Learn how UpToDate can help you.
Select the option that best describes you
- Medical Professional
- Resident, Fellow, or Student
- Hospital or Institution
- Group Practice
- Patient or Caregiver
- Find in topic

RELATED TOPICS
INTRODUCTION
The clinical manifestations and diagnosis of KD are discussed in this topic review. The epidemiology, etiology, treatment, and complications of KD, including cardiac sequelae, are presented separately. Incomplete (atypical) KD and unique features in infants and adults are also reviewed separately. (See "Kawasaki disease: Epidemiology and etiology" and "Kawasaki disease: Initial treatment and prognosis" and "Cardiovascular sequelae of Kawasaki disease: Clinical features and evaluation" and "Incomplete (atypical) Kawasaki disease" and "Kawasaki disease: Complications" .)
CLINICAL MANIFESTATIONS
Variations in age have the greatest impact on a patient's likelihood of developing mucocutaneous manifestations of KD. Oral mucous membrane findings are seen in approximately 90 percent of cases of KD, polymorphous rash in 70 to 90 percent, extremity changes in 50 to 85 percent, ocular changes in >75 percent, and cervical lymphadenopathy in 25 to 70 percent [ 7,10-12 ].
These findings are often not present at the same time, and there is no typical order of appearance. As an example, some patients have only developed fever and cervical lymphadenopathy by the time of admission (so-called KD with isolated cervical lymphadenopathy, KDiL) [ 13 ]. In one case series, these patients tended to be older and to have a more severe course, with increased risk of coronary artery (CA) disease and lack of response to intravenous immune globulin (IVIG). Thus, repeated histories and physical examinations are important both for making a timely diagnosis of KD in children who fail to meet diagnostic criteria, as well as for appropriate consideration of alternative diagnoses. (See 'Diagnosis' below.)

- My presentations
Auth with social network:
Download presentation
We think you have liked this presentation. If you wish to download it, please recommend it to your friends in any social system. Share buttons are a little bit lower. Thank you!
Presentation is loading. Please wait.
Kawasaki disease: Difficult case scenario and guidelines
Published by Morgan Phillips Modified over 5 years ago
Similar presentations
Presentation on theme: "Kawasaki disease: Difficult case scenario and guidelines"— Presentation transcript:

Hatem Eleishi, MD Rheumatologist STILL’S DISEASE.

Kawasaki disease is a rare condition. It is most common in children under five years old and most cases occur in children aged between nine months and.

CASE PRESENTATION Abhilash Sailendra GPST 1. AN 18 MONTH OLD WITH FEVER AND RASH AN 18 MONTH OLD WITH FEVER AND RASH High fever for 4 days. Four days.

Journal Club General Medicine C- 4/3/14

Viral Myocarditis.

Juvenile Rheumatoid Arthritis B. Paul Choate, M.D.

Kawasaki Disease Danielle Hann ST2 GPVTS Kawasaki Disease 80% cases aged 6/12 to 5 years Acute inflammatory vasculitis of medium sized arteries.

Acquired Heard Diseases MSN.Khitam moh ’ d Outline 1. Congestive Heart Disease 2. Rheumatic fever 3. Kawasaki Disease.

From Pediatric M&M Fort Carson MEDDAC

DR.IBTISAM JALI MEDICAL DEMONSTRATOR

KAWASAKI’S DISEASE By: Madeline Dixon and Megan Curry.

Kawasaki Disease Dr Paul A Brogan Senior Lecturer in vasculitis
What is Kawasaki Disease? Kawasaki Disease (KD) also known as Kawasaki Syndrome. An unusual and serious illness of young children. It is an autoimmune.

COURAGE: Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation Purpose To compare the efficacy of optimal medical therapy (OMT)

Kawasaki Disease: An Update

Update on Kawasaki Disease June 7 th, 2010 Aaron S. Miller, MD, MSPH Division of Hospitalist Medicine St. Louis Children’s Hospital.

Mucocutaneous lymph node syndrome Ma Lian

A 25 year old farmer with joint pain Laura Zakowski, MD* * No financial disclosures.

The Sick Infant: Five Deadly Misconceptions Todd Wylie, MD University of Florida Department of Emergency Medicine June , 2009.

Immunoglobulin plus prednisolone in severe Kawaski disease (RAISE study) Steph Borg 22 November 2012 SCH Journal Club.
About project
© 2024 SlidePlayer.com Inc. All rights reserved.
- Patient Care & Health Information
- Diseases & Conditions
- Kawasaki disease
Kawasaki disease causes swelling, called inflammation, in the walls of small to medium-sized blood vessels that carry blood throughout the body. Kawasaki disease most often affects the heart arteries in children. Those arteries supply oxygen-rich blood to the heart.
Kawasaki disease is sometimes called mucocutaneous lymph node syndrome. That's because it also causes swelling in glands, called lymph nodes, and mucous membranes inside the mouth, nose, eyes and throat.
Children with Kawasaki disease might have high fever, swollen hands and feet with skin peeling, and red eyes and tongue. But Kawasaki disease is often treatable. With early treatment, most children get better and have no long-lasting problems.
Products & Services
- A Book: Mayo Clinic Family Health Book, 5th Edition
- Newsletter: Mayo Clinic Health Letter — Digital Edition
Symptoms of Kawasaki disease include a fever greater than 102.2 degrees Fahrenheit (39 degrees Celsius) for five or more days. And the child has at least four of the following symptoms.
- A rash on the main part of the body or in the genital area.
- An enlarged lymph node in the neck.
- Very red eyes without a thick discharge.
- Red, dry, cracked lips and a red, swollen tongue.
- Swollen, red skin on the palms of the hands and the soles of the feet. Later the skin on fingers and toes peels.
The symptoms might not happen at the same time. Let your child's healthcare professional know about a symptom that has gone away.
Other symptoms might include:
- Belly pain.
- Joint pain.
Some children get a high fever for five or more days but have fewer than four of the symptoms needed for a diagnosis of Kawasaki disease. They might have what's called incomplete Kawasaki disease. Children with incomplete Kawasaki disease are still at risk of damage to the heart arteries. They still need treatment within 10 days of when symptoms appear.
Kawasaki disease can have symptoms like those of a condition called multisystem inflammatory syndrome in children. The syndrome happens in children with COVID-19.
When to see a doctor
If your child has a fever that lasts more than three days, contact your child's healthcare professional. Treating Kawasaki disease within 10 days of when it began may reduce the chances of lasting damage to the arteries that supply the heart.
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
From Mayo Clinic to your inbox
Sign up for free and stay up to date on research advancements, health tips, current health topics, and expertise on managing health. Click here for an email preview.
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing!
You'll soon start receiving the latest Mayo Clinic health information you requested in your inbox.
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
No one knows what causes Kawasaki disease. But experts don't believe the disease spreads from person to person. Some think that Kawasaki disease happens after a bacterial or viral infection, or that it's linked to factors in the environment. Certain genes might make children more likely to get Kawasaki disease.
Risk factors
Three things are known to increase a child's risk of developing Kawasaki disease.
- Age. Children under 5 years old are at highest risk of Kawasaki disease.
- Sex. Children who are assigned male at birth are slightly more likely to get Kawasaki disease.
- Ethnicity. Children of Asian or Pacific Islander descent have higher rates of Kawasaki disease.
Kawasaki disease tends to occur seasonally. In North America and countries with like climates, it most often happens in the winter and early spring.
Complications
Kawasaki disease is a leading cause of heart disease in children who live in developed countries. But, with treatment, few children have lasting damage.
Heart complications include:
- Swelling of blood vessels, most often the arteries that send blood to the heart.
- Swelling of the heart muscle.
- Heart valve problems.
Any of these complications can damage the heart. Swelling of the heart arteries can weaken them and cause a bulge in the artery wall, called an aneurysm. Aneurysms raise the risk of blood clots. These can lead to a heart attack or cause bleeding inside the body.
Rarely, for children who get heart artery problems, Kawasaki disease can cause death.
Kawasaki disease care at Mayo Clinic
- Ferri FF. Kawasaki disease. In: Ferri's Clinical Advisor 2022. Elsevier; 2022. https://www.clinicalkey.com. Accessed Sept. 3, 2021.
- Elsevier Point of Care. Clinical overview: Kawasaki disease. https://www.clinicalkey.com. Accessed Aug. 10, 2023.
- AskMayoExpert. Kawasaki disease (child). Mayo Clinic; 2023.
- Sundel R. Kawasaki disease: Clinical features and diagnosis. https://www.uptodate.com/contents/search. Accessed Aug. 10, 2023.
- Sundel R. Kawasaki disease: Initial treatment and prognosis. https://www.uptodate.com/contents/search. Accessed Aug. 10, 2023.
- Rife E, et al. Kawasaki disease: An update. Current Rheumatology Reports. 2020; doi:10.1007/s11926-020-00941-4.
Associated Procedures
- Complete blood count (CBC)
- Echocardiogram
- Electrocardiogram (ECG or EKG)
Mayo Clinic in Rochester, Minnesota, has been recognized as one of the top Cardiology & Heart Surgery hospitals in the nation for 2023-2024 by U.S. News & World Report.
- Symptoms & causes
- Diagnosis & treatment
- Doctors & departments
- Care at Mayo Clinic
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
Your gift holds great power – donate today!
Make your tax-deductible gift and be a part of the cutting-edge research and care that's changing medicine.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 26 May 2020
Kawasaki disease: pathophysiology and insights from mouse models
- Magali Noval Rivas ORCID: orcid.org/0000-0001-5570-8928 1 , 2 &
- Moshe Arditi ORCID: orcid.org/0000-0001-9042-2909 1 , 2 , 3
Nature Reviews Rheumatology volume 16 , pages 391–405 ( 2020 ) Cite this article
45k Accesses
132 Citations
56 Altmetric
Metrics details
- Experimental models of disease
- Immunopathogenesis
- Inflammation
- Vasculitis syndromes
Kawasaki disease is an acute febrile illness and systemic vasculitis of unknown aetiology that predominantly afflicts young children, causes coronary artery aneurysms and can result in long-term cardiovascular sequelae. Kawasaki disease is the leading cause of acquired heart disease among children in the USA. Coronary artery aneurysms develop in some untreated children with Kawasaki disease, leading to ischaemic heart disease and myocardial infarction. Although intravenous immunoglobulin (IVIG) treatment reduces the risk of development of coronary artery aneurysms, some children have IVIG-resistant Kawasaki disease and are at increased risk of developing coronary artery damage. In addition, the lack of specific diagnostic tests and biomarkers for Kawasaki disease make early diagnosis and treatment challenging. The use of experimental mouse models of Kawasaki disease vasculitis has considerably improved our understanding of the pathology of the disease and helped characterize the cellular and molecular immune mechanisms contributing to cardiovascular complications, in turn leading to the development of innovative therapeutic approaches. Here, we outline the pathophysiology of Kawasaki disease and summarize and discuss the progress gained from experimental mouse models and their potential therapeutic translation to human disease.
Kawasaki disease is a childhood systemic vasculitis leading to the development of coronary artery aneurysms; it is the leading cause of acquired heart disease in children in developed countries.
The cause of Kawasaki disease is unknown, although it is suspected to be triggered by an unidentified infectious pathogen in genetically predisposed children.
Kawasaki disease might not be a normal immune response to an unusual environmental stimulus, but rather a genetically determined unusual and uncontrolled immune response to a common stimulus.
Although the aetiological agent in humans is unknown, mouse models of Kawasaki disease vasculitis demonstrate similar pathological features and have substantially accelerated discoveries in the field.
Genetic and transcriptomic analysis of blood samples from patients with Kawasaki disease and experimental evidence generated using mouse models have demonstrated the critical role of IL-1β in the pathogenesis of this disease and the therapeutic potential of targeting this pathway (currently under investigation in clinical trials).
Similar content being viewed by others

Long COVID: major findings, mechanisms and recommendations
Hannah E. Davis, Lisa McCorkell, … Eric J. Topol

Acute kidney injury
John A. Kellum, Paola Romagnani, … Hans-Joachim Anders

The cGAS–STING pathway as a therapeutic target in inflammatory diseases
Alexiane Decout, Jason D. Katz, … Andrea Ablasser
Introduction
Kawasaki disease is a systemic vasculitis that affects infants and young children 1 , 2 , 3 . Kawasaki disease is now the leading cause of acquired heart disease among children in North America, Europe and Japan 4 , 5 . The cardiovascular sequelae resulting from childhood Kawasaki disease are increasingly recognized to extend into adulthood, and the disease is no longer considered self-limiting 6 , 7 , 8 , 9 . The triggering agents for Kawasaki disease remain unidentified; however, results from our laboratory 10 , 11 and others 12 , 13 are consistent with the interpretation that a conventional antigen is probably responsible. Coronary arteritis and predominantly coronary artery aneurysms (CAAs) occur in up to 30% of untreated children, although this rate is reduced to 5–7% in children treated with high-dose intravenous immunoglobulin (IVIG) 3 , 14 , 15 . IVIG treatment leads to CAA regression in 60–75% of patients with Kawasaki disease 16 , 17 . However, the exact mechanisms by which IVIG reduces the rate of cardiovascular complications are unknown 18 . Up to 15–20% of patients with Kawasaki disease do not respond to IVIG treatment, and these individuals have an increased rate of CAA development 3 , 15 , 19 , 20 , 21 .
Kawasaki disease is associated with infiltration of the coronary artery wall by a broad variety of innate and adaptive immune cells. Immunohistochemical analysis of human post-mortem tissues shows accumulation in the arterial wall of monocytes, macrophages and neutrophils 22 , 23 , and the presence of activated CD8 + T cells 24 as well as IgA + plasma cells 25 , 26 . The release of pro-inflammatory cytokines, such as TNF and IL-1β, by infiltrating immune cells promotes vascular endothelial cell damage and the development of CAAs 27 , 28 .
However, understanding of Kawasaki disease pathophysiology is limited by the low availability of human tissues of the disease, failure to identify specific aetiological agents triggering the disease, and incomplete understanding of the molecular and cellular mechanisms leading to cardiovascular sequelae. Therefore, experimental animal models mimicking the human features of Kawasaki disease and their translational utility have been invaluable to investigation of this disease. In this Review, we discuss advances from human and mouse studies that have contributed to an improved understanding of Kawasaki disease pathophysiology and the cellular and molecular circuitries involved in disease development. We also outline how evidence obtained from experimental mouse models of Kawasaki disease vasculitis has paved the way for the development of new efficient therapeutics to treat human Kawasaki disease.
Aetiological agents
The causative agents initiating the disease have still not been identified >50 years after the first description of Kawasaki disease. However, the trigger is suspected to be of viral origin and to enter the body through the mucosal surfaces in the lung 29 (Fig. 1 ). This hypothesis is supported by the seasonality of Kawasaki disease outbreaks, which is similar to that of other respiratory infections. In Japan, two seasonal peaks have been observed, one in winter and another in summer, whereas in the USA, the incidence peaks are observed during spring and winter 30 . Development of Kawasaki disease is age specific, with children from 6 months to 5 years of age at greatest risk 3 , 30 , 31 , which suggests a protective maternal passive immunity against the causative agent from birth to 6 months of age and the importance of immune system maturation in children ≥6 years of age 29 .
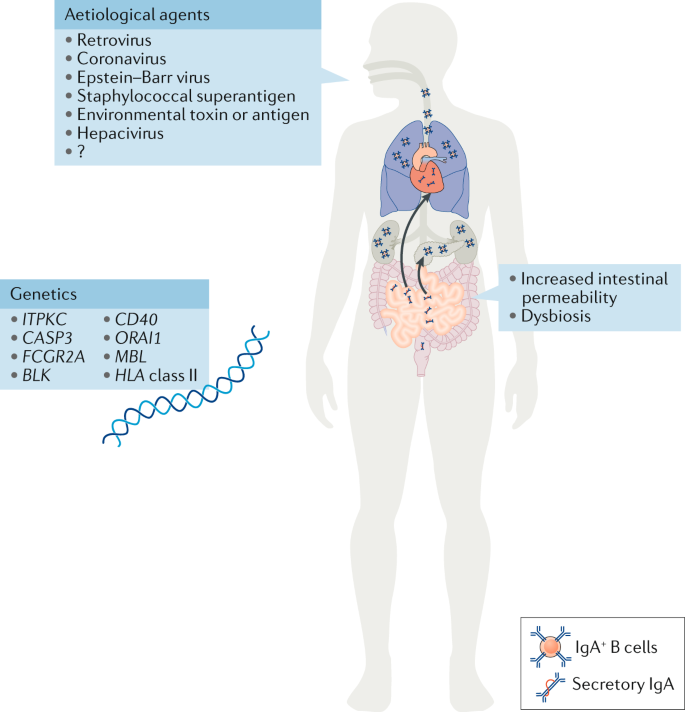
Different aetiological agents, from viruses to environmental toxins, have been proposed as triggering agents for Kawasaki disease; however, none has been corroborated, and the aetiological agent remains unidentified. Increased numbers of IgA + plasma cells have been detected in the pancreas, the kidneys, the coronary artery wall and the respiratory tract of patients with Kawasaki disease. Patients with Kawasaki disease have increased concentrations of secretory IgA in their serum, indicative of defective intestinal barrier function and increased intestinal permeability. Changes in the gut microbiota composition (dysbiosis) have also been suggested to have a role in the development of Kawasaki disease. Single nucleotide polymorphisms in the genes listed have been associated with susceptibility to Kawasaki disease and disease severity. The current understanding is that Kawasaki disease is triggered in genetically predisposed children by a ubiquitous environmental stimulus that typically would not result in an uncontrolled immune response and development of vasculitis.
The clinical features of Kawasaki disease, such as high fever, skin rash and peeling, conjunctivitis and intense release of pro-inflammatory cytokines, are reminiscent of other infectious diseases such as staphylococcal and streptococcal toxic shock syndromes 32 . Some studies have shown that, compared with healthy control individuals, patients with Kawasaki disease have a skewed Vβ T cell repertoire and increased frequencies of circulating Vβ2 + and Vβ8.1 + T cells, leading to the early suggestion that a superantigen toxin might have a role in triggering Kawasaki disease 33 , 34 , 35 . However, similar results were not reproduced in later studies 36 , 37 , leading to the more generalized hypothesis that the development of Kawasaki disease might be triggered by multiple conventional antigens.
Several early studies showed reduced prevalence of antibodies to the Epstein–Barr virus (EBV) capsid antigen in Japanese children with Kawasaki disease compared with age and sex-matched control patients 38 , 39 , 40 , suggesting the involvement of an abnormal immune response to EBV in disease development. However, this difference in EBV antibody seropositivity could not be reproduced in other studies 41 , 42 , 43 . A human coronavirus was detected more frequently in respiratory secretions of patients with Kawasaki disease than in control individuals 44 , although, again, other studies could not replicate this finding 45 , 46 , indicating that the original association might have been coincidental. The possibility that a retrovirus is the triggering agent for Kawasaki disease has also been proposed, owing to detection of retrovirus-specific reverse transcriptase activity in the co-culture supernatant of peripheral blood mononuclear cells (PBMCs) from patients with Kawasaki disease but not controls 47 , 48 . However, this result could not be replicated in later studies 49 , 50 , 51 . A peptide recognized by antibodies produced during the acute phase of Kawasaki disease has been identified in 2020 (ref. 52 ). Although the protein epitopes seem similar to hepaciviruses 53 , further studies are required to determine the specific gene sequence from which this peptide emerges.
Altogether, the absence of consistent and reproducible studies pinpointing a specific aetiological agent suggests that Kawasaki disease is caused not by one but by multiple infectious agents. Acute Kawasaki disease is associated with infiltration of IgA + plasma cells in the respiratory tract, implying that the upper airways act as a portal of entry 25 , 26 . One suggestion is that the triggering agent might be an environmental toxin or antigen transported by wind currents 54 ; however, this possibility cannot be rigorously assessed until precise identification of the aetiological agents is achieved 29 .
SNPs influencing susceptibility
Although Kawasaki disease has been observed around the world and in multiple ethnic groups, geographical differences exist in incidence. The highest incidence is in Asian countries such as Korea and Japan, where it has increased over the past decades and is now 10–20 times more prevalent than in North America and Europe 30 . This increased susceptibility in Asian children, as well as in children with Asian ancestry living in North America 31 , indicates that genetic components predispose to disease susceptibility. In Japan, siblings of children with Kawasaki disease are at increased risk of developing the disease 55 . Single nucleotide polymorphisms (SNPs) in multiple genes have been associated with increased susceptibility to Kawasaki disease (Fig. 1 ); however, mechanisms linking those SNPs with Kawasaki disease progression are not yet well understood and require more investigation.
Calcium signalling pathway
Inositol 1,4,5-trisphosphate 3-kinase C (ITPKC), a kinase that phosphorylates inositol 1,4,5-triphosphate (IP 3 ), is involved in many signalling processes in a wide array of cells. In T cells, IP 3 is released after T cell receptor stimulation, thus increasing levels of intracellular Ca 2+ through IP 3 receptors expressed on the endoplasmic reticulum and leading to nuclear translocation of nuclear factor of activated T cells (NFAT), IL-2 production and T cell activation 56 . By blocking the interaction of IP 3 with its receptor, ITPKC negatively regulates T cell activation. A functional SNP in ITPKC has been associated with increased risk of coronary artery lesions in Taiwanese 57 , Japanese and American patients with Kawasaki disease 58 . Mechanistically, this ITPKC polymorphism might directly contribute to T cell hyperactivity, and more importantly, it might promote NLRP3 inflammasome activation and increase production of IL-1β and IL-18 (ref. 59 ). ORAI1 is a membrane-bound Ca 2+ channel protein encoded by ORAI1 that is involved in the Ca 2+ –calcineurin–NFAT signalling pathway. Although no significant association between ORAI1 polymorphisms and Kawasaki disease susceptibility or IVIG treatment response was initially reported in the Taiwanese population 60 , an SNP in exon 2 of ORAI1 is associated with Kawasaki disease susceptibility in the Japanese population 61 , and interestingly this SNP is 20 times more frequent in the general Japanese population than in the general European population 61 . Another SNP in SLC8A1 , which encodes the Na + –Ca 2+ exchanger, is also associated with susceptibility to Kawasaki disease and aneurysm formation 62 , further highlighting the critical role of calcium signalling pathways in development of Kawasaki disease. Crucially, the Ca 2+ –NFAT signalling pathway is also key to intracellular Ca 2+ regulation and therefore to NLRP3 inflammasome activation and IL-1β production 63 , 64 .
CD40 ligand
CD40 ligand (CD40L) is a protein expressed by a large array of cells including activated T cells, B cells, monocytes and platelets. CD40L receptor, CD40, is expressed by antigen-presenting cells as well as endothelial cells 65 . CD40 engagement is associated with cell survival, activation, proliferation and cytokine production 65 . Compared with control patients with other febrile illnesses, patients with Kawasaki disease have increased CD40L expression on CD4 + T cells and platelets, which correlates with increased development of coronary artery lesions and is reduced by IVIG treatment 66 . An SNP in CD40L has been reported in Japanese patients with Kawasaki disease and is more frequent in male patients with coronary artery lesions than in female patients 67 . This polymorphism was not observed in a cohort of Taiwanese patients 68 ; however, another SNP in the CD40 gene has been reported in an independent cohort of Taiwanese patients and is associated with increased susceptibility to Kawasaki disease and development of coronary artery lesions 69 . These results indicate a role of the CD40–CD40L pathway in the development and severity of Kawasaki disease and highlight this pathway as a potential therapeutic target.
Mannose-binding lectin
Mannose-binding lectin (MBL), a pattern recognition molecule of the innate immune system, binds the surface of pathogenic organisms and activates the complement pathway 70 . A polymorphism in MBL2 was found to be an age-related risk factor for development of coronary artery lesions in a Dutch cohort of patients 71 , 72 . Another study in a cohort of Japanese patients with Kawasaki disease showed that codon 54 variants in MBL2 are significantly associated with susceptibility to Kawasaki disease 73 . Interestingly, in the Candida albicans water-soluble fraction (CAWS) mouse model of Kawasaki disease vasculitis, MBL-A and MBL-C deposition are observed in the aortic root, suggesting involvement of the MBL-dependent lectin pathway in this experimental model 74 . However, further studies are required to understand the pathogenic roles of those two proteins as well as their potential as therapeutic targets.
Fcγ receptors
Polymorphisms in genes encoding the receptors for the Fc portion of immunoglobulins, Fcγ receptors (FcγRs), have been associated with the development of autoimmune and infectious diseases 75 , 76 , 77 . As Kawasaki disease is considered an infectious disorder, several studies have investigated the potential association of FcγR SNPs with Kawasaki disease susceptibility and the development of coronary artery lesions. In a cohort of Dutch patients, no difference in FcγR SNP distribution was observed between healthy individuals and patients with Kawasaki disease, and no association was noted between SNPs in FcγR genes and Kawasaki disease susceptibility 78 . However, a later study with >2,000 patients with Kawasaki disease and 9,000 control patients from multiple independent cohorts across different populations highlighted a Kawasaki disease-associated polymorphism in the FCGR2A locus, which encodes FcγRIIA (CD32a), a member of the family of IgG receptors 79 . This polymorphism has important implications as the standard of care for Kawasaki disease is IVIG, a pool of plasma IgG that interacts with FcγRs on immune cells. Interestingly, 15–20% of patients with Kawasaki disease have IVIG-resistant disease and require another round of IVIG treatment or the use of adjunctive therapies 15 , 19 , 20 , 80 . The exact mechanisms by which IVIG mediates its therapeutic effect and how IVIG resistance develops remain unknown, and the potential involvement of this FcγRIIA polymorphism in IVIG resistance requires further investigation.
Pathophysiology of Kawasaki disease
The innate immune response.
The immune response associated with Kawasaki disease is complex and involves the activation and infiltration of the coronary artery wall by both innate and adaptive immune cells (Fig. 2 ). On the basis of studies of post-mortem tissue from patients with Kawasaki disease, Kawasaki disease vascular pathology has been classified into three sequential linked pathological processes 81 . Necrotizing arteritis develops in the first 2 weeks of the disease and is associated with neutrophilic infiltrations, which gradually destroy the coronary artery intima, media and some portions of the adventitia. Alarmins from the S100 protein family, which are present in the cytoplasm of neutrophils, monocytes and macrophages 82 , also participate in this inflammatory process. Concentrations of circulating S100A8/A9 heterodimers (calprotectin) and S100A12 are substantially higher in patients with Kawasaki disease during the acute phase than in control patients with other febrile illnesses and decline after IVIG treatment 83 , 84 , 85 . After the acute phase of Kawasaki disease, plasma concentrations of S100A8/A9 heterodimers only remain elevated in patients with giant CAAs 84 , highlighting its potential utility as a biomarker to monitor long-term persistence of inflammation. S100A12 also contributes to the acute inflammatory response by directly stimulating monocytes to produce IL-1β, which in turn activates coronary endothelial cells 85 . Necrotizing arteritis might result in the formation of CAAs and is followed by two other processes, subacute or chronic vasculitis and luminal myofibroblast proliferation (LMP), which occur simultaneously and might be observed for months to years after disease onset 81 . The inflammatory infiltrates are composed of CD8 + T cells, IgA + plasma cells, eosinophils and macrophages, which release pro-inflammatory cytokines contributing to cardiovascular pathology. Meanwhile, myofibroblasts, mainly derived from smooth muscle cells, and their matrix products progressively obstruct the coronary lumen 81 (Fig. 2 ). Persistent subacute and chronic vasculitis and LMP can lead to stenosis and thrombosis after acute illness 6 , 9 .
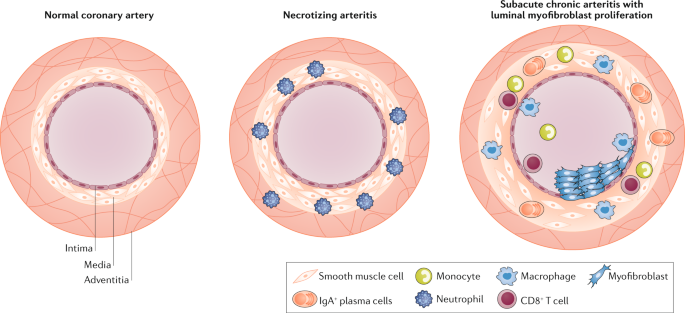
The normal coronary artery is composed of three general layers: the tunica intima, tunica media and tunica adventitia. The intima is mainly composed of endothelial cells, the media of smooth muscle cells and the adventitia of loose connective tissue. In Kawasaki disease, necrotizing arteritis develops in the first 2 weeks of the disease and is associated with neutrophilic infiltration, which gradually destroys the intima, media and some portions of the adventitia of the coronary artery. CD8 + T cells, IgA + plasma cells, monocytes and macrophages compose the inflammatory infiltrate during subacute chronic arteritis. These cells release pro-inflammatory cytokines such as IL-1β and TNF, which contribute to luminal myofibroblast proliferation, in which myofibroblasts, mainly derived from smooth muscle cells, and their matrix products progressively obstruct the coronary lumen.
Matrix metalloproteinases
Matrix metalloproteinases (MMPs; zinc-dependent endopeptidases that degrade extracellular matrix components) are known to have an important role in both inflammation and tissue remodelling processes 86 . Increased expression and activity of a diverse set of MMPs has been demonstrated in acute Kawasaki disease 87 , 88 , 89 . The expression levels of MMP3 and MMP9, both known to mediate vascular smooth muscle cell migration and neointimal formation 90 , are increased in patients with Kawasaki disease 91 , and the circulating levels of these MMPs correlate with the development of CAAs in these patients 92 . MMP3 SNPs are also associated with the development of CAAs 88 , and this protease is considered to be a driving factor allowing IL-1-induced signalling to lead to migration of vascular smooth muscle cells and their transition to proliferating myofibroblasts 93 , 94 , 95 . Whereas MMP9 has been studied and implicated in elastin breakdown in the Lactobacillus casei cell wall extract (LCWE)-induced Kawasaki disease mouse model 96 , 97 , information about the role of MMP3 in this mouse model is lacking.
MicroRNAs (miRNAs; a class of small non-coding RNAs that regulate mRNA expression) are emerging as critical gene regulators in a host of cellular processes, including inflammation 98 . Of human coding genes, 60–70% are estimated to be regulated by miRNAs 99 . Several studies attempting to discover Kawasaki disease biomarkers have found that the miRNA profiles of serum exosome or coronary artery tissues are associated with acute Kawasaki disease 100 , 101 , 102 , 103 , 104 . These miRNAs include miR-23a 100 , 101 , 102 , 103 , miR-27b 100 , miR-223 (refs 100 , 101 , 102 , 103 ) and miR-145 (ref. 103 ). These miRNAs might provide clues as to the molecular mechanisms involved in the development of the cardiovascular lesions associated with Kawasaki disease. For example, miR-145 is highly expressed in vascular smooth muscle cells and has been reported to promote their switching to neointimal proliferating cells 105 , 106 and to regulate the transforming growth factor-β signalling pathway 103 . Increased levels of miR-23a contribute to cardiomyocyte apoptosis and may promote inflammatory responses by blocking macrophage autophagy activity 107 , 108 . However, improved understanding and characterization of the molecular and cellular mechanisms underlying the different roles of miRs during Kawasaki disease require further studies with animal models.
Myocarditis
Most attention in Kawasaki disease research and clinical practice has focused on the development of CAAs and long-term complications of coronary artery stenosis and ischaemia 109 . However, the subacute and chronic inflammation of Kawasaki disease is also associated with the development of myocarditis 3 , 6 , 110 , 111 , 112 . Myocarditis has been described as the ‘hidden face of the moon’ in Kawasaki disease 110 . Reports indicate that myocarditis occurs frequently during acute Kawasaki disease 111 , and serial myocardial biopsy studies have documented that histological myocarditis develops in the majority of patients with Kawasaki disease, even in the absence of coronary aneurysms 113 , 114 . More recent data indicate that myocardial inflammation can be documented in 50–70% of patients using gallium citrate ( 67 Ga) scans and technetium-99 ( 99m Tc)-labelled white blood cell scans 115 . Another study has shown that myocardial inflammatory changes and myocardial oedema in Kawasaki disease occur even before coronary artery abnormalities and without concurrent ischaemic damage 112 .
Myocarditis in Kawasaki disease tends to develop early, and acute left ventricular dysfunction is generally transient and responds readily to anti-inflammatory treatment 116 . However, Kawasaki disease myocarditis might be associated with fatal arrhythmias in infants, and in certain cases might lead to long-term complications including myocardial fibrosis 81 , 117 . Therefore, myocarditis during Kawasaki disease and its potential consequences deserve serious investigation, and long-term studies into late adulthood are needed.
Complement and immune complexes
Kawasaki disease affects small and medium sized vessels, particularly the coronary arteries; however, dilatations and aneurysms can occur systemically, including in the axillary, subclavian, brachial, renal and iliac arteries as well as the abdominal aorta 23 , 118 , 119 , 120 . Post-mortem findings have revealed that 73% of patients with Kawasaki disease have renal artery involvement and acute kidney injury 121 involving glomerulonephritis with intracapillary changes and deposition of immune complex composed of IgA and complement component 3 (C3) 22 , 122 , 123 . These findings are comparable to those in two other human vasculitis diseases, IgA vasculitis (IgAV) and IgA nephropathy (IgAN), which are similarly characterized by IgA immune complexes with C3 deposition in kidney glomeruli (see below). Increased concentrations of circulating IgA and secretory IgA (sIgA) have been reported in the serum of children with Kawasaki disease during the acute phase 124 . IgA + plasma cells are present in the coronary artery wall and in non-vascular tissues, such as the kidney, trachea and pancreas of patients with Kawasaki disease 25 , 26 . This IgA response is oligoclonal, seems to be antigen driven and might be caused by Kawasaki disease-triggering agents 125 , 126 .
The IL-1 signalling pathway
Evidence from mouse models of Kawasaki disease 11 , 127 , 128 , as well as transcriptome analysis performed on whole blood of patients with Kawasaki disease during the acute or convalescent phase 129 , 130 , demonstrate the involvement of innate immune cells and inflammasome overactivation throughout the acute phase of the disease. In vitro cultured PBMCs isolated from patients with Kawasaki disease spontaneously release IL-1β into the supernatant, and this process is substantially reduced after IVIG treatment 28 . Serum concentrations of both IL-1β and IL-18 are also higher in children with acute Kawasaki disease than in control patients with other febrile illnesses, and markedly decrease during the convalescent phase 59 , supporting the concept of activation of the NLRP3 inflammasome complex. Similarly, IL-1 and NLRP3-related gene transcripts are upregulated in PBMCs from patients with acute Kawasaki disease and are decreased during the convalescent phase of the disease 59 , and an IL1B -gene-related signature is associated with acute phase disease and IVIG resistance 130 . Furthermore, a study has shown that differential expression of IL-1β and related signalling genes might have a role in mediating the sex-based differences seen in patients with Kawasaki disease 131 . In the LCWE mouse model of Kawasaki disease, the activation of caspase 1, IL-1α and IL-1β is key to the development of coronary arteritis, aneurysms, myocarditis and abdominal aorta aneurysms 127 , 128 , 132 . IL-1 has the capacity to expand and promote the differentiation of antigen-specific CD8 + T cells 133 , and indeed the frequencies of circulating CD4 + and CD8 + T cells are increased in patients with Kawasaki disease 134 . Infiltrations of mature dendritic cells as well as activated cytotoxic CD8 + T cells have been reported in arterial layers of coronary aneurysms 24 , 135 . Therefore, blocking the NLRP3–IL-1β pathway seems to be a valid therapeutic option in Kawasaki disease.
Role of the gastrointestinal tract
Intestinal permeability.
The intestinal barrier has a critical role in maintaining intestinal homeostasis and health by preventing harmful organisms and luminal antigens from entering the circulation. A dysfunctional intestinal barrier, characterized by increased intestinal permeability, is recognized as a pathogenic factor in many inflammatory diseases 136 . In Kawasaki disease, abdominal pain, diarrhoea and vomiting are often observed at the onset of acute illness, affecting up to 60% of diagnosed patients and indicating that the gastrointestinal tract is also affected 4 , 137 , 138 , 139 , 140 . A multicentre study of >300 patients revealed that gastrointestinal manifestations at onset of disease complicate diagnosis, delay adequate treatment and correlate with IVIG resistance and severity of CAAs 141 . Immunohistochemical studies have revealed higher numbers of activated CD4 + T cells and macrophages along with lower numbers of CD8 + T cells in the jejunum lamina propria in patients with Kawasaki disease than in control patients with diarrhoea from cows’ milk protein intolerance 142 . However, these cellular abnormalities are specific to the acute phase of the disease and return to normal during the convalescent phase 142 . IgA + plasma cells have also been observed in a variety of different vascular and non-vascular tissues in patients with Kawasaki disease 26 , and patients with Kawasaki disease also have increased concentrations of sIgA, which is produced at the intestinal mucosal surface, in their serum 124 . These studies indicate that the gastrointestinal tract is affected during Kawasaki disease and that mucosal immune activation might compensate and protect from defective intestinal barriers.
The role of gut-related immunity in the induction of inflammation in organ systems distant from the gut has been the subject of intensive investigation. We have observed increased intestinal permeability and a dysregulated intestinal immune response characterized by increased numbers of IgA + B cells in the Peyer’s patches in the LCWE-induced mouse model of Kawasaki disease 143 (Fig. 3 ). In this model, the excessive IL-1β release associated with LCWE injection acts on intestinal epithelial cells to open tight junctions, and administration of IVIG or pharmacological agents that block intestinal permeability significantly reduces disease development 143 . Altogether, these observations link increased intestinal permeability and defective intestinal barrier function with systemic IL-1β release in Kawasaki disease.
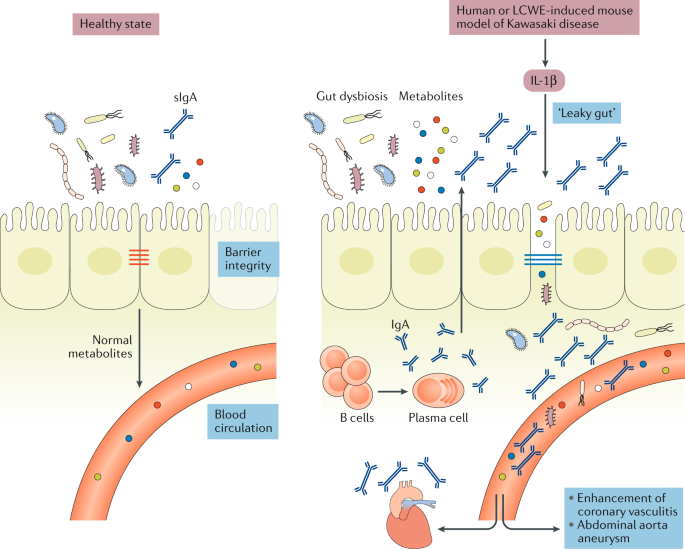
In healthy individuals, intestinal epithelial cells are sealed together by intestinal tight junctions, and the intestinal epithelium acts as a barrier that prevents the passage of commensal bacteria and pathogens while permitting intercellular flux of ions, molecules and metabolites. Lactobacillus casei cell wall extract (LCWE)-induced Kawasaki disease vasculitis and human Kawasaki disease are associated with increased IL-1β production, which leads to decreased expression of intestinal tight junctions, resulting in increased intestinal permeability. Differences in intestinal microbiota composition have been observed in patients with Kawasaki disease, and intestinal dysbiosis might contribute further to the inflammatory process. LCWE injection is also associated with a dysregulated intestinal immune response characterized by increased numbers of IgA + B cells in the gastrointestinal tract and elevated secretory IgA (sIgA) concentrations. Intestinal barrier dysfunction results in sIgA leakage to the systemic circulation and pathogenic IgA–C3 immune complex deposition in the vascular tissues.
The intestinal microbiome
Despite the strong connection between the intestinal microbiome and development of cardiovascular diseases 144 , 145 , only a few studies have investigated the role of the intestinal microbiome during development of Kawasaki disease or treatment resistance. Microbiological culture-based methods demonstrated that, compared with healthy control individuals, patients with Kawasaki disease have a different intestinal microbiota composition characterized by a lower incidence of the Lactobacillus genus 146 , 147 and increased Streptococcus and Staphylococcus 148 species. Lactobacilli have been reported to prevent diarrhoeal disorders 149 , 150 and to improve intestinal barrier function by increasing the expression of intestinal tight junctions 151 , 152 , enhancing the intestinal mucus layer 153 and modulating the intestinal microbiota composition 154 . Lactobacilli have also been shown to boost innate and immune functions against a variety of bacterial infections 155 , 156 , 157 , and their disappearance during acute Kawasaki disease might lead to the blooming of other bacterial pathogens, which might further promote intestinal barrier dysfunction and inflammation. Intriguingly, a retrospective study of 364 patients with Kawasaki disease showed that children who received microbiome-altering antibiotics in the week before Kawasaki disease diagnosis were substantially more likely to have IVIG-resistant disease than those who did not receive antibiotics 158 . Antibiotics alter the abundance, taxonomic richness and diversity of the bacterial 159 , 160 as well as fungal 161 intestinal microbiome, and those alterations might persist from weeks to years after treatment discontinuation 159 , 160 , 162 . A longitudinal metagenomic study of faecal samples derived from patients with Kawasaki disease showed a marked increase of five Streptococcus spp. during the acute phase of Kawasaki disease 163 ; however, all patients in that study were treated with antibiotics in the early stage of disease, therefore this observation might be reflective of antibiotic-induced dysbiosis and not Kawasaki disease itself. Nonetheless, how this intestinal dysbiosis occurs and how its effect on intestinal permeability affects the development of cardiovascular lesions during Kawasaki disease vasculitis remains unknown and under-appreciated.
Link with IgA vasculitis
IgAV, or Henoch-Schönlein purpura, is an IgA-mediated necrotizing vasculitis resulting in fibrinoid destruction of the affected small vessels. Renal involvement, characterized by IgA deposition in the kidney glomeruli, is also observed in IgAV 164 . IgAV nephritis is closely related to another glomerular disease, IgAN, wherein accumulation and deposition of IgA and IgA immune complexes in the kidney glomerular mesangium drive glomerular inflammation 165 . As IgA is mainly found at mucosal surfaces, a ‘gut–kidney axis’, influenced by a mix of genetic, microbial and dietary factors, has been suggested to be involved in the development of both IgAN 166 and IgAV in paediatric and adult patients 167 . We have demonstrated that the LCWE-induced mouse model of Kawasaki disease vasculitis is associated with the deposition of IgA and IgA–C3 immune complexes in vascular tissues, such as the inflamed coronary artery and abdominal aorta 143 . Deposited IgA and IgA–C3 immune complexes might result in overactivation of the immune cells present in the cardiovascular lesions and subsequent amplification of inflammation 143 . Substantial evidence indicates that immune complexes might promote vascular damage during human Kawasaki disease through the activation and aggregation of platelets, the release of vasoactive mediators, and the subsequent recruitment of neutrophils and leukocytes to the site of inflammation (reviewed elsewhere 168 ).
Interestingly, we have also observed IgA and C3 deposition in the kidney glomeruli of LCWE-injected mice developing Kawasaki disease 143 , and immune complex-mediated nephropathy has also been observed in Kawasaki disease 123 . However, to date IgA deposition has not been reported in CAAs of patients with Kawasaki disease. Given that availability of human tissue samples is limited, and those that are available are usually collected at the end stage of the disease, they might not be representative of active Kawasaki disease pathological features, and further studies are warranted. Like Kawasaki disease, IgAV develops mostly in children, affects males more than females, is more predominant in Asian countries such as Japan and Korea, and is also associated with abdominal pain, diarrhoea, skin rash and IgA deposition in the affected small vessels 169 . IgAN also shares pathological features with Kawasaki disease, such as increased intestinal permeability, low to moderate intestinal inflammation associated with activation of inflammatory cells in the small intestinal mucosa and colocalization of sIgA-complement in the glomerular mesangium 165 , 170 . Moreover, a polymorphism in the promoter of the lipopolysaccharide (LPS) receptor CD14 (CD14/159) is associated with coronary artery abnormalities in patients with Kawasaki disease 171 and has been linked to progression of IgAN to more severe renal disease 172 . IL-1β has a key pathogenic role during Kawasaki disease and also seems to be implicated in renal complications related to IgAV 173 and IgAN 174 . Altogether, given that Kawasaki disease shares clinical features and pathological mechanisms with both IgAV and IgAN, it is possible that Kawasaki disease is a form of IgAV. Similarly, treatments that have shown efficacy in Kawasaki disease, such as anakinra and IVIG, might be suitable and useful for treating IgAV 175 and IgAN.
Mouse models of Kawasaki disease
The lack of identification of specific aetiological agents and incomplete understanding of the molecular mechanisms involved in Kawasaki disease cardiovascular pathology have delayed the development of targeted and effective treatment options for this disease. In addition, the limited availability of tissue samples from patients with Kawasaki disease has considerably impeded progress in understanding the pathogenesis of the disease, making the availability of relevant animal models of Kawasaki disease extremely valuable. Kawasaki disease vasculitis can be induced in mice by injection of cell wall components from L. casei 176 , C. albicans 177 or nucleotide-binding oligomerization domain containing 1 (Nod1) ligand 178 (Table 1 ). These mouse models of Kawasaki disease have accelerated research and have enhanced understanding of the pathogenesis of this disease. However, no animal model perfectly recapitulates human disease. Particularly in the context of Kawasaki disease, given that the aetiology remains unknown, researchers must exercise caution in interpreting results based on experimental models and confirm findings in patient cohorts. Nevertheless, even though the extrapolation of preclinical mouse data to humans is far from straightforward, mouse models are still invaluable tools to study certain pathological aspects of human inflammatory diseases and gain mechanistic insights.
The LCWE mouse model
L. casei is a Gram-positive bacteria that colonizes the gastrointestinal and urogenital tracts of both human and animals 179 . More than 35 years ago, Lehman et al. 180 demonstrated that a single intraperitoneal injection of LCWE induces a dose-dependent and chronic polyarthritis in rats. However, when injected into mice, LCWE induces instead a focal coronary arteritis 176 . How and which element of LCWE triggers Kawasaki disease vasculitis is unknown. LCWE is mainly composed of peptidoglycans, contains high levels of rhamnose and is resistant to lysozyme degradation 176 .
The cardiovascular lesions induced in mice by LCWE are histologically similar to those observed in human disease. LCWE-induced Kawasaki disease vasculitis is characterized by infiltration of inflammatory cells in the aortic root, development of necrotizing arteritis in the coronary artery followed by luminal obstruction due to LMP that can lead to complete coronary artery stenosis 181 , recapitulating the three pathological processes of human Kawasaki disease described above (Fig. 4a – d ). In children with Kawasaki disease, thrombotic occlusion of the inflamed coronary artery leads to ischaemic heart disease 23 , 120 , and similarly, occluding organizing thrombus in the coronary artery can be observed in LCWE-injected mice (Fig. 4e ). Acute myocarditis and chronic scarring of the coronary arteries with the formation of stenotic fragments are also observed in LCWE-induced Kawasaki disease vasculitis (Fig. 4f ), even long after the acute phase 182 , which is similar to the fibrotic lesions that might lead children with Kawasaki disease to develop long-term cardiovascular sequelae in adulthood 8 , 9 . MRI and echocardiography in LCWE-injected mice demonstrate the presence of electrocardiographic changes (as observed in human Kawasaki disease) and myocardial dysfunction, which are responsive to anakinra therapy 183 , 184 .
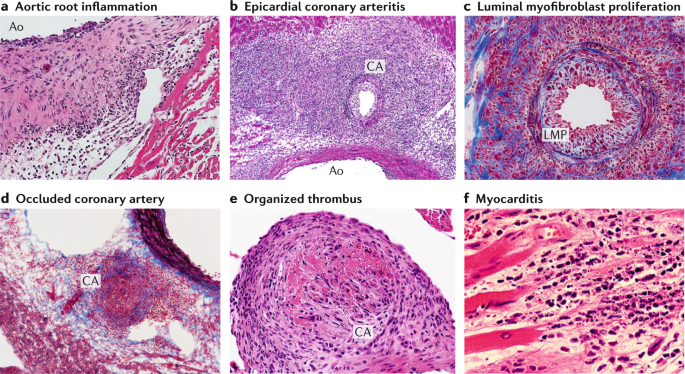
Wild-type mice underwent intraperitoneal injection with Lactobacillus casei cell wall extract (LCWE), and heart tissues were harvested 2 weeks later. Haematoxylin and eosin (H&E) and trichrome staining were performed on heart sections. a | Inflammatory cell infiltration in the aortic route (H&E staining; ×40). b | Arteritis development in epicardial muscular coronary artery (H&E staining; ×20). c | Luminal myofibroblast proliferation (LMP) and non-specific neointimal proliferation injury to the arterial wall (trichrome staining; ×200). d | Complete occlusion of the coronary artery by LMP (trichrome staining; ×20). e | Organized thrombus in the coronary artery (H&E staining; ×200). f | Myocarditis (H&E staining; ×200). Ao; aorta, CA; coronary artery.
The LCWE-induced Kawasaki disease vasculitis in mice is dependent on intact TLR2 and MyD88 signalling and the subsequent release of pro-inflammatory cytokines, including IL-1β, IL-6 and TNF 10 . Genetic depletion of the TNF receptor or pharmacological blockade of the TNF signalling pathway (with infliximab (monoclonal antibodies to TNF) or etanercept (soluble TNF receptors)) protects mice from LCWE-induced Kawasaki disease vasculitis 132 , 185 . This model is also T cell dependent, as Rag1 –/– mice develop fewer cardiovascular lesions 11 . CD8 + T cells are specifically required for LCWE-induced Kawasaki disease vasculitis as treatment of LCWE-injected mice with an anti-CD8-depleting antibody prevents the development of vasculitis 181 . This finding correlates with human disease, in which infiltrations of CD3 + T cells 135 , and particularly CD8 + T cells, are detected in the CAAs 24 . The LCWE model has also confirmed the importance of the ITPKC pathway in Kawasaki disease development and demonstrated that ITPKC deficiency is associated with increased Ca 2+ flux and levels of IL-1β in vitro 59 . Interestingly, the relatively mild development of coronary arteritis in LCWE-injected CBA/N mice — which are characterized by a defective B cell maturation process and poor humoral immune responses — suggests that the humoral immune response might participate in amplification of the disease 186 . IgA + plasma cells infiltrate vascular and non-vascular tissues during the acute phase of Kawasaki disease 25 , 26 , resulting in the development of an oligoclonal IgA response in the coronary artery 125 , 126 . Interestingly, we have observed increased numbers of IgA + plasmablasts in the spleen, Peyer’s patches and abdominal aorta draining lymph nodes of LCWE-injected mice, as well as increased concentrations of circulating IgA and IgA deposition in heart tissues, abdominal aorta and kidney glomureli 143 .
Mouse models also provide a useful opportunity to evaluate the efficacy of therapeutic regimens on the development and healing of cardiovascular lesions. When given up to 5 days after LCWE injection, IVIG substantially decreases the severity of cardiovascular lesions in mice 187 , mirroring the effects of IVIG treatment in humans. As described above, IL-1β signalling is higher in patients with Kawasaki disease than in age-matched control patients with other febrile illnesses 91 , 188 , and studies using the LCWE model helped lead to the discovery of the importance of this pathway in the pathogenesis of the disease and the therapeutic potential of IL-1 blockade. Depletion of macrophages or blocking the IL-1 pathway either genetically using IL1R −/− , IL1α −/− or IL1β −/− mice or with antibodies targeting IL-1α or IL-1β, or anakinra (IL1Ra), strongly reduces cardiovascular lesion development as well as myocardial dysfunction in LCWE-injected mice 128 , 132 , 184 .
The CAWS mouse model
C. albicans is a harmless commensal fungus normally present in the human gastrointestinal tract that can transition into a pathogen capable of inducing inflammation in immune-impaired hosts. In 1979, Murata demonstrated that an alkaline extract made from C. albicans isolated from faeces from a patient with Kawasaki disease induced coronary arteritis in mice 177 . CAWS is composed of polysaccharides, mainly β-glucans and α-mannan proteins of the yeast cell wall 189 , and needs to be injected intraperitoneally for five consecutive days in the first week of the disease to induce vasculitis in the aortic valves and the coronary arteries 189 , 190 . In this model, recognition of α-mannan proteins by the dectin-2 receptor seems to be essential, as CAWS-injected Dectin-2 −/− mice do not develop vasculitis 191 .
The CAWS model shares some histological similarities with human Kawasaki disease pathology in that inflammation affects both the aortic root and the proximal region of the coronary arteries 190 . Inflammation can also affect non-coronary artery sites in 25% of CAWS-injected mice and can be observed in the lymph nodes, the kidneys and the liver 190 , 192 . CAWS-induced coronary artery lesions resemble those of human Kawasaki disease and are typically proliferative, granulomatous and characterized by intimal thickening with destruction of the elastic lamina and media 190 . Echocardiography in CAWS-injected mice indicates a marked decrease of cardiac function, which can be restored by IL-10 supplementation 193 . IL-10 is a potent anti-inflammatory cytokine that might improve the outcome of CAWS-induced vasculitis by inhibiting the release of pro-inflammatory mediators, such as TNF and IL-1β, from tissue-infiltrating innate immune cells 194 . Interestingly, CAWS-induced Kawasaki disease vasculitis is also strain dependent, as CAWS injections lead to a high incidence of vasculitis in CD-1, C3H/HeN, DBA/2 and C57BL/6N mice, but the CBA/JN strain is resistant to coronary arteritis 190 , 195 . The DBA/2 strain is the most sensitive, with the highest mortality rate resulting from a more intense coronary arteritis 195 . The sensitivity of DBA/2 mice is associated with increased production of the pro-inflammatory cytokines TNF, IL-6 and IFNγ 195 , 196 , whereas resistance of CBA/JN mice is explained by increased levels of IL-10 production in that strain 197 .
Despite the presence of T cell and B cell infiltration in the inflamed coronary artery, mice lacking T cells still develop moderate to typical cardiac inflammation, indicating that T cells might not be required in the development of Kawasaki disease vasculitis in this particular model 198 , 199 . Absence of both T cells and B cells in Rag1 −/− mice leads to lower incidence of CAWS-induced Kawasaki disease vasculitis; reconstitution of Rag1 −/− mice with wild-type, but not CCR2 −/− , T cells and B cells restores cardiovascular lesions, suggesting roles for both T cells and B cells and the modulation of disease development by CCR2 expression 200 . The innate immune response also participates in vasculitis development; resident macrophages recognize the CAWS antigens through the dectin-2 receptor, leading to their activation, release of CCL2, and recruitment of neutrophils and inflammatory monocytes producing IL-1β in the aortic root 201 .
CAWS-induced vasculitis is also associated with the rapid production of granulocyte–monocyte colony-stimulating factor in the heart, which subsequently drives inflammatory myocarditis by activating tissue macrophages and promoting recruitment of neutrophils and monocytes 199 . TNF is also produced during the acute phase of CAWS-induced Kawasaki disease vasculitis and is essential for the development of acute myocarditis, as TNF receptor-deficient mice are protected from the development of CAWS vasculitis 202 . IVIG administration substantially reduces CAWS-induced heart vessel inflammation 203 . Like the LCWE model, the CAWS model is also dependent on the IL-1 pathway, as IL1R −/− , IL1β −/− , Asc −/− and Nlrp3 −/− mice are protected from induction of vasculitis, and treatment with anti-IL-1β agents substantially attenuates CAWS vasculitis 202 , 204 , 205 .
The Nod1 ligand mouse model
Endothelial cells are equipped to sense microbial components through Toll-like receptors and nucleotide-binding oligomerization domain-containing protein like receptors. Subcutaneous injection or oral delivery of FK565, a specific synthetic Nod1 ligand, in mice primed with LPS results in a diffuse cellular inflammation of the aortic root and transmural infiltration of inflammatory cells in the coronary artery wall 178 , 206 . Other arteries, such as the iliac and renal arteries, also show signs of inflammation associated with a thickening of the intima 206 .
The mechanisms by which FK565 induces coronary arteritis in mice remain unknown. When administered orally, FK565 does not induce intestinal mucosa inflammation, but specifically activates vascular cells to produce a diverse array of pro-inflammatory cytokines, including IL-1β 206 , and chemokines such as CCL2, resulting in the recruitment of inflammatory cells in the tissues 178 . This model seems to be independent of T cells, B cells and natural killer T cells, as LPS-primed Rag-1 −/− mice still develop aortitis and coronary arteritis after FK565 injection 207 . The inflammatory infiltrates observed around the inflamed aortic root and coronary arteries mainly comprise neutrophils and CD11c + cardiac macrophages; their specific depletion considerably reduces the development of FK565-induced Kawasaki disease vasculitis 178 , 207 . The concentration of circulating IL-1β is substantially increased in the serum of FK565-injected mice compared with control or CAWS-injected animals, and higher IL-1β levels correlate with a larger inflammation area 206 . However, specific studies further investigating the role of IL-1β in this model are needed.
Treatment of Kawasaki disease
Traditional and novel therapies in humans.
The current standard of care for Kawasaki disease is the use of high-dose IVIG together with aspirin. If given during the first 10 days of the disease, IVIG reduces the risk of development of coronary arteritis and aneurysms from about 30% to 5–7% 14 , 15 . The mechanisms by which IVIG treatment reduces the inflammatory responses are still unknown; however, IVIG is suspected to have a wide spectrum of action targeting multiple arms of the immune response 18 . IVIG has been shown to inhibit IL-1β production from in vitro stimulated macrophages and to stimulate the production of IL-1Ra 208 , 209 . During Kawasaki disease, IVIG reduces production of inflammatory cytokines and chemokines, and decreases the activation and number of circulating neutrophils, monocytes, macrophages and activated T cells by saturating Fc receptors 18 . The majority of patients with Kawasaki disease who are treated with IVIG improve and do not develop coronary artery damage; however, up to 20% of children with Kawasaki disease do not respond to treatment or have fever recurrence after initial IVIG treatment, and these patients are at the highest risk of developing coronary artery lesions 3 , 20 , 210 .
The involvement of pro-inflammatory cytokines in the acute phase of Kawasaki disease suggests that combinational therapy, composed of IVIG associated with TNF inhibitors, steroids, calcineurin inhibitors or anakinra, might be useful to treat patients with IVIG-resistant disease. The use of TNF inhibitors in combination with IVIG has had mixed results thus far. Infliximab was associated with decreased fever duration and reduced markers of inflammation (C-reactive protein and neutrophil counts), suggesting a possible improvement of coronary artery outcomes 211 ; however, etanercept treatment resulted in a substantial reduction in IVIG resistance only in patients >1 year old 212 .
An important area of research is the use of biomarkers to predict IVIG resistance in Kawasaki disease. The Kobayashi scoring system, based on a combination of laboratory test results (for example, C-reactive protein levels, neutrophil percentages, platelets counts and levels of aspartate and alanine aminotransferase) and demographic variables (sex, age and number of days of illness before the start of the treatment) has been successfully used to predict IVIG-resistance in Japanese patients 213 , but not in North American children with Kawasaki disease 214 . The combination of prednisolone and IVIG to treat Japanese patients with Kawasaki disease predicted to have IVIG-resistant disease according to the Kobayashi score (RAISE study) resulted in more rapid fever resolution, reduced development of CAAs and lower incidence of additional rescue treatment 215 compared with IVIG alone.
As discussed above, Kawasaki disease susceptibility and increased coronary artery lesion risk are associated with an SNP in ITPKC 58 that results in a lack of NFAT regulation and activation of the T cell compartment owing to increased IL-2 production 216 . CD8 + cytotoxic T cells are present in the inflamed arterial wall during Kawasaki disease 24 , 135 ; therefore, targeting T cell expansion might be an efficient approach to preventing CAAs during Kawasaki disease. A combination treatment of IVIG and ciclosporin, a calcineurin inhibitor that suppresses IL-2 production and T cell activation, was tested in a clinical trial in Japanese patients with Kawasaki disease predicted to have IVIG-resistant disease based on the Kobayashi score (KAICA trial) 217 . In this trial, the combination treatment was shown to be safe and associated with a lower incidence of CAAs; however, treatment was linked with increased risk of relapse 217 . Furthermore, the scoring system used to identify IVIG-non-responders is poorly predictive in European children with Kawasaki disease, limiting the conclusions of this study.
The important role of the IL-1β–IL-1 receptor pathway in Kawasaki disease development has been demonstrated in both human patients 27 , 28 , 129 , 130 and mouse models 127 , 132 , 202 , 204 . Therefore, clinical trials investigating IL-1 pathway inhibition by using anakinra, which blocks both IL-1α and IL-1β, have been initiated in North America (ANAKID; ClinicalTrials.gov identifier NCT02179853) 218 and Europe (Kawakinra; European Clinical Trials number 2014-002715-4) 219 . Already, multiple case reports exist of the successful use of anakinra to treat patients with IVIG-resistant Kawasaki disease 220 , 221 , 222 , 223 , 224 , indicating the promise of this second-line therapy.
Therapeutic insights from mouse models
Although no animal model can fully mimic human disease, the LCWE-induced Kawasaki disease mouse model has been accepted by many in the research community as a reliable experimental model providing novel insights that can be tested in patients. For example, IVIG efficiently prevents coronary arteritis development in LCWE-injected mice 187 as well as in the CAWS mouse model of Kawasaki disease 203 .
The effects of the calcineurin inhibitors ciclosporin and tacrolimus have been investigated in the Nod1 ligand-induced mouse model of Kawasaki disease vasculitis 225 . This approach was rational given the established role of T cells and calcium signalling in Kawasaki disease. However, contrary to the expected outcome, these inhibitors exacerbated the coronary arteritis 225 . Notably, however, this result was probably related to the choice of mouse model, as the Nod1 ligand-mediated mouse model of Kawasaki disease vasculitis has previously been shown to be T cell-independent 207 . Indeed, in an independent study using the CAWS mouse model, which is T cell dependent, ciclosporin suppressed CAWS-induced vasculitis 226 , emphasizing the importance of model selection in preclinical studies. Most importantly, results in human studies bear out the therapeutic potential of calcineurin inhibition, as the Japanese phase III trial (KAICA trial) showed that adding ciclosporin to IVIG in patients with Kawasaki disease who were at high risk of IVIG resistance was beneficial in diminishing overall incidence of CAAs 217 .
The role of TNF has been investigated in both the LCWE and the CAWS mouse models of Kawasaki disease vasculitis 185 . Initially, etanercept treatment or genetic deletion of TNF receptor 1 was shown to protect mice from LCWE-induced coronary arteritis 185 , 202 . Infliximab treatment also prevented the development of both LCWE-induced coronary arteritis and myocarditis 132 . Similar results were obtained in the CAWS mouse model of Kawasaki disease vasculitis, in which etanercept 226 , 227 suppressed the incidence and decreased the severity of vasculitis. Mechanistically, TNF has been proposed to be produced by myeloid cells in the acute phase and to promote myocarditis and recruitment of immune cells by acting on cardiac stromal cells 202 . However, infliximab and etanercept might not directly target the TNF signalling pathway, and their observed effects might be indirect. Indeed, infliximab is not able to bind mouse TNF 227 , 228 ; therefore, the anti-inflammatory effect of infliximab might be attributable to the binding of Fc receptors at the surface of activated cells 229 , 230 .
The overwhelming evidence for the critical role of IL-1β in promoting LCWE-induced Kawasaki disease vasculitis in mice 127 , 128 , 132 led to the initiation of clinical trials testing the effect of anakinra for blocking IL-1β as a second therapy option to treat children with IVIG-resistant Kawasaki disease. Multiple case reports now outline the successful use of anakinra to treat patients with IVIG-resistant Kawasaki disease 221 , 222 , 223 , 224 . Alternatively, direct inhibition of the NLRP3 inflammasome might be a more targeted therapeutic strategy to treat Kawasaki disease, as it would affect several pathways beyond IL-1β, including IL-1α and IL-18. Several NLRP3 inhibitors have been identified 231 and tested in mouse models of inflammatory diseases, such as experimental autoimmune encephalomyelitis and cryopyrin-associated periodic syndrome 232 . It would be interesting to determine if such drugs could be used to prevent and reduce the cardiovascular complications in mouse models of Kawasaki disease vasculitis.
Conclusions
Over the past 40 years, research has improved our understanding of Kawasaki disease pathology and the development of coronary vasculitis. However, some questions still remain unanswered, such as the identification of the aetiological agents, how the disease is triggered, and the specific immune pathways associated with coronary vasculitis development and IVIG resistance. Owing to the rarity of human tissues from patients with Kawasaki disease, the use of animal models reproducing human Kawasaki disease features is invaluable. Many advances have been made over the decades by combining biological observations in human samples with mechanistic insights from experimental animal models. This ‘bench to bedside’ approach successfully led to the identification of the critical role of IL-1β in Kawasaki disease and resulted in the development of clinical trials in which anakinra is being used to treat children with IVIG-resistant Kawasaki disease.
LCWE-injected mice exhibit a dysfunctional intestinal barrier, and the increased IgA response and elevated sIgA levels in both LCWE-injected mice and children with Kawasaki disease reveal the existence of a ‘gut–vascular’ axis 143 . In evaluating this model system and the role of IgA, it should not be forgotten that injection of identically prepared LCWE induces chronic polyarthritis in selected inbred rat strains 180 , 233 . This observation implies that a common immunogenetic pathway might underlie a variety of autoimmune illnesses, with disease expression moderated not by the inducing agent, but rather by host genetics. The fact that cell wall fragments of common gut bacteria can produce varying disease manifestations in the face of inflammation-induced increased gut permeability suggests that some autoimmune diseases might not in fact be induced by the normal response to an unusual agent, but rather an unusual response to a common agent. Similarly, we hypothesize that vasculitic diseases, including Kawasaki disease, are not a usual response to an unusual environmental stimulus, but rather an unusual response (genetically determined) to a common environmental stimulus. This hypothesis has major implications for understanding the aetiology and pathogenesis of not only Kawasaki disease but also IgA-mediated diseases and perhaps others. In addition, it strongly suggests that inhibition of IL-1β might be effective for the many chronic inflammatory diseases in which IgA deposition is a key finding.
Kawasaki, T., Kosaki, F., Okawa, S., Shigematsu, I. & Yanagawa, H. A new infantile acute febrile mucocutaneous lymph node syndrome (MLNS) prevailing in Japan. Pediatrics 54 , 271–276 (1974).
CAS PubMed Google Scholar
Newburger, J. W., Takahashi, M. & Burns, J. C. Kawasaki disease. J. Am. Coll. Cardiol. 67 , 1738–1749 (2016).
Article PubMed Google Scholar
McCrindle, B. W. et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation 135 , e927–e999 (2017).
Newburger, J. W. et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation 110 , 2747–2771 (2004).
Singh, S., Vignesh, P. & Burgner, D. The epidemiology of Kawasaki disease: a global update. Arch. Dis. Child. 100 , 1084–1088 (2015).
Gordon, J. B., Kahn, A. M. & Burns, J. C. When children with Kawasaki disease grow up: myocardial and vascular complications in adulthood. J. Am. Coll. Cardiol. 54 , 1911–1920 (2009).
Article PubMed PubMed Central Google Scholar
Daniels, L. B. et al. Prevalence of Kawasaki disease in young adults with suspected myocardial ischemia. Circulation 125 , 2447–2453 (2012).
Rizk, S. R. et al. Acute myocardial ischemia in adults secondary to missed Kawasaki disease in childhood. Am. J. Cardiol. 115 , 423–427 (2015).
Gordon, J. B. et al. The spectrum of cardiovascular lesions requiring intervention in adults after Kawasaki disease. JACC Cardiovasc. Interv. 9 , 687–696 (2016).
Rosenkranz, M. E. et al. TLR2 and MyD88 contribute to Lactobacillus casei extract-induced focal coronary arteritis in a mouse model of Kawasaki disease. Circulation 112 , 2966–2973 (2005).
Article CAS PubMed Google Scholar
Schulte, D. J. et al. Involvement of innate and adaptive immunity in a murine model of coronary arteritis mimicking Kawasaki disease. J. Immunol. 183 , 5311–5318 (2009).
Article CAS PubMed PubMed Central Google Scholar
Rowley, A. H., Baker, S. C., Orenstein, J. M. & Shulman, S. T. Searching for the cause of Kawasaki disease–cytoplasmic inclusion bodies provide new insight. Nat. Rev. Microbiol. 6 , 394–401 (2008).
Rowley, A. H. et al. Ultrastructural, immunofluorescence, and RNA evidence support the hypothesis of a new virus associated with Kawasaki disease. J. Infect. Dis. 203 , 1021–1030 (2011).
Burns, J. C., Capparelli, E. V., Brown, J. A., Newburger, J. W. & Glode, M. P. Intravenous gamma-globulin treatment and retreatment in Kawasaki disease. US/Canadian Kawasaki Syndrome Study Group. Pediatr. Infect. Dis. J. 17 , 1144–1148 (1998).
Tremoulet, A. H. et al. Resistance to intravenous immunoglobulin in children with Kawasaki disease. J. Pediatr. 153 , 117–121 (2008).
Kato, H. et al. Long-term consequences of Kawasaki disease. A 10- to 21-year follow-up study of 594 patients. Circulation 94 , 1379–1385 (1996).
Friedman, K. G. et al. Coronary artery aneurysms in Kawasaki disease: risk factors for progressive disease and adverse cardiac events in the US population. J. Am. Heart Assoc. 5 , e003289 (2016).
Article PubMed PubMed Central CAS Google Scholar
Burns, J. C. & Franco, A. The immunomodulatory effects of intravenous immunoglobulin therapy in Kawasaki disease. Expert. Rev. Clin. Immunol. 11 , 819–825 (2015).
Moffett, B. S., Syblik, D., Denfield, S., Altman, C. & Tejtel-Sexson, K. Epidemiology of immunoglobulin resistant Kawasaki disease: results from a large, national database. Pediatr. Cardiol. 36 , 374–378 (2015).
Skochko, S. M. et al. Kawasaki disease outcomes and response to therapy in a multiethnic community: a 10-year experience. J. Pediatr. 203 , 408–415 (2018).
Makino, N. et al. Nationwide epidemiologic survey of Kawasaki disease in Japan, 2015–2016. Pediatr. Int. 61 , 397–403 (2019).
Takahashi, K., Oharaseki, T., Yokouchi, Y., Hiruta, N. & Naoe, S. Kawasaki disease as a systemic vasculitis in childhood. Ann. Vasc. Dis. 3 , 173–181 (2010).
Takahashi, K., Oharaseki, T. & Yokouchi, Y. Histopathological aspects of cardiovascular lesions in Kawasaki disease. Int. J. Rheum. Dis. 21 , 31–35 (2017).
Article PubMed CAS Google Scholar
Brown, T. J. et al. CD8 T lymphocytes and macrophages infiltrate coronary artery aneurysms in acute Kawasaki disease. J. Infect. Dis. 184 , 940–943 (2001).
Rowley, A. H., Eckerley, C. A., Jack, H. M., Shulman, S. T. & Baker, S. C. IgA plasma cells in vascular tissue of patients with Kawasaki syndrome. J. Immunol. 159 , 5946–5955 (1997).
Rowley, A. H. et al. IgA plasma cell infiltration of proximal respiratory tract, pancreas, kidney, and coronary artery in acute Kawasaki disease. J. Infect. Dis. 182 , 1183–1191 (2000).
Leung, D. Y. et al. Two monokines, interleukin 1 and tumor necrosis factor, render cultured vascular endothelial cells susceptible to lysis by antibodies circulating during Kawasaki syndrome. J. Exp. Med. 164 , 1958–1972 (1986).
Leung, D. Y. et al. Endothelial cell activation and high interleukin-1 secretion in the pathogenesis of acute Kawasaki disease. Lancet 2 , 1298–1302 (1989).
Rowley, A. H. Is Kawasaki disease an infectious disorder? Int. J. Rheum. Dis. 21 , 20–25 (2018).
Uehara, R. & Belay, E. D. Epidemiology of Kawasaki disease in Asia, Europe, and the United States. J. Epidemiol. 22 , 79–85 (2012).
Holman, R. C. et al. Hospitalizations for Kawasaki syndrome among children in the United States, 1997–2007. Pediatr. Infect. Dis. J. 29 , 483–488 (2010).
PubMed Google Scholar
Faulkner, L., Cooper, A., Fantino, C., Altmann, D. M. & Sriskandan, S. The mechanism of superantigen-mediated toxic shock: not a simple Th1 cytokine storm. J. Immunol. 175 , 6870–6877 (2005).
Abe, J. et al. Selective expansion of T cells expressing T-cell receptor variable regions V beta 2 and V beta 8 in Kawasaki disease. Proc. Natl Acad. Sci. USA 89 , 4066–4070 (1992).
Abe, J. et al. Characterization of T cell repertoire changes in acute Kawasaki disease. J. Exp. Med. 177 , 791–796 (1993).
Curtis, N., Zheng, R., Lamb, J. R. & Levin, M. Evidence for a superantigen mediated process in Kawasaki disease. Arch. Dis. Child. 72 , 308–311 (1995).
Mancia, L. et al. Characterization of the T-cell receptor V-beta repertoire in Kawasaki disease. Scand. J. Immunol. 48 , 443–449 (1998).
Pietra, B. A., De Inocencio, J., Giannini, E. H. & Hirsch, R. TCR V beta family repertoire and T cell activation markers in Kawasaki disease. J. Immunol. 153 , 1881–1888 (1994).
Iwanaga, M. et al. Kawasaki disease and Epstein-Barr virus. Lancet 317 , 938–939 (1981).
Article Google Scholar
Kikuta, H. et al. Recurrence of Kawasaki disease and Epstein-Barr virus infection. J. Infect. Dis. 162 , 1215–1215 (1990).
Kikuta, H. et al. Epstein-Barr virus genome-positive T lymphocytes in a boy with chronic active EBV infection associated with Kawasaki-like disease. Nature 333 , 455–457 (1988).
Marchette, N. J. et al. Epstein-Barr virus and other herpesvirus infections in Kawasaki syndrome. J. Infect. Dis. 161 , 680–684 (1990).
Fuse, S. et al. Children with Kawasaki disease are not infected with Epstein-Barr virus. Pediatr. Infect. Dis. J. 29 , 286–287 (2010).
Lee, S. J., Lee, K. Y., Han, J. W., Lee, J. S. & Whang, K. T. Epstein-Barr virus antibodies in Kawasaki disease. Yonsei Med. J. 47 , 475–479 (2006).
Esper, F. et al. Association between a novel human coronavirus and Kawasaki disease. J. Infect. Dis. 191 , 499–502 (2005).
Ebihara, T., Endo, R., Ma, X., Ishiguro, N. & Kikuta, H. Lack of association between New Haven coronavirus and Kawasaki disease. J. Infect. Dis. 192 , 351–352 (2005).
Belay, E. D. et al. Kawasaki disease and human coronavirus. J. Infect. Dis. 192 , 352–353; author reply 353 (2005).
Shulman, S. T. & Rowley, A. H. Does Kawasaki disease have a retroviral aetiology? Lancet 2 , 545–546 (1986).
Burns, J. C. et al. Polymerase activity in lymphocyte culture supernatants from patients with Kawasaki disease. Nature 323 , 814–816 (1986).
Nigro, G. & Midulla, M. Retrovirus and Kawasaki disease. Lancet 2 , 1045 (1986).
Rauch, A. M., Fultz, P. N. & Kalyanaraman, V. S. Retrovirus serology and Kawasaki syndrome. Lancet 329 , 1431 (1987).
Okamoto, T. et al. Lack of evidence of retroviral involvement in Kawasaki disease. Pediatrics 81 , 599 (1988).
Rowley, A. H. et al. A protein epitope targeted by the antibody response to Kawasaki disease. J. Infect. Dis. https://doi.org/10.1093/infdis/jiaa066 (2020).
Rowley, A. H. et al. Monoclonal antibodies from children with Kawasaki disease (KD) recognize hepacivirus peptides [abstract]. Presented at the 2019 Pediatric Academic Societies Meeting https://www.xcdsystem.com/pas2019/program/2019/index.cfm?pgid=156&sid=1060 (2019).
Rodo, X. et al. Tropospheric winds from northeastern China carry the etiologic agent of Kawasaki disease from its source to Japan. Proc. Natl Acad. Sci. USA 111 , 7952–7957 (2014).
Fujita, Y. et al. Kawasaki disease in families. Pediatrics 84 , 666–669 (1989).
Jayaraman, T., Ondriasova, E., Ondrias, K., Harnick, D. J. & Marks, A. R. The inositol 1,4,5-trisphosphate receptor is essential for T-cell receptor signaling. Proc. Natl Acad. Sci. USA 92 , 6007–6011 (1995).
Kuo, H. C. et al. ITPKC single nucleotide polymorphism associated with the Kawasaki disease in a Taiwanese population. PLoS One 6 , e17370 (2011).
Onouchi, Y. et al. ITPKC functional polymorphism associated with Kawasaki disease susceptibility and formation of coronary artery aneurysms. Nat. Genet. 40 , 35–42 (2008).
Alphonse, M. P. et al. Inositol-triphosphate 3-kinase C mediates inflammasome activation and treatment response in Kawasaki disease. J. Immunol. 197 , 3481–3489 (2016).
Kuo, H. C. et al. Lack of association between ORAI1/CRACM1 gene polymorphisms and Kawasaki disease in the Taiwanese children. J. Clin. Immunol. 31 , 650–655 (2011).
Onouchi, Y. et al. Variations in ORAI1 gene associated with Kawasaki disease. PLoS One 11 , e0145486 (2016).
Shimizu, C. et al. Genetic variation in the SLC8A1 calcium signaling pathway is associated with susceptibility to Kawasaki disease and coronary artery abnormalities. Circ. Cardiovasc. Genet. 9 , 559–568 (2016).
Murakami, T. et al. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc. Natl Acad. Sci. USA 109 , 11282–11287 (2012).
Rossol, M. et al. Extracellular Ca2+ is a danger signal activating the NLRP3 inflammasome through G protein-coupled calcium sensing receptors. Nat. Commun. 3 , 1329 (2012).
Elgueta, R. et al. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol. Rev. 229 , 152–172 (2009).
Wang, C. L. et al. Expression of CD40 ligand on CD4+ T-cells and platelets correlated to the coronary artery lesion and disease progress in Kawasaki disease. Pediatrics 111 , E140–E147 (2003).
Onouchi, Y. et al. CD40 ligand gene and Kawasaki disease. Eur. J. Hum. Genet. 12 , 1062–1068 (2004).
Huang, F. Y. et al. Genetic polymorphisms in the CD40 ligand gene and Kawasaki disease. J. Clin. Immunol. 28 , 405–410 (2008).
Kuo, H.-C. et al. CD40 gene polymorphisms associated with susceptibility and coronary artery lesions of Kawasaki disease in the Taiwanese population. ScientificWorldJournal 2012 , 520865 (2012).
Turner, M. W. The role of mannose-binding lectin in health and disease. Mol. Immunol. 40 , 423–429 (2003).
Biezeveld, M. H. et al. Polymorphisms in the mannose-binding lectin gene as determinants of age-defined risk of coronary artery lesions in Kawasaki disease. Arthritis Rheum. 54 , 369–376 (2006).
Biezeveld, M. H. et al. Association of mannose-binding lectin genotype with cardiovascular abnormalities in Kawasaki disease. Lancet 361 , 1268–1270 (2003).
Sato, S. et al. Association of mannose-binding lectin gene polymorphisms with Kawasaki disease in the Japanese. Int. J. Rheum. Dis. 12 , 307–310 (2009).
Nakamura, A. et al. Involvement of mannose-binding lectin in the pathogenesis of Kawasaki disease-like murine vasculitis. Clin. Immunol. 153 , 64–72 (2014).
Manger, K. et al. Fcgamma receptor IIa polymorphism in Caucasian patients with systemic lupus erythematosus: association with clinical symptoms. Arthritis Rheum. 41 , 1181–1189 (1998).
Brun, J. G., Madland, T. M. & Vedeler, C. A. Immunoglobulin G fc-receptor (FcgammaR) IIA, IIIA, and IIIB polymorphisms related to disease severity in rheumatoid arthritis. J. Rheumatol. 29 , 1135–1140 (2002).
Bredius, R. G. et al. Fc gamma receptor IIa (CD32) polymorphism in fulminant meningococcal septic shock in children. J. Infect. Dis. 170 , 848–853 (1994).
Biezeveld, M. et al. The involvement of Fc gamma receptor gene polymorphisms in Kawasaki disease. Clin. Exp. Immunol. 147 , 106–111 (2007).
CAS PubMed PubMed Central Google Scholar
Khor, C. C. et al. Genome-wide association study identifies FCGR2A as a susceptibility locus for Kawasaki disease. Nat. Genet. 43 , 1241–1246 (2011).
Tremoulet, A. H. Adjunctive therapies in Kawasaki disease. Int. J. Rheum. Dis. 21 , 76–79 (2018).
Orenstein, J. M. et al. Three linked vasculopathic processes characterize Kawasaki disease: a light and transmission electron microscopic study. PLoS One 7 , e38998 (2012).
Austermann, J., Spiekermann, C. & Roth, J. S100 proteins in rheumatic diseases. Nat. Rev. Rheumatol. 14 , 528–541 (2018).
Abe, J. et al. Gene expression profiling of the effect of high-dose intravenous Ig in patients with Kawasaki disease. J. Immunol. 174 , 5837–5845 (2005).
Lech, M. et al. Circulating markers of inflammation persist in children and adults with giant aneurysms after Kawasaki disease. Circ. Genom. Precis. Med. 12 , e002433 (2019).
Armaroli, G. et al. Monocyte-derived interleukin-1β as the driver of S100A12-induced sterile inflammatory activation of human coronary artery endothelial cells: implications for the pathogenesis of Kawasaki disease. Arthritis Rheumatol. 71 , 792–804 (2019).
Siefert, S. A. & Sarkar, R. Matrix metalloproteinases in vascular physiology and disease. Vascular 20 , 210–216 (2012).
Senzaki, H. The pathophysiology of coronary artery aneurysms in Kawasaki disease: role of matrix metalloproteinases. Arch. Dis. Child. 91 , 847–851 (2006).
Shimizu, C. et al. Matrix metalloproteinase haplotypes associated with coronary artery aneurysm formation in patients with Kawasaki disease. J. Hum. Genet. 55 , 779–784 (2010).
Korematsu, S. et al. Cell distribution differences of matrix metalloproteinase-9 and tissue inhibitor of matrix metalloproteinase-1 in patients with Kawasaki disease. Pediatr. Infect. Dis. J. 31 , 973–974 (2012).
Johnson, J. L., Dwivedi, A., Somerville, M., George, S. J. & Newby, A. C. Matrix metalloproteinase (MMP)-3 activates MMP-9 mediated vascular smooth muscle cell migration and neointima formation in mice. Arterioscler. Thromb. Vasc. Biol. 31 , e35–e44 (2011).
Popper, S. J. et al. Gene-expression patterns reveal underlying biological processes in Kawasaki disease. Genome Biol. 8 , R261 (2007).
Matsuyama, T. Tissue inhibitor of metalloproteinases-1 and matrix metalloproteinase-3 in Japanese healthy children and in Kawasaki disease and their clinical usefulness in juvenile rheumatoid arthritis. Pediatr. Int. 41 , 239–245 (1999).
Bonin, P. D., Fici, G. J. & Singh, J. P. Interleukin-1 promotes proliferation of vascular smooth muscle cells in coordination with PDGF or a monocyte derived growth factor. Exp. Cell Res. 181 , 475–482 (1989).
Sasu, S. & Beasley, D. Essential roles of IκB kinases α and β in serum- and IL-1-induced human VSMC proliferation. Am. J. Physiol. Heart Circ. Physiol. 278 , H1823–H1831 (2000).
Alexander, M. R. et al. Genetic inactivation of IL-1 signaling enhances atherosclerotic plaque instability and reduces outward vessel remodeling in advanced atherosclerosis in mice. J. Clin. Invest. 122 , 70–79 (2012).
Lau, A. C., Duong, T. T., Ito, S. & Yeung, R. S. Matrix metalloproteinase 9 activity leads to elastin breakdown in an animal model of Kawasaki disease. Arthritis Rheum. 58 , 854–863 (2008).
Lau, A. C., Duong, T. T., Ito, S., Wilson, G. J. & Yeung, R. S. Inhibition of matrix metalloproteinase-9 activity improves coronary outcome in an animal model of Kawasaki disease. Clin. Exp. Immunol. 157 , 300–309 (2009).
Tahamtan, A., Teymoori-Rad, M., Nakstad, B. & Salimi, V. Anti-inflammatory microRNAs and their potential for inflammatory diseases treatment. Front. Immunol. 9 , 1377 (2018).
van Rooij, E. The art of microRNA research. Circ. Res. 108 , 219–234 (2011).
Chu, M. et al. Bone marrow-derived microRNA-223 works as an endocrine genetic signal in vascular endothelial cells and participates in vascular injury from Kawasaki disease. J. Am. Heart Assoc. 6 , e004878 (2017).
Kuo, H.-C. et al. Next-generation sequencing identifies micro-RNA-based biomarker panel for Kawasaki disease. J. Allergy Clin. Immunol. 138 , 1227–1230 (2016).
Rowley, A. H. et al. A study of cardiovascular miRNA biomarkers for Kawasaki disease. Pediatr. Infect. Dis. J. 33 , 1296–1299 (2014).
Shimizu, C. et al. Differential expression of miR-145 in children with Kawasaki disease. PLoS One 8 , e58159 (2013).
He, M. et al. miR-483 targeting of CTGF suppresses endothelial-to-mesenchymal transition: therapeutic implications in Kawasaki disease. Circ. Res. 120 , 354–365 (2017).
Rangrez, A. Y., Massy, Z. A., Meuth, V. M.-L. & Metzinger, L. miR-143 and miR-145: molecular keys to switch the phenotype of vascular smooth muscle cells. Circ. Cardiovasc. Genet. 4 , 197–205 (2011).
Parmacek, M. S. MicroRNA-modulated targeting of vascular smooth muscle cells. J. Clin. Invest. 119 , 2526–2528 (2009).
Si, X. et al. miR‑23a downregulation modulates the inflammatory response by targeting ATG12‑mediated autophagy. Mol. Med. Rep. 18 , 1524–1530 (2018).
Long, B., Gan, T.-Y., Zhang, R.-C. & Zhang, Y.-H. miR-23a regulates cardiomyocyte apoptosis by targeting manganese superoxide dismutase. Mol. Cells 40 , 542–549 (2017).
Suzuki, A. et al. Active remodeling of the coronary arterial lesions in the late phase of Kawasaki disease: immunohistochemical study. Circulation 101 , 2935–2941 (2000).
Dahdah, N. Not just coronary arteritis, Kawasaki disease is a myocarditis, too. J. Am. Coll. Cardiol. 55 , 1507 (2010).
Dionne, A. & Dahdah, N. Myocarditis and Kawasaki disease. Int. J. Rheum. Dis. 21 , 45–49 (2017).
Harada, M. et al. Histopathological characteristics of myocarditis in acute-phase Kawasaki disease. Histopathology 61 , 1156–1167 (2012).
Yutani, C. et al. Cardiac biopsy of Kawasaki disease. Arch. Pathol. Lab. Med. 105 , 470–473 (1981).
Takahashi, M. Myocarditis in Kawasaki syndrome. A minor villain? Circulation 79 , 1398–1400 (1989).
Kao, C. H., Hsieh, K. S., Wang, Y. L., Wang, S. J. & Yeh, S. H. The detection of ventricular dysfunction and carditis in children with Kawasaki disease using equilibrium multigated blood pooling ventriculography and 99Tcm-HMPAO-labelled WBC heart scans. Nucl. Med. Commun. 14 , 539–543 (1993).
Printz, B. F. et al. Noncoronary cardiac abnormalities are associated with coronary artery dilation and with laboratory inflammatory markers in acute Kawasaki disease. J. Am. Coll. Cardiol. 57 , 86–92 (2011).
Burns, J. C. Kawasaki disease update. Indian. J. Pediatr. 76 , 71–76 (2009).
Miyake, T., Yokoyama, T., Shinohara, T., Seto, S. & Oiki, M. Transient dilatation of the abdominal aorta in an infant with Kawasaki disease associated with thrombocytopenia. Acta Paediatr. Jpn. 37 , 521–525 (1995).
Canter, C. E., Bower, R. J. & Strauss, A. W. Atypical Kawasaki disease with aortic aneurysm. Pediatrics 68 , 885–888 (1981).
Amano, S. et al. General pathology of Kawasaki disease. On the morphological alterations corresponding to the clinical manifestations. Acta Pathol. Jpn. 30 , 681–694 (1980).
Watanabe, T. Kidney and urinary tract involvement in Kawasaki disease. Int. J. Pediatr. 2013 , 831834 (2013).
Watanabe, T. Clinical features of acute kidney injury in patients with Kawasaki disease. World J. Clin. Pediatr. 7 , 83–88 (2018).
Ohshio, G. et al. High levels of IgA-containing circulating immune complex and secretory IgA in Kawasaki disease. Microbiol. Immunol. 31 , 891–898 (1987).
Rowley, A. H. et al. Cloning the arterial IgA antibody response during acute Kawasaki disease. J. Immunol. 175 , 8386–8391 (2005).
Rowley, A. H., Shulman, S. T., Spike, B. T., Mask, C. A. & Baker, S. C. Oligoclonal IgA response in the vascular wall in acute Kawasaki disease. J. Immunol. 166 , 1334–1343 (2001).
Lee, Y. et al. IL-1 signaling is critically required in stromal cells in Kawasaki disease vasculitis mouse model: role of both IL-1α and IL-1β. Arterioscler. Thromb. Vasc. Biol. 35 , 2605–2616 (2015).
Wakita, D. et al. Role of interleukin-1 signaling in a mouse model of Kawasaki disease-associated abdominal aortic aneurysm. Arterioscler. Thromb. Vasc. Biol. 36 , 886–897 (2016).
Hoang, L. T. et al. Global gene expression profiling identifies new therapeutic targets in acute Kawasaki disease. Genome Med. 6 , 541 (2014).
Fury, W. et al. Transcript abundance patterns in Kawasaki disease patients with intravenous immunoglobulin resistance. Hum. Immunol. 71 , 865–873 (2010).
Porritt, R. A. et al. Iinterleukin-1-mediated sex differences in Kawasaki disease vasculitis development and response to treatment. Arterioscler. Thromb. Vasc. Biol. 40 , 802–818 (2020).
Lee, Y. et al. Interleukin-1β is crucial for the induction of coronary artery inflammation in a mouse model of Kawasaki disease. Circulation 125 , 1542–1550 (2012).
Ben-Sasson, S. Z. et al. IL-1 enhances expansion, effector function, tissue localization, and memory response of antigen-specific CD8 T cells. J. Exp. Med. 210 , 491–502 (2013).
Hirao, J. & Sugita, K. Circulating CD4+CD8+ T lymphocytes in patients with Kawasaki disease. Clin. Exp. Immunol. 111 , 397–401 (1998).
Yilmaz, A. et al. Activated myeloid dendritic cells accumulate and co-localize with CD3+ T cells in coronary artery lesions in patients with Kawasaki disease. Exp. Mol. Pathol. 83 , 93–103 (2007).
Bischoff, S. C. et al. Intestinal permeability–a new target for disease prevention and therapy. BMC Gastroenterol. 14 , 189 (2014).
Miyake, T. et al. Small bowel pseudo-obstruction in Kawasaki disease. Pediatr. Radiol. 17 , 383–386 (1987).
Yaniv, L., Jaffe, M. & Shaoul, R. The surgical manifestations of the intestinal tract in Kawasaki disease. J. Pediatr. Surg. 40 , e1–e4 (2005).
Baker, A. L. et al. Associated symptoms in the ten days before diagnosis of Kawasaki disease. J. Pediatr. 154 , 592–595 (2009).
Colomba, C. et al. Intestinal involvement in Kawasaki disease. J. Pediatr. 202 , 186–193 (2018).
Fabi, M. et al. Gastrointestinal presentation of Kawasaki disease: a red flag for severe disease? PLoS One 13 , e0202658 (2018).
Nagata, S., Yamashiro, Y., Maeda, M., Ohtsuka, Y. & Yabuta, K. Immunohistochemical studies on small intestinal mucosa in Kawasaki disease. Pediatr. Res. 33 , 557–563 (1993).
Noval Rivas, M. et al. Intestinal permeability and IgA provoke immune vasculitis linked to cardiovascular inflammation. Immunity 51 , 508–521 (2019).
Tang, W. H. W. & Hazen, S. L. The contributory role of gut microbiota in cardiovascular disease. J. Clin. Invest. 124 , 4204–4211 (2014).
Tang, W. H. W. & Hazen, S. L. The gut microbiome and its role in cardiovascular diseases. Circulation 135 , 1008–1010 (2017).
Takeshita, S., Kobayashi, I., Kawamura, Y., Tokutomi, T. & Sekine, I. Characteristic profile of intestinal microflora in Kawasaki disease. Acta Paediatr. 91 , 783–788 (2002).
Yamashiro, Y., Nagata, S., Ohtsuka, Y., Oguchi, S. & Shimizu, T. Microbiologic studies on the small intestine in Kawasaki disease. Pediatr. Res. 39 , 622–624 (1996).
Nagata, S. et al. Heat shock proteins and superantigenic properties of bacteria from the gastrointestinal tract of patients with Kawasaki disease. Immunology 128 , 511–520 (2009).
Goldenberg, J. Z. et al. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst. Rev. 12 , CD004827 (2015).
Google Scholar
Allen, S. J., Martinez, E. G., Gregorio, G. V. & Dans, L. F. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst. Rev. 11 , CD003048 (2010).
Karczewski, J. et al. Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 298 , G851–G859 (2010).
Han, X. et al. Lactobacillus rhamnosus GG prevents epithelial barrier dysfunction induced by interferon-gamma and fecal supernatants from irritable bowel syndrome patients in human intestinal enteroids and colonoids. Gut Microbes 10 , 59–76 (2019).
Mack, D. R., Michail, S., Wei, S., McDougall, L. & Hollingsworth, M. A. Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. Am. J. Physiol. 276 , G941–G950 (1999).
van Baarlen, P., Wells, J. M. & Kleerebezem, M. Regulation of intestinal homeostasis and immunity with probiotic lactobacilli. Trends Immunol. 34 , 208–215 (2013).
Belkacem, N. et al. Lactobacillus paracasei feeding improves immune control of influenza infection in mice. PLoS One 12 , e0184976 (2017).
Belkacem, N., Bourdet-Sicard, R. & Taha, M. K. Lactobacillus paracasei feeding improves the control of secondary experimental meningococcal infection in flu-infected mice. BMC Infect. Dis. 18 , 167 (2018).
Gebremariam, H. G. et al. Lactobacillus gasseri suppresses the production of proinflammatory cytokines in Helicobacter pylori-infected macrophages by inhibiting the expression of ADAM17. Front. Immunol. 10 , 2326 (2019).
Downie, M. L. et al. Variability in response to intravenous immunoglobulin in the treatment of Kawasaki disease. J. Pediatr. 179 , 124–130 (2016).
Dethlefsen, L. & Relman, D. A. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc. Natl Acad. Sci. USA 108 (Suppl. 1), 4554–4561 (2011).
Modi, S. R., Collins, J. J. & Relman, D. A. Antibiotics and the gut microbiota. J. Clin. Invest. 124 , 4212–4218 (2014).
Wheeler, M. L. et al. Immunological consequences of intestinal fungal dysbiosis. Cell Host Microbe 19 , 865–873 (2016).
Jernberg, C., Löfmark, S., Edlund, C. & Jansson, J. K. Long-term ecological impacts of antibiotic administration on the human intestinal microbiota. ISME J. 1 , 56–66 (2007).
Kinumaki, A. et al. Characterization of the gut microbiota of Kawasaki disease patients by metagenomic analysis. Front. Microbiol. 6 , 824 (2015).
Audemard-Verger, A., Pillebout, E., Guillevin, L., Thervet, E. & Terrier, B. IgA vasculitis (Henoch-Shönlein purpura) in adults: diagnostic and therapeutic aspects. Autoimmun. Rev. 14 , 579–585 (2015).
Floege, J. & Feehally, J. The mucosa-kidney axis in IgA nephropathy. Nat. Rev. Nephrol. 12 , 147–156 (2016).
Coppo, R. The gut-kidney axis in IgA nephropathy: role of microbiota and diet on genetic predisposition. Pediatr. Nephrol. 33 , 53–61 (2018).
Moja, P. et al. Is there IgA from gut mucosal origin in the serum of children with Henoch-Schönlein purpura? Clin. Immunol. Immunopathol. 86 , 290–297 (1998).
Menikou, S., Langford, P. R. & Levin, M. Kawasaki disease: the role of immune complexes revisited. Front. Immunol. 10 , 1156 (2019).
Oni, L. & Sampath, S. Childhood IgA vasculitis (Henoch Schonlein Purpura)–advances and knowledge gaps. Front. Pediatr. 7 , 257 (2019).
Oortwijn, B. D. et al. A pathogenic role for secretory IgA in IgA nephropathy. Kidney Int. 69 , 1131–1138 (2006).
Nishimura, S. et al. A polymorphism in the promoter of the CD14 gene (CD14/-159) is associated with the development of coronary artery lesions in patients with Kawasaki disease. J. Pediatr. 143 , 357–362 (2003).
Yoon, H. J. et al. Association of the CD14 gene -159C polymorphism with progression of IgA nephropathy. J. Med. Genet. 40 , 104–108 (2003).
Boyer, E. M., Turman, M. & O’Neil, K. M. Partial response to anakinra in life-threatening Henoch-Schönlein purpura: case report. Pediatr. Rheumatol. Online J. 9 , 21 (2011).
Chun, J. et al. NLRP3 localizes to the tubular epithelium in human kidney and correlates with outcome in IgA nephropathy. Sci. Rep. 6 , 24667 (2016).
Meinzer, U. et al. Interleukin-1 targeting drugs in familial Mediterranean fever: a case series and a review of the literature. Semin. Arthritis Rheum. 41 , 265–271 (2011).
Lehman, T. J., Walker, S. M., Mahnovski, V. & McCurdy, D. Coronary arteritis in mice following the systemic injection of group B Lactobacillus casei cell walls in aqueous suspension. Arthritis Rheum. 28 , 652–659 (1985).
Murata, H. Experimental candida-induced arteritis in mice. Relation to arteritis in the mucocutaneous lymph node syndrome. Microbiol. Immunol. 23 , 825–831 (1979).
Nishio, H. et al. Nod1 ligands induce site-specific vascular inflammation. Arterioscler. Thromb. Vasc. Biol. 31 , 1093–1099 (2011).
Hill, D. et al. The Lactobacillus casei group: history and health related applications. Front. Microbiol. 9 , 2107 (2018).
Lehman, T. J., Allen, J. B., Plotz, P. H. & Wilder, R. L. Polyarthritis in rats following the systemic injection of Lactobacillus casei cell walls in aqueous suspension. Arthritis Rheum. 26 , 1259–1265 (1983).
Noval Rivas, M. et al. CD8+ T cells contribute to the development of coronary arteritis in the Lactobacillus casei cell wall extract-induced murine model of Kawasaki disease. Arthritis Rheumatol. 69 , 410–421 (2017).
Matundan, H. H. et al. Myocardial fibrosis after adrenergic stimulation as a long-term sequela in a mouse model of Kawasaki disease vasculitis. JCI Insight 4 , e126279 (2019).
Article PubMed Central Google Scholar
Abe, M. et al. IL-1-dependent electrophysiological changes and cardiac neural remodeling in a mouse model of Kawasaki disease vasculitis. Clin. Exp. Immunol. 199 , 303–313 (2019).
Gorelik, M. et al. IL-1 receptor antagonist, anakinra, prevents myocardial dysfunction in a mouse model of Kawasaki disease vasculitis and myocarditis. Clin. Exp. Immunol. 198 , 101–110 (2019).
Hui-Yuen, J. S., Duong, T. T. & Yeung, R. S. M. TNF-α is necessary for induction of coronary artery inflammation and aneurysm formation in an animal model of Kawasaki disease. J. Immunol. 176 , 6294–6301 (2006).
Lehman, T. J., Warren, R., Gietl, D., Mahnovski, V. & Prescott, M. Variable expression of Lactobacillus casei cell wall-induced coronary arteritis: an animal model of Kawasaki’s disease in selected inbred mouse strains. Clin. Immunol. Immunopathol. 48 , 108–118 (1988).
Myones, B., Bathoria, J., Lehman, T. & Shulman, S. in Kawasaki Disease: Proceedings of the 5th International Kawasaki Disease Symposium (ed. Kato, H et al.) 252–256 (Elsevier, 1995).
Maury, C. P., Salo, E. & Pelkonen, P. Circulating interleukin-1β in patients with Kawasaki disease. N. Engl. J. Med. 319 , 1670–1671 (1988).
Ohno, N. Chemistry and biology of angiitis inducer, Candida albicans water-soluble mannoprotein-β-glucan complex (CAWS). Microbiol. Immunol. 47 , 479–490 (2003).
Takahashi, K. et al. Histopathological features of murine systemic vasculitis caused by Candida albicans extract–an animal model of Kawasaki disease. Inflamm. Res. 53 , 72–77 (2004).
Oharaseki, T. et al. Recognition of alpha-mannan by dectin 2 is essential for onset of Kawasaki disease-like murine vasculitis induced by Candida albicans cell-wall polysaccharide. Mod. Rheumatol. 30 , 350–357 (2020).
Yoshikane, Y. et al. JNK is critical for the development of Candida albicans-induced vascular lesions in a mouse model of Kawasaki disease. Cardiovasc. Pathol. 24 , 33–40 (2015).
Nakamura, J. et al. Adeno-associated virus vector-mediated interleukin-10 induction prevents vascular inflammation in a murine model of Kawasaki disease. Sci. Rep. 8 , 7601 (2018).
Sabat, R. et al. Biology of interleukin-10. Cytokine Growth Factor. Rev. 21 , 331–344 (2010).
Ohno, N. Murine model of Kawasaki disease induced by mannoprotein-beta-glucan complex, CAWS, obtained from Candida albicans. Jpn. J. Infect. Dis. 57 , S9–S10 (2004).
Miura, N. N. et al. IL-10 is a negative regulatory factor of CAWS-vasculitis in CBA/J mice as assessed by comparison with Bruton’s tyrosine kinase-deficient CBA/N mice. J. Immunol. 183 , 3417–3424 (2009).
Ohno, N. A murine model of vasculitis induced by fungal polysaccharide. Cardiovasc. Hematol. Agents Med. Chem. 6 , 44–52 (2008).
Nagi-Miura, N., Adachi, Y. & Ohno, N. Coronary arteritis induced by CAWS (Candida albicans water-soluble fraction) in various strains of mice. Nihon Ishinkin Gakkai Zasshi 49 , 287–292 (2008).
Stock, A. T., Hansen, J. A., Sleeman, M. A., McKenzie, B. S. & Wicks, I. P. GM-CSF primes cardiac inflammation in a mouse model of Kawasaki disease. J. Exp. Med. 213 , 1983–1998 (2016).
Martinez, H. G. et al. Important role of CCR2 in a murine model of coronary vasculitis. BMC Immunol. 13 , 56 (2012).
Miyabe, C. et al. Dectin-2-induced CCL2 production in tissue-resident macrophages ignites cardiac arteritis. J. Clin. Invest. 130 , 3610–3624 (2019).
Stock, A. T., Jama, H. A., Hansen, J. A. & Wicks, I. P. TNF and IL-1 play essential but temporally distinct roles in driving cardiac inflammation in a murine model of Kawasaki disease. J. Immunol. 202 , 3151–3160 (2019).
Takahashi, K. et al. Administration of human immunoglobulin suppresses development of murine systemic vasculitis induced with Candida albicans water-soluble fraction: an animal model of Kawasaki disease. Mod. Rheumatol. 20 , 160–167 (2010).
Hashimoto, Y. et al. Interleukin-1beta inhibition attenuates vasculitis in a mouse model of Kawasaki disease. J. Nippon. Med. Sch. 86 , 108–116 (2019).
Anzai, F. et al. Crucial role of NLRP3 inflammasome in a murine model of Kawasaki disease. J. Mol. Cell Cardiol. 138 , 185–196 (2019).
Ohashi, R. et al. Characterization of a murine model with arteritis induced by Nod1 ligand, FK565: a comparative study with a CAWS-induced model. Mod. Rheumatol. 27 , 1024–1030 (2017).
Motomura, Y. et al. Identification of pathogenic cardiac CD11c+ macrophages in Nod1-mediated acute coronary arteritis. Arterioscler. Thromb. Vasc. Biol. 35 , 1423–1433 (2015).
Iwata, M., Shimozato, T., Tokiwa, H. & Tsubura, E. Antipyretic activity of a human immunoglobulin preparation for intravenous use in an experimental model of fever in rabbits. Infect. Immun. 55 , 547–554 (1987).
Ruiz de Souza, V. et al. Selective induction of interleukin-1 receptor antagonist and interleukin-8 in human monocytes by normal polyspecific IgG (intravenous immunoglobulin). Eur. J. Immunol. 25 , 1267–1273 (1995).
Uehara, R. et al. Analysis of potential risk factors associated with nonresponse to initial intravenous immunoglobulin treatment among Kawasaki disease patients in Japan. Pediatr. Infect. Dis. J. 27 , 155–160 (2008).
Tremoulet, A. H. et al. Infliximab for intensification of primary therapy for Kawasaki disease: a phase 3 randomised, double-blind, placebo-controlled trial. Lancet 383 , 1731–1738 (2014).
Portman, M. A. et al. Etanercept with IVIg for acute Kawasaki disease: a randomized controlled trial. Pediatrics 143 , e20183675 (2019).
Kobayashi, T. et al. Prediction of intravenous immunoglobulin unresponsiveness in patients with Kawasaki disease. Circulation 113 , 2606–2612 (2006).
Sleeper, L. A. et al. Evaluation of Kawasaki disease risk-scoring systems for intravenous immunoglobulin resistance. J. Pediatr. 158 , 831–835 (2011).
Kobayashi, T. et al. Efficacy of immunoglobulin plus prednisolone for prevention of coronary artery abnormalities in severe Kawasaki disease (RAISE study): a randomised, open-label, blinded-endpoints trial. Lancet 379 , 1613–1620 (2012).
Lee, J., Kim, L. & Choi, J. Revisiting the concept of targeting NFAT to control T cell immunity and autoimmune diseases. Front. Immunol. 9 , 2747–2747 (2018).
Hamada, H. et al. Efficacy of primary treatment with immunoglobulin plus ciclosporin for prevention of coronary artery abnormalities in patients with Kawasaki disease predicted to be at increased risk of non-response to intravenous immunoglobulin (KAICA): a randomised controlled, open-label, blinded-endpoints, phase 3 trial. Lancet 393 , 1128–1137 (2019).
Tremoulet, A. H. et al. Rationale and study design for a phase I/IIa trial of anakinra in children with Kawasaki disease and early coronary artery abnormalities (the ANAKID trial). Contemp. Clin. Trials 48 , 70–75 (2016).
Koné-Paut, I. et al. KAWAKINRA: a phase IIA multicenter trial to assess the efficacy, and safety of anakinra in patients with intravenous immunoglobulin-resistant Kawasaki disease [abstract OP0147]. Ann. Rheum. Dis. 78 (Suppl. 2), 149 (2019).
Cohen, S. et al. A child with severe relapsing Kawasaki disease rescued by IL-1 receptor blockade and extracorporeal membrane oxygenation. Ann. Rheum. Dis. 71 , 2059–2061 (2012).
Shafferman, A., Birmingham, J. D. & Cron, R. Q. High dose anakinra for treatment of severe neonatal Kawasaki disease: a case report. Pediatr. Rheumatol. Online J. 12 , 26 (2014).
Guillaume, M. P., Reumaux, H. & Dubos, F. Usefulness and safety of anakinra in refractory Kawasaki disease complicated by coronary artery aneurysm. Cardiol. Young 28 , 739–742 (2018).
Sánchez-Manubens, J. et al. A child with resistant Kawasaki disease successfully treated with anakinra: a case report. BMC Pediatr. 17 , 102 (2017).
Blonz, G. et al. Severe late-onset Kawasaki disease successfully treated with anakinra. J. Clin. Rheumatol. 26 , e42–e43 (2020).
Murata, K. et al. Calcineurin inhibitors exacerbate coronary arteritis via the MyD88 signalling pathway in a murine model of Kawasaki disease. Clin. Exp. Immunol. 190 , 54–67 (2017).
Ohashi, R. et al. Etanercept suppresses arteritis in a murine model of Kawasaki disease: a comparative study involving different biological agents. Int. J. Vasc. Med. 2013 , 543141 (2013).
PubMed PubMed Central Google Scholar
Oharaseki, T. et al. The role of TNF-α in a murine model of Kawasaki disease arteritis induced with a Candida albicans cell wall polysaccharide. Mod. Rheumatol. 24 , 120–128 (2014).
Assas, B. M. et al. Anti-inflammatory effects of infliximab in mice are independent of tumour necrosis factor α neutralization. Clin. Exp. Immunol. 187 , 225–233 (2017).
Arora, T. et al. Differences in binding and effector functions between classes of TNF antagonists. Cytokine 45 , 124–131 (2009).
Vos, A. C. et al. Anti-tumor necrosis factor-α antibodies induce regulatory macrophages in an Fc region-dependent manner. Gastroenterology 140 , 221–230 (2011).
Mangan, M. S. J. et al. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat. Rev. Drug. Discov. 17 , 688 (2018).
Coll, R. C. et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat. Med. 21 , 248–255 (2015).
Lehman, T. J., Allen, J. B., Plotz, P. H. & Wilder, R. L. Lactobacillus casei cell wall-induced arthritis in rats: cell wall fragment distribution and persistence in chronic arthritis-susceptible LEW/N and -resistant F344/N rats. Arthritis Rheum. 27 , 939–942 (1984).
Download references
Acknowledgements
The work of M.A. is supported by the NIH Grant R01 AI072726 and M.N.R. is supported by the NIH grant R01 HL139766.
Author information
Authors and affiliations.
Departments of Pediatrics, Division of Infectious Diseases and Immunology, and Infectious and Immunologic Diseases Research Center (IIDRC), Department of Biomedical Sciences, Cedars-Sinai Medical Center, Los Angeles, CA, USA
Magali Noval Rivas & Moshe Arditi
Department of Pediatrics, David Geffen School of Medicine at UCLA, Los Angeles, CA, USA
Cedars-Sinai Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, CA, USA
Moshe Arditi
You can also search for this author in PubMed Google Scholar
Contributions
Both authors contributed equally to all aspects of the manuscript.
Corresponding authors
Correspondence to Magali Noval Rivas or Moshe Arditi .
Ethics declarations
Competing interests.
The authors declare no competing interests.

Additional information
Peer review information.
Nature Reviews Rheumatology thanks Ho-Chang Kuo, Isabelle Kone-Paut and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Noval Rivas, M., Arditi, M. Kawasaki disease: pathophysiology and insights from mouse models. Nat Rev Rheumatol 16 , 391–405 (2020). https://doi.org/10.1038/s41584-020-0426-0
Download citation
Accepted : 23 April 2020
Published : 26 May 2020
Issue Date : July 2020
DOI : https://doi.org/10.1038/s41584-020-0426-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Development of a prediction model for progression of coronary artery lesions in kawasaki disease.
- Ye-Shi Chen
- Xiao-Hui Li
Pediatric Research (2024)
Immunopathology of and potential therapeutics for secondary hemophagocytic lymphohistiocytosis/macrophage activation syndrome: a translational perspective
- Tram T. T. Nguyen
- Yoon Tae Kim
Experimental & Molecular Medicine (2024)
Markers of Endothelial Dysfunction in Kawasaki Disease: An Update
- Rajni Kumrah
- Surjit Singh
Clinical Reviews in Allergy & Immunology (2024)
[18F]FDG PET-MR characterization of aortitis in the IL1rn−/− mouse model of giant-cell arteritis
- Samuel Deshayes
- Caroline Baugé
- Achille Aouba
EJNMMI Research (2023)
Kawasaki disease: ubiquitin-specific protease 5 promotes endothelial inflammation via TNFα-mediated signaling
- Chengcheng Huang
Pediatric Research (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Achieve Mastery of Medical Concepts
Study for medical school and boards with lecturio.
USMLE Step 1 | USMLE Step 2 | COMLEX Level 1 | COMLEX Level 2 | ENARM | NEET
Kawasaki Disease
Kawasaki disease (KD), also known as mucocutaneous lymph node syndrome or infantile polyarteritis, is a medium-sized necrotizing vasculitis that predominantly affects children < 5 years of age. The e tiology is currently unknown, but it is postulated to involve a combination of environmental and genetic factors. Multiple systems are involved, but the disease displays a predilection for the coronary arteries, which may lead to serious complications. The diagnosis can be made based on clinical criteria. In cases where incomplete KD may be suspected, the diagnosis may be supported by laboratory studies and echocardiography. Management involves intravenous immunoglobulin and high-dose aspirin Aspirin The prototypical analgesic used in the treatment of mild to moderate pain. It has anti-inflammatory and antipyretic properties and acts as an inhibitor of cyclooxygenase which results in the inhibition of the biosynthesis of prostaglandins. Aspirin also inhibits platelet aggregation and is used in the prevention of arterial and venous thrombosis. Nonsteroidal Antiinflammatory Drugs (NSAIDs) . Follow-up requires serial echocardiograms to monitor for coronary artery Coronary Artery Truncus Arteriosus aneurysm Aneurysm An aneurysm is a bulging, weakened area of a blood vessel that causes an abnormal widening of its diameter > 1.5 times the size of the native vessel. Aneurysms occur more often in arteries than in veins and are at risk of dissection and rupture, which can be life-threatening. Thoracic Aortic Aneurysms .
Last updated: Nov 15, 2023
Epidemiology and Etiology
Pathophysiology, clinical presentation, complications, differential diagnosis.
Share this concept:
Epidemiology
- One of the most common vasculitides Vasculitides Vasculitides are a group of conditions characterized by vasculitis, ischemia, and damage to the organs supplied by the affected vessels. The affected arteries are of different sizes and locations and vary by the type of vasculitis. Vasculitides of childhood
- Boys are more commonly affected than girls.
- 80%–90% of cases are in children younger than 5 years of age.
- Highest incidence Incidence The number of new cases of a given disease during a given period in a specified population. It also is used for the rate at which new events occur in a defined population. It is differentiated from prevalence, which refers to all cases in the population at a given time. Measures of Disease Frequency in children who live in East Asia ASIA Spinal Cord Injuries or are of Asian ancestry living in other parts of the world
- Overall annual incidence Incidence The number of new cases of a given disease during a given period in a specified population. It also is used for the rate at which new events occur in a defined population. It is differentiated from prevalence, which refers to all cases in the population at a given time. Measures of Disease Frequency of 20 per 100,000 children younger than 5 years in the United States
- ¼ of adult cases occur in patients Patients Individuals participating in the health care system for the purpose of receiving therapeutic, diagnostic, or preventive procedures. Clinician–Patient Relationship with HIV HIV Anti-HIV Drugs .
The etiology of Kawasaki disease (KD) is unknown. There are several theories:
Immunologic response theory:
- Neutrophils Neutrophils Granular leukocytes having a nucleus with three to five lobes connected by slender threads of chromatin, and cytoplasm containing fine inconspicuous granules and stainable by neutral dyes. Innate Immunity: Phagocytes and Antigen Presentation
- CD8+ T cells T cells Lymphocytes responsible for cell-mediated immunity. Two types have been identified – cytotoxic (t-lymphocytes, cytotoxic) and helper T-lymphocytes (t-lymphocytes, helper-inducer). They are formed when lymphocytes circulate through the thymus gland and differentiate to thymocytes. When exposed to an antigen, they divide rapidly and produce large numbers of new T cells sensitized to that antigen. T cells: Types and Functions
- Eosinophils Eosinophils Granular leukocytes with a nucleus that usually has two lobes connected by a slender thread of chromatin, and cytoplasm containing coarse, round granules that are uniform in size and stainable by eosin. Innate Immunity: Phagocytes and Antigen Presentation
- IgA IgA Represents 15-20% of the human serum immunoglobulins, mostly as the 4-chain polymer in humans or dimer in other mammals. Secretory iga is the main immunoglobulin in secretions. Immunoglobulins: Types and Functions plasma Plasma The residual portion of blood that is left after removal of blood cells by centrifugation without prior blood coagulation. Transfusion Products cells
- Macrophages Macrophages The relatively long-lived phagocytic cell of mammalian tissues that are derived from blood monocytes. Main types are peritoneal macrophages; alveolar macrophages; histiocytes; kupffer cells of the liver; and osteoclasts. They may further differentiate within chronic inflammatory lesions to epithelioid cells or may fuse to form foreign body giant cells or langhans giant cells. Innate Immunity: Phagocytes and Antigen Presentation (unique among the vasculitides Vasculitides Vasculitides are a group of conditions characterized by vasculitis, ischemia, and damage to the organs supplied by the affected vessels. The affected arteries are of different sizes and locations and vary by the type of vasculitis. Vasculitides )
- Adrenomedullin
- Granulin
- The stimulus for this gene Gene A category of nucleic acid sequences that function as units of heredity and which code for the basic instructions for the development, reproduction, and maintenance of organisms. Basic Terms of Genetics expression and inflammatory infiltration is unknown.
Infection theory:
- Febrile exanthem Exanthem Diseases in which skin eruptions or rashes are a prominent manifestation. Classically, six such diseases were described with similar rashes; they were numbered in the order in which they were reported. Only the fourth (Duke’s disease), fifth (erythema infectiosum), and sixth (exanthema subitum) numeric designations survive as occasional synonyms in current terminology. Varicella-Zoster Virus/Chickenpox with lymphadenitis Lymphadenitis Inflammation of the lymph nodes. Peritonsillar Abscess and mucositis Mucositis Stomatitis is a general term referring to inflammation of the mucous membranes of the mouth, which may include sores. Stomatitis can be caused by infections, autoimmune disorders, allergic reactions, or exposure to irritants. The typical presentation may be either solitary or a group of painful oral lesions. Stomatitis
- ↑ Incidence Incidence The number of new cases of a given disease during a given period in a specified population. It also is used for the rate at which new events occur in a defined population. It is differentiated from prevalence, which refers to all cases in the population at a given time. Measures of Disease Frequency during winter Winter Pityriasis Rosea and summer
- Occurs in epidemics Epidemics Sudden outbreaks of a disease in a country or region not previously recognized in that area, or a rapid increase in the number of new cases of a previous existing endemic disease. Epidemics can also refer to outbreaks of disease in animal or plant populations. Influenza Viruses/Influenza
- Boys > girls
- ↑ Incidence Incidence The number of new cases of a given disease during a given period in a specified population. It also is used for the rate at which new events occur in a defined population. It is differentiated from prevalence, which refers to all cases in the population at a given time. Measures of Disease Frequency in younger children
- Adenovirus Adenovirus Adenovirus (member of the family Adenoviridae) is a nonenveloped, double-stranded DNA virus. Adenovirus is transmitted in a variety of ways, and it can have various presentations based on the site of entry. Presentation can include febrile pharyngitis, conjunctivitis, acute respiratory disease, atypical pneumonia, and gastroenteritis. Adenovirus
- Cytomegalovirus Cytomegalovirus CMV is a ubiquitous double-stranded DNA virus belonging to the Herpesviridae family. CMV infections can be transmitted in bodily fluids, such as blood, saliva, urine, semen, and breast milk. The initial infection is usually asymptomatic in the immunocompetent host, or it can present with symptoms of mononucleosis. Cytomegalovirus
- Rotavirus Rotavirus A genus of Reoviridae, causing acute gastroenteritis in birds and mammals, including humans. Transmission is horizontal and by environmental contamination. Seven species (rotaviruses A through G) are recognized. Rotavirus
- Bacterial: Mycoplasma Mycoplasma Mycoplasma is a species of pleomorphic bacteria that lack a cell wall, which makes them difficult to target with conventional antibiotics and causes them to not gram stain well. Mycoplasma bacteria commonly target the respiratory and urogenital epithelium. Mycoplasma pneumoniae (M. pneumoniae), the causative agent of atypical or “walking” pneumonia. Mycoplasma
Genetic predisposition theory:
- Asian populations
- Family members (siblings of those affected with KD have a higher likelihood of also developing KD than the general population)
- Variations and polymorphisms of genes Genes A category of nucleic acid sequences that function as units of heredity and which code for the basic instructions for the development, reproduction, and maintenance of organisms. DNA Types and Structure related to immune regulation and vascular homeostasis Homeostasis The processes whereby the internal environment of an organism tends to remain balanced and stable. Cell Injury and Death have been associated with KD.
Environmental factors theory:
- Mercury Mercury A silver metallic element that exists as a liquid at room temperature. It has the atomic symbol Hg (from hydrargyrum, liquid silver), atomic number 80, and atomic weight 200. 59. Mercury is used in many industrial applications and its salts have been employed therapeutically as purgatives, antisyphilitics, disinfectants, and astringents. It can be absorbed through the skin and mucous membranes which leads to mercury poisoning. Because of its toxicity, the clinical use of mercury and mercurials is diminishing. Renal Tubular Acidosis
- Dust mites Mites Any arthropod of the subclass acari except the ticks. They are minute animals related to the spiders, usually having transparent or semitransparent bodies. They may be parasitic on humans and domestic animals, producing various irritations of the skin (mite infestations). Many mite species are important to human and veterinary medicine as both parasite and vector. Mites also infest plants. Scabies
- Rug shampoo
- Pollen
- Lacks supporting evidence
Related videos
Kawasaki disease is a systemic, inflammatory illness that affects medium-sized arteries Arteries Arteries are tubular collections of cells that transport oxygenated blood and nutrients from the heart to the tissues of the body. The blood passes through the arteries in order of decreasing luminal diameter, starting in the largest artery (the aorta) and ending in the small arterioles. Arteries are classified into 3 types: large elastic arteries, medium muscular arteries, and small arteries and arterioles. Arteries: Histology , especially the coronary arteries Arteries Arteries are tubular collections of cells that transport oxygenated blood and nutrients from the heart to the tissues of the body. The blood passes through the arteries in order of decreasing luminal diameter, starting in the largest artery (the aorta) and ending in the small arterioles. Arteries are classified into 3 types: large elastic arteries, medium muscular arteries, and small arteries and arterioles. Arteries: Histology .
- Multiple organs and tissues are involved, but long-term sequelae occur only in arteries Arteries Arteries are tubular collections of cells that transport oxygenated blood and nutrients from the heart to the tissues of the body. The blood passes through the arteries in order of decreasing luminal diameter, starting in the largest artery (the aorta) and ending in the small arterioles. Arteries are classified into 3 types: large elastic arteries, medium muscular arteries, and small arteries and arterioles. Arteries: Histology .
- Early-stage : vascular media and endothelium Endothelium A layer of epithelium that lines the heart, blood vessels (vascular endothelium), lymph vessels (lymphatic endothelium), and the serous cavities of the body. Arteries: Histology become edematous
- Eosinophils Eosinophils Granular leukocytes with a nucleus that usually has two lobes connected by a slender thread of chromatin, and cytoplasm containing coarse, round granules that are uniform in size and stainable by eosin. Innate Immunity: Phagocytes and Antigen Presentation and macrophages Macrophages The relatively long-lived phagocytic cell of mammalian tissues that are derived from blood monocytes. Main types are peritoneal macrophages; alveolar macrophages; histiocytes; kupffer cells of the liver; and osteoclasts. They may further differentiate within chronic inflammatory lesions to epithelioid cells or may fuse to form foreign body giant cells or langhans giant cells. Innate Immunity: Phagocytes and Antigen Presentation can also be prominent.
- Multiple cytokines Cytokines Non-antibody proteins secreted by inflammatory leukocytes and some non-leukocytic cells, that act as intercellular mediators. They differ from classical hormones in that they are produced by a number of tissue or cell types rather than by specialized glands. They generally act locally in a paracrine or autocrine rather than endocrine manner. Adaptive Immune Response and matrix metalloproteinases Matrix metalloproteinases A family of zinc-dependent metalloendopeptidases that is involved in the degradation of extracellular matrix components. Hypertrophic and Keloid Scars are secreted by inflammatory cells that result in vascular damage.
- Fibrous Fibrous Fibrocystic Change connective tissue Connective tissue Connective tissues originate from embryonic mesenchyme and are present throughout the body except inside the brain and spinal cord. The main function of connective tissues is to provide structural support to organs. Connective tissues consist of cells and an extracellular matrix. Connective Tissue: Histology within the vascular wall can develop and cause a thickening of the intima, narrowing of the vessel lumen, and formation of a thrombus.
- Destruction of elastin and collagen Collagen A polypeptide substance comprising about one third of the total protein in mammalian organisms. It is the main constituent of skin; connective tissue; and the organic substance of bones (bone and bones) and teeth (tooth). Connective Tissue: Histology fibers can cause a loss of structural integrity of the arterial wall leading to dilation and aneurysm Aneurysm An aneurysm is a bulging, weakened area of a blood vessel that causes an abnormal widening of its diameter > 1.5 times the size of the native vessel. Aneurysms occur more often in arteries than in veins and are at risk of dissection and rupture, which can be life-threatening. Thoracic Aortic Aneurysms formation.
Commonly presenting symptoms
- > 101.3°F (38.5°C)
- Minimally responsive to antipyretics
- Presents with great variability (polymorphous)
- Usually NOT vesicular
- Found on trunk, extremities, and perineal regions
- Often described as “unconsolable”
- Decreased activity and appetite are common.
- Bilateral
- Nonexudative
- Limbic sparing
- Begins within days of fever Fever Fever is defined as a measured body temperature of at least 38°C (100.4°F). Fever is caused by circulating endogenous and/or exogenous pyrogens that increase levels of prostaglandin E2 in the hypothalamus. Fever is commonly associated with chills, rigors, sweating, and flushing of the skin. Fever
- Sometimes seen with photophobia Photophobia Abnormal sensitivity to light. This may occur as a manifestation of eye diseases; migraine; subarachnoid hemorrhage; meningitis; and other disorders. Photophobia may also occur in association with depression and other mental disorders. Migraine Headache
- Erythema Erythema Redness of the skin produced by congestion of the capillaries. This condition may result from a variety of disease processes. Chalazion of the lips Lips The lips are the soft and movable most external parts of the oral cavity. The blood supply of the lips originates from the external carotid artery, and the innervation is through cranial nerves. Lips and Tongue: Anatomy and oral mucosa Oral mucosa Lining of the oral cavity, including mucosa on the gums; the palate; the lip; the cheek; floor of the mouth; and other structures. The mucosa is generally a nonkeratinized stratified squamous epithelium covering muscle, bone, or glands but can show varying degree of keratinization at specific locations. Stomatitis
- “Strawberry tongue Tongue The tongue, on the other hand, is a complex muscular structure that permits tasting and facilitates the process of mastication and communication. The blood supply of the tongue originates from the external carotid artery, and the innervation is through cranial nerves. Lips and Tongue: Anatomy ”
- Swelling Swelling Inflammation and/or erythema Erythema Redness of the skin produced by congestion of the capillaries. This condition may result from a variety of disease processes. Chalazion on palms and soles
- Periungual desquamation Desquamation Staphylococcal Scalded Skin Syndrome (SSSS)
- Generally seen late in course of disease
- Least consistent manifestation of KD
- Tends to involve anterior cervical chain nodes
- Nonspecific prodrome Prodrome Symptoms that appear 24–48 hours prior to migraine onset. Migraine Headache of respiratory or gastrointestinal (GI) symptoms
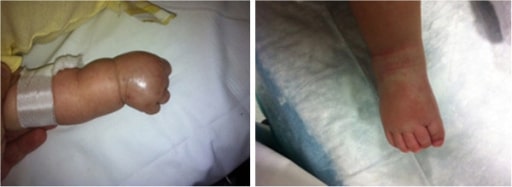
Edema and a polymorphous rash on the hands and feet in a 3-month-old patient with KD
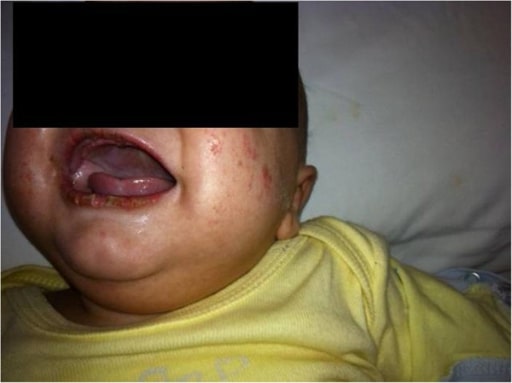
Lip fissuring alongside a polymorphous rash in KD
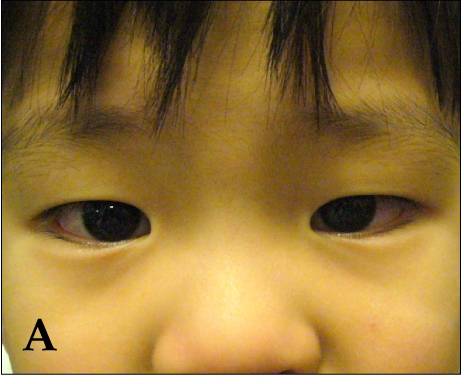
Bilateral, non-exudative conjunctivitis observed in a patient with KD
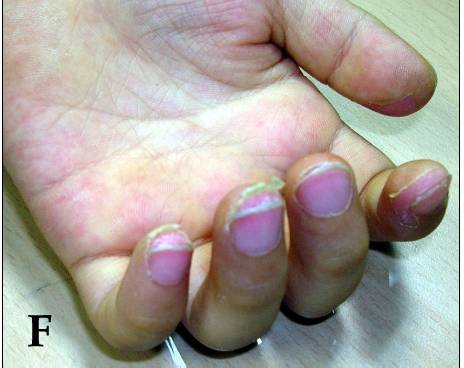
Desquamation of the fingertips observed in KD at 10–14 days
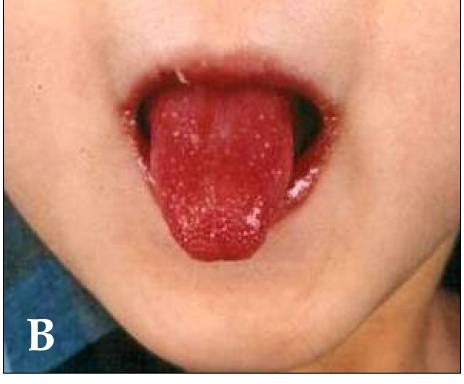
Strawberry tongue and bright-red, swollen lips with vertical cracking and bleeding in a patient with KD
Less common manifestations
Diagnostic criteria.
- Bilateral, non-exudative conjunctival injection
- Fissured lips Lips The lips are the soft and movable most external parts of the oral cavity. The blood supply of the lips originates from the external carotid artery, and the innervation is through cranial nerves. Lips and Tongue: Anatomy
- Strawberry tongue Tongue The tongue, on the other hand, is a complex muscular structure that permits tasting and facilitates the process of mastication and communication. The blood supply of the tongue originates from the external carotid artery, and the innervation is through cranial nerves. Lips and Tongue: Anatomy
- Injected pharynx Pharynx The pharynx is a component of the digestive system that lies posterior to the nasal cavity, oral cavity, and larynx. The pharynx can be divided into the oropharynx, nasopharynx, and laryngopharynx. Pharyngeal muscles play an integral role in vital processes such as breathing, swallowing, and speaking. Pharynx: Anatomy
- Erythema Erythema Redness of the skin produced by congestion of the capillaries. This condition may result from a variety of disease processes. Chalazion of palms/soles
- Edema Edema Edema is a condition in which excess serous fluid accumulates in the body cavity or interstitial space of connective tissues. Edema is a symptom observed in several medical conditions. It can be categorized into 2 types, namely, peripheral (in the extremities) and internal (in an organ or body cavity). Edema of hands/feet
- Erythematous polymorphous rash Rash Rocky Mountain Spotted Fever
- Cervical lymphadenopathy Lymphadenopathy Lymphadenopathy is lymph node enlargement (> 1 cm) and is benign and self-limited in most patients. Etiologies include malignancy, infection, and autoimmune disorders, as well as iatrogenic causes such as the use of certain medications. Generalized lymphadenopathy often indicates underlying systemic disease. Lymphadenopathy : at least 1 node > 1.5 cm in diameter
- If < 4 criteria → incomplete Kawasaki, supplement with laboratories below
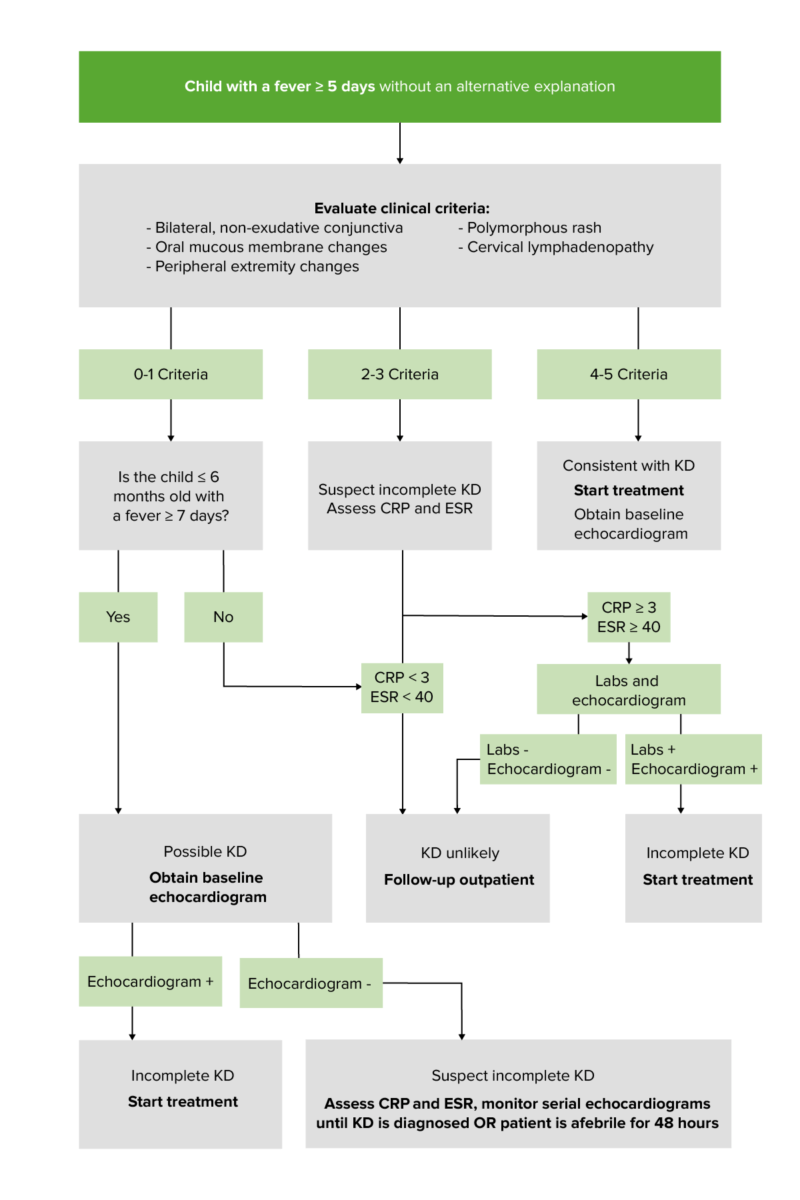
Diagnostic algorithm for children with fever/diagnosis of Kawasaki disease: Units: CRP in mg/dL; erythrocyte sedimentation rate (ESR) in mm/hr Positive labs (≥ 3 supplemental laboratory findings) include: Anemia (for age) ↑ Platelet count ↑ WBC ↓ Albumin ↑ ALT Pyuria
To recall the important clinical criteria for KD, remember CRASH and Burn :
C onjunctivitis R ash A denopathy S trawberry tongue Tongue The tongue, on the other hand, is a complex muscular structure that permits tasting and facilitates the process of mastication and communication. The blood supply of the tongue originates from the external carotid artery, and the innervation is through cranial nerves. Lips and Tongue: Anatomy H ands and feet Burn: fever Fever Fever is defined as a measured body temperature of at least 38°C (100.4°F). Fever is caused by circulating endogenous and/or exogenous pyrogens that increase levels of prostaglandin E2 in the hypothalamus. Fever is commonly associated with chills, rigors, sweating, and flushing of the skin. Fever
Laboratory and imaging studies
- ↑ Platelet counts Platelet counts The number of platelets per unit volume in a sample of venous blood. Coagulation Studies
- Anemia Anemia Anemia is a condition in which individuals have low Hb levels, which can arise from various causes. Anemia is accompanied by a reduced number of RBCs and may manifest with fatigue, shortness of breath, pallor, and weakness. Subtypes are classified by the size of RBCs, chronicity, and etiology. Anemia: Overview and Types
- ↑ Aspartate Aspartate One of the non-essential amino acids commonly occurring in the l-form. It is found in animals and plants, especially in sugar cane and sugar beets. It may be a neurotransmitter. Synthesis of Nonessential Amino Acids aminotransferase ( AST AST Enzymes of the transferase class that catalyze the conversion of l-aspartate and 2-ketoglutarate to oxaloacetate and l-glutamate. Liver Function Tests ) and alanine Alanine A non-essential amino acid that occurs in high levels in its free state in plasma. It is produced from pyruvate by transamination. It is involved in sugar and acid metabolism, increases immunity, and provides energy for muscle tissue, brain, and the central nervous system. Synthesis of Nonessential Amino Acids aminotransferase ( ALT ALT An enzyme that catalyzes the conversion of l-alanine and 2-oxoglutarate to pyruvate and l-glutamate. Liver Function Tests )
- ↓ Albumin Albumin Serum albumin from humans. It is an essential carrier of both endogenous substances, such as fatty acids and bilirubin, and of xenobiotics in the blood. Liver Function Tests
- ↑ Erythrocyte sedimentation rate Erythrocyte Sedimentation Rate Soft Tissue Abscess ( ESR ESR Soft Tissue Abscess )
- Urinalysis Urinalysis Examination of urine by chemical, physical, or microscopic means. Routine urinalysis usually includes performing chemical screening tests, determining specific gravity, observing any unusual color or odor, screening for bacteriuria, and examining the sediment microscopically. Urinary Tract Infections (UTIs) in Children : sterile Sterile Basic Procedures pyuria Pyuria The presence of white blood cells (leukocytes) in the urine. It is often associated with bacterial infections of the urinary tract. Pyuria without bacteriuria can be caused by tuberculosis, stones, or cancer. Urinary tract infections (UTIs)
- May demonstrate changes related to myocarditis Myocarditis Myocarditis is an inflammatory disease of the myocardium, which may occur alone or in association with a systemic process. There are numerous etiologies of myocarditis, but all lead to inflammation and myocyte injury, most often leading to signs and symptoms of heart failure. Myocarditis , pericarditis Pericarditis Pericarditis is an inflammation of the pericardium, often with fluid accumulation. It can be caused by infection (often viral), myocardial infarction, drugs, malignancies, metabolic disorders, autoimmune disorders, or trauma. Acute, subacute, and chronic forms exist. Pericarditis , or myocardial ischemia Myocardial ischemia A disorder of cardiac function caused by insufficient blood flow to the muscle tissue of the heart. The decreased blood flow may be due to narrowing of the coronary arteries (coronary artery disease), to obstruction by a thrombus (coronary thrombosis), or less commonly, to diffuse narrowing of arterioles and other small vessels within the heart. Coronary Heart Disease /infarction
- ↑ PR interval PR interval Electrocardiogram (ECG)
- Low voltage
- ↑ Size of left anterior descending artery or right coronary artery Right coronary artery Heart: Anatomy
- Coronary artery Coronary Artery Truncus Arteriosus aneurysm Aneurysm An aneurysm is a bulging, weakened area of a blood vessel that causes an abnormal widening of its diameter > 1.5 times the size of the native vessel. Aneurysms occur more often in arteries than in veins and are at risk of dissection and rupture, which can be life-threatening. Thoracic Aortic Aneurysms observed
- ↓ Left ventricular function
- Valvular dysfunction (e.g., regurgitation Regurgitation Gastroesophageal Reflux Disease (GERD) )
- Pericardial effusion Pericardial effusion Fluid accumulation within the pericardium. Serous effusions are associated with pericardial diseases. Hemopericardium is associated with trauma. Lipid-containing effusion (chylopericardium) results from leakage of thoracic duct. Severe cases can lead to cardiac tamponade. Pericardial Effusion and Cardiac Tamponade
- Should be performed in all patients Patients Individuals participating in the health care system for the purpose of receiving therapeutic, diagnostic, or preventive procedures. Clinician–Patient Relationship with KD
- Establishes a reference point for longitudinal follow-up
- Determines treatment efficacy
- Used to observe aneurysms already detected via echocardiogram Echocardiogram Transposition of the Great Vessels
- Not used for initial detection or diagnosis
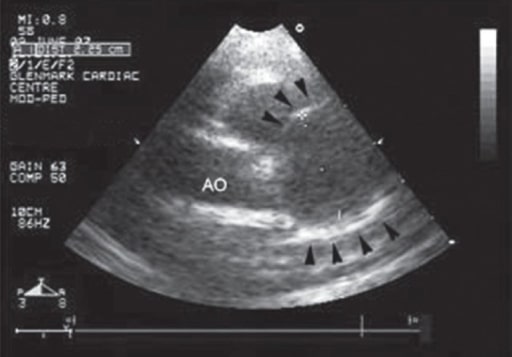
Echocardiography view at the level of aortic valve demonstrating an increase in the size of a coronary artery aneurysm (arrowheads) secondary to KD
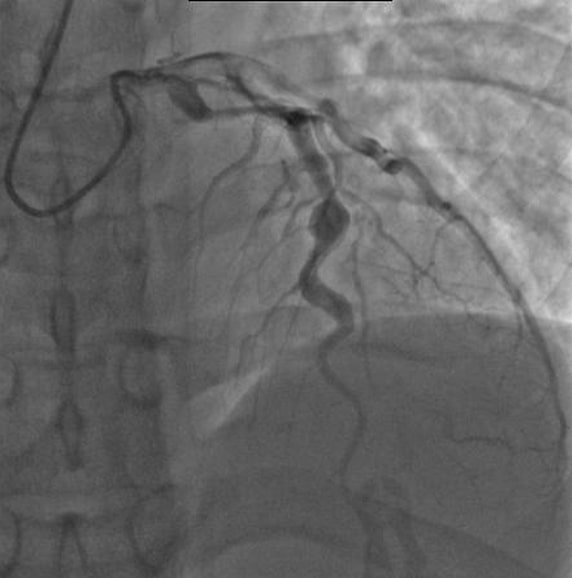
Angiography of a patient with KD showing ectatic left anterior descending coronary artery, with the largest aneurysm measuring 6.5 mm in diameter.
- Kawasaki disease is self-limited!
- Treatment is aimed at preventing complications and reducing symptoms.
- 2 g/kg administered as a single infusion over 8–12 hours
- Started within 10 days of fever Fever Fever is defined as a measured body temperature of at least 38°C (100.4°F). Fever is caused by circulating endogenous and/or exogenous pyrogens that increase levels of prostaglandin E2 in the hypothalamus. Fever is commonly associated with chills, rigors, sweating, and flushing of the skin. Fever onset, it reduces the risk of coronary artery Coronary Artery Truncus Arteriosus aneurysms.
- Observe for 24 hours following completion of IVIG IVIG Dermatomyositis infusion to confirm fever Fever Fever is defined as a measured body temperature of at least 38°C (100.4°F). Fever is caused by circulating endogenous and/or exogenous pyrogens that increase levels of prostaglandin E2 in the hypothalamus. Fever is commonly associated with chills, rigors, sweating, and flushing of the skin. Fever resolution.
- Patients Patients Individuals participating in the health care system for the purpose of receiving therapeutic, diagnostic, or preventive procedures. Clinician–Patient Relationship at high risk for IVIG IVIG Dermatomyositis resistance Resistance Physiologically, the opposition to flow of air caused by the forces of friction. As a part of pulmonary function testing, it is the ratio of driving pressure to the rate of air flow. Ventilation: Mechanics of Breathing are additionally treated with systemic glucocorticoids Systemic Glucocorticoids Glucocorticoids .
- 30–50 mg/kg daily divided into 4 doses
- Anti-inflammatory and antiplatelet effects
- Prevents thrombus in coronary arteries Arteries Arteries are tubular collections of cells that transport oxygenated blood and nutrients from the heart to the tissues of the body. The blood passes through the arteries in order of decreasing luminal diameter, starting in the largest artery (the aorta) and ending in the small arterioles. Arteries are classified into 3 types: large elastic arteries, medium muscular arteries, and small arteries and arterioles. Arteries: Histology
- Follow-up with serial echocardiograms at 2 and 6 weeks
- KD shock Shock Shock is a life-threatening condition associated with impaired circulation that results in tissue hypoxia. The different types of shock are based on the underlying cause: distributive (↑ cardiac output (CO), ↓ systemic vascular resistance (SVR)), cardiogenic (↓ CO, ↑ SVR), hypovolemic (↓ CO, ↑ SVR), obstructive (↓ CO), and mixed. Types of Shock syndrome (KDSS) is sustained systolic hypotension Hypotension Hypotension is defined as low blood pressure, specifically Hypotension or clinical signs of poor perfusion.
- Thrombocytosis
- Younger age
- Coronary artery Coronary Artery Truncus Arteriosus abnormalities
- Coronary artery Coronary Artery Truncus Arteriosus dilation, aneurysm Aneurysm An aneurysm is a bulging, weakened area of a blood vessel that causes an abnormal widening of its diameter > 1.5 times the size of the native vessel. Aneurysms occur more often in arteries than in veins and are at risk of dissection and rupture, which can be life-threatening. Thoracic Aortic Aneurysms , and/or stenosis Stenosis Hypoplastic Left Heart Syndrome (HLHS)
- Myocardial ischemia Myocardial ischemia A disorder of cardiac function caused by insufficient blood flow to the muscle tissue of the heart. The decreased blood flow may be due to narrowing of the coronary arteries (coronary artery disease), to obstruction by a thrombus (coronary thrombosis), or less commonly, to diffuse narrowing of arterioles and other small vessels within the heart. Coronary Heart Disease or infarction
- Ventricular dysfunction
- Valvular regurgitation Regurgitation Gastroesophageal Reflux Disease (GERD)
- Pericardial effusions
- Monitor with serial echocardiograms.
- Activation and proliferation of macrophages Macrophages The relatively long-lived phagocytic cell of mammalian tissues that are derived from blood monocytes. Main types are peritoneal macrophages; alveolar macrophages; histiocytes; kupffer cells of the liver; and osteoclasts. They may further differentiate within chronic inflammatory lesions to epithelioid cells or may fuse to form foreign body giant cells or langhans giant cells. Innate Immunity: Phagocytes and Antigen Presentation and T cells T cells Lymphocytes responsible for cell-mediated immunity. Two types have been identified – cytotoxic (t-lymphocytes, cytotoxic) and helper T-lymphocytes (t-lymphocytes, helper-inducer). They are formed when lymphocytes circulate through the thymus gland and differentiate to thymocytes. When exposed to an antigen, they divide rapidly and produce large numbers of new T cells sensitized to that antigen. T cells: Types and Functions
- Disseminated intravascular coagulopathy
- Cytopenias Cytopenias IPEX Syndrome
- Thrombosis Thrombosis Formation and development of a thrombus or blood clot in the blood vessel. Epidemic Typhus
- Caused by peripheral arterial obstruction
- Can involve the viscera or limbs
- Sterile Sterile Basic Procedures pyuria Pyuria The presence of white blood cells (leukocytes) in the urine. It is often associated with bacterial infections of the urinary tract. Pyuria without bacteriuria can be caused by tuberculosis, stones, or cancer. Urinary tract infections (UTIs) (common)
- Acute interstitial nephritis Acute interstitial nephritis Inflammation of the interstitial tissue of the kidney. This term is generally used for primary inflammation of kidney tubules and/or surrounding interstitium. For primary inflammation of glomerular interstitium, see glomerulonephritis. Infiltration of the inflammatory cells into the interstitial compartment results in edema, increased spaces between the tubules, and tubular renal dysfunction. Acute Kidney Injury
- Mild proteinuria Proteinuria The presence of proteins in the urine, an indicator of kidney diseases. Nephrotic Syndrome in Children
- Acute kidney injury Acute Kidney Injury Acute kidney injury refers to sudden and often reversible loss of renal function, which develops over days or weeks. Azotemia refers to elevated levels of nitrogen-containing substances in the blood that accompany AKI, which include BUN and creatinine. Acute Kidney Injury
- Hydrops Hydrops Cholecystitis of the gallbladder Gallbladder The gallbladder is a pear-shaped sac, located directly beneath the liver, that sits on top of the superior part of the duodenum. The primary functions of the gallbladder include concentrating and storing up to 50 mL of bile. Gallbladder and Biliary Tract: Anatomy is a common finding during KD’s acute phase Acute phase Short Bowel Syndrome
- Rapidly resolves upon IVIG IVIG Dermatomyositis administration
- Irritability is a common feature of KD’s acute phase Acute phase Short Bowel Syndrome
- Thought to be related to CSF pleocytosis Pleocytosis Tick-borne Encephalitis Virus
- Sensorineural hearing loss Sensorineural hearing loss Hearing loss resulting from damage to the cochlea and the sensorineural elements which lie internally beyond the oval and round windows. These elements include the auditory nerve and its connections in the brainstem. Hearing Loss : can occur in KD’s acute phase Acute phase Short Bowel Syndrome , but rarely persists
- Scarlet fever Scarlet fever Infection with group a Streptococci that is characterized by tonsillitis and pharyngitis. An erythematous rash is commonly present. Scarlet Fever : a disease that occurs as a result of a group A streptococcal infection, also known as Streptococcus Streptococcus Streptococcus is one of the two medically important genera of gram-positive cocci, the other being Staphylococcus. Streptococci are identified as different species on blood agar on the basis of their hemolytic pattern and sensitivity to optochin and bacitracin. There are many pathogenic species of streptococci, including S. pyogenes, S. agalactiae, S. pneumoniae, and the viridans streptococci. Streptococcus Pyogenes . Signs and symptoms include sore throat Sore throat Pharyngitis is an inflammation of the back of the throat (pharynx). Pharyngitis is usually caused by an upper respiratory tract infection, which is viral in most cases. It typically results in a sore throat and fever. Other symptoms may include a runny nose, cough, headache, and hoarseness. Pharyngitis , fever Fever Fever is defined as a measured body temperature of at least 38°C (100.4°F). Fever is caused by circulating endogenous and/or exogenous pyrogens that increase levels of prostaglandin E2 in the hypothalamus. Fever is commonly associated with chills, rigors, sweating, and flushing of the skin. Fever , headaches, swollen lymph nodes Lymph Nodes They are oval or bean shaped bodies (1 – 30 mm in diameter) located along the lymphatic system. Lymphatic Drainage System: Anatomy , a characteristic rash Rash Rocky Mountain Spotted Fever (red and sandpaper-like), and red/bumpy tongue Tongue The tongue, on the other hand, is a complex muscular structure that permits tasting and facilitates the process of mastication and communication. The blood supply of the tongue originates from the external carotid artery, and the innervation is through cranial nerves. Lips and Tongue: Anatomy . The exudative pharyngitis Pharyngitis Pharyngitis is an inflammation of the back of the throat (pharynx). Pharyngitis is usually caused by an upper respiratory tract infection, which is viral in most cases. It typically results in a sore throat and fever. Other symptoms may include a runny nose, cough, headache, and hoarseness. Pharyngitis in KD can be confused with streptococcal pharyngitis Streptococcal Pharyngitis Rheumatic Fever . The diagnosis of scarlet fever Scarlet fever Infection with group a Streptococci that is characterized by tonsillitis and pharyngitis. An erythematous rash is commonly present. Scarlet Fever is usually confirmed with rapid antigen detection Antigen detection Respiratory Syncytial Virus or throat Throat The pharynx is a component of the digestive system that lies posterior to the nasal cavity, oral cavity, and larynx. The pharynx can be divided into the oropharynx, nasopharynx, and laryngopharynx. Pharyngeal muscles play an integral role in vital processes such as breathing, swallowing, and speaking. Pharynx: Anatomy culture. Management is with penicillin Penicillin Rheumatic Fever or amoxicillin Amoxicillin A broad-spectrum semisynthetic antibiotic similar to ampicillin except that its resistance to gastric acid permits higher serum levels with oral administration. Penicillins .
- Measles Measles Measles (also known as rubeola) is caused by a single-stranded, linear, negative-sense RNA virus of the family Paramyxoviridae. It is highly contagious and spreads by respiratory droplets or direct-contact transmission from an infected person. Typically a disease of childhood, measles classically starts with cough, coryza, and conjunctivitis, followed by a maculopapular rash. Measles Virus : infection by the paramyxovirus Paramyxovirus Mumps Virus/Mumps that presents with fever Fever Fever is defined as a measured body temperature of at least 38°C (100.4°F). Fever is caused by circulating endogenous and/or exogenous pyrogens that increase levels of prostaglandin E2 in the hypothalamus. Fever is commonly associated with chills, rigors, sweating, and flushing of the skin. Fever , conjunctivitis Conjunctivitis Conjunctivitis is a common inflammation of the bulbar and/or palpebral conjunctiva. It can be classified into infectious (mostly viral) and noninfectious conjunctivitis, which includes allergic causes. Patients commonly present with red eyes, increased tearing, burning, foreign body sensation, and photophobia. Conjunctivitis , and a polymorphous rash Rash Rocky Mountain Spotted Fever that is highly contagious. Discrete intraoral lesions of KD can be confused with Koplik spots of measles Measles Measles (also known as rubeola) is caused by a single-stranded, linear, negative-sense RNA virus of the family Paramyxoviridae. It is highly contagious and spreads by respiratory droplets or direct-contact transmission from an infected person. Typically a disease of childhood, measles classically starts with cough, coryza, and conjunctivitis, followed by a maculopapular rash. Measles Virus . Diagnosis is made by viral polymerase chain reaction Polymerase chain reaction Polymerase chain reaction (PCR) is a technique that amplifies DNA fragments exponentially for analysis. The process is highly specific, allowing for the targeting of specific genomic sequences, even with minuscule sample amounts. The PCR cycles multiple times through 3 phases: denaturation of the template DNA, annealing of a specific primer to the individual DNA strands, and synthesis/elongation of new DNA molecules. Polymerase Chain Reaction (PCR) ( PCR PCR Polymerase chain reaction (PCR) is a technique that amplifies DNA fragments exponentially for analysis. The process is highly specific, allowing for the targeting of specific genomic sequences, even with minuscule sample amounts. The PCR cycles multiple times through 3 phases: denaturation of the template DNA, annealing of a specific primer to the individual DNA strands, and synthesis/elongation of new DNA molecules. Polymerase Chain Reaction (PCR) ), and management involves isolation and supportive treatment.
- Lyme disease Lyme disease Lyme disease is a tick-borne infection caused by the gram-negative spirochete Borrelia burgdorferi. Lyme disease is transmitted by the black-legged Ixodes tick (known as a deer tick), which is only found in specific geographic regions. Patient presentation can vary depending on the stage of the disease and may include a characteristic erythema migrans rash. Lyme Disease : an infectious disease caused by Borrelia burgdorferi Borrelia burgdorferi A specific species of bacteria, part of the borrelia burgdorferi group, whose common name is lyme disease spirochete. Borrelia , which spreads by ticks Ticks Blood-sucking acarid parasites of the order ixodida comprising two families: the softbacked ticks (argasidae) and hardbacked ticks (ixodidae). Ticks are larger than their relatives, the mites. They penetrate the skin of their host by means of highly specialized, hooked mouth parts and feed on its blood. Ticks attack all groups of terrestrial vertebrates. In humans they are responsible for many tick-borne diseases, including the transmission of rocky mountain spotted fever; tularemia; babesiosis; african swine fever; and relapsing fever. Coxiella/Q Fever . The most common sign is erythema Erythema Redness of the skin produced by congestion of the capillaries. This condition may result from a variety of disease processes. Chalazion migrans that appear at the site of a tick bite about a week after it occurred. Other symptoms are joint pain Pain An unpleasant sensation induced by noxious stimuli which are detected by nerve endings of nociceptive neurons. Pain: Types and Pathways , severe headache Headache The symptom of pain in the cranial region. It may be an isolated benign occurrence or manifestation of a wide variety of headache disorders. Brain Abscess , neck stiffness Neck Stiffness Meningitis or heart palpitations Palpitations Ebstein’s Anomaly . Diagnosis relies on clinical findings and tick exposure, and is supported by serologic testing. Antibiotics, such as doxycycline, are used for treatment.
- Rocky Mountain spotted fever Fever Fever is defined as a measured body temperature of at least 38°C (100.4°F). Fever is caused by circulating endogenous and/or exogenous pyrogens that increase levels of prostaglandin E2 in the hypothalamus. Fever is commonly associated with chills, rigors, sweating, and flushing of the skin. Fever : A bacterial infection that spreads by a bite from an infected tick. Symptoms include vomiting Vomiting The forcible expulsion of the contents of the stomach through the mouth. Hypokalemia , a sudden high fever Fever Fever is defined as a measured body temperature of at least 38°C (100.4°F). Fever is caused by circulating endogenous and/or exogenous pyrogens that increase levels of prostaglandin E2 in the hypothalamus. Fever is commonly associated with chills, rigors, sweating, and flushing of the skin. Fever , headache Headache The symptom of pain in the cranial region. It may be an isolated benign occurrence or manifestation of a wide variety of headache disorders. Brain Abscess , abdominal pain Abdominal Pain Acute Abdomen , rash Rash Rocky Mountain Spotted Fever , and muscle aches. Diagnosis is based on the clinical features, biopsy Biopsy Removal and pathologic examination of specimens from the living body. Ewing Sarcoma of the rash Rash Rocky Mountain Spotted Fever , and serologic testing. Treatment involves antibiotics, such as doxycycline.
- Rotavirus Rotavirus A genus of Reoviridae, causing acute gastroenteritis in birds and mammals, including humans. Transmission is horizontal and by environmental contamination. Seven species (rotaviruses A through G) are recognized. Rotavirus : Rotavirus Rotavirus A genus of Reoviridae, causing acute gastroenteritis in birds and mammals, including humans. Transmission is horizontal and by environmental contamination. Seven species (rotaviruses A through G) are recognized. Rotavirus is a common cause of severe gastroenteritis Gastroenteritis Gastroenteritis is inflammation of the stomach and intestines, commonly caused by infections from bacteria, viruses, or parasites. Transmission may be foodborne, fecal-oral, or through animal contact. Common clinical features include abdominal pain, diarrhea, vomiting, fever, and dehydration. Gastroenteritis . The nonspecific prodrome Prodrome Symptoms that appear 24–48 hours prior to migraine onset. Migraine Headache of gastrointestinal (GI) symptoms such as diarrhea Diarrhea Diarrhea is defined as ≥ 3 watery or loose stools in a 24-hour period. There are a multitude of etiologies, which can be classified based on the underlying mechanism of disease. The duration of symptoms (acute or chronic) and characteristics of the stools (e.g., watery, bloody, steatorrheic, mucoid) can help guide further diagnostic evaluation. Diarrhea , abdominal pain Abdominal Pain Acute Abdomen , and vomiting Vomiting The forcible expulsion of the contents of the stomach through the mouth. Hypokalemia that can be seen in KD can be confused with an infection due to rotavirus Rotavirus A genus of Reoviridae, causing acute gastroenteritis in birds and mammals, including humans. Transmission is horizontal and by environmental contamination. Seven species (rotaviruses A through G) are recognized. Rotavirus . Diagnostic testing for rotavirus Rotavirus A genus of Reoviridae, causing acute gastroenteritis in birds and mammals, including humans. Transmission is horizontal and by environmental contamination. Seven species (rotaviruses A through G) are recognized. Rotavirus is generally not required. Oral rehydration Rehydration Dengue Virus therapy is the mainstay of treatment.
- Pneumonia Pneumonia Pneumonia or pulmonary inflammation is an acute or chronic inflammation of lung tissue. Causes include infection with bacteria, viruses, or fungi. In more rare cases, pneumonia can also be caused through toxic triggers through inhalation of toxic substances, immunological processes, or in the course of radiotherapy. Pneumonia : an acute or chronic inflammation Chronic Inflammation Inflammation of lung tissue caused by infection with bacteria Bacteria Bacteria are prokaryotic single-celled microorganisms that are metabolically active and divide by binary fission. Some of these organisms play a significant role in the pathogenesis of diseases. Bacteriology , viruses Viruses Minute infectious agents whose genomes are composed of DNA or RNA, but not both. They are characterized by a lack of independent metabolism and the inability to replicate outside living host cells. Virology , or fungi Fungi A kingdom of eukaryotic, heterotrophic organisms that live parasitically as saprobes, including mushrooms; yeasts; smuts, molds, etc. They reproduce either sexually or asexually, and have life cycles that range from simple to complex. Filamentous fungi, commonly known as molds, refer to those that grow as multicellular colonies. Mycology that is considered a routine childhood illness. The nonspecific prodrome Prodrome Symptoms that appear 24–48 hours prior to migraine onset. Migraine Headache of respiratory and GI symptoms of KD can be mistaken for pneumonia Pneumonia Pneumonia or pulmonary inflammation is an acute or chronic inflammation of lung tissue. Causes include infection with bacteria, viruses, or fungi. In more rare cases, pneumonia can also be caused through toxic triggers through inhalation of toxic substances, immunological processes, or in the course of radiotherapy. Pneumonia . Diagnosis of pneumonia Pneumonia Pneumonia or pulmonary inflammation is an acute or chronic inflammation of lung tissue. Causes include infection with bacteria, viruses, or fungi. In more rare cases, pneumonia can also be caused through toxic triggers through inhalation of toxic substances, immunological processes, or in the course of radiotherapy. Pneumonia is based on the clinical history and examination, and supported by imaging (such as a chest X-ray X-ray Penetrating electromagnetic radiation emitted when the inner orbital electrons of an atom are excited and release radiant energy. X-ray wavelengths range from 1 pm to 10 nm. Hard x-rays are the higher energy, shorter wavelength x-rays. Soft x-rays or grenz rays are less energetic and longer in wavelength. The short wavelength end of the x-ray spectrum overlaps the gamma rays wavelength range. The distinction between gamma rays and x-rays is based on their radiation source. Pulmonary Function Tests ). Management involves supportive care and antimicrobial agents based on the etiology.
- Adenovirus Adenovirus Adenovirus (member of the family Adenoviridae) is a nonenveloped, double-stranded DNA virus. Adenovirus is transmitted in a variety of ways, and it can have various presentations based on the site of entry. Presentation can include febrile pharyngitis, conjunctivitis, acute respiratory disease, atypical pneumonia, and gastroenteritis. Adenovirus : causes mild upper respiratory infections Infections Invasion of the host organism by microorganisms or their toxins or by parasites that can cause pathological conditions or diseases. Chronic Granulomatous Disease in young children. The prodrome Prodrome Symptoms that appear 24–48 hours prior to migraine onset. Migraine Headache of nonspecific respiratory symptoms and accompanying nonexudative conjunctivitis Conjunctivitis Conjunctivitis is a common inflammation of the bulbar and/or palpebral conjunctiva. It can be classified into infectious (mostly viral) and noninfectious conjunctivitis, which includes allergic causes. Patients commonly present with red eyes, increased tearing, burning, foreign body sensation, and photophobia. Conjunctivitis in KD can be mistaken for a common upper respiratory infection Upper respiratory infection Rhinitis caused by adenovirus Adenovirus Adenovirus (member of the family Adenoviridae) is a nonenveloped, double-stranded DNA virus. Adenovirus is transmitted in a variety of ways, and it can have various presentations based on the site of entry. Presentation can include febrile pharyngitis, conjunctivitis, acute respiratory disease, atypical pneumonia, and gastroenteritis. Adenovirus . Diagnosis of adenovirus Adenovirus Adenovirus (member of the family Adenoviridae) is a nonenveloped, double-stranded DNA virus. Adenovirus is transmitted in a variety of ways, and it can have various presentations based on the site of entry. Presentation can include febrile pharyngitis, conjunctivitis, acute respiratory disease, atypical pneumonia, and gastroenteritis. Adenovirus is confirmed with PCR PCR Polymerase chain reaction (PCR) is a technique that amplifies DNA fragments exponentially for analysis. The process is highly specific, allowing for the targeting of specific genomic sequences, even with minuscule sample amounts. The PCR cycles multiple times through 3 phases: denaturation of the template DNA, annealing of a specific primer to the individual DNA strands, and synthesis/elongation of new DNA molecules. Polymerase Chain Reaction (PCR) and antigen Antigen Substances that are recognized by the immune system and induce an immune reaction. Vaccination testing. Most infections Infections Invasion of the host organism by microorganisms or their toxins or by parasites that can cause pathological conditions or diseases. Chronic Granulomatous Disease are self-limited, so management is generally supportive.
- Meningitis Meningitis Meningitis is inflammation of the meninges, the protective membranes of the brain, and spinal cord. The causes of meningitis are varied, with the most common being bacterial or viral infection. The classic presentation of meningitis is a triad of fever, altered mental status, and nuchal rigidity. Meningitis : inflammation Inflammation Inflammation is a complex set of responses to infection and injury involving leukocytes as the principal cellular mediators in the body’s defense against pathogenic organisms. Inflammation is also seen as a response to tissue injury in the process of wound healing. The 5 cardinal signs of inflammation are pain, heat, redness, swelling, and loss of function. Inflammation of the meninges Meninges The brain and the spinal cord are enveloped by 3 overlapping layers of connective tissue called the meninges. The layers are, from the most external layer to the most internal layer, the dura mater, arachnoid mater, and pia mater. Between these layers are 3 potential spaces called the epidural, subdural, and subarachnoid spaces. Meninges: Anatomy most commonly caused by bacteria Bacteria Bacteria are prokaryotic single-celled microorganisms that are metabolically active and divide by binary fission. Some of these organisms play a significant role in the pathogenesis of diseases. Bacteriology and viruses Viruses Minute infectious agents whose genomes are composed of DNA or RNA, but not both. They are characterized by a lack of independent metabolism and the inability to replicate outside living host cells. Virology . Irritability is a common manifestation of KD and meningitis Meningitis Meningitis is inflammation of the meninges, the protective membranes of the brain, and spinal cord. The causes of meningitis are varied, with the most common being bacterial or viral infection. The classic presentation of meningitis is a triad of fever, altered mental status, and nuchal rigidity. Meningitis . Other presenting signs and symptoms include fever Fever Fever is defined as a measured body temperature of at least 38°C (100.4°F). Fever is caused by circulating endogenous and/or exogenous pyrogens that increase levels of prostaglandin E2 in the hypothalamus. Fever is commonly associated with chills, rigors, sweating, and flushing of the skin. Fever , altered mental status Altered Mental Status Sepsis in Children , and nuchal rigidity Nuchal Rigidity Meningitis . Diagnosis is confirmed with CSF analysis CSF analysis Meningitis . Management includes immediate broad-spectrum Broad-Spectrum Fluoroquinolones antimicrobial therapy, which can be tailored once the offending pathogen is identified.
- Stevens-Johnson syndrome Stevens-Johnson syndrome Stevens-Johnson syndrome (SJS) is a cutaneous, immune-mediated hypersensitivity reaction that is commonly triggered by medications, including antiepileptics and antibiotics. The condition runs on a spectrum with toxic epidermal necrolysis (TEN) based on the amount of body surface area (BSA) involved. Stevens-Johnson Syndrome ( SJS SJS Stevens-Johnson syndrome (SJS) is a cutaneous, immune-mediated hypersensitivity reaction that is commonly triggered by medications, including antiepileptics and antibiotics. The condition runs on a spectrum with toxic epidermal necrolysis (TEN) based on the amount of body surface area (BSA) involved. Stevens-Johnson Syndrome ) : immune-complex mediated hypersensitivity reaction that can be triggered by infectious etiologies Infectious Etiologies High-Risk Headaches or the use of anticonvulsants, antibiotics, or other drugs. The syndrome is characterized by epidermal necrolysis, separation of the epidermis Epidermis The external, nonvascular layer of the skin. It is made up, from within outward, of five layers of epithelium: (1) basal layer (stratum basale epidermidis); (2) spinous layer (stratum spinosum epidermidis); (3) granular layer (stratum granulosum epidermidis); (4) clear layer (stratum lucidum epidermidis); and (5) horny layer (stratum corneum epidermidis). Skin: Structure and Functions from the dermis Dermis A layer of vascularized connective tissue underneath the epidermis. The surface of the dermis contains innervated papillae. Embedded in or beneath the dermis are sweat glands; hair follicles; and sebaceous glands. Skin: Structure and Functions , and the formation of skin Skin The skin, also referred to as the integumentary system, is the largest organ of the body. The skin is primarily composed of the epidermis (outer layer) and dermis (deep layer). The epidermis is primarily composed of keratinocytes that undergo rapid turnover, while the dermis contains dense layers of connective tissue. Skin: Structure and Functions blisters and bullae Bullae Erythema Multiforme on the face, lips Lips The lips are the soft and movable most external parts of the oral cavity. The blood supply of the lips originates from the external carotid artery, and the innervation is through cranial nerves. Lips and Tongue: Anatomy , throat Throat The pharynx is a component of the digestive system that lies posterior to the nasal cavity, oral cavity, and larynx. The pharynx can be divided into the oropharynx, nasopharynx, and laryngopharynx. Pharyngeal muscles play an integral role in vital processes such as breathing, swallowing, and speaking. Pharynx: Anatomy , and extremities. The bullous or vesicular rash Rash Rocky Mountain Spotted Fever in KD can be mistaken for SJS SJS Stevens-Johnson syndrome (SJS) is a cutaneous, immune-mediated hypersensitivity reaction that is commonly triggered by medications, including antiepileptics and antibiotics. The condition runs on a spectrum with toxic epidermal necrolysis (TEN) based on the amount of body surface area (BSA) involved. Stevens-Johnson Syndrome . The diagnosis of SJS SJS Stevens-Johnson syndrome (SJS) is a cutaneous, immune-mediated hypersensitivity reaction that is commonly triggered by medications, including antiepileptics and antibiotics. The condition runs on a spectrum with toxic epidermal necrolysis (TEN) based on the amount of body surface area (BSA) involved. Stevens-Johnson Syndrome is usually clinical, and management is largely supportive. Withdrawal of any causative agent is required.
- Epstein-Barr virus Epstein-Barr Virus Epstein-Barr virus (EBV) is a linear, double-stranded DNA virus belonging to the Herpesviridae family. This highly prevalent virus is mostly transmitted through contact with oropharyngeal secretions from an infected individual. The virus can infect epithelial cells and B lymphocytes, where it can undergo lytic replication or latency. Epstein-Barr Virus : causes infectious mononucleosis Mononucleosis Infectious mononucleosis (IM), also known as “the kissing disease,” is a highly contagious viral infection caused by the Epstein-Barr virus. Its common name is derived from its main method of transmission: the spread of infected saliva via kissing. Clinical manifestations of IM include fever, tonsillar pharyngitis, and lymphadenopathy. Mononucleosis and is associated with Burkitt’s lymphoma Lymphoma A general term for various neoplastic diseases of the lymphoid tissue. Imaging of the Mediastinum , hemophagocytic lymphohistiocytosis Hemophagocytic lymphohistiocytosis A group of related disorders characterized by lymphocytosis; histiocytosis; and hemophagocytosis. The two major forms are familial and reactive. Epstein-Barr Virus , and Hodgkin’s lymphoma Lymphoma A general term for various neoplastic diseases of the lymphoid tissue. Imaging of the Mediastinum . The generalized lymphadenopathy Generalized Lymphadenopathy Lymphadenopathy observed in KD can be mistaken for the generalized lymphadenopathy Generalized Lymphadenopathy Lymphadenopathy of infectious mononucleosis Mononucleosis Infectious mononucleosis (IM), also known as “the kissing disease,” is a highly contagious viral infection caused by the Epstein-Barr virus. Its common name is derived from its main method of transmission: the spread of infected saliva via kissing. Clinical manifestations of IM include fever, tonsillar pharyngitis, and lymphadenopathy. Mononucleosis . The diagnosis is clinical and confirmed through heterophile antibody testing or serology Serology The study of serum, especially of antigen-antibody reactions in vitro. Yellow Fever Virus . Management is supportive.
- Sundel, R. (2020). Kawasaki disease: Clinical features and diagnosis. In TePas, E. (Ed.), UpToDate. Retrieved February 15, 2022, from https://www.uptodate.com/contents/kawasaki-disease-clinical-features-and-diagnosis
- Sundel, R. (2020). Kawasaki disease: Epidemiology and etiology. In TePas, E. (Ed.), UpToDate. Retrieved February 15, 2022, from https://www.uptodate.com/contents/kawasaki-disease-epidemiology-and-etiology
- Sundel, R. (2020). Kawasaki disease: Initial treatment and prognosis Prognosis A prediction of the probable outcome of a disease based on a individual’s condition and the usual course of the disease as seen in similar situations. Non-Hodgkin Lymphomas . In TePas, E. (Ed.), UpToDate. Retrieved February 15, 2022, from https://www.uptodate.com/contents/kawasaki-disease-initial-treatment-and-prognosis
- Raab, C.P. (2021). Kawasaki disease. [online] MSD Manual Professional Version. Retrieved February 15, 2022, from https://www.msdmanuals.com/professional/pediatrics/miscellaneous-disorders-in-infants-and-children/kawasaki-disease
- Modesti, A.M., and Plewa, M.C. (2021). Kawasaki disease. [online] StatPearls. Retrieved February 15, 2022, from https://www.ncbi.nlm.nih.gov/books/NBK537163/
- Sosa, T.K., and Shah, S.S. (2018). Kawasaki disease. In Steele, R.W. (Ed.), Medscape. Retrieved February 15, 2022, from https://emedicine.medscape.com/article/965367-overview
Study with Lecturio for
Medical School
Nursing School
- Data Privacy
- Terms and Conditions
- Legal Information
USMLE™ is a joint program of the Federation of State Medical Boards (FSMB®) and National Board of Medical Examiners (NBME®). MCAT is a registered trademark of the Association of American Medical Colleges (AAMC). NCLEX®, NCLEX-RN®, and NCLEX-PN® are registered trademarks of the National Council of State Boards of Nursing, Inc (NCSBN®). None of the trademark holders are endorsed by nor affiliated with Lecturio.
Create your free account or log in to continue reading!
Sign up now and get free access to Lecturio with concept pages, medical videos, and questions for your medical education.
Log in to your account
User Reviews
Get Premium to test your knowledge
Lecturio Premium gives you full access to all content & features
Get Premium to watch all videos
Verify your email now to get a free trial.
Create a free account to test your knowledge
Lecturio Premium gives you full access to all contents and features—including Lecturio’s Qbank with up-to-date board-style questions.

KAWASAKI DISEASE
Jan 20, 2012
600 likes | 1.99k Views
KAWASAKI DISEASE. Learning about Kawasaki Disease and How It Affects Children. Kawasaki disease is a frightening and rare condition in children. Amount of cases reported monthly in the duration of 10 years since 1988-1998. What is Kawasaki Disease?.
Share Presentation
- kawasaki disease
- coronary arteries
- small aneurysms
- serum transaminase levels
- kawasaki disease recover completely

Presentation Transcript
KAWASAKI DISEASE Learning about Kawasaki Disease and How It Affects Children
Kawasaki disease is a frightening and rare condition in children.
Amount of cases reported monthly in the duration of 10 years since 1988-1998
What is Kawasaki Disease? • Kawasaki disease is a group of specific symptoms and physical signs. • Kawasaki disease is associated with swelling and damage of the blood vessels, or arteries. This swelling and damage is called inflammation. • Kawasaki disease often affects the arteries that supply the heart with blood. These are called the coronary arteries. • Children who get the disease are usually less than 5 years old. • It is very rare to get the disease after a child is 10 years old. • The risk of other children in the same family getting Kawasaki disease is very low. • The risk of a child getting Kawasaki disease for the second time is very rare.
What Causes Kawasaki Disease? • At present, no one actually knows what causes Kawasaki disease. Research is being done into the possible causes. • Researchers now think that the disease may be caused by an infection. Normally, the body's immune system quickly recognizes germs and destroys them. • But in the case of Kawasaki disease, a poison or toxin may be formed by the infection. This poison, or toxin, or perhaps the infection itself, makes certain individuals have an unusual, or abnormal reaction from the immunity system to the infection. However, we do not know what the infection or toxin is.
We also do not know what makes some individuals more likely to get this disease than others. There is a slight chance that the brothers and sisters of a child with Kawasaki disease may also get the disease. But this is very rare. Unfortunately, there is no way we know of to prevent a child from getting the disease.
Kawasaki Disease: Symptoms and Diagnosis • All patients have a continuous fever lasting 5 days or more. • 4 out of 5 of the following symptoms are also necessary before the doctor can know for sure, if a child has this disease.
Symptoms • Red eyes • Redness of the lips, tongue or mouth • Redness or swelling of the hands and feet • A body rash • Swelling of the glands in the neck
Kawasaki Disease Symptoms: Red Eyes • The below photo shows what the child’s eyes look like with Kawasaki disease.
Kawasaki Disease Symptoms: Changes in the Lips, Tongue and Mouth • Some changes which occur in the mouth and lips may be seen in the pictures below.
These changes of the mouth and lips include such things as: • redness, • drying with cracking of the lips, and • a red strawberry-like tongue.
Kawasaki Disease Symptoms: Changes to the Hands and Feet • The palms, or inside flat part of the hands, and the soles, or bottoms of the feet may turn red in colour. They may also appear swollen.
About a week after the fever breaks, the skin from the fingers and toes may peel off, sometimes in large pieces. When this happens, new, normal skin shows below the peeling skin.
Kawasaki Disease Symptoms: Body Rash • A body rash usually first appears with the fever. The skin appears red and will feel 'bumpy' to the touch.
A red rash can be seen in the groin or diaper area, particularly in young infants. This can also peel off later.
Kawasaki Disease Symptoms: Swelling of the Glands in the Neck • The picture below shows a child with swollen lymph nodes. You can sometimes feel these, just under the skin in the neck. Usually, they are soft, painless and about the size of a pea.
In a child with Kawasaki disease, these lymph nodes may become swollen or enlarged. They become easier to feel.
What is the Treatment for a Child with Kawasaki Disease? • There is no single medicine, which can treat or cure Kawasaki disease.
There are two medicines, which can be given to reduce the effects of Kawasaki disease • Aspirin. • Gamma Globulin.
What are the Possible Serious Problems for a Child with Kawasaki Disease? • 1. Changes in the Coronary Arteries: • The most serious problems from Kawasaki disease have to do with its effects on the heart. During the disease, the arteries, which supply the heart with blood, called coronary arteries, can become inflamed and damaged
2. Formation of Aneurysms: • Inflammation and damage to the coronary arteries could weaken their walls and cause the walls to develop pouches or sacs, called aneurysms. This is just like a weak spot in a tire or hose, that swells up with water or air.
What is an Aneurysm? • Normally, the walls of blood vessels are smooth and even. In Kawasaki disease, the muscular walls of the coronary arteries may become weakened. • The pressure of the blood flowing through the arteries may cause these weak spots to balloon-out, just like a weak spot in a tire or inner tube.
Abnormal Angiogram: Aneurysm • This is an angiogram of a heart with abnormal coronary arteries. The arrows point to some of the abnormalities. Here, the blood vessels are not smooth; they have ballooned out to form aneurysms. This can happen to a few children who have had Kawasaki disease.
Tests to Monitor Heart Complications • The following tests are used by a cardiologist to find out what possible effects Kawasaki disease has had on the heart and coronary arteries: • 1. Echocardiography • 2. Electrocardiography • 3. Angiography
Long Term Effects of Kawasaki Disease • About 95 out of every 100 children who have had Kawasaki disease recover completely. If no damage in the coronary arteries is seen on the echo tests, then complete recovery is most likely. This means that there is little chance of future problems.
If small aneurysms or dilations of the coronary artery are found, this is not serious. Small aneurysms tend to go away in about a year or two. Large or giant aneurysms do not tend to go away, and may lead to clots or narrowings. Although these children with severe coronary damage need to be followed closely, even these children tend to lead relatively normal lives
LINK TO CORONARY ARTERY DISEASE • There have been 74 reported cases of adult coronary artery disease attributed to childhood Kawasaki disease. The mean age at onset of symptoms of carditis and ischaemia was 25 (range 12 to 39) years with symptoms precipitated by exercise in 82% of these patients.
LABORATORY FINDINGS • Laboratory findings include neutrophilia, anaemia, thrombocytosis, elevated erythrocyte sedimentation rate, elevated serum transaminase levels, hypoalbuminemia and an elevated serum alpha1-antitrypsin level. • Unlike atherosclerosis, no fatty streaks or macrophage accumulations occur.
Other Significant Clinical and Laboratory Findings • Cardiovascular: On auscultation, gallop rhythm or distant heart sounds; ECG changes (arrhythmias, abnormal Q waves, prolonged PR and/or QT intervals, occasionally low voltage, or ST-T wave changes); chest X-ray abnormalities (cardiomegaly); echocardiographic changes (pericardial effusion, coronary aneurysms, or decreased contractility); mitral and/or aortic valvular insufficiency; and rarely, aneurysms of peripheral arteries (e.g., axillary), angina pectoris, or myocardial infarction
Gastrointestinal: Diarrhea, vomiting, abdominal pain, hydrops of gallbladder, paralytic ileus, mild jaundice, and mild increase of serum transaminase levels
Blood: Increased erythrocyte sedimentation rate, leukocytosis with left shift, positive C-reactive protein, hypoalbuminemia, and mild anemia in acute phase of illness (thrombocytosis in subacute phase)
Urine: Sterile pyuria of urethral origin and occasional proteinuria • Skin: Perineal rash and desquamation in subacute phase and transverse furrows of fingernails (Beau's lines) during convalescence
Respiratory: Cough, rhinorrhea, and pulmonary infiltrate • Joint: Arthralgia and arthritis • Neurological: Mononuclear pleocytosis in cerebrospinal fluid, striking irritability, and rarely, facial palsy
Prepared by Roshen John Group 27 of the 3rd course of medicine Ternopil State Medical University ThankYou!
- More by User

Kawasaki Motocycle Clothing
Cheap Discount Kawasaki Motocycle Clothing Shop, offers a wide range of top-quality Kawasaki Clothing.
387 views • 5 slides

Kawasaki Disease: An Update of diagnosis and treatment
Kawasaki Disease: An Update of diagnosis and treatment . What is Kawasaki Disease?. Idiopathic multisystem disease characterized by vasculitis of small & medium blood vessels, including coronary arteries. Diagnostic Criteria. Fever for at least 5 days At least 4 of the following 5 features:
783 views • 27 slides

Kawasaki Disease
Kawasaki Disease. Danielle Hann ST2 GPVTS 2010. Kawasaki Disease. 80% cases aged 6/12 to 5 years Acute inflammatory vasculitis of medium sized arteries Incidence varies worldwide England - 8 per 100 000 Japan – 184 per 100 000. Causes. ??
1.1k views • 14 slides

Kawasaki Disease. Acute febrile vasculitic syndrome of early childhoodAffecting all blood vessels in the body but mostly medium and small vessels with a preferential involvement of the coronary arteries. Exact etiology unknown but thought to be infectious in nature. Epidemiology . Race: Japanese >
653 views • 17 slides

Kawasaki Disease: An Update
Objectives. Review the historical
950 views • 56 slides

February 01, 2008. KAWASAKI. Kawasaki Hydraulic Deck Machinery . HYDRAULIC MARINE MACHINERY. KAWASAKI. Deck machinery. Steering gear. Deck crane. MARKET SHARE OF DECK MACHINERY. Market Share of Deck Machinery in Japan. KAWASAKI. AMOUNT OF PRODUCTION. KAWASAKI.
710 views • 22 slides

Guy Kawasaki
Guy Kawasaki. By Nagarjuna.R Enroll No: 8024. GIST(great ideas for starting things). Make Meaning Make Mantra Get going Define your business model Weave a MAT. Make Meaning.
405 views • 10 slides

Kawasaki Disease. Morning Report July 16 th , 2013. Epidemiology. Previously known as mucocutaneous lymph node syndrome Incidence is greatest in children in East Asia or are of Asian ancestry Boys more commonly affected than girls 80-90% of cases in children < 5 yo
826 views • 15 slides

KAWASAKI DISEASE. Carreon G., Carreon J., Casaul J.R., Coronado M.C., Corpuz D., Credo C., Danguilan M.C., De Gracia C. AIM:. Review the clinical presentations of K awasaki disease, its progression, serious complications and management. Discuss the D/ Dx of Kawasaki disease. S>.
608 views • 27 slides

Urine Proteomics in Kawasaki Disease
Urine Proteomics in Kawasaki Disease. Pawan Sharma 15 th October 2013. Kawasaki Disease. Uncommonly common systemic vasculitis. 6 months to 4 years age. Significant mortality and morbidity esp with delayed diagnosis. No pathognomic test for early diagnosis.
443 views • 21 slides
What is Kawasaki Disease?
What is Kawasaki Disease?. Kawasaki Disease (KD ) also known as Kawasaki Syndrome . • An unusual and serious illness of young children. It is an autoimmune disease that can effect any type of blood vessel in the body, including arteries, veins, and capillaries.
2.22k views • 13 slides

Kawasaki Disease risk-associated snps
Kawasaki Disease risk-associated snps. As Identified by Lee et al., 2012 Jennifer Przybylo. Typical presentation. Treatment. Aspirin! IV immunoglobulin G. BUT…. “Two new susceptibility loci for Kawasaki disease identified through genome-wide association analysis”.
201 views • 6 slides

Kawasaki Disease. Mucocutaneous lymph node syndrome Ma Lian. Introduction. Kawasaki disease (KD) is a common vasculitic disorder usually seen in children below 5 years of age The leading cause of acquired heart disease in children. Kawaski T.
1.06k views • 43 slides

Kawasaki Disease . Morning Report 7/13/09. Kawasaki Disease. Acute febrile vasculitic syndrome of early childhood Affecting all blood vessels in the body but mostly medium and small vessels with a preferential involvement of the coronary arteries.
1.07k views • 20 slides

Kawasaki Disease: An Update of diagnosis and treatment. What is Kawasaki Disease?. Idiopathic multisystem disease characterized by vasculitis of small & medium blood vessels, including coronary arteries. Diagnostic Criteria. Fever for at least 5 days At least 4 of the following 5 features:
547 views • 27 slides

Kawasaki Fairings
Kawasaki fairings sport bike Fairing kits are available at very affordable cost Oyo Cycle has a huge range of fairing kit. Oyo Cycle, a huge selection of top quality ABS aftermarket fairings for Kawasaki. These high quality fairings and body kits are manufactured by original kind of material ABS with injection mold technology.
58 views • 5 slides
Kawasaki z800 | Kawasaki
Kawasaki Z800 gained 6bhp in power to 113bhp with 3.7ftlb of peak torque, however peak performance was only the headline and it was throughout the entire rev range that the differences were really noticeable. Then, as a final touch, Kawasaki added two teeth to the rear sprocket, increasing it from 43 to 45-teeth to help boost the Zedu2019s acceleration.
58 views • 1 slides
Kawasaki Disease Vaishali Soneji Lafita, MD
Kawasaki Disease Vaishali Soneji Lafita, MD. Presentation – Patient 1. 10 years old male with Kawasaki Disease Possible mildly ectatic posterior descending coronary artery on Echo. CTA was performed. Heart rate control Contrast enhanced axial images of the coronary arteries
344 views • 28 slides

KAWASAKI ZX10R
Sprint Filter P08 is our standard performance air filter, ideal for street legal use or have a funny track day with a tuned motorcycle.https://www.sprintfilter.net/motorcycle/products/kawasaki/zx10r-/375
221 views • 17 slides

Kawasaki Disease Treatment Market Size, Key Drivers and Analysis by 2028
Kawasaki disease treatment market is expected to gain market growth in the forecast period of 2021 to 2028. Data Bridge Market Research analyses the market is growing at a CAGR of 6.80% in the above-mentioned research forecast period. Rapid rise in incidence of kawasaki disease in the emerging countries and increase in adoption of intravenous immunoglobulin therapy in order to prevent or reduce the coronary artery problem are some factors driving the market growth.
22 views • 2 slides
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.14(2); 2022 Feb

Incomplete Kawasaki Disease in an Infant: A Case Report and Literature Review
1 Pediatrics, Woodhull Medical Center, New York City, USA
Abdalaziz M Awadelkarim
2 Internal Medicine, Wayne State University Detroit Medical Center, Detroit, USA
Eltaib Saad
3 Internal Medicine, AMITA Saint Francis Hospital, Evanston, USA
Susan Beker
4 Pediatric Cardiology, New York University, New York, USA
The term "incomplete Kawasaki Disease (IKD)" was first used to describe patients with coronary complications who did not fulfill the classical diagnostic criteria for Kawasaki Disease (KD). The risk of coronary artery involvement is similar if not greater in cases of IKD. However, the recognition of IKD is challenging and often delayed, especially in infants. Multiple algorithms have been formulated to identify cases of IKD utilizing supplemental clinical, echocardiographic, and laboratory features. Although fever is not required for a diagnosis of KD in the Japanese guideline, most of the current guidelines, including those of the American Heart Association (AHA), consider the presence of fever for at least seven days a requirement for the diagnosis of both KD and IKD in infants.
We present a case of IKD in a four-month-old female who presented with fever for less than three days and did not follow the current AHA algorithm for IKD. An echocardiogram obtained 10 days later revealed a coronary artery aneurysm, and a retrospective diagnosis of IKD was made. A review of the literature identified similar cases with a growing consensus on the need to redefine the role of fever. Pediatricians should search for coronary artery lesions in cases of high clinical suspicion, even if the fever period is short, particularly in those less than six months. Additionally, further innovative research is directly needed to identify immunological and cellular markers that could be tested early in the course of the disease and guide the management.
Introduction
Kawasaki Disease (KD) is an acute, self-limited systemic vasculitis of small and medium vessels and occurs mainly between six months and five years of age. It was named after Tomisaku Kawasaki, a Japanese pediatrician who described this febrile vasculitis for the first time in 1967. Coronary artery complications occur in 25% of affected children. Currently, it is recognized as the major cause of acquired heart disease in developed countries [ 1 ].
The term "incomplete Kawasaki disease (IKD)" was at first used to describe patients with coronary complications who do not fulfill the full diagnostic criteria for KD. Multiple studies have shown that the risk of coronary artery involvement is similar if not greater in cases of IKD. However, the recognition of IKD is challenging and often delayed [ 2 - 3 ]. Infants less than six months represent a special age group, as they are more likely to lack classical manifestations and have a higher risk for coronary artery involvement [ 4 ]. Multiple algorithms have been formulated to identify cases of IKD utilizing supplemental clinical, echocardiographic, and laboratory features. Although fever is not required for a diagnosis of KD in the Japanese guideline, the majority of current guidelines, including the American Heart Association (AHA), consider the presence of fever for at least seven days a requirement for the diagnosis of both KD and IKD infants [ 5 ]. We present a case of Incomplete Kawasaki disease (IKD) in a four-month-old female infant who presented with fever for less than three days and did not follow the current AHA algorithm for IKD.
Case presentation
A previously healthy four-month-old Hispanic female presented to the emergency department (ED) with a fever of (102.1°F) and decreased oral intake of one-day duration. Physical examination was benign. Urine culture, chest X-ray, COVID-19, and influenza A&B polymerase chain reactions (PCRs) were all negative. She was discharged home on oral paracetamol. One day later (the second day of illness), she presented again to our ED with unremitting fever, irritability, bilateral eye redness, and rash in the torso. Physical examination was remarkable for fever 102.2°F, tachycardia, bilateral nonexudative conjunctivitis, erythematous cracked lips, and a polymorphic maculopapular rash covering her torso and extremities. Laboratory workup was significant for mild thrombocytosis (448,000/mm 3 ), an elevation of acute phase reactants (c-reactive protein (CRP) 63 mg/L, erythrocyte sedimentation rate (ESR) 90 mm/h), procalcitonin 0.49 ng/dl, positive respiratory viral panel for Rhino/Enterovirus. Urine analysis, serum albumin, AST, and ALT were normal. Blood culture and urine showed no growth.
Screening for viral exanthem, including Adenovirus, RSV, parvovirus, measles, and EBV, was negative. Based on existent clinical and laboratory findings, the patient was admitted and was initially treated as a viral illness with intravenous hydration and antipyretics. The patient had a good response to supportive measures: fever subsided on the day of admission, inflammatory markers trended down, and the patient was discharged on day four of hospitalization (sixth day of illness). The patient was evaluated by cardiology and didn't fulfill the clinical or laboratory criteria for IKD. However, it was still considered as a differential diagnosis, and an echocardiogram was scheduled for outpatient follow-up.
On clinic follow-up three days after discharge (ninth day of illness), she had a persistent red tongue and lips; additionally, new swelling of both hands and feet was noticed. Her skin rash and conjunctival injection had resolved. The repeat platelet count was 750,000/mm. The EKG was normal. Echocardiogram showed normal ventricular function, significantly dilated left main coronary artery (LMCA) (Z score = 3.4), mild dilatation of the right coronary artery (RCA) (Z score = 2.5) and left coronary artery (LAD) (Z score = 2.6) (Figure 1 ).

In combination with clinical history and echocardiogram findings, she was readmitted and received intravenous immunoglobulin (IVIG), steroids, and aspirin. The patient had an uncomplicated hospital stay and was discharged three days later. A follow-up echocardiogram obtained six weeks into the illness showed resolution of coronary artery abnormalities (Figure 2 ). She remains in good health, and no further coronary artery abnormalities were identified on a three-month follow-up echocardiogram. The patient remained afebrile throughout her follow-up visits.

Complete KD occurs predominantly in the age group of six months to five years. The diagnostic criteria include fever for more than five days along with at least four of the following clinical features: erythema of the lips and oral mucosa, bilateral nonexudative conjunctival injection, polymorphous skin rash, changes in the extremities, and unilateral painless cervical lymphadenopathy. In the presence of ≥4 principal clinical criteria, mainly when redness and swelling of the hands and feet are present, the diagnosis may be made with only four days of fever. Typically, the clinical features are not all present at a single point in time, and it is generally challenging to establish the diagnosis very early in the course. If coronary artery abnormalities are detected, the diagnosis of KD is considered confirmed in most cases [ 5 - 6 ]. Echocardiography should be obtained at the time of diagnosis, one to two weeks later, and six weeks post-discharge. CT, MR, or conventional angiography should be considered for further risk stratification and complete coronary assessment in children who have significant coronary artery aneurysms on echocardiogram [ 6 ].
However, pediatricians sometimes encounter febrile children who do not meet the complete clinical picture but have several findings compatible with those of KD. In this situation, the identification of IKD is a clinical challenge, which can lead to delays in diagnosis and management. Echocardiographic changes take time to develop, and they are usually identified after the first week of illness [ 5 ]. It is well-established that the prompt recognition of KD and early initiation of IVIG, specifically within seven days, can decrease the incidence of coronary artery aneurysms from 25% to 4% [ 7 - 8 ]. Multiple studies have shown that the risk of coronary artery involvement is similar, if not greater, in cases of IKD [ 3 ]. Therefore, the current diagnostic criteria for KD are insensitive indicators for having or developing coronary complications.
A significant proportion of the burden of the disease occurs in infants less than six months of age (10%) [ 9 ]. This age group tends to have fewer typical clinical manifestations and has a higher prevalence of incomplete and atypical KD (40%) compared to older patients (10-12%) [ 10 - 11 ]. Fever and excessive irritability may be the only clinical manifestations of KD in infants. The presence of fever and pyuria in an infant can be mistakenly attributed to a urinary tract infection. Due to the elusive nature of the presentation, it's easy to understand why the diagnosis, and subsequently the treatment, of KD gets delayed in the infant group. As a result, they are usually diagnosed late and have a higher risk of coronary artery abnormalities, coronary artery aneurysms, and cardiac complications, including giant coronary artery aneurysms, shock, and death. Morbidity and mortality in this age group are highest compared to any other age group [ 6 , 12 - 13 ].
Park et al. compared patients with KD younger and older than six months and reported a higher incidence of coronary artery abnormalities (21.0% vs. 18.7%) and coronary artery aneurysms (4.7% vs. 3.1%) among the younger group [ 14 ]. Mastrangelo et al., in their recent single-center cohort of 113 patients younger than one year with KD, reported that infants with incomplete KD seem to have more severe disease and a greater predilection for coronary involvement compared to infants with complete KD [ 13 ].
The most recent algorithm of IKD by the AHA for infants includes ≥7 days of unexplained fever plus either three or more supplemental laboratory findings or typical echocardiographic findings (Z-score of the left anterior coronary artery (LAD) or right coronary artery (RCA) is ≥2.5). Additionally, the diagnosis may be considered in the following situations in infants less than six months: (1) prolonged fever and irritability; (2) prolonged fever and unexplained aseptic meningitis; (3) prolonged fever and unexplained or culture-negative shock; (4) prolonged fever and cervical lymphadenitis unresponsive to antibiotic therapy; (5) prolonged fever and retropharyngeal or parapharyngeal phlegmon unresponsive to antibiotic therapy [ 5 ].
The duration of fever has been confirmed as an important risk factor of coronary artery abnormalities [ 6 , 15 ]. There is a paucity of large-scale studies exclusively on KD below the age of 12 months regarding this topic. Non-fever KD has been reported in the available literature; nevertheless, it remains poorly defined with no clear consensus on its natural history and prognosis. From the scant reported cases, it appears to be more common in toddlers, and most cases had a mild course and few coronary artery abnormalities were identified [ 16 - 17 ]. In contrast, we were able to identify only two cases of infantile non-fever KD in the English literature. All reported cases, including our case, had significant echocardiographic findings reported [ 16 , 18 ].
The majority of existing guidelines consider the presence of fever for at least seven days a requirement for the diagnosis of both KD and IKD in infants. Therefore, it may induce a delay of management and postpone the diagnosis of IKD until the confirmation of coronary artery abnormalities, similar to our case. A decreased ability to mount a fever response may be present in some young infants, further contributing to the difficulty of diagnosing KD in this age group. Salgado et al., Singh et al., and Pilania et al. have all expressed similar concerns [ 12 , 19 - 20 ]. We do believe that the role of fever and the duration required to fulfill the criteria (especially in infants) needs to be redefined in the future.
There are many treatment options in the management of IKD, but no consensus has yet been established. The backbone of therapy includes IVIG and aspirin. Adjunctive therapies in the treatment included corticosteroids and biologic agents [ 5 - 6 ]. In our case, the patient was treated with IVIG as soon as the diagnosis of IKD was made; the patient had an excellent response with reduction of coronary artery dilatation and absence of new aneurysms on follow-up echocardiogram two weeks later. This case highlights that the diagnosis of IKD should be considered in infants presenting with unexplained fever even in the absence of the principal clinical findings of KD, particularly in those less than six months.
Conclusions
Presently, the diagnosis of incomplete Kawasaki Disease might be made in cases with fewer classical diagnostic criteria and with several compatible clinical, laboratory, or echocardiographic findings. However, the diagnosis of IKD in infants less than six months of age remains a clinical challenge. This results in an increased rate of delayed diagnosis and subsequent risk of developing cardiac complications among this age group. Pediatricians should search for coronary artery lesions in cases of high clinical suspicion, even if the fever period is short, particularly in those less than six months. Additionally, further innovative research is very much needed to identify immunological and cellular markers that could be tested early in the course of the disease and guide management.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study

IMAGES
COMMENTS
The clinical presentation of Kawasaki disease can be divided into 3 stages. The acute stage begins with the onset of fever and lasts 1 to 2 weeks. ... From here, the disease spreads to the perineal region (see next slide). A few days after the appearance of the eruption shown in the previous slide, the disease may affect the perineal region ...
Document presentation format: On-screen Show ... Arial Wingdings Calibri Orbit Orbit Kawasaki Disease Introduction Etiology Epidemiology Clinical History Physical Diagnosis Physical Diagnosis Physical Diagnosis Slide 9 Clinical course Slide 11 Aneurysms Risk factors for aneurysms Slide 14 Differential Diagnosis Workup Imaging Studies Imaging ...
Kawasaki disease' presentation also overlaps with other immunologic reactions such as multiple drug hypersensitivity reactions, juvenile idiopathic arthritis, infantile polyarteritis nodosa, and systemic lupus erythematosus. These can be differentiated from KD by the absence of classic clinical criteria and by chronicity and the number of ...
The clinical features of KD reflect widespread inflammation of primarily medium-sized muscular arteries. Diagnosis is based upon evidence of systemic inflammation (eg, fever) in association with signs of mucocutaneous inflammation. The characteristic bilateral nonexudative conjunctivitis, erythema of the lips and oral mucosa, rash, extremity ...
Kawasaki Disease (KD ) also known as Kawasaki Syndrome . • An unusual and serious illness of young children. It is an autoimmune disease that can effect any type of blood vessel in the body, including arteries, veins, and capillaries. Download Presentation. specific virus.
Kawasaki disease is the most common cause of multisystem vasculitis in childhood. ... Download ppt "Kawasaki disease: Difficult case scenario and guidelines" Similar presentations . Hatem Eleishi, MD Rheumatologist STILL'S DISEASE. Kawasaki disease is a rare condition. It is most common in children under five years old and most cases occur in ...
Kawasaki Disease.ppt - Free download as Powerpoint Presentation (.ppt), PDF File (.pdf), Text File (.txt) or view presentation slides online. Scribd is the world's largest social reading and publishing site.
AIM: • Review the clinical presentations of Kawasaki disease, its progression, serious complications and management. • Discuss the D/Dx of Kawasaki disease. S> • 14 m/o, M, ... KAWASAKI. AMOUNT OF PRODUCTION. KAWASAKI. 691 views • 22 slides. Kawasaki Ninja 250R. Kawasaki Ninja 250R. Background on the Ninja 250R. An entry-level 250cc ...
Kawasaki disease is sometimes called mucocutaneous lymph node syndrome. That's because it also causes swelling in glands, called lymph nodes, and mucous membranes inside the mouth, nose, eyes and throat. Children with Kawasaki disease might have high fever, swollen hands and feet with skin peeling, and red eyes and tongue.
Kawasaki disease symptoms can include: Fever lasting for at least five days. Irritability. Red or pink eyes without discharge. Redness or cracking of your child's lips or tongue. Swelling and/or redness of your child's hands or feet. Peeling of your child's skin, usually beginning around their nails.
Kawasaki disease is an acute systemic vasculitis that was first reported in 1961. Over the last 5 decades multiple papers have been published to further understand this disease. ... Dr. Kawasaki observed 50 cases with similar presentations, following which he published a paper entitled 'Acute febrile musculocutaneous lymph node syndrome ...
Presentation Transcript. 1. Kawasaki Disease. 2. Kawasaki Disease Acute febrile vasculitic syndrome of early childhood Affecting all blood vessels in the body but mostly medium and small vessels with a preferential involvement of the coronary arteries. Exact etiology unknown but thought to be infectious in nature. 3.
Kawasaki disease is an acute febrile illness and systemic vasculitis of unknown aetiology that predominantly afflicts young children, causes coronary artery aneurysms and can result in long-term ...
Kawasaki disease (KD), also known as mucocutaneous lymph node syndrome or infantile polyarteritis, is a medium-sized necrotizing vasculitis that predominantly affects children < 5 years of age. The etiology is currently unknown, but it is postulated to involve a combination of environmental and genetic factors.
KAWASAKI DISEASE. Learning about Kawasaki Disease and How It Affects Children. Kawasaki disease is a frightening and rare condition in children. ... KAWASAKI. AMOUNT OF PRODUCTION. KAWASAKI. 710 views • 22 slides. Guy Kawasaki. Guy Kawasaki. By Nagarjuna.R Enroll No: 8024. ... C., De Gracia C. AIM:. Review the clinical presentations of K ...
Kawasaki disease (KD) is the commonest cause of acquired heart disease in children in the developed world and is increasingly being reported from developing countries. ... Gordon J, Singh S. Presentation of missed childhood Kawasaki disease in adults: experience from a tertiary care center in North India. Int J Rheum Dis. 2017; 20:1023-1027 ...
Kawasaki Disease (KD) is an acute, self-limited systemic vasculitis of small and medium vessels and occurs mainly between six months and five years of age. ... Case presentation. A previously healthy four-month-old Hispanic female presented to the emergency department (ED) with a fever of (102.1°F) and decreased oral intake of one-day duration ...