- PRO Courses Guides New Tech Help Pro Expert Videos About wikiHow Pro Upgrade Sign In
- EDIT Edit this Article
- EXPLORE Tech Help Pro About Us Random Article Quizzes Request a New Article Community Dashboard This Or That Game Popular Categories Arts and Entertainment Artwork Books Movies Computers and Electronics Computers Phone Skills Technology Hacks Health Men's Health Mental Health Women's Health Relationships Dating Love Relationship Issues Hobbies and Crafts Crafts Drawing Games Education & Communication Communication Skills Personal Development Studying Personal Care and Style Fashion Hair Care Personal Hygiene Youth Personal Care School Stuff Dating All Categories Arts and Entertainment Finance and Business Home and Garden Relationship Quizzes Cars & Other Vehicles Food and Entertaining Personal Care and Style Sports and Fitness Computers and Electronics Health Pets and Animals Travel Education & Communication Hobbies and Crafts Philosophy and Religion Work World Family Life Holidays and Traditions Relationships Youth
- RANDOM QUIZ
- Browse Articles
- Learn Something New
- Quizzes Hot
- This Or That Game New
- Train Your Brain
- Explore More
- Support wikiHow
- About wikiHow
- Log in / Sign up
- Education and Communications
- Chemistry Calculations

How to Calculate the Concentration of a Solution
Last Updated: March 20, 2024 Fact Checked
This article was co-authored by Chris Hasegawa, PhD and by wikiHow staff writer, Hunter Rising . Dr. Chris Hasegawa was a Science Professor and the Dean at California State University Monterey Bay. Dr. Hasegawa specializes in teaching complex scientific concepts to students. He holds a BS in Biochemistry, a Master’s in Education, and his teaching credential from The University of California, Davis. He earned his PhD in Curriculum and Instruction from The University of Oregon. Before becoming a professor, Dr. Hasegawa conducted biochemical research in Neuropharmacology at the National Institute of Health. He also taught physical and life sciences and served as a teacher and administrator at public schools in California, Oregon, and Arizona. There are 8 references cited in this article, which can be found at the bottom of the page. This article has been fact-checked, ensuring the accuracy of any cited facts and confirming the authority of its sources. This article has been viewed 2,119,022 times.
In chemistry, a solution’s concentration is how much of a dissolvable substance, known as a solute, is mixed with another substance, called the solvent. The standard formula is C = m/V, where C is the concentration, m is the mass of the solute dissolved, and V is the total volume of the solution. If you have a small concentration, find the answer in parts per million (ppm) to make it easier to follow. In a lab setting, you may be asked to find the molarity , or molar concentration, of the solution instead.
Using the Mass per Volume Equation

- If the solute you’re using is a liquid, then you can also calculate the mass using the density formula, where density D = m/V, where m is the mass of the liquid and V is the volume. To find the mass, multiply the density of the liquid by the volume.
Tip: If you need to use a scale, subtract the mass of the container you’re using to hold the solute or else your calculations will be off.

- If you aren’t measuring the volume yourself, you may need to convert the mass of the solute into volume using the density formula.
- For example, if you’re finding the concentration of 3.45 grams of salt in 2 liters of water, you would find the volume of salt using the density formula. Look up the density of salt either in a textbook or online and solve the formula for m. In this case, the density of salt is 2.16 g/mL. The formula would read 2.16 g/mL = (3.45 g)/V. Multiply each side by V to get V(2.16 g/mL) = 3.45 g. Then divide the each side by 2.16 to find the volume, or V = (3.45 g)/(2.16 g/mL) = 1.60 mL.
- Add the volume of the solute to the volume of your solvent, ma. So in this example, 2 L + 1.6 mL = 2,000 mL + 1.6 mL = 2,001.6 mL. You can either leave the measurement in milliliters or convert it back to liters to get 2.002 L.

- In our example for the concentration of 3.45 grams of salt in 2 liters of water, your equation would be C = (3.45 g)/(2.002 L) = 1.723 g/L.
- Certain problems may ask for your concentration in specific units. Be sure to convert the units before putting them in your final formula.
Finding Concentration in Percentage or Parts per Million

- If your solute is a liquid, you may need to calculate the mass using the formula D = m/V, where D is the liquid’s density, m is the mass, and V is the volume. Look up the density of the liquid in a textbook or online and then solve the equation for the mass.

- For example, if you want to find the concentration of 10 g of cocoa powder mixed with 1.2 L of water, you would find the mass of the water using the density formula. The density of water is 1,000 g/L, so your equation would read 1,000 g/L = m/(1.2 L). Multiply each side by 1.2 L to solve the mass in grams, so m = (1.2 L)(1,000 g/L) = 1,200 g. Add the mass of the cocoa powder to get 1,210 g.

- In our example, C = (10 g)/(1,210 g) = 0.00826.

- In this example, the percent concentration is (0.00826)(100) = 0.826%.

- In our example, the ppm = (0.00826)(1,000,000) = 8,260 ppm.
Tip: Parts per million is usually used for very small concentrations since it’s easier to write and understand than a percentage.
Calculating Molarity

- For example, if your solute is potassium hydroxide (KOH), find the atomic masses for potassium, oxygen, and hydrogen and add them together. In this case molar mass = 39 +16 + 1 = 56 g/mol.
- Molarity is used mainly in chemistry when you know the chemical makeup of the solute you’re using.

- For example, if you want to find the number of moles in 25 g of potassium hydroxide (KOH), then the equation is mol = (25 g)/(56 g/mol) = 0.45 mol
- Convert the mass of your solute to grams if it isn’t already listed in grams.
- Moles are used to represent the number of atoms in the solution.

- In this example, if you’re using 400 mL of water, then divide it by 1,000 to convert it to liters, which is 0.4 L.
- If your solvent is already listed in liters, then you can skip this step.
Tip: You don’t need to include the volume of the solute since it doesn’t usually affect the volume that much. If there is a visible change in volume when you mix the solute with the solvent, then use the total volume instead.

- In this example, M = (0.45 mol)/(0.4 L) = 1.125 M.
Calculator, Practice Problems, and Answers

Community Q&A
- If you are in a lab and don’t know how much of a solute was added, you can perform a titration test using other reactive chemicals. You do need to learn how to balance chemical equations with stoichiometry . Thanks Helpful 0 Not Helpful 0

You Might Also Like

- ↑ https://www.physiologyweb.com/calculators/mass_per_volume_solution_concentration_calculator.html
- ↑ https://www.omnicalculator.com/conversion/ppm
- ↑ https://sciencing.com/calculate-concentration-ppm-6935286.html
- ↑ https://chem.libretexts.org/Courses/Los_Angeles_Trade_Technical_College/Chem_51/15%3A_Solutions/15.03%3A_Solution_Concentration_-_Molality_Mass_Percent_ppm_and_ppb
- ↑ https://chem.libretexts.org/Bookshelves/General_Chemistry/Map%3A_Chemistry_-_The_Central_Science_(Brown_et_al.)/04%3A_Reactions_in_Aqueous_Solution/4.05%3A_Concentration_of_Solutions
- ↑ Chris Hasegawa, PhD. Retired Science Professor & Dean. Expert Interview. 29 July 2021.
- ↑ https://www.khanacademy.org/science/chemistry/states-of-matter-and-intermolecular-forces/mixtures-and-solutions/a/molarity
- ↑ https://www.inchcalculator.com/convert/milliliter-to-liter/
About This Article

To calculate the concentration of a solution, start by converting the solute, or the substance being dissolved, into grams. If you're converting from milliliters, you may need to look up the solute's density and then multiply that by the volume to convert to grams. Next, convert the solvent to liters. Finally, divide the solvent by the solute to find the concentration of the solution. To learn how to calculate the concentration of a solution as a percentage or parts per million, scroll down! Did this summary help you? Yes No
- Send fan mail to authors
Reader Success Stories
Brenda Horn
Oct 19, 2020
Did this article help you?

Jan 9, 2017
Nov 19, 2017
Ejiga Victor
Jun 28, 2017
Katelyn Bosh
May 15, 2017

Featured Articles

Trending Articles

Watch Articles

- Terms of Use
- Privacy Policy
- Do Not Sell or Share My Info
- Not Selling Info
Don’t miss out! Sign up for
wikiHow’s newsletter
Concentration of Solutions
This is a series of lectures in videos covering Chemistry topics taught in High Schools. These lessons look at calculating the concentration of a solution.
Related Pages IGCSE Chemistry High School Chemistry More Lessons for Chemistry
Concentrations Calculations (% weight/volume) A tutorial on calculating the concentration of a solution in % weight-volume.
Example: You dissolve 2.5 moles of strantium acetate into 1.0 L of water. Determine the concentration of the solution in %W/C.
Concentration of Solutions: Volume/Volume % (v/v) A volume/volume percent (v/v) gives the volume of solute divided by the volume of the solution (expressed as a percent). The following video looks at calculating concentration of solutions. We will look at a sample problem dealing with volume/volume percent (v/v)%
Example: Rubbing alcohol is commonly used as an antiseptic for small cuts. It is sold as 70% (v/v) solution of isopropyl alcohol in water. What volume of isopropyl alcohol is used to make 500 mL of rubbing alcohol?
Concentration of Solutions: mass/volume % (m/v)% The following video looks at calculating concentration of solutions. We will look at a sample problem dealing with mass/volume percent (m/v)%.
Example: Many people use a solution of sodium phosphate (Na 3 PO 4 - commonly called TSP), to clean walls before putting up wallpaper. The recommended concentration is 1.7%(m/v). What mass of TSP is needed to make 2.0 L of solution?
Concentration of Solutions: Mass/Mass % (m/m)% A mass/mass percent gives the mass of a solute divided by the mass of solution (expressed as a percent) The following video looks at calculating concentration of solutions. We will look at a sample problem dealing with mass/mass percent (m/m)%
Example: CaCl 2 is used to melt ice on roads. To determine how much CaCl 2 has been used, you take a sample of slush to analyze. The sample had a mass of 23.47g. When the solution was evaporated, the residue had a mass of 4.58g. What was the mass/mass percent of CaCl 2 in the slush? How many grams of CaCl 2 were present in 100g of solution?

We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.
Concentration with Examples
Concentration
Concentration is the amount of solute in given solution. We can express concentration in different ways like concentration by percent or by moles.
1) Concentration by Percent:
It is the amount of solute dissolves in 100 g solvent. If concentration of solution is 20 %, we understand that there are 20 g solute in 100 g solution.
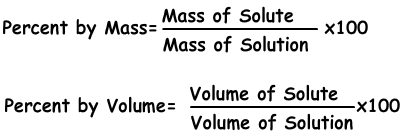
Example: 10 g salt and 70 g water are mixed and solution is prepared. Find concentration of solution by percent mass.
Mass of Solute: 10 g
Mass of Solution: 10 + 70 = 80 g
80 g solution includes 10 g solute
100 g solution includes X g solute
¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯
Or using formula;
Percent by mass=10.100/80=12,5 %
Example: If concentration by mass of 600 g NaCl solution is 40 %, find amount of solute by mass in this solution.
100 g solution includes 40 g solute
600 g solution includes X g solute
X=240 g NaCl salt dissolves in solution.
Example: If we add 68 g sugar and 272 g water to 160 g solution having concentration 20 %, find final concentration of this solution.
Mass of solution is 160 g before addition sugar and water.
100 g solution includes 20 g sugar
160 g solution includes X g sugar
X=32 g sugar
Mass of solute after addition=32 + 68=100 g sugar
Mass of solution after addition=272 +68 + 160=500 g
500 g solution includes 100 g sugar
100 g solution includes X g sugar
X= 20 % is concentration of final solution.
2) Concentration by Mole:
We can express concentration of solutions by moles. Number of moles per liter is called molarity shown with M.
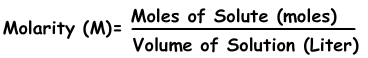
Example: Using 16 g NaOH, 200 ml solution is prepared. Which ones of the following statements are true for this solution?(Molar mass of NaOH is 40 g)
I. Concentration of solution is 2 molar
II. Volume of the water in solution is 200 ml
III. If we add water to solution, moles of solute decreases.
Solution: Moles of NaOH
I. n NaOH =16/40=0,4 mole
V=200 mL= 0,2 Liters
Molarity=0,4/0,2=2 molar
II. Since volume of solution is 200 mL, volume of water is smaller than 200 mL. II is false.
III. If we add water to solution, volume of solution increases but moles of solute does not change.
Example: 4,4 g XCl 2 salt dissolves in water and form 100 ml 0,4 molar XCl 2 solution. Find molar mass of X. (Cl=35)
Molarity=n/V
n=M.V where V=100mL=0,1 L and M=0,4 molar
n=0,1.0,4=0,04 mole
If 0,04 mole XCl 2 is 4,4 g
1 mole XCl 2 is ? g
?=110 g XCl 2
Molar mass of XCl 2 =X+2.(35)=110
X=40 g/mole
3) Molality:
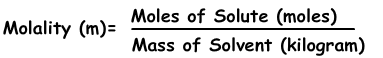
4) Normality:
We can express concentration in another way with normality using equivalents of solutes.

Equivalents can be defined as; number of moles of H + ion in acids and OH - ion in base reactions. For example; 1 mole H 2 SO 4 gives 2 H + ion, equivalent of H 2 SO 4 is 2. We find equivalent weight;
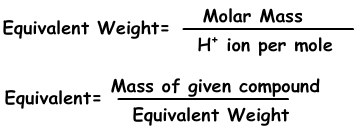
Solutions Exams and Problem Solutions
Forgot password? New user? Sign up
Existing user? Log in
Solving Mixture Problems
Already have an account? Log in here.
Mixture problems involve combining two or more things and determining some characteristic of either the ingredients or the resulting mixture. For example, we might want to know how much water to add to dilute a saline solution, or we might want to determine the percentage of concentrate in a jug of orange juice.
Introduction to Mixtures
Using a table to problem solve, practice problems.
We can use fractions, ratios, or percentages to describe quantities in mixtures.
If 200 grams of a saline solution has 40 grams of salt, what percentage of the solution is salt? Answer \(\frac{40}{200} = 0.20 = 20\%\) of the solution is salt.
If each can of orange juice concentrate contains the same amount of concentrate, which recipe will make the drink that is most orangey?
There is a general strategy for solving these mixture problems that uses simple algebra organized with a chart.
How much 40% rubbing alcohol do we need to add to 90% rubbing alcohol to make a 50% solution of rubbing alcohol? We could organize the data we are given in the following chart: Solution Type Concentration Amount of Solution Amount of Pure Alcohol 40% rubbing alcohol 0.4 ? liters ? liters 90% rubbing alcohol 0.9 ? liters ? liters 50% rubbing alcohol 0.5 10 liters \(0.5(10) = 5\) liters In general, the rows of the chart are the mixture types that you have. The columns describe the amount of each compound you have and the concentration of that component in each mixture (represented as a decimal). When you don't have some of the information needed to fill in the cart, use a variable instead. Solution Type Concentration Amount of Solution Amount of Pure Compound 40% rubbing alcohol 0.4 \(x\) \(0.4(x)\) 90% rubbing alcohol 0.9 \(10-x\) \(0.9(10-x)\) 50% rubbing alcohol 0.5 10 \(0.5(10) = 5\) The amount of 40% solution that we'll need is unknown (so make it \(x\)). The amount of 90% solution that we'll need is also unknown, but must be \(10-x\) liters so that we'll, in total, make 10 liters of the final solution. The amount of alcohol that each part of the mixture adds to the final result is equal to the amount of each solution mixed in, times the fraction of alcohol that that solution is made from. To use this chart to solve the problem, we will use the fourth column as an equation to solve for \(x.\) The 10 liters of our final mixture must have a total volume of 5 liters of alcohol in it in order to be 50% alcohol. Those 5 liters must come from a combination of the amount of 40% solution we mix in and the amount of 90% solution. If the volume (in liters) of 40% solution that we mix in is \(x,\) then \(0.4x\) will be the volume (in liters) of the amount of alcohol contributed. Similarly, \(0.9(10-x)\) will be the amount of alcohol contributed by the \(10-x\) liters of 90% alcohol solution that we add. Therefore, in total, \(0.4x + 0.9(10-x)\) must be equal to the 5 total liters we'll need in the final solution to make it 50% alcohol. Solving the equation, \[\begin{align} 0.4x + (9 - 0.9x) &= 5\\ -0.5x + 9 &= 5\\ 0.5x &= 4\\ \Rightarrow x &= \frac{4}{0.5}= 8. \end{align}\] Therefore, we need \(x = 8\) liters of the 40% solution.
How many grams of pure water must be added to 40 grams of a 10% saline solution to make a saline solution that is 5% salt? Answer Let's set up a table to solve this problem, using \(x\) to represent the number of grams of water that must be added. Solution Type Concentration Amount of Solution Total Amount of Salt water 0 \(x\) 0 10% solution 0.1 40 \((0.1)(40)\) 5% solution 0.05 40+\(x\) \((0.05)(40+x)\) Because the total amount of salt remains the same after we add the water, we can set up and solve the following equation: \[\begin{align} (0.1)(40)&=(0.05)(40+x) \\ 4 &= 2 + 0.05x \\ x&=40.\end{align}\] We need to add \(40 \) grams of pure water to create the solution that is \(5\%\) salt.
I have a 100 ml mixture that is 20% isopropyl alcohol (80 ml of water and 20 ml of isopropyl alcohol).
How much more alcohol do I need to add to make the mixture 25% alcohol?
There is a 40 litre solution of milk and water in which the concentration of milk is 72%. How much water must be added to this solution to make it a solution in which the concentration of milk is 60% ?
Let's practice using the strategies from above on a variety of problems.
Jill mixes 100 liters of A-beverage that contains 45% juice with 200 liters of B-beverage. The resulting C-beverage is 30% fruit juice. What is the percent of fruit juice in the 200 liters of the B-beverage? Answer Let's begin by making a table to show what we know. Beverage Liters of Beverage Concentration Liters of Juice Beverage A 100 0.45 \((0.45)(100) = 45\) Beverage B 200 \(x\) \(200x\) Beverage C 300 0.30 \((0.30)(300)=90\) The total number of liters of juice in Beverages A and B must equal 90, so \[\begin{align} 45 + 200x &= 90 \\ 200x &= 45 \\ 0.225 &= x.\end{align}\] The concentration of juice in Beverage B is 22.5%.
Unequal amounts of 40% and 10% acid solutions were mixed and the resulting mixture was 30% concentrated. However, the required concentration is 25%, so the Chemist added 300 cubic meters of 20% acid solution in order to get the required concentration. What was the original amount of 40% acid solution?
Write only the quantity without the units (cubic meters).
Legend: wt = weight
Strawberries contain about 15 wt% solids and 85 wt% water. To make strawberry jam, crushed strawberries and sugar are mixed in a 45:55 mass ratio, and the mixture is heated to evaporate water until the residue contains one-third water by mass.
Question: 1.) Calculate how many pounds of strawberries are needed to make a pound of jam.
In a mixture of 60 litres, the ratio of milk and water 2 : 1. If this ratio is to be 1 : 2, then the quanity of water to be further added is:
Problem Loading...
Note Loading...
Set Loading...
If you're seeing this message, it means we're having trouble loading external resources on our website.
If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
To log in and use all the features of Khan Academy, please enable JavaScript in your browser.
UP Class 12 Chemistry
Course: up class 12 chemistry > unit 1.
- Concentration of a solution_Worked Problem
Calculating concentration of a solution
- (Choice A) χ fructose = 0.98 , χ water = 1.02 A χ fructose = 0.98 , χ water = 1.02
- (Choice B) χ fructose = 0.017 , χ water = 0.983 B χ fructose = 0.017 , χ water = 0.983
- (Choice C) χ fructose = 0.97 , χ water = 0.018 C χ fructose = 0.97 , χ water = 0.018
- (Choice D) χ fructose = 0.08 , χ water = 0.92 D χ fructose = 0.08 , χ water = 0.92
Your browser is not supported
Sorry but it looks as if your browser is out of date. To get the best experience using our site we recommend that you upgrade or switch browsers.
Find a solution
- Skip to main content
- Skip to navigation

- Back to parent navigation item
- Primary teacher
- Secondary/FE teacher
- Early career or student teacher
- Higher education
- Curriculum support
- Literacy in science teaching
- Periodic table
- Interactive periodic table
- Climate change and sustainability
- Resources shop
- Collections
- Post-lockdown teaching support
- Remote teaching support
- Starters for ten
- Screen experiments
- Assessment for learning
- Microscale chemistry
- Faces of chemistry
- Classic chemistry experiments
- Nuffield practical collection
- Anecdotes for chemistry teachers
- On this day in chemistry
- Global experiments
- PhET interactive simulations
- Chemistry vignettes
- Context and problem based learning
- Journal of the month
- Chemistry and art
- Art analysis
- Pigments and colours
- Ancient art: today's technology
- Psychology and art theory
- Art and archaeology
- Artists as chemists
- The physics of restoration and conservation
- Ancient Egyptian art
- Ancient Greek art
- Ancient Roman art
- Classic chemistry demonstrations
- In search of solutions
- In search of more solutions
- Creative problem-solving in chemistry
- Solar spark
- Chemistry for non-specialists
- Health and safety in higher education
- Analytical chemistry introductions
- Exhibition chemistry
- Introductory maths for higher education
- Commercial skills for chemists
- Kitchen chemistry
- Journals how to guides
- Chemistry in health
- Chemistry in sport
- Chemistry in your cupboard
- Chocolate chemistry
- Adnoddau addysgu cemeg Cymraeg
- The chemistry of fireworks
- Festive chemistry
- Education in Chemistry
- Teach Chemistry
- On-demand online
- Live online
- Selected PD articles
- PD for primary teachers
- PD for secondary teachers
- What we offer
- Chartered Science Teacher (CSciTeach)
- Teacher mentoring
- UK Chemistry Olympiad
- Who can enter?
- How does it work?
- Resources and past papers
- Top of the Bench
- Schools' Analyst
- Regional support
- Education coordinators
- RSC Yusuf Hamied Inspirational Science Programme
- RSC Education News
- Supporting teacher training
- Interest groups

- More from navigation items
Calculating and comparing solution concentrations | 16-18 years
- No comments
Practise calculating the concentration of a solution from the mass of solute and the volume of water using this lesson plan with activities for 16–18 year olds
In this activity, students begin by comparing the concentrations of several solutions and reflecting on their current level of understanding. They then work in pairs using cards to link a mass of solute, volume of water and concentration of a solution, exploring the process of calculating concentration.
The lesson plan includes an extension activity giving students additional practise calculating the concentration of solutions.
Learning objectives
Students will understand:
- How to calculate the concentration of a solution.
Sequence of activities
Introduction and demonstration.
- Issue ‘traffic light’ cards to all students.
- Compare the concentration and number of moles of solute in solutions.
- Use the ‘traffic light’ cards to indicate their view: green for ‘the same’, red for ‘different’ and yellow for ‘unsure’.
- Pour 100 cm 3 of copper(II) sulfate solution into each of two beakers A and B. Pour half of the solution from beaker A into a third beaker C.
- The number of moles of copper(II) sulfate in beakers B and C.
- The concentration of copper(II) sulfate in beakers B and C.
- Use their indications as an aid to sharing the learning objective.
Explaining concentrations
Give each student an ‘Explaining concentrations’ sheet. Organise the students to:
- Work individually to complete the explanations and the ‘can do’ / ‘can’t do’ / ‘not sure’ boxes.
- Join with another student.
- Compare responses and convert any ‘can’t do’ or ‘not sure’ responses to ‘can do’.
- Join with another pair of students if there are still any ‘can’t do’ or ‘not sure’ responses.
Card matching: stage 1
- Move students back into pairs.
- Give a set of ‘Concentration cards’ to each pair and an ‘Answers’ sheet to each student.
- Group cards together showing the mass of sodium hydroxide and volume of water needed to produce the concentration shown on one of the cards.
- Record their answers on the ‘Answers’ sheet.
- Explain the general approach to calculating concentrations (on the ‘Answers’ sheet).
Card matching: stage 2
When pairs have recorded and shown the correct answers, give them a set of ‘Blank Concentration cards’ and a solute chosen from:
- Sodium carbonate.
- Sulfuric acid.
- Potassium hydroxide.
- Calcium bromide.
- Copper(II) sulfate.
Circulate and support with prompts while pairs:
- Devise their own set of concentration cards using the solute given to them so that all cards are used up when the mass of solute, volume of water and concentration of solute or ions in solution are matched up.
- Join up with another pair.
- Exchange the cards they have devised.
- Match up and record the cards devised by the other pair on their ‘Answers’ sheet.
- Help each other pair to select appropriate cards where this is necessary.
Extension activity
As an extension, set the following problem and work through the solution in a plenary.
Calculate the final concentrations in mol dm -3 of H + , Na + , Cl - and SO 4 2- , when the following three solutions are mixed together to give a total volume of 2 dm 3 :
- 1000 cm 3 of 0.1 mol dm -3 HCl
- 500 cm 3 of 0.2 mol dm -3 NaCl
- 500 cm 3 of 0.2 mol dm -3 Na 2 SO 4
Before finishing
Give each student a ‘Review’ sheet to complete and hand in.
Give written feedback that acknowledges achievement and leads students to recognise their next steps and how to take them.
The snapshot of student confidence, at the start of the session, gives the students a baseline as well as informing the teacher.
By writing explanations of how to do simple calculations and discussing their competence in a structured way the students are helped to recognise their own strength and weaknesses. Their learning is embedded when they set a further card matching exercise for their peers.
The final review guides students through an assessment that will reinforce confidence and help them to interpret feedback from the teacher.
Practical notes
- Beakers, 250 cm 3 , x3
- Copper(II) sulfate solution 0.1 mol dm -3 , 200 cm 3
- Water, 50 cm 3
Health, safety and technical notes
- Read our standard health and safety guidance .
Other equipment
- A set of ‘traffic light’ cards for each student
Card matching
Total volume = 2 dm 3 (ie 2000 cm 3 )
Assume all species are strong electrolytes and are fully dissociated in aqueous solution.
Final solution contains:
- 0.05 mol dm -3 HCl – ie 0.05 mol dm -3 H + and 0.05 mol dm -3 Cl -
- 0.05 mol dm -3 NaCl – ie 0.05 mol dm -3 Na + and 0.05 mol dm -3 Cl -
- 0.05 mol dm -3 Na 2 SO 4 – ie 0.10 mol dm -3 Na + and 0.05 mol dm -3 SO 4 2-
- Concentration of H + = 0.05 mol dm -3
- Concentration of Cl - = 0.05 + 0.05 = 0.10 mol dm -3
- Concentration of Na + = 0.05 + 0.10 = 0.15 mol dm -3
- Concentration of SO 4 2- = 0.05 mol dm -3
Explaining concentrations sheet
Answers sheet, review sheet, concentration cards, blank concentration cards, additional information.
This lesson plan was originally part of the Assessment for Learning website, published in 2008.
Assessment for Learning is an effective way of actively involving students in their learning. Each session plan comes with suggestions about how to organise activities and worksheets that may be used with students.
Acknowledgement
K. Crawford and A. Heaton, Problem solving in analytical chemistry, Section 1 , Calculating concentrations . London: Royal Society of Chemistry, 1999.
- 16-18 years
- Demonstrations
- Formative assessment
- Lesson planning
- Quantitative chemistry and stoichiometry
Specification
- Solutions. Expression of solution concentration in mol l⁻¹ (molarity), g l⁻¹ and also in % (w/v), % (v/v) % (w/w)
- WS.4.5 Interconvert units.
- The concentration of a solution can be measured in mol/dm³.
- The amount in moles of solute or the mass in grams of solute in a given volume of solution can be calculated from its concentration in mol/dm³.
- Students should be able to explain how the concentration of a solution in mol/dm³ is related to the mass of the solute and the volume of the solution.
- (HT only) Explain how the mass of a solute and the volume of the solution is related to the concentration of the solution.
- WS4.5 Interconvert units.
- IaS2.5 when processing data interconvert units
- C5.4.2 explain how the mass of a solute and the volume of the solution is related to the concentration of the solution and calculate concentration using the formula: concentration (g/dm³) = (mass of solute (g)) / (volume (dm³))
- C5.4.3 explain how the concentration of a solution in mol/ dm³ is related to the mass of the solute and the volume of the solution and calculate the molar concentration using the formula: concentration (mol/dm³) = (number of moles in solute) / (volume (d…
- C5.3.1 explain how the mass of a solute and the volume of the solution is related to the concentration of the solution and calculate concentration using the formula: concentration (g/dm³) = (mass of solute (g)) / (volume (dm³))
- C5.3.2 explain how the concentration of a solution in mol/ dm3 is related to the mass of the solute and the volume of the solution and calculate the molar concentration using the formula: concentration (mol/dm³) = (number of moles in solute) / (volume (d…
- C1.3j explain how the mass of a solute and the volume of the solution is related to the concentration of the solution
- C5.1a explain how the concentration of a solution in mol/dm3 is related to the mass of the solute and the volume of the solution
- C5.1f explain how the mass of a solute and the volume of the solution is related to the concentration of the solution
- For solutions, the mass of solute (grams or g), the number of moles of solute (moles or mol), the volume of solution (litres or l) or the concentration of the solution (moles per litre or mol l⁻¹) can be calculated from data provided
- (f) acid-base titrations
- (g) concept of concentration and its expression in terms of grams or moles per unit volume (including solubility)
- (j) titration as a method to prepare solutions of soluble salts and to determine relative and actual concentrations of solutions of acids/alkalis
- (k) the concentration of a solution in mol dm⁻³
- 2.6.1 calculate the concentration of a solution in mol/dm³ given the mass of solute and volume of solution;
- 2.6.2 calculate the number of moles or mass of solute in a given volume of solution of known concentration;
- 2.6.5 calculate the concentration of a solution in mol/dm³ given the mass of solute and volume of solution;
- 2.6.6 calculate the number of moles or mass of solute in a given volume of solution of known concentration;
Related articles

Why I use video to teach chemistry concepts
2024-02-27T08:17:00Z By Helen Rogerson
Helen Rogerson shares why and when videos are useful in the chemistry classroom

Why I don’t use video to explain chemistry concepts
2024-02-16T05:00:00Z By Adam Boxer
Discover Adam Boxer’s reasons for favouring a teacher’s explanation over a video

Build successful lesson plans using AI
2024-02-12T05:00:00Z By Colin McGill
Discover how AI can empower you to become a better teacher
No comments yet
Only registered users can comment on this article., more from lesson plans.

Determining the structure of compounds | 16–18 years
Examine data relating to the structure and complexity of compounds, including mass, infrared and 1 H NMR spectra

How do scientists grow protein crystals? | 14-16 years
Discover the methods and conditions used by chemical scientists to grow protein crystals in this lesson plan with activities for 14–16 year olds.

How does sodium react with chlorine? | 14-16 years
Investigate the reaction of sodium with chlorine, using students’ understanding of atoms, ions and lattice structure, in this lesson plan for 14–16 year olds.
- Contributors
- Email alerts
Site powered by Webvision Cloud
Calculating the Concentration of a Chemical Solution
How to Calculate Concentration
- Chemical Laws
- Periodic Table
- Projects & Experiments
- Scientific Method
- Biochemistry
- Physical Chemistry
- Medical Chemistry
- Chemistry In Everyday Life
- Famous Chemists
- Activities for Kids
- Abbreviations & Acronyms
- Weather & Climate
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
Concentration is an expression of how much solute is dissolved in a solvent in a chemical solution . There are multiple units of concentration . Which unit you use depends on how you intend to use the chemical solution. The most common units are molarity, molality, normality, mass percent, volume percent, and mole fraction. Here are step-by-step directions for calculating concentration, with examples showing the math and tips on when to use the units.
How to Calculate Molarity of a Chemical Solution
Yucel Yilmaz / Getty Images
Molarity is one of the most common units of concentration. It is used when the temperature of an experiment won't change. It's one of the easiest units to calculate. You get the mass of solute for the solution, mix the solute with a known volume of solvent, and divide mass by volume for concentration.
Calculate Molarity : moles solute per liter of solution ( not volume of solvent added since the solute takes up some space)
M = moles / liter
Example : What is the molarity of a solution of 6 grams of NaCl (~1 teaspoon of table salt) dissolved in 500 milliliters of water?
First, convert grams of NaCl to moles of NaCl.
From the periodic table:
- Na = 23.0 g/mol
- Cl = 35.5 g/mol
- NaCl = 23.0 g/mol + 35.5 g/mol = 58.5 g/mol
- Total number of moles = (1 mole / 58.5 g) * 6 g = 0.62 moles
Now determine moles per liter of solution:
M = 0.62 moles NaCl / 0.50 liter solution = 1.2 M solution (1.2 molar solution)
Note that I assumed dissolving the 6 grams of salt did not appreciably affect the volume of the solution. When you prepare a molar solution, avoid this problem by adding solvent to your solute to reach a specific volume.
How to Calculate Molality of a Solution
Molality is used to express the concentration of a solution when you are performing experiments that involve temperature changes or are working with colligative properties. Note that with aqueous solutions at room temperature, the density of water is approximately 1 kg/L, so M and m are nearly the same.
Calculate Molality : moles solute per kilogram solvent
m = moles / kilogram
Example : What is the molality of a solution of 3 grams of KCl (potassium chloride) in 250 ml of water?
First, determine how many moles are present in 3 grams of KCl. Start by looking up the number of grams per mole of potassium and chlorine on a periodic table . Then add them together to get the grams per mole for KCl.
- K = 39.1 g/mol
- KCl = 39.1 + 35.5 = 74.6 g/mol
For 3 grams of KCl, the number of moles is:
(1 mole / 74.6 g) * 3 grams = 3 / 74.6 = 0.040 moles
Express this as moles per kilogram solution. Now, you have 250 ml of water, which is about 250 g of water (assuming a density of 1 g/ml), but you also have 3 grams of solute, so the total mass of the solution is closer to 253 grams than 250. Using 2 significant figures, it's the same thing. If you have more precise measurements, don't forget to include the mass of solute in your calculation!
- 250 g = 0.25 kg
- m = 0.040 moles / 0.25 kg = 0.16 m KCl (0.16 molal solution)
How to Calculate Normality of a Chemical Solution
Normality is similar to molarity, except it expresses the number of active grams of a solute per liter of solution. This is the gram equivalent weight of solute per liter of solution.
Normality is often used in acid-base reactions or when dealing with acids or bases.
Calculate Normality : grams active solute per liter of solution
Example : For acid-base reactions, what would be the normality of 1 M solution of sulfuric acid (H 2 SO 4 ) in water?
Sulfuric acid is a strong acid that completely dissociates into its ions, H + and SO 4 2- , in aqueous solution. You know there are 2 moles of H+ ions (the active chemical species in an acid-base reaction) for every 1 mole of sulfuric acid because of the subscript in the chemical formula. So, a 1 M solution of sulfuric acid would be a 2 N (2 normal) solution.
How to Calculate Mass Percent Concentration of a Solution
Mass percent composition (also called mass percent or percent composition) is the easiest way to express the concentration of a solution because no unit conversions are required. Simply use a scale to measure the mass of the solute and the final solution and express the ratio as a percentage. Remember, the sum of all percentages of components in a solution must add up to 100%
Mass percent is used for all sorts of solutions but is particularly useful when dealing with mixtures of solids or anytime physical properties of the solution are more important than chemical properties.
Calculate Mass Percent : mass solute divided by mass final solution multiplied by 100%
Example : The alloy Nichrome consists of 75% nickel, 12% iron, 11% chromium, 2% manganese, by mass. If you have 250 grams of nichrome, how much iron do you have?
Because the concentration is a percent, you know a 100-gram sample would contain 12 grams of iron. You can set this up as an equation and solve for the unknown "x":
12 g iron / 100 g sample = x g iron / 250 g sample
Cross-multiply and divide:
x= (12 x 250) / 100 = 30 grams of iron
How to Calculate Volume Percent Concentration of a Solution
Volume percent is the volume of solute per volume of solution. This unit is used when mixing together volumes of two solutions to prepare a new solution. When you mix solutions, the volumes aren't always additive , so volume percent is a good way to express concentration. The solute is the liquid present in a smaller amount, while the solution is the liquid present in a larger amount.
Calculate Volume Percent : volume of solute per volume of solution ( not volume of solvent), multiplied by 100%
symbol : v/v %
v/v % = liters/liters x 100% or milliliters/milliliters x 100% (doesn't matter what units of volume you use as long as they are the same for solute and solution)
Example : What is the volume percent of ethanol if you dilute 5.0 milliliters of ethanol with water to obtain a 75-milliliter solution?
v/v % = 5.0 ml alcohol / 75 ml solution x 100% = 6.7% ethanol solution, by volume.
How to Calculate Mole Fraction of a Solution
Mole fraction or molar fraction is the number of moles of one component of a solution divided by the total number of moles of all chemical species. The sum of all mole fractions adds up to 1. Note that moles cancel out when calculating mole fraction, so it is a unitless value. Note some people express mole fraction as a percent (not common). When this is done, the mole fraction is multiplied by 100%.
symbol : X or the lower-case Greek letter chi, χ, which is often written as a subscript
Calculate Mole Fraction : X A = (moles of A) / (moles of A + moles of B + moles of C...)
Example : Determine the mole fraction of NaCl in a solution in which 0.10 moles of the salt is dissolved in 100 grams of water.
The moles of NaCl is provided, but you still need the number of moles of water , H 2 O. Start by calculating the number of moles in one gram of water, using periodic table data for hydrogen and oxygen:
- H = 1.01 g/mol
- O = 16.00 g/mol
- H 2 O = 2 + 16 = 18 g/mol (look at the subscript to note there are 2 hydrogen atoms)
Use this value to convert the total number of grams of water into moles:
(1 mol / 18 g ) * 100 g = 5.56 moles of water
Now you have the information needed to calculate mole fraction.
- X salt = moles salt / (moles salt + moles water)
- X salt = 0.10 mol / (0.10 + 5.56 mol)
- X salt = 0.02
More Ways to Calculate and Express Concentration
There are other easy ways to express the concentration of a chemical solution. Parts per million and parts per billion are used primarily for extremely dilute solutions.
g/L = grams per liter = mass of solute / volume of solution
F = formality = formula weight units per liter of solution
ppm = parts per million = ratio of parts of solute per 1 million parts of the solution
ppb = parts per billion = ratio of parts of solute per 1 billion parts of the solution.
- Calculating Concentrations with Units and Dilutions
- Concentration Definition (Chemistry)
- Convert Molarity to Parts Per Million Example Problem
- How to Calculate Mass Percent Composition
- Mass Percentage Definition and Example
- Here's How to Calculate pH Values
- Normality Definition in Chemistry
- Molarity Definition in Chemistry
- Solubility Definition in Chemistry
- Calculating the Number of Atoms and Molecules in a Drop of Water
- Topics Typically Covered in Grade 11 Chemistry
- Calculate Empirical and Molecular Formulas
- Molecules and Moles in Chemistry
- Mole Ratio: Definition and Examples
- Experimental Determination of Avogadro's Number
- What Is a Mole Fraction?
- TPC and eLearning
- Read Watch Interact
- What's NEW at TPC?
- Practice Review Test
- Teacher-Tools
- Subscription Selection
- Seat Calculator
- Ad Free Account
- Edit Profile Settings
- Classes (Version 2)
- Student Progress Edit
- Task Properties
- Export Student Progress
- Task, Activities, and Scores
- Metric Conversions Questions
- Metric System Questions
- Metric Estimation Questions
- Significant Digits Questions
- Proportional Reasoning
- Acceleration
- Distance-Displacement
- Dots and Graphs
- Graph That Motion
- Match That Graph
- Name That Motion
- Motion Diagrams
- Pos'n Time Graphs Numerical
- Pos'n Time Graphs Conceptual
- Up And Down - Questions
- Balanced vs. Unbalanced Forces
- Change of State
- Force and Motion
- Mass and Weight
- Match That Free-Body Diagram
- Net Force (and Acceleration) Ranking Tasks
- Newton's Second Law
- Normal Force Card Sort
- Recognizing Forces
- Air Resistance and Skydiving
- Solve It! with Newton's Second Law
- Which One Doesn't Belong?
- Component Addition Questions
- Head-to-Tail Vector Addition
- Projectile Mathematics
- Trajectory - Angle Launched Projectiles
- Trajectory - Horizontally Launched Projectiles
- Vector Addition
- Vector Direction
- Which One Doesn't Belong? Projectile Motion
- Forces in 2-Dimensions
- Being Impulsive About Momentum
- Explosions - Law Breakers
- Hit and Stick Collisions - Law Breakers
- Case Studies: Impulse and Force
- Impulse-Momentum Change Table
- Keeping Track of Momentum - Hit and Stick
- Keeping Track of Momentum - Hit and Bounce
- What's Up (and Down) with KE and PE?
- Energy Conservation Questions
- Energy Dissipation Questions
- Energy Ranking Tasks
- LOL Charts (a.k.a., Energy Bar Charts)
- Match That Bar Chart
- Words and Charts Questions
- Name That Energy
- Stepping Up with PE and KE Questions
- Case Studies - Circular Motion
- Circular Logic
- Forces and Free-Body Diagrams in Circular Motion
- Gravitational Field Strength
- Universal Gravitation
- Angular Position and Displacement
- Linear and Angular Velocity
- Angular Acceleration
- Rotational Inertia
- Balanced vs. Unbalanced Torques
- Getting a Handle on Torque
- Torque-ing About Rotation
- Properties of Matter
- Fluid Pressure
- Buoyant Force
- Sinking, Floating, and Hanging
- Pascal's Principle
- Flow Velocity
- Bernoulli's Principle
- Balloon Interactions
- Charge and Charging
- Charge Interactions
- Charging by Induction
- Conductors and Insulators
- Coulombs Law
- Electric Field
- Electric Field Intensity
- Polarization
- Case Studies: Electric Power
- Know Your Potential
- Light Bulb Anatomy
- I = ∆V/R Equations as a Guide to Thinking
- Parallel Circuits - ∆V = I•R Calculations
- Resistance Ranking Tasks
- Series Circuits - ∆V = I•R Calculations
- Series vs. Parallel Circuits
- Equivalent Resistance
- Period and Frequency of a Pendulum
- Pendulum Motion: Velocity and Force
- Energy of a Pendulum
- Period and Frequency of a Mass on a Spring
- Horizontal Springs: Velocity and Force
- Vertical Springs: Velocity and Force
- Energy of a Mass on a Spring
- Decibel Scale
- Frequency and Period
- Closed-End Air Columns
- Name That Harmonic: Strings
- Rocking the Boat
- Wave Basics
- Matching Pairs: Wave Characteristics
- Wave Interference
- Waves - Case Studies
- Color Addition and Subtraction
- Color Filters
- If This, Then That: Color Subtraction
- Light Intensity
- Color Pigments
- Converging Lenses
- Curved Mirror Images
- Law of Reflection
- Refraction and Lenses
- Total Internal Reflection
- Who Can See Who?
- Formulas and Atom Counting
- Atomic Models
- Bond Polarity
- Entropy Questions
- Cell Voltage Questions
- Heat of Formation Questions
- Reduction Potential Questions
- Oxidation States Questions
- Measuring the Quantity of Heat
- Hess's Law
- Oxidation-Reduction Questions
- Galvanic Cells Questions
- Thermal Stoichiometry
- Molecular Polarity
- Quantum Mechanics
- Balancing Chemical Equations
- Bronsted-Lowry Model of Acids and Bases
- Classification of Matter
- Collision Model of Reaction Rates
- Density Ranking Tasks
- Dissociation Reactions
- Complete Electron Configurations
- Elemental Measures
- Enthalpy Change Questions
- Equilibrium Concept
- Equilibrium Constant Expression
- Equilibrium Calculations - Questions
- Equilibrium ICE Table
- Ionic Bonding
- Lewis Electron Dot Structures
- Limiting Reactants
- Line Spectra Questions
- Measurement and Numbers
- Metals, Nonmetals, and Metalloids
- Metric Estimations
- Metric System
- Molarity Ranking Tasks
- Mole Conversions
- Name That Element
- Names to Formulas
- Names to Formulas 2
- Nuclear Decay
- Particles, Words, and Formulas
- Periodic Trends
- Precipitation Reactions and Net Ionic Equations
- Pressure Concepts
- Pressure-Temperature Gas Law
- Pressure-Volume Gas Law
- Chemical Reaction Types
- Significant Digits and Measurement
- States Of Matter Exercise
- Stoichiometry Law Breakers
- Stoichiometry - Math Relationships
- Subatomic Particles
- Spontaneity and Driving Forces
- Gibbs Free Energy
- Volume-Temperature Gas Law
- Acid-Base Properties
- Energy and Chemical Reactions
- Chemical and Physical Properties
- Valence Shell Electron Pair Repulsion Theory
- Writing Balanced Chemical Equations
- Mission CG1
- Mission CG10
- Mission CG2
- Mission CG3
- Mission CG4
- Mission CG5
- Mission CG6
- Mission CG7
- Mission CG8
- Mission CG9
- Mission EC1
- Mission EC10
- Mission EC11
- Mission EC12
- Mission EC2
- Mission EC3
- Mission EC4
- Mission EC5
- Mission EC6
- Mission EC7
- Mission EC8
- Mission EC9
- Mission RL1
- Mission RL2
- Mission RL3
- Mission RL4
- Mission RL5
- Mission RL6
- Mission KG7
- Mission RL8
- Mission KG9
- Mission RL10
- Mission RL11
- Mission RM1
- Mission RM2
- Mission RM3
- Mission RM4
- Mission RM5
- Mission RM6
- Mission RM8
- Mission RM10
- Mission LC1
- Mission RM11
- Mission LC2
- Mission LC3
- Mission LC4
- Mission LC5
- Mission LC6
- Mission LC8
- Mission SM1
- Mission SM2
- Mission SM3
- Mission SM4
- Mission SM5
- Mission SM6
- Mission SM8
- Mission SM10
- Mission KG10
- Mission SM11
- Mission KG2
- Mission KG3
- Mission KG4
- Mission KG5
- Mission KG6
- Mission KG8
- Mission KG11
- Mission F2D1
- Mission F2D2
- Mission F2D3
- Mission F2D4
- Mission F2D5
- Mission F2D6
- Mission KC1
- Mission KC2
- Mission KC3
- Mission KC4
- Mission KC5
- Mission KC6
- Mission KC7
- Mission KC8
- Mission AAA
- Mission SM9
- Mission LC7
- Mission LC9
- Mission NL1
- Mission NL2
- Mission NL3
- Mission NL4
- Mission NL5
- Mission NL6
- Mission NL7
- Mission NL8
- Mission NL9
- Mission NL10
- Mission NL11
- Mission NL12
- Mission MC1
- Mission MC10
- Mission MC2
- Mission MC3
- Mission MC4
- Mission MC5
- Mission MC6
- Mission MC7
- Mission MC8
- Mission MC9
- Mission RM7
- Mission RM9
- Mission RL7
- Mission RL9
- Mission SM7
- Mission SE1
- Mission SE10
- Mission SE11
- Mission SE12
- Mission SE2
- Mission SE3
- Mission SE4
- Mission SE5
- Mission SE6
- Mission SE7
- Mission SE8
- Mission SE9
- Mission VP1
- Mission VP10
- Mission VP2
- Mission VP3
- Mission VP4
- Mission VP5
- Mission VP6
- Mission VP7
- Mission VP8
- Mission VP9
- Mission WM1
- Mission WM2
- Mission WM3
- Mission WM4
- Mission WM5
- Mission WM6
- Mission WM7
- Mission WM8
- Mission WE1
- Mission WE10
- Mission WE2
- Mission WE3
- Mission WE4
- Mission WE5
- Mission WE6
- Mission WE7
- Mission WE8
- Mission WE9
- Vector Walk Interactive
- Name That Motion Interactive
- Kinematic Graphing 1 Concept Checker
- Kinematic Graphing 2 Concept Checker
- Graph That Motion Interactive
- Two Stage Rocket Interactive
- Rocket Sled Concept Checker
- Force Concept Checker
- Free-Body Diagrams Concept Checker
- Free-Body Diagrams The Sequel Concept Checker
- Skydiving Concept Checker
- Elevator Ride Concept Checker
- Vector Addition Concept Checker
- Vector Walk in Two Dimensions Interactive
- Name That Vector Interactive
- River Boat Simulator Concept Checker
- Projectile Simulator 2 Concept Checker
- Projectile Simulator 3 Concept Checker
- Hit the Target Interactive
- Turd the Target 1 Interactive
- Turd the Target 2 Interactive
- Balance It Interactive
- Go For The Gold Interactive
- Egg Drop Concept Checker
- Fish Catch Concept Checker
- Exploding Carts Concept Checker
- Collision Carts - Inelastic Collisions Concept Checker
- Its All Uphill Concept Checker
- Stopping Distance Concept Checker
- Chart That Motion Interactive
- Roller Coaster Model Concept Checker
- Uniform Circular Motion Concept Checker
- Horizontal Circle Simulation Concept Checker
- Vertical Circle Simulation Concept Checker
- Race Track Concept Checker
- Gravitational Fields Concept Checker
- Orbital Motion Concept Checker
- Angular Acceleration Concept Checker
- Balance Beam Concept Checker
- Torque Balancer Concept Checker
- Aluminum Can Polarization Concept Checker
- Charging Concept Checker
- Name That Charge Simulation
- Coulomb's Law Concept Checker
- Electric Field Lines Concept Checker
- Put the Charge in the Goal Concept Checker
- Circuit Builder Concept Checker (Series Circuits)
- Circuit Builder Concept Checker (Parallel Circuits)
- Circuit Builder Concept Checker (∆V-I-R)
- Circuit Builder Concept Checker (Voltage Drop)
- Equivalent Resistance Interactive
- Pendulum Motion Simulation Concept Checker
- Mass on a Spring Simulation Concept Checker
- Particle Wave Simulation Concept Checker
- Boundary Behavior Simulation Concept Checker
- Slinky Wave Simulator Concept Checker
- Simple Wave Simulator Concept Checker
- Wave Addition Simulation Concept Checker
- Standing Wave Maker Simulation Concept Checker
- Color Addition Concept Checker
- Painting With CMY Concept Checker
- Stage Lighting Concept Checker
- Filtering Away Concept Checker
- InterferencePatterns Concept Checker
- Young's Experiment Interactive
- Plane Mirror Images Interactive
- Who Can See Who Concept Checker
- Optics Bench (Mirrors) Concept Checker
- Name That Image (Mirrors) Interactive
- Refraction Concept Checker
- Total Internal Reflection Concept Checker
- Optics Bench (Lenses) Concept Checker
- Kinematics Preview
- Velocity Time Graphs Preview
- Moving Cart on an Inclined Plane Preview
- Stopping Distance Preview
- Cart, Bricks, and Bands Preview
- Fan Cart Study Preview
- Friction Preview
- Coffee Filter Lab Preview
- Friction, Speed, and Stopping Distance Preview
- Up and Down Preview
- Projectile Range Preview
- Ballistics Preview
- Juggling Preview
- Marshmallow Launcher Preview
- Air Bag Safety Preview
- Colliding Carts Preview
- Collisions Preview
- Engineering Safer Helmets Preview
- Push the Plow Preview
- Its All Uphill Preview
- Energy on an Incline Preview
- Modeling Roller Coasters Preview
- Hot Wheels Stopping Distance Preview
- Ball Bat Collision Preview
- Energy in Fields Preview
- Weightlessness Training Preview
- Roller Coaster Loops Preview
- Universal Gravitation Preview
- Keplers Laws Preview
- Kepler's Third Law Preview
- Charge Interactions Preview
- Sticky Tape Experiments Preview
- Wire Gauge Preview
- Voltage, Current, and Resistance Preview
- Light Bulb Resistance Preview
- Series and Parallel Circuits Preview
- Thermal Equilibrium Preview
- Linear Expansion Preview
- Heating Curves Preview
- Electricity and Magnetism - Part 1 Preview
- Electricity and Magnetism - Part 2 Preview
- Vibrating Mass on a Spring Preview
- Period of a Pendulum Preview
- Wave Speed Preview
- Slinky-Experiments Preview
- Standing Waves in a Rope Preview
- Sound as a Pressure Wave Preview
- DeciBel Scale Preview
- DeciBels, Phons, and Sones Preview
- Sound of Music Preview
- Shedding Light on Light Bulbs Preview
- Models of Light Preview
- Electromagnetic Radiation Preview
- Electromagnetic Spectrum Preview
- EM Wave Communication Preview
- Digitized Data Preview
- Light Intensity Preview
- Concave Mirrors Preview
- Object Image Relations Preview
- Snells Law Preview
- Reflection vs. Transmission Preview
- Magnification Lab Preview
- Reactivity Preview
- Ions and the Periodic Table Preview
- Periodic Trends Preview
- Reaction Rates Preview
- Ammonia Factory Preview
- Stoichiometry Preview
- Gaining Teacher Access
- Tasks and Classes
- Tasks - Classic
- Subscription
- Subscription Locator
- 1-D Kinematics
- Newton's Laws
- Vectors - Motion and Forces in Two Dimensions
- Momentum and Its Conservation
- Work and Energy
- Circular Motion and Satellite Motion
- Thermal Physics
- Static Electricity
- Electric Circuits
- Vibrations and Waves
- Sound Waves and Music
- Light and Color
- Reflection and Mirrors
- About the Physics Interactives
- Task Tracker
- Usage Policy
- Newtons Laws
- Vectors and Projectiles
- Forces in 2D
- Momentum and Collisions
- Circular and Satellite Motion
- Balance and Rotation
- Electromagnetism
- Waves and Sound
- Forces in Two Dimensions
- Work, Energy, and Power
- Circular Motion and Gravitation
- Sound Waves
- 1-Dimensional Kinematics
- Circular, Satellite, and Rotational Motion
- Einstein's Theory of Special Relativity
- Waves, Sound and Light
- QuickTime Movies
- About the Concept Builders
- Pricing For Schools
- Directions for Version 2
- Measurement and Units
- Relationships and Graphs
- Rotation and Balance
- Vibrational Motion
- Reflection and Refraction
- Teacher Accounts
- Task Tracker Directions
- Kinematic Concepts
- Kinematic Graphing
- Wave Motion
- Sound and Music
- About CalcPad
- 1D Kinematics
- Vectors and Forces in 2D
- Simple Harmonic Motion
- Rotational Kinematics
- Rotation and Torque
- Rotational Dynamics
- Electric Fields, Potential, and Capacitance
- Transient RC Circuits
- Light Waves
- Units and Measurement
- Stoichiometry
- Molarity and Solutions
- Thermal Chemistry
- Acids and Bases
- Kinetics and Equilibrium
- Solution Equilibria
- Oxidation-Reduction
- Nuclear Chemistry
- NGSS Alignments
- 1D-Kinematics
- Projectiles
- Circular Motion
- Magnetism and Electromagnetism
- Graphing Practice
- About the ACT
- ACT Preparation
- For Teachers
- Other Resources
- Newton's Laws of Motion
- Work and Energy Packet
- Static Electricity Review
- Solutions Guide
- Solutions Guide Digital Download
- Motion in One Dimension
- Work, Energy and Power
- Frequently Asked Questions
- Purchasing the Download
- Purchasing the CD
- Purchasing the Digital Download
- About the NGSS Corner
- NGSS Search
- Force and Motion DCIs - High School
- Energy DCIs - High School
- Wave Applications DCIs - High School
- Force and Motion PEs - High School
- Energy PEs - High School
- Wave Applications PEs - High School
- Crosscutting Concepts
- The Practices
- Physics Topics
- NGSS Corner: Activity List
- NGSS Corner: Infographics
- About the Toolkits
- Position-Velocity-Acceleration
- Position-Time Graphs
- Velocity-Time Graphs
- Newton's First Law
- Newton's Second Law
- Newton's Third Law
- Terminal Velocity
- Projectile Motion
- Forces in 2 Dimensions
- Impulse and Momentum Change
- Momentum Conservation
- Work-Energy Fundamentals
- Work-Energy Relationship
- Roller Coaster Physics
- Satellite Motion
- Electric Fields
- Circuit Concepts
- Series Circuits
- Parallel Circuits
- Describing-Waves
- Wave Behavior Toolkit
- Standing Wave Patterns
- Resonating Air Columns
- Wave Model of Light
- Plane Mirrors
- Curved Mirrors
- Teacher Guide
- Using Lab Notebooks
- Current Electricity
- Light Waves and Color
- Reflection and Ray Model of Light
- Refraction and Ray Model of Light
- Classes (Legacy Version)
- Teacher Resources
- Subscriptions

- Newton's Laws
- Einstein's Theory of Special Relativity
- About Concept Checkers
- School Pricing
- Newton's Laws of Motion
- Newton's First Law
- Newton's Third Law
Chemistry: Molarity and Solutions
Talk to our experts
1800-120-456-456
- Concentration of Solution

An introduction to Concentration of Solution
Everyone talks about the concentration of solutions. They may also talk about the concentration of coffee or tea. Everyone has a particular view of what is meant by the concentration of a solution. You must have noticed that whenever you make coffee, if you add a lot of powder, you will end up with a concentrated drink, whereas if you add little, it will result in a dilute solution. Therefore, it is essential that you understand what the concentration of the solution is. In this chapter, we will learn about what is meant by the concentration of a solution; we will also see how to find the concentration of a solution and the different methods of expressing the concentration of the solution.
What is Concentration of a Solution?
In an aqueous solution, two parts exist, namely solute and solvent. They are the two basic solution concentration terms that you need to know. We always need to keep an account of the amount of solute in the solution. In chemistry, we define the concentration of solution as the amount of solute in a solvent. When a solution has more solute in it, we call it a concentrated solution. Whereas when the solution has more solvent in it, we call it a dilute solution.
(Image will be uploaded soon)
Now that you understand the concept of what is the concentration of solution let's move on to the different methods of expressing concentration.
Methods of Expressing the concentration of Solution
There are various methods of expressing the concentration of a solution. You will usually see Chemists working with the number of moles. Pharmacists will use percentage concentrations instead of the number of moles. Hence it is important to understand all the methods of expressing the concentration of solutions.
The concentration of the solution formula is given as follows.
Concentration of solution = \[\frac{\text{Weight of the solute in gram}}{\text{volume in Litres}}\]
We will also see other methods on how to calculate the concentration of a solution based on the different methods of expressing concentrations.
Concentration in Parts per Million
It is expressed in terms of weight. The formula for parts per million is given as follows:
ppm(A)= \[\frac{\textrm{Mass of A}}{\textrm{Total mass of the solution}}\]x10\[^{6}\]
Mass Percentage (w/w)
It is expressed in terms of mass percentage of solute to the solution. The formula for mass percentage is given as follows.
Mass percentage of A = \[\frac{\text{Mass of component A}}{\text{Total mass of the soution}}\]x100
e.g. CH 3 COOH 33% w/w, and H 2 SO 4 98.0% w/w.
Volume Percentage (V/V)
It is expressed in terms of the volume percentage of solute to the solvent. The formula for volume percentage is given as follows.
Volume percentage of A = \[\frac{\text{Volume of component A}}{\text{Total volume of the solution}}\]x100
Mass by Volume Percentage (w/V)
Percentage weight in volume expresses the number of grams of solute in 100 ml of product.
e.g. BaCl 2 solution 10% w/v, and H 2 O 2 solution 5-7% w/v.
Molarity (M)
It is the number of moles of solute contained in 1000 ml of solution. It is a commonly used method for expressing concentrations.
Molarity = \[\frac{\text{Mass of solute}}{\text{volume of solution in litres}}\]
Molality (m)
The molality is expressed as the number of moles of a solute contained in 1000 gm of a solvent. The formula for molality is given as follows.
Molality (m) = \[\frac{\text{Mass of solute}}{\text{Mass of solvent in Kg}}\]
Normality (N)
We can define it as the number of equivalents of the solute present in the solution, and it is also called equivalent concentration. The formula for normality is given as follows.
Normality (N) = \[\frac{\text{Weight of solute in grams}}{\text{Equivalent mass} \times \text{Volume in litre}}\]
Mole Fraction:
The mole fraction (X) of a component in a solution is defined as the ratio of the number of moles of that component to the total number of moles of all components in the solution. The mole fraction of A is expressed as X A with the help of the following equation in a solution consisting of A, B, C, … we can calculate X A .
X\[_{A}\] = \[\frac{\text{moles of A}}{\text{mole of A + mole of B + momle of C +.... }}\]
Similarly, we can calculate the mole fraction of B, X B with the help of the following formula.
X\[_{B}\] = \[\frac{\text{moles of B}}{\text{mole of A + mole of B + momle of C +.... }}\]
Now that you know how to find the concentration of a solution using various concentrations of solution formulas, we will try to solve some concentrations of solution questions.
Solved Problems
Question 1) 2 ml of water is added to 4 g of a powdered drug. The final volume is 3ml. Find the mass by volume percentage of the solution?
Answer 1) Given, Mass of solute = 4g
Volume of solution = 3ml
Mass by volume percentage = \[\frac{\text{Mass of solute}}{\text{Volume of solution}}\]x100 = \[\frac{4g}{3ml}\] = 133%
Therefore, the mass by volume percentage is 133 %.
Question 2) Many people use a solution of Na 3 PO 4 to clean walls before putting up wallpaper. The recommended concentration is 1.7 % (m/v). Find the mass of Na 3 PO 4 needed to make 2.0 L of the solution?
Answer 2) Given,
Mass/Volume percentage = 1.7 %
Volume of Solution = 2000 ml
Mass by volume percentage = \[\frac{\text{Mass of solute}}{\text{Volume of solution}}\] × 100
1.7 % = \[\frac{\text{Mass of solute}}{2000ml}\] ×100
Mass of solute = 34 g
Therefore, the mass required is 34 g.
In chemistry, we are often required to calculate the concentration of the solution. The above-mentioned methods of expressing the concentration of a solution are important. The solved examples are helpful for a better understanding of the concept of concentration of a solution.

FAQs on Concentration of Solution
1. Does solution concentration change when solution volume changes?
The answer to this question depends on how we define concentration. If we talk about molarity, then yes it does change. If we take concentration by mass into consideration, it will still change, unless the substance is with an undefined density. That's because the mass of a substance will change with its volume, and so the concentration changes. But, if both the solute and solvent are either increasing or decreasing in volume/mass/moles in an equal ratio, the concentration and molarity will remain the same.
2. How do I convert from molarity to a weight percentage?
The first step is to multiply the molarity by the molar mass of the solute to get grams of solute per litre. The second step is to divide the concentration expressed as grams of solute per litre by the density of the solution in grams per litre. Finally, multiply it by 100% to convert it to percentage.
- Thermodynamics
- Analytical Chemistry
- Atomic Structure
- Classification of Organic Compounds
- Periodic Table of Elements
- Nuclear Force
- Importance of Chemistry
- Chemistry Notes Class 12
- Chemistry Notes Class 8
- Chemistry Notes Class 9
- Chemistry Notes Class 10
- Chemistry Notes Class 11
- CBSE Class 9 Chemistry Notes
Chapter 1: Matter in our Surroundings
- Matter is Made of Tiny Particles
- Why Solids, Liquids and Gases Have Different Properties
- Classification of Matter
- Brownian Movement
- States of Matter
- Evaporation
- Effects of Relative Humidity and Wind Speed
- How Does Evaporation Cause Cooling?
- Effect of Change of Temperature
- Melting Point
- What is Vaporization?
- Condensation
- Effects of Change of Pressure
- Difference between Rigidity and Fluidity of Matter
- Prove That Liquids have No fixed Shape but have a Fixed Volume
- Diffusion in Solids, Liquids, and Gases
- What is the Unit of Temperature?
- What is the Relationship Between Celsius and Kelvin Scale of Temperature?
- Liquification of Gases
- How to demonstrate the Presence of Water Vapour in Air?
- What is Plasma and Bose-Einstein Condensate?
Chapter 2: Is Matter Around Us Pure?
- Solution: Properties of Solution
- Saturated and Unsaturated Solutions
Concentration of a Solution
- Suspensions
- How will you distinguish a Colloid from a Solution?
- Classification of Colloids
- Tyndall Effect
- Separation of Mixtures
- How to separate a Mixture of Two Solids?
- Separation by a suitable solvent
- Separation of Mixtures using Sublimation and Magnets
- How to Separate a Mixture of a Solid and a Liquid?
- Water Purification
- Centrifugation
- How to Separate Cream from milk?
- Heterogeneous and Homogeneous Mixtures
- Difference Between Compound and Mixture
- Factors affecting Solubility
- Separation by Evaporation
- Crystallization
- Chromatography
- Distillation
- Separation of Mixtures of Two or More Liquids
- Fractional Distillation
- Pure and Impure Substances
- What is an Element?
- Metals, Non-Metals and Metalloids
- Properties of Metals and Non-Metals
Chapter 3: Atoms and Molecules
- Laws of Chemical Combination
- Law of Conservation of Mass
- Verification of the Law of Conservation of Mass in a Chemical Reaction
- Law of Constant Proportions
- Atomic Mass
- How Do Atoms Exist?
- Cations vs Anions
- What are Ionic Compounds?
- What are Monovalent Ions?
- What are Divalent Ions?
- Trivalent Ions - Cations and Anions
- Polyatomic Ions
- Formulas of Ionic Compounds
- Chemical Formula Of Common Compounds
- Chemical Formula of Common Compounds
- Molecular Mass
- Mole Concept
- Problems Based on Mole Concepts
- Dalton's Atomic Theory
- Drawbacks of Dalton's Atomic Theory
- Significance of the Symbol of Elements
- Difference Between Molecules and Compounds
- How to Calculate Valency of Radicals?
- What is the Significance of the Formula of a Substance?
- Gram Atomic and Gram Molecular Mass
Chapter 4: Structure of the Atom
- Charged Particles in Matter
- Thomson's Atomic Model
- Rutherford Atomic Model
- Drawbacks of Rutherford's Atomic Model
- Bohr's Model of an Atom
- Valence Electrons
- Mass Number
- Relation Between Mass Number and Atomic Number
- Why do all the Isotopes of an Element have similar Chemical Properties?
- Why Isotopes have different Physical Properties?
- What is Fractional Atomic Mass?
- Radioactive Isotopes
- Discovery of Electrons
- What is a Proton?
- Rutherford's Alpha Scattering Experiment
- Atomic Nucleus
- How did Neil Bohr explained the Stability of Atom?
- Electron Configuration
- Potassium and Calcium - Atomic Structure, Chemical Properties, Uses
- What is meant by Chemical Combination?
- Difference between Electrovalency and Covalency
Concentration of Solution is a measure of the amount of solute that has been dissolved in the given amount of solvent. In simple words, it means determining how much of one substance is mixed with another substance. As Concentration is a frequently used term in chemistry and other relevant fields, although it is most commonly used in the context of solutions, where it refers to the quantity of solute dissolved in a solvent. Concentration can be expressed in both qualitative or quantitative (numerically) terms.
Concentration of a Solution Definition
Concentration of a solution is defined as the amount of solute dissolved in the solution. It is given by the ratio of the amount of solute to the amount of solution or solvent sometimes. However, we can express it in percentages, Parts per Million, and several other ways. The concentration of a solution can be expressed both qualitatively and quantitatively which we will see in the below topics.
Qualitative Expressions of Concentration
To qualitatively express concentration, a solution can be classified as a dilute solution or a concentrated solution, which are explained as follows:
Dilute Solution
Dilute Solution contains a smaller proportion of solute than the proportion of solvent. For example, if 2 grams of salicylic acid is dissolved in 100 ml of water and in another container, 8 grams of salicylic acid is dissolved in the same amount of water then a 2-gram solution of salicylic acid is a dilute solution compared to 8 grams solution of salicylic acid.
Concentrated Solution
Concentrated Solution contains a much greater proportion of solute than the proportion of solvent. For example, if 2 grams of salicylic acid is dissolved in 100 ml of water and in another container, 8 grams of salicylic acid is dissolved in the same amount of water then 8 grams solution of salicylic acid is a concentrated solution compared to 2 grams solution of salicylic acid.

Figure 1. Dilute (left) and Concentrated (right) solutions
Semi-Qualitative Expressions of Concentration
To semi-qualitatively express concentration, a solution can be classified as a saturated solution or an unsaturated solution , which are explained as follows:
Saturated Solution
A saturated solution is one in which the greatest quantity of solute is dissolved in a solvent at a given temperature. When a solution reaches saturation, it can no longer dissolve any more solute at that temperature. Undissolved chemicals settle to the bottom. The saturation point is determined as the point at which no more solute can be dissolved in the solvent.
Unsaturated Solution
An unsaturated solution is one that contains less solute than the maximum possible solute it can dissolve before the solution reaches the saturation level. When more solute is dissolved in this solution, there are no residual substances at the bottom, indicating that all of the solutes have been dissolved in the solvent. An unsaturated solution is a chemical solution in which the solute concentration is less than the corresponding equilibrium solubility.

Figure 2. Unsaturated (left) and Saturated (right) solutions
Solubility is defined as the greatest amount of solute that may dissolve in a certain quantity of solvent at a given temperature.
A solution is a liquid that is a homogeneous combination of one or more solutes and a solvent. A typical example of a solution is, sugar cubes added to a cup of tea or coffee. Here, solubility is the characteristic that allows sugar molecules to dissolve.
As a result, the term solubility may be defined as a substance’s (solute’s) ability to dissolve in a particular solvent.
Quantitative Expressions of Concentration
Qualitative expressions of concentration are relative terms, which do not provide the exact concentration of the solution. To characterize the concentrations of various solutions around us in an accurate and precise manner, we require quantitative expressions of concentration.
Generally, concentration is represented in both ways: Concentration = Quantity of Solute / Quantity of Solution or Concentration = Quantity of Solute / Quantity of Solvent
To quantitatively express concentration, we use the following terms:
- Mass Percentage
- Volume Percentage
- Mass by Volume Percentage
- Parts per Million and Parts per Billion
Mole Fraction
Mass percentage (w/w%).
Mass percentage which is also called weight by weight concentration of solute and is defined as the amount of solute (in grams) present in 100 gm of the solution.
Mass Percentage = (Mass of Solute / Mass of Solution) × 100
Mass percentage has no unit as it is the ratio of the mass of solute and solution.
Volume Percentage (v/v%)
Volume Percentage which is also called volume by volume concentration of solute and is defined as the amount of solute (in ml) present in 100 ml of the solution.
Volume Percentage = (Volume of Solute / Volume of Solution) × 100
Volume percentage has no unit as it is the ratio of the volume of solute and solution.
Mass by Volume Percentage(w/v %)
It is defined as the amount of solute (in grams) present in the 100ml of the solution.
Mass by Volume Percentage = (Mass of Solute(in gm) / Volume of Solution(in ml) × 100
Unit of mass by volume percentage is gram per milliliter as it is the ratio of the mass of the solute and volume of the solution.
Parts per Million (PPM)
Parts Per Million or PPM is used to measure the very small amount of solute dissolved in the Solvent. Drinking water, air, soils, etc. are the solvents that have very fewer amounts of solutes in them, which can’t be measured in percentage as a percentage only calculates the concentration out of 100. If we represent concentrations of these solvents in percentage it looks like 0.00002 %, which is not an effective way. That’s why parts per million were introduced to make a representation of these concentrations.
PPM of solute = Mass of solute (in milligrams)/Mass of solution(In Kg)
Parts per Billion
Like, Parts per million, Parts Per Billion are also used to represent solute available in trace quantities. Parts Per Billion represents the amount of solute in 1 billion parts of the solution.
PPB of solute = Mass of solute (in micrograms)/Mass of solution(In Kg)
Molarity of a given solution is defined as the number of moles present in the 1 liter of solution. For example, if 2 moles of NaCl are dissolved in 1 liter of water, the molarity of the resulting solution would be 2M, and the Formula for Molarity is given as follows:
Molarity (M) = Moles of solute /Volume of solution(in Liter)
Molality of a given solution is defined as the number of moles present in 1 kg of solution. For example, if 3 moles of KOH are dissolved in 3 Liters of water (density of water 1 kg/L), the molality of the resulting solution would be 1 m, as there is 1 mole of KOH present in each Kg of water. The formula for molality is given as follows:
Molality (m) = Moles of solute / Mass of solvent(in Kg)
Mole fraction i.e., X is defined as the ratio of the number of moles of one component to the total number of moles present in the solution. It is a dimensionless quantity. The mole fraction of solute A in a solution containing n components such as A, B, C, . . ., N can be calculated using the following formula:
Mole fraction of A (X A ) = Moles of A / (Moles of A + Moles of B + . . . + Moles of N)
The mole fraction of other solvents (B, C, D, . . .N) in a solution can be calculated using a similar formula.
Temperature Dependence of Quantitative Expressions of Concentration
The following table shows the temperature dependence of the Quantitative Expressions of Concentration.
Mole Concept Heterogeneous and Homogeneous Mixtures Ideal and Non-Ideal Solutions
Sample Problems on Concentration of Solution
Problem 1: 15 g of common salt is dissolved in 400 g of water. Calculate the concentration of the solution by expressing it in Mass by Mass percentage (w/w%).
Given that, Mass of solute (common salt) = 15 g …(1) Mass of Solvent (water) = 400 g …(2) It is known that, Mass of Solution = Mass of Solute + Mass of Solvent …(3) So, Substituting (1) and (2) in (3), we obtain the following, Mass of Solution = 15 g + 400 g = 415 g …(4) From Figure 4, we know Mass by Mass Percentage = ( Mass of Solute / Mass of Solution ) × 100 …(5) Substituting (1) and (4) in (5), we obtain the following, Mass by Mass Percentage = ( 15 g / 415 g ) × 100 = ( 0.0361 ) × 100 = 3.61 Answer is: ( w / w % ) = 3.61
Problem 2: 15 g of common salt is dissolved in a solution of 300 mL, calculate the Mass by Volume percentage (w/v%).
Given that, Mass of solute (common salt) = 15 g . . . (1) Mass of Solution (salt solution) = 300 mL . . . (2) From Figure 5, we know Mass by Volume Percentage = ( Mass of Solute / Volume of Solution ) × 100 . . . (3) Substituting (1) and (2) in (3), we obtain the following, Mass by Volume Percentage = ( 15 g / 300 mL ) × 100 = ( 0.05 ) × 100 = 5 g/mL Answer is: ( w / v % ) = 5 g/mL
Problem 3: Richard dissolved 70 g of sugar in 750 mL of sugar solution. Calculate the Mass by Volume percentage (w/v%).
Given that, Mass of solute (common salt) = 70 g . . . (1) Mass of Solution (salt solution) = 750 mL . . . (2) From Figure 5, we know Mass by Volume Percentage = ( Mass of Solute / Volume of Solution ) × 100 . . . (3) Substituting (1) and (2) in (3), we obtain the following, Mass by Volume Percentage = ( 70 g / 750 mL ) * 100 = ( 0.933 ) × 100 = 93.3 g/mL Answer is: ( w / v % ) = 93.3 g/mL
Problem 4: What is the molarity of a solution containing 0.5 moles of NaCl dissolved in 500 mL of water?
Given, Moles of NaCl = 0.5, Volume of Solution = 500 mL = 0.5 Liter As, Molarity (M) = moles of solute / liters of solution ⇒ M = 0.5 moles / 0.5 liters = 1 M So the molarity of the solution is 1 M.
Problem 5: What is the molality of a solution containing 20 g of glucose dissolved in 500 g of water?
Given: Mass of Glucose = 20 g, Mass of water = 500 g = 0.5 kg, Molar mass of glucose (C 6 H 12 O 6 ) = 180 g/mol Number of Moles = Mass/Molar Mass ⇒ Moles of Glucose = 20 / 180 = 1/9 ≈ 0.111 moles of glucose As, Molality (m) = moles of solute / kilograms of solvent ⇒ m = 0.111 / 0.5 = 0.222 mol/kg So the molality of the solution is 0.222 mol/kg.
Problem 6:How many moles of HCl are present in 250 mL of a 0.2 M HCl solution?
Given: Molarity of solution = 0.2 M, Volume of solution = 250 mL = 0.25 liters Molarity (M) = moles of solute / liters of solution ⇒ Moles of solute = Molarity x liters of solution ⇒ Moles of HCl = 0.2 M x 0.25 L = 0.05 moles So there are 0.05 moles of HCl present in 250 mL of the solution.
Problem 7:What is the ppm of lead in a sample that contains 20 mg of lead in 10 L of water?
Given : mass of solute(in mg) = 20 mg and Volution of solvent = 10 L Mass of solution = Mass of water = 10 L x 1 Kg/L = 10 Kg (density of water is 1Kg/L or 1g/mL) ppm (parts per million) = Mass of solute(in mg)/Mass of solution (in Kg) ⇒ ppm = 20 / 10 = 2 So the ppm of lead in the sample is 2ppm.
Problem 8: What is the ppb of mercury in a sample that contains 0.01 g of mercury in 1000 L of air?
Given : mass of solute(in mg) = 0.01 g = 10,000 μg and Volution of solvent = 1000 L Mass of solution = Mass of water = 1000 L x 1 Kg/L = 1000 Kg (density of water is 1Kg/L or 1g/mL) ppb (parts per million) = Mass of solute(in μg)/Mass of solution (in Kg) ⇒ ppm = 10000 / 1000 = 10 So the ppm of lead in the sample is 10 ppb.
FAQs on Concentration of Solution
Q1: what is solubility, q2: what is a dilute solution.
A dilute solution is solution which contains a smaller proportion of solute as compared to the proportion of solvent.
Q3: What is the difference between PPM and PPB?
PPM and PPB are both represents the concentration of very small scale and only difference between them is that PPM is 1000 times greater scale then PPB or PPB is 1000 times smaller scale then PPM i.e., 1 PPM = 1000 PPB
Q4: What is the difference between Molarity and Molality?
Molarity is the number of moles of solute per liter of solution, whereas molality is the number of moles of solute per kilogram of solvent.
Please Login to comment...
- Chemistry-Class-11
- Chemistry-Class-12
- Chemistry-Class-9
- Chemistry-Formulas
- Physical-Chemistry
- School Chemistry
- School Learning
- WhatsApp To Launch New App Lock Feature
- Node.js 21 is here: What’s new
- Zoom: World’s Most Innovative Companies of 2024
- 10 Best Skillshare Alternatives in 2024
- 30 OOPs Interview Questions and Answers (2024)
Improve your Coding Skills with Practice
What kind of Experience do you want to share?
Mixture Problems With Solutions
Mixture problems and their solutions are presented along with their solutions. Percentages are also used to solve these types of problems.
Problem 1: How many liters of 20% alcohol solution should be added to 40 liters of a 50% alcohol solution to make a 30% solution?
Solution to Problem 1: Let x be the quantity of the 20% alcohol solution to be added to the 40 liters of a 50% alcohol. Let y be the quantity of the final 30% solution. Hence x + 40 = y We shall now express mathematically that the quantity of alcohol in x liters plus the quantity of alcohol in the 40 liters is equal to the quantity of alcohol in y liters. But remember the alcohol is measured in percentage term. 20% x + 50% * 40 = 30% y Substitute y by x + 40 in the last equation to obtain. 20% x + 50% * 40 = 30% (x + 40) Change percentages into fractions. 20 x / 100 + 50 * 40 / 100= 30 x / 100 + 30 * 40 / 100 Multiply all terms by 100 to simplify. 20 x + 50 * 40 = 30 x + 30 * 40 Solve for x. x = 80 liters 80 liters of 20% alcohol is be added to 40 liters of a 50% alcohol solution to make a 30% solution.
Problem 2: John wants to make a 100 ml of 5% alcohol solution mixing a quantity of a 2% alcohol solution with a 7% alcohol solution. What are the quantities of each of the two solutions (2% and 7%) he has to use?
Solution to Problem 2: Let x and y be the quantities of the 2% and 7% alcohol solutions to be used to make 100 ml. Hence x + y = 100 We now write mathematically that the quantity of alcohol in x ml plus the quantity of alcohol in y ml is equal to the quantity of alcohol in 100 ml. 2% x + 7% y = 5% 100 The first equation gives y = 100 - x. Substitute in the last equation to obtain 2% x + 7% (100 - x) = 5% 100 Multiply by 100 and simplify 2 x + 700 - 7 x = 5 * 100 Solve for x x = 40 ml Substitute x by 40 in the first equation to find y y = 100 - x = 60 ml
Problem 3: Sterling Silver is 92.5% pure silver. How many grams of Sterling Silver must be mixed to a 90% Silver alloy to obtain a 500g of a 91% Silver alloy?
Solution to Problem 3: Let x and y be the weights, in grams, of sterling silver and of the 90% alloy to make the 500 grams at 91%. Hence x + y =500 The number of grams of pure silver in x plus the number of grams of pure silver in y is equal to the number of grams of pure silver in the 500 grams. The pure silver is given in percentage forms. Hence 92.5% x + 90% y = 91% 500 Substitute y by 500 - x in the last equation to write 92.5% x + 90% (500 - x) = 91% 500 Simplify and solve 92.5 x + 45000 - 90 x = 45500 x = 200 grams. 200 grams of Sterling Silver is needed to make the 91% alloy.
Problem 4: How many Kilograms of Pure water is to be added to 100 Kilograms of a 30% saline solution to make it a 10% saline solution.
Solution to Problem 4: Let x be the weights, in Kilograms, of pure water to be added. Let y be the weight, in Kilograms, of the 10% solution. Hence x + 100 = y Let us now express the fact that the amount of salt in the pure water (which 0) plus the amount of salt in the 30% solution is equal to the amount of salt in the final saline solution at 10%. 0 + 30% 100 = 10% y Substitute y by x + 100 in the last equation and solve. 30% 100 = 10% (x + 100) Solve for x. x = 200 Kilograms.
Problem 5: A 50 ml after-shave lotion at 30% alcohol is mixed with 30 ml of pure water. What is the percentage of alcohol in the new solution?
Solution to Problem 5: The amount of the final mixture is given by 50 ml + 30 ml = 80 ml The amount of alcohol is equal to the amount of alcohol in pure water ( which is 0) plus the amount of alcohol in the 30% solution. Let x be the percentage of alcohol in the final solution. Hence 0 + 30% 50 ml = x (80) Solve for x x = 0.1817 = 18.75%
Problem 6: You add x ml of a 25% alcohol solution to a 200 ml of a 10% alcohol solution to obtain another solution. Find the amount of alcohol in the final solution in terms of x. Find the ratio, in terms of x, of the alcohol in the final solution to the total amount of the solution. What do you think will happen if x is very large? Find x so that the final solution has a percentage of 15%.
Solution to Problem 6: Let us first find the amount of alcohol in the 10% solution of 200 ml. 200 * 10% = 20 ml The amount of alcohol in the x ml of 25% solution is given by 25% x = 0.25 x The total amount of alcohol in the final solution is given by 20 + 0.25 x The ratio of alcohol in the final solution to the total amount of the solution is given by [ ( 20 + 0.25 x ) / (x + 200)] If x becomes very large in the above formula for the ratio, then the ratio becomes close to 0.25 or 25% (The above function is a rational function and 0.25 is its horizontal asymptote). This means that if you increase the amount x of the 25% solution, this will dominate and the final solution will be very close to a 25% solution. To have a percentage of 15%, we need to have [ ( 20 + 0.25 x ) / (x + 200)] = 15% = 0.15 Solve the above equation for x 20 + 0.25 x = 0.15 * (x + 200) x = 100 ml
- Chemistry Articles
- Concentration Of Solution And The Calculation
Expression of Concentration of Solutions
We always discuss a solution being diluted or concentrated; this is a qualitative way of expressing the concentration of the solution. A dilute solution means the quantity of solute is relatively very small, and a concentrated solution implies that the solution has a large amount of solute. But these are relative terms and do not give us the quantitative concentration of the solution.

Table of Contents
Concentration.
- Recommended Video
- Concentration in parts per million
- Mass percentage
- Volume percentage
- Mass by Volume percentage
- Mole Fraction
- Solutions of Solid in Liquid
Solubility of Gases
Frequently asked questions – faqs.
So, to quantitatively describe the concentrations of various solutions around us, we commonly express levels in the following way:
It is the amount of solute present in one litre of solution. It is denoted by C or S.
Recommended Videos
Introduction to solutions – concentration terms.

Is Matter Around us Pure?

Effect of Change in Concentration

1. Concentration in Parts Per Million (ppm)
The parts of a component per million parts (10 6 ) of the solution.
2. Mass Percentage (w/w):
When the concentration is expressed as the percent of one component in the solution by mass it is called mass percentage (w/w). Suppose we have a solution containing component A as the solute and B as the solvent, then its mass percentage is expressed as:
Mass % of A = \(\begin{array}{l} \frac {Mass \space of \space component \space A ~ in ~the ~ solution}{Total ~ mass ~of~ the~ solution } × 100 \end{array} \)
3. Volume Percentage (V/V):
Sometimes we express the concentration as a percent of one component in the solution by volume, it is then called as volume percentage and is given as:
volume % of A = \(\begin{array}{l} \frac {Volume ~of~ component~ A~ in~ the ~solution}{Total ~ volume ~ of ~ the ~ solution } × 100 \end{array} \)
For example, if a solution of NaCl in water is said to be 10 % by volume that means a 100 ml solution will contain 10 ml NaCl.
4. Mass by Volume Percentage (w/V):
This unit is majorly used in the pharmaceutical industry. It is defined as the mass of a solute dissolved per 100mL of the solution.
% w/V = (Mass of component A in the solution/ Total Volume of the Solution)x 100
5. Molarity (M):
One of the most commonly used methods for expressing the concentrations is molarity. It is the number of moles of solute dissolved in one litre of a solution. Suppose a solution of ethanol is marked 0.25 M, this means that in one litre of the given solution 0.25 moles of ethanol is dissolved.
Molarity (M) = Moles of Solute/Volume of Solution in litres
6. Molality (m):
Molality represents the concentration regarding moles of solute and the mass of solvent. It is given by moles of solute dissolved per kg of the solvent . The molality formula is as given-
\(\begin{array}{l} Molality (m) = \frac {Moles~ of ~solute}{Mass~ of~ solvent~ in~ kg}\end{array} \)
7. Normality
It is the number of gram equivalents of solute present in one litre of the solution and it is denoted by N.
The relation between normality and molarity.
- N x Eq.Wt = Molarity x Molar mass
- N = Molarity x Valency
- N = Molarity x Number of H + or OH – ion.
8. Formality
It is the number of gram formula units present in one litre of solution. It is denoted by F.
It is applicable in the case of ionic solids like NaCl.
9. Mole Fraction:
If the solution has a solvent and the solute, a mole fraction gives a concentration as the ratio of moles of one component to the total moles present in the solution. It is denoted by x. Suppose we have a solution containing A as a solute and B as the solvent. Let n A and n B be the number of moles of A and B present in the solution respectively. So, mole fractions of A and B are given as:
\(\begin{array}{l}~~~~~~~~~~~~~~~\end{array} \) \(\begin{array}{l} x_A = \frac {n_A}{n_A + n_B}\end{array} \) \(\begin{array}{l}~~~~~~~~~~~~~~~\end{array} \) \(\begin{array}{l} x_B = \frac {n_B}{n_A + n_B} \end{array} \)
The above-mentioned methods are commonly used ways of expressing the concentration of solutions. All the methods describe the same thing that is, the concentration of a solution, each of them has its own advantages and disadvantages. Molarity depends on temperature while mole fraction and molality are independent of temperature. All these methods are used on the basis of the requirement of expressing the concentrations.
Solutions of Solids in Liquids
- A saturated solution is a solution that remains in contact with an excess of solute.
- The amount of solute dissolved per 100g of solvent in a saturated solution at a specific temperature represents the solubility of the solute.
- For exothermic substances such as KOH, CaO, Ca(OH) 2 , M 2 CO 3 , M 2 SO 4 , etc, solubility is inversely proportional to temperature.
- For endothermic substances such as NaCl, KNO 3 , NaNO 3 , glucose, etc., solubility is directly proportional to temperature.
The solubility of gases is mostly expressed in terms of the absorption coefficient,k that is the volume of the gas dissolved by unit volume of solvent at 1 atm pressure and a specific temperature.
The solubility of a gas in a liquid depends upon
- Temperature solubility is inversely proportional to temperature as the dissolution of gas is exothermic in most cases.
- Nature of gas – Gases having a higher value of van der Waals force of attraction that is gases that are more easily liquefied are more soluble. For example, SO 2 and CO 2 are more soluble in water than O 2 , N 2 , and H 2 .
- Nature of solvent – Gases which can ionise in an aqueous solution are more stable in water as compared to the other solvents.
How do you change the concentration of a solution?
Sometimes, by modifying the quantity of solvent, a worker would need to modify the concentration of a solution. Dilution is the addition of a solvent that reduces the solute concentration of the solution. Concentration is solvent elimination, which increases the solute concentration in the solution.
What is a high concentration?
A concentration of persons means that in one place there are more of them. A high concentration of a material in a solution means there’s a lot of it compared to the volume: because of the high concentration of salt, the Great Salt Lake has very little fish.
Is dilute acid dangerous?
In general, a mild condensed acid is more harmful than a solid diluted acid. Although concentrated acetic acid is much less reactive, you do not want to touch the skin or mucous membranes because it is corrosive.
What is the concentration of a solution?
A solution concentration is a measure of the quantity of solute that has been dissolved in a given quantity of solvent or solution. One that contains a relatively high volume of dissolved solute is a concentrated solution. That that contains a relatively minimal volume of dissolved solute is a dilute solution.
How do you prepare a solution of known concentration?
Solutions of known concentration can be prepared either by dissolving the known mass of the solvent solution and diluting it to the desired final volume or by diluting it to the desired final volume by diluting the acceptable volume of the more concentrated solution (the stock solution).

Put your understanding of this concept to test by answering a few MCQs. Click ‘Start Quiz’ to begin!
Select the correct answer and click on the “Finish” button Check your score and answers at the end of the quiz
Visit BYJU’S for all Chemistry related queries and study materials
Your result is as below
Request OTP on Voice Call
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Post My Comment
- Share Share
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.

End-to-End Verification for Subgraph Solving
- Stephan Gocht Lund University, Lund, Sweden University of Copenhagen, Copenhagen, Denmark
- Ciaran McCreesh University of Glasgow, Glasgow, Scotland
- Magnus O. Myreen Chalmers University of Technology, Gothenburg, Sweden
- Jakob Nordström University of Copenhagen, Copenhagen, Denmark Lund University, Lund, Sweden
- Andy Oertel Lund University, Lund, Sweden University of Copenhagen, Copenhagen, Denmark
- Yong Kiam Tan Institute for Infocomm Research (I2R), A*STAR, Singapore

- Video/Poster
How to Cite
- Endnote/Zotero/Mendeley (RIS)
Information
- For Readers
- For Authors
- For Librarians
Developed By
Subscription.
Login to access subscriber-only resources.
Part of the PKP Publishing Services Network
Copyright © 2024, Association for the Advancement of Artificial Intelligence


Semiconductors at scale: New processor achieves remarkable speedup in problem solving
A nnealing processors are designed specifically for addressing combinatorial optimization problems, where the task is to find the best solution from a finite set of possibilities. This holds implications for practical applications in logistics, resource allocation, and the discovery of drugs and materials.
In the context of CMOS (a type of semiconductor technology), it is necessary for the components of annealing processors to be fully "coupled." However, the complexity of this coupling directly affects the scalability of the processors.
In a new IEEE Access study led by Professor Takayuki Kawahara from Tokyo University of Science, researchers have developed and successfully tested a scalable processor that divides the calculation into multiple LSI chips. The innovation was also presented in IEEE 22nd World Symposium on Applied Machine Intelligence and Informatics (SAMI 2024) on 25 January 2024.
According to Prof. Kawahara, "We want to achieve advanced information processing directly at the edge, rather than in the cloud or performing preprocessing at the edge for the cloud. Using the unique processing architecture announced by the Tokyo University of Science in 2020, we have realized a fully coupled LSI (Large Scale Integration) on one chip using 28nm CMOS technology. Furthermore, we devised a scalable method with parallel-operating chips and demonstrated its feasibility using FPGAs (Field-Programmable Gate Arrays) in 2022."
The team created a scalable annealing processor. It used 36 22nm CMOS calculation LSI (Large Scale Integration) chips and one control FPGA. This technology enables the construction of large-scale, fully coupled semiconductor systems following the Ising model (a mathematical model of magnetic systems) with 4096 spins.
The processor incorporates two distinct technologies developed at the Tokyo University of Science. This includes a "spin thread method" that enables 8 parallel solution searches, coupled with a technique that reduces chip requirements by about half compared to conventional methods. Its power needs are also modest, operating at 10MHz with a power consumption of 2.9W (1.3W for the core part). This was practically confirmed using a vertex cover problem with 4096 vertices.
In terms of power performance ratio, the processor outperformed simulating a fully coupled Ising system on a PC (i7, 3.6GHz) using annealing emulation by 2,306 times. Additionally, it surpassed the core CPU and arithmetic chip by 2,186 times.
The successful machine verification of this processor suggests the possibility of enhanced capacity. According to Prof. Kawahara, who holds a vision for the social implementation of this technology (such as initiating a business, joint research, and technology transfer), "In the future, we will develop this technology for a joint research effort targeting an LSI system with the computing power of a 2050-level quantum computer for solving combinatorial optimization problems."
"The goal is to achieve this without the need for air conditioning, large equipment, or cloud infrastructure using current semiconductor processes. Specifically, we would like to achieve 2M (million) spins by 2030 and explore the creation of new digital industries using this."
In summary, researchers have developed a scalable, fully coupled annealing processor incorporating 4096 spins on a single board with 36 CMOS chips. Key innovations, including chip reduction and parallel operations for simultaneous solution searches, played a crucial role in this development.
More information: Taichi Megumi et al, Scalable Fully-Coupled Annealing Processing System Implementing 4096 Spins Using 22nm CMOS LSI, IEEE Access (2024). DOI: 10.1109/ACCESS.2024.3360034
Provided by Tokyo University of Science
- Skip to main content
- Keyboard shortcuts for audio player
Aliens attack science in '3 Body Problem,' a new adaptation of a Chinese sci-fi novel

Eric Deggans

The new Netflix series brings to life a sprawling, successful Chinese novel outlining a new kind of alien invasion. Above, Zine Tseng in 3 Body Problem. Maria Heras/Netflix hide caption
The new Netflix series brings to life a sprawling, successful Chinese novel outlining a new kind of alien invasion. Above, Zine Tseng in 3 Body Problem.
My favorite kind of science fiction involves stories rooted in real science — much as I love a good lightsaber or phaser fight, there is something special about seeing characters wrestle with concepts closer to our current understanding of how the universe works.
That's why I enjoy so much of what happens in Netflix's 3 Body Problem , the TV series which brings to life a sprawling, successful Chinese novel rooted in science, outlining a new kind of alien invasion.

Book Reviews
'three-body problem' asks a classic sci-fi question, in chinese.
3 Body Problem actually starts with two problems. First, we meet investigators tackling a string of unexplained suicides by scientists, including one who had a bizarre countdown written on the walls of his home in blood with his eyes gouged out. (Fortunately, viewers only see the horrific aftermath.) Benedict Wong plays one of those investigators, continually lightening the show's ominous vibe with his spot-on portrayal of a world-weary gumshoe tracking the world's biggest mystery with a healthy dose of gallows humor.

Benedict Wong plays Da Shi in 3 Body Problem. Ed Miller/Netflix hide caption
Benedict Wong plays Da Shi in 3 Body Problem.
"One of the betting sites had him picked as a favorite for the next Nobel Prize in physics," Wong's assistant tells him of the scientist who died.
"You can bet on that?" Wong's character replies, looking over the gruesome scene.
Tracking why science is broken
The other problem which surfaces immediately is that science seems to have stopped working. Researchers are reporting results from experiments in supercolliders that make no sense, putting the lie to all our accepted theories of physics. Saul Durand — played by Jovan Adepo, Durand is one among a group of brilliant, young scientist friends at the center of the story — notes simply, "science is broken."

Jovan Adepo and Jin Cheng in 3 Body Problem. Ed Miller/Netflix hide caption
Jovan Adepo and Jin Cheng in 3 Body Problem.
This all adds up to a unique attack on humanity's scientific progress. But who – or what – is behind these bizarre occurrences, involving events which don't seem possible in the modern world?
Netflix's show takes its time unveiling the full scope of the story and answering these questions, which leads to the third problem here. It takes a while for the series' narrative to really gain momentum – my advice is to hang on through the first three episodes (yes, I also hate streaming shows which ask this of beleaguered viewers; but in this case, it's worth it).
The pacing may not be a surprise, given that two of the series' three creators are David Benioff and D.B. Weiss, former showrunners of HBO's Game of Thrones , which had its own problems with narrative flow at times (the third creator is former True Blood writer/executive producer Alexander Woo). Once the show does find its groove, the series builds into an epic science fiction tale with eye-popping special effects – the tragic destruction of a huge ship packed with people is one that stuck with me long after viewing — and a timeline stretching from China's 1960s-era cultural revolution to the present day.
Bringing a Chinese sci fi-literary triumph to TV
Netflix's 3 Body Problem is based on a 2008 novel from Chinese engineer and science fiction writer Liu Cixin; the original novel became a book series touted by big names like Barack Obama. It managed the neat trick of popularizing Chinese science fiction internationally while delivering compelling observations on the nature of humanity's societal and technological progress, some of which actually find their way into the TV show.

Cultural Revolution-Meets-Aliens: Chinese Writer Takes On Sci-Fi
It makes sense that a story like this — which crosses between Western and Chinese culture to tell the story of a planet under threat – would be cracked by Netflix. The streaming service has educated a generation of American customers to appreciate smart, entertaining TV from South Korea, Latin America, Europe and elsewhere across the globe.
So kicking off 3 Body Problem with a scene showing a young Chinese scientist watching an angry mob murder her father – who is also a scientist – during the purges of China's cultural revolution feels daring and entirely on brand. Later on, that younger scientist, fueled by hate and loss, will make a decision that puts the entire planet at risk, showing how disappointment in humanity's missteps can lead to desperate, misguided solutions.
Fans of the books will find some tweaks here to make for better television, amping up the thriller elements of the story to ask a compelling question: How to fight an alien enemy targeting the world's scientific progress?
As the characters in 3 Body Problem lurch toward answers, we all get to bask in an ambitious narrative fueling an ultimately impressive tale. Just remember to be patient as the series sets the stage early on.
What to know about the crisis of violence, politics and hunger engulfing Haiti

A long-simmering crisis over Haiti’s ability to govern itself, particularly after a series of natural disasters and an increasingly dire humanitarian emergency, has come to a head in the Caribbean nation, as its de facto president remains stranded in Puerto Rico and its people starve and live in fear of rampant violence.
The chaos engulfing the country has been bubbling for more than a year, only for it to spill over on the global stage on Monday night, as Haiti’s unpopular prime minister, Ariel Henry, agreed to resign once a transitional government is brokered by other Caribbean nations and parties, including the U.S.
But the very idea of a transitional government brokered not by Haitians but by outsiders is one of the main reasons Haiti, a nation of 11 million, is on the brink, according to humanitarian workers and residents who have called for Haitian-led solutions.
“What we’re seeing in Haiti has been building since the 2010 earthquake,” said Greg Beckett, an associate professor of anthropology at Western University in Canada.

What is happening in Haiti and why?
In the power vacuum that followed the assassination of democratically elected President Jovenel Moïse in 2021, Henry, who was prime minister under Moïse, assumed power, with the support of several nations, including the U.S.
When Haiti failed to hold elections multiple times — Henry said it was due to logistical problems or violence — protests rang out against him. By the time Henry announced last year that elections would be postponed again, to 2025, armed groups that were already active in Port-au-Prince, the capital, dialed up the violence.
Even before Moïse’s assassination, these militias and armed groups existed alongside politicians who used them to do their bidding, including everything from intimidating the opposition to collecting votes . With the dwindling of the country’s elected officials, though, many of these rebel forces have engaged in excessively violent acts, and have taken control of at least 80% of the capital, according to a United Nations estimate.
Those groups, which include paramilitary and former police officers who pose as community leaders, have been responsible for the increase in killings, kidnappings and rapes since Moïse’s death, according to the Uppsala Conflict Data Program at Uppsala University in Sweden. According to a report from the U.N . released in January, more than 8,400 people were killed, injured or kidnapped in 2023, an increase of 122% increase from 2022.
“January and February have been the most violent months in the recent crisis, with thousands of people killed, or injured, or raped,” Beckett said.

Armed groups who had been calling for Henry’s resignation have already attacked airports, police stations, sea ports, the Central Bank and the country’s national soccer stadium. The situation reached critical mass earlier this month when the country’s two main prisons were raided , leading to the escape of about 4,000 prisoners. The beleaguered government called a 72-hour state of emergency, including a night-time curfew — but its authority had evaporated by then.
Aside from human-made catastrophes, Haiti still has not fully recovered from the devastating earthquake in 2010 that killed about 220,000 people and left 1.5 million homeless, many of them living in poorly built and exposed housing. More earthquakes, hurricanes and floods have followed, exacerbating efforts to rebuild infrastructure and a sense of national unity.
Since the earthquake, “there have been groups in Haiti trying to control that reconstruction process and the funding, the billions of dollars coming into the country to rebuild it,” said Beckett, who specializes in the Caribbean, particularly Haiti.
Beckett said that control initially came from politicians and subsequently from armed groups supported by those politicians. Political “parties that controlled the government used the government for corruption to steal that money. We’re seeing the fallout from that.”

Many armed groups have formed in recent years claiming to be community groups carrying out essential work in underprivileged neighborhoods, but they have instead been accused of violence, even murder . One of the two main groups, G-9, is led by a former elite police officer, Jimmy Chérizier — also known as “Barbecue” — who has become the public face of the unrest and claimed credit for various attacks on public institutions. He has openly called for Henry to step down and called his campaign an “armed revolution.”
But caught in the crossfire are the residents of Haiti. In just one week, 15,000 people have been displaced from Port-au-Prince, according to a U.N. estimate. But people have been trying to flee the capital for well over a year, with one woman telling NBC News that she is currently hiding in a church with her three children and another family with eight children. The U.N. said about 160,000 people have left Port-au-Prince because of the swell of violence in the last several months.
Deep poverty and famine are also a serious danger. Gangs have cut off access to the country’s largest port, Autorité Portuaire Nationale, and food could soon become scarce.
Haiti's uncertain future
A new transitional government may dismay the Haitians and their supporters who call for Haitian-led solutions to the crisis.
But the creation of such a government would come after years of democratic disruption and the crumbling of Haiti’s political leadership. The country hasn’t held an election in eight years.
Haitian advocates and scholars like Jemima Pierre, a professor at the University of British Columbia, Vancouver, say foreign intervention, including from the U.S., is partially to blame for Haiti’s turmoil. The U.S. has routinely sent thousands of troops to Haiti , intervened in its government and supported unpopular leaders like Henry.
“What you have over the last 20 years is the consistent dismantling of the Haitian state,” Pierre said. “What intervention means for Haiti, what it has always meant, is death and destruction.”

In fact, the country’s situation was so dire that Henry was forced to travel abroad in the hope of securing a U.N. peacekeeping deal. He went to Kenya, which agreed to send 1,000 troops to coordinate an East African and U.N.-backed alliance to help restore order in Haiti, but the plan is now on hold . Kenya agreed last October to send a U.N.-sanctioned security force to Haiti, but Kenya’s courts decided it was unconstitutional. The result has been Haiti fending for itself.
“A force like Kenya, they don’t speak Kreyòl, they don’t speak French,” Pierre said. “The Kenyan police are known for human rights abuses . So what does it tell us as Haitians that the only thing that you see that we deserve are not schools, not reparations for the cholera the U.N. brought , but more military with the mandate to use all kinds of force on our population? That is unacceptable.”
Henry was forced to announce his planned resignation from Puerto Rico, as threats of violence — and armed groups taking over the airports — have prevented him from returning to his country.

Now that Henry is to stand down, it is far from clear what the armed groups will do or demand next, aside from the right to govern.
“It’s the Haitian people who know what they’re going through. It’s the Haitian people who are going to take destiny into their own hands. Haitian people will choose who will govern them,” Chérizier said recently, according to The Associated Press .
Haitians and their supporters have put forth their own solutions over the years, holding that foreign intervention routinely ignores the voices and desires of Haitians.
In 2021, both Haitian and non-Haitian church leaders, women’s rights groups, lawyers, humanitarian workers, the Voodoo Sector and more created the Commission to Search for a Haitian Solution to the Crisis . The commission has proposed the “ Montana Accord ,” outlining a two-year interim government with oversight committees tasked with restoring order, eradicating corruption and establishing fair elections.
For more from NBC BLK, sign up for our weekly newsletter .
CORRECTION (March 15, 2024, 9:58 a.m. ET): An earlier version of this article misstated which university Jemima Pierre is affiliated with. She is a professor at the University of British Columbia, Vancouver, not the University of California, Los Angeles, (or Columbia University, as an earlier correction misstated).
Patrick Smith is a London-based editor and reporter for NBC News Digital.
Char Adams is a reporter for NBC BLK who writes about race.
Generalized fuzzy difference method for solving fuzzy initial value problem
- Open access
- Published: 27 March 2024
- Volume 43 , article number 129 , ( 2024 )
Cite this article
You have full access to this open access article
- S. Soroush 1 ,
- T. Allahviranloo ORCID: orcid.org/0000-0002-6673-3560 1 , 2 ,
- H. Azari 3 &
- M. Rostamy-Malkhalifeh 3
We are going to explain the fuzzy Adams–Bashforth methods for solving fuzzy differential equations focusing on the concept of g -differentiability. Considering the analysis of normal, convex, upper semicontinuous, compactly supported fuzzy sets in \(R^n\) and also convergence of the methods, the general expression of solutions is obtained. Finally, we demonstrate the importance of our method with some illustrative examples. These examples are provided aiming to solve the fuzzy differential equations.
Avoid common mistakes on your manuscript.
1 Introduction
According to the most recent published papers, the fuzzy differential equation was introduced in 1978. Moreover, Kandel ( 1980 ) and Byatt and Kandel ( 1978 ) present the fuzzy differential equation and have rapidly expanded literature. First-order linear fuzzy differential equations emerge in modeling the uncertainty of dynamical systems. The solutions of first-order linear fuzzy differential equations have been widely considered (e. g., see Chalco-Cano and Roman-Flores 2008 ; Buckley and Feuring 2000 ; Seikkala 1987 ; Diamond 2002 ; Song and Wu 2000 ; Allahviranloo et al. 2009 ; Zabihi et al. 2023 ; Allahviranloo and Pedrycz 2020 ).
The most famous numerical solutions of order fuzzy differential equations are investigated and analyzed under the Hukuhara and gH -differentiability (Safikhani et al. 2023 ). It is widely believed that the common Hukuhara difference and so Hukuhara derivative between two fuzzy numbers are accessible under special circumstances (Kaleva 1987 ; Diamond 1999 , 2000 ). The gH -derivative, however, is available in less restrictive conditions, even though this is not always the case (Dubois et al. 2008 ). To overcome these serious defects of the concepts mentioned above, Bede and Stefanini (Dubois et al. 2008 ) describe g -derivative. In 2007, Allahviranloo used the predictor–corrector under the Seikkala-derivative method to propose a numerical solution of fuzzy differential equations (Allahviranloo et al. 2007 ).
Here, we investigate the Adams–Bashforth method to solve fuzzy differential equations focusing on g -differentiability. We restrict our study on normal, convex, upper semicontinuous, and compactly supported fuzzy sets in \(\mathbb {R}^n\) .
This paper has been arranged as mentioned below: firstly, in Sect. 2 , we recall the necessary definitions to be used in the rest of the article, after a preliminary section in Sect. 3 , which is dedicated to the description of the Adams–Bashforth method to fix the purposed equation. The convergence theorem is formulated and proved in Sect. 4 . For checking the accuracy of the method, three examples are presented. In Sect. 5 , their solutions are compared with the exact solutions. In the last section, some conclusions are given.
2 Preliminaries
Definition 2.1.
(Mehrkanoon et al. 2009 ) A fuzzy subset of the real line with a normal, convex, and upper semicontinuous membership function of bounded support is a fuzzy number \(\tilde{w}\) . The family of fuzzy numbers is indicated by F .
We show an arbitrary fuzzy number with an ordered pair of functions \((\underline{w}(\gamma ),\overline{w}(\gamma ))\) , \(0\le \gamma \le 1\) which provides the following:
\(\underline{w}(\gamma )\) is a bounded left continuous non-decreasing function over [0, 1], corresponding to any \(\gamma \) .
\(\overline{w}(\gamma )\) is a bounded left continuous non-decreasing function over [0, 1], corresponding to any \(\gamma \) .
Then, the \(\gamma \) -level set
is a closed bounded interval, which is denoted by:
Definition 2.2
(Bede and Stefanini 2013 ) The g -difference is defined as follows:
In Bede and Stefanini ( 2013 ), the difference between g -derivative and q -derivative has been fully investigated.
Definition 2.3
(Bede and Stefanini 2013 ; Diamond 1999 , The Hausdorff distance ) The Hausdorff distance is defined as follows:
where \(|| \cdot ||=D(\cdot , \cdot )\) and the gH -difference \(\circleddash _{gH}\) is with interval operands \([u]^\gamma \) and \([v]^\gamma \)
By definition, D is a metric in \(R_F\) which has the subsequent properties:
\(D(w+t, z+t)=D(w,z ) \qquad \forall w, z, t \in R_F\) ,
\(D(rw,rz)=|r|D(w,z)\qquad \forall w, z\in \ R_F, r\in R\) ,
\(D(w+t,z+d)\le D(w,z)+ D(t, d)\qquad \forall w, z, t, d \in R_F\) .
Then, \((D, R_F)\) is called a complete metric space.
Definition 2.4
(Bede and Stefanini 2013 ) Neumann’s integral of \(k{:}\, [m, n] \rightarrow R_F\) is defined level-wise by the fuzzy
Definition 2.5
(Bede and Stefanini 2013 ) Suppose \(k{:}\, [m,n] \rightarrow R_F\) is a function with \([k(y)]^{\gamma }=[\underline{k}_{\gamma }(y), \overline{k}_{\gamma }(y)]\) . If \(\underline{k}_{\gamma }(y)\) and \(\overline{k}_{\gamma }(y)\) are differentiable real-valued functions with respect to y , uniformly for \(\gamma \in [0, 1]\) , then k ( y ) is g -differentiable and we have
Definition 2.6
(Bede and Stefanini 2013 ) Let \(y_0 \in [m, n]\) and t be such that \(y_0+t \in ]m, n[\) , then the g -derivative of a function \(k{:}\, ]m, n[ \rightarrow R_F\) at \(y_0\) is defined as
If there exists \(k'_g(y_0)\in R_F\) satisfying ( 7 ), we call it generalized differentiable ( g -differentiable for short) at \(y_0\) . This relation depends on the existence of \(\circleddash _g\) , and there exists no such guarantee for this desire.
Theorem 2.7
Suppose \(k{:}\,[m,n]\rightarrow R_F\) is a continuous function with \([k(y)]^{\gamma }=[k^{-}_{\gamma }(y), k^{+}_{\gamma }(y)]\) and g -differentiable in [ m , n ]. In this case, we obtain
To show the assertion, it is enough to show their equality in level-wise form, suppose k is g -differentiable, so we have
\(\square \)
Definition 2.8
(Kaleva 1990 , fuzzy Cauchy problem ) Suppose \(x'_g(s)=k(s,x(s))\) is the first-order fuzzy differential equation, where y is a fuzzy function of s , k ( s , x ( s )) is a fuzzy function of the crisp variable s , and the fuzzy variable x and \(x'\) is the g -fuzzy derivative of x . By the initial value \(x(s_0)=\gamma _0\) , we define the first-order fuzzy Cauchy problem:
Proposition 2.9
Suppose \(\textit{k, h}{:}\, [\textit{a}, \textit{b}] {\rightarrow } R_F\) are two bounded functions, then
Since \(k(y)\le \textrm{sup}_A k\) and \(k(y)\le \textrm{sup}_A k\) for every \(y \in [m,n]\) , one can obtain \(k(y)+h(y)\le \textrm{sup}_A k+\textrm{sup}_A h\) . Thus, \(k+h\) is bounded from above by \(\textrm{sup}_A k+\textrm{sup}_A h\) , so \(\textrm{sup}_A( k+h) \le \textrm{sup}_A k+ \textrm{sup}_A h\) . The proof for the infimum is similar. \(\square \)
Definition 2.10
Let \(\{\widetilde{q}_m\}^\infty _{m=0}\) be a fuzzy sequence. Then, we define the backward g -difference \(\nabla _g \widetilde{q}_m\) as follows
So, we have
Consequently,
Proposition 2.11
For a given fuzzy sequence \({\left\{ {\widetilde{q}}_m\right\} }^{\infty }_{m=0}\) , by supposing backward g -difference, we have
We prove proposition by induction that, for all \(n \in \mathbb {Z}^+\) ,
Using Definition 2.10 , for base case, \(n = 1\) , we have
Induction step : Let \( k \in \mathbb {Z}^+\) be given and suppose ( 14 ) is true for \(n=k\) . Then,
Conclusion : For all \(m\in \mathbb {Z}^+\) , ( 14 ) is correct, by the principle of induction. \(\square \)
Definition 2.12
( Switching Point ) The concept of switching point refers to an interval where fuzzy differentiability of type-(i) turns into type-(ii) and also vice versa.
3 Fuzzy Adams–Bashforth method
To derivative of a fuzzy multistep method, we consider the solution of the initial-value problem:
To obtain the approximation \(t_{j+1}\) at the mesh point \(s_{j+1}\) , where initial values
are assumed.
If integrated over the interval \([s_j,s_{j+1}]\) , we get
but, without knowing \(\widetilde{x}(s)\) , we cannot integrate \(\widetilde{k}(s,\widetilde{x}(s))\) , one can apply an interpolating polynomial \(\widetilde{q}(s)\) to \(\widetilde{k}(s, \widetilde{x}(s))\) , which is computed by the data points \(\left( s_0, {\widetilde{t}}_0\right) , \left( s_1,{\widetilde{t}}_1\right) ,\ldots \left( s_j,{\widetilde{t}}_j\right) \) . These data were obtained in Sect. 2 .
Indeed, by supposing that \(\widetilde{x}\left( s_j\right) \approx \ {\widetilde{t}}_j\ \) , Eq. ( 17 ) is rewritten as
To take a fuzzy Adams–Bashforth explicit m -step method under the notion of g -difference, we construct the backward difference polynomial \({\widetilde{q}}_{n-1}(s)\) ,
We assume that the n th derivatives of the fuzzy function k exist. This means that all derivatives are g -differentiable. As \({\widetilde{q}}_{n-1}(s)\) is an interpolation polynomial of degree \(n-1\) , some number \({\xi }_j\) in \(\left( s_{j+1-n}, s_j\right) \) exists with
where the corresponding notation \({\widetilde{k}}^{(n)}_g (s, \widetilde{x}(s)),n\in \mathbb {N},\) exists. Moreover, it can be mentioned that the existence of this corresponding formula based on the existence of \({\circleddash }_g\) , and while \({\circleddash }_g\) exist this relation always exists.
We introduce the \(s=s_j+\beta h\) , with \(\textrm{d}s=h \textrm{d}\beta \) , substituting these variable into \({\widetilde{q}}_{n-1}(s)\) and the error term indicates
So, we will get
Obviously, the product of \(\beta \ \left( \beta +1\right) \cdots \left( \beta +n-1\right) \) does not change sign on [0, 1], so the Weighted Mean Value Theorem for some number \({\mu }_j\) , where \(s_{j+1-n}< {\mu }_j< s_{j+1}\) , can be applied to the last term in Eq. ( 22 ), hence it becomes
So, it simplifies to
So, Eq. ( 20 ) is written as
It is also worth mentioning that the notions \(\Delta _{g}\) and \(\oplus \) are extensively utilized solving the problems of sup and inf existence.
To illustrate this method, we discuss solving the fuzzy initial value problem \({\widetilde{x}}'\left( s\right) =\widetilde{k}(s,\widetilde{x}\left( s\right) )\) by Adams–Bashforth’s three-step method. To derive the three-step Adams–Bashforth technique, with \(n= 3\) , We have
For \(m=2, 3,\ldots , N-1.\) So
Here, we also describe our model as introduced models \(\Delta _{g}\) and \(\oplus \) .
By considering
As a consequence
from which we obtain
From ( 24 ) and ( 29 ), we get
if we suppose that
Then, we have
Similarly, we have
Then, we can say
4 Convergence
We begin our dissection with definitions of the convergence of multistep difference equation and consistency before discussing methods to solve the differential equation.
Definition 4.1
The differential fuzzy equation with initial condition
and similarly, the other models can be derived as
is the \(\left( j+1\right) \) st step in a multistep method. At this step, it has a fuzzy local truncation error as follows
Exists N that for all \(j= n-1, n, \ldots N-1\) , and \(h=\frac{b-a}{N}\) , where
And \(\widetilde{x}\left( s_j\right) \) indicates the exact value of the solution of the differential equation. The approximation \({\widetilde{t}}_j\) is taken from the different methods at the j th step.
Definition 4.2
A multistep method with local truncation error \({\widetilde{\nu }}_{j+1}\left( h\right) \) at the \((j+1)\) th step is called consistent with the differential equation approximation if
Theorem 4.3
Let the initial-value problem
be approximated by a multistep difference method:
Let a number \(h_0>0\) exist, and \(\phi \left( s_j,\widetilde{t}\left( s_j\right) \right) \) be continuous, with meets the constant Lipschitz T
Then, the difference method is convergent if and only if it is consistent. It is equal to
We are aware of the concept of convergence for the multistep method. As the step size approaches zero, the solution of the difference equation approaches the solution to the differential equation. In other words
For the multistep fuzzy Adams–Bashforth method, we have seen that
using Proposition 2.11 , \(\nabla ^l_g{\widetilde{k}}_m=h^l\widetilde{k}^{(l)}_{m_g}\) , and substituting it in Eq. ( 66 ), we have
under the hypotheses of paper, \(\widetilde{k}({(s}_j,\widetilde{x}(s_j))\in R_F\) , and by definition g -differentiability \(\widetilde{k}^{(n)}({(s}_j,\widetilde{x}(s_j))\in R_F\) so by Definition 2.1 \(\ {\widetilde{k}}^{(n)}\left( {(s}_j,\widetilde{x}\left( s_j\right) \right) \in R_F\) for \(j\ge 0\) are bounded, thus exists M such that
When \(h\rightarrow 0\) , we will have \(Z\rightarrow 0\) so
So, we see that it satisfied the first condition of Definition 4.2 . The concept of the second part is that if the one-step method generating the starting values is also consistent, then the multistep method is consistent. So our method is consistent; therefore according to Theorem 4.3 , this difference method is convergent.
Example 5.1
Consider the initial-value problem
Obviously, one can check the exact solution as follows:
Indeed, the solution is a triangular number
So, the exact solution in mesh point \(s=0.01\) is
On the other hand with the proposed method, the approximated solution in \(s=0.01\) is as follows:
where \(\tilde{t}^\gamma \) is a approximated of \(\tilde{x}\) .
The maximum error in \(s=0.1\) , \(s=0.2, \ldots , s=1\) , also shows the errors (Table 1 ).
Thus, we have
where ( 90 ) are real values. Suppose
By ( 85 ), ( 86 ), ( 92 ), we obtain
According to the previous sections, this example has been solved by the two-step Adams–Bashforth method with \(t=0.1\) and \(N=10\) . We use the following relations to solve it.
Example 5.2
First, we solve the problem with the gH -differentiability. The initial-value problem on [0, 1] is \([(i)-gH]\) -differentiable and \([(i)-gH]\) -differentiable on (1, 2]. By solving the following system, the \([(i)-gH]\) -differentiable solution will be achieved
By solving the following system, the \([(ii)-gH]\) -differentiable solution will be achieved
If we apply the Euler method to the approximate solution of the initial-value problem by
The results are presented in Table 2 .
In the calculations of this method, we need to consider the \(i-gh\) -differentiability and \(ii-gH\) -differentiability. But when we use g -differentiability, we do not need to check the different states of the differentiability. To solve using the method mentioned in the article, we have:
Or we have \(x^\gamma (0)-[\gamma , 2-\gamma ]\) . The exact solution is as follows:
The results of the solution using the Adams–Bashforth two-step method for \(h = 1\) and calculating the approximate value of the solution and the error of the method can be seen in the Table 3 .
Consider the initial-value problem \(\tilde{x'}=(s\ominus 1)\odot \tilde{x}^2\) , where \(s\in [-1,1]\)
the exact solution is
6 Conclusion
In the present paper, the proposed method, which is based on the concept of g-differentiability, provides a fuzzy solution. This solution is related to a set of equations from the family of Adams-Bashforth differential equations, which coincide with the solutions derived by fuzzy differential equations.
The gH -difference is a powerful and versatile fuzzy differential operator that is more flexible, robust, and computationally efficient, making it a good choice for solving a wide range of fuzzy differential equations. It does not need i and ii -differentiability. In Examples, we compare g -differentiability and gH -differentiability.
G-differentiability allows for capturing gradual changes in a fuzzy-valued function. G-differentiable functions exhibit certain degrees of smoothness and continuity, which can be useful in modeling and analyzing fuzzy systems. The choice of the parameter g in g -differentiability is crucial and depends on the specific problem. Determining an appropriate value for g requires careful consideration and analysis. H -differentiability combines the gradual reduction of fuzziness (via the parameter g ) with the Hukuhara difference ( H -difference). It provides a more refined analysis of fuzzy-valued functions. gH -differentiability offers enhanced modeling capabilities by considering both the gradual reduction of fuzziness and the separation between fuzzy numbers or fuzzy sets. But gH -differentiability introduces an additional level of complexity compared to g -differentiability or H -differentiability alone. The combination of gradual reduction and H -difference requires careful understanding and analysis to ensure proper application.
Data availability
There is no data available for this research.
Allahviranloo T, Pedrycz W (2020) Soft numerical computing in uncertain dynamic systems. Academic Press, New York
Google Scholar
Allahviranloo T, Ahmady N, Ahmady E (2007) Numerical solution of fuzzy differential equations by predictor-corrector method. Inf Sci 177(7):1633–1647
Article MathSciNet Google Scholar
Allahviranloo T, Kiani NA, Motamedi N (2009) Solving fuzzy differential equations by differential transformation method. Inf Sci 179(7):956–966
Bede B, Stefanini L (2013) Generalized differentiability of fuzzy-valued functions. Fuzzy Sets Syst 230:119–141. https://doi.org/10.1016/j.fss.2012.10.003
Buckley JJ, Feuring T (2000) Fuzzy differential equations. Fuzzy Sets Syst 110(1):43–54
Byatt W, Kandel A (1978) Fuzzy differential equations. In: Proceedings of the international conference on Cybernetics and Society, Tokyo, Japan, vol 1
Chalco-Cano Y, Roman-Flores H (2008) On new solutions of fuzzy differential equations. Chaos Solitons Fractals 38(1):112–119
Diamond P (1999) Time-dependent differential inclusions, cocycle attractors and fuzzy differential equations. IEEE Trans Fuzzy Syst 7(6):734–740
Article Google Scholar
Diamond P (2000) Stability and periodicity in fuzzy differential equations. IEEE Trans Fuzzy Syst 8(5):583–590
Diamond P (2002) Brief note on the variation of constants formula for fuzzy differential equations. Fuzzy Sets Syst 129(1):65–71
Dubois D, Lubiano MA, Prade H, Gil MA, Grzegorzewski P, Hryniewicz O (2008) Soft methods for handling variability and imprecision, vol 48. Springer, Berlin
Book Google Scholar
Kaleva O (1987) Fuzzy differential equations. Fuzzy Sets Syst 24(3):301–317
Kaleva O (1990) The Cauchy problem for fuzzy differential equations. Fuzzy Sets Syst 35(3):389–396. https://doi.org/10.1016/0165-0114(90)90010-4
Kandel A (1980) Fuzzy dynamical systems and the nature of their solutions. In: Fuzzy sets. Springer, Berlin, pp 93–121
Mehrkanoon S, Suleiman M, Majid Z (2009) Block method for numerical solution of fuzzy differential equations. In: International mathematical forum, vol 4, Citeseer, pp 2269–2280
Safikhani L, Vahidi A, Allahviranloo T, Afshar Kermani M (2023) Multi-step gh-difference-based methods for fuzzy differential equations. Comput Appl Math 42(1):27
Seikkala S (1987) On the fuzzy initial value problem. Fuzzy Sets Syst 24(3):319–330
Song S, Wu C (2000) Existence and uniqueness of solutions to Cauchy problem of fuzzy differential equations. Fuzzy Sets Syst 110(1):55–67
Zabihi S, Ezzati R, Fattahzadeh F, Rashidinia J (2023) Numerical solutions of the fuzzy wave equation based on the fuzzy difference method. Fuzzy Sets Syst 465:108537. https://doi.org/10.1016/j.fss.2023.108537
Download references
Acknowledgements
The authors are thankful to the area editor and referees for giving valuable comments and suggestions.
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and affiliations.
Department of Mathematics, Science and Research Branch, Islamic Azad University, Tehran, Iran
S. Soroush & T. Allahviranloo
Research Center of Performance and Productivity Analysis, Istinye University, Istanbul, Turkey
T. Allahviranloo
Department of Applied Mathematics, Faculty of Mathematics Sciences, Shahid Beheshti University, Tehran, Iran
H. Azari & M. Rostamy-Malkhalifeh
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to T. Allahviranloo .
Additional information
Communicated by Marcos Eduardo Valle.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Soroush, S., Allahviranloo, T., Azari, H. et al. Generalized fuzzy difference method for solving fuzzy initial value problem. Comp. Appl. Math. 43 , 129 (2024). https://doi.org/10.1007/s40314-024-02645-2
Download citation
Received : 30 March 2023
Revised : 12 January 2024
Accepted : 14 February 2024
Published : 27 March 2024
DOI : https://doi.org/10.1007/s40314-024-02645-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Fuzzy differential equation
- Generalized differentiability
- Adams–Bashforth method
- Fuzzy difference equations
Mathematics Subject Classification
- Find a journal
- Publish with us
- Track your research
- Share full article
Advertisement
Supported by
Guest Essay
A Solution on North Korea Is There, if Biden Will Only Grasp It

By John Delury
Dr. Delury is a professor of Chinese studies and an expert on North Korea.
How do you solve a problem like North Korea?
Since the end of the Cold War, it seems that every formula, from threatening war to promising peace, has been tried. And yet, despite being under more sanctions than just about any other country, North Korea developed a nuclear arsenal estimated at 50 warheads and sophisticated missiles that can, in theory, deliver those weapons to targets in the continental United States.
President Biden’s administration has taken a notably more ambivalent approach toward North Korea than his predecessor Donald Trump, who alternately railed at and courted its leader, Kim Jong-un. But we shouldn’t stop trying to come up with bold ways to denuclearize North Korea, improve the lives of its people or lessen the risks of conflict, even if that means making unpalatable choices. On the contrary, there is more urgency now than there has been for years.
As the analyst Robert Carlin and the nuclear scientist Siegfried Hecker, two experienced North Korea watchers, warned in January, Mr. Kim has shifted away from pursuing better relations with the United States and South Korea and closer to President Vladimir Putin of Russia and may be preparing for war. Just days after the two experts issued their warning, Mr. Kim disavowed the long-cherished goal of peaceful reconciliation between the two Koreas, and he called for “completely occupying, subjugating and reclaiming” the South if war breaks out.
It might seem preposterous, even suicidal, for Mr. Kim to seek war. But many people in Ukraine doubted that Mr. Putin would launch a full invasion, right up until the rockets began landing in February 2022, and Hamas caught Israel completely by surprise in October. Both conflicts have had devastating human tolls and are severely taxing America’s ability to manage concurrent crises. The people of both Koreas certainly don’t need war, and neither does the United States.
Mr. Kim’s grandfather started the Korean War, and his father was a master of brinkmanship. Mr. Kim is cut from the same cloth and could instigate a limited conflict by, for example, launching an amphibious assault on South Korean-controlled islands in disputed waters of the Yellow Sea, less than 15 miles off North Korea’s coast. North Korea shelled one of the islands in 2010, killing two South Korean military personnel and two civilians and triggering an exchange of artillery with the South. Just two months ago, Pyongyang fired more than 200 shells into waters near the islands.
Mr. Kim may believe he can manage escalation of such a crisis — threatening missile or even nuclear attack to deter retaliation, perhaps taking the islands, then spinning it as a great propaganda victory and demanding a redrawing of maritime boundaries and other security concessions.
If anything like that scenario came to pass, Mr. Biden would have to explain another outbreak of war on his watch to weary American voters. And it would provide Mr. Trump an opportunity to trumpet his willingness to engage with Mr. Kim.
The mutual distrust between Washington and Pyongyang has only deepened under Mr. Biden, making a breakthrough seem unlikely. Yet there are two underappreciated dynamics at play in North Korea where the United States might find leverage.
The first is China. Despite the veneer of Communist kinship, Mr. Kim and President Xi Jinping of China are nationalists at heart, and they watch each other warily. I have made numerous visits to both nations’ capitals and met with officials and policy shapers. The sense of deep mutual distrust is palpable. Many Chinese look down on neighboring North Korea as backward and are annoyed by its destabilizing behavior. Many North Koreans resent China’s success and resist its influence; Pyongyang could allow much more Chinese investment but doesn’t want to be indebted to Chinese capital. And Mr. Kim seems to delight in timing provocations for maximum embarrassment in Beijing, including testing weapons — prohibited by U.N. sanctions — in the lead-up to sensitive Chinese political events .
Mr. Kim waited six years after becoming the paramount leader in 2011 before making a trip to Beijing to meet Mr. Xi. When Covid emerged, North Korea was among the first countries to shut its borders with China, and ties atrophied during those nearly three years of closure . Last year Mr. Kim chose Mr. Putin, not Mr. Xi, for his first postpandemic summit, skipping China to travel to Russia’s far east. Mr. Kim’s distrust of China is an opening for the United States.
The second point is Mr. Kim’s economic ambitions. For every speech mentioning nukes, he talks at much greater length about the poor state of his nation’s economy while promising to improve it. It was the prospect of American-led economic sanctions being lifted that persuaded him to make the 60-hour train ride from Pyongyang to Hanoi to meet then-President Trump for their second summit in 2019. Mr. Kim explicitly offered to dismantle his main nuclear weapons complex, but Mr. Trump demanded the North also turn over all of its nuclear weapons, material and facilities. The talks collapsed, and Mr. Trump seemed to lose interest in dealing with Mr. Kim. A rare opportunity was wasted, leaving Mr. Kim embittered.
The key to any new overture to North Korea is how it is framed. The White House won’t like to hear this, but success will probably depend on Mr. Biden putting his fingerprints all over the effort, by, for example, nominating a new White House envoy with the stature of someone like John Kerry and announcing a sweeping policy on North Korea and an intelligence review. Only the president can get through to Mr. Kim, and only Mr. Kim can change North Korean policy.
Mr. Biden also would need to use radically different language in framing a new overture as an effort to improve relations and aid North Korea’s economy — not to denuclearize a country that in 2022 passed a law declaring itself a nuclear weapons state. Yes, that would be a bitter pill for America to swallow: Denuclearization has been a guiding principle of U.S. policy toward North Korea for decades. But it is unrealistic to pretend that Pyongyang will surrender its nuclear weapons anytime soon. Disarmament can remain a long-term goal but is impossible if the two sides aren’t even talking.
Mr. Biden’s Republican opponents might accuse him of appeasement by engaging with Mr. Kim, but that is precisely what Mr. Trump tried. Mr. Kim, likewise, might mistake boldness for weakness. But it would be easy enough for the United States to pull back from diplomacy if it goes nowhere.
The United States must be realistic. The world is very different from when the United States, China, Russia, Japan and the two Koreas came together in the 2000s for negotiations to denuclearize North Korea. The country is now a formidable nuclear power, and its leader sounds increasingly belligerent. The president needs to get the wheels of diplomacy turning before it’s too late.
John Delury (@JohnDelury) is a professor of Chinese studies at Yonsei University in Seoul, the Tsao fellow at the American Academy in Rome and the author of “Agents of Subversion: The Fate of John T. Downey and the CIA’s Covert War in China.”
The Times is committed to publishing a diversity of letters to the editor. We’d like to hear what you think about this or any of our articles. Here are some tips . And here’s our email: [email protected] .
Follow The New York Times Opinion section on Facebook , Instagram , TikTok , X and Threads .

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

11.3: Solution Concentration - Molarity
- Last updated
- Save as PDF
- Page ID 465574
Learning Objectives
- To describe the concentrations of solutions quantitatively
Many people have a qualitative idea of what is meant by concentration . Anyone who has made instant coffee or lemonade knows that too much powder gives a strongly flavored, highly concentrated drink, whereas too little results in a dilute solution that may be hard to distinguish from water. In chemistry, the concentration of a solution is the quantity of a solute that is contained in a particular quantity of solvent or solution. Knowing the concentration of solutes is important in controlling the stoichiometry of reactants for solution reactions. Chemists use many different methods to define concentrations, some of which are described in this section.
The most common unit of concentration is molarity , which is also the most useful for calculations involving the stoichiometry of reactions in solution. The molarity (M) is defined as the number of moles of solute present in exactly 1 L of solution . It is, equivalently, the number of millimoles of solute present in exactly 1 mL of solution:
\[ molarity = \dfrac{moles\: of\: solute}{liters\: of\: solution} = \dfrac{mmoles\: of\: solute} {milliliters\: of\: solution} \label{4.5.1} \]
The units of molarity are therefore moles per liter of solution (mol/L), abbreviated as \(M\). An aqueous solution that contains 1 mol (342 g) of sucrose in enough water to give a final volume of 1.00 L has a sucrose concentration of 1.00 mol/L or 1.00 M. In chemical notation, square brackets around the name or formula of the solute represent the molar concentration of a solute. Therefore,
\[[\rm{sucrose}] = 1.00\: M \nonumber \]
is read as “the concentration of sucrose is 1.00 molar.” The relationships between volume, molarity, and moles may be expressed as either
\[ V_L M_{mol/L} = \cancel{L} \left( \dfrac{mol}{\cancel{L}} \right) = moles \label{4.5.2} \]
\[ V_{mL} M_{mmol/mL} = \cancel{mL} \left( \dfrac{mmol} {\cancel{mL}} \right) = mmoles \label{4.5.3} \]
Figure \(\PageIndex{1}\) illustrates the use of Equations \(\ref{4.5.2}\) and \(\ref{4.5.3}\).
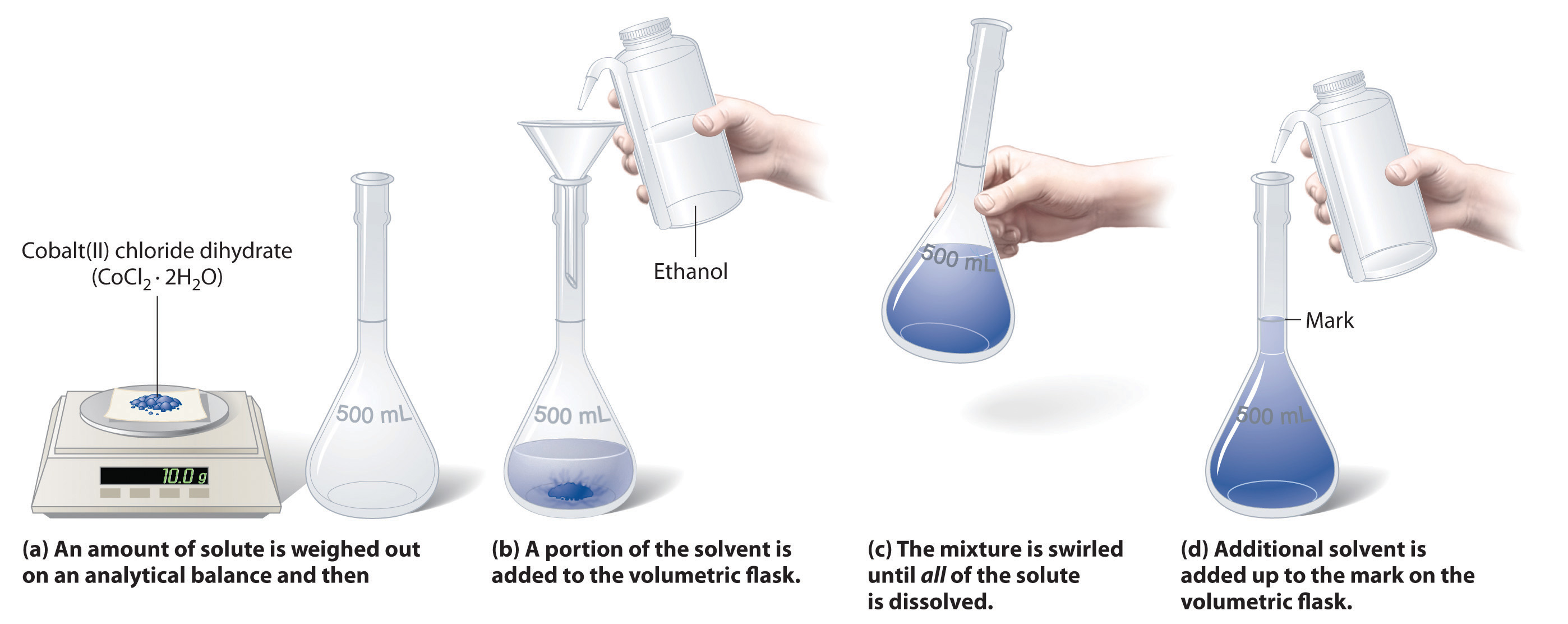
Example \(\PageIndex{1}\): Calculating Moles from Concentration of NaOH
Calculate the number of moles of sodium hydroxide (NaOH) in 2.50 L of 0.100 M NaOH.
Given: identity of solute and volume and molarity of solution
Asked for: amount of solute in moles
Use either Equation \ref{4.5.2} or Equation \ref{4.5.3}, depending on the units given in the problem.
Because we are given the volume of the solution in liters and are asked for the number of moles of substance, Equation \ref{4.5.2} is more useful:
\( moles\: NaOH = V_L M_{mol/L} = (2 .50\: \cancel{L} ) \left( \dfrac{0.100\: mol } {\cancel{L}} \right) = 0 .250\: mol\: NaOH \)
Exercise \(\PageIndex{1}\): Calculating Moles from Concentration of Alanine
Calculate the number of millimoles of alanine, a biologically important molecule, in 27.2 mL of 1.53 M alanine.
Calculations Involving Molarity (M): Calculations Involving Molarity (M), YouTube(opens in new window) [youtu.be]
Concentrations are also often reported on a mass-to-mass (m/m) basis or on a mass-to-volume (m/v) basis, particularly in clinical laboratories and engineering applications. A concentration expressed on an m/m basis is equal to the number of grams of solute per gram of solution; a concentration on an m/v basis is the number of grams of solute per milliliter of solution. Each measurement can be expressed as a percentage by multiplying the ratio by 100; the result is reported as percent m/m or percent m/v. The concentrations of very dilute solutions are often expressed in parts per million ( ppm ), which is grams of solute per 10 6 g of solution, or in parts per billion ( ppb ), which is grams of solute per 10 9 g of solution. For aqueous solutions at 20°C, 1 ppm corresponds to 1 μg per milliliter, and 1 ppb corresponds to 1 ng per milliliter. These concentrations and their units are summarized in Table \(\PageIndex{1}\).
The Preparation of Solutions
To prepare a solution that contains a specified concentration of a substance, it is necessary to dissolve the desired number of moles of solute in enough solvent to give the desired final volume of solution. Figure \(\PageIndex{1}\) illustrates this procedure for a solution of cobalt(II) chloride dihydrate in ethanol. Note that the volume of the solvent is not specified. Because the solute occupies space in the solution, the volume of the solvent needed is almost always less than the desired volume of solution. For example, if the desired volume were 1.00 L, it would be incorrect to add 1.00 L of water to 342 g of sucrose because that would produce more than 1.00 L of solution. As shown in Figure \(\PageIndex{2}\), for some substances this effect can be significant, especially for concentrated solutions.
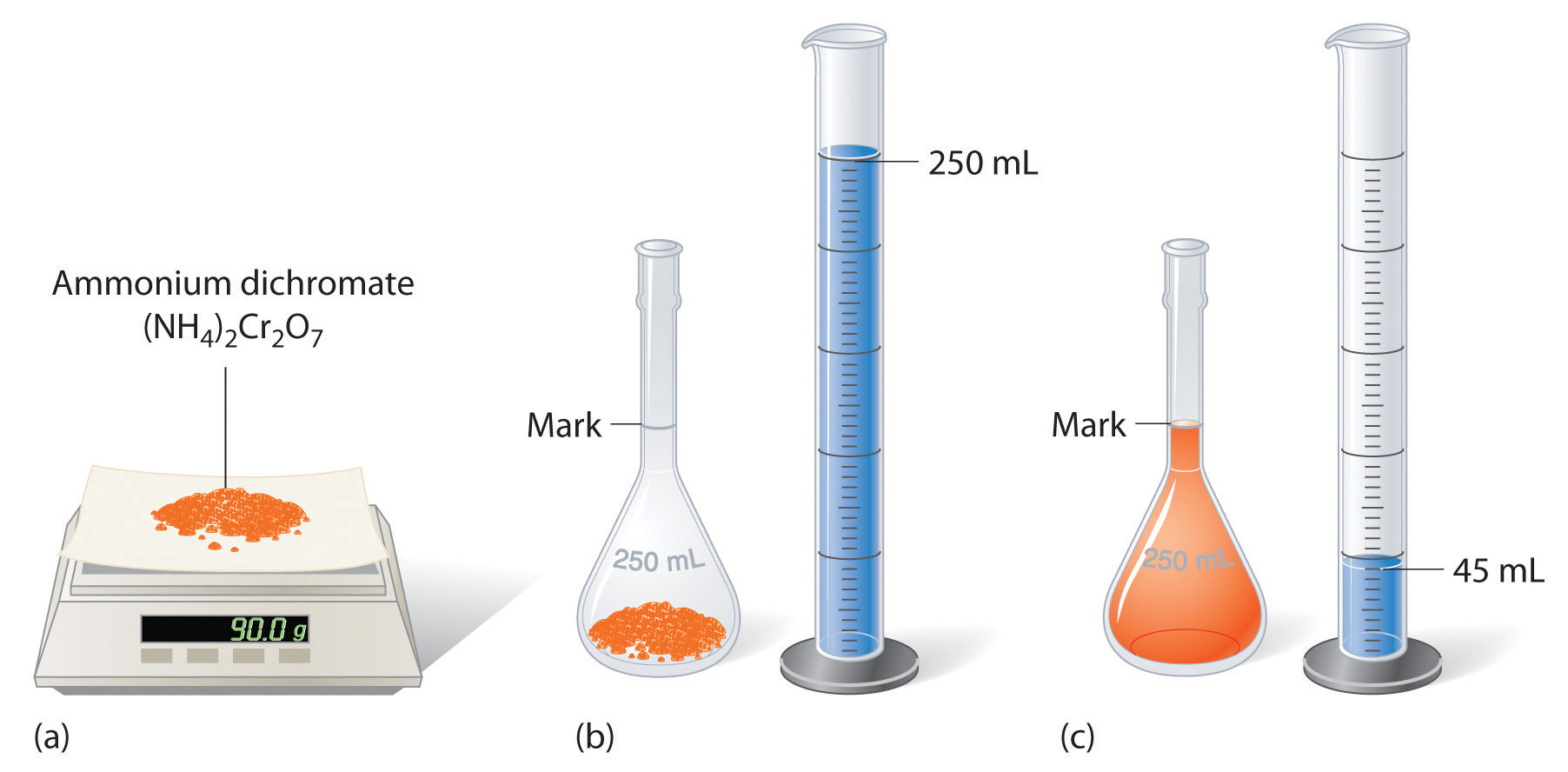
Example \(\PageIndex{2}\)
The solution contains 10.0 g of cobalt(II) chloride dihydrate, CoCl 2 •2H 2 O, in enough ethanol to make exactly 500 mL of solution. What is the molar concentration of \(\ce{CoCl2•2H2O}\)?
Given: mass of solute and volume of solution
Asked for: concentration (M)
To find the number of moles of \(\ce{CoCl2•2H2O}\), divide the mass of the compound by its molar mass. Calculate the molarity of the solution by dividing the number of moles of solute by the volume of the solution in liters.
The molar mass of CoCl 2 •2H 2 O is 165.87 g/mol. Therefore,
\[ moles\: CoCl_2 \cdot 2H_2O = \left( \dfrac{10.0 \: \cancel{g}} {165 .87\: \cancel{g} /mol} \right) = 0 .0603\: mol \nonumber \]
The volume of the solution in liters is
\[ volume = 500\: \cancel{mL} \left( \dfrac{1\: L} {1000\: \cancel{mL}} \right) = 0 .500\: L \nonumber \]
Molarity is the number of moles of solute per liter of solution, so the molarity of the solution is
\[ molarity = \dfrac{0.0603\: mol} {0.500\: L} = 0.121\: M = CoCl_2 \cdot H_2O \nonumber \]
Exercise \(\PageIndex{2}\)
The solution shown in Figure \(\PageIndex{2}\) contains 90.0 g of (NH 4 ) 2 Cr 2 O 7 in enough water to give a final volume of exactly 250 mL. What is the molar concentration of ammonium dichromate?
\[(NH_4)_2Cr_2O_7 = 1.43\: M \nonumber \]
To prepare a particular volume of a solution that contains a specified concentration of a solute, we first need to calculate the number of moles of solute in the desired volume of solution using the relationship shown in Equation \(\ref{4.5.2}\). We then convert the number of moles of solute to the corresponding mass of solute needed. This procedure is illustrated in Example \(\PageIndex{3}\).
Example \(\PageIndex{3}\): D5W Solution
The so-called D5W solution used for the intravenous replacement of body fluids contains 0.310 M glucose. (D5W is an approximately 5% solution of dextrose [the medical name for glucose] in water.) Calculate the mass of glucose necessary to prepare a 500 mL pouch of D5W. Glucose has a molar mass of 180.16 g/mol.
Given: molarity, volume, and molar mass of solute
Asked for: mass of solute
- Calculate the number of moles of glucose contained in the specified volume of solution by multiplying the volume of the solution by its molarity.
- Obtain the mass of glucose needed by multiplying the number of moles of the compound by its molar mass.
A We must first calculate the number of moles of glucose contained in 500 mL of a 0.310 M solution:
\( V_L M_{mol/L} = moles \)
\( 500\: \cancel{mL} \left( \dfrac{1\: \cancel{L}} {1000\: \cancel{mL}} \right) \left( \dfrac{0 .310\: mol\: glucose} {1\: \cancel{L}} \right) = 0 .155\: mol\: glucose \)
B We then convert the number of moles of glucose to the required mass of glucose:
\( mass \: of \: glucose = 0.155 \: \cancel{mol\: glucose} \left( \dfrac{180.16 \: g\: glucose} {1\: \cancel{mol\: glucose}} \right) = 27.9 \: g \: glucose \)
Exercise \(\PageIndex{3}\)
Another solution commonly used for intravenous injections is normal saline, a 0.16 M solution of sodium chloride in water. Calculate the mass of sodium chloride needed to prepare 250 mL of normal saline solution.
A solution of a desired concentration can also be prepared by diluting a small volume of a more concentrated solution with additional solvent. A stock solution is a commercially prepared solution of known concentration and is often used for this purpose. Diluting a stock solution is preferred because the alternative method, weighing out tiny amounts of solute, is difficult to carry out with a high degree of accuracy. Dilution is also used to prepare solutions from substances that are sold as concentrated aqueous solutions, such as strong acids.
The procedure for preparing a solution of known concentration from a stock solution is shown in Figure \(\PageIndex{3}\). It requires calculating the number of moles of solute desired in the final volume of the more dilute solution and then calculating the volume of the stock solution that contains this amount of solute. Remember that diluting a given quantity of stock solution with solvent does not change the number of moles of solute present. The relationship between the volume and concentration of the stock solution and the volume and concentration of the desired diluted solution is therefore
\[(V_s)(M_s) = moles\: of\: solute = (V_d)(M_d)\label{4.5.4} \]
where the subscripts s and d indicate the stock and dilute solutions, respectively. Example \(\PageIndex{4}\) demonstrates the calculations involved in diluting a concentrated stock solution.
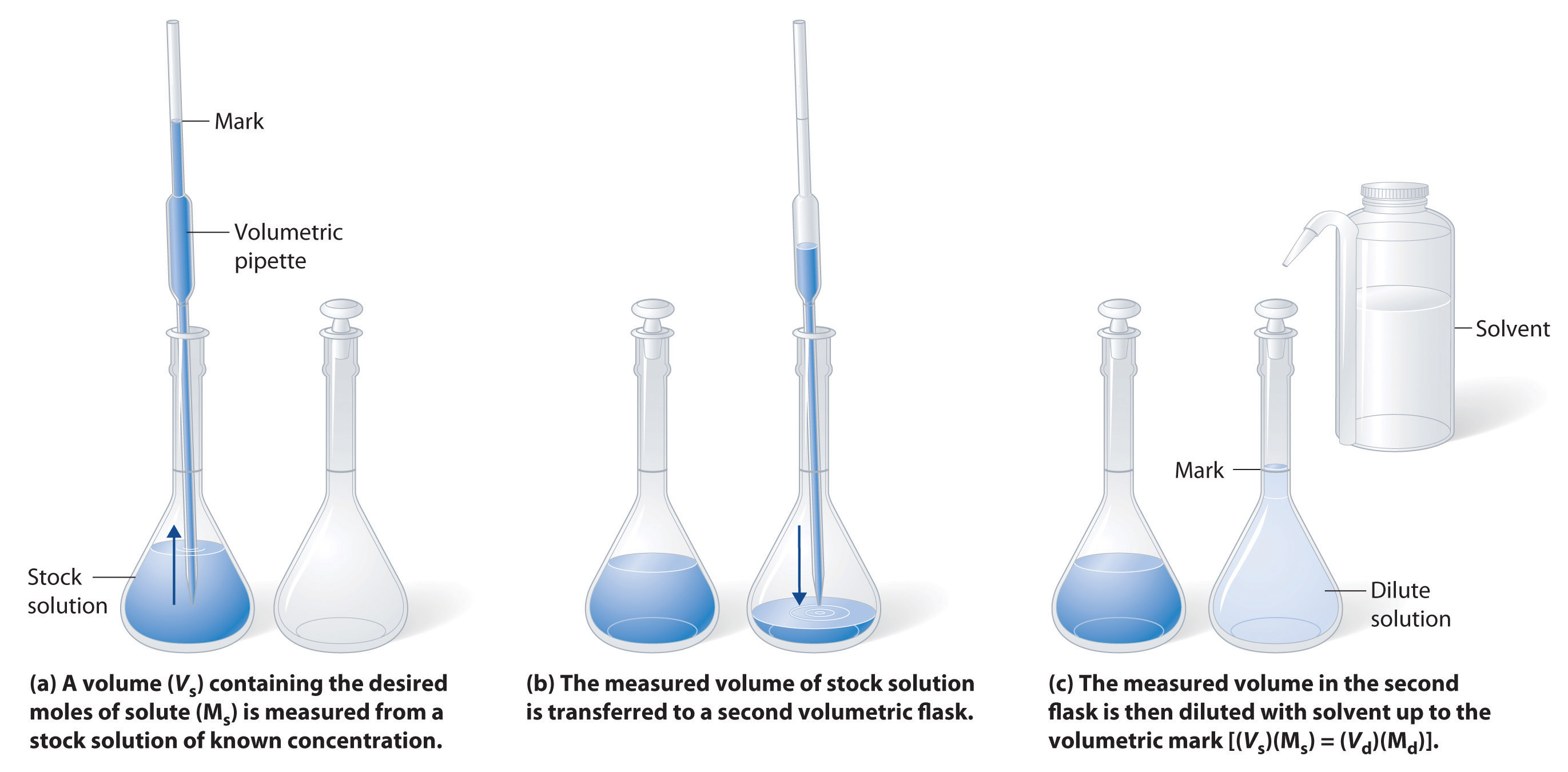
Example \(\PageIndex{4}\)
What volume of a 3.00 M glucose stock solution is necessary to prepare 2500 mL of the D5W solution in Example \(\PageIndex{3}\)?
Given: volume and molarity of dilute solution
Asked for: volume of stock solution
- Calculate the number of moles of glucose contained in the indicated volume of dilute solution by multiplying the volume of the solution by its molarity.
- To determine the volume of stock solution needed, divide the number of moles of glucose by the molarity of the stock solution.
A The D5W solution in Example 4.5.3 was 0.310 M glucose. We begin by using Equation 4.5.4 to calculate the number of moles of glucose contained in 2500 mL of the solution:
\[ moles\: glucose = 2500\: \cancel{mL} \left( \dfrac{1\: \cancel{L}} {1000\: \cancel{mL}} \right) \left( \dfrac{0 .310\: mol\: glucose} {1\: \cancel{L}} \right) = 0 .775\: mol\: glucose \nonumber \]
B We must now determine the volume of the 3.00 M stock solution that contains this amount of glucose:
\[ volume\: of\: stock\: soln = 0 .775\: \cancel{mol\: glucose} \left( \dfrac{1\: L} {3 .00\: \cancel{mol\: glucose}} \right) = 0 .258\: L\: or\: 258\: mL \nonumber \]
In determining the volume of stock solution that was needed, we had to divide the desired number of moles of glucose by the concentration of the stock solution to obtain the appropriate units. Also, the number of moles of solute in 258 mL of the stock solution is the same as the number of moles in 2500 mL of the more dilute solution; only the amount of solvent has changed . The answer we obtained makes sense: diluting the stock solution about tenfold increases its volume by about a factor of 10 (258 mL → 2500 mL). Consequently, the concentration of the solute must decrease by about a factor of 10, as it does (3.00 M → 0.310 M).
We could also have solved this problem in a single step by solving Equation 4.5.4 for V s and substituting the appropriate values:
\[ V_s = \dfrac{( V_d )(M_d )}{M_s} = \dfrac{(2 .500\: L)(0 .310\: \cancel{M} )} {3 .00\: \cancel{M}} = 0 .258\: L \nonumber \]
As we have noted, there is often more than one correct way to solve a problem.
Exercise \(\PageIndex{4}\)
What volume of a 5.0 M NaCl stock solution is necessary to prepare 500 mL of normal saline solution (0.16 M NaCl)?
Ion Concentrations in Solution
In Example \(\PageIndex{2}\), the concentration of a solution containing 90.00 g of ammonium dichromate in a final volume of 250 mL were calculated to be 1.43 M. Let’s consider in more detail exactly what that means. Ammonium dichromate is an ionic compound that contains two NH 4 + ions and one Cr 2 O 7 2 − ion per formula unit. Like other ionic compounds, it is a strong electrolyte that dissociates in aqueous solution to give hydrated NH 4 + and Cr 2 O 7 2 − ions:
\[ (NH_4 )_2 Cr_2 O_7 (s) \xrightarrow {H_2 O(l)} 2NH_4^+ (aq) + Cr_2 O_7^{2-} (aq)\label{4.5.5} \]
Thus 1 mol of ammonium dichromate formula units dissolves in water to produce 1 mol of Cr 2 O 7 2 − anions and 2 mol of NH 4 + cations (see Figure \(\PageIndex{4}\)).
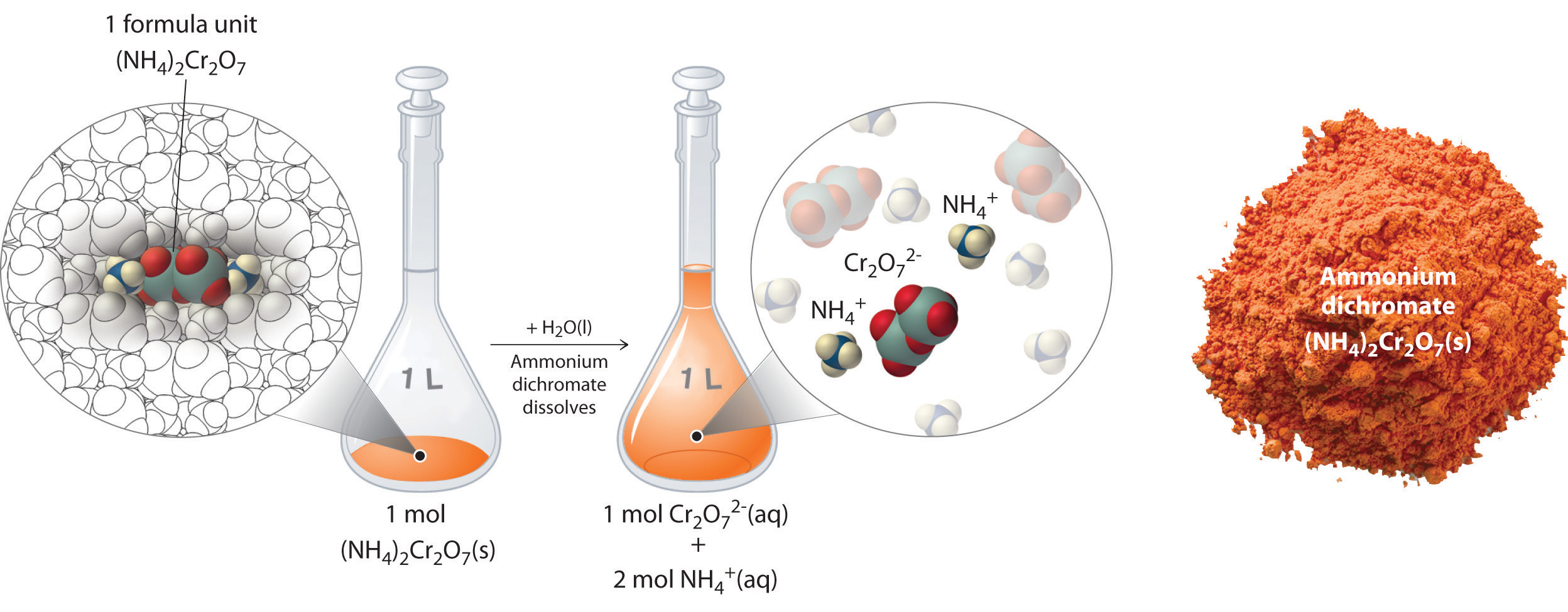
When carrying out a chemical reaction using a solution of a salt such as ammonium dichromate, it is important to know the concentration of each ion present in the solution. If a solution contains 1.43 M (NH 4 ) 2 Cr 2 O 7 , then the concentration of Cr 2 O 7 2 − must also be 1.43 M because there is one Cr 2 O 7 2 − ion per formula unit. However, there are two NH 4 + ions per formula unit, so the concentration of NH 4 + ions is 2 × 1.43 M = 2.86 M. Because each formula unit of (NH 4 ) 2 Cr 2 O 7 produces three ions when dissolved in water (2NH 4 + + 1Cr 2 O 7 2 − ), the total concentration of ions in the solution is 3 × 1.43 M = 4.29 M.
Concentration of Ions in Solution from a Soluble Salt: Concentration of Ions in Solution from a Soluble Salt, YouTube(opens in new window) [youtu.be]
Example \(\PageIndex{5}\)
What are the concentrations of all species derived from the solutes in these aqueous solutions?
- 0.21 M NaOH
- 3.7 M (CH 3 ) 2 CHOH
- 0.032 M In(NO 3 ) 3
Given: molarity
Asked for: concentrations
A Classify each compound as either a strong electrolyte or a nonelectrolyte.
B If the compound is a nonelectrolyte, its concentration is the same as the molarity of the solution. If the compound is a strong electrolyte, determine the number of each ion contained in one formula unit. Find the concentration of each species by multiplying the number of each ion by the molarity of the solution.
B Because each formula unit of NaOH produces one Na + ion and one OH − ion, the concentration of each ion is the same as the concentration of NaOH: [Na + ] = 0.21 M and [OH − ] = 0.21 M.
B The only solute species in solution is therefore (CH 3 ) 2 CHOH molecules, so [(CH 3 ) 2 CHOH] = 3.7 M.
\( In(NO _3 ) _3 (s) \xrightarrow {H_ 2 O(l)} In ^{3+} (aq) + 3NO _3^- (aq) \)
B One formula unit of In(NO 3 ) 3 produces one In 3 + ion and three NO 3 − ions, so a 0.032 M In(NO 3 ) 3 solution contains 0.032 M In 3 + and 3 × 0.032 M = 0.096 M NO 3 – —that is, [In 3 + ] = 0.032 M and [NO 3 − ] = 0.096 M.
Exercise \(\PageIndex{5}\)
- 0.0012 M Ba(OH) 2
- 0.17 M Na 2 SO 4
- 0.50 M (CH 3 ) 2 CO , commonly known as acetone
\([Ba^{2+}] = 0.0012\: M; \: [OH^-] = 0.0024\: M\)
\([Na^+] = 0.34\: M; \: [SO_4^{2-}] = 0.17\: M\)
\([(CH_3)_2CO] = 0.50\: M\)
Solution concentrations are typically expressed as molarities and can be prepared by dissolving a known mass of solute in a solvent or diluting a stock solution.
- definition of molarity: \[ molarity = \dfrac{moles\: of\: solute}{liters\: of\: solution} = \dfrac{mmoles\: of\: solute} {milliliters\: of\: solution} \nonumber \]
- relationship among volume, molarity, and moles : \[ V_L M_{mol/L} = \cancel{L} \left( \dfrac{mol}{\cancel{L}} \right) = moles \nonumber \]
- relationship between volume and concentration of stock and dilute solutions : \[(V_s)(M_s) = moles\: of\: solute = (V_d)(M_d) \nonumber \]
The concentration of a substance is the quantity of solute present in a given quantity of solution. Concentrations are usually expressed in terms of molarity , defined as the number of moles of solute in 1 L of solution. Solutions of known concentration can be prepared either by dissolving a known mass of solute in a solvent and diluting to a desired final volume or by diluting the appropriate volume of a more concentrated solution (a stock solution ) to the desired final volume.
Contributors and Attributions
Modified by Joshua Halpern ( Howard University )

IMAGES
VIDEO
COMMENTS
PROBLEM 6.1.1.6 6.1.1. 6. Calculate the molarity of each of the following solutions: (a) 0.195 g of cholesterol, C 27 H 46 O, in 0.100 L of serum, the average concentration of cholesterol in human serum. (b) 4.25 g of NH 3 in 0.500 L of solution, the concentration of NH 3 in household ammonia.
Answer. PROBLEM \ (\PageIndex {14}\) Calculate the molality of each of the following solutions: 0.710 kg of sodium carbonate (washing soda), Na 2 CO 3, in 10.0 kg of water—a saturated solution at 0°C. 125 g of NH 4 NO 3 in 275 g of water—a mixture used to make an instant ice pack.
CHEMFILE MINI-GUIDE TO PROBLEM SOLVING General Plan for Solving Molarity Problems Mass of solute in g 1 Amount of solute in mol M moles solute liter solution 2 Volume of solution in L 3 Molar concentration, M 4 Convert using the molar mass of the solute. MOLARITY Molarity is the most common way to express concentration in chemistry.
Set up your equation so the concentration C = mass of the solute/total mass of the solution. Plug in your values and solve the equation to find the concentration of your solution. In our example, C = (10 g)/ (1,210 g) = 0.00826. 4. Multiply your answer by 100 if you want to find the percent concentration.
The following video looks at calculating concentration of solutions. We will look at a sample problem dealing with mass/volume percent (m/v)%. Example: Many people use a solution of sodium phosphate (Na 3 PO 4 - commonly called TSP), to clean walls before putting up wallpaper. The recommended concentration is 1.7% (m/v).
1) Concentration by Percent: It is the amount of solute dissolves in 100 g solvent. If concentration of solution is 20 %, we understand that there are 20 g solute in 100 g solution. Example: 10 g salt and 70 g water are mixed and solution is prepared. Find concentration of solution by percent mass.
Course: Class 9 Chemistry (India) > Unit 1. Lesson 1: Mixtures and solution. Types of mixtures. Concentration of a solution. Concentration of a solution.
To use this chart to solve the problem, we will use the fourth column as an equation to solve for \ (x.\) The 10 liters of our final mixture must have a total volume of 5 liters of alcohol in it in order to be 50% alcohol. Those 5 liters must come from a combination of the amount of 40% solution we mix in and the amount of 90% solution.
Calculating concentration of a solution. A solution of fructose ( C 6 H 12 O 6 ) in water is 15 % (w/w) .
Compare the concentration and number of moles of solute in solutions. Use the 'traffic light' cards to indicate their view: green for 'the same', red for 'different' and yellow for 'unsure'. Pour 100 cm 3 of copper (II) sulfate solution into each of two beakers A and B. Pour half of the solution from beaker A into a third beaker C.
Concentrations of Solutions. Concentrations of Solutions. There are a number of ways to express the relative amounts of solute and solvent in a solution. This page describes calculations for four different units used to express concentration: Percent Composition (by mass) Molarity. Molality.
Because the concentration is a percent, you know a 100-gram sample would contain 12 grams of iron. You can set this up as an equation and solve for the unknown "x": 12 g iron / 100 g sample = x g iron / 250 g sample. Cross-multiply and divide: x= (12 x 250) / 100 = 30 grams of iron.
We have 14 ready-to-use problem sets on the topic of Molarity and Solutions. These problem sets focus on the use of the concept of concentration (most specifically molarity) in the analysis of situations involving solution formation, dilution, and solution stoichiometry. Problems will range from the very easy plug-and-chug to the more difficult ...
In chemistry, the concentration of a solution describes the quantity of a solute that is contained in a particular quantity of solvent or solution. Knowing the concentration of solutes is important in controlling the stoichiometry of reactants for reactions that occur in solution. Chemists use many different ways to define concentrations, some ...
Percent Concentration. One way to describe the concentration of a solution is by the percent of the solution that is composed of the solute. This percentage can be determined in one of three ways: (1) the mass of the solute divided by the mass of solution, (2) the volume of the solute divided by the volume of the solution, or (3) the mass of the solute divided by the volume of the solution.
Consequently, the concentration of the solute must decrease by about a factor of 10, as it does (3.00 M → 0.310 M). We could also have solved this problem in a single step by solving Equation 4.5.4 for Vs and substituting the appropriate values: Vs = (Vd)(Md) Ms = (2.500L)(0.310M) 3.00M = 0.258L.
Hence it is important to understand all the methods of expressing the concentration of solutions. The concentration of the solution formula is given as follows. Concentration of solution =. Weight of the solute in gram volume in Litres. We will also see other methods on how to calculate the concentration of a solution based on the different ...
Ideal and Non-Ideal Solutions; Sample Problems on Concentration of Solution. Problem 1: 15 g of common salt is dissolved in 400 g of water. Calculate the concentration of the solution by expressing it in Mass by Mass percentage (w/w%). Solution: Given that, Mass of solute (common salt) = 15 g …(1)
Qualitative Expressions of Concentration. A solution can be qualitatively described as. dilute: a solution that contains a small proportion of solute relative to solvent, or. concentrated: a solution that contains a large proportion of solute relative to solvent. Microscopic view of a dilute solution of liquid Br 2 dissolved in liquid water.
Solution to Problem 5: The amount of the final mixture is given by 50 ml + 30 ml = 80 ml The amount of alcohol is equal to the amount of alcohol in pure water ( which is 0) plus the amount of alcohol in the 30% solution. Let x be the percentage of alcohol in the final solution. Hence 0 + 30% 50 ml = x (80) Solve for x x = 0.1817 = 18.75%.
A dilute solution means the quantity of solute is relatively very small, and a concentrated solution implies that the solution has a large amount of solute. But these are relative terms and do not give us the quantitative concentration of the solution. Table of Contents. Concentration; Recommended Video; Concentration in parts per million; Mass ...
Moreover, the proposed algorithm achieves the lowest average of the best so-far solutions the fastest for most functions. This fast convergence to the (near)-optimal solution is noticed and makes the proposed mWSO algorithm a promising optimization algorithm to solve problems that require fast computation, such as online optimization problems.
In this work, we present the first formally verified toolchain capable of full end-to-end verification for subgraph solving, which closes both of these trust gaps. We have built encoder frontends for various graph problems together with a 0-1 ILP (a.k.a. pseudo-Boolean) proof checker, all implemented and formally verified in the CakeML ecosystem.
Annealing processors are designed specifically for addressing combinatorial optimization problems, where the task is to find the best solution from a finite set of possibilities. This holds ...
3 Body Problem actually starts with two problems.First, we meet investigators tackling a string of unexplained suicides by scientists, including one who had a bizarre countdown written on the ...
Chaos has gutted Port-au-Prince and Haiti's government, a crisis brought on by decades of political disruption, a series of natural disasters and a power vacuum left by the president's assassination.
We are going to explain the fuzzy Adams-Bashforth methods for solving fuzzy differential equations focusing on the concept of g-differentiability.Considering the analysis of normal, convex, upper semicontinuous, compactly supported fuzzy sets in \(R^n\) and also convergence of the methods, the general expression of solutions is obtained. Finally, we demonstrate the importance of our method ...
Peggy Lawson (Oxbow Prairie Heights School). Funded by Saskatchewan Educational Technology Consortium. 5.4: Solution Concentration- Molarity is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts. Another way of expressing concentration is to give the number of moles of solute per unit volume of solution.
A Solution on North Korea Is There, if Biden Will Only Grasp It. March 16, 2024. ... How do you solve a problem like North Korea? Since the end of the Cold War, it seems that every formula, from ...
Use either Equation 11.3.2 or Equation 11.3.3, depending on the units given in the problem. Solution: Because we are given the volume of the solution in liters and are asked for the number of moles of substance, Equation 11.3.2 is more useful: molesNaOH = VLMmol / L = (2.50L)(0.100mol L) = 0.250molNaOH.