Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 19 July 2018

Endometriosis
- Krina T. Zondervan 1 , 2 ,
- Christian M. Becker 1 ,
- Kaori Koga 3 ,
- Stacey A. Missmer 4 , 5 ,
- Robert N. Taylor 6 &
- Paola Viganò 7
Nature Reviews Disease Primers volume 4 , Article number: 9 ( 2018 ) Cite this article
21k Accesses
678 Citations
118 Altmetric
Metrics details
- Infertility
- Inflammation
- Reproductive disorders
- Reproductive techniques
Endometriosis is a common inflammatory disease characterized by the presence of tissue outside the uterus that resembles endometrium, mainly on pelvic organs and tissues. It affects ~5–10% of women in their reproductive years — translating to 176 million women worldwide — and is associated with pelvic pain and infertility. Diagnosis is reliably established only through surgical visualization with histological verification, although ovarian endometrioma and deep nodular forms of disease can be detected through ultrasonography and MRI. Retrograde menstruation is regarded as an important origin of the endometrial deposits, but other factors are involved, including a favourable endocrine and metabolic environment, epithelial–mesenchymal transition and altered immunity and inflammatory responses in genetically susceptible women. Current treatments are dictated by the primary indication (infertility or pelvic pain) and are limited to surgery and hormonal treatments and analgesics with many adverse effects that rarely provide long-term relief. Endometriosis substantially affects the quality of life of women and their families and imposes costs on society similar to those of other chronic conditions such as type 2 diabetes mellitus, Crohn’s disease and rheumatoid arthritis. Future research must focus on understanding the pathogenesis, identifying disease subtypes, developing non-invasive diagnostic methods and targeting non-hormonal treatments that are acceptable to women who wish to conceive.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
92,52 € per year
only 92,52 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Rethinking mechanisms, diagnosis and management of endometriosis
Real world data on symptomology and diagnostic approaches of 27,840 women living with endometriosis

Endometriosis and chronic pelvic pain have similar impact on women, but time to diagnosis is decreasing: an Australian survey
Marsh, E. E. & Laufer, M. R. Endometriosis in premenarcheal girls who do not have an associated obstructive anomaly. Fertil. Steril. 83 , 758–760 (2005).
PubMed Google Scholar
Halme, J., Hammond, M. G., Hulka, J. F., Raj, S. G. & Talbert, L. M. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet. Gynecol. 64 , 151–154 (1984).
CAS PubMed Google Scholar
Horton, J. D., Dezee, K. J., Ahnfeldt, E. P. & Wagner, M. Abdominal wall endometriosis: a surgeon’s perspective and review of 445 cases. Am. J. Surg. 196 , 207–212 (2008).
Goldberg, J. & Davis, A. Extrapelvic endometriosis. Semin. Reprod. Med. 35 , 98–101 (2016).
Hirsch, M. et al. Diagnosis and management of endometriosis: a systematic review of international and national guidelines. BJOG 125 , 556–564 (2018).
American Society for Reproductive Medicine. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil. Steril. 67 , 817–821 (1997).
Google Scholar
Vercellini, P. et al. Association between endometriosis stage, lesion type, patient characteristics and severity of pelvic pain symptoms: a multivariate analysis of over 1000 patients. Hum. Reprod. 22 , 266–271 (2006).
Nnoaham, K. E. et al. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil. Steril. 96 , 366–373.e8 (2011). This paper presents the largest multicentre prospective study to date describing the effects of endometriosis; in women undergoing their first laparoscopy, a significantly reduced physical (but not mental) HRQOL, which was associated with delay in diagnosis, was reported in those with endometriosis compared with endometriosis-free symptomatic and asymptomatic women .
PubMed PubMed Central Google Scholar
Peres, L. C. et al. Racial/ethnic differences in the epidemiology of ovarian cancer: a pooled analysis of 12 case-control studies. Int. J. Epidemiol. 47 , 460–472 (2018).
Eskenazi, B. & Warner, M. L. Epidemiology of endometriosis. Obstet. Gynecol. Clin. North Am. 24 , 235–258 (1997).
Buck Louis, G. M. et al. Incidence of endometriosis by study population and diagnostic method: the ENDO study. Fertil. Steril. 96 , 360–365 (2011).
Janssen, E. B., Rijkers, A. C. M., Hoppenbrouwers, K., Meuleman, C. & D’Hooghe, T. M. Prevalence of endometriosis diagnosed by laparoscopy in adolescents with dysmenorrhea or chronic pelvic pain: a systematic review. Hum. Reprod. Update 19 , 570–582 (2013).
Zondervan, K. T., Cardon, L. R. & Kennedy, S. H. What makes a good case–control study? Hum. Reprod. 17 , 1415–1423 (2002).
Adamson, G. D., Kennedy, S. & Hummelshoj, L. Creating solutions in endometriosis: global collaboration through the World Endometriosis Research Foundation. J. Endometr. Pelvic Pain Disord. 2 , 3–6 (2010).
Gemmell, L. C. et al. The management of menopause in women with a history of endometriosis: a systematic review. Hum. Reprod. Update 23 , 481–500 (2017).
CAS PubMed PubMed Central Google Scholar
Leibson, C. L. et al. Incidence and characterization of diagnosed endometriosis in a geographically defined population. Fertil. Steril. 82 , 314–321 (2004).
Missmer, S. A. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. Am. J. Epidemiol. 160 , 784–796 (2004).
Nnoaham, K. E., Webster, P., Kumbang, J., Kennedy, S. H. & Zondervan, K. T. Is early age at menarche a risk factor for endometriosis? A systematic review and meta-analysis of case-control studies. Fertil. Steril. 98 , 702–712.e6 (2012).
Missmer, S. A. et al. Reproductive history and endometriosis among premenopausal women. Obstet. Gynecol. 104 , 965–974 (2004).
Peterson, C. M. et al. Risk factors associated with endometriosis: importance of study population for characterizing disease in the ENDO Study. Am. J. Obstet. Gynecol. 208 , 451.e1–451.e11 (2013).
Prescott, J. et al. A prospective cohort study of endometriosis and subsequent risk of infertility. Hum. Reprod. 31 , 1475–1482 (2016).
Buck Louis, G. M. et al. Women’s reproductive history before the diagnosis of incident endometriosis. J. Womens Health 25 , 1021–1029 (2016).
Farland, L. V. et al. History of breast feeding and risk of incident endometriosis: prospective cohort study. BMJ 358 , j3778 (2017).
Leeners, B., Damaso, F., Ochsenbein-Kölble, N. & Farquhar, C. The effect of pregnancy on endometriosis — facts or fiction? Hum. Reprod. Update 24 , 290–299 (2018).
Shah, D. K., Correia, K. F., Vitonis, A. F. & Missmer, S. A. Body size and endometriosis: results from 20 years of follow-up within the Nurses’ Health Study II prospective cohort. Hum. Reprod. 28 , 1783–1792 (2013).
Farland, L. V. et al. Associations among body size across the life course, adult height and endometriosis. Hum. Reprod. 32 , 1732–1742 (2017).
McCann, S. E. et al. Endometriosis and body fat distribution. Obstet. Gynecol. 82 , 545–549 (1993).
Rahmioglu, N. et al. Genome-wide enrichment analysis between endometriosis and obesity-related traits reveals novel susceptibility loci. Hum. Mol. Genet. 24 , 1185–1199 (2015).
de Ridder, C. M. et al. Body fat mass, body fat distribution, and plasma hormones in early puberty in females. J. Clin. Endocrinol. Metab. 70 , 888–893 (1990).
Cramer, D. W. The relation of endometriosis to menstrual characteristics, smoking, and exercise. JAMA 255 , 1904–1908 (1986).
Baron, J. A., La Vecchia, C. & Levi, F. The antiestrogenic effect of cigarette smoking in women. Am. J. Obstet. Gynecol. 162 , 502–514 (1990).
Ohtake, F., Fujii-Kuriyama, Y., Kawajiri, K. & Kato, S. Cross-talk of dioxin and estrogen receptor signals through the ubiquitin system. J. Steroid Biochem. Mol. Biol. 127 , 102–107 (2011).
Parazzini, F. Selected food intake and risk of endometriosis. Hum. Reprod. 19 , 1755–1759 (2004).
Trabert, B., Peters, U., De Roos, A. J., Scholes, D. & Holt, V. L. Diet and risk of endometriosis in a population-based case–control study. Br. J. Nutr. 105 , 459–467 (2010).
Missmer, S. A. et al. A prospective study of dietary fat consumption and endometriosis risk. Hum. Reprod. 25 , 1528–1535 (2010).
Savaris, A. L. & do Amaral, V. F. Nutrient intake, anthropometric data and correlations with the systemic antioxidant capacity of women with pelvic endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 158 , 314–318 (2011).
Mozaffarian, D. et al. Dietary intake of trans fatty acids and systemic inflammation in women. Am. J. Clin. Nutr. 79 , 606–612 (2004).
Lebovic, D. I., Mueller, M. D. & Taylor, R. N. Immunobiology of endometriosis. Fertil. Steril. 75 , 1–10 (2001).
Rier, S. E. et al. Endometriosis in rhesus monkeys (Macaca mulatta) following chronic exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Sci. 21 , 433–441 (1993).
CAS Google Scholar
Smarr, M. M., Kannan, K. & Buck Louis, G. M. Endocrine disrupting chemicals and endometriosis. Fertil. Steril. 106 , 959–966 (2016).
Kvaskoff, M. et al. Endometriosis: a high-risk population for major chronic diseases? Hum. Reprod. Update 21 , 500–516 (2015). This article presents a meta-analysis of all data and a critical methodologic review suggesting that patients with endometriosis are at higher risk of ovarian and breast cancers, cutaneous melanoma, asthma and some autoimmune, cardiovascular and atopic diseases and are at decreased risk of cervical cancer .
Benagiano, G., Brosens, I. & Habiba, M. Structural and molecular features of the endomyometrium in endometriosis and adenomyosis. Hum. Reprod. Update 20 , 386–402 (2014).
Kunz, G. et al. Adenomyosis in endometriosis — prevalence and impact on fertility. Evidence from magnetic resonance imaging. Hum. Reprod. 20 , 2309–2316 (2005).
Pearce, C. L. et al. Association between endometriosis and risk of histological subtypes of ovarian cancer: a pooled analysis of case–control studies. Lancet Oncol. 13 , 385–394 (2012).
Kim, H. S., Kim, T. H., Chung, H. H. & Song, Y. S. Risk and prognosis of ovarian cancer in women with endometriosis: a meta-analysis. Br. J. Cancer 110 , 1878–1890 (2014).
Farland, L. V. et al. Endometriosis and the risk of skin cancer: a prospective cohort study. Cancer Causes Control 28 , 1011–1019 (2017).
Sinaii, N. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum. Reprod. 17 , 2715–2724 (2002).
Nielsen, N. M., Jorgensen, K. T., Pedersen, B. V., Rostgaard, K. & Frisch, M. The co-occurrence of endometriosis with multiple sclerosis, systemic lupus erythematosus and Sjogren syndrome. Hum. Reprod. 26 , 1555–1559 (2011).
Harris, H. R. et al. Endometriosis and the risks of systemic lupus erythematosus and rheumatoid arthritis in the Nurses’ Health Study II. Ann. Rheum. Dis. 75 , 1279–1284 (2015).
Mu, F. et al. Association between endometriosis and hypercholesterolemia or hypertensionnovelty and significance. Hypertension 70 , 59–65 (2017).
Nyholt, D. R. et al. Genome-wide association meta-analysis identifies new endometriosis risk loci. Nat. Genet. 44 , 1355–1359 (2012).
Borghese, B., Zondervan, K. T., Abrao, M. S., Chapron, C. & Vaiman, D. Recent insights on the genetics and epigenetics of endometriosis. Clin. Genet. 91 , 254–264 (2017).
Treloar, S. A. et al. Genomewide linkage study in 1,176 affected sister pair families identifies a significant susceptibility locus for endometriosis on chromosome 10q26. Am. J. Hum. Genet. 77 , 365–376 (2005).
Zondervan, K. T. et al. Significant evidence of one or more susceptibility loci for endometriosis with near-Mendelian inheritance on chromosome 7p13–15. Hum. Reprod. 22 , 717–728 (2006).
Rahmioglu, N., Montgomery, G. W. & Zondervan, K. T. Genetics of endometriosis. Womens Health 11 , 577–586 (2015).
Sapkota, Y. et al. Meta-analysis identifies five novel loci associated with endometriosis highlighting key genes involved in hormone metabolism. Nat. Commun. 8 , 15539 (2017).
Lee, S. H. et al. Estimation and partitioning of polygenic variation captured by common SNPs for Alzheimer’s disease, multiple sclerosis and endometriosis. Hum. Mol. Genet. 22 , 832–841 (2012).
Uimari, O. et al. Genome-wide genetic analyses highlight mitogen-activated protein kinase (MAPK) signaling in the pathogenesis of endometriosis. Hum. Reprod. 32 , 780–793 (2017).
Lu, Y. et al. Shared genetics underlying epidemiological association between endometriosis and ovarian cancer. Hum. Mol. Genet. 24 , 5955–5964 (2015).
Painter, J. N. et al. Genetic overlap between endometriosis and endometrial cancer: evidence from cross-disease genetic correlation and GWAS meta-analyses. Cancer Med. 7 , 1978–1987 (2018).
Sapkota, Y. et al. Analysis of potential protein-modifying variants in 9000 endometriosis patients and 150000 controls of European ancestry. Sci. Rep. 7 , 11380 (2017).
Visscher, P. M., Brown, M. A., McCarthy, M. I. & Yang, J. Five years of GWAS discovery. Am. J. Hum. Genet. 90 , 7–24 (2012).
Fung, J. N. et al. The genetic regulation of transcription in human endometrial tissue. Hum. Reprod. 32 , 1–12 (2017).
Becker, C. M. et al. World Endometriosis Research Foundation Endometriosis Phenome and Biobanking Harmonisation Project: I. Surgical phenotype data collection in endometriosis research. Fertil. Steril. 102 , 1213–1222 (2014).
Vitonis, A. F. et al. World Endometriosis Research Foundation Endometriosis Phenome and Biobanking Harmonization Project: II. Clinical and covariate phenotype data collection in endometriosis research. Fertil. Steril. 102 , 1223–1232 (2014).
Rahmioglu, N. et al. World Endometriosis Research Foundation Endometriosis Phenome and Biobanking Harmonization Project: III. Fluid biospecimen collection, processing, and storage in endometriosis research. Fertil. Steril. 102 , 1233–1243 (2014).
Fassbender, A. et al. World Endometriosis Research Foundation Endometriosis Phenome and Biobanking Harmonisation Project: IV. Tissue collection, processing, and storage in endometriosis research. Fertil. Steril. 102 , 1244–1253 (2014).
Wiegand, K. C. et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N. Engl. J. Med. 363 , 1532–1543 (2010).
Yamamoto, S., Tsuda, H., Takano, M., Tamai, S. & Matsubara, O. Loss of ARID1A protein expression occurs as an early event in ovarian clear-cell carcinoma development and frequently coexists with PIK3CA mutations. Mod. Pathol. 25 , 615–624 (2011).
Anglesio, M. S. et al. Cancer-associated mutations in endometriosis without cancer. N. Engl. J. Med. 376 , 1835–1848 (2017).
Kato, S., Lippman, S. M., Flaherty, K. T. & Kurzrock, R. The conundrum of genetic ‘drivers’ in benign conditions. J. Natl Cancer Inst. 108 , djw036 (2016).
Guo, S.-W. Epigenetics of endometriosis. Mol. Hum. Reprod. 15 , 587–607 (2009).
Wu, Y., Starzinski-Powitz, A. & Guo, S.-W. Trichostatin A, a histone deacetylase inhibitor, attenuates invasiveness and reactivates E-cadherin expression in immortalized endometriotic cells. Reprod. Sci. 14 , 374–382 (2007).
Dyson, M. T. et al. Genome-wide DNA methylation analysis predicts an epigenetic switch for GATA factor expression in endometriosis. PLoS Genet. 10 , e1004158 (2014).
Burney, R. O. et al. MicroRNA expression profiling of eutopic secretory endometrium in women with versus without endometriosis. Mol. Hum. Reprod. 15 , 625–631 (2009).
Saare, M. et al. Challenges in endometriosis miRNA studies — from tissue heterogeneity to disease specific miRNAs. Biochim. Biophys. Acta 1863 , 2282–2292 (2017).
Sampson, J. A. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am. J. Obstet. Gynecol. 14 , 422–469 (1927).
Vercellini, P. et al. Asymmetry in distribution of diaphragmatic endometriotic lesions: evidence in favour of the menstrual reflux theory. Hum. Reprod. 22 , 2359–2367 (2007).
D’Hooghe, T. M., Bambra, C. S., Raeymaekers, B. M. & Koninckx, P. R. Increased prevalence and recurrence of retrograde menstruation in baboons with spontaneous endometriosis. Hum. Reprod. 11 , 2022–2025 (1996).
Witz, C. A., Cho, S., Centonze, V. E., Montoya-Rodriguez, I. A. & Schenken, R. S. Time series analysis of transmesothelial invasion by endometrial stromal and epithelial cells using three-dimensional confocal microscopy. Fertil. Steril. 79 (Suppl. 1), 770–778 (2003).
Reis, F. M., Petraglia, F. & Taylor, R. N. Endometriosis: hormone regulation and clinical consequences of chemotaxis and apoptosis. Hum. Reprod. Update 19 , 406–418 (2013).
Sanchez, A. M. et al. The endometriotic tissue lining the internal surface of endometrioma: hormonal, genetic, epigenetic status, and gene expression profile. Reprod. Sci. 22 , 391–401 (2015).
Borghese, B., Zondervan, K. T., Abrao, M. S., Chapron, C. & Vaiman, D. Recent insights on the genetics and epigenetics of endometriosis. Clin. Genet. 91 , 254–264 (2016).
Meyer, R. Zur Frage der heterotopen Epithelwucherung, insbesondere des Peritonealepithels und in die Ovarien [German]. Virch Arch. Path. Anat. Phys. 250 , 595–610 (1924).
Ferguson, B. R., Bennington, J. L. & Haber, S. L. Histochemistry of mucosubstances and histology of mixed müllerian pelvic lymph node glandular inclusions. Evidence for histogenesis by müllerian metaplasia of coelomic epithelium. Obstet. Gynecol. 33 , 617–625 (1969).
Figueira, P. G. M., Abrão, M. S., Krikun, G. & Taylor, H. Stem cells in endometrium and their role in the pathogenesis of endometriosis. Ann. NY Acad. Sci. 1221 , 10–17 (2011).
Du, H. & Taylor, H. S. Contribution of bone marrow-derived stem cells to endometrium and endometriosis. Stem Cells 25 , 2082–2086 (2007).
Gargett, C. E. & Masuda, H. Adult stem cells in the endometrium. Mol. Hum. Reprod. 16 , 818–834 (2010).
Matsuzaki, S. & Darcha, C. Epithelial to mesenchymal transition-like and mesenchymal to epithelial transition-like processes might be involved in the pathogenesis of pelvic endometriosis. Hum. Reprod. 27 , 712–721 (2012).
Somigliana, E. et al. Association rate between deep peritoneal endometriosis and other forms of the disease: pathogenetic implications. Hum. Reprod. 19 , 168–171 (2004).
Zheng, W. et al. Initial endometriosis showing direct morphologic evidence of metaplasia in the pathogenesis of ovarian endometriosis. Int. J. Gynecol. Pathol. 24 , 164–172 (2005).
Troncon, J. K. et al. Endometriosis in a patient with mayer-rokitansky-küster-hauser syndrome. Case Rep. Obstet. Gynecol. 2014 , 376231 (2014).
Taguchi, S., Enomoto, Y. & Homma, Y. Bladder endometriosis developed after long-term estrogen therapy for prostate cancer. Int. J. Urol. 19 , 964–965 (2012).
Halban, J. Hysteroadenosis metastatica. Zentralbl. Gyndkoi 7 , 387–391 (1925).
Mechsner, S. et al. Estrogen and progestogen receptor positive endometriotic lesions and disseminated cells in pelvic sentinel lymph nodes of patients with deep infiltrating rectovaginal endometriosis: a pilot study. Hum. Reprod. 23 , 2202–2209 (2008).
Gargett, C. E. et al. Potential role of endometrial stem/progenitor cells in the pathogenesis of early-onset endometriosis. Mol. Hum. Reprod. 20 , 591–598 (2014).
Witz, C. A. et al. Short-term culture of peritoneum explants confirms attachment of endometrium to intact peritoneal mesothelium. Fertil. Steril. 75 , 385–390 (2001).
Zeitoun, K. M. & Bulun, S. E. Aromatase: a key molecule in the pathophysiology of endometriosis and a therapeutic target. Fertil. Steril. 72 , 961–969 (1999).
Pellegrini, C. et al. The expression of estrogen receptors as well as GREB1, c-MYC, and cyclin D1, estrogen-regulated genes implicated in proliferation, is increased in peritoneal endometriosis. Fertil. Steril. 98 , 1200–1208 (2012).
Plante, B. J. et al. G protein-coupled estrogen receptor (GPER) expression in normal and abnormal endometrium. Reprod. Sci. 19 , 684–693 (2012).
Han, S. J. et al. Estrogen receptor β modulates apoptosis complexes and the inflammasome to drive the pathogenesis of endometriosis. Cell 163 , 960–974 (2015).
Vercellini, P., Viganò, P., Somigliana, E. & Fedele, L. Endometriosis: pathogenesis and treatment. Nat. Rev. Endocrinol. 10 , 261–275 (2013). For this review, the best-quality evidence was selected to describe the performance of diagnostic tools and the effectiveness of approaches to address endometriosis-associated symptoms and infertility .
Patel, B. et al. Role of nuclear progesterone receptor isoforms in uterine pathophysiology. Hum. Reprod. Update 21 , 155–173 (2014).
Al-Sabbagh, M., Lam, E. W.-F. & Brosens, J. J. Mechanisms of endometrial progesterone resistance. Mol. Cell. Endocrinol. 358 , 208–215 (2012).
La Marca, A., Carducci Artenisio, A., Stabile, G., Rivasi, F. & Volpe, A. Evidence for cycle-dependent expression of follicle-stimulating hormone receptor in human endometrium. Gynecol. Endocrinol. 21 , 303–306 (2005).
Zondervan, K. et al. Beyond endometriosis genome-wide association study: from genomics to phenomics to the patient. Semin. Reprod. Med. 34 , 242–254 (2016).
Jiang, Y., Chen, L., Taylor, R. N., Li, C. & Zhou, X. Physiological and pathological implications of retinoid action in the endometrium. J. Endocrinol. 236 , R169–R188 (2018).
Orvis, G. D. & Behringer, R. R. Cellular mechanisms of Müllerian duct formation in the mouse. Dev. Biol. 306 , 493–504 (2007).
Vigano, P. et al. Time to redefine endometriosis including its pro-fibrotic nature. Hum. Reprod. 33 , 347–352 (2018).
Yu, J. et al. Endometrial stromal decidualization responds reversibly to hormone stimulation and withdrawal. Endocrinology 157 , 2432–2446 (2016).
Yin, X., Pavone, M. E., Lu, Z., Wei, J. & Kim, J. J. Increased activation of the PI3K/AKT pathway compromises decidualization of stromal cells from endometriosis. J. Clin. Endocrinol. Metab. 97 , E35–E43 (2012).
Du, Y., Liu, X. & Guo, S.-W. Platelets impair natural killer cell reactivity and function in endometriosis through multiple mechanisms. Hum. Reprod. 32 , 1–17 (2017).
Lessey, B., Lebovic, D. & Taylor, R. Eutopic endometrium in women with endometriosis: ground zero for the study of implantation defects. Semin. Reprod. Med. 31 , 109–124 (2013).
Cominelli, A. et al. Matrix metalloproteinase-27 is expressed in CD163+/CD206+ M2 macrophages in the cycling human endometrium and in superficial endometriotic lesions. Mol. Hum. Reprod. 20 , 767–775 (2014).
Takebayashi, A. et al. Subpopulations of macrophages within eutopic endometrium of endometriosis patients. Am. J. Reprod. Immunol. 73 , 221–231 (2015).
Nisenblat, V. et al. Blood biomarkers for the non-invasive diagnosis of endometriosis. Cochrane Database Syst. Rev. 5 , CD012179 (2016).
Wang, X.-Q. et al. The high level of RANTES in the ectopic milieu recruits macrophages and induces their tolerance in progression of endometriosis. J. Mol. Endocrinol. 45 , 291–299 (2010).
McKinnon, B. D., Kocbek, V., Nirgianakis, K., Bersinger, N. A. & Mueller, M. D. Kinase signalling pathways in endometriosis: potential targets for non-hormonal therapeutics. Hum. Reprod. Update 22 , 382–403 (2016).
Morotti, M., Vincent, K. & Becker, C. M. Mechanisms of pain in endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 209 , 8–13 (2017).
Berkley, K. J. The pains of endometriosis. Science 308 , 1587–1589 (2005).
Taylor, R. N. & Lebovic, D. I. in Yen and Jaffe’s Reproductive Endocrinology (eds Strauss, J. F. & Barbieri, R. L.) 565–585 (Saunders Elsevier, 2014). This book chapter discusses the diagnosis and management of endometriosis, with a particularly well-referenced review of theories of aetiology and pathogenesis
Gnecco, J. S. et al. Compartmentalized culture of perivascular stroma and endothelial cells in a microfluidic model of the human endometrium. Ann. Biomed. Eng. 45 , 1758–1769 (2017).
Sanchez, A. M. et al. The endometriotic tissue lining the internal surface of endometrioma. Reprod. Sci. 22 , 391–401 (2014).
Ryan, I. P., Schriock, E. D. & Taylor, R. N. Isolation, characterization, and comparison of human endometrial and endometriosis cells in vitro. J. Clin. Endocrinol. Metab. 78 , 642–649 (1994).
Greaves, E., Critchley, H. O. D., Horne, A. W. & Saunders, P. T. K. Relevant human tissue resources and laboratory models for use in endometriosis research. Acta Obstet. Gynecol. Scand. 96 , 644–658 (2017).
Korch, C. et al. DNA profiling analysis of endometrial and ovarian cell lines reveals misidentification, redundancy and contamination. Gynecol. Oncol. 127 , 241–248 (2012).
Grümmer, R. Translational animal models to study endometriosis-associated infertility. Semin. Reprod. Med. 31 , 125–132 (2013). Given the ethical limitations of clinical studies, this is a comprehensive review of the use of animal models to investigate factors contributing to the fertility-compromising effects of endometriosis .
Bruner-Tran, K. L., McConaha, M. E. & Osteen, K. G. in Endometriosis. Science and Practice (eds Giudice, L. C., Evers, J. L. H. & Healy, D. L.) 270–283 (Wiley-Blackwell, 2012).
Mariani, M. et al. The selective vitamin D receptor agonist, elocalcitol, reduces endometriosis development in a mouse model by inhibiting peritoneal inflammation. Hum. Reprod. 27 , 2010–2019 (2012).
Fazleabas, A. in Endometriosis: Science and Practice (eds Giudice, L. C., Evers, J. L. & Healy, D. L.) 285–291 (Wiley-Blackwell, 2012).
Ngô, C. et al. Antiproliferative effects of anastrozole, methotrexate, and 5-fluorouracil on endometriosis in vitro and in vivo. Fertil. Steril. 94 , 1632–1638.e1 (2010).
Bruner-Tran, K. L., Osteen, K. G. & Duleba, A. J. Simvastatin protects against the development of endometriosis in a nude mouse model. J. Clin. Endocrinol. Metab. 94 , 2489–2494 (2009).
Pullen, N. et al. The translational challenge in the development of new and effective therapies for endometriosis: a review of confidence from published preclinical efficacy studies. Hum. Reprod. Update 17 , 791–802 (2011).
Greaves, E. et al. A novel mouse model of endometriosis mimics human phenotype and reveals insights into the inflammatory contribution of shed endometrium. Am. J. Pathol. 184 , 1930–1939 (2014).
Stilley, J. A. W., Woods-Marshall, R., Sutovsky, M., Sutovsky, P. & Sharpe-Timms, K. L. Reduced fecundity in female rats with surgically induced endometriosis and in their daughters: a potential role for tissue inhibitors of metalloproteinase 11. Biol. Reprod. 80 , 649–656 (2009).
Zondervan, K. T. Familial aggregation of endometriosis in a large pedigree of rhesus macaques. Hum. Reprod. 19 , 448–455 (2004).
Lebovic, D. I. et al. Peroxisome proliferator-activated receptor-γ receptor ligand partially prevents the development of endometrial explants in baboons: a prospective, randomized, placebo-controlled study. Endocrinology 151 , 1846–1852 (2010).
D’Hooghe, T. M. Clinical relevance of the baboon as a model for the study of endometriosis. Fertil. Steril. 68 , 613–625 (1997).
Fazleabas, A. Progesterone resistance in a baboon model of endometriosis. Semin. Reprod. Med. 28 , 75–80 (2010).
D’Hooghe, T. M. et al. Nonhuman primate models for translational research in endometriosis. Reprod. Sci. 16 , 152–161 (2009).
Chapron, C. et al. Surgical complications of diagnostic and operative gynaecological laparoscopy: a series of 29,966 cases. Hum. Reprod. 13 , 867–872 (1998).
Chung, M. K., Chung, R. R., Gordon, D. & Jennings, C. The evil twins of chronic pelvic pain syndrome: endometriosis and interstitial cystitis. JSLS 6 , 311–314 (2002).
Redwine, D. B. Diaphragmatic endometriosis: diagnosis, surgical management, and long-term results of treatment. Fertil. Steril. 77 , 288–296 (2002).
Fedele, L. et al. Ileocecal endometriosis: clinical and pathogenetic implications of an underdiagnosed condition. Fertil. Steril. 101 , 750–753 (2014).
DiVasta, A. D., Vitonis, A. F., Laufer, M. R. & Missmer, S. A. Spectrum of symptoms in women diagnosed with endometriosis during adolescence versus adulthood. Am. J. Obstet. Gynecol. 218 , 324.e1–324.e11 (2018).
Ballard, K., Lane, H., Hudelist, G., Banerjee, S. & Wright, J. Can specific pain symptoms help in the diagnosis of endometriosis? A cohort study of women with chronic pelvic pain. Fertil. Steril. 94 , 20–27 (2010).
Becker, C. M., Gattrell, W. T., Gude, K. & Singh, S. S. Reevaluating response and failure of medical treatment of endometriosis: a systematic review. Fertil. Steril. 108 , 125–136 (2017).
Brawn, J., Morotti, M., Zondervan, K. T., Becker, C. M. & Vincent, K. Central changes associated with chronic pelvic pain and endometriosis. Hum. Reprod. Update 20 , 737–747 (2014).
Morotti, M., Vincent, K., Brawn, J., Zondervan, K. T. & Becker, C. M. Peripheral changes in endometriosis-associated pain. Hum. Reprod. Update 20 , 717–736 (2014).
Brosens, I., Gordts, S. & Benagiano, G. Endometriosis in adolescents is a hidden, progressive and severe disease that deserves attention, not just compassion. Hum. Reprod. 28 , 2026–2031 (2013).
Laufer, M. R., Sanfilippo, J. & Rose, G. Adolescent endometriosis: diagnosis and treatment approaches. J. Pediatr. Adolesc. Gynecol. 16 , S3–S11 (2003).
Davis, G. D., Thillet, E. & Lindemann, J. Clinical characteristics of adolescent endometriosis. J. Adolesc. Health 14 , 362–368 (1993).
Saraswat, L. et al. Pregnancy outcomes in women with endometriosis: a national record linkage study. BJOG 124 , 444–452 (2017).
Sanchez, A. M. et al. Is the oocyte quality affected by endometriosis? A review of the literature. J. Ovarian Res. 10 , 43 (2017).
Simón, C. et al. Outcome of patients with endometriosis in assisted reproduction: results from in-vitro fertilization and oocyte donation. Hum. Reprod. 9 , 725–729 (1994).
Senapati, S., Sammel, M. D., Morse, C. & Barnhart, K. T. Impact of endometriosis on in vitro fertilization outcomes: an evaluation of the Society for Assisted Reproductive Technologies Database. Fertil. Steril. 106 , 164–171.e1 (2016).
Taniguchi, F. et al. Analysis of pregnancy outcome and decline of anti-Müllerian hormone after laparoscopic cystectomy for ovarian endometriomas. J. Obstet. Gynaecol. Res. 42 , 1534–1540 (2016).
Lessey, B. A. & Kim, J. J. Endometrial receptivity in the eutopic endometrium of women with endometriosis: it is affected, and let me show you why. Fertil. Steril. 108 , 19–27 (2017).
Miravet-Valenciano, J., Ruiz-Alonso, M., Gómez, E. & Garcia-Velasco, J. A. Endometrial receptivity in eutopic endometrium in patients with endometriosis: it is not affected, and let me show you why. Fertil. Steril. 108 , 28–31 (2017).
Díaz, I. et al. Impact of stage iii–iv endometriosis on recipients of sibling oocytes: matched case-control study. Fertil. Steril. 74 , 31–34 (2000).
Prapas, Y. et al. History of endometriosis may adversely affect the outcome in menopausal recipients of sibling oocytes. Reprod. Biomed. Online 25 , 543–548 (2012).
Burney, R. O. et al. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology 148 , 3814–3826 (2007).
Dunselman, G. A. J. et al. ESHRE guideline: management of women with endometriosis. Hum. Reprod. 29 , 400–412 (2014). This guideline discusses the management of general endometriosis .
Weijenborg, P. T. M., ter Kuile, M. M. & Jansen, F. W. Intraobserver and interobserver reliability of videotaped laparoscopy evaluations for endometriosis and adhesions. Fertil. Steril. 87 , 373–380 (2007).
Tuttlies, F. et al. ENZIAN-score, a classification of deep infiltrating endometriosis [German]. Zentralbl. Gynakol. 127 , 275–281 (2005).
Johnson, N. P. et al. World Endometriosis Society consensus on the classification of endometriosis. Hum. Reprod. 32 , 315–324 (2017).
Nisolle, M. & Donnez, J. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil. Steril. 68 , 585–596 (1997).
Fukuda, S. et al. Thoracic endometriosis syndrome: comparison between catamenial pneumothorax or endometriosis-related pneumothorax and catamenial hemoptysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 225 , 118–123 (2018).
Rousset-Jablonski, C. et al. Catamenial pneumothorax and endometriosis-related pneumothorax: clinical features and risk factors. Hum. Reprod. 26 , 2322–2329 (2011).
Redwine, D. B. Ovarian endometriosis: a marker for more extensive pelvic and intestinal disease. Fertil. Steril. 72 , 310–315 (1999).
Hudelist, G. et al. Diagnostic accuracy of transvaginal ultrasound for non-invasive diagnosis of bowel endometriosis: systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 37 , 257–263 (2011).
Holland, T. K. et al. Ultrasound mapping of pelvic endometriosis: does the location and number of lesions affect the diagnostic accuracy? A multicentre diagnostic accuracy study. BMC Womens Health 13 , 43 (2013).
Noventa, M. et al. Ultrasound techniques in the diagnosis of deep pelvic endometriosis: algorithm based on a systematic review and meta-analysis. Fertil. Steril. 104 , 366–383.e2 (2015).
Bazot, M. et al. European Society of Urogenital Radiology (ESUR) guidelines: MR imaging of pelvic endometriosis. Eur. Radiol. 27 , 2765–2775 (2016).
Exacoustos, C., Manganaro, L. & Zupi, E. Imaging for the evaluation of endometriosis and adenomyosis. Best Pract. Res. Clin. Obstet. Gynaecol. 28 , 655–681 (2014).
Stratton, P. Diagnostic accuracy of laparoscopy, magnetic resonance imaging, and histopathologic examination for the detection of endometriosis. Fertil. Steril. 79 , 1078–1085 (2003).
Kennedy, S. et al. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum. Reprod. 20 , 2698–2704 (2005).
Wykes, C. B., Clark, T. J. & Khan, K. S. REVIEW: Accuracy of laparoscopy in the diagnosis of endometriosis: a systematic quantitative review. BJOG 111 , 1204–1212 (2004).
Balasch, J. et al. Visible and non-visible endometriosis at laparoscopy in fertile and infertile women and in patients with chronic pelvic pain: a prospective study. Hum. Reprod. 11 , 387–391 (1996).
Lessey, B. A., Higdon, H. L., Miller, S. E. & Price, T. A. Intraoperative detection of subtle endometriosis: a novel paradigm for detection and treatment of pelvic pain associated with the loss of peritoneal integrity. J. Vis. Exp. https://doi.org/10.3791/4313 (2012).
Lue, J. R., Pyrzak, A. & Allen, J. Improving accuracy of intraoperative diagnosis of endometriosis: role of firefly in minimal access robotic surgery. J. Minim. Access Surg. 12 , 186–189 (2016).
Practice Committee of American Society for Reproductive Medicine. Treatment of pelvic pain associated with endometriosis. Fertil. Steril. 90 , S260–S269 (2008). This article is a guideline for the management of pelvic pain associated with endometriosis .
Duffy, J. M. N. et al. Laparoscopic surgery for endometriosis. Cochrane Database Syst. Rev. 4 , CD011031 (2014).
Practice Committee of the American Society for Reproductive Medicine. Endometriosis and infertility: a committee opinion. Fertil. Steril. 98 , 591–598 (2012). This paper is a guideline for the management of infertility associated with endometriosis .
Tanbo, T. & Fedorcsak, P. Endometriosis-associated infertility: aspects of pathophysiological mechanisms and treatment options. Acta Obstet. Gynecol. Scand. 96 , 659–667 (2017).
Osuga, Y. et al. Role of laparoscopy in the treatment of endometriosis-associated infertility. Gynecol. Obstet. Invest. 53 , 33–39 (2002).
Hart, R. J., Hickey, M., Maouris, P., Buckett, W. & Garry, R. Excisional surgery versus ablative surgery for ovarian endometriomata. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD004992.pub2 (2005).
Benaglia, L. et al. Rate of severe ovarian damage following surgery for endometriomas. Hum. Reprod. 25 , 678–682 (2010).
Vercellini, P. et al. Effect of patient selection on estimate of reproductive success after surgery for rectovaginal endometriosis: literature review. Reprod. Biomed. Online 24 , 389–395 (2012).
Nyangoh Timoh, K., Ballester, M., Bendifallah, S., Fauconnier, A. & Darai, E. Fertility outcomes after laparoscopic partial bladder resection for deep endometriosis: retrospective analysis from two expert centres and review of the literature. Eur. J. Obstet. Gynecol. Reprod. Biol. 220 , 12–17 (2018).
Adamson, G. D. & Pasta, D. J. Endometriosis fertility index: the new, validated endometriosis staging system. Fertil. Steril. 94 , 1609–1615 (2010).
Werbrouck, E., Spiessens, C., Meuleman, C. & D’Hooghe, T. No difference in cycle pregnancy rate and in cumulative live-birth rate between women with surgically treated minimal to mild endometriosis and women with unexplained infertility after controlled ovarian hyperstimulation and intrauterine insemination. Fertil. Steril. 86 , 566–571 (2006).
National Institute for Health and Care Excellence. Fertility problems: assessment and treatment. NICE https://www.nice.org.uk/guidance/cg156/chapter/Recommendations#intrauterine-insemination (2013).
de Ziegler, D., Borghese, B. & Chapron, C. Endometriosis and infertility: pathophysiology and management. Lancet 376 , 730–738 (2010).
Steures, P. et al. Prediction of an ongoing pregnancy after intrauterine insemination. Fertil. Steril. 82 , 45–51 (2004).
Reindollar, R. H. et al. A randomized clinical trial to evaluate optimal treatment for unexplained infertility: the fast track and standard treatment (FASTT) trial. Fertil. Steril. 94 , 888–899 (2010).
Eijkemans, M. J. C. et al. Cost-effectiveness of ‘immediate IVF’ versus ‘delayed IVF’: a prospective study. Hum. Reprod. 32 , 999–1008 (2017).
Hamdan, M., Omar, S. Z., Dunselman, G. & Cheong, Y. Influence of endometriosis on assisted reproductive technology outcomes. Obstet. Gynecol. 125 , 79–88 (2015).
Benschop, L., Farquhar, C., van der Poel, N. & Heineman, M. J. Interventions for women with endometrioma prior to assisted reproductive technology. Cochrane Database Syst. Rev. 11 , CD008571 (2010).
de Ziegler, D. et al. Use of oral contraceptives in women with endometriosis before assisted reproduction treatment improves outcomes. Fertil. Steril. 94 , 2796–2799 (2010).
Donnez, J., García-Solares, J. & Dolmans, M.-M. Fertility preservation in women with ovarian endometriosis. Minerva Ginecol. https://doi.org/10.23736/S0026-4784.18.04229-6 (2018).
Somigliana, E. et al. Surgical excision of endometriomas and ovarian reserve: a systematic review on serum antimüllerian hormone level modifications. Fertil. Steril. 98 , 1531–1538 (2012).
Bianchi, P. H. M. et al. Extensive excision of deep infiltrative endometriosis before in vitro fertilization significantly improves pregnancy rates. J. Minim. Invasive Gynecol. 16 , 174–180 (2009).
Zullo, F. et al. Endometriosis and obstetrics complications: a systematic review and meta-analysis. Fertil. Steril. 108 , 667–672.e5 (2017).
Vigano, P., Corti, L. & Berlanda, N. Beyond infertility: obstetrical and postpartum complications associated with endometriosis and adenomyosis. Fertil. Steril. 104 , 802–812 (2015).
Donnez, J. & Squifflet, J. Complications, pregnancy and recurrence in a prospective series of 500 patients operated on by the shaving technique for deep rectovaginal endometriotic nodules. Hum. Reprod. 25 , 1949–1958 (2010).
Zanelotti, A. & Decherney, A. H. Surgery and endometriosis. Clin. Obstet. Gynecol. 60 , 477–484 (2017).
Olive, D. L. Medical therapy of endometriosis. Semin. Reprod. Med. 21 , 209–222 (2003).
Harada, T., Momoeda, M., Taketani, Y., Hoshiai, H. & Terakawa, N. Low-dose oral contraceptive pill for dysmenorrhea associated with endometriosis: a placebo-controlled, double-blind, randomized trial. Fertil. Steril. 90 , 1583–1588 (2008).
Vercellini, P. et al. Treatment of symptomatic rectovaginal endometriosis with an estrogen–progestogen combination versus low-dose norethindrone acetate. Fertil. Steril. 84 , 1375–1387 (2005).
Harada, T. et al. Dienogest is as effective as intranasal buserelin acetate for the relief of pain symptoms associated with endometriosis — a randomized, double-blind, multicenter, controlled trial. Fertil. Steril. 91 , 675–681 (2009).
Casper, R. F. Progestin-only pills may be a better first-line treatment for endometriosis than combined estrogen-progestin contraceptive pills. Fertil. Steril. 107 , 533–536 (2017).
Abou-Setta, A. M., Houston, B., Al-Inany, H. G. & Farquhar, C. Levonorgestrel-releasing intrauterine device (LNG-IUD) for symptomatic endometriosis following surgery. Cochrane Database Syst. Rev. 1 , CD005072 (2013).
Brown, J., Pan, A. & Hart, R. J. Gonadotrophin-releasing hormone analogues for pain associated with endometriosis. Cochrane Database Syst. Rev. 12 , CD008475 (2010).
Sagsveen, M. et al. Gonadotrophin-releasing hormone analogues for endometriosis: bone mineral density. Cochrane Database Syst. Rev. 4 , CD001297 (2003).
Bedaiwy, M. A., Allaire, C. & Alfaraj, S. Long-term medical management of endometriosis with dienogest and with a gonadotropin-releasing hormone agonist and add-back hormone therapy. Fertil. Steril. 107 , 537–548 (2017).
Taylor, H. S. et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N. Engl. J. Med. 377 , 28–40 (2017).
Hornstein, M. D. An oral GnRH antagonist for endometriosis — a new drug for an old disease. N. Engl. J. Med. 377 , 81–83 (2017).
Bedaiwy, M. A., Alfaraj, S., Yong, P. & Casper, R. New developments in the medical treatment of endometriosis. Fertil. Steril. 107 , 555–565 (2017).
Shakiba, K., Bena, J. F., McGill, K. M., Minger, J. & Falcone, T. Surgical treatment of endometriosis. Obstet. Gynecol. 111 , 1285–1292 (2008).
Vercellini, P. Laparoscopic uterosacral ligament resection for dysmenorrhea associated with endometriosis: results of a randomized, controlled trial. Fertil. Steril. 68 , S3 (1997).
Meuleman, C. et al. Surgical treatment of deeply infiltrating endometriosis with colorectal involvement. Hum. Reprod. Update 17 , 311–326 (2011).
Koga, K., Takamura, M., Fujii, T. & Osuga, Y. Prevention of the recurrence of symptom and lesions after conservative surgery for endometriosis. Fertil. Steril. 104 , 793–801 (2015).
Gao, X. et al. Health-related quality of life burden of women with endometriosis: a literature review. Curr. Med. Res. Opin. 22 , 1787–1797 (2006).
Simoens, S. et al. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum. Reprod. 27 , 1292–1299 (2012). This prospective study calculates the average annual costs and HRQOL per woman with endometriosis-associated symptoms, showing it to be similar to other chronic conditions .
Jones, G., Kennedy, S., Barnard, A., Wong, J. & Jenkinson, C. Development of an endometriosis quality-of-life instrument. Obstet. Gynecol. 98 , 258–264 (2001). This study describes the only validated endometriosis-specific quality-of-life outcome tool developed, measuring endometriosis-related health status on five scales. The tool has since been shown to be sensitive to changes in symptoms, making it a useful tool in endometriosis-specific clinical trials .
Jones, G., Jenkinson, C. & Kennedy, S. Development of the short form endometriosis health profile questionnaire: the EHP-5. Qual. Life Res. 13 , 695–704 (2004).
Jones, G., Jenkinson, C. & Kennedy, S. Evaluating the responsiveness of the endometriosis health profile questionnaire: the EHP-30. Qual. Life Res. 13 , 705–713 (2004).
Hirsch, M. et al. Variation in outcome reporting in endometriosis trials: a systematic review. Am. J. Obstet. Gynecol. 214 , 452–464 (2016).
Khan, K. The CROWN Initiative: journal editors invite researchers to develop core outcomes in women’s health. BJOG 123 , 103–104 (2016).
van Nooten, F. E., Cline, J., Elash, C. A., Paty, J. & Reaney, M. Development and content validation of a patient-reported endometriosis pain daily diary. Health Qual. Life Outcomes 16 , 3 (2018).
Rogers, P. A. W. et al. Research priorities for endometriosis. Reprod. Sci. 24 , 202–226 (2017).
Butrick, C. W. Patients with chronic pelvic pain: endometriosis or interstitial cystitis/painful bladder syndrome? JSLS 11 , 182–189 (2007).
Sørlie, T. et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc. Natl Acad. Sci. USA 100 , 8418–8423 (2003).
Cancer Genome Atlas Network. et al. Comprehensive molecular portraits of human breast tumours. Nature 490 , 61–70 (2012).
Gupta, D. et al. Endometrial biomarkers for the non-invasive diagnosis of endometriosis. Cochrane Database Syst. Rev. 4 , CD012165 (2016).
Nisenblat, V. et al. Combination of the non-invasive tests for the diagnosis of endometriosis. Cochrane Database Syst. Rev. 7 , CD012281 (2016).
Nisenblat, V., Bossuyt, P. M. M., Farquhar, C., Johnson, N. & Hull, M. L. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst. Rev. 2 , CD009591 (2016).
May, K. E. et al. Peripheral biomarkers of endometriosis: a systematic review. Hum. Reprod. Update 16 , 651–674 (2010).
May, K. E., Villar, J., Kirtley, S., Kennedy, S. H. & Becker, C. M. Endometrial alterations in endometriosis: a systematic review of putative biomarkers. Hum. Reprod. Update 17 , 637–653 (2011).
van der Zanden, M. & Nap, A. W. Knowledge of, and treatment strategies for, endometriosis among general practitioners. Reprod. Biomed. Online 32 , 527–531 (2016).
Seear, K. The etiquette of endometriosis: stigmatisation, menstrual concealment and the diagnostic delay. Soc. Sci. Med. 69 , 1220–1227 (2009).
Greene, R., Stratton, P., Cleary, S. D., Ballweg, M. L. & Sinaii, N. Diagnostic experience among 4,334 women reporting surgically diagnosed endometriosis. Fertil. Steril. 91 , 32–39 (2009).
Horne, A. W., Saunders, P. T. K., Abokhrais, I. M. & Hogg, L. Top ten endometriosis research priorities in the UK and Ireland. Lancet 389 , 2191–2192 (2017).
Hans Evers, J. L. H. Is adolescent endometriosis a progressive disease that needs to be diagnosed and treated? Hum. Reprod. 28 , 2023 (2013).
Neal, D. M. & McKenzie, P. J. Putting the pieces together: endometriosis blogs, cognitive authority, and collaborative information behavior. J. Med. Libr. Assoc. 99 , 127–134 (2011).
Uno, S. et al. A genome-wide association study identifies genetic variants in the CDKN2BAS locus associated with endometriosis in Japanese. Nat. Genet. 42 , 707–710 (2010).
Painter, J. N. et al. Genome-wide association study identifies a locus at 7p15.2 associated with endometriosis. Nat. Genet. 43 , 51–54 (2011).
Albertsen, H. M., Chettier, R., Farrington, P. & Ward, K. Genome-wide association study link novel loci to endometriosis. PLoS ONE 8 , e58257 (2013).
Steinthorsdottir, V. et al. Common variants upstream of KDR encoding VEGFR2 and in TTC39B associate with endometriosis. Nat. Commun. 7 , 12350 (2016).
Sobalska-Kwapis, M. et al. New variants near RHOJ and C2, HLA-DRA region and susceptibility to endometriosis in the Polish population — the genome-wide association study. Eur. J. Obstet. Gynecol. Reprod. Biol. 217 , 106–112 (2017).
Download references
Acknowledgements
R.N.T. acknowledges funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development through grants R01-HD33238, U54-HD37321, U54-HD55787, R01-HD55379, U01-HD66439 and R21-HD78818. S.A.M. is also affiliated with the Boston Center for Endometriosis, Boston Children’s Hospital and Brigham and Women’s Hospital and the Division of Adolescent and Young Adult Medicine, Department of Medicine, Boston Children’s Hospital and Harvard Medical School. The authors thank N. Moore (Oxford University Hospitals Foundation Trust, UK) for providing MRI images, D. Barber (Oxford Endometriosis CaRe Centre, UK) for providing ultrasonography pictures and J. Malzahn (Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford, UK) for providing the picture of the histology slide in Fig. 5.
Reviewer information
Nature Reviews Disease Primers thanks I. Brosens, M. J. Canis, S. Ferrero, C. Nezhat, V. Remorgida, M. Simões Abrão and other anonymous referee(s) for the peer review of this work.
Author information
Authors and affiliations.
Oxford Endometriosis CaRe Centre, Nuffield Department of Women’s & Reproductive Health, University of Oxford, Oxford, UK
Krina T. Zondervan & Christian M. Becker
Wellcome Centre for Human Genetics, University of Oxford, Oxford, UK
Krina T. Zondervan
Department of Obstetrics and Gynecology, The University of Tokyo, Tokyo, Japan
Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA
Stacey A. Missmer
Department of Obstetrics, Gynecology and Reproductive Biology, College of Human Medicine, Michigan State University, Grand Rapids, MI, USA
Department of Obstetrics and Gynecology, University of Utah School of Medicine, Salt Lake City, UT, USA
Robert N. Taylor
Division of Genetics and Cell Biology, San Raffaele Scientific Institute, Milano, Italy
Paola Viganò
You can also search for this author in PubMed Google Scholar
Contributions
Introduction (K.T.Z.); Epidemiology (S.A.M.); Mechanisms/pathophysiology (R.N.T. and P.V.); Diagnosis, screening and prevention (C.M.B.); Management (K.K.); Quality of life (K.T.Z.); Outlook (K.T.Z.); Overview of the Primer (K.T.Z.).
Corresponding author
Correspondence to Krina T. Zondervan .
Ethics declarations
Competing interests.
K.T.Z. has received grant funding from the Wellcome Trust, Medical Research Council UK, the US NIH, the European Union and the World Endometriosis Research Foundation (WERF). She also has scientific collaborations with, and has received grant funding from, Bayer AG, MDNA Life Sciences, Roche Diagnostics and Volition Rx and has served as a scientific consultant to AbbVie and Roche Diagnostics. She is Secretary of the World Endometriosis Society (WES), the European Society of Human Reproduction and Embryology (ESHRE) Special Interest Group in Endometriosis and Endometrial Disorders and Wellbeing of Women, and she is Chair of the WES Research Directions Working Group. C.M.B. is a member of the independent data monitoring group for a clinical endometriosis trial by ObsEva. He has received research grants from Bayer AG, MDNA Life Sciences, Volition Rx and Roche Diagnostics as well as from Wellbeing of Women, Medical Research Council UK, the NIH, the UK National Institute for Health Research and the European Union. He is the current Chair of the Endometriosis Guideline Development Group of the ESHRE and was a co-opted member of the Endometriosis Guideline Group by the UK National Institute for Health and Care Excellence (NICE). K.K. has received grant funding from the Ministry of Education, Culture, Sports Science and Technology Japan, the Ministry of Health, Labour and Welfare Japan, Takeda Research Support and MSD. She has also served as a scientific consultant to Bayer AG. She is an ambassador of the WES and a member of the Guideline Development Group of the Japan Society of Obstetrics and Gynecology. S.A.M. has received grant funding from the NIH and the Marriott family foundations and has served as an adviser to and has scientific collaborations with AbbVie, Celmatix and Oratel Diagnostics. She is a treasurer of the WES, Secretary of the WERF, Chair of the American Society of Reproductive Medicine Endometriosis Special Interest Group and a member of the NIH Reproductive Medicine Network Data Safety and Monitoring Board. R.N.T. has received grant funding from Bayer AG, Ferring Research Institute, the NIH and Pfizer and has served as a scientific consultant or adviser to AbbVie, Allergan, the NIH, ObsEva SA and the Population Council. He is the immediate past honorary secretary of the WES. P.V. has received grant funding from Bayer AG and Merck Serono and has served as a scientific consultant to Ferring Pharmaceuticals and Roche Diagnostics. She is a board member of the WES.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
WERF Endometriosis Phenome and Biobanking Harmonisation Project: https://endometriosisfoundation.org/ephect/
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Zondervan, K.T., Becker, C.M., Koga, K. et al. Endometriosis. Nat Rev Dis Primers 4 , 9 (2018). https://doi.org/10.1038/s41572-018-0008-5
Download citation
Published : 19 July 2018
DOI : https://doi.org/10.1038/s41572-018-0008-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Impact of oil-based contrast agents in hysterosalpingography on fertility outcomes in endometriosis: a retrospective cohort study.
- Yingqin Huang
Reproductive Biology and Endocrinology (2024)
Efficacy and safety of a novel pain management device, AT-04, for endometriosis-related pain: study protocol for a phase III randomized controlled trial
- Hiroshi Ishikawa
- Osamu Yoshino
Reproductive Health (2024)
Surge in endometriosis research after decades of underfunding could herald new era for women’s health
- Clare Watson
Nature Medicine (2024)
Mining phase separation-related diagnostic biomarkers for endometriosis through WGCNA and multiple machine learning techniques: a retrospective and nomogram study
- Qiuyi Liang
- Shengmei Yang
Journal of Assisted Reproduction and Genetics (2024)
The roles of chromatin regulatory factors in endometriosis
Quick links.
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- Pathophysiology,...
Pathophysiology, diagnosis, and management of endometriosis
- Related content
- Peer review
- Andrew W Horne , professor of gynaecology and reproductive sciences 1 ,
- Stacey A Missmer , professor of obstetrics, gynaecology, and reproductive biology , adjunct professor of epidemiology 2 3
- 1 EXPPECT Edinburgh and MRC Centre for Reproductive Health, University of Edinburgh, Edinburgh, UK
- 2 Michigan State University, Grand Rapids, MI, USA
- 3 Harvard T.H. Chan School of Public Health, Boston, MA, USA
- Correspondence to: A W Horne andrew.horne{at}ed.ac.uk
Endometriosis affects approximately 190 million women and people assigned female at birth worldwide. It is a chronic, inflammatory, gynecologic disease marked by the presence of endometrial-like tissue outside the uterus, which in many patients is associated with debilitating painful symptoms. Patients with endometriosis are also at greater risk of infertility, emergence of fatigue, multisite pain, and other comorbidities. Thus, endometriosis is best understood as a condition with variable presentation and effects at multiple life stages. A long diagnostic delay after symptom onset is common, and persistence and recurrence of symptoms despite treatment is common. This review discusses the potential genetic, hormonal, and immunologic factors that lead to endometriosis, with a focus on current diagnostic and management strategies for gynecologists, general practitioners, and clinicians specializing in conditions for which patients with endometriosis are at higher risk. It examines evidence supporting the different surgical, pharmacologic, and non-pharmacologic approaches to treating patients with endometriosis and presents an easy to adopt step-by-step management strategy. As endometriosis is a multisystem disease, patients with the condition should ideally be offered a personalized, multimodal, interdisciplinary treatment approach. A priority for future discovery is determining clinically informative sub-classifications of endometriosis that predict prognosis and enhance treatment prioritization.
Introduction
Endometriosis is a chronic, inflammatory, gynecologic disease marked by the presence of endometrial-like tissue outside the uterus, which affects approximately 10% of women during their reproductive years—190 million women worldwide. 1 In many patients, it is associated with chronic painful symptoms and other comorbidities, including infertility. 2 The health burden of endometriosis includes chronic pain and significant lifetime costs of $27 855 per year per patient, 3 accumulating to annual healthcare costs for endometriosis of approximately $22bn in the US alone and £12.5bn in the UK in treatment, work loss, and healthcare costs. 4 Although more than 50% of adults diagnosed as having endometriosis report onset of severe pelvic pain during adolescence, 5 most young women with endometriosis do not receive timely treatment. Almost 60% of women will see three or more clinicians before a diagnosis of endometriosis is made after an average of seven years with symptoms. 6 Women with endometriosis lose on average 11 hours of work per week, similar to other chronic conditions including type 2 diabetes, Crohn’s disease, and rheumatoid arthritis. 7 Adolescents are at risk of having inadequately remediated symptoms during prime years for social development and life planning, 8 and women must be resilient against inadequately remediated symptoms and emerging comorbidities. Women, healthcare providers, and scientists would benefit from conceptualizing endometriosis as a condition that can affect the whole woman. This includes a better understanding of the risk of subsequent development of autoimmune disease, cancer, and cardiovascular disease and a whole health approach to monitoring and wellbeing. 9
This review is aimed at general practitioners and pediatric specialists who are most likely to interact with patients as signs and symptoms of endometriosis first emerge and from whom early attention and empiric treatment may dramatically shorten the burden; gynecology specialists for whom myths must be dispelled and who must be aware of state of the art knowledge about patient centered treatments; endometriosis specialists who care for women’s endometriosis associated symptoms across the life course; and clinical researchers and scientists who must be inspired to bring their expertise and creativity to answer the fundamental enigmas of endometriosis etiology, informative sub-phenotyping, and novel patient centered treatment.
In this review, we use the terms “woman” and “women.” However, it is important to note that endometriosis can affect all people assigned female at birth.
Sources and selection criteria
We searched PubMed for studies using the term “endometriosis.” We considered all peer reviewed studies published in the English language between 1 January 2010 and 28 February 2022. We also identified references from international guidelines on endometriosis published during this time period. We selected relevant publications outside this timeline on the basis of review of the bibliography. We predefined the priority of study selection for this review according to the level of the evidence (meta-analyses, systematic or scoping reviews, randomized controlled trials (RCTs), prospective cohort studies, case-control studies, cross sectional studies; a priori exclusion of case series and case reports), by sample size (we prioritized studies with larger sample size as well as studies providing precision statistics), by population sampling (we prioritized studies with more diverse populations or with declared sub-population design over narrow population samples), and publication date (we prioritized more recent studies).
Overall quality of evidence
Much of the knowledge on endometriosis is based on concepts in early stages of evidence development or on sparse literature. Many studies include single hospital or clinic population samples with small total sample sizes and disproportionately representing patients presenting with infertility compared with endometriosis associated pain. 10
Beyond the limitations of the existing literature, fundamental problems with the diagnosis of endometriosis must be overcome before we can adequately define endometriosis, its prevalence, biologically and clinically informative sub-phenotypes, and its response to treatment and long term prognosis. 11 The lack of a non-invasive diagnostic modality creates insurmountable diagnostic biases driven by characteristics of those patients who can and those who cannot access a definitive surgical or imaging diagnosis and at what point in their endometriosis journey the condition is diagnosed.
Ovarian endometrioma or deep endometriosis can be diagnosed through imaging if the patient is geographically, economically, and socially able to achieve referral to and evaluation from an experienced imaging specialist. 12 13 For women with superficial peritoneal disease, definitive diagnosis by means of surgical evaluation is limited to those with symptoms deemed sufficiently severe and life affecting and resistant to empiric treatment to justify the inherent risks of surgery. Even among patients with symptoms deemed to have enough of an effect to warrant referral for a surgical evaluation, stigma, 14 disbelief and misperceptions of pain or fertility that can be driven by racism or elitism, 15 and geographic and economic barriers to accessing endometriosis focused surgeons remain.
Beyond access to an appropriate, skilled physician, the wide range of symptoms associated with endometriosis—many of which are stigmatized or normalized 14 16 —reduces the likelihood of referral and increases time to referral to appropriate specialists. 5 6 11 17 The bias in diagnosis itself may be influenced by variations in clinical symptoms among different populations not adequately captured or appreciated by standard clinical definitions or may represent implicit bias in healthcare, leading to an alternate interpretation of the same symptoms affecting the likelihood of diagnosis. This delay to diagnosis affects patients directly, but it also results in most scientific studies capturing patients’ characteristics, biologic samples, and biomarker measurements far into the natural pathophysiologic progression of the disease. Moreover, studies from African and Asian countries are considerably under-represented compared with European and North American countries. 10 High quality studies from these regions and development of a sensitive non-invasive diagnostic tool might alter existing global prevalence and incidence estimates and may reveal a more comprehensive view of what early milieu, signs and symptoms, and long term health outcomes are truly attributable to endometriosis.
Definition, symptoms, and classification
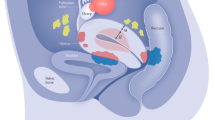
Among women with the condition, endometriosis has a highly heterogeneous presentation of visualized endometriotic lesions, multisystem symptom presentation, and comorbid conditions ( fig 1 ).

Highly varied presentation of endometriosis
- Download figure
- Open in new tab
- Download powerpoint
Surgically visualized macro sub-phenotypes of endometriosis
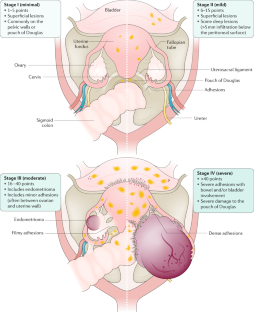
Endometriosis is defined by the presence of endometrium-like epithelium and/or stroma (lesions) outside the endometrium and myometrium, usually with an associated inflammatory process. 18 Most endometriosis is found within the abdominal cavity, and it exists as three subtypes: superficial peritoneal endometriosis (accounting for around 80% of endometriosis), ovarian endometriosis (cysts or “endometrioma”), and deep endometriosis 1 19 ( box 1 ; fig 2 ). All forms of endometriosis can be found together, not solely as separate entities. Although not a subtype, endometriosis situated inside the bowel wall is termed “bowel endometriosis.” It mostly affects the rectosigmoid area, but lesions can also be found in other parts of the gastrointestinal system, including the appendix. Endometriosis involving the detrusor muscle and/or the bladder epithelium is termed “bladder endometriosis.” Extra-abdominal (replacing the older term “extra-pelvic”) endometriosis is used to describe any endometriosis lesions found outside of the abdomen (for example, thoracic endometriosis). 20 Iatrogenic endometriosis describes endometriosis thought to be arising from direct or indirect dissemination of endometrium following surgery (for example, cesarean scar endometriosis).
Nomenclature 18
Superficial peritoneal endometriosis.
Endometrium-like tissue lesions involving the peritoneal surface with multiple appearances
Ovarian endometriosis
Endometrium-like tissue lesions in the form of ovarian cysts containing endometrium-like tissue and dark blood stained fluid (endometrioma or “chocolate cysts”)
Deep endometriosis
Endometrium-like tissue lesions extending on or infiltrating the peritoneal surface (usually nodular, invading into adjacent structures, and associated with fibrosis)
Extra-abdominal endometriosis
Endometrium-like tissue outside the abdominal cavity (for example, thoracic, umbilical, brain endometriosis)
Iatrogenic endometriosis
Direct or indirect dissemination of endometrium following surgery (for example, cesarean scar endometriosis)

Surgical images of endometriosis sub-phenotypes
Adenomyosis is not a sub-phenotype of endometriosis, 21 although it is characterized by endometrial tissue surrounded by smooth muscle cells within the myometrium. 22 Symptoms include dysmenorrhea and heavy menstrual and/or abnormal uterine bleeding, 23 and a heterogeneous adenomyosis presentation is visualized with radiologic imaging or at hysterectomy that lacks an agreed terminology or classification system. 24 25 Evidence is emerging of tissue injury and repair mechanisms mediated by estradiol and inflammation. 25 26
Endometriosis associated symptoms
Endometriosis is often associated with a range of painful symptoms that include chronic pelvic pain (cyclical and non-cyclical), painful periods (dysmenorrhea), painful sex (dyspareunia), and pain on defecation (dyschezia) and urination (dysuria). 1 27 Their severity can range from mild to debilitating. Some women have no symptoms, others have episodic pelvic pain, and still others experience constant pain in multiple body regions. 28 A related observation is that some women transition between these categories, progressing from episodic and localized pain to that which is chronic, complex, and more difficult to treat. Furthermore, women with disease that is anatomically “severe” can have minimal symptoms and women with “minimal” evidence of endometriosis can have severe, life affecting symptoms. 1 19 In common with other chronic pain conditions, women with endometriosis often report experiencing fatigue and depression. Infertility is significantly more common in patients with endometriosis, with a doubling of risk compared with women without endometriosis. 2 Endometriosis is discovered in 30-50% of women who present for assisted reproductive treatment. 29 30
Endometriosis associated or high risk comorbidities
Endometriosis is certainly a multisystem condition, perhaps as a result of common pathogenesis or as a consequence of the chronic endogenous response to the presence of endometriotic lesions. 9 Although pelvic pain is the most common symptom of possible endometriosis, women with endometriosis also have a high risk of co-occurring or evolving multisite pain. 28 Patients with endometriosis have a higher risk of presentation with comorbid chronic pain conditions such as fibromyalgia, 31 32 33 migraines, 34 35 and also rheumatoid arthritis, 33 36 psoriatic arthritis, 37 and osteoarthritis. 36 38 Reports of back, bladder, or bowel pain are prevalent, 16 39 with dyschezia being potentially predictive of endometriosis. 40 Nearly 50% of women with bladder pain syndrome or interstitial cystitis have endometriosis. 41 42 Irritable bowel syndrome is a common co-occurring diagnosis that reinforces the importance of awareness of endometriosis among gastroenterologists. 43 44 45 These conditions may share a common cause, 46 they may arise together owing to shared environmental or genetic factors, and/or the occurrence of comorbid pain conditions could be due to changes in pain perception after repeated sensitization. 47 Research focused on disentangling the overlapping and independent pathways of these frequently co-occurring pain associated conditions is essential. 48 49
Women with endometriosis have a greater risk of presenting with other non-malignant gynecologic diseases, including uterine fibroids and adenomyosis. 50 51 They are also at greater risk of a subsequent diagnosis of malignancies, autoimmune diseases, early natural menopause, and cerebrovascular and cardiovascular conditions. 36 52 53 54 55 56 57 The hypothesized causal mechanisms for endometriosis discussed below are all thought to be enhanced by and/or result in chronic inflammation. Local and systemic chronic inflammation can directly activate afferent nociceptive fibers and promote pelvic pain, 58 although this does not entirely explain the heterogeneity in types and severity of painful symptoms that patients experience. Furthermore, endometriosis induced chronic inflammation and immune dysregulation may also contribute to the endometriosis associated subsequent risk of each of these comorbid conditions. 59 60
Although this multisystem effect reinforces the importance of knowledge of and attention to endometriosis from general practitioners and a myriad specialists for whole healthcare, the most prominent association, and the focus of the greatest volume of comorbidity research, is the elevated risk of ovarian cancer among women with endometriosis. A recent meta-analysis confirmed this association, 53 finding a nearly twofold greater relative risk of ovarian cancer among patients with endometriosis (summary relative risk (SRR) 1.93, 95% confidence interval 1.68 to 2.22; n=24 studies) that was strongest for clear cell (3.44, 2.82 to 4.42; n=5 studies) and endometrioid (2.33, 1.82 to 2.98; n=5 studies) histotypes. However, among these 24 studies, significant evidence existed of both heterogeneity across studies and publication bias (Egger’s and Begg’s P values <0.01). Clinicians need to reinforce that ovarian cancer is rare regardless of women’s endometriosis status 61 : the absolute lifetime risk in the general population is 1.3%, 62 and applying the risk estimate from the meta-analysis (SRR 1.9) gives an absolute lifetime risk for women with endometriosis of 2.5%, which is 1.2% higher than the absolute risk for women without endometriosis and still very low.
We should also recognize that coexisting gynecologic conditions such as adenomyosis and uterine fibroids, 50 as well as associations with endometrial cancer, 53 can be influenced by diagnostic biases and failure to distinguish between diagnoses in women undergoing hysterectomy and those in women with an intact uterus. 11 51 When attempting to infer a causal relation between endometriosis and other conditions, applying rigorous prospective temporality (rather than cross sectional co-occurrence) is particularly important for valid subsequent risk associations. 53 These studies need large study populations with well documented longitudinal data. A large impediment is the lack of routine, harmonized documentation of the characteristics of endometriosis and its absence from international classification of diseases coding. 63 64
Endometriosis classification systems
Several classification, staging, and reporting systems have been developed; 22 systems were published between 1973 and 2021. 65 The three most commonly used systems are the revised American Society for Reproductive Medicine (rASRM) classification (stages I-IV; where stage I is equivalent to “minimal” disease and stage 4 to “severe” disease), the ENZIAN (and newer #ENZIAN) classification, and the Endometriosis Fertility Index (EFI). 66 67 68 69 Many validation studies and reports on the implementation of the different systems have been published. The rASRM system (scored at surgery on the basis of the extent of visualized superficial peritoneal lesions, endometriomas, and adhesions) has been shown to have poor correlation with pain, 70 fertility outcomes, and prognosis, and the ENZIAN system (which additionally includes deep endometriosis) has been shown to have poor correlation with symptoms and infertility. 71 72 73 The EFI is a well validated clinical tool that predicts pregnancy rates after surgical staging of endometriosis, with ongoing evaluation to determine the predictive importance of the individual parameters included in the scoring algorithm as well as the effect of completeness of surgical treatment on pregnancy prediction. 74 Unfortunately, no international agreement exists on how to describe endometriosis or how to classify it. As most systems show no, or very little, correlation with patients’ symptoms and outcomes, this is further evidence of our lack of understanding of the physiology underlying the symptoms associated with endometriosis.
Epidemiology
The exact prevalence of endometriosis is unknown given diagnostic delays and barriers, and—perhaps consequently—it is extremely varied depending on the population and the indication for evaluation. A recent meta-analysis identified 69 studies describing the prevalence and/or incidence of endometriosis, among which 26 studies were general population samples, 17 were from regional/national hospitals or insurance claims systems, and the remaining 43 studies were conducted in single clinic or hospital settings. 10 The prevalence reported in general population studies ranged from 0.7% to 8.6%, whereas that reported in single clinic or hospital based studies ranged from 0.2% to 71.4%.
When defined by indications for diagnosis, the prevalence of endometriosis ranged from 15.4% to 71.4% among women with chronic pelvic pain, from 9.0% to 68.0% among women presenting with infertility, and from 3.7% to 43.3% among women undergoing tubal sterilization. Few studies have investigated the incidence and prevalence of endometriosis specifically among adolescents. The reported prevalence of visually confirmed endometriosis among adolescents with pelvic pain ranges from 25% to 100%, with an average of 49% among adolescents with chronic pelvic pain and 75% among those unresponsive to medical treatment. 75 The Ghiasi meta-analysis reported a decrease in recorded prevalence across the past 30 years. 10 Speculating, this may be due to more rapid and more ubiquitous embracing of empiric treatment of symptom, forgoing or delaying definitive imaging or surgical diagnosis, a patient centered approach that has been ratified by the most recent European endometriosis guideline. 13 This hypothesis is supported by a recent report from a large US health system’s electronic medical records database that observed a decline from 2006 through 2015 in incidence rates for endometriosis (from 30.2 per 10 000 person years in 2006 to 17.4 per 10 000 person years in 2015) but an increase in documentation of chronic pelvic pain diagnoses (from 3.0% to 5.6%). 76
Pathophysiology
Heritability and genetics.
Estimates from twin studies suggest 47-51% total heritability of endometriosis, with 26% estimated to be from genetic variation. 77 78 79 To date, nine genome-wide association studies have been reported. 59 The largest study so far, using 17 045 cases and 191 596 controls, has identified 19 single nucleotide polymorphisms, most of which were more strongly associated with rASRM stage III/IV, rather than stage I/II, explaining 1.75% of risk for endometriosis. 80 Consistent with other complex diseases with multifactorial origins, no high penetrance susceptibility genes for endometriosis have yet been identified. 62 The loci discovered to date are almost all located in intergenic regions that are known to play a role in the regulation of expression of target genes yet to be identified. The critical next steps in genetic discovery are to identify additional genes that reveal novel pathophysiological pathways and also emerge to better define the underpinnings of variation in symptoms (in particular, pain types and infertility and treatment response predictors) and also gene expression correlated with comorbid autoimmune, cancer, and cardiovascular conditions. 62
Reflux of endometrial tissue fragments/cells and protein rich fluid through the fallopian tubes into the pelvis during menstruation is considered the most likely explanation for why endometriotic lesions form within the peritoneal cavity, although this mechanism is not sufficient as nearly all women experience retrograde menstruation. 81 82 Additional postulated origins include celomic metaplasia and lymphatic and vascular metastasis. Scientific avenues exploring contributions of interacting endocrine, immunologic, proinflammatory, and proangiogenic processes are drawing curiosity and expertise from varied disciplines with application of state of the art technologies. 59 Retrograde menstruation of stem cells contributes to the establishment of endometriosis, 83 whereas bone marrow stem cells contribute to the continued growth of endometriosis lesions. 84 85 Bone marrow derived stem cells may be responsible for those cases of endometriosis outside of the abdominal cavity. 86
Studies exploring why lesions develop in some, but not all, women have detected changes in the endometrial tissue as well as in the peritoneal fluid and cells lining the cavity. Eutopic endometrial tissue has a significantly different immune profile in women with endometriosis compared with those without it. 87 However, the extent to which this inflammation is a cause or an effect of endometriosis remains unclear. Aberrant inflammation could have an effect on the development of endometriosis lesions and disease progression in various ways, including immune angiogenesis and immune-endocrine interaction. 59 60 Specifically, some of the proposed pathways include altered production of inflammatory cytokines by immune cells in lesions or by endometrial cells themselves involving decreased immune clearance of abnormal endometrial cells and consequent seeding and development of lesions, increased likelihood of adhesion to mesothelial cells due to pro-invasion inflammatory milieu, inflammation promoted proliferation of endometrial cells, and inflammation promoted reduction in apoptosis of endometrial cells. 88 Analysis of eutopic endometrium from women with endometriosis has identified altered expression of genes implicated in the inflammation/immune response, angiogenesis, and steroid responsiveness (progesterone “resistance”). 89 Shed menstrual tissue contains high concentrations of pro-inflammatory cytokines, proteases, and immune cells, all of which may influence the peritoneal microenvironment after reflux. Stem/progenitor cells have been identified in the endometrium and are thought to survive and implant onto the peritoneum, contributing to lesions. 83 Mesothelial cells line the pelvic peritoneal cavity, and changes in their function in women with endometriosis, including altered morphology and metabolism (switch to aerobic glycolysis) 90 and production of factors that promote immune cell recruitment and angiogenesis are all thought to favor survival and establishment of lesions. 91 Physiological hormonal fluctuations in women induce cyclical episodes of cell proliferation, inflammation, injury, and repair within lesions that favor fibroblast to myofibroblast differentiation and fibrosis.
Mechanisms of endometriosis associated pain
The development of a new blood supply and associated nerves (neuroangiogenesis) is considered key to the establishment of endometriotic lesions and the activation of peripheral pain pathways ( fig 3 ). 92 Sensory C, sensory Ad, cholinergic, and adrenergic nerve fibers have all been detected in lesions. Estrogens can promote crosstalk between immune cells and nerves within lesions, increasing expression of nociceptive ion channels such as the transient receptor potential cation channel subfamily V member 1. 93 Factors that promote inflammation and nerve growth, such as nerve growth factor, tumor necrosis factor α, and interleukin 1-β, are increased in the peritoneal fluid of women with endometriosis and may exacerbate a neuroinflammatory cascade. Consistent with other conditions associated with chronic pain, endometriosis is associated with unique, and sometimes disease specific, alterations in the peripheral and central nervous systems, including changes in the volume of regions of the brain and in brain biochemistry. 94 Increased risk of central sensitization may partially explain why approximately 30% of patients with endometriosis will develop chronic pelvic pain that is unresponsive to conventional treatments, including surgery. 95 Through this central process, patients can experience reduced pain thresholds, increased responsiveness and length of aftereffects to noxious stimuli, and expansion of the receptive field so that input from non-injured tissue may elicit pain. 46 47 Among endometriosis patients with central sensitization, the removal of the endometriotic lesions is unlikely to result in adequate pain remediation owing to continued activation of the central nervous system. 96 97 Thus, endometriosis associated pain does not neatly fall into one of the three main categories of chronic pain (that is, nociceptive, neuropathic, or nociplastic), 98 99 100 and it likely has a mixed pain phenotype or sits somewhere along a continuum of these pain phenotypes. For example, some patients have primarily nociceptive or neuropathic pain, others have primarily nociplastic pain, and the rest have a mixed phenotype with variable contributions of nociceptive, neuropathic, and nociplastic pain.

Pathophysiology of endometriosis: (1) potential factors contributing to endometriosis associated pain; (2) potential mechanisms of endometriosis associated infertility; (3) local factors involved in the development of an endometriosis lesion; (4) role of the eutopic endometrium in the development of an endometriosis lesion. CNS=central nervous system; PNS=peripheral nervous system
Mechanisms of endometriosis associated infertility
Endometriosis may impair fertility through multiple pathways, including peritoneal inflammation and endocrine derangements, which interfere with the follicular environment and consequently affect ovarian function and ultimately reduce oocyte competence. 101 Several studies of women undergoing in vitro fertilization have documented lower oocyte yield or ovarian reserve among women with endometriosis compared with those with other infertility diagnoses. 102 103 A recent study observed lower oocyte yield among endometrioma affected ovaries but not among the contralateral ovaries that were unaffected by endometriosis compared with unexposed ovaries from women with no evidence of endometriosis. 104 In addition, although unproven, anatomical distortion and adhesions caused by endometriosis, particularly in stage III-IV disease, seem likely to reduce the chance of natural conception.
No way to prevent endometriosis is known. Enhanced awareness, followed by early diagnosis and management, may slow or halt the natural progression of the disease and reduce the long term burden of painful symptoms, including possibly the risk of central sensitization, but no cure exists. Furthermore, the evidence for modifiable risk factors for endometriosis remains unacceptably sparse. 12 Critically needed are large scale longitudinal studies that can quantify modifiable exposures in girls and young women in the pre-diagnostic, and ideally the pre-symptomatic, window that are then explored further in humans, 105 106 107 as well as in experimental models, to determine the physiologic pathways defined by causal effects on the epigenome, transcriptome, proteome, and metabolome. To date, few risk factors have been robustly replicated in multiple populations, with the most consistently associated with endometriosis including müllerian anomalies, low birth weight and lean body size, early age at menarche, short menstrual cycles, and nulliparity. 1 11 Less research has supported associations with endocrine disrupting toxins including diethystilbestrol. 108
Clinical course
A critical aspect of care for women with endometriosis is that associated symptoms progress and recede over the life course, sometimes in response to treatment and sometimes with age or altered environment in pathways that we do not yet understand ( fig 4 ). For example, pain remediation is often a priority among adolescents, 109 whereas older women may be focused on fertility or on life affecting fatigue. 8 110 Furthermore, a long held belief that endometriosis and its symptoms do not occur in adolescents and end at menopause was erroneous. However, the years of perimenopause can be a time of increased pelvic pain, 111 112 with particular attention needing to be paid to symptom management that may include an unexpected return of pain in those patients for whom a treatment regimen had been successful during premenopause. 113 Clinicians need to focus across the life course on patient centered care, engaging in a dialogue to capture evolving symptomatology but also to collaborate on what symptoms are of most importance to the patient at this life stage. 110 Importantly, all that we believe we know about endometriosis is limited to the characteristics and natural history of those women who successfully obtain a diagnosis. To whatever extent asymptomatic or incidental findings have influenced the diagnostic population or to whatever extent health disparities or biases regarding symptom belief or access to pain or infertility care have limited or skewed those diagnosed, our elucidation of the true signs and symptoms and prognosis of endometriosis will evolve as care and access to it improves. 11

Endometriosis risk, establishment, and multisystem effects encompassing evolution across the life course
Diagnosis and monitoring
Although endometriosis has a highly variable presentation, steps can be recommended for decision making by general practitioners and gynecologists to approach a “working diagnosis” of probable endometriosis, implement treatment to remediate endometriosis associated symptoms, and consider multi-specialty collaboration for patient centered whole healthcare ( fig 5 ).

Flowchart for a step-by-step approach to patients with suspected endometriosis (adapted from flowcharts in the NICE 114 and ESHRE 13 endometriosis guidelines). *Imaging does not rule out endometriosis; if “negative” imaging but symptoms highly suggestive of endometriosis, consider “working diagnosis” of probable endometriosis. †General practitioners should monitor for emergence of signs of conditions associated with endometriosis and involve/refer to appropriate specialist (eg, gastroenterologist, cardiologist, rheumatologist, psychologist, oncologist). ‡Ideally within accredited specialist endometriosis center
“Red flag” symptoms and signs
The diagnosis of endometriosis should be considered in women (including girls aged 17 and under) presenting with one (or more) of the following symptoms or signs: chronic pelvic pain with or without cyclic flares, dysmenorrhea (affecting daily activities and quality of life), deep dyspareunia, cyclical gastrointestinal symptoms (particularly dyschezia), cyclical urinary symptoms (particularly hematuria or dysuria), or infertility in association with one (or more) of the preceding symptoms or signs. 114 Shoulder tip pain (pain under the shoulder blade), catamenial pneumothorax, cyclical cough/hemoptysis/chest pain, and cyclical scar swelling/pain can indicate endometriosis at extra-abdominal sites. 13 40 115 116 Fatigue is commonly reported by women with endometriosis. An abdomino-pelvic examination may help to identify ovarian and deep disease. 117
Diagnostic biomarkers
Many research studies and Cochrane reviews have assessed potential biomarkers for endometriosis, 118 119 120 with the ultimate goal of reducing the delay that exists in diagnosing endometriosis. Unfortunately, all of the candidates investigated to date have proven non-specific or unreliable, making them inappropriate for routine clinical use.
Imaging to diagnose endometriosis
Ultrasonography and magnetic resonance imaging (ideally two dimensional, T2 weighted sequences without fat suppression) can be used to diagnose endometriosis preoperatively, but the absence of findings on imaging does not exclude endometriosis, particularly superficial peritoneal disease. 121 122 Nevertheless, the ENDO Study enrolled 131 women from the general population who had not presented for gynecologic evaluation, among whom magnetic resonance imaging was used to diagnose endometriosis in 11%. 123 Although the sensitivity of transvaginal ultrasonography is maximized only for endometriomas, technological and training advances are improving detection of all sub-phenotypes of endometriotic lesions. 124 125 Saline infusion sonoPODography is a novel technique that may be able to diagnose superficial peritoneal endometriosis on ultrasonography, although it needs to be validated. 126
Laparoscopic diagnosis and appearance of endometriosis
In patients with suspected endometriosis, in whom imaging has shown no obvious pelvic pathology or for whom empirical treatment has been unsuccessful, laparoscopy is recommended for diagnosis. Laparoscopy for endometriosis should always involve a comprehensive exploration of the abdominal and pelvic contents. Histopathological confirmation is ideal; however, histologic definitions for endometriosis have remained stagnant for decades, with a lower than expected sensitivity, 12 particularly among younger women with endometriosis. 127 Superficial peritoneal endometriosis has been described as having a black (powder burn) or dark bluish appearance from the accumulation of blood pigments ( fig 2 ). 128 However, lesions can appear as white opacifications, red flame-like lesions, or yellow-brown patches in earlier, active stages of disease. 129 Ovarian endometriomas have a distinct morphology classically described as a “chocolate cysts,” containing old menstrual blood, necrotic fluid, and other poorly defined components that give their contents a dark brown appearance. Adhesions are often found in association with endometriomas and consist of fibrous scar tissue resulting from chronic inflammation. In many cases, endometriosis is present at the site of ovarian fixation. 130 Deep endometriosis appears as multifocal nodules and may infiltrate the surrounding viscera and peritoneal tissue. 131 Almost 40% of laparoscopies done for pelvic pain do not identify any pathology. 99 Clinicians should always consider other pelvic and non-pelvic visceral and somatic structures, as well as centrally mediated pain factors, that could be generating or contributing to the pain. 99
“Working diagnosis” of probable endometriosis
In women with a high suspicion of endometriosis, in whom imaging has not shown obvious pelvic pathology and a laparoscopy has not been done or is awaited, giving a “working diagnosis” of probable endometriosis and instigating early medical treatment without waiting for a more definitive diagnosis can be helpful. 13 114 132 133 This is an emerging concept for which some people use the terms “working” and “clinical” diagnosis interchangeably.
Delayed diagnosis
Endometriosis can occur at any age, with some patients reporting that pelvic pain symptoms arose at or soon after thelarche or menarche. Among women with endometriosis diagnosed in adulthood, nearly a fifth report that their symptoms began before age 20 and two thirds report onset before age 30. 5 The exact time of disease onset is unknown for endometriosis, as symptoms must emerge and be sufficiently life affecting to gain referral for definitive diagnosis. Furthermore, non-specific symptoms such as dysmenorrhea have often been treated with hormonal drugs without consideration of endometriosis, whereas the current recommendation is to be aware and consider a working diagnosis of probable endometriosis. Thus, varied non-specific symptomatology, normalization of pelvic pain, clinicians’ awareness of endometriosis, and economic and geographic access to care all contribute to a delay averaging seven years from symptom onset to surgical diagnosis. 1 5
Long term monitoring of endometriosis
Follow-up, including psychological support, should be considered in women with confirmed endometriosis, with renewed evaluation and a revised treatment plan if symptoms emerge, recur, or worsen over time. However, no evidence exists of benefit of regular long term monitoring (for example, imaging) for early detection of endometriotic lesion recurrence, complications, or malignant transformation, in the absence of complex ovarian masses or endometriosis with deep bowel effect. 134 135 Given growing evidence of risk of multisystem involving conditions ( fig 4 ), patient centered whole healthcare dictates that monitoring by general practitioners for emergence of signs and symptoms of mental health conditions, cardiovascular disease, immunologic and autoimmune disorders, gastrointestinal conditions, or multifocal pain conditions should be heightened and referral to a non-gynecologic specialist should be considered as needed.
Management of endometriosis associated pain
The growing recognition that endometriosis associated pain has a mixed pain phenotype (or occupies different points on a continuum) supports a personalized, multimodal, interdisciplinary treatment approach, 13 which might include surgical ablation/excision of lesions, analgesics, hormonal treatments, non-hormonal treatments including neuromodulators, and non-drug therapies (or a combination of the above). 1 The evidence supporting different surgical, pharmacologic, and non-pharmacologic approaches to treating endometriosis is examined below.
Surgical management of endometriosis associated pain
The most recent guidelines for endometriosis (for example, the National Institute for Health and Care Excellence (NICE), ESHRE) recommend surgery as a treatment option to reduce endometriosis associated pain. 13 114 However, only a limited number of RCTs have assessed pain outcomes after surgery (and most are small, offer little detail on endometriosis sub-phenotypes visualized at surgery, and have a follow-up period of less than 12 months). Furthermore, the authors of the most recent Cochrane review of surgery for endometriosis associated pain concluded that they were “uncertain of the effect of laparoscopic surgery on pain and quality of life” owing to the low quality of the available studies. 136 They included only two of the published RCTs (comparing surgical treatment of endometriosis with diagnostic laparoscopy alone) in their analysis of laparoscopic excision to improve pain and quality of life. 137 138 One trial of 16 participants experiencing pain associated with endometriosis assessed “overall pain” scores at 12 months (mean difference on 0-100 visual analog scale (VAS) 1.65, 95% confidence interval 1.11 to 2.19), and the other trial of 39 participants assessed quality of life at six months measured using the EuroQol-5D (mean difference 0.03, –0.12 to 0.18). The evidence of benefit for specific subtypes is discussed in more detail below.
Surgery for superficial peritoneal endometriosis
Little evidence shows that surgery to treat isolated superficial peritoneal endometriosis improves overall symptoms and quality of life. The uncertainty around surgical management of this subtype is compounded by the limited evidence to allow an informed selection of specific surgical modalities to remove the lesions (for example, laparoscopic ablation versus laparoscopic excision). 139 140
Surgery for ovarian endometriosis
To our knowledge, no RCTs have compared cystectomy versus no treatment in women with endometrioma and measured the effect on painful symptoms. Also, no published data indicate a threshold cyst size below which surgery may be safely withheld in the absence of suspicious features on imaging (surgery is the only means by which a tissue specimen can be obtained to rule out ovarian malignancy). Thus, surgical excision is generally considered the optimal treatment for ovarian endometriosis. Cystectomy, instead of drainage and coagulation, is the preferred surgical approach as it reduces recurrence of endometrioma and endometriosis associated pain. 141 Cystectomy should be chosen with caution for women who desire fertility, as a risk of fertility affecting diminished ovarian reserve exists, and a highly skilled conservative approach should be applied to minimize ovarian damage. 142
Surgery for deep endometriosis
Surgical treatment to completely excise deep disease is generally considered to be the treatment of choice. 143 144 Nevertheless, most of the studies that have reported improvements in quality of life following surgical excision of deep endometriosis (typically involving the bowel) have been done in small cohorts of women, usually from single centers, without a comparator arm, and this affects the precision and generalizability of the results. The largest multicenter prospective non-randomized study published to date reported the six, 12, and 24 month follow-up outcomes on nearly 5000 women undergoing laparoscopic excision of deep rectovaginal endometriosis. 143 This showed clinically and statistically significant reductions in premenstrual, menstrual, and non-cyclical pelvic pain, deep dyspareunia, dyschezia, low back pain, and bladder pain at 24 months (data from 524-560 participants for each symptom) with a corresponding improvement in quality of life (575 participants, median score on EuroQol-5D 76, 95% confidence interval 75 to 80). Although the results should be interpreted with caution, because data were missing for >70% of patients at 24 months, assigned score methods suggest that evidence of improvement remained statistically significant.
Hysterectomy for endometriosis
No RCTs on hysterectomy for the treatment of endometriosis associated pain have been done. Most published articles are retrospective case series, and only a few prospective studies have been reported. Hysterectomy (with or without oophorectomy) with removal of all visible endometriosis lesions should be reserved for women who no longer wish to conceive and who have not responded to more conservative management. Women with endometriosis should be informed that hysterectomy is not a “cure” for endometriosis and that it is best reserved for women with coexisting adenomyosis (which does occur inside the uterus) or for women with severe pain who have exhausted all other options to improve their symptoms. 145 Recent longitudinal studies have not found a benefit of bilateral oophorectomy for long term pain management. 145 146 Of note, BIPOC (black, indigenous, and people of color) women are more likely to have complications of hysterectomy, in part because they are more likely to undergo laparotomy rather than minimally invasive laparoscopy. 147 Women should be informed that hysterectomy is associated with long term morbidity, 148 including cardiovascular disease, 56 among those with and without surgically induced menopause. 149 150
Recurrence or progression of endometriosis after surgery
The reported recurrence rate of painful symptoms attributed to endometriosis is high, estimated as 21.5% at two years and 40-50% at five years. 146 151 However, although a purist’s definition of “endometriosis recurrence” calls for “second look” laparoscopy, it is most often diagnosed in the real world on the basis of recurrence of symptoms alone. In addition, no robust evidence exists to support an ordered progression of endometriotic lesions. In prospective studies of repeat surgeries, lesions progressed (in 29% of cases), regressed (in 42%), or were static (in 29%). 152 Surgical treatment of certain subtypes of endometriosis could also exacerbate painful symptoms. 153 154
Preoperative and postoperative hormone treatment
Preoperative hormone treatment has not been shown to improve the immediate outcome of surgery for pain, or reduce recurrence, in women with endometriosis. 155 A meta-analysis of 340 participants found that compared with surgery alone, postoperative hormone treatment of endometriosis reduced pelvic pain after 12 months (standardized mean difference on VAS −0.79, −1.02 to −0.56), but the evidence is very low quality. 155 Women with endometriosis who undergo hysterectomy with oophorectomy should be advised to start continuous combined hormone replacement therapy (HRT) for at least the first few years after surgery. 156 This may be changed later to estrogen alone, but this needs to be balanced with the theoretical risk of reactivation and malignant transformation of any residual endometriosis, which can occur many years later.
Pharmacologic management of endometriosis associated pain
Most women with suspected or known endometriosis use over-the-counter drugs, such as non-steroidal anti-inflammatory drugs (NSAIDs). However, the available evidence to support their use is scarce. The data on the benefit of NSAIDs are limited to one small RCT. 157 They can be useful as “breakthrough medication” in the management of a pain flare.
Hormonal treatments
Hormone treatments for endometriosis include combined contraceptives, progestogens, gonadotrophin releasing hormone (GnRH) agonists, GnRH antagonists, and aromatase inhibitors ( table 1 ). All of these hormone treatments (except the newer GnRH antagonists, which have not been so extensively studied) have been included in a multivariate network meta-analysis of the outcomes “menstrual pain” and “non-menstrual pelvic pain” (pain relief on VAS; total of 1680 participants). 114 All treatments led to a clinically significant reduction in pain on the VAS compared with placebo. The magnitude of this treatment effect is similar for all treatments, suggesting that little difference exists between them in their capacity to reduce pain. Furthermore, symptoms return after cessation of treatment and hormone treatments used to manage endometriosis all have side effects. In addition, although the contraceptive properties of the hormones may be welcome if the woman does not wish to become pregnant, they may be unwanted if fertility is desired.
Hormone treatments
- View inline
Non-hormonal treatments
Analgesic tricyclic antidepressants (for example, amitriptyline, nortriptyline), selective serotonin uptake inhibitors (for example, duloxetine) and anticonvulsants (for example, gabapentin and pregabalin) are sometimes used clinically in the treatment of endometriosis associated pain without a strong evidence base. 167 These “neuromodulatory drugs” differ from conventional analgesics, such as NSAIDs, in that they primarily affect the central nervous system’s modulation of pain rather than peripheral mediators of inflammation. However, in a recent RCT for the management of chronic pelvic pain (in the absence of endometriosis), gabapentin was not shown to be superior to placebo and was associated with dose limiting side effects. 168
Non-drug management of endometriosis associated pain
Pelvic physiotherapy.
An increasing number of women with endometriosis report anecdotal benefit from pelvic physiotherapy. Physiotherapists may support women with activity management (for example, exercises, pacing strategies, and goal setting) and/or use complementary approaches to manage their pelvic pain symptoms (for example, massage and trigger point release therapy). Establishing the independent benefit of standalone physiotherapy is difficult, because most studies have assessed it in combination with psychological and medical management. 169 Two small pilot studies assessed the outcome of manipulations and massage for relief of endometriosis associated pain specifically, but they included specific patient groups and need expansion and replication to support recommendations for care of endometriosis patients. 170 171
The most common psychologically based intervention for chronic pain is cognitive behavioral therapy (CBT). Most of the studies of CBT in women with endometriosis are of low quality, designed using different methods and based on different psychological frameworks (making separation of effects difficult). However, given that CBT has been evaluated across a spectrum of other chronic pain disorders and shown to be effective for developing pain coping strategies, 98 99 172 it should be integrated into individualized treatment plans when needed.
Dietary intervention
Diet has been postulated to affect symptoms of endometriosis. However, very few studies (all of limited quality) have evaluated the benefit of dietary interventions and their effect on endometriosis symptoms. Supplements, such as omega-3 polyunsaturated fatty acids (O-PUFAs), have been investigated as a way of reducing inflammation and pain in endometriosis. 173 174 In a recent review, decreased pain scores were observed in women with endometriosis after use of O-PUFAs, which were not seen in controls. 175 Clinicians should be aware that women with endometriosis have an increased risk of co-presenting with irritable bowel syndrome concomitant with endometriosis associated dyschezia. 44 Patients are not uncommonly referred for gastroenterology evaluation without consideration of potential endometriosis. 45
Treatment of endometriosis associated infertility
Hormonal/medical therapies.
No evidence exists of benefit of suppression of ovarian function in women with endometriosis associated infertility who wish to conceive. 176 Following surgery for endometriosis, women seeking pregnancy should not be treated with postoperative hormone suppression with the sole purpose of enhancing future pregnancy rates.
Surgery to increase chance of natural pregnancy
Moderate quality evidence from a Cochrane meta-analysis of three RCTs in a total of 528 participants shows that laparoscopic treatment (ablation or excision) of superficial peritoneal endometriosis increases viable intrauterine pregnancy rates compared with diagnostic laparoscopy only (odds ratio 1.89, 95% confidence interval 1.25 to 2.86). 136 We found no data on live birth rates, and the effect on ectopic pregnancy and miscarriage rates is unclear. No published RCTs have assessed fertility outcomes after surgery for ovarian or deep disease, and surgery is generally recommended only in the presence of painful symptoms. 13 Use of the Endometriosis Fertility Index to support decision making for the most appropriate option to achieve pregnancy after surgery (for example, women who may benefit from medically assisted reproduction) has been recently suggested. 13 68
Medically assisted reproduction
Low quality evidence shows that viable intrauterine pregnancy rates are increased in women with superficial peritoneal endometriosis if they undergo intrauterine insemination with ovarian stimulation, instead of expectant management or intrauterine insemination alone. In one RCT of 103 participants randomized either to ovarian stimulation with gonadotrophins and intrauterine insemination treatment or to expectant management, the live birth rate was 5.6 (95% confidence interval 1.18 to 17.4) times higher in the treated couples. 177 In women with ovarian or deep endometriosis, the benefit of ovarian stimulation with intrauterine insemination is unclear. No RCTs have evaluated the efficacy of assisted reproductive technology (ART) versus no intervention in women with endometriosis. Recommendations in guidelines suggesting that ART may be effective for endometriosis associated infertility have been based on meta-analyses of observational studies comparing the outcomes of ART in women with and without endometriosis. 13 178 179 Doing surgery before ART for infertility associated with superficial peritoneal endometriosis is not recommended, as the evidence suggesting benefit is based on a single retrospective study of low quality 180 (and is not supported by indirect evidence from multiple studies comparing outcomes in women with surgically treated endometriosis and those managed without surgery 179 ). Doing surgery for ovarian endometrioma before ART to improve live birth rates is also not recommended. Current evidence shows no benefit, and surgery is likely to have a negative effect on ovarian reserve. 181 182 In addition, no evidence shows that doing surgical excision of deep endometriosis before ART improves reproductive outcomes, and this should be reserved for women with concomitant painful symptoms.
Specialist endometriosis centers
Specialist centers were first formally proposed in 2006, 183 and this model of care has been successfully implemented in the UK and several other European countries such as Denmark, Germany, and France. 184 185 The role of specialist endometriosis centers should be to offer a coordinated, holistic, multidisciplinary, multimodal approach to women with complex symptoms of endometriosis that are experienced and evolve across the life course ( fig 5 ). Although relevant surgical expertise is important, the role of a center is not to focus solely on surgical treatment to eradicate lesions. Thus, a specialist center should offer an integrated service, including gynecologists, colorectal surgeons, urologists, endometriosis specialist nurses, pain medicine specialists, psychologists, physiotherapists, fertility specialists, and imaging experts.
Various national and international organizations have issued guidelines for the assessment and management of endometriosis. We reviewed and compared nine of these guidelines (including the recent 2022 update of the ESHRE guideline). 186 187 188 189 190 191 192 193 All of the guidelines recommend the combined oral contraceptive pill and progestogens for endometriosis associated pain, but they differ in the recommendations around “second line” medical treatments. All of the guidelines recommend laparoscopic surgery for the management of endometriosis associated pain, although some acknowledge the lack of evidence for surgery in the management of pain associated with superficial peritoneal endometriosis specifically. 13 114 No clear consensus exists regarding surgical treatment for endometriosis associated infertility, especially with regard to the management of an endometrioma before assisted reproduction.
Emerging diagnostic tools and treatments
Most endometriosis research studies to date have been underfunded and on a small scale, and have involved poorly defined populations of women and samples captured from those who receive a diagnosis well along in their endometriosis journey. However, real hope exists of a breakthrough in the development of a biomarker to diagnose endometriosis closer to emergence and earlier in its natural progression, and to predict response to treatment, owing to the establishment of globally harmonized endometriosis protocols for clinical data and human tissue collection. 105 106 107 The biomarker field will also hopefully benefit from new insights being gained from the study of serum microRNAs and metabolomics. 194 195 Preclinical studies of new non-hormonal medical treatments have offered insights by focusing on inflammation, pain, and metabolism as the platform for repurposing of drugs already approved for other conditions. 19 90 196 Increasing evidence also suggests that the “gut-brain axis” could be a novel therapeutic target for pain symptom relief in endometriosis. 197 Microbiomes likely play a role in the gut-brain axis, are associated with the spectrum of symptoms associated with endometriosis, and are an exciting putative therapeutic target. Lastly, although randomized evaluations of surgical interventions for endometriosis have been rare (and some interventions have been adopted without rigorous evaluation), we are witnessing important collaboration between research and surgical communities to conduct large scale, appropriate, and well designed trials (for example, PRE-EMPT ( https://www.birmingham.ac.uk/research/bctu/trials/womens/pre-empt/index.aspx ), REGAL ( https://w3.abdn.ac.uk/hsru/REGAL/Public/Public/index.cshtml ), ESPriT2 ( https://www.ed.ac.uk/centre-reproductive-health/esprit2 ), and DIAMOND ( https://w3.abdn.ac.uk/hsru/DIAMOND/Public/Public/index.cshtml )). Surgical trials are difficult to undertake successfully and pose practical and methodological challenges. However, the inherent value of a well conducted RCT to predict the outcomes and/or success rates of surgical treatments for endometriosis should not be overlooked.
Endometriosis is a prevalent, often life affecting condition that in most women emerges during adolescence and can evolve to include symptoms and conditions encompassing multiple systems. Endometriosis demands to be known, considered, and tackled by all practitioners—general and specialist—who treat female patients at all stages across the life course. Patient centered whole healthcare requires a dialog between a woman and her healthcare practitioners to monitor symptom remediation, persistence, or recurrence and to prioritize the focus of care—for example, fatigue remediation when sports participation is paramount, fertility when family building is desired, a revision of medical treatment during perimenopause, or early response to signs of cardiovascular changes. Stigma around menstrual health and chronic pain remain all too ubiquitous barriers to high quality healthcare. Awareness in the general public and among healthcare providers is essential.
Once their symptoms are acknowledged and treated, most patients with endometriosis do well. However, despite overcoming diagnostic delays and access to state of the art treatment, some experience persistence or progression of symptoms. Critical next steps for discovery include defining sub-phenotypes of endometriosis that classify patients into groups that are predictive of prognosis and the natural course of the condition and indicate selection of treatments most likely to be successful to restore high quality of life. We must also answer foundational questions that remain about the causes and natural progression of endometriosis that need expanded funding and attraction of multidisciplinary scientists from all areas of population and bench science. Recommendations to permit a “working diagnosis” of probable endometriosis are having an effect on patient centered care and faster symptom remediation. Through the work of endometriosis associations, non-governmental organizations, and the endometriosis community across the globe, awareness of endometriosis has increased in recent years, along with some increases in funding. We are early on the necessary trajectory, but the journey is gaining speed.

Questions for future research
What causes endometriosis?
Can a non-invasive screening tool be developed to aid the diagnosis of endometriosis?
What are the most effective ways of maximizing and/or maintaining fertility in women with confirmed or suspected endometriosis?
What are the most effective ways of managing the emotional, psychological, and/or fatigue related impact of living with endometriosis?
Can we predict the outcomes and/or success rates for surgical or medical treatments for endometriosis?
What are the most effective non-surgical ways of managing endometriosis related pain and/or symptoms?
Adapted from the James Lind Alliance “Top ten research priorities for endometriosis in the UK and Ireland” 198
Patient involvement
We consulted Emma Cox, chief executive of Endometriosis UK, a nationally recognized representative and voice of patients with endometriosis, in the development of this review, and she commented on the draft and final manuscript. No patients were involved directly in the preparation of this article.
Acknowledgments
In addition to invaluable insight provided by Emma Cox, we thank Naoko Sasamoto and Marzieh Ghiasi for early design of figures 1 and 4, which were further adapted by SAM for this review; Kevin Kuan for designing figure 3 in BioRender; and Dan Martin for contributing image 1 to figure 2.
Series explanation: State of the Art Reviews are commissioned on the basis of their relevance to academics and specialists in the US and internationally. For this reason they are written predominantly by US authors
Contributors: AWH and SAM contributed equally to the planning, analysis, and writing of the article. AWH is the guarantor.
Funding: AWH is supported by an MRC Centre Grant (MRC G1002033) and an NIHR Project Grant (NIHR129801).
Competing interests: We have read and understood BMJ policy on declaration of interests and declare the following interests: AWH’s institution (University of Edinburgh) has received payment for consultancy and grant funding from Roche Diagnostics to assist in the early development of a possible blood diagnostic biomarker for endometriosis. AWH has received grant funding from the MRC and NIHR for endometriosis research; he is a board member of the World Endometriosis Society and Society for Endometriosis and Uterine Disorders, is co-editor in chief of Reproduction and Fertility , has been a member of the NICE and ESHRE Endometriosis Guideline Groups, and is a trustee and medical adviser to Endometriosis UK. SAM has received payment for consultancy and grant funding from AbbVie, LLC, for population based research unrelated to product development and has received grant funding from the US National Institutes of Health, US Department of Defense, and the Marriott Family Foundations for endometriosis research. SAM is a board member of the World Endometriosis Society, World Endometriosis Research Foundation, American Society for Reproductive Medicine Endometriosis Special Interest Group, and the European Society for Human Reproduction and Embryology Special Interest Group on Endometriosis and Endometrial Disorders; a member of the Interdisciplinary Network on Female Pelvic Health of the Society for Women’s Health Research; and is a statistical advisory board member for Human Reproduction and field chief editor for Frontiers in Reproductive Health .
Provenance and peer review: Commissioned; externally peer reviewed.
- Zondervan KT ,
- Becker CM ,
- Prescott J ,
- Farland LV ,
- Tobias DK ,
- Soliman AM ,
- Simoens S ,
- Dunselman G ,
- Dirksen C ,
- Nnoaham KE ,
- Hummelshoj L ,
- Webster P ,
- World Endometriosis Research Foundation Global Study of Women’s Health consortium
- Stratton P ,
- Cleary SD ,
- Ballweg ML ,
- Missmer SA ,
- Agarwal SK ,
- Kulkarni MT ,
- Shafrir AL ,
- Taylor HS ,
- Adamson GD ,
- Diamond MP ,
- Heikinheimo O ,
- ESHRE Endometriosis Guideline Group
- DiVasta AD ,
- Vitonis AF ,
- Laufer MR ,
- Ballard K ,
- Tomassetti C ,
- Johnson NP ,
- Petrozza J ,
- International Working Group of AAGL, ESGE, ESHRE and WES
- Saunders PTK ,
- Andres MP ,
- Arcoverde FVL ,
- Souza CCC ,
- Fernandes LFC ,
- Halvorson LM
- Isaacson K ,
- Halvorson LM ,
- Giudice LC ,
- Vannuccini S ,
- Petraglia F ,
- Bourdon M ,
- Santulli P ,
- Jeljeli M ,
- Saunders PTK
- Williams C ,
- Bedaiwy MA ,
- Counsellor VS
- Eskenazi B ,
- Coloma JL ,
- Martínez-Zamora MA ,
- Collado A ,
- Larrosa Pardo F ,
- Bondesson E ,
- Schelin MEC ,
- Palmor MC ,
- Fourquet J ,
- Miller JA ,
- Shigesi N ,
- Kvaskoff M ,
- Kirtley S ,
- Harris HR ,
- Korkes KMN ,
- Costenbader KH ,
- Kennedy SH ,
- Jenkinson C ,
- World Endometriosis Research Foundation Women’s Health Symptom Survey Consortium
- Rodríguez MA ,
- Buchwald DS ,
- National Institute of Diabetes and Digestive and Kidney Diseases Working Group on Urological Chronic Pelvic Pain
- Tirlapur SA ,
- Chaliha C ,
- Chiaffarino F ,
- Cipriani S ,
- Zimmerman LA ,
- Fadayomi AB ,
- Morotti M ,
- Pogatzki-Zahn EM ,
- Liedgens H ,
- IMI-PainCare PROMPT consensus panel
- Nunez-Badinez P ,
- Laux-Biehlmann A ,
- Gallagher CS ,
- Mäkinen N ,
- 23andMe Research Team
- Mahamat-Saleh Y ,
- Thombre Kulkarni M ,
- Shafrir A ,
- Degnan WJ 3rd . ,
- Rich-Edwards J ,
- Spiegelman D ,
- Forman JP ,
- Vincent K ,
- Taylor RN ,
- Symons LK ,
- Miller JE ,
- Rahmioglu N ,
- Morris AP ,
- WERF EPHect Working Group
- Whitaker LHR ,
- Vermeulen N ,
- Einarsson JI ,
- International working group of AAGL, ESGE, ESHRE and WES
- ↵ Revised American Society for Reproductive Medicine classification of endometriosis: 1996 . Fertil Steril 1997 ; 67 : 817 - 21 . doi: 10.1016/S0015-0282(97)81391-X pmid: 9130884 OpenUrl CrossRef PubMed Web of Science
- Tuttlies F ,
- Keckstein J ,
- Saridogan E ,
- Ulrich UA ,
- Schliep KC ,
- Mumford SL ,
- Peterson CM ,
- Shamiyeh A ,
- World Endometriosis Society Sao Paulo Consortium
- Borrelli GM ,
- Maheux-Lacroix S ,
- Nesbitt-Hawes E ,
- Janssen EB ,
- Rijkers AC ,
- Hoppenbrouwers K ,
- Meuleman C ,
- D’Hooghe TM
- Christ JP ,
- Schulze-Rath R ,
- Grafton J ,
- Treloar SA ,
- O’Connor DT ,
- O’Connor VM ,
- Pettersson HJ ,
- Svedberg P ,
- Nyholt DR ,
- ANZGene Consortium ,
- International Endogene Consortium ,
- Genetic and Environmental Risk for Alzheimer’s disease Consortium
- Sapkota Y ,
- Steinthorsdottir V ,
- iPSYCH-SSI-Broad Group
- Hammond MG ,
- Cousins FL ,
- Figueira PG ,
- Critchley HOD ,
- Babayev E ,
- Gajbhiye R ,
- McKinnon B ,
- Mortlock S ,
- Mueller M ,
- Montgomery G
- Saunders PT ,
- Greaves E ,
- Esnal-Zufiurre A ,
- Mechsner S ,
- Saunders PT
- As-Sanie S ,
- Harris RE ,
- Napadow V ,
- Coccia ME ,
- Rizzello F ,
- Palagiano A ,
- Scarselli G
- Sutton CJ ,
- Whitelaw N ,
- Khachikyan I ,
- Carrillo J ,
- Di Tucci C ,
- Di Feliciantonio M ,
- Senapati S ,
- Sammel MD ,
- Barnhart KT
- Carvalho LFP ,
- Fassbender A ,
- Buck Louis GM
- Gallagher JS ,
- Angioni S ,
- Gemmell LC ,
- Webster KE ,
- VanBuren WM ,
- Kuznetsov L ,
- Dworzynski K ,
- Overton C ,
- Guideline Committee
- Ballard KD ,
- Seaman HE ,
- de Vries CS ,
- Bonsignore L ,
- Samuels S ,
- Vercellini P
- Rouzier R ,
- Thomassin-Naggara I ,
- Nisenblat V ,
- Bossuyt PM ,
- Farquhar C ,
- Johnson N ,
- Moura APC ,
- Ribeiro HSAA ,
- Bernardo WM ,
- Buck Louis GM ,
- Hediger ML ,
- ENDO Study Working Group
- Guerriero S ,
- Condous G ,
- van den Bosch T ,
- Leonardi M ,
- Mestdagh W ,
- Stamatopoulos N ,
- Watkins JC ,
- Mettler L ,
- Schollmeyer T ,
- Lehmann-Willenbrock E ,
- Jansen RP ,
- Koninckx PR ,
- Adamyan L ,
- Wattiez A ,
- Barraclough K ,
- Chapron C ,
- Andreotti RF ,
- Vercellini P ,
- Sergenti G ,
- Frattaruolo MP ,
- Beebeejaun Y ,
- Bosteels J ,
- Jarrell J ,
- Mohindra R ,
- Taenzer P ,
- Daniels J ,
- DeSarno M ,
- Findley J ,
- Maouris P ,
- Shaltout MF ,
- Elsheikhah A ,
- BSGE Endometriosis Centres
- Bendifallah S ,
- Sandström A ,
- Johansson M ,
- Bäckström T ,
- Shakiba K ,
- McGill KM ,
- Orlando MS ,
- Luna Russo MA ,
- Richards EG ,
- Stewart EA ,
- Laughlin-Tommaso SK ,
- Weaver AL ,
- Hans Evers JL
- Schrepf AD ,
- Zakhari A ,
- Delpero E ,
- McKeown S ,
- Tomlinson G ,
- Choudhry AJ ,
- ↵ British Menopause Society. Tools for Clinicians: induced menopause in women with endometriosis. 2019. https://thebms.org.uk/publications/tools-for-clinicians/ .
- Kauppila A ,
- di Tucci C ,
- Achilli C ,
- Benedetti Panici P
- Kettner LO ,
- Lessey BA ,
- Tanimoto M ,
- Kusumoto T ,
- Arjona Ferreira JC ,
- Hewitt CA ,
- GaPP2 collaborative
- Nordling J ,
- Jaszczak P ,
- Deboute O ,
- Zacharopoulou C ,
- Valiani M ,
- Ghasemi N ,
- Bahadoran P ,
- Williams ACC ,
- Eccleston C
- Abokhrais IM ,
- Denison FC ,
- Nodler JL ,
- Collins JJ ,
- Fedorkow DM ,
- Vandekerckhove P
- Tummon IS ,
- Martin JS ,
- Gallos ID ,
- Coomarasamy A
- Opøien HK ,
- Fedorcsak P ,
- Nickkho-Amiry M ,
- Majumder K ,
- Edi-O’sagie E ,
- D’Hooghe T ,
- Hummelshoj L
- van Hanegem N ,
- van Kesteren P ,
- Kalaitzopoulos DR ,
- Samartzis N ,
- Kolovos GN ,
- Dunselman GA ,
- European Society of Human Reproduction and Embryology
- Collinet P ,
- Revel-Delhom C ,
- Buchweitz O ,
- German and Austrian Societies for Obstetrics and Gynecology
- Leyland N ,
- Laberge P ,
- Practice Committee of the American Society for Reproductive Medicine
- World Endometriosis Society Montpellier Consortium
- Moustafa S ,
- Mamillapalli R ,
- Nematian S ,
- Koscielniak M ,
- Williams L ,
- Salliss ME ,
- Mahnert ND ,
- Herbst-Kralovetz MM
- Endometriosis Priority Setting Partnership Steering Group (appendix)

Image credit: Shutterstock
Global study shows the experience of Endometriosis is rooted in genetics
Researchers at the University of Oxford, in collaboration with 25 teams across the world, have published the largest study to date of the genetic basis of endometriosis.
Their study included DNA from 60,600 women with endometriosis and 701,900 without. It revealed compelling evidence of a shared genetic basis for endometriosis and other types of pain seemingly unrelated to endometriosis, including migraine, back pain and multi-site pain. The study has also revealed that ovarian endometriosis has a different genetic basis from other disease manifestations. The results open up new avenues for designing new medical treatments targeting subtypes of endometriosis, or even the repurposing of existing pain treatments for endometriosis.
Endometriosis has enormous implications on the quality of a woman’s life. This severe inflammatory condition occurring in 5-10% of women of reproductive age (190 million globally) can cause constant and intense pelvic pain, fatigue, depression, anxiety, and infertility. It is characterized by the presence of tissue that resembles the uterus lining (endometrium) outside the uterus. The location of these endometriotic deposits is primarily on organs within the pelvis (e.g. ovaries, pelvic surfaces and ligaments, bowel or bladder), although more rarely it can also be found outside of the pelvis. The huge impact on the health of many women is compounded by the fact that endometriosis can only reliably be diagnosed through surgery and sometimes imaging, and often takes many years to diagnose (eight years on average from first symptoms). Treatment is limited to repeated surgeries, and hormonal treatments with many side-effects that do not allow women to get pregnant.
It is known that endometriosis can run in families, and therefore that genetic factors (heritability) play a role in how it develops in some women but not in others. Very little is known about the causes of endometriosis, and studying genetics - by comparing the DNA code in women with and without the disease - can give us clues to the biological processes that are the basis for onset and progression.
By conducting the largest genetic study ever conducted, the researchers found 42 areas across the genome that harbour variants that increase risk of endometriosis. By linking these variants to the profiles of molecules in endometrium and blood, they identified a range of genes that were differently expressed in these tissues and therefore had a likely role in disease development. This list of genes is important for further work to develop of new treatments, better targeted to subtypes of disease. For instance, they found that some genetic variants were more associated with ovarian ‘cystic’ endometriosis than superficial disease spread throughout the pelvis.
What they noted in particular is that many of the implicated genes play a role in pain perception and maintenance. Indeed, they found that there was a shared genetic basis for endometriosis and a range of other chronic pain types such migraine, back pain, and multi-site pain. This could be related to so-called sensitisation of the central nervous system, which makes individuals suffering from chronic pain more prone to other types of pain. These findings open up the possibility of designing new pain-focussed non-hormonal treatments, or repurposing existing pain treatments, for endometriosis.
Dr Nilufer Rahmioglu , Senior Research Scientist at the Wellcome Centre for Human Genetics , University of Oxford, and first author of the study commented: 'Using different datasets of women with and without endometriosis, some of which had unprecedented detailed data on surgical findings and pain experience collected using standardised criteria, allowed us to generate a treasure trove of new information about genetically driven endometriosis subtypes and pain experience.'
Professor Krina Zondervan , Co-Director of the Endometriosis CaRe Centre , and Head of the Nuffield Department of Women’s & Reproductive Health , University of Oxford, senior author on the paper, commented: 'Endometriosis is now recognised as a major health issue affecting women’s lives. This study involved the analysis of DNA from more than 60,000 women with endometriosis worldwide, in an unprecedented collaboration of 25 academic and industry groups contributing their data and time. It has provided a wealth of new knowledge on the genetics underlying endometriosis, which will help the research community in their efforts to come up with new treatments and possibly new ways of diagnosing the disease benefiting millions of women worldwide.'
The full paper, ' The genetic basis of endometriosis and comorbidity with other pain and inflammatory conditions ', is published in Nature Genetics .
DISCOVER MORE
- Support Oxford's research
- Partner with Oxford on research
- Study at Oxford
- Research jobs at Oxford
You can view all news or browse by category
- International edition
- Australia edition
- Europe edition
Researchers optimistic about potential new treatment for endometriosis
UK trial of first non-hormonal drug for condition may lead to ‘long overdue’ innovation in relieving often debilitating pain
- ‘Worse than childbirth’: women with endometriosis call for better treatments
Women will be given a potential new treatment for endometriosis in a groundbreaking clinical trial that doctors hope will pave the way for the first new class of drug for the condition in 40 years.
The trial will involve 100 women in Edinburgh and London and will assess whether the drug, dichloroacetate, helps relieve pain. If successful, it would be the first non-hormonal, non-surgical treatment for endometriosis, which affects roughly one in 10 women of reproductive age.
“We know women with endometriosis desperately want more treatment options and better ways to manage the often-debilitating pain that it causes,” said Dr Lucy Whitaker, a clinical lecturer in obstetrics and gynaecology at the University of Edinburgh, who is leading the research. “Our research so far shows promising results that dichloroacetate can make a huge difference. I hope our new trial will confirm this and give women hope that new treatments and a better quality of life are on the horizon.”
Endometriosis affects 1.5 million women in the UK and occurs when tissue similar to the lining of the womb grows elsewhere in the body. During a woman’s period, these cells bleed, causing inflammation, pain and the formation of scar tissue. A lack of awareness of the condition, compounded by the requirement for a diagnostic laparoscopy, means that women in the UK typically wait eight years for a diagnosis after first experiencing symptoms.
Current treatment options include conventional pain relief, hormonal contraceptives and surgery. However, hormone-based treatments – typically the pill or a contraceptive implant – have side-effects and are not suitable for everyone, including those trying to conceive. Surgery carries risks and is not always effective in the long term, with studies showing that about half of those who have surgery experience a return of symptoms within five years.
Janet Lindsay, the chief executive of Wellbeing of Women, a women’s health charity that is funding the trial with the Scottish government, said that progress in treating endometriosis was “long overdue”.
“It is completely unacceptable that there have been no new treatments for endometriosis in 40 years,” she said. “Too many women and girls are suffering from debilitating symptoms, such as chronic pelvic pain, fatigue and even fertility problems, and current hormonal and surgical treatments aren’t suitable for everyone.”
The latest trial builds on previous research showing that cells from the pelvic wall of women with endometriosis produce higher amounts of lactate, a potentially harmful waste product that is normally produced by muscles and red blood cells when the body is running low on oxygen during exercise. In endometriosis, lab-based experiments suggested the lactate was creating an environment that fuelled the development and growth of endometrial tissue.
When cells were treated with dichloroacetate, in the lab and in mouse experiments, lactate production decreased to normal levels and the size of the endometriosis lesions was reduced. The drug is already licensed as a medicine to treat rare childhood metabolic disorders and various cancers, meaning that it has an established safety profile. In a pilot study, with 30 women, the main side-effects were a slightly upset stomach on starting the medication and a tingling sensation in the fingers.
In the latest trial, which will start recruiting this autumn, half of the women will receive dichloroacetate and half will be given a placebo and they will take the tablets for 12 weeks. The participants will complete a series of questionnaires and give blood samples over the course of two-and-a-half years, to determine whether the treatment is effective for relieving pain and other symptoms.
Dr Ranee Thakar, the president of the Royal College of Obstetricians and Gynaecologists, welcomed the trial. “We know current endometriosis treatment options don’t work well for everyone, leaving many women with symptoms that can have a serious impact on their quality of life, affecting their physical and mental health,” she said. “We look forward to the results of this trial and it’s potential to improve the day-to-day lives of women and people living with endometriosis.”
- Endometriosis
- Women's health
- Medical research
Scientists map cellular changes linked to endometriosis
Women who were tall and lean in childhood more at risk of endometriosis – study

How to move: with endometriosis

Liz Lea: Red review – the pain and power of living with endometriosis
New Zealand introduces endometriosis guidelines for doctors
Endometriosis study 'sheds light on links to infertility' say scientists

Endometriosis: 20 things every woman (and every doctor) should know

'My endometriosis diagnosis took 20 years': readers on their struggles for help
Most viewed.
Clinical Trials
Endometriosis.
Displaying 11 studies
The purpose of this study is to examine the gene expression within endometriosis.
The purpose of this study is to map the female reproductive tract microbiome in patients with and without endometriosis to seek correlations of the microbiome to endometriosis, to determine if lower reproductive tract microbiome is predictive of upper reproductive tract changes by comparing composition of the microbiome in the lower and upper reproductive tracts, to explore immunologic differences in patients with and without endometriosis, compare presence of immunologic factors and cells between ectopic and eutopic endometrium, and to compare systemic immune system characteristics in patients with and without endometriosis using mass cytometry of peripheral blood samples.
The purpose of this study is to evaluate the diagnosis of endometriosis by gross visualization and compare to histologic evaluation during laparoscopic resection. By better understanding the polymorphic appearance of endometriosis, it will improve our diagnostic potential without having to rely on pathology. With this knowledge, it will improve patient centered care and possibly expedite treatment.
The objective of this study is to conduct a prospective randomized controlled trial of robotic-assisted versus conventional laparoscopy for the treatment of endometriosis.
The purpose of this study is to demonstrate the feasibility of F18- FES PET/MRI in patients with endometriosis, to establish the normal bio-distribution of F18- FES in pre-menopausal women, and to compare the diagnostic performance of F18- FES PET/MRI with that of conventional imaging (ultrasound and MRI alone) using surgical findings as the reference standard.
The purpose of this study is to analytically validate a cluster of specific biomarkers for endometriosis diagnosis and disease recurrence. This signature will be tested on endometrium and blood from 975 patients, divided in two groups : 550 patients affected by endometriosis and 225 patients unaffected (controls). EndoSearch is not about drug or medical device assessment but a research study for biomarker analytical validation purpose.
The purpose of this study is to investigate if complete pelvic peritonectomy is associated with improved quality of life in patients with chronic pelvic pain without histologic diagnosis of endometriosis as compared to patients with histologic diagnosis of endometriosis.
The purpose of this study is to investigate the value of ultrasound imaging of microscopic blood vessels as a new biomarker for identifying the difference between benign and cancerous uterine pathologies, and to compare ultrasound imaging to the diagnostics of MRI and (if available), surgical pathology.
The purpose of this study is to investigate the safety and effectiveness of FE 999049 compared to placebo in women aged 35-42 years old.
The purpose of this study is to investigate the safety and effectiveness of FE 999049 compared to placebo in women aged 18-34 years old.
The aim is the characterization of the microbiome of subjects experiencing benign reproductive tract disorders and its comparison to the microbiome present in individuals lacking that disorder.
Mayo Clinic Footer
- Request Appointment
- About Mayo Clinic
- About This Site
Legal Conditions and Terms
- Terms and Conditions
- Privacy Policy
- Notice of Privacy Practices
- Notice of Nondiscrimination
- Manage Cookies
Advertising
Mayo Clinic is a nonprofit organization and proceeds from Web advertising help support our mission. Mayo Clinic does not endorse any of the third party products and services advertised.
- Advertising and sponsorship policy
- Advertising and sponsorship opportunities
Reprint Permissions
A single copy of these materials may be reprinted for noncommercial personal use only. "Mayo," "Mayo Clinic," "MayoClinic.org," "Mayo Clinic Healthy Living," and the triple-shield Mayo Clinic logo are trademarks of Mayo Foundation for Medical Education and Research.
- Open access
- Published: 23 April 2024
Endometriosis in infertile women: an observational and comparative study of quality of life, anxiety, and depression
- Lilian Pagano Mori ORCID: orcid.org/0000-0002-0391-7231 1 ,
- Victor Zaia ORCID: orcid.org/0000-0003-2930-1870 1 , 2 ,
- Erik Montagna ORCID: orcid.org/0000-0001-6834-0261 1 ,
- Fabia Lima Vilarino ORCID: orcid.org/0000-0003-4088-1693 2 &
- Caio Parente Barbosa ORCID: orcid.org/0000-0002-2922-0264 1 , 2
BMC Women's Health volume 24 , Article number: 251 ( 2024 ) Cite this article
Metrics details
A women’s chances of getting pregnant decreases in cases of infertility, which may have several clinical etiologies. The prevalence of infertility is estimated as 10–15% worldwide. One of the causes of infertility is endometriosis, defined as the presence of an endometrial gland and/or stroma outside the uterus, inducing a chronic inflammatory reaction. Thus, infertility and endometriosis are diagnoses that significantly affect women’s mental health. This study accessed and compared the levels of depression, anxiety, and quality of life in infertile women with and without endometriosis.
was an observational and cross-sectional study which included 201 infertile women, 81 of whom were also diagnosed with endometriosis. The STROBE Guidelines was used. The data were collected using validated scales: Hamilton D Questionnaire, Beck Depression Inventory, and Fertility Quality of Life Questionnaire; The data were collected at the Ideia Fertil Institute (Santo Andre, Brazil), between February 28 and June 8, 2019.
the infertile women with endometriosis reported higher presence of depressive symptoms and a lower quality of life compared to women with infertility only. Similar presence of anxiety symptoms was observed regardless of being diagnosed with endometriosis. Women with infertility and endometriosis presented lower levels in quality-of-life domains when compared to women with infertility only - Mind and Body (58.33 × 79.17, p < 0.001), Relational (75 × 81.25, p = 0.009), Social (66.67 × 77.08, p = 0.001), Emotional (50.62 × 67.43, p < 0.001).
the findings indicate the need for increased psychosocial support care for women suffering from infertility and endometriosis to assist them in maintaining and managing their own mental health and achieving their reproductive goals.
Peer Review reports
Estimates show that healthy young women, under 25 years old, have the best chances of becoming pregnant, with a progressive decline in fertility ranging from 4.5% (25 years old) to 100% (50 years old) [ 1 ]. However, this percentage decreases greatly in cases of infertility, which may have several clinical etiologies [ 2 ]. The prevalence of infertility is estimated as 10–15% worldwide [ 3 ]. One of the causes of infertility is endometriosis, defined as the presence of an endometrial gland and/or stroma outside the uterus, inducing a chronic inflammatory reaction [ 4 ], with a prevalence ranging from 5 to 10% among women of reproductive age [ 5 ]. .
Women with infertility lose control over reproductive decisions and experience feelings of guilt, sadness, shame, and social isolation [ 6 , 7 ]. These feelings reduce quality of life and negatively affect mental health [ 8 , 9 ]. The relationship between endometriosis and infertility is expressive, about 40% of women with endometriosis are infertile, and between 25% and 50% of infertile women have endometriosis [ 10 ]. In addition, clinical symptoms of endometriosis such as menstrual irregularity, chronic pelvic pain (CPP), dysmenorrhea, and dyspareunia can emotionally affect patients [ 11 , 12 ].
Some of the disorders associated with endometriosis include depression and anxiety [ 12 , 13 ]. A meta-analysis indicated that the magnitude of the difference in the occurrence of these two symptoms between healthy women and those with endometriosis is 0.71 for depression and 0.60 for anxiety, with both showing greater prevalence in the group of women with endometriosis [ 14 ]. Another study conducted in the United Kingdom with data from 202,276 women found that the group with endometriosis had a higher prevalence of depression (9.8%) and anxiety (3.6%) compared to the group of healthy women [ 15 ]. Additionally, endometriosis can impair women’s functional capacity [ 16 ], particularly in cases with dyspareunia [ 17 ].
Consequently, women diagnosed with endometriosis experience a reduction in quality of life (QoL) [ 18 , 19 , 20 ], which is defined as an individual’s perception of their own life, taking into account their cultural background, values, aspirations, and expectations [ 21 , 22 , 23 ]. A study [ 24 ] comparing QoL levels between healthy women and those with endometriosis revealed an average decrease of 30 points in QoL among participants with endometriosis.
Previous studies [ 9 , 11 , 12 , 13 , 25 ] demonstrated that endometriosis and infertility negatively affect QoL and favor increased levels of anxiety and depression. To enable a more personalized and specific understanding of this demographic, this study uniquely identified and compared anxiety, depression, and QoL levels among infertile women both with and without endometriosis, while also examining the correlations between these variables.
Participants and setting
This was an observational and cross-sectional. Internationally validated and self-applicable scales were used. This study used the STROBE [ 26 ] for the reporting of observational studies.
Sample size was calculated using the G*Power software, a significance value of 5% and a minimum test power of 95% were used. The analysis indicated a minimum of 71 participants per group. A larger number of participants were invited to ensure the minimum number was met, accounting for possible participant loss. The participants were subdivided into two groups: Comparator group (A): 120 patients with infertility diagnosis only, and Endometriosis group (B): 81 patients with infertility and endometriosis diagnosed by video-laparoscopy and confirmed with histopathology. Patients included were at the earlier stage of the treatment, after the first consultation or during the clinical testing before the first ovulatory induction cycle and in their first assisted reproduction treatment.
This study was conducted at the Ideia Fertil Reproductive Health Institute, located in São Paulo, Brazil. The sample was characterized as non-probabilistic type. The data were collected between February 28 and June 8, 2019. A total of 230 women were invited to participate. However, 29 of these women declined their participation, indicating no interest or no time. There were 201 infertile women who met the inclusion criteria: [ 1 ]age equal to or above 18 years and [ 2 ]diagnosis of infertility. The exclusion criteria were: [ 1 ]diagnosis of a psychiatric disorder [ 2 ], psychotherapy in the last six months [ 3 ], psychotropic medication in the last six months [ 4 ], history of fibromyalgia [ 5 ], neuropathy [ 6 ], osteopathy, and [ 7 ]presence of malignant tumors. The participants were invited in person and individually exclusively by the author LPM to reduce possible biases while they waited for a medical consultation at that Institute.
Sociodemographic Questionnaire - developed ad-hoc for this study, included questions to characterize the participants, such as age, partner’s age, infertility time.
Fertility Quality of Life (FertiQol) [ 23 ] − 26 items in four domains: Mind-Body, Relational, Social, and Emotional. The answers are on a five-point Likert scale. Higher scores mean higher QoL. The Brazilian version utilized in this study is official and accessible on the authors’ website (Cardiff University), which was adapted from the Portuguese language validation process [ 27 ]. Cronbach’s Alpha of the Fertiqol was 0.921.
Hospital Anxiety and Depression Scale (HADS), validated in Brazilian Portuguese [ 28 ] − 14 items, seven of which cover anxiety symptoms and seven cover depression. Each question is scored on a scale (0–3), composing a maximum score of 21 points for each scale. Higher scores indicate higher levels of anxiety and depression, and the scale has a cutoff: up to or equal to seven points indicates no anxiety/depression, and eight or higher points indicates the presence of anxiety/depression. Cronbach’s Alpha of the HADS (alpha = 0.809).
Beck Depression Inventory II (BDI-II), validated in Brazilian Portuguese [ 29 ] - measures depressive symptoms and consists of 21 items, each corresponding to a specific category of symptoms and attitude, such as sadness, pessimism, loss of pleasure, guilty feelings, and other aspects [ 23 ]. Each question is scored on a scale (0–3), with a total score ranging from 0 to 63. A score of 0–10 points indicates no depressive symptoms, 11–63 points indicates the presence of depressive symptoms. Cronbach’s Alpha of the BDI (alpha = 0.877).
Statistical analysis
R 4.2.1 used for data transcription and analysis. Basic and Psych Packages were performed. The data were independently typed by two researchers (LPM and VZ) and then combined to avoid transcription errors. Missing data were checked and not found. The distribution of normality of continuous variables was verified using the Kolmogorov-Smirnov test. For the aim of identifying and describing the sample, levels of anxiety, depression, and QoL, we conducted descriptive statistical analyses (e.g., percentile, mean/median) for each group (A and B).
Reliability measures of the psychometric scales were verified using the Cronbach’s Alpha with a rigorous value (cutoff ≥ 0.80) [ 30 ], which indicated the exclusion of the depression dimension in HADS (alpha = 0.787).
For comparing variables between groups, we conducted the chi-square test for categorical variables (e.g., presence of anxiety and group) and the Mann-Whitney U test for subgroups comparison (A and B). The FertiQoL emotional domain was the only variable showing a normal distribution, for which the t-test was applied to compare groups. Additionally, to explore correlations between study variables, we conducted Spearman correlation analysis (for continuous scoring of psychometric variables – QoL, anxiety, and depression).
A significance value of 5% was used. Correlation and Cohen coefficient values were considered as small (< 0.30), medium (0.30–0.49), or large (≥ 0.50) [ 31 ].
The population was subdivided into two groups: 120 patients (59.7%) were allocated to group A (with exclusive diagnosis of infertility) and 81 patients (40.3%) to group B (with diagnosis of infertility and endometriosis). The groups were homogeneous for all sociodemographic variables tested: age (34.61 ±4.78), infertility time (4.43 ±3.11), partner’s age (36.71 ±6.31) and primary infertility (90.5%) (Table 1 ).
A significant difference was observed between groups A and B for levels of depressive symptoms ( p = 0.002) and anxiety ( p = 0.026), being greater for group B, (infertility and endometriosis). Both groups showed statistically significant differences in relation to QoL, with group A having better levels in all areas of QoL. Moreover, the effect sizes between the groups were significant, except for anxiety, indicating a medium effect for depression (higher levels in Group B), QoL Relation and Social (both with higher scores for Group A), and a large effect for QoL Mind and Body, and Emotional (both with higher scores in Group A) – as shown in Table 2 .
Correlations between the psychometric variables studied were verified, all of which were significant ( p ≤ 0.001), indicating inverse correlations of moderate level between the relational domain in FertiQoL and anxiety (rho= -0.360) and depression (rho= -0.412), and between the social domain in FertiQoL and anxiety (rho= -0.420). The other correlations between depression, anxiety, and the domains of QoL remained inverse and strong. Depression and anxiety were positively highly correlated (rho = 0.620).
Considering the division between Group A and B, a stronger inverse correlation between depressive symptoms and quality of life is observed in the group with endometriosis compared to the group with infertility only (Table 3 ).
Summary of findings
This study measured QoL, and depressive and anxiety symptoms in women with infertility, verifying the possible impact between the psychological variables and the double diagnosis: infertility and endometriosis. The findings indicate that women with an overlapping diagnosis (endometriosis-infertility) have higher levels of depressive symptoms and lower QoL than women with infertility only. In addition, lower QoL levels were related to higher levels of anxiety and depressive symptoms.
Data from the literature suggests that sociodemographic variables (e.g., age, infertility duration, partner’s age, and type of infertility) may influence QoL, anxiety, and depression [ 24 , 32 , 33 ], potentially introducing confounding factors in the psychometric measures used [ 34 , 35 ]. However, since our groups did not show differences in these variables, we suggest that they may not have been determining factors for the differences found in this study.
The levels of depressive symptoms found were higher than those in the general population, estimated at 4.4% according to a study by the World Health Organization [ 16 ], corresponding to less than a quarter of the presence of depressive symptoms in the population studied. Furthermore, higher levels of depressive symptoms were observed in participants with both diagnoses: endometriosis and infertility. A similar result was found in a study involving women with endometriosis, which demonstrated a correlation between depression and various comorbidities, including infertility, indicating a stronger link between depressive symptoms and the diagnosis of infertility in women with endometriosis than with other morbidities [ 36 ]. Additionally, such findings may be corroborated by the influence of clinical symptoms of endometriosis, beyond infertility, on an individual’s mental health [ 19 , 20 , 23 ].
QoL levels in both groups were lower than those of the general population, consistent with previous studies examining the impact of infertility on QoL [ 37 , 38 ]. Specifically, lower QoL levels were observed in infertile participants with endometriosis compared to infertile women without endometriosis, aligning with prior research that identifies endometriosis as a factor exacerbating the decline in quality of life and mental health [ 3 , 8 , 32 , 39 , 40 , 41 , 42 ]. This further supports a trend in the group with infertility alone towards higher quality of life and reduced levels of depressive and anxiety symptoms, as evidenced in this study through Cohen’s d, when contrasted with the group of women with endometriosis and infertility.
Endometriosis and infertility are associated with clinical conditions that cause emotional morbidity, affecting social, sexual, and professional lives [ 4 , 43 ]. The unregulated immune and inflammatory reactions of endometriosis, which generate CPP, may explain a higher QoL decrease, and more depressive symptoms compared to women with only infertility [ 12 ]. To partially restore this impairment, clinical or surgical treatment has proven to be effective in relieving pain [ 32 ], but emotional aspects must also be respected and treated by specialists, such as psychologists [ 20 ].
Another potential explanation for the correlation between low QoL and mental health in the group with endometriosis and infertility is the connection of endometriosis with psychological factors [ 33 , 36 ], such as perceived pain and stress, sleep quality [ 33 , 44 ], anxiety, and depression [ 37 , 45 ].
Moderate correlations were found between the emotional domain of QoL and depression, with a stronger correlation observed in the infertility with endometriosis group compared to the infertility group alone. This reinforces the connection between impaired mental health and reduced quality of life in situations of heightened anxiety and depression, as seen in infertile women with endometriosis. The findings also indicate that higher levels of anxiety and depression are linked to lower QoL, consistent with previous studies investigating these variables [ 12 , 25 , 45 ].
There are few studies [ 19 , 25 , 36 ] that address psychological aspects in women with both infertility and endometriosis diagnoses, and this study contributes to that field. The data indicates that infertile women with endometriosis exhibit more severe depressive symptoms, anxiety, and decreased quality of life compared to women solely diagnosed with infertility.
Clinical implications
These results emphasize the relevance of patient-centered education and psychological support for women struggling with endometriosis and infertility to help them manage possible mental health problems and achieve their reproductive goals successfully [ 13 , 45 ]. Thus, is it possible to question what changes in the reproductive treatment routine which may provide support to patients with infertility and endometriosis. Based on our findings, one of the possibilities would be to include a psychologist in the reproductive team to support patients in maintaining or re-establishing their mental health.
Strengths and limitations
Some limitations of the present study need to be discussed. First, the study population comprised women diagnosed with infertility and endometriosis at various time intervals since diagnosis. This variation could potentially influence the overall levels of anxiety, quality of life, and depression examined [ 8 , 37 ], thereby limiting the generalizability of findings to similar populations. Future studies should differentiate the time of diagnosis of each participant. Second, the numerical difference between the groups of infertile women with and without endometriosis is a limiting factor, which hinders comparisons between the two sub-samples. However, the statistical tests used account for these differences in sample sizes, as well as satisfying the minimum sample size outlined by the power calculation.
The use of validated instruments to measure QoL, anxiety and depression in patients is an important strength of the present study, allowing a robust and internationally comparable measurement [ 11 , 18 , 46 , 47 ], which is important particularly when considering populations with higher vulnerability to psychiatric disorders such as individuals with infertility [ 29 , 30 , 37 , 48 ] and endometriosis [ 23 , 28 , 39 ]. Another important aspect of this study was differentiating the variables studied for the diagnostic overlap between endometriosis and infertility, which improves the knowledge of the emotional aspects of these populations, considering the high co-occurrence of infertility and endometriosis [ 10 ]. Additionally, the findings provide information that supports better emotional support and care in reproductive treatment.
In conclusion, QoL in infertile women is impaired by increased depressive symptoms and anxiety. Compared to women exclusively diagnosed with infertility, infertile women with endometriosis are characterized by a significantly worse emotional state in terms of depressive symptoms and QoL. This suggests the need for care and emotional support in infertility management, especially when associated with endometriosis.
Data availability
The data of the present study can be requested from the correspondence author.
Abbreviations
II-Beck Depression Inventory II
chronic pelvic pain
Fertility Quality of Life
Hospital Anxiety and Depression Scale
Quality of Life
Vander Borght M, Wyns C. Fertility and infertility: definition and epidemiology. Clin Biochem. 2018;62:2–10.
Article PubMed Google Scholar
Tarin JJ, Garcia-Perez MA, Hamatani T, Cano A. Infertility etiologies are genetically and clinically linked with other diseases in single meta-diseases. Reproductive Biology Endocrinology: RB&E. 2015;13:31.
Article Google Scholar
WHO WHO. Reproductive health indicators: guidelines for their generation, interpretation and analysis for global monitoring. 2006.
Greil AL, Slauson-Blevins KS, Lowry MH, McQuillan J. Concerns about treatment for infertility in a probability-based sample of US women. J Reprod Infant Psychol. 2019:1–9.
Taylor HS, Kotlyar AM, Flores VA. Endometriosis is a chronic systemic disease: clinical challenges and novel innovations. Lancet (London England). 2021;397(10276):839–52.
Article CAS PubMed Google Scholar
Kamel RM. Management of the infertile couple: an evidence-based protocol. Volume 8. Reproductive biology and endocrinology: RB&E; 2010. p. 21.
Google Scholar
Rockliff HE, Lightman SL, Rhidian E, Buchanan H, Gordon U, Vedhara K. A systematic review of psychosocial factors associated with emotional adjustment in in vitro fertilization patients. Hum Reprod Update. 2014;20(4):594–613.
Chachamovich J, Chachamovich E, Fleck MP, Cordova FP, Knauth D, Passos E. Congruence of quality of life among infertile men and women: findings from a couple-based study. Hum Reprod (Oxford England). 2009;24(9):2151–7.
Article CAS Google Scholar
G PG, MG VH CVMZ. C C, CP B. Sexual Satisfaction among Involuntarily Childless Women: A Cross-Cultural Study in Italy and Brazil. 2016.
Evans MB, Decherney AH. Fertility and endometriosis. Clin Obstet Gynecol. 2017;60(3):497–502.
Young K, Fisher J, Kirkman M. Clinicians’ perceptions of women’s experiences of endometriosis and of psychosocial care for endometriosis. Aust N Z J Obstet Gynaecol. 2017;57(1):87–92.
Lagana AS, La Rosa VL, Rapisarda AMC, Valenti G, Sapia F, Chiofalo B, et al. Anxiety and depression in patients with endometriosis: impact and management challenges. Int J Womens Health. 2017;9:323–30.
Article PubMed PubMed Central Google Scholar
Facchin F, Barbara G, Dridi D, Alberico D, Buggio L, Somigliana E, et al. Mental health in women with endometriosis: searching for predictors of psychological distress. Hum Reprod (Oxford England). 2017;32(9):1855–61.
van Barneveld E, Manders J, van Osch FHM, van Poll M, Visser L, van Hanegem N, et al. Depression, anxiety, and correlating factors in endometriosis: a systematic review and Meta-analysis. J Womens Health (Larchmt). 2022;31(2):219–30.
Koller D, Pathak GA, Wendt FR, Tylee DS, Levey DF, Overstreet C, et al. Epidemiologic and genetic associations of Endometriosis with Depression, anxiety, and eating disorders. JAMA Netw Open. 2023;6(1):e2251214.
WHO WHO. Depression and other Common Mental disorders: Global Health estimates. Geneve: WHO; 2017. p. 23.
Pessoa de Farias Rodrigues M, Lima Vilarino F, de Souza Barbeiro Munhoz A, da Silva Paiva L, de Alcantara Sousa LV, Zaia V, et al. Clinical aspects and the quality of life among women with endometriosis and infertility: a cross-sectional study. BMC Womens Health. 2020;20(1):124.
Aarts JW, van Empel IW, Boivin J, Nelen WL, Kremer JA, Verhaak CM. Relationship between quality of life and distress in infertility: a validation study of the Dutch FertiQoL. Hum Reprod (Oxford England). 2011;26(5):1112–8.
Mori L, Vilarino F, Pádua M, Zaia V, Barbosa C. Depression and quality of life in patients with endometriosisand infertility. Fertil Steril. 2017.
Brasil DL, Montagna E, Trevisan CM, La Rosa VL, Lagana AS, Barbosa CP, et al. Psychological stress levels in women with endometriosis: systematic review and meta-analysis of observational studies. Minerva Med. 2020;111(1):90–102.
WHOQOL-GROUP. The World Health Organization Quality of Life assessment (WHOQOL): position paper from the World Health Organization. Social science & medicine. (1982). 1995;41(10):1403-9.
Fleck MP, Louzada S, Xavier M, Chachamovich E, Vieira G, Santos L, et al. [Application of the Portuguese version of the abbreviated instrument of quality life WHOQOL-bref]. Rev Saude Publica. 2000;34(2):178–83.
Boivin J, Takefman J, Braverman A. The fertility quality of life (FertiQoL) tool: development and general psychometric properties. Hum Reprod (Oxford England). 2011;26(8):2084–91.
Rossi V, Tripodi F, Simonelli C, Galizia R, Nimbi FM. Endometriosis-associated pain: a review of quality of life, sexual health and couple relationship. Minerva Obstet Gynecol. 2021;73(5):536–52.
Wu MH, Su PF, Chu WY, Lin CW, Huey NG, Lin CY, et al. Quality of life among infertile women with endometriosis undergoing IVF treatment and their pregnancy outcomes. J Psychosom Obstet Gynaecol. 2021;42(1):57–66.
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of Observational studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495–9.
Melo C, Gameiro S, Canavarro MC, Boivin J. Does the FertiQoL assess quality of life? Results from the validation of the Portuguese version of the FertiQoL. Hum Reprod. 2012;27.
Freire MÁ, Figueiredo VLMd, Gomide A, Jansen K, Silva RA. Da MPdS. [Hamilton Scale: study of the psychometric characteristics in a sample from Southern Brazil]. Jornal Brasileiro De Psiquiatria. 2014;63(4):281–9.
Gomes-Oliveira MH, Gorenstein C, Lotufo Neto F, Andrade LH, Wang YP. Validation of the Brazilian Portuguese version of the Beck Depression Inventory-II in a community sample. Braz J Psychiatry. 2012;34(4):389–94.
Streiner D, Norman G, Cairney J. Health measurement scales: a practical guide to their development and use (5th edition). Australian and New Zealand journal of public health. 2016;40(3):294-5.
Cohen J. Statistical power analysis for the behavioral sciences. New York: Academic; 2013.
Book Google Scholar
Matasariu RD, Mihaila A, Iacob M, Dumitrascu I, Onofriescu M, Crumpei Tanasa I, et al. Psycho-Social aspects of Quality of Life in women with endometriosis. Acta Endocrinol (Buchar). 2017;13(3):334–9.
Rees M, Kiemle G, Slade P. Psychological variables and quality of life in women with endometriosis. J Psychosom Obstet Gynaecol. 2020:1–8.
Liu J, Brodley CE, Healy BC, Chitnis T. Removing confounding factors via constraint-based clustering: an application to finding homogeneous groups of multiple sclerosis patients. Artif Intell Med. 2015;65(2):79–88.
Choi BY. Instrumental variable estimation of truncated local average treatment effects. PLoS ONE. 2021;16(4):e0249642.
Article CAS PubMed PubMed Central Google Scholar
Skegro B, Bjedov S, Mikus M, Mustac F, Lesin J, Matijevic V, et al. Endometriosis, Pain and Mental Health. Psychiatria Danubina. 2021;33(Suppl 4):632–6.
PubMed Google Scholar
Terzioglu F, Turk R, Yucel C, Dilbaz S, Cinar O, Karahalil B. The effect of anxiety and depression scores of couples who underwent assisted reproductive techniques on the pregnancy outcomes. Afr Health Sci. 2016;16(2):441–50.
Ashraf DM, Ali D, Azadeh DM. Effect of infertility on the quality of life, a cross- sectional study. J Clin Diagn Research: JCDR. 2014;8(10):OC13–5.
Chen LC, Hsu JW, Huang KL, Bai YM, Su TP, Li CT, et al. Risk of developing major depression and anxiety disorders among women with endometriosis: a longitudinal follow-up study. J Affect Disord. 2016;190:282–5.
Pope CJ, Sharma V, Sharma S, Mazmanian D. A Systematic Review of the Association between Psychiatric Disturbances and endometriosis. Journal of obstetrics and gynaecology Canada: JOGC = Journal d’obstetrique. et gynecologie du Can : JOGC. 2015;37(11):1006–15.
Kocas HD, Rubin LR, Lobel M. Stigma and mental health in endometriosis. Eur J Obstet Gynecol Reprod Biol X. 2023;19:100228.
Thiel PS, Bougie O, Pudwell J, Shellenberger J, Velez MP, Murji A. Endometriosis and mental health: a population-based cohort study. Am J Obstet Gynecol. 2024.
Cavaggioni G, Lia C, Resta S, Antonielli T, Benedetti Panici P, Megiorni F, et al. Are mood and anxiety disorders and alexithymia associated with endometriosis? A preliminary study. Biomed Res Int. 2014;2014:786830.
Jia SZ, Leng JH, Shi JH, Sun PR, Lang JH. Health-related quality of life in women with endometriosis: a systematic review. J Ovarian Res. 2012;5(1).
Massarotti C, Gentile G, Ferreccio C, Scaruffi P, Remorgida V, Anserini P. Impact of infertility and infertility treatments on quality of life and levels of anxiety and depression in women undergoing in vitro fertilization. Gynecol Endocrinology: Official J Int Soc Gynecol Endocrinol. 2019;35(6):485–9.
Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica. 1983;67(6):361–70.
Thomas FS, Stanford JB, Sanders JN, Gurtcheff SE, Gibson M, Porucznik CA, et al. Development and initial validation of a fertility experiences questionnaire. Reprod Health. 2015;12:62.
Raguz N, McDonald SW, Metcalfe A, O’Quinn C, Tough SC. Mental health outcomes of mothers who conceived using fertility treatment. Reprod Health. 2014;11(1):19.
Download references
Acknowledgements
We would like to thank the participants who took part in the study and the research team.
This work was supported by the FAPESP under Grant 2019/17853-2.
Author information
Authors and affiliations.
Programa de Pós-graduação em Ciências da Saúde, Centro Universitário FMABC, Av. Lauro Gomes, Santo André, 2000, 09060-870, SP, Brazil
Lilian Pagano Mori, Victor Zaia, Erik Montagna & Caio Parente Barbosa
Instituto Ideia Fertil de Saúde Reprodutiva, , Santo Andre - SP, Brasil
Victor Zaia, Fabia Lima Vilarino & Caio Parente Barbosa
You can also search for this author in PubMed Google Scholar
Contributions
VZ, LPM, FLV, CPB, conceived and designed the study. VZ and EM analyzed the data and drafted the manuscript. VZ, EM and LPM interpreted the data and criticized the manuscript for important intellectual content. All authors have read and approved the final version of the manuscript. This article is the work of the authors. All authors had full access to all the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Correspondence to Victor Zaia .
Ethics declarations
Ethics approval and consent to participate, informed consent.
was obtained from all subjects, and all participated voluntarily. Anonymity was assured. This study was approved by the Research Ethics Committee of Centro Universitario FAMBC (Number: 999.283/2015) and all assessments were in accordance with The Helsinki Declaration.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Authors’ information
LPM is an obstetric gynecologist, specialized in Assisted Human Reproduction (AHR) and has a master’s in science in Health Science. VZ is a psychologist, a specialist in psychometrics, data analysis and has a PhD in Health Psychology. EM is a pharmacist and biochemist, specialist in health data analysis, holds a PhD in Biological Science with an emphasis on education. FLV is an obstetric gynecologist, specialized in AHR and holds a PhD in Health Science. CPB is an obstetric gynecologist, specialized in AHR and holds a PhD in Medicine.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Mori, L.P., Zaia, V., Montagna, E. et al. Endometriosis in infertile women: an observational and comparative study of quality of life, anxiety, and depression. BMC Women's Health 24 , 251 (2024). https://doi.org/10.1186/s12905-024-03080-5
Download citation
Received : 30 June 2023
Accepted : 07 April 2024
Published : 23 April 2024
DOI : https://doi.org/10.1186/s12905-024-03080-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Endometriosis
- Infertility
- Quality of life
BMC Women's Health
ISSN: 1472-6874
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Determinants of Conception and Adverse Pregnancy Outcomes in Women with Endometriosis: A Longitudinal Study
- Endometriosis: Short Communications
- Published: 23 April 2024
Cite this article

- Hrishikesh Munshi ORCID: orcid.org/0000-0002-6808-5368 1 ,
- Tabassum Khan ORCID: orcid.org/0009-0009-5308-4969 1 ,
- Shagufta Khan 1 ,
- Pramathes DasMahapatra 2 ,
- Sheila Balakrishnan 3 ,
- Chelana Nirmala 4 ,
- Vinita Das 5 ,
- Ketki Kulkarni 6 ,
- Bimal M John 7 ,
- Amiya Majumdar 2 ,
- CV Sowmini 4 ,
- Aarti Srivastava 5 ,
- Komal Khade 8 &
- Rahul K Gajbhiye ORCID: orcid.org/0000-0003-3864-1818 1
Endometriosis, affecting approximately 10% of reproductive-aged women globally, poses significant challenges, including chronic pelvic pain, dysmenorrhea, and infertility. In low- and middle-income countries like India, accessibility to affordable infertility care remains a concern. This multicenter prospective cohort study, conducted across six tertiary care hospitals in India from 2017 to 2022, aims to explore the natural progression of conception and pregnancy outcomes in women with endometriosis. Of the 257 participants, 19.1% conceived during the study, revealing significant geographic and income-based variations ( p < 0.001, p = 0.01). Dysmenorrhea ( p < 0.001) and dyspareunia ( p =0.027) were correlated with conception, while no such associations were found with chronic pelvic pain or menstrual factors. Lesion type, number, and severity showed no conclusive link with conception. Natural conception occurred in 70% of cases, with an average post-surgery conception time of 282.1 days. Live birth rate was 85.7%, while complications included placenta previa (16.4%), preeclampsia (4.1%), and preterm births (4.1%). This study, one of the first in India on endometriosis-related fertility progression, emphasizes the need for comprehensive understanding and management of conception and pregnancy outcomes. Considering India's substantial endometriosis burden, the study recommends prioritizing larger multicenter investigations for a better understanding and effective strategies for infertility management.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Data Availability
The data that support the findings of this study are available from the corresponding author (RKG), upon reasonable request. Data containing information that could compromise participant identity and confidentiality will not be shared.
Abbreviations
Artificial Reproductive Technology
Deep Infiltrating Endometriosis
Endometriosis Fertility Index
Gestational Diabetes Mellitus
Institutional Ethics Committee
Intrauterine Growth Restriction
Ovarian Endemetrioma
Placenta previa
Revised American Fertility Society
Superficial Peritoneal Endometriosis
World Endometriosis Research Foundation Endometriosis Phenome and Biobanking Harmonisation Project
Gajbhiye RK, Montgomery G, Pai MV, Phukan P, Shekhar S, Padte K, et al. Protocol for a case-control study investigating the clinical phenotypes and genetic regulation of endometriosis in Indian women: the ECGRI study. BMJ Open. 2021;11:e050844. https://doi.org/10.1136/bmjopen-2021-050844 .
Article PubMed PubMed Central Google Scholar
Macer ML, Taylor HS. Endometriosis and Infertility. Obstet Gynaecol Clin N Am. 2012;39:535–49. https://doi.org/10.1016/j.ogc.2012.10.002 .
Article Google Scholar
Farland LV, Prescott J, Sasamoto N, Tobias DK, Gaskins AJ, Stuart JJ, et al. Endometriosis and Risk of Adverse Pregnancy Outcomes. Obstet Gynecol. 2019;134:527–36. https://doi.org/10.1097/AOG.0000000000003410 .
Saraswat L, Ayansina D, Cooper K, Bhattacharya S, Miligkos D, Horne A, et al. Pregnancy outcomes in women with endometriosis: a national record linkage study. BJOG: Int J Obstet Gy. 2017;124:444–52. https://doi.org/10.1111/1471-0528.13920 .
Article CAS Google Scholar
Bhurke AV, DasMahapatra P, Balakrishnan S, Khan SA, Mortlock S, Das V, et al. Clinical characteristics and surgical management of endometriosis-associated infertility: A multicenter prospective cohort study. Int J Gynaecol Obstet. 2022;159:86–96. https://doi.org/10.1002/ijgo.14115 .
Tahmasbi Rad M, Akpinar-Isci D, Nobs T, Gasimli K, Becker S. Pregnancy after laparoscopic surgery for endometriosis: How long should we wait? A retrospective study involving a long-term follow up at a university endometriosis center. Intl J Gynecology & Obste. 2023;163:108–14. https://doi.org/10.1002/ijgo.14849 .
Qiao X, Wu L, Liu D, Pei T, Huang W. Existence of chronic endometritis and its influence on pregnancy outcomes in infertile women with minimal/mild endometriosis. Intl J Gynecology & Obste. 2023;160:628–34. https://doi.org/10.1002/ijgo.14326 .
Matsuzaki S, Nagase Y, Ueda Y, Lee M, Matsuzaki S, Maeda M, et al. The association of endometriosis with placenta previa and postpartum hemorrhage: a systematic review and meta-analysis. Am J Obstet Gynecol MFM. 2021;3:100417. https://doi.org/10.1016/j.ajogmf.2021.100417 .
Article CAS PubMed Google Scholar
Drummond K, Danesh NM, Arseneault S, Rodrigues J, Tulandi T, Raina J, et al. Association between Endometriosis and Risk of Preeclampsia in Women Who Conceived Spontaneously: A Systematic Review and Meta-analysis. J Minimal Invasive Gynecol. 2023;30:91–9. https://doi.org/10.1016/j.jmig.2022.11.008 .
Salmeri N, Li Piani L, Cavoretto PI, Somigliana E, Viganò P, Candiani M. Endometriosis increases the risk of gestational diabetes: a meta-analysis stratified by mode of conception, disease localization and severity. Sci Rep. 2023;13:8099. https://doi.org/10.1038/s41598-023-35236-y .
Article CAS PubMed PubMed Central Google Scholar
Brønd M, Breintoft K, Forman A, Henriksen TB, Ramlau-Hansen CH, Rytter D, et al. Measures of fetal growth and preterm birth and risk of endometriosis and adenomyosis in adult life: a systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2023;102:986–99. https://doi.org/10.1111/aogs.14594 .
Salmeri N, Farina A, Candiani M, Dolci C, Bonavina G, Poziello C, et al. Endometriosis and Impaired Placentation: A Prospective Cohort Study Comparing Uterine Arteries Doppler Pulsatility Index in Pregnancies of Patients with and without Moderate-Severe Disease. Diagnostics (Basel). 2022;12:1024. https://doi.org/10.3390/diagnostics12051024 .
Scala C, Leone Roberti Maggiore U, Racca A, Barra F, Vellone VG, Venturini PL, et al. Influence of adenomyosis on pregnancy and perinatal outcomes in women with endometriosis. Ultrasound Obstet Gynecol. 2018;52:666–71. https://doi.org/10.1002/uog.18989 .
Didziokaite G, Biliute G, Gudaite J, Kvedariene V. Oxidative Stress as a Potential Underlying Cause of Minimal and Mild Endometriosis-Related Infertility. Int J Mol Sci. 2023;24:3809. https://doi.org/10.3390/ijms24043809 .
Buyalos RP, Agarwal SK. Endometriosis-associated infertility. Curr Opin Obstet Gynecol. 2000;12:377–81. https://doi.org/10.1097/00001703-200010000-00006 .
Download references
Acknowledgement
The authors thank Dr. Smita Mahale, Dr. Geetanjali Sachdeva, Dr. Neeta Warty, Dr Shahina Begum, Dr. Periyasamy Kuppusamy, Ms. Aishwarya Bhurke, Ms. Merlin Pious, Ms. Sana Shaikh, Ms. Akhila S., staff of HRRC Lucknow, and staff of HRRC Trivandrum. Data collection was facilitated by and conducted in compliance with the World Endometriosis Research Foundation Endometriosis Phenome and Biobanking Harmonization Project (WERF EPHect). Rahul K Gajbhiye is an awardee of the DBT Wellcome Trust India Alliance Clinical and Public Health Intermediate Fellowship (Grant no. IA/CPHI/18/1/503933).
The study was partially funded by Department of Science and Technology, Government of India (EEQ/2016/000206), and ICMR–National Institute for Research in Reproductive and Child Health, Mumbai (ICMR-NIRRCH/MS/RA/1613/09–2023). The funders had no role in designing the study; in the collection, analysis and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication.
Author information
Authors and affiliations.
Clinical Research Laboratory, ICMR-National Institute for Research in Reproductive and Child Health, Mumbai, India
Hrishikesh Munshi, Tabassum Khan, Shagufta Khan & Rahul K Gajbhiye
Spectrum Clinic and Endoscopy Research Institute, Kolkata, India
Pramathes DasMahapatra & Amiya Majumdar
Department of Reproductive Medicine, Sree Avittam Thirunal (SAT) Hospital, Thiruvananthapuram, India
Sheila Balakrishnan
Department of Obstetrics and Gynecology, Sree Avittam Thirunal (SAT) Hospital, Thiruvananthapuram, India
Chelana Nirmala & CV Sowmini
Department of Obstetrics and Gynecology, King George’s Medical University (KGMU), Lucknow, India
Vinita Das & Aarti Srivastava
Nowrosjee Wadia Maternity Hospital, Mumbai, India
Ketki Kulkarni
Minimally Invasive Surgery Unit, Credence Hospital - Multispecialty Women’s Hospital and IVF Center, Thiruvananthapuram, India
Bimal M John
Molecular Endocrinology Laboratory, ICMR-National Institute for Research in Reproductive and Child Health, Mumbai, India
Komal Khade
You can also search for this author in PubMed Google Scholar
Contributions
Rahul K. Gajbhiye had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Conceptualization and funding acquisition: Rahul K. Gajbhiye. Investigation at respective centers: Pramathes Das Mahapatra, Sheila Balakrishnan, Chelana Nirmala, Vinita Das, Ketki Kulkarni, Bimal M John, Amiya Majumdar, CV Sowmini, Aarti Srivastava. Study coordination: Shagufta Khan, Komal Khade, Rahul K. Gajbhiye. Literature review and data analysis: Hrishikesh Munshi, Tabassum Khan. Preparation of original draft of manuscript: Hrishikesh Munshi, Tabassum Khan, Rahul K. Gajbhiye. Revision, editing and approval of final version of manuscript for submission: Hrishikesh Munshi, Tabassum Khan, Shagufta Khan, Pramathes DasMahapatra, Sheila Balakrishnan, Chelana Nirmala, Vinita Das, Ketki Kulkarni, Bimal M John, Amiya Majumdar, CV Sowmini, Aarti Srivastava, Komal Khade, Rahul K Gajbhiye.
Corresponding author
Correspondence to Rahul K Gajbhiye .
Ethics declarations
Ethics approval.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Institutional Ethics Committee (IEC) of the ICMR-National Institute for Research in Reproductive and Child Health, Mumbai (D/ICEC/Sci-106/143/2016) as well as by the IECs of all participating study sites. Written informed consent were obtained from all participating women.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
(DOCX 30 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Munshi, H., Khan, T., Khan, S. et al. Determinants of Conception and Adverse Pregnancy Outcomes in Women with Endometriosis: A Longitudinal Study. Reprod. Sci. (2024). https://doi.org/10.1007/s43032-024-01569-w
Download citation
Received : 14 December 2023
Accepted : 18 April 2024
Published : 23 April 2024
DOI : https://doi.org/10.1007/s43032-024-01569-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Endometriosis
- Infertility
- Multicenter study
- Pregnancy outcomes
- Find a journal
- Publish with us
- Track your research
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts

Endometriosis: Epidemiology, Diagnosis and Clinical Management
Parveen parasar.
1 Boston Center for Endometriosis, Boston Children’s and Brigham and Women’s Hospitals, 333 and 221 Longwood Avenue, Boston, MA 02115, USA
2 Center for Infertility and Reproductive Surgery, Department of Obstetrics, Gynecology, and Reproductive Biology, Brigham and Women’s Hospital and Harvard Medical School, 75 Francis Street, Boston, MA 02115, USA
Pinar Ozcan
Kathryn l. terry.
3 Obstetrics and Gynecology Epidemiology Center, Brigham and Women’s Hospital and Harvard Medical School, 221 Longwood Avenue, Boston, MA 02115, USA
4 Harvard T.H. Chan School of Public Health, 677 Huntington Avenue, Boston, MA 02115, USA
Purpose of review
Endometriosis is a disease of adolescents and reproductive-aged women characterized by the presence of endometrial tissue outside the uterine cavity and commonly associated with chronic pelvic pain and infertility. Here we review the epidemiology of endometriosis as well as potential biomarkers for detection and with the goal of highlighting risk factors that could be used in combination with biomarkers to identify and treat women with endometriosis earlier..
Recent findings
Early age at menarche, shorter menstrual length, and taller height are associated with a higher risk of endometriosis while parity, higher body mass index (BMI) and smoking are associated with decreased risk. Endometriosis often presents as infertility or continued pelvic pain despite treatment with analgesics and cyclic oral contraceptive pills.
Despite a range of symptoms, diagnosis of endometriosis is often delayed due to lack of non-invasive, definitive and consistent biomarkers for diagnosis of endometriosis. Hormone therapy and analgesics are used for treatment of symptomatic endometriosis. However, the efficacy of these treatments are limited as endometriosis often recurs. In this review, we describe potential diagnostic biomarkers and risk factors that may be used as early non-invasive in vitro tools for identification of endometriosis to minimize diagnostic delay and improve reproductive health of patients.
Introduction
Endometriosis is defined as the presence of endometrial glands and stroma like lesions outside of the uterus [ 1 ]. The lesions can be peritoneal lesions, superficial implants or cysts on the ovary, or deep infiltrating disease [ 2 ]. While there is no definitive etiology of endometriosis, there are several hypotheses regarding how endometriotic lesions develop. One possible mechanism is retrograde menstruation, a feature of the menstrual cycle in women and non-human primates, which is an outflow of the endometrial lining through the patent fallopian tubes into the pelvic space. This retrograde flow, along with potential hematogenous or lymphatic circulation, may result in the seeding of endometrial tissue in ectopic sites. However, retrograde menstruation is common (perhaps universal among menstruating women) while endometriosis is much less common. Therefore, other factors, such as hormonal, inflammatory, or immunologic milieu may determine whether lesions deposited in the pelvic cavity implant and persist [ 3 – 6 ]. Alternatively, endometriosis lesions may arise from Müllerian remnants that did not properly differentiate or migrate during fetal development or from circulating blood cells that transdifferentiate into endometriosis [ 7 – 9 ]. Similarly, the characteristics of the local environment would influence the maintenance of these endometriotic lesions. When considering these etiologic hypotheses, it is important to recognize that endometriotic lesions are antigenically similar to eutopic endometrium but not necessarily endometrium.
Endometriosis affects 10–15% of all women of reproductive age [ 1 ] and 70% of women with chronic pelvic pain [ 10 ]. Unfortunately, for many of these women there is often a delay in diagnosis of endometriosis resulting in unnecessary suffering and reduced quality of life. In patients aged 18–45 years, the average delay is 6.7 years [ 11 ]. As most women with endometriosis report the onset of symptoms during adolescence, early referral, diagnosis, identification of disease and treatment may mitigate pain, prevent disease progression and thus preserve fertility [ 12 – 14 ]. Barriers to early diagnosis include the high cost of diagnosis and treatment in adolescent patients and presentation of confounding symptoms such as cyclic and acyclic pain. Thus, a non-invasive tool to diagnose endometriosis could facilitate earlier diagnosis and intervention that could ultimately improve quality of life and preserve fertility.
The immunologic, genetic, and serum markers proposed to date for endometriosis diagnosis are not sufficiently sensitive and specific to justify their use as a screening test. In this review, we will discuss the epidemiology of endometriosis and current diagnostic tools and available potential diagnostic biomarkers for endometriosis that may be used to better clinically manage the disease to improve the quality of life of adult and adolescent patients.
Presentation and clinical course of endometriosis
Clinical presentation of endometriosis varies in women. Patients often present with symptoms such as intermenstrual bleeding, painful periods (dysmenorrhea), painful intercourse (dyspareunia), painful defecation (dyschezia) and painful urination (dysuria) [ 15 ]. Pelvic pain may present before menstruation begins. Often, endometriosis can be asymptomatic, only coming to a clinician’s attention during evaluation for infertility.
Classification of endometriosis associated pain symptoms have been established by the American Society for Reproductive Medicine (ASRM) based on the morphology of peritoneal and pelvic implants such as red, white and black lesions, percentage of involvement of each lesion should be included. The pelvis is inspected in clockwise or counterclockwise fashion. Number, size, and location endometrial implants, plaques, endometriomas and adhesions should be noted. Endometriosis in bowel, urinary tract, fallopian tube, vagina, cervix, skin, or other locations should be documented per ASRM guidelines. Stages of endometriosis according to ASRM guidelines are stage I, II, III, and IV determined based on the point scores and correspond to minimal, mild, moderate and severe endometriosis [ 16 ].
Epidemiology and risk factors
Several reproductive factors have been consistently associated with risk for endometriosis ( Table 1 ), suggesting hormonal variation may have a significant impact on the risk of developing endometriosis. For instance, early age at menarche ( 17 , 18 – 20 , 33 ) and short menstrual cycle length ( 19 – 23 ) are associated with an increased risk, while parity ( 20 , 24 – 26 ) and current oral contraceptive use ( 27 ) are associated with a decreased risk. Circulating estradiol and estrone, which stimulate ectopic and eutopic endometrial tissue, are higher among women with an earlier age at menarche and in nulliparous women ( 28 – 32 ). Though not a reproductive risk factor, a consistent inverse association has also been observed between body mass index (BMI) and endometriosis ( 17 , 18 – 19 , 22 , 33 – 38 ) may also relate to hormonal differences between heavy and lean women.
Risk factors for endometriosis
Unfortunately, the evaluation of tubal ligation, parity, and oral contraceptive use in relation to endometriosis risk have been plagued by methodologic issues. Tubal ligation has been hypothesized to decrease endometriosis risk through blocking retrograde menstruation from reaching the pelvic cavity. However, the association between tubal ligation and endometriosis is difficult to interpret since endometriosis is characterized by infertility and women who seek a tubal ligation are more likely to be parous than the general population ( 3 , 39 , 40 ). The association between oral contraceptive use and endometriosis risk is mixed with most ( 27 , 41 ) but not all showing a decreased risk for current users but an increased risk for past users. However, oral contraceptives are used to treat endometriosis-associated pain and, therefore, this association may reflect suppression of endometriosis symptoms while on oral contraceptives that reappear after the oral contraceptives are stopped.
The association between smoking and endometriosis is unclear. Although smoking is deleterious to many other aspects of health, smoking is associated with a decreased risk of endometriosis in some ( 42 , 19 , 22 ) but not all ( 43 , 44 , 26 , 37 ) studies. Interestingly, exposure to cigarette smoke in utero is associated with an 80% reduction endometriosis risk, but passive smoking exposure during childhood increases risk ( 45 – 47 ). Although the mechanism is unknown, circulating estrogens are known to be lower in women who smoke ( 48 ) and could inhibit the growth and persistence of endometriotic tissue.
The association between alcohol and caffeine consumption is similarly mixed and may depend on fertility status. Among infertile women, several studies have reported increased risk with higher alcohol or caffeine intake ( 49 – 52 ). Increased bioavailable estrogen levels in women who consume moderate amounts of alcohol lend biologic credibility to the association. However, studies not restricted to infertile women have shown no association ( 33 , 53 – 55 ).
Other lifestyle factors and dietary patterns that influence endometriosis risk may relate to their ability to mitigate inflammation. Physical activity and omega-3 dietary fatty acids may reduce levels of tumor necrosis factor alpha (TNFα), interleukin 6 (IL6) and other inflammatory markers [ 56 – 60 ]. While the association between physical activity and endometriosis is unclear ( 43 ), higher intake of long-chain omega-3 fatty acids has been associated with reduced endometriosis risk [ 61 ].
Despite recent advances in identifying risk factors for endometriosis, the field continues to be limited by requiring surgical diagnosis of the disease, often done laproscopically to confirm effected cases and appropriate controls (those that are sampled from the same base population as the cases). Validation is needed in large cohorts of women with laproscopically-confirmed endometriosis and appropriate control groups. Furthermore, as reproductive and lifestyle factors change, such as changes in contraception formulations and patterns of use as well as delayed childbearing, newer cohorts of young women are needed to understand how changes in established factors may influence endometriosis incidence as well as aid in the discovery of novel risk factors. Ultimately, the establishment of a defined set of endometriosis risk factors could lead to the identification of a group of women and girls with a high enough risk profile to warrant screening. Furthermore, these risk factors can also provide new insights into the etiology of the disease, which could lead to important advances in identifying potential screening biomarkers and treatment targets.
Diagnosis of endometriosis
Preliminary diagnosis of endometriosis is usually done on the basis of clinical history since most women show normal results of physical examination. Clinicians palpate for uterine or adnexal tenderness, a retroverted fixture, nodulating uterosacral ligament, and any pelvic masses. A tenderness on palpation of posterior fornix is the most common finding. Pelvic pain is also a symptom of other diseases such as pelvic adhesions, adenomyosis, and gastrointestinal or urologic disorders; therefore, differential diagnosis is important ( 7 ). Other causes of pelvic pain should be ruled out by carrying out appropriate diagnostic tests like urinalysis, Pap smear, pregnancy test, vaginal and endocervical swabs. Pelvic ultrasound scans are performed to facilitate diagnosis of an endometrioma, fibroids and ovarian cysts.
Pelvic masses are visualized by the use of transvaginal and transabdominal ultrasound. Transvaginal ultrasound is used to better visualize endometrium and uterine cavity and detect ovarian endometriotic cysts but does not rule out peritoneal endometriosis, endometriosis-associated adhesions and deep infiltrating endometriosis [ 66 – 70 ]. Occasionally, a magnetic resonance imaging and computed tomography scans are conducted to characterize the pelvic masses.
Despite the aforementioned tentative tests available, gold standard for confirmatory diagnosis of endometriosis is laparoscopic inspection with histologic confirmation after biopsy [ 66 ]. Endometriotic lesions are visualized by the use of laparoscope; however, the correlation between clinical symptoms and disease burden is poor [ 66 , 71 ].
Since laparoscopy is not practical as a first line diagnostic tool, investigators have sought to identify non-invasive tools for early diagnosis that might prevent or delay progression of endometriosis ( Table 2 ). Despite the range of blood tests that have been evaluated, a reliable test has yet to be identified for the diagnosis of endometriosis [ 72 , 73 ]. A change in levels of analytes, proteins, microRNAs, and other markers corresponding to a disease state could be the basis for identifying novel biomarkers. Women with endometriosis show altered levels of CA-125, cytokines, angiogenic and growth factors compared to normal women, but none of the markers have been proven to be definitive clinical tool for diagnosis of endometriosis.
Potential diagnostic biomarkers for endometriosis
Regulated upon Activation, Normal T-cell Expressed and Secreted (RANTES), Monocyte chemotactic protein 1 (MCP-1), Vascular endothelial growth factor (VEGF), Microvessel density (MVD), Focal adhesion kinase (FAK), Insulin-like growth (IGF), Hepatocyte growth factor (HGF), Matrix metalloproteinase (MMP), Tissue inhibitors of metalloproteinases (TIMPs), Pak-1 (p21 activated kinase-1), 17 β hydroxysteroid dehydrogenase (17 βHSD), Estrogen receptors (ERs)
Biomarkers for the diagnosis of endometriosis
Current guidelines recommend that the histological examination of specimens collected from the suspicious areas during the visual inspection of the pelvis at laparoscopy is the gold standard for diagnosis of endometriosis [ 71 ]. However, laparoscopy may not be appropriate for all women with a history and physical examination suggestive of endometriosis. Therefore, care has been given to identify simple and reliable biomarkers of endometriosis for early noninvasive or semi-invasive diagnosis of this disease. Many studies have evaluated the diagnostic value of biomarkers for endometriosis but to date there is no reliable recommended biomarkers in endometrial tissue, menstrual or uterine fluids and immunologic markers in blood or urine for clinical use as a diagnostic test for endometriosis yet [ 74 ].
By using semi or non-invasive diagnostic tools to evaluate biomarkers from blood, urine, or menstrual fluid, a surgical procedure could be avoided and women with endometriosis, who could benefit from surgery to increase fertility and decrease pain, could be identified. Moreover, it provides data early in the disease process that could aid in treatment or prevent the progression of disease in particular for women with minimal-mild disease [ 75 ]. A list of candidate biomarkers for endometriosis diagnosis and progression are summarized in ( Table 2 ). A combination of these biomarkers may improve the sensitivity and specificity over any single biomarker [ 74 ]. Moreover, stem cell, proteomic and genomic studies could provide advanced opportunities for discovery of the potentially new reliable diagnostic biomarkers with high sensitivity for endometriosis.
Clinical Management practices for associated pain and infertility
The management of endometriosis requires a multidisciplinary approach with [i] surgical diagnosis and debulking of disease load, [ii] hormonal treatment to suppress and delay recurrence and progression of disease, [iii] pain managment strategies best provided by a pain center clinic that develops individualized care plans and pelvic therapy. Symptomatic endometriosis is typically treated by surgical or medical treatment both equally effective. Despite the availability of treatments of associated pain, recurrence of endometriosis is not uncommon. Choice of medical treatments is done based on side effect profile, cost and personal preference. Non-steroidal anti-inflammatory drugs (NSAIDs) and low-dose combined oral contraceptive pills (COCPs) such as ethyl estradiol and progestins are the first choice drugs [ 91 ]. If patients do not respond to NSAIDs in three months a second line of treatments is used which includes progestins (oral, injectable and intra-uterine), androgens, and gonadotropin releasing hormone agonists (GnRH) which reduce moderate to severe pain of endometriosis [ 92 – 94 ].
Surgical techniques include excision or removal of endometrial implants, ablation of uterosacral nerves by employment of endocoagulation, electrocautery or laser treatment, presacral neurectomy, and hysterectomy with bilateral salpingooophorectomy [ 95 , 96 ]. They have 50–80% success rate in reducing symptoms. Unfortunately, endometriosis recurs in 5 to 15% of cases even after hysterectomy and bilateral oophorectomy.
The primary benefit of surgery for infertility associated with endometriosis is to enhance the probability of natural conception [ 97 ]. Surgery for infertility or pain increases the spontaneous post-operative pregnancy rate [ 98 ]. On the other hand, surgery for endometrioma could lead to reduced ovarian function and the possible loss of the ovary. Therefore, the decision of surgery should be made carefully, particularly in women with advanced age, bilateral disease, impaired ovarian reserve, who had previous surgery for endometriomas, or long-term infertility, who are incompatible with natural conception due to tubal or male factors.
Future Perspectives
With the advancement of technologies and novel research findings, novel markers have been reported which can potentially be developed as therapeutic targets of endometriosis. In this class, immunomodulators such as interferon alpha 2 (IFN- α 2) and tumor necrosis factor (TNF) – α inhibitors have been tested in animal models [ 99 ]. In one study [ 100 ], visceral sensitivity was measured in endometriosis patients and compared with patients with irritable bowel syndrome (IBS) and identified that patients who had pain associated with endometriosis had greater visceral hypersensitivity compared to IBS patients. This not only gives a way to differentially diagnose endometriosis patients but also provides a novel target for therapy of endometriosis. A recent study has shown that inflammation leads to elevation of components of signaling pathways such as mitogen-activated protein kinase (MAPK) in endometriosis [ 101 , 102 ] and could be a potential targets of therapy for endometriosis. A combination of unique and specific diagnostic biomarkers and novel therapeutic targets will pave a path for better early diagnosis and more effective treatment of endometriosis.
Conclusions
In summary, endometriosis is a debilitating disease that impacts the quality of life of adult and adolescent patients. Diagnostic delays are common and may lead to a decline in reproductive potential and fertility. A semi/non-invasive diagnostic biomarker would be a useful tool to identify patients early in the disease process and thus improving outcomes, including less pain and better fertility. A myriad of biomarkers have been associated with endometriosis; however, they are not sensitive and specific enough for use in screening. These potential biomarkers would reduce the cost of surgical intervention by early diagnosing the cases and thus improve clinical management of the disease. Therefore, more research is needed in this area of medicine.
Conflict of Interest
Parveen Parasar, Pinar Ozcan, and Kathryn L. Terry declare that they have no conflict of interest.
Compliance with Ethics Guidelines
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Papers of particular interest, published recently, have been highlighted as:
• Of importance •• Of major importance

IMAGES
COMMENTS
Endometriosis affects roughly 10% (190 million) of reproductive age women and girls globally. It is a chronic disease associated with severe, life-impacting pain during periods, sexual intercourse, bowel movements and/or urination, chronic pelvic pain, abdominal bloating, nausea, fatigue, and sometimes depression, anxiety, and infertility.
Scottish and UK governments have introduced women's health plans in which endometriosis features highly; Connecticut passed a historic bill to create a state-wide endometriosis research program ...
GWAS research in endometriosis is still ongoing, providing new results. Literature reports on 998 Belgian patients with endometriosis and 783 controls showed that the polymorphisms rs7521902, rs13394619 and rs6542095 may be associated with this disease .
Endometriosis is a common disease affecting 5-10% of women of reproductive age globally. However, despite its prevalence, diagnosis is typically delayed by years, misdiagnosis is common, and delivery of effective therapy is prolonged. Identification and prompt treatment of endometriosis are essential and facilitated by accurate clinical diagnosis. Endometriosis is classically defined as a ...
The prevalence levels of endometriosis in women with infertility, chronic pelvic pain and asymptomatic were 31 (95% CI: 15-48), 42 (95% CI: 25-58) and 23 per cent (95% CI: 19-26), respectively. Interpretation & conclusions: The results of this study showed that the prevalence of endometriosis in developing countries was high.
About 10% of reproductive-age women have endometriosis. Symptoms can include severe pain, dysmenorrhea, dyspareunia, dysuria, infertility, and fatigue. The pathogenesis is unclear. Hormonal therapy...
Endometriosis is a condition defined by the presence of endometrial-like tissue outside the uterus. 1 It typically occurs in women of reproductive age but prepubertal endometriosis has been reported. Endometriosis is also reported in women after menopause but is thought to have developed prior to menopause. 2 3 Globally, it affects 190 million ...
PubMed and Google Scholar were used to review on clinical diagnosis of endometriosis. The search strategy contained the terms "endometriosis" and "clinical diagnosis.". All research articles published between 1960 and 2021 were included in the search. The findings were then categorized to summarize the evidence.
Endometriosis is a common inflammatory disease characterized by the presence of tissue outside the uterus that resembles endometrium, mainly on pelvic organs and tissues. It affects ~5-10% of ...
Endometriosis affects approximately 190 million women and people assigned female at birth worldwide. It is a chronic, inflammatory, gynecologic disease marked by the presence of endometrial-like tissue outside the uterus, which in many patients is associated with debilitating painful symptoms. Patients with endometriosis are also at greater risk of infertility, emergence of fatigue, multisite ...
Endometriosis, the presence of endometrial-like tissue outside the uterus, is a common clinical entity between women of reproductive age, with a prevalence of about 10%. Due to the variety of endometriosis-associated symptoms, a great variety of treatments have been implemented. The aim of this review is to give an overview on therapeutical approaches of eight national and international widely ...
Endometriosis can have a profound impact on women's lives, including associated pain, infertility, decreased quality of life, and interference with daily life, relationships, and livelihood. The first step in alleviating these adverse sequelae is to diagnose the underlying condition. For many women, the journey to endometriosis diagnosis is long and fraught with barriers and misdiagnoses.
Researchers at the University of Oxford, in collaboration with 25 teams across the world, have published the largest study to date of the genetic basis of endometriosis. Their study included DNA from 60,600 women with endometriosis and 701,900 without. It revealed compelling evidence of a shared genetic basis for endometriosis and other types ...
Endometriosis is a chronic inflammatory disease that is defined as the presence of endometrium-like tissues outside the uterus causing pain symptoms (dysmenorrhea, dyspareunia, chronic pelvic pain) and infertility [].This gynecological disease affects approximately 2-10% of the reproductive-aged and 50% of infertile women, and women with endometriosis have increased risk of obstetric outcome [].
Doctors typically use DCA to help treat metabolic disorders in children. Recent research has shown that people with endometriosis tend to have excess lactate in their pelvis. The drug DCA seems to ...
Wed 31 May 2023 22.51 EDT. Last modified on Thu 1 Jun 2023 10.55 EDT. Sydney researchers have made a world-first leap forward that could change the treatment of endometriosis and improve the ...
Women will be given a potential new treatment for endometriosis in a groundbreaking clinical trial that doctors hope will pave the way for the first new class of drug for the condition in 40 years.
Endometriosis research deserves the funding and attention that befits a disease with its substantial prevalence, effects, and economic costs. This funding could improve patient outcomes by introducing less invasive and more timely methods for diagnosis and treatment, including options such as novel biomarkers, nanomedicine, and microbiome ...
This signature will be tested on endometrium and blood from 975 patients, divided in two groups : 550 patients affected by endometriosis and 225 patients unaffected (controls). EndoSearch is not about drug or medical device assessment but a research study for biomarker analytical validation purpose.
Endometriosis is "a systemic disease that is often painful and chronic.". Current estimates suggest it affects 176 million reproductive-age women worldwide. Symptoms can include: In severe ...
Specifically, lower QoL levels were observed in infertile participants with endometriosis compared to infertile women without endometriosis, aligning with prior research that identifies endometriosis as a factor exacerbating the decline in quality of life and mental health [3, 8, 32, 39,40,41,42]. This further supports a trend in the group with ...
Endometriosis, affecting approximately 10% of reproductive-aged women globally, poses significant challenges, including chronic pelvic pain, dysmenorrhea, and infertility. In low- and middle-income countries like India, accessibility to affordable infertility care remains a concern. This multicenter prospective cohort study, conducted across six tertiary care hospitals in India from 2017 to ...
We aimed to establish a diagnostic model of endometriosis (EM) based on disulfidptosis-related genes (DRGs). Materials and Methods. The mRNA expression data of EM were downloaded from the gene expression omnibus database and subjected to differential analysis, and co-expression analysis was performed based on 10 disulfidptosis genes to acquire ...
Endometriosis (EM) is a chronic, ... In the current research, utilizing a public database, we assessed both the individual and combined impacts of CDAI and EM on RA risk among women of childbearing age. The study revealed a correlation between a lower CDAI score and an increased risk of RA, and an association between EM and risk of RA. ...
Apr 9, 2024. The Society of Radiologists in Ultrasound (SRU) has issued a new expert consensus statement that aims to improve the evaluation of endometriosis. The consensus, published April 9 in Radiology, provides recommendations for improving routine pelvic ultrasounds through additional maneuvers and imaging to improve diagnosis of deep ...
Endometriosis is a disease of adolescents and reproductive-aged women characterized by the presence of endometrial tissue outside the uterine cavity and commonly associated with chronic pelvic pain and infertility. ... With the advancement of technologies and novel research findings, novel markers have been reported which can potentially be ...
Coping methods for acyclic pelvic pain associated with endometriosis vary by age group, with adolescents reporting sleep and music as most helpful and exercise as less helpful, researchers ...
SAN ANTONIO--(BUSINESS WIRE)-- Hera Biotech is pleased to report the interim results for its proof-of-concept multi-center clinical trial of the MetriDx™ endometriosis diagnostic test.Per protocol data from the first 38 patients who have been enrolled in the 60 patient study shows that the diagnostic test is performing with 92% sensitivity, 95% specificity and an overall accuracy (AUC) of 94 ...
Research has indicated that women with endometriosis had higher prevalence levels of Mycoplasma genitalium in their pelvic fluid. Although the exact pathogenic mechanisms elucidating the relationship between Mycoplasma genitalium and endometriosis remain unclear, the organism's presence in the pelvic cavity raises the possibility of a role in ...
Last year, Seattle's Rivkin Center and Washington state's Andy Hill Cancer Research Endowment (CARE) Fund joined forces to earmark $1 million in funding for ovarian cancer research.. This week, they began to dole those funds out. Fred Hutch Cancer Center and UW Medicine researchers Holly Harris, MPH, ScD, and Elizabeth Swisher, MD, each received $200,000 from the organizations — nearly ...