Study on Synthesis of Dibutyl Maleate with Solid Superacid Catalyst
Wan Shun-mei
Influential Citations
Journal of Xinxiang Education College

Key Takeaway : Dibutyl maleate synthesis with solid superacid catalyst offers higher yield, simple procedure, shorter reaction time, non-corrosive, and non-pollution advantages.
The optimum technological conditions were studied on the synthesis of Dibutyl maleate by the reaction of maleic acid and n-butanol with SO_4~2-/ Fe_2O_3-TiO_2 as catalyst.The results showed that the yield was over 96% when molar ratio of n-butanol and maleic acid was 4.0:1,reaction time of reflux was 3.0h mass of catalyst was 5% of maleic acid.This process has many advantages such as higher yield,simple procedure,shorter reaction time,non-corrosive,non-pollution,etc.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

Synthesis and Characterization of Organotin Containing Copolymers: Reactivity Ratio Studies
Organotin monomers containing dibutyltin groups – dibutyltin citraconate (DBTC) as a new monomer and dibutyltin maleate (DBTM) – were synthesized. Free radical copolymerizations of the organotin monomers with styrene (ST) and butyl acrylate (BA) were performed. The overall conversion was kept low (≤15% wt/wt) for all studied samples and the copolymers composition was determined from tin analysis using the Gillman and Rosenberg method. The reactivity ratios were calculated from the copolymer composition using the Fineman-Ross (FR) method. The synthesized monomers were characterized by elemental analysis, 1 H-, 13 C-NMR and FTIR spectroscopy.
1. Introduction
Copolymerization is one of the most important means to improve the performance of polymers. Copolymers are extensively used in industrial processes, because their physical properties, such as elasticity, permeability, glass transition temperature ( T g ) and solvent diffusion kinetics can be varied within wide limits [ 1 , 2 ]. Knowledge of a copolymer’s composition is an important factor in the evaluation of its utility [ 3 , 4 ]. Controlling the polymer property parameters, such as copolymer composition, copolymer sequence distribution and molecular weight averages, is of particular importance in copolymerization processes. This is because copolymer density and viscosity, which are two of the most important property measures used by polymer manufacturers, depend on these parameters [ 5 ]. Reactivity ratios are among the most important parameters for the composition equation of copolymers, which can offer information such as the relative reactivity of monomer pairs and help estimate the copolymer composition [ 3 ]. To calculate the polymerization rate or polymer productivity and copolymer composition, monomer reactivity ratios must be known. The method which is used most often nowadays for estimating monomer reactivity ratios is to perform a low conversion copolymerization at various initial monomers feed compositions. Subsequently, the copolymer composition is determined for each reaction [ 5 ]. Reactivity ratio values may be evaluated by various procedures: linear procedures, nonlinear procedures, and other copolymer composition equations [ 6 , 7 , 8 , 9 ].
Organotin derivatives of a compound containing bioactive alkyltin groups have considerable interest as biocides [ 10 ]. Organotin compounds have important applications in several areas and hence they are made industrially on a large scale [ 11 ]. The organotin moiety is attached to the monomers and copolymers via O-Sn and/or N-Sn bonds [ 10 , 11 , 12 , 13 , 14 , 15 ]. Acrylic copolymers with pendant organotin moieties find widespread applications as antifouling agents, [ 10 , 16 ] wood preservatives, [ 10 ] fungicides, pesticides, mosquito larvacides, [ 10 , 17 ] heat and light stabilizers in the manufacture of poly(vinyl chloride). [ 10 ] and biological activities against various species [ 10 , 18 ].
The present article investigates the synthesis, and structural characterization of copolymers of dibutyltin citraconate (DBTC), and dibutyltin maleate (DBTM), with styrene (ST) and butyl acrylate (BA) as well as the reactivity ratios in the copolymerization. For this purpose, reactivity ratios for the classical copolymerization model were determined using the linearization methods of Finemann–Ross (FR method) [ 19 ].
2. Results and Discussion
2.1. synthesis of organotin monomers.
Organotin monomers, (I) and (II), were prepared by the reaction of dibutyltin oxide (DBTO) with maleic anhydride or citraconic anhydride in equimolecular ratio as shown in Scheme 1 and Scheme 2 .
The structures were elucidated by elemental analysis, FTIR, 1 H and 13 C-NMR spectroscopy. In addition, the purity of the prepared monomers was checked by Thin Layer Chromatography (TLC) using ethyl acetate/cyclohexane (2:1). Generally, elemental microanalyses, as shown in Table 1 , are in a good agreement with the calculated values.
Elemental microanalysis of synthesized monomers I and II .
*Sn was estimated using the Gilman and Rosenberg method [ 20 ].
2.1.1. Dibutyltin Maleate (DBTM, I )
The FT-IR spectrum of dibutyltin maleate (DBTM, I ) showed characteristic peaks at: 2,854, 2,868, 2,926, and 2,958 cm -1 assigned to C-H stretching (-CH 2 CH 2 CH 2 CH 3 , and -CH=CH-), 1,615 cm -1 assigned to C=O stretching and 1,582 cm -1 assigned to C=C stretching. The 1 H-NMR spectrum (CDCl 3 ) showed peaks at δ 0.86 (triplet, -CH 2 CH 2 CH 2 C H 3 ), δ 1.30–1.36 (multiplet, -CH 2 C H 2 CH 2 CH 3 ), δ 1.65 (multiplet, -C H 2 CH 2 CH 2 CH 3 ), δ 1.74 (triplet, -CH 2 CH 2 C H 2 CH 3 ), δ 6.22 (singlet, -C H =C H -). The 13 C-NMR spectrum (CDCl 3 ) showed peaks at δ 13.60, 25.79, 26.54, 26.73 (- C H 2 C H 2 C H 2 C H 3 ), δ 129.61 (- C H= C H-), δ 175.01 (- C =O).
2.1.2. Dibutyltin Citraconate (DBTC, II )
The FT-IR spectrum of dibutyltin citroconate (DBTC, II ) showed peaks at: 2,857, 2,868, 2,927, and 2,956 cm -1 assigned to C-H stretching (-CH 2 CH 2 CH 2 CH 3 , & -CH=CH-), 1,610 and 1,667 cm -1 assigned to C=O stretching, 1,561 cm -1 assigned to C=C stretching. The 1 H-NMR spectrum showed peaks at δ 0.80 (triplet, -CH 2 CH 2 CH 2 C H 3 ), δ 1.26 (multiplet, -CH 2 C H 2 CH 2 CH 3 ), δ 1.59 (multiplet, -C H 2 CH 2 CH 2 CH 3 ), δ 1.61 (triplet, -CH 2 CH 2 C H 2 CH 3 ), δ 1.96 (singlet, -CH=C-C H 3 ), δ 5.77 (singlet, -C H =C-CH 3 ). The 13 C-NMR spectrum showed peaks at δ 13.59, 25.46, 26.62, 26.71 (- C H 2 C H 2 C H 2 C H 3 ), δ 20.81 (-CH=C- C H 3 ), δ 120.91 (- C H=C-CH 3 ), δ 145.22 (-CH= C -CH 3 ), δ 174.80 (-CH=CCH 3 - C =O), δ 177.92 (- C O-CH=C-CH 3 ).
2.2. Copolymerization Method
Copolymerization of DBTM or DBTC with styrene (ST) and butyl acrylate (BA) was done in solution using benzoyl peroxide as initiator via the free radical technique ( Scheme 3 and Scheme 4 ).
Copolymerization was done at 70 ºC in benzene with a total concentration of 2 mol/L at different time intervals. The formed copolymer was precipitated in an excess amount (20 fold), of the corresponding solvent, and was purified by washing with excess precipitation solvent or by reprecipitation from benzene, chloroform, or acetone, depending on the type of copolymer. All samples were dried in an oven under vacuum at 40–60 ºC. Different copolymers with different ratios were prepared and the percentage of tin was determined in each sample [ 20 ] ( Table 2 ).
Tin percentage of copolymers (III-VI) with different ratios.
a DBTM/ST; b DBTM/BA; c DBTC/ST; d DBTC/BA
2.3. Overall Conversion and Structural Characterization
The aim of the copolymerization was to study the copolymerization behavior of the monomers DBTM and DBTC, with ST and BA. The overall conversion of monomers to poly(DBTM- co -ST) ( III ), poly(DBTM- co -BA) ( IV ), poly(DBTC- co -ST) ( V ) and poly(DBTC- co -BA) ( VI ), after 2 h, was found to be 4.09, 34.01%, 4.52 and 24.93%, respectively. After 8 h, the overall conversion of monomers to ( III ), ( IV ), ( V ) and ( VI ) was found to be 13.89, 36.8%, 16.55 and 38.40%, respectively.
So the copolymerization of DBTM or DBTC with BA showed the highest overall conversion, compared to ST. Based on the percentage of tin ( Table 3 ), the copolymer composition showed that the content of DBTM in ( III ) is higher than in ( IV ) and the copolymer composition showed that the content of DBTC in ( V ) is lower than in ( VI ) after 4 h [ 21 ].
Copolymers composition after 4 h.
a Copolymer Composition.
The structural characterizations of ( III ), ( IV ), ( V ) and ( VI ) were done by FTIR and 1 H-NMR spectroscopy. The FTIR spectrum of ( III ), ( IV ), ( V ) and ( VI ) with overall conversion after 8 hrs of 13.89, 36.80, 16.55 and 38.40%, respectively, was characterized by the disappearance of C=C stretching bands at 1,582 and 1,610–1,640 cm -1 of DBTM or DBTC with ST or BA, respectively which confirm the formation of the copolymer.
Generally, the FTIR spectrum showed peaks at 1,452, 1,492, 1,583, and 1,601 cm -1 assigned to C=C stretching of the aromatic ring of ST [ 8 ]. The FTIR spectrum also showed peaks at 3,024, 3,058, and 3,080 cm -1 assigned to C-H stretching of the aromatic ring, and peaks at 2,848, 2872, 2931, 2,914, and 2957cm -1 assigned to aliphatic C-H stretching. On the other hand, the FTIR showed characteristic peaks at 1,605 and 1,736 cm -1 assigned to C=O stretching of DBTM and BA, respectively.
The 1 H-NMR spectrum of ( III ) and ( IV ) was characterized by the disappearance of peaks at δ 5.49–6.23 ppm (-C H =C H - and C H 2 =C H -) or (-C H =CCH 3 - and C H 2 =C H -) of DBTM or DBTC with ST or BA, respectively, which confirm the formation of the copolymer.
The 1 H-NMR spectrum of ( III ) was characterized by the appearance of peaks at δ 0.91–2.17 ppm (-C H 2 C H 2 C H 2 C H 3 , -C H 2 -CPh-, and -C H -C H -), and at δ 6.58–7.25 ppm ( H arom ). The 1 H-NMR spectrum of ( IV ) was characterized by the appearance of peaks at δ 0.92–1.59 ppm (-C H 2 C H 2 C H 2 C H 3 , -COOCH 2 C H 2 C H 2 C H 3 , -C H -CHCOO-) and at δ 4.03 ppm (-C H -C H -, -CH-C H COO-).
The 1 H-NMR spectrum of ( V ) was characterized by the appearance of peaks at δ 0.90–1.83 ppm (-C H 2 C H 2 C H 2 C H 3 , -C H 2 -C-COO-, and -CH-CHC H 3 -), and at δ 6.57–7.25 ppm ( H arom ). The 1 H-NMR spectrum of ( VI ) was characterized by the appearance of peaks at δ 0.89–1.57 ppm (-C H 2 C H 2 C H 2 C H 3 , -COOCH 2 C H 2 C H 2 C H 3 , -C H -CHCOO-, -CH-CHC H 3 -) and at δ 4.01 ppm (-C H -C H CH 3 -, -CH-C H COO-).
2.4. Reactivity Ratio Determination
2.4.1. poly(dbtm-co-st) ( iii ).
Poly(DBTM- co -ST) ( III ) was prepared using different ratios of DBTM and ST with BPO as initiator and the polymerization was stopped at an overall conversion ≤15 wt/wt%. The copolymer was precipitated in excess methanol. The percentage of tin was calculated according to the Gilman and Rosenberg method [ 20 ], and subsequently the copolymer composition (f) was determined as shown in ( Table 4 ). The monomers reactivity ratios and the content of the reaction mixture and the copolymer was calculated according to the FR method [ 22 , 23 , 24 ] ( Table 5 ). The FR parameters for DBTM and ST ( Table 6 ) were calculated by plotting the relation between F(f-1)/f and F 2 /f. From the values of the experimental reactivity ratio, r 1 ( k 11 /k 12 ) is smaller than r 2 ( k 22 /k 21 ), it is evident that monomer DBTM (r 1 = 0.099) is less reactive towards the addition of its units compared to the addition of ST units. On the other hand, the route of ST (r 2 = 9.9065) is more reactive towards the addition of its units compared to the addition of DBTM units. As r 1 r 2 < 1, so the copolymer tends to random distribution of its monomer units [ 15 ].
The parameters of poly(DBTM- co -ST) (III) composition.
a Mole fraction of DBTM in reaction mixture; b Molar ratio of DBTM to ST in reaction mixture; c Mole fraction of DBTM in copolymer; d Molar ratio of DBTM to ST in copolymer; e Overall conversion.
The monomer reactivity ratios and the FR parameters of poly(DBTM- co -ST) ( III ).
Experimental reactivity ratios according to the FR method.
a Reactivity Ratio of DBTM or DBTC; b Reactivity Ratio of vinyl monomers (ST and BA).
2.4.2. Poly(DBTM-co-BA) ( IV )
Poly(DBTM- co -BA) ( IV ) was prepared using different ratios of DBTM and BA with BPO as initiator and the polymerization was stopped at an overall conversion ≤15 wt/wt%. The copolymer was precipitated in excess methanol and was purified by reprecipitation in chloroform. The percentage of tin was calculated according to the Gilman and Rosenberg method [ 20 ], and subsequently the copolymer composition (f) was determined as shown in Table 7 . The monomers reactivity ratios and the content of the reaction mixture and the copolymer was calculated according to FR method [ 12 , 13 , 14 ] ( Table 8 ). The FR parameters for DBTM and BA ( Table 6 ) were calculated by plotting the relation between F(f-1)/f and F 2 /f. From the values of the experimental reactivity ratio, r 1 ( k 11 /k 12 ) is smaller than r 2 ( k 22 /k 21 ), it is evident that monomer DBTM (r 1 = 0.0248) is less reactive towards the addition of its units compared to the addition of BA units. On the other hand, the route of BA (r 2 = 24.431) is more reactive towards the addition of its units compared to the addition of DBTM units. As r 1 < 1 and r 2 > 1, so the copolymer will contain blocks of BA with low random units of DBTM due to the high reactivity of BA with its high reactivity ratio compared to DBTM [ 25 ]. Moreover, as r 1 r 2 < 1, so the copolymer tends to random distribution of its monomer units.
The parameters of poly(DBTM- co -BA) (IV) composition.
a Mole fraction of DBTM in reaction mixture; b Molar ratio of DBTM to BA in reaction mixture; c Mole fraction of DBTM in copolymer; d Molar ratio of DBTM to BA in copolymer; f Overall conversion.
The monomers reactivity ratios and the FR parameters of poly(DBTM- co -BA) (IV).
2.4.3. Poly(DBTC-co-ST) ( V )
Poly(DBTC- co -ST) ( V ) was prepared using different ratios of DBTC and ST using BPO as initiator and the copolymerization was stopped at overall conversion ≤15 wt/wt%. The copolymer was precipitated in excess methanol. The percentage of tin was calculated according to the Gilman and Rosenberg method [ 20 ], and subsequently the copolymer composition (f) was determined ( Table 9 ). The monomers reactivity ratios and the content of the reaction mixture and the copolymer was calculated according to FR method [ 22 , 23 , 24 ] ( Table 10 ). The FR parameters for DBTC and ST ( Table 6 ) were calculated by plotting the relation between F(f-1)/f and F 2 /f.
The parameters of poly(DBTC- co -ST) (V) composition.
a Mole fraction of DBTC in reaction mixture; b Molar ratio of DBTC to ST in reaction mixture; c Mole fraction of DBTC in copolymer; d Molar ratio of DBTC to ST in copolymer; e Overall conversion.
The monomers reactivity ratios and the FR parameters of poly(DBTC- co -ST) (V).
From the values of the experimental reactivity ratio, r 1 ( k 11 /k 12 ) is smaller than r 2 ( k 22 /k 21 ), it is evident that the monomer ST prefers the addition of its units compared to the addition of DBTC units. As r 1 < 1 and r 2 > 1, so the copolymer will contain blocks of ST with low random units of DBTC due to the high reactivity of ST with its high reactivity ratio compared to DBTC [ 25 ]. Finally, when r 1 < 1, the copolymerization is preferred and when r 2 > 1, ST will tend to homopolymerizations.
2.4.4. Poly(DBTC-co-BA) (VI)
Poly(DBTC- co -BA) (VI) was prepared using different ratios of DBTC and BA using BPO as initiator and the polymerization was stopped at overall conversion ≤15 wt/wt%. The copolymer was precipitated in excess methanol and was purified by reprecipitation from acetone. The percentage of Tin was calculated according to Gilman and Rosenberg method [ 20 ], and subsequently the copolymer composition (f) was determined as shown in ( Table 11 ). The monomers reactivity ratios and the content of the reaction mixture and the copolymer was calculated according to FR method [ 22 , 23 , 24 ] ( Table 12 ). The FR parameters for DBTC and BA ( Table 6 ) were calculated by plotting the relation between F(f-1)/f and F 2 /f.
The parameters of poly(DBTC-co-BA) (VI) composition.
a Mole fraction of DBTC in reaction mixture; b Molar ratio of DBTC to BA in reaction mixture; c Mole fraction of DBTC in copolymer; d Molar ratio of DBTC to BA in copolymer; f Overall conversion.
The monomer reactivity ratios and the FR parameters of poly(DBTC-co-BA) (VI).
From the values of the experimental reactivity ratio, r 1 ( k 11 /k 12 ) is smaller than r 2 ( k 22 /k 21 ), it is evident that monomer DBTC (r 1 = 0.2727) is less reactive towards the addition of its units compared to the addition of BA units. On the other hand, the route of BA (r 2 = 33.611) is more reactive towards the addition of its units compared to the addition of DBTC units. As r 1 < 1 and r 2 > 1, so the copolymer will contain blocks of BA with low random units of DBTC due to the high reactivity of BA with its high reactivity ratio compared to DBTC [ 25 ]. Moreover, when r 1 < 1, the copolymerization is preferred and when r 2 > 1, BA will tend to homopolymerization.
3. Experimental
3.1. materials.
Dibutyltin (IV) oxide was purchased from Sigma-Aldrich. Citraconic anhydride and styrene (ST) were purchased from Fluka. Maleic anhydride 99% and benzoyl peroxide (BPO) were purchased from BDH. Butyl acrylate and 2, 2’-azobisisobutyronitrile (AIBN) were purchased from Riedel-de-Haen. All solvents were purchased from BDH and were used as received.
3.2. Characterization
1 H- and 13 C-NMR Spectra were recorded on a Jeol (400 MHz) instrument. FTIR Spectra were recorded on a Perkin Elmer 883. Elemental analyses were performed at Perkin Elmer Series II CHN/O Analyzer 2400. Thin-layer chromatography (TLC) was performed using the ascending technique with silica gel 60F 254 precoated aluminum sheets.
3.3. Synthesis of Organotin Monomers
3.3.1. synthesis of dibutyltin maleate (dbtm, i ).
In a 500 mL round bottom flask, maleic anhydride (4.90 g, 50.0 mmol) was added to dibutyltin oxide (12.44 g, 50.0 mmol) in dry benzene (170 mL). The mixture was heated under gentle reflux for 9 h. The formed precipitate was removed by filtration, and the solvent was totally evaporated on a rotavapor to give an oily residue. The oily residue was dissolved in diethyl ether (100 mL) under gentle heating with stirring. The solution was filtered and the filtrate was concentrated to one third its volume on a rotavapor. The solution was cooled to room temperature and then was kept in freezer (-10 ºC) for 72 h to give a white precipitate. The formed precipitate was filtered, recrystallized from diethyl ether [ 26 ] and was dried under vacuum at 40 ºC for 24 h to give 10.20 g, 58.8% yield and m.p. 127–129 ºC. The product I was characterized by elemental analysis ( Table 1 ), FTIR and 1 H- and 13 C NMR spectroscopy.
3.3.2. Synthesis of Dibutyltin Citraconate (DBTC, II )
The dibutyltin citraconate (DBTC, II ) was prepared as described earlier for DBTM ( I ) using the following quantities and conditions: dibutyltin oxide (12.44 g, 50.0 mmol), citraconic anhydride (5.60 g, 50.0 mmol) in dry benzene (170 mL) for 10 hrs. The product II was recrystallized from n -hexane and was dried under vacuum at 40 ºC for 24 h to give 9.10 g, 50.4% yield and m.p. 107–110 ºC. The product II was characterized by elemental analysis ( Table 1 ), FTIR and 1 H- and 13 C-NMR spectroscopy.
3.4. General Procedure for Copolymerization
Copolymerizations were carried out in a three neck round bottomed flask. Copolymerization was done in solution by dissolving benzoyl peroxide (BPO) (1% mol) in 2 mL of the corresponding solvent, and then the calculated molar quantities of the monomers were added. The reaction mixture was bubbled with nitrogen to expel oxygen. Copolymerization was done at 70 ºC for the desired period of time. The formed copolymer was precipitated in excess amount (20 fold), of the corresponding solvent. All samples were dried in oven under vacuum at 40–60 ºC. For reactivity ratio determination, the copolymerization was stopped at overall conversion below 15% wt/wt [ 25 ] from the total weight of both monomers by changing the time of polymerization.
3.5. Overall Conversion
The overall conversion in copolymerization [ 25 , 27 , 28 ] of monomers DBTM and DBTC with ST and BA was studied by taking a fixed number of moles (20 mmol), and composition of 20% mol of monomer DBTM or DBTC and 80% mol of ST and BA, in benzene with a total concentration of 2 mol/L at different time intervals. The overall conversion by weight (wt/wt%) was determined using Equation (1):

3.6. Reactivity Ratios Determination
For reactivity ratio determination, copolymerizations were performed with different initial feed ratios while maintaining the monomer conversion below 15%. The Fineman–Ross (FR) method was employed. The initiator concentration was kept at 1% relative to the total monomers concentration in benzene. Monomer reactivity ratios can be calculated from the experimental results depending on the copolymer composition. Copolymer composition can be expressed as following;
Where m 1 & m 2 are the mole fractions of DBTM or DBTC and vinyl monomer in the copolymer, respectively, and f 1 & f 2 are its molar ratios in the copolymer.
Moreover, the feed composition of the reaction mixture is known in advance, so feed composition was used in the calculations of the reactivity ratios and can be expressed as follows:
where M 1 & M 2 are the mole fractions of DBTM or DBTC and vinyl monomer in the reaction mixture, respectively, and F 1 & F 2 are its Molar ratios in the feed composition.
In this research, the calculations were based on the tin content in the copolymer composition [ 29 ]. The Fineman–Ross (FR) [ 30 ] method is based on the use of copolymer composition and the content of the polymerization mixture. Based on the calculations of the copolymer composition and feed composition and according to equation (2):

A plot of (F 2 /f) on X-axis vs {F/f(f-1)} on Y-axis gave a straight line, the intercept is r 2 and the slope is r 1 .

4. Conclusions
The organotin monomers dibutyltin citraconate (DBTC) as a new monomer, and dibutyltin maleate (DBTM), were synthesized. The organotin monomers were copolymerized with styrene (ST) and butyl acrylate (BA) using a free radical technique. The overall conversion was kept low (≤15% wt/wt) for all studied samples and the copolymers composition was determined from tin analysis. From the values of the experimental reactivity ratio, r 1 ( k 11 /k 12 ) is smaller than r 2 ( k 22 /k 21 ), it is evident that poly(DBTM- co -ST) ( III ), poly(DBTM- co -BA) ( IV ) tend to random distribution of its monomer units as r 1 r 2 < 1. For poly(DBTC- co -ST) ( V ), r 1 < 1 and r 2 > 1, so the copolymer will contain blocks of ST with low random units of DBTC due to the high reactivity of ST with its high reactivity ratio compared to DBTC. For poly(DBTC- co -BA) ( VI ), r 1 < 1 and r 2 > 1, so the copolymer will contain blocks of BA with low random units of DBTC due to the high reactivity of BA with its high reactivity ratio compared to DBTC.

Synthesis of dibutyl maleate (DTBM, I ).

Synthesis of dibutyltin citraconate (DBTC, II)

Copolymerization of DBTM with ST and BA.

Copolymerization of DBTC with ST and BA.
Sample Availability: Samples of the compounds are available from authors.
Study of the Structure of Polybutylene Succinate Modified with Malic Acid and Its Ester
- POLYCONDENSATION
- Published: 30 November 2022
- Volume 64 , pages 636–643, ( 2022 )
Cite this article
- N. S. Kuz’mina 1 ,
- A. A. Prokhorova 1 ,
- S. V. Portnova 1 &
- E. L. Krasnykh 1
59 Accesses
Explore all metrics
A number of multifunctional biodegradable copolyesters have been synthesized using natural renewable products: succinic acid, 1,4-butanediol, malic acid, and its ester. The chemical structure and properties of the resulting polymers were characterized using 1 H and 13 C nuclear magnetic resonance spectroscopy, Fourier-transform infrared spectroscopy, dilute solution viscometry, and differential scanning calorimetry.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others
Bio-Based Poly(Butylene succinate-co-dodecylene succinate) Derived from 1,12-Dodecanediol: Synthesis and Characterization
Guoqiang Wang, Xingyu Hao, … Li Zhang

Effect of molecular weight on the properties of poly(butylene succinate)
Tian-xiang Jin, Mi Zhou, … Yi Fu
Synthesis and Characterization of Poly(ester amide)s Consisting of Poly(L-lactic acid) and Poly(butylene succinate) Segments with 2,2′-Bis(2-oxazoline) Chain Extending
Jun Zou, Yingzhen Qi, … Haiqing Xu
Q. Zhang, M. Song, Y. Xu, W. Wang, Z. Wang, L. Zhang, Prog. Polym. Sci. 120 , 101430 (2021).
H. Song and S. Y. Lee, Enzyme Microbiol. Technol. 39 , 352 (2006).
Article CAS Google Scholar
C. Pateraki, M. Patsalou, A. Vlysidis, N. Kopsahelis, C. Webb, A. A. Koutinas, M. Koutinas, Biochem. Eng. J. 112 (3), 285 (2016).
I.-N. Georgousopoulou, S. Vouyiouka, P. Dole, and C. D. Papaspyrides, Polym. Degrad. Stab. 128 , 182 (2016).
J. Zhang, X. Wang, F. Li, and J. Yu, Fibers Polym. 13 (10), 1233 (2012).
H. Shirali, M. Rafizadeh, and F. A. Taromi, Macromol. Res. 23 (8), 755 (2015).
E. Zakharova, C. Lavilla, A. Alla, A. M. Ilarduya, S. de Muñoz-Guerra, Eur. Polym. J. 61 , 263 (2014).
J. Xu and B.-H. Guo, Biotechnol. J. 5 (11), 1149 (2010).
S. N. Sheikholeslami, M. Rafizadeh, F. A. Taromi, H. Shirali, E. Jabbari, Polymer 98 , 70 (2016).
G. Z. Papageorgiou and D. N. Bikiaris, Polymer 46 (26), 12081 (2005).
D. N. Bikiaris, G. Z. Papageorgiou, and D. S. Achilias, Polym. Degrad. Stab. 91 (1), 31 (2006).
P. Parcheta and J. Datta, Polym. Degrad. Stab. 155 , 238 (2018).
K. Zhang and Z. Qiu, Polym. Test. 90 , 106755 (2020).
A. K. Schrock, H. S. C. Hamilton, N. D. Johnson, C. del Rosario, B. D. Thompson, K. Ulrich, W. D. Coggio, Polymer 101 , 233 (2016).
T. Debuissy, E. Pollet, and L. Avérous, Eur. Polym. J. 93 , 103 (2017).
Z. Sun, Z. Jiang, and Z. Qiu, Polymer 213 , 123197 (2021).
B. Bharathiraja, I. A. E. Selvakumari, J. Jayamuthunagai, R. P. Kumar, S. Varjani, A. Pandey, E. Gnansounou, Sci. Total. Environ. 709 , 136206 (2020).
S. Harun, A. Luthfi, P. Abdul, N. Bukhari, J. Jahim, Value-Chain of Biofuels: Fundamentals, Technology, and Standardization , ed. by S. Yusup and N. A. Rashidi (Elsevier, Amsterdam, 2022), p. 249.
Google Scholar
B. He, J. Bei, and S. Wang, Polym. Adv. Technol. 14 (9), 645 (2003).
J. Wang, C. Ni, Y. Zhang, M. Zhang, W. Li, B. Yao, L. Zhang, Colloids Surf., B 115 , 275 (2014).
T. Kajiyama, T. Taguchi, H. Kobayashi, K. Kataoka, J. Tanaka, Polym. Degrad. Stab. 81 (3), 525 (2003).
R. Yang, M. Li, X. Zhang, and J. Li, Ind. Crops Prod. 169 , 113648 (2021).
R. Yang, B. Wang, M. Li, X. Zhang, J. Li, Ind. Crops Prod. 136 , 121 (2019).
T. Yang, W. Li, X. Duan, L. Zhu, L. Fan, Y. Qiao, H. Wu, PLOS ONE 11 (9) (2016).
S. Zhang, J. Yang, X. Liu, J. Chang, A. Cao, Biomacromolecules 4 (2), 437 (2003).
G. Li, D. Yao, and M. Zong, Eur. Polym. J. 44 (4), 1123 (2008).
H. Wang, K. Liu, X. Chen, and M. Wang, Chemosphere 272 , 129543 (2021).
N. S. Kuz’mina, S. V. Portnova, and E. L. Krasnykh, Izv. Vyssh. Usebn. Zaved., Khim. Khim. Tekhnol. 64 (5), 71 (2021).
N. S. Kuz’mina, S. V. Portnova, and E. L. Krasnykh, Tonk. Khim. Tekhnol. 2 (15), 47 (2020).
D. N. Bikiaris and D. S. Achilias, Polymer 49 (17), 3677 (2008).
T. Taguchi, H. Kobayashi, K. Kataoka, and J. Tanaka, Polym. Bull. 50 (1), 69 (2003).
Article Google Scholar
T. Kajiyama, H. Kobayashi, K. Morisaku, T. Taguchi, K. Kataoka, J. Tanaka, Polym. Degrad. Stab. 84 (1), 151 (2004).
Download references
ACKNOWLEDGMENTS
The 1 H, 13 C NMR study was carried out using the equipment of the Shared Use Center “Investigation of the physicochemical properties of substances and materials” at the Samara State Technical University.
This work was supported by the Russian Foundation for Basic Research, project no. 20-08-01050 a.
Author information
Authors and affiliations.
Samara State Technical University, 443100, Samara, Russia
N. S. Kuz’mina, A. A. Prokhorova, S. V. Portnova & E. L. Krasnykh
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to N. S. Kuz’mina .
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by S. Zatonsky
Rights and permissions
Reprints and permissions
About this article
Kuz’mina, N.S., Prokhorova, A.A., Portnova, S.V. et al. Study of the Structure of Polybutylene Succinate Modified with Malic Acid and Its Ester. Polym. Sci. Ser. B 64 , 636–643 (2022). https://doi.org/10.1134/S156009042270052X
Download citation
Received : 17 July 2022
Revised : 13 September 2022
Accepted : 05 October 2022
Published : 30 November 2022
Issue Date : October 2022
DOI : https://doi.org/10.1134/S156009042270052X
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Find a journal
- Publish with us
- Track your research
- Architecture and Design
- Asian and Pacific Studies
- Business and Economics
- Classical and Ancient Near Eastern Studies
- Computer Sciences
- Cultural Studies
- Engineering
- General Interest
- Geosciences
- Industrial Chemistry
- Islamic and Middle Eastern Studies
- Jewish Studies
- Library and Information Science, Book Studies
- Life Sciences
- Linguistics and Semiotics
- Literary Studies
- Materials Sciences
- Mathematics
- Social Sciences
- Sports and Recreation
- Theology and Religion
- Publish your article
- The role of authors
- Promoting your article
- Abstracting & indexing
- Publishing Ethics
- Why publish with De Gruyter
- How to publish with De Gruyter
- Our book series
- Our subject areas
- Your digital product at De Gruyter
- Contribute to our reference works
- Product information
- Tools & resources
- Product Information
- Promotional Materials
- Orders and Inquiries
- FAQ for Library Suppliers and Book Sellers
- Repository Policy
- Free access policy
- Open Access agreements
- Database portals
- For Authors
- Customer service
- People + Culture
- Journal Management
- How to join us
- Working at De Gruyter
- Mission & Vision
- De Gruyter Foundation
- De Gruyter Ebound
- Our Responsibility
- Partner publishers

Your purchase has been completed. Your documents are now available to view.
Effects of polypropylene-g-dibutyl maleate on mechanical and rheological properties of PP/PA6 blends
The effects of polypropylene-grafted-dibutyl maleate (PP- g -DBM) used as compatibilizer on mechanical, rheological and morphological properties of polypropylene (PP) and nylon 6 (PA6) blends was systematically investigated in this paper. The results of Molau test, solvent extraction and differential scanning calorimetry (DSC) indicated the formation of PP- g -PA6 in vicinity of interfaces during melting extrusion. Owing to the reaction between the reactive groups of PPg- DBM and amine (-NH 2 ) end groups of PA6, the tensile and impact strength of the PP- g -DBM compatibilized PP/PA6 blends were much higher than that of the uncompatibilized PP/PA6 blends. Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) results indicated that the domain sizes of the dispersed phase in the compatibilized PP/PA6 blends decreased and the interfaces become more indistinct, which indicated a clear compatibiliting effect was induced by the PP- g -DBM in the immiscible PP/PA6 blends, i.e. the PP- g -DBM was an effective compatibilizer for the PP/PA6 blends. In addition, the rheological measurements showed the PP- g -DBM compatibilized PP/PA6 blends possessed higher pseudo plasticity, melt viscosity and flow activation energy.
© 2013 by Walter de Gruyter GmbH & Co.
- X / Twitter
Supplementary Materials
Please login or register with De Gruyter to order this product.
Journal and Issue
Articles in the same issue.

IMAGES
VIDEO
COMMENTS
Study on Synthesis of Dibutyl Maleate. Dibutyl maleate was prepared from maleic anhydride and n-butyl alcohol in the presence of p-toluene sulphonic acid.The yield of the ester could reach 95.6% under amount of substance ratio of maleic anhydride,n-butyl alcohol and p-toluene sulphonic acid were 1∶4∶0.16,refluxing and water segrating for 70 ...
Abstract. Dibutyl maleate was prepared from maleic anhydride and n - butyl alcohol in the presence of p - toluene sulphonic acid. The yield of the ester could reach 95.6% under amount of substance ...
Inorganic composite - solid acid catalysts SO42- /ZrO2-MoO3 were prepared and used in synthesizing dibutyl maleate. Optimal conditions were: for preparation of SO42-/ZrO2 - MoQ3 rinsing ZrO2 - MoO3 by 0.05mol/L H2SO4,calcining temperature 500 ℃ ,dosage of catalyst 0.5 g per mole acid anhydride,mole ratio of alcohol/acid anhydride 4 , and reaction time 4 h. Esterification degree was above 99 % .
Comparison of conventional and microwave irradiated synthesis of dibutyl maleate. The experimental results obtained by MW synthesis of DBM were compared with the conventional method reported earlier [6]. As inferred from Table 5, it reveals that traditional reaction was carried out for 240 min while MW-assisted reaction took place for 60 min.
Scheme 1 displays the reaction scheme for the synthesis of dibutyl maleate (Scheme 1 a) and fumarate (Scheme 1 b) plasticizers. DBM was synthesized according to a modified procedure from Yadav et. al. [29].Maleic acid (17.9 g, 0.154 mol) was dissolved in 211 mL of anhydrous butanol and the Amberlyst 15 in protonated form (heterogenous catalyst) (8.5 g) was added.
1. Rationale. Esterification is a prime reaction having wide applications in the production of flavors and fragrances, solvents, plasticizers, pesticides, and herbicides [1], [2], [3], [4].Dibutyl maleate (DBM), an unsaturated ester produced with the reaction of maleic acid and n-butanol, is used for creating sulfosuccinate surfactants in detergents and paints.
The present research investigates the kinetics of ultrasound‐assisted synthesis of dibutyl maleate using a heterogeneous catalyst (Amberlyst‐15) in solvent‐free system. Reaction parameters were optimized based on conversion by varying the various parameters such as n‐butanol to maleic acid mole ratio, temperature, molecular sieves ...
The optimum technological conditions were studied on the synthesis of Dibutyl maleate by the reaction of maleic acid and n-butanol with SO_4~2-/ Fe_2O_3-TiO_2 as catalyst.The results showed that the yield was over 96% when molar ratio of n-butanol and maleic acid was 4.0:1,reaction time of reflux was 3.0h mass of catalyst was 5% of maleic acid.This process has many advantages such as higher ...
Abstract. Abstract: Dibutyl maleatewas prepared from maleic anhydride and n-butyl alcohol in the presence of p-toluene sulphonic acid. The yield of the ester could reach 95.6% under amount of substance ratio of maleic anhydride, n-butyl alcohol and p-toluene sulphonic acid were 1:4:0. 16, refluxing and water segrating for 70 min.The ...
The optimum technological conditions were studied on the synthesis of Dibutyl maleate by the reaction of maleic acid and n-butanol with SO_4~2-/ Fe_2O_3-TiO_2 as catalyst.The results showed that the yield was over 96% when molar ratio of n-butanol and maleic acid was 4.0:1,reaction time of reflux was 3.0h mass of catalyst was 5% of maleic acid ...
Abstract Dibutyl maleate is a perfumery ester used as an intermediate in the production of paints, adhesives, and copolymers. Esterification of maleic acid and butanol was studied in presence of acidic cation exchange resin as a catalyst. The objective of this work was to test the suitability and efficacy of heterogeneous catalysts such as Indion 225H and Amberlyst-15 in the synthesis of ...
Dibutyl maleate is a perfumery ester used as an intermediate in the production of paints, adhesives, and copolymers. Esterification of maleic acid and butanol was studied in presence of acidic ...
The organotin monomers dibutyltin citraconate (DBTC) as a new monomer, and dibutyltin maleate (DBTM), were synthesized. The organotin monomers were copolymerized with styrene (ST) and butyl acrylate (BA) using a free radical technique. The overall conversion was kept low (≤15% wt/wt) for all studied samples and the copolymers composition was ...
Dibutyl maleate. Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). Dibutyl maleate is an organic compound with the formula (CHCO 2 Bu) 2 (Bu = butyl ). It is the diester of the unsaturated dicarboxylic acid maleic acid. It is a colorless oily liquid, although impure samples can ...
The novel vinyl acetate (VAc)/vinyl neo-decanoate (VeoVa10)/dibutyl maleate/triethoxy vinyl silane quadripolymer latex was prepared via the semi-continuous seeded emulsion polymerization emulsified with disodium laureth sulfosuccinate (AEMES) as anionic surfactant and beta-cyclodextrin (β-CD) mixture as emulsifier and initiated with potassium persulfate (KPS), respectively.
Abstract: Dibutyl maleatewas prepared from maleic anhydride and n-butyl alcohol in the presence of p-toluene sulphonic acid. The yield of the ester could reach 95.6% under amount of substance ratio of maleic anhydride, n-butyl alcohol and p-toluene sulphonic acid were 1:4:0. 16, refluxing and water segrating for 70 min.The catalytic activities of p- toluene sulphonic acid, sulfuric acid ...
Free radical solution copolymerization of vinyl acetate (VAc) and dibutyl maleate (DBM) initiated by AIBN was performed in the presence of chloroform as both solvent and chain transfer agent at various temperatures, comonomer mixture composition and initiator concentrations. Structures of the copolymers were characterized by FT-IR and 1H-NMR techniques. Effect of the above-mentioned variables ...
We examined the emulsion copolymerization of vinyl acetate (VAc) with dibutyl maleate (DBM) (VAc:DBM 4:1 w/w) in the presence of three dyes chosen for fluorescence resonance energy transfer (ET) experiments. The donor dyes were 9-acryloxymethylphenanthrene (PheMA) and 9-methacryloxymethylphenanthrene (PheMMA), and the nonfluorescent acceptor dye was 2'-acryloxy-4'-methyl-4-(N,N ...
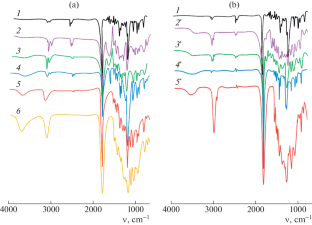
Samples 2'-5' produce this signal due to the presence of 1.2% dibutyl maleate and dibutyl fumarate, the unsaturated derivatives of malic acid dibutyl ester. The 13 C NMR spectra show chemical shifts corresponding to carbonyl carbon . Signals at 170.5 and 172.4 ppm belong to the carbonyl groups of malic and succinic acids, respectively, in the ...
In the present study, films of PLA were plasticized with the structural isomers dibutyl maleate and dibutyl fumarate (DBF) at varying compositions using extrusion and thermopressing. The impact of the additives on the thermal and mechanical properties of PLA was investigated by dynamic mechanic analysis (DMA), differential scanning calorimetry ...
Conventional dimethyl maleate (DMM) synthesis relies on the use of sulfuric acid as a catalyst which requires water washing and produces a large amount of wastewater that harms the environment. The use of this method is expensive since it involves numerous processes such as neutralization of sulfuric acid, washing with water, distillation etc. and the yield of dimethyl maleate is not very high.
In support of this idea, ionization energy of dibutyl maleate has been reported as 7.41 eV in a paper penned by Xu and coworkers [36]. 4. Conclusions. In the present paper, the effect of dibutyl maleate (DBM) of the glass transition temperature (Tg) of polymethyl methacrylate (PMMA) was analyzed with the help of experimental and theoretical ...
The effects of polypropylene-grafted-dibutyl maleate (PP- g -DBM) used as compatibilizer on mechanical, rheological and morphological properties of polypropylene (PP) and nylon 6 (PA6) blends was systematically investigated in this paper. The results of Molau test, solvent extraction and differential scanning calorimetry (DSC) indicated the formation of PP- g -PA6 in vicinity of interfaces ...