Your Account
Manage your account, subscriptions and profile.
MyKomen Health
ShareForCures
In Your Community
In Your Community
View resources and events in your local community.
Change your location:

Susan G. Komen®
One moment can change everything.

Current Funding Opportunities
Through our Research Program, Komen evaluates and invests in science and technology that will accelerate research discoveries to change the standard of breast cancer care and improve the delivery of that care. We’re focused on conquering aggressive and metastatic breast cancers and eliminating breast cancer disparities. Our goal: to advance personalized medicine and improve health outcomes for everyone.
Each year, we release Request for Applications or Letter of Intent (LOI) Announcements to communicate which grant mechanisms and scientific focus areas are available for funding. The types of mechanisms and the scientific focus areas may vary from year to year.
2023-2024 (FY24) Letter of Intent Announcement
• LOI Announcement Release Date: June 15, 2023 • LOI Submission system open date: June 15, 2023 • LOI Submission deadline: July 14, 2023 at 1:00pm EST • Invitation to submit an Application: July 25, 2023 at 1:00pm EST
CAREER TRANSITION AWARD (CTA)
This new Susan G. Komen grant mechanism aims to help outstanding senior postdoctoral fellows and clinical fellows working under the guidance of a mentor to launch their competitive, independent breast cancer research careers. The Career Transition Awards provide up to five years of funding in two phases: Phase 1 supports the final years of mentored, postdoctoral training and Phase 2 supports the independent research of the early career, tenure-track investigators.
GRANT TERMS: Applicant/PI may request up to 5 years of funding totaling up to $650,000.00 over the two phases of the Award. Up to $100,000.00 per year (direct costs only) for up to two years may be requested for the first phase of the Career Transition Award to support the postdoctoral work. For the second phase of the Career Transition Award, the Applicant/PI may request up to $150,000.00 per year (direct and indirect costs) for up to three years to support independent research.
Eligibility: Individuals pursuing independent breast cancer research careers who are in the final years of mentored postdoctoral research training positions with no more than five years of total postdoctoral research experience at the time of Letter of Intent submission (July 14, 2023 ). For this application clinical fellows are considered eligible and equal to the postdoctoral rank.
Research Focus: Research projects must be hypothesis-driven, breast cancer-focused studies. They may be considered basic, translational, clinical and/or population science in nature and should align with Komen’s research goals and priorities and mission to save lives from breast cancer.
Download the current Career Transition Award LOI Announcement (PDF) for more details
Komen Career Transition Award Review Process
Susan G. Komen® utilizes a multi-step approach to grant application and review that first requires submission of a Letter of Intent and upon invitation only, submission of an Application.
Applicants/PIs will be notified of Letter of Intent review decisions via email. Applicants/PIs invited to submit an Application will then be granted access to the Application site in proposalCENTRAL.
All institutions and industry partners must agree to adhere to Komen’s Policies and Procedures for Research and Training Grants. A copy of the latest version of these awards is found with the other documents for the LOI in proposalCENTRAL.
ASPIRE : A Supplement to Promote Inclusion for Research Excellence
Despite progress in breast cancer research and patient care, not everyone is benefitting from the scientific advances and improvements in breast cancer care and outcomes. This new Susan G. Komen grant is intended to enhance the diversity of the breast cancer research workforce by providing established breast cancer scientists with supplemental funding to support research trainees from communities historically minoritized and marginalized in research. A breast cancer research workforce that represents the breast cancer community is one way to work towards health equity and better outcomes for all. By supporting these promising trainees early in their research careers, Komen seeks to ensure that a diverse group of highly trained scientists who reflect the communities we serve will emerge as the next generation of leaders in breast cancer research and end breast cancer forever.
Award amount : up to $100,000 per year to cover a trainee’s salary for one year or two years.
Eligibility: Lead mentor must have a currently funded breast cancer research project that has undergone a rigorous peer review (see request for application for link of organizations that fall under this category). Trainees supported with this supplement are from groups shown to be historically minoritized and marginalized in biomedical research from National Science Foundation data. This includes individuals who identify as Black or African American, Latino or Hispanic, American Indian or Alaska Native, Native Hawaiian and other Pacific Islanders; individuals with disabilities; and individuals from disadvantaged backgrounds according to the criteria used by the NIH ( https://diversity.nih.gov/about-us/population-underrepresented ). Eligibility for disadvantaged background will be measured using the same characteristics as the NIH, i.e., at least two of the criteria must be met to be included.
Download the current ASPIRE Request for Applications for more details here .
All institutions must agree to adhere to Komen’s Policies and Procedures for Research and Training Grants.
Key Dates :
Applications due November 1, 2023
Award notification on or around April 15, 2024.
Grantee Resources
View current funding opportunities for Scientific Research Grants.
Funded Grants
View currently funded Scientific Research Grants.
Research Accomplishments
See how Komen researchers are making difference.
Clinical Trials We Are Funding
See what types of clinical trials Komen research has supported.
Bringing the Patient Voice to Research
Learn how breast cancer survivors and advocates are involved in our scientific research.
TOOLS & RESOURCES
Useful Link
Submit a Letter of Intent
Give For Metastatic Breast Cancer Research
Clinical Trials
Breast cancer.
Displaying 356 studies
The purpose of this study is to show that improvements in the molecular breast imaging (MBI) technology will allow reduction of the administered dose of Tc-99m sestamibi while maintaining a sensitivity of 90% for tumor detection.
The purpose of this study is to determine whether treatment with alpelisib plus fulvestrant will lengthen progression-free survival compared to fulvestrant and a placebo in men and postmenopausal women who have hormone receptor positive (HR+), HER2-negative advanced breast cancer.
This study aims to compare an additional support program (text message reminders and/or telephone-based counseling) with usual care in making sure breast cancer patients take their endocrine therapy medication as prescribed (medication adherence).
This randomized phase II trial studies how well carboplatin and paclitaxel with or without atezolizumab before surgery works in treating patients with newly diagnosed, stage II-III triple negative breast cancer. Monoclonal antibodies, such as atezolizumab, may block tumor growth in different ways by targeting certain cells. Drugs used in chemotherapy, such as carboplatin and paclitaxel, work in different ways to stop the growth of tumor cells, either by killing the cells, by stopping them from dividing, or by stopping them from spreading. Giving carboplatin and paclitaxel with or without atezolizumab before surgery may make the tumor smaller and reduce the ...
The purpose of this study is to identify subtype-specific signatures for breast cancer using genomic positioning of plasma DNA fragments, and to validate changes in ctDNA levels as a biomarker for treatment monitoring in patients with metastatic breast cancer.
RATIONALE: Estrogen can cause the growth of breast cancer cells. Hormone therapy using tamoxifen citrate, goserelin acetate, leuprolide acetate, anastrozole, letrozole, or exemestane, may fight breast cancer by lowering the amount of estrogen the body makes. Everolimus may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. It is not yet know whether hormone therapy is more effective when given with or without everolimus in treating breast cancer.
PURPOSE: This randomized phase III trial studies how well giving hormone therapy together with or without everolimus work in treating patients with breast cancer.
The purpose of this study is to further advance the ability to practice personalized medicine by learning which new drug agents are most effective with which types of breast cancer tumors and by learning more about which early indicators of response (tumor analysis prior to surgery via magnetic resonance imaging (MRI) images along with tissue and blood samples) are predictors of treatment success.
RATIONALE: Estrogen can stimulate the growth of breast cancer cells. Hormone therapy using letrozole may fight breast cancer by reducing the production of estrogen.
PURPOSE: This randomized phase III trial is studying letrozole to see how well it works in treating women with breast cancer who have received tamoxifen for at least 5 years.
The purpose of this study is to to evaluate the integration of cancer genetic testing into a mammography practice aimed toward women at intermediate- to-high lifetime risk of breast cancer. If successful, this will provide an opportunity for cancer risk stratification and individualized screening.
The primary purpose of this study is to evaluate the incremental invasive cancer yield in patients with a negative mammogram who are intermediate or high-risk for breast cancer and get supplemental screening with Contrast-Enhanced Digital Mammography (CEDM).
The goal of this clinical research study is to learn how often breast cancer recurs (returns after treatment) in the breast in patients who have been treated with chemotherapy and have had follow-up radiation therapy (but not surgery) and are in complete remission (no evidence of disease). This is an investigational study. Radiation therapy is delivered using FDA-approved and commercially available methods. The study doctor can explain how radiation therapy is designed to work. About 120 participants will be enrolled on this multicenter study. Up to 90 may take part at MD Anderson.
This randomized phase III trial studies how well aspirin works in preventing the cancer from coming back (recurrence) in patients with human epidermal growth factor receptor 2 (HER2) breast cancer after chemotherapy, surgery, and/or radiation therapy. Aspirin is a drug that reduces pain, fever, inflammation, and blood clotting. It is also being studied in cancer prevention. Giving aspirin may reduce the rate of cancer recurrence in patients with breast cancer.
The purpose of this study is to determine the recommended Phase 2 dose twice weekly of berzosertib administered concurrently with conventionally fractionated radiation therapy to the breast/chest wall and regional nodes.
RATIONALE: Drugs used in chemotherapy use different ways to stop tumor cells from dividing so they stop growing or die. Combining more than one drug and giving them in different ways after surgery may kill more tumor cells. It is not yet known which chemotherapy regimen is more effective in treating older women with breast cancer. PURPOSE: This randomized phase III trial is studying different combination chemotherapy regimens to see how well they work in treating older women who have undergone surgery for breast cancer.
The primary purpose of this study is to examine the effects of abemaciclib on the CD8/FOXP3 ratio in chemotherapy-resistant triple negative breast cancer (TNBC) patients following neoadjuvant chemotherapy.
This is a 3 arm Phase 3 study to evaluate the safety and efficacy of the addition of veliparib plus carboplatin versus the addition of carboplatin to standard neoadjuvant chemotherapy versus standard neoadjuvant chemotherapy in subjects with early stage TNBC.
The purpose of this registry is to collect and maintain samples of breast tissue from women and men undergoing surgery for a breast related concern at Mayo Clinic Rochester, to create a biospecimen resource for the study of benign and cancerous breast conditions.
The purpose of this study is designed to evaluate the effectiveness and safety of AL101 monotherapy in subjects with Notch-activated recurrent or metastatic Triple Negative Breast Cancer (TNBC); Notch activation will be determined by a Next Generation Sequencing (NGS) test.
This study will evaluate the efficacy, safety and tolerability of trastuzumab deruxtecan compared with investigator's choice chemotherapy in human epidermal growth factor receptor (HER)2-low, hormone receptor (HR) positive breast cancer patients whose disease has progressed on endocrine therapy in the metastatic setting.
The purpose of this study is to evaluate an investigational drug as a possible treatment for breast cancer that is positive for the protein Human Epidermal Growth Factor Receptor 2, also known as HER2-positive breast cancer. The drug involved in this study is: -ado-trastuzumab emtansine (T-DM1)
RATIONALE: Drugs used in chemotherapy, such as doxorubicin, cyclophosphamide, and paclitaxel, work in different ways to stop the growth of tumor cells, either by killing the cells or by stopping them from dividing. Monoclonal antibodies, such as trastuzumab, can block tumor growth in different ways. Some block the ability of tumor cells to grow and spread. Others find tumor cells and help kill them or carry tumor-killing substances to them. Lapatinib may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. Giving combination chemotherapy together with trastuzumab and lapatinib after surgery may kill ...
This randomized phase III trial studies combination chemotherapy and trastuzumab to see how well they work compared to combination chemotherapy alone in treating women with breast cancer. Drugs used in chemotherapy use different ways to stop tumor cells from dividing so they stop growing or die. Monoclonal antibodies such as trastuzumab can locate tumor cells and either kill them or deliver tumor-killing substances to them without harming normal cells. It is not yet known whether combination chemotherapy is more effective with or without trastuzumab in treating breast cancer.
This study is an open label, randomized, multicenter trial of MM-302 plus trastuzumab. The trial is designed to demonstrate whether MM-302 plus trastuzumab is more effective than the chemotherapy of physician's choice (CPC) plus trastuzumab in locally advanced/metastatic HER2-positive breast cancer patients. Patients may not have been previously treated with an anthracycline in any setting. Patients must have received prior treatment with trastuzumab in any setting, have either progressed or are intolerant to ado-trastuzumab emtansine in the metastatic or locally advanced setting, have either progressed or are intolerant to pertuzumab in the metastatic or locally advanced setting or had disease ...
The no show rate for mammography screening is high among Navajo women. One barrier to preventive screening is a lack of cancer literacy including low knowledge and cultural attitudes (e.g., fatalism) about screening. The investigators will examine the potential feasibility and acceptability of a cancer literacy intervention for families of Navajo women who have no showed for three consecutive times to mammography screening who have never or rarely been screened in the past.
This open label, randomised, controlled, multi-centre phase III study will assess the efficacy and safety of single agent olaparib vs standard of care based on physician's choice of capecitabine, vinorelbine or eribulin in metastatic breast cancer patients with gBRCA 1/2 mutations.
The purpose of this study is to gain an understanding of the lived experiences of symptoms and quality of life issues experienced by women with metastatic breast cancer.
The purpose of this trial is to compare denosumab to placebo for the prevention of breast cancer in women with a BRCA1 germline mutation. A germline mutation is an inherited gene change which, in the BRCA1 gene, is associated with an increased risk of breast and other cancers. Denosumab is a monoclonal antibody that is used to treat bone loss in order to reduce the risk of bone fractures in healthy people, and to reduce new bone growths in cancer patients whose cancer has spread to their bones. Research has shown that denosumab may also reduce the risk of developing ...
The purpose of this study is to compare and identify different forms/regimens of chemotherapy/hormone therapy in breast cancer patients who underwent mastectomy and subsequent immediate breast reconstruction (tissue expander or direct-to-implant). Subsequently, correlate these findings to assess overall surgical and clinical outcomes.
We are doing this research study to compare radiation dermatitis severity in exposed skin protected by a thin film dressing versus skin that is not protected during radiation treatment. We will also be comparing two types of film; a perforated thin film dressing versus the standard thin film.
The primary aim of this study is to determine if the addition of an individual polygenic risk score (PRS) in addition to the Breast Cancer Risk Assessment Tool (BCRAT) or Tyrer-Cuzick (IBIS) score will aid women at risk of breast cancer in making a decision to take (or not take) medications to prevent breast cancer.
The primary purpose of this study is to identify the therapeutic effect of Adipose-Induced Regeneration (AIR) in radiation-induced skin injury of post-mastectomy breast cancer patients.
The purpose of this study is to determine the safety of 5 fraction vs. 25 fraction radiation to the whole breast or post-mastectomy chest wall/reconstructed chest with regional nodal radiation.
The purpose of this study is to find out if using probiotics will help the body's immune system react to breast cancer. New studies showed that diverse species of bacteria inside the bowel might help improve immune system, particularly the ability of immune system to recognize cancer. This study will investigate how probiotics will affect the subjects' immune system on breast cancer.
The purpose of this study is to assess outcomes, satisfaction and aesthetics of two different breast reconstruction techniques (Goldilocks alone, and Goldilocks with Implant-Based Reconstruction) and compare its safety, patient satisfaction, aesthetic evaluation and complications.
The purpose of this study is to compare the safety and efficacy of nab-paclitaxel in combination with either gemcitabine or carboplatin to the combination of gemcitabine and carboplatin as first line treatment in female subjects with triple negative metastatic breast cancer (TNMBC) or metastatic triple negative breast cancer.
The purpose of this study is to compare the progression-free survival (PFS) between sacituzumab govitecan-hziy (SG) and pembrolizumab versus treatment of physician's choice (TPC) and pembrolizumab in participants with previously untreated, locally advanced inoperable or metastatic triple-negative breast cancer, whose tumors express programmed cell death ligand 1 (PD-L1).
This randomized phase II trial studies how well cisplatin works with or without veliparib in treating patients with triple-negative breast cancer and/or BRCA mutation-associated breast cancer that has come back or has or has not spread to the brain. Drugs used in chemotherapy, such as cisplatin, work in different ways to stop the growth of tumor cells, either by killing the cells, by stopping them from dividing, or by stopping them from spreading. Veliparib may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. It is not yet known if cisplatin is more ...
The purpose of this trial is to determine how well estradiol works in treating patients with estrogen receptor beta (ER beta) positive, triple negative breast cancer that has spread to nearby tissue or lymph nodes (locally advanced) or other places in the body (metastatic). Hormone receptors like ER beta allow the body to respond appropriately to hormones. Triple negative means that the breast cancer does not express other hormone receptors called ER alpha, progesterone, and HER2. In some people with triple negative breast cancer, ER beta is overexpressed. Tumor cells that overexpress ER beta grow slower in the laboratory and ...
The purpose of this study is to determine the correlation between HER2 specific T-cell response in HER2-positive breast cancer patients with stage I-IV who receive anti-HER2 therapies, such as trastuzumab, pertuzumab, lapatinib, or neratinib and clinical responses.
This partially randomized phase Ib/II trial studies the side effects and best dose of taselisib when given together with enzalutamide and to see how well they work in treating patients with androgen receptor positive triple-negative breast cancer that has spread to other places in the body. Taselisib is a PI3K inhibitor. The PI3K pathway is involved is cancer growth. Androgen may cause the growth of tumor cells. Enzalutamide may stop the growth of tumor cells by blocking the androgen receptor from working. Giving taselisib with enzalutamide may be a better treatment for patients with breast cancer.
This randomized phase II trial studies how well olaparib and atezolizumab work either alone or in combination in treating patients with stage III-IV triple negative breast cancer. Olaparib may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. Monoclonal antibodies, such as atezolizumab, may interfere with the ability of tumor cells to grow and spread. It is not known whether giving olaparib and atezolizumab either alone or in combination would work better in patients with triple negative breast cancer.
The purpose of this study is to evaluate if changes made to the design of the table used specifically for breast Positron Emission Mammography (PEM), will help improve the images of breast tissue close to the chest wall.
The purpose of this study is to evaluate T cell and antibody immunity in patients with HER2+ breast cancer who will receive trastuzumab with standard chemotherapy in order to address whether the immunity is associated with the patient’s response to treatment.
The purpose of this study is to evaluate a new treatment using a potent Poly polymerase (PARP) inhibitor known as Niraparib. Niraparib will be combined with trastuzumab, a HER2-targeted agent, to evaluate the safety and tolerability in patients with metastatic HER2 positive breast cancer. It is anticipated that the combination of drugs will improve survival and have few side effects.
The human epidermal growth factor receptor 2 (HER2) regulates cell growth and survival. Approximately 15-20% of all breast cancers are HER2-positive, which are an aggressive and fast-growing subtype of breast cancer.
The purpose of this study is to determine if tucatinib works better than placebo when given with other drugs to treat participants with HER2-positive breast cancer. A placebo is a pill that looks the same as tucatinib but has no medicine in it. This study will also test what side effects happen when participants take this combination of drugs. A side effect is anything a drug does to the body besides treating your disease. Participants will have cancer that has spread in the body near where it started (locally advanced) and cannot be removed (unresectable) or has spread through the body ...
This study will examine the safety and effectiveness of pertuzumab combined with high-dose trastuzumab in adult patients who have HER2-positive breast cancer that has spread to the central nervous system and the brain following radiation therapy.
The primary purpose of the Safety-Run-in Cohort 1 of this study is to evaluate the safety, tolerability, and recommended Phase 2 dose of magrolimab in combination with nab-paclitaxel or paclitaxel. In Phase 2 Cohort 1, the study will compare the efficacy of magrolimab in combination with nab-paclitaxel or paclitaxel versus nab-paclitaxel or paclitaxel alone as determined by progression-free survival (PFS) by investigator assessment
The primary purpose of the Safety-Run-in Cohort 2 of this study is to evaluate the safety, tolerability, and recommended Phase 2 dose of magrolimab in combination with sacituzumab govitecan. In Cohort 2 (Safety Run-in Cohort 2 and Phase 2 Cohort 2) the study will evaluate the efficacy of magrolimab in combination ...
The purpose of this study is to understand the causes of breast cancer, in particular the connection between genetic variations and breast density or how tissue is distributed on a mammogram.
The purpose of this study is to evaluate the efficacy of venetoclax in combination with fulvestrant compared with fulvestrant alone for HER2-negative breast cancer.
The study is intended to show superiority of AZD9833 in combination with CDK4/6 inhibitor (palbociclib, abemaciclib or ribociclib) versus aromatase inhibitors (anastrozole or letrozole) in combination with CDK4/6 inhibitor in patients with hormone receptor-positive (HR-positive), human epidermal growth factor receptor 2-negative (HER2-negative) metastatic breast cancer with detectable ESR1 mutation.
The investigators hypothesize that knowledge of the functional behavior of areas of mammographic density will enable more specific identification of dense tissue at-risk for breast cancer, ultimately providing predictive information on an individual's risk of developing breast cancer.
The purpose of this study is to assess whether elevated fasting plasma proneurotensin levels are common in patients genetically predisposed to breast cancer.
The purpose of this trial is to assess the safety and effectiveness of the combination of dapagliflozin plus metformin extended release (XR) compared with metformin XR during treatment with alpelisib plus fulvestrant in participants with HR-positive, HER2-negative advanced breast cancer with a PIK3CA mutation following progression on or after endocrine-based therapy.
RATIONALE: Drugs used in chemotherapy use different ways to stop tumor cells from dividing so they stop growing or die. It is not yet known which regimen of chemotherapy is more effective for breast cancer.
PURPOSE: Randomized phase III trial to compare the effectiveness of two different regimens of combination chemotherapy in treating women who have stage II or stage IIIA breast cancer that has spread to the lymph nodes.
Researchers at Mayo Clinic are developing a Biobank of adult stem cell-rich breast organoids, a new research resource to facilitate normal and cancer stem cell research. Subjects in the Biobank will provide samples of excess breast tissue, complete a health questionnaire, and allow access to medical records now and in the future. The Biobank serves as a library for researchers; instead of having to look for volunteers for each new project, researchers can use samples from the Biobank as well as share information already collected.
This study will use proteomic and genomic profiling to analyze tumor tissue to see if treatment selected by this analysis will benefit patients.
RATIONALE: Testosterone may help relieve moderate or severe arthralgia associated with the use of aromatase inhibitors, such as anastrozole or letrozole.
PURPOSE: This randomized phase III trial studies testosterone to see how well it works compared to placebo in treating postmenopausal patients with arthralgia caused by anastrozole or letrozole.
This randomized phase III trial studies standard or comprehensive radiation therapy in treating patients with early-stage breast cancer who have undergone surgery. Radiation therapy uses high-energy x rays to kill tumor cells. It is not yet known whether comprehensive radiation therapy is more effective than standard radiation therapy in treating patients with breast cancer.
This pilot study involves very frequent monitoring of breast cancer patient blood levels of hs-cTnT Troponin and n-t-BNP (Brain Natriuretic Peptide) before and after initiation of chemotherapy with either adriamycin or trastuzumab in order to define the kinetics of both biomarkers during the first two cycles of chemotherapy. Cardiac troponins and BNP are frequently elevated after experimental chemotherapy in animal models. Their behavior in humans has been inconsistent, with occasional elevations seen, usually within 30 days of therapy. Assays for troponin with sensitivity into the pg/ml range have now been introduced. A majority of patients greater than age 50 have ...
The purpose of this study is to identify a (Z)-endoxifen dose that achieves (Z)-endoxifen steady-state plasma concentrations (Css) between 500-1000 ng/mL. Dosing will begin with the (Z)-endoxifen 40 mg/day dose and may additionally explore either a lower (20 mg/day) or higher (80 mg/day) dose level based on (Z)-endoxifen Css as well as toxicity.
The purpose of this study is to collect user and subject feedback on the design, use and operation of Affirm® Contrast Biopsy.
Utilizing CellSearch® technology, the ability to both enumerate and reliably and reproducibly characterize circulating tumor cells (CTC) for tumor markers that predict endocrine sensitivity (estrogen receptor [ER] and Bcl-2) and resistance (HER2 and Ki67) has been demonstrated. An algorithm for a CTC-Endocrine Therapy Index (CTC-ETI) has been constructed that can be calculated for each patient using the CTC enumeration and marker results. The primary goal of this study is to determine a CTC-ETI in ER positive, HER2 negative metastatic breast cancer patients before the initiation of a new endocrine therapy for the identification of patients that will progress rapidly.
The purpose of this retrospective study is to evaluate the complication rate of prophylactic open NSM procedures through 42 days follow-up from retrospective chart review at the same investigators and institutions as those included under IDE Study protocol G190065/A001.
Olaparib treatment in patients with germline BRCA1/2 mutations and high risk HER2 negative primary breast cancer who have completed definitive local treatment and neoadjuvant or adjuvant chemotherapy
The purpose of this study is to study to collect tissue samples from patients with early stage hormone receptor-positive HER2-negative breast cancer.
The objective of this study is to determine if the diagnostic performance of a dedicated breast-specific positron emission mammography (PEM) system, is superior to that obtained with a conventional PET/CT scanner and capable of producing acceptable image quality at a low-radiation dose level.
The purpose of this research study is to better understand the reasons why or why not breast cancers are destroyed by standard chemotherapy. This information will be used to develop new and better cancer therapies.
The purpose of this study is to compare three different combination chemotherapy regimens and evaluate how well they work in treating women who have undergone surgery for node-positive breast cancer. Drugs used in chemotherapy, such as docetaxel, doxorubicin, cyclophosphamide, paclitaxel, and gemcitabine work in different ways to stop tumor cells from dividing so they stop growing or die. Giving combination chemotherapy after surgery may kill any remaining tumor cells.
The purpose of this study to assess the effectiveness of proton vs. photon therapy in reducing major cardiovascular events (MCE), defined as atherosclerotic coronary heart disease or other heart disease death, myocardial infarction, coronary revascularization, or hospitalization for major cardiovascular event (heart failure, valvular disease, arrhythmia, or unstable angina or other major cardiovascular event) in patients with locally-advanced breast cancer.
This is an open label phase II study to determine the safety and efficacy of a novel 3 fraction daily dosing regimen for accelerated partial breast irradiation (APBI) for early invasive and noninvasive breast cancer. The three techniques utilized are recognized as standard options for the delivery of APBI, and there is no evidence that either technique is superior or inferior to any other. The APBI technique utilized will be at the physician's discretion and will be based on technical considerations, availability at the treating radiation facility, insurance coverage, as well as patient preference.
The objective of this study is to provide preliminary data to support the development of selected technologies for the efficient and reliable analyses of cell free DNA (cfDNA) and circulating tumor cells (CTCs) in the setting of metastatic breast cancer.
The purpose of this study is to look at the safety and immune response to a vaccine used in patients previously treated for HER2 (human epidermal growth factor receptor 2) positive breast cancer.
This research study is a Phase II clinical trial. Phase II clinical trials test the effectiveness of an investigational drug to learn whether the drug works in treating a specific cancer. "Investigational" means that the drug is still being studied and that research doctors are trying to find out more about it-such as the safest dose to use, the side effects it may cause, and if the drug is effective for treating different types of cancer. It also means that the FDA has not approved this drug for use patients undergoing adjuvant treatment for HER2+ breast cancer. Trastuzumab emtansine (T-DM1) ...
The objective of this study is to assess efficacy and safety of radium-223 dichloride in subjects with human epidermal growth factor receptor 2 negative (HER2 negative) hormone receptor positive breast cancer with bone metastases treated with hormonal treatment background therapy.
The purpose of this study is to evaluate how well paclitaxel, trastuzumab, and pertuzumab with or without atezolizumab works in treating patients with breast cancer that has spread to other parts of the body. Drugs used in chemotherapy, such as paclitaxel, work in different ways to stop the growth of tumor cells, either by killing the cells, by stopping them from dividing, or by stopping them from spreading. Immunotherapy with trastuzumab, pertuzumab, and atezolizumab, may induce changes in body's immune system and may interfere with the ability of tumor cells to grow and spread. It is not yet known whether giving ...
The purpose of this study is to apply new microvasculature imaging for differentiation of breast lesions, and prediction of response to preoperative chemotherapy.
The purpose of the study is to determine whether treatment with a PI3K inhibitor plus letrozole leads to an increase in pathologic clinical response and Objective Response Rate compared to treatment with placebo plus letrozole in patients with Breast cancer
The purpse of this study is to evaluate the safety and tolerability of the lasofoxifene and abemaciclib combination for the treatment of pre- and postmenopausal women with locally advanced or metastatic ER+/HER2− breast cancer who have disease progression on first and/or 2nd lines of hormonal treatment for metastatic disease and have an ESR1 mutation.
The purpose of this study is to collect (i) pre-treatment (at the time of breast cancer surgery) normal skin and (ii) on-treatment (around the third week of treatment with breast radiation) irradiated skin with clinical hallmarks of radiation dermatitis (erythema and/or dry desquamation, documented by a photograph and graded by clinical criteria).
The purpose of this study is to determine the usefulness and effectiveness of Muse, a brain sensing headband intervention, to affect quality of life, stress, fatigue and sleep in newly diagnosed breast cancer patients who are undergoing surgical treatment.
The primary objective of this study is to determine the correlation between the distribution of F-18 FES within ER+ breast tumors as seen on Positron Emission Mammography (PEM) images of the breast, and the distribution of cells stained ER+ within the tumor by immunohistochemistry (IHC) measurements at surgical pathology. The secondary aim is to determine if the correlation (or lack of) between F-18 FES uptake and F-18 FDG uptake as imaged by PEM, is an accurate representation of the heterogeneity of ER expression in the tumor.
The primary purposes of this trial are to determine the safety and tolerability of RBX7455 given for at least 2 weeks and not more than 4 weeks prior to surgery, and to evaluate intratumoral immune system resonse, including TILs, CD4, and CD8 T cells, in operable breast cancer patients.
This is a combination Phase I and Phase II study, with an aim to evaluate the combination of GSK525762 and fulvestrant in women with advanced or metastatic ER+ breast cancer, who have disease that has progressed after prior treatment with at least one line of endocrine therapy. The objectives of the study are to first identify, in open-label single-arm Phase I, a recommended Phase II dose of GSK525762 that may be combined safely with fulvestrant. Phase I will follow a modified toxicity probability interval (mTPI) design, and a sentinel group will be evaluated first for dose-limiting toxicity and further expanded ...
The treatment for postmenopausal women diagnosed with hormone receptor positive (HR+) metastatic breast cancer (mBr) includes endocrine therapy However, de novo or acquired resistance to endocrine therapy remains an important clinical problem. Many hormone receptor positive breast cancers demonstrate overexpression of cyclin D. Cyclin D interacts directly with cyclin-dependent kinases 4 and 6 (CDK4/6) in an active protein complex that promotes cell proliferation; and consequently, CDK4/6 represents a potential therapeutic target for HR+ breast cancers. LY2835219 represents a selective and potent small molecule inhibitor of CDK4/6. LY2835219 demonstrates suitable physical and pharmacokinetic (PK) properties, an acceptable toxicity profile in ...
RATIONALE: It is not yet know whether higher per daily radiation therapy is equally as effective as standard per daily radiation therapy in treating breast cancer.
PURPOSE: This randomized phase III trial studies how well an accelerated course of higher per daily radiation therapy with concomitant boost works compared to standard per daily radiation therapy with a sequential boost in treating patients with early-stage breast cancer that was removed by surgery.
In the light of the pandemic, institutions have had to take greater precautions and instigate procedures to aim to improve safety and reduce risk for patients undergoing surgery. One intiative was designed to implement a same day discharge for patients undergoing mastectomy with or without alloplastic reconstruction. This study aims to evaluate the outcomes and patient satisfaction with same day mastectomy with or without alloplastic reconstruction following COVID-19 and compare satisfaction and outcomes (e.g complications) with patients pre-COVID 19. This is part of a quality improvement project.
This is a phase 1b/2 study of the safety and efficacy of MLN0128 in combination with exemestane or fulvestrant therapy in women with estrogen receptor positive/human epidermal growth factor receptor 2 negative (ER+/HER2-) advanced or metastatic breast cancer that has progressed on treatment with everolimus in combination with exemestane or fulvestrant.
The purpose of this study is to see if having different kinds of bacteria genes in breast tissue may be connected to the risk of getting breast cancer.
The purpose of this study is to investigate the mechanical properties of breast tissue on MRE (including stiffness, elasticity, viscosity, and volumetric stain) to find any correlation between these variables with breast density and background parenchymal enhancement, both of which are considered independent risk factors for breast carcinoma. We can also assess any suspicious lymph nodes noted on diagnostic MRI and correlate with stiffness values of the lymph nodes on MRI.
The purpose of this study is to assess the effectiveness of capivasertib + fulvestrant vs placebo + fulvestrant for the treatment of patients with locally advanced (inoperable) or metastatic HR+/HER2- breast cancer following recurrence or progression on or after Aromatase Inhibitor (AI) therapy.
The purpose of this study is to evaluate the addition of 2 years of palbociclib to standard adjuvant endocrine therapy for patients with HR+ / HER2- early breast cancer to determine whether the addition of palbociclib will improve outcomes over endocrine therapy alone.
To determine the maximally tolerated dose (MTD) of intratumoral administration of an Edmonston strain measeles virus genetically engineered to express NAP (MV-s-NAP) in patients with metastatic breast cancer; to determine the safety and toxicity of on-time and serial administration of MV-s-NAP in patients with metastic breast cancer.
This research study is being done to determine if a low vitamin D level and mammographic density may be risk factors for developing breast cancer.
The purpose of this study is to compare the detection sensitivity of positron emission mammography to contrast-enhanced breast MRI in women with a high suspicion of breast cancer.
The purpose of this study is to evaluate a low-cost Contrast Enhanced Digital Mammogram (CEDM) protocol as a supplemental screening method to standard mammographic screening in women at intermediate lifetime-risk (and not undergoing annual MR surveillance) for breast cancer.
The purpose of this study is to evaluate the safety and effectiveness of the da Vinci Surgical Systems in Nipple Sparing Mastectomy procedures.
The purposes of this study are (i) to obtain and study biospecimens from patients with breast cancer that has either spread out of the breast or recurred after initial treatment(s), such as surgery, chemotherapy, and/or radiation, and (ii) to collect information about patients, treatments, and the behavior of the underlying cancer. Research involving biospecimens that are linked to related medical information is one way to learn more about diseases. In this case, we seek to understand the mechanism of tumor spread and determine why people respond differently to specific cancer treatments. In general terms, scientists will study the cells, DNA, ...
The aim of this study is to use the combine clinical risk assessment models that are already used in routine clinical practice with information derived from polygenic risk score (PRS) testing in women of racial minorities to see if this can improve adherence to recommended breast cancer screening and prevention strategies.
The purpose of this study is to determine whether a supervised exercise-training program, initiated prior to chemotherapy induction (pre-conditioning) and continued throughout chemotherapy treatment, can preserve short- and long-term cardiovascular performance, skeletal muscle function, cognitive ability and quality of life better than current standard or care recommendations for exercise during chemotherapy.
The objective of this study is to assess efficacy and safety of radium 223 dichloride in subjects with human epidermal growth factor receptor 2 (HER2) negative hormone receptor positive breast cancer with bone metastases treated with exemestane and everolimus.
Estrogen can cause the growth of breast cancer cells. Hormone therapy using tamoxifen citrate may fight breast cancer by blocking the use of estrogen by the tumor cells
This phase IIb trial studies how well low-dose tamoxifen citrate works in reducing breast cancer risk in radiation-induced cancer survivors.
This trial studies how well paclitaxel, trastuzumab, and pertuzumab work in eliminating further chemotherapy after surgery in patients with HER2-positive stage II-IIIa breast cancer who have no cancer remaining at surgery (either in the breast or underarm lymph nodes) after pre-operative chemotherapy and HER2-targeted therapy. Drugs used in chemotherapy, such as paclitaxel, work in different ways to stop the growth of tumor cells, either by killing the cells, by stopping them from dividing, or by stopping them from spreading. Trastuzumab and pertuzumab are both a form of "targeted therapy" because they work by attaching themselves to specific molecules (receptors) on ...
The purpose of this study is to determine whether treatment with alpelisib in combination with nab-paclitaxel is safe and effective in subjects with advanced triple negative breast cancer (aTNBC) who carry either a PIK3CA mutation (Study Part A) or have PTEN loss without PIK3CA mutation (Study Parts B1 and B2)
The primary objective of this study is to demonstrate that the combination of palbociclib with anti-HER2 therapy plus endocrine therapy is superior to anti-HER2-based therapy plus endocrine therapy alone in improving the outcomes of subjects with hormone receptor-positive, HER2+ metastatic breast cancer.
The purpose of this trilal is to study a combination of neoadjuvant radiotherapy (RT), immunotherapy (pembrolizumab) and chemotherapy for lymph node-positive, triple negative (TN) or hormone receptor positive/HER2-negative breast cancer.
The primary purpose of this study is to investigate the relationship between a technology-assisted diet and exercise program which is easily implemented in an outpatient setting and the levels of biomarkers that have been associated with breast cancer recurrence risk in overweight women with stage 0, I, or II breast cancer.
The purpose of this study is to explore the effectiveness of massage therapy combined with acupuncture in breast cancer patients recovering from autologous tissue reconstruction with the hope that the combination will augment the benefit obtained by massage therapy alone.
The optimal dose and fractionation regimen for whole breast irradiation, whole breast and regional nodal irradiation, and postmastectomy radiotherapy remains unknown. The goal of this phase II randomized controlled trial is to determine whether the hypofractionated proton regimens proposed are non-inferior compared with standard fractionated proton radiotherapy and therefore worthy of further investigation.
This randomized phase III trial has several primary objectives. One primary objective is to compare the efficacy of 3 different endocrine therapies, the estrogen receptor down regulator fulvestrant and the aromatase inhibitor anastrozole, either alone or in combination, in reducing cancer growth before surgery (neoadjuvant) in postmenopausal women with clinical stage II-III estrogen receptor positive and HER2 negative breast cancer. Another primary objective is to evaluate whether patients who achieved a modified PEPI (Preoperative Endocrine Prognostic Index) score of 0, defined by tumor size
The purpose of this study is to determine the feasibility and perceived effectiveness of acupuncture for breast cancer-related symptoms.
The purpose of this study is to evaluate digital tomosynthesis (3-D) mammography and digital mammography in screening patients for breast cancer. Screening for breast cancer with tomosynthesis mammography may be superior to digital mammography for breast cancer screening and may help reduce the need for additional imaging or treatment.
The purpose of this study is to examine the effectiveness and safety of pembrolizumab as first line or above treatment in patients with metastatic triple-negative breast cancer.
The primary purposes of this study are to is to assess safety and tolerability of IV administration of TVH vaccine alone and in combination with HER2 antibodies in patients with advanced cancer.
This research is being done to determine if early changes on a type of imaging procedure called PET (Positron Emission Tomography) can predict which patients are most likely to respond to the combination of trastuzumab and pertuzumab when given prior to surgery.
This randomized, double-blind, placebo-controlled, two-arm study will assess the safety and efficacy of pertuzumab in addition to chemotherapy plus Herceptin (t rastuzumab) as adjuvant therapy in patients with operable HER2-positive primary breast cancer. After surgery, patients will be randomized to receive either pertuzumab or placebo intravenously (iv) every 3 weeks for one year, in addition to 6-8 cycles of chemotherapy and 1 year of Herceptin (trastuzumab) iv every 3 wee ks. Anticipated time on study treatment is 52 weeks. This study will be carried out in collaboration with the Breast International Group (BIG).
The primary objective of this study is to compare progression-free survival (PFS) in patients with advanced HER2-positive breast cancer treated with T-DM1 and abemaciclib vs. T-DM1 monotherapy.
The purpose of this study is to evaluate combining endocrine therapy with CDK4/6 inhibition along with trastuzumab in ER+/ human epidermal growth factor receptor 2 (HER2)+ early stage breast cancer in order to influence estrogen receptor (ER) signaling.
The purpose of this study is to determine if the invasive disease-free survival (iDFS) with T-DM1 and tucatinib is superior to the iDFS in the control arm (T-DM1 + placebo) when administered to high risk patients with HER2-positive breast cancer and residual disease after neoadjuvant HER2-directed therapy.
This phase II trial studies how well FASN inhibitor TVB-2640 and trastuzumab plus either paclitaxel or endocrine therapy with an aromatase inhibitor work in treating patients with HER2 positive breast cancer that has spread to other places in the body. FASN inhibitor TVB-2640 may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. Drugs used in chemotherapy, such as paclitaxel and trastuzumab, work in different ways to stop the growth of tumor cells, either by killing the cells, by stopping them from dividing, or by stopping them from spreading. Endcocrine therapy helps reduce ...
The purpose of this study is to test if a culturally tailored virtual navigation program (Second-Life) meet the needs of Black women in learning more about breast health and care for cancer prevention, and mammography screening. We are asking for feedback on the Second-Life experience to determine if the information is easy to understand, relevant, and valuable to the community. The goal is to develop virtual community navigation that helps Black women make informed decisions about their breast healthcare since Black women have high mortality rates from breast cancer compared to other races or ethnicities.
The purpose of this study is to assess the effect of acculturation, socio-economic status (SES) and place of residence (urban vs. rural) on the level of participation in breast cancer screening programs and on the breast cancer knowledge and beliefs among Asian American women in Olmsted and surrounding counties.
This phase II trial studies how well alisertib with or without fulvestrant works in treating patients with endocrine-resistant breast cancer that has spread to other places in the body. Alisertib may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. Hormone therapy using fulvestrant may fight breast cancer by blocking the use of estrogen by the tumor cells or reducing the amount of estrogen made by the body. Giving alisertib with or without fulvestrant may be better in treating patients with breast cancer.
The purpose of this study is to assess the antitumor activity of TAS-120 as monotherapy or in combination with Fulvestrant in the treatment of patients with metastatic breast cancer harboring fibroblast growth factor receptor (FGFR) amplifications.
The purpose of this study is to evaluate pre-operative therapy that is specifically targeted for breast cancer in individuals with BRCA mutations. The drugs are Niraparib (Zejula) and Dostarlimab.
The purpose of the study is to alleviate the occurrence of chemotherapy-induced nausea (CIN) and to improve chemotherapy treatment outcomes. Recent research has shown that changes in the functions performed by the gut microbiome can cause the occurrence of chemotherapy-induced symptoms that include chemotherapy-induced nausea.
This randomized phase III trial studies how well hypofractionated radiation therapy works in preventing recurrence in patients with stage IIa-IIIa cancer who have undergone mastectomy. Hypofractionated radiation therapy delivers higher doses of radiation therapy over a shorter period of time and may kill more tumor cells that remain after surgery and have fewer side effects.
This research study is studying Ruxolitinib as possible treatment for Inflammatory Breast Cancer (IBC). The Following drugs will be use in combination with Ruxolinitinib. - Paclitaxel (also called Taxol) - Doxorubicin also called Adriamycin - Cyclophosphamide, also called Cytoxan
The significance of this study is that it will be the first prospective trial to compare MBI, a relatively low-cost functional breast imaging technique, to DBT, the new standard anatomic breast cancer screening technique in women with dense breasts. This study is also the first to evaluate two consecutive annual MBI scans to assess change in advanced cancer presentation after introduction of a functional imaging technique. These data will inform individualized decisions on supplemental screening and determine if a functional technique that is relatively low in cost and complexity of interpretation can eliminate the reservoir of clinically important breast cancers ...
The purpose of this study is to evaluate whether or not treatment with alternating 17B-estradiol / anti-estrogen therapies on a defined 8-week / 16-week schedule will more effectively prevent cancer growth than continuous treatment with either type of therapy in patients with metastatic anti-estrogen-resistant ER+ breast cancer.
The purpose of this study is to evaluate the change of vulvovaginal symptoms score from baseline to 6 months in premenopausal women with stage 0-III breast cancer treating with ovarian suppression in combination with aromatase inhibitors.
The purpose of this trial is to determine the safety of 15 fraction vs 25 fraction pencil beam scanning proton radiotherapy after mastectomy in patients requiring regional nodal irradiation. Proton therapy is recognized as a standard option for the delivery of radiotherapy for breast cancer.
This study is being performed to better understand the mechanisms behind severe radiation toxicity of a patient with severe fibrosis after breast radiation.
The investigators are conducting a longitudinal cohort study of young women with breast cancer. The investigators identify women age 40 and younger with newly-diagnosed breast cancer from academic and community healthcare institutions. After women consent to the study, they fill-out surveys and give blood samples, and the investigators collect tissue from their breast cancer tumor after it is removed. Women are surveyed every 6 months for the first 3 years after diagnosis, then yearly thereafter for an additional 7 years (for a total follow-up of at least 10 years following diagnosis). The study investigates short and long-term disease and treatment ...
A Phase II study to investigate the potential utility of PD 0332991 in the treatment of early stage ER+ Human epidermal growth factor receptor 2 (HER2)- breast cancer, to investigate whether the combination of PD 0332991 and anastrozole is able to: 1) improve the pathologic complete response rate when compared to the historical control of single agent aromatase inhibitors, 2) result in fewer patients with on therapy Ki67>10% compared to historical control.
This study is being done to determine the long-term effects of different kinds of breast surgeries on women’s health-related quality of life from the patient’s perspective. The information will also provide information to assist in improving the quality of care given to patients.
The purpose of this study is to determine if exercise improves cardiac remodeling as a way to mitigate the negative effects of chemotherapy and radiation therapy which lead to reduced exercise tolerance.
The purpose of this study is to culture human mammary cells to identify cellular characteristics associated with lobular involution status.
RATIONALE: Estrogen can cause the growth of breast cancer cells. Hormone therapy using letrozole may fight breast cancer by lowering the amount of estrogen the body makes. It is not yet known whether letrozole is more effective than a placebo in treating patients with hormone receptor-positive breast cancer.
PURPOSE: This randomized phase III trial is studying letrozole to see how well it works compared with a placebo in treating postmenopausal women who have received hormone therapy for hormone receptor-positive breast cancer.
Collection of blood to track serial circulating tumor cells (CTCs) in subjects with metastatic breast cancer (MBC). Study will also collect data from investigators are Mayo Clinic and the Mayo Clinic Health Systems to determine effectiveness of the proposed process.
The purpose of this study is:
- To assess whether a team based care model applied to distressed breast cancer patients will result in lower distress at 3, 6, 9 & 12 months compared to treatment as usual.
- To assess whether health promotion tools such as psychoeducation applied to non-distressed breast cancer patients will result in lower distress at 3, 6, 9 & 12 months compared to treatment as usual.
The purpose of this study is to evaluate how well multi-epitope folate receptor alpha peptide vaccine, sargramostim, and cyclophosphamide work in treating patients with triple negative breast cancer. Vaccines made from a person's white blood cells mixed with tumor proteins may help the body build an effective immune response to kill tumor cells. Drugs used in chemotherapy, such as cyclophosphamide, work in different ways to stop the growth of tumor cells, either by killing the cells, by stopping them from dividing, or by stopping them from spreading. Giving multi-epitope folate receptor alpha peptide vaccine, sargramostim, and cyclophosphamide may work better ...
The goal of this project is to develop an AI system for early cancer detection on routine screening tomosynthesis mammograms. There will be significant impact on clinical practices if a mammography AI tool can be built. Such a tool will improve efficiency and decrease false positives and false negatives in practice.
This protocol captures the details for the reader study involving Aidoc, ScreenPoint, and iCAD. Mayo Clinic Breast Imaging Radiologists will evaluate the software tools that both ScreenPoint and iCAD have developed for assisting in reading tomosynthesis scans. Aidoc is facilitating this study by providing their user ...
The goal of this research is to identify risk profiles of women (with particular emphasis on Hispanic women) for breast cancer based on family history, breast density and other factors known to impact risk such as age, weight, age at menarche, age at birth of first child, etc.
Rationale: Gathering medical information and tumor samples from patients with male breast cancer may help doctors learn more about the disease.
Purpose retrospective part: to perform a large international retrospective analysis of clinical and biological data of male BC patients treated in the participating centers from 1990 to 2010.
Purpose prospective part: to create a registry of men with breast cancer for a period of 30 months (starting early 2014).
The purpose of this study is to improve the interpretation of mutations in breast cancer predisposition genes. This will be accomplished by recruiting members of families found to carry deleterious (mainly protein truncating) mutations and evaluating co-segregation of the mutations with cancer within families.
This phase II study will test cancer to see if it has a HER2 mutation and, if so, see how HER2 mutated cancer responds to treatment with neratinib.
The purpose of this study is to evaluate the accuracy of diagnosis with contrast enhanced digital mammography when used in addition to standard mammography or ultrasound in patients with suspicious findings.
The purpose of this study is to measure the expression and frequency of the tumor tissue biomarkers (the genetics) of breast cancer, specifically the decreased presence and amount of a specific protein (Human epidermal growth factor receptor-2 [HER2]), how often genetic mutations occur, and why the cancer might or might not respond to monoclonal antibody therapy, such as trastuzumab emtansine (T-DM1) and/or pertuzumab.
The purpose of this study is to determine the diagnostic performance of the nonlinear elasticity parameter mapping method by associating its results with pathology in a population of patients with suspicious breast masses.
The purpose of this study is to determine the ability to capture PROs during patients’ radiation and during 3 months after.
Additionally, the study plans to conduct a retrospective chart review of prospectively accrued patients and ask subjects to complete a questionnaire.
The purpose of this study is to evaluate if Magseed is a viable alternative to radioactive seed as a localization method for biopsy proven metastatic breast carcinoma following neoadjuvant chemotherapy.
A pragmatic randomized clinical trial of patients with locally advanced breast cancer randomized to either proton or photon therapy and followed longitudinally for cardiovascular morbidity and mortality, health-related quality of life, and cancer control outcomes. Quality of life is the outcome measure for the estimated primary completion date of November, 2020.
The purpose of this study is to evaluate the use of molecular breast imaging as an accurate way to assess the response of breast cancer tumors to chemotherapy or hormone therapy that is newly supplemental to the standard treatment.
Efficacy and safety of treatment with alpelisib plus endocrine therapy in patients with HR+, HER2-negative aBC, with PIK3CA mutations, whose disease has progressed on or after CDK 4/6 treatment with an aromatase inhibitor (AI) or fulvestrant
The purpose of this study is to:
- To assess patient feelings and opinions on their variants
- To evaluate how the discovery of this variant has impacted relatives
- To evaluate how individuals would incorporate this knowledge into reproductive planning
- To create practice guidelines for handling this information in the future
The purpose of this study is to compare the effectiveness and safety of elacestrant to the standard of care (SoC) options of fulvestrant or an aromatase inhibitor (AI) in women and men with breast cancer whose disease has advanced on at least one endocrine therapy including a CDK4/6 inhibitor in combination with fulvestrant or an aromatase inhibitor (AI) .
The purpose of this study is to evaluate the impact of blood collection tube type and processing methods on ctDNA, evaluate the impact of long-term storage of plasma and extracted DNA, and evaluate ctDNA levels at baseline and during treatment for patients with Stage I-III breast cancer.
GRAIL is using high-intensity sequencing of circulating cell-free nucleic acids (cfNAs) to develop blood tests to detect cancer early. The purpose of this study is to train and validate an assay to detect invasive breast cancer in patients undergoing mammography.
There is evidence that mindfulness-based interventions (MBIs) such as meditation, mindfulness-based stress reduction (MBSR) and yoga might improve Quality of Life (QOL) and reduce stress in breast cancer survivors. These interventions are becoming increasingly popular in cancer survivors. However, little is known about the feasibility and effect of MBIs administered during the interval of time of chemotherapy, on QOL and stress. The investigators are planning a MBI intervention study developed specifically for breast cancer survivors receiving chemotherapy (usually 4-5 months) at the investigators institution, for at least 8 sessions combined with at least 8 weeks of home-practice, in 25 women ...
This study is being done to:
• Test an investigational stiffness measurement and imaging method on the lymph node found in the underarm area.
• Compare investigational imaging to sonography images.
• Compare investigational information to FDA approved US elasticity imaging conducted by SuperSonic Imaging (SSI) machine on the same lymph node in your underarm area.
• Compare to FDA approved ultrasound stiffness imaging system (GE Logiq E9)
• Compare to ultrasound images using Alpinion clinical ultrasound platform, FDA approved ECUBE 12and a non-FAD approved ECUBE 12R
The aim of this study is to examine alterations in the skin microbiome that occur during radiation therapy. The study design will examine changes secondary to ionizing radiation, and correlate these changes with the development and severity of radiation dermatitis. The goal is to improve understanding of the mechanism of radiation dermatitis.
The overall goal of this project is to study a new 3D ultrasound imaging technology for evaluation of axillary lymph nodes in patients with breast cancer.
The purpose of this study is to analyze the impact of active participation of patients in producing tones in combination with breathing technique; i.e., TBT to reduce aromatase inhibitor induced musculoskeletal symptoms.
Tonation Breathing Techniques (TBT) is a set of diverse, mostly non-strenuous, specialized breathing techniques with the addition of Tonation; i.e., controlled exhalation through nostrils or lips while producing and sustaining a constant sound frequency as is comfortable to the participant as instructed in Musopathy sessions and/or videos.
This randomized phase II/III trial studies how well standard of care therapy with stereotactic radiosurgery and/or surgery works and compares it to standard of care therapy alone in treating patients with breast cancer that has spread to one or two locations in the body (limited metastatic) that are previously untreated. Standard of care therapy comprising chemotherapy, hormonal therapy, biological therapy, and others may help stop the spread of tumor cells. Radiation therapy and/or surgery is usually only given with standard of care therapy to relieve pain; however, in patients with limited metastatic breast cancer, stereotactic radiosurgery, also known as stereotactic ...
This project will investigate whether ctDNA analysis in newly diagnosed stage I, II, III breast cancer patients treated with neoadjuvant systemic therapy can predict pathological Complete Response (pCR).
RATIONALE: Breast-conserving surgery is a less invasive type of surgery for breast cancer and may have fewer side effects and improve recovery. Radiation therapy uses high-energy x rays to kill tumor cells. Giving radiation therapy after surgery may kill any tumor cells that remain after surgery.
PURPOSE: This phase II trial studies how well breast-conserving surgery and radiation therapy work in treating patients with multiple ipsilateral breast cancer
The purpose of this study is to test whether a short course of aspirin can change the markers of inflammation in patients who have a benign finding within five years of their last pregnancy, and possibly reduce their risk of future breast cancer.
The purpose of this study is to interview patients and providers at Phoenix Indian Medical Center and Mayo Clinic Arizona to identify perceptions, experiences, and perceived factors influencing referrals and enrollment on clinical trials in the Department of Radiation Oncology at Mayo Clinic Arizona.
The overall goal of this line of research is to enhance the hospitality, cultural responsiveness, and efficiency with which a leading cancer center can collaborate with a neighboring treatment hub for an important, underserved population within that cancer center’s catchment area.
American Indian and Alaska Native people experience higher rates of cancer ...
The purpose of this study is to extend the follow up on the BEAUTY study (MC1137) cohort and collect additional blood samples to evaluate for minimal residual disease and tissue at the time of any breast cancer recurrence.
The purpose of this research is to evaluate the efficacy of a new breast tissue assessment tool that provides new diagnostic information about breast masses and potential for early classification of malignant masses.
• Test an investigational breast imaging system;
• Image the breast and see if we can differentiate lesions by using the investigational imaging system;
• Test an investigational breast stiffness measurement method
• Test and compare to an FDA approved ultrasound stiffness imaging system (GE LOGIQ E9 (LE9)
• Compare to ultrasound images using Alpinion ...
The purpose of this study is to gather qualitative information about patient comfort during MBI examinations. The primary aim is to assess patient comfort during MBI, relative to comfort during a mammogram. We also wish to identify factors that contribute to discomfort and patients’ willingness to have MBI in the future.
The short-term goal of the proposed research is to develop a new method for viscoelasticity imaging of breast that can work with any type of wave, and not restricted to plane shear waves.
The long-term goal of this project is to develop an ultrasound-based breast imaging technique to improve the diagnostic specificity in breast cancer.
A Phase I, Multicenter, Open-label, Dose-Escalation, Safety, Pharmacokinetic and Pharmacodynamic Study of Minnelide™Capsules given daily for 21 days followed by 7 days off schedule in patients with Advanced Solid Tumors
This study proposes to combine data from six large existing epidemiology studies to examine the association of MD and clinical risk factors with breast cancer subtypes.
This randomized phase III trial studies whether weight loss in overweight and obese women may prevent breast cancer from coming back (recurrence). Previous studies have found that women who are overweight or obese when their breast cancer is found (diagnosed) have a greater risk of their breast cancer recurring, as compared to women who were thinner when their cancer was diagnosed. This study aims to test whether overweight or obese women who take part in a weight loss program after being diagnosed with breast cancer have a lower rate of cancer recurrence as compared to women who do not take ...
This is a two arm Phase III trial in first and second-line HER2 negative patients with locally recurrent or metastatic breast cancer. The primary endpoint is overall survival (OS), and the objective is to test for the superiority of eribulin mesylate over standard weekly paclitaxel. Patients will be randomized between the experimental and control arm with equal allocation (1:1) within strata defined by prior adjuvant taxanes, hormone receptor status, and line of therapy. Subjects will continue protocol directed therapy until documentation of disease progression, development of unacceptable toxicity, or withdrawal of consent. Those who discontinue study treatment without radiological progression ...
The purpose of this study is to evaluate whether engineering gut microbiome using probiotics will alter host immunological response to breast and lung cancers.
This randomized phase II trial studies how well a controlled low calorie diet works in reducing side effects and increasing response to chemotherapy in patients with breast or prostate cancer. Drugs used in chemotherapy work in different ways to stop the growth of cancer cells, either by killing the cells or by stopping them from dividing. Eating a special diet with low calories may reduce the side effects of chemotherapy and improve the response to treatment
The purpose of this study is to gather measurements of mechanical properties of BI-RADS 4 and 5 lesions in order to test the effectiveness of MR elastography (MRE) in differentiating benign versus malignant disease. We will also test the mechanical properties of bilateral breast tissue on MRE to find any correlation with breast density on mammograms.
The purpose of this study is to help women in their late 30s and in their 40s, make decisions regarding breast cancer screening that align with each women’s values and preferences.
The purpose of this study is to to bring molecular risk prediction for breast cancer into the clinical arena through: the establishment of a large tissue repository from a retrospective cohort of women with benign breast disease with complete and long-term clinical follow-up to identify those who developed breast cancer (cases) and those who did not (controls); the application of potential biomarkers of risk to this archival tissue set; and, the discovery of new, potentially relevant biomarkers of risk in fresh and frozen specimens of benign breast disease.
This is an open-label, two-part, multiple study to evaluate the safety and tolerability of DS-8201a in patients with advanced solid malignant tumors.
The purpose of this study is to determine whether contrast-enhanced ultrasound (CEUS) can be used in diagnostic evaluation of breast lesions that cannot be seen using contrast-enhanced MRI (CEMR) and contrast- enhanced dual energy mammography (CEDM). If so, patients can undergo US guided biopsy which is more comfortable for patients and more cost effective.
The purpose of this research study is to understand the views and experiences of African American women with a diagnosis or who support a family member with breast or ovarian cancer. We also want to know participant thoughts on genetic testing for cancer risk.
This is a qualitative interview study. Study participation involves talking on the phone or videoconference (e.g., Zoom) for about 1 hour with a researcher.
The purpose of this study is to identify the recommended Phase 2 dose (RP2D) of LY3484356 administered as monotherapy and in combination with other anticancer therapies in patients with locally advanced or metastatic ER+ breast cancer or ER+ recurrent, persistent, or metastatic endometrial endometrioid cancer (EEC).
The purpose of this study is to find out if ginseng decreases fatigue in people who were treated for cancer.
The purpose of this study is to offer pre-approval drug access of iniparib combined with gemcitabine and carboplatin, in order to provide potential clinical benefit to patients who have ER-, PR-, and HER2-negative metastatic breast cancer.
The purpose of this study is to evaluate whether breast conservation surgery and endocrine therapy results in a non-inferior rate of invasive or non-invasive ipsilateral breast tumor recurrence (IBTR) compared to breast conservation with breast radiation and endocrine therapy.
The goal of the project is to develop and test a new 3D Doppler technology, with enhanced vessel sensitivity than conventional Doppler, to employ tumor vascularity as a differential biomarker for improved cancer diagnosis and reduced unnecessary biopsy on benign tumors
The primary objective of this study is to determine if exercise, fasting, or eating prior to the molecular breast imaging study will have an effect on the uptake of the tracer in the breast tissue.
This phase I/II trial studies the best dose of pembrolizumab and binimetinib and how well it works when giving together with pembrolizumab in treating patients with triple negative breast cancer that has spread to other parts of the body. Monoclonal antibodies, such as pembrolizumab, may block tumor growth in different ways by targeting certain cells. Binimetinib may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. Giving pembrolizumab and binimetinib may work better in treating patients with triple negative breast cancer.
An Open-Label, First-in-Human Study of the Safety, Tolerability, and Pharmacokinetics (PK) of M6620 in Combination With Cytotoxic Chemotherapy in Subjects With Advanced Solid Tumors
This study will examine the safety and tolerability of SGN-LIV1A in patients with metastatic breast cancer. SGN-LIV1A will be given every 3 weeks alone or in combination with trastuzumab.
This phase II trial is studying giving veliparib together with carboplatin to see how well they work compared to veliparib alone in treating patients with stage III or stage IV breast cancer. Veliparib may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. Drugs used in chemotherapy, such as carboplatin, work in different ways to stop the growth of tumor cells, either by killing the cells or by stopping them from dividing. It is not yet known whether veliparib is more effective with or without carboplatin in treating breast cancer.
The purpose of this study is to evaluate the safety and effectiveness of combined treatment with niraparib and pembrolizumab (MK-3475) in patients who have triple-negative breast cancer that is advanced or has spread, or ovarian cancer that has returned after previous treatment.
The purpose of this study is to determine the safety and tolerability of OBT076, and to define the maximum tolerated dose (MTD) and/or the RP2D of OBT076.
The purpose of this research is to determine if previously adding a medication by the name of bevacizumab to the current standard chemotherapy of cancer-reducing medications, namely doxorubicin, cyclophosphamide and paclitaxel, reduces the risk of recurrence (called disease-free survival) compared to standard chemotherapy alone.
The purpose of this research study is to see if Atorvastatin(Lipitor) 40 mg by mouth daily decreases the chance of developing heart problems in women who are receiving anthracycline-based chemotherapy for breast cancer.
The purpose of this study is to explore, through surveys of providers and patients, the frequency and nature of shared decision making aspects in the cardiovascular care of cancer patients seen in the cardio-oncology clinic referred by an oncology provider.
The purpose of this study is to evaluate how well the combination of avelumab with liposomal doxorubicin with or without binimetinib, or the combination of avelumab with sacituzumab govitecan works in treating patients with triple negative breast cancer that is stage IV or is not able to be removed by surgery (unresectable) and has come back (recurrent).
The purpose of this study is to learn about potential side effects facing people who are undergoing treatments for their cancer, specifically, hair loss. While this is not a well-documented side effect of hormone-blocking medications (such as tamoxifen, letrozole, anastrozole, or exemestane), we have preliminary evidence that it is a problem for some patients getting this treatment. This study will include some patients receiving the hormone therapy and some patients who are not, so we can better understand whether patients getting the hormonal therapy have more hair loss than patients who are not getting such.
RATIONALE: Drugs used in chemotherapy use different ways to stop tumor cells from dividing so they stop growing or die. Combining more than one drug may kill more tumor cells. It is not yet known which combination chemotherapy regimen is more effective for breast cancer.
PURPOSE: Randomized phase III trial to compare the effectiveness of two combination chemotherapy regimens in treating women with breast cancer who have undergone surgery to remove the tumor.
The purpose of this study is to to establish a minimally-invasive blood based test for the detection of clinically actionable genetic changes in breast cancer patients.
The study is designed as an open-label, randomized, parallel, two arm, multicenter, international Phase 3 study in patients with recurrent or metastatic breast cancer previously treated with cytotoxic chemotherapy regimens.
The primary study objective is to compare overall survival of patients who receive NKTR-102 given once every 21 days to patients who receive treatment of Physician's Choice selected from a list of seven single-agent intravenous therapies.
The purpose of this study is to collect and correlate the data from various cardiovascular risk asessments to successfully measure the risk of heart toxicity in patients receiving chemotherapy for breast cancer.
The purpose of this study is to determine if Brain Natriuretic Peptide (NT-pro-BNP) values increase over time in breast cancer survivors and correlate with cardiac dysfunction. This study will define the average NT-pro-B-natriuretic peptide values in female breast cancer patients 1, 2, 3, 4, and 5 years out from anthracycline-based chemotherapy.
This phase I trial studies the side effects and the best dose of Z-endoxifen hydrochloride in treating patients with estrogen receptor-positive (ER+) breast cancer that has spread to other places in the body (metastatic) or has come back at or near the same place as the original tumor (locally recurrent). Estrogen can cause the growth of breast cancer cells. Hormone therapy using Z-endoxifen hydrochloride may fight breast cancer by blocking the use of estrogen by tumor cells.
This research trial studies genetic profiles in blood and tumor samples from patients with estrogen receptor positive and HER2 negative breast cancer that has spread to other places in the body who are receiving palbociclib and endocrine therapy. Examine the genetic changes associated with the cancer and comparing the genetic material from the cancer tissue with the genetic material found in the blood may help doctors to develop customized treatment for breast cancer.
The best available evidence suggests that pregnancy after breast cancer does not increase a woman's risk of developing a recurrence from her breast cancer. In particular, the most recent data suggest that this is the case also in women with a hormone receptor-positive breast cancer. There is also no indication of increased risk for delivery complications or for the newborn. The aim of the study is to investigate if temporary interruption of endocrine therapy, with the goal to permit pregnancy, is associated with a higher risk of breast cancer recurrence.The study aims also to evaluate different specific indicators related to ...
Lymphaticovenous anstomosis is an effective surgery to treat lymphedema in the upper extremities secondary to cancer treatment. A crucial step is to identify patent lymphatic channels. Contrast enhanced ultrasound (CEUS) with intradermal injection of microbubbles is a promising method for lymphatic mapping in the upper extrmeities with lymphedema. The goals of the study are(1) to establish the preferred FDA approved microbubble agent (Lumason, Optison, Definity) for CEUS lymphatic mapping, (2) to identify lymphatic channels with CEUS and high-frequency ultrasound in patients receiving lymphaticovenous anastomosis surgery for upper extremity lymphedema, (3) and to validate the use of shear wave elastography for detecting improving in ...
This is an international, multi-center, open-label, randomized, Phase III study in patients with metastatic TNBC refractory or relapsing after at least 2 prior chemotherapies (including a taxane) for their metastatic disease. Patients meeting eligibility will be randomized 1:1 to receive either sacituzumab govitecan or treatment of physician choice (TPC), which needs to be selected prior to randomization from one of the 4 allowed regimens. Randomization will be stratified by number of prior chemotherapies for advanced disease (2-3 vs > 3) and geographical location (North America vs Europe). Patients will be treated until progression, unacceptable toxicity, study withdrawal, or death, whichever ...
The purpose of this study is to better understand the anatomy of the lymphatic structure and the molecular process that leads to the over production of lymph fluid. This proposal will begin intense lymphedema screening and identify baseline characteristics potentially predisposing someone to lymphedema, and identify molecular markers that might be altered to prevent lymphedema.
The purpose of this study is to examine the capability of contrast enhanced breast PCD-CT in staging breast cancer within the breasts and regional nodes of human subjects. Developing and using a PCD-CT imaging technique and postprocessing algorithms, dedicated for breast cancer detection.
The main purpose of this study is to evaluate how effective nonsteroidal aromatase inhibitors (NSAI) plus abemaciclib are in postmenopausal women with breast cancer.
The purpose of this study is to collect blood samples and fresh tissue from biopsies of metastatic lesions from Mayo Clinic patients with metastatic breast cancer. The biospecimens will be used for analysis of genetic alterations in germline and tumor DNA and for tracking of response to therapy using blood-based liquid biopsy approaches.
The Primary Aim of this study is to quantify BDTC in breast cancer patients at different stages of cancer. As part of this aim we will establish the proliferation status of the tumor cells. We will in parallel examine CTC to determine the correlation between BDTC and CTC. The Second Aim is to determine role of tumor associated immune responses in maintaining tumor dormancy. Knowledge gained will provide the rationale for an in depth study of breast cancer tumor dormancy and immune response. Ultimately, the information gained will help us to design of immune intervention strategies that prevent cancer recurrence.
The purpose of this studyis to assess how well tamoxifen citrate works in patients with metastatic or recurrent breast cancer. Estrogen can cause the growth of breast cancer cells. Hormone therapy using tamoxifen citrate may fight cancer by blocking the use of estrogen by tumor cells.
The purpose of this study is to evaluate the effectiveness of abemaciclib plus trastuzumab with or without fulvestrant or chemotherapy in women with hormone receptor positive (HR+), human epidermal growth factor receptor 2 positive (HER2+) locally advanced or metastatic breast cancer after prior exposure to at least two HER2-directed therapies for advanced disease.
A pragmatic randomized clinical trial of patients with locally advanced breast cancer randomized to either proton or photon therapy and followed longitudinally for cardiovascular morbidity and mortality, health-related quality of life, and cancer control outcomes. Quality of life is the outcome measure for the estimated primary completion date of November, 2020."
Objective: To determine the Overall Response Rate (ORR) to Imprime PGG + pembrolizumab in subjects with advanced melanoma or metastatic TNBC
Safety: To characterize the safety of Imprime PGG + pembrolizumab given in combination
Hypothesis: Restore (for melanoma) or enhance (for TNBC) sensitivity to checkpoint inhibitors (CPI) by appropriate and effective stimulation of the subject's innate and adaptive immune systems in those subjects who have failed 1st line therapy
The study will incorporate Simon's optimal 2-stage design with sample size fixed at 12 subjects each in Stage 1 for advanced melanoma and for Triple Negative Breast Cancer ...
The treating physician/investigator contacts Lilly when, based on their medical opinion, a patient meets the criteria for inclusion in the expanded access program.
The purpose of this study is to correlate the parathyroid hormone-related peptide (PTHrp) levels in the current and new assays in patients with known disease and calcium status.
RATIONALE: Drugs used in chemotherapy use different ways to stop tumor cells from dividing so they stop growing or die. Combining more than one drug may kill more tumor cells. Monoclonal antibodies such as trastuzumab can locate tumor cells and either kill them or deliver tumor-killing substances to them without harming normal cells.
PURPOSE: Phase II trial to study the effectiveness of combining paclitaxel, carboplatin, and trastuzumab in treating women who have metastatic breast cancer that overexpresses HER2.
The purpose of this study is to look at the effects cancer and melanoma have on the immune cells found in lymph nodes.
The purpose of this study is to evaluate different strategies of cardiovascular therapy with Carvedilol, aiming to reduce the incidence of left ventricular ejection fraction (LVEF) decline and heart failure (HF) in patients undergoing curative intent Trastuzumab for breast cancer.
The purpose of this study is to assess the side effects and best dose of ruxolitinib phosphate when given together with pembrolizumab for treating patients with stage IV triple negative breast cancer that has spread to other places in the body. Monoclonal antibodies, such as pembrolizumab, may interfere with the ability of tumor cells to grow and spread. Ruxolitinib phosphate may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. Giving pembrolizumab and ruxolitinib phosphate together may work better in treating patients with stage IV triple negative breast cancer.
The goal of this study is to perform a quantitative measure of lobular involution (qLI) from breast biopsy samples obtained at baseline (surgery) and 12 months or at time of study discontinuation. The study will determine breast density (MMG) and correlate with qLI. The study will also determine the influence of chemoprevention therapies such as tamoxifen and letrozole on qLI.
This phase I trial studies the side effects and best dose of olaparib and onalespib when given together in treating patients with solid tumors that have spread to other places in the body or cannot be removed by surgery or ovarian, fallopian tube, primary peritoneal, or triple-negative breast cancer that has come back. Olaparib and onalespib may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth.
The purpose of this study is to assess the side effects and effectiveness of s-adenosyl-L-methionine for treating hot flashes in women who have a history of breast cancer or who do not wish to take estrogen due to a perceived increased risk of breast cancer.
The purpose of this research is to see how well fruquintinib works in combination with tislelizumab in participants with metastatic colorectal cancer (mCRC).
The purpose of part one of this study is to determine the maximum tolerated dose (MTD), dose-limiting toxicities (DLTs), and safety profile of Q901 monotherapy when administered via intravenous (IV) infusion once-weekly (QW) for 4 weeks and once every 2 weeks (Q2W) thereafter. Also, to establish for future clinical development the recommended Phase 2 dose (RP2D) of Q901 monotherapy when administered via IV infusion QW for 4 weeks and Q2W thereafter.
The purpose of part two of this study is to evaluate safety and tolerability and evidence of anticancer activity of Q901 as monotherapy and in combination with pembrolizumab. In Part 2 Cohort 1, ...
The purpose of this study is to compare conventional assessment of systolic ventricular function on a 2D heart echo with an assessment of more immediate changes in heart mechanics using 2D and 3D measurements of deformation and strain in patients undergoing infusion of Trastuzumab for breast cancer.
The purpose of this study is to evaluate SV-BR-1-GM in metastatic or locally recurrent breast cancer patients, in combination with the PD-1 inhibitor INCMGA00012 and the IDO inhibitor epacadostat. Patients who with advanced breast cancer who have failed prior therapies will be eligible to enroll in this study. The study will evaluate SV-BR-1-GM in combination with INCMGA00012 and epacadostat. Treatment cycles will be every 3 weeks with evaluations for tumor progression or response every 6-12 weeks.
To evaluate the safety and tolerability of AMG 650 in adult participants and to determine the maximum tolerated dose (MTD) and/or recommended phase 2 dose (RP2D).
The purpose of this study is to evaluate the safety and tolerability of LYL797, a ROR1-targeted CAR T-cell therapy, in patients with ROR1+ relapsed or refractory triple negative breast cancer (TNBC) or non-small cell lung cancer (NSCLC). The first part of the study will determine the safe dose for the next part of the study, and will enroll TNBC patients only. The second part of the study will test that dose in additional TNBC patients and NSCLC patients.
The overall goal is to investigate the value of ultrasound imaging of small vasculature as a new biomarker for cancer characterization and early treatment evaluation.
The purpose of this study is to compare the effectiveness of two different combinations of chemotherapy in treating women who have stage II or stage IIIA breast cancer that has spread to the lymph nodes. Drugs used in chemotherapy use different ways to stop tumor cells from dividing so they stop growing or die. It is not yet known which regimen of chemotherapy is more effective for breast cancer.
The purpose of this study is to evaluate the safety and feasibility of the spinal array in treatment of patients with leptomeningeal metastases within the spine
The median survival of patients with LM with treatment is generally less than 5 months. There are four FDA-approved drugs for intra-CSF use in LM, but all have shown limited activity with no clear increase in survival outcome with treatment. Intra-CSF treatment is also invasive, involving either surgical placement of an intraventricular reservoir, or treatment (intrathecal) via repetitive lumbar punctures, and there is risk of adverse events including vomiting, headache, arachnoiditis and ...
The purpose of this study is to analyze the effect of sacituzumab govitecan in treating patients with HER2-negative breast cancer that has spread to the brain (brain metastases). Sacituzumab govitecan is a monoclonal antibody, called sacituzumab, linked to a chemotherapy drug, called govitecan. Sacituzumab is a form of targeted therapy because it attaches to specific molecules on the surface of cancer cells, known as Trop-2 receptors, and delivers govitecan to kill them. Giving sacituzumab govitecan may shrink the cancer in the brain and/or extend the time until the cancer gets worse.
The purpose of this study is to evaluate different strategies of cardiovascular therapy with carvedilol aiming to reduce the incidence of heart function declines and heart failure in at-risk breast cancer patients while on trastuzumab therapy.
The purpose of this study is to analyze the prevalence of mood disorders in newly-diagnosed breast cancer patients with use of specific questionnaires, aimed to diagnose clinically significant depression and anxiety, at a rural community hospital.
This study is being done to gather information. The study will provide important information related to the safety and the effect of the vaccine on a patient's immune system. What researchers learn from this study could possibly be used in the future to prevent or delay recurrence of breast or ovarian cancers.
The purpose of this study is to evaluate dose escalation and expansion of MT-5111 (a recombinant fusion protein) in subjects with HER2-positive solid tumors.
The purpose of this study is to assess the safety of PH FDC SC when administered at home by an HHNP. Patients will be assessed for safety by regular evaluation of AEs, vital signs, routine clinical laboratory tests (hematology, blood chemistry), LVEF assessments, and by physical examinations.
This study is a process evaluation of a digital storytelling (DST) intervention. Because the DST intervention has not yet been developed, it is appropriate to use qualitative methods to assess the process.
This study will recruit gifted storytellers for a digital storytelling intervention on breast, cervical, and colorectal cancer. A digital storytelling intervention will be developed to improve breast, cervical, and colorectal cancer screening rates among Hispanic, Spanish speaking individuals. Additionally, to conduct a qualitative assessment of the digital storytelling workshop.
The purpose of this study is to determine the maximum dose of LDE225 and BKM120 that can be safely given together to patients and/or the dose that will be used in future studies. This study will also learn more about how the combination of these two investigational drugs may work for patients with certain cancers (specifically metastatic breast cancer, advanced pancreatic adenocarcinoma, metastatic colorectal cancer and recurrent glioblastoma multiforme).
The purpose of this study is to estimate the circulating tumor DNA (ctDNA)detection rate and mutational load in breast cancer patients with indications for regional nodal irradiation.
The purpose of this study is to analyze breast tissue changes after a short course of Tamoxifen (Tam).
The purpose of this study is to to map out the extent of Skin Angiosarcoma disease using ultrasound. This will be compared to the MRI and or PET/CT and with clinical and photographic determination of disease extent confirmed with clinically requested punch biopsies. The patient will be scanned with a commercially avilable GE Logiq 10 machine and then with the 25-30 MHz linear microvessel transducer for microvessel imaging. These scans will be obtained pretreatment, after 2 cylces of neoadjuvant chemotherapy and after completion of neoadjuvant chemotherapy and radiation therapy prior to surgery.
This phase II trial studies how well biopsy of breast after chemotherapy works in predicting pathologic response in patients with stage II-IIIA breast cancer undergoing breast conserving surgery. Tumor tissue collected from biopsy before surgery may help to check if chemotherapy destroyed the breast cancer cells and may be compared to the tumor removed during surgery to check if they are the same.
The purpose of this research study is to determine how well neratinib works in treating breast cancer that has spread to the brain. Neratinib is a recently discovered oral drug that may stop breast cancer cells from growing abnormally by inhibiting (or blocking) members of a family of proteins that include Human Epidermal Growth Factor Receptor 2 (HER2).
In this research study, the investigators are looking to see how well neratinib works to decrease the size of or stabilize breast cancer that has spread to the brain. The investigators are also looking at how previous treatments have affected your ...
The purpose of this study is to compare the use of Bioimpedance Spectroscopy versus tape measurements for follow-up arm measurements after regional treatment for breast cancer. Catching the smallest increases in fluid buildup and intervening early may result in a decrease in the rate of progressions to chronic lymphedema.
The purpose of this study is to assess safety of nab-paclitaxel based chemotherapy regimens administered prior to and/or in combination with nivolumab in Pancreatic Cancer, Non Small Cell Lung Cancer (NSCLC) and Metastatic Breast Cancer (mBC).
The purpose of this study is to determine the safety, effcicacy and tolerability of H2NVAC in patients with HER2-expressing DCIS in order to prevent future invasive breast cancer among patients who are diagnosed with DCIS.
The purpose of this study is to assess the use of an audio recording containing positive suggestion as a means to provide needed psychological support to critically ill patients in a feasible and reliable manner.
The purpose of this study is to investigate the antitumor activity of navicixizumab monotherapy or in combination with paclitaxel or irinotecan in patients with advanced solid tumors including:
- Cohort A: Colorectal cancer (CRC);
- Cohort B: Gastric and gastroesophageal junction (GEJ) cancer;
- Cohort C: Triple-negative breast cancer (TNBC);
- Cohort D: Platinum-resistant/refractory epithelial ovarian, primary peritoneal, or fallopian tube cancer (ovarian cancer).
We aim to determine if Molecular Breast Imaging (a new nuclear medicine technique developed at Mayo) can identify malignant breast lesions in women who have atypical ductal hyperplasia, atypical lobular hyperplasia, or lobular carcinoma in situ.
The purposes of this study are to to determine the physical, cognitive, emotional, and social health outcomes and trajectory of recovery in a population of children post-critical illness, to determine the baseline health, presenting problem, and PICU factors associated with impaired physical, cognitive, emotional, and social outcomes among PICU survivors, and to determine the emotional and social health outcomes in parents and siblings of PICU survivors. Our primary goal is to explicate the impact of pediatric critical illness over a two-year period of time to guide future intervention research to optimize child and family outcomes. Our overall goal is to improve ...
A Phase 1a/1b Open-Label Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of PY314 as a Single Agent and In Combination with Pembrolizumab in Subjects with Advanced Solid Tumors
This study looks at the risks and benefits of active surveillance (AS) compared to guideline concordant care (GCC) in the setting of a pragmatic prospective randomized trial for low risk DCIS. Our overarching hypothesis is that management of low-risk Ductal Carcinoma in Situ (DCIS) using an AS approach does not yield inferior cancer or quality of life outcomes compared to GCC.
The purpose of this study is to evaluate the timing of AVB-620 administration relative to surgery on the fluorescence and accuracy of the AVB-620 imaging data to distinguish between malignant and nonmalignant tissues in women undergoing surgery with primary, nonrecurrent and nonmetastatic breast cancer.
The purpose of this study is to determine the recommended Phase 2 dose (RP2D) of TBio-6517 when administered by direct injection into tumor(s) alone and when combined with pembrolizumab in patients with solid tumors (RIVAL-01).
In this research study, the investigators are testing a new type of breast camera, called Molecular Breast Imaging, to see if it can find tumors in the subject's breast.
This randomized phase IIB trial studies how well tamoxifen or afimoxifene works in treating patients with estrogen receptor positive breast cancer. Estrogen can cause the growth of breast cancer cells. Hormone therapy using tamoxifen citrate or afimoxifene may fight breast cancer by blocking the use of estrogen by the tumor cells.
Over a third of those who survive critical illness suffer from symptoms of anxiety, depression, or post-traumatic stress disorder (PTSD) after leaving the intensive care unit (ICU). This is twice as high as the rates of PTSD in combat veterans. The strongest risk factor is memories of frightening experiences and delusions (something that is very common during critical illness, when patients feel that something is real when it is actually not). Patients can hear speech even when sedated, yet there is no systematic communication with the critically ill while they receive life-saving medical treatments. We think that lack of real-time communication ...
The purpose of this study is to evaluate whether post-ICU assessment predicts PICS at 3 months and to validate a screening tool to identify patients at risk for PICS.
The purpose of this study is to develop a breast cancer survivor (BCS) multiscale omics database that includes a complete fecal metagenome (gut microbiome) and fecal metabolome characterization.
The purpose of this study is to assess the safety of in vivo in host drug sensitivity testing in patients with breast cancer and patients with lymphoma (nodal, extranodal masses, or cutaneous lesions).
RATIONALE: Estrogen can cause the growth of breast cancer cells. Hormone therapy using letrozole may fight breast cancer by blocking the use of estrogen by the tumor cells or by lowering the amount of estrogen the body makes.
PURPOSE: This phase II trial is studying how well letrozole works in treating women with ductal carcinoma in situ.
RATIONALE: Giving calcium together with magnesium may stop or delay the development of peripheral neuropathy in patients with cancer who are receiving treatment with ixabepilone. It is not yet known whether calcium and magnesium are more effective than a placebo in preventing peripheral neuropathy caused by ixabepilone.
PURPOSE: This randomized phase III trial is studying calcium given together with magnesium to see how well it works compared with a placebo in preventing peripheral neuropathy caused by ixabepilone in patients with breast cancer.
This is a Phase I study to understand the biodistribution of MM-398 and to determine the feasibility of using Ferumoxytol as a tumor imaging agent.
This randomized phase II study aims to investigate whether the addition of bevacizumab to standard corticosteroid therapy results in greater improvement in symptoms and less treatment-induced symptoms compared with standard corticosteroid therapy for patients with symptomatic brain radionecrosis following radiosurgery. It is hypothesized that the addition of bevacizumab to standard care corticosteroids will reduce treatment-induced toxicities and improve neurologic impairments in patients with brain radionecrosis following radiosurgery for brain metastases.
The purpose of this study is to evaluate the safety, tolerability, feasibility, and preliminary effectiveness of the administration of genetically-modified autologous T cells (CART-TnMUC1 cells) engineered to express a chimeric antigen receptor (CAR) capable of recognizing the tumor antigen, TnMUC1 and activating the T cell (CART- TnMUC1 cells).
The purpose of this trial is to evaluate the safety of GEN1046 in patients with malignant solid tumors.
The purpose of this research study is to test whether metformin, a drug commonly used to treat diabetes, is able to get rid of atypia (early cell changes that are thought to be a marker of breast cancer risk) in women at increased risk for breast cancer. There will be testing for the presence of atypia in the breast after metformin is given to see if it can get rid of atypia. The study will compare the effects, good and/or bad, of metformin or placebo on atypia to find out which is better. Note: The standard drug used for the ...
This is a Phase 1 multi-center study to evaluate the clinical safety and immune response of ID-LV305 when injected intradermally in patients with advanced cancer. ID-LV305 is a novel immunotherapy agent designed to target dendritic cells and stimulate the body's immune system to fight the spread and growth of cancer for patients whose tumors express the NY-ESO-1 protein. Patients with melanoma, sarcoma, ovarian cancer, or non-small cell lung cancer that express NY-ESO-1 may be considered for the trial. Selected sites will be evaluating ID-LV305 with pembrolizumab for patients with melanoma who have inadequately responded to anti-PD-1 therapy.
The purpose of this study is to determine if counseling patients about low dose tamoxifen will influence the decision to take (or not take) preventive therapy among women at increased risk for breast cancer.
This phase I trial studies the side effects and the best dose of stereotactic body radiation therapy in treating patients with breast cancer, non-small cell lung cancer, or prostate cancer that has spread to other parts of the body. Stereotactic body radiation therapy delivers fewer, tightly-focused, high doses of radiation therapy to all known sites of cancer in the body while minimizing radiation exposure of surrounding normal tissue.
The purpose of this study is to test the safety and side effects of a drug called SGN-B7H4V in participants with solid tumors. A side effect is anything a drug does to the body besides treating the disease. Participants will have cancer that has spread in the body near where it started (locally advanced) and cannot be removed (unresectable) or has spread through the body (metastatic). This study will have three parts. Parts A and B of the study will find out how much SGN-B7H4V should be given to participants. Part C will use the dose found in Parts A ...
The purpose of this trial is to compare the effectiveness of psychological support based on positive suggestions (PSBPS) vs. usual care on mental health morbidity and cognitive function in survivors of critical illness.
The purpose of this study is to compare the effectiveness of fulvestrant to anastrozole or tamoxifen in treating invasive lobular breast cancer, by measuring the level of the biomarker Ki67 found in tumor tissue before and then after treatment.
The purpose of this study is to determine the safety, tolerability, activity, and drug/body interactions of Oradoxel for the treatment of patients who have advanced malignancies.
The purpose of this study is to determine if the use of Gilenya® can reduce neuropathy caused by paclitaxel.
The primary purpose of this study is to obtain de-identified, clinically characterized, whole blood specimens to evaluate biomarkers associated with cancer for diagnostic assay development.
THe purpose of this study is to examine the current and (potential) future therapeutic relevance of pharmacogenomics (PGx) testing for a cohort of cancer patients in order to improve quality of life (QOL) in patients receiving clinical care at Mayo Clinic.
The purpose for this study is to find out if MEDI5395 and durvalumab will work and be safe for the treatment of solid tumors.
The primary objectives for this study are:
- The percentage of subjects who can enroll on an A2 CAR T-cell therapy study within approximately 6 months of documentation of HLA-A LOH status
- The percentage of subjects who can enroll on an A2 CAR T-cell therapy study within approximately 12 months of documentation of HLA-A LOH status
- The percentage of subjects who can enroll on an A2 CAR T-cell therapy study within approximately 18 months of documentation of HLA-A LOH status
- The percentage of subjects who can enroll on an A2 CAR T-cell therapy study within approximately 24 months of HLA-A LOH status
- Percentage of screened subjects experiencing loss ...
The ultimate goal of this biobank will be to provide the resource to initiate an exploration of human saliva as a potential liquid biopsy for cancer detection and surveillance.
This randomized phase III trial studies how well oxybutynin chloride works in managing hot flashes in patients who are not candidates for, or not interested in hormone replacement therapy. Previous studies have shown that oxybutynin is effective in managing hot flashes, however doses used in prior studies have resulted in side effects. This trial is evaluating lower doses of oxybutynin with the goal of determining if they are efficacious with less side effects.
This study will recruit breast cancer patients and survivors to take part in an ongoing study of the issues and concerns surrounding breast cancer survivorship. We will recruit both newly diagnosed patients as well as patients diagnosed within the past 5 years. Those who consent to the study will be asked to provide a series of questionnaires and blood samples over time. These data/samples will create a repository that will enable us to address many specific hypotheses both now and in the future. As part of the study DNA samples will be genotyped for common genetic variants ...
The purpose of this multicenter prospective observational case-control study is to train and validate Adela’s cfMeDIP-seq based methylome profiling platform to detect and differentiate multiple cancer subtypes. In addition, this study includes longitudinal follow-up for a subset of participants to train and validate the methylome profiling platform to detect minimal residual disease and recurrence.
The purpose of this study is to demonstrate that rivaroxaban is superior to placebo for reducing the risk of lower extremity proximal deep vein thrombosis (DVT), asymptomatic lower extremity proximal DVT, symptomatic upper extremity DVT, symptomatic non-fatal pulmonary embolism (PE), incidental PE, and venous thromboembolism (VTE)-related death in ambulatory adult patients with various cancer types receiving systemic cancer therapy who are at high risk of developing a VTE.
Tinostamustine (EDO-S101) is a new chemical entity, an AK-DAC (a first-in-class alkylating deacetylase inhibiting molecule) that, in preclinical studies, has been shown to simultaneously improve access to the DNA strands within cancer cells, break them and block damage repair. This Phase 1/2 study will enroll patients with various advanced solid tumors.
The goal of this study is to further evaluate the effect of magnesium on the symptoms of menopause, specifically vasomotor symptoms (VMS) in breast cancer patients and/or women at an elevated risk of breast cancer.
The purpose of this study is to assess the safety, tolerability and pharmacokinetics (PK) of CYT-0851 in patients with relapsed/refractory B-cell malignancies and advanced solid tumors and to identify a recommended Phase 2 dose for evaluation in these patients.
The purpose of this study is to collect clinical and biomarker data from patients receiving neurotoxic chemotherapy who are at risk for developing Chemotherapy-induced Peripheral Neuropathy (CIPN).
The purpose of this study is to collect blood and tissue samples from patients with and without cancer to evaluate laboratory tests for early cancer detection which may help researchers develop tests for the early detection of cancers.
The purpose of this study is to characterize TRPC6 risk variants for doxorubicin-related cardiotoxicity in prospectively collected samples from breast cancer patients.
Breast cancer patients are more than three times at risk for developing congestive heart failure (CHF), compared with patients who did not have cancer. The increased risk of HF is observed as early as one year from diagnosis of cancer and overall, 7% of patients develop CHF (median follow-up 8.5 years)
GRAIL is using deep sequencing of circulating cell-free nucleic acids (cfNAs) to develop assays to detect cancer early in blood. The purpose of this study is to collect biological samples from donors with a new diagnosis of cancer (blood and tumor tissue) and from donors who do not have a diagnosis of cancer (blood) in order to characterize the population heterogeneity in cancer and non-cancer subjects and to develop models for distinguishing cancer from non-cancer.
The goal of the study is to create a database of clinical information and a repository of biological specimens for genetic, molecular and microbiological research to better understand hereditary cancer and help develop new therapies and preventive strategies.
The purpose of this study is to develop a biorepository of blood samples from cancer patients participating in the Gemini (IRB 19-006717) protocol. These samples will be used for future biomarker discovery and other translational studies.
This is a Phase I, open label study to evaluate the safety, tolerability, and immunogenicity of INO-1400 alone or in combination with INO-9012, delivered by electroporation in subjects with high-risk solid tumor cancer with no evidence of disease after surgery and standard therapy. Subjects will be enrolled into one of six treatment arms. Subjects will be assessed according to standard of care. Restaging and imaging studies will be performed to assess disease relapse per NCCN guidelines. RECIST will be used to validate the findings in cases of relapse.
The purpose of this study is to determine the prevalence of genetic mutations in cancer patients from various ethnic populations seeking care at Mayo Clinic cancer clinics.
The purpose of this study is to sequence patient germline and tumor samples, and nominate top neoantigen candidates using an in-house developed bioinformatics pipeline, and to validate the neoantigen candidates by laboratory assays using patient peripheral blood immune cells or serum.
The purpose of this study is to provide a large database and platform for prospective sub-studies and eventually develop additional collaborations with a platform for clinical studies and trials following the initial pilot phase.
The purpose of this study is to evaluate the challenges, behavioral patterns, and preferences of minority patient participation in clinical trials. Also, to develop and validate a personalized clinical trial educational platform to boost participation among underserved cancer patients.
The purpose of this study is to evaluate the safety, tolerability, and preliminary anti-tumor activity of SAR444881 alone and in combination with pembrolizumab or with cetuximab. The study will enroll advanced cancer patients with unresectable or metastatic disease who are refractory to or are not candidates for standard approved therapy and will be comprised of two parts - an initial "3 + 3" dose escalation phase (Part 1) with Sub-Parts 1A (monotherapy SAR444881), 1B (SAR444881 in combination with pembrolizumab) and 1C (SAR444881 in combination with cetuximab) followed by a dose optimization/expansion phase (Part 2), including Sub-Part 2A (Dose Optimization) with Cohorts A1 (SAR444881 in ...
Falls are common and catastrophic in cancer patients. Cancer patients are vulnerable to falls due to muscle loss. In prescribing exercise in a data driven manner to cancer patients, our hypothesis is this "prescription" for exercise will eventually be demonstrated to reduce the occurrence of injurious falls.
Mayo Clinic Footer
- Request Appointment
- About Mayo Clinic
- About This Site
Legal Conditions and Terms
- Terms and Conditions
- Privacy Policy
- Notice of Privacy Practices
- Notice of Nondiscrimination
- Manage Cookies
Advertising
Mayo Clinic is a nonprofit organization and proceeds from Web advertising help support our mission. Mayo Clinic does not endorse any of the third party products and services advertised.
- Advertising and sponsorship policy
- Advertising and sponsorship opportunities
Reprint Permissions
A single copy of these materials may be reprinted for noncommercial personal use only. "Mayo," "Mayo Clinic," "MayoClinic.org," "Mayo Clinic Healthy Living," and the triple-shield Mayo Clinic logo are trademarks of Mayo Foundation for Medical Education and Research.
Breast Cancer Research Results and Study Updates
See Advances in Breast Cancer Research for an overview of recent findings and progress, plus ongoing projects supported by NCI.
Some people with no evidence of cancer in nearby lymph nodes after presurgical chemotherapy can skip radiation to that area without increasing the risk of the cancer returning, a clinical trial found. But some experts caution that more details are needed.
For women in their 70s and older, the risk of overdiagnosis with routine screening mammography is substantial, a new study suggests. The findings highlight the need for conversations between older women and their health care providers about the potential benefits and harms of continuing screening mammography.
Many young women who are diagnosed with early-stage breast cancer want to become pregnant in the future. New research suggests that these women may be able to pause their hormone therapy for up to 2 years as they try to get pregnant without raising the risk of a recurrence in the short term.
For younger women with advanced breast cancer, the combination of ribociclib (Kisqali) and hormone therapy was much better at shrinking metastatic tumors than standard chemotherapy treatments, results from an NCI-funded clinical trial show.
In a large clinical trial, a condensed course of radiation therapy was as effective and safe as a longer standard course for those with higher-risk early-stage breast cancer who had a lumpectomy. This shorter radiation course makes treatment less of a burden for patients.
Adding the immunotherapy drug pembrolizumab (Keytruda) to chemotherapy can help some patients with advanced triple-negative breast cancer live longer. In the KEYNOTE-355 trial, overall survival improved among patients whose tumors had high levels of the PD-L1 protein.
People with metastatic breast cancer whose tumors had low levels of HER2 protein lived longer after treatment with trastuzumab deruxtecan (Enhertu) than those treated with standard chemotherapy, results of the DESTINY-Breast04 clinical trial show.
NCI researchers have shown that an experimental form of immunotherapy that uses an individual’s own tumor-fighting immune cells could potentially be used to treat people with metastatic breast cancer who have exhausted all other treatment options.
Most breast cancer risk tools were developed with data mainly from White women and don’t work as well for Black women. A new tool that estimates risk for Black women may help identify those who might benefit from earlier screening, enabling earlier diagnosis and treatment.
In people with metastatic HER2-positive breast cancer, the targeted drug trastuzumab deruxtecan (Enhertu) markedly lengthened progression-free survival compared with trastuzumab emtansine (Kadcycla), new study results show.
In a large clinical trial, women with HR-positive, HER2-negative metastatic breast cancer treated with ribociclib (Kisqali) and letrozole (Femara) as their initial treatment lived approximately 1 year longer than women treated with letrozole only.
Women with early-stage breast cancer who had one or both breasts surgically removed (a unilateral or bilateral mastectomy) had lower scores on a quality-of-life survey than women who had breast-conserving surgery, a new study has found.
For women undergoing chemotherapy for breast cancer, meeting the national physical activity guidelines may help alleviate cognitive issues, a new study suggests. The benefits may be even greater for patients who were physically active before treatment.
Sacituzumab govitecan (Trodelvy) now has regular FDA approval for people with locally advanced or metastatic triple-negative breast cancer (TNBC). The update follows last year’s accelerated approval of the drug for people with TNBC.
For some people with ER-positive breast cancer, a new imaging test may help guide decisions about receiving hormone therapy, according to a new study. The test can show whether estrogen receptors in tumors are active and responsive to estrogen.
The test, which helps guide treatment decisions, was not as good at predicting the risk of death from breast cancer for Black patients as for White patients, a new study has found. The findings highlight the need for greater racial diversity in research studies.
The drug abemaciclib (Verzenio) may be a new treatment option for people with the most common type of breast cancer, with new study findings suggesting that it can reduce the risk of the cancer returning.
Fertility preservation for young women with breast cancer doesn’t increase their risk of dying in the ensuing decades, a new study affirmed. Experts said the findings support routinely offering fertility preservation to patients who want it.
Some postmenopausal women with HR-positive, HER2-negative breast cancer may not benefit from chemotherapy and can safely forgo the treatment, according to clinical trial results presented at the San Antonio Breast Cancer Symposium.
A heart-related event, like a heart attack, may make breast cancer grow faster, a new study suggests. In mice, heart attacks accelerated breast tumor growth and human studies linked cardiac events with breast cancer recurrence, researchers reported.
FDA has approved sacituzumab govitecan (Trodelvy) for the treatment of triple-negative breast cancer that has spread to other parts of the body. Under the approval, patients must have already undergone at least two prior treatment regimens.
Women with high-risk breast cancer who engaged in regular exercise before their cancer diagnosis and after treatment were less likely to have their cancer return or to die compared with women who were inactive, a recent study found.
Researchers have developed a “microscaled” approach to analyze the proteins and genetic changes (proteogenomics) of a tumor that uses tissue from a core needle biopsy. The analyses can provide important information that may help guide treatment.
Tucatinib improved survival for women in the HER2CLIMB trial, including some whose cancer had spread to the brain. Trastuzumab deruxtecan improved survival and shrank many tumors in the DESTINY-Breast01 trial, which led to its accelerated approval.
A TAILORx analysis shows women with early-stage breast cancer and high recurrence scores on the Oncotype DX who received chemotherapy with hormone therapy had better long-term outcomes than what would be expected from hormone therapy alone.
Men with breast cancer may be more likely to die of the disease than women, particularly during the first 5 years after diagnosis, a new study suggests. The higher likelihood of death was linked in part to undertreatment and later diagnosis.
In a survey of nearly 600 breast cancer survivors, researchers found that the cost of care factored into the decisions the women made about what type of surgery to get. Many women also reported never discussing costs with their physicians.
FDA has expanded the approved use of the drug ado-trastuzumab emtansine (Kadcyla), also called T-DM1, to include adjuvant treatment in some women with early-stage HER2-positive breast cancer.
Many women diagnosed with ovarian and breast cancer are not undergoing tests for inherited genetic mutations that can provide important information to help guide decisions about treatment and longer-term cancer screening, a new study has found.
FDA has approved atezolizumab (Tecentriq) in combination with chemotherapy for the treatment of some women with advanced triple-negative breast cancer. This is the first FDA-approved regimen for breast cancer to include immunotherapy.
The build-up of connective tissue around some types of cancer can act as a barrier to immunotherapy. A new study uses a bone marrow transplant drug, plerixafor, to break down this barrier and improve the efficacy of immune checkpoint inhibitors in animal models of breast cancer.
A new study in mice shows that disrupting the relationship between breast cancer cells that spread to bone and normal cells surrounding them makes the cancer cells sensitive to treatment.
In women with early-stage breast cancer, two clinical trials have shown that both whole- and partial-breast radiation therapy are effective at preventing the cancer from returning after breast-conserving surgery.
Researchers are testing a topical-gel form of the drug tamoxifen to see if it can help prevent breast cancer as effectively as the oral form of the drug but with fewer side effects.
Findings from a clinical study and a mouse study may shed light on genetic risk factors for developing cancer-related cognitive problems in older breast cancer survivors. The results suggest a gene associated with Alzheimer’s disease may play a role.
Arsenic trioxide and retinoic acid work together to target the master regulator protein Pin1, a new study shows. In cancer cell lines and mice, the drug combination slowed the growth of triple-negative breast cancer tumors.
FDA has expanded the approved uses of ribociclib (Kisqali) for women with advanced breast cancer, including new uses in pre- and postmenopausal women. It’s the first approval under a new FDA program to speed the review of cancer drugs.
Using a liquid biopsy to test for tumor cells circulating in blood, researchers found that, in women with breast cancer, the presence of these cells could identify women at risk of their cancer returning years later.
Findings from the TAILORx clinical trial show chemotherapy does not benefit most women with early breast cancer. The new data, released at the 2018 ASCO annual meeting, will help inform treatment decisions for many women with early-stage breast cancer.
Do cancer study participants want to receive their genetic test results? A recent study involving women with a history of breast cancer tested an approach for returning genetic research results and evaluated the impact those results had on the women.
Researchers compared the risk of death for women with breast cancer who had low skeletal muscle mass, or sarcopenia, at the time of their cancer diagnosis and women who had adequate muscle mass.
Some people who have been treated for breast cancer or lymphoma have a higher risk of developing congestive heart failure than people who haven’t had cancer, results from a new study show.
FDA has approved the CDK4/6 inhibitor abemaciclib (Verzenio) as a first-line treatment in some women with advanced or metastatic breast cancer. Under the approval, the drug must be used in combination with an aromatase inhibitor.
A new study in mice raises the possibility that using microscopic, oxygen-carrying bubbles may improve the effectiveness of radiation therapy in the treatment of breast cancer.
The drug olaparib (Lynparza®) is the first treatment approved by the Food and Drug Administration for patients with metastatic breast cancer who have inherited mutations in the BRCA1 or BRCA2 genes.
Joint pain caused by aromatase inhibitors in postmenopausal women with breast cancer can cause some women to stop taking the drugs. Reducing their symptoms may translate into better adherence to therapy.
A new study suggests that the cells in treatment-resistant tumors in women with metastatic breast cancer share important characteristics that could potentially make tumors vulnerable to therapies that otherwise might not have been considered.
A large nationwide clinical trial called TMIST has been launched to compare two techniques used for mammograms: tomosynthesis, often called 3D mammography, and standard 2D digital mammography.
FDA approved abemaciclib (Verzenio™) for the treatment of some people with advanced or metastatic HR-positive, HER2-negative breast cancer whose disease has progressed after treatment with hormone therapy.
Long-term results from a large clinical trial confirm that, for some women with early-stage breast cancer who have lumpectomy as their surgical treatment, a less extensive lymph node biopsy approach is sufficient.
When given at the same time, two immune checkpoint inhibitors were ineffective against breast cancer growth in mice, a new study found. The combination was more effective and safer if the two inhibitors were given in a specific sequence.
FDA has expanded its approval of fulvestrant (Faslodex®) as a standalone treatment for postmenopausal women with advanced HR-positive, HER2-negative breast cancer who have not previously undergone endocrine therapy.
Many women who receive taxane-based chemotherapy to treat breast cancer experience long-term nerve damage, or peripheral neuropathy, data from a large clinical trial show.
Researchers recognized the potential of endoxifen as a treatment for breast cancer and, with NCI support, developed the compound into a drug now being tested in clinical trials.
Researchers have used modified stem cells to deliver a cancer drug selectively to metastatic breast cancer tumors in mice. The stem cells target metastatic tumors by homing in on the stiff environment that typically surrounds them.
FDA has approved neratinib for patients with early-stage HER2-positive breast cancer who have finished at least 1 year of adjuvant therapy with trastuzumab.
Many survivors of early-stage breast cancer prefer that their oncologist handle aspects of routine medical care usually overseen by primary care practitioners, leading to concerns about gaps in care.
Results from the first large prospective study of breast and ovarian cancer risk in women with inherited mutations in the BRCA 1 or BRCA2 genes confirm the high risks estimated from earlier, retrospective studies.
Two clinical trials show that trastuzumab emtansine (T-DM1) improves survival compared with other standard treatments for patients with HER2-positive metastatic breast cancer that has progressed after treatment with other HER2-targeted drugs.
Using one of the largest collections of tumor samples from African Americans with breast cancer, researchers tried to assess the extent to which the molecular characteristics on these tumors might help to explain breast cancer disparities.
A new study shows that the number of women in the United States living with distant metastatic breast cancer (MBC), the most severe form of the disease, is growing. This is likely due to the aging of the U.S. population and improvements in treatment.
In a randomized trial, low-income women who role-played talking with their doctor about their survivorship care plan in a counseling session reported receiving more of their recommended care than women who did not get counseling.
The FDA has approved a new targeted therapy, ribociclib, and expanded its earlier approval of another targeted therapy, palbociclib, for some women with metastatic breast cancer.
Researchers have found that duloxetine (Cymbalta®), a drug most commonly used to treat depression, may also reduce joint pain caused by aromatase inhibitors in some women being treated for early-stage breast cancer.
- Open access
- Published: 26 July 2023
The relation between obesity and breast cancer risk in women by considering menstruation status and geographical variations: a systematic review and meta-analysis
- Tania Dehesh 1 , 2 ,
- Shohreh Fadaghi 3 ,
- Mehrnaz Seyedi 4 ,
- Elham Abolhadi 2 ,
- Mehran Ilaghi 5 ,
- Parisa Shams 6 ,
- Fatemeh Ajam 2 ,
- Mohammad Amin Mosleh-Shirazi 7 , 8 &
- Paria Dehesh 9
BMC Women's Health volume 23 , Article number: 392 ( 2023 ) Cite this article
2313 Accesses
3 Citations
76 Altmetric
Metrics details
Given the increase in the incidence of breast cancer during the past decades, several studies have investigated the effects of variables on breast cancer, especially obesity. This systematic review and meta-analysis aims to evaluate any effects of obesity on breast cancer risk in women, before and after menopause, and in different continents.
All forms of relevant literature examining any association between obesity and breast cancer, including cohort, case–control, and cross-sectional studies, were identified in the PubMed, Scopus, EMBASE, and Web of Science databases from January 1, 1990 until January 13, 2023. Body mass index (BMI) > 30 was used to indicate obesity. Every type of breast cancer was examined as outcome factors. The quality of the papers was evaluated using the Newcastle–Ottawa scale checklist. The Egger and Begg test was used to evaluate publication bias. To assess any extra impact of each research on the final measurement, a sensitivity analysis was carried out.
One hundred and two studies were included in this meta-analysis. Respectively, 48 and 67 studies reported associations between obesity and breast cancer in pre and post menopausal women. Combining all studies, the pooled OR of the association between obesity and breast cancer in pre-menopausal women was OR = 0.93 CI: (0.85–1.1), (I 2 = 65.4%), and for post-menopausal woman, OR = 1.26 CI: (1.19–1.34), (I 2 = 90.5%).
Obesity has a protective role in breast cancer among pre-menopausal women, but this relationship is statistically significant only in European women. The chance of developing breast cancer increases in post-menopausal women who are obese. This relationship is significant among Asian, North American, African and European women.
Peer Review reports
Introduction
The most frequent malignancy among women worldwide is breast cancer; 2.3 million new diagnoses of breast cancer in women, which represents 11.7% of all new cancer cases in the world, were made in 2020. Breast cancer, with 685,000 fatalities in a year, is the fifth cause of deaths among different cancers (1 in 6 cancer deaths) [ 1 ]. Environmental factors, including smoking, alcohol consumption, diet and eating habits, inactivity, obesity, excessive exposure to sunlight, and infection with some viruses and bacteria, have an important role in the emergence of breast cancer in females [ 2 ].
Obesity and overweightness are common and serious risk factors for many chronic diseases and cancers. According to the World Health Organization's (WHO) definition of obesity, people with a body mass index (BMI) > 30 are considered obese. The relationship between obesity and the occurrence of some chronic diseases and cancer has been clarified [ 3 ].
More than 50 research studies have looked into obesity and its role in the prognosis of breast cancer. A study revealed that the 5-year breast cancer survival rate for obese or overweight women was 55.6%, whereas it was 79.9% for women with normal weight. This study also demonstrated that obese women with breast cancer are more likely to have bigger tumors and invasive lymph nodes [ 4 ]. Many researchers have investigated the association between high BMI or obesity and developing breast cancer in women [ 5 , 6 ], but there is some discordance between the findings of the studies. According to the findings of several observational studies, obesity raises the risk of getting breast cancer both before and after menopause [ 7 , 8 , 9 ]. The results of some studies show that obesity has a protective role in developing cancer in the pre-menopause period, while, it constitutes a risk factor in the post-menopause period [ 10 , 11 ]. Pre-menopausal obesity in European and American women has been reported to decrease the risk of breast cancer [ 12 , 13 ], while the positive association of pre-menopausal obesity with breast cancer incidence in Asian populations has been reported across the studies [ 14 , 15 ].
It has been several years since systematic reviews and meta-analyses were done on the association between breast cancer and obesity. The meta-analysis study on the relationship between breast cancer and obesity needs to be updated as the numerous publications that have been published in recent years. According to our knowledge, no meta- analyse study has been conducted to investigate the relationship between breast cancer and obesity in different continents simultaneously. Since weight gain and breast cancer are both worldwide health concerns, this systematic review and meta-analysis aims to investigate the association between obesity and breast cancer risk in the pre- and post-menopause periods and also demonstrate any geographical variations by examining this association in different continents.
The MOOSE and STROBE recommendations for reviews of observational studies were used in this systematic review [ 16 , 17 ]. The protocol of this systematic review and meta-analysis has been recorded in Prospero (ID: CRD42021261448).
Search method
Global databases including Scopus, Web of Sciences, Medline (PubMed), and EMBASE were searched to find all original published papers. No articles were found by searching through the Cochrane database. The papers listed in the retrieved studies were manually searched to increase search sensitivity. For January 1990 to January 2023, searches were carried out without linguistic constraints using "Obesity," "Body Weight," "Breast Neoplasms," "Breast Tumors," "Breast Carcinomas," and "Breast Cancer" as the the keywords. Only human observational studies were selected. Many of the articles from the original search results were eliminated after a review of the titles and abstracts. Two researchers separately identified the inclusion and exclusion criteria.
Eligibility criteria
A study was not included unless it satisfied the following requirements: original publication, cross-sectional/case–control/cohort research, human studies, the main independent variable being obesity (BMI > 30), and the outcome being breast cancer. Review papers, editorials, letters, case reports, conference abstracts, studies that were not peer-reviewed, and articles on animals or genetics, were all eliminated from the study. All discrepancies found during the stages of data collection, compilation, and analysis were investigated and resolved by the authors.
Data extraction
Three researchers conducted separate evaluations of each included paper. A fourth author reviewed the work and helped them determine whether there were any differences of opinion. The two independent, matched reviewers who were responsible for gathering the data did so by using an Excel worksheet. Authors, publication year, study type, location, sample size, BMI, breast cancer type, and confonder variables of the association were listed in that order. After that, information from a systematic checklist was gathered. Figure 1 showed the PRISMA flowchart. In this flowchart, the number of initial search results in different databases was shown, and in the next step, the titles of the articles were checked, and finally, some articles were removed due to inappropriate titles and abstracts. According to the Fig. 1 , about 10,662 articles were found in fiest setps search with sepecial keywords in international databases. Om the next setep, duplicates and articles that were not related to the topic in terms of title were removed, 1002 studies remained. In the next step, 527 articles were removed after reviewing the abstract, finally 102 articles were were entered into the meta-analysis.
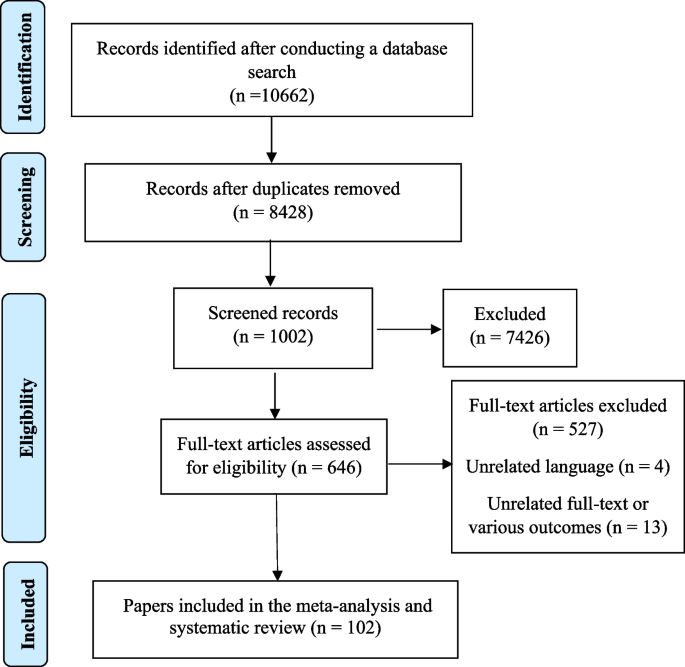
PRISMA Flow chart of the search and selection of the studies
Risk of bias assessment
The obsevestional studies quality were evaluated by the Newcastle–Ottawa Scale (NOS) from 0 to 9 stars [ 18 ]. The articles based on the Newcastle–Ottawa Scale score were divided into three groups of 0–3 (fair), 4–6 (moderate), and 7–9 (good).
Statistical analysis
This meta-analysis included all subtypes of breast cancer, regardless of clinical features or tumor stage. If several studies were published using the same data, the last study was selected and included in the meta-analysis [ 19 ]. The indicators used to measure the relationship in this meta-analysis were odds ratios (ORs) for case–control and cross-sectional studies, and risk ratios, relative risks, and hazard ratios for cohort studies. Most of the research conducted on the relationship between obesity and breast cancer were case–control studies, therfore, for better clarity, the OR has been considered as the primary index in this investigation. The results were determined according to the type of studies. ORs with standard error logarithm and logarithmic base were used for the meta-analysis. To estimate the pooled OR with a 95% confidence interval (CI 95%) due to heterogeneity between studies, random models were used using DerSimonian and Laird techniques [ 20 ]. Adjusted OR, where available, was preferred to sloppy measures. If several measures were reported in a study to investigate the association between obesity and breast cancer, the approach of combining the effect size reported in that study was used. If the heterogeneity test was significant, random effects models were employed to estimate the combined effect size. The Cochran's Q test and the I 2 statistic were employed to determine statistical heterogeneity across papers in this research [ 21 ].
A meta-regression and subgroup analysis were also carried out to identify the reason for research heterogeneity. Moreover, the funnel plot and Egger and Begg's tests were performed to assess publication bias [ 22 ]. The trim and fill test was used to investigate publication bias further [ 23 ]. The statistical analysis was conducted by using Stata 14.0, and the threshold of statistical significance was established at p < 0.05.
Identification and description of the studies
One hundred and two studies were included in this meta-analysis. Forty eight studies reported an association between obesity and breast cancer in pre-menopausal women, and 67 studies showed a relationship between obesity and breast cancer in post-menopausal women. 34 studies (4 cross-sectional,18 case–control, and 12 cohort) with a total sample size of 60,985 cases and 48,720 controls were used to evaluate the relation between obesity and breast cancer, without distinguishing between pre-menopausal and post-menopausal women.
Fifteen studies were conducted in North America, 21 in Asia, 8 in Europe, 2 in Africa, 1 in South America for the pre-menopause period. Twenty three studies were conducted in North America, 27 in Asia, 10 in Europe, 2 in Africa, 3 in South America and 1 in Oceania for the post-menopause period. We aimed to pool the findings of the research in this analysis individually depending on the subgroup of studies that measured the relation between obesity and breast cancer before and after menstruation. Table 1 s describes the features of each study included in this systematic review and meta-analysis.
Quality assessment and risk of bias
In this meta-analysis, the quality of case–control, cohort, and cross-sectional studies was examined by using the Newcastle–Ottawa scale (NOS), the results of which were presented in the checklists completed based on the type of study. The quality assessment checklists of the case–control and cohort studies showed that most of these studies had a moderate to high-quality score (Table 1 s).
Relationship between obesity and breast cancer risk in pre-menopausal women
Forty eight studies (two cross-sectional, 35 case–control, and 11 cohort) with a total sample size of 66,999 cases and 845,612 controls were included in the evaluation of the association between obesity and breast cancer in pre-menopausal women. Regardless of the type of study, the lowest and highest ORs were equal to 0.44 (95% CI; 0.19 – 1.01) and 2.73 (95% CI; 1.79 – 4.18), respectively. After combining all studies, the pooled OR of the association between obesity (with case group BMI > 30) and breast cancer in pre-menopause was OR = 0.93 CI: (0.85–1.1), I2 = 65.4%, P < 0.001 (Fig. 2 ). It showed that obesity was not a significant protective factor for breast cancer in pre-menopause women. Visual inspection of the funnel plot in Fig. 5 a, indicated asymmetry, but Egger's tests (β =—0.071, P -value = 0.85), did not show significant publication bias. When the trim and fill approach was used to further evaluate publication bias, no study was imputed. The result of the meta-regression analysis described that the study quality and a sample size of more than 1000 had no significant linear relationship with the OR of obesity and breast cancer risk, so these variables were not the reason for the heterogeneity (Table 1 ).
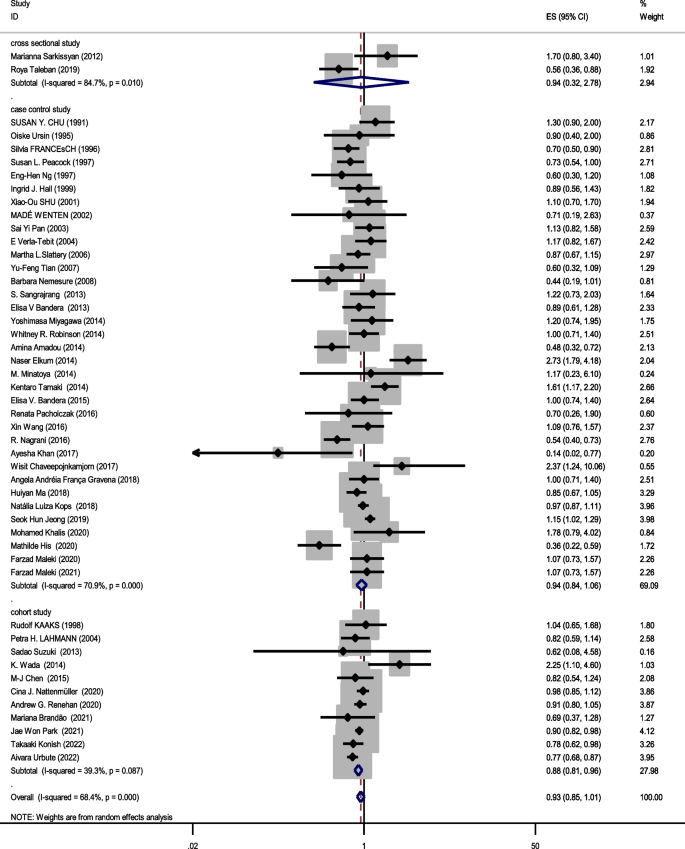
Meta-analysis of the relation between obesity and breast cancer risk in pre-menopausal women
Sensitivity analysis showed that the pooled effect size on the relationship between obesity and breast cancer risk in pre-menopause women did not depend on a single study (CI range: 0.84- 1.01).
Relationship between obesity and breast cancer risk in post-menopausal women
Sixty seven studies (three cross-sectional, 46 case–control, and 18 cohort) with a total sample size of 262,434 cases and 1,501,879 controls were used in the evaluation of the association between obesity and breast cancer risk in post-menopausal women. Regardless of the type of study, the lowest and highest ORs were equal to 0.56 (95% CI; 0.33 – 0.99) and 3.26 (95% CI; 1.54 – 6.9), respectively. After combining all studies, the pooled OR of the relation between obesity and breast cancer risk in post-menopausal women was OR = 1.26 CI: (1.19–1.34), I2 = 90.5%, P < 0.001(Fig. 3 ). It showed a higher probability of developing breast cancer by up to 26% in obese post-menopausal women. The funnel plots (Fig. 5 b) for these studies provided substantial evidence of publication bias, but with the Egger test, a significant publication bias was not seen (β = 0. 78, P -value = 0.103). The trim-and-fill method used for more certainty (Fig. 6 a), showed that 11 assumptive missing papers have been imputed, and the "adjusted" point estimate indicated a comparable OR compared to the original analysis (OR = 1.20, 95% CI: 1.13–1.27). The result of the meta-regression analysis indicated that the probability of having breast cancer in obese women increased with an increase in study quality and sample size, but this association was not statistically significant. Therefore, study quality and a sample size of more than 1000 were not the reasons for the heterogeneity.

Meta-analysis of the relation between obesity and breast cancer risk in post-menopausal women
Sensitivity analysis showed that the pooled effect size on the relationship between obesity and breast cancer risk in post-menopausal women did not depend on a single study (CI range: 1.18–1.34).
Relationship between obesity and breast cancer risk regardless of menstrual status
The data from 34 studies (Four cross-sectional,18 case–control, and 12 cohort) with a total sample size of 34,962 cases and 2,076,826 controls were included in the evaluation of the relation between obesity and breast cancer risk, without distinguishing between pre-menopausal and post-menopausal women. After combining all studies, the pooled OR of the association between obesity and breast cancer was OR = 1.29 CI: (1.18–1.42), I2 = 84.9%, P < 0.001(Fig. 4 ). We observed an asymmetry based on a visual assessment of the funnel plot (Fig. 5 c); however, no significant publication bias was indicated using Egger's test (β = 0.45, P -value = 0.75). Using the trim-and-fill procedure showed that 10 assumptive missing papers have been imputed, and after being included, the "adjusted" point estimate indicated a similar OR to the original analysis (OR = 1.13, 95% CI: 1.02—1.24) (Fig. 6 b). According to the meta-regression analysis, study quality and a sample size of more than 1000 were not the reasons for the heterogeneity.
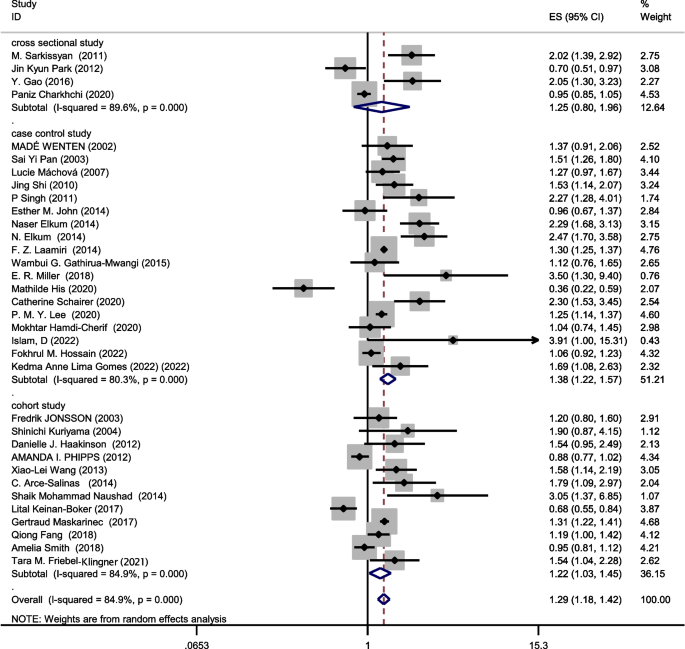
Meta-analysis of the relation between obesity and breast cancer risk in all women regardless of their menstrual status
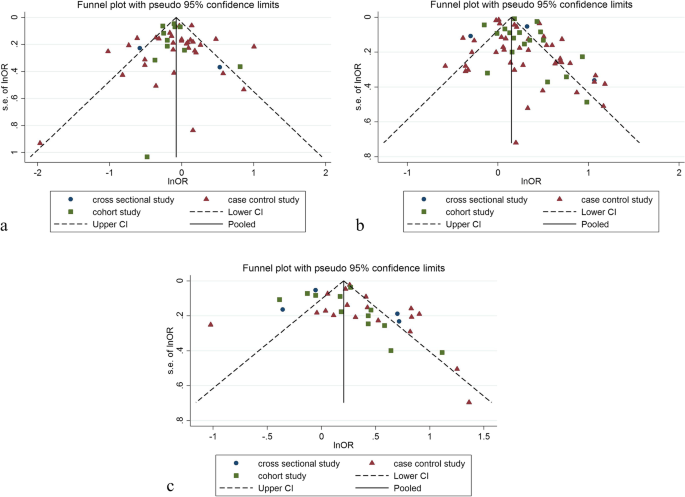
Funnel plots for publication bias in pre-menopause studies ( a ), in post-menopause studies ( b ) and studies regardless of menstrual status ( c )
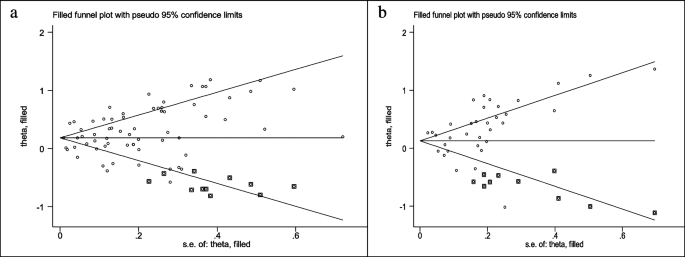
Trim-filled funnel plots for post-menopausal studies ( a ) and studies regardless of menstrual status ( b )
Sensitivity analysis indicated that the pooled effect size on the relationship between obesity and breast cancer risk did not depend on a single study (CI range: 1.17–1.42).
Subgroup analysis based on combining menstrual status
Figures 2 , 3 and 4 shows subgroup analysis irrespective of the type of study. The findings showed that obesity was linked to a decreased risk of breast cancer in pre-menopausal women across all study types. In the case–control papers, obese women had a 6% lower risk of breast cancer than normal-weight women (OR = 0.94, 95% CI: 0.84–1.06; I2: 70.9%; P -value 0.001), although this difference was not statistically significant. Also, in the cross-sectional studies (OR = 0.94, 95% CI: 0.32–2.78; I2: 84.7%; P -value < 0.001), obese women had a 6% lower risk of breast cancer than normal-weight women. There is a strong link between obesity and breast cancer in the pre-menopause period, according to cohort studies (OR = 0.88, 95% CI: 0.81–0.96; I2: 39.3%; P -value = 0.0.87). Case–control studies that explored the correlation between obesity and breast cancer risk in post-menopausal women reported that being overweight significantly increases the probability of developing the disease (OR = 1.27, 95% CI: 1.17–1.37; I2: 87.3%; P -value 0.001). It means that obese women have a 27% more chance of breast cancer in post-menopause relative to normal-weight women. Moreover, cross-sectional studies and cohort studies both showed a stronger link between obesity and breast cancer risk in post-menopausal women (OR = 1.31, 95% CI: 0.75–2.27; I2: 97.3%; P -value 0.001), and OR = 1.29, 95% CI: 1.16–1.43, respectively.
Combining the results of the studies that showed the relation between obesity and breast cancer risk regardless of menstrual status, the results of subgroups showed that case–control studies (OR = 1.38, 95% CI: 1.22–1.57; I 2 : 80.3%; P -value < 0.001), cohort studies (OR = 1.22, 95% CI: 1.03–1.45; I 2 : 84.9%; P -value = 0.0001) and cross-sectional studies (OR = 1.25, 95% CI: 0.8–1.96; I 2 : 89.6%; P -value < 0.001) report increased association between obesity and breast cancer risk.
Table 2 shows the results of the relation between obesity and breast cancer risk by geographic location of where the study was conducted. The result showed that Asian (OR = 1.49, 95% CI: 1.32–1.69; I2: 94.7%; P -value < 0.001) and North American (OR = 1.21, 95% CI: 1.07–1.36; I2: 75.5%; P -value < 0.001) obese woman in post-menopause were more likely to develop breast cancer than the European ones (OR = 1.10, 95% CI: 0.97–1.25; I2: 85%; P-value = 0.0001). The relation between obesity and breast cancer risk in post-menopause was not significant in European women. According to the different I 2 tests in subgroup analysis based on geographic location, the difference in the geographical location of the studies is one of the reasons for the heterogeneity. Obesity was not a significant factor for breast cancer risk in pre-menopause in Asians (OR = 1.00, 95% CI: 0.87–1.15; I 2 : 76.71%; P -value < 000.1), and North Americans (OR = 1.07, 95% CI: 0.42–2.69; I 2 : 69.7%; P -value = 0.069). In European women, obesity was a significant protective factor for breast cancer risk in pre-menopause (OR = 0.88, 95% CI: 0.79–0.98 I 2 : 44.8%; P -value = 0.081).
The breast cancer burden is substantial in terms of incidence, mortality and taking treatment resources. For example, radiotherapy centers normally deliver a large number of breast cancer treatments every day (usually larger than any other type of cancer) using a variety of techniques. [ 1 , 24 , 25 , 26 ]. Any knowlwdge gained from detailed identification of risk factors that can be used toward improving breast cancer prevention is, therefore, highly useful and important. This systematic review and meta-analysis contributes to that effort.
The findings of this study divided the association between obesity and breast cancer risk into three categories: pre-menopause, post-menopause, and both together. This relation was also reported among the different continents in which the studies where conducted. The findings demonstrated that when combining those studies that did not separate the menopause period, obesity is shown to increase the risk of breast cancer. More specifically, obese women have a 1.29-fold higher chance of developing breast cancer than women of normal weight. The increased leptin in obese women has been associated with diagnosis of colon, breast, prostate and ovarian cancers in a previous study [ 27 ]. However, evidence for an association between obesity and breast cancer risk is controversial. Leptin is a fat-derived hormone that regulates appetite and energy homeostasis [ 28 ]. Studies have described that leptin receptors (LEPR) exist in breast cancer patients [ 29 , 30 ].
Obesity not only increases the chance of breast cancer, but it also has a significant effect on the disease development process. Obese women with breast cancer are more susceptible to having larger tumors. These women may become resistant to hormone treatment and have a greater incidence of metastases. Clinical studies have shown that obese breast cancer patients receiving chemotherapeutic or estrogen inhibitors have a lower chance of recurrence than those who have normal weight [ 31 , 32 ].
Obesity and risk of breast cancer in pre-menopausal women
The dose–response meta-analysis conducted by Liu et al. reported that a 5 unit increase BMI leads to a 2% increase in the probability of breast cancer. Also, that study's findings revealed that women with greater BMIs before menopause had a lower chance of getting breast cancer [ 6 ]. The results of the current meta-analysis also show that obesity is a protective factor against breast cancer in pre-menopause.
It has been demonstrated that increased endogenous estrogen exposure in women raises the probability of developing breast cancer. Therefore, long-term exposure to estrogen for various reasons such as early-age menstruation or prolonged menopause may raise the probability of developing breast cancer [ 33 ]. Due to a possible hormonal interaction during ovulatory cycles, young obese women have a higher reduction in progesterone levels at the end of the menstruation cycle compared to the normal woman, and progesterone levels come down later in obese women [ 34 ]. Obese pre-menopausal women have longer periods than women who have normal weight before menopause. As a result, the duration of exposure to estrogen in these people is less, which could reduce the chance of breast cancer in these people [ 33 ].
Obesity and risk of breast cancer in post-menopausal women
According to the results of several studies, post-menopausal obesity increases the risk of breast cancer. This may be due to producing more estrogen hormone from adipose tissue in obese women in post-menopause [ 35 ].
The results of several studies show that the serum level of sex hormones such as estrogen is higher in women with breast cancer compared to healthy women of the same age [ 36 , 37 , 38 ]. Reduction in sex hormone-binding globulin receptors leads to a higher level of estrogen in obese women compared to normal-weight women in post-menopause [ 33 ]. As a result, it is thought that the primary location of estrogen manufacture in obese post-menopausal women occurs in excess adipose tissue, resulting in a multiplication of the estrogen hormone in the blood. Thus, increasing estrogen levels may increase the probability of breast cancer in post-menopausal women [ 33 ].
Subgroup meta-analysis
After performing the subgroup analysis in each continent, it was found that obesity during pre-menopause is a significant protective factor among the population of European women. This protective relation was also found in the other continents, but without statistical significant. The findings of this study are in contrast with those of another meta-analysis that identified a significant positive relation between obesity and breast cancer risk in pre-menopausal Asian women [ 39 ]. The dose–response meta-analysis revealed that every 5 kg/m 2 increase was significantly and negatively associated with the risk of breast cancer during the pre-menopause period among Africans (RR = 0.95, 95% CI: 0.91, 0.98) [ 15 ].
Throughout this meta-analysis, heterogeneity was found in studies that show the relation between obesity and breast cancer risk in pre- and post-menopausal women. This heterogeneity was found in different study designs. The relation between breast cancer risk and obesity may have been confounded by a factor such as the status of taking hormonal drugs, the type of breast cancer, geographical location, sample size, physical activity, etc. To investigate the sources of heterogeneity observed among the studies, subgroup analyses, and meta-regression were performed for some of these confounding factors. The results of subgroup analyses showed that geographical location may be one of the reasons for heterogeneity. The meta-regression results showed that sample size and the quality of the studies do not have a significant effect on creating heterogeneity.
One of the strengths of this meta-analysis compared to previous meta-analyses is combining different study designs separately and performing sensitivity analysis. Also, the results of the relationship between obesity and breast cancer risk have been reported separately before and after menopause.
Limitations
There were several limitations in our study. First, obesity was calculated using BMI by measuring height and weight at the time of diagnosis. The association between obesity and breast cancer did not take into account long-term variations in weight and body composition, and several other weight-determining factors (including waist circumference and waist-to-hip ratio). Second, BMI grouping in some articles was not based on WHO standards. Third, the type of breast cancer was not specified in many studies. Fourth, few studies have been done on obesity and breast cancer risk in Oceania, South America, and Africa. Therefore, generalizing the results of this meta-analysis in these continents should be done with caution. Finally, important confounders of breast cancer and obesity were ignored in some studies and this may affect the result of the current analysis.
Conclusions
Obesity has a protective role in breast cancer among pre-menopausal women, but this relationship is statistically significant only in European women. The chance of developing breast cancer increases in post-menopausal women who are obese. This relationship is significant among Asian, North American, African and European women. Therefore, it is recommended that post-menopausal women prevent overweight and obesity by having sufficient physical activity and suitable nutrition.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Davoodi H, Esmaeili S, Mortazavian A. Effects of milk and milk products consumption on cancer: a review. Comprehensive Rev Food Sci Food Safety. 2013;12(3):249–64.
Article CAS Google Scholar
Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–78.
Article PubMed Google Scholar
Ligibel J. Obesity and breast cancer. Oncology. 2011;25(11):994.
PubMed Google Scholar
Abolmaali S, Shirzaei S. A comparative study of SIR model, linear regression, logistic function and ARIMA Model for forecasting COVID-19 cases. AIMS Public Health. 2021;8(4):598.
Article PubMed PubMed Central Google Scholar
Liu K, Zhang W, Dai Z, Wang M, Tian T, Liu X, et al. Association between body mass index and breast cancer risk: Evidence based on a dose–response meta-analysis. Cancer Manag Res. 2018;10:143.
Barlow WE, White E, Ballard-Barbash R, Vacek PM, Titus-Ernstoff L, Carney PA, et al. Prospective breast cancer risk prediction model for women undergoing screening mammography. J Natl Cancer Inst. 2006;98(17):1204–14.
Sonnenschein E, Toniolo P, Terry MB, Bruning PF, Kato I, Koenig KL, et al. Body fat distribution and obesity in pre-and postmenopausal breast cancer. Int J Epidemiol. 1999;28(6):1026–31.
Article CAS PubMed Google Scholar
Tian Y-F, Chu C-H, Wu M-H, Chang C-L, Yang T, Chou Y-C, et al. Anthropometric measures, plasma adiponectin, and breast cancer risk. Endocr Relat Cancer. 2007;14(3):669–77.
Montazeri A, Sadighi J, Farzadi F, Maftoon F, Vahdaninia M, Ansari M, et al. Weight, height, body mass index and risk of breast cancer in postmenopausal women: a case-control study. BMC Cancer. 2008;8:1–7.
Article Google Scholar
Palmer JR, Adams-Campbell LL, Boggs DA, Wise LA, Rosenberg L. A prospective study of body size and breast cancer in black women. Cancer Epidemiol Biomark Prev. 2007;16(9):1795–802.
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108.
Group PBCC, Schoemaker MJ, Nichols HB, Wright LB, Brook MN, Jones ME, O’Brien KM, Adami H. O, Baglietto L, Bernstein L, et al Association of body mass index and age with subsequent breast cancer risk in premenopausal women. JAMA Oncol. 2018;4:e181771.
Nitta J, Nojima M, Ohnishi H, Mori M, Wakai K, Suzuki S, et al. Weight gain and alcohol drinking associations with breast cancer risk in Japanese postmenopausal women-results from the Japan Collaborative Cohort (JACC) Study. Asian Pac J Cancer Prev. 2016;17(3):1437–43.
Amadou A, Ferrari P, Muwonge R, Moskal A, Biessy C, Romieu I, et al. Overweight, obesity and risk of premenopausal breast cancer according to ethnicity: a systematic review and dose-response meta-analysis. Obes Rev. 2013;14(8):665–78.
Poorolajal J, Cheraghi Z, Irani AD, Rezaeian S. Quality of cohort studies reporting post the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement. Epidemiol Health. 2011;33:1–4.
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283(15):2008–12.
Cook DA, Reed DA. Appraising the quality of medical education research methods: the medical education research study quality instrument and the Newcastle–Ottawa scale-education. Acad Med. 2015;90(8):1067–76.
Tawfik GM, Dila KAS, Mohamed MYF, Tam DNH, Kien ND, Ahmed AM, et al. A step by step guide for conducting a systematic review and meta-analysis with simulation data. Tropical medicine and health. 2019;47(1):1–9.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58.
Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
Article CAS PubMed PubMed Central Google Scholar
Duval S, Tweedie R. Trim and fill: a simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–63.
Heer E, Harper A, Escandor N, Sung H, McCormack V, Fidler-Benaoudia MM. Global burden and trends in premenopausal and postmenopausal breast cancer: a population-based study. Lancet Glob Health. 2020;8(8):e1027–37.
Haussmann J, Corradini S, Nestle-Kraemling C, Bölke E, Njanang FJD, Tamaskovics B, et al. Recent advances in radiotherapy of breast cancer. Radiat Oncol. 2020;15:1–10.
Zeinali RB, Mosleh SMA, Faghihi R, Mosalaei A, Omidvari S, Hadad K, et al. Breast cancer and its radio therapeutic methods. Iran J Med Phys. 2012;75–85.
Davoodi SH, Malek-Shahabi T, Malekshahi-Moghadam A, Shahbazi R, Esmaeili S. Obesity as an important risk factor for certain types of cancer. Iran J Cancer Prev. 2013;6(4):186.
CAS PubMed PubMed Central Google Scholar
Cohen P, Zhao C, Cai X, Montez JM, Rohani SC, Feinstein P, et al. Selective deletion of leptin receptor in neurons leads to obesity. J Clin Investig. 2001;108(8):1113–21.
Jardé T, Perrier S, Vasson M-P, Caldefie-Chézet F. Molecular mechanisms of leptin and adiponectin in breast cancer. Eur J Cancer. 2011;47(1):33–43.
Snoussi K, Strosberg AD, Bouaouina N, Ahmed SB, Helal AN, Chouchane L. Leptin and leptin receptor polymorphisms are associated with increased risk and poor prognosis of breast carcinoma. BMC Cancer. 2006;6(1):1–10.
Santa-Maria CA, Yan J, Xie X-J, Euhus DM. Aggressive estrogen-receptor-positive breast cancer arising in patients with elevated body mass index. Int J Clin Oncol. 2015;20:317–23.
Gerard C, Brown KA. Obesity and breast cancer–Role of estrogens and the molecular underpinnings of aromatase regulation in breast adipose tissue. Mol Cell Endocrinol. 2018;466:15–30.
Bianchini F, Kaaks R, Vainio H. Overweight, obesity, and cancer risk. Lancet Oncol. 2002;3(9):565–74.
Mohanty SS, Mohanty PK. Obesity as potential breast cancer risk factor for postmenopausal women. Genes Dis. 2021;8(2):117–23.
Geczik AM, Falk RT, Xu X, Ansong D, Yarney J, Wiafe-Addai B, et al. Measured body size and serum estrogen metabolism in postmenopausal women: the Ghana Breast Health Study. Breast Cancer Res. 2022;24(1):9.
Thomas HV, Reeves GK, Key TJ. Endogenous estrogen and postmenopausal breast cancer: a quantitative review. Cancer Causes Control. 1997;8:922–8.
Geyer FC, Rodrigues DN, Weigelt B, Reis-Filho JS. Molecular classification of estrogen receptor-positive/luminal breast cancers. Adv Anat Pathol. 2012;19(1):39–53.
Toniolo PG, Levitz M, Zeleniuch-Jacquotte A, Banerjee S, Koenig KL, Shore RE, et al. A prospective study of endogenous estrogens and breast cancer in postmenopausal women. J Natl Cancer Institute. 1995;87(3):190–7.
Nindrea RD, Aryandono T, Lazuardi L, Dwiprahasto I. Association of overweight and obesity with breast cancer during premenopausal period in Asia: A meta-analysis. Int J Prev Med. 2019;10;192.
Download references
Acknowledgements
Not applicable.
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and affiliations.
Modeling in Health Research Center, Institute for Futures Studies in Health, Kerman University of Medical Sciences, Kerman, Iran
Tania Dehesh
Department of Biostatistics and Epidemiology, School of Public Health, Kerman University of Medical Sciences, Kerman, Iran
Tania Dehesh, Elham Abolhadi & Fatemeh Ajam
Department of Immunology, School of Medicine, Kerman University of Medical Sciences, Kerman, Iran
Shohreh Fadaghi
Department of Health of Management and Medical Information Sciencese, Kerman University of Medical Sciences, Kerman, Iran
Mehrnaz Seyedi
Institute of Neuropharmacology, Kerman Neuroscience Research Center, Kerman University of Medical Sciences, Kerman, Iran
Mehran Ilaghi
Department of Anatomical Sciences, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran
Parisa Shams
Ionizing and Non-Ionizing Radiation Protection Research Center (INIRPRC), School of Paramedical Sciences, Shiraz University of Medical Sciences, Shiraz, Iran
Mohammad Amin Mosleh-Shirazi
Department of Radio-Oncology, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran
Department of Epidemiology, School of Public Health, University of Medical Sciences, Tehran, Iran
Paria Dehesh
You can also search for this author in PubMed Google Scholar
Contributions
T.D and P.D conceptualization, methodology, analysis, interpreting of data, writing original draft, MA.S writing review and editing. F.A, P.S, Sh.F, M.S, M.I and E.A search studies in database and data extraction. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Paria Dehesh .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Dehesh, T., Fadaghi, S., Seyedi, M. et al. The relation between obesity and breast cancer risk in women by considering menstruation status and geographical variations: a systematic review and meta-analysis. BMC Women's Health 23 , 392 (2023). https://doi.org/10.1186/s12905-023-02543-5
Download citation
Received : 10 May 2023
Accepted : 13 July 2023
Published : 26 July 2023
DOI : https://doi.org/10.1186/s12905-023-02543-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Breast neoplasms
- Meta-analysis
BMC Women's Health
ISSN: 1472-6874
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Advertisement
Racial Disparities in Breast Cancer: from Detection to Treatment
- Published: 15 December 2023
- Volume 26 , pages 10–20, ( 2024 )
Cite this article
- JC Chen 1 ,
- Daniel G. Stover 2 ,
- Tarah J. Ballinger 3 ,
- Jose G. Bazan 4 ,
- Bryan P. Schneider 3 ,
- Barbara L. Andersen 5 ,
- William E. Carson 1 &
- Samilia Obeng-Gyasi ORCID: orcid.org/0000-0002-5330-7247 1 , 6
490 Accesses
13 Altmetric
Explore all metrics
Purpose of Review
Update on current racial disparities in the detection and treatment of breast cancer.
Recent Findings
Breast cancer remains the leading cause of cancer death among Black and Hispanic women. Mammography rates among Black and Hispanic women have surpassed those among White women, with studies now advocating for earlier initiation of breast cancer screening in Black women. Black, Hispanic, Asian, and American Indian and Alaskan Native women continue to experience delays in diagnosis and time to treatment. Further, racial discrepancies in receipt of guideline-concordant care, access to genetic testing and surgical reconstruction persist. Disparities in the initiation, completion, toxicity, and efficacy of chemotherapy, endocrine therapy, and targeted drug therapy remain for racially marginalized women.
Efforts to evaluate the impact of race and ethnicity across the breast cancer spectrum are increasing, but knowledge gaps remain and further research is necessary to reduce the disparity gap.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others

Health and Racial Disparity in Breast Cancer

The impact of race and ethnicity in breast cancer—disparities and implications for precision oncology
Kelly A. Hirko, Gabrielle Rocque, … Yeon Hee Park

Racial disparities in breast cancer persist despite early detection: analysis of treatment of stage 1 breast cancer and effect of insurance status on disparities
Ethan J. Hoppe, Lala R. Hussain, … Barbara A. Wexelman
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• Giaquinto AN, Sung H, Miller KD, Kramer JL, Newman LA, Minihan A, et al. Breast Cancer Statistics, 2022. CA Cancer J Clin. 2022;72(6):524–41. https://doi.org/10.3322/caac.21754 . American Cancer Society’s most recent update on breast cancer statistics including incidence, screening behaviors, mortality, and survival.
Article PubMed Google Scholar
Giaquinto AN, Miller KD, Tossas KY, Winn RA, Jemal A, Siegel RL. Cancer statistics for African American/Black People 2022. CA Cancer J Clin. 2022;72(3):202–29. https://doi.org/10.3322/caac.21718 .
Duffy SW, Tabár L, Yen AM, Dean PB, Smith RA, Jonsson H, et al. Mammography screening reduces rates of advanced and fatal breast cancers: results in 549,091 women. Cancer. 2020;126(13):2971–9. https://doi.org/10.1002/cncr.32859 .
Ahmed AT, Welch BT, Brinjikji W, Farah WH, Henrichsen TL, Murad MH, et al. Racial disparities in screening mammography in the United States: a systematic review and meta-analysis. J Am Coll Radiol. 2017;14(2):157-65.e9. https://doi.org/10.1016/j.jacr.2016.07.034 .
Hensing WL, Poplack SP, Herman CR, Sutcliffe S, Colditz GA, Ademuyiwa FO. Racial differences in no-show rates for screening mammography. Cancer. 2021;127(11):1857–63. https://doi.org/10.1002/cncr.33435 .
Cancer Prevention & Early Detection Facts & Figures 2023-2024. Atlanta: American Cancer Society; 2023-2024.
Swami N, Nguyen T, Dee EC, Franco I, Baez YA, Lapen K, et al. Disparities in primary breast cancer stage at presentation among Hispanic subgroups. Ann Surg Oncol. 2022;29(13):7977–87. https://doi.org/10.1245/s10434-022-12302-9 .
•• Fayanju OM, Edmonds CE, Reyes SA, Arciero C, Bea VJ, Crown A, et al. The landmark series-addressing disparities in breast cancer screening: new recommendations for black women. Ann Surg Oncol. 2023;30(1):58–67. https://doi.org/10.1245/s10434-022-12535-8 . An overview of epidemiologic factors associated with breast cancer among Black women and their implications on screening, specifically reviewing evidence behind their recommendations to stratify risk and start screening earlier for Black women.
Chen T, Kharazmi E, Fallah M. Race and ethnicity-adjusted age recommendation for initiating breast cancer screening. JAMA Netw Open. 2023;6(4): e238893. https://doi.org/10.1001/jamanetworkopen.2023.8893 .
Article PubMed PubMed Central Google Scholar
La Frinere-Sandoval QNNB, Cubbin C, DiNitto DM. Racial and ethnic disparities in cervical and breast cancer screenings by nativity and length of U.S. residence. Ethn Health. 2023:1–17. https://doi.org/10.1080/13557858.2023.2174254 .
Lawson MB, Bissell MCS, Miglioretti DL, Eavey J, Chapman CH, Mandelblatt JS, et al. Multilevel Factors Associated With Time to Biopsy After Abnormal Screening Mammography Results by Race and Ethnicity. JAMA Oncol. 2022;8(8):1115–26. https://doi.org/10.1001/jamaoncol.2022.1990 .
Nguyen KH, Pasick RJ, Stewart SL, Kerlikowske K, Karliner LS. Disparities in abnormal mammogram follow-up time for Asian women compared with non-Hispanic white women and between Asian ethnic groups. Cancer. 2017;123(18):3468–75. https://doi.org/10.1002/cncr.30756 .
Article CAS PubMed Google Scholar
Ramirez AG, Pérez-Stable EJ, Talavera GA, Penedo FJ, Carrillo JE, Fernandez ME, et al. Time to definitive diagnosis of breast cancer in Latina and non-Hispanic white women: the six cities study. Springerplus. 2013;2(1):84. https://doi.org/10.1186/2193-1801-2-84 .
Dontchos BN, Achibiri J, Mercaldo SF, Wang GX, Lamb LR, Miles RC, et al. Disparities in same-day diagnostic imaging in breast cancer screening: impact of an immediate-read screening mammography program implemented during the COVID-19 pandemic. AJR Am J Roentgenol. 2022;218(2):270–8. https://doi.org/10.2214/AJR.21.26597 .
Dontchos BN, Narayan AK, Seidler M, Mercaldo SF, Miles RC, Ebert E, et al. Impact of a same-day breast biopsy program on disparities in time to biopsy. J Am Coll Radiol. 2019;16(11):1554–60. https://doi.org/10.1016/j.jacr.2019.05.011 .
Jackson DK, Li Y, Eskander MF, Tsung A, Oppong BA, Bhattacharyya O, et al. Racial disparities in low-value surgical care and time to surgery in high-volume hospitals. J Surg Oncol. 2021;123(2):676–86. https://doi.org/10.1002/jso.26320 .
Zaveri S, Nevid D, Ru M, Moshier E, Pisapati K, Reyes SA, et al. Racial disparities in time to treatment persist in the setting of a comprehensive breast center. Ann Surg Oncol. 2022;29(11):6692–703. https://doi.org/10.1245/s10434-022-11971-w .
Sukniam K, Kasbi AA, Ashary MA, Popp K, Attwood K, George A, et al. Disparities in time to treatment for breast cancer. Anticancer Res. 2022;42(12):5813–8. https://doi.org/10.21873/anticanres.16088 .
Stabellini N, Cullen J, Cao L, Shanahan J, Hamerschlak N, Waite K, et al. Racial disparities in breast cancer treatment patterns and treatment related adverse events. Sci Rep. 2023;13(1):1233. https://doi.org/10.1038/s41598-023-27578-4 .
Article ADS CAS PubMed PubMed Central Google Scholar
Corradini S, Reitz D, Pazos M, Schönecker S, Braun M, Harbeck N, et al. Mastectomy or breast-conserving therapy for early breast cancer in real-life clinical practice: outcome comparison of 7565 cases. Cancers (Basel). 2019;11(2). https://doi.org/10.3390/cancers11020160 .
Chervu N, Darbinian K, Sakowitz S, Verma A, Bakhtiyar SS, Shuch BM, et al. Disparate utilization of breast conservation therapy in the surgical management of early-stage breast cancer. Clin Breast Cancer. 2023. https://doi.org/10.1016/j.clbc.2023.04.008 .
Relation T, Obeng-Gyasi S, Bhattacharyya O, Li Y, Eskander MF, Tsung A, et al. Racial differences in response to neoadjuvant chemotherapy: impact on breast and axillary surgical management. Ann Surg Oncol. 2021;28(11):6489–97. https://doi.org/10.1245/s10434-021-09657-w .
Fwelo P, Nwosu KOS, Adekunle TE, Afolayan O, Ahaiwe O, Ojaruega AA, et al. Racial/ethnic and socioeconomic differences in breast cancer surgery performed and delayed treatment: mediating impact on mortality. Breast Cancer Res Treat. 2023;199(3):511–31. https://doi.org/10.1007/s10549-023-06941-z .
• Yu AYL, Thomas SM, DiLalla GD, Greenup RA, Hwang ES, Hyslop T, et al. Disease characteristics and mortality among Asian women with breast cancer. Cancer. 2022;128(5):1024–37. https://doi.org/10.1002/cncr.34015 . Evaluation of breast cancer characteristics and outcomes amongst Asian subgroups, highlighting the necessity to disaggregate Asian women.
Advani P, Bondy M, Thompson PA, Martínez ME, Nodora JN, Vernon SW, et al. Impact of acculturation on breast cancer treatment and survivorship care among Mexican American patients in Texas. J Cancer Surviv. 2018;12(5):659–68. https://doi.org/10.1007/s11764-018-0703-y .
Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Costantino JP, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol. 2010;11(10):927–33. https://doi.org/10.1016/S1470-2045(10)70207-2 .
Black DM, Jiang J, Kuerer HM, Buchholz TA, Smith BD. Racial disparities in adoption of axillary sentinel lymph node biopsy and lymphedema risk in women with breast cancer. JAMA Surg. 2014;149(8):788–96. https://doi.org/10.1001/jamasurg.2014.23 .
Grimmer L, Liederbach E, Velasco J, Pesce C, Wang CH, Yao K. Variation in contralateral prophylactic mastectomy rates according to racial groups in young women with breast cancer, 1998 to 2011: a report from the national cancer data base. J Am Coll Surg. 2015;221(1):187–96. https://doi.org/10.1016/j.jamcollsurg.2015.03.033 .
Scheepens JCC, Veer LV, Esserman L, Belkora J, Mukhtar RA. Contralateral prophylactic mastectomy: a narrative review of the evidence and acceptability. Breast. 2021;56:61–9. https://doi.org/10.1016/j.breast.2021.02.003 .
Kim Y, McCarthy AM, Bristol M, Armstrong K. Disparities in contralateral prophylactic mastectomy use among women with early-stage breast cancer. NPJ Breast Cancer. 2017;3:2. https://doi.org/10.1038/s41523-017-0004-z .
Verdial FC, Bartek MA, Anderson BO, Javid SH. Genetic testing and surgical treatment after breast cancer diagnosis: results from a national online cohort. J Surg Oncol. 2021;123(7):1504–12. https://doi.org/10.1002/jso.26372 .
Nikolaidis C, Duquette D, Mendelsohn-Victor KE, Anderson B, Copeland G, Milliron KJ, et al. Disparities in genetic services utilization in a random sample of young breast cancer survivors. Genet Med. 2019;21(6):1363–70. https://doi.org/10.1038/s41436-018-0349-1 .
Reid S, Cadiz S, Pal T. Disparities in genetic testing and care among black women with hereditary breast cancer. Curr Breast Cancer Rep. 2020;12(3):125–31. https://doi.org/10.1007/s12609-020-00364-1 .
Peterson JM, Pepin A, Thomas R, Biagi T, Stark E, Sparks AD, et al. Racial disparities in breast cancer hereditary risk assessment referrals. J Genet Couns. 2020;29(4):587–93. https://doi.org/10.1002/jgc4.1250 .
Centers for Medicare & Medicaid Services. Women's Health and Cancer Rights Act (WHCRA) Women's Health and Cancer Rights Act (WHCRA). 2023. CMS.gov. Retrieved November 10, 2023, from https://www.cms.gov/cciio/programs-and-initiatives/other-insurance-protections/whcra_factsheet
Sergesketter AR, Thomas SM, Lane WO, Orr JP, Shammas RL, Fayanju OM, et al. Decline in racial disparities in postmastectomy breast reconstruction: a surveillance, epidemiology, and end results analysis from 1998 to 2014. Plast Reconstr Surg. 2019;143(6):1560–70. https://doi.org/10.1097/PRS.0000000000005611 .
Article CAS PubMed PubMed Central Google Scholar
Lee C, Sunu C, Pignone M. Patient-reported outcomes of breast reconstruction after mastectomy: a systematic review. J Am Coll Surg. 2009;209(1):123–33. https://doi.org/10.1016/j.jamcollsurg.2009.02.061 .
Cordova LZ, Hunter-Smith DJ, Rozen WM. Patient reported outcome measures (PROMs) following mastectomy with breast reconstruction or without reconstruction: a systematic review. Gland Surg. 2019;8(4):441–51. https://doi.org/10.21037/gs.2019.07.02 .
Xie Y, Tang Y, Wehby GL. Federal health coverage mandates and health care utilization: the case of the women’s health and cancer rights act and use of breast reconstruction surgery. J Womens Health (Larchmt). 2015;24(8):655–62. https://doi.org/10.1089/jwh.2014.5057 .
Hamad A, Li Y, Tsung A, Oppong B, Eskander MF, Bhattacharyya O, et al. Hispanic ethnicity and breast cancer: disaggregating surgical management and mortality by race. J Racial Ethn Health Disparities. 2022;9(4):1568–76. https://doi.org/10.1007/s40615-021-01096-3 .
Johnstone T, Thawanyarat K, Rowley M, Francis S, Camacho JM, Singh D, et al. Racial Disparities in Postoperative Breast Reconstruction Outcomes: A National Analysis. J Racial Ethn Health Disparities. 2023. https://doi.org/10.1007/s40615-023-01599-1 .
Mets EJ, Chouairi FK, Gabrick KS, Avraham T, Alperovich M. Persistent disparities in breast cancer surgical outcomes among hispanic and African American patients. Eur J Surg Oncol. 2019;45(4):584–90. https://doi.org/10.1016/j.ejso.2019.01.016 .
Comprehensive Breast Reconstruction Act of 2021, H.R.469, 117th Congress, 2021.
Dehal A, Abbas A, Johna S. Racial disparities in clinical presentation, surgical treatment and in-hospital outcomes of women with breast cancer: analysis of nationwide inpatient sample database. Breast Cancer Res Treat. 2013;139(2):561–9. https://doi.org/10.1007/s10549-013-2567-1 .
Akinyemiju TF, Vin-Raviv N, Chavez-Yenter D, Zhao X, Budhwani H. Race/ethnicity and socio-economic differences in breast cancer surgery outcomes. Cancer Epidemiol. 2015;39(5):745–51. https://doi.org/10.1016/j.canep.2015.07.010 .
Oskar S, Nelson JA, Hicks MEV, Seier MSKP, Tan KS, Chu JJ, et al. The impact of race on perioperative and patient-reported outcomes following autologous breast reconstruction. Plast Reconstr Surg. 2022;149(1):15–27. https://doi.org/10.1097/PRS.0000000000008633 .
Montagna G, Zhang J, Sevilimedu V, Charyn J, Abbate K, Gomez EA, et al. Risk factors and racial and ethnic disparities in patients with breast cancer-related lymphedema. JAMA Oncol. 2022;8(8):1195–200. https://doi.org/10.1001/jamaoncol.2022.1628 .
Deldar RM, Spoer DM, Gupta NM, Towfighi PB, Boisvert MM, Wehner PM, et al. Prophylactic lymphovenous bypass at the time of axillary lymph node dissection decreases rates of lymphedema. Ann Surg Open. 2023;4(2): e278. https://doi.org/10.1097/AS9.0000000000000278 .
Jørgensen MG, Toyserkani NM, Sørensen JA. The effect of prophylactic lymphovenous anastomosis and shunts for preventing cancer-related lymphedema: a systematic review and meta-analysis. Microsurgery. 2018;38(5):576–85. https://doi.org/10.1002/micr.30180 .
Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233–41. https://doi.org/10.1056/NEJMoa022152 .
Yeboa DN, Xu X, Jones BA, Soulos P, Gross C, Yu JB. Trend in age and racial disparities in the receipt of postlumpectomy radiation therapy for stage I breast cancer: 2004–2009. Am J Clin Oncol. 2016;39(6):568–74. https://doi.org/10.1097/COC.0000000000000094 .
Parekh A, Fu W, Hu C, Shen CJ, Alcorn S, Rao AD, et al. Impact of race, ethnicity, and socioeconomic factors on receipt of radiation after breast conservation surgery: analysis of the national cancer database. Breast Cancer Res Treat. 2018;172(1):201–8. https://doi.org/10.1007/s10549-018-4881-0 .
Wakefield DV, Carnell M, Dove APH, Edmonston DY, Garner WB, Hubler A, et al. Location as destiny: identifying geospatial disparities in radiation treatment interruption by neighborhood, race, and insurance. Int J Radiat Oncol Biol Phys. 2020;107(4):815–26. https://doi.org/10.1016/j.ijrobp.2020.03.016 .
Lamm R, Woodward SG, Varshney K, Lyons W, Anne PR, George BJ, et al. A comparison of timely completion of hypofractionated and traditional adjuvant radiation therapy in early-stage breast cancer: evidence of impact on reducing racial and socioeconomic disparities. Surgery. 2022;172(1):31–40. https://doi.org/10.1016/j.surg.2022.03.019 .
Dee EC, Taunk NK, Chino FL, Deville C, McClelland S, Muralidhar V, et al. Shorter radiation regimens and treatment noncompletion among patients with breast and prostate cancer in the united states: an analysis of racial disparities in access and quality. JCO Oncol Pract. 2023;19(2):e197–212. https://doi.org/10.1200/OP.22.00383 .
• Chapman CH, Jagsi R, Griffith KA, Moran JM, Vicini F, Walker E, et al. Mediators of racial disparities in heart dose among whole breast radiotherapy patients. J Natl Cancer Inst. 2022;114(12):1646–55. https://doi.org/10.1093/jnci/djac120 . This study uses a statewide database to evaluate racial differences in receipt of breast radiotherapy, ultimately finding that Black and Asian women receive higher cardiac doses leading to greater cardiac events and deaths compared to White women.
Jagsi R, Griffith KA, Vicini F, Boike T, Burmeister J, Dominello MM, et al. Toward improving patients’ experiences of acute toxicity from breast radiotherapy: insights from the analysis of patient-reported outcomes in a large multicenter cohort. J Clin Oncol. 2020;38(34):4019–29. https://doi.org/10.1200/JCO.20.01703 .
Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351(27):2817–26. https://doi.org/10.1056/NEJMoa041588 .
Paik S, Tang G, Shak S, Kim C, Baker J, Kim W, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24(23):3726–34. https://doi.org/10.1200/JCO.2005.04.7985 .
Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med. 2018;379(2):111–21. https://doi.org/10.1056/NEJMoa1804710 .
• Kantor O, King TA, Freedman RA, Mayer EL, Chavez-MacGregor M, Korde LA, et al. Racial and ethnic disparities in locoregional recurrence among patients with hormone receptor-positive, node-negative breast cancer: a post hoc analysis of the TAILORx randomized clinical trial. JAMA Surg. 2023;158(6):583–91. https://doi.org/10.1001/jamasurg.2023.0297 . Post hoc analysis of TAILORx clinical trial noting racial discrepancies in locoregional recurrence rate, even upon adjustment for patient, tumor, and treatment factors, overall suggesting persistent differences despite similar access to care.
Hoskins KF, Danciu OC, Ko NY, Calip GS. Association of race/ethnicity and the 21-gene recurrence score with breast cancer-specific mortality among US women. JAMA Oncol. 2021;7(3):370–8. https://doi.org/10.1001/jamaoncol.2020.7320 .
Albain KS, Gray RJ, Makower DF, Faghih A, Hayes DF, Geyer CE, et al. Race, ethnicity, and clinical outcomes in hormone receptor-positive, HER2-negative, node-negative breast cancer in the randomized TAILORx trial. J Natl Cancer Inst. 2021;113(4):390–9. https://doi.org/10.1093/jnci/djaa148 .
Jasem J, Fisher CM, Amini A, Shagisultanova E, Rabinovitch R, Borges VF, et al. The 21-gene recurrence score assay for node-positive, early-stage breast cancer and impact of RxPONDER trial on chemotherapy decision-making: have clinicians already decided? J Natl Compr Canc Netw. 2017;15(4):494–503. https://doi.org/10.6004/jnccn.2017.0049 .
Abdou Y, WE Barlow, Gralow JR. Race and clinical outcomes in the RxPONDER Trial (SWOG S1007): clinical trial Rx for positive node, endocrine responsive breast cancer. 2022 San Antonio Breast Cancer Symposium. San Antonio, TX. 2022.
•• Reid S, Haddad D, Tezak A, Weidner A, Wang X, Mautz B, et al. Impact of molecular subtype and race on HR+, HER2- breast cancer survival. Breast Cancer Res Treat. 2021;189(3):845–52. https://doi.org/10.1007/s10549-021-06342-0 . This study evaluates PAM50-based genomic signatures amongst HR+, HER2- breast cancer, overall finding racial differences in subtypes that may contribute to mortality discrepancies. These findings also suggest the possible need to re-structure the current classification of breast cancer.
Killelea BK, Yang VQ, Wang SY, Hayse B, Mougalian S, Horowitz NR, et al. Racial differences in the use and outcome of neoadjuvant chemotherapy for breast cancer: results from the national cancer data base. J Clin Oncol. 2015;33(36):4267–76. https://doi.org/10.1200/JCO.2015.63.7801 .
Shubeck S, Zhao F, Howard FM, Olopade OI, Huo D. Response to treatment, racial and ethnic disparity, and survival in patients with breast cancer undergoing neoadjuvant chemotherapy in the US. JAMA Netw Open. 2023;6(3): e235834. https://doi.org/10.1001/jamanetworkopen.2023.5834 .
Terman E, Sheade J, Zhao F, Howard FM, Jaskowiak N, Tseng J, et al. The impact of race and age on response to neoadjuvant therapy and long-term outcomes in black and white women with early-stage breast cancer. Breast Cancer Res Treat. 2023;200(1):75–83. https://doi.org/10.1007/s10549-023-06943-x .
Zhao F, Miyashita M, Hattori M, Yoshimatsu T, Howard F, Kaneva K, et al. Racial disparities in pathological complete response among patients receiving neoadjuvant chemotherapy for early-stage breast cancer. JAMA Netw Open. 2023;6(3): e233329. https://doi.org/10.1001/jamanetworkopen.2023.3329 .
Knisely AT, Michaels AD, Mehaffey JH, Hassinger TE, Krebs ED, Brenin DR, et al. Race is associated with completion of neoadjuvant chemotherapy for breast cancer. Surgery. 2018;164(2):195–200. https://doi.org/10.1016/j.surg.2018.03.011 .
Vo JB, Ramin C, Lawrence WR, Barac A, Ho KL, Rhee J, et al. Racial and ethnic disparities in treatment-related heart disease mortality among US breast cancer survivors. JNCI Cancer Spectr. 2023;7(2). https://doi.org/10.1093/jncics/pkad024 .
Schneider BP, Shen F, Jiang G, O'Neill A, Radovich M, Li L, et al. Impact of genetic ancestry on outcomes in ECOG-ACRIN-E5103. JCO Precis Oncol. 2017;2017. https://doi.org/10.1200/PO.17.00059 .
Hu X, Kaplan CM, Martin MY, Walker MS, Stepanski E, Schwartzberg LS, et al. Race differences in patient-reported symptoms during chemotherapy among women with early-stage hormone receptor-positive breast cancer. Cancer Epidemiol Biomarkers Prev. 2023;32(2):167–74. https://doi.org/10.1158/1055-9965.EPI-22-0692 .
Yee MK, Sereika SM, Bender CM, Brufsky AM, Connolly MC, Rosenzweig MQ. Symptom incidence, distress, cancer-related distress, and adherence to chemotherapy among African American women with breast cancer. Cancer. 2017;123(11):2061–9. https://doi.org/10.1002/cncr.30575 .
Samuel CA, Schaal J, Robertson L, Kollie J, Baker S, Black K, et al. Racial differences in symptom management experiences during breast cancer treatment. Support Care Cancer. 2018;26(5):1425–35. https://doi.org/10.1007/s00520-017-3965-4 .
Emerson MA, Achacoso NS, Benefield HC, Troester MA, Habel LA. Initiation and adherence to adjuvant endocrine therapy among urban, insured American Indian/Alaska Native breast cancer survivors. Cancer. 2021;127(11):1847–56. https://doi.org/10.1002/cncr.33423 .
Sheppard VB, de Mendoza AH, He J, Jennings Y, Edmonds MC, Oppong BA, et al. Initiation of adjuvant endocrine therapy in black and white women with breast cancer. Clin Breast Cancer. 2018;18(5):337-46.e1. https://doi.org/10.1016/j.clbc.2017.12.002 .
Sheppard VB, Sutton AL, Hurtado-de-Mendoza A, He J, Dahman B, Edmonds MC, et al. Race and patient-reported symptoms in adherence to adjuvant endocrine therapy: a report from the women’s hormonal initiation and persistence study. Cancer Epidemiol Biomarkers Prev. 2021;30(4):699–709. https://doi.org/10.1158/1055-9965.EPI-20-0604 .
Yanez B, Gray RJ, Sparano JA, Carlos RC, Sadigh G, Garcia SF, et al. Association of modifiable risk factors with early discontinuation of adjuvant endocrine therapy: a post hoc analysis of a randomized clinical trial. JAMA Oncol. 2021;7(8):1–7. https://doi.org/10.1001/jamaoncol.2021.1693 .
Hu X, Walker MS, Stepanski E, Kaplan CM, Martin MY, Vidal GA, et al. Racial differences in patient-reported symptoms and adherence to adjuvant endocrine therapy among women with early-stage, hormone receptor-positive breast cancer. JAMA Netw Open. 2022;5(8): e2225485. https://doi.org/10.1001/jamanetworkopen.2022.25485 .
Wheeler SB, Spencer J, Pinheiro LC, Murphy CC, Earp JA, Carey L, et al. Endocrine therapy nonadherence and discontinuation in black and white women. J Natl Cancer Inst. 2019;111(5):498–508. https://doi.org/10.1093/jnci/djy136 .
Sadigh G, Gray RJ, Sparano JA, Yanez B, Garcia SF, Timsina LR, et al. Breast cancer patients’ insurance status and residence zip code correlate with early discontinuation of endocrine therapy: an analysis of the ECOG-ACRIN TAILORx trial. Cancer. 2021;127(14):2545–52. https://doi.org/10.1002/cncr.33527 .
Sadigh G, Gray RJ, Sparano JA, Yanez B, Garcia SF, Timsina LR, et al. Assessment of racial disparity in survival outcomes for early hormone receptor-positive breast cancer after adjusting for insurance status and neighborhood deprivation: a post hoc analysis of a randomized clinical trial. JAMA Oncol. 2022;8(4):579–86. https://doi.org/10.1001/jamaoncol.2021.7656 .
Hwang GS, Paranjpe R, Opsomer C, Lu K, Abajue U, Abughosh S, et al. Oral endocrine therapy agent, race/ethnicity, and time on therapy predict adherence in breast cancer patients in a large academic institution. Clin Breast Cancer. 2020;20(6):520–6. https://doi.org/10.1016/j.clbc.2020.06.004 .
Daly B, Olopade OI, Hou N, Yao K, Winchester DJ, Huo D. Evaluation of the quality of adjuvant endocrine therapy delivery for breast cancer care in the United States. JAMA Oncol. 2017;3(7):928–35. https://doi.org/10.1001/jamaoncol.2016.6380 .
Reeder-Hayes K, Peacock Hinton S, Meng K, Carey LA, Dusetzina SB. Disparities in use of human epidermal growth hormone receptor 2-targeted therapy for early-stage breast cancer. J Clin Oncol. 2016;34(17):2003–9. https://doi.org/10.1200/JCO.2015.65.8716 .
Al-Sadawi M, Hussain Y, Copeland-Halperin RS, Tobin JN, Moskowitz CS, Dang CT, et al. Racial and socioeconomic disparities in cardiotoxicity among women with her2-positive breast cancer. Am J Cardiol. 2021;147:116–21. https://doi.org/10.1016/j.amjcard.2021.02.013 .
Litvak A, Batukbhai B, Russell SD, Tsai HL, Rosner GL, Jeter SC, et al. Racial disparities in the rate of cardiotoxicity of HER2-targeted therapies among women with early breast cancer. Cancer. 2018;124(9):1904–11. https://doi.org/10.1002/cncr.31260 .
Lao C, Lawrenson R, Edwards M, Campbell I. Treatment and survival of Asian women diagnosed with breast cancer in New Zealand. Breast Cancer Res Treat. 2019;177(2):497–505. https://doi.org/10.1007/s10549-019-05310-z .
Alvarez A, Bernal AM, Anampa J. Racial disparities in overall survival after the introduction of cyclin-dependent kinase 4/6 inhibitors for patients with hormone receptor-positive, HER2-negative metastatic breast cancer. Breast Cancer Res Treat. 2023;198(1):75–88. https://doi.org/10.1007/s10549-022-06847-2 .
Schreier A, Munoz-Arcos L, Alvarez A, Sparano JA, Anampa JD. Racial disparities in neutrophil counts among patients with metastatic breast cancer during treatment with CDK4/6 inhibitors. Breast Cancer Res Treat. 2022;194(2):337–51. https://doi.org/10.1007/s10549-022-06574-8 .
Lee KWC, Lord S, Finn RS, Lim E, Martin A, Loi S, et al. The impact of ethnicity on efficacy and toxicity of cyclin D kinase 4/6 inhibitors in advanced breast cancer: a meta-analysis. Breast Cancer Res Treat. 2019;174(1):271–8. https://doi.org/10.1007/s10549-018-5054-x .
Sohn YJ, Chang CY, Miles RC. Current gaps in breast cancer screening among Asian and Asian American women in the United States. J Am Coll Radiol. 2021;18(10):1376–83. https://doi.org/10.1016/j.jacr.2021.06.002 .
Taparra K, Dee EC, Dao D, Patel R, Santos P, Chino F. Disaggregation of Asian American and Pacific Islander women with stage 0-ii breast cancer unmasks disparities in survival and surgery-to-radiation intervals: a national cancer database analysis from 2004 to 2017. JCO Oncol Pract. 2022;18(8):e1255–64. https://doi.org/10.1200/OP.22.00001 .
Cobb RJ, Thomas CS, Laster Pirtle WN, Darity WA. Self-identified race, socially assigned skin tone, and adult physiological dysregulation: assessing multiple dimensions of “race” in health disparities research. SSM Popul Health. 2016;2:595–602. https://doi.org/10.1016/j.ssmph.2016.06.007 .
Chen JC, Pawlik T, Kelly EP, Obeng-Gyasi S. Intersectionality in patients with cancer: who should care and why? Future Oncol. 2022;18(38):4137–40. https://doi.org/10.2217/fon-2022-0992 .
Download references
Author information
Authors and affiliations.
Division of Surgical Oncology, Department of Surgery, The Ohio State University Wexner Medical Center and James Cancer Hospital, Columbus, OH, USA
JC Chen, William E. Carson & Samilia Obeng-Gyasi
Department of Internal Medicine, The Ohio State University Wexner Medical Center and James Cancer Hospital, Columbus, OH, USA
Daniel G. Stover
Department of Internal Medicine, Indiana University, Indianapolis, IN, USA
Tarah J. Ballinger & Bryan P. Schneider
Department of Radiation Oncology, City of Hope, Duarte, CA, USA
Jose G. Bazan
Department of Psychology, The Ohio State University, Columbus, OH, USA
Barbara L. Andersen
The Ohio State University, N924 Doan Hall, 410 West 10th, Columbus, OH, 43210, USA
Samilia Obeng-Gyasi
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Samilia Obeng-Gyasi .
Ethics declarations
Conflict of interest.
Samilia Obeng-Gyasi and JC Chen are funded by The Ohio State University Comprehensive Cancer Center Pelotonia Grant. Samilia Obeng-Gyasi is also funded by the Paul Calabresi Career Development Award (K12 CA133250), Conquer Cancer Breast Cancer Research Foundation Advanced Clinical Research Award for Diversity and Inclusion in Breast Cancer Research, The Society of University Surgeons, and The American Cancer Society (RSG-22-106-01-CSCT). Tarah Ballinger reports personal fees from Medscape, MDEdge, and TerSera.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Chen, J., Stover, D.G., Ballinger, T.J. et al. Racial Disparities in Breast Cancer: from Detection to Treatment. Curr Oncol Rep 26 , 10–20 (2024). https://doi.org/10.1007/s11912-023-01472-8
Download citation
Accepted : 15 October 2023
Published : 15 December 2023
Issue Date : January 2024
DOI : https://doi.org/10.1007/s11912-023-01472-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Health inequities
- Breast cancer
- Find a journal
- Publish with us
- Track your research
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Br J Cancer
- v.63(2); 1991 Feb
A proposal for short-term quality control in breast cancer screening.
Current proposals for a monitoring and evaluation system in breast cancer screening programmes focus on mortality reduction. Here emphasis is laid on the prevention of too high a number of false-positive screening results, i.e. no subsequent demonstration of malignancy. By comparing the specificity of the screening test, the positive predictive value and the detection rate with reference values, the screening performance can be measured in a very early phase of the programme, even before the registration results on interval cancers become available. The proposed average reference values for the first screening round are 99.2%, 40% and 5.4/1000, respectively. Measures specifically for the age groups 45-49, 50-59 and 60-69 will be given, thus allowing improvements to be made if necessary.
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (730K), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References .
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander FE, Roberts MM, Huggins A, Muir BB. Use of risk factors to allocate schedules for breast cancer screening. J Epidemiol Community Health. 1988 Jun; 42 (2):193–199. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Beahrs OH, Smart CR. Diagnosis of minimal breast cancers in the BCDDP: the 66 questionable cases. Cancer. 1979 Mar; 43 (3):848–850. [ PubMed ] [ Google Scholar ]
- Brecht JG, Robra BP. A graphic method of estimating the specificity of screening programmes from incomplete follow-up data. Methods Inf Med. 1987 Jan; 26 (1):53–58. [ PubMed ] [ Google Scholar ]
- Chamberlain J, Clifford RE, Nathan BE, Price JL, Burn I. Repeated screening for breast cancer. J Epidemiol Community Health. 1984 Mar; 38 (1):54–57. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Day NE, Williams DR, Khaw KT. Breast cancer screening programmes: the development of a monitoring and evaluation system. Br J Cancer. 1989 Jun; 59 (6):954–958. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Elston CW. Pathological aspects of the UK Breast Screening Project with special reference to minimal and 'borderline' lesions. Aust N Z J Surg. 1984 Jun; 54 (3):201–204. [ PubMed ] [ Google Scholar ]
- Knox EG. Evaluation of a proposed breast cancer screening regimen. BMJ. 1988 Sep 10; 297 (6649):650–654. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Peeters PH, Verbeek AL, Hendriks JH, van Bon MJ. Screening for breast cancer in Nijmegen. Report of 6 screening rounds, 1975-1986. Int J Cancer. 1989 Feb 15; 43 (2):226–230. [ PubMed ] [ Google Scholar ]
- Peeters PH, Verbeek AL, Straatman H, Holland R, Hendriks JH, Mravunac M, Rothengatter C, Van Dijk-Milatz A, Werre JM. Evaluation of overdiagnosis of breast cancer in screening with mammography: results of the Nijmegen programme. Int J Epidemiol. 1989 Jun; 18 (2):295–299. [ PubMed ] [ Google Scholar ]
- Peeters PH, Verbeek AL, Hendriks JH, Holland R, Mravunac M, Vooijs GP. The occurrence of interval cancers in the Nijmegen screening programme. Br J Cancer. 1989 Jun; 59 (6):929–932. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Tabar L, Fagerberg G, Duffy SW, Day NE. The Swedish two county trial of mammographic screening for breast cancer: recent results and calculation of benefit. J Epidemiol Community Health. 1989 Jun; 43 (2):107–114. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- van der Maas PJ, de Koning HJ, van Ineveld BM, van Oortmarssen GJ, Habbema JD, Lubbe KT, Geerts AT, Collette HJ, Verbeek AL, Hendriks JH, et al. The cost-effectiveness of breast cancer screening. Int J Cancer. 1989 Jun 15; 43 (6):1055–1060. [ PubMed ] [ Google Scholar ]
- Witcombe JB. A licence for breast cancer screening? Br Med J (Clin Res Ed) 1988 Mar 26; 296 (6626):909–911. [ PMC free article ] [ PubMed ] [ Google Scholar ]

Request an appointment Clinical Trials Give Now
Ginny L. Clements Breast Cancer Research Institute
Are you in search of a post-doctoral fellowship in cancer research?

The T32 offers career-making mentorship and transformational training
For more than 45 years, the University of Arizona Cancer Center has had an invaluable training grant through the National Cancer Institute known as the T32 .
This year, the Cancer Center has two T32 openings for postdoctoral fellows with doctoral degrees relevant to cancer prevention and control (CPC) science who are interested in advancing their research program to address cancer prevention and control and cancer related health disparities.
“The T32 provides fellows with critical protected time to establish a focused research idea and related programming,” said Cancer Center member Cynthia Thomson , PhD, RDN, T32 corresponding principal investigator. “It provides them with specific training opportunities to hone their skills and knowledge. It is also a critical time for developing professional relationships and collaborations to carry their research agenda forward in a meaningful way.”
Dr. Thomson, a professor in the UArizona College of Public Health, Nutritional Sciences and Wellness, and the BIO5 Institute , was a T32 trainee from 1998-2000 before joining the university faculty.
She is one of three multiple principal investigators on the T32, including Cancer Center members Robin B. Harris , PhD, MPH, and Terry Badger , PhD, RN.
The benefits of being a T32 fellow

The program is a two-year traineeship with exceptional mentors in cancer prevention and control science. Selected fellows engage in innovative research, submit research funding proposals, publish in peer-reviewed scientific journals, and have several opportunities to present their research at scientific conferences around the globe. The program offers preparation for independent cancer prevention academic research careers with a unique population in an environment that addresses health disparities.
“Mentorship is central to our success,” Dr. Thomson said. “Having a strong mentoring opportunity for the fellow is an important criterion for acceptance into the training program.”
Each trainee has a primary mentor, responsible for supporting the individual development plan with the student and other secondary or content-specific mentors. Dr. Thomson said the fellows’ primary mentor will be at the university, but others may come from across the U.S. or internationally.
One example of the program’s success includes former fellow Celina I. Valencia , DrPH, who is now an assistant professor in the UArizona Family and Community Medicine. She is a health equity scientist focused on developing novel methods for measuring the role of social determinants of health on the breast cancer mortality disparity experienced by women from historically marginalized communities in the U.S.
For her fellowship training, Dr. Valencia’s focused on training in epidemiology and data science analytical approaches to identify and evaluate cancer disparities. Dr. Valencia worked with Dr. Harris as her mentor.
“They provide a unique and carefully curated scientific training experience that is strategically designed to ensure that the trainee successfully accomplishes their training goal,” said Dr. Valencia of the program’s multiple principal investigators. “Additionally, they provide professional development experiences to ensure that the trainee is well positioned to launch to the next career level.”
Joining the newest fellows
Two of the four postdoctoral fellowship positions are already filled. The selected T32 scholars currently in training are Sarah Yeo , PhD, MPP, and Zachary Compton , PhD.
Dr. Yeo said her research involves elucidating multilevel factors influencing cancer prevention, including modifiable risk factors, cancer screening behaviors, and healthcare access among refugee populations. Her T32 mentor is Cancer Center member Scott Carvajal , PhD, MPH.
“I selected my mentor because our research interests closely intersected,” she said. “He possesses substantial expertise in health disparities among Hispanic immigrants through community-based participatory research.”
Dr. Carvajal’s project, “Understanding Ecologic Stress, Risk, and Health Resiliencies in Mexican-Origin Adults Living in a High Poverty Rural Community,” explores factors influencing health outcomes among foreign-born Hispanic immigrants.
Dr. Yeo said that by collaborating with Dr. Carvajal on this project, she anticipates gaining invaluable insights into cancer prevention among foreign-born immigrants, improving her skills in data analysis, and gaining familiarity with NIH-funded research.
“Participating in a career development award such as T32 is an accomplishment in itself, which can be perceived favorably by future reviewers,” she said. “More importantly, it offers fellows increased flexibility and autonomy to discern and prioritize their career goals and essential development needs. Additionally, I think that fellows have better access to resources and learning opportunities as they are considered as a significant part of the program.”

Through his T32 training program, Dr. Compton is developing methods to measure tumor phenotypic plasticity and evolvability as a means of predicting progression in pre-malignant models of oral squamous cell carcinoma.
His mentor is Carlos Caulin , PhD, associate professor in the UArizona Department of Otolaryngology and director of Translational Head/Neck Cancer Research.
“Although Dr. Caulin didn’t have any direct experience in my specific line of work, he has so many of the traits a young career scientist should seek out in a mentor,” Dr. Compton said. “I think Dr. Caulin is second to none at the university when it comes to NIH grant writing. He and I have different but matching, foci in our research, and the mentor/mentee relationship is wonderfully complementary.”
To qualify for the training program, applicants must be a U.S. citizen or permanent resident. Applicants representing diverse groups are encouraged to apply.
To learn more about applying, complete the T32 Interest Form or email Rebecca Crocker, PhD at [email protected] . View the Cancer Center T32 main page for general information.

Recommendations
Public comments and nominations, about the uspstf.
- Recommendation In Progress
- Draft Recommendation: Breast Cancer: Screening
Draft Recommendation Statement
Breast cancer: screening, may 09, 2023.
Recommendations made by the USPSTF are independent of the U.S. government. They should not be construed as an official position of the Agency for Healthcare Research and Quality or the U.S. Department of Health and Human Services.
- Update in Progress for Breast Cancer: Screening
Breast Cancer Screening Saves Lives: New Draft Available
The Task Force is now recommending that all women get screened every other year starting at age 40. The draft recommendation also urgently calls for research in key areas.
Explore this page to learn more about the latest Task Force draft recommendation on screening for breast cancer.
Dr. Carol Mangione shares key information about the draft.
Frequently asked questions.
In this draft recommendation statement, the Task Force recommends that all women get screened for breast cancer every other year starting at age 40 to reduce their risk of dying from this disease. This is a B grade .
We are also urgently calling for more research that will allow us to build on our existing recommendations and help all women live longer and healthier lives. Specifically, we need to know how best to address the health disparities across screening and treatment experienced by Black, Hispanic, Latina, Asian, Pacific Islander, Native American, and Alaska Native women.
We also need studies showing how additional screening with breast ultrasound or MRI might help women with dense breasts and evidence on the benefits and harms of screening in older women. These are I statements .
New and more inclusive science about breast cancer in people younger than 50 has enabled us to expand our prior recommendation and encourage all women to get screened in their 40s. We have long known that screening for breast cancer saves lives, and the science now supports all women getting screened, every other year, starting at age 40.
Nearly half of all women have dense breasts, which increases their risk for breast cancer and means that mammograms do not work as well for them. Women are generally told by their clinician that they have dense breasts after they've had a mammogram. These women deserve to know whether and how additional screening might help them stay healthy. Unfortunately, there is not yet enough evidence for the Task Force to recommend for or against additional screening with breast ultrasound or MRI. We are urgently calling for more research on whether and how additional screening might help women with dense breasts find cancers earlier.
Black women are 40 percent more likely to die from breast cancer than White women and too often get aggressive cancers at young ages. Ensuring Black women start screening at 40 is an important first step, yet it is not enough to improve these inequities. It's important that healthcare professionals involve patients in a conversation on how best to support them to ensure equitable follow-up after screening and timely and effective treatment of breast cancer.
We are urgently calling for more evidence to better understand whether Black women could potentially be helped by different screening strategies.
Get the Facts
- May 25, 2023 | MedPage Today (USPSTF Opinion Piece) USPSTF: What Our Patients With Dense Breasts Deserve to Know May 9, 2023 | USPSTF Task Force Issues Draft Recommendation Statement on Screening for Breast Cancer May 9, 2023 | PBS News Hour New guidelines recommend earlier mammograms amid rise in breast cancer among younger women May 9, 2023 | The Washington Post Health panel recommends women get screening mammograms at age 40
Media Contact
- Email: [email protected]
- Phone: (301) 951-9203
- Visit the Newsroom
Recommendation Summary
Pathway to benefit.
To achieve the benefit of screening and mitigate disparities in breast cancer mortality by race and ethnicity, it is important that all persons with abnormal screening mammography receive equitable and appropriate followup evaluation and additional testing, inclusive of indicated biopsies, and that all persons diagnosed with breast cancer receive effective treatment.
Additional Information
- Supporting Evidence and Research Taxonomy
- Related Resources & Tools
- Draft Modeling Report (May 09, 2023)
- Draft Evidence Review (May 09, 2023)
- Final Research Plan (May 06, 2021)
- Draft Research Plan (January 21, 2021)
- Screening for Breast Cancer (Consumer Guide): Draft Recommendation | Link to File
Recommendation Information
Full recommendation:.
The US Preventive Services Task Force (USPSTF) makes recommendations about the effectiveness of specific preventive care services for patients without obvious related signs or symptoms to improve the health of people nationwide.
It bases its recommendations on the evidence of both the benefits and harms of the service and an assessment of the balance. The USPSTF does not consider the costs of providing a service in this assessment.
The USPSTF recognizes that clinical decisions involve more considerations than evidence alone. Clinicians should understand the evidence but individualize decision-making to the specific patient or situation. Similarly, the USPSTF notes that policy and coverage decisions involve considerations in addition to the evidence of clinical benefits and harms.
The USPSTF is committed to mitigating the health inequities that prevent many people from fully benefiting from preventive services. Systemic or structural racism results in policies and practices, including health care delivery, that can lead to inequities in health. The USPSTF recognizes that race, ethnicity, and gender are all social rather than biological constructs. However, they are also often important predictors of health risk. The USPSTF is committed to helping reverse the negative impacts of systemic and structural racism, gender-based discrimination, bias, and other sources of health inequities, and their effects on health, throughout its work.
Among all U.S. women, breast cancer is the second most common cancer and the second most common cause of cancer death. In 2022, an estimated 43,250 women died of breast cancer. 1 Non-Hispanic White women have the highest incidence of breast cancer (5-year age-adjusted incidence rate, 137.6 cases per 100,000 women) and non-Hispanic Black women have the second highest incidence rate (5-year age-adjusted incidence rate, 129.6 cases per 100,000 women). 2 Incidence has gradually increased among women ages 40 to 49 years from 2000 to 2015 but increased more noticeably from 2015 to 2019, with a 2.0% average annual increase. 3 Despite having a similar or higher rate of mammography screening, 4 Black women are more likely to be diagnosed with breast cancer beyond stage 1 than other racial and ethnic groups, are more likely to be diagnosed with triple-negative cancers (i.e., ER-, PR-, and HER2-), which are more aggressive tumors, compared with White women, 5 and are approximately 40% more likely to die from breast cancer compared with White women. 6
The U.S. Preventive Services Task Force (USPSTF) concludes with moderate certainty that biennial screening mammography in women ages 40 to 74 years has a moderate net benefit .
The USPSTF concludes that the evidence is insufficient to determine the balance of benefits and harms of screening mammography in women age 75 years or older.
The USPSTF concludes that the evidence is insufficient to determine the balance of benefits and harms of supplemental screening for breast cancer with breast ultrasound or MRI, regardless of breast density.
Go to Table 1 for more information on the USPSTF recommendation rationale and assessment. For more details on the methods the USPSTF uses to determine the net benefit, see the USPSTF Procedure Manual. 7
Patient Population Under Consideration
These recommendations apply to cisgender women and all other persons assigned female at birth (including transgender men and nonbinary persons) age 40 years or older at average risk of breast cancer. This is because the net benefit estimates are driven by sex (i.e., female) rather than gender identity, although the studies reviewed for this recommendation generally used the term “women.” These recommendations apply to persons with a family history of breast cancer (i.e., those with a first-degree relative with breast cancer) and to persons who have other risk factors such as having dense breasts. They do not apply to persons who have a genetic marker or syndrome associated with a high risk of breast cancer (e.g., BRCA1 or BRCA2 genetic mutations), a history of high-dose radiation therapy to the chest at a young age, or previous breast cancer or a high-risk breast lesion on previous biopsies.
Screening Tests
Both digital mammography (DM) and digital breast tomosynthesis (DBT or “3D mammography”) are effective mammographic screening modalities. DBT must be accompanied by traditional DM or synthetic DM, which is a two-dimensional image constructed from DBT data; 8 , 9 hereafter, references to DBT will imply concurrent use with DM or synthetic DM. In general, studies have reported small increases in positive predictive value with DBT compared with DM. Trials reporting on at least two consecutive rounds of screening have generally found no statistically significant difference in breast cancer detection or in tumor characteristics (tumor size, histologic grade, or node status) when comparing screening with DBT vs. DM. 4
The Breast Cancer Surveillance Consortium (BCSC) is a network of six active breast imaging registries and two historic registries, providing a large observational database related to breast cancer screening. 10 Collaborative modeling, using inputs from BCSC data, suggests similar benefits and fewer false-positive results with DBT compared with DM. 11
Screening Interval
Available evidence suggests a more favorable trade-off of benefits vs. harms with biennial vs. annual screening. BCSC data showed no difference in detection of stage IIB+ cancers and cancers with less favorable prognostic characteristics with annual vs. biennial screening interval for any age group, 12 and modeling data estimate that biennial screening has a more favorable balance of benefits to harms compared with annual screening. 11
Treatment or Intervention
Breast cancer treatment regimens are highly individualized according to each patient’s clinical status, cancer stage, tumor biomarkers, clinical subtype, and personal preferences. 13 Ductal carcinoma in situ (DCIS) is a noninvasive condition with abnormal cells in the breast duct lining and there is uncertainty regarding the prognostic importance of DCIS. Consequently, there is clinical variability in the treatment approach when DCIS is identified at screening. It is unknown what proportion of screen-detected DCIS represents overdiagnosis (i.e., a lesion that would not have led to health problems in the absence of detection by screening). In general, DCIS treatment, which may include surgery, radiation, and endocrine treatment, is intended to reduce the risk for future invasive breast cancer.
Disparities in Breast Cancer Outcomes and Implementation Considerations
Mortality from breast cancer is highest for Black women even when accounting for differences in age and stage at diagnosis; mortality is approximately 40% higher for Black women compared with White women. 6 While the underlying causes of this disparity are complex, the National Institute of Minority Health and Disparities has developed a framework that recognizes multiple determinants, including the healthcare system, the sociocultural and built environments, behavioral factors, and genetic factors, that can contribute to health inequities. 14 Inequities in breast cancer mortality can be examined at each step along the cancer screening, diagnosis, treatment, and survival pathway with these factors in mind. The higher mortality rate for Black women diagnosed with breast cancer in the United States aligns with other health inequities that are attributed to the effects of structural racism, which include inequalities in resources, harmful exposures, and access to and delivery of high-quality healthcare. 15-17 Racial and economic residential segregation driven by discriminatory housing policies has been associated with poorer breast cancer survival. Residential segregation also increases exposure to toxic environments such as air pollution, industrial waste, and built environments that do not support health, and stressful life conditions, which can increase cancer risk. 18-20
Black women have a higher incidence of breast cancer with at least one negative molecular marker, and the incidence of triple-negative cancers (i.e., ER-, PR-, and HER2-) is twice as high compared with White women (24.1 vs. 12.4 cases per 100,000 women). 5 The higher incidence of negative hormonal receptor (HR) status leads to worse outcomes because these subtypes are less readily detected through screening and less responsive to current therapy, 21 and triple-negative cancers are more likely to be aggressive and diagnosed at later stages than other subtypes. It is important to note that observed regional differences in the incidence of HR-negative cancer within and between racial groups suggest that environmental factors and social determinants of health, including racism, are largely responsible for the differential risk of developing HR-negative cancer. 22 , 23 Although differences in the incidence of cancer subtypes explain some of the differences in breast cancer mortality, racial differences in mortality within subtypes point to barriers to obtaining high-quality healthcare and disparities in screening followup and treatment initiation as contributors. 22
Of note, Black women have a similar or higher rate of self-reported mammography screening as all women (84.5% vs 78%, respectively, in the past 2 years). 4 However, benefits from mammography screening require initiation and completion of appropriate and effective followup evaluation and treatment. Both screening and guideline-concordant treatment are essential for reducing breast cancer mortality, 24 highlighting the importance of timely and effective treatment at the earliest stage of diagnosis. Delays and inadequacies in the diagnostic and treatment pathway downstream from screening likely contribute to increased mortality compared with women receiving prompt, effective care.
Disparities in followup after screening and treatment have been observed for Black, Hispanic, and Asian women. 25-34 Adjuvant endocrine therapy reduces the risk of cancer recurrence among individuals with HR-positive cancers, but long-term adherence can be difficult. Black women are more likely to discontinue adjuvant endocrine therapy compared with White women, in part due to greater physical (vasomotor, musculoskeletal, or cardiorespiratory) and psychological (distress or despair) symptom burdens. 33 , 34 Improvements in access to effective healthcare, removal of financial barriers, and use of support services to ensure equitable followup after screening and timely and effective treatment of breast cancer have the potential to reduce mortality for individuals experiencing disparities related to racism, rural location, 35 low income, or other factors associated with lower breast cancer survival.
Suggestions for Practice Regarding the I Statement
Potential preventable burden.
Breast cancer incidence increases with age and peaks among persons ages 70 to 74 years, though rates in persons age 75 years or older remain high (460.2 and 416.5 cases per 100,000 women ages 75–79 and 80–84 years, respectively, compared with 477.7 cases per 100,000 women ages 70–74 years), and mortality from breast cancer increases with increasing age. 36 , 37 However, no randomized clinical trials (RCTs) of breast cancer screening included women age 75 years or older. 4 Collaborative modeling suggests that screening in women age 75 years or older is of benefit, 11 but a trial emulation found no benefit with breast cancer screening in women ages 75 to 84 years. 38 Thus, there is insufficient evidence to recommend for or against screening mammography in women age 75 years or older.
There is insufficient evidence about the effect of supplemental screening using breast ultrasonography or MRI on health outcomes such as breast cancer morbidity and mortality in women with dense breasts who have an otherwise normal screening mammogram. Dense breasts are associated with both reduced sensitivity and specificity of mammography and with an increased risk of breast cancer. 39 , 40 However, increased breast density itself is not associated with higher breast cancer mortality among women diagnosed with breast cancer, after adjustment for stage, treatment, method of detection, and other risk factors, according to data from the BCSC. 41
Potential Harms
Potential harms of screening mammography include false-positive results, which may lead to psychological harms, additional testing, and invasive followup procedures; overdiagnosis and overtreatment of lesions that would not have led to health problems in the absence of detection by screening; and radiation exposure.
Current Practice
Centers for Disease Control and Prevention data show that as of 2015, over 50% of women age 75 years or older reported having a mammogram within the past 2 years. 42 At the present time, 38 states and the District of Columbia require patient notification of breast density when mammography is performed; in some states, legislation also includes notification language informing women that they should consider adjunctive screening. 43 Starting in September 2024, the U.S. Food and Drug Administration will require mammography centers to notify patients of their breast density, inform them that dense breast tissue raises the risk of breast cancer and makes it harder to detect on a mammogram, and that other imaging tests may help to find cancer. 44
Additional Tools and Resources
The National Cancer Institute has information on breast cancer screening for healthcare professionals ( https://www.cancer.gov/types/breast/hp/breast-screening-pdq ) and for patients ( https://www.cancer.gov/types/breast/patient/breast-screening-pdq ).
The Centers for Disease Control and Prevention has information on breast cancer screening ( https://www.cdc.gov/cancer/breast/basic_info/screening.htm ).
Other Related USPSTF Recommendations
The USPSTF has made recommendations about the use of medications to reduce women’s risk for breast cancer, 45 as well as risk assessment, genetic counseling, and genetic testing for BRCA1 - or BRCA2 -related cancer. 46
When final, this recommendation will update the 2016 recommendation on breast cancer screening. In 2016, the USPSTF recommended biennial screening mammography for women ages 50 to 74 years and individualizing the decision to undergo screening for women ages 40 to 49 years, based on factors such as individual risk and personal preferences and values. The USPSTF concluded that the evidence was insufficient to assess the benefits and harms of DBT as a primary screening method; the balance of benefits and harms of adjunctive screening for breast cancer using breast ultrasonography, MRI, or DBT in women identified to have dense breasts on an otherwise negative screening mammogram; and the balance of benefits and harms of screening mammography in women age 75 years or older. 47 For the current draft recommendation, the USPSTF recommends biennial screening mammography for women ages 40 to 74 years. The USPSTF again finds that the evidence is insufficient to assess the balance of benefits and harms of supplemental screening for breast cancer using breast ultrasonography or MRI in women identified to have dense breasts on an otherwise negative screening mammogram and the balance of benefits and harms of screening mammography in women age 75 years or older. Current evidence suggests that both DM and DBT are effective primary screening modalities.
Scope of Review
To update its 2016 recommendation, the USPSTF commissioned a systematic review on the comparative effectiveness of different mammography-based breast cancer screening strategies by age to start and stop screening, screening interval, modality, use of supplemental imaging, or personalization of screening for breast cancer on the incidence and progression to advanced breast cancer, breast cancer morbidity, and breast cancer–specific or all-cause mortality. The review also assessed the harms of different breast cancer screening strategies. 4 Evidence from the trials that established breast cancer screening effectiveness has not been updated, as there are no new studies that include a group that is not screened. Analyses from prior reviews of that evidence were considered foundational evidence for the current recommendation.
In addition to the systematic evidence review, the USPSTF commissioned collaborative modeling studies from CISNET (Cancer Intervention and Surveillance Modeling Network) to provide information about the benefits and harms of breast cancer screening strategies that vary by the ages to begin and end screening, screening modality, screening interval, and by race. 11 The modeling studies complement the evidence that the systematic review provides.
In alignment with the USPSTF’s commitment to improve health equity, the evidence review included contextual questions on the drivers behind and approaches to address disparities in health outcomes related to breast cancer, particularly the higher mortality in Black women, and the CISNET collaborative modeling investigated outcomes of screening for Black women.
Benefits and Comparative Benefits of Early Detection and Treatment
Randomized trials that began enrolling participants more than 30 to 40 years ago have established the effectiveness of screening mammography to reduce breast cancer mortality. A meta-analysis conducted in support of the 2016 USPSTF breast cancer screening recommendation found that screening mammography was associated with relative risk (RR) reductions in breast cancer mortality of 0.88 (95% confidence interval [CI], 0.73 to 1.00; 9 trials) for women ages 39 to 49 years, 0.86 (95% CI, 0.68 to 0.97; 7 trials) for women ages 50 to 59 years, 0.67 (95% CI, 0.54 to 0.83; 5 trials) for women ages 60 to 69 years, and 0.80 (95% CI, 0.51 to 1.28; 3 trials) for women ages 70 to 74 years, 48 and an updated analysis of three Swedish screening trials reported a 15% relative reduction in breast cancer mortality for women ages 40 to 74 years (RR, 0.85 [95% CI, 0.73 to 0.98]). 49 Only one of these trials enrolled a significant proportion of Black women. 50 None of the trials nor the combined meta-analysis demonstrated a difference in all-cause mortality with screening mammography. The current USPSTF review focused on the comparative benefits of different screening strategies.
Age to Start or Stop Screening
The USPSTF did not identify any RCTs designed to test the comparative effectiveness of different ages to start or stop screening that reported morbidity, mortality, or quality of life outcomes. One trial emulation study (N=264,274), using a random sample from Medicare claims data, estimated the effect of women stopping screening at age 70 years compared with those who continued annual screening after age 70 years. Based on survival analysis, this study reported that continued screening between the ages of 70 and 74 years was associated with a 22% decrease in the risk of breast cancer mortality, compared with a cessation of screening at age 70 years, and there was no difference in the hazard ratio or absolute rates of breast cancer mortality with continued screening vs. discontinued screening from ages 75 to 84 years. 38
Collaborative modeling data estimated that compared with biennial screening from ages 50 to 74 years, biennial screening starting at age 40 years until 74 years would lead to 1.3 additional breast cancer deaths averted per 1,000 women screened over a lifetime of screening for all women. Modeling also estimated that screening benefits for Black women are similar for breast cancer mortality reduction and greater for life-years gained and breast cancer deaths averted compared with all women. Thus, biennial screening starting at age 40 years would result in 1.8 additional breast cancer deaths averted per 1,000 women screened for Black women. 11 Epidemiologic data has shown that the incidence rate of invasive breast cancer for 40- to 49-year-old women has increased an average of 2.0% annually between 2015 and 2019, a higher rate than in previous years. 3 These factors led the USPSTF to conclude that screening mammography in women ages 40 to 49 years has a moderate benefit in reducing the risk of breast cancer mortality.
The USPSTF did not identify any randomized trials directly comparing annual vs. biennial screening that reported morbidity, mortality, or quality of life outcomes. One controlled trial (N=14,765) conducted in Finland during the years 1985 to 1995 assigned participants ages 40 to 49 years to annual or triennial screening invitations based on birth year (even birth year: annual; odd birth year: triennial) and reported similar mortality from incident breast cancer and for all-cause mortality between the two groups, with followup to age 52 years. 51
A nonrandomized study using BCSC data (N=15,440) compared the tumor characteristics of cancers detected following annual vs. biennial screening intervals. 12 The relative risk of being diagnosed with a stage IIB or higher cancer and cancer with less favorable characteristics was not statistically different for biennially vs. annually screened women in any of the age categories. The risk of a stage IIB or higher cancer diagnosis and of having a tumor with less favorable prognostic characteristics were higher for premenopausal women screened biennially vs. annually (RR, 1.28 [95% CI, 1.01 to 1.63] and RR, 1.11 [95% CI, 1.00 to 1.22], respectively). However, this study did not conduct formal tests for interaction in the subgroup comparisons and did not adjust for multiple comparisons.
One RCT (n=76,022) conducted between 1989 and 1996 randomized individuals to annual or triennial screening and reported on breast cancer incidence. The number of screen-detected cancers was higher in the annual screening study group (RR, 1.64 [95% CI, 1.28 to 2.09]). However, the total number of cancers diagnosed either clinically or with screening was similar after 3 years of screening. Cancers occurring in the annual screening group (including clinically diagnosed cancers) did not differ by prognostic features such as tumor size, node positivity status, or histologic grade compared with those in the triennial screening group. 52
Collaborative modeling estimated that biennial screening results in greater incremental life-years gained and mortality reduction per mammogram and has a more favorable balance of benefits to harms for all women and for Black women, compared with annual screening. While modeling suggests that screening Black women annually and screening other women biennially would reduce the disparity in breast cancer mortality, 11 trial or observational evidence is lacking that screening any group of women annually compared with biennial screening improves mortality from breast cancer. 4
The USPSTF did not identify any RCTs or observational studies that compared screening with DBT vs. DM and reported morbidity, mortality, or quality of life outcomes.
Three RCTs 53-55 and one nonrandomized study 56 compared detection of invasive cancer over two rounds of screening with DBT vs. DM. These trials screened all participants with the same screening modality at the second screening round—DM in two trials and the nonrandomized study, and DBT in one trial. Stage shift or differences in tumor characteristics across screening rounds could offer indirect evidence of potential screening benefit. The trials found no statistically significant difference in detection at the second screening round (pooled RR, 0.87 [95% CI, 0.73 to 1.05]; 3 trials; n=105,064). 4 The nonrandomized study (n=92,404) found higher detection at round one for the group screened with DBT and higher detection at round two for the group screened with DM at both rounds. There were no statistically significant differences in tumor diameter, histologic grade, and node status at the first or second round of screening in any of these studies.
Collaborative modeling data estimated that the benefits of DBT are similar to the estimated benefits of DM (e.g., approximately 5 to 6 more life-years gained per 1,000 women screened). 11
Supplemental Screening With MRI or Ultrasonography, or Personalized Screening
The USPSTF found no studies of supplemental screening with MRI or ultrasonography, or studies of personalized (e.g., risk-based) screening strategies, that reported on morbidity or mortality or on cancer detection and characteristics over multiple rounds of screening. 4 Collaborative modeling studies did not investigate the effects of screening with MRI or ultrasonography. Modeling generally estimated that the benefits of screening mammography would be greater for persons at modestly increased risk (e.g., the risk of breast cancer associated with a first-degree family history of breast cancer). 11
Harms of Screening
For this recommendation, the USPSTF also reviewed the harms of screening for breast cancer and whether the harms varied by screening strategy. Potential harms of screening for breast cancer include false-positive and false-negative results, need for additional imaging and biopsy, overdiagnosis, and radiation exposure.
The most common harm is a false-positive result, which can lead to psychological harms, as well as additional testing and invasive followup procedures without the potential for benefit. Collaborative modeling data estimated that a strategy of screening biennially from ages 40 to 74 years would result in 1,376 false-positive results per 1,000 women screened over a lifetime of screening. 11
Overdiagnosis occurs when breast cancer that would never have become a threat to a person’s health, or even apparent, during their lifetime is found due to screening. It is not possible to directly observe for any individual person whether they have or do not have an overdiagnosed tumor; it is only possible to indirectly estimate the frequency of overdiagnosis that may occur across a screened population. Estimates of overdiagnosis from RCTs that had comparable groups at baseline, had adequate followup, and did not provide screening to the control group at the end of the trial range from approximately 11% to 19%. 4 Collaborative modeling data estimate that a strategy of screening biennially from ages 40 to 74 years would lead to 14 overdiagnosed cases of breast cancer per 1,000 persons screened over the lifetime of screening, though with a very wide range of estimates (4 to 37 cases) across models. 11
One trial emulation (n=264,274) compared discontinuation of mammography screening at age 70 years or older with continued annual screening beyond this age. 38 Overall, the 8-year cumulative risk of a breast cancer diagnosis was higher for the continued annual screening strategy after age 70 years (5.5% overall; 5.3% in women ages 70–74 years; 5.8% in women ages 75–84 years) compared with the stop screening strategy (3.9% overall; same proportion for both age groups). Fewer cancers were diagnosed under the stop screening strategy (ages 70 to 84 years); consequently, there was a lower risk of undergoing followup and treatment. For women aged 75 to 84 years, additional diagnoses did not contribute to a difference in the risk of breast cancer mortality, raising the possibility that the additionally diagnosed cancers represent overdiagnosis.
Collaborative modeling data estimated that lowering the age to start screening to 40 years from 50 years would result in about a 60% increase in false-positive results, and 2 additional overdiagnosed cases of breast cancer (range, 0–4) per 1,000 women over a lifetime of screening. 11
Rates of interval cancers (cancer diagnosis occurring between screening) reported in screening studies reflect a combination of cancers that were missed during previous screening examinations (false-negative results) and incident cancers emerging between screening rounds. Evidence from studies comparing various intervals and reporting on the effect of screening interval on the rate of interval cancers is mixed. One RCT comparing annual vs. triennial screening reported that the rate of interval cancers was significantly lower in the annual invitation group (1.84 cases per 1,000 women initially screened) than in the triennial invitation group (2.70 cases per 1,000 women initially screened) (RR, 0.68 [95% CI, 0.50 to 0.92]), 52 while a second quasirandomized study, also comparing annual vs. triennial screening, found no difference in the number of interval cancers between the two groups. 51
Based on two studies, false-positive recall was more likely to occur with annual screening compared with longer intervals between screening. 57 , 58 One of these studies, using data from the BCSC, reported that biennial screening led to a 5% absolute decrease in the 10-year cumulative false-positive biopsy rate compared with annual screening, whether screening was conducted with DBT or DM. 57 Collaborative modeling estimated that annual screening results in more false-positive results and breast cancer overdiagnosis. For example, a strategy of screening annually from ages 40 to 74 years would result in about 50% more false-positive results and 50% more overdiagnosed cases of breast cancer compared with biennial screening for all women and a similar increase in false-positive results and a somewhat smaller increase in overdiagnosed cases for Black women. 11
Three RCTs did not show statistically significant differences in the risk of interval cancer following screening with DBT or DM (pooled RR, 0.87 [95% CI, 0.64 to 1.17]; 3 trials; n=130,196). 4 Five nonrandomized studies generally support the RCT findings. Three of the nonrandomized studies found no significant difference in the rate of interval cancers diagnosed following screening with DBT or DM, 56 , 59 , 60 while one study found a slight increased risk with DBT screening, 61 and one study found an unadjusted decreased risk with DBT screening. 62
A pooled analysis of three RCTs (n=105,244) comparing screening with DBT vs. DM did not find a difference in false-positive recalls at the second round of screening. 4 A nonrandomized study using BCSC data reported that the estimated cumulative probability of having at least one false-positive recall over 10 years of screening was generally lower with DBT screening compared with DM screening (annual screening: 10-year cumulative probability of a false-positive recall was 49.6% with DBT and 56.3% with DM; biennial screening: 10-year cumulative probability of a false-positive recall was 35.7% for DBT and 38.1% for DM). The risk of having a biopsy over 10 years of screening was slightly lower when comparing annual screening with DBT vs. DM but did not differ between DBT and DM for biennial screening (annual screening: 10-year cumulative probability of a false-positive biopsy was 11.2% with DBT and 11.7% with DM; biennial screening: 10-year cumulative probability of a false-positive biopsy was 6.6% for DBT and 6.7% for DM). When results were stratified by breast density, the difference in false-positive recall probability with DBT vs. DM was largest for women with nondense breasts and was not significantly different among women with extremely dense breasts. 57 Collaborative modeling, using inputs from BCSC data, estimated that screening women ages 40 to 74 years with DBT would result in 167 fewer false-positive results (range, 166 to 169) per 1,000 persons screened, compared with DM. 11
In the three RCTs cited above, rates of DCIS detected did not differ between persons screened with DBT and DM. 53-55
Screening with DBT includes evaluation of a two-dimensional image, generated either with DM or using the DBT scan to produce a synthetic DM image. 8 , 9 Studies using DBT with DM screening reported radiation exposure approximately two times higher compared with the DM-only control group. 53 , 55 , 63 Differences in radiation exposure were smaller in studies using DBT/synthetic DM compared with DM. 64 , 65
Supplemental Screening With Ultrasonography or MRI
The DENSE RCT, which compared invitation to screening with DM plus MRI compared with DM alone in participants ages 50 to 75 years with extremely dense breasts and a negative mammogram, reported a significantly lower rate of invasive interval cancers—2.2 cases per 1,000 women invited to screening with DM plus MRI, compared with 4.7 cases per 1,000 women invited to screening with DM only (RR, 0.47 [95% CI, 0.29 to 0.77]). 66
In this trial, the rate of recall among participants who underwent additional imaging with MRI was 94.9 per 1,000 screens, the false-positive rate was 79.8 per 1,000 women screened, and the rate of biopsy was 62.7 per 1,000 women screened. 67 In a nonrandomized study using U.S. insurance claims data, individuals who had an MRI compared with those receiving only a mammogram were more likely in the subsequent 6 months to have additional cascade events related to extramammary findings (adjusted difference between groups, 19.6 per 100 women screened [95% CI, 8.6 to 30.7]), mostly additional healthcare visits. 68
In an RCT comparing screening with DM plus ultrasonography vs. DM alone conducted in persons ages 40 to 49 years and not specifically among persons with dense breasts, the interval cancer rates reported were not statistically significantly different between the two groups (RR, 0.58 [95% CI, 0.31 to 1.08]); 69 similarly, in a nonrandomized study comparing DM plus ultrasonography vs. DM alone using BCSC data, there was no difference in interval cancers (adjusted RR, 0.67 [95% CI, 0.33 to 1.37]) (72), though in both studies the confidence intervals were wide for this uncommon outcome. In the BCSC analysis, the rates of referral to biopsy and false-positive biopsy recommendations were twice as high and short interval followup was three times higher for the group screened with ultrasonography. 70
See Table 2 for research needs and gaps related to screening for breast cancer.
The American Cancer Society recommends that women with an average risk of breast cancer should undergo regular screening mammography starting at age 45 years. It suggests that women ages 45 to 54 years should be screened annually, that women age 55 years or older should transition to biennial screening or have the opportunity to continue screening annually, that women should have the opportunity to begin annual screening between the ages of 40 and 44 years, and that women should continue screening mammography as long as their overall health is good and they have a life expectancy of 10 years or longer. 71
The American College of Obstetricians and Gynecologists recommends that women at average risk of breast cancer should be offered screening mammography starting at age 40 years, using shared decision making, and if they have not initiated screening in their 40s, they should begin screening mammography by no later than age 50 years. It recommends that women at average risk of breast cancer should have screening mammography every 1 or 2 years and should continue screening mammography until at least age 75 years. Beyond age 75 years, the decision to discontinue screening mammography should be based on shared decision making informed by the woman’s health status and longevity. 72
The American Academy of Family Physicians supports the current USPSTF recommendation on screening for breast cancer. 73
1. National Cancer Institute; Surveillance Epidemiology and End Results Program. Cancer Stat Facts: Female Breast Cancer. Accessed April 20, 2023. https://seer.cancer.gov/statfacts/html/breast.html 2. National Cancer Institute; Surveillance Epidemiology and End Results Program. Breast: SEER 5-Year Age-Adjusted Incidence Rates, 2016-2020, by Race/Ethnicity, Female, All Ages, All Stages. Accessed April 20, 2023. https://seer.cancer.gov/statistics-network/explorer/application.html?site=55&data_type=1&graph_type=10&compareBy=race&chk_race_6=6&chk_race_5=5&chk_race_4=4&chk_ race_9=9&chk_race_8=8&series=9&sex=3&age_range=1&stage=101&advopt_precision=1&advopt_show_ci=on&hdn_view=0#resultsRegion0 3. National Cancer Institute; Surveillance Epidemiology and End Results Program. SEER*Stat Database: Incidence - SEER Research Limited-Field Data with Delay-Adjustment, 22 Registries, Malignant Only, Nov 2021 Sub (2000-2019) - Linked To County Attributes - Time Dependent (1990-2019) Income/Rurality, 1969-2020 Counties. Bethesda, MD: National Cancer Institute; 2022. 4. Henderson JT, Webber, EM, Weyrich M, Miller M, Melnikow J. Screening for Breast Cancer: A Comparative Effectiveness Review for the U.S. Preventive Services Task Force. Evidence Synthesis No. 231. Rockville, MD: Agency for Healthcare Research and Quality; 2023. AHRQ Publication No. 23-05303-EF-1. 5. National Cancer Institute; Surveillance Epidemiology and End Results Program. Breast: SEER 5-Year Age-Adjusted Incidence Rates, 2016-2020, by Subtype, Female, All Races/Ethnicities, All Ages, All Stages. Accessed April 20, 2023. https://seer.cancer.gov/statistics-network/explorer/application.html?site=55&data_type=1&graph_type=10&compareBy=subtype&chk_subtype_55=55&chk_subtype_622=622&chk_subtype_ 623=623&chk_subtype_620=620&chk_subtype_621=621&series=9&sex=3&race=1&age_range=1&stage=101&advopt_precision=1&advopt_show_ci=on&hdn_view=0 6. Giaquinto AN, Sung H, Miller KD, et al. Breast cancer statistics, 2022. CA Cancer J Clin . 2022;72(6):524-541. 7. U.S. Preventive Services Task Force. Procedure Manual. Accessed April 20, 2023. https://www.uspreventiveservicestaskforce.org/uspstf/about-uspstf/methods-and-processes/procedure-manual 8. Ciatto S, Houssami N, Bernardi D, et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol . 2013;14(7):583-589. 9. Skaane P, Bandos AI, Eben EB, et al. Two-view digital breast tomosynthesis screening with synthetically reconstructed projection images: comparison with digital breast tomosynthesis with full-field digital mammographic images. Radiology . 2014;271(3):655-663. 10. Breast Cancer Surveillance Consortium. About the BCSC. Accessed April 20, 2023. https://www.bcsc-research.org/about 11. Trentham-Dietz A, Chapman CH, Jinani J, et al. Breast Cancer Screening With Mammography: An Updated Decision Analysis for the U.S. Preventive Services Task Force. Rockville, MD: Agency for Healthcare Research and Quality; 2023. AHRQ Publication No. 23-05303-EF-2. 12. Miglioretti DL, Zhu W, Kerlikowske K, et al. Breast tumor prognostic characteristics and biennial vs annual mammography, age, and menopausal status . JAMA Oncol . 2015;1(8):1069-1077. 13. National Comprehensive Cancer Network. Breast Cancer Screening and Diagnosis. 2019. 14. Alvidrez J, Castille D, Laude-Sharp M, Rosario A, Tabor D. The National Institute on Minority Health and health disparities research framework. Am J Public Health . 2019;109(S1):S16-S20. 15. Williams DR, Priest N, Anderson NB. Understanding associations among race, socioeconomic status, and health: patterns and prospects. Health Psychol . 2016;35(4):407-411. 16. Bailey ZD, Krieger N, Agénor M, Graves J, Linos N, Bassett MT. Structural racism and health inequities in the USA: evidence and interventions. Lancet . 2017;389(10077):1453-1463. 17. Zavala VA, Bracci PM, Carethers JM, et al. Cancer health disparities in racial/ethnic minorities in the United States. Br J Cancer . 2021;124(2):315-332. 18. Bemanian A, Beyer KM. Measures matter: the local exposure/isolation (LEx/Is) metrics and relationships between local-level segregation and breast cancer survival. Cancer Epidemiol Biomarkers Prev . 2017;26(4):516-524. 19. Goel N, Westrick AC, Bailey ZD, et al. Structural racism and breast cancer-specific survival: impact of economic and racial residential segregation. Ann Surg . 2022;275(4):776-783. 20. Siegel SD, Brooks MM, Lynch SM, Sims-Mourtada J, Schug ZT, Curriero FC. Racial disparities in triple negative breast cancer: toward a causal architecture approach. Breast Cancer Res . 2022;24(1):37. 21. Niraula S, Biswanger N, Hu P, Lambert P, Decker K. Incidence, characteristics, and outcomes of interval breast cancers compared with screening-detected breast cancers. JAMA Netw Open . 2020;3(9):e2018179. 22. Jatoi I, Sung H, Jemal A. The emergence of the racial disparity in U.S. breast-cancer mortality. New Engl J Med . 2022;386(25):2349-2352. 23. Davis Lynn BC, Chernyavskiy P, Gierach GL, Rosenberg PS. Decreasing incidence of estrogen receptor-negative breast cancer in the United States: trends by race and region. J Natl Cancer Inst . 2022;114(2):263-270. 24. Plevritis SK, Munoz D, Kurian AW, et al. Association of screening and treatment with breast cancer mortality by molecular subtype in US women, 2000-2012. JAMA . 2018;319(2):154-164. 25. Fayanju OM, Ren Y, Stashko I, et al. Patient-reported causes of distress predict disparities in time to evaluation and time to treatment after breast cancer diagnosis. Cancer . 2021;127(5):757-768. 26. Selove R, Kilbourne B, Fadden MK, et al. Time from screening mammography to biopsy and from biopsy to breast cancer treatment among black and white, women Medicare beneficiaries not participating in a health maintenance organization. Womens Health Issues . 2016;26(6):642-647. 27. Nguyen KH, Pasick RJ, Stewart SL, Kerlikowske K, Karliner LS. Disparities in abnormal mammogram follow-up time for Asian women compared with non-Hispanic white women and between Asian ethnic groups. Cancer . 2017;123(18):3468-3475. 28. Warner ET, Tamimi RM, Hughes ME, et al. Time to diagnosis and breast cancer stage by race/ethnicity. Breast Cancer Res Treat . 2012;136(3):813-821. 29. Kovar A, Bronsert M, Jaiswal K, et al. The waiting game: how long are breast cancer patients waiting for definitive diagnosis? Ann Surg Oncol . 2020;27(10):3641-3649. 30. Elmore JG, Nakano CY, Linden HM, Reisch LM, Ayanian JZ, Larson EB. Racial inequities in the timing of breast cancer detection, diagnosis, and initiation of treatment. Med Care . 2005;43(2):141-148. 31. Emerson MA, Golightly YM, Aiello AE, et al. Breast cancer treatment delays by socioeconomic and health care access latent classes in Black and White women. Cancer . 2020;126(22):4957-4966. 32. Lawson MB, Bissell MC, Miglioretti DL, et al. Multilevel factors associated with time to biopsy after abnormal screening mammography results by race and ethnicity. JAMA Oncol . 2022;8(8):1115-1126. 33. Hu X, Walker MS, Stepanski E, et al. Racial differences in patient-reported symptoms and adherence to adjuvant endocrine therapy among women with early-stage, hormone receptor-positive breast cancer. JAMA Netw Open . 2022;5(8):e2225485. 34. Hu X, Chehal PK, Kaplan C, et al. Characterization of clinical symptoms by race among women with early-stage, hormone receptor-positive breast cancer before starting chemotherapy. JAMA Netw Open . 2021;4(6):e2112076. 35. Clemons K, Blackford AL, Gupta A, et al. Geographic disparities in breast cancer mortality and place of death in the United States from 2003 to 2019. J Clin Oncol . 2022;40(16 Suppl):12034. 36. National Cancer Institute; Surveillance Epidemiology and End Results Program. Breast: SEER Incidence Rates by Age at Diagnosis, 2016-2020, by Sex, Delay-Adjusted SEER Incidence Rate, All Races/Ethnicities. Accessed April 20, 2023. https://seer.cancer.gov/statistics-network/explorer/application.html?site=55&data_type=1&graph_type=3&compareBy=sex&chk_sex_3=3&rate_type=2&race=1&advopt_precision=1&advopt_ show_ci=on&hdn_view=0#resultsRegion0 37. National Cancer Institute; Surveillance Epidemiology and End Results Program. Breast: U.S. Mortality Rates by Age at Death, 2016-2020, by Sex, All Races/Ethnicities. Accessed April 20, 2023. https://seer.cancer.gov/statistics-network/explorer/application.html?site=55&data_type=2&graph_type=3&compareBy=sex&chk_sex_3=3&race=1&advopt_precision=1&advopt_show_ci=on&hdn_ view=0#resultsRegion0 38. García-Albéniz X, Hernán MA, Logan RW, Price M, Armstrong K, Hsu J. Continuation of annual screening mammography and breast cancer mortality in women older than 70 years. Ann Intern Med . 2020;172(6):381-389. 39. Kerlikowske K, Zhu W, Tosteson AN, et al. Identifying women with dense breasts at high risk for interval cancer: a cohort study. Ann Intern Med . 2015;162(10):673-681. 40. Price ER, Hargreaves J, Lipson JA, et al. The California breast density information group: a collaborative response to the issues of breast density, breast cancer risk, and breast density notification legislation. Radiology . 2013;269(3):887-892. 41. Gierach GL, Ichikawa L, Kerlikowske K, et al. Relationship between mammographic density and breast cancer death in the Breast Cancer Surveillance Consortium. J Natl Cancer Inst . 2012;104(16):1218-1227. 42. Centers for Disease Control and Prevention. Health, United States, 2018. Accessed April 20, 2023. https://www.cdc.gov/nchs/data/hus/hus18.pdf 43. Dense Breast-info. State Legislation Map. Accessed April 20, 2023. https://densebreast-info.org/legislative-information/state-legislation-map/ 44. Mammography Quality Standards Act, 21 C.F.R. § 900 (2023). 45. US Preventive Services Task Force. Medication use to reduce risk of breast cancer: US Preventive Services Task Force recommendation statement. JAMA . 2019;322(9):857-867. 46. US Preventive Services Task Force. Risk assessment, genetic counseling, and genetic testing for BRCA-related Cancer: US Preventive Services Task Force recommendation statement . JAMA . 2019;322(7):652-665. 47. US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med . 2016;164(4):279-296. 48. Nelson HD, Cantor A, Humphrey L, et al. Screening for Breast Cancer: A Systematic Review to Update the 2009 U.S. Preventive Services Task Force Recommendation. Evidence Synthesis No. 124. Rockville, MD: Agency for Healthcare Research and Quality; 2016. AHRQ Publication No. 14-05201-EF-1. 49. Nyström L, Bjurstam N, Jonsson H, Zackrisson S, Frisell J. Reduced breast cancer mortality after 20+ years of follow-up in the Swedish randomized controlled mammography trials in Malmö, Stockholm, and Göteborg. J Med Screen . 2017;24(1):34-42. 50. Jones BA, Patterson EA, Calvocoressi L. Mammography screening in African American women: evaluating the research. Cancer . 2003;97(1 Suppl):258-272. 51. Parvinen I, Chiu S, Pylkkänen L, Klemi P, Immonen-Räihä P, Kauhava L, et al. Effects of annual vs triennial mammography interval on breast cancer incidence and mortality in ages 40-49 in Finland. Br J Cancer . 2011;105:1388-91. 52. The frequency of breast cancer screening: results from the UKCCCR randomised trial. United Kingdom Co-ordinating Committee on Cancer Research. Eur J Cancer . 2002;38(11):1458-1464. 53. Armaroli P, Frigerio A, Correale L, et al. A randomised controlled trial of digital breast tomosynthesis versus digital mammography as primary screening tests: screening results over subsequent episodes of the Proteus Donna study. Int J Cancer . 2022;151(10):1778-1790. 54. Hofvind S, Moshina N, Holen ÅS, et al. Interval and subsequent round breast cancer in a randomized controlled trial comparing digital breast tomosynthesis and digital mammography screening. Radiology . 2021;300(1):66-76. 55. Pattacini P, Nitrosi A, Giorgi Rossi P, et al. A randomized trial comparing breast cancer incidence and interval cancers after tomosynthesis plus mammography versus mammography alone. Radiology . 2022;303(2):256-266. 56. Hovda T, Holen ÅS, Lång K, et al. Interval and consecutive round breast cancer after digital breast tomosynthesis and synthetic 2d mammography versus standard 2d digital mammography in BreastScreen Norway. Radiology . 2020;294(2):256-264. 57. Ho TH, Bissell MC, Kerlikowske K, et al. Cumulative probability of false-positive results after 10 years of screening with digital breast tomosynthesis vs digital mammography. JAMA Netw Open . 2022;5(3):e222440 58. McGuinness JE, Ueng W, Trivedi MS, et al. Factors associated with false positive results on screening mammography in a population of predominantly Hispanic women. Cancer Epidemiol Biomarkers Prev . 2018;27(4):446-453. 59. Conant EF, Beaber EF, Sprague BL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography compared to digital mammography alone: a cohort study within the PROSPR consortium. Breast Cancer Res Treat . 2016;156(1):109-116. 60. Kerlikowske K, Su YR, Sprague BL, et al. Association of screening with digital breast tomosynthesis vs digital mammography with risk of interval invasive and advanced breast cancer. JAMA . 2022;327(22):2220-2230. 61. Richman IB, Long JB, Hoag JR, et al. Comparative effectiveness of digital breast tomosynthesis for breast cancer screening among women 40-64 years old. J Natl Cancer Inst . 2021;113(11):1515-1522. 62. Johnson K, Lang K, Ikeda DM, et al. Interval breast cancer rates and tumor characteristics in the prospective population-based Malmö breast tomosynthesis screening trial. Radiology . 2021;299(3):559-567. 63. Zackrisson S, Lång K, Rosso A, et al. One-view breast tomosynthesis versus two-view mammography in the Malmö Breast Tomosynthesis Screening Trial (MBTST): a prospective, population-based, diagnostic accuracy study. Lancet Oncol . 2018;19(11):1493-1503. 64. Heindel W, Weigel S, Gerß J, et al. Digital breast tomosynthesis plus synthesized mammography versus digital screening mammography for the detection of invasive breast cancer (TOSYMA): a multicentre, open-label, randomised, controlled, superiority trial. Lancet Oncol . 2022;23(5):601-611. 65. Aase HS, Holen ÅS, Pedersen K, et al. A randomized controlled trial of digital breast tomosynthesis versus digital mammography in population-based screening in Bergen: interim analysis of performance indicators from the To-Be trial. Eur Radiol. 2019;29(3):1175-1186. 66. Bakker MF, de Lange SV, Pijnappel RM, et al. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med . 2019;381(22):2091-2102. 67. Veenhuizen SG, de Lange SV, Bakker MF, et al. Supplemental breast MRI for women with extremely dense breasts: results of the second screening round of the DENSE trial. Radiology . 2021;299(2):278-286. 68. Ganguli I, Keating NL, Thakore N, Lii J, Raza S, Pace LE. downstream mammary and extramammary cascade services and spending following screening breast magnetic resonance imaging vs mammography among commercially insured women. JAMA Netw Open . 2022;5(4):e227234. 69. Ohuchi N, Suzuki A, Sobue T, et al. Sensitivity and specificity of mammography and adjunctive ultrasonography to screen for breast cancer in the Japan Strategic Anti-cancer Randomized Trial (J-START): a randomised controlled trial. Lancet . 2016;387(10016):341-348. 70. Lee JM, Arao RF, Sprague BL, et al. Performance of screening ultrasonography as an adjunct to screening mammography in women across the spectrum of breast cancer risk. JAMA Intern Med . 2019;179(5):658-667. 71. Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA . 2015;314(15):1599-1614. 72. Committee on Practice Bulletins—Gynecology. Practice Bulletin Number 179: breast cancer risk assessment and screening in average-risk women. Obstet Gynecol . 2017;130(1):e1-e16. 73. American Academy of Family Physicians. Clinical Preventive Service Recommendation: Breast Cancer. Accessed April 20, 2023. https://www.aafp.org/family-physician/patient-care/clinical-recommendations/all-clinical-recommendations/breast-cancer.html
Abbreviations: MRI=magnetic resonance imaging; USPSTF=U.S. Preventive Services Task Force.
To fulfill its mission to improve health by making evidence-based recommendations for preventive services, the USPSTF routinely highlights the most critical evidence gaps for creating actionable preventive services recommendations. The USPSTF often needs additional evidence to create the strongest recommendations for everyone, especially those with the greatest burden of disease. In some cases, clinical preventive services have been well studied, but there are important evidence gaps that prevent the USPSTF from making recommendations for specific populations. In Table 2, the USPSTF summarizes the gaps in the evidence for screening for breast cancerand emphasizes health equity gaps that need to be addressed to advance the health of the nation. Although the health equity gaps focus on Black women because they have the poorest health outcomes from breast cancer, it is important to note that all studies should actively recruit enough women of all racial and ethnic groups, including Black, Hispanic, Asian, Native American/Alaska Native, and Native Hawaiian/Pacific Islander participants, to investigate whether the effectiveness of screening, diagnosis, and treatment vary by group.
Abbreviations: DBT=digital breast tomosynthesis; DCIS=ductal carcinoma in situ; DM=digital mammography; MRI=magnetic resonance imaging.
University of South Florida
Main Navigation

Emanuelle M. Dias, PhD, MPH, CPH. (Photo courtesy of Dias)
From tragedy to triumph: One alum’s path to the U.S. Army
- Liz Bannon, College of Public Health
- April 19, 2024
- Alumni & Development
USF College of Public Health (COPH) alumna Emanuelle Dias is a proud native Floridian who grew up in South Florida. When she was 14 years old, her mother passed away from breast cancer which inspired her healing journey through education, research and cancer prevention.
Dias began her education at the University of Florida as a pre-med student. She was halfway through her first year when she started exploring different paths. “I took an introduction to health disparities class and knew in that moment it was exactly what I wanted to have a career in,” she said.
After graduating, Dias decided to pursue her master of public health at the COPH. “I knew that it was the number one program in the state and I would be able to work with my mentor, former COPH professor Dr. Alicia Best,” Dias said. “We had very similar backgrounds, both losing our mothers to breast cancer, and that was part of our public health journey that connected us. It just felt right to be at USF.”
Dias said one of the most memorable experiences she had was in March of 2019 with the International Health Service Collaborative , a group of USF Health students, faculty and professionals focused on promoting sustainable health in underserved communities abroad.
“We went on a medical mission trip to Jamaica for a week during spring break,” she said. “It was incredible because one of the students was from Jamaica and helped make many connections and contacts there for us. We were able to squeeze in some fun time too.”

Dias, pictured front row and second from the left, conducted a one-week medical mission trip to Montego Bay, Jamaica in March 2019 with the International Health Service Collaboration. (Photo courtesy of Dias)
Dias points to two people who inspired her during her time at the COPH.
“ Dr. Dinorah "Dina" Martinez Tyson embodies being a community-engaged researcher. Her cancer survivorship camp motivated and inspired me to be the type of researcher that I'm still working on being. The kind of public health professional that I strive to be,” Dias said. “Then, Dr. Mahmooda Pasha believed in me and encouraged me to take on a PhD.”
Dias also participated in a leadership training that promoted Latino researchers to get their PhD's in public health. “After that, I knew I wanted to do research in the cancer prevention space,” she said. Dias became involved with the Florida Prevention Research Center with Dr. Pasha and Professor Dr. Claudia Parvanta . “The center allowed me to build strong research experience, work on presentations and publications and allowed me to become a competitive applicant going into a PhD program,” Dias said. Dias was accepted to the University of Texas Health Science Center in Houston for her PhD where she is currently residing. “Houston is very much a cancer prevention research hub,” Dias said. “It aligned with my journey and so I grew my research skills, solidified myself as a scientist and was able to expand and become a research professional.”
During her PhD program, Dias said she was funded by a National Cancer Institute predoctoral fellowship, which allowed her to focus on her dissertation.
Dias graduated in December 2023 with a PhD in public health, with a focus in health promotion and behavioral sciences.
“After I graduated, I had to decide what was next,” she said. “I could have gone the typical route and stayed in academia and go through with the postdoctoral fellowship, but I decided to leave to explore some other sectors.”
In January 2024, Dias was offered a position as an Oak Ridge Institute for Science and Education fellow with the U.S. Department of the Army.
“This was something I honestly never envisioned myself doing. No one in my family has been enlisted and I don’t know a lot of Army folks, but it has been a really rewarding experience. I’ve even visited Washington, D.C., and the Pentagon through my work!”

D ias, pictured front row on the far left, in the Pentagon Library and Conference Center, March 2024 with the Integrated Prevention Advisory Group, the U.S. Army's new primary prevention workforce. (Photo courtesy of Dias)
“I'm part of an integrated prevention division,” Dias said. “We are working on preventing harmful behaviors including sexual assault, suicide, substance misuse, child mistreatment and domestic partnership violence.” “I’m working at the strategic level to build this workforce,” Dias said. “This infrastructure is solidifying a research agenda. As this has never existed, we're starting from scratch. I’ve been working on literature review and scanning strategic initiatives at the Centers for Disease Control and Prevention and other government organizations to align with our organization.” For the future, Dias says she will most likely stay in the government sector but she’s also interested in going into the private sector, like a research institution. No matter where she ends up, she says the journey she started after her mom’s passing feels complete.
“There's so much opportunity and public health continues to grow. The U.S. Army is developing a public health infrastructure, and I know we will continue to see these various roles across different sectors.”

Dias presented a poster exploring the association between maternal and paternal characteristics and the risk of colorectal cancer in adult offspring at the American Society of Preventive Oncology in March 2023 in San Diego, California. (Photo courtesy of Dias)
Fast Five What did you dream of becoming when you were young? I wanted to be a medical doctor. Where would we find you on the weekend? I am very active, so I love weightlifting and yoga.
What is the last book you read? “Everything I Know About Love” by Dolly Alderton. What superpower would you like to have? Teleportation, so I can get to places quickly.
What is your all-time favorite movie? “The Proposal” with Sandra Bullock. It’s one of the last movies that I watched with my mom before she passed.
Return to article listing
Emanuelle Dias
Explore More Categories
- Awards & Honors
- College News
- Community Engagement
- In the Media
- Research & Innovation
- Student Life
- USF SafetyFlorida
About Department News
Welcome to the USF COPH news page. Our marketing and communications team is entrusted with storytelling. Through written stories, photography, video and social media we highlight alumni, faculty, staff and students who are committed to passionately solving problems and creating conditions that allow every person the universal right to health and well-being. These are our stories.
- Share full article

Should Alcoholic Beverages Have Cancer Warning Labels?
Ireland will require them starting in 2026, and there are nascent efforts elsewhere to add more explicit labeling about the health risks of drinking.
An example of a label that will be added in 2026 to all beer, wine and liquor sold in Ireland, emphasizing ties between alcohol use and liver disease or cancer. Credit... Alcohol Action Ireland
Supported by
By Ted Alcorn
- Published April 9, 2024 Updated April 11, 2024
Fifteen words are roiling the global alcohol industry.
Beginning in 2026, containers of beer, wine and liquor sold in Ireland will be required by law to bear a label in red capital letters with two warnings: “THERE IS A DIRECT LINK BETWEEN ALCOHOL AND FATAL CANCERS” and “DRINKING ALCOHOL CAUSES LIVER DISEASE.”
The requirement, signed into law last year, is backed by decades of scientific research and goes much further than any country has thus far communicated the health risks of alcohol consumption. It has sparked fierce opposition from alcohol businesses worldwide, but it is also inspiring a push in some other countries to pursue similar measures.
“It’s an important step,” said Dr. Timothy Naimi, the director of the Canadian Institute for Substance Use Research at the University of Victoria. “People who drink should have the right to know basic information about alcohol, just as they do for other food and beverage products.”
In Thailand, the government is in the final stages of drafting a regulation requiring alcohol products to carry graphic images accompanied by text warnings such as “alcoholic beverages can cause cancer,” according to The Bangkok Post .
A bill has been introduced in the Canadian Parliament that would require labels on all alcoholic beverages to communicate a “direct causal link between alcohol consumption and the development of fatal cancers.”
Last week, the Alaska State Legislature held a committee hearing on a bill that would require businesses selling alcohol to post signs carrying a cancer warning.
Norway, which already heavily regulates the sale of alcohol, is developing proposals for introducing cancer warning labels. The country’s state secretary, Ole Henrik Krat Bjorkholt, who followed Ireland’s effort with great interest, said in an interview, “I think it’s probable that we will implement something similar.”
Ireland has been a trailblazer in setting aggressive public health policies before. In 2004, it became the first country to ban smoking in indoor workplaces, including bars and restaurants, a policy since adopted in over 70 countries. The warning label requirement for alcohol could be the start of a similar change in how beverages are packaged, and a vehicle for raising awareness about the dangers of drinking, however small the amount.
A long fight
The evidence linking drinking and cancer is well established. In 1988, the World Health Organization’s International Agency for Research on Cancer concluded that alcohol is carcinogenic to humans. Research in the decades since has only strengthened the conclusion, including for breast, liver, colorectal and esophageal cancers. In November, the W.H.O. and the I.A.R.C. declared in a joint statement : “No safe amount of alcohol consumption for cancers can be established.”
Despite this, the connection between alcohol and cancer isn’t well known. In the United States, a recent nationwide survey found that about one in three Americans was aware that drinking increased the risk of cancer.
Globally, only a quarter of countries require any kind of health warning on alcohol, according to a recent study , and the mandated language is generally imprecise. The United States last altered its warning labels in 1989 , when it introduced language that discouraged drinking during pregnancy, or before driving or operating heavy machinery, and that vaguely acknowledged that alcohol “may cause health problems.”

It took over a decade for Ireland’s labeling requirement to become a reality, according to Sheila Gilheany, chief executive of the advocacy organization Alcohol Action Ireland, who described it as “the most contested piece of legislation in Irish history.” She said that the effort began in 2012, when a steering group assigned to address the country’s high rate of alcohol-related deaths recommended a raft of measures, including warning labels.
Many of the recommendations were watered down by the time they became law in 2018, but the labeling requirement made it through unscathed. It took another four years for lawmakers to hammer out the specific wording and the design that would be required.
As those details were decided, alcohol companies stepped up their protests. In late 2022, a group of major alcohol-exporting European countries submitted formal objections to the European Commission, the European Union’s executive branch, arguing that Ireland’s labels impeded free trade and were not appropriate or proportionate to the objective of reducing alcohol’s harms.
When the commission raised no objection, Antonio Tajani, Italy’s foreign minister, called the Irish proposal “an attack on the Mediterranean diet.” The language in the labels “doesn’t take into account the difference between moderate consumption and alcohol abuse,” he said on Twitter .
Coordinated industry opposition
Alcohol businesses are fighting on multiple fronts to keep the Irish labeling requirement from taking effect. At committee meetings of the World Trade Organization in June and November, trade groups and eleven alcohol-exporting countries, including the United States, expressed concerns, questioned the scientific validity of the cancer warning and argued that Ireland’s labels would infringe on free trade.
In comments submitted to the World Trade Organization, the Distilled Spirits Council of the United States called the labels “inaccurate” and “misleading.” The group also suggested that “this important public health objective would be best managed” as part of a parallel effort to address cancer in the European Union, an area where the alcohol industry has proved to have greater influence.
The European Commission was supposed to propose language for alcohol health warnings as part of the its Beating Cancer Plan by the end of 2023 but failed to meet that deadline. In December, over the objections of the World Health Organization , the European Parliament approved a report that did not affirm the need for warning labels, instead calling for information on “moderate and responsible drinking.”
In the final report, its authors repeatedly watered down language about alcohol’s role in disease, narrowly warning only about “harmful” or “excess” consumption.
Size and design
Cormac Healy, the director of Drinks Ireland, a trade group, said that his organization wasn’t entirely utterly opposed to health warnings. But he said that the mandated size of the labels would be impractical for use on smaller products, picking a 50-milliliter bottle up from his desk to demonstrate. And the warning language itself was “disproportionate and inaccurate,” he said, and primarily geared toward scaring people.
“To inform, to educate — you can’t really do that on a label,” he added.
In the United States, alcohol warning labels are typically on the back of the bottle or can, where they blend in with other graphic features. Dr. Marissa Hall, an assistant professor in the department of health behavior at the University of North Carolina at Chapel Hill, said that the labels would be more effective at catching a purchaser’s eye if they were on the front, included an image or icon, and featured one of a rotating group of brief messages.
Dr. Hall recently received a grant from the National Institutes of Health to test the impact of stronger design features. When she tells friends about her research, many are surprised to learn the United States requires warning labels at all, she said, because the existing ones so easily go unnoticed.
“They have no idea,” she said.
In the last 15 years, a few countries have proposed stronger alcohol warning labels, but each has been met with fierce opposition, said Paula O’Brien, a professor of law at the University of Melbourne. In 2010, Thailand proposed requiring a rotating group of warnings accompanied by graphic color imagery; O’Brien called it “the high-water mark for alcohol labeling.” But at the World Trade Organization, other countries raised concerns that the labels would restrain free trade, and the measure stalled.
In 2016, South Korea overcame similar objections to mandate a group of warning labels, some of which link alcohol with cancer, that alcohol makers can choose from to put on their products.
Even research on the topic has been contentious. In 2017, Yukon, a sparsely populated territory in northwest Canada, forged a partnership with scientists to introduce and test the impact of brightly colored warning labels, one of which included the phrase “alcohol can cause cancer.” But after alcohol trade groups complained, the local government paused the study out of fear it would face a lawsuit that it could not afford to fight.
“I was a bit surprised about the strength of the reaction,” said Dr. Erin Hobin, a scientist at Public Health Ontario who led the project in Yukon.
When the researchers resumed the study several months later, on the condition that the cancer warning be omitted, they found that people buying alcoholic beverages featuring the labels were still more likely to notice the messages, and reported reducing their drinking. Sales of products carrying the labels also fell by around 7 percent during the intervention and several months that followed.
Most importantly, Dr. Hobin said, as drinkers grew more informed about the link between alcohol and cancer, they also became more likely to support policies for controlling alcohol availability, pricing and marketing, which have been shown to reduce drinking even more.
If the alcohol industry dissuaded the European Union from adopting warning labels, it would keep Ireland isolated and out of harmony with European law. That could ultimately form a basis for challenging the labeling requirement in Irish courts, said Dr. Ollie Bartlett, an assistant professor of law at Maynooth University in Ireland. But he said that such efforts were unlikely to prevail because Ireland’s alcohol warning labels are “proportionate to the objective of protecting public health.”
Observers say the European Union isn’t likely to take any further action until after parliamentary elections this summer. And there’s no indication that Ireland will retreat from its commitment to require the labels starting in May 2026.
Dr. Gauden Galea, a strategic adviser at the World Health Organization, said he was confident that broader labeling efforts would eventually succeed. At 63, he’s old enough to recall how cigarette companies once advertises on the front pages of newspapers, he added.
Eventually, he hopes, “People will not remember the time when you needed a warning on pesticides, but could sell an unlabeled carcinogen like alcohol with impunity.”
The Fight Against Cancer
We asked experts what to know about melanoma symptoms, treatment and prevention. Here’s how to avoid one of the deadliest forms of skin cancer.
Colon and rectal cancers are increasing among people younger than 50. Experts have a few ideas about why .
Should alcoholic beverages have cancer warning labels? Ireland will require them starting in 2026, and there are nascent efforts elsewhere .
Risk calculators can offer a more personalized picture of an individual patient’s breast cancer risk. But experts warn that the results need to be interpreted with the help of a doctor .
The human papillomavirus vaccine provides powerful protection against the leading cause of cervical cancer and against a strong risk factor for anal cancer. Here’s what to know about the shot .
A recent study adds to growing evidence that exercise is an important part of preventing prostate cancer , the second most common and second most fatal cancer in the United States for men.
Advertisement

IMAGES
COMMENTS
prevalent cases of cancer and about 35,000 new cases are added to this every year. Based on the. (18.5%) it ranks second to cervical cancer. The burden of breast cancer is increasing in both. rate ...
A breast cancer research workforce that represents the breast cancer community is one way to work towards health equity and better outcomes for all. By supporting these promising trainees early in their research careers, Komen seeks to ensure that a diverse group of highly trained scientists who reflect the communities we serve will emerge as ...
Pre-op Pembro + Radiation Therapy in Breast Cancer (P-RAD) Rochester, MN. The purpose of this trilal is to study a combination of neoadjuvant radiotherapy (RT), immunotherapy (pembrolizumab) and chemotherapy for lymph node-positive, triple negative (TN) or hormone receptor positive/HER2-negative breast cancer.
Vision - A world without breast cancer. The BCRP challenges the scientific community to design research that will address the urgency of ending breast cancer. Specifically, the BCRP seeks to accelerate high-impact research with clinical relevance, encourage innovation and stimulate creativity, and facilitate productive collaborations.
Request for Proposals . June 4, 2021 . I. Introduction Conquer Cancer, the ASCO Foundation and Pfizer Global Medical Grants are collaborating to offer a research grant funding opportunity to promote the advancement of scientific knowledge concerning equitable care of metastatic breast cancer (mBC) patients. This research grant
PDF | Breast cancer is the most common diagnosed malignancy in women worldwide (22%) and in India (18.5%) it ranks second to cervical cancer. ... Research Proposal PDF Available. Breast Cancer ...
Introduction. Therapy with curative intent for breast cancer achieves optimal outcomes when it is administered to completion within a defined timeframe; its success depends on appropriate referrals for timely and personalized multimodality treatment after the receipt of a definitive diagnosis. 1 The provision of cancer treatment requires an organized, multidisciplinary approach in which ...
Sample size calculations for the pilot study showed that, assuming an OR for breast cancer mortality of 0.7 and a number of discordant pairs of 33%, two controls per case with 800 breast cancer ...
New research questions emerge as medical needs continue to evolve and as we improve our understanding of cancer biology and treatment of malignancies. Although significant advances have been made in some areas of breast cancer research resulting in improvements in therapies and outcomes over the last few decades, other areas have not benefited to the same degree and we continue to have many ...
Most women who develop breast cancer in a high-income country will survive; the opposite is true for women in most low-income and many middle-income countries . In 2020 breast cancer mortality-to-incidence ratio (MIR) as a representative indicator of 5-year survival rates was 0.30 globally . Taking into consideration the clinical extent of ...
Breast cancer is the most common malignant tumor in women in the world. Breast cancer patients account for as much as 36% of oncological patients. An estimated 2.089 million women were diagnosed with breast cancer in 2018 [, ]. The incidence of this malignant tumor is increasing in all regions of the world, but the highest incidence occurs in ...
NCI is funding a large-scale randomized breast screening trial, the Tomosynthesis Mammographic Imaging Screening Trial (TMIST), to compare the number of advanced cancers detected in women screened for 5 years with 3-D mammography with the number detected in women screened with 2-D mammography. Two concerns in breast cancer screening, as in all ...
More than half a million of the world's women died from breast cancer in 2012, making it one of the leading (BC) causes of cancer-related deaths in women across the world (Ferlay et al., 2015). The phosphatidylinositol 3-kinase (PI3K)/Akt pathway represents a complex signalling network that integrates numerous upstream stimuli to
Every year, millions of women are diagnosed with breast cancer worldwide, and 2019 estimates from the National Cancer Institute indicate that in the triennium between 2020 and 2022, 200,000 new cases of the disease are expected to be diagnosed in Brazil (INCA 2019).. In the last three decades, the mortality rate of the disease has increased, which is most likely due to increased incidence due ...
Posted: January 20, 2023. Many young women who are diagnosed with early-stage breast cancer want to become pregnant in the future. New research suggests that these women may be able to pause their hormone therapy for up to 2 years as they try to get pregnant without raising the risk of a recurrence in the short term.
Department of Defense Breast Cancer Research Program, should supply clear, public statements describing the benefit of including patient advocates in the research process. All disease-related
In addition, breast cancer is one of the most common types of cancer. For example, from 2012 to 2016, breast cancer had the highest incidence rate in the United States . Naturally, breast cancer and research on it are of great concern to the public and healthcare professionals alike. Second, lots of funding flows into breast cancer research.
The Pamela Delp Polashenski M.D. Breast Cancer Research Trainee Grant in the amount of $25,000; The grants are awarded with the potential to yield significant medical breakthroughs in the cause and prevention of breast cancer, prevention of metastasis, and cure. Research proposals are solicited from regional medical and research institutions ...
We are committed to supporting young investigators in breast cancer research and encourage applications from talented new postdoctoral researchers. 2. Eligibility Please note the following: ... research proposal for a different award (for example, a rejected fellowship resubmitted as a
BCH Research proposal - approved & clearance received from institutional review board (irb, USA) and institutional ethics committee (IEC, India). ... DR LOPAMUDRA DAS ROY's Research contributions. CLICK HERE TO DOWNLOAD. Breast Cancer Hub Epidemiological Research & Clinical Data Analysis Study: Research approved by WCG IRB (Institutional Review ...
1. Background. Cancer is a group of diseases that causes by the uncontrolled growth and spread of abnormal cells anywhere in the body (Diabate et al., Citation 2018.Breast cancer is a type of malignant tumor, which it starts in the cells of the breast tissue that is made up of glands for milk production, called lobules, and the ducts that connect the lobules to the nipple.
The Lancet Breast Cancer Commission—a diverse, multidisciplinary international group—are unanimous in our determination to improve the lives of all people who live with or are at risk of breast cancer. We came together in July, 2021, and are committed to raising the standard of breast cancer care to close the equity gap that exists between and within countries. Over a 2-year period, we ...
Given the increase in the incidence of breast cancer during the past decades, several studies have investigated the effects of variables on breast cancer, especially obesity. This systematic review and meta-analysis aims to evaluate any effects of obesity on breast cancer risk in women, before and after menopause, and in different continents.All forms of relevant literature examining any ...
Purpose of Review Update on current racial disparities in the detection and treatment of breast cancer. Recent Findings Breast cancer remains the leading cause of cancer death among Black and Hispanic women. Mammography rates among Black and Hispanic women have surpassed those among White women, with studies now advocating for earlier initiation of breast cancer screening in Black women. Black ...
Abstract. Current proposals for a monitoring and evaluation system in breast cancer screening programmes focus on mortality reduction. Here emphasis is laid on the prevention of too high a number of false-positive screening results, i.e. no subsequent demonstration of malignancy. By comparing the specificity of the screening test, the positive ...
The program is a two-year traineeship with exceptional mentors in cancer prevention and control science. Selected fellows engage in innovative research, submit research funding proposals, publish in peer-reviewed scientific journals, and have several opportunities to present their research at scientific conferences around the globe.
A meta-analysis conducted in support of the 2016 USPSTF breast cancer screening recommendation found that screening mammography was associated with relative risk (RR) reductions in breast cancer mortality of 0.88 (95% confidence interval [CI], 0.73 to 1.00; 9 trials) for women ages 39 to 49 years, 0.86 (95% CI, 0.68 to 0.97; 7 trials) for women ...
USF College of Public Health (COPH) alumna Emanuelle Dias is a proud native Floridian who grew up in South Florida. When she was 14 years old, her mother passed away from breast cancer which inspired her healing journey through education, research and cancer prevention.
UF Health Cancer Center members Carma Bylund, Ph.D., a professor in the department of health outcomes and biomedical informatics, and Susan Bluck, Ph.D., a lifespan developmental psychologist and gerontologist in the UF College of Liberal Arts and Sciences' department of psychology, recently co-hosted a summit at UF that delved into the science of how people care for one another ...
Norway, which already heavily regulates the sale of alcohol, is developing proposals for introducing cancer warning labels. The country's state secretary, Ole Henrik Krat Bjorkholt, who followed ...