Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article

More than 75 percent decline over 27 years in total flying insect biomass in protected areas
Roles Conceptualization, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliation Radboud University, Institute for Water and Wetland Research, Animal Ecology and Physiology & Experimental Plant Ecology, PO Box 9100, 6500 GL Nijmegen, The Netherlands
Roles Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Resources, Writing – review & editing
Affiliation Entomological Society Krefeld e.V., Entomological Collections Krefeld, Marktstrasse 159, 47798 Krefeld, Germany
Roles Conceptualization, Funding acquisition, Investigation, Supervision, Writing – review & editing
Roles Conceptualization, Investigation, Supervision, Writing – review & editing
Roles Formal analysis, Resources, Software, Validation
Roles Data curation, Funding acquisition, Investigation, Methodology, Project administration, Writing – review & editing
Roles Data curation, Funding acquisition, Methodology, Project administration, Writing – review & editing
Roles Data curation, Project administration, Resources, Writing – review & editing
Roles Data curation, Investigation, Methodology, Resources, Writing – review & editing
Roles Conceptualization, Investigation, Writing – review & editing
Affiliation University of Sussex, School of Life Sciences, Falmer, Brighton BN1 9QG, United Kingdom
Roles Conceptualization, Funding acquisition, Investigation, Methodology, Resources, Supervision, Writing – review & editing
- Caspar A. Hallmann,
- Martin Sorg,
- Eelke Jongejans,
- Henk Siepel,
- Nick Hofland,
- Heinz Schwan,
- Werner Stenmans,
- Andreas Müller,
- Hubert Sumser,

- Published: October 18, 2017
- https://doi.org/10.1371/journal.pone.0185809
- Reader Comments
Global declines in insects have sparked wide interest among scientists, politicians, and the general public. Loss of insect diversity and abundance is expected to provoke cascading effects on food webs and to jeopardize ecosystem services. Our understanding of the extent and underlying causes of this decline is based on the abundance of single species or taxonomic groups only, rather than changes in insect biomass which is more relevant for ecological functioning. Here, we used a standardized protocol to measure total insect biomass using Malaise traps, deployed over 27 years in 63 nature protection areas in Germany (96 unique location-year combinations) to infer on the status and trend of local entomofauna. Our analysis estimates a seasonal decline of 76%, and mid-summer decline of 82% in flying insect biomass over the 27 years of study. We show that this decline is apparent regardless of habitat type, while changes in weather, land use, and habitat characteristics cannot explain this overall decline. This yet unrecognized loss of insect biomass must be taken into account in evaluating declines in abundance of species depending on insects as a food source, and ecosystem functioning in the European landscape.
Citation: Hallmann CA, Sorg M, Jongejans E, Siepel H, Hofland N, Schwan H, et al. (2017) More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 12(10): e0185809. https://doi.org/10.1371/journal.pone.0185809
Editor: Eric Gordon Lamb, University of Saskatchewan, CANADA
Received: July 28, 2017; Accepted: September 19, 2017; Published: October 18, 2017
Copyright: © 2017 Hallmann et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All relevant data are within the paper and its Supporting Information files.
Funding: CH and EJ were supported by the Netherlands Organization for Scientific Research (NWO grants 840.11.001 and 841.11.007), and NH by the Triodos Foundation. The investigations of the Entomological Society Krefeld and its members are spread over numerous individual projects at different locations and in different years. Grants and permits that have made this work possible are listed below: Bezirksregierungen Düsseldorf & Köln, BfN - Bundesamt für Naturschutz, Land Nordrhein-Westfalen - Europäische Gemeinschaft ELER, Landesamt für Agrarordnung Nordrhein-Westfalen, Landesamt für Natur, Umwelt und Verbraucherschutz Nordrhein-Westfalen, Landesamt für Umwelt Brandenburg, Landesamt für Umwelt Rheinland-Pfalz, LVR - Landschaftsverband Rheinland, Naturschutzbund Deutschland, Nordrhein-Westfalen Stiftung, RBN Bergischer Naturschutzverein, RVR - Regionalverband Ruhr, SGD Nord Rheinland-Pfalz, Universitäten Bonn, Duisburg-Essen & Köln, Untere Landschaftsbehörden: Kreis Düren, Kreis Heinsberg, Kreis Kleve, Kreis Viersen, Kreis Wesel & AGLW, Stadt Düsseldorf, Stadt Köln, Stadt Krefeld, Rheinisch Bergischer Kreis, Rhein Kreis Neuss & Rhein-Sieg-Kreis. Members of the Entomological Society Krefeld and cooperating botanists and entomologists that were involved in the empirical investigations are greatly acknowledged: U.W. Abts, F. Bahr, A. Bäumler, D. & H. Beutler, P. Birnbrich, U. Bosch, J. Buchner, F. Cassese, K. Cölln, A.W. Ebmer, R. Eckelboom, B. Franzen, M. Grigo, J. Günneberg, J. Gusenleitner, K. Hamacher, F. Hartfeld, M. Hellenthal, J. Hembach, A. Hemmersbach, W. Hock, V. Huisman-Fiegen, J. Illmer, E. Jansen, U. Jäckel, F. Koch, M. Kreuels, P. Leideritz, I. Loksa, F. B. Ludescher, F. J. Mehring, G. Milbert, N. Mohr, P. Randazzo, K. Reissmann, S. Risch, B. Robert, J. de Rond, U. Sandmann, S. Scharf, P. Scherz, J. Schiffer, C. Schmidt, O. & W. Schmitz, B. P. & W. Schnell, J. L. Schönfeld, E. Schraetz, M. Schwarz, R. Seliger, H. W. Siebeneicher, F. & H. Sonnenburg W. J. S. & P. Sorg, A. Ssymank, H. Sticht, M. Weithmann, W. Wichard and H. Wolf.
Competing interests: The authors have declared that no competing interests exist.
Introduction
Loss of insects is certain to have adverse effects on ecosystem functioning, as insects play a central role in a variety of processes, including pollination [ 1 , 2 ], herbivory and detrivory [ 3 , 4 ], nutrient cycling [ 4 ] and providing a food source for higher trophic levels such as birds, mammals and amphibians. For example, 80% of wild plants are estimated to depend on insects for pollination [ 2 ], while 60% of birds rely on insects as a food source [ 5 ]. The ecosystem services provided by wild insects have been estimated at $57 billion annually in the USA [ 6 ]. Clearly, preserving insect abundance and diversity should constitute a prime conservation priority.
Current data suggest an overall pattern of decline in insect diversity and abundance. For example, populations of European grassland butterflies are estimated to have declined by 50% in abundance between 1990 and 2011 [ 7 ]. Data for other well-studied taxa such as bees [ 8 – 14 ] and moths [ 15 – 18 ] suggest the same trend. Climate change, habitat loss and fragmentation, and deterioration of habitat quality have been proposed as some of the prime suspects responsible for the decline [ 9 – 11 , 13 , 18 – 22 ]. However, the number of studies on insect trends with sufficient replication and spatial coverage are limited [ 10 , 23 – 25 ] and restricted to certain well-studied taxa. Declines of individual species or taxa (e.g. [ 7 , 26 ]) may not reflect the general state of local entomofauna [ 27 ]. The total insect biomass would then be a better metric for the status of insects as a group and its contribution to ecosystem functioning, but very few studies have monitored insect biomass over an extensive period of time [ 28 ]. Hence, to what extent total insect biomass has declined, and the relative contribution of each proposed factor to the decline, remain unresolved yet highly relevant questions for ecosystem ecology and conservation.
Here, we investigate total aerial insect biomass between 1989 and 2016 across 96 unique location-year combinations in Germany, representative of Western European low-altitude nature protection areas embedded in a human-dominated landscape ( S1 Fig ). In all years we sampled insects throughout the season (March through October), based on a standardized sampling scheme using Malaise traps. We investigated rate of decline in insect biomass, and examined how factors such as weather, habitat and land use variables influenced the declines. Knowledge on the state of insect biomass, and it’s direction over time, are of broad importance to ecology and conservation, but historical data on insect biomass have been lacking. Our study makes a first step into filling this gap, and provides information that is vital for the assessment of biodiversity conservation and ecosystem health in agricultural landscapes.
Materials and methods
Biomass data..
Biomass data were collected and archived using a standardized protocol across 63 unique locations between 1989 and 2016 (resulting in 96 unique location-year combinations) by the Entomological Society Krefeld. The standardized protocol of collection has been originally designed with the idea of integrating quantitative aspects of insects in the status assessment of the protected areas, and to construct a long-term archive in order to preserve (identified and not-identified) specimens of local diversity for future studies. In the present study, we consider the total biomass of flying insects to assess the state of local entomofauna as a group.
All trap locations were situated in protected areas, but with varying protection status: 37 locations are within Natura2000 sites, seven locations within designated Nature reserves, nine locations within Protected Landscape Areas (with funded conservation measures), six locations within Water Protection Zones, and four locations of protected habitat managed by Regional Associations. For all location permits have been obtained by the relevant authorities, as listed in the S1 Appendix . In our data, traps located in nutrient-poor heathlands, sandy grasslands, and dune habitats provide lower quantities of biomass as compared to nutrient nutrient-rich grasslands, margins and wastelands. As we were interested in whether the declines interact with local productivity, traps locations were pooled into 3 distinct habitat clusters, namely: nutrient-poor heathlands, sandy grassland, and dunes (habitat cluster 1, n = 19 locations, Fig 1A ), nutrient-rich grasslands, margins and wasteland (habitat cluster 2, n = 41 locations, Fig 1B ) and a third habitat cluster that included pioneer and shrub communities (n = 3 locations).
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0185809.g001
Most locations (59%, n = 37) were sampled in only one year, 20 locations in two years, five locations in three years, and one in four years, yielding in total 96 unique location-year combinations of measurements of seasonal total flying insect biomass. Our data do not represent longitudinal records at single sites, suitable to derive location specific trends (e.g. [ 28 ]). Prolonged trapping across years is in the present context (protected areas) deemed undesirable, as the sampling process itself can negatively impact local insect stocks. However, the data do permit an analysis at a higher spatial level, i.e. by treating seasonal insect biomass profiles as random samples of the state of entomofauna in protected areas in western Germany.
Malaise traps were deployed through the spring, summer and early autumn. They operated continuously (day and night), and catches were emptied at regular intervals, on average every 11.2 days (sd = 6.3). We collected in total 1503 trap samples, with an average of 16 (4–35) successive catches per location-year combination ( Table 1 ). Between 1989 and 2016, a total of 53.54kg of invertebrates have been collected and stored, over a total trap exposure period of 16908 days, within an average of 176 exposure days per location-year combination. Malaise traps are known to collect a much wider diversity of insect species (e.g. [ 29 – 31 ]) as compared to suction traps (e.g. [ 28 ]) and are therefore considered superior as a method of collecting flying insects. On the basis of partial assessments, we can assume that the total number of insects included in 53.54 kg biomass represents millions of individuals.
For each year, the number of locations sampled, the number of location re-sampled, total number of samples, as well as mean and standard deviation of exposure time at the trap locations (in days) are presented.
https://doi.org/10.1371/journal.pone.0185809.t001
The sampling was standardized in terms of trap construction, size and design (identical parts), colors, type of netting and ground sealing, trap orientation in the field as well as slope at the trap location. Hence none of the traps differed in any of these field aspects. Our trap model was similar to the bi-colored malaise trap model by Henry Townes [ 32 , 33 ]. The traps, collecting design, and accompanying methods of biomass measurement as designed and applied by the Entomological Society Krefeld are described elsewhere [ 34 – 36 ] and in S2 Appendix .
Trap catches were stored in 80% ethanol solution, prior to weighing, and total insect biomass of each catch (bottle) was obtained based on a standardized measurement protocol by first subtracting fluid content. In order to optimally preserve samples for future species determination, the insects were weighed in an alcohol-wet state. First, the alcohol concentration in the vessels was stabilized to 80%, while this concentration was controlled with an areometer over a period of at least two days. In order to obtain biomass per sample with sufficient accuracy and comparability, the measuring process was fixed using a standardized protocol [ 34 ]. For this purpose the insects of a sample were poured onto a stainless steel sieve (10cm diameter) of 0.8 mm mesh width. This sieve is placed slightly obliquely (30 degrees) over a glass vessel. The skew position accelerates the first runoff of alcohol and thus the whole measuring procedure. The drop sequence is observed with a stopwatch. When the time between two drops has reached 10 seconds for the first time, the weighing process is performed with a laboratory scale. For the determination of the biomass, precision scales and analytical scales from Mettler company were used with an accuracy of at least 0.1g and controlled with calibrated test weights at the beginning of a new weighing series. In a series of 84 weightings of four different samples repeating this measurement procedure, an average deviation from the mean value of the measurement results of 0.4 percent was observed (unpublished results).
Weather data.
Climate change is a well-known factor responsible for insect declines [ 15 , 18 , 21 , 37 ]. To test if weather variation could explain the observed decline, we included mean daily temperature, precipitation and wind speed in our analysis, integrating data from 169 weather stations [ 38 ] located within 100km to the trap locations. We examined temporal trends in each weather variable over the course of the study period to assess changes in climatic conditions, as a plausible explanation for insect decline. Estimates of each weather variable at the trap locations were obtained by interpolation of each variable from the 169 climate stations.
We initially considered mean daily air temperature, precipitation, cloud cover, relative air moisture content, wind speed, and sunshine duration. However, only temperature, precipitation and wind speed were retained for analysis, as the other variables were significantly correlated with the selected variables [R(temperature, cover) = −43.2%, R(temperature, sunshine) = 53.4%, R(precipitation, moisture) = −47.3%] and because we wanted to keep the number of covariates as low as possible. Additionally, we calculated the number of frost days and the sum of precipitation in the months November- February preceding a sampling season. We used spatio-temporal geostatistical models [ 39 , 40 ] to predict daily values for each weather variable to each trap location. Amongst other methods, the geostatistical approach is considered a superior interpolation method in order to derive weather variables to trap locations [ 41 ]. Uncertainty in interpolated variables such as wind speed is usually associated with altitude differences. However, as our trap locations are all situated in lowland areas with little altitude variation, we do not expect a large error in our interpolations at trap locations.
Land use data.
Land use variables (and changes therein) were derived from aerial photographs [ 44 ] taken within two distinct time periods (between 1989–1994, and between 2012–2015), and allowed us to characterize land use composition at surroundings of the traps, as well as changes over time. We distinguished cover of forests, agricultural areas, natural grassland, and surface water. For each trap location, aerial photographs were manually processed, polygons extracted and categorized, and their surface area calculated with a radius of 200 meter. Preliminary analysis of the relationship between log biomass and landuse variables, on a subset of the trap locations, indicated that land use elements at 200m radius better predicted insect biomass than elements at 500 and 1000m radius, similar to findings elsewhere for wild bees [ 45 ]. Land use variables were measured at a coarse temporal resolution, but fortunately cover the temporal span of insect sampling. To link the cover of a given land use variable to the insect biomass samples in a particular year, we interpolated coverage between the two time points to the year of insect sampling using generalized linear models with a binomial error distribution, a logit link, and an estimated dispersion parameter. Mean distributions of land use at each of the two time points are depicted in S3A & S3B Fig .
Habitat data.
Plant inventories were conducted in the immediate surroundings (within 50m) of the trap, in the same season of insect sampling. These data permitted the assessment of plant species richness (numbers of herbs, shrubs and trees) and environmental conditions based on average Ellenberg values [ 46 – 48 ], as well as changes therein over time. Each Ellenberg indicator (we considered nitrogen, pH, light, temperature and moisture) was averaged over all species for each location-year combination. We examined annual trends in each of the above-mentioned variables in order to uncover potential structural changes in habitat characteristics over time. Species richness was analyzed using mixed-effects generalized linear models [ 49 ] with a random intercept for trap location and assuming a Poisson distribution for species richness, and a normal distribution for mean Ellenberg indicator values. Although a Poisson distribution fitted tree and shrub species adequately, (residual deviance/ degree of freedom = 0.94 and 1.04 respectively), severe overdispersion was found for herb species richness (residual deviance/ degree of freedom = 2.16). Trend coefficients of richness over time between a Poisson mixed effects model and a negative binomial model were comparable but differed in magnitude (Poisson GLMM: −0.034 (se = 0.003), vs NB GLMM −0.027 (se = 0.006)). Although the fit is not perfect in the case of herb richness, we believe our trend adequately describes direction of change over time. Mean changes in plant species richness are depicted in S3C Fig .
Insect biomass model
We developed a series of models each consisting of a set of explanatory variables that measure aspects of climate, land use and local habitat characteristics. Significant explanatory variables in these models were combined into a final model, which was then reduced to exclude insignificant effects. An overview of which covariates were included in each model is given in Table 2 .
The year covariate yields the annual trend coefficient.
https://doi.org/10.1371/journal.pone.0185809.t002
Weather effects explored were daily temperature, precipitation and wind speed, as well as the number of frost days and sum of precipitation in the preceding winter. Habitat effects explored tree and herb species richness, as well as average Ellenberg values for nitrogen, pH, light, temperature and moisture, per location-year combination. Land use effects explored the fractions of agricultural area, forest, grass, and surface water in a radius of 200m around the plot location.
Parameter values are obtained by the use of Markov chain Monte Carlo (MCMC) methods by the aid of JAGS (Just Another Gibbs Sampler [ 50 ]) invoked through R [ 51 ] and the R2Jags package [ 52 ]. JAGS model scripts are given in S1 Code , while data are given in S1 and S2 Dataset. For each model, we ran 3 parallel chains each consisting of 24000 iterations (first 4000 discarded), and kept every 10 th value as a way to reduce within chain autocorrelation. We used vague priors for all parameters, with uniform distributions for the residual and random effect variance components, and flat normal distributions (with very high variance) for all other parameters. Covariates in X were standardized prior to model fitting, with the exception of year (values 1–26), and land use variables (proportions within 0–1 range).
For each model, the number of parameters, the Deviance Information Criterion, the effective number of parameters (pD), calculated R 2 and difference in DIC units between each model and the model with lowest ΔDIC. See Table 2 for covariates included in each model.
https://doi.org/10.1371/journal.pone.0185809.t003
Our basic model included habitat cluster (3 levels), a quadratic effect for day number, an annual trend coefficient measuring the rate of biomass change, and the interactions between the annual trend coefficient and the day number variables. Next we developed 3 models each consisting of either weather variables ( S1 Table ), land use variables ( S2 Table ), or habitat variables. Because interactions between the annual rate of change and land use variables seemed plausible, a fourth model was developed to include these interactions ( S3 Table ). Finally, all significant variables were combined into our final model ( Table 4 ), which included effects of an annual trend coefficient, season (linear and quadratic effect of day number), weather (temperature, precipitation, number of frost days), land use (cover of grassland and water, as well as interaction between grassland cover and trend), and habitat (number of herb and tree species as well as Ellenberg temperature).
For each included variable, the corresponding coefficient mean, standard deviation and 95% credible intervals are given. P-values were calculated empirically based on posterior distributions of coefficients.
https://doi.org/10.1371/journal.pone.0185809.t004
Our estimate of decline is based on our basic model, from which we can derive seasonal estimates of daily biomass for any given year. The basic model includes only a temporal (annual and seasonal effects, as well as interactions) and a basic habitat cluster distinction (additive effects only) as well as a random trap location effect. We here report the annual trend coefficient, as well as a weighted estimate of decline that accounts for the within season differences in biomass decline. The weighted insect biomass decline was estimated by projecting the seasonal biomass (1-April to 30-October) for years 1989 and 2016 using coefficients our basic model, and then dividing the summed (over the season) biomass of 2016 by the summed biomass over 1989.
Using our final model, we assessed the relative contribution (i.e. net effect) of the explanatory variables to the observed decline, both combined and independently. To this aim we projected the seasonal daily biomass for the years 1989 and 2016 twice: first we kept covariates at their mean values during the early stages of the study period, and second we allowed covariate values to change according to the observed mean changes (see S2 and S3 Figs). Difference in the total biomass decline between these two projections are interpreted as the relative contribution of the explanatory variables to the decline. The marginal (i.e. independent) effects of each covariate were calculated by projecting biomass increase/decline as result of the observed temporal developments in each variable separately, and expressing it as percentual change.
Our data provide repetitions across years for only a subset of locations (n = 26 out of 63). As such, spatial variation in insect biomass may confound the estimated trend. To verify that this is not the case, we fitted our basic model (but excluding the day number and year interaction to avoid overparameterization) to the subset of our data that includes only locations that were sampled in more than one year. Seasonal profiles of daily biomass values are depicted in S4 Fig . Finally, we reran our basic model for the two (of the three) habitat clusters (for which sufficient data existed; see Biomass Data) separately in order to compare the rate of decline between them ( S5 Fig ).
Following corrections for seasonal variation and habitat cluster (basic model, see Materials and methods ), the annual trend coefficient of our basic model was significantly negative (annual trend coefficient = −0.063, sd = 0.002, i.e. 6.1% annual decline). Based on this result, we estimate that a major (up to 81.6% [79.7–83.4%]) decline in mid-summer aerial insect biomass has taken place since 1989 ( Fig 2A ). However, biomass loss was more prominent in mid-summer as compared to the start and end of the season ( Fig 3A ), indicating that the highest losses occur when biomass is highest during the season ( Fig 2B ). As such, a seasonally weighted estimate (covering the period 1-April to 30-October; see methods) results in an overall 76.7% [74.8–78.5%] decline over a 27 year period. The pattern of decline is very similar across locations that were sampled more than once ( Fig 4 ), suggesting that the estimated temporal decline based on the entire dataset is not confounded by the sampling procedure. Re-estimation of the annual decline based on 26 locations that have been sampled in more than one year ( S4 Fig ), revealed a similar rate of decline (76.2%[73.9–78.3%]).
(A) Boxplots depict the distribution of insect biomass (gram per day) pooled over all traps and catches in each year (n = 1503). Based on our final model, the grey line depicts the fitted mean (+95% posterior credible intervals) taking into account weather, landscape and habitat effects. The black line depicts the mean estimated trend as estimated with our basic model. (B) Seasonal distribution of insect biomass showing that highest insect biomass catches in mid summer show most severe declines. Color gradient in both panels range from 1989 (blue) to 2016 (orange).
https://doi.org/10.1371/journal.pone.0185809.g002
(A) Seasonal decline of mean daily insect biomass as estimated by independent month specific log-linear regressions (black bars), and our basic mixed effects model with interaction between annual rate of change and a quadratic trend for day number in season. (B), Seasonal phenology of insect biomass (seasonal quantiles of biomass at 5% intervals) across all locations revealing substantial annual variation in peak biomass (solid line) but no direction trend, suggesting no phenological changes have occurred with respect to temporal distribution of insect biomass.
https://doi.org/10.1371/journal.pone.0185809.g003
(A) Daily biomass (mean ±1 se) across 26 locations sampled in multiple years (see S4 Fig for seasonal distributions). (B) Distribution of mean annual rate of decline as estimated based on plot specific log-linear models (annual trend coefficient = −0.053, sd = 0.002, i.e. 5.2% annual decline).
https://doi.org/10.1371/journal.pone.0185809.g004
Insect biomass was positively related to temperature and negatively to precipitation ( S1 Table ). Including lagged effects of weather revealed no effect of either number of frost days, or winter precipitation, on the biomass in the next season ( S1 Table ). The overall model fit improved as compared to our basic model (R 2 = 65.4%, Table 3 ), explaining within and between year variation in insect biomass, but not the overall decline (log( λ ) = −0.058, sd = 0.002). Over the course of the study period, some temporal changes occurred in the means of the weather variables ( S2 Fig ), most notably an increase by 0.5°C in mean temperature and a decline 0.2 m/sec in mean wind speed. Yet, these changes either do not have an effect on insect biomass (e.g. wind speed) either are expected to positively affected insect biomass (e.g. increased temperature). Furthermore, a phenological shift with peak biomass earlier in the season could have resulted in lower biomass in the mid-season ( Fig 3A ), but this does not appear to be the case as none of the seasonal distribution quantiles in biomass showed any temporal trend ( Fig 3B ).
There was substantial variation in trapped insect biomass between habitat clusters (see Materials and methods ), with nutrient-rich grasslands, margins and wasteland containing 43% more insect biomass than nutrient-poor heathland, sandy grassland, and dunes. Yet, the annual rate of decline was similar, suggesting that the decline is not specific to certain habitat types ( S5 Fig ). To further characterize trap locations, we used past (1989–1994) and present (2012–2015) aerial photographs and quantified land use cover within 200m around the trap locations. On average, cover of arable land decreased, coverage of forests increased, while grassland and surface water did not change much in extent over the last three decades ( S3 Fig ). Overall, adding land use variables alone did not lead to a substantial improvement of the model fit (R 2 = 61.3%, Table 3 ), nor did it affect the annual trend coefficient (log( λ ) = −0.064, sd = 0.002). While presence of surface water appeared to significantly lower insect biomass, none of the other variables were significantly related to biomass. However, including interactions between the annual trend coefficient and land use variables increased the model fit slightly ( Table 3 ), and revealed significant interactions for all variables except coverage of surface water ( S2 Table ). These interactions, which were retained in our final model ( Table 4 ), revealed higher rates of decline where coverage of grassland was higher, while lower declines where forest and arable land coverage was higher.
We hypothesized that successional changes in plant community [ 55 ] or changes in environmental conditions [ 9 , 18 ], could have affected the local insect biomass, and hence explain the decline. Plant species inventories that were carried out in the immediate vicinity of the traps and in the same season of trapping, revealed that species richness of trees, shrubs and herbs declined significantly over the course of the study period ( S3 Fig ). Including species richness in our basic model, i.e. number of tree species and log number of herb species, revealed significant positive and negative effects respectively on insect biomass, but did not affect the annual trend coefficient ( S3 Table ), explaining some variation between locations rather than the annual trend coefficient. Moreover, and contrary to expectation, trends in herb species richness were weakly negatively correlated with trends in insect biomass, when compared on per plot basis for plots sampled more than once. Ellenberg values of plant species provide a reliable indicator for the environmental conditions such as pH, nitrogen, and moisture [ 46 , 47 ]. Around trap locations, mean indicators (across all locations) were stable over time, with changes in the order of less than 2% over the course of the study period. Adding these variables to our basic model revealed a significant positive effect of nitrogen and light, and a significant negative effect of Ellenberg temperature on insect biomass, explaining a major part of the variation between the habitat types. However, Ellenberg values did not affect the insect biomass trend coefficient (log( λ ) = −0.059, sd = 0.003, S3 Table ) and only marginally improved the model fit (R 2 = 61.9%, Table 3 ). All habitat variables were considered in our final model ( Table 4 ), with the exception of of pH and moisture.
Our final model, based on including all significant variables from previous models, revealed a higher trend coefficient as compared to our basic model (log( λ ) = −0.081, sd = 0.006, Table 4 ), suggesting that temporal developments in the considered explanatory variables counteracted biomass decline to some degree, leading to an even more negative coefficient for the annual trend. The marginal net effect of changes in each covariate over time (see Analysis), showed a positive contribution to biomass growth rate of temporal developments in arable land, herb species richness, and Ellenberg Nitrogen, while negative effects of developments of tree species richness and forest coverage ( Fig 5 ). For example, the negative effect of arable land on biomass ( Table 4 ), in combination with a decrease in coverage of arable land ( S3 Fig ), have resulted in a net positive effect for biomass ( Fig 5 ). Projections of our final model, while fixing the coefficient for the temporal annual trend log( λ ) to zero, suggest insect biomass would have remained stable, or even increased by approximately 8% (mean rate = 1.075, 0.849–1.381) over the course of the study period.
Each bar represents the rate of change in total insect biomass, as the combined effect of the relevant coefficient ( Table 4 ) and the temporal development of each covariate independently ( S2 and S3 Figs).
https://doi.org/10.1371/journal.pone.0185809.g005
Our results document a dramatic decline in average airborne insect biomass of 76% (up to 82% in midsummer) in just 27 years for protected nature areas in Germany. This considerably exceeds the estimated decline of 58% in global abundance of wild vertebrates over a 42-year period to 2012 [ 56 , 57 ]. Our results demonstrate that recently reported declines in several taxa such as butterflies [ 7 , 25 – 27 , 58 ], wild bees [ 8 – 14 ] and moths [ 15 – 18 ], are in parallel with a severe loss of total aerial insect biomass, suggesting that it is not only the vulnerable species, but the flying insect community as a whole, that has been decimated over the last few decades. The estimated decline is considerably more severe than the only comparable long term study on flying insect biomass elsewhere [ 28 ]. In that study, 12.2m high suction traps were deployed at four locations in the UK over the time period 1973–2002, and showed a biomass decline at one of the four sites only. However, the sampling designs differ considerably between the two studies. Suction traps mainly target high-flying insects, and in that study the catches were largely comprised of flies belonging to the Bibionidae family. Contrary, malaise traps as used in the present study target insects flying close to the ground surface (up to 1 meter), with a much wider diversity of taxa. Future investigations should look into how biomass is distributed among insect species, and how species trends contribute to the biomass decline.
Although the present dataset spans a relatively large number of years (27) and sites (63), the number of repetitions (i.e. multiple years of seasonal distributions at the same locations) was lower (n = 26). We are however confident that our estimated rate of decline in total biomass resembles the true rate of decline, and is not an artifact of site selection. Firstly, our basic model (including an annual rate of decline) outperformed the null-model (without an annual rate of decline; ΔDIC = 822.62 units; Table 3 ), while at the same time, between-plot variation (i.s. σ site ) and residual variation ( v ) decreased by 44.3 and 9.7% respectively, after incorporating an annual rate of decline into the models. Secondly, using only data from sites at which malaise traps were operating in at least two years, we estimated a rate of decline similar to using the full dataset ( Fig 4 ), with the pattern of decline being congruent across locations ( S4 Fig ). Taken together, there does not seem to be evidence that spatial variation (between sites) in this dataset forms a confounding factor to the estimated temporal trend, and conclude that our estimated biomass decline is representative for lowland protected areas in west Germany.
In light of previously suggested driving mechanisms, our analysis renders two of the prime suspects, i.e. landscape [ 9 , 18 , 20 ] and climate change [ 15 , 18 , 21 , 37 ], as unlikely explanatory factors for this major decline in aerial insect biomass in the investigated protected areas. Habitat change was evaluated in terms of changes in plant species composition surrounding the standardized trap locations, and in plant species characteristics (Ellenberg values). Land use changes was evaluated in terms of proportional surface changes in aerial photographs, and not for example changes in management regimes. Given the major decline in insect biomass of about 80%, much stronger relationships would have been expected if changes in habitat and land use were the driving forces, even with the somewhat crude parameters that were at our disposal.
The decline in insect biomass, being evident throughout the growing season, and irrespective of habitat type or landscape configuration, suggests large-scale factors must be involved. While some temporal changes in climatic variables in our study area have taken place, these either were not of influence (e.g. wind speed), or changed in a manner that should have increased insect biomass (e.g temperature). However, we have not exhaustively analysed the full range of climatic variables that could potentially impact insect biomass. For example prolonged droughts, or lack of sunshine especially in low temperatures might have had an effect on insect biomass [ 59 – 62 ]. Agricultural intensification [ 17 , 20 ] (e.g. pesticide usage, year-round tillage, increased use of fertilizers and frequency of agronomic measures) that we could not incorporate in our analyses, may form a plausible cause. The reserves in which the traps were placed are of limited size in this typical fragmented West-European landscape, and almost all locations (94%) are enclosed by agricultural fields. Part of the explanation could therefore be that the protected areas (serving as insect sources) are affected and drained by the agricultural fields in the broader surroundings (serving as sinks or even as ecological traps) [ 1 , 63 – 65 ]. Increased agricultural intensification may have aggravated this reduction in insect abundance in the protected areas over the last few decades. Whatever the causal factors responsible for the decline, they have a far more devastating effect on total insect biomass than has been appreciated previously.
The widespread insect biomass decline is alarming, ever more so as all traps were placed in protected areas that are meant to preserve ecosystem functions and biodiversity. While the gradual decline of rare insect species has been known for quite some time (e.g. specialized butterflies [ 9 , 66 ]), our results illustrate an ongoing and rapid decline in total amount of airborne insects active in space and time. Agricultural intensification, including the disappearance of field margins and new crop protection methods has been associated with an overall decline of biodiversity in plants, insects, birds and other species in the current landscape [ 20 , 27 , 67 ]. The major and hitherto unrecognized loss of insect biomass that we report here for protected areas, adds a new dimension to this discussion, because it must have cascading effects across trophic levels and numerous other ecosystem effects. There is an urgent need to uncover the causes of this decline, its geographical extent, and to understand the ramifications of the decline for ecosystems and ecosystem services.
Supporting information
S1 appendix. malaise trap permissions..
https://doi.org/10.1371/journal.pone.0185809.s001
S2 Appendix. Malaise traps.
https://doi.org/10.1371/journal.pone.0185809.s002
https://doi.org/10.1371/journal.pone.0185809.s003
S1 Dataset.
https://doi.org/10.1371/journal.pone.0185809.s004
S2 Dataset.
https://doi.org/10.1371/journal.pone.0185809.s005
S1 Fig. Map of study area.
Insect trap locations (yellow points) in Nordrhein-Westfalen (n = 57), Rheinland-Pfalz (n = 1) and Brandenburg (n = 5), as well as weather stations (crosses) used in the present analysis.
https://doi.org/10.1371/journal.pone.0185809.s006
S2 Fig. Temporal variation in weather variables.
Annual means (A-C), daily means (D-F), and mean daily residual values (G-I) of temperature, precipitation and wind speed respectively. In all panels, black lines depict data while blue and red lines represent long term and seasonal fitted means of the variables, respectively.
https://doi.org/10.1371/journal.pone.0185809.s007
S3 Fig. Land use and plant species richness changes.
Mean land use in 1989–1994 (A) and 2012–2014 (B), based on aerial photograph analysis at 63 protected areas show a decrease of arable land and an increase in forested area over the past 25 years. (C) Changes in plants species richness for herbs (black) shrubs (red) and trees (blue). Annual means as well as mean trends are depicted in the corresponding colors. Linear trends are based on generalized linear mixed effects models with a Poisson error distribution and a random intercept effect for location. Note, zero values for tree and shrub species not depicted.
https://doi.org/10.1371/journal.pone.0185809.s008
S4 Fig. Seasonal profiles of daily biomass across 26 locations.
For each location, different colors represent different years, with time color-coded from green (1989) to red (2016). X-axis represents day number (January 1 = 0).
https://doi.org/10.1371/journal.pone.0185809.s009
S5 Fig. Daily biomass of insects over time for two habitat clusters.
Boxplots depict the distribution of insect biomass pooled over all traps and catches in each year at trap locations in nutrient-poor heathland, sandy grassland, and dunes (A), and in nutrient-rich grasslands, margins and wasteland (B). Grey lines depict the fitted mean (+95% posterior credible intervals), while the black lines the mean estimated trend. Estimated annual decline amounts to 7.5%(6.6–8.4) for habitat cluster 1, as compared to 5.2% (4.8–5.5) habitat cluster 2. Models fitted independently for each habitat location. Color gradient in all panels range from 1989 (blue) to 2016 (orange).
https://doi.org/10.1371/journal.pone.0185809.s010
S1 Table. Posterior parameter estimates of the mixed effects model including weather variables.
For each included variable, the corresponding coefficient posterior mean, standard deviation and 95% credible intervals are given. P-values are calculated empirically based on posterior distributions of coefficients.
https://doi.org/10.1371/journal.pone.0185809.s011
S2 Table. Posterior parameter estimates of the mixed effects model including land use variables and interactions.
https://doi.org/10.1371/journal.pone.0185809.s012
S3 Table. Posterior parameter estimates of the mixed effects model including habitat variables.
https://doi.org/10.1371/journal.pone.0185809.s013
Acknowledgments
CH and EJ were supported by the Netherlands Organization for Scientific Research (NWO grants 840.11.001 and 841.11.007), and NH by the Triodos Foundation. The investigations of the Entomological Society Krefeld and its members are spread over numerous individual projects at different locations and in different years. Grants and permits that have made this work possible are listed below:
Bezirksregierungen Düsseldorf & Köln, BfN—Bundesamt für Naturschutz, Land Nordrhein-Westfalen—Europäische Gemeinschaft ELER, Landesamt für Agrarordnung Nordrhein-Westfalen, Landesamt für Natur, Umwelt und Verbraucherschutz Nordrhein-Westfalen, Landesamt für Umwelt Brandenburg, Landesamt für Umwelt Rheinland-Pfalz, LVR—Landschaftsverband Rheinland, Naturschutzbund Deutschland, Nordrhein-Westfalen Stiftung, RBN—Bergischer Naturschutzverein, RVR—Regionalverband Ruhr, SGD Nord Rheinland-Pfalz, Universitäten Bonn, Duisburg-Essen & Köln, Untere Landschaftsbehörden: Kreis Düren, Kreis Heinsberg, Kreis Kleve, Kreis Viersen, Kreis Wesel & AGLW, Stadt Düsseldorf, Stadt Köln, Stadt Krefeld, Rheinisch Bergischer Kreis, Rhein Kreis Neuss & Rhein-Sieg-Kreis. Members of the Entomological Society Krefeld and cooperating botanists and entomologists that were involved in the empirical investigations are greatly acknowledged: U.W. Abts, F. Bahr, A. Bäumler, D. & H. Beutler, P. Birnbrich, U. Bosch, J. Buchner, F. Cassese, K. Cölln, A.W. Ebmer, R. Eckelboom, B. Franzen, M. Grigo, J. Günneberg, J. Gusenleitner, K. Hamacher, F. Hartfeld, M. Hellenthal, J. Hembach, A. Hemmersbach, W. Hock, V. Huisman-Fiegen, J. Illmer, E. Jansen, U. Jäckel, F. Koch, M. Kreuels, P. Leideritz, I. Loksa, F. B. Ludescher, F. J. Mehring, G. Milbert, N. Mohr, P. Randazzo, K. Reissmann, S. Risch, B. Robert, J. de Rond, U. Sandmann, S. Scharf, P. Scherz, J. Schiffer, C. Schmidt, O. & W. Schmitz, B. P. & W. Schnell, J. L. Schönfeld, E. Schraetz, M. Schwarz, R. Seliger, H. W. Siebeneicher, F. & H. Sonnenburg W. J. S. & P. Sorg, A. Ssymank, H. Sticht, M. Weithmann, W. Wichard and H. Wolf.
- View Article
- Google Scholar
- PubMed/NCBI
- 38. Deutscher Wetterdienst. Recent and historical daily NKDZ climate station data; 2015. http://www.deutscher-wetterdienst.de .
- 43. Jarvis A, Reuter HI, Nelson A, Guevara E. Hole-filled seamless SRTM data V4, International Centre for Tropical Agriculture (CIAT); 2008. http://srtm.csi.cgiar.org .
- 44. TIM-online. Karten NRW; 2016. http://www.tim-online.nrw.de/tim-online/initParams.do .
- 46. Ellenberg H. Zeigerwerte der Gefäßpflanzen Mitteleuropas. Scripta Geobotanica 9. Goltze, Göttingen. 1974;.
- 47. Ellenberg H, Weber HE, Düll R, Wirth V, Werner W, Paulißen D, et al. Zeigerwerte von pflanzen in Mitteleuropa. E. Goltze; 1992.
- 50. Plummer M, et al. JAGS: A program for analysis of Bayesian graphical models using Gibbs sampling. In: Proceedings of the 3rd International Workshop on Distributed Statistical Computing. vol. 124. Vienna; 2003. p. 125.
- 51. R Core Team. R: A Language and Environment for Statistical Computing; 2015. Available from: https://www.R-project.org/ .
- 52. Su YS, Yajima M. R2jags: Using R to Run JAGS; 2015. Available from: http://CRAN.R-project.org/package=R2jags .
- 56. WWF International. Living Planet Report 2016: Risk and resilience in a new era. Gland, Switzerland. 2016;.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

Biodiversity seen through the perspective of insects: 10 simple rules on methodological choices and experimental design for genomic studies
Pável matos-maraví.
1 Department of Biological and Environmental Sciences, University of Gothenburg, Gothenburg, Sweden
2 Gothenburg Global Biodiversity Centre, Gothenburg, Sweden
3 Institute of Entomology, Biology Centre CAS, České Budějovice, Czech Republic
Camila Duarte Ritter
4 Department of Eukaryotic Microbiology, University of Duisburg-Essen, Essen, Germany
Christopher J. Barnes
5 Natural History Museum of Denmark, University of Copenhagen, Copenhagen, Denmark
Martin Nielsen
6 Section for Evolutionary Genomics, Department of Biology, University of Copenhagen, Copenhagen, Denmark
Urban Olsson
Niklas wahlberg.
7 Department of Biology, Lund University, Lund, Sweden
Daniel Marquina
8 Department of Bioinformatics and Genetics, Swedish Museum of Natural History, Stockholm, Sweden
9 Department of Zoology, Stockholm University, Stockholm, Sweden
Ilari Sääksjärvi
10 Biodiversity Unit, University of Turku, Turku, Finland
Alexandre Antonelli
11 Royal Botanical Garden, Kew, Richmond, Surrey, UK
Associated Data
The following information was supplied regarding data availability:
This article conducted a literature review and retrieved data from Web of Science. The raw data and input data used for the analyses are provided as Supplemental Files .
Massively parallel DNA sequencing opens up opportunities for bridging multiple temporal and spatial dimensions in biodiversity research, thanks to its efficiency to recover millions of nucleotide polymorphisms. Here, we identify the current status, discuss the main challenges, and look into future perspectives on biodiversity genomics focusing on insects, which arguably constitute the most diverse and ecologically important group among all animals. We suggest 10 simple rules that provide a succinct step-by-step guide and best-practices to anyone interested in biodiversity research through the study of insect genomics. To this end, we review relevant literature on biodiversity and evolutionary research in the field of entomology. Our compilation is targeted at researchers and students who may not yet be specialists in entomology or molecular biology. We foresee that the genomic revolution and its application to the study of non-model insect lineages will represent a major leap to our understanding of insect diversity.
Introduction
The global decline in biodiversity is unquestionable ( Barnosky et al., 2011 ). The rate of species diversity loss is comparable to those of ancient mass-extinction events ( Ceballos et al., 2015 ). However, our understanding of the mechanisms that form and maintain species diversity and the impact of environmental disturbances on biodiversity remains limited. Not only do the current methodologies to quantify biodiversity at different temporal and spatial scales need to be profoundly revised ( Vellend, 2017 ), but also a multi-disciplinary effort is necessary to fully understand species diversity and its evolution. In order to maximize efforts when analyzing biodiversity, large datasets need to be generated for hundreds or thousands of specimens with as few steps as possible, following easy-to-implement protocols. Massively parallel DNA sequencing, also called high-throughput sequencing or next-generation sequencing, has been one of the leading technologies for the generation of molecular data since the mid 2000s ( Metzker, 2010 ; Mardis, 2017 ; Shendure et al., 2017 ). By using a multiplexing approach, massively parallel sequencing outperforms automated Sanger sequencing in efficiency to recover genomic information, which can be used to understand species diversity variation in time and space.
In this article, we aim to review and to provide a practical guideline on the use of massively parallel DNA sequencing technologies with a focus on one of the largest biotic radiations on Earth: insects. These six-legged invertebrates represent more than half of all known eukaryotic species ( Grimaldi & Engel, 2005 ; Mora et al., 2011 ; Stork et al., 2015 ; Stork, 2018 ) and they are one of the most important components of eukaryotic biodiversity in terms of abundance and ecology. However, as much as 80% of insect diversity, and therefore much of the Earth’s biodiversity, remains to be formally described ( Hamilton et al., 2010 ; Scheffers et al., 2012 ; Stork, 2018 ). While there is so much undescribed insect diversity in nature, a significant number may already be deposited within museum collections in need of formal description ( Suarez & Tsutsui, 2004 ; Veijalainen et al., 2012 ). Therefore, the study of biodiversity through massively parallel sequencing applied to insects, using both mass-sampling techniques in the field and the archived material at public and private collections, is timely and represents a significant opportunity to advance our understanding of life on Earth.
This article fills a gap in the literature in the form of a simple, concise and hopefully easy-to-follow guideline to study biodiversity using insects and massively parallel sequencing. Accordingly, this review is primarily targeted at researchers and students who may not yet be experts in entomology or molecular biology.
Survey methodology
The authors of this paper are familiar with entomological mass-sampling techniques, specimen preservation and storage for genomic work, massively parallel sequencing and tools for post-sequencing bioinformatics. We discussed the relevant literature on these topics during a two-day workshop titled “Insect diversity and evolution on the era of genomics,” held on the February 27th and 28th, 2017 in Gothenburg, Sweden. During this meeting, we reviewed published literature related to biodiversity and evolutionary research using insects, including but not limited to methods, reviews and original articles. In order to unveil the number of publications using insects and high-throughput sequencing over years, the most popular sequencing platforms and library preparations, we ensured an unbiased procedure by searching the literature stored in the Web of Science ™ Core Collection on November 22nd, 2018. We used 12 combinations of the keywords: “insect” + “biodiversity”/“museum”/“metabarcoding”/“phylogenom*” + “next generation sequencing”/“high throughput sequencing”/“single molecule sequencing.” We searched for publications from 2006, the year of release of the first truly high-throughput sequencing platform ( Goodwin, McPherson & McCombie, 2016 ), to November 2018. We retrieved a total of 118 publications ( File S1 ) and we filtered this list by type of article (original article, review, others). In addition, based on our expertise, we added to this list 18 relevant original articles that were not retrieved in our search using Web of Science. In total, we selected 91 original articles that generated sequence data by massively parallel sequencing for discussion below ( File S2 ). We acknowledge that this is not a complete list of studies on this topic, but we consider it to be representative for the work being conducted in the last years.
Ten simple steps to study biodiversity through insect genomics
We structure this article in 10 simple rules ( Fig. 1 ), formulated in a way that we hope will be accessible for readers who may not yet be familiar with entomological or massively parallel sequencing approaches. Based on these recommendations, we hope that readers will eventually be capable of (1) better interpret the results and conclusions coming from published insect biodiversity research, and (2) start planning a multi-dimensional study of biodiversity using insects as target group and high-throughput sequencing. Overall, we briefly review the current state in biodiversity and evolutionary research through the study of insect diversity. We identify a series of limitations and challenges currently faced by these studies, but we also find hopeful approaches to study biodiversity patterns through the perspective of insects.

Rule 1: Define the questions and scope of the study
Producing genomic data is no longer a major challenge for many research groups. Instead, many researchers seem to be producing large amounts of data, without always having a clear idea of how to properly use them afterward. Although it may seem obvious, we consider important to stress that careful thinking and planning is required to define the research questions and hypothesis of any study, and how to best address them. This is particularly important when dealing with a data-rich, novel technology such as massively parallel DNA sequencing. A few projects might be totally discovery-driven with no prior expectations, but in general it is preferable to clearly define the hypotheses to be tested a priori , and how. This will then inform on the whole chain of methods and analyses. There is no “one size fits all” methodology when it comes to biodiversity and evolutionary studies.
With massively parallel DNA sequencing, the study of evolutionary relations can be complemented with fast quantification of diversity and abundances. It also facilitates research on species interactions such as studies on ecological networks through metabarcoding ( Toju, 2015 ), and in environmental samples ( Shokralla et al., 2012 ) or even from the ethanol used for preservation of historical specimens ( Linard et al., 2016 ). However, economical limitations exist regarding the number of specimens and the extent of their genomes that can be sequenced in a typical project ( Wachi, Matsubayashi & Maeto, 2018 ). Therefore, researchers should choose from a series of available sequencing approaches that best suits their research questions (see Rule 7). For example, if the focus is on finding potential loci involved in adaptation and speciation, a reduced representation of the genomes might be cost-efficient because several individuals from different populations could be pooled in one sequencing experiment. If the aim is instead to profile many organisms within insect communities, DNA metabarcoding may provide a fast quantification of diversity.
Rule 2: Set up your collaborations strategically
A major challenge in the study of evolution from populations to species is the lack of non-genomic data, including taxonomic, paleontological, and ecological information. Despite the abundance of genomic information that can nowadays be generated, major challenges remain to (1) increase field expeditions in search of the unknown diversity, (2) incorporate fossil data in phylogenies based on molecular data, and (3) study the phenotypes and life history data in specimen collections. Naturally, the most efficient direction to integrate such different perspectives is to establish and strengthen a collaborative network. For example, working along with paleontologists might bring a temporal perspective in the study of evolution and biodiversity dynamics ( Marshall, 2017 ). Collaborating closely with ecologists would strengthen the study of adaptation and the mechanisms of speciation. A comprehensive knowledge of life history data, insect ecologies, or common garden experiments are ideal to tease apart adaptive from non-adaptive variation. Moreover, natural history museums (NHMs) are the repositories of our natural world and include not only archived specimens but also valuable historical, demographic, life-history, and genetic data that can add additional dimensions to evolutionary research ( Burrell, Disotell & Bergey, 2015 ; Buerki & Baker, 2016 ). For example, population range expansion in historical times ( Ryan et al., 2018 ), host-parasite interaction changes after human disturbances ( Gottdenker et al., 2016 ), or the effect of current climate change on the structure of populations ( Basset et al., 2015 ), are topics that could be directly benefited by incorporating the information from NHM collection records ( Burrell, Disotell & Bergey, 2015 ).
Collaborative networks are also very important to be more efficient at planning budgets and to set the standards for whole-genome sequencing. For example, the Vertebrate Genomes Project ( https://vertebrategenomesproject.org/ ) is a large collaborative network with the aim to sequence and annotate high-quality genome sequences of all 66,000 extant vertebrate species. Although such large collaborative networks are yet missing for the insect research community, large projects focusing on insect diversity and evolution have been successful at disentangling phylogenetic relationships (e.g., the 1KITE project; http://www.1kite.org/ ) and for the coordination of efforts for whole genome sequencing among research groups ( Sadd et al., 2015 ).
Rule 3: Go to the field
We are worried that the rapid increase of genetic data in public databases might discourage students and researchers from generating novel data. Instead, we argue that field work is absolutely essential to the advancement of our field, and should be part of every biologist’s education as well as part of the routine of more senior researchers. Fieldwork will also benefit museum collections, and vice-versa: museum collections—through genetic and morphological studies based on specimens—will benefit fieldwork. Of course, there might be lines of research that do not demand fieldwork, but even taxonomists, method developers, and researchers in other disciplines may profit from the experience of regularly studying and responsibly collecting specimens or samples in nature. Extensive field surveys are often required to obtain a representative inventory of insect assemblages at both local and regional scales; but such surveys represent only a minority of all entomological field studies. This is problematic given the high species richness and varying abundance, habits and seasonality of insects, including parasitoids, predators, scavengers, leaf-chewers, sap-suckers, among others ( Stork, 2018 ). A careful selection of field sampling methods, along with proper understanding of their function and targeted groups, is thus critical ( Noyes, 1989 ) (see Table 1 for an overview of main mass-sampling methods and Fig. 2 ).
This is not a comprehensive list and is only aimed at providing an overview of available possibilities of widespread use. In Costs (equipments and consumables per sampling effort), we roughly categorized them as Low (approx. < US $50), Medium (approx. US $50—$100), High (approx. > US $100).

(A) Van Someren-Rydon trap, which targets fruit-feeding butterflies. (B) Pitfall trap, which is used to collect forest floor insects—photograph within the red frame depicts the content of pitfall trap. (C) Winkler, an insect collecting device for species inhabiting the leaf litter and soil. (D) Malaise trap, which targets strong-flying insects. (E) The content deposited in the collecting vessel of a Malaise trap. (F) Flight interception, which collects insects flying into the barrier. Photo credits: A, Phil DeVries; B, Martin Nielsen; C, Matthias Seidel; D, Martin Nielsen; E, Daniel Marquina; and F, Emmanuel Arriaga-Varela.
For some cases, such as in biodiversity assessments, it may be enough to conduct simple and rapid field surveys. However, in other cases, such as in exhaustive inventories or when studying diversity dynamics through time and space, greater mass-sampling efforts may be needed. Such campaigns require a combination of multiple methods, longer term inventories and wide expertise, together with effective ways to estimate true species richness based on collected samples ( Vogel, 2017 ). For example, in a recent tropical large-scale species inventory, Borkent & Brown (2015) investigated local species richness of cloud forest Diptera (true flies) for more than 1 year by using two Malaise traps and a wide range of supplementary collecting methods. In addition to these, a 1-week intensive “Diptera-Blitz” was conducted by a large network of experts, inspired on the BioBlitz concept ( Lundmark, 2003 ) which aims at recording most of biodiversity at one locality during a short time period. In another case study, Gómez et al. (2018) sampled the Western Amazonian local parasitoid wasp diversity by using 41 Malaise traps in three separate field campaigns and seasons, with a total sampling effort of 230 Malaise-trap months scattered throughout 1998–2011 (one Malaise-trap month corresponds to one trap collecting in the field for a period of 1 month). In this case, despite the massive sampling effort, cumulative species curves suggested that a significant portion of the local parasitoid diversity remained unobserved; a fact that can be generalized for many other tropical insect groups. Reviews of entomological collection methods for both mass-sampling and group-specific research are available in the literature and are essential reading before field collections ( Agosti et al., 2000 ; Basset et al., 2003 ; Lamarre et al., 2012 ; Larsen, 2016 ).
Needless to say, be a sensible collector! Many insects are rare and threatened, so every collecting effort should be associated with a risk assessment, even informally if not required by law. There are also many federal and international regulations to follow, such as those stipulated under the Nagoya Protocol under the Convention on Biological Diversity ( https://www.cbd.int/abs/about/ ) and the CITES legislation ( https://www.cites.org/ ). In addition, researchers should follow all good practices for Access and Benefit Sharing (e.g., https://naturwissenschaften.ch/organisations/biodiversity/abs/goodpractice ), and deposit their specimens in public NHMs.
Rule 4: Treat your specimens well to enhance their use
The amount and quality of isolated genomic DNA from insect collections depend on a myriad of factors, including killing reagents, method of preservation of specimens in the field, and final voucher storage conditions ( Kanda et al., 2015 ; Short, Dikow & Moreau, 2018 ). For example, Dillon, Austin & Bartowsky (1996) (see also Reiss, Schwert & Ashworth, 1995 ; Gilbert et al., 2007b ) found that specimens killed with ethanol yielded significantly higher quantities of high quality DNA compared to other killing/preservation agents such as ethyl acetate vapor, formalin or ethylene glycol. Moreover, rapid and effective drying of the specimens in the field, especially in the tropics, are important for voucher preservation and may be an alternative to freezing-based preservation ( Prendini, Hanner & DeSalle, 2002 ); cryopreservation is the formal name for the technique that uses very low temperatures to preserve tissues and specimens. Initiatives to establish large cryobanks are important ( Koebler, 2013 ), although these technologies are currently limited to very few large and well-funded NHMs ( Corthals & Desalle, 2005 ). Preservation of specimens in ethanol and at low temperatures is ideal, but may cause logistic problems during transportation and would make the collections highly flammable. Propylene glycol may be a safer alternative and logistically easier to transport than ethanol ( Ferro & Park, 2013 ), and it might even be used to attract certain arthropod species ( Höfer et al., 2015 ). The use of ethylene glycol may provide reasonable amounts of DNA regardless of specimen age, and with lesser risks in the field ( Dillon, Austin & Bartowsky, 1996 ).
The age of specimens seems not to be a critical factor for obtaining DNA for massively parallel sequencing (e.g., as in snakes archived in museum collections, ( Ruane & Austin, 2017 ); see also Table 2 for an overview of published studies using archived insects). DNA fragmentation increases with time, while the median fragment sizes decrease, but these changes do not happen linearly over time ( Sawyer et al., 2012 ). Rather than age, preservation and storage methods are in fact better predictors of DNA quality isolated from old specimens ( Burrell, Disotell & Bergey, 2015 ). Evidently, due to the fragmented nature of ancient DNA, PCR-based techniques are overall not successful to recover genetic data. Fortunately, evidence suggests that fragmented DNA due to age or preservation reagents does not dramatically affect the performance of PCR-free, massively parallel sequencing ( Tin, Economo & Mikheyev, 2014 ; Timmermans et al., 2016 ; Carøe et al., 2018 ).
This is a selection of studies covering a variety of taxonomic groups, sampling strategies and sequencing approaches.
Despite the advantages of using massively parallel DNA sequencing over Sanger when dealing with old specimens, the success of current sequencing approaches still depends in some cases on the quality of isolated DNA, such as in RAD-seq and single-molecule sequencing. For these reasons, minimal specimen damage in the field and during storage is always strongly advisable.
Rule 5: Work closely with taxonomists
The tasks of taxonomists, including the identification, description, and classification of species in meaningful groupings, are unfortunately sometimes neglected. The high diversity and density of insects, coupled with laborious taxonomic assessment and lack of resources for taxonomists, makes the morphological identification of every specimen sampled by mass-collecting techniques a difficult and high resource-consuming task. The so-called “taxonomic impediment” ( Di Castri, Vernhes & Younes, 1992 ) encompasses two general difficulties: (1) not enough resources and training are allocated to taxonomic work and (2) few people are working in taxonomy thus slowing down the rate of species discovery, identification, and classification ( Wheeler, Raven & Wilson, 2004 ; De Carvalho et al., 2007 ; Ebach, Valdecasas & Wheeler, 2011 ; Audisio, 2017 ).
We may be in the midst of a revolution in taxonomy to cope with recent technological advances ( Dubois, 2011 ; Ceríaco et al., 2016 ; Garnett & Christidis, 2017 ; Raposo et al., 2017 ; Thorpe, 2017 ). In the meantime, entomological research must use complementary approaches to reliably estimate diversity through time and among localities. Therefore, taxonomists should be part of any biodiversity studies using insect genomics, and the DNA sequences generated by such studies should be seen as a necessary supplement to the traditional work of taxonomists.
Rule 6: Isolate DNA in the right way
Most recent studies using massively parallel DNA sequencing, even those on ancient insects, have used commercial kits for DNA isolation, thus reducing time, complexity, and health risks in laboratory procedures ( Staats et al., 2013 ; Heintzman et al., 2014 ; Kanda et al., 2015 ; Blaimer et al., 2016 ; Pitteloud et al., 2017 ). However, in-house methods might be more effective than commercial kits when working with old samples having little and low-quality DNA (e.g., see laboratory protocols in Gilbert et al., 2007c ; Meyer et al., 2016 ). Whenever possible, non-destructive protocols for DNA isolation are preferable when working with valuable, archived specimens or with bulk samples such as those coming from insect mass-collecting techniques that later need to be taxonomically curated. However, there is surprisingly little data available comparing the efficiency of destructive vs. non-destructive protocols applied to insects (but see Gilbert et al., 2007a ; Nieman et al., 2015 ). A number of non-destructive DNA isolation protocols have been proposed ( Thomsen et al., 2009 ; Castalanelli et al., 2010 ; Tin, Economo & Mikheyev, 2014 ), but in general they vary depending on the targeted insect group. For example, insects whose external structure are not delicate, including Diptera, Hymenoptera and Coleoptera, tend to be more resistant to submergence of whole specimen in extraction buffers, giving higher DNA yields ( Heintzman et al., 2014 ; Tin, Economo & Mikheyev, 2014 ). In other more delicate groups such as Lepidoptera, the use of abdomens is advisable, given that in many cases the abdomens need to be removed from the individual for genitalia preparation ( Knölke et al., 2005 ). In other insect groups that hold sufficient starting material for DNA isolation in particular tissues, such as muscles in the massive legs of Orthoptera (grasshoppers, locusts, crickets) and large beetles, grinding one leg might not be a significant loss to the collection ( Tagliavia et al., 2011 ). Inminute insects such asmicrohymenopterans (tiny wasps in the superfamily Chalcidoidea), the use of non-destructive DNA extraction protocols can be complemented with whole genome amplification prior to library preparation for highthroughput sequencing ( Cruaud et al., 2018 , 2019 ).
Many curators at NHMs may be reluctant to provide specimens for molecular studies, with valid reasons, since most species might consist of singletons or very rare collections ( Lim, Balke & Meier, 2012 ). The design of selective sampling, minimizing the damage of collections, is therefore crucial. As a side note, there has not been any discussion in the literature about the suitability for massively parallel sequencing using the hundreds of thousands, or perhaps millions, DNA aliquots generated in the past three decades for Sanger-sequencing work. In principle, old DNA aliquots of low quantities and potentially fragmented may face the same constraints of using archived specimens from NHMs or other collections, and might thus be processed using laboratory protocols designed for old specimens (e.g., library preparation, sequencing approach) ( Tin, Economo & Mikheyev, 2014 ; Kanda et al., 2015 ; Suchan et al., 2016 ; Timmermans et al., 2016 ).
Highly-degraded DNA material, such as those coming from museum specimens, might not be suitable for single-molecule DNA sequencing or by certain short-read sequencing protocols such as RADseq (but see protocols that use whole genome amplification prior to reduced-representation sequencing, Cruaud et al., 2018 , and targeted sequencing, Cruaud et al., 2019 ). High molecular weight is only ensured from fresh specimens that have been stored at low temperatures. Moreover, in single-molecule sequencing technologies such as PacBio ® (see Rule 7), the required DNA quantity may demand the use of more than one individual when insects are tiny ( Pacific Biosciences, 2018 ). Additionally, dissections of insects prior to genomic DNA isolation might be necessary in single-molecule DNA sequencing, in order to avoid inadvertently sequencing the DNA of symbionts, or when the focus of the study is on a particular insect microbiome (e.g., the gut microbiota).
Rule 7: Revise your DNA sequencing approach
At this point, you should already have decided which sequencing approach will be best suitable to address your research question(s), but now you should carefully evaluate the quality of DNA that you de facto were able to obtain, and decide on which sequencing approach to really follow.
Reviews on massively parallel DNA sequencing approaches can be found in the literature ( Mamanova et al., 2010 ; Metzker, 2010 ; Mardis, 2017 ). Below, we categorize and briefly describe available massively parallel DNA sequencing technologies of potential interest for entomological biodiversity research (see Table 3 for a summary of such methods and key publications). The current leading short-read DNA sequencing technology is from Illumina, Inc.: approximately 68% of the studies we were able to find that used high-throughput sequencing on insects were conducted using this platform ( Fig. 3A ). We have grouped the main approaches used in the study of entomological biodiversity into three categories ( Table 3 ): (1) targeted-sequencing, (2) non-targeted, reduced-representation of whole genome, and (3) whole-genome skimming. In addition, emerging single-molecule DNA sequencing technologies, such as those developed by Oxford Nanopore Technologies Ltd. and PacBio (Pacific Biosciences of California, Inc., Menlo Park, CA, USA), can accelerate the amount of DNA data recovery in real time ( Thompson & Milos, 2011 ). We consider these technologies as promising, despite the fact that they have only been recently implemented for the study of insect diversity (e.g., in the genome assembly of a firefly, Coleoptera, ( Fu et al., 2017 )). Below we provide a summary of these techniques.
These studies were among the first that used high-throughput methods to investigate insect diversity. A more comprehensive list of published studies is presented in File S2 .

(A) The main sequencing platforms (SM stands for single-molecule, including those from PacBio and Oxford Nanopore technologies). (B) The main library preparation methods used for high-throughput sequencing (WG stands for whole-genome sequencing). (C) Number of publications by year (**our search was conducted on November 22nd, 2018). (D) Cumulative publications over time (number of publications in logarithmic scale). In general, about 68% of the studies we were able to find ( File S2 ) were conducted in Illumina platform, whereas about 65% of all studies have used some form of targeted sequencing.
Targeted sequencing: This is a highly-efficient approach when the aim is to recover DNA markers with a particular rate of evolution (fast and slow) or under different selective pressures ( Lemmon & Lemmon, 2013 ). Moreover, because it targets only a tiny subset of the whole genome, targeted sequencing is cost-effective as tens or hundreds of specimens can be pooled together in a single sequencing experiment ( Mamanova et al., 2010 ). In fact, about 65% of published studies focusing on insects or their symbionts have used some form of targeted sequencing ( Fig. 3B ). Targeted sequencing is particularly useful when working with environmental samples, such as those coming from mass-sampling techniques ( Morinière et al., 2016 ). For example, metabarcoding, an approach that targets a barcoding region such as the COI mitochondrial gene, can be useful in the study of evolution among environments and in biodiversity assessments. This is because metabarcoding might be more reliable, faster and replicable than traditional biodiversity surveys ( Ji et al., 2013 ; Zhou et al., 2013 ; Vesterinen et al., 2016 ), although they should rather be seen as complimentary ( Ritter et al., 2019 ).
There are two usual ways to target particular loci: (1) through PCR or (2) by using “baits”-based in-vitro capture. PCR has the advantage of being cheap but the development of universal primers is the main limitation because sequence specificity to desired loci decreases through mutation and long divergence times among lineages. Nevertheless, PCR-based amplicon sequencing is so far the main method used in published studies with a focus on insects or their symbionts (ca. 60% of reviewed studies; Fig. 3B ). On the other hand, target capture using hybridizing baits instead of PCR can be expensive (baits need to be specially synthesized) but has the advantages of (1) simplify laboratory procedures (one can pool several specimens for the capture experiment), (2) target a wider range of lineages despite evolutionary distance among them, (3) reduce amplification biases due to PCR primer design and relative abundance of DNA molecules in a pool of specimens, and (4) it might still work with highly fragmented DNA such as those coming from archived specimens at NHMs.
Prior genomic information, either published annotated genomes or transcriptomes, is needed in order to design target-enrichment probes, which are the hybridizing baits that pull out the targeted loci for sequencing. Probe kits targeting conserved regions primarily for phylogenomic purposes have been published for those insect orders having good genomic reference databases ( Faircloth et al., 2015 ; Faircloth, 2016a ; Young et al., 2016 ; Breinholt et al., 2018 ). Recent attempts to integrate baits-based capture into metabarcoding have had different degrees of success, such as the sequencing of non-target organisms or pseudogenes on the negative side ( Shokralla et al., 2016 ), or the recovery of sequences of very rare species in a pool of samples and the quantification of relative abundance and biomass on the positive side ( Dowle et al., 2016 ).
Random reduced-representation of genome: Restriction-site-associated DNA (RAD) sequencing has proven to be a cost-efficient approach to generate millions of single nucleotide polymorphisms (SNPs), both neutral and under selection ( Andrews et al., 2016 ) RAD-seq is a versatile approach as it has been used in studies on phylogeography (e.g., postglacial range expansions, Emerson et al., 2010 ), ecology (e.g., habitat association and differentiation of populations, Nice et al., 2019 ), and evolution (e.g., inference of phylogenetic relationships and genome-wide introgression, Dasmahapatra et al., 2012 ). However, there are two possible caveats.
Firstly, restriction enzyme sites may not be evolutionarily conserved. Thus, RAD-seq seems to be restricted to populations or closely-related species. However, a recent protocol targeting RAD-seq markers (hyRAD) may ameliorate the lack of phylogenetic conservation of restriction enzyme sites across divergent lineages ( Suchan et al., 2016 ).
Secondly, the amount and quality of DNA might impose a limitation to RAD-seq. For example, Tin, Economo & Mikheyev (2014) , using ant specimens as old as 100 years, were able to recover SNPs, but were unsuccessful at genome mapping due to the extremely short DNA fragments and imprecise DNA size selection. Long DNA fragments are needed for an efficient restriction enzyme activity. An alternative reduced-representation method called MIG-seq ( Suyama & Matsuki, 2015 ) might work with moderately fragmented DNA, because it is based on PCR without restriction enzyme digestion steps.
Whole-genome skimming: This is the simplest approach in terms of sequence library preparation. It consists of randomly, shallowly sequencing the whole-genome of an individual, including both mitochondrial and nuclear content. Furthermore, when working with historical specimens with highly fragmented DNA, one can skip the step of fragmentation (usually through sonication) during library preparation ( Suchan et al., 2016 ; Timmermans et al., 2016 ). Whole-genome skimming has been applied in a number of insect studies, proving that the method is fast and can recover entire mitochondrial genomes from even old museum material ( Staats et al., 2013 ), and low-copy nuclear protein-coding genes ( Maddison & Cooper, 2014 ; Kanda et al., 2015 ).
With the expected decrease in sequencing prices, target sequencing approaches may no longer be a cost-effective choice in the future. For instance, recent studies have identified the benefits of mitochondrial metagenomics (MMG). This technique produces longer barcodes with larger numbers of SNPs, because it uses mitogenomes instead of only the COI fragment, and PCR-free library preparation ( Crampton-Platt et al., 2015 ). This in turn allows the use of highly-fragmented DNA from old specimens, and permits a more reliable quantification of relative abundance (i.e., biomass) in mass-sampling collections ( Crampton-Platt et al., 2015 , 2016 ; Cicconardi et al., 2017 ; Gómez-Rodríguez et al., 2017 ). However, it has been noted that having a reference genome is important to improve mapping and discovery of homologous SNPs in the nuclear genome ( Tin, Economo & Mikheyev, 2014 ), which may yet restrict the use of whole-genome skimming and the recovery of nuclear data in insect groups with poor genomic information.
Single-molecule sequencing approaches such as those developed by PacBio ® and Oxford Nanopore Technologies, Ltd. The portability of some devices (e.g., Oxford Nanopore MinION ™ , Oxford, United Kingdom) that can generate DNA sequences in real-time and in virtually any place in the world is a main advantage of these technologies. Indeed, DNA sequencing has already been performed in remote field locations, dealing with for example vertebrates ( Menegon et al., 2017 ) and plants ( Parker et al., 2017 ). The use of MinION ™ in DNA barcoding in insects has proven to be fast (ca. 2 h), cheap (<USD 2 per sample) and reliable when correction pipelines are used to overcome the still high basecall error rates (>10%) ( Mardis, 2017 ; Shendure et al., 2017 ; Srivathsan et al., 2018 ).
Taxonomic biases in bulk material coming from mass-sampling techniques have been reported when working with rDNA amplicons, perhaps associated with the different fragment lengths across insect orders ( Krehenwinkel et al., 2018 ). On the other hand, laboratory protocols are simplified and DNA amplification is not necessary in single-molecule sequencing, which is beneficial for a more accurate quantification of DNA molecules present in the sample pool ( Thompson & Milos, 2011 ). Single-molecule sequencing also promises to drastically reduce costs, meaning that the time when having complete genome sequences for any living insect might be even closer than previously thought ( Kelley et al., 2014 ). Finally, the long reads that single-molecule sequencing approaches generate might help resolve long repeat elements in the genome, thus providing invaluable scaffold for short reads to improve accuracy in assembly and annotation of insect genomes (see Richards & Murali, 2015 ).
The quality of reference genomes and chromosome-scale scaffolds can be improved by combining long-range and short-read sequencing technologies. For example, PacBio and Nanopore sequencing can overcome repetitive elements by sequencing long DNA fragments, while more accurate short-read sequencing technologies like Illumina can sort out the high error rate of long-range sequencing platforms. For instance, this approach has led to 200-fold increases in contig assembly length and the filling of many gaps in genomes left by short-read approaches only (for example, in avian genome assemblies, Korlach et al., 2017 ).
Rule 8: Choose the most suitable tools for data analyses
Although genomic sequencing is becoming easier and more affordable, processing the data generated remains a major bottleneck in many projects. Bioinformatic pipelines have been implemented during the past two decades of massively parallel sequencing, thus researchers nowadays count with standard procedures to analyze genomic DNA. For example, packages for cleaning and assembling reads exist for bait-based targeted sequencing, such as PHYLUCE ( Faircloth, 2016b ) and SECAPR ( Andermann et al., 2018 ), as well as for RADseq analysis, such as iPyRAD ( Eaton, 2014 ; Eaton & Overcast, 2016 ) and Stacks ( Rochette & Catchen, 2017 ). However, there remain limitations and challenges. For example, missing data in supermatrices for phylogenomic studies might hinder statistical power in the inference of species relationships, but their effects in systematic biases are yet unclear ( Misof et al., 2014a , 2014b ). Moreover, taxonomic sampling in phylogenomics is usually lower than in published Sanger-sequencing work, which may bias systematic inference in insect higher-level phylogenies ( Behura, 2015 ). In general, phylogenomic dataset sizes increase as sequencing costs per base pair decreases over time ( Bravo et al., 2018 ).
A number of pipelines have been published for analyzing amplicon-based, target-sequencing data from environmental samples ( Schloss et al., 2009 ; Caporaso et al., 2010 ; Boyer et al., 2016 ). Such programs provide a delimitation of operational taxonomic units (OTUs), the analogs of species, derived from sequence similarity of typically 97%. However, assigning thresholds to define analogs of species is problematic because (1) there is a risk to artificially increase or decrease local diversity as compared to morphology-based taxonomic assessments, (2) inflated OTU richness might be related to sequence chimeras and sequencing errors (but see recent methods to alleviate this; Frøslev et al., 2017 ), and (3) there is a lack of standardization of threshold values in the literature, reducing the comparability potential of results across studies ( Huse et al., 2010 ; Oliver et al., 2015 ; Alberdi et al., 2018 ). The shortcomings of using thresholds to define OTUs might even escalate when studying the entomofauna of hyperdiverse regions such as the tropics. In those cases, there are usually no good estimates of genetic variability between species and a large portion of tropical insects are not represented in reference databases. In any case, the preservation and morphological study of vouchers are critical to validate taxonomic assignments and thresholds.
Mitochondrial metagenomics could in principle improve OTU assignments and species delimitation because contigs span different barcode regions (COI, ND2, 16S rDNA) ( Tedersoo et al., 2015 ; Liu et al., 2016 ; Srivathsan et al., 2016 ) and risks of primer-related biases are ameliorated ( Taberlet et al., 2012 ; Tang et al., 2014 ). Whilst approaches such as log-binomial normalizations (through DeSeq2 and CSS) have attempted to normalize metabarcoding data ( McMurdie & Holmes, 2014 ), results via PCR-based approaches remain semi-quantitative at best ( Pawluczyk et al., 2015 ). However, metagenomic studies of insects have generally been limited only to their microbiomes ( Cox-Foster et al., 2007 ; Suen et al., 2010 ; Shi et al., 2013 ). It is difficult to assess the convenience of metagenomics in more complex environmental insect samples because (1) de novo assembly of mixed mitogenomes remains challenging due to the scarcity of reference mitogenomes, and (2) as the number of individuals in a pool increases, sequencing depth needs to be significantly increased in order to get large enough k-mers/contigs to partition different mitogenomes (but see some exceptions in Crampton-Platt et al., 2015 , 2016 ; Cicconardi et al., 2017 ; Gómez-Rodríguez et al., 2017 ).
Rule 9: Make your data and results publicly available
From a practical viewpoint, what is not in a database does not exist (or nearly so). Databases are not only the repositories of genomic information, but also an indispensable tool in the study of biodiversity and evolution. They also allow the reproduction of results and use for other purposes such as in biodiversity assessments. Biodiversity and evolutionary studies might benefit from the hundreds of insect genome projects already published and registered in GenBank ( Yeates et al., 2016 ) and InsectBase ( Yin et al., 2016 ). In the study of species interactions, such as in host-parasite and feeding habits, a reference database is important because in many cases the identification of taxa through morphological comparison becomes impossible. Examples include the study of internal parasites ( Schoonvaere et al., 2016 ), gut microbiota ( Hammer et al., 2017 ), and highly-degraded organic material as in dietary content ( Pompanon et al., 2012 ).
Initiatives such as BOLD ( Ratnasingham & Hebert, 2007 ) and the widespread usage of the COI barcode will certainly contribute to the assignments of OTU thresholds when studying tropical communities ( García-Robledo et al., 2013 ). However, building local databases that include several markers would complement metabarcoding studies in the identification and delimitation of species ( Deagle et al., 2014 ). Several national reference databases have been implemented or are underway, such as the newly initiated DNAmark project in Denmark ( https://www.dnamark.ku.dk/ ). That initiative aims to provide a reference database for 1,000 species with full mitochondrial sequences, along with nuclear sequences derived from shotgun sequencing. Other initiatives to catalogue national biodiversity have also been put forward in Germany ( Hendrich et al., 2015 ), Norway (NorBOL; http://www.norbol.org/ ) and Finland (FinBOL; http://www.finbol.org/ ), which together are further expanding the BOLD project worldwide.
Rule 10: Disseminate your findings
Research articles are the standard way to communicate results to the scientific community. However, misinterpretations of scientific findings can be common in the literature aimed for the general public and decision-makers. Thus, public outreach should be explicitly considered as part of project design. Moreover, because scientific research is a collaborative enterprise (see Rule 2), it is important to discuss and reach a consensus with collaborators before spreading findings to the general public. This is particularly important given the recent misunderstandings on biodiversity research that have been reported, and the urge to include both factual evidence and ethical arguments in communications to the general public ( Antonelli & Perrigo, 2018 ).
Given that diversity estimates can fluctuate significantly depending on the way data are produced and analyzed (e.g., as in metabarcoding; Frøslev et al., 2017 ; Alberdi et al., 2018 ) special care should be taken when presenting these findings. In general, we advocate for approaches that do not artificially inflate diversity estimates. Furthermore, the access of scientific knowledge and data by governmental bodies is still restricted, especially in low and lower-middle income countries. Biodiversity is a cornerstone in Environmental Impact Assessments, but animal groups such as insects remain underrepresented in biodiversity assessments in species-rich countries ( Ritter et al., 2017 ).
Perspectives and Conclusions
In this article we have identified general challenges, including: (1) insufficient evaluation of non-destructive methods applied to insects , in order to generate DNA of high quantity and quality from fresh, mass-collections and archived specimens, (2) limitations to genomic data analyses , including missing genomic information from datasets and methods for estimating diversity and abundance in environmental samples, and (3) limited taxonomic, ecological, and life history knowledge , which is not being produced at the same pace as genomic data.
Insects are ideal study organisms because they show remarkable diversity in species number and ecology, being the dominant eukaryotic group in most terrestrial and freshwater environments. The integration of ecology and evolution is achievable with the new massively parallel sequencing approaches, which offer the possibility to generate datasets that can be used in the study of biodiversity at different spatiotemporal scales. For example, the evolutionary framework of local insect communities can now be inferred in a single sequencing effort ( Crampton-Platt et al., 2015 ), while the study of populations and speciation using massively parallel sequencing can be better understood with a comprehensive knowledge of local variations ( Jiggins, 2016 ). Altogether, we expect that the increase of molecular data together with more taxonomic and ecological studies will allow a better understanding of biodiversity and evolution.
Supplemental Information
Supplemental information 1.
Our Literature Review was based on an unbiased search in Web of Science. More details can be found in the main text.
Supplemental Information 2
Data was retrieved from Web of Science. The analyses were performed only on Original research. Other type of articles retrieved by our search in Web of Science can be found in the raw data file in Supporting Material.
Acknowledgments
We thank the Academic editor Joseph Gillespie and three anonymous reviewers for comments and suggestions that contributed to improving this manuscript. This article is a product of the workshop organized by the Gothenburg Global Biodiversity Centre (GGBC), in Gothenburg, Sweden, in February 2017. We are grateful to Sven Toresson for his help in organizing the logistics of the workshop and many colleagues for discussions and feedback.
Funding Statement
Funding to Pável Matos-Maraví was provided by a Marie Skłodowska-Curie fellowship (project MARIPOSAS-704035). Camila Duarte Ritter received support from CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico–Brazil-249064/2013-8) and the Alexander von Humboldt Foundation. Christopher Barnes was funded by the Aage V. Jensen Naturfond of Denmark (1121721001). Daniel Marquina and Niklas Wahlberg received funding from European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No. 642241 (BIG4 project). Niklas Wahlberg received funding from the Swedish Research Council. Alexandre Antonelli is supported by a grant from the Knut and Alice Wallenberg Foundation, the Swedish Research Council (B0569601), the Swedish Foundation for Strategic Research, the Faculty of Sciences at the University of Gothenburg, and the David Rockefeller Center for Latin American Studies at Harvard University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
The authors declare that they have no competing interests.
Pável Matos-Maraví conceived and designed the experiments, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Camila Duarte Ritter conceived and designed the experiments, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Christopher J. Barnes prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Martin Nielsen prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Urban Olsson prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Niklas Wahlberg prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Daniel Marquina prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Ilari Sääksjärvi prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Alexandre Antonelli conceived and designed the experiments, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
We can help you reset your password using the email address linked to your BioOne Complete account.

- BioOne Complete
- BioOne eBook Titles
- By Publisher
- About BioOne Digital Library
- How to Subscribe & Access
- Library Resources
- Publisher Resources
- Instructor Resources
- DOWNLOAD PAPER SAVE TO MY LIBRARY
Insects are not only the most species—rich group on Earth, they also play numerous crucial roles in ecosystem functioning and the global economy. The conservation of insect diversity is therefore a topic of global importance. However, insects are mostly ignored by “biodiversity” research; for example, relationships between insect diversity and vegetation or climate change remain widely unknown. This paper makes suggestions for further research into insect diversity patterns based on two typical forest types in northern China: Changbai Mountain, northeast China and Dongling Mountain, central northern China. Plants and climate have been well studied in these areas but substantial knowledge gaps prevail in relation to insect diversity patterns. It is therefore important to investigate insect biodiversity patterns in these regions.

KEYWORDS/PHRASES
Publication title:, collection:, publication years.

Vol. 48 No. 2

Vol. 48 No. 1

Vol. 47 No. 2

Vol. 47 No. 1

Vol. 46 No. 2
Open access.
Journal of Insect Biodiversity is a hybrid journal and has published many open access papers:
- View Open Access Papers
We encourage the open access of papers as they are more widely read and cited. Our open access option has one of the lowest article processing fees.
Announcements
The 2022 journal impact factor is 0.4 2023-06-28.
The journal impact fatcor of Journal of Insect Biodiversity is 0.4 according to June 2023 release of Journal Citation Reports.
Web of Science Indexes 2021-06-24
Seasonality and composition of the simuliidae fauna (insecta: diptera) from the altitudinal gradient in itatiaia national park, brazil.
- IVYN KARLA LIMA-DE-SOUSA
- OSCAR S. MOLINA
- LEONARDO H. GIL-AZEVEDO
- RONALDO FIGUEIRÓ
Two new species of grass flies (Diptera, Chloropidae) from Sweden
- PAULA RAILE RICCARDI
Redescription of Graphomya adumbrata (Wiedemann, 1824) (Diptera: Muscidae), including male and female terminalia
- NANDAN JANA
- PRAVAS HAZARI
- SHUVRA KANTI SINHA
A review of long-legged flies from the Republic of Guinea (Diptera:Dolichopodidae) with description of a new Lichtwardtia species
- IGOR YA. GRICHANOV
- DMITRIY V. DUBROVSKIY
A new species of Notorhabdium N. Ohbayashi & Shimomura, 1986 from Yunnan, China, with annotated catalogue for the genus (Coleoptera: Cerambycidae: Lepturinae)
- CHENG-BIN WANG
most viewed
Lathrobium motonari , a new soil-inhabiting species of the genus lathrobium (coleoptera: staphylinidae: paederinae) from western honshu, japan : lathrobium motonari sp. nov. from western honshu, japan.
- YOSHIHIRO SENDA
Diversity, abundance and distribution of grasshopper species (Orthoptera: Acrididea) in three different types of vegetation with different levels of anthropogenic disturbances in the Littoral Region of Cameroon
- YETCHOM-FONDJO JEANNE AGRIPPINE
- KEKEUNOU - SÉVILOR
- KENNE - MARTIN
- MISSOUP ALAIN DIDIER
- SHENG-QUAN XU

Summary of native geographic distribution of all 3,341 species of the most speciose animal genus Agrilus (Coleoptera: Buprestidae)
- EDUARD JENDEK
- VASILY V. GREBENNIKOV
New Afrotropical and Oriental elements in the weevil fauna of Iran (Coleoptera: Curculionidae) with discussion on their origin
- NIKOLAI YUNAKOV
- HIVA NASSERZADEH
- NASTARAN REZAEI
- SARA ZARGHAMI
Contributions to the Heteroptera (Hemiptera) fauna of Anatolia with new records for Turkey
- BARIŞ ÇERÇİ
- NESLİHAN GÜLTEKİN
- CELALETTİN GÖZÜAÇIK
- MELEK GÜDEK GÜÇLÜ
- DİLEK DOĞAN
About the Journal
Journal of Insect Biodiversity ( JIB ) is an international, peer-reviewed scientific journal of Biodiversity Application & Research Center of the Atatürk University. JIB is dedicated to...
Author Guidelines
The Journal of Insect Biodiversity is dedicated to publishing high quality novel scientific data on insect biodiversity . It publishes research articles, reviews articles and short...
Recent Issues
- Volume 48, Issue 2
- Volume 48, Issue 1
- Volume 47, Issue 2
Related Journals

Advertisement
Modern rose hybrids have a worrying lack of genetic diversity
Intensive breeding since the 19 th century has created thousands of varieties of rose, but a reduction in genetic diversity could leave them vulnerable to diseases and climate change
By James Woodford
25 April 2024

Hybrid tea roses, which include varieties such as Charles de Gaulle, are the most popular class of modern garden rose
Fir Mamat / Alamy
Modern commercial roses have lost over a quarter of their genetic diversity compared with older varieties, which could leave them vulnerable to diseases or climate change.
Roses have been admired and grown since antiquity, when people in Asia and Europe began cultivating ancient varieties from a dozen wild rose species. There were less than 100 varieties before 1800, but by 1900 there were around 8000 varieties of crossed Asian and European…
Sign up to our weekly newsletter
Receive a weekly dose of discovery in your inbox! We'll also keep you up to date with New Scientist events and special offers.
To continue reading, subscribe today with our introductory offers
No commitment, cancel anytime*
Offer ends 2nd of July 2024.
*Cancel anytime within 14 days of payment to receive a refund on unserved issues.
Inclusive of applicable taxes (VAT)
Existing subscribers
More from New Scientist
Explore the latest news, articles and features
Saving the world's largest flowers in the Philippines
Environment, insects thrive on solar farms planted with native flowers.
Subscriber-only
Male orchid bees craft unique perfumes from flowers – now we know why
Odd parasitic plant with fleshy flowers identified as new species, popular articles.
Trending New Scientist articles
Scientists push new paradigm of animal consciousness, saying even insects may be sentient

Bees play by rolling wooden balls — apparently for fun . The cleaner wrasse fish appears to recognize its own visage in an underwater mirror . Octopuses seem to react to anesthetic drugs and will avoid settings where they likely experienced past pain.
All three of these discoveries came in the last five years — indications that the more scientists test animals, the more they find that many species may have inner lives and be sentient. A surprising range of creatures have shown evidence of conscious thought or experience, including insects, fish and some crustaceans.
That has prompted a group of top researchers on animal cognition to publish a new pronouncement that they hope will transform how scientists and society view — and care — for animals.
Nearly 40 researchers signed “ The New York Declaration on Animal Consciousness ,” which was first presented at a conference at New York University on Friday morning. It marks a pivotal moment, as a flood of research on animal cognition collides with debates over how various species ought to be treated.
The declaration says there is “strong scientific support” that birds and mammals have conscious experience, and a “realistic possibility” of consciousness for all vertebrates — including reptiles, amphibians and fish. That possibility extends to many creatures without backbones, it adds, such as insects, decapod crustaceans (including crabs and lobsters) and cephalopod mollusks, like squid, octopus and cuttlefish.
“When there is a realistic possibility of conscious experience in an animal, it is irresponsible to ignore that possibility in decisions affecting that animal,” the declaration says. “We should consider welfare risks and use the evidence to inform our responses to these risks.”
Jonathan Birch, a professor of philosophy at the London School of Economics and a principal investigator on the Foundations of Animal Sentience project, is among the declaration’s signatories. Whereas many scientists in the past assumed that questions about animal consciousness were unanswerable, he said, the declaration shows his field is moving in a new direction.
“This has been a very exciting 10 years for the study of animal minds,” Birch said. “People are daring to go there in a way they didn’t before and to entertain the possibility that animals like bees and octopuses and cuttlefish might have some form of conscious experience.”
From 'automata' to sentient
There is not a standard definition for animal sentience or consciousness, but generally the terms denote an ability to have subjective experiences: to sense and map the outside world, to have capacity for feelings like joy or pain. In some cases, it can mean that animals possess a level of self-awareness.
In that sense, the new declaration bucks years of historical science orthodoxy. In the 17th century, the French philosopher René Descartes argued that animals were merely “material automata” — lacking souls or consciousness.
Descartes believed that animals “can’t feel or can’t suffer,” said Rajesh Reddy, an assistant professor and director of the animal law program at Lewis & Clark College. “To feel compassion for them, or empathy for them, was somewhat silly or anthropomorphizing.”
In the early 20th century, prominent behavioral psychologists promoted the idea that science should only study observable behavior in animals, rather than emotions or subjective experiences . But beginning in the 1960s, scientists started to reconsider. Research began to focus on animal cognition, primarily among other primates.
Birch said the new declaration attempts to “crystallize a new emerging consensus that rejects the view of 100 years ago that we have no way of studying these questions scientifically.”
Indeed, a surge of recent findings underpin the new declaration. Scientists are developing new cognition tests and trying pre-existing tests on a wider range of species, with some surprises.
Take, for example, the mirror-mark test, which scientists sometimes use to see if an animal recognizes itself.
In a series of studies, the cleaner wrasse fish seemed to pass the test .
The fish were placed in a tank with a covered mirror, to which they exhibited no unusual reaction. But after the cover was lifted, seven of 10 fish launched attacks toward the mirror, signaling they likely interpreted the image as a rival fish.
After several days, the fish settled down and tried odd behaviors in front of the mirror, like swimming upside down, which had not been observed in the species before. Later, some appeared to spend an unusual amount of time in front of the mirror, examining their bodies. Researchers then marked the fish with a brown splotch under the skin, intended to resemble a parasite. Some fish tried to rub the mark off.
“The sequence of steps that you would only ever have imagined seeing with an incredibly intelligent animal like a chimpanzee or a dolphin, they see in the cleaner wrasse,” Birch said. “No one in a million years would have expected tiny fish to pass this test.”
In other studies, researchers found that zebrafish showed signs of curiosity when new objects were introduced into their tanks and that cuttlefish could remember things they saw or smelled . One experiment created stress for crayfish by electrically shocking them , then gave them anti-anxiety drugs used in humans. The drugs appeared to restore their usual behavior.
Birch said these experiments are part of an expansion of animal consciousness research over the past 10 to 15 years. “We can have this much broader canvas where we’re studying it in a very wide range of animals and not just mammals and birds, but also invertebrates like octopuses, cuttlefish,” he said. “And even increasingly, people are talking about this idea in relation to insects.”
As more and more species show these types of signs, Reddy said, researchers might soon need to reframe their line of inquiry altogether: “Scientists are being forced to reckon with this larger question — not which animals are sentient, but which animals aren’t?”
New legal horizons
Scientists’ changing understanding of animal sentience could have implications for U.S. law, which does not classify animals as sentient on a federal level, according to Reddy. Instead, laws pertaining to animals focus primarily on conservation, agriculture or their treatment by zoos, research laboratories and pet retailers.
“The law is a very slow moving vehicle and it really follows societal views on a lot of these issues,” Reddy said. “This declaration, and other means of getting the public to appreciate that animals are not just biological automatons, can create a groundswell of support for raising protections.”

State laws vary widely. A decade ago, Oregon passed a law recognizing animals as sentient and capable of feeling pain, stress and fear, which Reddy said has formed the bedrock of progressive judicial opinions in the state.
Meanwhile, Washington and California are among several states where lawmakers this year have considered bans on octopus farming, a species for which scientists have found strong evidence of sentience.
British law was recently amended to consider octopuses sentient beings — along with crabs and lobsters .
“Once you recognize animals as sentient, the concept of humane slaughter starts to matter, and you need to make sure that the sort of methods you’re using on them are humane,” Birch said. “In the case of crabs and lobsters, there are pretty inhumane methods, like dropping them into pans of boiling water, that are very commonly used.”
Evan Bush is a science reporter for NBC News. He can be reached at [email protected].
What caused Dubai floods? Experts cite climate change, not cloud seeding
- Medium Text
DID CLOUD SEEDING CAUSE THE STORM?

CAN'T CREATE CLOUDS FROM NOTHING
Sign up here.
Reporting by Alexander Cornwell; editing by Maha El Dahan and Alexandra Hudson
Our Standards: The Thomson Reuters Trust Principles. New Tab , opens new tab
China General Nuclear Power Group (CGN) has secured its first yuan-denominated trade finance to import Chinese PV modules for a solar plant in Brazil, official media said on Friday.

World Chevron

Ceasefire monitoring centre in Nagorno-Karabakh shuts as Russian peacekeepers withdraw
The Russian and Turkish flags were lowered on Friday to mark the closure of a centre set up to monitor a ceasefire in Nagorno-Karabakh, as Russian peacekeepers ended a multi-year deployment in the strategic South Caucasus region.

Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- NEWS AND VIEWS
- 30 October 2019
Robust evidence of declines in insect abundance and biodiversity
- William E. Kunin 0
William E. Kunin is in the School of Biology, University of Leeds, Leeds LS2 9JT, UK.
You can also search for this author in PubMed Google Scholar
There are certain times in life — whether in our relationships, personal health or scientific research — when we think that we know something but the evidence is less than conclusive. An accumulation of clues or symptoms might suggest a particular interpretation without being strong enough to clinch the argument. In such situations, it can be a relief to finally get a definitive answer, even if the news is bad. Once we know that a problem definitely exists, we may be able to do something about it. Readers might feel the same way when they read the results reported in Nature by Seibold et al . 1 , which provide compelling evidence of a major problem — large-scale declines in the numbers and diversity of insects and other jointed invertebrates known as arthropods.
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
185,98 € per year
only 3,65 € per issue
Rent or buy this article
Prices vary by article type
Prices may be subject to local taxes which are calculated during checkout
Nature 574 , 641-642 (2019)
doi: https://doi.org/10.1038/d41586-019-03241-9
Seibold, S. et al. Nature 574 , 671–674 (2019).
Article Google Scholar
Hallmann, C. A., Foppen, R. P. B., van Turnhout, C. A. M., de Kroon, H. & Jongejans, E. Nature 511 , 341–343 (2014).
Article PubMed Google Scholar
Biesmeijer J. C. et al. Science 313 , 351–354 (2006).
Thomas, J. A., Morris, M. G. & Hambler, C. Phil. Trans. R. Soc. B 344 , 47–54 (1994).
Maes, D. & Van Dyck, H. Biol. Conserv. 99 , 263–276 (2001).
Sánchez-Bayo, F. & Wyckhuys, K. A. G. Biol. Conserv. 232 , 8–27 (2019).
Isaac, N. J. B., van Strien, A. J., August, T. A., de Zeeuw, M. P. & Roy, D. B. Methods Ecol. Evol. 5 , 1052–1060 (2014).
Winfree, R., Fox, J. W., Williams, N. M., Reilly, J. R. & Cariveau, D. P. Ecol. Lett. 18 , 626–635 (2015).
Hallmann, C. A. et al. PLoS ONE 12 , e0185809 (2017).
Fischer, M. et al. Basic Appl. Ecol. 11 , 473–485 (2010).
Noleppa, S. et al. Banning neonicotinoids in the European Union . Research paper 01/2017 (HFFA Research GmbH, 2017).
Google Scholar
Download references
Reprints and permissions
Related Articles

- Conservation biology
Director of the Czech Advanced Technology and Research Institute of Palacký University Olomouc
The Rector of Palacký University Olomouc announces a Call for the Position of Director of the Czech Advanced Technology and Research Institute of P...
Czech Republic (CZ)
Palacký University Olomouc
Course lecturer for INFH 5000
The HKUST(GZ) Information Hub is recruiting course lecturer for INFH 5000: Information Science and Technology: Essentials and Trends.
Guangzhou, Guangdong, China
The Hong Kong University of Science and Technology (Guangzhou)
Suzhou Institute of Systems Medicine Seeking High-level Talents
Full Professor, Associate Professor, Assistant Professor
Suzhou, Jiangsu, China
Suzhou Institute of Systems Medicine (ISM)
Postdoctoral Fellowships: Early Diagnosis and Precision Oncology of Gastrointestinal Cancers
We currently have multiple postdoctoral fellowship positions within the multidisciplinary research team headed by Dr. Ajay Goel, professor and foun...
Monrovia, California
Beckman Research Institute, City of Hope, Goel Lab
Postdoctoral Research Fellow Positions, Division of Rheumatology
We seek two postdoctoral fellows to join the Mustelin/Najjar lab in the Rheumatology division, University of Washington, Seattle, USA, to lead and ...
Seattle, Washington State
University of Washington, Department of Medicine, Division of Rheumatology
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Genetics studies have a diversity problem that researchers struggle to fix
Lack of diversity in genetics research has real health care implications, by lauren sausser.
This article originally appeared on KFF Health News .
CHARLESTON, S.C. — When he recently walked into the dental clinic at the Medical University of South Carolina donning a bright-blue pullover with “In Our DNA SC” embroidered prominently on the front, Lee Moultrie said, two Black women stopped him to ask questions.
“It’s a walking billboard,” said Moultrie, a health care advocate who serves on the community advisory board for In Our DNA SC, a study underway at the university that aims to enroll 100,000 South Carolinians — including a representative percentage of Black people — in genetics research. The goal is to better understand how genes affect health risks such as cancer and heart disease.
Moultrie, who is Black and has participated in the research project himself, used the opportunity at the dental clinic to encourage the women to sign up and contribute their DNA. He keeps brochures about the study in his car and at the barbershop he visits weekly for this reason. It’s one way he wants to help solve a problem that has plagued the field of genetics research for decades: The data is based mostly on DNA from white people.
Project leaders in Charleston told KFF Health News in 2022 that they hoped to enroll participants who reflect the demographic diversity of South Carolina, where just under 27% of residents identify as Black or African American. To date, though, they’ve failed to hit that mark. Only about 12% of the project’s participants who provided sociodemographic data identify as Black, while an additional 5% have identified as belonging to another racial minority group.
“We’d like to be a lot more diverse,” acknowledged Daniel Judge, principal investigator for the study and a cardiovascular genetics specialist at the Medical University of South Carolina.
"We need to be even more creative in how we obtain people to help contribute to studies,” she said."
Lack of diversity in genetics research has real health care implications. Since the completion more than 20 years ago of the Human Genome Project, which mapped most human genes for the first time, close to 90% of genomics studies have been conducted using DNA from participants of European descent, research shows . And while human beings of all races and ancestries are more than 99% genetically identical, even small differences in genes can spell big differences in health outcomes.
“Precision medicine” is a term used to describe how genetics can improve the way diseases are diagnosed and treated by considering a person’s DNA, environment, and lifestyle. But if this emerging field of health care is based on research involving mostly white people, “it could lead to mistakes, unknowingly,” said Misa Graff, an associate professor in epidemiology at the University of North Carolina and a genetics researcher.
In fact, that’s already happening. In 2016, for example, research found that some Black patients had been misdiagnosed with a potentially fatal heart condition because they’d tested positive for a genetic variant thought to be harmful. That variant is much more common among Black Americans than white Americans, the research found, and is considered likely harmless among Black people. Misclassifications can be avoided if “even modest numbers of people from diverse populations are included in sequence databases,” the authors wrote.
The genetics research project in Charleston requires participants to complete an online consent form and submit a saliva sample, either in person at a designated lab or collection event or by mail. They are not paid to participate, but they do receive a report outlining their DNA results. Those who test positive for a genetic marker linked to cancer or high cholesterol are offered a virtual appointment with a genetics counselor free of charge.
Some research projects require more time from their volunteers, which can skew the pool of participants, Graff said, because not everyone has the luxury of free time. “We need to be even more creative in how we obtain people to help contribute to studies,” she said.
Moultrie said he recently asked project leaders to reach out to African American media outlets throughout the Palmetto State to explain how the genetics research project works and to encourage Black people to participate. He also suggested that when researchers talk to Black community leaders, such as church pastors, they ought to persuade those leaders to enroll in the study instead of simply passing the message along to their congregations.
“We have new ideas. We have ways we can do this,” Moultrie said. “We’ll get there.”
Other ongoing efforts are already improving diversity in genetics research. At the National Institutes of Health, a program called “All of Us” aims to analyze the DNA of more than 1 million people across the country to build a diverse health database. So far, that program has enrolled more than 790,000 participants. Of these, more than 560,000 have provided DNA samples and about 45% identify as being part of a racial or ethnic minority group.
“Diversity is so important,” said Karriem Watson, chief engagement officer for the All of Us research program . “When you think about groups that carry the greatest burden of disease, we know that those groups are often from minoritized populations.”
Diverse participation in All of Us hasn’t come about by accident. NIH researchers strategically partnered with community health centers, faith-based groups, and Black fraternities and sororities to recruit people who have been historically underrepresented in biomedical research.
In South Carolina, for example, the NIH works with Cooperative Health, a network of federally qualified health centers near the state capital that serve many patients who are uninsured and Black, to recruit patients for All of Us. Eric Schlueter, chief medical officer of Cooperative Health, said the partnership works because their patients trust them.
“We have a strong history of being integrated into the community. Many of our employees grew up and still live in the same communities that we serve,” Schlueter said. “That is what is part of our secret sauce.”
So far, Cooperative Health has enrolled almost 3,000 people in the research program, about 70% of whom are Black.
“Our patients are just like other patients,” Schlueter said. “They want to be able to provide an opportunity for their children and their children’s children to have better health, and they realize this is an opportunity to do that.”
Theoretically, researchers at the NIH and the Medical University of South Carolina may be trying to recruit some of the same people for their separate genetics studies, although nothing would prevent a patient from participating in both efforts.
The researchers in Charleston acknowledge they still have work to do. To date, In Our DNA SC has recruited about half of the 100,000 people it hopes for, and of those, about three-quarters have submitted DNA samples.
Caitlin Allen, a program investigator and a public health researcher at the medical university, acknowledged that some of the program’s tactics haven’t succeeded in recruiting many Black participants.
For example, some patients scheduled to see providers at the Medical University of South Carolina receive an electronic message through their patient portal before an appointment, which includes information about participating in the research project. But studies show that racial and ethnic minorities are less likely to engage with their electronic health records than white patients, Allen said.
“We see low uptake” with that strategy, she said, because many of the people researchers are trying to engage likely aren’t receiving the message.
The study involves four research coordinators trained to take DNA samples, but there’s a limit to how many people they can talk to face-to-face. “We’re not necessarily able to go into every single room,” Allen said.
That said, in-person community events seem to work well for enrolling diverse participants. In March, In Our DNA SC research coordinators collected more than 30 DNA samples at a bicentennial event in Orangeburg, South Carolina, where more than 60% of residents identify as Black. Between the first and second year of the research project, Allen said, In Our DNA SC doubled the number of these community events that research coordinators attended.
“I would love to see it ramp up even more,” she said.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF—an independent source of health policy research, polling, and journalism. Learn more about KFF .
Subscribe to KFF Health News' free Morning Briefing.
about health disparities
- Black women are vital to science and health journalism
- Racism derails Black men’s health, even as education levels rise
- Modern-day segregation in hospitals is killing Black patients
Related Topics ------------------------------------------
Related articles.
Species diversity and extinction risk of vertebrate pollinators in India
- Original Research
- Open access
- Published: 25 April 2024
Cite this article
You have full access to this open access article

- Ratheesh Kallivalappil ORCID: orcid.org/0009-0001-9646-0655 1 ,
- Florencia Grattarola ORCID: orcid.org/0000-0001-8282-5732 2 ,
- Dilkushi de Alwis Pitts 1 , 3 ,
- Sheena C. Cotter 1 &
- Daniel Pincheira-Donoso 4
Animal pollinators underpin the functioning and persistence of ecosystems globally. However, the vital role of pollination is being progressively eroded by the worldwide decline of pollinator species caused by human-induced environmental degradation, resulting in rising costs to biodiversity, agriculture, and economy. Most studies quantifying pollinator diversity and declines have focused on insects, whereas vertebrate pollinators remain comparatively neglected. Here, we present the first comprehensive study quantifying the macroecological patterns of species richness and extinction risk of bird and mammal pollinators in India, a region of extremely high biodiversity and increasing anthropogenic pressure. Our results reveal that hotspots of mammal pollinator diversity are restricted to the south of the Western Ghats, whereas bird pollinator diversity hotspots are scattered throughout the country. Analyses of hotspots of threatened species (based on the IUCN Red List) show that only mammal pollinators are currently classified as threatened in India, whereas multiple hotspots of population declines were observed for birds, and primarily in the Southwest for mammal pollinators. Our analyses failed to identify a role for species traits as drivers of these patterns, whereas most pollinators appear to be threatened by agriculture, logging and hunting for food, and medicinal purposes. Pollinator endangerment has widescale ecological and economic implications such as reduced food production, plant extinction, loss of functional and genetic diversity, and economic damage. We suggest protection of vertebrate pollinators should be emphasised in active conservation agendas in India.
Avoid common mistakes on your manuscript.
Introduction
The progressive accumulation of the human industrial footprint has degraded environments globally, triggering an alarming erosion of biodiversity from across the tree of life (Parmesan 2006 ; Davidson et al. 2009 ; Hoffmann et al. 2010 ; Stork 2010 ; Dirzo et al. 2014 ; Urban 2015 ; Ripple et al. 2016 , 2017 ; Finn et al. 2023 ). Among species undergoing declines, pollinators have attracted increasing attention given the diverse and widespread costs expected to result from their loss (Biesmeijer et al. 2006 ; National Research Council 2007 ; Potts et al. 2010 ; vanEngelsdorp et al. 2010 ). Both invertebrates and vertebrates pollinate a variety of angiosperms, thus playing a crucial role in the functioning and persistence of ecosystems and the biodiversity they sustain (Proctor et al. 1996 ). Vertebrate pollination is a common phenomenon across the tropics, therefore, the dependency of plants on vertebrate pollinators is high in these regions (Fleming and Muchhala 2008 ; Fleming et al. 2009 ; Ratto et al. 2018 ). Vertebrates, particularly birds, pollinate approximately 5% of a region’s flora and it can be as much as 10% in island ecosystems (Anderson 2003 ; Kato and Kawakita 2004 ; Bernardello et al. 2006 ), and bats are believed to be the main pollinators of nearly 1000 economically important plants species in the tropics (Bumrungsri et al. 2008 ; Aziz et al. 2017 ; Sritongchuay et al. 2019 ; Tremlett et al. 2020 ). However, most research on the decline of animal pollinators has been focused on insect pollinators (Biesmeijer et al. 2006 , Kleijn and Raemakers 2008 , Potts et al. 2010 , vanEngelsdorp et al. 2010 , Roulston and Goodell 2011 , Carvalheiro et al. 2013 , Vanbergen and Initiative 2013 , Ollerton et al. 2014 , Scheper et al. 2014 , Goulson et al. 2015 , Powney et al. 2019 ), whereas the study of extinctions across vertebrate pollinators remains comparatively neglected. Therefore, some significant gaps about the diversity, spatial distribution and ‘hotspots’ of extinction risk among vertebrate pollinators are still to be closed by empirical research, especially at large taxonomic and geographic scales.
Vertebrates pollinate a wide variety of economically and ecologically important plants across tropical and subtropical regions (Law and Lean 1999 ; Yumoto 2000 ; Ollerton et al. 2011 ; Maas et al. 2013 ). From among these regions, the highly biodiverse subcontinent of India is known to host a range of vertebrate species that pollinate a wide variety of plant species of significant economic importance for the country (Atluri et al. 2000 ; Nathan et al. 2005 ; Sinu et al. 2011 ; Erancheri et al. 2013 ; Raju et al. 2013 ; Khanduri and Kumar 2017 ). Remarkably, although the territory of India covers ~ 2% of the world’s landmass, this country hosts ~ 7.5% of animal species globally (ZSI 2017 ; Venkataraman and Sivaperuman 2018 ), among which many are pollinators. Between 2014 and 2015, the commercial demand for herbal raw drugs in India was estimated at 512,000 metric tonnes, and the estimated export of these drugs, including extracts, at 134,500 metric tonnes (Goraya and Ved 2017 ). Vertebrate pollinators pollinate some of these plants.
Conservation planning and investment in India are based on large charismatic animals. Whilst this can be beneficial: these animals tend to have large ranges and therefore need large protected areas for their protection, small animals and other taxa are often neglected, regardless of their ecological importance (Treves and Karanth 2003 ; Smith et al. 2012 ; Macdonald et al. 2015 ; Srivathsa et al. 2020 ). Pollinators tend to be small, often inconspicuous animals, and are not considered in terms of conservation planning, despite their importance, both in agricultural and natural ecosystems.
The key roles that vertebrate pollinators play in the functioning and economy of ecosystems in India strongly contrast with the lack of large-scale data on their biodiversity, patterns of distribution and extinction risks. Therefore, to prevent pollinator biodiversity loss and maintain the pollination services (and their associated economic benefits) contributed by vertebrate pollinators that are not redundant with those provided by insects (i.e., the economic contribution from insect pollination to Indian agriculture, at an estimated US$ 22.52 billion during 2012–2013; Chaudhary and Chand 2017 ), we quantify the spatial organisation of pollinator species richness (i.e., biodiversity hotspots) to then identify patterns of spatial distribution of species classified as threatened and those undergoing population declines according to the IUCN Red List. This information is critical to enhance capacities for effective conservation and policy implementation. We expect that both bird and mammal pollinators would show different hotspots of species richness patterns across the geographical regions of India due to differences in their ability to move between habitats. We also predict that the risk of extinction and population declines of bird and mammal pollinators will increase with body size given the increased probability of hunting in association with the tendency of large-sized endotherms for low reproductive rates, which impacts on their chances of rapid demographic recovery (Purvis et al. 2000 ; Cardillo et al. 2005 ; Hutchings et al. 2012 ; Comeros-Raynal et al. 2016 ; Verde Arregoitia 2016 ; Ripple et al. 2017 , Pincheira-Donoso et al. 2021 ). Similarly, we predict that species with small geographic range sizes and low-density populations are more predisposed to extinction risk since they are more vulnerable to local catastrophes and demographic stochasticity, as well as inbreeding events (Kattan 1992 ; Manne et al. 1999 ; Gaston and Fuller 2009 ; Pincheira-Donoso and Hodgson 2018 ; Chichorro et al. 2019 ; Shuai et al. 2021 ). Finally, we investigate the extinction risk of pollinators from anthropogenic activities due to habitat loss and their unsustainable exploitation for bushmeat in the markets.
Materials and methods
Pollinator species name collection.
We collated from the primary literature a de novo database containing all species of vertebrate pollinators of India from mainstream search engines (e.g., Web of Science, Google Scholar). We also retrieved data from additional sources of literature available at the Biodiversity Heritage Library (biodiversitylibrary.org) using the author’s name and year of publication for Singh 1929 , Ali 1931 , McCann 1931 , McCann 1933 , and Davidar 1985 and emailed authors where papers could not be accessed. We used four groups of search terms in Web of Science and Google Scholar: (1) vertebrate, bird, avian, mammal, bat; (2) pollination, flowers, plant; (3) interaction, reproduction; and (4) India. Search methods included one word from each group, where relevant, and all possible four combinations were used to retrieve bird and mammal pollinator species (see Tables S1 & S2 ; accessed until 15th June 2022). Our initial search on the Web of Science yielded 1,178 articles. However, for Google Scholar, we adopted a methodology of selecting the first 20 pages of each keyword thus yielding 2,800 articles. Hence, we retrieved 3,978 articles through both databases and ten articles through other sources (Biodiversity Heritage Library and authors). We screened the titles and abstracts of all the articles for relevance. After eliminating spurious results, 294 appropriate studies were assessed for eligibility. We read the full text of all the remaining articles to determine their suitability to be included in the database, resulting in 57 eligible studies for the synthesis of the vertebrate pollinator database (see Fig. S1 for full PRISMA report). We reviewed literature written in English only, therefore, we might underrepresent the overall published studies if published in other Indian vernacular languages. These studies altogether provided 67 bird and 18 mammal pollinators, resulting in a total of 85 vertebrate pollinator species for the study (see Supplementary Material for a full list of retrieved papers used to compile the pollinator dataset).
For birds, we classified as pollinators those species that regularly make contact with the stigma and anthers of flowers in the process of collecting nectar/pollen (Tandon et al. 2003 ), or identified as pollinators by assessing pollen load on the stigma or fruit production through pollination efficiency experiments (Schemske and Horvitz 1984 ), or animal agents reported to pollinate flowers that do not open naturally, and they require manipulation by visitors for opening unless flowers fall off without pollination (Davidar 1983 ).
For mammals, we classified them as pollinators based on the descriptions of foraging behaviour, such as regularly observed sucking or licking flowers’ nectar, carrying pollen load on fur, or fruit/seed production through pollination efficiency experiments (Regan et al. 2015 ).
To avoid duplication of species due to taxonomic variants, we used the Handbook of the Birds of the World and BirdLife International 2017 (Hoyo et al. 2014 ) and the IUCN Red List mammal taxonomy (IUCN 2018 ) as the taxonomic authority for bird and mammal scientific names.
Collecting species’ threat status and ecological data
We used the function rl_search from the package rredlist (Gearty and Chamberlain 2022 ) to retrieve the IUCN risk status and population trend data for each species. We manually collected information on anthropogenic threats, pet and food trades and their usage in various markets from the IUCN portal.
Assessing extinction risk and population trends of pollinators
To investigate threats to bird and mammal pollinators we categorized species into threatened or non-threatened as per the IUCN categories, where Vulnerable (VU), Endangered (EN) and Critically Endangered (CR) species were considered as threatened, and the remaining categories (Least Concern (LC) and Near Threatened (NT)) non-threatened. We then employed the IUCN ‘consensus approach’ explained below to calculate the proportion of threatened species (PropThreat) (Clausnitzer et al. 2009 ; Hoffmann et al. 2010 ; Böhm et al. 2013 , Pincheira-Donoso et al. 2021 , 2023 ). As per the formula: PropThreat = (CR + EN + VU)/(N – DD), where N is the total number of species in the sample per category and DD is the number of species in the Data Deficient category.
Additionally, we estimated the proportion of species with decreasing populations as: PropDecr = DecreaseN/(N − UnknownN) where DecreaseN is the number of decreasing species, UnknownN is the number of unknown-trend species, and N is the total number of species in all four population trends (Finn et al. 2023 ). We accounted for the unknown-trend species by assuming that no unknown-trend species are decreasing [lower margin; PropDecrL = DecreaseN/N] but all unknown-trend species are decreasing [upper margin; PropDecrU = (DecreaseN + UnknownN)/N]. We also estimated the proportion for species with stable and increasing population trends.
Spatial distribution of species richness
We collected occurrence records for all 67 bird and 18 mammal pollinator species identified using Global Biodiversity Information Facility (GBIF.org 2023 ), iNaturalist web portal (iNaturalist 2023 ), and India Biodiversity portal (Vattakaven et al. 2016 ). We additionally collected 136 occurrence records for 17 mammal pollinator species from various published literature sources (see Supplementary Material : pollinator occurrences reference list), as these species were not sufficiently represented in the main databases. Using the R package CoordinateCleaner (Zizka et al. 2019 ), we cleaned the inaccurate geographic records, such as records in the sea, species outliers, duplicates and invalid coordinates (Table S3 ).
Using the function convexHull from the package alphahull (Pateiro-Lopez et al. 2022 ) and spatialEco (Evans and Murphy 2023 ), we created alphahull polygons for each species of bird and mammal pollinator (Coordinate Reference System: Kalianpur 1975/UTM Zone 44N, EPSG: 24344; length unit in metre). For bird pollinators, we used an alpha-value of 0.1 for the species who had sufficient occurrence data ( N = 65 out of 67). For mammals, we used an alpha-value of 0.5 ( N = 16 out of 18). We used alpha-values of 2.7 and 0.4 for two bird pollinators ( Dicrurus adsimilis and Rubigula melanictera ), and an alpha-value of 2 for two mammal pollinators ( Platacanthomyus lasiurus and Tophozous longimanus ) based on the minimum occurrence points required to create polygons. We removed overlapping polygons of each species by using the function st_union from the sf package (Pebesma 2018 ), and each cleaned individual polygon was then combined for bird and mammal pollinator groups.
We imported India’s shapefile using the function st_read from the R package sf (Pebesma 2018 ) and created a 0.1 × 0.1-degree grid for India using the function st_make_grid . Using the function st_intersection from the above package, we intersected the grids with India map boundaries. Using the same function, we imported the species’ shapefiles and spatially joined them with the gridded India map using the function st_join from the sf package (Pebesma 2018 ). Then we counted the number of overlapping polygons (i.e., species) for each grid cell, resulting in a species richness count. We used the R package tmap (Tennekes 2018 ) to visualize the gridded species richness patterns. All distribution maps were reported on Kalianpur 1975/UTM Zone 44N, EPSG: 24,344 coordinate reference system. To create the maps of species richness, we organised species into a range of different groups: (i) all species (bird = 67, mammal = 18), (ii) species with decreasing population trends according to the IUCN Red List (bird = 16, mammal = 10), (iii) species classified as threatened by the IUCN Red List (bird = 0, mammal = 5), (iv) species for which population trends are unknown (bird = 9, mammals = 2), and (v) endemic species (bird = 4, mammal = 8).
Additionally, we investigated the hotspots of the proportion of species for which population is decreasing or threatened across India. For this, we transferred the variables of decreasing/threatened shapefile to all species shapefile (0.1-degree gridded shapefile obtained from our species richness mappings) by using the function match from the R software (R Core Team 2020 ). We then calculated the proportion for each grid cell by dividing the decreasing/threatened richness by all species’ richness using the same software. We used the package tmap (Tennekes 2018 ) to visualise the patterns.
Estimating variations in spatial distribution of species richness
To investigate spatial variation in spatial patterns between bird and mammal pollinators, we employed the proportions of grid cells with the presence of at least one pollinator species. We compared the proportions of bird and mammal pollinators presence (at least single species in a grid cell) with their total grid cells using a Chi-square test in R (function prop.test ).
Spatial correlations between decreasing/threatened species and species with unknown population trend
To evaluate the risk of population decrease/threat in the species for which population trends are unknown, we assessed the spatial association between the richness of species that are decreasing (for birds) and/or threatened (for mammals) and the richness of species with unknown population trends using Tjøstheim's coefficient with the function cor.spatial (nonparametric) from the SpatialPack package (Vallejos et al. 2020 ). A positive correlation between spatial patterns of species with population decrease/threatened and species with unknown population trend indicates that those poorly evaluated species are likely to be at risk (assuming species experience similar anthropogenic threat). Similarly, using the function cor.test (nonparametric Spearman), we also analysed the latitudinal trend in bird and mammal pollinator richness.
Estimation of species range sizes
To explore extinction risk among Indian bird and mammal pollinators based on their range size, we imported the shapefiles (which we created for the spatial richness mapping) and intersected them across the geographic boundary of India using the function st_intersection from the R package sf (Pebesma 2018 ). We then calculated EOO (extension of occurrence) as the area (km 2 ) of polygons of each species using the function st_area of the sf package (Pebesma 2018 ). We considered a species range size as the sum of all polygons of each species.
After estimating range sizes, species were categorised into range size quartiles (see Jetz and Rahbek 2002 ; Szabo et al. 2009 ; Geng et al. 2012 ), using the function quantile in R (R Core Team 2020 ). We consider the species’ ranges falling in the first quartile (< 25%) as narrow-ranged and species’ ranges falling above this quartile (> 25%) as broad-ranged species (the estimation and categorisation of narrow and broad-ranged species was based on the ranges obtained from data collected in Indian territory only, but the overall range size of species can be distinct from our estimation). As a result, the bird group contains 50 broad and 17 narrow-ranged species, and the mammal group contains 13 broad and 5 narrow-ranged species (25% of quartile range breaks for bird was 26,786.42 km 2 and, mammal was 5030.54 km 2 ; Table S4 ).
Predicting extinction risk
To predict extinction risk based on intrinsic traits in pollinators, we used the phylogenetic generalised least squares regression model (PGLS) from the package caper (Orme et al. 2018 ) with a phylogenetic variance/covariance matrix to account for the evolutionary non-independency between species (for birds, phylogeny obtained from Jetz et al. ( 2012 ) and for mammals from Upham et al. ( 2019 )). We used the IUCN risk status (threatened/not-threatened) and/or population trend (decreasing/not-decreasing) as the dependent variable, and body mass (adult body mass in grams), clutch (number of eggs laid in a single brood), or litter size (number of offspring at a birth; data from Amniote life-history database (Myhrvold et al. 2015 ), and other research databases: see Table S5 ) and range size (extension of occurrences in km 2 ) as predictor variables. This approach allows us to predict the probability of being classified as threatened or registering a decreasing population trend. Using the function pgls , we fitted the models of PGLS to the testing data. We used a Pagel’s lambda transformation with λ optimised by maximum likelihood (ML) (Orme et al. 2018 ), to account for the strength of phylogenetic signal in the evolutionary correlations of pollinator species (Pagel 1999 ). We analysed the significance of each predictor variable individually and combined after log transforming the predictor variables.
We could not find sufficient data for further variables to analyse the impact on species threat or population trends. Thus, we decided to include the variables that have sufficient data points. We used a sample size of 58 bird species (from a total of N = 67) for population decreasing and 18 ( N = 18) and 16 ( N = 18) mammal species for threat and population decreasing analyses after removing data deficient species (we treat as data deficient the species that were labelled as “unknown” in other sections of the methods). Using the same model, we further tested the effect of body mass on the food trade of pollinators (based on the species being used in local, national, and international markets). We used a sample size of 67 bird and 18 mammal species for the analyses.
Our final dataset consisted of 67 bird (6 orders, 27 families) and 18 mammal (4 orders, 6 families) species that met our criteria for them to be classified as pollinators in India. These species represented 6% and 4.2% of the total Indian bird and mammal species, respectively. No studies have reported reptiles as pollinators in India.
Macroecological patterns of species richness distribution
Our results showed that the distribution of bird and mammal pollinators varied spatially across India (Fig. 1 a-b). However, bird pollinators have a broader distributional pattern (proportion test: Chi-squared = 1096.1, df = 1, p = 0.001; Table S6 ) relative to mammals (12,728/33,012 (38.6%) of grid cells in birds; 8,742/33,012 (26.5%) for mammals; Fig. S2 ). The hotspots of bird pollinators occurred along the Western Ghats and Himalayan biodiversity hotspots of India (Fig. 1 a), whereas the hotspots of mammal pollinators largely occurred south of the Western Ghats (Fig. 1 b). The lowest species richness of pollinators occurred in many regions of India, including the Indian and Pakistani controlled Kashmir region (Fig. 1 a-b). The result also showed a scattered pattern of population decrease of pollinators across India. The hotspots of population decrease for bird pollinators were across the Western Ghats and Himalayan biodiversity hotspots (Fig. 1 c). Whereas the hotspots of population decrease and threat of mammal pollinator diversity were in the Western Ghats biodiversity hotspot (Fig. 1 d-f). In addition, we found that the proportion of species undergoing population declines and species classified as threatened overlapped with the Himalayan (for birds) and Western Ghats biodiversity hotspots (for mammals), respectively (Fig. S3 a, S3 b & S3 c).
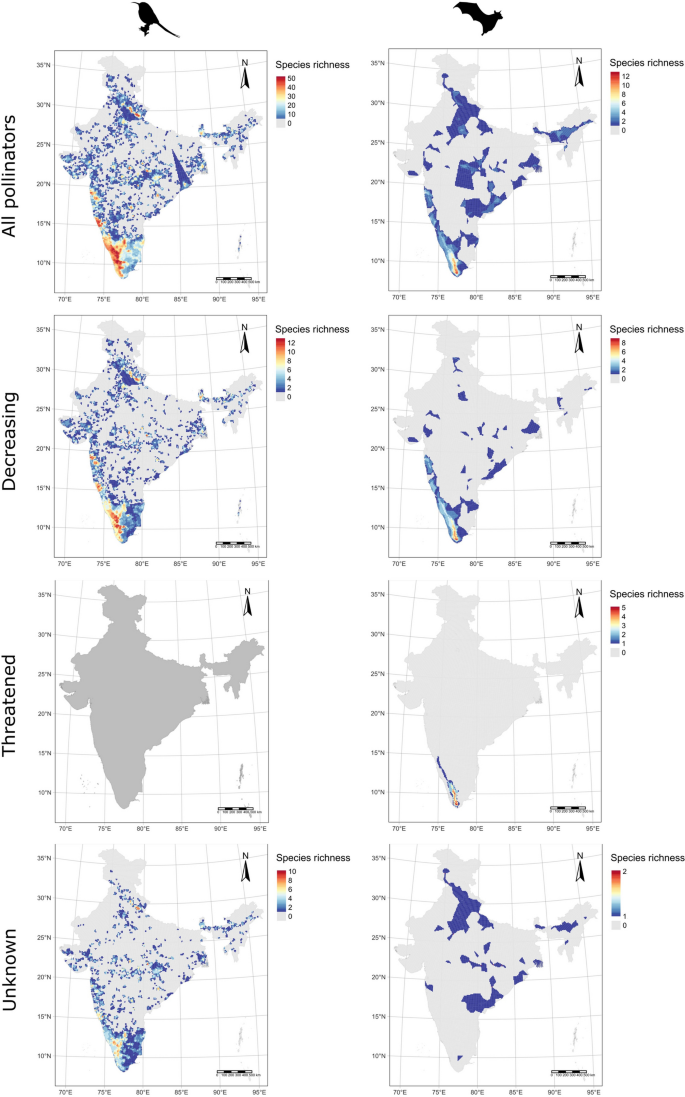
Species richness maps of pollinators for ( a , b ) all pollinators, ( c , d ) species with decreasing populations, ( e , f ) threatened and ( g - h ) population-unknown species for ( a , c , e , g ) birds and ( b , d , f , h ) mammal pollinators of India. No bird pollinators are currently threatened in India ( e ). The maps were created using a spatial resolution of 0.1-degree (approximately 10 km) longitudinal and latitudinal grids
Our dataset showed that 9 bird and 2 mammalian pollinator species do not have population trend data. The distributional patterns of these species showed that the hotspots of bird pollinators occurred across the Western Ghats and Himalayan biodiversity hotspots and certain isolated parts of central India (Fig. 1 g). However, the hotspot of mammal pollinators occurred in the Himalayan and eastern parts of India (Fig. 1 h). Similarly, the Western Ghats biodiversity hotspot was the hottest hotspot for the Indian endemic bird and mammal pollinator diversity (Fig. 2 a-b).
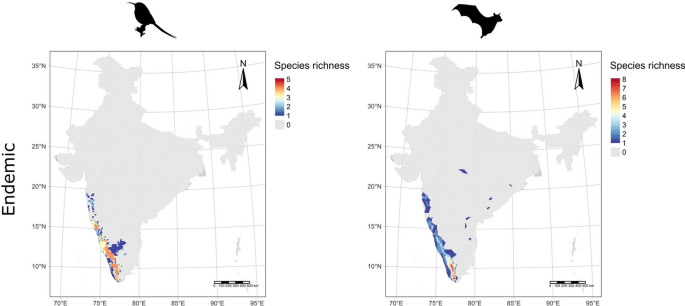
Species richness maps of endemic bird ( a ) and mammal ( b ) pollinators of India. The maps were created using a spatial resolution of 0.1-degree (approximately 10 km) longitudinal and latitudinal grids
Spatial correlations between decreasing/threatened and species with unknown population trend
The spatial patterns of decreasing and/or threatened species richness were not correlated with those of unknown population trend for bird (Tjøstheim's coefficient = 0.0002 ± 0.00) and mammal pollinators (Tjøstheim's coefficient =—0.065 ± 0.0001). Further, we found a weak decline in all bird ( r =—0.315, p = 0.001; Fig. S4 a) and mammal ( r =—0.183, p = 0.001; Fig. S4 b) pollinators richness with increasing latitude.
Extinction risk
Our assessment of the extinction risk of Indian bird and mammal pollinators showed bird pollinators were less threatened than mammal pollinators (Table 1 ). Among mammal pollinators, the narrow-ranged pollinators were more threatened than the broad-ranged pollinators, however, this was statistically not significant (Table 1 ).
The species undergoing population decrease followed the same pattern as extinction risk, where many more mammal pollinators (63%) were significantly decreasing compared to bird pollinators (28%; Table 2 ). Similarly, many broad and narrow-ranged pollinators were decreasing in India. In birds and mammals, the population of narrow-ranged pollinators was more likely to be decreasing relative to broad-ranged pollinators, but for neither group was this statistically significant (Table 2 ).
Susceptibility to extinction risk as a function of species traits
No model supported a significant effect of any predictor on threat levels or population decrease when taken individually (see Supplementary Material: PGLS models ) or combined (Tables S7 , S8 & S9 ).
Extrinsic drivers of extinction risk
In addition to the agriculture expansion and other threats, most Indian mammal pollinators were significantly more threatened by biological resource use such as logging, hunting, poaching, catching and snaring than bird pollinators (Table S10 ). Our results showed that both bird and mammal pollinators are used in various industries, including food, where both groups were used equally (Table 3 ). In addition, analyses suggest that the likelihood for a species to be used as a food resource increases with increasing body size in bird and mammal pollinators (Table S11 & S12 ). We noticed that significantly more bird species were used in national pet markets relative to mammals. In contrast, significantly more mammalian pollinators were used for local medicinal purposes (Table S13 ).
Further, we identified that mammal pollinators were more widely hunted (Fisher’s Exact Test: odds ratio = 115.96, p = 0.001) across India than bird pollinators, where only a single bird species was hunted in accordance with the IUCN data (see Supplementary Material : Pollinator Dataset). The larger mammal pollinators were highly vulnerable for hunting ( R 2 = 0.312, F = 7.25, p = 0.02; Table S14 ).
Using a novel dataset, our study presents the first comprehensive analysis of the broad scale patterns of vertebrate pollinator distribution and risk of extinction throughout India. Our analyses reveal that bird pollinators aggregate in geographically large biodiversity hotspots, compared to the smaller extent of mammal pollinator hotspots. We show that only mammal pollinators are currently classified as threatened with extinction in India, but we acknowledge that an important proportion of both bird and mammal pollinators are showing a population decrease. We identified the biodiversity hotspots, especially the Western Ghats biodiversity hotspot, as a centre of richness, declining, and threatened biodiversity for bird and mammal pollinators. Contrary to our initial expectation, our analyses failed to identify a role for species traits as drivers of extinction risk. Instead, most pollinators appear to be threatened by anthropogenic activities, such as hunting and logging.
We found that the 67 species of bird pollinators we recorded in India were more widespread than the 18 mammalian pollinator species, with hotspots of species richness across the Western Ghats and Himalayan regions. In contrast, the mammalian pollinator hotspot was only in the southern region of the Western Ghats. Whilst no bird pollinators were threatened with extinction (according to the IUCN), several mammalian pollinators were threatened, and these also predominated in the southern Western Ghats. However, a significant proportion/numbers of species in both groups showed decreasing populations, for the mammals, again in the southern Western Ghats, but for birds, decreasing species peaked across the Western Ghats biodiversity hotspot and in Himachal Pradesh, Sikkim and Assam regions of the Himalayan biodiversity hotspot (Fig. 3 ). For all pollinators, the regions of India and Pakistan controlled Kashmir have the lowest species richness, but this is most likely due to deficient data from this region for geopolitical reasons (Bhatnagar et al. 2009 ; Singh et al. 2020 ).
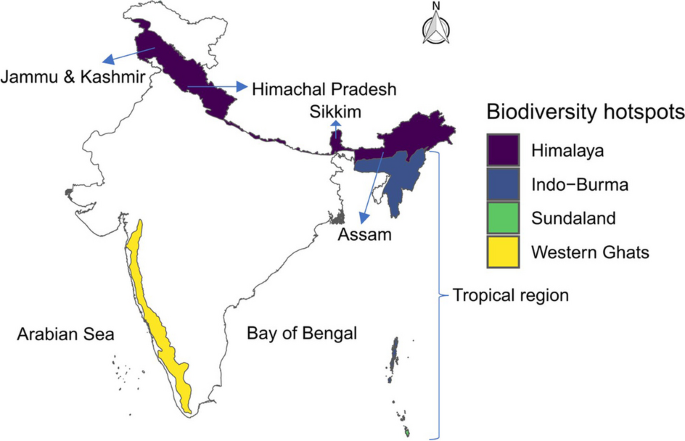
The geographical distribution of four biodiversity hotspots found in India. The names marked with arrows indicate the approximate position of the Indian states explained in the main texts
Hotspots of pollinator richness
The higher species richness of both pollinator groups in the Western Ghats hotspot is potentially due to its tropical climate, a greater topographical variation, a higher diversity of plant species that support the survival of pollinator species by providing higher resource diversity, and its Gondwana origin (Ramesh et al. 1997 , Myers et al. 2000 , Briggs 2003 , Gimaret-Carpentier et al. 2003 , Pascal et al. 2004 , Steffan-Dewenter et al. 2006 , Davidar et al. 2007 , Gunawardene et al. 2007 , Prasad et al. 2009 , Majewska and Altizer 2020 , Kral-O’Brien et al. 2021 , Wei et al. 2021 ). A recent global meta-analysis reported that plant dependence on vertebrate pollinators for fruit or seed production was greater in the tropical regions than at higher latitudes and, specifically, the bat-pollinated plants were more dependent on their vertebrate pollinators (Ratto et al. 2018 ). The higher diversity of mammalian pollinators, especially bats, is likely a result of mutual dependency of plants and their pollinators in this region, because studies found that the birds and bats are effective pollinators in terms of pollen deposition and long-distance pollens dispersal in tropical regions (Stiles 1978 ). Specifically, many bat pollinators and their flowers occur in tropical forest habitats, therefore, they may be more reliable pollinators for plant species and hence plant fitness in this region (Fleming et al. 2009 ; Muchhala et al. 2009 ).
Latitudinal pattern in species’ spatial distribution
Species richness in both bird and mammal pollinators showed a tendency to decline towards higher latitudes. This was consistent with previous findings reported in India for plants (Behera and Roy 2019 ; Page and Shanker 2020 ) and, more generally, with the widespread observation species diversity increases towards the tropics, known as the latitudinal diversity gradient (Pianka 1966 ; Willig et al. 2003 ; Hillebrand 2004 ; Mittelbach et al. 2007 ). It is believed that the tropical regions often provide year-round food supply relative to temperate regions (where plant species experience a period of dormancy due to cold season), hence allowing both specialised and opportunistic pollinators to survive (Cronk and Ojeda 2008 ).
Spatial distribution of population decreases and threats in pollinators
Besides examining the general patterns of species richness, we also considered where species were most threatened and where populations were decreasing. Our results show that the population sizes of both bird and mammal pollinators are decreasing across India. Approximately 28% (range 24 to 37%) of bird pollinator species, whose populations have been assessed by the IUCN, are decreasing. For mammal pollinators, it is 63% (range 56 to 67%). This peak of bird and mammal pollinator species with decreasing populations in the Himalaya, Indo-Burma and Western Ghats biodiversity hotspots indicates widespread bird and mammal exploitation and anthropogenic activities, including forest transformation to agricultural land in this region (Chatterjee et al. 2006 ; Kumara and Sinha 2009 ; Velho et al. 2012 ; Behera et al. 2014 ; Wordley et al. 2017 ; Ghosh-Harihar et al. 2019 ; Murugan et al. 2020 ). A high intensity of pollinators’ population decrease will trigger many ecological vulnerabilities in these hotspots as they host exceptional biodiversity (Chatterjee et al. 2006 ; Joppa et al. 2013 ; Huang et al. 2017 ). Globally, several studies have investigated the potential consequences of decreasing pollinator population on plants and ecosystem services. For example, with a meta-analysis using 126 experiments, Ratto et al. ( 2018 ) showed that the exclusion of bird pollinators caused, on average, a reduction of 46% in fruit/seed production, but this was 83% for bat pollinators. In the Himalayan biodiversity hotspot, studies showed that birds pollinate 10 Rhododendron (Ericaceae) species (Huang et al. 2017 ; Basnett et al. 2019 ), where the exclusion of birds caused decreased seed production in plants (Zhang et al. 2012 ; Huang et al. 2017 ), highlighting that the decline of pollinators would have major consequences for ecosystem functioning. However, more research to understand the effect of pollinators' population decrease and the consequences of reduced fruit/seed set on recruitment in future generations of plants is important in India, particularly in the tropical forests where many birds and mammals are actively involved in pollination and seed dispersal, respectively (Davidar 1983 ; Devy and Davidar 2003 ; Raju et al. 2005 ; Raju 2005 ; Raghuram et al. 2011 ).
According to the IUCN, the only group of pollinators in India currently facing extinction is mammal pollinators, with a peak hotspot of threat in the Western Ghats, indicating the imperilled status of this region. This threat is mainly caused by habitat loss from high human population densities and associated issues like agricultural expansion and developmental activities (Prasad 1998 ; Cincotta et al. 2000 ; Jha et al. 2000 ; Giriraj et al. 2008 ; Reddy et al. 2016 ; Ghosh-Harihar et al. 2019 ). A high proportion of threatened species (28%) may have severe consequences for the provision of ecosystem services (Dobson et al. 2006 ; Whelan et al. 2015 ; Pyšek et al. 2017 ). For example, In New Zealand , Anderson et al. ( 2011 ) found that functional extinction of bird pollinators reduced pollination, seed production and plant density, reducing seed production by 84% and juvenile plants by 55% per adult. Interestingly, the five threatened mammal pollinators (1 bat, 2 monkeys, 2 rodents) are geographically restricted to the Western Ghats region, and some of them have very low reproductive potential, mostly one or two offspring per litter (Ross and Jones 1999 ; Brunet-Rossinni and Austad 2004 ; Dhawale and Sinha 2022 ). Therefore, the reduction in pollinator species can have negative consequences on dependent plant species. Importantly, the geographically restricted species often exhibit distinct traits that may put ecosystems at risk if they are wiped out (Mouillot et al. 2013 ; Loiseau et al. 2020 ).
The level of threat in this region is a major biodiversity concern because there is a high specialisation of plant and vertebrate pollinators in this region. Devy and Davidar ( 2003 ) found that about 75% of the tree species in this region are specialised to just 2–3 species of pollinators and several plants have a single pollinator species (for example, Helicia nilagirica by Platacanthomys lasiurus, and Syzygium mundagam by Cynopterus sphinx ). Such increased specialisation, as we note for many plant species (for example, 31 plant species with a single pollinator species; see Tables S15 & S16 ; Fig. S5 ), raises the risk of extinction, and higher rates of specialised pollinator loss will have a cascading effect on dependent species (Dunn et al. 2009 ), because many plants have lost the ability to pollinate themselves and are entirely dependent on these pollinators to thrive (Cox and Elmqvist 2000 ). A recent simulation study from Indian Himalayan biodiversity hotspot suggested that the plant-pollinator network collapses faster when the most connected pollinators are removed, rather than the most connected plants (Rather et al. 2023 ), emphasising the importance of pollinators for plants survival. We encourage more research to understand this trend across India at various spatial–temporal levels.
We did not find a spatial correlation between the decreasing/threatened and unknown population trend of bird and mammal pollinators, suggesting less likelihood of population decrease/threat in their ranges. However, these relationships are likely to change as the scale of analysis changes (Keil et al. 2011 ; Viladomat et al. 2014 ).
Drivers of extinction risk
Our result did not support the fact that body mass, clutch, litter, or range size predicted threat and decline of species, in contrast to what has been found previously (Cardillo 2003 ; Cardillo et al. 2005 ; Hutchings et al. 2012 ; Comeros-Raynal et al. 2016 ; Ripple et al. 2017 ). However, biological resource use (e.g., logging, hunting) was the biggest threat for both birds and mammals, with agriculture also being an important threat for mammals. In terms of trade, most mammals were used as food, whilst birds were mostly collected as pets. It is therefore likely to be the level of human impact that bird and mammal pollinators are exposed to that makes them vulnerable independently from other intrinsic characteristics of the species (Purvis et al. 2000 ; Cardillo et al. 2005 ). This suggests strict policies on hunting and land use should be implemented and practiced in hotspot regions.
Our study on the pattern of distribution and extinction risk of Indian pollinators brings unique information on this group. Both bird and mammal pollinators show different biodiversity patterns across India with hotspots of species richness overlapping the Himalayan (for birds) and Western Ghats (for birds and mammals) biodiversity hotspots. The decline and threat of pollinators across these regions show the impact of anthropogenic disturbances. Our assessment using the IUCN data shows that 1 in 4 mammal pollinators is currently threatened with extinction in India, but the population trend shows that 1 in 4 bird and 1 in 2 mammal pollinators are currently decreasing. However, the insufficient population data for some species is a conservation concern and future research should be focused on them. We failed to find a relationship between species traits and threat/decrease. Instead, we showed that human activities threaten vertebrate pollinators mostly through habitat loss and hunting. We encourage more research to evaluate the relationship between these traits and threats with a large species pool. We believe that community education and marketing campaigns are essential along with the policy on hunting and land use to mitigate the extinction of vertebrate pollinators in India.
Data availability
The datasets generated during the current study can be accessed in the Zenodo repository: https://doi.org/10.5281/zenodo.10926527 .
Ali S (1931) The role of sunbirds and flowerpeckers in the propagation and distribution of the tree-parasite, Loranthus longiflorus Dest., in the Konkan (W. India). J Bombay Nat Hist Soc 35:144–149
Google Scholar
Anderson SH (2003) The relative importance of birds and insects as pollinators of the New Zealand flora. N Z J Ecol 27:83–94
Anderson SH, Kelly D, Ladley JJ, Molloy S, Terry J (2011) Cascading effects of bird functional extinction reduce pollination and plant density. Science 331:1068–1071
Article CAS Google Scholar
Atluri J, Rao SP, Reddi CS (2000) Pollination ecology of Helicteres isora Linn. (Sterculiaceae). Curr Sci 78:713–718
Aziz SA, Clements GR, McConkey KR, Sritongchuay T, Pathil S, Abu Yazid MNH, Campos-Arceiz A, Forget PM, Bumrungsri S (2017) Pollination by the locally endangered island flying fox (Pteropus hypomelanus) enhances fruit production of the economically important durian (Durio zibethinus). Ecol Evol 7:8670–8684
Article PubMed PubMed Central Google Scholar
Basnett S, Ganesan R, Devy SM (2019) Floral traits determine pollinator visitation in Rhododendron species across an elevation gradient in the Sikkim Himalaya. Alp Bot 129:81–94
Article Google Scholar
Behera M, Patidar N, Chitale V, Behera N, Gupta D, Matin S, Tare V, Panda S, Sen D (2014) Increase in agricultural patch contiguity over the past three decades in Ganga River Basin, India. Curr Sci 107:502–511
Behera MD, Roy PS (2019) Pattern of distribution of angiosperm plant richness along latitudinal and longitudinal gradients of India. Biodivers Conserv 28:2035–2048
Bernardello G, Anderson GJ, Stuessy TF, Crawford DJ (2006) The angiosperm flora of the Archipelago Juan Fernandez (Chile): origin and dispersal. Botany 84:1266–1281
Bhatnagar YV, Ahmad R, Kyarong SS, Ranjitsinh M, Seth C, Lone IA, Easa P, Kaul R, Raghunath R (2009) Endangered markhor Capra falconeri in India: through war and insurgency. Oryx 43:407–411
Biesmeijer JC, Roberts SP, Reemer M, Ohlemuller R, Edwards M, Peeters T, Schaffers A, Potts SG, Kleukers R, Thomas C (2006) Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science 313:351–354
Article CAS PubMed Google Scholar
Böhm M, Collen B, Baillie JE, Bowles P, Chanson J, Cox N, Hammerson G, Hoffmann M, Livingstone SR, Ram M (2013) The conservation status of the world’s reptiles. Biol Cons 157:372–385
Briggs JC (2003) The biogeographic and tectonic history of India. J Biogeogr 30:381–388
Brunet-Rossinni AK, Austad SN (2004) Ageing studies on bats: a review. Biogerontology 5:211–222
Bumrungsri S, Harbit A, Benzie C, Carmouche K, Sridith K, Racey P (2008) The pollination ecology of two species of Parkia (Mimosaceae) in southern Thailand. J Trop Ecol 24:467–475
Cardillo M (2003) Biological determinants of extinction risk: why are smaller species less vulnerable? In animal conservation forum, vol 6. Cambridge University Press, pp 63–69
Cardillo M, Mace GM, Jones KE, Bielby J, Bininda-Emonds OR, Sechrest W, Orme CDL, Purvis A (2005) Multiple causes of high extinction risk in large mammal species. Science 309:1239–1241
Carvalheiro LG, Kunin WE, Keil P, Aguirre-Gutiérrez J, Ellis WN, Fox R, Groom Q, Hennekens S, Van Landuyt W, Maes D (2013) Species richness declines and biotic homogenisation have slowed down for NW-European pollinators and plants. Ecol Lett 16:870–878
Chatterjee S, Saikia A, Dutta P, Ghosh D, Pangging G, Goswami AK (2006) Biodiversity significance of North east India for the study n natural resources, water and environment nexus or development and growth in northeastern India (Background Paper No: 13). WWF-India, Technical report, New Delhi, pp 1–71
Chaudhary O, Chand R (2017) Economic benefits of animal pollination to Indian agriculture. Indian J Agric Sci 87:1117–1138
Chichorro F, Juslén A, Cardoso P (2019) A review of the relation between species traits and extinction risk. Biol Cons 237:220–229
Cincotta RP, Wisnewski J, Engelman R (2000) Human population in the biodiversity hotspots. Nature 404:990–992
Clausnitzer V, Kalkman VJ, Ram M, Collen B, Baillie JE, Bedjanič M, Darwall WR, Dijkstra K-DB, Dow R, Hawking J (2009) Odonata enter the biodiversity crisis debate: the first global assessment of an insect group. Biol Cons 142:1864–1869
Comeros-Raynal MT, Polidoro BA, Broatch J, Mann BQ, Gorman C, Buxton CD, Goodpaster AM, Iwatsuki Y, MacDonald TC, Pollard D (2016) Key predictors of extinction risk in sea breams and porgies (Family: Sparidae). Biol Cons 202:88–98
Cox PA, Elmqvist T (2000) Pollinator extinction in the Pacific Islands. Conserv Biol 14:1237–1239
Cronk Q, Ojeda I (2008) Bird-pollinated flowers in an evolutionary and molecular context. J Exp Bot 59:715–727
Davidar P (1983) Similarity between flowers and fruits in some flowerpecker pollinated mistletoes. Biotropica 15:32–37
Davidar P (1985) Ecological interactions between mistletoes and their avian pollinators in south India. J Bombay Nat Hist Soc 82:45–60
Davidar P, Arjunan M, Mammen PC, Garrigues J, Puyravaud JP, Roessingh K (2007) Forest degradation in the Western Ghats biodiversity hotspot: resource collection, livelihood concerns and sustainability. Curr Sci 93:1573–1578
Davidson AD, Hamilton MJ, Boyer AG, Brown JH, Ceballos G (2009) Multiple ecological pathways to extinction in mammals. Proc Natl Acad Sci 106:10702-10705
Devy MS, Davidar P (2003) Pollination systems of trees in Kakachi, a mid-elevation wet evergreen forest in Western Ghats, India. Am J Bot 90:650–657
Article PubMed Google Scholar
Dhawale AK, Sinha A (2022) Gemini calling! First occurrence of successful twinning in wild, endangered lion-tailed macaques Macaca silenus in the Anamalai hills of the Western Ghats, India. bioRxiv:2022.2008.2003.502728. https://doi.org/10.1101/2022.08.03.502728
Dirzo R, Young HS, Galetti M, Ceballos G, Isaac NJ, Collen B (2014) Defaunation in the Anthropocene. Science 345:401–406
Dobson A, Lodge D, Alder J, Cumming GS, Keymer J, McGlade J, Mooney H, Rusak JA, Sala O, Wolters V (2006) Habitat loss, trophic collapse, and the decline of ecosystem services. Ecology 87:1915–1924
Dunn RR, Harris NC, Colwell RK, Koh LP, Sodhi NS (2009) The sixth mass coextinction: are most endangered species parasites and mutualists? Proc R Soc B: Biol Sci 276:3037-3045
Erancheri P, Sunojkumar P, Dilsha Das M (2013) Bat pollination in medicinally important Cochlospermum religiosum. Ann Plant Sci 2:292–295
Evans JS, Murphy M (2023) spatialEco. R package version 2.0–2. https://github.com/jeffreyevans/spatialEco . Accessed Dec 2023
Finn C, Grattarola F, Pincheira-Donoso D (2023) More losers than winners: investigating Anthropocene defaunation through the diversity of population trends. Biol Rev 98:1732–1748
Fleming TH, Geiselman C, Kress WJ (2009) The evolution of bat pollination: a phylogenetic perspective. Ann Bot 104:1017–1043
Fleming TH, Muchhala N (2008) Nectar-feeding bird and bat niches in two worlds: pantropical comparisons of vertebrate pollination systems. J Biogeogr 35:764–780
Gaston KJ, Fuller RA (2009) The sizes of species’ geographic ranges. J Appl Ecol 46:1–9
GBIF.org (2023) GBIF occurrence download https://doi.org/10.15468/dd.3grghf
Gearty W, Chamberlain S (2022) rredlist:‘IUCN’Red List client. R package version 0.7. https://CRAN.R-project.org/package=rredlis t, https://github.com/ropensci/rredlist (devel), https://docs.ropensci.org/rredlist (docs)
Geng Y, Wang Z, Liang C, Fang J, Baumann F, Kühn P, Scholten T, He JS (2012) Effect of geographical range size on plant functional traits and the relationships between plant, soil and climate in Chinese grasslands. Glob Ecol Biogeogr 21:416–427
Ghosh-Harihar M, An R, Athreya R, Borthakur U, Chanchani P, Chetry D, Datta A, Harihar A, Karanth KK, Mariyam D (2019) Protected areas and biodiversity conservation in India. Biol Cons 237:114–124
Gimaret-Carpentier C, Dray S, Pascal JP (2003) Broad-scale biodiversity pattern of the endemic tree flora of the Western Ghats (India) using canonical correlation analysis of herbarium records. Ecography 26:429–444
Giriraj A, Irfan-Ullah M, Murthy MSR, Beierkuhnlein C (2008) Modelling spatial and temporal forest cover change patterns (1973–2020): A case study from South Western Ghats (India). Sensors 8:6132–6153
Goraya G, Ved D (2017) Medicinal plants in India: an assessment of their demand and supply. National Medicinal Plants Board, Ministry of AYUSH, Government of India, New Delhi and Indian Council of Forestry Research and Education, Dehradun. ISBN 978-81-211-0628-3
Goulson D, Nicholls E, Botías C, Rotheray EL (2015) Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347:1255957
Gunawardene NR, Daniels A, Gunatilleke I, Gunatilleke C, Karunakaran P, Nayak KG, Prasad S, Puyravaud P, Ramesh B, Subramanian K (2007) A brief overview of the Western Ghats-Sri Lanka biodiversity hotspot. Curr Sci 00113891:93
Hillebrand H (2004) On the generality of the latitudinal diversity gradient. Am Nat 163:192–211
Hoffmann M, Hilton-Taylor C, Angulo A, Böhm M, Brooks TM, Butchart SH, Carpenter KE, Chanson J, Collen B, Cox NA (2010) The impact of conservation on the status of the world’s vertebrates. Science 330:1503–1509
Hoyo JD, Collar NJ, Christie DA, Elliott A, Fishpool LD, Allen R (2014) Handbook of the birds of the world and birdlife international illustrated checklist of the birds of the world. Lynx Editions BirdLife International, Barcelona, Spain and Cambridge, UK
Huang Z-H, Song Y-P, Huang S-Q (2017) Evidence for passerine bird pollination in Rhododendron species. AoB plants 9:plx062
Hutchings JA, Myers RA, García VB, Lucifora LO, Kuparinen A (2012) Life-history correlates of extinction risk and recovery potential. Ecol Appl 22:1061–1067
iNaturalist (2023) Available from https://www.inaturalist.org . Accessed December 2023
IUCN (2018) The IUCN red list of threatened species. Version 2018‐1. International union for conservation of nature and natural resources gland. Available from https://www.iucnredlist.org/ . Accessed Aug 2022
Jetz W, Rahbek C (2002) Geographic range size and determinants of avian species richness. Science 297:1548–1551
Jetz W, Thomas GH, Joy JB, Hartmann K, Mooers AO (2012) The global diversity of birds in space and time. Nature 491:444–448
Jha C, Dutt C, Bawa K (2000) Deforestation and land use changes in Western Ghats, India. Curr Sci 79:231–238
Joppa L, Visconti P, Jenkins C, Pimm S (2013) Achieving the convention on biological diversity’s goals for plant conservation. Science 341:1100–1103
Kato M, Kawakita A (2004) Plant-pollinator interactions in New Caledonia influenced by introduced honey bees. Am J Bot 91:1814–1827
Kattan GH (1992) Rarity and vulnerability: the birds of the Cordillera Central of Colombia. Conserv Biol 6:64–70
Keil P, Biesmeijer JC, Barendregt A, Reemer M, Kunin WE (2011) Biodiversity change is scale-dependent: an example from Dutch and UK hoverflies (Diptera, Syrphidae). Ecography 34:392–401
Khanduri VP, Kumar KS (2017) Reproductive effort and success in Bombax ceiba L. in a tropical forest of Mizoram, Indo-Burma region of North-East India. Braz J Bot 40:157–166
Kleijn D, Raemakers I (2008) A retrospective analysis of pollen host plant use by stable and declining bumble bee species. Ecology 89:1811–1823
Kral-O’Brien KC, O’Brien PL, Hovick TJ, Harmon JP (2021) Meta-analysis: Higher plant richness supports higher pollinator richness across many land use types. Ann Entomol Soc Am 114:267–275
Kumara HN, Sinha A (2009) Decline of the endangered lion-tailed macaque Macaca silenus in the Western Ghats, India. Oryx 43:292–298
Law BS, Lean M (1999) Common blossom bats (Syconycteris australis) as pollinators in fragmented Australian tropical rainforest. Biol Cons 91:201–212
Loiseau N, Mouquet N, Casajus N, Grenié M, Guéguen M, Maitner B, Mouillot D, Ostling A, Renaud J, Tucker C (2020) Global distribution and conservation status of ecologically rare mammal and bird species. Nat Commun 11:5071
Article CAS PubMed PubMed Central Google Scholar
Maas B, Clough Y, Tscharntke T (2013) Bats and birds increase crop yield in tropical agroforestry landscapes. Ecol Lett 16:1480–1487
Macdonald E, Burnham D, Hinks A, Dickman A, Malhi Y, Macdonald D (2015) Conservation inequality and the charismatic cat: Felis felicis. Glob Ecol Conserv 3:851–866
Majewska AA, Altizer S (2020) Planting gardens to support insect pollinators. Conserv Biol 34:15–25
Manne LL, Brooks TM, Pimm SL (1999) Relative risk of extinction of passerine birds on continents and islands. Nature 399:258–261
McCann C (1931) On the fertilisation of the flowers of the sausage tree (Kigelia pinnata) by bats. J Bombay Nat Hist Soc 35:467–471
McCann C (1933) The flying fox (P. giganteus) and the palm squirrel (F. tristiatus) as agents of pollinization in (Grevillea robusta A. Cunn.) the silky oak. J Bombay Nat Hist Soc 36:761–764
Mittelbach GG, Schemske DW, Cornell HV, Allen AP, Brown JM, Bush MB, Harrison SP, Hurlbert AH, Knowlton N, Lessios HA (2007) Evolution and the latitudinal diversity gradient: speciation, extinction and biogeography. Ecol Lett 10:315–331
Mouillot D, Bellwood DR, Baraloto C, Chave J, Galzin R, Harmelin-Vivien M, Kulbicki M, Lavergne S, Lavorel S, Mouquet N (2013) Rare species support vulnerable functions in high-diversity ecosystems. PLoS Biol 11:e1001569
Muchhala N, Caiza A, Vizuete JC, Thomson JD (2009) A generalized pollination system in the tropics: bats, birds and Aphelandra acanthus. Ann Bot 103:1481–1487
Murugan CM, Bhavan PS, Mahandran V, Nathan PT (2020) Hunting bats for bushmeat despite Nipah concerns in Idukki, Kerala, India. Ecotropica 22:202006–202006
Myers N, Mittermeier RA, Mittermeier CG, Da Fonseca GA, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858
Myhrvold NP, Baldridge E, Chan B, Sivam D, Freeman DL, Ernest SM (2015) An amniote life-history database to perform comparative analyses with birds, mammals, and reptiles: Ecological Archives E096–269. Ecology 96:3109–3109
Nathan PT, Raghuram H, Elangovan V, Karuppudurai T, Marimuthu G (2005) Bat pollination of kapok tree, Ceiba pentandra. Curr Sci 88:1679–1681
National Research Council (2007) Status of pollinators in North America. Status Pollinators North Am: Natl Acad Press 10:11761
Ollerton J, Erenler H, Edwards M, Crockett R (2014) Extinctions of aculeate pollinators in Britain and the role of large-scale agricultural changes. Science 346:1360–1362
Ollerton J, Winfree R, Tarrant S (2011) How many flowering plants are pollinated by animals? Oikos 120:321–326
Orme D, Freckleton R, Thomas G, Petzoldt T, Fritz S, Isaac N, Pearse W (2018) The caper package: comparative analysis of phylogenetics and evolution in R. R package version 1.0.1. 5:1-36. https://CRAN.R-project.org/package=caper . Accessed Jan 2024
Page NV, Shanker K (2020) Climatic stability drives latitudinal trends in range size and richness of woody plants in the Western Ghats, India. Plos One 15:e0235733
Article CAS PubMed Central Google Scholar
Pagel M (1999) Inferring the historical patterns of biological evolution. Nature 401:877–884
Parmesan C (2006) Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol Syst 37:637–669
Pascal J, Ramesh B, Franceschi DD (2004) Wet evergreen forest types of the southern Western Ghats, India. Trop Ecol 45:281–292
Pateiro-Lopez B, Rodriguez-Casal A, Pateiro-Lopez MB (2022) alphahull: Generalization of the Convex Hull of a Sample of Points in the Plane. R package version 2.5. https://CRAN.R-project.org/package=alphahull . Accessed Jan 2023
Pebesma E (2018) Simple features for R [R package sf version 0.6–0]. https://CRAN.R-project.org/package=sf . Accessed Oct 2022
Pianka ER (1966) Latitudinal gradients in species diversity: a review of concepts. Am Nat 100:33–46
Pincheira-Donoso D, Hodgson DJ (2018) No evidence that extinction risk increases in the largest and smallest vertebrates. Proc Natl Acad Sci 115:E5845-E5846
Pincheira-Donoso D, Harvey LP, Cotter SC, Stark G, Meiri S, Hodgson DJ (2021) The global macroecology of brood size in amphibians reveals a predisposition of low-fecundity species to extinction. Glob Ecol Biogeogr 30:1299–1310
Pincheira-Donoso D, Harvey LP, Johnson JV, Hudson D, Finn C, Goodyear LE, Guirguis J, Hyland EM, Hodgson DJ (2023) Genome size does not influence extinction risk in the world’s amphibians. Funct Ecol 37:190–200
Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, Kunin WE (2010) Global pollinator declines: trends, impacts and drivers. Trends Ecol Evol 25:345–353
Powney GD, Carvell C, Edwards M, Morris RK, Roy HE, Woodcock BA, Isaac NJ (2019) Widespread losses of pollinating insects in Britain. Nat Commun 10:1018
Prasad SN (1998) Conservation planning for the Western Ghats of Kerala: II. Assessment of habitat loss and degradation. Curr Sci 75:228–235
Prasad V, Farooqui A, Tripathi S, Garg R, Thakur B (2009) Evidence of late Palaeocene-early Eocene equatorial rain forest refugia in southern Western Ghats, India. J Biosci 34:777–797
Proctor M, Yeo P, Lack A (1996) The natural history of pollination. Harper Collins Publishers, New York, NY
Purvis A, Gittleman JL, Cowlishaw G, Mace GM (2000) Predicting extinction risk in declining species. Proc R Soc London Ser B: Biol Sci 267:1947–1952
Pyšek P, Blackburn TM, García-Berthou E, Perglová I, Rabitsch W (2017) Displacement and local extinction of native and endemic species. Impact Biol Invasions Ecosyst Serv 12:157–175
R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/ . Accessed Aug 2022
Raghuram H, Singaravelan N, Nathan PT, Emmanuvel K, Rajan K, Marimuthu G (2011) Foraging ecology of pteropodid bats: pollination and seed dispersal. Zupan JL, and Mlakar (Eds.), SL, Bats: biology, behavior and conservation. Nova Science Publishers Inc, New York, NY
Raju AJ, Rao SP, Rangaiah K (2005) Pollination by bats and birds in the obligate outcrosser Bombax ceiba L. (Bombacaceae), a tropical dry season flowering tree species in the Eastern Ghats forests of India. Ornithol Sci 4:81–87
Raju AJS (2005) Passerine bird pollination and seed dispersal in Woodfordia floribunda Salisb. (Lythraceae), a common low altitude woody shrub in the Eastern Ghats forests of India. Ornithol Sci 4:103–108
Raju AJS, Lakshmi PV, Ramana KV (2013) Entomophily, ornithophily and anemochory in the highly self-incompatible Boswellia ovalifoliolata Bal. & Henry (Burseraceae), an endemic and endangered medicinal tree species. Biol Sci-PJSIR 56:1–10
CAS Google Scholar
Ramesh B, Menon S, Bawa KS (1997) A vegetation based approach to biodiversity gap analysis in the Agastyamalai region, Western Ghats, India. Ambio 26:529–536
Rather ZA, Ollerton J, Parey SH, Ara S, Watts S, Paray MA, Khuroo AA (2023) Plant-pollinator meta-network of the Kashmir Himalaya: Structure, modularity, integration of alien species and extinction simulation. Flora 298:152197
Ratto F, Simmons BI, Spake R, Zamora-Gutierrez V, MacDonald MA, Merriman JC, Tremlett CJ, Poppy GM, Peh KSH, Dicks LV (2018) Global importance of vertebrate pollinators for plant reproductive success: a meta-analysis. Front Ecol Environ 16:82–90
Reddy CS, Jha CS, Dadhwal VK (2016) Assessment and monitoring of long-term forest cover changes (1920–2013) in Western Ghats biodiversity hotspot. J Earth Syst Sci 125:103–114
Regan EC, Santini L, Ingwall-King L, Hoffmann M, Rondinini C, Symes A, Taylor J, Butchart SH (2015) Global trends in the status of bird and mammal pollinators. Conserv Lett 8:397–403
Ripple WJ, Chapron G, López-Bao JV, Durant SM, Macdonald DW, Lindsey PA, Bennett EL, Beschta RL, Bruskotter JT, Campos-Arceiz A (2016) Saving the world’s terrestrial megafauna. Bioscience 66:807–812
Ripple WJ, Wolf C, Newsome TM, Hoffmann M, Wirsing AJ, McCauley DJ (2017) Extinction risk is most acute for the world’s largest and smallest vertebrates. Proc Natl Acad Sci 114:10678-10683
Ross C, Jones KE (1999) Socioecology and the evolution of primate reproductive rates. Comp Primate Socioecol 22:73–110
Roulston TH, Goodell K (2011) The role of resources and risks in regulating wild bee populations. Ann Rev Entomol 56:293–312
Schemske DW, Horvitz CC (1984) Variation among floral visitors in pollination ability: a precondition for mutualism specialization. Science 225:519–521
Scheper J, Reemer M, van Kats R, Ozinga WA, van der Linden GT, Schaminée JH, Siepel H, Kleijn D (2014) Museum specimens reveal loss of pollen host plants as key factor driving wild bee decline in The Netherlands. Proc Natl Acad Sci 111:17552-17557
Shuai L, Chen C, Liu W, Xu W, Wang Y, Zeng Z, Zhang Z, Zhao L, Wang Y (2021) Ecological correlates of extinction risk in Chinese terrestrial mammals. Divers Distrib 27:1294–1307
Singh PB, Mainali K, Jiang Z, Thapa A, Subedi N, Awan MN, Ilyas O, Luitel H, Zhou Z, Hu H (2020) Projected distribution and climate refugia of endangered Kashmir musk deer Moschus cupreus in greater Himalaya, South Asia. Sci Rep 10:1511
Singh T (1929) A preliminary note on the pollination of the coral tree (Erythrina indica Lamk.). J Bombay Nat Hist Soc 33:460–462
Sinu PA, Kuriakose G, Shivanna K (2011) Is the bumblebee (Bombus haemorrhoidalis) the only pollinator of large cardamom in central Himalayas, India? Apidologie 42:690–695
Smith RJ, Veríssimo D, Isaac NJ, Jones KE (2012) Identifying Cinderella species: uncovering mammals with conservation flagship appeal. Conserv Lett 5:205–212
Sritongchuay T, Hughes AC, Bumrungsri S (2019) The role of bats in pollination networks is influenced by landscape structure. Glob Ecol Conserv 20:e00702
Srivathsa A, Majgaonkar I, Sharma S, Singh P, Punjabi GA, Chawla MM, Banerjee A (2020) Opportunities for prioritizing and expanding conservation enterprise in India using a guild of carnivores as flagships. Environ Res Lett 15:064009
Steffan-Dewenter I, Klein AM, Gaebele V, Alfert T, Tscharntke T (2006) Bee diversity and plant-pollinator interactions in fragmented landscapes. In: Waser NM, Ollerton J (eds) Specialization and generalization in plant-pollinator interactions. University of Chicago Press
Stiles FG (1978) Ecological and evolutionary implications of bird pollination. Am Zool 18:715–727
Stork NE (2010) Re-assessing current extinction rates. Biodivers Conserv 19:357–371
Szabo ND, Algar AC, Kerr JT (2009) Reconciling topographic and climatic effects on widespread and range-restricted species richness. Glob Ecol Biogeogr 18:735–744
Tandon R, Shivanna K, Mohan Ram H (2003) Reproductive biology of Butea monosperma (Fabaceae). Ann Bot 92:715–723
Tennekes M (2018) tmap: thematic maps in R. J Stat Softw 84:1–39
Tremlett CJ, Moore M, Chapman MA, Zamora-Gutierrez V, Peh KSH (2020) Pollination by bats enhances both quality and yield of a major cash crop in Mexico. J Appl Ecol 57:450–459
Treves A, Karanth KU (2003) Human-carnivore conflict and perspectives on carnivore management worldwide. Conserv Biol 17:1491–1499
Upham NS, Esselstyn JA, Jetz W (2019) Inferring the mammal tree: species-level sets of phylogenies for questions in ecology, evolution, and conservation. PLoS Biol 17:e3000494
Urban MC (2015) Accelerating extinction risk from climate change. Science 348:571–573
Vanbergen AJ, Initiative TIP (2013) Threats to an ecosystem service: pressures on pollinators. Front Ecol Environ 11:251–259
vanEngelsdorp D, Hayes J Jr, Underwood RM, Pettis JS (2010) A survey of honey bee colony losses in the United States, fall 2008 to spring 2009. J Apic Res 49:7–14
Vattakaven T, George RM, Balasubramanian D, Réjou-Méchain M, Muthusankar G, Ramesh BR, Prabhakar R (2016) India Biodiversity Portal: An integrated, interactive and participatory biodiversity informatics platform. Biodivers Data J 4:e10279. https://doi.org/10.3897/BDJ.3894.e10279
Velho N, Karanth KK, Laurance WF (2012) Hunting: A serious and understudied threat in India, a globally significant conservation region. Biol Cons 148:210–215
Vallejos R, Osorio F, Bevilacqua M (2020) Spatial relationships between two georeferenced variables: with applications in R. Springer Nature, Berlin/Heidelberg, Germany. https://doi.org/10.1007/978-3-030-56681-4
Venkataraman K, Sivaperuman C (2018) Biodiversity hotspots in India. Indian Hotspots: Vertebr Faunal Divers Conserv Manag 1:1–27
Verde Arregoitia LD (2016) Biases, gaps, and opportunities in mammalian extinction risk research. Mammal Rev 46:17–29
Viladomat J, Mazumder R, McInturff A, McCauley DJ, Hastie T (2014) Assessing the significance of global and local correlations under spatial autocorrelation: a nonparametric approach. Biometrics 70:409–418
Wei N, Kaczorowski RL, Arceo-Gómez G, O’Neill EM, Hayes RA, Ashman T-L (2021) Pollinators contribute to the maintenance of flowering plant diversity. Nature 597:688–692
Whelan CJ, Şekercioğlu ÇH, Wenny DG (2015) Why birds matter: from economic ornithology to ecosystem services. J Ornithol 156:227–238
Willig MR, Kaufman DM, Stevens RD (2003) Latitudinal gradients of biodiversity: pattern, process, scale, and synthesis. Annu Rev Ecol Evol Syst 34:273–309
Wordley CF, Sankaran M, Mudappa D, Altringham JD (2017) Bats in the Ghats: Agricultural intensification reduces functional diversity and increases trait filtering in a biodiversity hotspot in India. Biol Cons 210:48–55
Yumoto T (2000) Bird-pollination of three Durio species (Bombacaceae) in a tropical rainforest in Sarawak, Malaysia. Am J Bot 87:1181–1188
Zhang FP, Cai XH, Wang H, Ren ZX, Larson-Rabin Z, Li DZ (2012) Dark purple nectar as a foraging signal in a bird-pollinated Himalayan plant. New Phytol 193:188–195
Zizka A, Silvestro D, Andermann T, Azevedo J, Duarte Ritter C, Edler D, Farooq H, Herdean A, Ariza M, Scharn R (2019) CoordinateCleaner: standardized cleaning of occurrence records from biological collection databases. Methods Ecol Evol 10:744–751
ZSI (2017) Animal discoveries of 2016. New species and new records. Zoological Survey of India, Kolkata. Director, Zoological Survey of India (eds): Survey of India, pp 1–95
Download references
Acknowledgements
We thank those who have conducted research in pollination biology for making data openly available for data extraction. We express our sincere gratitude to the Ministry of Social Justice and Empowerment, Government of India for financial support through National Overseas Scholarship for this study awarded to RK. We extend our thanks to the University of Lincoln for facilities and technical support for the completion of this study. We also thank the associate editor and the anonymous referee for the constructive comments to improve our manuscript.
This work was supported by the Ministry of Social Justice and Empowerment, Government of India. Author Ratheesh Kallivalappil has received research support through the National Overseas Scholarship for PhD education.
Author information
Authors and affiliations.
School of Life and Environmental Sciences, Joseph Banks Laboratories, University of Lincoln, Brayford Campus, Lincoln, LN6 7DL, UK
Ratheesh Kallivalappil, Dilkushi de Alwis Pitts & Sheena C. Cotter
Faculty of Environmental Sciences, Czech University of Life Sciences Prague, Kamýcká 129, Prague-Suchdol, 165 00, Czech Republic
Florencia Grattarola
College of Interdisciplinary Studies, Zayed University, P.O. Box 144534, Abu Dhabi, United Arab Emirates
Dilkushi de Alwis Pitts
MacroBiodiversity Lab, School of Biological Sciences, Queen’s University Belfast, 19 Chlorine Gardens, Belfast, BT9 5DL, UK
Daniel Pincheira-Donoso
You can also search for this author in PubMed Google Scholar
Contributions
All authors contributed to the study conception and design. Literature search and data collection were performed by Ratheesh Kallivalappil. The analysis was performed by Ratheesh Kallivalappil, Florencia Grattarola, and Sheena C. Cotter. The first draft of the manuscript was written by Ratheesh Kallivalappil, Florencia Grattarola, Dilkushi de Alwis Pitts, Sheena C. Cotter and Daniel Pincheira-Donoso and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Correspondence to Sheena C. Cotter or Daniel Pincheira-Donoso .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Communicated by Jens Dauber.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (DOCX 37 KB)
Supplementary file2 (xls 39 kb), supplementary file3 (doc 1093 kb), rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Kallivalappil, R., Grattarola, F., de Alwis Pitts, D. et al. Species diversity and extinction risk of vertebrate pollinators in India. Biodivers Conserv (2024). https://doi.org/10.1007/s10531-024-02848-3
Download citation
Received : 22 July 2023
Revised : 07 April 2024
Accepted : 15 April 2024
Published : 25 April 2024
DOI : https://doi.org/10.1007/s10531-024-02848-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Pollinators
- Distributions
- Find a journal
- Publish with us
- Track your research

IMAGES
VIDEO
COMMENTS
Analysing >5,000 population abundance time series for insects and other arthropods from 68 sites within the US Long Term Ecological Research network, the authors find high variation but no ...
Insect diversity was estimated using spatially explicit earth observation data and insect species assemblages across microhabitats in two agro-ecological zones in Africa. ... according to research ...
Most of the studies on insect diversity examined one a single measure, such as biomass or proxies expressing the level of diversity, such as species richness 4,19. However, modern sampling methods ...
Insect diversity is the evolutionary basis that enables species adaptation and survival, but also the essential pillar of ecosystem services and homeostasis. In this chapter, we discuss four types of diversity (genetic, behavioral, species, and ecosystem) and justify their relevance to insect conservation. We focus on behavioral variability ...
The insect apocalypse. In 2017, an article reporting a 75% decline over 27 years in flying insect biomass in nature reserves in Germany [7], was picked up by the global media and sparked a flurry of public interest and much-debated research (e.g. 8, 9, 10).This led to insect conservation entering the spotlight, with multiple academic papers and news articles citing the ongoing and impending ...
Abstract. Insect 'diversity' is assessed most commonly by enumeration of species, but many such entities cannot be recognised or defined consistently by non-specialists. Practical approximations are used widely in interpreting insect diversity in ecological samples and to overcome the logistic barriers to full interpretation at species level.
Global declines in insects have sparked wide interest among scientists, politicians, and the general public. Loss of insect diversity and abundance is expected to provoke cascading effects on food webs and to jeopardize ecosystem services. Our understanding of the extent and underlying causes of this decline is based on the abundance of single species or taxonomic groups only, rather than ...
Abstract Insects are the most speciesrich group on the Earth, hence it play numerous. crucial roles in ecosystem functioning and the global economy. The. conservation of insect diversity is ...
Insect declines and biodiversity loss have attracted much attention in recent years, but lack of comprehensive data, conflicting interests among stakeholders and insufficient policy guidance hinder progress in preserving biodiversity. The project DINA (Diversity of Insects in Nature protected Areas) investigates insect communities in 21 nature reserves in Germany. All selected conservation ...
Emerging evidence suggests that insect populations may be declining at local and global scales, threatening the sustainability of the ecosystem services that insects provide. Insect declines are of particular concern in the Neotropics, which holds several of the world's hotspots of insect endemism and diversity. Conservation policies are one way to prevent and mitigate insect declines, yet ...
Explore the latest full-text research PDFs, articles, conference papers, preprints and more on INSECT DIVERSITY. Find methods information, sources, references or conduct a literature review on ...
Insects are an important component of the ecosystem and fast dwindling of its diversity is reported globally. The International Union for Conservation of Nature has assessed a total of 77,435 ...
Phylogenomics captures only a snapshot of insect evolution, turning a blind eye to the immense diversity of extinct insect lineages. ... Support for the present study was provided by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB26000000), the National Natural Science Foundation of China (41672011 and 41688103 ...
Overview of published studies focusing on insect diversity and evolution using massively parallel sequencing. (A) The main sequencing platforms (SM stands for single-molecule, including those from PacBio and Oxford Nanopore technologies). ... Research articles are the standard way to communicate results to the scientific community. However ...
INTRODUCTION. Conservation of insects in urban environments is a key global challenge (Luke et al., 2023).The continued expansion of urban areas across the globe (Angel et al., 2011) has raised concerns about how this impacts biodiversity and the field of urban ecology has grown rapidly in recent decades (Niemelä et al., 2011).To date, urban animal studies have, however, largely focused on ...
Insects are not only the most species—rich group on Earth, they also play numerous crucial roles in ecosystem functioning and the global economy. The conservation of insect diversity is therefore a topic of global importance. However, insects are mostly ignored by "biodiversity" research; for example, relationships between insect diversity and vegetation or climate change remain widely ...
Although research has shown that biodiversity changes are driven primarily by land-use change and increasingly by climate change6,7, the potential for interaction between these drivers and insect ...
Journal of Insect Biodiversity is a hybrid journal and has published many open access papers: ... Diversity, abundance and distribution of grasshopper species (Orthoptera: Acrididea) in three different types of vegetation with different levels of anthropogenic disturbances in the Littoral Region of Cameroon ... It publishes research articles ...
Biodiversity plays an important role in maintaining the ecological balance of the earth. The study of traditional knowledge related to biological resources is a hot issue in the field of international biodiversity conservation. Xishuangbanna is a key area of biodiversity and a cultural hotspot of international significance. According to the Technical Regulation for Classification ...
The increase in insect diversity was observed until April, while a declining trend was visible between November, December, and January (Fig. 3A). We observed maximum similarity in insect communities between March and April, while less similarity was observed in December and January (Fig. 3B). Relative humidity was found to have a significant effect on the composition of insect assemblages ...
Intensive breeding since the 19 th century has created thousands of varieties of rose, but a reduction in genetic diversity could leave them vulnerable to diseases and climate change By James Woodford
of Life Sciences, Bharathiar University, Coimbatore - 641 046, T amil Nadu, India, Email [email protected]. Received: September 25, 2018 Published: November 06, 2018. Abstract. In the present ...
In this study, we first re-examine the global insect fossil record to account for the changes in family-level insect diversity 3,4,5,24 and analytical methods 29,30 that have occurred in the last ...
Gene editing has the potential to solve fundamental challenges in agriculture, biotechnology, and human health. CRISPR-based gene editors derived from microbes, while powerful, often show significant functional tradeoffs when ported into non-native environments, such as human cells. Artificial intelligence (AI) enabled design provides a powerful alternative with potential to bypass ...
The current research has three study objectives. (1) the Collection and identification of flying insect fauna from wheat crop. ... This study highlights the richness, abundance and diversity of the insect fauna in Sialkot. The number of sampled insects showed numerical variations in different months. Identified orders were also reported by Ruby ...
Far more animals than previously thought likely have consciousness, top scientists say in a new declaration — including fish, lobsters and octopus. Recent research backs them up.
A storm hit the United Arab Emirates and Oman this week bringing record rainfall that flooded highways, inundated houses, grid-locked traffic and trapped people in their homes.
Insect declines have been implicated as possible drivers of declines in insect-eating birds 2 and in animal-pollinated plants 3. Thus, massive losses in insect diversity (Fig. 1) and abundance ...
Lack of diversity in genetics research has real health care implications. Since the completion more than 20 years ago of the Human Genome Project, which mapped most human genes for the first time ...
Animal pollinators underpin the functioning and persistence of ecosystems globally. However, the vital role of pollination is being progressively eroded by the worldwide decline of pollinator species caused by human-induced environmental degradation, resulting in rising costs to biodiversity, agriculture, and economy. Most studies quantifying pollinator diversity and declines have focused on ...