reduced to avoid hypoglycemia in the first trimester. In the second trimester, increasing placental hormones increase insulin resistance, and the dosage of insulin may have to be increased. Insulin requirements may decrease again at 38 weeks gestation. Insulin Aspart and lispro are fast-acting insulins that are highly effective if given before meals.
Monitor serum blood glucose levels (fasting blood sugar, 1-hour postprandial) on the first visit, and then as indicated by the client’s condition. The client should obtain fasting and 1-hour postprandial values four times a day, and goals include fasting numbers of 90 mg/dL and below and postprandial values less than 140 mg/dL. The client monitors her blood glucose levels by using a glucometer. The results should be documented by the client and presented to her healthcare provider to determine if any adjustments in her insulin or oral diabetic regimen are necessary.
Obtain results of glycosylated hemoglobin (HbA1c) every 2-4weeks. The measurement of HbA1c, the amount of glucose attached to hemoglobin, is used to detect the degree of hyperglycemia present. Measuring HbA1c is advantageous not just because it offers a present value of glucose but because it reflects the average blood glucose level over the past 4 to 6 weeks.
Administer intravenous fluids and insulin additives or oral diabetic agents as prescribed. Correcting blood glucose is vital to both maternal and fetal well-being. Insulin therapy is needed by clients who cannot control their blood glucose levels with diet or oral therapy. Short-acting insulin may be used alone or with an intermediate type. The use of insulin pumps has also proved great value for glucose control in pregnant and nonpregnant clients with diabetes mellitus and reduces hypoglycemic events.
Coordinate multispecialty care conferences as appropriate. Provides an opportunity to review the management of both pregnancy and diabetic conditions and plan for special needs during intrapartum and postpartum periods. A dietitian can determine foods to meet her needs and help find solutions to adhering to the diet. Referral to a diabetes management center can also be helpful. During birth, neonatal nurses and a neonatologist are often present.
Prepare for hospitalization if diabetes is not controlled. Assist the client in transfer to the hospital unit. Infant morbidity is linked to maternal hyperglycemia-induced fetal hyperinsulinemia. Continuous monitoring is necessary to detect if uterine contractions and preterm birth were halted.
Nutrition requirements of women diagnosed with gestational diabetes mellitus are similar to non-GDM pregnancies. Still, they require a special focus on dietary modification to ensure healthy and mindful eating to achieve and maintain maternal euglycemia. Change preparedness is high during pregnancy, as emotion is increased because of perceived risk but with the possibility of improved outcome with change. There is also greater motivation, a sense of self-efficacy, and a willingness to acquire new skills. This is particularly relevant for GDM pregnancy, where nutrition and lifestyle change provides the bedrock for managing the condition (Kapur et al., 2021).
Assess and record dietary patterns and caloric intake using a 24-hour recall. Dietary patterns help evaluate the client’s understanding and compliance to a strict dietary regimen. Identifying both the good eating habits and not-so-good eating habits in the current eating pattern of the client can help individualize diet advice (Kapur et al., 2021).
Weigh the client every prenatal visit . Encourage the client to monitor weight at home between visits periodically. The combination of high pre-pregnancy body mass index (BMI) and excessive weight gain during pregnancy increases the risk for GDM, preeclampsia , large for gestational age infants, and complications for both the mother and the newborn at delivery. According to a WHO report, high-risk women who follow lifestyle change interventions (both diet and exercise) reduce the risk of excessive gestational weight gain (GWG), thereby reducing the risk of perinatal complications (Kapur et al., 2021).
Evaluate the influence of cultural factors. Identify what the client perceives as normal dietary practices and food preferences, and dietary patterns that can be strengthened or altered, as indicated. For instance, in South East Asia, rice is the staple food, and this may pose major challenges for women from this background to curtail their rice intake. The diet for South Asians is similarly heavily reliant on carbohydrates, and multiple sources of carbohydrates are often included in any one meal (Yuen & Wong, 2015).
Educate the client and family members about the importance of regular meals and snacks (e.g., three meals or four snacks) when taking insulin. Eating very frequent small meals improves insulin function. A balanced food intake is divided among meals and at least three to four snacks throughout the day to maintain stable blood glucose levels. The timing and content of meals and snacks may require adjustment to prevent early-morning hypoglycemia. Foods that release glucose slowly are preferred to avoid rapid changes in blood glucose. The bedtime snack is important to minimize the risk of hypoglycemia.
Educate the client on easy-to-implement tools to make nutrition counseling more effective. The T-shaped plate model, especially for the main meals, is effective as a basic teaching tool to control portion size and plan meals more effectively. Visuals of portion size and use of household containers (cups and glasses) as measures of food quantity are practical and easy teaching tools to help improve adherence to the quantity of food consumed. Writing a food journal and analyzing them together with the nurse or healthcare provider help to understand the effect of different foods on glucose levels, adjust the diet to change the portion size of carbohydrates in different meals, and improve glycemic control (Kapur et al., 2021).
Instruct the client about proper carbohydrate restriction. Carbohydrate restriction remains the most common approach for medical nutrition therapy in GDM. According to the American College of Obstetricians and Gynaecologists (ACOG) and the endocrine society, restricting carbohydrates in all GDM clients on the medical nutrition program is recommended (Kapur et al., 2021). A 1,800 to 2,400 calorie diet, divided into three meals and three snacks to try and keep carbohydrates evenly distributed during the day, so the glucose level remains constant, is a typical nutrition regimen during pregnancy. Ideally, 20% of dietary calories should be from protein, 40% to 50% from carbohydrates, and up to 30% from fat. Urge the client, however, not to reduce their intake to below 1,800 calories during pregnancy as an intake this low in carbohydrates can lead to fat breakdown and acidosis.
Encourage the client to include fiber and reduce saturated fats and cholesterol in her diet. Dietary fiber decreases postprandial hyperglycemia and lowers insulin requirements. Increased consumption of saturated and total fats could worsen insulin requirements. During pregnancy, adequate protein intake is essential to prevent the depletion of maternal stores and prevent muscle breakdown from supplying fetal needs (Kapur et al., 2021). Urge the client to make her final snack of day one of protein and a complex carbohydrate (egg and whole grain toast, hummus, and whole-grain crackers) to allow slow digestion during the night.
Adjust diet or insulin regimen to meet individual needs. Prenatal metabolic needs change throughout the trimesters, and adjustment is determined by weight gain and laboratory test results. Typically, insulin dosage may have to be reduced to avoid hypoglycemia in the first trimester, when nausea decreases appetite and physical activity may be reduced. Increasing placental hormones increase insulin resistance in the second trimester, and the insulin dosage may increase. Insulin requirements decrease again at 38 weeks gestation.
Recommend monitoring urine ketones on awakening and when a planned meal or snack is delayed . Insufficient caloric intake is reflected by ketonuria, indicating a need for an increased intake of carbohydrates or additional snacks in the dietary plan (e.g., recurrent presence of ketonuria on awakening may be eliminated by 3 am a glass of milk). Adjustment of insulin type, dosage, and frequency must be required. However, ketonuria accompanied by hyperglycemia requires prompt evaluation for diabetic ketoacidosis, rapidly fatal for the fetus.
Refer to a dietician for an individualized diet plan and counsel dietary questions. A dietician can provide an optimum nutrition plan for the client.
In diabetes mellitus, cells essentially starve because they cannot obtain glucose. The body compensates by metabolizing protein and fat for energy, which causes ketones and acids to accumulate. Estrogen , progesterone, insulinase (an enzyme produced by the placenta ), and increased prolactin levels increase the resistance of cells to insulin and increase insulin breakdown, resulting in the accumulation of glucose in the bloodstream. This puts the mother at risk for hyperglycemia, gestational hypertension , cesarean birth , preterm labor, abruption placenta, spontaneous abortion , and diabetic ketoacidosis.
Assess the client for signs of placenta abruption, such as vaginal bleeding and abdominal tenderness. Placental abruption , the premature detachment of the placenta from the uterine wall before birth and after 20 weeks of gestation, is one of the most significant determinants of maternal morbidity and perinatal morbidity and mortality. Vascular changes associated with diabetes that causes placental dysfunction and separation from the uterine wall place the client at risk for abruption placenta (Downes et al., 2017).
Assess and monitor for signs of edema . In the early stage of insulin resistance, hyperinsulinemia would cause sodium reabsorption by the kidney tubules, which causes edema. The increased vasoconstriction leads to the smooth muscle of the small artery proliferation and narrowing of the blood vessels, resulting in increased systolic pressure (Diao et al., 2021).
Determine fundal height; check for edema of extremities and dyspnea . Polyhydramnios is a pathologic excess of amniotic fluid volume (AFV) in pregnancy. In cases of maternal hyperglycemia, fetal urine excretion is thought to be due to the increase of osmotic diuresis contributing to increased AFV production (Hwang & Bordoni, 2021).
Monitor for signs and symptoms of preterm labor. Hydramnios may predispose the client to early labor. The client may present in preterm labor or premature rupture of membranes, and excessive amniotic fluid may result in a non-vertex fetal presentation or cord prolapse. Delivery is recommended at a tertiary facility due to potential maternal and neonatal morbidity and mortality associated with severe polyhydramnios (Hwang & Bordoni, 2021).
Note White’s classification for diabetes. Assess the degree of diabetic control (Pederson’s Criteria). Priscilla White developed white’s classification in 1949, which was revised in 1980. It estimates the risks of “perinatal wastage” in pregnancies complicated by diabetes. Multiple studies have evaluated the validity of White’s classification, including Pederson et al., who set out to improve the methods for identifying diabetic pregnancies at risk for adverse outcomes. They created a new system by identifying four “prognostically bad signs of pregnancy,” which included clinical pyelonephritis ; precoma and severe acidosis; toxemia; and maternal neglecters (late prenatal care, poor social circumstances) (Bennett et al., 2015).
Closely monitor the client’s vital signs, fetal heart rate (FHR), and uterine contractions. Helps determine the client’s progress by monitoring and physical exam. Inform the client and the healthcare provider of any signs of beginning labor. An increased FHR is a sign of fetal distress, and uterine contractions could mark the beginning of preterm labor.
Educate the client on performing home blood glucose monitoring four times/day. The client must keep her blood glucose levels as close to normal as possible and be taught the signs and symptoms of hypoglycemia and hyperglycemia. Additionally, the client should monitor her blood glucose levels several times a day as instructed by the healthcare provider to allow greater accuracy than urine testing because the renal threshold for glucose is lowered during pregnancy and facilitates tighter control of serum glucose levels.
Monitor for ketones in the urine daily. Urine ketones may be checked to identify the need for more carbohydrates. If the client’s carbohydrate intake is insufficient, she may metabolize protein and fat to produce glucose, resulting in ketonuria. However, ketonuria accompanied by hyperglycemia requires prompt evaluation for diabetic ketoacidosis. Ketoacidosis can be rapidly fatal to the fetus.
Monitor the client closely if tocolytic drugs are used to arrest labor. Tocolytic drugs such as beta-agonists stimulate sympathetic nerves , causing hyperglycemia. Atosiban, an oxytocin antagonist, contrasts with oxytocin, which suppresses cortisol, thereby increasing the serum level of cortisol and subsequently increasing blood glucose levels (Ko et al., 2021).
Monitor serum glucose level each visit. The client must be aware of being hypoglycemic and ketoacidosis caused by the fetus’s constant use of glucose. Therefore, regular monitoring of blood glucose levels should be performed by the nurse or the client herself at home.
Monitor hematocrit and hemoglobin levels on the initial visit, then during the second trimester and at term. Hemoglobin measurements during the first antenatal visit have become a standard for pregnant women to determine possible risks of feto-maternal complications. Iron is said to interfere with the action of insulin and impairs glucose entry into the adipocytes resulting in overt hyperglycemia. Therefore, it is suggested that the level of body iron stores may play a role in GDM development due to its effect on insulin secretion and function (Tiongco et al., 2018).
Obtain HbA1c every three months, as indicated. Glycosylated hemoglobin (HbA1c) is performed every three months to indicate long-term (4 to 6 weeks) glucose control; lower values indicate successful glucose management of the pregnant diabetic. The normal upper level of HbA1c is 6% of total hemoglobin.
Monitor for total protein excretion, creatinine clearance, BUN, and uric acid levels. The risk of renal morbidity in GDM is reported and associated with overt diabetes. Elevated serum creatinine in GDM clients may be a warning sign of possible renal disease since kidney-related impairments remain clinically silent until an advanced stage (Mishra et al., 2021). A creatinine clearance test may be ordered each trimester. A normal creatinine clearance rate suggests a woman’s vascular system is intact because kidney function is normal. By default, this also implies uterine perfusion is also adequate.
Prepare the client for ultrasonography at 28 and 36 to 38 weeks of gestation as indicated. An ultrasound examination may be taken at week 28 and then again at weeks 36 to 38 to determine fetal growth, amniotic fluid volume, placental location, and biparietal diameter. Oligohydramnios may indicate fetal growth restriction or a fetal renal abnormality, whereas polyhydramnios may indicate gastrointestinal malformation or poorly controlled disease.
Schedule for ophthalmologic examination during the first trimester for all clients and in second and third trimesters if clients are at class D, E, F. An ophthalmic examination should be done once during the pregnancy for a woman with gestational diabetes and at each trimester for women with known diabetes because of common background retinal changes that are common in diabetes, such as increased exudate, dot hemorrhage , and macular edema, can progress or originate during pregnancy. Laser coagulation therapy may improve the client’s condition and reduce optic fibrosis.
Administer glucagon as an IV infusion or subcutaneously in case of insulin shock. Glucagon can be administered as an IV infusion or subcutaneously. Glucagon is available as a dehydrated powder termed a “Glucagon Emergency Kit,” which is reconstituted with sterile water. Severe insulin shock or hypoglycemia is life-threatening, and glucagon has attractive traits in the diabetic population due to its simplicity of use and safe administration (Morris & Baker, 2021).
Administer intravenous fluids and insulin therapy as indicated to correct diabetic ketoacidosis (DKA). Fluid resuscitation, maintenance, and insulin therapy are mainstays of management in diabetic ketoacidosis. In clients with DKA, the fluid deficit could be up to 10-15% of the body weight. Immediate fluid resuscitation is vital to correct hypovolemia , restore tissue perfusion , and clear ketones. Intravenous insulin by continuous infusion is the standard of care. In uncomplicated, mild DKA, subcutaneous injection of insulin lispro hourly may be safer and more cost-effective than regular intravenous insulin (Lizzo et al., 2021).
Infants of women with poorly controlled diabetes tend to be large (>10 lb) because the increased insulin the fetus must produce to counteract the overload of glucose they receive acts as a growth stimulant. A macrosomic infant may create birth problems at the end of the pregnancy because of cephalopelvic disproportion or an increased risk for shoulder dystocia . There is also a high incidence of congenital anomaly, spontaneous miscarriage, and stillbirth in women with uncontrolled diabetes.
Determine White’s classification for diabetes; explain classification and significance to client/couple. The fetus is at less risk if White’s classification is A, B, or C. The client with classification D, E, or F who develops kidney or acidotic problems or gestational hypertension is at high risk. As a means of determining prognosis for the perinatal outcome, White’s classification has been used in conjunction with (1) evaluation of diabetic control or lack of control and (2) presence or absence of Pedersen’s prognostically bad signs of pregnancy (PBSP), which includes acidosis, mild/severe toxemia, and pyelonephritis (Bennett et al., 2015).
Review the client’s diabetic control before conception. Strict control (normal HbA1c levels) before conception helps reduce the risk of fetal mortality and congenital abnormalities. The presence of fasting hyperglycemia (>105 mg/dL or >5.8 mmol/L) may be associated with an increase in the risk of intrauterine fetal death during the last 4-8 weeks of gestation. GDM of any severity increases the risk of fetal macrosomia (American Diabetes Association, 2022).
Assess for signs of hypertensive disorders of pregnancy (edema, hypertension, proteinuria ). About 12-13% of diabetic individuals develop hypertensive disorders owing to cardiovascular changes associated with diabetes. Bennett et al. (2015) revealed in their study that hypertension is associated with a significantly increased incidence of small for gestational age (SGA) infants, preeclampsia, preterm delivery, and composite perinatal outcome. The presence of hypertension appeared to have a significant effect on adverse outcomes.
Assess the client’s fundal height each visit. Monitoring the client’s fundal height is useful in identifying abnormal growth patterns (macrosomia or IUGR, small or large gestational age [SGA/LGA]). Hydramnios may develop because a high glucose concentration causes extra fluid to shift and enlarge the amount of amniotic fluid. A macrosomic infant may create birth problems at the end of the pregnancy because of cephalopelvic disproportion.
Assess fetal movement and fetal heart rate each visit as indicated. Encourage the client to periodically record fetal movements at about 18 weeks gestation, then daily from 34 weeks gestation. Fetal movement and fetal heart rate may be negatively affected when placental insufficiency and maternal ketosis occur. The client may be asked to self-monitor fetal well-being by recording how many movements occur an hour (usually about ten fetal kicks. Be certain that the client understands that fetal activity varies depending on her activity and meal patterns to prevent her from becoming frightened by normal variations.
Monitor the client’s urine for ketones. Irreparable CNS damage or fetal death can occur due to maternal ketonemia, especially in the third trimester. Urine ketone monitoring may be useful in detecting insufficient caloric or carbohydrate intake in clients treated with calorie restriction (American Diabetes Association, 2022).
Assess the client for fruity or acetone breath. A fruity or acetone breath is indicative of diabetic ketoacidosis (DKA). Ketoacidosis can be rapidly fatal to the fetus. Early detection and prompt treatment of DKA in pregnancy results in successful fetal outcomes (Edo et al., 2019).
Assess HbA1c every 4-6 weeks, as indicated. The incidence of congenitally malformed infants is increased in women with high HbA1c levels (greater than 8.5%) early in pregnancy or before conception. HbA1c indicates long-term glucose control; lower values indicate successful glucose management. However, HbA1c monitoring cannot be used as a guide to adjust daily insulin needs during pregnancy, but it may warn of risk for fetal anomalies.
Assess glycosylated albumin level at 24-28 weeks’ gestation, especially for a client in a high-risk category. Serum test for glycosylated albumin reflects glycemia over several days. It may gain acceptance as a screening tool in determining GDM because it does not involve potentially harmful glucose loading with OGTT. Current studies suggest that glycosylated albumin is more associated with infant complications related to gestational diabetes. Glycosylated albumin also responds better to situations of postprandial hyperglycemia and fluctuations in blood glucose. Thus, it could reflect the glycemic status of GDM clients more precisely (Liu et al., 2021).
Assess the client’s creatinine clearance levels periodically. There is a slight parallel between renal vascular damage and impaired uterine blood flow. A creatinine clearance test may be ordered each trimester. A normal creatinine clearance rate suggests a woman’s vascular system is intact because the kidney function is normal. By default, it also implies uterine perfusion is also adequate.
Educate the client about the possible effects of diabetes on fetal growth and development. Educating the client helps them make informed decisions about managing regimens and may increase cooperation. The expectant mother may be anxious about the outcome for herself and her child. Therapeutic communication helps her to express her frustrations and fears.
Educate the client about home blood glucose monitoring and diabetic management. The ACOG recommends fasting values below 95 mg/dL (5.27 mmol/L), 1-h postprandial values below 130-140 mg/dL (7.22-7.77 mmol/L), and 2-h postprandial values below 120 mg/dL (6.66 mmol/L). Studies revealed lower perinatal mortality rates for diabetic pregnancies when mean glucose concentrations were kept in that reference interval. Postprandial testing was associated with lower rates for large-for-gestational-age offspring, fewer cesarean births, and less neonatal hypoglycemia (Coustan, 2013).
Explain the implications of performing periodic nonstress tests (NSTs). Fetal activity and movement are good predictors of fetal wellness. Activity level decreases before alterations in FHR occur. Routine monitoring includes twice-weekly non-stress tests and amniotic fluid index, a strategy that has been shown to reduce stillbirth in pregnancies with pre-gestational GDM or GDM (Dickens & Thomas, 2019).
Educate the client about the importance of amniocentesis using the lecithin-sphingomyelin ratio (L/S) ratio and the presence of phosphatidylglycerol (PG). A lecithin/sphingomyelin ratio by amniocentesis is usually performed by week 36 of pregnancy to assess fetal maturity. In pregnancies complicated by diabetes, this ratio tends not to show maturity as early as in other pregnancies, probably because the synthesis of phosphatidylglycerol, the compound that stabilizes surfactant, is delayed if hyperglycemia is present. Because lung surfactant does not appear to form as early in these fetuses as in others, the presence of phosphatidylglycerol at amniocentesis is used to indicate lung maturity for these infants.
Monitor labor contractions and fetal heart rate during labor. Labor contractions and fetal heart sounds need to be conscientiously monitored during labor to ensure early detection of placental dysfunction.
Assist in obtaining alpha-fetoprotein (AFP) levels are at 15-17 weeks’ gestation. Because women with diabetes tend to have infants with a higher than normal incidence of birth anomalies, a client will have a serum alpha-fetoprotein level obtained at 15 to 17 weeks to assess for a neural tube defect.
Perform nonstress test (NST) and oxytocin challenge test (OCT)/contraction stress test (CST), as appropriate. NST and OST assess fetal well-being and adequacy of placental perfusion. Placental functioning may be assessed by a weekly nonstress test or biophysical profile during the last trimester of pregnancy if a woman is in good control, or a daily nonstress test if her regulation is poor. GDM mothers with risk factors may begin twice-weekly nonstress tests and an amniotic fluid index between 32 and 36 weeks (Coustan, 2013).
Prepare the client for ultrasonography, as indicated. Ultrasonography is useful in confirming gestation dates and helps to evaluate intrauterine growth restriction (IUGR), macrosomia, and excess amniotic fluid. Many practitioners perform third-trimester ultrasounds in all women with GDM to assess fetal weight and identify cases where elective induction or cesarean birth may be indicated to prevent birth complications (Dickens & Thomas, 2019).
Assist as necessary with biophysical profile (BPP) assessment. BPP provides a score to assess fetal well-being/risk. The criteria include NST results, fetal breathing movements, amniotic fluid volume, fetal tone, and fetal body movements. For each criterion met, a score of 2 is given. A total score of 8-10 is reassuring, a score of 4-7 indicates a need for further evaluation and retesting, and a score of 0-3 is ominous. BPP evaluation carried out twice a week can prevent fetal death in diabetic pregnant women (Pandraklakis & Pappa, 2019).
Assist with preparation for delivery of fetus vaginally or surgically if test results indicate placental aging and insufficiency. American College of Obstetricians and Gynecologists (ACOG) recommends that for women with GDM well controlled on nutritional therapy alone, delivery should not be induced before 39 weeks. Expectant management up to 40 6/7 weeks is reasonable with appropriate monitoring. For women with GDM requiring medication, delivery between 39 0/7 and 39 6/7 weeks is recommended. In preparation for scheduled cesarean birth or labor induction, the long-acting insulin dose should be reduced by 50%. On the morning of the surgery or induction, insulin or OAD should be held. Intrapartum glycemic control can be maintained by rotating fluid protocol which uses 5% dextrose in normal saline infusion for maternal glucose <100 mg/dL, lactated Ringer’s or normal saline for glucose >101 mg/dL, and rapid-acting infusion for glucose >140 mg/dL titrated to goal glucose 100 mg/dL (Dickens & Thomas, 2019).
Pregnant women with gestational diabetes mellitus are at increased risk for infections of the genital tract. Diabetic pregnant women are more likely to acquire genital infections because of poor metabolic control, higher body mass index (BMI), and potentially impaired leukocyte function. Moreover, pregnancy itself harbors an immunocompromised state, leading to an increased risk of vaginal Candida colonization. As insulin resistance increases along with gestational age, the susceptibility for infections in diabetic pregnant women may rise with the duration of pregnancy and poor glycemic control (Marschalek et al., 2016).
Assess the client for urinary tract infection (UTI) signs. Observe the client for any signs of infection and inflammation, such as fever , flushed appearance, or cloudy urine. Early detection of UTI may prevent the occurrence of pyelonephritis, which can contribute to premature labor. Diabetes mellitus has been associated with the reduced response of T cells , neutrophil function, and disorders of humoral immunity. (Alves et al., 2012).
Determine the nature of any vaginal discharge. If glycosuria is present, a client is more likely to develop monilial vulvovaginitis, caused by Candida albicans and may lead to oral thrush in the newborn. Candida and yeast normally reside in the vagina, and their unchecked growth is restricted by lactobacillus. The common complaints of vaginal thrush are abnormal vaginal discharge, vaginal itch, painful micturition, and dyspareunia (Sadaqat et al., 2020).
Inspect the client’s feet, noting the presence of ulcers, infected ingrown toenails, or other problems requiring interventions. Foot injuries, sensory neuropathy, and impaired circulation are associated with many complications in diabetics, including skin and soft tissue infections. The initial assessment should evaluate the foot and the general situation of the client. Local signs of infection are increased temperature, erythema, pain , lack of functionality, and edema (Nikoloudi et al., 2018).
Assess the client’s vital signs, especially temperature. Local and systemic signs of infection may be present and include fever, chills, tachycardia, hypotension , increased respiratory rate, fatigue, and metabolic disorders. While treated as an outpatient, careful monitoring is needed, and infections should be reassessed in 2-4 days or immediately if the situation worsens (Nikoloudi et al., 2018).
Promote good handwashing by staff, the client, and family members. Good hand hygiene reduces the risk of cross-contamination. Healthcare-associated infections (HAIs) affect hundreds of millions of individuals worldwide. Performing hand hygiene is widely accepted as a key strategy of infection prevention and control to prevent HAIs, as healthcare workers’ contaminated hands are the vehicle most often implicated in the cross-contamination of pathogens in healthcare (Vermeil et al., 2019).
Caution the client not to self-medicate with vaginal creams available over-the-counter. Choice of self-treatment may be inappropriate/mask infection. Self-medication carries a serious risk of drug interactions, polypharmacy, misdiagnosis, excessive drug dosage use, prolonged drug use, incorrect drug choice, rare but severe adverse events, dependence or abuse , and increased antimicrobial resistance. The Food and Drug Authority (FDA) classified drugs in categories according to their safety in pregnancy, from class A (the safest) to class X (the teratogenic group). Unfortunately, only a few drugs (40%) are listed in this classification, indicating that only a few drugs are safe during pregnancy because self-medication is common among pregnant women (Marwa et al., 2018).
Maintain aseptic technique for invasive procedures such as IV and catheter insertion. High glucose in the blood creates an excellent medium for bacterial growth. The main pathogenic mechanisms are hyperglycemic environment increasing the virulence of some pathogens; lower production of interleukins in response to infection; glycosuria; gastrointestinal and urinary dysmotility (Alves et al., 2012).
Provide catheter and perineal care when indicated. Teach the client to clean from front to back after elimination to minimize the risk of UTI. Candida organisms probably access the vagina via migration across the perianal area from the rectum. Nearly 10% of women in their reproductive age have this recurrent infection, translating to about 140 million women being affected worldwide (Sadaqat et al., 2020).
Encourage adequate dietary and fluid intake (at least 2500 mL daily if not contraindicated). Increased fluid intake reduces the client’s susceptibility to infection. Increased urinary flow prevents stasis and aids in maintaining urine acidity, reducing bacteria growth, and flushing organisms out of the system (Perrier et al., 2020).
Obtain urinalysis and urine culture. Asymptomatic bacteriuria (ASB) is a positive urine culture without any clinical manifestation. Unlike the general female population, ASB in pregnant women always requires treatment to reduce the possible maternal and fetal risks. Strains such as Group B Streptococcus carries a high risk for premature rupture of membranes preterm labor and significantly increase the risk for neonatal infection by 25-folds (Abou Heidar et al., 2019).
Administer antibiotics as indicated. The cornerstone of any bacterial infection, including UTI, is antimicrobial therapy. Since UTIs are very common, especially in women, controlled use of antibiotics must be initiated for treatment (Abou Heidar et al., 2019). Fosfomycin is an acceptable antibiotic for pregnant women with UTIs. Women with asymptomatic bacteriuria should be treated by the standard regimen of antibiotics for seven days, except for recurrent infections where treatment should last for 10-14 days (Kalinderi et al., 2018).
Obtain culture of vaginal discharge, if present. Candida vulvovaginitis can cause oral thrush in the newborn. Discharge is a prominent feature in clients with vulvovaginitis. Fungal cultures should be performed in all pregnant females to confirm the presence of candida (Sadaqat et al., 2020).
Educate the client on how they can recognize signs of infection. It is important to seek medical help early to avoid further complications. Educate the client about common signs of infection such as fever, chills, tachycardia, hypotension , increased respiratory rate, and fatigue. Wound infections in diabetic clients can result in gruesome complications. Symptoms may include increased temperature, erythema, pain, lack of functionality, and edema (Nikoloudi et al., 2018).
The insulin requirement progressively rises during pregnancy, explaining the higher incidence of DKA in the second and third trimesters (Sharma et al., 2020). Fluid and electrolyte losses in clients with diabetes, particularly DKA, are predominantly caused by hyperglycemia with glycosuria and osmotic diuresis. Additionally, the kidney has a low threshold for ketoacids, which are excreted into the urine with electrolytes , further exacerbating the electrolyte loss. Intravascular dehydration occurs because of a relatively large sodium loss with polyuria and polydipsia (Inward & Chambers, 2002).
Assess and monitor the client’s vital signs. Hypovolemia may be manifested by hypotension and tachycardia. Lungs remove carbonic acid through respiration, producing a compensatory respiratory alkalosis or ketoacidosis. This results in acetone breath, which is due to the breakdown of acetoacetic acid. Correction of hyperglycemia and acidosis will cause the respiratory rate and pattern to approach normal. Fever accompanied by flushed, dry skin may reflect dehydration (Lizzo et al., 2021).
Assess the client’s peripheral pulses, capillary refill, skin turgor, and mucous membranes. These are good indicators of hydration and adequacy of circulating volume. As the client becomes volume-depleted, they may experience decreased urine output, dry mouth, or decreased sweating indicative of dehydration . The client may have signs of dehydration, including poor capillary refill, skin turgor, and dry mucous membranes (Lizzo et al., 2021).
Weigh the client daily. The weight provides one of the best assessments of current fluid status and adequacy of fluid replacement. The client may complain about many other symptoms, including anorexia , nausea, vomiting, abdominal pain, and weight loss (Lizzo et al., 2021).
Monitor the client’s intake and output ; note urine-specific gravity. Measuring the client’s intake and output provides an ongoing estimate of volume replacement needs, kidney function, and effectiveness of the therapy. Diuresis induced by hyperglycemia, dehydration, hyperosmolarity, and electrolyte imbalance decreases glomerular filtration. Due to worsening renal function, hyperosmolality worsens (Lizzo et al., 2021).
Assess for changes in mentation and level of consciousness. Changes in mentation can be due to abnormally high or low glucose, electrolyte abnormalities, decreased cerebral perfusion, acidosis, or developing hypoxia. Arterial pH is the prime determinant of mental status in DKA, which appeared to act synergistically with hyperosmolarity to produce depressed consciousness (Nyenwe et al., 2010).
Promote a comfortable environment. Cover the client with light sheets to avoid overheating, promoting further fluid loss. According to Richard et al., fluid loss through sweats up to 1.4% of the body weight can still be tolerated without serious problems. However, if fluid loss reaches 3-6% of body weight, body productivity will be affected (Zulkarnain et al., 2019).
Encourage increased fluid intake unless contraindicated. Diabetic pregnant women may need more fluid intake due to physiological changes in the mother and fetal growth. Thus, the risk of being dehydrated for pregnant women is relatively high due to insufficient fluid intake. For pregnant diabetic women, the endocrine system shows considerable changes, which may affect water metabolism and balance, and thus, hydration state (Zhang et al., 2020).
Monitor laboratory studies, such as hematocrit, BUN, creatinine, serum osmolality, sodium, and potassium . Hematocrit assesses the level of hydration, and it is often elevated because of hemoconcentration associated with osmotic diuresis. Elevated values of BUN and creatinine may reflect cellular breakdown from dehydration. Elevated serum osmolality occurs due to hyperglycemia and dehydration. Decreased sodium levels reflect the shifting of fluids from the intracellular compartment as with osmotic diuresis. Hyperkalemia initially occurs in response to metabolic acidosis, but as this potassium is lost in the urine, the absolute potassium level in the body is depleted (Eledrisi & Elzouki, 2020).
Insert and maintain indwelling urinary catheter, as indicated. An indwelling catheter provides for accurate and ongoing measurement of urinary output as necessary, however, this should be removed immediately once the client is stable enough to reduce the risk of infection. Unnecessary or overlong catheterization is a further risk factor, with poor urethral orifice asepsis as a predisposing factor. The use of urinary catheters is the most common source of infections and Gram-negative bacteremia in the hospital setting. (Storme et al., 2019).
Administer intravenous fluids, as ordered. Intravenous solutions replace intravascular and extravascular fluids and replenish electrolyte losses. They also dilute both the levels of glucose and circulating counterregulatory hormones. Fluids that may be used include isotonic (0.9%) or lactated Ringer’s solution (Eledrisi & Elzouki, 2020).
Administer potassium and other electrolytes intravenously or by oral route, as indicated. Potassium should be added to the IV as soon as the urinary flow is adequate to prevent hypokalemia. The amount and timing of potassium replacement depend on the serum potassium concentration. The levels should be monitored closely because the entry of potassium in cells would be facilitated by volume expansion, resolution of acidosis, and insulin therapy, which, in turn, would result in decreased concentration of serum potassium (Eledrisi & Elzouki, 2020).
Because blood glucose levels near normal help minimize the risk of maternal and fetal complications, both women with gestational diabetes and those with overt diabetes need more frequent prenatal visits than usual to ensure close monitoring of their condition and that of the fetus. However, some factors may hinder the client’s adherence to the prescribed regimen, which in turn may endanger her and the infant’s lives.
Assess the client’s knowledge about the disease process and the treatment regimen. Providing the client with the basic knowledge about the disease will help them understand the necessity of the treatment regimen and how it will help them achieve a normal, safe delivery.
Assess the client’s dietary plan. The client’s dietary plan should include avoiding sugar, limiting the intake of fat, salt, and alcohol, and intake of complex carbohydrates high in fiber. Medical nutrition therapy encourages clients to make meal choices based on their individual needs. Awareness of the importance of dietary control aids the client in planning meals and adhering to the regimen. Limited caloric intake has been widely recommended for obese women with GDM. ACOG recommends a reduction of approximately 24 kcal/kg per day for pregnant women with >120% of the normal body weight (Dirar & Doupis, 2017).
Assess the client’s knowledge about potential complications of GDM. Awareness helps the client be more consistent with care and may prevent or delay the onset of complications. Assess for the client’s knowledge about acute and chronic complications of the disease, which may include visual disturbances, neurosensory and cardiovascular changes, renal impairment, hypertension, and DKA. Provide information about fetal and neonatal complications, such as macrosomia, congenital abnormalities, birth injury , neonatal hypoglycemia, etc.
Therapeutically communicate with the client by creating an environment of trust, listening to their concerns, and being available. Rapport and respect need to be established before the client will be willing to participate in the learning process. Employ the use of therapeutic communication skills to help build rapport.
Include the client while creating the care plan and the goals for learning. Participation in planning promotes enthusiasm and cooperation with the principles learned. Farahani Dastjani et al. (2015) observed an inverse relationship and a significant difference between the lack of motivation and adherence to pharmacotherapy. Including the client during the care plan creation will boost their motivation to adhere to their own set of standards (Moradi et al., 2021).
Determine the appropriate teaching strategy that will suit the client’s knowledge level and learning capacity. Select the appropriate teaching strategy for the client, such as demonstrating needed skills and having the client do a return demonstration or incorporating new skills into the hospital routine. The use of different means of accessing information promotes information retention. According to previous studies, guideline dissemination alone does not change the client’s practice. Therefore, the assessment of barriers and implementation design must be theory -driven (Salama & Abushaikha, 2018).
Educate the client and family members about the glucose self-monitoring system and have them demonstrate the procedure in return. Explain glucose self-monitoring to the client and the family members, such as the fingerstick testing. Afterward, have them return demonstrate obtaining of sample and operating the glucometer until proficient. This is performed four times a day by pricking a finger and using a glucometer to determine the blood glucose level. The client should then document the results and bring them for the next prenatal check-up to determine if any adjustments to the regimen are needed.
Review the client’s medication regimen. Determine the client’s agents in her treatment regimen and ascertain their adherence to the therapy. Drugs for diabetes may work in different ways to ensure a normal blood glucose level. Combination drugs may contain more than one type of diabetes medication. The dose and frequency of insulin injections are tailored to the client’s individual needs. It is often administered on a sliding scale, in which the client varies her dose of insulin based on each blood glucose level.
Educate the client about self-administration of insulin and let them perform a return demonstration. Review the client’s knowledge about self-administration of insulin and clarify or enforce misunderstood information. Have the client demonstrate the procedure by drawing up and injecting insulin, using the insulin pen technique, or using a continuous insulin pump. This will either confirm that the client is proficient in skills or requires assistance or full care to manage the necessary procedures. Follow-up observation and documentation are essential parts of insulin administration.
Establish a regular exercise pattern or activity schedule. In GDM, exercise can help control blood glucose levels, and diet and exercise can minimize the need for insulin. The client with GDM should be counseled that exercise after meals is preferred because glucose levels are higher at that time. Blood glucose levels should be monitored before, during, and after exercise, and hard candy should be on hand to deal with hypoglycemia.
Educate the client about the symptoms of hypoglycemia. Symptoms of hypoglycemia include hunger trembling. Weakness, lethargy, irritability, headache, sweating, pale, cool, moist skin, blurred vision , or loss of consciousness. The client and family members should be aware of the signs and symptoms of hypoglycemia and educated about ways to manage it. Hypoglycemia often results from diabetes management errors which should be detected early to limit its effects.
Educate the partner or family members about the emergency use of glucagon. Glucagon is given to treat severe hypoglycemia when the client is unable to take oral carbohydrates. Prompt intervention may prevent more serious complications from developing. Clients with decreased levels of consciousness cannot safely consume the oral carbohydrates needed to raise their blood glucose without risk of aspiration , and obtaining IV access can be problematic in the diabetic population. A pre-filled glucagon injection has received approval, similar to epinephrine auto-injectors. An intranasal spray may be commonly encountered for layperson use, which contains glucagon as a dehydrated powder (Morris & Baker, 2021).
Discuss with the client the importance of follow-up care. Follow-up visits help maintain tighter control of the disease process and may prevent exacerbations of DM, retarding the development of systemic complications. Antenatal care is an effective measure that provides care to pregnant women. Providers should be trained and supported to adhere to the guidelines during care to all pregnant women (Salama & Abushaikha, 2018).
Educate the client about the importance of routine foot examination and proper foot care. Diabetes is the leading cause of nontraumatic lower-extremity amputations in the United States. A diabetic foot ulcer is often the result of poor glycemic control, underlying neuropathy, or poor foot care. Prevention and early treatment of diabetic foot injuries or ulcers are critical. Demonstrate ways to examine the feet, inspect shoes for fit, and care for toenails, calluses, and corns. Advise the client to avoid going barefoot (Dreyer, 2021).
Pregnant women with diabetes mellitus often find living with glucose monitoring, diet control, and frequent insulin administration bothersome, especially if they do not understand their rationale. The expectant mother may be anxious about the outcome for herself and her infant. Therefore, the nurse should educate them and evaluate for understanding to ensure that they have enough foundation to become adherent with their therapeutic regimen.
Assess the client’s and/or couple’s knowledge of the disease condition and treatment, including relationships between diet, exercise, stress, illness, and insulin requirements. When there is a clear understanding of both the disease condition and rationale for each management, it helps the client and/or couple make informed decisions. Once a GDM diagnosis is established, the mainstays of therapy include medical nutrition, physical activity, weight management, and glucose monitoring. GDM can be managed with lifestyle interventions alone in up to 80-90% of clients (Dickens & Thomas, 2019).
Assess the client’s readiness to learn and individual learning needs. Ascertain the client’s level of knowledge, including anticipatory needs and their ability to learn. They may not be physically, emotionally, or mentally capable at this time to receive new knowledge, especially if there are factors that hinder the learning process.
Assess for factors that are pertinent to the learning process. Factors such as age, social/cultural influences, religion, life experiences, level of education, and sense of powerlessness may affect the client or couple’s willingness to learn. Determine if there are blocks to learning, such as language barriers or physical factors, and resolve them early into the nurse-client relationship to reach your goals successfully. Barriers to GDM care for minority and low-income women have been explored in small cohorts with focus groups. Communication, personal/environmental barriers, and type of quality and healthcare were identified as areas for improvement (Dickens & Thomas, 2019).
Educate the client on how to perform serum glucose monitoring at home using a glucometer and the need to record readings (usually at least 2-4 times/day). Self-monitoring of blood glucose should initially be performed at least four times daily: fasting and 1-2 h postprandial. Glycemic targets include fasting glucose <95 mg/dL, 1-h postprandial glucose <140 mg/dL, and 2-h postprandial glucose <120 mg/dL. For clients with GDM well controlled with diet alone, frequency of self-monitoring may be reduced but generally should still be performed at least twice daily (Dickens & Thomas, 2019).
Explain the difference between normal and abnormal weight gain during pregnancy. Facilitate home visits to check and monitor the weight. Monitoring weight changes are important to ensure dietary therapy adequacy and maintain a weight gain within the recommended rates. The Institute of Medicine (IOM) in America recommended a weight gain during pregnancy of 12.5-18 kg (27.5-39.6 lbs) or underweight (BMI <19.8 kg/m²), 11.5-16 kg (25.4-35.3 lbs) for healthy women (BMI 19.8-26.0 kg/m²), 7-11 kg (15.4-24.3 lbs) for overweight (BMI 26.0-29.0 kg/m²), and at least 7 kg (15.4 lbs) for obese (BMI >29.0 kg/m²) (Dirar & Doupis, 2017).
Explain the importance of understanding the maternal and fetal effects of oral agents for GDM. Systematic reviews and meta-analyses of several studies testing oral agents or insulin treatment in GDM have shown that both strategies present comparable safety and efficacy. However, metformin was associated with more risk of preterm birth and neonatal hypoglycemia compared with insulin treatment. Glyburide has shown similar efficacy and outcomes with insulin. However, it demonstrated an increased risk of macrosomia and neonatal hypoglycemia in women treated with glyburide than insulin (Dirar & Doupis, 2017).
Educate the client regarding the use and action of insulin. As indicated, demonstrate how to administer insulin (by injection, nasal spray, or an insulin pump). The dose and frequency of insulin injections are tailored to a woman’s individual needs. Insulin is often administered on a sliding scale, in which the woman varies her dose of insulin based on each blood glucose level. Typically, insulin dosage may be reduced to avoid hypoglycemia in the first trimester. In the second trimester, the insulin dosage may have to be increased due to increased insulin resistance. Insulin requirements may decrease again at 38 weeks gestation. The use of an insulin pump has proved of great value for glucose control in pregnant and nonpregnant clients with diabetes mellitus and reduces hypoglycemic events.
Educate the client regarding medical nutrition therapy (MNT) and provide instructions for appropriate dietary intake for GDM. MNT aims to attain normal glycemic control without ketosis and fetal compromise and maintain adequate weight gain based on prenatal BMI. ACOG recommends reducing caloric intake to approximately 24 kcal/kg per day for pregnant women with >120% of the normal body weight. The limited caloric intake had been widely recommended for obese women with GDM (Dirar & Doupis, 2017).
Provide information about the appropriate carbohydrate intake for GDM. Women with diabetes need to be aware of how much carbohydrate they eat daily by estimating the total carbohydrate each anticipated meal will contain and then administering several units of insulin before that based on a predetermined insulin-to-carbohydrate ratio. 1800 to 2400-calorie diet (or one calculated at 30 kcal/kg of ideal weight), divided into three meals and three snacks to try and keep carbohydrates evenly distributed during the day.
Educate the client on preventing and treating hyperglycemia or hypoglycemia at home. The client’s diet should include a reduced amount of saturated fats and cholesterol and increased dietary fiber. Increasing fiber decreases postprandial hyperglycemia, and so lowers insulin requirements. Urge the client to make her final snack of day one of protein and a complex carbohydrate (an egg and whole grain toast, hummus, and whole-grain crackers) to allow slow digestion during the night, when women are extremely vulnerable to hypoglycemia.
Instruct the client on how to incorporate exercise into her regimen. Exercise is another mechanism that lowers serum glucose levels and the need for insulin. Educate the client that she may notice excessive glucose fluctuations first when she begins an exercise program. With exercise, blood glucose levels decrease because the muscles increase their need for glucose, an effect that lasts for at least 12 hours after exercise. Instruct the client to eat a snack consisting of protein and complex carbohydrates before exercise and maintain a consistent exercise program. She should not do aerobic exercises one day and then none the next, but rather do 30 minutes of walking every day.
Provide information regarding the impact of pregnancy on the diabetic condition and future expectations. Sufficient knowledge can decrease the fear of the unknown, increase the likelihood of participation, and help reduce fetal/maternal complications. Educate the client that following delivery, most women return to their previous pre-gestational glycemic levels soon afterward. However, some women may continue with hyperglycemia, possibly representing the category of undiagnosed T2DM.
Explain the significance of breastfeeding for GDM clients during the postpartum period. Breastfeeding improves weight and glucose tolerance and should be encouraged. According to the Nurses’ Health Study (NHS), it has been shown to reduce the risk of type 2 diabetes at 14-15% yearly. Breastfeeding has additional short and long-term beneficial effects on offspring metabolic health (Dickens & Thomas, 2019).
Assist client and family to learn glucagon administration. Glucagon is used to manage and treat hypoglycemia as an antidote to beta-blocker and calcium channel blocker overdose. Glucagon is a reliable method of raising the client’s glucose and relieving severe hypoglycemia long enough for more definitive correction of the client’s glucose levels by mouth. A pre-filled glucagon injection has received approval, injecting into the client’s thigh. The intranasal powder requires no preparation, and administration is via a spray into the client’s nose while holding the other nare closed. (Morris & Baker, 2021).
Encourage the client to maintain a diary of home assessment of serum glucose levels, insulin dosage, reactions, general well-being, diet, exercise, and other thoughts related to the disease condition. A diary can help the health care provider evaluate and alter the therapy provided as indicated. Persistent glucose intolerance is present in up to 20% of women at postpartum follow-up, and subsequent risk for type 2 diabetes is significantly increased (Dickens & Thomas, 2019).
Administering medications and providing pharmacologic support is an important aspect of managing patients with gestational diabetes mellitus (GDM). GDM is a type of diabetes that occurs during pregnancy and requires careful monitoring and treatment to maintain stable blood sugar levels. Pharmacotherapy aims to control blood glucose levels and prevent complications for both the mother and the developing baby.
1. Insulin Therapy Insulin is the primary medication used to manage GDM when lifestyle modifications alone are insufficient to control blood sugar levels. Insulin helps regulate blood glucose levels by facilitating the uptake of glucose into cells. It is safe for both the mother and the baby during pregnancy. The type, dosage, and frequency of insulin administration will be determined by the healthcare provider based on the patient’s specific needs and blood glucose levels.
2. Oral Antidiabetic Agents In some cases, oral antidiabetic agents, such as metformin or glyburide, may be prescribed to manage blood sugar levels in GDM if insulin therapy is not feasible or contraindicated. These medications help lower blood glucose levels by improving insulin sensitivity or reducing hepatic glucose production. The use of oral antidiabetic agents during pregnancy requires careful monitoring and should be guided by an experienced healthcare provider.
3. Self-Monitoring of Blood Glucose (SMBG) Patients with GDM are often advised to regularly monitor their blood glucose levels using a glucometer at home. SMBG allows patients to track their blood sugar levels and make appropriate adjustments to their diet, exercise, and medication regimen. It helps ensure that blood glucose levels are within the target range, minimizing the risk of complications for both the mother and the baby.
Regular monitoring of diagnostic and laboratory results helps healthcare providers evaluate blood glucose control, detect any potential complications, and make informed treatment decisions for patients with GDM. It allows for timely interventions to optimize maternal and fetal health outcomes.
1. Blood Glucose Monitoring Self-Monitoring of Blood Glucose (SMBG). Patients with GDM are often advised to regularly check their blood glucose levels using a glucometer at home. They measure their blood glucose levels by pricking their finger and applying a small drop of blood to a test strip. SMBG provides real-time information about blood glucose levels, allowing patients and healthcare providers to monitor and adjust treatment strategies accordingly. Regular monitoring helps ensure that blood glucose levels are within the target range and minimizes the risk of complications for both the mother and the baby.
2. Glycated Hemoglobin (HbA1c) Testing HbA1c is a laboratory test that provides an average measurement of blood glucose levels over the past two to three months. It reflects long-term blood glucose control. HbA1c testing provides an indication of overall blood glucose control and helps assess the effectiveness of treatment interventions over time. It is particularly useful for assessing long-term glycemic control in patients with GDM.
3. Oral Glucose Tolerance Test (OGTT) The OGTT is a diagnostic test used to evaluate glucose metabolism. It involves drinking a glucose solution, followed by blood glucose measurements at specific time intervals. OGTT helps assess insulin resistance, glucose tolerance, and the body’s ability to metabolize glucose. It is commonly used to diagnose GDM and may be repeated periodically to monitor blood glucose control during pregnancy.
4. Urine Testing Urine testing for glucose and ketones may be performed to assess renal glucose excretion and detect any signs of uncontrolled blood glucose levels or ketosis. Urine testing can provide additional information about blood glucose control and the presence of ketones, which may indicate poor glycemic control or the need for adjustments in treatment.
5. Fetal Monitoring Fetal ultrasound and non-stress test (NST) may be used to monitor the well-being of the developing baby and assess any potential complications related to maternal blood glucose levels. Fetal monitoring helps detect any fetal growth abnormalities, signs of distress, or other complications associated with uncontrolled blood glucose levels in the mother.
Gestational diabetes can increase the risk of various complications for both the mother and the baby. Regular assessment and monitoring for potential complications in patients with GDM allow for early detection and timely intervention, reducing the risk of adverse outcomes for both the mother and the baby.
1. Maternal Assessment
- Blood Pressure Monitoring. Regular monitoring of blood pressure helps identify the development of gestational hypertension or preeclampsia, which can occur in women with GDM.
- Weight Monitoring. Monitoring weight gain helps identify excessive weight gain, which may increase the risk of complications such as macrosomia (large baby) or cesarean delivery.
- Symptom Assessment. Assessing for symptoms such as excessive thirst, frequent urination, blurred vision, or signs of infection is important to detect any complications or worsening of GDM.
2. Fetal Assessment
- Fetal Movement Counting. Encouraging the mother to monitor fetal movements regularly can help identify any changes or abnormalities in fetal activity.
- Fetal Growth Assessment. Regular ultrasound examinations can assess fetal growth and detect any signs of macrosomia or intrauterine growth restriction.
- Non-Stress Test (NST). This test measures the baby’s heart rate in response to movement. It is used to assess fetal well-being and detect any signs of fetal distress.
3. Blood Glucose Monitoring Regular self-monitoring of blood glucose levels, as discussed earlier, helps assess blood glucose control and prevent complications for both the mother and the baby.
- Assessing Hypoglycemia. Monitoring for signs and symptoms of hypoglycemia, such as sweating, shakiness, dizziness, or confusion, is essential, as hypoglycemia can occur in patients with GDM who are on insulin or oral antidiabetic medications.
4. Urine Testing Regular urine testing for proteinuria and ketones helps assess kidney function and detect any signs of uncontrolled blood glucose levels or ketosis, which may indicate poor glycemic control or the need for adjustments in treatment.
5. Education and Counseling Providing education and counseling to the patient about potential complications associated with GDM, such as macrosomia, preterm birth, or the development of type 2 diabetes in the future, is crucial. Ensuring that the patient understands the importance of self-care , including maintaining a healthy diet, regular physical activity, and adherence to medication and blood glucose monitoring, is essential for optimal outcomes.
Recommended nursing diagnosis and nursing care plan books and resources.
Disclosure: Included below are affiliate links from Amazon at no additional cost from you. We may earn a small commission from your purchase. For more information, check out our privacy policy .
Ackley and Ladwig’s Nursing Diagnosis Handbook: An Evidence-Based Guide to Planning Care We love this book because of its evidence-based approach to nursing interventions. This care plan handbook uses an easy, three-step system to guide you through client assessment, nursing diagnosis, and care planning. Includes step-by-step instructions showing how to implement care and evaluate outcomes, and help you build skills in diagnostic reasoning and critical thinking.

Nursing Care Plans – Nursing Diagnosis & Intervention (10th Edition) Includes over two hundred care plans that reflect the most recent evidence-based guidelines. New to this edition are ICNP diagnoses, care plans on LGBTQ health issues, and on electrolytes and acid-base balance.

Nurse’s Pocket Guide: Diagnoses, Prioritized Interventions, and Rationales Quick-reference tool includes all you need to identify the correct diagnoses for efficient patient care planning. The sixteenth edition includes the most recent nursing diagnoses and interventions and an alphabetized listing of nursing diagnoses covering more than 400 disorders.

Nursing Diagnosis Manual: Planning, Individualizing, and Documenting Client Care Identify interventions to plan, individualize, and document care for more than 800 diseases and disorders. Only in the Nursing Diagnosis Manual will you find for each diagnosis subjectively and objectively – sample clinical applications, prioritized action/interventions with rationales – a documentation section, and much more!

All-in-One Nursing Care Planning Resource – E-Book: Medical-Surgical, Pediatric, Maternity, and Psychiatric-Mental Health Includes over 100 care plans for medical-surgical, maternity/OB, pediatrics, and psychiatric and mental health. Interprofessional “patient problems” focus familiarizes you with how to speak to patients.

Other recommended site resources for this nursing care plan:
- Nursing Care Plans (NCP): Ultimate Guide and Database MUST READ! Over 150+ nursing care plans for different diseases and conditions. Includes our easy-to-follow guide on how to create nursing care plans from scratch.
- Nursing Diagnosis Guide and List: All You Need to Know to Master Diagnosing Our comprehensive guide on how to create and write diagnostic labels. Includes detailed nursing care plan guides for common nursing diagnostic labels.
Other care plans related to the care of the pregnant mother and her baby:
- Abortion (Termination of Pregnancy) | 8 Care Plans
- Cervical Insufficiency (Premature Dilation of the Cervix) | 4 Care Plans
- Cesarean Birth | 11 Care Plans
- Cleft Palate and Cleft Lip | 7 Care Plans
- Gestational Diabetes Mellitus | 8 Care Plans
- Hyperbilirubinemia (Jaundice) | 4 Care Plans
- Labor Stages, Induced, Augmented, Dysfunctional, Precipitous Labor | 45 Care Plans
- Neonatal Sepsis | 8 Care Plans
- Perinatal Loss (Miscarriage, Stillbirth) | 6 Care Plans
- Placental Abruption | 4 Care Plans
- Placenta Previa | 4 Care Plans
- Postpartum Hemorrhage | 8 Care Plans
- Postpartum Thrombophlebitis | 5 Care Plans
- Prenatal Hemorrhage (Bleeding in Pregnancy) | 9 Care Plans
- Preeclampsia and Gestational Hypertension | 6 Care Plans
- Prenatal Infection | 5 Care Plans
- Preterm Labor | 7 Care Plans
- Puerperal & Postpartum Infections | 5 Care Plans
- Substance Abuse in Pregnancy | 9 Care Plans
Recommended journals, books, and other interesting materials to help you learn more about gestational diabetes mellitus nursing care plans and nursing diagnosis:
- Abou Heidar, N. F., Degheili, J. A., Yacoubian, A. A., & Khauli, R. B. (2019, October). Management of urinary tract infection in women: A practical approach for everyday practice. Urology Annals, 11(4), 339-346.
- Alves, C., Casqueiro, J., & Casqueiro, J. (2012). Infections in patients with diabetes mellitus: A review of pathogenesis. NCBI. Retrieved January 26, 2022 .
- American Diabetes Association. (2004, January). Gestational Diabetes Mellitus. Diabetes Care, 27(Supplement 1).
- American Diabetes Association. (2022). Standards of Medical Care in Diabetes—2022 Abridged for Primary Care Providers. Clinical diabetes, 40(1), 10-38.
- Bennett, S. N., Tita, A., Owen, J., Biggio, J. R., & Harper, L. M. (2015, May). Assessing White’s Classification of Pregestational Diabetes in a Contemporary Diabetic Population. Obstetrics and Gynecology, 125(5), 1217-1223.
- Coustan, D. R. (2013). Gestational Diabetes Mellitus. Clinical Chemistry, 59(9), 1310-1321.
- Diao, D., Diao, F., Xiao, B., Liu, N., Li, F., & Yang, X. (2021, July 30). Causation between Gestational Diabetes Mellitus(GDM) and Pregnancy Induced Hypertension(PIH): A Statistic Case Study in Harbin, China. Research Square.
- Dickens, L. T., & Thomas, C. C. (2019, May 9). Updates in Gestational Diabetes Prevalence, Treatment, and Health Policy. Economics and Policy in Diabetes, 19(33).
- Dirar, A. M., & Doupis, J. (2017, December 15). Gestational Diabetes from A to Z. World Journal of Diabetes, 8(12), 489-506.
- Downes, K. L., Grantz, K. L., & Shenassa, E. D. (2017, August). Maternal, Labor, Delivery, and Perinatal Outcomes Associated with Placental Abruption: A Systematic Review. Am J Perinatol, 34(10), 935-957.
- Dreyer, M. A. (2021, August 19). Diabetic Foot Ulcer – StatPearls. NCBI. Retrieved January 28, 2022 .
- Edo, A.E., Ohenhen, O.A., Adejumo, O.A., & Aisien, A.O. (2019). Diabetic ketoacidosis complicating gestational diabetes mellitus: A case report. Annals of Biomedical Sciences , 18(1).
- Eledrisi, M. S., & Elzouki, A.-N. (2020, August 20). Management of Diabetic Ketoacidosis in Adults: A Narrative Review. Saudi Journal of Medicine and Medical Sciences, 8(3), 165-173.
- Gabbe, S. G., Niebyl, J. R., Simpson, J. L., Landon, M. B., Galan, H. L., Jauniaux, E. R.M., Driscoll, D. A., Berghella, V., & Grobman, W. A. (2017). Obstetrics: Normal and Problem Pregnancies (7th ed.). Elsevier.
- Hwang, D. S., & Bordoni, B. (2021, September 2). Polyhydramnios. Statpearls. Retrieved January 19, 2022.
- Kalinderi, K., Delkos, D., Kalinderis, M., Athanasiadis, A., & Kalogiannidis, I. (2018, February 6). Urinary tract infection during pregnancy: current concepts on a common multifaceted problem. Journal of Obstetrics and Gynaecology, 38(4), 448-453.
- Kapur, K., Kapur, A., & Hod, M. (2021, February 1). Nutrition Management of Gestational Diabetes Mellitus. Annals of Nutrition and Metabolism, 76(suppl 3), 17-29.
- Ko, H. J., Hong, S. Y., & Bae, J. Y. (2021, April 15). Pregnancy and neonatal outcomes of hyperglycemia caused by atosiban administration during pregnancy. Clinical and Experimental Obstetrics and Gynecology, 48(2), 257-262.
- Leifer, G. (2018). Introduction to Maternity and Pediatric Nursing (8th ed.). Elsevier.
- Liu, X., Wu, N., & Al-Mureish, A. (2021, March 30). A Review on Research Progress in the Application of Glycosylated Hemoglobin and Glycated Albumin in the Screening and Monitoring of Gestational Diabetes. International Journal of General Medicine, 14, 1155-1165.
- Lizzo, J. M., Goyal, A., & Gupta, V. (2021, November 20). Adult Diabetic Ketoacidosis – StatPearls. NCBI. Retrieved January 27, 2022 .
- Marcovecchio, M. L., & Chiarelli, F. (2012, October 23). The effects of acute and chronic stress on diabetes control. Science Signaling, 5(247).
- Marwa, K. J., Njalika, A., Ruganuza, D., Katabalo, D., & Kamugisha, E. (2018, January 8). Self-medication among pregnant women attending antenatal clinic at Makongoro health centre in Mwanza, Tanzania: a challenge to health systems. B MC Pregnancy and Childbirth, 18(16).
- Mishra, J., Srivastava, S. K., & Pandey, K. B. (2021, April 1). Compromised Renal and Hepatic Functions and Unsteady Cellular Redox State during Preeclampsia and Gestational Diabetes Mellitus. Archives of Medical Research, 1(38).
- Moradi, M., Salarfard, M., Abedian, Z., Mazloum, S. R., & Farkhani, E. M. (2021, February 1). The Relationship Between Underlying Factors and Treatment Adherence in Women With Gestational Diabetes. Journal of Arak University of Medical Sciences, 23(6).
- Morris, C. H., & Baker, J. (2021, July 21). Glucagon – StatPearls. NCBI. Retrieved January 19, 2022 .
- Nikoloudi, M., Eleftheriadou, I., Tentolouris, A., Kosta, O. A., & Tentolouris, N. (2018, August 1). Diabetic Foot Infections: Update on Management. Current Infectious Disease Reports, 20(40).
- Nyenwe, E. A., Razavi, L. N., Kitabchi, A. E., Khan, A. N., & Wan, J. Y. (2010, May 18). Acidosis: The Prim e Determinant of Depressed Sensorium in Diabetic Ketoacidosis. Diabetes Care, 33(8), 1837-1839.
- Pandraklakis, A., & Pappa, K. (2019, January-March). The management of the macrosomic fetus and the assessment of wellbeing in gestational diabetes mellitus. HJOG An Obstetrics and Gynecology International Journal, 18(1), 11-20.
- Perrier, E. T., Armstrong, L. E., Bottin, J. H., Clark, W. F., Dolci, A., Guelinckx, I., Iroz, A., Kavouras, S. A., Lang, F., Lieberman, H. R., Melander, O., Morin, C., Seksek, I., Stookey, J. D., Tack, I., Vanhaecke, T., Vecchio, M., & Peronnet, F. (2020, July 6). Hydration for health hypothesis: a narrative review of supporting evidence. European Journal of Nutrition, 60, 1167-1180.
- Pillitteri, A., & Silbert-Flagg, J. (2018). Maternal & Child Health Nursing: Care of the Childbearing & Childrearing Family (8th ed.). Wolters Kluwer.
- Sadaqat, A., Batool, H., & Ambreen, A. (2020, January). Diabetes in Pregnancy: High Risk for Vulvovaginal Candidiasis. Annals of King Edward Medical University, 26(1), 72-76.
- Salama, N. I., & Abushaikha, L. (2018, October). Adherence to Clinical Practice Guidelines during Antenatal Management of Gestational Diabetes Mellitus: An Integrative Review. Open Journal of Nursing, 8(10).
- Storme, O., Saucedo, J. T., Mora, A. G.-., Davila, M. D.-., & Naber, K. G. (May, 2019). Risk factors and predisposing conditions for urinary tract infection. Therapeutic Advances in Urology, 11.
- Tiongco, R. E., Arceo, E., Clemente, B., & Cortel, M. R. P. (2018, October 12). Association of maternal iron deficiency anemia with the risk of gestational diabetes mellitus: a meta-analysis. Archives of Gynecology and Obstetrics, 299, 89-95.
- Vermeil, T., Peters, A., Kilpatick, C., Pires, D., Allegranzi, B., & Pittet, D. (2019, April). Hand Hygiene in hospitals: Anatomy of a revolution. Journal of Hospital Infection, 101(4), 383-392.
- Yuen, L., & Wong, V. W. (2015, July 25). Gestational diabetes mellitus: Challenges for different ethnic groups. World Journal of Diabetes, 6(8), 1024-1032.
- Zhang, N., Zhang, F., Chen, S., Han, F., Lin, G., Zhai, Y., He, H., Zhang, J., & Ma, G. (2020, February 7). Associations between hydration state and pregnancy complications, maternal-infant outcomes: protocol of a prospective observational cohort study. BMC Pregnancy and Childbirth, 20(82).
- Zulkarnain, M., Flora, R., Faisya, A. F., Martin, S., & Aguscik. (2019). Dehydration Index and Fatigue Level of Workers Laboring in Heat Exposed Environments. Advances in Health Sciences Research, 25.
Reviewed and updated by M. Belleza, R.N.
Leave a Comment Cancel reply
- Open access
- Published: 11 January 2024
Experiences and self-care of pregnant nurses with gestational diabetes mellitus: a qualitative study
- Jing He ORCID: orcid.org/0000-0001-7654-1872 1 , 2 , 3 ,
- Hui Wang 1 &
- Xiaoli Chen 3
BMC Nursing volume 23 , Article number: 33 ( 2024 ) Cite this article
1011 Accesses
Metrics details
Pregnant nurses are at high risk of developing gestational diabetes mellitus (GDM), and nurses diagnosed with GDM face challenges in balancing disease management and work, which affects maternal and child health and the quality of care. GDM requires significant changes to lifestyle and physical activity to control blood glucose levels, which is key to reducing adverse pregnancy outcomes. However, few studies have focused on the experiences of pregnant nurses with GDM. This study aimed to gain insight into the experiences of pregnant nurses with GDM in China in terms of their illness, work burdens, and self-care.
This qualitative study used an interpretative phenomenological analysis. Face-to-face semi-structured in-depth interviews were conducted with pregnant nurses with GDM to investigate their experiences and self-care. The study was performed at Chongqing’s maternal and child health hospital in China. A purposive sampling was used. Nine pregnant nurses diagnosed with GDM were recruited and interviewed.
The interview data generated four themes and 11 sub-themes. The four themes were ‘the perceptions and feelings of GDM’, ‘experiences of lifestyle changes’, ‘social support needs’, and ‘health expectations and risk perception.’
Many factors such as the unique occupational environment, overwork, occupational pressure, shift work, family status, and education level may lead to difficulties in managing blood glucose in nurses with GDM. These findings suggest that managers should pay more attention to nurses with GDM and develop personalized medical care and work arrangements. These measures can improve the self-care and well-being of nurses with GDM and promote the health of nurses and their offspring.
Peer Review reports
Introduction
According to Chinese health statistics, there were approximately 5.02 million registered nursing staff in the country in 2021. Women accounted for 96.7% of these, and 85% of female nurses were of childbearing age between the ages of 18 to 44 years [ 1 ]. There were 8.47 billion visits to medical and health institutions in China in 2021 while the proportion of registered nurses per 1,000 population was 3.56, indicating a high demand on nursing teams and nursing service quality [ 1 ]. Shen et al. observed that the status quo of nurses’ clinical work represented continuous busyness with regular overtime involving shift relief and the writing up of nursing records and quality control information) [ 2 ]. Occupational challenges, as well as the physical and mental changes occurring during pregnancy, make it difficult for nurses to maintain a healthy lifestyle; these challenges include long working hours, night work, stress, and working in a COVID-19 background, amongst others [ 3 , 4 ].
Studies have shown that specific aspects of nursing work, such as long shifts, can worsen insulin resistance during pregnancy [ 5 ]. Shan et al. and Pan et al. found that night shifts and job-related psychosocial stress were independent risk factors for type 2 diabetes mellitus (T2DM) in nurses [ 6 , 7 ]. In addition, sleep disturbances and stress may lead to insulin resistance, impaired glucose regulation, and the development of T2DM [ 8 , 9 ]. The pathological mechanism of gestational diabetes mellitus (GDM) is similar to that of T2DM and is associated with insulin resistance and islet cell dysfunction [ 10 ]. The results of these studies suggest that pregnant nurses are likely to be at high risk of developing GDM; however, there is little information on the occurrence of GDM in nurses.
Once a pregnant nurse is diagnosed with GDM, daily work tasks and blood glucose management during pregnancy become more challenging. These complex scenarios during pregnancy can exacerbate burnout and job dissatisfaction, as well as limiting effective glycemic management and self-care during pregnancy [ 4 , 11 ]. Weschenfelder et al. found that poor sleep status in women with GDM was independently related to the need to use long-acting insulin at night [ 12 ]. Circadian disruption in shift work can impair blood glucose regulation [ 13 ], and a study from China found that reduced durations of nighttime sleep were associated with poor blood sugar control in women with GDM [ 14 ]. Moreover, GDM increases the risk of short-term adverse perinatal outcomes and long-term metabolic diseases [ 15 , 16 ].
Guidelines from the American Diabetes Association (ADA) recommend that adopting a healthy lifestyle during GDM significantly reduces the risk of adverse outcomes, indicating the importance of blood sugar control and management [ 17 ]. Unfortunately, although nurses with GDM may understand the importance of such requirements, they may not be able to obtain regular exercise, adequate stress management sleep, or eat a healthy diet [ 18 ]. Nurses may also consider it too difficult to balance busy work schedules with regular self-care behaviors during pregnancy [ 4 ]. In addition, nurses with GDM need to practice proper blood glucose management and the self-care of a healthy lifestyle during pregnancy to ensure the health and well-being of both themselves and the fetus [ 4 ]. However, there are no current studies addressing the work experience, blood glucose management, and self-care of pregnant nurses with GDM.
It is thus important to investigate the experiences of nurses with GDM to determine the problems they face and their ability to manage both the disease and self-care during pregnancy to identify and mitigate the possible risks and problems in this field. This study aimed to investigate and analyze the experiences of pregnant nurses with GDM. The findings can provide a comprehensive understanding of the needs and difficulties of nurses with GDM, help to develop personalized nursing management plans, reduce the risk of metabolic diseases caused by GDM, and promote the health of nurses and their offspring.
Study design
This study was undertaken as an interpretative phenomenological analysis (IPA) of qualitative research to explore the experiences and feelings of pregnant nurses with GDM. The purpose of an IPA is to explore how participants understand their own personal and social worlds [ 19 ]. This method can be used to elaborate on the interviewee’s personal views and understanding of the object or events in a specific life experience. Our view of the world consists of the interaction between the raw material of the world and the complex mental framework developed by personal backgrounds and life experiences [ 20 ]. This interaction helps us construct unique interpretations of the world. Researchers need to identify how participants try to make sense of their personal and social worlds in a particular cultural context. The researcher (JH) who conducted the interviews was a female nursing postgraduate student and qualified Psychological Consultant with expert psychological knowledge and interview experience.
This study was approved by the Ethics Committee of the Chongqing Health Center for Women and Children (Number: 2020-022), and participants provided signed informed consent to participate in the study before taking part. The Consolidated Criteria for Reporting Qualitative Studies (COREQ) checklist was used in the study [ 21 ].
Sampling and participants
The study participants were selected using the purposive sampling approach combined with maximum variation (in terms of age, parity, pre-pregnancy body mass index, educational background, and clinical department) [ 22 ]. The pregnant nurses with GDM were interviewed and information was collected. The study used Information Power to determine the sample size; this includes four specifications, namely, a narrow study aim, dense sample specificity, strong dialogue quality, and case strategy [ 23 ]. The obstetrics GDM specialist outpatient nurse was responsible for recruiting participants who met the criteria at Chongqing Health Center for Women and Children. One researcher (JH) was responsible for contacting and identifying participants willing to participate in the study and making a telephone appointment to be interviewed at the next outpatient obstetric visit after the diagnosis of GDM.
The inclusion criteria were as follows; pregnant nurses with professional qualifications, and patients using the 75-gram Oral Glucose Tolerance Test (OGTT) who met the diagnostic criteria of the International Association of Diabetes in Pregnancy Study Group (IADPSG). Any abnormalities in these indicators were diagnosed as GDM. The exclusion criteria were patients with other pregnancy complications or other underlying medical conditions.
Data collection
The data were collected through personal face-to-face semi-structured interviews using the Chinese language. Before the research interview, we introduced the purpose, significance, methods and content of the research to the participants and established a familiar relationship. Written informed consent was obtained from the participants for the recording and taking of notes. The collection site was a quiet office with only one participant and one researcher (JH) in the clinic. The subjects were anonymized with letters and numbers (e.g., G1 = Participant 1).
A total of 20 eligible participants were recruited and 11 nurses declined to participate. Nine pregnant nurses did not have time for the interview and two pregnant nurses did not want to talk about GDM. Each participant was interviewed only once. The interviews were completed from May to October 2020 and lasted on average 41.2 min. The nurses were asked to describe their experiences and feelings after being diagnosed with GDM. The interview questions are shown in Table 1 . All information relating to the interview was kept on secure computers and password protected by two researchers (JH and XC). One researcher translated the Chinese quotations into English while another, a Chinese person living in an English-speaking country, translated them back to ensure that the original meaning was preserved.
Data analysis
The analysis followed the procedures outlined by the IPA [ 24 ]. The specific steps of analysis were: (1) Repeated reading of the transcribed text; (2) Analysis of each code line by line, looking for descriptive, linguistic, and conceptual points of interest, and forming preliminary comments and analysis; (3) Transformation of the initial notes into emerging emotional themes that capture the core of the participants’ experience, leading to the proposal of themes; (4) Cases are collected and converted into a separate topic table, and correlations between the topics are identified; (5) Analysis of subsequent cases, grouping and converting each case into a separate topic table; (6) Comparison of the topics by searching for convergence and divergence, that is, looking for thematic patterns between individual cases. The IPA endorses a two-stage interpretive process, implying that researchers are required to construct meaning by understanding participants [ 24 ].
NVivo12 (QSR International Pty Ltd. Version 10, 2014) was used in the management, shaping, and analysis of the text of qualitative data. The results of data transcription and analysis were returned to the interview subject for confirmation and questions or ambiguities were clarified. Two researchers (JH and XC) acquired, analyzed, summarized, and supplemented the recording materials and field notes within 24 h. The recorded material was transcribed verbatim into text. The participants listened to the recorded material, compared the transcribed material, and added and recorded nonverbal information. Three researchers (JH, HW, and XC) were involved in the coding of the data. HW and XC are nursing professors working in a hospital and university, respectively, with rich experience in nursing management and obstetric care. Different analyses by different researchers could potentially extract different elements from the participants’ accounts. Therefore, a collaborative approach to analysis ensures the comprehensiveness and credibility of the final report [ 25 ].
Participant characteristics
Nine pregnant nurses that had been diagnosed with GDM were interviewed. The participants were between 25 and 33 years old with an average age of 29 years. The interviewees were included five primiparas and four multiparas with pre-pregnancy body mass index (BMI) of 19.5–28.9 kg/m 2 . Three pregnant nurses were educated to junior college level and six nurses were educated to undergraduate level. The characteristics of the participants are summarized in Table 2 .
In-depth interviews were conducted on the experiences and self-care of nine pregnant nurses diagnosed with GDM, and the interview data comprising 571 nodes were analysed and coded. In addition, the interview data formed 11 sub-themes and four themes. The findings were presented as four core themes: the perception and feelings of GDM, experiences of lifestyle changes, social support needs, and health expectations and risk perception (Table 3 ).
Fasting, fasting plasma glucose of oral glucose tolerance test (OGTT); 1-h, OGTT 1-h plasma glucose; 2-h, OGTT 2-h plasma glucose (mmol/L); Pre-BMI, Pre-pregnant body mass index.
The perceptions and feelings of GDM
The first topic of the study was the perception and feelings associated with GDM. Pregnant nurses reported emotional shock at the diagnosis of GDM, which caused them to question their health status. Pregnant nurses with GDM immediately reflected on the influence of diet and exercise on their blood glucose levels based on their medical knowledge. In addition, nurses also expressed perceptions of GDM, including issues such as controllability and disease stigma. Three sub-themes were identified in relation to this topic.
Emotional response to the diagnosis
Pregnant nurses with GDM were interviewed for this study. The pregnant nurses with GDM were psychologically shocked, amazed, lost, and sad when they became aware of their blood glucose results. However, as nurses, as they had medical knowledge and understood that GDM was controllable, these adverse emotional reactions were rapidly alleviated. At this stage, the nurses considered that excessive anxiety over the controllability of blood glucose was unnecessary and that maintaining a good state of mind was important for fetal health. In addition, some nurses said a diagnosis of GDM was a “wake-up call” to past unhealthy eating habits. Typical statements conveying their emotions are shown below.
“My OGTT results were only 0.2 (mmol/l) above the diagnostic criteria 2-h plasma glucose. However, the doctor said it was GDM, although I was reluctant to accept the result… (sighs).” (G1) . “When I got the OGTT results, I was so upset. I couldn’t control my emotions and cried for a while. I began to resist anything sweet and dared not eat fruit.” (G5) .
Self-reflection
Pregnant nurses reported self-reflection on the possible causes of the abnormal OGTT results immediately after the diagnosis, reflecting their professional knowledge and understanding of health. They considered the risk factors for GDM in a medical context, including overindulgence in fruit and sweets, drinking soft drinks, insufficient exercise, obesity, and eating takeaways.
“I know that my pre-pregnancy weight (overweight) and PCOS (polycystic ovary syndrome) are high-risk factors for GDM. I need to stay in a good mood and control my diet, including limiting anything too sweet or salty.” (G3) . “I order takeout at work. I know takeout is unhealthy (a GDM risk), but I was so busy at work that I didn’t have time to choose other healthy foods.” (G5) .
Attitudes and perceptions of GDM
All pregnant nurses with GDM in the study described GDM as a controllable disease, and understood that adverse effects on the fetus could be reduced if the blood glucose is controlled. Several nurses said that GDM is a widespread condition and they should not worry about adverse pregnancy outcomes. Other nurses were reluctant to talk about GDM and felt guilty and stigmatized for their poor blood glucose control. Some nurses interpreted GDM as a threat to their health and wanted to maintain a healthy lifestyle during pregnancy and the postpartum period.
“It (diet management of GDM) is excruciating. I always say, after delivery, I want to eat cake and fruit. I try to control myself deliberately at present.” (G5) .
Experiences of lifestyle changes
The second theme of this study was the indication by pregnant nurses with GDM that lifestyle changes based on existing medical knowledge could help them cope with the complex glycemic management of GDM. Nevertheless, they described the significant challenges involved in maintaining a healthy lifestyle and the difficulty in balancing their busy clinical work and self-care. Regular blood glucose monitoring was unsatisfactory in terms of time and brought emotional turmoil and self-reproach due to pain and abnormal blood glucose levels. Three sub-themes were identified under this theme.
Conflict between diet management and clinical work
We found that pregnant nurses with GDM could appreciate the relationship between the blood glucose results and daily diet and could actively change and adjust their diets. However, many nurses said that that the adjustment to the GDM lifestyle required significant effort especially as they had to balance the proper diet with busy clinical work and shift work. They had to give up dietary management during working hours because strategies to reduce the amount and portion size of meals are difficult to implement. The pregnant nurses with GDM also reported guilt, anxiety, and frustration about the health of the fetus when the lack of dietary management resulted in abnormal blood glucose levels.
“I’m always busy with clinical work and don’t have time to stop. There’s no way to get much time to eat (sigh of resignation). So, I need to eat quickly and eat filling food every time I eat.” (G8) .
Fatigue from physical labor
In this study, pregnant nurses with GDM reported significant levels of fatigue resulting in physical and emotional labor, shift work, and the intense pace of their daily clinical work, with no extra time for physical activity. At the same time, they also indicated that the physical labor of clinical work was heavy, and that the amount of walking done was more than 10,000 steps per day, which they believed met the energy consumption required for GDM. Exhausted from nursing work, nurses described going home and wanting to lie and rest or sleep. In addition, nurses with specific pregnancy conditions, such as artificial insemination or pregnant women with scars for a short time, were cautious and worried about clinical work and exercise.
“I am busy at work (as a nurse). I do approximately 15,000 steps per day. I rarely schedule a time to exercise alone anymore. I am tired, mentally and physically.” (G6) .
Blood glucose monitoring and emotional shock
Pregnant nurses with GDM reported understanding the importance of self-monitoring blood glucose as they were caregivers for others, as well as having a clear understanding of the impact of a healthy lifestyle. Some nurses said blood glucose measurement was convenient in hospital work. At the same time, they hoped to get help from colleagues but did not want to increase the burden on colleagues.
Furthermore, all pregnant nurses with GDM reported that their daily emotions, sleep patterns, and lifestyles were affected by the blood glucose-monitoring results. Meanwhile, the nurses also described guilt and remorse when their blood glucose was abnormal, and they were at a loss. We also found that nurses were resistant to the use of insulin. Even though endocrinologists emphasized the safety of the drug’s use, they were still worried and fearful about possible side effects of the drug on fetal health. This corresponds to the Chinese proverb that drugs are seven parts effective and three parts toxic.
“The doctor advised me to be hospitalized and injected insulin to adjust my blood glucose. I’m scared. But then I got my blood glucose under control with diet and physical activity, and I was so glad.” (G7) . “It’s hard for me to find time to test blood glucose in my clinical work. I occasionally measure my blood glucose and find it extremely high, leaving me devastated and overwhelmed.” (G9) .
Social support needs
The third theme of this study was the stated need by pregnant nurses with GDM for social support and the observation that clinical work stress, such as overburdening with work and specific shift systems, conflicted with the lifestyle management required. Nurses have subject to high levels of expectation for humanistic care from leaders. However, most of the time, family and professional support could help them cope with a complex condition. Three sub-themes were identified under this theme.
Humanistic care needs
Nurses expressed concern that overcrowded working conditions and the need to deal with emergencies resulted in high levels of mental stress, especially during COVID-19, with little energy left over to pay attention to pregnancy status and blood glucose. At the same time, they were also concerned that the working hours of the shift system, especially the night shift, could harm the health of both themselves and the fetus. Most of the nurses in the study said they received care and help from the nursing managers to reduce their work burden as much as possible. For example, nursing managers would reduce clinical work schedules, reduce night shifts, arrange easy administrative work, and other ways to take humanistic care of nurses with GDM.
“We must work until 37 weeks before we can take maternity leave, and most jobs involve lifting patients. So, my nursing work is very stressful and busy. I can’t feel like a pregnant woman, and it was hard (crying). I also didn’t take the time to manage my blood glucose.” (G1) . “Working night shifts during pregnancy was tiring, and the disturbed sleep was terrible for my blood glucose. After work, I want to sleep and do nothing. I am worried about my baby’s health, but in this profession, there is no other way (helpless sigh).” (G9) .
Family support needs
The pregnant nurses with GDM agreed that family support helped with pregnancy and blood glucose management and eased their anxiety. They reported that care and encouragement from their partners provided both emotional support and assistance or participation in encouraging blood glucose management behaviors among nurses. Some pregnant nurses with GDM described feeling happy to be recognized for sharing a healthy lifestyle with family members.
“He (husband) encouraged me to control it (blood glucose).” (G8) . “I share healthy eating ways with my family. They recognize my expertise and eat the same food as I do.” (G6) .
Some pregnant nurses with GDM expressed that ingrained traditional dietary beliefs affected the relationships between the participants and their mothers-in-law. For example, conventional cultural habits believe that eating rich food (such as high carbohydrate foods and the soup of the day) and more fruit is necessary during pregnancy to promote fetal development. The nurses said they understood the elders’ concerns, but that the recommendations would result in overnourishment. Nurses are knowledgeable about nutrition and can adequately self-care in this area.
“I don’t like hearing my mother-in-law’s lessons, such as eating more (high-fat) soup, rice, and fruit, which can promote foetal development. I think these perceptions are problematic. However, they are concerned and ask me to do what they want (helpless smile, sigh).” (G7) .
Available professional support
The pregnant nurses with GDM had a different willingness to learn new GDM knowledge. All nurses were self-rated that they had a basic knowledge of GDM, blood glucose control, and its adverse effects on the fetus and themselves. Most pregnant nurses were not willing to participate in or occasionally participate in the GDM courses offered by the hospital. They explained that they were too busy at work and believed that their medical knowledge was sufficient. However, the study found that pregnant nurses were not sufficiently clear about their knowledge of GDM and were not enthusiastic about professional support. Other nurses stated that it was easier to get help from obstetricians in the hospital, which reduced negative emotions about adverse fetal outcomes. In addition, they could share their blood glucose status with colleagues at any time to get encouragement and comfort.
“I occasionally attend classes at the diabetes day clinic, and also dos online study on GDM. I think that I have adequate knowledge on blood glucose control so this does not need to be learned.” (G7) .
A few other pregnant nurses with GDM actively acquired knowledge of GDM and had good medical knowledge. These nurses felt reassured that enough professional information could help manage the illness and maintain good mental equilibrium. In addition, two nurses expressed horror because they were well aware of the adverse risks of GDM to the fetus and their own high risk of diabetes.
“Although I am a nurse, I do not work in endocrinology or obstetrics and thus I don’t have specific knowledge of GDM. I have taken online courses to learn about GDM. This information and understanding eased my anxiety.” (G4) .
Health expectations and risk perception
The fourth theme identified in this study was the nurses’ perception of the threat of GDM to their own health and that of the fetus. They stated that blood glucose management was the responsibility of the mother and the health of the fetus was the driver of lifestyle change. In addition, pregnant nurses with GDM had concerns and fears about weight, shame, and future health risks. Two sub-themes were identified under this theme.
The responsibilities and expectations of motherhood
Successful behavioral change depends on many factors, and the fetus is the most crucial factor in blood glucose management. The pregnant nurses with GDM expressed responsibility for the health of their babies. We found that pregnant women with GDM had more had an increased knowledge of the adverse pregnancy outcomes that added to their worries concerning their children’s health.
“When I feel terrible about my diet and lack of exercise, I’m very scared. Because I underwent IVF (In-vitro fertilization) many times. Now I have GDM, which makes me even more worried about the effect on the baby.” (G4) . “I’m a little worried, worried… polyhydramnios, macrosomia, miscarriage, premature delivery. Hypoglycaemia after birth… I’m mainly worried about my baby.” (G9) .
Awareness of self-health and image
Most nurses with GDM said they were mainly worried about the baby’s health not themselves. They generally believed that the blood glucose levels would naturally return to normal after childbirth. In addition, some nurses with GDM were well aware that a history of GDM carries a high risk of developing T2DM and felt afraid of financial burden of diabetes. Some GDM nurses also expressed dissatisfaction and anxiety about the impact of excessive weight gain during pregnancy on their self-image, and there was weight shame.
“I’m afraid I will get diabetes. People with diabetes have daily blood glucose tests and insulin injections, and there is no cure.” (G5) . “I’m worried I’ll still be this fat postpartum.” (G2) .
This study explored the experiences and feelings of pregnant nurses in China after a diagnosis of GDM. The results showed that pregnant nurses with GDM have medical knowledge to help them understand the disease and blood glucose management. Most nurses said they understood the importance of a healthy lifestyle for blood glucose but found balancing busy clinical work with blood glucose management challenging. In addition, as nurses take care of others, they spend most of their time taking care of patients, so they often lack the ability or motivation to care for themselves. At the same time, nurses felt helpless about the adverse effects of work overload, especially the demanding working conditions, long working hours, and shift work, on managing GDM and the fetus. Pregnant nurses with GDM expressed their desire to receive more care and special care from nursing managers to minimize adverse pregnancy outcomes.
Pregnant nurses experienced strong feelings of shock, fear, sadness, guilt, and anxiety when diagnosed with GDM. In a review study, women were found to suffer from emotional disorders [ 15 ], and some women experienced excessive fear, helplessness, stigma, and self-blame due to information gaps after a GDM diagnosis [ 26 ]. In this study, patients with medical knowledge of GDM were more inclined to evaluate and analyze their physical condition based on diagnostic criteria and blood glucose levels. Compared to pregnant women with less knowledge of GDM, women with a medical background had a shorter interval between the shock of diagnosis and emotional plateau and had confidence in proper glycaemic control.
Pregnant nurses with GDM face many challenges in learning to adjust their diet and exercise habits. Social and cultural backgrounds also influence eating habits. For example, rice and pasta are the main meals in China, so it is difficult to adjust the intake of staple foods [ 27 ]. However, it was found to be often difficult for nurses with GDM to balance dietary management and demanding clinical work during pregnancy. Failure to manage blood glucose levels adequately can significantly increase the anxiety and stress during pregnancy [ 15 , 28 ].
Physical activity is another crucial factor in controlling blood glucose levels. The nurses said they took over 10,000 steps daily during their clinical work. Most nurses with GDM stated that they were physically and mentally exhausted due to the high work load and long-term mental stress and were not able to arrange extra time for physical activities [ 5 ]. Moreover, all pregnant nurses with GDM recognized the importance and convenience of monitoring blood glucose. However, only a few nurses with GDM conducted regular blood glucose monitoring, and the busy work pace was an obstacle to blood glucose monitoring. However, women with GDM found that abnormal blood glucose readings increased their feelings of guilt and self-blame [ 29 ]. Both nurses and women in general with GDM were found to poor compliance with routine monitoring, which is significant for neonatal outcomes [ 30 ].
In the study, all nurses with GDM used lifestyle changes to control blood glucose, and expressed rejection, worry, and fear about the use of insulin. The pros, cons, and importance of drug therapy and blood glucose management should be emphasized and refined in GDM health guidance [ 15 , 31 ]. Furthermore, nurses with GDM experience pregnancy stress and emotional stress in addition to their demanding clinical work and shift-work loads, which can further worsen their islet function [ 12 ]. Pregnant nurses experience various difficulties due to the physical and mental changes occurring during pregnancy, and the demanding clinical working environment requires greater humanistic care [ 3 ]. Support from partners and other family members can ease a woman’s anxiety during pregnancy and increase her confidence and enthusiasm in managing GDM [ 32 ].
Studies have shown that educational courses about GDM were considered feasible for learning about glycemic management and risk perception [ 15 , 33 ]. While the nurses with GDM lacked precise relevant knowledge, the study found that they took the initiative to acquire knowledge about GDM when needed and could easily understand it. Poor management of GDM in women in general is associated with a lack of adequate and understandable health education [ 34 ]. In addition, Dayyani et al. emphasized that a false sense of security should not arise by informing the offspring of the possibility of blood glucose recovery after birth [ 26 ].
We found that women’s focus and concern for fetal health is the key promoter of glucose management, which agrees with many previous studies [ 35 , 36 ]. However, some nurses with GDM described fear and stigma associated with the possibility of adverse risk outcomes, and expressed guilt or anxiety about poor glycemic management behavior [ 37 , 38 ]. The stigma associated with body image resulting from excess weight gain during pregnancy and obesity is also widely recognized [ 39 ]. During the interview, we learned that most women with GDM are aware of the risk of type 2 diabetes, but the risk awareness is related to education level and medical background. In general, health awareness and risk consciousness are related to the level of knowledge of GDM [ 15 ].
Pregnancy and GDM experienced by nurses, who are core providers of health services, should not be seen as problems that individuals suffer in isolation [ 18 ]. Pregnant nurses with GDM should receive care from nursing managers, active improvements to their working environment, and encouragement for self-care and the promotion of their well-being experience and the ability of nurses with GDM to self-care, we can lay a foundation for providing accurate and personalized medical services and management measures.
Study limitations
Our study had several limitations. Although the researchers and participants were in a quiet and undisturbed office during the interview, it was inevitable that participants would be interrupted by calls they needed to answer, which may have impacted their thinking during the interview. As with all qualitative studies, the intent of these results is not to generalize, as they are specific to the participants’ experiences, which were those of a relatively small number of nurses with GDM. The physical and mental burdens of pregnant nurses with GDM increased during COVID-19 with the additional tasks of nucleic acid testing and high levels of patient management. The findings provide an understanding of the experiences of pregnant nurses during that disastrous era.
Conclusions
The study’s results provide information on nurses’ emotional responses, disease experiences, and self-care abilities after being diagnosed with GDM. In addition, many factors, such as unique occupational environment, demanding and pressured work often leading to overwork, long hours and shift work, family status, and education level may lead to difficulties in the management of blood glucose levels in nurses with GDM. Based on these findings, we strongly encourage nurses and healthcare managers to pay more attention to pregnant nurses with GDM and develop personalized medical care and work arrangements. These measures could improve self-care and well-being in nurses with GDM and promote the health of both the nurses and their offspring.
Data availability
All data generated or analyzed during this study are included in this published article.
National Health Commission of the People’s Republic of China. China health statistical yearbook(2022). Beijing: China Union Medical College Press: National Health Commission; 2022.
Google Scholar
Shen L, Bao L, Huang Y. Nurses’ experiences of time pressure in clinical work: a qualitative study. Chin Nurs Manage. 2023;23:599–603. https://doi.org/10.3969/j.issn.1672-1756.2023.04.023 .
Article Google Scholar
Lee H, Chang HE, Ha J. Nurses’ Clinical Work Experience during Pregnancy. Healthc (Basel). 2020;9. https://doi.org/10.3390/healthcare9010016 .
Rainbow JG, Dolan HR, Farland L. Nurses’ experiences of working while pregnant: A qualitative descriptive study. Int J Nurs Stud. 2021;124104092. https://doi.org/10.1016/j.ijnurstu.2021.104092 .
Wallace DA, Reid K, Grobman WA, Facco FL, Silver RM, Pien GW, et al. Associations between evening shift work, irregular sleep timing, and gestational Diabetes in the Nulliparous pregnancy outcomes study: monitoring mothers-to-be (nuMoM2b). Sleep. 2022. https://doi.org/10.1093/sleep/zsac297 .
Article PubMed PubMed Central Google Scholar
Shan Z, Li Y, Zong G, Guo Y, Li J, Manson JE, et al. Rotating night shift work and adherence to unhealthy lifestyle in predicting risk of type 2 Diabetes: results from two large US cohorts of female nurses. BMJ. 2018;363k4641. https://doi.org/10.1136/bmj.k4641 .
Pan KY, Xu W, Mangialasche F, Fratiglioni L, Wang HX. Work-related psychosocial stress and the risk of type 2 Diabetes in later life. J Intern Med. 2017;281:601–10. https://doi.org/10.1111/joim.12615 .
Article CAS PubMed Google Scholar
Li Y, Gao X, Winkelman JW, Cespedes EM, Jackson CL, Walters AS, et al. Association between sleeping difficulty and type 2 Diabetes in women. Diabetologia. 2016;59:719–27. https://doi.org/10.1007/s00125-015-3860-9 .
Article CAS PubMed PubMed Central Google Scholar
Heraclides AM, Chandola T, Witte DR, Brunner EJ. Work stress, obesity and the risk of type 2 Diabetes: gender-specific bidirectional effect in the Whitehall II study. Obes (Silver Spring). 2012;20:428–33. https://doi.org/10.1038/oby.2011.95 .
American Diabetes Association. 2. Classification and diagnosis of Diabetes: standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44:15–s33. https://doi.org/10.2337/dc21-S002 .
Miller E, Hutzel-Dunham E. Prioritizing self-care of nurses. Pain Manag Nurs. 2022;23:689–90. https://doi.org/10.1016/j.pmn.2022.10.004 .
Article PubMed Google Scholar
Weschenfelder F, Lohse K, Lehmann T, Schleußner E, Groten T. Circadian rhythm and gestational Diabetes: working conditions, sleeping habits and lifestyle influence insulin dependency during pregnancy. Acta Diabetol. 2021;58:1177–86. https://doi.org/10.1007/s00592-021-01708-8 .
McHill AW, Wright KP. Jr. Role of sleep and circadian disruption on energy expenditure and in metabolic predisposition to human obesity and metabolic Disease. Obes Rev. 2017;18(Suppl 1):15–24. https://doi.org/10.1111/obr.12503 .
Li Y, Gong Y. Correlation between sleep lasting time and blood glucose control in patients with gestational Diabetes Mellitus. Chongqing Med. 2017;46:4350–4. https://doi.org/10.3969/j.issn.1671-8348.2017.31.010 .
He J, Chen X, Wang Y, Liu Y, Bai J. The experiences of pregnant women with gestational Diabetes Mellitus: a systematic review of qualitative evidence. Rev Endocr Metab Disord. 2021;22:777–87. https://doi.org/10.1007/s11154-020-09610-4 .
McIntyre HD, Catalano P, Zhang C, Desoye G, Mathiesen ER, Damm P. Gestational Diabetes Mellitus. Nat Rev Dis Primers. 2019;548. https://doi.org/10.1038/s41572-019-0104-1 .
ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D et al. 15. Management of Diabetes in Pregnancy: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46,S254-s266. https://doi.org/10.2337/dc23-S015 .
Ross A, Yang L, Wehrlen L, Perez A, Farmer N, Bevans M. Nurses and health-promoting self-care: do we practice what we preach? J Nurs Manag. 2019;27:599–608. https://doi.org/10.1111/jonm.12718 .
Smith JA. Interpretative phenomenological analysis: getting at lived experience. J Posit Psychol. 2017;12:303–4. https://doi.org/10.1080/17439760.2016.1262622 .
Birtchnell J. The interpreted world: an introduction to phenomenological psychology (second edition). Psychol Psychotherapy-Theory Res Pract. 2005;78:568–9. https://doi.org/10.1348/147608305x71486 .
Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19:349–57. https://doi.org/10.1093/intqhc/mzm042 .
Yunxain Z. Quality of nursing research: theory and cases. Zhejiang: Zhejiang University Press. ;; 2017. (Chinese book).
Malterud K, Siersma VD, Guassora AD. Sample size in qualitative interview studies: guided by Information Power. Qual Health Res. 2016;26:1753–60. https://doi.org/10.1177/1049732315617444 .
Smith J, Flowers P, Larkin M. Interpretative phenomenological analysis: theory, Method and Research. London: SAGE; 2009.
Brocki JM, Wearden AJ. A critical evaluation of the use of interpretative phenomenological analysis (IPA) in health psychology. Psychol Health. 2006;21:87–108. https://doi.org/10.1080/14768320500230185 .
Dayyani I, Maindal T, Rowlands H, Lou G. A qualitative study about the experiences of ethnic minority pregnant women with gestational Diabetes. Scand J Caring Sci. 2019;33:621–31. https://doi.org/10.1111/scs.12655 .
Cui M, Li X, Yang C, Wang L, Lu L, Zhao S, et al. Effect of carbohydrate-restricted dietary pattern on insulin treatment rate, lipid metabolism and nutritional status in pregnant women with gestational Diabetes in Beijing, China. Nutrients. 2022;14. https://doi.org/10.3390/nu14020359 .
Martis R, Brown J, McAra-Couper J, Crowther CA. Enablers and barriers for women with gestational Diabetes Mellitus to achieve optimal glycaemic control - a qualitative study using the theoretical domains framework. BMC Pregnancy Childbirth. 2018;1891. https://doi.org/10.1186/s12884-018-1710-8 .
Dingena CF, Holmes MJ, Campbell MD, Cade JE, Scott EM, Zulyniak MA. Observational assessments of the relationship of dietary and pharmacological treatment on continuous measures of dysglycemia over 24 hours in women with gestational Diabetes. Front Endocrinol. 2023;141065985–1065985. https://doi.org/10.3389/fendo.2023.1065985 .
He J, Song J, Zou Z, Fan X, Tian R, Xu J, et al. Association between neonatal hyperbilirubinemia and hypoglycemia in Chinese women with Diabetes in pregnancy and influence factors. Sci Rep. 2022;1216975. https://doi.org/10.1038/s41598-022-21114-6 .
Hjelm K, Bard K, Apelqvist J. A qualitative study of developing beliefs about health, Illness and healthcare in migrant African women with gestational Diabetes living in Sweden. BMC Womens Health. 2018;1834. https://doi.org/10.1186/s12905-018-0518-z .
Neven ACH, Lake AJ, Williams A, O’Reilly SL, Hendrieckx C, Morrison M, et al. Barriers to and enablers of postpartum health behaviours among women from diverse cultural backgrounds with prior gestational Diabetes: a systematic review and qualitative synthesis applying the theoretical domains framework. Diabet Med. 2022;39. https://doi.org/10.1111/dme.14945 .
Helmersen M, Sørensen M, Lukasse M, Laine HK, Garnweidner-Holme L. Women’s experience with receiving advice on diet and self-monitoring of blood glucose for gestational Diabetes Mellitus: a qualitative study. Scand J Prim Health Care. 2021;39:44–50. https://doi.org/10.1080/02813432.2021.1882077 .
Mukuve A, Noorani M, Sendagire I, Mgonja M. Magnitude of screening for gestational Diabetes Mellitus in an urban setting in Tanzania; a cross-sectional analytic study. BMC Pregnancy Childbirth. 2020;20418. https://doi.org/10.1186/s12884-020-03115-3 .
Banerjee AT, McTavish S, Ray JG, Gucciardi E, Lowe J, Feig D, et al. Reported Health Behaviour changes after a diagnosis of Gestational Diabetes Mellitus among ethnic minority women living in Canada. J Immigr Minor Health. 2016;18:1334–42. https://doi.org/10.1007/s10903-015-0266-1 .
Youngwanichsetha S, Phumdoung S. Lived experience of blood glucose self-monitoring among pregnant women with gestational Diabetes Mellitus: a phenomenological research. J Clin Nurs. 2017;26:2915–21. https://doi.org/10.1111/jocn.13571 .
Nahum Sacks K, Friger M, Shoham-Vardi I, Abokaf H, Spiegel E, Sergienko R, et al. Prenatal exposure to gestational Diabetes Mellitus as an Independent risk factor for long-term neuropsychiatric morbidity of the offspring. Am J Obstet Gynecol. 2016;215:380e381–387. https://doi.org/10.1016/j.ajog.2016.03.030 .
Davidsen E, Maindal HT, Rod MH, Olesen K, Byrne M, Damm P, et al. The stigma associated with gestational Diabetes Mellitus: a scoping review. eClinicalMedicine. 2022;52. https://doi.org/10.1016/j.eclinm.2022.101614 .
Sercekus P, Isbir GG, Bakan G. Being overweight or obese during pregnancy: a qualitative study. J Maternal-Fetal Neonatal Med. 2021. https://doi.org/10.1080/14767058.2021.1946777 .
Download references
Acknowledgements
We thank all pregnant nurses for participating and sharing their experiences.
This work was supported by the Wuhan Nursing Association in China (Number WHHL202201) and the Research Major Project for Tongji Hospital of Tongji Medical College of Huazhong University of Science and Technology (Number 2022C06).
Author information
Authors and affiliations.
Nursing department, Tongji Hospital of Tongji Medical College of Huazhong, University of Science and Technology, NO.1095 Jiefang Avenue, Wuhan, China
Jing He & Hui Wang
School of Nursing, Tongji Medical College of Huazhong, University of Science and Technology, NO.13 Aviation Road, Wuhan, China
School of Nursing, Wuhan University, NO. 115 Donghu Road, Wuhan, China
Jing He & Xiaoli Chen
You can also search for this author in PubMed Google Scholar
Contributions
J.H. drafted the manuscript. J.H., X.C., and H.W. contributed to the concept and design of the study. J.H., H.W., and X.C. have contributed to the acquisition, analysis, or interpretation of data. X.C. provided research supervision. H.W. critically modified the content of the manuscript. They have complete access to all the data of the study, and the patients are responsible for the integrity of the data and the accuracy of the data analysis.
Corresponding authors
Correspondence to Hui Wang or Xiaoli Chen .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Ethics approval and consent to participate
All procedures involving human participants in this study comply with institutional and national Research Council ethical standards and the Declaration of the Helsinki Medical Society. This study involves human participants and was approved by the Ethics Committee of Chongqing Health Center for Women and Children (Number: 2020-022). Participants gave informed consent and signature to participate in the study before taking part.
Consent for publication
Not applicable.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
He, J., Wang, H. & Chen, X. Experiences and self-care of pregnant nurses with gestational diabetes mellitus: a qualitative study. BMC Nurs 23 , 33 (2024). https://doi.org/10.1186/s12912-023-01679-x
Download citation
Received : 30 April 2023
Accepted : 20 December 2023
Published : 11 January 2024
DOI : https://doi.org/10.1186/s12912-023-01679-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Gestational diabetes mellitus
- Qualitative research
- Disease experiences
BMC Nursing
ISSN: 1472-6955
- General enquiries: [email protected]
Maternal hemodynamic changes in gestational diabetes: a prospective case–control study
- Maternal-Fetal Medicine
- Published: 26 October 2021
- Volume 306 , pages 357–363, ( 2022 )
Cite this article
- Federico Mecacci 1 , 2 ,
- Serena Ottanelli 2 ,
- Silvia Vannuccini ORCID: orcid.org/0000-0001-5790-587X 2 ,
- Sara Clemenza 1 ,
- Federica Lisi 1 ,
- Caterina Serena 2 ,
- Marianna Pina Rambaldi 2 ,
- Serena Simeone 2 ,
- Ilaria Pisani 3 ,
- Felice Petraglia 1 na1 &
- Herbert Valensise 3 na1
350 Accesses
6 Citations
Explore all metrics
The aim of the study is to compare maternal hemodynamic adaptations in gestational diabetes (GDM) versus healthy pregnancies.
A prospective case–control study was conducted, comparing 69 singleton pregnancies with GDM and 128 controls, recruited between September 2018 and April 2019 in Maternal–Fetal Medicine Unit, Careggi University Hospital, Florence, Italy. Hemodynamic assessment by UltraSonic Cardiac Output Monitor (USCOM) was performed in both groups in four gestational age intervals: 17–20 weeks (only in early GDM cases), 26–30 weeks, 32–35 weeks and 36–39 weeks. We evaluated six hemodynamic parameters comparing GDM cases versus controls: cardiac output (CO), cardiac index (CI), stroke volume (SV), total vascular resistance (TVR), inotropy index (INO) and potential to kinetic energy ratio (PKR).
GDM group had significantly lower values of CO and SV than controls from the early third trimester (26–30 weeks) until term ( p < 0.001). CI is significantly lower in GDM women already at the first evaluation ( p = 0.002), whereas TVR and PKR were significantly higher in GDM ( p < 0.001). GDM women showed also lower INO values than controls in all assessments.
Conclusions
A hemodynamic maternal maladaptation to pregnancy can be detected in GDM women. The effect of hyperglycemia on vascular system or a poor pre-pregnancy cardiovascular (CV) reserve could explain this hemodynamic maladaptation. The abnormal CV response to pregnancy in GDM women may reveal a predisposition to develop CV disease later in life and might help in identifying patients who need a CV follow‐up.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others
Evaluation of fetal cardiac function in pregnancies with well-controlled gestational diabetes
F. D’Ambrosi, G. Rossi, … E. Ferrazzi
Maternal cardiovascular hemodynamics in normotensive versus preeclamptic pregnancies: a prospective longitudinal study using a noninvasive cardiac system (NICaS™)
Anat Lavie, Maya Ram, … Ariel Many
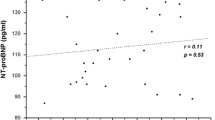
Relationship between maternal hemodynamics and plasma natriuretic peptide concentrations during pregnancy complicated by preeclampsia and fetal growth restriction
S R Giannubilo, A Pasculli, … A Ciavattini
Metzger BE, Lowe LP, Dyer AR et al (2008) Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 358:1991–2002. https://doi.org/10.1056/NEJMoa0707943
Article PubMed Google Scholar
Bryson CL, Ioannou GN, Rulyak SJ, Critchlow C (2003) Association between gestational diabetes and pregnancy-induced hypertension. Am J Epidemiol 158:1148–1153. https://doi.org/10.1093/aje/kwg273
Ostlund I, Haglund B, Hanson U (2004) Gestational diabetes and preeclampsia. Eur J Obstet Gynecol Reprod Biol 113:12–16. https://doi.org/10.1016/j.ejogrb.2003.07.001
Schneider S, Freerksen N, Röhrig S et al (2012) Gestational diabetes and preeclampsia–similar risk factor profiles? Early Hum Dev 88:179–184. https://doi.org/10.1016/j.earlhumdev.2011.08.004
Kessous R, Shoham-Vardi I, Pariente G et al (2013) An association between gestational diabetes mellitus and long-term maternal cardiovascular morbidity. Heart 99:1118–1121. https://doi.org/10.1136/heartjnl-2013-303945
Tobias DK, Stuart JJ, Li S et al (2017) Association of history of gestational diabetes with long-term cardiovascular disease risk in a large prospective cohort of US women. JAMA Intern Med 177:1735–1742. https://doi.org/10.1001/jamainternmed.2017.2790
Article PubMed PubMed Central Google Scholar
Mosca L, Benjamin EJ, Berra K et al (2011) Effectiveness-based guidelines for the prevention of cardiovascular disease in women–2011 update: a guideline from the American Heart Association. J Am Coll Cardiol 57:1404–1423. https://doi.org/10.1016/j.jacc.2011.02.005
Weissgerber TL, Mudd LM (2015) Preeclampsia and diabetes. Curr Diab Rep 15:9. https://doi.org/10.1007/s11892-015-0579-4
Article CAS PubMed Google Scholar
Thilaganathan B (2018) Pre-eclampsia and the cardiovascular-placental axis. Ultrasound Obstet Gynecol 51:714–717. https://doi.org/10.1002/uog.19081
Foo FL, Mahendru AA, Masini G et al (2018) Association between prepregnancy cardiovascular function and subsequent preeclampsia or fetal growth restriction. Hypertens (Dallas, Tex 1979) 72:442–450
Article CAS Google Scholar
Valensise H, Vasapollo B, Gagliardi G, Novelli GP (2008) Early and late preeclampsia: two different maternal hemodynamic states in the latent phase of the disease. Hypertens (Dallas, Tex 1979) 52:873–880
Melchiorre K, Sharma R, Thilaganathan B (2014) Cardiovascular implications in preeclampsia: an overview. Circulation 130:703–714. https://doi.org/10.1161/CIRCULATIONAHA.113.003664
Tiralongo GM, Pisani I, Vasapollo B et al (2018) Effect of a nitric oxide donor on maternal hemodynamics in fetal growth restriction. Ultrasound Obstet Gynecol 51:514–518. https://doi.org/10.1002/uog.17454
Mecacci F, Avagliano L, Lisi F et al (2020) Fetal growth restriction: does an integrated maternal hemodynamic-placental model fit better? Reprod Sci. https://doi.org/10.1007/s43032-020-00393-2
Savvidou MD, Anderson JM, Kaihura C, Nicolaides KH (2010) Maternal arterial stiffness in pregnancies complicated by gestational and type 2 diabetes mellitus. Am J Obstet Gynecol 203:274.e1–7. https://doi.org/10.1016/j.ajog.2010.06.021
Article Google Scholar
Moodley S, Arunamata A, Stauffer KJ et al (2018) Maternal arterial stiffness and fetal cardiovascular physiology in diabetic pregnancy. Ultrasound Obstet Gynecol 52:654–661. https://doi.org/10.1002/uog.17528
Metzger BE, Gabbe SG, Persson B et al (2010) International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 33:676–682. https://doi.org/10.2337/dc09-1848
Kager CCM, Dekker GA, Stam MC (2009) Measurement of cardiac output in normal pregnancy by a non-invasive two-dimensional independent Doppler device. Aust N Z J Obstet Gynaecol 49:142–144. https://doi.org/10.1111/j.1479-828x.2009.00948.x
Smith BE, Madigan VM (2013) Non-invasive method for rapid bedside estimation of inotropy: theory and preliminary clinical validation. Br J Anaesth 111:580–588. https://doi.org/10.1093/bja/aet118
Osman MW, Nath M, Khalil A et al (2018) Haemodynamic differences amongst women who were screened for gestational diabetes in comparison to healthy controls. Pregnancy Hypertens 14:23–28. https://doi.org/10.1016/j.preghy.2018.07.007
Webber J, Charlton M, Johns N (2015) Diabetes in pregnancy: management of diabetes and its complications from preconception to the postnatal period (NG3). Br J Diabetes 15:107–111
Heitritter SM, Solomon CG, Mitchell GF et al (2005) Subclinical inflammation and vascular dysfunction in women with previous gestational diabetes mellitus. J Clin Endocrinol Metab 90:3983–3988. https://doi.org/10.1210/jc.2004-2494
Kramer CK, Campbell S, Retnakaran R (2019) Gestational diabetes and the risk of cardiovascular disease in women: a systematic review and meta-analysis. Diabetologia 62:905–914. https://doi.org/10.1007/s00125-019-4840-2
Retnakaran R, Shah BR (2019) Glucose screening in pregnancy and future risk of cardiovascular disease in women: a retrospective, population-based cohort study. Lancet Diabetes Endocrinol 7:378–384. https://doi.org/10.1016/S2213-8587(19)30077-4
Franzago M, Fraticelli F, Stuppia L, Vitacolonna E (2019) Nutrigenetics, epigenetics and gestational diabetes: consequences in mother and child. Epigenetics 14:215–235. https://doi.org/10.1080/15592294.2019.1582277
Monteiro LJ, Norman JE, Rice GE, Illanes SE (2016) Fetal programming and gestational diabetes mellitus. Placenta 48(Suppl 1):S54–S60. https://doi.org/10.1016/j.placenta.2015.11.015
Rao R, Sen S, Han B et al (2014) Gestational diabetes, preeclampsia and cytokine release: similarities and differences in endothelial cell function. Adv Exp Med Biol 814:69–75. https://doi.org/10.1007/978-1-4939-1031-1_6
Carpenter MW (2007) Gestational diabetes, pregnancy hypertension, and late vascular disease. Diabetes Care 30(Suppl 2):S246–S250. https://doi.org/10.2337/dc07-s224
Mastrogiannis DS, Spiliopoulos M, Mulla W, Homko CJ (2009) Insulin resistance: the possible link between gestational diabetes mellitus and hypertensive disorders of pregnancy. Curr Diab Rep 9:296–302. https://doi.org/10.1007/s11892-009-0046-1
Yogev C, Hod, et al (2010) Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study: preeclampsia. Am J Obstet Gynecol 202:255.e1–7. https://doi.org/10.1016/j.ajog.2010.01.024
Hauth JC, Clifton RG, Roberts JM et al (2011) Maternal insulin resistance and preeclampsia. Am J Obstet Gynecol 204:327.e1–6. https://doi.org/10.1016/j.ajog.2011.02.024
Khalil A, Garcia-Mandujano R, Chiriac R et al (2012) Maternal hemodynamics at 11–13 weeks’ gestation in gestational diabetes mellitus. Fetal Diagn Ther 31:216–220. https://doi.org/10.1159/000336692
Hartling L, Dryden DM, Guthrie A et al (2013) Benefits and harms of treating gestational diabetes mellitus: a systematic review and meta-analysis for the U.S. Preventive Services Task Force and the National Institutes of Health Office of Medical Applications of Research. Ann Intern Med 159:123–129. https://doi.org/10.7326/0003-4819-159-2-201307160-00661
Spaight C, Gross J, Horsch A, Puder JJ (2016) Gestational diabetes mellitus. Endocr Dev 31:163–178. https://doi.org/10.1159/000439413
Download references
Acknowledgements
The authors have no acknowledgements to make.
The author declares no funding sources.
Author information
Felice Petraglia and Herbert Valensise have contributed equally to this work.
Authors and Affiliations
Department of Biomedical, Experimental and Clinical Sciences, University of Florence, Careggi University Hospital, Florence, Italy
Federico Mecacci, Sara Clemenza, Federica Lisi & Felice Petraglia
High Risk Pregnancy Unit, Careggi University Hospital, Largo Brambilla 3, 50134, Florence, Italy
Federico Mecacci, Serena Ottanelli, Silvia Vannuccini, Caterina Serena, Marianna Pina Rambaldi & Serena Simeone
Department of Surgery, University “Tor Vergata”, Policlinico Casilino, Rome, Italy
Ilaria Pisani & Herbert Valensise
You can also search for this author in PubMed Google Scholar
Contributions
FM contributed to the conception and design of the study. SC, FL, GM, SC, MPR, SS, IP contributed to patient recruitment and collection of data. SO, SV, GM and SC collaborated on data analysis and first draft writing. FM substantially contributed to the interpretation of data. FM, FP and HV revised the manuscript for important intellectual content. All authors approved the final version.
Corresponding author
Correspondence to Silvia Vannuccini .
Ethics declarations
Conflict of interest.
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This study was approved by the Careggi University Hospital Research Ethics Committee in September 2018 (study number 13623).
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Mecacci, F., Ottanelli, S., Vannuccini, S. et al. Maternal hemodynamic changes in gestational diabetes: a prospective case–control study. Arch Gynecol Obstet 306 , 357–363 (2022). https://doi.org/10.1007/s00404-021-06288-0
Download citation
Received : 26 December 2020
Accepted : 13 October 2021
Published : 26 October 2021
Issue Date : August 2022
DOI : https://doi.org/10.1007/s00404-021-06288-0

Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Gestational diabetes
- Maternal hemodynamics
- Cardiovascular disease
- Cardiac output
- Total vascular resistance
- Inotropy index
- Find a journal
- Publish with us
- Track your research
Gestational diabetes
The two case studies presented here provide an overview of gestational diabetes (GDM). The scenarios cover the screening, identification and management of GDM, as well as the steps that should be taken to screen for, and ideally prevent, development of type 2 diabetes in the long term post-pregnancy. By actively engaging with the case studies, readers will feel more confident and empowered to manage GDM effectively in the future.
Useful resources
At a glance factsheet: Diabetes before, during and after pregnancy. Diabetes & Primary Care 23 : 73–4
Bellamy L, Casas JP, Hingorani AD, Williams D (2009) Type 2 diabetes mellitus after gestational diabetes: A systematic review and meta-analysis. Lancet 373 : 1773–9
Catalano PM (2014) Trying to understand gestational diabetes. Diabet Med 31 : 273–81
ElSayed NA, Aleppo G, Aroda VR et al; American Diabetes Association (2023a) 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes–2023. Diabetes Care 46 (Suppl 1): S19–40
ElSayed NA, Aleppo G, Aroda VR et al; American Diabetes Association (2023b) 15. Management of Diabetes in Pregnancy: Standards of Care in Diabetes–2023. Diabetes Care 46 (Suppl 1): S254–66
Horvath K, Koch K, Jeitler K et al (2010) Effects of treatment in women with gestational diabetes mellitus: Systematic review and meta-analysis. BMJ 340 : c1395
Iftakhar R (2012) Benefit of metformin in reducing weight gain and insulin requirements in pregnancies complicated by gestational diabetes. Diabesity in Practice 3 : 108–13
Knowler WC, Barrett-Connor E, Fowler SE et al; Diabetes Prevention Program Research Group (2002) Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346 : 393–403
Lindström J, Louheranta A, Mannelin M et al; Finnish Diabetes Prevention Study Group (2003) The Finnish Diabetes Prevention Study (DPS): Lifestyle intervention and 3-year results on diet and physical activity. Diabetes Care 26 : 3230–6
McGovern A, Butler L, Munro M, de Lusignan S (2014) Gestational diabetes mellitus follow-up in primary care: A missed opportunity. Diabetes & Primary Care 16 : 60
Meltzer SJ (2010) Treatment of gestational diabetes. BMJ 340 : c1708
NICE (2017) Type 2 diabetes: prevention in people at high risk [PH38]. Available at: https://www.nice.org.uk/guidance/ph38
NICE (2020) Diabetes in pregnancy: management from preconception to the postnatal period [NG3]. Available at: https://www.nice.org.uk/guidance/ng3
NICE (2022) Type 2 diabetes in adults: management [NG28]. Available at: https://www.nice.org.uk/guidance/ng28
Noctor E, Dunne F (2017) A practical guide to pregnancy complicated by diabetes. Diabetes & Primary Care 19 : 35–4
Rowan JA, Hague WM, Gao W et al; MiG Trial Investigators (2008) Metformin versus insulin for the treatment of gestational diabetes. N Engl J Med 358 : 2003–15
Silverman BL, Rizzo TA, Cho NH, Metzger BE (1998) Long-term effects of the intrauterine environment. The Northwestern University Diabetes in Pregnancy Center. Diabetes Care 21 (Suppl 2): B142–9
Questions Summary
0 of 17 Questions completed
Information
You have already completed the questions before. Hence you can not start it again.
Questions is loading…
You must sign in or sign up to start the questions.
You must first complete the following:
Questions complete. Results are being recorded.
Time has elapsed
- Not categorized 0%
1 . Question
Section 1 – holly.
Holly is a 31-year-old lady who is now 26 weeks into her first pregnancy. She sees you with a 3-day history of dysuria and frequency of micturition. There is no history of abdominal pain or fever.
A urine dipstick reveals a positive test for nitrites and the presence of white cells. It also shows glycosuria ++.
What is your assessment of Holly’s situation?
This response will be awarded full points automatically, but it can be reviewed and adjusted after submission.
2 . Question
Holly appears to have a lower urinary tract infection (UTI). The glycosuria suggests she may have gestational diabetes (GDM), although it is possible she may have entered the pregnancy with undiagnosed type 2 diabetes.
What factors would you look for in Holly’s history that could suggest she is at high risk of gestational diabetes?
3 . Question
Risk factors for developing GDM include (NICE, 2020):
- BMI >30 kg/m 2 .
- Previous GDM.
- Previous macrosomic baby weighing 4.5 kg or more.
- Family history of diabetes (first-degree relative).
- Ethnic background with high prevalence of diabetes (South or East Asian, African–Caribbean, Middle Eastern).
Further considerations regarding the likelihood of GDM include advanced maternal age (>35 years), a history of polycystic ovarian syndrome (a condition of increased insulin resistance) and previous unexplained fetal death.
Holly had a pre-pregnancy BMI of 29.4 kg/m 2 but no family history of type 2 diabetes.
What action would you take?
4 . Question
In addition to treating the UTI, Holly needs to be investigated for possible GDM.
Holly is prescribed a course of cephalexin (a safe and effective antibiotic in pregnancy) and encouraged to drink plenty of fluids. A mid-stream urine sample is sent off for laboratory analysis. A fingerprick glucose reading demonstrates a glucose level of 11.7 mmol/L. A blood sample is sent off for HbA 1c assessment (HbA 1c will not accurately reflect recent onset of diabetes in a pregnancy but could point toward the likelihood of pre-existing undiagnosed type 2 diabetes when entering the pregnancy).
NICE (2020) recommends formal testing for GDM if pregnant women have:
- Glycosuria of ++ or above on one occasion.
- Glycosuria of + or above on more than one occasion.
In Holly’s case, local maternity services are contacted and an oral glucose tolerance test (OGTT) is arranged to ascertain the diagnosis of GDM. In the meantime, the importance of controlling blood glucose levels in pregnancy is explained to her and she is advised on eating a healthy diet and taking exercise to help achieve this.
Can you explain why gestational diabetes arises?
5 . Question
GDM is defined as diabetes diagnosed during the second and third trimester of pregnancy that was not clearly overt diabetes prior to gestation (ElSayed et al, 2023). As pregnancy progresses, insulin resistance rises and this is normally countered by increasing insulin production. However, women with GDM inherently have a greater degree of insulin resistance compared to those without GDM, and this coupled with reduced beta-cell capacity to produce the required insulin response leads to maternal hyperglycaemia (Catalano, 2014).
GDM is a more common cause of diabetes in pregnancy than pre-existing diabetes, accounting for nearly 90% of cases, and the prevalence is increasing in line with the demographic rise in body mass index (Noctor and Dunne, 2017).
Why is it important to identify gestational diabetes?
6 . Question
Maternal hyperglycaemia from poorly controlled GDM leads to fetal macrosomia. The consequence at delivery is an increased risk of obstructed delivery from shoulder dystocia, clavicular fracture and brachial plexus injury (Melzer, 2010).
Fetal hyperglycaemia is associated with a rise in congenital abnormalities. The incidence of neonatal respiratory distress, meconium aspiration and jaundice are all raised following GDM, and the carry-over of fetal hyperinsulinaemia post-delivery can lead to neonatal hypoglycaemia. All of the above are common reasons for admission to the neonatal intensive care unit (Noctor and Dunne, 2017).
For the mother with GDM, the incidence of pre-eclampsia, polyhydramnios, pre- and post-partum haemorrhage, and infection are all increased. Failure to progress with labour and delivery by caesarean section are more frequent with pregnancy complicated by GDM (Noctor and Dunne, 2017).
In the longer term, there is an association between offspring exposed to in utero GDM and the later occurrence of obesity and glucose intolerance (Silverman et al, 1998). Women who have had GDM are at increased risk of developing type 2 diabetes (Bellamy et al, 2009).
It is important, therefore, that primary and community care practitioners can appropriately advise women at high risk of GDM, understand the management of GDM and, as indicated later in this case study, assume responsibility for post-pregnancy follow-up of GDM (McGovern et al, 2014).
What treatments for diabetes are considered safe and effective in gestational diabetes?
7 . Question
Whilst the joint diabetes and antenatal clinic will assume responsibility for the majority of treatment decisions, primary healthcare workers need to be aware of GDM management. Effective treatment of GDM has been demonstrated to reduce fetal macrosomia, fetal and maternal birth trauma, and perinatal death (Horvath et al, 2010).
Lifestyle change constitutes the first-line treatment of GDM. NICE advises that a trial of diet and exercise should be offered to women with GDM without complications who have a fasting plasma glucose (FPG) level below 7 mmol/L at diagnosis (NICE, 2020). If dietary change and physical exercise prove ineffective in achieving blood glucose targets within 1–2 weeks, medical treatment should be commenced. In the absence of contraindications, this would usually be metformin.
For those women with an FPG of 7 mmol/L or above at diagnosis, and for women with an FPG of 6.0–6.9 mmol/L who have a complication such as fetal macrosomia or hydramnios, immediate medical treatment with insulin (with or without metformin), alongside lifestyle measures, is recommended (NICE, 2020).
Metformin, although not licensed for use in pregnancy, is considered safe and has gained ground as an option for treating GDM when lifestyle measures are insufficient. The risk of perinatal complications appears to be no higher than in insulin-treated patients (Rowan et al, 2008) and there is the advantage of less weight gain, less monitoring and reduced risk of hypoglycaemia in comparison to insulin (Iftakhar, 2012). Should gastrointestinal side effects prove troublesome with metformin, the modified-release preparation may be tried. However, if glycaemic control is inadequate then insulin should be promptly initiated.
Insulin therapy has traditionally been regarded as the first-line medical treatment in GDM and remains the preferred treatment of the American Diabetes Association (ElSayed et al, 2022b). Often a basal–bolus insulin regimen will be required, and insulin doses can be adjusted on a frequent basis, noting that requirements are likely to rise as pregnancy progresses, reflecting increasing insulin resistance. The rapid-acting insulin analogues (e.g. insulin aspart and insulin lispro) offer advantages in improved glycaemic control and reduced hypoglycaemia compared with human soluble insulins (NICE, 2020).
- Mark as read
8 . Question
Holly’s OGTT revealed an FPG of 6.7 mmol/L and a 2-hour plasma glucose of 9.4 mmol/L, confirming the diagnosis of GDM (thresholds for diagnosis: either FPG ≥5.6 mmol/L or 2-hour plasma glucose ≥7.8 mmol/L), and she was reviewed in the joint diabetes and antenatal clinic.
The need to carefully control blood glucose levels was explained to Holly and she was offered lifestyle advice, instructed on glucose monitoring, referred to a dietitian and subsequently commenced on metformin.
What frequency of glucose monitoring should be advised for Holly, who is using metformin for her gestational diabetes?
9 . Question
Holly, as a person using oral medication to control GDM, should be encouraged to take fasting and 1-hour post-meal readings to guide treatment. This monitoring pattern would also be appropriate for those on dietary control alone and if insulin is administered as a basal injection alone.
10 . Question
Section 10 – nadia.
Nadia is a 34-year-old lady of Indian ethnic origin who is now 24 weeks into her second pregnancy, her last pregnancy being 7 years ago. Nadia’s BMI is 32.4 kg/m 2 and her father has type 2 diabetes. GDM was not, however, diagnosed during her first pregnancy and her first baby was born at term weighing 3.8 kg.
How would you assess Nadia’s risk of acquiring gestational diabetes?
11 . Question
Nadia’s ethnic origin, obesity and family history of type 2 diabetes place her at high risk of GDM.
These risk factors were identified by the midwives at booking, and Nadia is scheduled for an OGTT at 28 weeks’ gestation.
There are conflicting recommendations over screening arrangements and diagnostic criteria for GDM. NICE (2020) recommends an OGTT between 24 and 28 weeks’ gestation for those at high risk of GDM (or as soon as possible after booking if previous GDM), with the following diagnostic criteria:
- Fasting plasma glucose of 5.6 mmol/L or more.
- 2-hour plasma glucose of 7.8 mmol/L or more.
The American Diabetes Association now recommends screening for diabetes (with an HbA 1c or fasting plasma glucose test) in those women with risk factors who are planning a pregnancy, so that those found to have diabetes can optimise their glucose control ahead of pregnancy (ElSayed et al, 2022a).
12 . Question
A diagnosis of GDM is made from Nadia’s OGTT (FPG 8.1 mmol/L; 2-hour plasma glucose 12.7 mmol/L). Nadia is accordingly referred to the joint diabetes and antenatal clinic.
Nadia is quickly established on a basal–bolus insulin regimen of Lantus and NovoRapid in the diabetes/antenatal clinic, provided with a meter to self-monitor her capillary glucose and set targets for glycaemic control. NovoRapid is licensed for use in pregnancy and the SmPC of Lantus advises that Lantus may be considered during pregnancy if clinically needed.
What frequency of glucose monitoring might you expect Nadia to undertake?
13 . Question
Intensive glucose monitoring is advised during a pregnancy affected by diabetes, and you should be prepared to issue more glucose test strips. For Nadia, on a basal–bolus insulin regimen, NICE (2020) recommends capillary blood glucose monitoring prior to meals (including fasting), 1-hour post-meals and before bedtime.
NICE (2020) recommends setting the same capillary plasma glucose target levels for women with GDM as for those with pre-existing diabetes. Thus, ideal glucose targets would be the following:
- Fasting: 5.3 mmol/L.
- 1 hour after meals: 7.8 mmol/L; or 2 hours after meals: 6.4 mmol/L.
In practice, targets will need to be individualised, recognising the need to avoid problematic hypoglycaemia. In the case of women using insulin, such as Nadia, capillary glucose levels should be kept above 4 mmol/L. Women at risk of hypoglycaemia should be advised to carry a fast-acting form of glucose at all times.
It should be mentioned that continuous subcutaneous insulin infusion (an insulin pump) is an alternative to a basal–bolus insulin regimen for pregnant women who do not achieve adequate glucose control without troublesome hypoglycaemia. Real-time or intermittently scanned (flash) glucose monitoring is an option where there is severe hypoglycaemia (especially if there is hypoglycaemia unawareness) or where unstable glucose readings are problematic (NICE, 2020).
What role does primary care have in managing women with gestational diabetes?
14 . Question
Whilst management of GDM will primarily occur in the diabetes/antenatal clinic, women with GDM may, in addition to the usual pregnancy-related health issues, need support from practice nurses and GPs in blood glucose monitoring and interpretation, and reassurance that maintaining good glycaemic control will improve outcomes for their babies and themselves. Advice regarding medication use and side-effects, and in particular the recognition and treatment of hypoglycaemia for those using insulin, may be necessary.
Nadia is frequently reviewed in the diabetes/antenatal clinic and achieves satisfactory glycaemic control with her insulin regimen. She delivers a healthy girl at 39 weeks’ gestation without significant problems. Nadia’s insulin is stopped after delivery and glucose levels are checked and seen to be running below 10 mmol/L. It is also important to check baby’s glucose levels, as there is a risk of neonatal hypoglycaemia.
How should Nadia’s glucose control be assessed in the post-partum period?
15 . Question
Whilst diabetes medications used for GDM are usually stopped at delivery in the expectation that glucose levels will fall to pre-pregnancy levels, there is the possibility that hyperglycaemia will persist (and hence the need for glucose checks immediately following delivery).
Nadia should have a formal test for hyperglycaemia within 3 months of delivery, and there needs to be clarity as to who assumes responsibility for this.
NICE (2020) advises that women with GDM (whose blood glucose levels return to normal after delivery) should be offered a fasting plasma glucose (FPG) test at 6–13 weeks postpartum to exclude diabetes (pragmatically, this could be at the 6-week postnatal review); otherwise, beyond 13 weeks postpartum, an HbA 1c test can be offered.
- If FPG is ≥7.0 mmol/L or HbA 1c is ≥48 mmol/mol, a confirmatory test for type 2 diabetes should be carried out and type 2 diabetes pathways (NICE, 2022) should then be followed.
- If FPG is 6.0–6.9 mmol/L or HbA 1c is 39–47 mmol/mol, there is a high risk of developing type 2 diabetes. Lifestyle advice and an offer to refer to the NHS Diabetes Prevention Programme should follow.
- Note these are different HbA 1c risk thresholds than for “prediabetes” in the NICE (2017) PH38 advice, because they refer to a different population.
Nadia has an FPG test at 6 weeks post-delivery, which reveals an FPG <6.0 mmol/L, suggesting that she does not have prediabetes or type 2 diabetes.
What about Nadia’s future risk of developing type 2 diabetes?
16 . Question
Nadia’s GDM is a marker for insulin resistance and places her at increased risk of developing prediabetes and type 2 diabetes in the future compared to women without GDM (relative risk 7.4; Bellamy et al, 2009). Nadia should be alerted to the likelihood of recurrence of GDM in future pregnancies and of the possibility of type 2 diabetes in the future.
Nadia sees you at the clinic following her normal FPG test and asks about her GDM and its future implications.
What advice would you offer Nadia for the future, and what arrangements would you set in place for future screening of diabetes?
17 . Question
It is helpful to think that GDM confers “prediabetic status”. There is good evidence that lifestyle adjustment can prevent or delay the onset of diabetes (Knowler et al, 2002; Lindström et al, 2003). Encourage Nadia to be careful with her diet, take regular exercise and aim for weight loss – similar principles as for those with type 2 diabetes – as detailed in NICE public health guidance on prevention of type 2 diabetes (NICE, 2017). She is offered referral to the Healthier You NHS Diabetes Prevention Programme.
Nadia should be placed on annual recall, checking HbA 1c , lipids, renal function, weight and blood pressure.
With her history of GDM, in any future pregnancy Nadia should be offered early self-monitoring of blood glucose or an OGTT as soon as possible after booking (NICE, 2020).
In reality, there is a disappointingly low rate of both short-term and longer-term review of GDM, with rates of both around 20% in one study (McGovern et al, 2014). Possible reasons for this poor follow-up include lack of awareness amongst women with GDM, poor communication between secondary and primary care, a lack of consensus over responsibility for post-natal tests and missed opportunities in primary care for the annual review.
Share this article + Add to reading list – Remove from reading list
- At-a-glance factsheets
- How to... series
- Diabetes Distilled
- Supplements
Dyslipidaemia and type 2 diabetes. Part 2: Statin-induced myalgia, secondary prevention of cardiovascular disease and using non-statin agents
Statin intolerance pathways and non-statin drugs for both primary and secondary prevention.
12 Feb 2024
Dyslipidaemia and type 2 diabetes. Part 1: Primary prevention of cardiovascular disease and use of statins
Assessing cardiovascular risk and using statins for primary prevention.
16 Nov 2023
Obesity and type 2 diabetes
Assessing and managing obesity in the context of type 2 diabetes.
20 Oct 2023
Early-onset type 2 diabetes in adults
The clinical implications, diagnosis and management of early-onset type 2 diabetes.
Erectile dysfunction in type 2 diabetes
Geoffrey has type 2 diabetes and is seeking help for erectile dysfunction (ED). What examinations, investigations and treatments would you consider?
Heart failure and type 2 diabetes
Mark and Jane present with symptoms suggestive of heart failure. How would you establish the diagnosis and which therapies would be effective?
The elderly and type 2 diabetes
Three elderly people require very different levels of care for their type 2 diabetes. How should you treat each of them appropriately?
Problems with the diabetic foot
Glenda, Sam and Proma all have established type 2 diabetes. Each presents with different foot problems. How would you interpret their symptoms?
25 Oct 2022
Recognition and management of pancreatogenic (type 3c) diabetes
Sarah was diagnosed with type 2 diabetes two years ago. Recently her glycaemic control has deteriorated and she is unwell. What are the implications?
18 Aug 2022
Pre-pregnancy care in diabetes
Georgia wants advice before trying for pregnancy, while Emily is determined to go ahead. Both have type 2 diabetes. What advice would you provide?
30 May 2022
Managing type 2 diabetes during Ramadan
Aabid and Jameela both have type 2 diabetes. Should they fast during Ramadan and, if so, how can they do it safely?
10 Mar 2022
Managing hypertension in diabetes – tricky cases
Winston, Lily and Mark have elevated blood pressure but, given their different case histories, how should each be treated?
Managing hypertension in diabetes – the basics
Brian is 52 years old. He has type 2 diabetes, is obese and his blood pressure was recently recorded as 167/97 mmHg.
Fatty liver disease and type 2 diabetes
Cassie, a 49-year-old with type 2 diabetes, is overweight, has hypertension and her liver function test results are elevated.
11 Oct 2021
Hypoglycaemia and type 2 diabetes
Four mini-scenarios to help build your skills in managing hypoglycaemia in people with type 2 diabetes.
12 Aug 2021
Steroid-induced hyperglycaemia
3 mini-case studies to take you through what it is necessary to consider in identifying and managing steroid-induced hyperglycaemia.
14 Jul 2021
Making a diagnosis of type 2 diabetes
Colin, Rao and Rachael each have signs of elevated blood glucose, but how should correct diagnoses be made?
18 May 2021
Type 2 diabetes and diabetic nephropathy
Meet Louise, a 56-year-old schoolteacher with type 2 diabetes and now showing signs of possible diabetic nephropathy.
The thin person with T2D Meet Jim, a 47-year-old electrician diagnosed with type 2 diabetes four years ago, now presenting with elevated HbA1c.
17 May 2021
Sign up to all DiabetesontheNet journals
- CPD Learning
- Diabetes & Primary Care
- Journal of Diabetes Nursing
- Diabetes Care for Children & Young People
- The Diabetic Foot Journal
- Diabetes Digest
Useful information
- Terms and conditions
- Privacy policy
- Editorial policies and ethics

By clicking ‘Subscribe’, you are agreeing that DiabetesontheNet.com are able to email you periodic newsletters. You may unsubscribe from these at any time. Your info is safe with us and we will never sell or trade your details. For information please review our Privacy Policy .
Are you a healthcare professional? This website is for healthcare professionals only. To continue, please confirm that you are a healthcare professional below.
We use cookies responsibly to ensure that we give you the best experience on our website. If you continue without changing your browser settings, we’ll assume that you are happy to receive all cookies on this website. Read about how we use cookies .
Username or Email Address
Remember Me

User Details: UserFields
- Receive all email newsletters and site updates
- Diabetes & Primary Care (DPC)
- Journal of Diabetes Nursing (JDN)
- Diabetes Care for Children & Young People (DCCYP)
- The Diabetic Foot Journal (DFJ)
- Diabetes Digest (DD)
Registration confirmation will be emailed to you.

NurseStudy.Net
Nursing Education Site

Gestational Diabetes Nursing Diagnosis and Nursing Care Plans
Last updated on May 16th, 2022 at 12:47 pm
Gestational Diabetes Nursing Care Plans Diagnosis and Interventions
Gestational Diabetes NCLEX Review and Nursing Care Plans
Gestational Diabetes is a pregnancy-related type of diabetes. It causes elevated blood sugar level which can be detrimental to both the mother and baby’s health during pregnancy.
Like any other complications of pregnancy, gestational diabetes is seemingly alarming but risks may be reduced by controlling the blood sugar level of the mother.
This can be achieved by modifying diet and appropriate exercise.
Medications are likely needed if these interventions are not enough. It is essential to keep blood sugar at normal level to ensure healthy pregnancy and safe delivery.
Gestational diabetes usually disappears after giving birth. However, women who have had gestational diabetes are at risk for recurrence in next pregnancies and even developing Type 2 diabetes in the near future.
A regular blood sugar level check is necessary to note any changes.
Signs and Symptoms of Gestational Diabetes
- Polydipsia – increased thirst
- Polyuria – increased urinary frequency
- mouth dryness
- fatigue or tiredness
The mother can be asymptomatic and the condition can only be diagnosed when she goes to her prenatal visits.
Causes of Gestational Diabetes
The exact cause of gestations diabetes is still unknown.
However, the risk factors that contribute to its development include: being overweight or obese , previous gestational diabetes or prediabetes, a lack of physical activity, diabetes in an immediate family member, polycystic ovary syndrome (PCOS), and previously delivering a baby weighing more than 9 pounds (4.1 kilograms).
In addition to these, women who are Black, Hispanic, American Indian and Asian American have a higher risk of developing gestational diabetes.
Complications of Gestational Diabetes
Failure to manage gestational diabetes may cause elevation in blood sugar levels which can greatly affect the mother and her baby.
It may also increase the likelihood of delivering thru Cesarean section .
The fetus may be at risk for having the following conditions:
- Fetal macrosomia. This term used for excessive birth weight, typically weighs 9 pounds or more which makes them at risk for birth injuries. It also increases the need for surgical delivery
- Early preterm birth. High blood sugar level may precipitate early labor and delivery prior to the expected delivery date
- Serious breathing disorders such as newborn respiratory distress syndrome (NRDS) which are common in preterm newborns
- Hypoglycemia . Low blood sugar after birth and risk for having type 2 diabetes and obesity later in life
- Stillbirth or fetal death before or shortly after delivery
The mother may be at risk for having the following conditions:
- Hypertension . Elevated blood pressure can lead to a serious complication such as preeclampsia that may put the mother and the baby’s life at risk.
- Delivery via C-Section. Macrosomia can cause the baby to become wedged in the birth canal causing difficulty in vaginal delivery.
- Diabetes. It can be either developed on the next pregnancy or as the mother gets older.
Diagnosis of Gestational Diabetes
- Screening tests – usually done during the second trimester which is between 24- and 28-weeks of pregnancy and during the prenatal visit for those who are at high risk.
- Initial glucose challenge test- a blood sugar below 140 mg/dL (7.8 mmol/L) can be considered normal
- Follow-up glucose tolerance testing
Treatment of Gestational Diabetes
The following may help in prevention and treatment of gestational diabetes:
- Blood sugar monitoring. Gestational diabetes can be treated through lifestyle modification. Blood sugar monitoring (one in the morning and after meals) also helps in managing blood sugar levels. An individual’s lifestyle plays an important role in maintaining their blood sugar at a normal level. The mother’s food choices and daily activities can improve or negatively affect her blood sugar. It’s important to set a pregnancy weight gain goal with the dietitian.
- Proper Nutrition. It’s important to get the daily nutrition by consuming foods that are high in nutrients such as fruits, vegetables, whole grains and lean protein. Foods that are high in fat and highly refined sugars should be avoided. A meal plan based on one’s preference, food habits and blood sugar can be of great help.
- Regular Exercise. Exercise not only relieves pregnancy discomfort but also helps a lot in lowering blood sugar. Everyday activities such as walking, doing household chores and gardening are also beneficial.
- Insulin administration. If the lifestyle modifications are inadequate then insulin injections may be incorporated in the management. Close monitoring of the baby’s condition thru ultrasounds and other diagnostics will be done throughout the pregnancy.
Nursing Care Plans for Gestational Diabetes
Diabetes is a medical condition that involves excessive glucose (sugar) levels in the blood due to the little or no production of the hormone insulin, or the presence of insulin resistance.
Despite not having a cure, diabetes can be controlled by effective medical and nursing management, as well as the patient’s strict adherence to prescribed medication, lifestyle changes, and blood sugar monitoring.
The following nursing care plans can be used to assess, plan, manage, and monitor the symptoms and effects of diabetes to a patient.
Gestational Diabetes Nursing Care Plan 1
Nursing Diagnosis: Deficient Knowledge related to new diagnosis of gestational diabetes as evidenced by patient’s verbalization of “I want to know more about my new diagnosis and care”
Desired Outcome: At the end of the health teaching session, the patient will be able to demonstrate sufficient knowledge of gestational diabetes and its management.
Gestational Diabetes Nursing Care Plan 2
Nursing Diagnosis: Fatigue related to decreased metabolic energy production as evidenced by overwhelming lack of energy, verbalization of tiredness, generalized weakness, blood sugar level of 210 mg/dL, and shortness of breath upon exertion
Desired Outcome : The patient will demonstration active participation in necessary and desired activities and demonstrate increase in activity levels.
Gestational Diabetes Nursing Care Plan 3
Risk for Imbalanced Nutrition: Less than Body Requirements
Nursing Diagnosis: Risk for Imbalanced Nutrition: Less than Body Requirements related to lack of ability to make use of nutrients appropriately secondary to gestational diabetes.
Desired Outcomes:
- The patient will express an understanding of the treatment management process and the necessity of regular self-assessment.
- The patient will attain the required fasting blood sugar levels, between 60 to 100 mg/dl, and no higher than 140 mg/dl after meals.
- The patient will increase body weight by at least 24 to 30 pounds prenatally or according to the recommended pre-pregnancy weight.
- The patient will not develop diabetic ketoacidosis and signs and symptoms such as weakness, fruity-scented breath, excessive thirst, frequent urination, confusion , and complications.
Gestational Diabetes Nursing Care Plan 4
Risk for Maternal Injury
Nursing Diagnosis: Risk for Maternal Injury related to changes in diabetic control secondary to gestational diabetes.
- The patient will maintain a normal blood sugar level.
- The patient will be free of signs and symptoms of maternal injury related to gestational diabetes.
Gestational Diabetes Nursing Care Plan 5
Risk for Fetal Injury
Nursing Diagnosis: Risk for Fetal Injury related to elevated maternal serum blood glucose levels secondary to gestational diabetes.
- The fetus will remain safe and pregnancy is maintained until it reaches maturity.
- The fetus will display a reactive normal stress test, a negative result in OCT and CST.
Nursing References
Ackley, B. J., Ladwig, G. B., Makic, M. B., Martinez-Kratz, M. R., & Zanotti, M. (2020). Nursing diagnoses handbook: An evidence-based guide to planning care . St. Louis, MO: Elsevier. Buy on Amazon
Gulanick, M., & Myers, J. L. (2022). Nursing care plans: Diagnoses, interventions, & outcomes . St. Louis, MO: Elsevier. Buy on Amazon
Ignatavicius, D. D., Workman, M. L., Rebar, C. R., & Heimgartner, N. M. (2018). Medical-surgical nursing: Concepts for interprofessional collaborative care . St. Louis, MO: Elsevier. Buy on Amazon
Silvestri, L. A. (2020). Saunders comprehensive review for the NCLEX-RN examination . St. Louis, MO: Elsevier. Buy on Amazon
Disclaimer:
Please follow your facilities guidelines and policies and procedures. The medical information on this site is provided as an information resource only and is not to be used or relied on for any diagnostic or treatment purposes.
This information is not intended to be nursing education and should not be used as a substitute for professional diagnosis and treatment.
Leave a Comment Cancel reply
This site uses Akismet to reduce spam. Learn how your comment data is processed .
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts

Case 6–2020: A 34-Year-Old Woman with Hyperglycemia
Presentation of case.
Dr. Max C. Petersen (Medicine): A 34-year-old woman was evaluated in the diabetes clinic of this hospital for hyperglycemia.
Eleven years before this presentation, the blood glucose level was 126 mg per deciliter (7.0 mmol per liter) on routine laboratory evaluation, which was performed as part of an annual well visit. The patient could not recall whether she had been fasting at the time the test had been performed. One year later, the fasting blood glucose level was 112 mg per deciliter (6.2 mmol per liter; reference range, <100 mg per deciliter [<5.6 mmol per liter]).
Nine years before this presentation, a randomly obtained blood glucose level was 217 mg per deciliter (12.0 mmol per liter), and the patient reported polyuria. At that time, the glycated hemoglobin level was 5.8% (reference range, 4.3 to 5.6); the hemoglobin level was normal. One year later, the glycated hemoglobin level was 5.9%. The height was 165.1 cm, the weight 72.6 kg, and the body-mass index (BMI; the weight in kilograms divided by the square of the height in meters) 26.6. The patient received a diagnosis of prediabetes and was referred to a nutritionist. She made changes to her diet and lost 4.5 kg of body weight over a 6-month period; the glycated hemoglobin level was 5.5%.
Six years before this presentation, the patient became pregnant with her first child. Her prepregnancy BMI was 24.5. At 26 weeks of gestation, the result of a 1-hour oral glucose challenge test (i.e., the blood glucose level obtained 1 hour after the oral administration of a 50-g glucose load in the nonfasting state) was 186 mg per deciliter (10.3 mmol per liter; reference range, <140 mg per deciliter [<7.8 mmol per liter]). She declined a 3-hour oral glucose tolerance test; a presumptive diagnosis of gestational diabetes was made. She was asked to follow a meal plan for gestational diabetes and was treated with insulin during the pregnancy. Serial ultrasound examinations for fetal growth and monitoring were performed. At 34 weeks of gestation, the fetal abdominal circumference was in the 76th percentile for gestational age. Polyhydramnios developed at 37 weeks of gestation. The child was born at 39 weeks 3 days of gestation, weighed 3.9 kg at birth, and had hypoglycemia after birth, which subsequently resolved. Six weeks post partum, the patient’s fasting blood glucose level was 120 mg per deciliter (6.7 mmol per liter), and the result of a 2-hour oral glucose tolerance test (i.e., the blood glucose level obtained 2 hours after the oral administration of a 75-g glucose load in the fasting state) was 131 mg per deciliter (7.3 mmol per liter; reference range, <140 mg per deciliter). Three months post partum, the glycated hemoglobin level was 6.1%. Lifestyle modification for diabetes prevention was recommended.
Four and a half years before this presentation, the patient became pregnant with her second child. Her prepregnancy BMI was 25.1. At 5 weeks of gestation, she had an elevated blood glucose level. Insulin therapy was started at 6 weeks of gestation, and episodes of hypoglycemia occurred during the pregnancy. Serial ultrasound examinations for fetal growth and monitoring were performed. At 28 weeks of gestation, the fetal abdominal circumference was in the 35th percentile for gestational age, and the amniotic fluid level was normal. Labor was induced at 38 weeks of gestation; the child weighed 2.6 kg at birth. Neonatal blood glucose levels were reported as stable after birth. Six weeks post partum, the patient’s fasting blood glucose level was 133 mg per deciliter (7.4 mmol per liter), and the result of a 2-hour oral glucose tolerance test was 236 mg per deciliter (13.1 mmol per liter). The patient received a diagnosis of type 2 diabetes mellitus; lifestyle modification was recommended. Three months post partum, the glycated hemoglobin level was 5.9% and the BMI was 30.0. Over the next 2 years, she followed a low-carbohydrate diet and regular exercise plan and self-monitored the blood glucose level.
Two years before this presentation, the patient became pregnant with her third child. Blood glucose levels were again elevated, and insulin therapy was started early in gestation. She had episodes of hypoglycemia that led to adjustment of her insulin regimen. The child was born at 38 weeks 5 days of gestation, weighed 3.0 kg at birth, and had hypoglycemia that resolved 48 hours after birth. After the birth of her third child, the patient started to receive metformin, which had no effect on the glycated hemoglobin level, despite adjustment of the therapy to the maximal dose.
One year before this presentation, the patient became pregnant with her fourth child. Insulin therapy was again started early in gestation. The patient reported that episodes of hypoglycemia occurred. Polyhydramnios developed. The child was born at 38 weeks 6 days of gestation and weighed 3.5 kg. The patient sought care at the diabetes clinic of this hospital for clarification of her diagnosis.
The patient reported following a low-carbohydrate diet and exercising 5 days per week. There was no fatigue, change in appetite, change in vision, chest pain, shortness of breath, polydipsia, or polyuria. There was no history of anemia, pancreatitis, hirsutism, proximal muscle weakness, easy bruising, headache, sweating, tachycardia, gallstones, or diarrhea. Her menstrual periods were normal. She had not noticed any changes in her facial features or the size of her hands or feet.
The patient had a history of acne and low-back pain. Her only medication was metformin. She had no known medication allergies. She lived with her husband and four children in a suburban community in New England and worked as an administrator. She did not smoke tobacco or use illicit drugs, and she rarely drank alcohol. She identified as non-Hispanic white. Both of her grandmothers had type 2 diabetes mellitus. Her father had hypertension, was overweight, and had received a diagnosis of type 2 diabetes at 50 years of age. Her mother was not overweight and had received a diagnosis of type 2 diabetes at 48 years of age. The patient had two sisters, neither of whom had a history of diabetes or gestational diabetes. There was no family history of hemochromatosis.
On examination, the patient appeared well. The blood pressure was 126/76 mm Hg, and the heart rate 76 beats per minute. The BMI was 25.4. The physical examination was normal. The glycated hemoglobin level was 6.2%.
A diagnostic test was performed.
DIFFERENTIAL DIAGNOSIS
Dr. Miriam S. Udler: I am aware of the diagnosis in this case and participated in the care of this patient. This healthy 34-year-old woman, who had a BMI just above the upper limit of the normal range, presented with a history of hyperglycemia of varying degrees since 24 years of age. When she was not pregnant, she was treated with lifestyle measures as well as metformin therapy for a short period, and she maintained a well-controlled blood glucose level. In thinking about this case, it is helpful to characterize the extent of the hyperglycemia and then to consider its possible causes.
CHARACTERIZING HYPERGLYCEMIA
This patient’s hyperglycemia reached a threshold that was diagnostic of diabetes 1 on two occasions: when she was 25 years of age, she had a randomly obtained blood glucose level of 217 mg per deciliter with polyuria (with diabetes defined as a level of ≥200 mg per deciliter [≥11.1 mmol per liter] with symptoms), and when she was 30 years of age, she had on the same encounter a fasting blood glucose level of 133 mg per deciliter (with diabetes defined as a level of ≥126 mg per deciliter) and a result on a 2-hour oral glucose tolerance test of 236 mg per deciliter (with diabetes defined as a level of ≥200 mg per deciliter). On both of these occasions, her glycated hemoglobin level was in the prediabetes range (defined as 5.7 to 6.4%). In establishing the diagnosis of diabetes, the various blood glucose studies and glycated hemoglobin testing may provide discordant information because the tests have different sensitivities for this diagnosis, with glycated hemoglobin testing being the least sensitive. 2 Also, there are situations in which the glycated hemoglobin level can be inaccurate; for example, the patient may have recently received a blood transfusion or may have a condition that alters the life span of red cells, such as anemia, hemoglobinopathy, or pregnancy. 3 These conditions were not present in this patient at the time that the glycated hemoglobin measurements were obtained. In addition, since the glycated hemoglobin level reflects the average glucose level typically over a 3-month period, discordance with timed blood glucose measurements can occur if there has been a recent change in glycemic control. This patient had long-standing mild hyperglycemia but met criteria for diabetes on the basis of the blood glucose levels noted.
Type 1 and Type 2 Diabetes
Now that we have characterized the patient’s hyperglycemia as meeting criteria for diabetes, it is important to consider the possible types. More than 90% of adults with diabetes have type 2 diabetes, which is due to progressive loss of insulin secretion by beta cells that frequently occurs in the context of insulin resistance. This patient had received a diagnosis of type 2 diabetes; however, some patients with diabetes may be given a diagnosis of type 2 diabetes on the basis of not having features of type 1 diabetes, which is characterized by autoimmune destruction of the pancreatic beta cells that leads to rapid development of insulin dependence, with ketoacidosis often present at diagnosis.
Type 1 diabetes accounts for approximately 6% of all cases of diabetes in adults (≥18 years of age) in the United States, 4 and 80% of these cases are diagnosed before the patient is 20 years of age. 5 Since this patient’s diabetes was essentially nonprogressive over a period of at least 9 years, she most likely does not have type 1 diabetes. It is therefore not surprising that she had received a diagnosis of type 2 diabetes, but there are several other types of diabetes to consider, particularly since some features of her case do not fit with a typical case of type 2 diabetes, such as her age at diagnosis, the presence of hyperglycemia despite a nearly normal BMI, and the mild and nonprogressive nature of her disease over the course of many years.
Less Common Types of Diabetes
Latent autoimmune diabetes in adults (LADA) is a mild form of autoimmune diabetes that should be considered in this patient. However, there is controversy as to whether LADA truly represents an entity that is distinct from type 1 diabetes. 6 Both patients with type 1 diabetes and patients with LADA commonly have elevated levels of diabetes-associated autoantibodies; however, LADA has been defined by an older age at onset (typically >25 years) and slower progression to insulin dependence (over a period of >6 months). 7 This patient had not been tested for diabetes-associated autoantibodies. I ordered these tests to help evaluate for LADA, but this was not my leading diagnosis because of her young age at diagnosis and nonprogressive clinical course over a period of at least 9 years.
If the patient’s diabetes had been confined to pregnancy, we might consider gestational diabetes, but she had hyperglycemia outside of pregnancy. Several medications can cause hyperglycemia, including glucocorticoids, atypical antipsychotic agents, cancer immunotherapies, and some antiretroviral therapies and immunosuppressive agents used in transplantation. 8 However, this patient was not receiving any of these medications. Another cause of diabetes to consider is destruction of the pancreas due to, for example, cystic fibrosis, a tumor, or pancreatitis, but none of these were present. Secondary endocrine disorders — including excess cortisol production, excess growth hormone production, and pheochromocytoma — were considered to be unlikely in this patient on the basis of the history, review of symptoms, and physical examination.
Monogenic Diabetes
A final category to consider is monogenic diabetes, which is caused by alteration of a single gene. Types of monogenic diabetes include maturity-onset diabetes of the young (MODY), neonatal diabetes, and syndromic forms of diabetes. Monogenic diabetes accounts for 1 to 6% of cases of diabetes in children 9 and approximately 0.4% of cases in adults. 10 Neonatal diabetes is diagnosed typically within the first 6 months of life; syndromic forms of monogenic diabetes have other abnormal features, including particular organ dysfunction. Neither condition is applicable to this patient.
MODY is an autosomal dominant condition characterized by primary pancreatic beta-cell dysfunction that causes mild diabetes that is diagnosed during adolescence or early adulthood. As early as 1964, the nomenclature “maturity-onset diabetes of the young” was used to describe cases that resembled adult-onset type 2 diabetes in terms of the slow progression to insulin use (as compared with the rapid progression in type 1 diabetes) but occurred in relatively young patients. 11 Several genes cause distinct forms of MODY that have specific disease features that inform treatment, and thus MODY is a clinically important diagnosis. Most forms of MODY cause isolated abnormal glucose levels (in contrast to syndromic monogenic diabetes), a manifestation that has contributed to its frequent misdiagnosis as type 1 or type 2 diabetes. 12
Genetic Basis of MODY
Although at least 13 genes have been associated with MODY, 3 genes — GCK , which encodes glucokinase, and HNF1A and HNF4A , which encode hepatocyte nuclear factors 1A and 4A, respectively — account for most cases. MODY associated with GCK (known as GCK-MODY) is characterized by mild, nonprogressive hyperglycemia that is present since birth, whereas the forms of MODY associated with HNF1A and HNF4A (known as HNF1A-MODY and HNF4A-MODY, respectively) are characterized by the development of diabetes, typically in the early teen years or young adulthood, that is initially mild and then progresses such that affected patients may receive insulin before diagnosis.
In patients with GCK-MODY, genetic variants reduce the function of glucokinase, the enzyme in pancreatic beta cells that functions as a glucose sensor and controls the rate of entry of glucose into the glycolytic pathway. As a result, reduced sensitivity to glucose-induced insulin secretion causes asymptomatic mild fasting hyperglycemia, with an upward shift in the normal range of the fasting blood glucose level to 100 to 145 mg per deciliter (5.6 to 8.0 mmol per liter), and also causes an upward shift in postprandial blood glucose levels, but with tight regulation maintained ( Fig. 1 ). 13 This mild hyperglycemia is not thought to confer a predisposition to complications of diabetes, 14 is largely unaltered by treatment, 15 and does not necessitate treatment outside of pregnancy.

Key features suggesting maturity-onset diabetes of the young (MODY) in this patient were an age of less than 35 years at the diagnosis of diabetes, a strong family history of diabetes with an autosomal dominant pattern of inheritance, and hyperglycemia despite a close-to-normal body-mass index. None of these features is an absolute criterion. MODY is caused by single gene–mediated disruption of pancreatic beta-cell function. In MODY associated with the GCK gene (known as GCK-MODY), disrupted glucokinase function causes a mild upward shift in glucose levels through-out the day and does not necessitate treatment. 13 In the pedigree, circles represent female family members, squares male family members, blue family members affected by diabetes, and green unaffected family members. The arrow indicates the patient.
In contrast to GCK-MODY, the disorders HNF1A-MODY and HNF4A-MODY result in progressive hyperglycemia that eventually leads to treatment. 16 Initially, there may be a normal fasting glucose level and large spikes in postprandial glucose levels (to >80 mg per deciliter [>4.4 mmol per liter]). 17 Patients can often be treated with oral agents and discontinue insulin therapy started before the diagnosis of MODY. 18 Of note, patients with HNF1A-MODY or HNF4A-MODY are typically sensitive to treatment with sulfonylureas 19 but may also respond to glucagon-like peptide-1 receptor agonists. 20
This patient had received a diagnosis of diabetes before 35 years of age, had a family history of diabetes involving multiple generations, and was not obese. These features are suggestive of MODY but do not represent absolute criteria for the condition ( Fig. 1 ). 1 Negative testing for diabetes-associated autoantibodies would further increase the likelihood of MODY. There are methods to calculate a patient’s risk of having MODY associated with GCK , HNF1A , or HNF4A . 21 , 22 Using an online calculator ( www.diabetesgenes.org/mody-probability-calculator ), we estimate that the probability of this patient having MODY is at least 75.5%. Genetic testing would be needed to confirm this diagnosis, and in patients at an increased risk for MODY, multigene panel testing has been shown to be cost-effective. 23 , 24
DR. MIRIAM S. UDLER’S DIAGNOSIS
Maturity-onset diabetes of the young, most likely due to a GCK variant.
DIAGNOSTIC TESTING
Dr. Christina A. Austin-Tse: A diagnostic sequencing test of five genes associated with MODY was performed. One clinically significant variant was identified in the GCK gene (NM_000162.3): a c.787T→C transition resulting in the p.Ser263Pro missense change. Review of the literature and variant databases revealed that this variant had been previously identified in at least three patients with early-onset diabetes and had segregated with disease in at least three affected members of two families (GeneDx: personal communication). 25 , 26 Furthermore, the variant was rare in large population databases (occurring in 1 out of 128,844 European chromosomes in gnomAD 27 ), a feature consistent with a disease-causing role. Although the serine residue at position 263 was not highly conserved, multiple in vitro functional studies have shown that the p.Ser263Pro variant negatively affects the stability of the glucokinase enzyme. 26 , 28 – 30 As a result, this variant met criteria to be classified as “likely pathogenic.” 31 As mentioned previously, a diagnosis of GCK-MODY is consistent with this patient’s clinical features. On subsequent testing of additional family members, the same “likely pathogenic” variant was identified in the patient’s father and second child, both of whom had documented hyperglycemia.
DISCUSSION OF MANAGEMENT
Dr. Udler: In this patient, the diagnosis of GCK-MODY means that it is normal for her blood glucose level to be mildly elevated. She can stop taking metformin because discontinuation is not expected to substantially alter her glycated hemoglobin level 15 , 32 and because she is not at risk for complications of diabetes. 14 However, she should continue to maintain a healthy lifestyle. Although patients with GCK-MODY are not typically treated for hyperglycemia outside of pregnancy, they may need to be treated during pregnancy.
It is possible for a patient to have type 1 or type 2 diabetes in addition to MODY, so this patient should be screened for diabetes according to recommendations for the general population (e.g., in the event that she has a risk factor for diabetes, such as obesity). 1 Since the mild hyperglycemia associated with GCK-MODY is asymptomatic (and probably unrelated to the polyuria that this patient had described in the past), the development of symptoms of hyperglycemia, such as polyuria, polydipsia, or blurry vision, should prompt additional evaluation. In patients with GCK-MODY, the glycated hemoglobin level is typically below 7.5%, 33 so a value rising above that threshold or a sudden large increase in the glycated hemoglobin level could indicate concomitant diabetes from another cause, which would need to be evaluated and treated.
This patient’s family members are at risk for having the same GCK variant, with a 50% chance of offspring inheriting a variant from an affected parent. Since the hyperglycemia associated with GCK-MODY is present from birth, it is necessary to perform genetic testing only in family members with demonstrated hyperglycemia. I offered site-specific genetic testing to the patient’s parents and second child.
Dr. Meridale V. Baggett (Medicine): Dr. Powe, would you tell us how you would treat this patient during pregnancy?
Dr. Camille E. Powe: During the patient’s first pregnancy, routine screening led to a presumptive diagnosis of gestational diabetes, the most common cause of hyperglycemia in pregnancy. Hyperglycemia in pregnancy is associated with adverse pregnancy outcomes, 34 and treatment lowers the risk of such outcomes. 35 , 36 Two of the most common complications — fetal overgrowth (which can lead to birth injuries, shoulder dystocia, and an increased risk of cesarean delivery) and neonatal hypoglycemia — are thought to be the result of fetal hyperinsulinemia. 37 Maternal glucose is freely transported across the placenta, and excess glucose augments insulin secretion from the fetal pancreas. In fetal life, insulin is a potent growth factor, and neonates who have hyperinsulinemia in utero often continue to secrete excess insulin in the first few days of life. In the treatment of pregnant women with diabetes, we strive for strict blood sugar control (fasting blood glucose level, <95 mg per deciliter [<5.3 mmol per liter]; 2-hour postprandial blood glucose level, <120 mg per deciliter) to decrease the risk of these and other hyperglycemia-associated adverse pregnancy outcomes. 38 – 40
In the third trimester of the patient’s first pregnancy, obstetrical ultrasound examination revealed a fetal abdominal circumference in the 76th percentile for gestational age and polyhydramnios, signs of fetal exposure to maternal hyperglycemia. 40 – 42 Case series involving families with GCK-MODY have shown that the effect of maternal hyperglycemia on the fetus depends on whether the fetus inherits the pathogenic GCK variant. 43 – 48 Fetuses that do not inherit the maternal variant have overgrowth, presumably due to fetal hyperinsulinemia ( Fig. 2A ). In contrast, fetuses that inherit the variant do not have overgrowth and are born at a weight that is near the average for gestational age, despite maternal hyperglycemia, presumably because the variant results in decreased insulin secretion ( Fig. 2B ). Fetuses that inherit GCK-MODY from their fathers and have euglycemic mothers appear to be undergrown, most likely because their insulin secretion is lower than normal when they and their mothers are euglycemic ( Fig. 2D ). Because fetal overgrowth and polyhydramnios occurred during this patient’s first pregnancy and neonatal hypoglycemia developed after the birth, the patient’s first child is probably not affected by GCK-MODY.

Pathogenic variants that lead to GCK-MODY, when carried by a fetus, change the usual relationship of maternal hyperglycemia to fetal hyperinsulinemia and fetal overgrowth. GCK-MODY–affected fetuses have lower insulin secretion than unaffected fetuses in response to the same maternal blood glucose level. In a hyperglycemic mother carrying a fetus who is unaffected by GCK-MODY, excessive fetal growth is usually apparent (Panel A). Studies involving GCK-MODY–affected hyperglycemic mothers have shown that fetal growth is normal despite maternal hyperglycemia when a fetus has the maternal GCK variant (Panel B). The goal of treatment of maternal hyperglycemia when a fetus is unaffected by GCK-MODY is to establish euglycemia to normalize fetal insulin levels and growth (Panel C); whether this can be accomplished in the case of maternal GCK-MODY is controversial, given the genetically determined elevated maternal glycemic set point. In the context of maternal euglycemia, GCK-MODY–affected fetuses may be at risk for fetal growth restriction (Panel D).
In accordance with standard care for pregnant women with diabetes who do not meet glycemic targets after dietary modification, 38 , 39 the patient was treated with insulin during her pregnancies. In her second pregnancy, treatment was begun early, after hyperglycemia was detected in the first trimester. Because she had not yet received the diagnosis of GCK-MODY during any of her pregnancies, no consideration of this condition was given during her obstetrical treatment. Whether treatment affects the risk of hyperglycemia-associated adverse pregnancy outcomes in pregnant women with known GCK-MODY is controversial, with several case series showing that the birth weight percentile in unaffected neonates remains consistent regardless of whether the mother is treated with insulin. 44 , 45 Evidence suggests that it may be difficult to overcome a genetically determined glycemic set point in patients with GCK-MODY with the use of pharmacotherapy, 15 , 32 and affected patients may have symptoms of hypoglycemia when the blood glucose level is normal because of an enhanced counterregulatory response. 49 , 50 Still, to the extent that it is possible, it would be desirable to safely lower the blood glucose level in a woman with GCK-MODY who is pregnant with an unaffected fetus in order to decrease the risk of fetal overgrowth and other consequences of mildly elevated glucose levels ( Fig. 2C ). 46 , 47 , 51 In contrast, there is evidence that lowering the blood glucose level in a pregnant woman with GCK-MODY could lead to fetal growth restriction if the fetus is affected ( Fig. 2D ). 45 , 52 During this patient’s second pregnancy, she was treated with insulin beginning in the first trimester, and her daughter’s birth weight was near the 16th percentile for gestational age; this outcome is consistent with the daughter’s ultimate diagnosis of GCK-MODY.
Expert opinion suggests that, in pregnant women with GCK-MODY, insulin therapy should be deferred until fetal growth is assessed by means of ultrasound examination beginning in the late second trimester. If there is evidence of fetal overgrowth, the fetus is presumed to be unaffected by GCK-MODY and insulin therapy is initiated. 53 After I have counseled women with GCK-MODY on the potential risks and benefits of insulin treatment during pregnancy, I have sometimes used a strategy of treating hyperglycemia from early in pregnancy using modified glycemic targets that are less stringent than the targets typically used during pregnancy. This strategy attempts to balance the risk of growth restriction in an affected fetus (as well as maternal hypoglycemia) with the potential benefit of glucose-lowering therapy for an unaffected fetus.
Dr. Udler: The patient stopped taking metformin, and subsequent glycated hemoglobin levels remained unchanged, at 6.2%. Her father and 5-year-old daughter (second child) both tested positive for the same GCK variant. Her father had a BMI of 36 and a glycated hemoglobin level of 7.8%, so I counseled him that he most likely had type 2 diabetes in addition to GCK-MODY. He is currently being treated with metformin and lifestyle measures. The patient’s daughter now has a clear diagnosis to explain her hyperglycemia, which will help in preventing misdiagnosis of type 1 diabetes, given her young age, and will be important for the management of any future pregnancies. She will not need any medical follow-up for GCK-MODY until she is considering pregnancy.
FINAL DIAGNOSIS
Maturity-onset diabetes of the young due to a GCK variant.
Acknowledgments
We thank Dr. Andrew Hattersley and Dr. Sarah Bernstein for helpful comments on an earlier draft of the manuscript.
This case was presented at the Medical Case Conference.
No potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org .

VIDEO
COMMENTS
Gestational diabetes is defined as "any degree of carbohydrate intolerance with onset first recognized during pregnancy. This definition applies whether insulin ... is used for treatment and whether or not the condition persists after pregnancy."1 Risk assessment is done early in the pregnancy, with average-risk women being tested at 24-28 weeks' gestation and low-risk women requiring ...
Abstract. Gestational diabetes mellitus (GDM) is defined as any degree of glucose intolerance with onset or first recognition during pregnancy. Screening for GDM is usually done at 24-28 weeks of gestation. In this case, we report a 31-year-old woman who developed gestational diabetes at 6 weeks in two successive pregnancies.
The clinical manifestations of gestational diabetes mellitus coincide with the signs and symptoms of the other types of diabetes mellitus. These are popularly known as the "3 P's" or polydipsia (excessive thirst), polyphagia (excessive hunger), and polyuria (frequent urination). Aside from these manifestations, there are also other signs ...
This study provides a summary of best practices regarding the diagnosis, screening and nursing management of gestational diabetes mellitus that provide guidance for nurse-midwives on maternal and postpartum follow‐up care for women at risk or diagnosed with gestational diabetes mellitus.
Page: 47. This series of interactive case studies is aimed at all healthcare professionals in primary and community care who would like to broaden their understanding of diabetes. These two cases provide an overview of gestational diabetes (GDM). The scenarios cover the screening, identification and management of GDM, as well as the steps that ...
Gestational Diabetes Mellitus (GDM) is glucose intolerance with onset during pregnancy. In true GDM, glucose usually returns to normal by six weeks postpartum, although women with GDM have an increased risk of developing type 2 diabetes mellitus later in life. The primary concern for any woman with this disorder is controlling the balance ...
The pathological mechanism of gestational diabetes mellitus (GDM) is similar to that of T2DM and is associated with insulin resistance and islet cell dysfunction . The results of these studies suggest that pregnant nurses are likely to be at high risk of developing GDM; however, there is little information on the occurrence of GDM in nurses.
Background Pregnant nurses are at high risk of developing gestational diabetes mellitus (GDM), and nurses diagnosed with GDM face challenges in balancing disease management and work, which affects maternal and child health and the quality of care. GDM requires significant changes to lifestyle and physical activity to control blood glucose levels, which is key to reducing adverse pregnancy ...
Gestational diabetes mellitus (GDM) can be defined as any degree of glucose intolerance with onset or first recognition during pregnancy. Screening for GDM is done usually at 24-28 weeks of ...
Pregnancies complicated by both obesity and gestational diabetes mellitus (GDM) increase the risk of maternal and fetal complications, including but not limited to gestational hypertension, cesarean surgical birth, fetal macrosomia and postpartum hemorrhage. Because of the increased maternal and fetal risks associated with maternal obesity and GDM, the development of evidence‐based ...
Randomized Trial of Gestational Diabetes Screening. 02:20. Gestational diabetes mellitus, one of the most common complications of pregnancy, 1,2 affects 6 to 25% of pregnant women (depending on ...
Purpose The aim of the study is to compare maternal hemodynamic adaptations in gestational diabetes (GDM) versus healthy pregnancies. Methods A prospective case-control study was conducted, comparing 69 singleton pregnancies with GDM and 128 controls, recruited between September 2018 and April 2019 in Maternal-Fetal Medicine Unit, Careggi University Hospital, Florence, Italy. Hemodynamic ...
25 weeks of gestational age, when she weighed 67 Kg of BW and had a BMI of 27.9 Kg.m-2. At the OGTT: The fasting serum glycemia was Abstract Background: How best to define Gestational Diabetes Mellitus (GDM) is the object of debate, with International Association of Diabetes in Pregnancy Study Groups criteria (IADPSGc) differing
1.1. Need for developing case definitions and guidelines for data collection, analysis, and presentation for gestational diabetes mellitus as an adverse event following immunization. Gestational diabetes mellitus (GDM) is a common condition in pregnancy that can result in significant morbidity and mortality to both mother and fetus.
The two case studies presented here provide an overview of gestational diabetes (GDM). The scenarios cover the screening, identification and management of GDM, as well as the steps that should be taken to screen for, and ideally prevent, development of type 2 diabetes in the long term post-pregnancy. By actively engaging with the case studies ...
Diagnosis of Gestational Diabetes. Screening tests - usually done during the second trimester which is between 24- and 28-weeks of pregnancy and during the prenatal visit for those who are at high risk. Initial glucose challenge test- a blood sugar below 140 mg/dL (7.8 mmol/L) can be considered normal.
Gestational Diabetes - Case Study #5 - Copy. nursing. Course. Foundations of Nursing Practice 2 (Nur0081) 78 Documents. Students shared 78 documents in this course. University ... Chapter 01 Overview of Professional Nursing Concepts for Med; Case Study #129 - Antepartum (Prenatal) Preview text.
It is important to screen the GDM and for the case to be managed by nurses. Nurses are needed to care for pregnant women with GDM, and the work experience of nurses is directly related to the ...
One of the main forms of diabetes is gestational diabetes mellitus (GDM), which is recognized as glucose intolerance, and is diagnosed initially during pregnancy. ... Considering the importance of early detection and appropriate GDM diagnosis, the International Association of Diabetes and Pregnancy Study Groups (IADPSG) (6) has identified new ...
Y. is diagnosed with gestational diabetes mellitus (GDM). What is GDM? Glucose intolerance with onset during pregnancy. In true GDM, glucose usually returns to normal by 6 weeks postpartum, although women with GDM have increased risk of developing type 2 diabetes mellitus later in life. List 5 risk factors for GDM.
the mother and fetus. Appropriate interventions are needed in providing nursing care to patients with gestational diabetes mellitus to reduce complications in childbirth so that appropriate nursing actions can be taken. This case study aimed to examine the implementation of "Normal Childbirth Nursing Care with Gestational Diabetes
The ongoing Treatment of Booking Gestational Diabetes Mellitus study, ... In a UK prospective case-control study (n = 1024), women with undiagnosed GDM based on a fasting glucose level ≥ 5.6mmol/L (≥100 mg/dL) had a 4-fold greater risk of late stillbirth (defined as occurring ≥28 weeks' gestation) ...
Background and Objectives: The escalating prevalence of gestational diabetes mellitus (GDM) and the limitations associated with utilizing body mass index (BMI) as a predictive measure underscore the imperative need for identifying an optimal early pregnancy predictor. ... This prospective case-control study measured adipose thickness with ...
Dr. Max C. Petersen (Medicine): A 34-year-old woman was evaluated in the diabetes clinic of this hospital for hyperglycemia. Eleven years before this presentation, the blood glucose level was 126 mg per deciliter (7.0 mmol per liter) on routine laboratory evaluation, which was performed as part of an annual well visit.