

How To Do Secondary Research or a Literature Review
- Secondary Research
- Literature Review
- Step 1: Develop topic
- Step 2: Develop your search strategy
- Step 3. Document search strategy and organize results
- Systematic Literature Review Tips
- More Information
Search our FAQ
Make a research appointment.
Schedule a personalized one-on-one research appointment with one of Galvin Library's research specialists.
What is Secondary Research?
Secondary research, also known as a literature review , preliminary research , historical research , background research , desk research , or library research , is research that analyzes or describes prior research. Rather than generating and analyzing new data, secondary research analyzes existing research results to establish the boundaries of knowledge on a topic, to identify trends or new practices, to test mathematical models or train machine learning systems, or to verify facts and figures. Secondary research is also used to justify the need for primary research as well as to justify and support other activities. For example, secondary research may be used to support a proposal to modernize a manufacturing plant, to justify the use of newly a developed treatment for cancer, to strengthen a business proposal, or to validate points made in a speech.
Why Is Secondary Research Important?
Because secondary research is used for so many purposes in so many settings, all professionals will be required to perform it at some point in their careers. For managers and entrepreneurs, regardless of the industry or profession, secondary research is a regular part of worklife, although parts of the research, such as finding the supporting documents, are often delegated to juniors in the organization. For all these reasons, it is essential to learn how to conduct secondary research, even if you are unlikely to ever conduct primary research.
Secondary research is also essential if your main goal is primary research. Research funding is obtained only by using secondary research to show the need for the primary research you want to conduct. In fact, primary research depends on secondary research to prove that it is indeed new and original research and not just a rehash or replication of somebody else’s work.
- Next: Literature Review >>
- Last Updated: Dec 21, 2023 3:46 PM
- URL: https://guides.library.iit.edu/litreview
- USC Libraries
- Research Guides
Organizing Your Social Sciences Research Paper
- Secondary Sources
- Purpose of Guide
- Design Flaws to Avoid
- Independent and Dependent Variables
- Glossary of Research Terms
- Reading Research Effectively
- Narrowing a Topic Idea
- Broadening a Topic Idea
- Extending the Timeliness of a Topic Idea
- Academic Writing Style
- Choosing a Title
- Making an Outline
- Paragraph Development
- Research Process Video Series
- Executive Summary
- The C.A.R.S. Model
- Background Information
- The Research Problem/Question
- Theoretical Framework
- Citation Tracking
- Content Alert Services
- Evaluating Sources
- Primary Sources
- Tiertiary Sources
- Scholarly vs. Popular Publications
- Qualitative Methods
- Quantitative Methods
- Insiderness
- Using Non-Textual Elements
- Limitations of the Study
- Common Grammar Mistakes
- Writing Concisely
- Avoiding Plagiarism
- Footnotes or Endnotes?
- Further Readings
- Generative AI and Writing
- USC Libraries Tutorials and Other Guides
- Bibliography
In the social sciences, a secondary source is usually a scholar book, journal article, or digital or print document that was created by someone who did not directly experience or participate in the events or conditions under investigation. Secondary sources are not evidence per se, but rather, provide an interpretation, analysis, or commentary derived from the content of primary source materials and/or other secondary sources.
Value of Secondary Sources
To do research, you must cite research. Primary sources do not represent research per se, but only the artifacts from which most research is derived. Therefore, the majority of sources in a literature review are secondary sources that present research findings, analysis, and the evaluation of other researcher's works.
Reviewing secondary source material can be of valu e in improving your overall research paper because secondary sources facilitate the communication of what is known about a topic. This literature also helps you understand the level of uncertainty about what is currently known and what additional information is needed from further research. It is important to note, however, that secondary sources are not the subject of your analysis. Instead, they represent various opinions, interpretations, and arguments about the research problem you are investigating--opinions, interpretations, and arguments with which you may either agree or disagree with as part of your own analysis of the literature.
Examples of secondary sources you could review as part of your overall study include: * Bibliographies [also considered tertiary] * Biographical works * Books, other than fiction and autobiography * Commentaries, criticisms * Dictionaries, Encyclopedias [also considered tertiary] * Histories * Journal articles [depending on the discipline, they can be primary] * Magazine and newspaper articles [this distinction varies by discipline] * Textbooks [also considered tertiary] * Web site [also considered primary]
- << Previous: Primary Sources
- Next: Tiertiary Sources >>
- Last Updated: Mar 26, 2024 10:40 AM
- URL: https://libguides.usc.edu/writingguide

- Teesside University Student & Library Services
- Learning Hub Group
Research Methods
Secondary research.
- Primary Research
What is Secondary Research?
Advantages and disadvantages of secondary research, secondary research in literature reviews, secondary research - going beyond literature reviews, main stages of secondary research, useful resources, using material on this page.
- Quantitative Research This link opens in a new window
- Qualitative Research This link opens in a new window
- Being Critical This link opens in a new window
- Subject LibGuides This link opens in a new window

Secondary research
Secondary research uses research and data that has already been carried out. It is sometimes referred to as desk research. It is a good starting point for any type of research as it enables you to analyse what research has already been undertaken and identify any gaps.
You may only need to carry out secondary research for your assessment or you may need to use secondary research as a starting point, before undertaking your own primary research .
Searching for both primary and secondary sources can help to ensure that you are up to date with what research has already been carried out in your area of interest and to identify the key researchers in the field.
"Secondary sources are the books, articles, papers and similar materials written or produced by others that help you to form your background understanding of the subject. You would use these to find out about experts’ findings, analyses or perspectives on the issue and decide whether to draw upon these explicitly in your research." (Cottrell, 2014, p. 123).
Examples of secondary research sources include:.
- journal articles
- official statistics, such as government reports or organisations which have collected and published data
Primary research involves gathering data which has not been collected before. Methods to collect it can include interviews, focus groups, controlled trials and case studies. Secondary research often comments on and analyses this primary research.
Gopalakrishnan and Ganeshkumar (2013, p. 10) explain the difference between primary and secondary research:
"Primary research is collecting data directly from patients or population, while secondary research is the analysis of data already collected through primary research. A review is an article that summarizes a number of primary studies and may draw conclusions on the topic of interest which can be traditional (unsystematic) or systematic".
Secondary Data
As secondary data has already been collected by someone else for their research purposes, it may not cover all of the areas of interest for your research topic. This research will need to be analysed alongside other research sources and data in the same subject area in order to confirm, dispute or discuss the findings in a wider context.
"Secondary source data, as the name infers, provides second-hand information. The data come ‘pre-packaged’, their form and content reflecting the fact that they have been produced by someone other than the researcher and will not have been produced specifically for the purpose of the research project. The data, none the less, will have some relevance for the research in terms of the information they contain, and the task for the researcher is to extract that information and re-use it in the context of his/her own research project." (Denscombe, 2021, p. 268)
In the video below Dr. Benedict Wheeler (Senior Research Fellow at the European Center for Environment and Human Health at the University of Exeter Medical School) discusses secondary data analysis. Secondary data was used for his research on how the environment affects health and well-being and utilising this secondary data gave access to a larger data set.
As with all research, an important part of the process is to critically evaluate any sources you use. There are tools to help with this in the Being Critical section of the guide.
Louise Corti, from the UK Data Archive, discusses using secondary data in the video below. T he importance of evaluating secondary research is discussed - this is to ensure the data is appropriate for your research and to investigate how the data was collected.
There are advantages and disadvantages to secondary research:
Advantages:
- Usually low cost
- Easily accessible
- Provides background information to clarify / refine research areas
- Increases breadth of knowledge
- Shows different examples of research methods
- Can highlight gaps in the research and potentially outline areas of difficulty
- Can incorporate a wide range of data
- Allows you to identify opposing views and supporting arguments for your research topic
- Highlights the key researchers and work which is being undertaken within the subject area
- Helps to put your research topic into perspective
Disadvantages
- Can be out of date
- Might be unreliable if it is not clear where or how the research has been collected - remember to think critically
- May not be applicable to your specific research question as the aims will have had a different focus
Literature reviews
Secondary research for your major project may take the form of a literature review . this is where you will outline the main research which has already been written on your topic. this might include theories and concepts connected with your topic and it should also look to see if there are any gaps in the research., as the criteria and guidance will differ for each school, it is important that you check the guidance which you have been given for your assessment. this may be in blackboard and you can also check with your supervisor..
The videos below include some insights from academics regarding the importance of literature reviews.
Secondary research which goes beyond literature reviews
For some dissertations/major projects there might only be a literature review (discussed above ). For others there could be a literature review followed by primary research and for others the literature review might be followed by further secondary research.
You may be asked to write a literature review which will form a background chapter to give context to your project and provide the necessary history for the research topic. However, you may then also be expected to produce the rest of your project using additional secondary research methods, which will need to produce results and findings which are distinct from the background chapter t o avoid repetition .
Remember, as the criteria and guidance will differ for each School, it is important that you check the guidance which you have been given for your assessment. This may be in Blackboard and you can also check with your supervisor.
Although this type of secondary research will go beyond a literature review, it will still rely on research which has already been undertaken. And, "just as in primary research, secondary research designs can be either quantitative, qualitative, or a mixture of both strategies of inquiry" (Manu and Akotia, 2021, p. 4) .
Your secondary research may use the literature review to focus on a specific theme, which is then discussed further in the main project. Or it may use an alternative approach. Some examples are included below. Remember to speak with your supervisor if you are struggling to define these areas.
Some approaches of how to conduct secondary research include:
- A systematic review is a structured literature review that involves identifying all of the relevant primary research using a rigorous search strategy to answer a focused research question.
- This involves comprehensive searching which is used to identify themes or concepts across a number of relevant studies.
- The review will assess the q uality of the research and provide a summary and synthesis of all relevant available research on the topic.
- The systematic review LibGuide goes into more detail about this process (The guide is aimed a PhD/Researcher students. However, students on other levels of study may find parts of the guide helpful too).
- Scoping reviews aim to identify and assess available research on a specific topic (which can include ongoing research).
- They are "particularly useful when a body of literature has not yet been comprehensively reviewed, or exhibits a complex or heterogeneous nature not amenable to a more precise systematic review of the evidence. While scoping reviews may be conducted to determine the value and probable scope of a full systematic review, they may also be undertaken as exercises in and of themselves to summarize and disseminate research findings, to identify research gaps, and to make recommendations for the future research." (Peters et al., 2015) .
- This is designed to summarise the current knowledge and provide priorities for future research.
- "A state-of-the-art review will often highlight new ideas or gaps in research with no official quality assessment." (Baguss, 2020) .
- "Bibliometric analysis is a popular and rigorous method for exploring and analyzing large volumes of scientific data." (Donthu et al., 2021)
- Quantitative methods and statistics are used to analyse the bibliographic data of published literature. This can be used to measure the impact of authors, publications, or topics within a subject area.
The bibliometric analysis often uses the data from a citation source such as Scopus or Web of Science .
- This is a technique used to combine the statistic results of prior quantitative studies in order to increase precision and validity.
- "It goes beyond the parameters of a literature review, which assesses existing literature, to actually perform calculations based on the results collated, thereby coming up with new results" (Curtis and Curtis, 2011, p. 220)
(Adapted from: Grant and Booth, 2009, cited in Sarhan and Manu, 2021, p. 72 )
- Grounded Theory is used to create explanatory theory from data which has been collected.
- "Grounded theory data analysis strategies can be used with different types of data, including secondary data." ( Whiteside, Mills and McCalman, 2012 )
- This allows you to use a specific theory or theories which can then be applied to your chosen topic/research area.
- You could focus on one case study which is analysed in depth, or you could examine more than one in order to compare and contrast the important aspects of your research question.
- "Good case studies often begin with a predicament that is poorly comprehended and is inadequately explained or traditionally rationalised by numerous conflicting accounts. Therefore, the aim is to comprehend an existent problem and to use the acquired understandings to develop new theoretical outlooks or explanations." ( Papachroni and Lochrie, 2015, p. 81 )
Main stages of secondary research for a dissertation/major project
In general, the main stages for conducting secondary research for your dissertation or major project will include:
Click on the image below to access the reading list which includes resources used in this guide as well as some additional useful resources.

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License .
- << Previous: Primary Research
- Next: Quantitative Research >>
- Last Updated: Aug 11, 2022 3:41 PM
- URL: https://libguides.tees.ac.uk/researchmethods
- Search This Site All UCSD Sites Faculty/Staff Search Term
- Contact & Directions
- Climate Statement
- Cognitive Behavioral Neuroscience
- Cognitive Psychology
- Developmental Psychology
- Social Psychology
- Adjunct Faculty
- Non-Senate Instructors
- Researchers
- Psychology Grads
- Affiliated Grads
- New and Prospective Students
- Honors Program
- Experiential Learning
- Programs & Events
- Psi Chi / Psychology Club
- Prospective PhD Students
- Current PhD Students
- Area Brown Bags
- Colloquium Series
- Anderson Distinguished Lecture Series
- Speaker Videos
- Undergraduate Program
- Academic and Writing Resources
Writing Research Papers
- Research Paper Structure
Whether you are writing a B.S. Degree Research Paper or completing a research report for a Psychology course, it is highly likely that you will need to organize your research paper in accordance with American Psychological Association (APA) guidelines. Here we discuss the structure of research papers according to APA style.
Major Sections of a Research Paper in APA Style
A complete research paper in APA style that is reporting on experimental research will typically contain a Title page, Abstract, Introduction, Methods, Results, Discussion, and References sections. 1 Many will also contain Figures and Tables and some will have an Appendix or Appendices. These sections are detailed as follows (for a more in-depth guide, please refer to " How to Write a Research Paper in APA Style ”, a comprehensive guide developed by Prof. Emma Geller). 2
What is this paper called and who wrote it? – the first page of the paper; this includes the name of the paper, a “running head”, authors, and institutional affiliation of the authors. The institutional affiliation is usually listed in an Author Note that is placed towards the bottom of the title page. In some cases, the Author Note also contains an acknowledgment of any funding support and of any individuals that assisted with the research project.
One-paragraph summary of the entire study – typically no more than 250 words in length (and in many cases it is well shorter than that), the Abstract provides an overview of the study.
Introduction
What is the topic and why is it worth studying? – the first major section of text in the paper, the Introduction commonly describes the topic under investigation, summarizes or discusses relevant prior research (for related details, please see the Writing Literature Reviews section of this website), identifies unresolved issues that the current research will address, and provides an overview of the research that is to be described in greater detail in the sections to follow.
What did you do? – a section which details how the research was performed. It typically features a description of the participants/subjects that were involved, the study design, the materials that were used, and the study procedure. If there were multiple experiments, then each experiment may require a separate Methods section. A rule of thumb is that the Methods section should be sufficiently detailed for another researcher to duplicate your research.
What did you find? – a section which describes the data that was collected and the results of any statistical tests that were performed. It may also be prefaced by a description of the analysis procedure that was used. If there were multiple experiments, then each experiment may require a separate Results section.
What is the significance of your results? – the final major section of text in the paper. The Discussion commonly features a summary of the results that were obtained in the study, describes how those results address the topic under investigation and/or the issues that the research was designed to address, and may expand upon the implications of those findings. Limitations and directions for future research are also commonly addressed.
List of articles and any books cited – an alphabetized list of the sources that are cited in the paper (by last name of the first author of each source). Each reference should follow specific APA guidelines regarding author names, dates, article titles, journal titles, journal volume numbers, page numbers, book publishers, publisher locations, websites, and so on (for more information, please see the Citing References in APA Style page of this website).
Tables and Figures
Graphs and data (optional in some cases) – depending on the type of research being performed, there may be Tables and/or Figures (however, in some cases, there may be neither). In APA style, each Table and each Figure is placed on a separate page and all Tables and Figures are included after the References. Tables are included first, followed by Figures. However, for some journals and undergraduate research papers (such as the B.S. Research Paper or Honors Thesis), Tables and Figures may be embedded in the text (depending on the instructor’s or editor’s policies; for more details, see "Deviations from APA Style" below).
Supplementary information (optional) – in some cases, additional information that is not critical to understanding the research paper, such as a list of experiment stimuli, details of a secondary analysis, or programming code, is provided. This is often placed in an Appendix.
Variations of Research Papers in APA Style
Although the major sections described above are common to most research papers written in APA style, there are variations on that pattern. These variations include:
- Literature reviews – when a paper is reviewing prior published research and not presenting new empirical research itself (such as in a review article, and particularly a qualitative review), then the authors may forgo any Methods and Results sections. Instead, there is a different structure such as an Introduction section followed by sections for each of the different aspects of the body of research being reviewed, and then perhaps a Discussion section.
- Multi-experiment papers – when there are multiple experiments, it is common to follow the Introduction with an Experiment 1 section, itself containing Methods, Results, and Discussion subsections. Then there is an Experiment 2 section with a similar structure, an Experiment 3 section with a similar structure, and so on until all experiments are covered. Towards the end of the paper there is a General Discussion section followed by References. Additionally, in multi-experiment papers, it is common for the Results and Discussion subsections for individual experiments to be combined into single “Results and Discussion” sections.
Departures from APA Style
In some cases, official APA style might not be followed (however, be sure to check with your editor, instructor, or other sources before deviating from standards of the Publication Manual of the American Psychological Association). Such deviations may include:
- Placement of Tables and Figures – in some cases, to make reading through the paper easier, Tables and/or Figures are embedded in the text (for example, having a bar graph placed in the relevant Results section). The embedding of Tables and/or Figures in the text is one of the most common deviations from APA style (and is commonly allowed in B.S. Degree Research Papers and Honors Theses; however you should check with your instructor, supervisor, or editor first).
- Incomplete research – sometimes a B.S. Degree Research Paper in this department is written about research that is currently being planned or is in progress. In those circumstances, sometimes only an Introduction and Methods section, followed by References, is included (that is, in cases where the research itself has not formally begun). In other cases, preliminary results are presented and noted as such in the Results section (such as in cases where the study is underway but not complete), and the Discussion section includes caveats about the in-progress nature of the research. Again, you should check with your instructor, supervisor, or editor first.
- Class assignments – in some classes in this department, an assignment must be written in APA style but is not exactly a traditional research paper (for instance, a student asked to write about an article that they read, and to write that report in APA style). In that case, the structure of the paper might approximate the typical sections of a research paper in APA style, but not entirely. You should check with your instructor for further guidelines.
Workshops and Downloadable Resources
- For in-person discussion of the process of writing research papers, please consider attending this department’s “Writing Research Papers” workshop (for dates and times, please check the undergraduate workshops calendar).
Downloadable Resources
- How to Write APA Style Research Papers (a comprehensive guide) [ PDF ]
- Tips for Writing APA Style Research Papers (a brief summary) [ PDF ]
- Example APA Style Research Paper (for B.S. Degree – empirical research) [ PDF ]
- Example APA Style Research Paper (for B.S. Degree – literature review) [ PDF ]
Further Resources
How-To Videos
- Writing Research Paper Videos
APA Journal Article Reporting Guidelines
- Appelbaum, M., Cooper, H., Kline, R. B., Mayo-Wilson, E., Nezu, A. M., & Rao, S. M. (2018). Journal article reporting standards for quantitative research in psychology: The APA Publications and Communications Board task force report . American Psychologist , 73 (1), 3.
- Levitt, H. M., Bamberg, M., Creswell, J. W., Frost, D. M., Josselson, R., & Suárez-Orozco, C. (2018). Journal article reporting standards for qualitative primary, qualitative meta-analytic, and mixed methods research in psychology: The APA Publications and Communications Board task force report . American Psychologist , 73 (1), 26.
External Resources
- Formatting APA Style Papers in Microsoft Word
- How to Write an APA Style Research Paper from Hamilton University
- WikiHow Guide to Writing APA Research Papers
- Sample APA Formatted Paper with Comments
- Sample APA Formatted Paper
- Tips for Writing a Paper in APA Style
1 VandenBos, G. R. (Ed). (2010). Publication manual of the American Psychological Association (6th ed.) (pp. 41-60). Washington, DC: American Psychological Association.
2 geller, e. (2018). how to write an apa-style research report . [instructional materials]. , prepared by s. c. pan for ucsd psychology.
Back to top
- Formatting Research Papers
- Using Databases and Finding References
- What Types of References Are Appropriate?
- Evaluating References and Taking Notes
- Citing References
- Writing a Literature Review
- Writing Process and Revising
- Improving Scientific Writing
- Academic Integrity and Avoiding Plagiarism
- Writing Research Papers Videos
Root out friction in every digital experience, super-charge conversion rates, and optimize digital self-service
Uncover insights from any interaction, deliver AI-powered agent coaching, and reduce cost to serve
Increase revenue and loyalty with real-time insights and recommendations delivered to teams on the ground
Know how your people feel and empower managers to improve employee engagement, productivity, and retention
Take action in the moments that matter most along the employee journey and drive bottom line growth
Whatever they’re are saying, wherever they’re saying it, know exactly what’s going on with your people
Get faster, richer insights with qual and quant tools that make powerful market research available to everyone
Run concept tests, pricing studies, prototyping + more with fast, powerful studies designed by UX research experts
Track your brand performance 24/7 and act quickly to respond to opportunities and challenges in your market
Explore the platform powering Experience Management
- Free Account
- For Digital
- For Customer Care
- For Human Resources
- For Researchers
- Financial Services
- All Industries
Popular Use Cases
- Customer Experience
- Employee Experience
- Employee Exit Interviews
- Net Promoter Score
- Voice of Customer
- Customer Success Hub
- Product Documentation
- Training & Certification
- XM Institute
- Popular Resources
- Customer Stories
Market Research
- Artificial Intelligence
- Partnerships
- Marketplace
The annual gathering of the experience leaders at the world’s iconic brands building breakthrough business results, live in Salt Lake City.
- English/AU & NZ
- Español/Europa
- Español/América Latina
- Português Brasileiro
- REQUEST DEMO
- Experience Management
- Secondary Research
Try Qualtrics for free
Secondary research: definition, methods, & examples.
19 min read This ultimate guide to secondary research helps you understand changes in market trends, customers buying patterns and your competition using existing data sources.
In situations where you’re not involved in the data gathering process ( primary research ), you have to rely on existing information and data to arrive at specific research conclusions or outcomes. This approach is known as secondary research.
In this article, we’re going to explain what secondary research is, how it works, and share some examples of it in practice.
Free eBook: The ultimate guide to conducting market research
What is secondary research?
Secondary research, also known as desk research, is a research method that involves compiling existing data sourced from a variety of channels . This includes internal sources (e.g.in-house research) or, more commonly, external sources (such as government statistics, organizational bodies, and the internet).
Secondary research comes in several formats, such as published datasets, reports, and survey responses , and can also be sourced from websites, libraries, and museums.
The information is usually free — or available at a limited access cost — and gathered using surveys , telephone interviews, observation, face-to-face interviews, and more.
When using secondary research, researchers collect, verify, analyze and incorporate it to help them confirm research goals for the research period.
As well as the above, it can be used to review previous research into an area of interest. Researchers can look for patterns across data spanning several years and identify trends — or use it to verify early hypothesis statements and establish whether it’s worth continuing research into a prospective area.
How to conduct secondary research
There are five key steps to conducting secondary research effectively and efficiently:
1. Identify and define the research topic
First, understand what you will be researching and define the topic by thinking about the research questions you want to be answered.
Ask yourself: What is the point of conducting this research? Then, ask: What do we want to achieve?
This may indicate an exploratory reason (why something happened) or confirm a hypothesis. The answers may indicate ideas that need primary or secondary research (or a combination) to investigate them.
2. Find research and existing data sources
If secondary research is needed, think about where you might find the information. This helps you narrow down your secondary sources to those that help you answer your questions. What keywords do you need to use?
Which organizations are closely working on this topic already? Are there any competitors that you need to be aware of?
Create a list of the data sources, information, and people that could help you with your work.
3. Begin searching and collecting the existing data
Now that you have the list of data sources, start accessing the data and collect the information into an organized system. This may mean you start setting up research journal accounts or making telephone calls to book meetings with third-party research teams to verify the details around data results.
As you search and access information, remember to check the data’s date, the credibility of the source, the relevance of the material to your research topic, and the methodology used by the third-party researchers. Start small and as you gain results, investigate further in the areas that help your research’s aims.
4. Combine the data and compare the results
When you have your data in one place, you need to understand, filter, order, and combine it intelligently. Data may come in different formats where some data could be unusable, while other information may need to be deleted.
After this, you can start to look at different data sets to see what they tell you. You may find that you need to compare the same datasets over different periods for changes over time or compare different datasets to notice overlaps or trends. Ask yourself: What does this data mean to my research? Does it help or hinder my research?
5. Analyze your data and explore further
In this last stage of the process, look at the information you have and ask yourself if this answers your original questions for your research. Are there any gaps? Do you understand the information you’ve found? If you feel there is more to cover, repeat the steps and delve deeper into the topic so that you can get all the information you need.
If secondary research can’t provide these answers, consider supplementing your results with data gained from primary research. As you explore further, add to your knowledge and update your findings. This will help you present clear, credible information.
Primary vs secondary research
Unlike secondary research, primary research involves creating data first-hand by directly working with interviewees, target users, or a target market. Primary research focuses on the method for carrying out research, asking questions, and collecting data using approaches such as:
- Interviews (panel, face-to-face or over the phone)
- Questionnaires or surveys
- Focus groups
Using these methods, researchers can get in-depth, targeted responses to questions, making results more accurate and specific to their research goals. However, it does take time to do and administer.
Unlike primary research, secondary research uses existing data, which also includes published results from primary research. Researchers summarize the existing research and use the results to support their research goals.
Both primary and secondary research have their places. Primary research can support the findings found through secondary research (and fill knowledge gaps), while secondary research can be a starting point for further primary research. Because of this, these research methods are often combined for optimal research results that are accurate at both the micro and macro level.
Sources of Secondary Research
There are two types of secondary research sources: internal and external. Internal data refers to in-house data that can be gathered from the researcher’s organization. External data refers to data published outside of and not owned by the researcher’s organization.
Internal data
Internal data is a good first port of call for insights and knowledge, as you may already have relevant information stored in your systems. Because you own this information — and it won’t be available to other researchers — it can give you a competitive edge . Examples of internal data include:
- Database information on sales history and business goal conversions
- Information from website applications and mobile site data
- Customer-generated data on product and service efficiency and use
- Previous research results or supplemental research areas
- Previous campaign results
External data
External data is useful when you: 1) need information on a new topic, 2) want to fill in gaps in your knowledge, or 3) want data that breaks down a population or market for trend and pattern analysis. Examples of external data include:
- Government, non-government agencies, and trade body statistics
- Company reports and research
- Competitor research
- Public library collections
- Textbooks and research journals
- Media stories in newspapers
- Online journals and research sites
Three examples of secondary research methods in action
How and why might you conduct secondary research? Let’s look at a few examples:
1. Collecting factual information from the internet on a specific topic or market
There are plenty of sites that hold data for people to view and use in their research. For example, Google Scholar, ResearchGate, or Wiley Online Library all provide previous research on a particular topic. Researchers can create free accounts and use the search facilities to look into a topic by keyword, before following the instructions to download or export results for further analysis.
This can be useful for exploring a new market that your organization wants to consider entering. For instance, by viewing the U.S Census Bureau demographic data for that area, you can see what the demographics of your target audience are , and create compelling marketing campaigns accordingly.
2. Finding out the views of your target audience on a particular topic
If you’re interested in seeing the historical views on a particular topic, for example, attitudes to women’s rights in the US, you can turn to secondary sources.
Textbooks, news articles, reviews, and journal entries can all provide qualitative reports and interviews covering how people discussed women’s rights. There may be multimedia elements like video or documented posters of propaganda showing biased language usage.
By gathering this information, synthesizing it, and evaluating the language, who created it and when it was shared, you can create a timeline of how a topic was discussed over time.
3. When you want to know the latest thinking on a topic
Educational institutions, such as schools and colleges, create a lot of research-based reports on younger audiences or their academic specialisms. Dissertations from students also can be submitted to research journals, making these places useful places to see the latest insights from a new generation of academics.
Information can be requested — and sometimes academic institutions may want to collaborate and conduct research on your behalf. This can provide key primary data in areas that you want to research, as well as secondary data sources for your research.
Advantages of secondary research
There are several benefits of using secondary research, which we’ve outlined below:
- Easily and readily available data – There is an abundance of readily accessible data sources that have been pre-collected for use, in person at local libraries and online using the internet. This data is usually sorted by filters or can be exported into spreadsheet format, meaning that little technical expertise is needed to access and use the data.
- Faster research speeds – Since the data is already published and in the public arena, you don’t need to collect this information through primary research. This can make the research easier to do and faster, as you can get started with the data quickly.
- Low financial and time costs – Most secondary data sources can be accessed for free or at a small cost to the researcher, so the overall research costs are kept low. In addition, by saving on preliminary research, the time costs for the researcher are kept down as well.
- Secondary data can drive additional research actions – The insights gained can support future research activities (like conducting a follow-up survey or specifying future detailed research topics) or help add value to these activities.
- Secondary data can be useful pre-research insights – Secondary source data can provide pre-research insights and information on effects that can help resolve whether research should be conducted. It can also help highlight knowledge gaps, so subsequent research can consider this.
- Ability to scale up results – Secondary sources can include large datasets (like Census data results across several states) so research results can be scaled up quickly using large secondary data sources.
Disadvantages of secondary research
The disadvantages of secondary research are worth considering in advance of conducting research :
- Secondary research data can be out of date – Secondary sources can be updated regularly, but if you’re exploring the data between two updates, the data can be out of date. Researchers will need to consider whether the data available provides the right research coverage dates, so that insights are accurate and timely, or if the data needs to be updated. Also, fast-moving markets may find secondary data expires very quickly.
- Secondary research needs to be verified and interpreted – Where there’s a lot of data from one source, a researcher needs to review and analyze it. The data may need to be verified against other data sets or your hypotheses for accuracy and to ensure you’re using the right data for your research.
- The researcher has had no control over the secondary research – As the researcher has not been involved in the secondary research, invalid data can affect the results. It’s therefore vital that the methodology and controls are closely reviewed so that the data is collected in a systematic and error-free way.
- Secondary research data is not exclusive – As data sets are commonly available, there is no exclusivity and many researchers can use the same data. This can be problematic where researchers want to have exclusive rights over the research results and risk duplication of research in the future.
When do we conduct secondary research?
Now that you know the basics of secondary research, when do researchers normally conduct secondary research?
It’s often used at the beginning of research, when the researcher is trying to understand the current landscape . In addition, if the research area is new to the researcher, it can form crucial background context to help them understand what information exists already. This can plug knowledge gaps, supplement the researcher’s own learning or add to the research.
Secondary research can also be used in conjunction with primary research. Secondary research can become the formative research that helps pinpoint where further primary research is needed to find out specific information. It can also support or verify the findings from primary research.
You can use secondary research where high levels of control aren’t needed by the researcher, but a lot of knowledge on a topic is required from different angles.
Secondary research should not be used in place of primary research as both are very different and are used for various circumstances.
Questions to ask before conducting secondary research
Before you start your secondary research, ask yourself these questions:
- Is there similar internal data that we have created for a similar area in the past?
If your organization has past research, it’s best to review this work before starting a new project. The older work may provide you with the answers, and give you a starting dataset and context of how your organization approached the research before. However, be mindful that the work is probably out of date and view it with that note in mind. Read through and look for where this helps your research goals or where more work is needed.
- What am I trying to achieve with this research?
When you have clear goals, and understand what you need to achieve, you can look for the perfect type of secondary or primary research to support the aims. Different secondary research data will provide you with different information – for example, looking at news stories to tell you a breakdown of your market’s buying patterns won’t be as useful as internal or external data e-commerce and sales data sources.
- How credible will my research be?
If you are looking for credibility, you want to consider how accurate the research results will need to be, and if you can sacrifice credibility for speed by using secondary sources to get you started. Bear in mind which sources you choose — low-credibility data sites, like political party websites that are highly biased to favor their own party, would skew your results.
- What is the date of the secondary research?
When you’re looking to conduct research, you want the results to be as useful as possible , so using data that is 10 years old won’t be as accurate as using data that was created a year ago. Since a lot can change in a few years, note the date of your research and look for earlier data sets that can tell you a more recent picture of results. One caveat to this is using data collected over a long-term period for comparisons with earlier periods, which can tell you about the rate and direction of change.
- Can the data sources be verified? Does the information you have check out?
If you can’t verify the data by looking at the research methodology, speaking to the original team or cross-checking the facts with other research, it could be hard to be sure that the data is accurate. Think about whether you can use another source, or if it’s worth doing some supplementary primary research to replicate and verify results to help with this issue.
We created a front-to-back guide on conducting market research, The ultimate guide to conducting market research , so you can understand the research journey with confidence.
In it, you’ll learn more about:
- What effective market research looks like
- The use cases for market research
- The most important steps to conducting market research
- And how to take action on your research findings
Download the free guide for a clearer view on secondary research and other key research types for your business.
Related resources
Market intelligence 10 min read, marketing insights 11 min read, ethnographic research 11 min read, qualitative vs quantitative research 13 min read, qualitative research questions 11 min read, qualitative research design 12 min read, primary vs secondary research 14 min read, request demo.
Ready to learn more about Qualtrics?
- Privacy Policy
Buy Me a Coffee

Home » Research Paper – Structure, Examples and Writing Guide
Research Paper – Structure, Examples and Writing Guide
Table of Contents

Research Paper
Definition:
Research Paper is a written document that presents the author’s original research, analysis, and interpretation of a specific topic or issue.
It is typically based on Empirical Evidence, and may involve qualitative or quantitative research methods, or a combination of both. The purpose of a research paper is to contribute new knowledge or insights to a particular field of study, and to demonstrate the author’s understanding of the existing literature and theories related to the topic.
Structure of Research Paper
The structure of a research paper typically follows a standard format, consisting of several sections that convey specific information about the research study. The following is a detailed explanation of the structure of a research paper:
The title page contains the title of the paper, the name(s) of the author(s), and the affiliation(s) of the author(s). It also includes the date of submission and possibly, the name of the journal or conference where the paper is to be published.
The abstract is a brief summary of the research paper, typically ranging from 100 to 250 words. It should include the research question, the methods used, the key findings, and the implications of the results. The abstract should be written in a concise and clear manner to allow readers to quickly grasp the essence of the research.
Introduction
The introduction section of a research paper provides background information about the research problem, the research question, and the research objectives. It also outlines the significance of the research, the research gap that it aims to fill, and the approach taken to address the research question. Finally, the introduction section ends with a clear statement of the research hypothesis or research question.
Literature Review
The literature review section of a research paper provides an overview of the existing literature on the topic of study. It includes a critical analysis and synthesis of the literature, highlighting the key concepts, themes, and debates. The literature review should also demonstrate the research gap and how the current study seeks to address it.
The methods section of a research paper describes the research design, the sample selection, the data collection and analysis procedures, and the statistical methods used to analyze the data. This section should provide sufficient detail for other researchers to replicate the study.
The results section presents the findings of the research, using tables, graphs, and figures to illustrate the data. The findings should be presented in a clear and concise manner, with reference to the research question and hypothesis.
The discussion section of a research paper interprets the findings and discusses their implications for the research question, the literature review, and the field of study. It should also address the limitations of the study and suggest future research directions.
The conclusion section summarizes the main findings of the study, restates the research question and hypothesis, and provides a final reflection on the significance of the research.
The references section provides a list of all the sources cited in the paper, following a specific citation style such as APA, MLA or Chicago.
How to Write Research Paper
You can write Research Paper by the following guide:
- Choose a Topic: The first step is to select a topic that interests you and is relevant to your field of study. Brainstorm ideas and narrow down to a research question that is specific and researchable.
- Conduct a Literature Review: The literature review helps you identify the gap in the existing research and provides a basis for your research question. It also helps you to develop a theoretical framework and research hypothesis.
- Develop a Thesis Statement : The thesis statement is the main argument of your research paper. It should be clear, concise and specific to your research question.
- Plan your Research: Develop a research plan that outlines the methods, data sources, and data analysis procedures. This will help you to collect and analyze data effectively.
- Collect and Analyze Data: Collect data using various methods such as surveys, interviews, observations, or experiments. Analyze data using statistical tools or other qualitative methods.
- Organize your Paper : Organize your paper into sections such as Introduction, Literature Review, Methods, Results, Discussion, and Conclusion. Ensure that each section is coherent and follows a logical flow.
- Write your Paper : Start by writing the introduction, followed by the literature review, methods, results, discussion, and conclusion. Ensure that your writing is clear, concise, and follows the required formatting and citation styles.
- Edit and Proofread your Paper: Review your paper for grammar and spelling errors, and ensure that it is well-structured and easy to read. Ask someone else to review your paper to get feedback and suggestions for improvement.
- Cite your Sources: Ensure that you properly cite all sources used in your research paper. This is essential for giving credit to the original authors and avoiding plagiarism.
Research Paper Example
Note : The below example research paper is for illustrative purposes only and is not an actual research paper. Actual research papers may have different structures, contents, and formats depending on the field of study, research question, data collection and analysis methods, and other factors. Students should always consult with their professors or supervisors for specific guidelines and expectations for their research papers.
Research Paper Example sample for Students:
Title: The Impact of Social Media on Mental Health among Young Adults
Abstract: This study aims to investigate the impact of social media use on the mental health of young adults. A literature review was conducted to examine the existing research on the topic. A survey was then administered to 200 university students to collect data on their social media use, mental health status, and perceived impact of social media on their mental health. The results showed that social media use is positively associated with depression, anxiety, and stress. The study also found that social comparison, cyberbullying, and FOMO (Fear of Missing Out) are significant predictors of mental health problems among young adults.
Introduction: Social media has become an integral part of modern life, particularly among young adults. While social media has many benefits, including increased communication and social connectivity, it has also been associated with negative outcomes, such as addiction, cyberbullying, and mental health problems. This study aims to investigate the impact of social media use on the mental health of young adults.
Literature Review: The literature review highlights the existing research on the impact of social media use on mental health. The review shows that social media use is associated with depression, anxiety, stress, and other mental health problems. The review also identifies the factors that contribute to the negative impact of social media, including social comparison, cyberbullying, and FOMO.
Methods : A survey was administered to 200 university students to collect data on their social media use, mental health status, and perceived impact of social media on their mental health. The survey included questions on social media use, mental health status (measured using the DASS-21), and perceived impact of social media on their mental health. Data were analyzed using descriptive statistics and regression analysis.
Results : The results showed that social media use is positively associated with depression, anxiety, and stress. The study also found that social comparison, cyberbullying, and FOMO are significant predictors of mental health problems among young adults.
Discussion : The study’s findings suggest that social media use has a negative impact on the mental health of young adults. The study highlights the need for interventions that address the factors contributing to the negative impact of social media, such as social comparison, cyberbullying, and FOMO.
Conclusion : In conclusion, social media use has a significant impact on the mental health of young adults. The study’s findings underscore the need for interventions that promote healthy social media use and address the negative outcomes associated with social media use. Future research can explore the effectiveness of interventions aimed at reducing the negative impact of social media on mental health. Additionally, longitudinal studies can investigate the long-term effects of social media use on mental health.
Limitations : The study has some limitations, including the use of self-report measures and a cross-sectional design. The use of self-report measures may result in biased responses, and a cross-sectional design limits the ability to establish causality.
Implications: The study’s findings have implications for mental health professionals, educators, and policymakers. Mental health professionals can use the findings to develop interventions that address the negative impact of social media use on mental health. Educators can incorporate social media literacy into their curriculum to promote healthy social media use among young adults. Policymakers can use the findings to develop policies that protect young adults from the negative outcomes associated with social media use.
References :
- Twenge, J. M., & Campbell, W. K. (2019). Associations between screen time and lower psychological well-being among children and adolescents: Evidence from a population-based study. Preventive medicine reports, 15, 100918.
- Primack, B. A., Shensa, A., Escobar-Viera, C. G., Barrett, E. L., Sidani, J. E., Colditz, J. B., … & James, A. E. (2017). Use of multiple social media platforms and symptoms of depression and anxiety: A nationally-representative study among US young adults. Computers in Human Behavior, 69, 1-9.
- Van der Meer, T. G., & Verhoeven, J. W. (2017). Social media and its impact on academic performance of students. Journal of Information Technology Education: Research, 16, 383-398.
Appendix : The survey used in this study is provided below.
Social Media and Mental Health Survey
- How often do you use social media per day?
- Less than 30 minutes
- 30 minutes to 1 hour
- 1 to 2 hours
- 2 to 4 hours
- More than 4 hours
- Which social media platforms do you use?
- Others (Please specify)
- How often do you experience the following on social media?
- Social comparison (comparing yourself to others)
- Cyberbullying
- Fear of Missing Out (FOMO)
- Have you ever experienced any of the following mental health problems in the past month?
- Do you think social media use has a positive or negative impact on your mental health?
- Very positive
- Somewhat positive
- Somewhat negative
- Very negative
- In your opinion, which factors contribute to the negative impact of social media on mental health?
- Social comparison
- In your opinion, what interventions could be effective in reducing the negative impact of social media on mental health?
- Education on healthy social media use
- Counseling for mental health problems caused by social media
- Social media detox programs
- Regulation of social media use
Thank you for your participation!
Applications of Research Paper
Research papers have several applications in various fields, including:
- Advancing knowledge: Research papers contribute to the advancement of knowledge by generating new insights, theories, and findings that can inform future research and practice. They help to answer important questions, clarify existing knowledge, and identify areas that require further investigation.
- Informing policy: Research papers can inform policy decisions by providing evidence-based recommendations for policymakers. They can help to identify gaps in current policies, evaluate the effectiveness of interventions, and inform the development of new policies and regulations.
- Improving practice: Research papers can improve practice by providing evidence-based guidance for professionals in various fields, including medicine, education, business, and psychology. They can inform the development of best practices, guidelines, and standards of care that can improve outcomes for individuals and organizations.
- Educating students : Research papers are often used as teaching tools in universities and colleges to educate students about research methods, data analysis, and academic writing. They help students to develop critical thinking skills, research skills, and communication skills that are essential for success in many careers.
- Fostering collaboration: Research papers can foster collaboration among researchers, practitioners, and policymakers by providing a platform for sharing knowledge and ideas. They can facilitate interdisciplinary collaborations and partnerships that can lead to innovative solutions to complex problems.
When to Write Research Paper
Research papers are typically written when a person has completed a research project or when they have conducted a study and have obtained data or findings that they want to share with the academic or professional community. Research papers are usually written in academic settings, such as universities, but they can also be written in professional settings, such as research organizations, government agencies, or private companies.
Here are some common situations where a person might need to write a research paper:
- For academic purposes: Students in universities and colleges are often required to write research papers as part of their coursework, particularly in the social sciences, natural sciences, and humanities. Writing research papers helps students to develop research skills, critical thinking skills, and academic writing skills.
- For publication: Researchers often write research papers to publish their findings in academic journals or to present their work at academic conferences. Publishing research papers is an important way to disseminate research findings to the academic community and to establish oneself as an expert in a particular field.
- To inform policy or practice : Researchers may write research papers to inform policy decisions or to improve practice in various fields. Research findings can be used to inform the development of policies, guidelines, and best practices that can improve outcomes for individuals and organizations.
- To share new insights or ideas: Researchers may write research papers to share new insights or ideas with the academic or professional community. They may present new theories, propose new research methods, or challenge existing paradigms in their field.
Purpose of Research Paper
The purpose of a research paper is to present the results of a study or investigation in a clear, concise, and structured manner. Research papers are written to communicate new knowledge, ideas, or findings to a specific audience, such as researchers, scholars, practitioners, or policymakers. The primary purposes of a research paper are:
- To contribute to the body of knowledge : Research papers aim to add new knowledge or insights to a particular field or discipline. They do this by reporting the results of empirical studies, reviewing and synthesizing existing literature, proposing new theories, or providing new perspectives on a topic.
- To inform or persuade: Research papers are written to inform or persuade the reader about a particular issue, topic, or phenomenon. They present evidence and arguments to support their claims and seek to persuade the reader of the validity of their findings or recommendations.
- To advance the field: Research papers seek to advance the field or discipline by identifying gaps in knowledge, proposing new research questions or approaches, or challenging existing assumptions or paradigms. They aim to contribute to ongoing debates and discussions within a field and to stimulate further research and inquiry.
- To demonstrate research skills: Research papers demonstrate the author’s research skills, including their ability to design and conduct a study, collect and analyze data, and interpret and communicate findings. They also demonstrate the author’s ability to critically evaluate existing literature, synthesize information from multiple sources, and write in a clear and structured manner.
Characteristics of Research Paper
Research papers have several characteristics that distinguish them from other forms of academic or professional writing. Here are some common characteristics of research papers:
- Evidence-based: Research papers are based on empirical evidence, which is collected through rigorous research methods such as experiments, surveys, observations, or interviews. They rely on objective data and facts to support their claims and conclusions.
- Structured and organized: Research papers have a clear and logical structure, with sections such as introduction, literature review, methods, results, discussion, and conclusion. They are organized in a way that helps the reader to follow the argument and understand the findings.
- Formal and objective: Research papers are written in a formal and objective tone, with an emphasis on clarity, precision, and accuracy. They avoid subjective language or personal opinions and instead rely on objective data and analysis to support their arguments.
- Citations and references: Research papers include citations and references to acknowledge the sources of information and ideas used in the paper. They use a specific citation style, such as APA, MLA, or Chicago, to ensure consistency and accuracy.
- Peer-reviewed: Research papers are often peer-reviewed, which means they are evaluated by other experts in the field before they are published. Peer-review ensures that the research is of high quality, meets ethical standards, and contributes to the advancement of knowledge in the field.
- Objective and unbiased: Research papers strive to be objective and unbiased in their presentation of the findings. They avoid personal biases or preconceptions and instead rely on the data and analysis to draw conclusions.
Advantages of Research Paper
Research papers have many advantages, both for the individual researcher and for the broader academic and professional community. Here are some advantages of research papers:
- Contribution to knowledge: Research papers contribute to the body of knowledge in a particular field or discipline. They add new information, insights, and perspectives to existing literature and help advance the understanding of a particular phenomenon or issue.
- Opportunity for intellectual growth: Research papers provide an opportunity for intellectual growth for the researcher. They require critical thinking, problem-solving, and creativity, which can help develop the researcher’s skills and knowledge.
- Career advancement: Research papers can help advance the researcher’s career by demonstrating their expertise and contributions to the field. They can also lead to new research opportunities, collaborations, and funding.
- Academic recognition: Research papers can lead to academic recognition in the form of awards, grants, or invitations to speak at conferences or events. They can also contribute to the researcher’s reputation and standing in the field.
- Impact on policy and practice: Research papers can have a significant impact on policy and practice. They can inform policy decisions, guide practice, and lead to changes in laws, regulations, or procedures.
- Advancement of society: Research papers can contribute to the advancement of society by addressing important issues, identifying solutions to problems, and promoting social justice and equality.
Limitations of Research Paper
Research papers also have some limitations that should be considered when interpreting their findings or implications. Here are some common limitations of research papers:
- Limited generalizability: Research findings may not be generalizable to other populations, settings, or contexts. Studies often use specific samples or conditions that may not reflect the broader population or real-world situations.
- Potential for bias : Research papers may be biased due to factors such as sample selection, measurement errors, or researcher biases. It is important to evaluate the quality of the research design and methods used to ensure that the findings are valid and reliable.
- Ethical concerns: Research papers may raise ethical concerns, such as the use of vulnerable populations or invasive procedures. Researchers must adhere to ethical guidelines and obtain informed consent from participants to ensure that the research is conducted in a responsible and respectful manner.
- Limitations of methodology: Research papers may be limited by the methodology used to collect and analyze data. For example, certain research methods may not capture the complexity or nuance of a particular phenomenon, or may not be appropriate for certain research questions.
- Publication bias: Research papers may be subject to publication bias, where positive or significant findings are more likely to be published than negative or non-significant findings. This can skew the overall findings of a particular area of research.
- Time and resource constraints: Research papers may be limited by time and resource constraints, which can affect the quality and scope of the research. Researchers may not have access to certain data or resources, or may be unable to conduct long-term studies due to practical limitations.
About the author
Muhammad Hassan
Researcher, Academic Writer, Web developer
You may also like

How to Cite Research Paper – All Formats and...

Data Collection – Methods Types and Examples

Delimitations in Research – Types, Examples and...

Research Paper Format – Types, Examples and...

Research Process – Steps, Examples and Tips


Research Design – Types, Methods and Examples
Dissertation Structure & Layout 101: How to structure your dissertation, thesis or research project.
By: Derek Jansen (MBA) Reviewed By: David Phair (PhD) | July 2019
So, you’ve got a decent understanding of what a dissertation is , you’ve chosen your topic and hopefully you’ve received approval for your research proposal . Awesome! Now its time to start the actual dissertation or thesis writing journey.
To craft a high-quality document, the very first thing you need to understand is dissertation structure . In this post, we’ll walk you through the generic dissertation structure and layout, step by step. We’ll start with the big picture, and then zoom into each chapter to briefly discuss the core contents. If you’re just starting out on your research journey, you should start with this post, which covers the big-picture process of how to write a dissertation or thesis .
*The Caveat *
In this post, we’ll be discussing a traditional dissertation/thesis structure and layout, which is generally used for social science research across universities, whether in the US, UK, Europe or Australia. However, some universities may have small variations on this structure (extra chapters, merged chapters, slightly different ordering, etc).
So, always check with your university if they have a prescribed structure or layout that they expect you to work with. If not, it’s safe to assume the structure we’ll discuss here is suitable. And even if they do have a prescribed structure, you’ll still get value from this post as we’ll explain the core contents of each section.
Overview: S tructuring a dissertation or thesis
- Acknowledgements page
- Abstract (or executive summary)
- Table of contents , list of figures and tables
- Chapter 1: Introduction
- Chapter 2: Literature review
- Chapter 3: Methodology
- Chapter 4: Results
- Chapter 5: Discussion
- Chapter 6: Conclusion
- Reference list
As I mentioned, some universities will have slight variations on this structure. For example, they want an additional “personal reflection chapter”, or they might prefer the results and discussion chapter to be merged into one. Regardless, the overarching flow will always be the same, as this flow reflects the research process , which we discussed here – i.e.:
- The introduction chapter presents the core research question and aims .
- The literature review chapter assesses what the current research says about this question.
- The methodology, results and discussion chapters go about undertaking new research about this question.
- The conclusion chapter (attempts to) answer the core research question .
In other words, the dissertation structure and layout reflect the research process of asking a well-defined question(s), investigating, and then answering the question – see below.
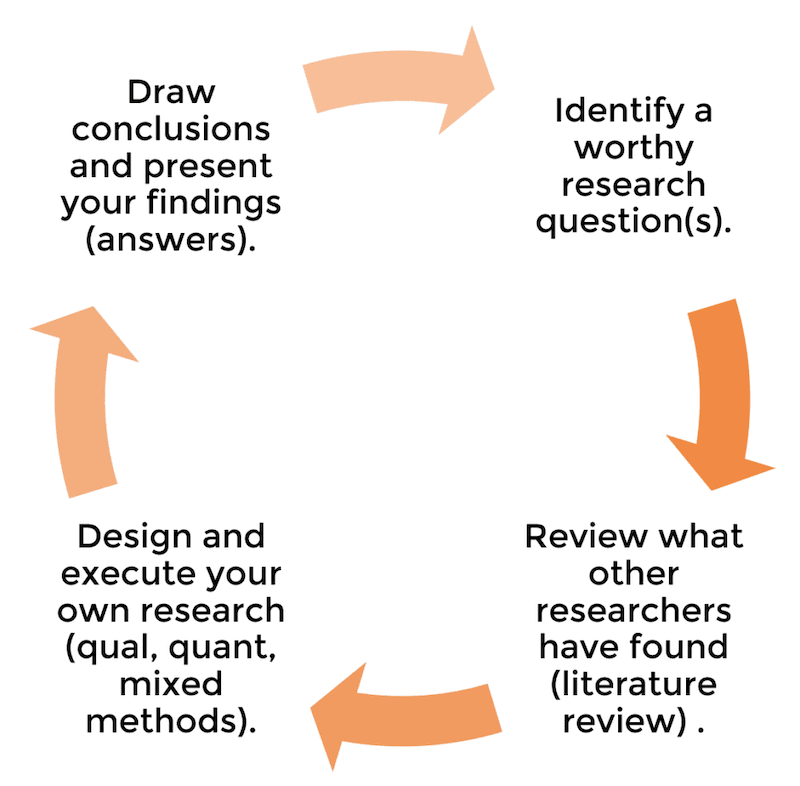
To restate that – the structure and layout of a dissertation reflect the flow of the overall research process . This is essential to understand, as each chapter will make a lot more sense if you “get” this concept. If you’re not familiar with the research process, read this post before going further.
Right. Now that we’ve covered the big picture, let’s dive a little deeper into the details of each section and chapter. Oh and by the way, you can also grab our free dissertation/thesis template here to help speed things up.
The title page of your dissertation is the very first impression the marker will get of your work, so it pays to invest some time thinking about your title. But what makes for a good title? A strong title needs to be 3 things:
- Succinct (not overly lengthy or verbose)
- Specific (not vague or ambiguous)
- Representative of the research you’re undertaking (clearly linked to your research questions)
Typically, a good title includes mention of the following:
- The broader area of the research (i.e. the overarching topic)
- The specific focus of your research (i.e. your specific context)
- Indication of research design (e.g. quantitative , qualitative , or mixed methods ).
For example:
A quantitative investigation [research design] into the antecedents of organisational trust [broader area] in the UK retail forex trading market [specific context/area of focus].
Again, some universities may have specific requirements regarding the format and structure of the title, so it’s worth double-checking expectations with your institution (if there’s no mention in the brief or study material).

Acknowledgements
This page provides you with an opportunity to say thank you to those who helped you along your research journey. Generally, it’s optional (and won’t count towards your marks), but it is academic best practice to include this.
So, who do you say thanks to? Well, there’s no prescribed requirements, but it’s common to mention the following people:
- Your dissertation supervisor or committee.
- Any professors, lecturers or academics that helped you understand the topic or methodologies.
- Any tutors, mentors or advisors.
- Your family and friends, especially spouse (for adult learners studying part-time).
There’s no need for lengthy rambling. Just state who you’re thankful to and for what (e.g. thank you to my supervisor, John Doe, for his endless patience and attentiveness) – be sincere. In terms of length, you should keep this to a page or less.
Abstract or executive summary
The dissertation abstract (or executive summary for some degrees) serves to provide the first-time reader (and marker or moderator) with a big-picture view of your research project. It should give them an understanding of the key insights and findings from the research, without them needing to read the rest of the report – in other words, it should be able to stand alone .
For it to stand alone, your abstract should cover the following key points (at a minimum):
- Your research questions and aims – what key question(s) did your research aim to answer?
- Your methodology – how did you go about investigating the topic and finding answers to your research question(s)?
- Your findings – following your own research, what did do you discover?
- Your conclusions – based on your findings, what conclusions did you draw? What answers did you find to your research question(s)?
So, in much the same way the dissertation structure mimics the research process, your abstract or executive summary should reflect the research process, from the initial stage of asking the original question to the final stage of answering that question.
In practical terms, it’s a good idea to write this section up last , once all your core chapters are complete. Otherwise, you’ll end up writing and rewriting this section multiple times (just wasting time). For a step by step guide on how to write a strong executive summary, check out this post .
Need a helping hand?
Table of contents
This section is straightforward. You’ll typically present your table of contents (TOC) first, followed by the two lists – figures and tables. I recommend that you use Microsoft Word’s automatic table of contents generator to generate your TOC. If you’re not familiar with this functionality, the video below explains it simply:
If you find that your table of contents is overly lengthy, consider removing one level of depth. Oftentimes, this can be done without detracting from the usefulness of the TOC.
Right, now that the “admin” sections are out of the way, its time to move on to your core chapters. These chapters are the heart of your dissertation and are where you’ll earn the marks. The first chapter is the introduction chapter – as you would expect, this is the time to introduce your research…
It’s important to understand that even though you’ve provided an overview of your research in your abstract, your introduction needs to be written as if the reader has not read that (remember, the abstract is essentially a standalone document). So, your introduction chapter needs to start from the very beginning, and should address the following questions:
- What will you be investigating (in plain-language, big picture-level)?
- Why is that worth investigating? How is it important to academia or business? How is it sufficiently original?
- What are your research aims and research question(s)? Note that the research questions can sometimes be presented at the end of the literature review (next chapter).
- What is the scope of your study? In other words, what will and won’t you cover ?
- How will you approach your research? In other words, what methodology will you adopt?
- How will you structure your dissertation? What are the core chapters and what will you do in each of them?
These are just the bare basic requirements for your intro chapter. Some universities will want additional bells and whistles in the intro chapter, so be sure to carefully read your brief or consult your research supervisor.
If done right, your introduction chapter will set a clear direction for the rest of your dissertation. Specifically, it will make it clear to the reader (and marker) exactly what you’ll be investigating, why that’s important, and how you’ll be going about the investigation. Conversely, if your introduction chapter leaves a first-time reader wondering what exactly you’ll be researching, you’ve still got some work to do.
Now that you’ve set a clear direction with your introduction chapter, the next step is the literature review . In this section, you will analyse the existing research (typically academic journal articles and high-quality industry publications), with a view to understanding the following questions:
- What does the literature currently say about the topic you’re investigating?
- Is the literature lacking or well established? Is it divided or in disagreement?
- How does your research fit into the bigger picture?
- How does your research contribute something original?
- How does the methodology of previous studies help you develop your own?
Depending on the nature of your study, you may also present a conceptual framework towards the end of your literature review, which you will then test in your actual research.
Again, some universities will want you to focus on some of these areas more than others, some will have additional or fewer requirements, and so on. Therefore, as always, its important to review your brief and/or discuss with your supervisor, so that you know exactly what’s expected of your literature review chapter.

Now that you’ve investigated the current state of knowledge in your literature review chapter and are familiar with the existing key theories, models and frameworks, its time to design your own research. Enter the methodology chapter – the most “science-ey” of the chapters…
In this chapter, you need to address two critical questions:
- Exactly HOW will you carry out your research (i.e. what is your intended research design)?
- Exactly WHY have you chosen to do things this way (i.e. how do you justify your design)?
Remember, the dissertation part of your degree is first and foremost about developing and demonstrating research skills . Therefore, the markers want to see that you know which methods to use, can clearly articulate why you’ve chosen then, and know how to deploy them effectively.
Importantly, this chapter requires detail – don’t hold back on the specifics. State exactly what you’ll be doing, with who, when, for how long, etc. Moreover, for every design choice you make, make sure you justify it.
In practice, you will likely end up coming back to this chapter once you’ve undertaken all your data collection and analysis, and revise it based on changes you made during the analysis phase. This is perfectly fine. Its natural for you to add an additional analysis technique, scrap an old one, etc based on where your data lead you. Of course, I’m talking about small changes here – not a fundamental switch from qualitative to quantitative, which will likely send your supervisor in a spin!
You’ve now collected your data and undertaken your analysis, whether qualitative, quantitative or mixed methods. In this chapter, you’ll present the raw results of your analysis . For example, in the case of a quant study, you’ll present the demographic data, descriptive statistics, inferential statistics , etc.
Typically, Chapter 4 is simply a presentation and description of the data, not a discussion of the meaning of the data. In other words, it’s descriptive, rather than analytical – the meaning is discussed in Chapter 5. However, some universities will want you to combine chapters 4 and 5, so that you both present and interpret the meaning of the data at the same time. Check with your institution what their preference is.
Now that you’ve presented the data analysis results, its time to interpret and analyse them. In other words, its time to discuss what they mean, especially in relation to your research question(s).
What you discuss here will depend largely on your chosen methodology. For example, if you’ve gone the quantitative route, you might discuss the relationships between variables . If you’ve gone the qualitative route, you might discuss key themes and the meanings thereof. It all depends on what your research design choices were.
Most importantly, you need to discuss your results in relation to your research questions and aims, as well as the existing literature. What do the results tell you about your research questions? Are they aligned with the existing research or at odds? If so, why might this be? Dig deep into your findings and explain what the findings suggest, in plain English.
The final chapter – you’ve made it! Now that you’ve discussed your interpretation of the results, its time to bring it back to the beginning with the conclusion chapter . In other words, its time to (attempt to) answer your original research question s (from way back in chapter 1). Clearly state what your conclusions are in terms of your research questions. This might feel a bit repetitive, as you would have touched on this in the previous chapter, but its important to bring the discussion full circle and explicitly state your answer(s) to the research question(s).

Next, you’ll typically discuss the implications of your findings? In other words, you’ve answered your research questions – but what does this mean for the real world (or even for academia)? What should now be done differently, given the new insight you’ve generated?
Lastly, you should discuss the limitations of your research, as well as what this means for future research in the area. No study is perfect, especially not a Masters-level. Discuss the shortcomings of your research. Perhaps your methodology was limited, perhaps your sample size was small or not representative, etc, etc. Don’t be afraid to critique your work – the markers want to see that you can identify the limitations of your work. This is a strength, not a weakness. Be brutal!
This marks the end of your core chapters – woohoo! From here on out, it’s pretty smooth sailing.
The reference list is straightforward. It should contain a list of all resources cited in your dissertation, in the required format, e.g. APA , Harvard, etc.
It’s essential that you use reference management software for your dissertation. Do NOT try handle your referencing manually – its far too error prone. On a reference list of multiple pages, you’re going to make mistake. To this end, I suggest considering either Mendeley or Zotero. Both are free and provide a very straightforward interface to ensure that your referencing is 100% on point. I’ve included a simple how-to video for the Mendeley software (my personal favourite) below:
Some universities may ask you to include a bibliography, as opposed to a reference list. These two things are not the same . A bibliography is similar to a reference list, except that it also includes resources which informed your thinking but were not directly cited in your dissertation. So, double-check your brief and make sure you use the right one.
The very last piece of the puzzle is the appendix or set of appendices. This is where you’ll include any supporting data and evidence. Importantly, supporting is the keyword here.
Your appendices should provide additional “nice to know”, depth-adding information, which is not critical to the core analysis. Appendices should not be used as a way to cut down word count (see this post which covers how to reduce word count ). In other words, don’t place content that is critical to the core analysis here, just to save word count. You will not earn marks on any content in the appendices, so don’t try to play the system!
Time to recap…
And there you have it – the traditional dissertation structure and layout, from A-Z. To recap, the core structure for a dissertation or thesis is (typically) as follows:
- Acknowledgments page
Most importantly, the core chapters should reflect the research process (asking, investigating and answering your research question). Moreover, the research question(s) should form the golden thread throughout your dissertation structure. Everything should revolve around the research questions, and as you’ve seen, they should form both the start point (i.e. introduction chapter) and the endpoint (i.e. conclusion chapter).
I hope this post has provided you with clarity about the traditional dissertation/thesis structure and layout. If you have any questions or comments, please leave a comment below, or feel free to get in touch with us. Also, be sure to check out the rest of the Grad Coach Blog .

Psst… there’s more (for free)
This post is part of our dissertation mini-course, which covers everything you need to get started with your dissertation, thesis or research project.
You Might Also Like:

36 Comments
many thanks i found it very useful
Glad to hear that, Arun. Good luck writing your dissertation.
Such clear practical logical advice. I very much needed to read this to keep me focused in stead of fretting.. Perfect now ready to start my research!
what about scientific fields like computer or engineering thesis what is the difference in the structure? thank you very much
Thanks so much this helped me a lot!
Very helpful and accessible. What I like most is how practical the advice is along with helpful tools/ links.
Thanks Ade!
Thank you so much sir.. It was really helpful..
You’re welcome!
Hi! How many words maximum should contain the abstract?
Thank you so much 😊 Find this at the right moment
You’re most welcome. Good luck with your dissertation.
best ever benefit i got on right time thank you
Many times Clarity and vision of destination of dissertation is what makes the difference between good ,average and great researchers the same way a great automobile driver is fast with clarity of address and Clear weather conditions .
I guess Great researcher = great ideas + knowledge + great and fast data collection and modeling + great writing + high clarity on all these
You have given immense clarity from start to end.
Morning. Where will I write the definitions of what I’m referring to in my report?
Thank you so much Derek, I was almost lost! Thanks a tonnnn! Have a great day!
Thanks ! so concise and valuable
This was very helpful. Clear and concise. I know exactly what to do now.
Thank you for allowing me to go through briefly. I hope to find time to continue.
Really useful to me. Thanks a thousand times
Very interesting! It will definitely set me and many more for success. highly recommended.
Thank you soo much sir, for the opportunity to express my skills
Usefull, thanks a lot. Really clear
Very nice and easy to understand. Thank you .
That was incredibly useful. Thanks Grad Coach Crew!
My stress level just dropped at least 15 points after watching this. Just starting my thesis for my grad program and I feel a lot more capable now! Thanks for such a clear and helpful video, Emma and the GradCoach team!
Do we need to mention the number of words the dissertation contains in the main document?
It depends on your university’s requirements, so it would be best to check with them 🙂
Such a helpful post to help me get started with structuring my masters dissertation, thank you!
Great video; I appreciate that helpful information
It is so necessary or avital course
This blog is very informative for my research. Thank you
Doctoral students are required to fill out the National Research Council’s Survey of Earned Doctorates
wow this is an amazing gain in my life
This is so good
How can i arrange my specific objectives in my dissertation?
Trackbacks/Pingbacks
- What Is A Literature Review (In A Dissertation Or Thesis) - Grad Coach - […] is to write the actual literature review chapter (this is usually the second chapter in a typical dissertation or…
Submit a Comment Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
- Print Friendly
Structure of a Research Paper

Structure of a Research Paper: IMRaD Format
I. The Title Page
- Title: Tells the reader what to expect in the paper.
- Author(s): Most papers are written by one or two primary authors. The remaining authors have reviewed the work and/or aided in study design or data analysis (International Committee of Medical Editors, 1997). Check the Instructions to Authors for the target journal for specifics about authorship.
- Keywords [according to the journal]
- Corresponding Author: Full name and affiliation for the primary contact author for persons who have questions about the research.
- Financial & Equipment Support [if needed]: Specific information about organizations, agencies, or companies that supported the research.
- Conflicts of Interest [if needed]: List and explain any conflicts of interest.
II. Abstract: “Structured abstract” has become the standard for research papers (introduction, objective, methods, results and conclusions), while reviews, case reports and other articles have non-structured abstracts. The abstract should be a summary/synopsis of the paper.
III. Introduction: The “why did you do the study”; setting the scene or laying the foundation or background for the paper.
IV. Methods: The “how did you do the study.” Describe the --
- Context and setting of the study
- Specify the study design
- Population (patients, etc. if applicable)
- Sampling strategy
- Intervention (if applicable)
- Identify the main study variables
- Data collection instruments and procedures
- Outline analysis methods
V. Results: The “what did you find” --
- Report on data collection and/or recruitment
- Participants (demographic, clinical condition, etc.)
- Present key findings with respect to the central research question
- Secondary findings (secondary outcomes, subgroup analyses, etc.)
VI. Discussion: Place for interpreting the results
- Main findings of the study
- Discuss the main results with reference to previous research
- Policy and practice implications of the results
- Strengths and limitations of the study
VII. Conclusions: [occasionally optional or not required]. Do not reiterate the data or discussion. Can state hunches, inferences or speculations. Offer perspectives for future work.
VIII. Acknowledgements: Names people who contributed to the work, but did not contribute sufficiently to earn authorship. You must have permission from any individuals mentioned in the acknowledgements sections.
IX. References: Complete citations for any articles or other materials referenced in the text of the article.
- IMRD Cheatsheet (Carnegie Mellon) pdf.
- Adewasi, D. (2021 June 14). What Is IMRaD? IMRaD Format in Simple Terms! . Scientific-editing.info.
- Nair, P.K.R., Nair, V.D. (2014). Organization of a Research Paper: The IMRAD Format. In: Scientific Writing and Communication in Agriculture and Natural Resources. Springer, Cham. https://doi.org/10.1007/978-3-319-03101-9_2
- Sollaci, L. B., & Pereira, M. G. (2004). The introduction, methods, results, and discussion (IMRAD) structure: a fifty-year survey. Journal of the Medical Library Association : JMLA , 92 (3), 364–367.
- Cuschieri, S., Grech, V., & Savona-Ventura, C. (2019). WASP (Write a Scientific Paper): Structuring a scientific paper. Early human development , 128 , 114–117. https://doi.org/10.1016/j.earlhumdev.2018.09.011

3 Structure of a Scientific Research Paper
A primary way that scientists communicate with one another is through scientific papers. We will model our Biocore lab reports on the format most commonly used by scientific journals. Your lab reports should follow the guidelines described below unless the lab manual or your TA specifically tells you otherwise. Some lab reports have a modified format or require only a subset of the standard sections listed below.
The figure below indicates the four main sections (Intro, Methods, Results and Discussion) that form the body of a scientific paper. Each section of the paper (except for “Title”) should begin with one of these terms as a heading These main sections are bookended on the front end with a Title and Abstract summarizing the whole document and on the back end by a Literature Cited and Appendices (optional) in support of the document.
Other classes and some scientific journals deviate from this format, and you should always consult the guidelines specified before preparing a paper for another class (or submitting a manuscript for publication ).
Introduction
Methods and materials.
- Results (including figures and tables)
Literature Cited
The Methods and Results are specific to your hypothesis and the experiment you performed.
Then the Discussion starts more narrowly focused on whether you support or reject your hypothesis, but then broadens to integrate your findings into the existing literature, and finishes with a conclusion that is based on the experimental evidence you present.
The title is a clear, specific statement of the subject of your report. Think of the words in your title as key search terms. It introduces the reader to your paper and lets them know what to expect.
Titles should:
- Be concise and informative and need not be complete sentences.
- Avoid filler words like “Studies on” or “Investigations of” and opening words like A, An, or The.
- Be as specific as possible.
- Avoid abbreviations and jargon.
- state the results.
*If your report constitutes the results of an experiment where you manipulated variables and analyzed the result, include the independent and dependent variables, the direction of your results, as well as the study organism/ subject in your title.
How will titles be evaluated? To see our expectations for your Title, see the Biocore Research Paper Rubric in this Writing Manual.
In scientific journal articles, the first author listed is the primary author, and subsequent authors are listed according to the magnitude of their contribution to the study. Research mentors such as principal investigators (PI’s) of labs, are typically listed last. If all authors have made equivalent contributions to the article, then the paper will state that authors’ names are listed in alphabetical order.
In Biocore you will work within teams to do independent research projects, but we usually ask for individual lab reports because we want to give you many opportunities to work on your writing and thinking skills. At other times we will ask you to submit group posters and PowerPoint presentations. Here is how you should list teammates for various Biocore assignments:
- Individual papers or mini-posters: List yourself first as the primary author under your title, then list teammates as contributors at the top of the page in alphabetical order. Also list your lab section and TA.
- Group posters or PowerPoint presentations: We assume that all of you have made equivalent contributions to these collaborative group assignments, so include all researchers’ names as authors in alphabetical order.
*Not all Biocore lab reports require abstracts! Research proposals generally do not require abstracts, but check assignment description for details.
The abstract forces the author to distill the essence of the paper to a very brief summary (100-200 words) . Think of the abstract as the two-minute version of your entire experiment. Many readers use the abstract to decide whether they want to find and read the entire paper.
You must be concise. One way to do this is to summarize, in one or two sentences each :
- the rationale behind the experiment (goal of your experiment, model system, most important background information)
- your hypothesis
- the approach you took (how and what you actually tested)
- results or expected results
- conclusions/implications
Other tips:
- Always write the abstract last, after you thoroughly understand the experiment and its meaning.
- Abstracts should be understandable without referring to the rest of the paper.
- You do not cite references in an abstract. General and/or specifically applicable knowledge is assumed or is cited elsewhere in your paper.
Example Abstract From Systematic Observation Study
Adapted from paper by Kristin Magliocco (Fall 2009)
Phosphorus in the runoff to urban streams such as Willow Creek can lead to phosphorus build up and ultimately eutrophication of larger bodies of water. Rain gardens have been constructed on the UW Madison campus adjacent to Willow Creek to prevent accumulation of phosphorus in the creek itself. [Background] By slowing and delaying runoff from reaching the creek, the rain gardens are intended to retain phosphorus and, therefore decrease the amount of phosphorus that reaches the creek. [BR] To test the efficacy of the rain gardens, we hypothesized that there would be no significant difference in the phosphorus concentrations of the water in Willow Creek upstream and downstream of the boundaries of the northeast rain garden. [Hypothesis]We selected four replicate locations in the rain garden itself and in Willow Creek, both upstream and downstream of the rain garden, where we used a Hach phosphorus colorimeter to measure phosphorus concentration. [Approach] Our data supported our hypothesis, with the upstream mean concentration of 0.07335 ± 0.00471 mg/L and the downstream mean concentration of 0.08213 ± 0.0139 mg/L showing no statistically significant difference. [Results]We cautiously concluded that the rain gardens near Willow Creek do prevent further phosphorus accumulation in the stream, but pointed toward future studies focusing on amount of rainfall as an important factor in rain garden efficiency. [Conclusion]
How will abstracts be evaluated? To see our expectations for your Abstract, see the Biocore the Biocore Research Paper Rubric in this Writing Manual.
This section provides guidelines on how to construct a solid introduction to a scientific paper including background information, study question , biological rationale, hypothesis , and general approach . If the Introduction is done well, there should be no question in the reader’s mind why and on what basis you have posed a specific hypothesis.
Broad Question: based on an initial observation (e.g., “I see a lot of guppies close to the shore. Do guppies like living in shallow water?”). This observation of the natural world may inspire you to investigate background literature on previous research by others or gather some initial data/ observations as a pilot study. Broad questions are not always included in your written text, but are essential for establishing the direction of your research.
Background information: key issues, concepts, terminology, and definitions are needed to understand the biological rationale for the experiment. The background often includes a summary of findings from previous, relevant studies that introduce the study system, the independent and dependent variable. Remember to cite references, be concise, and only include relevant information given your audience and your experimental design. Your concise summary of background information should lead to specific scientific knowledge gaps that still exist. (e.g., “No studies on lake guppy distribution to date have examined whether guppies do indeed spend more time in shallow water.”)
Testable Question : these questions are much more focused than the initial broad question, are specific to the knowledge gap identified, and can be addressed with data. (e.g., “Do guppies spend different amounts of time in water less than 1 meter deep as compared to their time in water that is greater than 1 meter deep?”)
View testable question diagram as pdf
Biological Rationale (BR): The BR explains why you expect your independent variable(s) to affect your dependent variable(s) in the way your hypothesis indicates. After you have summarized the background information relevant to the study, the “BR” provides the logic and reasoning for your hypothesis and experimental approach, describing the biological mechanism that connects your independent and dependent variables and the assumptions that provides evidence for why your hypothesis should be supported. The biological rationale is based on your interpretation of the scientific literature, your personal observations, and the underlying assumptions you are making about how you think the system works. If you have written your biological rationale logically and clearly, your reader should see your hypothesis in your introduction section and say to themselves— “Of course this hypothesis is supportable. It seems very logical based on the rationale presented.”
Steps for Developing a BR—Based on your background information:
- Dependent Variable(s)- List key aspects of the dependent variable (DV) that are known (based on the scientific literature) and those that are unknown that you may need to assume or may be associated with a knowledge gap.
- Independent Variable(s)- List key aspects of the independent variable (IV) that are known (based on the scientific literature) and those that are unknown that you may need to assume or may be associated with a knowledge gap.
- Connection between DV and IV- List what is known and what you are assuming about the ways (mechanisms or relationships) in which the IV influences the DV , either directly or indirectly, either in the system you are studying, in a similar system, or a more distant dissimilar system. If possible, note literature that support any assumptions. The biological link between your IV and DV(s) is central piece of your BR.
- Based on #3, articulate the specific knowledge gap you hope to fill in this study.
- Generate a draft hypothesis based on steps 1-4.
Once you have done steps 1-5, start to sketch out your reasoning using a conceptual or graphic model
In Biocore, we will ask you to construct two different types of models as you are learning to develop your BR:
- Conceptual Model – a logical flow of ideas utilizing boxes and arrows to indicate how variables are connected and support your hypothesis. Conceptual models are helpful for developing logical thought progression but are generally not included in a paper or final presentation.
- Graphic or Visual Model – A cartoon or graphic depiction for how variables interact to result in your predicted outcome. Graphic models are often included in scientific posters and Powerpoint presentations, and sometimes in scientific papers.
See following sections for examples of Biological Rationale in the form of Conceptual and Graphic Models
Conceptual Model
In the Conceptual Model example below, the biological rationale is depicted as a logical flow of statements beginning with a testable question and ending with a hypothesis.
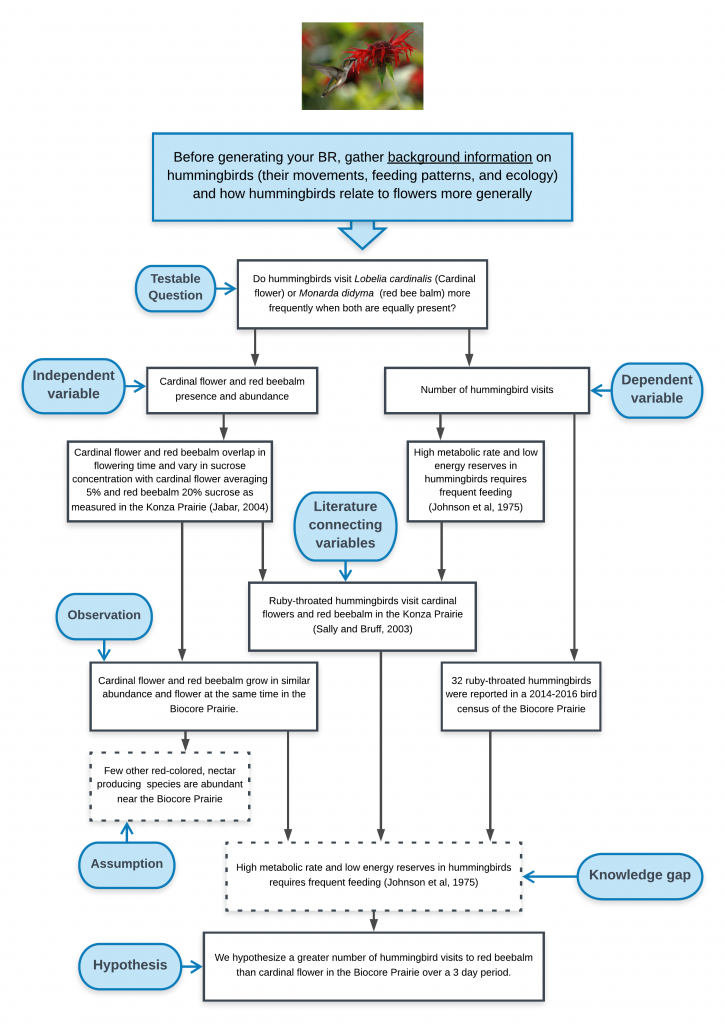
View conceptual model as a pdf
Graphic or Visual Model
Graphic or Visual Model uses cartoon diagrams and symbols to communicate the predicted interaction among variables and the mechanism by which they interact. Visual models use shorthand literature citations (superscript numbers) to indicate literature references that are further discussed in an oral presentation (poster or PowerPoint) or written narrative (paper).
Example Graphic Model of Biological Rationale appropriate for diagram in a paper, poster, or presentation. Adapted from poster by McKenna DeFoer, Sadie Gugel, Evan Polce, Kyrie Sellnow in Biocore 486, Organismal Biology lab.
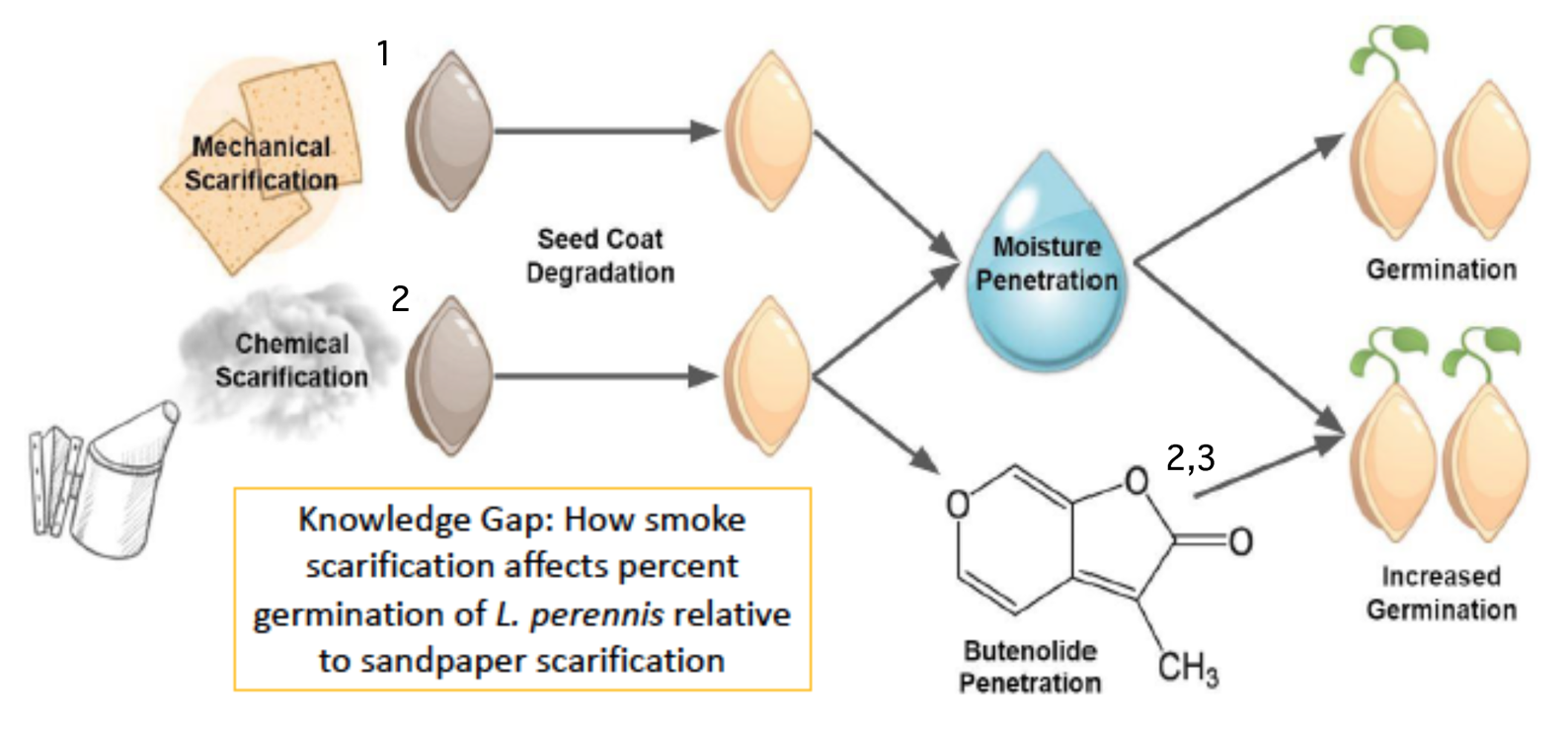
Narrative : Scarification using sandpaper abates the seed coat of L. perennis. This process allows moisture to permeate the seed coat during stratification and initiates the biochemical pathway for germination (1. Diboll 2008). Similarly, exposing seeds to cellulose-derived smoke causes chemical scarification (2. Egerton-Warburton 1997). This type of smoke contains butenolide, a compound synthesized during the combustion of plant material that has been found to further stimulate germination (3. Keeley and Fotheringham 1997).
More on Biological Rationale:
- A thorough rationale defines your knowledge gap about the system that has not been revealed in scientific literature or from previous observation. The knowledge gap is the knowledge we are attempting to create. The interpretation of your experimental data and the integration of literature will fill or partially fill the knowledge gap. In order to fill the knowledge gap, you may need to make assumptions about how your system operates. Assumptions are aspects of the system that you are not testing directly, but you think are particularly important since they drive the direction of your specific hypothesis or general predictions. Sometimes students confuse the knowledge gap and assumptions. Data gathered during the experiment can address the knowledge gap but generally do not provide direct evidence to support or refute assumptions.
- Defining the BR is probably the most critical task for a writer, as it tells your reader why your research is biologically meaningful. It may help to think about the rationale as a link between your independent and dependent variables, because the rationale answers these questions— how is this investigation related to what we know, what assumptions am I making about what we don’t yet know, AND how will this experiment add to our knowledge?
- Expect to spend time and mental effort on your BR. You may have to do considerable digging into the scientific literature to define how your experiment fits into what is already known and why it is relevant to pursue.
- Be open to the possibility that as you work with and think about your data, you may develop a deeper, more accurate understanding of the experimental system. You may find the original rationale needs to be revised to reflect your new, more sophisticated understanding.
- As you progress through Biocore and upper level biology courses, your rationale should become more focused and matched with the level of study i.e ., cellular, biochemical, or physiological mechanisms that underlie the rationale. Achieving this type of understanding takes effort, but it will lead to better communication of your science.
Hypothesis / Predictions: specific prediction(s) that you will test during your experiment. For manipulative experiments , the hypothesis should include the independent variable (what you manipulate), the dependent variable(s) (what you measure), the organism or system , the direction of your results, and comparison to be made. See the following examples.
If you are doing a systematic observation , your hypothesis presents a variable or set of variables that you predict are important for helping you characterize the system as a whole, or predict differences between components/areas of the system that help you explain how the system functions or changes over time.
Note that hypotheses/ predictions you develop in Biocore lab are much more specific than the general hypotheses that guide the research questions you encounter in scientific literature or in faculty research labs. That is because the research projects you do in Biocore are short-term, small(er) in scale or context specific, and therefore require greater specification to be testable within our class context.
Experimental Approach: Briefly gives the reader a general sense of the experiment, the type of data it will yield, and the kind of conclusions you expect to obtain from the data. Do not confuse the experimental approach with the experimental protocol . The experimental protocol consists of the detailed step-by-step procedures and techniques used during the experiment that are to be reported in the Methods and Materials section.
***Some Final Tips on Writing an Introduction***
- As you progress through the Biocore sequence for instance, from organismal level of Biocore 381/382 to the cellular level in Biocore 383/384, we expect the contents of your “Introduction” paragraphs to reflect the level of your coursework and previous writing experience. For example, in Biocore 384 (Cell Biology Lab) biological rationale should draw upon assumptions we are making about cellular and biochemical processes.
- Be Concise yet Specific: Remember to be concise and only include relevant information given your audience and your experimental design. As you write, keep asking, “Is this necessary information or is this irrelevant detail?” For example, if you are writing a paper claiming that a certain compound is a competitive inhibitor to the enzyme alkaline phosphatase and acts by binding to the active site, you need to explain (briefly) Michaelis-Menton kinetics and the meaning and significance of Km and Vmax. This explanation is not necessary if you are reporting the dependence of enzyme activity on pH because you do not need to measure Km and Vmax to get an estimate of enzyme activity.
- Another example: if you are writing a paper reporting an increase in water flea heart rate upon exposure to caffeine you need not describe the reproductive cycle of water fleas unless it is germane to your results and discussion. Be specific and concrete, especially when making introductory or summary statements.
Where do you discuss Pilot Studies? Many times it is important to do pilot studies to help you get familiar with your experimental system or to improve your experimental design. If your pilot study influences your biological rationale or hypothesis, you need to describe it in your Introduction. If your pilot study simply informs the logistics or techniques, but does not influence your rationale, then the description of your pilot study belongs in the Materials and Methods section.
How will introductions be evaluated? To see our expectations for your Introduction, see the Biocore Research Paper Rubric in this Writing Manual.
Example Introductions
Example introduction from systematic observation study.
Adapted from a paper by Will Klein 2009
Throughout history, humans have discovered and used chemicals derived from plant extracts as antimicrobial compounds for medicinal purposes. Although useful to humans, why would a plant create an antimicrobial defense that affects the growth of bacteria? [broad study question] As non-mobile organisms, plants have evolved mutually beneficial associations with beneficial microbes (Brooker et al. 2011) and a full arsenal of adaptations for defense against pathogenic microorganisms (bacteria, viruses, fungi). Borchardt et al. (2008) did an antimicrobial screening of 339 plant species growing in Minnesota and Wisconsin, many of which are prairie plant species. The researchers tested aerial plant parts (leaves, stems, flowers) for growth inhibition of one, two or three common mammalian pathogens ( Escherichia coli , Staphylococcus aureus, Candida albicans) and found 109 species inhibited growth of at least one microorganism. Leave extracts of Silphium sp. , a species found in the Biocore Prairie, contains antimicrobial compounds that inhibit the growth of many types of Gram-negative and Gram-positive bacteria (Kowalski and Kedzia, 2007; Kowalski, 2008). [background information]
Plants may produce chemical defense in the form of antimicrobial compounds contained in stems, roots, leaves, bark, flowers or fruits. [BR: assumption] By investing energy to generate these antimicrobial compounds, the plant maximizes its likelihood to succeed in its particular ecological niche (i.e. the Biocore Prairie) and improves its biological fitness. [BR: assumption ] No studies howver have directly examined the effect of native Biocore prairie plant extracts on indigenous soil bacteria growth. [testable question]
Through preliminary investigations in the Biocore Prairie during summer 2010, we sought to find prairie plant species and extracts from different plant parts (roots, leaves or stems) that would inhibit soil bacteria-bacteria cultured from soil that the prairie plants are growing in. Although most soil bacteria are beneficial or do nothing to affect prairie plants, we reasoned that plant species coexisting in the same environment with particular soil microbes may have efficient defense mechanisms towards pathogenic “prairie soil” bacteria. [BR: assumption] Huechera richardsonii, Monarda fistulosa, and Euphorbia corollata are three species common to the Biocore Prairie. Although leaf tissue of these three species have all been shown to contain antimicrobial properties against S. aureus (Borchart et al. 2008), how extracts from these species influence growth of bacteria indigenous to the Biocore Prairie is not known. [knowledge gap] We believe these plant species will contain antimicrobial properties in leaves to protect the tissue from microbial leaf pathogens that also occur in the soil. [BR: assumption]
We hypothesized that leaf extracts of Huechera richardsonii, Monarda fistulosa, and Euphorbia corollata would exhibit antimicrobial properties on the bacteria found in their native environment. [hypothesis] Our approach was to grow soil bacteria collected from the Biocore Prairie on agar plates, and then expose bacteria to leaf extracts absorbed on filter paper discs and measure the extent to which the extracts inhibited bacterial growth. [approach]
*Note: If you are a Biocore 382 student—do not worry if you don’t understand the scientific content in these two examples. We will get there! These examples are provided to refer to as you progress through the curriculum
Example 4: Good Introduction from manipulative experiment in Cell Biology Lab
(adapted from a poster by Kari Esselman, John Kinzfogl, Amber Kugel, & Katie Luettgen, Spring 2003)
In the yeast ( Saccharomyces cerevisiae ) mating signal transduction pathway, interaction of the complete –mating factor with
the G-protein-coupled receptor on a MAT-a cell induces cell cycle arrest in the G1 phase, morphological changes or “shmooing,” and activation of genes involved in the mating process (Hoopes et al., 1998). In Saccharomyces cerevisiae , the amino acids Trp1, Lys7 and Gln 10, the central ß –turn conformation, and the amino acids near the C-terminus are directly involved in the binding of the a–mating factor to the receptor (Saskiawan et al., 2002). Altering the structure of the a –factor produces a conformational change in the receptor that is distinct from the conformational change of the normal a –factor, consequentially altering or even inhibiting the mating cascade of events (Bukusoglu and Kemmess. 1996). Elimination of Lys7 and Gln10 from the a –mating factor results in greater than a 100 fold decrease in mating signal transduction (Xue et al., 1996). [all background info]
It is unclear whether elimination of amino acid residues other than Lys7 and Gln10 in the a –mating factor also decrease the yeast mating response. [broad question] When introduced to MAT-a Saccharomyces cerevisiae cells, this sort of a – factor fragment could: 1.bind to the receptor site and induce the same change that the complete a –mating factor would; 2. bind to the receptor site but not induce the same changes as the complete a –factor, or 3.not bind to the receptor site at all. [BR: assumed biological mechanism] If the mating response to this fragment is different than normal (BR: assumption) , this would indicate which amino acid side groups are important in binding the receptor. An examination of Saccharomyces cerevisiae response to an a –mating factor fragment missing amino acids other than Lys7 and Gln10 would thus increase our understanding of the specificity of the a –factor receptor for its ligand. [BR: study goal/broader implication]
We hypothesized that the introduction of an a –mating factor fragment missing amino acids 7 through 13 to MAT-a Saccharomyces cerevisiae cells would cause more budding and less mating gene transcription and shmooing, as compared to the response to the complete a –factor. [hypothesis] We tested this hypothesis by adding this a –factor fragment to yeast cells transformed with a plasmid containing the FUS1 promoter attached to the lacZ reporter gene and recording the resulting morphological changes (budding and shmooing) and ß-galactosidase (ß -gal) activity. [approach]
Example 5: Good Introduction from manipulative experiment in Organismal Biology Lab
(adapted from a paper by Matt Young, Fall 2003)
The diving response is a set of characteristic reactions following the immersion of certain body parts in water. It is observed primarily in diving mammals and ducks, but humans have also elicited the response, perhaps as a trait that was not selected against during their evolution (McCulloch et. al. 1995; Hlastala and Berger 2001). Gooden (1993) clearly demonstrated that the diving reflex prepares the animal’s body for the effects of long periods of apnea (breathing cessation) associated with being underwater. It does this by decreasing oxygen consumption and redirecting blood flow out of the peripheral structures and towards the central organs such as the heart and brain.
McCulloch et. al. (1995) showed that the diving response is initiated by the stimulation of the trigeminal (Vth cranial) nerve, a primary sensory supply from the face, including the nose and forehead areas. Stimulation of this nerve results in a complex series of sympathetic and parasympathetic nerve activations (Gooden 1994). Increased parasympathetic activity triggers the vagus nerve to inhibit the cardiac pacemaker, resulting in reduced heart rate (Andersson et. al. 2000). Limb vasoconstriction occurs in response to increased sympathetic nerve activity, which results in increased mean arterial blood pressure (MABP) (Andersson et. al. 2000; Gooden 1994). [all background info in previous paragraphs]
Along with submersion in water, apnea is believed to be a major component in eliciting a proper diving response. It is still not clear, however, how necessary apnea is for the induction of the diving response or the mechanism for this induction (Gooden 1994). [broad question] Campbell et. al. (1969) argued that apnea, whether voluntary or involuntary, is essential for a diving response to occur, while Andersson et. al. (2000) found that facial immersion with eupnea resulted in reduced, but noticeable, diving responses. [background info]
It is believed that apnea stimulates chemoreceptors and thoracic stretch receptors in order to exert its effects. The thoracic stretch receptors are sensitive to movements in the airways, while chemoreceptors are sensitive to the oxygen lack associated with breath-holding. Increased firing of these two receptors due to their respective stimuli is believed to be the method by which apnea influences the diving response, but the exact pathway this firing takes to exert such effects remains unclear. It may either directly affect the cardiovascular centers, or indirectly affect the cardiovascular system via the medulla (Gooden 1994). [background info which identifies knowledge gap]
Does apnea significantly increase the human diving response during facial submersion? [testable question] It seems plausible that simultaneous activation of the trigeminal nerve, thoracic stretch receptors, and arterial chemorecptors would produce a more pronounced cardiovascular diving response. (BR: biological assumption) The goal of this experiment is to examine whether the diving response in eupneic (normal breathing) situations is significantly different than that observed during apneic situations. [BR: study goal] We will focus on heart rate and blood pressure changes, two of the many responses associated with the diving response. If heart rate and blood pressure changes during apneic submersion are significantly greater than those observed during eupneic submersions, this would indicate that simultaneous stimulation of the trigeminal nerve, thoracic stretch receptors, and chemoreceptors produces a greater cardiovascular response than stimulation of the trigeminal nerve alone. [BR: assumed mechanism]
We hypothesized that diving responses in human participants would be more pronounced in those experiencing apnea during immersion compared to those experiencing eupnea. More specifically, we expected non-breathing participants’ heart rates to decrease and blood pressures to increase significantly more than breathing participants in response to facial immersion in cold water. [hypothesis]
We tested this hypothesis by having 12 human subjects immerse their foreheads, noses, and cheekbones in cold water. We used a paired analysis to determine whether the change in heart rate and blood pressure from just prior to immersion to the end of immersion was different during apneic as compared to eupneic submersions. [approach]
This section is often the easiest to write since it is simply a clear explanation of the specific procedures, techniques , and materials you used . In some cases ( e.g. , the projects carried out in the Biocore Prairie), it is necessary to include procedures carried out by previous classes as well. Provide enough details that a knowledgeable reader ( e.g ., a Biocore peer who is not enrolled in lab) could replicate the experiment. This will also allow him/her to evaluate whether to trust your findings. In the case of field investigations, include a description of the type of community and the location of the site studied.
Mathematical manipulations or statistical analyses applied to the data should be explained under a subheading, but keep these brief. Although calculations are not normally included in a scientific paper, we sometimes ask you to include examples to check whether you are doing them correctly. If this is the case, put them in an appendix at the end of the paper.
Focus on essentials that affect the results . For example, in a genetics experiment with flies, it is important to state whether the females used for the crosses were virgins; it is not necessary to list the type of food or anesthetic used. However, these details would be important if your experiment was testing how different diets affected fruit fly activity level or some other physiological parameter. In cases where detailed protocols are given in the lab manual, merely cite the appropriate chapter of the lab manual, note any details relevant to the experiment but not specified in the protocol ( e.g. , identify the particular strain of organism you and your teammates used when several were available), and describe any manipulations you made that are not outlined in the manual. Include only what is vital for the reader’s understanding of how the results were obtained. (E.g., Drawing white poker chips out of a 1 quart Babcock Vanilla flavored ice cream container to get two numbers to pace out and place quadrats is not as important as the fact that quadrat placement was random.) If you are having trouble deciding what to put in and what to leave out, consult with your TA, peers, or other instructional staff for guidance before handing in your final paper.
- Use subheadings, including one called “data analysis”
- Describe your schedule of procedures in chronological order (if it makes sense to do so)
- When writing a final paper, use the past tense for this section (because you refer to procedures that you carried out in the past). When writing a proposal, use future tense.
- Report final concentrations (in molar, millimolar, micromolar etc). rather than final volumes (see table below). Readers can replicate concentrations, but often find it difficult to discern concentrations when only volumes are reported.
Example of Good Methods text
(Excerpt adapted from a paper by Beth Theusch, Biocore 384, Spring 2003: Inorganic phosphate competitively inhibits alkaline phosphatase-catalyzed hydrolysis of p-nitrophenylphophate )
Pilot Study*
A pilot study using various Pi concentrations but a constant substrate concentration close to the Km value was conducted in order to determine a Na2HPO4 concentration that has a moderate effect on initial reaction velocity to use in the inhibitor kinetics study. We tested a range of concentrations between 2.5 uM and 200 uM Na2HPO4 in tubes containing 0.05 M Tris-HCl, pH 8.6, 0.05 mM pNPP (the approximate Km value), and 4 ug/ml bovine intestinal alkaline phosphatase in a total volume of 5 ml. There was a control with no Na2HPO4 added and a blank with no enzyme added.
Experimental Protocol
The inhibitor kinetics study involved two sets of replicated reactions over a 0-0.5 mM range of pNPP substrate concentrations. One set of reactions was conducted in the absence of inhibitor and used as a control. The other set of reactions had a uniform concentration of Pi inhibitor, which was determined to be 0.05 mM from the pilot study, added to each tube. All tubes had 0.05 M Tris pH 8.6, 4 ug/ml alkaline phosphatase, and the appropriate amount of distilled water to bring the total volume of each tube to 5 ml. In each case, there was a control with no substrate added and a blank with no enzyme added. The pH of the Na2HPO4 salt solution was checked to ensure that the pH was approximately the same in the uninhibited and the inhibited reactions. Four replicates were performed for both the inhibited reaction and non-inhibited reaction.
For a complete protocol of the non-inhibited experiment, refer to “Enzyme Catalysis” in the Biocore Cellular Biology Lab Manual (Becker, Metzenberg, Dehring, 2003). For the inhibitor kinetics study, the product concentrations were used to calculate the initial reaction velocities at each substrate concentration in the presence and absence of inhibitor. Michaelis-Menten curves and Lineweaver-Burk plots were then generated to compare the values of Km and Vmax for the inhibited and uninhibited reactions. Ki was determined using the relationship that the inhibited Km = (1 + [inhibitor] / Ki) times the uninhibited Km.
Statistical Analysis
We performed an independent sample T-test to determine whether the differences between the average Km and Vmax values between the inhibited and uninhibited reactions were statistically significant.
*Note: Not all papers require the inclusion of pilot studies in the Methods section. Discuss this with your instructors.
How will methods/materials be evaluated? To see our expectations for your Methods & Materials, see the Biocore Research Paper Rubric in this Writing Manual.
The Results section is a logically organized presentation of your observational and numeric data . This is an opportunity to emphasize points or trends that you will be focusing on in your discussion. In many cases the organization and subheadings of this section should be consistent with those of the Methods and Materials section.
Before you start writing, make sure you have discussed the data and have shared your plan for analysis with your group members. Your group should share a common data set and, therefore, should be working with the same mean, standard deviation, and other descriptive statistics. As long as all group members have the same raw data set, you may choose to display the data differently.
There are usually two parts to this section:
- tables and figures
Text : The key purpose of the text in the results section is to point out and emphasize patterns in your data. You may choose to illustrate some of these patterns, especially those that pertain to your hypothesis, in figures or tables. However, each figure and table needs accompanying text to point out the obvious—or sometimes the not so obvious.
- Briefly describe, but do NOT make conclusions about ( i.e ., interpret) your data here — save that for the Discussion section .
- Point out any trends. (Trends are relationships between one variable and another. e.g ., as variable one changes, variable 2 tends to change in a consistent way.)
- Note differences or similarities between treatment groups.
- If you perform statistical analyses, report any significant biological differences you found, followed by pertinent statistical summary information (test score such as a “t” or “F” value, degrees of freedom, one or two-tailed p-value; see Biocore Statistics Primer for more info).
Refer your reader to “Table 1” or “Figure 1” as you explicitly identify relationships, patterns, or general trends that you see in the data. Remember that relationships that are obvious to you may not be obvious to someone who has not carried out the experiment.
- Never write a sentence that just tells the reader where the data are. Point out to your reader the general trends in the data, then refer to the figure or table parenthetically.
- When using the term “significant” in your results section recognize that it has a specific connotation in science that reads “statistically significant.” Therefore, use the term “significant” when explaining differences you observe only if you found statistically significant differences.
The Results section should not be controversial since you are merely reporting findings, not saying what you think they mean. Avoid judging your data as “good” or “bad.” Data are facts and facts simply are what they are. Remember: you are not graded on whether your experiment “worked” or on your results; you are graded on how you handle them . Always report what you saw , not what you think you should have seen.
See the following excerpt from a good Results section describing data from a systematic study.
Example of a Good Results Section from a Systematic Observation Study
(excerpted from a Biocore 382 paper by Kim Treml, Fall 2003)
Water Quality
Water quality testing revealed a mean pH of 6.67 +/-0.07 pH units (Table 1). Mean dissolved oxygen and dissolved carbon dioxide were 3.4 +/- 0.4ppm and 55 +/-3ppm respectively. Also, the total phosphorus was measured as 0.51 +/-0.5mg.L and conductivity, measured in microsiemens, was 1,063 +/-17μs. All means were computed with n=45. Both conductivity and phosphorus fall far out of range of optimal water quality levels for a healthy aquatic ecosystem (Table 1). The measured phosphorus level is an order of magnitude larger than what is recommended by the EPA. Conductivity is twice as high as the ideal level in a freshwater ecosystem. [RESULTS TEXT]
Table 1. Water quality data obtained from the University Bay marsh in 2003. Each value represents the mean of 45 trials. The error margin is + or – 1 standard error. Optimal data ranges for a healthy aquatic ecosystem are shown for comparison. [TABLE LEGEND]
Macroinvertebrate Diversity
Macroinvertebrate species in the University Bay marsh were catalogued and presence or absence of each species was noted. Figure 3* depicts the calculated frequency of each species per 500mL. The species are approximately organized on the chart from left to right with increasing pollution tolerance as described on North Carolina State University’s water quality webpage (2003). The highest frequency in both 2002 and 2003 exists among organisms around the mid-range of pollution tolerance. Orb snails, scuds, backswimmers, copepods, seed and clam shrimp, nematodes and tubifex worms were present in over half of our samples in either 2002 or 2003. Species indicative of very high water quality or very low water quality were less frequent compared to species indicative of the mid range. Nonetheless, the data show an increase in the variety of species present from 18 species in 2002 to 26 in 2003. [RESULTS TEXT]
* Figure 3 not shown in this Writing Manual
Tables and Figures:
Tables and figures are key elements of a scientific paper.
- Tables are organized lists of numbers, ideas, or other data.
- Figures are graphs, charts, diagrams, or photos.
Why use tables and figures? First, they offer a concise way to present a large amount of information. Second, they carry the bulk of the experimental evidence needed to support your conclusions. Third, they offer the reader a chance to assess your data and determine whether or not your conclusions are valid. Finally, the values in them can be used by other scientists who wish to build on your work. Usually, summarized (e.g., averages and measures of variation) rather than raw data are included in a paper. Always make it clear whether you are presenting actual data or averages. (In some cases we will ask you to include raw data as an appendix.) Please refer to the Biocore Statistics Primer for directions on producing figures in Excel.
Each table or figure should be referred to in the text of your paper at least once. If you have nothing to note about a particular table or figure, leave it out. Identify and number tables or figures according to the order they appear in the text (Table 1, Table 2, Figure 1, Figure 2, etc.). This way the reader will know exactly what data you are discussing.
Tables and figures should be neat, logically organized, and informative. If properly prepared, they can stand independently of the paper. Always remember that readers are not familiar with your data. A table or figure that seems self-explanatory to you may not seem so to a reader.
Here are some rules for presentation of graphs and tables:
- Present your final data in table or graphical form. The choice of table or figure should be based on the type of data you have. If you are trying to show trends or simple comparisons it may be best to use a figure. If you have long lists or many comparisons to be made across groups a table may be more appropriate. [ DO NOT present the same data in both table and graphic form.]
- The most common way to present graphical data is either an XY scatterplot for continuous data or a bar chart for categorical data/ results of statistical comparison of the means of two or more groups.
- Keep it simple! The amount of time it takes a reader to interpret a figure is inversely proportional to how well those data are presented. Do not overuse transformations or ratios if they are unnecessary for accuracy and clarity of your results.
- Clearly label all axes or columns including units ( e.g. Time (min.), Concentration (mM), Mass (mg)). Describe any symbols you use in your graphs using a KEY (see figures below for examples of keys).
- POOR LEGEND: Enzyme activity vs. salt (Avoid using the term vs)
- BETTER : Average alkaline phosphatase activity for concentrations of NaCl from 0.1 to 1 mM. The substrate for the reaction was ATP at a concentration of 2 mM for a total reaction time of 3 minutes. Columns represent mean values (N=3) with error bars representing ± 1 SE.
- Put table legends above a table. Put figure legends below or to the side of a figure.
- Do NOT create titles for figures or tables. Instead of a title, use a simple legend numbering each table and figure consecutively is sufficient. Do not use titles like “Chart 1” that are automatically generated by Excel.
- For graphs that present an average value as a single point or bar, include error bars and state what they represent. Usually, this will be 1 standard deviation (SD) or 1 standard error (SE) on either side of the mean (see figure 1 below for an example).
- For tables presenting means, include some measure of variation (SD or SE). (See Table 1 above for an example of this).
- State the number of samples used to calculate an average. If you measured the height of 12 purple cone flower plants and reported an average height of 0.82m, indicate the number of samples used to generate that statistic as n=12.
- Do not connect the points on a line graph unless you really mean to say that the values in between the points shown should follow the line drawn. Trend lines have very limited predictive value or validity when connecting 3 points or less.
Drawing a diagram or presenting a photomicrograph: Drawn diagrams or photographs taken from a microscope and their legends should contain enough information that a reader can understand (as near as possible) what you actually observed and the conditions surrounding the observation. Diagrams must be large enough to show significant details of what you observed. In practice, this generally means that each diagram should cover at least a quarter of an 8.5×11” page . Indicate the type of microscopy used and the total magnification in your legend. Include a scale on your drawing. Define the experimental conditions and include notes on the process of your investigation. See Figures A-7, A-13, and A-14 in the World of the Cell’s “Principles & Techniques of Microscopy” for examples of good figure legends.
Example of Good Results bar graph
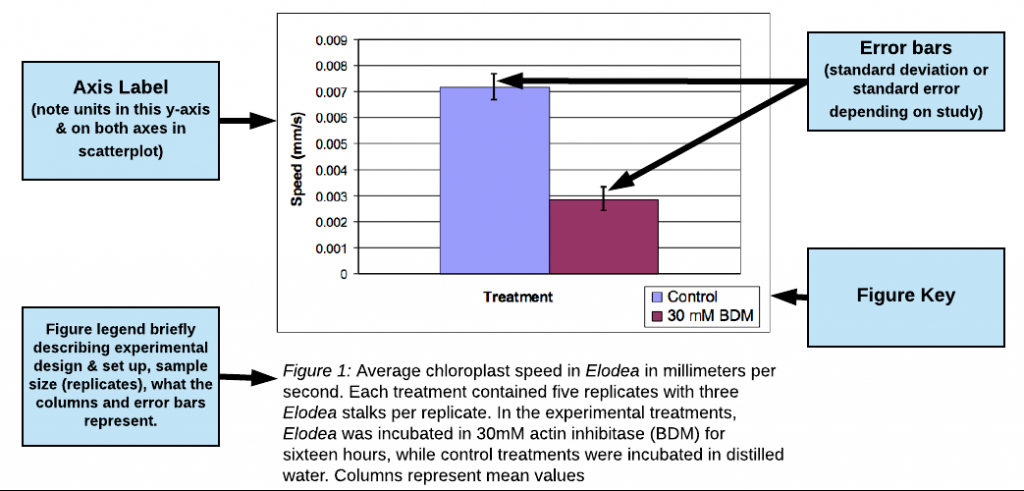
Example of Good Results scatterplot
(excerpted and adapted from a presentation by Jennifer Rowland, Beth Rollmann, Simona Rosu, and Christopher Luty, Biocore 384, Spring 2003; Gramicidin Decreases CO2 Consumption in Elodea)
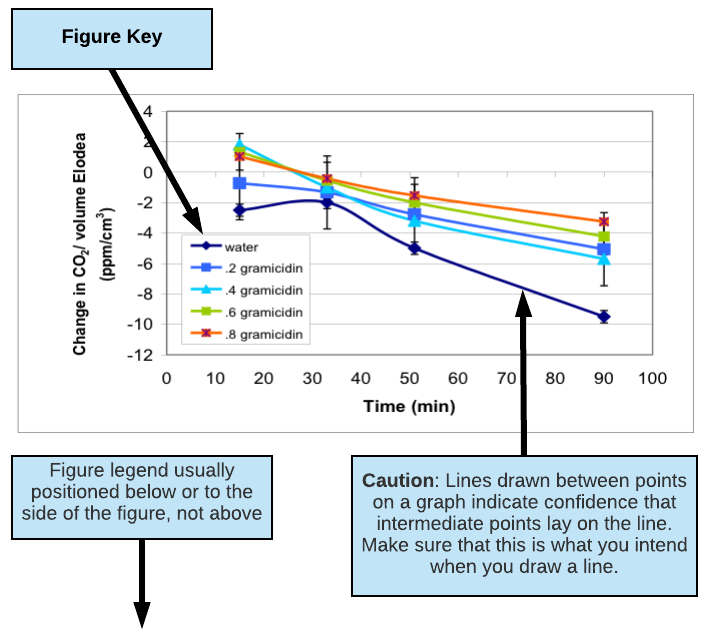
Figure 2: Change in dissolved CO2 levels in water surrounding six Elodea sprigs (6 cm in length) in 75 ml culture tubes over 100 minutes of light exposure. Dissolved gramicidin concentrations ranged from 0 to 0.8 µM. Each data point represents the mean of N=11-15 culture tubes for each gramicidin concentration plus/minus one standard error.
view this figure as a pdf
Example of Good Table
(adapted from Jenna Voegele paper on water quality in Willow Creek, Biocore 382, Fall 2004)
Table 1. Mean values of water chemistry tests from upstream and downstream sampling locations during a three day study period, Sept 14-16, 2004. Variation is shown as ± 1 SE next to each mean value, followed by sample size (in parentheses) in which varied for each test and sampling location. Note the smaller sample size for the nitrate-N test.
Writing a figure legend for a drawing or micrograph:
If you are including an image (drawing or photomicrograph) in your paper, highlight attributes of the image that are important for your paper and to your reader. If the reason for including the image is to highlight anatomy, you may want to label structures and include a description of movement or other important observations in the figure legend. When writing a figure legend to accompany a photo or drawing, include enough information so that a reader can understand (as near as possible) what you actually observed and the conditions surrounding the observation. This means that you should indicate the type of microscopy used (phase contrast, bright field, fluorescence, etc.) and any notes regarding the preparation (e.g., mounted in ProtoSlow, water or saliva, with coverslip, types of stains used, etc.). Also indicate the total magnification in your legend. Diagrams must be large enough to show significant details of what you observed. It is important to include a scale on your drawing.
Click on the three purple icons in the diagram below for more information about each element.
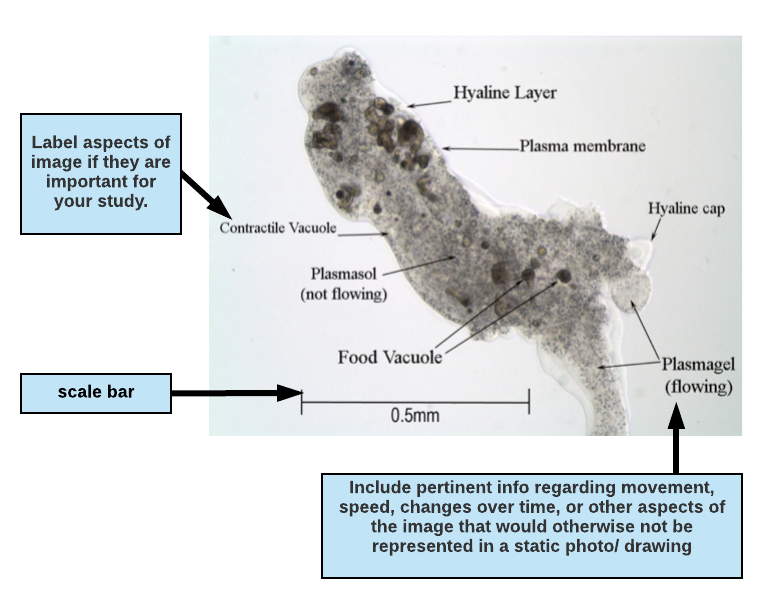
Figure 1.1 Micrograph of the protozoan Pelomyxa carolinensis viewed under phase contrast microscopy, magnification 100X. The specimen is mounted in ProtoSlow and coverslip to reduce its movement. Plasmagel streams readily into pseudopodia (seen at the bottom right of the photo) allowing the amoeba to slowly crawl across the field of view.
In the figure descripton above, the writer has indicated the type of microscopy (phase contrast microscopy, magnification 100X) and the total magnification (100X).
How will results (including text & figures/tables) be evaluated? To see our expectations for your Results, see the Biocore Research Paper Rubric in this Writing Manual.
This is where you interpret your results for the reader . It is the most important part of your paper and often one of the most difficult to write. The discussion section is NOT a restatement of your results, but rather where you provide your insight on the investigation through logical analysis. Key elements of your discussion section include:
- BROAD STUDY QUESTION that your research is trying to address
- SUPPORT/REJECT HYPOTHESIS
- INTERPRET the dependent variable measured (if multiple variables are measured you interpret each variable independently and then INTEGRATE variables for overall interpretation of data)
- Formulate argument for your conclusions, emphasizing how your data do or do not support your biological rationale & by comparing with relevant findings in the literature
- NEW KNOWLEDGE that your investigation has generated: highlight the knowledge gap that your data help address, and the implications of your work. Introduce at least one new paper from the scientific literature to help you discuss or support your findings.
- EVALUATE confidence in experimental design and reliability of data
- NEW QUESTIONS and FUTURE STUDIES that the new knowledge inspires
- UNEXPECTED OBSERVATIONS are unique observations not collected in rigorous way but still intriguing and could inspire new investigations
- CONCLUSION brief statement as summary.
The organization of your discussion section is not fixed but rather it is driven by the reliability of the data you collect. The discussion should complement the logic set up with your biological rationale in the Introduction.
The following is not an appropriate discussion section: “Our data supported the hypothesis. The results were what we expected (see Results section).” Instead, state specifically what you observed in your data, and the conclusions you feel confident you can make based on the evidence you gathered. The Discussion should formulate and support a logical argument , leading the reader through the specific conclusions drawn from the data to their more general implications beyond the experiment.
Elements in the Discussion Section
Broad Study Question
What is the broad question that your research is trying to address? State your question clearly in the opening paragraph.
Support or Reject Hypothesis :
- If you have conducted a manipulative study, restate your hypothesis and whether you support or reject your hypothesis referencing appropriate data . (Note that finding no difference between two treatments is a result).
- Critically evaluate your biological rationale, experimental design, data collection, and explicit/implicit assumptions throughout. After this evaluation, you should be able to support or reject your hypothesis….OR you may feel that you did not fully test your hypothesis after all. A key step here is to look at your controls and variation in your measurements. How much variation surrounds your controls? How reliable and accurate are your measurements?
- IMPORTANT NOTE: finding no difference between treatments is NOT an inconclusive result–No difference is a very valid result that contributes to a conclusion for either supporting or rejecting a hypothesis!

Interpreting Data : If you feel that your protocol allowed you to test your hypothesis,
- Interpret each piece of data presented in the results independently and evaluate the reliability of the data.
- Discuss how these data are similar (confirm) or contrast with what is reported in literature you presented in the introduction OR new literature you discovered after you completed your experiment. Explain the trends you feel are important to support your conclusion(s) and evaluate how this supports or contradicts the biological assumptions you outlined in your biological rationale. Be prepared to detach yourself from your original biological rationale in explaining or being critical of your results.
- Combine and integrate the multiple types of reliable data you collected and discuss how together they inform the broad question (only combine data you are confident in).
Generating New Knowledge
Describe how your experiment contributes to the knowledge gap you identified in your introduction. Cite similar, contrary and/or supportive literature.
- If your data supported your hypothesis: guide your readers through the steps in your reasoning referring back to your biological rationale to provide context. Present the arguments that explain how your experimental approach and the pieces of evidence (data) convince you of your conclusion. Explain how do your findings add to those that others have observed. Compare your findings with information from the literature (this often requires a post-experimental literature search), citing appropriate references that support for your results. These references include many that you cited in your Introduction section; briefly summarize them but avoid redundancy.
- If your results are contrary to your hypothesis , you need to speculate the reasons for this difference, continue your literature search to explain your alternative results. Are your results consistent or inconsistent with others findings—why or why not? Distance yourself from the project while writing and be reasonably critical of your data. What evidence do you have that your biological rationale is acting? Is the mechanism you propose in effect? Evaluate the key biological assumptions in your biological rationale which were not correct.
- Implications of your findings- How does your experiment add to the current body of knowledge? Speculate on the implications of your findings. It is essential that you refer back to your biological rationale . Implications are specific, reasonable extensions of your results or the meaning of your results for the larger picture. Be careful, however, with your choice of words: state implications as logical possibilities rather than as fact. Your results may lead to new insights about relationships in nature. An unexpected result (if it holds up on repeating the experiment) may yield insight to guide a more effective experimental approach.
Evaluate Confidence in Experimental Design and Data Reliability/Quality
- Evaluate the strengths and weakness of your experiment and your confidence (or lack of) in your experimental design. Explain how these factors allow you to gauge the strength of your conclusion(s). Always address whether your protocol allowed you to truly test your hypothesis (see special note about inconclusive results in ‘support or reject hypothesis’ section above). In some cases you may discover unexpected inaccuracies in your data or that the methods you used were not appropriate or precise enough to address your question or test your hypothesis. Address the errors, unresolved issues and speculate how the experimental approach might be improved. Inconclusive results may show that you weren’t asking a relevant question in the first place or that the experiment was not able to test the question you posed. This, in turn, can generate specific new questions and experimental approaches. Avoid making a laundry list of mistakes you made in carrying out your experiment. Only mention errors if they help explain unexpected data values and/or lead you to conclude that your methods did not allow you to test your hypothesis.
- Evaluate reliability of data – Once you have established that your experimental design was appropriate to address your original question, you must also evaluate how well you carried out your intended design and what that means to your data reliability ( e.g . evaluating whether the variation you see between samples is natural variation or experimenter’s error). How good are your data? Consider the variability in your data (variance, standard deviation, standard error). Did you have enough replicates? Did you have a large degree of experimental error? What are the implications of variability? Do not over-interpret your data . Recognize the magnitude of the variation within your data and the level of departure you would need to conclude true differences. In most cases you are trying to attach meaning to a group of numbers generated by some procedure. Help your readers make sense of these numbers by explaining how the patterns and relationships you observed reflect the biological concepts or issues you set out to explore. How do your data fit with your biological rationale?
New Questions and Future Studies : Science is built on an iterative cycle of questions, experiments, results and conclusions. Often it is appropriate to suggest the next step in the investigation. Be sure to include the reasoning that leads to your insights . Your experiment will likely provide many opportunities to ask new questions and suggest future studies.
Final Conclusion : End your paper strongly with a clear, brief conclusion that relates directly to the question, hypothesis, or knowledge gap you stated in the Introduction.
If you get stuck : The hard work of making meaning of data will be easier if you have a clear idea of what it was that you set out to do in the first place. Re-read your question and biological rationale. Do your results allow you to answer the question you posed in light of your biological rationale? A second reading of your BR after examining your data will often solve much of the confusion you may be experiencing. Be sure to discuss your results thoroughly with your research team. They may have some insight, intriguing literature for comparison, or thoughts about the data that could benefit your interpretation.
Other things you can do:
- Take a look at the example of good discussions on the next pages.
- Make a conceptual diagram for yourself or with your team. This is especially useful for seeing new connections, structuring ideas, and finding interactions at multiple levels.
- Explain the experiment and its significance to a friend who knows nothing about it. If you understand the full content, context, results and relevance of your experiment, you should be able to explain what was done and what it means. This should help provide some organization to your paper.
How will discussions be evaluated ? To see our expectations for your Discussion, see the Biocore Research Paper Rubric in this Writing Manual.
Example of Good Discussion
Adapted from a paper by Jeremiah Wilke, Biocore 382, Fall 2003 Practice Paper entitled “Queen Anne’s Lace ( Daucus carota ) Species Frequency Suggests Rototilling as Most Effective method for Control of Invasive Weeds in Prairie Restoration Projects
The results suggest that rototilling is the most effective method as mulching and mowing yielded frequency values approximately 5 fold greater. The greater effectiveness of rototilling over the other methods coincides with previous knowledge of Queen Anne’s lace as it is known to favor habitats in no-till fields (Rose and Sheaffer, 2003) and re-sprout stems even after being cut (Biocore 382, class 2001, unpublished data) . (setting up logical argument: referring back to biological rationale and comparing findings with the literature) . The frequency means suggest mowing to be slightly more effective than mulching; however, the distribution of the frequencies indicates little difference as the methods share common values. (Data interpretation- part of logical argument; Add re-statement of hypothesis and clearly state whether it was supported or rejected based on data interpretation)
Through rototilling seems to be the most efficacious for Queen Anne’s lace, several factors prevent us from making a definitive conclusion, most notably a small sample size. (Evaluating the validity and reliability of data) Frequency calculations can suggest patterns in the treatment, but they give no sense of the species density (number of a give species per quadrat). Examinations of the species frequency of Queen Anne’s lace in a control would also allow us to be more conclusive by gaining a sense of the improvement the methods made over untreated plots. (evaluating experimental design) Beyond our inability to decisively say which treatment is the most effective for Queen Anne’s lace, further work by the University of Wisconsin-Madison Biocore class of 2001 suggests we cannot generalize to other non-native species (Batzli, 2003). In their research, none of the methods demonstrated an appreciably greater capacity for weed control when tested on a variety of species. (discussion of other data makes our interpretation and argument more convincing) Species density calculations, measurements against a control, and the effectiveness of treatments on the other invasive plants therefore all necessitate future research. Mixing treatments has also been proposed (Batzli, 2003), while engineering novel methods deserves further study. (next steps)
(Final conclusion and brief discussion of implications of this research would help here)
Example of a Good Discussion that enumerates assumptions and how violating assumptions changes conclusions
Adapted from a poster by Beth Gausden, Katie Gielissen, Emily Gurnee, Jordan Mollet, and Carley Zeal, Biocore 384, Spring 2006
Addition of colchicines to MATa S. cerevisiae in vivo does not inhibit budding in the absence of α-factor but reduces shmooing and β-gal activity in response to α-factor
The results in Fig. 2 do not support our hypothesis (rejection of original hypothesis) that yeast exposed to colchicine in the absence of α-factor show a drastic decline in the incidence of budding as compared to controls. Our original hypothesis was based on the assumption that inhibition of mitotic division would prevent budding. (clear statement of key assumption in biological rationale) Although nuclear division is mediated by microtubules, pinching action and subsequent cytokinesis (budding) is controlled by actin filaments1. The tubulin-colchicine complex inhibits karygomy; however, bud formation can occur independently of nuclear division.1 Budding was still observed microscopically after three hours of incubation with colchicine (Fig. 2)- approximately two generations. These results indicate that bud formation was not inhibited by colchicine; (summary of how results do not support biological assumption) however, later generations incubated in colchicine may show complete cessation of budding as a result of aneuploidy, an irregular number of chromosomes.1 This occurs when a yeast cell undergoes successful cytokinesis but unsuccessful karyogamy; if this process is continuous or prolonged, cells will be unable to bud.
The results in Fig. 1 and Fig. 2 do not support our hypothesis that colchicine does not affect shmooing or the transcription of mating genes. We expected no change in the incidence of mating gene transcription as reported by the β-gal assay and percent of shmooing yeast in the yeast treated with colchicine compared to untreated yeast. The β-gal assay, Fig. 1, indicates a large decrease occurred in the transcription of mating genes in the presence of colchicine. Similarly, we observed a lower percentage of shmooing cells in the presence of colchicine. If nuclear division were inhibited by colchicine, then the portion of cells experiencing aneuploidy would be unable to respond to α-factor by shmooing or transcribing mating genes.
Our results suggest that colchicine does not inhibit bud formation (in the absence of α-factor) after 3 hours. We also observed decreased shmooing as well as β-galactosidase activity in yeast cells treated with colchicine and α-factor. The consistency of our results provides reasonable confidence in the methods. In future studies, longer incubation times, differing concentrations of colchicine, and chromosome and microtubule staining could be used to investigate the mechanism more thoroughly.
Adapted from a paper by Beth Theusch, Biocore 384, Spring 2003 Inorganic Phosphate Competitively Inhibits Alkaline Phosphatase-Catalyzed Hydrolysis of p-Nitrophenylphosphate
We hypothesized that inorganic phosphate (Pi) would act as a competitive inhibitor of the alkaline phosphatase-catalyzed pNPP hydrolysis reaction. Our data support this hypothesis. (re-statement of hypothesis and whether it was supported or rejected) As expected, we found that addition of inorganic phosphate increased the Km of the alkaline phosphatase-catalyzed pNPP hydrolysis reaction while the Vmax remained relatively unchanged. (setting up logical argument) After the addition of a concentration of Pi inhibitor approximately equal to the uninhibited Km substrate concentration, the apparent Km became 6-7 times as large (from 0.038 mM to 0.253 mM) as the uninhibited Km. Therefore, pNPP substrate molecules had to be almost 7 times as numerous as inhibitor molecules to access alkaline phosphatase’s active site and produce product equivalent to an initial uninhibited reaction velocity of 1/2 Vmax. These data indicate that Pi is quite an effective competitive inhibitor. One reason for its effectiveness as an inhibitor could be that the molecular weight (MW) of inorganic phosphate is about 96 g/mol, while the MW of pNPP, with its bulky nitrophenyl group, is almost 217 g/mol. Temperature is a measure of average molecular kinetic energy and is proportional to mv2. This means that lighter molecules have to move faster than heavy ones at 37oC in order to have the same kinetic energy as the large molecules. Molecules that move faster have more collisions, so it is likely that each Pi molecule had a greater chance of colliding with the alkaline phosphatase (AP) active site than did each pNPP substrate molecule during our experiment. (constructing new knowledge: references would help a lot here to show that the differences in molecular weight mentioned could significantly change kinetic energy) In addition, AP may have had a greater affinity for Pi than it did for the pNPP substrate, since alkaline phosphatases have a high affinity for inorganic phosphate (McComb et al ., 1979). The bulky phenyl group on pNPP may have sterically hindered the hydrolysis reaction more than the hydrogen on Pi, depending on the specific geometry of the active site. As we mentioned previously, AP generally hydrolyzes Pi at a slower rate than it hydrolyzes phosphomonoesters (Schwartz, 1963), and so it may be that Pi occupies the AP active site longer per hydrolysis and thus excludes available pNPP from subsequently binding. (constructing new knowledge: referring back to biological rationale and comparing findings with the literature)
At first glance, it might appear that some of the increase in apparent Km could be attributed to a slight change in pH, since the Km value is pH dependent. Dibasic Pi can act as a base by adding a proton and becoming h1PO4- and as an acid by losing a proton and forming PO43-, but phosphate is predominantly the dianion at a pH of 8.6. Since the pH of the 0.05 mM Na2HPO4 salt solution was 7.7, which is close to the targeted value of 8.6, it is a reasonable to assume that the buffer counteracted any fluctuations in pH and essentially kept the pH constant. (evaluating experimental design)
Although the Vmax did not change dramatically between uninhibited and inhibited reactions, there was some difference between the uninhibited value of 0.056 umol/min and the inhibited value of 0.070 umol/min. Since Vmax did not decrease, it was clear that Pi did not act as a noncompetitive inhibitor. Since Vmax increases in the presence of an activator, it is possible that slight changes in ionic strength resulting from the addition of the salt could have activated AP somewhat. However, previous studies at a pH of 10 have shown that the activities of mammalian alkaline phosphatases are either unaffected or diminished by an increase in ionic strength. Specifically, calf intestinal AP experienced no change in activity following the addition of 1M NaCl, a much higher concentration than the Na+ that we introduced in our experiment. In other systems, NaCl addition at a pH of 9.0, close to the 8.6 we used in our experiment, had little effect on maximum velocity and actually inhibited it at low substrate concentrations (McComb et al ., 1979). Since other variables in the experiment were held constant, the differences in Vmax values could simply be due to experimental error. (evaluating data reliability & experimental design)
The Ki value of 8.78 uM obtained from this study was comparable to but slightly greater than literature values for the Ki of E. coli AP. The values of 1 uM (O’Brien and Herschlag, 2001) and 0.6 uM (McComb et al ., 1979) for Pi inhibition of E. coli AP were both obtained at a pH of 8.0 and temperature of 25oC, while we used a pH of 8.6, a temperature of 37oC, and bovine intestinal AP in our study. Just like Km values, Ki values are pH dependent. It is generally recognized that competitive inhibitors of AP are more effective at lower pHs (McComb et al ., 1979). The pH difference alone could probably explain why our Ki was slightly larger and our inhibitor was slightly less effective than in the E. coli studies. In addition, bovine intestinal AP has a structure that is somewhat different from E. coli AP, so it is reasonable that the kinetics of the two enzymes could differ slightly. Some studies in rats have shown that only 1/10 as much Pi is needed to inhibit intestinal AP as compared to the amount that is needed to inhibit AP in other rat tissues (McComb et al ., 1979). (evaluating data reliability & experimental design) Perhaps there are lower Pi concentrations in intestinal cells as compared to cells in other tissues. It would be interesting to see if this is true for bovine and other mammalian AP as well. (New questions/Future Studies)
The inhibition of AP by Pi, the product of AP catalyzed hydrolysis reactions, is a substrate-level regulation mechanism (Becker, Kleinsmith, and Hardin, 2003). This allows the AP enzyme to be responsive to product concentrations, so it is not always functioning at its maximum rate. It is not in the best interest of the cell to convert all phosphomonoesters into Pi and an alcohol at once, and the competitive inhibition by Pi helps to prevent this. This is precisely why initial reaction velocities are used when studying enzyme kinetics; if products are allowed to accumulate, they are likely to have an inhibitory effect on the enzyme. (implications of results, referring back to biological rationale)
Overall, the results of this study indicate that Pi is indeed a competitive inhibitor of bovine intestinal AP, as we had hypothesized. Specifically, we found that the Km value increased from 0.038 mM to 0.253 mM while Vmax remained relatively constant. We also found that our Ki value of 8.78 uM was reasonably similar to that reported previously for this particular enzyme and inhibitor. (final conclusion)
Parenthetical Citations Within Text
- Cite all information that you use from published or unpublished sources in the body of your paper and provide full citations in the Literature Cited section at end of the paper.
- Parenthetical author-date format within a sentence or at the end of a block of text. Provide the last name of the author(s) and the date the work was published, both enclosed by parentheses. Example: Global warming is a looming threat to biodiversity (Peters and Lovejoy 1992).
- More than one source , list them in chronological order: e.g. (Jones 1992; Smith and Jacobs 1993; Torrez 1995). If a work has more than two authors, you may list the first followed by et al. (latin for “and others”) and the date: (Jones et al. 1995). However, the names of all of the authors must be included in the list of citations at the end of the paper.
- Unpublished information: If you cannot find a published citation you can site personal communication in the body of your text – NOT in the literature cited. The format for unpublished information or data communication to you by a colleague is the source followed by “personal communication” or “unpublished data”: e.g. (Maria Rodriguez, personal communication 2002; Biocore 382 class, unpublished data). ***Use these sparingly as sources usually are not formal and cannot be verified easily. DO NOT base the major foundation of your study on personal communication unless the information gained is unique and not found elsewhere.
List all works cited in the text – and no others – alphabetically in the References section at the end of your paper. The specific format used for references varies depending on each journal’s conventions, web-site format and the type of source to which you are referring. We would like you to use the format demonstrated below which follows the Name-Year system . Each reference should include the names of all the authors, the date the article or book was published and/or the date the website was accessed and its title. Regardless of the exact format used, make sure that you are consistent!
Here are some examples to follow:
Format as follows :
Author(s). year of publication. Title of the article (with only the first word capitalized). title of journal plus volume (issue): Inclusive page numbers.
One author example
Vitousek, P.M. 1994. Beyond global warming: ecology and global change. Ecology 75: 1861-1876.
Multiple author example
Post, W.M., Emanuel, W.R., Zinke, P.J., and Stangenberger, A.G. 1982. Soil carbon pools and world life zones. Nature 298: 156-159.
Internet Sources
A full discussion of number and types of internet resources is beyond the scope of this manual.
However, the following is a general guide for most articles that are published on the internet. As with all resources, especially those found on the internet, you must be wary of the source and its validity. If it doesn’t have an author or publication/ posting date BEWARE!
Format as follows :
Author(s). Year of publication. Title of the work. Title of the complete work or website or on-line journal plus volume (issue) if available/ applicable. Website URL or address (except for online journal or personal email). Date you accessed the web page.
Carbon, J.J. Physiology data. Personal email (7 July 2010).
Listserv or RSS feed newslist:
Blystone, R.V. 1994. Setting up a digital classroom and other stuff. [email protected] (accessed May 10, 1996).
World Wide Web: Basic form is: Author. Date. Title. URL (Access date)
Waterman, M., Stanley, E., Soderberg, P., and Jungck, J.R. 1999 Kingdoms entangled: molecules, malaria, and maise. BioQUEST Curriculum Consortium. http://bioquest.org/case.html (accessed April 12, 2012)
Macreal, H. 2001. Large Fish, Small Pond. http://www.bigfish.org/articles (accessed April 20, 2001)
Splice, G. 2000. Mutations are the Ultimate form of Variation. University Press Weekly vol 22. Electric Library. http://www.elibrary.com/ (accessed October 17, 2011).
*Note: Do not write out a website address (URL) as a parenthetic citation within the text of your paper—instead include the author and year of publication (e.g. Macreal 2001), just as you do with all other publications. Whenever possible, list the author. If you can’t find an author, list the organization that provided the information. If you can’t find the name of the organization, question the quality of your source.
Biocore Lab Manual
You will be citing one of your Biocore lab manuals in many of your research papers. To do this, look at the lab manual chapter to find the author(s) you wish to cite and the example format below. NOTE: This is an example for the Biocore Prairie chapter of the Biocore 382 lab manual.
Book Citations
Format as follows:
First author’s last name, First initials, subsequent authors’ name separated by commas, year of publication, title of book (italicized, with only the first word capitalized), edition number (if it is not the first edition), the publisher, the city of publication, and the state (omit the state for well known cities like New York).
Kuhn, T.S. 1962. The structure of scientific revolutions. University of Chicago Press, Chicago.
Purves, W.K., Sadava, D., Orians, G.H., and Heller, H.C. 2001. Life, the science of biology, 6th ed. Sinauer, Sunderland, MA.
Chapter in a Book
Naes, A. 1986. Intrinsic value: will the defenders of nature please rise? In Soulé, M.E., editor. Conservation biology: the science of scarcity and diversity. Sinauer Associates, Sunderland, MA. pp. 504-515.
Process of Science Companion: Science Communication Copyright © 2017 by University of Wisconsin-Madison Biology Core Curriculum (Biocore) is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License , except where otherwise noted.
Share This Book
Write Your Dissertation Using Only Secondary Research

Writing a dissertation is already difficult to begin with but it can appear to be a daunting challenge when you only have other people’s research as a guide for proving a brand new hypothesis! You might not be familiar with the research or even confident in how to use it but if secondary research is what you’re working with then you’re in luck. It’s actually one of the easiest methods to write about!
Secondary research is research that has already been carried out and collected by someone else. It means you’re using data that’s already out there rather than conducting your own research – this is called primary research. Thankfully secondary will save you time in the long run! Primary research often means spending time finding people and then relying on them for results, something you could do without, especially if you’re in a rush. Read more about the advantages and disadvantages of primary research .
So, where do you find secondary data?
Secondary research is available in many different places and it’s important to explore all areas so you can be sure you’re looking at research you can trust. If you’re just starting your dissertation you might be feeling a little overwhelmed with where to begin but once you’ve got your subject clarified, it’s time to get researching! Some good places to search include:
- Libraries (your own university or others – books and journals are the most popular resources!)
- Government records
- Online databases
- Credible Surveys (this means they need to be from a reputable source)
- Search engines (google scholar for example).
The internet has everything you’ll need but you’ve got to make sure it’s legitimate and published information. It’s also important to check out your student library because it’s likely you’ll have access to a great range of materials right at your fingertips. There’s a strong chance someone before you has looked for the same topic so it’s a great place to start.
What are the two different types of secondary data?
It’s important to know before you start looking that they are actually two different types of secondary research in terms of data, Qualitative and quantitative. You might be looking for one more specifically than the other, or you could use a mix of both. Whichever it is, it’s important to know the difference between them.
- Qualitative data – This is usually descriptive data and can often be received from interviews, questionnaires or observations. This kind of data is usually used to capture the meaning behind something.
- Quantitative data – This relates to quantities meaning numbers. It consists of information that can be measured in numerical data sets.
The type of data you want to be captured in your dissertation will depend on your overarching question – so keep it in mind throughout your search!
Getting started
When you’re getting ready to write your dissertation it’s a good idea to plan out exactly what you’re looking to answer. We recommend splitting this into chapters with subheadings and ensuring that each point you want to discuss has a reliable source to back it up. This is always a good way to find out if you’ve collected enough secondary data to suit your workload. If there’s a part of your plan that’s looking a bit empty, it might be a good idea to do some more research and fill the gap. It’s never a bad thing to have too much research, just as long as you know what to do with it and you’re willing to disregard the less important parts. Just make sure you prioritise the research that backs up your overall point so each section has clarity.
Then it’s time to write your introduction. In your intro, you will want to emphasise what your dissertation aims to cover within your writing and outline your research objectives. You can then follow up with the context around this question and identify why your research is meaningful to a wider audience.
The body of your dissertation
Before you get started on the main chapters of your dissertation, you need to find out what theories relate to your chosen subject and the research that has already been carried out around it.
Literature Reviews
Your literature review will be a summary of any previous research carried out on the topic and should have an intro and conclusion like any other body of the academic text. When writing about this research you want to make sure you are describing, summarising, evaluating and analysing each piece. You shouldn’t just rephrase what the researcher has found but make your own interpretations. This is one crucial way to score some marks. You also want to identify any themes between each piece of research to emphasise their relevancy. This will show that you understand your topic in the context of others, a great way to prove you’ve really done your reading!
Theoretical Frameworks
The theoretical framework in your dissertation will be explaining what you’ve found. It will form your main chapters after your lit review. The most important part is that you use it wisely. Of course, depending on your topic there might be a lot of different theories and you can’t include them all so make sure to select the ones most relevant to your dissertation. When starting on the framework it’s important to detail the key parts to your hypothesis and explain them. This creates a good foundation for what you’re going to discuss and helps readers understand the topic.
To finish off the theoretical framework you want to start suggesting where your research will fit in with those texts in your literature review. You might want to challenge a theory by critiquing it with another or explain how two theories can be combined to make a new outcome. Either way, you must make a clear link between their theories and your own interpretations – remember, this is not opinion based so don’t make a conclusion unless you can link it back to the facts!
Concluding your dissertation
Your conclusion will highlight the outcome of the research you’ve undertaken. You want to make this clear and concise without repeating information you’ve already mentioned in your main body paragraphs. A great way to avoid repetition is to highlight any overarching themes your conclusions have shown
When writing your conclusion it’s important to include the following elements:
- Summary – A summary of what you’ve found overall from your research and the conclusions you have come to as a result.
- Recommendations – Recommendations on what you think the next steps should be. Is there something you would change about this research to improve it or further develop it?
- Show your contribution – It’s important to show how you’ve contributed to the current knowledge on the topic and not just repeated what other researchers have found.
Hopefully, this helps you with your secondary data research for your dissertation! It’s definitely not as hard as it seems, the hardest part will be gathering all of the information in the first place. It may take a while but once you’ve found your flow – it’ll get easier, promise! You may also want to read about the advantages and disadvantages of secondary research .
You may also like
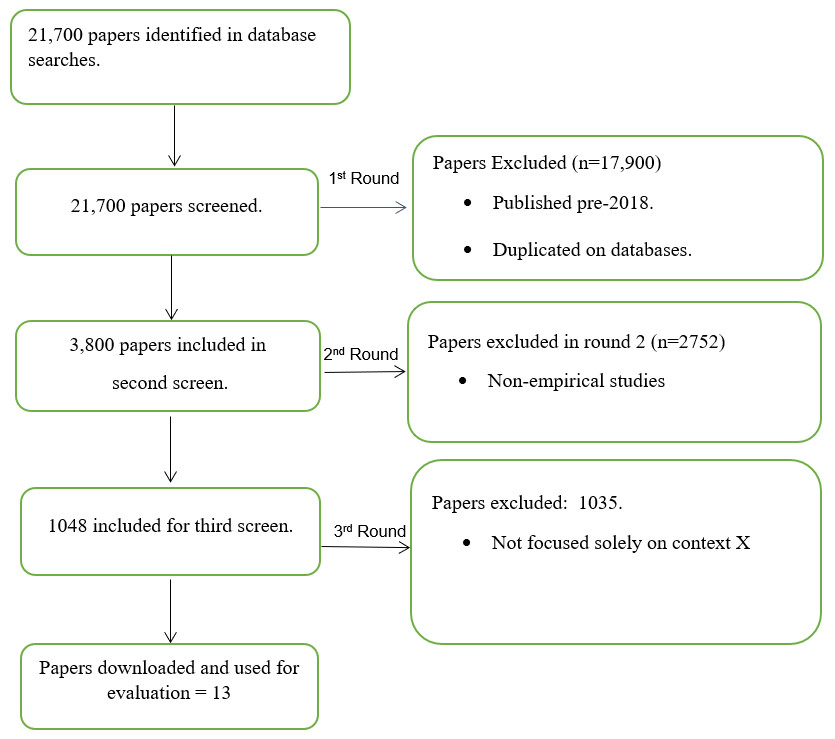
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Comput Struct Biotechnol J
Secondary structures in RNA synthesis, splicing and translation
Ilias georgakopoulos-soares.
a Department of Bioengineering and Therapeutic Sciences, University of California San Francisco, San Francisco, CA, USA
b Institute for Human Genetics, University of California San Francisco, San Francisco, CA, USA
Guillermo E. Parada
c Donnelly Centre for Cellular and Biomolecular Research, University of Toronto, Toronto, ON M5S 3E1, Canada
d Department of Molecular Genetics, University of Toronto, Toronto, ON M5A 1A8, Canada
Martin Hemberg
e Evergrande Center for Immunologic Diseases, Harvard Medical School and Brigham and Women’s Hospital, Boston, MA, USA
Graphical abstract

Even though the functional role of mRNA molecules is primarily decided by the nucleotide sequence, several properties are determined by secondary structure conformations. Examples of secondary structures include long range interactions, hairpins, R-loops and G-quadruplexes and they are formed through interactions of non-adjacent nucleotides. Here, we discuss advances in our understanding of how secondary structures can impact RNA synthesis, splicing, translation and mRNA half-life. During RNA synthesis, secondary structures determine RNA polymerase II (RNAPII) speed, thereby influencing splicing. Splicing is also determined by RNA binding proteins and their binding rates are modulated by secondary structures. For the initiation of translation, secondary structures can control the choice of translation start site. Here, we highlight the mechanisms by which secondary structures modulate these processes, discuss advances in technologies to detect and study them systematically, and consider the roles of RNA secondary structures in disease.
1. Introduction
mRNAs are essential molecules in the cell as they are key to extracting information stored in the DNA. Although the function of mRNA molecules is primarily determined by the nucleotide sequence, some properties are determined by secondary structures. Secondary structures are defined as distinct features, including hairpins, long range interactions, G-quadruplexes, R-loops and pseudoknots and they are formed as a consequence of the interactions of non-adjacent nucleotides. Their presence can impact various processes involving the mRNA, including synthesis, splicing and translation. Secondary structures are dynamic and can be modulated by multiple proteins, in particular RNA binding proteins (RBPs), and as they cannot be predicted solely from the primary sequence they are challenging to study. Nevertheless, several assays are available for both in vitro and in vivo profiling, and in this Review, we summarize these methods, provide an overview of some of the elucidated and putative functional roles of mRNA secondary structures, and finally we discuss their impact on disease. We discuss the consequences of secondary structure formation for splicing and translation, with particular focus in G-quadruplexes, hairpins and long range interactions. We also discuss the contribution of secondary structures in the regulation of mRNA splicing and in translation initiation and discuss the mechanisms involved.
2. RNA secondary structure formation
In RNA, intra and intermolecular long-range interactions, including hairpins, pseudoknots, and G-quadruplexes, are commonly observed. Hairpins are composed of a hybridized stem and a single stranded loop ( Fig. 1 a and b) and can contain mismatches and bulges. Pseudoknots contain nested stem-loop structures, with half of one stem intercalated between the two halves of another stem. G-quadruplex formation is driven by the inherent propensity of guanines to self-assemble, in the presence of monovalent cations, into planar structures known as G-quartets [1] . Each G-quartet is composed of four guanine nucleotides that interact with each other through Hoogsteen hydrogen-bonds. Consecutive runs of guanines (G-tracts) may lead to the formation of consecutive G-quartets that can stack with each other to form G-quadruplex structures ( Fig. 1 c). Biophysical properties such as the length of intervening loops between consecutive G-runs influence their formation dynamics. In addition, G-quadruplexes can be intramolecular or intermolecular. During transcription, dynamic hybrid structures between DNA and nascent RNA transcripts can be formed, such as R-loops ( Fig. 1 d) [2] . R-loops are three stranded hybrid structures in which an RNA molecule invades and hybridizes with one DNA strand, while displacing the other. The size of R-loops can range from <100 base pairs to >2000 base pairs [3] . Formation and stabilization of R-loops is particularly favorable when the non-template strand is G-rich, but it can also be promoted by DNA supercoiling, the presence of DNA nicks, and the formation of G-quartets [3] , [4] . The impact of R-loop formation, as well as the formation of DNA and RNA G-quadruplexes and other secondary structures, impacts transcript elongation rates and can have a kinetic repercussion on co-transcriptional events involved in RNA processing, such as alternative splicing [5] , [6] .

RNA and DNA-RNA hybrid secondary structures. A. Hairpin formation in which the stem hybridizes with hydrogen bonds while the loop remains single stranded. B. A long range interaction with an imperfect hairpin containing a bulge C. A G-quartet is formed by four guanines linked with Hoogsteen hydrogen bonds with each other (shown as squares in brown). Hoogsteen base pairing is a type of non-Watson–Crick base pairing. G-quadruplexes are formed by the stacking of multiple G-quartets. D. R-loops are three stranded DNA:RNA hybrid structures that can be formed co-transcriptionally at the template strand. The nascent RNA produced by the RNAPII ( shown in green ) hybridizes with the template strand to form an R-loop structure, while the non-template strand remains single-stranded. Phosphorylation events in the Carboxy-Terminal Domain (CTD) of RNA polymerase II are shown in yellow. In schematics A, B and D thicker lining of the mRNA indicates exonic regions whereas thinner lining indicates intronic regions. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
A number of methods that probe RNA structures have been developed. Methods such as selective 2′-hydroxyl acylation analyzed by primer extension (SHAPE)-seq [7] and parallel analysis of RNA structure (PARS) [8] were able to identify RNA structures in vitro, while more recent methods can deduce structures in vivo [9] , [10] . For instance, RNA in situ conformation sequencing (RIC-seq) [11] is a powerful new method that enables global detection of intra- and intermolecular RNA–RNA interactions, such as duplexes and long-range loop-loop interactions. Cross-linking immunoprecipitation high-throughput sequencing (CLIP-seq) enables the investigation of protein interactions with RNA molecules [12] from which many variant technologies have emerged. RNA G-quadruplexes can be characterized transcriptome-wide [13] , [14] using rG4-seq, which is a modified sequencing method that stalls at RNA G-quadruplexes, enabling identification of RNA G-quadruplexes in vitro , and RNA G-quadruplexes have also been visualized in cellulo using a specific antibody [15] . Moreover, researchers have developed small molecules, such as carboxy-pyridostatin, a cyanine dye called CyT and Thioflavin T [15] , [16] , [17] , [18] , [19] , that can shift the equilibrium between the folded and unfolded state of RNA G-quadruplexes and which display preference for RNA over DNA G-quadruplexes. Identification of R-loops has been enabled by usage of specific antibodies [20] , [21] , [22] , [23] and other nuclease-based methods [24] , [25] .
3. RNA polymerase speed and secondary structures
A variety of features are associated with RNAPII speed. For instance, the presence of introns and the length of the first intron are both positively correlated with RNAPII speed [26] , while nucleosome formation can reduce RNAPII speed [27] , [28] . Regions with high propensity of forming DNA, RNA, or hybrid secondary structures are also associated with RNAPII pausing or slower RNAPII speed ( Fig. 2 a and b) [29] , [30] , [31] . Another example of structure remodeling due to slower RNAPII speed is inhibition of hairpin formation due to competition with other alternative structures resulting in reduced binding by stem–loop-binding proteins [30] . In S. cerevisiae and S. pombe , folding energy and GC content in the transcription bubble have been correlated with RNA polymerase distribution, and RNA structures within nascent transcripts promote forward translocation of the polymerase and limit back-tracking [32] . This indicates how nascent RNA structures can promote the forward movement of an RNA polymerase molecule. Analyses of nascent RNAs have provided evidence that the formation of secondary structures within introns is associated with more efficient co-transcriptional splicing, which is favored under slower transcriptional rates [32] , [33] . Taken together, secondary structures will impact several processes, including promoter-proximal pausing, exon recognition, splicing and transcription termination, as they are all influenced by RNAPII speed.

Mechanisms by which structure formation influences splicing. A. In the absence of secondary structures, RNAPII elongation rate is higher, which disfavors the recruitment of splicing factors that promote assembly of the spliceosome and exon definition. In this situation exons flanked by weak splice sites may not be recognised, and they are consequently skipped. Exons flanked by strong splice sites can be efficiently recognized by small ribonucleoproteins (snRNPs) U1 and U2, leading to the formation of the pre-spliceosome (complex A) and promoting exon definition and inclusion in the mature mRNA transcripts. B. Formation of secondary structures at DNA and RNA can decrease RNAPII elongation speed. For example, during transcription R-loops formed at the 3′ of genes can be stabilized by non-template DNA G-quadruplex formation. Low transcription rates promote exon inclusion by allowing the formation of secondary structures and binding of proteins that can favor the recognition of weak splice sites that would not be recognized otherwise. An RBP that recognizes and binds to the secondary structure is shown in green whereas an RBP whose binding is inhibited by secondary structure formation is shown in red. C. RNA secondary structures can modulate mRNA interactions with RBPs either promoting or inhibiting their binding at the mRNA molecule. For example, G-quadruplexes formed at the DNA or RNA level can selectively recruit RBPs to influence splicing outcome. In schematics A, B and C, thicker lining of the mRNA indicates exonic regions whereas thinner lining is indicating intronic regions. The dashed line of mRNA molecules indicates that the length of the transcript can be longer than displayed. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. RNA splicing and secondary structures
Pre-mRNA splicing is a key biological process that enables the removal of introns and the joining of intervening exons, eventually resulting in a mature mRNA molecule. Alternative splicing affects approximately 90–95% of mRNA transcripts in humans [34] , [35] and most often occurs co-transcriptionally [33] , while for a minority of transcripts it occurs post-transcriptionally [36] . Splicing is a highly conserved mechanism [37] that is pivotal for a number of biological processes such as cell growth, differentiation, immune response, neuronal development [38] , [39] , [40] , while aberrant splicing is implicated in multiple diseases [41] including neurological disorders [42] and cancer [43] .
Splicing is mediated through the spliceosome complex which recognizes splice signals, the key members being the 5′ splice site (5′ss), the 3′ splice site (3′ss), and the branch point. The recognition of these consensus sequences is commanded by U1 and U2 small nuclear ribonucleoproteins (snRNPs) and other auxiliary protein factors that are involved in early spliceosomal assembly. Since higher-eukaryotic genes are often interrupted by long introns, early spliceosomal complex assembly over exons recognizes both splice sites during a process commonly known as exon definition [37] . Nevertheless, computational analyses of vertebrate splice sites have shown that the consensus splicing signals only account for approximately half of the information required to accurately define exon/intron boundaries [34] , suggesting that other regulatory elements such as RBP sites and secondary structures are crucial for splice site definition. Splice sites with sequences that are substantially different from the consensus signals lead to suboptimal recognition of splice sites (weak splice sites), and are often associated with alternative splicing events. Recent models using deep learning can predict to a large extent splicing events using the primary DNA sequence and can integrate the effects of mutations [44] , [45] .
Even though the RNA structural code has been less explored [46] , it is known that the effects of cis -regulatory elements can be modulated by the presence of RNA structures in nascent transcripts and in mature mRNAs [47] . Co-transcriptional transient RNA structure formation can impact splicing through RNAPII pausing and backtracking, which can have a direct kinetic effect over co-transcriptional splicing events [48] . One such example is the human ATE1 gene, where splicing of two mutually exclusive exons is regulated by competing long-range hairpin structures that span up to 30 kB [49] . Mutations that disrupted each of the secondary structures shift the equilibrium between the two exons indicating direct control of splicing outcome. Reduction of transcription rates can favor further formation of RNA secondary structures [30] and binding of splicing regulatory factors that can increase splicing efficiency therefore allowing the recognition of exons that are flanked by weak splice sites, which would otherwise be skipped [5] , [50] ( Fig. 2 a and b).
5. The interplay between RBPs and secondary structures
During the mRNA lifecycle, RBPs regulate to a significant extent diverse transcriptional and post-transcriptional stages including splicing, transportation, translation, stability and degradation. They bind to pre-mRNA molecules in the nucleus and regulate its maturation and transportation to the cytoplasm where they regulate translation and degradation. The number of proteins that can bind to RNA in humans is estimated to be more than 1,500, adding complexity to all the aforementioned programs [51] .
RBPs can facilitate or inhibit the recognition of splice sites thereby acting as splicing enhancers or splicing silencers [46] , [52] , [53] . The majority of RBP motifs are not bound in vivo as demonstrated by high-throughput experiments that identify the sites where RBPs bind to endogenous RNAs such as cross-linking immunoprecipitation followed by high-throughput sequencing (CLIP-seq). One possible explanation is that RNA structures provide additional contextual features beyond the primary motif sequences ( Fig. 2 b and c), and it has also been shown that RNA secondary structure is predictive of binding [54] , [55] . Several studies have shown that during pre-mRNA synthesis the formation of RNA structures influences alternative splicing by diverse mechanisms [56] , [57] , and that local RNA structure formation can impact splicing by modulating the accessibility of core splicing signals [58] , [59] , [60] as well as RBP binding sites [58] , [61] , [62] .
An example of how RNA secondary structures can dictate the binding of specific RBPs, is provided by MBNL1 and U2AF65 binding to influence exon inclusion in the fifth exon of TNNT2 [63] , [64] . MBNL1 favors hairpins and when bound inhibits U2AF65, which favors a linear structure, from binding the polypyrimidine tract resulting in exon skipping. Additional evidence from mice shows that MBNL1 also binds the hairpin structure of exon F in TNNT3. Another example is elF3, which recognizes and binds to hairpin structures at 5′UTR to exert translational activation or repression [65] . Other studies have shown preferential binding of RBPs at RNA G-quadruplex sites, e.g. CNBP, which prevents RNA G-quadruplex structure formation and promotes translation [66] and FMRP, which preferentially binds RNA G-quadruplex structures [66] , [67] . Secondary structures and RNA binding proteins have been systematically investigated, enabling the identification of preferences of structured RNA for particular proteins [68] , [69] . Interestingly, a recent genetic study showed that G-quadruplex sequences at 5′UTRs are selectively constrained and are enriched for eQTLs, loci containing genetic variants that result in changes of the expression level of a gene, and RBP sites [70] .
6. Helicases as key regulators of secondary structures
Structure formation is to a large extent modulated by enzymes such as eIF4A and DHX29, that can unwind them, and their importance is demonstrated by their pivotal role in translation initiation [71] , [72] . Similarly, the continuous activity of DNA/RNA helicases and ribonucleases H (RNAse H1 and H2) release R-loop structures [3] . Interestingly, R-loops and G-quadruplexes were both found to be unwound by the helicase DHX9 in humans [73] . DHX9 activity protects single-stranded DNA against damage and preserves genomic stability [74] . RNA G-quadruplexes are known to interact with several proteins [70] , [75] , [76] . For example, the RNA helicase RHAU (also known as DHX36) resolves mRNA G-quadruplexes [77] , [78] . One of its targets is a G-quadruplex at the 5′UTR of Nkx2-5 mRNA, and it has been shown that DHX36-mediated G-quadruplex structure unfolding is required for the gene to be expressed [79] . Another DHX36 target is Gnai2 mRNA, a key regulator of stem cell function and muscle regeneration [78] . DHX36 and DHX9 were also found to modulate translational efficiency by resolving 5′UTR RNA G-quadruplexes [80] , while several RBPs such as hnRNP H/F and helicases such as DDX21, DDX17 DDX3X, DDX5 and DDX1 have been found to unwind RNA G-quadruplexes and are also involved in transcription, splicing and translation regulation [81] , [82] , [83] , [84] . Similarly, multiple helicases have been shown to resolve hairpin structures. For instance, UPF1 can resolve RNA hairpins [85] , while DDX5 can resolve DNA and RNA G-quadruplexes as well as hairpin structures [86] , [87] ( Table 1 ).
Important helicases that play a role unwinding RNA and DNA secondary structures. G4s in the table refer to G-quadruplexes. This a non-exhaustive list of relevant DNA/RNA helicases. Additional examples are reviewed by [92] , [93] , [94] . Alternative gene names are listed between parenthesis and gene paralogs with homologous functions are separated by “/”.
The cellular mechanisms mediating the stabilization and resolution of RNA secondary structures remain incompletely understood, as are the interactions between secondary structures and protein complexes. In addition, the effect of perturbing these mechanisms and their relevance to disease progression is unclear. High throughput screens coupled with short hairpin RNAs (shRNAs) or CRISPR-based technologies have enabled systematic interrogation of the roles of diverse proteins, such as RBPs, helicases, and topoisomerases [88] , [89] , [90] , [91] . Furthermore, mutational analysis with CRISPR-Cas9 could be used to study the effects of secondary structure disruption in vivo or in cellulo . CRISPR-induced mutations that destroy the secondary structure motifs, for example the G-runs of G-quadruplexes or the stem sequence of hairpins, but leave other regulatory sequences such as RBP motifs unchanged, could advance the understanding of how secondary structures determine gene expression.
7. G-quadruplexes as regulators of alternative splicing
G-quadruplex sequences are enriched at promoters and they have been extensively studied in this context [131] . Additionally, G-quadruplexes have been related to splicing, 3′ processing, transcription termination, RNA localization and translation regulation [76] . Interestingly, it has been shown that G-quadruplex sequences have a high enrichment in the proximity of both 3′ and 5′ splice sites across a wide range of species. The effect is more pronounced at the non-template strand, suggesting that the G-quadruplexes are formed primarily by the RNA and that they may favor or block the binding of RBPs [132] .
One of the first exemplary cases of RNA G-quadruplex mediated regulation of alternative splicing was found in the hTERT gene, which encodes for the catalytic subunit of the telomerase enzyme, and one of its exon skipping events is promoted by the stabilization of intronic G-quadruplexes [133] . Gomez and colleagues hypothesized that RNA G-quadruplex formation can prevent RBP binding to intronic enhancers, leading to exon skipping. However, based on different functional assays, RNA G-quadruplex formation has also been proposed to promote RBP binding to splicing regulatory elements [134] , [135] , [136] . Since G-quadruplex-dependent splicing events were often demonstrated by introducing mutations at G-quadruplex motifs, it was unclear from these results whether the G-quadruplex structure or the linear form of these G-rich sequences act as a splicing enhancer. To disentangle these effects, Huang and colleagues showed that mutations that prevent intronic G-quadruplex formation but keep G tracts intact, led to exon exclusion of an alternative exon in the CD44 gene [137] . Since the CD44 intronic G-quadruplex motif sequence can be bound by two RBPs that have the opposite effect on exon exclusion, RNA G-quadruplex formation may function as a switch to promote the binding of one RBP over the other [138] . In another recent study where the role of wild-type and mutated G-quadruplex sequences in alternative splicing was tested using a minigene, it was also shown that the presence of an RNA G-quadruplex favors exon inclusion [132] , consistent with the aforementioned findings. There is also evidence of an interplay between RNA G-quadruplex stabilization and specific binding proteins such as HNRNP H/F [116] , [137] and HNRPU [139] and recent studies suggest that RNA G-quadruplex formation can modulate in vitro RBP binding to mRNA molecules [66] .
The genome-wide effect of RNA G-quadruplex formation over splicing factor binding remains unclear. High-throughput screening of chemical compounds via dual-color splicing reporters has identified two small molecules, emetine and cephaeline, that disrupt RNA G-quadruplex formation [140] . Genome-wide evaluation of emetine effects on alternative splicing showed substantial alternative splicing changes after treatment, with nearly 60% being exon skipping events. It was also shown that multiple RBPs colocalize with G-quadruplex motifs flanking splice junctions, suggesting an interplay between RBP binding and RNA G-quadruplex structure formation, which was further corroborated by loss of function experiments followed by RNA-seq, identifying consistent associations for 36 RBPs [132] , [137] .
8. Hairpins enable long range RNA interactions during splicing
Long range interactions are important for splicing modulation [141] , and they are more enriched at weak alternative acceptor splice sites [142] . Some long range interactions can span several kilobases and can bring in proximity otherwise distant splice sites. One of the best-characterized examples of regulation of splicing through RNA structures can be found in D. melanogaster for the DSCAM gene, where RNA-RNA interactions, mediated through multiple structures, regulate the selection of exons within arrays of mutually exclusive exons [143] , [144] . In this case, RNA looping can bring splicing elements situated thousands of bases away from each other into close proximity.
Hairpins may also directly affect exon skipping events by a mechanism known as “looping-out”, whereby inter-intronic base-pairing RNA interactions can loop out exons to promote their skipping [56] . This mechanism is supported by the enrichment of conserved complementary sequences present in intronic regions flanking exon skipping events [145] . Moreover, the artificial introduction of self-complementary regions across exons suppresses exon inclusion in yeast, suggesting a causal relationship between hairpins and exon skipping [146] . Interestingly, the expansion of self-complementary regions is related to the primate-specific Alu retrotransposon, which is enriched in regions flanking alternative exons, suggesting a role in splicing regulation [147] . During back-splicing, an unconventional splicing mechanism, the second nucleophilic attack is performed over an upstream 3′ splice leading to circular RNA (circRNAs) products. circRNAs are particularly abundant in the brain and RNA structures that favor back-splicing are often derived from complementary intronic sequences associated with Alu elements [148] . In zebrafish, hairpin formation between dinucleotide repeats that co-occur at opposite boundaries of an intron, mediate splicing without U2AF2, which is a major component of the spliceosome [149] .
The formation of RNA structures can also enhance RBP regulatory range by bringing distal regulatory elements in close proximity with their exon targets [150] . This can be particularly important for RBFOX2 regulated exons since more than half of RBFOX2-binding sites are found over 500 bp away from any annotated exons, and it has been shown that long-range RNA hairpin formation is necessary for the regulatory effect of distal binding sites [151] . It has also been shown that hairpin formation can influence splicing regulatory protein binding, with enhancers and silencers having a stronger effect when present in the loop relative to the stem [52] , [54] , suggesting that RBP binding is inhibited at the stem [58] , [61] . In an elegant set of experiments, it was shown that in the case of FGFR2 , the formation of a hairpin structure is required for efficient splicing from two mutually exclusive exons and its splicing effect is not dependent on its primary nucleotide composition as shown using minigene assays [152] .
The fibronectin EDA exon is controlled by seven hairpins and a key exonic splicing enhancer is found in the loop of one of the hairpins, which is in turn bound by splicing regulatory proteins such as SRSF1 [153] , [154] . Other examples include a hairpin which modulates the inclusion of the alternative exon 6B of the β-tropomyosin transcript in chicken [155] . It was also shown that a mutation in PS2 that deletes or destabilizes a hairpin in exon 5, results in higher levels of exon inclusion [156] . Importantly, the formation of hairpin structures could be dynamic and due to environmental changes, an example being temperature-dependent formation of a hairpin that controls splicing of APE2 gene in yeast [157] . In addition, alternatively spliced exons display an enrichment for secondary structures and evolutionary conservation of many of these structures indicates their important regulatory functions [57] . This is exemplified by conservation of secondary structures over the primary nucleotide sequence such as a conserved hairpin structure in RB1CC1 [57] . Advances in long-read RNA sequencing technologies will enable improved detection of long-range interactions and their impact in the regulation of alternative splicing events.
9. The role of RNA structures on RNA stability and decay
The half-life and decay rates of mRNA transcripts in human cells influence protein expression levels. A number of features determine transcript stability including GC content, transcript length, polyA tail length, RBP sites, microRNA binding sites, and mRNA secondary structures [158] , [159] , [160] , [161] , [162] , [163] . Structural features of mRNAs dictate to a large extent mRNA half-life with transcripts that have a structured coding sequence showing higher expression levels [159] . Hairpins in mRNA transcripts can result in increased stability [163] , [164] , [165] , such as when found at the 3′UTR near mRNA cleavage sites. The accessibility of microRNA sites influences mRNA half-life and secondary structure formation can change the microRNA binding efficiency [166] . For example, the introduction of a hairpin in the 5′UTR of a transcript, results in substantial increases in gene expression [167] , [168] . Constitutive decay elements are RNA motifs that mediate the destabilization and degradation of mRNA molecules, and contain a hairpin sequence [169] at which Roquin proteins bind to induce the decay of the transcript [170] .
Massively parallel reporter assays are high-throughput technologies that enable rapid measurements of thousands of sequences for their regulatory activity and have received widespread adoption in recent years [171] , [172] , [173] , [174] . Multiple variants of this technology have been implemented to study a plethora of gene regulatory elements, including promoters, enhancers, 5′ UTRs, and 3′ UTRs, by placing synthetic sequences in the appropriate location relative to a reporter gene. In this case massively parallel reporter assay experiments have shown that its destabilizing effects increase as a function of the hairpin length [165] .
10. Secondary structures in translation
Translation can be divided into four phases, initiation, elongation, termination and ribosome recycling [175] , [176] . Initiation is the rate limiting and most regulated step, consisting of several complex programs. The regulation of translation directly impacts protein levels with most regulatory mechanisms affecting the rate-limiting initiation step [177] , [178] , [179] . The multifarious effects of translational control can be observed across biological processes including development, differentiation, functions of the nervous system and disease [177] , [180] . Initiation can be either cap-dependent or cap-independent [181] , [182] . Cap-dependent translation is the most frequently used in eukaryotes and starts with the binding of eIF4E to the mRNA cap. The most common cap-independent initiation mechanism, often utilized by viral RNAs, involves an internal ribosome entry site (IRES) of structured mRNA. IRES structures can recruit ribosomal subunits and eukaryotic initiation factors [183] . RNA molecules fold in complex configurations with the presence of RNA secondary structures in the 5′UTR being a major determinant of the rate of translation ( Fig. 3 a and b) [184] , [185] , [186] . Moreover, the ribosome itself is a major remodeler of RNA structure [187] . Lower translation rates can not only limit protein abundance, but can also enable correct co-translational protein folding [188] , [189] . In addition, secondary structures can influence the recognition of the IRESs ( Fig. 3 c).

Mechanisms by which RNA structure formation influences translation. A. During cap-dependent translation, translation initiation factors (blue proteins) recognize the mRNA 5′ cap structure (purple circle) and bridge its interaction with the 3′ polyA tail, through polyA binding proteins (PABPs). During translation several helicases actively unwind the mRNA, which could remove secondary structures. This could lead to faster ribosome speeds, which may result in protein misfolding. B. Cap-dependent translation can be regulated by the dynamic formation of secondary structures in the 5′ UTR. Hairpin formation can limit the binding of the ribosome and translation initiation factors, thereby repressing protein translation. The presence of G-quadruplexes in the 5′ UTR may inhibit translation directly, activate upstream ORFs, or promote translation. C. Cap-independent translation can take place in the presence of IRESs, which require highly structured 5′UTR domains that indirectly interact with PBAPs to promote mRNA circularisation. Some IRES structures can be activated by RNA G-quadruplex formation. Further formation of RNA secondary structures across the ORF can limit the translation speed and favor a step-by-step modular folding. Additional details on Cap-dependent and Cap-independent mechanisms are comprehensively reviewed at [234] . (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Although the vast majority of eukaryotic translation start sites have an AUG codon, often the first AUG codon is bypassed, resulting in usage of more distal AUG codons and alternative protein isoforms. This process is referred to as leaky scanning, with a proportion of ribosomes initiating translation from downstream start codons. Leaky scanning and translational efficiency are influenced by the presence of secondary structures [8] , [190] , [191] , [192] . Moreover, there is a large proportion of suboptimal start sites that do not contain the canonical start codon. Microsatellite expansions can cause non-AUG initiation [193] . These non-AUG start sites are often associated with alternative translation start [194] , [195] . Ribosome profiling is one of the primary methods of identifying the occupancy of elongating ribosomes on mRNAs, therefore providing a direct readout of ribosome decoding rates [176] .
Secondary structures can conceal or expose binding sites for translation regulators, and it has been shown that certain RBPs bind preferentially at structured RNA while others have a preference for linear forms [196] . Moreover, formation of secondary structures can change the distance between translation-associated motifs, an example being the distance between the stem-loop and the cap [197] . Secondary structure formation can also promote cap-independent translation, and the disruption of an IRES hairpin can in turn reduce translation efficiency in viral [198] , [199] and eukaryotic [200] mRNAs.
Riboswitches are components of mRNA molecules that can bind a small molecule and directly control gene expression through RNA conformational changes, without proteins being involved. They are found in both prokaryotes and eukaryotes, with most discovered riboswitches being present in bacteria and archaea [201] . The aptamer is a receptor for a small molecule, and it is usually located in the 5′UTR of a mRNA where it forms a secondary structure that binds to the small molecule. The expression platform is the regulatory domain of the riboswitch and it modulates gene expression upon binding of the small molecule. Riboswitches have been found to regulate a number of processes including initiation of translation [202] , mRNA decay [203] , transcription termination [204] and splicing [205] , [206] . For instance, in E. coli the lysine riboswitch when lysine is present it restricts translation initiation and also exposes RNase E cleavage sites [203] .
RNA structures can directly interact with the translational machinery and influence the recognition of the translation start [207] . Note that the interaction is complicated by the fact that the translational machinery can unwind and remodel RNA structures [187] . There is also decreased translational efficiency at highly structured 5′UTRs [80] , [208] . For example, in the case of BRCA1 , a tumor suppressor gene, a longer 5′UTR isoform is expressed only in breast cancer cells, resulting in a 10-fold decrease of translational efficiency due to the formation of a stable complex secondary structure [208] . Finally, the interplay between RNA structure formation and unwinding influences ribosome initiation, scanning and elongation. Therefore, secondary structures can account for differences between mRNA and protein levels [209] .
11. Hairpins enable long range RNA interactions in translation initiation
Early studies indicated that hairpin formation can influence translation efficiency [210] . Hairpins with high thermal stability upstream of the translation start site resulted in reduced translation by up to 85–95%, whereas hairpin formation downstream of an AUG at specific positions resulted in an increase in translation rate by facilitating recognition of initiator codons by ribosomes [211] , [212] . Stem length and GC content, both of which increase thermal stability, inhibit translation, while more distant hairpins have a smaller inhibitory effect [213] . Other studies have also indicated that both the GC content of the stem and the positioning of the hairpin relative to the translation start site dramatically influence the translation efficiency [207] .
Hairpins at the 5′UTR of ferritin-H and ferritin-L mRNAs act as an iron-responsive element controlling iron levels and are highly dynamic response elements to environmental changes [214] . Another example is a hairpin structure in the c-JUN 5′ UTR which is recognized by eIF3 and is required for initiation of translation [215] . Another study generated a library of half a million 50 bp long 5′UTRs and identified hairpin structures to negatively impact protein levels, especially those with longer stems and shorter loops [216] .
12. G-quadruplexes in translation initiation
RNA G-quadruplexes are enriched at 5′UTRs (Huppert et al. 2005) where they show a higher frequency at the template strand, suggesting a relative depletion of G-quadruplexes at the RNA level [217] . There is also a difference in the density of G-quadruplexes, with the highest density being found within 50 bp of the start of the 5′UTR and a declining frequency moving away from it [217] . It has been shown that G-quadruplexes in the 5′UTR of mRNAs are inhibitory elements [218] , and several studies have since shown that G-quadruplexes at the 5′UTR interfere with the recognition by ribosomes [17] , [219] , [220] , [221] , [222] , [223] . Specifically, experiments involving luciferase plasmid vectors indicate that G-quadruplexes inhibit expression across 5′UTR regions, perhaps by interfering with ribosome scanning. However, in many of these experiments the researchers used controls where guanines had been substituted for uracils, potentially also interfering with RBP binding sites and the GC content [218] , [219] .
It has also been shown that G-quadruplexes at 5′UTRs of eukaryotic genes can promote translation by favoring recognition of the IRES [224] , [225] , [226] , [227] . In FGF-2, a gene that is associated with tissue development and repair, a G-quadruplex motif together with two hairpin sequences are found within the IRES, and they promote translation in a cap-independent translational program [225] . A G-quadruplex site in the RBP FMRP is a binding site for the protein itself, and it has been suggested that it could in this way control both its own expression levels [228] and its mRNA splicing [134] . In VEGF, an RNA G-quadruplex was shown to be essential for IRES-mediated translation initiation [227] , [229] , [230] ; however other studies have contended its role and provided evidence for inhibitory functions [231] , [232] .
A study that used massively parallel reporter assays to investigate mRNA translation found that G-quadruplexes in the 5′UTR act as translational inhibitors, and that knockdown of G-quadruplex resolving helicases aggravated these phenotypes [233] . It was also found that RNA G-quadruplex formation could promote the usage of an upstream translation start site by slowing down the pre-initiation complex scanning [80] . The role of secondary structures was systematically explored in a high-throughput experiment where half a million 50 bp randomly generated 5′UTRs were synthesized and tested in yeast. The results showed that several secondary structures, including RNA G-quadruplexes and hairpins, are important contributors to expression levels [216] . RNA G-quadruplexes can either restrict or promote the recognition by ribosomes and even though there are more studies indicating inhibitory functions, it is not clear which effect is more widespread and what features determine if the G-quadruplex will restrict or promote ribosomal recognition.
13. Splicing and translation associated secondary structures in disease
Regions that are predisposed to secondary structure formation, such as G-quadruplexes have an excess of germline and somatic mutations [235] , [236] . The functional role of these structures is supported by the observation that eQTLs are enriched at G-quadruplexes within 5′UTRs and splicing quantitative trait loci (sQTLs) are enriched at G-quadruplex motifs flanking splice sites [70] , [132] . The accumulation of R-loops is also associated with genomic instability [237] , [238] , [239] , [240] As secondary structure formation modulates diverse processes including splicing and translation initiation, changes in the mRNA structure have been associated with and can result in human disease.
Mutations of alternative splicing factors can lead to R-loop accumulation, which may compromise genomic stability and be relevant in the context of cancer pathogenesis [241] , [242] . RNA splicing perturbation by expression of U2AF1 or SRSF2 mutants, mutations that are commonly observed in myelodysplastic syndrome, results in the accumulation of R-loops [243] . In the MAPT gene, also known as tau, in the interface between exon 10 and intron 10, there is a hairpin structure which can mask the splice site [244] , [245] and DDX5 was found to be involved in the resolution of this hairpin structure controlling splicing of MAPT (tau) exon 10 [86] . Mutations at the hairpin result in its destabilization, causing inclusion of exon 10 due to increased association with U1 snRNP [244] and results in higher prevalence of neurodegeneration. Hairpin sequences were also identified in the 5′UTR of other transcripts including the amyloid precursor protein [246] and α-synuclein [247] , indicating the importance of structure-mediated control of expression levels. In spinal muscular atrophy, a stem-loop RNA structure overlaps with the 5′ splicing site of exon 7 of SMN2 and interference with the structure formation is a therapeutic target against the spinal muscular atrophy molecular phenotype [248] . Sulovari et al. showed that variable number tandem repeats were particularly enriched at Alu elements and found an association between genes differentially spliced or expressed between human and chimpanzee brains [249] .
RNA G-quadruplex structures have been identified in several cancer genes, including TP53 and TERT , where they can modulate splicing and protein isoforms [133] , [135] . In CD44 an RNA G-quadruplex in intron 8 functions as a splicing enhancer with roles in the control of the epithelial–mesenchymal transition [137] , a process that is important for cancer metastasis [250] . One of the canonical translation initiation factors, elF4A, is a DEAD-box RNA helicase that can unwind secondary structures, including RNA G-quadruplexes, and its activity is correlated with the number of secondary structures in the 5′UTR [251] . Perturbation of elF4A can contribute to oncogenesis as it results in formation of RNA G-quadruplexes in the 5′UTRs of mRNAs targeted by elF4A, including many oncogenes, transcription factors, and epigenetic regulators [252] .
The expansion of microsatellite repeats at 5′UTRs has been associated with aberrant translation and has been implicated in multiple disorders [193] , [253] . The mechanisms involve the formation of secondary structures that interfere with translation and repeat-associated non-AUG translation. One of the most well-studied examples is the expansion of the hexanucleotide GGGGGC in the first intron of the C9orf72 gene which results in frontotemporal dementia (FTD) and amyotrophic lateral sclerosis (ALS). These repeats form different secondary structures including G-quadruplexes, R-loops and hairpins [254] , [255] , [256] which leads to aborted transcription at the repeat site [254] . Expansion of these repeats results in repeat-associated non-AUG translation and the generation of toxic dipeptide proteins [257] , while reducing DHX36 levels in cells derived from C9orf72-linked ALS patients results in reduced dipeptide protein burden due to the formation of RNA G-quadruplexes [258] . In ALS and FTD, Nucleolin binds to the G-quadruplex forming hexanucleotide repeat, resulting in its mislocalization in the cell [254] . In addition, a number of other proteins associated with the ALS pathology such as TDP-43, FUS/TLS, hnRNPA1, hnRNPA2B1, hnRNPA3 and EWSR1 interact with the RNA G-quadruplex [259] , [260] , [261] , [262] , [263] , [264] . Encouragingly, G-quadruplex binding small molecules ameliorate the pathologies associated with ALS and FTD in model systems, indicating that RNA G-quadruplexes can pose as a therapeutic target [265] . Beta-amyloid precursor protein cleaving enzyme 1 (BACE1) encodes a protein that cleaves amyloid precursor protein resulting in the generation of amyloid-beta peptide, the accumulation of which is a hallmark of Alzheimer’s disease [266] . An RNA G-quadruplex in exon 3 of BACE1 modulates splicing by inhibiting the binding of hnRNP H, thereby promoting a shorter isoform without the proteolytic activity that creates the neurotoxic peptide [267] . ADAM-10 is also associated with Alzheimer’s disease due to its anti-amyloidogenic activity and a RNA G-quadruplex in its 5′UTR represses its expression [268] .
14. Concluding remarks
RNA secondary structures are pervasive, interact with RNA binding proteins and are linked to a large number of important functions, including transcription, splicing and translation. Even though the functional importance of secondary structures has been repeatedly demonstrated, the contribution of RNA structures in these processes remains incompletely understood due to the difficulties in identifying dynamic RNA structures and their mechanisms of action. High-throughput technologies enable the systematic investigation of RNA secondary structures and the design of experiments to quantify their contribution in transcription, splicing and translation enables directly testing their mechanisms of action. New methods to dynamically identify RNA secondary structures are gradually revealing their widespread and diverse contributions in gene regulation. However, it remains difficult to capture their dynamic changes across cellular conditions and their interplay with proteins. The degree to which RNA secondary structure formation is influenced by the tissue and cell type remains largely unstudied. The availability of large scale single cell assays will enable the investigation of associations between secondary structures, the presence of various sequence motifs, and expression levels of RBPs across different cell types. Even more interesting could be the combination of single cell technologies with different small molecules that stabilize specific structures.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank Dr Felipe Carvajal for discussions about regulation of protein translation mechanisms. IGS is supported by the National Human Genome Research Institute (1UM1HG009408, R01HG010333, 1R21HG010065, UM1HG011966, and 1R21HG010683) GEP was supported by Charles H. Best Postdoctoral Fellowship. MH was supported by start-up funding from the Evergrande Center.

IMAGES
VIDEO
COMMENTS
identify the impact of your research results (think "big picture") Methods: the "HOW" section (min: 1 page) Before you outline this section, ORGANIZE your 5-7 (or more) sources in some logical way (by risk factor studied, method, etc.) Review the research questions and types of study designs (survey, interview, focus groups, etc.)
Secondary research is a research method that uses data that was collected by someone else. In other words, whenever you conduct research using data that already exists, you are conducting secondary research. On the other hand, any type of research that you undertake yourself is called primary research. Example: Secondary research.
Secondary research is also used to justify the need for primary research as well as to justify and support other activities. For example, secondary research may be used to support a proposal to modernize a manufacturing plant, to justify the use of newly a developed treatment for cancer, to strengthen a business proposal, or to validate points ...
Therefore, the majority of sources in a literature review are secondary sources that present research findings, analysis, and the evaluation of other researcher's works. Reviewing secondary source material can be of valu e in improving your overall research paper because secondary sources facilitate the communication of what is known about a topic.
The structure of the paper is of the following: The paper first discusses the ethical and legal considerations. ... Unlike primary research, secondary research needs to minimize interpretations subjective to interview setting as they would be based on perceptions and assumptions of the secondary researcher introducing bias. Step 4. Data Filtering
Abstract. This article aims to provide an overview of the structure, form and content of systematic reviews. It focuses in particular on the literature searching component, and covers systematic database searching techniques, searching for grey literature and the importance of librarian involvement in the search.
Secondary research. Secondary research uses research and data that has already been carried out. It is sometimes referred to as desk research. It is a good starting point for any type of research as it enables you to analyse what research has already been undertaken and identify any gaps. You may only need to carry out secondary research for ...
Step 1: Identify the paragraph's purpose. First, you need to know the central idea that will organize this paragraph. If you have already made a plan or outline of your paper's overall structure, you should already have a good idea of what each paragraph will aim to do.. You can start by drafting a sentence that sums up your main point and introduces the paragraph's focus.
Research that falls into this category is somewhat less common than primary research publications and is called secondary research. In secondary research publications, field observations that have been published by other authors are re-interpreted and re-written as a secondary research article. Let us now look at secondary publications in more ...
A complete research paper in APA style that is reporting on experimental research will typically contain a Title page, Abstract, Introduction, Methods, Results, Discussion, and References sections. 1 Many will also contain Figures and Tables and some will have an Appendix or Appendices. These sections are detailed as follows (for a more in ...
A decimal outline is similar in format to the alphanumeric outline, but with a different numbering system: 1, 1.1, 1.2, etc. Text is written as short notes rather than full sentences. Example: 1 Body paragraph one. 1.1 First point. 1.1.1 Sub-point of first point. 1.1.2 Sub-point of first point.
So, rightly secondary research is also termed " desk research ", as data can be retrieved from sitting behind a desk. The following are popularly used secondary research methods and examples: 1. Data Available on The Internet. One of the most popular ways to collect secondary data is the internet.
The Structure of an Academic Paper www.communicate.gse.harvard.edu Academic papers are like hourglasses. The paper opens at its widest point; the introduction makes broad connections to the reader's interests, hoping they will be persuaded to follow along, then gradually narrows to a tight, focused, thesis statement.
This includes internal sources (e.g.in-house research) or, more commonly, external sources (such as government statistics, organizational bodies, and the internet). Secondary research comes in several formats, such as published datasets, reports, and survey responses, and can also be sourced from websites, libraries, and museums.
Definition: Research Paper is a written document that presents the author's original research, analysis, and interpretation of a specific topic or issue. It is typically based on Empirical Evidence, and may involve qualitative or quantitative research methods, or a combination of both. The purpose of a research paper is to contribute new ...
Time to recap…. And there you have it - the traditional dissertation structure and layout, from A-Z. To recap, the core structure for a dissertation or thesis is (typically) as follows: Title page. Acknowledgments page. Abstract (or executive summary) Table of contents, list of figures and tables.
II. Abstract: "Structured abstract" has become the standard for research papers (introduction, objective, methods, results and conclusions), while reviews, case reports and other articles have non-structured abstracts. The abstract should be a summary/synopsis of the paper. III. Introduction: The "why did you do the study"; setting the ...
3. Structure of a Scientific Research Paper. A primary way that scientists communicate with one another is through scientific papers. We will model our Biocore lab reports on the format most commonly used by scientific journals. Your lab reports should follow the guidelines described below unless the lab manual or your TA specifically tells you ...
assigned readings from the course syllabus) and research papers (typically requiring additional research in a library or archive on a topic of your own choosing). Different types of history papers naturally require different amounts of research, analysis, and interpretation. Despite this variety, historical arguments often assume a common form.
Just make sure you prioritise the research that backs up your overall point so each section has clarity. Then it's time to write your introduction. In your intro, you will want to emphasise what your dissertation aims to cover within your writing and outline your research objectives. You can then follow up with the context around this ...
General Steps of Protein Structure Prediction. 2.1. Conformation initialization. The starting point (input) of protein structure prediction is the one-dimensional amino acid sequence of target protein and the ending point (output) is the model of three-dimensional structures. The theoretically possible steric conformation for a protein sequence ...
2. RNA secondary structure formation. In RNA, intra and intermolecular long-range interactions, including hairpins, pseudoknots, and G-quadruplexes, are commonly observed. Hairpins are composed of a hybridized stem and a single stranded loop ( Fig. 1 a and b) and can contain mismatches and bulges. Pseudoknots contain nested stem-loop structures ...
Secondary Research Paper Structure. Our team of paper writers consists only of native speakers coming from countries such as the US or Canada. But being proficient in English isn't the only requirement we have for an essay writer. All professionals working for us have a higher degree from a top institution or are current university professors.