News: Teamscope joins StudyPages 🎉
Data collection in the fight against COVID-19

Query Management in Field Research
Query management finds data entries with issues and isolates them into a report. Learn how this functionality helps prevent data quality issues.
.jpg)
Choosing an app
.jpg)
Is data being collected according to the protocol design? Are there any data errors or fields that are being left empty? Which of my sites or team members are generating the most invalid data? These are the usual worries that every researcher has when conducting a clinical study yet most are only capable of answering them once data collection has ended, and they are performing data analysis. Correcting invalid data months after it's collected can be a nightmare and extremely consuming. Dirty data leads to weaker conclusions and sometimes the need to redo part, or all, of the data collection. Is there any way that a researcher can identify and resolve problems in their data while it's gathered?
The answer to this is a query management system (QMS). After reading this blog post, you should have a clear understanding of what queries are in clinical research and why automatic query management is a researcher's best friend.
What are Data Queries?
A data query is an error or discrepancy generated when a validation check, either done manually or by a computer program, detects a problem with the data. A query management system is a tool that tracks data queries so they can be adequately individualized and resolved. QMS substantially minimizes and even eliminates the risk of invalid data being unnoticed. When a data query (e.g., data issue) is created it should be persistent, which allows it to be tracked over time, and only be resolved in the following ways:
- by correcting the error, in other words entering a new value that is valid
- by marking the data in conflict as correct
Implementing a query management workflow
Clinical data collection may be done in different ways, some researchers use paper forms, excel while more experiences one's Electronic Data Capture, here we expose how query management can be set up with different data collection methods:
Paper Case Report Forms (CRFs)
When collecting data with paper Case Report Forms (CRFs) the task of transcribing the data from paper to a database software may be carried out by a separate team. This team is responsible for confirming that data has been correctly collected and will often do this twice in a process called double data entry, to minimize the possibility of mistakes being unnoticed and new errors being of created during data transcription. Any discrepancies that may be found during double data entry may be kept on a separate Excel file, so they are tracked and resolved by contacting the person that originated that issue. Collecting research data with paper forms is not only prone to mistakes with handwriting interpretation but also makes the task of validating data extremely time-consuming. We have published an article specifically on why researchers should not use paper forms .

Online survey tools.
Creating data validation rules with online questionnaires like Qualtrics and Survey Monkey is possible. Validation rules may be established to force or remind a user that a field is required or that a value is out of range. Data validation minimizes the possibility of errors and required fields being left empty. Although data validating can be automatic, isolating data issues with an online survey tool needs manual filtering and a high level of attention to assure no data problems are unnoticed.
Spreadsheets
Excel is often used for research data collection. Researchers value the fact that Excel is almost as ubiquitous as a computer and that it works without the need for internet. Excel’s data validation is very powerful; you can use formulas that nest arithmetic calculations and combine multiple fields or variables.
As mentioned above, data validation is only the first step in a query management workflow, and since Excel does not generate reports of data issues, to raise and resolve queries one has to rely on manual and time-consuming work.
Electronic Data Capture
Electronic Data Capture (EDC) is software specially designed for the collection of clinical data in electronic format, often for use in human clinical trials. EDCs, like Teamscope , have built-in query management and comply with Good Clinical Practice (GCP). We recommend to always use a validated EDC for collecting sensitive and research data. EDC eliminates the need for paper forms and drastically simplifies data monitoring.
How queries work on Teamscope
Teamscope’s query management is built on top of our data validation system.
Your first step is to add a Condition to any data field:
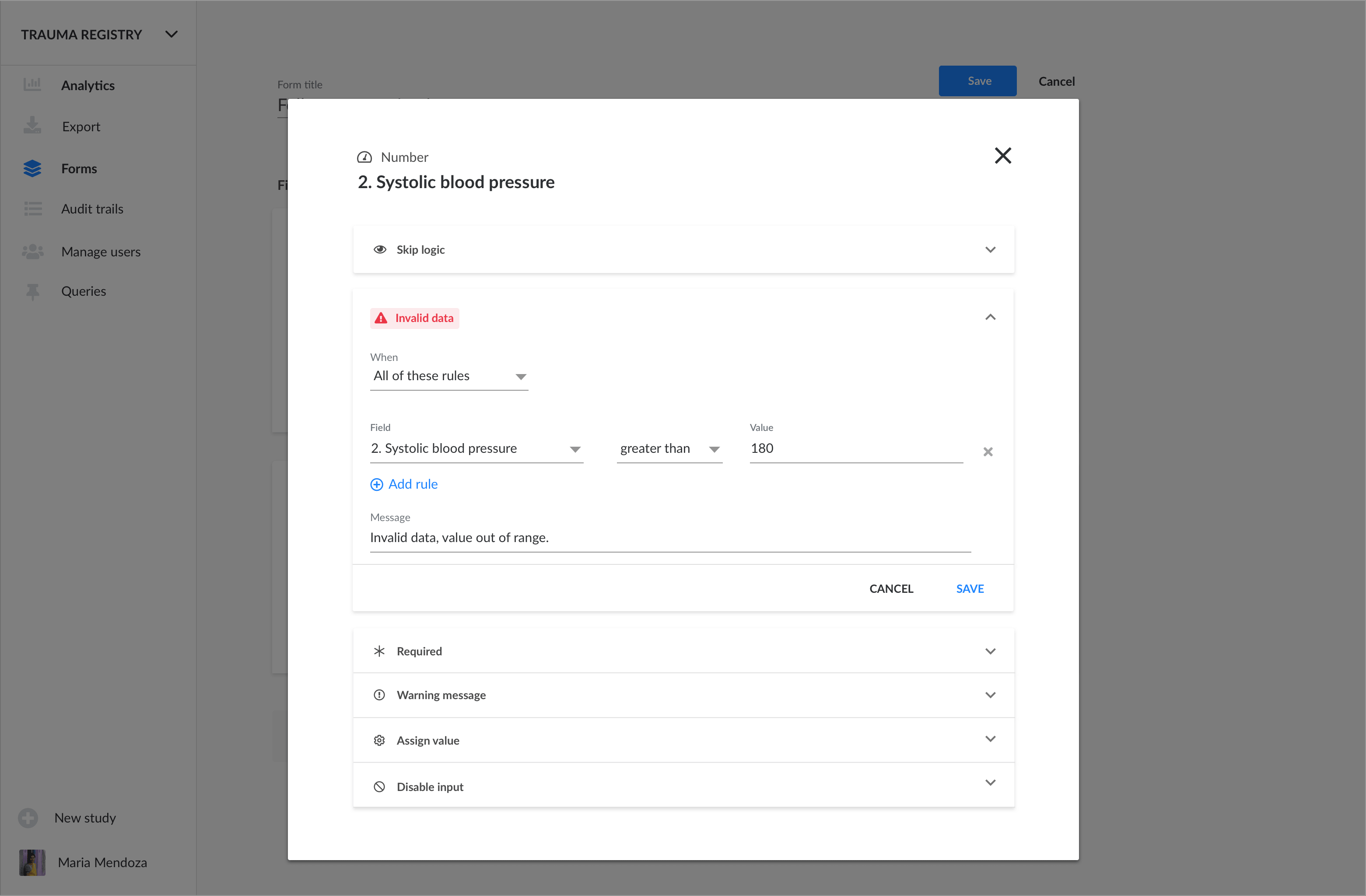
Data queries are automatically generated when a value is invalid , matches warning criteria or is required and has been left empty:
.png)
There is a smarter way to do research.
Build fully customizable data capture forms, collect data wherever you are and analyze it with a few clicks — without any training required.
From Teamscope Web you can see:
- Who in your team created this data query
- When it was originated
- The data that caused
- The condition that triggered it: Invalid, Warn or Required
- The status: Open or Resolved
By clicking on the status of a query you may change from Open to Resolved , and vice versa.
.png)
Alternatively, whenever the data issue is fixed, the platform automatically ✨changes the status of the query from Open to Resolved :
.png)
When a researcher finds out months or weeks after data collection that there are issues with his/her data, this not only means the studies’ outcome will be weaker but potentially data collection will have to be redone. The best way to mitigate this risk is to use an Electronic Data Capture system that has the capability of not only validating data as its inputted but most importantly keeping track of the data entries that have issues. A query management system (QMS) automatically isolates data issues and allows researchers to react to them immediately, giving them full control of data quality and accuracy.
Teamscope’s Query Management System is fully automated and easy to use. Want to have a custom demo with one of our specialists? Sign up here .
- Wikipedia contributors. (2018, October 3). Clinical data management. In Wikipedia, The Free Encyclopedia . Retrieved 20:36, January 2, 2019, from https://en.wikipedia.org/w/index.php?title=Clinical_data_management&oldid=862284831
- Bellary S, Krishnankutty B, Latha M S. Basics of case report form designing in clinical research. Perspect Clin Res 2014;5:159-66
More chapters from this guide
Choosing a mobile app
8 Apps for Field Data Collection
From paper to mobile
Getting started with Mobile Forms
Working offline
How to collect field data when working offline
Data Security
Data security and HIPAA compliance
Data visualization in mobile data collection.
Calculation fields
Using conditional logic & formulas on Mobile Forms
Your session is about to expire
Query management in clinical trials, definition of query management.
Query management describes the process of identifying, prioritizing, analyzing, and addressing (resolving) questions/issues that arise during clinical trials - most commonly discrepancies in clinical data. Developing and implementing a systematic approach for recording and resolving queries allows for transparency and accuracy throughout the entire trial process.
Importance of query management in clinical data management
Effective query management is critical in order to ensure that data gathered during clinical trials is accurate and reliable. High-quality query management procedures help safeguard against data errors or discrepancies that may occur during the collection, transcription, or processing of study data. Good query management practices also help maintain ethical standards by ensuring that all queries are addressed appropriately and documented correctly, which also has implications for participant safety and well-being. Relatedly, proper documentation of queries assists with analysis of the trial's progress and compliance by review boards and regulatory bodies.
Types of queries in clinical trials
Clinical trials involve collecting, analyzing, and interpreting a considerable amount of health data. In doing so, a variety of queries may arise which need to be addressed by the clinical trial team - either at sites, by a central monitor, or even by external stakeholders/auditors. Most commonly, queries are related to data discrepancies, protocol deviations, and participant safety.
Data discrepancy queries
Data entry queries are typically requests for clarification on data entered into a subject's clinical record, or case report form ( CRF ). They are generated to replace or adjust data that was entered incorrectly into the CRF, CDMS, or EDC or other system, or which is missing altogether. Data discrepancy queries involve identifying potential errors or discrepancies between what was entered into the system versus what was observed by researchers or investigators on site at the time of the visit (so-called “source data”), such as measurements of participants’ vital signs. This type of query requires review/correction by members of the clinical trial staff - generally by the person responsible for the original data entry.
Queries may be generated as part of routine data review, such as in source data verification ( SDV ), or when an investigator or the sponsor notices a suspicious data point, such as values that are incompatible with life (i.e., pulse rate 700 bpm).
Protocol queries
Protocol queries may arise when there is deviation from any step(s) outlined in the protocol (e.g., a change in visit sequence). Queries related to protocol deviations may require detailed investigation and responses from trial staff as well as regulatory bodies in order to ensure that trial operations remain compliant with ethical and legal regulations.
Safety queries
Queries related to participant safety could involve discrepancies between adverse events identified via monitoring activities versus those reported through standard practices like SAE reporting. Queries could also be generated directly during monitoring activities if data is identified that could indicate a potential adverse event or serious adverse event. This process could also be automated, such as in the case of risk-based monitoring, which may be set up to flag certain outlier data points that could indicate participant safety issues. Such queries will likely require a comprehensive review process involving the sponsor, monitor, and perhaps a study clinician.
Query management in clinical trials
Clinical trials are complex endeavors that require a structured approach and collaboration between numerous teams to ensure accuracy and compliance with protocol. Query management represents the formal system used for communicating and resolving discrepancies between the sponsor, investigator, sites, and research personnel. The clinical research coordinator plays an especially important role in query management by developing an appropriate query management plan and protocols.
The role of the clinical research coordinator in query management
A clinical research coordinator will typically be responsible for developing a query management plan that meets all regulatory requirements in order to minimize the risk of errors and compliance violations during a trial. This requires an understanding of applicable regulations and ethical standards, so that query management protocols are designed in such a way that queries are managed in line with these standards, regardless of who resolves the query.
Developing a query management plan
When creating a query management plan, it is important to consider how queries will be identified, documented, escalated (when needed), analyzed for accuracy and completeness, tracked until resolution is achieved, closed, and - perhaps most importantly - communicated between team members.
In order to developing the query management plan, the following protocol aspects need to be clarified:
- Determine whether manual processes or electronic (and possibly automated) systems (or even both) will be used for collecting data from different sources
- Decide whether queries will be managed manually or electronically (or in an automated fashion)
- Develop criteria for initiating audit trails when discrepancies are found
- Establish methods for communicating and tracking queries
- Decide how queries and query resolution will be documented throughout the trial, so they can easily be accessed later, if necessary, by external auditors or regulators
Methods of Managing Queries in Clinical Trials
There are different options available for managing queries; the appropriate query management process will depend heavily on the specific trial protocol, any technological systems used in operations, and the preferences of the sponsor and investigators.
A. Manual query management
Manual query management describes the traditional means for handling queries submitted during a clinical trial. It involves manually entering information related to each query into a system such as Microsoft Excel, or even on paper records, and tracking the status of each query throughout the review process. Although manual query management may be effective when performed carefully, it is time-consuming and prone to errors, which can lead to delays in resolving queries or even instances of non-compliance if not managed properly.
B. Automated/electronic: Query management system
Automated query management is an increasingly popular alternative to manual query management. Software solutions, such as an electronic quality management system ( eQMS ), enable sponsors and investigators to electronically manage queries without relying on outdated paper forms or spreadsheets. These systems enable faster recording and tracking of all data related to each query, and streamlined management of simultaneous queries. Electronic query management systems will require initial setup costs and ongoing maintenance in order to remain operational over the course of the trial, but the initial investment tends to pay off due to increased operational efficiency and reduced error rate.
Query resolution and documentation
Swift and appropriate resolution of queries is essential for the success of a clinical trial, as unresolved issues can lead to legal concerns or safety issues. Additionally, it is important that records be kept regarding when and how each query was resolved in order to facilitate audits and remain compliant.
Best practices for query resolution and documentation
As a clinical trial sponsor, there are a few best practices you might wish to follow in order to maximize the efficiency of query management and resolution. The following considerations can help you ensure smooth and swift query management.
Successful query management strategies
1. Leverage automation and electronic query management tools
Automated query generation tools and electronic query management systems can help streamline query generation, tracking, and resolution by reducing the risk of manual data entry mistakes, automating parts of the workflow (for example, automatic notifications), and increasing the speed of response times. These tools can be used in conjunction with other “eClinical” tools such as clinical trial management systems ( CTMS ), EDC, and risk-based monitoring tools.
2. Use clear language in queries and provide explicit instructions for users
Providing users with simple but clear instructions on how to properly respond to any given query can help ensure that they provide accurate answers in a timely manner and avoid unnecessary delays or complications due to misunderstandings. While a query may not suggest a specific solution, it should indicate exactly which data field/concept is problematic, and where/how it might be verified.
→ Poor example:
Subject 140 date of birth is unclear, should be 12/12/1990; please verify at source.
→ Better example:
Subject 140’s date of birth is unreadable. Please verify from the case report form and re-enter in the format DD/MM/YYYY.
3. Respond to user comments and feedback
Feedback or comments from the person assigned to resolving the query can be invaluable for identifying ways to improve the clarity of queries generated. For example, if an extensive back-and-forth discussion is required to resolve a simple query for correcting a single data field, this could be an indication that the instructions in the original query were not sufficiently clear.
4. Monitor query metrics to set targets for future improvements
Monitoring queries can provide insights into the frequency of discrepant data, how quickly queries are resolved, and the overall management of queries. This information can be used to identify where improvements can be made and make changes to enhance the management of queries in future trials.
Efficient query management procedures can accelerate trial timelines and enhance patient safety, while also ensuring the quality and integrity of trial data and results. Having consistent processes in place - and in particular, using electronic systems - reduces risk associated with errors due to human oversight or miscommunications.
As technology continues to advance, new opportunities will emerge for improving how queries are managed within clinical research. Advanced data analytics may provide deeper insights into trends across all aspects of a study, bringing further transparency into how queries are handled throughout different stages of development. Artificial intelligence (AI), which is already being increasingly applied in various aspects of clinical research , could reduce manual work associated with handling queries while cutting down response times even further. These technologies present opportunities for improving query management and overall trial efficiency, but they also present challenges that will require careful consideration when implementing them into existing workflows, particularly with regard to compliance and patient data privacy .
Other Trials to Consider
Pharmacist-led Hospital Discharge Care Intervention
Personalized trial abccba, matter intervention, effortful self-regulatory activities, educational intervention, supportive care interventions, structured interview and safety plan, contingency management, popular categories.
Glioblastoma Clinical Trials 2024
Pain Clinical Trials 2024
TMJ Clinical Trials 2024
Huntington Disease Clinical Trials 2024
Tymlos Clinical Trials
Clinical Trials in Tampa, FL
Clinical Trials in Albuquerque, NM
Clinical Trials in Long Beach, CA

Paid Clinical Trials in Tennessee
Hypertrophy Clinical Trials 2023
Popular guides.
Research Question 101 📖
Everything you need to know to write a high-quality research question
By: Derek Jansen (MBA) | Reviewed By: Dr. Eunice Rautenbach | October 2023
If you’ve landed on this page, you’re probably asking yourself, “ What is a research question? ”. Well, you’ve come to the right place. In this post, we’ll explain what a research question is , how it’s differen t from a research aim, and how to craft a high-quality research question that sets you up for success.
Research Question 101
What is a research question.
- Research questions vs research aims
- The 4 types of research questions
- How to write a research question
- Frequently asked questions
- Examples of research questions
As the name suggests, the research question is the core question (or set of questions) that your study will (attempt to) answer .
In many ways, a research question is akin to a target in archery . Without a clear target, you won’t know where to concentrate your efforts and focus. Essentially, your research question acts as the guiding light throughout your project and informs every choice you make along the way.
Let’s look at some examples:
What impact does social media usage have on the mental health of teenagers in New York?
How does the introduction of a minimum wage affect employment levels in small businesses in outer London?
How does the portrayal of women in 19th-century American literature reflect the societal attitudes of the time?
What are the long-term effects of intermittent fasting on heart health in adults?
As you can see in these examples, research questions are clear, specific questions that can be feasibly answered within a study. These are important attributes and we’ll discuss each of them in more detail a little later . If you’d like to see more examples of research questions, you can find our RQ mega-list here .

Research Questions vs Research Aims
At this point, you might be asking yourself, “ How is a research question different from a research aim? ”. Within any given study, the research aim and research question (or questions) are tightly intertwined , but they are separate things . Let’s unpack that a little.
A research aim is typically broader in nature and outlines what you hope to achieve with your research. It doesn’t ask a specific question but rather gives a summary of what you intend to explore.
The research question, on the other hand, is much more focused . It’s the specific query you’re setting out to answer. It narrows down the research aim into a detailed, researchable question that will guide your study’s methods and analysis.
Let’s look at an example:
Research Aim: To explore the effects of climate change on marine life in Southern Africa.
Research Question: How does ocean acidification caused by climate change affect the reproduction rates of coral reefs?
As you can see, the research aim gives you a general focus , while the research question details exactly what you want to find out.
Need a helping hand?
Types of research questions
Now that we’ve defined what a research question is, let’s look at the different types of research questions that you might come across. Broadly speaking, there are (at least) four different types of research questions – descriptive , comparative , relational , and explanatory .
Descriptive questions ask what is happening. In other words, they seek to describe a phenomena or situation . An example of a descriptive research question could be something like “What types of exercise do high-performing UK executives engage in?”. This would likely be a bit too basic to form an interesting study, but as you can see, the research question is just focused on the what – in other words, it just describes the situation.
Comparative research questions , on the other hand, look to understand the way in which two or more things differ , or how they’re similar. An example of a comparative research question might be something like “How do exercise preferences vary between middle-aged men across three American cities?”. As you can see, this question seeks to compare the differences (or similarities) in behaviour between different groups.
Next up, we’ve got exploratory research questions , which ask why or how is something happening. While the other types of questions we looked at focused on the what, exploratory research questions are interested in the why and how . As an example, an exploratory research question might ask something like “Why have bee populations declined in Germany over the last 5 years?”. As you can, this question is aimed squarely at the why, rather than the what.
Last but not least, we have relational research questions . As the name suggests, these types of research questions seek to explore the relationships between variables . Here, an example could be something like “What is the relationship between X and Y” or “Does A have an impact on B”. As you can see, these types of research questions are interested in understanding how constructs or variables are connected , and perhaps, whether one thing causes another.
Of course, depending on how fine-grained you want to get, you can argue that there are many more types of research questions , but these four categories give you a broad idea of the different flavours that exist out there. It’s also worth pointing out that a research question doesn’t need to fit perfectly into one category – in many cases, a research question might overlap into more than just one category and that’s okay.
The key takeaway here is that research questions can take many different forms , and it’s useful to understand the nature of your research question so that you can align your research methodology accordingly.

How To Write A Research Question
As we alluded earlier, a well-crafted research question needs to possess very specific attributes, including focus , clarity and feasibility . But that’s not all – a rock-solid research question also needs to be rooted and aligned . Let’s look at each of these.
A strong research question typically has a single focus. So, don’t try to cram multiple questions into one research question; rather split them up into separate questions (or even subquestions), each with their own specific focus. As a rule of thumb, narrow beats broad when it comes to research questions.
Clear and specific
A good research question is clear and specific, not vague and broad. State clearly exactly what you want to find out so that any reader can quickly understand what you’re looking to achieve with your study. Along the same vein, try to avoid using bulky language and jargon – aim for clarity.
Unfortunately, even a super tantalising and thought-provoking research question has little value if you cannot feasibly answer it. So, think about the methodological implications of your research question while you’re crafting it. Most importantly, make sure that you know exactly what data you’ll need (primary or secondary) and how you’ll analyse that data.
A good research question (and a research topic, more broadly) should be rooted in a clear research gap and research problem . Without a well-defined research gap, you risk wasting your effort pursuing a question that’s already been adequately answered (and agreed upon) by the research community. A well-argued research gap lays at the heart of a valuable study, so make sure you have your gap clearly articulated and that your research question directly links to it.
As we mentioned earlier, your research aim and research question are (or at least, should be) tightly linked. So, make sure that your research question (or set of questions) aligns with your research aim . If not, you’ll need to revise one of the two to achieve this.
FAQ: Research Questions
Research question faqs, how many research questions should i have, what should i avoid when writing a research question, can a research question be a statement.
Typically, a research question is phrased as a question, not a statement. A question clearly indicates what you’re setting out to discover.
Can a research question be too broad or too narrow?
Yes. A question that’s too broad makes your research unfocused, while a question that’s too narrow limits the scope of your study.
Here’s an example of a research question that’s too broad:
“Why is mental health important?”
Conversely, here’s an example of a research question that’s likely too narrow:
“What is the impact of sleep deprivation on the exam scores of 19-year-old males in London studying maths at The Open University?”
Can I change my research question during the research process?
How do i know if my research question is good.
A good research question is focused, specific, practical, rooted in a research gap, and aligned with the research aim. If your question meets these criteria, it’s likely a strong question.
Is a research question similar to a hypothesis?
Not quite. A hypothesis is a testable statement that predicts an outcome, while a research question is a query that you’re trying to answer through your study. Naturally, there can be linkages between a study’s research questions and hypothesis, but they serve different functions.
How are research questions and research objectives related?
The research question is a focused and specific query that your study aims to answer. It’s the central issue you’re investigating. The research objective, on the other hand, outlines the steps you’ll take to answer your research question. Research objectives are often more action-oriented and can be broken down into smaller tasks that guide your research process. In a sense, they’re something of a roadmap that helps you answer your research question.
Need some inspiration?
If you’d like to see more examples of research questions, check out our research question mega list here . Alternatively, if you’d like 1-on-1 help developing a high-quality research question, consider our private coaching service .

Psst… there’s more (for free)
This post is part of our dissertation mini-course, which covers everything you need to get started with your dissertation, thesis or research project.
You Might Also Like:

Submit a Comment Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
- Print Friendly
Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, generate accurate citations for free.
- Knowledge Base
- Starting the research process
A Beginner's Guide to Starting the Research Process
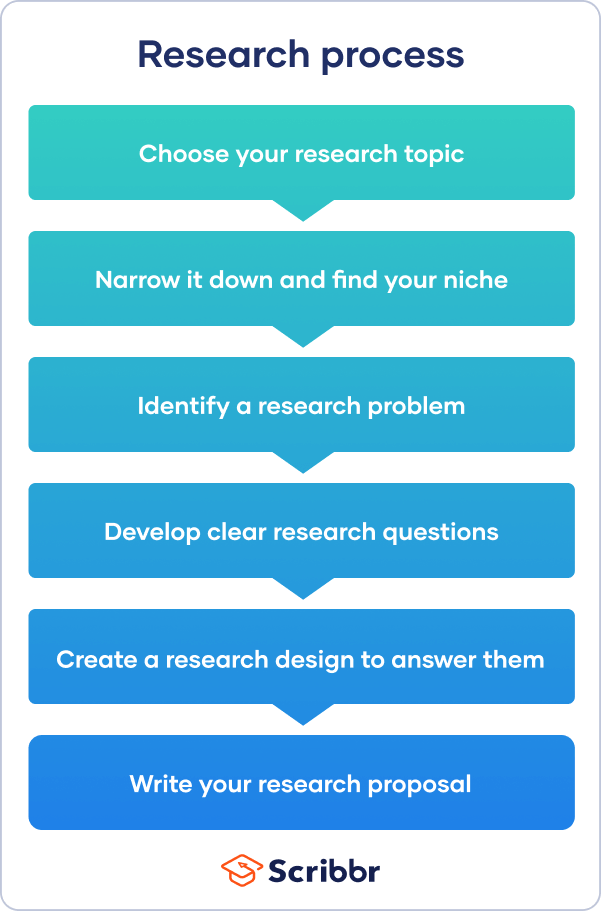
When you have to write a thesis or dissertation , it can be hard to know where to begin, but there are some clear steps you can follow.
The research process often begins with a very broad idea for a topic you’d like to know more about. You do some preliminary research to identify a problem . After refining your research questions , you can lay out the foundations of your research design , leading to a proposal that outlines your ideas and plans.
This article takes you through the first steps of the research process, helping you narrow down your ideas and build up a strong foundation for your research project.
Table of contents
Step 1: choose your topic, step 2: identify a problem, step 3: formulate research questions, step 4: create a research design, step 5: write a research proposal, other interesting articles.
First you have to come up with some ideas. Your thesis or dissertation topic can start out very broad. Think about the general area or field you’re interested in—maybe you already have specific research interests based on classes you’ve taken, or maybe you had to consider your topic when applying to graduate school and writing a statement of purpose .
Even if you already have a good sense of your topic, you’ll need to read widely to build background knowledge and begin narrowing down your ideas. Conduct an initial literature review to begin gathering relevant sources. As you read, take notes and try to identify problems, questions, debates, contradictions and gaps. Your aim is to narrow down from a broad area of interest to a specific niche.
Make sure to consider the practicalities: the requirements of your programme, the amount of time you have to complete the research, and how difficult it will be to access sources and data on the topic. Before moving onto the next stage, it’s a good idea to discuss the topic with your thesis supervisor.
>>Read more about narrowing down a research topic
Here's why students love Scribbr's proofreading services
Discover proofreading & editing
So you’ve settled on a topic and found a niche—but what exactly will your research investigate, and why does it matter? To give your project focus and purpose, you have to define a research problem .
The problem might be a practical issue—for example, a process or practice that isn’t working well, an area of concern in an organization’s performance, or a difficulty faced by a specific group of people in society.
Alternatively, you might choose to investigate a theoretical problem—for example, an underexplored phenomenon or relationship, a contradiction between different models or theories, or an unresolved debate among scholars.
To put the problem in context and set your objectives, you can write a problem statement . This describes who the problem affects, why research is needed, and how your research project will contribute to solving it.
>>Read more about defining a research problem
Next, based on the problem statement, you need to write one or more research questions . These target exactly what you want to find out. They might focus on describing, comparing, evaluating, or explaining the research problem.
A strong research question should be specific enough that you can answer it thoroughly using appropriate qualitative or quantitative research methods. It should also be complex enough to require in-depth investigation, analysis, and argument. Questions that can be answered with “yes/no” or with easily available facts are not complex enough for a thesis or dissertation.
In some types of research, at this stage you might also have to develop a conceptual framework and testable hypotheses .
>>See research question examples
The research design is a practical framework for answering your research questions. It involves making decisions about the type of data you need, the methods you’ll use to collect and analyze it, and the location and timescale of your research.
There are often many possible paths you can take to answering your questions. The decisions you make will partly be based on your priorities. For example, do you want to determine causes and effects, draw generalizable conclusions, or understand the details of a specific context?
You need to decide whether you will use primary or secondary data and qualitative or quantitative methods . You also need to determine the specific tools, procedures, and materials you’ll use to collect and analyze your data, as well as your criteria for selecting participants or sources.
>>Read more about creating a research design
Finally, after completing these steps, you are ready to complete a research proposal . The proposal outlines the context, relevance, purpose, and plan of your research.
As well as outlining the background, problem statement, and research questions, the proposal should also include a literature review that shows how your project will fit into existing work on the topic. The research design section describes your approach and explains exactly what you will do.
You might have to get the proposal approved by your supervisor before you get started, and it will guide the process of writing your thesis or dissertation.
>>Read more about writing a research proposal
If you want to know more about the research process , methodology , research bias , or statistics , make sure to check out some of our other articles with explanations and examples.
Methodology
- Sampling methods
- Simple random sampling
- Stratified sampling
- Cluster sampling
- Likert scales
- Reproducibility
Statistics
- Null hypothesis
- Statistical power
- Probability distribution
- Effect size
- Poisson distribution
Research bias
- Optimism bias
- Cognitive bias
- Implicit bias
- Hawthorne effect
- Anchoring bias
- Explicit bias
Is this article helpful?
Other students also liked.
- Writing Strong Research Questions | Criteria & Examples
What Is a Research Design | Types, Guide & Examples
- How to Write a Research Proposal | Examples & Templates
More interesting articles
- 10 Research Question Examples to Guide Your Research Project
- How to Choose a Dissertation Topic | 8 Steps to Follow
- How to Define a Research Problem | Ideas & Examples
- How to Write a Problem Statement | Guide & Examples
- Relevance of Your Dissertation Topic | Criteria & Tips
- Research Objectives | Definition & Examples
- What Is a Fishbone Diagram? | Templates & Examples
- What Is Root Cause Analysis? | Definition & Examples
"I thought AI Proofreading was useless but.."
I've been using Scribbr for years now and I know it's a service that won't disappoint. It does a good job spotting mistakes”

Want to create or adapt books like this? Learn more about how Pressbooks supports open publishing practices.
The search query

To construct the search query consider using some search techniques such as Boolean operators and phrase searching.
In considering the topic I am interested in the recycling of waste water in cities, three main aspects were identified, and a list of possible keywords (search terms) was compiled.
The search statement may look like the following:
(recycling OR reuse) AND (“waste water” OR sewerage OR “drain water”) AND (cities OR urban)
This search query has used:
- double quote marks enclosed around keywords that contain two words so they are searched as a phrase
- Boolean operator OR is used to combine keywords about the same aspect
- Boolean operator AND is used to combine keywords about different aspects.
The search features of library databases make constructing your search query easier, especially when using advanced search options. For example, the Boolean operators can usually be selected from a drop-down menu. This is evident in the example below, showing the search query where the Boolean operators of AND are selected.
Note: nearly all databases have these features, but they might look different. You may need to explore to find the options you need.
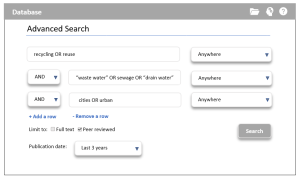
(Copyright © 2022 RMIT University)
Now it’s your turn to develop your search query for your own research topic.
- Write down your research topic and identify your main aspects.
- For each aspect list some alternative search terms (synonyms).
- Consider some different search techniques that you may need to use.
- Create a search query that combines your topic keywords and synonyms using Boolean operators AND and OR.
Research and Writing Skills for Academic and Graduate Researchers Copyright © 2022 by RMIT University is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License , except where otherwise noted.
Share This Book
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Perspect Clin Res
- v.4(3); Jul-Sep 2013
Qualitative research
Vibha pathak.
Department of Clinical Research, Bharti Hospital and BRIDE, Karnal, Haryana, India
Bijayini Jena
1 Department of Nutrition, Bharti Hospital and BRIDE, Karnal, Haryana, India
Sanjay Kalra
2 Department of Endocrinology, Bharti Hospital and BRIDE, Karnal, Haryana, India
Scientific research is based upon finding a solution to a particular problem one can identify. There are various methods of formulating a research design for the study. Two broad approaches of data collection and interpretation in research are qualitative and quantitative research. The elementary method of conducting research was quantitative, but recently, qualitative method of research has also gained momentum among researchers.
Qualitative research focuses in understanding a research query as a humanistic or idealistic approach. Though quantitative approach is a more reliable method as it is based upon numeric and methods that can be made objectively and propagated by other researchers. Qualitative method is used to understand people's beliefs, experiences, attitudes, behavior, and interactions. It generates non-numerical data. The integration of qualitative research into intervention studies is a research strategy that is gaining increased attention across disciplines. Although once viewed as philosophically incongruent with experimental research, qualitative research is now recognized for its ability to add a new dimension to interventional studies that cannot be obtained through measurement of variables alone.[ 1 ] Qualitative research was initially used in psychological studies when researchers found it tedious to evaluate human behavior in numeric. Since then, qualitative research is used in other research fields as well. In clinical research, qualitative approach can help view the data more extensively. It strengthens clinical trials by enhancing user involvement in it.
Three broad categories of qualitative research of interest exists in clinical research: Observational studies, interview studies and documentary/textual analysis of various written records.[ 2 ] Qualitative research gives voice to the participants in the study.[ 1 ] It permits the participants to share their experiences of the effects of the drug of interest. This can open our eyes to new aspects of the study and help modify the design of the clinical trial. Qualitative study enhances the involvement of everyone related to the study. The researcher works on the social parameters in addition to the quantitative measures in the study. The subjects also have an empowering experience in the study. They have an active role in the study and can voice their individual benefits and harms of the study. In addition, with qualitative methods, the relationship between the researcher and the participant is often less formal than in quantitative research.
Qualitative research can have a major contribution in health research. In clinical trials, qualitative research can have a great impact on data collection, its analysis and the interpretation of results. Qualitative studies should be well-designed and the aims, procedures of the study should be meticulously adjudicated. Study should have pre-determined methods to nullify research bias. When combined with quantitative measures, qualitative study can give a better understanding of health related issues. The perspectives in clinical research should highlight advances in qualitative research as well, to optimize quality and utility of this method of research.
Query Management in Field Research (Beginner’s Guide)
Table of Contents
Query Management in Clinical Data Management refers to designing, conducting, and analyzing surveys, questionnaires, and other data collection methods to gather information for research. Query Management in Clinical Data Management is essential in field research as it helps ensure that data is collected efficiently, accurately and in a way that meets the research objectives. Effective query management enables researchers to gather reliable data and draw valid conclusions. This beginner’s guide to query management in field research will cover the importance of query management, the basic steps involved, advanced techniques, & common challenges. The guide will provide a comprehensive understanding of query management for those new to the field.
Understanding the Importance of Query Management in Clinical Data Management
Why query management matters in field research.
Query Management in Clinical Data Management is important in field research as it helps to ensure that data is collected efficiently and accurately. Poorly managed data collection methods can result in accurate data, leading to reliable results and valid conclusions. On the other hand, effective query management enables researchers to gather high-quality data, leading to robust and reliable research findings.
The Benefits of Effective Query Management in Clinical Data Management
Effective query management in field research has several benefits, including:
- Better data quality and accuracy
- Improved data collection efficiency
- Increased response rates
- Improved data analysis
- Better data security
- Enhanced research validity and reliability
- Better insights and decision-making based on research findings.
By following best practices in query management, researchers can ensure that their data collection methods are efficient, accurate, and relevant to their research objectives, leading to high-quality research outcomes.
Basic Steps in Query Management
Preparation.
- Define Research Objectives: The first step in query management is to define the research objectives. This includes determining the research questions and the information needed to answer those questions.
- Identify Target Audience: Next, researchers must identify the target audience they wish to survey. This involves defining the population of interest and selecting a sample of individuals participating in the data collection.
- Develop Research Questionnaire: Researchers must develop a questionnaire based on the research objectives and target audience. This involves designing questions relevant to the research objectives and easy for the target audience to understand and respond to.
Implementation
- Choose Data Collection Mode: Researchers must choose the appropriate data collection mode, such as online surveys, in-person interviews, or telephone surveys. The choice of data collection mode will depend on the research objectives, target audience, and available resources.
- Ensure Data Quality: Once the data collection mode has been chosen, researchers must ensure that the data collected is high quality. This involves ensuring that the questionnaire is properly administered, that the data is accurate and complete, and that any anomalies or errors are identified and corrected.
- Monitor Progress: Finally, researchers must monitor the progress of the data collection process and make any necessary adjustments to ensure that the data collection is proceeding smoothly and efficiently.
Advanced Query Management Techniques
Data analysis.
- Descriptive Statistics: It is a method of summarizing and presenting data that has been collected. This includes calculating measures of central tendency (e.g., mean, median, mode) and measures of dispersion (e.g., standard deviation, range).
- Inferential Statistics: Inferential statistics is a method of making conclusions about a population based on a sample of data. This involves using statistical methods to estimate population parameters and test hypotheses.
Sampling Techniques
- Simple Random Sampling: It is a method of selecting a sample from a population where each individual has an equal chance of being selected. This method is used when the population is large, and the sample is small.
- Stratified Sampling: It is a method of selecting a sample from a population where the sample is divided into subgroups based on specific characteristics. When a sample needs to be relevant, and the population is diverse, the researcher employs this technique.
- Cluster Sampling: It is a method of selecting a sample from a population where the sample is divided into clusters based on geographical or other criteria. This method is used when the population is too large or dispersed to be efficiently surveyed using simple random sampling.
By incorporating these advanced techniques, researchers can enhance the accuracy and efficiency of their data collection and analysis methods, leading to more robust and reliable research outcomes.
Common Challenges in Query Management and Solutions
Common challenges.
- Low Response Rates: One of the most common challenges in query management is low response rates, which can impact the validity and reliability of research findings.
- Non-Response Bias: Non-response bias occurs when individuals who do not respond to a survey differ systematically from those who do respond. This can impact the validity and reliability of research findings.
- Measurement Error: Measurement error occurs when data is collected inaccurately, leading to incorrect results. This can be caused by poor questionnaire design, poor data collection methods, or the inability of respondents to answer questions accurately.
- Increase Response Rates: To increase response rates, researchers can use incentives, such as cash or prizes, to encourage individuals to participate in the survey. They can also use personalized emails or phone calls to follow up with non-responders.
- Minimize Non-Response Bias: To minimize non-response bias, researchers can use weighting techniques to adjust for differences between those who respond and those who do not respond. They can also use multiple follow-up methods, such as phone calls, emails, and mail, to increase response rates.
- Reduce Measurement Error: To reduce measurement error, researchers can use clear and concise questions, pre-testing the questionnaire, and ensure that the data collection process is properly administered. They can also use multiple data collection methods to maximize the accuracy of the data collected.
By addressing these common challenges, researchers can ensure that their data collection methods are efficient, accurate, and reliable, leading to more robust and valid research outcomes.
Element Technologies offers conventional Query Management in Clinical Data Management methods in addition to electronic data capture (EDC), and electronic patient-reported outcomes (ePRO) approaches, providing a range of data management options while consistently upholding strict standards for the accuracy and timeliness of the data.
- Customers solely rely on our professionals to handle all biometrics-related tasks, from quick data entry to final trial results. Our knowledge enables us to suggest the most effective economic strategy for any clinical research project.
- Over the past few years, our Query Management in Clinical Data Management team has accumulated a wealth of practical knowledge with various data management platforms.
Element Technologies can provide the flexibility that our clients want and need as a result and similarly to all other areas of our operations.
Post A Comment
Save my name, email, and website in this browser for the next time I comment.

IMAGES
VIDEO
COMMENTS
Electronic Data Capture (EDC) is software specially designed for the collection of clinical data in electronic format, often for use in human clinical trials. EDCs, like Teamscope, have built-in query management and comply with Good Clinical Practice (GCP). We recommend to always use a validated EDC for collecting sensitive and research data.
Clinical Trial Basics: Understanding Query Management - Vial
Definition of query management. Query management describes the process of identifying, prioritizing, analyzing, and addressing (resolving) questions/issues that arise during clinical trials - most commonly discrepancies in clinical data. Developing and implementing a systematic approach for recording and resolving queries allows for ...
If the query phrase is found in the list of indexed phrases, then PubMed ™ retrieves the set of records that contain the query phrase; otherwise PubMed ™ treats the search phrase as if it was entered without quotes. Handling a query as a phrase is very important; however, it is currently limited to the list of indexed phrases in PubMed ™.
A research aim is typically broader in nature and outlines what you hope to achieve with your research. It doesn’t ask a specific question but rather gives a summary of what you intend to explore. The research question, on the other hand, is much more focused. It’s the specific query you’re setting out to answer.
Step 4: Create a research design. The research design is a practical framework for answering your research questions. It involves making decisions about the type of data you need, the methods you’ll use to collect and analyze it, and the location and timescale of your research. There are often many possible paths you can take to answering ...
Research. A research question is "a question that a research project sets out to answer". [1] Choosing a research question is an essential element of both quantitative and qualitative research. Investigation will require data collection and analysis, and the methodology for this will vary widely.
Write down your research topic and identify your main aspects. For each aspect list some alternative search terms (synonyms). Consider some different search techniques that you may need to use. Create a search query that combines your topic keywords and synonyms using Boolean operators AND and OR. Previous: Developing a search strategy.
Qualitative research focuses in understanding a research query as a humanistic or idealistic approach. Though quantitative approach is a more reliable method as it is based upon numeric and methods that can be made objectively and propagated by other researchers. Qualitative method is used to understand people's beliefs, experiences, attitudes ...
Effective query management in field research has several benefits, including: Better data quality and accuracy. Improved data collection efficiency. Increased response rates. Improved data analysis. Better data security. Enhanced research validity and reliability. Better insights and decision-making based on research findings.