Why Quality Matters
By Bethany Simunich, Ph.D., QM Vice President of Innovation and Research
This piece was written as a response to a growing sense that fears related to loss of academic freedom and creativity are being fueled by misinformation, including articles and blog posts written by individuals who purport to be deeply and experientially familiar with QM and its tools when they are not. It’s my hope that it addresses misunderstandings about what QM is , how it is used , and the intended purpose of QM Rubrics. More broadly, I hope my experience and perspective can promote constructive dialogue about the need for and appropriate use of standards for online course quality.
I remember with painful acuity the first time I taught an online course. Having previously taught the course for years in a face-to-face (F2F) format, when asked if I’d teach it online, I quickly agreed, eager to explore this new-to-me modality. This was over 15 years ago…before many institutions understood the differences (and nuances) of teaching in the online classroom, and way before I understood anything about designing or teaching online courses. I thought my experience teaching the course and passion for the subject matter would be enough, and that the technology I’d need to “migrate” my course online would be relatively simple and straightforward, even for a Luddite like myself. I distinctly remember thinking, “ How hard can it be? ”
Teaching my first online course was, as you’ve likely guessed, very hard — much more difficult than I had anticipated. It remains to this day the biggest “ I didn’t know what I didn’t know ” experience of my career. Prior to teaching online, I had never deliberately and strategically designed a course, nor had any training on how to do so. Moving online, I quickly learned that I couldn’t create a course in the learning management system the same way I had created it for the on-ground classroom. Like most faculty, I “taught how I was taught,” which for me meant: selecting the textbook, creating lectures to augment that text, developing in-class activities to engage with students and allow them to engage with the material, and creating assignments and exams. I had never truly examined if these components supported one another, as well as my pedagogical goals, and I certainly never had to create a well-organized web-based layout for a course. Designing a great online course, I came to realize, was not only a skill set I didn’t yet have, it was also complex and incredibly time-intensive. In person, I could change things quickly and easily, and I never had to purposefully create a web-based learning path for students, or be transparent about the design of the course. Face-to-face design and teaching were concurrent and dynamically intertwined in a way that asynchronous online design and teaching were not.
Consider this : if our habit is to teach as we were taught, what does that mean for those of us who have never taken an online course? Or have never taken a well-designed online course taught by a great online instructor? I had no models. I had never taken an online course or even seen an example of a good one.
I realized too late in the process that teaching online was not simply digitizing and uploading materials from my F2F class. At this point, I didn’t yet know that uploading a bunch of documents doesn’t equal an online course, and when I realized that I couldn’t even create a “bad” online course easily, I was suddenly struck with an uncomfortable feeling of being unmoored in my teaching and frustrated by the lack of guidance and training. I also felt that I had let my students down before the semester even started. I was woefully unprepared.
I spent the next decade or so learning what I could about designing and teaching online courses, and continue to learn more every day. I left full-time teaching and, after taking additional courses and certificates (I had two masters degrees and a doctorate, but had never had a course on instructional design), accepted an entry-level position as an instructional designer. Since that time, I’ve worked with faculty at several institutions, helping design and revise hundreds of online courses. I’ve trained over a dozen instructional designers and led ID teams. I’ve spent many, many years doing faculty development work for online design and teaching, and have created and delivered dozens of workshops, several online courses, and over a hundred conference presentations on online learning topics. I’ve helped with the development of several fully-online degree programs and worked to bring administrators, faculty, and staff together through the entire process. I’ve conducted research focused on many aspects of online learning and spent time learning the history of the field, while keeping abreast of new research. I’ve surveyed, interviewed, and spoken with over 500 online students about their experiences. And I’ve continued to teach online.
Why am I sharing all of this? Because I believe it is crucial to understand the background and experience that anyone who is talking about quality in online learning brings to the table — what time have they spent striving to increase online quality in a variety of ways, in various institutional contexts, and what roles have they performed in implementing quality assurance at scale?

Humanizing Quality Assurance
Too often, we limit the quality assurance conversations to abstract examples that present quality standards as a phantom menace against creativity, while ignoring the actual people that are harmed by a lack of quality assurance efforts and preparation. Allow me to “humanize” quality for a moment. Achieving online quality means successfully addressing a wide range of real-life challenges such as:
- The fantastic face-to-face teacher who is afraid they’ll lose their connection to their students once they move online;
- The instructor who happily eschewed using technology for many years, but now needs it to support their online teaching;
- The faculty member who doesn’t have a clear idea of how to use web-based navigation and organization to create a learning path;
- The instructional designer tasked with revising dozens or hundreds of online courses, none of which may live up to the institutional reputation or the learning quality that students were promised;
- The administrator who has never taught online or learned online, but now leads the decision-making about online strategy;
- The colleague conducting a peer review of an online course who has never taught online themselves, and is required to use a form designed for F2F teaching evaluation;
- The instructional designers and faculty developers who receive panicked calls from faculty the week (or weekend) before the term begins, desperate for help for online courses that were assigned or not thought about until the last minute, and are not yet designed or built;
- The students who feel as though they’re “teaching themselves”, wondering where the professor is, why they aren’t answering emails, and why the course isn’t visible or ready.
I have experienced every single one of these, either as a faculty member or as online learning staff. It is the last one that especially, and regularly, breaks my heart. All of these situations reflect people in scary, frustrating, or terrible situations. These real-life situations are why we need to talk about the quality of the online learning that we offer our students and get beyond misassumptions that elevating quality can only lead to standardized, “cookie-cutter” courses or a curtailment of academic freedom (neither of which I have seen or been provided evidence of).
The Community Experience
While diving into online learning best practices over the past decade, I discovered Quality Matters (QM) — an international nonprofit dedicated to promoting and improving the quality of online education and student learning. If you are reading this, you likely know QM and are working with them in some capacity. In my former roles at higher ed institutions, I personally used and found QM’s tools and resources to be incredibly valuable. In 2020, I decided to join the team and currently serve as QM’s Director of Research and Innovation. But it wasn’t just about the tools and resources, it was about what QM truly is:
An organization founded by faculty and educational staff that creates and provides tools and processes for quality online learning, and whose work is continuously informed and improved by its members.
It was, in fact, the collegial community that first drew me to QM — it differed so much from the other educational organizations I interacted with. Instead of feeling nameless and overwhelmed at a conference, I felt included and mentored. I found a community of people who were passionate about creating the best online learning opportunities we could for students, including so many online faculty and instructional designers who shared their knowledge and tips. I felt connected and respected, even when I was new to online design and teaching.
Unfortunately, these aspects— and so many other important factors — are often lost in the conversations around QM.
The Quality Matters Rubrics
Those deeply familiar with the QM Rubric know that it inherently provides flexibility, laid out well in the Standard’s Annotations, for how the Specific Review Standards can be met. Over the years, I’ve heard many things that “can’t be done” in a course and still meet quality standards.
Some examples include:
- “I can’t include a video to welcome my students”
- “I can’t utilize ungrading”
- “I can’t make this work for my practicum course”
- “This doesn’t apply to my doctoral students”
- “I teach a [hands-on course, math course, science course, public speaking course], so these things don’t apply/can’t be done”
- “I can’t use a flexible, student-inclusive approach to design my course”
- “I can’t make changes to my course while it’s running/after it’s done”
- “This goes against my academic freedom — I can’t create the course I want to create, use the content I want, incorporate elements of small teaching, etc.”
I would say every single one of these is a false assumption, and points to a limited understanding of the Rubric, rather than a limitation of the Rubric itself. I am not saying that it’s impossible that there could be a situation or pedagogical approach that is limited by the Rubric, but I am saying that I have yet to discover one. One of my colleagues, for example, teaches his online course by embracing open pedagogy and allowing the students to co-author the assessment questions, suggest or create activities, curate and share content, and also select specific topics for exploration in the course. Nothing about that instructional approach would be prohibited or hindered by the QM Rubric so long as the intention and/or goal of that type of assignment is apparent to the student.
I understand that it can be easy and tempting to tear down tools for quality assurance…but my experience shows that these tools can help generate ideas for how we can improve and practice good online education. Too often, ill-informed assumptions and opinions steal valuable time from the conversation of “ How can this be done? ” and “ How can we collectively do this better? ” in order to dive into conversations that often result in defensiveness, posturing, and the marginalization of voices and experiences. I would love to spend more time listening, generating possibilities, and co-creating solutions, and much less time defending online quality assurance from hastily-made assumptions. It’s important to understand, though, what the tools can and cannot help us achieve.
The QM Higher Education Rubric is:
- The first rubric developed by faculty specifically for the evaluation of online courses, and developed with the intent of collegiality, continuous improvement, and flexible implementation;
- The only rubric regularly updated by online faculty, distance learning staff, and online experts to reflect the latest in online learning research and pedagogical practice — over 100 independent educators have participated in updating the Rubric, now in its sixth edition;
- A rubric continuously informed and improved based on usage and feedback from its community;
- A tool maintained and supported by an educational nonprofit staffed by 44 truly dedicated people, most of whom are former teachers, instructional designers, and educational staff.
Quality Matters has created a quality assurance tool — five tools , actually — that are usable and adaptable across all disciplines, all institution types, all online modalities, and all class sizes. How is that possible? Because at their core, the QM Rubrics are — more than anything else — flexible .
QM Rubrics:
- Do NOT require or prescribe a particular pedagogical approach or philosophy, specific teaching strategies or methods, and do not dictate types of instructional materials or assessments;
- Provide Annotations that offer a myriad of ways, though not exhaustive, to meet each Standard;
- Provide the opportunity to embed yourself in the student perspective.
In short, you simply cannot create and use inflexible, un-adaptable tools when you are serving over 1,500 unique educational institutions and over 100,000 educators around the globe. If the QM Rubrics were truly rigid, inflexible, or an impingement on creativity and freedom, then we wouldn’t see thousands of QM-Certified courses that span countless disciplines, course types, institutional cultures, faculty, and pedagogical strategies. We wouldn’t see a 99% satisfaction rate by faculty who engage in the QM-Certified review process, or data that shows 98% of faculty who engage with our professional development find the information so valuable, that they take it back to their F2F classroom as well.
It’s important to note, though, that while QM Rubrics reflect well-researched instructional design principles, they’re not a course design checklist, and to see it as such would likely create the assumption that online course design is prescriptive. For faculty who were looking for a design guide, however, and who especially need design assistance during the pandemic, QM developed the publicly available Bridge to Quality Design Guide .
It’s important to view the Rubric through the lens that you are applying it, whether via its original, intended use as a review tool for online quality assurance, or in an adaptation of that use — as a tool for information and ideas as you design your online course. Let me give an example:
Standard 5.3 reads: The instructor’s plan for interacting with learners during the course is clearly stated . If you’re reviewing a course, you’d then look to the Annotation, which provides more information, including having a clear plan for interacting with students in primary ways, such as responding to questions and providing feedback. It provides several, non-exhaustive examples of information that instructors might give to their online students, as well as several examples of where this information is commonly found. It doesn’t prescribe that you adhere to any particular type of grading approach or that you provide a specific type of feedback. It also includes specific information if one is reviewing a Competency-Based Course.
Let’s say that a given course includes information in the syllabus that lets students know that if they email or post a question, they’ll receive a reply within 24 hours during the week and 48 hours on the weekend. Additionally, the instructor lets students know that they can expect to receive feedback on course activities within a week after they submit their work. Students are also informed that their instructor makes the effort to provide feedback within one week so that they can use that feedback to improve their work on the next activity or assessment. The QM-Certified Peer Reviewer in this case isn’t asked if they agree with the policy — they might, for example, feel that a one-week turnaround time is an unreasonable promise, or that they themselves have a 24-hour response time for questions, even on weekends. The Reviewer can provide feedback and suggestions, but they are only evaluating the Standard in terms of whether they, from the student perspective, would understand some important ways their instructor is going to interact with them and respond to their needs in their asynchronous course. This is a great example of how the QM Rubric is not prescriptive… unless one disagrees with the idea that we should let students know when we’ll answer their questions or provide feedback.
However, if you’re adapting the Rubric as a guide for design , you might be inspired to ask colleagues how they think through their policy, and even what approaches seem to work better for the cohort of students that typically take your class. The Annotation provides a bit of the “why” as well, which can also prompt some good reflection as you design. One note, for example, says: “Frequent feedback from the instructor increases learners' sense of engagement in a course. Learners are better able to manage their learning activities when they know upfront when to expect feedback from the instructor.” This cues you into how this Standard is grounded in research and best practices for student engagement, as well as methods to elevate teaching presence. There are a variety of ways to meet this Standard in your online course, and there is no requirement that all policies look the same or be standardized.
Beyond Rubrics
While QM is often synonymous with its Rubrics, QM is actually a comprehensive, multi-faceted quality assurance framework, whose use and implementation are customizable to institutional needs and goals. In addition to the Rubric, QM offers multiple course review options as well as professional development opportunities and a number of publicly available free resources .
Just like there are a variety of ways to use the Rubrics, there’s no “one, right way” to evaluate review quality. QM does offer a pathway for certified, third-party reviews by faculty specifically experienced with online teaching and evaluation — an option rarely presented for face-to-face courses. But those reviews are only one of many ways to meet institutional and student goals for quality. There are also a variety of other review options and pathways available, including:
- Internal reviews that combine institutional standards with QM Standards
- Internal reviews that combine institutional standards with select QM Standards
- “Lite” QM Reviews that focus only on select QM Standards, as determined by the institution or faculty member
- Internal reviews that combine QM Standards with other rubric standards
- Self-reviews done by the faculty teaching the course
All of these options are available, and QM even has a tool to enable this flexibility called My Custom Reviews (MyCR). This tool, like so many of QM’s other resources, is designed to allow institutions to choose what standards they want to use and what processes they want to create for reviewing courses.
If you are engaging with official reviews, here are some important facts you need to know:
- Official Reviews are a collegial, collaborative, faculty-driven process;
- Review teams are made up of three individuals, all of whom have taught a for-credit online or blended course in the last 18 months;
- All Reviewers go through a rigorous professional development process;
- The review process is designed to be diagnostic and collegial, not evaluative and judgmental;
- The subjectivity of human judgment is embedded within the review process. Reviewers are encouraged to discuss and are not led to a forced agreement or unanimous decision;
- Instructors receive three independent pieces of feedback for each standard, which they could choose to apply or not, in a way that works for them and their students.
The Review process is, in fact, more collegial and collaborative than any classroom-based review that I’ve been a part of or witnessed. I often felt it shortchanged a F2F class to have a peer attend a single class session and make judgments from a templated checklist. The QM Peer Review process, on the other hand, begins with the instructor discussing the course, describing the design, their learning goals, their students, and more. As the review is conducted, Reviewers continue the dialogue with the instructor, and ask questions or make suggestions for quick fixes. The Review team itself represents a diversity of experiences and voices, comprised of three online teaching faculty — a great improvement, in my opinion, from the too-often singular review voice of an institutionally-based evaluation of a F2F course.
Quality Matters Implementation
The QM framework, consisting of the Rubric, supporting professional development, and options for internal and certified reviews, are resources, processes, and tools that are implemented by the institution. Quality assurance implementation, however, is often not given the consideration for the change-management initiative that it is, and institutions may experience missteps or disruptions if it’s not created as an inclusive process that reflects the institutional culture and goals. QM doesn’t prescribe how an institution implements the QM tools and resources for quality assurance. An individual faculty member has many choices about how they can use the QM Rubric, engage with professional development, or conduct course reviews. However, the most successful implementations occur when considered in conjunction with the institutional culture and context — including stated goals — and also when the implementation is inclusive, collaborative, and collegial in nature.
Additionally, we’re supporting this work with research to further explore best practices and key drivers in implementing online quality assurance within higher ed institutions. Current findings include choosing the right person/people to lead this effort, making it inclusive from the start, and embracing a bottom-up approach. If you believe that your institution is not meeting faculty, staff, student, and other stakeholder needs with regard to QA implementation, I encourage you to have crucial conversations about implementation efforts, to connect with campus offices and partners that support QA in online learning, and to connect with the resources and training that organizations like QM provide to help in these efforts. Oftentimes, implementation is intrinsically linked with accreditation efforts, and that is an additional place to begin, or continue, the dialogue. QM also provides professional development opportunities, including two free workshops seats for those coordinating implementation efforts, and additional resources to help faculty and institutions decide how implementation would work best on their campus.
The Student Experience
In all of the talk about rubrics, reviews, policies, and implementation, however, it’s important we don’t forget about those who are disadvantaged by lower quality online learning experiences. The human face of quality assurance is equally valid to academically-embedded conversations that never extend to online students. Students are the ones who are disadvantaged by lower-quality online learning experiences and need to be at the heart of the conversation. Consider the following real-life examples:
- The online student who struggled with a midterm assignment but did not reach out to his professor for help. Why? Because he felt like he didn’t really know his professor — he was worried that the professor wasn’t nice, and wouldn’t help. In truth, his professor was kind, engaging, and student-focused… but had never thought about creating an instructor introduction video. The student had never even seen his face or heard his voice.
- The online student who emailed me out of desperation, fearing she was failing her class and couldn’t afford to retake it. She accompanied her emails with screenshots of the course, convinced she was “too dumb” to figure things out. Emails to her instructor had gone unanswered. Looking at the screenshots, I could see her struggles were largely a result of how the course was organized. All the files had been uploaded into a single folder with no directions or guidance. I also discovered the instructor had not been in the course for several weeks.
- The online student who I found in tears in the campus library, devastated about their performance on a midterm exam. They thought they had prepared well by watching the instructor videos (on campus because their home internet did not have the bandwidth to handle the hour+ length of each video). They had read all the materials, highlighted key passages, and made review cards and a study guide. When I asked what they felt had “gone wrong,” they replied: “I didn’t know I wasn’t understanding the material. We had some quizzes, but they hadn’t been graded, so I didn’t know how I was doing. It wasn’t until a few questions into the midterm that I realized I had some big misunderstandings, and I wasn’t thinking about things like I should, but by then it was too late.”
This is the other side of online course standards and policies. It might seem like a great, freeing idea to not be clear with students about expectations, to not approach your design by also thinking about how you’ll connect with and interact with students, to not learn about good organization and navigation, to not tell your students how they can contact you and when they’ll receive a response, to not give students multiple chances and ways to check their understanding and gauge their learning process… but the reality is that not considering best practices such as this frequently disadvantages students .
I ended up meeting with all three of these faculty, and trust me when I say that they absolutely wanted to do the very best for their online students. They didn’t know, however, that it’s important to introduce themselves as an instructor (Standard 1.8), that they needed to consider before the course began how they would interact with students, including responding to questions and providing feedback (Standard 5.3), that navigation and organization are absolutely crucial in online design (Standard 8.1), that posting long videos causes technology and accessibility issues for students, and is often too much information to absorb or review at one time (Standard 8.4), or that online students need multiple opportunities to check their understanding and progress (Standard 3.5), and with prompt feedback.
These were caring, experienced instructors who just didn’t know what they didn’t know .
The Whole Quality Picture
Equally important as what we “don’t know,” is defining what it is we are talking about when we discuss quality — because it’s vital to understand that “quality” is not just one thing . It lies not only in the design of an online course but also in:
- The quality of the content the faculty chooses to create or curate for the course;
- The effectiveness of their teaching (as well as how well they’re supported in that teaching);
- The institutional infrastructure and readiness for quality online learning;
- The preparedness and support of our online students;
- The technology used, including how faculty and learners are supported in using the technology that supports good online learning.
Quality online learning is more than a rubric, or any single tool, and it is a privileged perspective to posit that we should not define what quality means for students, nor create tools and processes to support faculty in improvements that lead to greater quality. Objections fail to address equity, access, student preparedness, and the complex, real-life issues faced when trying to ensure that all students receive a quality learning experience, regardless of whether that course is online or face-to-face. We can’t ignore the very real institutional barriers embedded in the change management it requires to create and implement quality initiatives at scale, nor the reality that many instructional designers know: across the vast landscape of higher education there are, and long have-been, online courses that fail to meet basic student needs, whether for support or learning.
Be Part of the Conversation
I want to make clear that I am not claiming, nor do I believe, that the QM Rubric is perfect and should never be critiqued or improved. If I, or QM, felt that way, we wouldn’t bring together community experts, combined with expansive survey feedback from all our community members, to regularly revise the Rubric. We already intend, for example, to include more information about inclusive and culturally responsive design in the next Rubric revision.
You, as a member of our community, matter! And we want to invite you to share your thoughts. Please feel free to complete this short survey , which can be filled out anonymously. We continuously provide many avenues for the community to share their comments, ideas, and questions, and use that feedback to inform and improve the resources and services we provide. We also have options for individual consultations, and can join the conversation at your campus via a web meeting as well.
Thank you for all you do to ensure high-quality online learning for your students, and thank you, in advance, for sharing your experiences, ideas, and questions with us. I look forward to our continued collaboration and conversation.
Dr. Bethany Simunich is QM’s Director of Research and Innovation. She has worked in higher education for over 20 years and has over 15 years of experience in eLearning research, instructional design and online pedagogy. As QM's Director of Research and Innovation, she helps provide research-based tools, ideas and solutions to enable individuals and institutions to assess and achieve their quality assurance goals. Her research interests include presence in the online classroom, online student and instructor self-efficacy and satisfaction, and outcomes achievement in online courses. Connect with Dr. Simunich on Twitter or LinkedIn .

- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- News & Views
- How to improve...
How to improve healthcare improvement—an essay by Mary Dixon-Woods
Read the full collection.
- Related content
- Peer review
- Mary Dixon-Woods , director
- THIS Institute, Cambridge, UK
- director{at}thisinstitute.cam.ac.uk
As improvement practice and research begin to come of age, Mary Dixon-Woods considers the key areas that need attention if we are to reap their benefits
In the NHS, as in health systems worldwide, patients are exposed to risks of avoidable harm 1 and unwarranted variations in quality. 2 3 4 But too often, problems in the quality and safety of healthcare are merely described, even “admired,” 5 rather than fixed; the effort invested in collecting information (which is essential) is not matched by effort in making improvement. The National Confidential Enquiry into Patient Outcome and Death, for example, has raised many of the same concerns in report after report. 6 Catastrophic degradations of organisations and units have recurred throughout the history of the NHS, with depressingly similar features each time. 7 8 9
More resources are clearly necessary to tackle many of these problems. There is no dispute about the preconditions for high quality, safe care: funding, staff, training, buildings, equipment, and other infrastructure. But quality health services depend not just on structures but on processes. 10 Optimising the use of available resources requires continuous improvement of healthcare processes and systems. 5
The NHS has seen many attempts to stimulate organisations to improve using incentive schemes, ranging from pay for performance (the Quality and Outcomes Framework in primary care, for example) to public reporting (such as annual quality accounts). They have had mixed results, and many have had unintended consequences. 11 12 Wanting to improve is not the same as knowing how to do it.
In response, attention has increasingly turned to a set of approaches known as quality improvement (QI). Though a definition of exactly what counts as a QI approach has escaped consensus, QI is often identified with a set of techniques adapted from industrial settings. They include the US Institute for Healthcare Improvement’s Model for Improvement, which, among other things, combines measurement with tests of small change (plan-do-study-act cycles). 8 Other popular approaches include Lean and Six Sigma. QI can also involve specific interventions intended to improve processes and systems, ranging from checklists and “care bundles” of interventions (a set of evidence based practices intended to be done consistently) through to medicines reconciliation and clinical pathways.
QI has been advocated in healthcare for over 30 years 13 ; policies emphasise the need for QI and QI practice is mandated for many healthcare professionals (including junior doctors). Yet the question, “Does quality improvement actually improve quality?” remains surprisingly difficult to answer. 14 The evidence for the benefits of QI is mixed 14 and generally of poor quality. It is important to resolve this unsatisfactory situation. That will require doing more to bring together the practice and the study of improvement, using research to improve improvement, and thinking beyond effectiveness when considering the study and practice of improvement.
Uniting practice and study
The practice and study of improvement need closer integration. Though QI programmes and interventions may be just as consequential for patient wellbeing as drugs, devices, and other biomedical interventions, research about improvement has often been seen as unnecessary or discretionary, 15 16 particularly by some of its more ardent advocates. This is partly because the challenges faced are urgent, and the solutions seem obvious, so just getting on with it seems the right thing to do.
But, as in many other areas of human activity, QI is pervaded by optimism bias. It is particularly affected by the “lovely baby” syndrome, which happens when formal evaluation is eschewed because something looks so good that it is assumed it must work. Five systematic reviews (published 2010-16) reporting on evaluations of Lean and Six Sigma did not identify a single randomised controlled trial. 17 18 19 20 21 A systematic review of redesigning care processes identified no randomised trials. 22 A systematic review of the application of plan-do-study-act in healthcare identified no randomised trials. 23 A systematic review of several QI methods in surgery identified just one randomised trial. 56
The sobering reality is that some well intentioned, initially plausible improvement efforts fail when subjected to more rigorous evaluation. 24 For instance, a controlled study of a large, well resourced programme that supported a group of NHS hospitals to implement the IHI’s Model for Improvement found no differences in the rate of improvement between participating and control organisations. 25 26 Specific interventions may, similarly, not survive the rigours of systematic testing. An example is a programme to reduce hospital admissions from nursing homes that showed promise in a small study in the US, 27 but a later randomised implementation trial found no effect on admissions or emergency department attendances. 28
Some interventions are probably just not worth the effort and opportunity cost: having nurses wear “do not disturb” tabards during drug rounds, is one example. 29 And some QI efforts, perversely, may cause harm—as happened when a multicomponent intervention was found to be associated with an increase rather than a decrease in surgical site infections. 30
Producing sound evidence for the effectiveness of improvement interventions and programmes is likely to require a multipronged approach. More large scale trials and other rigorous studies, with embedded qualitative inquiry, should be a priority for research funders.
Not every study of improvement needs to be a randomised trial. One valuable but underused strategy involves wrapping evaluation around initiatives that are happening anyway, especially when it is possible to take advantage of natural experiments or design roll-outs. 31 Evaluation of the reorganisation of stroke care in London and Manchester 32 and the study of the Matching Michigan programme to reduce central line infections are good examples. 33 34
It would be impossible to externally evaluate every QI project. Critically important therefore will be increasing the rigour with which QI efforts evaluate themselves, as shown by a recent study of an attempt to improve care of frail older people using a “hospital at home” approach in southwest England. 35 This ingeniously designed study found no effect on outcomes and also showed that context matters.
Despite the potential value of high quality evaluation, QI reports are often weak, 18 with, for example, interventions so poorly reported that reproducibility is frustrated. 36 Recent reporting guidelines may help, 37 but some problems are not straightforward to resolve. In particular, current structures for governance and publishing research are not always well suited to QI, including situations where researchers study programmes they have not themselves initiated. Systematic learning from QI needs to improve, which may require fresh thinking about how best to align the goals of practice and study, and to reconcile the needs of different stakeholders. 38
Using research to improve improvement
Research can help to support the practice of improvement in many ways other than evaluation of its effectiveness. One important role lies in creating assets that can be used to improve practice, such as ways to visualise data, analytical methods, and validated measures that assess the aspects of care that most matter to patients and staff. This kind of work could, for example, help to reduce the current vast number of quality measures—there are more than 1200 indicators of structure and process in perioperative care alone. 39
The study of improvement can also identify how improvement practice can get better. For instance, it has become clear that fidelity to the basic principles of improvement methods is a major problem: plan-do-study-act cycles are crucial to many improvement approaches, yet only 20% of the projects that report using the technique have done so properly. 23 Research has also identified problems in measurement—teams trying to do improvement may struggle with definitions, data collection, and interpretation 40 —indicating that this too requires more investment.
Improvement research is particularly important to help cumulate, synthesise, and scale learning so that practice can move forward without reinventing solutions that already exist or reintroducing things that do not work. Such theorising can be highly practical, 41 helping to clarify the mechanisms through which interventions are likely to work, supporting the optimisation of those interventions, and identifying their most appropriate targets. 42
Research can systematise learning from “positive deviance,” approaches that examine individuals, teams, or organisations that show exceptionally good performance. 43 Positive deviance can be used to identify successful designs for clinical processes that other organisations can apply. 44
Crucially, positive deviance can also help to characterise the features of high performing contexts and ensure that the right lessons are learnt. For example, a distinguishing feature of many high performing organisations, including many currently rated as outstanding by the Care Quality Commission, is that they use structured methods of continuous quality improvement. But studies of high performing settings, such as the Southmead maternity unit in Bristol, indicate that although continuous improvement is key to their success, a specific branded improvement method is not necessary. 45 This and other work shows that not all improvement needs to involve a well defined QI intervention, and not everything requires a discrete project with formal plan-do-study-act cycles.
More broadly, research has shown that QI is just one contributor to improving quality and safety. Organisations in many industries display similar variations to healthcare organisations, including large and persistent differences in performance and productivity between seemingly similar enterprises. 46 Important work, some of it experimental, is beginning to show that it is the quality of their management practices that distinguishes them. 47 These practices include continuous quality improvement as well as skills training, human resources, and operational management, for example. QI without the right contextual support is likely to have limited impact.
Beyond effectiveness
Important as they are, evaluations of the approaches and interventions in individual improvement programmes cannot answer every pertinent question about improvement. 48 Other key questions concern the values and assumptions intrinsic to QI.
Consider the “product dominant” logic in many healthcare improvement efforts, which assumes that one party makes a product and conveys it to a consumer. 49 Paul Batalden, one of the early pioneers of QI in healthcare, proposes that we need instead a “service dominant” logic, which assumes that health is co-produced with patients. 49
More broadly, we must interrogate how problems of quality and safety are identified, defined, and selected for attention by whom, through which power structures, and with what consequences. Why, for instance, is so much attention given to individual professional behaviour when systems are likely to be a more productive focus? 50 Why have quality and safety in mental illness and learning disability received less attention in practice, policy, and research 51 despite high morbidity and mortality and evidence of both serious harm and failures of organisational learning? The concern extends to why the topic of social inequities in healthcare improvement has remained so muted 52 and to the choice of subjects for study. Why is it, for example, that interventions like education and training, which have important roles in quality and safety and are undertaken at vast scale, are often treated as undeserving of evaluation or research?
How QI is organised institutionally also demands attention. It is often conducted as a highly local, almost artisan activity, with each organisation painstakingly working out its own solution for each problem. Much improvement work is conducted by professionals in training, often in the form of small, time limited projects conducted for accreditation. But working in this isolated way means a lack of critical mass to support the right kinds of expertise, such as the technical skill in human factors or ergonomics necessary to engineer a process or devise a safety solution. Having hundreds of organisations all trying to do their own thing also means much waste, and the absence of harmonisation across basic processes introduces inefficiencies and risks. 14
A better approach to the interorganisational nature of health service provision requires solving the “problem of many hands.” 53 We need ways to agree which kinds of sector-wide challenges need standardisation and interoperability; which solutions can be left to local customisation at implementation; and which should be developed entirely locally. 14 Better development of solutions and interventions is likely to require more use of prototyping, modelling and simulation, and testing in different scenarios and under different conditions, 14 ideally through coordinated, large scale efforts that incorporate high quality evaluation.
Finally, an approach that goes beyond effectiveness can also help in recognising the essential role of the professions in healthcare improvement. The past half century has seen a dramatic redefining of the role and status of the healthcare professions in health systems 54 : unprecedented external accountability, oversight, and surveillance are now the norm. But policy makers would do well to recognise how much more can be achieved through professional coalitions of the willing than through too many imposed, compliance focused diktats. Research is now showing how the professions can be hugely important institutional forces for good. 54 55 In particular, the professions have a unique and invaluable role in working as advocates for improvement, creating alliances with patients, providing training and education, contributing expertise and wisdom, coordinating improvement efforts, and giving political voice for problems that need to be solved at system level (such as, for example, equipment design).
Improvement efforts are critical to securing the future of the NHS. But they need an evidence base. Without sound evaluation, patients may be deprived of benefit, resources and energy may be wasted on ineffective QI interventions or on interventions that distribute risks unfairly, and organisations are left unable to make good decisions about trade-offs given their many competing priorities. The study of improvement has an important role in developing an evidence-base and in exploring questions beyond effectiveness alone, and in particular showing the need to establish improvement as a collective endeavour that can benefit from professional leadership.
Mary Dixon-Woods is the Health Foundation professor of healthcare improvement studies and director of The Healthcare Improvement Studies (THIS) Institute at the University of Cambridge, funded by the Health Foundation. Co-editor-in-chief of BMJ Quality and Safety , she is an honorary fellow of the Royal College of General Practitioners and the Royal College of Physicians. This article is based largely on the Harveian oration she gave at the RCP on 18 October 2018, in the year of the college’s 500th anniversary. The oration is available here: http://www.clinmed.rcpjournal.org/content/19/1/47 and the video version here: https://www.rcplondon.ac.uk/events/harveian-oration-and-dinner-2018
This article is one of a series commissioned by The BMJ based on ideas generated by a joint editorial group with members from the Health Foundation and The BMJ , including a patient/carer. The BMJ retained full editorial control over external peer review, editing, and publication. Open access fees and The BMJ ’s quality improvement editor post are funded by the Health Foundation.
Competing interests: I have read and understood BMJ policy on declaration of interests and a statement is available here: https://www.bmj.com/about-bmj/advisory-panels/editorial-advisory-board/mary-dixonwoods
Provenance and peer review: Commissioned; not externally peer reviewed.
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/ .
- Neuburger J ,
- Hutchings A ,
- Stewart K ,
- Buckingham R
- Castelli A ,
- Verzulli R ,
- Allwood D ,
- Sunstein CR
- Healthcare Quality Improvement Partnership
- Shortell SM
- Martin GP ,
- Dixon-Woods M
- Donabedian A
- Himmelstein DU ,
- Woolhandler S
- Woolhandler S ,
- Himmelstein DU
- Dixon-Woods M ,
- Ioannidis JPA ,
- Marshall M ,
- Pronovost P ,
- Glasgow JM ,
- Scott-Caziewell JR ,
- Nicolay CR ,
- Deblois S ,
- Moraros J ,
- Lemstra M ,
- Amaratunga T ,
- Dobranowski J
- van Leijen-Zeelenberg JE ,
- Elissen AMJ ,
- Taylor MJ ,
- McNicholas C ,
- Nicolay C ,
- Morgan DJ ,
- Diekema DJ ,
- Sepkowitz K ,
- Perencevich EN
- Benning A ,
- Ouslander JG ,
- Huckfeldt P ,
- Westbrook JI ,
- Hooper TD ,
- Middleton S ,
- Anthony T ,
- Murray BW ,
- Sum-Ping JT ,
- Portela MC ,
- Pronovost PJ ,
- Woodcock T ,
- Ramsay AIG ,
- Hoffman A ,
- Richardson A ,
- Hibbert P ,
- Matching Michigan Collaboration & Writing Committee
- Tarrant C ,
- Pearson M ,
- Hemsley A ,
- Blackwell R ,
- Custerson L
- Goodman D ,
- Watson SI ,
- Taylor CA ,
- Chazapis M ,
- Gilhooly D ,
- Liberati EG ,
- Davidoff F ,
- Leviton L ,
- Johnston M ,
- Abraham C ,
- “Psychological Theory” Group
- Clay-Williams R ,
- Braithwaite J
- Bradley EH ,
- Willars J ,
- Mahajan A ,
- Aveling EL ,
- Thibaut BI ,
- Ramtale SC ,
- Boozary AS ,
- Shojania KG
- Pronovost PJ
- Armstrong N ,
- Herbert G ,
- Purkayastha S ,
- Greenhalgh A ,
Importance and seven principles of quality management

Checked : Mark A. , Abigail C.
Latest Update 20 Jan, 2024
Table of content
- Quality management: what exactly?
The importance of quality management for the company
The basic principles of quality management, the seven principles of quality management, 1 - customer orientation, 2 - management responsibility (leadership), 3 - staff involvement, 4 - process approach, 5 – improvement, 6 - evidence-based decision making, 7 - management of relations with interested parties.
Quality management is the process by which a company seeks to achieve its quality objectives. But how important is it really? Our article gives you an explanation.
Quality management: what exactly?
It is the set of strategies implemented by a company in order to establish a quality approach within it. This approach aims, for its part, to improve the quality of organization and production. To do this, it seeks to optimize the quality of management, products, and services offered to customers or the employee environment.
The ISO 9001 quality management approach calls on strategies, equipment, and actors who contribute to the implementation of concrete improvement actions. Therefore, these actions and the resources involved constitute the quality management system (QMS), governed by the ISO 9001 standard.
The quality management system represents a pillar of growth for the company. The reason is simple: its effectiveness is largely based on this system. It is indeed this process that allows the company to have an efficient organization in which employees participate in achieving development objectives. This quality approach is also essential to set up a service that meets customer expectations and achieves a high level of satisfaction.
In a word, quality management is the pivot of the company's competitiveness. Without this system, it will be difficult for him to make a profit from his activity and optimize his profits. Therefore, the development of an effective quality management system is crucial for any company that wishes to evolve in its environment.
An effective quality approach revolves around the seven principles set out in the ISO 9001 standard. Indeed, these principles are considered to be the main factors for the success of a QMS.
The ISO 9001: 2015 standards on quality management systems are based on general principles. There are 7 quality management principles are used, compared to 8 for the 2008 edition. These principles are developed in 2.3 of ISO 9000: 2015 ("Quality management systems - Essential principles and vocabulary"); part of this information is included in annex B of ISO 9001: 2015. This article uses these standards to present the "philosophy" of management principles. Each principle is illustrated with a quote for ease of understanding.
The titles are those of ISO / DIS 9000: 2014; they should not change in the final version. Some are obvious and natural, and all show common sense.
- Client orientation
- Management responsibility
- Staff involvement
- Process approach
- Improvement
- Evidence-based decision making
- Management of relations with interested parties
To convince you of the merits of this approach, let's imagine these seven principles, but taken in reverse:
- Customer operations
- Impassibility of management
- Staff impairment
- Rambling approach
- Deterioration
- Heads-up decision
- Disengagement from relationships with interested parties
There is only one boss: the customer. And he can fire all the staff, from the manager to the employee, simply by spending his money elsewhere. The challenge of this principle is to satisfy the customer to build customer loyalty. This is all the more important since today, with social networks and the internet in general; the customer can express his dissatisfaction/delight and be heard by everyone, immediately. What demolish the image of an organization or, on the contrary, forge an excellent reputation.
To strengthen its customer orientation, the organization must work on its customers' expectations: identify them (and even anticipate them) and make every effort to ensure that the products/services offered to meet them. Quality management must take an interest in customer needs in order to understand them better and set up services that adapt to their expectations.
The performance of a company depends on managers' ability to mobilize collective intelligence for the achievement of the objectives set.
In addition to not investing all of the company's profits in the renovation of its bachelor apartment, management is expected to:
- Define the direction of the body
- Ensures the availability of resources to achieve the objectives
- Involve staff
Thus, the body knows where it should go, has the means, and the desire.
Employee involvement:
Employee involvement and motivation are the pillars of the company's performance and growth.
The title of this principle is reductive:
In addition to being involved (due to its management's great work), the staff must be competent and feel valued. It is really a question of considering the individual under the blue of work. In this spirit, recognition must be expressed by communicating on the added value of the work of the staff and the initiatives taken. Personal skills need to be developed, which will improve the skills of the organization as a whole.
Managing resources and activities as a global process make it possible to obtain a more precise vision of its performance. Having a process approach means considering the activity of the organism as a set of correlated sub-activities. In this model, each process takes into account input data and produces output data. This data can go from one process to another. This approach makes it easier to tackle the different activities, their management, their needs, their objectives. It is, of course, natural that a company organizes itself in services, each managing processes.

We Will Write an Essay for You Quickly
This involves maintaining development actions continuously in order to improve the performance of the company constantly. The organization must constantly seek to improve (the famous continuous improvement), at least to maintain its performance levels, ideally to progress. Improvement applies to the principles already set out: the improvement of customer satisfaction and improvement of process performance. In ISO9001: 2015, reducing risks, seizing opportunities, or even correcting non-conformities are all sources of improvement.
The objective is to pay particular attention to the elements and factual results.
The idea is to reduce the inevitable uncertainty when making decisions by relying on objective data, where we look at the causes to understand the effects.
Supplier Relations is finding a way to maintain a strong relationship with suppliers.
The stakeholders include all factors that influence or are influenced by the organization's activities. They include, in particular: suppliers, bankers, regulation, and even the ISO9001 standard. It is by communicating with interested parties and taking their requirements into account that the organization will be able to improve its performance.
Have you any questions? Let us know in the comment box. And for business essay, don’t hesitate to contact us.
Looking for a Skilled Essay Writer?

- Ohio State University Bachelor's degree
No reviews yet, be the first to write your comment
Write your review
Thanks for review.
It will be published after moderation
Latest News

What happens in the brain when learning?
10 min read
20 Jan, 2024

How Relativism Promotes Pluralism and Tolerance

Everything you need to know about short-term memory
Why Is Quality Important for a Business?
- Small Business
- Business Communications & Etiquette
- Importance of Business Communication
- ')" data-event="social share" data-info="Pinterest" aria-label="Share on Pinterest">
- ')" data-event="social share" data-info="Reddit" aria-label="Share on Reddit">
- ')" data-event="social share" data-info="Flipboard" aria-label="Share on Flipboard">
What Are the Benefits of a Company With a Well-Executed Branding Strategy?
Penetration pricing advantages over skim pricing, what core competencies give an organization competitive advantage.
- The Advantages of Supply Chain Management Systems
- Marketing Environment & Competitor Analysis
With so many options available to customers, you may be wondering whether or not quality still matters. The answer is a resounding “yes,” and quality isn’t just about offering a product or service that exceeds the standard, but it’s also about the reputation you gain for consistently delivering a customer experience that is “above and beyond.” Managing quality is crucial for small businesses.
Quality products help to maintain customer satisfaction and loyalty and reduce the risk and cost of replacing faulty goods. Companies can build a reputation for quality by gaining accreditation with a recognized quality standard.
Meet Customer Expectations
Regardless of what industry you’re involved in, your customers aren’t going to choose you solely based on price, but often on quality. In fact, studies have shown that customers will pay more for a product or service that they think is made well or exceeds the standard. Your customers expect you to deliver quality products.
Quality is Critical to Satisfied Customers
If you fail to meet customers' expectation, they will quickly look for alternatives. Quality is critical to satisfying your customers and retaining their loyalty so they continue to buy from you in the future. Quality products make an important contribution to long-term revenue and profitability. They also enable you to charge and maintain higher prices.
Quality is a key differentiator in a crowded market. It’s the reason that Apple can price its iPhone higher than any other mobile phone in the industry – because the company has established a long history of delivering superior products.
Establish Your Reputation
Quality reflects on your company’s reputation. The growing importance of social media means that customers and prospects can easily share both favorable opinions and criticism of your product quality on forums, product review sites and social networking sites, such as Facebook and Twitter. A strong reputation for quality can be an important differentiator in markets that are very competitive. Poor quality or product failure that results in a product recall campaign can lead to negative publicity and damage your reputation.
If your business consistently delivers what it promises, your customers are much more likely to sing your praises on social media platforms. This not only helps drive your brand awareness, but it also creates the much-desired FOMO effect, which stands for “Fear of Missing Out.” Social-media users that see your company’s strong reputation will want to become part of the product or service you’re offering, which can boost your sales.
Meet or Exceed Industry Standards
Adherence to a recognized quality standard may be essential for dealing with certain customers or complying with legislation. Public-sector companies, for example, may insist that their suppliers achieve accreditation with quality standards. If you sell products in regulated markets, such as health care, food or electrical goods, you must be able to comply with health and safety standards designed to protect consumers.
Accredited quality control systems play a crucial role in complying with those standards. Accreditation can also help you win new customers or enter new markets by giving prospects independent confirmation of your company’s ability to supply quality products.
Manage Costs Effectively
Poor quality increases costs. If you do not have an effective quality-control system in place, you may incur the cost of analyzing nonconforming goods or services to determine the root causes and retesting products after reworking them.
In some cases, you may have to scrap defective products and pay additional production costs to replace them. If defective products reach customers, you will have to pay for returns and replacements and, in serious cases, you could incur legal costs for failure to comply with customer or industry standards.
- Reputation Management: Why a Great Company Reputation is Important
- Entrepreneur: 7 Ways Quality Boosts Business That Quantity Can’t Match
- CMO: Consumers Say They Will Pay More For a Better Experience
Sampson Quain is an experienced content writer with a wide range of expertise in small business, digital marketing, SEO marketing, SEM marketing, and social media outreach. He has written primarily for the EHow brand of Demand Studios as well as business strategy sites such as Digital Authority.
Related Articles
How to upload crisp facebook profile pictures for companies, retail industry and vendor relations, describe the factors used by marketers to position products, importance of containing quality costs, quality control of exports, what does elasticity mean in a company, what steps do companies take to maximize profit or minimize loss, quality management system goals & objectives, the importance of continuous improvement in marketing, most popular.
- 1 How to Upload Crisp Facebook Profile Pictures for Companies
- 2 Retail Industry and Vendor Relations
- 3 Describe the Factors Used by Marketers to Position Products
- 4 Importance of Containing Quality Costs
Essay on Quality Control of Products: Top 13 Essays
After reading this essay you will learn about:- 1. Meaning and Definitions of Quality Control 2. Quality Control Organisation 3. Advantages of Quality Control 4. Quality Control for Export 5. Indian Standard Institution 6. Quality Assurance 7. Causes of Quality Failures 8. Economics of Quality 9. Product Quality Analysis 10. Quality Planning 11. Quality Improvement 12. Quality Management System 13. Role of Top Management.
- Essay on the Role of Top Management towards Quality
Essay # 1. Meaning and Definitions of Quality Control :
Quality control in its simplest term, is the control of quality during manufacturing. Both quality control and inspection are used to assure quality. Inspection is a determining function which determines raw materials, supplies, parts or finished products etc. as acceptable or unacceptable.
As control becomes effective, the need for inspection decreases. Quality control determines the cause for variations in the characteristics of products and gives solutions by which these variations can be controlled. It is economic in its purpose, objective in its procedure, dynamic in its operation and helpful in its treatment.
ADVERTISEMENTS:
Since variations in raw materials have large effects on the quality of in-process materials, quality control includes statistical sampling and testing before acceptance. It also includes the examination of quality characteristics in finished products so as to assure satisfactory outgoing quality.
Cooperation between the quality control group and other departments such as production, planning and inspection is of vital importance. With proper managerial support and co-operation the quality control programme will be more successful.
Definitions :
In current quality control theory and practice, the meaning of “Quality” is closely allied to cost and customer needs. “Quality” may simply be defined as fitness for purpose at lowest cost.
“Quality” of any product is regarded as the degree to which it fulfills the requirements of the customer. “Quality” means degree of perfection. Quality is not absolute but it can only be judged or realized by comparing with standards. It can be determined by some characteristics namely, design, size, material, chemical composition, mechanical functioning, workmanship, finishing and other properties.
Quality of a product depends upon the application of materials, men, machines and manufacturing conditions. The systematic control of these factors is the quality control. The quality of a product differs greatly due to these factors. For example, a skilled worker will produce products of better quality and a less skilled worker will produce poor quality products.
Similarly better machines and better materials with satisfactory manufacturing conditions produce a better quality product. Thus, it is clear that to control the quality of product various factors which are responsible for quality are required to be controlled properly.
In the words of Alford and Beatly, “quality control” may be broadly defined as that “Industrial management technique by means of which products of uniform acceptable quality are manufactured.” Quality control is concerned with making things right rather than discovering and rejecting those made wrong.
“It may also be defined as the function or collection of duties which must be performed throughout the organisation in order to achieve its quality objective” or in the other words ‘Quality is every body’s business and not only the duty of the persons in the Inspection Staff.
Concluding, we can say that quality control is a technique of management for achieving required standard of products.
Factors Affecting Quality :
In addition to men, materials, machines and manufacturing conditions there are some other factors which affect the quality of product as given below:
(i) Market Research i.e. demand of purchaser.
(ii) Money i.e. capability to invest.
(iii) Management i.e. Management policies for quality level.
(iv) Production methods and product design.
Apart from these, poor packing, inappropriate transportation and poor after sales service are the areas which can cause damage to a company’s quality image. There are cases where goods of acceptable quality before transportation were downgraded on receipt by the retailer just because they had been damaged in transportation.
Modern quality control begins with an evaluation of the customer’s requirements and has a part to play at every stage from goods manufactured right through sales to a customer, who remains satisfied.
Essay # 2. Quality Control Organisation :
Over the years, the status of the quality control organisation changed from a function merely responsible for detecting inferior or standard material to a function that establishes what are termed preventive programmes.
These programmes are designed to detect quality problems in the design stage or at any point in the manufacturing process and to follow up on corrective action.
Immediate responsibility for quality products rest with the manufacturing departments. All the activities concerning product quality are usually brought together in the organisation which may be known as inspection, quality control, quality assurance department or any other similar name.
Quality control is a staff activity since it serves the line or production department by assisting them in managing quality. Since the quality control function has authority delegated by management to evaluate material produced by the manufacturing department, it should not be in a position to control or dictate to the quality activity.
The quality control organisation depending upon the type of product, method of quality is sufficient enough to carry out following activities:
1. Inspection of raw material, product or processes.
2. Salvage inspection to determine rejected part and assembly disposition.
3. Records and reports maintenance.
4. Statistical quality control.
5. Gauges for inspection.
6. Design for quality control and inspection.
7. Quality control system maintenance and development.
Functions of Quality Control Department :
Quality control department has the following important functions to perform:
1. Only the products of uniform and standard quality are allowed to be sold.
2. To suggest methods and ways to prevent the manufacturing difficulties.
3. To reject the defective goods so that the products of poor quality may not reach to the customers.
4. To find out the points where the control is breaking down and investigates the causes of it.
5. To correct the rejected goods, if it is possible. This procedure is known as rehabilitation of defective goods.
Essay # 3. Advantages of Quality Control :
There are many advantages by controlling the product quality.
Some of them are listed below:
1. Quality of product is improved which in turn increases sales.
2. Scrap rejection and rework are minimised thus reducing wastage. So the cost of manufacturing reduces.
3. Good quality product improves reputation.
4. Inspection cost reduces to a great extent.
5. Uniformity in quality can be achieved.
6. Improvement in manufacturer and consumer relations.
7. Improvement in technical knowledge and engineering data for process development and manufacturing design.
Essay # 4. Quality Control for Export :
Today we need foreign exchange for our requirements and for repayment of our debts and services. If our products are expensive and are of sub-standard quality then the customers abroad will not buy goods from us.
Therefore, we must be able to supply goods which may meet the requirements of foreign buyers. For this purpose quality and good packing determines to a large extent the continued acceptability of the product.
At present some organisations lite Export Inspection Council of India, the Indian Standards Institution, the Indian Society of Quality Control and the Indian Institute of Foreign Trade are helping about this problem of quality control.
Implementation of the Export Act 1963 and the work of Export Inspection Council (set up under Export Act) have helped in planned approach towards quality control. The advice of Export Inspection Council is very helpful for pre-shipment inspection of exportable goods.
These organisations have been authorised to issue a “Certificate of Quality” after satisfying themselves that the goods fulfill the minimum standards of quality laid down or that they are of the quality claimed by the exporter.
Essay # 5. Indian Standard Institution (I.S.I. Renamed as B.I.S.) :
To protect the interest of the consumers, Indian Standard Institution is serving in India. In most of the western countries, consumers nave formed their own associations to protect their interest. In some countries these associations, receive official support and guidance.
I.S.I, serves the consumers through Certification Marks Scheme. Under this scheme I.S.I, has been vested with the authority to grant licenses to manufacturers to apply the I.S.I, mark on their products in token of their conformity to the desired Indian Standards.
To control the quality, I.S.I, inspectors carry out sudden inspections of the factories of the licensee. Inspectors may check the incoming raw materials, outgoing finished products and may carry out necessary tests at different levels of control during production.
Thus I.S.I, mark gives guarantee to the purchaser that the goods with this mark have been manufactured under a well-defined system of quality control. From first April 1987 it has been renamed as Bureau of Indian Standards.
Essay # 6. Quality Assurance :
Inspection, quality control and quality assurance:.
Inspection is a process of sorting good from a lot. Whereas Quality Control is aimed at prevention of defects at the very source, relies on effective feedback system, and procedure for corrective action.
In Quality control programme, inspection data are used to take prompt corrective action to check the defects. For this purpose, detailed studies are conducted to find out that from where the defect is originated, and how to prevent it, may it be at manufacturing, design, purchase of raw materials, despatch or storage stage.
Quality Assurance means to provide the necessary confidence to the customer as well as to top management that all concerned are carrying out their job effectively and that the product quality is as per customer’s satisfaction with economy. Quality products can be produced only when all the departments fully participate and co-operate.
Presently, customers demand for higher quality and reliability. It has been felt that even a single defect whatever may be the reasons, result in economic loss.
These reasons have necessitated the need for total quality and reliability programmes to cover wide spectrum of functions and various areas of product design, production system design through various states of material, manufacture and commitment to efficient maintenance and operation of the system as a whole. This is necessary for quality assurance and reliability of the product. This assures the continuous failure free system to the customers.
Responsibilities of Quality Assurance Department :
i. Plan, develop and establish Quality policies.
ii. To assure that products of prescribed specification reaches to the customers.
iii. Regularly evaluate the effectiveness of the Quality programmes.
iv. Conduct studies and investigations related to the quality problems.
v. Liaise with different department, in and outside the organisation.
vi. Organise training programmes on quality.
vii. Plan and coordinate vendor quality surveys and evaluate their results.
viii. Develop Quality assurance system and regularly evaluate its effectiveness.
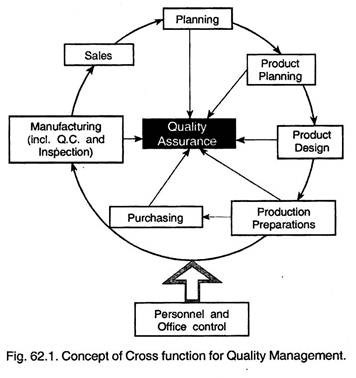
Quality Assurance System :
Quality assurance system should be developed incorporating the following aspects:
i. Formulate the quality control and manufacturing procedures.
ii. Percentage checking be decided.
iii. Procedures and norms for plant performances as regards to quality be developed.
iv. Rejection analysis and immediate feed-back system for corrective action.
v. Prepare a manual for quality assurance.
vi. Formulate plans for quality improvement, quality motivation and quality awareness in the entire organisation.

Essay # 7. Causes of Quality Failures :
Quality failures occur due to various causes, most of them are because of lack of involvement of men concerned with the quality. Studies have indicated that more than 50% of quality failures are due to human errors at various levels, such as understanding of customer’s requirements, manufacturing, inspection, testing, packaging and design etc.
Error affecting quality can be classified into following categories :
(a) Error Due to Inadvertence:
These are due to lack of knowledge of the product, and continue due to lack of information about quality deficiency. Such mistakes can be controlled, if a system for feedback is developed in which quality performance results are analysed in a regular and timely manner.
(b) Errors Due to Lack of Technique:
These errors are due to lack of knowledge, skill, technique etc. In such cases performance of ‘better’ operation are compared with those of ‘poor’ or ‘defect prone’ operations, and the process adopted by them are studied and reasons for errors are investigated.
(c) Willful Errors:
Sometimes quality is compromised due to early delivery schedules, reduction in cost, safety etc.
Reduction of Errors by Improved Motivation :
Quality motivational programmes are developed for getting quality product from the line staff so that they take interest in improving the quality. Motivational programmes are designed after identifying the sources/reasons of failures.
Operators are motivated by designing a campaign to secure alertness, awareness and new actions, and by observing the managers for their behaviours or reactions on any quality problem. Campaign can be launched through mass meetings, quality posters, exhibition of quality deficiencies etc.
Campaign may also invite operators to participate in analysing the causes of defects or the failure on the part of operation and/or systems. Trainings are very helpful in making the operators aware of the technological does and don’ts and the purpose behind each operation.
Essay # 8. Economics of Quality :
The good economic performance is the most essential for survival and growth of any organisation in the highly competitive environment. Therefore, one of the most common objections of every organisation is to attain excellence in its economic performance. The single most important factor which leads to good economic performance is the ‘quality’ of its products or services.
Therefore, in order to achieve economy, quality management system must contribute towards the establishment of customer-oriented quality discipline in the marketing, design, engineering, procurement, production, inspection, testing and other related servicing functions.
Everybody in the organisation must be involved in the production and delivery of quality product or services, consistently to meet the customer needs and satisfaction.
The production of defective output results in the costs of sorting, scrap, rework, dealing with customer complaints, replacement under warranty etc. It is more serious and very difficult to ascertain the cost associated with the loss of goodwill, following the sale of defective or non-conforming products.
Designers of economic models use following costs:
i. Fixed costs of sampling, inspecting, testing and measuring.
ii. Variable cost of sampling, measuring, calculating and plotting each sample value on control charts.
iii. Cost of correcting and assignable cause.
iv. Total loss in profit, when the process is running out of control.
It has been experienced that the savings due to control of poor quality products, better control over the quality of purchased product, use of more economical materials or methods due to their greater reliability, are sometime spectacular.
Quality is a dynamic phenomenon and is being improved continuously with the new developments in technology and management techniques.
Quality and Cost :
Studies have indicated that any reduction in quality results in a reduced level of satisfaction and decrease in customer goodwill toward the producer. This will lead to reduction in return on investment in the long run.
Following are the general principles of quality and cost relationship:
(i) Cost of poor quality are far larger than that had been recognised.
(ii) Appraisal costs are reduced by focussing on preventing errors at the source.
(iii) System be established for reducing the cost rather than reducing the quality.
(iv) By focussing on quality improvement overall, performance of the firm can be improved.
(v) Focus of quality improvement be shifted from product attributes to operational procedure.
Quality Cost (or Costs Associated with Quality) :
Quality cost means cost of poor quality goods or services.
Following are the main quality associated costs:
1. Failure Costs :
(A) Internal Failure Costs:
(i) Scrap and rework cost.
(ii) Costs involved in testing, inspecting and sorting for down-gradation.
(iii) Losses due to avoidable processing.
(iv) Expenditure in failure analysis.
(B) External Failure Costs:
(i) Warranty charges.
(ii) Redressal of complaints.
(iii) Loss of future sales.
(iv). Other expenses on return of materials, failure analysis outside the factory.
2. Appraisal/Detection Costs :
(i) Incoming test and inspection including materials, in-process and final quality sampling.
(ii) Quality audits.
(iii) Equipment calibration.
(iv) Evaluation of performance.
(v) Evaluation of customer satisfaction.
3. Prevention Costs :
(i) Quality planning.
(ii) New product review.
(iii) Process control.
(iv) Training and education.
(v) Process quality planning.
Quality Cost Control :
For the purpose of reducing the cost, when internal and external failure costs are cost down, the appraisal cost and preventive cost may slightly go up. Therefore, it is necessary for optimum balance to reduce failure cost with slight increase in appraisal and preventive cost, with the aim of substantial reduction in total quality cost without compromising with the quality.
Efforts for reducing quality cost must be continuous.
The cost reduction programme must be followed in following stages:
1. Identification of quality cost items.
2. Structuring of quality cost reporting, including related analysis and control, and
3. Maintenance of programme to ensure that the objectives of higher quality at lower cost.
Quality control and quality cost must be directed in such a way so as to provide the firm with major added business value.
Essay # 9. Product Quality Analysis :
It includes:
(i) The various functions to be performed by the manufactured product.
(ii) Life and durability of product.
(iii) Working conditions required during manufacturing.
(iv) Product specifications.
(v) Manufacturing processes and methods.
(vi) Maintenance and installation.
(Quality at level Y is the most economical. A drop of quality to level X reduces the cost by M but also reduces the quality value by N which is greater than M. A rise of quality to level Z increases the quality value by O and increases the cost by P, which is greater than O) — Refer Fig. 62.1.
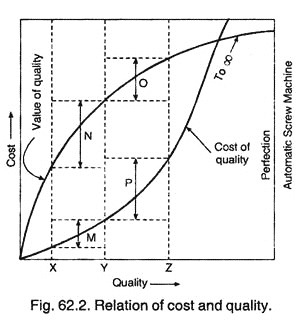
Essay # 10. Quality Planning :
Quality planning is done keeping the company needs and customer needs in view, and a comprehensive quality plan is prepared for implementation in the company.
Quality plan is a document setting out the specific quality practices, resources and activities relevant to a particular product, process, services, contract or project.
Quality planning is a systematic process for:
(i) Identifying customers,
(ii) Discovering customer needs,
(iii) Designing the responsive products,
(iv) Developing the process for creating and delivering the products, and
(v) Transferring the process and its contents to those who will perform the product or service.
Essay # 11. Quality Improvement :
Quality improvement is a structured process for reducing the deficiencies that are present in products, processes and services and/or improving performance whenever there is an opportunity to improve.
Quality problems are of following two types:
1. Sporadic Problems:
A sporadic problem is a sudden adverse change in the status quo, which requires remedy. The variations due to these problems are so significant that they trigger the alarm signals of the control system.
2. Chronic Problems:
Chronic problem is long-standing adverse situation which requires remedy through changing the status quo. For such problems, by adopting ‘continuous improvement concept’ better and better levels of performance can be achieved. These problems occur for a long time, and are often difficult to solve, as they are accepted as inevitable.
Essay # 12. Quality Management System :
A quality management system organises overall activities of the company in such a way that the technical, administrative and human factors affecting the quality of products or services are under control. The quality management system guides the cooperated actions of the people, machines and information to achieve the quality objectives.
1. Activities:
Activities of quality management system are:
(i) Marketing to evaluate customer needs and use requirements.
(ii) Design and engineering to translate the customer needs into product, process and material specifications.
(iii) Purchasing to select the competent vendors who can supply materials, components, sub-assemblies as per specifications.
(iv) Production to ensure that product is produced under controlled conditions in conformance to standards.
(v) Quality assurance to identify appropriate test methods and exercise quality control techniques.
(vi) Shipping to ensure proper packaging, transportation and distribution of material.
(vii) Documentation to maintain system and progress documents at each stage of operation.
(viii) Product development for innovation and improvement based on customer’s feedback.
(ix) Auditing to identify the non-conforming of the system and product, and follow up the corrective actions.
2. Benefits:
(i) To meet the customer requirements by providing quality products or services to satisfy the customer needs.
(ii) Good reputation helps in better marketability of the company’s products and services.
(iii) Confidence is created.
(iv) Consistivity in quality.
(v) Productivity improvement.
(vi) Better financial performance.
(vii) Brings clarity in working.
(viii) Better documentation.
(ix) Better monitoring.
(x) Increases export potential.
(xi) Human resources development.
3. Quality Function:
(i) Marketing and market research.
(ii) Design and product development.
(iii) Procurement.
(iv) Process planning and development.
(v) Production.
(vi) Inspection, testing and examination.
(vii) Packaging and storage.
(viii) Sales and distribution.
(ix) Installation and operation.
(x) Technical assistance and maintenance.
(xi) Disposal after use.
4. Quality and Top Management:
Responsibility for and commitment to quality always belong to the highest level of management.
Following action points are necessary to be adopted by top management to achieve quality objectives of the company:
(i) Define and state quality policy.
(ii) Appoint a management representation.
(iii) Define responsibility and authority.
(iv) Establish an internal verification system.
(v) Establish a quality system.
(vi) Review the functioning of quality system at regular intervals.
5. Installing the Quality System :
(A) Preparations:
(i) Analyse the existing status and identify what needs to be done? Prepare an action plan.
(ii) Develop an organisation structure.
(iii) Develop quality system documentation.
(iv) Prepare the material and machinery resources.
(B) Implementation:
(i) Implement the documented quality system.
(ii) Establish internal quality audit system.
(iii) Monitor, control and stabilise the quality system.
(iv) Hormonise the practices with the standards.
Essay # 13. Role of Top Management towards Quality :
Main roles of the top management towards quality are:
1. Define quality Control. Establish a Quality Council.
2. Establish quality policies.
3. Establish quality goals.
4. Provide the resources.
5. Provide problem-oriented training.
6. Serve on quality improvement teams which address chronic problems.
7. Stimulate improvement.
8. Provide for reward and recognition.
9. Top management is required to:
(a) Develop strategies for quality, and
(b) Provide leadership for implementation of these strategies.
Related Articles:
- Essay on Quality Control | Products | Production Management
- Essay on the Pricing of Products: Top 5 Essays | Marketing Management
- Acceptance Sampling: Meaning, Role and Quality Indices
- Essay on Materials Management: Top 7 Essays | Branches | Management
We use cookies
Privacy overview.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

Importance of medicine quality in achieving universal health coverage
Sachiko ozawa.
1 Division of Practice Advancement and Clinical Education, UNC Eshelman School of Pharmacy, University of North Carolina, Chapel Hill, NC, United States of America
2 Department of Maternal and Child Health, UNC Gillings School of Global Public Health, University of North Carolina, Chapel Hill, NC, United States of America
Colleen R. Higgins
Tatenda t. yemeke, jude i. nwokike.
3 Promoting the Quality of Medicines (PQM) Program, United States Pharmacopeial Convention (USP), Rockville, MD, United States of America
Lawrence Evans
Mustapha hajjou, victor s. pribluda, associated data.
Data for the indicators from the Global Health Observatory can be found at https://apps.who.int/gho/data/node.imr . Data for the World Bank Worldwide Governance Indicators can be found at https://info.worldbank.org/governance/wgi/ . Data on the prevalence of substandard and falsified medicines for each country analyzed here can be found in the Supporting Information of this manuscript.
To assess the importance of ensuring medicine quality in order to achieve universal health coverage (UHC).
We developed a systems map connecting medicines quality assurance systems with UHC goals to illustrate the ensuing impact of quality-assured medicines in the implementation of UHC. The association between UHC and medicine quality was further examined in the context of essential medicines in low- and middle-income countries (LMICs) by analyzing data on reported prevalence of substandard and falsified essential medicines and established indicators for UHC. Finally, we examined the health and economic savings of improving antimalarial quality in four countries in sub-Saharan Africa: the Democratic Republic of the Congo (DRC), Nigeria, Uganda, and Zambia.
A systems perspective demonstrates how quality assurance of medicines supports dimensions of UHC. Across 63 LMICs, the reported prevalence of substandard and falsified essential medicines was found to be negatively associated with both an indicator for coverage of essential services ( p = 0.05) and with an indicator for government effectiveness ( p = 0.04). We estimated that investing in improving the quality of antimalarials by 10% would result in annual savings of $8.3 million in Zambia, $14 million in Uganda, $79 million in two DRC regions, and $598 million in Nigeria, and was more impactful compared to other potential investments we examined. Costs of substandard and falsified antimalarials per malaria case ranged from $7 to $86, while costs per death due to poor-quality antimalarials ranged from $14,000 to $72,000.
Medicines quality assurance systems play a critical role in reaching UHC goals. By ensuring the quality of essential medicines, they help deliver effective treatments that lead to less illness and result in health care savings that can be reinvested towards UHC.
Introduction
The goal of Universal Health Coverage (UHC) is to ensure that all people obtain the health services they need without suffering financial hardship when paying for them [ 1 ]. Sustainable Development Goal 3.8 supports UHC by aiming to achieve “access to safe, effective, quality, and affordable essential medicines and vaccines for all” [ 2 ]. A successful UHC system is a result of the combination of quality health services and expanding coverage of affordable care.
Quality health services cannot be delivered without quality-assured medicines. Ensuring medicine quality is paramount in providing safe and effective health care and reducing overall health care costs. The World Health Organization (WHO) estimates that, on average, 1 in 10 medical products circulating in low- and middle-income countries (LMICs) is substandard or falsified [ 3 ]. WHO defines substandard medicines as “authorized medical products that fail to meet their quality standards, specifications, or both [ 4 ].” Medical products that “deliberately and fraudulently misrepresent their identity, composition, or source” are classified as falsified [ 4 ]. A recent meta-analysis found that 13.6% (95% CI, 11.0–16.3%) of essential medicines in LMICs were either substandard or falsified [ 5 ], with other literature reviews reporting a comparable range [ 3 , 6 – 8 ]. Large percentages of poor-quality antimalarials (19.1%) and antibiotics (12.4%) were found, with the highest reported prevalence observed in Africa (18.7%) and Asia (13.7%) [ 5 ]. Despite the growing evidence of the problem, the importance of quality-assured medicines and the challenge of ensuring their quality is rarely discussed in UHC planning.
The pharmaceutical system operates within a complex health system, where pharmaceutical good governance can be viewed as a component of health systems strengthening necessary to support UHC [ 9 ]. Ensuring the delivery of quality-assured medicines also requires strengthened governance of medicine procurement systems. Medicine procurement for the public sector is typically handled by the government, where purchase and supply chain delivery are shared between medicines and other health service commodities. Medicines are a leading source of health system inefficiency due to the pervasiveness of inappropriate use, variable quality on the market, and high-priced brand name medicines being preferred over generics despite their bioequivalence [ 9 ]. In addition, availability of unregistered medicines presents alternatives for patients to access and use medicines that are neither included in health systems nor covered on insurance plans. Improving financing schemes and strengthening governance of medicine procurement systems is essential to increasing financial protection and access to health services, which are core tenets of UHC [ 10 ]. Increased health care utilization alone will not result in better outcomes if the quality of services is low [ 11 ]. Similarly, the full benefits of expanded coverage may not be realized without also ensuring the quality of covered medicines [ 12 ].
Financing and procurement of medicines play an important role in UHC schemes, where medicine quality needs to be safeguarded [ 9 ]. Globally, a quarter of all health expenditures are spent on medicines [ 13 ]. The Lancet ’s Commission on Essential Medicines Policies estimated that between $13 and $25 per capita (US$77.4 to $151.9 billion) is required to finance a basic package of essential medicines in all LMICs [ 13 ]. However, the majority of low-income countries and over a quarter of middle-income countries spend less [ 13 ]. Moreover, medicines are often paid out-of-pocket in many countries, putting individuals and households at risk of having poor access to treatments and/or becoming poor due to their costs [ 14 – 16 ]. UHC can improve population health by providing quality health services and quality-assured medicines, while preventing catastrophic medical expenditures for the world’s poorest communities.
This study uses systems mapping to conceptually illustrate the benefits of ensuring medicine quality in UHC. We subsequently examined the association between UHC and medicine quality in the context of essential medicines in LMICs using data on substandard and falsified medicines prevalence and UHC indicators. The study also demonstrates the health and economic costs of poor-quality medicines that could be averted by quality assurance interventions where savings could be reinvested in UHC, using antimalarials as a case study.
Materials and methods
Systems map linking medicine quality with uhc.
Systems mapping has been increasingly used in health to help understand indirect effects in complex systems [ 17 – 20 ]. With more detail displaying associations between variables than in a conceptual framework, systems mapping can show how different parts of a system fit together and interact, which make it a useful tool for understanding linkages that are less frequently explored. An advantage of a systems approach, such as systems mapping, is that it takes the entire system into consideration, which can facilitate understanding of indirect effects and unintended consequences [ 17 ]. Taking a systems perspective is particularly useful for medicine quality due to the variety of processes and stakeholders involved, from medicine manufacturing, to purchase and utilization throughout the supply chain. As medicine quality is not commonly emphasized in UHC planning and policies, we use a systems approach to make a conceptual linkage between medicine quality assurance and UHC, allowing us to explore the potential secondary and tertiary effects of medicine quality assurance systems and interventions on UHC.
This research mapped the series of stages where quality assurance processes and interventions are necessary to ensure the quality of medicines reaching patients, illustrating the flow of medicines from manufacturing to utilization. We illustrated the linkages between health systems and health insurance processes, and the resulting benefits to patients. The systems map also illustrates the three dimensions of UHC, demonstrating how medicine quality assurance processes and interventions, and the ensuing benefits, relate to the UHC dimensions [ 10 ]. Finally, we depicted interventions that target quality assurance processes to illustrate some of the key levers required to ensure quality-assured products in UHC. We developed the systems map through an iterative process. First, the study team mapped the system map elements based on existing literature. We then solicited feedback on the map from external stakeholders with expertise in health systems and medicine quality assurance, including experts from the Promoting the Quality of Medicines (PQM) program at the United States Pharmacopeial Convention (USP), United States Agency for International Development (USAID), and academics at the University of North Carolina at Chapel Hill (UNC). We subsequently revised the systems map based on conceptual feedback by including additional content and linkages between the map elements, and made structural revisions to improve comprehension and readability of the map.
Association between UHC and medicine quality
To relate the conceptual linkage between UHC and medicine quality portrayed through our systems map to real world indicators, we investigated the potential association between UHC and medicine quality indicators utilizing existing data. Two indicators from the WHO were used to determine progress towards UHC: an indicator on essential services coverage; and an indicator on the proportion of the population with large household expenditures on health as a share of total household expenditures [ 21 ]. The WHO indicator on coverage of essential services was calculated from 16 tracer indicators measuring average insurance coverage of interventions in areas such as maternal and child health, infectious diseases, and non-communicable diseases [ 22 ]. The WHO indicator for large health expenditures measured the proportion of the population that spends over 10% of their household expenditures on health services.
In addition, two indicators for government effectiveness and regulatory quality were abstracted from the World Governance Indicators of the World Bank [ 23 ]. The government effectiveness measure combined perceptions of the quality of a country’s public and civil services, independence from political pressures, as well as formulation and commitment to policies. This indicator is also used by USAID to help inform strategic decisions and assess a country’s path to self-reliance [ 24 ]. The second regulatory quality indicator from the World Bank assessed the perception of the soundness of a government’s policies and control over private sector practices. We also retrieved under-five mortality rates for each country from the United Nations International Children's Emergency Fund (UNICEF) [ 25 ].
We examined how these indicators were associated with estimated prevalence of poor-quality medicines for each country, using data on reported prevalence of substandard and falsified essential medicines among 63 LMICs previously gathered from a systematic literature review and meta-analysis [ 5 ]. Substandard and falsified medicines prevalence was defined as the number of failed samples over the total number of samples of essential medicines chemically tested and publicly reported within each country ( S1 Table ). We examined the association between country specific reported prevalence of substandard and falsified medicines and the proportion of essential services covered, government effectiveness, regulatory quality, large health expenditures, and under-five mortality. We used simple linear regression models given the small sample size for a multi-country analysis, using prevalence of substandard and falsified medicines as the dependent variable. We conducted visual tests for linearity and Breusch Pagan tests for heteroskadasticity. Gross Domestic Product (GDP) per capita was assessed as a main confounder in each analysis.
Health and economic impact of poor-quality antimalarials
To illustrate the potential health and economic impact that can result from ensuring the quality of antimalarials, we analyzed the current landscape of the impact of poor-quality antimalarials in a case study. Four countries were included in our analysis: the Democratic Republic of the Congo (DRC), Nigeria, Uganda, and Zambia [ 26 – 28 ]. Malaria is associated with high levels of morbidity and mortality in LMICs, and medicines to treat malaria (i.e. antimalarials) are, within the therapies surveyed, one of the medications most commonly tested and found to be of poor quality [ 5 ]. UHC planning in malaria endemic countries would naturally entail decisions on what malaria medications and services to make available, and what populations will receive these benefits.
Estimates of country-specific impact were obtained from the Substandard and Falsified Antimalarial Research Impact (SAFARI) model, an agent-based model we built that simulates malaria disease progression, care seeking, and outcomes for children under age five [ 26 – 28 ]. The SAFARI model was adapted and run separately for each country using country-specific demographic and epidemiological inputs. Methods for the development of the SAFARI model and individual country data are described in detail in other publications [ 26 – 28 ]. We simulated child agents who sought malaria treatment and received either quality-assured or poor-quality antimalarials. Poor-quality antimalarials reduced treatment efficacy and increased the likelihood of agents progressing to severe malaria. The model tracked agent children’s health over the course of treatments leading to hospitalization, neurological sequelae, death, further treatment, or recovery ( S1 Fig ). The costs incurred from these events were recorded as both direct costs to patients and facilities for the treatment of malaria, as well as the indirect productivity losses incurred from time spent seeking treatment, life lived with a neurological disorder caused by severe malaria, and early death due to malaria.
In order to compare the impact of poor-quality antimalarials across countries, we calculated the potential savings in each country if the reported prevalence of substandard and falsified antimalarials were to be reduced by 10%. We estimated this impact by comparing a baseline scenario using the reported prevalence of substandard and falsified antimalarials, to a scenario where 10% more antimalarials were quality-assured. This provided an estimate of the health and economic benefits a health system would experience by investing in quality assurance mechanisms that increased the supply and utilization of quality-assured antimalarials and reduced use of substandard and falsified antimalarials by 10%. The savings are estimated in fewer deaths, fewer hospitalizations, lower costs of care, and productivity gains simulated when poor-quality antimalarials are replaced with quality-assured antimalarials. The economic impact of poor-quality antimalarials was further assessed at an individual level to demonstrate the cost of poor-quality medicines per malaria case, and the cost attributable to substandard and falsified medicines for each additional malaria death, hospitalization, and disability-adjusted life year (DALY). This was calculated by taking the total savings in direct costs and productivity losses averted from reducing the prevalence of substandard and falsified antimalarials by 10% and dividing it by estimated reductions in deaths, hospitalizations, and DALYs.
To contextualize the impact of improving medicine quality, we compared a scenario in which no poor-quality antimalarials were present, to other options that governments may consider in reducing malaria burden. We simulated two additional interventions: a scenario in which there were no stockouts of antimalarials, and one in which only Artemisinin-based Combination Therapies (ACTs) were taken as first-line therapy. Moreover, low-quality anti-infectives can induce further costs to health systems and society by contributing to the development of antimicrobial resistance. We simulated the impact of widespread ACT resistance in a hypothetical scenario where the efficacy of ACTs were reduced to efficacy of other malaria treatments, impacting the duration and severity of malarial illness among children.
Fig 1 presents the systems map of associations between medicine quality and essential components of UHC. We first mapped the essential steps and processes involved in order for beneficiaries to receive quality-assured medicines (blue ovals). This illustrates the movement of medicines from manufacturing, procurement, and through the supply chain to reach health facilities, after which beneficiaries who seek health care can obtain and utilize medicines. We then mapped the resulting benefits–when beneficiaries utilize quality-assured medicines, as opposed to no medicines or poor-quality medicines, beneficiaries can be healthier with a shorter duration of illness and milder symptoms, thus needing less additional health care and having the possibility to return to work earlier (pink rectangles) [ 26 – 29 ]. For example, using a substandard medicine with inadequate amounts of active pharmaceutical ingredients could add days to recovery time or be completely ineffective, requiring a patient to seek additional care or suffer a longer duration of illness without proper treatment, increasing the severity of disease [ 26 – 28 ]. With quality-assured medicines, beneficiaries may be cured of illness, and may even avert disability or death [ 26 – 28 ].

Quality-assured medicines bring further benefits (orange rectangles). Appropriate use of quality-assured medicines contributes to maintaining medicine efficacy by delaying the development of antimicrobial resistance [ 29 , 30 ]. When beneficiaries require less health care because they have access and utilize quality-assured medicines, they decrease the risk of becoming poor due to additional expenses for medicines and health care [ 31 ]. Healthier beneficiaries are also more productive in society, resulting in productivity gains. Ensuring medicine quality thus contributes to the overall goal of UHC to ensure health care access without suffering financial hardship when paying for them.
Averting the need for additional health care when beneficiaries are healthier with quality-assured medicines can trigger a number of health system mechanisms (purple rectangles). When beneficiaries require less health care, the health system is less burdened and the quality of health services could improve [ 32 ]. Healthier beneficiaries utilizing quality-assured medicines can also trigger UHC mechanisms (green rectangles). As beneficiaries require less care, health care costs for health insurers decrease, and those savings could be reinvested back into the system [ 33 ]. Health insurers can reinvest these savings into three dimensions of UHC–as illustrated by the cube in Fig 1 –by covering more medicines and services, covering more beneficiaries, or covering more costs incurred by individuals to reduce cost-sharing for medicines and health services.
Regulatory oversight and quality assurance mechanisms throughout the supply chain reinforce the system that ensures patients can obtain and use quality-assured medicines. These are the backbone of the key interventions (yellow rectangles) within the system, broken down to be those affecting medicine delivery, administration, or UHC. These interventions include best practices (e.g. good manufacturing, distribution, and storage practices), policies (e.g. for medicine registration, pre-qualification requirements, and procurement), regulations (e.g. post-market surveillance, including customs screening and inspections), and education (e.g. health education on medicines and advocacy campaigns) [ 34 – 36 ]. Within UHC, we include the role of formulary management, where bioequivalence studies can trigger changes in formularies [ 37 , 38 ]. This can subsequently change insurance coverage for generic medicines, affecting prescribing practices and costs to health insurers and beneficiaries [ 39 – 42 ]. Getting quality-assured generic medicines on the preferred list of formularies is an example of a medicine quality assurance intervention that can save costs to health insurers and affect UHC [ 41 , 42 ].
The systems map connects the mechanisms to build effective medicines quality assurance systems with the health and economic benefits that quality-assured medicines can bring to UHC, highlighting the need for investments in strengthening these systems to achieve UHC.
We sought evidence to further describe the linkages between medicine quality and UHC by examining the association between existing data on the reported prevalence of substandard and falsified medicines ( S1 Table ) and UHC indicators among 63 LMICs ( Table 1 ). Each individual indicator was first regressed on prevalence of substandard and falsified medicines while controlling for GDP per capita. This confounder was only found to yield a strong relationship when included with the indicator for large health expenditures. Visual tests for linearity were conducted and yielded no grounds to reject the linearity assumption. Breusch Pagan tests for heteroskadasticity were performed on each model, each resulting in insignificant p-values (>0.05). With no evidence to reject homoskedasticity, we present the results of simple linear regressions.
GDP: gross domestic product
1 Data from the most recent year available were used for each country for each indicator: coverage of essential services: 2015; large household expenditure: various years between 1998 and 2015; government effectiveness: 2017; regulatory quality: 2017; under-five mortality rate: 2017.
2 Data were retrieved from the Global Health Observatory repository
3 Measured on a linear scale between -2.5 and 2.5
Across countries, we found that coverage of essential services by health insurance schemes was found to be negatively associated with reported prevalence of poor-quality medicines ( p value 0.054; Fig 2B ). The direction of the relationship could indicate that countries that have higher coverage of essential services by health insurance were likely to have lower reported prevalence of poor-quality medicines. However, the effect size was small and not significant at a 0.05 alpha level. Countries with stronger capacity to provide greater health insurance coverage could be in a better position to effectively regulate the medicines supply chain, thus preventing the availability of poor-quality medicines. Coverage of essential services has a further benefit when combined with medicines quality through streamlined, well-regulated processes. This mitigates the need to seek potentially compromised medicines from the informal system.

Regulatory quality (C) and government effectiveness (D) are measured on a linear scale between -2.5 and 2.5.
A negative association was also found between reported prevalence of substandard and falsified medicines and indicators for government effectiveness and regulatory quality, where higher reported prevalence of poor-quality medicines was associated with lower governmental effectiveness ( p value 0.048; Fig 2C ) and lower regulatory quality ( p value 0.046; Fig 2D ). The direction of this association reflects the expected inverse relationship between these parameters, where an increase in regulatory quality or government effectiveness scores was associated with a decrease in prevalence of poor-quality medicines, though with a small effect size. In addition, reported prevalence of substandard and falsified medicines was shown to have a slightly positive but not significant correlation with under-five mortality ( p value 0.056; Fig 2A ).
No significant association was found between large health expenditures and reported prevalence of substandard and falsified medicines ( p value 0.335) after controlling for GDP per capita. This lack of observable association does not indicate that poor-quality medicines do not result in larger household expenditures. It instead suggests that, given the data available, poor-quality medicines may not be the main driver of enhanced expenditures where other factors are at play. Furthermore, data on catastrophic health spending was not available for all countries, resulting in a lower number of observations for this analysis compared to others (51 compared to 63 countries).
Table 2 summarizes the results of the SAFARI model, showing the monetary and health savings that could result if the prevalence of poor-quality antimalarials in each modeled country were reduced by 10%. The reported prevalence of substandard and falsified antimalarials in the four countries ranged from 10.3% in Zambia to 22% in Uganda [ 5 ]. By improving the quality of antimalarials through removing 1 in 10 poor-quality ones, we simulated fewer deaths annually– 8,255 averted in Nigeria, 507 in Uganda, 208 in Zambia, and 667 and 4,764 in the Kinshasa and Katanga regions in DRC. This means that ensuring that 10% more antimalarials are quality-assured can result in 22 fewer deaths per day in Nigeria and 3 fewer deaths a week in Zambia.
GDP: Gross domestic product, DRC: Democratic Republic of the Congo, SF: substandard and falsified, DALYs: disability adjusted life years
1 Table results were calculated by comparing baseline results for the health and economic burden of malaria to a scenario in which only high quality antimalarials were available.
2 Costs are presented in 2017 USD. Lifetime costs were discounted at 3%.
3 DALY estimates were not included in the DRC version of the SAFARI model.
4 Estimated by dividing the costs of substandard and falsified antimalarials by the number of malaria cases in each country.
We estimated that each country would experience substantial savings by improving the quality of antimalarials for children. By replacing 10% of the current substandard and falsified antimalarials with quality-assured antimalarials, we simulated savings of $8.3 million in Zambia, $14 million in Uganda, $598 million in Nigeria, $68 million in Katanga and $11 million in Kinshasa. Direct costs incurred by public facilities and by patients were estimated to contribute between 3.3% (Nigeria) and 23.8% (Kinshasa) of total savings ($813 thousand to $20 million) across countries. The model results suggest that assuring the quality of antimalarials would result in considerable savings in productivity, largely composed of the potential economic productivity that a child would contribute over a lifetime if death or disability due to poor-quality antimalarials were averted. We estimated that reducing poor-quality antimalarials by 10% would reduce productivity losses annually by $7.5 million in Zambia and $578 million in Nigeria.
We estimated that substandard and falsified antimalarials contribute between $7 per malaria case in Zambia, and $86 per malaria case in the Katanga region in DRC. Each additional death due to poor quality antimalarials approximately cost between $14,300 per death in Katanga, and $72,500 per death in Nigeria. Each additional pediatric malaria hospitalization attributable to substandard and falsified medicines cost between $584 per hospitalization in Kinshasa, and $26,800 per hospitalization in Nigeria. The costs at an individual level are heavily influenced by the cost of care, amount of care seeking, number of malaria cases, and the GDP per capita in each country.
Fig 3 compares the potential economic impact of three simulated interventions (no substandard and falsified antimalarials, no stockouts, and replacing all antimalarials with ACT treatments) and one negative scenario (emergence of antimalarial resistance) in each country. Among the different investments that governments could make towards malaria burden reduction, ensuring the quality of all antimalarials was found to be the most impactful course in DRC, Uganda, and Zambia, while in Nigeria it was second to preventing stockouts. In addition, the negative impact of antimalarial resistance, a potential consequence of recurrent use of poor-quality medicines, could have a substantial negative impact, costing countries nearly $10 million in Zambia to $839 million in Nigeria.

Nigeria is depicted separately due to scale. Costs are in 2017 USD.
Despite the many differences between countries, this study consistently observed that substandard and falsified essential medicines were associated with key components of UHC such as coverage of essential medicines, government effectiveness, and regulatory quality. Using systems mapping, we illustrated the conceptual link between UHC and regulatory measures to improve the quality of medicines. With the conceptual mapping as guidance, we demonstrated that a relationship can be observed between medicine quality and UHC indicators within existing data. We then showed the importance to a health system of investing in the quality of medicines, and the potential savings that could be reinvested in UHC dimensions (i.e. covering more beneficiaries in health insurance schemes, covering more services, or reducing out-of-pocket expenditures for patients). We show that improving antimalarial quality would not only save millions by improving outcomes—it can also help avert the immense costs associated with potential development of drug resistance [ 43 ]. Using high-quality medicines results in shorter and less severe illness, leading to fewer fatalities and less opportunity for the spread of contagious diseases. With less time spent sick, and less money spent on care resulting from poor-quality medicines, patients can be more economically productive and less likely to use up their resources to pay for health care. Moreover, improving medicine quality can have a dual impact by reducing inequities, since providing only high-quality antimalarials has been shown to have greater benefits among poor and rural populations [ 44 ].
Interventions aimed at ensuring medicine quality, such as good practices in manufacturing and distribution, or establishing an essential medicines list, strengthen institutions necessary for maintaining continued access to quality-assured medicines and quality services [ 45 – 48 ]. UHC financing schemes can create incentives for providers to prescribe and dispense medicines procured through systems or processes that ensure their quality. As coverage of services and populations increases, this system may require continuous strengthening to satisfy the increased demand for quality-assured medicines. Some quality assurance and regulatory interventions for medicines could also result in cost savings to health insurers, facilitating greater coverage of essential services. For example, data from bioequivalence studies of quality-assured generic medicines may guide and facilitate registration and subsequent procurement of cheaper generics [ 37 , 38 ], resulting in cost savings.
Recent literature has drawn attention to potential negative unintended consequences in UHC implementation, where incentives for procuring cheaper medicines to meet increased demand under UHC can lead to arbitrage opportunities and proliferation of suppliers of cheaper, non-quality-assured medicines [ 49 , 50 ]. Therefore, ensuring medicine quality within UHC requires planning and continued attention to regulatory processes such as good registration practices and post-market surveillance strategies, as well as robust quality assurance mechanisms. There are also concerns that the costs of medicine quality assurance activities could result in higher medicine prices, making them less affordable. However, other literature has argued that it is feasible to attain UHC with affordable quality medicines through a mix of quality assurance interventions and incentive schemes [ 51 ], citing successes such as the WHO prequalification program (WHO PQP) and the Medicines Patent Pool in increasing access to affordable, quality-assured medicines [ 52 , 53 ]. Our study builds upon this prior literature by illustrating the multiple conceptual linkages and connections between medicines quality assurance systems and UHC processes. Further, our study shows how quality-assured medicines can improve health outcomes and reduce the financial costs of implementing UHC by preventing costs associated with utilization of poor-quality medicines. Hence, evaluations of the potential costs of quality assurance systems in UHC schemes, including possible higher medicine costs, should include the value of the averted health and economic costs of substandard and falsified medicines that would ensue if there are no investments in quality assurance systems. Accrued savings in the health system from utilizing quality-assured medicines could then be reinvested in strengthening various UHC dimensions, including reducing out-of-pocket expenses for patients and making medicines more affordable.
Our analysis has a number of limitations. Agent-based models provide results that depend on the quality of data inputs. Because data on substandard and falsified medicines, care-seeking, and costs for malaria in LMICs are limited, we performed extensive literature searches and analysis of the most recent quality data for our inputs. Epidemiological data and cost inputs were probabilistically ranged to account for uncertainty in outcomes [ 26 ]. In addition, our data analysis was limited by data availability across countries where combined indicators were only available for 51 to 63 countries. Given this small number of data points, many confounders could not be controlled for, including country differences in underlying wealth, wealth distribution, population composition, distribution of health services, and political systems. Although the prevalence of substandard and falsified medicines was searched systematically, the prevalence we report were based on a limited number of medicine quality studies performed in each country. In addition, our literature analysis does not include data generated by medicines regulatory authorities through their own post-marketing surveillance processes, which are not readily made publically available.
Despite these limitations, this study contributes to emerging analyses regarding the role that medicines quality assurance systems play in UHC, and conceptually highlights the importance of ensuring medicine quality in order to achieve UHC goals. Our systems map can be used as a conceptual and advocacy tool to make a case for the importance of investing in medicine quality assurance with UHC among a broad range of stakeholders. The system mapping approach can also be adapted and customized to explore medicine quality assurance and UHC within local specific contexts. Cost-savings implications of ensuring medicine quality and providing UHC can assist policy makers, governments, civil society, and other stakeholders, when prioritizing health interventions and engaging with health insurers and other financing agencies. In addition, this analysis highlights the critical role that Medicines Regulatory Authorities play in UHC through several key functions, such as enforcement of good practices for manufacturing, registration, distribution and storage; establishing pharmacovigilance and medicines quality post-marketing surveillance programs; and enforcing regulatory actions to withdraw poor-quality medicines from circulation. Thus, ensuring the use of quality-assured medicines is critical in UHC and investing in medicine quality assurance systems and interventions is vital for the long-term success of UHC planning in LMICs.
Supporting information
Funding statement.
Research reported in this publication was supported by the United States Agency for International Development (USAID) through the Promoting the Quality of Medicines (PQM) Program via GHS-A-00-09-00003-00. Members of the PQM program are co-authors on this manuscript and contributed to its preparation and the analysis within.
Data Availability
The Key Dimensions of Quality Essay
Introduction.
Over time, several debates have been conducted by various scholars to aptly define quality. In essence, quality is a simple concept. However, there are many dynamics and aspects that are variably used by scholars to pin-point the exact meaning of quality. It is for this reason that quality means different things to different people and countless of debates are still ongoing to find an aptly fitting definition.
For the purpose of this paper, quality will be generally, yet representatively inclusive, defined as the ability of a product or service to meet the expected needs of its clients (Pereira, 2008). In this definition, several vital aspects such as performance, perceived quality, value and aesthetic value have to be put into consideration.
It is with this—and many other reasons in mind—that this paper seeks to define the key aspects of quality. In doing so, fitting examples from the field of Human Resource Management (HRM) will be used to exemplify each of the defined dimensions
Performance
According to Seidel et al. (2009), performance simply refers to the assessment of whether a product or service has performed as it is purposed to do. In assessing performance, an evaluation has to be done on the strengths and weaknesses of the product or service then an overall rating is done as to whether it performed or not.
For example, in HR Management, performance can be measured by assessing whether the workers of a company served its clients appropriately. From a managerial perspective, performance majorly entails profitability by the company, satisfaction of clients and satisfaction of the workers (Pilbeam & Corbridge, 2006).
Just like the name suggests, features refer to the specific attributes, traits or characteristics of a product or service that enables it to serve its intended purpose (Gibbs, 2010). Other than enabling a product or service to serve its purpose in terms of performance, features also play the irreplaceable role of defining a particular product or service in aspects such as brand management.
A good example of such is the difference in features of phones from companies like Nokia, Apple and Samsung. For efficacy in HR Management, there has to be close coordination between the managers, workers and clients so that products and services are tailored with the appropriate features that meet the needs of the target market (Seidel et al., 2009).
Reliability
With regards to HR Management, reliability emphasizes on the trustworthiness or dependability of a product of service over a given period of time. In assessing the quality, a product or service must be able to perform as is required over a given period of time while intermittently being able to avoid unnecessary brake-downs, failures and down-times (Gibbs, 2010).
For example, reliability of a HR manager can be assessed by his ability to perform his duties in a dependable way over a particular duration. Just like features, reliability goes a long way in portraying a company image thus should be keenly considered by those in charge of quality issues in the company.
Conformance
This dimension of quality answers the question; does a product or service conform to the required features or specifications? Conformance can be based on various aspects of quality. For example, some products or services are specifically designed to conform to reliability whereas others are simply for performance. In HR Management, conformance is sometimes used to refer to the ability of a product or service to match up with the requirements in the market (Bratton & Gold, 2007).
For instance, based on the ever increasing relevance and importance of IT (Information and Technology) issues in today’s business-oriented world; most recruitment personnel in companies have made it mandatory for the prospective workers to have basic knowledge in IT. In this regard, the need to have IT-compliant workers is viewed as a form of compliance to the elemental importance of knowledge in IT.
In most occasions, durability is viewed as a subset of reliability. This is majorly based on the relevance of the aspect of time. However, unlike reliability which stresses more-or-less on performance, durability is chiefly concerned with the quality of a product or service based on its ability to function effectively and last over a long period of time. Additionally, durability strongly considers issues such as tare and ware of a product.
The durability of a product hugely determines crucial aspects of quality such as warranty. As a general rule, products or services that are durable tend to have longer warranties than those with less durability. Based on such knowledge on durability, HR Managers can easily tailor their products and services to be durable so as to attract and retain as many customers as possible (Pilbeam & Corbridge, 2006).
Serviceability
Serviceability simply refers to the ease in maintenance of a product or service. Most products that are reliable and durable witness less functionality and performance problems thus easily serviceable. It is worth noting that, based on their simplistic features or components, some products are easy to maintain.
For example, vehicles tend to be more difficult to service when compared to motorcycles. This ease or difficulty of product maintenance is also referred to as serviceability. For HR Managers, serviceability is not only vital in terms of maintenance and repair of products for performance of these products but it also helps in cost-efficacy since well maintained products are less faulty thus less need to incur costs in repairing or maintaining them.
Aesthetics refer to the outward beauty of a product or service. In today’s world, beauty plays an important role in marketing of products or services. It is based on this reason that HR Managers in most, if not all, companies usually strive to create aesthetic products and services attract clients (Gibbs, 2010). Tare and ware often destroys the aesthetic value of a product. It is during such instances that aspects of quality such as serviceability come in handy in-terms of conducting maintenance and repairs.
Perceived Quality
The manner in which human beings perceive things greatly determines how they get to deal with day-to-day endeavors. Aptly put in the words of Pereira (2008), “perception is reality”, in other words, our perceptions determine our realities. Needless to say, different people perceive different things in different ways based on differences in cultures, beliefs or even religious backgrounds. These differences hugely determine how these people interpret the quality of certain products or services.
For example, conservative societies tend to appreciate “decent” clothing like full dresses for women. In the permissive societies, decency plays a very minor role and that’s the reason women in such societies can get to walk in the streets with very exposing clothes in the name of fashion. Consequently, HR Managers should preliminarily be aware of the perceptions of their clients. Once they have firm background knowledge on the people targeted by their products and services, it will be relatively easy to serve them fittingly.
In most instances, value and quality are often used synonymously and interchangeably since they tend to refer to the same thing. The value of a product or service is usually based on almost all the dimensions of quality that have been discussed above.
The distinguishing feature that separates value from quality is that, in a good number of instances, value hugely relies on individual perceptions and the sentimental or emotional element of products or services. On the other hand, quality tends to be a combination of all the aforementioned aspects balanced in a more-or-less universally accepted way.
Over time, HR Managers have been able to study the perceptions and values of individuals or groups of people in regards to quality and it has been found that the value of a product can be easily created by market controllers when compared to other aspects such as durability. Through such mechanisms, products or services whose quality is spiraling downwards can be easily rejuvenated by these HR Managers (Adams & Goldbard, 2002).
From the discussions above, it is eminently evident that all the key dimensions discussed herein are interrelated in one way or another. The success of one dimension hugely determines the success of the other. So, if overall efficacy is to be witnessed in terms of quality of products or services, HR Managers, and other concerned parties, must find a way of ensuring that all these dimensions of quality are equally facilitated.
Adams, D., & Goldbard, A. (2002). Community, culture and globalization . New York: Rockefeller Foundation.
Bratton, J., & Gold, J. (2007). Human resource management: theory and practice (4 th ed.). Basingstoke: Palgrave Macmillan.
Gibbs, G. (2010). Dimensions of quality . Web.
Pereira, R. (2008). 8 dimensions of quality. Web.
Pilbeam, S., & Corbridge, M. (2006). People resourcing: contemporary hrm in practice . London, UK: Prentice Hall.
Seidel, S., Tishman, S., Winner, E., Hetland, L., & Palmer, P. (2009). The qualities of quality: Understanding excellence in arts education . Web.
- Chicago (A-D)
- Chicago (N-B)
IvyPanda. (2020, May 25). The Key Dimensions of Quality. https://ivypanda.com/essays/the-key-dimensions-of-quality-essay/
"The Key Dimensions of Quality." IvyPanda , 25 May 2020, ivypanda.com/essays/the-key-dimensions-of-quality-essay/.
IvyPanda . (2020) 'The Key Dimensions of Quality'. 25 May.
IvyPanda . 2020. "The Key Dimensions of Quality." May 25, 2020. https://ivypanda.com/essays/the-key-dimensions-of-quality-essay/.
1. IvyPanda . "The Key Dimensions of Quality." May 25, 2020. https://ivypanda.com/essays/the-key-dimensions-of-quality-essay/.
Bibliography
IvyPanda . "The Key Dimensions of Quality." May 25, 2020. https://ivypanda.com/essays/the-key-dimensions-of-quality-essay/.
- Firm's Management Priorities: Quality Improvement
- Various dimensions of product and service quality
- Conference Panelist's, Coordinator's, Moderator's Roles
- Sulfate Attack: Durability of Concrete Structures
- HR Management: Role, Competencies, and Functions
- The MiniDisc's Durability, Portability and Convenience
- Strategic HR Management: Achieving Sustained Competitive Advantage
- HR Management Strategies at the WHO
- Durable Vinyl Siding Corporation's Procurement Costs
- Introduction to Strategic HR Management and Strategic Business Objectives
- Capital structure
- Start up Business in Saudi Arabia: Challenges of Finding Finance.
- Cirque du Soleil
- High Turnover of Nurses on General Surgical Floor
- English Furniture Market
Home — Essay Samples — Education — School Uniform — The Importance of Regulations in Education
The Importance of Regulations in Education
- Categories: School Uniform
About this sample

Words: 504 |
Published: Mar 20, 2024
Words: 504 | Page: 1 | 3 min read
Table of contents
Ensuring quality and standards, protecting students and teachers, accountability and transparency.

Cite this Essay
Let us write you an essay from scratch
- 450+ experts on 30 subjects ready to help
- Custom essay delivered in as few as 3 hours
Get high-quality help

Dr Jacklynne
Verified writer
- Expert in: Education

+ 120 experts online
By clicking “Check Writers’ Offers”, you agree to our terms of service and privacy policy . We’ll occasionally send you promo and account related email
No need to pay just yet!
Related Essays
5 pages / 2090 words
1 pages / 616 words
1 pages / 557 words
3 pages / 1514 words
Remember! This is just a sample.
You can get your custom paper by one of our expert writers.
121 writers online
Still can’t find what you need?
Browse our vast selection of original essay samples, each expertly formatted and styled
Related Essays on School Uniform
The debate over whether students should wear uniforms in schools has been a long-standing one. In this essay, we will explore the reasons why students should not wear uniforms, focusing on how uniforms can limit students' [...]
Alston K.G, Staden van J.G, Pretorius J.L. 2003. “The constitutional right to freedom of expression: How enforceable are school dress codes?”. SAJE .23(3):163-167. . Available at: http://dx.doi.org/10.4314/pelj.v14i6.
Maarman, J., & Lamont-Mbawuli, M. (2017). Learner mental health: A conceptual framework towards a successful curriculum reform in South African schools. South African Journal of Childhood Education, 7(1), a546.Biegel, D. E. [...]
School uniforms have long been a topic of debate in the education world. Advocates argue that they bring numerous benefits to students and schools, while critics raise concerns about limiting individual expression and [...]
Introduction to the debate on whether students should wear uniforms Mention of the reasons for and against school uniforms Discussion of the role of uniforms in enhancing school security Potential risks of [...]
Have you ever felt restricted by a dress code that dictates what you can and cannot wear? Many schools and workplaces enforce strict dress codes in the name of professionalism or modesty, but is this really necessary? In this [...]
Related Topics
By clicking “Send”, you agree to our Terms of service and Privacy statement . We will occasionally send you account related emails.
Where do you want us to send this sample?
By clicking “Continue”, you agree to our terms of service and privacy policy.
Be careful. This essay is not unique
This essay was donated by a student and is likely to have been used and submitted before
Download this Sample
Free samples may contain mistakes and not unique parts
Sorry, we could not paraphrase this essay. Our professional writers can rewrite it and get you a unique paper.
Please check your inbox.
We can write you a custom essay that will follow your exact instructions and meet the deadlines. Let's fix your grades together!
Get Your Personalized Essay in 3 Hours or Less!
We use cookies to personalyze your web-site experience. By continuing we’ll assume you board with our cookie policy .
- Instructions Followed To The Letter
- Deadlines Met At Every Stage
- Unique And Plagiarism Free

IMAGES
VIDEO
COMMENTS
As improvement practice and research begin to come of age, Mary Dixon-Woods considers the key areas that need attention if we are to reap their benefits. In the NHS, as in health systems worldwide, patients are exposed to risks of avoidable harm 1 and unwarranted variations in quality. 2 3 4 But too often, problems in the quality and safety of ...
Quality Improvement (QI) has being define by the Institute of Medicine (IOM) the way to which patient care services increase the possibility of desired health outcomes and are consistent with the professional knowledge. "The roots of the quality improvement movement can be traced back to the work of epic figures such as Ignaz Semmelweis, the ...
1) Quality of design. Quality of design measure the functionality of a product or services. It is the decision of designer to include or exclude certain production features. The customer really measures quality through appearance, operation and reliability. 2) Quality of performance.
Why Quality Matters. September 10, 2021. By Bethany Simunich, Ph.D., QM Vice President of Innovation and Research. This piece was written as a response to a growing sense that fears related to loss of academic freedom and creativity are being fueled by misinformation, including articles and blog posts written by individuals who purport to be ...
The Indian standard of quality must be met before the BIS certify any product. The objective of the scheme is to provide Third Party Guarantee of safety, reliability and quality of products to the consumers (BIS, 2007). The BIS is supported by legislation by Act of the Indian Parliament.
Costs and economic evaluations of quality improvement collaboratives in healthcare: A systematic review. BMC Health Services Research, 20 (1). This essay, "The Importance of Quality Healthcare" is published exclusively on IvyPanda's free essay examples database. You can use it for research and reference purposes to write your own paper.
6.1. General conclusions. Quality 2030 consists of five collectively designed themes for future QM research and practice: (a) systems perspectives applied, (b) stability in change, (c) models for smart self-organising, (d) integrating sustainable development, and (e) higher purpose as QM booster.
In quality management, change of organizational culture is the most important point to consider since it waylays quality into planning (Evans & Lindsay, 1995, p. 59). There is no point of addressing only those issues that can be easily corrected and then claim that six-sigma approach to quality management is viable.
1. Introduction. Quality is a multi-faceted and intangible construct (Charantimath, Citation 2011; Zhang, Citation 2001) that has been subject to many interpretations and perspectives in our everyday life, in academia, as well as in industry and the public domain.In industry, most organisations have well-established quality departments (Sousa & Voss, Citation 2002), but the method of ...
As improvement practice and research begin to come of age, Mary Dixon-Woods considers the key areas that need attention if we are to reap their benefits In the NHS, as in health systems worldwide, patients are exposed to risks of avoidable harm 1 and unwarranted variations in quality.234 But too often, problems in the quality and safety of healthcare are merely described, even "admired,"5 ...
The application of quality in business has been responsible for the development of new organizations and even industries. The connotation of quality in business focuses on eliminating errors throughout the operations, and producing at the ideal quality level desired by customers, while considering pricing and costs. Quality may not sound as ...
Therefore, these actions and the resources involved constitute the quality management system (QMS), governed by the ISO 9001 standard. The importance of quality management for the company . The quality management system represents a pillar of growth for the company. The reason is simple: its effectiveness is largely based on this system.
Quality reflects on your company's reputation. The growing importance of social media means that customers and prospects can easily share both favorable opinions and criticism of your product ...
After reading this essay you will learn about:- 1. Meaning and Definitions of Quality Control 2. Quality Control Organisation 3. Advantages of Quality Control 4. Quality Control for Export 5. Indian Standard Institution 6. Quality Assurance 7. Causes of Quality Failures 8. Economics of Quality 9. Product Quality Analysis 10. Quality Planning 11. Quality Improvement 12. Quality Management ...
Large percentages of poor-quality antimalarials (19.1%) and antibiotics (12.4%) were found, with the highest reported prevalence observed in Africa (18.7%) and Asia (13.7%) . Despite the growing evidence of the problem, the importance of quality-assured medicines and the challenge of ensuring their quality is rarely discussed in UHC planning.
The Importance Of Quality Management. With the development of science and technology and the needs of market competition, more and more companies begin to pay attention to quality management. In the process of enterprises' development, the quality is the key to success or failure of any companies (Mile, 2004).
Short Essay on Quality. The literal meaning of quality means the degree of excellence. It simply means that whenever we compare any 2 things for examples, when we go to buy something, we always compare those things. ... Such is the importance of quality in the chemical laboratories and the respected manufacturing companies. Quality assurance is ...
Seidel, S., Tishman, S., Winner, E., Hetland, L., & Palmer, P. (2009). The qualities of quality: Understanding excellence in arts education. Web. This essay, "The Key Dimensions of Quality" is published exclusively on IvyPanda's free essay examples database. You can use it for research and reference purposes to write your own paper.
The concept of quality has existed for many years, though its meaning has changed and evolved over time. In the early twentieth century, quality management meant inspecting products to ensure that they met specifications. In the 1940s, during World War II, quality became more statistical in nature.
Quality of the product is one of the most important characteristics that determine demand for the product and is of strategic importance for the economic health of companies as well as countries. In particular, quality affects a firm in the following ways. 1. Improved profitability: Improved quality results in improved profitability due to ...
Quality education reduces poverty. If all children in low-income countries left school able to read, global poverty would fall by 12 %. 1 If all adults completed secondary education, 420 million people could be lifted out of poverty, reducing the total number of poor people by more than half worldwide. 2 An extra year of school can increase men's income by at least 10 %, women's income by at ...
Importance Of Quality Over Quantity Essay. Isaac Newton once said, "We build too many walls and not enough bridges.". Had someone presented this quote to my young, tween, self, my life might have been very different. The saying "quality over quantity" was one that governed my younger years, voluntarily at certain times, and at others ...
Access to quality education positively impacts children as they get older by providing them with new opportunities and improving a country's economy. The first impact of access to quality education is that it opens up new opportunities for kids as they grow older. If a child learns information when they are young, it would be easier for them ...
However, the quality of education can vary significantly depending on the regulations and standards in place. Regulations in education are crucial for ensuring that students receive a high-quality and equitable education, and for maintaining the integrity and reputation of educational institutions. ... This essay will explore the importance of ...