- PRO Courses Guides New Tech Help Pro Expert Videos About wikiHow Pro Upgrade Sign In
- EDIT Edit this Article
- EXPLORE Tech Help Pro About Us Random Article Quizzes Request a New Article Community Dashboard This Or That Game Popular Categories Arts and Entertainment Artwork Books Movies Computers and Electronics Computers Phone Skills Technology Hacks Health Men's Health Mental Health Women's Health Relationships Dating Love Relationship Issues Hobbies and Crafts Crafts Drawing Games Education & Communication Communication Skills Personal Development Studying Personal Care and Style Fashion Hair Care Personal Hygiene Youth Personal Care School Stuff Dating All Categories Arts and Entertainment Finance and Business Home and Garden Relationship Quizzes Cars & Other Vehicles Food and Entertaining Personal Care and Style Sports and Fitness Computers and Electronics Health Pets and Animals Travel Education & Communication Hobbies and Crafts Philosophy and Religion Work World Family Life Holidays and Traditions Relationships Youth
- Browse Articles
- Learn Something New
- Quizzes Hot
- This Or That Game New
- Train Your Brain
- Explore More
- Support wikiHow
- About wikiHow
- Log in / Sign up
- Education and Communications
- College University and Postgraduate
- Academic Writing
- Research Papers

How to Write a Medical Research Paper
Last Updated: February 5, 2024 Approved
This article was co-authored by Chris M. Matsko, MD . Dr. Chris M. Matsko is a retired physician based in Pittsburgh, Pennsylvania. With over 25 years of medical research experience, Dr. Matsko was awarded the Pittsburgh Cornell University Leadership Award for Excellence. He holds a BS in Nutritional Science from Cornell University and an MD from the Temple University School of Medicine in 2007. Dr. Matsko earned a Research Writing Certification from the American Medical Writers Association (AMWA) in 2016 and a Medical Writing & Editing Certification from the University of Chicago in 2017. wikiHow marks an article as reader-approved once it receives enough positive feedback. In this case, 89% of readers who voted found the article helpful, earning it our reader-approved status. This article has been viewed 201,325 times.
Writing a medical research paper is similar to writing other research papers in that you want to use reliable sources, write in a clear and organized style, and offer a strong argument for all conclusions you present. In some cases the research you discuss will be data you have actually collected to answer your research questions. Understanding proper formatting, citations, and style will help you write and informative and respected paper.
Researching Your Paper

- Pick something that really interests you to make the research more fun.
- Choose a topic that has unanswered questions and propose solutions.

- Quantitative studies consist of original research performed by the writer. These research papers will need to include sections like Hypothesis (or Research Question), Previous Findings, Method, Limitations, Results, Discussion, and Application.
- Synthesis papers review the research already published and analyze it. They find weaknesses and strengths in the research, apply it to a specific situation, and then indicate a direction for future research.

- Keep track of your sources. Write down all publication information necessary for citation: author, title of article, title of book or journal, publisher, edition, date published, volume number, issue number, page number, and anything else pertaining to your source. A program like Endnote can help you keep track of your sources.
- Take detailed notes as you read. Paraphrase information in your own words or if you copy directly from the article or book, indicate that these are direct quotes by using quotation marks to prevent plagiarism.
- Be sure to keep all of your notes with the correct source.
- Your professor and librarians can also help you find good resources.

- Keep all of your notes in a physical folder or in a digitized form on the computer.
- Start to form the basic outline of your paper using the notes you have collected.
Writing Your Paper

- Start with bullet points and then add in notes you've taken from references that support your ideas. [1] X Trustworthy Source PubMed Central Journal archive from the U.S. National Institutes of Health Go to source
- A common way to format research papers is to follow the IMRAD format. This dictates the structure of your paper in the following order: I ntroduction, M ethods, R esults, a nd D iscussion. [2] X Research source
- The outline is just the basic structure of your paper. Don't worry if you have to rearrange a few times to get it right.
- Ask others to look over your outline and get feedback on the organization.
- Know the audience you are writing for and adjust your style accordingly. [3] X Research source

- Use a standard font type and size, such as Times New Roman 12 point font.
- Double-space your paper.
- If necessary, create a cover page. Most schools require a cover page of some sort. Include your main title, running title (often a shortened version of your main title), author's name, course name, and semester.

- Break up information into sections and subsections and address one main point per section.
- Include any figures or data tables that support your main ideas.
- For a quantitative study, state the methods used to obtain results.

- Clearly state and summarize the main points of your research paper.
- Discuss how this research contributes to the field and why it is important. [4] X Research source
- Highlight potential applications of the theory if appropriate.
- Propose future directions that build upon the research you have presented. [5] X Research source
- Keep the introduction and discussion short, and spend more time explaining the methods and results.

- State why the problem is important to address.
- Discuss what is currently known and what is lacking in the field.
- State the objective of your paper.
- Keep the introduction short.

- Highlight the purpose of the paper and the main conclusions.
- State why your conclusions are important.
- Be concise in your summary of the paper.
- Show that you have a solid study design and a high-quality data set.
- Abstracts are usually one paragraph and between 250 – 500 words.

- Unless otherwise directed, use the American Medical Association (AMA) style guide to properly format citations.
- Add citations at end of a sentence to indicate that you are using someone else's idea. Use these throughout your research paper as needed. They include the author's last name, year of publication, and page number.
- Compile your reference list and add it to the end of your paper.
- Use a citation program if you have access to one to simplify the process.

- Continually revise your paper to make sure it is structured in a logical way.
- Proofread your paper for spelling and grammatical errors.
- Make sure you are following the proper formatting guidelines provided for the paper.
- Have others read your paper to proofread and check for clarity. Revise as needed.
Expert Q&A

- Ask your professor for help if you are stuck or confused about any part of your research paper. They are familiar with the style and structure of papers and can provide you with more resources. Thanks Helpful 0 Not Helpful 0
- Refer to your professor's specific guidelines. Some instructors modify parts of a research paper to better fit their assignment. Others may request supplementary details, such as a synopsis for your research project . Thanks Helpful 0 Not Helpful 0
- Set aside blocks of time specifically for writing each day. Thanks Helpful 0 Not Helpful 0

- Do not plagiarize. Plagiarism is using someone else's work, words, or ideas and presenting them as your own. It is important to cite all sources in your research paper, both through internal citations and on your reference page. Thanks Helpful 4 Not Helpful 2
You Might Also Like

- ↑ http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3178846/
- ↑ http://owl.excelsior.edu/research-and-citations/outlining/outlining-imrad/
- ↑ http://china.elsevier.com/ElsevierDNN/Portals/7/How%20to%20write%20a%20world-class%20paper.pdf
- ↑ http://intqhc.oxfordjournals.org/content/16/3/191
- ↑ http://www.ruf.rice.edu/~bioslabs/tools/report/reportform.html#form
About This Article

To write a medical research paper, research your topic thoroughly and compile your data. Next, organize your notes and create a strong outline that breaks up the information into sections and subsections, addressing one main point per section. Write the results and discussion sections first to go over your findings, then write the introduction to state your objective and provide background information. Finally, write the abstract, which concisely summarizes the article by highlighting the main points. For tips on formatting and using citations, read on! Did this summary help you? Yes No
- Send fan mail to authors
Reader Success Stories
Joshua Benibo
Jun 5, 2018
Did this article help you?

Dominic Cipriano
Aug 16, 2016
Obiajulu Echedom
Apr 2, 2017
Noura Ammar Alhossiny
Feb 14, 2017
Dawn Daniel
Apr 20, 2017

Featured Articles

Trending Articles

Watch Articles

- Terms of Use
- Privacy Policy
- Do Not Sell or Share My Info
- Not Selling Info
wikiHow Tech Help Pro:
Develop the tech skills you need for work and life
- Affiliate Program

- UNITED STATES
- 台灣 (TAIWAN)
- TÜRKIYE (TURKEY)
- Academic Editing Services
- - Research Paper
- - Journal Manuscript
- - Dissertation
- - College & University Assignments
- Admissions Editing Services
- - Application Essay
- - Personal Statement
- - Recommendation Letter
- - Cover Letter
- - CV/Resume
- Business Editing Services
- - Business Documents
- - Report & Brochure
- - Website & Blog
- Writer Editing Services
- - Script & Screenplay
- Our Editors
- Client Reviews
- Editing & Proofreading Prices
- Wordvice Points
- Partner Discount
- Plagiarism Checker
- APA Citation Generator
- MLA Citation Generator
- Chicago Citation Generator
- Vancouver Citation Generator
- - APA Style
- - MLA Style
- - Chicago Style
- - Vancouver Style
- Writing & Editing Guide
- Academic Resources
- Admissions Resources
How to Write a Medical Abstract for Publication
Preparing Your Study, Review, or Article for Publication in Medical Journals
The majority of social, behavioral, biological, and clinical journals follow the conventional structured abstract form with the following four major headings (or variations of these headings):
OBJECTIVE (Purpose; Aim; Goal) : Tells reader the purpose of your research and the questions it intends to answer
METHODS (Setting; Study Design; Participants) : Explains the methods and process so that other researchers can assess, review, and replicate your study.
RESULTS (Findings; Outcomes) : Summarizes the most important findings of your study
CONCLUSIONS (Discussion; Implications; Further Recommendations) : Summarizes the interpretation and implications of these results and presents recommendations for further research
Sample Health/Medical Abstract

Structured Abstracts Guidelines *
- Total Word Count: ~200-300 words (depending on the journal)
- Content: The abstract should reflect only the contents of the original paper (no cited work)
* Always follow the formatting guidelines of the journal to which you are submitting your paper.
Useful Terms and Phrases by Abstract Section
Objective: state your precise research purpose or question (1-2 sentences).
- Begin with “To”: “We aimed to…” or “The objective of this study was to…” using a verb that accurately captures the action of your study.
- Connect the verb to an object phrase to capture the central elements and purpose of the study, hypothesis , or research problem . Include details about the setting, demographics, and the problem or intervention you are investigating.
METHODS : Explain the tools and steps of your research (1-3 sentences)
- Use the past tense if the study has been conducted; use the present tense if the study is in progress.
- Include details about the study design, sample groups and sizes, variables, procedures, outcome measures, controls, and methods of analysis.
RESULTS : Summarize the data you obtained (3-6 sentences)
- Use the past tense when describing the actions or outcomes of the research.
- Include results that answer the research question and that were derived from the stated methods; examine data by qualitative or quantitative means.
- State whether the research question or hypothesis was proven or disproven.
CONCLUSIONS : Describe the key findings (2-5 sentences)
- Use the present tense to discuss the findings and implications of the study results.
- Explain the implications of these results for medicine, science, or society.
- Discuss any major limitations of the study and suggest further actions or research that should be undertaken.
Before submitting your abstract to medical journals, be sure to receive proofreading services from Wordvice, including journal manuscript editing and paper proofreading , to enhance your writing impact and fix any remaining errors.
Related Resources
- 40 Useful Words and Phrases for Top-Notch Essays (Oxford Royale Academy)
- 100+ Strong Verbs That Will Make Your Research Writing Amazing (Wordvice)
- Essential Academic Writing Words and Phrases (My English Teacher.eu)
- Academic Vocabulary, Useful Phrases for Academic Writing and Research Paper Writing (Research Gate)
- How to Compose a Journal Submission Cover Letter (Wordvice/YouTube)
- How to Write the Best Journal Submission Cover Letter (Wordvice)

Professional Medical Writing & Editing Services

The Ultimate Guide for Medical Manuscript Writing
Medical Manuscript writing can be overwhelming, but there are some tried-and-true techniques and creative tips that can dramatically simplify the process.
We mined the literature for strategies plus revealed some tricks from our seasoned writers to help you get your manuscript written and published.
In this document, we focused our attention on manuscripts since they are one of the most common types of medical writing . However, these are techniques that can be useful for any medical writing project.
What is good medical manuscript writing?
When you’re writing for a scientific audience it’s important to write with three C’s in mind:
- Clear: Don’t be ambiguous or leave anything to the imagination.
- Concise: Use brief, simple language and avoid repetition/redundancy.
- Correct: Be accurate, and don’t overstate the significance of your results.
Good medical writing is never more complicated than it needs to be.
Make it easy for your audience by keeping your language clear and simple.

How is medical writing different?
Many people want to know how writing healthcare blog topics is different from other types of writing. The answer is simple: it isn’t.
Good writing has a goal and a target audience and they will influence how you write, regardless of what you’re writing. A good manuscript is rooted in a good story.
Even data-driven medical texts can be delivered in an engaging way. Most of us can think of examples of stand-out papers in our field of expertise.
At their best these papers are entertaining and thought-provoking even while they deliver complicated, data-heavy material.
Make an Outline for your Manuscript
Before you start writing you need to have a clear understanding of the type and scope of your writing.
For example, consider exactly what you are writing. Is it a case study, textbook chapter , or literature review? These distinctions have important implications for how you craft and present your material.
An outline should be an obvious place to start, but you’d be surprised at how often this step is skipped.
How to structure your medial manuscript outline
When possible, before you start your outline you should understand the formatting requirements for your targeted publisher.
Many publishers specify abstract headings and have specific requirements for what can (and can’t) be included in the body of your text.
Use the outline as a way to narrow down the research you’ll need to do as you write.
It is an unfortunate fact of life: the abstract is often the only portion of a paper that ever gets read. For this reason, your abstract needs to convey the most important points from your paper in 300 words or less. Note what these points are in your abstract.
Make sure you know what your publisher expects from your abstract. Some journals limit you to 150 words or require that you arrange your abstract using specific headers.
You may need to include an objective or a statement of impact as well.
Most publishers ask authors to provide some keywords. Think about the keywords you’d use to search for your paper and write them down.
Keywords that are more general will increase the number of search results your paper will appear in. For example, use “spinal cord stimulation” instead of “neuromodulation.”
Define your goal
Is your goal to present new research data or to provide a meta-analysis of existing data? By clarifying the goal of your manuscript you can streamline preparation and writing.
Defining the goal is one of the secrets of successful grants and manuscripts of top biomedical PIs . Having a well-defined goal will also help you find the most appropriate publisher.
Keep your target audience in mind as you define your goal.
Introduction/background
The outline for your intro should note the current state of the field and identify knowledge gaps.
A good way to understand how to arrange your intro is by looking at similar papers that have been published by your target journal.
A standard approach to an intro can be broken down as follows:
- First paragraph: Current knowledge and foundational referencesYou’re paving the way for your readers to understand your objective
- Second paragraph: Introduce your specific topic and identify knowledge gaps
- Third paragraph: Clearly identify your aim
Key references and identifying your hypothesis and aim(s).
Methods Briefly list your methods and timeframe, but don’t get too detailed. This is just an outline.
Results The results section can be the most challenging to organize.
To simplify the writing process, state your overall question and create subsections for each dataset.
List the experiments you did and your results.
In your outline, identify data that should be presented in a figure or table. Save any subjective interpretations for the discussion section.
Discussion Your outline for the discussion should pick up where the introduction left off.
For example, if your intro ends with an aim, your discussion should start by restating your aim and reminding your readers of the knowledge gap(s) that you are addressing.
Your discussion needs to address each set of experiments and your interpretation, but don’t simply restate your results section.
Timeline Make a timeline for your manuscript and specify a submission date to help keep you on track.
Questions to consider when making your discussion outline include:
- How do your data relate to your original question?
- Do they support your hypothesis?
- Are your results consistent with what other researchers have found?
- If you had unexpected results, is there an explanation for them?
- Consider your data from the perspective of a competitor. Can you punch holes in your argument?
- Address potential concerns about your data head on. Don’t try to hide them or gloss over them.
- If you weren’t able to fully address your question(s) or aim(s), what else do you need to do?
- How do your data fit into the big picture?
Include a discussion subsection for each of your results subsections where you can subjectively interpret your data. Your outline should include the points you want to make in each subsection as well as your overall goal.
Conclude your discussion with a one sentence summary of your conclusion and its relevance to the field.
Again, don’t forget to write to your target audience!
Additional Resources for Medical Writing
Templates for Building a Perfect Writing Plan:
- Scope of work guide
- 30 Scope of work templates
- Medical cover letter + Templates
- Detailed guide of medical manuscript Scope of Work
- Short guide of scope of work for a medical journal
Know the Literature Before You Write Anything
An effective medical or scientific manuscript provides compelling information that builds on the existing literature and advances what is currently known.
This means you need to have a thorough understanding of the relevant literature!
Your goal is to collect all relevant references into a structured document. Make note of the aim and conclusion of each reference. Use this as a foundation to refer back to when you’re writing your paper.
Organize your research into buckets.

When you find a relevant source, ask yourself:
- Are the data consistent with what’s already known?
- If not: why are they different and how do they affect what’s known?
- Do your data support or refute the data presented in the source?
- You’ll need to explicitly address inconsistencies and identify potential resolutions.
Find Scholarly Sources
If you are writing an original research article , how do your data fit into the broader topic?
Google searches don’t usually produce scholarly resources unless you know where to look.
There are numerous FREE and Paid online resources available to find the right sources.
Top Scholarly Databases for journals, news, and articles
These tools can be used to find all the reputable sources needed to flesh out quality medical writing.
PubMed (MEDLINE):
PUBMED is an extremely popular and free search engine hosted by the NIH (National institutes of Health and U.S. National Library of Medicine. It can be used to access a vast index of peer-reviewed biological and medical research.
EMBASE is a database of literature intended to aid in organizational adherence to prescription drug regulations. Whereas it does contain some references that are not returned by PUBMED, there is a subscription fee associated with EMBASE.
Cochrane Library
The Cochrane Library is a curated database of medical research reviews, protocols, and editorials. While a subscription is required, the Cochrane is a critical resource for evidence-based medicine.
Web of Science
The Web of Science is another subscription service similar to those that have already been mentioned, albeit with an expanded range of academic disciplines including the arts, social sciences, and others.
Google Scholar
Google Scholar leverages Google’s powerful search engine to retrieve published literature from the whole internet (rather than just biomedical journals). This means you’ll get textbooks, theses, conference proceedings, and other publications that won’t show up in PubMed or EMBASE searches. Google Scholar is a powerful tool but it lacks the curation of other search tools, so a careful vetting of any information from this source is important.
Other databases:
Faculty of 1000 (F1000) offers Faculty Opinions and F1000Research. Faculty Opinions are links to recommended life-science articles, while F1000Research is a database of open-source research papers and results.
EBSCO is an online library providing a wide range of services, including its research databases that allow powerful searches of journals in a variety of academic disciplines.
iSEEK Education:
iSeek Education is a search engine geared specifically for academics. The resources from iSeek are meant to be dependable and from reliable sources, such as government agencies and universities.
RefSeek is another popular option for academically oriented search engines. RefSeek is designed to pull results from a large number of sources but not commercial links.
Virtual LRC:
The Virtual Learning Resources Center is a modified Google search of academic information websites. Its index of websites has been chosen by qualified curators.
More journal databases for medical research
- 100+ journal databases
- Top Academic Search Engines
- 101 Free Journal and Research Databases
- List of Global scholarly sources
Organize Your References:
One other point on knowing the literature: find a strategy that helps you keep references organized.
If you’ve ever written a paper and couldn’t remember where on earth you saw that one, perfect reference you know how important this is!
Rather than putting things into a long word document start with a research template.
RESOURCE: Use our FREE research template to collect sources for your manuscript
You can also download free basic software to organize references.
5 Reference Organization Tools and Software
EndNote is the most popular reference organization tool for medical writers.
A basic version of EndNote is available for free, but paid subscriptions offer more options.
EndNote features include:
- Import, annotate, and search PDFs
- Ability to store reference libraries online, so you can access them from anywhere
- Collaboration is easy with shared libraries
- EndNote provides the most comprehensive citation style database, or you can create custom citation styles
- Easy to import/export references from databases using RIS, BibTex, and many other standard data schemes
One potential drawback of EndNote is that it’s not compatible with Linux.
Zotero Zotero is a free, open-source reference management and citation tool.
Features of Zotero include:
- Save screenshots and annotate them within your citation library
- Import and export references in many formats, including RIS, BibTeX and BibLateX, EndNote, RefWorks, and more
- Supports over 30 languages
- Zotero’s online bibliography tool ZoteroBib lets you generate bibliographies without installing Zotero or creating an account
- Drag-and-drop interface
- Linux compatible
Mendeley Mendeley is Elsevier’s “freemium” referencing software, meaning the basic package is available for free but more sophisticated versions require a paid subscription.
Features of Mendeley include:
- Extract metadata from PDFs
- Create private, shareable libraries
A free online reference tool, Citefast allows users to quickly generate a library in APA 6 or 7, MLA 7 or 8, or Chicago styles.
Citefast doesn’t require you to make an account, but if you don’t create one your references will be lost after 4 days of inactivity.
Another free online resource, BibMe lets you import references and offers MLA, APA, Chicago, and Turabian formatting styles.
BibMe can also check your spelling and grammar, as well as look for plagiarism.
List of MORE Online Software Tools for Academic and Medical Research
30+ research tools to make your life easier
5 best tools for academic research
31 Best Online Tools for Research
10 great tools for online research
Know your audience
It’s important to know your audience before you start writing. This will help you define your goals and create an outline.
For example, if you’re preparing a case study for specialists your manuscript will be different than one for a multidisciplinary audience.
Ask yourself what your message is and find out how it aligns with the goals of your readers to maximize your paper’s impact.
Formatting requirements
Whenever possible, find out the formatting requirements you’ll need to follow before you start writing. They will explicitly state the layout, word limits, figure/table formatting, use of abbreviations, and which reference style to use.
If you’re writing for a journal their website will have a Guide for Authors that specifies formatting. If you’re not sure what the requirements are, you should contact the editor or publisher and ask them.
Types of medical manuscripts
Knowing what kind of manuscript you’re writing will help you organize your material and identify which information you should present. In addition, many publishers have different formatting requirements for different types of articles.
Although each publisher has their own guidelines for authors, many journals encourage authors to follow reporting guidelines from the EQUATOR Network (Enhancing the QUAlity and Transparency Of health Research).
Original research
The goal of an original research article is to convey your research findings to an audience. These articles typically follow the same structure:
- Introduction
Methods & Materials
Examples of great original medical research manuscripts:
- Nowacki J, Wingenfeld K, Kaczmarczyk M, et al. Steroid hormone secretion after stimulation of mineralocorticoid and NMDA receptors and cardiovascular risk in patients with depression . Transl Psychiatry . 2020 Apr 20;10(1):109.
- Pfitzer A, Maly C, Tappis H, et al. Characteristics of successful integrated family planning and maternal and child health services: Findings from a mixed-method, descriptive evaluation . F1000Res . 2019 Feb 28;8:229.
- Yi X, Liu M, Luo Q, et al. Toxic effects of dimethyl sulfoxide on red blood cells, platelets, and vascular endothelial cells in vitro . FEBS Open Bio . 2017 Feb 20;7(4):485-494.
- Karsan N, Goadsby PJ. Imaging the Premonitory Phase of Migraine . Front Neurol . 2020 Mar 25;11:140.
- Chan SS, Chappel AR, Maddox KEJ, et al. Pre-exposure prophylaxis for preventing acquisition of HIV: A cross-sectional study of patients, prescribers, uptake, and spending in the United States, 2015-2016 . PLoS Med . 2020 Apr 10;17(4):e1003072.
Examples of great medical journal publications from The Med Writers :

Rapid communications
Rapid (or brief) communications are aimed at publishing highly impactful preliminary findings.
They are shorter than original research articles and focus on one specific result.
Many journals prioritize rapid communications, since they can provide paradigm-shifts in how we understand a particular topic.
5 Examples of Rapid Communications
- Rose D, Ashwood P. Plasma Interleukin-35 in Children with Autism . Brain Sci . 2019 Jun 27;9(7).
- Nash K, Johansson A, Yogeeswaran K. Social Media Approval Reduces Emotional Arousal for People High in Narcissism: Electrophysiological Evidence . Front Hum Neurosci . 2019 Sep 20;13:292.
- Su Q, Bouteau A, Cardenas J, et al. Long-term absence of Langerhans cells alters the gene expression profile of keratinocytes and dendritic epidermal T cells . PLoS One . 2020 Jan 10;15(1):e0223397.
- Nilsson I, Palmer J, Apostolou E, et al. Metabolic Dysfunction in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Not Due to Anti-mitochondrial Antibodies . Front Med . 2020 Mar 31;7:108.
- Rabiei S, Sedaghat F, Rastmanesh R. Is the hedonic hunger score associated with obesity in women? A brief communication . BMC Res Notes. 2019 Jun 10;12(1):330.
Case reports
Case reports detail interesting clinical cases that provide new insight into an area of research.
These are brief reports that chronicle a case, from initial presentation to prognosis (if known).
Importantly, when writing a case report, you need to clearly identify what makes your case unique and why it’s important. 5 Examples of Great Case Studies
- Scoles D, Ammar MJ, Carroll SE, et al. Cytomegalovirus retinitis in an immunocompetent host after complicated cataract surgery . Am J Ophthalmol Case Rep . 2020 Apr 6;18:100702.
- Yanagimoto Y, Ishizaki Y, Kaneko K. Iron deficiency anemia, stunted growth, and developmental delay due to avoidant/restrictive food intake disorder by restricted eating in autism spectrum disorder . Biopsychosoc Med . 2020 Apr 10;14:8.
- Pringle S, van der Vegt B, Wang X, et al. Lack of Conventional Acinar Cells in Parotid Salivary Gland of Patient Taking an Anti-PD-L1 Immune Checkpoint Inhibitor . Front Oncol . 2020 Apr 2;10:420.
- Crivelli P, Ledda RE, Carboni M, et al. Erdheim-Chester disease presenting with cough, abdominal pain, and headache . Radiol Case Rep . 2020 Apr 10;15(6):745-748.
- Tsai AL, Agustines D. The Coexistence of Oculocutaneous Albinism with Schizophrenia . Cureus . 2020 Jan 9;12(1):e6617.
Literature review
A good literature review provides a comprehensive overview of current literature in a new way. There are four basic types of literature review:
Traditional: Also known as narrative reviews, these reviews deliver a thorough synopsis of a body of literature. They may be used to highlight unanswered questions or knowledge gaps.
Li X, Geng M, Peng Y, Meng L, Lu S. Molecular immune pathogenesis and diagnosis of COVID-19 . J Pharm Anal . 2020 Mar 5.
Wardhan R, Kantamneni S. The Challenges of Ultrasound-guided Thoracic Paravertebral Blocks in Rib Fracture Patients . Cureus . 2020 Apr 10;12(4):e7626.
Lakhan, S.E., Vieira, K.F. Nutritional therapies for mental disorders . Nutr J 7, 2 (2008). https://doi.org/10.1186/1475-2891-7-2
A minireview is similar to a review, but confines itself to a specific subtopic:
Marra A, Viale G, Curigliano G. Recent advances in triple negative breast cancer: the immunotherapy era . BMC Med . 2019 May 9;17(1):90.
Systematic : These are rigorous, highly structured reviews that are often used to shed light on a specific research question. They are often combined with a meta-analysis or meta-synthesis.
Asadi-Pooya AA, Simani L. Central nervous system manifestations of COVID-19: A systematic review . J Neurol Sci . 2020 Apr 11;413:116832.
Katsanos K, Spiliopoulos S, Kitrou P, Krokidis M, Karnabatidis D. Risk of Death Following Application of Paclitaxel-Coated Balloons and Stents in the Femoropopliteal Artery of the Leg: A Systematic Review and Meta-Analysis of Randomized Controlled Trials . J Am Heart Assoc . 2018 Dec 18;7(24):e011245.
Meta-analysis: A meta-analysis analyzes data from multiple published studies using a standardized statistical approach. These reviews can help identify trends, patterns, and new conclusions.
Zhang J, Zhang X, Meng Y, Chen Y. Contrast-enhanced ultrasound for the differential diagnosis of thyroid nodules: An updated meta-analysis with comprehensive heterogeneity analysis . PLoS One . 2020 Apr 20;15(4):e0231775.
Meta-synthesis: A meta-synthesis is a qualitative (non-statistical) way to evaluate and analyze findings from several published studies.
Stuart R, Akther SF, Machin K, et al. Carers’ experiences of involuntary admission under mental health legislation: systematic review and qualitative meta-synthesis . BJPsych Open . 2020 Feb 11;6(2):e19.
At this point you’ve established three things for your manuscript:
- Your goal : Is your goal to convey the latest research? You should find a way to describe what you want to accomplish with this article.
- Your target audience : The most effective medical writing is done with a specific audience in mind.
- Type of manuscript : The type of article you’re writing will influence the format of the document you are writing.
You probably have a target journal or publisher in mind and you should have checked out their formatting requirements. Now it’s time to start writing!
Notably, many seasoned authors don’t write their articles from beginning to end. For example, if you’re preparing an original research manuscript they suggest writing the methods section first, followed by the results, discussion, introduction, and, lastly, the abstract. This will help you stay within the scope of the article.
Generally, the title for a medical document should be as succinct as possible while conveying the purpose of the article.
If you’re writing an original research article your title should convey your main finding as simply as possible.
Avoid using unnecessary jargon and ambiguity.
Some authors recommend including keywords that will help people find your writing in the title.
Your publisher may have a specific abstract format for you to follow. There are three general types of abstract:
- Indicative (descriptive) abstracts provide a clear overview of the topics covered. They are common in review articles and conference reports.
- Informative abstracts summarize the article based on structure (e.g. problem, methods, case studies/results, conclusions) but without headings.
- Structured abstracts use headings as specified by the publisher.
Good abstracts are clear, honest, brief, and specific. They also need to hook readers or your article will never be read (no pressure!).
Many publishers will ask you to come up with some keywords for your article. Make sure they’re specific and clearly represent the topic of your article.
If you’re not sure about your keywords the National Library of Medicine’s Medical Subject Headings ( MeSH ) website can help. Just type in a term and it will bring up associated subject headings and definitions.
Introduction
The goal of the introduction is to briefly provide context for your work and convince readers that it’s important. It is not a history lesson or a place to wax poetic about your love of medicine (unless you’re writing about history or your love of medicine). Everything in your introduction needs to be directly relevant to the overall goal of your manuscript.
Introductions vary in length and style between the different types of manuscript. The best way to understand what your publisher is looking for in an introduction is to read several examples from articles that are stylistically similar to yours.
Broadly speaking, an introduction needs to clearly identify the topic and the scope of the article. For an original research article this means you explicitly state the question you’re addressing and your proposed solution. For a literature review, the topic and its parameters should be stated.
Importantly, don’t mix the introduction with other sections. Methods and results don’t belong in the introduction.
Abbreviations
If you use terms that are abbreviated, some journals will ask you to include a section after the introduction where you define them. Consult the authors guide to learn how you should handle abbreviations. Also check to see if they have standard abbreviations that you don’t need to define in your manuscript.
A couple of tips for abbreviations:
· Terms that are only used once or twice should be spelled out, not abbreviated
· Don’t capitalize each word in an acronym unless it’s a proper noun (e.g. ubiquitin proteasome system (UPS), not Ubiquitin Proteasome System (UPS))
A good methods section will contain enough information that another researcher could reproduce the work. Clearly state your experimental design, what you did in chronological order, including equipment model numbers and specific settings you used. Make sure to include all equipment, materials, and products you used as they could account for future variability. Describe any statistical analyses.
The methods section should describe the following:
· Population and sampling methods
· Equipment and materials
· Procedures
· Time frame (if relevant)
· Analysis plan
· Approaches to ensure reliability/validity
· Any assumptions you used
· Scope and limitations
If you are using methods that have been described before you can refer to that publication or include them in your supplementary material, rather than re-writing them in the body of your text.
The results section is where your findings are objectively presented (save your interpretation of the results for the discussion section). Figure out which data are important for your story before you write the results section. For each important data set provide the results (preferably in a table or graph) and include a sentence or two that summarizes the results.
It’s easy to lose sight of the goal of the paper when you’re relaying numbers through the lens of statistics. Make sure to tie your results back to the biological aspects of your paper.
The discussion section is where you sell your interpretation of the data. Your discussion section needs to tie your introduction and your results sections together. A common strategy for the discussion section is to reiterate your main findings in light of the knowledge gaps you outlined in your introduction. How do your findings move the field forward?
Consider each of your results with respect to your original question and hypothesis. If there are multiple ways to interpret your data, discuss each of them. If your findings were not in line with your hypothesis, state this and provide possible explanations.
If your data are inconsistent with other published literature it’s important to consider technical and experimental differences before concluding that you’ve stumbled onto a groundbreaking medical discovery. Discuss all potential reasons for the divergent data.
Key points to include in your discussion section:
· What your results mean
· Whether your methods were successful
· How findings relate to other studies
· Limitations of your study
· How your work advances the field
· Applications
· Future directions
Don’t draw grand conclusions that aren’t supported by your data; some speculating is okay but don’t exaggerate the importance of your findings.
It’s important to remind your reader of your overall question and hypothesis throughout the discussion section, while you are providing your interpretation of the results. This will ensure that you stay on track while you’re writing and that your readers will understand exactly how your findings are relevant.
This is your final chance to convince your readers that your work is important.
Start your conclusion by restating your question and identify whether your findings support (or fail to support) your hypothesis.
Summarize your findings and discuss whether they agree with those of other researchers.
Finally, identify how your data advances the field and propose new or expanded ways of thinking about the question.
It’s important to avoid making unsupported claims or over-emphasize the impact of your findings. Even if you think your findings will revolutionize medicine as we know it, refrain from making that claim until you have the evidence to back it up.
Figures/Tables
Many readers will get the bulk of their information from your figures so make sure they are clear and informative. Your readers should be able to identify your key findings from figures alone.
Tips for figures and tables:
· Don’t repeat data in tables, figures and in the text
· Captions should sufficiently describe the figure so the reader could understand it even if the figure was absent
· Keep graphs simple! If a basic table will work there’s no need for a multi-colored graph
Acknowledgements
Use the acknowledgments section to identify people who made your manuscript possible. Include advisors, proofreaders, and financial backers. In addition, identify funding sources including grant or reference numbers.
Make sure to use the reference style specified by your target journal or publisher. Avoid too many references, redundant references, excessive self-referencing, and referencing for the sake of referencing. Personal communications, unpublished observations, and submitted, unaccepted manuscripts should generally be avoided.
It should go without saying that you need to be ethical when preparing medical manuscripts. Fabricating or falsifying data is never acceptable, and you put your career at risk. It’s not worth it.
Plagiarism is not a viable strategy for getting works published. Any indication that you’ve plagiarized will be investigated, and if you’re found to have plagiarized your career and scientific reputation are at stake. Any time you refer to published work you need to reference it, even if it was your own publication. Be very careful about self-plagiarizing!
To learn more about ethical writing take a look at the U.S. Department of Health and Human Services guide: Avoiding Plagiarism, Self-plagiarism, and Other Questionable Writing Practices: A Guide to Ethical Writing , by Dr. Miguel Roig.
Ethics standards require that you submit your manuscript to only one publisher at a time. If you’re caught submitting to multiple editors none of them will publish your work.
Traps to avoid
Seasoned writers told us some of the pitfalls they’ve learned how to watch out for:
Writing versus editing
Writing and editing are not the same. Get comfortable writing , that is, pouring out all of your ideas without editing yourself. Then go back and edit.
Lack of editing
One of the toughest parts of writing is opening yourself up to critique. As hard as it can be, the best way to get a polished and meaningful manuscript is to have other people read it. As writers we can get attached to particular phrases or styles that may not read as well to other people.
Scientific manuscript editing is the toughest of any manuscript editing but if you keep patience and edit honestly it will get easier over time. Imagine that you’re editing someone else’s document to help give you fresh eyes. If possible give yourself a couple of days without looking at the manuscript, then go back and read it.
Being unfamiliar with the literature
It’s important to be familiar with the current literature on the topic you’re writing about. A fatal flaw of any research manuscript is proposing a hypothesis that has already been tested or posing questions that have already been answered.
Not formatting properly
If your manuscript is not formatted properly, it is less likely to be accepted. Make sure your font and line spacing are correct, that you’ve adhered to word and figure limits, and that your references are in the correct style.
Useful tips
Here are some helpful tips that you can use to improve your writing:
Framing your manuscript
A common trope in outlining manuscripts is the inverted triangle approach, which starts generally and ends specifically. A more useful method is to consider an hourglass-shaped outline, which starts generally, specifically addresses your contribution to the field, then ties your contribution back to current knowledge and unanswered questions.
Passive and active voice
Medical writing has long used passive voice to communicate and, while this is still the status quo for many journals, don’t be afraid to get out of that mire. As journals begin to recognize that active voice is not only more economical but can also be more readable they are becoming more comfortable publishing articles that include active voice.
Don’t edit while you write
Get a first draft onto paper as quickly as possible and then edit. Don’t waste time trying to get a paragraph perfect the first time you write it.
Ask someone else to edit
Medical writing does have some unique challenges associated with it. Your audience may not be experts on the material you are delivering, so an ability to communicate complicated information in an accessible manner is very helpful. Improve on your skills by asking people outside of your field to provide constructive criticism on writing samples.
It can be a very useful practice to edit some manuscripts that other people have written. This will help you understand what editors are paying attention to.
Keep track of references
Make sure to keep detailed notes of where you got your references so that you can easily and accurately cite the literature you used. There’s nothing more frustrating than not being able to remember where you saw a really great reference.
Before you submit your manuscript
Ideally, you’ve left yourself plenty of time to proofread and have other people edit your document. At the very least make sure you budget some hours to carefully proofread. Triple check that your paper adheres to formatting requirements. You can learn how to proofread scientific manuscripts before submitting them for publication.
Cover letters
If you’re submitting an article for consideration you’ll need to write a cover letter. Take the time to find out who the editor is and address your letter to him or her. This is your chance to communicate with the editor! A generic “To whom it may concern” won’t impress anyone.
Your cover letter should be brief, but it needs to convey the value of your paper to the journal. Describe your main findings and their significance and why they’re a great fit for your publication of interest.
If you have conflicts of interest, disclose them in your cover letter. Also, if your paper has already been rejected, let them know. Include the reason (if known) and reviewer comments, as well as discussing changes you’ve made to improve the paper.
You can also suggest peer-reviewers or people who shouldn’t review your paper. Be cautious when suggesting reviewers! Some of the most critical reviews come from suggested reviewers.
Your cover letter is an excellent opportunity to prove that you know what the goals of the journal are and that your article furthers them. Don’t waste it!
Reviewer comments/Revisions
If the publisher asks you to address reviewer comments, take the time to do this seriously and thoughtfully. Understand reviewer comments and address them objectively and scientifically (be polite!). If you disagree with a comment, state why and include supporting references. When more experiments or computations are requested, do them. It will make your paper stronger.
When you resubmit your manuscript make sure to identify page/line numbers where changes were made.
What if you’re rejected?
Don’t despair! Rejection happens to every writer. Try to understand why your manuscript was rejected. Evaluate your manuscript honestly and take the opportunity to learn from your mistakes.
A rejected paper isn’t a dead paper. You’ll need to make some substantial revisions and may need to change your formatting before resubmitting to a new journal or publisher. In the cover letter to the new editor you’ll need to state that your manuscript was rejected. Include any information you got about why your manuscript was rejected and all reviewer comments. Identify changes you made to the paper and explain why you chose to submit to the new journal.
Medical writing can be very rewarding but it’s important that writers have a clear understanding of what publishers are looking for. High-quality, original works that advance the medical field are much more likely to be published than papers that are not original or that have little medical or scientific interest.
Quality medical writing should have clarity, economy of language, and a consistent theme. It’s important to always state the question or topic you’re addressing early and refer to it often. This will help you stay focused and within the scope of your article during the writing process and it will help your readers understand your intentions. Using an outline is a very helpful way to make sure your article is consistently on-topic.
Following the tips and techniques provided here will definitely improve your writing skills, but the most effective way to get better at medical writing is to do it. There is no single best way to prepare a medical manuscript and even professional writers are continuously tweaking their writing strategies.
Hopefully these tips have helped you create a great manuscript. If you’re feeling overwhelmed and want some help with your medical writing or editing, we at The Med Writers can help. Contact us to learn more about our writing and editing services.
Purdue Online Writing Lab Purdue OWL® College of Liberal Arts
Healthcare Writing

Welcome to the Purdue OWL
This page is brought to you by the OWL at Purdue University. When printing this page, you must include the entire legal notice.
Copyright ©1995-2018 by The Writing Lab & The OWL at Purdue and Purdue University. All rights reserved. This material may not be published, reproduced, broadcast, rewritten, or redistributed without permission. Use of this site constitutes acceptance of our terms and conditions of fair use.
Subsections
- Open access
- Published: 07 September 2020
A tutorial on methodological studies: the what, when, how and why
- Lawrence Mbuagbaw ORCID: orcid.org/0000-0001-5855-5461 1 , 2 , 3 ,
- Daeria O. Lawson 1 ,
- Livia Puljak 4 ,
- David B. Allison 5 &
- Lehana Thabane 1 , 2 , 6 , 7 , 8
BMC Medical Research Methodology volume 20 , Article number: 226 ( 2020 ) Cite this article
36k Accesses
52 Citations
58 Altmetric
Metrics details
Methodological studies – studies that evaluate the design, analysis or reporting of other research-related reports – play an important role in health research. They help to highlight issues in the conduct of research with the aim of improving health research methodology, and ultimately reducing research waste.
We provide an overview of some of the key aspects of methodological studies such as what they are, and when, how and why they are done. We adopt a “frequently asked questions” format to facilitate reading this paper and provide multiple examples to help guide researchers interested in conducting methodological studies. Some of the topics addressed include: is it necessary to publish a study protocol? How to select relevant research reports and databases for a methodological study? What approaches to data extraction and statistical analysis should be considered when conducting a methodological study? What are potential threats to validity and is there a way to appraise the quality of methodological studies?
Appropriate reflection and application of basic principles of epidemiology and biostatistics are required in the design and analysis of methodological studies. This paper provides an introduction for further discussion about the conduct of methodological studies.
Peer Review reports
The field of meta-research (or research-on-research) has proliferated in recent years in response to issues with research quality and conduct [ 1 , 2 , 3 ]. As the name suggests, this field targets issues with research design, conduct, analysis and reporting. Various types of research reports are often examined as the unit of analysis in these studies (e.g. abstracts, full manuscripts, trial registry entries). Like many other novel fields of research, meta-research has seen a proliferation of use before the development of reporting guidance. For example, this was the case with randomized trials for which risk of bias tools and reporting guidelines were only developed much later – after many trials had been published and noted to have limitations [ 4 , 5 ]; and for systematic reviews as well [ 6 , 7 , 8 ]. However, in the absence of formal guidance, studies that report on research differ substantially in how they are named, conducted and reported [ 9 , 10 ]. This creates challenges in identifying, summarizing and comparing them. In this tutorial paper, we will use the term methodological study to refer to any study that reports on the design, conduct, analysis or reporting of primary or secondary research-related reports (such as trial registry entries and conference abstracts).
In the past 10 years, there has been an increase in the use of terms related to methodological studies (based on records retrieved with a keyword search [in the title and abstract] for “methodological review” and “meta-epidemiological study” in PubMed up to December 2019), suggesting that these studies may be appearing more frequently in the literature. See Fig. 1 .
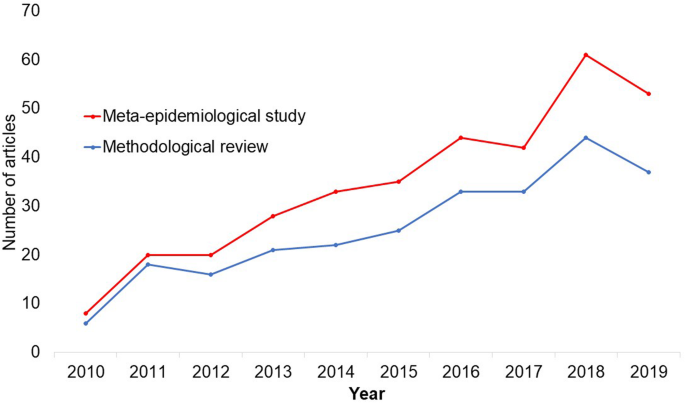
Trends in the number studies that mention “methodological review” or “meta-
epidemiological study” in PubMed.
The methods used in many methodological studies have been borrowed from systematic and scoping reviews. This practice has influenced the direction of the field, with many methodological studies including searches of electronic databases, screening of records, duplicate data extraction and assessments of risk of bias in the included studies. However, the research questions posed in methodological studies do not always require the approaches listed above, and guidance is needed on when and how to apply these methods to a methodological study. Even though methodological studies can be conducted on qualitative or mixed methods research, this paper focuses on and draws examples exclusively from quantitative research.
The objectives of this paper are to provide some insights on how to conduct methodological studies so that there is greater consistency between the research questions posed, and the design, analysis and reporting of findings. We provide multiple examples to illustrate concepts and a proposed framework for categorizing methodological studies in quantitative research.
What is a methodological study?
Any study that describes or analyzes methods (design, conduct, analysis or reporting) in published (or unpublished) literature is a methodological study. Consequently, the scope of methodological studies is quite extensive and includes, but is not limited to, topics as diverse as: research question formulation [ 11 ]; adherence to reporting guidelines [ 12 , 13 , 14 ] and consistency in reporting [ 15 ]; approaches to study analysis [ 16 ]; investigating the credibility of analyses [ 17 ]; and studies that synthesize these methodological studies [ 18 ]. While the nomenclature of methodological studies is not uniform, the intents and purposes of these studies remain fairly consistent – to describe or analyze methods in primary or secondary studies. As such, methodological studies may also be classified as a subtype of observational studies.
Parallel to this are experimental studies that compare different methods. Even though they play an important role in informing optimal research methods, experimental methodological studies are beyond the scope of this paper. Examples of such studies include the randomized trials by Buscemi et al., comparing single data extraction to double data extraction [ 19 ], and Carrasco-Labra et al., comparing approaches to presenting findings in Grading of Recommendations, Assessment, Development and Evaluations (GRADE) summary of findings tables [ 20 ]. In these studies, the unit of analysis is the person or groups of individuals applying the methods. We also direct readers to the Studies Within a Trial (SWAT) and Studies Within a Review (SWAR) programme operated through the Hub for Trials Methodology Research, for further reading as a potential useful resource for these types of experimental studies [ 21 ]. Lastly, this paper is not meant to inform the conduct of research using computational simulation and mathematical modeling for which some guidance already exists [ 22 ], or studies on the development of methods using consensus-based approaches.
When should we conduct a methodological study?
Methodological studies occupy a unique niche in health research that allows them to inform methodological advances. Methodological studies should also be conducted as pre-cursors to reporting guideline development, as they provide an opportunity to understand current practices, and help to identify the need for guidance and gaps in methodological or reporting quality. For example, the development of the popular Preferred Reporting Items of Systematic reviews and Meta-Analyses (PRISMA) guidelines were preceded by methodological studies identifying poor reporting practices [ 23 , 24 ]. In these instances, after the reporting guidelines are published, methodological studies can also be used to monitor uptake of the guidelines.
These studies can also be conducted to inform the state of the art for design, analysis and reporting practices across different types of health research fields, with the aim of improving research practices, and preventing or reducing research waste. For example, Samaan et al. conducted a scoping review of adherence to different reporting guidelines in health care literature [ 18 ]. Methodological studies can also be used to determine the factors associated with reporting practices. For example, Abbade et al. investigated journal characteristics associated with the use of the Participants, Intervention, Comparison, Outcome, Timeframe (PICOT) format in framing research questions in trials of venous ulcer disease [ 11 ].
How often are methodological studies conducted?
There is no clear answer to this question. Based on a search of PubMed, the use of related terms (“methodological review” and “meta-epidemiological study”) – and therefore, the number of methodological studies – is on the rise. However, many other terms are used to describe methodological studies. There are also many studies that explore design, conduct, analysis or reporting of research reports, but that do not use any specific terms to describe or label their study design in terms of “methodology”. This diversity in nomenclature makes a census of methodological studies elusive. Appropriate terminology and key words for methodological studies are needed to facilitate improved accessibility for end-users.
Why do we conduct methodological studies?
Methodological studies provide information on the design, conduct, analysis or reporting of primary and secondary research and can be used to appraise quality, quantity, completeness, accuracy and consistency of health research. These issues can be explored in specific fields, journals, databases, geographical regions and time periods. For example, Areia et al. explored the quality of reporting of endoscopic diagnostic studies in gastroenterology [ 25 ]; Knol et al. investigated the reporting of p -values in baseline tables in randomized trial published in high impact journals [ 26 ]; Chen et al. describe adherence to the Consolidated Standards of Reporting Trials (CONSORT) statement in Chinese Journals [ 27 ]; and Hopewell et al. describe the effect of editors’ implementation of CONSORT guidelines on reporting of abstracts over time [ 28 ]. Methodological studies provide useful information to researchers, clinicians, editors, publishers and users of health literature. As a result, these studies have been at the cornerstone of important methodological developments in the past two decades and have informed the development of many health research guidelines including the highly cited CONSORT statement [ 5 ].
Where can we find methodological studies?
Methodological studies can be found in most common biomedical bibliographic databases (e.g. Embase, MEDLINE, PubMed, Web of Science). However, the biggest caveat is that methodological studies are hard to identify in the literature due to the wide variety of names used and the lack of comprehensive databases dedicated to them. A handful can be found in the Cochrane Library as “Cochrane Methodology Reviews”, but these studies only cover methodological issues related to systematic reviews. Previous attempts to catalogue all empirical studies of methods used in reviews were abandoned 10 years ago [ 29 ]. In other databases, a variety of search terms may be applied with different levels of sensitivity and specificity.
Some frequently asked questions about methodological studies
In this section, we have outlined responses to questions that might help inform the conduct of methodological studies.
Q: How should I select research reports for my methodological study?
A: Selection of research reports for a methodological study depends on the research question and eligibility criteria. Once a clear research question is set and the nature of literature one desires to review is known, one can then begin the selection process. Selection may begin with a broad search, especially if the eligibility criteria are not apparent. For example, a methodological study of Cochrane Reviews of HIV would not require a complex search as all eligible studies can easily be retrieved from the Cochrane Library after checking a few boxes [ 30 ]. On the other hand, a methodological study of subgroup analyses in trials of gastrointestinal oncology would require a search to find such trials, and further screening to identify trials that conducted a subgroup analysis [ 31 ].
The strategies used for identifying participants in observational studies can apply here. One may use a systematic search to identify all eligible studies. If the number of eligible studies is unmanageable, a random sample of articles can be expected to provide comparable results if it is sufficiently large [ 32 ]. For example, Wilson et al. used a random sample of trials from the Cochrane Stroke Group’s Trial Register to investigate completeness of reporting [ 33 ]. It is possible that a simple random sample would lead to underrepresentation of units (i.e. research reports) that are smaller in number. This is relevant if the investigators wish to compare multiple groups but have too few units in one group. In this case a stratified sample would help to create equal groups. For example, in a methodological study comparing Cochrane and non-Cochrane reviews, Kahale et al. drew random samples from both groups [ 34 ]. Alternatively, systematic or purposeful sampling strategies can be used and we encourage researchers to justify their selected approaches based on the study objective.
Q: How many databases should I search?
A: The number of databases one should search would depend on the approach to sampling, which can include targeting the entire “population” of interest or a sample of that population. If you are interested in including the entire target population for your research question, or drawing a random or systematic sample from it, then a comprehensive and exhaustive search for relevant articles is required. In this case, we recommend using systematic approaches for searching electronic databases (i.e. at least 2 databases with a replicable and time stamped search strategy). The results of your search will constitute a sampling frame from which eligible studies can be drawn.
Alternatively, if your approach to sampling is purposeful, then we recommend targeting the database(s) or data sources (e.g. journals, registries) that include the information you need. For example, if you are conducting a methodological study of high impact journals in plastic surgery and they are all indexed in PubMed, you likely do not need to search any other databases. You may also have a comprehensive list of all journals of interest and can approach your search using the journal names in your database search (or by accessing the journal archives directly from the journal’s website). Even though one could also search journals’ web pages directly, using a database such as PubMed has multiple advantages, such as the use of filters, so the search can be narrowed down to a certain period, or study types of interest. Furthermore, individual journals’ web sites may have different search functionalities, which do not necessarily yield a consistent output.
Q: Should I publish a protocol for my methodological study?
A: A protocol is a description of intended research methods. Currently, only protocols for clinical trials require registration [ 35 ]. Protocols for systematic reviews are encouraged but no formal recommendation exists. The scientific community welcomes the publication of protocols because they help protect against selective outcome reporting, the use of post hoc methodologies to embellish results, and to help avoid duplication of efforts [ 36 ]. While the latter two risks exist in methodological research, the negative consequences may be substantially less than for clinical outcomes. In a sample of 31 methodological studies, 7 (22.6%) referenced a published protocol [ 9 ]. In the Cochrane Library, there are 15 protocols for methodological reviews (21 July 2020). This suggests that publishing protocols for methodological studies is not uncommon.
Authors can consider publishing their study protocol in a scholarly journal as a manuscript. Advantages of such publication include obtaining peer-review feedback about the planned study, and easy retrieval by searching databases such as PubMed. The disadvantages in trying to publish protocols includes delays associated with manuscript handling and peer review, as well as costs, as few journals publish study protocols, and those journals mostly charge article-processing fees [ 37 ]. Authors who would like to make their protocol publicly available without publishing it in scholarly journals, could deposit their study protocols in publicly available repositories, such as the Open Science Framework ( https://osf.io/ ).
Q: How to appraise the quality of a methodological study?
A: To date, there is no published tool for appraising the risk of bias in a methodological study, but in principle, a methodological study could be considered as a type of observational study. Therefore, during conduct or appraisal, care should be taken to avoid the biases common in observational studies [ 38 ]. These biases include selection bias, comparability of groups, and ascertainment of exposure or outcome. In other words, to generate a representative sample, a comprehensive reproducible search may be necessary to build a sampling frame. Additionally, random sampling may be necessary to ensure that all the included research reports have the same probability of being selected, and the screening and selection processes should be transparent and reproducible. To ensure that the groups compared are similar in all characteristics, matching, random sampling or stratified sampling can be used. Statistical adjustments for between-group differences can also be applied at the analysis stage. Finally, duplicate data extraction can reduce errors in assessment of exposures or outcomes.
Q: Should I justify a sample size?
A: In all instances where one is not using the target population (i.e. the group to which inferences from the research report are directed) [ 39 ], a sample size justification is good practice. The sample size justification may take the form of a description of what is expected to be achieved with the number of articles selected, or a formal sample size estimation that outlines the number of articles required to answer the research question with a certain precision and power. Sample size justifications in methodological studies are reasonable in the following instances:
Comparing two groups
Determining a proportion, mean or another quantifier
Determining factors associated with an outcome using regression-based analyses
For example, El Dib et al. computed a sample size requirement for a methodological study of diagnostic strategies in randomized trials, based on a confidence interval approach [ 40 ].
Q: What should I call my study?
A: Other terms which have been used to describe/label methodological studies include “ methodological review ”, “methodological survey” , “meta-epidemiological study” , “systematic review” , “systematic survey”, “meta-research”, “research-on-research” and many others. We recommend that the study nomenclature be clear, unambiguous, informative and allow for appropriate indexing. Methodological study nomenclature that should be avoided includes “ systematic review” – as this will likely be confused with a systematic review of a clinical question. “ Systematic survey” may also lead to confusion about whether the survey was systematic (i.e. using a preplanned methodology) or a survey using “ systematic” sampling (i.e. a sampling approach using specific intervals to determine who is selected) [ 32 ]. Any of the above meanings of the words “ systematic” may be true for methodological studies and could be potentially misleading. “ Meta-epidemiological study” is ideal for indexing, but not very informative as it describes an entire field. The term “ review ” may point towards an appraisal or “review” of the design, conduct, analysis or reporting (or methodological components) of the targeted research reports, yet it has also been used to describe narrative reviews [ 41 , 42 ]. The term “ survey ” is also in line with the approaches used in many methodological studies [ 9 ], and would be indicative of the sampling procedures of this study design. However, in the absence of guidelines on nomenclature, the term “ methodological study ” is broad enough to capture most of the scenarios of such studies.
Q: Should I account for clustering in my methodological study?
A: Data from methodological studies are often clustered. For example, articles coming from a specific source may have different reporting standards (e.g. the Cochrane Library). Articles within the same journal may be similar due to editorial practices and policies, reporting requirements and endorsement of guidelines. There is emerging evidence that these are real concerns that should be accounted for in analyses [ 43 ]. Some cluster variables are described in the section: “ What variables are relevant to methodological studies?”
A variety of modelling approaches can be used to account for correlated data, including the use of marginal, fixed or mixed effects regression models with appropriate computation of standard errors [ 44 ]. For example, Kosa et al. used generalized estimation equations to account for correlation of articles within journals [ 15 ]. Not accounting for clustering could lead to incorrect p -values, unduly narrow confidence intervals, and biased estimates [ 45 ].
Q: Should I extract data in duplicate?
A: Yes. Duplicate data extraction takes more time but results in less errors [ 19 ]. Data extraction errors in turn affect the effect estimate [ 46 ], and therefore should be mitigated. Duplicate data extraction should be considered in the absence of other approaches to minimize extraction errors. However, much like systematic reviews, this area will likely see rapid new advances with machine learning and natural language processing technologies to support researchers with screening and data extraction [ 47 , 48 ]. However, experience plays an important role in the quality of extracted data and inexperienced extractors should be paired with experienced extractors [ 46 , 49 ].
Q: Should I assess the risk of bias of research reports included in my methodological study?
A : Risk of bias is most useful in determining the certainty that can be placed in the effect measure from a study. In methodological studies, risk of bias may not serve the purpose of determining the trustworthiness of results, as effect measures are often not the primary goal of methodological studies. Determining risk of bias in methodological studies is likely a practice borrowed from systematic review methodology, but whose intrinsic value is not obvious in methodological studies. When it is part of the research question, investigators often focus on one aspect of risk of bias. For example, Speich investigated how blinding was reported in surgical trials [ 50 ], and Abraha et al., investigated the application of intention-to-treat analyses in systematic reviews and trials [ 51 ].
Q: What variables are relevant to methodological studies?
A: There is empirical evidence that certain variables may inform the findings in a methodological study. We outline some of these and provide a brief overview below:
Country: Countries and regions differ in their research cultures, and the resources available to conduct research. Therefore, it is reasonable to believe that there may be differences in methodological features across countries. Methodological studies have reported loco-regional differences in reporting quality [ 52 , 53 ]. This may also be related to challenges non-English speakers face in publishing papers in English.
Authors’ expertise: The inclusion of authors with expertise in research methodology, biostatistics, and scientific writing is likely to influence the end-product. Oltean et al. found that among randomized trials in orthopaedic surgery, the use of analyses that accounted for clustering was more likely when specialists (e.g. statistician, epidemiologist or clinical trials methodologist) were included on the study team [ 54 ]. Fleming et al. found that including methodologists in the review team was associated with appropriate use of reporting guidelines [ 55 ].
Source of funding and conflicts of interest: Some studies have found that funded studies report better [ 56 , 57 ], while others do not [ 53 , 58 ]. The presence of funding would indicate the availability of resources deployed to ensure optimal design, conduct, analysis and reporting. However, the source of funding may introduce conflicts of interest and warrant assessment. For example, Kaiser et al. investigated the effect of industry funding on obesity or nutrition randomized trials and found that reporting quality was similar [ 59 ]. Thomas et al. looked at reporting quality of long-term weight loss trials and found that industry funded studies were better [ 60 ]. Kan et al. examined the association between industry funding and “positive trials” (trials reporting a significant intervention effect) and found that industry funding was highly predictive of a positive trial [ 61 ]. This finding is similar to that of a recent Cochrane Methodology Review by Hansen et al. [ 62 ]
Journal characteristics: Certain journals’ characteristics may influence the study design, analysis or reporting. Characteristics such as journal endorsement of guidelines [ 63 , 64 ], and Journal Impact Factor (JIF) have been shown to be associated with reporting [ 63 , 65 , 66 , 67 ].
Study size (sample size/number of sites): Some studies have shown that reporting is better in larger studies [ 53 , 56 , 58 ].
Year of publication: It is reasonable to assume that design, conduct, analysis and reporting of research will change over time. Many studies have demonstrated improvements in reporting over time or after the publication of reporting guidelines [ 68 , 69 ].
Type of intervention: In a methodological study of reporting quality of weight loss intervention studies, Thabane et al. found that trials of pharmacologic interventions were reported better than trials of non-pharmacologic interventions [ 70 ].
Interactions between variables: Complex interactions between the previously listed variables are possible. High income countries with more resources may be more likely to conduct larger studies and incorporate a variety of experts. Authors in certain countries may prefer certain journals, and journal endorsement of guidelines and editorial policies may change over time.
Q: Should I focus only on high impact journals?
A: Investigators may choose to investigate only high impact journals because they are more likely to influence practice and policy, or because they assume that methodological standards would be higher. However, the JIF may severely limit the scope of articles included and may skew the sample towards articles with positive findings. The generalizability and applicability of findings from a handful of journals must be examined carefully, especially since the JIF varies over time. Even among journals that are all “high impact”, variations exist in methodological standards.
Q: Can I conduct a methodological study of qualitative research?
A: Yes. Even though a lot of methodological research has been conducted in the quantitative research field, methodological studies of qualitative studies are feasible. Certain databases that catalogue qualitative research including the Cumulative Index to Nursing & Allied Health Literature (CINAHL) have defined subject headings that are specific to methodological research (e.g. “research methodology”). Alternatively, one could also conduct a qualitative methodological review; that is, use qualitative approaches to synthesize methodological issues in qualitative studies.
Q: What reporting guidelines should I use for my methodological study?
A: There is no guideline that covers the entire scope of methodological studies. One adaptation of the PRISMA guidelines has been published, which works well for studies that aim to use the entire target population of research reports [ 71 ]. However, it is not widely used (40 citations in 2 years as of 09 December 2019), and methodological studies that are designed as cross-sectional or before-after studies require a more fit-for purpose guideline. A more encompassing reporting guideline for a broad range of methodological studies is currently under development [ 72 ]. However, in the absence of formal guidance, the requirements for scientific reporting should be respected, and authors of methodological studies should focus on transparency and reproducibility.
Q: What are the potential threats to validity and how can I avoid them?
A: Methodological studies may be compromised by a lack of internal or external validity. The main threats to internal validity in methodological studies are selection and confounding bias. Investigators must ensure that the methods used to select articles does not make them differ systematically from the set of articles to which they would like to make inferences. For example, attempting to make extrapolations to all journals after analyzing high-impact journals would be misleading.
Many factors (confounders) may distort the association between the exposure and outcome if the included research reports differ with respect to these factors [ 73 ]. For example, when examining the association between source of funding and completeness of reporting, it may be necessary to account for journals that endorse the guidelines. Confounding bias can be addressed by restriction, matching and statistical adjustment [ 73 ]. Restriction appears to be the method of choice for many investigators who choose to include only high impact journals or articles in a specific field. For example, Knol et al. examined the reporting of p -values in baseline tables of high impact journals [ 26 ]. Matching is also sometimes used. In the methodological study of non-randomized interventional studies of elective ventral hernia repair, Parker et al. matched prospective studies with retrospective studies and compared reporting standards [ 74 ]. Some other methodological studies use statistical adjustments. For example, Zhang et al. used regression techniques to determine the factors associated with missing participant data in trials [ 16 ].
With regard to external validity, researchers interested in conducting methodological studies must consider how generalizable or applicable their findings are. This should tie in closely with the research question and should be explicit. For example. Findings from methodological studies on trials published in high impact cardiology journals cannot be assumed to be applicable to trials in other fields. However, investigators must ensure that their sample truly represents the target sample either by a) conducting a comprehensive and exhaustive search, or b) using an appropriate and justified, randomly selected sample of research reports.
Even applicability to high impact journals may vary based on the investigators’ definition, and over time. For example, for high impact journals in the field of general medicine, Bouwmeester et al. included the Annals of Internal Medicine (AIM), BMJ, the Journal of the American Medical Association (JAMA), Lancet, the New England Journal of Medicine (NEJM), and PLoS Medicine ( n = 6) [ 75 ]. In contrast, the high impact journals selected in the methodological study by Schiller et al. were BMJ, JAMA, Lancet, and NEJM ( n = 4) [ 76 ]. Another methodological study by Kosa et al. included AIM, BMJ, JAMA, Lancet and NEJM ( n = 5). In the methodological study by Thabut et al., journals with a JIF greater than 5 were considered to be high impact. Riado Minguez et al. used first quartile journals in the Journal Citation Reports (JCR) for a specific year to determine “high impact” [ 77 ]. Ultimately, the definition of high impact will be based on the number of journals the investigators are willing to include, the year of impact and the JIF cut-off [ 78 ]. We acknowledge that the term “generalizability” may apply differently for methodological studies, especially when in many instances it is possible to include the entire target population in the sample studied.
Finally, methodological studies are not exempt from information bias which may stem from discrepancies in the included research reports [ 79 ], errors in data extraction, or inappropriate interpretation of the information extracted. Likewise, publication bias may also be a concern in methodological studies, but such concepts have not yet been explored.
A proposed framework
In order to inform discussions about methodological studies, the development of guidance for what should be reported, we have outlined some key features of methodological studies that can be used to classify them. For each of the categories outlined below, we provide an example. In our experience, the choice of approach to completing a methodological study can be informed by asking the following four questions:
What is the aim?
Methodological studies that investigate bias
A methodological study may be focused on exploring sources of bias in primary or secondary studies (meta-bias), or how bias is analyzed. We have taken care to distinguish bias (i.e. systematic deviations from the truth irrespective of the source) from reporting quality or completeness (i.e. not adhering to a specific reporting guideline or norm). An example of where this distinction would be important is in the case of a randomized trial with no blinding. This study (depending on the nature of the intervention) would be at risk of performance bias. However, if the authors report that their study was not blinded, they would have reported adequately. In fact, some methodological studies attempt to capture both “quality of conduct” and “quality of reporting”, such as Richie et al., who reported on the risk of bias in randomized trials of pharmacy practice interventions [ 80 ]. Babic et al. investigated how risk of bias was used to inform sensitivity analyses in Cochrane reviews [ 81 ]. Further, biases related to choice of outcomes can also be explored. For example, Tan et al investigated differences in treatment effect size based on the outcome reported [ 82 ].
Methodological studies that investigate quality (or completeness) of reporting
Methodological studies may report quality of reporting against a reporting checklist (i.e. adherence to guidelines) or against expected norms. For example, Croituro et al. report on the quality of reporting in systematic reviews published in dermatology journals based on their adherence to the PRISMA statement [ 83 ], and Khan et al. described the quality of reporting of harms in randomized controlled trials published in high impact cardiovascular journals based on the CONSORT extension for harms [ 84 ]. Other methodological studies investigate reporting of certain features of interest that may not be part of formally published checklists or guidelines. For example, Mbuagbaw et al. described how often the implications for research are elaborated using the Evidence, Participants, Intervention, Comparison, Outcome, Timeframe (EPICOT) format [ 30 ].
Methodological studies that investigate the consistency of reporting
Sometimes investigators may be interested in how consistent reports of the same research are, as it is expected that there should be consistency between: conference abstracts and published manuscripts; manuscript abstracts and manuscript main text; and trial registration and published manuscript. For example, Rosmarakis et al. investigated consistency between conference abstracts and full text manuscripts [ 85 ].
Methodological studies that investigate factors associated with reporting
In addition to identifying issues with reporting in primary and secondary studies, authors of methodological studies may be interested in determining the factors that are associated with certain reporting practices. Many methodological studies incorporate this, albeit as a secondary outcome. For example, Farrokhyar et al. investigated the factors associated with reporting quality in randomized trials of coronary artery bypass grafting surgery [ 53 ].
Methodological studies that investigate methods
Methodological studies may also be used to describe methods or compare methods, and the factors associated with methods. Muller et al. described the methods used for systematic reviews and meta-analyses of observational studies [ 86 ].
Methodological studies that summarize other methodological studies
Some methodological studies synthesize results from other methodological studies. For example, Li et al. conducted a scoping review of methodological reviews that investigated consistency between full text and abstracts in primary biomedical research [ 87 ].
Methodological studies that investigate nomenclature and terminology
Some methodological studies may investigate the use of names and terms in health research. For example, Martinic et al. investigated the definitions of systematic reviews used in overviews of systematic reviews (OSRs), meta-epidemiological studies and epidemiology textbooks [ 88 ].
Other types of methodological studies
In addition to the previously mentioned experimental methodological studies, there may exist other types of methodological studies not captured here.
What is the design?
Methodological studies that are descriptive
Most methodological studies are purely descriptive and report their findings as counts (percent) and means (standard deviation) or medians (interquartile range). For example, Mbuagbaw et al. described the reporting of research recommendations in Cochrane HIV systematic reviews [ 30 ]. Gohari et al. described the quality of reporting of randomized trials in diabetes in Iran [ 12 ].
Methodological studies that are analytical
Some methodological studies are analytical wherein “analytical studies identify and quantify associations, test hypotheses, identify causes and determine whether an association exists between variables, such as between an exposure and a disease.” [ 89 ] In the case of methodological studies all these investigations are possible. For example, Kosa et al. investigated the association between agreement in primary outcome from trial registry to published manuscript and study covariates. They found that larger and more recent studies were more likely to have agreement [ 15 ]. Tricco et al. compared the conclusion statements from Cochrane and non-Cochrane systematic reviews with a meta-analysis of the primary outcome and found that non-Cochrane reviews were more likely to report positive findings. These results are a test of the null hypothesis that the proportions of Cochrane and non-Cochrane reviews that report positive results are equal [ 90 ].
What is the sampling strategy?
Methodological studies that include the target population
Methodological reviews with narrow research questions may be able to include the entire target population. For example, in the methodological study of Cochrane HIV systematic reviews, Mbuagbaw et al. included all of the available studies ( n = 103) [ 30 ].
Methodological studies that include a sample of the target population
Many methodological studies use random samples of the target population [ 33 , 91 , 92 ]. Alternatively, purposeful sampling may be used, limiting the sample to a subset of research-related reports published within a certain time period, or in journals with a certain ranking or on a topic. Systematic sampling can also be used when random sampling may be challenging to implement.
What is the unit of analysis?
Methodological studies with a research report as the unit of analysis
Many methodological studies use a research report (e.g. full manuscript of study, abstract portion of the study) as the unit of analysis, and inferences can be made at the study-level. However, both published and unpublished research-related reports can be studied. These may include articles, conference abstracts, registry entries etc.
Methodological studies with a design, analysis or reporting item as the unit of analysis
Some methodological studies report on items which may occur more than once per article. For example, Paquette et al. report on subgroup analyses in Cochrane reviews of atrial fibrillation in which 17 systematic reviews planned 56 subgroup analyses [ 93 ].
This framework is outlined in Fig. 2 .
A proposed framework for methodological studies
Conclusions
Methodological studies have examined different aspects of reporting such as quality, completeness, consistency and adherence to reporting guidelines. As such, many of the methodological study examples cited in this tutorial are related to reporting. However, as an evolving field, the scope of research questions that can be addressed by methodological studies is expected to increase.
In this paper we have outlined the scope and purpose of methodological studies, along with examples of instances in which various approaches have been used. In the absence of formal guidance on the design, conduct, analysis and reporting of methodological studies, we have provided some advice to help make methodological studies consistent. This advice is grounded in good contemporary scientific practice. Generally, the research question should tie in with the sampling approach and planned analysis. We have also highlighted the variables that may inform findings from methodological studies. Lastly, we have provided suggestions for ways in which authors can categorize their methodological studies to inform their design and analysis.
Availability of data and materials
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Abbreviations
Consolidated Standards of Reporting Trials
Evidence, Participants, Intervention, Comparison, Outcome, Timeframe
Grading of Recommendations, Assessment, Development and Evaluations
Participants, Intervention, Comparison, Outcome, Timeframe
Preferred Reporting Items of Systematic reviews and Meta-Analyses
Studies Within a Review
Studies Within a Trial
Chalmers I, Glasziou P. Avoidable waste in the production and reporting of research evidence. Lancet. 2009;374(9683):86–9.
PubMed Google Scholar
Chan AW, Song F, Vickers A, Jefferson T, Dickersin K, Gotzsche PC, Krumholz HM, Ghersi D, van der Worp HB. Increasing value and reducing waste: addressing inaccessible research. Lancet. 2014;383(9913):257–66.
PubMed PubMed Central Google Scholar
Ioannidis JP, Greenland S, Hlatky MA, Khoury MJ, Macleod MR, Moher D, Schulz KF, Tibshirani R. Increasing value and reducing waste in research design, conduct, and analysis. Lancet. 2014;383(9912):166–75.
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet. 2001;357.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100.
Shea BJ, Hamel C, Wells GA, Bouter LM, Kristjansson E, Grimshaw J, Henry DA, Boers M. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J Clin Epidemiol. 2009;62(10):1013–20.
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, Moher D, Tugwell P, Welch V, Kristjansson E, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. Bmj. 2017;358:j4008.
Lawson DO, Leenus A, Mbuagbaw L. Mapping the nomenclature, methodology, and reporting of studies that review methods: a pilot methodological review. Pilot Feasibility Studies. 2020;6(1):13.
Puljak L, Makaric ZL, Buljan I, Pieper D. What is a meta-epidemiological study? Analysis of published literature indicated heterogeneous study designs and definitions. J Comp Eff Res. 2020.
Abbade LPF, Wang M, Sriganesh K, Jin Y, Mbuagbaw L, Thabane L. The framing of research questions using the PICOT format in randomized controlled trials of venous ulcer disease is suboptimal: a systematic survey. Wound Repair Regen. 2017;25(5):892–900.
Gohari F, Baradaran HR, Tabatabaee M, Anijidani S, Mohammadpour Touserkani F, Atlasi R, Razmgir M. Quality of reporting randomized controlled trials (RCTs) in diabetes in Iran; a systematic review. J Diabetes Metab Disord. 2015;15(1):36.
Wang M, Jin Y, Hu ZJ, Thabane A, Dennis B, Gajic-Veljanoski O, Paul J, Thabane L. The reporting quality of abstracts of stepped wedge randomized trials is suboptimal: a systematic survey of the literature. Contemp Clin Trials Commun. 2017;8:1–10.
Shanthanna H, Kaushal A, Mbuagbaw L, Couban R, Busse J, Thabane L: A cross-sectional study of the reporting quality of pilot or feasibility trials in high-impact anesthesia journals Can J Anaesthesia 2018, 65(11):1180–1195.
Kosa SD, Mbuagbaw L, Borg Debono V, Bhandari M, Dennis BB, Ene G, Leenus A, Shi D, Thabane M, Valvasori S, et al. Agreement in reporting between trial publications and current clinical trial registry in high impact journals: a methodological review. Contemporary Clinical Trials. 2018;65:144–50.
Zhang Y, Florez ID, Colunga Lozano LE, Aloweni FAB, Kennedy SA, Li A, Craigie S, Zhang S, Agarwal A, Lopes LC, et al. A systematic survey on reporting and methods for handling missing participant data for continuous outcomes in randomized controlled trials. J Clin Epidemiol. 2017;88:57–66.
CAS PubMed Google Scholar
Hernández AV, Boersma E, Murray GD, Habbema JD, Steyerberg EW. Subgroup analyses in therapeutic cardiovascular clinical trials: are most of them misleading? Am Heart J. 2006;151(2):257–64.
Samaan Z, Mbuagbaw L, Kosa D, Borg Debono V, Dillenburg R, Zhang S, Fruci V, Dennis B, Bawor M, Thabane L. A systematic scoping review of adherence to reporting guidelines in health care literature. J Multidiscip Healthc. 2013;6:169–88.
Buscemi N, Hartling L, Vandermeer B, Tjosvold L, Klassen TP. Single data extraction generated more errors than double data extraction in systematic reviews. J Clin Epidemiol. 2006;59(7):697–703.
Carrasco-Labra A, Brignardello-Petersen R, Santesso N, Neumann I, Mustafa RA, Mbuagbaw L, Etxeandia Ikobaltzeta I, De Stio C, McCullagh LJ, Alonso-Coello P. Improving GRADE evidence tables part 1: a randomized trial shows improved understanding of content in summary-of-findings tables with a new format. J Clin Epidemiol. 2016;74:7–18.
The Northern Ireland Hub for Trials Methodology Research: SWAT/SWAR Information [ https://www.qub.ac.uk/sites/TheNorthernIrelandNetworkforTrialsMethodologyResearch/SWATSWARInformation/ ]. Accessed 31 Aug 2020.
Chick S, Sánchez P, Ferrin D, Morrice D. How to conduct a successful simulation study. In: Proceedings of the 2003 winter simulation conference: 2003; 2003. p. 66–70.
Google Scholar
Mulrow CD. The medical review article: state of the science. Ann Intern Med. 1987;106(3):485–8.
Sacks HS, Reitman D, Pagano D, Kupelnick B. Meta-analysis: an update. Mount Sinai J Med New York. 1996;63(3–4):216–24.
CAS Google Scholar
Areia M, Soares M, Dinis-Ribeiro M. Quality reporting of endoscopic diagnostic studies in gastrointestinal journals: where do we stand on the use of the STARD and CONSORT statements? Endoscopy. 2010;42(2):138–47.
Knol M, Groenwold R, Grobbee D. P-values in baseline tables of randomised controlled trials are inappropriate but still common in high impact journals. Eur J Prev Cardiol. 2012;19(2):231–2.
Chen M, Cui J, Zhang AL, Sze DM, Xue CC, May BH. Adherence to CONSORT items in randomized controlled trials of integrative medicine for colorectal Cancer published in Chinese journals. J Altern Complement Med. 2018;24(2):115–24.
Hopewell S, Ravaud P, Baron G, Boutron I. Effect of editors' implementation of CONSORT guidelines on the reporting of abstracts in high impact medical journals: interrupted time series analysis. BMJ. 2012;344:e4178.
The Cochrane Methodology Register Issue 2 2009 [ https://cmr.cochrane.org/help.htm ]. Accessed 31 Aug 2020.
Mbuagbaw L, Kredo T, Welch V, Mursleen S, Ross S, Zani B, Motaze NV, Quinlan L. Critical EPICOT items were absent in Cochrane human immunodeficiency virus systematic reviews: a bibliometric analysis. J Clin Epidemiol. 2016;74:66–72.
Barton S, Peckitt C, Sclafani F, Cunningham D, Chau I. The influence of industry sponsorship on the reporting of subgroup analyses within phase III randomised controlled trials in gastrointestinal oncology. Eur J Cancer. 2015;51(18):2732–9.
Setia MS. Methodology series module 5: sampling strategies. Indian J Dermatol. 2016;61(5):505–9.
Wilson B, Burnett P, Moher D, Altman DG, Al-Shahi Salman R. Completeness of reporting of randomised controlled trials including people with transient ischaemic attack or stroke: a systematic review. Eur Stroke J. 2018;3(4):337–46.
Kahale LA, Diab B, Brignardello-Petersen R, Agarwal A, Mustafa RA, Kwong J, Neumann I, Li L, Lopes LC, Briel M, et al. Systematic reviews do not adequately report or address missing outcome data in their analyses: a methodological survey. J Clin Epidemiol. 2018;99:14–23.
De Angelis CD, Drazen JM, Frizelle FA, Haug C, Hoey J, Horton R, Kotzin S, Laine C, Marusic A, Overbeke AJPM, et al. Is this clinical trial fully registered?: a statement from the International Committee of Medical Journal Editors*. Ann Intern Med. 2005;143(2):146–8.
Ohtake PJ, Childs JD. Why publish study protocols? Phys Ther. 2014;94(9):1208–9.
Rombey T, Allers K, Mathes T, Hoffmann F, Pieper D. A descriptive analysis of the characteristics and the peer review process of systematic review protocols published in an open peer review journal from 2012 to 2017. BMC Med Res Methodol. 2019;19(1):57.
Grimes DA, Schulz KF. Bias and causal associations in observational research. Lancet. 2002;359(9302):248–52.
Porta M (ed.): A dictionary of epidemiology, 5th edn. Oxford: Oxford University Press, Inc.; 2008.
El Dib R, Tikkinen KAO, Akl EA, Gomaa HA, Mustafa RA, Agarwal A, Carpenter CR, Zhang Y, Jorge EC, Almeida R, et al. Systematic survey of randomized trials evaluating the impact of alternative diagnostic strategies on patient-important outcomes. J Clin Epidemiol. 2017;84:61–9.
Helzer JE, Robins LN, Taibleson M, Woodruff RA Jr, Reich T, Wish ED. Reliability of psychiatric diagnosis. I. a methodological review. Arch Gen Psychiatry. 1977;34(2):129–33.
Chung ST, Chacko SK, Sunehag AL, Haymond MW. Measurements of gluconeogenesis and Glycogenolysis: a methodological review. Diabetes. 2015;64(12):3996–4010.
CAS PubMed PubMed Central Google Scholar
Sterne JA, Juni P, Schulz KF, Altman DG, Bartlett C, Egger M. Statistical methods for assessing the influence of study characteristics on treatment effects in 'meta-epidemiological' research. Stat Med. 2002;21(11):1513–24.
Moen EL, Fricano-Kugler CJ, Luikart BW, O’Malley AJ. Analyzing clustered data: why and how to account for multiple observations nested within a study participant? PLoS One. 2016;11(1):e0146721.
Zyzanski SJ, Flocke SA, Dickinson LM. On the nature and analysis of clustered data. Ann Fam Med. 2004;2(3):199–200.
Mathes T, Klassen P, Pieper D. Frequency of data extraction errors and methods to increase data extraction quality: a methodological review. BMC Med Res Methodol. 2017;17(1):152.
Bui DDA, Del Fiol G, Hurdle JF, Jonnalagadda S. Extractive text summarization system to aid data extraction from full text in systematic review development. J Biomed Inform. 2016;64:265–72.
Bui DD, Del Fiol G, Jonnalagadda S. PDF text classification to leverage information extraction from publication reports. J Biomed Inform. 2016;61:141–8.
Maticic K, Krnic Martinic M, Puljak L. Assessment of reporting quality of abstracts of systematic reviews with meta-analysis using PRISMA-A and discordance in assessments between raters without prior experience. BMC Med Res Methodol. 2019;19(1):32.
Speich B. Blinding in surgical randomized clinical trials in 2015. Ann Surg. 2017;266(1):21–2.
Abraha I, Cozzolino F, Orso M, Marchesi M, Germani A, Lombardo G, Eusebi P, De Florio R, Luchetta ML, Iorio A, et al. A systematic review found that deviations from intention-to-treat are common in randomized trials and systematic reviews. J Clin Epidemiol. 2017;84:37–46.
Zhong Y, Zhou W, Jiang H, Fan T, Diao X, Yang H, Min J, Wang G, Fu J, Mao B. Quality of reporting of two-group parallel randomized controlled clinical trials of multi-herb formulae: A survey of reports indexed in the Science Citation Index Expanded. Eur J Integrative Med. 2011;3(4):e309–16.
Farrokhyar F, Chu R, Whitlock R, Thabane L. A systematic review of the quality of publications reporting coronary artery bypass grafting trials. Can J Surg. 2007;50(4):266–77.
Oltean H, Gagnier JJ. Use of clustering analysis in randomized controlled trials in orthopaedic surgery. BMC Med Res Methodol. 2015;15:17.
Fleming PS, Koletsi D, Pandis N. Blinded by PRISMA: are systematic reviewers focusing on PRISMA and ignoring other guidelines? PLoS One. 2014;9(5):e96407.
Balasubramanian SP, Wiener M, Alshameeri Z, Tiruvoipati R, Elbourne D, Reed MW. Standards of reporting of randomized controlled trials in general surgery: can we do better? Ann Surg. 2006;244(5):663–7.
de Vries TW, van Roon EN. Low quality of reporting adverse drug reactions in paediatric randomised controlled trials. Arch Dis Child. 2010;95(12):1023–6.
Borg Debono V, Zhang S, Ye C, Paul J, Arya A, Hurlburt L, Murthy Y, Thabane L. The quality of reporting of RCTs used within a postoperative pain management meta-analysis, using the CONSORT statement. BMC Anesthesiol. 2012;12:13.
Kaiser KA, Cofield SS, Fontaine KR, Glasser SP, Thabane L, Chu R, Ambrale S, Dwary AD, Kumar A, Nayyar G, et al. Is funding source related to study reporting quality in obesity or nutrition randomized control trials in top-tier medical journals? Int J Obes. 2012;36(7):977–81.
Thomas O, Thabane L, Douketis J, Chu R, Westfall AO, Allison DB. Industry funding and the reporting quality of large long-term weight loss trials. Int J Obes. 2008;32(10):1531–6.
Khan NR, Saad H, Oravec CS, Rossi N, Nguyen V, Venable GT, Lillard JC, Patel P, Taylor DR, Vaughn BN, et al. A review of industry funding in randomized controlled trials published in the neurosurgical literature-the elephant in the room. Neurosurgery. 2018;83(5):890–7.
Hansen C, Lundh A, Rasmussen K, Hrobjartsson A. Financial conflicts of interest in systematic reviews: associations with results, conclusions, and methodological quality. Cochrane Database Syst Rev. 2019;8:Mr000047.
Kiehna EN, Starke RM, Pouratian N, Dumont AS. Standards for reporting randomized controlled trials in neurosurgery. J Neurosurg. 2011;114(2):280–5.
Liu LQ, Morris PJ, Pengel LH. Compliance to the CONSORT statement of randomized controlled trials in solid organ transplantation: a 3-year overview. Transpl Int. 2013;26(3):300–6.
Bala MM, Akl EA, Sun X, Bassler D, Mertz D, Mejza F, Vandvik PO, Malaga G, Johnston BC, Dahm P, et al. Randomized trials published in higher vs. lower impact journals differ in design, conduct, and analysis. J Clin Epidemiol. 2013;66(3):286–95.
Lee SY, Teoh PJ, Camm CF, Agha RA. Compliance of randomized controlled trials in trauma surgery with the CONSORT statement. J Trauma Acute Care Surg. 2013;75(4):562–72.
Ziogas DC, Zintzaras E. Analysis of the quality of reporting of randomized controlled trials in acute and chronic myeloid leukemia, and myelodysplastic syndromes as governed by the CONSORT statement. Ann Epidemiol. 2009;19(7):494–500.
Alvarez F, Meyer N, Gourraud PA, Paul C. CONSORT adoption and quality of reporting of randomized controlled trials: a systematic analysis in two dermatology journals. Br J Dermatol. 2009;161(5):1159–65.
Mbuagbaw L, Thabane M, Vanniyasingam T, Borg Debono V, Kosa S, Zhang S, Ye C, Parpia S, Dennis BB, Thabane L. Improvement in the quality of abstracts in major clinical journals since CONSORT extension for abstracts: a systematic review. Contemporary Clin trials. 2014;38(2):245–50.
Thabane L, Chu R, Cuddy K, Douketis J. What is the quality of reporting in weight loss intervention studies? A systematic review of randomized controlled trials. Int J Obes. 2007;31(10):1554–9.
Murad MH, Wang Z. Guidelines for reporting meta-epidemiological methodology research. Evidence Based Med. 2017;22(4):139.
METRIC - MEthodological sTudy ReportIng Checklist: guidelines for reporting methodological studies in health research [ http://www.equator-network.org/library/reporting-guidelines-under-development/reporting-guidelines-under-development-for-other-study-designs/#METRIC ]. Accessed 31 Aug 2020.
Jager KJ, Zoccali C, MacLeod A, Dekker FW. Confounding: what it is and how to deal with it. Kidney Int. 2008;73(3):256–60.
Parker SG, Halligan S, Erotocritou M, Wood CPJ, Boulton RW, Plumb AAO, Windsor ACJ, Mallett S. A systematic methodological review of non-randomised interventional studies of elective ventral hernia repair: clear definitions and a standardised minimum dataset are needed. Hernia. 2019.
Bouwmeester W, Zuithoff NPA, Mallett S, Geerlings MI, Vergouwe Y, Steyerberg EW, Altman DG, Moons KGM. Reporting and methods in clinical prediction research: a systematic review. PLoS Med. 2012;9(5):1–12.
Schiller P, Burchardi N, Niestroj M, Kieser M. Quality of reporting of clinical non-inferiority and equivalence randomised trials--update and extension. Trials. 2012;13:214.
Riado Minguez D, Kowalski M, Vallve Odena M, Longin Pontzen D, Jelicic Kadic A, Jeric M, Dosenovic S, Jakus D, Vrdoljak M, Poklepovic Pericic T, et al. Methodological and reporting quality of systematic reviews published in the highest ranking journals in the field of pain. Anesth Analg. 2017;125(4):1348–54.
Thabut G, Estellat C, Boutron I, Samama CM, Ravaud P. Methodological issues in trials assessing primary prophylaxis of venous thrombo-embolism. Eur Heart J. 2005;27(2):227–36.
Puljak L, Riva N, Parmelli E, González-Lorenzo M, Moja L, Pieper D. Data extraction methods: an analysis of internal reporting discrepancies in single manuscripts and practical advice. J Clin Epidemiol. 2020;117:158–64.
Ritchie A, Seubert L, Clifford R, Perry D, Bond C. Do randomised controlled trials relevant to pharmacy meet best practice standards for quality conduct and reporting? A systematic review. Int J Pharm Pract. 2019.
Babic A, Vuka I, Saric F, Proloscic I, Slapnicar E, Cavar J, Pericic TP, Pieper D, Puljak L. Overall bias methods and their use in sensitivity analysis of Cochrane reviews were not consistent. J Clin Epidemiol. 2019.
Tan A, Porcher R, Crequit P, Ravaud P, Dechartres A. Differences in treatment effect size between overall survival and progression-free survival in immunotherapy trials: a Meta-epidemiologic study of trials with results posted at ClinicalTrials.gov. J Clin Oncol. 2017;35(15):1686–94.
Croitoru D, Huang Y, Kurdina A, Chan AW, Drucker AM. Quality of reporting in systematic reviews published in dermatology journals. Br J Dermatol. 2020;182(6):1469–76.
Khan MS, Ochani RK, Shaikh A, Vaduganathan M, Khan SU, Fatima K, Yamani N, Mandrola J, Doukky R, Krasuski RA: Assessing the Quality of Reporting of Harms in Randomized Controlled Trials Published in High Impact Cardiovascular Journals. Eur Heart J Qual Care Clin Outcomes 2019.
Rosmarakis ES, Soteriades ES, Vergidis PI, Kasiakou SK, Falagas ME. From conference abstract to full paper: differences between data presented in conferences and journals. FASEB J. 2005;19(7):673–80.
Mueller M, D’Addario M, Egger M, Cevallos M, Dekkers O, Mugglin C, Scott P. Methods to systematically review and meta-analyse observational studies: a systematic scoping review of recommendations. BMC Med Res Methodol. 2018;18(1):44.
Li G, Abbade LPF, Nwosu I, Jin Y, Leenus A, Maaz M, Wang M, Bhatt M, Zielinski L, Sanger N, et al. A scoping review of comparisons between abstracts and full reports in primary biomedical research. BMC Med Res Methodol. 2017;17(1):181.
Krnic Martinic M, Pieper D, Glatt A, Puljak L. Definition of a systematic review used in overviews of systematic reviews, meta-epidemiological studies and textbooks. BMC Med Res Methodol. 2019;19(1):203.
Analytical study [ https://medical-dictionary.thefreedictionary.com/analytical+study ]. Accessed 31 Aug 2020.
Tricco AC, Tetzlaff J, Pham B, Brehaut J, Moher D. Non-Cochrane vs. Cochrane reviews were twice as likely to have positive conclusion statements: cross-sectional study. J Clin Epidemiol. 2009;62(4):380–6 e381.
Schalken N, Rietbergen C. The reporting quality of systematic reviews and Meta-analyses in industrial and organizational psychology: a systematic review. Front Psychol. 2017;8:1395.
Ranker LR, Petersen JM, Fox MP. Awareness of and potential for dependent error in the observational epidemiologic literature: A review. Ann Epidemiol. 2019;36:15–9 e12.
Paquette M, Alotaibi AM, Nieuwlaat R, Santesso N, Mbuagbaw L. A meta-epidemiological study of subgroup analyses in cochrane systematic reviews of atrial fibrillation. Syst Rev. 2019;8(1):241.
Download references
Acknowledgements
This work did not receive any dedicated funding.
Author information
Authors and affiliations.
Department of Health Research Methods, Evidence and Impact, McMaster University, Hamilton, ON, Canada
Lawrence Mbuagbaw, Daeria O. Lawson & Lehana Thabane
Biostatistics Unit/FSORC, 50 Charlton Avenue East, St Joseph’s Healthcare—Hamilton, 3rd Floor Martha Wing, Room H321, Hamilton, Ontario, L8N 4A6, Canada
Lawrence Mbuagbaw & Lehana Thabane
Centre for the Development of Best Practices in Health, Yaoundé, Cameroon
Lawrence Mbuagbaw
Center for Evidence-Based Medicine and Health Care, Catholic University of Croatia, Ilica 242, 10000, Zagreb, Croatia
Livia Puljak
Department of Epidemiology and Biostatistics, School of Public Health – Bloomington, Indiana University, Bloomington, IN, 47405, USA
David B. Allison
Departments of Paediatrics and Anaesthesia, McMaster University, Hamilton, ON, Canada
Lehana Thabane
Centre for Evaluation of Medicine, St. Joseph’s Healthcare-Hamilton, Hamilton, ON, Canada
Population Health Research Institute, Hamilton Health Sciences, Hamilton, ON, Canada
You can also search for this author in PubMed Google Scholar
Contributions
LM conceived the idea and drafted the outline and paper. DOL and LT commented on the idea and draft outline. LM, LP and DOL performed literature searches and data extraction. All authors (LM, DOL, LT, LP, DBA) reviewed several draft versions of the manuscript and approved the final manuscript.
Corresponding author
Correspondence to Lawrence Mbuagbaw .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
DOL, DBA, LM, LP and LT are involved in the development of a reporting guideline for methodological studies.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.

Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Mbuagbaw, L., Lawson, D.O., Puljak, L. et al. A tutorial on methodological studies: the what, when, how and why. BMC Med Res Methodol 20 , 226 (2020). https://doi.org/10.1186/s12874-020-01107-7
Download citation
Received : 27 May 2020
Accepted : 27 August 2020
Published : 07 September 2020
DOI : https://doi.org/10.1186/s12874-020-01107-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Methodological study
- Meta-epidemiology
- Research methods
- Research-on-research
BMC Medical Research Methodology
ISSN: 1471-2288
- General enquiries: [email protected]
Research articles
Quality and safety of artificial intelligence generated health information, large language models and the generation of health disinformation, 25 year trends in cancer incidence and mortality among adults in the uk, cervical pessary versus vaginal progesterone in women with a singleton pregnancy, comparison of prior authorization across insurers, diagnostic accuracy of magnetically guided capsule endoscopy with a detachable string for detecting oesophagogastric varices in adults with cirrhosis, ultra-processed food exposure and adverse health outcomes, added benefit and revenues of oncology drugs approved by the ema, exposure to air pollution and hospital admission for cardiovascular diseases, short term exposure to low level ambient fine particulate matter and natural cause, cardiovascular, and respiratory morbidity, optimal timing of influenza vaccination in young children, effect of exercise for depression, association of non-alcoholic fatty liver disease with cardiovascular disease and all cause death in patients with type 2 diabetes, duration of cpr and outcomes for adults with in-hospital cardiac arrest, clinical effectiveness of an online physical and mental health rehabilitation programme for post-covid-19 condition, atypia detected during breast screening and subsequent development of cancer, publishers’ and journals’ instructions to authors on use of generative ai in academic and scientific publishing, effectiveness of glp-1 receptor agonists on glycaemic control, body weight, and lipid profile for type 2 diabetes, neurological development in children born moderately or late preterm, invasive breast cancer and breast cancer death after non-screen detected ductal carcinoma in situ, all cause and cause specific mortality in obsessive-compulsive disorder, acute rehabilitation following traumatic anterior shoulder dislocation, perinatal depression and risk of mortality, undisclosed financial conflicts of interest in dsm-5-tr, effect of risk mitigation guidance opioid and stimulant dispensations on mortality and acute care visits, update to living systematic review on sars-cov-2 positivity in offspring and timing of mother-to-child transmission, perinatal depression and its health impact, christmas 2023: common healthcare related instruments subjected to magnetic attraction study, using autoregressive integrated moving average models for time series analysis of observational data, demand for morning after pill following new year holiday, christmas 2023: christmas recipes from the great british bake off, effect of a doctor working during the festive period on population health: experiment using doctor who episodes, christmas 2023: analysis of barbie medical and science career dolls, christmas 2023: effect of chair placement on physicians’ behavior and patients’ satisfaction, management of chronic pain secondary to temporomandibular disorders, christmas 2023: projecting complete redaction of clinical trial protocols, christmas 2023: a drug target for erectile dysfunction to help improve fertility, sexual activity, and wellbeing, christmas 2023: efficacy of cola ingestion for oesophageal food bolus impaction, conservative management versus laparoscopic cholecystectomy in adults with gallstone disease, social media use and health risk behaviours in young people, untreated cervical intraepithelial neoplasia grade 2 and cervical cancer, air pollution deaths attributable to fossil fuels, implementation of a high sensitivity cardiac troponin i assay and risk of myocardial infarction or death at five years, covid-19 vaccine effectiveness against post-covid-19 condition, association between patient-surgeon gender concordance and mortality after surgery, intravascular imaging guided versus coronary angiography guided percutaneous coronary intervention, treatment of lower urinary tract symptoms in men in primary care using a conservative intervention, autism intervention meta-analysis of early childhood studies, effectiveness of the live zoster vaccine during the 10 years following vaccination, effects of a multimodal intervention in primary care to reduce second line antibiotic prescriptions for urinary tract infections in women, pyrotinib versus placebo in combination with trastuzumab and docetaxel in patients with her2 positive metastatic breast cancer, association of dcis size and margin status with risk of developing breast cancer post-treatment, racial differences in low value care among older patients in the us, pharmaceutical industry payments and delivery of low value cancer drugs, rosuvastatin versus atorvastatin in adults with coronary artery disease, clinical effectiveness of septoplasty versus medical management for nasal airways obstruction, ultrasound guided lavage with corticosteroid injection versus sham lavage with and without corticosteroid injection for calcific tendinopathy of shoulder, early versus delayed antihypertensive treatment in patients with acute ischaemic stroke, mortality risks associated with floods in 761 communities worldwide, interactive effects of ambient fine particulate matter and ozone on daily mortality in 372 cities, association between changes in carbohydrate intake and long term weight changes, future-case control crossover analysis for adjusting bias in case crossover studies, association between recently raised anticholinergic burden and risk of acute cardiovascular events, suboptimal gestational weight gain and neonatal outcomes in low and middle income countries: individual participant data meta-analysis, efficacy and safety of an inactivated virus-particle vaccine for sars-cov-2, effect of invitation letter in language of origin on screening attendance: randomised controlled trial in breastscreen norway, visits by nurse practitioners and physician assistants in the usa, non-erosive gastro-oesophageal reflux disease and oesophageal adenocarcinoma, venous thromboembolism with use of hormonal contraception and nsaids, food additive emulsifiers and risk of cardiovascular disease, balancing risks and benefits of cannabis use, promoting activity, independence, and stability in early dementia and mild cognitive impairment, effect of home cook interventions for salt reduction in china, cancer mortality after low dose exposure to ionising radiation, effect of a smartphone intervention among university students with unhealthy alcohol use, long term risk of death and readmission after hospital admission with covid-19 among older adults, mortality rates among patients successfully treated for hepatitis c, association between antenatal corticosteroids and risk of serious infection in children, the proportions of term or late preterm births after exposure to early antenatal corticosteroids, and outcomes, safety of ba.4-5 or ba.1 bivalent mrna booster vaccines, comparative effectiveness of booster vaccines among adults aged ≥50 years, third dose vaccine schedules against severe covid-19 during omicron predominance in nordic countries, private equity ownership and impacts on health outcomes, costs, and quality, healthcare disruption due to covid-19 and avoidable hospital admission, educational inequalities in mortality and their mediators among generations across four decades, prevalence and predictors of data and code sharing in the medical and health sciences, medicare eligibility and in-hospital treatment patterns and health outcomes for patients with trauma, therapeutic value of first versus supplemental indications of drugs in us and europe, hospital admissions linked to sars-cov-2 infection in children and adolescents, vitamin d supplementation and major cardiovascular events, menopausal hormone therapy and dementia, associations between modest reductions in kidney function and adverse outcomes in young adults, association between surgeon volume and patient outcomes after elective shoulder replacement surgery, risk prediction of covid-19 related death or hospital admission in adults testing positive for sars-cov-2, effectiveness of grace risk score in patients admitted to hospital with non-st elevation acute coronary syndrome, peer reviewers’ response to invitations by gender and geographical region, breast cancer mortality in women with early invasive breast cancer, mmr vaccine at age 6 months and hospitalisation for infection before age 12 months, recovery up to two years after sars-cov-2 infection, race-free estimated glomerular filtration rate equation in kidney transplant recipients, follow us on, content links.
- Collections
- Health in South Asia
- Women’s, children’s & adolescents’ health
- News and views
- BMJ Opinion
- Rapid responses
- Editorial staff
- BMJ in the USA
- BMJ in South Asia
- Submit your paper
- BMA members
- Subscribers
- Advertisers and sponsors
Explore BMJ
- Our company
- BMJ Careers
- BMJ Learning
- BMJ Masterclasses
- BMJ Journals
- BMJ Student
- Academic edition of The BMJ
- BMJ Best Practice
- The BMJ Awards
- Email alerts
- Activate subscription
Information
- Log In Username Enter your ACP Online username. Password Enter the password that accompanies your username. Remember me Forget your username or password ?
- Privacy Policy
- Career Connection
- Member Forums
© Copyright 2024 American College of Physicians, Inc. All Rights Reserved. 190 North Independence Mall West, Philadelphia, PA 19106-1572 800-ACP-1915 (800-227-1915) or 215-351-2600
If you are unable to login, please try clearing your cookies . We apologize for the inconvenience.
Preparing the Research Presentation
If you have never presented a paper at a scientific meeting, you should read this article. Even if you have presented before, it is likely that this article contains information that will improve your presentation. This article contains a set of practical, proven steps that will guide your preparation of the presentation. Our assumptions are that you will schedule appropriate planning and preparation time, are interested in doing the best job possible, and know that a quality presentation is a combination of good research and communication skills. This and subsequent articles will focus on planning, preparation, creating visual aids (slides), and presentation skills for a scientific presentation. The intent of this series of articles is to help you make a favorable impression at the scientific meeting and reap the rewards, personal and professional, of a job well done.
To begin with, you need to create an outline of the topics you might present at the meeting. Your outline should follow the IMRAC format (introduction, methods, results, and conclusion). This format is chosen because your audience understands it and expects it. If you have already prepared a paper for publication, it can be a rich source of content for the topic outline.
To get you started, we have prepared a generic outline to serve as an example. We recognize that a generic outline does not necessarily adapt to all research designs, but we ask you to think, "How can I adapt this to my situation?" To help you visualize the content you might include in the outline, two types of examples have been included, one that describes a cross-sectional study using a survey methodology (example A), and a second using a combination of a case-control and cohort designs (example B).
Use the Preparing the Research Presentation Checklist to assist you in preparing the topic outline.
How to Write a Research Paper Introduction (with Examples)

The research paper introduction section, along with the Title and Abstract, can be considered the face of any research paper. The following article is intended to guide you in organizing and writing the research paper introduction for a quality academic article or dissertation.
The research paper introduction aims to present the topic to the reader. A study will only be accepted for publishing if you can ascertain that the available literature cannot answer your research question. So it is important to ensure that you have read important studies on that particular topic, especially those within the last five to ten years, and that they are properly referenced in this section. 1 What should be included in the research paper introduction is decided by what you want to tell readers about the reason behind the research and how you plan to fill the knowledge gap. The best research paper introduction provides a systemic review of existing work and demonstrates additional work that needs to be done. It needs to be brief, captivating, and well-referenced; a well-drafted research paper introduction will help the researcher win half the battle.
The introduction for a research paper is where you set up your topic and approach for the reader. It has several key goals:
- Present your research topic
- Capture reader interest
- Summarize existing research
- Position your own approach
- Define your specific research problem and problem statement
- Highlight the novelty and contributions of the study
- Give an overview of the paper’s structure
The research paper introduction can vary in size and structure depending on whether your paper presents the results of original empirical research or is a review paper. Some research paper introduction examples are only half a page while others are a few pages long. In many cases, the introduction will be shorter than all of the other sections of your paper; its length depends on the size of your paper as a whole.
- Break through writer’s block. Write your research paper introduction with Paperpal Copilot
Table of Contents
What is the introduction for a research paper, why is the introduction important in a research paper, craft a compelling introduction section with paperpal. try now, 1. introduce the research topic:, 2. determine a research niche:, 3. place your research within the research niche:, craft accurate research paper introductions with paperpal. start writing now, frequently asked questions on research paper introduction, key points to remember.
The introduction in a research paper is placed at the beginning to guide the reader from a broad subject area to the specific topic that your research addresses. They present the following information to the reader
- Scope: The topic covered in the research paper
- Context: Background of your topic
- Importance: Why your research matters in that particular area of research and the industry problem that can be targeted
The research paper introduction conveys a lot of information and can be considered an essential roadmap for the rest of your paper. A good introduction for a research paper is important for the following reasons:
- It stimulates your reader’s interest: A good introduction section can make your readers want to read your paper by capturing their interest. It informs the reader what they are going to learn and helps determine if the topic is of interest to them.
- It helps the reader understand the research background: Without a clear introduction, your readers may feel confused and even struggle when reading your paper. A good research paper introduction will prepare them for the in-depth research to come. It provides you the opportunity to engage with the readers and demonstrate your knowledge and authority on the specific topic.
- It explains why your research paper is worth reading: Your introduction can convey a lot of information to your readers. It introduces the topic, why the topic is important, and how you plan to proceed with your research.
- It helps guide the reader through the rest of the paper: The research paper introduction gives the reader a sense of the nature of the information that will support your arguments and the general organization of the paragraphs that will follow. It offers an overview of what to expect when reading the main body of your paper.
What are the parts of introduction in the research?
A good research paper introduction section should comprise three main elements: 2
- What is known: This sets the stage for your research. It informs the readers of what is known on the subject.
- What is lacking: This is aimed at justifying the reason for carrying out your research. This could involve investigating a new concept or method or building upon previous research.
- What you aim to do: This part briefly states the objectives of your research and its major contributions. Your detailed hypothesis will also form a part of this section.
How to write a research paper introduction?
The first step in writing the research paper introduction is to inform the reader what your topic is and why it’s interesting or important. This is generally accomplished with a strong opening statement. The second step involves establishing the kinds of research that have been done and ending with limitations or gaps in the research that you intend to address. Finally, the research paper introduction clarifies how your own research fits in and what problem it addresses. If your research involved testing hypotheses, these should be stated along with your research question. The hypothesis should be presented in the past tense since it will have been tested by the time you are writing the research paper introduction.
The following key points, with examples, can guide you when writing the research paper introduction section:
- Highlight the importance of the research field or topic
- Describe the background of the topic
- Present an overview of current research on the topic
Example: The inclusion of experiential and competency-based learning has benefitted electronics engineering education. Industry partnerships provide an excellent alternative for students wanting to engage in solving real-world challenges. Industry-academia participation has grown in recent years due to the need for skilled engineers with practical training and specialized expertise. However, from the educational perspective, many activities are needed to incorporate sustainable development goals into the university curricula and consolidate learning innovation in universities.
- Reveal a gap in existing research or oppose an existing assumption
- Formulate the research question
Example: There have been plausible efforts to integrate educational activities in higher education electronics engineering programs. However, very few studies have considered using educational research methods for performance evaluation of competency-based higher engineering education, with a focus on technical and or transversal skills. To remedy the current need for evaluating competencies in STEM fields and providing sustainable development goals in engineering education, in this study, a comparison was drawn between study groups without and with industry partners.
- State the purpose of your study
- Highlight the key characteristics of your study
- Describe important results
- Highlight the novelty of the study.
- Offer a brief overview of the structure of the paper.
Example: The study evaluates the main competency needed in the applied electronics course, which is a fundamental core subject for many electronics engineering undergraduate programs. We compared two groups, without and with an industrial partner, that offered real-world projects to solve during the semester. This comparison can help determine significant differences in both groups in terms of developing subject competency and achieving sustainable development goals.
Write a Research Paper Introduction in Minutes with Paperpal
Paperpal Copilot is a generative AI-powered academic writing assistant. It’s trained on millions of published scholarly articles and over 20 years of STM experience. Paperpal Copilot helps authors write better and faster with:
- Real-time writing suggestions
- In-depth checks for language and grammar correction
- Paraphrasing to add variety, ensure academic tone, and trim text to meet journal limits
With Paperpal Copilot, create a research paper introduction effortlessly. In this step-by-step guide, we’ll walk you through how Paperpal transforms your initial ideas into a polished and publication-ready introduction.
How to use Paperpal to write the Introduction section
Step 1: Sign up on Paperpal and click on the Copilot feature, under this choose Outlines > Research Article > Introduction
Step 2: Add your unstructured notes or initial draft, whether in English or another language, to Paperpal, which is to be used as the base for your content.
Step 3: Fill in the specifics, such as your field of study, brief description or details you want to include, which will help the AI generate the outline for your Introduction.
Step 4: Use this outline and sentence suggestions to develop your content, adding citations where needed and modifying it to align with your specific research focus.
Step 5: Turn to Paperpal’s granular language checks to refine your content, tailor it to reflect your personal writing style, and ensure it effectively conveys your message.
You can use the same process to develop each section of your article, and finally your research paper in half the time and without any of the stress.
The purpose of the research paper introduction is to introduce the reader to the problem definition, justify the need for the study, and describe the main theme of the study. The aim is to gain the reader’s attention by providing them with necessary background information and establishing the main purpose and direction of the research.
The length of the research paper introduction can vary across journals and disciplines. While there are no strict word limits for writing the research paper introduction, an ideal length would be one page, with a maximum of 400 words over 1-4 paragraphs. Generally, it is one of the shorter sections of the paper as the reader is assumed to have at least a reasonable knowledge about the topic. 2 For example, for a study evaluating the role of building design in ensuring fire safety, there is no need to discuss definitions and nature of fire in the introduction; you could start by commenting upon the existing practices for fire safety and how your study will add to the existing knowledge and practice.
When deciding what to include in the research paper introduction, the rest of the paper should also be considered. The aim is to introduce the reader smoothly to the topic and facilitate an easy read without much dependency on external sources. 3 Below is a list of elements you can include to prepare a research paper introduction outline and follow it when you are writing the research paper introduction. Topic introduction: This can include key definitions and a brief history of the topic. Research context and background: Offer the readers some general information and then narrow it down to specific aspects. Details of the research you conducted: A brief literature review can be included to support your arguments or line of thought. Rationale for the study: This establishes the relevance of your study and establishes its importance. Importance of your research: The main contributions are highlighted to help establish the novelty of your study Research hypothesis: Introduce your research question and propose an expected outcome. Organization of the paper: Include a short paragraph of 3-4 sentences that highlights your plan for the entire paper
Cite only works that are most relevant to your topic; as a general rule, you can include one to three. Note that readers want to see evidence of original thinking. So it is better to avoid using too many references as it does not leave much room for your personal standpoint to shine through. Citations in your research paper introduction support the key points, and the number of citations depend on the subject matter and the point discussed. If the research paper introduction is too long or overflowing with citations, it is better to cite a few review articles rather than the individual articles summarized in the review. A good point to remember when citing research papers in the introduction section is to include at least one-third of the references in the introduction.
The literature review plays a significant role in the research paper introduction section. A good literature review accomplishes the following: Introduces the topic – Establishes the study’s significance – Provides an overview of the relevant literature – Provides context for the study using literature – Identifies knowledge gaps However, remember to avoid making the following mistakes when writing a research paper introduction: Do not use studies from the literature review to aggressively support your research Avoid direct quoting Do not allow literature review to be the focus of this section. Instead, the literature review should only aid in setting a foundation for the manuscript.
Remember the following key points for writing a good research paper introduction: 4
- Avoid stuffing too much general information: Avoid including what an average reader would know and include only that information related to the problem being addressed in the research paper introduction. For example, when describing a comparative study of non-traditional methods for mechanical design optimization, information related to the traditional methods and differences between traditional and non-traditional methods would not be relevant. In this case, the introduction for the research paper should begin with the state-of-the-art non-traditional methods and methods to evaluate the efficiency of newly developed algorithms.
- Avoid packing too many references: Cite only the required works in your research paper introduction. The other works can be included in the discussion section to strengthen your findings.
- Avoid extensive criticism of previous studies: Avoid being overly critical of earlier studies while setting the rationale for your study. A better place for this would be the Discussion section, where you can highlight the advantages of your method.
- Avoid describing conclusions of the study: When writing a research paper introduction remember not to include the findings of your study. The aim is to let the readers know what question is being answered. The actual answer should only be given in the Results and Discussion section.
To summarize, the research paper introduction section should be brief yet informative. It should convince the reader the need to conduct the study and motivate him to read further. If you’re feeling stuck or unsure, choose trusted AI academic writing assistants like Paperpal to effortlessly craft your research paper introduction and other sections of your research article.
1. Jawaid, S. A., & Jawaid, M. (2019). How to write introduction and discussion. Saudi Journal of Anaesthesia, 13(Suppl 1), S18.
2. Dewan, P., & Gupta, P. (2016). Writing the title, abstract and introduction: Looks matter!. Indian pediatrics, 53, 235-241.
3. Cetin, S., & Hackam, D. J. (2005). An approach to the writing of a scientific Manuscript1. Journal of Surgical Research, 128(2), 165-167.
4. Bavdekar, S. B. (2015). Writing introduction: Laying the foundations of a research paper. Journal of the Association of Physicians of India, 63(7), 44-6.
Paperpal is a comprehensive AI writing toolkit that helps students and researchers achieve 2x the writing in half the time. It leverages 21+ years of STM experience and insights from millions of research articles to provide in-depth academic writing, language editing, and submission readiness support to help you write better, faster.
Get accurate academic translations, rewriting support, grammar checks, vocabulary suggestions, and generative AI assistance that delivers human precision at machine speed. Try for free or upgrade to Paperpal Prime starting at US$19 a month to access premium features, including consistency, plagiarism, and 30+ submission readiness checks to help you succeed.
Experience the future of academic writing – Sign up to Paperpal and start writing for free!
Related Reads:
- Scientific Writing Style Guides Explained
- 5 Reasons for Rejection After Peer Review
- Ethical Research Practices For Research with Human Subjects
- 8 Most Effective Ways to Increase Motivation for Thesis Writing
Practice vs. Practise: Learn the Difference
Academic paraphrasing: why paperpal’s rewrite should be your first choice , you may also like, quillbot review: features, pricing, and free alternatives, what is an academic paper types and elements , should you use ai tools like chatgpt for..., publish research papers: 9 steps for successful publications , what are the different types of research papers, how to make translating academic papers less challenging, self-plagiarism in research: what it is and how..., 6 tips for post-doc researchers to take their..., presenting research data effectively through tables and figures, ethics in science: importance, principles & guidelines .

Writing a Medical Clinical Trial Research Paper – Example & Format
Hello, this is Sam from Ref-n-write. In this blog, I will explain how to write a clinical trial research paper for a medical journal. We will go through the basic components that make up a good clinical paper. The title of our research paper is “The Effects of Vitamin D Supplements on Obesity: A Randomized Clinical Trial Study” I must insist that this is not an actual research paper from an actual medical journal. This is just an example medical research paper we put together for the purpose of teaching the process of writing up clinical trials.
The Effects of Vitamin D Supplements on Obesity: A Randomized Clinical Trial Study Research Paper Title
1. Introduction Paragraph
Let’s start with the introduction paragraph. This is where you tell your readers what your topic is and why it is important. It is a good idea to start your intro paragraph with a hook. A hook is a powerful opening statement designed to grab the reader’s attention. This can be a fact, a statistic or a question. Since our study is about obesity, let’s give an interesting statistic about obesity. After starting with a broad statement, the next step is to narrow down the topic. In the second statement, we are dropping a hint that our paper is concerned with vitamin D and obesity. With the next statement, we are establishing the importance of the topic. We are saying that many people are dying due to obesity, and vitamin D is causing a lot of health issues, so we must do something about it. Then in the final statement of the intro paragraph, we explain how conducting research in this field will benefit the community. In our case, doctors will be able to prescribe better treatment options for obese patients. That concludes the introduction paragraph of our research paper.
Obesity is a Worldwide disease; In 2020, more than 2 billion adults, 18 years and older, were overweight. There is a general consensus in the research community that there is a strong association between obesity and Vitamin D. This represents an important and timely topic because obesity is currently fifth greatest risk of mortality, and Vitamin D deficiency has been associated with variety of chronic diseases. Better understanding of this link will greatly aid medical practitioners in effective treatment and management of obese patients. Introduction Paragraph
2. Literature Review
Let’s move on to the literature review. This is where you provide a comprehensive summary of previous research on this topic. Let’s start with a broad statement summarizing the research in the area. In the first statement, we are saying that many studies have confirmed some link between vitamin D and obesity. Now let’s move on to specific studies. In the second statement, we are reporting the results of a specific study that came out recently and talks about the link between vitamin D and obesity in western countries. Now let’s talk about some mixed evidence that casts some doubt on the current understanding of the topic. In the final statement, we are saying that some studies have shown that obesity causes vitamin D deficiency, whereas other studies have shown that it is in fact, vitamin D that causes obesity issues among people.
Several studies have reported an association between low vitamin D levels and obesity levels [1-3] . Recently John et al , reported high prevalence of Vitamin D deficiency in several western countries with high levels of obesity [1]. Some studies suggested that obesity increased the risk of vit D deficiency [15] whereas other studies have shown the opposite [3]. Literature Review
3. Research Gap and Research Statement
Now it is time to establish the research gap. The previous statements we made, nicely lead to this statement. We are saying that we lack clear evidence linking vitamin D to obesity. We are also saying that most of the existing studies were conducted on subjects with preexisting health conditions, so there is a research gap to be filled. Now you must define your research question and explain how it addresses the research gap you established in the literature review. We are saying here that the study’s main aim is to investigate the effect of vitamin D on weight loss among the healthy population. We are also defining a specific hypothesis that we will either prove or disprove towards the end of the paper.
Due to lack of clear evidence, the link between vitamin D and obesity remains unclear. Moreover, most studies were conducted on subjects with preexisting health conditions or of certain background. The aim of the study was to examine the effect of vitamin D supplementation on weight loss among healthy population. We hypothesized that vitamin D could enhance weight loss without side effects. Research Gap and Statement
4. Materials and Methods
Let’s move on to materials and methods. The ‘materials and methods’ is one of the most important parts of your paper. This section should have enough detail so that another researcher can reproduce your experiments and results.
4.1. Study Design and Ethical Approval
Let’s start with study design. In clinical trial papers, you must explain the study design employed in your work. In our case, it was a randomized, double-blinded placebo trial. Then you can talk about the location and period in which the clinical trial was conducted. Then provide details about the ethical approval that was obtained for the study. Nowadays, registering your clinical trial on the website clinicaltrial.org is a requirement before you begin recruiting patients for your study. You must also include the registration number. I must warn you that many journals will refuse to publish the results of your paper if the clinical trial is unregistered. Good clinical practice (GCP) is a set of internationally recognized quality standards that must be followed when conducting clinical trials involving people. It is a good idea to provide information about who is responsible for monitoring this for your trial.
The study was a randomized double-blind placebo-controlled clinical trial study. The study was conducted between April 2015 and March 2017 at five different hospitals in the central United Kingdom. This study was approved by the National Health Service ethical committee and registered on www.clinicaltrial.org as NC 34532. The trial was conducted according to the guidelines of good clinical practice (GCP) and monitored by the GCP unit at the hospital. Study Design, Ethical Approval & Good Clinical Practice
4.2. Participant Recruitment and Consent
It is very important to define the inclusion and exclusion criteria used for the study. The inclusion criteria define the characteristics that will make subjects eligible for the study. In our case, we only included non-smoking and nondiabetic subjects with BMI greater than 25. The exclusion criteria define the characteristics that make subjects ineligible for the study. In our case, we exclude subjects participating in weight loss programs and taking dietary supplements. Now let’s detail the characteristics of the cohort, such as sample size, age, gender etc. In our case, we recruited 50 subjects in the age range of 15-60.
Let’s provide some information about the recruitment procedure. In our case, there was a face-to-face interview to confirm eligibility. And also, the eligible participants were asked to fill in a questionnaire so that we could gather demographic information. Another important part of the recruitment process is to get informed consent from the participants. The participants should be given all the information about the trial, including the benefits and risks, so they can decide whether to participate in the trial or not.
Subjects were included if they met the following criteria: (1) BMI>25; (2) non-smoker; and (3) no history of diabetes. Subjects participating in weight loss programs were excluded from the study. A total of 50 subjects (25 male & 25 female) participated in the study at the age range of 15-60. The eligibility was evaluated by interview. They were asked to fill in a questionnaire to gather demographic information. An informed consent was obtained from all the participants. Inclusion/Exclusion Criteria, Patient Recruitment & Consent
4.3. Outcomes and Follow up
Let us now explain how the participants were divided into groups. In our study, the participants were randomly split into intervention and control groups. The intervention group was given vitamin D supplements, and the control group was given a placebo. Our study is double-blinded, which means neither the participants nor the researchers knew which group they belonged to. Let’s talk about the follow-up period. Choosing an appropriate follow-up period for your study is important because a shorter follow-up period leads to an underestimation of the effects being measured. On the other hand, a long follow-up period increases the risk of subjects dropping out of the study. In our case, we have chosen a follow-up period of 12 months, and the measurements were performed every 6 months.
Then we have to explain what parameters we are measuring on the participants during the course of the study. In our case, we measured BMI, waist circumference and blood pressure. BMI is the primary outcome. It means it is the most important outcome, and we will analyze the changes in BMI values to either prove or disprove our hypothesis. The secondary outcomes, such as waist circumference and BP, are additional measurements that we perform to provide supporting evidence for the main finding.
Participants were randomly divided into intervention and control groups, and received vit D supplements and placebo, respectively. Patients were assessed at 0, 6 and 12 months for a follow-up period of 1 year. BMI (primary outcome), waist circumference (secondary outcome) and BP (secondary outcome) were measured by a trained personal at each visit. Grouping, Outcome & Follow-up Period
4.4. Statistical Analysis
Let’s talk about the statistical analysis and tools used for the study. In our case, we are using an independent sample t-test for statistical analysis. The independent sample t-test compares the means of two groups, in our case, the vitamin D group and the placebo group. We also specify the definition of statistical significance; if the p-value is less than 0.05, then we will consider the difference to be statistically significant. We also give the format of the data presented in the paper. In our case, all the data will be represented in the format mean ± SE. We also mention the name of the statistical package used for the analysis. In our case, We are using SPSS statistical software, and the version number is 10.0.
Analyses were performed with independent t-test and paired t-test. All data were shown as mean ± SE. In all analysis, P value <0.05 was considered statistically significant. The data were analyzed using SPSS 10.0 (http://www.spss.com) software. Statistical Analysis
Let’s move on to the results section. This is where you present the core findings of your study. You have to present your results in a logical sequence. Do not interpret the results here. Instead, save them for the discussion section later. Before jumping into results, you must first let the audience know if you did any preprocessing or data cleanup before the analysis. In our case, four participants had to be excluded from the study due to health issues. And two participants dropped out due to personal reasons. So it means we are dealing with a slightly smaller sample size than we initially set out. Try to present your data in figures and tables, and only elaborate on the most important results in the main text. In our case, we are presenting the characteristics of both groups in a table. And we are plotting the change in BMI over time as a graph and presenting it as a figure in the paper.
From 50 participants, four subjects were excluded due to health issues. Two participants withdrew from the study due to personal reasons. Table 1 illustrates the characteristics of two groups participated in the study. In Figure 1, the BMI values are plotted as a function of duration for both groups. The results show that vitamin D supplementation caused a significant decrease in BMI (p<0.001). There was no significant difference in BP (p=0.71) between vitamin D (121 ± 3.1) and placebo groups (123 ± 4.2). Results
Now let’s start with the main finding. In our case, we found a significant drop in BMI among the cohort taking vitamin D supplements. Since we use the word significant, we have to provide a p-value. Let’s move on to the next result. We are reporting that there is no significant difference in blood pressure between the two groups. We are also providing actual values in the text. We have already mentioned in the methods section that the data will be in the format mean ± SE.
6. Discussion
Let’s move on to the discussion. This is where you interpret your findings and compare your results with previously published work in this domain. This is the place to talk about limitations and the future direction of your work. It is a good idea to start with the main result. In our case, we found that vitamin D reduces BMI, which supports the main hypothesis. Let’s also mention how these findings fit into existing research in the domain. In our case, these findings are in line with the results of previous studies published on this topic. Now, let’s move on to a negative result. In our case, we observed a negative association between waist circumference and vitamin D. Now give your interpretation of this negative result. We think it is because of the shorter duration of the study. Let’s report an unexpected result. In our case, we found that the blood pressure was a bit on the higher side with the cohort taking vitamin D supplements. Let’s give our interpretation of the result. We believe it is because of limited data.
Vitamin D supplementation significantly reduced the BMI over a 1 year period supporting the main hypothesis. The results agree well with the findings of existing studies [7-8]. A negative association was observed between waist length and vitamin D supplementation. The findings are in contrast with the previous studies [9-10]. This outcome is likely due to the shorter duration of the study. It was quite surprising to find that there was a slight increase in BP among the vitamin D group. The result must be interpreted with caution due to limited data. Interpretation of Results
Let’s talk about the implications of our research. This is where you describe the significance of your findings. You must explain how your findings will benefit society. You can also explain how your findings contribute to the existing body of knowledge and impact future research in the area. Then add one or two lines about the novelty of your research. Explain what is so unique about your research. In our case, this is the first study to be conducted on a healthy population. Let’s move on to limitations. Every study has limitations. If you hide your limitations, I can guarantee that reviewers will reject your paper. Be honest about the limitations and explain how future studies can rectify the shortcomings of your work. In our case, the major limitation is that our study uses a small number of participants. Let’s move on to the final statement of the paper. Finish your paper with one or two lines about the possible future direction of your research. In our case, we can conduct a much bigger study to reconfirm our findings in the future.
The results demonstrated in this work provides a new perspective on the link between vitamin D and obesity from a clinical treatment perspective. To the best of our knowledge, this is the largest study to date to be conducted on health population. One of the most important limitation of the study is the small sample size. Larger clinical trials are needed to confirm the findings. This should be considered in future studies. Implications, Limitations and Future work
Similar Posts

How to Write a Research Paper? A Beginners Guide with Useful Academic Phrases
This blog explains how to write a research paper and provides writing ideas in the form of academic phrases.

Useful Phrases and Sentences for Academic & Research Paper Writing
In this blog, we explain various sections of a research paper and give you an overview of what these sections should contain.

Research Paper Structure – Main Sections and Parts of a Research Paper
In this blog, we explain various topics and sub-topics to be included under each section of a research paper via word cloud diagrams.
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
Enter the email address you signed up with and we'll email you a reset link.
- We're Hiring!
- Help Center

The medical research paper: Structure and functions

1997, English for Specific Purposes
Related Papers
Journal of Language Teaching and Research
Morteza Amirsheibani
Since Swales’ CARS model (1981, 1990) work on the move structure of research article, studies on genre analysis have been carried out so far among which works on different parts of research article in various disciplines has gained a considerable literature. The present study aims to examine the move structure of research article conclusion sections in two fields of ELT and Nursing based on Yang and Allison’s (2003) model of move structure in conclusion sections. Each corpus of the current study contains 25 research articles related specifically to the field under study. The results of data analysis indicated that both corpora contained the moves proposed in Yang and Allison’s (2003) model and almost no significant differences were observed in the rhetorical structure of the afore-mentioned fields. Therefore, the authors of both ELT and Nursing had a great tendency to apply this model in the conclusion sections of their articles. The obtained findings of the current study can be use...
Science Park Research Organization & Counselling
Daniel Lees Fryer
Genre analysis can be used as a means of understanding the communicative practices of specific discourse communities and may therefore be of particular benefit to students in higher education for whom the interpretation and production of discipline-specific texts is paramount. This study takes global medical research as a case in point and examines the generic discourse features of the experimental medical research article (RA), using a systemic-functional and ‘structural moves analysis’ approach. Based on this novel, combined methodology, a sequence of generic rhetorical moves and steps across a series of medical RAs are described in terms of their function and lexicogrammar. The implications of the study are discussed in relation to previous research and their potential pedagogical and methodological applications.
Journal of Applied Linguistics and Applied Literature Dynamics and Advances
Since Swales' (1981, 1990) CARS model work on the move structure of research articles, studies on genre analysis have been carried out amongst which works on different parts of research articles in various disciplines has gained a considerable literature. This study aims to investigate the rhetorical structure of the Introduction sections of articles in two fields of English Language Teaching (ELT) and Nursing, based on two corpora. Each corpus contains 25 research articles related specifically to the field under study. This study initially identified the structural organization of the Introduction sections of the articles. The results of this analysis revealed that both corpora contained the moves proposed in CARS model and almost no significant differences were observed in the move structures of articles in the afore-mentioned fields. Therefore, there is a considerable tendency for both ELT and Nursing to use CARS model. The findings from the analysis could provide linguistic researchers in Foreign Language Teaching (FLT) with a holistic and unitary methodology as an authentic model of language in use through enriching their understandings and knowledge about the true nature and organization of different disciplines.
English for Specific Purposes
Joseph Adjei
Terminology
López Arroyo Belén
Genre analysis studies concerning the medical research article are limited, and the few studies that do exist tend to focus exclusively on the textual aspects of the genre, with little consideration for the context and discourse community in which texts are produced.
The British journal of general practice : the journal of the Royal College of General Practitioners
An increasing number of people involved in medicine are under pressure to publish research, but there is little understanding of how to describe structured writing. This paper aims to describe the structure of original research papers published in the British Journal of General Practice with a view to providing insight into the nature of such analyses, and particularly to help researchers and trainers to write and teach writing more successfully. A sample of 50 original papers published in the Journal between January 1989 and March 1993 were examined. The papers were subjected to a form of 'move structure analysis', a technique used in applied linguistics; move structure analysis assigns a tentative function to a piece of text, and identifies words/phrases associated with it. To be recognized, moves thus identified had to occur in the same section of the paper in 65% of the corpus, and/or appear in the same order relative to other moves in 50%. Fifteen moves were identified,...
Budsaba Kanoksilapatham
This study aims to develop the academic writing skills of biomedical engineers by analyzing a dataset of 37 discussion sections in biomedical engineering research articles written in English. Specifi c objectives of this study are twofold: 1) to identify the components that make up this section classifi ed as ‘moves’ and 2) to describe how each component of move is expressed. First, the analysis reveals that this section consists of three principal moves, and each move possibly entails a number of sub-units called ‘steps’. The analysis also demonstrates that these units of texts are organized in a particular pattern forming a typical sequence. Second, the examination to scrutinize how these textual units are written shows that a number of linguistic features are conventionally used to serve a particular purpose. This study bears pedagogical implications preparing scholars who are not competent in English to be able to disseminate their scientifi c achievements regionally and interna...
International Journal of Information Science and Management
raouf moini
RELATED PAPERS
Luis Jimena
Anales de Psicología
Fulgencio Marin
devki khanna
Journal of Eco-friendly Agriculture
Proceedings of the Australian Conference on Science and Mathematics Education
Rachel Wilson
Stratum plus, 4
Mastykova Anna
Brabha Nagaratnam
Applied Energy
Alhamra Jurnal Studi Islam
Mahfidhatul Khasanah
Giulia Gasparro
Journal for Research in Applied Sciences and Biotechnology
Abdullah Qadri
Faizan Zaidi
Anne Andermann
John Chatzis
Anais do X Brazilian Workshop on Social Network Analysis and Mining (BraSNAM 2021)
Jonatas Santos
Christian Rusli
Nigerian Journal of Medicine
nkeiruka ameh
Plant Pathology
herve Avenot
Corrosion Science
Zoran M Stevic
International Journal of Plant Genomics
Dr. SHAILESH KUMAR Yadav
Pangea. Revista de Red Académica Iberoamericana de Comunicación
Carmen López-Sánchez
Journal of Wildlife Diseases
Marcela Uhart
jhkghjf hfdgedfg
hjhggf jgffgd
Revista de la Sociedad Científica del Paraguay
Danilda Hufana-Duran
See More Documents Like This
RELATED TOPICS
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024
Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, generate accurate citations for free.
- Knowledge Base
- Starting the research process
- How to Write a Research Proposal | Examples & Templates
How to Write a Research Proposal | Examples & Templates
Published on October 12, 2022 by Shona McCombes and Tegan George. Revised on November 21, 2023.
A research proposal describes what you will investigate, why it’s important, and how you will conduct your research.
The format of a research proposal varies between fields, but most proposals will contain at least these elements:
Introduction
Literature review.
- Research design
Reference list
While the sections may vary, the overall objective is always the same. A research proposal serves as a blueprint and guide for your research plan, helping you get organized and feel confident in the path forward you choose to take.
Table of contents
Research proposal purpose, research proposal examples, research design and methods, contribution to knowledge, research schedule, other interesting articles, frequently asked questions about research proposals.
Academics often have to write research proposals to get funding for their projects. As a student, you might have to write a research proposal as part of a grad school application , or prior to starting your thesis or dissertation .
In addition to helping you figure out what your research can look like, a proposal can also serve to demonstrate why your project is worth pursuing to a funder, educational institution, or supervisor.
Research proposal length
The length of a research proposal can vary quite a bit. A bachelor’s or master’s thesis proposal can be just a few pages, while proposals for PhD dissertations or research funding are usually much longer and more detailed. Your supervisor can help you determine the best length for your work.
One trick to get started is to think of your proposal’s structure as a shorter version of your thesis or dissertation , only without the results , conclusion and discussion sections.
Download our research proposal template
Here's why students love Scribbr's proofreading services
Discover proofreading & editing
Writing a research proposal can be quite challenging, but a good starting point could be to look at some examples. We’ve included a few for you below.
- Example research proposal #1: “A Conceptual Framework for Scheduling Constraint Management”
- Example research proposal #2: “Medical Students as Mediators of Change in Tobacco Use”
Like your dissertation or thesis, the proposal will usually have a title page that includes:
- The proposed title of your project
- Your supervisor’s name
- Your institution and department
The first part of your proposal is the initial pitch for your project. Make sure it succinctly explains what you want to do and why.
Your introduction should:
- Introduce your topic
- Give necessary background and context
- Outline your problem statement and research questions
To guide your introduction , include information about:
- Who could have an interest in the topic (e.g., scientists, policymakers)
- How much is already known about the topic
- What is missing from this current knowledge
- What new insights your research will contribute
- Why you believe this research is worth doing
As you get started, it’s important to demonstrate that you’re familiar with the most important research on your topic. A strong literature review shows your reader that your project has a solid foundation in existing knowledge or theory. It also shows that you’re not simply repeating what other people have already done or said, but rather using existing research as a jumping-off point for your own.
In this section, share exactly how your project will contribute to ongoing conversations in the field by:
- Comparing and contrasting the main theories, methods, and debates
- Examining the strengths and weaknesses of different approaches
- Explaining how will you build on, challenge, or synthesize prior scholarship
Following the literature review, restate your main objectives . This brings the focus back to your own project. Next, your research design or methodology section will describe your overall approach, and the practical steps you will take to answer your research questions.
To finish your proposal on a strong note, explore the potential implications of your research for your field. Emphasize again what you aim to contribute and why it matters.
For example, your results might have implications for:
- Improving best practices
- Informing policymaking decisions
- Strengthening a theory or model
- Challenging popular or scientific beliefs
- Creating a basis for future research
Last but not least, your research proposal must include correct citations for every source you have used, compiled in a reference list . To create citations quickly and easily, you can use our free APA citation generator .
Some institutions or funders require a detailed timeline of the project, asking you to forecast what you will do at each stage and how long it may take. While not always required, be sure to check the requirements of your project.
Here’s an example schedule to help you get started. You can also download a template at the button below.
Download our research schedule template
If you are applying for research funding, chances are you will have to include a detailed budget. This shows your estimates of how much each part of your project will cost.
Make sure to check what type of costs the funding body will agree to cover. For each item, include:
- Cost : exactly how much money do you need?
- Justification : why is this cost necessary to complete the research?
- Source : how did you calculate the amount?
To determine your budget, think about:
- Travel costs : do you need to go somewhere to collect your data? How will you get there, and how much time will you need? What will you do there (e.g., interviews, archival research)?
- Materials : do you need access to any tools or technologies?
- Help : do you need to hire any research assistants for the project? What will they do, and how much will you pay them?
If you want to know more about the research process , methodology , research bias , or statistics , make sure to check out some of our other articles with explanations and examples.
Methodology
- Sampling methods
- Simple random sampling
- Stratified sampling
- Cluster sampling
- Likert scales
- Reproducibility
Statistics
- Null hypothesis
- Statistical power
- Probability distribution
- Effect size
- Poisson distribution
Research bias
- Optimism bias
- Cognitive bias
- Implicit bias
- Hawthorne effect
- Anchoring bias
- Explicit bias
Once you’ve decided on your research objectives , you need to explain them in your paper, at the end of your problem statement .
Keep your research objectives clear and concise, and use appropriate verbs to accurately convey the work that you will carry out for each one.
I will compare …
A research aim is a broad statement indicating the general purpose of your research project. It should appear in your introduction at the end of your problem statement , before your research objectives.
Research objectives are more specific than your research aim. They indicate the specific ways you’ll address the overarching aim.
A PhD, which is short for philosophiae doctor (doctor of philosophy in Latin), is the highest university degree that can be obtained. In a PhD, students spend 3–5 years writing a dissertation , which aims to make a significant, original contribution to current knowledge.
A PhD is intended to prepare students for a career as a researcher, whether that be in academia, the public sector, or the private sector.
A master’s is a 1- or 2-year graduate degree that can prepare you for a variety of careers.
All master’s involve graduate-level coursework. Some are research-intensive and intend to prepare students for further study in a PhD; these usually require their students to write a master’s thesis . Others focus on professional training for a specific career.
Critical thinking refers to the ability to evaluate information and to be aware of biases or assumptions, including your own.
Like information literacy , it involves evaluating arguments, identifying and solving problems in an objective and systematic way, and clearly communicating your ideas.
The best way to remember the difference between a research plan and a research proposal is that they have fundamentally different audiences. A research plan helps you, the researcher, organize your thoughts. On the other hand, a dissertation proposal or research proposal aims to convince others (e.g., a supervisor, a funding body, or a dissertation committee) that your research topic is relevant and worthy of being conducted.
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the “Cite this Scribbr article” button to automatically add the citation to our free Citation Generator.
McCombes, S. & George, T. (2023, November 21). How to Write a Research Proposal | Examples & Templates. Scribbr. Retrieved March 21, 2024, from https://www.scribbr.com/research-process/research-proposal/
Is this article helpful?
Shona McCombes
Other students also liked, how to write a problem statement | guide & examples, writing strong research questions | criteria & examples, how to write a literature review | guide, examples, & templates, "i thought ai proofreading was useless but..".
I've been using Scribbr for years now and I know it's a service that won't disappoint. It does a good job spotting mistakes”
Custom Essay, Term Paper & Research paper writing services
- testimonials
Toll Free: +1 (888) 354-4744
Email: [email protected]
Writing custom essays & research papers since 2008
Medical research paper topics: ideas for students.
When pursuing medical studies, students are required to write research papers on different medical topics. It’s not surprising that medical research paper topics are among the most searched phrases by students online. Perhaps, that’s because the grades of students in medical schools are largely dependent on their performance on research papers. The choice of a topic on medical science varies depending on the specific subject or area of study or interest.
But, whether it’s medical conditions or alternative medicine, the goal is generally to choose a topic that will grab the reader’s attention and compel them to read further. Medical research is very important because it enhances innovation and analysis. It also promotes a better understanding of the profession by students.
Nevertheless, developing or choosing medical research paper topics is sometimes challenging than the actual writing task. In some cases, professors provide a scope within which to choose topics. What’s more, there are different categories of interesting medical topics for research paper for students to choose from. These are some of the things that make choosing the topics to write about challenging for most students.
Best Medical Topics for Research Paper
Medical school professors are knowledgeable about most medical topics. Their reason for asking students to write research papers is to check their understanding and knowledge of the taught material. They also assign students writing tasks to stimulate their creative thinking ability. Nevertheless, the choice of a topic can affect the ability of a student to write a quality paper.
The best topic should be narrow and specific. However, it should enable the writer to present and discuss different issues. The topic should also be captivating enough to capture the reader’s attention. But most importantly, it should be a topic you are interested in.
Categories of Medical Research Paper Topics for College Students
Research topics for medical papers fall into different categories. When choosing the topic to write about, therefore, students should consider these categories. Here are the major categories of medical topics for writing research papers.
Controversial Medical Topics for Research Paper
University and college professors want students to tackle existing controversies in the medical field (for instance, regarding the problem of abortion ) by analyzing the published evidence and then drawing conclusions based on the investigated evidence. Here are examples of medical controversial topics for research paper. All of them are quite complicated, so you may need to ask professional writers for help.
- Is Cloning a Complication or a Solution?
- Does Cloning amount to Trespassing on a Divine Territory?
- Is there a Room for Medical Mistakes when it comes to Euthanasia?
- Can Death Penalty Be Executed in a Humane Way?
- Where is the Line Drawn Between Societal Security and Right to Privacy?
- Can a Person be compelled to Donate Organs Under any Condition?
- Is It Acceptable for Parents to be allowed to Influence the Future of their Children by Predetermining their Hair Color or Sex?
- Who Should Decide to Donate a Dead Person’s Organ?
- Is Prenatal Illness a Justifiable Ground for Parents and Health Workers to opt for Abortion?
- Is Being a Vegetarian a Healthy Option?
- Is Anti-Smoking an Effective Campaign from a Medical Viewpoint?
Some controversial topics can also be considered medical ethics paper topics. That’s because they also touch on medical ethics.
Argumentative Health Research Paper Topics
Argumentative research topics enable students to get a wider outlook on different medical problems. It’s also by conducting research and writing on these topics that students make informed decisions and take positions regarding them. Here are examples of argumentative health-related topics for research paper.
- Why it’s Important to Involve Trained Dogs in Neurological Patients Treatment
- How Breastfeeding Affects the Future Development of a Child
- Why it’s Important to Increase the Awareness of the Public about Bipolar Disorder
- Social Factors that Affect Schizophrenia Development and Eventual Progression
- What is the Family Role in Sleeping Disorders’ Treatment?
- How Does Formula Feeding Relate to Early Child Obesity?
- What’s the Role of Physical Exercises in Heart-Related Diseases Prevention?
- How Does Stress Affect Health Development?
- What’s the New Problem Created by Electronic Cigarettes?
- Should Eating Disorders be Considered Medical Illnesses?
- Is Shifting Scientific Research towards Adult Stem Cells instead of Embryonic Stem Cells Critical?
Medical History Topics
In most cases, medical history topics try to address issues that have existed for a while. Some of them can be medical ethics research paper topics trying to address existing ethical issues. There are also cases where students are allowed to come up with topics. Here are examples of medical history topics for research papers.
- Doctors in Totalitarian Regimes Service- Murderers or System Victims?
- Frequently Abused Drugs Application in Medical Practice
- Hygiene Introduction into Medical Practice- A Revolutionary Development
- History of Modern Psychiatry Development from Radical Surgery to Fine Medicines that Have Minimal Side Effects
- Historical Alterations of Nurses Roles, Responsibilities, and Duties
- History of Osteopathic Medicine Development
- History of Medical Testing Using Living Creatures Effects of Pandemic Diseases on Human Society and Medicine Development
- History of X-Ray Photography and its Effectiveness in the Diagnosis of the Pulmonary Disease
- Ultrasonic Research History
Medical Sociology Topics
Most public health topics for research paper fall into this category. Sociology is a more specialized branch of medical sciences. However, you may still want to write your paper on a topic in this category. Here are examples of sociology medical topics.
- What’s the Role of the Government in the Health of its Citizens from a Statistical Standpoint?
- Why is Centralized Effort Important for Public Health?
- Social Factors Involved in Euthanasia Legalization
- How popular are Unconventional Medical Practices?
- How Sociological Data Influences Nursing Practices
- The Effect of Medical Factors Like Sociological Data
- Statistical Evidence Against or Supporting the Relationship between Weight Control and Physical Exercises
- How Traditional Chinese Medicine Influences Modern Medical Practices
Some of these subjects are ideal health care policy paper topics. You can also use them as your inspiration to formulate your topics.
Current and Recent Events Medical Research Topics
The latest events related to the medical field can also be an inspiration for formulating research topics. For instance, increased alcohol and drug abuse in contemporary society have led to more cases of mental health problems. This trend can be an inspiration for formulating mental health research paper topics. Here are examples of interesting medical research topics related to current or recent events.
- Why the Modern Lifestyle is to Blame for the Increasing Cancer Cases
- How Technology is Increasing Autism Cases
- Could Modern Diseases Be a Result of Science Negligence?
- The Growing Concern Over the Increasing Cases of Insulin Resistance
- Psychological Consequences of Prolonged Use of Mobile Phone
- How the growing Refugees Number is Affecting the Public Health Sector
- Rising Terrorism and Mental Health
- Health Costs and Risk of Social Anxiety
- Should the Society Turn to Alternative Medicine?
- Should the World Open an Organ Market?
- Is the Focus on HIV/AIDS Dying?
Whether you choose medical history or medical ethics research paper topics, it’s important to ensure that you can support your argument with authoritative facts. Therefore, choose a topic for which you can find credible sources of information and medical facts. Don’t forget to avoid confusing or misleading your readers when writing your paper.
The Bottom Line
The most interesting medical topics address issues that affect society. Therefore, follow the news, read medical publications, and consider your interests when choosing a topic for your medical research paper. This will surely give you topic ideas for your medical research paper.
Find it hard to cope with your college paper? Great news! Use promo “ mypaper20 ” and enjoy 20% discount on a medical writing assignment from our profs!

Featured Clinical Reviews
- Screening for Atrial Fibrillation: US Preventive Services Task Force Recommendation Statement JAMA Recommendation Statement January 25, 2022
- Evaluating the Patient With a Pulmonary Nodule: A Review JAMA Review January 18, 2022
Select Your Interests
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
- Download PDF
- Share X Facebook Email LinkedIn
- Permissions
Reporting Use of AI in Research and Scholarly Publication—JAMA Network Guidance
- 1 Executive Managing Editor, JAMA and JAMA Network
- 2 Associate Editor, JAMA
- 3 University of California, San Francisco
- 4 Yale School of Medicine, New Haven, Connecticut
- 5 JAMA and JAMA Network
- 6 Editor in Chief, JAMA and JAMA Network
- Editorial Guidance for Authors, Peer Reviewers, and Editors on Use of AI Annette Flanagin, RN, MA; Jacob Kendall-Taylor, BA; Kirsten Bibbins-Domingo, PhD, MD, MAS JAMA
- Editorial JAMA Call for Papers for AI in Medicine Rohan Khera, MD, MS; Atul J. Butte, MD, PhD; Michael Berkwits, MD, MSCE; Yulin Hswen, ScD, MPH; Annette Flanagin, RN, MA; Hannah Park; Gregory Curfman, MD; Kirsten Bibbins-Domingo, PhD, MD, MAS JAMA
Reports on the use of artificial intelligence (AI) and machine learning, including large language models (LLM), in medical research have intensified in the last year. Although machine learning research began 70 years ago with the conceptual development of artificial neural network algorithms, 1 AI research and use in clinical practice and health care are relatively recent advances. Throughout these developments, JAMA has sought to define the broad scope of discovery and innovation in medical applications of AI and to address potential challenges in its implementation. The journal’s 2016 publication of a study of deep learning algorithms for the detection of diabetic retinopathy from fundal photographs is a useful example. 2 The study represented a novel tool that could enable large-scale screening for a key vision-threatening disorder across the world. However, the accompanying Editorial 3 highlighted important challenges, spanning the need for broader patient representativeness, the investment necessary in validating the model in the context of its deployment and subsequent implementation, and whether clinicians would entrust decision-making to AI tools. The Editorial also called attention to concerns regarding AI eventually replacing humans in clinical systems. Although published before the recent AI boom, 4 this study and the comments about it augured many of today’s promises and concerns regarding AI in clinical research and practice. 5 - 11
Over the past decade, advances in AI have enabled many innovations that facilitate rapid research and resulted in tools for disease prediction, diagnosis, and prognostication. During the last year, JAMA provided guidance to authors and peer reviewers on the transparent, appropriate, and accountable use of AI. 12 , 13 Herein, we provide more detailed recommendations for authors and researchers. Editors and readers rely on authors to report their use of AI and to be fully accountable for such use. Transparency is crucial in employing AI technologies given its rapid development and yet-unknown functionalities. The guidance below is general and based on some common problems we have seen in submitted manuscripts; reporting guidelines will provide more specific guidance. This information has been added to the Instructions for Authors for JAMA and the JAMA Network journals. 14
AI Used in Manuscript Preparation
When traditional and generative AI technologies are used to create, review, revise, or edit any of the content in a manuscript, authors should report in the Acknowledgment section the following:
Name of the AI software platform, program, or tool
Version and extension numbers
Manufacturer
Date(s) of use
A brief description of how the AI was used and on what portions of the manuscript or content
Confirmation that the author(s) takes responsibility for the integrity of the content generated
Note that this guidance does not apply to basic tools for checking grammar, spelling, references, and similar.
AI Used in Research
When AI (eg, LLM or natural language processing [NLP], supervised or unsupervised machine learning for predictive/prescriptive or clustering tasks, chatbots, or similar technologies) is used as part of a scientific study, authors should
Follow relevant reporting guidelines for specific study designs when they exist (see examples in the Box ) and report each recommended guideline element with sufficient detail to enable reproducibility. 15 - 25
Avoid inclusion of identifiable patient information in text, tables, and figures.
Be aware of copyright and intellectual property concerns if including content (text, images) generated by AI, and indicate rights or permissions to publish that content as determined by the AI service or owner.
Also address the following:
Examples of AI-Related Reporting Guidelines
CONSORT-AI for clinical trial reports evaluating interventions with an AI component 15
SPIRIT-AI for clinical trial protocols evaluating interventions with an AI component 16
MI-CLAIM for studies including clinical AI modeling 17
CLAIM for studies describing applications of AI in medical imaging 18
MINIMAR (MINimumInformation for Medical AIReporting) for studies of AI in health care 19
DECIDE-AI for studies describing the early-stage live clinical evaluation of AI-based decision support systems 20
Recommendations for Reporting Machine Learning Analyses in Clinical Research for studies of machine learning analyses 21
Other AI reporting extensions and guidelines (under development):
STARD-AI for AI-centered diagnostic test accuracy studies 22
TRIPOD-AI for prediction model studies based on machine learning techniques 23
PROBAST-AI for risk of bias assessment of machine learning–based prediction model studies 23
CANGARU for ethical use, disclosure, and reporting of AI in scholarly publication 24
CHART for studies assessing use of chatbots and LLMs for health information 25
Methods Section
Include the study design and, if a relevant reporting guideline exists ( Box ), indicate how it was followed, with sufficient detail to enable reproducibility.
Describe how AI was used for specific aspects of the study (eg, to generate or refine study hypotheses, assist in the generation of a list of adjustment variables, create graphs to show visual relationships).
For studies using LLMs, provide the name of the platform or program, tool, version, and manufacturer; specify dates and prompt(s) used and their sequence and any revisions to prompts in response to initial outputs.
For studies reporting machine learning and algorithm development, include details about datasets used for development, training, and validation. Clearly state if algorithms were trained and tested only on previously collected or existing data sets or if the study includes prospective deployment. Include the machine learning model and describe the variables and outcome(s) and selection of the fine-tuning parameters. Describe any assumptions involved (eg, log linearity, proportionality) and how these assumptions were tested.
Indicate the metric used to evaluate the performance of the algorithms, including bias, discrimination, calibration, reclassification, and others as appropriate.
Indicate the methods used to address missing data.
Indicate institutional review board/ethics review, approval, waiver, or exemption.
Describe methods or analyses included to address and manage AI-related methodologic bias and inaccuracy of AI-generated content.
Indicate, when appropriate, if sensitivity analyses were performed to explore the performance of the AI model in vulnerable or underrepresented subgroups.
Provide a data sharing statement, including if code will be shared. 26
Results Section
When reporting comparisons, provide performance assessments (eg, against standard of care), include effect sizes and measures of uncertainty (eg, 95% CIs) and other measurements such as likelihood ratios, and include information about performance errors, inaccurate or missing data, and sufficient detail for others to reproduce the findings.
Report the results of analyses to address methodologic bias and population representation.
If examples of generated text or content are included in tables or figures, be sure to indicate the source and licensing information, as noted above.
Discussion Section
Discuss the potential for AI-related bias and what was done to identify and mitigate such bias.
Discuss the potential for inaccuracy of AI-generated content and what was done to identify and manage this.
Discuss generalizability of findings across populations and results of analyses performed to explore the performance of the AI model in vulnerable or underrepresented subgroups.
Conclusions
We welcome feedback on these recommendations. We will continue to monitor reporting guideline development and update these recommendations and our Instructions for Authors with the aim of providing authors, researchers, reviewers, and editors with information to help guide the transparent and accountable reporting of use of AI in research and publication.
Corresponding Author: Annette Flanagin, RN, MA, JAMA and JAMA Network ( [email protected] ).
Published Online: March 7, 2024. doi:10.1001/jama.2024.3471
Conflict of Interest Disclosures: Dr Khera reported receiving grants from NHLBI, Doris Duke Charitable Foundation, Bristol Myers Squibb, and Novo Nordisk, and serving as cofounder of Evidence2Health and Ensight-AI, outside the submitted work. Dr Pirracchio reported receiving personal fees from Philips and AOP outside the submitted work. No other disclosures were reported.
See More About
Flanagin A , Pirracchio R , Khera R , Berkwits M , Hswen Y , Bibbins-Domingo K. Reporting Use of AI in Research and Scholarly Publication—JAMA Network Guidance. JAMA. Published online March 07, 2024. doi:10.1001/jama.2024.3471
Manage citations:
© 2024
Artificial Intelligence Resource Center
Cardiology in JAMA : Read the Latest
Browse and subscribe to JAMA Network podcasts!
Others Also Liked
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
Increased Suicide in Emergency Departments Research Paper
Introduction.
The topic of this study is the prevention of suicide attempts leading to the death of patients and medical personnel in emergency department. The main concern of the study is the fact that patient or staff suicide has a high chance of occurring in the emergency department, and effective measures must be taken to prevent such cases. The emergency department must comply with all safety measures, including for people with mental health problems. However, suicide can occur due to a chaotic environment, high workload, confirmation of the diagnosis, and lack of care.
Providing timely intervention and protection for patients and staff exhibiting behavioral despair is a challenge in the emergency department due to a shortage of staff and supplies. The main goal of this study is to develop a system for immediate response and suicide prevention in the emergency department. To develop a system, it is necessary to find ways to recognize early signs of suicidal behavior. The latest statistical data and theoretical literature are used to confirm the significance and relevance of the study.
Theoretical Base Justification
Research needs to focus on a theoretical framework that provides statistical and descriptive data regarding suicide attempts in emergency departments. It is necessary to rely on existing approaches in assessing the suicidality of individual groups of people and the overall indicator. These data are required to develop appropriate scoring models to reduce the level of suicidal risk. The theoretical basis for communication with individuals who are at risk of suicidal behavior is necessary to justify the measures to reduce the number of suicides, providing an understanding of the reasons for such cases.
A separate body of the studied literature concerns the specifics of suicidal behavior among medical personnel in the United States. One concept for further study is post-traumatic stress disorder, described in the theoretical literature. Turning to such a source will allow the research to rely on the possible prerequisites for suicidal behavior in more detail. Literature on the management of suicidal tendencies is needed to develop a future strategy for help and prevention. It is necessary to draw on the literature on suicide and mental illness among physicians, including the emergency department. From such studies, it is possible to trace the main risk factors for an increase in suicides and develop a strategy for leveling them. It is worth emphasizing that the work used both general studies of suicidal behavior and private ones, concerning high risks of suicides in emergency departments.
Significance and Relevance of the Problem
Suicide is one of the most common causes of death in the United States, and therefore is a global problem that requires special attention. A lot of research has been devoted to reducing suicidal risks, but the specificity of suicides in the emergency department has not yet been clearly and precisely defined. The research problem is relevant, since the topic of suicide is extremely important, and the protection of patients and doctors should be a main task. The emergency room should be the safest place possible, and identifying the causes of increased risk and finding ways to resolve them should be a priority. The study will make a significant contribution to the practice of patient care, as it will allow the development of a rapid response system to ensure the safety of clients. The development of a system for identifying and reducing risks will be an additional layer of protection. The study will develop an effective system that promotes the safety of doctors. In this way, public health will become safer for all parties involved.
Literature Review
Basic concepts.
The main concepts of the studied literature include the risks of developing traumatic and suicidal behavior in patients and doctors, the specifics of the dynamics in the emergency department, and the methodology of work and possible solutions to this problem. All researchers recognize the high risks of increased suicidal behavior. For patients, the risks may be associated with stress and adverse news, or a severe course of illness. The risk of suicide in doctors increases due to high workload, high responsibility, stress and reduced motivation. Young and old people are most at risk of forming suicidal behavior. The problem requires immediate intervention and the development of a concept for patients and medical staff protection.
Generalization on the Subject of Suicide
Medical staff and patients in emergency departments have a high rate of suicidal behavior associated with a complex and multifactorial phenomenon. A study by Canner et al. (2018) attempts to recognize the causes of the high number of suicide attempts resulting in the death of patients and staff in emergency departments and to propose preventive solutions. Young people aged 15–19 were the most affected by age demographics, while women were the most affected in terms of gender (Canner et al., 2018). Older people may be at a separate risk group due to a greater number of comorbidities, affecting the moral state.
Emergency Department
Researchers highlight the seriousness of the problem of increased suicidal risk in the emergency department. Individuals presenting to the emergency department show symptoms of intentional self-harm and suicidal behavior. A study by Canner et al. (2018), using samples from the National Emergency Management Services (NEDS), found that between 2006 and 2013, there were 3,567,084 suicide attempts associated with emergency room visits. The results of the study inform of the severity of emergency department-related self-harm among patients who have been attending hospitals over the years (Canner et al., 2018). A further research question should concern causative factors and indicators of suicide among patients who have been in the emergency department.
The main risk group among patients are the elderly and young people. Older people are more receptive to changing situations due to greater somatic receptivity. Costanza et al. (2020) describe why older people visiting emergency departments are a high-risk factor for suicide based on enrollment cohorts. Psychiatric comorbidities are common among the elderly and play a significant role (Costanza et al., 2020). The high cost of emergency hospitalization causes depression and suicidal thoughts in older patients. Costanza et al. (2019) shows that the elderly are at high risk due to comorbidities and existing health complications. Drawing on the literature, the study will propose technical and policy solutions for managing the prevalence of suicide among patients following emergency department visits.
Medical Professionals
Studies have shown that not only patients visiting emergency rooms are at high suicidal risk. Stehman et al. (2019) found that emergency physicians are at the highest risk of suicide among healthcare workers. Burnout is a major cause of other psychological problems that lead to suicide. Intolerance for errors in emergency departments, irregular work hours, and duty expose emergency personnel to high rates of burnout. Although burnout is a psychological syndrome, it is not the only risk factor for suicidal thoughts in emergency room staff.
Determining the factors behind high suicide rates among emergency room workers will highlight the administrative and environmental aspects that lead to high suicide rates. An evidence-based review by Harvey et al. (2019) highlights that physicians’ unregulated work environment, workload, support organizations, and mental well-being have led to an increase in reports of suicide. Harvey et al. (2019) provide an overview of comprehensive aspects of the physician workplace, ethical and mental stability, ranging from training to organizational level interventions. DeLucia et al. (2019) found that suicide rates among healthcare workers are affected by workplace stressors and traumatic events. Emergency medical workers deal with critically injured patients, which can be highly psychologically traumatic experience.
Research Context, Venue and Methodology
The basis of research on the topic of suicidal risks among patients and doctors in the emergency department is statistics. Canner et al. (2018) and Costanza et al. (2020) provide statistics on the prevalence of suicide among patients leaving the emergency department and the underlying causes. The researches highlight possible ways to reduce the existing problem. Using the virtual collaborative suicide assessment and management system is a viable tool to promote technology-assisted care to reduce suicide rates among patients and emergency department staff (Dimeff et al., 2020). Both studies by Dimeff et al. (2020) and DeLucia et al. (2019) concluded that screening for symptoms of suicidal behavior and getting to the emergency room contributes to thoughts of self-harm among patients. All of the studies take place in the emergency department and report the same high suicidal risk. The methodology includes mainly quantitative studies to identify the causes of suicide and increased risk. Likewise, further research will rely on statistical data to identify possible solutions. An effective tool is the satisfaction survey of patients and medical employees.
Design and Methodology
Methodology.
To conduct the research, a clear methodology is needed to defend the general concepts of the proposed study to reduce suicidal risks. Firstly, the study involves the use of a quantitative method. It is proposed to compile and conduct a survey on suicidal risks among patients and medical personnel of the emergency department. This method will identify the real causes and gradually eliminate them. It is necessary to record the number of people in a depressed emotional state and analyze the reasons for such conditions.
Method of Data Collection and Analysis
The method of data collection is a questionnaire survey of patients and professionals of the emergency department. Additional psychological testing, such as the Beck Depression Scale, is suggested to determine whether there are suicidal risks associated with depression. Psychological testing will be preceded in a format of a questionnaire survey. Questionnaires will be different for patients and physicians in an emergency department. The list of questions for doctors is proposed to include:
- Do you experience stress associated with working in the emergency department?
- What motivates you to continue working?
- Have you ever thought about leaving this job?
- What do you think is the main reason for your stress in the workplace?
- Do you feel that you are experiencing professional burnout?
Questions for the patients are proposed to be slightly different:
- Are you satisfied with the level of attention provided in the emergency department?
- Do you experience stress from being in an emergency care unit?
- Do you see an increase in anxiety levels before and after being admitted to the hospital?
- Do you think your stress level will decrease after living the emergency department?
- What specifically might cause you anxiety when you arrive at the hospital?
The analysis of the obtained results is supposed to be interpreted in quantitative terms. The number of positive and negative responses will be obtained to determine if there is a general trend. Interpretation will also be given in the qualitative response: it is expected that patients and doctors will answer questions about what causes stress in the emergency department. In this way, the main factors that can create suicidal risks will be identified. The result of psychological testing will be obtained in quantitative terms, it will be possible to identify the average risk of depression among both patients and doctors.
Method Justification
The main principle of determining the method for the study is to protect the participants of the survey from undesirable consequences. Questionnaires can provoke participants to suicidal thoughts and reflect on their experience in a negative way. That is the reason why the questionnaire does not directly specify whether participants have suicidal thoughts. Therefore, it is proposed to use the Beck Scale, aimed at identifying depression and not directly asking about thoughts that increase suicidal risks. This technique simultaneously helps protect participants and obtain research-relevant data.
Research Design
Conditions, sampling, and defining variables.
The main variable for the study is the satisfaction of emergency department staff and patients. The level of satisfaction is planned to be measured in correlation with the stress experienced, both in the case of patients and staff. The sample will consist of long-term working doctors and patients at risk. The risk group includes young people under 30 years and elderly patients with concomitant diseases that can have a depressing effect on the psychological state. The sample should be at least 10 people for each category, 20 patients and doctors respectively. Tests for physicians and patients should differ significantly while maintaining a common focus. The questionnaires of doctors will include questions about professional duty, the level of pressure, the ability to cope with responsibility. Patients will be asked about their experience of being in an emergency care unit. The subjects are invited to undergo psychological testing aimed at identifying depressive disorders. Such tests will show how the risks differ, whether there are common features between patients and doctors, what are the main causes of this condition.
Obtaining and Forming Informed Consent
For any medical research involving participants, the informed consent of all those involved is required. Informed consent should be obtained immediately prior to any manipulation. The consent procedure will include a description of the goals and objectives of the study, the requirements for participants, and the benefits derived from the experiment. The informed consent form should reflect the items contained in the participant information that are important to investigators. Items on the informed consent form begin with the participant’s name. When asking a participant for permission to use specific data, this item should be reflected in the text of the Informed Consent Form. The text should contain information about signing two copies of the document.
Informed Consent Form
For the participant of the scientific pilot study “Increased Suicide in Emergency Departments”
- I have read and understood the information provided in this document about this medical research study. I had enough time to decision making; the supervisors in this study explained unclear information to me and fully answered all my questions.
- I hereby certify that I have been fully informed of the risks and potential benefits of my participation in the study.
- I voluntarily agree to participate in this study without any pressure.
- I authorize the researcher and/or research team to process my medical data for research purposes, as well as the transfer of this data in an anonymized form.
- I agree to make my medical records available to official authorized persons, subject to the rules of confidentiality of personal data.
- I have been provided with a signed and dated copy of the Participant Information Sheet and Informed Consent Form for participation in the study. I understand that the record of my consent to participate in the study will be kept in medical records.
Reliability
The study will be trustable because it relies on a sufficient ranking of participants. A broad theoretical base allows the research to state that this technique is reliable for obtaining relevant results. Data will be collected directly from people in emergency department conditions. This will clearly identify the reasons why there is an increased risk of suicidal behavior among medical staff and patients. Subsequently, this will help the development of a clear intervention strategy that will be based on data from real people experiencing relevant cases.
Limitations
The study has limitations based on the subjectivity of the questionnaire data obtained. The varying levels of emotional resilience and ability to cope with stress in participants may underestimate the resulting overall level of suicidal risk. Participants may be afraid to openly reflect on such topics, which provokes people involved not to be completely honest. The number of participants may be too small to get a wide range of results.
Ethical Considerations
Every research, for ethical reasons, should aim not only to obtain relevant results, but to ensure the safety of research participants. Therefore, before questioning and psychological testing, it is necessary to fully identify the risks of participating in the study. Participants should be clearly explained that testing may be associated with an increased risk of suicidal behavior. To comply with ethical standards, it is necessary to ensure the complete anonymity of participants. Anonymity will allow participants to feel safe and be more open about personal questions. The results of the research must be used solely for medical purposes and for the benefit of the community.
Suicidal behavior among patients and physicians in emergency department is a serious problem requiring intervention. To address this problem, it is proposed to conduct a study based on a survey of those involved in the functioning of the emergency department. The questionnaire will be aimed at identifying common suicidal risks, as well as the reasons that may cause such thoughts and actions. For more relevant research results, participants in the experiment will take the Beck Depression Scale, which will show a quantitative result. Once the actual level of suicidal risk and possible causes have been identified, a strategy is needed to address this problem.
Canner, J. K., Giuliano, K., Selvarajah, S., Hammond, E. R., & Schneider, E. B. (2018). Emergency department visits for attempted suicide and self-harm in the USA: 2006–2013 . Epidemiology and psychiatric sciences , 27 (1), 94-102. Web.
Costanza, A., Amerio, A., Radomska, M., Ambrosetti, J., Di Marco, S., Prelati, M., Aguglia, A., Serafini, G., Amore, M., Bondolfi G. & Pompili, M. (2020). Suicidality assessment of the elderly with physical illness in the emergency department . Frontiers in psychiatry , 11 , 558974. Web.
DeLucia, J. A., Bitter, C., Fitzgerald, J., Greenberg, M., Dalwari, P., & Buchanan, P. (2019). Prevalence of post-traumatic stress disorder in emergency physicians in the United States . Western journal of emergency medicine , 20 (5), 740. Web.
Dimeff, L. A., Jobes, D. A., Chalker, S. A., Piehl, B. M., Duvivier, L. L., Lok, B. C., & Koerner, K. (2020). A novel engagement of suicidality in the emergency department: Virtual Collaborative Assessment and Management of Suicidality. General hospital psychiatry , 63 , 119-126.
Harvey, S. B., Epstein, R. M., Glozier, N., Petrie, K., Strudwick, J., Gayed, A., Dean, K. & Henderson, M. (2021). Mental illness and suicide among physicians . The Lancet , 398 (10303), 920-930. Web.
Stehman, C. R., Testo, Z., Gershaw, R. S., & Kellogg, A. R. (2019). Burnout, drop out, suicide: physician loss in emergency medicine, part I . Western Journal of Emergency Medicine , 20 (3), 485. Web.
- Chicago (A-D)
- Chicago (N-B)
IvyPanda. (2024, March 23). Increased Suicide in Emergency Departments. https://ivypanda.com/essays/increased-suicide-in-emergency-departments/
"Increased Suicide in Emergency Departments." IvyPanda , 23 Mar. 2024, ivypanda.com/essays/increased-suicide-in-emergency-departments/.
IvyPanda . (2024) 'Increased Suicide in Emergency Departments'. 23 March.
IvyPanda . 2024. "Increased Suicide in Emergency Departments." March 23, 2024. https://ivypanda.com/essays/increased-suicide-in-emergency-departments/.
1. IvyPanda . "Increased Suicide in Emergency Departments." March 23, 2024. https://ivypanda.com/essays/increased-suicide-in-emergency-departments/.
Bibliography
IvyPanda . "Increased Suicide in Emergency Departments." March 23, 2024. https://ivypanda.com/essays/increased-suicide-in-emergency-departments/.
- Suicidal Behavior: Triggers and Solutions
- Suicidal Ideation as Ethical Dilemma in Nursing
- Instruments Measuring Suicidal Ideation
- Suicide, Suicidal Ideations and Suicide Attempts
- Suicidal Ideations and Public Health Interventions
- Suicide Education and Prevention
- The Suicide Warning Signs List
- Children and Adolescent Suicide Behavior
- Suicide: Theories, Dynamism, and Assessment
- Suicide and Social Influences on Behavior
- Trauma Treatment Planning for a Youngster
- Psychiatry: Somatic Symptom Disorder
- Unreliability of Biological Evidence for Psychiatry
- Dynamic Group Psychotherapy and Its Benefits for Depression
- Depression Assessment Report and Interpretation of Results
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- GMS J Med Educ
- v.34(1); 2017

Language: English | German
Accepted standards on how to give a Medical Research Presentation: a systematic review of expert opinion papers
Anerkannte standards zum halten medizinischer vorträge: eine systematische Übersicht publizierter experteneinschätzungen, christine blome.
1 University Medical Center Hamburg-Eppendorf (UKE), Institute for Health Services Research in Dermatology and Nursing (IVDP), German Center for Health Services Research in Dermatology (CVderm), Hamburg, Germany
Hanno Sondermann
Matthias augustin.
Background: This systematic review aimed to extract recommendations from expert opinion articles on how to give a medical research presentation on a scientific conference and to determine whether the experts agree on what makes an effective or poor presentation.
Methods: Presentation-related terms were searched within article titles listed in PubMed, restricting the search to English-language articles published from January 1975 to July 2015. Recommendations were extracted from the articles, grouped by content, and analyzed for frequency. Ninety-one articles were included. Among 679 different recommendations, 29 were given in more than 20% of articles each. The five most frequent recommendations were to keep slides simple, adjust the talk to the audience, rehearse, not read the talk from slides or a manuscript, and make eye contact.
Results: No article gave advice that was the complete opposite of the 29 most frequent recommendations with the exception of whether a light or dark background should be used for slides.
Conclusions: Researchers should comply with these widely accepted standards to be perceived as effective presenters.
Zusammenfassung
Hintergrund: Ziel dieser systematischen Übersichtsarbeit war es, aus publizierten Expertenstellungnahmen Empfehlungen zur Vorgehensweise bei medizinischen Präsentationen auf wissenschaftlichen Fachtagungen zu extrahieren und abzuleiten, ob Experten in der Frage übereinstimmen, was eine gute oder schlechte Präsentation ausmacht.
Methoden: Präsentationsbezogene Schlagwörter wurden in den Titeln englischsprachiger, in PubMed geführter und zwischen Januar 1975 und Juli 2015 erschienener Artikel gesucht. Aus den gefundenen Expertenartikeln wurden Empfehlungen extrahiert, inhaltlich gruppiert und nach Häufigkeit ausgewertet. Einundneunzig Artikel wurden eingeschlossen. Von insgesamt 679 unterschiedlichen Empfehlungen fanden sich 29 jeweils in mindestens 20% der Artikel. Die fünf häufigsten Empfehlungen lauteten: Einfache Folien verwenden; die Zuhörerschaft kennen; Augenkontakt halten; die Präsentation üben; nicht von Folien oder Manuskript ablesen.
Ergebnisse: In keinem Artikel wurde eine Empfehlung gegeben, die das klare Gegenteil einer der 29 häufigsten Empfehlungen darstellten, bis auf die Frage, ob ein heller oder dunkler Folienhintergrund verwendet werden sollte.
Schlussfolgerung: Wissenschaftler sollten sich an die hier gefundenen, weithin akzeptierten Empfehlungen halten, damit ihre Präsentationen positiv wahrgenommen werden.
1. Introduction
Some presentations at medical conferences are easy to follow, engaging, and even inspire changes in the way patients are treated or scientific work is conducted. Conversely, others induce the audience to check their mobile phones or take a nap because they are so difficult to concentrate on.
What exactly makes great medical research presentations great? Empirical or even experimental data on this question are scarce [ 1 ], [ 2 ], [ 3 ], [ 4 ]. However, more than 80 authors of expert opinion articles have described what they believe a medical presenter should or should not do. The aim of this review was to extract all recommendations from these articles and determine whether the experts agree on what makes a medical research presentation either effective or poor.
Parts of this study were obtained from a previous dissertation by Sondermann, 2014 [ 5 ].
Presentation-related terms were searched within the titles of articles listed in PubMed, restricting the search to English-language articles published from January 1975 to July 2015. The search terms were:
(scientific[ti] AND presentation*[ti]) OR (conference[ti] AND presentation*[ti]) OR (oral[ti] AND presentation*[ti]) OR (research[ti] AND presentation*[ti]) OR (scientific[ti] AND meeting*[ti]) OR (public[ti] AND speaking[ti]) OR (public[ti] AND speech[ti]) OR (Power[ti] AND Point[ti]) OR PowerPoint[ti] OR (scientific[ti] AND talk*[ti]) OR lecturing[ti] OR lectures[ti] OR (scientific[ti] AND conference*[ti]) OR (medical[ti] AND presentation*[ti]) OR (paper[ti] AND presentation*[ti]) AND "1975/01/01"[PDAT]:"2015/07/31"[PDAT] AND English[lang]
The bibliographies of eligible articles were reviewed for further references.
We included expert opinion articles and editorials that provided advice on how to give a medical research presentation at scientific conferences. We excluded articles exclusively referring to lectures to students, continued medical education, or health care management.
Recommendations were extracted from each article, including both direct (e.g., “You should…”) and indirect recommendations (e.g., “Remember the audience’s time (…) should not be abused by presentation of uninteresting preliminary material” [ 6 ]). Mere suggestions were not extracted; these were typically signaled by words such as “consider.” We also excluded recommendations on abstract writing, use of outdated technology (e.g., diapositives), radiologic images (for being too specific), and technical aspects (e.g., choice of software).
Differently worded advice from two authors was regarded as the same recommendation if equal in content (e.g., “initially, rehearse alone” [ 7 ] and “initially, practice the talk alone” [ 8 ]). Similar recommendations were grouped into more general but still concrete advice. For example, “limit the number of lines on a slide to six” [ 9 ] and “no more than seven lines per slide” [ 10 ] were grouped into “limit the number of lines per slide.” Finally, we determined the frequency of recommendations, counting those given in two articles by the same first author only once.
The PubMed search delivered 4,140 hits, 91 of which met the inclusion criteria [ 6 ], [ 7 ], [ 8 ], [ 9 ], [ 10 ], [ 11 ], [ 12 ], [ 13 ], [ 14 ], [ 15 ], [ 16 ], [ 17 ], [ 18 ], [ 19 ], [ 20 ], [ 21 ], [ 22 ], [ 23 ], [ 24 ], [ 25 ], [ 26 ], [ 27 ], [ 28 ], [ 29 ], [ 30 ], [ 31 ], [ 32 ], [ 33 ], [ 34 ], [ 35 ], [ 36 ], [ 37 ], [ 38 ], [ 39 ], [ 40 ], [ 41 ], [ 42 ], [ 43 ], [ 44 ], [ 45 ], [ 46 ], [ 47 ], [ 48 ], [ 49 ], [ 50 ], [ 51 ], [ 52 ], [ 53 ], [ 54 ], [ 55 ], [ 56 ], [ 57 ], [ 58 ], [ 59 ], [ 60 ], [ 61 ], [ 62 ], [ 63 ], [ 64 ], [ 65 ], [ 66 ], [ 67 ], [ 68 ], [ 69 ], [ 70 ], [ 71 ], [ 72 ], [ 73 ], [ 74 ], [ 75 ], [ 76 ], [ 77 ], [ 78 ], [ 79 ], [ 80 ], [ 81 ], [ 82 ], [ 83 ], [ 84 ], [ 85 ], [ 86 ], [ 87 ], [ 88 ], [ 89 ], [ 90 ], [ 91 ], [ 92 ], [ 93 ], [ 94 ], [ 95 ], [ 96 ]. Of the 91 articles, 63 were from the medical field and 28 from related fields such as nursing. We found 3 to 103 different recommendations in each article, totaling 3,135 recommendations. Identification of identical recommendations and grouping similar ones resulted in 679 different recommendations. Of these, 349 were given in only one article each; for example, “remain in the hall from the start of the session until your talk” [ 94 ].
The most frequent advice, given in 62.9% of articles, was to keep slides simple. In particular, authors stated that one should not overload slides or include too much detail, but use clear, concise, simply designed visuals instead. Simplicity of visuals was also the subject of 5 of the 29 most frequent recommendations (see Table 1 (Tab. 1) ), including limiting the number of lines per slide (42.9%) and number of words per line (28.6%), using simple tables and graphs (34.1%), using animations carefully (27.5%), and putting phrases, not sentences, on slides (24.2%).

The second most frequent advice, to know one’s audience (52.7%), referred to who the audience is (e.g., profession, size, age, education), what they already know of the topic, or why they are there (i.e., what their expectations, attitudes, and interests are). Authors advised adjusting the presentation accordingly instead of using canned talks.
Making eye contact was the third most frequent advice (46.2%). This was specified by some authors as making eye contact with many or all persons, making eye contact with persons in all sections of the audience, or making continuous eye contact.
Rehearsal of the presentation was recommended in 44.0% of the articles. In addition, one-third of the articles advised rehearsal in front of other persons. Taken together, 56.0% of the articles gave at least one of these two recommendations. Timing the presentation beforehand – recommended by 38.5% – can ensure that the presenter will stick to the allotted time, an advice given by 40.7%. Further advice calling for thorough preparation was to know one’s topic “like the back of one’s hand” (31.9%), to develop an objective for the talk (28.6%), to and prepare for questions (20.9%). All equipment should be tested beforehand (27.5%).
When delivering the presentation, one should not read the talk from either slides or a manuscript (44.0%). For this purpose (and for simplicity) slides should contain words or phrases instead of complete sentences (24.2%).
The presenter should vary the presentation of his or her voice instead of speaking monotonously (29.7%), not speak too fast (24.2%), face the audience (23.1%), and show some enthusiasm, excitement, or energy (20.9%). To enhance understanding, one should keep the presentation clear and simple (26.4%), be logical (23.1%), and end with a summary (26.4%). The number of slides should be limited (27.5%); most articles specified one slide per minute (n=7, 7.7%).
The slides should be readable (42.9%), referring to both text and visuals. This was probably also the reason for recommending large font sizes (this advice was not included in the 29 most frequent recommendations, however; n=18, 19.8%). Authors generally disagreed regarding the exact size to be used, which ranged from 18 to 32 points; a font size of 24 points was recommended most frequently (n=8, 8.8%).
Authors agreed that the slide design should be consistent throughout the presentation (20.9%) and that contrasting colors should be used (20.9%). Most authors recommended using a dark background (26.4%), while only few recommended using a light background (n=3, 3.3%), arguing that this makes slides easier to read [ 15 ], [ 46 ]; one paper [ 89 ] recommended light background for charts and graphs, but not for text slides (without giving reasons).
None of the included articles gave advice that was the complete opposite of these 29 most frequent recommendations (except for the light versus dark background). However, limiting advice was occasionally given, such as not to practice too much in order to save some enthusiasm [ 62 ] or not to exceed >10% of the original time [ 19 ]. Authors also disagreed on a few topics that did not make it to the 29 most frequent recommendations, including whether clipart or cartoons should be included, whether using a pointer is recommended, and whether information should be added sequentially on a slide.
4. Conclusions
This review extracted recommendations from 91 expert opinion articles on how to give a medical research presentation. We found a high degree of concordance among authors, with 29 recommendations given in more than one-fifth of articles each and very little explicit discordance.
Our findings are limited by the fact that we restricted the literature search to one database and to article titles (without the latter, our search would have yielded 195,766 hits). Nevertheless, we included 91 articles on the presentation of medical research and thus considerably more than two previous reviews, which included 9 expert opinion articles on podium presentations each [ 97 ], [ 98 ].
In addition, the distinction between what authors meant to be recommendations versus mere suggestions was a matter of interpretation; the same is true for decisions on whether recommendations were similar enough to be grouped.
The fact that many authors recommend a behavior does not necessarily mean it will indeed be effective. This can be tested in experimental studies that systematically vary a presenter’s behavior. As in clinical studies, the outcome of interest would need to be defined, which is rarely done in expert opinion articles. We propose as “presenter-relevant outcomes” a) to induce learning effects (i.e., comprehension and retention [ 99 ]), b) to change attitudes, c) to interest and entertain, and d) to improve the presenter’s reputation (e.g., by appearing competent).
To our knowledge, experimental studies have only been done for presentations other than medical research presentations. Surprisingly, the recommendation given most often in this study, “keep your slides simple”, has not been supported with regard to the amount of text on a slide (an aspect also related to further recommendations, like “limit the number of lines per slide”, “limit the number of words per line”, and “put phrases, not sentences, on slides”). A number of studies in students did not find significant differences in retention of information after presentations with concise slides as compared to presentations with more detailed slides [ 100 ], [ 101 ], [ 102 ], as would have been expected by cognitive load theory. This theory states that information will not be encoded adequately if the capacity of our working memory is overloaded [ 103 ], [ 104 ], for example when trying to understand detailed slides and at the same time listen to the presenter. These surprising findings underline the necessity of experimental research on presentation techniques. However, simple slides have been found to be more effective with regard to a different aspect: that is, whether they include pictures not related to the content of the talk. Here, recall was better in students who attended a presentation using slides with irrelevant pictures [ 105 ].
The third most frequent advice, to make eye contact, was found to be effective in one study: Not only did students consider a speaker who made eye contact to be more credible and his talk to be more comprehensible, but they actually learned more as indicated by a subsequent multiple-choice test [ 102 ]. In this study, the “eye contact” condition also differed from the control condition in that the presentation was more lively (recommendation no. 13: “vary your voice“) and in that the presenter did not read from written text only but also made colloquial interjections (recommendation no. 5: “do not read the talk from slides or a manuscript”).
It is quite possible that empirical studies will contradict the advice found in this opinion-based study. For example, there is reason to assume that dark backgrounds (recommended by 24 experts as compared to 3 experts recommending light background) may have disadvantages. For example, they may require dimming the lights so that the audience can read the slides, which in turn may lead to reduced levels of attention due to increased tiredness.
In addition, findings from previous studies may not be generalizable to medical conference presentations where the audience may differ in important aspects from students (which have been the subjects of many of the experiments [ 106 ]) – for example with regard to their reasons for attendance and their prior knowledge of the topic. Future experimental studies should therefore investigate whether the recommendations found in this study are indeed effective, looking at different audiences and contexts, and focusing also on rarely explored aspects related to the preparation of the presentation, like adjustment of the talk to the specific audience (recommendation no. 2) and rehearsal (recommendation no. 4).
Probably one of the main reasons that a particular piece of advice was given in the expert opinion papers is that the authors believed that many presenters did not yet follow it. The 29 most frequent recommendations can thus be interpreted as the 29 most common mistakes made by conference presenters. Most of them appear to be common sense and are generally well known [ 99 ]; therefore, why are flaws so common, even in senior presenters [ 98 ]? Researchers may be unwilling to invest time in thorough preparation [ 107 ], or perhaps they have competing interests such as drawing the audience’s attention away from themselves or using slides as a memory aid [ 104 ]. However, if presenters want their talk to be inspiring and practice-changing, they should adhere to the agreed advice found in this review.
Future experimental studies should investigate the effectiveness of the recommendations found in this opinion-based review.
Funding sources
The authors have no funding sources to declare.
Authors' contributions
CB conceived of the study, participated in its design, conduction, and analysis, and drafted the manuscript. HS participated in the study design, conduction, and analysis and helped draft the manuscript. MA participated in the study design. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.

IMAGES
VIDEO
COMMENTS
Include your main title, running title (often a shortened version of your main title), author's name, course name, and semester. 3. Compile your results. Divide the paper into logical sections determined by the type of paper you are writing.
Move 2. Research Context: a. Compare and contrast your findings with those of other published results. b. Explain any discrepancies and unexpected findings. c. State the limitations, weaknesses, and assumptions of your study. Move 3. Closing the paper: a. Summarize the answers to the research questions. b.
In this paper, a simple methodology for classifying simple composite wastes has been proposed. Repetition and Redundancy. Avoid circular sentences. In order to compare the differences in the two analytical methods, the dependent variable was set to concentration, in order to determine if changes had occurred.
A clinician should continuously strive to increase knowledge by reviewing and critiquing papers, thoughtfully considering how to integrate new data into practice. This is the essence of evidence-based medicine (EBM).[1] When new clinical queries arise, one should seek answers in the published literature. The ability to read a scientific or medical manuscript remains vitally important ...
Structure of a medical research paper: key content elements, writing tips and examples of reporting guidelines from the EQUATOR website ... the research study / paper Examples include: Results Report results of the investigations described in the Methods section (in same order) using text, tables, figures, and
WRITING PROMPTS: 1. Summarise what the paper is about in two to three sentences: Example: "This is an interview study of 53 people living with metastatic cancer about their perspective on physicians' use of the computer during follow-up visits. The findings are similar to other studies the authors cite (basically, most patients don't seem to mind when doctors are using the computer).
METHODS: Explain the tools and steps of your research (1-3 sentences) Use the past tense if the study has been conducted; use the present tense if the study is in progress. Include details about the study design, sample groups and sizes, variables, procedures, outcome measures, controls, and methods of analysis. Element.
Even data-driven medical texts can be delivered in an engaging way. Most of us can think of examples of stand-out papers in our field of expertise. At their best these papers are entertaining and thought-provoking even while they deliver complicated, data-heavy material. Make an Outline for your Manuscript
In this video we will teach you how to write a medical research paper for publishing in a high impact journal. We will go through an example medical research...
Healthcare Writing. These OWL resources will help you write in medical, healthcare, and/or scientific contexts. This section includes original research articles and samples of the healthcare writing produced for a more general, lay-population audience.
Background Methodological studies - studies that evaluate the design, analysis or reporting of other research-related reports - play an important role in health research. They help to highlight issues in the conduct of research with the aim of improving health research methodology, and ultimately reducing research waste. Main body We provide an overview of some of the key aspects of ...
Original research studies that can improve decision making in clinical medicine, public health, health care policy, medical education, or biomedical research.
To help you visualize the content you might include in the outline, two types of examples have been included, one that describes a cross-sectional study using a survey methodology (example A), and a second using a combination of a case-control and cohort designs (example B). Use the Preparing the Research Presentation Checklist to assist you in ...
Abstract. 1 Basics of Medical Research Research in any field is an enterprise that carries its own risks and benefits. One may make heavy investment in terms of time, money and expertise yet the ...
The present paper presents a brief historical background to medical publication and practical guidelines for writing scientific papers for acceptance in good journals. ... One example is the De Humani Corporis Fabrica by Vesalius which contains hundreds of illustrations of human dissection. ... For clinical research papers, you have to provide ...
Define your specific research problem and problem statement. Highlight the novelty and contributions of the study. Give an overview of the paper's structure. The research paper introduction can vary in size and structure depending on whether your paper presents the results of original empirical research or is a review paper.
The title of our research paper is "The Effects of Vitamin D Supplements on Obesity: A Randomized Clinical Trial Study" I must insist that this is not an actual research paper from an actual medical journal. This is just an example medical research paper we put together for the purpose of teaching the process of writing up clinical trials.
This study takes global medical research as a case in point and examines the generic discourse features of the experimental medical research article (RA), using a systemic-functional and 'structural moves analysis' approach. Based on this novel, combined methodology, a sequence of generic rhetorical moves and steps across a series of ...
Research proposal examples. Writing a research proposal can be quite challenging, but a good starting point could be to look at some examples. We've included a few for you below. Example research proposal #1: "A Conceptual Framework for Scheduling Constraint Management" Example research proposal #2: "Medical Students as Mediators of ...
Do not use a period after your title or after any heading in the paper (e.g., Works Cited). Begin your text on a new, double-spaced line after the title, indenting the first line of the paragraph half an inch from the left margin. Fig. 1. The top of the first page of a research paper.
Here are examples of argumentative health-related topics for research paper. Why it's Important to Involve Trained Dogs in Neurological Patients Treatment. How Breastfeeding Affects the Future Development of a Child. Why it's Important to Increase the Awareness of the Public about Bipolar Disorder.
Purpose and Importance of the Literature Review. An understanding of the current literature is critical for all phases of a research study. Lingard 9 recently invoked the "journal-as-conversation" metaphor as a way of understanding how one's research fits into the larger medical education conversation. As she described it: "Imagine yourself joining a conversation at a social event.
Examples of AI-Related Reporting Guidelines. CONSORT-AI for clinical trial reports evaluating interventions with an AI component 15. SPIRIT-AI for clinical trial protocols evaluating interventions with an AI component 16. MI-CLAIM for studies including clinical AI modeling 17. CLAIM for studies describing applications of AI in medical imaging 18. MINIMAR (MINimumInformation for Medical ...
For any medical research involving participants, the informed consent of all those involved is required. Informed consent should be obtained immediately prior to any manipulation. ... It contains thousands of paper examples on a wide variety of topics, all donated by helpful students. You can use them for inspiration, an insight into a ...
Background: This systematic review aimed to extract recommendations from expert opinion articles on how to give a medical research presentation on a scientific conference and to determine whether the experts agree on what makes an effective or poor presentation. Methods: Presentation-related terms were searched within article titles listed in PubMed, restricting the search to English-language ...