REVIEW article
Epileptic seizure detection and experimental treatment: a review.

- 1 Department of Computer Science, University of Colorado, Boulder, CO, United States
- 2 Department of Computer Science and Engineering, University of Texas at Arlington, Arlington, TX, United States
- 3 Department of Computer Science, University of Oxford, Oxford, United Kingdom
One-fourths of the patients have medication-resistant seizures and require seizure detection and treatment continuously to cope with sudden seizures. Seizures can be detected by monitoring the brain and muscle activities, heart rate, oxygen level, artificial sounds, or visual signatures through EEG, EMG, ECG, motion, or audio/video recording on the human head and body. In this article, we first discuss recent advances in seizure sensing, signal processing, time- or frequency-domain analysis, and classification algorithms to detect and classify seizure stages. Then, we show a strong potential of applying recent advancements in non-invasive brain stimulation technology to treat seizures. In particular, we explain the fundamentals of brain stimulation approaches, including (1) transcranial magnetic stimulation (TMS), (2) transcranial direct current stimulation (tDCS), (3) transcranial focused ultrasound stimulation (tFUS), and how to use them to treat seizures. Through this review, we intend to provide a broad view of both recent seizure diagnoses and treatments. Such knowledge would help fresh and experienced researchers to capture the advancements in sensing, detection, classification, and treatment seizures. Last but not least, we provide potential research directions that would attract seizure researchers/engineers in the field.

1. Introduction
Epileptic seizure is a transient occurrence of signs or symptoms due to abnormal excessive or synchronous neuronal activity in the brain ( 1 ). Currently, about 2.3 million adults and more than 450,000 children and adolescents in the United States live with epilepsy. About 150,000 people are diagnosed with epileptic seizures each year ( 2 ). Epileptic seizures all start in the brain with sudden abnormal electrical discharges 1 . Among patients with epileptic seizures, two-thirds can control seizures through anti-epileptic medication, and another 8-10% could benefit from surgery. The remaining 25% have medication-resistant epileptic seizures and experience sudden seizure symptoms ( 3 ). Therefore, it is essential to notify the patient's medication-resistant epileptic seizure to the caretaker and analyze the pattern of related signals before, during, and after the seizure onset.
This article contributes to organizing seizure detection, classification, and treatment. We also provide potential research directions that would attract seizure researchers/engineers in the field. The existing seizure surveys reviewed seizure detection ( 4 ), classification ( 5 – 8 ), or treatment ( 9 , 10 ). This paper discusses state-of-the-art techniques for (1) capturing the physiology signals of seizures, (2) detecting and classifying types of seizures, (3) seizures therapy, and (4) the challenges and potential seizure-related research directions.
First, accurately and reliably capturing physiology signals related to seizure is a critical step for designing robust seizure detection systems. Monitoring brain activity signal (e.g., Electroencephalogram, EEG) is the most common method to detect seizures. The EEG recording of patients with epileptic seizures has two categories of abnormal activity: interictal, abnormal signals recorded between epileptic seizures, and ictal, the activity recorded during an epileptic seizure ( 6 ). We focused on epileptic seizure detection and considered interictal and ictal EEG signals except postictal state to detect abnormal EEG signals. The EEG signature of an inter-ictal activity is occasional transient waveforms, while that of an ictal activity is composed of a continuous discharge of polymorphic waveforms of variable amplitude and frequency ( 11 ). There are two kinds of traditional EEG recording techniques: Invasive EEG and scalp EEG. The invasive EEG recording is necessary to do surgery to implant the electrodes in the brain. In the case of the scalp EEG, the user is required to attach multiple electrodes that are connecting to a monitoring device through many wires. Therefore, Patients have to suffer the inconvenience of inserting something into the body or attaching multiple electrodes. Also, for the scalp EEG, a trained physician does such a complicated setup, and the studies are often conducted in hospitals. Besides the traditional EEG-based approach, epileptic seizures can also be detected through eye (lid) movement, heart rate, blood pressure, arterial oxygenation ( SpO 2 ), respiration, sweating, and so on ( 4 ). These activities can be captured from physiology signals, including Electrooculography (EOG), electrocardiography (ECG), electromyography (EMG), electrodermal activity (EDA), motion, audio/video recording, and multimodality sensing approaches ( 4 , 7 ).
We also discuss in detail the key components of these state of the art systems to provide a detailed picture of recent efforts on extracting these physiological signals for seizure detection. These systems often include some essential components as following: (1) signal acquisition, (2) signal processing, (3) feature extraction. The signal acquisition component is designed to capture physiological signals that are directly or indirectly related to seizures ( 4 , 12 ). These signals often contain a lot of noises, which will be processed further using novel, yet complex algorithms to extract the signal of interests ( 13 , 14 ). Next, many recent efforts have focused on building a stable setup features representing the presence of seizures to improve the detection accuracy ( 15 – 18 ). Hybrid time-frequency analysis features are often used to overcome the impact of human motion artifacts as well as to improve the system sensitivities ( 19 – 21 ). Specifically, wavelet transform analysis (WT) approaches are employed ( 22 ) to provide detailed resolutions of the seizure-related signatures on both time and frequency domains ( 23 ).
Second, after capturing the physiology signals, it is important to accurately detect and classify the type of detected seizures ( 5 , 6 , 24 ). Existing seizure classification methods primarily include classical machine learning approaches [e.g., support vector machine (SVM)] and novel deep-learning solutions [e.g., artificial neural network (ANN) ( 7 )]. SVM divides data belonging to two groups into a hyperplane ( 25 , 26 ). The original SVM is a binary classification, whereas the class for seizure is divided into at least three (focal seizure, generalized seizure, and healthy). State of the art SVM-approaches only can classify two classes of seizures (seizure vs. non-seizure) with high accuracy ( 27 , 28 ). It is not sufficient for seizure classification. Multiclass SVM methods have been used by splitting one multiclass problem into several binary classification problems ( 29 , 30 ). Although many related works have used multiclass SVM to classify various seizure types, it is impractical due to the low classification accuracy and many false alarms ( 29 , 31 , 32 ). Many recent efforts have focused on developing more complex learning algorithms. Especially, deep-learning solutions to detect a variety of seizures attract much attention from researchers ( 33 ). The classification performance depends on how the system structures hidden layers, such as multilayer perceptron neural network (MLPNN), adaptive neuro-fuzzy inference system (ANFIS), radial basis function neural network (RBFNN), convolutional neural network (CNN), and recurrent neural network (RNN) ( 34 ). ANN is the preferred method over SVM because it is not affected by the number of classes.
Third, after detecting and classifying different types of seizures, treatment methods need to be developed to reduce or remove the impact of seizures on patients' normal life. Even though it is difficult to find existing works in this direction, we believe that these can be done by exploring the uses of state of the art brain stimulation technique. We also discuss how the recent development in brain stimulation and interventions would help to treat seizures, such as decreasing cortical excitability with low-frequency magnetic stimulation ( 35 ) or counterbalancing the neuronal hyper-excitation through electric neural modulation ( 36 ). In particular, brain stimulation has been noted as an alternative to drug therapy to decrease the frequency of seizure or reduce the symptom. It is mostly divided into invasive and non-invasive. Although the invasive brain stimulation stimulates the problematic seizure part of the brain directly and provides a fast and accurate effect, it is necessary to do surgery to implant the stimulator inside the brain. It is very costly and may damage the brain during the operation. Thus, many patients are reluctant to this type of therapy. For non-invasive brain stimulation, there are two principal methods: transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS) ( 10 ). TMS uses the principle of electromagnetic induction to focus induced current in the brain ( 37 ). The magnetic fields generated by TMS penetrate human tissue painlessly and induces electric currents that can depolarize neurons or their axons in the brain ( 38 ). tDCS is one of transcranial electrical stimulation (tES) and applies low-amplitude direct currents via scalp electrodes and penetrate the skull to enter the brain ( 37 ). Unlike other tES methods, tDCS delivers a sustained current ( 39 ) and can make the therapeutic effect through the sustained current. However, TMS and tDCS provide low spatial resolutions, which lead to modulate neuronal activity not only in the target but also in surrounding circuits ( 40 ). Transcranial focused ultrasound (tFUS) is emerging as a method that can complement the low degree of spatial focality of TMS and tDCS. We examine how brain stimulation can reduce seizures based on these three approaches.
Last but not least, inspiring from the recent development in seizure detection and classification method, we found that more efforts are needed to put into the following research direction to realize a complete, reliable, and low-cost seizure detection systems. First, we believe that the state-of-the-art seizure detection system performance is sufficient to build a robust and reliable wearable device that could be used for daily seizure monitoring and classification. Second, as the seizure signatures are detected and monitor, the recent brain-stimulation techniques can be used to reduce seizure. We also suggest different directions on how to build reliable and wearable seizure therapy systems. Lastly, we discuss how to build an integrated monitoring and stimulating seizure.
In the following, we first describe the state-of-the-art approach to capture physiological signals related to seizures in section 2 reliably. Next, we discuss recent efforts on building machine learning techniques to detect and classify seizures in section 3. In section 4, we discuss the different approaches to seizure therapy. Lastly, we summarize the overall contents of this article and provide the prospect of future research.
2. Analyzing Physiology Signals of Epileptic Seizure
Seizure detection and therapy systems generally consist of five processes: (1) signal acquisition, (2) signal processing, (3) feature extraction, (4) classification, and (5) therapy ( 5 , 6 , 24 ). The processes mentioned above are illustrated in Figure 1 . In this section, we discuss the needed signal processing steps to analyze the captured physiology signals of epileptic seizures. Upon the processed data, detection and classification algorithms could be built.
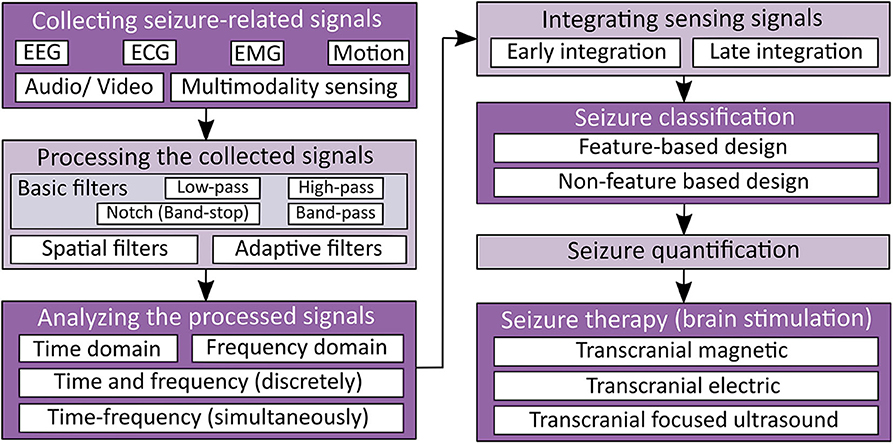
Figure 1 . Seizure detection and therapy overview.
2.1. Collecting Seizure-Related Signals
A seizure can be detected by monitoring various physiological signals from the human body through (1) EEG, (2) EMG, (3) ECG, (4) motion, and (5) audio/video recording ( 4 , 7 ). Among these physiological signals, EEG is the most popular choice because of its advantages, such as (1) the ability to capture the neural activation of the brain, (2) high temporal, and (3) spatial resolutions. However, the main limitation of traditional EEG measurement lies in its obtrusiveness and complicated setup, so it can only be performed in a controlled environment by a specialized technician. Also, some kinds of seizures like generalized onset motor seizures can be detected more clearly by measuring body movements or other physiological signals ( 18 , 41 ). Thus, researchers have developed seizure detection devices using various non-EEG signals as well as EEG signals ( 17 , 18 ). In the following discussion, we discuss how these recorded signals are used to detect seizure events by dividing into EEG and non-EEG methods.
2.1.1. Electroencephalogram (EEG)-Based Approach
EEG recording is the most common method to get the biosignals for seizure detection. It measures the electrical activity of the brain. Since epileptic seizure activities appear as abnormal signal patterns on the EEG, we can use the EEG signal variation to detect seizures. EEG signals with paroxysmal abnormality show spikes, spike-and-slow waves, and sharp waves in Figure 2A . Spikes are the primary form, and their time length is 20–70 ms. The spike-and-slow waves appear after spike-wave, and their time length is 200–500 ms. Sharp waves are similar to spike-wave, but their time length is 70–200 ms ( 5 ).
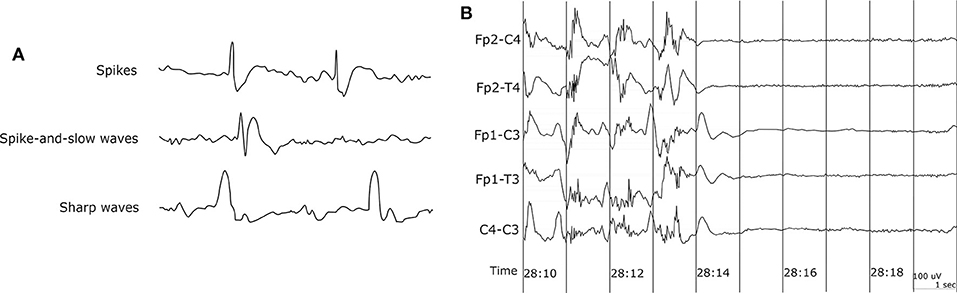
Figure 2 . EEG waveform. (A) EEG waveform with paroxysmal abnormality. (B) Sudden death in epilepsy recorded in ambulatory EEG ( 42 ).
The EEG recordings of patients with epileptic seizures show two categories of abnormal activity. Interictal has the abnormal signals recorded between epileptic seizures, and ictal is the activity recorded during an epileptic seizure ( 6 ). We focused on epileptic seizure detection and considered interictal and ictal EEG signals except postictal state to detect abnormal EEG signals. The EEG signature of an inter-ictal activity is occasional transient waveforms, while that of an ictal activity is composed of a continuous discharge of polymorphic waveforms of variable amplitude and frequency ( 11 ).
Many studies have been carried out for seizure detection using scalp EEG. Among them, we have selected and summarized some studies from past to recent which clearly explained the seizure detection procedure, as shown in Table 1 . Attaching EEG electrodes on all parts of the scalp is reasonable because there are many types of seizures, and the initial location which was generated the abnormal EEG signal is different. However, it causes mobility impairment, increases the cost of the measuring device, and is inappropriate for patients who need continuous seizure monitoring.
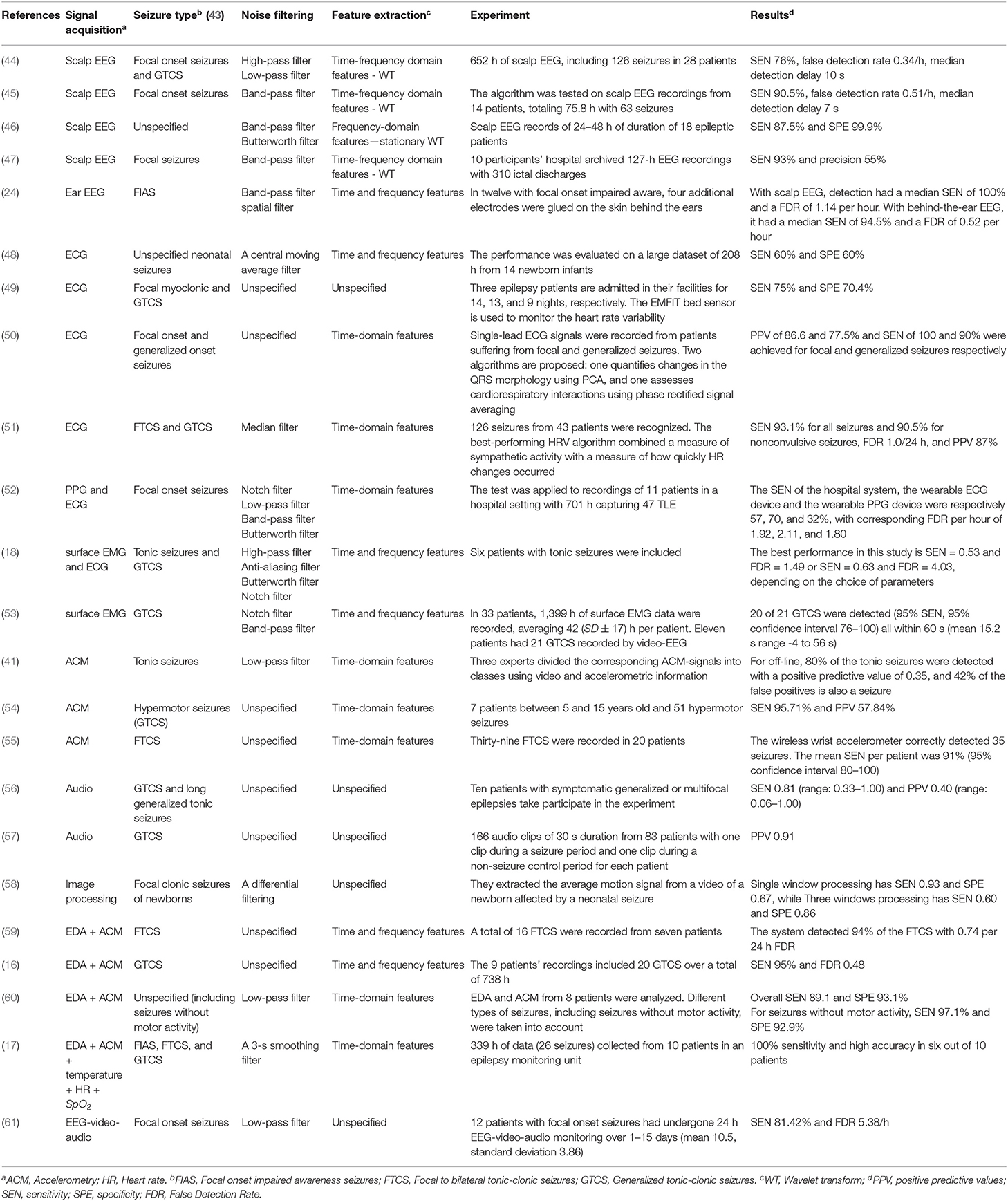
Table 1 . Seizure detection depending on the signal types.
Capturing EEG signals around the ear is a promising finding which can minimize the obtrusiveness of conventional EEG methods. The evoked responses from the ear-EEG are typically 10–20 dB lower in amplitude than those of traditional scalp EEG recordings while maintaining a similar signal-to-noise ratio (SNR) ( 62 ). Mikkelsen et al. ( 63 ) compared 32 conventional scalp electrodes with 12 ear electrodes. The measured signal from the ear electrodes reflects the same cortical activity as that from nearby scalp electrodes. Bleichner et al. ( 63 ) also worked for the comparison between a traditional EEG cap setup and their around-the-ear electrode array (cEEGrid). They have shown that their system can capture meaningful EEG signals such as eye-closing alpha wave, sleep spindles, and epileptic spike-wave. Gu et al. ( 24 ) utilized the cross-head and unilateral channels from the behind-the-ear EEG. Temporal waveform and frequency content during seizures from behind-the-ear EEG visually resembled those from scalp EEG. Especially, this paper provides the coherence between the behind-the-ear EEG channel and the best match-up scalp EEG channel on 12 patients like Figure 3 . McLean et al. ( 42 ) reported the sudden death in epilepsy recorded in ambulatory EEG. In Figure 2B , the seizure activity abruptly terminated, and the EEG became a flat line. The EEG variation graph of Figure 2B shows that these EEG channels may have significant patterns for detecting a seizure. Also, the EOG from LT-LC and RT-RC have similar morphology to that from Fp1-F7 and Fp2-F8, respectively.
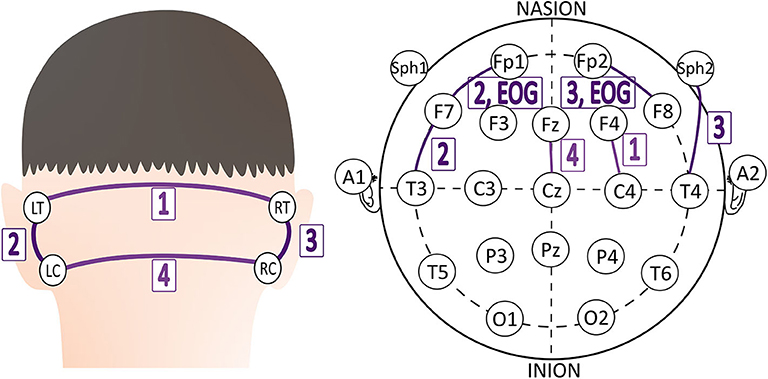
Figure 3 . The best match-up scalp EEG channel of each behind-the-ear EEG channel on 12 patients ( 24 ).
2.1.2. ECG, EMG, Motion, Audio, and Video-Based Approach
EEG-based measurement usually implies that the sensors need to be attached to a human head for seizure defections. Also, EEG monitoring is prone to errors in interpreting complex signals of EEG and is mainly used to detect seizures from temporal lobe epilepsies ( 2 , 64 ). Therefore, researchers have developed seizure detection devices with various other methods. Among the relatively recent studies, we tried to select papers, which use other signals more actively than EEG and follow the clear seizure detection procedure, as shown in Table 1 . For example, contactless sensing devices such as mattress sensor [Emfit 2 , MP5 3 ], carry-on devices such as smartwatches or wrist devices [Cogan ( 65 ), Embrace ( 59 ), Inspyre 4 ], smart textiles (Neuronaute 5 ), and temporary tattoos ( 4 ) can be used to detect epilepsy. We found that ECG, EMG, motion, and audio/video recording approaches have been used to monitor epilepsy.
Electrocardiography (ECG) monitoring measures the electrical properties of the heart and detects heart rate (HR) and heart rate variability (HRV). Most of the generalized tonic-clonic seizures (GTCS) cause an increase in HR ( 66 ). Such events subsequently increase the risk of sudden unexpected death in epilepsy (SUDEP) ( 42 ). HRV is also useful to distinguish focal seizures with physical exercise ( 51 ). The most common pattern of HRV associated with focal onset impaired awareness seizures is an initial steep acceleration at the onset of the seizure ( 67 ). The HRV in temporal lobe seizures is different from that in psychogenic non-epileptic seizures ( 68 ). ECG can be used to detect a seizure. However, the accuracy and ability to detect a seizure early are still very limited.
Electromyography (EMG) monitoring measures electrical activity in response to a nerve's stimulation of the muscle. Motion is detected using accelerometers measuring the accelerations of objects in motion along reference axes ( 69 ). Both signals could be useful to detect generalized onset motor seizures.
For Audio/video recording , Arends et al. ( 56 ) evaluated the performance of audio-based detection of primary seizures (tonic-clonic and long generalized tonic). They adapted the sound threshold by training during the first week. Recognizable sounds over the threshold occurred in 23 of the 45 significant seizures. This result signifies the use of only audio recording has definite limitations. Ntonfo et al. ( 58 ) proposed the image processing approach to detect the focal clonic seizures of newborns, which are related to the periodic movements of parts of the body. They extracted an average luminance signal representative of the body movements from a video of a newborn. Single window processing has high sensitivity ( T P T P + F N , where TP : True Positive, FN : False Negative) and low specificity, ( T N T N + F P , where TN : True Negative, FP : False Positive), while multiple interlaced window processing has low sensitivity and high specificity. It is necessary to apply the advanced window protocol to improve performance.
2.1.3. Multimodality Sensing Approach
The multimodality sensing approach may improve sensitivity and lower false-positive alarms by combining the profits of each sensor, like sensing EMG signals for tonic seizure detection ( 12 ). We have chosen a number of multimodality sensing studies for the purpose of dealing more with studies that use other signals more actively than EEG, as shown in Table 1 . Electrodermal activity (EDA) refers to the variation of the electrical properties of the skin in response to sweat secretion ( 70 ). EDA is mainly used with other sensors to detect seizures, especially with ACM ( 59 ).
Cogan et al. ( 65 ) detected epileptic seizures using wrist-worn bio-sensors, which detect heart rate (HR), arterial oxygenation ( SpO 2 ), ACM, EDA, and temperature. They observed the seizure pattern of HR ↑ ⇒ SpO 2 ↓⇒ EDA ↑ . Using EEG and non-EEG signals together could be more appropriate to employ the seizure detection system precisely and extensively. Pauri et al. ( 61 ) applied EEG-video-audio monitoring to 12 patients with refractory focal seizures using 15-channel EEGs (video-cassettes). Greene et al. ( 71 ) combined EEG monitoring with ECG monitoring simultaneously for the robust detection of neonatal seizures.
2.2. Processing the Collected Signals
To collect signals, we can use wet electrodes or dry electrodes. Although the use of dry electrodes is suitable for continuous signal collection, we still need to rely on the conductive paste and gripping force of the earpieces to address the gap between the electrodes and the user's skin ( 72 ). Therefore, wet electrodes are used to maintain signal quality. And gold-plated copper electrodes are proper material due to the resistance to skin oil and sweat and rare skin allergy ( 73 ). Signal processing is necessary to get the clear biosignal waveform in the most significant frequency range (1–35 Hz) without signal distortion. Raw signal is influenced by noises from power-line and other equipment, and the signal is a mixture of several biological signals, including EEG, EOG, and EMG signals. Thus, we need to use filters.
2.2.1. Basic Filters
Typical four types of the basic filter have been used to get the clear biosignal: a low-pass, high-pass, band-pass, and notch (= band-stop) filter. In the United States, the notch filter is set at 60 Hz 6 because the 60 Hz power-line frequency noise from wires, light fluorescent, and other equipment can contaminate biosignal records. The high-pass filter can remove the low-frequency artifacts noise due to poor contact state of electrodes or the sweat of the patient under the electrodes. Furthermore, the median filter can reduce noise and high-frequency oscillations in signal data ( 74 ). However, these filters do not preserve all designated frequencies and cannot extract the specific biosignal among the overlapped biosignals spectrum ( 75 ). For example, EOG signals by eye movements or blinks propagate to the scalp electrodes creating noises in the recorded EEG signals.
2.2.2. Spatial Filters
The spatial filter technique, such as Independent Components Analysis (ICA), is a promising solution to solve the challenge of overlapped artifacts in EEG recording. Jung et al. ( 76 ) applied the spatial filters derived by ICA, which can separate and remove ocular artifacts from the recorded EEG signals. ICA technique, however, requires the use of multiple EEG electrodes to provide spatial information with the captured signals. In other words, ICA can decompose the independent components only when the number of data channels is more than that of signal sources ( 77 ). Also, ICA does not work when the training data set is too small ( 76 ).
The regression-based technique is a proposed solution to overcome the limitation of ICA. We can apply the regression approach to any number of EEG channels. Regression-based noise filtering has two phases. First, the calibration phase determines the transfer coefficients between other biosignal channels and EEG channels. Second, the correction phase estimates the noise component in the EEG recording ( 78 ). Due to this procedure, it is challenging to apply this filter in real-time. The coefficients should be controlled in the normal range. Once the coefficient is out of the range, it is not trivial for the calibration phase to turn them over to the normal range.
The wavelet-based technique is another denoising method that has been proposed for EEG signals. The wavelet-based technique compares each wavelet coefficient to a predetermined threshold and sets it to zero if its magnitude is less than the threshold ( 78 ). This technique can work in real-time and does not require the prior data of the artifacts ( 79 ). However, choosing the threshold level is a complicated process.
Lastly, if EEG recordings have multi-channel, they give blurred images of brain activity due to the volume conduction ( 80 ). In this situation, spatial filters can improve the SNR using the common spatial pattern (CSP) algorithm. The CSP extracts new time series whose variances are optimal for the discrimination of two populations of EEG based on the simultaneous diagonalization of two covariance matrices ( 81 ). Several related works have demonstrated the performance of spatial filters for multi-channel EEG ( 80 , 81 ).
2.2.3. Adaptive Filters
Adaptive filter adapts the coefficients of the filter to generate a signal similar to the noise ( 75 ). A linear adaptive filter is made up of a primary signal (= corrupted signal) d ( n ), a secondary signal (= reference signal) x ( n ), an adjustable filter H ( z ), an output from the adjustable filter y ( n ), and an error e ( n ) in Figure 4 ( 82 ). The adaptive filter usually adjusts the coefficients of filter to minimize the squared error between d ( n ) and y ( n ) ( 83 ). Correa et al. ( 84 ) arranged a cascade of three adaptive filters to remove multiple artifacts and got the EEG signal from EEG + artifacts (EOG, ECG, and power-line frequencies). However, the linear adaptive filter cannot deal with non-linear signals.
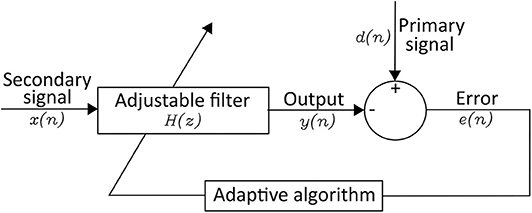
Figure 4 . Structure of an Linear Adaptive Filter.
Researchers have developed a neural network (NN) and fuzzy network (FN) to control non-linear signals. NN is made up of an input layer, hidden layer, and output layer, and users do not know the hidden layers. Fuzzy logic analyzes analog data as logical variables having continuous values between 0 and 1 ( 85 ). Each method has the following limitation. The structure of NN is challenging to decide, and the learning efficiency of FN is lower than that of NN ( 86 ). Combining them as a fuzzy neural network (FNN) is one solution to complement each drawback. However, FNN requires the training data in advance for the backpropagation, making the real-time application difficult ( 87 ).
3. Classifying and Detecting Epileptic Seizure
Seizure classification mainly categorizes the input data into one of two groups: seizure and non-seizure. Under specific requirements, the group of seizures can break down into sub-categories depending on the location of the source and symptoms. Those are considered as multiclass classification. Feature-based approaches, including feature extraction and conventional machine learning techniques, have been widely adopted to identify epileptic seizures ( 7 ). For each specific data set, the studies listed in Table 2 imposes different classifier configurations and features. Although we tried to cover recent studies in Table 2 , we also introduced some previously published papers to represent typical classification methods or data that were used well in the past. After the recent success of Deep Learning, many researchers start applying Deep Learning for medical problems, especially epileptic seizure detection/classification ( 33 ).
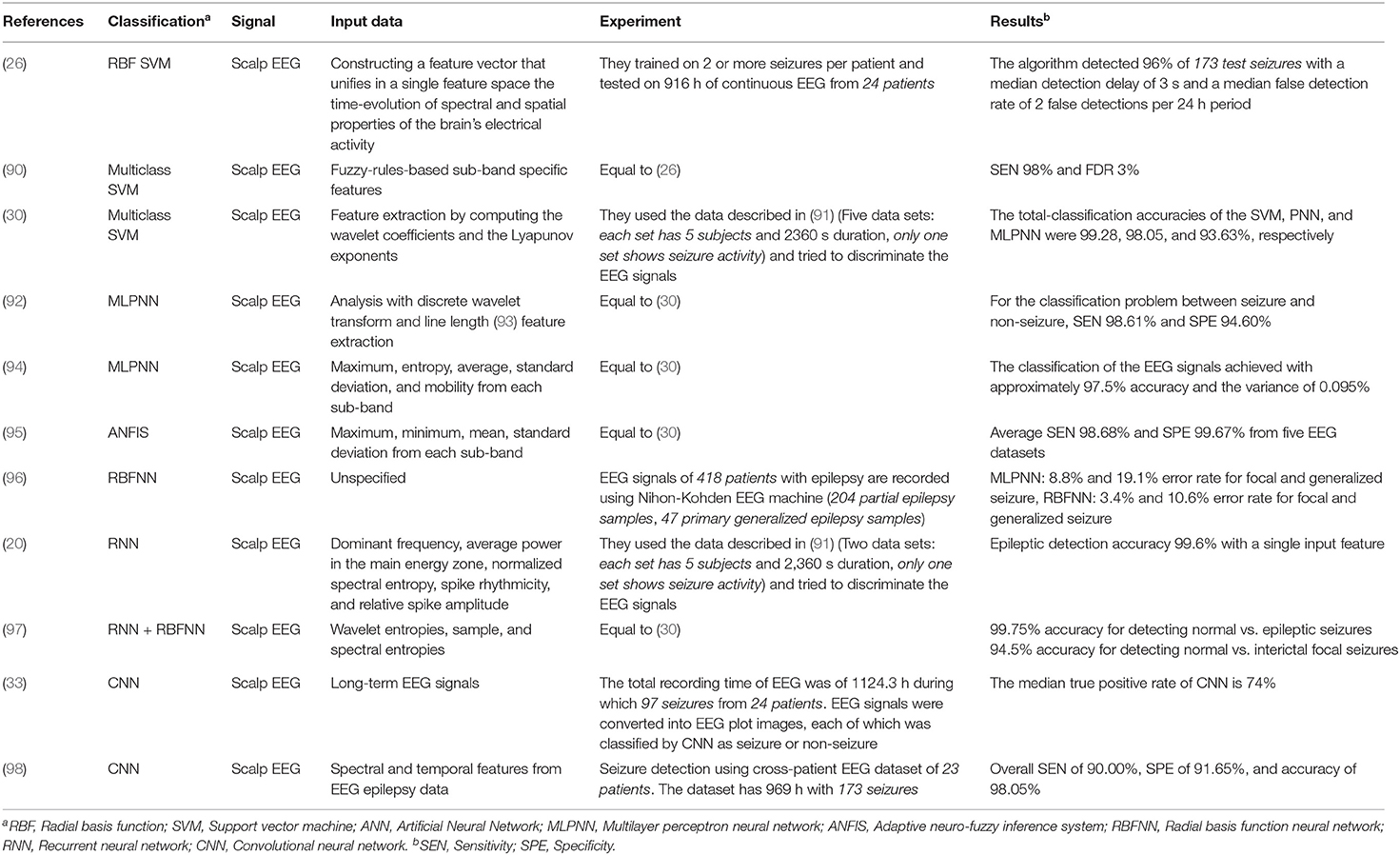
Table 2 . Classification for seizure detection [recommended comprehensive analysis: ( 88 , 89 )].
3.1. Feature-Based Design
In this protocol, the number and type of features have a significant impact on seizure detection performance. There are several feature extraction methods, including time-domain features, frequency-domain features, time and frequency features (discretely), and time-frequency domain features (simultaneously).
3.1.1. Feature Extraction
3.1.1.1. time-domain analysis.
Time-domain analysis works for the stationary signals, but biosignals are non-stationary. One method to quantify a non-stationary time series is to consider it as a large number of stationary segments ( 99 ). There are 12 key features in three categories: (1) mean and standard deviation for a time series with symmetric distribution; (2) median, mode, range, first quartile , and third quartile to measure the locations of a time series; (3) maximum, minimum, variation, skewness, kurtosis to pull out the shape characteristics of a time series ( 99 ). Besides, existing works have used slope sign change, Willison amplitude ( 100 ), Lyapunov exponents ( 13 ), and Hjorth parameters ( 19 ) to extract features from EEG signals.
3.1.1.2. Frequency-domain analysis
There are three basic techniques for frequency-domain analysis: Fast Fourier transform (FFT), Eigenvector, and Autoregressive ( 101 ). FFT decomposes a function (signal) of time into a frequency component fast by rearranging the input elements in a bit-reversed order and building the decimation in time ( 102 ). Fourier transform is only suitable when we are interested in what frequency components exist, not times occurring the frequency components ( 23 ). However, the time that a specific frequency component happens is essential to analyze biosignals. To solve this problem, a short-time Fourier transform (STFT) uses the idea that some part of a non-stationary signal at any given interval of time is a stationary signal. Johnson et al. ( 103 ) extracted relative power spectral density (PSD) value for each 1 Hz bin from EEG 1–40 Hz to check the state of drowsiness.
Eigenvectors are employed to calculate the signal's frequency and power from artifact dominated measurements ( 101 ). These methods are based on an eigen decomposition of the correlation matrix of the noise-corrupted signal and produce high-resolution frequency spectra even when the SNR is low ( 14 ). There are three eigenvector methods with higher resolution (Pisarenko, MUSIC, and Minimum-Norm) ( 104 ). The Pisarenko algorithm is particularly useful for estimating a spectrum containing sharp peaks at the expected frequencies ( 105 ). The MUSIC method eliminates the effects of spurious zeros by using the averaged spectra of all of the eigenvectors corresponding to the noise subspace ( 106 ). The Minimum-Norm method puts false zeros inside the unit circle and calculates the desired noise subspace vector from the eigenvectors ( 107 ).
Autoregressive methods estimate the PSD of the EEG signal using a parametric approach. These methods solve the spectral leakage problem and yield better frequency resolution ( 101 ). Yule-Walker method may lead to incorrect parameter estimates in the case of nearly periodic signals ( 108 ). As an alternative, Burg's method first estimates the reflection coefficients, and then the parameter estimates are determined using the Levinson-Durbin algorithm ( 108 ).
3.1.1.3. Time and frequency features
Using both time- and frequency-domain features can improve seizure classification performance. Srinivasan et al. ( 20 ) used three frequency-domain features (dominant frequency, average power in the primary energy zone, and normalized spectral entropy) and two time-domain features (spike rhythmicity and relative spike amplitude). Iscan et al. ( 109 ) combined time and frequency features to distinguish between seizure and healthy EEG segments. They got time-domain features using the cross-correlation method and frequency-domain features calculating the PSD.
3.1.1.4. Time-frequency domain analysis
Time-frequency domain analysis studies a signal in both the time and frequency-domains simultaneously. Time-frequency distribution (TFD) and wavelet transform analysis (WT) are the principal techniques of time-frequency domain analysis.
The basic idea of TFD is to devise a joint distribution of time and frequency that describes the energy density or intensity of a signal simultaneously in time and frequency ( 110 ). In this distribution, we can calculate the fraction of energy in a specific frequency and time range, and the distribution of frequency at a particular time. It is done by constructing a joint time-frequency function with the desired attributes and then obtaining the signal that produces the distribution ( 110 ). Boashash et al. ( 111 ) performed TFD feature extraction on multi-channel recordings for seizure detection in newborn EEG signals. They selected the optimal subset of TFD features using the wrapper method with sequential forward feature selection.
WT is an alternative to STFT. STFT gives information about the spectral components at any given interval of time, but not at a specific time instant ( 23 ). It causes a problem of resolution. WT gives a variable resolution using the characteristics that high frequencies are better resolved in time-domain, and low frequencies are in frequency-domain ( 23 ). WT can capture very minute details, sudden changes, and similarities in the EEG signals ( 22 ). It is more effective than other methods because biosignals are non-stationary ( 112 ). WT transforms a small wave (a mother wavelet) as a pattern and expresses an arbitrary waveform on the scale of magnification and reduction. WT classified into continuous wavelet transform (CWT) and discrete wavelet transform (DWT). The CWT Y ( j, k ) is defined by the following equation for any fixed-function Ψ j, k ( t ) in Equation (1). The mother wavelet (Ψ j, k ( t )) is shifted by a small interval of j in the x-axis, and correlation coefficients are computed. This procedure is repeated for various scaling factors k (dilations) in the y-axis ( 22 ).
CWT is computed by changing the scale of the analysis window, shifting the window in time, multiplying by the signal, and integrating overall times ( 23 ). However, the CWT has disadvantages such as severe redundancy of coefficients, difficulty in managing infinite wavelets, and lack of analytical methods that can easily calculate for most functions. The DWT solves these disadvantages by scaling and moving discretely, not continuously. The DWT employs two sets of functions called scaling functions and wavelet functions. These functions are related to the low-pass filter [ g ( n ), the mirror vision] and high-pass filter [ h ( n ), the discrete mother wavelet], respectively ( 113 ). In the sub-band decomposition of DWT, each stage consists of two digital filters and two downsamplers by 2. The first stage receives a signal x ( n ) and provides the detail D 1 and the approximation A 1 ( 114 ). The first approximation is further decomposed continuously. Many related works have used WT to extract features ( 115 ).
3.1.1.5. Integrating sensing signals from multiple channels
Shoeb et al. ( 116 ) extracted four features ( m = 4) representing waveform morphology on each of 21 channels ( n = 21). Then they assembled these features into a feature vector by concatenating them orderly called the early integration (EI) architecture. They also studied the performance of a patient-specific detector with an alternative architecture called the late integration (LI) architecture. In this structure, the m features of each channel assembled into a distinct feature vector and are assigned to the individual class (seizure or non-seizure). LI allows for the independent classification of activity on each channel, whereas EI summarizes interrelations between channels.
3.1.1.6. Lesson learned
Recording EEG signals is crucial because almost all seizures start from the brain. However, EEG measurement requires attaching many electrodes on the scalp with mobility impairment and making continuous measurement difficult. Therefore, we look forward to developing the devices which measure EEG signals without causing discomfort. Recording EEG signals around the ear is an emerging method to record EEG signals on the scalp. We confirmed some possibilities by comparing the similarity between EEG signal measurements around the ear and those on the scalp from many related works. Furthermore, we can get various biosignals as well as EEG around the ear ( 117 ).
The future seizure detection system is necessary to improve the signal processing procedure using spatial and adaptive filters. Basic filters do not entirely remove noise and not preserve all designated frequencies and cannot extract the specific biosignal among the overlapped biosignals spectrum ( 75 ). As we saw in Figure 3 , EEG recordings have multi-channels even around the ear. Spatial filters can improve the SNR among several channels and surrounding noises using the CSP algorithm. Adaptive filters reflect the previous signal error through the self-developed adaptive algorithm. Linear or non-linear signals are controlled depending on the adaptive algorithm.
Recent papers have applied WT to the seizure detection system to analyze the processed signals. Time-domain analysis and frequency-domain analysis are easy to use and give clearly defined features. However, they may not catch the minute features for seizure because biosignals are non-stationary. Even though there are alternatives like making a large number of stationary segments from any given interval of time, they still have a problem of resolution. Meanwhile, WT can capture very minute details, sudden changes, and similarities in the EEG signals ( 22 ). WT classified into CWT and DWT. CWT has disadvantages about the redundancy of coefficients, difficulty in managing infinite wavelets, and lack of analytical methods. DWT is usually used to solve these problems. Daubechies wavelet is the most commonly used wavelet for DWT, and the interested reader for the wavelet-based EEG processing can refer to ( 22 ) for more details.
3.1.2. Feature Classification Algorithms
3.1.2.1. support vector machine (svm).
SVM is a linear classifier that uses a hyperplane ( 25 ) to separate the data space. The mathematical expression of a hyperplane is the general form of a linear equation in multi-dimensional space.
which must have at least one a i other than zero. Given a dataset, there could be many hyperplanes that separate the data. SVM maximizes the distance between the nearest points from each group toward the hyperplane, as described in Figure 5A . This distance is called a margin. Conventional linear SVM has a limitation due to the non-linear changes of biosignals. A non-linear SVM classifier using an RBF kernel is potentially a proper approach because the seizure and non-seizure classes are not linearly separable. This approach detected 96% of 173 test seizures in a median detection delay of 3 s ( 26 ). When the categories of seizure are divided into more than two groups (e.g., focal seizure, generalized seizure, healthy), the binary classification is not sufficient to distinguish data. In this case, the SVM method for dealing with multiclass is applied to handle the problem.
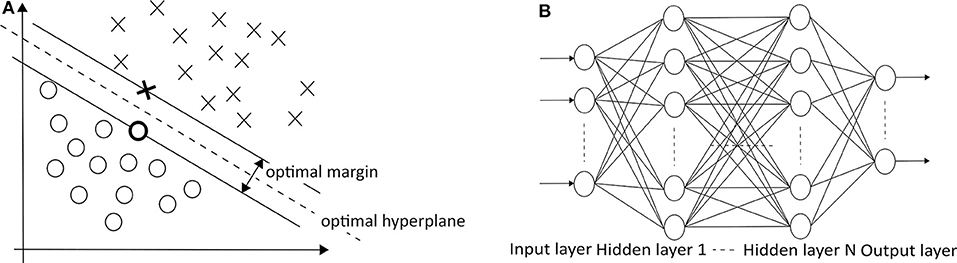
Figure 5. (A) SVM (O: positive cases, X: negative cases) ( 25 ) and (B) MLPNN ( 113 ) architectures.
The development of multiclass SVM follows two approaches. One vs. rest approach is a method of binarizing the i-th class and the remaining M −1 classes. This process is repeated in the same operation for the other classes. A total of M hyperplanes are created. On the other hand, one vs. one approach is to select two of the M classes to create a hyperplane, then select the other two class combinations and repeat the same operation. A total of M ( M −1)/2 hyperplanes are created. The one vs. rest approach has an imbalance in the size of the two sets, unlike the one vs. one approach. However, the one vs. rest approach is mainly used because the total number of hyperplanes increases linearly with the number of classes. Many related works have used multiclass SVM to classify seizure states ( 29 , 30 ).
3.1.2.2. Multilayer perceptron neural network (MLPNN)
In MLPNNs , each neuron in the hidden layer sums its input signals after multiplying them by each link weights and computes its output as an activation function of the sum, as shown in Figure 5B ( 113 ). The activation function can be the rectified linear unit (ReLU), hyperbolic tangent, and so on. Guo et al. ( 92 ) used Bayesian regularization back-propagation to train MLPNN, which updates the weights and biases depending on Levenberg-Marquardt optimization. It minimizes a combination of squared errors and weights and then determines the correct combination to produce a network that generalizes well. Their network structure has one input layer with five neurons, one hidden layer with 10 neurons, and one output layer with one neuron (0—the normal/non-seizure EEG, 1—the seizure EEG). Naghsh and Aghashahi imported the feature vectors into an MLPNN system to classify the signal into three states of normal (healthy), a seizure-free interval (interictal), and a full seizure interval (ictal) ( 94 ).
3.1.2.3. Adaptive neuro-fuzzy inference system (ANFIS)
Neuro-fuzzy systems utilize the mathematical properties of ANNs in tuning rule-based fuzzy systems to approximate the way humans process information ( 95 ). Especially, ANFIS ( 118 ) has shown significant results in modeling non-linear functions. A type-3 ANFIS has five layers like Figure 6A . A circle and square indicate a fixed and adaptive node, respectively. In layer 1, the input values pass through the selected fuzzy membership function (μ A i and μ B i , i = 1, 2). This function could be a bell-shaped with a maximum equal to 1. Premise parameters { a i , b i , c i } in the function change during the training process. In layer 2, each simple multiplier multiplies the output values of layer 1 [ w i = μ A i ( x )μ B i ( y ), i = 1, 2]. In layer 3, each normalization function produces w ¯ i = w i w 1 + w 2 , i = 1 , 2 . In layer 4, the output values of layer 3 go into the Takagi and Sugeno's first-order function ( 120 ). Consequent parameters { p i , q i , r i } in the function are determined during the training process. Lastly, one single node computes the overall output as the summation of all incoming signals ( ∑ i w ¯ i f i = ∑ i w i f i ∑ i w i ). Güler and Übeyli executed a detailed classification between set A (healthy volunteer, eyes open), set B (healthy volunteer, eyes closed), set C (seizure-free intervals of five parents from hippocampal formation of opposite hemisphere), set D (seizure-free intervals of five patients from epileptogenic zone), and set E (epileptic seizure segments) using ANFIS and got the classification accuracy 98.68% ( 95 ).
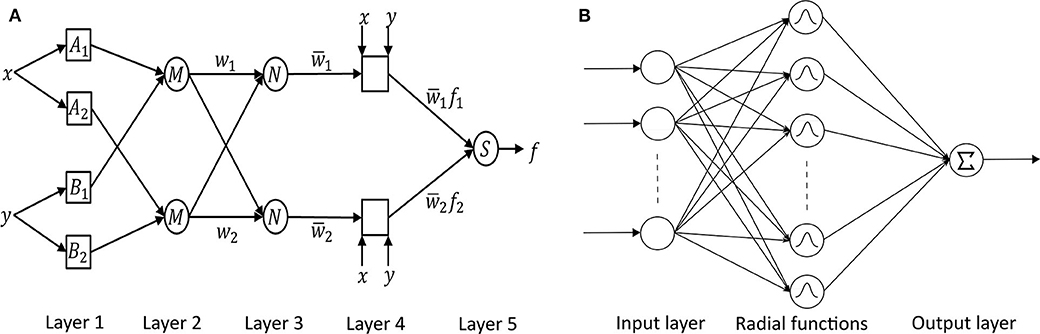
Figure 6. (A) ANFIS ( 118 ) and (B) RBFNN ( 119 ) architectures.
3.1.2.4. Radial basis function neural network (RBFNN)
RBFNN is feed-forward like MLPNN but has only one hidden layer with a non-linear radial basis function (RBF) in Figure 6B ( 119 ). RBF is a real-valued function whose value depends only on the distance from the origin. RBFNN has the advantages of a simple topological structure, its locally tuned neurons, and fast learning compared to MLPNN. Aslan et al. ( 96 ) compared MLPNN with RBFNN. In the case of MLPNN, 18 out of 204 focal seizure samples were classified as a generalized seizure (8.8% error rate for focal seizure), and 9 out of 47 generalized seizure samples were classified as a focal seizure (19.1% error rate for generalized seizure). RBFNN, on the other hand, showed 3.4% and 10.6% error rate for focal and generalized seizures, respectively. However, RBFNN requires to set correct initial states. Therefore, many seizure classification papers have focused on MLPNN.
3.2. Non-feature Based Design
According to the symptoms of seizures, various types of signal patterns appear, and it is difficult to understand all of them with specific features. Thus, no existing hand-crafted features appear universally applicable so far ( 33 ). Deep learning methods can analyze the EEG signal and learn related characteristics automatically in a supervised learning framework ( 121 ). Although there are existing works that use the classification methods described as feature-based ( 20 , 98 ), we summarize these techniques in terms of non-feature based design.
3.2.1. Convolutional Neural Network (CNN)
CNN takes the raw image data and calculates the convolution by iterating over the input data according to the filter size specified to extract the feature of the data. The shape of output data changes depending on filter size, stride, padding, max-pooling size, and so on. The classifier can perform supervised learning by matching the output data and answer classes. CNN, with its high recognition performance in medical images ( 122 ), can be as good as an epileptologist in classifying seizures by analyzing EEG plot images as being observed by Emami et al. ( 33 ). In their work, they applied CNN to long-term EEG that included epileptic seizure states. In particular, EEG data were divided into short segments based on a given time window (ranging from 0.5–10 s) and converted into EEG plot images (224 × 224 pixels), each of which was classified by CNN as seizure or non-seizure. They used VGG-16 ( 123 ) because small size convolution filters (3 × 3) are capable of detecting small EEG waves. VGG-16 is also computationally efficient and can handle non-stationary objectives. This work is meaningful because the study is the first comprehensive attempt to evaluate EEG as plot images. However, the median true positive rate of CNN 74% is still low, so we cannot use this classifier for real patients.
3.2.2. Recurrent Neural Network (RNN)
In RNN , in which a network's output state depends on an arbitrary number of previous inputs like Figure 7A . However, RNN has not been widely used in applications due to the lack of an efficient and universal training method ( 124 ). Other attempts have been made to overcome these limitations. Srinivasan et al. ( 20 ) used a special type of RNN as Elman network (EN) to detect epileptic seizures. An EN has the additional set called “context layer” as shown in Figure 7B . The hidden layer is connected to these context units. Kumar et al. ( 97 ) incorporated recurrent EN and RBFNN to detect epileptic seizures with the wavelet entropy features. They showed 99.75 and 94.5% accuracy for detecting normal vs. epileptic seizures and interictal focal seizures, respectively.
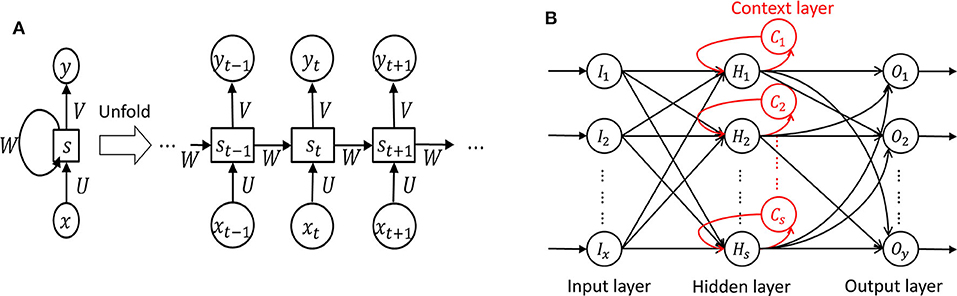
Figure 7 . Recurrent neural network ( 124 ). (A) Unfolded basic. (B) Elman network.
3.3. Seizure Quantification
Biosignal quantification is necessary to make the correlations between biosignals and actual seizures more accurate ( 125 ). Adeli et al. ( 126 ) utilized the correlation dimension (CD, representing system complexity) and the largest Lyapunov exponent (LLE, representing system chaoticity) to quantify the nonlinear dynamics of the original EEGs. They analyzed three groups: group H (healthy subjects), group E (epileptic subjects during a seizure-free interval), and group S (epileptic subjects during the seizure). For the CD values from the band-limited EEGs (0–60 Hz), group S (5.3) differs from the other two groups H (6.9) and E (6.7). For the LLE values, group H (0.089), group E (0.041), and group S (0.070) differ from each other. CD and LLE have shown the possibility of being used for classification. However, to the best of our knowledge, there is no concrete explanation between the biosignal and the severity of symptoms.
4. Experimental Non-invasive Anti-seizure Treatments
We have discussed the physiological signals related to seizure as well as how to use these signals to monitor and detect seizures. The next logical step is to build a system to reduce the impact of seizure. In this section, we discuss different non-invasive brain stimulation methods that can potentially be used for seizure therapy. While we try to describe the detailed specifications, working principles, advantages, and disadvantages of different brain stimulation techniques, we leave the discussions on how to design a proper seizure therapy for future works. In particular, we discuss in detail recent non-invasive brain stimulation efforts on Transcranial Magnetic Stimulation (TMS), Transcranial Direct Current Stimulation (tDCS) ( 10 ), and Transcranial Focused Ultrasound Stimulation (tFUS) ( 40 , 127 ). Since Vagus Nerve Stimulation (VNS) overlaps tDCS in terms of electrical stimulation methods and was previously introduced primarily as invasive stimulation, it was not included in the larger category. However, recently, invasive VNS therapy for drug-resistant epilepsy patients received FDA approval 7 . VNS is also a promising seizure therapy method.
4.1. Transcranial Magnetic Stimulation
Transcranial Magnetic Stimulation (TMS) uses the principle of electromagnetic induction to focus induced current in the brain, as shown in Figure 8A ( 37 ). Strong electric currents, circulating within a coil resting on the scalp, generate short and intense magnetic fields. These magnetic fields penetrate human tissue painlessly and induce electric currents that can depolarize neurons or their axons in the brain ( 38 ). TMS techniques include single-pulse TMS (spTMS), paired-pulse TMS (ppTMS), and repetitive TMS (rTMS), as shown in Figure 9 ( 131 ). In general, single- and paired-pulse TMS are used to verify brain functions, and rTMS induces changes in brain activity that can last beyond the stimulation period ( 132 ). While spTMS and ppTMS were reported to induce unexpected seizures during multiple experiments ( 133 ), rTMS is currently a better and safer approach for seizure therapy.
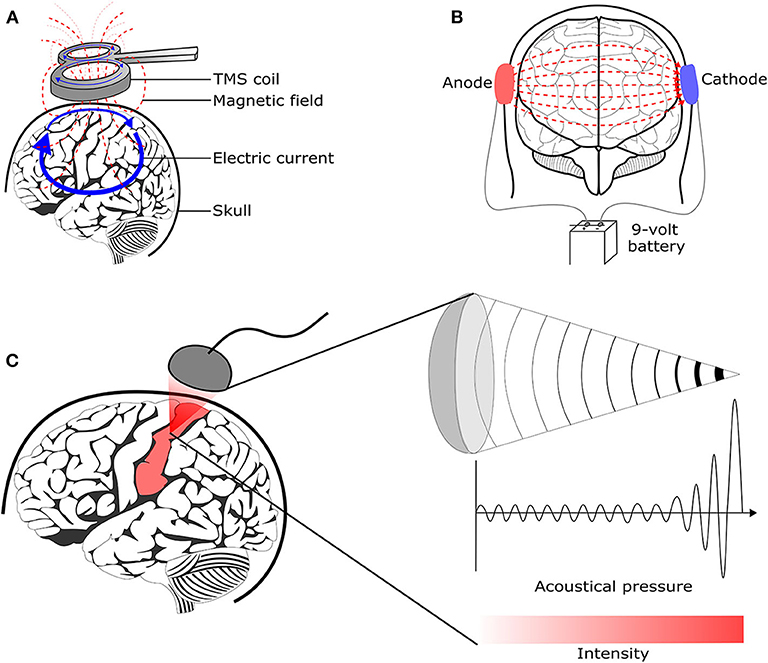
Figure 8 . Brain stimulation methods to reduce the seizure symptoms. (A) Transcranial magnetic stimulation ( 128 ) (B) Transcranial direct current stimulation ( 129 ) (C) Transcranial focused ultrasound stimulation ( 130 ).
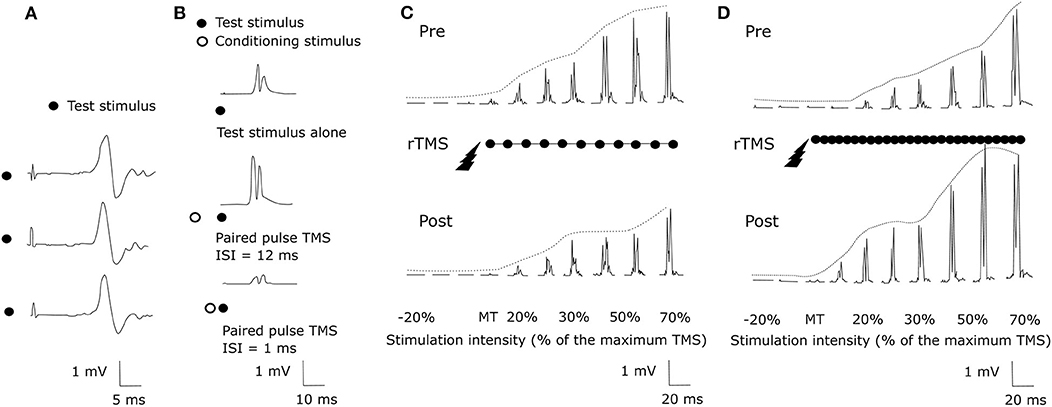
Figure 9 . TMS methods ( 131 ). (A) spTMS (B) ppTMS (C) low frequency rTMS (D) high frequency rTMS.
rTMS stimulates a single scalp site repeatedly and modulates cortical excitability. Figures 9C,D show examples tested before and after an rTMS regime. It consists of a long pattern of low (1 Hz) or high (20 Hz) frequency rTMS delivered to the left hemisphere's primary motor cortex during 28 min ( 131 , 134 ). rTMS has greater effects than single-pulse TMS but also has the potential to cause seizures ( 38 ). The FDA cleared an rTMS device as a treatment to alleviate symptoms of mildly treatment-resistant depression 8 . It shows the possibility of rTMS as a treatment for relieving various symptoms.
The effect of rTMS depends on the stimulation frequency, intensity, number of trains, inter-train interval, and number of sessions ( 135 ). We exclude the stimulus location element because it is a factor that varies depending on the symptom. The number of pulses per second of rTMS trains typically ranges between 1 and 50 Hz. One hertz paradigms are commonly applied continuously for several minutes, while higher frequency paradigms are applied in a patterned fashion like Figure 10 ( 136 ). Low-frequency rTMS produces a transient reduction in cortical excitability. High-frequency rTMS produces a local increase in cortical excitability and increases in MEP size ( 137 ). Specifically, this transient reduction effect of Low-frequency rTMS occurs in the motor cortex ( 138 ) and in the occipital cortex ( 139 ). High-frequency rTMS can improve cognitive processing to the dorsolateral prefrontal cortex ( 140 ). To compare low and high-frequency rTMS, Speer et al. ( 141 ) showed that 1-Hz rTMS was associated only with decreases in absolute regional cerebral blood flow (rCBF), while twenty-Hertz rTMS over the left prefrontal cortex was associated only with increases in rCBF.
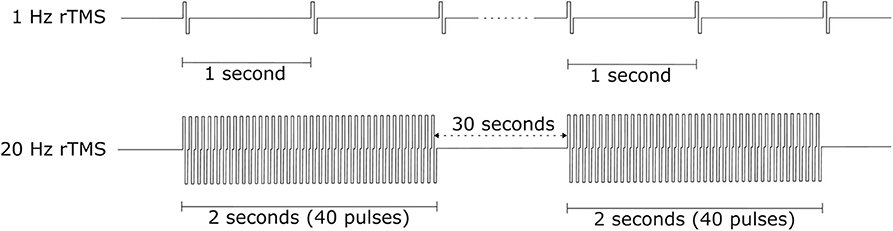
Figure 10 . rTMS protocol example.
The stimulation intensity is usually expressed as a percentage of MT. The MT is usually determined before each session by applying the TMS coil over the primary motor cortex ( 135 ). Pulse trains are the typical form to use rTMS. If TMS stimulates the brain continuously, it can increase the possibility of generating a seizure ( 142 ) and cause heating of the electrodes. Flitman et al. ( 143 ) reported the occurrence of focal to bilateral tonic-clonic seizure in one subject after three consecutive stimulated trials with 20% above MT and pulse train lasted 750 ms at 15 Hz. Dobek et al. ( 144 ) also found 25 reports of rTMS-induced seizures in their review. Therefore, we should follow the safety guidelines for rTMS ( 145 ). Based on the international workshop on the safety of rTMS in 1996, Wassermann et al. ( 146 ) introduced the guideline for the use of rTMS: at least 5 s intervals between 20 Hz trains with intensities of up to 1.1x the MEP threshold. A longer interval is necessary for the case of higher frequencies and intensities. Bae et al. investigated the risk of seizures associated with rTMS in patients with epilepsy and reported that only 4 of 280 patients experienced seizures during or after rTMS ( 147 ).
In animal studies, low-frequency rTMS (1,000 pulses at 0.5 Hz) decreased susceptibility to pentylenetetrazol-induced seizures in rats ( 148 ). Rotenberg et al. ( 149 ) suppressed seizures in rats injected with the kainic acid using EEG-guided 0.5 and 0.75 Hz rTMS, but 0.25 Hz rTMS was not effective. In human studies, 0.3 Hz low-frequency rTMS decreased interictal EEG epileptiform abnormalities in one-third of drug-resistant epilepsy patients but was not better than a placebo for seizure reduction ( 150 ). Instead, Cincotta et al. ( 151 ) suggested that 0.3 Hz rTMS produces a relatively long-lasting enhancement of the inhibitory mechanisms responsible for the cortical silent period. Low-frequency rTMS decreased the number of seizures in patients with focal neocortical epilepsy ( 35 ) and refractory epilepsy ( 152 ).
4.2. Transcranial Direct Current Stimulation
tDCS is one of transcranial electrical stimulation (tES) methods and applies low-amplitude direct currents via scalp electrodes and penetrate the skull to enter the brain, as shown in Figure 8B ( 37 ). The principal difference between tDCS and other tES techniques is the waveform to the brain, like Figure 11 . tDCS is the only class of neuromodulation technique that delivers a sustained direct current (DC) like Figure 11A ( 39 ). Transcranial alternating current stimulation (tACS) has a variety of stimulation with different frequencies (1–45 Hz), like Figure 11B ( 153 ). tACS enables the study of causal links between brain rhythms and specific aspects of behavior. Transcranial random noise stimulation (tRNS) follows a white-noise band-limited waveform (0.1–640 Hz) like Figure 11C ( 154 ). tRNS focuses on the link between behavior and frequency-specific noise inherent in neural processing ( 127 ). tACS and tRNS are usually used to identify or compare frequency-specific characteristics. They are not actively used as therapeutic methods to obtain actual effects compared to tDCS. Besides, tDCS is easier to use, cheaper, and more tolerable than TMS. However, tDCS is still an experimental form of brain stimulation and is not an FDA-approved treatment 9 . tDCS does not induce neuronal action potentials because static fields do not yield the rapid depolarization required to produce action potentials in neural membranes ( 155 ). Thus, it is a pure neuromodulatory intervention. tDCS could modulate cortical excitation and cortical inhibition by anodal polarity and cathodal polarity, respectively. By varying the current intensity and duration, the strength and duration of the after-effects could be controlled.
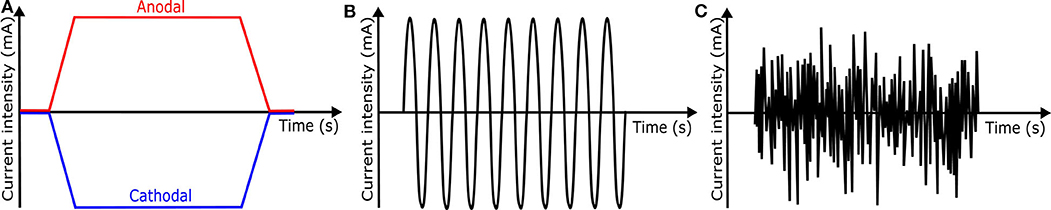
Figure 11 . Waveforms of different tES techniques. (A) tDCS waveform (B) tACS waveform (C) tRNS waveform.
4.2.1. Factors
The effect of tDCS depends on current density, stimulation duration, the orientation of the electric field (the electrodes' positions and polarity), electrode configuration (material and size), the patient, deep of the target, and intensity of the current ( 156 , 157 ). Long-lasting stimulation largely influenced the durability of after-effects to humans ( 158 ). tDCS protocols should specify electrode position and current direction because these elements cause different stimulation results. The electrodes for tDCS are usually a pair of electrodes covered by sponges filled with a contact medium such as NaCl solution or conductive cream ( 155 ). For the electrode size, although large electrodes expand the area of the excitability modification, small electrodes are better to increase tDCS focality ( 155 ).
4.2.2. Case Studies
Cathodal tDCS leads to a reduction of cortical excitability by decreasing the neuronal firing rate and inducing long-term depression (LTD) of neuronal excitability ( 159 ). In animal studies, cathodal tDCS at 100 μA for 60 min resulted in a duration of more than 2 h with an increasing threshold of focal onset seizure activity, while anodal tDCS had no significant effect on TLS in the rat ( 160 ). In human studies, cathodal tDCS may be effective to reduce seizures' frequency as shown in Table 3 . Most tDCS related works applied 1–2 mA cathodal tDCS for 20–60 min. Yook et al. ( 174 ) placed a tDCS cathode at the midpoint of P4 and T4, where the 11-year-old female seizure patient showed the abnormal EEG wave. After 2 mA cathodal tDCS for 20 min, the number of seizure occurrence and the duration of each seizure episode were reduced.
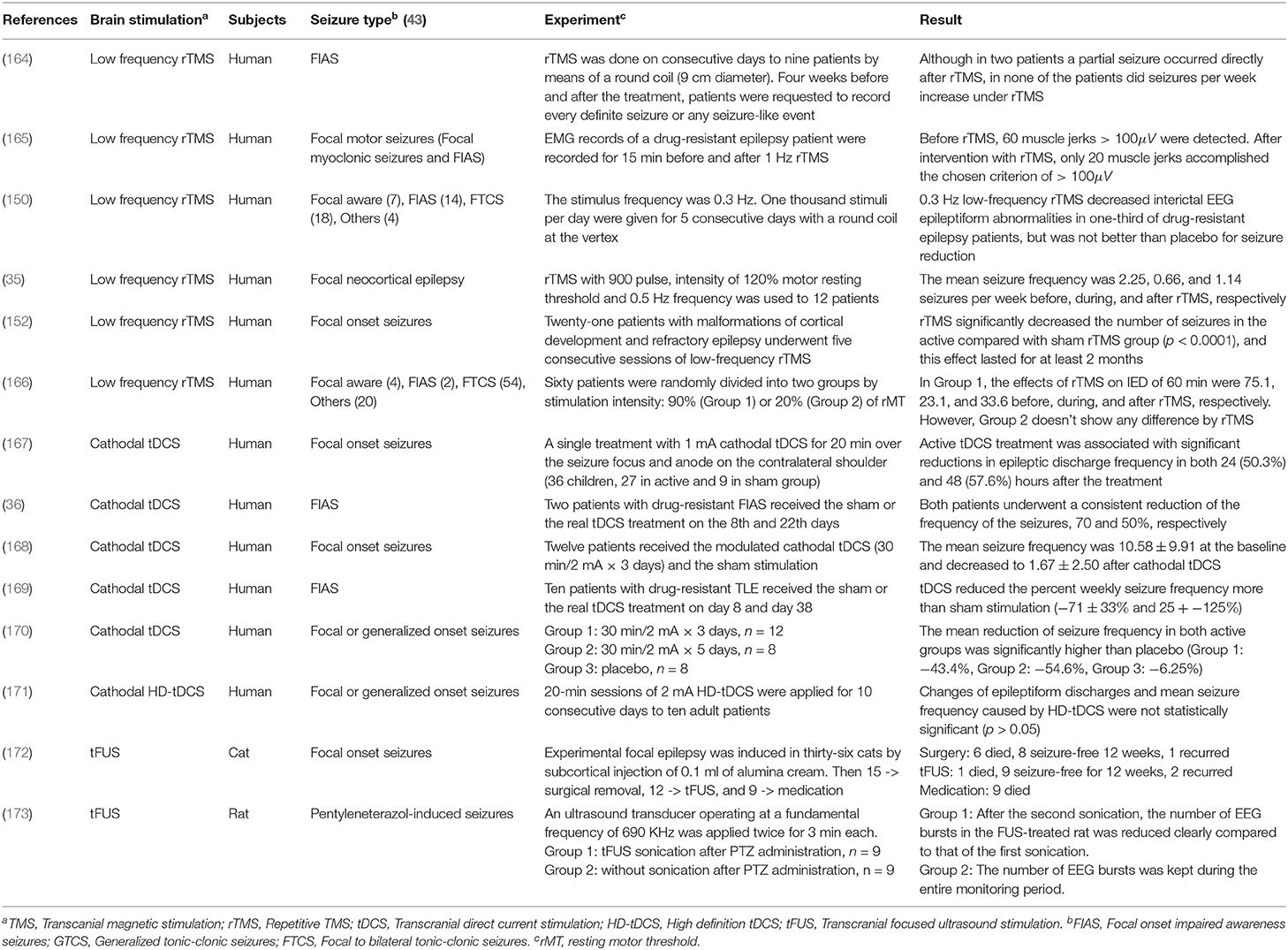
Table 3 . Experimental non-invasive neuromodulation treatments for epilepsy [Reviews for readers who want to know more about neuromodulation treatments: ( 161 – 163 )].
4.2.3. Safety
Several metrics, including current density, duration, and the charge, should be controlled carefully to prevent serious adverse effects. Bikson et al. ( 175 ) investigated the related papers for the safety of tDCS and offered the criterion of tDCS protocol: current density (6.3−13 A / m 2 ) from the animal models, and others (≤ 40 min , ≤ 4 mA , ≤ 7.2 Coulombs ) from the human trials. Under this condition, there were no reports of severe side effects across over 33, 200 sessions and 1,000 subjects ( 175 ).
4.2.4. Deep Brain Stimulation of tDCS
tDCS can only directly stimulate in cortical regions. To overcome this limitation, Grossman et al. ( 176 ) suggested a new protocol called temporal interference non-invasive brain stimulation (TI-NIBS). TI delivers multiple electric fields to the brain at frequencies which are too high to recruit neural firing. These multiple electric fields differ by a frequency within the dynamic range of neural firing. They applied TI-NIBS to a living mouse brain and demonstrated the effects of TI-NIBS by stimulating neurons in subcortical structures ( 176 ). While the current experiment has not yet been applied to humans, we believe this is one of the most potential approaches for seizure therapy in the future due to its capability of providing high spatial and temporal resolution. In addition, since the stimulation is only effective at the locations where all the beams are constructive, the beams may not harm the brain cells that are not located in the targeted areas.
4.3. Transcranial Focused Ultrasound Stimulation
tFUS is emerging as a method to improve the relatively low degree of spatial locality offered by TMS and tDCS ( 127 ). It is important because a low degree of spatial locality leads to modulating neuronal activity not only in the target but also in surrounding circuits. tFUS uses acoustic energy to stimulate the brain like Figure 8C . tFUS can both excite and suppress brain neuronal activity and has millimeter spatial resolutions ( 177 ). In 1988, Colemann and Lizzi developed the Sonocare CST-100, which is the first high intensity focused ultrasound and received the FDA pre-market approval 10 . The device was designed for the treatment of glaucoma.
4.3.1. Factors
There are some factors to control the effect of tFUS: acoustic frequencies, intensities, and modes of transmission ( 178 ). First, Ultrasound (US) has a frequency above 20 kHz. Most medical US frequency range is between 1 and 15 MHz, and therapeutic US application operates around 1 MHz or less ( 179 ). Second, therapeutic US can be divided into low power (<0.5 Wcm −2 ) or high power (>100 Wcm −2 ) depending on the acoustic intensity ( 178 ). Low power intensity US is used in physiotherapy, non-thermal actions, and so on, whereas high power intensity US is used in lithotripsy and the thermal ablation of tissue ( 180 ). The therapeutic US usually has low power intensity. Lower energy US increased the action potential, while higher energy US reduced the action potential due to the ultrasonic thermal effects. Third, the US has two modes of transmission: continuous wave (CW) and pulsed wave (PW) ( 178 ). CW stimuli are more effective than PW stimuli in eliciting responses ( 181 ). Therapeutic US is usually delivered as CW or long pulse exposures ( 180 ).
4.3.2. Current Progress
In animal studies, Manlapaz et al. ( 172 ) reported ultrasonic irradiation relieved the seizures of cats. They compared fifteen cats treated by surgical removal of the epileptogenic focus with twelve cats treated by ultrasonic irradiation. The ultrasonic approach showed less postoperative complications than those of surgery. Min et al. ( 173 ) injected pentylenetetrazol to rats to induce acute epilepsy and applied tFUS to the rat's brain twice for 3 min. Epileptic EEG signals of the rats decreased visibly after tFUS compared to the other group that did not receive any tFUS. In human studies, to the best of our knowledge, there are no existing works to handle the relationship between seizure and tFUS. Instead, we look at studies that relate the human brain to tFUS. Legon et al. ( 40 ) evaluated if tFUS is capable of modulating brain activity in the human primary somatosensory cortex. From the experiment, tFUS remarkably reduced the amplitude of somatosensory evoked potential. Lee et al. ( 182 ) reported tFUS of the primary visual cortex. The tFUS induced activation both from the sonicated brain area and from the visual or cognitive network regions. However, tFUS beam might potentially harm brain cells when it passes through them.
4.3.3. Safety
It is difficult to establish tFUS protocol for safety now because there is no enough medical data about tFUS. Although Legon et al. ( 183 ) applied low power tFUS to 120 participants who did not report any neurological impairment and reported that none of the participants experienced serious adverse effects, it does not prove the safety of tFUS. This is because ultrasound at high intensities can cause irreversible tissue damage ( 40 ). The safety protocol could be established from a variety of tFUS related experiments. US studies are necessary to be conducted on primate brains such as monkeys having a skull with similar thickness and size to that of humans ( 184 ).
5. Potential Research Directions
We have discussed state-of-the-arts sensing and stimulation technologies that are suitable for seizure monitoring and therapy. In this section, we present potential research directions that require more attention to building a robust, wearable, safe, sensing, and stimulation systems.
5.1. Robust and Wearable Seizure Detection System
5.1.1. monitoring seizures from the brain with high resolution.
Current technologies only allow us to monitor the whole brain. However, we envision a more robust sensing technology that could sense precisely where the seizure signal occurs on the human brain. The future sensor can be implemented as an array of electrodes to form a beam-forming receiver to capture only the brain area of interest. It will improve the performance of the sensing system by efficiently removing the interference signals from many non-related brain areas. TI-NIBS ( 176 ) design can be considered as the closest reference.
5.1.2. Improving Seizure Quantification
Most existing seizure detection systems have only focused on differentiating between seizure and non-seizure. Therefore, we could not find a concrete explanation between the biosignal and the severity of symptoms from the related works. The biggest problem is that there are no clear criteria for quantifying seizures, and it is difficult to obtain the ground truth data. Once reasonable standards are established, and researchers' consent is received, the seizure quantification will be applied to the seizure detection system quickly.
5.1.3. Making Seizure Monitoring System Become Wearable
We predict the core location of recording EEG signals will be around the ear. The reason is that the device around the ear can still acquire clear EEG signals and does not restrict the user's mobility. Dry electrodes could be applied to the ear-cover part ( 185 ) of the device to improve usability. Different biosignals, including EOG, EMG, and EDA, also could be detected with EEG signals around the ear. A headband with EEG electrodes is also used to detect seizures from the frontal lobe or other locations which could not be detected by seizure detection devices around the ear. The wearable devices can deliver biosignals to a smartphone through communication technology. The application for seizure detection on the smartphone will extract features and classify seizure types.
5.2. Safe, Accurate, and Reliable Seizure Stimulation
5.2.1. high spacial resolution stimulation.
The stimulation device needs to localize the target area of the brain and stimulate it accurately. Existing TMS and tDCS have a low degree of spatial locality. We introduced several approaches to solve this problem. Hesed coil design of TMS attaches several strips on the specific part of the head intensively with wires that induce stimulation in the desired direction ( 186 ). TI-NIBS, as an alternative of tDCS, delivers multiple electric fields to the brain at frequencies that are too high to recruit neural firing ( 176 ). In addition, new tDCS algorithms allow a better focal treatment using multi-target electrodes and smaller electrodes in High-Definition tDCS (HD-tDCS) ( 187 ). These approaches are likely to advance to deep brain stimulation and require additional studies because they are in the proposal stage.
5.2.2. Safe and Reliable Stimulation Device
Safety is the most crucial aspect of designing the stimulation system. A practical system should prioritize the safety aspect, for example, long term and short term side effects. Unlike TMS achieving some degree of safety, tDCS and tFUS are necessary to establish the safety protocol. Antal et al. ( 188 ) introduce detail information about the safety of tDCS, including long stimulation duration, montages with (multiple) small electrodes, and limiting the maximum current. Although there are a lot of works for tDCS, tDCS still only happens in the lab environment and is not an FDA-approved treatment solutions 9 . Rather, as another electrical stimulation approach, non-invasive VNS therapy for drug-resistant epilepsy patients received FDA approval 7 . In the near future, we believe the establishment of a tDCS safety protocol for humans by integrating its new experiment with existing work. Meanwhile, there are a few related works about tFUS. Many tests for tFUS are necessary before establishing a safety protocol.
5.2.3. Making Seizure Therapy System Become Wearable
We believe tDCS could be integrated with the seizure detection system, especially around the ear. tDCS applies low-amplitude direct currents via two scalp electrodes like Figure 8B . Each scalp electrode could be connected to the surrounding area of the left ear and right ear, respectively. When designing a circuit, we need to consider the difference between the battery used for the existing seizure detection device and that used for tDCS. Unless a new design is available, it seems difficult to create a wearable device that incorporates TMS or tFUS and a seizure detection device. The fact that both brain stimulation methods are applied with a small gap between the brain and the device makes it difficult to make a wearable device.
5.3. An Integrated Sensing and Stimulation System
Even when the seizure monitoring and stimulation systems are reliable, there are many challenges remaining in integrating these two components to produce a reliable integrated system in a wearable form.
6. Conclusion
. In this paper, we systematically categorized recent efforts on building seizure monitoring, detection, and therapy systems. We explained the overall systems and components that can be used to monitor the reliable physiological signals of seizures. We presented different techniques for extracting physiological seizure signals from the noises. Then, we discussed in detail recent effort on classifying/detecting seizure events using machine learning and deep learning. Next, we presented different seizure therapy techniques, including TMS, tDCS, and tFUS. Last but not least, we discussed potential future research directions on building a wearable seizure detection and therapy system based on our experience in building comprehensive health solutions.
Author Contributions
TK surveyed and wrote all parts of the manuscript. TK and PN discussed and wrote Lesson Learned and section 6. TK, PN, NP, NB, HT, SH, and TV revised the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. ^ https://www.epilepsysociety.org.uk/epileptic-seizures#.XsfrI2hKiHs
2. ^ https://www.safetysystemsdistribution.co.uk/emfit-tonic-clonic-seizure-monitor-basic/
3. ^ https://medpage-ltd.com/epileptic-tonic-clonic-seizure-alarm-MP5
4. ^ https://smart-monitor.com/about-smartwatch-inspyre-by-smart-monitor/
5. ^ https://www.bioserenity.com
6. ^ https://www.iec.ch/worldplugs/list_bylocation.htm
7. ^ https://www.mobihealthnews.com/content/fda-approves-sentiva-nerve-stimulation-device-epilepsy-therapy
8. ^ https://www.fisherwallace.com/
9. ^ https://www.hopkinsmedicine.org
10. ^ https://www.fusfoundation.org/the-technology/timeline-of-focused-ultrasound
1. Fisher RS, Boas WVE, Blume W, Elger C, Genton P, Lee P, et al. Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia . (2005) 46:470–2. doi: 10.1111/j.0013-9580.2005.66104.x
PubMed Abstract | CrossRef Full Text | Google Scholar
2. NINDS. The Epilepsies and Seizures: Hope through Research . Department of Health & Human Services, NIH, National Institute of Health (2015).
3. Mormann F, Andrzejak RG, Elger CE, Lehnertz K. Seizure prediction: the long and winding road. Brain . (2006) 130:314–33. doi: 10.1093/brain/awl241
4. Van de Vel A, Cuppens K, Bonroy B, Milosevic M, Jansen K, Van Huffel S, et al. Non-EEG seizure detection systems and potential SUDEP prevention: state of the art: review and update. Seizure . (2016) 41:141–53. doi: 10.1016/j.seizure.2016.07.012
5. Song Y. A review of developments of EEG-based automatic medical support systems for epilepsy diagnosis and seizure detection. J Biomed Sci Eng . (2011) 4:788. doi: 10.4236/jbise.2011.412097
CrossRef Full Text | Google Scholar
6. Tzallas AT, Tsipouras MG, Tsalikakis DG, Karvounis EC, Astrakas L, Konitsiotis S, et al. Automated epileptic seizure detection methods: a review study. In: Epilepsy-Histological, Electroencephalographic and Psychological Aspects . Ioannia: InTech (2012). p. 75–98.
Google Scholar
7. Ramgopal S, Thome-Souza S, Jackson M, Kadish NE, Fernández IS, Klehm J. Seizure detection, seizure prediction, and closed-loop warning systems in epilepsy. Epilepsy Behav . (2014) 37:291–307. doi: 10.1016/j.yebeh.2014.06.023
8. Gadhoumi K, Lina JM, Mormann F, Gotman J. Seizure prediction for therapeutic devices: a review. J Neurosci Methods . (2016) 260:270–82. doi: 10.1016/j.jneumeth.2015.06.010
9. Theodore WH, Fisher RS. Brain stimulation for epilepsy. Lancet Neurol . (2004) 3:111–8. doi: 10.1016/S1474-4422(03)00664-1
10. Nitsche MA, Paulus W. Noninvasive brain stimulation protocols in the treatment of epilepsy: current state and perspectives. Neurotherapeutics . (2009) 6:244–50. doi: 10.1016/j.nurt.2009.01.003
11. McGrogan N. Neural Network Detection of Epileptic Seizures in the Electroencephalogra . Department of Engineering Science Probationary Research Transfer Report, Oxford University (1999).
12. Leijten FS, Consortium DT, van Andel J, Ungureanu C, Arends J, Tan F, et al. Multimodal seizure detection: a review. Epilepsia . (2018) 59:42–7. doi: 10.1111/epi.14047
13. Übeyli ED. Lyapunov exponents/probabilistic neural networks for analysis of EEG signals. Expert Syst Appl . (2010) 37:985–92. doi: 10.1016/j.eswa.2009.05.078
14. Übeyli ED. Analysis of EEG signals by implementing eigenvector methods/recurrent neural networks. Digital Signal Process . (2009) 19:134–43. doi: 10.1016/j.dsp.2008.07.007
15. Baumgartner C, Koren JP. Seizure detection using scalp-EEG. Epilepsia . (2018) 59:14–22. doi: 10.1111/epi.14052
16. Regalia G, Onorati F, Migliorini M, Picard R. An improved wrist-worn convulsive seizure detector based on accelerometry and electrodermal activity sensors. In: American Epilepsy Society Annual Meeting . (2015).
PubMed Abstract | Google Scholar
17. Cogan D, Birjandtalab J, Nourani M, Harvey J, Nagaraddi V. Multi-biosignal analysis for epileptic seizure monitoring. Int J Neural Syst . (2017) 27:1650031. doi: 10.1142/S0129065716500313
18. Larsen SN, Conradsen I, Beniczky S, Sorensen HB. Detection of tonic epileptic seizures based on surface electromyography. In: Engineering in Medicine Biology Society (EMBC), 36th Annual International Conference of the IEEE. Chicago, IL: IEEE (2014). p. 942–5. doi: 10.1109/EMBC.2014.6943747
19. Vidaurre C, Krämer N, Blankertz B, Schlögl A. Time domain parameters as a feature for EEG-based brain-computer interfaces. Neural Netw . (2009) 22:1313–9. doi: 10.1016/j.neunet.2009.07.020
20. Srinivasan V, Eswaran C, Sriraam N. Artificial neural network based epileptic detection using time-domain and frequency-domain features. J Med Syst . (2005) 29:647–60. doi: 10.1007/s10916-005-6133-1
21. Hassanpour H, Mesbah M, Boashash B. Time-frequency feature extraction of newborn EEG seizure using SVD-based techniques. EURASIP J Appl Signal Process . (2004) 2004:2544–54. doi: 10.1155/S1110865704406167
22. Faust O, Acharya UR, Adeli H, Adeli A. Wavelet-based EEG processing for computer-aided seizure detection and epilepsy diagnosis. Seizure . (2015) 26:56–64. doi: 10.1016/j.seizure.2015.01.012
23. Polikar R. The Wavelet Tutorial . Rowan University (1996).
24. Gu Y, Cleeren E, Dan J, Claes K, Van Paesschen W, Van Huffel S, et al. Comparison between scalp EEG and behind-the-ear EEG for development of a wearable seizure detection system for patients with focal epilepsy. Sensors . (2018) 18:29. doi: 10.3390/s18010029
25. Cortes C, Vapnik V. Support-vector networks. Mach Learn . (1995) 20:273–7. doi: 10.1007/BF00994018
26. Shoeb AH, Guttag JV. Application of machine learning to epileptic seizure detection. In: Proceedings of the 27th International Conference on Machine Learning (ICML-10) . Haifa (2010). p. 975–82.
27. Tzimourta K, Tzallas A, Giannakeas N, Astrakas L, Tsalikakis D, Tsipouras M. Epileptic seizures classification based on long-term EEG signal wavelet analysis. In: Maglaveras N, Chouvarda I, de Carvalho P, editors. Precision Medicine Powered by pHealth and Connected Health. Thessaloniki: Springer (2018). p. 165–9. doi: 10.1007/978-981-10-7419-6_28
28. Subasi A, Kevric J, Canbaz MA. Epileptic seizure detection using hybrid machine learning methods. Neural Comput. Appl . (2019) 31:317–25. doi: 10.1007/s00521-017-3003-y
29. Übeyli ED. Analysis of EEG signals by combining eigenvector methods and multiclass support vector machines. Comput. Biol. Med . (2008) 38:14–22. doi: 10.1016/j.compbiomed.2007.06.002
30. Guler I, Ubeyli ED. Multiclass support vector machines for EEG-signals classification. IEEE Trans Inform Technol Biomed . (2007) 11:117–26. doi: 10.1109/TITB.2006.879600
31. Teixeira CA, Direito B, Bandarabadi M, Le Van Quyen M, Valderrama M, Schelter B, et al. Epileptic seizure predictors based on computational intelligence techniques: a comparative study with 278 patients. Comput Methods Programs Biomed . (2014) 114:324–36. doi: 10.1016/j.cmpb.2014.02.007
32. Direito B, Teixeira CA, Sales F, Castelo-Branco M, Dourado A. A realistic seizure prediction study based on multiclass SVM. Int J Neural Syst . (2017) 27:1750006. doi: 10.1142/S012906571750006X
33. Emami A, Kunii N, Matsuo T, Shinozaki T, Kawai K, Takahashi H. Seizure detection by convolutional neural network-based analysis of scalp electroencephalography plot images. Neuroimage Clin . (2019) 22:101684. doi: 10.1016/j.nicl.2019.101684
34. Orhan U, Hekim M, Ozer M. EEG signals classification using the K-means clustering and a multilayer perceptron neural network model. Expert Syst. Appl . (2011) 38:13475–81. doi: 10.1016/j.eswa.2011.04.149
35. Santiago-Rodríguez E, Cárdenas-Morales L, Harmony T, Fernández-Bouzas A, Porras-Kattz E, Hernández A. Repetitive transcranial magnetic stimulation decreases the number of seizures in patients with focal neocortical epilepsy. Seizure . (2008) 17:677–83. doi: 10.1016/j.seizure.2008.04.005
36. Assenza G, Campana C, Formica D, Schena E, Taffoni F, Di Pino G, et al. Efficacy of cathodal transcranial direct current stimulation in drug-resistant epilepsy: a proof of principle. In: Engineering in Medicine and Biology Society (EMBC). 2014 36th Annual International Conference of the IEEE . Chicago, IL: IEEE (2014). p. 530–3. doi: 10.1109/EMBC.2014.6943645
37. Wagner T, Valero-Cabre A, Pascual-Leone A. Noninvasive human brain stimulation. Annu Rev Biomed Eng . (2007) 9:527–65. doi: 10.1146/annurev.bioeng.9.061206.133100
38. Hallett M. Transcranial magnetic stimulation and the human brain. Nature. (2000) 406:147–50. doi: 10.1038/35018000
39. Gebodh N, Esmaeilpour Z, Adair D, Schestattsky P, Fregni F, Bikson M. Transcranial direct current stimulation among technologies for low-intensity transcranial electrical stimulation: classification, history, and terminology. In: Knotkova H, Nitsche MA, Bikson M,Woods AJ, editors. Practical Guide to Transcranial Direct Current Stimulation. Cham: Springer. (2019). p. 3–43. doi: 10.1007/978-3-319-95948-1_1
40. Legon W, Sato TF, Opitz A, Mueller J, Barbour A, Williams A, et al. Transcranial focused ultrasound modulates the activity of primary somatosensory cortex in humans. Nat Neurosci . (2014) 17:322. doi: 10.1038/nn.3620
41. Nijsen TM, Aarts RM, Arends JB, Cluitmans PJ. Automated detection of tonic seizures using 3-D accelerometry. In: International Federation for Medical and Biological Engineering. Antwerp: Springer (2009) p. 188–91. doi: 10.1007/978-3-540-89208-3_47
42. McLean B, Wimalaratna S. Sudden death in epilepsy recorded in ambulatory EEG. J Neurol Neurosurg Psychiatry . (2007) 78:1395–7. doi: 10.1136/jnnp.2006.088492
43. Fisher RS, Cross JH, French JA, Higurashi N, Hirsch E, Jansen FE, et al. Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia . (2017) 58:522–30. doi: 10.1111/epi.13670
44. Saab M, Gotman J. A system to detect the onset of epileptic seizures in scalp EEG. Clin Neurophysiol . (2005) 116:427–42. doi: 10.1016/j.clinph.2004.08.004
45. Zandi AS, Javidan M, Dumont GA, Tafreshi R. Automated real-time epileptic seizure detection in scalp EEG recordings using an algorithm based on wavelet packet transform. IEEE Trans Biomed Eng . (2010) 57:1639–51. doi: 10.1109/TBME.2010.2046417
46. Orosco L, Correa AG, Diez P, Laciar E. Patient non-specific algorithm for seizures detection in scalp EEG. Comput Biol Med . (2016) 71:128–34. doi: 10.1016/j.compbiomed.2016.02.016
47. Wang Y, Long X, van Dijk H, Aarts RM, Wang L, Arends JB. False alarms reduction in non-convulsive status epilepticus detection via continuous EEG analysis. Physiol Meas . (2020) doi: 10.1088/1361-6579/ab8cb3
48. Doyle O, Temko A, Marnane W, Lightbody G, Boylan G. Heart rate based automatic seizure detection in the newborn. Med Eng Phys . (2010) 32:829–39. doi: 10.1016/j.medengphy.2010.05.010
49. Massé F, Bussel MV, Serteyn A, Arends J, Penders J. Miniaturized wireless ECG monitor for real-time detection of epileptic seizures. ACM Trans Embed Comput Syst . (2013) 12:102. doi: 10.1145/2485984.2485990
50. Varon C, Jansen K, Lagae L, Van Huffel S. Can ECG monitoring identify seizures? J Electrocardiol . (2015) 48:1069–74. doi: 10.1016/j.jelectrocard.2015.08.020
51. Jeppesen J, Fuglsang-Frederiksen A, Johansen P, Christensen J, Wüstenhagen S, Tankisi H, et al. Seizure detection based on heart rate variability using a wearable electrocardiography device. Epilepsia . (2019) 60:2105–13. doi: 10.1111/epi.16343
52. Vandecasteele K, De Cooman T, Gu Y, Cleeren E, Claes K, Paesschen WV, et al. Automated epileptic seizure detection based on wearable ECG and PPG in a hospital environment. Sensors . (2017) 17:2338. doi: 10.3390/s17102338
53. Szabó CÁ, Morgan LC, Karkar KM, Leary LD, Lie OV, Girouard M, et al. Electromyography-based seizure detector: Preliminary results comparing a generalized tonic-clonic seizure detection algorithm to video-EEG recordings. Epilepsia . (2015) 56:1432–7. doi: 10.1111/epi.13083
54. Van de Vel A, Cuppens K, Bonroy B, Milosevic M, Van Huffel S, Vanrumste B, et al. Long-term home monitoring of hypermotor seizures by patient-worn accelerometers. Epilepsy Behav . (2013) 26:118–25. doi: 10.1016/j.yebeh.2012.10.006
55. Beniczky S, Polster T, Kjaer TW, Hjalgrim H. Detection of generalized tonic-clonic seizures by a wireless wrist accelerometer: a prospective, multicenter study. Epilepsia . (2013) 54:e58–61. doi: 10.1111/epi.12120
56. Arends JB, van Dorp J, van Hoek D, Kramer N, van Mierlo P, van der Vorst D, et al. Diagnostic accuracy of audio-based seizure detection in patients with severe epilepsy and an intellectual disability. Epilepsy Behav . (2016) 62:180–5. doi: 10.1016/j.yebeh.2016.06.008
57. Shum J, Fogarty A, Dugan P, Holmes MG, Leeman-Markowski BA, Liu AA, et al. Sounds of seizures. Seizure . (2020) 78:86–90. doi: 10.1016/j.seizure.2020.03.008
58. Ntonfo GMK, Ferrari G, Raheli R, Pisani F. Low-complexity image processing for real-time detection of neonatal clonic seizures. IEEE Trans Inform Technol Biomed . (2012) 16:375–82. doi: 10.1109/TITB.2012.2186586
59. Poh MZ, Loddenkemper T, Reinsberger C, Swenson NC, Goyal S, Sabtala MC, et al. Convulsive seizure detection using a wrist-worn electrodermal activity and accelerometry biosensor. Epilepsia . (2012) 53:e93–7. doi: 10.1111/j.1528-1167.2012.03444.x
60. Heldberg BE, Kautz T, Leutheuser H, Hopfengärtner R, Kasper BS, Eskofier BM. Using wearable sensors for semiology-independent seizure detection-towards ambulatory monitoring of epilepsy. In: Engineering in Medicine Biology Society (EMBC), 37th Annual International Conference of the IEEE . Milan: IEEE (2015). p. 5593–6. doi: 10.1109/EMBC.2015.7319660
61. Pauri F, Pierelli F, Chatrian GE, Erdly WW. Long-term EEG-video-audio monitoring: computer detection of focal EEG seizure patterns. Electroencephalogr Clin Neurophysiol . (1992) 82:1–9. doi: 10.1016/0013-4694(92)90175-H
62. Kidmose P, Looney D, Mandic DP. Auditory evoked responses from Ear-EEG recordings. In: Engineering in Medicine and Biology Society (EMBC). 2012 Annual International Conference of the IEEE . San Diego, CA: IEEE (2012). p. 586–9. doi: 10.1109/EMBC.2012.6345999
63. Mikkelsen KB, Kappel SL, Mandic DP, Kidmose P. EEG recorded from the ear: characterizing the ear-EEG method. Front Neurosci . (2015) 9:438. doi: 10.3389/fnins.2015.00438
64. Panayiotopoulos CP. Epileptic Syndromes and their Treatment. Neonatal Seizures . 2 nd ed. London: Springer (2007). p. 185–206.
65. Cogan D, Nourani M, Harvey J, Nagaraddi V. Epileptic seizure detection using wristworn biosensors. In: Engineering in Medicine Biology Society (EMBC), 37th Annual International Conference of the IEEE . IEEE (2015). p. 5086–9. doi: 10.1109/EMBC.2015.7319535
66. Blumhardt L, Smith P, Owen L. Electrocardiographic accompaniments of temporal lobe epileptic seizures. Lancet . (1986) 327:1051–56. doi: 10.1016/S0140-6736(86)91328-0
67. Smith P, Howell S, Owen L, Blumhardt L. Profiles of instant heart rate during partial seizures. Electroencephalogr Clin Neurophysiol . (1989) 72:207–17. doi: 10.1016/0013-4694(89)90245-9
68. Ponnusamy A, Marques JL, Reuber M. Comparison of heart rate variability parameters during complex partial seizures and psychogenic nonepileptic seizures. Epilepsia . (2012) 53:1314–21. doi: 10.1111/j.1528-1167.2012.03518.x
69. Yang CC, Hsu YL. A review of accelerometry-based wearable motion detectors for physical activity monitoring. Sensors . (2010) 10:7772–88. doi: 10.3390/s100807772
70. Benedek M, Kaernbach C. A continuous measure of phasic electrodermal activity. J Neurosci Methods . (2010) 190:80–91. doi: 10.1016/j.jneumeth.2010.04.028
71. Greene BR, Boylan GB, Reilly RB, de Chazal P, Connolly S. Combination of EEG and ECG for improved automatic neonatal seizure detection. Clin Neurophysiol . (2007) 118:1348–59. doi: 10.1016/j.clinph.2007.02.015
72. Pham N, Dinh T, Raghebi Z, Kim T, Bui N, Nguyen P, et al. WAKE: a behind-the-ear wearable system for microsleep detection. In: Proceedings of the 18th Annual International Conference on Mobile Systems, Applications, and Services. Toronto, ON: ACM (2020) doi: 10.1145/3386901.3389032
73. Rapson W. Skin contact with gold and gold alloys. Contact Dermatitis . (1985) 13:56–65. doi: 10.1111/j.1600-0536.1985.tb02505.x
74. Meier R, Dittrich H, Schulze-Bonhage A, Aertsen A. Detecting epileptic seizures in long-term human EEG: a new approach to automatic online and real-time detection and classification of polymorphic seizure patterns. J Clin Neurophysiol . (2008) 25:119–31. doi: 10.1097/WNP.0b013e3181775993
75. Reddy AG, Narava S. Artifact removal from EEG signals. Int J Comput Appl. (2013) 77:17–19. doi: 10.5120/13543-1175
76. Jung TP, Makeig S, Westerfield M, Townsend J, Courchesne E, Sejnowski TJ, et al. Removal of eye activity artifacts from visual event-related potentials in normal and clinical subjects. Clin Neurophysiol . (2000) 111:1745–58. doi: 10.1016/S1388-2457(00)00386-2
77. Makeig S, Jung TP, Ghahremani D, Sejnowski TJ. Independent Component Analysis of Simulated ERP Data . Institute for Neural Computation, University of California. Technical report INC-9606. (1996).
78. Jafarifarmand A, Badamchizadeh MA. Artifacts removal in EEG signal using a new neural network enhanced adaptive filter. Neurocomputing . (2013) 103:222–31. doi: 10.1016/j.neucom.2012.09.024
79. Zikov T, Bibian S, Dumont GA, Huzmezan M, Ries C. A wavelet based de-noising technique for ocular artifact correction of the electroencephalogram. In: Engineering in Medicine and Biology. Vol. 1. Houston, TX: IEEE (2002). p. 98–105.
80. Blankertz B, Tomioka R, Lemm S, Kawanabe M, Muller KR. Optimizing spatial filters for robust EEG single-trial analysis. IEEE Signal Process Mag . (2008) 25:41–56. doi: 10.1109/MSP.2008.4408441
81. Ramoser H, Muller-Gerking J, Pfurtscheller G. Optimal spatial filtering of single trial EEG during imagined hand movement. IEEE Trans Rehabil Eng . (2000) 8:441–6. doi: 10.1109/86.895946
82. Honig ML. Adaptive Filters. Structures, Algorithms and Applications . Kluwer (1984). p. 144–246.
83. Widrow B, Stearns S. Adaptive Signal Processing . Englewood Cliffs, NJ: Prentice-Hall, Inc. (1985).
84. Correa AG, Laciar E, Patiño H, Valentinuzzi M. Artifact removal from EEG signals using adaptive filters in cascade. J Phys . (2007) 90:012081. doi: 10.1088/1742-6596/90/1/012081
85. Pedrycz W. Fuzzy Control and Fuzzy Systems . 2nd ed. Taunton, UK: Research Studies Press Ltd. (1993).
86. Chen CH, Lin CJ, Lin CT. A functional-link-based neurofuzzy network for nonlinear system control. IEEE Trans Fuzzy Syst . Taunton (2008) 16:1362–78. doi: 10.1109/TFUZZ.2008.924334
87. Er MJ, Li Z. Adaptive noise cancellation using online self-enhanced fuzzy filters with applications to multimedia processing. In: Intelligent Multimedia Processing with Soft Computing. Springer (2005) p. 389–414. doi: 10.1007/3-540-32367-8_18
88. Daoud H, Bayoumi MA. Efficient epileptic seizure prediction based on deep learning. IEEE Trans Biomed Circ Syst . (2019) 13:804–13. doi: 10.1109/TBCAS.2019.2929053
89. Craik A, He Y, Contreras-Vidal JL. Deep learning for electroencephalogram (EEG) classification tasks: a review. J Neural Eng . (2019) 16:031001. doi: 10.1088/1741-2552/ab0ab5
90. Ramakrishnan S, Murugavel AM. Epileptic seizure detection using fuzzy-rules-based sub-band specific features and layered multi-class SVM. Pattern Anal Appl . (2019) 22:1161–76. doi: 10.1007/s10044-018-0691-6
91. Andrzejak RG, Lehnertz K, Mormann F, Rieke C, David P, Elger CE. Indications of nonlinear deterministic and finite-dimensional structures in time series of brain electrical activity: dependence on recording region and brain state. Phys Rev E . (2001) 64:061907. doi: 10.1103/PhysRevE.64.061907
92. Guo L, Rivero D, Dorado J, Rabunal JR, Pazos A. Automatic epileptic seizure detection in EEGs based on line length feature and artificial neural networks. J Neurosci Methods . (2010) 191:101–9. doi: 10.1016/j.jneumeth.2010.05.020
93. Esteller R, Echauz J, Tcheng T. Comparison of line length feature before and after brain electrical stimulation in epileptic patients. In: The 26th Annual International Conference of the IEEE Engineering in Medicine and Biology Society. San Francisco, CA: IEEE (2004). p. 4710–3.
94. Naghsh-Nilchi AR, Aghashahi M. Epilepsy seizure detection using eigen-system spectral estimation and Multiple Layer Perceptron neural network. Biomed Signal Process Control . (2010) 5:147–57. doi: 10.1016/j.bspc.2010.01.004
95. Güler I, Übeyli ED. Adaptive neuro-fuzzy inference system for classification of EEG signals using wavelet coefficients. J Neurosci Methods . (2005) 148:113–21. doi: 10.1016/j.jneumeth.2005.04.013
96. Aslan K, Bozdemir H, Şahin C, Oğulata SN, Erol R. A radial basis function neural network model for classification of epilepsy using EEG signals. J Med Syst . (2008) 32:403–8. doi: 10.1007/s10916-008-9145-9
97. Kumar SP, Sriraam N, Benakop P, Jinaga B. Entropies based detection of epileptic seizures with artificial neural network classifiers. Expert Syst Appl . (2010) 37:3284–91. doi: 10.1016/j.eswa.2009.09.051
98. Hossain MS, Amin SU, Alsulaiman M, Muhammad G. Applying deep learning for epilepsy seizure detection and brain mapping visualization. ACM Trans Multimedia Comput Commun Appl . (2019) 15:10. doi: 10.1145/3241056
99. Diykh M, Li Y, Wen P. EEG sleep stages classification based on time domain features and structural graph similarity. IEEE Trans Neural Syst Rehabil Eng . (2016) 24:1159–68. doi: 10.1109/TNSRE.2016.2552539
100. Khorshidtalab A, Salami M, Hamedi M. Robust classification of motor imagery EEG signals using statistical time-domain features. Physiol Meas . (2013) 34:1563. doi: 10.1088/0967-3334/34/11/1563
101. Al-Fahoum AS, Al-Fraihat AA. Methods of EEG signal features extraction using linear analysis in frequency and time-frequency domains. ISRN Neurosci . (2014) 2014:7. doi: 10.1155/2014/730218
102. Weisstein EW. “Fourier Transform.” From MathWorld–A Wolfram Web Resource . Available online at: https://mathworld.wolfram.com/FourierTransform.html
103. Johnson RR, Popovic DP, Olmstead RE, Stikic M, Levendowski DJ, Berka C. Drowsiness/alertness algorithm development and validation using synchronized EEG and cognitive performance to individualize a generalized model. Biol Psychol . (2011) 87:241–250. doi: 10.1016/j.biopsycho.2011.03.003
104. Akay M, Semmlow JL, Welkowitz W, Bauer MD, Kostis JB. Noninvasive detection of coronary stenoses before and after angioplasty using eigenvector methods. IEEE Biomed Eng . (1990) 37:1095–104. doi: 10.1109/10.61035
105. Pisarenko VF. The retrieval of harmonics from a covariance function. Geophys J Int . (1973) 33:347–66. doi: 10.1111/j.1365-246X.1973.tb03424.x
106. Schmidt R. Multiple emitter location and signal parameter estimation. IEEE Trans Antennas Propagat . (1986) 34:276–80. doi: 10.1109/TAP.1986.1143830
107. Kumaresan R, Tufts DW. Estimating the angles of arrival of multiple plane waves. IEEE Trans Aerospace Electron Syst. (1983) AES-19:134–9. doi: 10.1109/TAES.1983.309427
108. De Hoon M, Van der Hagen T, Schoonewelle H, Van Dam H. Why Yule-Walker should not be used for autoregressive modelling. Ann Nuclear Energy . (1996) 23:1219–28. doi: 10.1016/0306-4549(95)00126-3
109. Iscan Z, Dokur Z, Demiralp T. Classification of electroencephalogram signals with combined time and frequency features. Expert Syst Appl . (2011) 38:10499–505. doi: 10.1016/j.eswa.2011.02.110
110. Cohen L. Time-frequency distributions-a review. Proc IEEE . (1989) 77:941–81. doi: 10.1109/5.30749
111. Boashash B, Ouelha S. Automatic signal abnormality detection using time-frequency features and machine learning: a newborn EEG seizure case study. Knowl Based Syst . (2016) 106:38–50. doi: 10.1016/j.knosys.2016.05.027
112. Rosso OA, Blanco S, Yordanova J, Kolev V, Figliola A, Schürmann M, et al. Wavelet entropy: a new tool for analysis of short duration brain electrical signals. J Neurosci Methods . (2001) 105:65–75. doi: 10.1016/S0165-0270(00)00356-3
113. Subasi A. EEG signal classification using wavelet feature extraction and a mixture of expert model. Expert Syst Appl . (2007) 32:1084–93. doi: 10.1016/j.eswa.2006.02.005
114. Subasi A. Epileptic seizure detection using dynamic wavelet network. Expert Syst Appl . (2005) 29:343–55. doi: 10.1016/j.eswa.2005.04.007
115. Acharya UR, Sree SV, Ang PCA, Yanti R, Suri JS. Application of non-linear and wavelet based features for the automated identification of epileptic EEG signals. Neural Syst . (2012) 22:1250002. doi: 10.1142/S0129065712500025
116. Greene B, Marnane W, Lightbody G, Reilly R, Boylan G. Classifier models and architectures for EEG-based neonatal seizure detection. Physiol Meas . (2008) 29:1157. doi: 10.1088/0967-3334/29/10/002
117. Nguyen P, Bui N, Nguyen A, Truong H, Suresh A, Whitlock M, et al. Tyth-typing on your teeth: tongue-teeth localization for human-computer interface. In: Proceedings of the 16th Annual International Conference on Mobile Systems, Applications, and Services. Munich: ACM (2018). p. 269–82. doi: 10.1145/3210240.3210322
118. Jang JS. ANFIS: adaptive-network-based fuzzy inference system. IEEE Trans Syst Man Cybernet . (1993) 23:665–85. doi: 10.1109/21.256541
119. Moody J, Darken CJ. Fast learning in networks of locally-tuned processing units. Neural Comput . (1989) 1:281–94. doi: 10.1162/neco.1989.1.2.281
120. Takagi T, Sugeno M. Derivation of fuzzy control rules from human operator's control actions. IFAC Proc Vol . (1983) 16:55–60. doi: 10.1016/S1474-6670(17)62005-6
121. LeCun Y, Bengio Y, Hinton G. Deep learning. Nature . (2015) 521:436–44. doi: 10.1038/nature14539
122. Litjens G, Kooi T, Bejnordi BE, Setio AAA, Ciompi F, Ghafoorian M, et al. A survey on deep learning in medical image analysis. Med Image Anal . (2017) 42:60–88. doi: 10.1016/j.media.2017.07.005
123. Simonyan K, Zisserman A. Very deep convolutional networks for large-scale image recognition. arXiv preprint arXiv:14091556 (2014).
124. Petrosian A, Prokhorov D, Homan R, Dasheiff R, Wunsch D II. Recurrent neural network based prediction of epileptic seizures in intra-and extracranial EEG. Neurocomputing . (2000) 30:201–18. doi: 10.1016/S0925-2312(99)00126-5
125. Gotman J, Gloor P. Automatic recognition and quantification of interictal epileptic activity in the human scalp EEG. Electroencephalogr Clin Neurophysiol . (1976) 41:513–29. doi: 10.1016/0013-4694(76)90063-8
126. Adeli H, Ghosh-Dastidar S, Dadmehr N. A wavelet-chaos methodology for analysis of EEGs and EEG subbands to detect seizure and epilepsy. IEEE Trans Biomed Eng . (2007) 54:205–11. doi: 10.1109/TBME.2006.886855
127. Polania R, Nitsche MA, Ruff CC. Studying and modifying brain function with non-invasive brain stimulation. Nat Neurosci. (2018) 21: 174–87. doi: 10.1038/s41593-017-0054-4
128. Ridding MC, Rothwell JC. Is there a future for therapeutic use of transcranial magnetic stimulation? Nat Rev Neurosci . (2007) 8:559. doi: 10.1038/nrn2169
129. Higgins ES, George MS. Brain Stimulation Therapies for Clinicians . Sullivan's Island, SC: American Psychiatric Publication (2019).
130. Kim J, Lee S. Development of a wearable robotic positioning system for noninvasive transcranial focused ultrasound stimulation. IEEE/ASME Trans Mechatron . (2016) 21:2284–93. doi: 10.1109/TMECH.2016.2580500
131. Valero-Cabré A, Amengual JL, Stengel C, Pascual-Leone A, Coubard OA. Transcranial magnetic stimulation in basic and clinical neuroscience: a comprehensive review of fundamental principles and novel insights. Neurosci Biobehav Rev . (2017) 83:381–404. doi: 10.1016/j.neubiorev.2017.10.006
132. Klomjai W, Katz R, Lackmy-Vallée A. Basic principles of transcranial magnetic stimulation (TMS) and repetitive TMS (rTMS). Ann Phys Rehabil Med . (2015) 58:208–13. doi: 10.1016/j.rehab.2015.05.005
133. Schrader LM, Stern JM, Koski L, Nuwer MR, Engel J Jr. Seizure incidence during single-and paired-pulse transcranial magnetic stimulation in individuals with epilepsy. Clin Neurophysiol . (2004) 115:2728–37. doi: 10.1016/j.clinph.2004.06.018
134. Gangitano M, Valero-Cabré A, Tormos JM, Mottaghy FM, Romero JR, Pascual-Leone Á. Modulation of input-output curves by low and high frequency repetitive transcranial magnetic stimulation of the motor cortex. Clin Neurophysiol . (2002) 113:1249–57. doi: 10.1016/S1388-2457(02)00109-8
135. Spronk D, Arns M, Fitzgerald PB. Repetitive transcranial magnetic stimulation in depression: protocols, mechanisms, and new developments. In: Neurofeedback and Neuromodulation Techniques and Applications . Academic Press (2011). p. 257–91. doi: 10.1016/B978-0-12-382235-2.00010-X
136. Oberman L. Repetitive transcranial magnetic stimulation (rTMS) protocols. In: Rotenberg A, Horvath JC, Pascual-Leone A, editors. Transcranial Magnetic Stimulation . New York, NY: Springer (2014). p. 129–39. doi: 10.1007/978-1-4939-0879-0_7
137. Chen R, Seitz RJ. Changing cortical excitability with low-frequency magnetic stimulation. Neurology . (2001) 57:379–80. doi: 10.1212/WNL.57.3.379
138. Plewnia C, Lotze M, Gerloff C. Disinhibition of the contralateral motor cortex by low-frequency rTMS. Neuroreport . (2003) 14:609–12. doi: 10.1097/00001756-200303240-00017
139. Thut G, Theoret H, Pfennig A, Ives J, Kampmann F, Northoff G, et al. Differential effects of low-frequency rTMS at the occipital pole on visual-induced alpha desynchronization and visual-evoked potentials. Neuroimage . (2003) 18:334–47. doi: 10.1016/S1053-8119(02)00048-4
140. Rollnik JD, Huber TJ, Mogk H, Siggelkow S, Kropp S, Dengler R, et al. High frequency repetitive transcranial magnetic stimulation (rTMS) of the dorsolateral prefrontal cortex in schizophrenic patients. Neuroreport . (2000) 11:4013–5. doi: 10.1097/00001756-200012180-00022
141. Speer AM, Kimbrell TA, Wassermann EM, Repella JD, Willis MW, Herscovitch P, et al. Opposite effects of high and low frequency rTMS on regional brain activity in depressed patients. Biol Psychiatry . (2000) 48:1133–41. doi: 10.1016/S0006-3223(00)01065-9
142. Hallett M. Transcranial magnetic stimulation: a primer. Neuron . (2007) 55:187–99. doi: 10.1016/j.neuron.2007.06.026
143. Flitman S, Grafman J, Wassermann E, Cooper V, O'grady J, Pascual-Leone A, et al. Linguistic processing during repetitive transcranial magnetic stimulation. Neurology . (1998) 50:175–81. doi: 10.1212/WNL.50.1.175
144. Dobek CE, Blumberger DM, Downar J, Daskalakis ZJ, Vila-Rodriguez F. Risk of seizures in transcranial magnetic stimulation: a clinical review to inform consent process focused on bupropion. Neuropsychiatr Dis Treat . (2015) 11:2975. doi: 10.2147/NDT.S91126
145. Rossi S, Hallett M, Rossini PM, Pascual-Leone A Safety of TMS Consensus Group. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol . (2009) 120:2008–39. doi: 10.1016/j.clinph.2009.08.016
146. Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5-7, 1996. Electroencephalogr Clin Neurophysiol Evoked Potent Sec . (1998) 108:1–16. doi: 10.1016/S0168-5597(97)00096-8
147. Bae EH, Schrader LM, Machii K, Alonso-Alonso M, Riviello JJ Jr, Pascual-Leone A, et al. Safety and tolerability of repetitive transcranial magnetic stimulation in patients with epilepsy: a review of the literature. Epilepsy Behav . (2007) 10:521–8. doi: 10.1016/j.yebeh.2007.03.004
148. Akamatsu N, Fueta Y, Endo Y, Matsunaga K, Uozumi T, Tsuji S. Decreased susceptibility to pentylenetetrazol-induced seizures after low-frequency transcranial magnetic stimulation in rats. Neurosci Lett . (2001) 310:153–6. doi: 10.1016/S0304-3940(01)02116-4
149. Rotenberg A, Muller P, Birnbaum D, Harrington M, Riviello JJ, et al. Seizure suppression by EEG-guided repetitive transcranial magnetic stimulation in the rat. Clin Neurophysiol . (2008) 119:2697–702. doi: 10.1016/j.clinph.2008.09.003
150. Cantello R, Rossi S, Varrasi C, Ulivelli M, Civardi C, Bartalini S, et al. Slow repetitive TMS for drug-resistant epilepsy: clinical and EEG findings of a placebo-controlled trial. Epilepsia . (2007) 48:366–74. doi: 10.1111/j.1528-1167.2006.00938.x
151. Cincotta M, Borgheresi A, Gambetti C, Balestrieri F, Rossi L, Zaccara G, Pascual-Leone A, et al. Suprathreshold 0.3 Hz repetitive TMS prolongs the cortical silent period: potential implications for therapeutic trials in epilepsy. Clin Neurophysiol . (2003) 114:1827–33. doi: 10.1016/S1388-2457(03)00181-0
152. Fregni F, Otachi PT, Do Valle A, Boggio PS, Thut G, Rigonatti SP, et al. A randomized clinical trial of repetitive transcranial magnetic stimulation in patients with refractory epilepsy. Ann Neurol . (2006) 60:447–55. doi: 10.1002/ana.20950
153. Herrmann CS, Rach S, Neuling T, Strüber D. Transcranial alternating current stimulation: a review of the underlying mechanisms and modulation of cognitive processes. Front Hum Neurosci . (2013) 7:279. doi: 10.3389/fnhum.2013.00279
154. Reato D, Salvador R, Bikson M, Opitz A, Dmochowski J, Miranda PC. Principles of transcranial direct current stimulation (tDCS): introduction to the biophysics of tDCS. In: Knotkova H, Nitsche MA, Bikson M, Woods AJ, editors. Practical Guide to Transcranial Direct Current Stimulation . Cham: Springer (2019). p. 45–80. doi: 10.1007/978-3-319-95948-1_2
155. Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, et al. Transcranial direct current stimulation: state of the art (2008). Brain Stimul . (2008) 1:206–23. doi: 10.1016/j.brs.2008.06.004
156. Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol . (2000) 527:633–9. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x
157. Peterchev AV, Wagner TA, Miranda PC, Nitsche MA, Paulus W, Lisanby SH, et al. Fundamentals of transcranial electric and magnetic stimulation dose: definition, selection, and reporting practices. Brain Stimul . (2012) 5:435–53. doi: 10.1016/j.brs.2011.10.001
158. Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology . (2001) 57:1899–901. doi: 10.1212/WNL.57.10.1899
159. Palm U, Segmiller FM, Epple AN, Freisleder FJ, Koutsouleris N, Schulte-Körne G, et al. Transcranial direct current stimulation in children and adolescents: a comprehensive review. J Neural Trans . (2016) 123:1219–34. doi: 10.1007/s00702-016-1572-z
160. Liebetanz D, Klinker F, Hering D, Koch R, Nitsche MA, Potschka H, et al. Anticonvulsant effects of transcranial direct-current stimulation (tDCS) in the rat cortical ramp model of focal epilepsy. Epilepsia . (2006) 47:1216–24. doi: 10.1111/j.1528-1167.2006.00539.x
161. Schulze-Bonhage A. Brain stimulation as a neuromodulatory epilepsy therapy. Seizure . (2017) 44:169–75. doi: 10.1016/j.seizure.2016.10.026
162. Larkin M, Meyer RM, Szuflita NS, Severson MA, Levine ZT. Post-traumatic, drug-resistant epilepsy and review of seizure control outcomes from blinded, randomized controlled trials of brain stimulation treatments for drug-resistant epilepsy. Cureus . (2016) 8:16. doi: 10.7759/cureus.744
163. Li MC, Cook MJ. Deep brain stimulation for drug-resistant epilepsy. Epilepsia . (2018) 59:273–90. doi: 10.1111/epi.13964
164. Tergau F, Naumann U, Paulus W, Steinhoff BJ. Low-frequency repetitive transcranial magnetic stimulation improves intractable epilepsy. Lancet . (1999) 353:2209. doi: 10.1016/S0140-6736(99)01301-X
165. Rossi S, Ulivelli M, Bartalini S, Galli R, Passero S, Battistini N, et al. Reduction of cortical myoclonus-related epileptic activity following slow-frequency rTMS. A case study. Neuroreport . (2004) 15:293–96. doi: 10.1097/00001756-200402090-00016
166. Sun W, Mao W, Meng X, Wang D, Qiao L, Tao W, et al. Low-frequency repetitive transcranial magnetic stimulation for the treatment of refractory partial epilepsy: a controlled clinical study. Epilepsia . (2012) 53:1782–9. doi: 10.1111/j.1528-1167.2012.03626.x
167. Auvichayapat N, Rotenberg A, Gersner R, Ngodklang S, Tiamkao S, Tassaneeyakul W, et al. Transcranial direct current stimulation for treatment of refractory childhood focal epilepsy. Brain Stimul . (2013) 6:696–700. doi: 10.1016/j.brs.2013.01.009
168. Tekturk P, Erdogan ET, Kurt A, Vanli-yavuz EN, Ekizoglu E, Kocagoncu E, et al. The effect of transcranial direct current stimulation on seizure frequency of patients with mesial temporal lobe epilepsy with hippocampal sclerosis. Clin Neurol Neurosurg . (2016) 149:27–32. doi: 10.1016/j.clineuro.2016.07.014
169. Assenza G, Campana C, Assenza F, Pellegrino G, Di Pino G, Fabrizio E, et al. Cathodal transcranial direct current stimulation reduces seizure frequency in adults with drug-resistant temporal lobe epilepsy: a sham controlled study. Brain Stimul Basic Transl Clin Res Neuromodul . (2017) 10:333–5. doi: 10.1016/j.brs.2016.12.005
170. San-Juan D, López DAE, Gregorio RV, Trenado C, Gonzalez-Aragon MF, Morales-Quezada L, et al. Transcranial direct current stimulation in mesial temporal lobe epilepsy and hippocampal sclerosis. Brain Stimul . (2017) 10:28–35. doi: 10.1016/j.brs.2016.08.013
171. Karvigh SA, Motamedi M, Arzani M, Roshan JHN. HD-tDCS in refractory lateral frontal lobe epilepsy patients. Seizure . (2017) 47:74–80. doi: 10.1016/j.seizure.2017.03.005
172. Manlapaz J, Åström K, Ballantine H Jr, Lele P. Effects of ultrasonic radiation in experimental focal epilepsy in the cat. Exp Neurol . (1964) 10:345–56. doi: 10.1016/0014-4886(64)90005-6
173. Min BK, Bystritsky A, Jung KI, Fischer K, Zhang Y, Maeng LS, et al. Focused ultrasound-mediated suppression of chemically-induced acute epileptic EEG activity. BMC Neurosci . (2011) 12:23. doi: 10.1186/1471-2202-12-23
174. Yook SW, Park SH, Seo JH, Kim SJ, Ko MH. Suppression of seizure by cathodal transcranial direct current stimulation in an epileptic patient-a case report. Ann Rehabil Med . (2011) 35:579–82. doi: 10.5535/arm.2011.35.4.579
175. Bikson M, Grossman P, Thomas C, Zannou AL, Jiang J, Adnan T, et al. Safety of transcranial direct current stimulation: evidence based update (2016). Brain Stimul . (2016) 9:641–61. doi: 10.1016/j.brs.2016.06.004
176. Grossman N, Bono D, Dedic N, Kodandaramaiah SB, Rudenko A, Suk HJ, et al. Noninvasive deep brain stimulation via temporally interfering electric fields. Cell . (2017) 169:1029–41. doi: 10.1016/j.cell.2017.05.024
177. Yang T, Chen J, Yan B, Zhou D. Transcranial ultrasound stimulation: a possible therapeutic approach to epilepsy. Med Hypotheses . (2011) 76:381–3. doi: 10.1016/j.mehy.2010.10.046
178. Tufail Y, Yoshihiro A, Pati S, Li MM, Tyler WJ. Ultrasonic neuromodulation by brain stimulation with transcranial ultrasound. Nat Protoc . (2011) 6:1453. doi: 10.1038/nprot.2011.371
179. O B̀rien WD Jr. Ultrasound-biophysics mechanisms. Biophys Mol Biol . (2007) 93:212–255. doi: 10.1016/j.pbiomolbio.2006.07.010
180. Ter Haar G. Therapeutic applications of ultrasound. Biophys Mol Biol . (2007) 93:111–29. doi: 10.1016/j.pbiomolbio.2006.07.005
181. King RL, Brown JR, Newsome WT, Pauly KB. Effective parameters for ultrasound-induced in vivo neurostimulation. Ultrasound Med Biol . (2013) 39:312–31. doi: 10.1016/j.ultrasmedbio.2012.09.009
182. Lee W, Kim HC, Jung Y, Chung YA, Song IU, Lee JH, et al. Transcranial focused ultrasound stimulation of human primary visual cortex. Sci Rep . (2016) 6:34026. doi: 10.1038/srep34026
PubMed Abstract | CrossRef Full Text
183. Legon W, Bansal P, Ai L, Mueller JK, Meekins G, Gillick B. Safety of transcranial focused ultrasound for human neuromodulation. bioRxiv [Preprint] . (2018) 314856. doi: 10.1101/314856
184. Rezayat E, Toostani IG. A review on brain stimulation using low intensity focused ultrasound. Basic Clin Neurosci . (2016) 7:187. doi: 10.15412/J.BCN.03070303
185. Bleichner MG, Mirkovic B, Debener S. Identifying auditory attention with ear-EEG: cEEGrid versus high-density cap-EEG comparison. J Neural Eng . (2016) 13:066004. doi: 10.1088/1741-2560/13/6/066004
186. Roth Y, Zangen A, Hallett M. A coil design for transcranial magnetic stimulation of deep brain regions. J Clin Neurophysiol . (2002) 19:361–70. doi: 10.1097/00004691-200208000-00008
187. Edwards D, Cortes M, Datta A, Minhas P, Wassermann EM, Bikson M. Physiological and modeling evidence for focal transcranial electrical brain stimulation in humans: a basis for high-definition tDCS. Neuroimage . (2013) 74:266–75. doi: 10.1016/j.neuroimage.2013.01.042
188. Antal A, Alekseichuk I, Bikson M, Brockmöller J, Brunoni AR, Chen R, et al. Low intensity transcranial electric stimulation: safety, ethical, legal regulatory and application guidelines. Clin Neurophysiol . (2017) 128:1774–809. doi: 10.1016/j.clinph.2017.06.001
Keywords: seizure detection, biosignal processing, biosignal classification, brain stimulation, EEG
Citation: Kim T, Nguyen P, Pham N, Bui N, Truong H, Ha S and Vu T (2020) Epileptic Seizure Detection and Experimental Treatment: A Review. Front. Neurol. 11:701. doi: 10.3389/fneur.2020.00701
Received: 11 February 2020; Accepted: 09 July 2020; Published: 21 July 2020.
Reviewed by:
Copyright © 2020 Kim, Nguyen, Pham, Bui, Truong, Ha and Vu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tam Vu, tam.vu@colorado.edu ; tam.vu@cs.ox.ac.uk
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.

Epilepsy-Definition, Classification, Pathophysiology, and Epidemiology
Affiliation.
- 1 Department of Neurology and Neurological Sciences, Stanford University School of Medicine, Palo Alto, California.
- PMID: 33155183
- DOI: 10.1055/s-0040-1718719
Seizures affect the lives of 10% of the global population and result in epilepsy in 1 to 2% of people around the world. Current knowledge about etiology, diagnosis, and treatments for epilepsy is constantly evolving. As more is learned, appropriate and updated definitions and classification systems for seizures and epilepsy are of the utmost importance. Without proper definitions and classification, many individuals will be improperly diagnosed and incorrectly treated. It is also essential for research purposes to have proper definitions, so that appropriate populations can be identified and studied. Imprecise definitions, failure to use accepted terminology, or inappropriate use of terminology hamper our ability to study and advance the field of epilepsy. This article begins by discussing the pathophysiology and epidemiology of epilepsy, and then covers the accepted contemporary definitions and classifications of seizures and epilepsies.
Thieme. All rights reserved.
Publication types
- Epilepsy* / classification
- Epilepsy* / epidemiology
- Epilepsy* / etiology
- Epilepsy* / physiopathology
- Seizures* / classification
- Seizures* / epidemiology
- Seizures* / etiology
- Seizures* / physiopathology

Focus On Epilepsy Research
The epilepsies are a set of disorders characterized by recurring seizures, or disturbances in the electrical activity of the brain. Epilepsy affects people of all ages, from infants to the aged, and can result from many causes, including genetic variations, illness, head injury, or abnormal brain development. NIH / NINDS and its community-based research partners are dedicated to finding cures for the epilepsies and/or preventing epilepsy in individuals at risk for seizures.
Featured NINDS Epilepsy Research Initiatives The NINDS established the Centers Without Walls program in 2010 to rapidly advance epilepsy research through promoting interdisciplinary, collaborative research. Four centers have been funded: Image The Epilepsy 4000 (Epi4K) collaborative has examined genetic data from 4,000 individuals in order to understand the genes underlying epilepsy. See an NIH news release about the center . Image The Center for SUDEP Research ( CSR ) brings together extensive expertise to understand Sudden Unexplained Death in Epilepsy. See an NIH news release about the center . Image The Epilepsy Bioinformatics Study for Antiepileptogenic Therapy ( EpiBiosS4Rx ) will use studies of animals and patients with traumatic brain injury (TBI) leading to post-traumatic epilepsy (PTE) in order to develop future clinical trials of epilepsy prevention therapies. Image The Channelopathy-Associated Epilepsy Research Center ( CAERC ) will combine high-throughput technologies and high-content model systems to investigate the functional consequences of genetic variants in channelopathy-associated epilepsy. Image The Epilepsy Multiplatform Variant Prediction ( EpiMVP ) Center Without Walls will develop a modular, highly integrated platform approach to accelerate determination of the functional, pharmacological, neuronal network and whole animal consequences of genetic variants among a range of clinical epilepsy types.
Estimates of Funding for Various Research, Condition, and Disease Categories
Additional funding information on epilepsy research projects funded by the ICARE members, including federal and nonprofit organizations can be accessed at the Interagency Collaborative to Advance Research in Epilepsy Research Portfolio.
Related Federal Programs
Other federal partners with epilepsy research programs include:
- The Department of Defense's Epilepsy Research Program .
- The CDC's Epilepsy Program .
Proceedings & Outcomes
Status Epilepticus after Benzodiazepines: Seizures and Improving Long Term Outcomes This virtual workshop convened preclinical and clinical researchers, as well as relevant stakeholders to discuss and define the indications of potential therapeutics needed to improve outcomes following SE. Topics of discussion will include refractory SE, post SE neuropathology, current clinical trials, gaps in the research for follow-on treatments and barriers to transitioning therapies to the clinic. Outcomes of the workshop will include a clearer understanding of the unmet therapeutic needs and identification of key gaps in the research, increasing the potential for new therapeutics development.
- Workshop Summary
Post-Traumatic Epilepsy: Models, Common Data Elements and Optimization The conference will set the stage to optimize preclinical and clinical research to prevent epileptogenesis following TBI. The results will help improve biomedical research in posttraumatic epilepsy.
ICARE: Interagency Collaborative to Advance Research in Epilepsy, 2021 Epilepsy research needs reach across the missions of multiple NIH Institutes and Centers and across many organizations outside the NIH. As the primary NIH Institute for epilepsy research, NINDS leads this working group, with broad representation from the NIH, other Federal agencies, and the research and patient advocacy communities. Annual meetings provide a forum for sharing information about ongoing and planned epilepsy research activities, highlighting advances and discussing needs and opportunities, and promoting increased collaboration toward common research goals.
- Meeting Summary
Curing the Epilepsies 2021 This conference, held January 4-6, 2021, was an opportunity for all epilepsy research stakeholders to provide input on the transformative research priorities for the field, and to come together to find ways to move forward "Curing the Epilepsies"
- Conference Summary
Accelerating the Development of Therapies for Anti-Epileptogenesis and Disease Modification The “Accelerating the Development of Therapies for Anti-Epileptogenesis and Disease Modification” workshop, on August 6-7, 2018, brought together experts in the field of epilepsy to optimize and accelerate the development of therapies for anti-epileptogenesis and disease-modification in the epilepsies.
Benchmarks for Epilepsy Research
- 2021 Benchmarks for Epilepsy Research
- 2020 Editorial: The Benchmarks: Progress and Emerging Priorities in Epilepsy Research
- Epilepsy Benchmarks Area I: Understanding the Causes of the Epilepsies and Epilepsy-Related Neurologic, Psychiatric, and Somatic Conditions
- Epilepsy Benchmarks Area II: Prevent Epilepsy and Its Progression
- Epilepsy Benchmarks Area III: Improved Treatment Options for Controlling Seizures and Epilepsy-Related Conditions Without Side Effects
- Epilepsy Benchmarks Area IV: Limit or Prevent Adverse Consequence of Seizures and Their Treatment Across the Life Span
- 2014 Benchmarks for Epilepsy Research
- Epilepsy Research Benchmarks Progress Update 2007-2009
- 2007 Epilepsy Research Benchmarks
Resources and Tools
Adam Hartman, M.D. | Program Director, Office of Clinical Research [email protected]
George K. Essien Umanah, Ph.D. | Program Director, Channels, Synapses, and Circuits [email protected]
Miriam Leenders, Ph.D. | Program Director, Channels Synapses & Circuits [email protected]
Vicky Whittemore, Ph.D. | Program Director, Channels Synapses & Circuits [email protected]
Ben Churn, Ph.D. | Program Director, Channels Synapses & Circuits [email protected]
Brian Klein, Ph.D. | Program Director, Epilepsy Therapy Screening Program [email protected]
Funding Opportunities
Epilepsy Funding Opportunities
Related Topics PANAChE Database This resource contains public and non-confidential chemical structures and biological data for compounds which have been screened for efficacy and toxicity in animal models of epilepsy and related seizure disorders as part of the Epilepsy Therapy Screening Program (ETSP) at the National Institute of Neurological Disorders and Stroke. Epilepsy Therapy Screening Program (ETSP) This resource contains public and non-confidential chemical structures and biological data for compounds which have been screened for efficacy and toxicity in animal models of epilepsy and related seizure disorders as part of the Epilepsy Therapy Screening Program (ETSP) at the National Institute of Neurological Disorders and Stroke. ICARE: Interagency Collaborative to Advance Research in Epilepsy ICARE provides an interagency forum for sharing information about ongoing and planned epilepsy research activities. Epilepsy Common Data Elements The NINDS epilepsy common data elements provide data standards for clinical research in order to improve data quality and facilitate comparison and combination of data across studies. The Epilepsy Research Connection (ERC) The ERC provides information about grant and funding opportunities from non-profit and government organizations focused on epilepsy related research.
- Open access
- Published: 25 May 2020
A review of epileptic seizure detection using machine learning classifiers
- Mohammad Khubeb Siddiqui ORCID: orcid.org/0000-0001-6699-6216 1 na1 ,
- Ruben Morales-Menendez 1 ,
- Xiaodi Huang 2 na1 &
- Nasir Hussain 3
Brain Informatics volume 7 , Article number: 5 ( 2020 ) Cite this article
20k Accesses
200 Citations
1 Altmetric
Metrics details
Epilepsy is a serious chronic neurological disorder, can be detected by analyzing the brain signals produced by brain neurons. Neurons are connected to each other in a complex way to communicate with human organs and generate signals. The monitoring of these brain signals is commonly done using Electroencephalogram (EEG) and Electrocorticography (ECoG) media. These signals are complex, noisy, non-linear, non-stationary and produce a high volume of data. Hence, the detection of seizures and discovery of the brain-related knowledge is a challenging task. Machine learning classifiers are able to classify EEG data and detect seizures along with revealing relevant sensible patterns without compromising performance. As such, various researchers have developed number of approaches to seizure detection using machine learning classifiers and statistical features. The main challenges are selecting appropriate classifiers and features. The aim of this paper is to present an overview of the wide varieties of these techniques over the last few years based on the taxonomy of statistical features and machine learning classifiers—‘black-box’ and ‘non-black-box’. The presented state-of-the-art methods and ideas will give a detailed understanding about seizure detection and classification, and research directions in the future.
1 Introduction
The word epilepsy originates from the Latin and Greek word ‘epilepsia’ which means ‘seizure’ or ‘to seize upon’. It is a serious neurological disorder with unique characteristics, tending of recurrent seizures [ 1 ]. The context of epilepsy, found in the Babylonian text on medicine, was written over 3000 years ago [ 2 , 3 ]. This disease is not limited to human beings, but extends to cover all species of mammals such as dogs, cats and rats. However, the word epilepsy does not give any types of clues about the cause or severity of the seizures; it is unremarkable and uniformly distributed around the world [ 1 , 4 ].
Several theories about the cause are already available. The main cause is electrical activity disturbance inside a brain [ 1 , 5 , 6 ], which could be originated by several reasons [ 7 ] such as malformations, shortage of oxygen during childbirth, and low sugar level in blood [ 8 , 9 ]. Globally, epilepsy affects approximately 50 million people, with 100 million being affected at least once in their lifetime [ 5 , 10 ]. Overall, it accounts for 1% of the world’s burden of diseases, and the prevalence rate is reported at 0.5–1% [ 4 , 11 ]. The main symptom of epilepsy is to experience more than one seizure by a patient. It causes a sudden breakdown or unusual activity in the brain that impulses an involuntary alteration in a patient’s behaviour, sensation, and loss of momentary consciousness. Typically, seizures last from seconds to a few minute(s), and can happen at any time without any aura. This leads to serious injuries including fractures, burns, and sometimes death [ 12 ].
1.1 Seizure type
Based on the symptoms, seizures are categorized by neuro-experts into two main categories—partial and generalized [ 7 , 13 ]—as shown in Fig. 1 . Partial seizure, also called ‘focal seizure’, causes only a section of the cerebral hemisphere to be affected. There are two types of Partial seizure: simple-partial and complex-partial. In the simple-partial, a patient does not lose consciousness but cannot communicate properly. In the complex-partial, a person gets confused about the surroundings and starts behaving abnormally like chewing and mumbling; this is known as ‘focal impaired awareness seizure’. On the contrary, in the generalized seizures, all regions of the brain suffer and entire brain networks get affected quickly [ 14 ]. Generalized seizures are of many types, but they are broadly divided into two categories: convulsive and non-convulsive.
Types of seizure. Showing types of seizure and its sub-types
1.2 Main contributions of the paper
In brief, the contributions of this paper are as follows:
We have done the review according to five main dimensions. First, researchers who adopted the EEG, ECoG or both for seizure detection; second, significant features; third, machine learning classifiers; fourth, the performance of the classifier during a seizure, and last, knowledge discovery (e.g., seizure localization).
Through study, it has been explored that an ensemble of decision trees (i.e., decision forest–random forest) classifier outperforms other classifiers (ANN, KNN, SVM, single Decision Tree).
We also suggest, how decision forest algorithms could be more effective for other knowledge discovery tasks besides seizure detection.
This study will help the researchers with their data science backgrounds to identify which statistical and machine learning classifiers are more relevant for further improvement to the existing methods for seizure detection.
The study will also help the readers for understanding about the publicly available epilepsy datasets.
In the end, we have provided our observations by the current review and suggestions for future research in this area.
The structure of the paper is organized as follows. “ Role of data scientists in epileptic seizure detection ” section gives the overview of machine learning experts in EEG datasets. The preliminaries requirements are provided in “ A framework for seizure detection ” section; it presents a general model of seizure detection and explains each step in a subsequent manner. “ Publicly available datasets ” section provides the details of benchmark datasets with their description. “ Seizure detection based on statistical features and machine learning classifiers ” section explains the review of literature work done on seizure detection using different machine learning classifiers, with a detailed comparison. “ Seizure localization ” section reviews the work done in identifying the affected lobes of the brain using machine learning classifiers. In “ Problems identified in existing literature ” section, we have explored the issues in the previous work and highlighted the gap. Overall, “ observation about capable classifiers and statistical features ” section reports our observations from the review about a suitable classifier and feature. “ Research directions in seizure detection ” section emphasizes the future directions in this research area, followed by “ Conclusion ” section on the summary of the paper.
2 Role of data scientists in epileptic seizure detection
Applications of machine learning are significantly seen on health and biological data sets for better outcomes [ 15 , 16 ]. Researchers/scientists on different areas, specifically, data mining and machine learning, are actively involved in proposing solutions for better seizure detection. Machine learning has been significantly applied to discover sensible and meaningful patterns from different domain datasets [ 17 , 18 ]. It plays a significant and potential role in solving the problems of various disciplines like healthcare [ 17 , 19 , 20 , 21 , 22 , 23 , 24 , 25 ]. Applications of machine learning can also be seen on brain datasets for seizure detection, epilepsy lateralization, differentiating seizure sates, and localization [ 26 , 27 , 28 , 29 ]. This has been done by various machine learning classifiers such as ANN, SVM, decision tree, decision forest, and random forest [ 26 , 28 ].
Certainly, in the past, numerous reviews have been carried out on seizure detection along with applied features, classifiers, and claimed accuracy [ 27 , 30 , 31 , 32 , 33 ] without focusing on the challenges faced by the data scientists whilst doing research on datasets of neurological disorders. Therefore, this article provides a detailed study of machine learning applications on epileptic seizure detection and other related knowledge discovery tasks. In this review, the collected articles are from well-known journals of their relevant field. These references are either indexed by SCOPUS or Web of Science (WOS) . Besides, we also considered some good ranked conference papers. Extensive literature is available covering the deep analysis of different features and classifiers applied on EEG datasets for seizure detection [ 31 , 34 , 35 ]. Both, feature extraction and applying classification techniques are challenging tasks. Previous literature reveals that for the past few years, interest has been increased in the application of machine learning classifiers for extracting meaningful patterns from EEG signals, which helps for detecting seizures, its location in the brain, and other impressive related knowledge discoveries [ 28 , 36 , 37 ]. Three decades ago, Jean Gotman [ 6 , 38 , 39 , 40 ], analyzed and proposed the model for effective usage of EEG signals by applying different computational and statistical techniques for automatic seizure detection. Furthermore, the research has been carried out by different signal processing methods and data science methods to provide better outcomes [ 27 , 34 , 41 , 42 , 43 , 44 , 45 , 46 , 47 ].
3 A framework for seizure detection
In this section, we present a pictorial framework of the model used for seizure detection from an EEG/ECoG seizure dataset, illustrated in Fig. 2 . The process comprises four steps: Data Collection, Data Preparation, Applying Machine Learning Classifiers and Performance Evaluation.
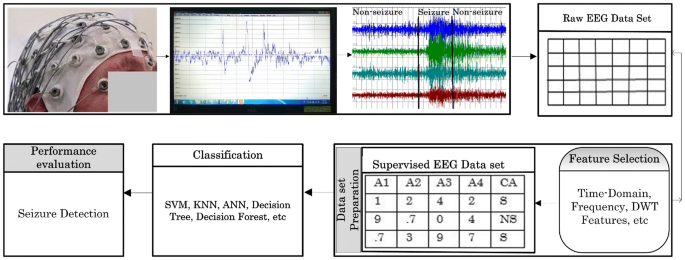
Basic model of epileptic seizure detection. This explains the basic steps to collect the dataset by EEG medium, display of raw EEG signals, transform EEG signals to two-dimensional table, feature selection, prepare the dataset with seizure (S) and non-seizure (NS) , apply machine learning classifier(s) and seizure detection, or other related tasks
3.1 Data collection
The initial requirement is to collect the dataset of brain signals. For this, different monitoring tools are used. Typically, the mostly used devices are EEG and ECoG, because their channels or electrodes are implanted by glue on the surface of the scalp as per 10–20 International system [ 48 ] at different lobes. Each of them has a wire connection to the EEG device, providing timely information about the variations in voltage, along with temporal and spatial information [ 49 ]. As highlighted in Fig. 2 , the EEG channels are placed on the subject’s scalp, and the electrical signals are read by the EEG monitoring tool and it displays these raw signals over the screen. Further, these raw signals have been carefully monitored by the analyst and classified into ‘seizure’ and ‘non-seizure’ states.
3.2 Data transformation
After data collection, the next crucial step is to transform the signal data into a 2-D Table format. The reason for this is to make it easier for analysis and provide necessary knowledge like seizure detection. This datum is raw because it has not been processed yet. Therefore, it will not be suitable to give relevant information. To do the processing, different feature selection modalities have been applied. This step also presents the dataset as supervised, which means that it provides the class attribute with possible class-values.
3.3 Dataset preparation
For data transformation, data processing is a decisive step to extract meaningful information from the collected raw dataset. As such, different feature extraction techniques have been used; as shown in Table 1 . These methods are generally applied to the extracted EEG signal dataset [ 31 , 34 ]. The raw dataset becomes rich in terms of different statistical measure values.
After feature extraction processing, the dataset becomes more informative that it ultimately helps the classifier for retrieving better knowledge.
3.4 Applying machine learning classifiers and performance evaluation
To achieve a high accuracy of seizure detection rate and explore relevant knowledge from the EEG processed dataset, different supervised and unsupervised machine learning have been used.
3.4.1 Classification
In classification, a dataset D has a set of ‘non-class attributes’, and a ‘class attribute’. They are the principal components and their pertinent knowledge is very important, as both have a strong association for potential classification. The target attribute is defined as the ‘class attribute’ C , and it comprises more than one class values, e.g., seizure and non-seizure . On the contrary, attributes \(A=\{A_1,A_2.A_3 \ldots A_n\}\) are known as ‘non-class attributes’ or predictors [ 50 , 51 ]. The following classifiers have been popularly used in seizure detection. Common classifiers such as SVM [ 52 ], decision tree [ 53 ] and decision forest [ 54 ] are applied to the processed EEG dataset for seizure detection.
3.4.2 Performance evaluation
The accuracy of the obtained results is used to evaluate different methods. The most popular training approach is tenfold cross-validation [ 55 ], where each fold, i.e., one horizontal segment of the dataset is considered to be the testing dataset and the remaining nine segments are used as the training dataset [ 56 , 57 ].
Except for the accuracy, the performance of the classifiers is commonly measured by the following metrics such as precision, recall, and f-measure [ 58 ]. These are based on four possible classification outcomes—True-Positive (TP), True-Negative (TN), False-Positive (FP), and False-Negative (FN) as presented in Table 2 .
Precision is the ratio of true-positives to the total number of cases that are detected as positive (TP+FP). It is the percentage of selected cases that are correct, as shown in Eq. 1 . High precision means the low false-positive rate.
Recall is the ratio of true-positive cases to the cases that are actually positive. Equation 2 shows the percentage of corrected cases that are selected.
Despite getting the high Recall results of the classifier, it does not indicate that the classifier performs well in terms of precision. As a result, it is mandatory to calculate the weighted harmonic mean of Precision and Recall; this measure is known as F-measure score, shown in Eq. 3 . The false-positives and the false-negatives are taken into account. Generally, it is more useful than accuracy, especially when the dataset is imbalanced.
4 Publicly available datasets
For data scientists and researchers, a dataset used is important for evaluating the performance of their proposed models. Similarly, in epileptic seizure detection, we need to capture the brain signals. EEG recording is the most used method for monitoring brain activity. These recordings play a vital role in machine learning classifiers to explore the novel methods for seizure detection in different ways such as onset seizure detection, quick seizure detection, patient seizure detection, and seizure localization. The significance of publicly available datasets is that they provide a benchmark to analyze and compare the results to others. In the following section, we will describe the popular datasets that are widely used on epilepsy.
4.1 Children Hospital Boston, Massachusetts Institute of Technology—EEG dataset
This dataset is publicly available on a physionet server and prepared at Children Hospital Boston, Massachusetts Institute of Technology (CHB-MIT) [ 59 , 60 ]. It can be collected easily via Cygwin tool which interacts with the physionet server. It contains the number of seizure and non-seizure EEG recordings for each patient of the CHB [ 61 ]. The dataset comprises 23 patients; 5 males, aged 3–22 years, and 17 females aged 1.5–19. Each patient contains multiple seizure and non-seizure recording files in European data format (.edf), representing the spikes with seizure start and end time, which is easily visible at a browser called an ‘EDFbrowser’. The primary datasets are in the 1-D format, containing EEG signals that are obtained through the different types of channels that were placed on the surface of the brain as per 10-20 International System. All these signals of the dataset were sampled at the frequency of 256Hz.
4.2 ECoG Dataset, Epilepsy Centre, University of California
This is a publicly available dataset of electrocorticogram (ECoG) signals from an epileptic patient, which was collected from the Epilepsy Center, University of California, San Francisco (UCSF) [ 62 ]. It was originally collected by implanting 76 electrodes on the scalp in both invasive (12-electrodes) and non-invasive manner (64-electrodes). It comprises 16 files altogether. Out of these, eight files ( \(F1, F2, \cdots F8\) ) are classified as ‘pre-ictal’ meaning the stage before the seizure. The rest of the files ( \(F9, F10, F11, \cdots F16\) ) represent the ‘ictal’ stage data. The collected data are sampled at the frequency of 400 Hz (i.e., 400 cycles/s) and the total duration is 10 s. As a result, there are (400 cycles/s \(\times\) 10 s) 4000 cycles in each file [ 63 ].
4.3 The Freiburg—EEG dataset
This dataset was collected from the invasive EEG recordings of 21 patients (8 males aged 13–47 years, 13 females aged 10–50 years) suffering from medically intractable focal epilepsy. It was recorded during an invasive pre-surgical epilepsy monitoring at the Epilepsy Centre of the University Hospital of Freiburg, Germany [ 64 ]. Out of 21 patients, 13 patients had 24 h of recordings, and 8 patients had less than 24 h. These recordings are inter-ictal, and together they provide 88 seizures.
4.4 Bonn University—EEG dataset
The dataset comprises five subsets, where each one denoted as (A–E) contains 100 single-channels recording, and each of them has a 23.6 s duration, captured by the international 10–20 electrode placement scheme. All the signals are recorded with the same 128-channel amplifier system channel [ 65 ].
4.5 BERN-BARCELONA—EEG dataset
This dataset comprised EEG recordings derived from five pharmacoresistant temporal lobe epilepsy patients with 3750 focal and 3750 non-focal bivariate EEG files. Three patients were seizure-free, with two patients only having auras but no other seizures following surgery. The multichannel EEG signals were recorded with an intracranial strip and depth electrodes. The 10–20 positioning was used for the electrodes’ implantation. EEG signals were either sampled at 512 or 1024 Hz, depending on whether they were recorded with more or less than 64 channels. According to the intracranial EEG recordings, they were able to localize the brain areas where seizures started for all five patients [ 66 ]. This dataset is good for the seizure localization purpose.
5 Seizure detection based on statistical features and machine learning classifiers
This section explains the comprehensive detail of work on seizure detection using statistical features, classifiers—‘black-box’ and ‘non-black-box’. They are illustrated in Table 3 . In brief, the ‘black-box’ classifiers are those which provide the accuracy without mentioning the reasons behind the results such as ANN and SVM [ 67 ]. They are unable to explain their classification steps. Whereas, ‘non-black-box’ classifiers such as decision forest and random forest can able to explain each step of the processing, which is human-understandable. As a result, it helps in human-interpretable knowledge with high accuracy [ 68 ].
5.1 Seizure detection based on statistical features
If we apply machine learning classifier(s) directly to raw EEG/ECoG datasets, it may not produce enough sensible patterns. Therefore, selecting significant and capable statistical features from EEG and ECoG raw datasets is one of the challenges and a crucial task. The nature of EEG and ECoG signals is very complex, non-stationary and time-dependent [ 105 , 106 , 107 ]. As such, we can apply the machine learning classifier(s) to the processed datasets, which will ultimately assist to solve various neurological problems; for example, identifying seizure’s stages, accurate seizure detection, fast detection, etc. In Table 3 , we summarize a review of several studies.
The significant statistical features were extracted by different types of transformation techniques; discrete wavelet transformations (DWT), continuous wavelet transformation (CWT), Fourier transformation (FT), discrete cosine transformation (DCT), singular value decomposition (SVD), intrinsic mode function (IMF), and time–frequency domain from EEG datasets [ 34 , 71 , 79 , 108 ]. Logesparan et al. [ 34 ] used different types of feature extraction methods for seizure detection, but they reported that two features—‘line length’ and ‘relative power’—are the good performers for seizure detection. Guerrero-Mosquera [ 109 ] applied three time-domain features—line length, frequency, and energy on the raw EEG dataset. These features claim to be suitable for seizure detection and other brain-related applications such as computer interface (BCI). The claimed performance was evaluated using the following metrics such as sensitivity, specificity, F-score, receiver operating characteristics (ROC) curve, and percentile bootstrap measures. Duo Chen [ 84 ] used DWT with the SVM classifier on two benchmark datasets—CHB-MIT and Bonn University, achieved seizure detection accuracies of 92.30% and 99.33%, respectively. Ramy Hussein et al. [ 100 ] proposed a new featured L1-penalized robust regression (L1PRR) for seizure detection, the issue with their approach is computational complexity. Zavid and Paul [ 99 ] focused on classifying the ‘ictal’ and ‘inter-ictal’ states, where they used four features DCT, DCT-DWT, SVD, and IMF; the obtained signals are further classified by LS-SVM due to less computational cost.
Several researchers have contributed to seizure detection using a single feature [ 108 , 110 ]. The feature ‘line length’ [ 108 , 110 ] was applied to an EEG dataset; approximately 4.1 s of mean detection latency is recorded at a false alarm rate of 0.051 Fp/h. Further, Guo et al. [ 69 ] also used ‘line length’ but with the ANN for classifying the records obtained by EEG signals. Their automated seizure detection accuracy is 99.6%. A system was proposed by Koolen et al. [ 70 ] to detect seizures from EEG recordings. This detection system uses a single feature—‘line length’. The performance of this system shows 84.27% accuracy, 84.00% sensitivity and 85.70% specificity, which are comparatively lower than the results of Guo et al. [ 69 ].
After 3 years of study on several of statistical features [ 34 ], Logesparan et al. [ 71 ] proposed the ‘line length’ feature for normalization and discrimination of class values from EEG datasets. It is noted that ‘line length’ could be taken as the strongest feature and provides considerable output. Based on previous studies, the ‘line length’ can be taken with other features, and the result would be more promising, specifically in machine learning. This is because the dataset dimension would also increase with meaningful statistical information in the attributes.
Some other studies on seizure detection based on a single feature, i.e., entropy and its sub-types such as approximate entropy (AE) and sample entropy (SE), have also been done [ 45 , 72 , 73 , 111 ]. The entropy feature helps to find the random behaviour of EEG signals and takes depth benefits in measuring the impurity of the signals [ 112 , 113 ]. The entropy feature has been used widely where data are in the form of signals such as ECG, [ 114 ], EEG, and ECoG [ 36 ]. This helps in further steps of the detection model.
Acharya et al. [ 111 ] used four different types of entropy-based features: sample entropy, approximate entropy, phase entropy (S1), and phase entropy (S2) of the EEG datasets. The processed dataset from these entropy features was used for seizure detection. In another study, Chen et al. [ 90 ] used eight different kinds of entropy feature—approximate, sample, spectral, fuzzy, permutation, Shannon, conditional and correction conditional on a raw EEG dataset; further, the processed data were classified into three class values: ‘ictal’, ‘inter-ictal’ and ‘normal stage’, and their accuracy is 99.50%. A tool was proposed by Selvakumari et al. [ 89 ] using four features—entropy, root mean square (RMS), variance, and energy. Based on these features, the detection was done using SVM and naïve Bayesian classifiers with a reported accuracy of 95.63%. The tool is also able to find the seizure region in the brain; however, they did not mention the exact percentage of seizure location. Song and Li [ 72 ] built classification models by two classifiers—Extreme Learner Machine (ELM) and the back-propagation neural network (BPNN). Overall, their findings show 95.6% of classification accuracy with less execution time. Yong Zhang et al. [ 73 ] applied two entropy features—AE and SE on two different classifiers—ELM and SVM for processing EEG dataset. The SE features with ELM provide good classification accuracy compared to the AE feature whilst detecting the seizure.
The energy feature has been significantly used in seizure detection [ 115 ]. It plays a vital role particularly when the seizure is detected by the epoch- or windows-based method. This means that the EEG signals are divided into various segments [ 79 , 94 ]. An exponential energy feature has been introduced by Fasil and Rajesh [ 97 ], which helps in identifying the irregularities in amplitude EEG signals.
Observations This section has provided an overview of the contributions of statistical features to seizure detection and their importance. Some researchers detect seizures using multiple sets of features, whilst others select a single feature such as ‘line length’. We recommend the ‘line length’ feature to be in the list of the set of suitable features for seizure detection because it is helpful in measuring the EEG signals complexity. It plays a sensitive role in the changes at the frequency and amplitude of signals. As a result, it helps to discriminate against the ‘seizure’ and ‘non-seizure’ cases. However, from the data science point of view, it is very important to see the various perspectives of each brain signals by observing other statistical features. Furthermore, we also suggest not to use the irrelevant feature(s) as they will unnecessarily increase the dataset size which results in an increase in computational time and gives insensible patterns too. As a result, it becomes a hassle to machine learning classifiers and users rather than providing the benefit. Some researchers [ 95 , 98 , 101 ] used a large number of features, which increases the attribute size, and results in more computational time and less accuracy. So, if we take the fewer features as previous researchers have done [ 71 , 73 , 79 ] this will give the low-dimensional dataset, which will not be fruitful for the knowledge discovery process. The next section illustrates the seizure detection by ‘black-box’ classifiers. As far as the classification purpose is concerned, it would be better to take more relevant statistical features, which can be integrated into knowledge discovery and a good performance rate.
5.2 Seizure detection based on black-box classifiers
The classifiers such as SVM, ANN, and KNN are considered as prominent ones due to their remarkable performances in different domains [ 67 , 116 ]. Each technique has its pros and cons, and ‘black-box’ methods are not an exception to this [ 104 ]. Even though these classifiers contribute well to brain datasets, some of the relevant works on seizure detection using these classifiers are reported here.
The study of Satapathy et al. [ 85 ] was based on two ‘black-box’ approaches—SVM and Neural networks using different kernel methods for seizure detection against a large EEG dataset. The performance of each classifier is measured independently by the majority voting system, and it was found that SVM was more capable than other neural networks. Subasi et al. [ 87 ] proposed the solution to detect seizure using a hybrid approach of SVM, genetic algorithm (GA), and particle swarm optimization (PSO). The method achieved impressive accuracy, i.e., 99.38%, but the problem is that the classifier trains the dataset twice, one for SVM-GA and another for SVM-PSO. This could be a time-consuming.
Shoeb and Guttag [ 41 ] performed seizure detection on their arranged dataset of Child Hospital Bostan, MIT (CHB-MIT) [ 60 ] using SVM with the vector feature and achieved the estimated accuracy of 96%. Dorai and Ponnambalam [ 42 ] came with an idea of the epoch, which means dividing the dataset into smaller time frames. Further, they applied an ensemble of four ‘black-box’ approaches—LDA, KNN, CVE, and SVM on these epoch EEG datasets. This approach provides the prediction of onset seizures 65 s earlier. Classifying the EEG data into two class ‘ ‘seizure” and ‘non-seizure’, Birjandtalab et al. [ 117 ] used a Gaussian mixture model (GMM) before detecting the seizure, and obtained 90% accuracy with 85.1% F-measure. They also raised the issue of class imbalance in their dataset. Tzallas et al. [ 103 ] used time–frequency-domain features with ANN for the EEG dataset and obtained 100% accuracy for the ‘seizure’ and ‘non-seizure’ classification problem; with epochs’ datasets the accuracy is 97.7% from (A, B, C, and D) for ‘non-seizure’ and set E for ‘seizure’ epoch classes. Amin et al. [ 79 ] extracted relative energy features from the DWT method, and four classifiers—SVM, MLP, KNN, and Naïve Bayes—were applied for the classification purpose, the result shows 98% of SVM accuracy, which outperforms remaining classifiers. A framework had been proposed by K. Abualsaud et al. [ 118 ] using the ensemble of ‘black-box’ classifiers for automated seizure detection on noisy EEG signals, and the reported classification accuracy is 95%. However, the ensemble approach did not provide good accuracy as desired because all four classifiers were ‘black-box’.
In 2018, Lahmiri et al. [ 92 ] used generalized Hurst exponent (GHE) and KNN, to propose a system for identifying the ‘seizure’ and ‘non-seizure’ classes from intracranial EEG recordings, detection rate, with 100% accuracy rate. Further, Lahmiri et al. [ 43 ] exploited GHE with SVM, to classify the ‘seizure’ and ‘non-seizure’, and also they found 100% accuracy in less time. Here, the good indication is that authors claim the good accuracy in less time for seizure detection. But, the authors did not clearly define how many times the seizure can be detected. In another study by Al Ghayab et al. [ 88 ], the obtained accuracy is 100% as a result of using the concept of Information gain theory, to extract and rank the meaningful features from EEG signal dataset. The least square-support vector machine (LS-SVM) is then applied to classify the seizure cases. Moreover, due to the ‘black-box’’s nature of applied classifiers, the authors could not explore any other related aspects in terms of Knowledge discovery. Zabihi et al. [ 81 ] did patient-specific seizure detection using SVM classifier on the processed dataset with a good set of features, comprising time-domain, frequency-domain, time–frequency domain, and non-linear feature. The performance of their model has achieved an average of 93.78% sensitivity and a specificity of 99.05%. Here, it is noteworthy that they skip an important feature—‘line length’, from the available literature, which is prominently used in seizure detection. We also argue that CHB-MIT dataset [ 60 ] is imbalanced because, in an hour(s) of recording, a seizure time span is for a few seconds.
Observations
The main issue with ‘black-box’ classifiers is that they only make prediction without providing logic rules or patterns. That is why, they are not recommended for extracting sensible knowledge. For example, for class imbalance issues in EEG datasets, insufficient related literature is found, and the researchers who attempted to work on this problem did not provide a conceivable solution as to how to solve the class imbalance issue whilst detecting the seizure.
5.3 Seizure detection based on non-black-box classifiers
‘Black-box’ classifiers are unable to express their classification procedure for human interpretation [ 67 , 104 , 116 ]. Consequently, there are fewer chances for knowledge discovery and better accuracy performance. Therefore, the concept of ‘non-black-box’ classifiers such as decision trees, and decision forests came into practice.
Chen et al. [ 119 ] first introduced the decision tree to the EEG dataset for seizure detection. Kemal and Saleh [ 120 ] used a C5.0 decision tree [ 121 ] algorithm to explore the logic rules for seizure detection, with an average accuracy of 75%. When the same C5.0 was applied to the same dataset processed by Fourier transformation the obtained accuracy with cross-validation was, however, 98.62%. A few related works are been available, where only a decision tree method is applied seizure detection because of less accuracy and a limited number of patterns obtained from the logic rules of a decision tree [ 122 ]. As a result, both the knowledge discovery and accuracy suffer. However, this gap can be filled by applying decision forest approaches instead [ 51 , 57 , 123 ].
Through the literature, it is found that the decision forest approaches are more effective than the single decision tree [ 57 , 124 ], because the decision tree often gives a confined set of rules and overfitting issue is also raised [ 68 ]. The rules are extracted from training data by a decision tree that generates either limited or a single set of logic rules (Say, wherever C2_Entropy value \(\le 101.01\) then \(Class\_value=seizure\) ) and stops growing the tree further records in the training dataset once the rule is accepted. However, if we generate a decision forest on the training data, we can achieve multiple sets of decision trees with the combination of sensible logic rules and a higher accuracy rate due to the majority voting method [ 57 ]. Decision forest classifiers [ 54 , 68 ] are the type of ensemble methods that are used frequently. These are also used in seizure detection as they provide a high accuracy rate which depends on the majority voting method from the ensemble of decision trees. Moreover, they produce more logic rules as multiple decision trees from the training data ( D ) [ 123 ]. These logic rules are humanly interpretable, and data scientists can easily interrelate them with other seizure-related information from EEG datasets.
Siddiqui and Islam [ 125 ] used Systematic Forest (SySFor) to detect the seizure on ECoG without epoch reduction. Further, Siddiqui et al. [ 63 ] applied two decision forests—Systematic Forest (SysFor) [ 123 ] and Forest CERN [ 51 ] on nine statistical features for quick seizure detection using the concept of epoch length reduction. It is based on dividing the size of training dataset D into \(D_1, D_2\) , ... \(D_n\) and testing the accuracy at every epoch of the dataset. These sub-datasets are in descending order in terms of time duration. If the seizure can be detected in a shorter epoch length without a decline in accuracy, then we can use the same one, which results in fast seizure detection. They achieved 100% accuracy. The limitation of this work is that authors have taken the dataset of a single patient, this could be tested for more patients. Several researchers have taken the advantages of random forest classifier for detecting the seizures [ 76 , 78 , 82 , 126 ]. Because researchers/data scientists are able to see the logic rules and interpret them correspondingly. Moreover, it also provides good accuracy [ 44 , 76 , 77 , 78 , 80 , 82 ]. Donos et al. [ 44 ] applied decision forest classifier—random forest, on time and frequency domains’ feature, which was extracted from an IEEG (Intra-cranial EEG) dataset. It helped in selecting the intra-cranial channels for early seizure detection in a closed-loop circuit. The results claimed that the system can detect the seizure with 93.8% sensitivity. Wang et al. [ 94 ] developed the greedy approach of random forest, i.e., forest-grid search optimization (RF-GSO), with this method and they found 96.7% accuracy. The shortcoming of this technique is that the performance could decline if EEG signals are too noisy. Tzimourta et al. [ 93 ] applied random forest to monitor seizure activities on the two benchmark epilepsy datasets [ 64 , 65 ], the reported performance is 99.74%. Pinto-Orellana and Fábio R. Cerqueira [ 76 ] also used the random forest on the processed CHB-MIT dataset by a Spectro-temporal feature, and 70s, and the accuracy of each block is 98.30%.
Truong ND et al. [ 82 ] had carried out novel work of channel selection whilst detecting the seizure. Their key contribution is that they also focus on channels contributing mostly to automatic seizure detection. They used the random forest to solve channel selection and seizure detection, and which achieving 96.94% area under the curve (AUC). In another work, Mursalin et al. [ 80 ] proposed a method for seizure detection by selecting features with an Improved Correlation-based Feature Selection(ICFS). Basically it is a fusion of time and frequency domain. Then, a random forest classifier was applied for the seizure detection model. The obtained average classification accuracy by this approach was 98.75%.
Some other works have used an ensemble of ‘non-black-box’ classifiers such as boosting, bagging and random subspace [ 78 , 127 ]. Yan et al. [ 78 ] applied a boosting classifier achieving 94.26% of accuracy, although the results were not as impressive as the ones obtained by [ 44 ], which used a random forest classifier. Hosseini [ 128 ] used Random subspace classifier along with an SVM classifier, to classify and detect seizures. Here, the benefit of applying a subspace on big datasets is to divide them into sub-datasets based on the random subspace concept, and then the SVM classifier was applied to each sub-dataset. Ensemble accuracy (EA) was calculated by the majority voting method, which was 95%. Apart from this study, the same authors of Hosseini et al. [ 126 ] recently did another research using an ensemble of classifiers. First, they created bootstrap samples using a random subspace method, and then applied classifiers such as SVM, KNN, extended nearest neighbor (ENN), and multilayer perceptron (MLP) obtaining 97% accuracy. Hussein et al. [ 100 ], proposed a novel feature extraction method, i.e., L1-penalized robust regression (L1PRR), which uses three common symptoms during seizures—muscles artifacts, eyes movement, and white noise. Inputting these features help the random forest classifier to obtain 100% accuracy.
Observations In comparison to decision trees, decision forest classifiers are tremendously used on brain datasets for exploring different research goals. It is difficult to suggest a particular classifier whilst dealing with a high-dimensional dataset, but a random forest classifier can be a capable classifier. However, it also criticizes that not all the ‘non-black-box’ classifiers are peculiar to detect seizures and have also pointed out the objection on the drawback of using a single decision tree classifier.
5.4 Seizure detection based on black-box and non-black-box machine learning classifiers
From the literature, it is found that just a single machine learning classifier is not sufficient. Therefore, to take advantage of both ‘black-box’ and ‘non-black-box’ classifiers, some researchers utilized them in their experiments. This section provides a comprehensive review of classifiers applied together to detect the seizure.
Acharya et al. [ 111 ] used the ensemble of seven different classifiers—Fuzzy surgeon classifier (FSC), SVM, KNN, Probabilistic neural network, GMM, decision tree and Naïve Bayes for distinguishing the three states of a patient as ‘normal, ‘pre-ictal’ and ‘ictal’. The overall accuracy is 98.1%. Fergus et al. [ 83 ] also used distinct classifiers such as linear discriminant analysis (LDA), quadratic discriminant classifier (QDC), logistic classifier, uncorrelated normal density-based classifier (UDC), polynomial classifier, KNN, PARZEN, SVM, and decision tree on the processed data with seven features such as entropy, RMS, skewness, and variance. They contributed that the detected patient is suffering from a ‘Generalize seizure’ (means affecting whole brain region) across different patients without prior information about the seizure focal points. Mursalin et al. [ 101 ] proposed a method to reduce the data size, statistical sampling technique called optimum sample allocation technique, and to reduce the features they develop a feature selection algorithm. The analysis was done on the combination of five classifiers—SVM, KNN, NB, Logistic Model Trees (LMT) and Random forest.
Rand and Sriram [ 95 ] used four classifiers such as SVM, KNN, random forest, and Adaboost on a high-dimensional dataset prepared by 28 features. Their result shows that SVM outperforms on the cubic kernel. In another study, Manzouri et al. [ 98 ] used SVM and random forest on the dataset produced by 10-time and frequency features. In comparison to SVM-based detector, random forest classifier outperforms. Subasi et al. [ 96 ] achieved 100% of accuracy using four machine learning classifiers such as ANN, KNN, SVM, and random forest on two popular datasets—Freiburg and CHB-MIT to classify the three different states of seizures ‘pre-ictal’, ‘ictal’, and ‘inter-ictal’. Sharma et al. [ 102 ] proposed an automated system using iterative filtering and random forest for classifying the EEG signals. This work achieved classification accuracies of 99.5% on BONN dataset (A-E), for A versus E subsets, 96% for D versus E subsets, and 98.4% for ABCD versus E classes of EEG signals. Birjandtalab et al. [ 77 ] used two classifiers for different purposes; KNN is used to discriminate the ‘seizure’ and ‘non-seizure’ classes, whereas random forest is used to explore the significant channels. Here, the random forest also helps in the dimension reduction problem. The main benefit of selecting suitable channels is that it helps in providing relevant required information from the chosen channels, and reduces the computational cost of a classifier too. However, the authors did not mention here the important information from channel selection like finding the seizure location from the brain scalp. The main critic in [ 95 , 98 , 101 ] is that because of a large number of features, the attribute size of dataset will increases, and as a result the accuracy and computation time suffer.
5.4.1 Observations
We observe that some work used an ensemble of distinguished classifiers to take the benefits separately. For example, influential channel selection can be independently done using decision forest classifiers like a random forest. But authors used other classifiers such as SVM and KNN for classifying the seizure records with good accuracy.
6 Seizure localization
After a successful seizure detection, localization is an essential task for epileptic surgery [ 129 , 130 , 131 ]. Typically, localized seizures can be cured by surgery which arises either from the left or right region of the brain. The seizure monitoring tools such as ECoG and EEG are prominently helpful to identify the seizure location. The electrodes/channels are implanted in a non-invasive (for EEG) and an invasive manner (for ECoG). Their positioning is based on the 10/20 (10–20) International system, which helps in identifying the seizure location [ 132 ]. The concept of seizure localization means identifying the region of the brain affected by a seizure. Though some types of seizures such as ‘tonic-clonic’ are cured by anti-epileptic drugs (AED), patients with partial seizures in some cases might go for surgery [ 13 ]. To solve this problem, finding the seizure location is an essential and challenging task for neurologists and neurosurgeon [ 129 , 130 ]. The surgical target is to find a point/location/focal area from where a seizure is originating. The 10–20 positioning system gives some clues for identifying the location of a seizure. Recently, computational and machine learning methods have been applied to identify a seizure location [ 130 , 133 ].
Acar et al. [ 133 ] used trucker and non-linear multi-way Trucker kernels, and claimed that other classifiers such as SVD and principal component analysis (PCA) were unable to localize a seizure. Ghannad-Rezaie [ 134 ] applied an advanced swarm intelligence algorithm to seizure data for finding seizure location. Their study produced some appreciable results, and explored whether the patient’s temporal lobe was affected by a seizure or not. They also suggested that SVM might be able to detect the seizure location. Moreover, they also focused on the reduction of ECoG electrodes. Mansouri et al. [ 135 ] proposed an algorithm for Seizure localization, which was tested on 10 sec of EEG dataset from Karuniya University. Here, they have taken the small-size dataset, because recording usually takes several hours. If they had tested on a big dataset, it would have been much better. Fakhraei et al. [ 130 ] calculated the sensitivity of each region of the brain. The confident prediction rate (CPR) was compared with the AUC of ROC plots obtained by six classifiers from the dataset of 79 patients (31 males, 48 females) with 197 medical features. The study found that CPR was more suitable than ROC. They also explored that 43 patients had the temporal lobe epilepsy (TLE) on their left sides whilst 36 patients had it on the right sides of their brains. Likewise, Rai et al. [ 136 ] proposed a method for identifying the focal points of the seizure by applying two entropy-based features—‘renyi entropy’ and ‘negentropy’ with the neural network classifier. Siddiqui et al. [ 63 ] localize the seizure using two decision forest classifiers, and their results showed that the left hemisphere of a brain was more affected by the seizures.
Observation
It is found that compared to seizure detection, machine learning classifiers have not been extensively applied for seizure localization. But some literature exist on this problem. In these reported works, authors did not mention the percentage of the affected region of the brain by a seizure, and they were not able to identify the exact location at the lobes such as occipital, frontal, parietal left and parietal right. Although, it is not our primary objective in this review paper, whilst discussing the related published research, we found some interesting clues for seizure localization.
7 Problems identified in existing literature
One of the most significant and decisive steps is to select suitable statistical features because each channel or electrode implanted on the brain provides different statistical measures. Undoubtedly, earlier researchers made their consistent efforts to find the best features. Whilst some researchers used many features [ 34 , 79 ], the others applied a few features [ 31 , 36 , 108 , 112 , 137 ] for detecting the seizure. As a data scientist, it is very important to see the different statistical perspectives of each brain signal by analyzing the statistical properties of the features such as entropy, energy, and skewness. And we must not focus on taking irrelevant feature(s) as such since it will unnecessarily increase the dataset size. Consequently, it will be more a burden to machine learning classifiers than a benefit, and if we take few features as previous researchers did [ 71 , 73 , 79 ], this will give the low-dimensional dataset and it will not be beneficial for an effective knowledge discovery process. Therefore, we should select those potential features that can to provide logical results. Hence, it is advisable to select a group of features to avoid a burden to the machine learning classifiers and to get help in related knowledge discovery.
Each classifier has its own merits and demerits, depending on the dataset attributes and requirements [ 138 ]. In general, it is very difficult to point out which classifier was the most effective for brain datasets. To identify the capable classifier, several classifiers have been tested on EEG datasets and their performance has been evaluated, and the one which performs well is to be considered in solving seizure detection and imparting knowledge discovery. The literature reveals that previous researchers had applied different approaches, most of which were from ‘black-box’ such as ANN, KNN and SVM. The biggest shortcoming in them is that they are unable to provide the appropriate explanations for patterns and the logic rules hidden inside the models. That is why, they are not suggested for remarkable knowledge discovery process. Data scientists may not explore the internal processing of patterns [ 51 , 104 ]. However, from the literature, it is noted that the ‘non-black-box’ approach, especially, random forest, is widely used for seizure detection [ 44 , 76 , 77 ], because of its nature of generating bootstrap samples [ 124 , 139 ] whilst building a decision forest. An analysis has been done to estimate the performance of machine learning classifiers on EEG datasets and has been found that ensemble non-black-classifiers performs effectively [ 104 ]. We argue that the random forest is based on bootstrap samples and it misses some influential attributes, because it randomly selects the attribute and sometimes generates the same set of logic rules also. As a result, sometimes, it creates irrelevant information too. To overcome this issue, we also suggest some other decision forest algorithms such as SysFor [ 123 ] and Forest CERN [ 51 ] methods in seizure detection.
All these findings on seizure detection raise few interesting research questions such as selecting suitable statistical features and machine learning classifiers to take less computation time as dataset has a high volume with high dimension, and the most significant missing information from machine learning classifiers is locating the accurate point of seizure at the brain lobe(s).
7.1 Class imbalance issue in seizure detection
Class imbalance is one of the serious problems [ 140 ] in machine learning and the majority is seen in medical datasets [ 141 ], particularly in EEG signals. This is because the duration of EEG recording is long, time-consuming and seizure duration is for a few seconds, which results in being prone to errors [ 91 ]. As a result, the dataset becomes highly imbalanced. Previous researchers have focused on seizure detection. Over the last few years, researchers have been focusing on the class imbalance challenge whilst detecting the seizures, and attempting to solve it by applying different conventional approaches with some novelties. Javad Birjandtalab et al. [ 91 ] used ANN with a weighted cost function to imbalanced EEG dataset, by achieving 86% F-measure. El Saadi et al. [ 142 ] obtained 97.3% accuracy using the under-sampling method with the SVM classifier. In another work by Saadullah and Awais [ 143 ], they used a combination of SMOTE and RUSTBOST techniques for detecting seizure to imbalance seizure data with 97% accuracy. However, the research done by Yuan Qi et al. [ 86 ] was very close to the satisfactory result as they assigned the heavy weights to a minority class of the data to maintain the effective balance and solved the biasing issue. The main critique of this work is that the authors did not mentioned what weights were assigned and what was their threshold level? Here, we argue that despite of EEG data are highly imbalanced as a result of their long-hour EEG recordings, the recordings continue until the seizure is detected. The seizure(s) time spans from only seconds to minute(s). Although researchers [ 76 , 86 , 117 , 143 ] made their efforts in addressing this issue using both ‘black-box’ and ‘non-black-box’ classifiers, they did not propose any justifiable solutions, in terms of how big weights should be assigned to the minority (seizure) classes.
8 Overall observation about capable classifiers and statistical features
It is challenging to suggest that a specific classifier should be capable for seizure detection. If we discuss classifiers, three constraints are very important whilst selecting a classifier—able to handle the high-dimensional dataset, high accuracy of the model, and able to retrieve the sensible knowledge. Not all machine learning classifiers are suitable for seizure detection and knowledge discovery tasks, mainly because of their black-box nature. This means that the logic rules/patterns are not visible and understandable to data scientists. In ‘non-black-box’ classifiers amongst decision trees [ 53 ] and decision forests [ 54 ], only decision forest algorithms are more capable, because the logic rules and knowledge discovered by a single decision tree are often limited and insufficient. For example, if we build a decision tree on a training dataset—it provides a limited or single set of logic rules and stops growing the tree further as all the data points in the training set accept that rule. On the other hand, if we build a decision forest on the same training set, we get multiple decision trees with more sensible logic rules. Siddiqui et al. [ 104 ] have done the analysis on CHB-MIT dataset to know which classifier performs better. For this, they applied two black-box (SVM and KNN) and two non-black-box (decision tree and ensemble of trees i.e., bagging, random subspace, boosting); they found non-black box classifier (ensemble) outperforms compared to other classifiers of black-box. Even ensemble also performs better than a single decision tree which is a non-black box classifier. Siddiqui et al. [ 63 ] applied two decision forests—Systematic Forest (SysFor) and Forest CERN for quick seizure detection using the concept of epoch length reduction. They achieved 100% of accuracy. Similarly, Hussein et al. [ 100 ] also achieved 100% accuracy using decision forest–random forest approach.
The literature reveals that in the last few years, ‘non-black-box’ classifiers, particularly decision forest approach, were widely used on brain datasets of EEG and ECoG for different research goals [ 76 , 82 , 94 , 144 ]. The reasons for using the decision forest for seizure detection are as follows:
A decision forest overcomes some of the disadvantages of a decision tree. A decision tree discovers only a single set of logic rules from an input dataset. The logic rules that are discovered by a single decision tree may fail to correctly predict and classify the class values;
A decision forest can produce more set of logic rules/patterns compared to a single decision tree and there is a high chance of good prediction/classification compared to a single decision tree;
Able to handle high-dimensional sets;
Due to its ensemble nature a decision forest mostly produces a high accuracy compared to a single tree and other classifiers [ 54 ];
Less computational time (specifically for Random forest);
Logic rules are clear and humanly interpretable such as analysts/domain experts can easily understand and suggest best opinions. For example, affected brain lobe by seizure, identifying suitable statistical features, etc.
Furthermore, many statistical features have been used for seizure detection. However, a comparison between them is difficult because of their heterogeneous nature. Some researchers used a single feature such as energy and entropy. On the other hand, a combination of statistical features such as energy, kurtosis, line length, entropy, skewness, max, standard deviation, and min may produce promising outcomes. Most research [ 34 , 46 , 92 , 100 , 109 , 145 ] have achieved better results using these features. The novelty of [ 29 , 63 , 104 , 125 ] is the selected nine statistical features are able to assist in seizure detection with high accuracy, i.e., 100%. This also provides the clue about seizure localization with the help of sensible logical rules. Hence, the selected group of features will not be a burden to the machine learning classifier but it will assist in related knowledge discovery.
9 Research directions in seizure detection
In this research analysis, we surveyed different machine learning classifiers used for seizure detection. No doubt, the progress of the persistent attempt has been found in this topic but few interesting research questions are also raised. In this section, we identify significant challenges which can uplift the future research in this area.
Selecting suitable statistical features and machine learning classifiers to take less computation time as the dataset has a high volume with a high dimension.
Accurate seizure detection on imbalanced datasets of long duration EEG recording datasets.
Quick seizure detection on long-hour EEG recording.
Whilst selecting the machine classifier it should be kept in mind that the classifier does not miss any necessary EEG channel/electrode.
Knowledge discovery from machine learning classifiers such as seizure localization which exactly points affected brain lobe(s), channel importance, and based on participating channels in seizure a knowledge could be provided to neurologist or neurosurgeon for suggesting epilepsy category.
10 Conclusion
With the increase of epilepsy, its accurate detection becomes increasingly important. A major challenge is to detect seizures correctly from a large volume of data. Due to the complexity of EEG signals in such datasets, machine learning classifiers are suitable for accurate seizure detection. Selecting suitable classifiers and features are, however, crucial.
As such, this paper has comprehensively reviewed machine learning approaches for seizure detection. As a result, we conclude that ‘non-black-box’ classifiers—decision forest (ensemble of decision trees)—is most effective. This is because it can produce multiple sensible, explanatory logic rules with high accuracy of prediction. Further, it can help discover some relevant information such as seizure localization and exploring seizure types. On the contrary, ‘black-box’ classifiers cannot generate logic rules, although they can achieve high predictive accuracy. As for selecting suitable features, we should select those that can provide logical results. By the review of the literature, the use of the features such as entropy, line length, energy, skewness, kurtosis, and standard deviation can achieve 100% accuracy in the classifiers. We suggest not to use the irrelevant features as the dimension of the data increases. This is because the computation cost of a classifier will grow high, and it may also produce insensible patterns. If we use just one or two features such as line length and energy, the low-dimensional dataset will be generated. However, this dataset will not be fruitful for the knowledge discovery process.
This review paper has provided new perspectives to data scientists who are working on epileptic seizure detection using EEG signals. In summary, this paper focuses on the review of selecting machine learning classifiers and suitable features.
Availability of data and materials
Not applicable.
Organization WH (2006) Neurological disorders: public health challenges. World Health Organization, New York
Google Scholar
Chaudhary UJ, Duncan JS, Lemieux L (2011) A dialogue with historical concepts of epilepsy from the babylonians to hughlings jackson: persistent beliefs. Epilepsy Behav 21(2):109–114
Article Google Scholar
Reynolds EH (2009) Milestones in epilepsy*. Epilepsia 50(3):338–342. https://doi.org/10.1111/j.1528-1167.2009.02050.x
WHO (2005) Atlas: Epilepsy care in the world. World Health Organization, Geneva
Alarcón G, Valentín A (2012) Introduction to Epilepsy. Cambridge University Press, Cambridge
Book Google Scholar
Qu H, Gotman J (1993) Improvement in seizure detection performance by automatic adaptation to the eeg of each patient. Electroencephalogr Clin Neurophysiol 86(2):79–87
Sazgar M, Young MG (2019) Seizures and Epilepsy. In: Absolute Epilepsy and EEG rotation review, pp. 9–46. Springer, New York
Chapter Google Scholar
Schachter S, Shafer P, Sirven J (2013) What causes epilepsy and seizures. Epilepsy Foundation
Delanty N, Vaughan CJ, French JA (1998) Medical causes of seizures. Lancet 352(9125):383–390
WHO: Media Center Epilepsy, (Fact sheet N999). http://www.who.int/mediacentre/factsheets/fs999/en/ (2015) Accessed 15 July 2019
Shafer PO, Sirven JI (2014) Epilepsy statistics. Epilepsy Foundation
Hannah JA, Brodie MJ (1998) Epilepsy and learning disabilities—a challenge for the next millennium? Seizure 7(1):3–13
Thurman DJ, Beghi E, Begley CE, Berg AT, Buchhalter JR, Ding D, Hesdorffer DC, Hauser WA, Kazis L, Kobau R et al (2011) Standards for epidemiologic studies and surveillance of epilepsy. Epilepsia 52(s7):2–26
Fisher RS (2017) The new classification of seizures by the international league against epilepsy 2017. Curr Neurol Neurosci Rep 17(6):48
Mahmud M, Kaiser MS, Hussain A, Vassanelli S (2018) Applications of deep learning and reinforcement learning to biological data. IEEE Trans Neural Networks Learn Syst 29(6):2063–2079
Article MathSciNet Google Scholar
Mahmud M, Kaiser MS, Hussain A (2020) Deep learning in mining biological data. arXiv:2003.00108
Fayyad UM, Piatetsky-Shapiro G, Smyth P, Uthurusamy R (eds.) (1996) Advances in Knowledge Discovery and Data Mining. American Association for Artificial Intelligence, Menlo Park, CA, USA
Yin Y, Kaku I, Tang J, Zhu J (2011) Data mining: concepts, methods and applications in management and engineering design. Springer, New York
Islam MZ, D’Alessandro S, Furner M, Johnson L, Gray D, Carter L (2016) Brand switching pattern discovery by data mining techniques for the telecommunication industry in australia. Aust J Inform Syst. https://doi.org/10.3127/ajis.v20i0.1420
Aljumah AA, Ahamad MG, Siddiqui MK (2013) Application of data mining: diabetes health care in young and old patients. J King Saud Univ Comput Inform Sci 25(2):127–136
Aljumah A, Siddiqui M (2016) Data mining perspective: prognosis of life style on hypertension and diabetes. Int Arab J Inf Technol 13(1)
Siddiqui MK, Morales-Menendez R, Gupta PK, Iqbal HM, Hussain F, Khatoon K, Ahmad S (2020) Correlation between temperature and COVID-19 (suspected, confirmed and death) cases based on machine learning analysis. J Pure Appl Microbiol 14(Spl Edn.)
Aljumah AA, Siddiqui MK (2014) Hypertension interventions using classification based data mining. Res J Appl Sci Eng Technol 7(17):3593–3602
Almazyad AS, Ahamad MG, Siddiqui MK, Almazyad AS (2010) Effective hypertensive treatment using data mining in Saudi Arabia. J Clin Monit Comput 24(6):391–401
Singh GA, Gupta PK (2019) Performance analysis of various machine learning-based approaches for detection and classification of lung cancer in humans. Neural Comput Appl 31(10):6863–6877
Fu T-c (2011) A review on time series data mining. Eng Appl Artif Intell 24(1):164–181
Tzallas AT, Tsipouras MG, Tsalikakis DG, Karvounis EC, Astrakas L, Konitsiotis S, Tzaphlidou M (2012) Automated epileptic seizure detection methods: a review study. In: Epilepsy-histological, electroencephalographic and psychological aspects. InTech
Abbasi B, Goldenholz DM (2019) Machine learning applications in epilepsy. Epilepsia
Siddiqui MK. Brain data mining for epileptic seizure-detection. Doctoral Dissertation, Charles Sturt University, Australia
Paul Y (2018) Various epileptic seizure detection techniques using biomedical signals: a review. Brain Inform 5(2):6
Boonyakitanont P, Lek-uthai A, Chomtho K, Songsiri J (2020) A review of feature extraction and performance evaluation in epileptic seizure detection using eeg. Biomed Signal Process Control 57:101702
Sharmila A, Geethanjali P (2019) A review on the pattern detection methods for epilepsy seizure detection from EEG signals. Biomed Eng /Biomedizinische Technik. 64(5):507–17
van Westrhenen A, De Cooman T, Lazeron RH, Van Huffel S, Thijs RD (2019) Ictal autonomic changes as a tool for seizure detection: a systematic review. Clin Auton Res 29(2):161–181
Logesparan AJC Lojini, Rodriguez-Villegas E (2012) Optimal features for online seizure detection. Med Biol Eng Comput 50(7):659–669
Ramgopal S, Thome-Souza S, Jackson M, Kadish NE, Fernández IS, Klehm J, Bosl W, Reinsberger C, Schachter S, Loddenkemper T (2014) Seizure detection, seizure prediction, and closed-loop warning systems in epilepsy. Epilepsy Behav 37:291–307
Quintero-Rincón A, D’Giano C, Batatia H (2019) Seizure onset detection in eeg signals based on entropy from generalized gaussian pdf modeling and ensemble bagging classifier. In: Digital Health Approach for Predictive, Preventive, Personalised and Participatory Medicine, pp. 1–10. Springer, New York
Greene B, Faul S, Marnane W, Lightbody G, Korotchikova I, Boylan G (2008) A comparison of quantitative eeg features for neonatal seizure detection. Clin Neurophysiol 119(6):1248–1261
Gotman J, Ives J, Gloor P (1979) Automatic recognition of inter-ictal epileptic activity in prolonged eeg recordings. Electroencephalogr Clin Neurophysiol 46(5):510–520
Gotman J (1982) Automatic recognition of epileptic seizures in the eeg. Electroencephalogr Clin Neurophysiol 54(5):530–540
Koffler D, Gotman J (1985) Automatic detection of spike-and-wave bursts in ambulatory eeg recordings. Electroencephalogr Clin Neurophysiol 61(2):165–180
Shoeb A, Guttag J (2010) Application of machine learning to epileptic seizure detection. In: 2010 the 27th International Conference on Machinelearning, Haifa, Israel
Dorai A, Ponnambalam K (2010) Automated epileptic seizure onset detection. In: Autonomous and Intelligent Systems (AIS), 2010 International Conference On, pp. 1–4 . IEEE
Lahmiri S (2018) An accurate system to distinguish between normal and abnormal electroencephalogram records with epileptic seizure free intervals. Biomed Sign Process Control 40:312–317
Donos C, Dümpelmann M, Schulze-Bonhage A (2015) Early seizure detection algorithm based on intracranial EEG and random forest classification. Int J Neur Syst 25(05):1550023
Ocak H (2009) Automatic detection of epileptic seizures in eeg using discrete wavelet transform and approximate entropy. Exp Syst Appl 36(2):2027–2036
Thomas E, Temko A, Lightbody G, Marnane W, Boylan G (2011) Advances in automated neonatal seizure detection. New Adv Intell Sign Process 1:93–113
Al Ghayab HR, Li Y, Abdulla S, Diykh M, Wan X (2016) Classification of epileptic EEG signals based on simple random sampling and sequential feature selection. Brain Inform 3(2):85–91
Herwig U, Satrapi P, Schönfeldt-Lecuona C (2003) Using the international 10–20 eeg system for positioning of transcranial magnetic stimulation. Brain Topogr 16(2):95–99
Misulis KE (2013) Atlas of EEG, seizure semiology, and management. Oxford University Press, Oxford
Sammut C, Webb GI (2017) Encyclopedia of machine learning and data mining. Springer, New York
Book MATH Google Scholar
Adnan MN, Islam MZ (2016) Forest CERN: A new decision forest building technique. In: Pacific-Asia Conference on Knowledge Discovery and Data Mining, pp. 304–315. Springer, New York
Cortes C, Vapnik V (1995) Support-vector networks. Mach Learn 20(3):273–297
MATH Google Scholar
Quinlan JR (1993) C4.5: Programs for machine learning. Morgan Kaufmann Publishers Inc., San Francisco
Breiman L (2001) Random forests. Mach Learn 45(1):5–32. https://doi.org/10.1023/A:1010933404324
Article MATH Google Scholar
Arlot S, Celisse A et al (2010) A survey of cross-validation procedures for model selection. Statistics surveys 4:40–79
Article MathSciNet MATH Google Scholar
Kurgan LA, Cios KJ (2004) CAIM discretization algorithm. IEEE Trans Knowl Data Eng 16(2):145–153
Li J, Liu H (2003) Ensembles of cascading trees. In: Data Mining, 2003. ICDM 2003. Third IEEE International Conference On, pp. 585–588. IEEE
Powers DM (2011) Evaluation: from precision, recall and f-measure to roc, informedness, markedness and correlation
Goldberger A, Amaral L, Glass L, Hausdorff J, Ivanov PC, Mark R, Mietus J, Moody G, Peng C, Stanley H (2000) PhysioBank, PhysioToolkit, and PhysioNet: components of a new research resource for complex physiologic signals. Circulation 101(23):e215–e220
CHB-MIT Scalp EEG Database. https://physionet.org/pn6/chbmit/ . Accessed 20 Jun 2015
Shoeb A, Edwards H, Connolly J, Bourgeois B, Treves ST, Guttag J (2004) Patient-specific seizure onset detection. Epilepsy Behav 5(4):483–498
Kramer MA, Kolaczyk ED, Kirsch HE (2008) Emergent network topology at seizure onset in humans. Epilepsy Res 79(2):173–186
Siddiqui MK, Islam MZ, Kabir MA (2018) A novel quick seizure detection and localization through brain data mining on ecog dataset. Neural Computing and Applications 1–14
Freiburg seizure prediction project. Freiburg, Germany. http://epilepsy.uni-freiburg.de/freiburg-seizure-prediction-project/eeg-database (2003)
Andrzejak RG, Lehnertz K, Mormann F, Rieke C, David P, Elger CE (2001) Indications of nonlinear deterministic and finite-dimensional structures in time series of brain electrical activity: Dependence on recording region and brain state. Phys Rev E 64(6):061907
Andrzejak RG, Schindler K, Rummel C (2012) Nonrandomness, nonlinear dependence, and nonstationarity of electroencephalographic recordings from epilepsy patients. Phys Rev E 86(4):046206
Lee H, Kim S (2016) Black-box classifier interpretation using decision tree and fuzzy logic-based classifier implementation. Int J Fuzzy Logic Intell Syst 16(1):27–35
Adnan MN, Islam MZ (2017) Forex++: A new framework for knowledge discovery from decision forests. Aust J Inform Syst. https://doi.org/10.3127/ajis.v21i0.1539
Guo L, Rivero D, Dorado J, Rabunal JR, Pazos A (2010) Automatic epileptic seizure detection in eegs based on line length feature and artificial neural networks. J Neurosci Methods 191(1):101–109
Koolen N, Jansen K, Vervisch J, Matic V, De Vos M, Naulaers G, Van Huffel S (2014) Line length as a robust method to detect high-activity events: automated burst detection in premature eeg recordings. Clin Neurophysiol 125(10):1985–1994
Logesparan L, Rodriguez-Villegas E, Casson AJ (2015) The impact of signal normalization on seizure detection using line length features. Med Biol Eng Comput 53(10):929–942
Song Y, Liò P (2010) A new approach for epileptic seizure detection: sample entropy based feature extraction and extreme learning machine. J Biomed Sci Eng 3(06):556
Zhang Y, Zhang Y, Wang J, Zheng X (2014) Comparison of classification methods on EEG signals based on wavelet packet decomposition. Neural Comput Appl 26(5):1217–1225. https://doi.org/10.1007/s00521-014-1786-7
Ahmad MA, Khan NA, Majeed W (2014) Computer assisted analysis system of electroencephalogram for diagnosing epilepsy. In: Pattern Recognition (ICPR), 2014 22nd International Conference On, pp. 3386–3391 . IEEE
Gill AF, Fatima SA, Akram MU, Khawaja SG, Awan SE (2015) Analysis of eeg signals for detection of epileptic seizure using hybrid feature set. In: Theory and Applications of Applied Electromagnetics, pp. 49–57. Springer
Orellana MP, Cerqueira F (2016) Personalized epilepsy seizure detection using random forest classification over one-dimension transformed EEG data. bioRxiv, 070300
Birjandtalab J, Pouyan MB, Cogan D, Nourani M, Harvey J (2017) Automated seizure detection using limited-channel eeg and non-linear dimension reduction. Comput Biol Med 82:49–58
Yan A, Zhou W, Yuan Q, Yuan S, Wu Q, Zhao X, Wang J (2015) Automatic seizure detection using stockwell transform and boosting algorithm for long-term eeg. Epilepsy Behav 45:8–14
Amin HU, Malik AS, Ahmad RF, Badruddin N, Kamel N, Hussain M, Chooi W-T (2015) Feature extraction and classification for eeg signals using wavelet transform and machine learning techniques. Austr Phys Eng Sci Med 38(1):139–149
Mursalin M, Zhang Y, Chen Y, Chawla NV (2017) Automated epileptic seizure detection using improved correlation-based feature selection with random forest classifier. Neurocomputing 241:204–214
Zabihi M, Kiranyaz S, Ince T, Gabbouj M (2013) Patient-specific epileptic seizure detection in long-term eeg recording in paediatric patients with intractable seizures
Truong ND, Kuhlmann L, Bonyadi MR, Yang J, Faulks A, Kavehei O (2017) Supervised learning in automatic channel selection for epileptic seizure detection. Exp Syst Appl
Fergus P, Hussain A, Hignett D, Al-Jumeily D, Abdel-Aziz K, Hamdan H (2016) A machine learning system for automated whole-brain seizure detection. Appl Comput Inform 12(1):70–89
Chen D, Wan S, Xiang J, Bao FS (2017) A high-performance seizure detection algorithm based on discrete wavelet transform (dwt) and eeg. PLoS ONE 12(3):0173138
Satapathy SK, Jagadev AK, Dehuri S (2017) Weighted majority voting based ensemble of classifiers using different machine learning techniques for classification of eeg signal to detect epileptic seizure. Informatica 41(1):99
MathSciNet Google Scholar
Yuan Q, Zhou W, Zhang L, Zhang F, Xu F, Leng Y, Wei D, Chen M (2017) Epileptic seizure detection based on imbalanced classification and wavelet packet transform. Seizure
Subasi A, Kevric J, Canbaz MA (2019) Epileptic seizure detection using hybrid machine learning methods. Neural Comput Appl 31(1):317–325
Al Ghayab HR, Li Y, Siuly S, Abdulla S (2019) Epileptic seizures detection in eegs blending frequency domain with information gain technique. Soft Comput 23(1):227–239
Selvakumari RS, Mahalakshmi M, Prashalee P (2019) Patient-specific seizure detection method using hybrid classifier with optimized electrodes. J Med Syst 43(5):121
Chen S, Zhang X, Chen L, Yang Z (2019) Automatic diagnosis of epileptic seizure in electroencephalography signals using nonlinear dynamics features. IEEE Access 7:61046–61056
Birjandtalab J, Jarmale VN, Nourani M, Harvey J (2018) Imbalance learning using neural networks for seizure detection. In: 2018 IEEE Biomedical Circuits and Systems Conference (BioCAS), pp. 1–4 . IEEE
Lahmiri S, Shmuel A (2018) Accurate classification of seizure and seizure-free intervals of intracranial eeg signals from epileptic patients. IEEE Trans Instrum Meas 68(3):791–796
Tzimourta KD, Tzallas AT, Giannakeas N, Astrakas LG, Tsalikakis DG, Angelidis P, Tsipouras MG (2019) A robust methodology for classification of epileptic seizures in eeg signals. Health Technol 9(2):135–142
Wang X, Gong G, Li N, Qiu S (2019) Detection analysis of epileptic EEG using a novel random forest model combined with grid search optimization. Front Human Neurosci 13:12
Raghu S, Sriraam N (2018) Classification of focal and non-focal eeg signals using neighborhood component analysis and machine learning algorithms. Exp Syst Appl 113:18–32
Alickovic E, Kevric J, Subasi A (2018) Performance evaluation of empirical mode decomposition, discrete wavelet transform, and wavelet packed decomposition for automated epileptic seizure detection and prediction. Biomed Sign Process Contr 39:94–102
Fasil O, Rajesh R (2019) Time-domain exponential energy for epileptic eeg signal classification. Neurosci Lett 694:1–8
Manzouri F, Heller S, Dümpelmann M, Woias P, Schulze-Bonhage A (2018) A comparison of machine learning classifiers for energy-efficient implementation of seizure detection. Front Syst Neurosci 12: 43
Parvez MZ, Paul M (2014) Epileptic seizure detection by analyzing eeg signals using different transformation techniques. Neurocomputing 145:190–200
Hussein R, Elgendi M, Wang ZJ, Ward RK (2018) Robust detection of epileptic seizures based on l1-penalized robust regression of EEG signals. Exp Syst Appl 104:153–167
Mursalin M, Islam SS, Noman MK (2019) Epileptic seizure classification using statistical sampling and a novel feature selection algorithm. arXiv preprint arXiv:1902.09962
Sharma RR, Varshney P, Pachori RB, Vishvakarma SK (2018) Automated system for epileptic EEG detection using iterative filtering. IEEE Sens Lett 2(4):1–4
Tzallas AT, Tsipouras MG (2007) Fotiadis DI (2007) Automatic seizure detection based on time-frequency analysis and artificial neural networks. Comput Intell Neurosci 2007:80510
Siddiqui MK, Islam MZ, Kabir MA (2017) Analyzing performance of classification techniques in detecting epileptic seizure. In: International Conference on Advanced Data Mining and Applications, pp. 386–398. Springer, New York
Sanei S, Chambers JA (2013) EEG signal processing. Wiley, New York
Chaovalitwongse WA, Prokopyev OA, Pardalos PM (2006) Electroencephalogram (eeg) time series classification: applications in epilepsy. Ann Ope Res 148(1):227–250
Moselhy HF (2011) Psychosocial and cultural aspects of epilepsy. In: Novel Aspects on Epilepsy. InTech
Esteller R, Echauz J, Tcheng T, Litt B, Pless B (2001) Line length: an efficient feature for seizure onset detection. In: Engineering in Medicine and Biology Society, 2001. Proceedings of the 23rd Annual International Conference of the IEEE, vol. 2, pp. 1707–1710 . IEEE
Guerrero-Mosquera C, Trigueros AM, Franco JI, Navia-Vázquez Á (2010) New feature extraction approach for epileptic eeg signal detection using time-frequency distributions. Med Biol Eng Comput 48(4):321–330
Olsen DE, Lesser RP, Harris JC, Webber WRS, Cristion JA (1994) Automatic detection of seizures using electroencephalographic signals. Google Patents. US Patent 5,311, 876
Acharya UR, Molinari F, Sree SV, Chattopadhyay S, Ng K-H, Suri JS (2012) Automated diagnosis of epileptic EEG using entropies. Biomed Sign Process Contr 7(4):401–408
Kannathal N, Choo ML, Acharya UR, Sadasivan P (2005) Entropies for detection of epilepsy in eeg. Comput Meth Progr Biomed 80(3):187–194
Kumar Y, Dewal M (2011) Complexity measures for normal and epileptic eeg signals using apen, sampen and sen. IJCCT 2(7):6–12
Shimizu M, Iiya M, Fujii H, Kimura S, Suzuki M, Nishizaki M (2019) Left ventricular end-systolic contractile entropy can predict cardiac prognosis in patients with complete left bundle branch block. Journal of Nuclear Cardiology 1–10
Pal PR, Panda R (2010) Classification of eeg signals for epileptic seizure evaluation. In: Students’ Technology Symposium (TechSym), 2010 IEEE, pp. 72–76. IEEE
Cepukenas J, Lin C, Sleeman D (2015) Applying rule extraction and rule refinement techniques to (blackbox) classifiers. In: Proceedings of the 8th international conference on knowledge capture, p. 27. ACM
Birjandtalab J, Pouyan MB, Nourani M (2016) Unsupervised eeg analysis for automated epileptic seizure detection. In: Proceedings of the first international workshop on pattern recognition, international society for optics and photonics, pp. 100110–100110
Abualsaud K, Mahmuddin M, Saleh M, Mohamed A (2015) Ensemble classifier for epileptic seizure detection for imperfect eeg data. Sci World J 2015:945689
Chen C, Liu J, Syu J (2012) Application of chaos theory and data mining to seizure detection of epilepsy. Proc Conf. IPCSIT/Hong Kong 25:23–28
Polat K, Güneş S (2007) Classification of epileptiform eeg using a hybrid system based on decision tree classifier and fast fourier transform. Appl Math Comput 187(2):1017–1026
MathSciNet MATH Google Scholar
Quinlan R (2004) Data mining tools see5 and c5. 0
Cios KJ, Pedrycz W, Swiniarski RW, Kurgan LA (2007) Data mining: a knowledge discovery approach. Springer, New York
Islam MZ, Giggins H (2011) Knowledge discovery through sysfor: a systematically developed forest of multiple decision trees. In: Proceedings of the Ninth Australasian Data Mining Conference-Volume 121, pp. 195–204. Australian Computer Society, Inc
Ho TK (1998) The random subspace method for constructing decision forests. IEEE Trans Patt Analy Mach Intell 20(8):832–844
Siddiqui MK, Islam MZ (2016) Data mining approach in seizure detection. In: 2016 IEEE Region 10 Conference (TENCON), Singapore, pp. 3579–3583. Institute of Electrical and Electronics Engineers (IEEE), https://doi.org/10.1109/tencon.2016.7848724
Hosseini M-P, Pompili D, Elisevich K, Soltanian-Zadeh H (2018) Random ensemble learning for eeg classification. Artif Intell Med 84:146–158
Hassan AR, Siuly S, Zhang Y (2016) Epileptic seizure detection in eeg signals using tunable-q factor wavelet transform and bootstrap aggregating. Comput Meth Prog Biomed 137:247–259
Hosseini M-P, Hajisami A, Pompili D (2016) Real-time epileptic seizure detection from eeg signals via random subspace ensemble learning. In: Autonomic Computing (ICAC), 2016 IEEE International Conference On, pp. 209–218 . IEEE
Jurcak V, Tsuzuki D, Dan I (2007) 10/20, 10/10, and 10/5 systems revisited: Their validity as relative head-surface-based positioning systems. NeuroImage 34(4):1600–1611. https://doi.org/10.1016/j.neuroimage.2006.09.024
Fakhraei S, Soltanian-Zadeh H, Fotouhi F, Elisevich K (2011) Confidence in medical decision making: Application in temporal lobe epilepsy data mining. In: Proceedings of the 2011 Workshop on Data Mining for Medicine and Healthcare. DMMH ’11, pp. 60–63. ACM, New York, NY, USA . https://doi.org/10.1145/2023582.2023593
Keraudren K, Kainz B, Oktay O, Kyriakopoulou V, Rutherford M, Hajnal JV, Rueckert D (2015) Automated localization of fetal organs in mri using random forests with steerable features. In: International conference on medical image computing and computer-assisted Intervention, pp. 620–627. Springer, New York
Trans-Cranial-Technologies: 10/20 System Positioning. https://www.trans-cranial.com/local/manuals/10_20_pos_man_v1_0_pdf.pdf . Accessed 7 Oct 2015
Acar E, Bingöl CA, Bingöl H, Yener B (2006) Computational analysis of epileptic focus localization. In: Proceedings of the 24th IASTED International Conference on Biomedical Engineering. BioMed’06, pp. 317–322. ACTA Press, Anaheim, CA, USA. http://dl.acm.org/citation.cfm?id=1166506.1166562
Ghannad-Rezaie M, Soltanain-Zadeh H, Siadat MR, Elisevich KV (2006) Medical data mining using particle swarm optimization for temporal lobe epilepsy. In: 2006 IEEE International conference on evolutionary computation, pp. 761–768. https://doi.org/10.1109/CEC.2006.1688388
Mansouri A, Singh SP, Sayood K (2019) Online eeg seizure detection and localization. Algorithms 12(9):176
Rai K, Bajaj V, Kumar A (2015) Features extraction for classification of focal and non-focal eeg signals. Inform Sci Appl Lect Notes Electr Eng 339:599–605
Hadoush H, Alafeef M, Abdulhay E (2019) Brain complexity in children with mild and severe autism spectrum disorders: Analysis of multiscale entropy in eeg. Brain topography 1–8
Fernández-Delgado M, Cernadas E, Barro S, Amorim D (2014) Do we need hundreds of classifiers to solve real world classification problems? J Mach Learn Res 15(1):3133–3181
Breiman L (1996) Bagging predictors. Mach Learn 24(2):123–140
Galar M, Fernandez A, Barrenechea E, Bustince H, Herrera F (2012) A review on ensembles for the class imbalance problem: bagging-, boosting-, and hybrid-based approaches. IEEE Trans Syst Man Cybern C 42(4):463–484
Yang F, Wang H-Z, Mi H, Cai W-W et al (2009) Using random forest for reliable classification and cost-sensitive learning for medical diagnosis. BMC Bioinform 10(1):22
El Saadi H, Al-Sadek AF, Fakhr MW (2012) Informed under-sampling for enhancing patient specific epileptic seizure detection. Int J Comput Appl 57:16
Amin S, Kamboh AM (2016) A robust approach towards epileptic seizure detection. In: Machine Learning for Signal Processing (MLSP), 2016 IEEE 26th International Workshop On, pp. 1–6 . IEEE
Yang Z, Choupan J, Reutens D, Hocking J (2015) Lateralization of temporal lobe epilepsy based on resting-state functional magnetic resonance imaging and machine learning. Front Neurol 6:184
Tito M, Cabrerizo M, Ayala M, Jayakar P, Adjouadi M (2009) Seizure detection: an assessment of time-and frequency-based features in a unified two-dimensional decisional space using nonlinear decision functions. J Clin Neurophysiol 26(6):381–391
Download references
Acknowledgements
We acknowledge the contribution of Dr. Khudeja Khatoon, MD, Faculty member of the Hayat Unani Medical College & Research Centre, India for carefully looking the medical terminologies in the paper. Also, thankful to Mr. Mohammad Arshad, English language expert, Shoumou Investment and Trading Company, KSA for proofreading the paper.

Author information
Mohammad Khubeb Siddiqui and Xiaodi Huang contributed equally to this work
Authors and Affiliations
School of Engineering and Sciences, Tecnologico de Monterrey, Av. E. Garza Sada 2501, Monterrey, Nuevo Leon, Mexico
Mohammad Khubeb Siddiqui & Ruben Morales-Menendez
School of Computing and Mathematics, Charles Sturt University, 2640, Albury, NSW, Australia
Xiaodi Huang
College of Applied Studies and Community Service, King Saud University, Riyadh, Kingdom of Saudi Arabia
Nasir Hussain
You can also search for this author in PubMed Google Scholar
Contributions
MKS: acquisition of related works, machine learning study, comparative study, arguments, writeup. RMM reviewed the overall paper and provided comments. XH: review the article on Machine Learning perspective, main contributions and direction of research. NH: discussion and table analysis. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Ruben Morales-Menendez .
Ethics declarations
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Siddiqui, M.K., Morales-Menendez, R., Huang, X. et al. A review of epileptic seizure detection using machine learning classifiers. Brain Inf. 7 , 5 (2020). https://doi.org/10.1186/s40708-020-00105-1
Download citation
Received : 24 December 2019
Accepted : 09 May 2020
Published : 25 May 2020
DOI : https://doi.org/10.1186/s40708-020-00105-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Applications of machine learning on epilepsy
- Statistical features
- Seizure detection
- Seizure localization
- Black-box and non-black-box classifiers
- EEG signals
- Open access
- Published: 28 June 2023
Therapeutic efficacy of voltage-gated sodium channel inhibitors in epilepsy
- John Agbo 1 ,
- Zainab G. Ibrahim 1 ,
- Shehu Y. Magaji 1 ,
- Yahkub Babatunde Mutalub 1 ,
- Philemon Paul Mshelia 2 &
- Daniel H. Mhyha 3
Acta Epileptologica volume 5 , Article number: 16 ( 2023 ) Cite this article
2186 Accesses
Metrics details
Epilepsy is a neurological disease characterized by excessive and abnormal hyper-synchrony of electrical discharges of the brain and a predisposition to generate epileptic seizures resulting in a broad spectrum of neurobiological insults, imposing psychological, cognitive, social and also economic burdens to the sufferer. Voltage-gated sodium channels (VGSCs) are essential for the generation and propagation of action potentials throughout the central nervous system. Dysfunction of these channels has been implicated in the pathogenesis of epilepsy. VGSC inhibitors have been demonstrated to act as anticonvulsants to suppress the abnormal neuronal firing underlying epileptic seizures, and are used for the management and treatment of both genetic-idiopathic and acquired epilepsies. We discuss the forms of idiopathic and acquired epilepsies caused by VGSC mutations and the therapeutic efficacy of VGSC blockers in idiopathic, acquired and pharmacoresistant forms of epilepsy in this review. We conclude that there is a need for better alternative therapies that can be used alone or in combination with VGSC inhibitors in the management of epilepsies. The current anti-seizure medications (ASMs) especially for pharmacoresistant epilepsies and some other types of epilepsy have not yielded expected therapeutic efficacy partly because they do not show subtype-selectivity in blocking sodium channels while also bringing side effects. Therefore, there is a need to develop novel drug cocktails with enhanced selectivity for specific VGSC isoforms, to achieve better treatment of pharmacoresistant epilepsies and other types of epileptic seizures.
Epilepsy is characterized by recurrent seizures due to aberrant excessive discharges of cortical neurons [ 1 ]. Epilepsy is a chronic brain disease that affects about 70 million people all over the world [ 2 , 3 , 4 , 5 ]. Nearly 80% of individuals with epilepsy live in low-and middle-income countries with limited resources and high poverty rate (sub-Saharan Africa, Latin America, southeast Asia), where the rate of new cases is more than two-fold higher than that in developed countries [ 6 ]. Epilepsies are divided into two categories [ 1 ] genetic epilepsy with no known structural, gross neuroanatomic, or neuropathologic abnormalities or predisposing factors but being primarily due to underlying genetic mutations [ 7 ], and [ 2 ] symptomatic acquired epilepsy, which is associated with gross anatomic and pathologic abnormalities, resulting from structural or metabolic perturbations in the brain [ 8 ]. Seizures can have multifactorial mechanisms, and they often appear so diverse that one would suspect that there is no common connotation. However, it is commonly believed that seizures arise when the homeostatic mechanisms are disrupted, causing an imbalance between excitation and inhibition. Normally, there are checkpoints that keep neurons from excessive action potential (AP) discharging, and also mechanisms that facilitate neuronal firing so that the nervous system can function normally. Homeostatic disruption of the checkpoints or promotion of the mechanisms that enhance excitation can lead to seizures. Currently, there is no cure for epilepsy. VGSC inhibitors and other anti-seizure medications (ASMs) are only aimed to suppress seizures. In addition, some VGSC inhibitors such as phenytoin, carbamazepine, and lamotrigine as well as other types of ASMs are contraindicated for some forms of genetic epilepsy that are caused by mutations in the α and β subunits of sodium channels. They do so by blocking sodium currents entering the neurons. Examples of such epilepsies include Dravet syndrome (also known as severe myoclonic epilepsy in infancy, SMEI), generalized (genetic) epilepsy with febrile seizures plus (GEFS +) and benign familial neonatal infantile seizures (BFNIS). Therefore, it is imperative to enhance recognition of the disease mechanisms, the molecular structure of sodium channel and physiological roles of VGSC subtypes, in order to develop new drugs that can modulate sodium currents and change the inactivation characteristics.
Physiological and molecular architecture of VGSCs
VGSCs are of great significance to the initiation of APs in neurons and other excitable cells [ 9 ], and their dysfunction causes epilepsy, inherited diseases of hyperexcitability and related channelopathies. VGSCs function by transiently increasing the membrane permeability to sodium ions during membrane depolarization. At resting states these membranes are usually closed. The depolarization of the membrane induces a conformational change of the α subunit through movements of the voltage-sensing domains, prompting the opening of the sodium-selective channel pore. Voltage-gated sodium channels open very fast within 1–2 ms, which is required for repetitive AP firing in neural circuits and for control of excitability in nerve and muscle cells [ 10 ]. Within a few milliseconds, the channels rapidly shift to a nonconducting inactivated state, mediated by the triad isoleucine-phenylalanine-methionine (IFM) motif of the α subunit [ 11 ]. Conversely, slow channel inactivation ensues as a result of long depolarization of nerve and muscle fibers mediated by inward trains of currents, with concomitant repetitive neuronal firing for a period of seconds due to the long-term changes in the resting membrane potential. Usually, VGSCs are highly dynamic transient channels that are inactivated and closed within milliseconds. However, under certain conditions, deficits or attenuation of fast inactivation can greatly enhance the amplitude of persistent sodium currents, resulting in bursts of APs as seen in paroxysmal epileptic seizures, pain and even cardiac arrhythmia.
Since the groundbreaking chemical characterization of VGSCs by Beneski and Catterall [ 12 ], efforts have been made to unravel structures and physiological roles of sodium channels. The mammalian VGSC is a complicated composed of a large, pore-forming α subunit of 260 kDa and one or two smaller β (auxiliary) subunits of 34–40 kDa. The ion-conducting pore, also known as the pore-forming domain, is contained within the α subunit. The α subunit, as the dominant subunit of VGSC, is responsible for normal electrophysiological function and mediates the fundamental physiologic properties of VGSC, including rapid inactivation. The sodium channel α subunit family have nine members, encoded by nine genes and expressed in different excitable tissues [ 13 ]. Mutations in these channels can result in genetic epilepsy [ 14 ] and other channelopathies [ 15 ]. The tenth VGSC is involved in salt-sensing and is not voltage-gated [ 16 ]. The 10 homologous α subunits of VGSCs found in humans are designated Nav1.1–Nav1.9 and NaX, encoded by ten different genes ( SCN1A-SCN11A, SCN6A and SCN7A represent the same gene). Nav1.1, Nav1.2, Nav1.3 and Nav1.6 ( SCN1A, SCN2A, SCN3A, SCN8A ) are expressed in the central nervous system (CNS). Nav1.7, Nav1.8 and Nav1.9 ( SCN9A, SCN10A, SCN11A ) are expressed in the peripheral nervous system. Nav1.4 ( SCN4A ) is the primary sodium channel in skeletal muscle, while Nav1.5 ( SCN5A ) is the canonical subtype in the heart [ 17 ]. The tenth isoform NaX ( SCN7A ) is voltage-insensitive and is considered atypical as it contains key distinguishing features in DI/III/IV S4 of voltage-sensor domains (VSDs) and DIII-IV linker sequence. Therefore, NaX is classified as a different type of Nav [ 18 ]. Apart from the primary tissue of expression, most of the sodium channels have expressions in other tissues.
The α subunit is the core subunit of VGSC, and is composed of three parts: (1) four highly homologous transmembrane domains designated as DI-DIV, with each domain harboring six nearly identical transmembrane segments with sequence homology greater than 50% [ 18 ]; (2) three intracellular loops (2 long loops, L1 and L2, and 1 short loop, L3); and (3) the N- and C-termini (NT and CT). The CT of NaV1.7 is reported to be involved in orchestrating the process of fast inactivation, which forms two electrostatic bridges with gating charges in VSD4 (switch 1) and DIII-DIV linker (switch 2), respectively [ 19 ]. Each domain comprises six water-filled α-helical transmembrane segments named S1-S6. Transmembrane segments S1-S4 of the sodium voltage-gated channel α subunits from each of the four domains form the voltage-sensing domain, an essential structure that function in the modulation of channel opening upon depolarization of the membrane. The VSD can move easily due to the presence of positively charged amino acid residues arginine and lysine at S4, which are exceedingly sensitive to the change of the membrane potential. For that reason, segment S4 is called the voltage sensor of the VGSC. The intracellular loop L3 connecting the homologous domains DIII-DIV constitute the inactivation gate that serves as a hinged lid and folds into the intracellular mouth of the pore during fast inactivation [ 20 ]. Defective fast inactivation of this hinged lid or if for any reason the hinged lid is left ajar for more than the required milliseconds, trains of Na + will rush in, causing excessive depolarization of the membrane and excessive neuronal firing (persistent sodium currents). Both of the CT and NT of the subunit also modulate the VGSCs, for example, the CT plays a crucial role in inactivation. Many mutations causing human diseases related to inactivation are identified in the CT of the sodium channels [ 21 , 22 , 23 , 24 , 25 ].
The α subunit of the VGSC is coupled to one or two β subunits called auxiliary subunits. The β subunits have unique functions independent of the α subunit: cell adhesion and intracellular/extracellular signaling [ 26 , 27 , 28 , 29 , 30 ].
Roles of VGSCs in genetic and acquired epilepsies
Based on the site of origin, genetic epilepsy (also known as idiopathic epilepsy (ies)) can be either focal or generalized epileptic seizures. Although these seizures lack a known cause, they are considered to be genetically determined. Genetic epilepsies do not show lesional neuropathologic abnormality, and have normal brain imaging presentation. They are estimated to represent about 47% of all epilepsies [ 31 ]. These types of epilepsies are mostly precipitated by gene mutations encoding ion channels or their ancillary subunits. The genetic defects can be either monogenic or polygeneic, with monogenic defects accounting for a small proportion (~ 2%). A good example of monogenic etiology is the benign familial neonatal seizures (BFNS), which are the first discovered CNS channelopathy and the best known genetically determined human epilepsies. Polygenic defects with convoluted polygenic traits account for a high percentage of idiopathic genetic epilepsies (IGE). Although numerous genetic mutations have been found to cause some genetic-idiopathic epilepsies in humans [ 32 , 33 , 34 , 35 , 36 ], some forms of idiopathic epilepsies still have unclear causes. However, ion channel defects are widely recognized as one of the major causes of idiopathic epilepsies. Genetic-idiopathic epilepsies can be caused by dysfunctions of voltage-gated ion channels (VGICs) which are essential for AP generation and maintenance of resting membrane potentials, or by ligand-gated ion channels (LGICs) which are mainly responsible for synaptic transmission. Mutations in VGICs (Na + , K + , Cl − , Ca 2+ channels) and LGICs (N-methyl-D-aspartate receptors, nicotinic acetylcholine receptors [nAChRs], γ-Aminobutyric acid sub-type A [GABA A ] receptors) can cause neuronal hyperexcitability through several pathogenic mechanisms. The CNS is abundantly enriched with VGICs, which are responsible for the generation, propagation, regulation of neuroexcitability and are therefore regarded as key players in the pathogenesis of epilepsy especially when the homeostatic mechanism goes awry. Idiopathic epilepsies are predominately due to the genes mutation encoding for ion channels. Although ion channel genes mutation contributes to only a small fraction (27%) of all genetic epilepsies (LGICs 10%, VGICs 17%) [ 37 ], they have received much attention from studies on genetic epilepsies and channelopathies. Understanding the role of ion channels in epilepsy can provide insight into the disease mechanisms, precision diagnosis and classification of epileptic syndromes, and promote drug design and development, validation of new drug target as well as development of pharmacotherpeutic strategies and interventions. Most of our understanding of molecular signatures of epilepsy in general came to the fore in 1995 when Steinlein and Colleagues reported for the first time that a missense mutation in the neuronal nACHR α4 subunit corresponds with autosomal-dominant nocturnal frontal lobe epilepsy (ADNFLE) [ 38 ]. Apart from nACHRs, mutations in a plethora of other genes have also been implicated in the epileptogenesis of ADNFLE, including DEPDC5 (22q12.3), CRH (8q13) and CABP4 (11q13.2). Examples of epilepsy caused by genetic mutations of ion channels include epilepsy caused by VGSC mutations. The important roles of VGSCs in neurohyperexactibility have made them potential candidates for episodic neurological disorders as seen in epileptic seizures. Usually, the VGSCs become permeable to sodium when the channels are open, and sodium ions flow into the intracellular space from the extracellular space (activated state). The opening of the channel is orchestrated by the DI–DIII S4 voltage sensors, which undergo rapid movement in response to altered electric field across the cell membrane due to depolarization, resulting in a conformational change in the protein [ 39 ]. After a few milliseconds, inactivation occurs, mediated by the IFM triplet located in the highly conserved intracellular linker connecting domains DIII and DIV. The inactivation gate plays the role of hinged seal and folds into the channel pore during fast inactivation [ 11 ]. Unfortunately, however, incomplete inactivation in some neurons either by mutations or temperature (fast and slow inactivation) have been involved in the pathogenesis of epilepsy, through generation of persistent current, a current activated at subthreshold voltages that enhances epileptic burst firing by decreasing the threshold for AP generation, sustaining repeated firing and augmenting depolarizing synaptic currents [ 40 ]. Mutations in VGSCs (such as in SCN1A, SCN2A, SCN3A, and SCN8A ) can lead to defects in inactivation gating, enhancing persistent sodium currents ( I NaP ) and firing of neurons, resulting in epilepsy and ataxia [ 41 ].
There are two major types of genetic epilepsy associated with VGSC dysfunction or mutation, the idiopathic generalized epilepsy (IGE) and idiopathic focal epilepsy. IGE is believed to be polygenic, and encompasses a continuum of epileptic seizures like absence seizures, myoclonic seizures and generalized tonic–clonic seizures. Two well-known examples of IGE with VGSC mutation implications are Dravet syndrome ( SCN1A ) and genetic epilepsy with febrile seizure plus GEFS + ( SCN1A, SCN1B ). BFNIS is a classic example of idiopathic focal epilepsy caused by VGSC mutations. Other rare monogenic idiopathic epilepsy syndromes like BFNS and ADNFLE are not caused by mutations of VGSCs and will not be discussed further here. Although there are fewer global genetic phenotypes or syndromes caused by VGSC mutations than by other VGICs, a particular VGSC gene can harbor plenty of mutations. For example, the Na V 1.1-encoding SCN1A gene, whose missense mutation causes DS, has been found with ~ 600 mutations in its sequenced coding sequences, representing 70% of cases [ 42 ]. The importance of VGSCs is not only because that they are responsible for the generation of APs but also that they harbor mutations that are responsible for the epileptogenesis of rarer genetic epileptic syndromes and many epileptic encephalopathies that are intractable and pharmacoresistant to ASMs. Most importantly, most notable ASMs exert their effects by modulating or manipulating the VGSCs. Among the genetic-idiopathic epilepsies in which VGSCs are implicated, Dravet syndrome (also known as SMEI) is a type of highly debilitating, recalcitrant and pharmacoresistant epilepsy resulted from missense mutations in the VGSC protein NaV1.1 encoded by the SCN1A gene [ 43 ]. Other forms of epileptic syndromes caused by mutations in VGSCs are generalized (genetic) epilepsy with febrile seizure plus + (GEFS), a milder form of epilepsy compared to Dravet syndrome, resulted from the mutations of SCN1A and SCN1B (which encodes the β1 subunit of nACHR); and BFNIS, which is caused by mutations in SCN2A , a gene encoding one of the α-subunits of VGSCs. Intractable childhood epilepsy generalized tonic-colonic is another type of epileptic seizure caused by VGSC mutations, which is similar to SMEI in many aspects, including pharmacoresistance, intractability, age onset, fever association and learning disability [ 44 , 45 ].
Unlike genetic idiopathic epilepsies that present no structural lesions or other predisposing causes, acquired epilepsies are characterized by visible structural lesions and neuroanatomic features. Acquired epilepsies start from a particular point around the structural lesion and therefore have a focal origin of bursting. The electroencephalogram pattern and clinical presentation of acquired epilepsies depend on the particular brain region where the seizures start and spread and can range from mild, moderate to severe. Acquired epilepsies are triggered by neuropathological insults and about 50% of all epilepsies are acquired. Examples of common brain injuries or insults that trigger acquired epilepsies are traumatic brain injury, hippocampal sclerosis, tumors, stroke and status epilepticus. Although VGSC mutations are mostly implicated in genetic epilepsies, evidence shows that aberrant functions and mutations of VGSCs are involved in the pathogenic mechanism of acquired epilepsies. This is because acquired epilepsies are mostly triggered through the process of epileptogenesis, a process of transformation from a functional balance between excitation and inhibition to hyperexcitability of neurons [ 46 ]. VGSCs have been implicated in acquired epilepsies through acquired channelopathies via generation of aberrant large persistent sodium current ( I NaP ) as observed in genetically normal rodents with acquired epilepsies [ 47 ]. It is already known that mutations in any of the genes for Nav1.1, Nav1.2, Nav1.3 and Nav1.6 that are present in the CNS result in diverse forms of genetic epilepsies including the severe refractory epilepsy like Dravet syndrome. However, what is fascinating now is that mutations of these genes causing the elevations of I NaP also result in acquired epilepsies through epileptic encephalopathy syndromes more lethal and severe than Dravet syndrome such as Lennox-Gastaut Syndrome (LGS) and sudden unexpected death in epilepsy (SUDEP) [ 48 ].
Mechanisms of action of ASMs that act through VGSCs
A majority of anti-seizure agents are designed to create a balance that favor inhibition over excitation and therefore stop or prevent seizure activity [ 49 ]. Although there is no permanent cure for epilepsy, the symptomatic remission or relief from seizures by ASMs occurs through various mechanisms and interactions with different cellular targets [ 50 , 51 ]. The mechanisms of ASMs can be classified into four major types: 1) modulation of VGICs such as calcium, sodium, and potassium channels; 2) potentiation of GABA-mediated inhibition through effects on GABA A receptors, GABA transporter 1, GABA-synthesizing enzyme glutamic acid decarboxylase, or the GABA-metabolizing enzyme GABA transaminase; 3) direct modulation of synaptic release through effects on components of the release machinery, including synaptic vesicle protein 2A; and 4) inhibition of synaptic excitation mediated by ionotropic glutamate receptors including AMPA receptors. ASMs act through VGSCs because the flow of cations across cell membranes is mediated via VGICs. VGSC mediates the rising phase of APs, during which the channel allows increased influx of sodium ions into the cell. Enormous neuronal excitation and excessive electrical discharge result in epileptic seizures. Therefore, VGSCs have been studied as a therapeutic target for epilepsy. ASMs acting as sodium channel inhibitors stabilize sodium channels by preventing them to return to the active state and potentiating the inactive state, thereby preventing repetitive firing of axons and neuronal depolarization. ASMs such as phenytoin, carbamazepine, oxcarbazepine, zonisamide and lamotrigine, inhibit abnormal epileptiform activities by blocking the fast inactivation state of VGSCs [ 52 ]. They bind to the inactivated voltage-gated channels after depolarization and modify their permeability to sodium ions, thereby reducing inward sodium movement. This leads to an enhancement in the inactivation (or refractory) period of frequently firing neurons. ASMs can manipulate either the fast- (phenytoin, carbamazepine, Fosphenytoin, oxcarbazepine, primidone, zonisamide, and valproic acid [VPA]) or the slow-inactivation (lacosamide and eslicarbazepine) gate or state of the VGSCs.
The role of phosphorylation of VGSCs in the pathogenesis and treatment of epilepsy
Phosphorylation is one of the most common post-translation modifications (PTMs) at the proteomic level, and together with N-glycosylation, is considered as the most abundant PTM [ 53 ]. Phosphorylation is also the most widely studied PTM in sodium channels. Although the molecular mechanisms of aberrant expression, localization, as well as function of Nav channels in the development of epilepsy is poorly understood, it is considered that it may be caused by altered PTMs. Phosphorylation modulating VGSC gating, and has been thought to be the cause of acquired insensitivity of Nav channels to anti-seizure medications in epileptic neurons. Neverthless, whether the changes of PTMs of specific Nav channels occur sharply during epileptic seizures remain unclear. Several sites of phosphorylation have been identified by proteomic profiling and mass spectrometry, although there is paucity of data on which protein kinase(s) catalyse the phosphorylation. Specifically, latest mass spectrometry-based proteomic analyses of Nav1.2 purified from rat brain [ 54 ] or present in whole mouse brain phosphoproteome fractions. Two different monoclonal antibodies one specific for Nav1.2, and one with pan-VGSC specificity, have been used in parallel immunopurification and MS analyses of rat brain VGSC phosphorylation. These studies characterized fifteen phosphosites on Nav1.2, and three on Nav1.1, making Nav1.2 the VGSC with the highest phosphosites [ 55 , 56 ] have identified > 60 in vivo phosphorylation sites on brain Nav1.2, much more than those identified on any other Nav channels. Nonetheless, cAMP-dependent kinase (PKA) and protein kinase C (PKC) have long been known to phosphorylate brain VGSC [ 57 , 58 , 59 ]. The intracellular domains of the VGSC are targets for phosphorylation at multiple sites [ 60 , 61 ]. Apart from PKA and PKC, other kinases for brain VGSC are glycogen synthase kinase 3 (GSK3) [ 62 , 63 ], a kinase-anchoring protein 15 [ 64 ], Fyn tyrosine kinase [ 65 ], as well as p38 mitogen-kinase activated protein kinase [ 66 ]. Many of these phosphorylations are related to the pathogenesis of genetic and acquired epilepsies. Therefore, identifying the signal pathway of dysfunction in epilepsy might supply new targets for anti-seizure medications [ 67 ].
The electrophysiological effects of phosphorylation on VGSC are often dependent on the specific isoform. PKA and PKC phosphorylation of Nav1.2 causes defective channel trafficking to the cell surface, resulting in attenuation of Nav1.2 currents [ 59 ]. Increased phosphorylation of Nav1.2 in the ID I-II linker region is usually related to the decrease of Nav current [ 57 , 68 , 69 ]. As evidence shows some effects of topiramate (TPM) on AMPA/kainate receptors are affected by the phosphorylation state of the receptors, TPM may bind to the phosphorylation sites of these receptors in the inner loop, thereby modulating ionic conductance via the channels allosterically. TPM may also prevent PKA and PKC from phosphorylating the channels. This suggests the crucial role of phosphorylation in the pathogenesis of epilepsy and its manipulation to exert anti-seizure effects [ 70 ]. Phosphorylation signaling pathways such as the p38 MAPK-JNK signaling are important regulators of cellular function and may be a target for drug design and development [ 71 , 72 ].
Therapeutic effectiveness of VGSC inhibitors in genetic and acquired epilepsies
ASMs, formerly referred as anti-epileptic drugs or anticonvulsant, are the main treatment for both genetic and acquired epilepsies. To date, most of the mutations established to be related to epilepsy locate in genes encoding VGSCs. Mutations in the 9 different α isoforms of VGSC (NaV1.1-NaV1.9) are reported to cause channelopathies. Specifically, mutations of genes for NaV1.1 ( SCN1A ), NaV1.2 ( SCN2A ), NaV1.3 ( SCN3A ), NaV1.6 ( SCN8A ) and NaV1.7 ( SCN9A ) are related to both genetic and acquired epilepsies because of their abundant presence in the CNS. Seizures are precipitated by bursts of high-frequency APs, and ASMs might inhibit seizures by impeding the bursts by gradually inhibitng VGSCs. The VGSCs can transit through multiple states, and ASMs have varying affinities to the channel depending on the state [ 73 ]. All these modulations and manipulations are possible because the inactivation of VGSC is typically characterized with fast and slow components. The fast component occurs within 5–10 ms, while the slow component may take hundreds of milliseconds to initiate. Considering the central role of VGSCs in regulating neuronal excitability, many common ASMs exert their putative actions by targeting VGSC function. Therefore, blocking or inhibiting VGSCs during excessive hyperexcitation seems to be a sensible way to repress or suppress seizures. However, some epilepsies are believed to arise from specific loss of VGSCs in inhibitory neurons, leading an imbalanced excitatory-inhibitory (E-I) ratio. In such cases, an activator of VGSC could restore the channel function in inhibitory neurons. Compounds that stimulate or selectively activate NaV1.1 are new targets to achieve this goal. Given the fact that NaV1.1 expressed predominantly in inhibitory interneurons, NaV1.1 activation is assumed to enhance overall inhibition and prevent seizures potentially [ 74 ]. The main mechanism of action of these ASMs seems to be use-dependent block, that is, when the membrane potential experiences repeated reach to depolarized levels more frequently, inhibition of sodium currents is stronger, exposing novel drug-binding sites and selectively blocking of channels was only allowed when they are in the active neurons [ 75 ]. Dysfunction of many subtypes of VGSCs may lead to the development of epilepsy. Below we discuss and summarize how dysfunction of some subtypes of VGSCs leads to the pathogenesis of epileptic seizures and how biophysical manipulations of these VGSCs could be used as an approach to the treatment of genetic, acquired, Dravet syndrome, Lennox Gastaut syndrome and other pharmacoresistant epilepsies.
Putative roles of Nav1.1 and Nav 1.2 in genetic and acquired epilepsies and epilepsy management
It is already known that VGSCs take charge of the initiation of APs in neurons, and inhibitors of sodium channels are used for treatment of epilepsy. Sodium channel activators were not considered to be therapeutically relevant due to their toxicity and side effects. However, selective activators of the NaV1.1 sodium channel might be potentially therapeutic for diseases, including epilepsy [ 76 ]. Like Nav1.2, Nav1.3, and Nav1.6, Nav1.1 also has high expression level in the CNS. It is well known that in the process of modulating GABAergic inhibitory interneuron physiology, Nav1.1 plays a vital role. Many mutations in sodium channels can cause inherited epilepsy syndromes of different severities, and among these mutations, the NaV1.1 channel encoded by the SCN1A gene is the most common target [ 77 , 78 ]. SCN1A , which encodes the Nav1.1 subtype of the pore-forming α subunit of the VGSCs, has been identified with 200 epilepsy mutations [ 79 , 80 ]. In fact, of all known mutations of epilepsy genes, SCN1A mutations are the most diversely implicated in both hereditary and acquired seizure pathogenesis [ 8 , 81 ]. Most Dravet syndrome and GEFS + cases have mutations in SCN1A, which suggests in the context of epilepsies, this channel plays a role. NaV1.1 causes epilepsy either by gain or by loss of function of sodium channels that either increase or decrease neuronal excitability via a widespread dysfunction of network inhibition. It is hypothesized that in the context of epilepsy, Due to its ability and propensity to attenuate Nav1.1 sodium current and resulting in reducing the excitability of inhibitory neurons, Nav1.1 mutations, regardless of being missense or nonsense, gain-of-function or loss-of-function, and their association with GEFS + or SMEI, all stem from it. In fact, reflecting upon individual genetic differences, a spectrum of diseases from GEFS + to SMEI, all reflect a certain extent of Nav1.1 attenuation, whether it be partial or complete. Several lines of evidence suggest that loss-of-function mutations in VSGCs cause epileptic disorders [ 82 , 83 ]. SMEI (or Dravet's Syndrome) is caused by complete loss-of-function mutations in NaV1.1, which is a severe and intractable epilepsy with comorbid ataxia and cognitive impairment [ 84 , 85 ]. Different lines of evidence support the notion that epilepsy is a condition characterized by network hyperexcitability. Epilepsy mutations are proposed to alter sodium channel behaviors by increasing the excitability of neurons expressing mutant channels. Consistently, studies have demonstrated the effects on sodium channel behavior [ 86 , 87 ]. Amongst various types of GABAergic interneurons, NaV1.1 serves as the principal voltage-gated Na + channel. Decreased activity of NaV1.1 can reduce excitability and decrease the GABAergic tone. Mutations in NaV1.1 may be responsible for epilepsy. The potential of modulating the function of sodium channels has been increasingly supported by evidence as a potential therapeutic approach. Interneurons, which synthesize and release the inhibitory neurotransmitter GABA, are inhibitory in nature. They regulate the secure synchronized activity and the excitability of neuronal subpopulations. Categorization of interneurons into subclasses is determined by physiological properties, neurochemical markers, and connectivity patterns. For the parvalbumin-expressing subclass of inhibitory neurons (fast-spiking interneurons), the NaV1.1 channel significantlt contributes the sodium current, which is crucial for AP generation and sustained excitability. Therefore, specifically increasing the function of NaV1.1 channels can potentially enhance the function of fast-spiking GABAergic interneurons, leading to a consequential impact on the excitability in the central nervous system. Therefore, activation of NaV1.1 channels using pharmacological methods is considered as a viable treatment option for SCN1A haploinsufficiency and other diseases associated with defective function of fast-spiking GABAergic parvalbumin interneurons. It has been well established that the sodium channel is a crucial target of drugs. Small-molecule inhibitors targeting sodium channels have been deployed in clinical settings to treat various conditions linked with abnormal cellular excitability, such as epilepsy and pain, comprising the first generation of such inhibitors [ 88 ]. As the NaV1.1 channels are responsible for the modulation of electrical excitability through inhibitory interneurons, the use of non-selective sodium channel inhibitors is contraindicated to GEFS + syndromes or SMEI, as it might exacerbate the disease by further suppressing the NaV1.1 channels [ 89 , 90 , 91 ]. Clobazam, as the first-line drug therapy treating epilepsy associated with SCN1A mutations, which increases transmission of postsynaptic GABAergic signals with allosteric modulation of GABA A receptors; and VPA, which increases GABA concentration in the synaptic gap through enhancement of GABA production and reduction of GABA degradation. Increasing the mRNA level of SCN1A using antisense nucleotides (ASO) has emerged as a promising approach for genetic disorders involving haploinsufficiency [ 92 ]. Alternative therapeutic options, such as ketogenic diets, may prove beneficial for cases of pharmacoresistant Dravet syndrome [ 93 , 94 ]. Nav1.1 blockers as anti-seizure medications exert function by stabilizing neuronal membranes through inhibiting the initiation or propagation of abnormal synchronous electrical activity within neurons, thus attenuating the spread of seizure activity emerging from a particular focus or source [ 95 ]. Numerous studies have indicated that sodium channel blockers might be the optimum choice for individuals suffering from SCN8A encephalopathy [ 96 , 97 , 98 , 99 ].
Unlike SCN1A , where epilepsy stems almost exclusively from loss-of-function variants that impair channel function, which is caused by deficits in circuit disinhibition and inhibitory interneuron excitability [ 100 , 101 , 102 ], the SCN2A gene which encodes Nav1.2 is associated with seizures through both gain-of function and loss-of-function mutations. Nav1.2 is expressed mainly in excitatory pyramidal neurons, contrary to Nav1.1. Apart from seizure pathology, NaV1.2 loss-of-function mutations are also strongly associated with intellectual disability and autism spectrum disorder. More specifically, Nav1.2 is found in high density locating in the proximal region of axon initial segments (AIS),in which it is considered pivotal for the backpropagation of APs into the neuronal soma [ 103 , 104 , 105 ]. During embryonic development, at immature nodes of Ranvier, high levels of Nav1.2 exists. As the time of nodes maturation, Nav1.6 gradually supersedes Nav1.2 [ 106 , 107 ]. Mutations in SCN2A are associated with inherited epilepsies including BFNIS. Specifically, in cases of BFNIS, the identification of missense mutations in SCN2A has been reported. [ 108 ]. Mutations in Nav1.2 also cause acquired form of seizures, such as febrile and afebrile seizures [ 109 , 110 ]. Seizures caused by VGSC dysfunctions are intractable. In some cases, even various anti-seizure medications cannot controlled seizures caused by mutations of NaV1.2 [ 111 ]. Gain in channel function resulting from Nav1.2 mutations is believed to be a possible mechanism for epilepsy pathogenesis. Sodium channel inhibitors, such as carbamazepine, phenytoin, oxcarbazepine, lamotrigine and TPM, are generally expected to be effective in treating epilepsy patients who have a mutation in SCN2A (Nav1.2). Despite the success of carbamazepine and high dose of phenytoin in certain cases of epilepsy resulting from SCN2A mutations, a considerable number of patients remain pharmaceutically intractable, even when treated with other traditional anti-seizure medications. As a result, there is an urgent requirement for innovative, more targeted pharmaceutical agents for these patients. Peters, et al.investigated the effects of ranolazine, a pharmaceutical substance commercially utilized as an anti-anginal medication on NaV1.2 channels. This study found that ranolazine suppressed macroscopic currents and extended the recuperation time of both rapid and slow inactivation of NaV1.2 channels. Further consensus studies should be geared towards making ranolazine an approved and efficacious therapy for epilepsy but presently this is highly unlikely because it exacerbates seizure semiology [ 112 ]. It’s crucial to develop pharmacotherapeutic agents for epilepsy associated with pathogenic SCN2A mutation, which function primarily by amplifying resurgent and/or persistent currents, and may get involved in selective suppression of the aberrantly enhanced resurgent and/or persistent currents.
Putative roles of Nav1.6 channel in both genetic and acquired epilepsies and its management.
The Nav1.6 channel encoded by the gene SCN8A is associated with over 300 cases of epileptic encephalopathy and ~ 200 putative spots of mutation have been characterized. Despite being one of the most massively expressed voltage-gated sodium channels in the CNS, Nav1.6 is the least studied of the Nav family. This gene has maximum expression in pyramidal cells of the hippocampal and the Purkinje cells and granule cells of the cerebellum [ 113 ]. This channel is particularly abundant in the distal part of AIS and in the nodes of Ranvier of myelinated axons, although it is also prevalent throughout the peripheral nervous systems and CNS, in both inhibitory and excitatory neurons [ 114 ]. Normal brain function is heavily reliant on the exquisite initiation and spread of APs, and this activity is crucially dependent on Nav1.6. Epilepsy-causing mutations in Nav1.6 occur through the entire structure of the channel and only 10% of these have been characterized at the molecular level. The majority of these mutations are gain-of-function mutations. Upregulation of Nav1.6 in the AIS is shown to result in an upsurge in spontaneous and repetitive firing of cortical neurons, a plausible explanation for why SCN8A mutations in patients with epilepsy are mainly gain-of-function and impact the AP threshold [ 115 ]. Although the function of Nav1.6 in inhibitory interneurons is still illusive, mounting evidence has indicated Nav1.6 plays a part in establishing synaptic inhibition within the thalamic network, corroborating the loss-of-function outcomes brought on missense mutations in the mature protein [ 116 , 117 ], which result in various network effects in different circuits of the nervous system. There are three classical forms of hereditary epilepsies associated with mutations of SCN8A , early infantile epileptic encephalopathy type 13 (EIEE 13), benign familial infantile seizures-5 and paroxysmal dyskinesia with SUDEP, which is the primary cause of epilepsy-related death from mutations of SCN8A . One possible explanation for the relationship between SCN8A -related epilepsy and SUDEP is the broad expression of NaV1.6 in ventricular myocytes and cardiac tissues [ 118 , 119 ]. Therefore, an accumulation of respiratory, neurological and cardiac factors lead to a “perfect storm” and thus result in death. Single and null mutations may have negative effect on the heart function, causing cardiorespiratory depression and, consequently, death [ 120 ]. EIEE 13 is a phenotypically complex early-onset epilepsy, with seizure onset before 18 months of age [ 121 , 122 , 123 ]. Examples of infantile epileptic encephalopathies are Lennox-Gastaut syndrome (LGS), Landau-Kleffner syndrome, myoclonic-astatic epilepsy, West syndrome, Ohtahara syndrome, and Dravet syndrome. Temporal lobe epilepsy (TLE) is one of the most common forms of adult-acquired epilepsy caused by gain-of-function mutations of SCN8A . Gain-of-function mutations of SCN8A are responsible for causing one of the most frequent types of acquired epilepsy (temporal lobe epilepsy, TLE) in adults. TLE can have myriad of etiologies. Seizures arising spontaneously from the temporal lobe are the hallmark feature of TLE. TLE is a complex and heterogeneous group of disorders with seizure initiation and invasion in the temporal lobe; however, variabilities exist amongst patients regarding their age of onset, etiologies, and response to various treatment approaches [ 124 ].
It is well established that VGSCs make the substantial contribution in modulating neuronal proexcitatory and physiology changes to these channels facilitate neuronal hyperexcitability in TLE. In TLE patients, significant changes in VGSC mRNAs are observed in the hippocampus [ 125 ] and noteworthy recording resulting from human TLE subiculum neurons reveal a marked upsurge of persistent sodium currents [ 126 ]. Animal models of TLE have recapitulated corresponding proexcitatory modifications to sodium channels. 4,9- anhydro-tetrodotoxin (4,9-ah-TTX), a toxin with a more significant binding affinity for Nav1.6 compared to other VGSC isoforms, can reveal the critical function of Nav1.6 in promoting neuronal hyperexcitability [ 127 ]. Previous studies have shown that the inhibition of Nav1.6 with 4,9-ah-TTX can effectively dampen neuronal hyperexcitability and reduce upregulated persistent and resurgent sodium currents in TLE [ 128 ]. Due to the role in driving hyperexcitability of neurons, Nav1.6 has been an attractive target for preventing or decreasing the occurrence of seizures in TLE animals. For SCN8A encephalopathy, no guidelines for treatment is available. Current treatments are aimed to control seizures through poly-drug therapies, while uncontrolled seizures increase the risk of SUDEP and permanent injury in patients [ 129 , 130 ]. Pharmacoresistance is typical among a number of SCN8A patients but some show prolonged seizure-free periods [ 131 ]. Many studies show that sodium channel inhibitors might be the most effective treatment approach for individuals with SCN8A encephalopathy [ 132 ]. A common pathogenic mechanism across SCN8A mutations is the disruption of sodium channel inactivation, and drugs that have greater affinity towards the inactivated state of these channels may offer advantage in treating patients. Phenytoin is one such drug that is believed to have higher affinity for the inactivated state [ 133 ]. Phenytoin can effectively attenuate proexcitatory alterations in the physiology of mutant channel [ 134 ] and offer improved seizure freedom in patients with SCN8A encephalopathy [ 135 ]. Although the treatment options with proven efficacy do exist for individuals with SCN8A encephalopathy, reports from several patients suggest that levetiracetam is ineffective at controlling seizures and may even worsen the symptoms [ 136 ]. TLE patients frequently exhibit pharmacoresistance and up to 30% of cases fail to attain seizure freedom only with ASMs [ 137 , 138 ]. Recent studies have suggested that carbamazepine and valproate, inhibitors of sodium channel, are the most promising ASM options for TLE patients [ 139 ]. The efficacy of ASMs in treating individuals with TLE is limited by the modified pharmacology caused by epileptogenesis. In animal models of temporal lobe epilepsy, kindled animals show acute reduction of the hypoexcitatory effects of carbamazepine compared to controls. Additionally, the EC50 is significantly increased in cells from kindled animals compared to controls [ 140 ]. In brain tissues from carbamazepine-resistant TLE patients, the blocking effect of carbamazepine significantly diminished [ 141 ]. Further, the reduced effects of carbamazepine on the recovery from inactivation have been observed in animal models with TLE [ 142 ]. The reduced efficacy is not just seen with carbamazepine, but also with phenytoin and lamotrigine [ 143 ]. However, the effects of valproate exhibit little change in cells derived from epileptic patients and kindling animal models. [ 144 ]. More researches are needed to investigate if Nav1.6 is a promising target for future ASMs (Fig. 1 ).
Primary structures of the subunits of voltage-gated sodium channel
VGSC-inhibitors in the treatment of Dravet syndrome and other pharmacoresistant epilepsies
Although there is currently no cure for epilepsy, early treatment can lead to a substantial remission and make a big difference. Pharmacoresistance is a broad term that encompasses refractory, intractable or recalcitrant type of epilepsy such as Dravet syndrome, Ohtahara syndrome, Rasmussen encephalitis, LGS, and infantile spams. Pharmacoresistant epilepsy, as defined by The International League Against Epilepsy, refer to the failure of a patient to respond to at least two ASMs which are suitably chosen and adminisitered for an adequate period of time, either as a monotherapy or as a polytherapy [ 145 , 146 , 147 ]. The etiology of pharmacoresistant epilepsy can be attributed to various factors, comprising genetic and environmental factors, along with drug- and disease-related factors. Although about 30 ASMs have been approved for about three decades for the treatment of epilepsy, unfortunately some patients do not exhibit a positive response to medical interventions. Some VGSC blockers like lamotrigine and carbamazepine are the most effective and commonly used ASMs, they are surprisingly unable to cause remission in intractable epileptic encephalopathy like Dravet syndrome. In fact, they are contraindicated for Dravet syndrome because they exacerbate seizures [ 148 , 149 ].
A pharmacoresistant form of epilepsy that emerges in infants, Dravet syndrome, leads to comorbidities of cognitive incapacity, psychomotor retardation, ataxia and premature mortality. Dravet syndrome represents the prototypical pharmacoresistant epilepsy. Seizures remain inadequately managed in a majority of patients, even with the use of multiple ASMs or polypharmacy. Aras et al. found that 45% of Dravet syndrome patients receiving inadequate treatment modalities persist in encountering over 4 tonic–clonic seizures each month [ 150 ]. Although Dravet syndrome is caused by polygenic mutations as mutations of other genes encoding calcium, potassium, and hyperpolarization-activated cyclic nucleotide-gated channels are also implicated in its pathogenesis, this disease is mainly caused by reduced sodium currents and impaired excitability of GABAergic interneurons (primarily defects in AP firing in fast-spike parvalbumin and somatostatin interneurons) in the hippocampus as well as to a lesser degree impairment of other classes of GABAergic interneurons. Agents that inhibit sodium channels are the drugs of choice for epileptic seizures, including lamotrigine, VPA, phenytoin, carbamazepine, and clobazam. Unfortunately, in fact, some of these standard sodium channel blockers worsen seizures in both mice and children with Dravet syndrome [ 151 , 152 ]. Although some of them (such as VPA, TPM, rufinamide, cenobamate and eslicarbazepine) are effective for Dravet syndrome remission either as a monotherapy or in a polytherapy [ 153 , 154 , 155 ], most of the standard VGSC therapies are contraindicated in Dravet syndrome [ 156 ]. LGS is another important type of drug-resistant epilepsy. It is a rare and severe epileptic encephalopathy of childhood onset with heterogeneous etiology, in which 65–75% of patients have known causes (genetic, structural, or metabolic) while others have unknown causes [ 157 , 158 ]. It is considered as one of the most severe and devastating type of epileptic syndromes in infancy and early childhood [ 159 , 160 , 161 ]. Even though VPA is not specifically licensed for application in LGS, owing to its broad spectrum and low potential for exacerbating seizures, it is widely recommended as an ideal first-line medicine [ 162 , 163 ]. TPM is another broad-spectrum VGSC inhibitor that is used in the treatment of LGS. During long-term therapeutic studies, TPM demonstrated well tolerance and effectiveness in managing the drug-resistant drop attacks (sudden falls) and seizures linked with LGS [ 164 , 165 ]. TPM is able to tackle seizure semiologies like tonic seizures, characterized by greatly increased muscle tone and abrupt stiffening movement in the limbs and body, which is commonly observed in LGS, as well as atonic seizures that manifest as sudden loss in muscle strength and tone [ 165 , 166 , 167 , 168 , 169 , 170 ]. Most of the ASMs available are used in polytherapies in the management of LGS, and emerging drugs are being re-directed to develop LGS-specific treatments. As per recent research, rufinamide is among the latest adjunctive drugs [ 171 , 172 , 173 , 174 ]. Both open-label studies and randomized controlled trials have suggested that rufinamide could be highly efficacious in mitigating a range of seizures, especially tonic-atonic seizures and those leading to falls observed in patients with LGS. Rufinamide appears to have an advantageous tolerability and safety profile, together with largely mild side effects as well as a good interaction profile with other ASMs. Other VGSC inhibitors in the treatment of LGS include ZNS, which is indicated as adjunctive safe and effective treatment in pediatric LGS patients [ 175 , 176 , 177 , 178 ]. Lamotrigine (LCM) was specifically approved for the management of LGS by US and EU after a double-blind, placebo-controlled, randomized controlled trial, which certified that LCM is efficacious in the treatment of LGS [ 179 , 180 ]. According to recent research, the application of LCM exhibits the potential to decrease the huge number of spike-and-wave events that are commonly observed in LGS [ 181 , 182 ], and also demonstrates efficacy as an adjunctive therapy for treating refractory epilepsy [ 183 ]. Although phenytoin can exacerbate atypical absences and myoclonic-seizures in LGS [ 184 ], it still plays a pivotal role in the management of LGS as it decreases tonic–clonic seizures and reduces tonic seizures.
Another pharmacoresistant type of epilepsy that can benefit from treatment with VGSC inhibitors is the early infantile epileptic encephalopathy (EIEE). EIEE is a childhood age-dependent disease of the brain, with pathological hallmarks including loss of neurologic function over time, abnormal electroencephalographic findings, and seizures. Although EIEE seizures are devastating, debilitating, intractable and pharmacoresistant to ASMs, some patients respond positively to high-dose VPA [ 185 ]. VPA also showed good efficacy in a EIEE patient caused by PACS2 gene mutation [ 186 ]. Unfortunately, however, VPA did not show beneficial effect in early diagnosis and treatment of an infant with epileptic encephalopathy caused by cytoplasmic FMRP interacting protein 2 mutation [ 187 ]. VGSC inhibitors are typically considered the first-line treatment for confirmed or suspected epileptic encephalopathies related to SCN2A . In severe cases with compatible electro-clinical features, carbamazepine used following a high-dose intravenous phenytoin (in the case of a positive response to phenytoin) may be a more suitable treatment algorithm for long-term maintenance treatment [ 188 ]. Carbamazepine as a VGSC inhibitor is also effective for RHOBTB2-related paroxysmal dyskinesia resulting from early infantile SCN1A epileptic encephalopathy [ 188 ] (Table 1 ).
Monotherapy vs. rational polytherapy with VGSC inhibitors in the management of pharmacoresistant and other forms of epilepsy
At present, treatment selections for epilepsy primarily addresses symptoms. After the first two drug regimens, most patients can achieve seizure freedom. Otherwise, they are defined as pharmacoresistant. Due to the various benefits including minimal side-effects, lack of drug-drug interactions, better adherence and lower cost, monotherapy is considered as the preferable treatment approach in epilepsy [ 189 ]. Another major impetus to achieve or maintain monotherapy is the fact that it decreases addictive neurotoxic and cognitive side effects. Carbamazepine and VPA are two well-known effective monotherapeutic ASMs and have been in use for the management of seizures for decades with remarkable success. One of the initial ASMs to be promoted for application as monotherapy was VPA. In a previously open monotherapy investigation, VPA was found to be effective in regulating all primary generalized seizure types in 83% of 118 patients evaluated in both adults and children, some of whom had failed to respond to the former treatment [ 190 ]. Carbamazepine has also robustly demonstrated effectiveness in monotherapy for seizures. Another dibenzazepine family member, eslicarbazepine acetate, has been sanctioned by the European Medicines Agency (EMA) and United States Food and Drug Administration (FDA) for monotherapy in adults with newly diagnosed epilepsy [ 191 , 192 , 193 , 194 ]. Apart from the aforementioned VGSC inhibitors, several other inhibitors have also demonstrated their potency and efficacy as monotherapeutic agents against epilepsy. Unfortunately, drug-resistant epilepsy occurs in at least 30% of people with epilepsy, who remain refractory to traditional pharmacological therapies, necessitating multiple drugs to be used simultaneously [ 195 , 196 ]. In addition, despite the introduction of new ASMs over the past two decades with advances in the field, management of pharmacoresistant epilepsies is still complicated and leaves a lot of unsolved questions. Also, a study has demonstrated that 30–40% of patients treated with an traditional ASM including carbamazepine and VPA as monotherapy experience adverse effects that contribute to therapeutic failure [ 197 ]. Over the past 20 years, monotherapy has been considered as the gold standard in epilepsy treatment, partly due to the heightened toxicity associated with polytherapy. Nevertheless, some people with pharmacoresistant epilepsy, such as LGS and Dravet syndrome, have not shown expected response to monotherapy. Such patients may require polytherapy and should be carefully evaluated taking into account the risk/benefit ratio in terms of tolerability, potency/efficacy and patient compliance. Rationally designed polytherapies can achieve better seizure control, maximize the efficacy, minimize drug interactions, drug load, and side effects, and control multiple seizure types that react to various therapeutic drugs [ 198 , 199 ].
The aims of using polytherapy in pharmacoresistant and other types of epilepsy are to maximize efficacy and minimize side effects [ 200 ]. Another aim of polytherapy and its practice as the first-line of treatment for refractory epilepsy is to achieve robust synergistic impact or lower drug toxicity with less doses of two medications instead of higher doses of a single drug. [ 201 ]. The usefulness of combination therapy should be an anti-seizure supra-additive effect (synergy effect) and possibly neurotoxic antagonism or neurotoxic infra-additive effect [ 202 ].
Polytherapy is highly desirable in the treatment of pharmacoresistant and other forms of epilepsies. In many non-randomized open studies, the efficacy of CBZ and VPA in combination has been established in patients who exhibited poor response to monotherapy [ 203 , 204 ]. Robust data have shown that the combination of VPA and lamotrigine exerts the best synergism in human studies. Multiple studies have reported on the synergistic relationship between these anti-seizure medications, highlighting the substantial response rate generated through the incorporation of lamotrigine as an add-on therapy to VPA, in contrast to addition of lamotrigine to phenytoin or carbamazepine [ 205 ]. Polytherapy of LTG-TPM and VPA is also useful in adults [ 206 , 207 , 208 ]. Combinational therapies of trio-ASMs including zonisamide, gabapentin, and eslicarbazepine acetate have also shown to be effective as additional drugs in treating drug-resistant epilepsy [ 209 , 210 , 211 ]. VPA, a VGSC inhibitor, is still considered as the primary ASM treating recently diagnosed drug-resistant epilepsy like DS and LGS. If VPA fails to cause seizure freedom, then another VGSC inhibitor lamotrigine can be considered. Duotherapies combining other VGSC inhibitors like rufinamide and TPM have shown effectiveness against LGS and drop attacks [ 168 , 212 , 213 , 214 , 215 ]. The combinational therapy of TPM and CLB has also shown effectiveness. FFA, perampanel, LEV and zonisamide may have an efficacy in LGS [ 216 ]. Polytherapy holds the future ace in the management of epilepsy, especially drug-resistant epilepsy, if the therapeutic factors and variables are strictly adhered to during these combinations. A recent review proposes that novel ASMs are preferred candidates for combinational therapy as they possess fewer pharmacokinetic interactions, predominantly weak enzyme inhibitors or inducers, and exhibit superior tolerability profiles [ 217 ]. Based on this, ASMs with fewest pharmacological interactions (TPM and zonisamide) are the best to be used in polytherapy for optimal results and few adverse events. In fact, some researchers propose that the occurrence of adverse events in polytherapy is not solely linked to the higher number of drugs but rather to the type and the dosage of the ASMs as well as individual vulnerability [ 218 , 219 ]. In addition, physicians should consider epilepsy syndromes and seizure types before selecting the best combination of ASMs. It is also crucial to take into account various other factors, such as pharmacokinetic and pharmacodynamic aspects of anti-seizure medications, along with patient-related elements including concomitant medications, pharmacogenomics, age, comorbidities, and compliance. It is pertinent to state that the well-defined pharmacoresistant epilepsies such as DS, LGS, EIEE and Rasmussen encephalitis need combinational therapies (such as polytherapy of TPM and VPA) especially where monotherapy is unable to lead to remission.
Conclusions
ASMs that inhibit VGSCs represent a fundamental aspect of treating epilepsy. Manipulations of VGSCs are the primary and most important mechanisms through which virtually all ASMs exert their antiepileptic potentials. Experimental data and evidence gathered over the past two to three decades suggest that VGSC inhibitors acting by blockade of sodium channels are the most effective ASMs in use today. In addition, some other ASMs whose main mechanisms of action are not directly related to VGSCs, have been shown to interact with voltage-gated sodium currents, called multimodality therapy that could be efficacious in the treatment of pharmacoresistant and other types of epilepsies. Presently available ASMs that function through inhibition of VGSCs are often effective in controling seizures in many patients. Unsurprisingly, however, seizure freedom is not totally achieved as seizures persist in a large number of epileptic patients due to drug resistance. Pharmacoresistant epilepsies are a type of highly refractory epilepsies in which a substantial proportion (about 30%) of epilepsy individuals exhibit treatment resistance to any of the three first-line ASMs, despite being administered in an optimal and monitored regimen. Despite efforts by epileptologists and other related researchers to unravel the molecular and cellular mechanisms underpinning pharmacoresistant epilepsies, a complete understanding has so far remained elusive. For the majority of the patients with epilepsy, polytherapy is still the reality. However, investigations conducted in animal models have not revealed evidently about the mechanisms underlying the effectiveness of polytherapy in humans, epileptologists may engage in pharmacogenomics that might provide other instructions as to which combinations or polytherapy could be efficacious potentially via the development of personalized therapeutic plans. Some VGSC inhibitors are not only ineffective in treating some forms of pharmacoresistant epilepsies as seen in DS and LGS, but are also contraindicated as they worsen seizures. The ineffectiveness of VGSC inhibitors in pharmacoresistance epilepsy like DS is not too surprising considering the fact that the pathogenesis of DS is not purely a consequence of epilepsy but is precipitated as a result of genetic mutations encoding mainly VGSCs and other channels like potassium and calcium channels modulated by genetic and non-genetic factors. In an effort to treat and mitigate the burden of these pharmacoresistant epilepsies, some combinatorial have all been approved by relevant regulatory bodies (FDA & EMA) for the treatment of pharmacoresistant epilepsies. Although VGSC inhibitors remain the standard therapy and mainstay in epilepsy treatments over the years, present VGSC inhibitors have discrimination among different VGSC isoforms, because of polygenic and heterogenous nature and thus selective blockers development might enhance their clinical utility. Therefore, there is a need to develop novel drug cocktails with higher selectivity for specific VGSC subtypes, which may be effective in treating several types of epileptic seizures. Despite recent breakthroughs, with approval of the polytherapy treatments for pharmacoresistant epilepsies, patients still encounter significant chanllenges due to the multifactorial nature and limited understanding of pathogenesis and mechanisms of these pharmacoresistant epilepsies. Therefore, more studies are needed to advance our understanding of the pathogenesis of pharmacoresistant epilepsies, which could provide further insight into their precise treatment. Also, research efforts should gear towards discovery, designing and development of optimal combination of VGSC blockers to achieve the maximum therapeutic effectiveness with minimum side effects. Alternatively, non-pharmacological methods such as ketogenic diet therapy and electrical stimulation are also showing emerging potentials.
Availability of data and materials
Not applicable.
Abbreviations
Autosomal dominant nocturnal frontal lobe epilepsy
Anti-seizure medication
α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
Benign familial neonatal infantile seizures
Benign familial neonatal seizures
Dravet syndrome
γ-Aminobutyric acid
Genetic epilepsy with febrile seizures plus
Isoleucine-phenylalanine-methionine
Idiopathic genetic epilepsies
Persistent sodium currents
Ligand gated ion channel
Lennox Gastuat syndrome
Nicotinic acetylcholine receptors
CAMP-dependent protein kinase
Protein Kinase C
Post-translational modification
Serve myoclonic epilepsy in infancy
Transient receptor potential vanilloid-1
Voltage- gated ion channel
Voltage- gated sodium channel
Voltage-sensing domain
Lozovaya N, Gataullina S, Tsintsadze T, Tsintsadze V, Pallesi-Pocachard E, Minlebaev M, et al. Selective suppression of excessive GluN2C expression rescues early epilepsy in a tuberous sclerosis murine model. Nat Commun. 2014;5:4563.
Article CAS PubMed Google Scholar
Goldenberg MM. Overview of drugs used for epilepsy and seizures: etiology, diagnosis, and treatment. P T. 2010;35(7):392–415.
PubMed PubMed Central Google Scholar
Jacoby A, Snape D, Baker GA. Epilepsy and social identity: the stigma of a chronic neurological disorder. Lancet Neurol. 2005;4(3):171–8.
Article PubMed Google Scholar
Rincon N, Barr D, Velez-Ruiz N. Neuromodulation in drug resistant epilepsy. Aging Dis. 2021;12(4):1070.
Article PubMed PubMed Central Google Scholar
Yemadje LP, Houinato D, Quet F, Druet-Cabanac M, Preux PM. Understanding the differences in prevalence of epilepsy in tropical regions. Epilepsia. 2011;52(8):1376–81.
Stafstrom CE, Carmant L. Seizures and epilepsy: an overview for neuroscientists. Cold Spring Harb Perspect Med. 2015;5(6):a022426.
Guerrini R, Marini C, Mantegazza M. Genetic epilepsy syndromes without structural brain abnormalities: clinical features and experimental models. Neurotherapeutics. 2014;11:269–85.
Lerche H, Shah M, Beck H, Noebels J, Johnston D, Vincent A. Ion channels in genetic and acquired forms of epilepsy. J Physiol. 2013;591(4):753–64.
Mantegazza M, Catterall WA. Voltage-Gated Na+ Channels: Structure, Function, and Pathophysiology. 4th ed. Jasper’s Basic Mechanisms of the Epilepsies. 2012.
Catterall WA, Swanson TM. Structural basis for pharmacology of voltage-gated sodium and calcium channels. Mol Pharmacol. 2015;88(1):141–50.
Article CAS PubMed PubMed Central Google Scholar
Catterall WA. From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron. 2000;26(1):13–25.
Beneski DA, Catterall WA. Covalent labeling of protein components of the sodium channel with a photoactivable derivative of scorpion toxin. Proc Natl Acad Sci U S A. 1980;77(1):639–43.
Yu FH, Catterall WA. Overview of the voltage-gated sodium channel family. Genome Biol. 2003;4(3):207.
Shi X, Yasumoto S, Nakagawa E, Fukasawa T, Uchiya S, Hirose S. Missense mutation of the sodium channel gene SCN2A causes Dravet syndrome. Brain and Dev. 2009;31(10):758–62.
Article Google Scholar
O’Malley HA, Isom LL. Sodium channel β subunits: emerging targets in channelopathies. Annu Rev Physiol. 2015;10(77):481–504.
Catterall WA. Voltage-gated sodium channels at 60: structure, function and pathophysiology. J Physiol. 2012;590(11):2577–89.
de Lera RM, Kraus RL. Voltage-gated sodium channels: structure, function, pharmacology, and clinical indications. J Med Chem. 2015;58(18):7093–118.
Noda M, Hiyama TY. The Na(x) channel: what it is and what it does. Neuroscientist. 2015;21(4):399–412.
Clairfeuille T, Cloake A, Infield DT, Llongueras JP, Arthur CP, Li ZR, et al. Structural basis of α-scorpion toxin action on Nav channels. Science. 2019;363(6433):eaav8573.
Catterall WA, Goldin AL, Waxman SG. International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol Rev. 2005;57(4):397–409.
Chung S, Skinner J, Rees M. Molecular genetics of arrhythmias. Principles and Practice of Clinical Cardiovascular Genetics. Oxford: Oxford University Press; 2010. p. 239–49.
Google Scholar
Lupoglazoff JM, Cheav T, Baroudi G, Berthet M, Denjoy I, Cauchemez B, et al. Homozygous SCN5A mutation in long-QT syndrome with functional two-to-one atrioventricular block. Circ Res. 2001;89(2):E16-21.
Baroudi G, Chahine M. Biophysical phenotypes of SCN5A mutations causing long QT and Brugada syndromes. FEBS Lett. 2000;487(2):224–8.
Baroudi G, Carbonneau E, Pouliot V, Chahine M. SCN5A mutation (T1620M) causing Brugada syndrome exhibits different phenotypes when expressed in Xenopus oocytes and mammalian cells. FEBS Lett. 2000;467(1):12–6.
Rivolta I, Abriel H, Tateyama M, Liu H, Memmi M, Vardas P, et al. Inherited Brugada and long QT-3 syndrome mutations of a single residue of the cardiac sodium channel confer distinct channel and clinical phenotypes. J Biol Chem. 2001;276(33):30623–30.
Isom LL, De Jongh KS, Catterall WA. Auxiliary subunits of voltage-gated ion channels. Neuron. 1994;12(6):1183–94.
Brackenbury WJ, Isom LL. Na+ Channel β Subunits: Overachievers of the Ion Channel Family. Front Pharmacol. 2011;2:53.
Chahine M, O’Leary ME. Regulatory role of voltage-gated Na+ channel β subunits in sensory neurons. Front Pharmacol. 2011;2:70.
Namadurai S, Balasuriya D, Rajappa R, Wiemhöfer M, Stott K, Klingauf J, et al. Crystal structure and molecular imaging of the Nav channel β3 subunit indicates a trimeric assembly. J Biol Chem. 2014;289(15):10797–811.
Waxman SG. Axonal conduction and injury in multiple sclerosis: the role of sodium channels. Nat Rev Neurosci. 2006;7(12):932–41.
Freitag CM, May TW, Pfäfflin M, König S, Rating D. Incidence of epilepsies and epileptic syndromes in children and adolescents: a population-based prospective study in Germany. Epilepsia. 2001;42(8):979–85.
Steinlein OK. Genetic mechanisms that underlie epilepsy. Nat Rev Neurosci. 2004;5(5):400–8.
Weber YG, Lerche H. Genetic mechanisms in idiopathic epilepsies. Dev Med Child Neurol. 2008;50(9):648–54.
Wallace RH, Wang DW, Singh R, Scheffer IE, George AL, Phillips HA, et al. Febrile seizures and generalized epilepsy associated with a mutation in the Na+-channel ß1 subunit gene SCN1B. Nat Genet. 1998;19(4):366–70.
Buchhalter JR. Animal models of inherited epilepsy. Epilepsia. 1993;34:S31-41.
Stein RE, Kaplan JS, Li J, Catterall WA. Hippocampal deletion of NaV1. 1 channels in mice causes thermal seizures and cognitive deficit characteristic of Dravet Syndrome. Proc Natl Acad Sci U S A. 2019;116(33):16571–6.
Oyrer J, Maljevic S, Scheffer IE, Berkovic SF, Petrou S, Reid CA. Ion channels in genetic epilepsy: from genes and mechanisms to disease-targeted therapies. Pharmacol Rev. 2018;70(1):142–73.
Steinlein OK, Mulley JC, Propping P, Wallace RH, Phillips HA, Sutherland GR, et al. A missense mutation in the neuronal nicotinic acetylcholine receptor α4 subunit is associated with autosomal dominant nocturnal frontal lobe epilepsy. Nat Genet. 1995;11(2):201–3.
Alabi AA, Bahamonde MI, Jung HJ, Kim JI, Swartz KJ. Portability of paddle motif function and pharmacology in voltage sensors. Nature. 2007;450(7168):370–5.
Stafstrom CE. Persistent sodium current and its role in epilepsy. Epilepsy Curr. 2007;7(1):15–22.
Eijkelkamp N, Linley JE, Baker MD, Minett MS, Cregg R, Werdehausen R, et al. Neurological perspectives on voltage-gated sodium channels. Brain. 2012;135(9):2585–612.
Oliva M, Berkovic SF, Petrou S. Sodium channels and the neurobiology of epilepsy. Epilepsia. 2012;53(11):1849–59.
Marini C, Scheffer IE, Nabbout R, Suls A, De Jonghe P, Zara F, et al. The genetics of Dravet syndrome. Epilepsia. 2011;52:24–9.
Fujiwara T, Sugawara T, Mazaki-Miyazaki E, Takahashi Y, Fukushima K, Watanabe M, et al. Mutations of sodium channel α subunit type 1 (SCN1A) in intractable childhood epilepsies with frequent generalized tonic–clonic seizures. Brain. 2003;126(3):531–46.
DeLorenzo RJ, Sun DA, Deshpande LS. Erratum to “Cellular mechanisms underlying acquired epilepsy: the calcium hypothesis of the induction and maintenance of epilepsy.” Pharmacol Ther. 2005;105(3):229–66 Pharmacol Ther. 2006;111(1):288-325.
Wengert ER, Patel MK. The role of the persistent sodium current in epilepsy. Epilepsy Curr. 2021;21(1):40–7.
Stafstrom CE. Severe epilepsy syndromes of early childhood: the link between genetics and pathophysiology with a focus on SCN1A mutations. J Child Neurol. 2009;24(8_suppl):15S-23S.
Maljevic S, Lerche H. Potassium channels: a review of broadening therapeutic possibilities for neurological diseases. J Neurol. 2013;260:2201–11.
Rogawski MA, Löscher W. The neurobiology of antiepileptic drugs. Nat Rev Neurosci. 2004;5(7):553–64.
Sills GJ, Rogawski MA. Mechanisms of action of currently used antiseizure drugs. Neuropharmacology. 2020;168:107966.
Brodie MJ. Sodium channel blockers in the treatment of epilepsy. CNS Drugs. 2017;31(7):527–34.
Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen Hauser W, Mathern G, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51(6):1069–77.
Khoury GA, Baliban RC, Floudas CA. Proteome-wide post-translational modification statistics: frequency analysis and curation of the swiss-prot database. Sci Rep. 2011;1:90.
Berendt FJ, Park KS, Trimmer JS. Multisite phosphorylation of voltage-gated sodium channel α subunits from rat brain. J Proteome Res. 2010;9(4):1976–84.
Baek JH, Cerda O, Trimmer JS. Mass spectrometry-based phosphoproteomics reveals multisite phosphorylation on mammalian brain voltage-gated sodium and potassium channels. Semin Cell Dev Biol. 2011;22(2):153–9.
Li M, West JW, Numann R, Murphy BJ, Scheuer T, Catterall WA. Convergent regulation of sodium channels by protein kinase C and cAMP-dependent protein kinase. Science. 1993;261(5127):1439–42.
West JW, Numann R, Murphy BJ, Scheuer T, Catterall WA. A phosphorylation site in the Na+ channel required for modulation by protein kinase C. Science. 1991;254(5033):866–8.
Numann R, Catterall WA, Scheuer T. Functional modulation of brain sodium channels by protein kinase C phosphorylation. Science. 1991;254(5028):115–8.
Li M, West JW, Lai Y, Scheuer T, Catterall WA. Functional modulation of brain sodium channels by cAMP-dependent phosphorylation. Neuron. 1992;8(6):1151–9.
Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev. 2005;57(4):411–25.
Cantrell AR, Catterall WA. Neuromodulation of Na+ channels: An unexpected form of cellular platicity. Nat Rev Neurosci. 2001;2(6):397–407.
James TF, Nenov MN, Wildburger NC, Lichti CF, Luisi J, Vergara F, et al. The Nav1. 2 channel is regulated by GSK3. Biochim Biophys Acta. 2015;1850(4):832–44.
Hien YE, Montersino A, Castets F, Leterrier C, Filhol O, Vacher H, et al. CK2 accumulation at the axon initial segment depends on sodium channel Nav1. FEBS Lett. 2014;588(18):3403–8.
Few WP, Scheuer T, Catterall WA. Dopamine modulation of neuronal Na+ channels requires binding of A kinase-anchoring protein 15and PKA by a modified leucine zipper motif. Proc Natl Acad Sci U S A. 2007;104(12):5187–92.
Beacham D, Ahn M, Catterall WA, Scheuer T. Sites and molecular mechanisms of modulation of NaV1. 2 channels by Fyn tyrosine kinase. J Neurosci. 2007;27(43):11543–51.
Wittmack EK, Rush AM, Hudmon A, Waxman SG, Dib-Hajj SD. Voltage-gated sodium channel Nav1. 6 is modulated by p38 mitogen-activated protein kinase. J Neurosci. 2005;25(28):6621–30.
Tai TY, Warner LN, Jones TD, Jung S, Concepcion FA, Skyrud DW, et al. Antiepileptic action of c-Jun N-terminal kinase (JNK) inhibition in an animal model of temporal lobe epilepsy. Neurosci. 2017;349:35–47.
Article CAS Google Scholar
Bréchet A, Fache MP, Brachet A, Ferracci G, Baude A, Irondelle M, et al. Protein kinase CK2 contributes to the organization of sodium channels in axonal membranes by regulating their interactions with ankyrin G. J Cell Biol. 2008;183(6):1101–14.
Baek JH, Rubinstein M, Scheuer T, Trimmer JS. Reciprocal changes in phosphorylation and methylation of mammalian brain sodium channels in response to seizures. J Biol Chem. 2014;289(22):15363–73.
Shank RP, Maryanoff BE. Molecular pharmacodynamics, clinical therapeutics, and pharmacokinetics of topiramate. CNS Neurosci Ther. 2008;14(2):120–42.
Cohen P. Protein kinases–the major drug targets of the twenty-first century? Nat Rev Drug Discov. 2002;1(4):309–15.
Chico LK, Van Eldik LJ, Watterson DM. Targeting protein kinases in central nervous system disorders. Nat Rev Drug Discov. 2009;8(11):892–909.
Hondeghem LM. Antiarrhythmic agents: modulated receptor applications. Circulation. 1987;75(3):514–20.
Jensen HS, Grunnet M, Bastlund JF. Therapeutic potential of Na(V)1.1 activators. Trends Pharmacol Sci. 2014;35(3):113–8.
Adler EM, Yaari Y, David G, Selzer ME. Frequency-dependent action of phenytoin on lamprey spinal axons. Brain Res. 1986;362(2):271–80.
Mantegazza M, Curia G, Biagini G, Ragsdale DS, Avoli M. Voltage-gated sodium channels as therapeutic targets in epilepsy and other neurological disorders. Lancet Neurol. 2010;9(4):413–24.
Maljevic S, Reid CA, Petrou S. Models for discovery of targeted therapy in genetic epileptic encephalopathies. J Neurochem. 2017;143(1):30–48.
Cheah CS, Yu FH, Westenbroek RE, Kalume FK, Oakley JC, Potter GB, et al. Specific deletion of NaV1. 1 sodium channels in inhibitory interneurons causes seizures and premature death in a mouse model of Dravet syndrome. Proc Natl Acad Sci. 2012;109(36):14646–51.
Ogiwara I, Miyamoto H, Morita N, Atapour N, Mazaki E, Inoue I, et al. Nav1. 1 localizes to axons of parvalbumin-positive inhibitory interneurons: a circuit basis for epileptic seizures in mice carrying an Scn1a gene mutation. J Neurosci. 2007;27(22):5903–14.
Catterall WA. Sodium channels, inherited epilepsy, and antiepileptic drugs. Annu Rev Pharmacol Toxicol. 2014;54:317–38.
Ademuwagun IA, Rotimi SO, Syrbe S, Ajamma YU, Adebiyi E. Voltage gated sodium channel genes in epilepsy: mutations, functional studies, and treatment dimensions. Front Neurol. 2021;12:600050.
Catterall WA, Kalume F, Oakley JC. NaV1.1 channels and epilepsy. J Physiol. 2010;588(Pt 11):1849–59.
Suls A, Jaehn JA, Kecskés A, Weber Y, Weckhuysen S, Craiu DC, et al. De novo loss-of-function mutations in CHD2 cause a fever-sensitive myoclonic epileptic encephalopathy sharing features with Dravet syndrome. Am J Hum Genet. 2013;93(5):967–75.
Oakley JC, Kalume F, Catterall WA. Insights into pathophysiology and therapy from a mouse model of Dravet syndrome. Epilepsia. 2011;52:59–61.
Wu YW, Sullivan J, McDaniel SS, Meisler MH, Walsh EM, Li SX, et al. Incidence of Dravet syndrome in a US population. Pediatrics. 2015;136(5):e1310–5.
Kaplan DI, Isom LL, Petrou S. Role of sodium channels in epilepsy. Cold Spring Harb Perspect Med. 2016;6(6):a022814.
Armstrong CM, Bezanilla F. Currents related to movement of the gating particles of the sodium channels. Nature. 1973;242(5398):459–61.
Nardi A, Damann N, Hertrampf T, Kless A. Advances in targeting voltage-gated sodium channels with small molecules. ChemMedChem. 2012;7(10):1712–40.
Oakley JC, Cho AR, Cheah CS, Scheuer T, Catterall WA. Synergistic GABA-enhancing therapy against seizures in a mouse model of Dravet syndrome. J Pharmacol Exp Ther. 2013;345(2):215–24.
Kalume F, Westenbroek RE, Cheah CS, Frank HY, Oakley JC, Scheuer T, et al. Sudden unexpected death in a mouse model of Dravet syndrome. J Clin Invest. 2013;123(4):1798–808.
Cheah CS, Westenbroek RE, Roden WH, Kalume F, Oakley JC, Jansen LA, et al. Correlations in timing of sodium channel expression, epilepsy, and sudden death in Dravet syndrome. Channels. 2013;7(6):468–72.
Han Z, Chen C, Christiansen A, Ji S, Lin Q, Anumonwo C, et al. Antisense oligonucleotides increase Scn1a expression and reduce seizures and SUDEP incidence in a mouse model of Dravet syndrome. Sci Transl Med. 2020;12(558):eaaz6100.
Sankar R, de Menezes MS. Metabolic and endocrine aspects of the ketogenic diet. Epilepsy Res. 1999;37(3):191–201.
Chiron C. Current therapeutic procedures in Dravet syndrome. Dev Med Child Neurol. 2011;53:16–8.
Jaworski T. Control of neuronal excitability by GSK-3beta: Epilepsy and beyond. Biochim Biophys Acta Mol Cell Res. 2020;1867(9):118745.
Cutts A, Savoie H, Hammer MF, Schreiber J, Grayson C, Luzon C, et al. Clinical characteristics and treatment experience of individuals with SCN8A developmental and epileptic encephalopathy (SCN8A-DEE): Findings from an online caregiver survey. Seizure. 2022;97:50–7.
Møller RS, Johannesen KM. Precision medicine: SCN8A encephalopathy treated with sodium channel blockers. Neurotherapeutics. 2016;13(1):190–1.
Orsini A, Esposito M, Perna D, Bonuccelli A, Peroni D, Striano P. Personalized medicine in epilepsy patients. J Transl Genet Genom. 2018;2:16.
Johnson JP, Focken T, Khakh K, Tari PK, Dube C, Goodchild SJ. NBI-921352, a first-in-class, NaV1. 6 selective, sodium channel inhibitor that prevents seizures in Scn8a gain-of-function mice, and wild-type mice and rats. Elife. 2022;11:e72468.
Bender AC, Morse RP, Scott RC, Holmes GL, Lenck-Santini PP. SCN1A mutations in Dravet syndrome: impact of interneuron dysfunction on neural networks and cognitive outcome. Epilepsy Behav. 2012;23(3):177–86.
Scheffer IE, Nabbout R. SCN1A-related phenotypes: epilepsy and beyond. Epilepsia. 2019;60:S17-24.
Layer N, Sonnenberg L, Pardo González E, Benda J, Hedrich UBS, Lerche H, et al. Dravet Variant SCN1AA1783V impairs interneuron firing predominantly by altered channel activation. Front Cell Neurosci. 2021;15:754530.
Kotler O, Khrapunsky Y, Shvartsman A, Dai H, Plant LD, Goldstein SA, et al. SUMOylation of NaV1. 2 channels regulates the velocity of backpropagating action potentials in cortical pyramidal neurons. Elife. 2023;12:e81463.
Spratt PW, Alexander RP, Ben-Shalom R, Sahagun A, Kyoung H, Keeshen CM, et al. Paradoxical hyperexcitability from NaV1 2 sodium channel loss in neocortical pyramidal cells. Cell Rep. 2021;36(5):109483.
Hu W, Tian C, Li T, Yang M, Hou H, Shu Y. Distinct contributions of Na(v)1.6 and Na(v)1.2 in action potential initiation and backpropagation. Nat Neurosci. 2009;12(8):996–1002.
Schafer DP, Custer AW, Shrager P, Rasband MN. Early events in node of Ranvier formation during myelination and remyelination in the PNS. Neuron Glia Biol. 2006;2(2):69–79.
Shah BS, Stevens EB, Pinnock RD, Dixon AK, Lee K. Developmental expression of the novel voltage-gated sodium channel auxiliary subunit β3, in rat CNS. J Physiol. 2001;534(Pt 3):763–76.
Misra SN, Kahlig KM, George AL Jr. Impaired NaV1. 2 function and reduced cell surface expression in benign familial neonatal-infantile seizures. Epilepsia. 2008;49(9):1535–45.
Sugawara T, Tsurubuchi Y, Agarwala KL, Ito M, Fukuma G, Mazaki-Miyazaki E, et al. A missense mutation of the Na+ channel alpha II subunit gene Na(v)1.2 in a patient with febrile and afebrile seizures causes channel dysfunction. Proc Natl Acad Sci U S A. 2001;98(11):6384–9.
Scalmani P, Rusconi R, Armatura E, Zara F, Avanzini G, Franceschetti S, et al. Effects in neocortical neurons of mutations of the Na(v)1.2 Na+ channel causing benign familial neonatal-infantile seizures. J Neurosci. 2006;26(40):10100–9.
Que Z, Olivero-Acosta MI, Zhang J, Eaton M, Tukker AM, Chen X, et al. Hyperexcitability and pharmacological responsiveness of cortical neurons derived from human iPSCs carrying epilepsy-associated sodium channel Nav 1. 2–L1342P genetic variant. J Neurosci. 2021;41(49):10194–208.
Zavala-Tecuapetla C, Manjarrez-Marmolejo J, Ramírez-Jarquín JO, Rivera-Cerecedo CV. Eslicarbazepine, but not lamotrigine or ranolazine, shows anticonvulsant efficacy in carbamazepine-resistant rats developed by window-pentylenetetrazole kindling. Brain Sci. 2022;12(5):629.
Vacher H, Mohapatra DP, Trimmer JS. Localization and targeting of voltage-dependent ion channels in mammalian central neurons. Physiol Rev. 2008;88(4):1407–47.
Hill AS, Nishino A, Nakajo K, Zhang G, Fineman JR, Selzer ME, et al. Ion channel clustering at the axon initial segment and node of Ranvier evolved sequentially in early chordates. PLoS Genet. 2008;4(12):e1000317.
Menezes LF, Sabiá Júnior EF, Tibery DV, Carneiro LD, Schwartz EF. Epilepsy-related voltage-gated sodium channelopathies: a review. Front Pharmacol. 2020;11:1276.
Blumenfeld H, Lampert A, Klein JP, Mission J, Chen MC, Rivera M, et al. Role of hippocampal sodium channel Nav1. 6 in kindling epileptogenesis. Epilepsia. 2009;50(1):44–55.
Hargus NJ, Nigam A, Bertram EH III, Patel MK. Evidence for a role of Nav1. 6 in facilitating increases in neuronal hyperexcitability during epileptogenesis. J Neurophysiol. 2013;110(5):1144–57.
Johannesen KM, Gardella E, Scheffer I, Howell K, Smith DM, Helbig I, et al. Early mortality in SCN8A-related epilepsies. Epilepsy Res. 2018;143:79–81.
Wang J, Gao H, Bao X, Zhang Q, Li J, Wei L, et al. SCN8A mutations in Chinese patients with early onset epileptic encephalopathy and benign infantile seizures. BMC Med Genet. 2017;18(1):1.
Meisler MH, Helman G, Hammer MF, Fureman BE, Gaillard WD, Goldin AL, et al. SCN8A encephalopathy: research progress and prospects. Epilepsia. 2016;57(7):1027–35.
Arafat A, Jing P, Ma Y, Pu M, Nan G, Fang H, et al. Unexplained early infantile epileptic encephalopathy in Han Chinese children: next-generation sequencing and phenotype enriching. Sci Rep. 2017;7(1):46227.
Mastrangelo M, Leuzzi V. Genes of early-onset epileptic encephalopathies: from genotype to phenotype. Pediatr Neurol. 2012;46(1):24–31.
Gardella E, Marini C, Trivisano M, Fitzgerald MP, Alber M, Howell KB, Darra F, Siliquini S, Bölsterli BK, Masnada S, Pichiecchio A. The phenotype of SCN8A developmental and epileptic encephalopathy. Neurology. 2018;91(12):e1112–24.
Blair RD. Temporal lobe epilepsy semiology. Epilepsy Res Treat. 2012;2012:751510.
Whitaker WR, Faull RL, Dragunow M, Mee EW, Emson PC, Clare JJ. Changes in the mRNAs encoding voltage-gated sodium channel types II and III in human epileptic hippocampus. Neuroscience. 2001;106(2):275–85.
Vreugdenhil M, Hoogland G, Van Veelen CW, Wadman WJ. Persistent sodium current in subicular neurons isolated from patients with temporal lobe epilepsy. Eur J Neurosci. 2004;19(10):2769–78.
Rosker C, Lohberger B, Hofer D, Steinecker B, Quasthoff S, Schreibmayer W. The TTX metabolite 4,9-anhydro-TTX is a highly specific blocker of the Na(v1.6) voltage-dependent sodium channel. Am J Physiol Cell Physiol. 2007;293(2):C783-9.
Hargus NJ, Merrick EC, Nigam A, Kalmar CL, Baheti AR, Bertram EH III, et al. Temporal lobe epilepsy induces intrinsic alterations in Na channel gating in layer II medial entorhinal cortex neurons. Neurobiol Dis. 2011;41(2):361–76.
Wagnon JL, Meisler MH. Recurrent and non-recurrent mutations of SCN8A in epileptic encephalopathy. Front Neurol. 2015;6:104.
Maguire MJ, Jackson CF, Marson AG, Nevitt SJ. Treatments for the prevention of Sudden Unexpected Death in Epilepsy (SUDEP). Cochrane Database Syst Rev. 2016;7(7):CD011792.
PubMed Google Scholar
Larsen J, Carvill GL, Gardella E, Kluger G, Schmiedel G, Barisic N, et al. The phenotypic spectrum of SCN8A encephalopathy. Neurology. 2015;84(5):480–9.
Boerma RS, Braun KP, van de Broek MP, van Berkestijn FM, Swinkels ME, Hagebeuk EO, et al. Remarkable phenytoin sensitivity in 4 children with SCN8A-related epilepsy: a molecular neuropharmacological approach. Neurotherapeutics. 2016;13:192–7.
Kuo CC, Bean BP. Slow binding of phenytoin to inactivated sodium channels in rat hippocampal neurons. Mol Pharmacol. 1994;46(4):716–25.
CAS PubMed Google Scholar
Barker BS, Ottolini M, Wagnon JL, Hollander RM, Meisler MH, Patel MK. The SCN8A encephalopathy mutation p. Ile1327Val displays elevated sensitivity to the anticonvulsant phenytoin. Epilepsia. 2016;57(9):1458–66.
Braakman HM, Verhoeven JS, Erasmus CE, Haaxma CA, Willemsen MH, Schelhaas HJ. Phenytoin as a last-resort treatment in SCN 8A encephalopathy. Epilepsia Open. 2017;2(3):343–4.
Ko A, Kang HC. Frequently identified genetic developmental and epileptic encephalopathy: a review focusing on precision medicine. Ann Child Neurol. 2019;27(1):2–12.
Löscher W, Klein P. The pharmacology and clinical efficacy of antiseizure medications: from bromide salts to cenobamate and beyond. CNS Drugs. 2021;35(9):935–63.
Fattorusso A, Matricardi S, Mencaroni E, Dell’Isola GB, Di Cara G, Striano P, et al. The pharmacoresistant epilepsy: an overview on existant and new emerging therapies. Front Neurol. 2021;12:674483.
Androsova G, Krause R, Borghei M, Wassenaar M, Auce P, Avbersek A, et al. Comparative effectiveness of antiepileptic drugs in patients with mesial temporal lobe epilepsy with hippocampal sclerosis. Epilepsia. 2017;58(10):1734–41.
Vreugdenhil M, Wadman WJ. Modulation of sodium currents in rat CA1 neurons by carbamazepine and valproate after kindling epileptogenesis. Epilepsia. 1999;40(11):1512–22.
Remy S, Gabriel S, Urban BW, Dietrich D, Lehmann TN, Elger CE, et al. A novel mechanism underlying drug resistance in chronic epilepsy. Ann Neurol. 2003;53(4):469–79.
Schaub C, Uebachs M, Beck H. Diminished response of CA1 neurons to antiepileptic drugs in chronic epilepsy. Epilepsia. 2007;48(7):1339–50.
Remy S, Urban BW, Elger CE, Beck H. Anticonvulsant pharmacology of voltage-gated Na+ channels in hippocampal neurons of control and chronically epileptic rats. Eur J Neurosci. 2003;17(12):2648–58.
Jeub M, Beck H, Siep E, Rüschenschmidt C, Speckmann EJ, Ebert U. Effect of phenytoin on sodium and calcium currents in hippocampal CA1 neurons of phenytoin-resistant kindled rats. Neuropharmacology. 2002;42(1):107–16.
Alexopoulos AV. Pharmacoresistant epilepsy: definition and explanation. Epileptology. 2013;1(1):38–42.
Sourbron J, Auvin S, Arzimanoglou A, Cross JH, Hartmann H, Pressler R, et al. Medical treatment in infants and young children with epilepsy: Off-label use of antiseizure medications. Survey Report of ILAE Task Force Medical Therapies in Children. Epilepsia Open. 2023;8(1):77–89.
Asadi-Pooya AA, Beniczky S, Rubboli G, Sperling MR, Rampp S, Perucca E. A pragmatic algorithm to select appropriate antiseizure medications in patients with epilepsy. Epilepsia. 2020;61(8):1668–77.
Nolan SJ, Tudur Smith C, Weston J, Marson AG. Lamotrigine versus carbamazepine monotherapy for epilepsy: an individual participant data review. Cochrane Database Syst Rev. 2016;11(11):CD001031.
Gambardella A, Labate A, Colosimo E, Ambrosio R, Quattrone A. Monotherapy for partial epilepsy: focus on levetiracetam. Neuropsychiatr Dis Treat. 2008;4(1):33–8.
Aras LM, Isla J, Mingorance-Le MA. The European patient with Dravet syndrome: results from a parent-reported survey on antiepileptic drug use in the European population with Dravet syndrome. Epilepsy Behav. 2015;44:104–9.
Barker-Haliski M, White HS. Validated animal models for antiseizure drug (ASD) discovery: advantages and potential pitfalls in ASD screening. Neuropharmacology. 2020;167:107750.
Santulli L, Coppola A, Balestrini S, Striano S. The challenges of treating epilepsy with 25 antiepileptic drugs. Pharmacol Res. 2016;107:211–9.
Gaily E, Anttonen AK, Valanne L, Liukkonen E, Träskelin AL, Polvi A, et al. Dravet syndrome: new potential genetic modifiers, imaging abnormalities, and ictal findings. Epilepsia. 2013;54(9):1577–85.
Dressler A, Trimmel-Schwahofer P, Reithofer E, Mühlebner A, Gröppel G, Reiter-Fink E, et al. Efficacy and tolerability of the ketogenic diet in Dravet syndrome–comparison with various standard antiepileptic drug regimen. Epilepsy Res. 2015;109:81–9.
Inoue Y, Ohtsuka Y, Study Group. Long-term safety and efficacy of stiripentol for the treatment of Dravet syndrome: a multicenter, open-label study in Japan. Epilepsy Res. 2015;113:90–7.
Xu C, Zhang Y, Gozal D, Carney P. Channelopathy of Dravet syndrome and potential neuroprotective effects of cannabidiol. J Cent Nerv Syst Dis. 2021;13:11795735211048044.
Amrutkar C, Riel-Romero RM. Lennox Gastaut Syndrome. In: StatPearls. Treasure Island: StatPearls Publishing; 2023.
Auvin S, Damera V, Martin M, Holland R, Simontacchi K, Saich A. The impact of seizure frequency on quality of life in patients with Lennox-Gastaut syndrome or Dravet syndrome. Epilepsy Behav. 2021;123:108239.
Abu Saleh T, Stephen L. Lennox gastaut syndrome, review of the literature and a case report. Head Face Med. 2008;4:1–7.
Camfield PR. Definition and natural history of Lennox-Gastaut syndrome. Epilepsia. 2011;52:3–9.
Nordli RD. Epileptic encephalopathies in infancy and early childhood: Overview. Atlas of Epilepsies. Oxford: Springer-Verlag London Limited; 2010. p. 881–3.
Romoli M, Mazzocchetti P, D’Alonzo R, Siliquini S, Rinaldi VE, Verrotti A, et al. Valproic acid and epilepsy: from molecular mechanisms to clinical evidences. Curr Neuropharmacol. 2019;17(10):926–46.
Sazgar M, Bourgeois BF. Aggravation of epilepsy by antiepileptic drugs. Pediatr Neurol. 2005;33(4):227–34.
Devi N, Madaan P, Ameen R, Sahu JK, Bansal D. Short-term and long-term efficacy and safety of antiseizure medications in Lennox Gastaut syndrome: a network meta-analysis. Seizure. 2022;99:164–75.
Guerreiro MM, Manreza ML, Scotoni AE, Silva EA, Guerreiro CA, Souza EA, et al. A pilot study of topiramate in children with Lennox-Gastaut syndrome. Arq Neuropsiquiatr. 1999;57(2A):167–75.
Coppola G, Caliendo G, Veggiotti P, Romeo A, Tortorella G, De Marco P, et al. Topiramate as add-on drug in children, adolescents and young adults with Lennox-Gastaut syndrome: an Italian multicentric study. Epilepsy Res. 2002;51(1–2):147–53.
Tartara A, Sartori I, Manni R, Galimberti CA, Di Fazio M, Perucca E. Efficacy and safety of topiramate in refractory epilepsy: a long-term prospective trial. Ital J Neurol Sci. 1996;17:429–32.
Grosso S, Franzoni E, Iannetti P, Incorpora G, Cardinali C, Toldo I, et al. Efficacy and safety of topiramate in refractory epilepsy of childhood: long-term follow-up study. J Child Neurol. 2005;20(11):893–7.
Conradsen I, Wolf P, Sams T, Sorensen HB, Beniczky S. Patterns of muscle activation during generalized tonic and tonic–clonic epileptic seizures. Epilepsia. 2011;52(11):2125–32.
Langtry HD, Gillis JC, Davis R. Topiramate: a review of its pharmacodynamic and pharmacokinetic properties and clinical efficacy in the management of epilepsy. Drugs. 1997;54:752–73.
Balagura G, Riva A, Marchese F, Verrotti A, Striano P. Adjunctive rufinamide in children with Lennox-Gastaut syndrome: a literature review. Neuropsychiatr Dis Treat. 2020;16:369–79.
Kluger G, Bauer B. Role of rufinamide in the management of Lennox-Gastaut syndrome (childhood epileptic encephalopathy). Neuropsychiatr Dis Treat. 2007;3(1):3–11.
Article CAS PubMed Central Google Scholar
Kim SH, Eun SH, Kang HC, Kwon EJ, Byeon JH, Lee YM, et al. Rufinamide as an adjuvant treatment in children with Lennox-Gastaut syndrome. Seizure. 2012;21(4):288–91.
Coppola G, Grosso S, Franzoni E, Veggiotti P, Zamponi N, Parisi P, et al. Rufinamide in children and adults with Lennox-Gastaut syndrome: first Italian multicenter experience. Seizure. 2010;19(9):587–91.
You SJ, Kang HC, Kim HD, Lee HS, Ko TS. Clinical efficacy of zonisamide in Lennox-Gastaut syndrome: Korean multicentric experience. Brain Dev. 2008;30(4):287–90.
Cross JH, Auvin S, Falip M, Striano P, Arzimanoglou A. Expert opinion on the management of Lennox-Gastaut syndrome: treatment algorithms and practical considerations. Front Neurol. 2017;8:505.
Yamauchi T, Aikawa H. Efficacy of zonisamide: our experience. Seizure. 2004;13:S41–8.
Karimzadeh P, Ashrafi MR, Bali MK, Nasehi MM, Otaghsara SM, Taghdiri MM, et al. Zonisamide efficacy as adjunctive therapy in children with refractory epilepsy. Iran J Child Neurol. 2013;7(2):37.
Motte J, Trevathan E, Arvidsson JF, Barrera MN, Mullens EL, Manasco P, et al. Lamotrigine for generalized seizures associated with the Lennox-Gastaut syndrome. N Engl J Med. 1997;337(25):1807–12.
Schapel GJ, Beran RG, Vajda FJ, Berkovic SF, Mashford ML, Dunagan FM, et al. Double-blind, placebo controlled, crossover study of lamotrigine in treatment resistant partial seizures. J Neurol Neurosurg Psychiatry. 1993;56(5):448–53.
Buchanan N. The efficacy of lamotrigine on seizure control in 34 children, adolescents and young adults with intellectual and physical disability. Seizure. 1995;4(3):233–6.
Marciani MG, Spanedda F, Bassetti MA, Maschio M, Gigli GL, Mattia D, et al. Effect of lamotrigine on EEG paroxysmal abnormalities and background activity: a computerized analysis. Br J Clin Pharmacol. 1996;42(5):621–7.
Boas J, Dam M, Friis ML, Kristensen O, Pedersen B, Gallagher J. Controlled trial of lamotrigine (Lamictal®) for treatment-resistant partial seizures. Acta Neurol Scand. 1996;94(4):247–52.
Schmidt D, Bourgeois B. A risk-benefit assessment of therapies for Lennox-Gastaut syndrome. Drug Saf. 2000;22(6):467–77.
Saitsu H, Kato M, Okada I, Orii KE, Higuchi T, Hoshino H, et al. STXBP1 mutations in early infantile epileptic encephalopathy with suppression-burst pattern. Epilepsia. 2010;51(12):2397–405.
Wu MJ, Hu CH, Ma JH, Hu JS, Liu ZS, Sun D. Early infantile epileptic encephalopathy caused by PACS2 gene variation: three cases report and literature review. Zhonghua Er Ke Za Zhi. 2021;59(7):594–9 (Chinese).
Zhong M, Liao S, Li T, Wu P, Wang Y, Wu F, et al. Early diagnosis improving the outcome of an infant with epileptic encephalopathy with cytoplasmic FMRP interacting protein 2 mutation: case report and literature review. Medicine(Baltimore). 2019;98(44):e17749.
Dilena R, Striano P, Gennaro E, Bassi L, Olivotto S, Tadini L, et al. Efficacy of sodium channel blockers in SCN2A early infantile epileptic encephalopathy. Brain Dev. 2017;39(4):345–8.
Guberman A. Monotherapy or polytherapy for epilepsy? Can J Neurol Sci. 1998;25(S4):S3-8.
Morris JC, Dodson WE, Hatlelid JM, Ferrendelli JA. Phenytoin and carbamazepine, alone and in combination: anticonvulsant and neurotoxic effects. Neurology. 1987;37(7):1111.
Sperling MR, Harvey J, Grinnell T, Cheng H, Blum D, 045 Study Team. Efficacy and safety of conversion to monotherapy with eslicarbazepine acetate in adults with uncontrolled partial-onset seizures: a randomized historical-control phase III study based in North America. Epilepsia. 2015;56(4):546–55.
Jacobson MP, Pazdera L, Bhatia P, Grinnell T, Cheng H, Blum D, et al. Efficacy and safety of conversion to monotherapy with eslicarbazepine acetate in adults with uncontrolled partial-onset seizures: a historical-control phase III study. BMC Neurol. 2015;15:46.
Sperling MR, French J, Jacobson MP, Pazdera L, Gough M, Cheng H, et al. Conversion to eslicarbazepine acetate monotherapy: a pooled analysis of 2 phase III studies. Neurology. 2016;86(12):1095–102.
Krauss G, Biton V, Harvey JH, Elger C, Trinka E, da Silva PS, et al. Influence of titration schedule and maintenance dose on the tolerability of adjunctive eslicarbazepine acetate: an integrated analysis of three randomized placebo-controlled trials. Epilepsy Res. 2018;139:1–8.
Jallon P, Picard F. Bodyweight gain and anticonvulsants: a comparative review. Drug Saf. 2001;24:969–78.
Kwan P, Brodie MJ. Drug treatment of epilepsy: when does it fail and how to optimize its use? CNS Spectr. 2004;9(2):110–9.
Mattson RH, Cramer JA, Collins JF, Smith DB, Delgado-Escueta AV, Browne TR, et al. Comparison of carbamazepine, phenobarbital, phenytoin, and primidone in partial and secondarily generalized tonic–clonic seizures. N Engl J Med. 1985;313(3):145–51.
Lee JW, Dworetzky B. Rational polytherapy with antiepileptic drugs. Pharmaceuticals. 2010;3(8):2362–79.
St Louis EK. Truly “rational” polytherapy: maximizing efficacy and minimizing drug interactions, drug load, and adverse effects. Curr Neuropharmacol. 2009;7(2):96–105.
Deckers CL, Hekster YA, Keyser A, Van Lier HJ, Meinardi H, Renier WO. Monotherapy versus polytherapy for epilepsy: a multicenter double-blind randomized study. Epilepsia. 2001;42(11):1387–94.
Reynolds EH, Shorvon SD. Monotherapy or poly therapy for epilepsy? Epilepsia. 1981;22(1):1.
Czuczwar SJ, Borowicz KK. Polytherapy in epilepsy: the experimental evidence. Epilepsy Res. 2002;52(1):15–23.
Armour DJ, Veitch GB. Is valproate monotherapy a practical possibility in chronically uncontrolled epilepsy? J Clin Pharm Ther. 1988;13(1):53–64.
Harden CL, Zisfein J, Atos-Radzion EC, Tuchman AJ. Combination valproate—carbamazepine therapy in partial epilepsies resistant to carbamazepine monotherapy. J Epilepsy. 1993;6(2):91–4.
Mj B. Yuen AWC and the 105-study group lamotrigine substitution study: evidence for synergism with sodium valproate. Epilepsy Res. 1997;26:423–32.
Stephen LJ, Sills GJ, Brodie MJ. Lamotrigine and topiramate may be a useful combination. Lancet. 1998;351(9107):958–9.
Read CL, Stephen LJ, Stolarek IH, Paul A, Sills GJ, Brodie MJ. Cognitive effects of anticonvulsant monotherapy in elderly patients: a placebo-controlled study. Seizure. 1998;7(2):159–62.
Brodie MJ. Pharmacological treatment of drug-resistant epilepsy in adults: a practical guide. Curr Neurol Neurosci Rep. 2016;16(9):82.
Chang XC, Yuan H, Wang Y, Xu HQ, Hong WK, Zheng RY. Eslicarbazepine acetate add-on therapy for drug-resistant focal epilepsy. Cochrane Database Syst Rev. 2021;6(6):CD008907.
Al‐Bachari S, Pulman J, Hutton JL, Marson AG. Gabapentin add‐on for drug‐resistant partial epilepsy. Cochrane Database Syst Rev. 2013(7):CD001415.
Pulman J, Jette N, Dykeman J, Hemming K, Hutton JL, Marson AG. Topiramate add-on for drug-resistant partial epilepsy. Cochrane Database Syst Rev. 2014;2:CD001417.
Levisohn PM. Safety and tolerability of topiramate in children. J Child Neurol. 2000;15(Suppl 1):S22–6.
Angehagen M, Rönnbäck L, Hansson E, Ben-Menachem E. Topiramate reduces AMPA-induced Ca(2+) transients and inhibits GluR1 subunit phosphorylation in astrocytes from primary cultures. J Neurochem. 2005;94(4):1124–30.
Korinthenberg R, Schreiner A. Topiramate in children with west syndrome: a retrospective multicenter evaluation of 100 patients. J Child Neurol. 2007;22(3):302–6.
van Rijckevorsel K. Treatment of Lennox-Gastaut syndrome: overview and recent findings. Neuropsychiatr Dis Treat. 2008;4(6):1001–19.
Verrotti A, Striano P, Iapadre G, Zagaroli L, Bonanni P, Coppola G, et al. The pharmacological management of Lennox-Gastaut syndrome and critical literature review. Seizure. 2018;63:17–25.
Kwan P, Brodie MJ. Combination therapy in epilepsy: when and what to use. Drugs. 2006;66:1817–29.
Canevini MP, De Sarro G, Galimberti CA, Gatti G, Licchetta L, Malerba A, et al. Relationship between adverse effects of antiepileptic drugs, number of coprescribed drugs, and drug load in a large cohort of consecutive patients with drug-refractory epilepsy. Epilepsia. 2010;51(5):797–804.
Lammers MW, Hekster YA, Keyser A, Meinardi H, Renier WO, Van Lier H. Monotherapy or polytherapy for epilepsy revisited: a quantitative assessment. Epilepsia. 1995;36(5):440–6.
Download references
Acknowledgements
We sincerely thank Professor Nuhu D. Mohammed for critical reading of the manuscript before submission. We are equally grateful to Professor Sambo Zailani, the Provost, College of Medical Sciences, Abubakar Tafawa Balewa University for his professional advice during the draft of this manuscript.
There is no funding for this article.
Author information
Authors and affiliations.
Department of Clinical Pharmacology and Therapeutics, Faculty of Basic Clinical Sciences, College of Medical Sciences, Abubakar Tafawa Balewa University, Bauchi, 740272, Nigeria
John Agbo, Zainab G. Ibrahim, Shehu Y. Magaji & Yahkub Babatunde Mutalub
Department of Physiology, Faculty of Basic Medical Science, College of Medical Sciences, Abubakar Tafawa Balewa University, Bauchi, 740272, Nigeria
Philemon Paul Mshelia
Department of Medical Biochemistry, Faculty of Basic Medical Science, College of Medical Sciences, Abubakar Tafawa Balewa University, Bauchi, 740272, Nigeria
Daniel H. Mhyha
You can also search for this author in PubMed Google Scholar
Contributions
JA put forward the conception of the review and wrote the manuscript. ZI, SM, YM, PM and DM participated in the proposal of the concept and revised the manuscript. JA anchored and wrote the final revised manuscript. All authors approved the submitted version.
Corresponding author
Correspondence to John Agbo .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
We declare no conflicts of financial interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Agbo, J., Ibrahim, Z.G., Magaji, S.Y. et al. Therapeutic efficacy of voltage-gated sodium channel inhibitors in epilepsy. Acta Epileptologica 5 , 16 (2023). https://doi.org/10.1186/s42494-023-00127-2
Download citation
Received : 26 December 2022
Accepted : 21 June 2023
Published : 28 June 2023
DOI : https://doi.org/10.1186/s42494-023-00127-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Voltage-gated sodium channels
- Idiopathic epilepsy
- Acquired epilepsy
- Anti-seizure medications
- Pharmacoresistant epilepsy
Acta Epileptologica
ISSN: 2524-4434
- Submission enquiries: Access here and click Contact Us
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Discoveries (Craiova)
- v.8(2); Apr-Jun 2020
Epileptic seizures
Haleema anwar.
1 CMH Lahore Medical College & Institute of Dentistry, Lahore, Pakistan
Qudsia Umaira Khan
Natasha nadeem, iqra pervaiz, muhammad ali, fatima fayyaz cheema.
Epilepsy is a condition marked by abnormal neuronal discharges or hyperexcitability of neurons with synchronicity and is recognized as a major public health concern. The pathology is categorized into three subgroups: acquired, idiopathic, and epilepsy of genetic or developmental origin. There are approximately 1000 associated genes and the role of γ-aminobutyric acid (GABA) mediated inhibition, as well as glutamate mediated excitation, forms the basis of pathology. Epilepsy is further classified as being of focal, general or unknown onset. Genetic predisposition, comorbidities and novel biomarkers are useful for prediction. Prevalent postictal symptoms are postictal headache and migraine, postictal psychosis and delirium, postictal Todd’s paresis and postictal automatisms. Diagnostic methods include electroencephalography (EEG), computed tomography scan, magnetic resonance imaging (MRI), positron emission tomography, single photon emission computed tomography and genetic testing; EEG and MRI are the two main techniques. Clinical history and witness testimonies combined with a knowledge of seizure semiology helps in distinguishing between seizures. Clinical information and patient history do not always lead to a clear diagnosis, in which case EEG and 24-hour EEG monitoring with video recording (video-EEG/vEEG) help in seizure differentiation. Treatment includes first aid, therapeutics such as anti-epileptic drugs, surgery, ketogenic diet and gene therapy. In this review, we are focusing on summarizing published literature on epilepsy and epileptic seizures, and concisely apprise the reader of the latest cutting-edge advances and knowledge on epileptic seizures.
1. Introduction
2. pathology and etiology, 3. types of epileptic seizures, 3.1. focal seizures, 3.2. generalized seizures, 4. prediction and prevention, 5. post seizure symptoms, 6. diagnosis, 7. differential diagnosis, 8. treatment, 8.1. first aid, 8.2. drug-based therapeutics, 8.3. surgery, 8.4. ketogenic diet, 8.5. gene therapy, 9. conclusion.
The World Health Organization (WHO) and its partners have recognized epilepsy as a major public health concern. Epilepsy occurs due to hyperexcitability and an imbalance between excitation and inhibition, leading to seizures 1 . According to the WHO, around fifty million people worldwide are affected by epilepsy, making it one of the most common neurological diseases globally. Epilepsy is a neurological disorder characterized by recurrent seizures caused by sudden surge in electrical activity of the brain. This is due to abnormal neuronal discharges or hyperexcitability of neurons with synchronicity. However, the frequency of these seizures varies for different people.
Epilepsy is a multifactorial neuronal disorder. Epileptic seizures are abnormal jerky or trembling movements in the body due to abnormal neuronal activity and can result in damage to the brain or other parts of the body. Even a single seizure can cause changes in neural development and can lead to behavioural and cognitive changes. Epileptic seizures have adverse clinical characteristics 2 . These seizures have a negative impact on the lives of patients especially those who have frequent reoccurrence. The epileptic seizures cause emotional, behavioural and neurological disturbances in patients. Seizures can occur in various regions of the brain and the degree of effectiveness depends upon the characteristic area, types of seizures and the area where abnormal neuronal activity is occurring 1 . Epileptic patients suffer from social stigma and discrimination; misconception and negative attitudes of society towards this disorder may prevent epileptic patients from seeking treatment and leading a confident life.
This review briefly covers the pathology and classification of epileptic seizures. It also highlights prediction and prevention, diagnosis, differential diagnosis and the various available treatments, including drugs, surgical excision, dietary therapy and gene therapy for epileptic seizures.
Epilepsy is classified into three categories based on the etiology, named acquired, idiopathic, and epilepsy of genetic or developmental origin. Idiopathic epilepsy is without neurological signs, and its onset is in childhood. Some examples of idiopathic epilepsies are childhood absence epilepsy and juvenile myoclonic epilepsy. Acquired epilepsy is due to identifiable structural abrasions of our brain. The causes of acquired epilepsy are cerebral trauma, cerebral tumor, cerebral infection, hippocampal sclerosis, cerebrovascular disorders, cerebral immunological disorders and perinatal and infantile causes. Some examples are epilepsy caused by open head surgery, viral meningitis, meningioma, cavernous hemangioma and cerebral infarction. Cryptogenic epilepsy has an unknown etiology. Among acute and remote causes, etiology can be difficult to identify 3 . In modern studies, the term cryptogenic is discouraged because it conveys unclear implications. It is replaced with probably symptomatic, which provides clear implications 4 . Most studies reveal that 40 out of 100 cases of epilepsy have known etiology that includes ischemic stroke, infections in the central nervous system, brain injury, prolonged symptomatic seizures intracerebral hemorrhage, and neurodegenerative diseases.
A research study published in 2016 revealed 977 epilepsy-associated genes. These were grouped into four categories, according to the phenotype. These genes are controlling the ion-channel, enzyme or enzyme regulator genes, transporter and other aspects of the cell, such as cell adhesion. 84 genes are associated with syndromes that have epilepsy as the core feature, 73 are neurodevelopment-associated genes related to brain developmental problems which can cause epilepsy, 536 epilepsy-related genes are associated with metabolic errors or other systemic abnormalities where epilepsy is not the central symptom, but rather it is one of the many clinical manifestations and 284 were potential epilepsy-associated genes that require further investigation 5 . This has been shown in Figure 1 .

(as determined by Wang J et al. 5 )
Epileptogenesis is fundamentally a biological process which leads to the appearance of the initial recurring epileptiform event, as well as to spontaneous seizures after brain insult. It involves the development and progression of epilepsy in the patient. Epileptogenesis involves biological processes, structural, as well as functional changes. Several neurotransmitters play an important role in the epileptic mechanism. The most important neurotransmitters are serotonin, dopamine, γ-aminobutyric acid (GABA), glutamate, and noradrenaline.
Two neurotransmitters are usually studied in reference to epilepsy: GABA and glutamate. In epilepsy, neuronal hyperexcitability is due to variation in GABA mediated inhibition as well as in glutamate-mediated excitation. Glutamate can depolarize the neurons, generating excitatory post-synaptic potentials. During the initiation and progression of epilepsy, specific glutamatergic molecular mechanisms occur, which includes an increase in the concentration of extracellular glutamate, upregulation of the glutamate receptors, and certain abnormalities in the glutamate transporters. These mechanisms cause hyperexcitability due to excessive glutamatergic activity.
GABA is one of the primary inhibitory neurotransmitters. It generates the presynaptic potentials through hyperpolarizing the neurons. It plays a vital role in counterbalancing the neuronal excitation, as well as in suppressing epileptiform discharges. Two GABA receptors named GABAA and GABAB are involved in the epileptogenesis 6 . Loss of GABAergic mechanisms can increase the risk of epilepsy in an individual. One of the primary transporters of GABA is GAT-1. It is accountable in re-uptake of GABA from the synapse. A mutation in the GABA transporter is also a cause of epilepsy with myoclonic, atonic seizures in many individuals 7 .
International League Against Epilepsy (ILAE) proposed a classification of epilepsies and epileptic syndrome in 1989. This classification was based on symptoms which grouped the epilepsies as either generalized or focal. The classification was also made based on etiology into two groups: idiopathic epilepsies and symptomatic epilepsies. Idiopathic epilepsies were due to genetic causes and were characterized by a normal background electroencephalography (EEG) and no brain lesions. Symptomatic epilepsies, as opposed to idiopathic, were characterized by brain lesions (either focal or diffused). In 2006, a new base for categorizing epilepsies was devised by ILAE Task Force on Classification and Terminology. It included seizure type, age of onset and interictal EEG. Subcategories of epilepsy syndrome according to age of onset were neonatal period, childhood, adolescence, special epilepsy conditions, and conditions with epileptic seizures that do not require diagnosis (e.g. febrile seizures). According to the type of seizure, the subcategories were self-limiting epileptic seizures (which included generalized onset, focal onset and neonatal seizures) and status epilepticus.
The classification was further extended in 2010 1,8 . Currently, the latest revision was published in 2017. This revision was made because some important seizures were not included in previous classification, few seizures can have both generalized and focal onset and some seizures were not classified on the basis of shallow knowledge about their onset.
In the 2017 revision, certain terms were added. Hyperkinetic seizures were added to focal seizure category, cognitive was used instead of ‘psychic’ (so that cognitive disabilities during seizures can be identified e.g. aphasia) 1,8 .The 2017 classification provides a much more lucid nomenclature and includes previously missing seizure types. An overview of the 2017 revised classification is shown in Figure 2 .

In all these modifications to classification over the years, the terms focal and generalized epilepsies have not been disturbed. These are discussed as following.
Focal seizures are the seizures that occur in a small localized region of the cerebral cortex or in a deeper structure of the cerebrum. A phenomenon called ‘jacksonian march’ is associated with this type of seizures. Jacksonian march is a progressive ‘march’ of muscle contraction caused by spread of neuronal discharge over the motor cortex. The muscle contraction occurs on the opposite side of the body and the direction may be from mouth region to the legs or vice versa.
In ILAE classification (according to the latest revision), focal seizures have the following major subcategories: aware, impaired awareness, motor onset, non-motor onset, focal to bilateral tonic-clonic seizures. Motor onset includes automatism, atonic, clonic, epileptic spasms, hyperkinetic, myoclonic and tonic seizures. Nonmotor onset comprises autonomic, behavior arrest, cognitive, emotional and sensory seizures.
In focal impaired awareness seizures, the person must reorient himself, whereas in focal aware seizure the person is fully aware during the seizure. This can be easily understood by a clinical scenario. A woman in her twenties experiences a seizure. During the seizure, she can hear other people talking. However, after the episode is over, she cannot recall what was being said. This is classified as focal impaired awareness. A man in his twenties has a seizure during which he is aware. He feels as if he is being flashed. This is a scenario of focal aware autonomic seizure 9 .
Focal clonic seizures can be understood by the following case: a baby boy has rhythmical jerks of the arm on one side 9 . On repositioning the jerks do not remit and EEG displays right frontal ictal rhythms. As the EEG displays a localization of the electrical discharge, therefore the seizure is focal. There is no knowledge about the awareness. Thus, awareness is not involved in the classification. As clonic seizures are a subtype of motor onset seizures, this can also be named focal motor onset clonic seizures.
These seizures affect both hemispheres of the brain. In the 2017 classification, they have been broadly classified into two categories: motor and non-motor. Motor seizures include tonic-clonic seizures, clonic, tonic, myoclonic, myoclonic-tonic-clonic, myotonic-atonic, atonic and epileptic spasms. Non-motor seizures include typical, atypical, myoclonic and eyelid myoclonia.
Currently, there is no reliable non-ictal biomarker capable of tracking epileptogenesis and concurrent human acquired epilepsies with reliable accuracy and specificity 10-12 . The most relevant markers are based on the EEG, particularly pathologic high‐frequency oscillations (pHFOs) which are brief EEG events in the range of 100 to 600 Hertz. These are speculated to reflect summated action potentials in hyperexcitable neurons. Yet, to improve the scope of prediction and prevention (by means of reoccurrence or otherwise), further discussion on the genetic predisposition, associated and indicated comorbidities, as well as other novel biomarkers is considered relevant.
There is a strong genetic component to the occurrence of epilepsy, with estimated hundreds of genes contributing to the pathogenesis. Identification of a novel CHRNA4 (OMIM 118504) variant in a pedigree with benign childhood epilepsy with centrotemporal spikes (BECTS) broadens the scope for prediction of at-risk subjects by means of genetic screening 13 . Epileptogenesis is the process by which a normal brain develops epilepsy. This phase of genesis is marked by changes, mostly in neurons and glial cells, particularly in methylation of epilepsy-relevant genes HDAC11, SPP1, GAL, DRD1 and SV2C 14 . Furthermore, abnormal methylation of RASgrf1 and its subsequent downregulation has been observed in the temporal neo-cortex of epileptics 14,15 . The methylation of genes coincides with the incidence of general epilepsy, whereby it serves both as a novel target for treatment and an indicator for the likelihood of seizure occurrence.
Also, under speculation is the involvement of ion channels in genetic epilepsy where an estimated 25% of genes in epilepsy code for ion channels 16 . Hence, this furthers the claim in observed literature of the association of a hyper-excitable visuo-motor system to the development of photosensitive epilepsy. The underlying difference between photosensitive epilepsy and those without sensitivity is the decreased alpha inhibition of the visuo-motor system due to a difference in the generation of alpha oscillations by the cortical-subcortical system. Thus, the identification of the “alpha phenotype” may be useful in predicting and preventing photosensitive epileptic reoccurrences/occurrences in those that show a genetic inclination 17 .
The mouse model of investigating post-infection (malarial) acquired epilepsy has proven useful in furthering the claim of a brain-heart interaction as a biomarker bearing relevance to epileptogenesis. On a beat to beat interval the aberrant cortical discharge precedes the change in heart activity, with this deviation from the norm being identifiable 2-14 weeks before the seizures 10 .
Epilepsy-associated comorbidities (intellectual disability (ID), anxiety disorder and attention-deficit/hyperactivity disorder (ADHD), depression & autism spectrum disorders) may share common pathological mechanisms with epileptogenesis and in certain cases have been increasingly recognized as an early symptom of the developing epilepsy. However, they have yet to be investigated and quantified in their predictive value 12 .
Trigger avoidance may as well be a hallmark of preventing seizures, whereby common triggers to be avoided include sleep deprivation, alcohol consumption, physical and mental exhaustion, metabolic disturbances and missed dosage of an antiepileptic drug (in treated patients). On the topic of reflex epilepsies, prevention is based on the avoidance of relevant triggers for the subject on a case by case basis 18 . Common triggers can be external (flashing lights, hot water), internal (emotional thinking) or both. It would be advised to make informed changes in lifestyle with flickering lights, distance to screens, intensity of images and the refresh rate of television screens (a 100 Hertz television display being less liable to inducing a seizure as compared to a 50 Hertz one) in known photosensitive subjects.
Identification of biomarkers is limited by an ill-defined window of time (e.g.: EEG changes in the acute time following a traumatic insult). Genetic predispositions are unlikely to be the primary predictor, since the patients will mostly come to clinicians after onset of symptoms of seizure. A single biomarker might prove to be insufficient in predicting the epileptogenesis or a preictal period, while multiple biomarkers may be the necessary requirement.
Postictal state is an abnormal condition, occurring from the end of an epileptic seizure and lasting until the return to a normal neurological function.The postictal period is characterized by changes in behaviour, motor function and neuropsychological performance. The type, intensity and duration of these symptoms is variable, differing even in the same patient from seizure to seizure 19 .
A systematic review determined that postictal symptom duration ranged from 3 seconds for postictal automatisms to about 12.3 days for postictal depression and anxiety. A 2019 meta-analysis found up to 31 postictal symptoms including depression, anxiety, irritability, hypersalivation, euphoria, hypomania, sleep, coma, lethargy, fatigue, vomiting, anorexia, laughter and sighing 20 . The more prevalent postictal symptoms are postictal headache and migraine, postictal psychosis and delirium, postictal Todd’s paresis, postictal aphasia, postictal cognitive deficits (confusion and memory loss) and postictal automatisms.
Postictal migraines are the second-most frequently occurring with a mean weighted overall estimate of 16.0% of epileptic patients experiencing them. Postictal headaches and migraines occur more frequently in children than in adults and postictal migraines occur more often in women than in men (63.0% vs 33.3%) 21 . The mean duration of postictal headaches was found to be 14 hours. This symptom generally manifests within 3 hours of an epileptic seizure and can continue up to 72 hours.
A study in 2018 found that the clinical pattern of headache correlated to seizure onset and epileptic patients demonstrated different types of headaches. Its results showed that tension-type headaches occurred in 73.3% of patients who experienced postictal headaches 22 .
A systematic review included a finding in which 66.4% of patients who experienced postictal headaches experienced this symptom after every single seizure. It has been speculated seizures can result in cerebral edema which greatly increases intracranial pressure; this indicates a potential cause for postictal headaches and migraines.
Postictal delirium typically lasts for hours but may continue up to 1 to 2 days. It is often of the hypoactive type but may evolve into the hyperactive type. During delirium, patients may become agitated or aggressive resulting in postictal violence. This symptom often occurs after complex partial or generalized epileptic seizures. Postictal psychosis is associated with hallucinations, delusions, delirium and amnesia 23 .
Todd’s paralysis (loss of motor function in the regions of the body involved during the seizure) has a good lateralizing value, pointing to the contralateral hemisphere as the site of seizure onset 24 . Practically, the concept of “Todd’s paralysis” has been broadened to include manifestations other than motor function, including postictal sensory deficits that present as somatosensory, oculomotor or auditory symptoms. The inability of patients to speak in the postictal state is referred to as postictal aphasia and lateralizes to a seizure originating in the speech-dominant temporal lobe. The postictal aphasia occurs only when the epileptic activity spreads to language areas, hence, it has limited localizing value.
The length of the aphasic period is dependent on the hemisphere of seizure onset and, in a 2017 retrospective study, was found to occur with left hemisphere seizure onset or with seizures spreading from right to the left hemisphere 25 . Postictal aphasia frequently occurs following left temporal lobe epilepsy.
Postictal automatisms are not a voluntary response and may involve the mouth (lip smacking, swallowing, chewing) or vocalization (grunts, repetition of phrases). Hand automatisms are a prominent sign of focal seizures, often manifesting unilaterally. Repetitive movements include postictal nose wiping and indicate an ipsilateral focus for seizure onset 26,27 .
With adequate treatment most epileptic patients can live a normal and healthy life, but some patients develop serious mental illnesses. Therefore, continuous medical assistance may be needed . Early diagnosis can improve the medical condition of the patients. However, even in developed countries, 10% of patients do not get appropriate treatment, whereas in low-income countries, the percentage is 75% 28 .
Several methods are used for the diagnosing epileptic seizures. These methods include, EEG, computed tomography (CT) scan, magnetic resonance imaging (MRI), positron emission tomography (PET), single photon emission computed tomography (SPECT) and genetic testing. Simple blood tests are also done as they can be a helpful tool for describing the etiology of toxic and metabolic encephalopathies 29 . Studies suggest that EEG and MRI are the two principle techniques used in the diagnosis of epileptic seizures. Additional techniques help to confirm diagnosis and can even identify false negative results.
EEG is considered to be the most effective method to diagnose epilepsy. Whenever an unusual EEG is observed, it helps in identifying whether the seizure is a focal or generalized epileptic seizure and it may also rule out the patient’s epilepsy syndrome. Thus, it can help in the prognosis of the disease and control of further seizures, allowing for better treatment choices to be made. When nothing abnormal is observed in brain imaging, surgery is opted as a treatment of choice after Video-EEG monitoring (vEEG) confirms the injured areas of the brain 30 .
CT scan also proves helpful, but the detection rate of the focal lesions in the patients is only 30%. When neurosurgical treatment is being opted, neuroimaging is of critical significance in the evaluation of epilepsy. With progress in MRI technology, acquisition protocols and methods, brain imaging has improved to a great extent. Structural MRI is the main neuroimaging technique which is used for the identification of an epileptogenic lesion 31 . However, there are chances of false negatives in about 15% to 30% of patients suffering from refractory focal epilepsy.
If a patient has no distinct lesions on MRI then the latest MRI hardware and techniques are used, which may show some minute abnormality. However, this should be handled with great vigilance, since there is a possibility of a false-positive result 32 . There are certain reasons to use MRI as a means of investigation of the disease. MRI can help in determination of the cause and evaluation of the condition before a surgery 33 .
PET and SPECT are functional imaging studies that aid in identifying the epileptogenic zone and to allow pre- surgical evaluation. In order to find the cause of some types of epilepsies, genetic testing is used. However, there are a lot of limitations, including the relatively high cost and lack of availability 34 . PET imaging and SPECT imaging are techniques used to localize the area of cortex responsible for initiation of seizures, especially in patients with focal epilepsy who have a normal MRI, patients with multiple abnormalities, or in patients with inconsistencies between MRI and EEG. Information regarding changes in the cerebral perfusion is also provided by SPECT imaging technique 35 .
There are three different types of seizures: epileptic seizures (ES), psychogenic nonepileptic seizures (PNES) and physiological nonepileptic events 36 (syncope, transient ischemic attacks, parasomnias, migraines with aura, paroxysmal extrapyramidal movement disorders, vestibular syndromes, drop attacks, hypoglycemia, panic attacks, paroxysmal sleep disorders).
The main misdiagnoses of epilepsy are PNES and syncope 37 . The most common misdiagnosis is PNES. PNES presents as paroxysmal signs and symptoms imitating epileptic seizures which makes it difficult to distinguish it from epileptic seizures. 10% to 15% of patients with long-term PNES turn out to additionally have epilepsy 36 .
Differential diagnosis between epileptic and nonepileptic events of psychogenic and physiological origin can be done on the basis of clinical history and witness testimonies. Clinical history combined with a knowledge of seizure semiology helps in distinguishing between seizures 38 .
History helps in identifying syncope and ruling out epileptic seizures. If the patient’s seizure happens shortly after standing up or diaphoresis occurs before the seizure, then vasovagal syncope should be considered 39 . Semiological characteristics are especially important for differentiation between ES and PNES and clinical signs. Symptoms associated with these seizures are contrasted in Table 1 .
However, clinical information and patient history do not always lead to a clear diagnosis, in which case EEG and 24-hour vEEG help in seizure differentiation. A normal EEG recording cannot rule out epilepsy because some epileptic seizures, such as simple partial epileptic seizures and frontal lobe epileptic (FLE) seizures, may show scalp-negative EEG findings. Therefore the “gold standard” for differential diagnosis of epileptic seizures is a combination of video recording, EEG and electrocardiography (ECG). vEEG recordings are a reliable way of differentiating between ES, PNES and non-epileptic seizures of psychological origin. When EEG is normal prior to, during and following the epileptic episode and vEEG recordings support clinical features that are associated with PNES, then a diagnosis of PNES can be made. Similarly, dampened amplitude and slowing of the brain waves on EEG indicate a non-epileptic seizure of physiological, possibly cardiac, origin, while positive EEG activity with supported clinical history indicates an epileptic seizure 39 .
However, vEEG may not always capture the necessary events needed for differential diagnosis and may not be able to distinguish between PNES and FLE seizures. Combining vEEG with seizure semiology makes for a more accurate diagnosis.
Other measures for differential diagnosis include prolactin levels, neuropsychological testing, provocative testing and single photon emission computed tomography. Neuropsychological test scores can differentiate between ES and PNES. Standardized tests – capable of measuring intellectual functioning, attention, memory, language skills, processing speed, executive function and emotional functioning – are carried out as part of a comprehensive neuropsychological battery 40 . PNES patients have been shown to have significantly higher intellectual functioning. This method of psychometric testing is a potential tool for differential diagnosis, especially in uncertain cases of patients with non-diagnostic vEEG.
Neurophysiological assays can also be used. They can distinguish between complex partial epileptic seizures and PNES. This is due to the association of epileptic seizures with elevated serum prolactin levels. The increase depends on which limbic region was involved during the seizure. However, one drawback is that neurophysiological assays cannot differentiate between syncope and epileptic seizures, or between PNES and partial epileptic seizures 39 .
However, these alternative techniques will likely not replace vEEG and ECG as the “gold standard” for differential diagnosis, though they may be used as complementary screening and diagnostic tools. In short, a combined electroclinical analysis that utilizes clinical semiology, ECG and vEEG findings is effective for the differential diagnosis of epileptic seizures.
The treatment for epileptic seizure includes first aid, therapeutics, gene therapy, ketogenic diet and surgery. These have been outlined in Figure 3 .

First aid management refers to the action taken by people witnessing a patient who experiences an epileptic seizure. This includes helping others cope with the situation.
The negative attitudes and stigmas against people with epilepsy are more confusing than the disease. Misconceptions make people frightened when encountering an individual having an epileptic seizure. The actions of such witnesses play a key role. The most important task in this situation is to keep calm and help the patient. There are evidences that seizures can be safely managed by patients and their families if they have the correct training. Also, self-management intervention improves the confidence of patient and the situation is more effectively managed 41 . Self-management courses provided to patients at the epileptic centers help them reduce fear of seizure and improve their self-management skills 41 . The vital managing steps to be taken for handling an episode of seizure are shown in Table 2 .
It is important to know that seizure is not an emergency in which there is a need to call an ambulance immediately 42 . The Table 3 highlights the situations where there is dire need to call for rescue to take the patient to the hospital.
After the duration of the seizure is monitored, the following must be checked: patients’ blood glucose level, pulse rate and respiration. Cyanosis may occur due to paralysis of respiratory muscles, but it must be made sure that it is temporary and normalizes when seizure subsides. The convulsive period of epileptic seizure rarely lasts longer than two to three minutes and rarely poses any life-threatening effects. Should, however, the seizure last for more than five minutes or if seizures occur with succession without the patient gaining consciousness in between, then it is an emergency situation and an ambulance must be immediately called. After the seizure ends, it is best to relax the patient and make him rest for some time. The patient will be sleeping for hours or even for a day after the seizure because of restlessness he faced during the attack 42 .
The most important treatment option for epileptic seizures is thought to be the administration of the anti-epileptic drugs (AED) or anticonvulsive drugs. Many conventional AEDs are in use and newer ones are also undergoing experimental studies to determine their efficacy. About 65% of children have been known to be completely cured by AED if they are administrated at the initial stage of the disorder. There are many people who are AED-resistant and need alternative treatment. While taking the AEDs, special cautions must be taken to avoid interaction with drugs that can be harmful 43 . The AEDs must be taken only when prescribed by a doctor with a comprehensive look on the mechanism of interaction of the drug, its possible side effects and its proper dosage 43 . The tolerability, safety and efficacy of AEDs are important in determining their profile; these parameters help in practical use of AEDs against epileptic seizures. Although these drugs have different mechanisms of action to act against seizures, most of them are inhibitors which act on ion channels (like sodium or calcium channels) or neurotransmitters like GABA. Examples of AEDs include bumetanide, felbamate, ganxolone, regtibine, parampanel and carbamazepine 44 .Their mechanism, side effects and characteristics have been shown in Table 4 .
New AEDs are being synthesized for seizures that are not efficiently treated with current ones. In some cases, newer AEDs are given in combination with conventional AEDs. These AEDs may be effective for drug resistant epilepsy (DRE). All of them, however, should not be used unprescribed. Some are already available like lamotrigine, vigabatrin, lacosamide, oxcarbazepine and some are in development 45 . These drugs are described in the Table 5 .
Most AEDs are metabolized in liver, whereas some in the kidney. There are certain factors that alter the action and effect of antiepileptic drugs as shown in Table 6 .
AEDs can have adverse effects on different systems of the body affecting function and efficiency. The most common side effects of AEDs include headache, ataxia, behavioural changes and allergic reactions. Several CNS functions are also affected 43 . The adverse effects are listed in Table 7 .
A patient manifesting epilepsy not responding to AEDs is said to have drug resistant epilepsy. The actual degree and pervasiveness of DRE and its mechanism is not yet clearly understood. Several hypotheses regarding DRE suggest that it is multifactorial, some are listed in the Table 8 .
Comorbidity is seen in epileptic patients. This includes depression, suicidal thoughts, hepatic diseases, renal disease, osteoarthritis, attention deficit hypersensitive disorder, intellectual disability, psychiatric and behavioral comorbidities and neuropsychiatric disorders.
Several drugs, registered in the database of the USA National Institute of Health, are under clinical trials for epilepsy treatment. Several main examples are shown in Table 9 The type of study for all trials in the table is interventional.
Surgical intervention should only be considered when the subject does not respond to non-invasive therapies or medication. There is variation in surgical intervention, depending on the area of the brain where the seizure may occur or originate. Procedures include:
- Focal resection of the area causing the seizure is a procedure reserved for non-critical areas of the brain, as is the case with temporal lobe epilepsy (TLE), in medically intractable scenarios.
- Lesionectomy involves the removal of a well-formed brain tissue aberration (localized tumour or vascular deformation). Patients commonly present with glioneuronal tumours or meningiomas. Tumours have a high chance of being causative agents for epilepsy, and they present a good probability of seizure freedom with surgical intervention.
- A less invasive alternative may be found in laser interstitial thermal therapy (LIIT), which · depends on an MRI to map out the seizure focus and hence, guide a laser to eliminate that area of the brain only. LITT, as a treatment option for mesiotemporal epilepsy, shows a need for further improvement in parameters for surgery, because variations in anatomy between patients’ needs to be accounted for on a case-by-case basis.
- Corpus callosotomy involves severing the connection between both hemispheres and is reserved for severe generalized epilepsy (marked by tonic-clonic seizures and frequent seizure related falls). The procedure offers rather unreliable results, with only a fifth achieving seizure freedom post-surgery as a meta-analysis showed.
- Cases ill-suited for resection or ablation might be treated with the implantation of neurostimulation devices, but they confer palliative treatment as seizure freedom rates are reported as low. Regardless, the procedure has been determined to be safe and somewhat effective.
Ketogenic diet (KD) is one which contains high fat content, low carbohydrates content and adequate protein content. This diet consists of high-fat in the form of long-chain triglycerides. As the fat metabolizes, it produces ketone bodies. The different ketone bodies, such as acetoacetate and beta hydroxybutyrate, are experimentally observed to have anticonvulsive role. Despite the new antiepileptic drugs, ketogenic diet is turning up to be a non-pharmacological substitute for treatment of epilepsy 51 .
The classic ketogenic diet is one that has calculated ratio of grams of fat to grams of carbohydrates plus protein. The most feasible ratios calculated until now are 3:1 or 4:1, with about 80-90% of the energy provided by fats and 10% by carbohydrates and proteins collectively 52 .
KD is most effective when taken after fasting or when the body's calorie levels are low. This is because the brain normally uses glucose as the source of energy, not fats. However, glucose levels are low in the fasting state, thus, the brain utilizes fats as the source of energy in this instance. Ketogenic diet has been used as a treatment for epileptic seizures due to its proven anticonvulsive role, and several research groups reported that KD therapy decreases the number of epileptic seizures in approximately 30-40% of children. Additionally, KD has also been seen to be effective for infantile epileptic seizures therapy 51 . It is mainly used as treatment in those patients who have DRE or for those patients who cannot undergo surgical excision procedure. Some guidelines concerning ketogenic therapy are shown in Table 10 .
KD is not fully effective because it can cause several disorders, including but not limited to gastrointestinal disturbances and heart pathologies. Before initiating KD, the patient must be screened for disorders concerning fatty acid oxidation and metabolism 52 .
The exact mechanism of how a ketogenic diet performs anticonvulsive activity is still unclear. However, researches have shown that it decreases threshold level of a seizure. A KD affects both neurotransmitters and the neuronal membranes when acting as an anticonvulsant.
GABA is an inhibitory neurotransmitter widely distributed in neurons. Ketogenic diets have a role in synthesizing and maintaining high levels of GABA, which may be effective in treating epileptic seizures 51 . It is hypothesized that a KD decreases aspartate levels (induced by ketone bodies), which will facilitate the conversions of glutamate to glutamine and glutamine to GABA.
Ketogenic diet in neuronal membranes alters the vesicular glutamate transporter (VGLUTS). These are functioning by filling presynaptic vesicles and are chloride ion-dependent 1 . Ketone bodies, particularly acetoacetate, competitively inhibit chloride ion channels. The exact relation between VGLUTS and KD is still unknown. This ultimately leads to an increased in the inhibitory neurotransmitter GABA, and a decreased excitatory neurotransmitter glutamate.
KD therapies have shown efficacy for epilepsy. However, in order to use ketogenic diets for treatment, the strict protocols for usage must be followed as recommended by neurologists 52 . KD is in use for epilepsy and further clinical trials are being performed.
Many new antiepileptic drugs have been introduced in the past few years. However, almost one-third of epileptic patients still suffer seizures despite the use of AEDs 53 . Approximately 30% of people remain resistant to pharmacotherapy even with optimal treatment. Surgical removal of epileptogenic zone can prevent seizures, but it is unsuitable for more than 90% of patients with refractory epilepsy. Surgical intervention especially in case of focal neocortical epilepsy (FNE) has a lot of complications, as the functional areas of cortex can be damaged during the surgical procedure. Only a minority of the pharmacoresistant epilepsy patients can undergo a curative surgery. Gene therapy can be considered a treatment strategy for such intractable cases. This technique is very specific, since the therapeutic genes are introduced into the abnormal tissues only. In addition to the resistance of patients to AEDs, another problem observed is the development of several harmful side effects by the use of these drugs, which include mental retardation and lack of emotions, i.e. numbness. According to the FDA, some AEDs can even induce suicidal tendencies. Neurosurgery for the removal of the epileptogenic focus is the ultimate solution for patients with drug-refractory epilepsy, however, even then, the cure rate is not satisfactory. For such intractable cases, gene therapy can be considered as a treatment strategy 54 . Some genes, such as the neuropeptide Y and galanin, have shown a positive effect on the seizure activity. For the gene therapy treatment to be successful, the therapeutic gene must be accurately delivered to the target neurons.
The idea of gene therapy seems simple. Deoxyribonucleic acid (DNA) encoding therapeutic protein(s) is transferred via a vector into the abnormal cells in order to repair them permanently. Some genetic forms of epilepsy can be treated by transferring a healthy gene in place of the defective gene. Defective genes can also be repaired by the use of different gene editing technologies, including CRISPR (clustered regularly interspaced short palindromic repeats), Cas9-mediated genetic modification and CRISPRa (clustered regularly interspaced short palindromic repeats activation) or CRISPRi (clustered regularly interspaced short palindromic repeats inhibition). The transfer of genes capable of altering the function of the cell in relation to its hyperexcitability is another option which can be considered. There are several options available and several strategies could be adopted, but their practical application requires further investigation 55 .
Non-viral vectors have a lower tendency of provoking an immune response as compared to viral vectors. However, the major drawback of the non-viral vectors is low transduction efficiency. Experiments on different viruses for gene therapy have shown that adeno-associated viruses, lentiviruses, and herpes viruses are the most suitable for CNS application 56 . However, the complexity and diversity of the tissue being targeted and the presence of the BBB (Blood-Brain Barrier) pose hurdles for the in vivo human use of these viral vectors.
So far, the process of gene therapy for the treatment of epilepsy is done on animals through the focal application of vectors. Gene therapy has become a popular topic in the clinical world. However, its clinical application and practice in treating CNS dysfunctions faces many challenges. With rapid advancements of this field, it is not too far away the time until a successful gene therapy for epilepsy is performed 57 .
- Epilepsy is one of the most common neurological disorders affecting about fifty million people worldwide.
- There has been progress in classification of the epilepsy subtypes (based on the cause and commonalities) while etiology of acute epileptic conditions is still difficult to identify.
- While new tools and procedures have been added to aid diagnosis as well as differential diagnosis, the cornerstone of epilepsy related investigation remains vEEG combined with ECG. This possesses its own limitations, leaving a lot up to the skill of the clinician, history taking and witness testimonies.
- This article has revealed that the very nature of epilepsy is challenging to predict, since the patients only present to the clinicians upon suffering from a seizure or other relevant symptoms, yet headway is being made in the way of identifying biomarkers for the preictal period.
- Treatment with anticonvulsants is found to be well established, yet the refining of minimally invasive procedures for those with DRE remains to be desired.
- All in all, this review finds that leading a better life with epilepsy is feasible for most cases and can be improved with further breakthroughs in diagnostic and treatment procedures.
Acknowledgments
We express gratitude to our principal Major General Khaliq Naveed and our host institution, CMH Lahore Medical and Dental College, Lahore, Pakistan, for supporting our research endeavors.
Conflict of interests: The authors declare no conflicts of interest.
Abbreviations World Health Organization (WHO); γ-aminobutyric acid (GABA); GABA transporter-1 (GAT-1); International League Against Epilepsy (ILAE); electroencephalography (EEG); pathologic high‐frequency oscillations (pHEOs); benign childhood epilepsy with centrotemporal spikes (BECTS); intellectual disability (ID); attention-deficit/hyperactivity disorder (ADHD); computed tomography (CT); magnetic resonance imaging (MRI); positron emission tomography (PET); single photon emission computed tomography (SPECT); video-EEG monitoring (vEEG); epileptic seizures (ES); pyschogenic nonepileptic seizures (PNES); frontal lobe epileptic (FLE); electrocardiography (ECG); anti-epileptic drugs (AEDs); Food and Drug Administration (FDA); α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPA); drug resistant epilepsy (DRE); central nervous system (CNS); gastrointestinal tract (GIT); diazepam buccal soluble film (DBSF); anterior nucleus (of thalamus) deep brain stimulation (AN-DBS); repetitive transcranial magnetic stimulation (rTMS); temporal lobe epilepsy (TLE); laser interstitial thermal therapy (LITT); ketogenic diet (KD); vesicular glutamate transporter (VGLUTS); focal neocortical epilepsy (FNE); deoxyribonucleic acid (DNA); clustered regularly interspaced short palindromic repeats (CRISPR); clustered regularly interspaced short palindromic repeats activation (CRISPRa); clustered regularly interspaced short palindromic repeats inhibition (CRISPRi); blood-brain barrier (BBB).
DISCOVERIES is a peer-reviewed, open access, online, multidisciplinary and integrative journal, publishing high impact and innovative manuscripts from all areas related to MEDICINE, BIOLOGY and CHEMISTRY
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 03 April 2024
Insights into epileptogenesis from post-traumatic epilepsy
- Matthew Pease ORCID: orcid.org/0000-0001-6125-9858 1 ,
- Kunal Gupta ORCID: orcid.org/0000-0002-9847-0099 2 ,
- Solomon L. Moshé 3 , 4 , 5 ,
- Daniel J. Correa ORCID: orcid.org/0000-0002-6490-9331 3 ,
- Aristea S. Galanopoulou ORCID: orcid.org/0000-0002-0472-2903 3 , 4 ,
- David O. Okonkwo 6 ,
- Jorge Gonzalez-Martinez 6 ,
- Lori Shutter 6 , 7 , 8 ,
- Ramon Diaz-Arrastia 9 &
- James F. Castellano ORCID: orcid.org/0000-0003-1052-8725 8
Nature Reviews Neurology ( 2024 ) Cite this article
289 Accesses
6 Altmetric
Metrics details
- Brain injuries
- Risk factors
Post-traumatic epilepsy (PTE) accounts for 5% of all epilepsies. The incidence of PTE after traumatic brain injury (TBI) depends on the severity of injury, approaching one in three in groups with the most severe injuries. The repeated seizures that characterize PTE impair neurological recovery and increase the risk of poor outcomes after TBI. Given this high risk of recurrent seizures and the relatively short latency period for their development after injury, PTE serves as a model disease to understand human epileptogenesis and trial novel anti-epileptogenic therapies. Epileptogenesis is the process whereby previously normal brain tissue becomes prone to recurrent abnormal electrical activity, ultimately resulting in seizures. In this Review, we describe the clinical course of PTE and highlight promising research into epileptogenesis and treatment using animal models of PTE. Clinical, imaging, EEG and fluid biomarkers are being developed to aid the identification of patients at high risk of PTE who might benefit from anti-epileptogenic therapies. Studies in preclinical models of PTE have identified tractable pathways and novel therapeutic strategies that can potentially prevent epilepsy, which remain to be validated in humans. In addition to improving outcomes after TBI, advances in PTE research are likely to provide therapeutic insights that are relevant to all epilepsies.
Post-traumatic epilepsy (PTE) is highly prevalent after traumatic brain injury, impairing neurological recovery and leading to worse functional outcomes.
Current epilepsy therapeutics symptomatically treat seizures but do not modify epileptogenesis, the process by which brain tissue becomes prone to seizures.
The unique nature of PTE, occurring after a well-defined epileptogenic insult, makes it a promising model system for understanding epileptogenesis.
Future research in individuals with PTE populations might not only reveal novel mechanisms of epileptogenesis but also enable anti-epileptogenic therapies to be tested.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Psychedelics for acquired brain injury: a review of molecular mechanisms and therapeutic potential
Josh Allen, Shannon S. Dames, … Sandy R. Shultz

Recovery from disorders of consciousness: mechanisms, prognosis and emerging therapies
Brian L. Edlow, Jan Claassen, … David M. Greer

Transcriptomic alterations in cortical astrocytes following the development of post-traumatic epilepsy
John Leonard, Xiaoran Wei, … Michelle H. Theus
Popescu, C., Anghelescu, A., Daia, C. & Onose, G. Actual data on epidemiological evolution and prevention endeavours regarding traumatic brain injury. J. Med Life 8 , 272–277 (2015).
CAS PubMed PubMed Central Google Scholar
Frieden, T. R., Houry, D. & Baldwin, G. Report to Congress on traumatic brain injury in the United States: epidemiology and rehabilitation. Centers for Disease Control and Prevention . https://www.cdc.gov/traumaticbraininjury/pdf/TBI_Report_to_Congress_Epi_and_Rehab-a.pdf (2015).
GBD 2016 Traumatic Brain Injury and Spinal Cord Injury Collaborators. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 18 , 56–87 (2019).
Article Google Scholar
Farrell, J. S., Wolff, M. D. & Teskey, G. C. Neurodegeneration and pathology in epilepsy: clinical and basic perspectives. Adv. Neurobiol. 15 , 317–334 (2017).
Article PubMed Google Scholar
Sharma, R. et al. Neuroinflammation in post-traumatic epilepsy: pathophysiology and tractable therapeutic targets. Brain Sci. 9 , 318 (2019).
Article CAS PubMed PubMed Central Google Scholar
Pease, M. et al. Association of posttraumatic epilepsy with long-term functional outcomes in individuals with severe traumatic brain injury. Neurology 100 , e1967–e1975 (2023).
Burke, J. et al. Association of posttraumatic epilepsy with 1-year outcomes after traumatic brain injury. JAMA Netw. Open 4 , e2140191 (2021).
Article PubMed PubMed Central Google Scholar
Fordington, S. & Manford, M. A review of seizures and epilepsy following traumatic brain injury. J. Neurol. 267 , 3105–3111 (2020).
Lucke-Wold, B. P. et al. Traumatic brain injury and epilepsy: underlying mechanisms leading to seizure. Seizure 33 , 13–23 (2015).
Annegers, J. F., Hauser, W. A., Coan, S. P. & Rocca, W. A. A population-based study of seizures after traumatic brain injuries. N. Engl. J. Med. 338 , 20–24 (1998).
Article CAS PubMed Google Scholar
Pease, M. et al. Risk factors and incidence of epilepsy after severe traumatic brain injury. Ann. Neurol. 92 , 663–669 (2022).
Schmidt, D., Friedman, D. & Dichter, M. A. Anti-epileptogenic clinical trial designs in epilepsy: issues and options. Neurotherapeutics 11 , 401–411 (2014).
Pitkänen, A., Lukasiuk, K., Dudek, F. E. & Staley, K. J. Epileptogenesis. Cold Spring Harb. Perspect. Med. 5 , a022822 (2015).
Löscher, W., Potschka, H., Sisodiya, S. M. & Vezzani, A. Drug resistance in epilepsy: clinical impact, potential mechanisms, and new innovative treatment options. Pharmacol. Rev. 72 , 606–638 (2020).
Kehne, J. H., Klein, B. D., Raeissi, S. & Sharma, S. The National Institute of Neurological Disorders and Stroke (NINDS) Epilepsy Therapy Screening Program (ETSP). Neurochem. Res. 42 , 1894–1903 (2017).
French, J. A. et al. Antiepileptogenesis and disease modification: clinical and regulatory issues. Epilepsia Open 6 , 483–492 (2021).
Löscher, W. & Schmidt, D. Modern antiepileptic drug development has failed to deliver: ways out of the current dilemma. Epilepsia 52 , 657–678 (2011).
World Health Organization. Epilepsy: a public health imperative. WHO . https://iris.who.int/bitstream/handle/10665/325440/WHO-MSD-MER-19.2-eng.pdf (2019).
Gugger, J. J. et al. Multimodal quality of life assessment in post-9/11 veterans with epilepsy: impact of drug resistance, traumatic brain injury, and comorbidity. Neurology 98 , E1761–E1770 (2022).
Raymont, V. et al. Correlates of posttraumatic epilepsy 35 years following combat brain injury. Neurology 75 , 224–229 (2010).
Vespa, P. M. et al. The epilepsy bioinformatics study for anti-epileptogenic therapy (EpiBioS4Rx) clinical biomarker: study design and protocol. Neurobiol. Dis. 123 , 110–114 (2019).
Saletti, P. G. et al. Tau phosphorylation patterns in the rat cerebral cortex after traumatic brain injury and sodium selenate effects: an Epibios4rx Project 2 study. J. Neurotrauma 41 , 222–243 (2024).
Saletti, P. G. et al. Early preclinical plasma protein biomarkers of brain trauma are influenced by early seizures and levetiracetam. Epilepsia Open 8 , 586–608 (2023).
Engel, J. J. Epileptogenesis, traumatic brain injury, and biomarkers. Neurobiol. Dis. 123 , 3–7 (2019).
Correa, D. J. et al. Applying participatory action research in traumatic brain injury studies to prevent post-traumatic epilepsy. Neurobiol. Dis. 123 , 137–144 (2019).
Duncan, D. et al. Big data sharing and analysis to advance research in post-traumatic epilepsy. Neurobiol. Dis. 123 , 127–136 (2019).
Christensen, J. The epidemiology of posttraumatic epilepsy. Semin. Neurol. 35 , 218–222 (2015).
Thapa, A. et al. Post-traumatic seizures — a prospective study from a tertiary level trauma center in a developing country. Seizure 19 , 211–216 (2010).
Ritter, A. C. et al. Incidence and risk factors of posttraumatic seizures following traumatic brain injury: a Traumatic Brain Injury Model Systems Study. Epilepsia 57 , 1968–1977 (2016).
Haltiner, A. M., Temkin, N. R. & Dikmen, S. S. Risk of seizure recurrence after the first late posttraumatic seizure. Arch. Phys. Med. Rehabil. 78 , 835–840 (1997).
Ngugi, A. K., Bottomley, C., Kleinschmidt, I., Sander, J. W. & Newton, C. R. Estimation of the burden of active and life-time epilepsy: a meta-analytic approach. Epilepsia 51 , 883–890 (2010).
Thijs, R. D., Surges, R., O’Brien, T. J. & Sander, J. W. Epilepsy in adults. Lancet 393 , 689–701 (2019).
Angeleri, F. et al. Posttraumatic epilepsy risk factors: one-year prospective study after head injury. Epilepsia 40 , 1222–1230 (1999).
Englander, J. et al. Analyzing risk factors for late posttraumatic seizures: a prospective, multicenter investigation. Arch. Phys. Med. Rehabil. 84 , 365–373 (2003).
Ferguson, P. L. et al. A population-based study of risk of epilepsy after hospitalization for traumatic brain injury. Epilepsia 51 , 891–898 (2010).
Laing, J. et al. Risk factors and prognosis of early posttraumatic seizures in moderate to severe traumatic brain injury. JAMA Neurol. 79 , 334–341 (2022).
Christensen, J. et al. Long-term risk of epilepsy after traumatic brain injury in children and young adults: a population-based cohort study. Lancet 373 , 1105–1110 (2009).
Karlander, M., Ljungqvist, J. & Zelano, J. Post-traumatic epilepsy in adults: a nationwide register-based study. J. Neurol. Neurosurg. Psychiatry 92 , 617–621 (2021).
DeGrauw, X. et al. Epidemiology of traumatic brain injury-associated epilepsy and early use of anti-epilepsy drugs: an analysis of insurance claims data, 2004–2014. Epilepsy Res. 146 , 41–49 (2019).
Annegers, J. F. et al. Seizures after head trauma: a population study. Neurology 30 , 683–689 (1980).
Mahler, B. et al. Unprovoked seizures after traumatic brain injury: a population-based case–control study. Epilepsia 56 , 1438–1444 (2015).
Santos, S., Murphy, G., Baxter, K. & Robinson, K. M. Organisational factors affecting the quality of hospital clinical coding. Heal. Inf. Manag. 37 , 25–37 (2008).
Google Scholar
O’Malley, K. J. et al. Measuring diagnoses: ICD code accuracy. Health Serv. Res. 40 , 1620–1639 (2005).
Langlois, J. A., Rutland-Brown, W. & Wald, M. M. The epidemiology and impact of traumatic brain injury: a brief overview. J. Head Trauma Rehabil. 21 , 375–378 (2006).
Pugh, M. J. V. et al. The prevalence of epilepsy and association with traumatic brain injury in veterans of the Afghanistan and Iraq wars. J. Head Trauma Rehabil. 30 , 29–37 (2015).
Lolk, K., Dreier, J. W. & Christensen, J. Repeated traumatic brain injury and risk of epilepsy: a Danish nationwide cohort study. Brain 144 , 875–884 (2021).
Tubi, M. A. et al. Early seizures and temporal lobe trauma predict post-traumatic epilepsy: a longitudinal study. Neurobiol. Dis. 123 , 115–121 (2019).
Salazar, A. M. et al. Epilepsy after penetrating head injury. I. Clinical correlates: a report of the Vietnam Head Injury Study. Neurology 35 , 1406–1414 (1985).
The World Bank. World bank country and lending groups. Worldbank.org . https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups (2023).
Ogunrin, O. A. & Adeyekun, A. A. Profile of post-traumatic epilepsy in Benin City, Nigeria. West Afr. J. Med . 29 , 153–157 (2011).
Rabiu, T. B. & Adetunmbi, B. Posttraumatic seizures in a rural Nigerian neurosurgical service. World Neurosurg. 104 , 367–371 (2017).
Wang, X. P. et al. Development and external validation of a predictive nomogram model of posttraumatic epilepsy: a retrospective analysis. Seizure 88 , 36–44 (2021).
Espinosa-Jovel, C., Toledano, R., Aledo-Serrano, Á., García-Morales, I. & Gil-Nagel, A. Epidemiological profile of epilepsy in low income populations. Seizure 56 , 67–72 (2018).
Gennarelli, T., Champion, H., Copes, W. & Sacco, W. Comparison of mortality, morbidity, and severity of 59,713 head injured patients with 114,447 patients with extracranial injuries. J. Trauma 37 , 962–968 (1994).
Eagle, S. R., Pease, M., Nwachuku, E., Deng, H. & Okonkwo, D. O. Prognostic models for traumatic brain injury have good discrimination but poor overall model performance for predicting mortality and unfavorable outcomes. Neurosurgery 92 , 137–143 (2023).
Eagle, S. R. et al. Performance of CRASH and IMPACT prognostic models for traumatic brain injury at 12 and 24 months post-injury. Neurotrauma Rep. 4 , 118–123 (2023).
Hanna Siig Hausted, J. F. N. & Odgaard, L. Epilepsy after severe traumatic brain injury: frequency and injury severity. Brain Inj. 34 , 889–894 (2020).
Ritter, A. C. et al. Prognostic models for predicting posttraumatic seizures during acute hospitalization, and at 1 and 2 years following traumatic brain injury. Epilepsia 57 , 1503–1514 (2016).
Xu, T. et al. Risk factors for posttraumatic epilepsy: a systematic review and meta-analysis. Epilepsy Behav. 67 , 1–6 (2017).
Temkin, N. R. Risk factors for posttraumatic seizures in adults. Epilepsia 44 , 18–20 (2003).
Arefan, D., Pease, M., Eagle, S. R., Okonkwo, D. O. & Wu, S. Comparison of machine learning models to predict long-term outcomes after severe traumatic brain injury. Neurosurg. Focus 54 , E14 (2023).
Dijkland, S. A. et al. Prognosis in moderate and severe traumatic brain injury: a systematic review of contemporary models and validation studies. J. Neurotrauma 37 , 1–13 (2020).
Steyerberg, E. W. et al. Predicting outcome after traumatic brain injury: development and international validation of prognostic scores based on admission characteristics. PLoS Med 5 , 1251–1261 (2008).
Immonen, R. et al. Imaging biomarkers of epileptogenecity after traumatic brain injury — preclinical frontiers. Neurobiol. Dis. 123 , 75–85 (2019).
Garner, R. et al. Imaging biomarkers of posttraumatic epileptogenesis. Epilepsia 60 , 2151–2162 (2019).
Prince, D. A. et al. Epilepsy following cortical injury: cellular and molecular mechanisms as targets for potential prophylaxis. Epilepsia 50 , 30–40 (2009).
Jennett, W. B. & Lewin, W. Traumatic epilepsy after closed head injuries. J. Neurol. Neurosurg. Psychiatry 23 , 295–301 (1960).
Al-Haddad, S. A. & Kirollos, R. A 5-year study of the outcome of surgically treated depressed skull fractures. Ann. R. Coll. Surg. Engl. 84 , 196–200 (2002).
PubMed PubMed Central Google Scholar
De Reuck, J. Risk factors for late-onset seizures related to cerebral contusions in adults with a moderate traumatic brain injury. Clin. Neurol. Neurosurg. 113 , 469–471 (2011).
La Rocca, M. et al. Distribution and volume analysis of early hemorrhagic contusions by MRI after traumatic brain injury: a preliminary report of the Epilepsy Bioinformatics Study for Antiepileptogenic Therapy (EpiBioS4Rx). Brain Imaging Behav. 15 , 2804–2812 (2021).
Messori, A., Polonara, G., Carle, F., Gesuita, R. & Salvolini, U. Predicting posttraumatic epilepsy with MRI: prospective longitudinal morphologic study in adults. Epilepsia 46 , 1472–1481 (2005).
Gupta, P. K. et al. Subtypes of post-traumatic epilepsy: clinical, electrophysiological, and imaging features. J. Neurotrauma 31 , 1439–1443 (2014).
Won, S. Y. et al. A systematic review of epileptic seizures in adults with subdural haematomas. Seizure 45 , 28–35 (2017).
La Rocca, M. et al. Multiplex networks to characterize seizure development in traumatic brain injury patients. Front. Neurosci. 14 , 591662 (2020).
Lutkenhoff, E. S. et al. Early brain biomarkers of post-traumatic seizures: initial report of the multicentre epilepsy bioinformatics study for antiepileptogenic therapy (EpiBioS4Rx) prospective study. J. Neurol. Neurosurg. Psychiatry 91 , 1154–1157 (2020).
Manninen, E. et al. Acute thalamic damage as a prognostic biomarker for post-traumatic epileptogenesis. Epilepsia 62 , 1852–1864 (2021).
Graham, N. S. N., Cole, J. H., Bourke, N. J., Schott, J. M. & Sharp, D. J. Distinct patterns of neurodegeneration after TBI and in Alzheimer’s disease. Alzheimers Dement. 19 , 3065–3077 (2023).
Dinkel, J. et al. Long-term white matter changes after severe traumatic brain injury: a 5-year prospective cohort. Am. J. Neuroradiol. 35 , 23–29 (2014).
Zelano, J. & Westman, G. Epilepsy after brain infection in adults. Neurology 95 , e3213–e3220 (2020).
Yu, T., Liu, X., Sun, L., Wu, J. & Wang, Q. Clinical characteristics of post-traumatic epilepsy and the factors affecting the latency of PTE. BMC Neurol. 21 , 301 (2021).
Candy, N., Tsimiklis, C., Poonnoose, S. & Trivedi, R. The use of antiepileptic medication in early post traumatic seizure prophylaxis at a single institution. J. Clin. Neurosci. 69 , 198–205 (2019).
DJohn, J., Ibrahim, R., Patel, P., DeHoff, K. & Kolbe, N. Administration of levetiracetam in traumatic brain injury: is it warranted? Cureus 12 , e9117 (2020).
Pease, M. et al. Multicenter and prospective trial of anti-epileptics for early seizure prevention in mild traumatic brain injury with a positive computed tomography scan. Surg. Neurol. Int. 13 , 241 (2022).
Lee, S. T., Lui, T. N., Wong, C. W., Yeh, Y. S. & Tzaan, W. C. Early seizures after moderate closed head injury. Acta Neurochir. 137 , 151–154 (1995).
Temkin, N. R. et al. A randomized, double-blind study of phenytoin for the prevention of post-traumatic seizures. N. Engl. J. Med. 323 , 497–502 (1990).
Gugger, J. J. & Diaz-Arrastia, R. Early posttraumatic seizures — putting things in perspective. JAMA Neurol. 79 , 325–326 (2022).
Jennett, B. Early traumatic epilepsy: incidence and significance after nonmissile injuries. Arch. Neurol. 30 , 394–398 (1974).
Glaser, A. C. et al. The effect of antiseizure medication administration on mortality and early posttraumatic seizures in critically ill older adults with traumatic brain injury. Neurocrit. Care 37 , 538–546 (2022).
Zhao, Y., Wu, H., Wang, X., Li, J. & Zhang, S. Clinical epidemiology of posttraumatic epilepsy in a group of Chinese patients. Seizure 21 , 322–326 (2012).
Bakr, A. & Belli, A. A systematic review of levetiracetam versus phenytoin in the prevention of late post-traumatic seizures and survey of UK neurosurgical prescribing practice of antiepileptic medication in acute traumatic brain injury. Br. J. Neurosurg. 32 , 237–244 (2018).
Carney, N. et al. Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery 80 , 6–15 (2017).
Wilson, C. D. et al. Early and late posttraumatic epilepsy in the setting of traumatic brain injury: a meta-analysis and review of antiepileptic management. World Neurosurg. 110 , e901–e906 (2018).
Sundararajan, K., Milne, D., Edwards, S., Chapman, M. J. & Shakib, S. Anti-seizure prophylaxis in critically ill patients with traumatic brain injury in an intensive care unit. Anaesth. Intensive Care 43 , 646–651 (2015).
Sun, Y. et al. Early post-traumatic seizures are associated with valproic acid plasma concentrations and UGT1A6/CYP2C9 genetic polymorphisms in patients with severe traumatic brain injury. Scand. J. Trauma Resusc. Emerg. Med 25 , 85 (2017).
Temkin, N. R. et al. Valproate therapy for prevention of posttraumatic seizures: a randomized trial. J. Neurosurg. 91 , 593–600 (1999).
Wang, B. C. et al. Comparative efficacy of prophylactic anticonvulsant drugs following traumatic brain injury: a systematic review and network meta-analysis of randomized controlled trials. PLoS ONE 17 , e0265932 (2022).
Kwon, S. J. et al. Lacosamide versus phenytoin for the prevention of early post traumatic seizures. J. Crit. Care 50 , 50–53 (2019).
Herman, S. T. et al. Consensus statement on continuous EEG in critically ill adults and children, part I: indications. J. Clin. Neurophysiol. 32 , 87–95 (2015).
Vespa, P. et al. Metabolic crisis occurs with seizures and periodic discharges after brain trauma. Ann. Neurol. 79 , 579–590 (2016).
Khor, D. et al. Early seizure prophylaxis in traumatic brain injuries revisited: a prospective observational study. World J. Surg. 42 , 1727–1732 (2018).
Inglet, S. et al. Seizure prophylaxis in patients with traumatic brain injury: a single-center study. Cureus 8 , 6–8 (2016).
Hazama, A. et al. The effect of Keppra prophylaxis on the incidence of early onset, post-traumatic brain injury seizures. Cureus 10 , e2674 (2018).
Pingue, V., Mele, C. & Nardone, A. Post-traumatic seizures and antiepileptic therapy as predictors of the functional outcome in patients with traumatic brain injury. Sci. Rep. 11 , 4708 (2021).
Zaccara, G. et al. Do antiepileptic drugs increase the risk of infectious diseases? A meta-analysis of placebo-controlled studies. Br. J. Clin. Pharm. 83 , 1873–1879 (2017).
Article CAS Google Scholar
Carpay, J. A., Aldenkamp, A. P. & van Donselaar, C. A. Complaints associated with the use of antiepileptic drugs: results from a community-based study. Seizure 14 , 198–206 (2005).
Lu, X. & Wang, X. Hyponatremia induced by antiepileptic drugs in patients with epilepsy. Expert Opin. Drug Saf. 16 , 77–87 (2017).
Aarabi, B., Taghipour, M., Haghnegahdar, A., Farokhi, M. & Mobley, L. Prognostic factors in the occurrence of posttraumatic epilepsy after penetrating head injury suffered during military service. Neurosurg. Focus 8 , e1 (2000).
Galanopoulou, A. S. et al. Antiepileptogenesis and disease modification: progress, challenges, and the path forward — Report of the Preclinical Working Group of the 2018 NINDS-sponsored antiepileptogenesis and disease modification workshop. Epilepsia Open 6 , 276–296 (2021).
Pingue, V. et al. Impact of seizures and their prophylaxis with antiepileptic drugs on rehabilitation course of patients with traumatic or hemorrhagic brain injury. Front. Neurol. 13 , 1060008 (2022).
Pugh, M. J. et al. The military injuries: understanding post-traumatic epilepsy study: understanding relationships among lifetime traumatic brain injury history, epilepsy, and quality of life. J. Neurotrauma 38 , 2841–2850 (2021).
Juengst, S. B. et al. Post-traumatic epilepsy associations with mental health outcomes in the first two years after moderate to severe TBI: a TBI Model Systems analysis. Epilepsy Behav. 73 , 240–246 (2017).
Mazzini, L. et al. Posttraumatic epilepsy: neuroradiologic and neuropsychological assessment of long-term outcome. Epilepsia 44 , 569–574 (2003).
Semple, B. D., Zamani, A., Rayner, G., Shultz, S. R. & Jones, N. C. Affective, neurocognitive and psychosocial disorders associated with traumatic brain injury and post-traumatic epilepsy. Neurobiol. Dis. 123 , 27–41 (2020).
Ngadimon, I. W. et al. An interplay between post-traumatic epilepsy and associated cognitive decline: a systematic review. Front. Neurol. 13 , 827571 (2022).
Mukherjee, S. et al. Neuroinflammatory mechanisms of post-traumatic epilepsy. J. Neuroinflammation 17 , 193 (2020).
La Rocca, M. et al. Functional connectivity alterations in traumatic brain injury patients with late seizures. Neurobiol. Dis. 179 , 106053 (2023).
Pease, M. et al. Outcome prediction in patients with severe traumatic brain injury using deep learning from head CT scans. Radiology 304 , 385–394 (2022).
Lutkenhoff, E. S. et al. The subcortical basis of outcome and cognitive impairment in TBI: a longitudinal cohort study. Neurology 95 , E2398–E2408 (2020).
Walker, W. C. et al. Global outcome and late seizures after penetrating versus closed traumatic brain injury: a NIDRR TBI Model Systems study. J. Head Trauma Rehabil. 30 , 231–240 (2015).
Bushnik, T., Englander, J., Wright, J. & Kolakowsky-Hayner, S. A. Traumatic brain injury with and without late posttraumatic seizures: what are the impacts in the post-acute phase: a NIDRR traumatic brain injury model systems study. J. Head Trauma Rehabil. 27 , 36–44 (2012).
Yu, T. et al. Predicting global functional outcomes among post-traumatic epilepsy patients after moderate-to-severe traumatic brain injury: development of a prognostic model. Front. Neurol. 13 , 874491 (2022).
Uski, J., Lamusuo, S., Teperi, S., Löyttyniemi, E. & Tenovuo, O. Mortality after traumatic brain injury and the effect of posttraumatic epilepsy. Neurology 91 , e878–e883 (2018).
Karlander, M., Ljungqvist, J., Sörbo, A. & Zelano, J. Risk and cause of death in post-traumatic epilepsy: a register-based retrospective cohort study. J. Neurol. 269 , 6014–6020 (2022).
Englander, J., Bushnik, T., Wright, J. M., Jamison, L. & Duong, T. T. Mortality in late post-traumatic seizures. J. Neurotrauma 26 , 1471–1477 (2009).
Rayner, G., Jackson, G. D. & Wilson, S. J. Two distinct symptom-based phenotypes of depression in epilepsy yield specific clinical and etiological insights. Epilepsy Behav. 64 , 336–344 (2016).
Ponsford, J. Anxiety and depression following TBI. in Neurobehavioural Disability and Social Handicap Following Traumatic Brain Injury 2nd edn (eds McMillan, T. M. & Wood, R. L. L.) 167–177 (Taylor & Francis, 2017).
Foreman, B. et al. Seizures and cognitive outcome after traumatic brain injury: a post hoc analysis. Neurocrit. Care 36 , 130–138 (2022).
Lee, H. et al. Continuous electroencephalography after moderate to severe traumatic brain injury. Crit. Care Med 47 , 574–582 (2019).
He, X. et al. Resective surgery for drug-resistant posttraumatic epilepsy: predictors of seizure outcome. J. Neurosurg. 133 , 1568–1575 (2019).
Chartrain, A. G. et al. Antiepileptics for post-traumatic seizure prophylaxis after traumatic brain injury. Curr. Pharm. Des. 23 , 6428–6441 (2017).
Zimmermann, L. L., Martin, R. M. & Girgis, F. Treatment options for posttraumatic epilepsy. Curr. Opin. Neurol. 30 , 580–586 (2017).
Hamed, R., Hussein, R., Ibraheem, S. & Jumaily, M. Comparative study between leviteracetam, phenytoin & carbamazepine in treating post traumatic epilepsy. NeuroQuantology 18 , 1–5 (2020).
Scheffer, I. E. et al. ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia 58 , 512–521 (2017).
Hitti, F. L. et al. Surgical outcomes in post-traumatic epilepsy: a single institutional experience. Oper. Neurosurg. 18 , 12–18 (2020).
Marks, D. A., Kim, J., Spencer, D. D. & Spencer, S. S. Seizure localization and pathology following head injury in patients with uncontrolled epilepsy. Neurology 45 , 2051–2057 (1995).
Hakimian, S. et al. Long-term outcome of extratemporal resection in posttraumatic epilepsy. Neurosurg. Focus 32 , E10 (2012).
Schuele, S. U. & Lüders, H. O. Intractable epilepsy: management and therapeutic alternatives. Lancet Neurol. 7 , 514–524 (2008).
Ferreira, L. D., Tabaeizadeh, M. & Haneef, Z. Surgical outcomes in post-traumatic temporal lobe epilepsy: a systematic review and meta-analysis. J. Neurotrauma 41 , 319–330 (2024).
Wiebe, S., Blume, W. T., Girvin, J. P. & Eliasziw, M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N. Engl. J. Med. 345 , 311–318 (2001).
Lee, H.-O. et al. Effect of vagus nerve stimulation in post-traumatic epilepsy and failed epilepsy surgery: preliminary report. J. Korean Neurosurg. Soc. 44 , 196–198 (2008).
Shen, C.-C. & Jiang, J.-F. Auricular electroacupuncture for late posttraumatic epilepsy after severe brain injury: a retrospective study. Evid. Based Complement Altern. Med. 2019 , 5798912 (2019).
Chen, Y. et al. Quantitative epileptiform burden and electroencephalography background features predict post-traumatic epilepsy. J. Neurol. Neurosurg. Psychiatry 94 , 245–249 (2023).
Kim, J. A. et al. Epileptiform activity in traumatic brain injury predicts post-traumatic epilepsy. Ann. Neurol. 83 , 858–862 (2018).
Faghihpirayesh, R. et al. Automatic detection of EEG epileptiform abnormalities in traumatic brain injury using deep learning. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2021 , 302–305 (2021).
Di Sapia, R. et al. ECoG spiking activity and signal dimension are early predictive measures of epileptogenesis in a translational mouse model of traumatic brain injury. Neurobiol. Dis. 185 , 106251 (2023).
Kumar, U., Li, L., Bragin, A. & Engel, J. J. Spike and wave discharges and fast ripples during posttraumatic epileptogenesis. Epilepsia 62 , 1842–1851 (2021).
Li, L. et al. Spatial and temporal profile of high-frequency oscillations in posttraumatic epileptogenesis. Neurobiol. Dis. 161 , 105544 (2021).
Pease, M. et al. Predicting post-traumatic epilepsy using admission electroencephalography after severe traumatic brain injury. Epilepsia 64 , 1842–1852 (2023).
Ghaith, H. S. et al. A literature review of traumatic brain injury biomarkers. Mol. Neurobiol. 59 , 4141–4158 (2022).
Misra, S. et al. Common pathways of epileptogenesis in patients with epilepsy post-brain injury: findings from a systematic review and meta-analysis. Neurology 101 , e2243–e2256 (2023).
Cotter, D., Kelso, A. & Neligan, A. Genetic biomarkers of posttraumatic epilepsy: a systematic review. Seizure 46 , 53–58 (2017).
Nam, J.-W. et al. Global analyses of the effect of different cellular contexts on microRNA targeting. Mol. Cell 53 , 1031–1043 (2014).
Yu, Y. et al. The role of exosomal microRNAs in central nervous system diseases. Mol. Cell. Biochem. 476 , 2111–2124 (2021).
Gitaí, D. L. G. et al. Extracellular vesicles in the forebrain display reduced miR-346 and miR-331-3p in a rat model of chronic temporal lobe epilepsy. Mol. Neurobiol. 57 , 1674–1687 (2020).
Chen, S.-D. et al. Circulating microRNAs from serum exosomes may serve as a putative biomarker in the diagnosis and treatment of patients with focal cortical dysplasia. Cells 9 , 1867 (2020).
Brennan, G. P. & Henshall, D. C. microRNAs in the pathophysiology of epilepsy. Neurosci. Lett. 667 , 47–52 (2018).
Wang, Y. et al. Circulating microRNAs from plasma small extracellular vesicles as potential diagnostic biomarkers in pediatric epilepsy and drug-resistant epilepsy. Front. Mol. Neurosci. 15 , 823802 (2022).
Heiskanen, M. et al. Discovery and validation of circulating microRNAs as biomarkers for epileptogenesis after experimental traumatic brain injury — the EPITARGET Cohort. Int. J. Mol. Sci. 24 , 2823 (2023).
Kumar, P. miRNA dysregulation in traumatic brain injury and epilepsy: a systematic review to identify putative biomarkers for post-traumatic epilepsy. Metab. Brain Dis. 38 , 749–765 (2023).
Karnati, H. K. et al. Neuronal enriched extracellular vesicle proteins as biomarkers for traumatic brain injury. J. Neurotrauma 36 , 975–987 (2019).
Lin, Z. et al. Serum exosomal proteins F9 and TSP-1 as potential diagnostic biomarkers for newly diagnosed epilepsy. Front. Neurosci. 14 , 737 (2020).
Karttunen, J., Heiskanen, M., Lipponen, A., Poulsen, D. & Pitkänen, A. Extracellular vesicles as diagnostics and therapeutics for structural epilepsies. Int. J. Mol. Sci. 20 , 1259 (2019).
Upadhya, D. & Shetty, A. K. Promise of extracellular vesicles for diagnosis and treatment of epilepsy. Epilepsy Behav. 121 , 106499 (2021).
Redell, J. B., Moore, A. N., Ward, N. H. III, Hergenroeder, G. W. & Dash, P. K. Human traumatic brain injury alters plasma microRNA levels. J. Neurotrauma 27 , 2147–2156 (2010).
Qin, X. et al. Expression profile of plasma microRNAs and their roles in diagnosis of mild to severe traumatic brain injury. PLoS ONE 13 , e0204051 (2018).
Ko, J. et al. Diagnosis of traumatic brain injury using miRNA signatures in nanomagnetically isolated brain-derived extracellular vesicles. Lab Chip 18 , 3617–3630 (2018).
Hicks, S. D. et al. Overlapping microRNA expression in saliva and cerebrospinal fluid accurately identifies pediatric traumatic brain injury. J. Neurotrauma 35 , 64–72 (2018).
Di Pietro, V. et al. MicroRNAs as novel biomarkers for the diagnosis and prognosis of mild and severe traumatic brain injury. J. Neurotrauma 34 , 1948–1956 (2017).
Bhomia, M., Balakathiresan, N. S., Wang, K. K., Papa, L. & Maheshwari, R. K. A panel of serum miRNA biomarkers for the diagnosis of severe to mild traumatic brain injury in humans. Sci. Rep. 6 , 28148 (2016).
Yan, S. et al. Altered microRNA profiles in plasma exosomes from mesial temporal lobe epilepsy with hippocampal sclerosis. Oncotarget 8 , 4136–4146 (2017).
Raoof, R. et al. Dual-center, dual-platform microRNA profiling identifies potential plasma biomarkers of adult temporal lobe epilepsy. eBioMedicine 38 , 127–141 (2018).
Medel-Matus, J.-S. et al. Susceptibility to epilepsy after traumatic brain injury is associated with preexistent gut microbiome profile. Epilepsia 63 , 1835–1848 (2022).
Medel-Matus, J.-S. et al. Modification of post-traumatic epilepsy by fecal microbiota transfer. Epilepsy Behav. 134 , 108860 (2022).
French, J. A. et al. Characteristics of medial temporal lobe epilepsy: I. Results of history and physical examination. Ann. Neurol. 34 , 774–780 (1993).
Löscher, W. The search for new screening models of pharmacoresistant epilepsy: is induction of acute seizures in epileptic rodents a suitable approach? Neurochem. Res. 42 , 1926–1938 (2017).
Dudek, F. E. & Staley, K. J. The time course and circuit mechanisms of acquired epileptogenesis. in Jasper’s Basic Mechanisms of the Epilepsies 4th edn (eds Noebels, J. L. et al.) (Oxford Univ. Press, 2012).
Santhakumar, V., Ratzliff, A. D., Jeng, J., Toth, Z. & Soltesz, I. Long-term hyperexcitability in the hippocampus after experimental head trauma. Ann. Neurol. 50 , 708–717 (2001).
Dudek, F. E. & Spitz, M. Hypothetical mechanisms for the cellular and neurophysiologic basis of secondary epileptogenesis: proposed role of synaptic reorganization. J. Clin. Neurophysiol. 14 , 90–101 (1997).
Cantu, D. et al. Traumatic brain injury increases cortical glutamate network activity by compromising GABAergic control. Cereb. Cortex 25 , 2306–2320 (2015).
Lowenstein, D. H., Thomas, M. J., Smith, D. H. & McIntosh, T. K. Selective vulnerability of dentate hilar neurons following traumatic brain injury: a potential mechanistic link between head trauma and disorders of the hippocampus. J. Neurosci. 12 , 4846–4853 (1992).
Raible, D. J., Frey, L. C., Cruz Del Angel, Y., Russek, S. J. & Brooks-Kayal, A. R. GABA(A) receptor regulation after experimental traumatic brain injury. J. Neurotrauma 29 , 2548–2554 (2012).
Gupta, A., Elgammal, F. S., Proddutur, A., Shah, S. & Santhakumar, V. Decrease in tonic inhibition contributes to increase in dentate semilunar granule cell excitability after brain injury. J. Neurosci. 32 , 2523–2537 (2012).
Webster, K. M. et al. Inflammation in epileptogenesis after traumatic brain injury. J. Neuroinflammation 14 , 10 (2017).
Hunt, R. F., Scheff, S. W. & Smith, B. N. Synaptic reorganization of inhibitory hilar interneuron circuitry after traumatic brain injury in mice. J. Neurosci. 31 , 6880–6890 (2011).
Bolkvadze, T., Puhakka, N. & Pitkänen, A. Epileptogenesis after traumatic brain injury in Plaur-deficient mice. Epilepsy Behav. 60 , 187–196 (2016).
Kumar, P. et al. Single-cell transcriptomics and surface epitope detection in human brain epileptic lesions identifies pro-inflammatory signaling. Nat. Neurosci. 25 , 956–966 (2022).
Srinivasan, D., Yen, J.-H., Joseph, D. J. & Friedman, W. Cell type-specific interleukin-1beta signaling in the CNS. J. Neurosci. 24 , 6482–6488 (2004).
Shiozaki, T. et al. Cerebrospinal fluid concentrations of anti-inflammatory mediators in early-phase severe traumatic brain injury. Shock 23 , 406–410 (2005).
Fan, L. et al. Experimental brain injury induces expression of interleukin-1 beta mRNA in the rat brain. Brain Res. Mol. Brain Res. 30 , 125–130 (1995).
Clausen, F. et al. Neutralization of interleukin-1beta modifies the inflammatory response and improves histological and cognitive outcome following traumatic brain injury in mice. Eur. J. Neurosci. 30 , 385–396 (2009).
Scaffidi, P., Misteli, T. & Bianchi, M. E. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418 , 191–195 (2002).
Shi, Y., Zhang, L., Teng, J. & Miao, W. HMGB1 mediates microglia activation via the TLR4/NF-κB pathway in coriaria lactone induced epilepsy. Mol. Med. Rep. 17 , 5125–5131 (2018).
Chiavegato, A., Zurolo, E., Losi, G., Aronica, E. & Carmignoto, G. The inflammatory molecules IL-1β and HMGB1 can rapidly enhance focal seizure generation in a brain slice model of temporal lobe epilepsy. Front. Cell. Neurosci. 8 , 155 (2014).
Semple, B. D., Kossmann, T. & Morganti-Kossmann, M. C. Role of chemokines in CNS health and pathology: a focus on the CCL2/CCR2 and CXCL8/CXCR2 networks. J. Cereb. Blood Flow Metab. 30 , 459–473 (2010).
Gosselin, R. D. et al. Constitutive expression of CCR2 chemokine receptor and inhibition by MCP-1/CCL2 of GABA-induced currents in spinal cord neurones. J. Neurochem. 95 , 1023–1034 (2005).
Patabendige, A. & Janigro, D. The role of the blood–brain barrier during neurological disease and infection. Biochem. Soc. Trans. 51 , 613–626 (2023).
Marchi, N., Granata, T., Alexopoulos, A. & Janigro, D. The blood–brain barrier hypothesis in drug resistant epilepsy. Brain 135 , e211 (2012).
Bargerstock, E. et al. Is peripheral immunity regulated by blood–brain barrier permeability changes? PLoS ONE 9 , e101477 (2014).
Dadas, A. & Janigro, D. Breakdown of blood–brain barrier as a mechanism of post-traumatic epilepsy. Neurobiol. Dis. 123 , 20–26 (2019).
Weissberg, I. et al. Albumin induces excitatory synaptogenesis through astrocytic TGF-β/ALK5 signaling in a model of acquired epilepsy following blood–brain barrier dysfunction. Neurobiol. Dis. 78 , 115–125 (2015).
Ivens, S. et al. TGF-beta receptor-mediated albumin uptake into astrocytes is involved in neocortical epileptogenesis. Brain 130 , 535–547 (2007).
Saletti, P. G. et al. In search of antiepileptogenic treatments for post-traumatic epilepsy. Neurobiol. Dis. 123 , 86–99 (2019).
Kendirli, M. T., Rose, D. T. & Bertram, E. H. A model of posttraumatic epilepsy after penetrating brain injuries: effect of lesion size and metal fragments. Epilepsia 55 , 1969–1977 (2014).
Takei, N. & Nawa, H. mTOR signaling and its roles in normal and abnormal brain development. Front. Mol. Neurosci. 7 , 28 (2014).
Lee, D. Y. Roles of mTOR signaling in brain development. Exp. Neurobiol. 24 , 177–185 (2015).
Butler, C. R., Boychuk, J. A. & Smith, B. N. Effects of rapamycin treatment on neurogenesis and synaptic reorganization in the dentate gyrus after controlled cortical impact injury in mice. Front. Syst. Neurosci. 9 , 163 (2015).
Niu, L.-J., Xu, R.-X., Zhang, P., Du, M.-X. & Jiang, X.-D. Suppression of Frizzled-2-mediated Wnt/Ca 2+ signaling significantly attenuates intracellular calcium accumulation in vitro and in a rat model of traumatic brain injury. Neuroscience 213 , 19–28 (2012).
Mardones, M. D. & Gupta, K. Transcriptome profiling of the hippocampal seizure network implicates a role for Wnt signaling during epileptogenesis in a mouse model of temporal lobe epilepsy. Int. J. Mol. Sci. 23 , 12030 (2022).
Gupta, K. & Schnell, E. Neuronal network remodeling and Wnt pathway dysregulation in the intra-hippocampal kainate mouse model of temporal lobe epilepsy. PLoS ONE 14 , e0215789 (2019).
Raza, M. et al. Evidence that injury-induced changes in hippocampal neuronal calcium dynamics during epileptogenesis cause acquired epilepsy. Proc. Natl Acad. Sci. USA 101 , 17522–17527 (2004).
Powell, K. L., Cain, S. M., Snutch, T. P. & O’Brien, T. J. Low threshold T-type calcium channels as targets for novel epilepsy treatments. Br. J. Clin. Pharm. 77 , 729–739 (2014).
Conboy, K., Henshall, D. C. & Brennan, G. P. Epigenetic principles underlying epileptogenesis and epilepsy syndromes. Neurobiol. Dis. 148 , 105179 (2021).
Dębski, K. J. et al. Etiology matters — genomic DNA methylation patterns in three rat models of acquired epilepsy. Sci. Rep. 6 , 25668 (2016).
Nelson, E. D., Kavalali, E. T. & Monteggia, L. M. Activity-dependent suppression of miniature neurotransmission through the regulation of DNA methylation. J. Neurosci. 28 , 395–406 (2008).
Machnes, Z. M. et al. DNA methylation mediates persistent epileptiform activity in vitro and in vivo. PLoS ONE 8 , e76299 (2013).
Deutsch, S. I., Mastropaolo, J., Burket, J. A. & Rosse, R. B. An epigenetic intervention interacts with genetic strain differences to modulate the stress-induced reduction of flurazepam’s antiseizure efficacy in the mouse. Eur. Neuropsychopharmacol. 19 , 398–401 (2009).
Perez-Pinera, P. et al. RNA-guided gene activation by CRISPR-Cas9-based transcription factors. Nat. Methods 10 , 973–976 (2013).
Dominguez, A. A., Lim, W. A. & Qi, L. S. Beyond editing: repurposing CRISPR-Cas9 for precision genome regulation and interrogation. Nat. Rev. Mol. Cell Biol. 17 , 5–15 (2016).
Sasa, M. A new frontier in epilepsy: novel antiepileptogenic drugs. J. Pharmacol. Sci. 100 , 487–494 (2006).
Brady, R. D. et al. Modelling traumatic brain injury and posttraumatic epilepsy in rodents. Neurobiol. Dis. 123 , 8–19 (2020).
Ford, I. & Norrie, J. Pragmatic trials. N. Engl. J. Med. 375 , 454–463 (2016).
Casey, J. D., Beskow, L. M. & Brown, J. Use of pragmatic and explanatory trial designs in acute care research: lessons from COVID-19. Lancet Respir. Med. 10 , 700–714 (2022).
Braun, R. It’s been a TRACK-TBI LONG time coming but well worth the wait. Neurology 101 , 287–289 (2023).
McCrea, M. A. et al. Functional outcomes over the first year after moderate to severe traumatic brain injury in the prospective, longitudinal TRACK-TBI study. JAMA Neurol. 78 , 982–992 (2021).
Andrews, P. J. et al. Therapeutic hypothermia to reduce intracranial pressure after traumatic brain injury: the Eurotherm3235 RCT. Health Technol. Assess. 22 , 1–134 (2018).
Bastian, L. A. et al. Stakeholder engagement in pragmatic clinical trials: emphasizing relationships to improve pain management delivery and outcomes. Pain Med. 21 , S13–S20 (2020).
Morain, S. & Largent, E. Think pragmatically: investigators’ obligations to patient-subjects when research is embedded in care. Am. J. Bioeth. 23 , 10–21 (2023).
Diaz, V. Encouraging participation of minorities in research studies. Ann. Fam. Med. 10 , 372–373 (2012).
Boden-Albala, B. et al. Use of community-engaged research approaches in clinical interventions for neurologic disorders in the United States. Neurology 101 , S27–S46 (2023).
Griffith, D. M., Towfighi, A., Manson, S. M., Littlejohn, E. L. & Skolarus, L. E. Determinants of inequities in neurologic disease, health, and well-being: the NINDS Social Determinants of Health Framework. Neurology 101 , S75–S81 (2023).
Danziger, J. et al. Temporal trends in critical care outcomes in U.S. minority-serving hospitals. Am. J. Respir. Crit. Care Med 201 , 681–687 (2020).
Kanter, G. P., Segal, A. G. & Groeneveld, P. W. Income disparities in access to critical care services. Health Aff. 39 , 1362–1367 (2020).
Nayfeh, A. & Fowler, R. A. Understanding patient- and hospital-level factors leading to differences, and disparities, in critical care. Am. J. Respir. Crit. Care Med 201 , 642–644 (2020).
Brown, K. E., Fohner, A. E. & Woodahl, E. L. Beyond the individual: community-centric approaches to increase diversity in biomedical research. Clin. Pharmacol. Ther. 113 , 509–517 (2023).
Benizri, N., Hallot, S., Burns, K. & Goldfarb, M. Patient and family representation in randomized clinical trials published in 3 medical and surgical journals: a systematic review. JAMA Netw. Open 5 , e2230858 (2022).
Gill, M. et al. Patient and family member-led research in the intensive care unit: a novel approach to patient-centered research. PLoS ONE 11 , e0160947 (2016).
Fairley, R. et al. Increasing clinical trial participation of Black women diagnosed with breast cancer. J. Racial Ethn. Health Disparities https://doi.org/10.1007/s40615-023-01644-z (2023).
Barrett, N. J. et al. Factors associated with biomedical research participation within community-based samples across 3 National Cancer Institute-designated cancer centers. Cancer 126 , 1077–1089 (2020).
Miles, S. R. et al. Evolution of irritability, anger, and aggression after traumatic brain injury: identifying and predicting subgroups. J. Neurotrauma 38 , 1827–1833 (2021).
Driver, S., Reynolds, M. & Kramer, K. Modifying an evidence-based lifestyle programme for individuals with traumatic brain injury. Brain Inj. 31 , 1612–1616 (2017).
Sutton, K. M. et al. Engaging individuals with neurological conditions and caregivers in rural communities in a health research team. Prog. Community Health Partnersh. 13 , 129–139 (2019).
Shimia, M. et al. A placebo-controlled randomized clinical trial of amantadine hydrochloride for evaluating the functional improvement of patients following severe acute traumatic brain injury. J. Neurosurg. Sci. 67 , 598–604 (2023).
Morey, C. E., Cilo, M., Berry, J. & Cusick, C. The effect of Aricept in persons with persistent memory disorder following traumatic brain injury: a pilot study. Brain Inj. 17 , 809–815 (2003).
Jha, A. et al. A randomized trial of modafinil for the treatment of fatigue and excessive daytime sleepiness in individuals with chronic traumatic brain injury. J. Head Trauma Rehabil. 23 , 52–63 (2008).
Shultz, S. R. et al. Sodium selenate reduces hyperphosphorylated tau and improves outcomes after traumatic brain injury. Brain 138 , 1297–1313 (2015).
Liu, S.-J. et al. Sodium selenate retards epileptogenesis in acquired epilepsy models reversing changes in protein phosphatase 2A and hyperphosphorylated tau. Brain 139 , 1919–1938 (2016).
Li, Z. et al. Iron neurotoxicity and protection by deferoxamine in intracerebral hemorrhage. Front. Mol. Neurosci. 15 , 927334 (2022).
Terrone, G., Balosso, S., Pauletti, A., Ravizza, T. & Vezzani, A. Inflammation and reactive oxygen species as disease modifiers in epilepsy. Neuropharmacology 167 , 107742 (2020).
Serrano, G. E. et al. Ablation of cyclooxygenase-2 in forebrain neurons is neuroprotective and dampens brain inflammation after status epilepticus. J. Neurosci. 31 , 14850–14860 (2011).
Desjardins, P. et al. Induction of astrocytic cyclooxygenase-2 in epileptic patients with hippocampal sclerosis. Neurochem. Int. 42 , 299–303 (2003).
Löscher, W. & Friedman, A. Structural, molecular, and functional alterations of the blood–brain barrier during epileptogenesis and epilepsy: a cause, consequence, or both? Int. J. Mol. Sci. 21 , 591 (2020).
Henshall, D. C. & Kobow, K. Epigenetics and epilepsy. Cold Spring Harb. Perspect. Med. 5 , a022731 (2015).
Guo, D., Zeng, L., Brody, D. L. & Wong, M. Rapamycin attenuates the development of posttraumatic epilepsy in a mouse model of traumatic brain injury. PLoS ONE 8 , e64078 (2013).
Galanopoulou, A. S., Gorter, J. A. & Cepeda, C. Finding a better drug for epilepsy: the mTOR pathway as an antiepileptogenic target. Epilepsia 53 , 1119–1130 (2012).
Coulter, D. A. & Steinhäuser, C. Role of astrocytes in epilepsy. Cold Spring Harb. Perspect. Med. 5 , a022434 (2015).
Download references
Acknowledgements
The authors thank Christopher Brown (Indiana University) for his contributions to the design of the figures for this manuscript.
Author information
Authors and affiliations.
Department of Neurosurgery, Indiana University, Bloomington, IN, USA
Matthew Pease
Department of Neurosurgery, Medical College of Wisconsin, Milwaukee, WI, USA
Kunal Gupta
The Saul R. Korey Department of Neurology, Albert Einstein College of Medicine, New York, NY, USA
Solomon L. Moshé, Daniel J. Correa & Aristea S. Galanopoulou
Department of Neuroscience, Albert Einstein College of Medicine, New York, NY, USA
Solomon L. Moshé & Aristea S. Galanopoulou
Department of Paediatrics, Albert Einstein College of Medicine, New York, NY, USA
Solomon L. Moshé
Department of Neurosurgery, University of Pittsburgh, Pittsburgh, PA, USA
David O. Okonkwo, Jorge Gonzalez-Martinez & Lori Shutter
Department of Critical Care Medicine, University of Pittsburgh, Pittsburgh, PA, USA
Lori Shutter
Department of Neurology, University of Pittsburgh, Pittsburgh, PA, USA
Lori Shutter & James F. Castellano
Department of Neurology, University of Pennsylvania, Philadelphia, PA, USA
Ramon Diaz-Arrastia
You can also search for this author in PubMed Google Scholar
Contributions
M.P., K.G., S.M., A.G., D.O.O., J.G.-M., L.S. and J.F.C. researched data for the article. All authors contributed substantially to discussion of the content, wrote the article and reviewed and/or edited the manuscript before submission.
Corresponding author
Correspondence to Matthew Pease .
Ethics declarations
Competing interests.
S.M. is the Charles Frost Chair in Neurosurgery and Neurology and partially funded by grants from NIH U54 NS100064 (EpiBioS4Rx), R01-NS43209 and R01-NS127524, the US Department of Defense (W81XWH-22-1-0510, W81XWH-22-1-0210), a pilot grant from the National Institute of Child Health and Human Development (NICHD) centre grant (P50 HD105352) for the Rose F. Kennedy Intellectual and Developmental Disabilities Research Center (RFK-IDDRC), the Heffer Family and the Segal Family Foundations, the Isabelle Rapin and Harold Oaklander Child Neurology Research Fund in the Isabelle Rapin Child Neurology Division and the Abbe Goldstein/Joshua Lurie and Laurie Marsh/Dan Levitz families. He is on the editorial boards of Brain and Development, Paediatric Neurology, Annals of Neurology, MedLink and Physiological Research. He receives compensation from MedLink for his work as Associate Editor; and royalties from books he co-edited. A.G. acknowledges research grant support from NINDS R01-NS127524, US Department of Defense (W81XWH-22-1-0210, W81XWH-22-1-0510, EP220067), a pilot grant from the NICHD centre grant (P50 HD105352) for the RFK-IDDRC, R01-DA019473, R01-AI164864, the Heffer Family and the Segal Family Foundations, the Isabelle Rapin and Harold Oaklander Child Neurology Research Fund in the Isabelle Rapin Child Neurology Division and the Abbe Goldstein/Joshua Lurie and Laurie Marsh/Dan Levitz families. She is the Editor-in-Chief of Epilepsia Open and associate editor of Neurobiology of Disease and receives royalties from Elsevier, Walters Kluwer and MedLink for publications. J.G.-M. receives consulting fees for Zimmer Biomet. D.C. receives compensation as lead editor for the Brain and Life podcast for the American Academy of Neurology and is co-editor of a new textbook on health equity among neurological disorders including chapters on traumatic brain injury and epilepsy. J.F.C. and K.G. accept fees from NeuroOne Medical Technologies Corporation for consulting. The other authors declare no competing interests.
Peer review
Peer review information.
Nature Reviews Neurology thanks S. Shultz and D. Duncan for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
(AEMs). Therapies that ameliorate epileptogenesis, with lasting effects beyond the period of drug exposure.
(ASMs). Medications that treat seizures but do not modify the process of epileptogenesis or alter the disease course of epilepsy.
A common neurosurgical procedure to drain cerebrospinal fluid, usually from the ventricular system, thereby decreasing intracranial pressure.
A radiological finding denoting an area of brain tissue that has undergone liquefactive necrosis.
A classification scheme of seizure outcomes after epilepsy surgery using four classes: 1, free of disabling seizures; 2, rare disabling seizures; 3, worthwhile improvement; and 4, no worthwhile improvement.
(GOSE). An extension of the Glasgow Outcome Scale that subdivides the categories of severe disability, moderate disability and good recovery into lower and upper categories.
(GCS). A broadly utilized clinical scale describing the level of consciousness after traumatic injury.
(GOS). A global scale for functional outcome after brain injury that rates patient status using five categories: dead, vegetative state, severe disability, moderate disability and good recovery.
Traumatic brain injury with post-impact (may not need resuscitation) Glasgow Coma Scale score 13–15.
Traumatic brain injury with post-resuscitation Glasgow Coma Scale score 9–12.
Stereotypical movements of the trunk and extremities in response to stimuli, typically indicative of significant CNS injury.
Traumatic brain injury with post-resuscitation Glasgow Coma Scale score ≤ 8.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Pease, M., Gupta, K., Moshé, S.L. et al. Insights into epileptogenesis from post-traumatic epilepsy. Nat Rev Neurol (2024). https://doi.org/10.1038/s41582-024-00954-y
Download citation
Accepted : 07 March 2024
Published : 03 April 2024
DOI : https://doi.org/10.1038/s41582-024-00954-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Translational Research newsletter — top stories in biotechnology, drug discovery and pharma.

Main navigation
- Clinics and Programs
- How to get an appointment at The Neuro
- COVID-19 information for patients and visitors
- Your visit to The Neuro
- The Neuro-Patient Resource Centre
- Services for patients and visitors
- Patients' Rights
- Diagnostic Services (EEG, EMG, X-RAY)
- Get involved: Open Biobank and Clinical Trials
- 5th Annual Neuro Open Science in Action Symposium 2023
- Open Science Awards and Prizes
- Open Science Best Practices
- Open Science Resources
- Open Stories
- Tanenbaum Open Science Institute (TOSI)
- Neuro Open Science Grants
- Azrieli Centre for Autism Research (ACAR)
- Open Biobank
- Early Drug Discovery Unit (EDDU)
- Funding opportunities
- Research Groups
- Partnerships
- Animal Research
- Research Ethics Board
- Brain Tumour
- Cognitive Neuroscience
- Neural Circuits
- Neurodegenerative Disorders
- Neurodevelopmental Disorders
- Neuroimaging and Neuroinformatics
- Neuroimmunological Diseases
- Rare Neurological Diseases and Disorders
- Centre of Neurological Disease Models
- Video on demand
- Plan an event
- A Day in the Life
- Patient Stories
- Research Stories
- Open Science stories
- I Choose the Neuro stories
- Neuro XXceptional Stories
- Giving Stories
- Message from the Director
- Equity, Diversity and Inclusion
- Information for staff at The Neuro
- Awards at a Glance
- A Killam Institution
- Brenda Milner
- Neuro Advisory Board
- Volunteer at The Neuro
A more effective means to detect and treat epileptic seizures

- Add to calendar
- Tweet Widget
One percent of the world’s population has epilepsy and 30 percent of those are resistant to medication. Their seizures can be debilitating a nd life threatening. For some, the best o ption is sur gery .
If the source of the seizures is unclear, surgeons may resort to placing electrodes into the brain to directly record from specific areas. If seizure activity is confined to an area around the electrodes, surgeons can creat e a lesion in the brain to stop the abnormal electrical activity that causes the seizure.
Unfortunately, the lesions created tend to be very small , often too small to permanently stop the seizures. Dr. Jeff ery Hall and colleagues at The Neuro are working on perfecting a way to increase lesion size without damaging nei ghbouring brain tissue, which might cause physical or cognitive impairments. Additionally, they must navigate around blood vessels to avoid bleeding.
The Neuro was the first centre in Canada to employ robotic technology in this type of surgery , improving the precision and effectiveness of treatment . Dr. Hall’s research seeks to refine robotic targeting and allow larger lesions to permanently disrupt the source of seizures. By using neuroimaging data and developing advanced algorithms , he is striving to optimize t he placement o f electrodes to detect abnormal electrical activity . Once detected, a treatment may be offered by pass ing electrical current through the electrodes, coagulating the abnormal tissue to reduce or stop its capacity to generate further seizures.
Combined exploration and lesion ing surgery
Epilepsy surgery is an invasive procedure that involves a hospital stay and recovery time. There are sometim e s two surgeries required — one to find the source of the seizure and another to coagulate it . At The Neuro, surgeons like Dr. Hall believe one surgery is better than two. They combine both surgeries , reducing the risk of complication and impact on the patient’s life.
“If you have to do a second surgery, then you assume all the risks of a second intervention ,” he says. “If it's possible t o create lesions with the electrodes that are already implanted as part of the investigation, that is the best option.”
The goal is to minimize the toll epilepsy takes on people’s lives, both the seizures and interventions necessary to stop them, offering improved outcomes for individuals living with this condition.
Related Content
- Epilepsy at The Neuro: past and present
- Jeffery Alan Hall, MD, FRCS(C)
GET OUR NEWSLETTER

The Neuro (Montreal Neurological Institute-Hospital) is a bilingual academic healthcare institution. We are a McGill research and teaching institute; delivering high-quality patient care, as part of the Neuroscience Mission of the McGill University Health Centre. We are proud to be a Killam Institution, supported by the Killam Trusts.
Department and University Information
The neuro (montreal neurological institute-hospital).

- Coronavirus Information
- Clinics and programs
- Diagnostic Services
- Clinical Trials
- Neuro Audio Visual
- Reserve a Room
- Facilities Request Form
- Visual Identity Guide and Templates
- Social Media Policy
- Cellular Wi-Fi Calling
- COVID-19 Information
- Animal Care Committee
- Careers at The Neuro
Nguyen marries basic and translational approaches to ID new brain region responsible for epileptic seizures
Lorena Infante Lara
Apr 17, 2024, 12:00 PM
Epilepsy, a neurological condition that results in seizures, is highly prevalent and affects about 1.2% percent of the U.S. population . Worldwide, it affects tens of millions of people, but although its etiology is known for some, the cause is not always clear for others. Incoming Assistant Professor of Pharmacology Quynh Anh Nguyen sat down with us to discuss the results of her most recent paper in which she identified a new brain region associated with certain forms of epilepsy and in which she suggests that targeting that region for treatment may help some patients control their epileptic seizures.

The research, published in Nature Medicine , was spearheaded by Nguyen and neurosurgery resident Ryan Jamiolkowski under the tutelage of Vivek P. Buch and Ivan Soltesz, both faculty members in the Department of Neurosurgery at the Stanford University School of Medicine.
What issue/problem does your research address?
Epilepsy is one of the most prevalent neurological disorders in the world, with tens of millions of people burdened by the occurrence of chronic spontaneous seizures. About one-third of epilepsy patients do not achieve adequate seizure control with existing anti-seizure medications and often elect to undergo invasive surgical resection or ablation of their epileptic brain tissue.
For patients with mesial temporal lobe epilepsy, the most common form of drug-resistant epilepsy, the main surgical target has been the anterior hippocampus and amygdala. About one-third of patients who undergo surgical intervention still do not receive adequate seizure freedom. Thus, there is a critical need to identify novel targets for controlling seizures in this significant patient population.
What was unique about your approach to the research?
This research took a unique approach that bridged the realms of biochemical and molecular tool development, neuroscience basic research, and clinical care.
As a postdoc in Ivan Soltesz’s lab in the Department of Neurosurgery at Stanford University, I had worked alongside Alice Ting’s lab in the Departments of Genetics, Biology, and Chemistry at Stanford to develop a light- and calcium-gated molecular integrator that could be used to label neurons that were active during a designated temporal window. I used this tool to label neurons during seizures in mice, which allowed me to identify a novel brain region involved in epilepsy.
Around the time that I made this discovery, a neurosurgery resident, Ryan Jamiolkowski, had joined the Soltesz lab and shared with me that he had an epilepsy patient whose seizures seemed to come from this region. We put forth a combined effort to show that this region is an important seizure node and a viable target for intervention in both mice and humans with epilepsy.
Overall, within the span of about 2.5 years, we took a basic science molecular tool and used it to make a fundamental discovery that could directly impact patient care. As a new assistant professor at Vanderbilt, I will continue this interdisciplinary approach in my own lab, with basic scientists and clinicians working side by side to find ways to help improve the human condition.
What were your findings? How can non-expert readers understand their significance?
We found that an understudied region of the brain called the fasciola cinereum is highly active during seizures in both mice and humans with epilepsy. Considering that most clinical targets focus on the anterior hippocampus and that the FC is located in the posterior hippocampal tail, the FC has previously been overlooked as a potential site of seizure onset. Yet, when we inhibited the activity of the neurons in this region in mice specifically during seizures, we found a significant reduction in seizure duration.
In all six of our human epilepsy patients, we found that the FC was involved in most of their seizures. In a patient with previously uncontrolled epilepsy who had undergone a prior surgical ablation of their anterior hippocampus, we found that targeted lesioning of this region reduced their seizure burden by 83 percent.
Together, these results highlight the involvement of the hippocampal tail in epilepsy and suggest a fundamental change in the standard of care to include the FC for consideration as a potential target site of seizure localization.
What do you hope will be achieved with the research results on the short term?
In the short term, I hope that more clinicians will consider the FC region as a candidate site for seizure localization in their patients with epilepsy. In addition, my own lab will be focusing on characterizing the neurons in this region and their function both in the normal as well as epileptic conditions. Although more work is needed to determine whether targeting FC alone is a viable intervention in humans, more patient recordings of FC and further basic science characterization of FC will help shed light on this understudied brain region and how it goes awry in disease.
What are the long-term societal, environmental, or economic benefits of this research?
This research has opened new avenues of investigation into the FC region, both from a clinical as well as basic science perspective. There is much to be done to understand what this region is, how it functions normally, and how its dysfunction leads to disease. In addition, we don’t know whether this area is also involved in other types of neurological disorders.
We hope this research will lead to larger-scale clinical trials on FC as a potential target to treat uncontrolled seizures. This may fuel further development of novel approaches or tools to specifically modulate this region in humans. Together, these efforts may lead to a significant reduction in the proportion of patients with uncontrolled epilepsy.
Where is this research taking you next?
A central focus of my new research lab will be to uncover the basic characteristics and functions of the FC and how these properties are affected by and lead to neurological disorders such as epilepsy. I will continue to work with my collaborators at Stanford and the University of Cambridge to develop new tools that will help me study this region, and with clinicians both at Stanford and at Vanderbilt who can help translate my basic science findings from mice to humans.
The article “ The Fasciola Cinereum of the Hippocampal Tail as an Interventional Target in Epilepsy ” was published in Nature Medicine in April 2024.
FUNDING AND ACKNOWLEDGEMENTS
This study was funded by the Stanford Maternal & Child Health Research Institute, the LGS Foundation, the Stanford University School of Medicine, the Wellcome Trust, and the National Institutes of Health. The authors also appreciate help and materials from T. Takemori and A. Ishige at RIKEN, A. Ting at Stanford University, and C. Porter and U. Chon.
Explore Story Topics
- Research, News & Discoveries
- hippocampus
- Pharmacology
- Quynh Anh Nguyen
- School of Medicine Basic Sciences
- Wellcome Trust Fund

Wait! Try this Special Offer
Subscribe now and save 15%.
- Facebook . opens in new tab
- Twitter . opens in new tab
- LinkedIn . opens in new tab
- Open Athens/Shibboleth
- Activate Print Subscription
- Create Account
- Renew subscription

Stay Informed, with Concise, Evidence Based Information

Renew today to continue your uninterrupted access to NEJM Journal Watch.
Stay Informed
Subscribe Or Renew

Sign into your account
Forgot Password? Login via Athens or your Institution
Already a print subscriber? Activate your online access.
- SUMMARY AND COMMENT |
April 16, 2024
A Mental Health Intervention in Pediatric Epilepsy
Sarah Aminoff Kelley, MD , reviewing Bennett SD et al. Lancet 2024 Mar 30
Children with epilepsy and anxiety, depression, or behavioral disorders improved more with the Mental Health Intervention for Children with Epilepsy than with usual care.
Neurobehavioral comorbidities affect the quality of life of people living with epilepsy and can be more problematic to daily functioning than seizures themselves. Such comorbidities are present at higher rates in children with epilepsy than in generally healthy children ( Lancet Neurol 2008; 7:151) and those with other chronic medical conditions (Rutter M et al. A Neuropsychiatric Study in Childhood [Clinics in Developmental Medicine]. J. B. Lippincott Co; 1970). In this open-label, randomized, controlled trial, researchers tested a standardized telemedicine intervention of up to 20 sessions, plus 2 booster sessions, using the Mental Health Intervention for Children with Epilepsy (MICE), provided by clinicians not formally trained in psychological therapy, to 148 patients aged 3 to 18 years with epilepsy and comorbid depression, anxiety, or disruptive behavior.
Compared with 148 participants who received usual care, the MICE group had an adjusted decrease of 1.7 points on the caregiver-rated Strengths and Difficulties Questionnaire at 6 months (the primary outcome). These results were not affected by intellectual disability (excluding those who could not participate) or autism. Caregivers also demonstrated improvement in their own depression and anxiety ratings at 6 months, although improvement in caregiver depression was not sustained at 12 months.
These positive results apply to several psychiatric comorbidities, giving us tools for helping our patients. Providers not trained in psychology could complete the sessions with the patients. This is significant; however, determining how to make it a billable, efficient service will be the next important step. I look forward to the upcoming report on cost-effectiveness to help with strategies. One additional highly prevalent comorbidity that should be addressed in the future is attention-deficit/hyperactivity disorder. The effect of caregiver well-being also is critical to the well-being of the patient and too often neglected. Continued work on implementation of programs such as these are of critical benefit to the patients and families.
Dr. Kelley is Associate Professor of Clinical Neurology, Johns Hopkins Hospital, Baltimore.
Bennett SD et al. Clinical effectiveness of the psychological therapy Mental Health Intervention for Children with Epilepsy in addition to usual care compared with assessment-enhanced usual care alone: A multicentre, randomised controlled clinical trial in the UK. Lancet 2024 Mar 30; 403:1254. ( https://doi.org/10.1016/S0140-6736(23)02791-5 )
Facebook | Twitter | LinkedIn | Email | Copy URL
Latest In General Medicine
- Apr 11, 2024
Diagnosing Heparin-Induced Thrombocytopenia: How Accurate Is the Recommended Algorithm?
Daniel D. Dressler, MD, MSc, MHM, FACP
A “Drug Watch” Feature in NEJM Journal Watch?
Allan S. Brett, MD
Aprocitentan, a Newly Approved Drug for Resistant Hypertension
Managing acute pancreatitis.
Andrew S. Parsons, MD, MPH
- Apr 10, 2024
A Model for Managing Patients with Monoclonal Gammopathy of Undetermined Significance
More about serologic diagnosis of celiac disease.
David J. Bjorkman, MD, MSPH (HSA), SM (Epid.)
Stay Informed, with Concise, Evidence-Based Information

Plus 2 free gifts

Fralin Biomedical Research Institute team unpacks genetic mysteries behind childhood epilepsies
Matthew Weston, associate professor at the Fralin Biomedical Research Institute at VTC, will lead efforts to understand how gene variants linked to severe, treatment-resistant childhood epilepsy disorders alter electrical activity in the brain.
- Clayton Metz
11 Apr 2024
- Share on Facebook
- Share on Twitter
- Copy address link to clipboard
Epilepsy is a brain disorder that causes recurring seizures.
It is one of the most common neurological diseases, and it affects approximately 50 million people worldwide, according to the World Health Organization. In 2023, nearly 450,000 children in the United States were diagnosed with the disease.
Researchers at the Fralin Biomedical Research Institute at VTC are exploring how gene variants identified in children with severe epilepsy can have an impact on neurons, leading to abnormal electrical activity in the brain and recurrent seizures.
With two recently awarded grants totaling $2.4 million from the National Institute of Neurological Disorders and Stroke at the National Institutes of Health, scientists led by Matthew Weston will use mouse models expressing these epilepsy-associated gene variants to understand how they alter neuron behavior to cause seizures.
The Weston lab is particularly interested in a gene called KCNT1. This specific segment of DNA carries the instructions for a protein that forms an ion channel that acts like a tiny gate embedded in the membrane of neurons to control the flow of potassium ions.
This flow is essential to help neurons communicate properly and regulate the electrical activity in our brain, according to Weston, associate professor at the Fralin Biomedical Research Institute.
Changes in this gene affect normal nervous system function and can lead to seizures by causing a dysregulation of electrical stabilization in neurons that can spread across networks throughout regions of the brain. Earlier investigations by Weston’s team examined the influence of KCNT1 genetic abnormalities on the excitability of neurons, indicating their potential connection to epilepsy.
"We’re using mouse models with the exact same KCNT1 mutations that cause severe and untreatable epilepsy in kids,” said Weston, who is also an associate professor in Virginia Tech’s School of Neuroscience in the College of Science . “By closely examining these models, we hope to discover a path to therapeutic intervention.”
Weston is collaborating with Wayne Frankel, professor of Genetics and Development at Columbia University’s Institute for Genomic Medicine. Frankel recently designed new research models for this study: a model with the KCNT1 genetic mutation in all neurons and another model that allows the KCNT1 genetic mutation to be expressed only in a subpopulation of neurons to identify which neuron types are most important for the disease.

By looking at the neurons in the brains of these models, Weston aims to uncover fresh perspectives on the alterations in neuronal function induced by KCNT1 mutations, resulting in heightened excitability and seizure occurrence. More importantly, he hopes to pinpoint the neuron types most susceptible to these changes, potentially guiding the development of innovative treatment strategies.
“With these models, we’re hoping to find new mechanisms underlying the disease and point to new therapies,” Weston said.
Weston serves on the scientific advisory board for the KCNT1 Epilepsy Foundation , which supports research and drug development with the ultimate goal of finding an eventual cure for KCNT1-related epilepsies.
Amy Shore, a research scientist in Weston’s lab, finds inspiration in the connection with the foundation.
“Engaging with parents, and hearing stories about the devastation of this disease on their children and their daily lives, motivates us to focus and do our best to find answers that can translate into hope,” she said.
Leigh Anne Kelley
540-526-2002
- Center for Neurobiology Research
- Faculty of Health Sciences
- Fralin Biomedical Research Institute at VTC
- Fralin Biomedical Research Institute at VTC - top news
- Good Health and Well-Being
- Health Sciences and Technology (Roanoke)
- Roanoke, Va.
Related Content

New Brain Target Key to Easing Tough-to-Treat Epilepsy
By Dennis Thompson HealthDay Reporter

WEDNESDAY, April 17, 2024 (HealthDay News) -- Some people with tough-to-treat epilepsy might benefit if doctors target a brain region newly linked to the disorder, a new study suggests.
Seizures declined by 83% after a patient underwent surgery that removed almost all of the fasciola cinereum, a previously overlooked region of the hippocampus, researchers report April 17 in the journal Nature Medicine .
In practical terms, that means a patient who had been having one or two seizures a month now has one seizure every three months or so, results show.
The findings indicate that people with drug-resistant epilepsy might need the fasciola cinereum treated alongside other brain regions that are typically targeted, the researchers said.
U.S. Cities With the Most Homelessness

“The hippocampus is the best studied part of the brain by far, but there is shockingly little known about the fasciola cinereum,” said senior researcher Ivan Soltesz , a professor of neurosurgery and neurosciences at Stanford University School of Medicine.
The standard of care for epilepsy when drugs fail to work is surgery, researchers explained in background notes.
In one type, called mesial temporal lobe epilepsy, seizures originate in two specific brain regions: the amygdala, an almond-shaped structure involved in processing emotions, and the hippocampus, a region necessary for forming memories.
The brain is symmetrical, with an amygdala and a hippocampus on both the left and right side, and often seizures erupt from the structures on one side of the brain, researchers said.
So, doctors use electrode implants to figure out which regions are causing seizures, and then they remove those structures through surgery or by using a laser to burn them away, a process called ablation.
Because there’s an amygdala and hippocampus on both sides of the brain, people still retain the ability to form memories following the procedure and typically have minimal side effects, researchers said.
But even this approach, as dire as it sounds, fails a third of the time, researchers said.
To figure out why, researchers started using electrodes to map in detail patients’ brain seizure activity.
The hippocampus, located deep within each hemisphere of the brain near ear level, looks like a sea horse lying on its side with the head pointing toward the front of the brain, researchers said.
Electrode mapping of seizure activity noted that neurons in the fasciola cireneum -- the far tip of the sea horse’s tail -- were active during seizures in mice.
Further, mouse studies indicated that if neuron activity in the fasciola cireneum were shut down, it shortened the duration of seizures in mice.
“Seizure activity in this region could be a reason why these surgeries sometimes fail,” said co-lead researcher Dr. Ryan Jamiolkowski , a resident in neurosurgery at Stanford Medicine.
Researchers next turned to six human epilepsy patients, implanting electrodes to map seizure activity in their brains.
The fasciola cinereum contributed to the recorded seizures in all six patients, including some episodes in which the rest of the hippocampus remained silent.
One of the patients already had received laser treatment to burn away their amygdala and most of their hippocampus in the left brain, but nevertheless continued having seizures.
Electrode mapping showed that the only part of the hippocampus that remained, the fasciola cireneum, was involved in those seizures.
A follow-up laser procedure burned away the fasciola cireneum, and the patient’s seizures declined by 83%.
Because of the way the hippocampus is shaped, future patients whose seizures involve the fasciola cireneum might need two separate surgeries as well, researchers said.
“The hippocampus curves like a banana, and the optical fiber used for laser ablation is a straight line,” Jamiolkowski said in a Stanford news release.
To burn away the entire structure “requires different trajectories that are not currently feasible to combine into one procedure,” Jamiolkowski explained.
The fasciola cireneum also might be targeted in patients with seizures emanating from the amygdala and hippocampus on both sides of their brains, researchers added.
To preserve their ability to form memories, these patients have a device implanted in the hippocampus that provides electrical jolts to interrupt a seizure before it can start rolling.
“Knowing which patients have seizures involving the fasciola cinereum would let us target it with either ablation or neurostimulation, and help us treat patients better than a one-size-fits all approach,” Jamiolkowski said.
More information
The Mayo Clinic has more on epilepsy .
SOURCE: Stanford University, news release, April 17, 2024
Copyright © 2024 HealthDay . All rights reserved.
Join the Conversation
Tags: epilepsy
America 2024

Health News Bulletin
Stay informed on the latest news on health and COVID-19 from the editors at U.S. News & World Report.
Sign in to manage your newsletters »
Sign up to receive the latest updates from U.S News & World Report and our trusted partners and sponsors. By clicking submit, you are agreeing to our Terms and Conditions & Privacy Policy .
You May Also Like
The 10 worst presidents.
U.S. News Staff Feb. 23, 2024

Cartoons on President Donald Trump
Feb. 1, 2017, at 1:24 p.m.

Photos: Obama Behind the Scenes
April 8, 2022

Photos: Who Supports Joe Biden?
March 11, 2020

Boeing Called Out for ‘Defective’ Planes
Laura Mannweiler April 17, 2024

Senate Kills Mayorkas Impeachment
Aneeta Mathur-Ashton April 17, 2024

Fed: Strong Economy Stalling Rate Cuts
Tim Smart April 17, 2024

The Implications of Trump Legal Wins
Lauren Camera April 16, 2024

The Week in Cartoons April 15-19
April 17, 2024, at 3:38 p.m.

Justices Weigh Jan. 6 Obstruction Charge


IMAGES
VIDEO
COMMENTS
Epilepsy is one of the most common and disabling neurologic conditions, yet we have an incomplete understanding of the detailed pathophysiology and, thus, treatment rationale for much of epilepsy. This article reviews the clinical aspects of seizures and epilepsy with the goal of providing neuroscientists an introduction to aspects that might ...
The classification of seizures and epilepsies by the International League Against Epilepsy (ILAE), 2017 is the most recent classification model which aimed to simplify terminologies that patients and their caregivers can easily understand, identify seizures that have both focal and generalized onset and incorporate missing seizures.
Introduction. Epilepsy is the enduring predisposition of the brain to generate seizures, a condition that carries neurobiological, cognitive, psychological, and social consequences ().Over 50 million people worldwide are affected by epilepsy and its causes remain partially elusive, leaving physicians, and patients an unclear insight into the etiology of the disease and the best treatment ...
The diagnosis and treatment of seizures and epilepsy is a common task of the physician. Approximately 1 in 10 people will have a seizure during their lifetime. Epilepsy is the tendency to have unprovoked seizures. Epilepsy is the fourth most common neurological disorder and affects 1 in 26 people in the United States and 65 million people worldwide. Evaluation of a patient presenting with a ...
In 2022, epilepsy research has made advances across a range of clinically important areas, from self-management, genetics, imaging, and surgical planning to understanding febrile seizures and coma-related periodic patterns. Most notably, in May 2022, the World Health Assembly adopted the Intersectoral Global Action Plan on Epilepsy and Other Neurological Disorders, which aims to address gaps ...
Approximately 3.5 million Americans were estimated to have epilepsy as of 2015; of these, 470,000 were younger than 18 years of age (Zack & Kobau, 2017).In an analysis of 67,733 children (0-18 years of age) from 2005 to 2012, the prevalence of epilepsy was higher than that of food allergies or diabetes (Miller et al., 2016).Among all patients with epilepsy, 30% continue to have seizures ...
Epilepsy articles from across Nature Portfolio. Epilepsy refers to a group of neurological disorders of varying aetiology, characterized by recurrent brain dysfunction that result from sudden ...
Epilepsy Research provides for publication of high quality articles in both basic and clinical epilepsy research, with a special emphasis on translational research that ultimately relates to epilepsy as a human condition. The journal is intended to provide a forum for reporting the best and most … View full aims & scope $2940
With great anticipation, 2023 has seen many important advances in epilepsy research. Noteworthy progress has been achieved in understanding the intricate mechanisms of epilepsy, accompanied by important strides in developing new therapies. Historically, the efficacy of most second-generation and third-generation antiseizure medications in ...
2.1. Collecting Seizure-Related Signals. A seizure can be detected by monitoring various physiological signals from the human body through (1) EEG, (2) EMG, (3) ECG, (4) motion, and (5) audio/video recording (4, 7).Among these physiological signals, EEG is the most popular choice because of its advantages, such as (1) the ability to capture the neural activation of the brain, (2) high temporal ...
Headache Is a Common Aura in Patients with Generalized Seizures. Dong Won Kwack, Dong Wook Kim. J Epilepsy ... please visit the J Epilepsy Res e-submission management system at http ... please contact: [email protected]. Free archive: Anyone may access any past or current articles without logging in. Editorial Office KCC Parktown ...
The pharmacological armamentarium against epilepsy has expanded considerably over the last three decades, and currently includes over 30 different antiseizure medications. Despite this large armamentarium, about one third of people with epilepsy fail to achieve sustained seizure freedom with currently available medications. This sobering fact, however, is mitigated by evidence that clinical ...
Epilepsia is the leading journal for innovative clinical and basic science research for all aspects of epilepsy and seizures, aiming to improve diagnosis and treatment. Abstract Objectives To evaluate the efficacy of a specialized inpatient rehabilitation program in patients with newly diagnosed epilepsy (NDE), who had been referred within 1 ...
Epilepsia. Epilepsia is the leading, authoritative journal for innovative clinical and basic science research for all aspects of epilepsy and seizures. In addition, Epilepsia publishes critical reviews, opinion pieces, and guidelines that foster understanding and aim to improve the diagnosis and treatment of people with seizures and epilepsy.
Seizures affect the lives of 10% of the global population and result in epilepsy in 1 to 2% of people around the world. Current knowledge about etiology, diagnosis, and treatments for epilepsy is constantly evolving. As more is learned, appropriate and updated definitions and classification systems …
Focus On Epilepsy Research. The epilepsies are a set of disorders characterized by recurring seizures, or disturbances in the electrical activity of the brain. Epilepsy affects people of all ages, from infants to the aged, and can result from many causes, including genetic variations, illness, head injury, or abnormal brain development.
Case 34-2021: A 38-Year-Old Man with Altered Mental Status and New Onset of Seizures. A.J. Cole and OthersN Engl J Med 2021;385:1894-1902. A 38-year-old man was evaluated in the emergency ...
The word epilepsy originates from the Latin and Greek word 'epilepsia' which means 'seizure' or 'to seize upon'. It is a serious neurological disorder with unique characteristics, tending of recurrent seizures [].The context of epilepsy, found in the Babylonian text on medicine, was written over 3000 years ago [2, 3].This disease is not limited to human beings, but extends to cover ...
Abstract and Figures. This paper reviews advances in epilepsy in recent years with an emphasis on therapeutics and underlying mechanisms, including status epilepticus, drug and surgical treatments ...
Epilepsy is a neurological disease characterized by excessive and abnormal hyper-synchrony of electrical discharges of the brain and a predisposition to generate epileptic seizures resulting in a broad spectrum of neurobiological insults, imposing psychological, cognitive, social and also economic burdens to the sufferer. Voltage-gated sodium channels (VGSCs) are essential for the generation ...
Epileptic seizures are abnormal jerky or trembling movements in the body due to abnormal neuronal activity and can result in damage to the brain or other parts of the body. Even a single seizure can cause changes in neural development and can lead to behavioural and cognitive changes. ... Epilepsy Research. 2010; 89 (2-3):310-318. [Google ...
repercussions. Recently, ILAE stated a n ew. definition of epilepsy for clinical use which can be. considered if any of the following situations. experienced by patients: (a) Minimum occurring of ...
1 INTRODUCTION. Patients with uncontrolled seizures log their seizures in a diary for physicians to adjust and optimize the antiseizure medicine or to consider other treatment options such as surgery or dietary therapy. 1, 2 However, the seizure diaries are unreliable as patients and caregivers are often unable to recognize and report seizures, and more than half of seizures go unnoticed, 1 ...
Post-traumatic epilepsy is a major driver of disability associated with traumatic brain injury. This article reviews the epidemiology and clinical features of post-traumatic epilepsy and discusses ...
One percent of the world's population has epilepsy and 30 percent of those are resistant to medication. Their seizures can be debilitating and life threatening. For some, the best option is surgery. If the source of the seizures is unclear, surgeons may resort to placing electrodes into the brain to directly record from specific areas. If seizure activity is confined to an area around the ...
Assistant professor of pharmacology Quynh Anh Nguyen spearheaded research that points to a previously unidentified region of the hippocampus as responsible for epileptic seizures. The work could lead to new avenues of treatment to help epilepsy patients control their seizures.
In this open-label, randomized, controlled trial, researchers tested a standardized telemedicine intervention of up to 20 sessions, plus 2 booster sessions, using the Mental Health Intervention for Children with Epilepsy (MICE), provided by clinicians not formally trained in psychological therapy, to 148 patients aged 3 to 18 years with ...
Researchers at the Fralin Biomedical Research Institute are exploring how gene variants affect brain cells in children with severe epilepsy. Here, the scientists use green and red fluorescent proteins to identify different types of neurons in a mouse model expressing a mutated KCNT1 gene associated with seizures in children.
WEDNESDAY, April 17, 2024 (HealthDay News) -- Some people with tough-to-treat epilepsy might benefit if doctors target a brain region newly linked to the disorder, a new study suggests.