America’s Most Popular Drug Has a Puzzling Side Effect. We Finally Know Why.
The reason statins can make your muscles sore or weak was unclear—until scientists accidentally stumbled upon an answer.
Statins, one of the most extensively studied drugs on the planet, taken by tens of millions of Americans alone, have long had a perplexing side effect. Many patients—some 5 percent in clinical trials, and up to 30 percent in observational studies—experience sore and achy muscles, especially in the upper arms and legs. A much smaller proportion, less than 1 percent, develop muscle weakness or myopathy severe enough that they find it hard to “climb stairs, get up from a sofa, get up from the toilet,” says Robert Rosenson, a cardiologist at Mount Sinai. He’s had patients fall on the street because they couldn’t lift their leg over a curb.
But why should an anticholesterol drug weaken muscles in the arms and legs? Recently, two groups of scientists stumbled upon an answer. They didn’t set out to study statins. They weren’t studying cholesterol at all. They were hunting for genes behind a rare disease called limb girdle muscle dystrophy, in which muscles of the upper arms and legs—sound familiar?—become weak and waste away. After both teams tracked the disease through a handful of families in the U.S. and a Bedouin family in Israel , their suspicions separately landed on mutations in a gene encoding a particularly intriguing enzyme.
The enzyme is known as HMG-CoA reductase, and to doctors, it is not obscure. It is, in fact, the very enzyme that statins block in the process of halting cholesterol production. And so, the answers to two mysteries suddenly became clear at once: Dysfunction in this enzyme causes muscle weakness from both limb girdle muscular dystrophy and statins.
This connection between a rare disease and a common drug stunned the researchers. “It seemed too good to be true,” says Joel Morales-Rosado, a pathologist who worked on one of the studies as a postdoctoral researcher at the Mayo Clinic. “One of the first things you learn in medical school is association between statins and myopathy.” Now the answer as to why— along with a potential treatment for it—has emerged from the DNA of just a few patients living with a seemingly unrelated genetic disease.
The first patient the Mayo team studied had been showing signs of limb girdle muscular dystrophy since he was a child, and his symptoms worsened over time until he lost the ability to walk or breathe with ease. (The disease can also affect large muscles in the torso.) Now in his 30s, he wanted to know the genetic cause of his disease before having children and potentially passing it on to them. His two brothers had the disease as well. So the team looked for genes in which all three brothers had mutations in both copies, which is how they zeroed in on the gene for HMG-CoA reductase.
Six more patients from four other families confirmed the link. They too all had mutations in the same gene, and they too were all diagnosed with some degree of limb girdle muscular dystrophy. (Interestingly, for reasons we don’t entirely understand, they all have normal or low cholesterol.)
Unbeknownst to the Mayo team, a group of researchers halfway around the world was already studying a large Bedouin family with a history of limb girdle muscular dystrophy. This family also carried mutations in the gene encoding HMG-CoA reductase. Those afflicted began experiencing minor symptoms in their 30s, such as muscle cramps, that worsened over time. The oldest family members, in their late 40s or 50s, had lost all movement in their arms and legs. One bedridden woman had to be ventilated full-time through a hole in her windpipe. Another had died in their mid-50s, Ohad Birk, a geneticist and doctor at Ben-Gurion University of the Negev, in Israel, told me. When his team saw that this family had the mutations in HMG-CoA reductase, they too immediately recognized the potential link to statins.
This pair of studies in the U.S. and Israel “really strongly suggests” that statins cause muscle damage via the same HMG-CoA reductase pathway, says Andrew Mammen, a neurologist at the National Institutes of Health who was not involved in either study. The enzyme’s role had been suspected, he told me, but “it had never been proven, especially in humans.” (Questions still remain, however. The enzyme, for example, is found in tissues throughout the body, so why do these common side effects show up in muscles specifically?) Rosenson, at Mount Sinai, wondered if variations in this gene could explain why statins don’t affect everyone the same. Perhaps patients who suffer particularly severe muscle side effects already have less functional versions of the enzyme, which becomes problematic only when they start taking statins, which reduce its function even further. This research might end up concretely improving the life of at least some of the patients most severely affected by statins.
That’s because Birk’s team in Israel did not stop at simply identifying the mutation. For two decades, he and his colleagues have been studying genetic disorders in this Bedouin community in the Negev and developing genetic tests so parents can avoid passing them on to their children. (Cousin marriages are traditional there, and when two parents are related, they are more likely to carry and pass on the same mutation to a child.) With limb girdle muscular dystrophy, his team went one step further than usual: They found a drug to treat it. This drug, called mevalonolactone, allows muscle cells to function more normally even without the HMG-CoA reductase enzyme. Birk’s team first tested it in mice given doses of statins high enough to weaken their limbs; those also given mevalonolactone continued to crawl and even hang upside down on a wire just fine. They seemed to suffer no ill effects. When that experimental drug was given to the Bedouin woman bedridden with limb girdle muscular dystrophy, she also started regaining control of her arms and legs. She could eventually lift her arm, sit up by herself, raise her knees, and even feed her grandchild on her own. It was a dramatic improvement. Birk told me he has since heard about dozens of patients with limb girdle muscular dystrophy around the world who may benefit from this experimental drug.
Mammen and others think the drug could help a small subset of patients who take statins as well. However, the majority of patients—those with relatively minor pains or weaknesses that go away after they switch statins or have their dosage reduced—probably don’t need this new treatment. It probably even undermines the whole point of taking statins: Mevalonolactone eventually gets turned into cholesterol in the body, so “you’re basically supplying the building blocks for making more cholesterol,” Mammen said. But for some people, numbering in the thousands, severe muscle weakness does not go away even after they stop taking statins. These patients have developed antibodies to HMG-CoA reductase , which Mammen suspects continue to bind and disable the enzyme.
Mammen is eager for these patients to try mevalonolactone, and he’s been in touch with Birk, who unfortunately doesn’t have enough of the drug to share. In fact, he doesn’t even have enough to treat all of the other family members in Israel who are clamoring for it. “We’re not a factory. We’re a research lab,” Birk told me. Mevalonolactone is available as a research chemical, but that’s not pure and safe enough for human consumption. Birk’s graduate student Yuval Yogev had to manufacture the drug himself by genetically engineering bacteria to make mevalonolactone, which he then painstakingly purified. Making a drug to this standard is a huge amount of work, even for commercial labs. Birk is looking for a pharmaceutical company that could manufacture the drug at scale—for both patients with limb girdle muscular dystrophy and those with the most severe forms of statin-associated muscle damage.
Back in 1980, the very first person to receive an experimental dose of statins suffered muscle weakness so severe, she could not walk. (She had been given an extremely high dose.) Forty years later, muscle pain and weakness are still common reasons patients quit these very effective drugs. This recent breakthrough is finally pointing researchers toward a better understanding of statins’ toll on muscles, even if they still can’t fix it for everyone.


New study suggests benefit-to-harm balance of statins for healthy adults “generally favourable”
Findings should reassure patients and should not deter their use, say researchers
Statins are associated with a small increased risk of side effects in patients without a history of heart disease, but these effects are mild compared with the potential benefits of treatment in preventing major cardiovascular events, say researchers in The BMJ today.
They say their findings suggest that the benefit-to-harm balance of statins for adults without heart disease is generally favourable.
Statins are widely used to prevent heart disease, and severe side effects are rare, but many people are reluctant to take them because of the potential for milder effects such as muscle weakness and stiffness.
For people with existing heart disease, the benefits of statins far outweigh the risk of these effects, but when statins are used by people without a history of heart disease (known as primary prevention) the benefit-to-harm balance of treatment might be less favourable.
Yet recent guidelines have recommended wider use of statins for primary prevention.
So, a team of UK and US researchers set out to examine the associations between statins and adverse events in adults without a history of heart disease, and how they vary by type and dose of statins.
They analysed the results of 62 randomised controlled trials with 120,456 participants (average age 61; 40% women) followed up for an average of 3.9 years. The trials were designed differently, and were of varying quality, but the researchers were able to allow for that in their analysis.
Statins were associated with a slightly increased risk of self-reported muscle pain, liver and kidney problems, and certain eye conditions such as cataracts, but were not associated with clinically confirmed muscle disorders or diabetes.
These risks equated to 15 more instances of muscle symptoms, eight more liver events, 12 more kidney events, and 14 more eye conditions per 10,000 patients treated for a year.
However, these increased risks did not outweigh the reduction in the risk of major cardiovascular events. For example, statins were estimated to prevent 19 heart attacks, nine strokes, and eight deaths from cardiovascular disease per 10,000 patients treated for a year.
This suggests that the benefit-to-harm balance of statins for primary prevention of cardiovascular disease is favourable, say the researchers.
Analyses by type of statin showed that atorvastatin, lovastatin, and rosuvastatin were associated with some adverse events, but few significant differences were seen between the statins.
A possible modest dose-response relationship was identified for the effect of atorvastatin on liver dysfunction, but the dose-response relationships for the other types of statins and adverse effects were inconclusive.
This suggests that tailoring statin doses to deal with safety concerns when starting treatment is not currently needed, the researchers add.
This was a large study that was able to accurately assess the adverse effects of treatment with statins. But the researchers point to some limitations in trial design that may have led to events being underestimated or more severe long term events being missed.
However, they say the low risk of adverse events caused by statins reported in this review “should reassure patients and physicians that the potential harms of statins are small and should not deter their use for primary prevention of cardiovascular disease.”
They agree that routine monitoring of liver function during treatment is probably warranted in primary prevention, and say further studies are needed to help improve adherence to treatment and achieve more efficient monitoring.
[Ends] 14/07/21
Notes for editors Research: Associations between statins and adverse events in primary prevention of cardiovascular disease: systematic review with pairwise, network, and dose-response meta-analyses Journal: The BMJ
Funding: British Heart Foundation
Link to Academy of Medical Sciences press release labelling system: https://press.psprings. co.uk/AMSlabels.pdf
Peer reviewed? Yes Evidence type: Systematic review and meta-analysis Subjects: People
BMJ Expert Media Panel
If you are a journalist needing to speak to an expert, please click here.
BMJ IN THE NEWS
Latest coverage of BMJ in the national and international media
JOIN OUR MEDIA LIST
If you are a journalist who would like to receive our press releases, please provide your details.
CONTACT OUR MEDIA RELATIONS TEAM
Email the UK media relations team for more information.
- See us on facebook
- See us on twitter
- See us on youtube
- See us on linkedin
- See us on instagram
Researchers solve mystery of how statins improve blood vessel health
Statins designed to lower cholesterol have long been noted to work in mysterious ways to improve other aspects of cardiovascular health. A Stanford Medicine-led study uncovers how they do it.
May 8, 2023 - By Nina Bai
Researchers at Stanford Medicine and their colleagues have discovered how statins improve cardiovascular health beyond lowering cholesterol. roger ashford/Shutterstock.com
Using new genetic tools to study statins in human cells and mice, Stanford Medicine researchers and collaborators have uncovered how the cholesterol-lowering drugs protect the cells that line blood vessels.
The findings provide new insight into statins’ curiously wide-ranging benefits, for conditions ranging from arteriosclerosis to diabetes, that have long been observed in the clinic.
“The study gives us an understanding, at a very deep mechanistic level, of why statins have such a positive effect outside of reducing LDL,” said professor of medicine Joseph Wu , MD, PhD, referring to low-density lipoprotein, or “bad” cholesterol. “Given how many people take statins, I think the implications are pretty profound.”
Statins are the most prescribed medications in the country, with more than 40 million Americans taking them. Developed in the 1980s from compounds found in mold and fungi, statins target an enzyme that regulates cholesterol production in the liver. But clinical trials have shown that they also seem to safeguard against cardiovascular disease beyond their ability to lower cholesterol.
Heart failure patients who take statins, for example, are less likely to suffer a second heart attack. They have also been shown to prevent the clogging of arteries, reduce inflammation and even lower cancer risk. Yet these underlying mechanisms are poorly understood.
“Statins were invented to lower cholesterol by targeting the liver. But we didn’t know the targets or the pathways in the cardiovascular system,” said Chun Liu , PhD, an instructor at the Stanford Cardiovascular Institute and co-lead author of the study published May 8 in Nature Cardiovascular Research . Mengcheng Shen , PhD, and Wilson Tan, PhD, postdoctoral scholars at the Stanford Cardiovascular Institute, are the other co-lead authors, and Wu is the senior author.

Hints from a dish
To take a closer look at statins’ effect on blood vessels, Liu and colleagues tested a common statin, simvastatin, on lab-grown human endothelial cells derived from induced pluripotent stem cells. Endothelial cells make up the lining of blood vessels, but in many diseases they transform into a different cell type, known as mesenchymal cells, which are poor substitutes.
“Mesenchymal cells are less functional and make tissues stiffer so they cannot relax or contract correctly,” Liu said.
The researchers suspected that statins could reduce this harmful transition. Indeed, endothelial cells treated with simvastatin in a dish formed more capillary-like tubes, a sign of their enhanced ability to grow into new blood vessels.
RNA sequencing of the treated cells offered few clues. The researchers saw some changes in gene expression, but they “didn’t find anything interesting,” Liu said.
It was not until they employed a newer technique called ATAC-seq that the role of statins became apparent. ATAC-seq reveals what happens at the epigenetic level, meaning the changes to gene expression that do not involve changes to the genetic sequence.
They found that the changes in gene expression stemmed from the way strings of DNA are packaged inside the cell nucleus. DNA exists in our cells not as loose strands but as a series of tight spools around proteins, together known as chromatin. Whether particular DNA sequences are exposed or hidden in these spools determines how much they are expressed.
“When we adopted the ATAC-seq technology, we were quite surprised to find a really robust epigenetic change of the chromatin,” Liu said.

ATAC-seq revealed that simvastatin-treated cells had closed chromatin structures that reduced the expression of genes that cause the endothelial-to-mesenchymal transition. Working backward, the researchers found that simvastatin prevents a protein known as YAP from entering the nucleus and opening chromatin.
The YAP protein is known to play important roles in development, such as regulating the size of our organs, but also has been implicated in the abnormal cell growth seen in cancer.
A look at diabetes
To see the drug in context, the researchers tested simvastatin on diabetic mice. Diabetes causes subtle changes to blood vessels that mimic the damage commonly seen in people who are prescribed statins — older patients who do not have a cardiovascular condition, Liu said.
They found that after eight weeks on simvastatin, the diabetic mice had significantly improved vascular function, with arteries that more easily relaxed and contracted.
“If we can understand the mechanism, we can fine-tune this drug to be more specific to rescuing vascular function,” Liu said.
The findings also provide a more detailed picture of the vascular disease process, which could help doctors identify and treat early signs of vascular damage.
“I’ve been taking statins for the past 10 years to keep my cholesterol down. I also knew it has good vascular effects. I just didn’t know how it does it,” said Wu, the Simon H. Stertzer, MD, Professor who is also the director of the Stanford Cardiovascular Institute. “This study explains how.”
Researchers from the University of North Texas and the Ohio State University College of Medicine contributed to this study.
The study was supported by funding from the National Institutes of Health (grants R01 HL130020, R01 HL150693, R01 HL163680, R01 HL145676, P01 HL141084, R01 HL141371, R01 HL126527, R01 HL15864, R01 HL161002, R01 HL155282 and 18CDA34110293), an American Heart Association SFRN grant, an AHA Career Development Award and the Tobacco-Related Disease Research Program.

About Stanford Medicine
Stanford Medicine is an integrated academic health system comprising the Stanford School of Medicine and adult and pediatric health care delivery systems. Together, they harness the full potential of biomedicine through collaborative research, education and clinical care for patients. For more information, please visit med.stanford.edu .
Artificial intelligence
Exploring ways AI is applied to health care
New study suggests benefit-to-harm balance of statins for healthy adults 'generally favorable'
Findings should reassure patients and should not deter their use, say researchers.
Statins are associated with a small increased risk of side effects in patients without a history of heart disease, but these effects are mild compared with the potential benefits of treatment in preventing major cardiovascular events, say researchers in The BMJ today.
They say their findings suggest that the benefit-to-harm balance of statins for adults without heart disease is generally favourable.
Statins are widely used to prevent heart disease, and severe side effects are rare, but many people are reluctant to take them because of the potential for milder effects such as muscle weakness and stiffness.
For people with existing heart disease, the benefits of statins far outweigh the risk of these effects, but when statins are used by people without a history of heart disease (known as primary prevention) the benefit-to-harm balance of treatment might be less favourable.
Yet recent guidelines have recommended wider use of statins for primary prevention.
So, a team of UK and US researchers set out to examine the associations between statins and adverse events in adults without a history of heart disease, and how they vary by type and dose of statins.
They analysed the results of 62 randomised controlled trials with 120,456 participants (average age 61; 40% women) followed up for an average of 3.9 years. The trials were designed differently, and were of varying quality, but the researchers were able to allow for that in their analysis.
Statins were associated with a slightly increased risk of self-reported muscle pain, liver and kidney problems, and certain eye conditions such as cataracts, but were not associated with clinically confirmed muscle disorders or diabetes.
These risks equated to 15 more instances of muscle symptoms, eight more liver events, 12 more kidney events, and 14 more eye conditions per 10,000 patients treated for a year.
However, these increased risks did not outweigh the reduction in the risk of major cardiovascular events. For example, statins were estimated to prevent 19 heart attacks, nine strokes, and eight deaths from cardiovascular disease per 10,000 patients treated for a year.
This suggests that the benefit-to-harm balance of statins for primary prevention of cardiovascular disease is favourable, say the researchers.
Analyses by type of statin showed that atorvastatin, lovastatin, and rosuvastatin were associated with some adverse events, but few significant differences were seen between the statins.
A possible modest dose-response relationship was identified for the effect of atorvastatin on liver dysfunction, but the dose-response relationships for the other types of statins and adverse effects were inconclusive.
This suggests that tailoring statin doses to deal with safety concerns when starting treatment is not currently needed, the researchers add.
This was a large study that was able to accurately assess the adverse effects of treatment with statins. But the researchers point to some limitations in trial design that may have led to events being underestimated or more severe long term events being missed.
However, they say the low risk of adverse events caused by statins reported in this review "should reassure patients and physicians that the potential harms of statins are small and should not deter their use for primary prevention of cardiovascular disease."
They agree that routine monitoring of liver function during treatment is probably warranted in primary prevention, and say further studies are needed to help improve adherence to treatment and achieve more efficient monitoring.
- Cholesterol
- Heart Disease
- Multiple Sclerosis Research
- Diseases and Conditions
- Multiple Sclerosis
- Alzheimer's
- COX-2 inhibitor
- Deep brain stimulation
- Personalized medicine
- Heart failure
- Psychopharmacology
Story Source:
Materials provided by BMJ . Note: Content may be edited for style and length.
Journal Reference :
- Ting Cai, Lucy Abel, Oliver Langford, Genevieve Monaghan, Jeffrey K Aronson, Richard J Stevens, Sarah Lay-Flurrie, Constantinos Koshiaris, Richard J McManus, F D Richard Hobbs, James P Sheppard. Associations between statins and adverse events in primary prevention of cardiovascular disease: systematic review with pairwise, network, and dose-response meta-analyses . BMJ , 2021; n1537 DOI: 10.1136/bmj.n1537
Cite This Page :
Explore More
- Food in Sight? The Liver Is Ready!
- Acid Reflux Drugs and Risk of Migraine
- Do Cells Have a Hidden Communication System?
- Mice Given Mouse-Rat Brains Can Smell Again
- How Do Birds Flock? New Aerodynamics
- Cancer: Epigenetic Origin Without DNA Mutation
- Climate Change Driving Biodiversity Loss
- Why Can't Robots Outrun Animals?
- Evolution of Gliding in Marsupials
- Novel One-Dimensional Superconductor
Trending Topics
Strange & offbeat.
- Skip to main content
- Keyboard shortcuts for audio player
Your Health
- Treatments & Tests
- Health Inc.
- Public Health
Statins vs. supplements: New study finds one is 'vastly superior' to cut cholesterol

Allison Aubrey

Millions of Americans are prescribed statins to reduce the risk of heart disease, but many prefer to take supplements like fish oil, garlic and flaxseed. Peter Dazeley/Getty Images hide caption
Millions of Americans are prescribed statins to reduce the risk of heart disease, but many prefer to take supplements like fish oil, garlic and flaxseed.
If you were prescribed medicine to lower your risk of a heart attack or stroke, would you take it?
Millions of Americans are prescribed statins such as Lipitor, Crestor or generic formulations to lower their cholesterol. But lots of people are hesitant to start the medication.
Some people fret over potential side effects such as leg cramps, which may be — or may not be — linked to the drug. As an alternative, dietary supplements, often marketed to promote heart health, including fish oil and other omega-3 supplements (omega-3s are essential fatty acids found in fish and flaxseed), are growing in popularity .
So, which is most effective? Researchers at the Cleveland Clinic set out to answer this question by comparing statins to supplements in a clinical trial. They tracked the outcomes of 190 adults, ages 40 to 75. Some participants were given a 5 mg daily dose of rosuvastatin, a statin that is sold under the brand name Crestor for 28 days. Others were given supplements, including fish oil, cinnamon, garlic, turmeric, plant sterols or red yeast rice for the same period.

Choose the best diet for you
The maker of Crestor, Astra Zeneca sponsored the study, but the researchers worked independently to design the study and run the statistical analysis.
"What we found was that rosuvastatin lowered LDL cholesterol by almost 38% and that was vastly superior to placebo and any of the six supplements studied in the trial," study author Luke Laffin, M.D. of the Cleveland Clinic's Heart, Vascular & Thoracic Institute told NPR. He says this level of reduction is enough to lower the risk of heart attacks and strokes. The findings are published in the Journal of the American College of Cardiology .
"Oftentimes these supplements are marketed as 'natural ways' to lower your cholesterol," says Laffin. But he says none of the dietary supplements demonstrated any significant decrease in LDL cholesterol compared with a placebo. LDL cholesterol is considered the 'bad cholesterol' because it can contribute to plaque build-up in the artery walls – which can narrow the arteries, and set the stage for heart attacks and strokes.
"Clearly, statins do what they're intended to do," the study's senior author Steve Nissen, a cardiologist and chief academic officer of the Heart, Vascular & Thoracic Institute at Cleveland Clinic told NPR. By comparison, he says this research shows that supplements are not effective. "They do not promote heart health. They do not improve levels of the bad cholesterol."
Nissen says supplements can be expensive compared to statin medications. Depending on insurance, he says people may pay less than $5 a month out-of-pocket for rosuvastatin.

Shots - Health News
Cholesterol provides a clue about heart risks from sleep apnea.
"Statins are the most effective heart attack and stroke prevention drugs that we have really ever seen," says Michael Honigberg , a cardiologist and researcher at Massachusetts General Hospital who is not affiliated with the new study. He says the new findings add to an already large body of evidence showing statins lower LDL cholesterol, and he's not surprised to see that the supplements were not as effective.
However, he says, not everyone with a family history of heart disease or slightly elevated cholesterol should be on a statin. The American College of Cardiology and American Heart Association developed some prescription guidelines. Typically, if a person's LDL cholesterol (bad cholesterol) is 190 or higher, they're often advised to start a statin. Health care professionals use a risk calculator to estimate a person's risk of having a heart attack or stroke over the next 10 years. If the risk is high enough, based on factors including age, blood pressure and smoking status, then a statin may be recommended.
Honigberg says for people who have slightly elevated cholesterol, but are not at high enough risk to be prescribed a statin, he recommends that they focus on diet and exercise, rather than buying supplements. "I tell my patients to save their money and instead spend that money on eating heart healthy, high quality food."
He points to studies that show heart-healthy diets, including Mediterranean diets which emphasize healthy fats, lots of fruits, vegetables and whole grains and the DASH diet , significantly reduce the risk of heart disease. "I think a formulation that we perhaps don't use enough is that food is medicine and is probably a more effective medicine than supplements," says Honigberg.
The National Center for Complementary and Integrative Health, part of the National Institutes of Health, has also concluded, based on prior research, that omega-3 supplements do not reduce the risk of heart disease , but eating fish – which contains omega-3 fatty acids – is linked to a reduced risk. This suggests that omega-3 fatty acids are most beneficial as part of a healthy diet.
And it's worth noting that the NIH review concludes that omega-3 supplements may help relieve symptoms of rheumatoid arthritis. Omega-3s are also added to baby formulas to promote brain development. The NIH review also concludes that omega-3 supplements can lower triglycerides, a type of fat found in the blood. But Honigberg says this may be recommended for a "small subset of patients" with very high triglyceride levels.
As for people whose risk of heart disease is high enough to warrant a statin prescription, Honigberg says he spends a fair amount of time talking through concerns with patients.
"We talk about the excellent safety profile and the very, very low risk of side effects," he says. He describes the risk of serious side effects as "vanishingly small."
Sometimes patients stop taking a statin because they believe it's causing a certain side effect. But Honigberg points to a double-blind research study that showed when patients were given a placebo in place of a statin, patients reported feeling most of the same side effects.
"So the punch line of the trial is people blame statins for side effects the statins aren't really causing," he says.
- supplements
- heart disease

Image credit: Shutterstock
New study shows muscle pain is not due to statins in over 90% of those taking the treatment
Statin therapies are not the cause of muscle pain in over 90% of those who experience symptoms, according to a new study led by researchers from Oxford Population Health. The results were published today in The Lancet and presented at the European Society of Cardiology Congress.
The study demonstrated that muscle pain or weakness is common in adults, regardless of whether they take a statin tablet or not. 14 out of 15 reported cases of muscle pain or weakness were not due to statin therapy, and those cases that were due to statins occurred mainly within the first year of treatment.
The researchers gathered together data from 23 large-scale randomised studies from the Cholesterol Treatment Trialists’ Collaboration, including information from almost 155,000 individuals. They used this information to assess the effect of statin treatments on the frequency of muscle-related symptoms across many different types of patient, and found that muscle symptoms such as muscle pain or weakness were common, even in those allocated a placebo (or dummy) tablet. In 19 trials of statin therapy versus placebo, similar numbers of people reported such symptoms (16,835; 27.1%) in the statin group and (16,446; 26.6%) in the placebo group.
They also found that statin treatments marginally increased the frequency of muscle-related symptoms. In those taking statins, about 14 out of 15 reports of muscle symptoms were not attributed to statins, falling to about 9 in 10 for patients taking a high intensity treatment. This means that statins are not the cause of muscle pain in over 90% of people who report symptoms.
Most of the reports of muscle symptoms in those taking statins occurred within the first year of treatment. After the first year of starting treatment, low/moderate intensity statin therapy caused no increase in the frequency of muscle symptoms.
Higher intensity statin treatments (those designed to produce greater reductions in low‑density lipoprotein (LDL) cholesterol) were more likely than low/moderate intensity statins to increase the risk of muscle symptoms with some persistent effect after the first year. (Overall low/moderate intensity regimens yielded a 3% increase in first reports, with higher intensity regimens resulting in an 8% increase in first reports.) There was no evidence of a relationship between the statin dose and muscle symptoms.
For every 1000 people taking a moderate intensity statin, the treatment would cause 11 (generally mild) episodes of muscle pain or weakness. This means that the slightly increased risk of muscle symptoms is greatly outweighed by the previously known benefits of statin therapy in preventing cardiovascular disease, including heart attacks and strokes. For example, for every 1000 people taking a moderate intensity statin, the treatment would typically prevent 50 major vascular events (such as heart attacks and strokes) in those with pre-existing vascular disease (secondary prevention), and 25 major vascular events if used for primary prevention.
Professor Colin Baigent , Director of the Medical Research Council Population Health Research Unit at the University of Oxford, and joint lead author of the study, said ‘Cardiovascular disease including heart attacks and strokes, is the world’s largest killer, responsible for around 18 million deaths each year. High levels of LDL (‘bad’) cholesterol are a major risk factor. Statins lower LDL-cholesterol levels and have been repeatedly proven to reduce the risk of cardiovascular disease. However, there have been concerns about muscle pain, and statins can, rarely, cause severe muscle problems.
‘Our research shows that, for most people taking a statin, any muscle-related symptoms they experience will not in fact be due to the statin itself – and so the potential benefits of statin therapy are likely to outweigh the muscle pain risks. Previous reports that statins are a major cause of muscle pain are likely to have been the result of methodological problems in the studies giving rise to those reports.
Dr Christina Reith , Senior Clinical Research Fellow at Oxford Population Health and joint lead author of the study, said: ‘Rates of cardiovascular disease are rising rapidly, particularly in low- and middle-income countries. Statin therapies are affordable and widely available, and are a key tool in helping to prevent avoidable disability and death. We hope that these results will help doctors and patients to make informed decisions about whether to start or remain on statin therapy and that information provided to doctors and patients will be reviewed in light of our study results.’
All of the trials included in the analysis were large-scale (involving at least 1000 participants) and of reasonably long duration (tracking patient outcomes for at least two years). The trials were also all double-blind, meaning that neither the trial participants nor those managing the participants or leading the study knew who was receiving which treatment, to avoid potential biases due to knowledge of treatment allocation.
In 19 of the trials, participants were randomly allocated to receive either statin treatment or a placebo, whilst in four trials the comparison was of higher doses of statins versus lower/moderate doses. Around 124,000 participants (average age 63 years) were involved in the 19 trials comparing statins to a placebo.
The researchers looked at all data about any adverse effects reported by individuals taking part in the clinical trials, as well as data on the timing of and reasons for stopping study treatment, use of other (non-trial) medications, other health conditions, and laboratory results that would help in the interpretation of particular adverse events.
Professor Sir Nilesh Samani, Medical Director at the British Heart Foundation, which co-funded the study said: ‘This accumulation of data from many clinical trials provides a clear picture that while statins are associated with a small increase in risk of muscle pains or weakness, they do not cause the majority of muscle pain symptoms commonly associated with them. It reinforces the evidence that statins are safe, which should provide reassurance to the many people taking, or considering taking, these lifesaving drugs that have been proven to protect against heart attacks and strokes.
‘However, it also shows how common muscle pain symptoms are. Almost one quarter of patients who participated in the trials reported such symptoms whether they were taking statins or placebo. It is vital that the genuine concerns of people who do experience muscle symptoms are not dismissed and that doctors have continued consultations with these patients to ensure their medication is tailored to work best for them.’
The study was conducted by the Cholesterol Treatment Trialists’ (CTT) Collaboration , a joint initiative coordinated between the National Health and Medical Research Council Clinical Trials Centre, University of Sydney, Australia and the Clinical Trial Service Unit & Epidemiological Studies Unit, Oxford Population Health, on behalf of academic researchers representing major statin trials worldwide. The work was funded by the British Heart Foundation, UK Medical Research Council, and the Australian National Health and Medical Research Council. The work of the CTT is overseen by an Independent Oversight Panel.
The full paper, ' Effect of statin therapy on muscle symptoms: an individual participant data meta-analysis of large-scale, randomised, double-blind trials ', can be read in The Lancet.
DISCOVER MORE
- Support Oxford's research
- Partner with Oxford on research
- Study at Oxford
- Research jobs at Oxford
You can view all news or browse by category
Benefits of statins may have been overstated – new study
Researcher, Medicine and Health, RCSI University of Medicine and Health Sciences
Disclosure statement
Paula Byrne does not work for, consult, own shares in or receive funding from any company or organisation that would benefit from this article, and has disclosed no relevant affiliations beyond their academic appointment.
RCSI University of Medicine and Health Sciences provides funding as a member of The Conversation UK.
View all partners
Cholesterol-lowering statins are one of the world’s most commonly used medicines. They were first approved for people with a high risk of cardiovascular disease in 1987. By 2020, global sales were estimated to have approached US$1 trillion (£764 billion).
However, there has been an ongoing debate about whether or not statins are over-prescribed. Does everyone who takes them really benefit from them? To find out, my colleagues and I found 21 relevant clinical trials and analysed the combined data (over 140,000 participants) in what is known as a meta-analysis.
We asked two questions: is it best to lower LDL cholesterol (sometimes known as “bad” cholesterol) as much as possible to reduce the risk of heart attack, stroke or premature death? And, how do the benefits of statins compare when it comes to reducing the risk of these events?
In answer to the first question, we found a surprisingly weak and inconsistent relationship between the degree of reduction in LDL cholesterol from taking statins and a person’s chance of having a heart attack or stroke, or dying during the trial period. In some trials, reductions in LDL cholesterol were associated with significant reductions in the risk of dying, but in others, reductions in LDL cholesterol did not reduce this risk.
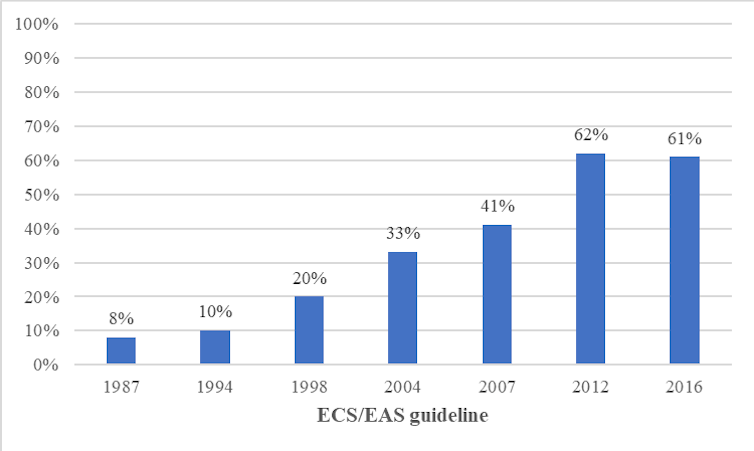
This is an important finding because clinical guidelines have expanded the proportion of people eligible for statins as “ideal” LDL cholesterol levels were incrementally lowered. For example, one study estimated a 600% increase in eligibility for statins between 1987 and 2016.
The proportion of people in Europe eligible for statins
Regarding the second question, we looked at two types of risk reduction: relative risk reduction and absolute risk reduction. Imagine your chance of dying from a certain condition prematurely is 0.2%, and there’s a drug that reduces your chance of dying to 0.1%. In relative terms (relative risk reduction), your chance of dying has been halved, or reduced by 50%. But in absolute terms (absolute risk reduction), your chance of dying has only gone down by 0.1%.
Although there is a 50% relative risk reduction, is it a meaningful difference? Would it be worthwhile changing to this drug, particularly if there are side-effects associated with it? Absolute risk reduction presents a clearer picture and makes it easier for people to make informed decisions.
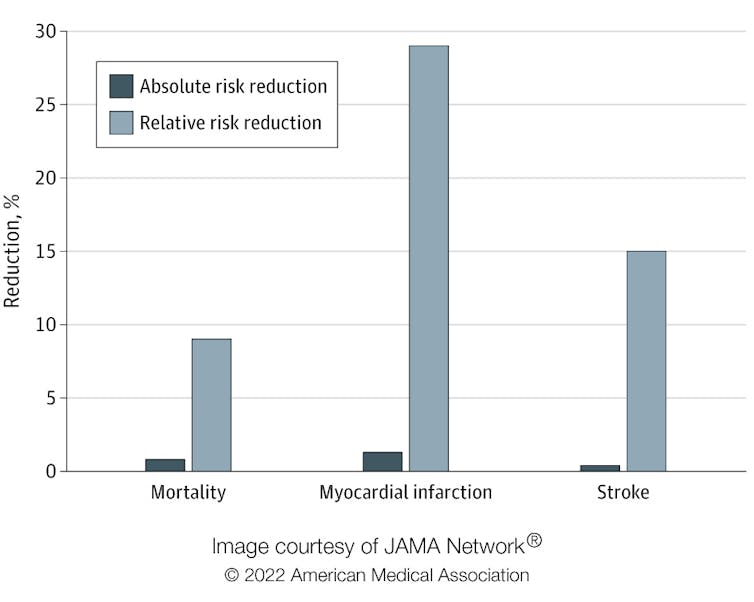
In our study, published in Jama Internal Medicine , we found that the absolute risk reduction from taking statins was modest compared with the relative risk reduction. The relative risk reduction for those taking statins compared with those who did not was 9% for deaths, 29% for heart attacks and 14% for strokes. Yet the absolute risk reduction of dying, having a heart attack or stroke was 0.8%, 1.3% and 0.4% respectively.
Absolute risk reduction compared with relative risk reduction
Individual differences
A further consideration is that trials report average outcomes across all included participants rather than for an individual. Clearly, people’s individual risk of disease varies depending on lifestyle and other factors. The baseline risk of cardiovascular disease can be estimated using an online calculator, such as QRisk , which takes a range of factors into account, such as weight, smoking, blood pressure, cholesterol and age.
The likelihood of a person developing cardiovascular disease in the next ten years is expressed as a percentage. For example, consider an overweight 65-year-old man who smokes, has high blood pressure and total cholesterol. He may be at high risk of cardiovascular disease, compared with a 45-year-old, non-smoking woman with slightly raised cholesterol and blood pressure and no other risk factors. If a doctor were to assess their risk of dying in the next ten years, the estimated risk for the man might be 38%, for example, whereas the woman’s risk might be only 1.4%.
Now consider the impact of taking statins for both. According to the study, statins would reduce the relative risk of dying by 9%. In absolute terms, the man would reduce his risk from 38% to 34.6%, and the woman from 1.4% to 1.3%.
Patients and their doctors need to consider whether they think these risk reductions are worthwhile in a trade-off between potential benefits and harms, including the inconvenience of taking a daily medicine, possibly for life. This is particularly salient for low-risk people for whom the benefits are marginal. However, people perceive risk differently based on their own experience and preferences, and what might look like a “good deal” to some may be seen as of little value to others.
Our study highlights that patients and doctors need to be supported to make decisions about treatments using evidence from all available studies and presented in a format that helps them understand potential benefits. Both patients and their doctors need to understand the true impact of medicines in order to make informed decisions. Relying on relative risk, which is numerically more impressive, instead of absolute, may lead both doctors and patients to overestimate the benefits of interventions.
For example, one study found that doctors rated a treatment as more effective and were more likely to prescribe it when the benefits were presented as relative rather than as absolute risk reductions. Another survey found that most respondents would agree to be screened for cancer if presented with relative risk reductions, whereas just over half would if presented with absolute risk reductions.
If you have been prescribed statins, don’t stop taking your medication without first consulting your doctor. Your risk profile might mean that they could benefit you. But if you’d like to reassess taking this drug, ask your doctor to explain your absolute risk reduction and then make a collaborative decision.
- Cardiovascular disease
- medical risk

Program Manager, Teaching & Learning Initiatives

Lecturer/Senior Lecturer, Earth System Science (School of Science)

Sydney Horizon Educators (Identified)

Deputy Social Media Producer

Associate Professor, Occupational Therapy
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
March 6, 2023
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
Study finds residual inflammation after statin therapy strongly predicts cardiovascular events, death
by Brigham and Women's Hospital

New evidence released today from a study of 31,245 patients already taking statin therapy indicates that inflammation may be a more powerful predictor of risk of future cardiovascular events—such as heart attack and stroke—than "bad" cholesterol.
Treatments that aggressively lower vascular inflammation need to be incorporated into daily practice if doctors are to maximize patient outcomes , according to the study's corresponding author, Paul Ridker, MD, a preventive cardiologist at Brigham and Women's Hospital, a founding member of the Mass General Brigham health care system. Ridker presented the findings at the American College of Cardiology meeting in New Orleans. Results are published simultaneously in The Lancet .
"The new data should be a wake-up call for preventive cardiologists and their patients," said Ridker. "Virtually all patients with or at risk for atherosclerotic disease are appropriately treated with aggressive statin therapy . Yet, in our study of patients already taking a statin, hsCRP—a measure of residual inflammatory risk—was a more powerful determinant of having a future heart attack or dying from cardiovascular disease than was LDL-cholesterol—a measure of residual cholesterol risk. The data are a powerful demonstration that to beat heart disease, we need to lower both cholesterol and inflammation, not just cholesterol alone."
Once a patient is on statin therapy, cardiologists typically describe two conditions: "residual cholesterol risk," which can be further reduced with additional lipid-lowering therapy, and "residual inflammatory risk," which can be further reduced with certain drugs that impact vascular inflammation. Whether clinicians should choose to focus on further lowering cholesterol or inflammation has been uncertain and controversial.
Ridker and colleagues examined data from three recently conducted clinical trials (PROMINENT, REDUCE-IT and STRENGTH) of patients with or at high risk for atherosclerotic disease to understand the relative importance of "residual inflammatory risk" as compared to "residual cholesterol risk" among contemporary statin-treated patients.
All patients were receiving aggressive guideline directed medical care including statins, usually at high doses. But cardiovascular event rates in all three trials approached 10 percent at five years. In all three trials, blood levels of high-sensitivity C-reactive protein (hs-CRP, a measure of vascular inflammation) were significantly associated with major adverse cardiovascular events (MACE), cardiovascular mortality and all-cause mortality.
Moreover, the researchers report that hs-CRP was a more potent predictor of future cardiovascular risk than LDL-cholesterol. For example, among aggressively treated patients already on higher intensity statins, the risks of cardiovascular death and all-cause mortality were more than twice as high among those with the highest levels of CRP when compared to those with the highest levels of cholesterol, differences that were highly statistically significant.
The data have immediate implications for patient care today and for future research, according to the authors.
"There is no doubt that lower is better for LDL-cholesterol and we need to aggressively reduce cholesterol whenever possible. But if cardiologists want to eliminate cardiovascular disease, they clearly must target inflammation as well," Ridker said.
Inflammation inhibition has been found in several clinical trials to reduce cardiovascular risk, yet uptake of anti-inflammatory therapy in daily practice has been limited. This has been particularly true for colchicine, an inexpensive anti-inflammatory therapy that reduced cardiovascular event rates in two major randomized trials with a benefit at least as large as that associated with much more expensive cholesterol -lowering drugs. Ridker notes the importance of weighing the potential benefits of anti-inflammatory agents, in addition to statin therapy and lifestyle modifications, to lower cardiovascular risk.
"Beyond statins and consideration of anti-inflammatory agents, physicians should not lose sight of diet, exercise, and smoking cessation, all of which lower vascular inflammation and save lives," Ridker said.
Explore further
Feedback to editors

Gene linked to epilepsy and autism decoded in new study
14 hours ago

Blood test finds knee osteoarthritis up to eight years before it appears on X-rays
15 hours ago

Researchers find pregnancy cytokine levels impact fetal brain development and offspring behavior

Study finds biomarkers for psychiatric symptoms in patients with rare genetic condition 22q

Clinical trial evaluates azithromycin for preventing chronic lung disease in premature babies
16 hours ago

Scientists report that new gene therapy slows down amyotrophic lateral sclerosis disease progression

Using stem cell-derived heart muscle cells to advance heart regenerative therapy
17 hours ago

Analysis identifies 50 new genomic regions associated with kidney cancer risk

Illusion demystifies the way vision works: Experiments imply brightness perception occurs deeper in brain than thought

How buildings influence the microbiome and human health
Related stories.
Study finds one in five patients at high risk of cardiovascular disease refuse statin therapy
Feb 28, 2023
'Active' statin selection process using risk assessment tools increases appropriate medication use by 50%: Study
Mar 6, 2023

Regular use of common cholesterol-lowering drug linked to reduction of COVID-19 severity, risk of death
Oct 22, 2022
Follow-up care with medication, testing after heart attack can prevent 94% of patients from having second cardiac event
Nov 7, 2022
Triglyceride-lowering trial neutral for cardiovascular event reduction

Inflammation reduction could cut risk of heart attack, stroke
Aug 28, 2017
Recommended for you

People with rare longevity mutation may also be protected from cardiovascular disease
18 hours ago

Link between depression and cardiovascular disease explained: They partly develop from same gene module
Apr 25, 2024

Researchers discover biology behind Fontan-operation-associated liver disease
Apr 24, 2024

Artificial intelligence can evaluate cardiovascular risk during CT scan

New study prompts call for considering cholesterol screening earlier in life
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
Featured Topics
Featured series.
A series of random questions answered by Harvard experts.
Explore the Gazette
Read the latest.

How old is too old to run?

America’s graying. We need to change the way we think about age.

Can we talk?
Don’t need high cholesterol to benefit from statins.
Miles Martin
BWH Communications
Studies find drug protects against heart disease in high-risk groups
Two new studies found that statins, the most prescribed class of drugs to treat high cholesterol, are protective for high-risk groups who haven’t yet had a heart attack or stroke but could be at risk of one, according to Harvard-affiliated Brigham and Women’s Hospital.
The results provide additional context to a longstanding debate among the medical community about whether there are benefits to initiating statin use in people who don’t already have high cholesterol or cardiovascular disease. The studies appear in the JAMA Network Open and the Journal of the American Geriatrics Society.
“Statins are a first line class of drugs that can lower cholesterol and lower the risk of a second heart attack or stroke in people who have already had one — there’s no question about that,” said corresponding author Ariela Orkaby of the Brigham’s Division of Aging . “However, many clinicians still don’t agree on whether statins should be used as a preventative treatment for people who haven’t had a heart attack or stroke yet but are at high risk due to age or other factors.
“Our findings demonstrate statins have a protective effect even in people who haven’t had their first major cardiac event, which means there are still benefits to prescribing these medications for primary prevention of heart disease,” said Orkaby, who is also an investigator in the Veterans Affairs Geriatric Research Education and Clinical Center.
For most people, statins are well-tolerated and don’t have significant side-effects. However, some doctors over the last few years have called for these medications to stop being prescribed for certain people, including those with chronic kidney disease. Notably, cardiovascular disease is the leading cause of death for older adults with kidney disease.
“Our results suggest that for statins, frailty status doesn’t decrease benefit, and it may be the frailest older adults who benefit the most.” Ariela Orkaby, Brigham and Women’s Hospital
“There has been some chatter about statins causing muscle pains but, for the vast majority of people, these are very safe and effective medications,” said Orkaby. “The problem is that we’re still missing a lot of clinical evidence about their effectiveness in certain groups, which has made some doctors deprescribe statins out of caution.”
In their study of 14,828 people with chronic kidney disease, the researchers found that starting statins was associated with 9 percent reduced mortality and a 4 percent lower risk of heart attack or stroke. The team also looked at a much larger group of older adults without kidney disease, of whom 12 percent were frail. Among this group of 710,313 people, they found that statin therapy was associated with a 39 percent lower risk of mortality and 14 percent lower risk of a first heart attack or stroke. Both studies used data from the Veteran’s Affairs Healthcare System.
Notably, the researchers found that these reductions in mortality and disease risk were independent of frailty, which the researchers measured through a score that accounted for dozens of age-related health conditions.
“When we’re talking about the risk-benefit analysis of using a certain medication in older populations, we need to consider frailty because medications may not work as well or may cause more side effects in people who are frailer,” said Orkaby. “Our results suggest that for statins, frailty status doesn’t decrease benefit, and it may be the frailest older adults who benefit the most.”
While the two studies benefited from the large patient population and long-term follow-up afforded by working with VA data, the researchers caution that their conclusions drawn from past patient data should be validated in new clinical trials that prospectively address these questions.
“We’re still missing some of the evidence we need to fully understand the scope of what these medications do,” said Orkaby. “However, these studies tell us that until we have clinical data that suggests otherwise, statins are safe and effective for older people and those with chronic kidney disease.”
Disclosures: Ariela Orkaby accepted personal fees from Anthos Therapeutics during this research, unrelated to this work. Luc Djosse reports current research funding from Novartis, unrelated to this work.
This research was supported by grants from the National Institute on Aging (R03-AG060169) and Veterans Affairs (IK2-CX001800, I01 CX001025) .
Share this article
You might like.
No such thing, specialist says — but when your body is trying to tell you something, listen

Experts say instead of disability, focus needs to shift to ability, health, with greater participation, economically and socially

Study finds that conversation – even online – could be an effective strategy to help prevent cognitive decline and dementia
When math is the dream
Dora Woodruff was drawn to beauty of numbers as child. Next up: Ph.D. at MIT.
Three will receive 2024 Harvard Medal
In recognition of their extraordinary service
So what exactly makes Taylor Swift so great?
Experts weigh in on pop superstar's cultural and financial impact as her tours and albums continue to break records.
Appointments at Mayo Clinic
Statin side effects: weigh the benefits and risks.
Statins are effective at lowering cholesterol and protecting against a heart attack and stroke, although they may lead to side effects for some people.
Health care professionals often prescribe statins for people with high cholesterol. Statins help lower total cholesterol and reduce the risk of a heart attack or stroke.
Statins include atorvastatin (Lipitor), fluvastatin (Lescol XL), lovastatin (Altoprev), pitavastatin (Livalo), pravastatin, rosuvastatin (Crestor) and simvastatin (Zocor).
Having too much cholesterol in the blood increases the risk of heart attacks and strokes. Statins block an enzyme the liver needs to make cholesterol. This causes the liver to remove cholesterol from the blood.
While statins are highly effective and safe for most people, they have been linked to muscle pain, digestive problems and mental fuzziness in some people. Rarely, they may cause liver damage.
If you think you're experiencing side effects from taking statins, don't just stop taking the pills. Talk to your health care team to see if a change in how much medicine you take or even a different type of medicine might be helpful.
What are statin side effects?
Muscle pain and damage.
One of the most common complaints of people taking statins is muscle pain. You may feel this pain as a soreness, tiredness or weakness in your muscles. The pain can be a mild discomfort, or it can be serious enough to make it hard to do your daily activities.
However, researchers have found a "nocebo" effect when it comes to people thinking they have muscle pain from statins. A "nocebo" effect means people who have negative expectations about a medicine report experiencing the potential side effect at higher rates than the drug should cause.
The real risk of developing muscle pain as a result of taking statins is about 5% or less compared with taking a pill that doesn't contain medicine, called a placebo. However, studies have found that nearly 30% of people stopped taking the pills because of muscle aches even when they were taking a placebo.
A strong predictor of if you'll experience muscle aches when taking statins could be whether or not you read about the potential side effect.
Very rarely, statins can cause life-threatening muscle damage called rhabdomyolysis (rab-doe-my-OL-ih-sis). Rhabdomyolysis can cause extreme muscle pain, liver damage, kidney failure and death. The risk of very serious side effects is extremely low. Only a few cases of rhabdomyolysis occur per million people taking statins. Rhabdomyolysis can occur when you take statins in combination with certain drugs or if you take a high dose of statins.
Liver damage
Occasionally, statin use could cause an increase in the level of enzymes in the liver. These enzymes signal inflammation. If the increase is only mild, you can continue to take the drug. Rarely, if the increase is severe, you may need to try a different statin.
Although liver problems are rare, your health care team may order a liver enzyme test before or shortly after you begin to take a statin. You won't need any further liver enzyme tests unless you begin to have symptoms of trouble with your liver.
Contact your health care professional immediately if you have unusual fatigue or weakness, loss of appetite, pain in your upper stomach, dark-colored urine, or yellowing of your skin or eyes.
Increased blood sugar or type 2 diabetes
It's possible that your blood sugar level, known as blood glucose, may increase when you take a statin. This may lead to developing type 2 diabetes. The risk is small but important enough that the Food and Drug Administration (FDA) has issued a warning on statin labels regarding blood glucose levels and diabetes.
The increase generally occurs when blood sugar levels are already higher than normal. People with prediabetes or diabetes may see their blood sugar levels rise when they start taking a statin.
But statins also prevent heart attacks in people with diabetes. The benefit of taking statins likely outweighs the small risk to have the blood sugar level go up. Talk to your health care team if you have concerns.
Neurological side effects
The FDA warns on statin labels that some people have developed memory loss or confusion while taking statins. These side effects reverse once you stop taking the medicines. There is limited evidence to prove a cause-effect relationship and several studies have found that statins have no effect on memory. Talk to your care team if you experience memory loss or confusion while taking statins.
There also has been evidence that statins may help with brain function — in people with dementia, for example. This is still being studied. Don't stop taking your statin medicine before talking to your health care professional.
Who's at risk of developing statin side effects?
Not everyone who takes a statin will have side effects, but some people may be at a greater risk. Risk factors include:
- Taking multiple medicines to lower your cholesterol.
- Taking medicines that interact with statins.
- Being female.
- Having a smaller body frame.
- Being age 80 or older.
- Having kidney or liver disease.
- Drinking too much alcohol.
- For some statins, drinking too much grapefruit juice.
- Having certain conditions such as hypothyroidism or neuromuscular disorders including amyotrophic lateral sclerosis (ALS).
Food and drugs that interact with statins
Grapefruit juice has a chemical that can interfere with the enzymes that break down the statins in your digestive system. While you won't need to cut grapefruit entirely from your diet, ask your health care expert about how much grapefruit you can have.
Some medicines that may interact with statins and increase your risk of side effects include:
- Amiodarone (Pacerone), a medicine for irregular heart rhythms.
- Gemfibrozil (Lopid), another variety of cholesterol drug.
- HIV treatments called protease inhibitors such as saquinavir and ritonavir (Norvir).
- Some antibiotic and antifungal medicines, such as clarithromycin and itraconazole (Sporanox).
- Some immunosuppressant medicines, such as cyclosporine (Sandimmune).
There are many drugs that may interact with statins, so be sure your health care professional is aware of all the medicines you take before starting with statins.
How to relieve statin side effects
To relieve side effects believed to be caused by statins, your health care team may recommend several options. Discuss these steps with your care team before trying them:
- Take a brief break from statin therapy. Sometimes it's hard to tell whether the muscle aches or other problems you're having are statin side effects or just part of the aging process. Taking a break can help you decide whether your aches and pains are due to statins or something else.
- Switch to another statin drug. It's possible, although unlikely, that one particular statin may cause side effects for you while another statin won't. It's thought that simvastatin (Zocor) may be more likely to cause muscle pain as a side effect than other statins when it's taken at high doses.
- Change your dose. Lowering your dose may reduce some of your side effects, but it may also reduce some of the cholesterol-lowering benefits your medicine has. Another option is to take the medicine every other day, especially if you take a statin that stays in the blood for several days. Talk to your health care professional to determine if this is appropriate for you.
- Take it easy when exercising. If you aren't used to exercising, this might increase your risk of muscle injury. It's best to make changes in your exercise routine gradually. Exercise causes muscle pain too, so it is sometimes difficult to know if the pain comes from the statin or the exercise in someone who just started an exercise program.
- Consider other cholesterol-lowering medicines. Although statins are the most effective medicines for lowering your cholesterol, other types of drugs also are available. Sometimes, taking a combination of cholesterol drugs can provide the same result with lower doses of statins.
- Try coenzyme Q10 supplements. Coenzyme Q10 supplements may help prevent statin side effects in some people, though more studies are needed to determine any benefits of taking it. Talk to your health care professional first to make sure the supplement won't interact with any of your other medicines.
Weigh the risks and benefits
Although side effects believed to be caused by statins can be annoying, consider the benefits of taking a statin before you decide to stop taking your medicine. Remember that statins can lower your risk of a heart attack or stroke, and the risk of life-threatening side effects from statins is very low.
If you have read about the potential side effects of statins, you may be more likely to blame your symptoms on the medicine, whether or not they're truly caused by the drug.
Even if your side effects are frustrating, don't stop taking your statin medicine without talking to your health care professional first. Your care team may be able make a different treatment plan that can help you lower your cholesterol without uncomfortable side effects.
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
From Mayo Clinic to your inbox
Sign up for free and stay up to date on research advancements, health tips, current health topics, and expertise on managing health. Click here for an email preview.
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing!
You'll soon start receiving the latest Mayo Clinic health information you requested in your inbox.
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
- Controlling cholesterol with statins. Food and Drug Administration. https://www.fda.gov/consumers/consumer-updates/controlling-cholesterol-statins. Accessed March 7, 2023.
- Rosenson RS. Statins: Actions, side effects and administration. https://www.uptodate.com/contents/search. Accessed March 7, 2023.
- Rosenson RS, et al. Statin muscle-related adverse events. https://www.uptodate.com/contents/search. Accessed March 7, 2023.
- Ferri FF. Statin-induced muscle syndromes. In: Ferri's Clinical Advisor 2023. Elsevier; 2023. https://www.clinicalkey.com. Accessed March 7, 2023.
- Blood cholesterol. National Heart, Lung, and Blood Institute. https://www.nhlbi.nih.gov/health/blood-cholesterol. Accessed March 7, 2023.
- US Preventive Services Task Force, et al. Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2022; doi:10.1001/jama.2022.13044.
- South-Paul JE, et al., eds. Dyslipidemias. In: Current Diagnosis & Treatment: Family Medicine. 5th ed. McGraw Hill; 2020. https://accessmedicine.mhmedical.com. Accessed March 7, 2023.
- What is cholesterol? American Heart Association. https://www.heart.org/en/health-topics/cholesterol/about-cholesterol. Accessed March 7, 2023.
- AskMayoExpert. Statin intolerance. Mayo Clinic; 2022.
- Libby P, et al., eds. Lipoprotein disorders and cardiovascular disease. In: Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. 12th ed. Elsevier; 2022. https://www.clinicalkey.com. Accessed March 7, 2023.
- van Hunsel F, et al. Media attention and the influence on the reporting odds ratio in disproportionality analysis: An example of patient reporting of statins. Pharmacoepidemiology and Drug Safety. 2010; doi:10.1002/pds.1865.
- Levenson JL. Psychological factors affecting other medical conditions: Management. https://www.uptodate.com/contents/search. Accessed May 16, 2023.
Products and Services
- A Book: Mayo Clinic Family Health Book, 5th Edition
- Nutritional Supplements at Mayo Clinic Store
- Newsletter: Mayo Clinic Health Letter — Digital Edition
- Arcus senilis: A sign of high cholesterol?
- Birth control pill FAQ
- Cholesterol level: Can it be too low?
- Cholesterol medications: Consider the options
- Cholesterol ratio or non-HDL cholesterol: Which is most important?
- Cholesterol test kits: Are they accurate?
- Cholesterol: Top foods to improve your numbers
- Cholesterol-lowering supplements may be helpful
- Coconut oil: Can it cure hypothyroidism?
- Congenital adrenal hyperplasia
- Prickly pear cactus
- Eggs and cholesterol
- Fasting diet: Can it improve my heart health?
- Hashimoto's disease
- HDL cholesterol: How to boost your 'good' cholesterol
- Herbal supplements and heart drugs
- High cholesterol
- High cholesterol in children
- High cholesterol treatment: Does cinnamon lower cholesterol?
- Hypothyroidism: Can calcium supplements interfere with treatment?
- Hypothyroidism diet
- Hypothyroidism and joint pain?
- Hypothyroidism: Should I take iodine supplements?
- Hypothyroidism symptoms: Can hypothyroidism cause eye problems?
- Hypothyroidism (underactive thyroid)
- Lowering Triglycerides
- Menus for heart-healthy eating
- Metabolic syndrome
- Niacin overdose: What are the symptoms?
- Niacin to improve cholesterol numbers
- Nuts and your heart: Eating nuts for heart health
- Is there a risk of rhabdomyolysis from statins?
- Soy: Does it reduce cholesterol?
- Soy: Does it worsen hypothyroidism?
- Statins: Do they cause ALS?
- Lifestyle changes to improve cholesterol
- Triglycerides: Why do they matter?
- VLDL cholesterol: Is it harmful?
- Mayo Clinic Minute: Out of shape kids and diabetes
- Mayo Clinic Minute: Weight loss surgery for kids
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
- Statin side effects Weigh the benefits and risks
Make twice the impact
Your gift can go twice as far to advance cancer research and care!
Advertisement
Latest statin risk-benefit analysis: more harm than good, a study sought to quantify benefits and harms but in so doing may serve only to reopen old grievances, one critic insists..

- Created with Sketch.

(UPDATED) A new study seeking to allay concerns about statin side effects in primary prevention may have the unintended effect of reopening a debate that many physicians have long considered as settled, a prominent cardiologist warns.
The stated aim of the meta-analysis, published in the BMJ , is to assess the benefit-to-harm ratio of the popular cholesterol-lowering drugs, given pervasive concerns about adverse events in people who haven’t already had a cardiovascular event.
“As patients get a little bit older, the potential harms of [statins and other medications] increase, and for some it may be the case that the harms of treatment might start to outweigh the potential benefits,” senior author James Sheppard, PhD (University of Oxford, England), explained to TCTMD. “And therefore, there may be some patients who are less likely to want to take these sorts of medications.
“But the problem for doctors in clinical practice is that they don't really know who these patients might be,” Sheppard continued, “and there's not great empirical evidence at the moment to the extent to which who has different outcomes. Particularly for statins, it's become quite a controversial area where you see some study saying that statins have lots of side effects and other studies saying that statins have no side effects. It becomes quite confusing quite quickly.”
However, for Steven Nissen, MD (Cleveland Clinic, OH), who commented on the analysis for TCTMD, the study “does a serious disservice to evidence-based medicine” because the researchers don’t go far enough in their wording of the title and conclusions to promote statin use.
The study by Sheppard and colleagues, with lead author Ting Cai, MPH (University of Oxford), estimated that statins prevent 19 MIs, nine strokes, and eight CV deaths per 10,000 patients treated per year. Side effects like muscle symptoms and liver dysfunction were “really, really rare, and from what we could see, they certainly weren't on the same level as the amount of benefits that you get from taking a statin,” Sheppard said.
The authors conclude their paper saying: “Statins were associated with a small increased risk of self-reported muscle symptoms, liver dysfunction, renal insufficiency, and eye conditions in patients without a history of cardiovascular disease. These adverse effects were mild compared with the potential benefits of treatment with statins in preventing major cardiovascular events, suggesting that the benefit-to-harm balance of statins for primary prevention of cardiovascular disease is generally favorable.”
That’s too soft a stance, says Nissen.
“What I'm worried about is when you get this kind of strange study, it really has the effect of getting people who are somewhat cautious about statins to be more cautious,” he stressed. “I think we ought to be more aggressive about giving statins because we know that LDL cholesterol is in fact strongly associated with morbidity and mortality. In general, if you read the manuscript closely, they are saying that the benefits do outweigh the harms, but they are really overemphasizing the harms. These harms are not important.”
In response, Sheppard argued that the issue is not as straightforward as Nissen suggests.
“Unfortunately, many patients (rightly or wrongly) have preconceived ideas about statin treatment and the potential for harm, which stop them from accepting treatment when it is offered,” he said. “To dismiss these ideas and say that the harms are not important I think is unhelpful. In our paper, we acknowledge that these side effects are real, but also mild and very rare. I believe these data are helpful for clinicians speaking to patients who are anxious about starting statin treatment, allowing them to reassure individuals that the potential for side effects is very small, and outweighed by the potential for benefit.”
On behalf of the BMJ editors, Associate Editor Joseph Ross, MD (Yale School of Medicine, New Haven, CT), told TCTMD in an email they “made the decision to publish this study because there remains outstanding uncertainty about the risk of adverse events attributable to statins when used for the primary prevention of cardiovascular disease.
“In contrast, there have been ample studies examining the benefits,” he continued. “Our hope is that because the authors have provided the evidence for risk alongside the evidence for benefits, doing their best to meta-analyze the literature using multiple methodological approaches, patients and their primary care physicians can make more informed choices about whether to use statins for primary prevention.”
Meta-analysis Findings
For the meta-analysis, Sheppard and colleagues included 62 statin trials comprised of more than 120,000 patients without a history of cardiovascular disease who were followed for a mean of 3.9 years.
Statins were associated with greater risks of the following side effects:
- Self-reported muscle symptoms (21 trials): OR 1.06; 95% CI 1.01-1.13
- Liver dysfunction (21 trials): OR 1.33; 95% CI 1.12-1.58
- Renal insufficiency (eight trials): OR 1.14; 95% CI 1.01-1.28
- Eye conditions (six trials): OR 1.23; 95% CI 1.04-1.47
Neither clinically confirmed muscle disorders nor diabetes were shown to be related to statin use.
On the other hand, statins significantly reduced the risks of MI (OR 0.72; 95% CI 0.66-0.78), stroke (OR 0.80; 95% CI 0.72-0.89), and CV death (OR 0.83; 95% CI 0.76-0.91) in 22, 17, and 22 trials, respectively.
“The population we looked at here were people who were at low risk of having a cardiovascular event, so the chances of benefitting from a statin were lower,” Sheppard said. “In this population, you might think actually if there were a risk you might be more cautious about starting a statin, but what we show in the results is that actually the harms associated with statins are so small that is still beneficial to take a statin even if you are at low risk of cardiovascular disease.”
What this analysis wasn’t able to show, however, is which patients are most likely to experience side effects and why, he added, saying this is where future research should focus.
Nissen also took issue with the term “liver dysfunction” used in the study, defined as a rise in serum concentration of aspartate transaminase or alanine transaminase to more than three times the upper limit of normal and other diagnosed liver disorders. “What actually happens with statins is there's a modest increase in liver enzymes that never leads to liver dysfunction,” he said. “So it's not liver dysfunction. It's isolated enzyme increases.”
Ultimately, to say statins are “generally favorable” is “an awfully weak statement of support,” Nissen said. “It's just bizarre that they would conclude that there's not clearly a benefit over a harm.”
Correction: When this story was first published, in 2021, the data in paragraph 6 were inadvertently reported as absolute reductions in risk, rather than absolute risk differences. The data have now been corrected.

Yael L. Maxwell is Senior Medical Journalist for TCTMD and Section Editor of TCTMD's Fellows Forum . She served as the inaugural…
Disclosures
Cai T, Abel L, Langford O, et al. Associations between statins and adverse events in primary prevention of cardiovascular disease: systematic review with pairwise, network, and dose-response meta-analyses. BMJ . 2021;374:n1537.
- This study was funded by a British Heart Foundation PhD Scholarship held by Cai.
- Sheppard is funded by a Wellcome Trust/Royal Society Sir Henry Dale Fellowship, and he also reports receiving funding from a National Institute for Health Research Oxford Biomedical Research Centre Senior Fellowship.
- Nissen reports no relevant conflicts of interest.
Never Miss a Beat
Stay up-to-date with breaking news, conference slides, and topical videos covering the spectrum of CVD. Join our newsletter!
New at TCTMD? Register today!
Forgot Password
Forgot Your Password?
Enter the email you used to register to reset your password.
Search TCTMD
Sign up for our newsletter.
Sign up to receive the most important cardiovascular news, research, and major meeting presentations.
Submit an Event
Become a premium member or log in to view exclusive content.
This content is available for meeting attendees and/or Platinum Members

REGISTER for free or LOG IN to view this content
- Type 2 Diabetes
- Heart Disease
- Digestive Health
- Multiple Sclerosis
- COVID-19 Vaccines
- Occupational Therapy
- Healthy Aging
- Health Insurance
- Public Health
- Patient Rights
- Caregivers & Loved Ones
- End of Life Concerns
- Health News
- Thyroid Test Analyzer
- Doctor Discussion Guides
- Hemoglobin A1c Test Analyzer
- Lipid Test Analyzer
- Complete Blood Count (CBC) Analyzer
- What to Buy
- Editorial Process
- Meet Our Medical Expert Board
This New Drug Lowers Cholesterol Without the Side Effects of Statins
Esperion Therapeutics, Inc
Key Takeaways
- Drugs called statins have been the go-to for helping people lower their cholesterol and reduce their risk of heart disease.
- Statins alone or with lifestyle changes are not always enough to help people get their cholesterol numbers down. Even when they work, the side effects of statins—particularly muscle pain—can be enough to make people stop taking the drugs.
- A new study has shown that another drug called Nexletol (bempedoic acid) can help people lower their cholesterol and prevent heart disease without causing the muscle pain that can come with taking statins.
While some people can lower their cholesterol through lifestyle changes alone, many people need to take medication to get their levels in check. But side effects of the most commonly prescribed medications to treat high cholesterol, called statins, can be enough of a problem that people stop taking the drugs, or won’t start taking them to begin with.
According to a new study, a non-statin medication can help people lower their cholesterol and prevent heart disease without causing muscle pain, which is a major side effect of statins. The drug could also be an add-on treatment for people already takings statins. Here’s what experts want you to know about the medication and whether it could be an option for you.
Bempedoic Acid: A Statin Alternative to Reduce Cholesterol
Statins reduce heart attacks and strokes, which is why they’re first-line therapy, but those side effects can be a problem for people.
The new study, published this month in the New England Journal of Medicine, shows a drug called bempedoic acid is a promising option for patients who can’t or won’t take statins.
Bempedoic acid , sold under the brand name Nexletol, is a first-of-its-kind drug called an adenosine triphosphate-citrate lyase (ACL) inhibitor. The drug works by preventing cholesterol synthesis in the liver.
Bempedoic acid is sold under the brand name Nexletol on its own and as a combination drug with ezetimibe called Nexlizet .
To find out if the drug could safely and effectively lower cholesterol, the researchers conducted a trial with almost 14,000 patients who could not or were not willing to take statins. All of the patients had a history of or were at high risk for getting heart disease. About 7,000 of the patients took Nexletol, and the rest took a placebo.
After about three years of follow-up, the researchers found that the group who took Nexletol experienced more of a cholesterol level decrease than the placebo group—about a 21% greater drop. They also had a lower risk for certain heart health-related events, like heart attacks.
What Are the Limitations of Nexletol?
Nexletol might be safe and effective and not cause muscle pain, but it’s not without side effects and risks.
Nexletol increases uric acid in the blood, which can cause gout and gallstones. The patients in the trial who took Nexletol had a higher incidence of gout than the placebo group. People who are prone to these health problems would need to talk to their providers about whether the statin alternative would be right for them.
What Is Gout?
Gout is a painful form of arthritis defined by inflammation and the accumulation of uric acid crystals in joints. It commonly affects the big toe.
Joshua Knowles, MD, PhD , associate professor of cardiovascular medicine at Stanford Health Care , told Verywell that while statins lower LDL cholesterol , or “bad” cholesterol, by an average of 50%, Nexletol lowers LDL by 25%, so it’s not as potent.
Ezetimibe or PCSK9 inhibitors are other options for patients who can’t take statins, but they can vary in effectiveness. Plus, injectable medications like PCSK9 inhibitors tend to be more expensive than oral medications.
Why Can’t Some People Take Statins?
Muscle aches are the best-known side effect of statin drugs and the most common reason people stop taking them.
While some studies suggest that between 5%–30% of people who take statins stop because they can’t handle the side effects, Knowles said that number is probably inflated—instead, many patients stop taking statins because they’re afraid of side effects.
According to Knowles, the actual number of patients who truly cannot take statins is probably closer to 2%–5%.
“Overcoming some of the limitations of statins was the major driver for this trial,” Michael Lincoff, MD , one of the study’s authors and a cardiologist with the Cleveland Clinic, told Verywell. In his experience, patients who perceive themselves to be statin-intolerant tolerate Nexletol pretty well.
Statins and bempedoic acid act on the same pathway the body uses to form cholesterol, but bempedoic acid is only active in the liver and does not affect the muscles. Researchers think that’s why the study participants who received bempedoic acid didn’t experience muscle pain.
Can You Take Statins and Nexletol Together?
Patients with an inherited predisposition to high cholesterol called familial hypercholesterolemia (also called “familial hypercholesteremia”) may have a hard time achieving a healthy LDL level if they’re only taking one medication.
“Only half of the patients with familial hypercholesteremia achieve goal LDL cholesterol with statins alone,” Knowles said. “Many patients with familial hypercholesteremia start with an LDL of 250 or 300. If you’ve already had a heart attack or stroke, you ideally need an LDL of 70 or lower.”
According to Knowles, providers usually want to reduce a patient’s LDL by over 50% to have a positive effect on their heart disease risk, but that’s beyond what can be achieved with statins alone.
Even with lifestyle modifications and maximum statin therapy, some patients still have high LDL. For these patients, Nexletol could be an effective add-on treatment.
“Some patients, even those that don’t have familial hypercholesteremia, don’t respond as well to statins,” Knowles said. “They might only have a 30% reduction in LDL with statins, so they need additional medication options.”
What’s Next for Nexletol?
The Food and Drug Administration (FDA) actually approved Nexletol back in 2020 for familial hypercholesterolemia and for patients with confirmed heart disease, but the latest study was the first to prove that the drug could be useful for preventing heart disease.
“Our trial proves that not only does bempedoic acid reduce LDL, it also reduces cardiovascular events,” Lincoff said. “That will hopefully enhance payer coverage for the drug and increase the motivation for practitioners to prescribe it.”
With new evidence in hand, Esperio, the company that manufactures Nexletol, will be able to seek permission to update drug labels to include the benefit of lowering heart disease risk.
While the recent trial showed promising evidence that Nexletol could have a role in high cholesterol treatment , statins still have the longest track record, so providers will probably keep using them as first-line treatment.
What This Means For You
Patients who can’t (or won’t) take statins or those who need another treatment in addition to statins and lifestyle changes to lower their cholesterol might benefit from Nexletol (bempedoic acid).
Nissen SE, Lincoff AM, Brennan D, et al. Bempedoic acid and cardiovascular outcomes in statin-intolerant patients . New England Journal of Medicine . Published online March 4, 2023. doi:10.1056/nejmoa2215024
National Library of Medicine: DailyMed. Nexletol—bempedoic acid tablet, film coated [drug label] .
National Library of Medicine: MedlinePlus. Ezetimibe .
Harvard Medical School. Are statins enough? When to consider PCSK9 inhibitors .
Cheeley MK, Saseen JJ, Agarwala A, et al. NLA scientific statement on statin intolerance: a new definition and key considerations for ASCVD risk reduction in the statin intolerant patient . J Clin Lipidol . 2022;16(4):361-375. doi:10.1016/j.jacl.2022.05.068
By Cyra-Lea Drummond, BSN, RN Drummond is a registered nurse and a writer specializing in heart health, cardiac care, pediatric health, and more.
- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español
You are here
Nih research matters.
April 23, 2024
Research in Context: Treating depression
Finding better approaches.
While effective treatments for major depression are available, there is still room for improvement. This special Research in Context feature explores the development of more effective ways to treat depression, including personalized treatment approaches and both old and new drugs.

Everyone has a bad day sometimes. People experience various types of stress in the course of everyday life. These stressors can cause sadness, anxiety, hopelessness, frustration, or guilt. You may not enjoy the activities you usually do. These feelings tend to be only temporary. Once circumstances change, and the source of stress goes away, your mood usually improves. But sometimes, these feelings don’t go away. When these feelings stick around for at least two weeks and interfere with your daily activities, it’s called major depression, or clinical depression.
In 2021, 8.3% of U.S. adults experienced major depression. That’s about 21 million people. Among adolescents, the prevalence was much greater—more than 20%. Major depression can bring decreased energy, difficulty thinking straight, sleep problems, loss of appetite, and even physical pain. People with major depression may become unable to meet their responsibilities at work or home. Depression can also lead people to use alcohol or drugs or engage in high-risk activities. In the most extreme cases, depression can drive people to self-harm or even suicide.
The good news is that effective treatments are available. But current treatments have limitations. That’s why NIH-funded researchers have been working to develop more effective ways to treat depression. These include finding ways to predict whether certain treatments will help a given patient. They're also trying to develop more effective drugs or, in some cases, find new uses for existing drugs.
Finding the right treatments
The most common treatments for depression include psychotherapy, medications, or a combination. Mild depression may be treated with psychotherapy. Moderate to severe depression often requires the addition of medication.
Several types of psychotherapy have been shown to help relieve depression symptoms. For example, cognitive behavioral therapy helps people to recognize harmful ways of thinking and teaches them how to change these. Some researchers are working to develop new therapies to enhance people’s positive emotions. But good psychotherapy can be hard to access due to the cost, scheduling difficulties, or lack of available providers. The recent growth of telehealth services for mental health has improved access in some cases.
There are many antidepressant drugs on the market. Different drugs will work best on different patients. But it can be challenging to predict which drugs will work for a given patient. And it can take anywhere from 6 to 12 weeks to know whether a drug is working. Finding an effective drug can involve a long period of trial and error, with no guarantee of results.
If depression doesn’t improve with psychotherapy or medications, brain stimulation therapies could be used. Electroconvulsive therapy, or ECT, uses electrodes to send electric current into the brain. A newer technique, transcranial magnetic stimulation (TMS), stimulates the brain using magnetic fields. These treatments must be administered by specially trained health professionals.
“A lot of patients, they kind of muddle along, treatment after treatment, with little idea whether something’s going to work,” says psychiatric researcher Dr. Amit Etkin.
One reason it’s difficult to know which antidepressant medications will work is that there are likely different biological mechanisms that can cause depression. Two people with similar symptoms may both be diagnosed with depression, but the causes of their symptoms could be different. As NIH depression researcher Dr. Carlos Zarate explains, “we believe that there’s not one depression, but hundreds of depressions.”
Depression may be due to many factors. Genetics can put certain people at risk for depression. Stressful situations, physical health conditions, and medications may contribute. And depression can also be part of a more complicated mental disorder, such as bipolar disorder. All of these can affect which treatment would be best to use.
Etkin has been developing methods to distinguish patients with different types of depression based on measurable biological features, or biomarkers. The idea is that different types of patients would respond differently to various treatments. Etkin calls this approach “precision psychiatry.”
One such type of biomarker is electrical activity in the brain. A technique called electroencephalography, or EEG, measures electrical activity using electrodes placed on the scalp. When Etkin was at Stanford University, he led a research team that developed a machine-learning algorithm to predict treatment response based on EEG signals. The team applied the algorithm to data from a clinical trial of the antidepressant sertraline (Zoloft) involving more than 300 people.

EEG data for the participants were collected at the outset. Participants were then randomly assigned to take either sertraline or an inactive placebo for eight weeks. The team found a specific set of signals that predicted the participants’ responses to sertraline. The same neural “signature” also predicted which patients with depression responded to medication in a separate group.
Etkin’s team also examined this neural signature in a set of patients who were treated with TMS and psychotherapy. People who were predicted to respond less to sertraline had a greater response to the TMS/psychotherapy combination.
Etkin continues to develop methods for personalized depression treatment through his company, Alto Neuroscience. He notes that EEG has the advantage of being low-cost and accessible; data can even be collected in a patient’s home. That’s important for being able to get personalized treatments to the large number of people they could help. He’s also working on developing antidepressant drugs targeted to specific EEG profiles. Candidate drugs are in clinical trials now.
“It’s not like a pie-in-the-sky future thing, 20-30 years from now,” Etkin explains. “This is something that could be in people's hands within the next five years.”
New tricks for old drugs
While some researchers focus on matching patients with their optimal treatments, others aim to find treatments that can work for many different patients. It turns out that some drugs we’ve known about for decades might be very effective antidepressants, but we didn’t recognize their antidepressant properties until recently.
One such drug is ketamine. Ketamine has been used as an anesthetic for more than 50 years. Around the turn of this century, researchers started to discover its potential as an antidepressant. Zarate and others have found that, unlike traditional antidepressants that can take weeks to take effect, ketamine can improve depression in as little as one day. And a single dose can have an effect for a week or more. In 2019, the FDA approved a form of ketamine for treating depression that is resistant to other treatments.
But ketamine has drawbacks of its own. It’s a dissociative drug, meaning that it can make people feel disconnected from their body and environment. It also has the potential for addiction and misuse. For these reasons, it’s a controlled substance and can only be administered in a doctor’s office or clinic.
Another class of drugs being studied as possible antidepressants are psychedelics. These include lysergic acid diethylamide (LSD) and psilocybin, the active ingredient in magic mushrooms. These drugs can temporarily alter a person’s mood, thoughts, and perceptions of reality. Some have historically been used for religious rituals, but they are also used recreationally.
In clinical studies, psychedelics are typically administered in combination with psychotherapy. This includes several preparatory sessions with a therapist in the weeks before getting the drug, and several sessions in the weeks following to help people process their experiences. The drugs are administered in a controlled setting.
Dr. Stephen Ross, co-director of the New York University Langone Health Center for Psychedelic Medicine, describes a typical session: “It takes place in a living room-like setting. The person is prepared, and they state their intention. They take the drug, they lie supine, they put on eye shades and preselected music, and two therapists monitor them.” Sessions last for as long as the acute effects of the drug last, which is typically several hours. This is a healthcare-intensive intervention given the time and personnel needed.
In 2016, Ross led a clinical trial examining whether psilocybin-assisted therapy could reduce depression and anxiety in people with cancer. According to Ross, as many as 40% of people with cancer have clinically significant anxiety and depression. The study showed that a single psilocybin session led to substantial reductions in anxiety and depression compared with a placebo. These reductions were evident as soon as one day after psilocybin administration. Six months later, 60-80% of participants still had reduced depression and anxiety.
Psychedelic drugs frequently trigger mystical experiences in the people who take them. “People can feel a sense…that their consciousness is part of a greater consciousness or that all energy is one,” Ross explains. “People can have an experience that for them feels more ‘real’ than regular reality. They can feel transported to a different dimension of reality.”
About three out of four participants in Ross’s study said it was among the most meaningful experiences of their lives. And the degree of mystical experience correlated with the drug’s therapeutic effect. A long-term follow-up study found that the effects of the treatment continued more than four years later.
If these results seem too good to be true, Ross is quick to point out that it was a small study, with only 29 participants, although similar studies from other groups have yielded similar results. Psychedelics haven’t yet been shown to be effective in a large, controlled clinical trial. Ross is now conducting a trial with 200 people to see if the results of his earlier study pan out in this larger group. For now, though, psychedelics remain experimental drugs—approved for testing, but not for routine medical use.
Unlike ketamine, psychedelics aren’t considered addictive. But they, too, carry risks, which certain conditions may increase. Psychedelics can cause cardiovascular complications. They can cause psychosis in people who are predisposed to it. In uncontrolled settings, they have the risk of causing anxiety, confusion, and paranoia—a so-called “bad trip”—that can lead the person taking the drug to harm themself or others. This is why psychedelic-assisted therapy takes place in such tightly controlled settings. That increases the cost and complexity of the therapy, which may prevent many people from having access to it.
Better, safer drugs
Despite the promise of ketamine or psychedelics, their drawbacks have led some researchers to look for drugs that work like them but with fewer side effects.
Depression is thought to be caused by the loss of connections between nerve cells, or neurons, in certain regions of the brain. Ketamine and psychedelics both promote the brain’s ability to repair these connections, a quality called plasticity. If we could understand how these drugs encourage plasticity, we might be able to design drugs that can do so without the side effects.

Dr. David Olson at the University of California, Davis studies how psychedelics work at the cellular and molecular levels. The drugs appear to promote plasticity by binding to a receptor in cells called the 5-hydroxytryptamine 2A receptor (5-HT2AR). But many other compounds also bind 5-HT2AR without promoting plasticity. In a recent NIH-funded study, Olson showed that 5-HT2AR can be found both inside and on the surface of the cell. Only compounds that bound to the receptor inside the cells promoted plasticity. This suggests that a drug has to be able to get into the cell to promote plasticity.
Moreover, not all drugs that bind 5-HT2AR have psychedelic effects. Olson’s team has developed a molecular sensor, called psychLight, that can identify which compounds that bind 5-HT2AR have psychedelic effects. Using psychLight, they identified compounds that are not psychedelic but still have rapid and long-lasting antidepressant effects in animal models. He’s founded a company, Delix Therapeutics, to further develop drugs that promote plasticity.
Meanwhile, Zarate and his colleagues have been investigating a compound related to ketamine called hydroxynorketamine (HNK). Ketamine is converted to HNK in the body, and this process appears to be required for ketamine’s antidepressant effects. Administering HNK directly produced antidepressant-like effects in mice. At the same time, it did not cause the dissociative side effects and addiction caused by ketamine. Zarate’s team has already completed phase I trials of HNK in people showing that it’s safe. Phase II trials to find out whether it’s effective are scheduled to begin soon.
“What [ketamine and psychedelics] are doing for the field is they’re helping us realize that it is possible to move toward a repair model versus a symptom mitigation model,” Olson says. Unlike existing antidepressants, which just relieve the symptoms of depression, these drugs appear to fix the underlying causes. That’s likely why they work faster and produce longer-lasting effects. This research is bringing us closer to having safer antidepressants that only need to be taken once in a while, instead of every day.
—by Brian Doctrow, Ph.D.
Related Links
- How Psychedelic Drugs May Help with Depression
- Biosensor Advances Drug Discovery
- Neural Signature Predicts Antidepressant Response
- How Ketamine Relieves Symptoms of Depression
- Protein Structure Reveals How LSD Affects the Brain
- Predicting The Usefulness of Antidepressants
- Depression Screening and Treatment in Adults
- Serotonin Transporter Structure Revealed
- Placebo Effect in Depression Treatment
- When Sadness Lingers: Understanding and Treating Depression
- Psychedelic and Dissociative Drugs
References: An electroencephalographic signature predicts antidepressant response in major depression. Wu W, Zhang Y, Jiang J, Lucas MV, Fonzo GA, Rolle CE, Cooper C, Chin-Fatt C, Krepel N, Cornelssen CA, Wright R, Toll RT, Trivedi HM, Monuszko K, Caudle TL, Sarhadi K, Jha MK, Trombello JM, Deckersbach T, Adams P, McGrath PJ, Weissman MM, Fava M, Pizzagalli DA, Arns M, Trivedi MH, Etkin A. Nat Biotechnol. 2020 Feb 10. doi: 10.1038/s41587-019-0397-3. Epub 2020 Feb 10. PMID: 32042166. Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: a randomized controlled trial. Ross S, Bossis A, Guss J, Agin-Liebes G, Malone T, Cohen B, Mennenga SE, Belser A, Kalliontzi K, Babb J, Su Z, Corby P, Schmidt BL. J Psychopharmacol . 2016 Dec;30(12):1165-1180. doi: 10.1177/0269881116675512. PMID: 27909164. Long-term follow-up of psilocybin-assisted psychotherapy for psychiatric and existential distress in patients with life-threatening cancer. Agin-Liebes GI, Malone T, Yalch MM, Mennenga SE, Ponté KL, Guss J, Bossis AP, Grigsby J, Fischer S, Ross S. J Psychopharmacol . 2020 Feb;34(2):155-166. doi: 10.1177/0269881119897615. Epub 2020 Jan 9. PMID: 31916890. Psychedelics promote neuroplasticity through the activation of intracellular 5-HT2A receptors. Vargas MV, Dunlap LE, Dong C, Carter SJ, Tombari RJ, Jami SA, Cameron LP, Patel SD, Hennessey JJ, Saeger HN, McCorvy JD, Gray JA, Tian L, Olson DE. Science . 2023 Feb 17;379(6633):700-706. doi: 10.1126/science.adf0435. Epub 2023 Feb 16. PMID: 36795823. Psychedelic-inspired drug discovery using an engineered biosensor. Dong C, Ly C, Dunlap LE, Vargas MV, Sun J, Hwang IW, Azinfar A, Oh WC, Wetsel WC, Olson DE, Tian L. Cell . 2021 Apr 8: S0092-8674(21)00374-3. doi: 10.1016/j.cell.2021.03.043. Epub 2021 Apr 28. PMID: 33915107. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, Alkondon M, Yuan P, Pribut HJ, Singh NS, Dossou KS, Fang Y, Huang XP, Mayo CL, Wainer IW, Albuquerque EX, Thompson SM, Thomas CJ, Zarate CA Jr, Gould TD. Nature . 2016 May 26;533(7604):481-6. doi: 10.1038/nature17998. Epub 2016 May 4. PMID: 27144355.
Connect with Us
- More Social Media from NIH
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
Latest science news, discoveries and analysis

Could a rare mutation that causes dwarfism also slow ageing?

Bird flu in US cows: is the milk supply safe?

Future of Humanity Institute shuts: what's next for ‘deep future’ research?

Judge dismisses superconductivity physicist’s lawsuit against university
Nih pay raise for postdocs and phd students could have us ripple effect, hello puffins, goodbye belugas: changing arctic fjord hints at our climate future, china's moon atlas is the most detailed ever made, ‘shut up and calculate’: how einstein lost the battle to explain quantum reality, ecologists: don’t lose touch with the joy of fieldwork chris mantegna.

Should the Maldives be creating new land?

Lethal AI weapons are here: how can we control them?

Algorithm ranks peer reviewers by reputation — but critics warn of bias

How gliding marsupials got their ‘wings’
Bird flu virus has been spreading in us cows for months, rna reveals, audio long read: why loneliness is bad for your health, nato is boosting ai and climate research as scientific diplomacy remains on ice, rat neurons repair mouse brains — and restore sense of smell.
Retractions are part of science, but misconduct isn’t — lessons from a superconductivity lab

Any plan to make smoking obsolete is the right step

Citizenship privilege harms science
European ruling linking climate change to human rights could be a game changer — here’s how charlotte e. blattner, will ai accelerate or delay the race to net-zero emissions, current issue.

The Maldives is racing to create new land. Why are so many people concerned?
Surprise hybrid origins of a butterfly species, stripped-envelope supernova light curves argue for central engine activity, optical clocks at sea, research analysis.

Ancient DNA traces family lines and political shifts in the Avar empire
A chemical method for selective labelling of the key amino acid tryptophan
Robust optical clocks promise stable timing in a portable package
Targeting RNA opens therapeutic avenues for Timothy syndrome
Bioengineered ‘mini-colons’ shed light on cancer progression, galaxy found napping in the primordial universe, tumours form without genetic mutations, marsupial genomes reveal how a skin membrane for gliding evolved.

Scientists urged to collect royalties from the ‘magic money tree’

Breaking ice, and helicopter drops: winning photos of working scientists

Shrouded in secrecy: how science is harmed by the bullying and harassment rumour mill
Want to make a difference try working at an environmental non-profit organization, how ground glass might save crops from drought on a caribbean island, books & culture.

How volcanoes shaped our planet — and why we need to be ready for the next big eruption

Dogwhistles, drilling and the roots of Western civilization: Books in brief

Cosmic rentals
Las borinqueñas remembers the forgotten puerto rican women who tested the first pill, dad always mows on summer saturday mornings, nature podcast.

Latest videos
Nature briefing.
An essential round-up of science news, opinion and analysis, delivered to your inbox every weekday.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Trying 'Magic Mushroom' Drug to Ease Depression? It Has Side Effects
Trying 'Magic Mushroom' Drug to Ease Depression? It Has Side Effects
By Ernie Mundell HealthDay Reporter

WEDNESDAY, April 24, 2024 (HealthDay News) -- Many people with tough-to-treat depression may be trying psilocybin, the active ingredient in magic mushrooms, as an alternative to antidepressants.
Thinking that it's a "natural" drug, folks might assume it comes without side effects.
That assumption would be wrong.
People in a new study who took psilocybin often experienced headache, nausea, anxiety, dizziness and elevated blood pressure -- side effects similar to those seen with regular antidepressants, according to a team from the University of Georgia in Athens (UGA).
U.S. Cities With the Most Homelessness

The good news: Such side effects were only temporary. It's less clear if longer-term side effects might emerge with time, the researchers noted.
The short-term side effects "are what we may expect from your traditional antidepressants because those medications work in a similar fashion to psilocybin. They both target serotonin receptors,” explained senior study author Dr. Joshua Caballero , an associate professor in UGA’s College of Pharmacy.
“It’s very encouraging," he added in a university news release, "because the studies we examined consist of just one or two doses per patient, and we’re finding that the beneficial effects of psilocybin may stay for months when treating depression.”
Psilocybin was shunned by the medical community for decades because, at higher doses, it can have hallucinatory properties. But used under the guidance and supervision of a therapist, the drug is having a comeback as a new form of antidepressant.
But what about any side effects?
To answer that question, Caballero's group looked at data from six different studies on the supervised use of single doses of psilocybin against depression. The studies included a total of 528 people.
They found a number of side effects, among which nausea, dizziness and elevated blood pressure were most common. These effects appeared to dissipate within 48 hours.
Importantly, "psilocybin use was not associated with risk of paranoia and transient thought disorder," the researchers said.
The findings were published recently in the journal JAMA Network Open .
“At some point, I do think that psilocybin will become a treatment option, and when it does, we need to know what the side effects and potential long-term complications are,” Caballero said.
Always use the drug under the supervision of a trusted therapist, he said. One recent study found this was key to successful treatment.
“I would urge caution for people that are thinking this is a magic cure and then go out and take excess mushrooms," Caballero said. "Without proper monitoring, you won’t know the concentration of psilocybin in those mushrooms and you could have a bad trip or other negative outcome.”
The researchers added that the longer-term effects, if any, of psilocybin therapy are unknown.
“There is still a lot we don’t know about the potential long-term side effects and more serious rare side effects of psilocybin use,” Caballero said. He noted that standard antidepressants already carry a boxed warning from the U.S. Food and Drug Administration regarding the potential for an increased risk of suicidal thoughts and suicide in young adults.
Could psilocybin use have a similar risk? It's just not clear yet, Cabellero and colleagues said.
Still, the overall news is good for folks battling tough depression.
"If we can safely use this drug in a controlled environment, I think it could be groundbreaking for a lot of patients that need it," Caballero said.
More information
Find out more about the use of psilocybin against depression at the U.S. National Institutes of Health.
SOURCE: University of Georgia, news release, April 23, 2024
Copyright © 2024 HealthDay . All rights reserved.
Join the Conversation
Tags: psychology , depression
America 2024

Health News Bulletin
Stay informed on the latest news on health and COVID-19 from the editors at U.S. News & World Report.
Sign in to manage your newsletters »
Sign up to receive the latest updates from U.S News & World Report and our trusted partners and sponsors. By clicking submit, you are agreeing to our Terms and Conditions & Privacy Policy .
You May Also Like
The 10 worst presidents.
U.S. News Staff Feb. 23, 2024

Cartoons on President Donald Trump
Feb. 1, 2017, at 1:24 p.m.

Photos: Obama Behind the Scenes
April 8, 2022

Photos: Who Supports Joe Biden?
March 11, 2020

RFK Jr.: By the Numbers
Laura Mannweiler April 26, 2024

Biden’s Student Loan Chief to Step Down
Lauren Camera April 26, 2024

What to Know: Bird Flu Virus in Milk
Cecelia Smith-Schoenwalder April 26, 2024

Inflation a Stubborn Foe for the Fed
Tim Smart April 26, 2024

The Curse of the Modern Vice President

‘A Rule for the Ages’
Lauren Camera April 25, 2024

An arthritis drug helps old dogs, but some owners worry about side effects
Around the country, veterinarians are prescribing a breakthrough new arthritis drug for dogs — Librela — which is helping aging pets get moving again by easing the aches and pains of osteoarthritis.
Ana Maria Cepeda, a veterinarian at North River Animal Hospital in Parrish, Fla., said her first patient was her 14-year-old pit bull mix who in the past has relied on a concoction of pain medication and supplements to cope with severe arthritis and joint issues. “It showed excellent promise on my own dog,” she said. “That gave me more confidence to start trying it in other dogs.”
But not everyone is convinced. Fears about a range of side effects have spread rapidly on social media. A Facebook group shares stories from pet owners alleging that after being given the drug, their dogs had trouble walking or suffered kidney failure . An online petition has been circulating pushing to recall the drug until more study is done. The Food and Drug Administration says its reviewing reports of adverse events.
Veterinarians and the drugmaker say Librela has been shown to be safe and effective. A variety of factors may explain concerns about side effects, including the fact that the drugs are often used in older dogs, who may have a range of health issues.
We spoke to experts about Librela. Here’s what to know.
What is Librela and how does it work?
Librela, made by Zoetis, is the brand name for a monthly injectable drug to treat canine osteoarthritis , a condition that affects an estimated 80 percent of all dogs 8 years or older and as many as 35 percent of dogs of all ages. The drug is an anti-nerve growth factor monoclonal antibody.
Get Well+Being tips straight to your inbox

The drug’s active ingredient is called bedinvetmab, a monoclonal antibody designed to target nerve growth factor, or NGF, a naturally produced protein that’s important for fetal and early development of the nervous system. In adulthood, NGF plays a role in pain transmission and the release of pro-inflammatory molecules. It’s found in high levels in dogs with osteoarthritis.
Librela works by neutralizing NGF in the joint, essentially shutting down the pain pathway and lowering the overall amount of NGF produced. “It reduces how many signals are going to the brain saying, ‘Hey, this hurts,’” said Katie Bennett, an anesthesia and pain management specialist at the Veterinary Specialty Center in Bannockburn, Ill. It also helps alleviate swelling, which causes discomfort.
It’s recommended that dogs receive a minimum of two doses 28 days apart to determine if it can help reduce their pain.
How long has Librela been available?
Librela launched in the United States in October , but it has been used in Europe for the last three years. A sister drug, Solensia, also by Zoetis, has been used to treat osteoarthritis in cats since 2022.
What are the known side effects?
According to Zoetis , side effects can include bacterial skin infections, dermatitis and renal and urinary disorders, including urinary tract infections. In Europe, elevated blood urea nitrogen, which may indicate a kidney issue, was a side effect.
More than 12 million doses of Librela have been sold globally over the last three years, and a fraction of the dogs using Librela (less than 0.20 percent) have experienced “an adverse event,” said Robert Polzer, president of research and development at Zoetis.
Polzer described Librela as “a very safe product.” The company takes adverse events “very seriously,” Polzer said, and continues to collect and analyze data. “To date, we haven’t seen any signals emerge as far as causation between Librela” and the negative outcomes being reported, he said.
“Every medication, whether a human or companion animal medication, has the potential for risk and adverse events,” Polzer said. “Certainly for any pet owner who’s had that experience with their pets, we are quite empathetic.”
On social media, dog owners say their pups’ mobility appeared to decline after using the drug, citing hind leg paralysis in some cases and an inability to walk. Others say their dogs experienced anorexia, lost control of their bowels or experienced kidney issues after using the drug.
Veterinarians said any adverse events should be reported. But they emphasize the drug’s largely positive results.
How much research has been conducted on Librela?
Two clinical trials in the United States and Europe recruited a total of 559 dogs and compared Librela with a placebo for three months each. Neither the dog owners nor the veterinary clinic personnel administering the doses knew if a pet was receiving the real treatment or a placebo.
In the U.S. study , 47.4 percent of dogs receiving Librela showed improvement after the first shot based on owner assessments, compared with 36.6 percent in the placebo group. In the European study , 43.5 percent improved after the first shot, compared with 16.9 percent on placebo. In both studies, the improvements were statistically meaningful. Adverse events were similar in the treatment and placebo groups. After three months of treatment in the European study, 89 dogs in the Librela group (63 percent) had responded positively based on owner and veterinarian assessments, and continued the treatment in a six-month open label phase of the study.
Anecdotally, veterinarians report success with the drug. Union Veterinary Clinic in Washington, D.C., has administered Librela to 49 dogs since October, and the majority are routinely receiving shots, according to Allison Gross, veterinarian and co-owner of the practice. Angell Animal Medical Center in Boston has administered approximately 350 shots, with largely positive results, said Susan O’Bell, veterinarian and internal medicine service director at Angell.
What animals should be given the drug?
Patient selection is important, vets say. The drug should only be prescribed to healthy dogs diagnosed with osteoarthritis and is not recommended for dogs with kidney or neurological issues. The drug is not recommended for dogs who have limping or lameness from another cause, such as a cruciate tear, bone tumors or back or disc injuries. Librela is not recommended for dogs under 12 months, who are pregnant or lactating.
Most veterinarians recommend bloodwork, a urinalysis and possibly other diagnostic tests before prescribing. “A lot is dependent too on the conversations with the client and what their ultimate goal is,” Cepeda said. “Some people, it’s a quality-of-life issue, some people have difficulty medicating, and some people have tried everything under the sun and are desperate for something else.”
Osteoarthritis is a progressive disease, and vets say controlling chronic pain requires time and patience. See how your dog responds to Librela, and discuss with your vet lowering the dosage of other medications.
“It is not a quick fix, not a magic bullet,” Bennett said. “Clients will have to work at it for a couple of months to learn a sweet spot.”
Weight management, water therapy, physiotherapy, laser treatments, acupuncture and a number of other therapeutics can collectively help manage not just joint pain but the disease’s overall effect on the body. “This is the long haul,” O’Bell said. Librela is “one more thing we have in our arsenal.”
How do you know if the drug is working?
Bennett asks owners to keep a daily journal of their dog’s progress and provides owners with a worksheet to measure improvement. Veterinarians suggest taking pictures and videos documenting changes in your dog’s body language, gait and demeanor.
Vets recommend trying two injections over two months before discontinuing Librela, which may not work for every patient. Some patients at Angell in Boston stopped Librela because owners did not see any big improvements, O’Bell said.
Bennett took her own senior dog off Librela after she developed a urinary tract infection. Not every dog will have a miraculous turn around, veterinarians caution. For some, the drug’s efficacy eventually wears off.
For dogs who regain their spark and spunk, experts advise easing them back into play. “Some dogs have become so active they injure themselves, because they go from not moving much at all to running around,” Gross said.
Alexandra E. Petri is a freelance writer based in New York who has worked for the Los Angeles Times and the New York Times. Petri is a two-time reporting fellow with the International Women’s Media Foundation.
Do you have a fitness question? Email [email protected] and we may answer your question in a future column.
Read more from Well+Being
Well+Being shares news and advice for living well every day. Sign up for our newsletter to get tips directly in your inbox.
Are you taking your meds wrong ? Many patients make these common mistakes.
Centenarians give their advice about everything.
The wall sit is a simple exercise that can lower your blood pressure.
Tart cherries — more specifically, tart cherry juice — may help with inflammation and pain.
Do you self-sabotage ? Here’s how to stop.
- Running or hiking solo? 9 ways to stay safer while exercising alone. April 24, 2024 Running or hiking solo? 9 ways to stay safer while exercising alone. April 24, 2024
- Want to run a fall marathon? It’s time to pick a training plan for your goals. April 10, 2024 Want to run a fall marathon? It’s time to pick a training plan for your goals. April 10, 2024
- How to start tai chi April 5, 2024 How to start tai chi April 5, 2024

An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts

Statin Adverse Effects: A Review of the Literature and Evidence for a Mitochondrial Mechanism
Beatrice a. golomb.
a Department of Medicine, University of California, San Diego, USA
b Department of Family & Preventive Medicine, University of California, San Diego, USA
Marcella A. Evans
c School of Medicine, University of California, Irvine, California, USA
HMG-CoA reductase inhibitors (statins) are a widely used class of drug, and like all medications have potential for adverse effects (AEs). Here we review the statin AE literature, first focusing on muscle AEs as the most reported problem both in the literature and by patients. Evidence regarding the statin muscle AE mechanism, dose effect, drug interactions, and genetic predisposition is examined. We hypothesize, and provide evidence, that the demonstrated mitochondrial mechanisms for muscle AEs have implications to other nonmuscle AEs in patients treated with statins. In meta-analyses of randomized controlled trials (RCTs), muscle AEs are more frequent with statins than with placebo. A number of manifestations of muscle AEs have been reported, with rhabdomyolysis the most feared. AEs are dose dependent, and risk is amplified by drug interactions that functionally increase statin potency, often through inhibition of the cytochrome P450 (CYP)3A4 system. An array of additional risk factors for statin AEs are those that amplify (or reflect) mitochondrial or metabolic vulnerability, such as metabolic syndrome factors, thyroid disease, and genetic mutations linked to mitochondrial dysfunction. Converging evidence supports a mitochondrial foundation for muscle AEs associated with statins, and both theoretical and empirical considerations suggest that mitochondrial dysfunction may also underlie many non-muscle statin AEs. Evidence from RCTs and studies of other designs indicates existence of additional statin-associated AEs, such as cognitive loss, neuropathy, pancreatic and hepatic dysfunction, and sexual dysfunction. Physician awareness of statin AEs is reportedly low even for the AEs most widely reported by patients. Awareness and vigilance for AEs should be maintained to enable informed treatment decisions, treatment modification if appropriate, improved quality of patient care, and reduced patient morbidity.
Introduction
HMG-CoA reductase inhibitors (statins) have been the best selling prescription drug class in the US and include atorvastatin, the best-selling prescription drug in the world – indeed in history. 1 - 3 These drugs are perceived to have a favorable safety profile 4 - 6 and have well documented benefits to cardiovascular disease in many groups, including persons who are younger and older, male and female, at moderate and high cardiovascular risk. In addition, benefits have been objectively shown to exceed risks on average for both total mortality and total morbidity (indexed by serious adverse events), specifically in clinical-trial equivalent middle-aged men who are at high cardiovascular risk. 7 - 9 Although many people treated with statins do well, no drug is without potential for adverse effects (AEs). There is need for awareness of risks as well as benefits of all drugs, particularly those that, like statins, are used on a wide scale where even uncommon effects can translate to significant public health impact.
Statins inhibit the enzyme HMG-CoA reductase, at a stage early in the mevalonate pathway. 10 This pathway generates a range of other products in addition to cholesterol, such as coenzyme Q10, heme-A, and isoprenylated proteins, 10 which have pivotal roles in cell biology and human physiology and potential relevance to benefits as well as risks of statins. 11 - 13 Additionally, cholesterol itself is not merely a final product (with its own range of vital roles) but also an intermediate to a suite of additional products of fundamental relevance to health and well-being, such as sex steroids, corticosteroids, bile acids and vitamin D, several of which have been shown to be affected with statin administration. 14 , 15 The biochemical influences of statins extend well beyond the lipid profile and its constituents (low-density lipoprotein cholesterol [LDL-C], high-density lipoprotein cholesterol [HDL-C], and triglycerides), and even beyond the direct products of the mevalonate pathway, to include a wide swath of products and functions modified through these as well as nonmevalonate effects of statins, ranging from nitric oxide and inflammatory markers 16 to polyunsaturated fatty acids, 17 among many others.
This report reviews evidence related to statin induction of AEs and evidence for a dose-response relationship, and describes reported drug interactions. Muscle AEs are emphasized as they are the best recognized AEs of statins (liver AEs are perhaps second most recognized), and the AEs on which much of the information on mechanism, drug interactions, and dose-response has been obtained – information that, as we show, has relevance to other statin AEs. 18 , 19 Statins lead to dose-dependent reductions in coenzyme Q10, 20 - 22 a key mitochondrial antioxidant and electron transport carrier that serves to help bypass existing mitochondrial respiratory chain defects. 23 - 25 We review convergent evidence supporting a role for mitochondrial predispositions and mechanisms for statin muscle AEs. We seek to place other statin AEs in the context this evidence provides, proposing that mitochondrial dysfunction may underlie additional AEs reported on statins.
Muscle Adverse Effects (AEs)
Myositis and myalgia.
The best recognized and most commonly reported AEs of statins are muscle AEs, 26 , 27 and include muscle pain, fatigue and weakness as well as rhabdomyolysis. While individual randomized controlled trials (RCTs) often fail to show an excess of muscle problems or symptoms, meta-analysis of randomized double-blind, placebo-controlled trials have shown increased myositis in patients receiving statins relative to placebo (odds ratio [OR] 2.56, 95% CI 1.12-5.85), with myositis there defined as creatinine kinase (CK) > 10 times the upper limit of normal with myalgia. 28
In contrast to myositis, myalgia was not increased on average based on meta-analysis of RCTs that compared statins to placebo (relative risk [RR] 1.09, 95% CI 0.97-1.23). 28 However, this does not necessarily mean that statins do not cause myalgia, and this point seems not to be uniformly appreciated. Rather, evidence has shown that statins reduce pain and improve walking distance in many individuals (for instance, but not confined to, persons with peripheral arterial disease), 29 an effect that may arise through improved blood flow deriving from endothelial function benefits in persons with endothelial dysfunction. 30 An overall null effect of statins on muscle pain in clinical trials may therefore indicate that, in the samples selected for these trials, statins caused muscle pain in approximately as many people as they relieved it.
In support of this view, triangulating evidence suggest that statins have a causal role in myalgia as well as muscle weakness in some people. For instance: A double-blind, placebo-controlled, crossover biopsy study showed partially reversible mitochondrial myopathy in persons reporting non-CK-elevating or minimally CK-elevating muscle symptoms on statins. 31 In a family in which multiple members experienced statin-associated non-CK elevating muscle pain, objective investigation affirmed myopathic findings. 32 Prior muscle symptoms on statins or other cholesterol drugs represent a predictor for future symptoms with statin rechallenge and may signal elevated risk for rhabdomyolysis on statins. 33 - 36 Patients who experienced muscle symptoms on statins (typically with normal or slightly elevated levels of CK), that reverse with discontinuation, most often re-experienced muscle symptoms if rechallenged with an equivalent or higher expected potency statin based on calculated potency equivalencies; in contrast, those rechallenged with a lower potency statin re-experience problems significantly less frequently, p<0.01). 37 These data support the view that muscle symptoms arising on statins and reversing with discontinuation are in many individual cases causally statin-associated, whether or not on average an increase in muscle symptoms occurs with statins.
This observation underscores a critically important point relevant to drug AEs in general, which merits emphasis and has relevance to other reported statin AEs. A significant increase in rates of a problem on drug vs placebo in RCTs supports a causal link between that drug and that AE, in some persons. However, absence of an average significant increase in a problem, or even presence of a significant average reduction in a problem, does not preclude causal occurrence of that problem in some individuals. Illustrating this point are the recognized occurrence of ‘paradoxical’ increases in blood pressure (BP) in some people with use of medicines designed to lower BP in most people, and ‘paradoxical’ increases in anxiety or aggression in some people who are given drugs designed to produce the opposite effect. 38 - 45
In the case of statins, a potential basis for opposing effects occurring in muscle and in other organs can be identified. Evidence supports the proposition that antioxidant effects of statins underlie (or contribute to) many fundamental statin benefits – including benefits to flow and oxygen delivery 46 - 48 and inflammation. 49 , 50 These effects may participate in improved walking distance in patients on statins, including benefits to muscle/walking in persons with and without peripheral artery disease. 29 Yet a subset of people reproducibly exhibit increases in markers of oxidation on statins, 51 and the occurrence of this increase has been tied to muscle pain on statins. 52 , 53
RCTs are important for evaluating average effects that may have relevance to use of a drug for treatment in a group overall. However, AEs are important to an individual even if they do not occur on average, and non-RCT data, including case-based data, have recognized importance in AE assessments. 54 - 57 Bearing this in mind, Table I shows a range of additional muscle problems that have been reported on statins beyond the classical ‘myalgia’ and ‘myositis.’ 17 , 29 , 51 , 58 - 78
Muscle Effects Reported on Statins
AChE = acetylcholinesterase; CK = creatine kinase; LDL-C = low density lipoprotein cholesterol.
Persistent Muscle Effects
Muscle effects arising on statins do not uniformly resolve fully with statin discontinuation. 155 Crossover biopsy studies show a partially reversible mitochondrial myopathy in persons presenting with recurrent muscle pain on statins. 31 Statins elevate the respiratory exchange ratio and do so even in asymptomatic persons, while persons who have been symptomatic on statins show an elevated off-statin respiratory exchange ratio. 156 - 158 This altered cell respiratory function in persons with AEs may represent a cause (predisposing to statin myopathy) and/or a consequence of statin myopathy; the relative contributions of each awaits prospective study.
A range of cases have now been reported in which statin use has “uncovered” previously clinically silent or clinically tolerated conditions, ranging from McArdle disease 159 , 160 to myotonic dystrophy 159 to acid maltase deficiency 161 to possible Kennedy disease. 159 Statins have also exacerbated known muscle conditions, such as myasthenia gravis. 78 In the case of mitochondrial myopathies, the relative degree to which statins have unmasked vs induced disease may not always be clear. 159 , 162
Rhabdomyolysis
Rhabdomyolysis is among the best-recognized and most feared complications of statins; it occurs when muscle damage is severe, leading to a marked elevation of CK (e.g. in excess of 10 times the upper limit of normal) often accompanied by evidence of renal dysfunction and occasionally renal failure and death. 81 , 94 , 163 - 166 Over 900 unique PubMed citations (as of January 2009) pair the keywords ‘rhabdomyolysis’ with terms referring to statins, i.e. ‘statins’, ‘HMG’, or each generic statin name individually. However, the recognition of rhabdomyolysis as a statin complication does not rest on randomized trial data, which even on meta-analysis do not support a significant increase (e.g. OR 1.59, 95% CI 0.54-4.70). 28
A case report has suggested that misinterpretation of evidence-based medicine from RCTs on statin rhabdomyolysis may have fatal consequences – and perhaps has had. 81 Underscoring the limitations of clinical trials for AE identification, cerivastatin was withdrawn from the market due to excess risk of rhabdomyolysis, although no cases of rhabdomyolysis occurred on cerivastatin in a meta-analysis of randomized trials. 28 In contrast, observational studies of real-world use reported that rhabdomyolysis occurred with substantially higher frequency on cerivastatin than other statins, 167 , 168 particularly for cerivastatin in combination with fibrates (and specifically gemfibrozil). 168 This was true for postmarketing surveillance data 169 and for claims data. 168 Illustrative of this, in one study, claims data rates per 10,000 person-years of treatment were 0.44 for simvastatin, atorvastatin or pravastatin alone (95% CI 0.20-0.84); 5.34 for cerivastatin (95% CI 1.46-13.68); 2.82 for fibrates (95% CI 0.58-8.24); and 0 for no lipid therapy (95% CI 0-0.48). With a modest number of total rhabdomyolysis cases, the difference approached but did not quite reach significance (p=0.056). Rates rose to 5.98 for statin (non-cerivastatin) combined with a fibrate (95% CI 0.72-216.0), and 1035 for cerivastatin-fibrate combinations (95% CI 389-2117). 168
Emphasizing that figures for a larger group need not apply to subgroups within that group, per year of therapy the number needed to treat, to see one case of rhabdomyolysis was 22,727 for statin (monotherapy) overall, but 484 for older patients with diabetes mellitus treated with combined statin and fibrate, and 9.7 to 12.7 for patients who received cerivastatin plus fibrate. 168 (As reviewed below, much of the excess in cases is attributable to high potency resulting specifically from gemfibrozil-cerivastatin interaction effects. 170 , 171 )
In the setting of statin rhabdomyolysis, other organs may also be severely affected. Renal failure is well recognized and is a consequence of the rhabdomyolysis, but concurrent heart, 96 , 109 , 110 pancreas, 96 , 105 liver, 96 , 106 - 108 , 172 bone marrow, 96 , 173 , 174 respiratory, 96 , 98 and CNS toxicity 96 , 112 – or all of the above 96 – are also reported.
Dose Response
A range of sources support a dose relation for statin AEs ( Table II 37 , 167 , 170 , 171 , 175 - 184 ), although there may exist AEs that are not dose dependent. Meta-analyses of RCTs comparing lower vs higher potency statins are of greatest relevance among the clinical trial data because these examine similar patients (within the same study) placed on drugs of different potencies. 176 , 178 Results of these meta-analyses have supported more total AEs with statin vs placebo 175 (although this may not be equally true in all settings), more total AEs with intensive vs nonintensive statin use, 176 and more AEs leading to dropouts with intensive vs nonintensive statin use. 176 (Dropout rates are not, however, necessarily greater for lower intensity statin use vs placebo in clinical trial samples. 28 ) In addition, CK elevations and liver function test (LFT elevations) occur more frequently with higher dose vs lower dose statins. 176 , 178
Evidence For, and Suggestive Of, Dose/Potency Dependence of Statin Adverse Effects (AEs)
ALT = alanine aminotransferase; CK = creatine kinase; CYP = cytochrome P450; HbA1c = glycosylated hemoglobin; JUPITER = Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin; LDL-C = low-density lipoprotein cholesterol; LFTs = liver function tests; OR = odds ratio; PROVE-IT–TIMI = Pravastatin or Atorvastatin Evaluation and Infection Therapy – Thrombolysis in Myocardial Infarction trial; RCT = randomized controlled trial; SEARCH = Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine; ULN = upper limit of normal; VA = US Department of Veterans Affairs.
Rechallenge data also support dose-related effects. This study design examines muscle symptom recurrence in persons with prior statin AEs. Patients rechallenged with same-or-higher potency statins (relative to the potency of the statin on which problems originally arose) usually re-experienced the problem, and did so significantly more frequently than those rechallenged with lower potency statins. 37 Examination of rechallenge data provides a highly efficient study design because at-risk patients are selected for, and by comparing subjects to themselves, erosion of power arising from cross-subject variability is reduced.
Although some investigators promote very low LDL-C targets, proposing that lower is better and no LDL-C is too low, 191 - 193 the US FDA has stated that “all statins… should be prescribed at the lowest dose that achieves the goals of therapy (e.g. target LDL-C level).” 180 Intensive statin treatment in RCTs does not improve mortality, even in patients with heart disease, relative to less intensive treatment (although it may do so in the setting of acute coronary syndrome). 194 Moreover, intensive treatment comes at the cost of an increased risk of adverse outcomes. 176 , 194
Drug Interactions
Fibrates, particularly gemfibrozil, amplify the risk of rhabdomyolysis on statins (most powerfully for cerivastatin 170 , 171 ), and are present in many statin rhabdomyolysis reports, 82 , 99 , 105, 109 , 195 - 221 likely due to their effect of impeding statin metabolism and perhaps their additional lipid-modifying effects. (Other cholesterol-lowering drugs have also been implicated in muscle toxicity 222 and in statin rhabdomyolysis cases, although less frequently. 223 - 225 ) However, lipid-lowering drugs are not the sole drug class that may increase risk of statin rhabdomyolysis and other statin AEs (see Table III, 105 155 , 169 - 171 , 179 , 195 - 199 , 202 , 210 , 223 - 233 ).
Drug Interactions Reported in Statin Rhabdomyolysis
AE = adverse effect; ALT = alanine aminotransferase; CAD = coronary artery disease; CK = creatine kinase; CYP = cytochrome P450; LDL-C = low-density lipoprotein cholesterol; SEARCH = Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine; ULN = upper limit of normal.
Drug interactions arise when drugs inhibit metabolic pathways of statins, compete for metabolism with statins, or cause similar or interacting toxicity. Additionally, apparent interactions may arise when drugs serve as markers for existing problems that signal vulnerability to statin AEs.
Several widely used statins – atorvastatin, simvastatin, and lovastatin (and previously cerivastatin, now off the market) – are metabolized by the cytochrome P450 (CYP)3A4 pathway. 318 (Simvastatin acid is also metabolized by CYP2C8; fluvastatin is primarily metabolized by the CYP2C9 pathway, while pravastatin and rosuvastatin are not metabolized by these systems. 318 ) Concurrent administration of statins with CYP3A4 inhibitors may raise statin concentrations and risk of toxicity, including rhabdomyolysis. 185 The CYP3A4 pathway is inhibited by a variety of agents including cyclosporin, erythromycin, azole antifungals, and antiretrovirals such as ritonavir. 318 , 319 (Antiretrovirals may also cause lipids to rise, thus creating both the need for lipid therapy and the setting in which it is more toxic. 302 ) Some agents, such as calcium channel blockers, are considered weaker CYP3A4 inhibitors and appear to increase statin rhabdomyolysis risk, perhaps to a lower degree. 186 , 318 , 320 However, interaction effects vary dramatically among statins as well as among subjects for a single statin. Regarding the former, increases in simvastatin concentrations may be several times greater than in atorvastatin concentrations with concurrent CYP3A4 inhibitors. 321 Regarding the latter, one study of 12 subjects showed more than tenfold interindividual variation in the extent of interaction between simvastatin and both erythromycin and verapamil as indexed by these drugs' effect on simvastatin concentration. 322 Of note, in a large study using administrative claims data, statin-associated muscle disorders including rhabdomyolysis were six-fold elevated in persons on concurrent CYP3A4 inhibitors. 167
Grapefruit juice and perhaps pomegranate juice inhibit CYP3A4 and have been presumptively linked to statin rhabdomyolysis. 230 , 323 (Combined rosuvastatin-ezetimibe therapy was involved in the report involving pomegranate juice. 230 ) Although some urge caution only with consumption of greater than a quart of grapefruit juice a day, 94 , 324 , 325 far smaller quantities of grapefruit juice can pose a potential risk in vulnerable subjects: less than a cup daily of grapefruit juice for three days, consumed prior to subjects' simvastatin dose, reportedly increased simvastatin concentrations by four-fold on average (range: ~two-fold to nine-fold, p<0.01). 326
The CYP3A pathway has a prominent role in drug metabolism in liver and intestine 327 and approximately half of prescription drugs are metabolized by CYP3A4. 328 For this reason polypharmacy may lead to competition for a common metabolic pathway. This competition may increase statin concentrations and the risk of dose-related statin AEs.
Individuals may differ in their response to individual statins, in terms of both efficacy and tolerability, due to pharmacogenomic differences, including those that affect statin hepatic uptake, clearance, and CYP pathways. 329 - 332 Differences in these pathways may also lead to differential vulnerability to drug interactions.
Fibrates have special relevance to statin AEs, and as noted above, gemfibrozil, in particular, interferes with statin metabolism (an effect that was found to be singularly powerful in combination with cerivastatin 170 , 171 ). Additionally, fibrates themselves may be linked to rhabdomyolysis. 168 Finally, fibrates may serve as markers for a population at risk for statin AEs – persons with high triglycerides and impaired fatty acid oxidation (those most likely to receive fibrates) may also be at amplified risk of statin AEs. 94 , 155 , 333
Risk Factors for Adverse Effects
In addition to dose and drug interactions, a multitude of other factors have been associated with an increased risk of statin AEs. Reported risk factors and corresponding citations are delineated in Table IV . 36 , 37 , 51 , 52 , 94 , 163 , 176 , 178 , 180 , 186 , 191 , 192 , 283 - 288 , 300 , 325 , 334 - 340
Risk Factors for Statin Adverse Effects (AEs)
ALT = alanine aminotransferase; BMI = body mass index; CK = creatine kinase; CYP = cytochrome P450; LDL-C = low-density lipoprotein cholesterol; OR = odds ratio; SEARCH = Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine; ULN = upper limit of normal.
Most risk factors depicted can be viewed as sharing one or both of two primary mediating pathways: increased statin exposure (e.g. dose, drug interactions, genetic variants or other factors that affect clearance or hepatic uptake) or mitochondrial derangement or vulnerability (with factors producing mitochondrial problems or serving as a marker for existing ones). Reduced concentrations of coenzyme Q10 are particularly a problem in the setting of existing mitochondrial dysfunction because ample coenzyme Q10 can bypass a range of respiratory chain defects, 23 - 25 fostering adequate ATP production and improving the redox state. Additionally, toxicity of certain interacting drugs may be mediated through mitochondrial mechanisms (as Table III shows), and mitochondrial-relevant genetic defects have been disproportionately found in patients who experience statin myopathy (reviewed below), strongly supporting mitochondrial vulnerability. Metabolic syndrome factors, particularly hypertension, are linked to increased risk of statin AEs; and these factors, including obesity, hypertriglyceridemia, hyperglycemia and particularly hypertension, have been linked to mitochondrial dysfunction and mitochondrial DNA defects. 377
Mitochondrial Effects
While a medley of potential mechanisms may cause or contribute to statin AEs (and these merit more full review in another venue), mitochondrial mechanisms have been repeatedly implicated in muscle AEs. Mitochondrial defects predispose to problems on statins (as shown in the second to last entry of Table IV , ‘Genetic mutations associated with mitochondrial dysfunction’). Additionally, statins predispose to mitochondrial defects ( Table V, 22 31 , 32 , 112 , 155 , 158 , 162 , 397 , 406 - 414 ) – in all users and, to a greater degree, in vulnerable individuals. Dose-dependent reductions in coenzyme Q10 20 - 22 can reduce cell energy, promote oxidation, 362 , 415 promote apoptosis, and unmask silent mitochondrial defects. 23 - 25 , 362 , 415 - 418 The mevalonate pathway, which statins inhibit, also produces heme-A, which has it own central involvement in mitochondrial electron transport. 419
Mitochondrial (mt) Effects Reported in Patients Treated with Statins a
AE = adverse effect; CK = creatine kinase; COX = cytochrome C oxidase; MRS = magnetic resonance spectroscopy; RER = respiratory exchange ratio; ULN = upper limit of normal.
Statins reduce 20 - 22 and coenzyme Q10 supplementation increases 420 - 422 serum coenzyme Q10 levels. The ability to demonstrate tissue changes in coenzyme Q10 with administration of either agent is more variable; however, irrespective of changes in tissue coenzyme Q10 levels, changes in tissue mitochondrial and respiratory function clearly occur (improved with coenzyme Q10, impaired with statins). 25 , 156 - 158 , 407 , 423 Indeed, a range of study types have shown mitochondrial and metabolic predispositions to statin AE vulnerability, and mitochondrial and metabolic effects of statins in animals 317 , 347 , 424 - 428 as well as humans, with mitochondrial effects in humans arising in all users or selectively in those who express AEs ( Tables TablesV V and andVI VI ).
Mitochondrial Adverse Effects (AEs) with Statins in Individuals and Families: Evidence from Case Reports a
CK = creatine kinase; MELAS = Mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes.
Non-muscle Statin Adverse Effects
Muscle is highly aerobically dependent and selectively vulnerable to mitochondrial pathology. 430 But given the evidence for mitochondrial vulnerability and pathology related to statin AEs, it merits note that other organs – including brain, liver, heart and kidney – can be affected by mitochondrial pathology as well, 430 and we suggest mitochondrial mechanisms may also be involved in a range of nonmuscle statin AEs. The occurrence of failure of other organs in concert with rhabdomyolysis is noteworthy in this regard, and multiple organ injury or failure has been reported in the context of statin rhabdomyolysis. 81 , 91 , 96 , 98 , 100 , 105 , 107 - 109 , 112 , 431
Cognitive problems are second only to muscle problems among patient reports of statin AEs. 432 Brain tissue shares with muscle tissue a high mitochondrial vulnerability as both are postmitotic tissue with high metabolic demand. 433 - 437 Muscle has a very high dynamic range of demand; and the brain, while reflecting only about 2-4% of (nonobese) body mass, accounts for approximately 20% of oxygen 438 and 50% of glucose utilization. 439 Muscle and brain are the organs most classically affected in mitochondrial disease (mitochondrial myopathy and encephalomyopathy are classical manifestations of respiratory chain diseases). For instance, mitochondrial encephalomyopathy resulting from heritable coenzyme Q10 deficiency classically produces fatigue, muscle symptoms, and cognitive problems, 440 although the cases referred for analysis are often relatively severe. 429 , 441 Gastrointestinal 26 and neurological symptoms, 432 , 442 psychiatric symptoms, 443 - 446 sleep problems, 444 , 447 glucose elevations, 182 and a range of other symptoms reported on statins also arise in mitochondrial dysfunction. 379 , 448 - 457
Table VII, 28 31 , 108 , 172 , 178 , 181 , 458 - 481 shows those AEs for which there is RCT support in some subject groups (and provides, in some cases, additional non-RCT evidence). Randomized trials have recognized limitations for AE detection, due in part to selection considerations. 482 Even among RCTs, studies that differ in selection criteria are expected to differ in expression of, and power for, AE occurrence due to effect modification. (This issue is not specific to statins, but is germane to assessment of risks and benefits for all drugs.)
Statin Adverse Effects (AEs) Supported by Evidence from Randomized Controlled Trials (RCTs) a
CK = creatine kinase; CVD = cardiovascular disease; HbA1c = glycosylated hemoglobin; HR = hazard ratio; JUPITER = Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin; LDL-C = low-density lipoprotein cholesterol; LFTs = liver function tests; PROVE-IT-TIMI = Pravastatin or Atorvastatin Evaluation and Infection Therapy – Thrombolysis in Myocardial Infarction trial; TIA = transient ischemic attack; ULN = upper limit of normal.
When the average effect (of drug on outcome) is harmful in RCTs, then it can be concluded that adverse consequences to that outcome occur in at least some individuals. However, when average effects are not harmful, AEs to that outcome are not on that basis excluded. Recall that randomized trials seek to determine the overall or average impact of a drug on an outcome (in the selected sample), in order to assess whether the drug may be used to benefit that outcome on average . It is worth re-emphasizing that harms in an individual are important even if benefits occur on average.
Case reports coupled with triangulating evidence can represent an important source of evidence regarding occurrence of specific AEs, and case reports and case series are reportedly the primary grounds upon which label changes with drugs occur. 54 - 57 For identification of AEs in an individual, the experience of the individual is the most relevant since average effects need not apply to an individual, whether average effects are determined by RCT or observational designs. Table VIII, 15 369 , 444 , 538 - 560 characterizes reported AEs that do not have identified RCT support.
Statin Adverse Effects (AEs) Supported or Suggested by Case Series, Case Studies and Observational Designs a , b
ALS = amyotrophic lateral sclerosis; CI = confidence interval; HRT = hormone-replacement therapy; LDL-C = low-density lipoprotein cholesterol; MELAS = Mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes; OR = odds ratio; RBC = red blood cells; RCT = randomized controlled trial; UCSD = University of California, San Diego.
Effect modification – leading to statins producing different effects on the same outcome in different individuals – is recognized in the context of statin (and other lipid-lowering drug) effects on lipids, 881 , 882 and has previously been discussed in relation to statin muscle effects (benefits to walking occur in some, 29 while detriments occur in others 113 ). As Table VII shows, a similar theme pervades other statin effects, with statins reported to benefit and worsen proteinuria and to benefit and worsen arrhythmia, cardiac function, and an array of other outcomes. We speculate that a common source of effect modification underlies many of these reported benefits and harms – with statin-induced antioxidant effects and improved flow benefiting many organs in some individuals; and statin-induced pro-oxidant effects and mitochondrial dysfunction adversely affecting a range of organs and outcomes in other individuals. Indeed, even RCT evidence has differed for the same outcome in different subject groups, generally along the lines this proposition predicts.
Prevention, Treatment, and Recovery of Statin Adverse Effects
Observational and limited randomized trial data variably suggest partial (though incomplete) benefit of coenzyme Q10 supplementation to muscle symptoms; and to other AEs of statins (observational data). 883 - 886 Additional studies are required to better understand the role of coenzyme Q10 supplementation in prevention and mitigation of statin AEs. It merits note that preparations of coenzyme Q10 vary widely in their bioavailability. 887
Randomized trial evidence has little to offer in understanding recovery profiles for statin AEs, although some evidence is beginning to emerge. While one study reported uniform recovery of statin muscle AEs, 888 a larger statin myopathy clinic including more objective data noted that recovery is often incomplete when objective measures are used. 889 Other evidence supports this, noting for muscle AEs that “variable persistent symptoms occurred in 68% of patients despite cessation of therapy.” 155 Incomplete resolution in some subjects has been reported for other AEs. Thus, in an analysis of data, presented in the Australian Adverse Drug Reaction Bulletin , it was noted that “Statin-associated peripheral neuropathy may persist for months or years after withdrawal of the statin… In two ADRAC (Adverse Drug Reactions Advisory Committee) cases of persistent peripheral neuropathy, motor and sensory conduction tests showed minimal recovery 4 and 12 months, respectively, after discontinuation of simvastatin, despite clinical improvement.” 561
Underrecognition of Statin Adverse Effects
As others have observed, “finding potential drug-safety problems requires skillful observation by clinicians who are attuned to the possibility of drug-related adverse events.” 890 and (according to FDA officials) “physicians need to think ‘adverse drug reactions’ when encountering unexpected symptoms in their patients.” 891
Even for the most commonly reported AEs involving statins, patients state that physicians often dismiss the possibility that their AE may be statin related. 432 Failure to recognize drug AEs can prevent needed reassessment of the risk-benefit profile for statin treatment – and where appropriate, modification of the treatment regimen, in the face of possible or probable statin AEs. This may reduce quality of patient care, reduce medication compliance relative to a modified regimen, and place patient safety in peril both for morbidity and mortality from not only the AEs, but also perhaps from the conditions the medication is designed to treat.
The converse is also true: awareness of statin AEs is vitally important as it may improve recognition of these effects when they arise, enable more informed treatment decisions by patient and provider, improve the quality of patient care – and reduce patient suffering and morbidity.
It has been observed that “as more information is learned through the results of clinical trials, LDL-C goals become more stringent and difficult to attain. Large doses of high-potency statins, sometimes given in combination with other lipid-lowering agents, are frequently necessary to achieve these goals. As a result, the frequency of AEs from statin therapy may be expected to increase, and less common AEs may occur more often.” 734 This increases the importance of recognition of statin AEs.
As reviewed here, AEs on statins may signal a mitochondrial vulnerability, which may alter or perhaps even reverse an otherwise favorable impact of statins on cell energetics. And AEs may signal occurrence of a net prooxidant rather than antioxidant effect of statins 53 with possible unfavorable implications for a range of statins' proposed pleiotropic effects. 892
When possible side effects arise in a patient on any drug, the risk-benefit balance of treatment should be reassessed. Statins are a linchpin of current approaches to cardiovascular protection: however, AEs of statins are neither vanishingly rare nor of trivial impact. For statins, as for all medications, vigilance for potential AEs is imperative. Recognition of potential statin AEs is needed and may be fostered by an improved awareness both of relevant literature and of its limitations.
Acknowledgements
The authors have no conflicts of interest to report. Work that contributed to this paper was funded by a Robert Wood Johnson Generalist Physician Faculty Scholar award to Dr Golomb. The study sponsor did not participate in study design; in the collection, analysis, or interpretation of data; in the writing of this report; or in the decision to submit this paper for publication. The authors would like to thank Hanh Nguyen and Jersey Neilson for kind assistance securing articles; and Sabrina Koperski for excellent editorial and administrative assistance.
- International edition
- Australia edition
- Europe edition

Cakes and drinks sweetener neotame can damage gut wall, scientists find
Industry’s sugar substitute E961 can have ‘toxic effect on health’, says study finding sweetener capable of damaging intestinal bacteria
A sweetener used in cakes, soft drinks and chewing gum can seriously damage people’s health by weakening the gut, a new study has found.
Consumption of even a small amount of the sweetener neotame can lead to someone starting to suffer irritable bowel syndrome, insulin resistance, and even sepsis, a condition that kills about 40,000 in Britain a year.
The findings underlined that some of a new generation of sweeteners that give food products a super-sweet taste can have a “toxic effect” on health, the researchers said.
Dr Havovi Chichger , the senior author of the study, said that while sweeteners could be a healthier alternative to sugar, some could harm consumers.
Neotame was developed in 2002 as a substitute for aspartame, a sweetener which has aroused concerns, and has become widely used in recent years in drinks and foodstuffs sold in the UK. It is often referred to as E961 on the list of ingredients found on labels of products.
Chichger, an associate professor at Anglia Ruskin University, and the study’s co-author, Dr Aparna Shil, of Jahangirnagar University, in Bangladesh, said neotame carried a threat to health because it could damage the intestine by causing “good bacteria” to become diseased and invade the gut wall. In the process that could lead to illness because the epithelial barrier, part of the gut wall, could break down.
They published their findings, which they said are the first to show that neotame can have that damaging impact on healthy gut bacteria, in the medical journal Frontiers in Nutrition .
Previous research, including by Chichger, found that other common sweeteners – such as saccharin, sucralose, and aspartame – can also have that harmful effect.
Chichger said: “There is now growing awareness of the health impacts of sweeteners such as saccharin, sucralose and aspartame, with our own previous work demonstrating the problems they can cause to the wall of the intestine and the damage to the ‘good bacteria’ which form in our gut.
“This can lead to a range of potential health issues including diarrhoea, intestinal inflammation, and even infections such as septicaemia if the bacteria were to enter the blood stream. Therefore, it is important to also study sweeteners that have been introduced more recently, and our new research demonstrates that neotame causes similar problems, including gut bacteria becoming diseased.”
The co-authors said further research was needed to look into “the toxic effects of some of the artificial sweeteners that have been developed more recently”, given their widespread use. Some of the newest sweeteners in use produce a sweet taste that is 1,000 times sweeter than sugar.
Even a low intake of neotame might be harmful, Chichger stressed. “Even when we studied neotame at very low concentrations, 10 times lower than the acceptable daily intake, we saw the breakdown of the gut barrier and a shift in bacteria to a more damaging behaviour, including increased invasion of healthy gut cells leading to cell death. This can be linked to issues such as irritable bowel diseases and sepsis,” she said.
The European Food Safety Authority ruled in 2007 that neotame was “safe for use”. It has since been approved for use in more than 35 countries. But Efsa is now reviewing the safety of neotame as part of what Chichger said is a series of evidence-based risk assessments which may lead to a reassessment of certain sweeteners.
- Food safety
- Soft drinks
- Irritable bowel syndrome
- Food & drink industry
- Health policy
Most viewed
Numbers, Facts and Trends Shaping Your World
Read our research on:
Full Topic List
Regions & Countries
- Publications
- Our Methods
- Short Reads
- Tools & Resources
Read Our Research On:
Mental health and the pandemic: What U.S. surveys have found

The coronavirus pandemic has been associated with worsening mental health among people in the United States and around the world . In the U.S, the COVID-19 outbreak in early 2020 caused widespread lockdowns and disruptions in daily life while triggering a short but severe economic recession that resulted in widespread unemployment. Three years later, Americans have largely returned to normal activities, but challenges with mental health remain.
Here’s a look at what surveys by Pew Research Center and other organizations have found about Americans’ mental health during the pandemic. These findings reflect a snapshot in time, and it’s possible that attitudes and experiences may have changed since these surveys were fielded. It’s also important to note that concerns about mental health were common in the U.S. long before the arrival of COVID-19 .
Three years into the COVID-19 outbreak in the United States , Pew Research Center published this collection of survey findings about Americans’ challenges with mental health during the pandemic. All findings are previously published. Methodological information about each survey cited here, including the sample sizes and field dates, can be found by following the links in the text.
The research behind the first item in this analysis, examining Americans’ experiences with psychological distress, benefited from the advice and counsel of the COVID-19 and mental health measurement group at Johns Hopkins Bloomberg School of Public Health.
At least four-in-ten U.S. adults (41%) have experienced high levels of psychological distress at some point during the pandemic, according to four Pew Research Center surveys conducted between March 2020 and September 2022.
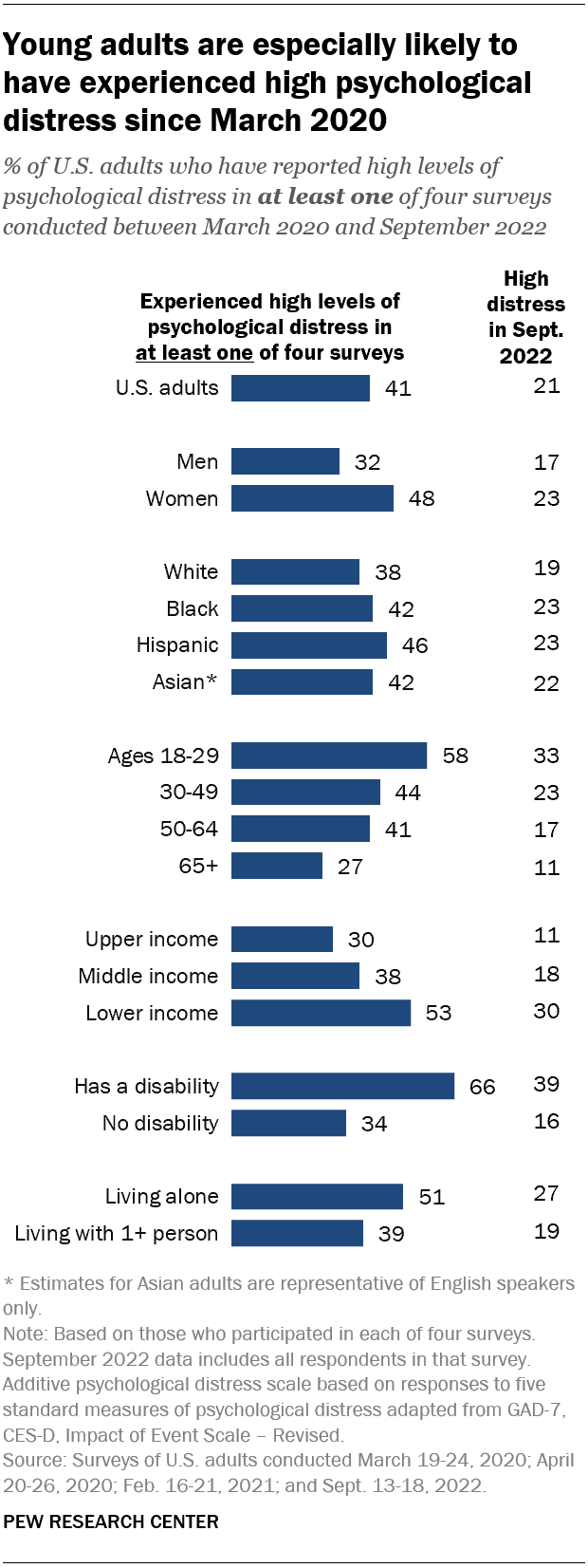
Young adults are especially likely to have faced high levels of psychological distress since the COVID-19 outbreak began: 58% of Americans ages 18 to 29 fall into this category, based on their answers in at least one of these four surveys.
Women are much more likely than men to have experienced high psychological distress (48% vs. 32%), as are people in lower-income households (53%) when compared with those in middle-income (38%) or upper-income (30%) households.
In addition, roughly two-thirds (66%) of adults who have a disability or health condition that prevents them from participating fully in work, school, housework or other activities have experienced a high level of distress during the pandemic.
The Center measured Americans’ psychological distress by asking them a series of five questions on subjects including loneliness, anxiety and trouble sleeping in the past week. The questions are not a clinical measure, nor a diagnostic tool. Instead, they describe people’s emotional experiences during the week before being surveyed.
While these questions did not ask specifically about the pandemic, a sixth question did, inquiring whether respondents had “had physical reactions, such as sweating, trouble breathing, nausea, or a pounding heart” when thinking about their experience with the coronavirus outbreak. In September 2022, the most recent time this question was asked, 14% of Americans said they’d experienced this at least some or a little of the time in the past seven days.
More than a third of high school students have reported mental health challenges during the pandemic. In a survey conducted by the Centers for Disease Control and Prevention from January to June 2021, 37% of students at public and private high schools said their mental health was not good most or all of the time during the pandemic. That included roughly half of girls (49%) and about a quarter of boys (24%).
In the same survey, an even larger share of high school students (44%) said that at some point during the previous 12 months, they had felt sad or hopeless almost every day for two or more weeks in a row – to the point where they had stopped doing some usual activities. Roughly six-in-ten high school girls (57%) said this, as did 31% of boys.
On both questions, high school students who identify as lesbian, gay, bisexual, other or questioning were far more likely than heterosexual students to report negative experiences related to their mental health.
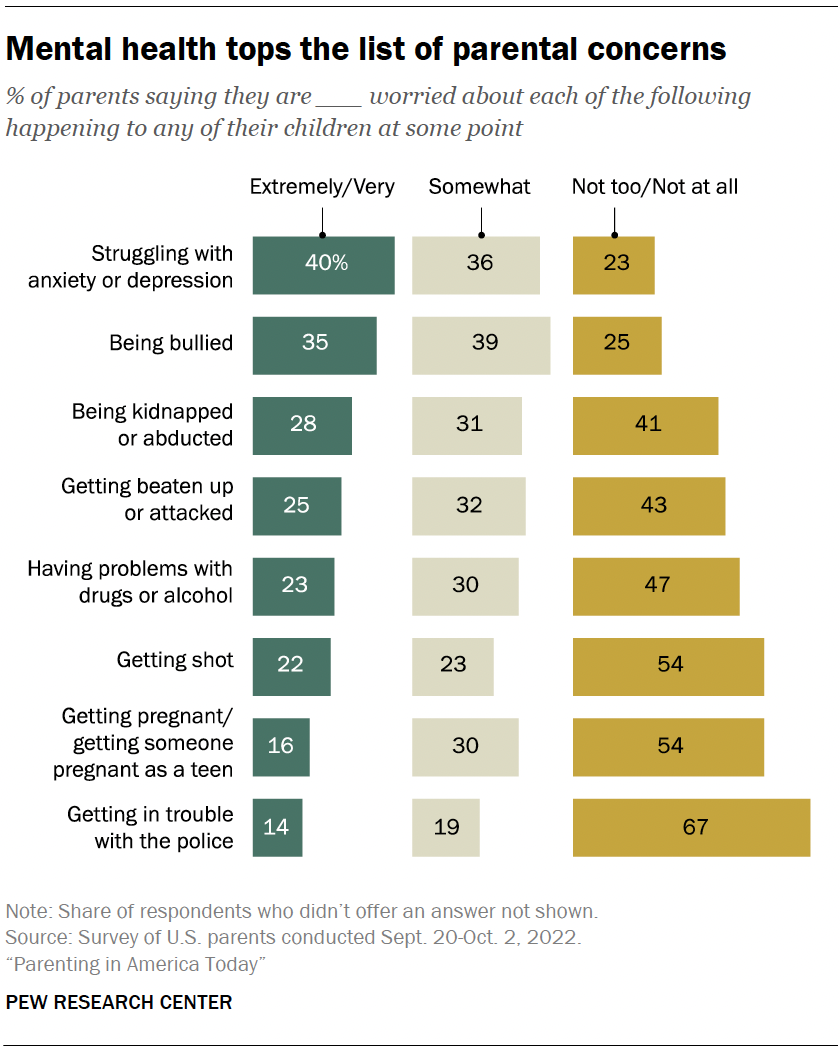
Mental health tops the list of worries that U.S. parents express about their kids’ well-being, according to a fall 2022 Pew Research Center survey of parents with children younger than 18. In that survey, four-in-ten U.S. parents said they’re extremely or very worried about their children struggling with anxiety or depression. That was greater than the share of parents who expressed high levels of concern over seven other dangers asked about.
While the fall 2022 survey was fielded amid the coronavirus outbreak, it did not ask about parental worries in the specific context of the pandemic. It’s also important to note that parental concerns about their kids struggling with anxiety and depression were common long before the pandemic, too . (Due to changes in question wording, the results from the fall 2022 survey of parents are not directly comparable with those from an earlier Center survey of parents, conducted in 2015.)
Among parents of teenagers, roughly three-in-ten (28%) are extremely or very worried that their teen’s use of social media could lead to problems with anxiety or depression, according to a spring 2022 survey of parents with children ages 13 to 17 . Parents of teen girls were more likely than parents of teen boys to be extremely or very worried on this front (32% vs. 24%). And Hispanic parents (37%) were more likely than those who are Black or White (26% each) to express a great deal of concern about this. (There were not enough Asian American parents in the sample to analyze separately. This survey also did not ask about parental concerns specifically in the context of the pandemic.)
Looking back, many K-12 parents say the first year of the coronavirus pandemic had a negative effect on their children’s emotional health. In a fall 2022 survey of parents with K-12 children , 48% said the first year of the pandemic had a very or somewhat negative impact on their children’s emotional well-being, while 39% said it had neither a positive nor negative effect. A small share of parents (7%) said the first year of the pandemic had a very or somewhat positive effect in this regard.
White parents and those from upper-income households were especially likely to say the first year of the pandemic had a negative emotional impact on their K-12 children.
While around half of K-12 parents said the first year of the pandemic had a negative emotional impact on their kids, a larger share (61%) said it had a negative effect on their children’s education.
- Coronavirus (COVID-19)
- Happiness & Life Satisfaction
- Medicine & Health
- Teens & Youth

John Gramlich is an associate director at Pew Research Center
How Americans View the Coronavirus, COVID-19 Vaccines Amid Declining Levels of Concern
Online religious services appeal to many americans, but going in person remains more popular, about a third of u.s. workers who can work from home now do so all the time, how the pandemic has affected attendance at u.s. religious services, economy remains the public’s top policy priority; covid-19 concerns decline again, most popular.
1615 L St. NW, Suite 800 Washington, DC 20036 USA (+1) 202-419-4300 | Main (+1) 202-857-8562 | Fax (+1) 202-419-4372 | Media Inquiries
Research Topics
- Age & Generations
- Economy & Work
- Family & Relationships
- Gender & LGBTQ
- Immigration & Migration
- International Affairs
- Internet & Technology
- Methodological Research
- News Habits & Media
- Non-U.S. Governments
- Other Topics
- Politics & Policy
- Race & Ethnicity
- Email Newsletters
ABOUT PEW RESEARCH CENTER Pew Research Center is a nonpartisan fact tank that informs the public about the issues, attitudes and trends shaping the world. It conducts public opinion polling, demographic research, media content analysis and other empirical social science research. Pew Research Center does not take policy positions. It is a subsidiary of The Pew Charitable Trusts .
Copyright 2024 Pew Research Center
Terms & Conditions
Privacy Policy
Cookie Settings
Reprints, Permissions & Use Policy

COMMENTS
June 27, 2023. Statins, one of the most extensively studied drugs on the planet, taken by tens of millions of Americans alone, have long had a perplexing side effect. Many patients—some 5 ...
They say their findings suggest that the benefit-to-harm balance of statins for adults without heart disease is generally favourable. Statins are widely used to prevent heart disease, and severe side effects are rare, but many people are reluctant to take them because of the potential for milder effects such as muscle weakness and stiffness.
Two widely used statins, rosuvastatin and atorvastatin, are equally effective at preventing heart attacks, strokes and death in people with coronary artery disease. But while rosuvastatin ...
To take a closer look at statins' effect on blood vessels, Liu and colleagues tested a common statin, simvastatin, on lab-grown human endothelial cells derived from induced pluripotent stem cells. Endothelial cells make up the lining of blood vessels, but in many diseases they transform into a different cell type, known as mesenchymal cells ...
Statins are associated with a small increased risk of side effects in patients without a history of heart disease, but these effects are mild compared with the potential benefits of treatment in ...
A meta-analysis of six statin trials that included 57,593 participants revealed a 13% increase in the relative risk of new-onset diabetes 3 — a more modest effect than that seen in the JUPITER ...
A new study shows statins are much more effective. ... based on prior research, ... "So the punch line of the trial is people blame statins for side effects the statins aren't really causing," he ...
Although large-scale meta-analyses confirmed that the frequency of clinically significant side effects associated with statin therapy is low, 10 more research is ... This meta-analysis sheds new light on the discussion of the relationship between statins and diabetes mellitus incidence and confirms that statin use is not associated with cancer ...
Statin therapies are not the cause of muscle pain in over 90% of those who experience symptoms, according to a new study led by researchers from Oxford Population Health. The results were published today in The Lancet and presented at the European Society of Cardiology Congress.
The pleiotropic effect of statins may potentially prevent brain damage after subarachnoid hemorrhage; thus, future large-cohort studies are desirable. In addition, recent research has focused on the rupture-preventive statin effects on unruptured cerebral aneurysms.
According to the study, statins would reduce the relative risk of dying by 9%. In absolute terms, the man would reduce his risk from 38% to 34.6%, and the woman from 1.4% to 1.3%. Patients and ...
New evidence released today from a study of 31,245 patients already taking statin therapy indicates that inflammation may be a more powerful predictor of risk of future cardiovascular events ...
Studies find drug protects against heart disease in high-risk groups. Two new studies found that statins, the most prescribed class of drugs to treat high cholesterol, are protective for high-risk groups who haven't yet had a heart attack or stroke but could be at risk of one, according to Harvard-affiliated Brigham and Women's Hospital.
Very rarely, statins can cause life-threatening muscle damage called rhabdomyolysis (rab-doe-my-OL-ih-sis). Rhabdomyolysis can cause extreme muscle pain, liver damage, kidney failure and death. The risk of very serious side effects is extremely low. Only a few cases of rhabdomyolysis occur per million people taking statins.
"Statins can be very helpful for long-term risk reduction and with very minimal side effects and great benefits. It's one of those key medications that have changed the face of medicine ...
Bempedoic acid, an ATP citrate lyase inhibitor, reduces low-density lipoprotein (LDL) cholesterol levels and is associated with a low incidence of muscle-related adverse events; its effects on card...
Statin-associated muscle symptoms. Statin-associated muscle symptoms (SAMS, the focus of a separate Consensus Statement) 12 are the predominant adverse effect encountered in clinical practice (Figure Figure1 1), and impact adherence and ultimately clinical outcomes (Box Box1 1). 13, 14 A much-debated issue is whether SAMS represent real or nocebo effects.
However, any adverse impact of these side-effects on major vascular events has already been taken into account in the estimates of the absolute benefits. Statin therapy may cause symptomatic adverse events (eg, muscle pain or weakness) in up to about 50-100 patients (ie, 0·5-1·0% absolute harm) per 10 000 treated for 5 years.
Statins are often discontinued because of side effects, 1,2 even though some blinded trials have not shown an excess of symptoms with statins as compared with placebo. 3,4 Patients who had ...
For the meta-analysis, Sheppard and colleagues included 62 statin trials comprised of more than 120,000 patients without a history of cardiovascular disease who were followed for a mean of 3.9 years. Statins were associated with greater risks of the following side effects: Self-reported muscle symptoms (21 trials): OR 1.06; 95% CI 1.01-1.13
Results. Myalgia is the most common side effect of statin use, with documented rates from 1-10%. Rhabdomyolysis is the most serious adverse effect from statin use, though it occurs quite rarely (less than 0.1%). The most common risk factors for statin-related myopathy include hypothyroidism, polypharmacy and alcohol abuse.
Even when they work, the side effects of statins—particularly muscle pain—can be enough to make people stop taking the drugs. A new study has shown that another drug called Nexletol (bempedoic acid) can help people lower their cholesterol and prevent heart disease without causing the muscle pain that can come with taking statins.
If you can't take statins because of the side effects, you're statin-intolerant. With certain statins, you should avoid grapefruit products because they can increase side effects. You should limit the amount of alcohol that you drink because combining alcohol and statin usage can increase your risk of liver damage.
Finding better approaches. While effective treatments for major depression are available, there is still room for improvement. This special Research in Context feature explores the development of more effective ways to treat depression, including personalized treatment approaches and both old and new drugs.
Find breaking science news and analysis from the world's leading research journal.
People in a new study who took psilocybin often experienced headache, nausea, anxiety, dizziness and elevated blood pressure -- side effects similar to those seen with regular antidepressants ...
Fears about a range of side effects have spread rapidly on social media. A Facebook group shares stories from pet owners alleging that after being given the drug, their dogs had trouble walking or ...
Abstract. HMG-CoA reductase inhibitors (statins) are a widely used class of drug, and like all medications have potential for adverse effects (AEs). Here we review the statin AE literature, first focusing on muscle AEs as the most reported problem both in the literature and by patients. Evidence regarding the statin muscle AE mechanism, dose ...
Previous research, including by Chichger, found that other common sweeteners - such as saccharin, sucralose, and aspartame - can also have that harmful effect.
At least four-in-ten U.S. adults (41%) have experienced high levels of psychological distress at some point during the pandemic, according to four Pew Research Center surveys conducted between March 2020 and September 2022. Young adults are especially likely to have faced high levels of psychological distress since the COVID-19 outbreak began: 58% of Americans ages 18 to 29 fall into this ...