Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals

Cancer immunotherapy articles from across Nature Portfolio
Cancer immunotherapy is a therapy used to treat cancer patients that involves or uses components of the immune system. Some cancer immunotherapies consist of antibodies that bind to, and inhibit the function of, proteins expressed by cancer cells. Other cancer immunotherapies include vaccines and T cell infusions.
Identification of dynamic microbiota signatures in patients with melanoma receiving ICIs: opportunities and challenges
The composition of the gut microbiota has emerged as a tumour-extrinsic factor that modulates response to immune-checkpoint inhibitors (ICIs), although the lack of consistency in microbiota signatures across studies has limited their value as reliable biomarkers. Herein, we discuss a recent study in which longitudinal microbiome profiling identified several taxa that are persistently enriched in patients with melanoma and a favourable response to ICIs.
- Saman Maleki Vareki
- Diwakar Davar
Latest Research and Reviews
An oncolytic virus delivering tumor-irrelevant bystander T cell epitopes induces anti-tumor immunity and potentiates cancer immunotherapy
Ye and colleagues show that an oncolytic virus that delivers tumor-irrelevant bystander T cell epitopes to tumor cells can exploit the abundant population of bystander T cells in the tumor for anti-tumor immunity and potentiate cancer immunotherapy.
- Xiangyu Chen
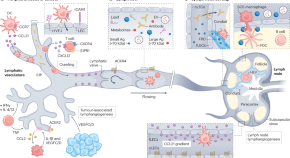
Lymphatic vessels in the age of cancer immunotherapy
Tumour-associated lymphatic growth and remodelling were once viewed as a passive means by which cancer cells could regionally spread to lymph nodes. However, recent data point to an active and contrasting role for lymphatic vessels and their transport in antitumour immune surveillance. In this Review, Karakousi et al. provide a working framework to define this role for the lymphatic system in tumour progression and present avenues for its therapeutic manipulation to improve cancer immunotherapy.
- Triantafyllia Karakousi
- Tenny Mudianto
- Amanda W. Lund
Single-cell sequencing reveals VEGFR as a potential target for CAR-T cell therapy in chordoma
- Huantong Wu
- Xinqiang Li
FOXO1 is a master regulator of memory programming in CAR T cells
The transcription factor FOXO1 has a key role in human T cell memory, and manipulating FOXO1 expression could provide a way to enhance CAR T cell therapies by increasing CAR T cell persistence and antitumour activity.
- Alexander E. Doan
- Katherine P. Mueller
- Evan W. Weber
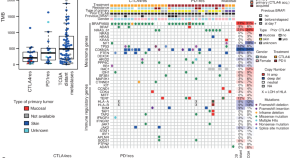
Molecular patterns of resistance to immune checkpoint blockade in melanoma
A large fraction of patients with melanoma still does not benefit from immune checkpoint blockade, associated with both primary and acquired resistance. Here the authors report genetic and immunological patterns of resistance in patients with melanoma after progression on anti-CTLA4 or anti-PD1 monotherapy.
- Martin Lauss
- Bengt Phung
- Göran Jönsson
An AI-based approach for modeling the synergy between radiotherapy and immunotherapy
- Casey Moore
- Robert Timmerman
News and Comment
Pembrolizumab plus chemoradiotherapy effective in locally advanced cervical cancer.
- Peter Sidaway
MEGA CRISPR rejuvenates exhausted CAR T cells
MEGA is a new CRISPR-based RNA-editing platform with the ability to enhance the fitness of CAR T cells; it may also overcome certain limitations of conventional DNA-targeting CRISPR–Cas9 systems.
- Karen O’Leary
Clinical outcomes of patients with lymphoid blastic phase of chronic myeloid leukemia treated with CAR T-cell therapy
Learning from cancer mutations.
- Nicholas J. Bernard
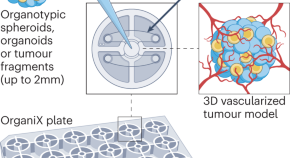
The OrganiX microfluidic system to recreate the complex tumour microenvironment
In this Tools of the Trade article, Giulia Adriani and Andrea Pavesi describe a new microfluidic device that supports the generation of vascularized 3D tumour models.
- Giulia Adriani
- Andrea Pavesi
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Suggestions or feedback?
MIT News | Massachusetts Institute of Technology
- Machine learning
- Social justice
- Black holes
- Classes and programs
Departments
- Aeronautics and Astronautics
- Brain and Cognitive Sciences
- Architecture
- Political Science
- Mechanical Engineering
Centers, Labs, & Programs
- Abdul Latif Jameel Poverty Action Lab (J-PAL)
- Picower Institute for Learning and Memory
- Lincoln Laboratory
- School of Architecture + Planning
- School of Engineering
- School of Humanities, Arts, and Social Sciences
- Sloan School of Management
- School of Science
- MIT Schwarzman College of Computing
New cancer treatment may reawaken the immune system
Press contact :, media download.
*Terms of Use:
Images for download on the MIT News office website are made available to non-commercial entities, press and the general public under a Creative Commons Attribution Non-Commercial No Derivatives license . You may not alter the images provided, other than to crop them to size. A credit line must be used when reproducing images; if one is not provided below, credit the images to "MIT."
Previous image Next image
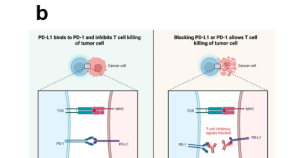
Immunotherapy is a promising strategy to treat cancer by stimulating the body’s own immune system to destroy tumor cells, but it only works for a handful of cancers. MIT researchers have now discovered a new way to jump-start the immune system to attack tumors, which they hope could allow immunotherapy to be used against more types of cancer.
Their novel approach involves removing tumor cells from the body, treating them with chemotherapy drugs, and then placing them back in the tumor. When delivered along with drugs that activate T cells, these injured cancer cells appear to act as a distress signal that spurs the T cells into action.
“When you create cells that have DNA damage but are not killed, under certain conditions those live, injured cells can send a signal that awakens the immune system,” says Michael Yaffe, who is a David H. Koch Professor of Science, the director of the MIT Center for Precision Cancer Medicine, and a member of MIT’s Koch Institute for Integrative Cancer Research.
In mouse studies, the researchers found that this treatment could completely eliminate tumors in nearly half of the mice.
Yaffe and Darrell Irvine, who is the Underwood-Prescott Professor with appointments in MIT’s departments of Biological Engineering and Materials Science and Engineering, and an associate director of the Koch Institute, are the senior authors of the study, which appears today in Science Signaling . MIT postdoc Ganapathy Sriram and Lauren Milling PhD ’21 are the lead authors of the paper.
T cell activation
One class of drugs currently used for cancer immunotherapy is checkpoint blockade inhibitors, which take the brakes off of T cells that have become “exhausted” and unable to attack tumors. These drugs have shown success in treating a few types of cancer but do not work against many others.
Yaffe and his colleagues set out to try to improve the performance of these drugs by combining them with cytotoxic chemotherapy drugs, in hopes that the chemotherapy could help stimulate the immune system to kill tumor cells. This approach is based on a phenomenon known as immunogenic cell death, in which dead or dying tumor cells send signals that attract the immune system’s attention.
Several clinical trials combining chemotherapy and immunotherapy drugs are underway, but little is known so far about the best way to combine these two types of treatment.
The MIT team began by treating cancer cells with several different chemotherapy drugs, at different doses. Twenty-four hours after the treatment, the researchers added dendritic cells to each dish, followed 24 hours later by T cells. Then, they measured how well the T cells were able to kill the cancer cells. To their surprise, they found that most of the chemotherapy drugs didn’t help very much. And those that did help appeared to work best at low doses that didn’t kill many cells.
The researchers later realized why this was so: It wasn’t dead tumor cells that were stimulating the immune system; instead, the critical factor was cells that were injured by chemotherapy but still alive.
“This describes a new concept of immunogenic cell injury rather than immunogenic cell death for cancer treatment,” Yaffe says. “We showed that if you treated tumor cells in a dish, when you injected them back directly into the tumor and gave checkpoint blockade inhibitors, the live, injured cells were the ones that reawaken the immune system.”
The drugs that appear to work best with this approach are drugs that cause DNA damage. The researchers found that when DNA damage occurs in tumor cells, it activates cellular pathways that respond to stress. These pathways send out distress signals that provoke T cells to leap into action and destroy not only those injured cells but any tumor cells nearby.
“Our findings fit perfectly with the concept that ‘danger signals’ within cells can talk to the immune system, a theory pioneered by Polly Matzinger at NIH in the 1990s, though still not universally accepted,” Yaffe says.
Tumor elimination
In studies of mice with melanoma and breast tumors, the researchers showed that this treatment eliminated tumors completely in 40 percent of the mice. Furthermore, when the researchers injected cancer cells into these same mice several months later, their T cells recognized them and destroyed them before they could form new tumors.
The researchers also tried injecting DNA-damaging drugs directly into the tumors, instead of treating cells outside the body, but they found this was not effective because the chemotherapy drugs also harmed T cells and other immune cells near the tumor. Also, injecting the injured cells without checkpoint blockade inhibitors had little effect.
“You have to present something that can act as an immunostimulant, but then you also have to release the preexisting block on the immune cells,” Yaffe says.
Yaffe hopes to test this approach in patients whose tumors have not responded to immunotherapy, but more study is needed first to determine which drugs, and at which doses, would be most beneficial for different types of tumors. The researchers are also further investigating the details of exactly how the injured tumor cells stimulate such a strong T cell response.
The research was funded, in part, by the National Institutes of Health, the Mazumdar-Shaw International Oncology Fellowship, the MIT Center for Precision Cancer Medicine, and the Charles and Marjorie Holloway Foundation.
Share this news article on:
Related links.
- Department of Biology
- Department of Biological Engineering
- Department of Materials Science and Engineering
- Koch Institute
- Ragon Institute
Related Topics
- Biological engineering
Related Articles
Cancer biologists identify new drug combo
A boost for cancer immunotherapy
Fighting cancer with the power of immunity
Previous item Next item
More MIT News

A biomedical engineer pivots from human movement to women’s health
Read full story →

MIT tops among single-campus universities in US patents granted

A new way to detect radiation involving cheap ceramics

A crossroads for computing at MIT

Growing our donated organ supply
New AI method captures uncertainty in medical images
- More news on MIT News homepage →
Massachusetts Institute of Technology 77 Massachusetts Avenue, Cambridge, MA, USA
- Map (opens in new window)
- Events (opens in new window)
- People (opens in new window)
- Careers (opens in new window)
- Accessibility
- Social Media Hub
- MIT on Facebook
- MIT on YouTube
- MIT on Instagram
Advertisement
The new progress in cancer immunotherapy
- Review Article
- Open access
- Published: 15 September 2022
- Volume 23 , pages 553–567, ( 2023 )
Cite this article
You have full access to this open access article
- Ajmeri Sultana Shimu 1 na1 ,
- Hua-xing Wei 2 na1 ,
- Qiangsheng Li 1 ,
- Xucai Zheng 1 &
- Bofeng Li 1
4985 Accesses
6 Citations
1 Altmetric
Explore all metrics
The cross talk between immune and non-immune cells in the tumor microenvironment leads to immunosuppression, which promotes tumor growth and survival. Immunotherapy is an advanced treatment that boosts humoral and cellular immunity rather than using chemotherapy or radiation-based strategy associated with non-specific targets and toxic effects on normal cells. Immune checkpoint inhibitors and T cell-based immunotherapy have already exhibited significant effects against solid tumors and leukemia. Tumor cells that escape immune surveillance create a major obstacle to acquiring an effective immune response in cancer patients. Tremendous progress had been made in recent years on a wide range of innate and adaptive immune checkpoints which play a significant role to prevent tumorigenesis, and might therefore be potential targets to suppress tumor cells growth. This review aimed to summarize the underlying molecular mechanisms of existing immunotherapy approaches including T cell and NK-derived immune checkpoint therapy, as well as other intrinsic and phagocytosis checkpoints. Together, these insights will pave the way for new innate and adaptive immunomodulatory targets for the development of highly effective new therapy in the future.
Similar content being viewed by others

Immunotherapy in Cancer: Immune Checkpoint Inhibitors; Changing Oncology Treatment Paradigm

Immune Checkpoint Therapy: A New Opportunity for Cancer Treatment
Next generation of immune checkpoint therapy in cancer: new developments and challenges.
Julian A. Marin-Acevedo, Bhagirathbhai Dholaria, … Yanyan Lou
Avoid common mistakes on your manuscript.
Introduction
The immune system is made up of a complex network of interconnected mechanisms that work together to provide an adaptive immune reaction against carcinogenesis and infection while maintaining immune tolerance to self-antigens [ 1 ]. On a cellular level, cancer is caused due to the accumulation of numerous genetic alterations that result in the breakdown of normal regulatory systems. Nowadays, immunotherapy is a well-known treatment strategy for cancer that can enhance the existing anti-tumor response so that immune cells can restrict the growth of malignant cells. Again, immunotherapy can effectively target the cancer cells while causing minimal adverse effects [ 2 , 3 ]. Moreover, to provide protective immunity against the tumor cells, a range of successive events take place in order. For example, the discharge of neo-antigens from malignant tumors in the first step is recorded by the dendritic cells [ 4 ]. The second step is known as the processing step where the release of pro-inflammatory cytokines and factors triggers the activation of effector T cells by presenting the dendritic cell-mediated captured antigens to T cells [ 5 ]. Then, the recognition and killing of cancer cells occur through the infiltration of activated T cells such as CD8 + effector T cells after trafficking into the tumor mass. After the death of cancer cells, more tumor-related proteins are secreted to increase the efficiency of successive anti-tumor immunological cycles [ 6 ]. Also, immunogenic cancer cells are killed by effector CD8 + T cells due to the expression of MHC-1 molecules on their cell surface, while NK cells can kill non-immunogenic cancer cells that do not contain MHC-1 molecules. Based on this understanding, cancer vaccines and immune checkpoint therapies are being created to attack immunogenic tumors that produce antigenic peptides to naive CD8 + T cells, whereas transfection of genetically modified T cell treatment can identify tumor cell-derived surface proteins that directly have evolved to target non-immunogenic tumors [ 7 ]. Therefore, it is critical to select an appropriate therapeutic technique depending on the tumor’s features to improve outcomes.
However, tumor progression occurs through the continuous development of an immune-suppressive channel by cancer cells that are mainly responsible for activating a variety of regulatory processes that allow immune evasion [ 8 , 9 ]. Indeed, cancer cells can evade the immune-surveillance pathway by displaying a wide range of co-inhibitory components such as programmed cell death ligand-1/2 (PD-L1/2), cytotoxic T lymphocyte antigen-4 (CTLA4), T cell immune receptor with Ig and ITIM domains (TIGIT), T cell immunoglobulin and mucin-domain containing-3 (TIM-3), and lymphocyte activating antigen-3 (LAG-3) [ 10 ]. However, the proliferation of diverse cell types that express inhibitory chemicals, such as TAMs (tumor-associated macrophages), Tregs (regulatory T cells), and MDSCs (myeloid-derived suppressor cells) also promotes the escape of effective immune response. On the other hand, the response of effector CD4 + and CD8 + T cells can be reduced by the interactions with these co-inhibitory proteins [ 11 , 12 ]. Furthermore, cancer cells can potentially avoid immune response by down-regulating MHC class I expression, resulting in a poor response of effector CD8 + cytotoxic T cells against tumors [ 13 ]. To suppress protective immunity, cancer cells also produce IL-10 (interleukin-10) and different growth factors such as VEGF (vascular endothelial growth factor) and TGF (transforming growth factor) [ 14 , 15 , 16 ].
Recently, numerous immune treatment methods, such as immune checkpoint blockade of co-inhibitory receptors using anti-PD1/PDL1 and anti-CTLA4, dendritic cell vaccines, and cytokine therapy, have been designed and therapeutically used to address tumor cell immune evasion [ 17 ]. Among these methods, ICB has made a breakthrough in developing long-lasting and effective anti-tumor immunity against melanoma, non-squamous non-small cell lung cancer, and metastatic bladder cancer [ 18 , 19 ]. Another type of cancer immunotherapy technique is known as ACT (adoptive cell transfer) which employs T cell receptor (TCR)-modified T cells or tumor-infiltrating T cells (TILs) or genetically engineered chimeric antigen receptor-specific T cells (CAR-T) to treat the cancerous cell more precisely and effectively [ 20 ]. Furthermore, combining effective treatments can improve the performance of immunotherapy, resulting in long-lasting anti-tumor immunomodulatory responses. In cancer immunotherapy, it is critical to understand the potential features of tumor immune-regulatory pathways so that they can provide an efficient immune system response [ 21 , 22 ]. To enhance anti-tumor immune response, the immunotherapy technique exerts the identification and programmed cell death mechanisms of malignant cells. Although immunotherapy is regarded as a promising therapeutic approach to treat cancer patients, there is still a need for successful new treatments because immunotherapy approaches are limited to a subset of cancer types and individuals with minor or no therapeutic benefits. Therefore, this review aimed to briefly elucidate the existing molecular and cellular mechanisms by focusing on how tumor cells escape the immune surveillance on T cells, macrophages, and NK cells, which will then lead to the development of a novel therapeutic approach to promote the anti-tumor immunity within close future.
T cell immune checkpoints-based immunotherapy
Immune checkpoints are important regulatory pathways in the immune system that are triggered by ligand-receptor interactions. Besides, they have a crucial role in the development of an effective immune response to eliminate infectious particles and tumor cells but no response against the self-antigens [ 5 ]. Generally, they can exhibit both co-stimulatory substances such as CD27, CD28, CD137, ICOS, 4-1BB, and OX-40 enact T cells to activate T cells and co-inhibitory molecules like PD-1, CTLA-4 to suppress the activity of T cells. Some of the inhibitory checkpoints are overexpressed within the tumor microenvironment to promote tumor cells-mediated immunosuppression.
To trigger therapeutic anti-tumor immunity, co-inhibitory immune checkpoint molecules can be blocked by antibodies or altered by recombinant forms of ligands or receptors [ 22 ]. The first immune therapeutics approved by the US Food and Drug Administration were antibodies against CTLA-4. Recently, more attention among the many other promising approaches due to its remarkable outcomes has been received [ 23 ]. Besides CTLA-4, now several immune checkpoint receptors (ICRs), such as PD‐1, LAG3, TIM-3, B7‐H3, and diacylglycerol kinase α, have been also identified to treat cancer patients effectively [ 24 , 25 ]. Additionally, the expression of these co-inhibitory receptors has been detected in tumor-infiltrating CD4/CD8 T eff cells and Tregs which are also involved to create tumor evasion [ 26 ]. Therefore, the targeting of CTLA-4 and PD-1 negative immune checkpoints-based immune suppression pathway along with some other important regulatory checkpoints such as Siglec factors and CD47-SIRPα may contribute to the upgrade of immune responses against cancer cells.
CTLA-4, also known as CD152, is the first detected co-inhibitory checkpoint receptor (ICR) that normally expressed on T cells and is linked to the suppression of endogenous anti-tumor immunity mediated by T cells [ 27 , 28 ]. Foxp3 and NFAT (nuclear factor of activated T cells) are the two regulatory factors that play an important role in the transcription of CTLA-4, which controls immunologic tolerance [ 29 ]. CTLA-4 deficiency in FoxP3 + cells impairs the inhibitory functions of Treg cells which are considered the biggest obstacle in immunotherapy targets due to their anti-tumor suppressing nature [ 30 ]. According to Buchbinder & Desai’s findings, CTLA-4 and CD28 have two similar ligands to outcompete each other including B7.1 (also known as CD80) and B7.2 (also known as CD86), whereas CTLA-4 has a more affinity to bind with those ligands than CD28 [ 31 ]. On the other hand, the interaction of CTLA-4 with its ligands not only suppresses T cells but also reduces the expression of dendritic cells-mediated immunologic signals [ 32 ]. However, the survival and activation of T cells occur when a high proportion of CD28 molecules are present in the tumor microenvironment to trigger the secretion of IL-2 cytokine which is responsible for the rise in metabolic rate [ 33 ]. Furthermore, various investigations on T cell signaling and activation have demonstrated that active SHP2 and PP2A proteins have the potential to neutralize the signaling kinases by stimulating CD28 and TCR [ 34 ]. When CTLA-4-derived inhibitory signals are significantly transferred into T cells, both CD80 and CD86 ligands are removed from the surface of APCs by disrupting their interaction with CD28 and TCR [ 35 ]. However, Eggermont et al. found that lowering the Tregs effective performance can increase the susceptibility to autoimmune diseases in patients with multiple sclerosis, type II polyglandular syndrome, type 1 diabetes, rheumatoid arthritis, psoriasis, and myasthenia gravis in comparison with healthy people [ 36 ]. In case of cancer immunotherapy, the use of monoclonal antibodies of CTLA-4 could increase T cell proliferation and their cytokine production. For example, the monoclonal antibodies ipilimumab and tremelimumab have been recently applied for the CTLA-4 blocking strategy which results in the growth of blood cells in the immune system causing cancer cells death [ 23 ]. Again, combining CTLA-4 therapeutic inhibition with cancer vaccines may provide effective anti-tumor immunity through the efficient induction of immune response against low immunogenic tumor cells [ 37 ].
PD-1 is another negative immune checkpoint receptor that is expressed on a variety of immune cells such as activated T cells and B cells, myeloid cells, NK cells, dendritic cells, and monocytes [ 38 ]. In addition, it belongs to the immunoglobulin superfamily as a monomer and has a vital suppressive role against T cell activation, similar to CTLA-4 [ 39 ]. After interacting with B7 family ligands, including PD‐L1 (B1‐H1) and PD‐L2 (B7‐DC), PD-1 inhibits the activation of T lymphocytes by limiting ZAP70/PI3K enzyme kinase activity via SHP2 phosphatase [ 40 ]. Active CD8 + T lymphocytes express PD-L1, while the expression of PD-L2 is primarily restricted to immune system cells. Like CTLA-4, it also stimulates Treg cell signaling, which is important for triggering immune suppression of effector T cells that control immune response homeostasis [ 41 ]. PD-1 ligands are more interesting than PD-L2 ligand because tumor cells can express PD-L1 as a result of inflammatory cytokines and oncogenic signaling pathways [ 32 ]. Furthermore, PD-1 interacts with its ligands immediately after TCR stimulation, resulting in phosphorylation of tyrosine receptors based on the inhibition of immune receptor tyrosine‐based inhibition motif [ITIM] and the switching of immune receptor tyrosine‐based switch motif [ITSM] [ 42 ].
To boost the activity of NK cells and PD-1 + B cells-mediated antibody production within the tumor microenvironment, blocking of the PD-1 pathway is used as a first method to improve the activity of effector T cells against tumor cells [ 43 , 44 , 45 ]. It is found that Treg cells are the primary target of PD-1 blockade method which can be detected in the peripheral blood in a study of stage IV melanoma patients [ 45 ]. Two studies have recently reported that combining PD-1/PD-L1 therapies with the cytokine TGF-β can enhance the efficacy of anti-tumor response through CTL infiltration to prevent the growth and metastatic spread of both murine EMT6 breast mammary carcinoma and orthotopic colorectal cancer vaccination models [ 22 , 46 ]. Taken together, these findings might provide the foundation for combining the PD-1 blocking pathway with other inhibitors to improve anti-tumor effector actions [ 47 ]. Several monoclonal antibody factors are recently used to block the PD-1 pathway, including FDA-approved nivolumab (a fully humanized IgG4) for treating melanoma and non-small cell lung cancer, another IgG4-based antibody, pembrolizumab, for treating skin cancer, and durvalumab (MEDI4736), which blocks PD-L1 and binds to PD-1 as well as CD80 [ 23 ].
Siglec-based immunotherapy
The development of acquired resistance in FDA-approved CTLA-4 and PD-1 immunosuppressive antibodies triggers to search for additional more effective therapeutic strategies so that the efficacy of PD-1 or PD-L1 blockade pathway can enhance T cell immune response in contrast to tumor cells [ 48 ]. To achieve this demand, another type of immune checkpoint receptors like Siglecs is needed to be targeted to promote innate and adaptive immune cells-mediated anti-tumor immunity. The Siglecs (sialic acid-binding immunoglobulin-like lectins) are type-1 immunoglobulin-like transmembrane immune cell receptors that bind a wide range of sialic acids ligands by using an amino-terminal V-set immunoglobulin domain and also display variable numbers (16 in the case of sialoadhesin) of C2-set immunoglobulin domains [ 49 , 50 ]. They can be divided into two groups such as CD33 related Siglecs (Siglec-3 (CD33), Siglec-5, Siglec-6, Siglec-7, Siglec-8, Siglec-9, Siglec-10, Siglec-11, Siglec-14, and Siglec-16) and conserved Siglecs (Siglec-1, Siglec-2, Siglec-4, and Siglec-15) based on sequence similarity and evolutionary conservation. By contrast to conserved Siglecs, CD33-related Siglecs have highly similar (~ 50–99%) sequences in their extracellular domains beyond their different composition property and also have one or more intracellular ITIMs which have the potential to suppress activation signals of immune cells through the recruitment of tyrosine and inositol phosphatases(Crocker et al., 2007) [ 51 ]. There are nine CD33-related Siglecs in humans, and they are mostly expressed by mature innate immune cells such as neutrophils, eosinophils, monocytes, macrophages, NK cells, DCs, and mast cells. Self-associated molecular patterns (SAMPs) are developed when Siglecs bind to diverse sialoglycan ligands, and individual Siglecs have variable binding preferences for sialoglycan ligands [ 52 , 53 ]. Current studies revealed that the inhibitory receptors of CD33r Siglecs can form a bridge between immune cells and tumor cells via a sialic acid-dependent mechanism [ 54 ]. Therefore, targeting the sialoglycan-SAMP/Siglec pathway in vitro and in vivo can open the door to increase anti-tumor immunity. Here, this study will discuss the CD33r Siglecs such as Siglec-9 and Siglec-10 along with Siglec-15.
Siglec-9 is an inhibitory immune checkpoint receptor of CD33r Siglec family prominently expressed on tumor-infiltrating lymphocytes (TILs) and macrophages [ 55 ]. The sufficient binding of sialylated ligands with Siglec-9 typically triggers the upregulation of Sia-SAMPs network that is essential for causing immune evasion and cancer metastasis [ 56 , 57 , 58 ]. Therefore, interruption of the Siglec-9/Sia-SAMPs pathway can suggest a therapeutic intervention to improve the effector T cell response against cancer [ 59 ]. Stanczak et al. reported the overexpression of Siglec-9 checkpoint receptor on the surface of TILs in the aspect of colorectal, NSCLC (non-small cell lung cancer), and ovarian cancer patients and also observed the co-expression of other inhibitory receptors like PD-1 [ 60 ]. It has been already proved that Siglec-9 inhibits NK cell-mediated tumor cell killing in vitro [ 61 ]. Again, the binding affinity of macrophage-derived Siglec-9 with sialylated glycoform can activate TAM (tumor-associated macrophage) to escape immune-regulatory mechanisms and further promote cancer progression [ 56 ]. Furthermore, Stanczak and his coworkers reported an increase in tumor cell growth due to Siglec-9 upregulation on CD4 + and CD8 + T cell-bearing mice when compared to control mice in their study. Besides that, when they used two full IgG Siglec-9 antibodies (191,240 and E10-286) to test the effect of Siglec-9 blockade on T cell activation in vitro , they found a dose-dependent inhibition of T cell activation [ 60 ]. Their study supports the theory that cancer-associated Sia-SAMPs interact with inhibitory Siglec-9 on TILs to promote immune evasion. In addition, the evidence demonstrated by other previous studies also supports their hypothesis in the aspect of tumor immunotherapy. However, it is still not clear what is the exact intracellular signaling pathway that regulates Siglec-9 to inhibit T cell activation. Therefore, the best immune-modulatory functions of Siglec-9 on different immune cells rather than T cells need to be studied further to suggest a potential therapeutic target for treatment.
Siglec-10 (sialic acid-binding Ig-like lectin 10) is TAM-expressed CD33 related inhibitory checkpoint receptor of Siglecs family and is considered a promising target for cancer immunotherapy [ 62 ]. It is well established that ‘don’t eat me’ signals are naturally generated by cancer cells to evade macrophage-mediated anti-tumor immunity. For example, tumor cells that expressed CD24 antigen, a glycosylated glycosylphosphatidylinositol-anchored surface protein, are capable of producing a ‘don’t eat me’ anti-phagocytic signal to protect cancer cells from macrophage-mediated phagocytosis via its interaction with the Siglec-10 receptor on TAM [ 51 , 63 ]. Again, the interaction of CD24 protein with Siglec-10 triggers the inhibition of inflammatory responses to liver damage [ 64 ], sepsis [ 65 ], infection [ 66 ], and chronic graft diseases [ 67 ]. Hence, the inhibitory signaling pathway is mediated by two SHP-1 and SHP-2 phosphatases that are connected with the cytoplasmic tail of Siglec-10 by using two ITIMs [ 51 , 68 ]. However, the overexpression of CD24 has been already observed in previous study reports in ovarian and breast cancer cells but it has not yet been declared which is the exact pathway that causes CD24-mediated immune resistance on TAM. Therefore, Barkal et al. used the single-cell RNA sequencing method to test the effects of CD24-Siglec-10 signaling cascade on macrophage-mediated tumor controlling ability within the tumor microenvironment, and they found the high expression of CD24 and Siglec-10 in several tumors that they analyzed at a cellular level which ultimately indicates the potential of CD24 as a tumor-specific marker [ 63 ]. Again, the upregulation of macrophage-derived Siglec-10 at a substantial amount was determined by FACS in the aspect of breast and ovarian cancer cells which elicits a strong affinity of CD24 to interact with Siglec-10 receptor. In addition, they obtained the enhancement of anti-tumor immunity by macrophages when monoclonal antibodies were used to block of CD24/Siglec-10 anti-phagocytic signal which strongly supports the Siglec-10 inhibition activity against phagocytosis. Therefore, the knockout of either Siglec-10 receptor, or CD24 protein, and antibody blockade of CD24/Siglec-10 cascade further showed the robust phagocytic expression of macrophages to restrict tumors growth [ 63 ]. These types of evidence strongly support that the blockade of CD24/Siglec-10 immune checkpoint pathway might provide an effective therapeutic target to improve anti-tumor immunity in the aspect of breast and ovarian cancer hosts.
Unlike Siglec-9 and Siglec-10, Siglec-15 belongs to the conserved gene family with a characteristic sialic acid-binding immunoglobulin type lectin structure [ 69 ]. Within the TME, Siglec-15 also contains the same domain composition as the PD-1/B7-H1 immune checkpoint and thereby suppresses immune responses against tumor progression, whereas the activation of suppressed T cells can restore defective immunity through the blockade of PD-1/B7-H1 signaling cascade with monoclonal antibodies which ultimately indicates the evidence of normalization theory in cancer immunotherapy [ 70 , 71 ]. However, other cellular or molecular mechanisms including the presence of dysfunctional T cells, TAM, and myeloid-derived suppressor compounds, the insufficient infiltration of immune cells, and the downregulation of immunomodulatory cells such as different cytokines and metabolites are critically involved to create immune evasion in TME along with the upregulation of PD-1/B7-H1 pathway based on the reports of numerous studies [ 72 , 73 , 74 , 75 ]. Hence, the selective target of myeloid cells-derived Siglec-15-mediated immune suppression can improve the therapeutic efficacy of PD-1/B7-H1 blockade strategy which then represents acquired resistance in the aspect of several human cancers [ 76 , 77 ]. A recent study by Jun Wang’s group represented the suppression of T cells activity in vivo and in vitro via the overexpression of macrophage-derived Siglec-15 in different human cancer cells. In addition, they also found the inhibition of tumor growth in multiple tumor models when they used antibodies to block Siglec-15. Furthermore, they confirmed that the M-CSF (macrophage colony-stimulating factor) induced overexpression of Siglec-15 on tumor-associated macrophages can be downregulated by IFN-γ which is a positive regulator of the B7-H1 family [ 78 ]. Collectively, the above results strongly support the potential of Siglec-15 as an immune-suppressive molecule in TME. Therefore, the antibody blockade technique of Siglec-15 expression may be used as an attractive therapeutic approach to target cancer patients who are resistant to current anti-PD-1/B7-H1 therapy [ 79 , 80 ]. However, the exact pathway that induces the overexpression of Siglec-15 in many cancers and/or tumor-infiltrating macrophages compared to normal tissues is still unknown which can be identified to enhance anti-tumor immunity.
CD47-SIRPα checkpoint-based immunotherapy
CD47-SIRPα checkpoint is a myeloid cell-derived immune checkpoint that also plays an important role in inducing immune evasion of cancer cells like other immune checkpoints [ 81 , 82 , 83 ]. Hence, tumor cells express CD47 protein that is then able to bind with SIRPα receptor on myeloid cells such as macrophages and neutrophils [ 84 ]. The interaction of the CD47 ligand with the SIRPα receptor finally triggers the cancer cells to develop a ‘don’t eat me’ signal toward the immune response of T cells [ 85 , 86 ]. Nowadays, this signaling pathway has been studied as a phagocytosis checkpoint [ 25 ] and the design of therapeutic strategies to disrupt the CD47-SIRPα axis through clinical investigations is ongoing [ 87 ]. However, prior research data strongly suggest that the blocking of CD47-SIRPα interaction with tumor opsonizing antibodies (such as anti-CD20 rituximab, or, anti-EGFR cetuximab, or anti-Her2 trastuzumab) can significantly lead to cancer cell clearance especially in case of liver cancer through suppressing cancer cell-expressed CD47 proteins [ 86 , 88 , 89 , 90 ] . Therefore, by using a haploid genetic screen, Logtenberg and his colleagues identified the presence of Golgi body and endoplasmic reticulum (ER) secreted QPCTL enzyme which belongs to the QPCT (Glutaminyl-peptide cyclotransferase) family and also stated that QPCTL is the critical regulator of the CD47-SIRPα axis. QPCTL is involved in the modification of CD47 protein due to the formation of pyroglutamate (pGU) residue at the N-terminus end of CD47 protein, and thereby it can interact with the SIRPα site [ 91 , 92 ]. In addition, another hypothesis arises from their study that the deletion or inhibition of QPCTL protein can provide strong evidence of macrophage and neutrophils-mediated tumor cell phagocytosis and cellular toxicity which is antibody dependent. Anyway, they did not show the effect of QPCTL inhibition on T cell responses. Collectively, the obtained results from their research data reveal that the development of novel QPCTL inhibitory molecules may be fused with antibody blockade of CD47 or SIRPα to promote both innate and adaptive immunity. Besides, targeting the CD47-SIRPα signaling cascade along with the existing therapeutic approach such as CTLA-4 and PD-1 may also contribute to the enhancement of immunotherapy efficacy in the future (Fig. 1 ).
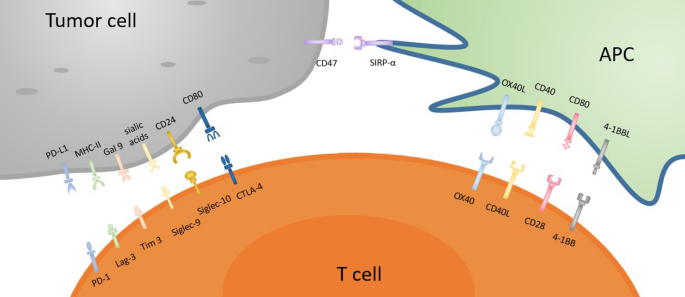
Inhibitory and stimulatory receptors on APC/T cells and tumor cells
Intrinsic molecular pathway based immunotherapy
Adoptive T cell therapy (ACT) has brought out unprecedented clinical outcomes in cancer immunotherapy as dysfunction of effector T cell is considered the central point for causing immune evasion by cancer cells. However, the main drawback of this method is the short-term immune response of adoptively transferred engineered T cells in some cancer patients [ 93 , 94 ]. Therefore, to enhance the therapeutic efficacy of ACT, it is further required the transformation of CD8 + T cells into long-lived effector T cells by targeting some regulatory proteins those act as T cell suppressors or activators in TME, including REGNASE-1, PAC1, and AGK [ 95 ].
REGNASE-1 is a major negative regulator of anti-tumor response mediated by T cells which are encoded by the Regnase 1 gene (also known as zc3h12a) with having RNA degrading properties) [ 96 , 97 ]. The exact role of its in TME is still not clear due to poor study of the fate of T cells although it has shown crucial effects on B cell homeostasis [ 98 ]. Jun and his group revealed that normal CD8 + T cells can be reprogrammed into effector T cells with long-term immunity by using the knowledge of CRISPR/cas9 mutagenesis approach for REGNASE-1 block which controls T cell activation. Again, they identified BATF as a key controlling agent of REGNASE-1 and confirmed the enhancement of therapeutic efficacy of REGNASE-1 deficient CD8 + T cells when they targeted BATF along with two other essential factors such as PTPN2 and SOCS1 by using genome-scale CRISPR screening technique [ 99 , 100 ]. However, their findings ultimately suggest that targeting CD8 + T cell-expressed REGNASE-1 with other immune checkpoint inhibitors may also open the door to new therapeutic approaches to provide effective anti-tumor immunity by CRISPR method.
PAC1 (Phosphatase of activated cell 1) is another negative immune checkpoint typically involved in the attenuation and exhaustion of CD8 + T cell-mediated anti-tumor immunity [ 101 , 102 ]. The upregulation of PAC1 has selectively occurred in exhausted TIL which is responsible for the poor prognosis of cancer patients [ 103 ]. Recently, the most promising approach to reprogramming exhausted T cell into effector T cell is combining checkpoint-based therapeutic strategies with chemical pathway as oxidative stresses such as ROS plays an important role to control T cell immune response against cancer [ 48 , 104 ]. Thus, it is needed to detect the specific pathway by which effector T cell activity is hampered. Besides, from the previous study, it has been already shown that excessive ROS production can exacerbate T cell exhaustion via the suppression of IL-17 and type-1 interferon production [ 105 , 106 ]. However, the direct way by which oxidative stress compounds regulate CD8 + T cell-specific gene expression is still elusive. Furthermore, Dan lu and his team found that the activation of EGR1 (redox transcription factor) mediated by oxidative stress generally induces the expression of PAC1 at a high rate in CD8 + T cells within the tumor microenvironment. Next, the upregulation of PAC1 triggers the recruitment of Mi-2β NuRD (nucleosome-remodeling-deacetylase) complex that is responsible for causing alteration of effector T cell genes expression, thereby ultimately leading to inactivation of T cell and further tumor progression [ 103 ]. Moreover, their research data collectively support that PAC1 serves as a T cell-specific negative immune checkpoint at the epigenetic level and thus targeting the ROS-EGR1-PAC1 signaling pathway may be a potential target of chemical-based immunotherapy which could offer an alternative way to boost the efficacy of T cell immune response in terms of cancer treatment and prevention.
Acylglycerol kinase (AGK) pathway
AGK (Acylglycerol kinase) is a positive regulator of CD8 + T cells glycolysis (glucose metabolism) which is a prerequisite for effector CD8 + T cells proliferation and activity, while CD8 + T cells play a pivotal role in adaptive anti-tumor immunity to eliminate cancer cells [ 107 ]. Indeed, AGK is an enzyme of lipid kinase property that is involved in the conversion of acylglycerol to lysophosphatidic acid (LPA) through phosphorylation and also regulates tumor expansion [ 108 ]. Bektas et al. reported that AGK is involved in the regulation of EGF (epidermal growth factor) signaling in prostate cancer cells [ 109 ]. Although it has been established from previous data that glycolytic metabolism plays a crucial role in the activation and functional fitness of CD8 + T cells, but the exact molecular mechanism that regulates the glycolysis of CD8 + T cells is still elusive. However, to unravel the AGK-mediated elevated CD8 + T cell glycolysis, Hu and his colleagues performed several in vivo and in vitro studies and it has been reported from their research data that AGK can also act as a protein kinase in the glycolytic metabolism of CD8 + T cells through the regulation of PTEN activity. Hence, PTEN is regarded as an inhibitor of PI3K (phosphatidylinositol-3-OH kinase)-mTOR (mammalian target of rapamycin) signaling which typically controls the metabolic switch of CD8 + T cell upon stimulation of TCR and CD28 molecules and that is why it is also known as an AGK-regulated checkpoint [ 110 , 111 ]. At a molecular level, they found that the stimulation of TCR and CD28 induces the recruitment of PTEN in the plasma membrane, and then, AGK-PTEN interaction triggers the phosphorylation of PTEN (at Ser380, Thrc382, and Thr383 positions) which restricts the phosphatase activity of PTEN, thereby promoting the activation of PI3K-mTOR signaling cascade and glycolysis of CD8 + T cells. On the other hand, the absence of AGK in CD8 + T cells showed the impairment of glycolysis and effective anti-tumor functions in vivo and in vitro respectively. Collectively, these findings demonstrate that AGK-mediated activation of the PI3K-mTOR pathway mainly contributes to the metabolic programming and functional fitness of CD8 + T cells [ 112 , 113 ]. Therefore, targeting the activity of AGK in CD8 + T cells may provide a better promising approach to promote the efficacy of T cell-mediated adaptive immune response.
NK cells-derived immune checkpoint-based immunotherapy
Even though T cell-based checkpoint immunotherapy has revolutionized cancer treatment, the poor immunogenic response of T cell-based immune checkpoint strategies against CTLA-4 and PD-L1 triggers the discovery of novel ‘checkpoints’ on other immune cells, particularly natural killer (NK) cells, which will be targeted in the future [ 114 , 115 , 116 ]. NK cells are the essential components of innate immunity that can release cytotoxic granules, such as perforin and granzymes to destroy cancer cells [ 115 , 117 ], or can cause cancer cell apoptosis through the production of TNF-α, FasL, and TRAIL genes [ 118 , 119 ]. The effector function of NK cells-mediated cytotoxicity is not only well recognized against blood tumors and metastatic tumors state but also significant patient outcomes have been confirmed in several solid tumors such as colon, lung, stomach cancer, and renal carcinoma [ 120 ], whereas the dysfunction of NK cells via knockout of IFN-γ or perforin genes has already shown a higher efficacy of carcinogenesis in mouse models of metastasis [ 121 ]. On the other hand, for enhancing the CD8 + T cells-mediated anti-tumor response, NK cells play an important role to facilitate the accumulation of cDC1 (conventional type of dendritic cells) into cancer cells via the production of chemo-attractants such as CCL5 and CXCL1 [ 122 ]. In addition, NK cells are generally able to eliminate the tumors that are not detected by the T cell immune-surveillance system due to the reduced expression of MHC-1 molecules. These promising features of NK cells highlight that they can be used as an emerging target of cancer immunotherapy along with boosting the efficacy of T cell-mediated anti-tumor response [ 123 ]. However, the effective anti-tumor function of NK cells mainly depends on the integration of some immune-activating and immune-suppressive signals/pathways. Among them, different types of extrinsic and intrinsic immune inhibitory receptors such as KIRS (killer inhibitory receptors), CD96, TIGIT, PD-1, CTLA-4, TIM-3, VISTA, and LAG-3, also known as checkpoint receptors, are responsible to prevent the tumor-killing potentiality of NK cells through their upregulation on NK cell surface which is typically mediated by IL-10 and TGF-β [ 120 ]. In this review, we will shed light on the brief discussion of the targeting strategy of NK-based checkpoint receptors especially CD96 and NKG2A receptors with monoclonal antibodies to exploit the potential anti-tumor activity of NK cells as a helper of T cell-based checkpoint immunotherapy.
CD96, a transmembrane checkpoint receptor of the immunoglobulin family, is normally expressed on the surface of NK cells and T cells and was previously shown to promote NK cell-mediated cancer cell cytolysis [ 124 ]. The deficiency of NK cells-derived CD96 checkpoint was showed increased tumor resistance in chemically produced tumor models of mice which were also dependent on NK and IFN-γ [ 125 ]. In contrast to NK cells from peripheral tumors, tumor-infiltrating NK cells had a higher proportion and intensity of CD96 on them, as well as a higher quantity of CD96 + NK cells in HCC patients. Later, the increased IL-10 and TGF-β production and the decreased IFN-γ and TNF-α production confirmed the dysfunction of CD96 + NK cells more than CD96 − NK cells. Interestingly, the higher expression of CD96 or its ligand CD155 resulted in poor clinical benefits in HCC cancer patients (H. Sun et al., 2019) [ 113 ]. However, the blockade of CD96 with monoclonal antibody further supports being a checkpoint receptor expressed on NK cells and the efficacy relies on the presence of CD226 or IFN-γ genes. Moreover, the combination of the CD96 blockade strategy with anti-CTLA-4 and anti-PD-1 immunotherapy demonstrated more significant effects to facilitate the infiltration of NK cells into tumor mass [ 120 , 126 ], which ultimately indicates that targeting of the CD96 checkpoint on NK cells might provide a potential immunotherapy strategy to enhance NK cells-mediated anti-tumor efficacy [ 127 ].
NKG2A is another checkpoint receptor that is present on both NK cells and T cells and also binds with CD94 to form a heterodimer [ 128 ]. In human blood and SCCHN (squamous cell carcinoma of the head and neck) tumors, the amount of NKG2A + NK cells is found as more than half of the quantity. Again, the stimulation of IL-15 further triggers the upregulation of NKG2A on the surface of NK cells [ 129 ], and importantly the expression of NKG2A subset along with PD-1 is also found among the NKG2A + NK cells. When NKG2A/CD94 binds to its ligand such as MHC-I and HLA-E (the ligand of NKG2A in humans), such kind of interaction reduces the effector anti-tumor activity of both NK cells and T cells via recruiting SHP-1 tyrosine phosphatase in the intercellular domain of NKG2A. However, blocking the expression of NKG2A reversed the HLA-E-mediated reduction of cytotoxicity and IFN-γ production by NK cells in vitro , and increased the efficacy of NK cells against HLA-E tumors in vivo after infusion [ 120 , 130 ]. Besides, the expression of NKG2A and HLA-E in hepatocellular carcinoma (HCC) tissues corresponded with poor prognosis in HCC patients, confirming these functional investigations [ 48 ]. Furthermore, the combined therapeutic blockade of NKG2A by monalizumab (a humanized anti-NKG2A antibody) with PD-1/PD-L1 demonstrated the improvement of NK cells-mediated cytotoxic activity and also increased immune response of CD8 + T cells against different tumors in compare to PD-1/PD-L1 blockade alone [ 129 ]. Collectively, this evidence indicates that the blockade of NKG2A can also stimulate the CD8 + T cells-dependent anti-tumor immunity [ 131 ], and therefore, it can be considered a promising immunotherapy approach to boost the effector functions of both NK cells and CD8 + T cells against the tumor (Fig. 2 ).
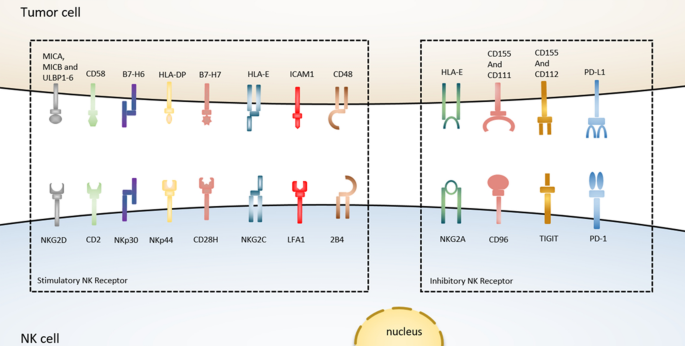
The interaction between NK cells and tumor cells
Targeting TAM to improve the therapeutic effectiveness
In tumor immunotherapy-based research, several clinical and preclinical tests are currently being conducted by combining the pre-existing therapies with novel therapies to get better outcomes for the improvement of immune system efficacy [ 132 , 133 ]. However, due to the immunogenic properties and microenvironment composition of the tumor, all techniques do not suit all tumors and the response of patients to different approaches also varies based on cancer subtype. For example, the administration of immune checkpoint-based antibodies against PD-1 or CTLA-4 receptors/ligands have shown dramatic effects in melanoma and lung cancer patients [ 134 ]. However, this therapeutic strategy does not fully respond to other types of solid tumors, such as pancreatic and breast cancer due to insufficient mutation that hinders the recognition of neo-antigens or tumor cell surface expressing antigenic peptide molecules by CD8 + T cells [ 135 ].
Solid tumors are formed because of non-hematopoietic stem cell mutations, and finally, they differentiate into malignant forms to cause cancer. In response to solid tumors, myeloid cells-derived immune cells such as regulatory T cells and tumor-associated macrophages (TAM) suppress the tumor-killing nature of cytotoxic lymphocytes such as CD8 + T cells and natural killer cells [ 136 , 137 ]. However, the preferential accumulations of tumor-associated macrophages on the surface of solid tumors limit the cytotoxic potentiality of CTL in vitro through either the direct expression of immune-suppressive molecules or indirect recruitment of Treg cells [ 138 , 139 , 140 ]. DeNardo found that the increasing anti-tumor immunity mediated by CD8 + T cells is mainly due to the depletion of TAM in a mouse model of breast cancer under chemotherapy treatment [ 141 ]. Therefore, TAM can be recommended as one of the vital therapeutic targets to increase the potentiality of immunotherapy. Besides, an effective therapeutic technique can be outlined by combining the immune-suppressive molecules along with CTL delivery within the tumor microenvironment. Recently, three therapeutic approaches such as depletion, reprogramming, and molecular targeting have been suggested to recover TAM-mediated immune suppression. It has already been proved that CCR2 triggers the differentiation of circulating monocytes into TAMs in the tumor microenvironment and another receptor that is required for the recruitment, differentiation, and survival of macrophages is called colony-stimulating factor-1 receptor (CSF1R) [ 142 ]. In the pancreatic mouse cancer model, the combination of CCR2 antagonist with anti-PD1 antibody therapy reduces the tumor growth, while single anti-PD1 antibody-mediated therapy is not successful [ 143 ]. Again, treatment with CSF1R antagonists (e.g., PLX3397, PLX73086, and BLZ945) also significantly decreases the number of TAMs in pancreatic, breast, cervical, mesothelioma, and ovarian mouse cancer models. Furthermore, immune checkpoint inhibitors-mediated blockade of CCR2 or CSF1R receptors can increase the efficacy of immune checkpoint therapy via the prevention of TAM infiltration into tumor [ 144 , 145 , 146 ]. Different in vitro studies on macrophages have already revealed that pro-inflammatory cytokines are released during the culture of IFN-γ and LPS. This type of cytokine then can help to become immune-activating TAMs from tumor suppressive form in response to certain environmental conditions [ 147 , 148 ], and thereby can enhance the efficacy of immunotherapy strategy.
Research carried out by Cassetta and Kitamura also supports that the efficacy of cancer vaccination is strongly related to the accumulation and activation of myeloid cells, specifically TAMs. Furthermore, it is also reported in their study that the efficacy of cancer vaccination can be improved by co-injection of immune adjuvants to enhance the host immune responses toward the toll-like receptor (TLR) ligands and DC-targeted antibodies. Additionally, TAM depletion can improve the effectiveness of the therapeutic vaccine with appropriate adjuvants [ 135 ]. In TAM reprogramming that mediates immune activation by activated CD8 + T cells, depletion of macrophages reduces the effectiveness of therapeutic cancer vaccination, and in this case, TAM targeting techniques need to be carefully combined [ 149 ]. For targeting the non-immunogenic cancer cells, the adoptive transfer of genetically engineered CD8 + T cells has been emerged as an attractive approach to exert effective tumoricidal ability via the expression of chimeric antigenic receptors (i.e., CAR-T cells). However, the tumor-killing activity of the chimeric antigen receptor (CAR) is restricted by TAM [ 150 , 151 , 152 ]. Genetic modulation of CAR-T cells to produce interleukin-12 (IL-12) has also been reported for increasing the effectiveness of immunotherapy via reprogramming of TAM which can express tumor necrosis factor-α (TNF-α) [ 153 ]. In the ID8 ovarian cancer model, TAMs found in ascites tumors express a large number of folate receptor β (FRβ) and thereby adoptive transfer of FRβ-specific CAR-T cells into the tumor-bearing mice results in in vitro enhanced cytotoxicity of NK cells [ 154 ]. These findings indicate that TAM depletion may increase the efficacy of NK cell infusion therapy (e.g., CCR2 or CSF1R antagonist therapy) by blocking TAM recruitment or survival signals (e.g., the cytotoxicity of CAR-T cell against tumors) [ 123 ]. Although the exact molecular mechanism by which TAM inhibits T cell-mediated tumor immunity is still not clear and collectively these studies indicate that either targeting TAM-derived suppressive molecules, or TAM depletion or changing the differentiation pattern can effectively eliminate TAM-mediated restriction of immunity.
Concluding remarks and future directions
For the treatment of cancer, numerous promising immunotherapy approaches have been emerged by targeting TME in the last few decades based on the knowledge of tumor-induced immune evasion. To escape T eff cell-based adaptive immune response, tumor cells continuously change their genetic materials and can express several suppressive molecules which ultimately to dampen immune response. Therefore, to achieve better clinical outcomes in different cancer patients, adaptive immune resistance-based immune evasion must be overcome in future studies. However, the FDA approval of anti-CTLA4 or anti-PD1 checkpoint strategy typically suggests that there is an urgent need to combine them with other therapeutic approaches such as cancer vaccines, different cytokines, TAM and TLR agonists to boost the efficacy of endogenous adaptive immune response against particular tumor sites without any complications or side effects. Basically, in the future newly designed immunotherapy techniques should not only focus on the enhancement of anti-tumor immunity but also need to identify the exact defects of each tumor so that malignant tumors can be transformed into a normalized state with no IREAs (immune-related adverse events) through using selective modification. Furthermore, the principles of different ICIs in the current studies will drive the development of more effective combinatorial tumor immunotherapy strategy by allowing the combinations of T cell-based checkpoint pathways with NK-derived immune checkpoint pathways against poorly immunogenic tumors. Besides, the understanding of cellular and molecular mechanisms of immune checkpoint-based inhibitory pathways may also provide better insight to target different cytotoxic factors such as IL-10, IL-15, VEGF, and TGF-β as well as Siglec-based checkpoints along with T cell and NK-based checkpoint inhibitors for an effective anti-tumor response. Again, to overcome adaptive resistance, ICIs or ACT could be combined with monoclonal antibodies based on specific tumor antigens that will block the functions of immune-suppressive cells including TAMs, Treg, MDSCs, etc. Hence, these types of combination-based immunotherapies may maximize the clinical efficacy in a subset of tumor patients with a long-lasting anti-tumor response rate. Moreover, targeting ICIs with phagocytosis checkpoints such as the CD47-SIRPα axis as well as epigenetic checkpoints like REGNASE-1, PAC-1, or PI3K-AGK pathways which are upregulated in some cancer cells may build up a strong connection between innate and adaptive immune cells to provide a more successful way for the treatments of cancer patients rather than conventional therapeutic approaches. Finally, for improving the combination-based therapeutic efficacy, promising biomarkers may be designed to predict specific tumor response rates for specific treatment strategies based on the disease history of cancer patients. Although our current review discusses the combination-based novel immune checkpoint strategies, a lot of further investigations about the regional properties of different immune cells will need to be unraveled in the future.
Whiteside TL. Immune suppression in cancer: Effects on immune cells, mechanisms and future therapeutic intervention. Semin Cancer Biol. 2006;16(1):3–15.
CAS PubMed Google Scholar
Harris, T. J.; Drake, C. G., Primer on tumor immunology and cancer immunotherapy. J Immuno Therapy Cancer 2013, 1.
Seledtsov VI, Goncharov AG, Seledtsova GV. Multiple-purpose immunotherapy for cancer. Biomed Pharmacother. 2015;76:24–9.
Tian T, Olson S, Whitacre JM, Harding A. The origins of cancer robustness and evolvability. Integr Biol. 2011;3(1):17–30.
CAS Google Scholar
Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–64.
CAS PubMed PubMed Central Google Scholar
Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10.
PubMed Google Scholar
Kershaw MH, Westwood JA, Darcy PK. Gene-engineered T cells for cancer therapy. Nat Rev Cancer. 2013;13(8):525–41.
Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3(11):991–8.
Teng MWL, Galon J, Fridman WH, Smyth MJ. From mice to humans: developments in cancer immunoediting. J Clin Investig. 2015;125(9):3338–46.
PubMed PubMed Central Google Scholar
Zam W, Ali L. Immune checkpoint inhibitors in the treatment of cancer. Curr Rev Clin Exp Pharmacol. 2021;17(2):103–13.
Google Scholar
Kumar P, Bhattacharya P, Prabhakar BS. A comprehensive review on the role of co-signaling receptors and Treg homeostasis in autoimmunity and tumor immunity. J Autoimmun. 2018;95:77–99.
Nishikawa H, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Curr Opin Immunol. 2014;27(1):1–7.
Garrido F, Aptsiauri N, Doorduijn EM, Garcia Lora AM, van Hall T. The urgent need to recover MHC class I in cancers for effective immunotherapy. Curr Opin Immunol. 2016;39:44–51.
Saleh R, Elkord E. Acquired resistance to cancer immunotherapy: role of tumor-mediated immunosuppression. Semin Cancer Biol. 2020;65:13–27.
Taylor A, Verhagen J, Blaser K, Akdis M, Akdis CA. Mechanisms of immune suppression by interleukin-10 and transforming growth factor-$$: The role of T regulatory cells. Immunology. 2006;117(4):433–42.
Voron T, Marcheteau E, Pernot S, Colussi O, Tartour E, Taieb J, Terme M. Control of the immune response by pro-angiogenic factors. Front Oncol. 2014;4:70.
Lerner A, Benzvi C. Checkpoint inhibitors and induction of celiac disease-like condition. Biomedicines. 2022;10(3):609.
Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng SL, Shen X, Boyd Z, Hegde PS, Chen DS, Vogelzang NJ. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515(7528):558–62.
Seidel JA, Otsuka A, Kabashima K. Anti-PD-1 and anti-CTLA-4 therapies in cancer: Mechanisms of action, efficacy, and limitations. Front Oncol. 2018;8:86.
Takeuchi Y, Nishikawa H. Roles of regulatory T cells in cancer immunity. Int Immunol. 2016;28(8):401–9.
Speiser DE, Ho PC, Verdeil G. Regulatory circuits of T cell function in cancer. Nat Rev Immunol. 2016;16(10):599–611.
Taefehshokr N, Baradaran B, Baghbanzadeh A, Taefehshokr S. Promising approaches in cancer immunotherapy. Immunobiology. 2020;225(2): 151875.
Sadreddini S, Baradaran B, Aghebati-Maleki A, Sadreddini S, Shanehbandi D, Fotouhi A, Aghebati-Maleki L. Immune checkpoint blockade opens a new way to cancer immunotherapy. J Cell Physiol. 2019;234(6):8541–9.
Abril-Rodriguez G, Ribas A. SnapShot: immune checkpoint inhibitors. Cancer Cell. 2017;31(6):848-848.e1.
Feng M, Jiang W, Kim BYS, Zhang CC, Fu YX, Weissman IL. Phagocytosis checkpoints as new targets for cancer immunotherapy. Nat Rev Cancer. 2019;19(10):568–86.
Kumar P, Saini S, Prabhakar BS. Cancer immunotherapy with check point inhibitor can cause autoimmune adverse events due to loss of Treg homeostasis. Semin Cancer Biol. 2020;64:29–35.
Brunet JF, Denizot F, Luciani MF, Roux-Dosseto M, Suzan M, Mattei MG, Golstein P. A new member of the immunoglobulin superfamily-CTLA-4. Nature. 1988;328(6127):267–70.
Yan, Q.; Zhang, B.; Ling, X.; Zhu, B.; Mei, S.; Yang, H.; Zhang, D.; Huo, J.; Zhao, Z., CTLA-4 facilitates DNA damage–induced apoptosis by interacting with PP2A. Front Cell Dev Biol 2022, 10.
Gibson HM, Hedgcock CJ, Aufiero BM, Wilson AJ, Hafner MS, Tsokos GC, Wong HK. Induction of the CTLA-4 gene in human lymphocytes is dependent on NFAT binding the proximal promoter. J Immunol. 2007;179(6):3831–40.
Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. J Immunol. 2017;198(3):981–5.
Buchbinder EI, Desai A. CTLA-4 and PD-1 pathways similarities, differences, and implications of their inhibition. Am J Clin Oncol Cancer Clin Trials. 2016;39(1):98–106.
Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, Linsley PS, Thompson CB, Riley JL. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25(21):9543–53.
Krummel MF, Allison JP. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J Exp Med. 1996;183(6):2533–40.
Schildberg FA, Klein SR, Freeman GJ, Sharpe AH. Coinhibitory pathways in the B7-CD28 ligand-receptor family. Immunity. 2016;44(5):955–72.
Collins AV, Brodie DW, Gilbert RJC, Iaboni A, Manso-Sancho R, Walse B, Stuart DI, Van Der Merwe PA, Davis SJ. The interaction properties of costimulatory molecules revisited. Immunity. 2002;17(2):201–10.
Eggermont AMM, Chiarion-Sileni V, Grob JJ, Dummer R, Wolchok JD, Schmidt H, Hamid O, Robert C, Ascierto PA, Richards JM, Lebb C, Ferraresi V, Smylie M, Weber JS, Maio M, Konto C, Hoos A, de Pril V, Gurunath RK, de Schaetzen G, Suciu S, Testori A. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16(5):522–30.
Rowshanravan B, Halliday N, Sansom DM. CTLA-4: A moving target in immunotherapy. Blood. 2018;131(1):58–67.
Keir ME, Liang SC, Guleria I, Latchman YE, Qipo A, Albacker LA, Koulmanda M, Freeman GJ, Sayegh MH, Sharpe AH. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203(4):883–95.
Li J, Jie HB, Lei Y, Gildener-Leapman N, Trivedi S, Green T, Kane LP, Ferris RL. PD-1/SHP-2 inhibits Tc1/Th1 phenotypic responses and the activation of t cells in the tumor microenvironment. Can Res. 2015;75(3):508–18.
Swaika A, Hammond WA, Joseph RW. Current state of anti-PD-L1 and anti-PD-1 agents in cancer therapy. Mol Immunol. 2015;67(2):4–17.
Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26(1):677–704.
Karunarathne DS, Horne-Debets JM, Huang JX, Faleiro R, Leow CY, Amante F, Watkins TS, Miles JJ, Dwyer PJ, Stacey KJ, Yarski M, Poh CM, Lee JS, Cooper MA, Rnia L, Richard D, McCarthy JS, Sharpe AH, Wykes MN. Programmed death-1 ligand 2-mediated regulation of the PD-L1 to PD-1 axis Is essential for establishing CD4+ T cell immunity. Immunity. 2016;45(2):333–45.
Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206(13):3015–29.
Velu V, Titanji K, Zhu B, Husain S, Pladevega A, Lai L, Vanderford TH, Chennareddi L, Silvestri G, Freeman GJ, Ahmed R, Amara RR. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458(7235):206–10.
Huang AC, Postow MA, Orlowski RJ, Mick R, Bengsch B, Manne S, Xu W, Harmon S, Giles JR, Wenz B, Adamow M, Kuk D, Panageas KS, Carrera C, Wong P, Quagliarello F, Wubbenhorst B, D’Andrea K, Pauken KE, Herati RS, Staupe RP, Schenkel JM, McGettigan S, Kothari S, George SM, Vonderheide RH, Amaravadi RK, Karakousis GC, Schuchter LM, Xu X, Nathanson KL, Wolchok JD, Gangadhar TC, Wherry EJ. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature. 2017;545(7652):60–5.
Tauriello DVF, Palomo-Ponce S, Stork D, Berenguer-Llergo A, Badia-Ramentol J, Iglesias M, Sevillano M, Ibiza S, Caellas A, Hernando-Momblona X. TGF$$ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature. 2018;554(7693):538–43.
Yi, M.; Zheng, X.; Niu, M.; Zhu, S.; Ge, H.; Wu, K., Combination strategies with PD-1/PD-L1 blockade: current advances and future directions. Molecular Cancer 2022, 21 (1).
Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541(7637):321–30.
Crocker PR. Siglecs: Sialic-acid-binding immunoglobulin-like lectins in cell-cell interactions and signalling. Curr Opin Struct Biol. 2002;12(5):609–15.
Varki A, Angata T. Siglecs - The major subfamily of I-type lectins. Glycobiology. 2006;16(1):1R-27R.
Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7(4):255–66.
Lubli H, Varki A. Sialic acid–binding immunoglobulin-like lectins (Siglecs) detect self-associated molecular patterns to regulate immune responses. Cell Mol Life Sci. 2020;77(4):593–605.
Padler-Karavani V, Hurtado-Ziola N, Chang YC, Sonnenburg JL, Ronaghy A, Yu H, Verhagen A, Nizet V, Chen X, Varki N, Varki A, Angata T. Rapid evolution of binding specificities and expression patterns of inhibitory CD33-related Siglecs in primates. FASEB J. 2014;28(3):1280–93.
Schwarz F, Landig CS, Siddiqui S, Secundino I, Olson J, Varki N, Nizet V, Varki A. Paired siglec receptors generate opposite inflammatory responses to a human-specific pathogen. EMBO J. 2017;36(6):751–60.
Jia Y, Yu H, Fernandes SM, Wei Y, Gonzalez-Gil A, Motari MG, Vajn K, Stevens WW, Peters AT, Bochner BS, Kern RC, Schleimer RP, Schnaar RL. Expression of ligands for Siglec-8 and Siglec-9 in human airways and airway cells. J Allergy Clin Immunol. 2015;135(3):799-810.e7.
Beatson R, Tajadura-Ortega V, Achkova D, Picco G, Tsourouktsoglou TD, Klausing S, Hillier M, Maher J, Noll T, Crocker PR, Taylor-Papadimitriou J, Burchell JM. The mucin MUC1 modulates the tumor immunological microenvironment through engagement of the lectin Siglec-9. Nat Immunol. 2016;17(11):1273–81.
Lubli H, Pearce OMT, Schwarz F, Siddiqui SS, Deng L, Stanczak MA, Deng L, Verhagen A, Secrest P, Lusk C, Schwartz AG, Varki NM, Bui JD, Varki A. Engagement of myelomonocytic Siglecs by tumor-associated ligands modulates the innate immune response to cancer. Proc Natl Acad Sci USA. 2014;111(39):14211–6.
Perdicchio M, Cornelissen LAM, Streng-Ouwehand I, Engels S, Verstege MI, Boon L, Geerts D, van Kooyk Y, Unger WWJ. Tumor sialylation impedes T cell mediated anti-tumor responses while promoting tumor associated-regulatory T cells. Oncotarget. 2016;7(8):8771–82.
Bull C, Boltje TJ, Balneger N, Weischer SM, Wassink M, van Gemst JJ, Bloemendal VR, Boon L, van der Vlag J, Heise T, den Brok MH, Adema GJ. Sialic Acid Blockade Suppresses Tumor Growth by Enhancing T-cell-Mediated Tumor Immunity. Cancer Res. 2018;78(13):3574–88.
Stanczak MA, Siddiqui SS, Trefny MP, Thommen DS, Boligan KF, Von Gunten S, Tzankov A, Tietze L, Lardinois D, Heinzelmann-Schwarz V, Von Bergwelt-Baildon M, Zhang W, Lenz HJ, Han Y, Amos CI, Syedbasha M, Egli A, Stenner F, Speiser DE, Varki A, Zippelius A, Lubli H. Self-associated molecular patterns mediate cancer immune evasion by engaging siglecs on T cells. J Clin Investig. 2018;128(11):4912–23.
Jandus C, Boligan KF, Chijioke O, Liu H, Dahlhaus M, Dmoulins T, Schneider C, Wehrli M, Hunger RE, Baerlocher GM, Simon HU, Romero P, Mnz C, Von Gunten S. Interactions between Siglec-7/9 receptors and ligands influence NK cell-dependent tumor immunosurveillance. J Clin Investig. 2014;124(4):1810–20.
Chen GY, Brown NK, Zheng P, Liu Y. Siglec-G/10 in self-nonself discrimination of innate and adaptive immunity. Glycobiology. 2014;24(9):800–6.
Barkal AA, Brewer RE, Markovic M, Kowarsky M, Barkal SA, Zaro BW, Krishnan V, Hatakeyama J, Dorigo O, Barkal LJ, Weissman IL. CD24 signalling through macrophage siglec-10 is a target for cancer immunotherapy. Nature. 2019;572(7769):392–6.
Chen GY, Tang J, Zheng P, Liu Y. CD24 and siglec-10 selectively repress tissue damage - Induced immune responses. Science. 2009;323(5922):1722–5.
Chen GY, Chen X, King S, Cavassani KA, Cheng J, Zheng X, Cao H, Yu H, Qu J, Fang D, Wu W, Bai XF, Liu JQ, Woodiga SA, Chen C, Sun L, Hogaboam CM, Kunkel SL, Zheng P, Liu Y. Amelioration of sepsis by inhibiting sialidase-mediated disruption of the CD24-SiglecG interaction. Nat Biotechnol. 2011;29(5):428–35.
Chen W, Han C, Xie B, Hu X, Yu Q, Shi L, Wang Q, Li D, Wang J, Zheng P, Liu Y, Cao X. Induction of siglec-G by RNA viruses inhibits the innate immune response by promoting RIG-I degradation. Cell. 2013;152(3):467–78.
Toubai T, Hou G, Mathewson N, Liu C, Wang Y, Oravecz-Wilson K, Cummings E, Rossi C, Evers R, Sun Y, Wu J, Choi SW, Fang D, Zheng P, Liu Y, Reddy P. Siglec-G-CD24 axis controls the severity of graft-versus-host disease in mice. Blood. 2014;123(22):3512–23.
Abram CL, Lowell CA. Shp1 function in myeloid cells. J Leukoc Biol. 2017;102(3):657–75.
Angata T, Tabuchi Y, Nakamura K, Nakamura M. Siglec-15: an immune system siglec conserved throughout vertebrate evolution. Glycobiology. 2007;17(8):838–46.
Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Cells E, Chen L. Tumor-associated B7–H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800.
Weiping, Z.; Jedd D., W.; Lieping, C., PD-L1 (B7-H1) and PD-1 Pathway Blockade for Cancer Therapy: MEchanisms, Response Biomarkers and Combinations. Science Translational Medicine 2016, 8 (328).
Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14(10):1014–22.
Kim TK, Herbst RS, Chen L. Defining and understanding adaptive resistance in cancer immunotherapy. Trends Immunol. 2018;39(8):624–31.
Mittal D, Gubin MM, Schreiber RD, Smyth MJ. New insights into cancer immunoediting and its three component phases-elimination, equilibrium and escape. Curr Opin Immunol. 2014;27(1):16–25.
Sanmamed MF, Chen L. A paradigm shift in cancer immunotherapy: from enhancement to normalization. Cell. 2018;175(2):313–26.
Hiruma Y, Hirai T, Tsuda E. Siglec-15, a member of the sialic acid-binding lectin, is a novel regulator for osteoclast differentiation. Biochem Biophys Res Commun. 2011;409(3):424–9.
Zhang Y, Chen L. Classification of advanced human cancers based on tumor immunity in the MicroEnvironment (TIME) for cancer immunotherapy. JAMA Oncol. 2016;2(11):1403–4.
Wang J, Sun J, Liu LN, Flies DB, Nie X, Toki M, Zhang J, Song C, Zarr M, Zhou X, Han X, Archer KA, O’Neill T, Herbst RS, Boto AN, Sanmamed MF, Langermann S, Rimm DL, Chen L. Siglec-15 as an immune suppressor and potential target for normalization cancer immunotherapy. Nat Med. 2019;25(4):656–66.
Chen X, Mo S, Zhang Y, Ma H, Lu Z, Yu S, Chen J. Analysis of a novel immune checkpoint, Siglec-15, in pancreatic ductal adenocarcinoma. J Pathol: Clin Res. 2022;8:268–78.
Stuible M, Moraitis A, Fortin A, Saragosa S, Kalbakji A, Filion M, Tremblay GB. Mechanism and function of monoclonal antibodies targeting Siglec-15 for therapeutic inhibition of osteoclastic bone resorption. J Biol Chem. 2014;289(10):6498–512.
Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs KD, van Rooijen N, Weissman IL. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138(2):286–99.
Matlung HL, Szilagyi K, Barclay NA, van den Berg TK. The CD47-SIRP$$ signaling axis as an innate immune checkpoint in cancer. Immunol Rev. 2017;276(1):145–64.
Zhao XW, Van Beek EM, Schornagel K, Van Der Maaden H, Van Houdt M, Otten MA, Finetti P, Van Egmond M, Matozaki T, Kraal G, Birnbaum D, Van Elsas A, Kuijpers TW, Bertucci F, Van Den Berg TK. CD47-signal regulatory protein- \((SIRP)\) interactions form a barrier for antibody-mediated tumor cell destruction. Proc Natl Acad Sci USA. 2011;108(45):18342–7.
Weiskopf K. Cancer immunotherapy targeting the CD47/SIRP$$ axis. Eur J Cancer. 2017;76:100–9.
Jaiswal S, Jamieson CHM, Pang WW, Park CY, Chao MP, Majeti R, Traver D, van Rooijen N, Weissman IL. CD47 Is Upregulated on circulating hematopoietic stem cells and Leukemia cells to avoid phagocytosis. Cell. 2009;138(2):271–85.
Willingham, S. B.; Volkmer, J. P.; Gentles, A. J.; Sahoo, D.; Dalerba, P.; Mitra, S. S.; Wang, J.; Contreras-Trujillo, H.; Martin, R.; Cohen, J. D.; Lovelace, P.; Scheeren, F. A.; Chao, M. P.; Weiskopf, K.; Tang, C.; Volkmer, A. K.; Naik, T. J.; Storm, T. A.; Mosley, A. R.; Edris, B.; Schmid, S. M.; Sun, C. K.; Chua, M. S.; Murillo, O.; Rajendran, P.; Cha, A. C.; Chin, R. K.; Kim, D.; Adorno, M.; Raveh, T.; Tseng, D.; Jaiswal, S.; Enger, P. y.; Steinberg, G. K.; Li, G.; So, S. K.; Majeti, R.; Harsh, G. R.; Van Rijn, M. D.; Teng, N. N. H.; Sunwoo, J. B.; Alizadeh, A. A.; Clarke, M. F.; Weissman, I. L., The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proceedings of the National Academy of Sciences of the United States of America 2012; 109 (17), 6662–6667.
Tan Y, Chen H, Zhang J, Cai L, Jin S, Song D, Yang T, Guo Z, Wang X. Platinum(IV) complexes as inhibitors of CD47-SIRP$$ axis for chemoimmunotherapy of cancer. Eur J Med Chem. 2022;229:114047.
Advani R, Flinn I, Popplewell L, Forero A, Bartlett NL, Ghosh N, Kline J, Roschewski M, LaCasce A, Collins GP, Tran T, Lynn J, Chen JY, Volkmer J-P, Agoram B, Huang J, Majeti R, Weissman IL, Takimoto CH, Chao MP, Smith SM. CD47 blockade by Hu5F9-G4 and rituximab in non-hodgkin’s lymphoma. N Engl J Med. 2018;379(18):1711–21.
Chao MP, Alizadeh AA, Tang C, Myklebust JH, Varghese B, Gill S, Jan M, Cha AC, Chan CK, Tan BT, Park CY, Zhao F, Kohrt HE, Malumbres R, Briones J, Gascoyne RD, Lossos IS, Levy R, Weissman IL, Majeti R. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-hodgkin lymphoma. Cell. 2010;142(5):699–713.
Ring NG, Herndler-Brandstetter D, Weiskopf K, Shan L, Volkmer JP, George BM, Lietzenmayer M, McKenna KM, Naik TJ, McCarty A, Zheng Y, Ring AM, Flavell RA, Weissman IL. Anti-SIRP$$ antibody immunotherapy enhances neutrophil and macrophage antitumor activity. Proc Natl Acad Sci USA. 2017;114(49):E10578–85.
Huang KF, Liu YL, Cheng WJ, Ko TP, Wang AHJ. Crystal structures of human glutaminyl cyclase, an enzyme responsible for protein N-terminal pyroglutamate formation. Proc Natl Acad Sci USA. 2005;102(37):13117–22.
Logtenberg MEW, Jansen JHM, Raaben M, Toebes M, Franke K, Brandsma AM, Matlung HL, Fauster A, Gomez-Eerland R, Bakker NAM, van der Schot S, Marijt KA, Verdoes M, Haanen JBAG, van den Berg JH, Neefjes J, van den Berg TK, Brummelkamp TR, Leusen JHW, Scheeren FA, Schumacher TN. Glutaminyl cyclase is an enzymatic modifier of the CD47- SIRP$$ axis and a target for cancer immunotherapy. Nat Med. 2019;25(4):612–9.
Kishton RJ, Sukumar M, Restifo NP. Metabolic regulation of T cell longevity and function in tumor immunotherapy. Cell Metab. 2017;26(1):94–109.
Lim WA, June CH. The Principles of Engineering Immune Cells to Treat Cancer. Cell. 2017;168(4):724–40.
Sade-Feldman M, Yizhak K, Bjorgaard SL, Ray JP, de Boer CG, Jenkins RW, Lieb DJ, Chen JH, Frederick DT, Barzily-Rokni M, Freeman SS, Reuben A, Hoover PJ, Villani AC, Ivanova E, Portell A, Lizotte PH, Aref AR, Eliane JP, Hammond MR, Vitzthum H, Blackmon SM, Li B, Gopalakrishnan V, Reddy SM, Cooper ZA, Paweletz CP, Barbie DA, Stemmer-Rachamimov A, Flaherty KT, Wargo JA, Boland GM, Sullivan RJ, Getz G, Hacohen N. Defining T cell states associated with response to checkpoint immunotherapy in melanoma. Cell. 2018;175(4):998-1013.e20.
Matsushita K, Takeuchi O, Standley DM, Kumagai Y, Kawagoe T, Miyake T, Satoh T, Kato H, Tsujimura T, Nakamura H, Akira S. Zc3h12a is an RNase essential for controlling immune responses by regulating mRNA decay. Nature. 2009;458(7242):1185–90.
Uehata T, Iwasaki H, Vandenbon A, Matsushita K, Hernandez-Cuellar E, Kuniyoshi K, Satoh T, Mino T, Suzuki Y, Standley DM, Tsujimura T, Rakugi H, Isaka Y, Takeuchi O, Akira S. XMalt1-induced cleavage of regnase-1 in CD4+ helper T cells regulates immune activation. Cell. 2013;153(5):1036.
Bhat N, Virgen-Slane R, Ramezani-Rad P, Leung CR, Chen C, Balsells D, Shukla A, Kao E, Apgar JR, Fu M, Ware CF, Rickert RC. Regnase-1 is essential for B cell homeostasis to prevent immunopathology. J Exp Med. 2021;218(5):20200971.
Shifrut E, Carnevale J, Tobin V, Roth TL, Woo JM, Bui CT, Li PJ, Diolaiti ME, Ashworth A, Marson A. Genome-wide CRISPR screens in primary human T Cells reveal key regulators of immune function. Cell. 2018;175(7):1958-1971.e15.
Wei J, Long L, Zheng W, Dhungana Y, Lim SA, Guy C, Wang Y, Wang YD, Qian C, Xu B, Kc A, Saravia J, Huang H, Yu J, Doench JG, Geiger TL, Chi H. Targeting REGNASE-1 programs long-lived effector T cells for cancer therapy. Nature. 2019;576(7787):471–6.
Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13(4):227–42.
Miller BC, Sen DR, Al Abosy R, Bi K, Virkud YV, LaFleur MW, Yates KB, Lako A, Felt K, Naik GS, Manos M, Gjini E, Kuchroo JR, Ishizuka JJ, Collier JL, Griffin GK, Maleri S, Comstock DE, Weiss SA, Brown FD, Panda A, Zimmer MD, Manguso RT, Hodi FS, Rodig SJ, Sharpe AH, Haining WN. Subsets of exhausted CD8+ T cells differentially mediate tumor control and respond to checkpoint blockade. Nat Immunol. 2019;20(3):326–36.
Lu D, Liu L, Sun Y, Song J, Yin Q, Zhang G, Qi F, Hu Z, Yang Z, Zhou Z, Hu Y, Zhang L, Ji J, Zhao X, Jin Y, McNutt MA, Yin Y. The phosphatase PAC1 acts as a T cell suppressor and attenuates host antitumor immunity. Nat Immunol. 2020;21(3):287–97.
Mahoney KM, Rennert PD, Freeman GJ. Combination cancer immunotherapy and new immunomodulatory targets. Nat Rev Drug Discov. 2015;14(8):561–84.
Wang Y, Swiecki M, Cella M, Alber G, Schreiber RD, Gilfillan S, Colonna M. Timing and magnitude of type i interferon responses by distinct sensors impact CD8 T cell exhaustion and chronic viral infection. Cell Host Microbe. 2012;11(6):631–42.
Wu T, Moseman EA, Xu HC, Manglani M, Kirby M, Anderson SM, Handon R, Kenyon E, Elkahloun A, Wu W, Lang PA, McGavern DB, Schwartzberg PL. The TCF1-Bcl6 axis counteracts type I interferon to repress exhaustion and maintain T cell stemness. Sci immunol. 2016;1(6):eaai8593.
Li J, Lee Y, Li Y, Jiang Y, Lu H, Zang W, Zhao X, Liu L, Chen Y, Tan H, Yang Z, Zhang MQ, Mak TW, Ni L, Dong C. Co-inhibitory molecule B7 superfamily member 1 expressed by tumor-infiltrating myeloid cells induces dysfunction of anti-tumor CD8+ T cells. Immunity. 2018;48(4):773-786.e5.
Waggoner DW, Johnson LB, Mann PC, Morris V, Guastella J, Bajjalieh SM. MuLK, a eukaryotic multi-substrate lipid kinase. J Biol Chem. 2004;279(37):38228–35.
Bektas M, Payne SG, Liu H, Goparaju S, Milstien S, Spiegel S. A novel acylglycerol kinase that produces lysophosphatidic acid modulates cross talk with EGFR in prostate cancer cells. J Cell Biol. 2005;169(5):801–11.
Powell JD, Pollizzi KN, Heikamp EB, Horton MR. Regulation of immune responses by mTOR. Annu Rev Immunol. 2012;30:39–68.
Ray JP, Craft J. PTENtiating autoimmunity through Treg cell deregulation. Nat Immunol. 2015;16(2):139–40.
Geltink RIK, Kyle RL, Pearce EL. Unraveling the complex interplay between T cell metabolism and function. Annu Rev Immunol. 2018;36:461–88.
Hu Z, Qu G, Yu X, Jiang H, Teng XL, Ding L, Hu Q, Guo X, Zhou Y, Wang F, Li HB, Chen L, Jiang J, Su B, Liu J, Zou Q. Acylglycerol Kinase maintains metabolic state and immune responses of CD8+ T Cells. Cell Metab. 2019;30(2):290-302.e5.
Chiossone L, Vienne M, Kerdiles YM, Vivier E. Natural killer cell immunotherapies against cancer: checkpoint inhibitors and more. Semin Immunol. 2017;31:55–63.
Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–74.
Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348(6230):56–61.
Souza-Fonseca-Guimaraes F, Cursons J, Huntington ND. The emergence of natural killer cells as a major target in cancer immunotherapy. Trends Immunol. 2019;40(2):142–58.
Chyuan IT, Tsai HF, Liao HJ, Wu CS, Hsu PN. An apoptosis-independent role of TRAIL in suppressing joint inflammation and inhibiting T-cell activation in inflammatory arthritis. Cell Mol Immunol. 2018;15(9):846–57.
Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9(5):503–10.
Bi J, Tian Z. NK cell dysfunction and checkpoint immunotherapy. Front Immunol. 1999;2019:10.
Smyth, M. J.; Thia, K. Y.; Cretney, E.; Kelly, J. M.; Snook, M. B.; Forbes, C. A.; Scalzo, A. A., Perforin is a major contributor to NK cell control of tumor metastasis. Journal of immunology (Baltimore, Md. : 1950) 1999, 162 (11), 6658–62.
Bottcher JP, Bonavita E, Chakravarty P, Blees H, Cabeza-Cabrerizo M, Sammicheli S, Rogers NC, Sahai E, Zelenay S, ReiseSousa C. NK cells stimulate recruitment of cDC1 into the tumor microenvironment promoting cancer immune control. Cell. 2018;172(5):1022–37.
Guillerey C, Huntington ND, Smyth MJ. Targeting natural killer cells in cancer immunotherapy. Nat Immunol. 2016;17(9):1025–36.
Fuchs A, Cella M, Giurisato E, Shaw AS, Colonna M. Cutting edge: CD96 (Tactile) Promotes NK cell-target cell adhesion by interacting with the poliovirus receptor (CD155). J Immunol. 2004;172(7):3994–8.
Chan CJ, Martinet L, Gilfillan S, Souza-Fonseca-Guimaraes F, Chow MT, Town L, Ritchie DS, Colonna M, Andrews DM, Smyth MJ. The receptors CD96 and CD226 oppose each other in the regulation of natural killer cell functions. Nat Immunol. 2014;15(5):431–8.
Blake SJ, Stannard K, Liu J, Allen S, Yong MCR, Mittal D, Aguilera AR, Miles JJ, Lutzky VP, de Andrade LF, Martinet L, Colonna M, Takeda K, Khnel F, Gurlevik E, Bernhardt G, Teng MWL, Smyth MJ. Suppression of metastases using a new lymphocyte checkpoint target for cancer immunotherapy. Cancer Discov. 2016;6(4):446–59.
Wang Y, Wang C, Qiu J, Qu X, Peng J, Lu C, Zhang M, Zhang M, Qi X, Li G, Hua K. Targeting CD96 overcomes PD-1 blockade resistance by enhancing CD8+ TIL function in cervical cancer. J Immunother Cancer. 2022;10(3): e003667.
Borrego F, Masilamani M, Kabat J, Sanni TB, Coligan JE. The cell biology of the human natural killer cell CD94/NKG2A inhibitory receptor. Mol Immunol. 2005;42(4):485–8.
Andr P, Denis C, Soulas C, Bourbon-Caillet C, Lopez J, Arnoux T, Blry M, Bonnafous C, Gauthier L, Morel A, Rossi B, Remark R, Breso V, Bonnet E, Habif G, Guia S, Lalanne AI, Hoffmann C, Lantz O, Fayette J, Boyer-Chammard A, Zerbib R, Dodion P, Ghadially H, Jure-Kunkel M, Morel Y, Herbst R, Narni-Mancinelli E, Cohen RB, Vivier E. Anti-NKG2A mAb Is a checkpoint inhibitor that promotes anti-tumor immunity by unleashing both T and NK Cells. Cell. 2018;175(7):1731-1743.e13.
Kamiya T, Seow SV, Wong D, Robinson M, Campana D. Blocking expression of inhibitory receptor NKG2A overcomes tumor resistance to NK cells. J Clin Investig. 2019;129(5):2094–106.
Ducoin K, Oger R, Bilonda Mutala L, Deleine C, Jouand N, Desfranois J, Podevin J, Duchalais E, Cruard J, Benlalam H, Labarrire N, Bossard C, Jarry A, Gervois-Segain N. Targeting NKG2A to boost anti-tumor CD8 T-cell responses in human colorectal cancer. OncoImmunology. 2022;11(1):2046931.
Bookstaver ML, Tsai SJ, Bromberg JS, Jewell CM. Improving vaccine and immunotherapy design using biomaterials. Trends Immunol. 2018;39(2):135–50.
Wraith DC. The future of immunotherapy: a 20-year perspective. Front Immunol. 2017;8:1668.
Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161(2):205–14.
Cassetta L, Kitamura T. Macrophage targeting: opening new possibilities for cancer immunotherapy. Immunology. 2018;155(3):285–93.
Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168(4):707–23.
Smyth MJ, Ngiow SF, Ribas A, Teng MWL. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat Rev Clin Oncol. 2016;13(3):143–58.
Gentles AJ, Newman AM, Liu CL, Bratman SV, Feng W, Kim D, Nair VS, Xu Y, Khuong A, Hoang CD, Diehn M, West RB, Plevritis SK, Alizadeh AA. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med. 2015;21(8):938–45.
Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51.
Zhang Q, Liu L, Gong C, Shi H, Zeng Y, Wang X, Zhao Y, Wei Y. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS ONE. 2012;7(12):50946.
DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, Gallagher WM, Wadhwani N, Keil SD, Junaid SA, Rugo HS, Shelley Hwang E, Jirstrm K, West BL, Coussens LM. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1(1):54–67.
Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14(7):399–416.
Janson C, Jung H, Ertl L, Liu S, Dang T, Zeng Y, Krasinski A, McMahon J, Zhang P, Charo I, Singh R, Schall TJ. Inhibition of CCR2 potentiates checkpoint inhibitor immunotherapy in murine model of pancreatic cancer. Can Res. 2017;77:5655–5655.
Kitamura T, Qian BZ, Soong D, Cassetta L, Noy R, Sugano G, Kato Y, Li J, Pollard JW. CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages. J Exp Med. 2015;212(7):1043–59.
Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475(7355):222–5.
Ries CH, Cannarile MA, Hoves S, Benz J, Wartha K, Runza V, Rey-Giraud F, Pradel LP, Feuerhake F, Klaman I, Jones T, Jucknischke U, Scheiblich S, Kaluza K, Gorr IH, Walz A, Abiraj K, Cassier PA, Sica A, Gomez-Roca C, DeVisser KE, Italiano A, LeTourneau C, Delord JP, Levitsky H, Blay JY, Rttinger D. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell. 2014;25(6):846–59.
Smith TD, Tse MJ, Read EL, Liu WF. Regulation of macrophage polarization and plasticity by complex activation signals. Integr Biol (UK). 2016;8(9):946–55.
Zhang YH, He M, Wang Y, Liao AH. Modulators of the balance between M1 and M2 macrophages during pregnancy. Front Immunol. 2017;8:120.
Qian H, Zhou T, Fu Y, Guo M, Yang W, Zhang D, Fang W, Yao M, Shi H, Chai C, Cheng W, Ding S, Chen T. Self-assembled tetrahedral framework nucleic acid mediates tumor-associated macrophage reprogramming and restores antitumor immunity. Mol Ther Nucl Acids. 2022;27:763–73.
Irving M, de Silly RV, Scholten K, Dilek N, Coukos G. Engineering chimeric antigen receptor T-cells for racing in solid tumors: don’t forget the fuel. Front Immunol. 2017;8:267.
June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science. 2018;359(6382):1361–5.
Khan ANH, Kolomeyevskaya N, Singel KL, Grimm MJ, Moysich KB, Daudi S, Grzankowski KS, Lele S, Ylagan L, Webster GA, Abrams SI, Odunsi K, Segal BH. Targeting myeloid cells in the tumor microenvironment enhances vaccine efficacy in murine epithelial ovarian cancer. Oncotarget. 2015;6(13):11310–26.
Portielje JEA, Gratama JW, Van Ojik HH, Stoter G, Kruit WHJ. IL-12: A promising adjuvant for cancer vaccination. Cancer Immunol Immunother. 2003;52(3):133–44.
Krneta T, Gillgrass A, Chew M, Ashkar AA. The breast tumor microenvironment alters the phenotype and function of natural killer cells. Cell Mol Immunol. 2016;13(5):628–39.
Download references
This work was supported by the National Natural Science Foundation of China (Grant No. 81974258) and the Fundamental Research Funds for the Central Universities (Grant No. WK3520000011).
Author information
Ajmeri Sultana Shimu and Hua-xing Wei have contributed equally to this work.
Authors and Affiliations
Department of Medical Oncology, Division of Life Sciences and Medicine, The First Affiliated Hospital of USTC, University of Science and Technology of China, Hefei, 230001, China
Ajmeri Sultana Shimu, Qiangsheng Li, Xucai Zheng & Bofeng Li
Department of Laboratory Medicine, Division of Life Sciences and Medicine, The First Affiliated Hospital of USTC, University of Science and Technology of China, Hefei, 230001, China
Hua-xing Wei
You can also search for this author in PubMed Google Scholar
Contributions
ASS and HW wrote the manuscript. Qiangsheng Li drew the figures. XZ and BL provided the guidance and revised the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding authors
Correspondence to Xucai Zheng or Bofeng Li .
Ethics declarations
Conflict of interest.
The authors declare no conflict of interest.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Shimu, A.S., Wei, Hx., Li, Q. et al. The new progress in cancer immunotherapy. Clin Exp Med 23 , 553–567 (2023). https://doi.org/10.1007/s10238-022-00887-0
Download citation
Received : 30 June 2022
Accepted : 30 August 2022
Published : 15 September 2022
Issue Date : July 2023
DOI : https://doi.org/10.1007/s10238-022-00887-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Immunotherapy
- Immune checkpoint
- Find a journal
- Publish with us
- Track your research
Watch Now : CRI’s Patient Immunotherapy Summit
Immune to Cancer: The CRI Blog

How CRI’s Immunotherapy Breakthroughs and Research are Shaping Cancer Treatment and Prevention
Today, cancer immunotherapy is the most forward-thinking and innovative form of cancer treatment for patients. Cancer immunotherapy is especially effective with treating melanoma, lung, breast, and several other types of cancer. When possible, however, there is a preferable option compared to treatment: the prevention of cancer entirely.
Want to do something big for cancer immunotherapy research? Make a donation today to the Cancer Research Institute .
CRI scientists are committed to groundbreaking cancer immunotherapy research that can benefit the lives of patients and potentially save lives. In addition to research regarding treating existing cancers, some CRI scientists are also working on forward-thinking research that can address cancer prevention and attack cancer at its roots.
CRI Scientists’ Innovative Work and Perspectives on Cancer Treatment and Prevention
1. Cancer Vaccine Discoveries
Elizabeth Jaffee, MD , deputy director of The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins and CRI Scientific Advisory Council associate director, serves on the panel for President Joe Biden’s Cancer Moonshot Initiative . Additionally, Dr. Jaffee’s research focuses on novel cancer vaccines, and she has patents for six of them. Dr. Jaffee foresaw the immense potential of cancer vaccines long before others did. At the 2023 CRI Patient Immunotherapy Summit , she succinctly outlined the transformative power of these vaccines.
“Vaccines are the biggest success story of the 20 th century other than penicillin,” Dr. Jaffee says. “We have suggested developing new technologies and new computational approaches that can take all of the new data we are generating and put it into a framework, biologically, that tells us which signals a tumor is sending out to cells around it to cause it to protect the tumor. Through that information we can develop drugs that can intercept those signals. We can now take vaccines, combine them with drugs, and we can make a difference. We are seeing vaccines close to approval for cancers like melanoma. We are going to see this happening more and more over the next five years.”
2. Measuring Immunotherapy Response in a Single Drop of Blood
Valsamo (Elsa) Anagnostou, MD, PhD , director of the thoracic oncology biorepository at Johns Hopkins, leader of Precision Oncology Analytics, co-leader of the Johns Hopkins Molecular Tumor Board, co-director of the Lung Cancer Precision Medicine Center of Excellence, CRI Torrey Coast Foundation GEMINI CLIP Investigator, and CRI Clinical Accelerator is at the forefront of leveraging cutting-edge technologies to advance diagnosis and therapy response. Her pioneering work has unleashed the power of liquid biopsies to test ctDNA (circulating tumor DNA) in patient blood, revolutionizing our ability to gauge patient responses to treatment. Liquid biopsies involve drawing small samples of blood from patients for testing. “ctDNA response is particularly informative to understand the complexity of stable disease on imaging, which represents a sizable fraction of patients in whom imaging fails to timely and accurately detect the magnitude of therapeutic response,” Dr. Anagnostou says. “ctDNA response correlated with tumor size seen on imaging, which is the gold standard for monitoring response to cancer treatments and seemed to be better correlated with survival.”
Liquid biopsies could be the first step in preventing excessive follow-up procedures and scans. This is a technology that can further be developed to test for markers in blood that can indicate presence of undetectable tumors or the presence of cancer cells even before they become large enough tumors to be detected using traditional scans.
3. The Tumor Microenvironment Holds the Answer to Cancer
Max Krummel, PhD, Robert E. Smith Endowed Chair in Experimental Pathology at the University of California San Francisco (UCSF), Professor, Department of Pathology at UCSF, and former CRI Investigator Award recipient, emphasizes the need to broaden our perspective on cancer prevention beyond the current focus on boosting T cell responses through checkpoint blockade. While acknowledging the significance of enhancing T cell activity, Dr. Krummel sees an equally promising avenue in understanding and targeting the tumor microenvironment (TME). The TME is comprised of the non-cancerous cells, blood vessels, and molecules that surround and sustain a tumor cell. He highlights, “We have started to think about the fundamental biology of the tumor and how to target [that].” This shift in focus towards comprehending the intricacies of the tumor microenvironment underscores the importance of exploring diverse approaches in our efforts to combat cancer effectively.
In the U.S. alone, about 600,000 people die from cancer annually. While treatment methods have improved in recent years, particularly with immunotherapy, there is no silver bullet for cancer prevention, there are several measures people can take to try and safeguard against a potential cancer diagnosis (via the Mayo Clinic).
Measures That can Help Prevent Cancer
1. Screen Early
Different populations are at greater risk of diagnosis depending on the type of cancer. For former and current smokers, screening against lung cancer is critical. Another example is that for women between 40-75 years old, having a mammogram every two years is greatly encouraged to guard against breast cancer .
Additionally, there are also tests that can detect specific cancer-related mutations that are routinely performed to determine if someone is at risk for cancer.
2. Limit Exposure to Harmful UV Rays
Limiting the amount of time your skin is exposed to the sun and avoiding tanning booths is a good way to safeguard against skin cancer. If you are going to be in the sun, applying sunscreen and covering your skin as best you can be good safety measures.
3. Consider Cancer Vaccines
There are currently four distinct preventative cancer vaccines for HPV and HBV-associated cancers that have been approved by the FDA. Viral infections have proven responsible for several cancers, and preventative cancer vaccines are an important tool to help thwart off cancer before it can develop.
Additionally, there are two approved therapeutic cancer vaccines for bladder and prostate cancers. These vaccines help the immune system identify cancer cells so they can be eliminated.
4. Maintain a Healthy Diet
Certain dietary measures, such as reducing one’s intake of red meat, can help reduce an individual’s risk of a cancer diagnosis . A healthy diet is one that is focused on fruits and vegetables, while avoiding refined sugars and excess animal fat.
Between new developments in cancer immunotherapy on the horizon and greater education of the public regarding preventative measures, there is potential for a greater collective focus on cancer prevention, and therefore, a world immune to cancer.
Let's spread the word about Immunotherapy! Click to share this page with your community.
This website uses tracking technologies, such as cookies, to provide a better user experience. If you continue to use this site, then you acknowledge our use of tracking technologies. For additional information, review our Privacy Policy .
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
January 17, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
New research tool seeks to accelerate hunt for cancer immunotherapy targets
by Cincinnati Children's Hospital Medical Center
An innovative computational tool dubbed "SNAF" may help the research world bring the emerging promise of cancer immunotherapy to a wider range of patients, according to a study published in Science Translational Medicine.
The research tool, called the Splicing Neo Antigen Finder (SNAF), was developed by a multidisciplinary team of researchers from Cincinnati Children's and the University of Virginia. The project was led by Guangyuan Li, Ph.D., and Nathan Salomonis, Ph.D., both with the Division of Biomedical Informatics at Cincinnati Children's.
The co-authors say the tool has already helped uncover shared immunogenic targets across various cancers, which could pave the way for a new wave of highly focused cancer treatments.
"The implications of this discovery are significant," says H. Leighton "Lee" Grimes, Ph.D., a co-author of the study and director of the Cancer Pathology Program at Cincinnati Children's. "By identifying shared splicing neoantigens present in up to 90% of cancer patients , SNAF not only presents new targets for therapy but also challenges and expands our understanding of cancer biology."
How the new tool finds new targets
Immunotherapy, which harnesses a patient's immune system to fight cancer, often targets neoantigens produced from genetic mutations. However, this approach typically benefits only those with a high mutational burden. SNAF aims to expand the universe of immunotherapy by identifying neoantigens arising from post-transcriptional modifications, particularly splicing errors, which have been largely untapped until now.
Using new artificial intelligence approaches, SNAF predicts immunogenic peptides that T cells can recognize and novel proteins with altered extracellular components that B cells can target. This dual approach is vital in developing comprehensive immunotherapies that engage both arms of the adaptive immune system.
Promising target emerges for melanoma
While cataloging all possible neoantigens produced from alternative mRNA pathways, the team found that the amount of splicing neoantigens is correlated with patient survival and responses to immunotherapy in patients with melanoma.
One such prediction, SLC45A2, has emerged as a particularly promising target due to its high tumor specificity and immunogenicity.
In addition to T-cell neoantigens, the team has discovered a novel class of tumor-specific extracellular neo-epitopes, termed ExNeoEpitopes, through their B-cell focused pipeline, SNAF-B. These ExNeoEpitopes show great promise for the development of monoclonal antibodies and CAR-T cell therapies.
"This is only the beginning," says Tamara Tilburgs, Ph.D., co-corresponding author and a researcher in the Division of Immunobiology. "The SNAF workflow's flexibility means it can be continuously adapted as we make further inroads into understanding and combating cancer ."
Salomonis and his team are now applying these tools in the most difficult-to-treat cancers to find the best targets for therapy development and understand their single-cell origins.
Explore further
Feedback to editors

Study finds that dopamine projections to the amygdala contribute to encoding identity-specific reward memories
29 minutes ago

Presence of specific lipids indicate tissue aging and can be decreased through exercise, study shows

PFAS exposure from high-seafood diets may be underestimated, finds study

Asia-Pacific gets new weapon in fight against drug-resistant TB
2 hours ago

Two key brain systems are central to psychosis, study finds
10 hours ago
COVID-19 vaccine effectiveness: Results from Norway demonstrate the reproducibility of federated analytics
14 hours ago
Elucidating the link between Guillain–Barré syndrome and Takotsubo cardiomyopathy
15 hours ago

Artificial intelligence can help people feel heard, study finds

A new diagnostic model offers hope for Alzheimer's
16 hours ago
New study validates prediction rules for pediatric intra-abdominal and traumatic brain injuries
Related stories.
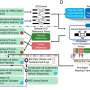
New computational tool identifies novel targets for cancer immunotherapy
May 17, 2023
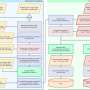
New machine-learning method may aid personalized cancer therapy
Aug 9, 2023

Epigenetically acting drugs could support cancer immunotherapy
Oct 24, 2023
Newly discovered class of molecules may boost cancer vaccine development
Aug 17, 2018

Algorithm offers new way to spot patients likely to respond to immunotherapy
Mar 10, 2023
Researchers identify potential formula for blood cancer vaccine
Dec 19, 2019
Recommended for you
Successful murine model of dermatomyositis reveals underlying immune system involvement
17 hours ago
New microfluidic device for cancer detection achieves precise separation of tumor entities
18 hours ago
Potential therapeutic target for small cell lung cancer discovered
20 hours ago

Researchers stimulate gene that enhances CAR T-cell treatments for solid tumors
Blood stem cells unlock clues for helping sepsis patients fight recurring infections
19 hours ago
Scientists uncover key resistance mechanism to Wnt inhibitors in pancreatic and colorectal cancers
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
- Skip to main content
- Keyboard shortcuts for audio player
- Your Health
- Treatments & Tests
- Health Inc.
- Public Health
After 40 years of smoking, she survived lung cancer thanks to new treatments

Yuki Noguchi

Denise Lee on her last day of chemo. In addition to chemo and surgery, she was treated with immunotherapy. She's currently in remission. Denise Lee hide caption
Denise Lee on her last day of chemo. In addition to chemo and surgery, she was treated with immunotherapy. She's currently in remission.
Denise Lee grew up in Detroit in the mid-1970s and went to an all-girls Catholic high school. She smoked her first cigarette at age 14 at school, where cigarettes were a popular way of trying to lose weight.
Instead, her nicotine addiction lasted four decades until she quit in her mid-50s.
"At some point it got up as high as 2.5 packs a day," Lee, 62, recalls.
Yet she didn't think about lung cancer risk — until she saw a billboard urging former smokers to get screened. Lee, a retired lawyer living in Fremont, Calif., used to drive past it on her way to work.
"The thing that caught my attention was the fact that it was an African American female on the front," she recalls.

Shots - Health News
The american cancer society says more people should get screened for lung cancer.
She eventually got the low-dose CT scan recommended for current and former smokers. When doctors found an early, but dangerous, tumor, Lee cried and panicked. Her mother had cared for her father, who'd died of prostate cancer. "My biggest concern was telling my mom," she says.
But that was six years ago, and Lee is cancer free today. Surgery removed the 2-inch tumor in her lung, then new treatments also boosted her immune system, fighting off any recurrence.
Lung cancer remains the most lethal form of the disease, killing about 135,000 Americans a year – more than breast, prostate and colon cancer combined – which is why many people still think of a diagnosis as synonymous with a death sentence. But with new treatments and technology, the survival rates from lung cancer are dramatically improving, allowing some patients with relatively late-stage cancers to live for years longer.
"If you're gonna have lung cancer, now is a good time," Lee says of the advances that saved her.

Denise Lee has been cancer-free for six years. She says she's grateful she got screened and caught her lung cancer early enough that treatment has been effective. Denise Lee hide caption
Denise Lee has been cancer-free for six years. She says she's grateful she got screened and caught her lung cancer early enough that treatment has been effective.
The key breakthrough, says Robert Winn, a lung cancer specialist at Virginia Commonwealth University, is the ability to better pinpoint the mutations of a patient's particular form of cancer. In the past, treatments were blunt tools that caused lots of collateral damage to healthy parts of the body while treating cancer.
"We've gone from that to molecular characterization of your lung cancer, and it has been a game changer," Winn says. "This is where science and innovation has an impact."
One of those game-changing treatments is called targeted therapy . Scientists identify genetic biomarkers in the mutated cancer cells to target and then deliver drugs that attack those targets, shrinking tumors.

CRISPR gene-editing may boost cancer immunotherapy, new study finds
Another is immunotherapy, usually taken as a pill, which stimulates the body's own defense system to identify foreign cells, then uses the immune system's own power to fight the cancer as if it were a virus.
As scientists identify new cancer genes, they're creating an ever-broader array of these drugs.
Combined, these treatments have helped increase national survival rates by 22% in the past five years – a rapid improvement over a relatively short time, despite the fact that screening rates are very slow to increase. Winn says as these treatments get cheaper and readily available, the benefits are even reaching rural and Black populations with historic challenges accessing health care.
The most remarkable thing about the drugs is their ability to, in some cases, reverse late-stage cancers. Chi-Fu Jeffrey Yang, a thoracic surgeon at Harvard, recalls seeing scans where large dark shadows of tumor would disappear: "It was remarkable to see the lung cancer completely melting away."
To Yang, such progress feels personal. He lost his beloved grandfather to the disease when Yang was in college. If he were diagnosed today, he might still be alive.
"Helping to take care of him was a big reason why I wanted to be a doctor," Yang says.
But the work of combating lung cancer is far from over; further progress in lung cancer survival hinges largely on getting more people screened.
Low-dose CT scans are recommended annually for those over 50 who smoked the equivalent of a pack a day for 20 years. But nationally, only 4.5% of those eligible get those scans , compared to rates of more than 75% for mammograms.
Andrea McKee, a radiation oncologist and spokesperson for the American Lung Association, says part of the problem is that lung cancer is associated with the stigma of smoking. Patients often blame themselves for the disease, saying: "'I know I did this to myself. And so I don't I don't think I deserve to get screened.'"
McKee says that's a challenge unique to lung cancer. "And it just boggles my mind when I hear that, because, of course, nobody deserves to die of lung cancer."
Denise Lee acknowledges that fear. "I was afraid of what they would find," she admits. But she urges friends and family to get yearly scans, anyway.
"I'm just so grateful that my diagnosis was early because then I had options," she says. "I could have surgery, I could have chemotherapy, I could be a part of a clinical trial."
And all of that saved her life.
- lung cancer screening
- immunotherapy
- lung cancer
REVIEW article
Car products from novel sources: a new avenue for the breakthrough in cancer immunotherapy.

- Department of Hematology, Sichuan Academy of Medical Sciences and Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu, China
Chimeric antigen receptor (CAR) T cell therapy has transformed cancer immunotherapy. However, significant challenges limit its application beyond B cell-driven malignancies, including limited clinical efficacy, high toxicity, and complex autologous cell product manufacturing. Despite efforts to improve CAR T cell therapy outcomes, there is a growing interest in utilizing alternative immune cells to develop CAR cells. These immune cells offer several advantages, such as major histocompatibility complex (MHC)-independent function, tumor microenvironment (TME) modulation, and increased tissue infiltration capabilities. Currently, CAR products from various T cell subtypes, innate immune cells, hematopoietic progenitor cells, and even exosomes are being explored. These CAR products often show enhanced antitumor efficacy, diminished toxicity, and superior tumor penetration. With these benefits in mind, numerous clinical trials are underway to access the potential of these innovative CAR cells. This review aims to thoroughly examine the advantages, challenges, and existing insights on these new CAR products in cancer treatment.
1 Introduction
Cancer represents a significant global public health challenge and remains one of the primary causes of mortality worldwide. According to the International Agency for Research on Cancer, there are approximately 19.3 million new cancer diagnoses and nearly 10.0 million cancer-related deaths each year ( 1 ). The development of cellular immunotherapy has profoundly changed cancer treatments. Inspired by allogeneic hematopoietic stem cell transplantation (HSCT), researchers have harnessed the immune system to target and eliminate cancer cells. This is achieved through the use of chimeric antigen receptors (CARs), synthetic receptors that redirect T cell specifically against cells with certain antigens. Thus, CAR T cell therapy has become a major breakthrough in immunotherapy ( 1 ).
Despite its notable successes, CAR T cells’ exceptional efficacy is mainly limited to B cell-driven hematological malignancies. Expanding its applicability to a wider range of cancers faces numerous obstacles ( 2 ). For instance, tumor cells frequently develop complex mechanisms to evade eradication, such as loss-of-antigen. It is reported that CD19-negative relapse accounts for around 50% of relapse after CD19-CAR T cell therapy in patients with B cell-driven malignancy ( 3 ). The tumor microenvironment (TME) frequently exhibits immunosuppressive characteristics. These include the presence of immunosuppressive cells and upregulated inhibitory immune checkpoints, which can compromise the effectiveness of CAR T cells in vivo . Continuous and intense stimulation may make T cells prone to exhaustion, raising concerns about the long-term efficacy and persistence of CAR T cells in tumors. Some clinical trials even reported that their mesothelin-CAR T cells can only persist in patient’s body for 28 days, resulting undesirable outcomes ( 4 ). In solid tumors, CAR T cells often exhibit suboptimal tumor infiltration. Besides, producing autologous cell products is labor-intensive and time-consuming. The starting material of the autologous products varies in each therapy, adding to the complexity. Importantly, CAR T cell therapy is associated with severe adverse effects. Life-threatening complications, such as cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS), often lead to treatment failures ( 2 ).
The immune system is a complex defense network, encompassing a wide range of immune cells. They come equipped with a variety of capacities, and these inherent properties may help overcome the challenges of conventional CAR T cell therapies. As a result, there is increasing interest in introducing CAR constructs into various immune cells. This review will focus on the latest and most promising advancements in new CAR cell therapies.
2 CAR cells generated from various T cell subtypes
The polyclonal nature of conventional CAR T cells’ endogenous TCRs introduces a heightened risk of off-target effects ( 5 ). Additionally, their antitumor activity is largely dependent on CAR receptors, which can be undermined by antigen loss, CAR downregulation, or the immunosuppressive effects of the tumor microenvironment (TME) ( 6 ). These factors raise significant concerns regarding the in vivo efficacy and potential toxicities of CAR T cell therapies. However, leveraging specific T cell subtypes to create CAR constructs could offer a solution. These new T cell-derived CAR cells might possess intrinsic, CAR-independent cytotoxic capabilities.
2.1 CAR NKT cells
2.1.1 properties and advantages.
Natural killer T (NKT) cells play a crucial role in innate tumor surveillance and exhibit significant antitumor activity ( 7 – 9 ). Type I or invariant NKT (iNKT) cells are the major subset of NKT cells with an identical or invariant T cell receptor (iTCR). This receptor uniquely recognizes lipid antigens presented by the non-polymorphic molecule CD1d, instead of traditional MHC molecules ( 10 – 12 ). This distinction provides several benefits for developing CAR cells from NKT cells. Specifically, the iTCR-CD1d interaction empowers CAR NKT cells to retain robust TCR signaling, enabling antitumor responses that extend beyond CAR activity ( 13 ). Additionally, the inherent CAR-independent cytotoxicity of iNKT-derived CAR NKT cells is naturally limited by CD1d expression ( 14 , 15 ). This restriction helps confine potential toxicities associated with iNKT cell activity primarily to CD1d-positive tissues ( 13 ). Moreover, CAR NKT cells may alter the immunosuppressive TME in a CD1d-dependent manner. Despite limited CD1d expression in most human tumors, tumor-associated macrophages (TAMs) in various cancers do express CD1d, allowing iNKT cells to either lyse ( 16 ) or remodel ( 17 , 18 ) these TAMs to enhance antitumor responses. Furthermore, in influenza A infections ( 19 ) or breast cancer ( 20 ), iNKT cells can inhibit myeloid-derived suppressor cells (MDSC) via CD1d interactions, mitigating immunosuppression ( Figure 1 ).
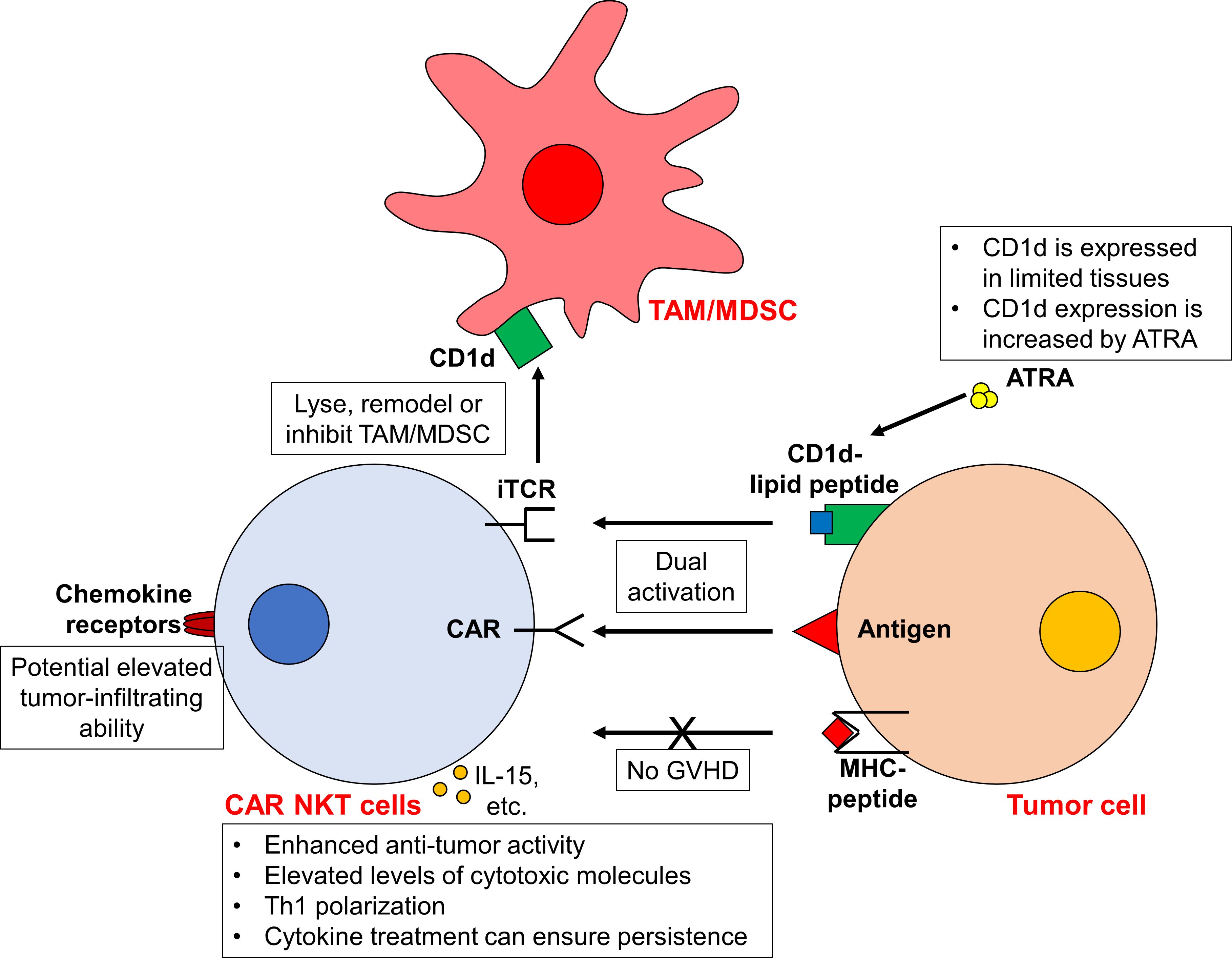
Figure 1 CAR NKT cells exhibit enhanced antitumor activity against tumor cells. Due to the unique iTCR-CD1d interaction, CAR NKT cells display both CAR-dependent and CAR-independent (i.e. iTCR-dependent) cytotoxicity, leading to enhanced antitumor activity. The expression of CD1d in tumor cells can be increased using ATRA, further boosting the iTCR-dependent activity. Furthermore, CAR NKT cells can target TAMs/MDSCs in a CD1d-dependent manner. Moreover, as the activity of iTCR is MHC-independent, the risk of GVHD is low. Upon activation, these CAR NKT cells are Th1 polarized and express higher cytotoxic molecules. The administration of cytokines (such as IL-15) may prolong the in vivo persistence of CAR NKT cells. In addition, the elevated expression of chemokine receptors on NKT cells may confer the ability of CAR NKT cells to infiltrate into tumors. However, it should be noted that CD1d is only expressed in certain tissues and cell types. While the toxicity from CAR NKT cells is limited, concerns arise that CAR NKT cells is probably only applicable to CD1d + tumors.
In addition to the CD1d-related advantages, iNKT cells also stand out for their enhanced ability to migrate towards tumors. This is due to their high levels of chemokine receptors such as CCR1, CCR2, CCR4, CCR5, CCR6, CXCR3 and CXCR4, compared to conventional T cells ( 21 ) ( Figure 1 ). For example, the expression of CCR4 facilitates the skin homing of T cells, which may direct CAR iNKT cells towards skin-associated malignancies ( 22 ). The high expression of CXCR4 on iNKT cells can promote bone marrow migration as well ( 23 ). Moreover, research has documented a notable presence of tumor-infiltrating human NKT cells in a murine lymphoid tumor model ( 24 ). Furthermore, increased NKT cell infiltration within tumors is correlated with improved clinical outcomes ( 25 – 30 ). However, a high degree of heterogeneity exists within iNKT cells. While CXCR3 and CXCR4 are expressed in over 90% of iNKT cells, only 30% of iNKT cells express CCR4 (which are mostly CD4 + iNKT cells). The expression of other chemokine receptors varies as well ( 21 ). Therefore, the tumor infiltration ability of each CAR NKT cell should be carefully assessed. The function of different iNKT cell subtypes is also different. For example, while CD4 + iNKT cells secrete both Th1 and Th2 cytokines, CD4 - CD8 - and CD8 + iNKT cells only generate Th1 cytokines ( 21 ). Whether this heterogeneity affect the activity of CAR iNKT cells awaits further investigation. It may be necessary to isolate subtypes of iNKT cells for CAR transduction.
Another promising benefit of CAR NKT cells is the potential allogeneic applications ( Figure 1 ). This is because NKT cell activity does not depend on MHC molecules. Researches have shown that donor-derived NKT cells can mitigate acute graft-versus-host disease (aGVHD) in animal models and clinical trials after HSCT ( 31 – 36 ). For instance, CD4 - NKT cells can suppress T cell proliferation and IFN-γ secretion through direct contact ( 34 ). Similarly, CD4 + NKT cells not only suppress effector T cell proliferation but also retain their graft-versus-tumor effects ( 33 ) and facilitate regulatory T cell expansion ( 31 ). Therefore, the risk of allogeneic CAR NKT cells inducing severe GVHD appears minimal. However, whether this Treg-like function affects the antitumor activity of CAR NKT cells and endogenous immune cells requires further investigation.
Peripheral blood mononuclear cells (PBMCs) are commonly used as the starting materials for generating CAR NKT cells. However, there is still no consensus on the specific procedure for processing NKT cells. For example, NKT cells can be isolated using TCRVα24 + TCRVβ11 + marker (0.13% of PBMCs) ( 13 ), Vα24-Jβ18 + marker (0.225% of PBMCs) ( 37 ), anti-iNKT microbeads (TCR α-chain Vα24-Jα18, percentage not reported) ( 38 ), or CD3 + CD56 + NKT cell isolation kit (2% of PBMCs) ( 39 ). Once isolated, NKT cells can be transduced directly or after initial expansion. Such expansion is achieved by using anti-CD3/CD28-mediated stimulation and/or iNKT cell agonist alpha-galactosylceramide (αGalCer)-pulsed cells, in the presence of IL-15 or IL-2 ( 13 , 38 , 39 ). Rotolo et al. conducted a study comparing different protocols and suggested that TCRVα24 + TCRVβ11 + selection, followed by CD3/CD28-activation in the presence of autologous antigen-presenting cells (APCs) and IL-15, can effectively generate viable CAR NKT cells ( 13 ). However, further investigations are still needed.
2.1.2 Current study
Exploiting the unique properties of NKT cells, numerous preclinical studies have focused on creating diverse CAR NKT cells to target various tumors. For instance, CD19 and CD1d are commonly expressed in various B cell malignancies ( 40 ). Building on this, Rotolo et al. engineered CD19-CAR NKT cells to specifically target CD19 + B cell lymphomas ( 13 ). These engineered cells achieved enhanced anti-lymphoma effects in vitro and in vivo by activating both the iTCR-CD1d and CAR-CD19 signaling pathways ( 13 ). Compared to conventional CD19-CAR T cells, CD19-CAR NKT cells manifested elevated levels of cytotoxic molecules such as IFNγ, perforin, and granzymes and displayed a pronounced Th1 polarization. Furthermore, the study also found that increasing CD1D expression in human B cells with the RARα ligand all-trans retinoic acid (ATRA) can increase the cytotoxicity of CD19-CAR NKT cells ( 13 ). Similarly, GD2-targeted CAR NKT cells designed for neuroblastoma exhibited inherent iTCR-dependent activity, including the ability to target TAMs, and demonstrated Th1 polarization ( 38 ). Additionally, Simon et al. highlighted that chondroitin sulfate proteoglycan 4 (CSPG4)-specific CAR NKT cells, designed for melanoma, maintained the ability to eliminate target cells through their endogenous TCRs, a mechanism independent from CAR-induced activity. These cells showed a comparable, if not superior, cytotoxicity relative to traditional CAR T cells ( 39 ). Likewise, CD38- and BCMA-CAR NKT cells exhibited dual CAR-dependent and iTCR-dependent activity against multiple myeloma (MM) cells ( 41 ). These examples underline the potential of CAR NKT cells for potentiated antitumor activity with reduced off-tumor effects ( 41 ).
The enhanced expression of chemokine receptors on CAR NKT cells potentially increases their tumor infiltration ability. Specifically, both CD19-CAR NKT cells and GD2-CAR NKT cells have demonstrated superior tumor infiltration ability in solid tumors when compared to the conventional CAR T cells ( 13 , 38 , 42 ). Moreover, the increased expression of ITGA4 and ITGB1 in CAR NKT cells facilitates the passage through the blood-brain barrier and supports the eradication of brain tumors ( 13 ).
For allogeneic or off-the-shelf applications, the infusion of humanized CAR NKT cells does not lead to clinically notable xenograft GVHD in mouse xenograft tumor models ( 13 , 38 ). Such complications are routinely observed following the administration of conventional CAR T cells ( 43 ). This observation suggests the potential for allogeneic CAR NKT cells to similarly avoid inducing acute GVHD in human. Furthermore, the inherent immunomodulatory properties of CAR NKT cells may help to reduce adverse effect elicited by endogenous immune cells. However, further studies are essential to substantiate this hypothesis ( Figure 1 ).
2.1.3 Challenges & solutions
While CAR NKT cells present a promising therapeutic intervention, two primary challenges persist. Firstly, the function of CAR NKT cells is somewhat dependent on CD1d, which is limited to specific cell types ( 14 , 15 ). While this reliance restricts off-target toxicity, it also confines their effectiveness largely to CD1d + tumors. Furthermore, loss-of-antigen relapse is a common cause of failure in CAR cell therapy ( 44 ). During CAR NKT cell treatment, tumor cells might downregulate their CD1d expression. However, this may not represent an insurmountable barrier for CAR NKT cells. Typically, B cell chronic lymphocytic leukemia (CLL) cells have low or absent CD1d expression ( 29 , 40 ). Yet, they can still be targeted by CD19-CAR NKT cells via both CAR and CD1d-dependent mechanism ( 13 ). The application of ATRA to modulate CD1d expression highlights a method to enhance both the efficacy and safety of CAR NKT cell treatments as well ( 13 ). Complementing this observation, Heczey et al. showed that GD2-CAR NKT cells could effectively target GD2 + CD1d - neuroblast cells ( 38 ). This suggests that CAR NKT cells can operate purely through CAR-induced signaling, akin to traditional CAR T cells. Nevertheless, whether the effectiveness of such CAR-mediated cytotoxicity is comparable with that of conventional CAR T cells requires further exploration.
Another primary challenge is the transient persistence of CAR NKT cells, often requiring repeat doses for sustain tumor control ( 38 ). Conventional CAR T cells benefit from design improvements, such as the tailored design of costimulatory domains, and the cytokines treatment like IL-15 ( 44 ). Similar efforts have been made for CAR NKT cells to extend their durability ( 38 , 42 ) ( Figure 1 ). Furthermore, CD62L + NKT cells exhibit markedly superior proliferative capabilities compared to the CD62L - NKT cells. Leveraging CD62L + cells for CAR NKT cell production results in enhanced persistence and antitumor effects, as evidenced in murine lymphoma and neuroblastoma models ( 45 ). Liu et al. further demonstrated that these memory-like CD62L + NKT cells can be induced upon cytokine administration ( 46 ). By incorporating IL-21 into B7H3-CAR NKT cells, they increased the frequency of CD62L + subsets in these CAR NKT cells. In mouse renal cancer xerograph models, such IL-21-armored B7H3-CAR NKT cells showed reduced exhaustion, significantly prolonged proliferation and extended persistence without obvious adverse effects ( 46 ). These insights suggest new avenues for optimizing the long-term efficacy of CAR NKT cells ( Table 1 ).
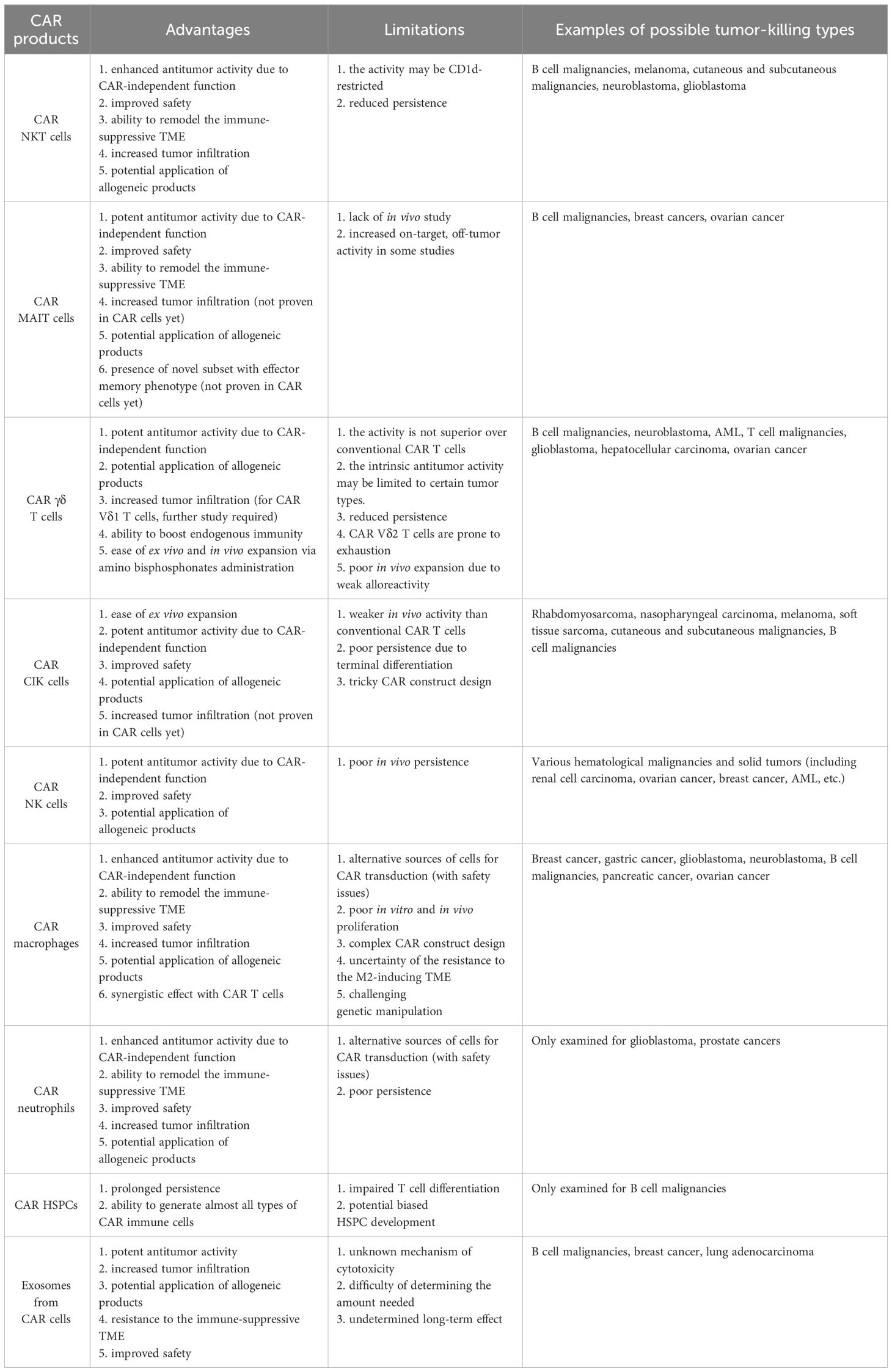
Table 1 Advantages and limitations of novel CAR products.
2.1.4 Ongoing clinical trials
Presently, two phase I clinical trials have published interim results: one evaluates autologous GD2-CAR NKT cells with IL-15 in children with relapsed or resistant neuroblastoma (NCT03294954), while the other focuses on allogeneic CD19-CAR NKT cells with IL-15 targeting relapse and refractory B cell malignancies (NCT03774654). Preliminary analysis of the former trial (in 2020) highlighted promising aspects such as in vivo expansion, tumor infiltration, and lack of dose-limiting toxicity associated with GD2-CAR NKT cells ( 47 ). However, merely one out of the 11 participants showed an objective response ( 47 ). In their latest interim reports (in 2023), the number of patients enrolled has increased to 12, and the objective response rate reached 25% (3/12, including 2 partial responses and 1 complete response) ( 48 ). It is noteworthy that the frequency of memory-like CD62L + subsets is positively correlated with the in vivo expansion and therapeutic outcomes, further suggesting that the persistence is one of the limiting factors for CAR NKT cell therapies. The team also identified that BTG anti-proliferation factor 1 (BTG1) as a key driver for hyporesponsiveness in CAR NKT cells ( 48 ). Meanwhile, allogeneic CD19-CAR NKT cells demonstrated both safety and in vivo expansion, yielding a complete response rate of 40% and a partial response rate of 40% ( 49 ). Another two clinical studies are actively assessing the efficacy of iNKT cells that co-express both CD19-CAR and IL-15, specifically targeting relapsed/refractory (r/r) or high-risk B cell tumors (NCT04814004 and NCT05487651) ( Table 2 ).
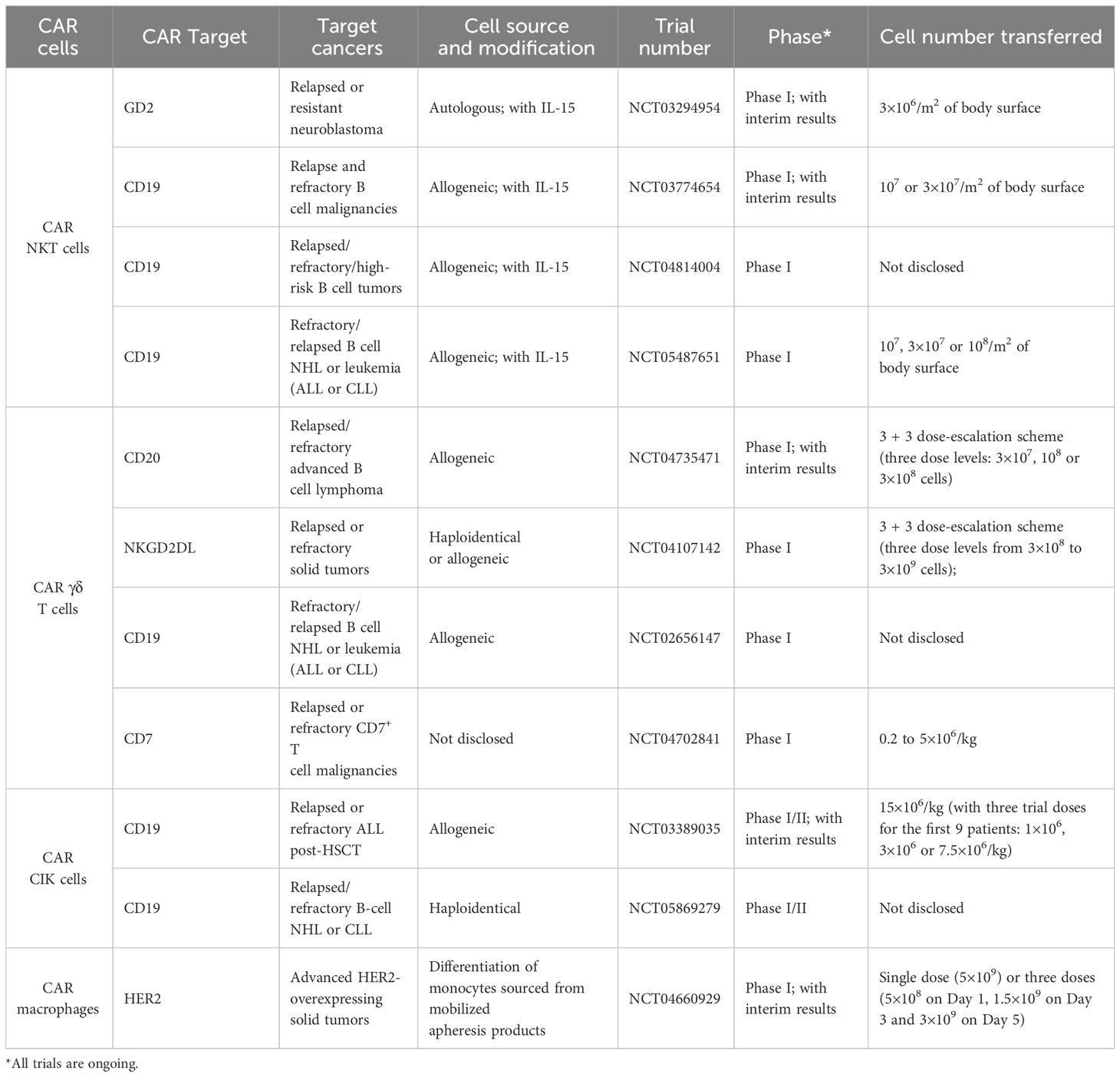
Table 2 Clinical trials of new CAR cells.
2.2 CAR MAIT cells
2.2.1 properties and advantages.
Similar to iNKT cells, mucosal-associated invariant T (MAIT) cells express a semi-invariant αβTCR with restricted repertoire. Instead of engaging with MHC molecules, the αβTCR of MAIT cells recognizes metabolite antigens presented by the MHC class I-like protein (MR1). These antigens are riboflavin-derived metabolites rather than peptides ( 50 ). MR1 is predominantly expressed in antigen-presenting cells, such as macrophages, dendritic cells, and monocytes, as well as epithelial cells. Upon activation, MAIT cells exhibit potent cytotoxic activity, directly killing target cells via the perforin/granzyme B pathways and the secretion of various proinflammatory cytokines ( 50 ). Beyond their MR1-restricted αβTCR, MAIT cells also express innate cell receptors such as Toll-like receptors (TLRs) and NK cell-activating receptors. This equips them with enhanced cytotoxic capabilities, even in the absence of MR1 ( 37 , 50 , 51 ). Thus, similar to CAR NKT cells, CAR MAIT cells may be activated through multiple mechanisms, culminating in augmented efficacy. Furthermore, MAIT cells proficiently target and eradicate MR1 + M2 polarized macrophages during co-culture, operating via a TCR-dependent and TCR-independent mechanism. This suggests a promising avenue for MAIT cells to remodel the immunosuppressive TME ( 37 ) ( Figure 2 ).
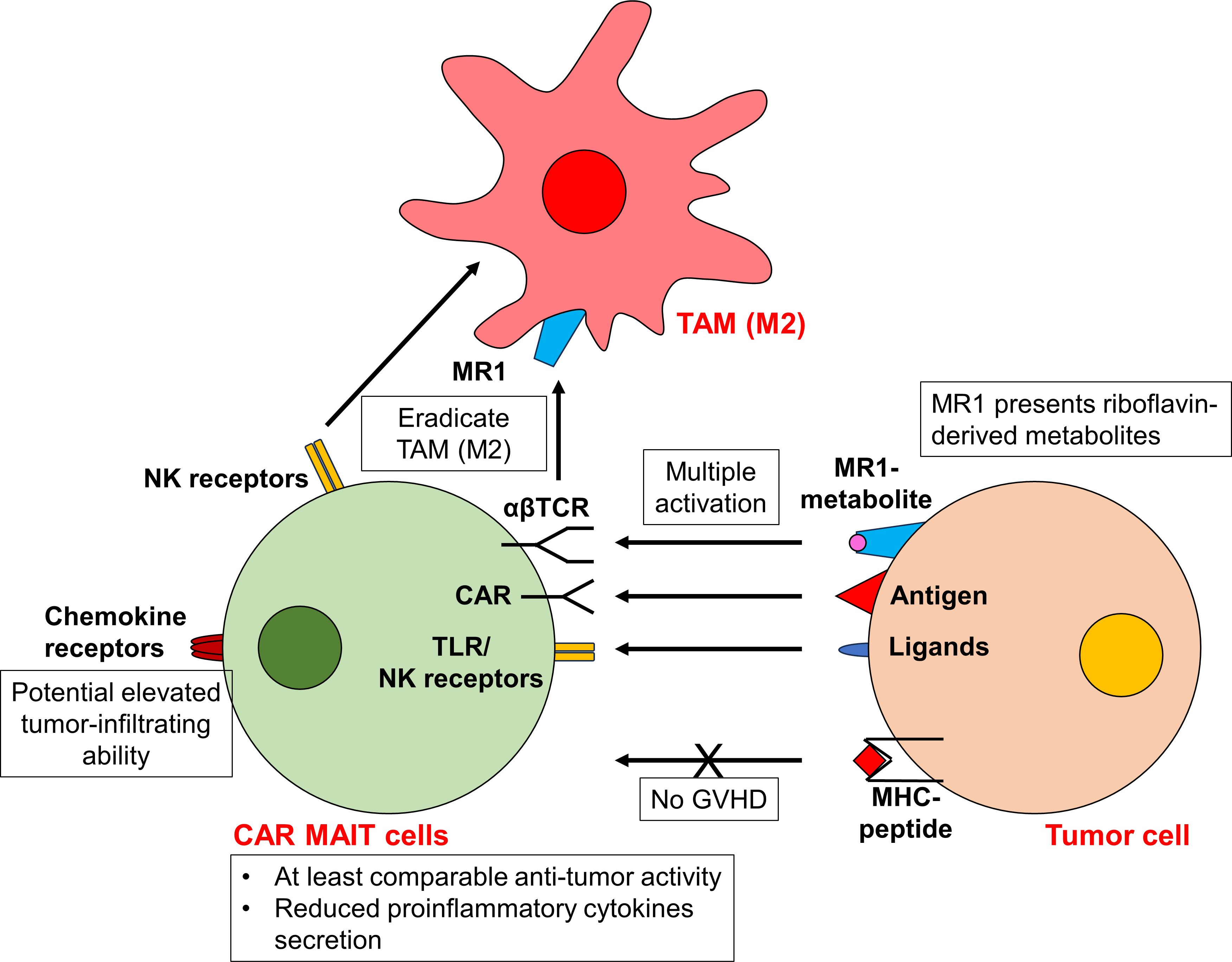
Figure 2 CAR MAIT cells display potent antitumor activity. Similar to CAR NKT cells, CAR MAIT cells can be activated via multiple mechanisms, in addition to conventional CAR-dependent signal. The αβTCR of CAR MAIT cells recognizes riboflavin-derived metabolites presented by the MHC class I-like protein MR1. CAR MAIT cells also express innate cell receptor TLRs and NK cell-activating receptors, facilitating their cytotoxicity even in the absence of MR1. As the activity of αβTCR is MHC-independent, it is unlikely for CAR MAIT cells to induce GVHD. Activated CAR MAIT cells exhibit at least comparable antitumor activity to conventional CAR T cells. They also release lower proinflammatory cytokines, suggesting an improved safety profile. In addition, CAR MAIT cells may be able to eliminate M2 macrophages via their αβTCR and NK cell-activating receptors. The highly expressed chemokine receptors on CAR MAIT cells also allow cells to infiltrate into peripheral tissues and tumors.
Apart from their CAR-independent cytotoxicity, MAIT cells offer another significant advantage: their high expression of chemokine receptors such as CXCR6 and CCR9 ( 52 , 53 ). This trait empowers them to efficiently migrate into peripheral tissues and tumors. Indeed, substantial infiltration of MAIT cells has been observed within the TME ( 54 – 56 ). Moreover, due to the non-polymorphic nature of MR1, MAIT cells are devoid of alloreactivity, similar to the behavior observed in NKT cells ( 57 ). Clinical data further supports this, showing an association between increased MAIT cells in grafts and reduced incidence of GVHD following HSCT ( 58 – 61 ). This positions MAIT cells as attractive candidates for allogeneic CAR cell therapies, with a lower risk of GVHD ( Figure 2 ).
These advantages are related to MR1-dependent activation, parallel those observed with CAR NKT cells, which rely on CD1d. Furthermore, a specific MAIT cell subset characterized as CD45RA − CD45RO + CD62L low CD161 + displays an effector memory phenotype, predisposing them for rapid expansion upon activation ( 53 , 62 ). This feature suggests that both MAIT cells and their engineered CAR counterparts may achieve prolonged persistence in vivo .
Similar to CAR NKT cells, CAR MAIT cells are also derived from PBMCs. MAIT cells are isolated based on the positive expression of TCR Vα7.2 (2.85% of PBMCs) ( 37 ) or Vα7.2 + CD161 + CD8 + expression (17.7% of CD8 + cells in PBMCs, although the authors used Vα7.2 + CD4 - expression as well) ( 63 ). After isolation, MAIT cells are activated and expanded in the presence of riboflavin-derived metabolites, such as 5-(2-oxopropylideneamino)-6-d-ribitylaminouracil (5-OP-RU) or 5-amino-6-d-ribitylaminouracil (5-ARU), as well as IL-2, IL-7 and/or IL-15 ( 37 , 63 ).
2.2.2 Current study
To date, CD19-, Her2- and mesothelin-specific CAR MAIT cells have been generated ( 37 , 63 , 64 ). In a comparative study, Dogan et al. observed that CD19-CAR MAIT cells displayed only marginally enhanced in vitro cytotoxicity against CD19 + cell lines (T2 and Nalm6) than conventional CAR T cells at specific effector: tumor (E:T) ratios ( 63 ). However, these CD19-CAR MAIT cells demonstrated pronounced cytotoxicity against primary B cells across all E:T ratios ( 63 ), raising concerns about potential on-target toxicity. In another aspect of their research, Dogan et al. developed Her2-CAR MAIT cells against breast cancers, and discovered that these cells displayed greater cytotoxicity than CAR T cells against MDA-231 cells at various E:T ratios ( 63 ). Notably, despite their comparable cytotoxic abilities, activated CAR MAIT cells secreted significantly lower levels of proinflammatory cytokines than CAR T cells ( 63 ). This suggests CAR MAIT cells might have an improved safety profile. Furthermore, CD19-CAR MAIT cells did not trigger GVHD in xenograft models ( 64 ), underscoring their potential allogeneic application. However, a comprehensive safety assessment is still needed. ( Figure 2 ).
Beyond CD19- and Her2-CAR MAIT cells, Li et al. generated mesothelin-specific CAR MAIT cells ( 37 ). These CAR MAIT cells proficiently targeted and eradicated the mesothelin-overexpressing ovarian cancer cell line, OVCAR3-FG. Their antitumor activity was further enhanced in the presence of 5-OP-RU ( 37 ). This enhancement highlights the dual activation mechanism for CAR MAIT cells, leveraging both CAR-dependent and MR1-restricted αβTCR-dependent pathways. The study also delved into the performance of mesothelin-CAR MAIT cells within a complex 3D organoid model, incorporating tumor cells, TAMs, and T cells. Remarkably, the mesothelin-CAR MAIT cells maintained their activation and cytotoxicity even in the presence of TAMs, showing their unique ability to counteract M2 macrophages ( 37 ). Overall, these findings reinforce the versatility of CAR MAIT cells capable of exerting cytotoxicity through both CAR-dependent and TCR-dependent mechanisms, and their efficacy may be preserved even amidst an immunosuppressive TME ( Figure 2 ).

2.2.3 Challenges & solutions
Preclinical in vivo investigations concerning CAR MAIT cells remain sparse, many aspects of their potential use in therapy are not fully understood. The detailed safety profiles, the capacity of tumor infiltration, and whether CAR MAIT cells maintain their effector memory subset in vivo are key questions that remain unanswered. The in vivo persistence of these CAR MAIT cells has yet been extensively studied. There is an imperative need for more researches to elucidate the functionality and safety of CAR MAIT cells. As of now, no clinical trials have been initiated to explore the use of CAR MAIT cells in treatment ( Table 1 ).
2.3 CAR γδ T cells
2.3.1 properties and advantages.
Gamma delta (γδ) T cells represent a unique subset of T cells characterized by their TCR γ and δ chains. They exhibit both innate and adaptive immune characteristics, including antibody-dependent cellular cytotoxicity (ADCC), direct cytotoxic effects, and antigen presentation ( 65 , 66 ). Depending on the specific γ and δ chains they express, γδ T cells can be further classified. The predominant γδ T cell subtype in human peripheral blood expresses Vγ9 and Vδ2, hence they are termed Vγ9Vδ2 T cells ( 67 ). The Vγ9Vδ2 TCR identifies phosphoantigens, which are small alkyl diphosphates synthesized by exogenous pathogens and diverse tumor cells. These phosphoantigens are presented via butyrophilin 3A1, instead of the conventional MHC ( 68 – 70 ). In contrast, Vδ1 T cells, predominantly resides in tissues, detect antigens presented by CD1c/d or the MHC class I-like molecules, MICA/B ( 67 ). Despite their differences in TCR engagement, both Vγ9Vδ2 and Vδ1 T cells exert potent cytotoxic activity against tumor cells ( 71 ). Additionally, γδ T cells can target tumor cells by employing mechanisms like engaging death ligands [e.g. TNF-related apoptosis-inducing ligand (TRAIL) and Fas ligand (FasL)] and NK receptors (e.g. NKG2D) ( 72 ). Beyond direct cytotoxicity, γδ T cells can upregulate CD16 and engage tumor cells through ADCC ( 72 ). Moreover, γδ T cells play an important role in orchestrating the immune response. Activated Vγ9Vδ2 T cells can function as professional APCs, enabling the cross-presentation of antigens to a broad range of immune cells ( 73 – 75 ). Furthermore, they also enhance NK cell-mediated cytotoxicity ( 76 ), promote dendritic cell maturation ( 77 , 78 ), and aid in B cell antibody secretion ( 79 ) ( Figure 3 ).
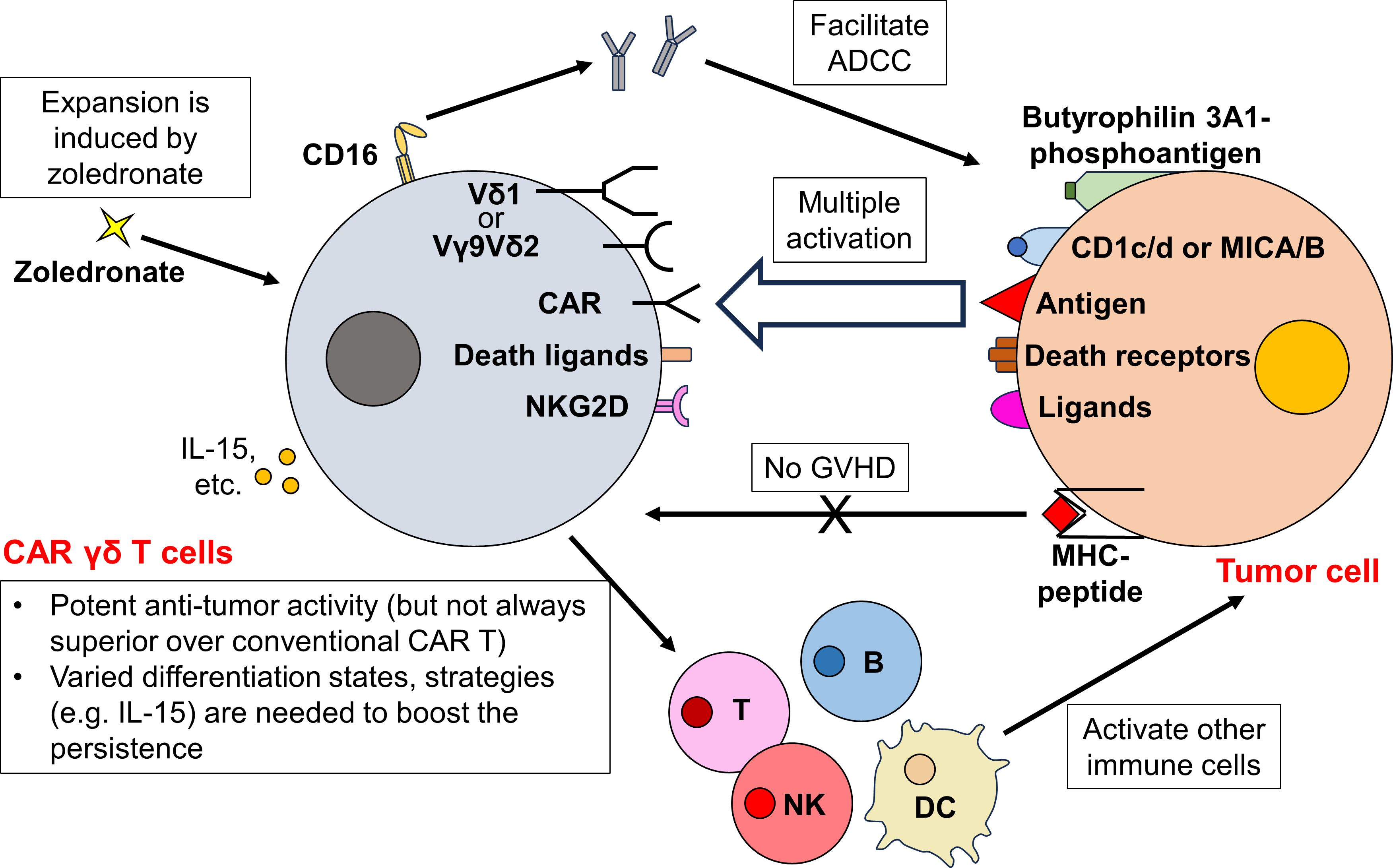
Figure 3 CAR γδ T cells orchestrate a complex immune response and exhibit potent antitumor activity. Besides the traditional CAR-dependent pathway, CAR γδ T cells can target tumor cells through multiple CAR-independent pathways. While Vγ9Vδ2 TCR recognizes phosphoantigens bound by butyrophilin 3A1, Vδ1 TCR targets antigens presented by CD1c/d or MHC class I-like MICA/B. Upon TCR engagement, CAR γδ T cells exert potent cytotoxicity towards tumor cells. Furthermore, CAR γδ T cells also kill tumor cells by expressing death ligands (TRAIL and FasL) and NK receptors (NKG2D). They also upregulate CD16 to eliminate tumor cells via ADCC. As these activation mechanisms are all MHC-independent, the risk of CAR γδ T cells to incur GVHD is low. After activation, CAR γδ T cells further boost the activity of other immune cells, orchestrating a complex antitumor immune response. Although the ex vivo and in vivo expansion of CAR γδ T cells can be easily achieved through zoledronate administration, the persistence of CAR γδ T cell therapy remains to be determined. Different subsets of CAR γδ T cells reside in different states of differentiation after CAR transduction and expansion, further strategies (e.g. IL-15 administration) are required to ensure a long-term efficacy of CAR γδ T cells.
Similar to other T cell subtypes discussed, γδ T cells recognize antigens via MHC-independent pathways. Therefore, the primary benefit of deriving CAR cells from γδ T cells is their potential to manifest increased antitumor responses beyond CAR-dependent activity. Additionally, since γδ T cell activation is MHC-independent, these cells are less likely to cause GVHD ( 80 ), allowing potential allogeneic application. Furthermore, the tissue-resident nature of Vδ1 T cells might offer enhanced tissue-targeting and tumor infiltration abilities. Notably, increased tumor infiltration by γδ T cells has been correlated with favorable prognostic outcomes ( 81 ). Moreover, γδ T cells are advantageous due to their ease of expansion, both ex vivo (during the manufacturing process) and in vivo following therapeutic administration. This proliferation can be effectively stimulated using amino bisphosphonates such as zoledronate ( 82 – 84 ) ( Figure 3 ).
Although different subsets of γδ T cell are enriched in various tissues, PBMCs are still used as the starting materials for CAR γδ T cell manufacturing. Unlike NKT cells and MAIT cells, γδ T cells within PBMCs can be expanded before isolation. For example, CD3 + Vδ2 + T cells consist only about 1.3% of PBMCs. After 10 days of expansion utilizing amino bisphosphonates and various cytokines (i.e. IL-2, IL-7 and/or IL-15), the purity of Vγ9Vδ2 T cells within cultured PBMCs reaches over 90%, making them suitable for CAR Vγ9Vδ2 T cells generation ( 83 , 84 ). Similarly, although Vδ1 T cells represent only 0.2-1% of PBMCs ( 85 ), they can be efficiently expanded using agonistic anti-Vδ1 antibody for CAR cell production ( 86 , 87 ). Additionally, αβTCR-depletion can be performed to further increase the purity before or after cell expansion for generating CAR cells from bulk γδ T cells [around 3.4% of CD3 + cells in PBMCs ( 88 )] or their subsets ( 88 , 89 ).
2.3.2 Current study
Researchers have developed a variety of CAR products from γδ T cells or their specific subtypes. For instance, Rozenbaum et al. engineered CD19-CAR γδ T cells from PBMCs, which demonstrated potent cytotoxicity against both CD19 + and CD19 - leukemia cells ( 88 ). This capability to target CD19 - cells suggests that CAR γδ T cells could offer a novel solution for overcoming antigen-loss relapses. Intriguingly, the cytotoxic activity of these cells was further enhanced by zoledronate. Deniger et al. developed another CD19-CAR γδ T cells demonstrating both CAR-specific and TCR-dependent cytotoxicity, effective against CD19 + tumor cells both in vitro and in vivo ( 90 ). Similarly, GD2-CAR γδ T cells, including both bulk population and individual subsets, showed potent cytotoxic effects against GD2 + neuroblastoma cell lines ( 91 ). Remarkably, upon expansion and activation, these GD2-CAR γδ T cells exhibited properties akin to professional APCs, facilitating CAR-independent tumor cell eradication. Further developments in this field include CD123-CAR Vδ1 T cells against acute myeloid leukemia (AML) ( 89 ), carcinoembryonic antigen (CEA)-CAR Vγ9Vδ2 T cells against xenograft mouse model with CEA + tumor ( 83 ), B7H3-CAR Vγ9Vδ2 T cells against glioblastoma ( 84 ), CD20-CAR Vγ9Vδ2 T cells with conjugated rituximab against B cell lymphoma ( 92 ), and HLA-G and PD-L1 multi-specific CAR Vδ2 T cells with the ability to secrete PD-L1/CD3ϵ bispecific T cell engagers (BiTEs, which recruit bystander T cells) against solid tumors ( 93 ).
Furthermore, the exploration of CAR γδ T cells in allogeneic setting is increasingly recognized. Makkouk et al. demonstrated the promise of this approach by engineering Glypican-3 (GPC-3)-CAR Vδ1 T cells ( 87 ). In a subcutaneous hepatocellular carcinoma xenograft mouse model, these CAR Vδ1 T cells demonstrated enhanced tumor migration and eradication capabilities without eliciting xenograft GVHD. Similarly, Nishimoto et al. observed that allogeneic CD20-CAR Vδ1 T cells displayed enhanced antitumor capabilities without inducing xenograft GVHD ( 86 ). Lee et al. advanced this concept by purposely selecting Vγ9Vδ2 T cell donors with high CD16 expression to enhance ADCC ( 94 ). The resulting mesothelin-CAR Vγ9Vδ2 T cells exhibited potent antitumor ability in mice intraperitoneal and subcutaneous ovarian cancer models without any signs of GVHD, while all mice treated with conventional CAR T cells died shortly from GVHD. Notably, these CAR Vγ9Vδ2 T cells were also capable of targeting TAMs in vitro , suggesting their potential ability of remodeling the TME ( 94 ).
Issues like T cell aplasia and fratricide (i.e. CAR T cells attack each other) usually occur in targeting T cell malignancies with conventional CAR T cell therapy. This is because the targeted antigens are regularly expressed on normal T cells and CAR T cells themselves ( 95 ). CAR γδ T cells offer an innovative solution to these problems through their MHC-independent cytotoxic mechanisms. Fleischer et al. highlighted this advantage by expressing CD5-targeted non-signaling CARs (NSCARs) in γδ T cells. Hence, the intrinsic cytotoxicity of these cells are redirected towards CD5 + T cell acute lymphoblastic leukemia (T-ALL) cell lines (although such antitumor activity is much weaker when compared to CD19-NSCAR γδ T cells against CD19 + B-ALL cell lines) ( 96 ). Given that CAR NKT cells and CAR MAIT cells also possess inherent MHC-independent antitumor capabilities, exploring their potential could broaden the therapeutic options for these challenging malignancies.
2.3.3 Challenges & solutions
While numerous preclinical studies highlight the potential of CAR γδ T cells in cancer therapy, yet their brief persistence poses a significant challenge. Rozenbaum et al. have shown a rapid decline in CAR γδ T cell numbers, noticeable just three days after injection, in stark contrast to the robust expansion observed with conventional CAR T cells ( 88 ). This limited persistence could be attributed to the inherent lack of alloreactivity in CAR γδ T cells. Makkouk et al. further emphasize this difference, revealing that while traditional CAR T cells exhibit substantial in vivo expansion, CAR Vδ1 T cells do not ( 87 ). Moreover, the persistence and propensity for exhaustion vary across CAR γδ T cell subtypes. Specifically, while Vδ1 T cells predominantly exhibit a naïve phenotype in PBMCs, Vδ2 T cells are mostly characterized as effector memory T cells ( 91 ). After CAR transduction and expansion, a significant number of CAR Vδ1 T cells retain their naïve state. This is in contrast to CAR Vδ2 T cells, which tend to differentiate further and display signs of exhaustion ( 86 , 91 ). It’s well-established that CAR T cells with a more naïve or less differentiated memory phenotype tend to persist longer and manifest an enhanced functional profile ( 97 , 98 ). Given the observed differences, it’s reasonable to suggest that CAR Vδ1 T cells may offer better in vivo longevity compared to CAR Vδ2 T cells. Indeed, this hypothesis is supported by several studies. For instance, Wang et al. reported that the effectiveness of CEA-CAR Vγ9Vδ2 T cells was limited in duration ( 83 ). Efforts to enhance the in vivo persistence of CAR T cells include the administration of IL-15 and the modulation of intracellular signaling pathways ( 44 ). Similar strategies are now being applied to CAR γδ T cells, aiming to extend their persistence ( 87 , 89 , 94 , 99 , 100 ) ( Figure 3 ). For example, Lee et al. engineered IL-15 into their CD16 high mesothelin-CAR Vγ9Vδ2 T cells (MCAR15-Vδ2T cells) ( 94 ). In mice models of intraperitoneal ovarian cancer, MCAR15-Vδ2T cells exhibited superior tumor control and prolonged persistence in the tumor and organs at day 57 (as compared to normal CAR Vγ9Vδ2 T cells without IL-15). Moreover, all 5/5 mice survived to day 180 with complete remission in MCAR15-Vδ2T cell group, while 3/5 mice died of relapse in normal CAR Vγ9Vδ2 T cell group ( 94 ).
Beyond the issue of limited persistence, CAR γδ T cells face several additional challenges that necessitate further investigation. One significant concern is their in vivo expansion capacity, which typically does not match that of conventional CAR T cells ( 87 ). Consequently, while CAR γδ T cells are theoretically predisposed to migrate to peripheral tissues, questions have been raised about their ability to accumulate in tumors in significant numbers. Moreover, data from in vitro transwell migration assays do not demonstrate significant differences in the migratory capacities of CAR Vδ1 T cells, CAR Vδ2 T cells, and conventional CAR T cells ( 91 ). This suggests that the superior ability of CAR γδ T cells to penetrate solid tumors requires more rigorous validation. When it comes to cytotoxic performance, CAR γδ T cells do not consistently outperform conventional CAR T cells. In certain instances, their efficacy is even surpassed by conventional CAR T cells ( 88 , 91 , 100 ). Additionally, the intrinsic antitumor responses of γδ T cells vary across different types of tumors, with only moderate activity observed against certain cancers such as ALL and non-Hodgkin lymphoma (NHL) ( 101 , 102 ). For example, NSCAR γδ T cells showed higher activity against B-ALL cell lines than T-ALL cell lines ( 96 ). Thus, CAR γδ T cells might be more effective against specific cancer types, potentially limiting their universal application in cancer therapy ( Table 1 ).
2.3.4 Ongoing clinical trials
Several clinical trials are currently underway. In study NCT04735471, the previously mentioned allogeneic CD20-CAR Vδ1 T cells have shown favorable tolerance in lymphoma patients. Of the six participants, four achieved complete remission, with no incidence of GVHD or severe adverse reactions recorded ( 103 ). Additionally, there are three ongoing phase I trials: NCT04107142 aims to evaluate the safety and efficacy of haploidentical or allogeneic NKGD2DL-specific CAR γδ T cells in patients with relapsed or refractory solid tumors; NCT02656147 assesses allogeneic CD19-CAR γδ T cells in patients diagnosed with high-risk or r/r B cell malignancies; and NCT04702841 examines CD7-CAR γδ T cells for patients with relapsed or refractory CD7 + T cell malignancies ( Table 2 ). As of now, results from these trials remain unpublished.
2.4 CAR CIK cells
2.4.1 properties and advantages.
Cytokine-induced killer (CIK) cells represent a heterogeneous group of T-NK killer lymphocytes. They are derived ex vivo from PBMCs, primarily from the CD3 + CD56 - CD8 + T cell subsets, after extensive culture in the presence of anti-CD3 antibodies, IFN-γ, and IL-2 ( 104 ). After 2-3 weeks of cultivation, the predominant phenotype among the expanded cells is CD3 + CD56 + , with a smaller fraction of CD3 + CD56 - cells ( 104 ). Intriguingly, these cells exhibit markers of NK cells (e.g. activating receptor NKG2D) to a variable extent, while also retaining hallmark T cell markers ( 105 ). This combination of markers empowers CIK cells with TCR-mediated cytotoxicity and MHC-independent NK cell-like functions ( 106 ). CIK cells demonstrate potent antitumor efficacy against various tumor cells. Activated CIK cells also upregulate the expression of FasL and perforin, partially mediated by NKG2D, facilitating tumor elimination ( 107 ). Additionally, CIK cells release a variety of pro-inflammatory cytokines, enhancing systemic immune responses against tumors ( 104 ).
As the predominant cytotoxic effector phenotype within CIK cells is CD3 + CD56 + , some studies suggest these cells could be categorized as NKT cells. However, it’s essential to clarify that these are not the previously mentioned iNKT cells, as CIK cells do not depend on CD1d for their activation ( 108 , 109 ). Despite this distinction, CIK cells’ MHC-independent cytotoxic activity aligns them with other T cell subtypes discussed earlier. Consequently, CAR CIK cells are believed to offer similar benefits, including robust endogenous antitumor activity, enhanced safety profiles, and reduced risk of GVHD ( 110 , 111 ). Furthermore, the nature of CIK cells ensures an ample source for CAR transduction. In addition, CIK cells have shown an ability to navigate to tumor sites following infusion, although a detailed evaluation of their tumor infiltration effectiveness remains to be conducted ( 110 , 112 ).
2.4.2 Current study
To date, numerous CAR CIK cell therapies utilizing first-, second-, or third-generation CAR constructs have been developed for various hematologic malignancies and solid tumors ( 113 – 118 ). A quintessential example is the human epidermal growth factor receptor 2 (HER2)-CAR CIK cells, created by Merker et al. These cells have demonstrated potent antitumor activity against rhabdomyosarcoma xenograft models without severe adverse effects ( 119 ). Yet, it’s worth noting that despite suggestions that HER2-CAR CIK cells have better tissue migration and persistence than wild-type CIK cells, histological evaluations using CD3 staining showed these cells to be scarce in most tissues. Other CAR CIK cells have demonstrated similarly potent cytotoxic abilities as well. For instance, 5T4-CAR CIK cells have been effective in eliminating nasopharyngeal carcinoma stem cell-like cells via both CAR-dependent and NKG2D-mediated CAR-independent mechanisms ( 120 ). Additionally, CD123-CAR CIK cells also exhibit inherent cytotoxic effects on CD123 - cells, complementing their CD123-targeted capabilities. Importantly, CD123-CAR CIK cells do not significantly increase their immunostimulatory cytokine release upon CARactivation compared to regular CIK cell activation. This suggests that CAR CIK cells might not trigger severe adverse effects ( 117 ). Research has also explored targeting both the leukemic cell marker CD33 and the mesenchymal stromal cell marker CD146 using CAR CIK cells ( 121 ). Delving deeper, this study has shown that mesenchymal stromal cells can attenuate the long-term activity of single-targeted CAR CIK cells. This reveals that CAR CIK cells, like CAR T cells, are vulnerable to the immunosuppressive TME.
2.4.3 Challenges & solutions
The therapeutic efficacy of CIK cell therapy is considered somewhat limited, leading to suggestions that a large number of CIK cells is required for optimal tumor elimination ( 122 , 123 ). Additionally, the in vivo antitumor activity of CAR CIK cells is reported to be less than that of CAR T cells ( 124 ) [Despite this, one study also proposed that CAR CIK cells and conventional CAR T cells have comparable in vitro cytotoxicity ( 125 )]. This reduced efficacy of CAR CIK cells is partially due to their diminished persistence. It has been reported that CSPG4-CAR CIK cells can only control tumor (i.e. soft tissue sarcoma) growth for 2 weeks ( 126 ), indicating a significantly low persistence of CAR CIK cells. Generally, CIK cells are characterized as terminally differentiated effector memory T cells (TEMRA). These cells have limited proliferative ability and are prone to apoptosis ( 127 ). Such profile is anticipated, given that CIK cells emerge from extensive ex vivo expansion. Notably, IL-15 is known to promote T cell activation and proliferation ( 128 , 129 ). Thus, the inclusion of IL-15 during the ex vivo cultivation process can markedly enhance the cytotoxic potential of CAR CIK cells ( 115 ). Interestingly, CAR CIK-like cells can be induced directly, bypassing the need of extensive ex vivo expansion and differentiation. This helps prolong the persistence of CAR cells. Hombach et al. integrated IL-12 into the exodomain of CAR ( 130 ). Upon activation, this IL-12-CAR then reprograms CD8 + T cells into heterogenous human leukocyte antigen E (HLA-E)-restricted NK-like cells. A major subset of these cells, characterized by the CD8 + CD56 + CD62L high expression, closely resembles CIK cells. In vitro test of these CEA-targeted IL-12-CAR T cells exert both antigen-dependent and -independent cytotoxicity against tumor cells. Importantly, such cytotoxicity persists upon repeated antigen stimulation (for 7 days). In contrast, the activity of conventional CAR T cells declines under the same condition. As a result, CEA-targeted IL-12-CAR T cells show superior antitumor activity (than conventional CAR T cells) in mice subcutaneous tumor models ( 130 ).
The proper design of CAR constructs plays a crucial role in ensuring the sustained efficacy of CAR CIK cells, similar to what is required for CAR T cells. Hombach et al. conducted a comparative analysis on the activity of CAR CIK cell using three generations of CAR constructs: the first-generation CD3ζ-CAR, the second-generation CD28-CD3ζ-CAR, and the third-generation CD28-CD3ζ-OX40 CAR ( 131 ). Surprisingly, the addition of costimulatory domains, such as CD28 alone or in combination with OX40, enhanced the initial activation and short-term activity of CAR CIK cells ( 122 , 131 ). However, the synergistic costimulation through both CD28 and OX40 led to accelerated maturation of these terminal CAR CIK cells. This acceleration promoted activation-induced cell death (AICD). As a result, this further maturation correspondingly attenuated the NKG2D-mediated MHC-independent cytotoxic activity of the CAR CIK cells. In contrast, CD28-mediated costimulation alone did not push the cells to mature more than those with the CD3ζ-CAR ( 131 ). Consequently, the long-term antitumor activity of CD28-CD3ζ-OX40 CAR CIK cells was significantly lower than that of the CD28-CD3ζ-CAR ( 131 ). Nonetheless, another study reported that the third-generation CD28-4-1BB-CD3ζ-CAR CIK cells showed superior long-term antitumor efficacy compared to both the first-generation CD3ζ-CAR and the second-generation CD28-CD3ζ-CAR ( 124 ). This underscores the significance of costimulatory domain optimization in engineering CAR CIK cells.
One critical aspect of CAR CIK cells is their potential toxicities. Although most CIK cells exhibit the CD3 + CD56 + phenotype, the CD3 + CD56 − subset of CIK cells also plays a significant role in cytotoxicity against tumor cells ( 132 ). Following CAR integration, it’s noteworthy that the majority of CAR CIK cells retain their TCRα/β signatures ( 133 ). Despite this, almost all preclinical studies report no or low adverse effects from CAR CIK cells. Another aspect requiring further investigation is the tumor infiltration ability of CAR CIK cells. As previously elucidated, the presence of CAR CIK cells in various tissues is limited ( 119 ). To improve the migration of CD33-CAR CIK cells to the bone marrow, Biondi et al. overexpressed CXCR4 in these CAR CIK cells. As a result, these CXCR4-overexpressing CAR CIK cells demonstrated not only an enhanced affinity for the bone marrow environment but also increased antileukemic activity ( 134 ) ( Table 1 ).
2.4.4 Ongoing clinical trials
NCT03389035 is a phase I/II clinical trial evaluating the safety and efficacy of allogeneic CD19-CAR CIK cells. These cells are engineered using the non-viral vector, the Sleeping Beauty transposon. Within the cohort of 27 patients with r/r ALL post-HSCT (including a group of 6 patients undergoing compassionate-use treatment), 18 patients achieved a complete response. The overall survival rate was 71.4% over a median follow-up of 2.8 years. No GVHD were reported. However, 11 patients experienced CRS or ICANS ( 135 ). Concurrently, another clinical trial, NCT05869279, is exploring the use of haploidentical CD19-CAR CIK cells against B cell NHL or CLL, though its outcomes have not been reported ( Table 2 ).
3 CAR cells generated from innate immune cells
In addition to T cells, innate immune cells offer a promising avenue for CAR product. Owing to their capacity to recognize and eliminate targets in an MHC-independent manner, CAR cells derived from innate cells may preserve this characteristic. Consequently, these CAR cells might demonstrate enhanced antitumor activity, not only from their engineered CAR-induced cytotoxicity but also from their inherent functions, akin to the various T cell subtypes discussed earlier. Currently, CAR NK cells, CAR macrophages and CAR neutrophils are garnering significant interest.
3.1 CAR NK cells
3.1.1 properties and advantages.
CAR NK cells are probably the second most recognized CAR-associated cell products. As components of the innate immune system, NK cells depend on a delicate balance of activating and inhibitory signals to target tumor cells. This is achieved through Perforin/Granzyme B pathways or apoptosis mechanisms ( 136 ). Numerous reviews have already delved into the research and clinical trials of CAR NK cells ( 136 – 139 ). As this review predominantly focuses on novel CAR-related treatments, only a brief overview of CAR NK cells is provided here.
CAR NK cells possess the innate ability to retain their endogenous activation receptors ( 137 ). This attribute makes them a powerful tool to reduce relapse due to loss of antigen. This feature also ensures CAR NK cell activity, even upon CAR downregulation. Furthermore, NK cells do not secrete key cytokines, such as IL-1 and IL-6, which are known to trigger CRS ( 137 ). Clinical trials have confirmed the safety of CAR NK cells, reporting minimal to no side effects. These trials also demonstrate their effectiveness against both solid and hematological malignancies ( 140 – 142 ). Another advantage of NK cell-based CAR therapies is their minimal alloreactivity ( 143 ), facilitating the development of allogenic CAR NK cell products. Sources of these non-autologous CAR NK cells include NK cell lines ( 141 , 144 ), cord blood sources ( 142 , 145 ), allogeneic NK cells ( 140 ), and allogeneic iPSC-derived NK cells ( 146 ).
3.1.2 Current study
Currently, the research focus of CAR NK cells is to explore strategies that can further enhance their antitumor activity. One approach to enhance the ability of CAR NK cells to infiltrate tumors is through the overexpression of various chemokines. For instance, by overexpressing CXCR1, NKG2D-CAR NK cells have been shown to migrate and infiltrate solid tumors in mouse models of ovarian cancer ( 147 ). In order to develop optimal and specific CAR constructs for NK cells, Li et al. conducted a screening of various CAR constructs that contained different signaling domains. They found that a CAR construct with the transmembrane domain of NKG2D, the 2B4 co-stimulatory domain, and the CD3ζ signaling domain was able to induce strong antigen-specific NK cell activity ( 148 ). To further enhance the antitumor activity, the researchers deleted cytokine-inducible Src homology 2-containing protein (CIS), the key negative regulator of IL-15 signaling, in CAR NK cells ( 149 ). As a result, the activity of IL-15-secreting CAR NK cells was enhanced through the Akt/mTORC1 and c-MYC pathways, which promote aerobic glycolysis.
3.1.3 Challenges & solutions
The in vivo persistence of CAR NK cells is a significant challenge for their broader clinical application. Notably, limited expansion of CAR NK cells has been observed in patients demonstrating suboptimal therapeutic outcomes ( 142 ). Addressing this issue may involve strategies used to enhance CAR T cells. For instance, integrating IL-15 into CAR T cell therapies has significantly improved persistence and proliferation in various murine studies ( 150 , 151 ). This approach has shown clinical benefits in a patient with B-ALL after failures with conventional and CAR T-cell treatments ( 152 ). Moreover, the costimulatory signals MyD88/CD40 have been found to boost CAR T cell proliferation and expansion ( 153 , 154 ). Inspired by these findings, efforts to prolong CAR NK cell persistence have employed IL-15 signaling or MyD88/CD40 pathways ( 149 , 155 ). Genetic engineering of CAR NK cells offers another strategy to enhance their therapeutic effectiveness ( 156 ) ( Table 1 ).
3.1.4 Ongoing/completed clinical trials
Currently, there are around 70 ongoing or completed clinical trials evaluating the effectiveness of CAR NK cells against tumors. A list of CAR NK cell-related clinical trials can be found in a recently published review ( 157 ). NCT02944162 is one of the earliest first-in-human phase I clinical trials of CAR NK cells, which assessed the safety of CD33-CAR NK cells in patients with r/r AML. Although the infusion of CD33-CAR NK cells at a dose of up to 5×10 9 cells per patient was deemed safe, all three enrolled patients either showed no response or relapsed at 15 months/5 years post-treatment ( 141 ). While this result are not satisfactory, other studies may yield better outcomes (although their follow-up period may not be long enough). For instance, in the phase I trial (NCT04623944), allogeneic NKG2D ligand-directed CAR NK cells exhibited a complete response rate of 67% in patients with r/r AML (3/6 achieved a complete response with hematologic recovery, and 1/6 had a complete response with incomplete hematologic recovery) ( 158 ). Similarly, in another trial (NCT05020678), 8 out of 14 patients with r/r NHL achieved a complete response after receiving allogeneic CD19-CAR NK cells (although 3 patients with indolent lymphoma experienced relapse after more than 6 months) ( 159 ). However, almost all 5 patients with B cell-driven leukemia (ALL and CLL) enrolled in the same trial did not show any response, except for one with CLL achieved stable disease. Nevertheless, no dose limiting toxicities, neurotoxicity, GVHD or long-lasting cytokine release syndrome (beyond 24 hours) were reported in all patients, further indicating the safety of CAR NK cells. More recent trials are assessing the efficacy of CAR NK cells with different modifications. For example, the phase I/IIa study NCT05410717 is evaluating the safety and efficacy of Claudin6, Glypican-3 (GPC3), mesothelin, or AXL-directed CAR NK cells in patients with Claudin6, GPC3, mesothelin, or AXL-positive advanced solid tumors. These CAR NK cells can secrete IL-7/CCL19 and/or scFvs against PD1/CTLA4/Lag3 to enhance their activity and prevent immunosuppression. In NCT05703854, CD70-CAR NK cells engineered with IL-15 are being assessed in patients with advanced renal cell carcinoma, mesothelioma and osteosarcoma as well. It is expected that these modified CAR NK cells will exhibit improved efficacy.
3.2 CAR macrophages
3.2.1 properties and advantages.
As the central regulator of innate immunity, macrophages are highly plastic innate immune cells with a broad spectrum of effector functions. These include direct phagocytosis, antigen presentation, and modulation of the TME ( 160 ). Introducing CAR constructs into macrophages aims to direct their phagocytic activity specifically towards cancer cells. TAMs comprise a substantial number of tumor-infiltrating immune cells ( 161 , 162 ), and this innate trait makes CAR macrophages promising candidates for penetrating solid tumors. Historically, the use of macrophages has been considered safe ( 163 ). This suggests that CAR macrophages are likely to be well-tolerated, with a low risk of adverse effects ( Figure 4 ).
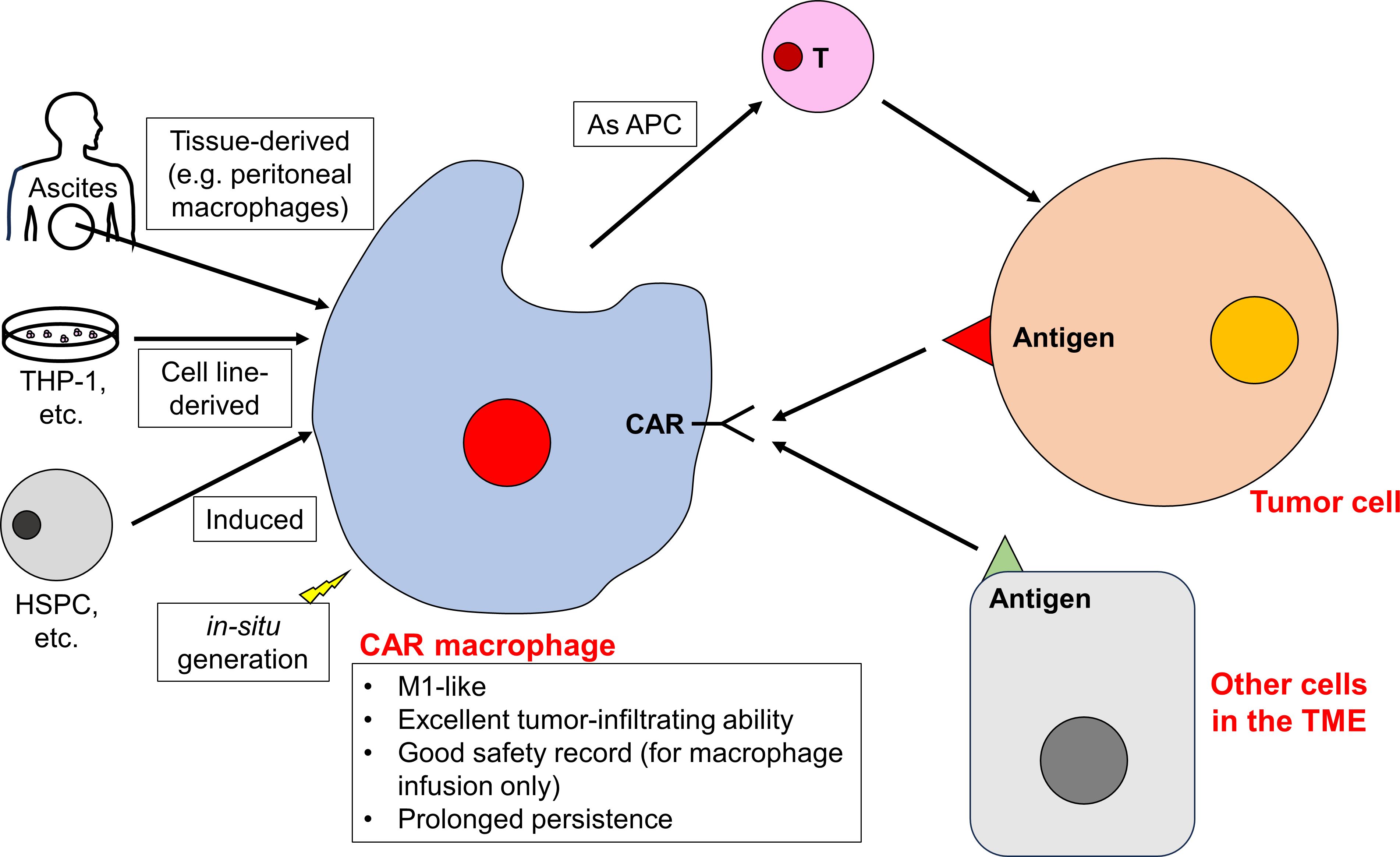
Figure 4 CAR macrophages play a pivotal role in reshaping the TME. CAR macrophages can be developed from tissue-resident macrophages, cell lines, differentiated progenitor cells, or generated in situ . CAR macrophages remain to be M1-like and exhibit excellent tumor-infiltrating ability, and they are able to target tumor cells and reshape the TME (including targeting other cells in the TME and facilitating the function of other immune cells).
Macrophages can be classified into two main polarization states: the classically activated, proinflammatory M1 and the alternatively activated, anti-inflammatory M2 ( 164 ). Within tumors, TAMs predominantly resemble the M2 phenotype ( 164 ). These TAMs contribute to an immunosuppressive TME by recruiting regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs), as well as suppressing cytotoxic effector cells ( 165 ). Given the significant role of macrophages in shaping immune microenvironments, there are efforts to introduce genetically engineered CAR macrophages, especially M1-like macrophages, into tumors.
While many CAR cells are derived from PBMCs due to their abundance and expandability, macrophages in PBMCs are relatively rare. This rarity makes direct developing CAR macrophages from PBMCs challenging. As a result, researchers have turned to alternative sources for macrophages, including tissue-derived macrophages, established macrophage cell lines, precursor or stem cell-derived macrophages, and the innovative concept of in situ CAR macrophage generation. For instance, ascites, which is rich in peritoneal macrophages, has emerged as a potential source ( 166 ). In murine tumor models, the mouse macrophage cell line, RAW 264.7, has been used to create CAR macrophages ( 167 – 169 ). In human settings, the THP-1 cell line, a macrophage/monocyte derivative, has been utilized ( 170 , 171 ). Macrophages can also be induced from hematopoietic stem and progenitor cells ( 172 – 176 ), human pluripotent stem cells ( 177 ), and iPSCs ( 175 , 178 – 180 ). In addition, there is interest in the in-situ generation of CAR macrophages using nanoparticles as well ( 181 – 184 ). These nanoparticles can induce CAR expression, direct phagocytic activity towards tumor cells, and prompt a shift from M2-like TAMs to M1-like macrophages. This shift creates a tumor suppressive immune microenvironment ( Figure 4 ). With in situ CAR macrophages production, there are no concerns about GVHD. Additionally, this method allows CAR macrophages to cross the blood-brain barrier and further enhance tumor infiltration. For example, Gao et al. locoregionally edited intratumoral macrophages (of brainstem gliomas) into M1-like HER2-specific CAR macrophages ( 182 ).
3.2.2 Current study
Due to their unique advantages in tumor infiltration, several CAR macrophages have been designed specifically to target solid tumors. These engineered macrophages exhibit dual capabilities of phagocytosis and immunomodulation. Notably, HER2-specific CAR macrophages targeting various tumor models have attracted significant attention ( 166 , 170 , 172 , 173 , 182 ). Dong et al. developed HER2-CAR macrophages for gastric cancers, utilizing human peritoneal macrophages ( 166 ). Upon activation, these CAR macrophages shift towards an M1-like phenotype with enhanced antigen-specific phagocytosis and antigen presentation to promote T cell proliferation. In multiple gastric cancer models, these CAR macrophages inhibited tumor growth without significant toxicity. They also exhibit synergistic effects with first-line chemotherapy ( 166 ). Klichinsky et al. engineered another HER2-CAR macrophage with an M1-like phenotype. It was derived from the THP-1 lineage or differentiated monocytes. This design aimed to amplify the endogenous immune response and increase the specificity of phagocytic activity ( 170 ). Characterized by prolonged persistence, these CAR macrophages show minimal toxicity, including rare occurrence of on-target, off-tumor toxicity ( 170 ). The M1 polarization of these HER2-CAR macrophages can be pre-conditioned in vitro using LPS and IFN-γ prior to therapeutic infusion, thereby further enhancing their antitumor activity ( 173 ). Remarkably, HER2-CAR macrophages can prevent antigen-negative relapses, suggesting their potential long-term activity ( 172 ). In another study, Chen et al. demonstrated that HER2-CAR macrophages promote the proliferation and activation of CD8 + cytotoxic T lymphocytes. They also remodel the overall macrophages within tumors (including the CAR macrophages themselves and TAMs) into an M1-like phenotype ( 171 ). It is proposed that the interaction between CAR macrophages and other immune cells in the TME may contribute to maintaining the M1-like phenotype of CAR macrophages ( 171 ) ( Figure 4 ).
In addition to HER2-CAR macrophages, researchers have developed a variety of other CAR macrophages. Examples include: CD133-CAR macrophages against glioblastoma ( 184 ), CD19-CAR macrophages against leukemia cell lines ( 178 ), mesothelin-CAR macrophages against ovarian or pancreatic cancer cell lines ( 178 ), GD2-CAR macrophages against neuroblastoma ( 177 ), GPC3-CAR macrophages against hepatocellular carcinoma ( 181 ), anaplastic lymphoma kinase (ALK)-CAR macrophages against neuroblastoma ( 183 ), and CEA-CAR macrophages against CEA + tumor cells (CEA is a cell adhesion protein upregulated in various solid tumors) ( 174 ).
Beyond macrophages engineered to directly target tumor cells, there is a growing interest in designing CAR macrophages to modulate the TME. For instance, Zhang et al. introduced the HER2-specific CAR-147 macrophage ( 167 ). Although the cell targets HER2, the HER2 antibody is linked to CD147, a membrane protein that promotes the expression of Matrix metalloproteinases (MMPs). While these CAR-147 macrophages did not show direct cytotoxicity against tumor cells in vitro , they demonstrated significant antitumor efficacy in vivo . Their effectiveness is not from direct cellular cytotoxicity but by degrading the tumor extracellular matrix via MMPs. This degradation enhanced T cell infiltration. A notable finding was the reduction of cytokines such as IFNγ, TNFα, and IL-6 in blood following CAR-147 macrophage therapy ( 167 ). Another similar approach focused on vascular endothelial growth factor receptor-2 (VEGFR2). VEGFR2 is essential for angiogenesis, and is highly expressed in the vascular endothelial cells of the TME ( 185 ). Upon activation, VEGFR2-CAR macrophages adopt an M1-like phenotype and demonstrate potent antitumor activities. Notably, their mechanism does not rely on the direct phagocytosis of tumor cells. Instead, they create an antitumor immune microenvironment to control tumor growth ( 169 ). Similarly, CCR7-CAR macrophages target lipid droplet-rich CCR7 + immunosuppressive cells within the tumor, with the potential to remodel the immunosuppressive TME ( 168 ) ( Figure 4 ).
The innate ability of CAR macrophages to boost the function of endogenous T cells suggests they could enhance CAR T cell activity. When co-cultured in vitro , CAR macrophages and CAR T cells exhibited remarkable synergy in cytotoxic activity against tumor cells ( 186 ). While CAR macrophages support the function of CAR T cells by upregulating costimulatory ligands (CD86 and CD80), CAR T cells also secrete proinflammatory cytokines that drive CAR macrophages towards an M1 polarization. These M1-polarized CAR macrophages further increase the expression of CD86 and CD80, creating a powerful and self-reinforcing loop of CAR cell activation ( 186 ). Exploring this synergistic dynamic in vivo represents a promising area of research.
3.2.3 Challenges & solutions
A major challenge in using CAR macrophages is the scarcity of macrophages available for CAR macrophages production. Adding to this challenge is the nature of macrophages themselves: they typically exhibit limited proliferation both in vitro and in vivo , though this observation has been debated ( 187 ). These limitations make it difficult to massively produce CAR macrophages, and they may require multiple administrations to maintain therapeutic levels within patients. As a solution, researchers have explored various macrophage sources, as previously mentioned. Yet, concerns regarding the potential tumorigenicity of products derived from progenitor cells, particularly those from iPSCs, pose significant barriers to their clinical utilization ( 188 , 189 ). It is crucial to rigorously evaluate the safety of CAR macrophages derived from these cells. Furthermore, studies examining the post-infusion in vivo phenotype of CAR macrophages are essential, with a particular emphasis on their persistence. Such research could offer key insights into the necessary dosages for CAR macrophage treatments, and also assess the possibility of maintaining prolonged antitumor effects with minimal therapeutic levels.
In addition to persistence, the ability of CAR macrophages to maintain their antitumor phenotype within the TME requires carefully study. While many studies have shown the M1-like polarization of CAR macrophages, these investigations were either conducted in vitro or over short period. Given that the TME contains numerous factors that induce TAMs towards an M2-like phenotype ( 164 ), it is crucial to verify if CAR macrophages can resist such modulation. This resistance is also essential for the sustained activity of CAR macrophages. Using an adenoviral vector, Ad5f35, Klichinsky et al. successful engineered CAR macrophages that exhibited a persistent M1-like phenotype even when exposed to M2-promoting factors ( 170 ). Moreover, Wang et al. identified ACOD1 as a key regulator of the pro-inflammatory M1 state in macrophages using CRISPR screening ( 180 ). ACOD1 knockout CAR macrophages derived from iPSCs exhibited enhanced and sustained M1 polarization. These cells had elevated ROS production, phagocytosis and cytotoxicity. In addition, proper CAR construct design might also help CAR macrophages resist M2 polarization. The “second-generation” CAR macrophages, with tandem domains of CD3ζ-TIR-CAR, maintained in M1-polarization due to the TIR domain in a nuclear factor kappa B (NF-κB)-dependent manner ( 179 ). However, this in vitro experiment lasted only 7 days. Therefore, comprehensive long-term in vivo evaluations are still needed.
The introduction of genes into macrophages emerges as another major challenge in CAR macrophage production. Macrophages naturally resist viral transfection, making effective genetic manipulation difficult ( 170 ). The aforementioned Ad5f35 is a replication-deficient adenoviral vector designed specifically for hematopoietic cells ( 190 ). In addition, Gao et al. generated a chimeric lentiviral vector (HIV-1-Vpx) by packing the HIV-2 accessory protein Vpx into the virus ( 191 ). Vpx promotes the degradation of SAMHD1, a myeloid-specific restriction factor that inhibits the virion cycle in macrophages. As a result, HIV-1-Vpx carrying the CAR construct can efficiently infect macrophages. Besides using specific vectors, Dong et al. enhanced the efficacy of lentiviral transduction by pre-treating macrophages with Vitamin D3 and NATE™ ( 166 ).
Optimal CAR construct design is crucial. Morrissey et al. select three distinct intracellular domains from phagocytic receptors for their CAR constructs. These included the common γ subunit of Fc receptors (FcRγ, termed CAR-FcRγ; FcR triggers the engulfment of antibody-bound particles), the intracellular domain of Megf10 (designated CAR-Megf10; murine phagocytic receptors for apoptotic cell recognition), and the CD19 cytoplasmic domain (named CAR-PI3K; for the recruitment of p85 subunit of PI3K) ( 192 ). While CAR-FcRγ and CAR-Megf1 exhibited robust trogocytosis, they were not effective in whole-cell phagocytosis. In contrast, the CAR-tandem construct, consisting of FcRγ and the CD19 cytoplasmic domain, demonstrated a superior ability for whole cell digestion. Further findings from their research indicated that the inclusion of TCR CD3ζ chain into the CAR construct and CD47 blockade enhanced the phagocytosis of CAR macrophages ( 192 ). Using the same CAR constructs, another study showed that these constructs provided CAR macrophages with varied cytotoxic and phagocytic capabilities ( 186 ). Additionally, the previously introduced second-generation iPSC-derived M1-polarized CAR macrophages contain tandem domains of CD3ζ-TIR-CAR ( 179 ). Upon CAR activation, the TIR domain (via the TLR4 signaling pathway) and the CD3ζ domain (to a lesser extent) both contribute to the production of pro-inflammatory cytokines. They induce M1-polarization and trigger CAR-dependent phagocytosis ( 179 ). Lei et al. further observed that these CD3ζ-TIR-CAR macrophages secrete TNF to induce tumor cell apoptosis, and these apoptotic cells are then cleared by CAR macrophages through efferocytosis. (It should be noted that Lei et al. refer to the CAR macrophages with CD3ζ-CAR as the first-generation CAR macrophages, as CD3ζ-CAR is originally designed for CAR T cells. CD3ζ-TIR-CAR is specifically designed for macrophages and is thus called the second-generation CAR. However, as previously mentioned, there are already numerous CAR constructs with different intracellular domains developed for macrophages. By this criterion, these CAR macrophages should also be classified as the second-generation CAR.) Beyond linking to intracellular domains of phagocytic receptors, CARs can incorporate other signaling domains. These include domains for T cell activation or immune microenvironment remodeling, like the CAR-147. There is still no consensus on the most suitable CAR macrophage constructs yet, and the design of CAR macrophage constructs needs optimization based on the purpose of the treatment ( Table 1 ).
3.2.4 Ongoing clinical trials
At present, only one clinical trial involving CAR macrophages has been conducted, using the HER2-CAR macrophages developed by Klichinsky et al. ( 170 ). This phase I, first-in-human study (NCT04660929) enrolled 7 patients with advanced HER2-overexpressing solid tumors, all of whom had failed prior treatment. The CAR macrophages were generated by differentiating monocytes from mobilized apheresis products. Remarkably, the CAR macrophages were well-tolerated by all participants, with no reports of severe organ damage or on-target, off-tumor toxicities. The study also noted increased tumor infiltration and activation of the TME. This activation was accompanied by improved T cell functions, including enhanced infiltration, proliferation, and activation. However, at 8 weeks, none of the 4 evaluated patients achieved remission: 3 patients had stable disease, and one patient experienced disease progression ( 193 ) ( Table 2 ).
3.3 CAR neutrophils
3.3.1 properties and advantages.
Neutrophils, constituting over 50% of human circulating leukocytes, are abundantly accumulated in various cancers ( 194 ). While they can facilitate tumor growth, by fostering angiogenesis and an immunosuppressive TME, they also display antitumor effects due to their high plasticity. Their antitumor activity includes direct cytotoxicity, activation of T cells and trogoptosis (neutrophil-mediated ADCC) ( 194 ). Therefore, harnessing the antitumor capabilities of neutrophils through CAR technology offers immense therapeutic potential. As neutrophils and macrophages are both key to innate immunity, the CAR cells derived from them have similar advantages and limitations. These include pronounced tumor infiltration, enhanced antitumor activities based on their intrinsic function, the ability of immunomodulation, a promising safety profile, and the potential for off-the-shelf applications.
3.3.2 Current study
Chang et al. developed chlorotoxin (CLTX)-directed CAR neutrophils for glioblastoma, derived from human pluripotent stem cells ( 195 ). These CAR neutrophils demonstrate remarkable mobility and selectively eliminate antigen-bearing tumor cells. They use a multifaceted approach for this: phagocytosis, reactive oxygen species (ROS) generation, and neutrophil extracellular trap formation (a process wherein neutrophils deploy their DNA to capture and eliminate targets). Like macrophages, neutrophils also exhibit antitumor N1 and pro-tumor N2 phenotypes within the hypoxic TME ( 196 ). While normal neutrophils shift to N2 phenotype under hypoxia in vitro , CLTX-CAR neutrophils remain N1-like under the same conditions ( 195 ). This suggests that CLTX-CAR neutrophils might maintain an antitumor N1-like phenotype in the hypoxic TME in vivo as well. Moreover, CLTX-CAR neutrophils can carry hypoxia-activated pro-drug to achieve precise drug delivery into glioblastoma ( 197 ). Using a similar approach, the same team developed prostate-specific membrane antigen (PSMA)-CAR neutrophil against prostate cancers as well (but have been only tested in vitro ) ( 198 ).
3.3.3 Challenges & solutions
A significant limitation of CAR neutrophils is their brief lifespan, typically just a few days ( 194 ). This short existence prevents the direct generation of CAR neutrophils from mature neutrophil. Instead, CAR neutrophils can only be developed from CAR-transduced human pluripotent stem cells or iPSCs. This raises safety concerns about these cells. However, generating neutrophils from CAR-transduced iPSCs bypasses the challenges of editing primary neutrophils’ genomes. Furthermore, the short lifespan of neutrophils also brings up questions about the duration and persistence of their therapeutic effects in vivo . This might necessitate repeated infusions for sustained treatment effectiveness. In addition, the ability of CAR neutrophils to persistently maintain the N1 phenotype within the TME needs further study ( Table 1 ).
4 CAR products generated from alternative sources
Beyond direct derivation from immune cells, there is an innovative approach wherein hematopoietic stem and progenitor cells (HSPCs) are engineered with CAR constructs prior to transplantation. These CAR HSPCs then act as progenitors for a variety of CAR immune cells. Additionally, exosomes secreted by CAR T cells have shown potent cytotoxicity against tumor cells, extending CAR-based therapy beyond cells.
4.1 CAR HSPCs
4.1.1 properties and advantages.
HSPCs can be isolated from bone marrow, peripheral blood post G-CSF mobilization, and umbilical cord blood ( 199 ). After HSCT, the transplanted HSPCs generate neutrophils within a month. NK cells appear between 1-3 months, T cells emerge after 100 days, and B cells develop within 1-2 years ( 200 ). Given their pluripotent nature, HSPCs, as immature multipotent stem cells for all hematopoietic cell lineages, can theoretically give rise to nearly all types of CAR immune cells after transplantation. A key advantage of CAR HSPCs is their potential for sustained antitumor effects, owed to the continuous generation of diverse CAR-immune effectors. Moreover, since HSCT is a cornerstone treatment for many hematological malignancies, processing of HSPCs (CD34 + cells) and incorporating CAR HSPCs into the HSCT framework is clinically feasible.
4.1.2 Current study
CD4-CAR HSPCs have been developed for human immunodeficiency virus (HIV) management in both humanized murine and macaque models ( 201 , 202 ). After transplantation, these CAR HSPCs differentiate into multiple hematopoietic lineages. These cells proliferate across various tissues and maintain their presence for nearly two years. Turning to cancer treatment, De Oliveira et al. demonstrated that CD19-CAR engineered HSPCs can differentiate into myeloid or NK cells in vitro ( 203 ). When tested in vivo , these CAR HSPCs behave like normal HSPCs. Remarkably, they can differentiate into multiple hematopoietic lineages and suppress tumor growth, even at 32 weeks after transplantation. The researchers further introduced a suicide gene, the herpes simplex virus thymidine kinase HSVsr39TK, into these CAR HSPCs ( 204 ). This suicide gene does not affect the engraftment, differentiation and antitumor activity of CAR HSPCs, and the differentiated CAR cells in mouse tissues can be eliminated after ganciclovir administration. However, not all gene-modified cells, especially those within bone marrows, can be fully removed. This is probably due to the protective effects of bone marrow. In addition to HSVsr39TK, the authors co-delivered truncated epidermal growth factor receptor (EGFRt) and CD19-CAR into HSPCs as well ( 205 ). These EGFRt-CD19-CAR modified cells can be removed by anti-CD19 antibody (cetuximab) treatment via ADCC. However, this ablation method is not as effective as the HSVsr39TK-ganciclovir system.
4.1.3 Challenges & solutions
There are concerns about the impact of CAR transduction into stem cells like HSPCs. Zhen et al. observed that CD4-CAR substitutes endogenous CD3 expression and TCR recombination during CD4-CAR HSPC-derived T cell differentiation. T cells with high CD4-CAR expression showed reduced CD3/TCR levels ( 202 ). This suggests that T cells generated from CAR HPSCs most likely lose their ability to combat various pathogens and cancers. A solution might be the concurrent transplantation of both CAR-integrated and unmodified HSPCs. However, co-injecting multiple types of CAR T cells may lead to growth competition ( 206 ). It remains unclear if such competition will arise among T cells derived from different HSPC variants. Moreover, the CD28 costimulatory domain in CAR seemingly augments the tendency of CD19-CAR HSPCs to differentiate into NK cells. It is crucial to determine if CAR-modified HSPCs can maintain a balanced developmental and differentiation pathway, and the design of CAR constructs should be optimized based on the target cells and treatment goals. Furthermore, the risk of tumorigenesis remains for gene-modified HSPCs, especially when suicide genes/strategies failed to ablate all CAR cells. Extended in vivo examinations are required to further assess the safety of CAR HSPCs ( Table 1 ).
4.2 CAR exosomes
4.2.1 properties and advantages.
Exosomes, which are extracellular vesicles spanning 40-160 nm in diameter, are ubiquitously secreted by a vast majority of eukaryotic cells. They are of endosomal origin and formed by the invagination of both plasma and endosomal membranes. Characterized by their profound heterogeneity, exosomes contain a variety of membrane-associated protein complexes from their progenitor cells. This makes them crucial in facilitating intercellular communications ( 207 ). Studies have elucidated that exosomes secreted by T cells mediate the interaction between cytotoxic T cells and their target cells. These T cell-released exosomes carry TCR, CD8, and cytotoxic molecules, such as perforin and granzymes, indicating their potential capability to killing cells in an antigen-specific manner ( 208 – 210 ). Given this background, it is logical that exosomes from CAR T cells have unique cytotoxic properties as well. Indeed, CAR T cell-derived exosomes express CAR and have high levels of cytotoxic molecules ( 211 ). Therefore, CAR exosomes can target and kill tumor cells in a CAR-specific manner.
CAR exosomes, as cell-independent CAR derivatives, present several compelling advantages. Foremost, exosomes can easily penetrate deep into tissues and cross various physiological barriers ( 212 ). This suggests that CAR exosomes may have enhanced tumor infiltration ability towards solid tumors. Additionally, CAR exosomes provide an off-the-shelf therapeutic option for CAR treatments, given their cell-free nature. Moreover, the cytotoxic potential of CAR exosomes is independent of T cells, making them resistant to the suppressive effects of the TME. In addition, CAR exosomes tend to be safer than CAR T cells. This safety applies both during production, where there is no risk of tumorigenesis (due to its cell-free nature), and after administration. Lastly, exosomes and extracellular vesicles are being recognized as promising drug delivery methods ( 213 ). With their tumor-tropic ability, CAR exosomes could serve as an effective platform for precise drug delivery.
4.2.2 Current study
Fu et al. developed exosomes with EGFR- and HER2-CARs from CAR-modified T cells ( 211 ). After antigen stimulation, these exosomes showed increased CAR expressions. Instead of undergoing uptake by target cells, these CAR exosomes selectively and directly lyse antigen-positive cells through granzyme B and perforin, displaying strong antitumor effects in vivo . Notably, these CAR exosomes did not express PD-1, making them resistant to PD-L1-mediated inhibition. In mouse models, the administration of these CAR exosomes resulted in no significant toxicities or CRS. Similarly, Yang et al. showed that mesothelin-CAR exosomes effectively suppressed tumor growth in vivo without evident toxicity ( 214 ). This effect comes from the direct killing of tumor cells by perforin and granzyme B on the CAR exosomes. Another study revealed that CD19-CAR exosomes induced contact-dependent cytotoxicity against CD19 + leukemia cell lines in vitro by upregulating pro-apoptotic genes ( 215 ). Interesting, this study noted that both CD19 + tumor cells and CD19 - control cells uptake CD19-CAR exosomes. This differs from Fu’s observation that target cells do not uptake CAR exosomes, However, only the uptake by CD19 + tumor cells led to a cytotoxic effect. It is postulated that cytotoxic effects occur only when CAR exosome entry is mediated by CD19 binding.
CAR exosomes have also been used to facilitate drug delivery. Zhu et al. developed a hybrid nanovesicle named Lip-CExo@PTX using exosomes from mesothelin- and PD-L1-bispecific CAR T cells. These exosomes were fused with lung-targeted liposomes and loaded with paclitaxel (PTX) ( 216 ). After intravenous administration into mice CT-26 metastatic lung cancer model, Lip-CExo@PTXs accumulated in the lung. There, they precisely released PTX and cytotoxic molecules towards mesothelin + tumors, leading to sequential targeted delivery. The anti-PD-L1 ability of Lip-CExo@PTX further prevent immune suppression. Besides intravenous administration, the authors also developed PTX-loaded CAR exosomes (PTX@CAR-Exos), which can be administered via inhalation ( 217 ). In PTX@CAR-Exos, PTX is encapsulated into mesothelin-directed CAR exosomes. In orthotopic lung cancer mouse models, inhaled PTX@CAR-Exos accumulated within tumors and exert antitumor activity. Moreover, PTX@CAR-Exos also remodeled the immunosuppressive TME.
Besides CAR exosomes derived from CAR T cells, a system (called ExoCAR/T7@Micelle) has been developed. This system uses exosomes from CAR NK cells for drug delivery ( 218 ). In this system, CAR exosomes are obtained from HER2-CAR NK cells. Similar to exosomes from CAR T cells, these NK cell-derived exosomes also express HER2-CAR and show high affinity towards HER2 + breast cancer cells. Then T7 peptide is inserted into the exosomes for the binding with the transferrin receptor (TfR) on cerebral vascular endothelial cells. This process helps the exosomes cross the blood-brain barrier. The CAR exosomes are also loaded with a ROS-responsive nanobomb (mPEG-TK-Ce6@RSL3). This nanobomb is triggered in tumors with high levels of ROS [ROS is further amplified by a photodynamic therapy (PDT)-based strategy]. Both the released RSL3 and ROS can induce ferroptosis, exerting toxicity towards HER2 + tumor cells. In mice with orthotopic HER2 + breast cancer brain metastasis, ExoCAR/T7@Micelle exhibited enhanced antitumor activity. However, the study only focused on using NK cell-derived CAR exosomes for drug delivery. It did not investigate whether the NK cell-derived CAR exosomes have cytotoxic activities of their own.
4.2.3 Challenges & solutions
While the aforementioned studies imply that the cytotoxicity of CAR exosomes relies on granzyme B and perforin, the precise mechanism remains unclear. Moreover, there is debate over whether target tumor cells uptake CAR exosomes. While Fu et al. demonstrated that CAR exosomes directly act on target cells without being taken up ( 211 ), other studies have reported that CAR exosomes are engulfed by target cells before releasing their cargo ( 215 , 218 ). This uncertainty complicates our understanding of CAR exosomes’ activity and potential toxicity. For instance, T cell-derived exosomes carry TCRs from their progenitor cells, it is possible that CAR T cell-derived exosomes might also display endogenous TCRs. Consequently, we cannot ignore the potential alloreactivity of CAR exosomes. A deeper understanding of signal transduction within exosomes might clarify whether TCRs on CAR exosomes trigger downstream signaling pathways and the risk of GVHD.
Determining the optimal dosage of CAR exosomes for desired antitumor effects poses another significant challenge, especially since CAR exosomes do not proliferate. Given the inherent differences between CAR exosomes and CAR T cells, their cytotoxic effects are not directly comparable. Fu et al. found that CAR exosomes and CAR T cells exhibit similar in vitro cytotoxicity when they express comparable levels of CAR proteins, as measured by ELISA ( 211 ). However, it is unclear if this similarity extends to in vivo antitumor activity. Furthermore, the non-proliferative nature of CAR exosomes leads to uncertainty about their sustained in vivo activity, suggesting that multiple administrations might be necessary ( Table 1 ).
5 Conclusions and future perspectives
While conventional CAR T cell therapy represents a pivotal advancement in cancer treatment, its clinical application is mainly limited to certain types of cancers. There are considerable challenges in improving the efficacy of CAR T cell therapy. These challenges also restrict its application to cancers beyond B cell-driven malignancies. Consequently, numerous studies have explored the use of CAR in different immune cells to overcome these challenges. Each cell type reviewed here have unique advantages that can potentially address the limitations of traditional CAR T cell therapy. A key feature of these cells is their ability to retain innate functions after CAR transduction. This enables them to exhibit CAR-independent antitumor activities alongside CAR-specific cytotoxicity. Another common feature is their MHC-independent activity, which reduces the risk of GVHD and pave ways for off-the-shelf CAR products. It is important to note that these CAR cells are reported safer than traditional CAR T cells. Yet, thorough in vivo studies are needed for a definitive safety assessment. While these CAR cells are theorized to have better tumor infiltration capabilities, verifying their actual accumulation within solid tumors is crucial. Lastly, many of the discussed CAR cells may modulate the immunosuppressive TME, but their effectiveness in the complex TME still needs to be proven ( Table 1 ).
As mentioned earlier, a significant advantage of CAR products is their potential for off-the-shelf application. In addition to the novel CAR products discussed, there have been efforts to create allogeneic CAR T cells. The main strategy involves deleting or downregulating the TCR complex on CAR T cells to prevent GVHD ( 219 – 225 ). For instance, Hu et al. genetically removed HLA class II expression and the TCR/CD3 complex from CAR T cells to avoid T cell-mediated alloreactivity. To protect these modified CAR T cells from being attacked by host NK cells, they added the extracellular and transmembrane domains of E-cadherin to the CD28 intracellular domain. This addition creates an inhibitory receptor for NK cells ( 219 ). Comparing allogeneic CAR T cells with CAR cells from other sources is challenging due to differences in CAR design and the targeted antigens or diseases. Generally, clinical trials have shown that patients receiving allogeneic CAR T cells either experienced no GVHD ( 219 , 224 , 225 ) or only mild GVHD (mostly at skin or gastrointestinal system) ( 221 , 223 , 225 ), similar to those aforementioned CAR cells. It should be noted that the lack of alloreactivity may lead to poor in vivo expansion and reduced persistence, as previously discussed. To address this, some studies have deleted CD52 in allogeneic CAR T cells to provide a survival advantage over host CD52 + immune cells. This advantage comes into play upon the administration of anti-CD52 antibodies ( 221 , 223 , 226 ). Despite this, allogeneic CAR T cells still failed to expand in some patients ( 219 , 221 ). Additionally, using anti-CD52 antibodies for lymphodepletion raises the risk of CMV reactivation ( 226 ). In contrast, the natural properties of other CAR cells might support their in vivo proliferation. For instance, the expansion of CAR γδ T cells can be promoted with amino bisphosphonates ( 82 – 84 ). However, the clinical application of this approach needs further exploration. Furthermore, gene deletion might diminish the effectiveness of CAR T cells ( 219 , 220 ). There are also safety concerns associated with CRISPR-based gene editing ( 227 ). In comparison, CAR cells from other T cell subtypes don’t need as much genetic alteration. Thus, they maintain their antitumor activity and sidestep potential safety issues.
Poor persistence is a common issue for many new CAR products. However, it is not always detrimental, as faster clearance makes it easier to manage adverse effects. The major challenge is to balance potency and safety. While CD19-CAR T cells have prolonged persistence and can establish memory in patients, patients may also suffer from lifelong B cell aplasia ( 228 , 229 ). Moreover, many of these new CAR cells can modulate the TME. Combining different CAR products can improve therapeutic efficacy or extend persistence (like the aforementioned synergistic effect of CAR macrophages and CAR T cells). For instance, since Th1 cells can convert TAMs to an M1 phenotype ( 17 ), and M1 macrophages can promote Th1 polarization ( 230 ), co-administering CAR macrophages with CAR NKT cells could be effective. M1-like CAR macrophages could enhance the activity and proliferation of Th1-like CAR NKT cells. In turn, Th1-like CAR NKT cells could help CAR macrophages retain their M1-like phenotype in the complex TME. This strategy can also be used by combining CAR cells derived from APCs like macrophages and γδ T cells with those from cytotoxic cells like T cells, NKT cells, and MAIT cells. Different CAR cells can amplify each other’s activity, fostering a sustained anti-tumor immune environment.
In the realm of CAR T cell therapy, concerted efforts have been directed towards improving its efficacy. These strategies could also benefit other CAR products, potentially leading to breakthroughs. To enhance persistence, IL-2 and IL-15 are often used. Similar approaches have been applied to CAR NKT cells, CAR γδ T cells, and CAR NK cells. Moreover, cytokines like IL-2, IL-7, and IL-15 can drive the expansion of CAR T cells ( 44 ). Using these cytokines in other CAR cells might boost their expansion in the absence of alloreactivity. Additionally, incorporating various interleukins can make CAR T cells more resilient to the immunosuppressive TME ( 44 ). This resilience could help CAR macrophages, neutrophils, and NKT cells maintain their anti-tumor phenotypes within the TME. Another challenge is that novel CAR cells tend to become exhausted. There have been many studies aimed at enhancing the fitness and stemness of CAR T cells, which may be helpful to other CAR cells as well. For example, designing CAR constructs carefully can minimize CAR T cell activation, reducing the risk of rapid exhaustion ( 3 , 44 ). Adjusting CAR T cell metabolism and boosting mitochondrial functions can also extend their activity ( 231 , 232 ). Although these new CAR cells are considered safe, concerns about potential on-target, off-tumor toxicity remain, such as CAR MAIT cells. The activity and potential toxicity of CAR T cells can be controlled through various logic-gating systems ( 233 ). Similar strategies could be adapted for CAR products from different cell sources. Combining these innovations in cellular immunotherapy with the unique features of these novel CAR cells, we look forward to future milestones in CAR cell therapy.
Author contributions
JWH: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. QY: Writing – review & editing. WW: Funding acquisition, Supervision, Writing – review & editing. JH: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was financially supported by National Natural Science Foundation of China (NSFC 81500173).
Acknowledgments
We acknowledge our colleagues in our department for their ongoing advices. We apologize to colleagues whose work we could not cite due to space limitations.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin . (2021) 71:209–49. doi: 10.3322/caac.21660
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J . (2021) 11:69. doi: 10.1038/s41408-021-00459-7
3. Luginbuehl V, Abraham E, Kovar K, Flaaten R, Muller AMS. Better by design: What to expect from novel CAR-engineered cell therapies? Biotechnol Adv . (2022) 58:107917. doi: 10.1016/j.biotechadv.2022.107917
4. Haas AR, Tanyi JL, O’Hara MH, Gladney WL, Lacey SF, Torigian DA, et al. Phase I study of lentiviral-transduced chimeric antigen receptor-modified T cells recognizing mesothelin in advanced solid cancers. Mol Ther . (2019) 27:1919–29. doi: 10.1016/j.ymthe.2019.07.015
5. Dotti G, Savoldo B, Brenner M. Fifteen years of gene therapy based on chimeric antigen receptors: “are we nearly there yet?”. Hum Gene Ther . (2009) 20:1229–39. doi: 10.1089/hum.2009.142
6. Li W, Qiu S, Chen J, Jiang S, Chen W, Jiang J, et al. Chimeric antigen receptor designed to prevent ubiquitination and downregulation showed durable antitumor efficacy. Immunity . (2020) 53:456–70 e6. doi: 10.1016/j.immuni.2020.07.011
7. Yamasaki K, Horiguchi S, Kurosaki M, Kunii N, Nagato K, Hanaoka H, et al. Induction of NKT cell-specific immune responses in cancer tissues after NKT cell-targeted adoptive immunotherapy. Clin Immunol . (2011) 138:255–65. doi: 10.1016/j.clim.2010.11.014
8. Vivier E, Ugolini S, Blaise D, Chabannon C, Brossay L. Targeting natural killer cells and natural killer T cells in cancer. Nat Rev Immunol . (2012) 12:239–52. doi: 10.1038/nri3174
9. Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol . (2007) 25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711
10. Uldrich AP, Patel O, Cameron G, Pellicci DG, Day EB, Sullivan LC, et al. A semi-invariant Valpha10+ T cell antigen receptor defines a population of natural killer T cells with distinct glycolipid antigen-recognition properties. Nat Immunol . (2011) 12:616–23. doi: 10.1038/ni.2051
11. Brigl M, van den Elzen P, Chen X, Meyers JH, Wu D, Wong CH, et al. Conserved and heterogeneous lipid antigen specificities of CD1d-restricted NKT cell receptors. J Immunol . (2006) 176:3625–34. doi: 10.4049/jimmunol.176.6.3625
12. Gadola SD, Dulphy N, Salio M, Cerundolo V. Valpha24-JalphaQ-independent, CD1d-restricted recognition of alpha-galactosylceramide by human CD4(+) and CD8alphabeta(+) T lymphocytes. J Immunol . (2002) 168:5514–20. doi: 10.4049/jimmunol.168.11.5514
13. Rotolo A, Caputo VS, Holubova M, Baxan N, Dubois O, Chaudhry MS, et al. Enhanced anti-lymphoma activity of CAR19-iNKT cells underpinned by dual CD19 and CD1d targeting. Cancer Cell . (2018) 34:596–610 e11. doi: 10.1016/j.ccell.2018.08.017
14. Ulanova M, Tarkowski A, Porcelli SA, Hanson LA. Antigen-specific regulation of CD1 expression in humans. J Clin Immunol . (2000) 20:203–11. doi: 10.1023/A:1006689514066
15. Brossay L, Chioda M, Burdin N, Koezuka Y, Casorati G, Dellabona P, et al. CD1d-mediated recognition of an alpha-galactosylceramide by natural killer T cells is highly conserved through mammalian evolution. J Exp Med . (1998) 188:1521–8. doi: 10.1084/jem.188.8.1521
16. Song L, Asgharzadeh S, Salo J, Engell K, Wu HW, Sposto R, et al. Valpha24-invariant NKT cells mediate antitumor activity via killing of tumor-associated macrophages. J Clin Invest . (2009) 119:1524–36. doi: 10.1172/JCI37869
17. Paul S, Chhatar S, Mishra A, Lal G. Natural killer T cell activation increases iNOS(+)CD206(-) M1 macrophage and controls the growth of solid tumor. J Immunother Cancer . (2019) 7:208. doi: 10.1186/s40425-019-0697-7
18. Cortesi F, Delfanti G, Grilli A, Calcinotto A, Gorini F, Pucci F, et al. Bimodal CD40/fas-dependent crosstalk between iNKT cells and tumor-associated macrophages impairs prostate cancer progression. Cell Rep . (2018) 22:3006–20. doi: 10.1016/j.celrep.2018.02.058
19. De Santo C, Salio M, Masri SH, Lee LY, Dong T, Speak AO, et al. Invariant NKT cells reduce the immunosuppressive activity of influenza A virus-induced myeloid-derived suppressor cells in mice and humans. J Clin Invest . (2008) 118:4036–48. doi: 10.1172/JCI36264
20. Gebremeskel S, Clattenburg DR, Slauenwhite D, Lobert L, Johnston B. Natural killer T cell activation overcomes immunosuppression to enhance clearance of postsurgical breast cancer metastasis in mice. Oncoimmunology . (2015) 4:e995562. doi: 10.1080/2162402X.2014.995562
21. Kim CH, Butcher EC, Johnston B. Distinct subsets of human Valpha24-invariant NKT cells: cytokine responses and chemokine receptor expression. Trends Immunol . (2002) 23:516–9. doi: 10.1016/S1471-4906(02)02323-2
22. Yoshie O. CCR4 as a therapeutic target for cancer immunotherapy. Cancers (Basel) . (2021) 13(21):5542. doi: 10.3390/cancers13215542
CrossRef Full Text | Google Scholar
23. Su L, Hu Z, Yang YG. Role of CXCR4 in the progression and therapy of acute leukaemia. Cell Prolif . (2021) 54:e13076. doi: 10.1111/cpr.13076
24. Bagnara D, Ibatici A, Corselli M, Sessarego N, Tenca C, De Santanna A, et al. Adoptive immunotherapy mediated by ex vivo expanded natural killer T cells against CD1d-expressing lymphoid neoplasms. Haematologica . (2009) 94:967–74. doi: 10.3324/haematol.2008.001339
25. Tang R, Liu X, Liang C, Hua J, Xu J, Wang W, et al. Deciphering the prognostic implications of the components and signatures in the immune microenvironment of pancreatic ductal adenocarcinoma. Front Immunol . (2021) 12:648917. doi: 10.3389/fimmu.2021.648917
26. Tachibana T, Onodera H, Tsuruyama T, Mori A, Nagayama S, Hiai H, et al. Increased intratumor Valpha24-positive natural killer T cells: a prognostic factor for primary colorectal carcinomas. Clin Cancer Res . (2005) 11:7322–7. doi: 10.1158/1078-0432.CCR-05-0877
27. Metelitsa LS, Wu HW, Wang H, Yang Y, Warsi Z, Asgharzadeh S, et al. Natural killer T cells infiltrate neuroblastomas expressing the chemokine CCL2. J Exp Med . (2004) 199:1213–21. doi: 10.1084/jem.20031462
28. Lundgren S, Warfvinge CF, Elebro J, Heby M, Nodin B, Krzyzanowska A, et al. The prognostic impact of NK/NKT cell density in periampullary adenocarcinoma differs by morphological type and adjuvant treatment. PloS One . (2016) 11:e0156497. doi: 10.1371/journal.pone.0156497
29. Gorini F, Azzimonti L, Delfanti G, Scarfo L, Scielzo C, Bertilaccio MT, et al. Invariant NKT cells contribute to chronic lymphocytic leukemia surveillance and prognosis. Blood . (2017) 129:3440–51. doi: 10.1182/blood-2016-11-751065
30. Fujii SI, Shimizu K. Immune networks and therapeutic targeting of iNKT cells in cancer. Trends Immunol . (2019) 40:984–97. doi: 10.1016/j.it.2019.09.008
31. Schneidawind D, Pierini A, Alvarez M, Pan Y, Baker J, Buechele C, et al. CD4+ invariant natural killer T cells protect from murine GVHD lethality through expansion of donor CD4+CD25+FoxP3+ regulatory T cells. Blood . (2014) 124:3320–8. doi: 10.1182/blood-2014-05-576017
32. Rubio MT, Bouillie M, Bouazza N, Coman T, Trebeden-Negre H, Gomez A, et al. Pre-transplant donor CD4(-) invariant NKT cell expansion capacity predicts the occurrence of acute graft-versus-host disease. Leukemia . (2017) 31:903–12. doi: 10.1038/leu.2016.281
33. Leveson-Gower DB, Olson JA, Sega EI, Luong RH, Baker J, Zeiser R, et al. Low doses of natural killer T cells provide protection from acute graft-versus-host disease via an IL-4-dependent mechanism. Blood . (2011) 117:3220–9. doi: 10.1182/blood-2010-08-303008
34. Chaidos A, Patterson S, Szydlo R, Chaudhry MS, Dazzi F, Kanfer E, et al. Graft invariant natural killer T-cell dose predicts risk of acute graft-versus-host disease in allogeneic hematopoietic stem cell transplantation. Blood . (2012) 119:5030–6. doi: 10.1182/blood-2011-11-389304
35. Pillai AB, George TI, Dutt S, Teo P, Strober S. Host NKT cells can prevent graft-versus-host disease and permit graft antitumor activity after bone marrow transplantation. J Immunol . (2007) 178:6242–51. doi: 10.4049/jimmunol.178.10.6242
36. Dellabona P, Casorati G, de Lalla C, Montagna D, Locatelli F. On the use of donor-derived iNKT cells for adoptive immunotherapy to prevent leukemia recurrence in pediatric recipients of HLA haploidentical HSCT for hematological Malignancies. Clin Immunol . (2011) 140:152–9. doi: 10.1016/j.clim.2010.11.015
37. Li YR, Brown J, Yu Y, Lee D, Zhou K, Dunn ZS, et al. Targeting immunosuppressive tumor-associated macrophages using innate T cells for enhanced antitumor reactivity. Cancers (Basel) . (2022) 14(11):2749. doi: 10.3390/cancers14112749
38. Heczey A, Liu D, Tian G, Courtney AN, Wei J, Marinova E, et al. Invariant NKT cells with chimeric antigen receptor provide a novel platform for safe and effective cancer immunotherapy. Blood . (2014) 124:2824–33. doi: 10.1182/blood-2013-11-541235
39. Simon B, Wiesinger M, Marz J, Wistuba-Hamprecht K, Weide B, Schuler-Thurner B, et al. The generation of CAR-transfected natural killer T cells for the immunotherapy of melanoma. Int J Mol Sci . (2018) 19(8):2365. doi: 10.3390/ijms19082365
40. Kotsianidis I, Nakou E, Spanoudakis E, Bouchliou I, Moustakidis E, Miltiades P, et al. The diagnostic value of CD1d expression in a large cohort of patients with B-cell chronic lymphoproliferative disorders. Am J Clin Pathol . (2011) 136:400–8. doi: 10.1309/AJCP2F2DOXOTXHZA
41. Poels R, Drent E, Lameris R, Katsarou A, Themeli M, van der Vliet HJ, et al. Preclinical evaluation of invariant natural killer T cells modified with CD38 or BCMA chimeric antigen receptors for multiple myeloma. Int J Mol Sci . (2021) 22(3):1096. doi: 10.3390/ijms22031096
42. Xu X, Huang W, Heczey A, Liu D, Guo L, Wood M, et al. NKT cells coexpressing a GD2-specific chimeric antigen receptor and IL15 show enhanced in vivo persistence and antitumor activity against neuroblastoma. Clin Cancer Res . (2019) 25:7126–38. doi: 10.1158/1078-0432.CCR-19-0421
43. Alcantar-Orozco EM, Gornall H, Baldan V, Hawkins RE, Gilham DE. Potential limitations of the NSG humanized mouse as a model system to optimize engineered human T cell therapy for cancer. Hum Gene Ther Methods . (2013) 24:310–20. doi: 10.1089/hgtb.2013.022
44. Huang J, Huang X, Huang J. CAR-T cell therapy for hematological Malignancies: Limitations and optimization strategies. Front Immunol . (2022) 13:1019115. doi: 10.3389/fimmu.2022.1019115
45. Tian G, Courtney AN, Jena B, Heczey A, Liu D, Marinova E, et al. CD62L+ NKT cells have prolonged persistence and antitumor activity in vivo. J Clin Invest . (2016) 126:2341–55. doi: 10.1172/JCI83476
46. Liu Y, Dang Y, Zhang C, Liu L, Cai W, Li L, et al. IL-21-armored B7H3 CAR-iNKT cells exert potent antitumor effects. iScience . (2024) 27:108597. doi: 10.1016/j.isci.2023.108597
47. Heczey A, Courtney AN, Montalbano A, Robinson S, Liu K, Li M, et al. Anti-GD2 CAR-NKT cells in patients with relapsed or refractory neuroblastoma: an interim analysis. Nat Med . (2020) 26:1686–90. doi: 10.1038/s41591-020-1074-2
48. Heczey A, Xu X, Courtney AN, Tian G, Barragan GA, Guo L, et al. Anti-GD2 CAR-NKT cells in relapsed or refractory neuroblastoma: updated phase 1 trial interim results. Nat Med . (2023) 29:1379–88. doi: 10.1038/s41591-023-02363-y
49. Ramos CA, Courtney AN, Robinson SN, Dakhova O, Lulla PD, Kamble R, et al. Allogeneic NKT cells expressing a CD19-specific CAR in patients with relapsed or refractory B-cell Malignancies: an interim analysis. Blood . (2021) 138:2819. doi: 10.1182/blood-2021-149712
50. Godfrey DI, Koay HF, McCluskey J, Gherardin NA. The biology and functional importance of MAIT cells. Nat Immunol . (2019) 20:1110–28. doi: 10.1038/s41590-019-0444-8
51. Ussher JE, van Wilgenburg B, Hannaway RF, Ruustal K, Phalora P, Kurioka A, et al. TLR signaling in human antigen-presenting cells regulates MR1-dependent activation of MAIT cells. Eur J Immunol . (2016) 46:1600–14. doi: 10.1002/eji.201545969
52. Sato T, Thorlacius H, Johnston B, Staton TL, Xiang W, Littman DR, et al. Role for CXCR6 in recruitment of activated CD8+ lymphocytes to inflamed liver. J Immunol . (2005) 174:277–83. doi: 10.4049/jimmunol.174.1.277
53. Dusseaux M, Martin E, Serriari N, Peguillet I, Premel V, Louis D, et al. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood . (2011) 117:1250–9. doi: 10.1182/blood-2010-08-303339
54. Sundstrom P, Szeponik L, Ahlmanner F, Sundquist M, Wong JSB, Lindskog EB, et al. Tumor-infiltrating mucosal-associated invariant T (MAIT) cells retain expression of cytotoxic effector molecules. Oncotarget . (2019) 10:2810–23. doi: 10.18632/oncotarget.v10i29
55. Sundstrom P, Ahlmanner F, Akeus P, Sundquist M, Alsen S, Yrlid U, et al. Human mucosa-associated invariant T cells accumulate in colon adenocarcinomas but produce reduced amounts of IFN-gamma. J Immunol . (2015) 195:3472–81. doi: 10.4049/jimmunol.1500258
56. Ling L, Lin Y, Zheng W, Hong S, Tang X, Zhao P, et al. Circulating and tumor-infiltrating mucosal associated invariant T (MAIT) cells in colorectal cancer patients. Sci Rep . (2016) 6:20358. doi: 10.1038/srep20358
57. Tourret M, Talvard-Balland N, Lambert M, Ben Youssef G, Chevalier MF, Bohineust A, et al. Human MAIT cells are devoid of alloreactive potential: prompting their use as universal cells for adoptive immune therapy. J Immunother Cancer . (2021) 9(10):e003132. doi: 10.1101/2021.04.29.21256184
58. Varelias A, Bunting MD, Ormerod KL, Koyama M, Olver SD, Straube J, et al. Recipient mucosal-associated invariant T cells control GVHD within the colon. J Clin Invest . (2018) 128:1919–36. doi: 10.1172/JCI91646
59. Kawaguchi K, Umeda K, Hiejima E, Iwai A, Mikami M, Nodomi S, et al. Influence of post-transplant mucosal-associated invariant T cell recovery on the development of acute graft-versus-host disease in allogeneic bone marrow transplantation. Int J Hematol . (2018) 108:66–75. doi: 10.1007/s12185-018-2442-2
60. Gao MG, Hong Y, Zhao XY, Pan XA, Sun YQ, Kong J, et al. The potential roles of mucosa-associated invariant T cells in the pathogenesis of gut graft-versus-host disease after hematopoietic stem cell transplantation. Front Immunol . (2021) 12:720354. doi: 10.3389/fimmu.2021.720354
61. Andrlova H, Miltiadous O, Kousa AI, Dai A, DeWolf S, Violante S, et al. MAIT and Vdelta2 unconventional T cells are supported by a diverse intestinal microbiome and correlate with favorable patient outcome after allogeneic HCT. Sci Transl Med . (2022) 14:eabj2829. doi: 10.1126/scitranslmed.abj2829
62. Konduri V, Oyewole-Said D, Vazquez-Perez J, Weldon SA, Halpert MM, Levitt JM, et al. CD8(+)CD161(+) T-cells: cytotoxic memory cells with high therapeutic potential. Front Immunol . (2020) 11:613204. doi: 10.3389/fimmu.2020.613204
63. Dogan M, Karhan E, Kozhaya L, Placek L, Chen X, Yigit M, et al. Engineering human MAIT cells with chimeric antigen receptors for cancer immunotherapy. J Immunol . (2022) 209:1523–31. doi: 10.4049/jimmunol.2100856
64. Bohineust A, Tourret M, Derivry L, Caillat-Zucman S. Mucosal-associated invariant T (MAIT) cells, a new source of universal immune cells for chimeric antigen receptor (CAR)-cell therapy. Bull Cancer . (2021) 108:S92–S5. doi: 10.1016/j.bulcan.2021.07.003
65. Godfrey DI, Uldrich AP, McCluskey J, Rossjohn J, Moody DB. The burgeoning family of unconventional T cells. Nat Immunol . (2015) 16:1114–23. doi: 10.1038/ni.3298
66. Sebestyen Z, Prinz I, Dechanet-Merville J, Silva-Santos B, Kuball J. Translating gammadelta (gammadelta) T cells and their receptors into cancer cell therapies. Nat Rev Drug Discovery . (2020) 19:169–84. doi: 10.1038/s41573-019-0038-z
67. Vantourout P, Hayday A. Six-of-the-best: unique contributions of gammadelta T cells to immunology. Nat Rev Immunol . (2013) 13:88–100. doi: 10.1038/nri3384
68. Vavassori S, Kumar A, Wan GS, Ramanjaneyulu GS, Cavallari M, El Daker S, et al. Butyrophilin 3A1 binds phosphorylated antigens and stimulates human gammadelta T cells. Nat Immunol . (2013) 14:908–16. doi: 10.1038/ni.2665
69. Sandstrom A, Peigne CM, Leger A, Crooks JE, Konczak F, Gesnel MC, et al. The intracellular B30.2 domain of butyrophilin 3A1 binds phosphoantigens to mediate activation of human Vgamma9Vdelta2 T cells. Immunity . (2014) 40:490–500. doi: 10.1016/j.immuni.2014.03.003
70. Harly C, Guillaume Y, Nedellec S, Peigne CM, Monkkonen H, Monkkonen J, et al. Key implication of CD277/butyrophilin-3 (BTN3A) in cellular stress sensing by a major human gammadelta T-cell subset. Blood . (2012) 120:2269–79. doi: 10.1182/blood-2012-05-430470
71. Park JH, Lee HK. Function of gammadelta T cells in tumor immunology and their application to cancer therapy. Exp Mol Med . (2021) 53:318–27. doi: 10.1038/s12276-021-00576-0
72. Zhao Y, Niu C, Cui J. Gamma-delta (gammadelta) T cells: friend or foe in cancer development? J Transl Med . (2018) 16:3. doi: 10.1186/s12967-017-1378-2
73. Khan MW, Curbishley SM, Chen HC, Thomas AD, Pircher H, Mavilio D, et al. Expanded human blood-derived gammadeltaT cells display potent antigen-presentation functions. Front Immunol . (2014) 5:344. doi: 10.3389/fimmu.2014.00344
74. Himoudi N, Morgenstern DA, Yan M, Vernay B, Saraiva L, Wu Y, et al. Human gammadelta T lymphocytes are licensed for professional antigen presentation by interaction with opsonized target cells. J Immunol . (2012) 188:1708–16. doi: 10.4049/jimmunol.1102654
75. Brandes M, Willimann K, Bioley G, Levy N, Eberl M, Luo M, et al. Cross-presenting human gammadelta T cells induce robust CD8+ alphabeta T cell responses. Proc Natl Acad Sci U S A . (2009) 106:2307–12. doi: 10.1073/pnas.0810059106
76. Maniar A, Zhang X, Lin W, Gastman BR, Pauza CD, Strome SE, et al. Human gammadelta T lymphocytes induce robust NK cell-mediated antitumor cytotoxicity through CD137 engagement. Blood . (2010) 116:1726–33. doi: 10.1182/blood-2009-07-234211
77. Munz C, Steinman RM, Fujii S. Dendritic cell maturation by innate lymphocytes: coordinated stimulation of innate and adaptive immunity. J Exp Med . (2005) 202:203–7. doi: 10.1084/jem.20050810
78. Conti L, Casetti R, Cardone M, Varano B, Martino A, Belardelli F, et al. Reciprocal activating interaction between dendritic cells and pamidronate-stimulated gammadelta T cells: role of CD86 and inflammatory cytokines. J Immunol . (2005) 174:252–60. doi: 10.4049/jimmunol.174.1.252
79. Huang Y, Getahun A, Heiser RA, Detanico TO, Aviszus K, Kirchenbaum GA, et al. gammadelta T cells shape preimmune peripheral B cell populations. J Immunol . (2016) 196:217–31. doi: 10.4049/jimmunol.1501064
80. Airoldi I, Bertaina A, Prigione I, Zorzoli A, Pagliara D, Cocco C, et al. gammadelta T-cell reconstitution after HLA-haploidentical hematopoietic transplantation depleted of TCR-alphabeta+/CD19+ lymphocytes. Blood . (2015) 125:2349–58. doi: 10.1182/blood-2014-09-599423
81. Gentles AJ, Newman AM, Liu CL, Bratman SV, Feng W, Kim D, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med . (2015) 21:938–45. doi: 10.1038/nm.3909
82. Fournie JJ, Sicard H, Poupot M, Bezombes C, Blanc A, Romagne F, et al. What lessons can be learned from gammadelta T cell-based cancer immunotherapy trials? Cell Mol Immunol . (2013) 10:35–41. doi: 10.1038/cmi.2012.39
83. Wang Y, Wang L, Seo N, Okumura S, Hayashi T, Akahori Y, et al. CAR-modified vgamma9Vdelta2 T cells propagated using a novel bisphosphonate prodrug for allogeneic adoptive immunotherapy. Int J Mol Sci . (2023) 24(13):10873. doi: 10.20944/preprints202305.1853.v1
84. Wang Y, Ji N, Zhang Y, Chu J, Pan C, Zhang P, et al. B7H3-targeting chimeric antigen receptor modification enhances antitumor effect of Vgamma9Vdelta2 T cells in glioblastoma. J Transl Med . (2023) 21:672. doi: 10.1186/s12967-023-04514-8
85. Davey MS, Willcox CR, Joyce SP, Ladell K, Kasatskaya SA, McLaren JE, et al. Clonal selection in the human Vdelta1 T cell repertoire indicates gammadelta TCR-dependent adaptive immune surveillance. Nat Commun . (2017) 8:14760. doi: 10.1038/ncomms14760
86. Nishimoto KP, Barca T, Azameera A, Makkouk A, Romero JM, Bai L, et al. Allogeneic CD20-targeted gammadelta T cells exhibit innate and adaptive antitumor activities in preclinical B-cell lymphoma models. Clin Transl Immunol . (2022) 11:e1373. doi: 10.1002/cti2.1373
87. Makkouk A, Yang XC, Barca T, Lucas A, Turkoz M, Wong JTS, et al. Off-the-shelf Vdelta1 gamma delta T cells engineered with glypican-3 (GPC-3)-specific chimeric antigen receptor (CAR) and soluble IL-15 display robust antitumor efficacy against hepatocellular carcinoma. J Immunother Cancer . (2021) 9(12):e003441. doi: 10.1136/jitc-2021-003441
88. Rozenbaum M, Meir A, Aharony Y, Itzhaki O, Schachter J, Bank I, et al. Gamma-delta CAR-T cells show CAR-directed and independent activity against leukemia. Front Immunol . (2020) 11:1347. doi: 10.3389/fimmu.2020.01347
89. Sanchez Martinez D, Tirado N, Mensurado S, Martinez-Moreno A, Romecin P, Gutierrez Aguera F, et al. Generation and proof-of-concept for allogeneic CD123 CAR-Delta One T (DOT) cells in acute myeloid leukemia. J Immunother Cancer . (2022) 10(9):e005400. doi: 10.1101/2022.03.15.484289
90. Deniger DC, Switzer K, Mi T, Maiti S, Hurton L, Singh H, et al. Bispecific T-cells expressing polyclonal repertoire of endogenous gammadelta T-cell receptors and introduced CD19-specific chimeric antigen receptor. Mol Ther . (2013) 21:638–47. doi: 10.1038/mt.2012.267
91. Capsomidis A, Benthall G, Van Acker HH, Fisher J, Kramer AM, Abeln Z, et al. Chimeric antigen receptor-engineered human gamma delta T cells: enhanced cytotoxicity with retention of cross presentation. Mol Ther . (2018) 26:354–65. doi: 10.1016/j.ymthe.2017.12.001
92. Li HK, Wu TS, Kuo YC, Hsiao CW, Yang HP, Lee CY, et al. A novel allogeneic rituximab-conjugated gamma delta T cell therapy for the treatment of relapsed/refractory B-cell lymphoma. Cancers (Basel) . (2023) 15(19):4844. doi: 10.3390/cancers15194844
93. Huang SW, Pan CM, Lin YC, Chen MC, Chen Y, Jan CI, et al. BiTE-secreting CAR-gammadeltaT as a dual targeting strategy for the treatment of solid tumors. Adv Sci (Weinh) . (2023) 10:e2206856. doi: 10.1002/advs.202206856
94. Lee D, Dunn ZS, Guo W, Rosenthal CJ, Penn NE, Yu Y, et al. Unlocking the potential of allogeneic Vdelta2 T cells for ovarian cancer therapy through CD16 biomarker selection and CAR/IL-15 engineering. Nat Commun . (2023) 14:6942. doi: 10.1038/s41467-023-42619-2
95. Fleischer LC, Spencer HT, Raikar SS. Targeting T cell Malignancies using CAR-based immunotherapy: challenges and potential solutions. J Hematol Oncol . (2019) 12:141. doi: 10.1186/s13045-019-0801-y
96. Fleischer LC, Becker SA, Ryan RE, Fedanov A, Doering CB, Spencer HT. Non-signaling Chimeric Antigen Receptors Enhance Antigen-Directed Killing by gammadelta T Cells in Contrast to alphabeta T Cells. Mol Ther Oncol . (2020) 18:149–60. doi: 10.1016/j.omto.2020.06.003
97. Lopez-Cantillo G, Uruena C, Camacho BA, Ramirez-Segura C. CAR-T cell performance: how to improve their persistence? Front Immunol . (2022) 13:878209. doi: 10.3389/fimmu.2022.878209
98. Arcangeli S, Bove C, Mezzanotte C, Camisa B, Falcone L, Manfredi F, et al. CAR T cell manufacturing from naive/stem memory T lymphocytes enhances antitumor responses while curtailing cytokine release syndrome. J Clin Invest . (2022) 132(12):e1508072. doi: 10.1172/JCI150807
99. Fisher J, Sharma R, Don DW, Barisa M, Hurtado MO, Abramowski P, et al. Engineering gammadeltaT cells limits tonic signaling associated with chimeric antigen receptors. Sci Signal . (2019) 12. doi: 10.1126/scisignal.aax1872
100. Fisher J, Abramowski P, Wisidagamage Don ND, Flutter B, Capsomidis A, Cheung GW, et al. Avoidance of on-target off-tumor activation using a co-stimulation-only chimeric antigen receptor. Mol Ther . (2017) 25:1234–47. doi: 10.1016/j.ymthe.2017.03.002
101. Wilhelm M, Kunzmann V, Eckstein S, Reimer P, Weissinger F, Ruediger T, et al. Gammadelta T cells for immune therapy of patients with lymphoid Malignancies. Blood . (2003) 102:200–6. doi: 10.1182/blood-2002-12-3665
102. Rischer M, Pscherer S, Duwe S, Vormoor J, Jurgens H, Rossig C. Human gammadelta T cells as mediators of chimaeric-receptor redirected anti-tumour immunity. Br J Haematol . (2004) 126:583–92. doi: 10.1111/j.1365-2141.2004.05077.x
103. Neelapu SS, Hamadani M, Miklos DB, Holmes H, Hinkle J, Kennedy-Wilde J, et al. A phase 1 study of ADI-001: Anti-CD20 CAR-engineered allogeneic gamma delta (γδ) T cells in adults with B-cell Malignancies. J Clin Oncol . (2022) 40:7509. doi: 10.1200/JCO.2022.40.16_suppl.7509
104. Gao X, Mi Y, Guo N, Xu H, Xu L, Gou X, et al. Cytokine-induced killer cells as pharmacological tools for cancer immunotherapy. Front Immunol . (2017) 8:774. doi: 10.3389/fimmu.2017.00774
105. Verneris MR, Baker J, Edinger M, Negrin RS. Studies of ex vivo activated and expanded CD8+ NK-T cells in humans and mice. J Clin Immunol . (2002) 22:131–6. doi: 10.1023/A:1015415928521
106. Pievani A, Borleri G, Pende D, Moretta L, Rambaldi A, Golay J, et al. Dual-functional capability of CD3+CD56+ CIK cells, a T-cell subset that acquires NK function and retains TCR-mediated specific cytotoxicity. Blood . (2011) 118:3301–10. doi: 10.1182/blood-2011-02-336321
107. Verneris MR, Karimi M, Baker J, Jayaswal A, Negrin RS. Role of NKG2D signaling in the cytotoxicity of activated and expanded CD8+ T cells. Blood . (2004) 103:3065–72. doi: 10.1182/blood-2003-06-2125
108. Joshi PS, Liu JQ, Wang Y, Chang X, Richards J, Assarsson E, et al. Cytokine-induced killer T cells kill immature dendritic cells by TCR-independent and perforin-dependent mechanisms. J Leukoc Biol . (2006) 80:1345–53. doi: 10.1189/jlb.0506305
109. Gutgemann S, Frank S, Strehl J, Schmidt-Wolf IG. Cytokine-induced killer cells are type II natural killer T cells. Ger Med Sci . (2007) 5:Doc07.
PubMed Abstract | Google Scholar
110. Nishimura R, Baker J, Beilhack A, Zeiser R, Olson JA, Sega EI, et al. In vivo trafficking and survival of cytokine-induced killer cells resulting in minimal GVHD with retention of antitumor activity. Blood . (2008) 112:2563–74. doi: 10.1182/blood-2007-06-092817
111. Baker J, Verneris MR, Ito M, Shizuru JA, Negrin RS. Expansion of cytolytic CD8(+) natural killer T cells with limited capacity for graft-versus-host disease induction due to interferon gamma production. Blood . (2001) 97:2923–31. doi: 10.1182/blood.V97.10.2923
112. Marin V, Dander E, Biagi E, Introna M, Fazio G, Biondi A, et al. Characterization of in vitro migratory properties of anti-CD19 chimeric receptor-redirected CIK cells for their potential use in B-ALL immunotherapy. Exp Hematol . (2006) 34:1219–29. doi: 10.1016/j.exphem.2006.05.004
113. Rotiroti MC, Buracchi C, Arcangeli S, Galimberti S, Valsecchi MG, Perriello VM, et al. Targeting CD33 in chemoresistant AML patient-derived xenografts by CAR-CIK cells modified with an improved SB transposon system. Mol Ther . (2020) 28:1974–86. doi: 10.1016/j.ymthe.2020.05.021
114. Leuci V, Casucci GM, Grignani G, Rotolo R, Rossotti U, Vigna E, et al. CD44v6 as innovative sarcoma target for CAR-redirected CIK cells. Oncoimmunology . (2018) 7:e1423167. doi: 10.1080/2162402X.2017.1423167
115. Oelsner S, Wagner J, Friede ME, Pfirrmann V, Genssler S, Rettinger E, et al. Chimeric antigen receptor-engineered cytokine-induced killer cells overcome treatment resistance of pre-B-cell acute lymphoblastic leukemia and enhance survival. Int J Cancer . (2016) 139:1799–809. doi: 10.1002/ijc.30217
116. Pizzitola I, Anjos-Afonso F, Rouault-Pierre K, Lassailly F, Tettamanti S, Spinelli O, et al. Chimeric antigen receptors against CD33/CD123 antigens efficiently target primary acute myeloid leukemia cells in vivo. Leukemia . (2014) 28:1596–605. doi: 10.1038/leu.2014.62
117. Tettamanti S, Marin V, Pizzitola I, Magnani CF, Giordano Attianese GM, Cribioli E, et al. Targeting of acute myeloid leukaemia by cytokine-induced killer cells redirected with a novel CD123-specific chimeric antigen receptor. Br J Haematol . (2013) 161:389–401. doi: 10.1111/bjh.12282
118. Giraudo L, Cattaneo G, Gammaitoni L, Iaia I, Donini C, Massa A, et al. CSPG4 CAR-redirected Cytokine Induced Killer lymphocytes (CIK) as effective cellular immunotherapy for HLA class I defective melanoma. J Exp Clin Cancer Res . (2023) 42:310. doi: 10.1186/s13046-023-02884-x
119. Merker M, Wagner J, Kreyenberg H, Heim C, Moser LM, Wels WS, et al. ERBB2-CAR-engineered cytokine-induced killer cells exhibit both CAR-mediated and innate immunity against high-risk rhabdomyosarcoma. Front Immunol . (2020) 11:581468. doi: 10.3389/fimmu.2020.581468
120. Guo X, Zheng H, Luo W, Zhang Q, Liu J, Yao K. 5T4-specific chimeric antigen receptor modification promotes the immune efficacy of cytokine-induced killer cells against nasopharyngeal carcinoma stem cell-like cells. Sci Rep . (2017) 7:4859. doi: 10.1038/s41598-017-04756-9
121. Alberti G, Arsuffi C, Pievani A, Salerno D, Mantegazza F, Dazzi F, et al. Engineering tandem CD33xCD146 CAR CIK (cytokine-induced killer) cells to target the acute myeloid leukemia niche. Front Immunol . (2023) 14:1192333. doi: 10.3389/fimmu.2023.1192333
122. Schlimper C, Hombach AA, Abken H, Schmidt-Wolf IG. Improved activation toward primary colorectal cancer cells by antigen-specific targeting autologous cytokine-induced killer cells. Clin Dev Immunol . (2012) 2012:238924. doi: 10.1155/2012/238924
123. Laport GG, Sheehan K, Baker J, Armstrong R, Wong RM, Lowsky R, et al. Adoptive immunotherapy with cytokine-induced killer cells for patients with relapsed hematologic Malignancies after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant . (2011) 17:1679–87. doi: 10.1016/j.bbmt.2011.05.012
124. Zuo S, Wen Y, Panha H, Dai G, Wang L, Ren X, et al. Modification of cytokine-induced killer cells with folate receptor alpha (FRalpha)-specific chimeric antigen receptors enhances their antitumor immunity toward FRalpha-positive ovarian cancers. Mol Immunol . (2017) 85:293–304. doi: 10.1016/j.molimm.2017.03.017
125. Circosta P, Donini C, Gallo S, Giraudo L, Gammaitoni L, Rotolo R, et al. Full chimaeric CAR.CIK from patients engrafted after allogeneic haematopoietic cell transplant: Feasibility, anti-leukaemic potential and alloreactivity across major human leukocyte antigen barriers. Br J Haematol . (2023) 200:64–9. doi: 10.1111/bjh.18469
126. Leuci V, Donini C, Grignani G, Rotolo R, Mesiano G, Fiorino E, et al. CSPG4-specific CAR.CIK lymphocytes as a novel therapy for the treatment of multiple soft-tissue sarcoma histotypes. Clin Cancer Res . (2020) 26:6321–34. doi: 10.1158/1078-0432.CCR-20-0357
127. Franceschetti M, Pievani A, Borleri G, Vago L, Fleischhauer K, Golay J, et al. Cytokine-induced killer cells are terminally differentiated activated CD8 cytotoxic T-EMRA lymphocytes. Exp Hematol . (2009) 37:616–28 e2. doi: 10.1016/j.exphem.2009.01.010
128. Zhang M, Wen B, Anton OM, Yao Z, Dubois S, Ju W, et al. IL-15 enhanced antibody-dependent cellular cytotoxicity mediated by NK cells and macrophages. Proc Natl Acad Sci U S A . (2018) 115:E10915–E24. doi: 10.1073/pnas.1811615115
129. Berraondo P, Sanmamed MF, Ochoa MC, Etxeberria I, Aznar MA, Perez-Gracia JL, et al. Cytokines in clinical cancer immunotherapy. Br J Cancer . (2019) 120:6–15. doi: 10.1038/s41416-018-0328-y
130. Hombach A, Barden M, Hannappel L, Chmielewski M, Rappl G, Sachinidis A, et al. IL12 integrated into the CAR exodomain converts CD8(+) T cells to poly-functional NK-like cells with superior killing of antigen-loss tumors. Mol Ther . (2022) 30:593–605. doi: 10.1016/j.ymthe.2021.10.011
131. Hombach AA, Rappl G, Abken H. Arming cytokine-induced killer cells with chimeric antigen receptors: CD28 outperforms combined CD28-OX40 “super-stimulation”. Mol Ther . (2013) 21:2268–77. doi: 10.1038/mt.2013.192
132. Sangiolo D, Martinuzzi E, Todorovic M, Vitaggio K, Vallario A, Jordaney N, et al. Alloreactivity and anti-tumor activity segregate within two distinct subsets of cytokine-induced killer (CIK) cells: implications for their infusion across major HLA barriers. Int Immunol . (2008) 20:841–8. doi: 10.1093/intimm/dxn042
133. Merker M, Pfirrmann V, Oelsner S, Fulda S, Klingebiel T, Wels WS, et al. Generation and characterization of ErbB2-CAR-engineered cytokine-induced killer cells for the treatment of high-risk soft tissue sarcoma in children. Oncotarget . (2017) 8:66137–53. doi: 10.18632/oncotarget.v8i39
134. Biondi M, Tettamanti S, Galimberti S, Cerina B, Tomasoni C, Piazza R, et al. Selective homing of CAR-CIK cells to the bone marrow niche enhances control of the acute myeloid leukemia burden. Blood . (2023) 141:2587–98. doi: 10.1182/blood.2022018330
135. Lussana F, Cavallaro G, De Simone P, Rambaldi A. Optimal use of novel immunotherapeutics in B-cell precursor ALL. Cancers (Basel) . (2023) 15(4):1349. doi: 10.3390/cancers15041349
136. Bald T, Krummel MF, Smyth MJ, Barry KC. The NK cell-cancer cycle: advances and new challenges in NK cell-based immunotherapies. Nat Immunol . (2020) 21:835–47. doi: 10.1038/s41590-020-0728-z
137. Maskalenko NA, Zhigarev D, Campbell KS. Harnessing natural killer cells for cancer immunotherapy: dispatching the first responders. Nat Rev Drug Discovery . (2022) 21(8):559–77. doi: 10.1038/s41573-022-00413-7
138. Shin MH, Kim J, Lim SA, Kim J, Kim SJ, Lee KM. NK cell-based immunotherapies in cancer. Immune Netw . (2020) 20:e14. doi: 10.4110/in.2020.20.e14
139. Marofi F, Saleh MM, Rahman HS, Suksatan W, Al-Gazally ME, Abdelbasset WK, et al. CAR-engineered NK cells; a promising therapeutic option for treatment of hematological Malignancies. Stem Cell Res Ther . (2021) 12:374. doi: 10.1186/s13287-021-02462-y
140. Xiao L, Cen D, Gan H, Sun Y, Huang N, Xiong H, et al. Adoptive transfer of NKG2D CAR mRNA-engineered natural killer cells in colorectal cancer patients. Mol Ther . (2019) 27:1114–25. doi: 10.1016/j.ymthe.2019.03.011
141. Tang X, Yang L, Li Z, Nalin AP, Dai H, Xu T, et al. First-in-man clinical trial of CAR NK-92 cells: safety test of CD33-CAR NK-92 cells in patients with relapsed and refractory acute myeloid leukemia. Am J Cancer Res . (2018) 8:1083–9.
142. Liu E, Marin D, Banerjee P, Macapinlac HA, Thompson P, Basar R, et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N Engl J Med . (2020) 382:545–53. doi: 10.1056/NEJMoa1910607
143. Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood . (2005) 105:3051–7. doi: 10.1182/blood-2004-07-2974
144. Leivas A, Valeri A, Cordoba L, Garcia-Ortiz A, Ortiz A, Sanchez-Vega L, et al. NKG2D-CAR-transduced natural killer cells efficiently target multiple myeloma. Blood Cancer J . (2021) 11:146. doi: 10.1038/s41408-021-00537-w
145. Teng KY, Mansour AG, Zhu Z, Li Z, Tian L, Ma S, et al. Off-the-shelf prostate stem cell antigen-directed chimeric antigen receptor natural killer cell therapy to treat pancreatic cancer. Gastroenterology . (2022) 162:1319–33. doi: 10.1053/j.gastro.2021.12.281
146. Arias J, Yu J, Varshney M, Inzunza J, Nalvarte I. Hematopoietic stem cell- and induced pluripotent stem cell-derived CAR-NK cells as reliable cell-based therapy solutions. Stem Cells Transl Med . (2021) 10:987–95. doi: 10.1002/sctm.20-0459
147. Ng YY, Tay JCK, Wang S. CXCR1 expression to improve anti-cancer efficacy of intravenously injected CAR-NK cells in mice with peritoneal xenografts. Mol Ther Oncol . (2020) 16:75–85. doi: 10.1016/j.omto.2019.12.006
148. Li Y, Hermanson DL, Moriarity BS, Kaufman DS. Human iPSC-derived natural killer cells engineered with chimeric antigen receptors enhance anti-tumor activity. Cell Stem Cell . (2018) 23:181–92 e5. doi: 10.1016/j.stem.2018.06.002
149. Daher M, Basar R, Gokdemir E, Baran N, Uprety N, Nunez Cortes AK, et al. Targeting a cytokine checkpoint enhances the fitness of armored cord blood CAR-NK cells. Blood . (2021) 137:624–36. doi: 10.1182/blood.2020007748
150. Krenciute G, Prinzing BL, Yi Z, Wu MF, Liu H, Dotti G, et al. Transgenic expression of IL15 improves antiglioma activity of IL13Ralpha2-CAR T cells but results in antigen loss variants. Cancer Immunol Res . (2017) 5:571–81. doi: 10.1158/2326-6066.CIR-16-0376
151. Ataca Atilla P, McKenna MK, Tashiro H, Srinivasan M, Mo F, Watanabe N, et al. Modulating TNFalpha activity allows transgenic IL15-Expressing CLL-1 CAR T cells to safely eliminate acute myeloid leukemia. J Immunother Cancer . (2020) 8(2):e001229. doi: 10.1136/jitc-2020-001229
152. Hurton LV, Singh H, Najjar AM, Switzer KC, Mi T, Maiti S, et al. Tethered IL-15 augments antitumor activity and promotes a stem-cell memory subset in tumor-specific T cells. Proc Natl Acad Sci U S A . (2016) 113:E7788–E97. doi: 10.1073/pnas.1610544113
153. Foster AE, Mahendravada A, Shinners NP, Chang WC, Crisostomo J, Lu A, et al. Regulated expansion and survival of chimeric antigen receptor-modified T cells using small molecule-dependent inducible myD88/CD40. Mol Ther . (2017) 25:2176–88. doi: 10.1016/j.ymthe.2017.06.014
154. Collinson-Pautz MR, Chang WC, Lu A, Khalil M, Crisostomo JW, Lin PY, et al. Constitutively active MyD88/CD40 costimulation enhances expansion and efficacy of chimeric antigen receptor T cells targeting hematological Malignancies. Leukemia . (2019) 33:2195–207. doi: 10.1038/s41375-019-0417-9
155. Wang X, Jasinski DL, Medina JL, Spencer DM, Foster AE, Bayle JH. Inducible MyD88/CD40 synergizes with IL-15 to enhance antitumor efficacy of CAR-NK cells. Blood Adv . (2020) 4:1950–64. doi: 10.1182/bloodadvances.2020001510
156. Biederstadt A, Rezvani K. Engineering the next generation of CAR-NK immunotherapies. Int J Hematol . (2021) 114:554–71. doi: 10.1007/s12185-021-03209-4
157. Page A, Chuvin N, Valladeau-Guilemond J, Depil S. Development of NK cell-based cancer immunotherapies through receptor engineering. Cell Mol Immunol . (2024) 21(4):315–31. doi: 10.1038/s41423-024-01145-x
158. Nkarta Inc. Nkarta updates clinical progress of car-nk cell therapy nkx101 for patients with relapsed or refractory acute myeloid leUKEMIA (2023). Available online at: https://ir.nkartatx.com/news-releases/news-release-details/nkarta-updates-clinical-progress-car-nk-cell-therapy-nkx101 .
Google Scholar
159. Dickinson M, Hamad N, Bryant C, Kothari N, Ojeras P, Vohra A, et al. S261: First in human data of nkx019, an allogeneic car nk for the treatment of relapsed/refractory (r/r) b-cell malignancies. HemaSphere . (2023) 7(Suppl):e37234fb2. doi: 10.1097/01.HS9.0000967956.37234.fb
160. Locati M, Curtale G, Mantovani A. Diversity, mechanisms, and significance of macrophage plasticity. Annu Rev Pathol . (2020) 15:123–47. doi: 10.1146/annurev-pathmechdis-012418-012718
161. Cassetta L, Pollard JW. Targeting macrophages: therapeutic approaches in cancer. Nat Rev Drug Discovery . (2018) 17:887–904. doi: 10.1038/nrd.2018.169
162. Anderson NR, Minutolo NG, Gill S, Klichinsky M. Macrophage-based approaches for cancer immunotherapy. Cancer Res . (2021) 81:1201–8. doi: 10.1158/0008-5472.CAN-20-2990
163. Andreesen R, Hennemann B, Krause SW. Adoptive immunotherapy of cancer using monocyte-derived macrophages: rationale, current status, and perspectives. J Leukoc Biol . (1998) 64:419–26. doi: 10.1002/jlb.64.4.419
164. Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci . (2008) 13:453–61. doi: 10.2741/2692
165. Pan Y, Yu Y, Wang X, Zhang T. Tumor-associated macrophages in tumor immunity. Front Immunol . (2020) 11:583084. doi: 10.3389/fimmu.2020.583084
166. Dong X, Fan J, Xie W, Wu X, Wei J, He Z, et al. Efficacy evaluation of chimeric antigen receptor-modified human peritoneal macrophages in the treatment of gastric cancer. Br J Cancer . (2023) 129:551–62. doi: 10.1038/s41416-023-02319-6
167. Zhang W, Liu L, Su H, Liu Q, Shen J, Dai H, et al. Chimeric antigen receptor macrophage therapy for breast tumours mediated by targeting the tumour extracellular matrix. Br J Cancer . (2019) 121:837–45. doi: 10.1038/s41416-019-0578-3
168. Niu Z, Chen G, Chang W, Sun P, Luo Z, Zhang H, et al. Chimeric antigen receptor-modified macrophages trigger systemic anti-tumour immunity. J Pathol . (2021) 253:247–57. doi: 10.1002/path.5585
169. Duan Z, Li Z, Wang Z, Chen C, Luo Y. Chimeric antigen receptor macrophages activated through TLR4 or IFN-gamma receptors suppress breast cancer growth by targeting VEGFR2. Cancer Immunol Immunother . (2023) 72(10):3243–57. doi: 10.1007/s00262-023-03490-8
170. Klichinsky M, Ruella M, Shestova O, Lu XM, Best A, Zeeman M, et al. Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat Biotechnol . (2020) 38:947–53. doi: 10.1038/s41587-020-0462-y
171. Chen Y, Zhu X, Liu H, Wang C, Chen Y, Wang H, et al. The application of HER2 and CD47 CAR-macrophage in ovarian cancer. J Transl Med . (2023) 21:654. doi: 10.1186/s12967-023-04479-8
172. Stefano P, Rashid G, Cecilia N, Alison W, Yumi O, Michael B, et al. 371 Chimeric antigen receptor macrophages (CAR-M) sensitize solid tumors to anti-PD1 immunotherapy. J ImmunoTher Cancer . (2022) 10:A390. doi: 10.1136/jitc-2022-SITC2022.0371
173. Huo Y, Zhang H, Sa L, Zheng W, He Y, Lyu H, et al. M1 polarization enhances the antitumor activity of chimeric antigen receptor macrophages in solid tumors. J Transl Med . (2023) 21:225. doi: 10.1186/s12967-023-04061-2
174. Paasch D, Meyer J, Stamopoulou A, Lenz D, Kuehle J, Kloos D, et al. Ex vivo generation of CAR macrophages from hematopoietic stem and progenitor cells for use in cancer therapy. Cells . (2022) 11(6):994. doi: 10.3390/cells11060994
175. Abdin SM, Paasch D, Kloos A, Oliveira MC, Jang MS, Ackermann M, et al. Scalable generation of functional human iPSC-derived CAR-macrophages that efficiently eradicate CD19-positive leukemia. J Immunother Cancer . (2023) 11(12):e007705. doi: 10.1136/jitc-2023-007705
176. Jin G, Chang Y, Bao X. Generation of chimeric antigen receptor macrophages from human pluripotent stem cells to target glioblastoma. Immunooncol Technol . (2023) 20:100409. doi: 10.1016/j.iotech.2023.100409
177. Zhang J, Webster S, Duffin B, Bernstein MN, Steill J, Swanson S, et al. Generation of anti-GD2 CAR macrophages from human pluripotent stem cells for cancer immunotherapies. Stem Cell Rep . (2023) 18:585–96. doi: 10.1016/j.stemcr.2022.12.012
178. Zhang L, Tian L, Dai X, Yu H, Wang J, Lei A, et al. Pluripotent stem cell-derived CAR-macrophage cells with antigen-dependent anti-cancer cell functions. J Hematol Oncol . (2020) 13:153. doi: 10.1186/s13045-020-00983-2
179. Lei A, Yu H, Lu S, Lu H, Ding X, Tan T, et al. A second-generation M1-polarized CAR macrophage with antitumor efficacy. Nat Immunol . (2024) 25:102–16. doi: 10.1038/s41590-023-01687-8
180. Wang X, Su S, Zhu Y, Cheng X, Cheng C, Chen L, et al. Metabolic Reprogramming via ACOD1 depletion enhances function of human induced pluripotent stem cell-derived CAR-macrophages in solid tumors. Nat Commun . (2023) 14:5778. doi: 10.1038/s41467-023-41470-9
181. Yang Z, Liu Y, Zhao K, Jing W, Gao L, Dong X, et al. Dual mRNA co-delivery for in situ generation of phagocytosis-enhanced CAR macrophages augments hepatocellular carcinoma immunotherapy. J Control Release . (2023) 360:718–33. doi: 10.1016/j.jconrel.2023.07.021
182. Gao L, Shi C, Yang Z, Jing W, Han M, Zhang J, et al. Convection-enhanced delivery of nanoencapsulated gene locoregionally yielding ErbB2/Her2-specific CAR-macrophages for brainstem glioma immunotherapy. J Nanobiotechnol . (2023) 21:56. doi: 10.1186/s12951-023-01810-9
183. Kang M, Lee SH, Kwon M, Byun J, Kim D, Kim C, et al. Nanocomplex-mediated in vivo programming to chimeric antigen receptor-M1 macrophages for cancer therapy. Adv Mater . (2021) 33:e2103258. doi: 10.1002/adma.202103258
184. Chen C, Jing W, Chen Y, Wang G, Abdalla M, Gao L, et al. Intracavity generation of glioma stem cell-specific CAR macrophages primes locoregional immunity for postoperative glioblastoma therapy. Sci Transl Med . (2022) 14:eabn1128. doi: 10.1126/scitranslmed.abn1128
185. Apte RS, Chen DS, Ferrara N. VEGF in signaling and disease: beyond discovery and development. Cell . (2019) 176:1248–64. doi: 10.1016/j.cell.2019.01.021
186. Liu M, Liu J, Liang Z, Dai K, Gan J, Wang Q, et al. CAR-macrophages and CAR-T cells synergistically kill tumor cells in vitro . Cells . (2022) 11(22):3692. doi: 10.3390/cells11223692
187. Pang J, Koh TJ. Proliferation of monocytes and macrophages in homeostasis, infection, injury and disease. J Leukoc Biol . (2023) 114(6):532–46. doi: 10.1093/jleuko/qiad093
188. Nori S, Okada Y, Nishimura S, Sasaki T, Itakura G, Kobayashi Y, et al. Long-term safety issues of iPSC-based cell therapy in a spinal cord injury model: oncogenic transformation with epithelial-mesenchymal transition. Stem Cell Rep . (2015) 4:360–73. doi: 10.1016/j.stemcr.2015.01.006
189. Blum B, Benvenisty N. The tumorigenicity of diploid and aneuploid human pluripotent stem cells. Cell Cycle . (2009) 8:3822–30. doi: 10.4161/cc.8.23.10067
190. Nilsson M, Ljungberg J, Richter J, Kiefer T, Magnusson M, Lieber A, et al. Development of an adenoviral vector system with adenovirus serotype 35 tropism; efficient transient gene transfer into primary Malignant hematopoietic cells. J Gene Med . (2004) 6:631–41. doi: 10.1002/jgm.543
191. Gao Y, Ju Y, Ren X, Zhang L, Yin X. Enhanced infection efficiency and cytotoxicity mediated by vpx-containing lentivirus in chimeric antigen receptor macrophage (CAR-M). Heliyon . (2023) 9:e21886. doi: 10.1016/j.heliyon.2023.e21886
192. Morrissey MA, Williamson AP, Steinbach AM, Roberts EW, Kern N, Headley MB, et al. Chimeric antigen receptors that trigger phagocytosis. Elife . (2018) 7:e36688. doi: 10.7554/eLife.36688
193. Reiss KA, Yuan Y, Ueno NT, Johnson ML, Gill S, Dees EC, et al. A phase 1, first-in-human (FIH) study of the anti-HER2 CAR macrophage CT-0508 in subjects with HER2 overexpressing solid tumors. J Clin Oncol . (2022) 40:2533. doi: 10.1200/JCO.2022.40.16_suppl.2533
194. Jaillon S, Ponzetta A, Di Mitri D, Santoni A, Bonecchi R, Mantovani A. Neutrophil diversity and plasticity in tumour progression and therapy. Nat Rev Cancer . (2020) 20:485–503. doi: 10.1038/s41568-020-0281-y
195. Chang Y, Syahirah R, Wang X, Jin G, Torregrosa-Allen S, Elzey BD, et al. Engineering chimeric antigen receptor neutrophils from human pluripotent stem cells for targeted cancer immunotherapy. Cell Rep . (2022) 40:111128. doi: 10.1016/j.celrep.2022.111128
196. Triner D, Shah YM. Hypoxic regulation of neutrophils in cancer. Int J Mol Sci . (2019) 20(17):418. doi: 10.3390/ijms20174189
197. Chang Y, Cai X, Syahirah R, Yao Y, Xu Y, Jin G, et al. CAR-neutrophil mediated delivery of tumor-microenvironment responsive nanodrugs for glioblastoma chemo-immunotherapy. Nat Commun . (2023) 14:2266. doi: 10.1038/s41467-023-37872-4
198. Harris JD, Chang Y, Syahirah R, Lian XL, Deng Q, Bao X. Engineered anti-prostate cancer CAR-neutrophils from human pluripotent stem cells. J Immunol Regener Med . (2023) 20:100074. doi: 10.1016/j.regen.2023.100074
199. Panch SR, Szymanski J, Savani BN, Stroncek DF. Sources of hematopoietic stem and progenitor cells and methods to optimize yields for clinical cell therapy. Biol Blood Marrow Transplant . (2017) 23:1241–9. doi: 10.1016/j.bbmt.2017.05.003
200. Ogonek J, Kralj Juric M, Ghimire S, Varanasi PR, Holler E, Greinix H, et al. Immune reconstitution after allogeneic hematopoietic stem cell transplantation. Front Immunol . (2016) 7:507. doi: 10.3389/fimmu.2016.00507
201. Barber-Axthelm IM, Barber-Axthelm V, Sze KY, Zhen A, Suryawanshi GW, Chen IS, et al. Stem cell-derived CAR T cells traffic to HIV reservoirs in macaques. JCI Insight . (2021) 6(1):e141502. doi: 10.1172/jci.insight.141502
202. Zhen A, Kamata M, Rezek V, Rick J, Levin B, Kasparian S, et al. HIV-specific immunity derived from chimeric antigen receptor-engineered stem cells. Mol Ther . (2015) 23:1358–67. doi: 10.1038/mt.2015.102
203. De Oliveira SN, Ryan C, Giannoni F, Hardee CL, Tremcinska I, Katebian B, et al. Modification of hematopoietic stem/progenitor cells with CD19-specific chimeric antigen receptors as a novel approach for cancer immunotherapy. Hum Gene Ther . (2013) 24:824–39. doi: 10.1089/hum.2012.202
204. Larson SM, Truscott LC, Chiou TT, Patel A, Kao R, Tu A, et al. Pre-clinical development of gene modification of haematopoietic stem cells with chimeric antigen receptors for cancer immunotherapy. Hum Vaccin Immunother . (2017) 13:1094–104. doi: 10.1080/21645515.2016.1268745
205. Kao RL, Truscott LC, Chiou TT, Tsai W, Wu AM, De Oliveira SN. A cetuximab-mediated suicide system in chimeric antigen receptor-modified hematopoietic stem cells for cancer therapy. Hum Gene Ther . (2019) 30:413–28. doi: 10.1089/hum.2018.180
206. Zah E, Lin MY, Silva-Benedict A, Jensen MC, Chen YY. T cells expressing CD19/CD20 bispecific chimeric antigen receptors prevent antigen escape by Malignant B cells. Cancer Immunol Res . (2016) 4:498–508. doi: 10.1158/2326-6066.CIR-15-0231
207. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science . (2020) 367(6478):eaau6977. doi: 10.1126/science.aau6977
208. Peters PJ, Geuze HJ, van der Donk HA, Slot JW, Griffith JM, Stam NJ, et al. Molecules relevant for T cell-target cell interaction are present in cytolytic granules of human T lymphocytes. Eur J Immunol . (1989) 19:1469–75. doi: 10.1002/eji.1830190819
209. Peters PJ, Geuze HJ, van der Donk HA, Borst J. A new model for lethal hit delivery by cytotoxic T lymphocytes. Immunol Today . (1990) 11:28–32. doi: 10.1016/0167-5699(90)90008-W
210. Peters PJ, Borst J, Oorschot V, Fukuda M, Krahenbuhl O, Tschopp J, et al. Cytotoxic T lymphocyte granules are secretory lysosomes, containing both perforin and granzymes. J Exp Med . (1991) 173:1099–109. doi: 10.1084/jem.173.5.1099
211. Fu W, Lei C, Liu S, Cui Y, Wang C, Qian K, et al. CAR exosomes derived from effector CAR-T cells have potent antitumour effects and low toxicity. Nat Commun . (2019) 10:4355. doi: 10.1038/s41467-019-12321-3
212. Lu J, Wu J, Tian J, Wang S. Role of T cell-derived exosomes in immunoregulation. Immunol Res . (2018) 66:313–22. doi: 10.1007/s12026-018-9000-0
213. Herrmann IK, Wood MJA, Fuhrmann G. Extracellular vesicles as a next-generation drug delivery platform. Nat Nanotechnol . (2021) 16:748–59. doi: 10.1038/s41565-021-00931-2
214. Yang P, Cao X, Cai H, Feng P, Chen X, Zhu Y, et al. The exosomes derived from CAR-T cell efficiently target mesothelin and reduce triple-negative breast cancer growth. Cell Immunol . (2021) 360:104262. doi: 10.1016/j.cellimm.2020.104262
215. Haque S, Vaiselbuh SR. CD19 chimeric antigen receptor-exosome targets CD19 positive B-lineage acute lymphocytic leukemia and induces cytotoxicity. Cancers (Basel) . (2021) 13(6):1401. doi: 10.3390/cancers13061401
216. Zhu T, Chen Z, Jiang G, Huang X. Sequential targeting hybrid nanovesicles composed of chimeric antigen receptor T-cell-derived exosomes and liposomes for enhanced cancer immunochemotherapy. ACS Nano . (2023) 17:16770–86. doi: 10.1021/acsnano.3c03456
217. Zheng W, Zhu T, Tang L, Li Z, Jiang G, Huang X. Inhalable CAR-T cell-derived exosomes as paclitaxel carriers for treating lung cancer. J Transl Med . (2023) 21:383. doi: 10.1186/s12967-023-04206-3
218. Tao B, Du R, Zhang X, Jia B, Gao Y, Zhao Y, et al. Engineering CAR-NK cell derived exosome disguised nano-bombs for enhanced HER2 positive breast cancer brain metastasis therapy. J Control Release . (2023) 363:692–706. doi: 10.1016/j.jconrel.2023.10.007
219. Hu Y, Zhou Y, Zhang M, Zhao H, Wei G, Ge W, et al. Genetically modified CD7-targeting allogeneic CAR-T cell therapy with enhanced efficacy for relapsed/refractory CD7-positive hematological Malignancies: a phase I clinical study. Cell Res . (2022) 32:995–1007. doi: 10.1038/s41422-022-00721-y
220. Xiang J, Devenport JM, Carter AJ, Staser KW, Kim MY, ON J, et al. An “off-the-shelf” CD2 universal CAR-T therapy for T-cell Malignancies. Leukemia . (2023) 37:2448–56. doi: 10.1038/s41375-023-02039-z
221. Ottaviano G, Georgiadis C, Gkazi SA, Syed F, Zhan H, Etuk A, et al. Phase 1 clinical trial of CRISPR-engineered CAR19 universal T cells for treatment of children with refractory B cell leukemia. Sci Transl Med . (2022) 14:eabq3010. doi: 10.1126/scitranslmed.abq3010
222. Wong XFA, Ng J, Zheng S, Ismail R, Qian H, Campana D, et al. Development of an off-the-shelf chimeric antigen receptor (CAR)-T cell therapy for T-cell acute lymphoblastic leukemia (T-ALL) without gene editing. Blood . (2022) 140:2358–9. doi: 10.1182/blood-2022-165822
223. Chiesa R, Georgiadis C, Syed F, Zhan H, Etuk A, Gkazi SA, et al. Base-edited CAR7 T cells for relapsed T-cell acute lymphoblastic leukemia. N Engl J Med . (2023) 389:899–910. doi: 10.1056/NEJMoa2300709
224. Wehbi VL, Tasken K. Molecular Mechanisms for cAMP-Mediated Immunoregulation in T cells - Role of Anchored Protein Kinase A Signaling Units. Front Immunol . (2016) 7:222. doi: 10.3389/fimmu.2016.00222
225. Srour S, Kotecha R, Curti B, Chahoud J, Drakaki A, Tang L, et al. Abstract CT011: A phase 1 multicenter study (TRAVERSE) evaluating the safety and efficacy of ALLO-316 following conditioning regimen in pts with advanced or metastatic clear cell renal cell carcinoma (ccRCC). Cancer Res . (2023) 83:CT011–CT. doi: 10.1158/1538-7445.AM2023-CT011
226. Mailankody S, Matous JV, Chhabra S, Liedtke M, Sidana S, Oluwole OO, et al. Allogeneic BCMA-targeting CAR T cells in relapsed/refractory multiple myeloma: phase 1 UNIVERSAL trial interim results. Nat Med . (2023) 29:422–9. doi: 10.1038/s41591-022-02182-7
227. Chehelgerdi M, Chehelgerdi M, Khorramian-Ghahfarokhi M, Shafieizadeh M, Mahmoudi E, Eskandari F, et al. Comprehensive review of CRISPR-based gene editing: mechanisms, challenges, and applications in cancer therapy. Mol Cancer . (2024) 23:9. doi: 10.1186/s12943-023-01925-5
228. Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med . (2011) 365:725–33. doi: 10.1056/NEJMoa1103849
229. Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med . (2011) 3:95ra73. doi: 10.1126/scitranslmed.3002842
230. Muraille E, Leo O, Moser M. TH1/TH2 paradigm extended: macrophage polarization as an unappreciated pathogen-driven escape mechanism? Front Immunol . (2014) 5:603. doi: 10.3389/fimmu.2014.00603
231. Ligtenberg MA, Mougiakakos D, Mukhopadhyay M, Witt K, Lladser A, Chmielewski M, et al. Coexpressed catalase protects chimeric antigen receptor-redirected T cells as well as bystander cells from oxidative stress-induced loss of antitumor activity. J Immunol . (2016) 196:759–66. doi: 10.4049/jimmunol.1401710
232. Atkins RM, Menges MA, Bauer A, Turner JG, Locke FL. Metabolically flexible CAR T cells (mfCAR-T), with constitutive expression of PGC-1α Resistant to post translational modifications, exhibit superior survival and function in vitro. Blood . (2020) 136:30. doi: 10.1182/blood-2020-143217
233. Williams JZ, Allen GM, Shah D, Sterin IS, Kim KH, Garcia VP, et al. Precise T cell recognition programs designed by transcriptionally linking multiple receptors. Science . (2020) 370:1099–104. doi: 10.1126/science.abc6270
Keywords: chimeric antigen receptors, cellular immunotherapy, cancer treatment, tumor microenvironment, toxicity, off-the-shelf products
Citation: Huang J, Yang Q, Wang W and Huang J (2024) CAR products from novel sources: a new avenue for the breakthrough in cancer immunotherapy. Front. Immunol. 15:1378739. doi: 10.3389/fimmu.2024.1378739
Received: 30 January 2024; Accepted: 27 March 2024; Published: 11 April 2024.
Reviewed by:
Copyright © 2024 Huang, Yang, Wang and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juan Huang, [email protected] ; Wen Wang, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Immunotherapy to Treat Cancer
Immunotherapy is a type of cancer treatment that helps your immune system fight cancer. The immune system helps your body fight infections and other diseases. It is made up of white blood cells and organs and tissues of the lymph system .
Immunotherapy is a type of biological therapy . Biological therapy is a type of treatment that uses substances made from living organisms to treat cancer.
How does immunotherapy work against cancer?
As part of its normal function, the immune system detects and destroys abnormal cells and most likely prevents or curbs the growth of many cancers. For instance, immune cells are sometimes found in and around tumors. These cells, called tumor-infiltrating lymphocytes or TILs, are a sign that the immune system is responding to the tumor. People whose tumors contain TILs often do better than people whose tumors don’t contain them.
Even though the immune system can prevent or slow cancer growth, cancer cells have ways to avoid destruction by the immune system. For example, cancer cells may:
- Have genetic changes that make them less visible to the immune system.
- Have proteins on their surface that turn off immune cells.
- Change the normal cells around the tumor so they interfere with how the immune system responds to the cancer cells.
Immunotherapy helps the immune system to better act against cancer.
What are the types of immunotherapy?
Get Answers >
Wondering if immunotherapy is an option for you? Connect with a Cancer Information Specialist.
Several types of immunotherapy are used to treat cancer. These include:
Learn more about immune checkpoint inhibitors .
T-cell transfer therapy may also be called adoptive cell therapy, adoptive immunotherapy, or immune cell therapy.
Learn more about T-cell transfer therapy .
Monoclonal antibodies may also be called therapeutic antibodies.
Learn more about monoclonal antibodies .
Learn more about cancer treatment vaccines .
Learn more about immune system modulators .
Which cancers are treated with immunotherapy?
Immunotherapy drugs have been approved to treat many types of cancer. However, immunotherapy is not yet as widely used as surgery , chemotherapy , or radiation therapy . To learn about whether immunotherapy may be used to treat your cancer, see the PDQ ® adult cancer treatment summaries and childhood cancer treatment summaries .
What are the side effects of immunotherapy?
Immunotherapy can cause side effects , many of which happen when the immune system that has been revved-up to act against the cancer also acts against healthy cells and tissues in your body.
Learn more about immunotherapy side effects .
How is immunotherapy given?
Different forms of immunotherapy may be given in different ways. These include:
- intravenous (IV) The immunotherapy goes directly into a vein .
- oral The immunotherapy comes in pills or capsules that you swallow.
- topical The immunotherapy comes in a cream that you rub onto your skin. This type of immunotherapy can be used for very early skin cancer.
- intravesical The immunotherapy goes directly into the bladder.
Where do you go for immunotherapy?
You may receive immunotherapy in a doctor’s office, clinic, or outpatient unit in a hospital. Outpatient means you do not spend the night in the hospital.
How often do you receive immunotherapy?
How often and how long you receive immunotherapy depends on:
- your type of cancer and how advanced it is
- the type of immunotherapy you get
- how your body reacts to treatment
You may have treatment every day, week, or month. Some types of immunotherapy given in cycles. A cycle is a period of treatment followed by a period of rest. The rest period gives your body a chance to recover, respond to immunotherapy, and build new healthy cells.
How can you tell if immunotherapy is working?
You will see your doctor often. He or she will give you physical exams and ask you how you feel. You will have medical tests, such as blood tests and different types of scans . These tests will measure the size of your tumor and look for changes in your blood work.
What is the current research in immunotherapy?
Researchers are focusing on several major areas to improve immunotherapy, including:
- Finding solutions for resistance. Researchers are testing combinations of immune checkpoint inhibitors and other types of immunotherapy, targeted therapy, and radiation therapy to overcome resistance to immunotherapy.
- Finding ways to predict responses to immunotherapy. Only a small portion of people who receive immunotherapy will respond to the treatment. Finding ways to predict which people will respond to treatment is a major area of research.
- Learning more about how cancer cells evade or suppress immune responses against them. A better understanding of how cancer cells get around the immune system could lead to the development of new drugs that block those processes.
- How to reduce the side effects of treatment with immunotherapy.
How do you find clinical trials that are testing immunotherapy?
To find clinical research studies that involve immunotherapy visit Find NCI-Supported Clinical Trials or call the Cancer Information Service, NCI’s contact center, at 1-800-4-CANCER (1-800-422-6237).
NCI’s list of cancer clinical trials includes all NCI-supported clinical trials that are taking place across the United States and Canada, including the NIH Clinical Center in Bethesda, MD.
Recent progress in cancer immunotherapy: Overview of current status and challenges
Affiliations.
- 1 Infectious Diseases and Tropical Medicine Research Center (IDTMRC), Department of Aerospace and Subaquatic Medicine, AJA University of Medicinal Sciences, Tehran, Iran. Electronic address: [email protected].
- 2 Student Research Committee, Semnan University of Medical Sciences, Semnan, Iran. Electronic address: [email protected].
- 3 School of Medicine, Tehran University of Medical Sciences, Tehran, Iran. Electronic address: [email protected].
- 4 Department of Infectious Disease, School of Medicine, Babol University of Medical Sciences, Babol, Iran. Electronic address: [email protected].
- 5 Department of Medicine, Kerman University of Medical Sciences, Kerman, Iran. Electronic address: [email protected].
- 6 Department of Digital Health, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran. Electronic address: [email protected].
- 7 Department of Biology and Anatomical Sciences, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran. Electronic address: [email protected].
- 8 Department of Pharmacology, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran. Electronic address: [email protected].
- 9 Department of Surgery, Faculty of Medicine, Kerman University of Medical Sciences, Kerman, Iran. Electronic address: [email protected].
- 10 Faculty of Medicine, Iran University of Medical Sciences (IUMS), Tehran, Iran. Electronic address: [email protected].
- PMID: 36543080
- DOI: 10.1016/j.prp.2022.154241
Cancer treatment is presently one of the most important challenges in medical science. Surgery, chemotherapy, radiotherapy, or combining these methods is used to eliminate the tumor. Hormone therapy, bone marrow transplantation, stem cell therapy as well as immunotherapy are other well-known therapeutic modalities. Immunotherapy, as the most important complementary method, uses the immune system for treating cancer followed by surgery, chemotherapy, and radiotherapy. This method is systematically used to prevent malignancies development mainly via potentiating antitumor immune cells activation and conversely compromising their exhaustion with the lowest negative effects on healthy cells. Active immunotherapy can be employed for cancer immunotherapy by directly using the ingredients of the immune system and activating immune responses. On the other hand, inactive immunotherapy is utilized by indirect induction and using immune cell-based products consisting of monoclonal antibodies. It has strongly been proved that combination therapy with immunotherapies and other therapeutic means, such as anti-angiogenic agents, could be a rational plan to treat cancer. Herein, we have focused on recent findings concerning the therapeutic merits of cancer therapy using immune checkpoint inhibitors (ICIs), adoptive cell transfer (ACT) and cancer vaccine alone or in combination with other approaches. Also, we offer a glimpse into the current challenges in this context.
Keywords: Cancer immunotherapy; Chimeric antigen receptor (CAR); Cytotoxic T-Lymphocyte; Myeloid-derived suppressor cells; Tumor-infiltrating lymphocytes.
Copyright © 2022. Published by Elsevier GmbH.
Publication types
- Angiogenesis Inhibitors
- Antibodies, Monoclonal / therapeutic use
- Immunotherapy*
- Immunotherapy, Adoptive
- Neoplasms* / drug therapy
- Antibodies, Monoclonal
Research could unlock more precise prognoses and targeted treatments for children with cancer
Neuroblastoma study identifies new subgroups with distinct prognoses and potential vulnerabilities to therapies.
Researchers have identified new variations in neuroblastoma that could lead to a more accurate prognosis and better-targeted treatments for this devastating childhood cancer.
A study published in the British Journal of Cancer reveals three new subgroups of the most common type of neuroblastoma, each with different genetic traits, expected outcomes, and distinguishing features that offer clues as to which treatments may be most effective.
Dr Yihua Wang from the University of Southampton, a senior author on the paper said: "This research represents a pivotal advancement in our understanding of MYCN non-amplified neuroblastomas. The results are striking. These kinds of neuroblastomas can be classified into three distinct subgroups, each demonstrating unique prognostic implications and varying vulnerabilities to investigational therapies."
Around 100 children are diagnosed with neuroblastoma each year in the UK, representing six to ten per cent of all childhood cancers. Neuroblastoma is a cancer that starts in a type of nerve cell called a neuroblast. It can present in the abdomen, chest neck or pelvis and can spread to other parts of the body.
The overall prognosis of the disease is poor, with just 20 per cent of patients still alive at 5 years after diagnosis, but the likelihood of the cancer being cured varies widely, with some tumours spontaneously regressing and others proving resistant to therapy and progressing.
One of the key indicators of risk is the amplification of a gene called MYCN , where tumours have too many of this type of gene. This occurs in around 20 per cent of cases and accounts for about 40 per cent of high-risk neuroblastomas.
Researchers from the University of Southampton and China wanted to find out more about cases where the MYCN gene isn't amplified to better understand the diversity of outcomes within these cases. Using advanced analytical techniques, the research team analysed over 1,500 biopsy samples from 16 different datasets sourced from Gene Expression Omnibus (GEO) and ArrayExpress.
The team were able to identify three distinct subtypes of these MYCN non-amplified cases based on their transcriptional signatures -- patterns of gene expression that can provide valuable insights into biological processes.
The first subgroup makes up around half of MYCN non-amplified cases and has the best prognosis, with a long-term survival rate of over 85 per cent, despite some cases being clinically classified as high risk.
Subgroup 2, representing a quarter of MYCN non-amplified cases, had the worst outcomes with a long-term survival rate of 50%. Interestingly, this group had a similar genetic signature to cases where MYCN is amplified. Researchers found a protein called Aurora Kinase A (AURKA) was expressed at significantly higher levels than in the other two subgroups. On further analysis, they found that AURKA mRNA levels alone could predict overall survival. This suggests that patients within the subgroup may benefit from treatment with AURKA inhibitors.
Meanwhile, Subgroup 3, which made up another quarter of MYCN non-amplified cases, is characterised by an 'inflamed' gene signature, with significantly higher levels of activity in immune cells. Further analysis indicates that patients in this subgroup were predicted to respond better to immunotherapy.
Dr Wang added: "This research opens new avenues for personalised medicine in the treatment of neuroblastomas. By leveraging transcriptional subtyping, we are now equipped to offer more precise prognosis and tailor therapies accordingly for patients with MYCN non-amplified neuroblastomas, potentially improving outcomes and quality of life."
The project was supported by the UK Medical Research Council and the Natural Science Foundation of China.
- Personalized Medicine
- Breast Cancer
- Diseases and Conditions
- Multiple Sclerosis
- Alzheimer's
- Huntington's Disease
- Stem cell treatments
- Breast cancer
- Personalized medicine
- Alzheimer's disease
- Sleep disorder
- Renal cell carcinoma
Story Source:
Materials provided by University of Southampton . Note: Content may be edited for style and length.
Journal Reference :
- Xiaoxiao Hu, Yilu Zhou, Charlotte Hill, Kai Chen, Cheng Cheng, Xiaowei Liu, Peiwen Duan, Yaoyao Gu, Yeming Wu, Rob M. Ewing, Zhongrong Li, Zhixiang Wu, Yihua Wang. Identification of MYCN non-amplified neuroblastoma subgroups points towards molecular signatures for precision prognosis and therapy stratification . British Journal of Cancer , 2024; DOI: 10.1038/s41416-024-02666-y
Cite This Page :
Explore More
- Ozone Removes Mating Barriers Between Fly ...
- Parkinson's: New Theory On Origins and Spread
- Clash of Stars Solves Stellar Mystery
- Secure Quantum Computing at Home
- Ocean Currents: Collapse of Antarctic Ice ...
- Pacific Cities Much Older Than Previously ...
- The Milky Way in Ancient Egyptian Mythology
- Physical Activity Best in the Evening
- How the Body Switches out of 'Fight' Mode
- New Drug Prevents Flu-Related Lung Damage
Trending Topics
Strange & offbeat.
Immunotherapy Before Surgery Might Boost Pancreatic Cancer Survival
By Carole Tanzer Miller HealthDay Reporter

MONDAY, April 8, 2024 (HealthDay News) -- Pancreatic cancer patients may do better if they receive an immunotherapy drug as well as chemotherapy in preparation for surgery, new research suggests.
Pancreatic cancer is one of the toughest to treat. Only 12% of patients live more than five years after diagnosis. Most therapies -- including chemo, targeted therapies and immunotherapies -- are unsuccessful.
For this study, researchers at UCLA Health Jonsson Comprehensive Cancer Center found three benefits from the pre-surgery regimen: a higher rate of successful tumor removal; a longer time before the cancer worsened; and longer survival compared to a comparison group that previously received traditional treatment.
"By treating patients before surgery, not only were we able to see whether the drug combination worked but by collecting surgical resection tissues, we went back to the lab to study why this combination does not always work," said senior study author Dr. Timothy Donohue , chief of surgical oncology at UCLA's David Geffen School of Medicine.
U.S. Cities With the Most Homelessness

First study author Dr. Zev Wainberg , co-director of the UCLA Health GI Oncology Program, noted that this is among the first trials reported using a PD1-inhibitor drug before pancreatic cancer surgery.
Researchers didn't say which PD1-inhibitor was used, but this class of drugs includes Keytruda and Opdivo . They work with the patient's immune system to thwart the growth and spread of cancer cells.
The new study included 28 pancreatic cancer patients with what doctors call borderline resectable tumors. That means their tumor may have grown into neighboring tissues or organs. It may be removable, but some cancer may remain.
For the study, 26 patients completed at least three cycles of the chemo/immunotherapy. All but two then had surgery.
Researchers compared results of genetic sequencing on 21 surgically removed tumors, six pre-treatment biopsies and nine tumors removed from patients not in the trial treated with chemotherapy alone.
At a median follow-up of 24 months, the median progression-free survival was 34.8 months, and the median overall survival was 35.1 months.
For patients whose pancreas was removed, the 18-month survival rate was 90%. Two patients had no signs of cancer in tissue samples removed after treatment and two had near complete responses to treatment.
Researchers said the findings point to the possibility of more effective treatment options for pancreatic cancer patients. The phase 2 trial is ongoing.
"We've identified some leads that will be the basis for subsequent studies, again in the preoperative setting," Donohue said in a UCLA news release. "Through these efforts, we are working to redefine the standard of care for pancreatic cancer."
Wainberg presented the findings Monday at the American Association for Cancer Research annual meeting in San Diego. Research presented at meetings is considered preliminary until published in a peer-reviewed journal.
More information
The American Cancer Society has more about immunotherapy .
SOURCE: UCLA Health Sciences, news release, April 5, 2024
Copyright © 2024 HealthDay . All rights reserved.
Join the Conversation
Tags: cancer , pancreatic cancer
America 2024

Health News Bulletin
Stay informed on the latest news on health and COVID-19 from the editors at U.S. News & World Report.
Sign in to manage your newsletters »
Sign up to receive the latest updates from U.S News & World Report and our trusted partners and sponsors. By clicking submit, you are agreeing to our Terms and Conditions & Privacy Policy .
You May Also Like
The 10 worst presidents.
U.S. News Staff Feb. 23, 2024

Cartoons on President Donald Trump
Feb. 1, 2017, at 1:24 p.m.

Photos: Obama Behind the Scenes
April 8, 2022

Photos: Who Supports Joe Biden?
March 11, 2020

Johnson Turns to Trump Amid GOP Dissent
Aneeta Mathur-Ashton April 11, 2024

A Reprieve for Inflation?
Tim Smart April 11, 2024

Embattled Johnson Bruised Again
Aneeta Mathur-Ashton April 10, 2024

What to Know About Biden's State Dinner
Laura Mannweiler April 10, 2024

Fed: Inflation Path ‘Somewhat Uneven’
Tim Smart April 10, 2024

No Letup From Inflation in March

Geneos cancer vaccine shrinks liver tumors in small trial
- Medium Text
- Company Geneos Therapeutics, Inc. Follow
- Company Merck & Co Inc Follow
- Company Moderna Inc Follow
Keep up with the latest medical breakthroughs and healthcare trends with the Reuters Health Rounds newsletter. Sign up here.
Reporting by Julie Steenhuysen Editing by Bill Berkrot
Our Standards: The Thomson Reuters Trust Principles. New Tab , opens new tab

Business Chevron

JPMorgan's profit rises on interest income strength
JPMorgan Chase's profit rose in the first quarter as it earned more from interest payments, the bank said on Friday.

What Is Immunotherapy for Cancer?

In recent years, the landscape of cancer treatments has witnessed a groundbreaking shift with the introduction of immunotherapy. This cutting-edge approach harnesses the body's own immune system to combat and conquer cancer cells. Let's dive into the details to learn more about this transformative technique and explore its impact on the fight against brain cancer .
What Is Immunotherapy?
Immunotherapy, often hailed as the "fifth pillar" of cancer treatment, stands out for its unique ability to empower the immune system. Unlike traditional treatments, which directly target cancer cells, immunotherapy stimulates the body's natural defenses, enhancing its capacity to identify and eliminate malignant cells.
How Does Immunotherapy Work?
Immunotherapy operates on a captivating principle, the immune system's inherent ability to recognize foreign entities, including cancer cells, is harnessed to enhance its proficiency in identifying and eliminating abnormal cells.
This involves utilizing substances like cytokines, antibodies, and immune checkpoint inhibitors. By unveiling cancer cells, the immune system becomes adept at identifying and eliminating them.
Within and around tumors, immune cells, specifically referred to as tumor-infiltrating lymphocytes (TILs), can be found. The presence of TILs serves as an indicator that the immune system is actively responding to the tumor.
Despite the immune system's ability to impede cancer progression, cancer cells employ various tactics to avoid its destructive effects. These strategies encompass genetic alterations that make them less detectable by the immune system, surface proteins that deactivate immune cells, and modifications to normal cells surrounding the tumor, disrupting the immune system's response to cancer cells. Immunotherapy plays a pivotal role in augmenting the immune system's capacity to effectively combat cancer.
In essence, immunotherapy harnesses the power of our immune system to target and combat cancer cells. By assisting the immune system in recognizing and attacking cancer cells, it plays a crucial role in the fight against cancer.
How successful Is immunotherapy for brain cancer?
Since immunotherapy is a fairly new treatment that is being tested for cancers such as brain cancer, there is not enough research to show how effective it is on the brain. Make sure to speak with one of our doctors to see if immunotherapy could be an option for you.
Types of Immunotherapy
There are many types of immunotherapy treatments being tested to help cure brain cancer. Here are some of the most popular treatments being researched:
Monoclonal Antibodies
Monoclonal Antibodies, often abbreviated as MABs, function by stimulating the immune system, aiding it in the fight against cancer. Some MABs operate with a specific focus, such as inhibiting signals that encourage cancer cells to divide. Antibodies, naturally occurring in our bloodstream, play a vital role in combating infections.
MAB therapies replicate these natural antibodies but are synthetically produced in a laboratory setting. The term "monoclonal" indicates that each MAB consists of numerous copies of a single type of antibody.
Immune Checkpoint Inhibitors
Immune Checkpoint Inhibitors operate through varied mechanisms, with some exhibiting multiple modes of action. As a subtype of immunotherapy, these inhibitors serve as a treatment for cancers such as melanoma skin cancer and lung cancer. Specifically, these drugs interfere with various checkpoint proteins, and they are often named after these specific proteins, such as CTLA-4 inhibitors, PD-1 inhibitors, and PD-L1 inhibitors.
These inhibitors play a crucial role in blocking proteins that would otherwise impede the immune system from effectively attacking cancer cells. It's worth noting that cancer drugs, including checkpoint inhibitors, don't always neatly fit into a single treatment category. Due to their diverse modes of action, some drugs belong to more than one group. In the case of checkpoint inhibitors, they are also classified as a type of monoclonal antibody or targeted treatment, showcasing the multifaceted approach these innovative therapies employ in the battle against cancer.
Cancer Vaccines
Preventative vaccines are designed to shield against the development of cancer. The concept underlying treatment vaccines is centered around the recognition of substances known as tumor-associated antigens within cancer cells. These antigens either don't exist in normal cells or, if present, are found at lower levels.
Treatment vaccines function by assisting the immune system in learning how to identify and respond to these specific antigens. By doing so, the immune system becomes better equipped to recognize and eliminate cancer cells that harbor these distinctive antigens.
Oncolytic Virus Therapy
Oncolytic Virus Therapy employs a modified virus with the capability to infect and eradicate tumor cells. Viruses, as microscopic particles, infiltrate or enter our cells, utilizing the cell's genetic machinery to replicate and then propagate to neighboring uninfected cells. T cells, a subset of white blood cells, play a crucial role in the immune system and originate from stem cells in the bone marrow.
In this therapeutic approach, researchers have engineered a cancer-infecting virus to obstruct the immune-suppressing protein TGF-beta and regulatory T cells (Tregs). This modification enhances the ability of T cells to mount an attack against the tumor, showcasing the innovative strides being made in leveraging viruses to target and combat cancer cells.
T-Cell Therapy
T-Cell Therapy, specifically CAR T-cell therapy, stands as a distinctive form of immunotherapy, also referred to as adoptive cell transfer. This intricate and specialized treatment involves a meticulous process wherein a specialist collects and modifies a small number of your T cells. Following this modification, a drip containing these altered T cells is reintroduced into your bloodstream after a few weeks. The modified CAR T-cells then possess the capability to recognize and mount an attack against cancer cells.
What Are The Side Effects of Immunotherapy?
The side effects can vary depending on many factors such as how healthy you are before beginning treatment, the dosage you are on, what type of immunotherapy you are on, how advanced your cancer is, and more.
However, here are some side effects that have been seen from immunotherapy:
- Nausea
- Joint Aches
- Low or High Blood Pressure
- Trouble Breathing
How Is Immunotherapy Given?
This can vary depending on the type of immunotherapy you are being given. Immunotherapy can be given intravenously (through a vein), subcutaneously (under the skin through an injection), or intramuscularly (into a muscle). At times, immunotherapy can even be given directly where the tumor is located.
How Long Does Immunotherapy Take To Work?
Since this is a new treatment, there is still debate over how long this medication can take to work. Many patients have been on immunotherapy for around two years. In general, it takes weeks or even months for immunotherapy treatments like checkpoint inhibitors to begin working. It depends on how the patient's immune system and the type of cancer they have respond to the treatment.
Doctors can track if the treatment is working by monitoring if there is any shrinking in tumor size or if the tumor is staying the same size. This, along with other factors can give doctors an idea of how fast or slow certain treatments take to work. There may be other signs that these treatments are affecting the immune system in other ways; however, since this is new in the field, there is still a lot of research needed to draw any firm conclusions.
Best Brain Tumor Treatment Center
Take the first step towards comprehensive care and a brighter future. Choose the Preston Robert Tisch Brain Tumor Center for personalized and advanced brain cancer treatment. Your journey to healing begins here.
Schedule a consultation with our expert team today or pay our offices in Durham, NC, a visit to book an appointment. Your well-being is our priority, and we look forward to helping you along your journey.
Related Readings:
- Radiation Therapy vs Chemotherapy
- "Brain Tumor Immunotherapy" Featuring Peter Fecci, MD, PhD
- Best Clinic for Brain Tumor Treatment
Commonly Asked Questions
At what stage of cancer is immunotherapy used?
Immunotherapy is typically recommended for patients with advanced cancer.
Is immunotherapy a last resort for cancer?
Immunotherapy is still a new treatment option in the medical field. It is typically used when other treatment options have failed to work.
How sick does immunotherapy make you?
This can vary depending on the patient’s unique circumstances; however, common side effects are chills, nausea, fatigue, headaches, fever, and more.
What type of cancer can be treated with immunotherapy?
While this is still being studied, research has shown that immunotherapy can be used to treat various forms of cancer such as brain cancer, breast cancer, bladder cancer, cervical cancer, kidney cancer, head and neck cancer, and more.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

A review of cancer immunotherapy: from the past, to the present, to the future
K. esfahani.
* Departments of Medicine and Oncology, Segal Cancer Centre, Sir Mortimer B. Davis Jewish General Hospital, Rossy Cancer Network, McGill University, Montreal, QC
N. Buhlaiga
S.v. del rincon.
† Department of Oncology, Lady Davis Institute, Sir Mortimer B. Davis Jewish General Hospital, McGill University, Montreal, QC
W.H. Miller, Jr
Compared with previous standards of care (including chemotherapy, radiotherapy, and surgery), cancer immunotherapy has brought significant improvements for patients in terms of survival and quality of life. Immunotherapy has now firmly established itself as a novel pillar of cancer care, from the metastatic stage to the adjuvant and neoadjuvant settings in numerous cancer types. In this review article, we highlight how the history of cancer immunotherapy paved the way for discoveries that are now part of the standard of care. We also highlight the current pitfalls and limitations of cancer checkpoint immunotherapy and how novel research in the fields of personalized cancer vaccines, autoimmunity, the microbiome, the tumour microenvironment, and metabolomics is aiming to solve those challenges.
INTRODUCTION
The field of immuno-oncology has been transformational in the care of cancer patients. William B. Coley, now widely accepted as the father of immunotherapy, first attempted to harness the power of the immune system for treating cancer in the late 19th century. As an orthopedic surgeon who operated on patients with bone sarcomas, he noticed that some patients with significant postoperative wound infections—a common occurrence when aseptic technique had not yet been optimized—would undergo spontaneous regression of their unresected tumours. Beginning in 1891, Coley injected more than a thousand patients with mixtures of live and inactivated bacteria such as Streptococcus pyogenes and Serratia marcescens with the hope of inducing sepsis and strong immune and antitumour responses. His cocktail of bacteria became widely known as “Coley’s toxin” and represents the first documented active cancer immunotherapy intervention 1 . Coley achieved durable complete remissions in several types of malignancies, including sarcoma, lymphoma, and testicular carcinoma. However, the lack of a known mechanism of action for Coley’s toxin and the risks of deliberately infecting cancer patients with pathogenic bacteria caused oncologists to adopt surgery and radiotherapy as alternative standard treatments early in the 20th century 2 .
It would take more than half a century before a better understanding of the key mediators of sepsis would shed some light on the mechanisms of action of Coley’s toxin. Those mediators constitute a cytokine family including interleukins, interferons, and chemokines 3 . Once again, the race was on to apply those novel discoveries to cancer therapy 4 . Physicians and researchers achieved modest success with this novel approach, occasionally inducing clinical remissions with high-dose interleukin 2 ( il -2) in metastatic renal cell carcinoma 5 and debatable responses with interferon in stages iii and iv melanoma 6 . Those modest successes were often counterbalanced with significant adverse events. Although novel methods of delivery such as pegylation would abate some of the toxicities, the sporadic and unpredictable immune responses seen with those therapies meant that only a small, carefully selected subgroup of cancer patients would benefit.
The next revolutionary wave in cancer immunotherapy came with the better understanding of the process of immune surveillance, by which innate immune cells eliminate cancer cells. The recent discovery of T cell immune checkpoints, such as ctla -4 and PD-1, propelled the field of immuno-oncology into its current era and saw the awarding of the 2018 Nobel prize in Physiology or Medicine to Drs. Allison and Honjo. Those hardwired signals have the crucial task of maintaining a fine balance between immune surveillance against foreign pathogens or abnormal cells and autoimmunity. Blocking those T cell surface receptors results in enhanced autoimmunity that induces an immune response against tumours, but can also increase the chance of autoimmune reactions.
In this review article, we highlight the current standards of care in cancer immunotherapy, with a strong focus on immune checkpoint inhibitors ( ici s), their limitations and pitfalls, and promising novel approaches.
Overview of Checkpoint Inhibitors
Cancer immuno-editing is the process by which various immune system components protect the host against primary tumour development or enhance tumour escape, or both, either by sculpting tumour immunogenicity or attenuating antitumour immune responses 7 . The process is tightly regulated by immune checkpoints, which are immune-cell surface receptors controlling either the activation or the inhibition of immune responses. Activation of the immune system is, on the one hand, the desired outcome to achieve tumour control, but on the other hand, responsible for autoimmunity. The discovery and development of monoclonal antibodies against the inhibitory immune checkpoints ctla -4 and PD-1 have resulted in dramatic antitumour responses by the up-regulation of immune activation at various stages of the immune cycle.
Immune checkpoint inhibitor therapies are now widely indicated in numerous cancer types ( Table I ). Furthermore, numerous ongoing clinical trials are assessing the potential of other agonistic or inhibitory checkpoints to affect tumour-related outcomes ( Table II ). The checkpoints are not equal in their potential. For example, the agonistic OX40 antibody has modest clinical activity, but the CD28 antibody—even at very subtherapeutic doses—resulted in massive cytokine syndrome and the intensive-care hospitalization of the first 6 healthy volunteers treated 8 . In that light, finding the right combination of ici therapy to induce the optimal amount of immune activation remains an active area of clinical research.
Indications for immune checkpoint inhibitors in advanced-stage cancers, as currently approved by Health Canada a
NSCLC = non-small-cell lung cancer; RCC = renal cell carcinoma (clear cell); SCCHN = squamous-cell carcinoma of head and neck; Tx = treatment; ASCT = autologous stem-cell transplantation; CTxRT = chemoradiotherapy.
Agonistic and antagonistic immune checkpoint modulators currently under investigation

Poly-ICLC = polyinosinic-polycytidylic acid–poly–L-lysine carboxymethylcellulose.
Modulating and Predicting Immune Toxicity for Better Efficacy
Immunotherapies are often limited by their immune-related adverse events (ir ae s), an immune activation and inflammatory response against the host’s healthy tissues. Immune activation against the host’s tumour is the desired outcome, but ir ae s are challenging to predict, diagnose, and treat. In the setting of metastatic melanoma, the addition of a ctla -4 antibody to PD-1 blockade is associated with only an incremental increase in survival, but at the cost of more than double the rate of serious ir ae s 9 . A recent meta-analysis reported a fatality rate of up to 1 patient in every 77 treated using an ici combination 10 . For specific ir ae s, such as immune-related myocarditis, the mortality rate is as high as 50% in treated patients 11 . Numerous predictors of ir ae s have been proposed (baseline lymphopenia and eosinophilia, B cell changes, T cell repertoire, circulating il -17, and gut microbiota changes 12 – 17 ), but few have been prospectively validated.
For serious ir ae s, guidelines recommend broad immunosuppression consisting of corticosteroids, followed by one or more biologics (tumour necrosis factor inhibitors) or T cell suppressants (such as mycophenolate mofetil) 18 – 20 . Very little prospective knowledge has been developed about the consequences of those therapies for cancer-related outcomes. An analysis of the baseline use of corticosteroids in patients with lung cancer reported an association with worse survival outcomes 21 . Similarly, the use of high-dose steroids in the setting of immune-related hypophysitis in patients with metastatic melanoma was also associated with worse survival 22 . On the other hand, the use of corticosteroids in other clinical settings in which patients experience ir ae s was not associated with a reduced response to ici therapy or with survival 23 . More studies are needed to assess the optimal immunosuppression regimen to be used with ici s to avoid impairing their efficacy. The use of m tor (mechanistic target of rapamycin) inhibitors shows promise to abate toxicities without impairing ici efficacy in the specific setting of organ transplantation 24 – 26 .
Modulating cytokines in the setting of an ici is a dualedged sword. Many of those soluble factors, such as tumour necrosis factor α and il -17, are called “pleiotropic cytokines” for the dual roles they play in immunity: on the one hand, they promote tumour surveillance; on the other hand, they can be key mediators of autoimmune reactions. As discussed earlier, circulating il -17 is a biomarker for the prediction of ici -induced colitis 16 . The addition of the il -17 monoclonal antibody secukinumab for the treatment of immune-related colitis and psoriasis, while effective at abating immune toxicities, has been reported to induce tumour escape 27 . The same effect has not yet been reported for tumour necrosis factor α or il -6. Tumour necrosis factor blockade seems not only to be safe for the treatment of ici -related colitis, but in animal models of melanoma, also adds synergistic antitumour efficacy to PD-1 inhibition 28 , 29 . Those early observations collectively highlight the importance of further study of the role of various cytokines and immune cells in the pathogenesis of ir ae s.
A New Era for Tumour-Specific Vaccines in Combination with ICIs
Despite promising results with ici s, single-agent PD-1 inhibitor has an objective response rate that varies from almost nonexistent in pancreatic cancer and microsatellite-stable colonic adenocarcinoma, to an average of 15%–30% in most other tumour types, but 50%–80% in melanoma, Hodgkin lymphoma, squamous-cell carcinoma of the skin, and Merkel cell carcinoma. The addition of an anti– ctla -4 agent increases the response rate, but comes with a significantly higher toxicity rate. A rational approach to achieving a higher response rate without increasing autoimmunity has been to combine an ici with a therapy that can sensitize the host’s immune system to the tumour in advance. Recent studies have shown that personalized neoantigen-based tumour-specific vaccines hold considerable promise.
Unlike hematologic malignancies, in which a common antigen is uniformly expressed on the surface of all malignant cells, making them amenable to targeted therapies such as therapy with chimeric antigen receptor T cells, solid tumours either lack such an antigen or undergo mutations under natural selection when exposed to therapeutic interventions such as monoclonal antibodies. Traditional cancer vaccines have failed for a number of potential reasons, including improper selection of a target antigen, lack of immunogenicity, or inadequate patient selection. In the new era of cancer vaccines, efficacy relies on computational pipelines geared to identify personal candidate neoantigens in real time. Comprehensive mutation analysis is performed by whole-exome sequencing, and based on affinity predictions, neo-epitopes encoded by somatic mutations in the tumour are selected given their probability of being presented by the individual’s major histocompatibility class molecules 30 – 34 . One of the most commonly used prediction algorithms for major histocompatibility class i binding, NetMHCpan (DTU Health Tech, Technical University of Denmark, Kongens Lyngby, Denmark), relies on state-of-the-art neural networks, putting the spotlight on the current power of bioinformatics for guiding precision immuno-oncology 35 . The concept has been translated to multiple phase i clinical trials evaluating neoantigen-based vaccines 36 – 38 . Other clinical trials are testing this novel vaccination strategy in combination with ipilimumab ( {"type":"clinical-trial","attrs":{"text":"NCT02950766","term_id":"NCT02950766"}} NCT02950766 at https://ClinicalTrials.gov/ ) or nivolumab ( {"type":"clinical-trial","attrs":{"text":"NCT02897765","term_id":"NCT02897765"}} NCT02897765 ), or as a personalized messenger rna mutatome vaccine in combination with the PD-L1 inhibitor atezolizumab ( {"type":"clinical-trial","attrs":{"text":"NCT03289962","term_id":"NCT03289962"}} NCT03289962 ).
The Crucial Role of the Tumour Microenvironment
An important advance in the field of immuno-oncology came from the increased understanding of the crucial role of the tumour microenvironment in the modulation of anticancer immune responses. In colorectal cancers, immune cell infiltration into the tumour microenvironment has been correlated with a strong immune response to treatment with ici s, with even better correlation than for microsatellite instability 39 , 40 . Based on those findings, the concept of “immune contexture” has been proposed and validated, with tumours classified into four proposed categories (hot, excluded, immunosuppressed, and cold) 41 , 42 . Apart from the presence of tumour-infiltrating lymphocytes, additional features such as the expression of anti–PD-L1 on tumour-associated immune cells, genomic instability, and the presence of a pre-existing antitumour immune response have been described as characteristics of “hot” tumours, which are associated with a good response to ici s 43 . Conversely, apart from being poorly infiltrated, “cold” tumours have also been described to be immunologically “ignorant” (scarcely expressing PD-L1) and characterized by high proliferation with a low mutational burden (low expression of neoantigens) and by low expression of antigen presentation machinery markers such as major histocompatibility class i 43 . Transforming “cold” tumours into fertile “hot” tumours responsive to ici s is an active area of investigation.
Radiotherapy and chemotherapy have both been used in combination with ici s to increase the antigenicity and priming potential of tumours, which in turn could be applied to turn “cold” tumours into “hot” ones. Ionizing radiation–induced immunogenic cell death and antigen release could potentially turn tumour cells into an in situ vaccine 44 . The outcome of that approach is not only local tumour control, but possibly a response at distant tumour sites through the abscopal effect 45 . On the other hand, chemotherapy can induce mutations, leading to the generation of neo-epitopes and therefore increasing the antigenicity of tumours 46 . Other approaches with proven benefit have been the local injection of oncolytic viruses into tumour beds. These native or genetically modified viruses selectively infect and replicate within tumour cells, eventually leading to tumour cell lysis and antigen release 47 . Again, that process results in local priming of the immune system, with responses seen both locally and systemically. The effect is accentuated when those therapies are combined with ici s 48 .
Another key targetable characteristic of “cold” tumours is strong expression of mesenchymal and collagen barrier molecules that prevent the migration of tumour-infiltrating lymphocytes to the tumour bed 49 , 50 . As an example, inhibition of transforming growth factor β, a key player in the formation of the mesenchymal barrier, when combined with an ici resulted in a strong antitumour response in mouse models 51 . That approach is now being tested in clinical trials.
Finally, another strategy that can convert a “cold” to a “hot” tumour microenvironment uses inhibitors of oncogenic kinases (reviewed in Guo et al. 52 ). The pi 3 k / akt pathway, glycogen synthase kinase 3α/β, and Mnk1 and Mnk2 are often aberrantly activated in cancer, and appreciation for their tumour-extrinsic effects in the cells of the tumour microenvironment to promote immune suppression is growing. For example, Mnk1 and Mnk2, which are critical regulators of messenger rna translation, have important immunomodulatory antitumor effects. Inhibitors of Mnk1 and Mnk2 can block the expression of secreted factors such as Nodal and Angptl4 53 , 54 , inhibiting the survival of neutrophils 55 and suppressing the expression of PD-L1 56 . The Mnk1 and Mnk2 inhibitors are actively being pursued in the clinic (see {"type":"clinical-trial","attrs":{"text":"NCT04261218","term_id":"NCT04261218"}} NCT04261218 , {"type":"clinical-trial","attrs":{"text":"NCT03616834","term_id":"NCT03616834"}} NCT03616834 , and {"type":"clinical-trial","attrs":{"text":"NCT03258398","term_id":"NCT03258398"}} NCT03258398 at https://ClinicalTrials.gov/ ).
Targeting Tumour Metabolism in the Tumour Microenvironment
There is growing evidence that the tumour microenvironment supports inappropriate metabolic reprogramming, negatively affecting T cell function and resulting in attenuated antitumour immune responses 57 , 58 . In that context, targeting both tumour and T cell metabolism can beneficially enhance immunity in an inhospitable microenvironment and markedly improve the success of immunotherapies. As discussed earlier, til s in the tumour microenvironment have significant prognostic and predictive significance. Their function is limited not only by immune checkpoints, but also by increasingly recognized “metabolic checkpoints” 59 .
Rapidly dividing tumour cells show complex and dynamic metabolic reprogramming and high glycolytic activity, a phenomenon called the “Warburg effect,” which is recognized as one of the hallmarks of carcinogenesis 60 . Thus, tumour cells impede the access of T cells to nutrients necessary for their activation and generate high levels of lactate. The resulting scarcity of nutrients and accumulation of metabolic waste products in the tumour microenvironment lead to a til metabolic switch that impairs optimal proliferation and function 61 .
Recent evidence suggests that ici s might directly sculpt the metabolic landscape in the tumour microenvironment, thus affecting the functioning of effector T cells. On the one hand, ctla -4 and PD-1 binding to their respective ligands impairs the metabolic til phenotype by inhibiting glycolysis 62 , thus causing reduced cytokine secretion and leading to an exhausted effector T cell phenotype 63 . On the other hand, ici s also have the opposite effect on metabolic reprogramming of cancer cells. Ligation of PD-L1 directly upregulates glycolysis in cancer cells by promoting glucose uptake and production of lactate, thus promoting tumour growth and metastasis 64 , 65 . Many therapeutic strategies have been proposed to tackle that imbalance.
The pi 3 k / akt /m tor pathway is well known to play a critical role in integrating the metabolism signals of cancer and immune cells. Recent preclinical evidence suggests that rapamycin, in combination with ici s, augments cytotoxic and memory T cell function 24 , 66 . Another promising therapeutic is metformin in combination with ici s. Metformin is known to target the mitochondrial respiratory complex i and to activate ampk pathway signal transduction, a key pathway in T cell regulatory and metabolic functioning 67 , 68 . In a cohort of patients with metastatic melanoma who received metformin in combination with ici s, favourable treatment-related outcomes (objective response rate, disease control rate, median progression-free survival, and median overall survival) were observed 69 . Those findings await further validation in larger randomized studies.
Besides glycolysis, another key element of metabolism dictating immune function in the tumour microenvironment is amino-acid catabolism. It is well established that l -arginine, tryptophan (Trp), and glutamine play fundamental roles in tumour progression and immunity 70 . Targeting those amino acids and their metabolic pathways in cancer therapy therefore becomes a promising strategy for the development of novel therapeutic agents. As one example, the depletion of tryptophan and the increase in kynurenine (Kyn) exert an important immunosuppressive function by activating T regulatory cells and suppressing the functioning of effector T cells 71 . The catabolic ido 1 enzyme in the Trp–Kyn–AhR metabolic pathway thus became an interesting therapeutic target. Despite promising results in early-phase clinical trials in a range of tumour types, a phase iii study of the ido 1-selective inhibitor epacadostat in combination with pembrolizumab in metastatic melanoma showed no difference between the epacadostat–pembrolizumab group and the placebo–pembrolizumab group 72 . That resulted in a diminution of interest in ido 1 inhibitors; however, other approaches to inhibiting that pathway continue to be considered. Novel Trp–Kyn–AhR pathway inhibitors such as Kyn-degrading enzymes, direct AhR antagonists, and Trp mimetics are advancing in early-stage or preclinical development 73 . Despite the uncertainty surrounding ido 1 inhibition, ample preclinical evidence supports the continued development of Trp–Kyn–AhR pathway inhibitors to enhance ici efficacy.
The Microbiome As a Master Regulator of Both ICI Efficacy and Toxicity
The host microbiome plays an important role in the efficacy of vaccine immune responses 74 , the promotion of carcinogenesis 75 – 81 , and the efficacy and toxicity of anticancer treatments 82 – 84 , including ici s 82 , 85 . A foundational study in mice showed that manipulation of the baseline flora of the gut microbiome affects melanoma growth kinetics and can enhance ici efficacy 86 . Other preclinical studies showed that the efficacy of anti– ctla -4 therapy can be compromised by antibiotic-induced dysbiosis or use of germ-free mice 87 . The efficacy of the ici could be restored in antibiotic-treated mice after gavage with Bacteroides fragilis or Bacteroides thetaiotaomicron (order Burkholderiales), or both, through an enhanced il -12–dependent type 1 T helper immune response 87 . Furthermore, analysis of stool from patients with ipilimumab-treated melanoma demonstrated selective enrichment of B. fragilis in clinical responders, possibly suggesting the presence of a ctla -4 blockade–induced gut dysbiosis.
Other key studies have focused on identifying human microbiota signatures predictive of clinical ici responses 88 – 92 . Patients with metastatic melanoma who were responders to anti–PD-1 therapy were shown to have enrichment of Bifidobacterium longum, Collinsella aerofaciens , and Enterococcus faecium in pre-treatment stool samples 89 . Subsequent fecal microbiota transplantation ( fmt ) of “responder” gut flora into germ-free mice was associated with improved melanoma tumour control through a CD8+ T cell immune response 89 . Furthermore, the ratio of “beneficial” to “non-beneficial” bacteria species in patients was the best predictor of an antitumour clinical response 89 . Another group identified enrichment of Akkermansia muciniphila in the microbiota of responders to anti–PD-1 or PD-L1 ici s in 3 cancer subtypes. They further demonstrated that oral supplementation with A. muciniphila, Alistipes indistinctus , or Enterococcus hirae could restore ici antitumour efficacy in germ-free mice colonized with bacterial species from non-responder fmt 88 . A third fundamental study showed that patients with melanoma responding to ici had greater alpha diversity in their gut flora, with selective enrichment in order Clostridiales family Ruminococcaceae , especially genus Faecalibacterium 90 . On the other hand, the microbiomes in ici non-responders showed a shift toward order Bacteroidales. Taken together, those studies demonstrate that the antitumour immune response depends on the composition of the gut microbiome and that antibiotic-induced dysbiosis is associated with reduced immune priming and primary resistance to immunotherapy. The deleterious effect of antibiotics given close to the time of ici treatment was confirmed in retrospective analyses of ici -treated patients with various cancers (renal cancer, non-small-cell lung cancer, urothelial cancer, and melanoma) 88 , 93 – 95 . Thus far, a modest overlap in key microbiome mediators of response to anti–PD-1 or PD-L1 therapies has been observed across cohorts, with an apparent common responder signature enriched in A. muciniphila , Clostridiales, E. faecium, Eubacterium species, the Firmicutes, and Ruminococcus species 96 . Although poor overlap between study cohorts might be a result of differences in technique or tumour type, additional heterogeneity of the gut microbiome linked to genetics, geography, lifestyle, or prior antibiotic and other drug exposure must be considered. Ultimately, “responder” gut profiles will likely reflect combinations of taxonomic orders and families rather than the presence or absence of one or a few particular species.
Besides priming the immune response to ici s, the microbiome also modulates ir ae s 17 , 91 . For instance, metagenomic sequencing found members of the Bacteroidetes phylum (families Bacteroidaceae, Rikenellaceae , and Barnesiellaceae) to be more abundant in stools obtained before treatment from patients with melanoma who did not develop ipilimumab-induced colitis, implying a protective effect of those microorganisms 17 . Importantly, specific microbial metabolic pathways (polyamine transport and vitamin B synthesis) were found to be predictive of resistance to ctla -4 blockade–induced colitis 17 . Another group corroborated the protective effect of a Bacteroidetes-rich phylotype against ctla -4 blockade–induced colitis in patients with melanoma. They further demonstrated that favourable clinical responses and susceptibility to colitis were both correlated with baseline microbiota enrichment in phylum Firmicutes (unclassified Ruminococcaceae, Clostridium cluster XIVa, and Blautia) and, in particular, Faecalibacterium , which is known to exert an anti-inflammatory role in the gut 91 . Further, the resolution of refractory ici -associated colitis in 2 patients with cancer was achieved by fmt from a healthy donor 97 . Thus, a critical goal of microbiome manipulation is to disentangle the modulation of toxicity from the preservation or enhancement of ici efficacy 98 .
There is a strong push to translate those newly acquired basic science findings about the microbiota into therapeutic clinical tools. Several trials are evaluating safety, efficacy, and immune profile changes in patients with ici -resistant cancer treated with “complete responder” fmt (see {"type":"clinical-trial","attrs":{"text":"NCT03353402","term_id":"NCT03353402"}} NCT03353402 , {"type":"clinical-trial","attrs":{"text":"NCT03341143","term_id":"NCT03341143"}} NCT03341143 , and {"type":"clinical-trial","attrs":{"text":"NCT03637803","term_id":"NCT03637803"}} NCT03637803 at https://ClinicalTrials.gov/ ). The safety of fmt is particularly important and under scrutiny, given that fmt or bacterial colonization experiments in mice have revealed a potential for transfer of chronic diseases 99 , 100 or increased risk of tumorigenesis 76 , 101 . Probiotics—loosely defined as health-promoting live organisms or fermented foods—are so far being assessed mostly in clinical trials aiming to reduce anticancer treatment toxicities; only one registered trial is testing their efficacy in the context of ici s ( {"type":"clinical-trial","attrs":{"text":"NCT03829111","term_id":"NCT03829111"}} NCT03829111 ). The optimal probiotic cocktail for immunotherapy remains to be determined and might vary with the ici or the tumour type. Additionally, reliable preparation of probiotics will be essential, likely requiring changes to their regulation. Ultimately, clinical studies of the effects of the microbiome on ici efficacy or toxicity will have to consider other confounding factors known to affect the commensal microbiome, such as concomitant radiation therapy 102 , exposure to antibiotics or other drugs (proton-pump inhibitors, antipsychotics, antimetabolites) 103 , and diet (including method and composition).
Cancer immunotherapy has dramatically changed survival and quality of life for patients. However, not all cancers are equal, and very few predictors of response and toxicity currently exist. Despite the rapid advances made in the field, immuno-oncology is still in its relative infancy, with numerous challenges and hurdles yet to be overcome. Over time, a realization grew that the standard tools used to assess choice of treatments in the era of chemotherapy and targeted therapies might not be valid for the new immunotherapies. As an example, the Response Evaluation Criteria in Solid Tumors ( recist ) used to assess response to treatments were modified to create i recist , which accounts for the novel patterns of response seen during immunotherapy, including tumour pseudoprogression 104 . In the same way that TNM staging has been crucial in guiding treatments in the era of chemotherapy, novel tools are required in the era of cancer immunotherapy. The Immunoscore has already been validated as adding important prognostic information to TNM staging in colon cancer 39 . The fact that T cells are currently widely recognized as the key mediators of antitumour efficacy with ici treatment suggests that use of the Immunoscore is an attractive option to help guide treatment selection in other cancer types as well. Still, that option does not exclude the possible use of additional parameters that might provide further insights into the specifics of each case.
It is becoming more challenging to increase the efficacy of combination therapies already established in clinical practice. In metastatic melanoma, combined ctla -4 and PD-1 blockade has achieved an unprecedented five-year overall survival above 50% 105 . In metastatic renal cell carcinoma, the same combination has been associated with an overall survival rate exceeding 60% at 3 years in the intention-to-treat population 106 , 107 . In the large landscape of ongoing early-phase clinical trials, few novel combinations have achieved a level of efficacy rivalling those new standards of care. What certainly remains to be improved is their safety profiles.
The approved induction and regimen dose of combination ici s (ipilimumab 3 mg/kg and nivolumab 1 mg/kg every 3 weeks) in the setting of melanoma is associated with a 59% rate of grades 3–4 toxicities 108 . Preliminary results from CheckMate 511, which used alternative dosing (ipilimumab 1 mg/kg and nivolumab 3 mg/kg every 3 weeks), showed a significant improvement in toxicity without loss of efficacy 109 . Given that ir ae s can sometimes be associated with mortality and significant lifelong morbidity (for example, de novo insulin-dependent diabetes, persistent pituitary dysfunction, or immune-related inflammatory arthropathies), predictors and novel strategies to abate those toxicities are urgently needed.
Another area of urgent need is to find novel treatments both for patients who are primary non-responders to ici s and for those who develop secondary resistance to those therapies. Beyond ici failure, very few treatments have been studied, and physicians often rely on previously validated standards of care for each specific cancer. Early observational data suggest that exposure to ici s might modulate the response to standard treatments received after progression. For instance, exceptionally high response rates to chemotherapy have occasionally been documented after ici failure 110 , 111 . Those observations might be secondary to immunotherapy having removed the inhibition initially exerted by tumour cells or other immune cells, followed by cytotoxic chemotherapy–mediated killing of tumour cells. On the other hand, progression-free survival and the adverse event profiles associated with exposure to targeted therapies (such as braf inhibition in melanoma) might be adversely affected by first-line exposure to ici s 112 .
To summarize, the future of cancer immunotherapy could rely on combination therapies using checkpoint inhibitors not with other novel checkpoint inhibitors, but rather with personalized cancer vaccines and novel targeted therapies directed at the tumour microenvironment, tumour glycosylation, and the host microbiome, as outlined in the present review. Advances in those fields will allow movement away from the current broad “shotgun” approach, which exposes all comers within the approved indications to ici s, to treatments tailored to the factors that make each cancer and host a unique pairing.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’ s policy on disclosing conflicts of interest, and we declare the following interests: WHM reports personal fees from Bristol–Myers Squibb, Merck, Roche, Novartis, and Amgen outside the submitted work; and KE reports personal fees from Bristol–Myers Squibb outside the submitted work. LR and NB have no conflicts of interest to disclose.
- Sustainability
- Latest News
- News Reports
- Documentaries & Shows
- TV Schedule
- CNA938 Live
- Radio Schedule
- Singapore Parliament
- Mental Health
- Interactives
- Entertainment
- Style & Beauty
- Experiences
- Remarkable Living
- Send us a news tip
- Events & Partnerships
- Business Blueprint
- Health Matters
- The Asian Traveller
Trending Topics
Follow our news, recent searches, commentary: 'curing cancer' is a loaded term, advertisement.
Cancer cases worldwide are set to jump by more than 75 per cent by 2050. Making treatment safer, more tolerable, and having access to drugs are ways to improve survival rates, says cancer specialist Dr Peter Ang from OncoCare Cancer Centre.
Crippling nausea, frailty and severe hair loss may be improved with new cancer therapies. (Photo: iStock/Pornpak Khunatorn)
This audio is AI-generated.

SINGAPORE: When Kate Middleton , Britain’s Princess of Wales, recently revealed that she was undergoing treatment for cancer , it reignited conversations surrounding this disease.
Despite decades of research, significant advancements in treatment and remarkable breakthroughs in our understanding of the disease, the quest for a universal cure for cancer continues to elude us. Why is it so hard to find a cure for cancer?
Curing cancer is a loaded term to use in oncology.
Put simply, cancer cannot be treated with a single cure as it is not a single disease and doesn’t have a single cause. There are more than 200 types of cancer, and there is no one-size-fits-all treatment. Cancer cells can also become resistant to treatments and begin growing again.
As the world's population continues to grow, cancer cases are projected to rise. In 2022, about 20 million around the world received a cancer diagnosis while 9.7 million people died from cancer. According to the World Health Organization, more than 35 million new cancer cases are predicted in 2050, a 77 per cent increase from 2022.
Commentary: ‘Living funerals’ help the dying and their loved ones live more meaningfully

Commentary: Kate Middleton is having ‘preventive chemotherapy’ for cancer. What does this mean?
The best way to beat cancer is prevention.
In Singapore, an average of 46 people were diagnosed with cancer daily and 16 people died of cancer each day from 2017 to 2021, according to the Singapore Cancer Society. One in four people may develop cancer in their lifetime.
Although the cancer survival rate in Singapore has increased to almost 60 per cent, 23.9 per cent of the 26,891 deaths in 2022 was caused by cancer - making it the leading cause of death in Singapore.
Improvements in cancer diagnosis, treatment and prevention continue to lead the way in reducing cancer deaths. The best odds for survival is detecting the cancer early in a premalignant stage so that it can be removed.
However, in many parts of the world, cancer is detected when the patient already has symptoms.
Historically, the primary treatment is to remove the cancer lump with surgery or destroy it with radiation therapy. Chemotherapy works by stopping or slowing the growth of all rapidly growing cells in the body, even if they are healthy, while targeted therapy attacks specific proteins on cancer cells.
However, tumours can recur or metastasize to sites distant from where the original tumour was. As such, adjuvant therapy is often used after primary treatment to remove unseen micrometastatic cancer cells to reduce the chance of cancer coming back.
USING OUR OWN IMMUNE SYSTEM TO FIGHT CANCER
A promising area of development in cancer treatment is immunotherapy, which uses the body’s own immune system to kill cancer cells.
It has been observed that in some cancers, spontaneous regression of the metastatic disease occurs although it is rare. Under the microscope, these cancer cells seem to have large numbers of immune cells around them. That was a clue that the immune system may be involved intimately in such cancers.
Our immune system comprises the white blood cells, the organs, and tissues of the lymph system, like the bone marrow. The immune system is massively powerful and the ability to harness this has seen significant improvements in survival rates in melanoma, kidney, lung, breast, and other cancers.
Immunotherapy has several interesting features. It may overcome resistance that happens with conventional chemotherapy. It has the potential to reach sanctuary sites such as the brain that medications may not penetrate well.
Unlike chemotherapy, immunotherapy can work for an extended period of time, sometimes after the treatment is stopped. This is because the body’s immune system “remembers” which cancer cells to look for and remains activated. Some cancers that do not respond well to conventional chemotherapy have a remarkable response with immunotherapy.

Commentary: I’ve just been diagnosed with cancer, now what?
Commentary: Singapore women shouldn’t put off breast cancer screening
Immunotherapy studies have shown that median survival for patients with unresectable stage 4 melanoma increased from six months to almost six years.
Immunotherapy is also a promising option for a triple-negative breast cancer, a particularly aggressive group of invasive breast cancer that is oestrogen receptor-negative, progesterone receptor-negative and HER2-negative.
In a study published in The New England Journal of Medicine in 2020, researchers noted that the pathological complete response rate for patients treated with immune checkpoint inhibitor pembrolizumab in conjunction with chemotherapy was 64.8 per cent. This compared with 51.2 per cent for the group that was given a placebo with chemotherapy. A higher pathological complete response rate increases the likelihood of breast conservation and long-term survival.
Subsequently, the treatment was approved in October 2022 by the Health Sciences Authority in Singapore and added to the Cancer Drug List in March 2024. Breast cancer is the most common cancer in women in Singapore with more than 1,100 new cases a year. Triple-negative breast cancers accounts for 10 to 20 per cent of cases.

The Big Read: For young adults with cancer, battling an ‘old person’s disease’ is a lonely journey

Commentary: ‘Mummy is dying because I misbehaved’ – when children’s grief turns to guilt
Continued research needed.
While immunotherapy offers hope for cancer patients, it is important to acknowledge that not all individuals will benefit equally from these treatments.
Immunotherapy medication is given as an intravenous infusion every few weeks. It can be associated with side effects although they may not be typical of what most patients would associate with chemotherapy such as nausea, vomiting or hair loss.
The side effects of immunotherapy are often related to immune activation, and some can be serious, necessitating early recognition and timely intervention.
For immune checkpoint inhibitors to work well, a healthy immune system is necessary and so the earlier use of such treatments might be better.
It remains to be seen if there will ever be a cure for cancer. But making treatment safer, more tolerable, and having access to drugs are ways to improve cancer cure rates. We can strive towards a future where cancer is no longer a feared diagnosis but a manageable condition.
Dr Peter Ang is a cancer specialist at OncoCare Cancer Centre.
Related Topics
Also worth reading, this browser is no longer supported.
We know it's a hassle to switch browsers but we want your experience with CNA to be fast, secure and the best it can possibly be.
To continue, upgrade to a supported browser or, for the finest experience, download the mobile app.
Upgraded but still having issues? Contact us

IMAGES
VIDEO
COMMENTS
Cancer immunotherapy is a therapy used to treat cancer patients that involves or uses components of the immune system. Some cancer immunotherapies consist of antibodies that bind to, and inhibit ...
Immunotherapy helps a person's immune system to target tumor cells. Recent advances in cancer immunotherapy, including immune checkpoint inhibition, chimeric antigen receptor T-cell therapy and cancer vaccination, have changed the landscape of cancer treatment. These approaches have had profound success in certain cancer types but still fail ...
Cancer immunotherapy represents a major advance in the cure of cancer following the dramatic advancements in the development and refinement of chemotherapies and radiotherapies. In the recent decades, together with the development of early diagnostic techniques, immunotherapy has significantly contributed to improving the survival of cancer patients. The immune-checkpoint blockade agents have ...
Recent Advances in Cancer Immunotherapy. The strategy to use the immune system to fight cancer is not a novel concept; in 1891, Coley reported the treatment of three cases of sarcoma by inoculation with erysipelas [ 1 ]. However, less than 10 years have passed since cancer immunotherapy began attracting a great deal of attention from clinicians ...
As of April 15, 2022, there were 2,756 active cell therapy agents in the global immuno-oncology pipeline, an increase of 36% over the 2021 landscape analysis that identified 2,031 such agents, but also a modest deceleration compared to 43% growth in the prior year. CAR-T therapeutics continue to dominate the cell therapy pipeline with growth of ...
Associated Data. Immunotherapy has opened a new era in cancer treatment. Drugs represented by immune checkpoint inhibitors have led to important breakthroughs in the treatment of various solid tumors, greatly improving the survival rate of cancer patients. Many types of immunotherapeutic drugs have become widely available; however, their ...
Abstract. Cancer treatment is presently one of the most important challenges in medical science. Surgery, chemotherapy, radiotherapy, or combining these methods is used to eliminate the tumor. Hormone therapy, bone marrow transplantation, stem cell therapy as well as immunotherapy are other well-known therapeutic modalities.
Immunotherapy is a promising strategy to treat cancer by stimulating the body's own immune system to destroy tumor cells, but it only works for a handful of cancers. MIT researchers have now discovered a new way to jump-start the immune system to attack tumors, which they hope could allow immunotherapy to be used against more types of cancer.
The cross talk between immune and non-immune cells in the tumor microenvironment leads to immunosuppression, which promotes tumor growth and survival. Immunotherapy is an advanced treatment that boosts humoral and cellular immunity rather than using chemotherapy or radiation-based strategy associated with non-specific targets and toxic effects on normal cells. Immune checkpoint inhibitors and ...
CRI scientists are committed to groundbreaking cancer immunotherapy research that can benefit the lives of patients and potentially save lives. In addition to research regarding treating existing cancers, some CRI scientists are also working on forward-thinking research that can address cancer prevention and attack cancer at its roots.
In recent years, cancer immunotherapy, exemplified by PD-1 and its ligand PD-L1 blockade, has made remarkable advances. But while immunotherapy drugs offer new treatment possibilities, only about ...
Credit: Cincinnati Children's. An innovative computational tool dubbed "SNAF" may help the research world bring the emerging promise of cancer immunotherapy to a wider range of patients, according ...
An experimental form of immunotherapy that uses an individual's own tumor-fighting immune cells could potentially be used to treat people with metastatic breast cancer, according to results from an ongoing clinical trial led by researchers at the National Cancer Institute's (NCI) Center for Cancer Research, part of the National Institutes of Health.
Scientific advances in immunotherapy and new targeted therapies have increased survival rates. But screening among former and current smokers still needs to improve to save more lives.
1 Introduction. Cancer represents a significant global public health challenge and remains one of the primary causes of mortality worldwide. According to the International Agency for Research on Cancer, there are approximately 19.3 million new cancer diagnoses and nearly 10.0 million cancer-related deaths each year ().The development of cellular immunotherapy has profoundly changed cancer ...
The emergence of immunotherapy has changed the standard and concept of tumor treatment. This article focuses on the latest clinical progress in cancer immunotherapy, including monoclonal antibodies (mAbs), small molecule drugs, adoptive cell therapy, oncolytic viruses, and cancer vaccines ( Figure 1 ). We discuss limitations, immune resistance ...
Immunotherapy is a type of cancer treatment that helps your immune system fight cancer. The immune system helps your body fight infections and other diseases. It is made up of white blood cells and organs and tissues of the lymph system.. Immunotherapy is a type of biological therapy.Biological therapy is a type of treatment that uses substances made from living organisms to treat cancer.
Abstract. Cancer treatment is presently one of the most important challenges in medical science. Surgery, chemotherapy, radiotherapy, or combining these methods is used to eliminate the tumor. Hormone therapy, bone marrow transplantation, stem cell therapy as well as immunotherapy are other well-known therapeutic modalities.
New Research Shows a Link Between Cell Identities and Childhood Cancer Type Neuroblastoma Sep. 7, 2021 — Neuroblastoma is a type of childhood cancer that develops in infants and young children.
MONDAY, April 8, 2024 (HealthDay News) -- Pancreatic cancer patients may do better if they receive an immunotherapy drug as well as chemotherapy in preparation for surgery, new research suggests ...
Nearly a third of patients with advanced liver cancer who received a personalized vaccine developed by Geneos Therapeutics along with an immunotherapy drug in a small, early trial saw their tumors ...
Liver cancer is the sixth most common cancer in the world. Researchers estimate 905,700 people were diagnosed with liver cancer in 2020 and that number is expected to hit 1.4 million by 2040. ...
By assisting the immune system in recognizing and attacking cancer cells, it plays a crucial role in the fight against cancer. How successful Is immunotherapy for brain cancer? Since immunotherapy is a fairly new treatment that is being tested for cancers such as brain cancer, there is not enough research to show how effective it is on the brain.
The next revolutionary wave in cancer immunotherapy came with the better understanding of the process of immune surveillance, by which innate immune cells eliminate cancer cells. The recent discovery of T cell immune checkpoints, such as ctla -4 and PD-1, propelled the field of immuno-oncology into its current era and saw the awarding of the ...
Curing cancer is a loaded term to use in oncology. Put simply, cancer cannot be treated with a single cure as it is not a single disease and doesn't have a single cause. There are more than 200 ...