Select "Patients / Caregivers / Public" or "Researchers / Professionals" to filter your results. To further refine your search, toggle appropriate sections on or off.

American Association for Cancer Research (AACR)
Investing in Innovation: The AACR grants program sparks innovation in basic, translational, and clinical research by providing critical support to promising investigators at all career levels.

Support Lifesaving Cancer Research. Donate Now.
Cancer is not a single disease, but rather a collection of diseases all characterized by the uncontrolled proliferation of cells.

Screening for Early Detection: Using evidence-based guidelines to screen for cancer can help find aberrations at the earliest possible detectable phase of cancer development and progression.

More than 299,000 men in the U.S. are expected to be diagnosed with prostate cancer this year. Read about prevention, screening, and treatment options for this type of cancer.

Get updates on the AACR Annual Meeting 2024 recently held in San Diego on the AACR Blog.
The report highlights the AACR’s impact on the cancer community during the past year while advancing our mission to prevent and cure all cancers.

Whether honoring a special person or a special day, a donation to the American Association for Cancer Research has a lasting impact.

The official news website of the AACR Annual Meeting 2024. Stay up to date on the latest developments from the most important cancer meeting in the world.
The AACR Cancer Progress Report 2023 provides a comprehensive overview of the latest research-driven advances against the collection of devastating diseases called cancer.
The AACR and its more than 58,000 members worldwide are advancing a scientifically bold agenda against the collection of diseases we call cancer.
A new wave of research-driven discoveries and technological innovations are delivering – and will propel additional – transformative advances to save more lives from cancer..
By the Numbers
percent decrease of the overall age-adjusted cancer death rate in the U.S. from 1991 to 2020
therapeutics were approved for new or expanded uses by the FDA from Aug. 1, 2022, to July 31, 2023
million cancer survivors in the U.S. are living with, through, and beyond their disease thanks to research
cancer diagnoses in the United States are associated with preventable risk factors
Your donation to the American Association for Cancer Research helps our more than 58,000 members worldwide drive progress against cancer.

- The Progression of Cancer
- AACR Project GENIE®: Powering Precision Medicine
- Patient Advocacy
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 24 March 2021
Advancing Cancer Therapy
Nature Cancer volume 2 , pages 245–246 ( 2021 ) Cite this article
15k Accesses
34 Citations
4 Altmetric
Metrics details
Cancer therapies have evolved considerably in recent decades, substantially improving the quality of life and survival of patients with cancer. In this issue, we launch our Series on Cancer Therapy, exploring current paradigms and recent advances and challenges in this field, through specially commissioned articles.
The earliest evidence of cancer treatment can be traced back to an ancient Egyptian medical text, written around 3000 BC and known widely as the ‘Edwin Smith Papyrus’, that described the cauterization of breast tumors for which, according to the text, there was no cure . The situation is very different now, as, depending on breast cancer subtype, stage and demographic factors, the 5-year survival rates for this disease can surpass 90% in developed countries. For cancer types that are responsive to therapy, including certain subtypes of breast, blood and prostate malignancies, patients now face the management of a chronic disease, rather than a fatal one, owing to the rapid advances in clinical oncology over recent decades. Similarly, the prognosis for several other cancer types has also been improving. For example, patients with melanoma, which used to be considered a deadly disease, have much better prospects thanks to the breakthroughs in targeted and immune-based therapies.
These advances reflect the focus placed on cancer research and oncology by governments, funders and research institutes across the globe over the past several decades. In the USA, 2021 marks the 50-year anniversary of the signing of the National Cancer Act into law, which marked the beginning of a concerted effort to address cancer as a leading cause of death in the USA at the federal level. The National Cancer Program that arose from this initiative resulted in a profound institutional reorganization within the National Institutes of Health, with the overarching goal of developing the infrastructures required ‘for the treatment, cure, and elimination of cancer’. Other countries and international agencies also adopted cancer-focused initiatives over the years, including, for example, the PRIME scheme of the European Medicines Agency, which supports the development of medicines that target an unmet medical need, including cancer, through accelerated planning, evaluation and approval processes.
Thus, substantial progress has been made across first-line cancer therapy modalities. Surgery continues to be a first-line treatment for many cancer types, but it now includes precision and minimally invasive surgery, molecular imaging support and, more recently, robot- or artificial intelligence–assisted surgical procedures. The clinical use of one of the most widely used treatment modalities, chemotherapy, has been improved through better dosing regimens, neoadjuvant or adjuvant administration, and combination therapies. Similarly, radiation oncology has been advanced through precision radiotherapy. First-line recommendations depend on the cancer type and stage at diagnosis, and have continued to be modified as new therapeutic modalities have become available. The advent of targeted therapy and immunotherapy has revolutionized the treatment of cancer, especially with the development and availability of sophisticated diagnostic and molecular characterization technologies. Among these, ‘-omics’ techniques stand out for increasingly enabling a more precise and granular molecular characterization of cancer types and subtypes and the identification of biological correlates of response to specific therapies, thereby enriching the roster of biomarkers at the disposal of clinicians.
Targeted therapies have swiftly taken a prominent position in cancer research and clinical oncology in recent decades, thanks to the molecular insights into oncogenic processes and mechanisms gained from fundamental research and technological development. A key example of how basic research on oncogenic alterations translated into substantial clinical benefits for a large number of patients is BCR-ABL1 tyrosine-kinase inhibitors for chronic myeloid leukemia. The first BCR-ABL1 tyrosine-kinase inhibitor was discovered through drug screens in 1992, and in 2001 it became the first-line therapy with long-term remission rates for BCR-ABL–driven chronic myeloid leukemia 1 ; second-generation tyrosine-kinase inhibitors, rationally designed to circumvent acquired resistance, earned approval from the US Food and Drug Administration as frontline therapies only a decade later. More recently, the announcement of the two first-in-class inhibitors of the mutant kinase KRAS G12C was a milestone in the decades-long efforts to study and treat tumors bearing these, up-to-now considered undruggable, KRAS mutations 2 . However, not every effort in precision oncology and targeted therapy is yielding similarly positive results, especially given the issue of adaptive and acquired resistance, a complication of therapy that a large part of the cancer-research community is striving to address. It should also be noted that advances in sophisticated cancer therapeutics are sometimes associated with a high financial burden for patients, a pressing societal issue tied to the complexities of addressing the challenge of cancer 3 .
In light of the progress made so far and the goals and challenges ahead, we are pleased to launch in this issue of Nature Cancer a Series on Cancer Therapy comprising specially commissioned Review, Perspective, News and Comment articles and a collection of relevant primary research articles published in Nature Cancer . The series is housed in a dedicated page on the Nature Cancer website and will be continually updated with additional content from key opinion leaders discussing novel therapeutic opportunities, the path to drug discovery, and how these advances are transforming clinical practice.
Our series launches with two Review articles that focus on different but important aspects of cancer treatment. Whereas substantial achievements have been witnessed in the treatment of primary tumors, progress has been more modest for metastatic disease. Yibin Kang and colleagues discuss the clinical challenge of treating metastatic disease, and how preclinical and mechanistic knowledge accumulated over the years is being translated into tangible clinical benefits for disseminated disease 4 . The authors also discuss the challenges of running clinical trials for metastatic disease, and the different degrees of success of clinical trials in the metastatic setting. In a separate Review, Frank McCormick and colleagues discuss the multiple and complex links between oncogenic KRAS—one of the most frequently mutated and, as noted above, hard-to-target cancer drivers—and metabolism, highlighting the potentially targetable vulnerabilities that arise at the interface of the two 5 . Although various aspects of targeting KRAS-dependent cancer metabolism have been explored extensively in preclinical settings, ongoing and future clinical trials will hopefully shed light on the translatability of these approaches to the clinic.
Despite the many milestones achieved in cancer treatment, much remains to be addressed. In future issues we will present additional pieces focusing on a breadth of topics under this theme, including key pathways deregulated in cancer, such as EGFR or PI3K, and ongoing clinical approaches for preventing and bypassing therapy resistance. Future issues will also discuss progress in radiotherapy, immunotherapy and therapy combinations, as well as new therapeutic modalities, such as bispecific antibodies, and innovative drug-development approaches through the implementation of artificial intelligence.
Through this selection of commissioned and primary research publications, we aim to underscore how much cancer therapy has advanced over the past several decades, which goals need to be prioritized, and the challenges that should be overcome to continue improving quality of life and outcomes for patients with cancer. We thank our authors and referees for their valuable contributions and hope that our readers will find this Series on Cancer Therapy informative and inspiring.
Druker, B. J. et al. N. Engl. J. Med. 344 , 1038–1042 (2001).
Article CAS Google Scholar
Bar-Sagi, D., Knelson, E. H. & Sequist, L. V. Nat. Cancer 1 , 25–27 (2020).
Article Google Scholar
Gruber, K. Nat. Cancer 1 , 1136–1139 (2020).
Esposito, M. et al. Nat. Cancer https://doi.org/10.1038/s43018-021-00181-0 (2021).
Mukhopadhyay, S. et al. Nat. Cancer https://doi.org/10.1038/s43018-021-00184-x (2021).
Download references
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Advancing Cancer Therapy. Nat Cancer 2 , 245–246 (2021). https://doi.org/10.1038/s43018-021-00192-x
Download citation
Published : 24 March 2021
Issue Date : March 2021
DOI : https://doi.org/10.1038/s43018-021-00192-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
A comprehensive review of cancer drug–induced cardiotoxicity in blood cancer patients: current perspectives and therapeutic strategies.
- Vincenzo Costanzo
- Yashwant Kumar Ratre
- Henu Kumar Verma
Current Treatment Options in Oncology (2024)
All-Rounder Liposomes in Cancer Immunotherapy: Strategies and Design Applications of Engineered Liposomal Nanomaterials
- Yonghyun Choi
- Jonghoon Choi
BioChip Journal (2024)
The mechanism of ferroptosis and its related diseases
- Shijian Feng
Molecular Biomedicine (2023)
Therapy resistance and metastasis
- Jonathan P. Sleeman
Clinical & Experimental Metastasis (2023)
Nanoparticle-mediated cancer cell therapy: basic science to clinical applications
- Caaisha Warsame
- Saurav Goel
Cancer and Metastasis Reviews (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Mayo Clinic Comprehensive Cancer Center — Research
- Adjunct Faculty
About the Cancer Center
- Cancer Center Blog
- Research Programs
- Shared Resources: Core Facilities and Resources for Research
- Specialized Programs of Research Excellence (SPORE) Grants
- Support Cancer Research
- Early Cancer Therapeutics Group
- Cancer Clinical Trials
Every day, more than 350 outstanding physicians and scientists at Mayo Clinic Comprehensive Cancer Center dedicate themselves to finding answers for the unmet medical needs of cancer patients.
Collaborating across the full spectrum of cancer research, these Mayo Clinic cancer experts are committed to understanding the biology of cancer, to discovering new ways to predict, prevent, diagnose and treat cancer, and to transforming the quality of life for cancer patients today and in the future.
Setting the bar for excellence
Cheryl Willman, M.D. , is the Stephen and Barbara Slaggie Executive Director of Mayo Clinic Cancer Programs and director of Mayo Clinic Comprehensive Cancer Center. Dr. Willman also is recognized with the distinction of being the David A. Ahlquist, M.D., Professor of Cancer Research.
Dr. Willman partners with Jolene M. Summer Bolster, associate administrator of the Cancer Center, to lead the Cancer Center and cancer programs in the Midwest, Arizona and Florida, and the newly developing Mayo Clinic global cancer programs in London, England, and Abu Dhabi, United Arab Emirates.
Dr. Willman continues the Cancer Center's tradition of excellence in leadership. She is a pioneer in the field of cancer precision medicine. Her research focuses on using genomics, next-generation genome sequencing and computational technologies to discover novel cancer-causing genomic mutations that can be translated to better cancer diagnostics and therapeutics.
Meeting rigorous National Cancer Institute standards
Mayo Clinic Comprehensive Cancer Center is designated as a National Cancer Institute (NCI) cancer center . This NCI designation recognizes Mayo Clinic's scientific leadership and resources; the depth and breadth of its basic, clinical and population science; and its substantial transdisciplinary research.
The Cancer Center receives about $119 million in competitive peer-review grants annually, including the NCI's highly sought Specialized Programs of Research Excellence (SPORE) grants .
Mayo Clinic Comprehensive Cancer Center has four NCI SPORE grants:
- Breast cancer
- Hepatobiliary cancer
- Multiple myeloma
- Ovarian cancer
Designed to rapidly move research discoveries into clinical settings for cancer patient care, SPORE grants support collaboration between basic researchers and clinical researchers on projects that result in new and diverse approaches to the prevention, early detection, diagnosis and treatment of cancer.
Offering extensive clinical trial options
At Mayo Clinic, research drives everything we do for patients. Mayo Clinic Comprehensive Cancer Center researchers relentlessly pursue discoveries that deliver hope and better health today and for generations to come.
Mayo Clinic Comprehensive Cancer Center patients have access to hundreds of clinical trials in all phases led by Mayo Clinic scientists. Trials are also available through cooperative research agreements with the National Cancer Institute, the Alliance for Clinical Trials in Oncology and other clinical trials groups.
- Find a cancer clinical trial to join .
Transforming cancer patient lives
Mayo Clinic's unique culture of collaboration and teamwork speeds the transformation of promising laboratory discoveries into lifesaving treatments for patients with cancer. Because of this research, more than 150,000 cancer patients come to Mayo Clinic each year seeking medical answers they haven't found anywhere else.
Mayo Clinic Comprehensive Cancer Center has nine major cancer research programs focused on providing medical solutions for cancer patients. These research programs benefit from the crucial infrastructure and scientific support of 13 shared resources .
- Learn more about cancer treatment at Mayo Clinic .
- Read the Cancer Center Blog .
Reaching new heights in cancer research
Our team-based, patient-centered research brings together renowned physicians, researchers and scientists to develop the latest technologies and treatments to address unmet patient needs.
This work expands beyond the nine research programs within the Cancer Center to include research with other cancer collaborative groups, research on health disparities, a focus on native populations, and integration with cancer research laboratories and other areas of cancer research at Mayo Clinic.
Cooperative groups and collaborations
Researchers in Mayo Clinic Comprehensive Cancer Center work with scientists and clinicians in other organizations worldwide through cancer cooperative groups and a range of collaborations. These networks help advance cancer breakthroughs and expand access for our patients. Read more .
Laboratories and other areas of cancer research
Many Mayo Clinic Comprehensive Cancer Center physicians and scientists lead their own laboratories and groups that conduct research related to cancer. These lab-based research efforts include numerous types of cancer and related conditions and span the spectrum of cancer research, including understanding the most basic biology of cancer development, diagnosis, treatment and finding new ways to deliver targeted cancer treatment. Explore research labs .
More about research at Mayo Clinic
- Research Faculty
- Laboratories
- Core Facilities
- Centers & Programs
- Departments & Divisions
- Clinical Trials
- Institutional Review Board
- Postdoctoral Fellowships
- Training Grant Programs
- Publications
Mayo Clinic Footer
- Request Appointment
- About Mayo Clinic
- About This Site
Legal Conditions and Terms
- Terms and Conditions
- Privacy Policy
- Notice of Privacy Practices
- Notice of Nondiscrimination
- Manage Cookies
Advertising
Mayo Clinic is a nonprofit organization and proceeds from Web advertising help support our mission. Mayo Clinic does not endorse any of the third party products and services advertised.
- Advertising and sponsorship policy
- Advertising and sponsorship opportunities
Reprint Permissions
A single copy of these materials may be reprinted for noncommercial personal use only. "Mayo," "Mayo Clinic," "MayoClinic.org," "Mayo Clinic Healthy Living," and the triple-shield Mayo Clinic logo are trademarks of Mayo Foundation for Medical Education and Research.
Home > AACR Cancer Progress Report > AACR Cancer Progress Report 2022: Contents > Impacting the Future of Cancer Research and Patient Care Through Evidence-Based Policies
Impacting the Future of Cancer Research and Patient Care Through Evidence-Based Policies
In this section, you will learn:
- Continued funding for NIH and NCI is vital to accelerate the pace of new scientific breakthroughs against cancer and build programs focused on the training and retention of a diverse cancer research workforce.
- Federal policy advancements from FDA, CDC, and NCI improve diversity and access to clinical trials, access to cancer screening, and cancer outcomes.
- Disparities in the cancer burden must be addressed through equitable access to health care, insurance, optimal nutrition, and physical activity.
- Public health resources are essential for understanding the impact of cancer health disparities, and these systems need additional investment to truly support the communities they serve.
Investments in Research Fuel a Healthier Future
Building a diverse cancer research workforce drives innovation, improving regulatory science to ensure safety and efficacy of cancer therapies.
- Diversifying and Decentralizing Clinical Trials
Advancing Policy to Strengthen Cancer Prevention and Screening Programs
Leveraging policy to reduce tobacco-related illness, accelerating progress against pediatric cancers, building health equity by addressing cancer disparities, learning from covid-19 to strengthen digital health infrastructure for cancer care.
Investments and policies enacted by Congress and programs implemented by federal agencies including NIH, NCI, CDC, and FDA are essential to making progress against the collection of diseases we know as cancer.

The 21st Century Cures Act, which was signed into law in December 2016, authorized $1.8 billion to fund the Cancer Moonshot, an initiative led by NCI with the goal of accelerating the pace of progress against cancer through prevention, screening, scientific discovery, collaboration, and data sharing, over a seven-year period. Congress has continued to appropriate full funding for the Cancer Moonshot (see sidebar on The Cancer Moonshot ).
The 21st Century Cures Act also established the FDA’s Oncology Center of Excellence (OCE). OCE was established to support the development of anticancer therapies with an emphasis on facilitating active collaboration between OCE and other FDA centers. These efforts have focused on diversifying and decentralizing clinical trials to improve minority representation when developing new therapeutic options with the goal of achieving patient-centered regulatory decision making through innovation and collaboration. OCE plays a crucial role in reviewing new breakthrough treatments to ensure they are safe and effective for patients with cancer.
In February 2022, President Joseph R. Biden, Jr., announced a reignited Cancer Moonshot with a mission to “reduce the death rate from cancer by at least 50 percent over the next 25 years and to improve the experience of people and their families living with and surviving cancer—and, by doing this and more, end cancer as we know it today.” The Cancer Moonshot, along with many other cancer-based initiatives, marks the continued commitment of Congress and the Executive Branch to cancer research and improving patient outcomes. To realize the goals of the reignited Cancer Moonshot, the full reach of the federal government, including NIH, NCI, FDA, and CDC, will be utilized to better prevent, detect, and treat cancer.
The Biden administration has also proposed the creation of an Advanced Research Projects Agency for Health (ARPA-H), designed to prioritize high-risk, high-reward approaches to prevent, diagnose, and cure diseases such as cancer. In the Fiscal Year (FY) 2022 funding bill, ARPA-H received $1 billion in start-up capital to begin creation of this new medical research authority, which is proposed to be housed within NIH. As Congress continues to debate the structure and location of ARPA-H, it is imperative that funding for ARPA-H supplement, and not supplant, funding for NIH’s core research functions.
The CDC’s Division of Cancer Prevention and Control (DCPC) is another important federal partner in fueling progress against cancer. DCPC brings science-driven public health interventions, including cancer screening and prevention programs, to communities across the country. DCPC works with state health agencies, territories, tribes and tribal organizations, and other key organizations to develop, implement, and promote effective cancer prevention and control practices.
President Biden’s vision of ending cancer as we know it will not be realized without robust, sustained, and predictable funding for basic research. Significant annual funding increases are essential for NIH, NCI, FDA, CDC, and other agencies to continue their vital work against cancer. Meanwhile, legislation aimed at increasing and diversifying participation in clinical trials; expanding access to quality, affordable health care; and accelerating progress against pediatric cancers will be vital to reducing cancer disparities and achieving health equity. In addition, investments in access to preventive care, reduction of tobacco-related illness through strong federal regulations, and access to healthy lifetime nutrition are some of the ways in which we can decrease cancer incidence and improve outcomes.
Remarkable advances in medical research have led to significant improvements in cancer prevention and reductions in cancer mortality. In the years since the enactment of the National Cancer Act in 1971, cancer mortality has dropped by 27 percent ( 3 ) Kratzer TB, et al. Progress against cancer mortality 50 years after passage of the National Cancer Act. JAMA Oncol 2022;8:156-9. [LINK NOT AVAILABLE] . This progress is a result of NCI investments in research that developed state-of-the-art anticancer therapies and more effective screening tools to detect cancers in earlier stages, as well as initiatives through CDC to raise awareness of cancer prevention and the importance of cancer screenings. As a result of these efforts, there are now more than 18 million cancer survivors living in the United States ( 2 ) Miller KD, et al. Cancer Treatment and Survivorship Statistics, 2022. CA Cancer J Clin 2022;0:1-28. [LINK NOT AVAILABLE] .
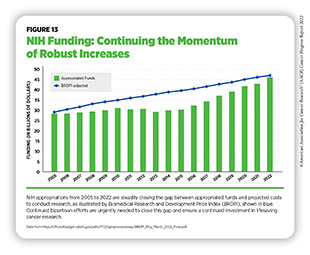
To continue progress against cancer, significant federal investments will be needed. Beginning in FY 2005, a decade of stalled funding at NIH caused budgets to be eroded by inflation. As a result, NIH’s purchasing power—the amount each dollar invested can buy—was reduced by nearly 25 percent compared to the previous decade ( 586 ) Extramural Nexus. One nation in support of biomedical research? Accessed: July 14, 2022.[cited 2020 Jul 15]. . This had a devastating impact on the ability of NIH to adequately fund research. Thanks to strong bipartisan support, Congress has made investments in medical research a top priority, increasing NIH funding by $14.9 billion over the last seven fiscal years, an increase of roughly 49 percent since FY 2015 (see Figure 13 ).
In FY 2022 alone, congressional leaders provided an increase of $2.25 billion for NIH and an increase of $353 million for NCI. As a result, NIH’s funding for medical research has almost returned to the capacity last seen in FY 2005, as measured by the Biomedical Research and Development Price Index (BRDPI) (see Figure 13 ). In particular, Chair Rosa DeLauro (D-CT), Ranking Member Tom Cole (R-OK), Chair Patty Murray (D-WA), and Ranking Member Roy Blunt (R-MO) have demonstrated remarkable leadership and commitment to medical research in their roles on the Labor, Health and Human Services, Education, and Related Agencies Appropriations Subcommittees in the House and Senate, respectively.
NIH funding increases also benefited NCI, which received an increase of $1.96 billion over the last seven fiscal years, to $6.912 billion, an increase of nearly 40 percent. With these funds, NCI provides support through a competitive process to research grants that can cover anything from basic laboratory science to clinical research. Unfortunately, despite seven consecutive years of bipartisan congressional support for investments in medical research, NCI still faces significant funding pressures that limit the amount of support it can provide for meritorious investigator-initiated research.
The percentage of approved research grant applications that receive funding are referred to as the success rate. In 1999, success rates reached 32 percent across NIH and 28 percent at NCI. These generous levels of funded grants fueled cancer discoveries at unprecedented rates and contributed to the advances in cancer care that we benefit from today ( 587 ) NIH Data Book. NIH Data Book – Success rates: R01-equivalent and research project grants. Accessed: July 14, 2022.[cited 2020 Jul 15]. . However, NCI funding has not kept pace with the subsequent exponential growth of applications. Between 2013 and 2018, NCI received a nearly 46 percent increase in grant applications, overshadowing the increase of other institutes at NIH which only increased by 4.9 percent.
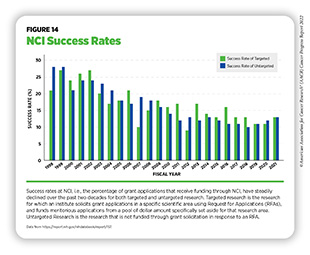
Despite the funding provided by Congress, NCI’s success rate in FY 2021 was only 13 percent, less than half the rate of two decades ago ( 587 ) NIH Data Book. NIH Data Book – Success rates: R01-equivalent and research project grants. Accessed: July 14, 2022.[cited 2020 Jul 15]. (see Figure 14 ). NCI’s success rate is also among the lowest of all institutes at NIH. Currently, fewer than one in seven approved grant applications is funded, leaving well-reviewed science unfunded and jeopardizing the United States’ position as a global leader in cancer research. In addition, lack of funding can potentially have far-reaching consequences for the cancer research community and the ability to recruit, train, and retain the next generation of cancer scientists. These trends can result in fewer women and underrepresented minorities (URMs) choosing careers in Science, Technology, Engineering, Mathematics, and Medicine (STEMM). By meeting the NCI Director’s Professional Judgment Budget level of $7.766 billion in FY 2023, NCI can increase the availability of research grants and accelerate the path to discoveries that will save lives.
CDC’s Division of Cancer Prevention and Control (DCPC) works with state and local governments, community organizations, and health providers to promote cancer prevention and early detection. These collaborations include funding for central cancer registries; comprehensive cancer control, which includes state, tribal, local, and territorial organization cancer planning; the National Breast and Cervical Cancer Early Detection Program; and initiatives focused on colorectal, skin, prostate, and ovarian cancer, as well as HPV-associated cancers.
Despite the importance of these public health-related programs, increased investments in CDC’s DCPC have also been minimal. Between FY 2010 and FY 2022, funding for these vital initiatives increased by a total of $8 million, or just 2.9 percent. This amounts to an estimated $100 million deficit relative to what the funding would be if adjusted for inflation from FY 2010 ( 588 ) One Voice Against Cancer. More funding needed for CDC cancer programs. Accessed: July 14, 2022.[cited 2020 Jul 15]. . As more than 40 percent of cancer cases in the United States each year are linked to modifiable risk factors and can be prevented, these initiatives and collaborations are critical to reduce the cancer burden.
Congress has made a clear and decisive commitment to medical research over the last seven fiscal years, returning NIH to a trajectory of steady funding growth. However, more must be done to expand opportunities in medical research, cancer prevention, and cancer treatment. With so many scientific opportunities to make progress against cancer and other diseases, it is imperative that our elected leaders continue to provide robust, sustained, and predictable increases in funding for medical research and cancer prevention at NIH, NCI, CDC, and FDA.
To prevent and cure all cancers, the next generation of cancer researchers will require thoughtful education, training, and support throughout their career paths. To realize the full potential of our medical research enterprise, research institutions must be proactive in recruiting, supporting, and retaining a cancer research workforce that reflects the diversity of our society. As described in the AACR Cancer Disparities Progress Report 2022, the amount of diversity within the cancer research workforce lags behind that of the general U.S. population. Also, complex, interrelated factors contribute to the low rates of URMs in STEMM. Proposed methods to overcome cancer disparities include increasing diversity early and consistently throughout the cancer research and care workforce. Furthermore, additional training in mentorship for successful senior scientists helps support the professional development of their trainees. Formal training programs, incentives, and compensation for excellence in mentorship have been shown to increase retention of URM trainees and scientists. NIH and NCI play important roles in fostering development of young researchers into becoming the scientific and clinical leaders of the future.
Encouraging early childhood interest in STEMM improves the likelihood of earning a higher degree ( 589 ) Reynolds AJ, et al. A multicomponent, preschool to third grade preventive intervention and educational attainment at 35 years of age. JAMA Pediatrics. Volume 172: American Medical Association; 2018. p 247-56. [LINK NOT AVAILABLE] . NIH sponsors the Science, Education, Partnership Awards (SEPA) Program, which facilitates partnerships between medical and clinical researchers, preK-12th grade teachers, schools, and other educational organizations ( 590 ) National Institutes of Health. Science Education Partnership Award. Accessed: July 14, 2022.[cited 2020 Jul 15]. . For example, the SEPA-sponsored high school program at the University of Arizona, Q-Cubed, has been instrumental to increasing the percentage of high school students that attend college. Since the launch of Q-Cubed, 98 percent of the program participants either attended or graduated from two- to four-year colleges ( 591 ) The University of Arizone. Statistics & evaluation | Q-cubed. Accessed: July 28, 2022.[cited 2020 Jul 15]. . These programs and awards provide valuable early exposures to the world of medical research and showcase the benefits of a career in research.
The NCI Center to Reduce Cancer Health Disparities provides funding support for URMs beginning in middle school and continuing to junior tenure-track faculty positions through the Continuing Umbrella of Research Experiences (CURE) program. Between 2001 and 2012, CURE supported more than 3,000 early-career researchers, who generated greater than 1,700 peer-reviewed publications ( 592 ) NCI Center to Reduce Cancer Health Disparities. National Cancer Institute’s (NCI’s) Continuing Umbrella of Research Experiences (CURE). Accessed: July 28, 2022.[cited 2020 Jul 15]. . In addition, the Intramural Continuing Umbrella of Research Experiences (iCURE) program brings undergraduate students, post-baccalaureate and post-master’s degree individuals, graduate students, and postdoctoral fellows into the NCI research community and supports mentored research experiences. iCURE particularly encourages the participation of individuals from underrepresented populations and aims to further NCI’s interest in increasing diversity in cancer research workforce.

Within the cancer research and care workforce, early-career researchers are instrumental in making advances against cancers as they bring innovative ideas and highly original perspectives to their research projects. Graduate students and postdoctoral fellows are the largest share of the academic research workforce. Trainees can be supported under their advisors’ grants, competitive institutional “T” awards, or individual “F” and “K” awards, as well as competitive philanthropic awards. These awards cover stipend and research costs of promising pre- and postdoctoral scientists, which enables them to take on more ambitious research. Some of these awards are focused on trainees that are URM in STEMM, while others, like the K99/R00 award, are designed to transition postdoctoral researchers into independent investigator positions. NCI and NIH have created several funding mechanisms to directly support URM early-stage investigators (ESIs). For example, the K01, K99/R00, and R21 grant mechanisms support the transition of postdoctoral early-career scientists into becoming independent researchers and some K01 and R21 grants are focused on supporting URM scientists ( 593 ) National Cancer Institute. NCI mentored research scientist development award to promote diversity (K01). Accessed: July 14, 2022.[cited 2020 Jul 15]. ( 594 ) National Cancer Institute. Exploratory grant award to promote workforce diversity in basic cancer research (R21). Accessed: July 14, 2022. [LINK NOT AVAILABLE] . Additionally, NIH Institutes and Centers issued 171 student loan repayment awards in FY 2020 totaling almost $13 million for investigators involved in health disparities research ( 595 ) NIH Extramural Nexus. What’s new with the NIH loan repayment programs: FY 2022 applications, anniversaries, and a new program. Accessed: July 14, 2022.[cited 2020 Jul 15]. . Focused approaches to funding ESIs, and women researchers identifying as URMs, should be a priority, as this could improve recruitment and retention within the cancer research workforce (see sidebar on NIH and NCI Initiatives to Promote Workforce Diversity and Outreach ).
NCI has also taken steps to support junior tenure-track research faculty. For example, NCI has helped ESI applicants establish independent laboratories by extending R01 paylines to the 16th percentile, instead of the standard 11th percentile ( 596 ) National Cancer Institute. NCI full year funding policy for RPG awards FY 2022. Accessed: July 14, 2022.[cited 2020 Jul 15]. . Additionally, ESI R01 applications within the 11th percentile are eligible for the R37 Method to Extend Research in Time (MERIT) award, which provides funding for up to seven years instead of the traditional five years ( 597 ) National Cancer Institute. MERIT Award (R37). Accessed: July 14, 2022.[cited 2020 Jul 15]. . The additional time provided by R37 MERIT awards enables further data collection for a second grant application and also supports the awardee through the tenure process, which lasts approximately seven years.
The influx of innovative ideas from young scientists continues to be critical for future breakthroughs against cancer and other deadly diseases. As Congress considers appropriations for NIH and NCI, it will be vital to invest in additional resources to support early-career researchers. Robust, sustained, and predictable funding increases for NIH and NCI are critical to ensure that these programs continue.
Regulatory review by FDA ensures medical research delivers safe and effective anticancer therapies for patients. To provide efficient oversight, FDA’s processes, staff, and technology must keep pace with the rapid advances in new target discovery and drug development to treat cancer. User fees paid by the industry when submitting applications and congressionally appropriated funds are both essential sources of support to FDA’s mission. Investments from Congress support critical regulatory science programs that help improve the regulatory process and shorten the time it takes for new advances in medicine to reach patients in need.

As one example, FDA OCE was established in 2017 by the 21st Century Cures Act to support development of anticancer therapies and improve regulatory efficiency in oncology. OCE facilitates collaborations between staff members with oncology expertise from other FDA centers, including the Center for Drug Evaluation and Research, Center for Biologics Evaluation, and Center for Devices and Radiological Health.
Diverisfying and Decentralizing Clinical Trials
The types of cancer included in clinical trials see the greatest advances in treatment and survival ( 598 ) Bleyer A, et al. Role of clinical trials in survival progress of American adolescents and young adults with cancer—and lack thereof. Pediatric Blood and Cancer 2018;65. [LINK NOT AVAILABLE] ( 599 ) Hunger SP, et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: A report from the children’s oncology group. Journal of Clinical Oncology 2012;30. [LINK NOT AVAILABLE] . Clinical trial participants often experience better clinical outcomes compared to nonparticipants (600). On average, 55 percent of adult patients with cancer join a trial when asked ( 601 ) Unger JM, et al. “When offered to participate”: A systematic review and meta-analysis of patient agreement to participate in cancer clinical trials. Journal of the National Cancer Institute. Volume 1132021. [LINK NOT AVAILABLE] . Unfortunately, overall participation in clinical trials is very low; only 8 percent of adult patients and 19.9 percent of pediatric and adolescent patients with cancer participate in clinical trials in the United States ( 602 ) Unger JM, et al. Systematic review and meta-analysis of the magnitude of structural, clinical, and physician and patient barriers to cancer clinical trial participation. Journal of the National Cancer Institute. Volume 1112019. [LINK NOT AVAILABLE] ( 603 ) Faulk KE, et al. Assessment of enrollment characteristics for Children’s Oncology Group (COG) upfront therapeutic clinical trials 2004-2015. PLoS ONE 2020;15. [LINK NOT AVAILABLE] . While academic medical centers tend to have above average trial participation rates ( 602 ) Unger JM, et al. Systematic review and meta-analysis of the magnitude of structural, clinical, and physician and patient barriers to cancer clinical trial participation. Journal of the National Cancer Institute. Volume 1112019. [LINK NOT AVAILABLE] , most patients with cancer are seen at community clinics or hospitals where trials are less prevalent. Another key reason for low trial participation is that more than 75 percent of patients with cancer either do not have a trial available for their specific disease or the strict eligibility criteria exclude them because of comorbidities or prior treatments ( 602 ) Unger JM, et al. Systematic review and meta-analysis of the magnitude of structural, clinical, and physician and patient barriers to cancer clinical trial participation. Journal of the National Cancer Institute. Volume 1112019. [LINK NOT AVAILABLE] . Additional challenges for clinical trials include communities without any health care facilities, patients not being asked to join a trial, lack of trust in medical research, dependent care needs, and costs and time related to participation ( 604 ) Nipp RD, et al. Addressing the financial burden of cancer clinical trial participation: Longitudinal effects of an equity intervention. The Oncologist. Volume 24: Wiley; 2019. p 1048-55. [LINK NOT AVAILABLE] ( 605 ) Valecha G, et al. Clinical trial awareness in oncology patients of diverse ethnic background: A single-institution analysis. Journal of Clinical Oncology. Volume 382020. [LINK NOT AVAILABLE] ( 606 ) Institute of Medicine. Barriers to patient recruitment and physician participation. Accessed: June 30, 2022.[cited 2020 Jul 15]. . These challenges disproportionately impact racial and ethnic minorities, contributing to disparities in clinical trial participation rates.
Improving representation of racial and ethnic minorities in oncology clinical trials is a key priority of OCE. In April 2022, OCE released draft voluntary guidance on creating prospective diversity action plans when submitting Investigational New Drug or marketing applications ( 607 ) U.S. Food and Drug Administration. Diversity plans to improve enrollment of participants from underrepresented racial and ethnic populations in clinical trials guidance for industry. Accessed: July 14, 2022.[cited 2020 Jul 15]. .
Voluntary FDA guidance is an important first step to improving clinical trial participation and representation. Additional authority to issue and enforce requirements in clinical trials could greatly enhance positive changes to the drug development process. The Diverse and Equitable Participation in Clinical Trials (DEPICT, H.R. 6584) Act would help accomplish these goals by allowing FDA to require diverse representation ( 608 ) Congress.Gov. H.R.6584 – 117th Congress (2021-2022): DEPICT Act. Accessed: June 30, 2022.[cited 2020 Jul 15]. . The Diversifying Investigations Via Equitable Research Studies for Everyone (DIVERSE) Trials Act (H.R. 5030/S. 2706) would also support greater trial participation by allowing trial sponsors to reimburse patients for transportation costs and increasing the use of telemedicine and remote data collection ( 609 ) Congress.Gov. S.2706 – 117th Congress (2021-2022): DIVERSE Trials Act. Accessed: June 30, 2022.[cited 2020 Jul 15]. . Furthermore, engaging patients and other stakeholders is critical to identify creative solutions in the policy making process and build trust in medical research.
Improving Anticancer Therapy Access for Older Adults
FDA is also working to improve trial access and outcomes for patients with cancer older than 65 years old. These patients represent more than half of all patients with cancer ( 610 ) National Cancer Institute. Risk Factors: Age. Accessed: August 12, 2022.[cited 2020 Jul 15]. , but are often excluded from clinical trials due to explicit age eligibility criteria or exclusion criteria for having other medical conditions or taking medications. As a result, oncology clinical trials include patients that are on average 6.49 years younger than the patient population affected ( 611 ) Ludmir EB, et al. Factors Associated With Age Disparities Among Cancer Clinical Trial Participants. JAMA Oncol 2019;5:1769-73. [LINK NOT AVAILABLE] .
FDA’s Project Silver is a global regulatory effort to highlight drug development programs with indications particularly impacting patients 65 years old and older. This public health initiative promotes increased enrollment of geriatric patients in clinical trials for anticancer therapeutics ( 612 ) U.S. Food and Drug Administration. Project Silver. Accessed: August 12, 2022.[cited 2020 Jul 15]. . As part of Project Silver, FDA issued final voluntary guidance in March 2022 to encourage trial sponsors to broaden the age range to increase the number of participants over the age of 65 in oncology clinical trials ( 613 ) U.S. Food and Drug Administration. Inclusion of Older Adults in Cancer Clinical Trials. Accessed: August 12, 2022.[cited 2020 Jul 15]. . The guidance emphasizes the importance of including older adult patients in early phase trials to analyze safety with co-morbid conditions and other medications. Another key recommendation was to add older adult patients to standard randomized clinical trials as an additional trial arm. This would allow trial sponsors to keep primary endpoints focused on outcomes of younger adult patients, and secondary endpoints could include data from older adult patients while expanding access to investigational therapies.
Congress recently enacted legislation intended to benefit older adults, including those with cancer, who receive health coverage under Medicare. This new law limits the out-of-pocket amount that a Medicare beneficiary would pay for prescription drugs to $2,000 per year beginning in 2025. It also allows the federal government to negotiate the price of some high-cost prescription drugs with manufacturers. Together, these policies are intended to reduce the cost of prescription drugs and make lifesaving treatments and more accessible and affordable.

Preventable risk factors, including tobacco use, infections, and UV exposure, account for approximately 40 percent of cancer cases in the United States (see Preventing Cancer: Identifying Risk Factors ). Detecting cancer early through routine screenings for common cancers also greatly improves treatment options and outcomes (see Screening for Early Detection ). Inequities in access to cancer screenings and follow-up treatment are major contributors to late-stage diagnoses among underinsured and uninsured patients. CDC’s National Breast and Cervical Cancer Early Detection Program and Colorectal Cancer Control Program help provide underserved patients with routine cancer screenings (see sidebar on CDC and NCI Cancer Screening Programs ). Unfortunately, limited funds result in continuing gaps in access to cancer screenings ( 614 ) Tangka F, et al. The eligibility and reach of the national breast and cervical cancer early detection program after implementation of the affordable care act. Cancer Causes Control 2020;31:473-89. [LINK NOT AVAILABLE] . Additional federal investment for these programs would improve equity in cancer screening and follow-up care. Growing evidence suggests expanding Medicaid has resulted in early detection of breast cancers ( 615 ) LeBlanc JM, et al. Association of medicaid expansion under the affordable care act with breast cancer stage at diagnosis. JAMA Surg 2020;155:752-8. [LINK NOT AVAILABLE] ; thus, Medicaid expansion is another substantive approach to achieving health equity.
HPV infections can lead to six types of cancer, including nearly every case of cervical cancer (see Prevent and Eliminate Infection with Cancer-causing Pathogens ) ( 616 ) Centers for Disease Control and Prevention. How many cancers are linked with HPV each year? Accessed: July 14, 2022.[cited 2020 Jul 15]. . Guideline-concordant HPV vaccination, cervical cancer screenings, and timely follow-up care are effective strategies to prevent cancer and potentially eliminate all cases of cervical cancer. However, uptake of HPV vaccination has been suboptimal; among eligible U.S. teens in 2020, less than 60 percent were fully vaccinated against HPV ( 194 ) Pingali C, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years – United States, 2020. MMWR Morb Mortal Wkly Rep 2021;70:1183-90. [LINK NOT AVAILABLE] . State-level policies requiring vaccines for other diseases, such as measles, have been particularly effective at nearly eradicating the viruses that cause them. However, only Hawaii, Rhode Island, Virginia, Puerto Rico, and Washington, DC, require HPV vaccination for attending public school ( 617 ) National Conference of State Legislatures. HPV vaccine: State legislation and regulation. Accessed: July 14, 2022.[cited 2020 Jul 15]. . Eliminating HPV-related cancers will only be achieved by coordinated strategies among all stakeholders to build confidence in vaccination and improve screening and treatment for HPV-related lesions.
Smoking rates among U.S. adults are at a historic low following decades of awareness campaigns and effective tobacco control policies. Adult smoking rates peaked in the 1960s when nearly half of adults smoked. In 2020, 19 percent of U.S. adults regularly used any tobacco product ( 87 ) Cornelius ME, et al. Tobacco Product Use Among Adults – United States, 2020. MMWR Morb Mortal Wkly Rep 2022;71:397-405. [LINK NOT AVAILABLE] , and only 12.5 percent of adults regularly smoked cigarettes. Concerningly, tobacco use remained higher among U.S. youth in 2020, including 23.6 percent of high school students who used tobacco products, primarily flavored e-cigarettes ( 618 ) Truth Initiative. E-cigarettes drive overall youth tobacco use to highest rate in decades. Accessed: July 15, 2022.[cited 2020 Jul 15]. ( 619 ) U.S. Food and Drug Administration. Results from the annual national youth tobacco survey. Accessed: July 15, 2022.[cited 2020 Jul 15]. . The ongoing epidemic of youth nicotine addiction threatens to reverse progress made against tobacco-related disease. Additional tobacco control policies across all levels of government remain important to continue reducing tobacco-related cancers as smoking remains the number one preventable cause of cancer.

In April 2022, FDA unveiled two proposals that would prohibit menthol cigarettes, as well as all flavored cigars ( 620 ) Federal Register. Tobacco product standard for menthol in cigarettes. Accessed: July 15, 2022.[cited 2020 Jul 15]. ( 621 ) Federal Register. Tobacco product standard for characterizing flavors in cigars. Accessed: July 15, 2022.[cited 2020 Jul 15]. . These proposals were welcomed by public health organizations, including AACR, that have advocated for a menthol cigarette ban for nearly 10 years. A large body of evidence, including studies from the tobacco industry, demonstrates that menthol increases smoking initiation, nicotine exposure, and the difficulty of tobacco use cessation ( 622 ) Villanti AC, et al. Menthol cigarettes and the public health standard: a systematic review. BMC Public Health 2017 17:1 2017;17:1-13. [LINK NOT AVAILABLE] (see Figure 15 ). Decades of predatory advertising practices for menthol cigarettes in predominantly racial and ethnic minority communities are responsible for large tobacco-related health disparities ( 623 ) Campaign for Tobacco-free Kids. Stopping menthol, saving lives. Accessed: July 15, 2022.[cited 2020 Jul 15]. .
Additionally, FDA also announced in June 2022 that it would pursue a new proposal to limit the amount of nicotine in combustible tobacco products ( 625 ) U.S. Food and Drug Administration. FDA announces plans for proposed rule to reduce addictiveness of cigarettes and other combusted tobacco products. Accessed: July 15, 2022.[cited 2020 Jul 15]. . This rule is estimated to prevent eight million tobacco-related deaths during the next 80 years ( 626 ) Apelberg BJ, et al. Potential public health effects of reducing nicotine levels in cigarettes in the united states. https://doiorg/101056/NEJMsr1714617. Volume 378: Massachusetts Medical Society; 2018. p 1725-33. [LINK NOT AVAILABLE] . If finalized, this could be one of the most powerful regulations ever implemented by FDA to protect public health.

In an effort to address the negative public health impacts of e-cigarettes, especially among youth, FDA deemed e-cigarettes to be classified as tobacco products and therefore under FDA’s authority. Following this classification, manufacturers were required to submit premarket tobacco product applications (PMTAs) for e-cigarettes to FDA for regulatory review. The Family Smoking Prevention and Tobacco Control Act places the responsibility on the manufacturers to provide scientific evidence within PMTAs proving that their products are appropriate for the protection of public health. More than 6.6 million PMTAs were submitted to FDA by the September 2020 deadline ( 627 ) U.S. Food and Drug Administration. FDA issues decisions on additional e-Cigarette products. Accessed: July 15, 2022.[cited 2020 Jul 15]. . FDA has since reached decisions on 99 percent of the submitted products, and almost all were denied marketing orders. In 2022, FDA reached decisions on several e-cigarette brands with large market shares. While FDA authorized several VUSE and NJOY branded e-cigarettes, they decided to remove JUUL-branded e-cigarettes from the market, pending an appeal ( 628 ) U.S. Food and Drug Administration. FDA denies authorization to market JUUL products. Accessed: July 15, 2022.[cited 2020 Jul 15]. . JUUL e-cigarettes comprised approximately 75 percent of the e-cigarette market in 2019 and were a major contributor to a doubling of the youth e-cigarette use between 2017 and 2019 ( 629 ) Wang TW, et al. Tobacco product use and associated factors among middle and high school students – United States, 2019. MMWR Surveill Summ 2019;68:1-22. [LINK NOT AVAILABLE] ( 630 ) technavio Blog. JUUL market share in 2019: Dominating the US e-cigarette market. Accessed: July 15, 2022.[cited 2020 Jul 15]. . JUUL’s intentional marketing to youth and addicting millions to nicotine demonstrate that its products are not appropriate for public health.
Further policies that could reduce tobacco-related illness include expanding flavor prohibitions to all tobacco products; increasing restrictions on tobacco product advertising and promotions; and increasing funding for awareness and cessation programs within FDA, NCI, and CDC’s Office on Smoking and Health.
Pediatric cancers are the leading cause of disease-related deaths in children up to the age of 14 years (1). Advances in cancer treatments over the last few decades have resulted in an increase in survival rates for pediatric cancer to 85 percent ( 1 ) Siegel RL, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [LINK NOT AVAILABLE] . However, there are many types of pediatric cancers with significantly poorer outcomes and for which there are no effective treatments. Additionally, children who survive cancer face long-term side effects from their treatment, as well as life-threatening late effects of childhood cancer (see Challenges Faced by Cancer Survivors ). It is imperative to develop policies that support identifying new treatments for pediatric cancers and advocate for survivors of childhood cancers. This is critical to ensuring the best outcome for every child impacted by cancer. The most comprehensive childhood cancer legislation to date was passed by Congress in 2018, the Childhood Cancer Survivorship, Treatment, Access, and Research (STAR) Act. Congress has consistently appropriated $30 million per year to fund programs created by the STAR Act. Numerous provisions within the STAR Act have been implemented to improve data collection, tracking, and survivorship support related to childhood cancers, such as:
- Awarding NCI grants to support and expand the collection of biospecimens from children, adolescents, and young adults diagnosed with cancer;
- Expanding childhood cancer surveillance programs at CDC by developing a new cloud-based data reporting system;
- Supporting research that will investigate the late effects of pediatric cancer treatments, improve collaboration among health care providers, and identify novel methods of care for pediatric cancer survivors; and
- Mandating the inclusion of at least one pediatric oncologist on the National Cancer Advisory Board.
Continued full appropriations will be essential to realizing the potential of the STAR Act. The Childhood STAR Reauthorization Act (H.R. 7630/S. 4120) was introduced in the House and the Senate in April 2022. Congress will need to reauthorize the STAR Act before its expiration at the end of FY 2023 to continue NCI-supported research and further development of biorepositories, identify and train pediatric cancer researchers, and strengthen infrastructure to capture pediatric cancer incidences.
The Childhood Cancer Data Initiative (CCDI) is another NCI program designed to improve data collection and research sharing related to pediatric cancers. The goals are to better understand cancer biology specific to children and to improve prevention, treatment, quality of life, and survivorship. CCDI funding is proposed for 10 years, from FY 2020 to FY 2029, with $50 million to be allocated each year. Congress fully funded the initiative in both FY 2020 and FY 2021. NCI has granted CCDI funds for pediatric cancers and research activities and has also engaged the entire childhood cancer community in the implementation of the initiative. In March 2022, the CCDI Molecular Characterization Initiative was launched to characterize tumors and develop biomarker testing in children ( 631 ) National Cancer Institute. Molecular characterization initiative for childhood cancers. Accessed: July 15, 2022.[cited 2020 Jul 15]. . These data will allow researchers to develop better clinical trials, identify the drivers of pediatric cancers, and support development of novel treatments for some pediatric cancers that currently lack effective treatments.
Molecularly targeted therapies have shown remarkable success for the treatment of adults with specific mutations that fuel cancer development. Many pediatric cancers exhibit the same mutations as adult cancers. However, designing clinical trials only for pediatric cancers with specific mutations is difficult because all pediatric cancers are rare. The low availability of molecularly targeted trials for pediatric patients means that targeted drugs approved to treat adult forms of cancer often do not get approved for children even when there is a strong potential of benefit. To address this issue, Congress passed key provisions for the Research to Accelerate Cures and Equity (RACE) for Children Act as part of the FDA Reauthorization Act of 2017 to amend the Pediatric Research Equity Act (PREA). In August 2021, the RACE Act came into full effect. It requires drug manufacturers to study molecularly targeted therapeutics developed for adult patients with cancer in pediatric populations with the same mutations. In response to these provisions, FDA developed a Pediatric Molecular Target List to provide guidance to companies as they plan for new drug and biologic submissions ( 632 ) U.S. Food and Drug Administration. Pediatric oncology. Accessed: July 15, 2022.[cited 2020 Jul 15]. . Additionally, applications submitted to FDA for therapies that meet the RACE Act criteria must have agency-approved pediatric study plans ( 633 ) U.S. Food and Drug Administration. Pediatric study plans: content of and process for submitting initial pediatric study plans and amended initial pediatric study plans. Accessed: July 15, 2022.[cited 2020 Jul 15]. .
New discoveries in understanding the biology of pediatric cancers and the connection to birth defects are also being supported by The Gabriella Miller Kids First Pediatric Research Program (Kids First) at NIH. Funding for this program was established in the Gabriella Miller Kids First Research Act, passed by Congress in 2014. As of 2021, the program had completed genome sequencing of more than 20,000 participants within 44 childhood cancer and structural birth defect cohorts for whole genome sequencing and is in the process of selecting additional cohorts for 2022. More than $75 million has been invested in pediatric research through this initiative. The bipartisan Gabriella Miller Kids First Research Act 2.0 was introduced in the House in January 2021 and would redirect penalties against pharmaceutical, cosmetic, supplement, and medical device companies for specified violations to the Kids First program, which is part of the NIH Common Fund. NIH would make allocations from this fund to support lifesaving pediatric research that does not duplicate existing activities.
The central sources of health insurance coverage for more than half of the children in the United States are Medicaid and the Children’s Health Insurance Program (CHIP) ( 634 ) Medicaid.Gov. Federal Fiscal Year (FFY) 2020 Statistical Enrollment Data System (SEDS) reporting. Accessed: July 15, 2022.[cited 2020 Jul 15]. ( 635 ) ChildStats.Gov. POP1 Child population: Number of children (in millions) ages 0-17 in the United States by age, 1950-2020 and projected 2021-2050. Accessed: July 15, 2022.[cited 2020 Jul 15]. . Coverage is limited to providers in the child’s home state. If out-of-state care is necessary for treatment, the health care provider and his or her team are required to undergo screening and enrollment within the Medicaid program in the child’s home state. In addition to funding research to identify novel treatments for pediatric cancers, it is imperative for Congress to reduce the regulatory hurdles for eligible health care providers to treat children enrolled in Medicaid or CHIP across state lines. The Accelerating Kids’ Access to Care Act would improve access to time-sensitive care by allowing eligible out-of-state providers to enroll in multiple state Medicaid programs without undergoing additional state-by-state screening.
As described in the AACR Cancer Disparities Progress Report 2022 and discussed by Congresswoman Nikema Williams ), systemic disadvantages greatly contribute to poorer health outcomes for medically underserved populations. Centuries of policies that restrict housing, educational, and employment opportunities for racial and ethnic minorities have led to lower health insurance coverage rates, lower utilization of preventive health services, poor nutrition, and inadequate access to quality health care. Additionally, the underrepresentation of high-quality health care facilities in low-income neighborhoods and rural communities results in a lower quality of care even for those who can afford it. Reducing cancer health disparities will require a long-term, multipronged approach that supports individuals, communities, health care centers, and federal agencies, as well as local, tribal, and state governments. Recent policy developments related to cancer screening, clinical trial participation, nutrition, and health insurance have demonstrated that progress in addressing cancer health disparities is occurring.
Routine cancer screenings are necessary to detect precancerous lesions as early as possible in cancer development; however, variability along the cancer screening continuum contributes to cancer health disparities. In 2021, USPSTF broadened lung cancer screening requirements and eligibility, reducing previously identified disparities ( 636 ) Pu CY, et al. Comparison between the 2021 USPSTF lung cancer screening criteria and other lung cancer screening criteria for racial disparity in eligibility. JAMA Oncol 2022;8:374-82. [LINK NOT AVAILABLE] . Unfortunately, follow-up care is less likely to occur in minority populations for many reasons, including being uninsured or underinsured, decreased access to care, health care system bias, and miscommunication with health care providers ( 270 ) Kaiser Family Foundation. Racial disparities in cancer outcomes, screening, and treatment. Accessed: July 15, 2022.[cited 2020 Jul 15]. . To address the health care needs of medically underserved populations, the Affordable Care Act provided states the option to expand Medicaid coverage to families earning 138 percent of the federal poverty line or less. In June 2022, the North Carolina Senate passed House Bill 149 that would expand Medicaid no later than July 2023 ( 637 ) General Assembly of North Carolina. House Bill 149. Accessed: July 15, 2022.[cited 2020 Jul 15]. . If House Bill 149 passes the North Carolina House of Representatives and is signed into law, North Carolina will join 38 other states (and Washington, DC) in having expanded Medicaid coverage ( 638 ) Kaiser Family Foundation. Status of state medicaid expansion decisions: Interactive map. Accessed: July 15, 2022.[cited 2020 Jul 15]. . Uninsured rates in those states have decreased by nearly half in states that have expanded Medicaid compared to those that have not ( 638 ) Kaiser Family Foundation. Status of state medicaid expansion decisions: Interactive map. Accessed: July 15, 2022.[cited 2020 Jul 15]. . Medicaid expansion has been particularly beneficial for young adult survivors of cancer ( 639 ) Su CT, et al. Affordable care act and cancer survivors’ financial barriers to care: Analysis of the national health interview survey, 2009-2018. JCO Oncology Practice2021. [LINK NOT AVAILABLE] ( 640 ) Nathan NH, et al. Evaluating Medicaid expansion benefits for patients with cancer: National Cancer Database analysis and systematic review. J Cancer Policy 2021;29:100292. [LINK NOT AVAILABLE] , who have seen dramatic increases in the ability to afford health care and are therefore less likely to skip medications or delay refills.
Food security—having reliable access to affordable and nutritious food—is instrumental to cancer treatment adherence and survival ( 641 ) Gany F, et al. Do our patients have enough to eat?: Food insecurity among urban low-income cancer patients. J Health Care Poor Underserved 2014;25:1153-68. [LINK NOT AVAILABLE] . The United States Department of Agriculture has two categories for food insecurity: low and very low. Low food security is reported reduced quality, variety, or desirability of diet without any indication of decreased food intake. Very low food security is a disruption of eating patterns with reduced food intake ( 642 ) HealthyPeople.Gov. Food insecurity. Accessed: July 15, 2022.[cited 2020 Jul 15]. ( 643 ) USDA Economic Research Services. Definitions of food security. Accessed: July 15, 2022.[cited 2020 Jul 15]. . Low food security can contribute to obesity, a known risk factor for many different cancers ( 644 ) Centers for Disease Control and Prevention. Obesity and cancer. Accessed: July 15, 2022.[cited 2020 Jul 15]. . Very low food security is a gap in navigating cancer management as patients with cancer and survivors of cancer may be without reliable access to a sufficient quantity of affordable, nutritious food ( 645 ) Patel KG, et al. Food insecurity screening: A missing piece in cancer management. Cancer 2019;125:3494-501. [LINK NOT AVAILABLE] .
Addressing the nutritional needs of patients with cancer and survivors has the potential to decrease cancer disparities and promote healthy outcomes. One of the encouraging efforts from CDC is the Racial and Ethnic Approaches to Community Health initiative ( 646 ) Centers for Disease Control and Prevention. Racial and ethnic approaches to community health. Accessed: July 15, 2022.[cited 2020 Jul 15]. . This program funds local, culturally appropriate public health efforts that promote reaching one’s full health potential. That includes promoting exercise and ensuring underserved individuals have options for good nutrition across their lifespan.
Several additional initiatives organized by NIH, NCI, the National Institute on Minority Health and Health Disparities (NIMHD), and CDC are designed to address cancer disparities. For example, NIH’s All of Us program aims to improve precision medicine research by building one of the largest and most diverse health databases. To date, over 400,000 people have joined the research program. The NCI Community Oncology Research Program is a national network that brings cancer clinical trials and care delivery studies to people in their own communities ( 647 ) National Cancer Institute. The NCI Community Oncology Research Program (NCORP). Accessed: July 28, 2022.[cited 2020 Jul 15]. . Additionally, the NCI Center to Reduce Cancer Health Disparities supports disparities research within NCI and reinforces training a diverse cancer research workforce. NIMHD is NIH’s core institute to support research on the many factors that contribute to disparate health outcomes, including socioeconomics, politics, discrimination, culture, and environment. Several NIMHD-promoted funding opportunities will support the investigation of underlying factors contributing to disparities in liver and lung cancer in medically underserved populations ( 648 ) National Institute of Minority Health and Health Disparities. Solicited and investigator-initiated research. Accessed: July 15, 2022.[cited 2020 Jul 15]. . CDC’s National Program of Cancer Registries is essential for understanding the scope of cancer disparities by tracking cancer rates and incidence across the United States.
A robust public health infrastructure is vital for building capacity to prevent chronic diseases, such as cancer, promote healthy living, and prepare for and respond to emergencies. Every public health service relies on basic infrastructure and staffing to understand and respond to the needs of a community. However, chronic underfunding of public health efforts has left federal, state, and local public health agencies with limited staff and obsolete technology ( 649 ) Maani N, et al. COVID-19 and underinvestment in the public health infrastructure of the United States. Milbank Q 2020;98:250-9. [LINK NOT AVAILABLE] ( 650 ) Kaiser Family Foundation. Hollowed-out public health system faces more cuts amid virus. Accessed: July 15, 2022.[cited 2020 Jul 15]. . Federal funds cover roughly one quarter of public health spending in the United States, while the remaining three quarters comes from state and local governments ( 650 ) Kaiser Family Foundation. Hollowed-out public health system faces more cuts amid virus. Accessed: July 15, 2022.[cited 2020 Jul 15]. . Unfortunately, per capita state public health funding decreased 16 percent between 2010 and 2020 and local funding decreased 18 percent. Rural communities are especially affected by public health divestment ( 650 ) Kaiser Family Foundation. Hollowed-out public health system faces more cuts amid virus. Accessed: July 15, 2022.[cited 2020 Jul 15]. ( 651 ) New York Times. ‘Small town, no hospital’: Covid-19 is overwhelming rural west Texas. Accessed: July 15, 2022.[cited 2020 Jul 15]. . Public health departments have struggled to quickly hire staff and replace outdated technology ( 652 ) Kaiser Family Foundation. States have yet to spend hundreds of millions of federal dollars to tackle covid health disparities. Accessed: July 15, 2022.[cited 2020 Jul 15]. . Challenges during the COVID-19 pandemic for cancer screening and prevention programs have clearly demonstrated that robust and sustainable investments are needed to strengthen public health infrastructure to eliminate disparities in access to cancer services ( 653 ) Trust for America’s Health. The impact of chronic underfunding on America’s public health system: Trends, risks, and recommendations, 2021. Accessed: Dec 17, 2021.[cited 2020 Jul 15]. ( 654 ) Fedewa SA, et al. Changes in cancer screening in the US during the COVID-19 pandemic. JAMA Netw Open 2022;5:e2215490. [LINK NOT AVAILABLE] .

Effective public health programs for cancer prevention depend on high-quality data to identify which communities and population groups are most impacted. However, public health data-reporting systems and quality of data collected vary greatly across geography and facility type ( 655 ) Politico. Bad state data hides coronavirus threat as Trump pushes reopening. Accessed: July 15, 2022.[cited 2020 Jul 15]. ( 656 ) MedPage Today. Nursing homes shocked at ‘insanely wrong’ CMS data on COVID-19. Accessed: Nov 12, 2021.[cited 2020 Jul 15]. . It is concerning that many states continue to rely on outdated fax machines to report public health data ( 657 ) Kaiser Family Foundation. Faxes and snail mail: Will pandemic-era flaws unleash improved health technology? Accessed: July 15, 2022.[cited 2020 Jul 15]. . Fortunately, Congress appropriated an initial $50 million for CDC Data Modernization activities in FY 2020 ( 658 ) Centers for Disease Control and Prevention. Surveillance and data strategy: Notable milestones. Accessed: July 15, 2022.[cited 2020 Jul 15]. ; an additional $1 billion was included in the CARES Act and the American Rescue Plan as well as $50 million in FY 2021 and $100 million in FY22 appropriations ( 659 ) Centers for Disease Control and Prevention. FY 2022 operating plan. Accessed: July 15, 2022.[cited 2020 Jul 15]. . These funds represent a down payment on the first ever national automated public health reporting system. This system could greatly improve the efficiency of monitoring public health issues, such as cancer incidence and risk factors like obesity, as well as support real-world evidence studies to analyze population-level efficacy of cancer treatments, screenings, and prevention programs.
The growing use of telehealth during the COVID-19 pandemic by patients with cancer has demonstrated the importance of reliable and fast Internet connections for cancer care (see sidebar on What Is Telemedicine? ). Unfortunately, approximately 42 million Americans lack access to Internet fast enough to stream video ( 660 ) Broadband Now Research. Broadbandnow estimates availability for all 50 states; confirms that more than 42 million americans do not have access to broadband. Accessed: July 15, 2022.[cited 2020 Jul 15]. . Historically marginalized urban and rural communities disproportionately experience limited access to Internet services. In FY 2020, Congress appropriated $8 billion for efforts to expand Internet and telehealth infrastructure ( 661 ) Universal Services Administrative Co. 2020 annual report. Accessed: July 15, 2022.[cited 2020 Jul 15]. . Furthermore, the 2021 Bipartisan Infrastructure law included an additional $65 billion for Internet access and to subsidize subscription costs for low-income families ( 662 ) U.S. Department of Commerce. Fact sheet: Department of Commerce’s use of bipartisan infrastructure deal funding to help close the digital divide. Accessed: July 15, 2022.[cited 2020 Jul 15]. . Continued support for increased Internet access and digital public health infrastructure at the federal and local levels will be vital for addressing public health challenges.
- A Message from AACR
- Executive Summary
- A Snapshot of a Year in Progress
- Cancer in 2023
- Understanding the Path to Cancer Development
- Reducing the Risk of Cancer Development
- Screening for Early Detection
- Advancing the Frontiers of Cancer Science and Medicine
- Spotlight on Immunotherapy: Pushing the Frontier of Cancer Medicine
- Perspective: Looking to the Future of Immunology
- Supporting Cancer Patients and Survivors
- Envisioning the Future of Cancer Science and Medicine
- Advancing the Future of Cancer Research and Care Through Evidence-based Policies
- AACR Call to Action
- AACR President’s Vision: Future of Cancer Research and Care
- AACR Cancer Progress Report 2023: Steering Committee
Your donation to the American Association for Cancer Research helps our more than 58,000 members worldwide drive progress against cancer.

NIH researchers develop AI tool with potential to more precisely match cancer drugs to patients
- Posted: April 18, 2024
240-760-6600
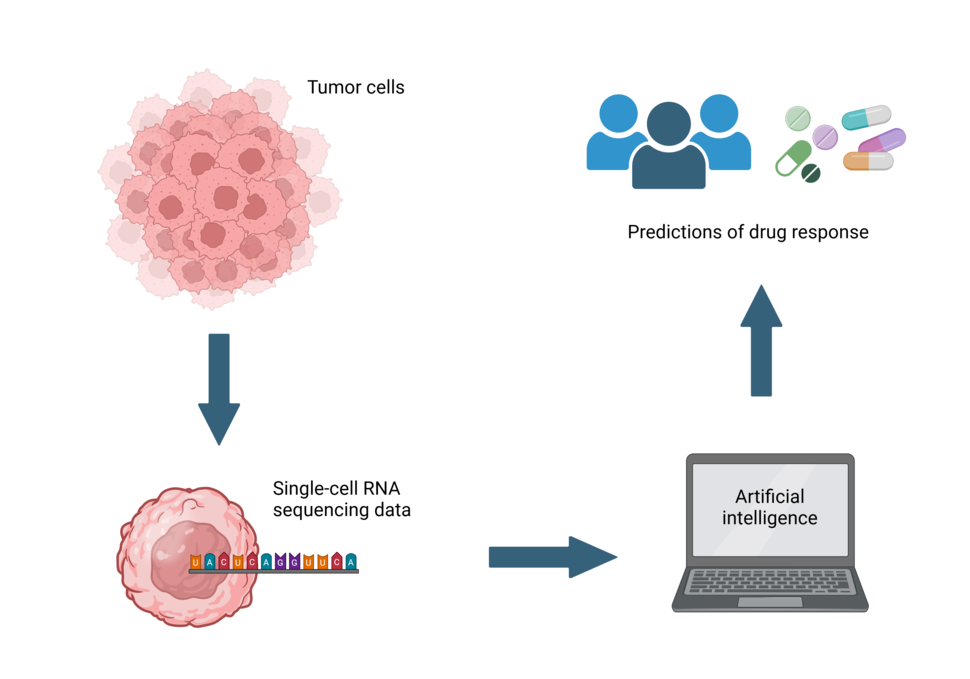
Researchers have used high-resolution gene expression data from individual tumor cells to fine-tune the ability of an AI tool called PERCEPTION to predict drug responses.
In a proof-of-concept study, researchers at the National Institutes of Health (NIH) have developed an artificial intelligence (AI) tool that uses data from individual cells inside tumors to predict whether a person’s cancer will respond to a specific drug. Researchers at the National Cancer Institute (NCI), part of NIH, published their work on April 18, 2024, in Nature Cancer, and suggest that such single-cell RNA sequencing data could one day be used to help doctors more precisely match cancer patients with drugs that will be effective for their cancer.
Current approaches to matching patients to drugs rely on bulk sequencing of tumor DNA and RNA, which takes an average of all the cells in a tumor sample. However, tumors contain more than one type of cell and in fact can have many different types of subpopulations of cells. Individual cells in these subpopulations are known as clones. Researchers believe these subpopulations of cells may respond differently to specific drugs, which could explain why some patients do not respond to certain drugs or develop resistance to them.
In contrast to bulk sequencing, a newer technology known as single-cell RNA sequencing provides much higher resolution data, down to the single-cell level. Using this approach to identify and target individual clones may lead to more lasting drug responses. However, single-cell gene expression data are much more costly to generate than bulk gene expression data and not yet widely available in clinical settings.
In the new study, the researchers investigated whether they could use a machine learning technique called transfer learning to train an AI model to predict drug responses using widely available bulk RNA sequencing data, but then fine-tune that model using single-cell RNA sequencing data. Using this approach on published cell-line data from large-scale drug screens, the researchers built AI models for 44 Food and Drug Administration–approved cancer drugs. The AI models accurately predicted how individual cells would respond to both single drugs and combinations of drugs.
The researchers then tested their approach on published data for 41 patients with multiple myeloma treated with a combination of four drugs and 33 patients with breast cancer treated with a combination of two drugs. The researchers discovered that if just one clone were resistant to a particular drug, the patient would not respond to that drug, even if all the other clones responded. In addition, the AI model successfully predicted the development of resistance in published data from 24 patients treated with targeted therapies for non-small cell lung cancer.
The researchers cautioned that the accuracy of this technique will improve if single-cell RNA sequencing data become more widely available. In the meantime, the researchers have developed a research website and a guide for how to use the AI model, called Personalized Single-Cell Expression-based Planning for Treatments In Oncology (PERCEPTION), with new datasets.
This work was conducted by NCI’s Center for Cancer Research and led by Alejandro Schaffer, Ph.D., and Sanju Sinha, Ph.D., previously at NCI, now at Sanford Burnham Prebys. NCI's Eytan Ruppin, M.D., Ph.D., supervised the work.
Eytan Ruppin, M.D., Ph.D., Center for Cancer Research , National Cancer Institute
“Predicting patient response and resistance to treatment from single-cell transcriptomics of their tumors via the PERCEPTION computational pipeline” appears April 18, 2024, in Nature Cancer.
About the National Cancer Institute (NCI): NCI leads the National Cancer Program and NIH’s efforts to dramatically reduce the prevalence of cancer and improve the lives of people with cancer. NCI supports a wide range of cancer research and training extramurally through grants and contracts. NCI’s intramural research program conducts innovative, transdisciplinary basic, translational, clinical, and epidemiological research on the causes of cancer, avenues for prevention, risk prediction, early detection, and treatment, including research at the NIH Clinical Center—the world’s largest research hospital. Learn more about the intramural research done in NCI’s Center for Cancer Research . For more information about cancer, please visit the NCI website at cancer.gov or call NCI’s contact center at 1-800-4-CANCER (1-800-422-6237).
About the National Institutes of Health (NIH): NIH, the nation's medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit nih.gov .
Advertisement
The Supportive Care Needs of Cancer Patients: a Systematic Review
- Open access
- Published: 25 January 2021
- Volume 36 , pages 899–908, ( 2021 )
Cite this article
You have full access to this open access article

- Madeleine Evans Webb 1 ,
- Elizabeth Murray ORCID: orcid.org/0000-0002-8932-3695 2 ,
- Zane William Younger 2 ,
- Henry Goodfellow 2 &
- Jamie Ross 2
8395 Accesses
33 Citations
4 Altmetric
Explore all metrics
Cancer, and the complex nature of treatment, has a profound impact on lives of patients and their families. Subsequently, cancer patients have a wide range of needs. This study aims to identify and synthesise cancer patients’ views about areas where they need support throughout their care. A systematic search of the literature from PsycInfo, Embase and Medline databases was conducted, and a narrative. Synthesis of results was carried out using the Corbin & Strauss “3 lines of work” framework. For each line of work, a group of key common needs were identified. For illness-work, the key needs idenitified were; understanding their illness and treatment options, knowing what to expect, communication with healthcare professionals, and staying well. In regards to everyday work, patients wanted to maintain a sense of normalcy and look after their loved ones. For biographical work, patients commonly struggled with the emotion impact of illness and a lack of control over their lives. Spiritual, sexual and financial problems were less universal. For some types of support, demographic factors influenced the level of need reported. While all patients are unique, there are a clear set of issues that are common to a majority of cancer journeys. To improve care, these needs should be prioritised by healthcare practitioners.
Similar content being viewed by others
Mapping a decade of interventions to address the supportive care needs of individuals living with or beyond cancer: a scoping review of reviews
Early integration of palliative/supportive cancer care—healthcare professionals’ perspectives on the support needs of cancer patients and their caregivers across the cancer treatment trajectory.

Use of the supportive care framework to explore haematological cancer survivors’ unmet needs: a qualitative study
Avoid common mistakes on your manuscript.
Introduction
Over 42 million people worldwide are currently living with cancer [ 1 ]. A cancer diagnosis often results in biographical disruption [ 2 ] and distress [ 3 ], sometimes lasting years post-treatment [ 4 , 5 ]. As survival rates continue to increase, more individuals will have to live with the long-term implications of cancer. It is therefore important that the support offered to cancer patients improves to meet this growing demand.
Cancer care pathways are often spread across multiple facilities and delivered by healthcare practitioners (HCP), which make it challenging for a patient’s wider support needs to be met. This has an impact on patient wellbeing [ 6 ] and survival outcomes [ 7 , 8 ]. Many studies focus on the needs of specific patient groups, defined by diagnosis, treatment or demographics, but there is no broad consensus on how common or dissimilar patients’ supportive care needs are across types of cancer and populations. The aim of this study was to synthesise existing data on the support needs of cancer patients across populations. Identifying the common underlying needs of cancer patients, as well as needs that are specific to a patient’s diagnosis or background, will help HCPs provide comprehensive support more efficiently.
Eligibility Criteria
Inclusion criteria: Any patient undergoing treatment for any form of cancer. Patients in remission or recovery were eligible only if they had not been in remission for longer than 5 years, a key milestone in cancer survivorship [ 9 ].
Intervention and Comparator
Patients that had received any form of treatment, be it curative or palliative, could be included. As this review was not assessing the effectiveness of an intervention program, there was no appropriate comparator or control group.
The primary outcome was the identification of any supportive care needs, categorised into emotional, informational, spiritual, social or “other”. Needs could be specifically identified, or could be inferred from reported distress, e.g. patients reporting high levels of loneliness would be categorised as having an emotional need.
Inclusion criteria: Any study design which included collection of primary data, quantitative or qualitative, was eligible for inclusion.
Exclusion criteria: Papers which did not include new primary data (e.g. reviews, meta-analyses, editorials), had not been peer reviewed or were not available in English.
The search strategy was the keywords: [emotional need] or [spiritual need] or [social need] or [emotional need] AND [Neoplasm(s)] either appearing in the title, abstract, subject heading, keyword heading, protocol supplementary concept, rare disease supplementary concept or as a unique identifier.
The search was carried out on PsycInfo, Embase and Medline databases, on 24 April 2018. This selection was based on a review of which databases have the highest recall rate, while also needing to produce a manageable number of results [ 10 ].
Reference lists of included papers were searched for potentially eligible studies.
Study Selection
Titles and abstracts were screened against the inclusion/exclusion criteria, and 10% of papers were also screened by a second author. For any paper that could not be confidently excluded, the full paper was read to determine whether it should be included. There was 100% agreement between the screeners about which papers should be excluded.
Data Extraction and Management
Data were extracted into an extraction form, which was piloted and refined. Data extracted from each paper were as follows: title, year of publication, country of study setting, study design, population studied, methods of data collection and analysis and results. The needs identified in each paper were classified as informational, emotional, spiritual, social or other. For quantitative data, scores or rankings for each need were recorded, along with whether needs differed between sub-groups. For qualitative data, overarching themes, subthemes and illustrative quotes were extracted.
Data Synthesis
Data were analysed using a narrative synthesis method [ 11 ]; this allowed for the synthesis of qualitative and quantitative data and analysis of whether medical or demographic factors shaped patient needs [ 11 , 12 ].
The first step was to group the needs identified in the papers into the categories specified in the primary literature. Seven categories of need were identified in the included papers: emotional, sexual, spiritual, social, financial, daily living, nutritional and informational. The second step was to map these categories onto the Corbin & Strauss “Three lines of work” model of chronic disease management. The model identifies three types of work associated with managing a long-term condition: illness-related work, everyday life work and biographical work [ 13 ]. Within each group, the relative importance and prevalence of all the needs identified in the primary literature were compared to identify which were the most common and urgent.
Our goal was to clarify the commonality of the experience of “cancer”, irrespective of the type of cancer, thus providing an overview of the common and important support needs faced by people with cancer, and hence an understanding of where supportive care is most needed. In instances where there was conflicting evidence in the primary literature on the importance of a specific need, clinical and demographic differences between study populations were reviewed in order to understand the potential reasons for this conflict.
The Corbin & Strauss model was chosen because the categories of need identified in the primary literature clearly corresponded to the types of work in the model (Fig. 2 ). Using the model as a framework to synthesise the data allowed us to compare the relative importance of needs from different categories that fell under the same type of work. The simplicity of the model meant it could be consistently applied to needs that were identified and categorised using a number of different methodologies.
In total, 2535 papers were identified, and 540 duplicates were removed. After screening against the criteria, 1829 papers were removed, and the remaining 80 papers were read in full (Fig. 1 ). Forty-six papers were found to be eligible for inclusion in this review.
PRISMA flow diagram of the paper identification process
Study Characteristics
Of the 46 studies, 34 were quantitative, 10 were qualitative and two were mixed methods. Study population sizes ranged from 7 to 1059 participants. Fifteen papers focused on patients with a specific type of cancer, with breast and colorectal cancer being the most common. Three studies looked at patients from specific ethnic backgrounds. Eight papers focused on patients receiving a specific form of care/treatment. Three papers focused on children or young adults. Three papers looked at adults within specific age groups. Eleven studies only included patients at a certain stage of cancer or time since diagnosis. Thirty-nine studies took place in high-income countries, 6 were from middle income countries and 1 took place in a low-income country.
Needs of Cancer Patients
Thirty-two papers mentioned informational needs, 31 mentioned emotional needs, 24 mentioned spiritual needs and 19 mentioned social needs. Thirty-five papers mentioned needs in at least one of these other categories: nutritional, sexual, daily living or financial.
The resulting needs identified were grouped according to the different forms of chronic disease “work” defined by the Corbin & Strauss framework (Fig. 2 ).
Illustration of how the different domains of need identified fit into Corbin and Strauss’ 3 lines of work model of managing chronic illness
Illness-Related Work
Illness-related work, defined by Corbin & Strauss, is “the tasks of controlling symptoms; monitoring, preventing crises; carrying out regimens and managing limitations of activity” [ 13 ]. The central goal of illness-related work for patients is to understand their illness and treatment, and subsequently the need for information is consistently reported as a high priority [ 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 ]. The only paper that did not find a high level of informational need specifically measured unmet need [ 30 ].
Most frequently patients wanted to know what treatment they were receiving and how it worked [ 20 , 26 , 27 , 29 , 31 , 32 , 33 , 34 , 35 , 36 ], why that treatment had been selected, its effectiveness and its pros and cons [ 14 , 20 , 21 , 22 , 23 , 24 , 26 , 35 ]. Patients also frequently searched for more specific information about their diagnosis and prognosis [ 15 , 20 , 21 , 22 , 23 , 24 , 25 , 29 , 31 , 33 , 34 ].
Patients wanted to know what to expect from their illness and treatment [ 15 , 16 , 31 , 33 , 34 , 35 , 37 , 38 ] (Box 1). This included knowing about the chance of a relapse [ 26 ], the length of their hospital stay [ 32 ] and when their life would return to “normal” [ 26 , 31 , 33 , 39 ]. One paper reported that being given “vague” answers by HCPs frustrated patients [ 38 ].
In regard to treatment, patients most often wanted to know what the possible side effects were [ 16 , 18 , 21 , 22 , 25 , 27 , 31 , 33 , 37 , 38 , 40 , 41 ] and how they could manage or relieve them [ 14 , 17 , 19 , 22 , 23 , 24 , 26 , 31 , 33 , 35 , 42 , 43 ]. The importance of this information may depend on the stage of the patient’s treatment, as patients receiving follow-up or palliative care placed less importance on symptom management [ 25 , 27 ].
Wanting to minimise the impact of side effects speaks to a commonly reported desire among patients to be as healthy as possible [ 14 , 15 , 18 , 19 , 22 , 23 , 24 , 26 , 27 , 28 , 29 , 33 , 38 , 39 , 40 ]. This aim is also seen in the nutritional needs of patients [ 16 , 20 , 33 , 40 , 41 , 44 ]. Rather than receiving generic information about healthy diets, patients wanted more specific advice around foods that could aid recovery or minimise side effects [ 16 , 40 , 41 ]. Nutritional needs had an outsized importance in studies involving Native American patients and colorectal cancer patients [ 16 , 40 ]. For colorectal cancer patients, nutritional needs are likely higher as their cancer directly affects their digestive system. Within the Native American population, there was a strong interest in information about traditional foods, possibly due to culturally specific reasons [ 40 ].
Generally, patients wanted their test results as soon as possible [ 21 , 22 , 24 , 27 , 33 , 43 ] and wanted the meaning of the results explained to them [ 21 , 22 , 26 , 34 , 43 ]. The importance of this information to patients could be due to a desire to have some say in the treatment they are given [ 18 , 33 , 34 , 45 ], although the level of interest in alternative treatments varied significantly [ 14 , 18 , 24 , 40 , 44 ] (Box 2). The only study where information about tests was less important involved newly diagnosed patients [ 18 ].
The final area of illness-related work highlighted by this review was communication. Patients wanted to be able to communicate with their HCPs [ 18 , 27 , 34 , 40 ] but often felt unsure of when or who to direct questions to [ 24 , 26 , 35 , 36 , 38 ]. Having a single HCP who they could talk to about all aspects of treatment was a high priority [ 19 , 21 , 22 , 23 , 28 , 43 ]. Less important was the need to talk to a professional counsellor [ 25 , 27 , 36 , 43 ].
Although a general need for information was consistent across all included studies, not all patients wanted a high volume of information. A significant minority of patients only wanted to know essential information or did not want to receive bad news [ 29 , 31 , 46 ]. Age may play a role in this dynamic, as multiple papers reported older people wanted less information [ 14 , 15 , 20 , 22 , 25 , 26 , 31 , 44 ], while only a couple found no relationship [ 28 , 34 ]. Timing could also be a factor, as some patients felt the amount of information received when diagnosis was overwhelming and preferred receiving information as it became relevant [ 34 , 37 , 39 , 41 ].
Everyday Life Work
This area of need encapsulates “the daily round of tasks that helps keep a household going”, which includes the practical tasks involved in managing an illness, along with trying to maintain the structure of life pre-diagnosis [ 13 ]. The most frequently reported social needs were about patients’ concern for their family [ 17 , 20 , 21 , 26 , 39 , 46 , 47 , 48 ]. The importance of maintaining relationships with their partner, children or friends were all mentioned [ 15 , 29 , 37 , 42 , 45 , 47 ], although notably not among patients with incurable cancer [ 27 ]. There was no consensus on whether patients wanted to discuss their cancer with loved ones; some papers found this to be highly important, others did not [ 20 , 31 , 36 , 42 , 49 ]. While there were no clear demographic or medical factors connected to this variation, Kent (2013) reported that patients whose existing relationships had been heavily affected by their diagnosis were more likely to want to talk about cancer [ 49 ].
Patients wanted to live a life they consider “normal”, reflected by the importance placed on daily living needs. The most common difficulties patients faced were coping with a lack of energy [ 17 , 19 , 21 , 27 , 28 , 30 , 36 , 38 , 43 ] and wanting to do the things they used to do [ 19 , 21 , 26 , 28 , 31 , 39 ] (Box 3). Patients placed a high value on socialising and leisure time [ 15 , 26 , 32 , 45 ] and reported a fear of being isolated or abandoned [ 16 , 18 , 20 ]. The importance of maintaining a job was influenced by age, with younger patients being more interested in how cancer will affect their career and their employment rights [ 15 , 18 , 20 , 26 , 29 , 33 , 39 , 42 ].
The final practical need identified was financial, though the level of need was highly dependent on location. Patient populations with greater access to healthcare placed lower importance on financial needs [ 25 , 27 , 29 , 30 , 33 ] (Box 4). The needs in these groups related to wanting financial stability and informational support [ 45 , 50 ], with low levels of interest in economic aid [ 34 ]. Patients in countries with more limited access to healthcare reported higher levels of financial stress and reliance on family for monetary support [ 32 , 46 ]. This was true in all US-based studies, apart from one in which the mean income of participants was high [ 18 , 20 , 24 , 28 ]. For these populations, financial concerns included managing bills [ 18 , 24 ], bankruptcy assistance [ 18 ], paying for care [ 20 , 32 , 46 ] and homelessness [ 46 ]. A few financial needs were common across healthcare systems, being able to maintain a basic standard of living [ 27 , 30 , 45 , 46 ] and helping understanding financial systems and resources [ 18 , 25 , 26 , 34 ], though again the level of importance varied.
Biographical Work
Biographical work is defined as “the work involved in defining and maintaining an identity” [ 13 ]. This involves coming to terms with and contextualising a diagnosis within a persons’ identity [ 42 , 45 ]. Patients wanted to be treated as individuals [ 16 , 19 , 21 , 22 , 34 ], be reassured [ 19 , 34 ], have their feelings acknowledged [ 19 , 22 ], be respected [ 34 , 45 ] and have their dignity preserved [ 47 ] (Box 5).
Biographical work includes dealing with the emotional impact of cancer. Feelings of despair or depression were common [ 19 , 21 , 23 , 28 , 30 , 42 , 51 , 52 ], as well as distress and anxiety [ 16 , 21 , 28 , 30 , 35 , 38 , 43 ]. Patients also reported a range of fears including cancer itself [ 17 , 21 , 23 , 31 , 43 , 51 ], their treatment [ 35 , 37 ], dying [ 17 , 19 , 42 , 52 ] and pain [ 27 ]. Physical changes also negatively affected patients’ sense of self [ 26 , 27 , 29 , 30 , 38 , 42 , 45 , 46 , 47 ]. Consequently, the need for relaxation and stress management was high [ 23 , 24 , 33 , 48 , 52 ].
Patients struggled to deal with the uncertainty [ 15 , 17 , 19 , 28 , 30 , 42 , 43 , 46 , 51 ] and expressed a desire for more control [ 17 , 19 , 20 , 21 , 27 , 28 , 30 , 42 , 43 , 53 ]. To cope, patients placed a lot of importance on receiving support from loved ones [ 14 , 18 , 32 , 35 , 36 , 42 , 51 ]. However, this directly conflicted with their fear of being a burden and a perceived pressure to “stay strong” [ 20 , 27 , 37 , 45 , 46 , 51 , 53 ] (Box 6). Other patients were identified as a source of support for some [ 18 , 34 , 37 , 49 , 52 ], but others, especially those who were receiving follow-up or palliative care, were less interested in talking to other patients [ 25 , 35 , 45 , 47 ]. This aligns with a reported need among terminal cancer patients to discuss things other than illness [ 54 ].
Sexuality is another part of identity that can be impacted by cancer. Patients wanted to know how cancer would impact their sex drive, sexuality [ 14 , 17 , 20 , 26 , 29 ] and their intimate relationships [ 14 , 17 , 30 , 31 ] but often felt uncomfortable discussing these needs with their HCPs [ 14 , 18 , 20 , 26 ] (Box 7). When ranked alongside other needs, sexuality was reported to be of lesser importance to most patients [ 17 , 19 , 21 , 22 , 35 , 42 , 43 ], apart from prostate cancer patients, who reported the impact on their sex drive and sexual activity as some of the most significant changes they faced [ 14 , 30 , 38 ] (Box 7). Higher sexuality-related needs were also identified in patients with colorectal and breast cancer, although not at the same level [ 22 , 30 ]. Of the papers that looked, five out of the six studies found a relationship between age and importance of sexual identity, with younger patients having a greater need for information on sex [ 17 , 22 , 26 , 31 ] and individuals over 40 wanting more guidance on fertility [ 18 ]. One study involving younger patients did report limited interest in sexuality; however, the majority of patients were under 18 and therefore were less likely to be sexually active [ 42 ].
Much like with sexual identity, patients’ spiritual needs were not highly important when ranked alongside other domains [ 23 , 24 , 27 , 28 , 33 , 34 , 40 , 45 ], but papers that focused solely on spirituality reported widespread need [ 47 , 48 , 52 , 54 , 55 , 56 , 57 , 58 ]. There was no consensus on the importance of accessing religious resources, some papers reported a strong need for religious support [ 23 , 32 , 45 , 55 , 56 , 59 ], but more papers reported low levels of interest [ 24 , 28 , 33 , 48 , 51 , 52 , 54 , 57 , 60 ]. In line with this, the most commonly reported spiritual needs were not explicitly religious. This included maintaining a sense of calm [ 45 , 46 , 47 , 48 , 52 , 53 , 55 , 56 , 58 , 60 ], staying positive or hopeful [ 23 , 24 , 32 , 45 , 47 , 48 , 57 , 58 , 59 ] and being able to appreciate or find meaning in life [ 32 , 45 , 47 , 48 , 55 , 56 , 57 , 59 , 60 ]. Generally, there was little reported interest in discussing death or dying [ 23 , 24 , 27 , 42 , 45 , 48 , 52 , 60 ] or making sense of why this happened [ 34 , 55 , 56 , 57 ]. Much like the importance of family relationships in everyday work, being with loved ones was important for patients’ spiritual wellbeing [ 47 , 51 , 53 , 54 , 55 , 56 , 57 , 58 , 60 ]. However, some patients reported that being part of a religious community gave them similar support [ 46 , 51 , 53 , 55 , 60 ].
The most commonly reported religious need for patients was to pray or be prayed for [ 32 , 46 , 48 , 55 , 56 , 57 , 59 ]. The fact that prayer was also important for non-religious participants suggests that it may be seen as a spiritual practice for some patients. A small number of papers reported that having a relationship with God was important to patients [ 15 , 48 , 56 , 57 , 59 ], with some patients viewing God as a saviour from illness [ 40 , 46 , 59 ], while others felt that God caused their illness as punishment or as a test of faith [ 46 , 47 , 51 ] (Box 8).
Cultural factors may also influence spiritual needs. The afterlife was found to be an important concern for some patients [ 52 , 53 , 55 ], but not if their culture had little belief in the concept [ 47 ]. In the same way, having a legacy was a key need in one paper due to the importance of continuity after death in that culture [ 53 ].
This is the first review to synthesise data about cancer patients’ supportive needs across all populations and cancer types. There was remarkable consistency in the needs identified, and these were well explained by the Corbin & Strauss model of managing a chronic condition [ 13 ]. Almost all studies confirmed patients’ need for high-quality, comprehensible and timely information about their illness, treatments and how best to manage their symptoms. Such information was necessary for patients to undertake illness-related, everyday living and biographical work. In addition, patients needed support in dealing with emotional issues, including existential uncertainty, changing relationships with friends and family and practical support with everyday tasks.
Previous Literature
This review confirms the findings of previous reviews focused on specific types of need or specific populations. The most common needs identified as illness-related work in this study correspond to key informational needs highlighted in previous reviews [ 61 , 62 ]. The spiritual needs discussed have also been found to be key in improving psycho-spiritual wellbeing in other research [ 63 ]. While our review did not assess the ability of current care models to meet these needs, it is noteworthy that the key needs we identified have been found to frequently go unmet in other research [ 64 , 65 ].
Strengths and Limitations
The main strength of this study is its inclusive nature, looking across all populations and all types of cancer. This, combined with the theoretical underpinning and use of the Corbin & Strauss model, provides reassurance about the overall transferability of these findings to other clinical populations.
The main limitation pertains to the scope of the primary literature, with most of the studies coming from high-income countries, and only 7 papers from low- or middle-income countries. While the nature of patients’ financial needs were clearly dependent on country, setting may also influence other needs in less direct ways, limiting how universal the findings are. Additionally, the majority of studies used opportunistic sampling so may not accurately capture the needs of the general cancer population. Most included studies were not longitudinal and therefore could not analyse how patients’ concerns changed over time. Finally, potentially relevant demographic information was not always collected. For example, only one of the papers that examined sexuality collected information about sexual orientation, and only a couple of studies that measured financial need recorded socioeconomic status.
Conclusions
This review highlights a number of underlying issues that affect cancer patients. These findings are consistent with the previous literature and fit well with multiple chronic illness frameworks, which suggests that they are robust enough to inform best practice. The most common needs identified support the argument for empowering people with cancer through a patient-centred form of care.
Priorities for practice should be to ensure patients understand their illness and what they can expect throughout their treatment pathway. Supportive care should work to enable patients to live a life they recognise as “normal” and help them maintain their closest relationships. HCPs should ensure that patients always feel that they are being treated as individuals and know who to go to when they have questions. These key needs should be addressed as a first step to provide a strong basis of care before providing more individualised support.
Further research should focus on how to ensure these needs are addressed effectively. Evaluation of supportive care interventions should remain focused on the experiences of patients to allow them to have a voice in their care. Additional research on when different needs arise over the disease progression would help ensure that resources are provided only when needed.
McGuire S (2016) World Cancer Report 2014. World Health Organization, International Agency for Research on Cancer, WHO Press, 2015. Oxford University Press, Geneva, Switzerland
Google Scholar
Park CL, Zlateva I, Blank TO (2009) Self-identity after cancer: “survivor”, “victim”, “patient”, and “person with cancer”. J Gen Intern Med 24(2):430–435
Article PubMed Central Google Scholar
Zabora J, BrintzenhofeSzoc K, Curbow B, Hooker C, Piantadosi S (2001) The prevalence of psychological distress by cancer site. Psycho-oncology. 10(1):19–28
Article CAS PubMed Google Scholar
Ferrell BR, Dow KH, Leigh S, Ly J, Gulasekaram P (1995) Quality of life in long-term cancer survivors. In: Oncology nursing forum
Stein KD, Syrjala KL, Andrykowski MA (2008) Physical and psychological long-term and late effects of cancer. Cancer. 112(S11):2577–2592
Article PubMed Google Scholar
Li HW, Lopez V, Chung OJ, Ho KY, Chiu SY (2013) The impact of cancer on the physical, psychological and social well-being of childhood cancer survivors. Eur J Oncol Nurs 17(2):214–219
Kroenke CH, Kubzansky LD, Schernhammer ES, Holmes MD, Kawachi I (2006) Social networks, social support, and survival after breast cancer diagnosis. J Clin Oncol 24(7):1105–1111
Brown KW, Levy AR, Rosberger Z, Edgar L (2003) Psychological distress and cancer survival: a follow-up 10 years after diagnosis. Psychosom Med 65(4):636–643
Welch HG, Schwartz LM, Woloshin S (2000) Are increasing 5-year survival rates evidence of success against cancer? Jama. 283(22):2975–2978
Bramer WM, Rethlefsen ML, Kleijnen J, Franco OH (2017) Optimal database combinations for literature searches in systematic reviews: a prospective exploratory study. Systematic reviews 6(1):245
Article PubMed PubMed Central Google Scholar
Popay J, Roberts H, Sowden A, Petticrew M, Arai L, Rodgers M et al (2006) Guidance on the conduct of narrative synthesis in systematic reviews. A product from the ESRC methods programme Version 1:b92
Barnett-Page E, Thomas J (2009) Methods for the synthesis of qualitative research: a critical review. BMC Med Res Methodol 9(1):59
Corbin JM, Strauss A. Unending work and care: managing chronic illness at home: Jossey-Bass; 1988
Boberg EW, Gustafson DH, Hawkins RP, Offord KP, Koch C, Wen K-Y, Kreutz K, Salner A (2003) Assessing the unmet information, support and care delivery needs of men with prostate cancer. Patient Educ Couns 49(3):233–242
Derdiarian AK (1986) Informational needs of recently diagnosed cancer patients. Nurs Res 35(5):276–281
Beaver K, Latif S, Williamson S, Procter D, Sheridan J, Heath J, Susnerwala S, Luker K (2010) An exploratory study of the follow-up care needs of patients treated for colorectal cancer. J Clin Nurs 19(23–24):3291–3300
Dubey C, De Maria J, Hoeppli C, Betticher D, Eicher M (2015) Resilience and unmet supportive care needs in patients with cancer during early treatment: a descriptive study. Eur J Oncol Nurs 19(5):582–588
Goldfarb M, Casillas J (2013) Unmet information and support needs in newly diagnosed thyroid cancer: comparison of adolescent/young adults (AYA) and older patients. J Cancer Surviv 8(3):394–401
Article Google Scholar
Hasegawa T, Goto N, Matsumoto N, Sasaki Y, Ishiguro T, Kuzuya N, Sugiyama Y (2016) Prevalence of unmet needs and correlated factors in advanced-stage cancer patients receiving rehabilitation. Support Care Cancer 24(11):4761–4767
Hawkins NA, Pollack LA, Leadbetter S, Steele WR, Carroll J, Dolan JG, Ryan EP, Ryan JL, Morrow GR (2008) Informational needs of patients and perceived adequacy of information available before and after treatment of cancer. J Psychosoc Oncol 26(2):1–16
Korner A, Garland R, Czajkowska Z, Coroiu A, Khanna M (2016) Supportive care needs and distress in patients with non-melanoma skin cancer: nothing to worry about? Eur J Oncol Nurs 20:150–155
Li WW, Lam WW, Au AH, Ye M, Law WL, Poon J et al (2013) Interpreting differences in patterns of supportive care needs between patients with breast cancer and patients with colorectal cancer. Psycho-Oncol 22(4):792–798
Article CAS Google Scholar
Moadel AB, Morgan C, Dutcher J (2007) Psychosocial needs assessment among an underserved, ethnically diverse cancer patient population. Cancer. 109(Suppl2):446–454
Moody K, Mannix MM, Furnari N, Fischer J, Kim M, Moadel A (2011) Psychosocial needs of ethnic minority, inner-city, pediatric cancer patients. Support Care Cancer 19(9):1403–1410
Ng CHR, Verkooijen HM, Ooi LL, Koh WP (2011) Unmet psychosocial needs among breast, colorectal and gynaecological cancer patients at the National Cancer Centre Singapore (NCCS). Support Care Cancer 20(5):1049–1056
O'Connor G, Coates V, O'Neill S (2010) Exploring the information needs of patients with cancer of the rectum. Eur J Oncol Nurs 14(4):271–277
Rainbird K, Perkins J, Sanson-Fisher R, Rolfe I, Anseline P (2009) The needs of patients with advanced, incurable cancer. Br J Cancer 101(5):759–764
Article CAS PubMed PubMed Central Google Scholar
Sanders SL, Bantum EO, Owen JE, Thornton AA, Stanton AL (2010) Supportive care needs in patients with lung cancer. Psycho-Oncology. 19(5):480–489
Whelan TJ, Mohide EA, Willan AR, Arnold A, Tew M, Sellick S, Gafni A, Levine MN (1997) The supportive care needs of newly diagnosed cancer patients attending a regional cancer center. Cancer. 80(8):1518–1524
White K, D'Abrew N, Katris P, O'Connor M, Emery L (2012) Mapping the psychosocial and practical support needs of cancer patients in Western Australia. Eur J Cancer Care. 21(1):107–116
Giacalone A, Blandino M, Talamini R, Bortolus R, Spazzapan S, Valentini M, Tirelli U (2007) What elderly cancer patients want to know? Differences among elderly and young patients. Psycho-Oncology. 16(4):365–370
Masika GM, Wettergren L, Kohi TW, von Essen L (2012) Health-related quality of life and needs of care and support of adult Tanzanians with cancer: a mixed-methods study. Health and Quality of Life Outcomes 10 (no pagination):(133)
Neumann M, Wirtz M, Ernstmann N, Ommen O, Langler A, Edelhauser F et al (2011) Identifying and predicting subgroups of information needs among cancer patients: an initial study using latent class analysis. Support Care Cancer 19(8):1197–1209
Tamburini M, Gangeri L, Brunelli C, Boeri P, Borreani C, Bosisio M et al (2003) Cancer patients’ needs during hospitalisation: a quantitative and qualitative study. BMC Cancer 3 (no pagination):(12)
van Weert JC, Bolle S, van Dulmen S, Jansen J (2013) Older cancer patients’ information and communication needs: what they want is what they get? Patient Educ Couns 92(3):388–397
Wong RK, Franssen E, Szumacher E, Connolly R, Evans M, Page B, Chow E, Hayter C, Harth T, Andersson L, Pope J, Danjoux C (2002) What do patients living with advanced cancer and their carers want to know? - a needs assessment. Support Care Cancer 10(5):408–415
Beaver K, Williamson S, Briggs J (2016) Exploring patient experiences of neo-adjuvant chemotherapy for breast cancer. Eur J Oncology Nurs 20:77–86
Grimsbo GH, Finset A, Ruland CM (2011) Left hanging in the air: experiences of living with cancer as expressed through e-mail communications with oncology nurses. Cancer Nurs 34(2):107–116
Heidari H, Mardani-Hamooleh M (2016) Cancer patients’ informational needs: qualitative content analysis. J Cancer Educ 31(4):715–720
Doorenbos AZ, Eaton LH, Haozous E, Towle C, Revels L, Buchwald D (2010) Satisfaction with telehealth for cancer support groups in rural American Indian and Alaska native communities. Clin J Oncol Nurs 14(6):765–770
James-Martin G, Koczwara B, Smith E, Miller M (2014) Information needs of cancer patients and survivors regarding diet, exercise and weight management: a qualitative study. Eur J Cancer Care 23(3):340–348
Decker C, Phillips CR, Haase JE (2004) Information needs of adolescents with cancer. J Pediatr Oncol Nurs 21(6):327–334
Beesley VL, Janda M, Goldstein D, Gooden H, Merrett ND, O'Connell DL, Rowlands IJ, Wyld D, Neale RE (2016) A tsunami of unmet needs: pancreatic and ampullary cancer patients’ supportive care needs and use of community and allied health services. Psycho-Oncology. 25(2):150–157
Choi KH, Park JH, Park SM (2011) Cancer patients’ informational needs on health promotion and related factors: a multi-institutional, cross-sectional study in Korea. Support Care Cancer 19(10):1495–1504
Buzgova R, Hajnova E, Sikorova L, Jarosova D (2014) Association between unmet needs and quality of life in hospitalised cancer patients no longer receiving anti-cancer treatment. Eur J Cancer Care. 23(5):685–694
Elsner F, Schmidt J, Rajagopal MR, Radbruch L, Pestinger M (2012) Psychosocial and spiritual problems of terminally ill patients in Kerala, India. Future Oncol 8(9):1183–1191
Hsiao SM, Gau ML, Ingleton C, Ryan T, Shih FJ (2011) An exploration of spiritual needs of Taiwanese patients with advanced cancer during the therapeutic processes. J Clin Nurs 20(7–8):950–959
Sharma RK, Astrow AB, Texeira K, Sulmasy DP (2012) The spiritual needs assessment for patients (SNAP): development and validation of a comprehensive instrument to assess unmet spiritual needs. J Pain Symptom Manag 44(1):44–51
Kent EE, Smith AW, Keegan TH, Lynch CF, Wu X-C, Hamilton AS et al (2013) Talking about cancer and meeting peer survivors: social information needs of adolescents and young adults diagnosed with cancer. J Adolesc Young Adult Oncol 2(2):44–52
O'Connor G, Coates V, O'Neill S (2010) Exploring the information needs of patients with cancer of the rectum. Eur J Oncol Nurs 14(4):271–277
Murray SA, Kendall M, Boyd K, Worth A, Benton T (2004) Exploring the spiritual needs of people dying of lung cancer or heart failure: a prospective qualitative interview study of patients and their carers. Palliat Med 18(1):39–45
Astrow AB, Wexler A, Texeira K, He MK, Sulmasy DP (2007) Is failure to meet spiritual needs associated with cancer patients’ perceptions of quality of care and their satisfaction with care? J Clin Oncol 25(36):5753–5757
Shih FJ, Lin HR, Gau ML, Chen CH, Hsiao SM, Shih SN, Sheu SJ (2009) Spiritual needs of Taiwan’s older patients with terminal cancer. Oncol Nurs Forum 36(1):E31–EE8
Hampton DM, Hollis DE, Lloyd DA, Taylor J, McMillan SC (2007) Spiritual needs of persons with advanced cancer. Am J Hospice Palliat Med 24(1):42–48
Dedeli O, Yildiz E, Yuksel S (2015) Assessing the spiritual needs and practices of oncology patients in Turkey. Holist Nurs Pract 29(2):103–113
Ghahramanian A, Markani AK, Davoodi A, Bahrami A (2016) Spiritual needs of patients with cancer referred to Alinasab and Shahid Ghazi Tabatabaie Hospitals of Tabriz, Iran. Asian Pacific journal of cancer prevention: APJCP 17(7):3105–3109
PubMed Google Scholar
Taylor EJ (2006) Prevalence and associated factors of spiritual needs among patients with cancer and family caregivers. Oncol Nurs Forum 33(4):729–735
Vilalta A, Valls J, Porta J, Vinas J (2014) Evaluation of spiritual needs of patients with advanced cancer in a palliative care unit. J Palliat Med 17(5):592–600
Taylor EJ (2003) Spiritual needs of patients with cancer and family caregivers. Cancer Nurs 26(4):260–266
Hocker A, Krull A, Koch U, Mehnert A (2014) Exploring spiritual needs and their associated factors in an urban sample of early and advanced cancer patients. European Journal of Cancer Care. 23(6):786–794
Rutten LJF, Arora NK, Bakos AD, Aziz N, Rowland J (2005) Information needs and sources of information among cancer patients: a systematic review of research (1980–2003). Patient Educ Couns 57(3):250–261
Fletcher C, Flight I, Chapman J, Fennell K, Wilson C (2017) The information needs of adult cancer survivors across the cancer continuum: a scoping review. Patient Educ Couns 100(3):383–410
Lin HR, Bauer-Wu SM (2003) Psycho-spiritual well-being in patients with advanced cancer: an integrative review of the literature. J Adv Nurs 44(1):69–80
Harrison JD, Young JM, Price MA, Butow PN, Solomon MJ (2009) What are the unmet supportive care needs of people with cancer? A systematic review. Support Care Cancer 17(8):1117–1128
Sanson-Fisher R, Girgis A, Boyes A, Bonevski B, Burton L, Cook P, Supportive Care Review Group (2000) The unmet supportive care needs of patients with cancer. Cancer. 88(1):226–237
Download references
Author information
Authors and affiliations.
UCL Research Department of Epidemiology & Public Health, 1-19 Torrington Place, London, WC1E 6BT, UK
Madeleine Evans Webb
Department of Primary Care and Population Health, Upper 3rd Floor, Royal Free Hospital, Rowland Hill Street, London, NW3 2PF, UK
Elizabeth Murray, Zane William Younger, Henry Goodfellow & Jamie Ross
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Elizabeth Murray .
Ethics declarations
Conflict of interest.
This work was part-funded by the MacMillan Cancer Support Research Grant number 6488115. Elizabeth Murray receives funding from the NIHR School for Primary Care Research and the NIHR Collaboration for Leadership in Applied Health Research and Care North Thames. Henry Goodfellow is funded through an NIHR Academic Clinical Fellowship. Jamie Ross is funded by an NIHR School for Primary Care Research fellowship.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
(DOCX 58 kb)
(DOCX 118 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Evans Webb, M., Murray, E., Younger, Z.W. et al. The Supportive Care Needs of Cancer Patients: a Systematic Review. J Canc Educ 36 , 899–908 (2021). https://doi.org/10.1007/s13187-020-01941-9
Download citation
Accepted : 06 December 2020
Published : 25 January 2021
Issue Date : October 2021
DOI : https://doi.org/10.1007/s13187-020-01941-9

Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Supportive care
- Patient needs
- Find a journal
- Publish with us
- Track your research
New study offers hope for a rare and devastating eye cancer

After more than a decade studying a rare eye cancer that produces some of the hardest-to-fight tumors, researchers from University of Pittsburgh Medical Center have found a treatment that works on some patients and, more importantly, a tool that can predict when it is likely to succeed.
The work, published in Nature Communications, is being validated in a clinical trial involving at least 30 patients. It could pave the way for similar methods designed to overcome one of the enduring frustrations of cancer care.
Because tumors differ, not only between patients but even inside the same patient, a treatment that works on one mass may fail on another, even when both are of the same cancer type.
The researchers in Pittsburgh tackled this problem in uveal melanoma, an eye cancer that afflicts only 5 people in a million, but that half the time spreads to other parts of the body, often the liver. The median survival once uveal melanoma has spread has been less than seven months, according to a 2018 study in the journal JAMA Ophthalmology.
“We chose this because it was one of the only cancers that 10 years ago when we started, there was nothing approved for it,” said Udai Kammula, who led the study and directs the Solid Tumor Cell Therapy Program at UPMC Hillman Cancer Center in Pittsburgh.
Scientists had long speculated that the reason uveal melanoma is so tough to fight is that something helps the tumor keep out T cells, a key part of the body’s immune system that develops in bone marrow. However, previous studies by Kammula and his colleagues showed that uveal melanoma tumors actually have T cells inside, and they are turned on.
The problem? The cells lie dormant instead of multiplying and reaching numbers large enough to overwhelm the tumor.
The culprit appears to reside somewhere inside the tumor’s ecosystem of cells, molecules and blood vessels, known formally as the tumor’s “microenvironment.” Kammula compares this ecosystem to the infrastructure that supports a city. Something in that infrastructure helps protect uveal melanoma tumors by preventing the critical T cells from multiplying.
“Ultimately, if we’re going to get rid of cancer, we have to get rid of this infrastructure,” Kammula said.
A tool for predicting success
He and his colleagues have had some success using a treatment known as adoptive cell therapy, which was developed in the 1980s by Steven Rosenberg at the National Institutes of Health.
The treatment involves removing the T cells from the tumor, where they have been unable to proliferate. Scientists then take those T cells and grow them outside the body in a lab dish. They treat patients with chemotherapy to kill off the last of their old immune systems. Finally, they reinfuse the lab-grown T cells into the patient’s blood stream and the cells, now in much greater numbers, go on to attack the tumor.
In this treatment, the T cells are often referred to as tumor-infiltrating leukocytes, or TILs.
Kammula said his team has found that tumors shrink partially or completely in about 35 percent of patients who receive the treatment. But they wanted to know why it doesn’t work in the majority of cases, and whether there might be some way to predict beforehand when it will succeed.
To find out, the researchers analyzed samples from 100 different uveal melanoma tumors that had spread to different parts of the body in 84 patients, seeking to examine all of the tumors’ genetic material.
“We basically put the tumor biopsy in a blender that had the stroma [supportive tissue], the blood vessels, the immune cells, the tumor cells. It had everything,” Kammula said, explaining that they then analyzed all of the tumor’s genetic material.
They found 2,394 genes that could have helped make the tumor susceptible to treatment, some of them genes that experts would regard as “the usual suspects” and others that were unexpected. Using this long list of genes, the scientists searched for characteristics that they shared.
The genes were predominantly involved in helping the body defend itself against viruses, bacteria and other foreign invaders by removing the invaders and helping tissue heal. Kammula and the study’s lead author, Shravan Leonard-Murali, a postdoctoral fellow in the lab, used the different activity levels of these genes to develop a clinical tool.
The tool, known as a biomarker, assigns a score to a uveal melanoma tumor based on the likelihood that it will respond well to the treatment ― removing T cells, growing them outside the body, then reinfusing them.
So far, Kammula said, the biomarker has been “extremely good,” in predicting when the treatment will be effective, though he added, “these findings will need confirmation in the current ongoing clinical trial.”
“I thought it was somewhat of a tour de force, honestly,” said Eric Tran, an associate member of the Earle A. Chiles Research Institute, a division of Providence Cancer Institute in Portland, Ore. Tran did not participate in the study.
He said that while it will be important to validate these results, “I was certainly encouraged by their studies. And from my perspective, I wonder if that sort of strategy can be deployed in other cancers.”
Ryan J. Sullivan, an oncologist at Massachusetts General Hospital and associate professor at Harvard Medical School who was not involved in the study, called the team’s work “timely” and said “it is even more significant that they appear to have a [tool] that appears to predict which patients will benefit.”
The team at UPMC is already investigating possible wider application of both the treatment and the biomarker in a second clinical trial that involves a dozen different cancers.

An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Cancers (Basel)

Physical Activity and Cancer Care—A Review
Weronika misiąg.
1 Student Research Club No. 180, Faculty of Medicine, Wroclaw Medical University, 50-367 Wroclaw, Poland
Anna Piszczyk
Anna szymańska-chabowska.
2 Department of Internal Medicine, Occupational Diseases, Hypertension and Clinical Oncology, Wroclaw Medical University, 50-556 Wroclaw, Poland
Mariusz Chabowski
3 Department of Nursing and Obstetrics, Faculty of Health Science, Wroclaw Medical University, 51-618 Wroclaw, Poland
4 Department of Surgery, 4th Military Teaching Hospital, 50-981 Wroclaw, Poland
Associated Data
All the data analysed during the current study are available from the corresponding author upon reasonable request.
Simple Summary
The aim of this paper is to outline the role and potential benefits of physical activity for cancer patients. We present a review of publications on the subject in order to compare the findings reported in the literature and draw general conclusions that could help clinicians who provide cancer care to develop a more comprehensive treatment approach. This review may also help patients overcome barriers and become more motivated to take up physical activity, which would improve their quality of life. We wish to demonstrate to patients that physical activity should not be regarded as a burdensome medical recommendation but rather as a factor that can reduce the risk of cancer mortality and recurrence.
In 2020, 19.3 million new cancer cases were diagnosed, and almost 10 million deaths from cancer were recorded. Cancer patients may experience fatigue, depression, anxiety, reduced quality of life and sleep problems. Cancer treatments cause numerous side effects and have a negative impact on all body systems. Physical activity is important for cancer patients. The aim of this review is to analyse recent studies on the role of physical activity in cancer patients and emphasize its importance. The review included 36 papers published in English between 2017 and 2021. The findings from these studies show that physical activity decreases the severity of side effects of cancer treatment, reduces fatigue, improves quality of life, has a positive impact on mental health and improves aerobic fitness in cancer patients. Moreover, it reduces the risk of cancer recurrence and death. Physical activity is recommended for patients with any type of cancer and at all stages of treatment. The type of physical activity should depend on the condition of the individual patient. It is extremely difficult to determine what type, intensity and duration of physical activity is likely to have the greatest effect.
1. Introduction
In 2020, 19.3 million new cancer cases were diagnosed, and almost 10 million deaths from cancer were recorded [ 1 ]. Cancer patients may experience fatigue, depression, anxiety, reduced quality of life (QoL) and sleep problems [ 2 , 3 , 4 ].
Cancer treatments have many side effects. They exert a negative impact on: the cardiovascular system, the endocrine system, the digestive system, the immune system, the nervous system, the respiratory system, systemic symptoms such as fatigue, which can persist for many years after treatment, and lymphedema [ 5 ].
Physical activity (PA) is important for cancer patients. The World Health Organization distinguishes between two types of physical activity: aerobic physical activity and anaerobic physical activity. Physical activity can be classified according to intensity as: light-intensity physical activity, 1.5–3 metabolic equivalents of task (METs), which does not result in a significant increase in heart rate or respiratory rate (one example of light-intensity physical activity is slow walking); moderate-intensity physical activity, 3–6 METs; and vigorous-intensity physical activity, more than 6 METs [ 6 ].
PA improves QoL, increases aerobic fitness, has a positive influence on mental health and reduces the side effects of cancer treatment, fatigue and mortality in cancer patients [ 2 , 3 , 7 , 8 , 9 ]. The type of physical activity should depend on the condition of the individual patient. A patient’s response to a given physical activity stimulus may vary due to the side effects of treatment, demographic factors (age), mobility restrictions or comorbidities [ 10 ]. However, patients should undertake physical activity unless the disturbances are severe enough to prevent them from exercising [ 11 ]. Moreover, a patient’s ability to tolerate exercise may vary during a disease. This is caused by the variability in the intensity of the symptoms [ 5 ].
With patients facing a life-threatening illness, recommending additional physical activity may seem to be unnecessarily burdensome or too simplistic, as it would require an investment of time and energy from the patient [ 10 ].
Although there are many research papers about the benefits of physical activity in cancer patients, in practice combining PA with treatment is rare. In 2020, as many as 35.5% of the cancer survivors aged 18 years and older reported physical inactivity [ 12 ]. Only 7% of cancer patients perform adequate exercises [ 13 ]. This manuscript analyses the recent studies from the last five years on the role of physical activity in cancer patients under active treatment and cancer survivors and emphasize its importance. The review summarizes the results gathered from 36 articles and presents the influence of PA in cancer care for different types of tumour and patient groups.
The aim of the study is to present the impact of physical activity on cancer patients and cancer survivors in order to reach the largest possible group of readers, both among healthcare professionals and oncological patients.
2. Material and Methods
We conducted a search of articles in the PubMed, Web of Science and EBSCO Information Services using the following keywords: cancer care, physical activity, survivors, quality of life, QoL. The inclusion criteria were as follows: articles in English, publication between 2017 and 2022. A total of 971 records were initially identified. We removed 381 duplicates and excluded articles to which we had no access, articles in a language other than English and articles not directly related to the subject of the review ( n = 326). During the eligibility assessment, we also excluded articles concerning cancer prevention, since the aim of this review was to evaluate the role of physical activity in patients already diagnosed with cancer, as well as articles with insufficient data ( n = 228). Thirty-six articles were ultimately identified as eligible for inclusion. These publications were meta-analyses, systematic reviews and randomised controlled trials. The identification process of eligible studies is shown in Figure 1 . Patient-reported outcomes were assessed using Functional Assessment of Cancer Therapy Scale (FACT) with a subscale for fatigue (FACT-F) [ 8 ]. The FACT Measurement System consists of over 250 questions, and it measures health-related QoL in patients with cancer and other chronic diseases. Patients are asked to answer about 60 questions, based on the general version FACT-G, then new questions could be added to focus specifically on the problems of a given disease [ 14 , 15 , 16 ].

Identification of studies via databases.
We carried out a review of 36 systematic reviews, meta-analyses and randomised controlled trials concerning physical activity and cancer care. The studies included in the review investigated the effects of physical activity, as measured by a number of questionnaires assessing the type, frequency and duration of particular activities. We presented the results in Table 1 . This table compares the influence of physical activity on cancer patients: both survivors and patients under active oncology treatment. It shows results depending on: the type of cancer, type of intervention and its intensity and the frequency and duration of PA. The main findings in Table 1 describes if the cancer care with PA is superior to the usual care of these patients. This paper presents the findings from studies investigating the impact of physical activity on particular areas of life in cancer patients, such as: QoL, mental health, physical fitness, muscle strength and impact on body weight. The survey describes the impact of PA on side effects, fatigue, mortality, survival and recurrence of cancer. A summary of the key points is presented in Figure 2 .
Comparison of the influence of physical activity on cancer survivors.
PA—physical activity; CARE—Combined Aerobic and Resistance Exercise; DELCaP—Diet, Exercise, Lifestyle and Cancer Prognosis Study; PAGA—Physical Activity Guidelines for Americans; RPA—recreational physical activity; MET—metabolic equivalent of task (minutes/hours); PCa—prostate cancer; PCSM—prostate-cancer-specific mortality; CRF—cancer-related fatigue.

A graphical abstract summarizing the presented results.
3.1. Side Effects of Cancer Treatment
Chemotherapy and radiotherapy inhibit physical activity due to their side effects, such as severe fatigue, lack of energy as well as hair loss and mental health problems [ 17 ]. Chemotherapy is more likely than chemoradiotherapy to cause fatigue and reduce motivation to exercise. While chemoradiotherapy involves a more intensive treatment schedule, it is better tolerated by patients [ 18 ]. Physical activity has been shown to reduce the side effects of treatment and fatigue in cancer patients. The reduction was seen in those patients who, despite the side effects of treatment, underwent physical activity [ 7 ]. Studies report that regular PA reduces disease-specific side effects in patients with MM [ 19 , 20 ]. However, there is no evidence that physical activity mitigates the cardiotoxicity induced by cytostatic drugs [ 21 ].
3.2. Fatigue
One study included in the review found that regular physical activity combined with an appropriate diet (the patients completed 71% of the aerobic exercise sessions of 41 ± 25 min and 58% of the resistance exercise sessions planned as part of the intervention) reduced the fatigue resulting from intensive cancer treatment. The QoL was improved as well as lower limb muscle mass and endurance in breast cancer patients undergoing chemotherapy or radiotherapy. An important finding from the study was that the beneficial effect on QoL and fatigue persisted one year after the intervention [ 2 ]. Combined aerobic and resistance exercise has been found to reduce fatigue in patients with breast cancer [ 8 ]. In a study by Singh et al. [ 3 ], analysing the findings from 19 clinical trials, physical activity was observed to have a significant effect on fatigue in patients with colorectal cancer as compared with usual cancer care. Physical activity reduces the level of fatigue in cancer patients. The association between exercise and reduced fatigue has been demonstrated in patients with breast, prostate, colon and lung cancers [ 4 ]. Moreover, moderate-intensity physical activity has been found to reduce cancer-related fatigue in patients with colorectal cancer [ 22 ].
3.3. Quality of Life
Physical activity improves physical and social QoL and reduces anxiety and depression in cancer patients [ 2 , 3 ]. Unlike moderate to vigorous intensity physical activity, sedentary time negatively affects QoL and wellbeing of cancer patients [ 4 ]. Findings from one randomised controlled trial showed that aerobic and resistance exercise improves QoL by reducing depression, fatigue and physical deconditioning, which are the most common symptoms reported by breast cancer survivors [ 23 ]. Combined aerobic and resistance exercise performed during chemotherapy results in better longer-term QoL outcomes in breast and colorectal cancer patients, improving sleep quality, reducing anxiety and depression and having a positive impact on happiness [ 3 , 8 ]. Our review also included studies investigating the effects of physical activity on QoL in paediatric cancer patients with the use of the Paediatric Quality of Life Inventory. The studies showed that exercise interventions significantly improved QoL in the patients [ 24 , 25 , 26 , 27 ], even patients with haematological malignancies such as multiple myeloma [ 28 , 29 , 30 ]. Physical activity has also been shown to improve QoL and reduce anxiety and depression in ovarian cancer patients [ 27 ]. The findings from one study indicated that physical activity improves QoL in cancer patients despite the bothersome side effects of cancer treatment [ 7 ].
3.4. Mental Health
Physical activity has a positive impact on the mental health of cancer patients and adds positivity to their daily life [ 7 ]. One study showed that aerobic, resistance and flexibility exercises undertaken by prostate cancer patients with bone metastases for 3 months resulted in self-reported improvements in physical functioning, which had a positive influence on the mental health of the patients studied [ 31 ]. Another study found that an 8-week exercise intervention programme consisting of twice-per-week sessions of 60 min of resistance, flexibility and cardiorespiratory exercises performed by patients with different types of cancer improved the capability of the patients to express positive emotions, improved their functional capacity and had a positive influence on their mental health [ 32 ].
3.5. Physical Fitness, Muscle Strength, Impact on Body Weight
Studies have shown that exercise improves aerobic fitness and upper-body strength and reduces BMI and body fat in colorectal cancer patients. The results of a meta-analysis conducted by Singh et al. showed a greater effect for exercise interventions lasting over 12 weeks and interventions conducted during chemotherapy in patients with colorectal cancer [ 3 ]. Combined aerobic and resistance exercise has been found to be associated with superior upper and lower body muscle endurance in breast cancer patients [ 8 ].
3.6. Mortality and Longer Survival
There is an association between greater physical activity and reduced mortality in colorectal, breast and prostate cancer patients, with 40–50% risk reductions observed among individuals undertaking physical activity [ 33 ]. A study by Palesh et al. found that engaging in moderate physical activity was associated with longer survival and reduced hazard of cancer-related mortality in patients with advanced breast cancer [ 34 ]. In their study, Di Maso et al. noted that only vigorous physical activity had the advantage over inactivity in terms of reduced risks of cardiovascular and cancer mortality [ 35 ]. The cohort studies referred to by the authors reported approximately 40% reduction in mortality from prostate cancer in physically active men. Physical activity has also been found to reduce the risk of mortality in breast and colorectal cancer patients [ 36 ]. Barnard et al. [ 37 , 38 ] reported that intense physical activity reduces insulin resistance and insulin levels, with greater effects observed for a combination of intense physical activity and a low-fat, high-fibre diet. One study reported that breast cancer patients who met the minimum physical activity guidelines (PAGAs) had lower hazards of mortality compared with physically inactive patients (HR = 0.74, 95%, CI = 0.56 to 0.96; HR—hazard ratio; CI—confidence interval) [ 9 ]. A cohort study carried out by Wang et al. [ 39 ] that investigated the effects of recreational physical activity in patients with non-metastatic prostate cancer found that engaging in ≥17.5 MET-h/week of recreational physical activity, compared with 3.5 ≤ 8.75 MET-h/week of recreational physical activity, was associated with a 31% lower risk of prostate cancer-specific mortality (HR 0.69, CI 95%, p = 0.006), with no differences between the TNM stage of a tumour.
3.7. Recurrence
Combined aerobic and resistance exercise reduces the incidence of metabolic syndrome in cancer survivors, particularly breast cancer survivors. Metabolic syndrome is a risk factor for breast cancer recurrence [ 23 , 40 ]. A randomised controlled trial conducted among 100 breast cancer survivors, assigned either to exercise or usual care, showed an improvement in BMI and levels of circulating biomarkers, i.e., insulin, IGF-1, adiponectin and leptin, in the exercise group after the exercise intervention. An improvement in all metabolic syndrome variables persisted at the 3-month follow-up in the exercise group. Another study found that breast cancer patients meeting the minimum PAGAs both before and after their diagnosis had >50% reduced hazards of recurrence in comparison with patients not meeting this minimum at either time point. The study also found reduced hazards of recurrence for patients not meeting the minimum physical activity guidelines prior to diagnosis but who reported meeting the guidelines after their treatment (2-year follow-up) [ 9 ].
4. Discussion
A diagnosis of cancer has a profound impact on the life of the patient. The fear of cancer progression, metastases and side effects of systemic treatment affects the quality of life as well as the mental and physical health of cancer patients. The anxiety, depression and bothersome somatic symptoms, such as fatigue, nausea, vomiting and hair loss, experienced by cancer patients significantly inhibit their physical activity. The barriers to undertaking physical activity faced by cancer patients are a very complex issue. They are associated with a number of factors. The nature, type and extent of cancer; the presence of metastases; cancer treatment and its side effects; the patient’s attitude to their illness and their coping strategy, as well as social and family support, have an enormous impact on the patient’s motivation and quality of life and thus their attempt to undertake regular physical activity. Moreover, cancer patients are often concerned that physical activity could have a negative impact on their illness, especially patients with diagnosed multiple myeloma, whom have the highest physical and mental impairments and a low QoL [ 20 , 45 ]. Furthermore, they are less willing to include exercise in their standard cancer treatment because of the fear that it will make them feel worse and due to a lack of knowledge of the benefits of physical activity. However, numerous studies have reported that standard cancer care combined with physical activity is superior to standard pharmacological care. Physical activity improves the daily functioning of cancer patients, reduces fatigue, side effects of intensive treatments, anxiety and depression and improves muscle endurance and mass, thereby allowing patients to perform their daily activities without difficulty. Moreover, the findings from the studies showed that physical activity is associated with a reduced risk of cancer of the breast, colon, stomach and endometrium (10–20% risk reduction). The studies manifest that PA reduces the risk of mortality by 40–50% for breast, colon and prostate cancers [ 33 ].
Cancer-related fatigue is a serious and complex problem that affects the quality of life and daily activities of cancer patients. Although, based on the results in the studies [ 2 , 3 , 4 ], it can be concluded that there is a correlation between fatigue and a tendency to have less PA, it cannot be considered as an unequivocal cause of decline in PA. Nevertheless, fatigue has a major impact on the functioning of cancer patients, and clinicians should aim to reduce fatigue levels. Numerous studies have shown that physical activity is associated with a significant reduction in fatigue in breast, colorectal, ovarian and prostate cancer patients and multiple myeloma patients [ 3 , 8 , 19 , 22 , 27 , 31 , 43 ]. A systematic review by Cataldi et al. found that aerobic exercise is more effective than other treatments in reducing cancer-related fatigue. Their review suggested that exercise should be performed at least 2 days per week for at least 8 weeks in order to achieve the best results and showed that the effects of low- to medium-intensity exercise did not differ between women and men [ 46 ].
According to the National Comprehensive Cancer Network (NCCN) and the American College of Sports Medicine (ACSM) (2018), physical activity improves QoL and physiological and psychological fitness in cancer patients [ 46 ].
Chemotherapy and radiotherapy have a negative impact on many aspects of the lives of cancer patients, reducing their interest in physical activity and decreasing the effectiveness of exercise. The side effects of treatment are bothersome, especially for patients with MM, and their intensity is much higher than people with other haematological cancers [ 20 , 47 ]. One study revealed that cancer patients found it very difficult to engage in physical activity in public places due to the side effects of their treatment, such as hair loss, as well as the fear of overheating and infection [ 17 ]. However, physical activity has been shown to reduce the side effects of cancer treatment. Importantly, the beneficial effect of an intervention involving physical activity in reducing such side effects of cancer treatment as fatigue persisted one year after the intervention [ 2 ]. Chemotherapy not only affects QoL and causes bothersome side effects, but it also has a direct impact on the patient’s physiology. It reduces mitochondrial function by impairing oxidative phosphorylation, resulting in sarcopenia. Moreover, it may reduce lung function [ 43 , 48 ]. It has been shown that aerobic exercise mitigates the impact of cancer treatment on physiological functions. Physical activity helps increase blood flow, activates the sympathetic nervous system, regulates the endocrine system and mobilises cytotoxic lymphocytes and NK cells, thus exerting antitumor effects. Moreover, it reduces the levels of lactate, which are a factor in promoting tumour growth [ 49 , 50 ].
The results from a study by Cannioto et al. [ 9 ] showed that breast cancer patients meeting the minimum guidelines for physical activity both before and after diagnosis had >50% reduced hazards of cancer recurrence and mortality. These findings are of great importance for the development of clinical oncology, as they suggest that clinicians should advise their patients to increase their physical activity immediately after a diagnosis, which would result in significant benefits. However, the benefits of regular engagement in physical activity are not only directly associated with cancer care but also translate into a reduced risk of comorbidities, improved cardiovascular function and physical fitness and thus improved wellbeing and better daily functioning.
Physical activity can improve immune system function by mobilizing leukocytes with increased functional capacities into the circulation. It helps with the elimination of dysfunctional T cells and improves the abundance of some T cell populations. PA may have an impact on CTLA-4 (inhibitory immune checkpoint) and provide to better response to immunotherapy in cancer patients [ 51 , 52 ].
As for incorporating exercise into cancer care and improving treatment outcomes, it is crucial to understand the role of the intensity, dose and mode of exercise in cancer patients. It is necessary to consider the individual needs of patients, the type of cancer they have as well as their treatment and health history. It has been found that the sooner physical activity is incorporated into a patient’s treatment plan after diagnosis, the more effective it is [ 53 ]. High-intensity exercise is not contraindicated for all cancer patients. Therefore, patients should not be restricted to exercise of low intensity. High-intensity exercise should be avoided by those who suffer from nausea and vomiting as well as those who have a blood clot related to a peripheral central catheter [ 54 ]. Positive effects of exercise are observed with sessions of at least 20 min on most days of the week (accounting for planned days of rest and unplanned days of inactivity [ 55 ] due to one of the following barriers: fatigue, pain, lack of motivation [ 54 ]). Recommendations from the ACSM, the NCCN and the Clinical Oncology Society of Australia (COSA) recommend participation in 150 min of moderate-intensity aerobic exercise, 3–5 sessions per week, as well as resistance training at least 2 days per week as part of a programme lasting 6–12 weeks [ 56 , 57 , 58 ]. It is also recommended that exercise interventions should, at least initially, be supervised by exercise trainers or physical therapists [ 59 ]. Cataldi et al. recommend increasing the quantity and quality of exercise in cancer patients by monitoring all parameters during exercise sessions. Persons responsible for cancer care should take into consideration the outcomes of studies on this subject, so as to best plan the intensity and volume of exercise for their patients [ 46 ]. Similarly, the World Cancer Research Fund (WCRF) and the American Institute for Cancer Research (AICR) recommend participation in at least 150 min of moderate-intensity exercise per week, including strength training exercises at least twice a week [ 60 ].
A major challenge for cancer patients is the very initiation of regular physical activity. This, in turn, is influenced by their strategy for coping with the illness. Strategies for coping with cancer can be constructive (e.g., fighting spirit, positive redefinition) or destructive (e.g., helplessness, hopelessness, anxious preoccupation). Choosing a constructive strategy will help initiate and maintain physical activity, whereas destructive strategies are a major barrier to the initiation of physical activity. The helplessness and anxiety associated with a diagnosis of cancer result in the patient giving in to the illness. This reduces the patient’s QoL, making it more difficult for them to maintain motivation for engaging in physical activity [ 61 ]. Other barriers to participation in physical activity reported by cancer patients include: fatigue, business and the associated lack of time [ 62 ], severe pain and social and environmental barriers—lack of an exercise partner, lack of exercise facilities, fear of injury, lack of willpower, lack of interest, lack of equipment and lack of experience [ 63 , 64 ]. A relatively large proportion of patients (approximately 17.9%) cite the lack of access to information about how to exercise and what type of exercise would be best for them as the reason for which they do not engage in physical activity [ 64 ]. Another reason why patients do not initiate physical activity is their concern that a given type of exercise is contraindicated for them due to their illness.
It is very difficult for patients to maintain the appropriate intensity of exercise, especially if they suffer from chronic comorbidities or experience bothersome side effects of cancer treatment. The occurrence of comorbidities such as hypertension, kidney disease, diabetes, liver disease or obesity is increasing in cancer survivors [ 65 ]. Obesity, which occurs particularly in colorectal and breast cancer survivors, increases the risk of heart diseases and hypercholesterolemia and has an influence on survival [ 66 ]. Attempts are being made to determine what training intensity would be most beneficial for such patients in terms of improving their QoL and maintaining their motivation for participating in physical activity. All members of the cancer care team should promote physical activity at all stages of cancer treatment. Exercise should be individualised, planned and tailored to the individual patient and adjusted to a specific type of cancer, as it offers major potential for reducing cancer morbidity and mortality [ 67 ]. Studies show that flexible time for PA sessions, low-cost and close location to home met with highest interest from patients and better compliance [ 20 ]. The literature discussed above suggests that physical activity has a significant multidimensional impact on the quality of life of cancer patients and plays a major role in improving cancer care, treatment outcomes, increasing survival time and reducing mortality in cancer patients. Therefore, it is important that clinical recommendations focus on educating patients and attempting to change their attitude to exercise [ 62 ]. It is extremely hard to find the best way to encourage patients to start and maintain physical activities. Nevertheless, the healthcare providers should aim to encourage patients to exercise. An adequate education and demonstration PA advantages may be the first step to motivate them. Healthcare professionals should devote their time to patients, list the barriers the cancer patients and cancer survivors are struggling with and should try to find a solution to reduce the barriers and recommend an appropriate intervention. Psychological help could be invaluable. The results presented in this study may be helpful to convince patients that PA can offer them many benefits for their QoL, everyday functioning and survival time.
5. Limitations
The study has potential limitations. The first limitation is the selection of articles only in English, which introduces a language bias. The reason for this limitation is the insufficient knowledge of other languages to discuss the results in the study with appropriate precision. The second limitation is exclusion of the papers which the authors had no access to, which may potentially have impact on the results. The third limitation is the lack of an unequivocal way to encourage patients to start and maintain PA. Our purpose is to motivate the authors of future studies to search for an effective method encouraging patients to exercise.
6. Conclusions
Physical activity improves quality of life, increases survival and reduces mortality, fatigue, side effects of treatment and the risk of recurrence.
Physical activity should be selected individually, depending on the type of cancer, treatment and comorbidities.
It is extremely difficult to determine what type, intensity and duration of physical activity is likely to have the greatest effect.
Funding Statement
This research received no external funding.
Author Contributions
W.M. wrote the manuscript; A.P. reviewed and drafted the manuscript; A.S.-C. analysed the data and supervised the manuscript; M.C. participated in supervision and project administration. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Conflicts of interest.
The authors declare no conflict of interest.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
April 25, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
Study reveals tai chi benefits for sleep quality in advanced lung cancer patients
by The University of Hong Kong

A research team from the School of Nursing, LKS Faculty of Medicine of the University of Hong Kong (HKUMed), found positive effects of tai chi and aerobic exercise on sleep quality, psychological well-being, physical function, and circadian rhythm in patients with advanced lung cancer.
The study, conducted over a four-year period, discovered additional advantages in terms of improving one-year survival rates and reducing fatigue.
The research has demonstrated remarkable potential of tai chi as a non-pharmacological intervention for improving survival in advanced lung cancer patients. It emphasizes the importance of integrating physical activity, particularly tai chi, into the treatment plan to enhance the holistic well-being of this vulnerable population. The study was published in JAMA Oncology .
Lung cancer has been the most prevalent cancer and the leading cause of cancer death in Hong Kong since 2019. Patients with advanced lung cancer often experience sleep disturbances and associated psychological symptoms, which impact their overall survival and quality of life. Pharmacological interventions can induce side effects that significantly worsen cancer-related symptoms.
To address these issues, HKUMed research team explored non-pharmacological interventions, with physical exercise being a promising option due to its safety, affordability, and diverse benefits.
The research team conducted a meta-analysis to examine the effects of aerobic and mind-body exercises, two widely recognized forms of physical exercises, which differ in intensity and modality. The study showed significant enhancement in sleep quality among cancer patients experiencing poor sleep. However, the comparative effects of these exercises in patients with advanced lung cancer remain unclear.
Research methods and findings
Between December 2018 and September 2022, HKUMed research team recruited 226 patients with advanced lung cancer in three public hospitals in Hong Kong. They were randomly assigned to one of the three groups: tai chi, aerobic exercise , or a self-management control group. The tai chi group attended classes twice a week for 16 weeks. The aerobic exercise group attended classes twice a month over the same 16-week period, engaging in activities such as treadmill walking, stationary bike riding, and resistance exercises.
The study assessed multiple factors, including subjective sleep quality, objective sleep parameters, psychological distress, fatigue, health-related quality of life, physical function, circadian rhythm, and one-year survival rates among advanced lung cancer patients. Assessments were conducted before the intervention classes, at the end of the 16-week intervention, and at week 52.
The results revealed that both the tai chi and aerobic exercise groups demonstrated a significant improvement in sleep quality, anxiety, depression, cardiorespiratory function, physical function, step count, and circadian rhythm at both week 16 and week 52 than the control group. Tai chi demonstrated superior benefits over aerobic exercise in terms of sleep quality, fatigue reduction, and balance.
The study found a remarkable 65% lower risk of mortality in the tai chi group compared to the control group, suggesting that engaging in tai chi may potentially offer better survival for patients with advanced lung cancer.
Led by Research Assistant Professor Dr. Naomi Takemura and supervised by Professor Chia-Chin Lin, both from the School of Nursing at HKUMed, this three-arm randomized controlled trial represents the largest study of its kind conducted to date, focusing specifically on patients with advanced lung cancer. The findings carry implications for the field of cancer care and highlight the potential benefits of tai chi, compared to conventional exercise.
"Tai chi's emphasis on the mind-body connection offers a holistic approach that goes beyond physical exercise alone. The meditative and mindful aspects of tai chi may help patients cope with psychological distress, reduce anxiety, and enhance their overall quality of life and one-year survival rate," said Dr. Naomi Takemura.
The study opens new avenues for supportive care in cancer management and highlights the importance of a multidimensional approach to address cancer symptoms. By incorporating tai chi into the treatment plan, health care providers can offer a safe, affordable, and potentially effective approach to alleviate the symptom burden and enhance the overall well-being of patients.
Explore further
Feedback to editors

Differentiating cerebral cortical neurons to decipher molecular mechanisms of neurodegeneration
4 minutes ago

National trial safely scales back prescribing of a powerful antipsychotic for the elderly
14 minutes ago

With hybrid brains, these mice smell like a rat

Premature mortality higher among sexual minority women, study finds

Creatine found to improve cognitive performance during sleep deprivation
19 minutes ago

Identifying a new liver defender: The role of resident macrophages
44 minutes ago

Researchers create an AI-powered digital imaging system to speed up cancer biopsy results

Understanding the cellular mechanisms of obesity-induced inflammation and metabolic dysfunction

Researchers publish final results of key clinical trial for gene therapy for sickle cell disease
2 hours ago

Using AI to improve diagnosis of rare genetic disorders
Related stories, exercise interventions in advanced lung cancer patients led to increased functionality.
Oct 18, 2017

Exercise shown to help patients with advanced breast cancer, especially if they are suffering with pain
Mar 19, 2024
More exercise may not help all cancer patients to the same extent
Oct 9, 2018

Exercise can reduce severity of breast cancer treatment side effects
Nov 21, 2022

Exercise may boost quality of life for patients with metastatic breast cancer
Dec 7, 2023

Q&A: How exercise can boost cancer treatment and recovery
Jan 29, 2024
Recommended for you

A closed-loop drug-delivery system could improve chemotherapy
18 hours ago

New potential avenues for cancer therapies through RNA-binding proteins
21 hours ago

Study highlights increased risk of second cancers among breast cancer survivors
15 hours ago

Newly discovered mechanism helps tumor cells evade the immune system early on
Apr 24, 2024

Mini-colons advance colorectal cancer research
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
- Skip to main content
- Keyboard shortcuts for audio player
- Your Health
- Treatments & Tests
Health Inc.
- Public Health
Oncologists' meetings with drug reps don't help cancer patients live longer
Sydney Lupkin

Drug companies often do one-on-one outreach to doctors. A new study finds these meetings with drug reps lead to more prescriptions for cancer patients, but not longer survival. Chris Hondros/Getty Images hide caption
Drug companies often do one-on-one outreach to doctors. A new study finds these meetings with drug reps lead to more prescriptions for cancer patients, but not longer survival.
Pharmaceutical company reps have been visiting doctors for decades to tell them about the latest drugs. But how does the practice affect patients? A group of economists tried to answer that question.
When drug company reps visit doctors, it usually includes lunch or dinner and a conversation about a new drug. These direct-to-physician marketing interactions are tracked as payments in a public database, and a new study shows the meetings work. That is, doctors prescribe about five percent more oncology drugs following a visit from a pharmaceutical representative, according to the new study published by the National Bureau of Economic Research this month.
But the researchers also found that the practice doesn't make cancer patients live longer.
"It does not seem that this payment induces physicians to switch to drugs with a mortality benefit relative to the drug the patient would have gotten otherwise," says study author Colleen Carey , an assistant professor of economics and public policy at Cornell University.
For their research, she and her colleagues used Medicare claims data and the Open Payments database , which tracks drug company payments to doctors.
While the patients being prescribed these new cancer drugs didn't live longer, Carey also points out that they didn't live shorter lives either. It was about equal.
The pharmaceutical industry trade group, which is known as PhRMA, has a code of conduct for how sales reps should interact with doctors. The code was most recently updated in 2022, says Jocelyn Ulrich, the group's vice president of policy and research .
"We're ensuring that there is a constant attention from the industry and ensuring that these are very meaningful and important interactions and that they're compliant," she explains.
The code says that if drug reps are buying doctors a meal, it must be modest and can't be part of an entertainment or recreational event. The goal should be education.
Ulrich also points out that cancer deaths in the U.S. have declined by 33 percent since the 1990s , and new medicines are a part of that.
- MUSC Health MyChart
- Schedule an Appointment
- Adult Patient Care
- Children's Health
- Education at MUSC
- Research at MUSC
- Latest News
- Patient Navigation
Student volunteers navigate cancer patients to resources through ACS CARES

Navigating the challenges of cancer care isn’t easy. Besides the obvious medical challenges, there’s also all of the “other stuff” – transportation, child care, food insecurity, insurance coverage and more, all of which can ultimately affect a patient’s care.
To help people to find solutions to these problems, five College of Charleston students are volunteering as navigators at MUSC Hollings Cancer Center through an American Cancer Society pilot program. They’re meeting with patients, listening to their dilemmas and connecting them to resources.
“A lot of them are future health care leaders in the field. And this is giving them experience that they might not get otherwise,” said Charlotte Waugh, program manager for the American Cancer Society Community Access to Resources, Education, and Support (ACS CARES) program. “This is really getting them hands on and thinking about health disparities and access to care and all these important things that they should be thinking about when they’re going into these fields.”

Hollings is one of three sites for the pilot program, but the ACS has big plans for this program. It intends to expand to nine more sites in the upcoming school year and 25 more sites in the school year after that, with the ultimate goal of expanding nationwide.
For the upcoming school year, meanwhile, program leaders at Hollings intend to recruit more student volunteers , recruit from other colleges in the area and, hopefully, expand placements beyond the main Hollings building on the peninsula to clinics in nearby communities like North Charleston.
“The plan is to continue to grow,” said Anne Puckett, survivorship program manager at Hollings. “It’s a great program for the students and the patients. As this program matures, we anticipate that the students will help to take some of the nonclinical aspects off of the nurse navigators so the nurse navigators can focus on the clinical piece.”
Abigail Ryan, a junior biochemistry and Spanish major who intends to apply to medical school, said the program has opened her eyes to the realities that many patients live every day.

“I didn’t have a concept of transportation being something that kept someone from getting care,” she said. “But I’ve had several conversations with people who were like, ‘I canceled my appointment last minute,’ or ‘I didn’t go because I couldn’t get there.’ To me, with cancer, the scary thing is the disease itself – not thinking about the burdens that it’s putting on your family’s finances, time or energy outside of that. So that’s been pretty eye-opening.”
For this pilot program, the student volunteers have been placed in a handful of clinics: radiation oncology, thoracic oncology, genitourinary oncology and the infusion suite. The students typically enter an exam room while the patient is waiting for the doctor; explain the program; conduct a needs assessment, if the patient wants to participate; and then get to work finding resources. Some patients don’t need much help, so the students offer a phone number if the patient wants to reach out. Other patients might need weekly check-ins, which the students can provide via phone call or an email messaging portal.
“I’d say the biggest thing is even when I just go and talk with patients, they feel so seen and heard. I also will express to them that this is not meant to be an extra thing added to your plate,” said Georgia Kern, a sophomore psychology major.

To quell any doubts about how a healthy young college student could possibly help, Kern also shares a bit of her story. She was a child when her little sister was diagnosed with a brain tumor. A child-life specialist worked with the family, inspiring Kern’s ultimate career goal of going into that field to help children and families.
“I wasn’t even the patient, but I still benefited so much from it. And that’s why I got into the ACS CARES program in the first place because I know it’s not just the patient that’s experiencing and affected by the diagnosis,” she said. “I’ll explain my family story and how I wish the ACS CARES program had existed. I think that there are so many patients that have been needing this for so long; I’m really glad that it’s an option now.”
Kern said that sometimes she meets people who are midway through treatment and have been muddling along, and she can make things a little bit easier. For example, she met a family that’s been traveling from the Myrtle Beach area for treatment. In cases like that, she can send them a few gas station gift cards to help with transportation costs. Another option is Road to Recovery, the American Cancer Society program of volunteer drivers who take people to appointments.
Waugh said that conducting the pilot program at three vastly different sites – the other pilot sites being the University of California, Los Angeles (UCLA) and the University of Iowa – is helping to smooth out the program as they prepare to expand.
“It’s been interesting how different it is at each site and what the different challenges are. I feel overall the students have a lot to gain from it,” she said. “And one of the main points that I'm proud of is the average interaction time among patients and students is over an hour for each interpersonal interaction, which I think is unique in the health care system.”
The students agree.
“It’s really rewarding to have a patient who knows how to get to an appointment, who knows that they can get to an appointment that they couldn’t previously,” Ryan said.

Outstanding Performance
The National Cancer Institute renewed MUSC Hollings Cancer Center's NCI designation, awarding the center its best score ever of “outstanding performance.”

OT for Oncology
Three MUSC occupational therapy students are passionate about elevating the profession's role in cancer care.

Fetter Collaboration
A Stand Up 2 Cancer grant will help to ensure that Fetter Health Care patients at risk of lung cancer get the screenings they need.
Related Links
More MUSC Hollings Cancer Center news
Subscribe to our newsletter
Hollings Horizons magazine
About the Author
Leslie Cantu MUSC Hollings Cancer Center
Categories: Cancer , Features

COMMENTS
Find research articles on cancer treatment, including news stories, clinical trials, blog posts, and descriptions of active studies. ... A comprehensive analysis of patients with cancer who had exceptional responses to therapy has revealed molecular changes in the patients' tumors that may explain some of the exceptional responses.
Population research (also known as epidemiological research) is the study of causes and patterns of occurrence of cancer and evaluation of risk. Population scientists, also known as epidemiologists, study the patterns, causes, and effects of health and diseases in defined groups. Population research is highly collaborative and can span the ...
Clinical Research. Clinical research involves the study of cancer in people. These cancer research studies are further broken down into two types: clinical trials and observational studies. Clinical trials are research studies that involve an intervention, which is a treatment or change that may affect the results of cancer.These can lead to new treatments, care, and improved results for ...
Dedicated to helping people who face cancer. Learn about cancer research, patient services, early detection, treatment and education at cancer.org. ... We've invested more than $5 billion in cancer research since 1946, all to find more - and better - treatments, uncover factors that may cause cancer, ...
NCI joins the cancer community in advancing the goals of the National Cancer Plan as part of its research programs. Studying cancer and its burden on a population-wide scale can provide information that directly affects the health of millions of people. NCI is the nation's leader in cancer research. Learn about key research areas, initiatives ...
Your donation to the American Association for Cancer Research helps our more than 58,000 members worldwide drive progress against cancer. The AACR is the first and largest cancer research organization. Our mission is to prevent and cure cancer through research, education, communication, collaboration, funding, and advocacy. Our 58,000 members ...
NCI has 30 divisions, offices, and centers who work together to build, maintain, and enhance a cohesive and comprehensive cancer research agenda. Advisory Boards and Review Groups NCI relies on advisory committees to provide advice on the National Cancer Program, NCI scientific priorities, and more.
Thanks to NCI-funded research, patients with cancer have a greater number of therapeutic options than ever before, many of which are more effective and less toxic than earlier options. But more research is needed to ensure the most effective treatment possible, including better ways to use existing therapies in combination, while maintaining ...
Cancer therapies have evolved considerably in recent decades, substantially improving the quality of life and survival of patients with cancer. In this issue, we launch our Series on Cancer ...
2021 Special Section: COVID-19 and Cancer. This year's special section focuses on the impact of the COVID-19 pandemic on people living with cancer, cancer surveillance capabilities, and the overall American health system. This section is intended to inform any person (which may include policy makers, researchers, clinicians, cancer control advocates, patients, and/or caregivers) with interest ...
The global oncology community will gather in Chicago to share and discuss the latest clinical cancer research impacting patient care at the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting. Treatment advances involving targeted therapies, immunotherapy, and new uses of technology, as well as research on improving patient quality of life and outcomes are among the topics that ...
Resources for Patients. We offer authoritative information about your type of cancer as well as information on a wide range of cancer topics and the latest cancer research. Also find NCI-supported clinical trials. For your questions about cancer or help navigating the NCI website, please contact our information specialists.
In select patients, omitting radiation to nearby lymph nodes didn't increase the risk of cancer returning, new clinical trial results show. In a clinical trial, participating in the classes reduced the risk of being hospitalized. Explore the many ways you can participate in cancer research, including treatment and non-treatment studies.
Among patients with cancers bearing the KRAS G12C mutation who received divarasib at a 400-mg dose, 56% with lung cancer, 36% with colorectal cancer, and 36% with other tumor types had a confirmed ...
About the Cancer Center. Every day, more than 350 outstanding physicians and scientists at Mayo Clinic Comprehensive Cancer Center dedicate themselves to finding answers for the unmet medical needs of cancer patients.. Collaborating across the full spectrum of cancer research, these Mayo Clinic cancer experts are committed to understanding the biology of cancer, to discovering new ways to ...
The Cancer Moonshot, along with many other cancer-based initiatives, marks the continued commitment of Congress and the Executive Branch to cancer research and improving patient outcomes. To realize the goals of the reignited Cancer Moonshot, the full reach of the federal government, including NIH, NCI, FDA, and CDC, will be utilized to better ...
NIH researchers develop AI tool with potential to more precisely match cancer drugs to patients. Posted: April 18, 2024. Contact: NCI Press Office. 240-760-6600. Researchers have used high-resolution gene expression data from individual tumor cells to fine-tune the ability of an AI tool called PERCEPTION to predict drug responses.
The American Cancer Society (ACS) has helped make possible almost every major cancer breakthrough since 1946. Since then, we've invested more than $5 billion in cancer research, making us the largest nonprofit funder of cancer research in the United States, outside of the federal government. We remain committed to finding more - and better ...
Cancer is a leading cause of death worldwide, accounting for nearly 10 million deaths in 2020, or nearly one in six deaths. The most common cancers are breast, lung, colon and rectum and prostate cancers. Around one-third of deaths from cancer are due to tobacco use, high body mass index, alcohol consumption, low fruit and vegetable intake, and ...
Cancer is a very complicated sequence of disease conditions progressing gradually with a generalized loss of growth control. 1 -3 There were only a few options of cancer treatment for patients for many decades which include surgery, radiation therapy, and chemotherapy as single treatments or in combination. 4,5 But recently, many pathways ...
Cancer, and the complex nature of treatment, has a profound impact on lives of patients and their families. Subsequently, cancer patients have a wide range of needs. This study aims to identify and synthesise cancer patients' views about areas where they need support throughout their care. A systematic search of the literature from PsycInfo, Embase and Medline databases was conducted, and a ...
Patients' quality of life has become a major objective of care in oncology. At the same time, it has become the object of increasing interest by researchers, working with both quantitative and qualitative methods. Progress in oncology has enabled more patients to survive longer, so that cancer is increasingly often a chronic disease that ...
1. Introduction. Cancer and its treatments can affect every aspect of an individual's life, giving rise to a range of supportive care needs that can include informational, physical, practical, social, spiritual, psychological, and emotional requirements [].When left unaddressed, these needs can impact capacity to tolerate or adhere to treatment, capacity to engage in treatment decision ...
Some reviews addressing cancer peer support have a broad research approach by including various formats of peer support like face-to-face groups, one-to-one, ... Peer led CSHGs can be an important source of nonprofessional support for cancer patients, but our findings show that participation in a CSHG requires an awareness of group dynamic ...
New study offers hope for a rare and devastating eye cancer. By Mark Johnson. April 22, 2024 at 5:00 a.m. EDT. Udai Kammula of University of Pittsburgh Medical Center stands with a patient who was ...
Although there are many research papers about the benefits of physical activity in cancer patients, in practice combining PA with treatment is rare. In 2020, as many as 35.5% of the cancer survivors aged 18 years and older reported physical inactivity . Only 7% of cancer patients perform adequate exercises . This manuscript analyses the recent ...
Research methods and findings. Between December 2018 and September 2022, HKUMed research team recruited 226 patients with advanced lung cancer in three public hospitals in Hong Kong.
Drug company reps commonly visit doctors to talk about new medications. A team of economists wanted to know if that helps patients live longer. They found that for cancer patients, the answer is no.
The students typically enter an exam room while the patient is waiting for the doctor; explain the program; conduct a needs assessment, if the patient wants to participate; and then get to work finding resources. Some patients don't need much help, so the students offer a phone number if the patient wants to reach out.
The research, published in Lancet Regional Health - Europe, found particular risks for younger breast cancer survivors. Eight out of 10 breast cancer cases are in women over the age of 50.