MRC Dyspnoea Scale
The mMRC (Modified Medical Research Council) Dyspnoea Scale is used to assess the degree of baseline functional disability due to dyspnoea.
It is useful in characterising baseline dyspnoea in patients with respiratory disease such as COPD. Whilst it moderately correlates with other healthcare-associated morbidity, mortality and quality of life scales (particularly in COPD) the scores are only variably associated with patients' perceptions of respiratory symptom burden. It is used as a component of the BODE Index, which predicts adverse outcomes, including mortality and risk of hospitalisation. The scale is easy and efficient to use.
The mMRC breathlessness scale ranges from grade 0 to 4. It is very similar to the original version and is now widely used in studies. It should be noted that the MRC clearly states on its website that it is unable to give permission for use of any modified version of the scale (including therefore, the mMRC scale). Use of the MRC questionnaire is free but should be acknowledged.
The modified MRC was developed by D A Mahler, see https://pubmed.ncbi.nlm.nih.gov/3342669/

Diagnostic testing
Your essential guide to respiratory diagnostic testing from FeNO and spirometry to CRP Point of Care Testing.
Clinical resources
Step by step guides, expert opinion, the latest insights and case studies - our resources cover a range of respiratory topics and a vital resource for any practitioner working in the delivery of respiratory healthcare
PCRS Respiratory Conference
The UK's leading respiratory conference for clinicians working primary, community and integrated care comes to Telford in September.
You may also be interested in...
Step by step guides, podcasts and webinars cover prevention, diagnosis, testing and management. They will help you to support your patients and improve their outcomes.
Inhaler devices
Inhaler devices may seem simple to use but they are often used incorrectly by patients and healthcare professionals alike.
Chronic Obstructive Pulmonary Disease (COPD) is the fifth leading cause of death in the UK. It's a serious condition which calls for a patient centric approach.
Join PCRS Today
Become part of the UK's largest network of dedicated respiratory professionals working in primary, community and integrated care settings.

MedicalCRITERIA.com
Unifying concepts, modified medical research council (mmrc) dyspnea scale.
The modified Medical Research Council (mMRC) scale is recommended for conducting assessments of dyspnea and disability and functions as an indicator of exacerbation.
The modified Medical Research Council (mMRC) scale
An mMRC scale grade of 3 have a significantly poorer prognosis and that the mMRC scale can be used to predict hospitalization and exacerbation.
References:
- Natori H, Kawayama T, Suetomo M, Kinoshita T, Matsuoka M, Matsunaga K, Okamoto M, Hoshino T. Evaluation of the Modified Medical Research Council Dyspnea Scale for Predicting Hospitalization and Exacerbation in Japanese Patients with Chronic Obstructive Pulmonary Disease. Intern Med. 2016;55(1):15-24. [Medline]
- Launois C, Barbe C, Bertin E, Nardi J, Perotin JM, Dury S, Lebargy F, Deslee G. The modified Medical Research Council scale for the assessment of dyspnea in daily living in obesity: a pilot study. BMC Pulm Med. 2012 Oct 1;12:61. [Medline]
Created Feb 10, 2021.
Related Posts
The muscle scale grades muscle power on a scale of 0 to 5 in relation…
The Hachinski ischemic score (HIS) is known to be a simple clinical tool, currently used…
The Karnofsky Performance Status Scale (KPS) was designed to measure the level of patient activity…
Instructions: This scale is intended to record your own assessment of any sleep difficulty you…
The FAST scale is a functional scale designed to evaluate patients at the more moderate-severe…

Users Online
Medical disclaimer, recent posts.
- Diagnostic Criteria for Catatonia
- Diagnostic Criteria for Dengue Hemorrhagic Fever (DHS) and Dengue Shock Syndrome (DSS)
- Diagnosis of Posterior Reversible Encephalopathy Syndrome (PRES)
- Manifestations of Right-Sided Heart Failure (RHF)
- Risk Factors of Budd-Chiari Syndrome
- About Us (1)
- Allergy (3)
- Anesthesiology (5)
- Cardiology (54)
- Critical Care (17)
- Dermatology (7)
- Diabetes (14)
- Endocrinology and Metabolism (20)
- Epidemiology (6)
- Family Practice (7)
- Gastroenterology (63)
- Hematology (40)
- Immumology (6)
- Infectious Disease (44)
- Internal Medicine (1)
- Nephrology (17)
- Neurology (79)
- Nutrition (29)
- Obstetrics & Gynecology (20)
- Oncology (13)
- Ophthalmology (4)
- Orthopedic (5)
- Otolaryngology (5)
- Pathologic (1)
- Pediatrics (14)
- Pharmacology (3)
- Physical Therapists (7)
- Psychiatry (50)
- Pulmonary (23)
- Radiology (5)
- Rheumatology (51)
- Surgery (8)
- Urology (5)
Popular of the Last Month
- DSM-5 Diagnostic Criteria for Schizophrenia 825 vistas
- Diagnostic Criteria for Anorexia Nervosa (DSM-V) 804 vistas
- DSM-5 Diagnostic Criteria for Panic Disorder 749 vistas
- Diagnostic Criteria for Bulimia Nervosa (DSM-5) 635 vistas
- DSM-5 Diagnostic Criteria for Major Depressive Disorder 504 vistas
- GLIM Criteria for the Diagnosis of Malnutrition 473 vistas
- Edmonton Obesity Staging System (EOSS) 365 vistas
- Stage Classification of Gastric Ulcer by Sakita-Miwa 354 vistas
- DSM-5 Diagnostic Criteria for Dyspraxia/Developmental Coordination Disorder 352 vistas
- Medical Research Council (MRC) Scale for Muscle Strength 310 vistas
Copyright by MedicalCriteria.com
Last Updated on 21 June, 2021 by Guillermo Firman
- Type 2 Diabetes
- Heart Disease
- Digestive Health
- Multiple Sclerosis
- COVID-19 Vaccines
- Occupational Therapy
- Healthy Aging
- Health Insurance
- Public Health
- Patient Rights
- Caregivers & Loved Ones
- End of Life Concerns
- Health News
- Thyroid Test Analyzer
- Doctor Discussion Guides
- Hemoglobin A1c Test Analyzer
- Lipid Test Analyzer
- Complete Blood Count (CBC) Analyzer
- What to Buy
- Editorial Process
- Meet Our Medical Expert Board
Measuring Shortness of Breath (Dyspnea) in COPD
How the Perception of Disability Directs Treatment
Dyspnea is the medical term used to describe shortness of breath, a symptom considered central to all forms of chronic obstructive pulmonary disease (COPD) including emphysema and chronic bronchitis.
As COPD is both a progressive and non-reversible, the severity of dyspnea plays a key role in determining both the stage of the disease and the appropriate medical treatment.
Challenges in Diagnosis
From a clinical standpoint, the challenge of diagnosing dyspnea is that it is very subjective. While spirometry tests (which measures lung capacity) and pulse oximetry (which measures oxygen levels in the blood) may show that two people have the same level of breathing impairment, one may feel completely winded after activity while the other may be just fine.
Ultimately, a person's perception of dyspnea is important as it helps ensure the person is neither undertreated nor overtreated and that the prescribed therapy, when needed, will improve the person's quality of life rather than take from it.
To this end, pulmonologists will use a tool called the modified Medical Research Council (mMRC) dyspnea scale to establish how much an individual's shortness of breath causes real-world disability.
How the Assessment Is Performed
The process of measuring dyspnea is similar to tests used to measure pain perception in persons with chronic pain. Rather than defining dyspnea in terms of lung capacity, the mMRC scale will rate the sensation of dyspnea as the person perceives it.
The severity of dyspnea is rated on a scale of 0 to 4, the value of which will direct both the diagnosis and treatment plan.
Role of the MMRC Dyspnea Scale
The mMRC dyspnea scale has proven valuable in the field of pulmonology as it affords doctors and researchers the mean to:
- Assess the effectiveness of treatment on an individual basis
- Compare the effectiveness of a treatment within a population
- Predict survival times and rates
From a clinical viewpoint, the mMRC scale correlates fairly well to such objective measures as pulmonary function tests and walk tests . Moreover, the values tend to be stable over time, meaning that they are far less prone to subjective variability that one might assume.
Using the BODE Index to Predict Survival
The mMRC dyspnea scale is used to calculate the BODE index , a tool which helps estimate the survival times of people living with COPD.
The BODE Index is comprised of a person's body mass index ("B"), airway obstruction ("O"), dyspnea ("D"), and exercise tolerance ("E"). Each of these components is graded on a scale of either 0 to 1 or 0 to 3, the numbers of which are then tabulated for a final value.
The final value—ranging from as low as 0 to as high as 10—provides doctors a percentage of how likely a person is to survive for four years. The final BODE tabulation is described as follows:
- 0 to 2 points: 80 percent likelihood of survival
- 3 to 4 points: 67 percent likelihood of survival
- 5 of 6 points: 57 percent likelihood of survival
- 7 to 10 points: 18 percent likelihood of survival
The BODE values, whether large or small, are not set in stone. Changes to lifestyle and improved treatment adherence can improve long-term outcomes, sometimes dramatically. These include things like quitting smoking , improving your diet and engaging in appropriate exercise to improve your respiratory capacity.
In the end, the numbers are simply a snapshot of current health, not a prediction of your mortality. Ultimately, the lifestyle choices you make can play a significant role in determining whether the odds are against you or in your favor.
Janssens T, De peuter S, Stans L, et al. Dyspnea perception in COPD: association between anxiety, dyspnea-related fear, and dyspnea in a pulmonary rehabilitation program . Chest. 2011;140(3):618-625. doi:10.1378/chest.10-3257
Manali ED, Lyberopoulos P, Triantafillidou C, et al. MRC chronic Dyspnea Scale: Relationships with cardiopulmonary exercise testing and 6-minute walk test in idiopathic pulmonary fibrosis patients: a prospective study . BMC Pulm Med . 2010;10:32. doi:10.1186/1471-2466-10-32
Esteban C, Quintana JM, Moraza J, et al. BODE-Index vs HADO-score in chronic obstructive pulmonary disease: Which one to use in general practice? . BMC Med . 2010;8:28. doi:10.1186/1741-7015-8-28
Chhabra, S., Gupta, A., and Khuma, M. " Evaluation of Three Scales of Dyspnea in Chronic Obstructive Pulmonary Disease. " Annals of Thoracic Medicine. 2009; 4(3):128-32. DOI: 10.4103/1817-1737.53351 .
Perez, T.; Burgel, P.; Paillasseur, J.; et al. " Modified Medical Research Council scale vs Baseline Dyspnea Index to Evaluate Dyspnea in Chronic Obstructive Pulmonary Disease. " International Journal of Chronic Obstructive Pulmonary Disease . 2015; 10:1663-72. DOI: 10.2147/COPD.S82408 .
By Deborah Leader, RN Deborah Leader RN, PHN, is a registered nurse and medical writer who focuses on COPD.
- Research article
- Open access
- Published: 01 October 2012
The modified Medical Research Council scale for the assessment of dyspnea in daily living in obesity: a pilot study
- Claire Launois 1 ,
- Coralie Barbe 2 ,
- Eric Bertin 3 ,
- Julie Nardi 1 ,
- Jeanne-Marie Perotin 1 ,
- Sandra Dury 1 ,
- François Lebargy 1 &
- Gaëtan Deslee 1
BMC Pulmonary Medicine volume 12 , Article number: 61 ( 2012 ) Cite this article
61k Accesses
74 Citations
2 Altmetric
Metrics details
Dyspnea is very frequent in obese subjects. However, its assessment is complex in clinical practice. The modified Medical Research Council scale (mMRC scale) is largely used in the assessment of dyspnea in chronic respiratory diseases, but has not been validated in obesity. The objectives of this study were to evaluate the use of the mMRC scale in the assessment of dyspnea in obese subjects and to analyze its relationships with the 6-minute walk test (6MWT), lung function and biological parameters.
Forty-five obese subjects (17 M/28 F, BMI: 43 ± 9 kg/m 2 ) were included in this pilot study. Dyspnea in daily living was evaluated by the mMRC scale and exertional dyspnea was evaluated by the Borg scale after 6MWT. Pulmonary function tests included spirometry, plethysmography, diffusing capacity of carbon monoxide and arterial blood gases. Fasting blood glucose, total cholesterol, triglyceride, N-terminal pro brain natriuretic peptide, C-reactive protein and hemoglobin levels were analyzed.
Eighty-four percent of patients had a mMRC ≥ 1 and 40% a mMRC ≥ 2. Compared to subjects with no dyspnea (mMRC = 0), a mMRC ≥ 1 was associated with a higher BMI (44 ± 9 vs 36 ± 5 kg/m 2 , p = 0.01), and a lower expiratory reserve volume (ERV) (50 ± 31 vs 91 ± 32%, p = 0.004), forced expiratory volume in one second (FEV 1 ) (86 ± 17 vs 101 ± 16%, p = 0.04) and distance covered in 6MWT (401 ± 107 vs 524 ± 72 m, p = 0.007). A mMRC ≥ 2 was associated with a higher Borg score after the 6MWT (4.7 ± 2.5 vs 6.5 ± 1.5, p < 0.05).
This study confirms that dyspnea is very frequent in obese subjects. The differences between the “dyspneic” and the “non dyspneic” groups assessed by the mMRC scale for BMI, ERV, FEV 1 and distance covered in 6MWT suggests that the mMRC scale might be an useful and easy-to-use tool to assess dyspnea in daily living in obese subjects.
Peer Review reports
Obesity, defined as a Body Mass Index (BMI) greater than or equal to 30 kg/m 2 , is a significant public health concern. According to the World Health Organization, worldwide obesity has more than doubled since 1980 and in 2008 there were about 1.5 billion overweight adults (25 ≤ BMI < 30 kg/m 2 ). Of these, over 200 million men and nearly 300 million women were obese [ 1 ].
Dyspnea is very frequent in obese subjects. In a large epidemiological study, 80% of obese patients reported dyspnea after climbing two flights of stairs [ 2 ]. In a series of patients with morbid obesity, Collet et al. found that patients with a BMI > 49 kg/m 2 had more severe dyspnea assessed with BDI (Baseline Dyspnea Index) than obese patients with a BMI ≤ 49 kg/m 2 [ 3 ]. The most frequent pulmonary function abnormalities associated with obesity [ 4 , 5 ] are a decrease in expiratory reserve volume (ERV) [ 6 – 8 ], functional residual capacity (FRC) [ 6 – 8 ], and an increase in oxygen consumption [ 9 ]. Although the mechanisms of dyspnea in obesity remain unclear, it is moderately correlated with lung function [ 3 , 10 – 16 ]. Of note, type 2 diabetes [ 17 ], insulin resistance [ 18 ] and metabolic syndrome [ 19 ] have been shown to be associated with reduced lung function in obesity. It must be pointed out that dyspnea is a complex subjective sensation which is difficult to assess in clinical practice. However, there is no specific scale to assess dyspnea in daily living in obesity. The modified Medical Research Council (mMRC) scale is the most commonly used validated scale to assess dyspnea in daily living in chronic respiratory diseases [ 20 – 22 ] but has never been assessed in the context of obesity without a coexisting pulmonary disease.
The objectives of this pilot study were to evaluate the use of the mMRC scale in the assessment of dyspnea in obese subjects and to analyze its relationships with the 6-minute walk distance (6MWD), lung function and biological parameters.
Adult obese patients from the Department of Nutrition of the University Hospital of Reims (France) were consecutively referred for a systematic respiratory evaluation without specific reason and considered for inclusion in this study. Inclusion criteria were a BMI ≥ 30 kg/m 2 and an age > 18 year-old. Exclusion criteria were a known coexisting pulmonary or neuromuscular disease or an inability to perform a 6MWT or pulmonary function testing. The study was approved by the Institutional Review Board (IRB) of the University Hospital of Reims, and patient consent was waived.
Clinical characteristics and mMRC scale
Demographic data (age, sex), BMI, comorbidities, treatments and smoking status were systematically recorded. Dyspnea in daily living was evaluated by the mMRC scale which consists in five statements that describe almost the entire range of dyspnea from none (Grade 0) to almost complete incapacity (Grade 4) (Table 1 ).
- Six-minute walk test
The 6MWT was performed using the methodology specified by the American Thoracic Society (ATS-2002) [ 23 ]. The patients were instructed that the objective was to walk as far as possible during 6 minutes. The 6MWT was performed in a flat, long, covered corridor which was 30 meters long, meter-by-meter marked. Heart rate, oxygen saturation and modified Borg scale assessing subjectively the degree of dyspnea graded from 0 to 10, were collected at the beginning and at the end of the 6MWT. When the test was finished, the distance covered was calculated.
Pulmonary function tests
Pulmonary function tests (PFTs) included forced expiratory volume in one second (FEV 1 ), vital capacity (VC), forced vital capacity (FCV), FEV 1 /VC, functional residual capacity (FRC), expiratory reserve volume (ERV), residual volume (RV), total lung capacity (TLC) and carbon monoxide diffusing capacity of the lung (DLCO) (BodyBox 5500 Medisoft Sorinnes, Belgium). Results were expressed as the percentage of predicted values [ 24 ]. Arterial blood gases were measured in the morning in a sitting position.
Biological parameters
After 12 hours of fasting, blood glucose, glycated hemoglobin (HbAIc), total cholesterol, triglyceride, N-terminal pro brain natriuretic peptide (NT-pro BNP), C-reactive protein (CRP) and hemoglobin levels were measured.
Statistical analysis
Quantitative variables are described as mean ± standard deviation (SD) and qualitative variables as number and percentage. Patients were separated in two groups according to their dyspnea: mMRC = 0 (no dyspnea in daily living) and mMRC ≥ 1 (dyspnea in daily living, ie at least short of breath when hurrying on level ground or walking up a slight hill).
Factors associated with mMRC scale were studied using Wilcoxon, Chi-square or Fisher exact tests. Factors associated with Borg scale were studied using Wilcoxon tests or Pearson’s correlation coefficients. A p value < 0.05 was considered statistically significant. All analysis were performed using SAS version 9.0 (SAS Inc, Cary, NC, USA).
Results and discussion
Demographic characteristics.
Fifty four consecutive patients with a BMI ≥ 30 kg/m 2 were considered for inclusion. Of these, 9 patients were excluded because of an inability to perform the 6MWT related to an osteoarticular disorder (n = 2) or because of a diagnosed respiratory disease (n = 7; 5 asthma, 1 hypersensitivity pneumonia and 1 right pleural effusion).
Results of 45 patients were considered in the final analysis. Demographic characteristics of the patients are presented in Table 2 . Mean BMI was 43 ± 9 kg/m 2 , with 55% of the patients presenting an extreme obesity (BMI ≥ 40 kg/m 2 , grade 3). Regarding smoking status, 56% of patients were never smokers and 11% were current smokers. The main comorbidities were hypertension (53%), dyslipidemia (40%) and diabetes (36%). Severe obstructive sleep apnea syndrome was present in 16 patients (43%).
Dyspnea assessment by the mMRC scale and 6MWT
Results of dyspnea assessment are presented in Table 3 . Dyspnea symptom assessed by the mMRC scale was very frequent in obese subjects with 84% (n = 38) of patients with a mMRC scale ≥ 1 and 40% (n = 18) of patients with a mMRC scale ≥ 2 (29% mMRC = 2, 9% mMRC = 3 and 2% mMRC = 4).
The mean distance covered in 6MWT was 420 ± 112 m. Sixteen percent of patients had a decrease > 4% of SpO2 during the 6MWT and one patient had a SpO2 < 90% at the end of the 6MWT (Table 4 ). The dyspnea sensation at rest was very slight (Borg = 1 ± 1.5) but severe after exertion (Borg = 5.4 ± 2.4). Fifty-three percent of patients exhibited a Borg scale ≥ 5 after the 6MWT which is considered as severe exertional dyspnea. No complication occurred during the 6MWT. Subjects with a mMRC score ≥ 2 had a higher Borg score after the 6MWT than subjects with a mMRC score < 2 (6.5 ± 1.5 vs 4.7 ± 2.5, p < 0.05).
Lung function tests
Results of spirometry, plethysmography and arterial blood gases are shown in Table 4 . Overall, the PFTs results remained in the normal range for most of the patients, except for ERV predicted values which were lower (ERV = 56 ± 34%). There were an obstructive ventilatory disorder defined by a FEV 1 /VC < 0.7 in 5 patients (11%) with 5 patients (13%) exhibiting a mMRC ≥ 1, a restrictive ventilatory disorder defined by a TLC < 80% in 5 patients (13%) with 5 patients (16%) exhibiting a mMRC ≥ 1, and a decrease in alveolar diffusion defined by DLCO < 70% in 10 patients (26%) with 9 patients (28%) exhibiting a mMRC ≥ 1. Arterial blood gases at rest were in the normal range with no hypoxemia < 70 mmHg and no significant hypercapnia > 45 mmHg.
Fifteen percent (n = 7) of patients presented anemia. All patients had a hemoglobin level ≥ 11 g/dL. Mean NT pro-BNP was 117 ± 285 pg/mL. Four patients (10%) had a pro-BNP > 300 pg/mL.Forty-five percent of patients had a fasting glucose level > 7 mmol/L, 51% a Hba1c > 6%, 29% a triglyceride level ≥ 1.7 mmol/L, 35% a total cholesterol level > 5.2 mmol/L and 31% a CRP level > 10 mg/L.
Relationships between the mMRC scale and clinical characteristics, PFTs and biological parameters
The comparisons between the mMRC scale and demographic, lung functional and biological parameters are shown in Table 5 . Subjects in the mMRC ≥ 1 group had a higher BMI (p = 0.01) (Figure 1 A), lower ERV (p < 0.005) (Figure 1 B), FEV 1 (p < 0.05), covered distance in 6MWT (p < 0.01) (Figure 1 C) and Hb level (p < 0.05) than subjects in the mMRC = 0 group. Of note, there was no association between the mMRC scale and age, sex, smoking history, arterial blood gases, metabolic parameters and the apnea/hypopnea index.
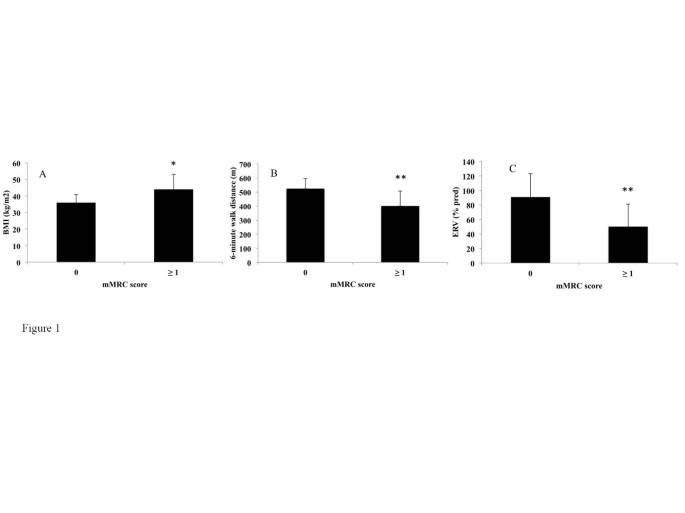
Differences in Body Mass Index (BMI) (A), Expiratory reserve volume (ERV) (B) and 6-minute walk distance (C) between non-dyspneic (modified Medical Research Council score = 0) and dyspneic (mMRC score ≥ 1) subjects. *p < 0.05, **p < 0.01. A Wilcoxon test was used.
The relationships between the Borg scale after 6MWT and demographic, lung functional and biological parameters were also analysed. The Borg score after 6MWT was correlated with a higher BMI (correlation coefficient = +0.44, p < 0.005) and a lower FEV 1 (correlation coefficient = -0.33, p < 0.05). No relationship was found between the Borg score after 6MWT and ERV or hemoglobin level. The Borg score after 6MWT was correlated with a higher fasting glucose (correlation coefficient = +0.46, p < 0.005) whereas this parameter was not associated with the mMRC scale (data not shown). We found no statistically different change in Borg scale ratings of dyspnea from rest to the end of the 6MWT between the two groups (p = 0.39).
In this study, 45 consecutive obese subjects were specifically assessed for dyspnea in daily living using the mMRC scale. Our study confirms the high prevalence of dyspnea in daily living in obese subjects [ 2 ] with 84% of patients exhibiting a mMRC scale ≥ 1 and 40% a mMRC scale ≥ 2. Interestingly, the presence of dyspnea in daily living (mMRC ≥ 1) was associated with a higher BMI and a lower ERV, FEV 1 , distance covered in 6MWT and hemoglobin level. Furthermore, a mMRC score ≥ 2 in obese subjects was associated with a higher Borg score after the 6MWT (data not shown).
The assessment of dyspnea in clinical practice is difficult. Regarding the mMRC scale, two versions of this scale have been used, one with 5 grades [ 20 ] as used in this study and an other with 6 grades [ 25 ] leading to some confusion. Other scales have been also used to assess dyspnea [ 26 ]. Collet at al. [ 3 ], Ofir et al. [ 11 ] and El-Gamal [ 27 ] et al provided some evidence to support the use of the BDI, Oxygen cost diaphragm (OCD) and Chronic Respiratory Disease Questionnaire (CRQ) to evaluate dyspnea in obesity. El-Gamal et al [ 27 ] demonstated the responsiveness of the CRQ in obesity as they did measurements before and after gastroplaty-induced weight loss within the same subjects. The Baseline Dyspnea Index (BDI) uses five grades (0 to 4) for 3 categories, functional impairment, magnitude of task and magnitude of effort with a total score from 0 to 12 [ 28 ]. The University of California San Diego Shortness of Breath Questionnaire comprises 24 items assessing dyspnea over the previous week [ 29 ]. It must be pointed out that these scores are much more time consuming than the mMRC scale and are difficult to apply in clinical practice.
To our knowledge, the mMRC scale has not been investigated in the assessment of dyspnea in daily living in obese subjects without a coexisting pulmonary disease. The mMRC scale is an unidimensional scale related to activities of daily living which is widely used and well correlated with quality of life in chronic respiratory diseases [ 20 ] such as chronic obstructive pulmonary disease (COPD) [ 21 ] or idiopathic pulmonary fibrosis [ 22 ]. The mMRC scale is easy-to-use and not time consuming, based on five statements describing almost the entire range of dyspnea in daily living. Our study provides evidence for the use of the mMRC scale in the assessment of dyspnea in daily living in obese subjects. Firstly, as expected, our results demonstrate an association between the mMRC scale and the BMI in the comparison between “dyspneic” and “non dyspneic” groups. Secondly, in our between-group comparisons, the mMRC scale was associated with pulmonary functional parameters (lower ERV, FEV 1 and distance walked in 6MWT) which might be involved in dyspnea in obesity. The reduction in ERV is the most frequent functional respiratory abnormality reported in obesity [ 6 – 8 ]. This decrease is correlated exponentially with BMI and is mainly due to the effect of the abdominal contents on diaphragm position [ 30 ]. While the FEV 1 might be slightly reduced in patients with severe obesity, the FEV 1 /VC is preserved as seen in our study [ 31 ]. The determination of the walking distance and the Borg scale using the 6MWT is known to be a simple method to assess the limitations of exercise capacity in chronic respiratory diseases [ 23 ]. Two studies have shown a good reproducibility of this test [ 32 , 33 ] but did not investigate the relationships between the 6MWD and dyspnea in daily living. Our study confirms the feasibility of the 6MWD in clinical practice in obesity and demonstrates an association between covered distance in 6MWT and the presence or the absence of dyspnea in daily living assessed by the mMRC scale. It must be pointed out that the 6MWT is not a standardized exercise stimulus. Exercise testing using cycloergometer or the shuttle walking test could be of interest to determine the relationships between the mMRC scale and a standardize exercise stimulus. In our between-group comparisons, BMI and FEV 1 were associated with the mMRC scale and correlated with the Borg scale after 6MWT. Surprisingly, the ERV was associated with the mMRC scale but not with the Borg scale. Moreover, the fasting glucose was correlated with the Borg scale after 6MWT but not associated with the mMRC scale. Whether these differences are due to a differential involvement of these parameters in dyspnea in daily living and at exercise, or simply related to a low sample size remains to be evaluated.
As type 2 diabetes, insulin resistance, metabolic syndrome [ 17 – 19 ], anemia and cardiac insufficiency have been shown to be associated with lung function and/or dyspnea, we also investigated the relationships between dyspnea in daily living and biological parameters. A mMRC scale ≥ 1 was associated with a lower hemoglobin level. However, all patients had a hemoglobin level > 11 g/dL and the clinical significance of the association between dyspnea in daily living and a mildly lower hemoglobin level has to be interpreted cautiously and remains to be evaluated. Of note, we did not find any associations between the mMRC scale and triglyceride, total cholesterol, fasting glucose, HbA1C, CRP or NT pro-BNP.
The strength of this study includes the assessment of the relationships between the mMRC scale and multidimensional parameters including exertional dyspnea assessed by the Borg score after 6MWT, PFTs and biological parameters. The limitations of this pilot study are as follows. Firstly, the number of patients included is relatively low. This study was monocentric and did not include control groups of overweight and normal weight subjects. Due to the limited number of patients, our study did not allow the analysis sex differences in the perception of dyspnea. Secondly, we did not investigate the relationships between the mMRC scale and other dyspnea scales like the BDI which has been evaluated in obese subjects and demonstrated some correlations with lung function [ 3 ]. Thirdly, it would have been interesting to assess the relationships between the mMRC scale and cardio-vascular, neuromuscular and psycho-emotional parameters which might be involved in dyspnea. Assessing the relationships between health related quality of life and dyspnea would also be useful. Finally, fat distribution (eg Waist circumferences or waist/hip ratios) has not been specifically assessed in our study but might be assessed at contributing factor to dyspnea. Despite these limitations, this pilot study suggests that the mMRC scale might be of value in the assessment of dyspnea in obesity and might be used as a dyspnea scale in further larger multicentric studies. It remains to be seen whether it is sensitive to changes with intervention.
Conclusions
This pilot study investigated the potential use of the mMRC scale in obesity. The differences observed between the “dyspneic” and the “non dyspneic” groups as defined by the mMRC scale with respect to BMI, ERV, FEV 1 and distance covered in 6MWT suggests that the mMRC scale might be an useful and easy-to-use tool to assess dyspnea in daily living in obese subjects.
Abbreviations
Body Mass Index
- Modified Medical Research Council scale
Expiratory volume in one second
Vital capacity
Forced vital capacity
Functional residual capacity
Expiratory reserve volume
Residual volume
Total lung capacity
Carbon monoxide diffusing capacity of the lung
Glycated hemoglobin
N-terminal pro brain natriuretic peptide
Serum C reactive protein.
WHO: Obesity and overweight. http://www.who.int/mediacentre/factsheets/fs311/en/ ,
Sjöström L, Larsson B, Backman L, Bengtsson C, Bouchard C, Dahlgren S, Hallgren P, Jonsson E, Karlsson J, Lapidus L: Swedish obese subjects (SOS). Recruitment for an intervention study and a selected description of the obese state. Int J Obes Relat Metab Disord. 1992, 16: 465-479.
PubMed Google Scholar
Collet F, Mallart A, Bervar JF, Bautin N, Matran R, Pattou F, Romon M, Perez T: Physiologic correlates of dyspnea in patients with morbid obesity. Int J Obes (Lond). 2007, 31: 700-706.
CAS Google Scholar
Salome CM, King GG, Berend N: Physiology of obesity and effects on lung function. J Appl Physiol. 2010, 108: 206-211. 10.1152/japplphysiol.00694.2009.
Article PubMed Google Scholar
Gibson GJ: Obesity, respiratory function and breathlessness. Thorax. 2000, 55 (Suppl 1): S41-S44.
Article PubMed PubMed Central Google Scholar
Ray CS, Sue DY, Bray G, Hansen JE, Wasserman K: Effects of obesity on respiratory function. Am Rev Respir Dis. 1983, 128: 501-506.
Article CAS PubMed Google Scholar
Jones RL, Nzekwu M-MU: The effects of body mass index on lung volumes. Chest. 2006, 130: 827-833. 10.1378/chest.130.3.827.
Biring MS, Lewis MI, Liu JT, Mohsenifar Z: Pulmonary physiologic changes of morbid obesity. Am J Med Sci. 1999, 318: 293-297. 10.1097/00000441-199911000-00002.
Babb TG, Ranasinghe KG, Comeau LA, Semon TL, Schwartz B: Dyspnea on exertion in obese women: association with an increased oxygen cost of breathing. Am J Respir Crit Care Med. 2008, 178: 116-123. 10.1164/rccm.200706-875OC.
Sahebjami H: Dyspnea in obese healthy men. Chest. 1998, 114: 1373-1377. 10.1378/chest.114.5.1373.
Ofir D, Laveneziana P, Webb KA, O’Donnell DE: Ventilatory and perceptual responses to cycle exercise in obese women. J Appl Physiol. 2007, 102: 2217-2226. 10.1152/japplphysiol.00898.2006.
Romagnoli I, Laveneziana P, Clini EM, Palange P, Valli G, de Blasio F, Gigliotti F, Scano G: Role of hyperinflation vs. deflation on dyspnoea in severely to extremely obese subjects. Acta Physiol (Oxf). 2008, 193: 393-402. 10.1111/j.1748-1716.2008.01852.x.
Article CAS Google Scholar
Jensen D, Webb KA, Wolfe LA, O’Donnell DE: Effects of human pregnancy and advancing gestation on respiratory discomfort during exercise. Respir Physiol Neurobiol. 2007, 156: 85-93. 10.1016/j.resp.2006.08.004.
Scano G, Stendardi L, Bruni GI: The respiratory muscles in eucapnic obesity: their role in dyspnea. Respir Med. 2009, 103: 1276-1285. 10.1016/j.rmed.2009.03.023.
Ora J, Laveneziana P, Ofir D, Deesomchok A, Webb KA, O’Donnell DE: Combined effects of obesity and chronic obstructive pulmonary disease on dyspnea and exercise tolerance. Am J Respir Crit Care Med. 2009, 180: 964-971.
Sava F, Laviolette L, Bernard S, Breton M-J, Bourbeau J, Maltais F: The impact of obesity on walking and cycling performance and response to pulmonary rehabilitation in COPD. BMC Pulm Med. 2010, 10: 55-10.1186/1471-2466-10-55.
Lecube A, Sampol G, Muñoz X, Hernández C, Mesa J, Simó R: Type 2 diabetes impairs pulmonary function in morbidly obese women: a case-control study. Diabetologia. 2010, 53: 1210-1216. 10.1007/s00125-010-1700-5.
Lecube A, Sampol G, Muñoz X, Lloberes P, Hernández C, Simó R: Insulin resistance is related to impaired lung function in morbidly obese women: a case-control study. Diabetes Metab Res Rev. 2010, 26: 639-645. 10.1002/dmrr.1131.
Leone N, Courbon D, Thomas F, Bean K, Jégo B, Leynaert B, Guize L, Zureik M: Lung function impairment and metabolic syndrome: the critical role of abdominal obesity. Am J Respir Crit Care Med. 2009, 179: 509-516. 10.1164/rccm.200807-1195OC.
Mahler DA, Wells CK: Evaluation of clinical methods for rating dyspnea. Chest. 1988, 93: 580-586. 10.1378/chest.93.3.580.
Hajiro T, Nishimura K, Tsukino M, Ikeda A, Koyama H, Izumi T: Analysis of Clinical Methods Used to Evaluate Dyspnea in Patients with Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 1998, 158: 1185-1189.
Nishiyama O, Taniguchi H, Kondoh Y, Kimura T, Kato K, Kataoka K, Ogawa T, Watanabe F, Arizono S: A simple assessment of dyspnoea as a prognostic indicator in idiopathic pulmonary fibrosis. Eur Respir J. 2010, 36: 1067-1072. 10.1183/09031936.00152609.
ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002, 166: 111-117.
Standardized lung function testing. Official statement of the European Respiratory Society. Eur Respir J Suppl. 1993, 16: 1-100.
Eltayara L, Becklake MR, Volta CA, Milic-Emili J: Relationship between chronic dyspnea and expiratory flow limitation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1996, 154: 1726-1734.
Gerlach Y, Williams MT, Coates AM: Weighing up the evidence-a systematic review of measures used for the sensation of breathlessness in obesity. Int J Obes. 2012
Google Scholar
El-Gamal H, Khayat A, Shikora S, Unterborn JN: Relationship of dyspnea to respiratory drive and pulmonary function tests in obese patients before and after weight loss. Chest. 2005, 128: 3870-3874. 10.1378/chest.128.6.3870.
Mahler DA, Weinberg DH, Wells CK, Feinstein AR: The measurement of dyspnea. Contents, interobserver agreement, and physiologic correlates of two new clinical indexes. Chest. 1984, 85: 751-758. 10.1378/chest.85.6.751.
Eakin EG, Resnikoff PM, Prewitt LM, Ries AL, Kaplan RM: Validation of a new dyspnea measure: the UCSD Shortness of Breath Questionnaire. University of California, San Diego. Chest. 1998, 113: 619-624. 10.1378/chest.113.3.619.
Parameswaran K, Todd DC, Soth M: Altered respiratory physiology in obesity. Can Respir J. 2006, 13: 203-210.
Sin DD, Jones RL, Man SFP: Obesity is a risk factor for dyspnea but not for airflow obstruction. Arch Intern Med. 2002, 162: 1477-1481. 10.1001/archinte.162.13.1477.
Beriault K, Carpentier AC, Gagnon C, Ménard J, Baillargeon J-P, Ardilouze J-L, Langlois M-F: Reproducibility of the 6-minute walk test in obese adults. Int J Sports Med. 2009, 30: 725-727. 10.1055/s-0029-1231043.
Larsson UE, Reynisdottir S: The six-minute walk test in outpatients with obesity: reproducibility and known group validity. Physiother Res Int. 2008, 13: 84-93. 10.1002/pri.398.
Pre-publication history
The pre-publication history for this paper can be accessed here: http://www.biomedcentral.com/1471-2466/12/61/prepub
Download references
Acknowledgements
We thank the personnel of the Department of Nutrition and Pulmonary Medicine of the University Hospital of Reims for the selection and clinical/functional assessment of the patients.
Author information
Authors and affiliations.
Service des Maladies Respiratoires, INSERM UMRS 903, Hôpital Maison Blanche, CHU de Reims, 45 rue Cognacq Jay 51092, Reims, Cedex, France
Claire Launois, Julie Nardi, Jeanne-Marie Perotin, Sandra Dury, François Lebargy & Gaëtan Deslee
Unité d'Aide Méthodologique, Pôle Recherche et Innovations, Hôpital Robert Debré, CHU de Reims, Reims, France
Coralie Barbe
Service d’Endocrinologie-Diabétologie-Nutrition, Hôpital Robert Debré, CHU de Reims, Reims, France
Eric Bertin
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Claire Launois .
Additional information
Competing interests.
None of the authors of the present manuscript have a commercial or other association that might pose a conflict of interest.
Authors’ contributions
CL, CB, EB, JN, JMP, SD, FL and GD conceived the study. CL acquired data. CB performed the statistical analysis. CL and GD drafted the manuscript. All authors read and approved the manuscript prior to submission.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Authors’ original file for figure 1
Rights and permissions.
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License ( http://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Reprints and permissions
About this article
Cite this article.
Launois, C., Barbe, C., Bertin, E. et al. The modified Medical Research Council scale for the assessment of dyspnea in daily living in obesity: a pilot study. BMC Pulm Med 12 , 61 (2012). https://doi.org/10.1186/1471-2466-12-61
Download citation
Received : 06 April 2012
Accepted : 22 September 2012
Published : 01 October 2012
DOI : https://doi.org/10.1186/1471-2466-12-61
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Lung function
BMC Pulmonary Medicine
ISSN: 1471-2466
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Learn how UpToDate can help you.
Select the option that best describes you
- Medical Professional
- Resident, Fellow, or Student
- Hospital or Institution
- Group Practice
- Patient or Caregiver
- Find in topic
Qualitative validation of the modified Medical Research Council (mMRC) dyspnoea scale as a patient-reported measure of breathlessness severity
Affiliations.
- 1 Respiratory Division, The George Institute for Global Health, UNSW Sydney, Sydney, Australia. Electronic address: [email protected].
- 2 The Woolcock Institute of Medical Research and The University of Sydney, Sydney, Australia.
- 3 Respiratory Division, The George Institute for Global Health, UNSW Sydney, Sydney, Australia. Electronic address: [email protected].
- PMID: 36179385
- DOI: 10.1016/j.rmed.2022.106984
Introduction: The modified Medical Research Council (mMRC) dyspnoea scale is a measure of breathlessness severity recommended by guidelines and utilised as an inclusion criterion or endpoint for clinical trials. No studies have been conducted to validate the categorical descriptors against the dyspnoea severity grade.
Methods: This study utilised cognitive interviews (Think Aloud method) to assess the content validity of the mMRC scale among 16 participants (13 with cardiac/respiratory disease). Participants were recruited to achieve representation across a variety of demographic factors. Interviews were conducted remotely via video conferencing and participants were presented with all 5 mMRC descriptors on screen in random order then asked to rank the statements "in order from the best breathing to the worst breathing".
Results: Mean age of participants was 57 years (range 22-84 years). Eleven had multimorbidity (≥2 comorbidities) including COPD, asthma, lung cancer, lung infection, interstitial lung disease, heart failure, depression, and anxiety. Length of time with breathlessness ranged between 2 weeks and >25 years. The median rank of the mMRC grade descriptors was concordant for mMRC grades 0, 1 and 4 but not grades 2 and 3. Even so, substantial heterogeneity was found in the distribution of responses for mMRC grade 0.
Conclusion: Our study found substantial heterogeneity in participant grading of the mMRC descriptors, particularly for grades 0, 2 and 3, indicating that mMRC might not be a good discriminator of difference or change in dyspnoea severity. This study demonstrates the importance of content validation even for long-established PROs like mMRC.
Keywords: Dyspnoea; Patient reported outcome measures; Validation study; mMRC dyspnoea scale.
Copyright © 2022 Elsevier Ltd. All rights reserved.
- Aged, 80 and over
- Biomedical Research*
- Dyspnea / diagnosis
- Dyspnea / psychology
- Middle Aged
- Patient Reported Outcome Measures
- Pulmonary Disease, Chronic Obstructive* / drug therapy
- Severity of Illness Index
- Young Adult
- Open access
- Published: 30 March 2024
Clinical characterization and outcomes of impulse oscillometry-defined bronchodilator response: an ECOPD cohort-based study
- Lifei Lu 1 na1 ,
- Fan Wu 1 , 2 na1 ,
- Jieqi Peng 1 , 2 ,
- Xiaohui Wu 1 ,
- Xiangqing Hou 2 ,
- Youlan Zheng 2 ,
- Huajing Yang 1 ,
- Zhishan Deng 1 ,
- Cuiqiong Dai 1 ,
- Ningning Zhao 1 ,
- Kunning Zhou 1 ,
- Gaoying Tang 1 ,
- Jiangyu Cui 1 ,
- Shuqing Yu 3 ,
- Xiangwen Luo 3 ,
- Changli Yang 4 ,
- Shengtang Chen 4 ,
- Pixin Ran 1 , 2 &
- Yumin Zhou 1 , 2
Respiratory Research volume 25 , Article number: 149 ( 2024 ) Cite this article
294 Accesses
1 Altmetric
Metrics details
The clinical significance of the impulse oscillometry-defined small airway bronchodilator response (IOS-BDR) is not well-known. Accordingly, this study investigated the clinical characteristics of IOS-BDR and explored the association between lung function decline, acute respiratory exacerbations, and IOS-BDR.
Participants were recruited from an Early Chronic Obstructive Pulmonary Disease (ECOPD) cohort subset and were followed up for two years with visits at baseline, 12 months, and 24 months. Chronic obstructive pulmonary disease (COPD) was defined as a post-bronchodilator forced expiratory volume in 1 s (FEV 1 )/forced vital capacity (FVC) ratio < 0.70. IOS-BDR was defined as meeting any one of the following criteria: an absolute change in respiratory system resistance at 5 Hz ≤ − 0.137 kPa/L/s, an absolute change in respiratory system reactance at 5 Hz ≥ 0.055 kPa/L/s, or an absolute change in reactance area ≤ − 0.390 kPa/L. The association between IOS-BDR and a decline in lung function was explored with linear mixed-effects model. The association between IOS-BDR and the risk of acute respiratory exacerbations at the two-year follow-up was analyzed with the logistic regression model.
This study involved 466 participants (92 participants with IOS-BDR and 374 participants without IOS-BDR). Participants with IOS-BDR had higher COPD assessment test and modified Medical Research Council dyspnea scale scores, more severe emphysema, air trapping, and rapid decline in FVC than those without IOS-BDR over 2-year follow-up. IOS-BDR was not associated with the risk of acute respiratory exacerbations at the 2-year follow-up.
Conclusions
The participants with IOS-BDR had more respiratory symptoms, radiographic structural changes, and had an increase in decline in lung function than those without IOS-BDR.
Trial registration
Chinese Clinical Trial Registry, ChiCTR1900024643. Registered on 19 July, 2019.
Introduction
Airflow limitation responsiveness is assessed with bronchodilator response (BDR) testing, which is a diagnostic tool for asthma [ 1 , 2 ]. BDR is commonly evaluated using spirometry and is known as spirometric BDR [ 3 , 4 , 5 ]. Previous studies revealed that 18.4–52.7% of participants with chronic obstructive pulmonary disease (COPD) exhibited spirometric BDR [ 6 , 7 , 8 ]. However, the clinical significance of spirometric BDR in patients with COPD remains controversial. Numerous studies have reported no association between spirometric BDR and exacerbations, mortality, or hospitalization rates in patients with COPD after adjusting for baseline function [ 6 , 7 , 9 ], however, a few studies have presented contrary conclusions [ 8 , 10 ].
Currently, spirometric BDR testing primarily reflects large airway obstruction responsiveness and correlates poorly with clinical symptoms [ 11 ]. Small airways are the predominant obstruction sites in COPD [ 12 , 13 ]. Nevertheless, the clinical significance of BDR in small airways in COPD is uncertain. Therefore, there is an urgent need for new tools to evaluate small airway BDR.
Impulse oscillometry (IOS) is more sensitive for detecting peripheral airways and small airway BDR changes than spirometry [ 14 , 15 , 16 , 17 , 18 ]. Since European Respiratory Society (ERS) guideline proposed a threshold for assessing small airway BDR using oscillation in healthy participants [ 19 , 20 ], several studies have explored different thresholds for BDR testing in small airways using IOS (IOS-BDR) [ 20 , 21 , 22 ]. In patients with COPD, many studies have only reported changes in IOS parameters after bronchodilator use or distinguished between asthma and COPD using IOS [ 23 , 24 , 25 ]. However, to the authors’ best knowledge, very few studies reported on clinical characterization and longitudinal prognosis of IOS-BDR using a fixed threshold. Alice M et al. had found that oscillation parameters were more sensitive in identifying poor asthma control than spirometry [ 14 ]. Henrik’s study showed that abnormal response in oscillation parameters had a higher prevalence of asthma and wheeze compared with participants with a normal response to bronchodilation [ 21 ]. These study more forced on effect of oscillation on symptoms and asthma control in patients with asthma, but the clinical significance of the IOS-BDR in COPD was not well-known. BDR is recognized as a “treatable traits” of COPD. Accordingly, identifying IOS-BDR clinical features would aid the formulation of a theoretical basis for COPD treatment.
Therefore, this study aimed to report clinical characteristics of IOS-BDR and the association between imaging changes, acute respiratory exacerbations, and lung function decline with IOS-BDR in participants through a prospective cohort study.
Materials and methods
Study participants.
The Early Chronic Obstructive Pulmonary Disease (ECOPD) cohort is a prospective observational study aimed at investigating COPD early occurrence and development (Chinese Clinical Trial Registry ChiCTR1900024643). The cohort rationale and design have been previously reported [ 26 ]. From July 2020 to December 2021, a subset of individuals aged 40–80 years from the ECOPD cohort was continuously recruited from the community in this study. These participants included participants with spirometry-defined COPD [post-bronchodilator FEV 1 /forced vital capacity [FVC] ratio < 0.70] and participants without spirometry-defined COPD [post-bronchodilator FEV 1 /FVC ratio ≥ 0.70]. The participants were followed up for two years with visits at baseline, 12 months, and 24 months.
The participants completed the questionnaires and underwent pre-bronchodilator IOS tests, pre-bronchodilator spirometry tests, post-bronchodilator IOS tests, and post-bronchodilator spirometry tests. Participants were excluded if they met any of the following criteria at baseline: (1) age < 40 years or > 80 years; (2) incomplete spirometry tests or IOS tests; (3) respiratory infection or exacerbations within four weeks prior to screening; (4) heart attack (myocardial infarction and malignant arrhythmia) in the past three months. The previous cohort design report contains more details [ 26 ].
This study adhered to the ethical guidelines outlined in the Declaration of Helsinki. The research protocol was approved by the First Affiliated Hospital of Guangzhou Medical University Ethics Committee (Approval No. 2018-53) prior to study initiation. Written informed consent was obtained from all participants prior to their enrollment in the study.
Questionnaire
The questionnaire in this study was revised in accordance with the Chinese COPD epidemiology study, including smoking status, pack-years, history of occupational exposure, family history of respiratory diseases, and history of asthma [ 27 , 28 ]. Biomass exposure was defined as cooking or heating using biomass (mainly wood, crop residues, charcoal, grass, and dung) for more than 1 year. History of occupational exposure to dust/gases/fumes was defined as having occupational exposure to dust/gases/fumes for more than 1 year over a participants’ lifetime. We defined family history of respiratory diseases as having parents, siblings, and children with respiratory diseases (chronic bronchitis, emphysema, asthma, COPD, cor pulmonale, bronchiectasis, lung cancer, interstitial lung disease, obstructive sleep apnea hypopnea syndrome). Current asthma was defined as self-reported physician diagnosed asthma in combination with current use of asthmatic medication and/or asthma attack within the last 12 months and as self-reported physician diagnosed asthma in combination with the participant reporting to still having asthma. The degree of dyspnea and the participants’ health status were assessed using modified Medical Research Council dyspnea scale (mMRC) scores and COPD assessment test (CAT) scores, respectively [ 29 ]. Acute respiratory exacerbation events/exacerbations of COPD were specifically characterized by the onset or aggravation of at least two of the following five symptoms: cough, sputum, purulent sputum, dyspnea, and wheeze > 2 days after excluding other diseases. Moderate and severe acute respiratory exacerbations were characterized based on symptom worsening requiring treatment with antibiotics and/or systemic corticosteroids or treatment in a clinic, emergency department, or hospital setting. Acute exacerbation events/exacerbations of COPD can be classified as mild, moderate, and severe. The severity of acute respiratory exacerbations was assessed and recorded by well-trained staff according to the following categories: mild exacerbations were defined as those resulting in domiciliary management with COPD medications alone. Moderate exacerbations were defined as those resulting in outpatient or emergency department visits and the need for COPD medication. Severe exacerbations were defined as those resulting in hospitalization [ 30 , 31 ].
Computed tomography (CT)
Quantitative CT image assessment was conducted using multidetector-row CT scanners (Siemens Definition AS Plus 128-slicers and United Imaging uCT 760 128-slicers) combined with 3D Slicer 4.11 software on Chest Imaging Platform [ 26 ]. Emphysema was quantified by measuring each patient’s emphysema index, which was defined as the percentage of low-attenuation areas below − 950 Hounsfield units (HU) on full-inspiration CT. Air trapping was defined as the percentage of low-attenuation areas below − 856 HU on full-expiration CT [ 32 ].
In accordance with ERS/American Thoracic Society (ATS) standards [ 33 ], the operator performed a 3-L volume spirometry calibration daily. The participants were instructed not to inhale any bronchodilator for at least 12 h and to avoid swallowing or air leakage during the operation and were required to complete at least three forced expiratory maneuvers until the largest and second-largest FEV 1 and FVC values were within 150 mL. BDR was tested after a 20-min administration of 400 µg salbutamol through a 500-mL spacer.
The mechanical properties of the respiratory system were measured using IOS [ 34 ]. Participants need breath lasting for more than 30 s and to avoid coughing, swallowing, and air leakage during tidal breathing [ 34 ]. The IOS parameters included respiratory system resistance at 5 Hz (R5), respiratory system resistance at 20 Hz (R20), the difference between R5 and R20 (R5-R20), respiratory system reactance at 5 Hz (X5), reactance area (AX), and resonant frequency (Fres). The absolute change was expressed as post-bronchodilator value minus pre-bronchodilator value, and IOS-BDR was defined as meeting any of the following criteria: absolute change in R5 ≤ − 0.137 kPa/L/s, absolute change in X5 ≥ 0.055 kPa/L/s, or absolute change in AX ≤ − 0.390 kPa/L [ 14 , 19 ].
Statistical analysis
Continuous variables with normal distribution are reported as the mean (standard deviation [SD]). Continuous variables that did not exhibit normal distribution are presented as the median [interquartile range (IQR)]. The differences in clinical characterization between participants with and without IOS-BDR were compared using Student’s t-test, the Wilcoxon rank-sum test, Fisher’s exact or chi-squared test. The difference between participants with and without IOS-BDR in terms of symptom scores (CAT scores), emphysema, and air trapping were examined with multivariable linear regression. The potential confounders considered were as follows: age, sex, body mass index (BMI), pack–years, smoking status, family history of respiratory diseases, occupational exposure, biomass exposure, and history of asthma. Associations between IOS-BDR and decline in lung function (FEV 1 , FVC, and FEV 1 /FVC ratio) were assessed using linear mixed-effects models, providing the mean change in lung function [ 35 ]. Baseline lung function was additionally included for confounding factor adjustment to analyze the rate of lung function decline. Baseline FEV 1 and past exacerbation history were the most important risk factors for acute respiratory exacerbations. Thus, logistic regression modeling was used to evaluate associations between acute respiratory exacerbations outcomes within 2-year follow-up and IOS-BDR. Exacerbations were modeled as a binary outcome (0 vs. ≥ 1 episode) in the aforementioned logistic models adjusting for the potential confounders (age, sex, BMI, pack–years, smoking status, family history of respiratory diseases, occupational exposure, biomass exposure, and history of asthma), exacerbations in the previous year, and baseline pre-bronchodilator FEV 1 .
Subsequently, subgroup analyses were conducted, where the participants were stratified by sex, smoking status, and COPD. All statistical analyses were conducted using IBM SPSS 27.0 and SAS 9.4 (SAS Institute, Inc.), and a P-value less than 0.05 was considered statistically significant.
Baseline characteristics
Figure 1 presents the inclusion and exclusion criteria for this study. Initially, 1862 participants completed pre-bronchodilator IOS tests at baseline in ECOPD cohort from July 2019 to August 2021, then only 466 participants underwent post-bronchodilator IOS tests. The participants in the present study were based on two parts: 333 participants underwent pre- and post-bronchodilator IOS tests in baseline from July 2020 to August 2021, and 133 participants underwent pre- and post-bronchodilator IOS tests in second-year followed-up from November 2021 to December 2021. Consequently, a final cohort of 466 participants was included for data analysis (92 participantss with IOS-BDR and 374 participants without IOS-BDR). These participants have completed a 2-year follow-up until December 2023. At baseline, the mean age of the total participants was 62.3 years (SD 8.0), 79.8% of the participants were males, and about 50% of the participants were current smokers. Compared with the participants without IOS-BDR, the participants with IOS-BDR had more chronic respiratory symptoms, such as cough (37.0% vs. 25.5%), wheeze (22.8% vs. 10.7%), and history of asthma (4.4% vs. 0.8%) (Table 1 ). Furthermore, the participants with IOS-BDR had more impaired lung function, more severe airflow limitation, higher airway resistance, and higher absolute change in IOS parameters than those without IOS-BDR (Table 2 ).
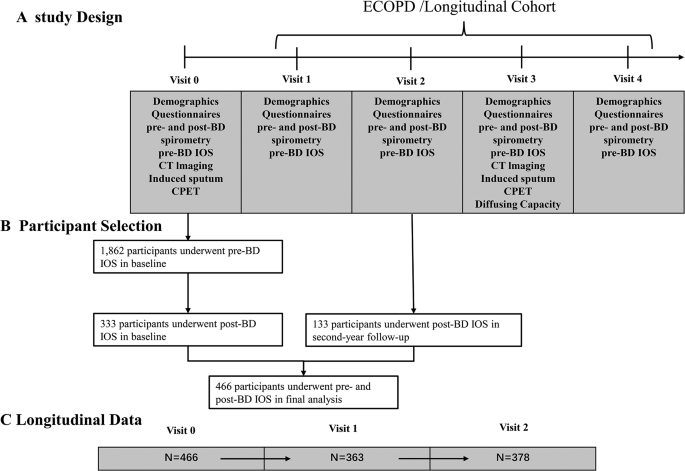
Study flow chart. Abbreviations: ECOPD = Early Chronic Obstructive Pulmonary Disease; IOS, Impulse oscillometry; CT, computed tomography; CPET, cardiopulmonary exercise testing. BD, bronchodilator
Proportion of IOS-BDR in participants stratified by sex, smoking status, and COPD
Figure 2 depicts the proportion of IOS-BDR in this study. Overall, the proportion of BDR assessed by R5 (R5-BDR), X5 (X5-BDR), AX (AX-BDR), and any of three IOS parameters (IOS-BDR) was 3.0%, 9.4%, 18.7%, and 19.7%, respectively (Fig. 2 A). Furthermore, the proportion of AX-BDR was larger than that of R5-BDR and X5-BDR. The participants with COPD had larger proportions of X5-BDR (14.5% vs. 3.7%), AX-BDR (26.9% vs. 9.2%), and IOS-BDR (27.7% vs. 10.6%) than the participants without COPD. However, the proportion of R5-BDR was not significantly different between the participants with and without COPD. In the participants with COPD, the proportions of X5-BDR, AX-BDR, and IOS-BDR increased with COPD severity, where approximately half of the participants with Global Initiative for Chronic Obstructive Lung Disease (GOLD)3–4 had AX-BDR or IOS-BDR. No difference existed in the proportion of IOS-BDR between the participants with GOLD1 and those without COPD. Moreover, the AX-BDR almost included BDR assessed by other indicators (R5-BDR, X5-BDR) in COPD participants with GOLD3–4 (Fig. 2 C, Table S1 ). Additionally, no difference existed in the proportions of R5-BDR, X5-BDR, AX-BDR, and IOS-BDR according to sex and smoking status (Fig. 2 B and D).
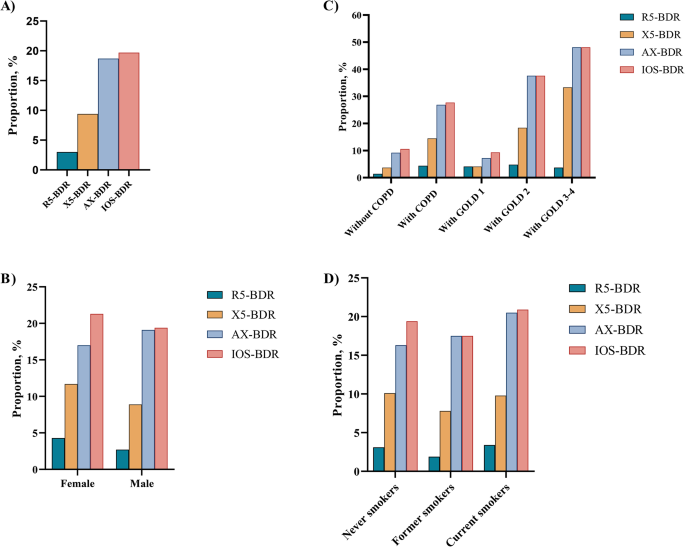
Proportion of IOS-BDR in participants stratified by sex, smoking status, and COPD. (A) in overall participants; (B) in male and female participants; (C) in participants with and without COPD. D)in participants with never smokers, former smokers, current smokers. R5-BDR, bronchodilator response assessed by R5; X5-BDR, bronchodilator response assessed by X5; AX-BDR, bronchodilator response assessed by AX; IOS-BDR, bronchodilator response assessed by one of three parameters (R5, X5, and AX)
Outcomes of participants with and without IOS-BDR
The differences between participants with and without IOS-BDR were investigated in terms of symptom scores and CT imaging changes. After adjusting for age, sex, BMI, pack–years, smoking status, family history of respiratory diseases, occupational exposure, biomass exposure, and history of asthma, multivariable linear regression of the participants overall demonstrated that the participants with IOS-BDR had higher CAT scores, more severe emphysema, and air trapping than those without IOS-BDR (Table 3 ).
Associations between lung function decline, acute respiratory exacerbations, and IOS-BDR
The associations between lung function decline, acute respiratory exacerbations, and IOS-BDR were observed. In the overall participants, linear mixed-effect model results demonstrated that the participants with IOS-BDR have an increase in decline in post-bronchodilator FVC (mean difference = − 209.1 mL, 95% CI: -329.7 mL, − 88.5 mL, P < 0.001) and FEV 1 /FVC (mean difference = − 1.0%, 95% CI: − 1.9%, − 0.2%, P = 0.013) than those without IOS-BDR over two visits. After adjusting for age, sex, BMI, pack–years, smoking status, family history of respiratory diseases, occupation exposure, biomass exposure, history of asthma, and post-bronchodilator baseline function (FEV 1 , FVC, FEV 1 /FVC), we found that participants with IOS-BDR have an increase in decline in post-bronchodilator FVC (adjusted mean difference = − 209.3 mL, 95% CI: -339.3 mL, − 79.4 mL, P = 0.002), but no difference between participants with and without IOS-BDR in decline in post-bronchodilator FEV 1 /FVC (adjusted mean difference = − 1.0%, 95% CI: -2.0%, 0.03%, P = 0.057). Logistic regression model results indicated no differences in any respiratory exacerbations or moderate to severe exacerbations at the 2-year follow-up between the participants with and without IOS-BDR (Table 4 ).
Subgroup analyses results
The associations between symptom scores, emphysema, air trapping, lung function decline, exacerbations, and IOS-BDR were examined with stratified analyses stratified by sex, smoking status, and COPD. The participants with IOS-BDR had higher symptom scores, more severe emphysema, and air trapping than those without IOS-BDR both male and ever-smoker participants (Table S4 , Table S6 ). The participants with IOS-BDR had more severe emphysema, air trapping than those without IOS-BDR both participants with COPD and female participants (Table S2 , Table S5 ). No difference existed between the participants with and without IOS-BDR in terms of symptom scores, emphysema, air trapping among never-smoker and the participants without COPD (Table S3 and Table S7 ). The participants with IOS-BDR had an increase in decline in post-bronchodilator FVC than those without IOS-BDR among male participants, never and ever-smoker participants, participants with and without COPD (Table S8 - 10 , S12 - 13 ). However, no difference existed between the participants with and without IOS-BDR in terms of decline in post-bronchodilator FVC, and acute respiratory exacerbations among female participants (Table S11 ).
This study describes the clinical characterization of IOS-BDR in participants from a general population. The participants with IOS-BDR exhibited more respiratory symptoms, emphysema, and air trapping than the participants without IOS-BDR. The longitudinal analysis demonstrated that IOS-BDR was associated with decline in lung function but unrelated to the risk of acute exacerbations.
In this study, the proportions of R5-BDR, X5-BDR, AX-BDR, and IOS-BDR were 3.0%, 9.4%, 18.7%, and 19.7%, respectively, in the overall participants. These results suggested that AX-defined BDR might better detecte more small airway responsiveness than R5-BDR and X5-BDR. Subsequently, the proportion of BDR assessed by IOS parameters was explored in different participants. No difference existed in the proportions of R5-BDR, X5-BDR, AX-BDR, and IOS-BDR when the participants were stratified by sex and smoking status. The proportion of IOS-BDR was 10.6% in the participants without COPD and was higher (27.7%) in the participants with COPD. BDR assessment using X5 and AX yielded similar results. However, the proportion of R5-BDR was not statistically significantly different between the participants with and without COPD. This results suggested that respiratory system reactance (Xrs) may be more sensitive than respiratory system resistance (Rrs) for detecting small airway responsiveness in COPD patients [ 22 ]. The reason may be that Xrs reflected stiffnesses of the lung and chest wall tissues, and may sensitivly detecte airway closure and severe narrowing in COPD [ 36 ].
The proportion of IOS-BDR gradually increased with COPD severity, where nearly half of the COPD participants with GOLD3–4 had IOS-BDR. However, the proportion of IOS-BDR between the COPD participants with GOLD 1 and participants without COPD was not statistically significantly different. The results revealed less IOS-BDR in early-stage COPD, especially in participants with mild COPD, but the IOS-BDR increased with disease progressions. Thus, IOS-BDR was associated with COPD severity.
In patients with advanced COPD, airway remodeling and emphysema, accompanied by loss of alveolar attachment, lead to early expiratory collapse of the small airway, followed by air trapping and dynamic hyperinflation. Stephen et al. reported that BDR assessed by forced oscillation was associated with hyperinflation and gas trapping in COPD [ 40 ]. An increased proportion of IOS-BDR closely reflects the progression of emphysema and small airway disease. The results of the present study confirmed this viewpoint, where the participants with IOS-BDR exhibited more severe emphysema and air trapping by high-resolution CT compared to those without IOS-BDR.
Alobaidi et al. reported that small airway BDR was defined based on a change in maximum mid-expiratory flow (MMEF) ≥ 30% and change ≥ 12% and absolute change ≥ 200 mL in the FEV 1 . Alobaidi et al. reported that MMEF detected a certain proportion of BDR in participants without BDR assessed by FEV 1 , suggesting that small airway BDR might benefit from the different treatable characteristics subtype [ 41 ].
To our knowledge, this is the first prospective study to reveal an association between respiratory symptoms, acute respiratory exacerbations, and decline in lung function and IOS-BDR. At baseline, the participants with IOS-BDR had more cough, wheeze, history of asthma, and medication use than those without IOS-BDR. These findings suggested that IOS-BDR was potentially associated with asthma. However, after adjusting for a history of asthma, the participants with IOS-BDR had higher mMRC and CAT scores than those without IOS-BDR. It is believed that IOS-BDR might reflect dynamic hyperinflation and premature airway closure, which can result in dyspnea. Accordingly, IOS-BDR might reflect the signs of early or subclinical COPD.
To confirm this hypothesis, the difference between participants with and without IOS-BDR in terms of lung function decline and acute respiratory exacerbations was analyzed. The participants with IOS-BDR had a rapid decline in FVC than those without IOS-BDR in the participants with COPD. This result indicated that IOS-BDR might reflect a special COPD subtype. Numerous studies demonstrated that patients with spirometric BDR experienced a rapid decline in lung function than patients without spirometric BDR. However, after adjusting for baseline FEV 1 , the spirometric BDR demonstrated no association with lung function decline [ 42 , 43 ]. Nevertheless, this study determined that, after adjusting for baseline lung function, the participants with IOS-BDR persistently exhibited a rapid decline in lung function compared with those without IOS-BDR. This result suggested that IOS-BDR might reflect different physiological characteristics compared with spirometric BDR.
Previous research demonstrated that BDR might indicate inflammation and be associated with eosinophil changes and increased exhaled nitric oxide [ 44 , 45 ]. Patients with IOS-BDR might respond well after inhaling corticosteroids. Therefore, early treatment with inhaled corticosteroids (ICS) in COPD patients with IOS-BDR might effectively impede the decline in lung function.
Among the participants without COPD, 10.6% paticipants had IOS-BDR. Here, Xrs exhibited more significant changes compared to Rrs after the administration of 400 µg salbutamol. This finding contradicted previous research that reported a decrease in Rrs but non-significant changes in Xrs in healthy participants after inhaling bronchodilators [ 19 , 46 ] A possible explanation is that an increase in the proportion of IOS-BDR might be associated with respiratory symptoms. While Oostveen et al. enrolled asymptomatic healthy participants without cardiopulmonary diseases, the present study enrolled some symptomatic participants, and the baseline results demonstrated that participants with IOS-BDR had more cough and wheezing symptoms, and higher CAT scores and mMRC scores than the participants without IOS-BDR. Jetmalani et al. also demonstrated a higher proportion of IOS-BDR in smoking individuals with respiratory symptoms than in asymptomatic smoking individuals, and the proportion of BDR assessed by Rrs and Xrs was similar in asymptomatic healthy participants ( ∼ 5.0%) [ 22 ] .
Previous study has identified differences in IOS parameters but spirometry indicators showed no differences before and after bronchodilator inhalation in health individuals. This result suggested that, in the early stages of COPD, IOS may be more sensitive in detecting airway responsiveness compared to traditional spirometry [ 23 ]. Our findings showed that in the participants without COPD, IOS-BDR was associated with lung function decline after adjusting for covariates. This result implied that individuals with IOS-BDR may be higher risk participants in pre-COPD. Early intervention may potentially slow down the decline in lung function and prevent progression to COPD. Similar to the spirometric BDR outcome in many studies, the present study detected no association between IOS-BDR and the risk of acute respiratory events/exacerbations in patients with COPD [ 6 , 47 ]. Further studies are warranted to identify the underlying mechanisms of IOS-BDR in patients without COPD.
This study had some limitations. First, IOS-BDR was defined as the absolute change in IOS parameters in our study. However, the absolute value strongly depended on the baseline value, increasing the proportion of IOS-BDR. The relative changes or Z-score changes in IOS parameters were recommended to greatly reflect BDR, but almost no participants with IOS-BDR defined based on the relative IOS parameter changes were detected in this study (not shown). In the present study, it is believed that many participants with mild to moderate COPD with low airway resistance after bronchodilator administration might not respond well. Accordingly, the recommended threshold of relative changes might be unsuitable for participants with COPD, and new thresholds should be explored for assessing IOS-BDR. Second, given the lack of information on ICS/long-acting β2-agonist (LABA) treatment, whether ICS/LABA use would affect the prognosis remained unclear. Thirdly, due to the greater variability of IOS compared with spirometric parameters [ 48 , 49 ], previous studies have reported that there was individual variability and day instability in spirometric BDR [ 3 , 50 ], however, the individual variability of IOS-BDR and whether IOS-BDR would identify a useful phenotype remained unclear. In addition, single IOS measurements was used in this study, different devices will be included to analyze the robustness of the results in future. Finally,We are sorry that design of the ECOPD cohort did not include the information related to Corona Virus Disease 2019 (COVID-19) infection, the reasons were as follow: (1) The contents of COVID-19 were not collected in design of the ECOPD cohort study. (2) At the end of 2022, there is no way to obtain accurate results due to none conditions for nasopharyngeal swab in some places. Although the contents of COVID-19 infection were not collected, we believe that COVID-2019 infection has little impact on the results of this study. at the end of 2022, it reported spread of the SARS-CoV2 Omicron variant in a very large population of very low pre-existing immunity, among hospitalized patients with Omicron infection olny had mild disease [ 51 , 52 ]. In addition, participants were required to perform lung function tests only when no acute exacerbation or acute upper respiratory tract infection occurred one month before the follow-up to ensure the accuracy of lung function.
IOS-BDR was prevalent in the participants with COPD, especially those with GOLD3–4. Participants with IOS-BDR had more respiratory symptoms, radiographic structural changes, and a rapid decline in lung function than those without IOS-BDR, suggesting that IOS-BDR might benefit from the different treatable characteristic subtypes.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- Impulse oscillometry
- Small airway dysfunction
Forced expiratory volume in one second
Forced vital capacity
- Bronchodilator response
Resistance at 5 Hz
R20-difference from R5 to R20
Reactance at 5 Hz
Area under the reactance curve
Resonant frequency
Calverley PM, Albert P, Walker PP. Bronchodilator reversibility in chronic obstructive pulmonary disease: use and limitations. Lancet Respir Med. 2013;1:564–73.
Article PubMed Google Scholar
Tuomisto LE, Ilmarinen P, Lehtimäki L, Tommola M, Kankaanranta H. Immediate bronchodilator response in FEV(1) as a diagnostic criterion for adult asthma. Eur Respir J. 2019;53(2):1800904.
Albert P, Agusti A, Edwards L, Tal-Singer R, Yates J, Bakke P, Celli BR, Coxson HO, Crim C, Lomas DA, et al. Bronchodilator responsiveness as a phenotypic characteristic of established chronic obstructive pulmonary disease. Thorax. 2012;67:701–8.
Hanania NA, Celli BR, Donohue JF, Martin UJ. Bronchodilator reversibility in COPD. Chest. 2011;140:1055–63.
Stanojevic S, Kaminsky DA, Miller MR, Thompson B, Aliverti A, Barjaktarevic I, et al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur Respir J. 2022;60(1):2101499.
Barjaktarevic IZ, Buhr RG, Wang X, Hu S, Couper D, Anderson W, Kanner RE, Paine Iii R, Bhatt SP, Bhakta NR, et al. Clinical significance of Bronchodilator Responsiveness evaluated by Forced Vital Capacity in COPD: SPIROMICS cohort analysis. Int J Chron Obstruct Pulmon Dis. 2019;14:2927–38.
Article PubMed PubMed Central Google Scholar
Janson C, Malinovschi A, Amaral AFS, Accordini S, Bousquet J, Buist AS, et al. Bronchodilator reversibility in asthma and COPD: findings from three large population studies. Eur Respir J. 2019;54(3):1900561.
Marín JM, Ciudad M, Moya V, Carrizo S, Bello S, Piras B, Celli BR, Miravitlles M. Airflow reversibility and long-term outcomes in patients with COPD without comorbidities. Respir Med. 2014;108:1180–8.
Hanania NA, Sharafkhaneh A, Celli B, Decramer M, Lystig T, Kesten S, Tashkin D. Acute bronchodilator responsiveness and health outcomes in COPD patients in the UPLIFT trial. Respir Res. 2011;12:6.
Article CAS PubMed PubMed Central Google Scholar
Kim J, Kim WJ, Lee CH, Lee SH, Lee MG, Shin KC, Yoo KH, Lee JH, Lim SY, Na JO, et al. Which bronchodilator reversibility criteria can predict severe acute exacerbation in chronic obstructive pulmonary disease patients? Respir Res. 2017;18:107.
Aburuz S, McElnay J, Gamble J, Millership J, Heaney L. Relationship between lung function and asthma symptoms in patients with difficult to control asthma. J Asthma. 2005;42:859–64.
Article CAS PubMed Google Scholar
van den Berge M, Ten Hacken NHT, Cohen J, Douma WR, Postma DS. Small airway disease in asthma and COPD: clinical implications. Chest. 2011;139:412–23.
Burgel P. The role of small airways in obstructive airway diseases. Eur Respiratory Rev. 2011;20:023–33.
Article Google Scholar
Cottee AM, Seccombe LM, Thamrin C, King GG, Peters MJ, Farah CS. Bronchodilator response assessed by the forced oscillation technique identifies poor asthma control with greater sensitivity than spirometry. Chest. 2020;157:1435–41.
Borrill ZL, Houghton CM, Woodcock AA, Vestbo J, Singh D. Measuring bronchodilation in COPD clinical trials. Br J Clin Pharmacol. 2005;59:379–84.
Yaegashi M, Yalamanchili VA, Kaza V, Weedon J, Heurich AE, Akerman MJ. The utility of the forced oscillation technique in assessing bronchodilator responsiveness in patients with asthma. Respir Med. 2007;101:995–1000.
Lu L, Peng J, Wu F, Yang H, Zheng Y, Deng Z, Zhao N, Dai C, Xiao S, Wen X, et al. Clinical characteristics of airway impairment assessed by impulse oscillometry in patients with chronic obstructive pulmonary disease: findings from the ECOPD study in China. BMC Pulm Med. 2023;23:52.
Lu L, Peng J, Zhao N, Wu F, Tian H, Yang H, Deng Z, Wang Z, Xiao S, Wen X, et al. Discordant spirometry and impulse oscillometry assessments in the diagnosis of small Airway Dysfunction. Front Physiol. 2022;13:892448.
Oostveen E, Boda K, van der Grinten CP, James AL, Young S, Nieland H, Hantos Z. Respiratory impedance in healthy subjects: baseline values and bronchodilator response. Eur Respir J. 2013;42:1513–23.
King GG, Bates J, Berger KI, Calverley P, de Melo PL, Dellacà RL, et al. Technical standards for respiratory oscillometry. Eur Respir J. 2020;55(2):1900753.
Johansson H, Wollmer P, Sundström J, Janson C, Malinovschi A. Bronchodilator response in FOT parameters in middle-aged adults from SCAPIS–normal values and relation to asthma and wheezing. Eur Respir J. 2021;58(3):2100229.
Jetmalani K, Brown NJ, Boustany C, Toelle BG, Marks GB, Abramson MJ, et al. Normal limits for oscillometric bronchodilator responses and relationships with clinical factors. ERJ open Res. 2021;7(4):00439–2021.
da Costa GM, Faria AC, Di Mango AM, Lopes AJ, Lopes de Melo P. Respiratory impedance and response to salbutamol in healthy individuals and patients with COPD. Respiration. 2014;88:101–11.
Park JH, Lee JH, Kim HJ, Jeong N, Jang HJ, Kim HK, Park CS. Usefulness of impulse oscillometry for the assessment of bronchodilator response in elderly patients with chronic obstructive airway disease. J Thorac Dis. 2019;11:1485–94.
Almeshari MA, Alobaidi NY, Sapey E, Usmani O, Stockley RA, Stockley JA. Small Airways Response to bronchodilators in adults with asthma or COPD: a systematic review. Int J Chron Obstruct Pulmon Dis. 2021;16:3065–82.
Wu F, Zhou Y, Peng J, Deng Z, Wen X, Wang Z, Zheng Y, Tian H, Yang H, Huang P, et al. Rationale and design of the early chronic obstructive Pulmonary Disease (ECOPD) study in Guangdong, China: a prospective observational cohort study. J Thorac Dis. 2021;13:6924–35.
Zhong N, Wang C, Yao W, Chen P, Kang J, Huang S, Chen B, Wang C, Ni D, Zhou Y, et al. Prevalence of chronic obstructive pulmonary disease in China: a large, population-based survey. Am J Respir Crit Care Med. 2007;176:753–60.
Zhou Y, Hu G, Wang D, Wang S, Wang Y, Liu Z, Hu J, Shi Z, Peng G, Liu S, et al. Community based integrated intervention for prevention and management of chronic obstructive pulmonary disease (COPD) in Guangdong, China: cluster randomised controlled trial. BMJ. 2010;341:c6387.
Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34:648–54.
Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GK, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 1987;106:196–204.
Kim V, Aaron SD. What is a COPD exacerbation? Current definitions, pitfalls, challenges and opportunities for improvement. Eur Respir J. 2018;52(5):1801261.
Regan EA, Lynch DA, Curran-Everett D, Curtis JL, Austin JH, Grenier PA, Kauczor H-U, Bailey WC, DeMeo DL, Casaburi RH. Clinical and radiologic disease in smokers with normal spirometry. JAMA Intern Med. 2015;175:1539–49.
Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005,26:319–338.
Oostveen E, MacLeod D, Lorino H, Farre R, Hantos Z, Desager K, Marchal F. Measurements ERSTFoRI: the forced oscillation technique in clinical practice: methodology, recommendations and future developments. Eur Respir J. 2003;22:1026–41.
Vestbo J, Edwards LD, Scanlon PD, Yates JC, Agusti A, Bakke P, Calverley PM, Celli B, Coxson HO, Crim C. Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med. 2011;365:1184–92.
Kaminsky DA, Simpson SJ, Berger KI, Calverley P, de Melo PL, Dandurand R, et al. Clinical significance and applications of oscillometry. Eur Respir Rev. 2022;31(163):210208.
Verbanck S. Physiological measurement of the small airways. Respiration. 2012;84:177–88.
Dellacà RL, Rotger M, Aliverti A, Navajas D, Pedotti A, Farré R. Noninvasive detection of expiratory flow limitation in COPD patients during nasal CPAP. Eur Respir J. 2006;27:983–91.
Dellacà RL, Santus P, Aliverti A, Stevenson N, Centanni S, Macklem PT, Pedotti A, Calverley PM. Detection of expiratory flow limitation in COPD using the forced oscillation technique. Eur Respir J. 2004;23:232–40.
Milne S, Hammans C, Watson S, Farah CS, Thamrin C, King GG. Bronchodilator responses in respiratory impedance, hyperinflation and gas trapping in COPD. Copd. 2018;15:341–9.
Alobaidi NY, Almeshari MA, Stockley JA, Stockley RA, Sapey E. The prevalence of bronchodilator responsiveness of the small airway (using mid-maximal expiratory flow) in COPD - a retrospective study. BMC Pulm Med. 2022;22:493.
Calverley PM, Burge PS, Spencer S, Anderson JA, Jones PW. Bronchodilator reversibility testing in chronic obstructive pulmonary disease. Thorax. 2003;58:659–64.
Anthonisen NR, Lindgren PG, Tashkin DP, Kanner RE, Scanlon PD, Connett JE. Bronchodilator response in the lung health study over 11 yrs. Eur Respir J. 2005;26:45–51.
Faul JL, Demers EA, Burke CM, Poulter LW. Alterations in airway inflammation and lung function during corticosteroid therapy for atopic asthma. Chest. 2002;121:1414–20.
Papi A, Romagnoli M, Baraldo S, Braccioni F, Guzzinati I, Saetta M, Ciaccia A, Fabbri LM. Partial reversibility of airflow limitation and increased exhaled NO and sputum eosinophilia in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;162:1773–7.
Bhatawadekar SA, Leary D, de Lange V, Peters U, Fulton S, Hernandez P, McParland C, Maksym GN. Reactance and elastance as measures of small airways response to bronchodilator in asthma. J Appl Physiol (1985). 2019;127:1772–81.
Janson C, Malinovschi A, Amaral AF, Accordini S, Bousquet J, Buist AS, et al. Bronchodilator reversibility in asthma and COPD: findings from three large population studies. Eur Respir J. 2019;54(3):1900561.
Crim C, Celli B, Edwards LD, Wouters E, Coxson HO, Tal-Singer R, Calverley PM. investigators E: Respiratory system impedance with impulse oscillometry in healthy and COPD subjects: ECLIPSE baseline results. Respir Med 2011, 105:1069–1078.
Xu J, Sun X, Zhu H, Cao Y, Pudasaini B, Yang W, Liu J, Guo J. Long-term variability of impulse oscillometry and spirometry in stable COPD and asthma. Respir Res. 2022;23:262.
Calverley PM, Albert P, Walker PP. Bronchodilator reversibility in chronic obstructive pulmonary disease: use and limitations. Lancet Respiratory Med. 2013;1:564–73.
Goldberg EE, Lin Q, Romero-Severson EO, Ke R. Swift and extensive omicron outbreak in china after sudden exit from ‘zero-COVID’ policy. Nat Commun. 2023;14(1):3888.
Wang B, Yu Y, Yu Y, et al. Clinical features and outcomes of hospitalized patients with COVID-19 during the omicron wave in Shanghai, China. J Infect. 2023;86(1):e27–e29.
Download references
Acknowledgements
We thank all the participants who participated in the study. We would like to express our appreciation to Xiang Wen, Shan Xiao, Peiyu Huang, Bijia Lin, Shaodan Wei, Xiaopeng Ling, Heshen Tian, Zihui Wang (State Key Laboratory of Respiratory Disease, National Clinical Research Center for Respiratory Disease, National Center for Respiratory Medicine, Guangzhou Institute of Respiratory Health, The First Affiliated Hospital of Guangzhou Medical University), and Jianhui Huang (Lianping County People’s Hospital) for their effort in collecting and verifying the data.
This work was supported by the Foundation of Guangzhou National Laboratory (SRPG22-018 and SRPG22-016), the National Natural Science Foundation of China (81970045, 81970038, and 82270043), and the Clinical and Epidemiological Research Project of State Key Laboratory of Respiratory Disease (SKLRD-L-202402).
Author information
Lifei Lu and Fan Wu contributed equally to this work.
Authors and Affiliations
State Key Laboratory of Respiratory Disease, National Clinical Research Center for Respiratory Disease, National Center for Respiratory Medicine, Guangzhou Institute of Respiratory Health, The First Affiliated Hospital of Guangzhou Medical University, Guangzhou, China
Lifei Lu, Fan Wu, Jieqi Peng, Xiaohui Wu, Huajing Yang, Zhishan Deng, Cuiqiong Dai, Ningning Zhao, Kunning Zhou, Qi Wan, Gaoying Tang, Jiangyu Cui, Pixin Ran & Yumin Zhou
Guangzhou National Laboratory, Guangzhou, China
Fan Wu, Jieqi Peng, Xiangqing Hou, Youlan Zheng, Pixin Ran & Yumin Zhou
Lianping County People’s Hospital, Heyuan, China
Shuqing Yu & Xiangwen Luo
Wengyuan County People’s Hospital, Shaoguan, China
Changli Yang & Shengtang Chen
You can also search for this author in PubMed Google Scholar
Contributions
P.X.R., Y.M.Z., F.W., and L.F.L designed the project and planned the statistical analysis. L.F.L drafted and revised the paper. L.F.L., F.W., J.Q.P., X.H.W., X.Q.H., Y.L.Z, H.J.Y., Z.S.D., C.Q.D., N.N.Z., K.N.Z, Q.W., G.Y.T., J.Y.C., S.Q.Y., X.W.L., C.L.Y and S.T.C collected and monitored the data collection. All authors approved the final draft of the manuscript for publication. L.F.L take responsibility for the integrity of the data and the accuracy of the data analysis. L.F.L is the study guarantors.
Corresponding authors
Correspondence to Pixin Ran or Yumin Zhou .
Ethics declarations
Ethics approval and consent to participate.
This study adhered to the ethical guidelines outlined in the Declaration of Helsinki. The research protocol received approval from the Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University (Approval No. 2018-53) prior to initiation. Written informed consent was obtained from all participants prior to their enrollment in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Lu, L., Wu, F., Peng, J. et al. Clinical characterization and outcomes of impulse oscillometry-defined bronchodilator response: an ECOPD cohort-based study. Respir Res 25 , 149 (2024). https://doi.org/10.1186/s12931-024-02765-7
Download citation
Received : 14 December 2023
Accepted : 11 March 2024
Published : 30 March 2024
DOI : https://doi.org/10.1186/s12931-024-02765-7

Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Decline in lung function
- Acute exacerbations
Respiratory Research
ISSN: 1465-993X
- General enquiries: [email protected]
Study record managers: refer to the Data Element Definitions if submitting registration or results information.
Search for terms

- Advanced Search
- See Studies by Topic
- See Studies on Map
- How to Search
- How to Use Search Results
- How to Find Results of Studies
- How to Read a Study Record

- Learn About Studies
- Other Sites About Studies
- Glossary of Common Site Terms

- Submit Studies to ClinicalTrials.gov PRS
- Why Should I Register and Submit Results?
- FDAAA 801 and the Final Rule
- How to Apply for a PRS Account
- How to Register Your Study
- How to Edit Your Study Record
- How to Submit Your Results
- Frequently Asked Questions
- Support Materials
- Training Materials

- Selected Publications
- Clinical Alerts and Advisories
- Trends, Charts, and Maps
- Downloading Content for Analysis

- ClinicalTrials.gov Background
- About the Results Database
- History, Policies, and Laws
- ClinicalTrials.gov Modernization
- Media/Press Resources
- Linking to This Site
- Terms and Conditions
- Search Results
- Study Record Detail

Study of Longidaze in the Prevention & Treatment of Pulmonary Fibrosis, Interstitial Lung Diseases Caused by COVID-19
- Study Details
- Tabular View
- No Results Posted

Change in the degree of dyspnea on the MMRC scale from baseline after 2.5 months and 6 months in patients of the Longidaze® group compared with the dynamic observation group.
MMRC scale (Modified Medical Research Council scale) 0 - no - Dyspnea does not bother, except for very intense exercise
- - mild - Shortness of breath bothers with brisk walking or climbing a small elevation
- - moderate to severe - Shortness of breath results in slower walking compared to other people of the same age, or need to stop while walking at normal pace on a level surface
- - Severe - Shortness of breath makes you stop when walking about 100 m or after a few minutes of walking on a flat surface
- - very severe - Shortness of breath makes it impossible to leave the house or appears when dressing and undressing
Inclusion Criteria:
- Patients with residual lung changes after complicated COVID-19
- Residual changes were detected no later than 2 months after the discharge after disease
- Treatment of COVID-19 was in accordance with the standard of the then current temporary guidelines for the treatment of COVID-19
- Age of patients over 18 years old
- Negative polymerase chain reaction (PCR) test COVID-19 at least 2 times in respiratory samples or based on serology results in blood samples
- Patients in the framework of routine clinical practice, in accordance with the instructions for use before inclusion in the study, were prescribed intramuscular treatment with Longidaze® at a dose of 3000 IU, 1 injection every 5 days for a total course of 15 injections or dynamic observation without the use of active therapy
- The patient did not participate in other drug clinical trials within 1 month prior to Visit 1.
- The patient or patient's caregiver agrees to participate in the trial and sign an informed consent form
- Patient understands and agrees to follow the planned procedures.
- Women with fertile potential must agree to use at least one method of contraception before completing participation in the study.
Exclusion Criteria:
- Women during pregnancy and lactation and women planning to become pregnant during the study period
- Severe background diseases, such as severe heart failure (class IV heart function), severe liver and kidney disease, severe bronchial asthma, severe chronic obstructive pulmonary disease, bronchiectasis, bullous emphysema and previously identified interstitial lung diseases, neurological diseases, tumors.
- Long-term bed rest, regardless of its cause
- Increased individual sensitivity to the components of the studied drug
- Pathological conditions that determine the impossibility of patient participation in the study (by the decision of the investigator)
- Medical history that, according to the investigator, does not allow the patient to be included in the study
- A burdened allergic anamnesis, which, according to the investigator, does not allow the patient to be included in the study
- For Patients and Families
- For Researchers
- For Study Record Managers
- Customer Support
- Accessibility
- Viewers and Players
- Freedom of Information Act
- HHS Vulnerability Disclosure
- U.S. National Library of Medicine
- U.S. National Institutes of Health
- U.S. Department of Health and Human Services
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
Prognostic indicators and outcomes of hospitalised COVID-19 patients with neurological disease: An individual patient data meta-analysis
Contributed equally to this work with: Bhagteshwar Singh, Suzannah Lant
Roles Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing
Affiliations National Institute for Health Research Health Protection Research Unit in Emerging and Zoonotic Infections, Institute of Infection, Veterinary and Ecological Sciences, University of Liverpool, Liverpool, United Kingdom, Tropical and Infectious Diseases Unit, Liverpool University Hospitals NHS Foundation Trust, Liverpool, United Kingdom, Department of Infectious Diseases, Christian Medical College, Vellore, India
Roles Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing
Affiliation National Institute for Health Research Health Protection Research Unit in Emerging and Zoonotic Infections, Institute of Infection, Veterinary and Ecological Sciences, University of Liverpool, Liverpool, United Kingdom
Affiliation Department of Health Data Science, University of Liverpool, Liverpool, United Kingdom
Roles Conceptualization, Investigation, Methodology, Validation, Visualization, Writing – review & editing
Roles Investigation, Writing – review & editing
Affiliations National Institute for Health Research Health Protection Research Unit in Emerging and Zoonotic Infections, Institute of Infection, Veterinary and Ecological Sciences, University of Liverpool, Liverpool, United Kingdom, Tropical and Infectious Diseases Unit, Liverpool University Hospitals NHS Foundation Trust, Liverpool, United Kingdom
Affiliation Queen Square Institute of Neurology, University College London, London, United Kingdom
Roles Writing – review & editing
Affiliations National Institute for Health Research Health Protection Research Unit in Emerging and Zoonotic Infections, Institute of Infection, Veterinary and Ecological Sciences, University of Liverpool, Liverpool, United Kingdom, The Walton Centre NHS Foundation Trust, Liverpool, United Kingdom
Affiliation Department of Neurology, Wayne State University, Detroit, Michigan, United States of America
Affiliation Instituto de Medicina Tropical, Universidade de São Paulo, São Paulo, Brazil
Affiliation Department of Medicine, King Saud University, Riyadh, Saudi Arabia
Affiliation Department of Clinical and Experimental Sciences, Neurology Unit, University of Brescia, Brescia, Italy
Affiliation Bangur Institute of Neurosciences, Institute of Post-Graduate Medical Education and Research, Kolkata, India
Roles Project administration, Writing – review & editing
Roles Data curation, Investigation, Writing – review & editing
Affiliation Homerton University Hospital NHS Foundation Trust, London, United Kingdom of Great Britain and Northern Ireland
Affiliation Department of Neurovirology, National Institute of Mental Health and Neurosciences, Bangalore, India
Affiliation Department of Infection, Manchester University NHS Foundation Trust, Manchester, United Kingdom of Great Britain and Northern Ireland
Affiliation Neurology Unit, Neuromotor & Rehabilitation Department, Azienda USL-IRCCS di Reggio Emilia, Reggio Emilia, Italy
Affiliation Liverpool University Hospitals NHS Foundation Trust, Liverpool, United Kingdom of Great Britain and Northern Ireland
Affiliation Department of Virology, UK Health Security Agency, Manchester University NHS Foundation Trust, Manchester, United Kingdom of Great Britain and Northern Ireland
Affiliation Barts Health NHS Trust, London, United Kingdom of Great Britain and Northern Ireland
Affiliation Department of Infectious Diseases & Tropical Medicine, North Manchester General Hospital, Manchester University Foundation NHS Trust, Manchester, United Kingdom of Great Britain and Northern Ireland
Affiliation Warrington Hospital, Warrington and Halton Teaching Hospitals NHS Foundation Trust, Warrington, United Kingdom of Great Britain and Northern Ireland
Affiliation Malawi-Liverpool-Wellcome Trust Clinical Research Programme, Blantyre, Malawi
Affiliation Kingston Hospital NHS Foundation Trust, Kingston upon Thames, United Kingdom of Great Britain and Northern Ireland
Affiliation Institute of Clinical Neurosciences, University of Bristol, Bristol, United Kingdom of Great Britain and Northern Ireland
Affiliation North Manchester General Hospital, Manchester University Foundation NHS Trust, Manchester, United Kingdom of Great Britain and Northern Ireland
Affiliation Epsom and St Helier University Hospitals NHS Foundation Trust, United Kingdom of Great Britain and Northern Ireland
Affiliation King’s College Hospital NHS Foundation Trust, London, United Kingdom of Great Britain and Northern Ireland
Affiliation Regional Infectious Diseases Unit, NHS Lothian, Edinburgh, United Kingdom of Great Britain and Northern Ireland
Affiliation Yerevan State Medical University named after Mkhitar Heratsi, Neuroscience Laboratory, Cobrain Center, Yerevan, Armenia
Affiliation St Vincent’s Hospital, Sydney, Australia
Affiliation Saint-Luc University Hospital, Brussels, Belgium
Affiliation Université de Mons, Mons, Belgium
Affiliation AZ Glorieux, Ronse, Belgium
Affiliation Hospital da Restauração, Recife, Brazil
Affiliation Hospital Federal dos Servidores do Estado, Rio de Janeiro, Brazil
Affiliation Hospital Dr. Sótero del Río, Santiago, Chile
Affiliation Universidad de Chile - Hospital Barros Luco Trudeau, Santiago, Chile
Affiliation The 940th Hospital of Joint Logistic Support Force of the People’s Liberation Army, Lanzhou, China
Affiliation Cairo University Hospital, Cairo, Egypt
Affiliation Kasr Alainy Teaching Hospital, Cairo, Egypt
Affiliation Mataria Teaching Hospital, Cairo, Egypt
Affiliation Fondation Rothschild, Paris, France
Affiliation Pitié Salpetriere Hospital, Paris, France
Affiliation Rennes University Hospital, Rennes, France
Affiliation Hôpitaux Universitaires de Strasbourg, Strasbourg, France
Affiliation Children’s Hospital, Dresden Municipal Hospital Teaching Hospital TUD, Dresden, Germany
Affiliation Medical Center University of Freiburg, Freiburg, Germany
Affiliation Department of Neurology, Technical University of Munich, Munich, Germany
Affiliation Mazandaran University of Medical Science, Sari, Islamic Republic of Iran
Affiliation Institute for Research in Fundamental Sciences (IPM), Tehran, Islamic Republic of Iran
Affiliation Iranian Research Center for HIV/AIDS, Tehran University of Medical Sciences, Tehran, Iran
Affiliation Fondazione Poliambulanza Istituto Ospedaliero, Brescia, Italy
Affiliation University of Brescia, Brescia, Italy
Affiliation San Gerardo Hospital ASST Monza, University of Milano Bicocca, Monza, Italy
Affiliation Fondazione Mondino IRCCS, Pavia, Italy
Affiliation Santa Maria delle Croci Hospital, AUSL Romagna, Ravenna, Italy
Affiliation Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy
Affiliation University Hospital of Rome Tor Vergata, Rome, Italy
Affiliation Healthcare Trust of the Autonomous Region of Trento, Rovereto, Italy
Affiliation Città della Salute e della Scienza di Torino, Regina Margherita Children’s Hospital, Turin, Italy
Affiliation University of Verona, Verona, Italy
Affiliation Halcyon Healthcare Limited, Nairobi, Kenya
Affiliation Hôpitaux Robert Schuman, Luxembourg, Luxembourg
Affiliation Leiden University Medical Center, Leiden, Netherlands
Affiliation Hospital Regional Docente de Trujillo, Trujillo, Peru
Affiliation Centro Hospitalar São João, Porto, Portugal
Affiliation Centro Hospitalar Universitário do Porto, Porto, Portugal
Affiliation Buyanov Moscow City Hospital, Moscow, Russian Federation
Affiliation Moscow Research and Clinical Center for Neuropsychiatry and Buyanov Moscow City Hospital, Moscow, Russian Federation
Affiliation National Neuroscience Institute, Singapore, Singapore
Affiliation Complejo Hospitalario Universitario de Albacete, Albacete, Spain
Affiliation Hospital Universitario Virgen de las Nieves, Granada, Spain
Affiliation University Hospital Sanchinarro, Madrid, Spain
Affiliation University Hospital Ramón y Cajal, Madrid, Spain
Affiliation Hospital Virgen de la Salud, Toledo, Spain
Affiliation Hospital del Río Hortega, Valladolid, Spain
Affiliation Hopitaux Universitaires de Genève, Geneva, Switzerland
Affiliation Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland
Affiliation Acibadem Mehmet Ali Aydinlar University Medical School, Istanbul, Turkey
Affiliation Ulster Hospital, Belfast, United Kingdom of Great Britain and Northern Ireland
Affiliation University of Bristol and North Bristol NHS Trust, Bristol, United Kingdom of Great Britain and Northern Ireland
Affiliation Gloucestershire Royal Hospital, Gloucester, United Kingdom of Great Britain and Northern Ireland
Affiliation Tropical and Infectious Diseases Unit, Liverpool University Hospitals NHS Foundation Trust, Liverpool, United Kingdom
Affiliation The Walton Centre NHS Foundation Trust, Liverpool, United Kingdom
Affiliation Great Ormond Street Hospital for Children, London, United Kingdom of Great Britain and Northern Ireland
Affiliation Imperial College London, London, United Kingdom of Great Britain and Northern Ireland
Affiliation The National Hospital for Neurology & Neurosurgery, London, United Kingdom of Great Britain and Northern Ireland
Affiliation University College London, London, United Kingdom of Great Britain and Northern Ireland
Affiliation University College London Queen Square Institute of Neurology, London, United Kingdom of Great Britain and Northern Ireland
Affiliation Eastern Pathology Alliance Department of Microbiology, Norfolk and Norwich University Hospitals NHS Foundation Trust, Norwich, United Kingdom of Great Britain and Northern Ireland
Affiliation Sheffield Teaching Hospitals Trust, Sheffield, United Kingdom of Great Britain and Northern Ireland
Affiliation Wessex Neurological Centre, Southampton, United Kingdom of Great Britain and Northern Ireland
Affiliation Emory University School of Medicine, Atlanta, Georgia, United States of America
Affiliation Massachusetts General Hospital / Harvard Medical School, Boston, Massachusetts, United States of America
Affiliation Yale New Haven Health Bridgeport Hospital, Bridgeport, Connecticut, United States of America
Affiliation Rush University Medical Center, Chicago, Illinois, United States of America
Affiliation University of Arkansas for Medical Sciences, Little Rock, Arkansas, United States of America
Affiliation Children’s Hospital Los Angeles and Keck School of Medicine at the University of Southern California, Los Angeles, California, United States of America
Affiliation Ochsner Medical Center, New Orleans, Los Angeles, United States of America
Affiliation Columbia University Irving Medical Center, New York, New York, United States of America
Affiliation New York University Grossman School of Medicine, New York, New York, United States of America
Affiliation Department of Neurology, National Neuroscience Institute, Singapore, Singapore
Affiliation Institute of Psychiatry, Psychology, and Neuroscience, King’s College London, London, United Kingdom
Affiliation Division of High-Consequence Pathogens and Pathology, National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia, United States of America
Roles Conceptualization, Data curation, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing
¶ Membership of The Brain Infections Global COVID-Neuro Network Study Group is provided in S1 Appendix .
¶ ‡ CTS and TS also contributed equally to this work.
- [ ... ],
Roles Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliations National Institute for Health Research Health Protection Research Unit in Emerging and Zoonotic Infections, Institute of Infection, Veterinary and Ecological Sciences, University of Liverpool, Liverpool, United Kingdom, Tropical and Infectious Diseases Unit, Liverpool University Hospitals NHS Foundation Trust, Liverpool, United Kingdom, The Walton Centre NHS Foundation Trust, Liverpool, United Kingdom
- [ view all ]
- [ view less ]
- Bhagteshwar Singh,
- Suzannah Lant,
- Sofia Cividini,
- Jonathan W. S. Cattrall,
- Lynsey C. Goodwin,
- Laura Benjamin,
- Benedict D. Michael,
- Ayaz Khawaja,
- Aline de Moura Brasil Matos,

- Published: June 2, 2022
- https://doi.org/10.1371/journal.pone.0263595
- Reader Comments
Neurological COVID-19 disease has been reported widely, but published studies often lack information on neurological outcomes and prognostic risk factors. We aimed to describe the spectrum of neurological disease in hospitalised COVID-19 patients; characterise clinical outcomes; and investigate factors associated with a poor outcome.
We conducted an individual patient data (IPD) meta-analysis of hospitalised patients with neurological COVID-19 disease, using standard case definitions. We invited authors of studies from the first pandemic wave, plus clinicians in the Global COVID-Neuro Network with unpublished data, to contribute. We analysed features associated with poor outcome (moderate to severe disability or death, 3 to 6 on the modified Rankin Scale) using multivariable models.
We included 83 studies (31 unpublished) providing IPD for 1979 patients with COVID-19 and acute new-onset neurological disease. Encephalopathy (978 [49%] patients) and cerebrovascular events (506 [26%]) were the most common diagnoses. Respiratory and systemic symptoms preceded neurological features in 93% of patients; one third developed neurological disease after hospital admission. A poor outcome was more common in patients with cerebrovascular events (76% [95% CI 67–82]), than encephalopathy (54% [42–65]). Intensive care use was high (38% [35–41]) overall, and also greater in the cerebrovascular patients. In the cerebrovascular, but not encephalopathic patients, risk factors for poor outcome included breathlessness on admission and elevated D-dimer. Overall, 30-day mortality was 30% [27–32]. The hazard of death was comparatively lower for patients in the WHO European region.
Interpretation
Neurological COVID-19 disease poses a considerable burden in terms of disease outcomes and use of hospital resources from prolonged intensive care and inpatient admission; preliminary data suggest these may differ according to WHO regions and country income levels. The different risk factors for encephalopathy and stroke suggest different disease mechanisms which may be amenable to intervention, especially in those who develop neurological symptoms after hospital admission.
Citation: Singh B, Lant S, Cividini S, Cattrall JWS, Goodwin LC, Benjamin L, et al. (2022) Prognostic indicators and outcomes of hospitalised COVID-19 patients with neurological disease: An individual patient data meta-analysis. PLoS ONE 17(6): e0263595. https://doi.org/10.1371/journal.pone.0263595
Editor: Patricia T. Bozza, Fundacao Oswaldo Cruz, BRAZIL
Received: October 14, 2021; Accepted: January 21, 2022; Published: June 2, 2022
This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Data Availability: All relevant data are within the paper and its Supporting information files.
Funding: The study was funded by the UK Medical Research Council’s Global Effort on COVID-19 Programme (MR/V033441/1) ( https://mrc.ukri.org/ ); UK National Institute for Health Research (NIHR)- funded Global Health Research Group on Acute Brain Infections (17/63/110) ( https://www.nihr.ac.uk/ ); and the NIHR Health Protection Research Unit in Emerging and Zoonotic Infections (NIHR200907), at University of Liverpool in partnership with Public Health England (PHE), in collaboration with Liverpool School of Tropical Medicine and the University of Oxford (Grant Nos. IS-HPU-1112-10117 and NIHR200907). These grants were awarded to TS. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: TS is part of the Data Safety Monitoring Committee of a study to evaluate the safety and immunogenicity of a candidate Ebola Vaccine in children - the GSK3390107A (ChAd3 EBO-Z) vaccine; he is a panel member of Covid-19 Vaccine Benefit Risk Expert Working Group for the Medicines and Healthcare Regulatory Agency (UK); he is a member of COVID-19 Therapeutics Advisory Panel for the UK Department of Health & Social Care; he is the Chair/Co-Chair of the COVID-19 Rapid Response and Rolling Funding Initiatives, which supported the development of the Oxford-AstraZeneca Covid-19 vaccine. In addition, Dr. Solomon has a diagnostic test for bacterial meningitis, based on a blood test, filed for patent pending.
Introduction
Since the first reported patients in December 2019, the COVID-19 pandemic has spread globally to cause more than 225 million cases, with over 4.5 million deaths [ 1 ]. SARS-CoV-2 virus principally causes respiratory disease, although neurological manifestations were also reported from early in the pandemic, including acute cerebrovascular events, other central and peripheral nervous system disease [ 2 ]. There have now been many such reports, but their use of standardised case definitions, detailed clinical and diagnostic evaluation has varied, making comparisons difficult; clinical outcomes and prognostic factors are often not well characterised. Several meta-analyses have also been published [ 3 – 8 ], but given they are based on these original reports, drawing firm conclusions is challenging. In July 2020 we published standardised case definitions for neurological COVID-19 disease [ 2 ], including an assessment of the strength of evidence for their association with SARS-CoV-2 infection, which are being used increasingly [ 2 , 9 – 11 ]. Using this framework and related data tools, we have now conducted an individual patient data (IPD) meta-analysis of patients with neurological COVID-19 disease from the global first wave. We aimed to firstly describe the spectrum of neurological disease in hospitalised COVID-19 patients using a uniform approach with standardised case definitions; secondly, characterise clinical outcomes; thirdly, investigate factors associated with a poor outcome; and finally, define how frequently acute neurological disease was observed as a proportion of all hospitalised COVID-19 patients. The protocol was registered prospectively on the PROSPERO registry (CRD42020196542).
Search strategy and selection criteria
We searched the following sources for articles published between 1st January 2020 and 3rd July 2020, without language restrictions: PubMed and Scopus; the preprint servers medRxiv and SSRN (Social Science Research Network); and the Brain Infections Global COVID-Neuro Resource and the Journal of Neurology, Neurosurgery and Psychiatry “Neurology and Neuropsychiatry of COVID-19” Blog. We used prespecified search terms modified as needed for each database (S1 Table in S1 Appendix ). We applied the following inclusion criteria to studies and then to individual patients: 1) hospitalised patients of any age; 2) diagnosed with COVID-19; and 3) acute onset of neurological symptoms, not explained fully by a pre-existing condition (e.g. progression of chronic neurological disease), with neurological illnesses classified according to our pre-defined syndromes [ 2 ], or a defined other neurological or neuropsychiatric diagnosis. Onset of neurological symptoms could have been before or after hospitalisation. We excluded studies that did not report original data, reported patients that were not hospitalised, or gave insufficient information. We selected abstracts and obtained full texts of potentially eligible studies. To compare the results of our IPD meta-analysis with other systematic reviews, meta-analyses and primary studies, including evidence from after the global second wave, we used the same search strategy to obtain articles published up to 30 th September 2021.
Data extraction and processing
We invited authors of published studies, and members of the COVID-Neuro Network of the Brain Infections Global Programme, to participate by providing IPD. Contributors ensured local ethical, regulatory and data sharing agreements were in place. We designed and piloted a standard data collection tool early in the pandemic (S2 Appendix, Section 1 to 3). Details included demographics, comorbidities and pre-admission medications; COVID-19 clinical features, including “typical” COVID-19 symptoms of cough, fever and breathlessness (patient-reported or clinician-assessed), the latter of which was taken as a proxy of COVID-19 severity (oxygen usage and ventilation were not chosen as proxies because access to these varied early in the pandemic, although these data were also collected); investigation results, including PCR (with cycle threshold if positive) and antibody testing for SARS-CoV-2 in blood and cerebrospinal fluid (CSF), with evidence of intrathecal production; COVID-19 disease severity as defined by the World Health Organization (WHO) [ 12 ]: neurological features and diagnosis; evidence for association between COVID-19 and neurological disease using pre-defined criteria (S2 Appendix, Section 3.3) [ 2 ]; dates of onset of typical and neurological COVID-19 symptoms (including symptoms that were part of the neurological diagnosis), hospital admission and discharge; treatment for COVID-19 (including maximum oxygen or respiratory support) and for neurological disease; admission to critical care, need for invasive ventilation, death, and modified Rankin Scale (mRS) score at discharge. We did not collect data for patients with no neurological disease.
Submitted datasets were cleaned and processed by at least two investigators from a core team of clinical reviewers. This was to harmonise data recording across studies in accordance with pre-defined variable types, descriptions and definitions; complete missing fields where details were available elsewhere in the dataset; and clarify outlying, unexpected or residual missing data with contributors where necessary. If a contributor was unable to harmonise their data with our format, we allowed original study data to be shared with a corresponding data code dictionary; these data were extracted by one reviewer and then checked fully by a second reviewer using an approach standardised through piloting and frequent team discussions.
Quality assessment
We designed and piloted a bespoke tool to classify study design (S1 Fig in S1 Appendix ) and assessed the quality of studies using an appropriate established assessment tool: for case reports and case series we used the Joanna Briggs Institute (JBI) critical appraisal tools [ 13 , 14 ]; for case-control, cohort and cross-sectional studies, we used the Newcastle-Ottawa Scale (NOS) [ 15 , 16 ]. Two independent reviewers appraised and assessed the quality of IPD studies, with disagreements resolved by consensus or involvement of a third reviewer.
Spectrum of neurological disease
Neurological syndromic diagnoses were made by contributors and checked by reviewers using standardised case definitions with levels of diagnostic certainty (S2 Appendix, Section 3.2) [ 2 ]. Pre-defined syndromic diagnoses included encephalopathy, encephalitis, meningitis, myelitis, acute disseminated encephalomyelitis (ADEM), and cerebrovascular events (including stroke, vasculitis, and cerebral venous sinus thrombosis). The definitions for encephalopathy (including delirium, coma, subsyndromal delirium and other encephalopathy not classified as delirium or coma, each defined as per the Ten Societies’ recommendations), and for encephalitis were combined for the purpose of the primary subgroup analysis [ 17 ]. A secondary analysis was performed for the encephalopathy subgroup excluding patients with encephalitis, who potentially have a different pathophysiological mechanism and so maybe different outcomes. We also included patients with Guillain-Barré syndrome (GBS) and variants, radiculopathy, cranial neuropathy, peripheral neuropathy, myopathy and myositis. Patients with a diagnosis outside our pre-defined criteria were categorised as ‘other neurological presentation’.
Clinical outcomes
Primary outcome..
We used the mRS to characterise outcome at hospital discharge, with a mRS score of 3 to 6 (moderate to severe disability or death) defined as a poor outcome.
Secondary outcomes.
- Mortality and days from hospital admission to death from any cause.
- Admission to critical care or receipt of invasive ventilation, referred to hereafter as “need for intensive care”.
- Length of stay in intensive care.
- Length of stay in hospital.
Statistical analysis
We used an ordinal logistic regression model with random effects to account for clustering within studies, and cumulative link function to estimate log cumulative odds of being at or above each mRS category, for all studies providing mRS for patients systematically. We fitted models for patients with any neurological syndrome, and then for the largest subgroups: cerebrovascular events, and encephalopathy. To identify factors associated with a ‘poor outcome’, an mRS of 3 to 6, we first fitted univariate models using a list of covariates. We then adjusted for a predefined subset of these covariates, which we considered important potential confounders, in multivariable logistic regression models (S2 Table in S1 Appendix ).
Mortality was analysed using Kaplan–Meier survival curves and marginal Cox regression model using the robust sandwich covariance estimates to account for the clustering of individuals within each study. For outcomes with competing risks (need for intensive care, length of stay in critical care and length of stay in hospital), the cumulative incidence curve for the event of interest in the presence of competing events (death) was estimated, and the subdistribution hazards for clustered data were modelled using the approach described by Zhou et al. [ 18 ]. For mortality and need for intensive care (i.e. admission to critical care or receipt of invasive ventilation), a pre-defined set of risk factors were explored in univariate regression models as well as in multivariable models to adjust for confounding factors (S2 Table in S1 Appendix ). We used a 5% significance level throughout. In a post-hoc analysis, we compared mortality and need for intensive care estimates between the two largest subgroups, encephalopathy, and cerebrovascular events, using the log-rank test (mortality) and Gray’s test (intensive care). In a post-hoc sensitivity analysis we compared the whole encephalopathy subgroup with a smaller subgroup of patients with encephalopathy that excluded those with a diagnosis of encephalitis.
Finally, we used data from cohort and cross-sectional studies providing verified totals of all patients hospitalised with COVID-19 in their respective centres, to estimate a pooled proportion of COVID-19 patients with neurological disease. Through inspection of study protocols, reports and other information provided by contributors, we ensured that the approaches used to screen and include participants, and to define denominators were similar across studies selected for meta-analysis.
The funders had no role in the study design, data collection, data analysis, data interpretation, or writing of this report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit it for publication.
Results and discussion
Study selection and ipd obtained.
We identified 4092 records by database searches. After screening these and adding a further 64 records from preprint servers and reference lists, 413 published studies were included ( Fig 1 ). We contacted all study authors, received responses from 128 and received 85 IPD study datasets (2505 patients), comprising 54 published studies, and 31 unpublished studies contributed by Global COVID-Neuro Network collaborators, five of which have now been published. Two studies (143 patients) were excluded as they did not meet inclusion criteria. When inclusion criteria were applied at individual patient-level to 83 studies, 383 patients were excluded, leaving 1979 patients for analyses ( Fig 1 ).
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
IPD = individual patient data.
https://doi.org/10.1371/journal.pone.0263595.g001
Characteristics of included studies
The make-up of the 83 studies is summarised in S3 Table in S1 Appendix . Case series accounted for the majority of studies (61 [73%] studies, 1049 patients); 26 [31%] studies collected data prospectively; patients were hospitalised across 101 sites; 75 studies included adult patients only (1844 [93%] patients); 1179 [60%] patients were male; and most were aged 60 years and above ( Fig 2A ). Nineteen (23%) studies reported from low- or middle-income countries (LMICs); 64 (77%) were from high-income countries (HICs). Most studies (53 [64%]) reported from the WHO European region; 16 (19%) from the Americas region; eight (10%) Eastern Mediterranean; three (4%) Western Pacific region; two (2%) Southeast Asian region; and one (1%) African region (S4 Table in S1 Appendix ). Fig 2B shows the distribution of age classes by WHO region and World Bank income group. The locations of the included studies are displayed in Fig 3 . For 11 of the 83 studies, all patients were on ICU; 17 studies had no patients on ICU; and 55 studies included some patients on ICU. Quality assessments were performed as described above for all studies. Most case reports and case series were of high methodological quality in most domains assessed: 11 of the 12 case reports had an answer of ‘Yes’ for the mandatory domains 1 to 6; and the majority of case series had positive responses for domains 1, 3 and 6–9 of their respective JBI assessment scales. The cohort and cross-sectional studies had lower quality in several domains (for complete assessments see S5 Table in S1 Appendix ).
https://doi.org/10.1371/journal.pone.0263595.g002
WHO regions are depicted in different colours. Countries from which we received IPD are depicted in a darker shade. Country names and numbers of patients for which we had IPD are displayed in boxes, grouped according to region.
https://doi.org/10.1371/journal.pone.0263595.g003
Spectrum of neurological disease in patients with COVID-19
First, we looked at the spectrum of neurological disease observed in patients with COVID-19 ( Table 1 ). From 83 studies, a total of 1979 patients had a syndromic or specific neurological diagnosis. The most commonly reported syndromes were encephalopathy (978 [49%]), and cerebrovascular events (506 [26%]); other important syndromes included smell or taste disturbance (13%), peripheral neuropathy (6%), GBS (3%) and neuropsychiatric disorders (2.5%). Less than 1% were reported to have each of meningitis, ADEM, myelitis, radiculitis, and myositis. For 1027 patients with both dates available, the median time from the onset of typical COVID-19 symptoms to the onset of neurological symptoms was 5 days (IQR 0–12). For patients with encephalopathy, this was 5 days (IQR 1–10); for cerebrovascular events, 7 days (IQR 0–15); peripheral neuropathy, 13 days (IQR 1–24); and GBS, 12 days (IQR 7–22). Of 807 patients for whom the dates of neurological symptom onset and admission were available, 532 (66%) had neurological symptom onset before the admission to hospital, and 275 (34%) after. This varied by neurological diagnosis: while a similar proportion of patients with encephalopathy (66%) and cerebrovascular events (68%) had neurological features at or before admission, the corresponding proportion was 77% for GBS and 38% for other peripheral neuropathy. The majority of patients (93% [1849/1979] of all patients; 95% [932/978] of the encephalopathy subgroup; 89% [450/506] of the cerebrovascular subgroup) had confirmation of COVID-19 by PCR of a respiratory sample for SARS-CoV-2. Two with myelitis had virus detected in the CSF by PCR; no patient had antibody detected in the CSF. The remaining 7% were either cases confirmed by antibody testing, or clinically probable or suspected cases, based on our prescribed definitions (S3.1 Table in S2 Appendix).
https://doi.org/10.1371/journal.pone.0263595.t001
Overall, 887 (45%) of the 1979 patients had severe or critical COVID-19, as per WHO definitions; this proportion was similar in the encephalopathy (47% [457/978]) and cerebrovascular event (51% [260/506]) subgroups ( Fig 2C and S6 Table in S1 Appendix ). Typical COVID-19 symptoms were present before admission in 747 (93%) of 807 patients.
The 978 (49%) encephalopathy cases were reported across 61 studies, of which 161 (16%) of 978 patients had delirium, 37 (4%) had coma, and 92 (9%) had possible or confirmed encephalitis; 688 (70%) of 978 had features of encephalopathy but did not meet criteria for the aforementioned subtypes and so were described as ‘encephalopathy other’, being not otherwise defined. Of the 506 (26%) patients with a cerebrovascular event, 308 (61%) had an ischaemic stroke, 90 (18%) of 506 haemorrhagic stroke, 2 (0.4%) vasculitis, and 106 (21%) another cerebrovascular event ( Table 1 ). Of these 506 patients, 90% (454 of 506) had neuroimaging that informed diagnosis.
According to our definitions for strength of evidence for an association between infection with SARS-CoV-2 and the development of neurological disease (S2 Appendix, Section 3.3), only two patients met criteria for confirmed association—both had myelitis with a positive CSF PCR test for SARS-CoV-2. Most patients were defined as having a probable association between neurological disease and COVID-19: this applied to 792 (96%) of 826 patients with encephalopathy or encephalitis. The majority of patients with cerebrovascular events for whom this assessment was available were classified as having a possible rather than probable association (362 of 454 [80%]) due to the presence of other pre-defined vascular risk factors. More complete details of the strength of association between neurological disease and infection in patients are provided in S8 Table in S1 Appendix . Four (5%) of the 83 studies included all consecutive patients with neurological COVID-19 disease in a given hospital or region (S10 Table in S1 Appendix ). For two of them encephalopathy was the most common presentation, accounting for 50% and 76% of patients, for two cerebrovascular disease predominated (both 64%).
A poor outcome (moderate to severe disability or death, mRS 3–6) was recorded for 50% (95% CI 41–59) of the 1052 patients in 73 studies reporting mRS systematically, after adjusting for clustering within studies ( Table 2 ). The predicted probability of having no symptoms at discharge (mRS 0) was estimated as 7%. Table 2 shows the probability of each mRS score for 413 patients with encephalopathy and 326 patients with cerebrovascular events, for whom an mRS score was available. There was a higher probability of a poor outcome for cerebrovascular patients (76% [95% CI 67–82]), than encephalopathy patients (54% [95% CI 42–65]). The crude probability of death at 30 days ( Fig 4A ) was estimated from a Kaplan-Meier analysis as 30% (95% CI 27–32) for all 1745 patients for whom the outcome was available and did not differ significantly for the encephalopathy and cerebrovascular subgroups. For the 1428 patients with adequate data, the crude cumulative incidence of need for intensive care by 30 days was 38% (95% CI 35–41; Fig 4B ); this was significantly higher for cerebrovascular patients (47% [95% CI 41–53]; 368 patients) than encephalopathy patients (38% [95% CI 34–42]; 617 patients; Gray’s test p = 0.03). The cumulative incidence of discharge from hospital by 30 days was 55% (95% CI 53–58; Fig 4D ) and did not differ significantly between subgroups. Outcomes for the encephalopathy subgroup excluding patients with a diagnosis of encephalitis were all similar to the outcomes of the whole encephalopathy subgroup (S9 Table in S1 Appendix ).
1. These figures show results of analyses for the whole IPD database (i.e., patients with any neurological disease diagnosis), and other than for A, the analyses use death as a competing risk. 2. A total of 1745 patients were included in this analysis. Of the 1979, 115 had no dates; 14 patients had no hospital admission date; 9 dead patients had no date of death; 88 alive patients had no discharge date; it was unknown if 8 patients were dead or alive. For time to death, individuals that were alive at discharge or last follow-up were censored. 3. This analysis uses date of hospital admission as day 0. A total of 1428 patients were included in this analysis: 404 patients had no dates; 17 had no hospital admission date; 123 (23 dead; 100 alive) patients had neither the date of admission to critical care or the date of commencement of invasive ventilation; 7 patients only had a hospital admission date, but it was unknown if they were dead or alive. For time to critical care admission, individuals who were alive at discharge or last follow-up and had not been admitted to intensive care were censored. Individuals who died without receiving critical care or invasive ventilation were treated as competing events in a competing risks analysis. 4. This analysis uses date of critical care admission as day 0. A total of 486 patients who were admitted critical care were included in this analysis: 1482 patients had no date of admission to critical care; 5 dead patients had no death date; 5 alive patients had no hospital discharge date; there were no dates for 1 patient. 5. For discharge from critical care, individuals that were alive and not yet discharged at last follow-up were censored. Individuals that died after admission to intensive care were treated as competing events in a competing risks analysis. 10. For length of hospital stay, individuals that were alive and not yet discharged at last follow-up were censored. Individuals that died were treated as competing events in a competing risks analysis.
https://doi.org/10.1371/journal.pone.0263595.g004
https://doi.org/10.1371/journal.pone.0263595.t002
Factors associated with clinical outcomes
On multivariable analysis, after adjusting for potential confounders (S3 Table in S1 Appendix ), we identified several factors associated with a poorer outcome (i.e. higher mRS score at hospital discharge) ( Table 3 ). For patients with any neurological diagnosis, these were: age (with the odds ratio [OR] up to 15.3 [95% CI 7.7–30.5] with increasing age); pre-existing dementia (OR 2.6 [1.2–5.7]); breathlessness on admission (OR 1.7 [1.1–2.4]); severely elevated initial blood D-dimer concentration (OR 2.5 [1.4–4.6] for >3000ng/mL vs. <500ng/mL); and corticosteroid use during admission (OR 2.8 [1.8–4.3]). For the encephalopathy subgroup, significant factors on multivariable analysis associated with a poor outcome were: age (OR 5.4 [95% CI 1.4–20.7] for 70–79 years; OR 12.2 [2.8–53.0] for ≥80 years); corticosteroid treatment in hospital (OR 3.6 [1.5–8.9]); anticoagulation in hospital (OR 3.1 [1.3–7.4]); and low initial lymphocyte count (OR 0.4 [0.2–0.9] for normal or high lymphocyte count). For patients with cerebrovascular events, the following were significant: age (OR 3.7 [1.2–11.2] for 60–69 years; OR 4.53 [1.59–12.9] for 70–79 years; OR 6.7 [2.2–20.7] for ≥80 years); elevated D-dimer (OR 2.8 [1.3–6.2] for 500-3000ng/mL; OR 3.5 [1.3–9.7] for ≥3000ng/mL); breathlessness on admission (OR 2.8 [1.4–5.5]); and corticosteroid use during admission (OR 4.8 [1.9–11.9]). For patients with cerebrovascular events, being in the WHO African/Eastern Mediterranean region was associated with a poor outcome relative to the WHO European region (OR 4.4 [1.4–14.4]), whereas being in the Southeast Asia/Western Pacific region was associated with a better outcome relative to the European region (OR 0.2 [0.1–0.9]).
https://doi.org/10.1371/journal.pone.0263595.t003
Hazard of death among patients with any neurological disease was found be associated with age, dementia, breathlessness at presentation, corticosteroid use in hospital, WHO Region (higher for all regions compared with Europe) and World Bank income group (higher for low- and lower-middle income countries), following adjustment for confounders in multivariable models ( Table 4 ). For patients with encephalopathy, age, dementia, corticosteroid use in hospital, WHO region and World Bank income group were statistically significant after adjustment for confounders. Adjusted multivariable models for the cerebrovascular patients found a significant association with increased hazard of death and low lymphocyte count, corticosteroid treatment, and WHO region, whereas anticoagulant use in hospital was protective.
https://doi.org/10.1371/journal.pone.0263595.t004
Multivariable regression analysis found a statistically significant association with requiring intensive care for male sex, breathlessness at presentation, pre-existing dementia or diabetes, increased CRP, elevated D-dimer, anticoagulant use, corticosteroid use, WHO region and World Bank income group ( Table 5 ). After fitting models for the encephalopathy subgroup, a statistically significant increased hazard of requiring intensive care was found for age (≥80 years), obesity, dementia, breathlessness, elevated CRP and D-dimer, corticosteroid use, WHO region, and World Bank income group. In the cerebrovascular event subgroup, pre-existing cardiac disease or dementia, corticosteroid treatment in hospital, income group and WHO region were significant after adjustment for confounders.
https://doi.org/10.1371/journal.pone.0263595.t005
Proportion of patients with neurological COVID-19 disease
Eight of the 83 studies included the total number of neurological and other hospitalised COVID-19 patients, admitted over a specified time period, in a comparable way which could be analysed. Five were case series. Fig 5 illustrates that, overall, 7.8% (95% CI 1.6–31.2) of hospitalised COVID-19 patients had neurological disease. The I 2 statistic showed a high degree of statistical heterogeneity among studies (100%). The studies contributing data are summarised in S11 Table in S1 Appendix . When one study, which included patients admitted to community isolation facilities as well as to hospitals, was excluded in a sensitivity analysis (S2 Fig in S1 Appendix ), the pooled percentage of hospitalised COVID-19 patients who had neurological disease was 14.7% (95% CI 4.7–37.8; I 2 98%).
Neurological disease = number of patients with neurological COVID-19 disease. All COVID-19 = number of patients with all COVID-19 disease hospitalised in the same centre over the same time period.
https://doi.org/10.1371/journal.pone.0263595.g005
Since the onset of the COVID-19 pandemic, there has been a plethora of studies reporting associated neurological disease, initially without the use of standardised case definitions, and often still without detailed clinical and diagnostic evaluation, investigation of prognostic markers or clinical outcomes. To some extent, this reflected the difficulties of studying a new highly infectious disease that was swamping health services, plus the desire to publish important information quickly [ 2 ]. Several meta-analyses have now also been published based on these original reports [ 3 – 8 ], but they may not accurately capture the true clinical picture, given the limitations of the original data.
In July 2020, we published standardised case definitions for neurological COVID-19 disease [ 2 ], which included assessment of the strength of evidence for an association, and have been modified and are being adopted by the Global Covid-19 Neuro Research Coalition and the WHO [ 19 ]. We therefore decided to perform an IPD meta-analysis of published and unpublished data from patients admitted to hospital during the first wave of the pandemic, using these case definitions and a standardised data collection tool. To date, no other published meta-analysis has included IPD for multiple pre-defined neurological diagnoses, though one large analysis combined data from two cohorts of patients with neurological COVID-19 and a third with or without neurological disease [ 20 ]. We received data on 1979 patients supplied from 83 studies (including 31 that were originally unpublished). Most previous systematic reviews described symptoms and diagnoses, with some estimating the proportion of COVID-19 patients that develop neurological disease. Here, we concentrated on detailed descriptions of the neurological diseases, their outcomes, and risk factors for a poor prognosis. This latter is especially important for neurologists and other hospital specialists who care for such patients. We also compared WHO regions and World Bank income groups, to initiate thinking about differences in outcomes across the global community.
The neurological syndromes seen most commonly were encephalopathy (49%), including encephalitis, coma, and delirium, and cerebrovascular events (26%), principally ischaemic stroke ( Table 1 ). There were also many patients with smell or taste disturbance (19%), and some with peripheral neuropathy (6%), GBS (3%) and neuropsychiatric disorders (2.5%). The cerebrovascular case definitions worked relatively well in terms of classifying patients. The encephalopathy definitions worked less well with 35% of patients being classed as “encephalopathy other” because they did not fit into the main categories of delirium, coma and encephalitis; although there has been considerable debate on encephalopathy case definitions among the neurology, geriatric, and psychiatric community [ 10 , 21 , 22 ] these results suggest clinicians may be unfamiliar with the definitions, or they may need further revision. Encephalopathy may be precipitated by many different factors, in the context of different diseases, and the spectrum of clinical features of this syndrome can make succinct classification a challenge. Assessment of patients with suspected delirium in our study was performed by clinicians, guided by the variables included in our data collection form; existing tools such as CAM-ICU, 4AT or AMT-4 could also be used to quantify neurocognitive features in more detail. In anticipation of other factors that can impact on conscious level, cognition, and behaviour, we also collected data on brain imaging and use of hypnotic and anxiolytic agents during hospital admission. For most encephalitis patients the aetiological link to SARS-CoV-2 was classed as “probable” or “possible”, because no virus was detected in their CSF. This is in contrast to herpes simplex virus encephalitis where virus is frequently detected, and there is marked inflammatory change on brain imaging or autopsy. Over a year into the pandemic, we now know that virus detection in the CSF is extremely rare, and the case definitions should probably be refined to reflect this, perhaps following the approach for enterovirus 71, which also causes severe brain disease with inflammatory changes despite virus rarely being detected in the CSF [ 23 , 24 ]. Of note, two myelitis patients had virus detected in CSF; we did not have the PCR cycle threshold values from this testing, but given the implications of true confirmed viral myelitis on management, this finding should be rigorously confirmed by by performing PCR for SARS-CoV-2 on CSF of myelitis patients who have concurrent or recent COVID-19, or who present during a pandemic wave.
In previous systematic reviews encephalopathy and cerebrovascular disease were the most commonly reported neurological presentations, though which of these was most important varied [ 25 – 29 ]. This likely reflects differences in study populations and case definitions. Even for the four studies in our analysis that recruited consecutive neurological patients, and where we could apply strict case definitions to the individual patient data, two studies had a predominance of patients with cerebrovascular events and the other two had a majority of patients with encephalopathy (S10 Table in S1 Appendix ). These differences may stem from varying approaches to screening for neurological symptoms and inclusion of hospitalised COVID-19 patients. In our database overall, we found encephalopathy was reported for about half the patients, and stroke for about a quarter. This is similar to one of the larger prospective series of 606 unselected neurological patients in New York, which found encephalopathy in 50% and stroke in 14% [ 30 ]. Another recent study combining COVID-19 and neurological disease patient registries reported encephalopathy in 49%, and stroke in 6% [ 20 ].
Approximately half of the 1052 patients with neurological COVID-19 disease and a mRS score available had a poor outcome on discharge from hospital, as determined by a mRS score of 3–6 (moderate to severe disability or death; Table 2 ); the proportion was higher in those with cerebrovascular events (76%) than encephalopathy (54%), and this was largely accounted for by those that died (33% versus 17%). Our findings highlight the degree of disability experienced by patients with COVID-19 and neurological disease; a recent report of hospitalised UK patients in the UK ISARIC-4C study found that functional outcomes are worse in those with neurological complications compared to those with other severe but non-neurological complications of COVID-19 [ 31 ]. In another study, the adjusted odds ratio of in-hospital death was 5.99 (95%CI: 4.33–8.28) for those with any neurological signs and/or syndromes compared to those without, though the odds ratio was greater for encephalopathy than stroke [ 20 ].
The mRS was devised for stroke and although it is not particularly reliable for brain injuries that result in cognitive disability [ 32 ], it is still widely used in this group. Future studies of neurological disability should use a more generic outcome score such as the Glasgow Outcome Scale which is equally simple to administer and may better capture the impact of neuropsychiatric manifestations [ 33 ]. Clinicians were not blinded to the patients neurological condition at the time of mRS assessment, but the outcome measure was clearly defined. Variable time to discharge (at which point mRS was calculated) may have affected our results, as we did not account for this in our multivariable models.
Overall, 30% of the 1745 neurological patients with outcome information available had died by 30 days, which is higher than the mortality of around 25% reported by meta-analyses of all hospitalised COVID-19 patients from North America, Europe, and China [ 34 , 35 ]. Our higher mortality rate is in keeping with the report of the ISARIC-4C study, which found patients with neurological COVID-19 complications (specifically meningitis, encephalitis, seizure, or stroke) had an increased hazard of mortality [ 31 ]. A previous systematic review of patients with neurological disease reported a lower mortality of 10%, but this study may have included non-hospitalised patients with neurological symptoms such as headache [ 3 ]. Nearly 40% of our patients needed intensive care (higher for those with a cerebrovascular event than for the encephalopathic patients). No previous systematic reviews of neurological COVID-19 patients have meta-analysed for these outcomes, though in the ISARIC-4C study, 22% of those with neurological complications were admitted to critical care compared with 14% of patients overall [ 31 ]. Approximately half of our neurological COVID-19 patients who required intensive care support still needed this at 30 days. Whilst we did not have a control group, this is considerably longer than what has been reported in published studies for all COVID-19 patients in intensive care: 12 days in one large UK cohort study [ 36 ], and 8 days in a meta-analysis [ 37 ]. 45% of our patients were still in hospital at 30 days. While previous systematic reviews of neurological COVID-19 do not report this, our estimate appears longer than studies reporting on all hospitalised COVID-19 patients: median length of stay was 12 days for one study of 1321 patients in France [ 38 ], and 8 days for 2005 patients in Germany [ 39 ] Collectively our results on the need for and duration of intensive care, length of hospital stay, and patient outcomes underscore the significant burden of neurological COVID-19 disease on health care resources, compared with COVID-19 disease as a whole. Post-acute COVID-19 neurological symptoms and outcomes are also an emerging and important issue, though longer-term data were not available for us to investigate this [ 40 ].
We found age, and markers of disease severity including breathlessness and elevated D-dimer were associated with a poor outcome among all patients with neurological disease ( Table 3 ). These same factors were also important for the subgroup with cerebrovascular events, but not those with encephalopathy. This is in keeping with an earlier report from the ISARIC-4C study indicating that patients presenting with encephalopathy, with or without typical COVID-19 symptoms, had a higher mortality [ 41 ]. Low initial lymphocyte count was associated with poor outcome in our encephalopathy patients, as has been shown in a meta-analysis of over 10,000 patients with COVID-19 [ 42 ]. Other biomarkers shown to be important in COVID-19 generally, such as neutrophil and platelet counts, were not available consistently for our patients [ 43 ].
Corticosteroid use in hospital was associated with a worse outcome in all neurological patients, as well as the cerebrovascular and encephalopathic subgroups ( Table 3 ). This is likely to be because clinicians were more inclined to use corticosteroids in these patients with severe disease. Anticoagulation use in hospital was also associated with a worse outcome in the encephalopathic patients, but intriguingly it was associated with a lower hazard of death in those with cerebrovascular events ( Table 4 ), suggesting it may be beneficial in these patients. Further work is needed to understand the role of anticoagulation in COVID-19 patients with stroke. While these variables might have been susceptible to immortal time bias, this is unlikely to have influenced the results significantly, as these drugs are usually started at admission.
Although international comparison was not the primary aim of our study, we could begin to explore differences in outcomes between different WHO regions, and World Bank income groups. We found that compared with neurological COVID-19 patients in the WHO European region, those in other regions had a higher hazard ratio for death ( Table 4 ); the hazard ratio was also higher for patients from low- and lower-middle-income countries compared to high-income countries (HICs), though with wide confidence intervals, reflecting fewer patients in the lower-income category. Differences in mRS scores were also seen across WHO regions ( Table 3 ), but only in patients with cerebrovascular disease and again with wide confidence intervals. Although these are only preliminary data, these differences may reflect broader public health approaches and capacities in different countries [ 44 ]. Further larger-scale studies including LMICs are needed to investigate these potential findings.
Although we applied standard case definitions (S2 Appendix, Section 3) and eligibility criteria to our IPD database, the original studies or case series had been conducted using different protocols, and many were small and did not capture all patients with neurological disease, potentially leading to selection bias. However, 47% of our patients were from cross-sectional and cohort studies, and the case series scored highly across several quality assessment domains. Our novel approach of capturing unpublished data through the Global Covid-Neuro Network meant we included patients, especially from LMICs, that would not otherwise have ever been included in a publication, thus improving accessibility and equity (S4 Table in S1 Appendix ). Despite this, just 6 (7%) of 83 studies providing 42 (2%) of the total 1979 patients were from low- or lower-middle income countries. Only 2% of our patients were children. This may reflect some degree of residual selection bias, despite not relying upon published literature. Finally, despite using multivariable analyses with pre-defined exposures and confounders, associations do not equate to causation; determining these would require further research.
Conclusions
We have shown that encephalopathy and stroke are the most commonly reported neurological manifestations of COVID-19, with the latter group having a worse outcome, as judged by the mRS. Nearly 40% of patients needed intensive care, and the burden in terms of prolonged intensive care and hospital stay was higher than for other hospitalised COVID-19 patients. Markers of disease severity such as breathlessness and elevated D-dimer were associated with poor outcome in the cerebrovascular event, but not the encephalopathic, patients, suggesting different disease mechanisms. For one third of the patients, the neurological symptoms started after hospital admission, providing a potential window for intervention if risk factors and neurological disease mechanisms were better understood. Prospective case-control studies across multiple WHO regions are needed to better understand the factors leading to neurological COVID-19 and point to potential interventions.
Supporting information
S1 checklist. prisma-ipd checklist..
https://doi.org/10.1371/journal.pone.0263595.s001
S1 Appendix. Author list, supplementary figures and tables.
https://doi.org/10.1371/journal.pone.0263595.s002
S2 Appendix. Data extraction tool and case definitions.
https://doi.org/10.1371/journal.pone.0263595.s003
Acknowledgments
Please see the S1 Appendix for details of the Group Authors, members of The Brain Infections Global COVID-Neuro Network. We acknowledge invaluable administrative support from Ms Clare Fotheringham in the Institute of Infection, Veterinary & Ecological Sciences and the Research Contracts Team at the University of Liverpool.
- View Article
- PubMed/NCBI
- Google Scholar
- 12. WHO. Coronavirus disease 2019 (COVID-19) Situation Report– 76. Geneva: World Health Organization; 2020.
- 13. Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, et al. Chapter 7: Systematic reviews of etiology and risk. Aromataris E, Z M, editors: Joanna Briggs Institute; 2017.
- 15. Wells G, Shea B, O’Connell D, Peterson j, Welch V, Losos M, et al. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 31. Drake TM RA, Fairfield CJ, et al, on behalf of the ISARIC4C investigators. Characterisation of in-hospital complications associated with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol UK: A prospective, multicentre cohort study2021 April 5, 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/962826/s1081-in-hospital-complications-isaric.pdf
- 43. Wijeratne T, Sales C, Karimi L, Jakovljevic M. Elevated Neutrophil to Lymphocyte Ratio Predicts In-hospital Mortality Among Stroke Patients in a Metropolitan hospital in Australia, Universal Value-added measure in Stroke Care. medRxiv [Preprint]. 2021 [posted 2021 Mar 3]. https://www.medrxiv.org/content/10.1101/2021.03.01.21252317v1
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Wiley - PMC COVID-19 Collection

Incidence and risk factors for persistent symptoms in adults previously hospitalized for COVID‐19
Daniel munblit.
1 Department of Paediatrics and Paediatric Infectious Diseases, Institute of Child’s Health, Sechenov First Moscow State Medical University (Sechenov University), Moscow Russia
2 Inflammation, Repair and Development Section, Faculty of Medicine, National Heart and Lung Institute, Imperial College London, London UK
3 Research and Clinical Center for Neuropsychiatry, Moscow Russia
Polina Bobkova
Ekaterina spiridonova, anastasia shikhaleva, aysylu gamirova, oleg blyuss.
4 School of Physics, Astronomy and Mathematics, University of Hertfordshire, Hatfield UK
Nikita Nekliudov
Polina bugaeva, margarita andreeva, audrey dunngalvin.
5 School of Applied Psychology, University College Cork, Cork City Ireland
Pasquale Comberiati
6 Department of Clinical and Experimental Medicine, Section of Pediatrics, University of Pisa, Pisa Italy
Christian Apfelbacher
7 Institute of Social Medicine and Health Systems Research, Faculty of Medicine, Otto von Guericke University Magdeburg, Magdeburg Germany
Jon Genuneit
8 Pediatric Epidemiology, Department of Pediatrics, Medical Faculty, Leipzig University, Leipzig Germany
Sergey Avdeev
9 Clinic of Pulmonology, Sechenov First Moscow State Medical University (Sechenov University), Moscow Russia
Valentina Kapustina
10 Department of Internal Medicine №1, Institute of Clinical Medicine, Sechenov First Moscow State Medical University (Sechenov University), Moscow Russia
Alla Guekht
Victor fomin.
11 Sechenov First Moscow State Medical University (Sechenov University), Moscow Russia
Andrey A. Svistunov
Peter timashev.
12 Institute for Regenerative Medicine, Sechenov First Moscow State Medical University (Sechenov University, Moscow Russia
Vladislav S. Subbot
13 Department of Oncology, Radiotherapy and Plastic Surgery, University Clinical Hospital No 1, Sechenov First Moscow State Medical University (Sechenov University), Moscow Russia
Valery V. Royuk
14 N.A. Semashko Department of Public Health and Healthcare, Sechenov First Moscow State Medical University (Sechenov University), Moscow Russia
Thomas M. Drake
15 Centre for Medical Informatics, University of Edinburgh, Edinburgh UK
Sarah Wulf Hanson
16 Institute for Health Metrics and Evaluation, University of Washington, Seattle USA
Laura Merson
17 Nuffield Department of Medicine, ISARIC Global Support Centre, University of Oxford, Oxford UK
Gail Carson
Peter horby, louise sigfrid, janet t. scott.
18 MRC‐University of Glasgow Centre for Virus Research, Glasgow UK
Malcolm G. Semple
19 Health Protection Research Unit in Emerging and Zoonotic Infections, Institute of Infection, Veterinary and Ecological Sciences, Faculty of Health and Life Sciences, University of Liverpool, Liverpool UK
20 Department of Respiratory Medicine, Alder Hey Children's Hospital, Liverpool UK
John O. Warner
Piero olliaro, petr glybochko, denis butnaru, associated data.
The data that support the findings of this study are available from the corresponding author, DM, upon reasonable request.
The long‐term sequalae of COVID‐19 remain poorly characterized. We assessed persistent symptoms in previously hospitalized patients with COVID‐19 and assessed potential risk factors.
Data were collected from patients discharged from 4 hospitals in Moscow, Russia between 8 April and 10 July 2020. Participants were interviewed via telephone using an ISARIC Long‐term Follow‐up Study questionnaire.
2,649 of 4755 (56%) discharged patients were successfully evaluated, at median 218 (IQR 200, 236) days post‐discharge. COVID‐19 diagnosis was clinical in 1291 and molecular in 1358. Most cases were mild, but 902 (34%) required supplemental oxygen and 68 (2.6%) needed ventilatory support. Median age was 56 years (IQR 46, 66) and 1,353 (51.1%) were women. Persistent symptoms were reported by 1247 (47.1%) participants, with fatigue (21.2%), shortness of breath (14.5%) and forgetfulness (9.1%) the most common symptoms and chronic fatigue (25%) and respiratory (17.2%) the most common symptom categories. Female sex was associated with any persistent symptom category OR 1.83 (95% CI 1.55 to 2.17) with association being strongest for dermatological (3.26, 2.36 to 4.57) symptoms. Asthma and chronic pulmonary disease were not associated with persistent symptoms overall, but asthma was associated with neurological (1.95, 1.25 to 2.98) and mood and behavioural changes (2.02, 1.24 to 3.18), and chronic pulmonary disease was associated with chronic fatigue (1.68, 1.21 to 2.32).
Conclusions
Almost half of adults admitted to hospital due to COVID‐19 reported persistent symptoms 6 to 8 months after discharge. Fatigue and respiratory symptoms were most common, and female sex was associated with persistent symptoms.
Word cloud showing persistent symptoms 6–8 months since hospital discharge in people previously hospitalised with COVID‐19.

KEY MESSAGES
- 6–8 months after hospital discharge, around a half of patients with Covid‐19 experienced persistent symptoms
- Chronic fatigue and respiratory problems were the commonest persistent symptoms, with 11.3% having multisystem involvement
- Female sex was associated with higher risk of persistent symptoms
1. INTRODUCTION
The emergence of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has placed a significant burden on health services and society worldwide. There have now been well over 100 million coronavirus disease 2019 (COVID‐19) cases reported with a mortality rate of around 2.2%globally. 1 The acute presentation of COVID‐19 has now been well investigated, with fever, cough, shortness of breath and anosmia among the most commonly reported symptoms. 2 , 3 , 4
It has become evident that a substantial proportion of people experience ongoing symptoms including fatigue and muscle weakness, joint and muscle pain, and breathlessness, months after the acute phase of COVID‐19. 5 , 6 , 7 This phenomenon is now commonly referred to as Long COVID but has also been described as post‐COVID syndrome, Post‐Acute Sequelae of SARS‐CoV‐2 infection (PASC), the post‐COVID‐19 condition 8 or patients have been labelled COVID long‐haulers. 9 , 10 There is still a paucity of long‐term follow‐up data, which means we have limited knowledge of the full range of symptoms, duration of disease and potential risk factors. Recently published data from China describing long‐term consequences of COVID‐19 show that 76% of previously hospitalized adult patients have at least one symptom 6 months after acute infection. 6 In a UK registry study of 47,780 previously hospitalized adults, 29.4% were readmitted and 12.3% died after initial discharge with multi‐organ dysfunction. 11
There is an urgent need for accurate long‐term follow‐up of COVID‐19 patients, 7 to inform future management plans and address the devastating impacts of this condition on the quality of life (QoL) of people affected. This observational cohort study aimed to investigate the incidence of long‐term consequences in adults previously hospitalized for COVID‐19 and to assess risk factors for Long COVID in Moscow, Russia. We used the standardized follow‐up data collection protocol of the International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC).
2.1. Study design, setting and participants
This is a longitudinal cohort study of patients with suspected or confirmed COVID‐19 infection admitted to Sechenov University Hospital Network (four tertiary hospitals) in Moscow, Russia. We collected the follow‐up data between 2 December 2020 and 14 January 2021 from patients discharged between 8 April 2020 and July, 2020. We included adult patients (≥18 years of age), with either reverse transcriptase polymerase chain reaction (RT‐PCR) confirmed SARS‐CoV‐2 infection and clinically confirmed infection, when the laboratory testing result is negative, inconclusive or unavailable.
The acute phase data, including comorbidities and disease severity, were extracted from electronic medical records (EMR) and the Local Health Information System (HIS) at the host institution using the modified and translated ISARIC WHO Clinical Characterisation protocol (CCP). 12 Details of the acute phase data collection are described elsewhere. 3 The study was approved by the Sechenov University Local Ethics Committee on 22 April 2020 (protocol number 08–20). A protocol amendment enabling serial follow‐up of the cohort was approved on 13 November 2020.
Information about the current condition and persistent symptoms was collected by telephone using the Tier 1 ISARIC Long‐term Follow‐up Study case report form (CRF) developed by the ISARIC Global COVID‐19 follow‐up working group, translated into Russian assessing the patients’ physical and mental health 9 ( Supplementary material ). Additional information was added from the WHO CRF for Post COVID conditions. 13 The participants were asked to report on dyspnoea, QoL and difficulties in functioning before the COVID‐19 illness and at the time of the interview. We used the British Medical Research Council (MRC) dyspnoea scale, the EuroQoL five‐dimension five‐level (EQ‐5D‐5L) questionnaire, the EuroQoL Visual Analogue Scale (EQ‐VAS) asking participants to score their QoL from 0 (worst imaginable health) to 100 (best imaginable health), UNICEF/Washington disability score and World Health Organisation Disability Assessment Schedule (WHODAS 2.0). The study was registered with EuroQoL as part of the ISARIC collaborative effort (EuroQoL ID 37035).
Data collection and entry were performed by a team of medical students who underwent training in basic data entry into REDCap and telephone interviews. Students have already had extensive data extraction experience gained from the previous research 3 and were supervised by senior academic staff members.
The research team members attempted to contact patients three times before declaring them lost to follow up. If available by telephone, the patients were asked to provide their verbal consent to the interview.
2.2. Data management
We used REDCap electronic data capture tools (Vanderbilt University, Nashville, TN, USA) hosted at Sechenov University and Microsoft Excel (Microsoft Corp) for data collection, storage and management. 11 , 12 The baseline characteristics, including demographics, symptoms on admission and comorbidities, had been extracted from EMRs and entered into REDCap previously.
2.3. Definitions
The acute disease severity was stratified in accordance with Arnold et al. 10 by a three‐category scale based on the degree of required supportive care during hospital stay: mild (no supplementary oxygen or intensive care), moderate (supplementary oxygen during hospitalization) and severe (need for non‐invasive respiratory modalities (NIV), invasive mechanical ventilation (IMV) and/or admission to intensive care unit (ICU)). A difference of 10 points at EQ‐VAS defined relevant change in the health status. 4
All comorbidities were reported by the patients and/or family members at the time of the hospital admission and subsequently double checked during the follow‐up telephone interview.
For the purpose of this study, we defined “persistent symptoms” (PS) as symptoms present since hospital discharge only.
PS present at the time of follow‐up were categorized into respiratory, gastrointestinal, dermatological, chronic fatigue, neurological, mood and behaviour, sensory (Table S1 ). Symptom categorization was based on previously published literature 14 , 15 and international expert group discussions.
2.4. Statistical analysis
Descriptive statistics were calculated for baseline characteristics. Continuous variables were summarized as median (with interquartile range) and categorical variables as frequency (percentage). The chi‐squared test or Fisher's exact test was used for testing differences in proportions between groups. The Wilcoxon rank‐sum test was used for testing the hypotheses about differences in means between the groups.
We performed multivariable logistic regression to investigate associations of demographic characteristics, comorbidities and severity of acute phase COVID‐19 with PS categories presence at the time of the follow‐up interview. To enhance the robustness of the effect estimates, only comorbidities that were present in at least 3% of the cohort were included in the modelling. Primary analysis was performed using the full data set, whereas sensitivity analysis included only a subset of people with RT‐PCR confirmed SARS‐CoV‐2 infection (ICD U07.1). We have previously found no significant differences in clinical signs, symptoms, laboratory test results and risk factors for in‐hospital mortality between clinically diagnosed patients and patients with positive RT‐PCR. 3 Therefore, primary analysis was performed using the full cohort. Robustness of findings was then investigated via sensitivity analysis which included only a subset with confirmed SARS‐CoV‐2 infection. We have not performed any imputation for missing data.
Venn diagrams were used to present the coexistence of the five most common persistent symptoms.
Two‐sided p‐values were reported for all statistical tests, a p‐value below 0.05 was considered to be statistically significant. Statistical analysis was performed using R version 3.5.1.
3.1. Description of study population
As outlined in Figure Figure1, 1 , out of 5,040 patients hospitalized with suspected COVID‐19 to the hospitals before 10 July 2020, 4,755 were discharged alive or transferred to another facility. Out of 4,019 patients with accurate contact information available, 2,649 were available for follow‐up (response rate 68.5%), 2,649 of whom had no missing baseline data in the electronic database and were included in the analysis. Of the 3,868 patients with contact information available 52 (1.3%) died after the hospital discharge.

Flow diagram of patients with COVID‐19 admitted to Sechenov University Hospital Network between April 8 and July 10, 2020. PCR, polymerase chain reaction
Analysis of the non‐response data was performed and Table S2 summarizes the differences between respondents and non‐respondents. A higher number of severe patients were among non‐respondents (4.2%) when compared with respondents (2.6%), while more individuals with asthma, type 2 diabetes and rheumatologic disorder were among respondents.
Out of 2,649 participants, 1,358 patients (51.3%) had RT‐PCR‐confirmed SARS‐CoV‐2 infection, whereas 1291 (48.7%) were clinically diagnosed with COVID‐19. In‐hospital case fatality ratio was 167/2674 (6.2%) in laboratory‐confirmed and 118/2360 (5%) in clinically diagnosed patients ( p = .09). The median age was 56 years (IQR, 46–66; range, 18–100 years), and 1,353 (51.1%) were women. Median follow‐up time post‐discharge was 217.5 days (IQR 200.4–235.5, range 18–100). 1,948 participants (77.4%) had a higher education; 1,531, (59.8%) of the participants were working part‐ or full‐time; and 830 (32.4%) were retired (Table 1 ).
Demographic characteristics of patients admitted to the Sechenov University Hospital Network
Data are n (%), n / N (%), or median (IQR). Statistically significant results ( p < .05) are highlighted in bold.
The most common pre‐existing comorbidity on admission was hypertension (1,219, 46.2%), followed by obesity (514, 19.6%) and type II diabetes (369, 14.1%). Most of the patients had mild COVID‐19 (1,637, 63.2%), with 902 (34%) classified as moderate and 68 (2.6%) as severe, respectively.
3.2. Symptoms at the time of follow‐up
At the time of the follow‐up interview, 1115 (42.1%) of the participants reported no symptoms, 444 (16.8%) reported one, 313 (11.8%) two and 777 (29.3%) three or more symptoms, with fatigue, shortness of breath, and forgetfulness being the most common. Just under half (1247;47.1%) reported one or more PS. Fatigue 551/2599 (21.2%), breathlessness 378/2614 (14.5%), forgetfulness 237/2597 (9.1%), muscle weakness 199/2592 (7.7%), problems seeing 198/2598 (7.6%), hair loss 183/2580 (7.1%) and problems sleeping 180/2583 (7%) were the most common PS reported at follow‐up. Detailed information on all the symptoms, including duration, is presented in Table S3 .
Although many patients had PS since discharge, some participants reported at least one symptom of a differing duration during follow‐up interview; 285 (10.8%) had experienced these symptoms for 3 to 6 months, 179 (6.8%) between 2 and 3 months, 157 (5.9%) between 1 and 2 months, 103 (3.9%) between 2 and 4 weeks and 140 (5.3%) between 1 and 2 weeks, respectively. The duration of the ten common symptoms at the time of the follow‐up is shown in Figure S1 .
A degree of overlap was found between the five most common PS, with 79/900 (8.8%) of patients experiencing both persistent fatigue and breathlessness, 54 (6%) persistent fatigue and muscle weakness. A smaller proportion of patients reported a combination of persistent fatigue, breathlessness and muscle weakness ‐ 26/900 (2.9%) with 16 (1.8%) patients having all five (Figure (Figure2 2 ).

Venn plot presenting coexistence of (A) five most common persistent symptoms and (B) five most common categories of persistent symptoms at the time of the follow‐up interview
3.3. Persistent symptom categories at the time of follow‐up
With regard to categories of PS, chronic fatigue was found to be the most common 658/2593 (25%) at the time of the follow‐up interview, followed by respiratory 451/2616 (17.2%), neurological 375/2586 (14.5%), mood and behaviour changes 284/2591 (11%) and dermatological 206/2583 (8%) symptoms. A smaller number of patients experienced gastrointestinal 110/2599 (4.2%) and sensory 70/2622 (2.7%) problems since discharge.
A small number of the PS categories were co‐existent: 174 (6.6%) participants reported PS from three different categories at the time of the follow‐up interview;88 (3.3%) reported four categories, and 37 (1.4%) reported five categories or more. Co‐existence of five most common categories of persistent symptoms at the time of the follow‐up interview is presented in the Figure Figure2 2 .
3.4. Risk factors associated with persistent symptom categories
Risk factors for all categories were assessed. In multivariable regression analysis, female sex was a predictor of “any” PS category with an odds ratio of 1.83 (95% confidence interval 1.55 to 2.17), chronic fatigue 1.67 (1.39 to 2.02), neurological (2.03, 1.60 to 2.58), mood and behaviour (1.83, 1.41 to 2.40), dermatological (3.26, 2.36 to 4.57), gastrointestinal (2.50, 1.64 to 3.89), sensory (1.73, 2.06 to 2.89) and respiratory (1.31, 1.06 to 1.62) PS categories, respectively. The effect of female sex remained unchanged in the sensitivity analyses, which included patients with RT‐PCR‐confirmed COVID‐19 only, for all categories except respiratory and sensory. Pre‐existing asthma was not associated with “any” PS category, but was consistently associated with neurological (1.95, 1.25 to 2.98) and mood and behavioural changes (2.02, 1.24 to 3.18) (Figures (Figures3 3 and and4), 4 ), with associations remaining significant in the sensitivity analyses. Chronic pulmonary disease was associated with “any” PS category (1.47, 1.08 to 1.99), chronic fatigue (1.68, 1.21 to 2.32) (Figure (Figure5) 5 ) and gastrointestinal (1.93, 1.02 to 3.43) PS categories development. However, an association with “any” and gastrointestinal symptoms was not confirmed in the sensitivity analysis. Rheumatological disorder was associated with the mood and behavioural PS (1.97, 1.12–3.33), but the effect was not confirmed in the sensitivity analysis. Confirmed RT‐PCR during acute phase was significantly associated with chronic fatigue, neurological, mood and behaviour and gastrointestinal categories, confirming importance of the sensitivity analyses. More details of primary and sensitivity analyses are presented in Table Table2, 2 , and forest plots are available as supplementary material .

Multivariable logistic regression model. Odds ratios and 95% CIs for “Neurological” category of persistent symptoms at the time of follow‐up. Abbreviation: CI, confidence interval. (A) primary analysis (age, sex, comorbidities, severity and RT‐PCR were included as potential risk factors); (B) sensitivity analysis (performed in a subgroup of RT‐PCR positive patients only)

Multivariable logistic regression model. Odds ratios and 95% CIs for “Mood and behaviour” category of persistent symptoms at the time of follow‐up. Abbreviation: CI, confidence interval. (A) primary analysis (age, sex, comorbidities, severity and RT‐PCR were included as potential risk factors); (B) sensitivity analysis (performed in a subgroup of RT‐PCR positive patients only)

Multivariable logistic regression model. Odds ratios and 95% CIs for “Chronic fatigue” category of persistent symptoms at the time of follow‐up. Abbreviation: CI, confidence interval. (A) primary analysis (age, sex, comorbidities, severity and RT‐PCR were included as potential risk factors); (B) sensitivity analysis (performed in a subgroup of RT‐PCR positive patients only)
Risk factors significantly associated with the different categories of persistent symptoms in the primary (age, sex, comorbidities, severity and RT‐PCR were included as potential risk factors) and sensitivity (performed in a subgroup of RT‐PCR positive patients only) multivariable regression analyses
Abbreviations: OR, odds ratio; CI, confidence interval; NA, not applicable; RT‐PCR “+,” real‐time polymerase chain reaction confirmed SARS‐CoV‐2 infection.
3.5. Dyspnoea scale and health state
Dyspnoea of different severity was reported by 318 (12%) patients during follow‐up with 194 (7.3%) equivalent to grade 3, 93 (3.5%) grade 4 and 31 (1.2%) grade 5 according to MRC Dyspnoea Scale (Table S4 ).
Participants reported lower scores (poorer health state) on the EuroQol visual analog scale at follow‐up compared with pre‐COVID‐19 onset, median 80 (IQR, 65–90) vs 85 (70–95) ( p < .001). Significant worsening of the health state compared with pre‐COVID‐19 was found across all symptom categories, with the highest median difference reported by patients with gastrointestinal (−15), mood and behaviour (−13) and neurological (−10.5) symptoms ( p < .001 for all) (Table S5 ). No statistically significant reduction in health state was found among patients reporting no symptoms. Participants falling into all symptom categories had significantly lower health state than those with no symptoms ( p < .001 for all).
4. DISCUSSION
This prospective cohort study with a large sample size and has to our knowledge one of the longest follow‐up duration, assessing the long‐term health and psycho‐social consequences of COVID‐19 in hospitalized adults. The cohort included a similar number of RT‐PCR‐confirmed COVID‐19 and those who were clinically diagnosed with COVID‐19. The clinical features, chest CT, and blood test results did not differ between test confirmed and clinically diagnosed patients. Clinical outcomes were also identical, as discussed elsewhere. 3 Patients were admitted to the hospitals during the first wave of the pandemic. At that time, local recommendations allowed for hospitalization of a milder patients than at present. This and much younger age of admitted patients, when compared with other cohorts, 2 may explain that most of the patients had mild‐to‐moderate disease at the time of the acute episode. We found that six of ten patients experienced at least one symptom of any duration 6 to 8 months after hospital discharge and almost a half of the patients reported at least one PS, with chronic fatigue and respiratory problems being the most frequent PS categories. One in ten patients reported multisystem impacts with three or more categories of PS symptoms present at follow‐up. PS were experienced by both sexes, with a higher risk amongst women. Pre‐existing chronic pulmonary disease was associated with chronic fatigue, and asthma with a higher risk of neurological symptoms and mood and behaviour problems.
4.1. Persistent symptoms
Other studies of previously hospitalized and non‐hospitalized COVID‐19 patients reported presence of short‐ and long‐term symptoms. 16 , 17 , 18 The majority of patients in our cohort experienced PS from the time of discharge, with a smaller number developing symptoms months following discharge. Persistent fatigue and breathlessness were the most frequent PS in our cohort, which is consistent with recent report from China. 6 Forgetfulness and vision problems were also common, while problems sleeping was less common (10.2%) compared to rates reported by follow‐up data from China (26%). 6
A novel finding relates to the development of symptoms, that were not present before COVID‐19 infection and/or at the time of discharge, weeks or months since recovery from COVID‐19. To our knowledge, this aspect has not been investigated in previous studies, as most of the cohorts did not collect data on the duration of the symptoms present at follow‐up. Patterns of the symptom development following COVID‐19 should be further investigated in future research.
4.2. Risk factors associated with persistent symptoms
Female sex was significantly associated with an increased risk of PS, regardless of symptom category, reflecting previous findings 6 and digital App 19 studies. Chronic pulmonary disease was a risk factor for the development of chronic fatigue. An association between chronic pulmonary disease and severe acute COVID‐19 was found in many studies, 20 but it has not been previously reported as a risk factor for COVID‐19 sequelae. The presence of chronic pulmonary disease has been previously associated with chronic fatigue syndrome. 21 The pandemic also had a significant adverse impact on care and support for patients with chronic pulmonary conditions, including a reduction in face‐to‐face clinic availability, lack of access to pulmonary rehabilitation sessions and hospital care during an exacerbation due to fear of COVID‐19 exposure. 22 The causality cannot be determined and we are unable to conclude if lack of follow‐up and involvement in rehabilitation programmes for chronic pulmonary conditions was the cause of ongoing symptoms. Future research should investigate COVID‐19 consequences in this group of patients in greater detail.
Data from the COVID Symptom Study app in the UK suggested that asthma is a risk factor for post‐COVID condition. 23 However, it did not separate ongoing respiratory symptoms which may have been due to incitement of the pre‐existing asthma from those in other systems. We found that asthma was associated with an increased risk of PS during follow‐up, specifically neurological and mood and behaviour. Although asthma has not been associated with a higher risk of hospital admission and/or in‐hospital mortality in COVID‐19 patients, 24 , 25 different results may be found when considering the long‐term consequences of infection. Recent research suggested that COVID‐19 sequelae may be associated with the mast cell activation syndrome 26 and the Th‐2 biased immunological response in asthmatic patients may be responsible for an increased risk of long‐term consequences from the infection. This finding may point to immune‐mediated mechanisms but requires confirmation in a larger sample size with a more detailed investigation, including in‐clinic visits.
4.3. Health state
Patients with all categories of PS reported significantly lower health state when compared with symptom‐free patients. They also considered the health state to be lower than before the COVID‐19 episode. This is consistent with previous reports from different countries. 5 , 6 , 27 This finding points to the multi‐factorial adverse effects of COVID‐19 and to the need for wide ranging and longer term support.
4.4. Strengths and limitations
A major strength of this study is the use of pre‐positioned data collection method using ISARIC Core CRF for acute phase data and ISARIC Long‐term Follow‐up Study CRF. Another strength is the large sample size, and this cohort has the lonest follow‐up assessment of hospitalized adults to date. Stratification to determine whether the symptoms were persistent following COVID‐19 was another novel aspect of the study. At the same time, this cohort study has some limitations. First, the study population only included patients within Moscow, although regional clustering is common to all major cohort studies published during the COVID‐19 pandemic. Second, acute data were collected from the electronic medical records with no access to additional information that could be potentially retrieved from the medical notes. The diagnoses of chronic pulmonary disease and asthma were reported by the patients/carers at the time of the hospital admission and subsequently verified during follow‐up telephone interview. Third, almost half of the patients in our cohort did not have RT‐PCR confirmed COVID‐19 infection, and however, our previous work 3 showed that clinical features of COVID‐19 and in‐hospital mortality were the same in COVID‐19 clinically diagnosed and laboratory‐confirmed cases. We also performed sensitivity analyses using data from the laboratory‐confirmed COVID‐19 patients only to ensure consistency and robustness of the findings. Fifth, some patients may have developed additional comorbidities or complications since the hospital discharge, which were not appropriately captured and could potentially affect the QoL and symptom prevalence and persistence. There is also a risk of recall bias in reporting quality of life and dyspnoea preceding COVID‐19. A third of potentially eligible participants were not enrolled, which is also a limitation, although most characteristics of those successfully interviewed were similar to those who were potentially eligible but not interviewed.
The study used to generate this data within the ISARIC WHO Clinical Characterisation Protocol initiative is a prospective pandemic preparedness protocol which is agnostic to disease and has a pragmatic design to allow recruitment during pandemic conditions. The reality of conducting research in outbreak conditions do not allow for appropriate co‐enrolment of a control group, which is not practical. One of the issues which has not been addressed so far in clinical research is what control group of individuals admitted to hospital during this period when hospitals were overwhelmed with COVID‐19 cases could provide a valid control group. The design of this study allows only to describe the feature of COVID‐19 survivors and cannot involve a control group. COVID‐19 is not just a respiratory tract infection so there is no one‐fit‐all control group.
5. CONCLUSION
At 6‐ to 8‐month follow‐up, many patients had experienced symptoms from the time of hospital discharge, with chronic fatigue and respiratory problems being the most common sequelae. Most patients reported symptoms at 6–8 months commencing from the time of discharge, although a subgroup reported symptoms limited to a few weeks and/or months after the acute phase. One in ten individuals had multisystem involvement at the time of the follow‐up. Female sex was the main risk factor for most of the long‐lasting symptom categories development, while chronic pulmonary disease was associated with a higher risk of chronic fatigue development and asthma with the neurological and mood and behaviour changes. Future studies should focus on patients with multisystem involvement and longer follow‐up of a large sample will allow for a better understanding of COVID‐19 sequelae and help with the phenotype recognition. Investigation of immunological aspects of the association between asthma and several long‐COVID outcomes may identify mechanisms and therapeutic targets for therapy to mitigate adverse consequences.
CONFLICT OF INTEREST
J. Genuneit reports working as a project manager of unrestricted research grants on the composition of breast milk to the Ulm University and Leipzig University. M.G. Semple reports grants from DHSC National Institute of Health Research UK, grants from Medical Research Council UK, grants from Health Protection Research Unit in Emerging & Zoonotic Infections, University of Liverpool outside the submitted work; he also reports a minority ownership at Integrum Scientific LLC, Greensboro, NC, USA outside the submitted work. T. Vos reports personal fees for work on Global Burden of Disease Study from Bill and Melinda Gates Foundation, outside the submitted work. All other authors report no relevant conflict of interests.
AUTHOR CONTRIBUTION
DM, DB, PBo, ES, AS, AG and OB conceptualized the project, formulated research goals and aims. DM, DB, OB, NN, PBu, PC, CA, JG, ADG, AG, TMD, SWH, LM, GC, PH, LS, JTS, MGS, JOW, TV and PO were responsible for the study design and methodology and participated in overall project design discussions. OB and SWH implemented the computer code and supporting algorithms and tested of existing code components. DM and OB tested hypotheses and discussed sensitivity analyses. OB performed statistical analysis. The StopCOVID Research Team, NN, PBu, MA, AG, AS, SA and VK conducted a research and investigation process, specifically performed data extraction, telephone interviews and data collection. VF, AAS, PT, VSS, VVR, DM, DB, PT, PG and SA provided study materials, access to patient data, laboratory data and computing resources. NN, PBu, PBo, ES and OB managed activities to annotate metadata and maintain research data for initial use and later reuse. OB, PBo, ES, AS and AG prepared visualization and worked on the data presentation. DM, DB and PG were responsible for the oversight and leadership for the research activity planning and execution. DM, DB, PBo, ES, AS, AG and OB provided management and coordination for the research activity planning and execution. VF, AAS, PT, VSS, VVR, DM, DB and PG were responsible for the acquisition of the financial support for the project leading to this publication. DM, PBu, NN, OB, JOW, PC and CA wrote original draft. All the authors critically reviewed and commented on the manuscript draft at both, pre‐ and post‐submission stages.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
We thank RFBR, grant 20‐04‐60063 for supporting the work. We would also like to thank UK Embassy in Moscow for providing a grant a grant INT 2021/RSM C 19 01 supporting our project. We are very grateful to the Sechenov University Hospital Network clinical staff and to the patients, carers and families for their kindness and understanding during these difficult times of COVID‐19 pandemic. We would like to express our very great appreciation to ISARIC Global COVID‐19 follow‐up working group for the survey development. We would like to thank Mr Maksim Kholopov for providing technical support in data collection and database administration. We are very thankful to Eat & Talk, Luch, Black Market, FLIP and Academia for providing us the work space in time of need. We are grateful to Ms Asmik Avagyan, Ms Daria Belykh, Ms Ekaterina Belyakova, Ms Anna Berbenyuk, Mr Dmitry Eliseev, Ms Mariia Grosheva, Ms Nelli Khusainova, Ms Maria Kislova, Ms Valeria Klishina, Ms Karina Kovygina, Ms Natalia Kogut, Ms Yana Kohanovskaya, Ms Anastasia Kuznetsova, Ms Elza Lidzhieva, Ms Nadezhda Markina, Mr Georgiy Novoselov, Ms Anna Pushkareva, Ms Olga Romanova, Ms Maria Shoshorina, Ms Jasmin Sibkhan, Ms Olga Spasskaya, Ms Anna Surkova, Ms Nailya Urmantaeva, Ms Ekaterina Varlamova, Ms Margarita Yegiyan, Ms Margarita Zaikina, Ms Anastasia Zorina, Ms Elena Zuikova, Prof Natalia V. Chichkova, Dr Anna V. Buchneva and Prof Natalya Serova for assistance in data extraction, document translation and help during the project. Finally, we would like to extend our gratitude to the Global ISARIC team and ISARIC Co‐ordinating Centre for their continuous support, expertise and for the development of the outbreak ready standardized protocols for the data collection.
Sechenov Stop COVID Research Team (Group authors)
Elina Abdeeva, 1 Nikol Alekseeva, 1 Elena Antsiferova, 1 Elena Artigas, 1 Anastasiia Bairashevskaia, 1 Anna Belkina, 1 Vadim Bezrukov, 1 Semyon Bordyugov, 1 Maria Bratukhina, 1 Jessica Chen, 2 Salima Deunezhewa, 1 Khalisa Elifkhanova, 1 Anastasia Ezhova, 1 Yulia Filippova, 1 Aleksandra Frolova, 1 Julia Ganieva, 1 Anastasia Gorina, 1 Yulia Kalan, 1 Bogdan Kirillov, 1 Mariia Korgunova, 1 Alexandra Krupina, 1 Anna Kuznetsova, 1 Ekaterina Listovskaia, 1 Margarita Mikheeva, 1 Aigun Mursalova, 1 Marina Ogandzhanova, 1 Callum Parr, 2 Mikhail Rumyantsev, 1 Denis Smirnov, 1 Nataliya Shishkina, 1 Yasmin El‐Taravi, 1 Maria Varaksina, 1 Maria Vodianova, 1 Anna Zezyulina 1
1 Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russia
2 Inflammation, Repair and Development Section, National Heart and Lung Institute, Faculty of Medicine, Imperial College London, London, UK
Munblit D, Bobkova P, Spiridonova E, et al. Incidence and risk factors for persistent symptoms in adults previously hospitalized for COVID‐19 . Clin Exp Allergy . 2021; 51 :1107–1120. 10.1111/cea.13997 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
Daniel Munblit, Polina Bobkova, Ekaterina Spiridonova, Anastasia Shikhaleva, Aysylu Gamirova, and Oleg Blyuss contributed equally to the paper.
Sechenov Stop COVID Research Team (Group authors) are present in Appendix.
Contributor Information
Sechenov StopCOVID Research Team: Elina Abdeeva , Nikol Alekseeva , Elena Antsiferova , Elena Artigas , Anastasiia Bairashevskaia , Anna Belkina , Vadim Bezrukov , Semyon Bordyugov , Maria Bratukhina , Jessica Chen , Salima Deunezhewa , Khalisa Elifkhanova , Anastasia Ezhova , Yulia Filippova , Aleksandra Frolova , Julia Ganieva , Anastasia Gorina , Yulia Kalan , Bogdan Kirillov , Mariia Korgunova , Alexandra Krupina , Anna Kuznetsova , Ekaterina Listovskaia , Margarita Mikheeva , Aigun Mursalova , Marina Ogandzhanova , Callum Parr , Mikhail Rumyantsev , Denis Smirnov , Nataliya Shishkina , Yasmin El‐Taravi , Maria Varaksina , Maria Vodianova , and Anna Zezyulina
Data Availability Statement

IMAGES
VIDEO
COMMENTS
The mMRC (Modified Medical Research Council) Dyspnoea Scale is used to assess the degree of baseline functional disability due to dyspnoea. It is useful in characterising baseline dyspnoea in patients with respiratory disease such as COPD. Whilst it moderately correlates with other healthcare-associated morbidity, mortality and quality of life ...
The modified Medical Research Council (mMRC) scale is recommended for conducting assessments of dyspnea and disability and functions as an indicator of exacerbation. The modified Medical Research Council (mMRC) scale. Grade. Description of Breathlessness. Grade 0. I only get breathless with strenuous exercise. Grade 1.
The mMRC dyspnea scale is used to calculate the BODE index, a tool which helps estimate the survival times of people living with COPD. The BODE Index is comprised of a person's body mass index ("B"), airway obstruction ("O"), dyspnea ("D"), and exercise tolerance ("E"). Each of these components is graded on a scale of either 0 to 1 or 0 to 3 ...
Background: In multidimensional Global Initiative for Chronic Obstructive Lung Disease (GOLD) classification, the choice of the symptom assessment instrument (modified Medical Research Council dyspnea scale [mMRC] or COPD assessment test [CAT]) can lead to a different distribution of patients in each quadrant. Considering that physical activities of daily living (PADL) is an important ...
other dyspnea measures, - 0.42 with FEV 1. N/A Grade Description of Breathlessness 0 I only get breathless with strenuous exercise. 1 I get short of breath when hurrying on level ground or walking up a slight hill. 2 On level ground, I walk slower than people of the same age because of breathlessness, or have to
The physical limitation or functional impact of breathlessness can be assessed using the Medical Research Council dyspnea scale (MRC; or modified MRC [mMRC] 39, 40 which is more widely used), 41 Dyspnea Exertion Scale (DES), 42 Oxygen Cost Diagram (OCD), 43 Baseline Dyspnea Index (BDI), 29 or Disability Related to COPD Tool (DIRECT). 44 The ...
The modified Medical Research Council (mMRC) dyspnea scale comprises five statements that describe a range of dyspnea effects in increasing order of severity. Use of this questionnaire is recommended in the GOLD 2020 report 5 ( Supplementary Table 1 ).
The modified Medical Research Council (mMRC) dyspnoea scale is a measure of breathlessness severity recommended by guidelines and utilised as an inclusion criterion or endpoint for clinical trials. No studies have been conducted to validate the categorical descriptors against the dyspnoea severity grade.
Introduction: Health-related quality of life (HRQoL) is an important patient-centred outcome in chronic obstructive pulmonary disease (COPD). The aim of the current study is to compare the discriminative capacity of the modified Medical Research Council (mMRC) dyspnoea scale and the Global Initiative for Chronic Obstructive Lung Disease (GOLD) spirometric classification of COPD on HRQoL, as ...
Background Dyspnea is very frequent in obese subjects. However, its assessment is complex in clinical practice. The modified Medical Research Council scale (mMRC scale) is largely used in the assessment of dyspnea in chronic respiratory diseases, but has not been validated in obesity. The objectives of this study were to evaluate the use of the mMRC scale in the assessment of dyspnea in obese ...
UpToDate is a trusted source of evidence-based medical information for clinicians and patients. This image shows the modified Medical Research Council (mMRC) scale for dyspnea, a simple tool to assess the severity of breathlessness in patients with respiratory diseases.
Calculator: Modified Medical Research Council (mMRC) scale for dyspnea - UpToDate.
Introduction: The modified Medical Research Council (mMRC) dyspnoea scale is a measure of breathlessness severity recommended by guidelines and utilised as an inclusion criterion or endpoint for clinical trials. No studies have been conducted to validate the categorical descriptors against the dyspnoea severity grade. Methods: This study utilised cognitive interviews (Think Aloud method) to ...
The degree of dyspnea and the participants' health status were assessed using modified Medical Research Council dyspnea scale (mMRC) scores and COPD assessment test (CAT) scores, respectively . Acute respiratory exacerbation events/exacerbations of COPD were specifically characterized by the onset or aggravation of at least two of the ...
1. Introduction. Breathlessness is a highly prevalent symptom [1] and a prognostic marker for many respiratory diseases [2, 3].Various scales are used to measure breathlessness severity; the modified Medical Research Council (MRC) dyspnoea scale ("mMRC") measures the effect of breathlessness on daily activities, and is recommended in respiratory guidelines [3] and as a core endpoint in ...
The modified Medical Research Council scale (mMRC scale) is largely used in the assessment of dyspnea in chronic respiratory diseases, but has not been validated in obesity. The objectives of this study were to evaluate the use of the mMRC scale in the assessment of dyspnea in obese subjects and to analyze its relationships with the 6-minute ...
Assessment of dyspnea in COPD patients relies in clinical practice on the modified Medical Research Council (mMRC) scale, whereas the Baseline Dyspnea Index (BDI) is mainly used in clinical trials. Little is known on the correspondence between the two methods.
Change in the degree of dyspnea on the MMRC scale from baseline after 2.5 months and 6 months in patients of the Longidaze® group compared with the dynamic observation group. MMRC scale (Modified Medical Research Council scale) 0 - no - Dyspnea does not bother, except for very intense exercise
We analysed features associated with poor outcome (moderate to severe disability or death, 3 to 6 on the modified Rankin Scale) using multivariable models. ... The study was funded by the UK Medical Research Council's Global Effort on COVID ... Markers of disease severity such as breathlessness and elevated D-dimer were associated with poor ...
We used the British Medical Research Council (MRC) dyspnoea scale, the EuroQoL five‐dimension five‐level (EQ‐5D‐5L) questionnaire, the EuroQoL Visual Analogue Scale (EQ‐VAS) asking participants to score their QoL from 0 (worst imaginable health) to 100 (best imaginable health), UNICEF/Washington disability score and World Health ...
The primary end point in the modified intention-to-treat population was the incidence of an unfavorable outcome, defined as treatment failure or disease relapse (clinical or bacteriologic) at 26 ...