
NECO Chemistry Questions and Answers 2022 (100% Sure) Theory & Obj Expo Answers
NECO Chemistry Questions and Answers 2022 is now release for the June/July 2022. NECO Chemistry Theory and Objective Answers (100%legit) Chemistry Essay verified Free (Expo) for National Examination Council. NECO Chemistry Questions For you to have good NECO result in Chemistry as well as repeated questions for free in this post. You will also understand how NECO Chemistry questions are set and how to answer them. The National Examination Council is an examination body in Nigeria that conducts the Senior Secondary Certificate Examination and the General Certificate in Education in June/July and November/December respectively.
For those that would like to see the question paper, you can click on the link below:
Click Here To see All NECO Chemistry Questions Paper & Answers
How To Join The Vip Mid-Night Chemistry Answers Group
Chemistry SUBSCRIPTION is #500 Chat me up on WhatsApp 09068683336 ===========================================
******* Payment For All art Subject : N3000 ***** [including practical] ****** Payment For All Science Subject: N3500 ***** [including practical] ===========================================
****** Payment For All Subject: N6000 ***** [including Science, art and Commercial] ===========================================
Chemistry SUBSCRIPTION is #500 Chat me up on WhatsApp 09068683336
Note : You can join our free NECO Questions and Answers 2022 expo Telegram Group To know when the questions and answers are available:
Click Here To Join Joberplanet Free NECO expo Telegram Group
Please Note that the NECO 2022 Chemistry Questions and Answers and any other NECO expo is provided by us for free. We understand that a lot of website charge of collect money from student to provide NECO expo Chemistry Answers to them. NECO questions and answers are provided for free. We will do same during Other Exam like WAEC GCE.
NECO Chemistry Questions and Answers 2022
NECO 2022 Chemistry Questions will be posted in this page. Our Team are right now with the question paper. It is under verification and once tthe verification process complete, we will go ahead to upload it here.

Click Here To See All Chemistry Question Paper
Be at alert! Keep refreshing this page for NECO Chemistry Theory Questions and Answers
2022 Chemistry Essay Questions Loading 93.8%
»» Click Here To Check Your JAMB Result »» Click Here To Check 2022 WAEC Result »» Click Here To Check 2022 NECO TIMETABLE
NECO Chemistry OBJECTIVES (OBJ) ANSWERS Loading
2022 VERIFIED Chemistry OBJ:…Loading
NECO Chemistry -OBJ-joberplanet.com Answers Loading…. Please Check back later. We are Joberplanet °°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°° °°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°
Chemistry+Obj! 1EBAAAAAACC 11BCBCACADCA 21AACBDDAACD 31CCACCACCDE 41EDCCDDBDAC 51CBACCDCBCD
NECO Chemistry Answers
NECO Chemistry Questions and Answers 2022 loading…
Please do not panic and fall into the right hand. 2022 Chemistry Answers will be posted for free here once ready.
We are right now getting things ready for you. The NECO 2022 Chemistry theory questions and answers will be posted any moment from now. All you need do is to keep refreshing this page until you see the answers.
NECO Chemistry -ESSAY-ANSWERS-joberplanet.com Answers Loading…. Please Check back later. We are Joberplanet °°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°° °°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°
(1ai) (i) KAl(SO₄)₂. 12H₂O (ii) Na₂SO₄
(1aii) Number of moles of sodium reascted = reacting mass/molar mass = 3.6/23 = 0.1565moles Number of moles of oxygen reacted = (1/4) x Number of sodium = 1/4 x (3.6/23) = 0.03913 Reacting mass of oxygen = Number of moles x molar mass = 0.03913 x (16×2) = 1.252grams
(1bi) Air in gaseous form is first passed through caustic soda to remove CO₂. It is compressed and cooled until ut becomes a liquid at -200°C. It is then led to a fractionating column. On distillation, Nitrogen which has a lowet boiling point of -196°C is evolced first leaving behind Oxygen in liquid form. Further heating converts the liquid to a gas at -183°C.
(1bii) (i) It is used for combustion (ii) It is used for artificial respiration
(1biii) This is because room temperature is warmer
(1biv) (i) Methyl orange (ii) Phenophthalein
(1ci) An ion is an atom or molecule which has a net electric charge due to the loss or gain of one or more electrons.
(1cii) HCl gas doesn’t contain ions
(1cii) (i) Noble gases: Neon, Helium (ii) Halogens: Iodine, Fluorine (iii) Alkaline earth metals: Calcium, Magnesium
(1civ) Halogens attain stable octet configuration by accepting an electron from donors Group I elements.
(1di) A sol is a colloid where solid particles are dispersed in liquid medium.
(1dii) Jelly
(1diii) (i) Acid-base titrations are used to determine the percentage purity of a substance (ii) Acid-base titrations are important to determine the number of water molecules in a hydrate.
More Answers Loading…. Keep Refreshing
If you have any questions about the NECO Chemistry theory & Obj 2022, kindly let us know in the comment box.
More On NECO Chemistry Questions and Answers 2022
We are happy you are here for the 2022 Chemistry answers. The complete solution will be made free in some minutes before the Chemistry examination.
NECO Chemistry questions 2022, NECO 2022 Chemistry questions and answers, Chemistry NECO 2022, NECO questions 2022/2023, History 2022,
Now Loading…94.5%
Subjects Related To NECO Chemistry Expo Questions & Answers
- NECO Store Management Questions and Answers
- NECO Agric Practical Specimen
- NECO Physics Practical Specimen
- NECO Chemistry Practical Specimen
- NECO Biology Practical Specimen
- NECO Fishery Practical Specimen
- NECO Technical Drawing Practical Specimen
- NECO Data Processing Practical Specimen
- NECO Visual Art Practical Specimen
- NECO Agric Science Questions and Answers
- NECO Geography Questions and Answers
- NECO Physics Questions and Answers
- NECO Further Questions and Answers
- NECO Chemistry Questions and Answers
- NECO CRS Questions and Answers
- WAEC Agric Practical Specimen
- WAEC Biology Practical Specimen
We want to hear from you concerning the NECO Chemistry Questions and Answers 2022 , kindly use the comment section below to get to us.
Related Posts
NECO Data Processing practical Questions and Answers 2024 (100% Sure) Theory Solution
WAEC Marketing Questions and Answers 2023 (100% Sure) Theory & Obj Solution
WAEC Visual Art Questions and Answers 2023 (100% Sure) Theory & Obj Solution
WAEC Physical Education Questions and Answers 2023 (100% Sure) Theory & Obj Solution
WAEC Music Questions and Answers 2023 (100% Sure) Theory & Obj Solution
WAEC IRK/IRS Questions and Answers 2023 (100% Sure) Theory & Obj Solution
Leave a reply.
Save my name, email, and website in this browser for the next time I comment.
Notify me of new posts by email.
Type above and press Enter to search. Press Esc to cancel.

- Form 1 Mathematics Notes
- Form 2 Mathematics Notes
- Form 3 Mathematics Notes
- Form 4 Mathematics Notes
- Form 1 Mathematics Topical Questions and Answers
- Form 2 Mathematics Topical Questions and Answers
- Form 3 Mathematics Topical Questions and Answers
- Form 4 Mathematics Topical Questions and Answers
- Form 1 Functional Writing Notes
- Form 2 Functional Writing Notes
- Form 3 Functional Writing Notes
- Form 4 Functional Writing Notes
- Poetry Notes
- Grammar Notes
- Oral Literature Notes
- Oral Skills Notes
- Guide to Blossoms of the Savannah Summarized Notes - Easy Elimu
- A Doll's House
- The Pearl Study Guide
- Memories We Lost and Other Stories Study Guide
- Inheritance Study Guide
- A Silent song and Other Stories Guide
- Fathers of Nations Guide
- An Artist of the Floating World Guide
- The Samaritan Guide
- Sarufi na Matumizi ya Lugha
- Isimu Jamii Notes
- Fasihi Notes
- Ushairi Notes
- Mwongozo wa Kuandika Insha
- Tumbo Lililoshiba na Hadithi Nyingine
- Mwongozo wa Kigogo
- Mwongozo wa Chozi La Heri - Chozi la Heri Notes PDF
- Mwongozo wa Bembea ya Maisha - Bembea ya Maisha Notes PDF
- Mwongozo wa Nguu za Jadi
- Mwongozo wa Mapambazuko ya Machweo na Hadithi Nyingine
- Biology Form 1 Notes
- Biology Form 2 Notes
- Biology Form 3 Notes
- Biology Form 4 Notes
- Biology Essays
- Form 1 Biology Topical Revision Questions and Answers
- Form 2 Biology Topical Revision Questions and Answers
- Form 3 Biology Topical Revision Questions and Answers
- Form 4 Biology Topical Revision Questions and Answers
- Form 1 Chemistry Notes
- Form 2 Chemistry Notes
- Form 3 Chemistry Notes
- Form 4 Chemistry Notes
- All Chemistry Practicals Notes for KCSE and MOCKS
- Form 1 Chemistry Topical Revision Questions and Answers
- Form 2 Chemistry Topical Revision Questions and Answers
- Form 3 Chemistry Topical Revision Questions and Answers
- Form 4 Chemistry Topical Revision Questions and Answers
- IRE Form 1 Notes
- IRE Form 2 Notes
- IRE Form 3 Notes
- IRE Form 4 Notes
- Physics Form 1 Notes
- Physics Form 2 Notes
- Physics Form 3 Notes
- Physics Form 4 Notes
- CRE Form 1 Notes
- CRE Form 2 Notes
- CRE Form 3 Notes
- CRE Form 4 Notes
- Geography Form 1 Notes
- Geography Form 2 Notes
- Geography Form 3 Notes
- Geography Form 4 Notes
- History Form 1 Notes
- History Form 2 Notes
- History Form 3 Notes
- History Form 4 Notes
- Business Studies Form 1 Notes
- Business Studies Form 2 Notes
- Business Studies Form 3 Notes
- Business Studies Form 4 Notes
- Home Science Form 2 Notes
- Home Science Form 3 Notes
- Home Science Form 4 Notes
- Home Science Form 1 Notes
- Agriculture Form 1 Notes
- Agriculture Form 2 Notes
- Agriculture Form 3 Notes
- Agriculture Form 4 Notes
- Agriculture KCSE 2019 Project
- Computer Studies Form 1 Notes
- Computer Studies Form 2 Notes
- Computer Studies Form 3 Notes
- Computer Studies Form 4 Notes
- KCSE 2017 Reports
- 2018 Pre-Mocks
- 2019 Pre-Mocks
- 2022 Pre Mocks
- 2021/2022 Pre-Mock Past Papers
- 2023 Pre Mocks
- 2017 Mock Past Papers
- 2019 Mock Past Papers
- 2020 Mock Past Papers
- Mock Exam Papers 2021/2022 - Easy Elimu
- Mock Exam 2022 Questions and Answers
- Alliance Boys High School
- Maranda High School
- Form 1 Past Papers
- Form 2 Past Papers
- Form 3 Past Papers
- Form 4 Past Papers
- 2019 KCSE Prediction Papers
- 2020 KCSE Prediction Papers
- 2021 KCSE Prediction Papers
- 2022 KCSE Prediction Questions and Answers - EasyElimu
- KCSE Prediction 2023
- 2020 Post Mock Past Papers
- 2021/2022 Post Mocks
- 2023 Post Mocks
- Play Group: Activities, Homework and Syllabus
- 2023 PP1 Exams
- 2023 PP2 Exams
- Grade 1 Notes
- 2023 Grade 1 Exams
- Grade 2 Notes
- 2023 Grade 2 Exams
- Grade 3 Notes
- 2023 Grade 3 Exams
- Grade 4 Notes
- 2023 Grade 4 Exams
- Grade 5 Notes
- 2023 Grade 5 Exams
- Grade 6 Notes
- KPSEA Exams
- 2023 Grade 6 Exams
- Class 6 : Notes, Revision Papers and Syllabus
- Class 7 : Notes, Revision Papers and Syllabus
- Class 8 Notes
- 2023 Class 8 Exams
- 2023 Kcpe Prediction
- Grade 7 Notes
- 2023 Grade 7 Exams
- The New EasyElimu Website
- Form 4 End Term 1 Exams
- Form 3 Exams 2024
- Form 2 End Term 1 Exams
- Form 1 End Term 1 Exams
- All Kiswahili setbook guides
- All English setbook guides
- Form 1 - 4 High School Notes
CHEMISTRY Paper 1 Questions and Answers - KCSE 2022 Past Papers
« Previous Topic KISWAHILI Paper 3 Questions and Answers - KCSE 2022 Past Papers
Next Topic » CHEMISTRY Paper 2 Questions and Answers - KCSE 2022 Past Papers
- State one property that can be used to distinguish between a proton and a neutron.(1 mark)
- Write the electron arrangement of the ion. (I mark)
- Identify the group and period in the Periodic Table to which the element belongs. Group .....................( 1 / 2 mark) Period. .....................( / 2 mark)
- Select from Table 1 an acidic chloride and write the equation for its reaction with water.(1 mark)
- Write a thermochemical equation for the formation of carbon(II) oxide. (1 mark)
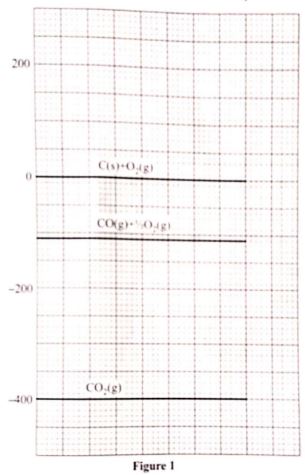
- formation of carbon(II) oxide (1 mark)
- combustion of carbon(II) oxide (1 mark)
- Give a reason why painting or galvanising iron sheets protects them from rusting (1 mark)
- Explain the advantage of galvanising over painting of iron sheets (2 marks)
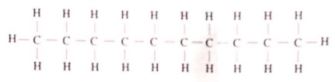
- name (1 mark)
- empirical formula (1 mark)
- Draw the structure of an alkanoic acid whose molecular formula is C,HO (1 mark)
- hydrogen in CaH 2 (1 mark)
- oxygen in OF 2 (1 mark)
- Write an ionic equation for the reaction between aqueous sodium hydrogen carbonate and ethanoic acid. (1 mark)
- molecular mass (1mark)
- molecular formula (1 mark)
- Draw a structure of the hydrocarbon in 8(a). (1 mark)
- write the formula of the product formed ( 1 / 2 mark)
- Name the type of bond formed ( 1 / 2 mark)
- The melting point of iodine is higher than that of chlorine. Explain. (2 marks)
- Write an equation for the reaction. (1 mark)
- State why the gas cannot be dried using anhydrous calcium chloride (1 mark)
- Name a suitable drying agent. (1 mark)
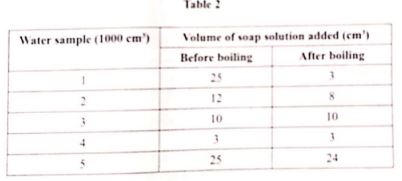
- temporary hardness ( 1 / 2 mark)
- no hardness( 1 / 2 mark)
- both temporary and permanent hardness ( 1 / 2 mark)
- Describe how water hardness can be removed using an ion exchange resin. (1 1 / 2 marks)
- State another factor that affects the products of electrolysis (1 mark)
- Carbon exhibits different boiling points. Explain. (1 mark)
- It takes 4-4 seconds for nitrogen(IV) oxide gas to effuse through an opening. Calculate how long it will take for an equal volume of chlorine gas to effuse through the same opening (N-14.0: 0-16.0: C1-35.5). (2 marks)
- cellulose material (1⁄2 mark)
- a hydrocarbon ( 1 / 2 marks)
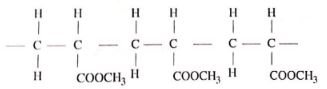
- Draw the structure of the monomer of perspex. (1 mark)
- Give two properties of perspex that make it suitable for use in making lenses. (1marks)
- Explain why the density of diamond is higher than that of graphite. (1mark)
- Graphite use ( 1 / 2 mark) property ( 1 / 2 mark)
- Diamond use ( 1 / 2 mark) property ( 1 / 2 mark)
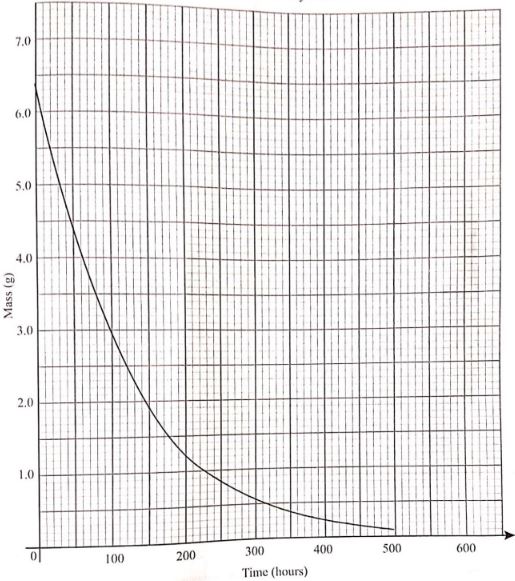
- half life of the radioactive isotope (1 mark)
- rate of decay at time 150 hours (1 mark)
- The half life of two radioactive isotopes A and B are 8 days and 5.2 years respectively Given that both of them emit beta radiation, explain why A would be more suitable in the treatment of a disease (1 mark)
- The formula of a hydrated salt of manganese is MnSO, XHO Given that the salt contains 24,7% manganese, determine the value of X (Mn-550, S-120.0-160, H1.0) (3 marks)
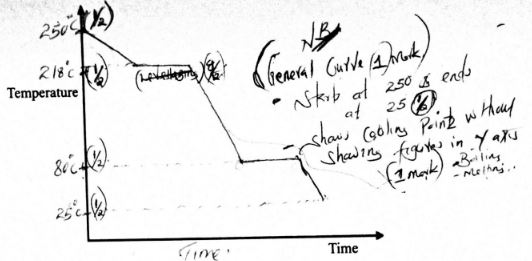
- Describe the correct procedure of heating a liquid in a test tube using a Bunsen burner. (3 marks)
- Draw a labelled diagram of a setup that can be used to prepare a dry sample of chlorine gas using potassium manganate(VII) and concentrated hydrochloric acid ( marks)
- hexane and butanol (1 1 / 2 marks)
- hexane and water (1 1 / 2 marks)
- Iron is extracted from haematite ore. If the ore contains oxides of silicon and aluminium, explain how these impurities are removed (2 marks)
- The extraction process of iron produces waste gases. State how these waste gases can be used to lower the operational cost of the extraction process (1 mark)
- solution smells strongly of chlorine (1 mark)
- addition of sodium hydroxide removes the smell (2 marks)
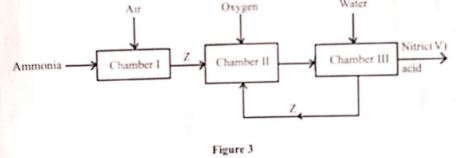
- Identify the chamber in which a catalyst is used. (1 mark)
- Name substance Z. (1 mark)
- Write an equation for the reaction that takes place in Chamber III.(1 mark)
- The fomula of complex ion formed when aqueous zinc sulphate reacts with aqueous sodium hydroxide is given as ;(Zn(OH) 4 ) Explain how the value of x is determined; (2marks)
- Name another reagent that can be used to obtain copper from copper(ii)oxide (1marks)
- The equation from the reaction with carbon(ii)oxide is ; CuO( S ) + CO( g ) → Cu( s ) +CO( g ) Calculate the maximum mass of copper that would be obtained using 200dm 3 of carbon(ii)oxide (Cu =63.5; Molar of gas =24.0dm 3) (2marks)
MARKING SCHEME .
- charge
- 2. 8.8 or 2.8 .8
- Identify the group and peariod in the periodic table to which the element belongs Group ii period 4
- PCl 3(l) + 3H 2 O (l) → H 3 PO 3 (aq) +3HCl (aq) PCl 5 (L) +4H 2 O(L) → H 3 PO4 (aq) + 5HCl (aq)
- Write a thermochemical equeation for the formation of carborn(ii)oxide (1mark) C(s) + 1/2O2 → CO(3) ;(ΔH°f)
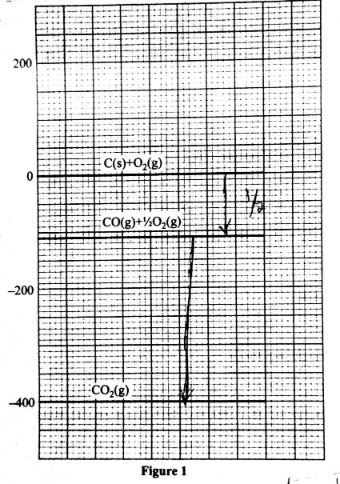
- formation of carbon|(ii0oxide ΔH°f = -110ki/mol
- combination of carbon(ii)oxide ΔH -400 -(-110) =-290kj
- both methods provides coating /that keeps iron from oxygen and water
- In galvanising , zinc acts as sacrificial metal source
- it is more reactive than iron thus prevent rusting ,
- name Decane (1mark)
- emperical fomula C 5 H 4 (1mark)

- hydrogen in CaH 2 (1mark) O.N of CaH 2 = +2+2H =0( 1 / 2 ) 2H = -2 O°N O of H =-1 ( 1 / 2 )
- oxygen in Of 2 O'No of OF2 = O +2(-1) O= +2
- CH3COOH(aq) = HCO 3 ‾(aq) → CH3COO‾(aq) =CO 2 +HO 2
- molecular 9.33 × 10 -23 × 6.0 × 10
- molecular formula (1mark) C 4 H 8
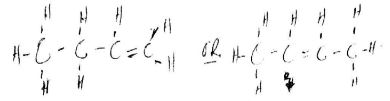
- Write the chemical fomular of the product formed ( 1 / 2 mark) H 3 O +
- Name the type of bond formed ( 1 / 2 marks) Co-ordinate
- Iodine has larger molecular mass the stronger vender walls forces of attractions then chlorine which has smaller mass .
- Write an equation for the reaction 2NH 4 Br (s) + Br(OH) 2(s) → BaBr 2(s) + 2NH 3(g) +2H 2 O 4(g)
- Ammonia reacts with calcium chloride to form CaCl 2 •2NH 3 which is a complex salt .
- CaO (s) /Calcium oxide
- Sample 2
- Hard water is run into a column containing the ion exchange resin
- Ca 2+ and Mg 2+ ion arte exchanged for Na + ion
- therefore water coming out is soft .
- Position of the ion/element in the ractevity searies
- Carbon exist in defferent crystoline forms same physical state hence different boiling point because of their different structure.

- Cotton wool ,wood ,paper
- Rubber

- Transparent - free from change of refraction index
- strong -consistant in refraction index
- Explain why the desity of diamond is higher than that of graphite . (1mark) Diamon has tetrahedral structure with all atoms forming four cobalent bond while in the graphite each atom form three covalent bonds in a layer structure which are far from each other .
- Lubricant / Electrode property ( 1 / 2 mrks)
- conduct of electricity
- tips od driling tips property ( 1 / 2 marks)
- Hardand abrasive
- cutting instrument
- Half ; life = 85 hours
- rate of decay at time 150hours (1mark) 3.0 - 0.5 / 80 -240 -0.016g/hrs
- A has a shoter half-life the B will clear from the body faster thus not expose the patient to radiation for a long time

- Hold the test tube with a test tube holder , keep it slanting with the open end facing away ,heat from top downwards and not from bottom to the top while rotating
- Fraction distilation put the two liquidin fractionating column ;heat the mixture gently ;hexone will distill ;
- separating funnel ;burette this are immiscible liquids ;hexane will float on water from the bottom of flask , hexane remove in the funnel
- they react with calcium oxide ;to form CaSiO 3 whic are removed as slag
- The waste gase are at high temp , the head can be recycle to pre-heat incoming air
- chlorine reacts partialy with water , there is strong smell due to presence of chlorine molecules .
- Addition of NaOH neutralize HCl (aq) and HOCl (aq) ,equilibrium shifts to the right chlorine molecule are consum hence the smell disappears.
- Nitrogen (ii)oxide (No)
- 3NO 2(g) + H 2 O (l) → 2HNO 3(aq)
- Oxidation state of zinc is =2
- OH‾ has a charge of -1
- +2 + (-1× 4) = X
- hydrogen gas
- CuO (s) + CO (g) → Cu (s) + CO 2(g) Calculate the maximum mass of copper that would be obtained using 200dm 3 of carbon(ii)oxide (Cu =63.5; Molar of gas =24.0dm 3) (2marks) CuO (s) + CO (g) → Cu 2(g) , Mole CO= mole cu = 200dm3 / 24dm3 mass = 200 / 24 × 63.5 =529.2
Download CHEMISTRY Paper 1 Questions and Answers - KCSE 2022 Past Papers .
Why download.
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students
Related items
- Music Paper 1 Questions and Answers - KCSE 2022 Past Papers
- Electricity Paper 2 Questions and Answers - KCSE 2021 Past Papers
- Drawing and Design Paper 1 Questions and Answers - KCSE 2021 Past Papers
- Art and Design Paper 1 Questions and Answers - KCSE 2021 Past Papers
- French Paper 1 Questions and Answers - KCSE 2022 Past Papers
access all the content at an affordable rate or Buy any individual paper or notes as a pdf via MPESA and get it sent to you via WhatsApp
What does our community say about us?
- KCSE Revision Questions
- Privacy Policy
- Mobile App Privacy Policy
- High Schools in Kenya
- Teacher Resources
- Questions and Answers
- Online Tuition and Classes in Kenya
Copyright © 2022 EasyElimu
- Terms and Conditions

EACH PAPER ==> N600 MTN TO: 09033919669
WAEC Chemistry Questions and Answers 2022
WAEC Chemistry Questions and Answers 2022 is now Available for the May/June 2022. WAEC Chemistry Theory and Objective Answers (100%legit) Chemistry 2 Essay verified Free (Expo) for West African Examinations Council.
WAEC Chemistry Questions For you to have accurate WAEC end result in Chemistry in addition to repeated questions free of charge on this post. You will even recognize how WAEC Chemistry questions are set and a way to solution them. The West African Examinations Council is an exam board hooked up via way of means of regulation to decide the examinations required within side the public hobby within side the English-speak me West African countries, to behavior the examinations and to award certificate akin to the ones of equal analyzing government internationally
Table of Contents
WAEC Chemistry Questions and Answers 2022 (Expo)
Chemistry 2 (Essay) – 2:00pm – 4:00pm Chemistry 1 (Objective) – 4:00pm – 5:00pm
CHEMISTRY OBJ: 1-10: ABADCCBDCA 11-20: DCABCBBCCB 21-30: ADCADAABCA 31-40: CBBCCBCCBB 41-50: CACBCBADCA

(1a) (i)An acid is any compound which, in a chemical reaction is able to attach itself to an unshared pair of electrons in another molecule-a base, to form a new compound.
(1b) Salting out is the process of adding concentrated sodium chloride solution to soap, to decrease the solubility of the soap. It consequently separates out as a hard cake on the surface or cooling.
(1c) Reagent: {Ammonical solution of Silver trioxonitrate(v) [AgNo₃]} Condition: {At room temperature}
(1d) The percentage abundance of an isotope is the percentage of all naturally occurring atoms with a specific atomic mass of an element.
(1e) (i)The element with atomic number 18 has electronic configuration 2,8,8. It has a completely filled valence shell. It is therefore inert. Hence it cannot have an oxide.
(ii)Being a covalent compound, the intermolecular forces between the Cl₂O molecules are weak and easily overcome.
(1f) Add barium chloride solution to H₂SO₄ and HNO₃ separately. A white precipitate is formed in H₂SO₄ and no such precipitation is formed in HNO₃.
(1g) (i)The main difference is that electrochemical cell does not need any external current for operation whereas electrolytic cells need external current to operate.
(ii)Also, Oxidation occurs at the positive electrode and reduction at the cathode, in the electrolytic cell. While the reverse is the case in electrochemical cells.
(1h) As we go down group 1 from top to bottom, it gets easier to remove the valence electron and form the positively charged cation. Hence group 1 elements increase in chemical reactivity as we go down the group
(1i) Molecular formula is a chemical formula that gives the total number of atoms of each element in each molecule of a substance.
(1j) (i)Ammonia, NH₃ will deviate more than hydrogen gas H₂

Answers Loading…
ENSURE YOU SUBSCRIBE TO THIS SUBJECT, I WON’T GUARANTEE YOU OF POSTING HERE.
( Chemistry – WAEC 2022) Direct Mobile/SMS: N1000 MTN CARD Password/Link: N500 MTN CARD
Forward Subject Name, MTN CARD PINS, Phone number to: 09033919669 via TEXT MESSAGE(SMS) ONLY.
NOTE: Do Not Subscribe On Whatsapp. Send via SMS ONLY. After sending, relax, and wait for your password.
CLICK HERE TO JOIN OUR TELEGRAM CHANNEL FOR ALL 2022 EXAM ANSWERS FOR FREE
OUR PAST WAEC Chemistry Questions and Answers 2022
We are happy you are here for the 2022 Chemistry answers. The complete solution will be made free in some minutes before the CRK examination.WAEC Chemistry questions 2022, WAEC 2022 CRK questions and answers, Chemistry WAEC 2022, WAEC questions 2022/2023, Chemistry 2022,
WAEC Chemistry 2022 Questions and Answers, Chemistry questions and answers pdf 2022, WAEC Christian Religious Studies 2022 Answer, Waec Chemistry 2022 Answers, waec Chemistry answers 2022 , Waec Chemistry 2022 question, Waec 2022 Chemistry Answers, Chemistry waec 2022, Here is the only legitimate website where can get the 100% Verified Waec 2022 Chemistry Answers. and also have the chance to score A’s , B’s and C’s in this ongoing Waec 2022 Chemistry Examination. We Assure you of getting the waec Chemistry questions and answers 2022 on time, only those that subscribed. WE DONT SCAM, ONLY A TRIAL WILL CONVINCE YOU.
How to Subscribe For WAEC Chemistry 2022 ANSWERS
Chemistry 2 (Essay) 2:00pm. – 4:00pm. Chemistry 1 (Objective) 4:00pm. – 5:00pm
Chemistry OBJ: 1-10: CDBAAAACAD 11-20: CBBCDCCADB 21-30: CACCABACBC 31-40: DBCBDCBABD 41-50: CBBCCABDDA
INSTRUCTION: YOU ARE TO ANSWER FOUR(4) QUESTIONS IN ALL.
QUESTION ONE(1) IN SECTION A & ANY THREE(3) QUESTIONS IN SECTION B.
NOTE: USE EITHER THE TYPED VERSION OR THE IMAGE VERSION… make your work look different.
(4bvi corrected) Take note

SECOND VERSION – TYPED
(1a) A molecular formula consists of the chemical symbols for the constituent followed by numeric subscripts showing the number of atoms of each element present while structural formula consists of symbols for the atoms connected by short lines that represent chemical bonds.
(1b) (i) The distance of the outermost electron (ii) The size of the positive nuclear charge (iii) The screening effect of the inner electron
(1c) (i) The reaction must proceed both forward and backward i.e reversible (ii) All the reactants and products are present at equilibrium
(1d) (i) B is the strongest reducing agent (ii) It has the least ionization energy among the three
(1e) (Pick one) Graham’s law of diffusion states that the rate of diffusion of a gas is inversely proportional to the square root of their densities provided other conditions remained constant
Graham’s law of diffusion states that at a constant temperature and pressure ,the rate of diffusion of a gas is inversely proportional to the square root of it’s density.
(1f) (i) Na2SO4, Mg(NO3)2 (ii) CaCO3
(1g) (i) Protein – condensational polymer (ii) Perspex – additional polymer (iii) Nylon – Condensation polymer
(1h) Atomic radius can be defined as the half distance between the nucleus of two covalently bonded atoms.
(1i) Ethanol has a higher boiling point than propane because it has stronger force of attraction than propane which has a weak van der waal’s force.
(1j) (i) It aids the digestion of food (ii) It aids the action of enzymes (iii) It controls the availability of nutrients
(3ai) H2SO4 (aq) + 2KOH(aq) —> K2SO4(aq) + 2H2O(l)
(3aii) Given, Va = 25.0cm³, Vb = 24.0cm³, Cb = 0.15mol/dm³, Ca =?, Na = 1 , Nb = 2 Using, CaVa/CbVb = Na/Nb Ca = CbVbNa/VaNb Ca = 0.15 × 24.0 × 1/25.0×2 Ca = 3.6/50 = 0.072mol/dm³
(3bi) (i) 2Mg(s) + CO2(g) —> 2MgO(aq) + C(g) (ii) This is because magnesium can reduce carbon (iv) oxide to black carbon and producing magnesium oxide
(3bii) Mg(NO3)2 M of Mg(NO3)2 = 24+(2×14) + (6×16) = 24 + 28 + 96 = 148g/mol % of N = 28/148 × 100/1 N = 18.9%
(3ci) (i) Reaction with KMnO4 (ii) Reaction with bromine water
(3cii) It turns them colourless
(3d) Boil the vegetable oil in sodium hydroxide solution, it breaks down releasing the organic acid and the alkanol. The process is known as saponification. The organic ester is immediately neutralized by the sodium hydroxide solution to form the sodium salt of the organic acid which is soap.
(4ai) (i) Graphite – Hexagonal (ii) Diamond – Octahedral
(4aii) Diamond us hard because of the strong force of attraction that hold them together and it can not conduct electricity because all the available electrons are used for bonding while Graphite is soft because it’s particles are in layers and can conduct electricity because not all the electrons are used for bonding
(4bi) (i) It helps to remove dissolved gases and oxidizes dissolved metals (ii) It helps to remove objects such as rags, paper, plastics, and clogging of downstream equipment (iii) It reduces particle concentration in water and minimizes the need for coagulation and flocculation
(4bii) (i) Calcium tetraoxosulphate (vi) (ii) Calcium hydrogen trioxocarbonate
(4biii) (i) Filtration (ii) Boiling
(4biv) It has a sweet taste and it builds the shells of lower organisms
(4ci) The ore (SnO2) is wasted out of the ground with water, powdered, washed and roasted to drive off oxides sulphur, arseric and antimony. The roasted one is now reduced by heating with coke in a reverberatory furnace and molten tin is tapped off from the bottom of the furnace. The tin obtained is impure and it is remelted on a slopping furnace in which the tin melts and run its moulds. The tin obtained is 99.9% pure
(4cii) SnO2(g) + 2C(s) —> Sn(l) + 2CO(g)
(4ciii) (i) Sn(g) + O2 —> SnO2(g) (ii) Sn(g) + 2Cl(g) —> SnCl4(g)
ATTENTION:- PLEASE AFTER SENDING YOUR CARD, DO NOT THROW THEM AWAY UNTIL YOU RECEIVE A CONFIRMATION MESSAGE FROM US.
( Waec Chemistry 2022 ) Whatsapp/Direct: N1000 MTN CARD. Password/Link: N500 MTN CARD. Forward Your Name, MTN PIN, Subject Name, Phone number to: 09033919669
NOTE : Direct Mobile Subscribers Get Answers Direct To Their Phone As Sms/Text Message, While Password/Link Get their Answers Online on Our Answer Page.
Online PIN MEANS The Pin to access our answers online via www.noniexpo.com will be sent to u at least 2hours before the exam to access our answers
SUBSCRIBING BEFORE THE EXAM DAY MAKES YOU SAFER BCOS YOU’LL GET PASSWORD EARLIER ON THAT EXAM DAY. ALWAYS SUBSCRIBE A DAY BEFORE EACH EXAM. NOTE:- All SMS Sent To The Above Number Are Attended To, Our Phone Number Might Be Diverted To Avoid Distraction. PLEASE DON’T CALL US!. WE ACCEPT ONLY TEXT MESSAGES. THE NUMBER IS NOT AVAILABLE FOR CALLS BUT CAN RECEIVE AND REPLY TEXT MASSAGES ONLY. Always Send Us SMS Of Your Complaint. WAEC Chemistry 2022 Answers. WAEC Chemistry Answers 2022. WAEC Chemistry 2022, WAEC Chemistry expo 2022, WAEC Chemistry questions and answers 2022, WAEC 2022 Chemistry Answers, WAEC Chemistry question and answer 2022, 2022 WAEC Chemistry obj and Theory Answers, WAEC Chemistry 2022, WAEC Chemistry questions 2022, 2022 WAEC Chemistry answers, Chemistry WAEC 2022 CHEMISTRY OBJ: 1-10: EECEBAEEBB 11-20: DDDBEACABB 21-30: CDECCDAAAB 31-40: BAECBBCDAC 41-50: CECCEBDBDC 51-60: CEBEEDDBAE ============================ (1) =========================== (2) ========================== (3) ========================== (4) ============================ (5) ========================== (6) Answers Loading… QUESTIONS ? 2022 NECO Chemistry Questions & Answers [28TH July, 2022] ENSURE YOU SUBSCRIBE TO THIS SUBJECT, I WON’T GUARANTEE YOU OF POSTING HERE. ANSWERS WILL BE AVAILABLE BY MIDNIGHT, ONLY FOR THOSE THAT SUBSCRIBE SUBSCRIBERS GET ANSWERS. ( Chemistry – NECO 2022) Direct Mobile/SMS: N800 MTN CARD Password/Link: N500 MTN CARD Forward Subject Name, MTN CARD PINS, Phone number to: 09033919669 via TEXT MESSAGE(SMS) ONLY. NOTE: Do Not Subscribe On Whatsapp. Send via SMS ONLY. After sending, relax, and wait for your password. QUESTIONS ?
Share this Post:
You may also like.

Neco English Language 2022 Answers

WAEC IRS Answers and Questions 2022 (100% Verified) Theory & Obj...

[Song] Flavour – Levels

[Mp3] Onyenze – Dis and Dat

[Mp3] Phyno – Stacks

[MP3] Joeboy – Alcohol
About the author.
The loyal guy from another planet, Whom always get what he always asked for. || Professional Blogger || The Main Man Behind Noniwap.com concept.
Leave a Comment X
Notify me of follow-up comments by email.
Notify me of new posts by email.
Join NONIEXPO Telegram Channel For All Exam Answers
- Login / Register

- International
- Entrepreneurship
- Find Scholarships
- Inter Studies
Join Our Newsletter
Join our subscribers list to get the latest news, updates and special offers directly in your inbox
WAEC Chemistry Questions and Answers 2022 Objectives and Essay
Waec chemistry 2022 answers. waec chemistry questions and answers 2022/2023 objective and essay. see the 2022 waec chemistry answers for both objective and theory below. get the waec chemistry objective and essay answers here. the 2022 waec obj and theory questions and answers are provided here for free. all you have to do is to […] the post waec chemistry questions and answers 2022 objectives and essay appeared first on study forum..
WAEC Chemistry 2022 answers. WAEC Chemistry questions and answers 2022/2023 objective and essay. See the 2022 WAEC Chemistry answers for both objective and theory below. Get the WAEC Chemistry objective and essay answers here.
The 2022 WAEC OBJ and theory questions and answers are provided here for free. All you have to do is to go through the questions and take note of the WAEC Chemistry answers 2022. Read on to find out.
WAEC Chemistry Questions and Answers 2022 Objective and Essay
Have you been searching on Google for the original WAEC Chemistry Questions and Answers 2022? If so, we have got you covered!
We have the 2022 WAEC Chemistry questions and our team of experts will soon upload the WAEC Chemistry questions and their accurate answers to help you pass the 2022 WAEC Chemistry examination.
The WAEC Chemistry answers 2022 will be uploaded any moment from now. So if you are searching for the WAEC Chemistry answers 2022 for objective and theory, then you are on the right page.
See WAEC Chemistry objective and essay questions and answers below.
WAEC Chemistry Questions and Answers 2022 Essay and Objective
The West Africa Examinations Council (WAEC) is an examination body in Nigeria that conducts the Senior Secondary Certificate Examination and the General Certificate in Education in May/June and November/December respectively.
The 2022 WAEC Chemistry questions are set from SS1 to SS3 Chemistry syllabus. So all the questions you will encounter in this year’s examination are in the syllabus, and nearly 90% of the questions are repeated.
You don’t have to worry about the 2022 WAEC Chemistry questions and answers (essay and objective). The WAEC Chemistry answers 2022 will be uploaded any moment from now. All you need to do is to keep refreshing this page so as not to miss out.
Once again, keep refreshing this page because we will upload the original WAEC Chemistry questions and answers for this year’s exams on this page at any moment from now.
If you have any questions about the WAEC Chemistry questions and answers 2022, feel free to use the comment box below or use the Chat With Us button and we will respond immediately.
The 2022 WAEC Chemistry answers will be posted here. Be patient. Keep checking and reloading this page for the correct answers. WAEC 2022 Chemistry answers loading…….
There is nothing like WAEC Chemistry Expo 2022 online. All students are advised to avoid all patronizing online fraudsters/vendors who claim to provide such services.
The post WAEC Chemistry Questions and Answers 2022 Objectives and Essay appeared first on Study Forum .
Previous Article
UITH CHO Training Institute Admission Form 2022/2023
Next Article
UNIBEN Post UTME Form 2022/2023 Academic Session
myschoolnews
Related Posts
BSUM notice on suspension of union activities and social...
myschoolnews Oct 13, 2021 0 19
Federal Polytechnic Ile-Oluji Post-UTME 2021: cut-off mark,...
myschoolnews Oct 18, 2021 0 22
EKSU Predegree Admission Form For 2021/2022 Session
myschoolnews Oct 21, 2021 0 18
Weekly data giveaway - check week 8 winners
myschoolnews Oct 25, 2021 0 19
FUTO JUPEB Admission List for 2021/2022 session
myschoolnews Oct 5, 2021 0 25
Study in Australia: Rustic Pathways Global Perspectives...
myschoolnews Oct 25, 2021 0 21
Popular Posts
National Common Entrance Past Questions & Answers [2011...
myschoolnews Dec 1, 2021 0 70
All About Saksham Scholarship
myschoolnews Aug 2, 2023 0 65
The Life Changer: 195 JAMB Novel Questions & Answers [PDF]
myschoolnews Jan 21, 2022 0 45
Download JAMB Past Questions & Answers In PDF [All Subjects]
myschoolnews Dec 13, 2021 0 35
Download BECE Past Questions and Answers PDF for All Subjects
myschoolnews Jan 5, 2022 0 34
Recommended Posts
Canadian Diploma College Scholarships in 2022 – Get Your...
myschoolnews May 7, 2022 0 430
Big Scholarship Programs Without IELTS 2022 | Fully Funded
myschoolnews May 7, 2022 0 423
10 Scholarships to Study in UK - Application Still in Progress..
myschoolnews May 7, 2022 0 536
Check How Students From Nigeria Study in UK Universities...
myschoolnews May 7, 2022 0 442
Top 8 Internship Abroad Scholarships & Grants to Apply...
myschoolnews May 7, 2022 0 391
Random Posts
University of pretoria tuks young research leadership program....
myschoolnews Apr 9, 2024 0 1
The TYRLP is an initiative of the University of Pretoria in partnership with the...
Welsh Government Wales & Africa Grant Scheme 2025 – Apply
myschoolnews Mar 12, 2024 0 6
Spread the loveEnabling community groups and organisations throughout Wales to access...
NYSC 2024 Batch ‘A’ Stream II Online Registration & Requirements...
myschoolnews Mar 25, 2024 0 5
National Youth Service Corps, NYSC Online Registration, Guide and Requirements for...
Apply Now: Stanbic IBTC Graduate Trainee Program 2024.
myschoolnews Apr 4, 2024 0 3
Spread the loveApplications are currently open for the Stanbic IBTC graduate trainee...
Npower Salary Structures [year]
myschoolnews Apr 18, 2023 0 211
Npower Salary [year] Structure. Checkout the current NPower [year] Salaries Structures...
Women for Africa Foundation (FMxA) Postgraduate Scholarship...
myschoolnews Apr 8, 2024 0 1
For yet another year, the long-awaited Learn Africa postgraduate scholarship program,...
UAT School Anthem for all Student | Full Lyrics and Audio...
myschoolnews Apr 9, 2024 0 0
UAT School Anthem for all Student | Full Lyrics and Audio Download,UAT School Anthem...
Osun State College of Technology Courses and Requirements...
myschoolnews Jan 24, 2024 0 8
Osun State College of Technology Courses and Requirements List 2024,Osun State College...

10+ Affordable Universities in the USA for International...
myschoolnews Mar 28, 2024 0 5
Affordable American Universities with Low Tuition Fee | An overview Thinking about...
New Zealand Visa Sponsorship Jobs 2024 | Work in New Zealand
myschoolnews Jan 10, 2024 0 28
Do you wish to migrate to New Zealand and take up job opportunities without having...
Voting Poll
How would you rate your teacher.
Vote View Results
Total Vote: 160
View Options
WAEC Chemistry Questions and Answers 2023: OBJ/Essay
The West African Examination Council (WAEC) conducts the West African Senior School Certificate Examination (WASSCE) for Chemistry. This examination tests students’ knowledge and skills in the subject, preparing them for higher education and career opportunities. In this comprehensive guide, we provide you with essential information, sample questions, and answers to help you excel in the Objectives and Essay sections of the WAEC Chemistry exam in 2023.

Overview of WAEC Chemistry Examination
The WAEC Chemistry examination consists of two main sections: Objectives and Essay. The Objectives section contains multiple-choice questions that test students’ basic understanding of Chemistry concepts. On the other hand, the Essay section requires students to provide detailed explanations and solutions to various Chemistry problems.
To excel in the WAEC Chemistry examination, you need to have a solid understanding of the subject matter. This guide will provide you with valuable insights and sample questions, covering both the Objectives and Essay sections of the exam.
WAEC Chemistry Objectives
The Objectives section consists of multiple-choice questions, which test your basic understanding of Chemistry concepts. In this section, we provide you with some sample questions and answers to help you prepare for the 2023 WAEC Chemistry Objectives.
Sample Objective Questions
- Which of the following elements will burn in excess oxygen to form a product that is neutral to litmus?
A. Carbon B. Hydrogen C. Sulphur D. Sodium
Answer: B. Hydrogen
- A solution of salt formed from HCl and NH3 solutions is:
A. Acidic B. Basic C. Complex D. Neutral
Answer: D. Neutral
- The boiling points of water, ethanol, methylbenzene, and butan-2-ol are 373.0K, 351.3K, 383.6K, and 372.5K respectively. Which liquid has the highest vapor pressure at 323.0K?
A. Water B. Methylbenzene C. Ethanol D. Butan-2-ol
Answer: C. Ethanol
WAEC Chemistry Essay
The Essay section requires students to provide detailed explanations and solutions to various Chemistry problems. In this section, we provide you with a comprehensive list of sample essay questions and answers to help you prepare for the 2023 WAEC Chemistry Essay.
Sample Essay Questions and Answers
- (a) Define the following terms:
(i) Atomicity (ii) Isotopes
(b) Elements P, Q, and R have atomic numbers 9, 16, and 20, respectively. Which of these elements would gain electron(s) during ionic bonding?
(c) Write the electron configuration of the following elements:
(i) 9F (ii) 20Ca
(a) (i) Atomicity refers to the number of atoms present in a molecule of an element or compound. For example, the atomicity of oxygen (O2) is 2, and that of ozone (O3) is 3.
(ii) Isotopes are varieties of an element that have the same atomic number but different mass numbers due to the different number of neutrons in their nuclei. For example, the isotopes of hydrogen are protium (1H), deuterium (2H), and tritium (3H).
(b) Elements with atomic numbers 9 (P) and 16 (Q) would gain electron(s) during ionic bonding because they have fewer than four electrons in their outermost shells.
(c) Electron configuration of:
(i) 9F: 1s² 2s² 2p⁵ (ii) 20Ca: 1s² 2s² 2p⁶ 3s² 3p⁶ 4s²
(i) Standard electrode potential (ii) Redox reaction
(b) Balance the following redox equation: MnO₄⁻ + I⁻ + H⁺ → Mn²⁺ + I₂ + H₂O
(a) (i) Standard electrode potential is the potential difference (voltage) between an electrode and its respective half-cell when all the species involved are in their standard states (298 K temperature, 1 atm pressure, and 1 mol/dm³ concentration).
(ii) A redox reaction is a chemical reaction in which the oxidation states of the atoms involved in the reaction change. One species is reduced (gains electrons), and another is oxidized (loses electrons).
(b) The balanced redox equation is: 2MnO₄⁻ + 10I⁻ + 16H⁺ → 2Mn²⁺ + 5I₂ + 8H₂O
Tips for Success in WAEC Chemistry Examination
- Understand the concepts: Develop a strong foundation in Chemistry by thoroughly understanding the basic concepts, principles, and theories.
- Practice regularly: Solve numerous practice questions and problems to improve your problem-solving skills and enhance your understanding of the subject.
- Manage your time effectively: Allocate adequate time for each section of the exam, ensuring you have enough time to read and understand the questions and provide well-thought-out answers.
- Learn from your mistakes: Review your practice questions and identify areas where you may be struggling, then focus on improving in these areas.
- Stay updated: Keep abreast of the latest developments in Chemistry and apply your knowledge to real-life situations.
In conclusion, preparing for the Objectives and Essay sections of the WAEC Chemistry examination requires dedication, persistence, and a strong understanding of the subject matter. By following the tips provided in this guide and practicing with the sample questions and answers, you will be well on your way to achieving success in the 2023 WAEC Chemistry examination.
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Related Articles

2024 NYSC MOBILIZATION: PCMS TO REGISTER WITH NATIONAL IDENTIFICATION NUMBERS

How to Apply for FG’s Student Loan in Nigeria

Federal University of Health Sciences Azare Post UTME Form 2022/2023
Neco Releases External Neco Result 2021

Kano State Polytechnic 2023/2024 Screening Procedure

Kano Scinece and Technical Board Sun Fara Bayar da Admission 2021/2022

Gurbin Daukan Aikin Koyarwa a Private School

Waec Clothing and Textiles Possible Question and Answer 2023

Gwamnatin Jihar Kano ta Bawa Dalibai Hutun Makaranta Domin Suyi Murmushi

Yadda Ake Rijistar Kano Science and Technical Schools Board || 2020

NECO Chemistry Practical Questions and Answers 2023 (Specimens)

BUK POST UTME PAST QUESTIONS AND ANSWERS (FACULTY OF SCIENCES)
St Charles Edu Services
Genuine Exam Past Questions and Answers Online Bookshop – PDF and MS Word Download
WAEC Chemistry Past Questions and Answers in 2023 PDF Download Objective & Theory
Are you writing the West Africa Examination Council WAEC Internal or External examination, if yes you need the WAEC Past Questions on Chemistry
we at stcharlesedu.com has compiled a good number of Chemistry WAEC Past Questions and Answers in Pdf Chemistry 2 – Theory/Essay Questions. Chemistry 1 – Objective Test Questions.
Our research has confirm that candidate that uses WASSCE Chemistry past questions to prepare is ten times better than those who do not.
Table of Contents
- 1.1 Chemistry WAEC Objective Questions
- 2 SSCE WAEC Chemistry Theory Questions
- 3 Chemistry WAEC Essay Questions
- 4 Free WAEC Chemistry Exam Past Questions Download
- 5 How to Get WASSCE Chemistry Exam Past Questions and Answers
SSCE WAEC Chemistry Objective Questions and Answers
CHEMISTRY Paper 1 (Objective Test Questions) Paper 1 will last for 1 hours Use HB pencil throughout.
Answer All Questions Each question is followed by four options lettered A to D. Find out the correct options for each question and shade in pencil on your answer sheet, the answer space which bears the same letter as the option you Chosen. Give only one answer to each question. An example is given below
What others are downloading WAEC Past Questions for all Subjects
Chemistry WAEC Objective Questions
Which of the following elements reacts with water? A. Carbon B. Iodine C. Sodium D. Sulphur
The correct answer is Sodium, which is lettered C and therefore answer space C would be shaded. [A] [ B ] [C] [ D ]
Think carefully before you shade the answer spaces; erase completely any answer you wish to change.
Which of the following raw materials is used in the plastic industry? A. Ethene B. Methane C. Sulphur D. Hydrogen
Which of the following organic compounds can undergo both addition and substitution reactions? A. Petane B. Benzene C. Propane D. Hexane
Which of the following equations represents a redox reaction? A. AgNO 3 (aq) + KCl(ag)->AgCl(s)+ KNO 3 (aq) B. HNO 3 (aq)+ NaOH(aq) -> NaNO 3 (aq) + H 2 O(l) C. CaCO 3 (s) -> CaO(s) + CO 2 (g) D. 2H 2 S(g) + SO 2 (g) -> 2H 2 O(I) + 3S(g)
T he process of extraction of iron from its ore is A. decomposition. B. oxidation. C. reduction. D. sublimation.
What is the solubility of a salt if 0.4 g of it is obtained on evaporating 200 cm3 of its saturated solution to dryness? A. 0.08 gdm -3 B. 2.00 gdm -3 C. 8.00 gdm -3 D. 80.00 gdm -3
An acidic salt has A. double anions in its aqueous solution. B. a single cation in its aqueous solution. C. hydrogen ions in its aqueous solution. D. hydrogen atoms in its aqueous solution.
A reaction is endothermic if the A. reaction vessel feels cool during the reaction. B. enthalpy change is negative. C. bond forming energy exceeds bond breaking energy. D. heat of formation of reactants exceeds heat of formation of products.
In which of the following compounds does hydrogen form ionic compounds? A. CH 4 B. HCl C. NH 3 D. NaH
Consider the following reaction equation: Br 2 + 2KI -> 2KBr + I 2 . Bromine is acting as A. an oxidizing agent. B. a reducing agent. C. an acid. D. a base.
An organic compound has the empirical formula CH 2 . If its molar mass is 42 gmol-1 what is its molecular formula? [H = 1.0, C = 12.0] A. C 2 H 4 B. C 3 H 4 C. C 3 H 6 D. C 4 H 8
Ethene is produced from ethanol by A. decomposition. B. hydrolysis. C. ozonolysis. D. dehydration.
Consider the following equilibrium reaction: 2 AB(g) + B 2 (g) -><- 2AB 3 (g) AH = -XkJmol -1 The backward reaction will be favored by A. a decrease in pressure. B. an increase in pressure. C. a decrease in temperature. D. an introduction of a positive catalyst.
What is the mass of solute in 500 cm 3 of 0.005 moldm -3 H 2 SO 4 ? [H =1.0, O = 16.0, S = 32.0] A. 0.490 g B. 0.049 g C. 0.245 g D. 0.0245 g
Pure water can be made to boil at a temperature lower than 100 °C by A. reducing its quantity. B. decreasing the external pressure. C. distilling it. D. increasing the external pressure.
Consider the following sketch of the solubility curve of some substances. Note: scroll down to download the free chemistry waec questions in pdf copy to view the sketch
At what temperature does the solubility of KNO 3, equal that of NaNO 3 ? A. 0°C B. 20 °C C. 30 °C D. 40 °C
When a salt is added to its saturated solution, the salt A. dissolves and the solution becomes super saturated. B. dissolves and the solution becomes unsaturated. C. precipitates and the solution remains unchanged. D. dissolves and crystals are formed.
When substance X was added to a solution of bromine water, the solution became colorless. X is likely to be A. propane. B. propanoic acid. C. propyne. D. propanol.
The preferential discharge of ions during electrolysis is influenced by the A. mechanism of electrolysis. B. electrolytic reactions. C. nature of the electrode. D. type of electrolytic cell.
The valence electrons of 12 Mg are in the A. 3s orbital. B. 2px orbital. C. 2s orbital. D. 1s orbital.
Stainless Steel is an alloy comprising of A. Fe and C. B. Fe and Ni. C. Fe, C and Ni. D. Fe, C and Al.
The number of hydrogen ions in 1.0 dm 3 of 0.02 moldm -3 tetraoxosulphate(VI) acid is [NA = 6.02 x 1023] A. 1.2 x 10 22 B. 1.2 x 10 23 . C. 2.4 x 10 22 . D. 2.4 x 10 23 .
The most suitable substance for putting out petrol fire is A. water. B. carbon(IV)oxide. C. fire blanket. D. sand.
The following factors would contribute to environmental pollution except A. production of ammonia. B. manufacture of cement. C. photosynthesis. D. combustion.
The position of equilibrium in a reversible reaction is affected by A. particle size of the reactants. B. vigorous stirring of the reaction mixture. C. presence of a catalyst. D. change in concentration of the reactants.
The diagram below illustrates a conical flask containing water and ice.
NOTE: scroll down and download the free chemistry pdf past questions to see the diagram
Which of the following statements about the diagram is correct? A. The water is at a lower temperature than the ice B. Energy is absorbed when the ice changes to water C. Energy is released when the ice changes to water D. The water molecules vibrate about a fixed point
Which of the following statements best explains the differences between a gas and a vapor? A. Unlike gases, vapors are liquids at room temperature B. Unlike gases, vapor can easily be condensed into liquids C. Unlike gases, vapour is readily converted into solids D. Vapours are generally denser than gases
Consider the following reaction equation: 2HCl + Ca(OH) 2 –> CaCl 2 + H 2 O. What is the volume of 0.1 moldrn -3 HCl that would completely neutralize 25cm 3 of 0.3 moldm -3 Ca(OH) 2 ? A. 150 cm 3 B. 75 cm 3 C. 30 cm 3 D. 25 cm 3
Cu and HNO 3 are not suitable for preparing hydrogen gas because of their A. reactivity and oxidation respectively. B. conductivity and corrosiveness respectively. C. melting point and reduction respectively. D. electro negativity and solubility respectively.
Which of the following formulae cannot be an empirical formula? A. CH B. CH2 C. P2O5 D. N204
One of the criteria for confirming the purity of benzene is to determine its A. heat capacity. B. boiling point. C. mass. D. colour.
Want more Chemistry Objective Test Questions like this? Get the Complete WAEC Chemistry Exam Past Questions and Answers (Obj and Essay) in PDF Format from us.
SSCE WAEC Chemistry Theory Questions
Chemistry Paper 2 Paper 2 will last for 2 hours This paper consists of two sections A and B. Answer one questions from Section A and three questions from Section B.
Credit will be given for clarity of expression and orderly presentation of material.
SECTION A (1ai) Define the term fermentation. (1aii) Name the catalyst that can be used for this process.
(b) Name two factors which determines the choice of an indicator for an acid-base titration. (c) Consider the following reaction equation: [Fe + H2S04 ] FeS04 + H2. Calculate the mass of unreacted iron when 5.0g of iron reacts with 10cm3 of 1.0 moldrrv3 H SO [Fe = 56.0] (d) Name one: (di) Heavy chemical used in electrolytic cells; (dii) Fine chemical used in textile industries.
(e) Explain briefly how a catalyst increases the rate of a chemical reaction. (f) (i) Write the chemical formula for the product formed when ethanoic acid reacts with ammonia. (ii) Give the name of the product formed in 1 (f) (i)..
(g) List three properties of aluminum that makes it suitable for the manufacture of drink can. (h) State two industrial uses of alkylalkanoates. (i) List two effects of global warming. (j) Name two steps involved in the crystallization of a salt from its solution.
Chemistry WAEC Essay Questions
SECTION B. 2ai. State the collision theory of reaction rates. 2aii.Using the collision theory, explain briefly how temperature can affect the rate of a chemical reaction.
bi. Sketch a graphical representation of Charles’s law. bii. Calculate the volume of oxygen that would be required for the complete combustion of 2.5moles of ethanol at s.t.p. [ molar volume at s.t.p = 22.4dm3]
ci. Define esterification. cii. Give two uses of alkanoates. ciii. Give the products of the alkaline hydrolysis of ethyl ethanoate.
d. A tin coated plate and a galvanized plate were exposed for the same length of time. di. Which of the two plates corrodes faster? dii. Explain briefly your answer in 2 (d) (i).
Want more Chemistry Theory Questions like this? Get the Complete WAEC Chemistry Exam Past Questions and Answer (Obj and Essay) in PDF Format from us.
Free WAEC Chemistry Exam Past Questions Download
Click to Download your free NECO Past Question on Painting and Decorating Paper 2 and 3
Link 1: WASSCE Chemistry Questions Booklet Link 2: WASSCE Chemistry Questions Booklet
How to Get WASSCE Chemistry Exam Past Questions and Answers
To get the complete and more recent copy of the West Africa Examination Council WAEC Past Questions and answer
Take Note of the following step
Make a Call Call or whatsapp us on 08051311885 for the account number to make payment and how to received your complete copy of the past questions to be sent directly to your email address or whatsapp number.
Mode of Payment. Mobile Transfer or Direct Bank Deposit.
After Payment send us the following Depositor Name: Name of Product Paid for: Valid email address.
DELIVERY ASSURANCE We will deliver the past question to you 10 mins after confirmation of payment to the email you will send to us.
Related Posts:
- WAEC Technical Drawing Past Questions PDF Download – Objective, Essay, Building Plan/Practical Drawing
- WAEC Government Past Questions and Answers in 2023 PDF Download Objective & Theory
- WAEC Financial Accounting Past Questions and Answer 2023 – Objective & Essay
- WAEC Visual Art Past Questions and Answers – Objective, Theory in 2023
- WASSCE/WAEC Electrical Installation & Maintenance Past Questions PDF – Objective/Essay
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
- 98494519845
- [email protected]
- Tyagal, Patan, Lalitpur

CHEMISTRY THEORY And OBJECTIVE: QUESTIONS & ANSWERS For 2022/2023 WAEC EXAM.
Chemistry Theory And Objective: Questions & Answers For 2022/2023 WAEC Exam. Are you a student who wants to make a good grade in Chemistry? Here are the WAEC Chemistry questions and everything you need to know about 2021 WAEC Chemistry. This post provides provided for you with all you need to know in Chemistry Paper 1 (Objective) and Chemistry 2 (Essay).
Table of Contents
Welcome to the Chemistry Theory and Objective: Questions & Answers for the 2022/2023 WAEC Exam. This comprehensive guide has been designed to assist students in their preparation for the upcoming WAEC Chemistry examination. It presents a collection of theory and objective questions along with their corresponding answers, covering the essential topics and concepts outlined in the WAEC syllabus. By studying and practicing these questions, you will gain a solid foundation in chemistry and improve your chances of achieving excellent results in the exam. So let’s delve into the world of chemistry and embark on a journey of learning and success.
The Question & Answers: CHEMISTRY THEORY And OBJECTIVE: QUESTIONS & ANSWERS For 2022/2023 WAEC EXAM.
Furthermore, below are the WAEC Chemistry questions. Read them properly. In fact, they will make you ready to score high in your WAEC Chemistry exam.
The West Africa Examination Council (WAEC) is an examination body in Nigeria. It has the statutory power to conduct the Senior Secondary Certificate Examination in May/June and the General Certificate in Education in November/December.
THE OBJECTIVE OF POST: CHEMISTRY THEORY And OBJECTIVE: QUESTIONS & ANSWERS For 2022/2023 WAEC EXAM.
Because people are asking about WAEC Chemistry and objective questions and answer 2022/2023. In fact, they are also asking for JAMB 2022 CHEMISTRY questions and answers pdf, The solutions are the objectives of this post. This post, therefore, takes care of those students who plan to do well in the WAEC exams this year.
The best way to read this post is to read it by clicking the highlighted topics for referencing. So check out these related topics that follow.
- Canada Visa Status
- Netherlands International Students guide
- 3 most demanded undergraduate courses in the Netherlands
- Most Popular Degree courses in Australia
- UK-approved graduate schools
- Most popular degree courses in the UK
- Approved Undergraduate – in UK
CHEMISTRY THEORY And OBJECTIVE: QUESTIONS & ANSWERS For 2022/2023 WAEC EXAM.
General tips and advice to help you prepare for your exams effectively..
- Study the syllabus: Familiarize yourself with the syllabus for your respective exams. Understand the topics and concepts that are likely to be covered and focus your preparation accordingly.
- Review past papers: Practice solving past exam papers to get an idea of the types of questions that may be asked. This will help you become familiar with the exam format and identify any areas where you need further improvement.
- Understand the concepts: Chemistry involves understanding fundamental concepts and principles. Ensure you have a strong foundation by studying the theory thoroughly. Take notes, create summaries, and use diagrams or visual aids to help you grasp complex concepts.
- Practice problem-solving: Chemistry often requires application and problem-solving skills. Practice solving numerical problems and chemical equations regularly to strengthen your problem-solving abilities. This will also help you become more familiar with the calculations and techniques needed for the exam.
- Seek clarification: If you come across any challenging topics or concepts, don’t hesitate to seek clarification from your teachers, classmates, or online resources. Understanding the material thoroughly will boost your confidence during the exam. CHEMISTRY THEORY And OBJECTIVE: QUESTIONS & ANSWERS For 2022/2023 WAEC EXAM..
- Create a study schedule: Plan your study sessions and allocate sufficient time to each topic based on its importance and your level of understanding. Break down your study material into manageable chunks and set realistic goals to stay motivated.
- Collaborate with peers: Consider forming study groups with classmates or friends who are also preparing for the exams. Discussing and explaining concepts to each other can enhance your understanding and retention of the material.
- Utilize available resources: Take advantage of textbooks, reference materials, online resources, and educational platforms that provide study materials, practice questions, and tutorials specific to your exam. These resources can provide valuable insights and additional practice opportunities. CHEMISTRY THEORY And OBJECTIVE: QUESTIONS & ANSWERS For 2022/2023 WAEC EXAM.
- Practice time management: During the exam, time management is crucial. Practice solving questions within the allocated time limits to improve your speed and accuracy. This will ensure that you can complete the exam within the given time frame.
- Stay focused and maintain a healthy lifestyle: Avoid distractions and create a conducive study environment. Take regular breaks, exercise, eat nutritious meals, and get enough sleep to keep your mind and body in optimal condition for studying.
Remember, success in any exam is a result of consistent effort, effective preparation, and a positive mindset. Good luck with your exams!
WAEC Chemistry Questions and Answers 2022:
Furthermore, the questions below are the WAEC Chemistry Questions. Going through them will make you ready to score high in your WAEC 2021 Chemistry Examination. Congratulations.
- How many alkoxy alkanes can be obtained from the molecular formula C4 H4O4
ANSWER: C (3)
- Element Y has two isotopes Y and Y present in the ratio 1:3. The relative atomic mass of Y would be
ANSWER: C (21.5)
- What condition favors the formation of the product for the endothermic reaction, N2O4(g) —><—– 2NO2(g)
A. Decrease in pressure
B. A decrease in volume
C. An increase in pressure
D. A constant volume
ANSWER: A ( Decrease in pressure)
- Elements X and Y have electronic configurations 1S22S22P4 and 1S22S22P63S23P1 respectively. When they combine the formula of the compound formed is
ANSWER: B (Y2X3)
CHEMISTRY THEORY And OBJECTIVE: QUESTIONS & ANSWERS For 2022/2023 WAEC EXAM
- A solution of 0.20 mole of NaBr and 0.20 mole of MgBr2 in 2.0 dm3 of water is to be analyzed. How many moles of Pb(NO3 )2 must be added to precipitate all the bromide as insoluble PbBr2
A. 0.30 mol
B. 0.10 mol
C. 0.20 mol
D. 0.40 mol
ANSWER: A (0.30 mol)
- Na2CO3 + HCl —-> NaHCO3 + NaCl. The indicator most suitable for this reaction should have pH equal to.
ANSWER: D (9)
- A saturated solution of silver trioxocarbonate (IV), was found to have a concentration of 1.30 x 10-5 moldm-3 . The solubility product of the trioxocarbonate (IV) is
A. 8.79 x 10-15
B. 1.69 x 10-10
C. 1.82 x 10-11
D. 9.84 x 10-10
ANSWER: A (8.79 x 10-15)
QUESTIONS & ANSWERS FOR THE 2022/2023 WAEC EXAM
- 100.0g of KClO3 was added to 40.0 cm^3 of water to give a saturated solution at 298K. If the solubility of the salt is 20.0 moldm^-3 at 298K, what percentage of the salt is left undissolved? {K= 39, Cl = 35.5, O = 16}
- Tetraoxosulphate (VI) ions are the final test using
A. acidified silver nitrate
B. acidic barium chloride
C. lime water
D. dilute hydrochloric acid
ANSWER: D (dilute hydrochloric acid)
- When platinum electrodes are used during the electrolysis of copper (II) tetraoxosulphate (IV) solution, the solution gets progressive
D. Atmospheric
ANSWER: A (Acidic)
WAEC 2022/2023 Chemistry Theory Questions
PAPER 2 (ESSAY) SECTION A
- (a) When calcium oxide and coke are heated in an electric furnace, the products are carbon (ii) oxide and calcium carbide (CaC2), write the equation for this reaction.
(b) The addition of water to calcium carbide leads to the formation of calcium hydroxide and ethyne. Write the equation for the production of ethyne.
- Calculate the percentage by mass of silicon tetrachloride. [2 marks]
- Ammonia, NH3, and phosphine, Ph3, are the hydrides of the first two elements in group 5. (a) Draw a dot and cross diagram for the ammonia molecule. [2 marks] (b) Sketch and explain the shape of the ammonia molecule. [3 marks]
- The first ionization energy of chlorine is +1260KJmol-1. (a) Define the term first ionization energy. (b) State and explain the general trend in the values of the first ionization energy for the elements across the period, sodium to argon in the periodic table.
- Compound A consists of carbon and hydrogen only. The compound was found to contain 80% carbon by mass. (a) Calculate the empirical formula of compound A using the data above. (b) The relative molecular mass of compound A was found to be 30. Use this information to deduce the molecular formula of compound A. [H = 1.00 C = 12.00]
- State two factors other than a change in temperature or the use of a catalyst that influence the rate of a chemical reaction.
- Identify the solid remaining when each of the following is heated. (a) lithium trioxonitrate (V) (b) potassium trioxonitrate (V) (c) calcium trioxonitrate (V)
CHEMISTRY THEORY And OBJECTIVE: QUESTIONS & ANSWERS For the 2022/2023 WAEC EXAM
- An aqueous solution has a pH of 4.0. (a) (i) What is the hydrogen ion concentration of the solution? (ii) What effect will it have on litmus paper? (iii) Which of the following salt solutions would have the same effect on litmus? Give a reason for your answer. NH4Cl(aq); NaCl(aq) ; CH3OON(aq). (b) (i) Differentiate between a fine chemical and a heavy chemical. (ii) Name two sources of air pollution. (iii) Suggest one way of reducing air pollution in cities
- (a) (i) Explain briefly the fermentation process. (ii) Write a balanced equation for the fermentation of glucose. (iii) What substance must be added to glucose solution to ferment it? (iv) Explain briefly why tightly corked glass filled to the brim with palm wine shatters on standing. (b) State one industrial application of each of the following methods of separation: (i) Crystallization; (ii) Fractional distillation. (c) Explain the following terms: (i) Saponification; (ii) Esterification. (d) Write a balanced equation to illustrate each of the terms in (c). (e) i) What is hydrocarbon compound? (ii) Name two principal sources of hydrocarbons.

QUESTIONS & ANSWERS FOR 2022/2023 WAEC EXAM
- (a) Two elements represented by the letters Q and R have atomic numbers 9 and 12 respectively. (i) Write the electron configuration of R. (ii) To what group does Q belong in the periodic table? (iii) Write the formula of the compound formed when Q combines with R. (iv) Explain briefly, why Q is a good oxidizing agent. (v) State whether R would be expected to form acidic or basic oxide. (b) (i) State two assumptions of the kinetic theory of gases. (ii) When some solids are heated, they change directly into the gaseous state. What name is given to this phenomenon? (iii) List two substances that exhibit the phenomenon mentioned in (ii). (iv) Write an expression to show the mathematical relationship between the rate of diffusion of a gas and its vapor.
READ ALSO FOR CHEMISTRY THEORY And OBJECTIVE: QUESTIONS & ANSWERS For 2022/2023 WAEC EXAM.
- Latest Secondary Education updates
- Physics 2021 WAEC Questions & Answers
- English Language for WAEC May/ June 2021
- How to Become a professional
- Secondary Education List of Subjects – WAEC approved
- Tips for Successful Professionals
- Tertiary Education updates
- Latest professional tips
- How the basic primary education works
- Professionals and Recruitment
HOW TO PARTNER WITH US: CHEMISTRY THEORY And OBJECTIVE: QUESTIONS & ANSWERS For 2022/2023 WAEC EXAM.
In conclusion, mastering the Chemistry theory and objectives is crucial for success in the upcoming 2022/2023 WAEC exam. By diligently studying and understanding the key concepts, practicing with a wide range of questions, and seeking clarification when needed, students can enhance their understanding of this fascinating subject. Remember, a strong foundation in Chemistry will not only boost your performance in the exam but also equip you with valuable knowledge for future academic pursuits. Stay focused, stay determined, and embark on this journey with confidence. Best of luck in your preparations and may you excel in the upcoming WAEC exam! CHEMISTRY THEORY And OBJECTIVE: QUESTIONS & ANSWERS For 2022/2023 WAEC EXAM.
Finally, for those who would want to win a scholarship and at times part-time studies, this website is for you. For career professional tutorials for ICAN, CITN, NBA exams, etc. this site is available for your current information. Therefore, send us your questions through the comment box. And keep in touch with us by filling in the email list box below for further updates. Our social media buttons will also help if you like us on then or follow us. Thanks Get ready with CHEMISTRY THEORY And OBJECTIVE: QUESTIONS & ANSWERS For 2022/2023 WAEC EXAM.
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Get the Most Legit Information and Guide on the Latest Jobs in Nigeria, Facebook and Education Here
WAEC GCE Chemistry Questions and Answers 2023/2024 (Essay and Objectives)
WAEC GCE Chemistry Questions and Answers 2023 . Welcome to 2023 WAEC Chemistry Questions and Answers. You will find WAEC GCE Chemistry Objective Answers, WAEC Chemistry Essay 2023, WAEC GCE 2023 Chemistry, and the tips you need to pass your WAEC GCE Chemistry examination with ease.

Table of Contents
WAEC GCE Chemistry Questions and Answers 2023 (Expo)
The 2023 WAEC GCE Chemistry expo will be posted here during the WAEC GCE Chemistry examination. Keep checking and reloading this page for the answers.
WAEC GCE 2023 Chemistry Answers Loading.. .
Today’s WAEC GCE Chemistry OBJ Answers:
———————————————————————————–
Note: The answers below are the 2020 Nov/Dec answers.
NOTE: Pls Trace It From Your Objective. [If you see any options here pick it from your objectives]
1 Involves the loss and gain of electrons 2 Polymerisation 3 Global warming 4 3 5 Zinc ions 6 +1.56V 7 Ethene 8 NH3 9 Propanol 10 28 11 Hydrolysis 12 d-orbital 13 Lowering the activation energy 14 Closeness between reactant particles 15 Remaining the same with time 16 Reaction vessel Fels cool during the reaction 17 Faster 18 Solvent extraction 19 Saturated Solution 20 2.75mol/dm³ 21 Partially dissociates in aqueous solution 22 138g 23 HI 24 2.00cm³ 25 hydrogen chloride 26 strong electrovalent bond between ions 27 is not ductile 28 Electrons 29 C2H4 30 Aluminium 31 0.010mol/dm³ 32 PbCO3 33 Linear 34 HCL and HOCL 35 +1 36 Ionic bond 37 have relatively low ionization energy 38 sour to taste 39 I,III and IV only 40 chrometography 41 2.00 dm 42 mole of solvent in 1dm³ of solution 43 does not contain neutron 44 1s²2s²2p⁶ 45 Ammonium chloride 46 IV 47 I,II and IV only 48 Quantum numbers of Electrons 49 -273⁰C 50 Mass number
1-10: DDDADBBABA
11-20: CDABDCCDDC
21-30: ACDBDCCDAB
31-40: BCADABDBAC
More Answers loading…
WAEC GCE Chemistry Theory Answers:
(i) Sodium trioxonitrate (v) decahydrate
NaNO₃ . 10H₂O
(ii) Sodium Oxide –> Na₂O
(iii) Potassium tetraoxophospate (v) –> K₃PO₄
Products formed are:
Hydrogen gas (a+ cathode)
Chlorine gas (a+ anode)
it decrease down the group
As the atomic radius increases down the group the attraction of the positive nucleus of the electron outer most electron, thus the ionization energy decreases
Nitrogen and carbon (ii) oxide
it is used to heat furnace
it is a source of nitrogen for the manufacture of ammonia
Sodium hydrogen – used in qualitative analysis
– Purification of bauxite
Sodium trioxocarbonate (iv)
-Manufacture of glass
-As a water soften
2-amino propane
(2ai) Percentage C5H12 of mass m = 7.2g Volume of O2 = 20.0dm³ (i) from the general combustion equation CxHy(g) + (x+y/4)O2 –> XCO2 + y/2H2O C5H12(l) + 802(g) –> 5CO2(g) + 6H2O(l)
(2aii) 1 mole of C5H12(72g) = 5 moles of CO2 At stop 7.2gC5H12 = x volume of CO2 X = 7.2g×5×22.4dm³/72g X = 5×2.224 = 11.2dm³ of CO2
(2aiii) Volume of oxygen left after the reaction from the equation of reaction 1 mole of C5H12(72g) = 8(22.4)dm³ 7.2g = x X = 7.2×8×22.4/72 = 17.92dm³ Volume of O2 left after the reaction = 20.0dm³ – 17.92dm³ = 2.08dm³
(2b) When molecules collide with one another they possess kinetic energy. As most energetic molecules (those with greater kinetic energy) try to escape. Their escape may be facilitated by heat or by passing a wave of air over the container or by increasing the surface area of the container. As they try to do this, some molecules will loose energy on collision and fall back to the container; as such the average kinetic of the molecules of the liquid in the container reduces which results to cooling effect.
(2ci) Avogadro’s Law states that the total number of atoms/molecules of a gas (i.e. the amount of gaseous substance) is directly proportional to the volume occupied by the gas at constant temperature and pressure.
(2cii) N2(g) + 3H2—> 2NH3 Where 1 mole = 30cm³ of gas
At constant temperature, the volume of a fixed mass of gas. When the volume of a cylinder or a container is increased, the gases have more space to travel and collide hence the pressure is reduced but as the volume is decreased or compressed the gases have less space to travel therefore more pressure is built up.
No (3ai) ¹³R, ⁸Q ¹³R=1s²,2s²,2p⁶,3s²,3p¹ ⁸Q=1s²,2s²,2p⁴
(3aii) ¹³R=2,8,3 Valency of ¹³R is 3 ⁸Q= 2,6 Valency of ⁸Q=2
(3di) 2H² SO4(aq)+4NaOH(aq)—>2Na² SO4(aq)+4H²O(s)
(3dii) Sodium teraoxosulphate (iv) salt and water
(3diii) The resulting solution NaSO4 is basic and will have no effect on litmus paper
(3div) When heated to dryness it can be used as a dehydrating agent
Typing….
—————————————————————————————————————–
The questions below are strictly for practice.
1. Which of the following statements best explains the difference between a gas and a vapour?
(a) Unlike gases, vapours are liquids at room temperature
(b) Unlike gases, vapour can easily be condensed into liquids
(c) Unlike gases, vapour is readily converted into solids
(d) Vapours are generally denser than gases
2. Consider the following reaction equation: 2HCI + Ca(OH) 2 → CaCI 2 + H 2 O. what is the volume of 0.1 moldm -3 , HCI that would completely neutralize 25 cm 3 or 0.3 moldm -3 Ca(OH) 2 ?
(a) 150 cm 3
(b) 75 cm 3
(c) 30 cm 3
(d) 25 cm 3
3. Cu and HNO 3 are not suitable for preparing hydrogen gas because of their
(a) Reactivity and oxidation respectively
(b) conductivity and corrosiveness respectively
(c) melting point and reduction respectively
(d) electronegativity and solubility respectively
4. Which of the following formulae cannot be an empirical formula?
(c) P 2 O 5
(d) N 2 O 4
5. One of the criteria for confirming the purity of benzene is to determine its
(a) Heat capacity
(b) boiling point
6. When chlorine is passed through a sample of water, the pH of the water sample would be (a) <7
(a) 1.20 x 10 23
(b) 2.41 x 10 23
(c) 3.62 x 10 23
(d) 4.82 x 10 23
8. The strength of metallic bonds depends on the
(a) charge density of the atoms
(b) ductility of the metal
(c) number of valence electrons
(d) total number of electrons in the atoms
9. When zinc is added to AgNO 3 solution, crystals of silver forms on the zinc surface. This indicates that zinc is
(a) oxidized
(b) reduced
(c) decomposed
(d) dissociated
(d) Cu 2 O 2
- WAEC GCE Mathematics Questions and Answers
- WAEC GCE Physics Questions and Answers
11. The change in the oxidation state of iron in the reaction represented by the equation below is 2FeCI 3 + H 2 S →2FeCI 2 + 2HCI + S
(a) +2 to +3
(b) +3 to +2
(c) 0 to +2
(d) +3 to 0
12. Which of the following methods can be used to separate blood cells from plasma?
(a) Centrifugation
(b) Filtration
(c) Chromatography
(d) Distillation
13. Which of the following statements about ionic radius is correct? ‘Ionic radius
(a) Increases as nuclear charge increases
(b) decreases as nuclear charge increases
(c) decreases as nuclear charges decreases.
(d) remains constant as nuclear charge increases
14. Analysis of a hydrocarbon shows that it contains 0.93 g of carbon per gram of the compound. The mole ratio of carbon to hydrogen in the compound is [H=1.0, C=12.0]
15. The law of definite proportions states that
(a) pure samples of same compound contain the same elements combined in the same proportion by mass
(b) pure samples of substances are in the same proportion by mass
(c) chemical compounds are pure because they contain the same elements
(d) matter can neither be created nor destroyed
17. Atoms are electrically neutral because they
(a) don not conduct electricity
(b) contain equal number of protons and electrons
(c) are composed of neutrons and electrons
(d) cannot be attracted by electromagnetic field
18. Common salt (NaC1) is used for preserving foods. Which of the following properties could be used to determine its purity before use?
(a) Solubility in water
(b) melting point
(c) Relative density
(d) Crystalline nature
19. Which of the following electron configurations represents the transition element chromium ( 24 Cr)?
(a) 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 4
(b) 1s 2 2 2s 2 2p 6 3s 2 3p 6 3d 6
(c) 1s 2 2s 2 2p 6 2s 2 3d 4 4s 1 (d) 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1 3d 5
20. The atomic number of an isotope of hydrogen is equal to its mass number because it
(a) has a totally filled valence shell
(b) has a high charge to mass ratio
(c) does not contain neutrons (d) exhibits isotopy
21. The total number of shared pair of electrons in the compound below is
22. The bonding pair of electrons in a hydrogen chloride molecule is pulled towards the chlorine atom because
(a) Chlorine has a larger atomic size
(b) chlorine has a large atomic mass
(c) chlorine is more electronegative
(d) there is no bonding orbitals within the hydrogen atom
23. The solubility of CO 2 in water can be accounted for by
(a) van der waal’s forces
(b) ionic attraction
(c) dipole attraction
(d) covalent bonding
24. Which of the following properties would not influence electrovalent bond information?
(a) Electronegativity
(b) Electron affinity
(c) Ionization potential
(d) Catalytic ability
25. Particles in a solid exhibit
(a) Vibrational motion only
(b) vibrational and translational motion
(c) vibrational and random motion
(d) random and translational motion
WAEC GCE Chemistry Essay 2023
The above questions are not exactly 2023 WAEC Chemistry questions and answers but likely WAEC Physics repeated questions and answers.
These questions are for practice. The 2023 WAEC GCE Chemistry expo will be posted on this page 30 minutes before the WAEC GCE Chemistry examination starts. Keep checking and refreshing this page for the answers.
If you have any questions about the WAEC GCE Chemistry questions and answers, kindly drop your questions in the comment box.
Last Updated on October 2, 2023 by Admin
Related posts:

8 thoughts on “WAEC GCE Chemistry Questions and Answers 2023/2024 (Essay and Objectives)”
How can I slove hard question in question
Pls i’m a candidate for gce advance level and i need chemistry papers 2023
Please I need mathematics, chemistry, physics and biology questions
Pls always post me from 2021till 2023 in 9 subject which is Econ, maths Igbo chemistry physics, Agric civic bio, Eng
I need waec English for today
I need past questions on all science subjects please
Pls I need the question for chemistry, physics, biology, agricultural science, english and mathematics
Okay heard you
Leave a Comment Cancel reply
Save my name, email, and website in this browser for the next time I comment.
Notify me of follow-up comments by email.
Notify me of new posts by email.


Home » WAEC Chemistry Answers 2024 Essay/OBJ Questions is Out
2024 WAEC Chemistry Questions & Answers for Essay and Objective Released.
The Waec chemistry answers 2024 essay and objective questions for the West African Examination Council (WAEC) Chemistry SSCE exam paper scheduled to be written on Tuesday, 12th December, 2023 can now be studied here.
The 2023 Chemistry Essay answer paper will start at 9:30 am and will last for 2hrs while the Objective exam will commence at 11:30 pm and will last for 1hr.
In this post, we will be posting the West African Senior School Certificate Examinations (WASSCE) Chemistry questions for candidates who will participate in the examination from past questions.

Continue reading below.
WAEC Chemistry Answers 2024.
PAPER 2 [Essay] Answer any FOUR questions. Write your answers on the answer booklet provided.
1. (a) (i) What is the common name given to the group VII elements? (ii) Name the hydrides of the first two elements in group VII. (iii) State three chemical properties of group VII elements.
(b) Copy and complete the following table
(c) (i) Define each of the following processes: nuclear fission; nuclear fusion. (ii) Give one use of each process in 1(c)(i)
(d) (i) List three types of radiation that are produced during radioactivity. (ii) Arrange the radiations listed in 1(d)(i) in order of increasing: penetrating power; ionizing power.
ANS: (a) (i) Halogens (ii) Hydrogen fluoride; Hydrogen chloride (iii) high electron affinity/strong oxidizing agents/electron acceptor – highly electronegative; – react with hydrogen to form acid; – form salts with metals; – react with alkalis to form salts; – react with water to form acids; – displacement of lower halogens from their acids/salts; – react with hydrocarbon to form alkyl halides. (b)
(c)(i) I. Nuclear fission – splitting of a heavy nucleus into two smaller nuclei of similar mass with the release of a large amount of energy and radiation. II. Nuclear fusion – a combination of smaller nuclei to form a large nucleus with the release of large amounts of energy and radiation. (ii) I. Used to generate electricity/nuclear bomb/production of new elements/production of radioisotopes. II. Used to produce nuclear weapons/atomic bombs/production of new elements/production radioisotopes. (d) (i) alpha, beta, gamma OR α , β, (ii) I. α < β < increasing penetrating power II. <β < α increasing ionizing power.
2. (a) (i) What is the structure of the atom as proposed by Rutherford? (ii) Distinguish between the atomic number and the mass number of an element. (iii) Explain briefly why the relative atomic mass of chlorine is not a whole number.
(b) (i) What is meant by first ionization energy? (ii) List three properties of electrovalent compounds (iii) Consider the following pairs of elements: 9F and 17CL; 12Mg and 20Ca. Explain briefly why the elements in each pair have similar chemical properties.
(c) Explain briefly the following terms using an appropriate example in each case (i) homologous series; (ii) heterolytic fission.
(d) State the indicator(s) which could be used to determine the end-point of the following titrations: (i) dilute hydrochloric acid against sodium hydroxide solution; (ii) dilute hydrochloric acid against ammonium hydroxide solution; (iii) ethanoic acid against sodium hydroxide solution.
(e) A solid chloride E which sublimed on heating reacted with an alkali F to give a choking gas G. G turned moist red litmus paper blue. Identify E, F and G.
ANS: (a) (i) The atom has a small/ tiny positively charged centre /nucleus with electrons surrounding the space around the centre. (ii) Atomic number of an element is the number of protons/electrons in an atom of the element while the mass number is the sum of the protons and neutrons in the atom of the element. (iii) Chlorine atom is made up of a mixture of isotopes and the relative atomic mass of chlorine is the average of its isotopic masses.
(b) (i) Is the (minimum) energy required to remove one mole of an electron from one mole of gaseous atom (to form one mole gaseous charged ion (ii) High melting /boiling point; Ability to conduct electricity in the molten state or in solution; Solid at room temperature; Soluble in water or polar solvents /insoluble in non-polar solvents. (iii) Atoms of the elements in each pair have the same number of electrons in their outer-most shell therefore similar chemical properties.
(c) (i) Is a family of organic compounds: – where successive members differ by –CH2 of the molar mass of 14; – with similar chemical properties; – which conform to the same general formula; – which show a gradation of physical properties; – which have the same general method of preparation. e.g alkanes, alkenes , alkanols. (ii) Is a process in which a (covalent) bond is broken in such a way that the electron pair is completely transferred to one of the atoms (resulting in the formation of ions) H ÷ CI → H+ + Cl-/ HCl ® H+ + Cl- (d)(i) Methyl orange/ methyl red/ phenolphthalein; (ii) Methyl orange/ methyl red; (iii) Phenolphthalein. (e) E – NH4Cl F – NaOH, KOH, or Ca (OH)2, Li OH, CsOH, Ba(oH)2, Mg(OH)2 G – NH3.
3. (a) (i) Define saturated solution. (ii) The solubility of KN03 at 20°C was 3.00 mol dm-3 If 67.0g of KN03 was added to 250cm3 of water and stirred at 20°C, determine whether the solution formed was saturated or not at that temperature.
(b) (i) Distinguish between the dative bond and covalent bond. (ii) Explain why sugar and common salt do not conduct electricity in the solid state. (iii) State the type of intermolecular forces present in I. hydrogen fluoride; II. argon. (iv) Consider the compounds with the following structures: S – H —-N and 0 – H —–N In which of the compounds is the hydrogen bond stronger? Give a reason for your answer.
(c) (i) State Dalton’s Law of Partial Pressure. (ii) If 200cm3 of carbon(IV) oxide were collected over water at 18°C and 700 mmHg, determine the volume of the dry gas at s.t.p.[ standard vapour pressure of water at 18°C = 15 mmHg]
ANS: (a) (i) Is a solution that contains the maximum amount of solute it can dissolve at a given temperature (in the presence of undissolved solute). (ii) Solubility of KN03 in in g dm-3 = 3.00 x 101 = 303 .. 1000cm3 of saturated solution = 303g 250cm3 of the solution = 303 x 250 1000 = 75.8 g Since the quantity of KN03 added (67.0) to 250 cnr’ of water is less than the maximum amount required to form a saturated solution, then the solution is unsaturated.
(b)(i) In a dative bond, only one of the participating atoms/ species donated electrons to be shared by both atoms while in a covalent bond both participating atoms/ species contribute equally to the electrons being shared. (ii) Sugar is covalent while common salt (NaCl) is electrovalent/ ionic. Electrical conductivity (in compounds) depends on the presence of mobile ions. (iii) The intermolecular forces present in hydrogen fluoride and argon were hydrogen bond and van der Waal’s forces respectively. (iv) 0 – H —- N has a stronger hydrogen bond because oxygen is more electronegative and smaller in size than sulphur.
(c)(i) the total pressure exerted by a mixture of gases that do not react chemically is equal to the sum of the individual partial pressures of the gases in the mixture. (ii) pressure of the dry gas (P 1) = 700 – 15 = 685 mmHg VI = 200cm 3 , TI = 18°C = 273 + 18 = 291K, P2 = 760 mmHg, T2 = 273 P1V1 = P2V2 T1 T2 V2 = P1V1T 2 = P2T1
= 685 x 200 x 273 760 x 291
= 169.1cm 3
4. (a) (i) Define nuclear fission (ii) A certain natural decay series starts with and ends with. Each step involves the loss of an alpha or a beta particle. Using the given information, deduce how many alpha and beta particles were emitted. (b) Consider the equilibrium reaction represented by the following equation: A2(g) + 3B2(g) 2AB3(g); H = + kJmol-1 Explain briefly the effect of each of the following changes on the equilibrium composition; (i) increase in the concentration of B; (ii) decrease in pressure of the system; (iii) addition of catalyst. (c) The lattice energies of three sodium halides are as follows:
Explain briefly the trend.
(d) State the property exhibited by nitrogen (IV) oxide in each of the following reactions: (i) 4Cu + 2NO2 4CuO + N2; (ii) H2O+ 2NO2 HNO3 + HNO2
(e) Iron is manufactured in a blast furnace using iron ore (Fe2O3), coke and limestone. Write the equation for the reaction(s) at the: (i) top of the furnace; (ii) middle of the furnace; (iii) bottom of the furnace. (f) (i) Name two products of destructive distillation of coal. (ii) Give one use of each product in 3(f)(i).
5. Copy and complete the following table:
(i) Mention one compound that makes water I. temporarily hard; II. permanently hard. (ii) State one method that could be used to remove I. only temporary hardness; II. permanent hardness. (iii) Write an equation to show the removal of: I. temporary hardness; II. permanent hardness. (c) (i) List three sources of water pollution. (ii) Mention two ways by which water pollution can be controlled. (d) state the function of each of the following substances in the purification of water for town supply: I. sodium aluminate (III) (NaAIO2); II. lime (calcium hydroxide); III. calculated mount of iodine; IV. sand bed.
6. (a) (i) What is meant by atomicity? (ii) Mention one element in each case which is I. monatomic, II. diatomic, III. tetratomic. (iii) Write the orbital electron configuration of I. 20Ca, II. 9F. a. In which group does each of the elements belong? b. How many unpaired electrons are present in 9F? c. How many electrons are present in 20Ca2+?
(b) (i) Write a balanced equation for the thermal decomposition of KCƖO3. (ii) Mention the catalyst that could be used to increase the rate of reaction in 6(b)(i). (ii) If 5.0 g of KCƖO3 was decomposed by heat, determine the volume of oxygen produced at s.t.p. [Molar gas volume at s.t.p. = 22.4dm3, K = 39, Cl = 35.5, O = 16]
(c) (i) Mention the products formed when each of the following substances is heated strongly: I. ZnCO3; II. CuSO4.5H2O. (ii) State the colour change observed when each of the residues in 1(d)(i) above is allowed to cool.
7. (a) Describe briefly how each of the following aqueous solutions could be identified in the laboratory: (i) Ammonium trioxocarbonate (IV); (ii)Ammonium chloride. (b) Arrange the following compounds in order of increasing boiling point and give reasons for your answer: CS2, NaF and CO2. (c) List two gases each that are: (i) acidic; (ii) highly soluble in water; (iii) oxidized by acidified KMnO4(aq). (d) In a tabular form, compare the elements silicon and sulphur under the following properties: (i) metallic character; (ii) physical state; (iii) conduction of electricity. (e) A cuboid piece of sodium metal measures 3 cm x 4 cm x 10 cm. If the density of sodium is 0.971 g cm-3, calculate the number of atoms in the sodium metal. [ Na = 23; Avogadro constant = 6.02 x 1023 mol_1 ]
8. (a) (i) Define standard electrode potential. (ii) State two factors that affect the value of standard electrode potential. (iii) Give two uses of the values of standard electrode potential. (iv) Draw and label a diagram for an electrochemical cell made up of Cu2+/Cu; = + 0.34 Zn2+/Zn; = – 0.76 (v) Calculate the e.m.f of the cell in 8(a)(iv) above (b) (i) In terms of electron transfer, define I. oxidation; II. oxidizing agent. (ii) Balance the following redox reaction: MnO4- + I- H+ I2Mn2+
(c) Classify each of the following oxides as basic, amphoteric, acidic or neutral: (i) Carbon (II) oxide; (ii) Sulphur(IV) oxide; (iii) Aluminium oxide; (iv) Lithium oxide.
(d) What is hydrogen bonding?
WAEC Chem Objective Questions 202 4 .
PAPER 1 [Essay] Answer All questions in this section. Write your answers on the answer sheet provided.
1. Two immiscible liquids with different boiling points can be separated by _____ A. The use of separating funnel B. Evaporation C. Distillation D. Decantation.
2. A mixture of CaCl2 and CaCO3 in water can be separated by ______ A. Evaporation B. Sublimation C. Distillation D. Decantation.
3. What is responsible for metallic bonding? A. sharing of electrons between the metal atoms B. attraction between the atomic nuclei and the cloud of electrons C. Transfer of electrons from one atom to another D. attraction between positive and negative ions.
4. 25cm3 of 1.5M solution of NaCl are added to 50cm3 of 3M NaCl. The molar concentration of the resulting solution is ________ A. 2.5M B. 3M C. 2.25M D. 4.5M
5. A solution of salt formed from HCl and NH3 solutions is _____ A. Acidic B. Basic C. complex D. Neutral
6. Which of the following elements will burn in excess oxygen to form a product that is neutral to litmus? A. carbon B. Hydrogen C. Sulphur D. Sodium
7. A current was passed for 10 mins and 0.2mole of Cu was deposited. How many grammes of Ag will it deposit? (Cu = 64, Ag = 108) A. 43.2g B. 21.6g C. 10.8g D. 5.4g
8. Pollution of underground water by metal ions is very likely in a soil that has high ________ A. Acidity B. Alkalinity C. Chloride content D. Nitrate content
9. Producer gas is a gas with low caloric value because it contains more ____ A. CO2 than O2 B. N2 than CO C. CO2 than N2 D. N2 than CO2
10. Silver chloride turns grey when exposed to sunlight because _____ A. The silver ion is reduced to silver B. The silver ion is oxidized to silver C. Silver is a transition metal D. The silver chloride forms complexes in the sun.
11. Which of these compounds exhibits resonance? A. Benzene B. Ethanol C. Propene D. Butyne
15. Hydrolysis of CH3COOCH2CH3 in dilute HCl produces ______ A. CH3COOH + CH3CH3 B. CH3CH2OH + CH3COCl C. CH3COOH + CH3CH2OH D. CH3COOH + CH3CH3
16. Calculate the volume of CO2 measured at s.t.p produced on heating 250g of potassium hydrogen trioxocarbonate (IV) strongly. (K = 39, H = 1, C = 12, O = 16) A. 28dm3 B. 2.8dm3 C.5.6dm3 D. 11.2dm3
17. The boiling points of water, ethanol, methylbenzene and butan-2-ol are 373.0K, 351.3K, 383.6K and 372.5K respectively. Which liquid has the highest vapour pressure at 323.0K? A. Water B. Methylbenzene C. Ethanol D. Butan-2-ol
18. The conclusion from Rutherford’s alpha scattering experiment is that ______ A. Atoms are mostly empty space with a small nucleus B. Emissions from radioactive substances consist of three main components C. There is a nuclear pull on orbital electrons D. Electrons are deflected by both magnetic and electric fields.
19. Elements P, Q and R have atomic numbers 9, 16 and 20 respectively. Which of them would gain electron(s) during ionic bonding A. Q and R B. P and R C. P and Q D. P, Q and R.
20. Which of the following has the lowest PH? A. 5cm3 of M/10 HCl B. 10cm3 of M/10 HCl C. 20cm3 of M/8 HCl D. 15cm3 of M/2 HCl
21. Which of the following is an acid salt? A. (NH4)2CO3 B. CHCOONa C. KHSO4 D. MgSO4.7H2O
22. Cr2O2-7 + 14H+ + 6I → 2Cr3+ + 3I2 + 7H2O. The change in the oxidation number of oxygen in the equation above is _______ A. 0 B. 1 C. 2 D. 7
23. During electrolysis of CuSO4 solution using platinum electrodes, which of the following occurs ______ A. Acidity increases at the cathode B. Oxygen is liberated at the cathode C. PH decreases at the cathode D. PH of solution increases.
24. Which of the following ions is a pollutant in drinking water even in trace quantities? A. Ca2+ B. Pb2+ C. Mg2+ D. Fe2+
25. The solubility of a salt of molar mass 100g at 20oC is 0.34mol/dm3. If 3.4g of that salt dissolved completely 250cm3 of water at that temperature, the resulting solution is ________ A. A suspension B. Saturated C. Unsaturated D. Supersaturated
26. Catalyst is important in in chemical industry in that ______ A. it affects the purity of the products B. it affects the quantity of the products C. it increases the time for reaching equilibrium D. Bond breaking is slowed down.
27. An alkanioc acid has a molecular mass of 88. Name the acid. (C = 12, O =16, H = 1) A. Propanioc acid B. Botanioc acid C. Pentanioc acid D. But-2-ionic acid
28. Ethyne undergoes the following reactions EXCEPT A. Polymerization B. Addition C. Combustion D. Etherification
Keep following, more questions and answers will be added soon.
PS: Once again, there is nothing like waec chemistry expo. Do not fall victim to scammers online trying to obtain money from you with fake promises of having access to live question paper before the exam. What we have on this page are likely exam questions from waec past questions and answers to serve as a revision guide.
« Zerry Dl – Puff & Pass | »
Home » NECO Answer » Neco Chemistry Answers 2022 (Objective And Theory)
Neco Chemistry Answers 2022 (Objective And Theory)

Are You Looking for NECO Chemistry Answers 2022? in this post am going to share with you NECO Chemistry Answers 2022 , make sure you read this article to the end, and don't forget to share it on social media […] A lot of people will collect money to publish the Neco Chemistry Answers 2022 For 25th July 2022, but our answers will be published here.
Neco Chemistry Answers 2022: here are the verified Neco 2022 Chemistry Objective & Essay questions and answers for SS3 students for Monday 25th July 2022. Neco 2022 Chemistry Objective & Essay Questions and Answers.
Table of Contents Toggle To get your Chemistry Objective And Theory 2022, WhatsApp me 09035742503, with Your 500 MTN card or transfer. Neco 2022 Chemistry Objective & Essay Questions and Answers Neco Chemistry Answers 2022 Neco 2022 Chemistry (Objective & Essay) Questions and Answers Neco Chemistry Answers 2022 (Objective And Theory) To get your Chemistry Objective And Theory 2022, WhatsApp me 09035742503, with Your 500 MTN card or transfer.
Neco Chemistry Answers 2022: EXPO: Neco Chemistry Objective & Essay Questions and Answers 2022/2023, 2022/2023 Neco EXPO ON Chemistry Objective & Essay QUESTIONS, 2022 Chemistry Objective & Essay Questions & Answers is Out.
Neco 2022 Chemistry Objective & Essay Questions and Answers
Neco Chemistry Objective & Essay Answers 2022: Welcome to the Expobite answer page where you will get verified and 100% authentic and correct Neco Chemistry Objective & Essay Answers 2022 for Monday 25th July 2022.
Examkey.net Chemistry Objective & Essay pin questions and answers for today, examkey, joberplanet, lasu-info, ceebook, noniwap, noniexpo, examspot, unn-edu, nairaland, utmeofficial, bestexamportal, loadedking.com literature Pin questions and answers for today.
Neco Chemistry Answers 2022
Neco Chemistry 2022 Answers & Questions for Practical, Neco Chemistry Objective & Essay Practical Questions and Answers 2022, 2022/2023 Neco EXPO ON Chemistry Objective & Essay QUESTIONS, Neco Chemistry Objective & Essay Questions and Answers for 2022/2023, Neco Chemistry Objective & Essay questions and answers, Neco Chemistry Objective & Essay Questions and Answers 2022/2023 Expo/Runs
I Will Send you the Correct 2022 Neco Chemistry (Objective & Essay) Questions and Answers, Neco 2022 Chemistry (Objective & Essay) Runs, Neco 2022 Chemistry (Objective & Essay) Expo, Neco 2022 Chemistry (Objective & Essay) Answers with Objective and Essay/Theory Exactly 5 hours Before the Exam. Neco 2022 Chemistry (Objective & Essay) Obj and Essay Questions & Answers.
Check Out: Neco Chemistry Answers 2022
Neco 2022 Chemistry (Objective & Essay) Questions and Answers
We are pleased to inform all Neco students sitting for the 2022 Neco Chemistry (Objective & Essay) Exam that we have the complete Neco 2022 Chemistry (Objective & Essay) Expo with Questions Paper for May/June Neco Examination.
Did you know a good Neco result is your one ticket to admission to any University or Polytechnic of your choice? If you're directed to this website pls subscribe now and smile while you write your exam.
Neco Chemistry (Objective & Essay) Answer 2022, Neco Chemistry (Objective & Essay) 2022, Neco Timetable 2022, Neco 2022 Syllabus, Neco Time Table Download, Neco May June exam Start When, Neco May/June 2022 Registration, Is Neco Registration Still on, Neco Chemistry (Objective & Essay) Nov Dec 2022.
TAGS: NECO , Pdf mentions
Do you find Naijalanded useful? Click here to give us five stars rating!

How To Check My WAEC Result April, 2024 | BEST WAEC Result Checker
Neco literature 2022 answers, neco mathematics 2022 answers for 2022 exam, neco biology practical 2022 answers for 2022 exam, neco gce expo 2022 free & verified questions and answers (all subject), 0 responses, leave a reply.
Name (required)
Email (required, but never shared)
Do you wish to initiate a DMCA takedown report?
Kindly send details to [email protected]
Home | Recent Posts | Pages
Advertise with us
To advertise with us, contact us at [email protected] or +2348066918208 . Visit [email protected] to learn more about NaijaLanded offerings and advertising options.
- Past Papers
- School Papers
- Applications
- A/L Marking Schemes
- O/L Marking Schemes
- Agriculture
- Combined Maths
- Business Studies
- Engineering Technology
- Study Materials

- 2022 (2023) A/L Chemistry Paper
- Download Pdf files – MCQ and 2nd paper
- MCQ answers for 2022 MCQ paper
“Download the 2022 (2023) Advanced level Chemistry paper in Sinhala medium as a PDF file. The file includes both the 1st and 2nd papers. Simply click on the links below to access and Download the files.”
MCQ answers for the 2022 (2023) Advanced level Chemistry paper in Sinhala medium are also available below 👇
——————————————————————–
————————————————————————-
MCQ Answers
Examination – G.C.E A/L Subject – Chemistry Year – 2022 (2023) Medium – Sinhala Medium
#PastPapers #2022 #Chemistry #BioApiLK
- McQ answers
- sinhala medium
G.C.E. O/L, A/L & Grade 5 Scholarship Exam Dates Announced – 2024
G.c.e. a/l exam 2023 (2024) – practical test time table & admission card, southern province chemistry 2019 grade 13 first term part i.
Tamil medium
LEAVE A REPLY Cancel reply
Save my name, email, and website in this browser for the next time I comment.
Most Popular
Presidential scholarship programme application 2024 for school students, national school graduate teaching exam admission card 2024 – download online, sabaragamuwa university (susl) – application for courses (2024 february), recent comments, gce a/l business studies past papers 2011 – 2015, north western province chemistry 2019 grade 13 first term part i, download resource books, popular posts, 2021 a/l biology mcq answers, download timetable and study plan, popular category.
- Past Papers 82
- Downloads 51
- Model Papers 42
- chemistry 39
- Provincial Papers / Model Papers 33
- A/L Marking Schemes 22
- Combined Mathematics 19
BioApi.Lk is a free resource site for O/L , A/L and other Students In Sri Lanka. Bio Api was founded in November 2016 as a Facebook Page. The main goal of this site is to provide Past Papers, Marking Schemes, Notes, and other Study materials that allow students to improve their knowledge. Stay with Us!
Contact us: [email protected]
© 2023 Bio Api | Designed by Sathsara

IMAGES
VIDEO
COMMENTS
Get Free Live 2022 NECO June/July Verified Chemistry (CHEM) OBJ & Essay Questions and Answers Free of Charge | NECO June/July Free Chemistry (Objectives and Theory) Questions and Answers EXPO Room (25th July, 2022).NECO JUNE/JULY 2022 FREE CHEMISTRY OBJ & ESSAY (CHEM) QUESTION AND ANSWER ROOM Monday 25th July 2022Chemistry (Objective & Essay)10:00am - 1:00pm
Questions 1-3 are long free-response questions that require about 23 minutes each to answer and are worth 10 points each. Questions 4-7 are short free-response questions that require about 9 minutes each to answer and are worth 4 points each. For each question, show your work for each part in the space provided after that part.
Get exam information and free-response questions with sample answers you can use to practice for the AP Chemistry Exam. ... The free-response section includes three long essay questions (worth 10 points each) and four short-answer questions (worth 4 points each). ... The CED was updated in the summer of 2022 to incorporate the change to the ...
NECO Chemistry Questions and Answers 2022 is now release for the June/July 2022. NECO Chemistry Theory and Objective Answers (100%legit) Chemistry Essay verified Free (Expo) for National Examination Council. NECO Chemistry Questions For you to have good NECO result in Chemistry as well as repeated questions for free in this post.
The 2022 Chemistry Essay paper will start by 9:30 am and will last for 2hrs while the Objective exam will commence at 11:30 am and will last for 1hr. In this post, we will be posting out the West African Senior School Certificate Examinations (WASSCE) Chemistry questions for candidates that will participate in the examination from past questions.
Water reacts with hydrogen ions: write the formula of the product formed ( 1 / 2 mark) Name the type of bond formed ( 1 / 2 mark) The melting point of iodine is higher than that of chlorine. Explain. (2 marks) A sample of ammonia gas can be prepared by heating a mixture of ammonium bromide and barium hydroxide.
How to Subscribe For WAEC Chemistry 2022 ANSWERS. Chemistry 2 (Essay) 2:00pm. - 4:00pm. INSTRUCTION: YOU ARE TO ANSWER FOUR (4) QUESTIONS IN ALL. QUESTION ONE (1) IN SECTION A & ANY THREE (3) QUESTIONS IN SECTION B. NOTE: USE EITHER THE TYPED VERSION OR THE IMAGE VERSION… make your work look different.
WAEC Chemistry 2022 answers. WAEC Chemistry questions and answers 2022/2023 objective and essay. See the 2022 WAEC Chemistry answers for both objective and theory below. Get the WAEC Chemistry objective and essay answers here. The 2022 WAEC OBJ and theory questions and answers are provided here for free. All you have to do is to […] The post WAEC Chemistry Questions and Answers 2022 ...
Sample Essay Questions and Answers. (a) Define the following terms: (i) Atomicity (ii) Isotopes. (b) Elements P, Q, and R have atomic numbers 9, 16, and 20, respectively. Which of these elements would gain electron (s) during ionic bonding? (c) Write the electron configuration of the following elements: (i) 9F (ii) 20Ca.
SSCE WAEC Chemistry Objective Questions and Answers. CHEMISTRY Paper 1 (Objective Test Questions) Paper 1 will last for 1 hours Use HB pencil throughout. Answer All Questions Each question is followed by four options lettered A to D. Find out the correct options for each question and shade in pencil on your answer sheet, the answer space which bears the same letter as the option you Chosen.
Download free-response questions from past exams along with scoring guidelines, sample responses from exam takers, and scoring distributions. AP Exams are regularly updated to align with best practices in college-level learning. Not all free-response questions on this page reflect the current exam, but the question types and the topics are ...
WAEC 2022/2023 Chemistry Theory Questions. PAPER 2 (ESSAY) SECTION A. (a) When calcium oxide and coke are heated in an electric furnace, the products are carbon (ii) oxide and calcium carbide (CaC2), write the equation for this reaction. (b) The addition of water to calcium carbide leads to the formation of calcium hydroxide and ethyne.
WAEC chemistry Questions and Answers 2022 is now release for the May/June 2022. WAEC chemistry Theory and Objective Answers (100%legit) chemistry 2 Essay verified Free (Expo) for West African Examinations Council. WAEC chemistry Questions For you to have good WAEC result in chemistry as well as repeated questions for free in this post.
PROCEDURE I (a) Place two test tubes in a test tube rack. To the first test tube, place about 2 cm3 of solution B. To the second test tube, place about 2 cm3 of solution CI. Add 2 drops of indicator solution A to each of the test tubes, shake and note the colour of each solution. Record the colours in Table 1.
NECO Chemistry Questions and Answers 2022. NECO 2022 Chemistry Questions will be posted in this page. Our Team are right now with the question paper. It is under verification and once tthe verification process complete, we will go ahead to upload it here. Be at alert! Keep refreshing this page for NECO Chemistry Theory Questions and Answers ...
Biology Essays; Form 1 Biology Topical Revision Questions and Answers; ... Chemistry Paper 2 Questions and Answers - KCSE 2022 Mock Exams Set 1. Download PDF for future reference Get on Whatsapp for 50/- ... Chemistry Paper 2 Questions and Answers - KCSE 2022 Mock Exams Set 1.
The 2023 answers will be posted here on 24th May during the exam. WAEC Chemistry OBJ Answers Loading…. 1-10: ACADABBDAC. 11-20: BCAABBCCBB. 21-30: ACBABDACBB. 31-40: ABBDAADCDD. 41-50: BDCDDCDDCD. (1a) A transition element, also known as a transition metal, is an element that belongs to the d-block of the periodic table.
The following NECO Chemistry questions are questions to expect in the 2023 NECO examination. 1. The minimum amount of energy required for effective collisions between reacting particles is known. A) Activation energy. B) Bond energy. C) Kinetic energy. D) Potential energy. 2.
WAEC GCE Chemistry Essay 2023. The above questions are not exactly 2023 WAEC Chemistry questions and answers but likely WAEC Physics repeated questions and answers. These questions are for practice. The 2023 WAEC GCE Chemistry expo will be posted on this page 30 minutes before the WAEC GCE Chemistry examination starts.
The 2023 Chemistry Essay answer paper will start at 9:30 am and will last for 2hrs while the Objective exam will commence at 11:30 pm and will last for 1hr. In this post, we will be posting the West African Senior School Certificate Examinations (WASSCE) Chemistry questions for candidates who will participate in the examination from past questions.
A lot of people will collect money to publish the Neco Chemistry Answers 2022 For 25th July 2022, but our answers will be published here. Neco Chemistry Answers 2022: here are the verified Neco 2022 Chemistry Objective & Essay questions and answers for SS3 students for Monday 25th July 2022. Neco 2022 Chemistry Objective & Essay Questions and ...
Download Pdf files - MCQ and 2nd paper. MCQ answers for 2022 MCQ paper. "Download the 2022 (2023) Advanced level Chemistry paper in Sinhala medium as a PDF file. The file includes both the 1st and 2nd papers. Simply click on the links below to access and Download the files.".
Biology Essays; Form 1 Biology Topical Revision Questions and Answers ... Chemistry Paper 1 Questions and Answers - KCSE 2022 Mock Exams Set 2. Next Topic » Chemistry Paper 3 Questions and Answers with Confidentials - KCSE 2022 Mock Exams Set 2. Print PDF for future reference . Published in KCSE 2022 Mock Exams Questions and Answers Set 2.