- Open access
- Published: 17 January 2024

Breast cancer survivorship needs: a qualitative study
- Rahimeh Khajoei ORCID: orcid.org/0000-0002-3770-6790 1 ,
- Payam Azadeh ORCID: orcid.org/0000-0003-1771-7377 2 ,
- Sima ZohariAnboohi ORCID: orcid.org/0000-0003-3422-9420 3 ,
- Mahnaz Ilkhani ORCID: orcid.org/0000-0002-5454-4041 3 &
- Fatemah Heshmati Nabavi ORCID: orcid.org/0000-0002-9842-966X 4
BMC Cancer volume 24 , Article number: 96 ( 2024 ) Cite this article
967 Accesses
Metrics details
Breast cancer rates and the number of breast cancer survivors have been increasing among women in Iran. Effective responses from healthcare depend on appropriately identifying survivors’ needs. This study investigated the experience and needs of breast cancer survivors in different dimensions.
In this qualitative content analysis, semi-structured in-depth interviews were conducted from April 2023 to July 2023. Data saturation was achieved after interviewing 16 breast cancer survivors (BCSs) and four oncologists using purposive sampling. Survivors were asked to narrate their experiences about their needs during the survivorship. Data were analyzed with an inductive approach in order to extract the themes.
Twenty interviews were conducted. The analysis focused on four central themes: (1) financial toxicity (healthcare costs, unplanned retirement, and insurance coverage of services); (2) family support (emotional support, Physical support); (3) informational needs (management of side effects, management of uncertainty, and balanced diet); and (4) psychological and physical issues (pain, fatigue, hot flashes, and fear of cancer recurrence).
Conclusions
This study provides valuable information for designing survivorship care plans. Identifying the survivorship needs of breast cancer survivors is the first and most important step, leading to optimal healthcare delivery and improving quality of life. It is recommended to check the financial capability of patients and take necessary measures for patients with financial problems. Additionally, support sources should be assessed and appropriate. Psychological interventions should be considered for patients without a support source. Consultation groups can be used to meet the information needs of patients. For patients with physical problems, self-care recommendations may also be useful in addition to doctors’ orders.
Peer Review reports
Introduction
According to the Globocan website, breast cancer is the most common cancer among women. Breast cancer was the most common cancer in Iran in 2020 in both sexes and the fifth cause of death in both sexes; however, it is the leading cause of death in women [ 1 ]. Due to early diagnosis and improvements in treatment methods, the prognosis and survival rate of women with breast cancer have enhanced significantly worldwide [ 2 ]. Breast cancer survivors face many problems and needs in different dimensions: psychological, physical, social, etc. [ 3 ]. These needs and issues during the survivorship period are different during the active phase of cancer treatment [ 4 ]. Physical and psychological symptoms include fatigue, pain, osteoporosis, premature menopause, fear of disease recurrence, sexual problems, and infertility [ 5 , 6 , 7 ]. To deal with these health needs, many patients require medical, psychological, and social care for more than ten years after diagnosis [ 8 , 9 ].
The growing population of survivors, along with their multiple needs, is a challenge for healthcare providers and health policymakers, who need to provide a good standard of care during the survival period and meet the different dimensions of the needs of these patients after treatment [ 3 , 10 ]. Failure to meet these needs is associated with negative consequences such as decreased satisfaction with care, poor adherence to treatment, decreased quality of life, and increased anxiety and depression [ 11 ]. The current trend in modern medicine is changing from a disease-based model to a patient-centered model in which patients are active and their preferences and needs are considered in care [ 12 ]. Therefore, the first step in planning supportive care services for cancer patients is to identify their care needs [ 13 ]. To date, several studies have investigated the needs and experiences of breast cancer survivors and obtained different results. The need for help in dealing with problems such as financial distress [ 14 ], Pain [ 15 ], Memory problems [ 16 ], Fear of disclosure [ 17 ], concerns relating to body image, femininity, altered physical appearance, and self-confidence [ 18 ] are among the needs experienced by breast cancer survivors. In 2023, a systematic study on the needs of breast cancer survivors showed that most of these patients had psychological and informational needs [ 19 ]. In assessing the needs of BCSs, it is essential to consider culture, ethnicity, socioeconomic status, birthplace, and native language. The elements of the survivorship journey may be somewhat similar for all women, yet we cannot assume that their experiences are monolithic, regardless of the context. Ethnic, racial, and cultural diversity may affect survivors’ needs. Iran is a country with unique cultural and religious characteristics and people’s behavior and attitude towards disease are different depending on their culture. As such, conducting research across cultures is necessary. For example, the family holds a valuable position in Iran. Cancer affects physical, mental, social, and economic dimensions of patients and their families. In this situation, preserving and consolidating the family foundation is important and may create different needs for patients. Therefore, considering the differences in the ethnic, cultural, and socioeconomic background of Iran, this study aimed to investigate the needs and experiences of breast cancer survivors in the country.
This qualitative study was conducted using a conventional content analysis approach in order to investigate the experiences and needs of breast cancer survivors. Qualitative research methods seek to discover and understand people’s inner worlds. As experiences form the structure of truth for each person, researchers can discover the meaning of phenomena from their perspective by taking into account people’s experiences [ 20 ]. In conventional content analysis, classes are extracted directly from the data text. This approach is used in studies whose purpose is to describe a phenomenon; this method was used in the present study [ 21 ].
Contributors
The qualitative research participants had deep experience of the phenomenon under study [ 19 ]. The participants in the study were breast cancer survivors (14 participants), patients’ families (two participants), and healthcare professionals (four participants). Women who had completed their treatment courses at a university hospital in Tehran between four and 18 months prior and were visiting the hospital for follow-up care were included in the study using targeted sampling. To achieve the maximum difference in the samples, participants with maximum diversity were included in the study over a period of three months. Participants of different ages, marriages, education levels, income, and ethnicities were included in the study. The sampling was continued until data saturation was achieved. The inclusion criteria were patients between 18 and 60 years of age, literacy, completion of treatment, non-metastatic cancer, and stable clinical conditions. The exclusion criteria were cancer recurrence or metastasis, secondary cancers, and cognitive impairment.
Collecting data
Two separate interview guides for survivors and healthcare professionals were developed by the research team, using expert input on the needs of cancer survivors. These guidelines are based on a literature review of the needs of survivors of breast cancer. In-depth semi-structured interviews were conducted in order to collect the data. After informed consent was obtained, an interview method with open-ended questions focusing on the study objectives was conducted. Each interview was conducted and analyzed. The interviews were scheduled at a convenient time and location for the interviewees. A total of 20 face-to-face and telephone interviews were conducted, lasting 45 min each. The data were collected between April 20 and July 30, 2023. The interviews began with an open-ended question relating to the needs and experiences of breast cancer survivors. Based on the interviewees’ answers, the interview process was directed towards achieving the main goal of the research. Exploratory questions were asked in order to attain a deeper understanding of the phenomena. The interviews continued until complete information saturation was reached. Full saturation occurs when classes and subclasses are completed, and new data do not add anything to these classes; at this point, no new data are obtained from the interviews and only previous information is repeated. Immediately after the end of the interviews, all interviews were recorded verbatim using the recording device and transcribed.
The interview guide questions included the following questions:
What were the most important issues you faced after completing your treatment?
What side effects did you experience and how did you deal with them?
What feelings did you experience during your recovery? Fear, anxiety, depression, despair, anger, fear of cancer returning, loss of self-confidence?
Explain your emotional life.
In addition, according to the participants’ answers, the following questions were addressed:
In what areas did you need more training and information?
What physical problems and complications do you currently face in need of support?
Do you need support in establishing relationships with others and being present in the community and workplace?
Do you feel that you need more support in your emotional and family relationships?
Do you need help from others in performing daily tasks?
Do you have trouble remembering and recalling events?
In addition, interview guide questions for healthcare providers include the following:
From your perspective, what are the most important issues and needs of breast cancer survivors? (in physical, mental, informational, cognitive dimensions, etc.)
Are there any issues that are more problematic for the Iranian recoveries?
The researcher further explored participants’ answers to each question. Furthermore, by restating and returning to the salient points or a summary of the participants’ answers, we confirmed the correctness of the data and increased the credibility of the results. At the end of each session, we asked questions such as “Is there anything else you would like to add?” and “Is there another question I should have asked?” Participants were asked to express any further experience or additional information. In addition, permission was obtained from the participants for future calls or interviews. At the end of each interview, the interviews were listened to several times at the earliest possible time and transcribed verbatim. The moods and characteristics of the participants were recorded along with the interviews. The mood and characteristics of the participants helped code the text of the interviews. That the codes accurately express a person’s understanding of the desired situation. The text of the interviews with pseudonyms was entered into MAXQDA 2020 software for data storage, retrieval, and analysis.
Data analysis
Data analysis was performed using conventional content analysis, based on the steps introduced by Graneheim and Lundman [ 22 ]. The sentences and phrases describing the needs and experiences of survivors were coded. The first and third researchers coded the texts of the interviews separately. The coded text was reviewed by a responsible researcher. Any disagreements were discussed to reach a consensus. Subsequently, the central concept of each class and the main and abstract concepts were defined. Following this, based on a constant comparison of similarities and differences, the codes that indicated a single topic were placed in a class, the subclasses and classes were categorized, and the core codes were formed. In the fifth step, the central concept of each class and the main and abstract concepts were defined.
Data integrity and robustness
To validate the research findings, the criteria of acceptability or validity, transferability, reliability, trust or stability, and ability, based on Lincoln and Guba, were used [ 23 ]. To ensure the validity of the data, in addition to allocating sufficient time for data collection and immersion, semi-structured interviews were conducted; the maximum variety in sampling was observed by interviewing people of different ages, education, income, and use at various stages of the disease. Sampling continued until the data reached saturation and the most appropriate semantic unit was selected. Internal validity of content analysis was condcuted; this was evaluated for its face validity. To validate the content, a panel of experts (research team) supported the generation of concepts or coding topics; these were also reviewed by the participants. For this purpose, the interview text and extracted codes were presented to participants, who commented on their accuracy [ 24 ]. In this study, a research audit, a detailed review of data by an external observer, was used to increase the stability of the research. In addition, the period of data collection (interviews) was carried out as quickly as possible and all participants were asked about the same topic [ 22 ]. To facilitate transferability, the researcher has provided a clear description of the platform, the method of selecting and characterizing the participants, the data collection, and the analysis process so that the reader can judge the applicability of the findings to other situations. In addition, rich and detailed findings with appropriate quotations and authentic documents are presented in order to increase transferability [ 25 ]. To increase the verifiability of the data, all research stages, methodology, and decisions taken in the research stages are explained in clear detail so that they can be followed by other researchers if necessary. In addition, all raw data and recorded notes, documents, and interviews have been retained for future review [ 26 ].
Ethical considerations
This study was approved by the Research Ethics Committee of Shahid Beheshti University of Medical Sciences in Tehran (IR. SBMU. PHARMACY. REC.1402.005). Informed consent was obtained from the research participants, and they were also informed about the confidentiality of their information.
Participants
The medical records of 450 BCSs were reviewed. 56 patients met the inclusion criteria for this study. 28 survivors could not be contacted, 12 declined to participate, and 16 participated in the interviews. Among the survivors, 64% were married and about half were between 30 and 50 years old. 60% had a higher education level and over 85% had an average income. Of the 12 oncologists who were invited to participate in this study, four agreed to participate, with a response rate of 33.3%.
The analysis focused on four central themes: (1) financial toxicity (healthcare costs, unplanned retirement, and insurance coverage of services); (2) family support (emotional support, Physical support); (3) informational needs (management of side effects, management of uncertainty, and balanced diet); and (4) psychological and physical issues (pain, fatigue, hot flashes, and fear of cancer recurrence).
Financial toxicity
The high cost of drugs and treatment, along with reduced work productivity and subsequent loss of income, impose a heavy financial burden on cancer patients, which in turn imposes a unique stress known as financial toxicity [ 27 ]. In this study, most patients mentioned financial problems as their most challenging issue.
Healthcare costs
One source of financial problems is the costs associated with cancer care services (e.g., medications, supplies, copayments, and transportation) [ 28 ]. Patients who report cancer-related financial problems or high healthcare costs are more likely to avoid or delay their medical care or prescribed medications. This may slow their healing process or aggravate the disease [ 29 ]. The participants in this study complained about the high costs of drugs and medical procedures. One participant said:
I had finished chemotherapy three months ago and was told to start radiation. But I couldn’t afford it, so I gave it up.
Coverage of insurance services
Another financial problem for the participants was that insurance did not cover the cost of some treatments. Obtaining health insurance did not fully protect against cancer-related financial problems. One participant said:
I am a worker and I have to work hard to improve my wife’s condition. Thank God, most of my wife’s treatment costs were paid by supplementary insurance. However, now the problem is that my wife has to undergo a mastectomy and she is very upset about the change in her appearance and wants to have breast implants. However, because it is considered a cosmetic procedure, unfortunately, insurance does not accept the payment and we are not able to pay the cost.
Unplanned retirement
One source of financial distress is reduced income due to loss of employment, missing work, or unplanned retirement [ 28 ]. In this study, it was found that some patients were forced to retire and had difficulty with paying the treatment costs. One participant said:
I am a teacher, and I was forced to retire due to illness. My pension is not sufficient to pay for my medicine and treatment. I really don’t know what to do to pay for the treatment.
Family support
Emotional support.
A remarkable finding obtained from the participants’ statements was that emotional support from their family, partner, and children played an important role in reducing their physical and psychological symptoms. Survivors who had a strong source of emotional support experienced fewer physical symptoms and a better mental state; among these patients, psychological symptoms such as stress, anxiety, and depression were far less common. Studies have shown that BCSs face various stresses during the course of the disease and need comprehensive support, including social and family support, to deal with these stressful factors and manage their resulting conditions better [ 30 ]. Family involvement increases their ability to cope [ 31 ]. Some of the participants’ statements support this:
After the radiation treatment, I felt much better because I went to my family in the city. They supported me a lot, making me feel better.
I live alone. Initially, my family supported me, but they left me little by little. Of course, they are right, our problem is ongoing, and I do not like to disturb anyone. I am struggling with several complications. The current problems include severe muscle pain, fatigue, weakness, and lethargy. I feel lonely. I often feel stressed and anxious.
The husband of one of the participants said:
My wife is not experiencing any particular problem at the moment because I am paying attention to her in every way. She is my life partner, and I should be by her side facing difficulties. I will do whatever I can to make her feel better. Thank God; there is no problem now.
Considering that partners play an essential role in providing emotional support, financial management, and decision-making regarding their spouses’ cancer treatment, there is a need to involve them in psychological interventions [ 32 ].
Physical support
Due to the complications of the treatment, the participants needed physical support from the family to fulfill their responsibilities. Patients who lacked physical support from the family had many problems in carrying out daily responsibilities and activities. One participant said:
I separated from my wife and live alone. My hand is swollen and painful, but I have to work with this condition; And I don’t have anyone to help me and this is really hard for me.
Informational needs
Almost all participants stated that they did not receive any information on what to expect. and would have liked to receive more information about management of side effects and balanced diet.
Management of side effects
Information needs were one of the most important needs for the survivors. Most participants said that they had not been given any training program. Many women reported that they had experienced persistent side effects from treatment for which they were poorly prepared. Moreover, they did not receive any related training. This lack of information confused them and exacerbated complications. One participant said:
I was not provided with any training. I was faced with cases where I did not know what to do. For example, because of menopause, I used to get hot flashes and I was very bothered. Later, they said you could use cool liquids like chicory, but I did not know what to do, and I tolerated it.
Management of uncertainty
One of the informational needs for BCSs was ensuring complete recovery. They were confused about being completely healed. Furthermore, they wanted more information about disease prevention for their daughters. One participant said:
My most important question during the recovery period is whether I have fully recovered. Does cancer have a chance of coming back? Is it possible for my daughter to develop cancer in the future?
One of the specialists stated:
Facing an uncertain future and the fear of the disease returning (especially the concern for children) is one of the most important concerns of BCSs. They should know that creating a suitable lifestyle and continuous follow-up can prevent the return of the disease.
Balanced diet
Another need expressed by patients was information about healthy eating or balanced eating (food to eat, food to avoid). One of the participants said:
In this regard, we did not receive sufficient training. I do not know what food is good for me. There is some information on the internet, but it is unreliable. I cannot always ask my doctor these questions. They should teach us about proper nutrition.
Psychological and physical issues
Most participants reported post-mastectomy, chest/arm, musculoskeletal, and lymphatic pain. Chronic pain is one of the most troublesome side effects of breast cancer treatment and affects patients’ quality of life [ 33 ]. Chronic pain causes discomfort and fatigue, reduces appetite and sleep, and interferes with many activities of daily living [ 34 ]. Some of the participants’ statements regarding pain are provided below. One of the specialists stated:
Survivors face long-term complications of treatment, such as myalgia, osteoporosis, and hot flashes due to hormonal treatments, respiratory disorders due to receiving radiotherapy, and paresthesias of limbs due to receiving chemotherapy drugs, and they should have the necessary information and background to face these complications.
Participants stated:
My main problem was that I experienced so much musculoskeletal pain that I could not move. I was admitted to the hospital a couple of times, and a bunch of drugs were injected into me, but it worked temporarily, and the pain recurred.
My left hand is in severe pain due to the removal of the lymph nodes, which makes it difficult for me to do my chores and I need the help of others.
Fatigue was another physical symptom mentioned by most participants. Fatigue is the most common and frequent symptom of BCSs after treatment [ 35 ]. Studies have shown that low physical activity, decreased muscle mass, and impaired physical fitness are potential causes of fatigue in BCSs [ 36 ]. One participant said:
Sometimes I am so tired that I have to take a break several times while doing a normal daily task and continue again.
Hot flashes
Most of the participants stated that they experienced hot flashes due to menopause. Hot flashes are a common problem in breast cancer survivors, affecting their work performance [ 37 ]. One of the participants stated:
I have night sweats and hot flashes due to menopause. It bothers me a lot; it starts with my head and then with the rest of my body. Anyone around me will notice that I am sweating profusely. When I get this condition at night, I wake up, get annoyed, and cannot sleep anymore. I drink cold liquids, and it gets better but not completely.
Fear of cancer recurrence
Fear of cancer recurrence is one of the most commonly reported concerns among cancer survivors [ 38 ]. Most participants experienced FCR; it was the most important source of stress and worry after breast cancer diagnosis and treatment, involving the fear of cancer returning to the breast, metastasizing, or returning as a second cancer. One of the contributors said:
I do not have much stress and anxiety because my doctor told me that my disease has progressed. My only concern is whether my cancer returns?
To the best of our knowledge, this is the first study to examine the needs and experiences of Iranian BCSs. The most important finding of this study was the patients’ experience with financial problems. Most patients had financial problems associated with cancer care services (e.g., therapeutic procedures, supplies, medications, and transportation) and reduced income due to loss of employment, missing work, or unplanned retirement. In Iranian government hospitals, all healthcare services are based on insurance. Patients who use these services in hospitals are required to pay a percentage of the treatment cost. Usually, cosmetic procedures are not included in these insurance programs. However, the increase in inflation and prices has caused most patients to experience financial problems. This finding is consistent with that of a study by Autade and Chauhan, in which all BCSs reported financial problems [ 39 ]. In addition, in a study by Carrera (2018), 28–48% of cancer survivors experienced financial toxicity based on monetary measures and 16–73% experienced financial toxicity based on subjective measures [ 40 ]. In a study by Barthakur et al. (2016), finances relating to treatment were a major concern for survivors and there was a need for information on reducing healthcare costs [ 41 ]. Over 8% reported that providing for the financial needs of their families was a severe problem [ 42 ]. Patients who report cancer-related financial problems or high costs may be more likely to forgo or delay prescription medications or medical care [ 29 ]. The inability to afford household expenses is one of the most commonly reported reasons for delayed medical care among cancer patients [ 43 ]. Financial problems, if not identified, can negatively affect the physical, psychological, and socioeconomic status of survivors and lead to poorer access to health services and consequently poorer health status and health-related quality of life [ 44 ]. Research shows that health professionals recognize patients’ financial concerns but may not be qualified to address them [ 44 ]. Healthcare providers can take several steps to reduce patients’ financial difficulties, including: (1) considering the cost of multiple treatment regimens with similar effects, (2) providing cancer cost estimates to patients, (3) considering treatment benefits, (4) assessing patients for financial toxicity, and (5) educating and assisting patients about insurance benefits and other financial assistance institutions that may be available to them [ 42 ].
Another finding of this study relates to patients’ experience of support resources. Consistent with Iranian culture, in this study family members were a valuable and main source of support for the BCSs. In the study of Lee et al., family members, including adult children and spouses, were the main source of support for all participants [ 45 ].
Upon receiving emotional support from their family, patients felt reassurance, acceptance, and attention. Those who had family support felt less anxious and depressed, and their physical symptoms were much fewer. The effective role of emotional support for patients has been reported in several studies [ 5 , 9 , 46 ]. Wilson et al. reported that support from friends and family is a positive strategy for reducing stress and anxiety and coping with illness [ 47 ]. In a study by Moradi et al. (2013), supportive care needs in women with breast cancer decreased after their husbands were educated about familiarity with breast cancer disease, symptoms and complications of the disease, and treatment. It has been suggested that caregivers use training sessions to reduce the needs of women with cancer [ 48 ]. These results were inconsistent, however, with those of Thewes (2004) and Arora (2007) [ 9 , 49 ]. They both concluded that friends and family members of patients with cancer tend to decrease or withdraw their support after treatment completion. The family’s role in improving patient outcomes cannot be overemphasized.
The study shows that patients who do not have access to a family support source have a greater tendency to use social support networks [ 50 ]. The researchers could create access to social support networks for patients who do not receive family support. Additionally, the role of support groups cannot be ignored. Support groups consisted of people with common experiences. In Iran, there are many support groups for cancer patients. Experiencers with any type of cancer can declare their readiness to participate in these groups by registering on related sites. These groups usually have weekly or monthly meetings. These meetings are held in person or virtually, according to the position of the group members. According to the needs of the members, the content of these meetings can be informative, reminiscent, fun, sports, cooking, or any topic desired by the majority of the members. Chou et al. (2016) showed that support groups help reduce psychological distress and increase the quality of life of patients with breast cancer. It is also possible to educate patients’ families about the needs of those who have recovered. Moghaddam Tabrizi et al. (2018) conducted an interventional study and trained patients’ families in family participation, optimism, coping with cancer, reducing uncertainty, and managing symptoms. The findings indicated a significant improvement in overall cancer coping scores on all subscales, including individual, positive focus, coping, deviation, planning, and interpersonal communication [ 31 ].
The participants also stated that they had unmet information needs in the areas of management of side effects, nutrition, and uncertainty management. Previous studies have also identified informational needs as the most common need for BCSs [ 3 , 5 , 51 ], even for survivors who have been diagnosed and treated for several years. These needs often include information on diet and nutrition, mental health counselling, infertility, and spiritual counselling [ 52 ]. Previous studies have indicated that BCSs often feel unready for the side effects that linger after therapy [ 17 , 18 ]. In a study by Pembroke et al., almost half of the participants reported informational needs associated with side effects of treatment [ 52 ]. In this study, patients met their information needs through the Internet; however, they had doubts regarding the use of this information. The high use of the Internet by patients with cancer suggests that healthcare providers may not be adequately meeting patients’ information needs [ 53 ].
Germino et al. (2013) conducted an intervention study with the aim of determining the impact of uncertainty management intervention on reducing uncertainty, better uncertainty management, fewer breast cancer-specific concerns and more positive psychological outcomes. The intervention consisted of a written CD and a manual with four weekly 20-minute training calls. The findings showed that BCS who received the intervention reported a reduction in uncertainty and significant improvement in behavioral and cognitive coping strategies for managing uncertainty, self-efficacy, and sexual dysfunction [ 54 ].
Physical problems were also reported by most participants. The patients mostly complained of pain, fatigue, and hot flashes. This finding is consistent with the results of the present study [ 9 , 16 , 17 , 39 ]. In the study by Bu et al. (2022), fatigue and pain were reported in 40.7% and 37.2% of the subjects respectively [ 55 ]. Studies have shown that the use of exercise-based interventions plays an effective role in reducing the pain and fatigue of breast cancer survivors [ 56 , 57 ].
Studies have also shown that mobile health education intervention improves cancer-related fatigue among breast cancer survivors [ 58 ]. mHealth interventions involve the adoption of mobile technologies to provide educational information, help users manage their own conditions and behaviors, and deliver healthcare to improve the health of users [ 59 ]. Integrating training to manage fatigue as part of routine care among breast cancer survivors is recommended [ 58 ].
The most important mental disorder experienced by patients was the fear of recurrence. The results of this study are similar to those of a previous study, which showed that the most common unmet need was help with coping with the fear of recurrence [ 60 , 61 , 62 ]. In the study of Brennan et al. (2010), fear of cancer recurrence was reported by 38% of the participants and emerged as the highest-ranked unmet need in all age groups [ 63 ]. In this study, fear of recurrence was identified as a key issue for cancer survivors [ 64 , 65 , 66 , 67 ].
The remarkable findings obtained from the interviews with health care professionals were that they mentioned the long-term complications caused by treatment procedures such as radiotherapy and chemotherapy in breast cancer survivors, including pain, fatigue, and hot flashes. Survivors have also reported these complications. According to healthcare professionals, facing an uncertain future, fear of cancer recurrence, and body image concerns are the most important concerns for breast cancer survivors. These results are consistent with those reported by Wells et al. [ 68 ].
Limitations
This study has several limitations. First, while the BCSs were diverse, all were recruited from a comprehensive cancer center in Tehran, where they were referred to receive survivorship care. They may differ in other centers. Second, we did not use random sampling to obtain a sample of study participants. Instead, we used purposive sampling, a sampling approach commonly used in qualitative research, in order to select participants who were particularly knowledgeable about the phenomenon under study. Third, a qualitative method was used in this study, which does not allow the testing of a specific hypothesis. Fourth, we used literate participants; different results may be obtained from illiterate individuals.
The results of this study can be used to design survival care programs for Iranian BCS. Therefore, patients’ support resources should be reviewed and measured. Appropriate psychological interventions could be used for patients who do not have a source of support. The financial ability of patients should be assessed, and necessary measures should be considered for patients with financial problems. All the patients were provided with an educational program tailored to their needs. Consultation groups could be used to meet the information needs of the patients. In patients with physical problems, self-care recommendations may also be useful in addition to doctors’ orders.
Data availability
The datasets analysed during the current study are not publicly available due to ethical restrictions. but are available from the corresponding author on reasonable request.
Abbreviations
Breast Cancer Survivor
Survivorship care plans
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. 2021;71(3):209–49.
Article Google Scholar
DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Goding Sauer A, et al. Breast cancer statistics, 2019. Cancer J Clin. 2019;69(6):438–51.
Cheng KKF, Cheng HL, Wong WH, Koh C. A mixed-methods study to explore the supportive care needs of breast cancer survivors. Psychooncology. 2018;27(1):265–71.
Article CAS PubMed Google Scholar
Joshi A, Larkins S, Evans R, Moodley N, Brown A, Sabesan S. Use and impact of breast cancer survivorship care plans: a systematic review. Breast Cancer. 2021;28(6):1292–317.
Article PubMed Google Scholar
Dsouza SM, Vyas N, Narayanan P, Parsekar SS, Gore M, Sharan K. A qualitative study on experiences and needs of breast cancer survivors in Karnataka, India. Clin Epidemiol Global Health. 2018;6(2):69–74.
Fong EJ, Cheah WL. Unmet supportive care needs among breast cancer survivors of community-based support group in Kuching, Sarawak. International journal of breast cancer. 2016;2016.
Hubbeling HG, Rosenberg SM, González-Robledo MC, Cohn JG, Villarreal-Garza C, Partridge AH, et al. Psychosocial needs of young breast cancer survivors in Mexico City, Mexico. PLoS ONE. 2018;13(5):e0197931.
Article PubMed PubMed Central Google Scholar
Lo-Fo‐Wong DN, de Haes HC, Aaronson NK, van Abbema DL, den Boer MD, van Hezewijk M, et al. Risk factors of unmet needs among women with breast cancer in the post‐treatment phase. Psycho‐Oncology. 2020;29(3):539–49.
Thewes B, Butow P, Girgis A, Pendlebury S. The psychosocial needs of breast cancer survivors; a qualitative study of the shared and unique needs of younger versus older survivors. Psychooncology. 2004;13(3):177–89.
Richter-Ehrenstein C, Martinez-Pader J. Impact of breast cancer diagnosis and treatment on work-related life and financial factors. Breast Care. 2021;16(1):72–6.
Mayer DK, Nasso SF, Earp JA. Defining cancer survivors, their needs, and perspectives on survivorship health care in the USA. Lancet Oncol. 2017;18(1):e11–e8.
Chae BJ, Lee J, Lee SK, Shin HJ, Jung SY, Lee JW, et al. Unmet needs and related factors of Korean breast cancer survivors: a multicenter, cross-sectional study. BMC Cancer. 2019;19(1):839.
Rahmani A, Ferguson C, Jabarzadeh F, Mohammadpoorasl A, Moradi N, Pakpour V. Supportive care needs of Iranian cancer patients. Indian J Palliat Care. 2014;20(3):224–8.
Buki LP, Rivera-Ramos ZA, Kanagui-Muñoz M, Heppner PP, Ojeda L, Lehardy EN, et al. I never heard anything about it: knowledge and psychosocial needs of Latina breast cancer survivors with lymphedema. Women’s Health. 2021;17:17455065211002488.
CAS PubMed PubMed Central Google Scholar
Gisiger-Camata S, Adams N, Nolan TS, Meneses K. Multi-level assessment to reach out to rural breast cancer survivors. Women’s Health. 2016;12(6):513–22.
PubMed PubMed Central Google Scholar
Gisiger-Camata S, Adams N, Nolan TS, Meneses K. Multi-level Assessment to Reach out to rural breast Cancer survivors. Womens Health (Lond). 2016;12(6):513–22.
Cappiello M, Cunningham RS, Tish Knobf M, Erdos D. Breast cancer survivors: information and support after treatment. Clin Nurs Res. 2007;16(4):278–93.
Galván N, Buki LP, Garcés DM. Suddenly, a carriage appears: social support needs of Latina breast cancer survivors. J Psychosoc Oncol. 2009;27(3):361–82.
Khajoei R, Ilkhani M, Azadeh P, Anboohi SZ, Nabavi FH. Breast cancer survivors–supportive care needs: systematic review. BMJ Supportive & Palliative Care. 2023;13(2):143–53.
Speziale HS, Streubert HJ, Carpenter DR. Qualitative research in nursing: advancing the humanistic imperative. Lippincott Williams & Wilkins; 2011.
Hsieh H-F, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277–88.
Graneheim UH, Lundman B. Qualitative content analysis in nursing research: concepts, procedures and measures to achieve trustworthiness. Nurse Educ Today. 2004;24(2):105–12.
Lincoln YS, Guba EG. But is it rigorous? Trustworthiness and authenticity in naturalistic evaluation. New Dir Program Evaluation. 1986;1986(30):73–84.
Corbin J, Strauss A. Strategies for qualitative data analysis. Basics of Qualitative Research Techniques and Procedures for Developing Grounded Theory. 2008;3(104135):9781452230153.
Google Scholar
Elo S, Kyngäs H. The qualitative content analysis process. J Adv Nurs. 2008;62(1):107–15.
Lietz CA, Langer CL, Furman R. Establishing trustworthiness in qualitative research in social work: implications from a study regarding spirituality. Qualitative Social work. 2006;5(4):441–58.
Lentz R, Benson AB III, Kircher S. Financial toxicity in cancer care: prevalence, causes, consequences, and reduction strategies. J Surg Oncol. 2019;120(1):85–92.
Perry LM, Hoerger M, Seibert K, Gerhart JI, O’Mahony S, Duberstein PR. Financial strain and physical and emotional quality of life in breast cancer. J Pain Symptom Manag. 2019;58(3):454–9.
Kent EE, Forsythe LP, Yabroff KR, Weaver KE, de Moor JS, Rodriguez JL, et al. Are survivors who report cancer-related financial problems more likely to forgo or delay medical care? Cancer. 2013;119(20):3710–7.
Hu RY, Wang JY, Chen WL, Zhao J, Shao CH, Wang JW, et al. Stress, coping strategies and expectations among breast cancer survivors in China: a qualitative study. BMC Psychol. 2021;9(1):26.
Moghaddam Tabrizi F, Alizadeh S. Family intervention based on the FOCUS Program effects on Cancer Coping in Iranian breast Cancer patients: a Randomized Control Trial. Asian Pac J Cancer Prev. 2018;19(6):1523–8.
PubMed Google Scholar
Sebri V, Pravettoni G. Tailored psychological interventions to manage body image: an Opinion study on breast Cancer survivors. Int J Environ Res Public Health. 2023;20(4).
Tait RC, Zoberi K, Ferguson M, Levenhagen K, Luebbert RA, Rowland K, et al. Persistent Post-mastectomy Pain: risk factors and current approaches to treatment. J Pain. 2018;19(12):1367–83.
Fitzgerald Jones K, Fu MR, McTernan ML, Ko E, Yazicioglu S, Axelrod D, et al. Lymphatic Pain in breast Cancer survivors. Lymphat Res Biol. 2022;20(5):525–32.
Schmidt ME, Chang-Claude J, Vrieling A, Heinz J, Flesch-Janys D, Steindorf K. Fatigue and quality of life in breast cancer survivors: temporal courses and long-term pattern. J Cancer Surviv. 2012;6(1):11–9.
Neil SE, Klika RJ, Garland SJ, McKenzie DC, Campbell KL. Cardiorespiratory and neuromuscular deconditioning in fatigued and non-fatigued breast cancer survivors. Support Care Cancer. 2013;21(3):873–81.
Harris PF, Remington PL, Trentham-Dietz A, Allen CI, Newcomb PA. Prevalence and treatment of menopausal symptoms among breast cancer survivors. J Pain Symptom Manage. 2002;23(6):501–9.
Thewes B, Lebel S, Seguin Leclair C, Butow P. A qualitative exploration of fear of cancer recurrence (FCR) amongst Australian and Canadian breast cancer survivors. Support Care Cancer. 2016;24(5):2269–76.
Autade YCG. Assess the prevalence for needs of breast cancer survivors’ in the oncology ward at a selected tertiary care hospital. JPRI. 2021;1(33):1–12.
Carrera PM, Kantarjian HM, Blinder VS. The financial burden and distress of patients with cancer: understanding and stepping-up action on the financial toxicity of cancer treatment. CA Cancer J Clin. 2018;68(2):153–65.
Barthakur MS, Sharma MP, Chaturvedi SK, Manjunath SK. Experiences of breast Cancer survivors with Oncology Settings in Urban India: qualitative findings. Indian J Surg Oncol. 2016;7(4):392–6.
Coughlin SS, Ayyala DN, Tingen MS, Cortes JE. Financial distress among breast cancer survivors. Curr Cancer Rep. 2020;2(1):48–53.
Knight TG, Deal AM, Dusetzina SB, Muss HB, Choi SK, Bensen JT et al. Financial toxicity in adults with Cancer: adverse outcomes and noncompliance. J Oncol Pract. 2018:Jop1800120.
Nipp RD, Shui AM, Perez GK, Kirchhoff AC, Peppercorn JM, Moy B, et al. Patterns in Health Care Access and Affordability among Cancer survivors during implementation of the Affordable Care Act. JAMA Oncol. 2018;4(6):791–7.
Lee S, Chen L, Ma GX, Fang CY, Oh Y, Scully L. Challenges and needs of Chinese and Korean American breast Cancer survivors: In-Depth interviews. N Am J Med Sci (Boston). 2013;6(1):1–8.
Shaw MD, Coggin C. Using a Delphi technique to determine the needs of African American breast cancer survivors. Health Promot Pract. 2008;9(1):34–44.
Wilson SE, Andersen MR, Meischke H. Meeting the needs of rural breast cancer survivors: what still needs to be done? J Womens Health Gend Based Med. 2000;9(6):667–77.
Moradi NAF, Rahmani AZV. Effects of husband’s education on meting supportive care needs of breast cancer patients: a clinical trial. Avicenna-J-Nurs-Midwifery-Care. 2013;21(3):40–50.
Arora NK, Finney Rutten LJ, Gustafson DH, Moser R, Hawkins RP. Perceived helpfulness and impact of social support provided by family, friends, and health care providers to women newly diagnosed with breast cancer. Psychooncology. 2007;16(5):474–86.
Namkoong K, Shah DV, Gustafson DH. Offline Social relationships and Online Cancer Communication: effects of Social and Family Support on Online Social Network Building. Health Commun. 2017;32(11):1422–9.
Liao MN, Chen SC, Chen SC, Lin YC, Hsu YH, Hung HC, et al. Changes and predictors of unmet supportive care needs in Taiwanese women with newly diagnosed breast cancer. Oncol Nurs Forum. 2012;39(5):E380–9.
Zebrack B. Information and service needs for young adult cancer survivors. Support Care Cancer. 2009;17(4):349–57.
Winzelberg AJ, Classen C, Alpers GW, Roberts H, Koopman C, Adams RE, et al. Evaluation of an internet support group for women with primary breast cancer. Cancer: Interdisciplinary International Journal of the American Cancer Society. 2003;97(5):1164–73.
Germino BB, Mishel MH, Crandell J, Porter L, Blyler D, Jenerette C, et al. Outcomes of an uncertainty management intervention in younger African American and caucasian breast cancer survivors. Oncol Nurs Forum. 2013;40(1):82–92.
Bu X, Jin C, Fan R, Cheng ASK, Ng PHF, Xia Y, et al. Unmet needs of 1210 Chinese breast cancer survivors and associated factors: a multicentre cross-sectional study. BMC Cancer. 2022;22(1):135.
Galiano-Castillo N, Cantarero‐Villanueva I, Fernández‐Lao C, Ariza‐García A, Díaz‐Rodríguez L, Del‐Moral‐Ávila R, et al. Telehealth system: a randomized controlled trial evaluating the impact of an internet‐based exercise intervention on quality of life, pain, muscle strength, and fatigue in breast cancer survivors. Cancer. 2016;122(20):3166–74.
Lin Y, Wu C, He C, Yan J, Chen Y, Gao L, et al. Effectiveness of three exercise programs and intensive follow-up in improving quality of life, pain, and lymphedema among breast cancer survivors: a randomized, controlled 6-month trial. Support Care Cancer. 2022;31(1):9.
Bandani-Susan B, Montazeri A, Haghighizadeh MH, Araban M. The effect of mobile health educational intervention on body image and fatigue in breast cancer survivors: a randomized controlled trial. Ir J Med Sci. 2022;191(4):1599–605.
Wei Y, Zheng P, Deng H, Wang X, Li X, Fu H. Design features for improving Mobile Health intervention user Engagement: systematic review and thematic analysis. J Med Internet Res. 2020;22(12):e21687.
Hodgkinson K, Butow P, Hunt GE, Pendlebury S, Hobbs KM, Wain G. Breast cancer survivors’ supportive care needs 2–10 years after diagnosis. Support Care Cancer. 2007;15(5):515–23.
Beatty L, Oxlad M, Koczwara B, Wade TD. The psychosocial concerns and needs of women recently diagnosed with breast cancer: a qualitative study of patient, nurse and volunteer perspectives. Health Expect. 2008;11(4):331–42.
Torres E, Dixon C, Richman AR. Understanding the breast Cancer Experience of survivors: a qualitative study of African American Women in Rural Eastern North Carolina. J Cancer Educ. 2016;31(1):198–206.
Brennan ME, Butow P, Spillane AJ, Boyle F. Patient-reported quality of life, unmet needs and care coordination outcomes: moving toward targeted breast cancer survivorship care planning. Asia‐Pacific J Clin Oncol. 2016;12(2):e323–e31.
Simard S, Thewes B, Humphris G, Dixon M, Hayden C, Mireskandari S, et al. Fear of cancer recurrence in adult cancer survivors: a systematic review of quantitative studies. J Cancer Surviv. 2013;7(3):300–22.
Crist JV, Grunfeld EA. Factors reported to influence fear of recurrence in cancer patients: a systematic review. Psychooncology. 2013;22(5):978–86.
Ellegaard MB, Grau C, Zachariae R, Bonde Jensen A. Fear of cancer recurrence and unmet needs among breast cancer survivors in the first five years. A cross-sectional study. Acta Oncol. 2017;56(2):314–20.
Fang SY, Fetzer SJ, Lee KT, Kuo YL. Fear of recurrence as a predictor of Care needs for long-term breast Cancer survivors. Cancer Nurs. 2018;41(1):69–76.
Wells KJ, Drizin JH, Ustjanauskas AE, Vázquez-Otero C, Pan-Weisz TM, Ung D, et al. The psychosocial needs of underserved breast cancer survivors and perspectives of their clinicians and support providers. Support Care Cancer. 2022;30:105–16.
Download references
Acknowledgements
Not applicable.
Not funded.
Author information
Authors and affiliations.
Student Research Committee, School of Nursing and Midwifery, Shahid Beheshti University of Medical Sciences, Tehran, IR, Iran
Rahimeh Khajoei
Radiation Oncology Department, School of Medicine, Imam Hossein Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Payam Azadeh
Department of Medical Surgical Nursing, School of Nursing and Midwifery, Shahid Beheshti University of Medical Sciences, Tehran, IR, Iran
Sima ZohariAnboohi & Mahnaz Ilkhani
Nursing and Midwifery Care Research Center, Mashhad University of Medical Sciences, Mashhad, Iran
Fatemah Heshmati Nabavi
You can also search for this author in PubMed Google Scholar
Contributions
R.K. and M.I. conceived and designed the study. R.K. conducted and transcribed interviews, analyzed the data, and wrote the manuscript. M.I., S.Z., P.A. and F.H. assisted in the analysis and interpretation of data, and in the revision of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Mahnaz Ilkhani .
Ethics declarations
Ethics approval and consent to participate.
This study was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences, Tehran with code IR.SBMU.PHARMACY.REC.1402.005. Informed consent was obtained from all subjects involved in the study.
Consent for publication
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Khajoei, R., Azadeh, P., ZohariAnboohi, S. et al. Breast cancer survivorship needs: a qualitative study. BMC Cancer 24 , 96 (2024). https://doi.org/10.1186/s12885-024-11834-5
Download citation
Received : 16 September 2023
Accepted : 03 January 2024
Published : 17 January 2024
DOI : https://doi.org/10.1186/s12885-024-11834-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Breast cancer
- Survivorship
ISSN: 1471-2407
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Open access
- Published: 19 May 2020
The mediating effect of social support on uncertainty in illness and quality of life of female cancer survivors: a cross-sectional study
- Insook Lee ORCID: orcid.org/0000-0001-6090-7999 1 &
- Changseung Park 2
Health and Quality of Life Outcomes volume 18 , Article number: 143 ( 2020 ) Cite this article
2971 Accesses
20 Citations
Metrics details
Cancer survivors have been defined as those living more than 5 years after cancer treatment with no signs of recurrence or further growth; however, the National Coalition for Cancer Survivorship of the United States defined cancer survivors as those undergoing treatment after being diagnosed with cancer or those considered to be fully cured. The National Cancer Institute of the United States established the Office of Cancer Survivorship, with the American Society of Clinical Oncology including “patient and survivor management” as its 2006 annual objective [ 1 ], indicating the importance of cancer survivor management as a major agenda item.
Typically, breast and thyroid cancer diagnoses occur among women in their 40s and 50s, and patients who receive treatment have high survival rates. The majority of breast and thyroid cancer survivors return to their daily lives within a relatively short timeframe [ 2 ], making quality of life after treatment and important factor in cancer treatment [ 3 , 4 , 5 ].
Cancer survivors have reported experiencing a variety of physical difficulties during or after treatment, including fatigue, pain, loss of energy, sleeping disorders, and constipation [ 6 , 7 ]. They also have psychological concerns, such as fear of the cancer spreading, concerns about treatment results, and uncertainty about the future [ 8 ], as well as financial difficulties, issues with their sex lives, decreased body image, difficulties in interpersonal relationships, role disorders, and difficulty returning to work [ 7 ]. Thus, cancer patients require a diverse range of healthcare services, plus emotional and socioeconomic support, with an international study of the quality of life and symptoms of cancer survivors reporting that the quality of life among Asian patients to be the lowest of those than other country [ 6 ]. Survivors of breast cancer have reported low quality of life after treatment [ 9 , 10 ], which is influenced by emotional and psychological factors such as uncertainty, body image, lack of self-respect, and depression [ 9 , 10 , 11 ], as well as social factors including social, family, and spouse support [ 10 , 11 ].
Uncertainty among women diagnosed with malignant illnesses was found to be higher than that among women with a lump in the breast [ 12 ]. Uncertainty among breast cancer patients continues for a long period because of the fear of recurrence [ 13 ] and reduced quality of life [ 11 ]. Similarly, thyroid cancer survivors also show higher levels of fatigue, depression, and anxiety compared to those with no experience of cancer [ 14 , 15 , 16 ].
Social support is a complex and multidimensional concept that is characterized by mutual benefits that include social, psychological, and material support provided by the social support network [ 17 ]. In other words, social support means help provided by social relationships such as family, friends, and significant others, and plays an important role in directly and indirectly reducing uncertainty [ 18 , 19 ]. For cancer survivors, the need for social support is varied and depends largely on the adaptive tasks they face [ 20 ]. Social support is closely related to breast cancer survivor prognosis [ 21 ]; breast cancer survivors’ uncertainty was found to lower their quality of life, but their recognition of social support was found to improve it [ 11 ]. Recognition of social support and uncertainty played a key role in managing and maintaining quality of life. Research has shown that social support differs according to survival stage, as patients who are undergoing treatment receive active support from healthcare professionals and their family, but this support declines notably after the treatment ends [ 14 , 22 , 23 ].
With the number of cancer survivors steadily increasing, there has been an increase in the number of studies published on cancer survivors internationally [ 22 , 24 ]. In Korea in particular, there have been studies on recurrence-prevention behaviors and quality of life [ 25 ], the factors influencing quality of life [ 10 ], fatigue and quality of life [ 26 ], distress and quality of life [ 27 ], and symptoms and quality of life among breast cancer survivors [ 8 ]. Most of these studies focused on breast cancer survivors, but few studies focus on overall quality of life and the related influential factors. Few studies have analyzed the relationships between uncertainty in illness, quality of life, and social support among female breast and thyroid cancer survivors.
This study aimed to identify the relationships among uncertainty in illness, social support, and quality of life in female cancer survivors, and to verify the mediating role of social support, in the relationship between uncertainty in illness and quality of life. Social support may act as a generative mechanism influencing how uncertainty in illness, the predictor variable, affects quality of life, the outcome variable (Fig. 1 ) [ 28 ]. Therefore, this study will provide foundational data for devising practical and helpful intervention strategies to raise the quality of life of cancer survivors.
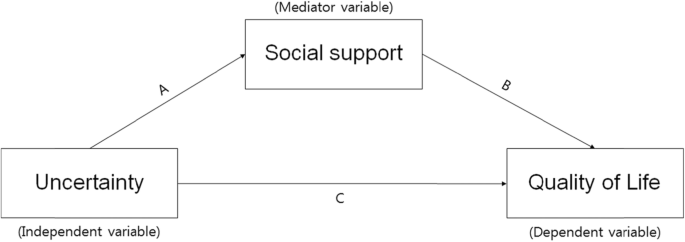
The theoretical research model showing the influence of uncertainty on quality of life and the mediating effect of social support
Participants
Participants were selected using convenience sampling of female cancer patients who were being treated by specialists in breast endocrinology at general hospitals located in J City of Korea. Among the 189 women (138 thyroid and 50 breast cancer patients) who agreed to participate, 156 surveys were collected (response rate: 82.5%). The final sample including 148 participants after excluding eight insincere responses. The data collection period was from April 21 to June 30, 2014. The completion of data collection through the mailed-in copies of surveys occurred on October 15, 2014. Participants were asked to complete the survey, put it in an opaque envelope, and seal it before returning it to the researchers. In cases in which on-site survey completion was difficult, participants were able to complete the survey at home and returned it by mail to the researcher.
The necessary sample size for the multiple regression analysis was confirmed utilizing G*power ver. 3.1.9 with a significance level (α) of .05; power of .80; effect size (f 2 ) of .15 (representing a medium effect size in the multiple regression analysis); and 13 independent variables (age, marital status, religion, level of education, occupation, satisfaction with economic status, smoking, drinking, diagnosis name, clinical stage of cancer, time passed since the end of treatment, uncertainty, and social support). The minimum sample size was determined to be 131. Since a maximum dropout rate of 40% was expected, information was collected from a total of 189 participants who fit the following inclusion criteria: 1) a diagnosis of cancer and no cognitive limitations; 2) the ability to understand and complete the survey in Korean; and 3) an understanding of the purpose of the study and consenting to participate. The exclusion criteria were those suffering from a mental illness, those with difficulties in communication, and those who did not wish to participate in the study.
Uncertainty in illness
Uncertainty occurs when an appropriate subjective interpretation of an illness or event is not formed. This study measured uncertainty using Mishel’s Uncertainty in Illness Scale (MUIS), which is composed of 33 items concerning uncertainty in illness. Mishel [ 19 ] originally developed the scale, and it has been translated into Korean by Lee [ 28 ]. The MUIS is a self-administered survey, with items scored on a 5-point scale from 5 ( strongly agree ) to 1 ( strongly disagree ). Positive items were measured backward, so that total scores ranged from 33 to 165. Higher scores indicated higher rates of uncertainty. The Cronbach’s α for the original 33-item tool was .91–.93; the Cronbach’s α for the tool used in the study of Korean breast cancer patients [ 29 ] was .83. In this study, the Cronbach’s α for the uncertainty scale was .88.
Social support
Social support was measured using Zimet et al.’s [ 30 ] Multidimensional Scale of Perceived Social Support (MSPSS). This 12-item measure is scored on a 7-point, Likert-type scale, and assessed the three dimensions of family, friends, and significant others. Its sub-domains are composed of four items. Overall social support scores are calculated by summing the scores for each item, with higher scores indicating higher levels of social support. At the time of development, the Cronbach’s α reliability was .91; Cronbach’s α for each subscale ranged from .90–.95. In this study, the Cronbach’s α of the social support scale was .95.
Quality of life
Quality of life was measured using a standardized tool that was translated into the Korean and verified validity of the Korean version of the EORTC QLQ-C30, which was developed through a process of international joint study from multiple countries, and it is the most widely used standardized tool to measure the quality of life of cancer patients. This tool is composed of three subdomains and 30 items. It includes two items on overall quality of life and five functional domains (i.e., physical, role, cognitive, emotional, and social functions) that include 15 items; three symptom domains (i.e., fatigue, pain, nausea/vomiting) that include seven items; and one item for each of the symptoms commonly reported by cancer patients (i.e., difficulties in breathing, loss of appetite, sleeping disorders, constipation, diarrhea, and financial hardship) [ 31 ]. The EORTC QLQ-C30 is converted into a score ranging between 0 and 100 points [ 31 ]; higher overall quality of life scores, higher functional domain scores, and lower symptom domain scores indicate higher quality of life. Moreover, overall quality of life can be understood as a measurement of comprehensive quality of life [ 31 ]. This study assess quality of life using the overall quality of life score. At the time of development, Cronbach’s α was .65–.73; the Cronbach’s α for the overall quality of life score in this study was .853.
Ethical considerations
This study was conducted after receiving approval of the research protocol from the Institutional Review Board (Approved number: 2014-L02–01). The purpose and method of the research was explained directly to participants by a trained research assistant. The participants then signed an informed consent form that stated the survey would be used for the purposes of the study only, and that their confidentiality would be safeguarded. The subjects who agreed to participate in the survey received a small amount of goods worth of KRW 3000, but there were no factors that could interfere with the answers in the survey.
Data analysis
The collected data were coded and analyzed using SPSS software (version 24.0; SPSS Inc., Chicago, IL) at .05 significance level. The analysis excluded missing data values. The general characteristics, illness-related characteristics, uncertainty, social support, and quality of life were measured using frequency, percentages, means, and standard deviations. The differences in uncertainty, social support, and quality of life in accordance with general and illness-related characteristics were analyzed using independent t -tests and one-way analysis of variance (ANOVA). Tukey’s post-hoc analysis was used for independent variables of more than three groups to identify which group contained the differences. Pearson’s correlation coefficient was calculated to identify correlations between uncertainty, social support, and quality of life. Multiple regression (stepwise method) was used to test the influence of uncertainty on social support and quality of life. To verify the mediating effects of social support in the relationship between uncertainty and quality of life, simple, and hierarchical multiple regression analyses were conducted as per the method proposed by Baron and Kenny [ 30 ]. The significance of the mediating effects of social support was verified using the Sobel test.
Demographic characteristics of subjects
The general characteristics of the female cancer survivors in this study indicated that their average age was 51.87 ( SD = 11.78) years, with the largest proportion (33.3%) of the population being in their 50s, followed by those in their 40s (30.1%), 60s and over (24.4%), and below 30 (12.2%). Most participants (76.2%) were married; 61.9% practiced a religion, and 66.7% had a high school education or below; 56.2% had jobs; 17.8% had lost their jobs as a result of their cancer diagnosis and treatment, and 73.1% indicated that their satisfaction with their financial status was average. They had an average of 2.26 ( SD = 1.19) children; 94.4% were non-smokers; 64.1% were non-drinkers; 29.1% of the subjects had breast cancer and 70.9% had thyroid cancer; 53.8% of the cancers were early stage, and 46.2% were advanced. The duration after cancer treatment averaged 17.64 ( SD = 31.30) months, with 70.3% reporting a duration of less than a year since they ended their treatment (Table 1 ).
Uncertainty in illness, social support, and quality of life
Average scores of uncertainty in illness, social support, and quality of life are given in Table 2 . The average uncertainty in illness score was 83.06 ( SD = 15.29; range: 44–127 points), and the average quality of life score was 66.90 ( SD = 20.32; range: 0–100 points). Average social support score was 62.62 ( SD = 17.09; range: 12–84 points), with family support being the highest (mean = 21.84, SD = 6.58), followed by support from significant others (mean = 21.28, SD = 5.93) and friends (mean = 19.45, SD = 6.70).
Differences in uncertainty in illness, social support, and quality of life according to general and illness-related characteristics
The results of the analysis of differences in uncertainty in illness, social support, and quality of life according to general and illness-related characteristics are given in Tables 3 and 4 . There were significant differences in uncertainty in illness by educational level ( t = 4.048, p < .001), satisfaction with financial status ( F = 3.760, p = .027), and smoking ( t = 2.195, p = .030). Uncertainty in illness was higher for subjects with less than a high school education, compared to those who had a university degree or higher, when they were dissatisfied with their financial status. Likewise, it was higher for smokers, compared to non-smokers.
Social support had statistically significant differences given satisfaction with financial status ( F = 5.151, p = .007) and duration since cancer treatment completion ( F = 4.292, p = .015). Social support was higher for subjects with average financial status satisfaction, and for subjects for whom it had been less than a year, or between 1 to 5 years, since they completed cancer treatment.
Quality of life significantly differed according to financial status satisfaction ( F = 6.648, p = .002). Participants had higher quality of life when they had high or average financial status satisfaction compared to dissatisfaction.
Correlation between uncertainty in illness, social support, and quality of life
The results of the correlation analyses indicated that uncertainty in illness had a significant negative correlation with social support ( r = −.335, p < .001) and quality of life ( r = −.312, p < .001); social support had a significant positive correlation with quality of life ( r = .321, p < .001). Correlations between the sub-factors of social support and quality of life indicate that there were significant positive correlations between quality of life and support from significant others ( r = .315, p < .001), friends ( r = .284, p = .001), and family ( r = .265, p = .001). Uncertainty and support from significant others ( r = −.326, p = .001), friends ( r = −.294, p = .002), and family ( r = −.244, p = .010) showed significant negative correlations; particularly, the highest correlation was between support from significant others and uncertainty (Table 5 ).
Mediating effect of social support
Four stages of regression analysis were conducted to verify whether social support had mediating effects in the process by which uncertainty in illness influenced quality of life. Prior to verifying the mediating effects of social support, this study examined the multicollinearity between variables. The residual limit was between 0.8–1.0, which is higher than 0.1; and the value of the variance inflation factor was between 1.0–1.2, which was lower than 10, indicating no issues with multicollinearity. Moreover, the Durbin-Watson test, which is the test of independence of residual error, indicated d = 1.903–1.944, which was close to two and met the independence condition, representing no issues with self-correlation.
Using the hierarchy regression, this confirmed the partial mediating effects of social support in the process of uncertainty influencing quality of life (Table 6 , Fig. 2 ). The first regression analysis indicated that the independent variable (uncertainty) had a statistically significant influence on the mediator variable (social support; β = − 0.335, p < .001), and the explanatory power for social support was 10.4%. The second stage regression analysis indicated that the mediator variable (social support) had a significant influence on the dependent variable (quality of life; β = 0.321, p < .001), and the explanatory power for quality of life was 9.7%. The third stage regression analysis indicated that the independent variable (uncertainty) had a significant influence on the dependent variable (quality of life; β = − 0.312, p = .001) with an explanatory power of 8.9%. At the fourth stage, this study aimed to test the influence of the independent variable (uncertainty) on the dependent variable (quality of life) with social support as the mediator variable. The results indicated that uncertainty (β = − 0.241, p = .014) and social support (β = 0.213, p = .030) were significant predictors of quality of life. When social support was set as the mediator variable, uncertainty was found to have a significant influence on quality of life; the unstandardized regression coefficient reduced from − 0.396 to − 0.398, indicating a partial mediation of social support. The explanatory power of these variables in terms of quality of life was 12.1%. This study executed the Sobel test to verify the significance of the mediating effects of social support, confirming that they were significant in the relationship between uncertainty and quality of life.
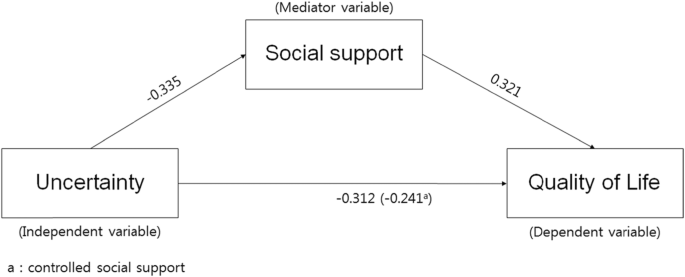
Model showing the influence of uncertainty on quality of life and the mediating effect of social support
Mullen divided the stages of cancer survival into three major classifications [ 32 , 33 ]. First is the acute stage, which marks the period after the cancer diagnosis. Second is the extended stage, in which the active treatment of cancer has ended and the patient is placed under tracking observation or engages in intermittent treatment. During this period, the majority of cancer survivors experience uncertainty toward their cancer treatment and fear recurrence, and they may experience physical and psychological issues. Lastly, the permanent stage marks a period in which the cancer is thought to be fully cured, or the patient is expected to survive long term, with a low risk of recurrence.
The participants in this study averaged a score of 66.90 for quality of life. As it is difficult to draw a direct comparison given the lack of research utilizing this measure, in converting quality of life into a scale of 100 points, this study’s results were similar to those found previously regarding post-hoc management following breast cancer treatment for 200 women [ 8 , 10 ]. However, a study covering regionally based, adult female breast cancer survivors between 6 months and 2 years after anti-cancer treatment completion reported lower scores (e.g., 60.13 points) compared to this study [ 27 ]. Likewise, a study of breast cancer survivors with completed surgeries and assistive treatments, breast cancer survivors whose treatment had ended had scores of 53.4 and 56.66 points, respectively for breast cancer survivors with completed surgeries and assistive treatments [ 25 , 26 ].
On the other hand, a report of 110 adult females with breast cancer or OB/GYN cancers [ 33 ] indicated that quality of life according to cancer survival stage was 58.7, 62.3, and 66.8 points during the acute, extended, and permanent stages, respectively. Quality of life in this study was similar to the level experienced by survivors during the permanent stage. Considering that the average time since treatment was 17.64 months, these results indicate a relatively high quality of life. While these differences cannot be accurately compared and discussed because of the lack of research covering the same variables, the majority of survivors had thyroid cancer (70.9%), and it is known that thyroid cancer has higher rates of survival. Going forward, it is important to develop interventions to improve quality of life by assessing survivors’ specific stages.
There were no significant relationships between quality of life and length of time since completing treatment. Existing research has suggested that quality of life was significantly higher for those surviving more than 5 years after cancer treatment completion [ 8 , 33 ], indicating that quality of life improves as duration of survival increases. The quality of life of these cancer survivors has been reported to improve with the passage of time [ 8 , 33 ]. Therefore, cross-sectional and longitudinal studies are required in the future to identify quality of life by survival stage and changes in quality of life over time.
On the other hand, qualitative studies of Korean female cancer survivors have indicated that the significant others and families of female cancer survivors wanted them to return to their pre-cancer lives to take care of their spouses and children, indicated the demands on female cancer survivors in Korea to fulfill their roles as wives and mothers before fully recovering from cancer [ 33 ]. Thus, customized interventions by survival stage for female cancer survivors are needed along with further research on the relationships between cultural specificity, role conflicts imposed on survivors because they are women, and their quality of life.
The uncertainty toward illness of the participants in this study was similar to existing research in breast cancer patients undergoing chemotherapy averaged 83.08 [ 34 ] and female thyroid cancer patients [ 35 ]. On the other hand, the level of uncertainty faced by cancer patients prior to surgery averaged 81.43 in a study of cancer patients hospitalized for breast, thyroid, and bladder cancer [ 36 ], which was slightly lower than the value found in this study. This appears to be because female cancer survivors in this study were mostly in the extended stage, which comes after the active treatment of their cancer [ 32 , 33 ]. Most cancer survivors face uncertainty toward cancer treatment and fear of recurrence [ 8 , 32 , 33 ]; thus, they experience a diverse range of physical and psychological problems [ 6 , 7 ]. On the other hand, a qualitative study of 25 breast cancer survivors aged over 30 who had undergone surgery and chemotherapy as their primary treatment for breast cancer [ 37 ] indicated that quality of life following treatment for breast cancer survivors saw a coexistence of anxiety and uncertainty about recurrence. A shorter duration of time since treatment led to higher confusion in their own health management efforts and health management in general.
These results indicate that there are limitations to comparing uncertainty results given the lack of domestic studies on cancer survivors; therefore, future studies are needed to fill this gap. Moreover, it is necessary to confirm uncertainty by cancer survival stage and develop interventions to reduce the uncertainty accompanying each stage.
Uncertainty in illness was higher for those with less than a high school education, compared to those with a university education or higher, when they were dissatisfied with their financial status, and for those who were smokers. These results were similar to previous research [ 36 ], which indicated high uncertainty for participants over 60 who had a low monthly income and low level of education. Therefore, it is necessary to consider these socioeconomic factors when developing uncertainty reducing strategies such as customized information delivery and communication.
The social support of female cancer survivors in this study was rather high, at 62.62 out of 84 points; family support was the highest, followed by support from significant others, and finally friends. Social support is known to play an important role in helping individuals reduce their levels of uncertainty [ 37 ]. Particularly, in Korea, family and healthcare professional support have been the most important support resources among all social support types [ 34 ]. The results of this study indicated that family support was the highest, which was in line with the results of existing studies. On the other hand, a qualitative study of 25 breast cancer survivors aged over 30 who had undergone surgery and chemotherapy as their primary breast cancer treatments [ 37 ] indicated that positive support and responses from family, patients with similar illnesses, and those surrounding them helped to strengthen positive self-suggestion, which also helped them to overcome their illnesses. Other studies have reported that patients undergoing treatment receive active support from healthcare professionals and their family, but they receive less support and interest from healthcare professionals, their family, and those surrounding them after the treatment ends [ 22 , 23 , 27 ]. Therefore, it is necessary to take a continuous interest in and facilitate social support for cancer survivors.
Social support was higher for participants with average satisfaction toward their financial status, and for those for whom less than a year, or between 1 and 5 years, had passed since the completion of their cancer treatment, compared to those for whom 5 years or more had passed since treatment. These results were similar to those of studies on cancer patients hospitalized for breast, thyroid, and bladder cancer surgery [ 36 ], which indicated social support differed according to the time that had passed since diagnosis. Moreover, these results are similar to those reporting that breast cancer survivors are required by their spouses or family to fulfill roles they had filled prior to their cancer diagnosis, and this was associated with decreasing support from family [ 38 ]. These results indicate that female cancer survivors require ongoing psychosocial support as well as education and access to information as they live out their lives.
According to Baik and Lim [ 20 ], who studied social support according to different stages of breast and gynecological cancer survival, the social support of patients in the acute stage was comparatively higher, but there were no significant differences in social support across the different stages, which was different from the findings of this study. While there were no significant differences, Baik and Lim [ 20 ] reported that the social support perceived by survivors decreased as they proceeded through the acute to the extended stage. The social support perceived by respondents decreased in the 2 years following the diagnosis but maintained the reduced rate through the permanent stage [ 20 ]. Long-term survivors had a greater need to meet other cancer patients and self-help groups [ 20 ]. Kwon and Yi [ 27 ] asserted that interest and support from family and the society in general are very important in raising breast cancer survivors’ quality of life and survival rates. Moreover, self-help groups were reported to be effective in providing emotional support for long- and short-term cancer survivors [ 39 ], which indicates the need for developing stage-specific social support interventions and various methods of facilitating social support groups. Moreover, further research is required concerning cancer survival stage-dependent social support and quality of life.
The results of this study showed that higher uncertainty in illness among female cancer survivors led to reduced social support and quality of life, while higher social support led to better quality of life. Support from others was found to be the most relevant aspect of the relationship between quality of life and uncertainty. These results were similar to those of studies on early-stage breast cancer patients [ 40 ] and on cancer patients hospitalized for breast, thyroid, and bladder cancer surgery [ 36 ], which indicated that perceived social support was lower as uncertainty increased.
Uncertainty was very influential on female cancer survivors’ quality of life. Higher uncertainty in illness among female cancer survivors led to lower social support and reduce quality of life; higher social support led to improved quality of life. The explanatory power of these variables on quality of life was 12.1%; uncertainty in illness and social support influenced the quality of life of female cancer survivors. Moreover, in the process of uncertainty in illness influencing subjects’ quality of life, social support was confirmed to play a significant, partially mediating, role in the relationship between uncertainty and quality of life. Higher uncertainty toward illness led to lower quality of life, higher social support led to higher quality of life, and social support influenced female cancer survivors’ quality of life by partially mediating its relationship with uncertainty. Social support plays an important role in directly and indirectly reducing uncertainty [ 18 , 19 ]. Social support is closely related to the prognosis of breast cancer survivors [ 21 ]. Uncertainty among breast cancer survivors has been found to lower their quality of life; however, social support has been found to improve quality of life [ 11 ]. Thus, the need for a diverse range of attempts, including developing and applying social support programs, to increase cancer survivors’ quality of life exists. On the other hand, the partially mediating effects of social support indicate that there are other mediating factors in uncertainty in illness’s influence on quality of life. Therefore, it is important for future studies to include other mediating factors in their examinations of what influences quality of life among female cancer survivors.
In Korea, studies on cancer survivors have been conducted since 2010, and the majority of these focused on breast cancer survivors. Particularly, as there has been no overall research into the healthy behaviors of cancer survivors, it is necessary to develop practical guidelines that befit Korea through studies concerning the development and application of health improvement programs based on the study of healthy behaviors, as per the assertion of Kim [ 32 ]. Moreover, attempts are needed to practically apply a diverse range of intervention studies to improve cancer survivors’ quality of life.
Moreover, future studies should include mediator variables other than social support that might influence quality of life. Additionally, both cross-sectional and longitudinal studies are needed to further investigate the quality of life and uncertainty according to the stages of survival.
Our results show that social support partial mediates the relationship between uncertainty and quality of life in female cancer survivors. The results of this study have great implications for improving cancer care, especially in how it relates to quality of life, and they also demonstrate how uncertainty can be decreased. Therefore, it is necessary to develop and apply intervention methods to improve social support thereby improving quality of life among female cancer survivors. A nurse-led social support program may especially contribute to enhancing the quality of life of cancer survivors by providing them with adequate health information and emotional support.
Availability of data and materials
The data used in this study were collected through questionnaires and analyzed by coding the original data. The datasets generated and/or analysed during the current study are not publicly available due [REASON WHY DATA ARE NOT PUBLIC] but are available from the corresponding author on reasonable request.
Abbreviations
Mishel’s Uncertainty in Illness Scale
Multidimensional Scale of Perceived Social Support
European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30
Obstetrics/Gynaecology
Lee JE, Shin DW, Cho BL. The current status of cancer survivorship care and a consideration of appropriate care model in Korea. Korean J Clin Oncol. 2014;10(2):58–62. https://doi.org/10.14216/kjco.14012 .
Article Google Scholar
Yoon HJ, Seok JH. Clinical factors associated with quality of life in patients with thyroid cancer. J Korean Thyroid Assoc. 2014;7(1):62–9. https://doi.org/10.11106/jkta.2014.7.1.62 .
Dow KH, Ferrell BR, Leigh S, Ly J, Gulasekaram P. An evaluation of the quality of life among long-term survivors of breast cancer. Breast Cancer Res Treat. 1996;39(3):261–73. https://doi.org/10.1007/BF01806154 .
Article CAS PubMed Google Scholar
Ferrell BR, Dow KH. Quality of life among long-term cancer survivors. Oncology. 1997;11:565–8.
CAS PubMed Google Scholar
Lee JE, Goo A, Lee KE, Park DJ, Cho B. Management of long-term thyroid cancer survivors in Korea. J Korean Med Assoc. 2016;59(4):287–93. https://doi.org/10.5124/jkma.2016.59.4.287 .
Article CAS Google Scholar
Molassiotis A, Yates P, Li Q, So WK, Pongthavornkamol K, Pittayapan P, et al. Mapping unmet supportive care needs, quality-of-life perceptions and current symptoms in cancer survivors across the Asia-Pacific region: results from the international STEP study. Ann Oncol. 2017;28(10):2552–8. https://doi.org/10.1093/annonc/mdx350 .
Yun YH, Kim YA, Sim JA, Shin AS, Chang YJ, Lee J, et al. Prognostic value of quality of life score in disease-free survivors of surgically-treated lung cancer. BMC Cancer. 2016;16(1):505–14. https://doi.org/10.1186/s12885-016-2504-x .
Article PubMed PubMed Central Google Scholar
Park JH, Jun EY, Kang MY, Joung YS, Kim GS. Symptom experience and quality of life in breast cancer survivors. J Korean Acad Nurs. 2009;39(5):613–21. https://doi.org/10.4040/jkan.2009.39.5.613 .
Article PubMed Google Scholar
Chae YR. Relationships of perceived health status, depression, and quality of life of breast cancer survivors. J Korean Adult Nurs. 2005;17:119–27.
Google Scholar
Kim YS, Tae YS. The influencing factors on quality of life among breast cancer survivors. J Korean Oncol Nurs. 2011;11(3):221–8. https://doi.org/10.5388/jkon.2011.11.3.221 .
Sammarco A, Konecny LM. Quality of life, social support, and uncertainty among Latina breast cancer survivors. Oncol Nurs Forum. 2008;35(5):844–9. https://doi.org/10.1188/08.ONF.844-849 .
Liao MN, Chen MF, Chen SC, Chen PL. Uncertainty and anxiety during the diagnostic period for women with suspected breast cancer. Cancer Nurs. 2008;31(4):274–83. https://doi.org/10.1097/01.NCC.0000305744.64452.fe .
Dirksen S, Erickson J. Well-being in Hispanic and non-Hispanic Caucasian survivors of breast cancer. Oncol Nurs Forum. 2002;29(5):820–6. https://doi.org/10.1188/02.ONF.820-826 .
Husson O, Haak HR, Buffart LM, Nieuwlaat WA, Oranje WA, Mols F, et al. Health-related quality of life and disease specific symptoms in long-term thyroid cancer survivors: a study from the population-based PROFILES registry. Acta Oncol. 2013;52(2):249–58. https://doi.org/10.3109/0284186X.2012.741326 .
Lee JI, Kim SH, Tan AH, Kim HK, Jang HW, Hur KY, et al. Decreased health-related quality of life in disease-free survivors of differentiated thyroid cancer in Korea. Health Qual Life Outcomes. 2010;8(1):101. https://doi.org/10.1186/1477-7525-8-101 .
Yang J, Yi M. Factors influencing quality of life in thyroid cancer patients with thyroidectomy. Asian Oncol Nurs. 2015;15(2):59–66. https://doi.org/10.5388/aon.2015.15.2.59 .
Oh KS. Social support as a prescription theory. J Nurs Query. 2006;15(1):134–54.
Lien CY, Lin HR, Kuo IT, Chen ML. Perceived uncertainty, social support and psychological adjustment in older patients with cancer being treated with surgery. J Clin Nurs. 2009;18(16):2311–9. https://doi.org/10.1111/j.1365-2702.2008.02549.x .
Mishel MH. Uncertainty in illness. Image J Nurs Sch. 1988;20(4):225–32. https://doi.org/10.1111/j.1547-5069.1988.tb00082.x .
Baik OM, Lim J. Social support in Korean breast and gynecological cancer survivors: comparison by the cancer stage at diagnosis and the stage of cancer survivorship. Korean J Family Soc Work. 2011;32(6):5–35.
Nausheen B, Kamal A. Familial social support and depression in breast cancer: an exploratory study on a Pakistani sample. Psychooncology. 2007;16(9):859–62. https://doi.org/10.1002/pon.1136 .
Alfano CM, Rowland JH. Recovery issues in cancer survivorship: a new challenge for supportive care. Cancer J. 2006;12(5):432–43. https://doi.org/10.1097/00130404-200609000-00012 .
Ashing KT, Padilla G, Tejero JS, Kagawa-Singer M. Understanding the breast cancer experience of Asian American women. Psychooncology. 2003;12(1):38–58. https://doi.org/10.1002/pon.632 .
Janz N, Mujahid M, Chung L, Lantz P, Hawley S, Morrow M, et al. Symptom experience and quality of life of women following breast cancer treatment. J Women's Health. 2007;16(9):1348–61. https://doi.org/10.1089/jwh.2006.0255 .
Min HS, Park SY, Lim JS, Park MO, Won HJ, Kim JI. A study on behaviors for preventing recurrence and quality of life in breast cancer survivors. J Korean Acad Nurs. 2008;38(2):187–94. https://doi.org/10.4040/jkan.2008.38.2.187 .
Kim GD. Impact of climacteric symptoms and fatigue on the quality of life in breast cancer survivors: the mediating effect of cognitive dysfunction. Asian Oncol Nurs. 2014;14(2):58–65. https://doi.org/10.5388/aon.2014.14.2.58 .
Kwon EJ, Yi M. Distress and quality of life in breast cancer survivors in Korea. Asian Oncol Nurs. 2012;12(4):289–96. https://doi.org/10.5388/aon.2012.12.4.289 .
Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–82. https://doi.org/10.1037/0022-3514.51.6.1173 .
Lee I. Uncertainty, appraisal and quality of life in patients with breast cancer across treatment phases. J Cheju Halla Collage. 2009;33:94–112.
Zimet GD, Powell SS, Farley GK, Werkman S, Berkoff KA. Psychometric characteristics of the multidimensional scale of perceived social support. J Pers Assess. 1990;55(3–4):610–7. https://doi.org/10.1080/00223891.1990.9674095 .
Fayers PM, Aaronson NK, Bjordal K, Groenvold M, Curran D, Bottomley A, On behalf of the EORTC Quality of Life Group. EORTC QLQ-C30 scoring manual. 3rd ed. Brussels: European Organization for Research and Treatment of Cancer; 2001. p. 2001.
Kim SH. Understanding cancer survivorship and its new perspectives. J Korean Oncol Nurs. 2010;10(1):19–29.
Lim J, Han I. Comparison of quality of life on the stage of cancer survivorship for breast and gynecological cancer survivors. Korean J Soc Welf. 2008;60(1):5–27. https://doi.org/10.20970/kasw.2008.60.1.001 .
Ahn JY. The influence of symptoms, uncertainty, family support on resilience in patients with breast cancer receiving chemotherapy [master’s thesis]. Seoul: The Seoul National University; 2014.
Lee I, Park CS. Convergent effects of anxiety, depression, uncertainty, and social support on quality of life in women with thyroid cancer. J Korea Convergence Soc. 2017;8(8):163–76. https://doi.org/10.15207/JKCS.2017.8.8.163 .
Park YJ. Uncertainty, anxiety and social support among preoperative patients of cancer: A correlational study [master’s thesis]. Seoul: The Seoul National University; 2015.
Yun M, Song M. A qualitative study on breast cancer survivors’ experiences. Perspect Nurs Sci. 2013;10:41–51.
Ashing-Giwa KT. The contextual model of HRQoL: a paradigm for expanding the HRQoL framework. Qual Life Res. 2005;14(2):297–307. https://doi.org/10.1007/s11136-004-0729-7 .
Adamsen L, Rasmussen J. Sociological perspectives on self-help groups: reflections on conceptualisation and social processes. J Adv Nurs. 2001;35(6):909–17. https://doi.org/10.1046/j.1365-2648.2001.01928.x .
Kim HY, So HS. A structural model for psychosocial adjustment in patients with early breast cancer. J Korean Acad Nurs. 2012;42(1):105–15. https://doi.org/10.4040/jkan.2012.42.1.105 .
Download references
Acknowledgements
We would like to thank Dr. Min, a physician of the Endocrine surgery at Cheju Halla Hospital, for collecting the data. And, we would like to thank the IRB who approved the research and the Editage Company who edited the English.
Conflict of interest
“No conflict of interest has been declared by the author(s).”
The authors did not receive any financial support for this study. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
“This research did not received any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.”
Author information
Authors and affiliations.
Department of Nursing, Changwon National University, C.P.O. Box 51140, Changwon, Republic of South Korea
Division of Nursing, Cheju Halla University, Jeju, Republic of South Korea
Changseung Park
You can also search for this author in PubMed Google Scholar
Contributions
IL designed the study, searched the literature, analyzed the data, conducted the interpretation of data, drafted and edited the manuscript, and submitted the manuscript for publishing. CSP collected the data, searched the literature, and critically revised the initial manuscript. And all authors read and approved the final manuscript.
Corresponding author
Correspondence to Insook Lee .
Ethics declarations
Ethics approval and consent to participate.
This study approved by IRB in Cheju Halla Hospital (IRB No. 2014-L02) and informed consent to participate.
Consent for publication
This manuscript has never been published in any other journal, and all authors agree to be published in this journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Lee, I., Park, C. The mediating effect of social support on uncertainty in illness and quality of life of female cancer survivors: a cross-sectional study. Health Qual Life Outcomes 18 , 143 (2020). https://doi.org/10.1186/s12955-020-01392-2
Download citation
Received : 08 December 2019
Accepted : 05 May 2020
Published : 19 May 2020
DOI : https://doi.org/10.1186/s12955-020-01392-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Health and Quality of Life Outcomes
ISSN: 1477-7525
- Submission enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Cancers (Basel)

Breast Cancer—Epidemiology, Classification, Pathogenesis and Treatment (Review of Literature)
Beata smolarz.
1 Laboratory of Cancer Genetics, Department of Pathology, Polish Mother’s Memorial Hospital Research Institute, Rzgowska 281/289, 93-338 Lodz, Poland; lp.pw@zciwonamor-annah
Anna Zadrożna Nowak
2 Department of Chemotherapy, Medical University of Lodz, Copernicus Memorial Hospital, 93-513 Lodz, Poland; [email protected]
Hanna Romanowicz
Simple summary.
Breast cancer is the most-commonly diagnosed malignant tumor in women in the world, as well as the first cause of death from malignant tumors. The incidence of breast cancer is constantly increasing in all regions of the world. For this reason, despite the progress in its detection and treatment, which translates into improved mortality rates, it seems necessary to look for new therapeutic methods, predictive and prognostic factors. The article presents a review of the literature on breast carcinoma - a disease affecting women in the world.
Breast cancer is the most-commonly diagnosed malignant tumor in women in the world, as well as the first cause of death from malignant tumors. The incidence of breast cancer is constantly increasing in all regions of the world. For this reason, despite the progress in its detection and treatment, which translates into improved mortality rates, it seems necessary to look for new therapeutic methods, and predictive and prognostic factors. Treatment strategies vary depending on the molecular subtype. Breast cancer treatment is multidisciplinary; it includes approaches to locoregional therapy (surgery and radiation therapy) and systemic therapy. Systemic therapies include hormone therapy for hormone-positive disease, chemotherapy, anti-HER2 therapy for HER2-positive disease, and quite recently, immunotherapy. Triple negative breast cancer is responsible for more than 15–20% of all breast cancers. It is of particular research interest as it presents a therapeutic challenge, mainly due to its low response to treatment and its highly invasive nature. Future therapeutic concepts for breast cancer aim to individualize therapy and de-escalate and escalate treatment based on cancer biology and early response to therapy. The article presents a review of the literature on breast carcinoma—a disease affecting women in the world.
1. Epidemiology
Breast cancer is the most common malignant tumor in women in the world. Breast cancer patients account for as much as 36% of oncological patients. An estimated 2.089 million women were diagnosed with breast cancer in 2018 [ 1 , 2 ]. The incidence of this malignant tumor is increasing in all regions of the world, but the highest incidence occurs in industrialized countries. Almost half of the cases on a global scale are in developed countries [ 2 , 3 ]. This trend is mainly due to the so-called Western lifestyle, associated with a poor diet, nicotinism, excessive stress and little physical activity [ 3 ]. In the case of breast cancer, mammography has become recognized as screening. The greatest value of mammography is observed in the group of women aged 50–69 years [ 1 , 3 ]. Classical mammography is characterized by 75–95% sensitivity and specificity at the level of 80–95% [ 4 ]. For women with suspected hereditary breast cancer, magnetic resonance mammography is used as a screening test. If a suspicious lesion is found in mammography, an ultrasound examination is performed and, if necessary, a thick needle biopsy along with a histopathological examination of the tumor.
In 2018, there were 234,087 cases of breast cancer in the United States (crude rate: 85/105), 55,439 in the United Kingdom (crude rate: 94/105), 56,162 in France (crude rate: 99/105), 71,888 in Germany (crude rate: 85.4/105) and 66,101 in Japan (crude rate: 58/105) [ 2 ]. The highest incidence rate in the world is found in Belgium (crude rate: 113/105), and among the continents—in Australia (crude rate: 94/105) [ 2 ]. In Poland, breast cancer is also the most-commonly diagnosed malignant tumor in women. There is a steady increase in cases (1990, 8000 new cases; 2018, 20,203 new cases) [ 2 ]. The average incidence rate in Europe is 84/105 [ 2 ]. The lowest incidence occurs in the countries of Southeast Asia and Africa, where the standardized incidence rate does not exceed 25/105 [ 2 ]. The lowest incidence rates in 2018 were recorded in Bhutan (crude rate: 5/105) and the Republic of The Gambia (crude rate: 6.5/105) [ 2 ]. Despite the greater effectiveness of initial diagnostics or the rapid development of pharmacotherapy in recent years, breast cancer is the first cause of death from malignant tumors in women in the world. In 2018, 626,679 people died from breast cancer. Unlike morbidity, the highest mortality from this malignant tumor is recorded in developing countries [ 2 ] (Fiji, crude rate 36/105, highest rate; Somalia, crude rate 29/105; Ethiopia, crude rate 23/105; Egypt, crude rate 21/105; Indonesia, crude rate 17/105; Papua New Guinea, crude rate 25/105) [ 2 ], in which as much as 60% of all deaths from breast cancer occur. This trend is mainly related to the lack of screening, which is less than in developed countries, the availability of diagnostics and modern methods of treatment [ 5 ]. In contrast, the standardized death crude rate in Belgium 16.3/105, in the United States 13/105, and in Japan 9.3/105 [ 2 ]. The number of breast cancer cases in Poland is much lower than in EU countries (in 2013, the standardized incidence rate for Polish—51.8, for the EU 106.6) [ 6 ]. The incidence of adult premenopausal women (20–49 years) has almost doubled over the past 30 years. Unfortunately, Polish women are still not very sensitive to prevention. They neglect their breasts and underestimate the importance of regular check-ups. Compared to other European countries, Polish women have a low incidence of preventative care—in the Netherlands, 80% of women report free mammogram prevention programs, in England 71%, and in Poland only 44% [ 6 ]. The percentage of 5-year survival due to breast cancer in Poland is 78.5%, differing significantly from, for example, the result of 90% achieved in the United States [ 7 ].
2. Risk Factors
The unambiguous cause of carcinogenesis has not yet been established, but several risk factors conducive to the development of breast cancer are known. One of the most important, as also indicated by the epidemiological data described above, are the gender, age, and degree of economic development of a given country. No less important are hormonal factors, mainly related to the time of exposure to estrogens, procreative factors, including the number of children born, the age of birth of the first child, or breastfeeding. Great importance in the development of breast cancer is attributed to genetic factors, the use of hormone replacement therapy, improper diet, and the resulting obesity. Among the significant risk factors for the development of breast cancer, hormonal contraception, alcohol consumption and exposure to ionizing radiation at a young age are also mentioned. Risk factors for breast cancer are presented in Table 1 .
Risk factors for breast cancer [ 8 ].
The vast majority of cases of breast cancer, reaching 99%, occur in women. Only 1% of cases of this malignant tumor affect men, for which the standardized incidence rate in Poland is 0.4/105. No more than 100 cases are reported each year [ 6 ]. However, the incidence of breast cancer in men, like that in women, shows a steady upward trend, which is most likely associated with obesity and longer survival [ 9 ].
Age is one of the most important risk factors for breast cancer. The global increase in the incidence of breast cancer is observed in all age groups and is highest in women under 50 years of age [ 9 ]. Although this malignant tumor is rare in this age group, it remains a significant clinical and social problem, due to its worse course—numerous studies indicate that breast cancer in young women is characterized by greater histological malignancy, marginal expression of steroid receptors, frequent overexpression of the HER-2 receptor or occurs as a molecular biological subtype “basal-like” (“triple negative”) [ 10 ]. Furthermore, the incidence of breast cancer in premenopausal women is increasing—within 30 years it has increased almost 2-fold [ 6 ].
2.3. Degree of Economic Development
As mentioned in the paragraph on epidemiology, the incidence of breast cancer and mortality from this malignant tumor is related to the economic development of a country. This relationship has been documented in many studies [ 3 , 11 , 12 ].
The incidence of breast cancer is increasing worldwide due to the continuous growth of the population and the ageing of the population [ 11 ]. The highest incidence rates are recorded in developed countries [ 3 , 11 , 12 ]. This phenomenon results from the so-called “Western lifestyle” described above. At the same time, it seems that soon the trend of high morbidity will also occur in developing countries. In these countries, along with economic development, access to public health care becomes easier, prevention and screening programs are introduced (which increases detection), maternal, infant and child mortality decreases [ 12 ]. On the other hand, the importance of factors conducive to the development of breast cancer is growing, such as late first birth, low number of babies born, use of hormone replacement therapy, obesity, lack of physical activity, or improper diet [ 11 , 12 ]. Currently, however, lower middle- and low-income countries are dominated by higher breast cancer mortality rates than in developed countries, despite lower incidence [ 3 , 5 , 11 , 12 ]. This trend is associated with frequent diagnosis of cancer at an advanced stage, which results from the lack of resources for the effective implementation of primary prevention programs, diagnostic tests (primarily mammography), and finally modern methods of treatment [ 5 , 11 , 12 ].
Approximately 645,000 cases of premenopausal breast cancer and 1.4 million cases of postmenopausal breast cancer were diagnosed worldwide in 2018, with more than 130,000 and 490,000 deaths in each menopausal group, respectively. Proportionally, countries with a low UNDP Human Development Index (HDI) faced a higher burden of premenopausal breast cancer for both new cases and deaths compared to higher-income countries [ 13 ]. Countries with very high HDI had the highest incidence of premenopausal and postmenopausal breast cancer (30.6 and 253.6 cases per 100,000, respectively), while countries with low and medium HDI had the highest premenopausal and postmenopausal mortality rates (5 and 53.3 deaths per 100,000, respectively). By studying trends in breast cancer, they noted significantly increasing age-standardized incidence rates (ASIRs) for premenopausal breast cancer in 20 of 44 populations and significantly increasing ASIRs for postmenopausal breast cancer in 24 of the 44 populations. Growth only in premenopausal age occurred mainly in high-income countries, while the increase in the burden of postmenopausal breast cancer was most noticeable in transition countries [ 13 ].
2.4. Hormonal Status
Factors related to a woman’s hormonal status seem to have a huge impact on the risk of developing breast cancer. The results of many studies indicate that the risk of developing breast cancer increases in proportion to the time of exposure to estrogen, which prolongs early menarche, late menopause, the age of birth of the first child and the number of children born [ 9 , 11 , 12 , 13 , 14 ].
Brinton et al. showed that the first menstruation that occurred at or after the age of 15 was associated with a 23% reduction in the risk of breast cancer compared to the first menstruation before the age of 12 (early menarche) [ 15 ]. Currently, it is believed that this reduction is about 30%. In turn, the Collaborative Group on Hormonal Factors in Breast Cancer published in 2012 in The Lancet Oncology the results of a meta-analysis, according to which the relative risk of developing breast cancer increased by 5% with each year of early menarche initiation [ 16 ].
In addition, it was found that early first menstruation was associated with a higher risk of developing breast cancer compared to late menopause—each year of late menopause increased the relative risk by 2.9%, with late menopause being believed to be for, achieved after the age of 54, increases the risk of breast cancer twice compared to the menopause achieved before the age of 45 [ 1 , 16 ].
The meta-analysis also showed that women who had not reached menopause had a higher risk of developing breast cancer compared to postmenopausal women of the same age. In this group of analyzed patients, the effect of the Body Mass Index on the risk of developing the disease was noticed—in premenopausal patients, obesity reduced this risk, and in postmenopausal patients it increased. This metanalysis also found that early menarche was associated with a higher risk of developing lobular breast cancer, as was late menopause [ 16 ]. Late menopause also predisposed to developing steroid-expressed breast cancer [ 16 ].
Other reproductive factors with an effect on breast cancer risk confirmed in numerous studies include the age at which the first child was born, the number of babies born and breastfeeding [ 14 ].
Studies indicate an increased risk of breast cancer in transgender women compared to cisgender men and a lower risk in transgender men compared to cisgender women [ 17 ]. In transgender women, the risk of breast cancer increases during a relatively short period of hormone treatment, and the characteristics of breast cancer are more like a female pattern. The results of the study suggest that guidelines for breast cancer screening for cisgender people are sufficient for transgender people using hormone treatment [ 17 ].
Recent studies indicate that the increased risk of breast cancer is associated with long-term use of estrogen-only therapy and combined estrogen-progestogen therapy [ 18 ]. The combination treatment associated with the least increase in risk is estradiol-dydrogesterone. Research suggests a lower increased risk of breast cancer associated with long-term hormone replacement therapy (HTR) use and a more noticeable decrease in risk after discontinuation of treatment [ 18 ].
2.5. Reproductive and Hormonal Risk Factors in Breast Cancer Patients
Estrogens play an important role in the pathogenesis of the development of breast cancer [ 19 ]. Breast cancer is considered a hormone-dependent tumor in which elevated estrogen levels and longer exposure to this hormone are associated with an increased risk of its development [ 19 ].
This is confirmed by epidemiological studies that increased exposure to endogenous and exogenous estrogens increases the risk of developing breast cancer [ 20 ].
In all postmenopausal women, high serum estrogen levels are associated with an increased risk of breast cancer. Both hormonal factors and reproductive factors are indisputably influencing the increase in the risk of breast cancer [ 20 ]. The duration of exposure to estrogen and the effect of pregnancy determined by parameters such as the age of the first menstrual bleeding, the age of the first pregnancy (especially exposure in women who gave birth to the first child after the age of 30), childlessness, or the age of onset of menopause change the individual risk of breast cancer [ 21 ].
Early onset of menstruation (12 years) and late termination (50 years) increases the risk twice compared to women who started menstruation late (15 years) and ended it early (40 years) [ 22 ].
Also, childlessness and the late age of the first pregnancy (over 30 years of age) are factors associated with prolonged exposure to estrogens [ 23 ].
Nulliparous women and those who became pregnant for the first time after the age of 30 have an increased risk of getting sick 2–5 times more. Spontaneous and artificial miscarriages (incomplete pregnancies) do not confer a protective effect as do full pregnancies, but they may increase the risk due to the lack of protective effect of progesterone in the second phase of pregnancy [ 24 ]. The effect of exogenous estrogen on breast cancer is a controversial issue and continues to be subjected to numerous studies.
The use of HRT is a significant risk factor for breast cancer. The first information on the adverse effects of HRT on the risk of developing breast cancer appeared in the nineteen nineties. In 1997, the Collaborative Group on Hormonal Factors in Breast Cancer published in The Lancet the results of a meta-analysis of 51 studies evaluating the relationship between HRT intake and breast cancer. This meta-analysis found that each year of HRT use increased the risk of breast cancer by 2.7% [ 25 ]. In 2019, the same society republished another meta-analysis in The Lancet, this time of 58 prospective studies evaluating the relationship between the type of HRT and the risk of developing breast cancer. This meta-analysis showed that HRT containing estrogens and progestogens increased the risk of breast cancer to the greatest extent, especially when progestogens were taken daily [ 25 ]. The use of HRT even for a short time (1–4 years) was also associated with an increased risk of breast cancer [ 25 ]. The risk of developing the disease was mainly related to breast cancer with the expression of steroid receptors [ 25 ]. The risk of developing the disease was slightly reduced if HRT was used after the age of 60 [ 24 ]. This risk was also lower for obese women, especially if they took HRT containing only estrogens [ 26 ].
Two-component HRT, used for 5 years from the age of 50, was associated with a 2% increase in the risk of breast cancer over 20 years in patients aged 50–69 (from 6.3% to 8.3%)—it is estimated that 1 in 50 women would develop cancer [ 26 ]. Similarly, the use of HRT with estrogens and progestogens taken intermittently increased the risk of breast cancer from 6.3% to 7.7% (1 in 70 women would get sick). In turn, single-component HRT (only with estrogens) used for 5 years was associated with the lowest increase in the risk of breast cancer in 20 years—from 6.3% to 6.8% (1 in 200 women would get sick) [ 26 ].
The relationship between hormonal contraceptive use and breast cancer risk has been demonstrated in two important papers—a reanalysis of 54 epidemiological studies by the Collaborative Group on Hormonal Factors in Breast Cancer published in The Lancet in 1996, and a prospective cohort study by Mørch et al. presented in the NEJM in 2017 [ 27 , 28 ]. Both studies found that long-term use of hormonal contraception adversely affects the relative risk of breast cancer. This risk was estimated at 1.20 (Danish study) and 1.24 (CGoHFiBC reanalysis). It was higher the longer the subjects took hormonal contraception (1.09 for hormonal contraception used for less than a year vs. 1.38 for women taking contraception for more than 10 years) [ 27 , 28 ].
In addition, this cohort study showed that the relative risk of developing breast cancer was elevated for at least 5 years after the end of hormonal contraception in women who took it for a long time (≥5 years). This trend was not noticed in women who used hormonal contraception for a short time (less than 5 years) [ 28 ].
The relative risk of developing breast cancer was also increased regardless of the type of contraception taken [ 27 ].
2.6. Genetic Factors, Family Occurrence
Only a small group of breast cancer cases (5–10%) are genetic. The best-known genetic mutations associated with this cancer include mutations in the BRCA1 and BRCA2 genes [ 29 ].
The BRCA1 gene, located on chromosome 17, is a suppressor gene that encodes nuclear protein, responsible for maintaining genome stability. Together with the products of other suppressor genes, signal transduction genes and DNA damage detection, this protein co-creates a protein complex that binds to RNA polymerase II and interacts with histone deacetylase, thus affecting the processes of transcription, DNA repair or recombination. The BRCA1 protein, together with the BRCA2 gene product, which is also a suppressor gene located on chromosome 13, is particularly involved in the repair of double DNA strand breaks by homologous recombination [ 30 ].
The presence of mutations in these genes occurs only in 3–5% of breast cancer patients. However, due to the high penetration of BRCA1/BRCA2 genes, these patients should be included in the prophylactic program. Carriers of the BRCA1/BRCA2 mutation are estimated to have a 10-fold higher risk of developing breast cancer [ 27 ]. The presence of BRCA1/BRCA2 gene mutations is associated with a cumulative risk of breast cancer at age of 70 of more than 60%, and the probability of developing this malignant tumor throughout life varies in the range of 41–90%. Mutations in the BRCA1 gene are associated with triple-negative cancer and in the BRCA2 gene for estrogen receptor-expressed breast cancer [ 31 , 32 , 33 ].
Other suppressor genes whose high-penetration mutations predispose to breast cancer are the TP53 (Li-Fraumeni syndrome) and PTEN (Cowden syndrome) genes. The cumulative risk of developing breast cancer at age 70 for women with Li-Fraumeni syndrome is 54%. In patients with Cowden’s syndrome, the risk of developing breast cancer throughout life is in the range of 25–50%. However, both genetic syndromes are very rare [ 34 , 35 ].
Mutations in the ATM , BRIP1 , CHEK2 and PALB2 genes show a moderate predisposition to breast cancer. Carriers of these mutations have a 2–3 times higher risk of developing this malignant tumor [ 35 ].
It is believed that <10% of breast cancers are genetically determined [ 36 ]. More than 90% of breast cancers, on the other hand, are formed because of sporadic somatic mutations. The risk of developing breast cancer increases twice in women whose closest relative (mother, sister) has been treated for the malignant tumor in question and by three to six times if the two closest relatives have been treated [ 1 ]. This risk decreased the older the relative was at the time of diagnosis of cancer [ 1 ].
2.7. Mild Breast Changes
Another factor that increases the risk of developing breast cancer is the presence of benign changes in the mammary glands. Some benign lesions—benign neoplasms, e.g., atypical ductal hyperplasia (ADH) or atypical lobular hyperplasia (ALH), which increase the risk by four or five times, and proliferative (proliferative) lesions without atypia (e.g., stellar scar or fibrotic adenoma) increasing the risk up to two times. The Hartmann et al. cohort study assessed the risk of breast cancer in patients with different types of benign lesions [ 33 ]. The relative risk of developing breast cancer for the entire study cohort was 1.56 (95% CI, 1.45–1.68) [ 37 ]. This risk was elevated for 25 years after the biopsy. For women with benign lesions without proliferation, the relative risk of developing breast cancer was 1.27 (95% CI, 1.15–1.41). In the presence of mild proliferating lesions, but without atypia, it was equal to 1.88 (95% CI, 1.66–2.12). The highest relative risk of developing breast cancer was for women with the presence of benign proliferating lesions with atypia (atypical ductal hyperplasia, atypical lobular hyperplasia, or both), amounting to as high as 4.24 (95% CI, 3.26–5.41). It was also found that the earlier benign changes were diagnosed (<55 years of age), the greater the risk of developing the malignant tumor in question [ 37 ].
In addition, it is believed that in women with atypical hyperplasia, whose first-degree relatives were treated for breast cancer, the risk of developing this malignant tumor is as much as nine times greater [ 38 ].
2.8. Ionizing Radiation
A recognized factor in the development of breast cancer is early exposure to ionizing radiation. In 2007, John et al. published an analysis of data from the Breast Cancer Family Registry assessing the relationship between exposure to ionizing radiation used in diagnosis and treatment and the risk of developing breast cancer [ 39 ]. This analysis showed that an increased risk of breast cancer was in women who had received radiation therapy in the past as part of cancer treatment and in women who underwent a control chest X-ray during treatment for tuberculosis and pneumonia [ 39 ]. The risk of developing breast cancer was highest in young patients whose exposure to ionizing radiation was multiple and in patients who had exposure at a very young age [ 39 ].
In the study Moskowitz et al. published in 2014, the risk of developing breast cancer was assessed depending on the dose and field of radiotherapy in women who were exposed to the chest area due to cancer (leukemia, Hodgkin’s or non-Hodgkin lymphoma, Wilms’ tumor, neuroblastoma, soft tissue sarcoma, bone malignant tumor, tumor of the central nervous system) before the age of 21 [ 39 ]. This study indicated that the highest risk of developing breast cancer was in patients who were treated with radiation therapy at lower doses (14 Gy) but for a large chest area (whole lung field), consequently covering a larger area of breast tissue [ 38 ]. The risk of developing breast cancer in women who received high-dose radiotherapy (30–40 Gy) for a smaller chest area (Mantle field) was comparable or lower (mediastinal field) but elevated compared to women who had not been irradiated in the past [ 39 ]. The risk of developing breast cancer was lower if the radiation field included the ovaries [ 39 ]. It was also shown that the cumulative risk of developing breast cancer at the age of 50 was 30%, with the highest (35%) in patients treated for Hodgkin lymphoma [ 40 ].
In a systematic review by Henderson et al., it was also found that the highest risk of developing breast cancer occurred in patients treated in the past for Hodgkin lymphoma. It was noted that the analyzed studies mostly concerned such patients [ 41 ].
In 2005, a paper by Travis et al. was published assessing only the relationship between breast cancer and radiotherapy received in the chest area for Hodgkin lymphoma. The study showed that the cumulative absolute risk of developing breast cancer increased with the patient’s age, sometimes after the diagnosis of cancer and the dose of irradiation [ 42 ].
It was mentioned above that performing a control chest X-ray during the treatment of tuberculosis and pneumonia increased the risk of developing breast cancer. As for other diagnostic methods, it is also believed that mammography performed in young women significantly increases the risk of breast cancer [ 1 ].
Ionizing radiation (IR) increases the risk of breast cancer, especially in women and when exposed at a younger age, and the evidence generally supports the linear dose-response relationship [ 43 ]. Ionizing radiation directly and indirectly causes DNA damage and increases the production of reactive oxygen and nitrogen species (RONS). The RONS lead to DNA damage and epigenetic changes leading to mutation and genomic instability. Proliferation of RONS enhances the effects of DNA damage and mutations leading to breast cancer. Separately, damage to reactive oxygen and nitrogen species and DNA also increases inflammation. Inflammation contributes to direct and indirect effects (effects in cells unattainable directly by IR) through positive feedback to RONS and DNA damage, and separately increases proliferation of breast cancer through pro-carcinogenic effects on cells and tissues. For example, changes in gene expression alter inflammatory mediators, resulting in improved cancer cell survival and growth and a more hospitable tissue environment [ 43 ]. All these events overlap at multiple points with events characteristic of “basic” breast cancer induction, including hormone-dependent proliferation, oxidative activity, and DNA damage. These overlays make the breasts particularly susceptible to ionizing radiation and confirm that these biological activities are important characteristics of carcinogens [ 43 ].
2.9. Alcohol Consumption
Numerous studies indicate a relationship between alcohol consumption and an increased risk of breast cancer [ 44 , 45 , 46 , 47 , 48 ]. This dependence results from several mechanisms —alcohol contributes to the increase in the concentration of estrogens in the blood by inhibiting their metabolism in the liver and by intensifying the conversion of androgens to estrogens. In addition, it has an inhibitory effect on the immune system, or DNA repair processes, may intensify cellular proliferation and migration. Finally, the metabolites of alcohol themselves are carcinogenic compounds [ 49 ]. It is estimated that for every consumption of 10 g of pure alcohol per day, there is an increase in the risk of breast cancer by 9% [ 1 ].
The influence of the type of diet used on the development of the cancer process has been the subject of numerous studies. The correlation between a low-varied diet, rich in saturated fats, including those of animal origin, and the risk of developing mainly colorectal cancer seems undeniable [ 50 ]. On the other hand, studies assessing the relationship between diet and the risk of breast cancer are not entirely consistent. Dandamudi et al. reviewed systematic studies published between 2013 and 2017. Ten of the seventeen publications evaluated looked at the impact of a so-called “unhealthy” diet on breast cancer risk. The basic products of the diet in question included: sweetened soft drinks, processed fruit juices, red and processed meats, hardened fats, saturated fats, salted products (chips, chips, peanuts), refined grains, sweetened products (sweets, desserts) [ 50 ]. In most, but not all, of the studies analyzed, a significant relationship was found between excessive consumption of the above-mentioned products and an increase in the risk of developing breast cancer. This relationship primarily concerned excessive consumption of red and processed meat, saturated fats, and sodium [ 51 ].
This systematic review also showed that a diet rich in vegetables, fruits, fish, legumes, oils, and vegetable oils reduces the risk of breast cancer [ 51 ].
Research suggests that nutrition affects the prognosis of breast cancer. Nevertheless, the level of evidence on the results is still insufficient to make recommendations. A healthy and balanced diet should be encouraged to reduce mortality in the world [ 52 ].
A healthy diet characterized by a high intake of unrefined grains, vegetables, fruits, nuts and olive oil, and a moderate/low intake of saturated fatty acids and red meat may improve overall survival after a diagnosis of breast cancer. Breast cancer patients undergoing chemotherapy and/or radiation therapy experience various symptoms that worsen patients’ quality of life. Studies on nutritional interventions during breast cancer treatment have shown that nutritional counseling and supplementation with certain dietary components, such as eicosapentaenoic (EPA) and docosahexaenoic (DHA) acids, may be useful in reducing drug-induced side effects as well as increasing therapeutic efficacy. Therefore, nutritional intervention in patients with BC can be considered an integral part of a multimodal therapeutic approach [ 53 ].
The link between breast cancer and diet is known to be complex, multifactorial, and nonlinear. Classical epidemiological studies on nutrition have shown conflicting results, showing little correlation between diet and breast cancer risk (except alcohol) [ 54 ]. It can be speculated that this may be due to the complexity of breast cancer, which is a multifaceted, highly heterogeneous disorder. Histological classifications, and more recently also molecular ones, have contributed to the formation of a rather complex picture.
Nutrigenomics and related disciplines can support advances in knowledge in this field by shedding light on the molecular basis of breast cancer formation and paving the way for personalized therapies.
2.11. Obesity
One of the risk factors for developing breast cancer, confirmed in many studies, is obesity. Jiralerspong and Goodwin compiled a pooled analysis of numerous publications evaluating the relationship between obesity and breast cancer prevalence in premenopausal and postmenopausal women. This analysis found that both overweight and obesity increased the risk of developing breast cancer, particularly steroid-receptor-expressed breast cancer, in postmenopausal women who did not use hormone replacement therapy [ 55 , 56 , 57 ].
Unlike postmenopausal patients, being overweight or obese in premenopausal women reduces the risk of developing hormone-dependent breast cancer. The authors of the analysis point out, however, that literature data indicate a relationship between obesity in premenopausal patients and the risk of developing triple-negative breast cancer [ 55 , 58 ].
This analysis also found that physical inactivity (combined with obesity) increases the risk of developing breast cancer regardless of menopausal status. Furthermore, according to the results of numerous studies, overweight and obesity are associated with a worse prognosis of breast cancer patients before and after menopause [ 55 , 59 , 60 ]. According to the authors, worse survival may be influenced by a greater stage of cancer at the time of diagnosis, as well as a more aggressive course of breast cancer in obese patients [ 51 ]. Obesity promotes the process of cancer through several mechanisms. Overdeveloped adipose tissue is a source of numerous cytokines, chemokines, endocrine factors, in particular proangiogenic and promitogenic leptin, which affects the immune environment of the described tissue [ 61 ]. There is a concentration of cells of the immune system of a pro-inflammatory nature, additionally secreting inflammatory cytokines. Excessive development of adipose tissue promotes the surrounding hypoxia, which leads to an increase in the secretion of leptin and VEGF factor, while inhibits the synthesis of antiangiogenic and antimitogenic adiponectin [ 62 ]. The NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) pathway is responsible for the development and maintenance of the inflammatory process within excessive adipose tissue, which through pro-inflammatory cytokines has an inhibitory effect on the process of apoptosis, and at a later stage promotes the proliferation of breast cancer cells, cancer invasion, angiogenesis, and metastasis [ 63 , 64 ].
Adipose tissue is also the main source of sex hormones in postmenopausal women. In this tissue, estrogens are formed in the process of aromatization of adrenal androgens. The accumulation of pro-inflammatory cytokines in overgrown adipose tissue, activation of the NF-κB pathway within adipose tissue, or dying adipocytes stimulate the activity of the aromatase complex, which in turn leads to excessive estrogen synthesis and promotes the development of breast cancer [ 62 ].
Furthermore, the metabolic syndrome that accompanies obesity is associated with insulin resistance, hyperinsulinemia, increased synthesis of insulin-like growth factor 1 (IGF-1). Studies have shown that insulin resistance and hyperinsulinemia are associated with poorer survival of breast cancer patients [ 65 ]. Breast cancer cells also often overexpress the IGF-1 receptor, making this factor considered their potential mitogen [ 61 ].
Obesity is a recognized risk factor for breast cancer and the development of relapses, even if patients are properly treated [ 66 ]. Obese women are less likely to undergo breast reconstruction than women of normal weight, and those who have undergone surgery experience more surgical complications. In obese women, systemic chemotherapy and hormone therapy are less effective. Obese women are at greater risk of local recurrence than women of normal weight. The effectiveness of cancer treatment is significantly lower in obese women who survive breast cancer [ 66 ].
Given the multidimensional effect of overgrown adipose tissue on the development of breast cancer, the campaign against obesity should form the basis for primary prevention of the malignant tumor in question.

2.12. Nicotinism
Research reports on the impact of chronic nicotinism on the increased risk of breast cancer are contradictory. However, a study by Jones et al. published in 2017 showed that smoking, especially at the beginning of early peripubertal age or adolescence, was associated with a moderate but statistically significant increase in the risk of developing breast cancer. The relative risk of breast cancer was higher with a positive family history [ 67 ]. Nicotine promotes breast cancer metastasis by stimulating N2 neutrophils and generating a pre-metastatic niche in the lung [ 68 ]. Chemoresistance effects of nicotine were demonstrated in breast cancer cells. These findings demonstrated the harmful effects of nicotine following metastasis of cancer, owing to the chemoresistance produced through uninterrupted smoking, which may impact the effectiveness of treatment [ 69 ].
3. Pathomorphology
The basis for the diagnosis of breast cancer remains standard pathomorphological diagnostics [ 70 ]. The result of histopathological examination should include not only the histological type of the tumor, its degree of histological malignancy, the degree of advancement according to the TNM classification, information on the completeness of the procedure, or infiltration by cancer cells of peritugal vessels, but also the expression of steroid receptors—estrogen and progesterone, HER-2 receptor, and cellular proliferation index Ki67 [ 71 ].
A reliable assessment of all the above parameters is possible thanks to the examination of material taken by means of a coarse needle biopsy or intra- and postoperative material [ 72 ]. The examination of the material obtained by fine needle biopsy does not allow to distinguish between infiltrating and pre-invasive cancer, as well as to assess the state of HER-2. The correct protocol of histopathological examination, considering the biological subtype of the tumor, determines the determination of recognized predictive and prognostic factors, and consequently the selection of appropriate, individual treatment for each patient.
A common classification of breast cancer is the WHO classification [ 73 ], for which in 2019, the 5th edition was published. This described cancers, both benign and malignant, of epithelial, mesenchymal, fibroepithelial origin, neuroendocrine neoplasms, breast wart and nipple areola tumors, in addition, breast lymphomas and metastatic changes in the mammary gland.
A simplified classification of epithelial precursor and invasive lesions is presented in Table 2 .
Epithelial precursor lesions and invasive lesions of the mammary gland [ 74 ].
The most common form of infiltrating cancer is cancer without a special type (NST), formerly called wired (70–80%) [ 1 ]. It is characterized by a large diversity in terms of cancer cell morphology and the presence of tubular or glandular structures. The second most common invasive breast cancer is lobular carcinoma (10%) [ 1 ]. This form of cancer is characterized by a small diversity of cancer cells, very frequent expression of steroid receptors and extremely rare overexpression of the Her-2 receptor [ 1 ].
The degree of histological malignancy (G, grade) was introduced due to the significant diversity of biological characteristics of breast cancers within the same histological type in the absence of characteristic morphological features. The classification used to correctly assess the degree of histological malignancy is, recommended by the WHO, the Bloom–Richardson–Scarff classification in Elston–Ellis modification ( Table 3 ).
Assessment of the degree of histological malignancy [ 75 ].
Originally, the assessment of the degree of histological malignancy concerned only invasive cancer without a special type (NST). Currently, it refers to any infiltrating cancer, excluding medullary and microinvasive cancer. In the case of heterogeneous cancer weaving, the fields with the highest degree of malignancy should be noted [ 76 ].
The current VIII edition of the TNM classification was published by the AJCC (American Joint Committee on Cancer) in 2018. According to this classification, histopathological examination should assess the size of the primary tumor (Tumor), the condition of the axillary lymph nodes (Nodes) and the presence of distant metastasis (Metastasis) ( Table 4 ). In the case of the N trait, the location and number of lymph nodes taken should be described, but it must not be less more than 10 nodes, as well as the number of lymph nodes affected by metastases, including micrometastases and isolated cancer cells. Correct assessment of all elements of the TNM classification makes it possible to determine the stage of cancer, which is the most important prognostic factor [ 68 ]. In countries where it is not possible to present a prognostic stage of advancement, containing the state of ER, PR and HER-2 receptors, its anatomical version should be used.
VIII edition of the pTNM classification.
4. Prognostic and Predictive Factors
As mentioned earlier, the stage of breast cancer is the most important prognostic factor. According to SEER data, 98.9% of patients with localized disease, 85.7% with regional advancement, and only 28.1% of patients with distant metastases will survive [ 7 ].
In addition, all the individual features of the TNM classification have a prognostic significance.
One of the most important prognostic factors is the condition of the lymph nodes (N). According to SEER data, the 5-year overall survival (OS) is 92% for patients with unoccupied regional lymph nodes, 81% with 1–3 lymph nodes occupied, and 57% when metastases were found in four or more lymph nodes. The presence of micrometastases and isolated cancer cells in regional lymph nodes is also of unfavorable prognostic importance [ 76 , 77 ].
The dimension of the primary tumor is also an important prognostic factor. SEER data indicate that 99% of women with a disease confined to the mammary gland and a tumor smaller than 1 cm, 89% with a tumor measuring 1–3 cm and 86% with a tumor of 3–5 cm will survive 5 years [ 77 ]. In addition, a tumor with an originally large size predisposes to the involvement of regional lymph nodes.
The current feature of T4 according to the TNM classification, i.e., invasion of the skin or chest wall, is also associated with a worse prognosis.
4.2. Degree of Histological Malignancy
Of slightly less prognostic significance are the histological type and the degree of histological malignancy. Less common cancers, such as tubular, papillary, and medullary, have a better prognosis with a 10% risk of recurrence with prolonged follow-up [ 78 ].
Determining the prognosis in the case of frequent cancers, infiltrating NST cancer and lobular cancer, facilitated the introduction of the degree of histological malignancy. Studies have shown that unfavorable prognostic significance is associated with low tumor differentiation (G3). However, it has not been clearly established what impact moderate differentiation has on the prognosis (G2) [ 79 ].
4.3. Hormonal Receptors
The expression of steroid receptors—estrogenic and progesterone—is particularly important due to the favorable value of both prognostic and predictive value for hormonal treatment. This expression is assessed by immunohistochemical method (IHC) in tissue material fixed in buffered formalin and embedded in paraffin. If tissue material cannot be obtained, the expression of the receptors is assessed in the cytological material fixed in alcohol. The tissue material should come from the infiltrating component of the primary tumor, prior to systemic treatment. Due to the frequent phenomenon of hormonal profile change in metastatic tumors, it is recommended to reassess the expression of steroid receptors in metastatic material.
The scale used to determine the expression of hormone receptors is the Allrad scale, according to which the percentage of stained nuclei of cancer cells (PS 0–5) and the strength of coloration (IS 0–1) should be assessed. The sum of both parameters is the total value of TS (TS = PS + IS 0–8). In practice, however, as justified by the recommendations of the International Breast Cancer Conference of St. Gallen, only the percentage of colored cell nuclei is considered. Any reaction in the ≥1% of cancer cells is considered positive [ 80 , 81 , 82 ].
In every patient with current steroid receptors, hormone therapy should be used, regardless of age, condition of regional lymph nodes or additional indications for chemotherapy. The efficacy of complementary treatment with tamoxifen and aromatase inhibitors in hormone-sensitive patients has been demonstrated in numerous randomized controlled trials. In turn, the first reports on the prognostic value of primarily the estrogen receptor were published in the second half of the twentieth century [ 83 , 84 , 85 , 86 , 87 , 88 , 89 ]. Steroid receptor expression is associated with better prognosis and lower sensitivity to chemotherapy.
4.4. HER-2 Receptor
The prognostic and predictive value for targeted treatment is also the overexpression of the HER-2 receptor or amplification of the HER-2 gene. The HER-2 state should be determined in the histological material. The assessment of the HER-2 status in the cytological material is of lower value because the staining reaction used in the determination of the receptor occurs in the cell membrane, which is easily damaged during a fine needle aspiration biopsy.
Determining the HER-2 status requires the use of two methods—immunopathological at each diagnosis of infiltrating cancer ( Table 5 ) and the method of in situ hybridization in immunohistochemically borderline cases (about 15–20% of cases). About 10% of ambiguous cases show amplification of the HER-2 gene after in situ hybridization (FISH or CISH), which is interpreted as a positive state. The in situ hybridization method involves counting a copy of the HER-2 gene (single probe) or a copy of the HER-2 gene and the number of centromeres of chromosome 17 (double probe). The test result is the average number of copies of the HER-2 gene per cell or the ratio of the number of copies of the HER-2 gene to the number of centromeres. Cases without amplification of the HER-2 gene are treated as negative.
HER-2 receptor IHC rating scale, interpretation.
The HER-2 receptor belongs to the family of four ERBB receptors. The first of these—the EGFR receptor (ERBB1), i.e., the epidermal growth factor receptor with tyrosine kinase properties, is a target for many molecularly targeted drugs. Its ligand is epidermal growth factor (EGF) and TGF-α. The HER-2 receptor (ERBB2), the second in the ERBB receptor family, does not have a specific ligand. Its role is to enhance signal transduction by heterodimerization with other ERBB receptors. Heterodimer with ERBB3 receptor is the strongest signal transducing complex. The presence of overexpression of the HER2 receptor or amplification of its gene is an unfavorable prognostic factor, and the introduction of drugs that block the HER-2 receptor, i.e., trastuzumab, T-DM1, pertuzumab, lapatinib, significantly improved the prognosis of patients. In the meta-analysis of phase III studies, min. HERA studies have shown that the addition of trastuzumab, a monoclonal antibody directed against the HER-2 receptor, to adjuvant chemotherapy is associated with a 40% reduction in the relative risk of recurrence and a relative risk of death of 34% compared to left chemotherapy alone [ 89 ].
Studies have shown that the improvement in prognosis also applies to patients treated palliatively. The best example of studies confirming the effectiveness of adjuvant trastuzumab therapy in patients with early HER2-positive breast cancer are 4 international randomized trials-HERA, NSABP-B31, NCCTG-N98 and BCIRG 00, of which the HERA study became a registration study in the above indication [ 90 , 91 , 92 , 93 , 94 ]. One of the first studies to assess the importance of trastuzumab in the first line of treatment for patients with advanced breast cancer was Slamon et al. [ 89 ]. The study showed that adding trastuzumab to chemotherapy (CHT) in the first line of treatment significantly improved prognosis.
Inhibition of HER2 in breast cancer with HER2 amplification is clinically effective, as demonstrated by the effectiveness of HER kinase inhibitors and HER2 antibody treatment. Although resistance to HER2 inhibition is common in the case of metastasis, specific programs that follow HER2 resistance have not been established. In the work of Smith et al. [ 95 ], through genomic profiling of 733 breast cancers with HER2 amplification, enrichment of somatic changes that promote MEK/ERK signaling in metastatic tumors with reduced progression-free survival after anti-HER2 therapy was identified. These mutations, including NF1 loss and ERBB2 activating mutations, are sufficient to mediate resistance to FDA-approved HER2 kinase inhibitors, including tucatinib and neratinib. Moreover, resistant cancers lose their dependence on AKT, undergoing dramatic sensitization to MEK/ERK inhibition. Mechanically, this driver path switch is the result of the activation of MEK-dependent CDK2 kinase. These results define the genetic activation of MAPK as a recurrent mechanism of resistance to anti-HER2 therapy that can be effectively combated with MEK/ERK inhibitors.
Although rare, HER2 mutations appear as important molecular changes that need to be identified, for example, in patients with metastasis, tumors with HER2 mutations may respond to specific tyrosine kinase inhibitors. HER2 mutation may also be a mechanism of resistance to anti-HER2 therapeutic compounds.
4.5. Proliferation Rate Ki67
The Ki67 protein, used in the evaluation of the cellular proliferation index, is a nuclear protein present in all phases of cell division, except the resting phase of G0, and therefore in all actively proliferating cells. The Ki67 protein is identified by immunohistochemical method. The percentage of colored testicles of cancer cells is the value of the cell proliferation index Ki67. However, the positive reaction criterion has not been fully established. It is assumed that 20% is the limit of low and high proliferation.
Currently, the assessment of the cellular proliferation index Ki67 is an essential element of the pathomorphological study, allowing to determine the final luminal subtype of cancer (A or B) and the degree of histological malignancy (G).
The high proliferation index has an unfavorable prognostic significance not only as a component of histological malignancy, but also as an independent prognostic factor [ 93 ].
4.6. Polygenic Prognostic Factors
The development of molecular biology and genetics allowed for the separation of many new prognostic factors (mainly genes), and the introduction of new technologies to create tools for their determination. These tools are multi-gene predictive tests, currently used to estimate the risk of relapse in individual patients and the benefits of the proposed treatments. In practice, these tests are primarily used to qualify patients with early luminal cancer for adjuvant chemotherapy, in addition to standard hormone therapy. The most well-known tests are Oncotype DX and Mammaprint, of which only Oncotype DX was included in the VIII edition of the TNM classification [ 28 ].
One of the prognostic factors widely commented on recently is the complete pathological response (pCR) obtained through induction chemotherapy. Evaluated in several studies, some contradictory, it has been meta-analyzed by Spring et al. and published in Clinical Cancer Research in 2020. This meta-analysis showed that the achievement of pCR as a result of preoperative systemic treatment was associated with an increase in event-free survival (HR = 0.31; 95% PI, 0.24–0.39), especially in the case of triple-negative cancer (HR = 0.18; 95% PI, 0.10–0.31) and HER2 positive (HR = 0.32; 95% PI, 0.21–0.47), as well as an increase in overall survival (HR = 0.22; 95% PI, 0.15–0.30) [ 86 ]. The results obtained were not dependent on subsequent adjuvant therapy. The pCR obtained through induction systemic therapy was considered a favorable prognostic factor for breast cancer [ 96 ].
5. Biological Types of Breast Cancer
Routinely determined elements of the pathomorphological examination seem insufficient to predict the clinical course of breast cancer, which makes it difficult to make appropriate therapeutic decisions. The diverse clinical course of cancers with similar morphological characteristics is due to their different gene profile.
The study of gene expression allowed the identification of five molecular subtypes of breast cancer, such as: luminal A, luminal B, HER-2 positive non-”luminal”, basal-like and special histological types. These surrogates correspond to the immunophenotypes of cancer cells determined according to pathological criteria.
The luminal type A is characterized by high expression of genes associated with the activity of estrogen receptors and at the same time low expression of genes associated with proliferation and genes associated with expressed by the HER2 receptor.
Luminal type B is characterized by a positive ER status associated with low expression of genes associated with this receptor and higher than in type A expression of genes associated with the assessed proliferation by marking Ki-67. A panel of panelists in St. Gallen recognized the meltdown and expression of the Ki-67 as factors that could be used to differentiate between tumors of luminal type A and subtype B [ 97 ]. This is important in the prognostic assessment, which is better in type A.
The next type is basal-like breast carcinoma, also called triple negative cancer due to the absence of estrogen and progesterone receptors and the lack of expression of the HER2 receptor- consequently, there is no expression of genes associated with these receptors. The group of patients with this type of cancer with metastases to the cerebellum is particularly interesting, in their case the use of biological markers (CK 5/6, HER1, c-KIT) can help in the restoration of the basal subtype similar and dissimilar, nevertheless, their clinical usefulness is ambiguous.
The molecular subtype of breast cancer HER2- positive is characterized by overexpression of HER2 combined with the absence of ER and PR.
Breast cancer is the most common cancer in women. Every year, the results of many clinical trials are published, only some of which cause a change in the standard of conduct. Treatment rules for patients with early breast cancer are updated every two years as part of a consensus set by experts St. Gallen International Breast Cancer Conference. Similarly, the European Society for Medical Oncology (ESMO) is developing its recommendations for the treatment of patients with breast cancer at an early stage. Recent Recommendations from St. Gallen (2019) highlight the progress that has been made, particularly in the management of HER2-positive and triple-negative breast cancers with residual disease after preoperative treatment [ 98 ].
6. Breast Cancer Treatment
The basic types of surgical procedures used in women treated for breast cancer are:
- - tumor excision;
- - mastectomy;
- - excision of the sentinel lymph node;
- - excision of the armpit lymphatic system.
Breast amputation is performed in patients who, due to the severity of the disease, do not qualify for breast-sparing treatment or do not agree to perform breast-sparing surgery. Breast amputation involves the removal of the entire breast and the entire skin covering the mammary gland (the exception is subcutaneous amputation). It is possible to make:
- - simple amputation—this is most often a palliative procedure in patients who are not eligible for radical treatment;
- - subcutaneous amputation, consisting in the removal of breast gland tissue and the nipple-areola complex, but leaving the skin;
- - modified radical mastectomy according to the Patey method, consisting in the removal of the mammary gland, lymph nodes of the axillary fossa, pectoral muscle minor and fascia of the pectoral muscle major;
- - modified radical mastectomy according to the Madden method, consisting in the removal of the mammary gland along with the fascia of the pectoral muscle major and the lymph nodes of the armpit in one tissue block;
- - radical mastectomy according to the Halsted method—performed in patients who have been diagnosed with infiltration of the cancer process on the pectoral muscles, consists in the removal of the mammary gland, lymph nodes of the axillary fossa, pectoral muscle larger. This treatment is currently rarely used [ 99 , 100 ].
Currently, breast conserving therapy (BCT), which is a method used in early forms of cancer, is becoming more and more widely used, and is characterized by the same effectiveness. Patients who meet the criteria for eligibility for sparing treatment, in accordance with the guidelines of the Association of Breast Surgery, should be offered the opportunity to choose between this treatment and mastectomy. Surgical treatment of breast cancer can take the form of sparing treatment consisting of:
- - removal of the tumor along with the margin of healthy tissues;
- - quadrantectomy;
- - surgery within the axillary fossa (all lymph nodes of the axillary fossa—axillary lymphadenectomy or sentinel lymph node).
The main purpose of surgery of patients treated for breast cancer is oncological completeness. Both in the case of radical mastectomy and breast conserving therapy, the appropriate cosmetic effect is important [ 98 ]. In the treatment of breast cancer, in addition to surgical intervention, adjuvant treatment is used consisting in the use of radiotherapy, chemotherapy, hormone therapy and immunotherapy or a combination of these methods. Radiation therapy is used in all patients treated with methods that spare the mammary gland, it reduces the risk of recurrence of the disease process. Indications for the use of adjuvant radiotherapy also include the occurrence of metastases in at least four axillary lymph nodes and the presence of positive tissue margins. The chest area and nodal fields are irradiated. Another type of adjuvant treatment is chemotherapy involving the use of cytostatics. This method is used in case of generalization of the disease process. It can be associated with radiation therapy. In breast amputees with indications for supplemental radiotherapy, it should be performed after the end of adjuvant chemotherapy. Hormone treatment is used in patients with breast cancer with estrogen receptor expression; it is used regardless of age and menopausal state. Another reason for using hormone therapy is to reduce the number of hormones secreted and alleviate the ailments and symptoms associated with cancer [ 101 ].
Thanks to advances in diagnostics, modern oncology can offer a personalized course of treatment—adapted to the characteristics of a given cancer. Doctors gained access to multigene tests, which, when used in a properly selected group of patients, are a valuable diagnostic tool. They help to plan the optimal treatment for a given patient and assess the likelihood of recurrence of the disease.
Diagnostic tests are used to measure the activity of a group of genes in breast cancer cells such as the Oncotype DX Breast Recurrence Score.
Its use was presented in the TAILORx clinical trial [ 102 ]. The study enrolled 10,273 breast cancer patients without lymph node metastases, estrogen receptor expression, and HER2 receptor expression. Based on the Oncotype DX test, patients with an intermediate risk of cancer recurrence were randomly assigned to an arm of the study in which only hormone therapy was used or to an arm in which patients were given chemotherapy together with hormone therapy. It was found that in the studied group of patients, independent hormone therapy is no less effective than combined chemotherapy with hormone therapy. Thanks to the results of a groundbreaking study, it is possible to safely avoid chemotherapy in the case of up to 70% of patients diagnosed with the most common form of breast cancer. The Oncotype DX Breast Recurrence Score is a diagnostic test which, based on the analysis of the expression of 21 genes, distinguishes three prognostic groups: with low, intermediate, and high risk of recurrence. The result of the study may help doctors make decisions about the optimal treatment of patients in the early stages of invasive breast cancer showing estrogen receptor activity (ER+), without expressing epidermal growth factor receptors (HER2-negative). The Oncotype DX test determines in this case the likelihood of a beneficial effect of the use of chemotherapy in combination with hormone therapy on treatment. Genetic tests in Oncotype DX work well in patients with Luminal A and B cancers, i.e., with positive estrogen and progestogen receptors, negative HER 2 and “clean” lymph nodes. Especially patients under 50 years of age are the group that can benefit most from the individualization of treatment. Patients with early breast cancer and good prognostic factors (ER+, PGR+, HER2−) are eligible for surgical treatment in the first place. After excision of the breast tumor and its histopathological evaluation and assessment of sentinel nodes (if there are no metastases in them), there is time to perform tests such as OncotypeDX or Mammaprint. The results of the tests make it easier to decide whether the patient should benefit from hormone therapy or chemotherapy will also be necessary and often put a dot over and in the qualification for treatment and allow the patient to be sure of the correctness of the choice of a specific therapy.
7. Recent Treatments for Triple Negative Breast Cancer
Among breast cancers, triple negative breast cancer (TNBC) is the most aggressive, and for its histochemical and molecular characteristics is also the one whose therapeutic opportunities are most limited. In case of breast cancer, a significant clinical problem is provided by the group of patients with no expression of any receptors, qualifying to hormonal therapy or target therapy against HER2 (the human epidermal growth factor receptor-2). The subtype of the disease, characterized by the lack of expression of estrogen receptors (ERs), the progesterone receptor (PR) and HER2, is referred to as the triple-negative breast cancer (TNBC). The triple-negative subtype constitutes approximately 15–20% of all breast cancer cases, its incidence being higher among younger women and is characterized by different biological features, unfavorable clinical course and poor prognosis. During the recent years, a thesis has been put forward that triple-negative breast cancer is a separate, heterogenic subtype of breast cancer, formed in the mechanism of different oncogenesis pathways, characterized by different prognoses and dependent on various clinical, pathological, and genetic factors. Despite its aggressive clinical course, the triple-negative breast cancer responds to chemical therapy, the response rate being very high. However, the disease recurrences are very frequent, while the lack of targeted therapy makes this cancer subtype very unfavorable from the prognostic point of view. No unequivocal principles of management have till now been proposed in the TNBC subgroup.
PARP inhibitors. So far, the Food and Drug Administration (FDA) has approved olaparib and thalasoparib for use in patients with advanced breast cancer with a germinal BRCA1/2 mutation [ 103 , 104 , 105 ]. The effectiveness of thalasoparib was proven in a phase III study, in which this drug was compared with standard chemotherapy, and its choice depended on the attending physician (in practice, capecitabine, vinorelbine, gemcitabine, eribulin). The I-row endpoint was progression-free survival (PFS). This study showed that thalasoparib was associated with a longer PFS duration (8.6 vs. 5.6 months, p < 0.001), thalasoparib was also better tolerated. Forty-five percent of patients were TNBC; 55% were HR+. Olaparib was validated based on a phase III study, with a very similar design to the EMBRACA study (with thalasoparib). The olaparib study showed that the use of this drug, compared to standard CHT, was associated with statistically significant PFS prolongation (7 vs. 4.2 months, p < 0.001). The tolerability of the treatment was also better. Patients eligible for the study, in addition to the current germline BRCA mutation, had to be HER2-. In both of the above studies, patients were previously treated (with anthracyclines and taxanes) and hormonotherapy [ 104 , 106 ].
Sacituzumab govitecan is a conjugate antibody directed against the surface tropoblast antigen Trop-2 along with the active metabolite irinotecan SN-38 [ 106 ]. A Phase 1/2 study evaluated the safety of sacituzumab govitecan in patients with advanced TNBC who had previously been treated with two chemotherapy (CHT) lines. Other endpoints included objective response rate, length of response, degree of clinical benefit, PFS, and OS. This study showed that the use of the above drug was associated with a benefit in the form of long-term objective responses. The 108 patients with triple-negative breast cancer had received a median of three previous therapies (range, 2 to 10). Four deaths occurred during treatment; three patients (2.8%) discontinued treatment because of adverse events. Grade 3 or 4 adverse events (in ≥10% of the patients) included anemia and neutropenia; 10 patients (9.3%) had febrile neutropenia. The response rate (3 complete and 33 partial responses) was 33.3% (95% confidence interval [CI], 24.6 to 43.1), and the median duration of response was 7.7 months (95% CI, 4.9 to 10.8); as assessed by independent central review, these values were 34.3% and 9.1 months, respectively. The clinical benefit rate was 45.4%. Median progression-free survival was 5.5 months (95% CI, 4.1 to 6.3), and overall survival was 13.0 months (95% CI, 11.2 to 13.7). The main adverse reaction was hematological toxicity.
Immunotherapy as monotherapy. Documented efficacy in TNBC has a doublet of atezolizumab along with nab-paclitaxel-study IMpassion130 [ 107 , 108 ]. Anti-PD-1 or anti-PD-L1 monotherapy is still under investigation, but the U.S. FDA has approved the use of pembrolizumab in previously treated BC patients, after exhaustion of therapy options that have shown microsatellite instability or dMMR (The National Comprehensive Cancer Network (NCCN) recommendations). Recently, the results of meta-analyses and systematic reviews have appeared, which indicate the possible effectiveness of anti-PD1 and anti-PD-L1 antibodies in patients with TNBC. Studies have evaluated the efficacy of pembrolizumab, atezolizumab and avelumab, including their effects on ORR, PFS, OS. The results are promising, mainly in the group of patients expressing PD1 or PD-L1, especially when immunotherapy is used in the 1st line of treatment. It seems important to confirm the effectiveness of immunotherapy in TNBC characterized by a particularly poor prognosis, the treatment of which is currently limited to standard CHT.
In the aforementioned 2019 IMpassion130 study, it was shown that the use of atezolizumab with nab-paclitaxel in 1 line of treatment compared to nab-paclitaxel and placebo in patients with advanced TNBC was associated with an increase in the median PFS from 7.2 to 5.5 months ( p = 0.002) in the ITT population, and in the PD-L1 expressing population 7.5 vs. 5.0 months ( p < 0.001). However, in August 2021, the company producing atezolizumab voluntarily withdrew this drug from the indications for the treatment of TNBC. Currently, the only registered checkpoint inhibitor is pembrolizumab—for neoadjuvant treatment along with CHT TNBC with a high risk of relapse, with follow-up adjuvant therapy—based on the Keynote 522 study, and for the treatment of advanced TNBC with PDL1 expression (CPS > 10) based on the Keynote 355 study, as well as in patients with MSI-H and dMMR. In the latter indication, dostarlimab-gxly is also registered. Research is ongoing on other checkpoint inhibitors, including the previously described monotherapy [ 109 , 110 ].
The work of Spini et al. [ 111 ] provides an overview of all evidence regarding the reuse of old, licensed non-cancer drugs for the treatment of TNBC, ranging from preclinical evidence to current clinical trials.
Beta-blockers (BBs) appear to be promising drugs for reuse in the treatment of TNBC. While BB has been shown to be beneficial in the treatment of TNBC, metformin, a promising molecule in preclinical studies, has shown no efficacy in treating women with TNBC. Metformin does not improve survival outcomes in the female population with TNBC compared to women who do not use TNBC. It is worth noting that two studies are underway on the use of metformin in clinical trials in patients with TNBC.
Articles by Shiao et al. [ 112 ] and Williams et al. [ 113 ] showed conflicting results for aspirin. While the first study showed a significant improvement in survival in Grade II/III women through the use of aspirin, Williams et al. did not demonstrate this benefit in the breast cancer study population (women with operative I-III TNBC at stage).
Recently, one phase II study on omeprazole activity in patients with operative TNBC was presented at the ASCO meeting, regardless of baseline fatty acid synthase expression (FASN) [ 114 ]. In vitro, proton pump inhibitors inhibit FASN activity and induce apoptosis in breast cancer cell lines. In this study, 42 patients were given omeprazole in combination with anthracycline-taxane (AC-T) until surgery and a complete pathological response (pCR) was investigated. A positive FASN score decreased significantly for omeprazole from 0.53 (SD = 0.25) at baseline to 0.38 (SD = 0.30; p = 0.02), and the drug was well tolerated without known Grade 3 or 4 toxicity. In addition, the percentage of pCT was 71.4% (95% CI: 51.3–86.8) in patients with FASN+ and 71.8% (95% CI: 55.1–85.0) in all enrolled patients, indicating that omeprazole, in addition to neoadjuvant AC-T, provides a promising PCR rate without adding toxicity. From the literature obtained, BBs seemed to be more promising drugs for repurposing.
Agents that target angiogenesis have shown limited efficacy for human triple-negative breast cancer (TNBC) in clinical trials [ 113 ]. Considering the recommendations of the National Comprehensive Cancer Network (NCCN), the only drug that improved the endpoints of studies evaluating the effectiveness of anti-VEGF drugs with chemotherapy was bevacizumab. Ramucirumab, sorafenib and sunitinib were also studied [ 115 ].
According to NCCN:
- - study E2100 (more than 700 patients) evaluated the combination of bevacizumab with paclitaxel vs. paclitaxel with placebo in line 1 treatment for breast cancer recurrence/spread. This study showed that the addition of bevacizumab allowed for prolongation of the median PFS;
- - a similar study (more than 700 patients) evaluated the doublet bevacizumab plus docetaxel vs. docetaxel with placebo and in this study also achieved improvements in PFS in the combination group (AVADO study);
- - in the RIBBON-1 study, bevacizumab was attached to capecitabine, to taxane (docetaxel or nab-paclitaxel), to anthracyclines—here also PFS elongation was achieved by adding bevacizumab to CHT (study with 2nd line of treatment; the next study with 2nd line was IMELDA-elongation and PFS and OS were shown)
- - the last study mentioned by the NCCN was the Phase III CALGB 4050 study, which evaluated the addition of bevacizumab to nab-paclitaxel in line 1 of advanced TNBC treatment and achieved a median PFS of 7.4 months. In general, research shows that the addition of bevacizumab has an effect on ORR and PFS, but not OS and QoL.
One study showed that bevacizumab used in neoadjuvant lengthened both PFS and OS slightly (NSABP B-40 study).
8. The Role of Non-Coding RNAs in Breast Cancer
The development of molecular biology has made it possible to conduct research at the level of the human genome. In 2003, its full sequence was published. Subsequently, it was discovered that only 1.2% of human genetic material encodes protein, with 93% of genes being transcribed. The huge pool of non-coding RNA molecules has aroused great interest among scientists. The subject of careful analysis in breast cancer became microRNAs, single-stranded RNA molecules with a length of 21 to 23 nucleotides, regulating the expression of other genes [ 116 , 117 , 118 , 119 ].
The first reports of the possible significance of altered miRNA expression in breast cancer were published in 2005. Over the past decade, several miRNA molecules involved in the initiation, progression, and metastasis of breast cancer have been identified [ 104 ]. The relationship between the expression of individual miRNAs and the clinical-pathological features of breast cancer, or the response to causal treatment of this malignant tumor,” has also been determined [ 115 ]. For example, studies have shown that in triple-negative breast cancer, there is an overexpression of oncogenic molecules miR-21, miR-210, miR-221, which is associated with a shorter disease-free time and worse survival [ 86 ]. Molecules with reduced expression, and therefore suppressor potential, are, for example, miR125-b in the case of HER-2 positive cancers, or miR-520 in hormone-dependent cancers [ 117 , 118 ].
Singh and Mo presented a review article on miRNA families, which play an important role in the course of the discussed malignant tumor. They paid attention to the miR-10 family, from which miR-10a and miR-10b are involved in the development and metastasis of breast cancer [ 116 ]. Overexpression of miR-10b is associated with a higher degree of cancer according to the TNM classification (larger size of the primary tumor, presence of metastases in the lymph nodes), a greater degree of cellular proliferation, overexpression, or amplification of the HER-2 receptor [ 117 ].
However, it is negatively correlated with the presence of steroid receptors and the concentration of E-cadherin, which seems to play a role in the suppression of the metastasis process in the EMT (Epithelial-mesenchymal transition) mechanism [ 105 ]. Metastasis, as well as a worse course of particularly ductal breast cancer and consequently shorter overall survival time is also associated with the oncogenic miR-21 family [ 104 ]. Among the families of suppressor miRNAs, with reduced expression in cancerous breast tissue compared to healthy tissue, the aforementioned authors mentioned the miR-200 family and miR-205 and miR-145.
MiR-200 and miR-205 probably inhibit the metastasis process associated with the EMT mechanism, and miR-145 affects cell apoptosis [ 119 ].
On the other hand, in a 2019 review article by Loh et al., the decisive oncogenic potential of the miR-200 family was described [ 120 ]. Increased concentrations of individual miR-200s were associated not only with breast cancer’s ability to form distant metastases, but also with resistance to chemotherapy [ 121 ].
The relationship between the expression of the rich miRNA family and the cell cycle, including the disturbed tumor cell cycle, certainly requires further analysis. However, there is no doubt that the molecules in question have a huge prognostic, predictive and therapeutic potential. Promising research results have prompted scientists to search for new regulatory molecules.
Of particular recent interest are long non-coding RNAs, or lncRNAs. An extensive description of lncRNA was presented by the authors of this article in a review paper Smolarz et al. [ 122 ].
The assessment of the achievements over recent years in the treatment of patients with breast cancer, with the simultaneous lack of fully satisfactory results and satisfactory solutions, suggests that further progress in the development of new methods of combating cancer will bring us closer to a new era in this field.
Funding Statement
This research has received no external funding.
Author Contributions
Conceptualization, B.S., A.Z.N. and H.R.; writing—original draft preparation, B.S.; writing—review and editing B.S.; revision and proofreading B.S. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Advertisement
A review of prognostic and predictive biomarkers in breast cancer
- Review Article
- Published: 15 January 2022
- Volume 23 , pages 1–16, ( 2023 )
Cite this article

- Elaheh Tarighati 1 ,
- Hadi Keivan 2 &
- Hojjat Mahani ORCID: orcid.org/0000-0003-2696-0758 3
4879 Accesses
29 Citations
2 Altmetric
Explore all metrics
Breast cancer (BC) is a common cancer all over the world that affects women. BC is one of the leading causes of cancer mortality in women, which today has decreased with the advancement of technology and new diagnostic and therapeutic methods. BCs are histologically divided into in situ and invasive carcinoma, and both of them can be divided into ductal and lobular. The main function after the diagnosis of invasive breast cancer is which patient should use chemotherapy, which patient should receive adjuvant therapy, and which should not. If the decision is for adjuvant therapy, the next challenge is to identify the most appropriate treatment or combination of treatments for a particular patient. Addressing the first challenge can be helped by prognostic biomarkers, while addressing the second challenge can be done by predictive biomarkers. Among the molecular markers related to BC, ER, PR, HER2, and the Mib1/Ki-67 proliferation index are the most significant ones and are tightly confirmed in the standard care of all primary, recurrent, and metastatic BC patients. CEA and CA-15–3 antigens are the most valuable markers of serum tumors in BC patients. Determining the series of these markers helps monitor response to the treatment and early detection of recurrence or metastasis. miRNAs have been demonstrated to be intricate in mammary gland growth, proliferation, and formation of BC known to be incriminated in BC biology. By combining established prognostic factors with valid prognostic/predicted biomarkers, we can start the journey to personalized treatment for every recently diagnosed BC patient.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price excludes VAT (USA) Tax calculation will be finalised during checkout.
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others

Prognostic and Predictive Factors in Breast Carcinoma

Biomarkers in Breast Carcinomas

Prognostic and Predictive Factors of Invasive Breast Cancer
Abbreviations.
Aromatase inhibitors
American society of clinical oncology
- Breast cancer
Cancer antigen
Cyclin-dependent kinase 4/6
Carcinoembryonic antigen
Circulating tumor cells
Circulating tumor DNA
Ductal carcinoma in situ
Disease-free survival
Estradiol 2
Epidermal growth factor receptor
European Group on Tumor Markers
Estrogen receptor
Mutational status of ER
Endocrine therapy
Human epidermal growth factor receptor
Immunohistochemistry
Infiltrating lobular carcinoma
In situ hybridization
Lobular carcinoma in situ
National Cancer Institute
Overall survival
Positron emission tomography/computed tomography
Protein interacting with never in mitosis A
Progesterone receptor
Selective ER down-regulator
Selective ER modulator
Triple-negative BC
Untranslated region
Variant allele frequency
Ali OS, Shabayek MI, Seleem MM, Abdelaziz HG, Makhlouf DO. MicroRNAs 182 and 375 sera expression as prognostic biochemical markers in breast cancer. Clin Breast Cancer. 2018;18(6):e1373–9.
Article CAS PubMed Google Scholar
Siegel RL, Miller KD, Goding Sauer A, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145–64.
Article PubMed Google Scholar
American Cancer Society. Breast Cancer Facts & Figures 2019-2020. Atlanta: American Cancer Society, Inc.; 2019.
Google Scholar
Anderson BO, Yip CH, Smith RA, et al. Guideline implementation for breast healthcare in low-income and middle-income countries: Overview of the Breast Health Global Initiative Global Summit 2007. Cancer. 2008;113(S8):2221–43.
Nicolini A, Ferrari P, Duffy MJ,. Prognostic and predictive biomarkers in breast cancer: past, present and future. Semin Cancer Biol. 2018;52(Pt 1):56–73.
Yao Y, Liu R, Gao C, et al. Identification of prognostic biomarkers for breast cancer based on miRNA and mRNA co-expression network. J Cell Biochem. 2019;120(9):15378–88.
Bertozzi S, Londero AP, Seriau L, Di Vora R, Cedolini C, Mariuzzi L. Biomarkers in Breast Cancer. In: Begum G, editor. Biomarker—Indicator of Abnormal Physiological Process. London: IntechOpen; 2018.
Khordadmehr M, Shahbazi R, Ezzati H, Jigari-Asl F, Sadreddini S, Baradaran B. Key microRNAs in the biology of breast cancer; emerging evidence in the last decade. J Cell Physiol. 2019;234(6):8316–26.
Nassar FJ, Nasr R, Talhouk R. MicroRNAs as biomarkers for early breast cancer diagnosis, prognosis and therapy prediction. Pharmacol Ther. 2017;172:34–49.
Sharma GN, Dave R, Sanadya J, Sharma P, Sharma K. Various types and management of breast cancer: an overview. J Adv Pharm Technol Res. 2010;1(2):109.
PubMed PubMed Central Google Scholar
Bodmer M, Meier C, Krähenbühl S, Jick SS, Meier CR. Long-term metformin use is associated with decreased risk of breast cancer. Diabetes Care. 2010;33(6):1304–8.
Article CAS PubMed PubMed Central Google Scholar
Page K, Guttery DS, Fernandez-Garcia D, et al. Next generation sequencing of circulating cell-free DNA for evaluating mutations and gene amplification in metastatic breast cancer. Clin Chem. 2017;63(2):532–41.
Akram M, Iqbal M, Daniyal M, Khan AU. Awareness and current knowledge of breast cancer. Biol Res. 2017;50(1):33.
Article PubMed PubMed Central Google Scholar
Ulaner GA, Riedl CC, Dickler MN, Jhaveri K, Pandit-Taskar N, Weber W. Molecular imaging of biomarkers in breast cancer. J Nucl Med. 2016;57(Suppl 1):53S.
Carrigan P, Krahn T. Impact of biomarkers on personalized medicine. New approaches to drug discovery. Berlin: Springer; 2015. p. 285–311.
Book Google Scholar
Goossens N, Nakagawa S, Sun X, Hoshida Y. Cancer biomarker discovery and validation. Transl Cancer Res. 2015;4(3):256.
CAS PubMed Google Scholar
Henry NL, Hayes DF. Cancer biomarkers. Mol Oncol. 2012;6(2):140–6.
Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351(27):2817–26.
Porika M, Malotu N, Veldandi UK, Yadala N, Abbagani S. Evaluation of tumor markers in southern Indian breast cancer patients. Asian Pac J Cancer Prev. 2010;11(1):157–9.
PubMed Google Scholar
Loibl S, von Minckwitz G, Schneeweiss A, et al. PIK3CA mutations are associated with lower rates of pathologic complete response to anti-human epidermal growth factor receptor 2 (her2) therapy in primary HER2-overexpressing breast cancer. J Clin Oncol. 2014;32:3212–20.
Krop I, Johnston S, Mayer IA. The FERGI phase II study of the PI3 K inhibitor pictilisib (GDC-0941) plus fulvestrant vs. fulvestrant plus placebo in patients with ER+, aromatase inhibitor (AI)- resistant advanced or metastatic breast cancer. San Antonio Breast Cancer Symposium 2014; Abstract No. S2–02.
Fasching PA, Brucker SY, Fehm TN, et al. Biomarkers in patients with metastatic breast cancer and the PRAEGNANT study network. Geburtsh Frauenheilk. 2015;75(1):41–50.
Zwart W, Theodorou V, Carroll JS. Estrogen receptor-positive breast cancer: a multidisciplinary challenge. Wiley Interdiscip Rev Syst Biol Med. 2011;3(2):216–30.
Kumar R, Zakharov MN, Khan SH, et al. The dynamic structure of the estrogen receptor. J Amino Acids. 2011;2011:812540.
Weigel MT, Dowsett M. Current and emerging biomarkers in breast cancer: prognosis and prediction. Endocr Relat Cancer. 2010;17(4):R245–62.
Jin C, Rajabi H, Pitroda S, Li A, et al. Cooperative interaction between the MUC1-C oncoprotein and the Rab31 GTPase in estrogen receptor-positive breast cancer cells. PLoS ONE. 2012;7(7):e39432.
Tecalco-Cruz AC, Ramírez-Jarquín JO. Mechanisms that increase stability of estrogen receptor alpha in breast cancer. Clin Breast Cancer. 2017;17(1):1–10.
Yuan S, Shi C, Liu L, Han W. MUC1-based recombinant Bacillus Calmette-Guerin vaccines as candidates for breast cancer immunotherapy. Expert Opin Biol Ther. 2010;10(7):1037–48.
Yuan S, Shi C, Ling R, Wang T, Wang H, Han W. Immunization with two recombinant Bacillus Calmette-Guerin vaccines that combine the expression of multiple tandem repeats of mucin-1 and colony stimulating-factor suppress breast tumor growth in mice. J Cancer Res Clin Oncol. 2010;136(9):1359–67.
Li X, Li L, Zhou Q, et al. Synthesis of the novel elemonic acid derivatives as Pin1 inhibitors. Bioorg Med Chem Lett. 2014;24(24):5612–5.
Kim JH, Jung JH, Kim SH, Jeong SJ. Decursin exerts anti-cancer activity in MDA-MB-231 breast cancer cells via inhibition of the Pin1 activity and enhancement of the Pin1/p53 association. Phytother Res. 2014;28(2):238–44.
Wei S, Kozono S, Kats L, et al. Active Pin1 is a key target of all-trans retinoic acid in acute promyelocytic leukemia and breast cancer. Nat Med. 2015;21(5):457–66.
Kim HM, Kim C-S, Lee J-H, et al. CG0009, a novel glycogen synthase kinase 3 inhibitor, induces cell death through cyclin D1 depletion in breast cancer cells. PLoS ONE. 2013;8(4):e60383.
Fowler AM, Solodin N, Preisler-Mashek MT, Zhang P, Lee AV, Alarid ET. Increases in estrogen receptor-α concentration in breast cancer cells promote serine 118/104/106-independent AF-1 transactivation and growth in the absence of estrogen. FASEB J. 2004;18(1):81–93.
Fowler AM, Solodin NM, Valley CC, Alarid ET. Altered target gene regulation controlled by estrogen receptor-α concentration. Mol Endocrinol. 2006;20(2):291–301.
Lumachi F, Brunello A, Maruzzo M, Basso U, M.M Basso S,. Treatment of estrogen receptor-positive breast cancer. Curr Med Chem. 2013;20(5):596–604.
Kabel AM, Elkhoely AA. Ameliorative potential of fluoxetine/raloxifene combination on experimentally induced breast cancer. Tissue Cell. 2016;48(2):89–95.
Han HH, Lee SH, Kim BG, Lee JH, Kang S, Cho NH. Estrogen receptor status predicts late-onset skeletal recurrence in breast cancer patients. Medicine. 2016;95(8):e2909.
Beije N, Onstenk W, Kraan J, et al. Prognostic impact of HER2 and ER status of circulating tumor cells in metastatic breast cancer patients with a HER2-negative primary tumor. Neoplasia. 2016;18(11):647–53.
Gulbahce HE, Blair CK, Sweeney C, Salama ME. Quantification of estrogen receptor expression in normal breast tissue in postmenopausal women with breast cancer and association with tumor subtypes. Appl Immunohistochem Mol Morphol. 2017;25(8):548–52.
Oh H, Eliassen AH, Wang M, et al. Expression of estrogen receptor, progesterone receptor, and Ki67 in normal breast tissue in relation to subsequent risk of breast cancer. NPJ Breast Cancer. 2016;2(1):1–3.
Article Google Scholar
Caruana D, Wei W, Martinez-Morilla S, Rimm DL, Reisenbichler ES. Association between low estrogen receptor positive breast cancer and staining performance. NPJ Breast Cancer. 2020;6(1):1–6.
Carroll J. EJE PRIZE 2016: mechanisms of oestrogen receptor (ER) gene regulation in breast cancer. Eur J Endocrinol. 2016;175(1):R41–9.
Bae SY, Kim S, Lee JH, et al. Poor prognosis of single hormone receptor-positive breast cancer: similar outcome as triple-negative breast cancer. BMC Cancer. 2015;15(1):138.
Ma CX, Reinert T, Chmielewska I, Ellis MJ. Mechanisms of aromatase inhibitor resistance. Nat Rev Cancer. 2015;15(5):261–75.
Jeselsohn R, Yelensky R, Buchwalter G, et al. Emergence of constitutively active estrogen receptor-α mutations in pretreated advanced estrogen receptor–positive breast cancer. Clin Cancer Res. 2014;20(7):1757–67.
Kumar S, Lindsay D, Chen QB, et al. Tracking plasma DNA mutation dynamics in estrogen receptor positive metastatic breast cancer with dPCR-SEQ. NPJ Breast Cancer. 2018;4(1):1–5.
Article CAS Google Scholar
Schiavon G, Hrebien S, Garcia-Murillas I, et al. Analysis of ESR1 mutation in circulating tumor DNA demonstrates evolution during therapy for metastatic breast cancer. Sci Transl Med. 2015;7(313):313ra182-313ra182.
Jeselsohn R, Buchwalter G, De Angelis C, Brown M, Schiff R. ESR1 mutations—a mechanism for acquired endocrine resistance in breast cancer. Nat Rev Clin Oncol. 2015;12(10):573.
Helena AY, Riely GJ, Lovly CM. Therapeutic strategies utilized in the setting of acquired resistance to EGFR tyrosine kinase inhibitors. Clin Cancer Res. 2014;20(23):5898–907.
Brady SW, Zhang J, Seok D, Wang H, Yu D. Enhanced PI3K p110α signaling confers acquired lapatinib resistance that can be effectively reversed by a p110α-selective PI3K inhibitor. Mol Cancer Ther. 2014;13(1):60–70.
Rosenzweig SA. Acquired resistance to drugs targeting receptor tyrosine kinases. Biochem Pharmacol. 2012;83(8):1041–8.
Carausu M, Bidard F-C, Callens C, et al. ESR1 mutations: a new biomarker in breast cancer. Expert Rev Mol Diagn. 2019;19(7):599–611.
Toy W, Shen Y, Won H, et al. ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nat Genet. 2013;45(12):1439.
De Santo I, McCartney A, Migliaccio I, Di Leo A, Malorni L. The emerging role of ESR1 mutations in luminal breast cancer as a prognostic and predictive biomarker of response to endocrine therapy. Cancers. 2019;11(12):1894.
Takeshita T, Yamamoto Y, Yamamoto-Ibusuki M, et al. Clinical significance of plasma cell-free DNA mutations in PIK3CA, AKT1, and ESR1 gene according to treatment lines in ER-positive breast cancer. Mol Cancer. 2018;17(1):1–6.
O’Leary B, Cutts RJ, Liu Y, et al. The genetic landscape and clonal evolution of breast cancer resistance to palbociclib plus fulvestrant in the PALOMA-3 trial. Cancer Discov. 2018;8(11):1390–403.
Reinert T, Coelho GP, Mandelli J, et al. Association of ESR1 mutations and visceral metastasis in patients with estrogen receptor-positive advanced breast cancer from Brazil. J Oncol. 2019;2019:1947215.
English DP, Roque DM, Santin AD. HER2 expression beyond breast cancer: therapeutic implications for gynecologic malignancies. Mol Diagn Ther. 2013;17(2):85–99.
Ishikawa T, Ichikawa Y, Shimizu D, et al. The role of HER-2 in Breast Cancer. J Surg Sci. 2014;2(1):4.
Winstanley J, Cooke T, Murray G, et al. The long term prognostic significance of c-erbB-2 in primary breast cancer. Br J Cancer. 1991;63(3):447.
Krishnamurti U, Silverman JF. HER2 in breast cancer: a review and update. Adv Anat Pathol. 2014;21(2):100–7.
Cao W, Zhang B, Liu Y, et al. High-level SLP-2 expression and HER-2/neu protein expression are associated with decreased breast cancer patient survival. Am J Clin Pathol. 2007;128(3):430–6.
Reix N, Malina C, Chenard M-P, et al. A prospective study to assess the clinical utility of serum HER2 extracellular domain in breast cancer with HER2 overexpression. Breast Cancer Res Treat. 2016;160(2):249–59.
Dieci MV, Miglietta F, Griguolo G, Guarneri V. Biomarkers for HER2-positive metastatic breast cancer: beyond hormone receptors. Cancer Treat Rev. 2020;88:102064.
Pegram MD, Konecny GE, O’Callaghan C, Beryt M, Pietras R, Slamon DJ. Rational combinations of trastuzumab with chemotherapeutic drugs used in the treatment of breast cancer. J Natl Cancer Inst Monogr. 2004;96(10):739–49.
Duffy M, Harbeck N, Nap M, et al. Clinical use of biomarkers in breast cancer: updated guidelines from the European Group on Tumor Markers (EGTM). Eur J Cancer. 2017;75:284–98.
Wu J-R, Zhao Y, Zhou X-P, Qin X. Estrogen receptor 1 and progesterone receptor are distinct biomarkers and prognostic factors in estrogen receptor-positive breast cancer: Evidence from a bioinformatic analysis. Biomed Pharmacother. 2020;121:109647.
Daniel AR, Hagan CR, Lange CA. Progesterone receptor action: defining a role in breast cancer. Expert Rev Endocrinol Metab. 2011;6(3):359–69.
Mohammed H, Russell IA, Stark R, et al. Progesterone receptor modulates ERα action in breast cancer. Nature. 2015;523(7560):313–7.
Carroll JS, Hickey TE, Tarulli GA, Williams M, Tilley WD. Deciphering the divergent roles of progestogens in breast cancer. Nat Rev Cancer. 2017;17(1):54–64.
Elledge RM, Green S, Pugh R, et al. Estrogen receptor (ER) and progesterone receptor (PgR), by ligand-binding assay compared with ER, PgR and pS2, by immuno-histochemistry in predicting response to tamoxifen in metastatic breast cancer: a Southwest Oncology Group study. Int J Cancer. 2000;89(2):111–7.
Chen X, Yuan Y, Gu Z, Shen K. Accuracy of estrogen receptor, progesterone receptor, and HER2 status between core needle and open excision biopsy in breast cancer: a meta-analysis. Breast Cancer Res Treat. 2012;134(3):957–67.
Fang C, Cao Y, Liu X, Zeng X-T, Li Y. Serum CA125 is a predictive marker for breast cancer outcomes and correlates with molecular subtypes. Oncotarget. 2017;8(38):63963.
Shao Y, Sun X, He Y, Liu C, Liu H. Elevated levels of serum tumor markers CEA and CA15–3 are prognostic parameters for different molecular subtypes of breast cancer. PLoS ONE. 2015;10(7):e0133830.
Gold P, Freedman SO. Specific carcinoembryonic antigens of the human digestive system. J Exp Med. 1965;122(3):467–81.
Wu S-g, He Z-y, Zhou J, et al. Serum levels of CEA and CA15–3 in different molecular subtypes and prognostic value in Chinese breast cancer. Breast. 2014;23(1):88–93.
Duffy MJ, Evoy D, McDermott EW. CA 15–3: uses and limitation as a biomarker for breast cancer. Clin Chim Acta. 2010;411(23–24):1869–74.
Li J, Liu L, Feng Z, et al. Tumor markers CA15–3, CA125, CEA and breast cancer survival by molecular subtype: a cohort study. Breast Cancer. 2020:1–10.
Molina R, Barak V, van Dalen A, et al. Tumor markers in breast cancer–European Group on Tumor Markers recommendations. Tumor Biol. 2005;26(6):281–93.
Marić P, Ozretić P, Levanat S, Orešković S, Antunac K, Beketić-Orešković L. Tumor markers in breast cancer-evaluation of their clinical usefulness. Coll Antropol. 2011;35(1):241–7.
Duffy MJ, Duggan C, Keane R, et al. High preoperative CA 15–3 concentrations predict adverse outcome in node-negative and node-positive breast cancer: study of 600 patients with histologically confirmed breast cancer. Clin Chem. 2004;50(3):559–63.
Kumpulainen EJ, Keskikuru RJ, Johansson RT. Serum tumor marker CA 15.3 and stage are the two most powerful predictors of survival in primary breast cancer. Breast Cancer Res Treat. 2002;76(2):95–102.
Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes—dealing with the diversity of breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22(8):1736–47.
Park B-W, Oh J-W, Kim J-H, et al. Preoperative CA 15–3 and CEA serum levels as predictor for breast cancer outcomes. Ann Oncol. 2008;19(4):675–81.
Molina R, Auge JM, Farrus B, et al. Prospective evaluation of carcinoembryonic antigen (CEA) and carbohydrate antigen 15.3 (CA 15.3) in patients with primary locoregional breast cancer. Clin Chem. 2010;56(7):1148–57.
Lee JS, Park S, Park JM, Cho JH, Kim SI, Park B-W. Elevated levels of serum tumor markers CA 15–3 and CEA are prognostic factors for diagnosis of metastatic breast cancers. Breast Cancer Res Treat. 2013;141(3):477–84.
Lee JS, Magbanua MJM, Park JW. Circulating tumor cells in breast cancer: applications in personalized medicine. Breast Cancer Res Treat. 2016;160(3):411–24.
Smerage JB, Barlow WE, Hortobagyi GN, et al. Circulating tumor cells and response to chemotherapy in metastatic breast cancer: SWOG S0500. J Clin Oncol. 2014;32(31):3483.
Le Du F, Ueno NT, Gonzalez-Angulo AM. Breast cancer biomarkers: utility in clinical practice. Curr Breast Cancer Rep. 2013;5(4):284–92.
Munzone E, Nolé F, Goldhirsch A, et al. Changes of HER2 status in circulating tumor cells compared with the primary tumor during treatment for advanced breast cancer. Clin Breast Cancer. 2010;10(5):392–7.
Pestrin M, Bessi S, Puglisi F, et al. Final results of a multicenter phase II clinical trial evaluating the activity of single-agent lapatinib in patients with HER2-negative metastatic breast cancer and HER2-positive circulating tumor cells. A proof-of-concept study. Breast Cancer Res Treat. 2012;134(1):283–9.
Cristofanilli M, Valero V, Mangalik A, et al. Phase II, randomized trial to compare anastrozole combined with gefitinib or placebo in postmenopausal women with hormone receptor-positive metastatic breast cancer. Clin Cancer Res. 2010;16(6):1904–14.
Nadal R, Fernandez A, Sanchez-Rovira P, et al. Biomarkers characterization of circulating tumour cells in breast cancer patients. Breast Cancer Res. 2012;14(3):1–12.
Soliman NA, Yussif SM. Ki-67 as a prognostic marker according to breast cancer molecular subtype. Cancer Biol Med. 2016;13(4):496.
Penault-Llorca F, Radosevic-Robin N. Ki67 assessment in breast cancer: an update. Pathology. 2017;49(2):166–71.
Feng X, Li H, Kornaga EN, et al. Low Ki67/high ATM protein expression in malignant tumors predicts favorable prognosis in a retrospective study of early stage hormone receptor positive breast cancer. Oncotarget. 2016;7(52):85798.
Pan Y, Yuan Y, Liu G, Wei Y. P53 and Ki-67 as prognostic markers in triple-negative breast cancer patients. PLoS ONE. 2017;12(2):e0172324.
Clarke RB. Steroid receptors and proliferation in the human breast. Steroids. 2003;68(10–13):789–94.
Harvey JA, Santen RJ, Petroni GR, et al. Histologic changes in the breast with menopausal hormone therapy use: correlation with breast density, estrogen receptor, progesterone receptor, and proliferation indices. Menopause. 2008;15(1):67.
Zhou C-j, Zhang Q-h, Zhang T-g, et al. Expression of ER, Ki-67 and cylinD1 in the pre-cancerous breast of Chinese patients. Pathol Oncol Res. 2009;15(2):153–8.
Petrelli F, Viale G, Cabiddu M, Barni S. Prognostic value of different cut-off levels of Ki-67 in breast cancer: a systematic review and meta-analysis of 64,196 patients. Breast Cancer Res Treat. 2015;153(3):477–91.
Li P-J, Jin T, Luo D-H, et al. Effect of prolonged radiotherapy treatment time on survival outcomes after intensity-modulated radiation therapy in nasopharyngeal carcinoma. PLoS ONE. 2015;10(10):e0141332.
Fabian CJ, Kimler BF, Zalles CM, et al. Reduction in proliferation with six months of letrozole in women on hormone replacement therapy. Breast Cancer Res Treat. 2007;106(1):75–84.
Okumura Y, Yamamoto Y, Zhang Z, et al. Identification of biomarkers in ductal carcinoma in situ of the breast with microinvasion. BMC Cancer. 2008;8(1):287.
Ringberg A, Anagnostaki L, Anderson H, Idvall I, Fernö M. Cell biological factors in ductal carcinoma in situ (DCIS) of the breast-relationship to ipsilateral local recurrence and histopathological characteristics. Eur J Cancer. 2001;37(12):1514–22.
Haroon S, Hashmi AA, Khurshid A, et al. Ki67 index in breast cancer: correlation with other prognostic markers and potential in Pakistani patients. Asian Pac J Cancer Prev. 2013;14(7):4353–8.
Nishimura R, Osako T, Okumura Y, Hayashi M, Toyozumi Y, Arima N. Ki-67 as a prognostic marker according to breast cancer subtype and a predictor of recurrence time in primary breast cancer. Exp Ther Med. 2010;1(5):747–54.
Ragab HM, Samy N, Afify M, Abd El Maksoud N, Shaaban HM. Assessment of Ki-67 as a potential biomarker in patients with breast cancer. J Genet Eng Biotechnol. 2018;16(2):479–84.
Yerushalmi R, Woods R, Ravdin PM, Hayes MM, Gelmon KA. Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol. 2010;11(2):174–83.
Eeckhoute J, Carroll JS, Geistlinger TR, Torres-Arzayus MI, Brown M. A cell-type-specific transcriptional network required for estrogen regulation of cyclin D1 and cell cycle progression in breast cancer. Genes Dev. 2006;20(18):2513–26.
Ortiz AB, Garcia D, Vicente Y, Palka M, Bellas C, Martin P. Prognostic significance of cyclin D1 protein expression and gene amplification in invasive breast carcinoma. PLoS ONE. 2017;12(11):e0188068.
Lamb R, Lehn S, Rogerson L, Clarke RB, Landberg G. Cell cycle regulators cyclin D1 and CDK4/6 have estrogen receptor-dependent divergent functions in breast cancer migration and stem cell-like activity. Cell Cycle. 2013;12(15):2384–94.
Roy PG, Pratt N, Purdie CA, et al. High CCND1 amplification identifies a group of poor prognosis women with estrogen receptor positive breast cancer. Int J Cancer. 2010;127(2):355–60.
Khordadmehr M, Shahbazi R, Sadreddini S, Baradaran B. miR-193: a new weapon against cancer. J Cell Physiol. 2019;234(10):16861–72.
Chan M, Liaw CS, Ji SM, et al. Identification of circulating microRNA signatures for breast cancer detection. Clin Cancer Res. 2013;19(16):4477–87.
Heneghan HM, Miller N, Kerin MJ. Circulating miRNA signatures: promising prognostic tools for cancer. J Clin Oncol. 2010;28(29):e573–4.
Kodahl AR, Lyng MB, Binder H, et al. Novel circulating microRNA signature as a potential non-invasive multi-marker test in ER-positive early-stage breast cancer: a case control study. Mol Oncol. 2014;8(5):874–83.
Shimomura A, Shiino S, Kawauchi J, et al. Novel combination of serum microRNA for detecting breast cancer in the early stage. Cancer Sci. 2016;107(3):326–34.
Hagrass HA, Sharaf S, Pasha HF, Tantawy EA, Mohamed RH, Kassem R. Circulating microRNAs-a new horizon in molecular diagnosis of breast cancer. Genes Cancer. 2015;6(5–6):281.
Bahmanpour Z, Sheervalilou R, Choupani J, Shekari Khaniani M, Montazeri V, Mansoori DS. A new insight on serum microRNA expression as novel biomarkers in breast cancer patients. J Cell Physiol. 2019;234(11):19199–211.
Wu Z-s, Wang C-q, Xiang R, et al. Loss of miR-133a expression associated with poor survival of breast cancer and restoration of miR-133a expression inhibited breast cancer cell growth and invasion. BMC Cancer. 2012;12(1):1–10.
Wu X, Somlo G, Yu Y, et al. De novo sequencing of circulating miRNAs identifies novel markers predicting clinical outcome of locally advanced breast cancer. J Transl Med. 2012;10(1):42.
Zheng R, Pan L, Gao J, et al. Prognostic value of miR-106b expression in breast cancer patients. J Surg Res. 2015;195(1):158–65.
Wang H, Tan G, Dong L, et al. Circulating MiR-125b as a marker predicting chemoresistance in breast cancer. PLoS ONE. 2012;7(4):e34210.
Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–74.
Guo H, Ding Q, Gong Y, et al. Comparison of three scoring methods using the FDA-approved 22C3 immunohistochemistry assay to evaluate PD-L1 expression in breast cancer and their association with clinicopathologic factors. Breast Cancer Res. 2020;22:69.
Muenst S, Schaerli AR, Gao F, et al. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2014;146:15–24.
Baptista MZ, Sarian LO, Derchain SFM, Pinto GA, Vassallo J. Prognostic significance of PD-L1 and PD-L2 in breast cancer. Hum Pathol. 2016;47:78–84.
Qin T, Zeng Y, Qin G, et al. High PD-L1expression was associated with poor prognosis in 870 Chinese patients with breast cancer. Oncotarget. 2015;6:33972–81.
Sabatier R, Finetti P, Mamessier E, et al. Prognostic and predictive value of PDL1 expression in breast cancer. Oncotarget. 2014;6:5449–64.
Article PubMed Central Google Scholar
Schmid P, Adams S, Rugo HS, et al. Atezolizumaband nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379:2108–21.
Wimberly H, Brown JR, Schalper K, et al. PD-L1 expression correlates with tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy in breast cancer. Cancer Immunol Res. 2015;3:326–32.
Salgado R, Denkert C, DeMaria S, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by anInternational TILs Working Group 2014. Ann Oncol. 2015;26:259–71.
Denkert C, Wienert S, Poterie A, et al. Standardized evaluation of tumor-infiltrating lymphocytes in breast cancer: Results of the ring studies of the international immuno-oncology biomarker working group. Mod Pathol. 2016;29:1155–64.
Denkert C, Von Minckwitz G, Darb-Esfahani S, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19:40–50.
Skriver SK, Jensen MB, Knoop AS, Ejlertsen B, Laenkholm AV. Tumour-infiltrating lymphocytes and response toneoadjuvant letrozole in patients with early oestrogen receptor-positive breast cancer: Analysis from a nationwide phase II DBCGtrial. Breast Cancer Res. 2020;22:46.
Harris L, Fritsche H, Mennel R, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25(33):5287–312.
Zervoudis S, Peitsidis P, Iatrakis G, et al. Increased levels of tumor markers in the follow-up of 400 patients with breast cancer without recurrence or metastasis: interpretation of false-positive results. J BUON. 2007;12(4):487.
Barrington SF, Mikhaeel NG, Kostakoglu L, et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol. 2014;32(27):3048.
van den Abbeele AD, Badawi RD. Use of positron emission tomography in oncology and its potential role to assess response to imatinib mesylate therapy in gastrointestinal stromal tumors (GISTs). Eur J Cancer. 2002;38:S60–5.
Burzykowski T, Buyse M, Piccart-Gebhart MJ, et al. Evaluation of tumor response, disease control, progression-free survival, and time to progression as potential surrogate end points in metastatic breast cancer. J Clin Oncol. 2008;26(12):1987–92.
Niikura N, Liu J, Hayashi N, et al. Loss of human epidermal growth factor receptor 2 (HER2) expression in metastatic sites of HER2-overexpressing primary breast tumors. J Clin Oncol. 2012;30(6):593.
Hoefnagel LD, van de Vijver MJ, van Slooten H-J, et al. Receptor conversion in distant breast cancer metastases. Breast Cancer Res. 2010;12(5):1–9.
Download references
The authors did not receive support from any organization for the submitted review.
Author information
Authors and affiliations.
Department of Medical Physics, Iran University of Medical Sciences, Tehran, Iran
Elaheh Tarighati
School of Paramedicine, Shahroud University of Medical Sciences, Shahroud, Iran
Hadi Keivan
Radiation Applications Research School, Nuclear Science and Technology Research Institute, P.O. Box: 14395-836, Tehran, Iran
Hojjat Mahani
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Hojjat Mahani .
Ethics declarations
Conflict of interest.
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable.
Informed consent
Additional information, publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Tarighati, E., Keivan, H. & Mahani, H. A review of prognostic and predictive biomarkers in breast cancer. Clin Exp Med 23 , 1–16 (2023). https://doi.org/10.1007/s10238-021-00781-1
Download citation
Received : 04 September 2021
Accepted : 01 December 2021
Published : 15 January 2022
Issue Date : February 2023
DOI : https://doi.org/10.1007/s10238-021-00781-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Tumor markers
- Find a journal
- Publish with us
- Track your research
Waiting time for radiation therapy in non-metastatic, surgically-treated breast cancer patients in Quebec
Contenu téléchargeable.
- Fortin, Bernard, 1970-
- Goldberg, Mark (Supervisor)
- The purpose of this study was to determine among surgically treated non-metastatic breast cancer patients in the province of Quebec the distribution of the time between surgery and post-operative radiation therapy (RT) as well as secular trends and other factors influencing waiting time. Using administrative records, I identified between 1992 and 1998 29,105 episodes of breast cancer and 17,704 of these contained an indication of receiving RT Hierarchical linear regression models were used to identify predictors of waiting time. The number of cases of breast cancer increased by 5.5% per year while the number of those receiving RT increased by 9%. Median post-surgery waiting time was 75 days in 1992 and by 1998 it had increased by 63% (95% Confidence Interval (CI) 35%--97%) among patients not requiring chemotherapy. In patients receiving chemotherapy, post-chemotherapy waiting time increased from 21 to 30 days (35% increase between 1998 and 1992 (95% CI -3%--88%)). In addition to a significant variability of waiting time according to radiation therapy centre, predictors of shorter waiting times were earlier year of treatment, localised cancer stage, breast conserving surgery, early consultation with a radiation oncologist, being operated in a centre with a radiation therapy facility, living close to a radiation therapy facility, and living in a higher socio-economic area. In conclusion, waiting time to start of radiation therapy after localised breast cancer increased substantially in Quebec from 1992 to 1998. Possible explanations include increased demand, insufficient resources and changes in the indications for breast conserving surgery and RT.
- Health Sciences, Public Health.
- Health Sciences, Oncology.
- McGill University
- https://escholarship.mcgill.ca/concern/theses/bg257f76f
- All items in eScholarship@McGill are protected by copyright with all rights reserved unless otherwise indicated.
- Department of Epidemiology, Biostatistics and Occupational Health
- Master of Science
- Theses & Dissertations
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
April 24, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
Study highlights increased risk of second cancers among breast cancer survivors
by University of Cambridge
Survivors of breast cancer are at significantly higher risk of developing second cancers, including endometrial and ovarian cancer for women and prostate cancer for men, according to new research studying data from almost 600,000 patients in England.
For the first time, the research has shown that this risk is higher in people living in areas of greater socioeconomic deprivation.
Breast cancer is the most commonly diagnosed cancer in the UK. Around 56,000 people in the UK are diagnosed each year, the vast majority (over 99%) of whom are women. Improvements in earlier diagnosis and in treatments mean that five year survival rates have been increasing over time, reaching 87% by 2017 in England.
People who survive breast cancer are at risk of a second primary cancer, but until now the exact risk has been unclear. Previously published research suggested that women and men who survive breast cancer are at a 24% and 27% greater risk of a non-breast second primary cancer than the wider population respectively. There have been also suggestions that second primary cancer risks differ by the age at breast cancer diagnosis .
To provide more accurate estimates, a team led by researchers at the University of Cambridge analyzed data from over 580,000 female and over 3,500 male breast cancer survivors diagnosed between 1995 and 2019 using the National Cancer Registration Dataset. The results of their analysis are published in The Lancet Regional Health—Europe .
First author Isaac Allen from the Department of Public Health and Primary Care at the University of Cambridge said, "It's important for us to understand to what extent having one type of cancer puts you at risk of a second cancer at a different site. The female and male breast cancer survivors whose data we studied were at increased risk of a number of second cancers. Knowing this can help inform conversations with their care teams to look out for signs of potential new cancers."
The researchers found significantly increased risks of cancer in the contralateral (that is, unaffected) breast and for endometrium and prostate cancer in females and males, respectively. Females who survived breast cancer were at double the risk of contralateral breast cancer compared to the general population and at 87% greater risk of endometrial cancer, 58% greater risk of myeloid leukemia and 25% greater risk of ovarian cancer .
Age of diagnosis was important, too—females diagnosed with breast cancer under the age of 50 were 86% more likely to develop a second primary cancer compared to the general population of the same age, whereas women diagnosed after age 50 were at a 17% increased risk. One potential explanation is that a larger number of younger breast cancer survivors may have inherited genetic alterations that increase risk for multiple cancers. For example, women with inherited changes to the BRCA1 and BRCA2 genes are at increased risk of contralateral breast cancer, ovarian and pancreatic cancer.
Females from the most socioeconomically deprived backgrounds were at 35% greater risk of a second primary cancer compared to females from the least deprived backgrounds. These differences were primarily driven by non-breast cancer risks, particularly for lung, kidney, head and neck, bladder, esophageal and stomach cancers. This may be because smoking, obesity, and alcohol consumption —established risk factors for these cancers—are more common among more deprived groups.
Allen, a Ph.D. student at Clare Hall, added, "This is further evidence of the health inequalities that people from more deprived backgrounds experience. We need to fully understand why they are at greater risk of second cancers so that we can intervene and reduce this risk."
Male breast cancer survivors were 55 times more likely than the general male population to develop contralateral breast cancer—though the researchers stress that an individual's risk was still very low. For example, for every 100 men diagnosed with breast cancer at age 50 or over, about three developed contralateral breast cancer during a 25 year period. Male breast cancer survivors were also 58% more likely than the general male population to develop prostate cancer.
Professor Antonis Antoniou from the Department of Public Health and Primary Care at the University of Cambridge, the study's senior author, said, "This is the largest study to date to look at the risk in breast cancer survivors of developing a second cancer. We were able to carry this out and calculate more accurate estimates because of the outstanding data sets available to researchers through the NHS."
Cancer Research UK's senior cancer intelligence manager, Katrina Brown, said, "This study shows us that the risk of second primary cancers is higher in people who have had breast cancer, and this can differ depending on someone's socioeconomic background. But more research is needed to understand what is driving this difference and how to tackle these health inequalities."
Explore further
Feedback to editors

Link between depression and cardiovascular disease explained: They partly develop from same gene module
3 hours ago

Opioids during pregnancy not linked to substantially increased risk of psychiatric disorders in children
9 hours ago

Research identifies pitfalls and opportunities for generative AI in patient messaging systems

A closed-loop drug-delivery system could improve chemotherapy
10 hours ago

It's easier now to treat opioid addiction with medication—but use has changed little, study finds

Solving the riddle of the sphingolipids in coronary artery disease

New AI technology estimates brain age using low-cost EEG device
11 hours ago

Alteration of brain network condition could predict painful vaso-occlusive crisis in patients with sickle cell disease
12 hours ago

Trials reveal that internet-based conversations help sustain brain function in older adults

Study finds that a dash of exercise can help students focus and enjoy university lectures
Related stories.

Assessing breast cancer risk
Mar 18, 2024

More targeted cancer prevention and early detection strategies needed in breast cancer survivorship
May 18, 2021

Health: Breast cancer in men
Nov 1, 2023
Can eating nuts have health-protective effects for breast cancer survivors?
Oct 20, 2021
Weight linked to risk of second cancer after breast cancer
Apr 12, 2021

Drinking alcohol not likely to increase risk of a breast cancer recurrence
Aug 9, 2023
Recommended for you

New potential avenues for cancer therapies through RNA-binding proteins
14 hours ago

Newly discovered mechanism helps tumor cells evade the immune system early on
16 hours ago

Mini-colons advance colorectal cancer research

CAR T cell therapy targeting HER2 antigen shows promise against advanced sarcoma in clinical trial
19 hours ago

Researchers identify novel gene networks associated with aggressive type of breast cancer
13 hours ago
Let us know if there is a problem with our content
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
Relationship With Partner Affects Outcomes for Breast Cancer Survivors
By Dennis Thompson HealthDay Reporter

MONDAY, April 22, 2024 (HealthDay News) -- A strong relationship can help a breast cancer survivor thrive in the aftermath of their terrible ordeal, a new study finds.
Diagnosis and treatment of breast cancer places tremendous stress on the women and their partners, researchers said.
Those women in a solid relationship with their partner tend to have less depression and fatigue following their treatment, as well as better physical functioning, the study results show.
For example, they were better able to carry groceries, walk around the block and perform other typical day-to-day tasks, researchers found.
U.S. Cities With the Most Homelessness

On the other hand, weaker relationships were associated with poor emotional and physical outcomes for breast cancer survivors.
“How the breast cancer survivor and partner communicated and handled stressful events, particularly those related to breast cancer, were linked to emotional and physical health for the survivor, with better agreement related to better outcomes,” said lead study author Eric Vachon . He's a research scientist with the Regenstrief Institute and Indiana University School of Nursing.
However, part of the strength of a relationship rests on a shared understanding between the partners, the study also found.
Couples where one person rated the relationship more highly than their partner tended to reap worse outcomes, results show.
“Interestingly, breast cancer survivors who rated their relationship satisfaction as high did not necessarily have better agreement with their partner or better well-being than those survivors who viewed their relationship less positively,” Vachon said. “It’s the communication and relationship between the survivor and partner that are determinant.”
For the study, researchers analyzed survey data from 387 couples, including 220 couples with a breast cancer survivor and 167 with no breast cancer. The average age of study participants was mid-40s.
“We knew from the literature that breast cancer survivors’ rating of their relationship satisfaction is linked with some poor physical and emotional outcomes,” Vachon said in an institute news release.
“We took that knowledge to the next level and combined the breast cancer survivors’ and partners’ views of relationship satisfaction and relationship agreement and determined impact on survivors’ health,” he added.
The satisfaction that breast cancer survivors had with their relationship was significantly associated with better physical function, ability to focus and sleep quality.
The findings were published recently in a special issue of the journal Healthcare .
“This work points to the critical importance of both members of the couple focusing on strengthening the relationship,” Vachon said. “Difficulties among couples can have devastating effects for your physical and emotional health.”
More information
Susan G. Komen has more on social support during breast cancer treatment .
SOURCE: Regenstrief Institute, news release, April 18, 2024
Copyright © 2024 HealthDay . All rights reserved.
Join the Conversation
Tags: breast cancer , marriage
America 2024

Health News Bulletin
Stay informed on the latest news on health and COVID-19 from the editors at U.S. News & World Report.
Sign in to manage your newsletters »
Sign up to receive the latest updates from U.S News & World Report and our trusted partners and sponsors. By clicking submit, you are agreeing to our Terms and Conditions & Privacy Policy .
You May Also Like
The 10 worst presidents.
U.S. News Staff Feb. 23, 2024

Cartoons on President Donald Trump
Feb. 1, 2017, at 1:24 p.m.

Photos: Obama Behind the Scenes
April 8, 2022

Photos: Who Supports Joe Biden?
March 11, 2020

A ‘Fork in the Road’ for Democracy
Lauren Camera April 24, 2024

Johnson at Columbia: ’Stop the Nonsense’
Aneeta Mathur-Ashton April 24, 2024

What to Know: Bird Flu Virus in Milk
Cecelia Smith-Schoenwalder April 24, 2024

High Court to Again Weigh Abortion Law
Laura Mannweiler April 23, 2024

Takeaways From Pecker’s Trump Testimony
Lauren Camera April 23, 2024

Biden, Trump: Trail v. Trial
Cecelia Smith-Schoenwalder April 23, 2024

Deprivation linked to higher second cancer risk among England breast cancer survivors
Cambridge study finds those from poorest areas have 35% higher risk of second non-breast cancer
- Share on Facebook
- Share on Twitter
- Share via Email
Female survivors of breast cancer living in the most deprived areas have a 35% higher risk of developing second, unrelated cancers, compared with those from the most affluent areas, research shows.
Breast cancer is the most commonly diagnosed cancer in the UK, with about 56,000 people being told they have it each year. Improved diagnosis and treatments mean that five-year survival rates are now 86% in England.
People who survive breast cancer have a greater likelihood of second primary (unrelated) cancer, but until now the exact risk has not been clear.
A team of researchers led by the University of Cambridge analysed NHS data from almost 600,000 patients in England and found, compared with the general female population, women who had survived breast cancer had an increased risk of developing 12 other primary cancers.
They had double the risk of developing cancer in the unaffected (contralateral) breast, an 87% higher risk of endometrial cancer, a 58% increased likelihood of myeloid leukaemia and a 25% higher risk of ovarian cancer.
The study, published in Lancet Regional Health – Europe, found that the risk of second primary cancers was higher in people living in areas of greater socioeconomic deprivation.
Compared with the most affluent, the least well-off female survivors of breast cancer had a 166% greater chance of developing lung cancer, a 78% higher risk of stomach cancer, more than 50% increased risk of bladder and oesophagus cancers, 48% higher risk of head and neck cancer and 43% increased risk of kidney cancer.
Overall, those from the most deprived areas had a 35% higher risk of a second non-breast cancer.
This may be because risk factors such as smoking, obesity and alcohol consumption are more common among more deprived groups. A 2023 study found that deprivation causes 33,000 extra cancer cases in the UK each year .
The first author, Isaac Allen, from the department of public health and primary care at the University of Cambridge, said: “This is the biggest study ever to examine second cancers after breast cancer and the first to show that women diagnosed with breast cancer in deprived regions are more likely to get second cancers. Many cancers are caused by deprivation, but more research is clearly needed to identify the specific factors driving the higher risks and how best to reduce these inequalities.”
In addition to data from more than 580,000 women, the authors examined the risk of second primary cancers for more than 3,500 male breast cancer survivors diagnosed between 1995 and 2019 using the national cancer registration dataset.
Male breast cancer survivors were 55 times more likely than the general male population to develop contralateral breast cancer, 58% more likely than the general male population to develop prostate cancer and had four times the risk of thyroid cancer, although the actual numbers of these cancers were low.
Responding to the findings, Prof Pat Price, a leading oncologist and co-founder of the Catch Up With Cancer campaign , said: “This highlights yet another instance of alarming inequalities within cancer, underscoring the urgent need for a dedicated cancer plan. Where one comes from or their socioeconomic status should not determine the chances of developing or surviving cancer.”
Dr Simon Vincent, the director of research, support and influencing at Breast Cancer Now, said while the higher risk of secondary cancer may occur due to genetic factors or the effects of initial breast cancer treatment, more research was needed into the causes of second primary cancers and how to follow up patients completing primary breast cancer treatment.
{{topLeft}}
{{bottomLeft}}
{{topRight}}
{{bottomRight}}
{{heading}}
- Breast cancer
- Women's health
- Social exclusion
- Share on LinkedIn
- Share on WhatsApp
- Share on Messenger
{{#isVideo}} {{/isVideo}}{{#isGallery}} {{/isGallery}}{{#isAudio}} {{/isAudio}} {{#isComment}} {{/isComment}} {{headline}}
- {{ title }}
- Sign in / Register
Switch edition
- {{ displayName }}

COMMENTS
3.3 Breast Cancer worldwide. Breast cancer is the most common type of cancer in women around the globe. It affects women from low, middle and high-income countries (Rohani, Abedi, Omranipour, & Langius-Eklöf, 2015, 2). Breast cancer prevalence has increased within the last few years in middle- and low-income countries.
Breast cancer is the second most commonly diagnosed cancer worldwide, accounting for an estimated 2.1 million new cases in 2018 [1]. Among women, breast cancer is the most commonly diagnosed cancer, accounting for almost 25% of cancer cases, and it is the leading cause of cancer deaths in women worldwide [1].
A common side effect of breast cancer treatment, is change in cognitive function, which research indicates is experienced by 30-60% of breast cancer survivors (Player, Mackenzie, Willis, & Loh, 2014). Symptoms may be subtle or dramatic, and include changes in memory, concentration, language, and executive function.
PHYSICAL ACTIVITY AND BODY IMAGE IN BREAST CANCER SURVIVORS Thesis is under the direction of Shannon L. Mihalko, Ph.D., Assistant Professor of Health and Exercise Science. With earlier detection and improved treatment techniques for breast cancer, more women are surviving breast cancer only to be presented with the challenge of coping with
respondents found that other breast cancer survivors had a better understanding of what they were going through. Breast cancer activism had positive effects on participants' healing processes. The importance of supporting other breast cancer survivors and of contributing to efforts to eradicate breast cancer was reflected in each of the women's
Introduction. The number of survivors of breast cancer (BrCa) has increased since 1990 because of advancements in biomedical technology and medical care that have increased early diagnosis and treatment (World Cancer Research Fund International, 2020).Worldwide, it has been reported that 2,261,419 new cases of BrCa were diagnosed among women, making BrCa the most common cancer worldwide ...
Breast cancer survivors face unique physical and emotional challenges after completing active treatment. This thesis analyzes questionnaires and semi-structured narrative interviews collected from 17 female BCS with the goal of understanding the factors that they deem most significant to their quality of life and identity after completing ...
Keywords Breast cancer · Survivorship · Qualitative · Meta-review · Systematic review Introduction Breast cancer (BC), while highly prevalent globally (highest incidence cancer among women in 159/185 countries) [], 1 has a high survival rateThe average ve-year survival rate in Australia for BC from 2013 to 2017 was 91.5%, compared to
and limited PA advice. The key findings illuminate the differences between the breast cancer survivors' and the breast cancer nurses' perceptions and attitudes. For example, the breast cancer survivors' meanings of PA were related to gaining a sense of 'normality' and control over their overall health and well-being. Whereas, the ...
Breast cancer (BC), while highly prevalent globally (highest incidence cancer among women in 159/185 countries) [], has a high survival rateThe average five-year survival rate in Australia for BC from 2013 to 2017 was 91.5%, compared to 69.7% for all cancer types combined [].Cancer survivors are defined as individuals diagnosed with cancer who have completed their initial cancer treatment ...
To summarise qualitative studies exploring the impact of losing the breast in women breast cancer survivors. Methods. We identify, appraise, and synthesise 12 qualitative studies from 2000 to 2015. Quality appraisal of the studies was examined using the Critical Appraisal Skill Programme Checklist and Sandelowski and Barroso's step to ...
At this time, breast cancer (BrCa) is the leading form of cancer diagnosed in women worldwide (66). Over the past two decades, advances in detection and anti-cancer treatments have led to improved survival rates and a shift in focus to post-treatment care of these patients.
The number of breast cancer survivors has increased since 1990 due to advances in biomedical technology that lead to an increase in early diagnosis and treatment. Research on survivorship has ...
Background: The number of breast cancer survivors has increased since 1990 due to advances in biomedical technology that lead to an increase in early diagnosis and treatment. Research on survivorship has focused on the psychological and treatment aspects of the disease. The goal of this study was focused on exploring the lived experiences of breast cancer survivors from diagnosis, treatment ...
Introduction. In 2020, there were estimated to be 19.3 million new cancer cases worldwide and almost 10 million cancer-related deaths. Female breast cancer was the most commonly diagnosed cancer, with 2.3 million new cases (11.7%), surpassing lung cancer. 1 Thanks to early diagnosis and improved treatment, the survival rate in patients with breast cancer has improved. 2 Almost 88% of patients ...
The number of breast cancer survivors has increased since 1990 due to advances in biomedical technology that lead to an increase in early diagnosis and treatment. Research on survivorship has focused on the psychological and treatment aspects of the disease. The goal of this study was focused on exploring the lived experiences of breast cancer ...
Breast cancer rates and the number of breast cancer survivors have been increasing among women in Iran. Effective responses from healthcare depend on appropriately identifying survivors' needs. This study investigated the experience and needs of breast cancer survivors in different dimensions. In this qualitative content analysis, semi-structured in-depth interviews were conducted from April ...
Likewise, a study of breast cancer survivors with completed surgeries and assistive treatments, ... [master's thesis]. Seoul: The Seoul National University; 2014. Lee I, Park CS. Convergent effects of anxiety, depression, uncertainty, and social support on quality of life in women with thyroid cancer. J Korea Convergence Soc. 2017;8(8):163-76.
Thesis submitted for the degree of Doctor of Philosophy by Wafa Almegewly Cardiff University School of Healthcare Sciences 2017. ii Declaration ... There is a debate in the literature about how breast cancer survivors perceive themselves and make sense of their experiences. This conflict has emerged from cancer advocacy
Breast cancer patients who had finished their primary oncologic treatment at least 6 months ago were randomized to an 8-week group intervention program of either mindful walking or moderate walking. Within the qualitative study part, semi-structured focus group interviews (2 interviews per study arm) were conducted and analyzed using a ...
Breast cancer patients account for as much as 36% of oncological patients. ... During the recent years, a thesis has been put forward that triple-negative breast cancer is a separate, heterogenic subtype of breast cancer, formed in the mechanism of different oncogenesis pathways, characterized by different prognoses and dependent on various ...
Breast cancer is the second most common cancer in women, surpassed only by skin cancer (National Institute of Health, 2019). In 2019, approximately 268,000 women were diagnosed with breast cancer (National Cancer Institute, 2020). Approximately seven out of a hundred women will develop breast cancer before the age of seventy (Centers for Disease
Second primary cancers (SPCs) after breast cancer (BC) present an increasing public health burden, with little existing research on socio-demographic, tumour, and treatment effects. We addressed this in the largest BC survivor cohort to date, using a novel linkage of National Disease Registration ...
A total of 387 women (220 breast cancer survivors on average six years out from time of diagnosis and 167 controls without previous cancer diagnosis) and 387 partners (all male, although both male ...
Breast cancer (BC) is a common cancer all over the world that affects women. BC is one of the leading causes of cancer mortality in women, which today has decreased with the advancement of technology and new diagnostic and therapeutic methods. BCs are histologically divided into in situ and invasive carcinoma, and both of them can be divided into ductal and lobular. The main function after the ...
Waiting time for radiation therapy in non-metastatic, surgically-treated breast cancer patients in Quebec. Skip to Content. Toggle navigation Hyrax: Discover theses, dissertations, articles, and more. eScholarship is McGill University's institutional digital repository featuring electronic, open access outputs of McGill researchers and ...
Background. Breast cancer is the most frequent malignancy affecting women across all ethnic groups in the United States. In 2013, an estimated 232 340 new cases of invasive breast cancer were expected to be diagnosed among women. 1 At the same time, the number of breast cancer survivors is increasing due primarily to advances in biomedical technology leading to an increase in early diagnosis ...
The female and male breast cancer survivors whose data we studied were at increased risk of a number of second cancers. Knowing this can help inform conversations with their care teams to look out ...
For the study, researchers analyzed survey data from 387 couples, including 220 couples with a breast cancer survivor and 167 with no breast cancer. The average age of study participants was mid-40s.
Female survivors of breast cancer living in the most deprived areas have a 35% higher risk of developing second, unrelated cancers, compared with those from the most affluent areas, research shows. Breast cancer is the most commonly diagnosed cancer in the UK, with about 56,000 people being told they have it each year.