

Summary of Awards to Date
Development of an infrared-functionalized microbalance sensor for cyclospora cayetanensis detection and differentiation.
Jan. 1, 2023 - Dec. 31, 2023
$100,756.00
Jenny Maloney, Ph.D. USDA - ARS
Monica Santin, Ph.D., Laurene Tetard, Ph.D.

Cyclospora cayetanensis (Cc) is a parasite which causes diarrheal illness in humans worldwide and is spread through contaminated food and water. Current procedures for Cc detection are expensive and time consuming. A detection system for Cc which is simple, fast, low-cost, and can be used in the field is needed. This proposal will test and develop a novel detection system for Cc by pairing infrared microscopy with cantilever-based microsensor technology. The sensing system will initially be developed using commercially available parasites and final testing stages will use oocysts of Cc. To determine if the sensing system has the sensitivity needed for testing produce and water samples, it will be compared to methods currently used for Cc detection. This project represents the first step toward producing a new tool which can be used by growers, processors, researchers, and testing laboratories to detect and quantify Cc quickly and cost-effectively. Such a tool could significantly improve our understanding of Cc risk and risk factor contributors and be used by growers, producers, and regulators to improve the safety of the fresh produce available to consumers.
Technical Abstract
Cyclospora cayetanensis (Cc) is a prevalent worldwide intestinal protozoan parasite of humans which is spread through contaminated food and water. Testing of food and water samples to understand the risk factors for Cc contamination is needed to limit transmission, develop control strategies, and improve food safety. Currently, microscopy and molecular techniques are used to detect Cc but are time intensive and require extensive sample preparation and personnel expertise. A method for Cc detection which is simple, fast, and low-cost with the potential for scalable implementation in the field would greatly enhance food safety. To build toward this goal, we will test and develop a sensing system which pairs infrared microscopy with cantilever-based microsensor technology to detect Cc. The initial design phase will employ commercially available protozoan parasites before testing the system on Cc oocysts from human samples. Comparisons between the sensitivity and specificity of the sensing system, microscopy, and PCR will be made to provide quantifiable measures of success of the platform. This project will provide the foundational data needed to further develop the sensing platform into a tool which can be used by growers, processors, Cc researchers, and testing laboratories to detect and quantify Cc quickly and cost-effectively. Such a tool could lead to significant improvements in understanding Cc risk and risk factor contributors, which can be used by growers, producers, and regulators to mitigate transmission risk and improve the safety of the fresh produce available to consumers.
- Grant Opportunities
- Funded Research Projects
- News Center
- Support CPS
- Annual Research Symposium
- Global Research Database
The Center for Produce Safety P: (530) 554-9706 [email protected] 1100 Main Street, Suite 210 Woodland, CA 95695
Stay Connected
Want to receive news, requests for proposals and find out about upcoming events?
Sign up for our mailing list here .
About CPS | Terms of Use
Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, generate accurate citations for free.
- Knowledge Base
- Starting the research process
- How to Write a Research Proposal | Examples & Templates
How to Write a Research Proposal | Examples & Templates
Published on October 12, 2022 by Shona McCombes and Tegan George. Revised on November 21, 2023.
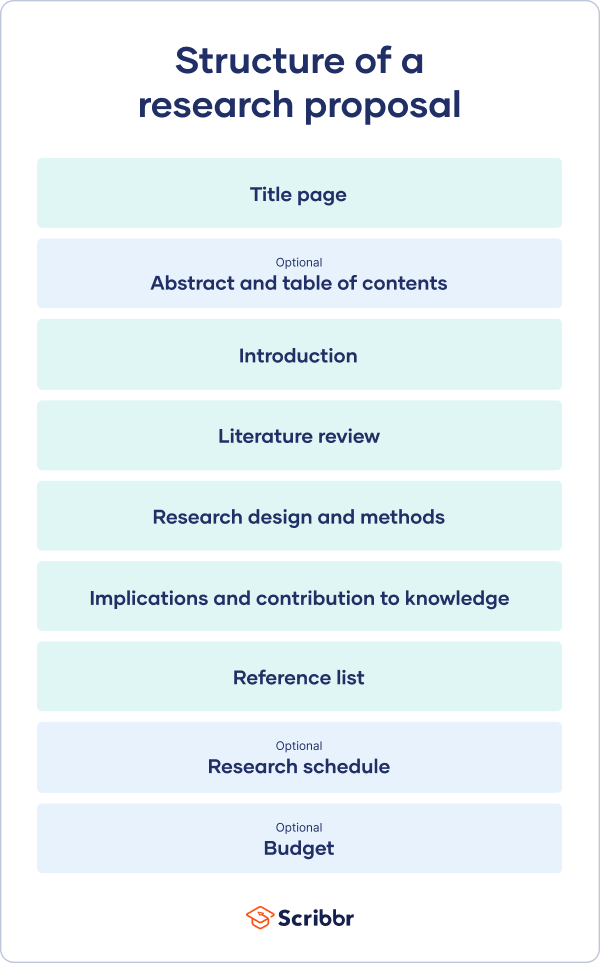
A research proposal describes what you will investigate, why it’s important, and how you will conduct your research.
The format of a research proposal varies between fields, but most proposals will contain at least these elements:
Introduction
Literature review.
- Research design
Reference list
While the sections may vary, the overall objective is always the same. A research proposal serves as a blueprint and guide for your research plan, helping you get organized and feel confident in the path forward you choose to take.
Table of contents
Research proposal purpose, research proposal examples, research design and methods, contribution to knowledge, research schedule, other interesting articles, frequently asked questions about research proposals.
Academics often have to write research proposals to get funding for their projects. As a student, you might have to write a research proposal as part of a grad school application , or prior to starting your thesis or dissertation .
In addition to helping you figure out what your research can look like, a proposal can also serve to demonstrate why your project is worth pursuing to a funder, educational institution, or supervisor.
Research proposal length
The length of a research proposal can vary quite a bit. A bachelor’s or master’s thesis proposal can be just a few pages, while proposals for PhD dissertations or research funding are usually much longer and more detailed. Your supervisor can help you determine the best length for your work.
One trick to get started is to think of your proposal’s structure as a shorter version of your thesis or dissertation , only without the results , conclusion and discussion sections.
Download our research proposal template
Receive feedback on language, structure, and formatting
Professional editors proofread and edit your paper by focusing on:
- Academic style
- Vague sentences
- Style consistency
See an example

Writing a research proposal can be quite challenging, but a good starting point could be to look at some examples. We’ve included a few for you below.
- Example research proposal #1: “A Conceptual Framework for Scheduling Constraint Management”
- Example research proposal #2: “Medical Students as Mediators of Change in Tobacco Use”
Like your dissertation or thesis, the proposal will usually have a title page that includes:
- The proposed title of your project
- Your supervisor’s name
- Your institution and department
The first part of your proposal is the initial pitch for your project. Make sure it succinctly explains what you want to do and why.
Your introduction should:
- Introduce your topic
- Give necessary background and context
- Outline your problem statement and research questions
To guide your introduction , include information about:
- Who could have an interest in the topic (e.g., scientists, policymakers)
- How much is already known about the topic
- What is missing from this current knowledge
- What new insights your research will contribute
- Why you believe this research is worth doing
As you get started, it’s important to demonstrate that you’re familiar with the most important research on your topic. A strong literature review shows your reader that your project has a solid foundation in existing knowledge or theory. It also shows that you’re not simply repeating what other people have already done or said, but rather using existing research as a jumping-off point for your own.
In this section, share exactly how your project will contribute to ongoing conversations in the field by:
- Comparing and contrasting the main theories, methods, and debates
- Examining the strengths and weaknesses of different approaches
- Explaining how will you build on, challenge, or synthesize prior scholarship
Following the literature review, restate your main objectives . This brings the focus back to your own project. Next, your research design or methodology section will describe your overall approach, and the practical steps you will take to answer your research questions.
To finish your proposal on a strong note, explore the potential implications of your research for your field. Emphasize again what you aim to contribute and why it matters.
For example, your results might have implications for:
- Improving best practices
- Informing policymaking decisions
- Strengthening a theory or model
- Challenging popular or scientific beliefs
- Creating a basis for future research
Last but not least, your research proposal must include correct citations for every source you have used, compiled in a reference list . To create citations quickly and easily, you can use our free APA citation generator .
Some institutions or funders require a detailed timeline of the project, asking you to forecast what you will do at each stage and how long it may take. While not always required, be sure to check the requirements of your project.
Here’s an example schedule to help you get started. You can also download a template at the button below.
Download our research schedule template
If you are applying for research funding, chances are you will have to include a detailed budget. This shows your estimates of how much each part of your project will cost.
Make sure to check what type of costs the funding body will agree to cover. For each item, include:
- Cost : exactly how much money do you need?
- Justification : why is this cost necessary to complete the research?
- Source : how did you calculate the amount?
To determine your budget, think about:
- Travel costs : do you need to go somewhere to collect your data? How will you get there, and how much time will you need? What will you do there (e.g., interviews, archival research)?
- Materials : do you need access to any tools or technologies?
- Help : do you need to hire any research assistants for the project? What will they do, and how much will you pay them?
If you want to know more about the research process , methodology , research bias , or statistics , make sure to check out some of our other articles with explanations and examples.
Methodology
- Sampling methods
- Simple random sampling
- Stratified sampling
- Cluster sampling
- Likert scales
- Reproducibility
Statistics
- Null hypothesis
- Statistical power
- Probability distribution
- Effect size
- Poisson distribution
Research bias
- Optimism bias
- Cognitive bias
- Implicit bias
- Hawthorne effect
- Anchoring bias
- Explicit bias
Once you’ve decided on your research objectives , you need to explain them in your paper, at the end of your problem statement .
Keep your research objectives clear and concise, and use appropriate verbs to accurately convey the work that you will carry out for each one.
I will compare …
A research aim is a broad statement indicating the general purpose of your research project. It should appear in your introduction at the end of your problem statement , before your research objectives.
Research objectives are more specific than your research aim. They indicate the specific ways you’ll address the overarching aim.
A PhD, which is short for philosophiae doctor (doctor of philosophy in Latin), is the highest university degree that can be obtained. In a PhD, students spend 3–5 years writing a dissertation , which aims to make a significant, original contribution to current knowledge.
A PhD is intended to prepare students for a career as a researcher, whether that be in academia, the public sector, or the private sector.
A master’s is a 1- or 2-year graduate degree that can prepare you for a variety of careers.
All master’s involve graduate-level coursework. Some are research-intensive and intend to prepare students for further study in a PhD; these usually require their students to write a master’s thesis . Others focus on professional training for a specific career.
Critical thinking refers to the ability to evaluate information and to be aware of biases or assumptions, including your own.
Like information literacy , it involves evaluating arguments, identifying and solving problems in an objective and systematic way, and clearly communicating your ideas.
The best way to remember the difference between a research plan and a research proposal is that they have fundamentally different audiences. A research plan helps you, the researcher, organize your thoughts. On the other hand, a dissertation proposal or research proposal aims to convince others (e.g., a supervisor, a funding body, or a dissertation committee) that your research topic is relevant and worthy of being conducted.
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the “Cite this Scribbr article” button to automatically add the citation to our free Citation Generator.
McCombes, S. & George, T. (2023, November 21). How to Write a Research Proposal | Examples & Templates. Scribbr. Retrieved April 15, 2024, from https://www.scribbr.com/research-process/research-proposal/
Is this article helpful?
Shona McCombes
Other students also liked, how to write a problem statement | guide & examples, writing strong research questions | criteria & examples, how to write a literature review | guide, examples, & templates, "i thought ai proofreading was useless but..".
I've been using Scribbr for years now and I know it's a service that won't disappoint. It does a good job spotting mistakes”
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- News Feature
- Published: 05 September 2012
ENCODE: The human encyclopaedia
- Brendan Maher
Nature volume 489 , pages 46–48 ( 2012 ) Cite this article
2346 Accesses
159 Citations
499 Altmetric
Metrics details
- Functional genomics
- Genetic databases
- Molecular biology
First they sequenced it. Now they have surveyed its hinterlands. But no one knows how much more information the human genome holds, or when to stop looking for it.
This is a preview of subscription content, access via your institution
Relevant articles
Open Access articles citing this article.
Improved and Flexible HDR Editing by Targeting Introns in iPSCs
- , Ya-Wen Fu
- … Chang-Kai Sun
Stem Cell Reviews and Reports Open Access 28 January 2022
LINC00665 promotes breast cancer progression through regulation of the miR-379-5p/LIN28B axis
- , Yu-Ling Diao
- … Yue Yu
Cell Death & Disease Open Access 06 January 2020
Access options
Subscribe to this journal
Receive 51 print issues and online access
185,98 € per year
only 3,65 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
The ENCODE Project Consortium Nature 489 , 57–74 (2012).
International Human Genome Sequencing Consortium Nature 431 , 931–945 (2004).
The ENCODE Project Consortium Nature 447 , 799–816 (2007).
Thurman, R. E. et al. Nature 489 , 75–82 (2012).
Article ADS CAS Google Scholar
Neph, S. et al. Nature 489 , 83–90 (2012).
Gerstein, M. B. et al. Nature 489 , 91–100 (2012).
Djebali, S. et al. Nature 489 , 101–108 (2012).
Sanyal, A., Lajoie, B. R., Jain, G. & Dekker, J. Nature 489 , 109–113 (2012).
Download references
You can also search for this author in PubMed Google Scholar
Related video
ENCODE: Encyclopedia of DNA Elements
Related links
Related links in nature research.
Spinning threads 2012-Sep-05
Presenting ENCODE 2012-Sep-05
Genomics: ENCODE explained 2012-Sep-05
The making of ENCODE: Lessons for big-data projects 2012-Sep-05
Human genome at ten: Life is complicated 2010-Mar-31
Genome project turns up evolutionary surprises 2007-Jun-13
Nature special: ENCODE
Nature News special: The human genome at ten
Scientfic American : Hidden treasures in ‘junk’ DNA
Related external links
Rights and permissions.
Reprints and permissions
About this article
Cite this article.
Maher, B. ENCODE: The human encyclopaedia. Nature 489 , 46–48 (2012). https://doi.org/10.1038/489046a
Download citation
Published : 05 September 2012
Issue Date : 06 September 2012
DOI : https://doi.org/10.1038/489046a

Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
- Chang-Kai Sun
Stem Cell Reviews and Reports (2022)
- Yu-Ling Diao
Cell Death & Disease (2020)
Digestion-ligation-only Hi-C is an efficient and cost-effective method for chromosome conformation capture
Nature Genetics (2018)
Towards precise reconstruction of gene regulatory networks by data integration
- Zhi‐Ping Liu
Quantitative Biology (2018)
Comparative transcriptomics in human and mouse
- Alessandra Breschi
- Thomas R. Gingeras
- Roderic Guigó
Nature Reviews Genetics (2017)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Translational Research newsletter — top stories in biotechnology, drug discovery and pharma.
HIST 489 Research Project Student Guide 2021 - History Programme School of History, Philosophy, Political Science and International Relations Te ...
- Download HTML
- Download PDF

- Home & Garden

- Uncategorized

- Current Events

- Health & Fitness

- Hobbies & Interests

- IT & Technique

- Government & Politics

- Arts & Entertainment

- World Around

- Food & Drink

- Style & Fashion

Research Project in Biochemistry Microbiology and Immunology
Subject: Bioch Micro Immuno Sciences Credit units: 6 Offered: Term 1 and 2 Weekly hours: 8 Practicum/Lab hours College: Medicine Department: Bioch Micro Immuno
Description
In this course, students have an exciting opportunity to carry out their own research project under the supervision of a faculty member and to further develop critical thinking skills. Students will learn how to review the scientific literature relevant to a specific research problem; how to develop a hypothesis and set specific objectives; and how to design and execute experiments. Students will also gain experience in analyzing and interpreting experimental data and learn how to write a scientific report. Students will also have an opportunity to present their project and findings to the department through an oral presentation.
Prerequisite(s): Admission to the Honours program in Biochemistry, Microbiology and Immunology. Note: Permission of the department is required. Students with credit for BIOC 489.6 or MCIM 491.6 may not take this course for credit.
Upcoming class offerings
For full details about upcoming courses, refer to the class search tool or, if you are a current student, the registration channel in PAWS.
- Class search
- Registration channel
The syllabus is a public document that provides detail about a class, such as the schedule of activities, learning outcomes, and weighting of assignments and examinations.
Once an instructor has made their syllabus publicly available on USask’s Learning Management System, it will appear below. Please note that the examples provided below do not represent a complete set of current or previous syllabus material. Rather, they are presented solely for the purpose of indicating what may be required for a given class. Unless otherwise specifically stated on the content, the copyright for all materials in each course belongs to the instructor whose name is associated with that course. The syllabus is the intellectual property of instructors or the university.
For more information, visit the Academic Courses Policy , the Syllabus page for instructors , or for students your Academic Advising office .
- Bookstore Textbook Search
- Library Reserves Search
- USask Tutoring Network
Jump to navigation

National Institute for Transportation and Communities
Search form.
- Latest News
- Media Coverage
- Join Our Mailing List
- Final Reports
- NITC Researchers
- Requirements and Forms
- Researcher Login
- Curriculum: K-12 and University
- A decade of NITC research
- Transportation and Land Use
- Multimodal Data and Modeling
- Walking and Biking
- New Mobility and Technology
- Equitable Mobility
- Transportation Resiliency
- Upcoming Events
- Annual Summit
- K-12 Programs
- Past Events
- Student Spotlights
- Student of the Year
- Dissertation Fellowships
- Student Groups
Wider Dissemination of Household Travel Survey Data Using Geographical Perturbation Methods
Principal investigator:, kelly clifton , portland state university, project details, downloadable products.
Other Products
- Clifton, Kelly J. and Gehrke, Steven R. "Application of Geographic Perturbation Methods to Residential Locations in the Oregon Household Activity Survey: Proof of Concept." Transportation Research Record: forthcoming (PUBLICATION)
On theory development in design science research: anatomy of a research project
- Special Issue Article
- Published: 18 November 2008
- Volume 17 , pages 489–504, ( 2008 )
Cite this article
- Bill Kuechler 1 &
- Vijay Vaishnavi 2
1960 Accesses
262 Citations
3 Altmetric
Explore all metrics
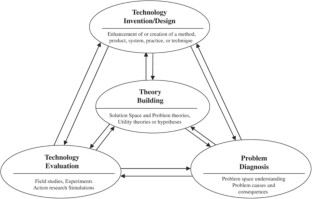
The common understanding of design science research in information systems (DSRIS) continues to evolve. Only in the broadest terms has there been consensus: that DSRIS involves, in some way, learning through the act of building . However, what is to be built – the definition of the DSRIS artifact – and how it is to be built – the methodology of DSRIS – has drawn increasing discussion in recent years. The relationship of DSRIS to theory continues to make up a significant part of the discussion: how theory should inform DSRIS and whether or not DSRIS can or should be instrumental in developing and refining theory. In this paper, we present the exegesis of a DSRIS research project in which creating a (prescriptive) design theory through the process of developing and testing an information systems artifact is inextricably bound to the testing and refinement of its kernel theory.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Baldwin D and Yadav S (1994) The process of research investigations in artificial intelligence – an unified view. IEEE Transactions on Systems, Man and Cybernetics 25 (5), 852–861.
Article Google Scholar
Canfora G, Casazza G and De Luca A (2000) A design rationale based environment for cooperative maintenance. International Journal of Software Engineering & Knowledge Engineering 10 (5), 627–646.
Carroll J and Kellogg W (1989) Artifact as theory nexus: hermeneutics meets theory-based design. In Proceedings of CHI ‘89 (B ICE K and L EWIS C, Eds), ACM Press, New York.
Cook D, Holder L and Youngblood C (2007) Graph-based analysis of human transfer learning using a game testbed. IEEE Transactions on Knowledge and Data Engineering 19 (11), 1465–1478.
Cysneiros L, Leite J and Nito J (2001) A framework for integrating non-functional requirements into conceptual models. Requirements Engineering 6 (2), 97–115.
Dasgupta S (1996) Technology and Creativity. Oxford University Press, New York.
Google Scholar
Davies I, Green P, Rosemann M, Indulska M and Gallo S (2006) How do practitioners use conceptual modeling in practice? Data & Knowledge Engineering 58, 358–380.
Fickas S and Helm R (1992) Knowledge representation and reasoning in the design of composite systems. IEEE Transactions on Software Engineering 18 (6), 470–482.
Gause D (2005) Why context matters – and what can we do about it? IEEE Software 22 (5), 13–15.
Gentner D (1983) Structure-mapping: a theoretical framework for analogy. Cognitive Science 7, 155–170.
Goldkuhl G (2004) Design theories in information systems – a need for multi-grounding. Journal of Information Technology Theory and Application 6 (2), 59–72.
Gotel O and Finkelstein A (1995) Contribution structures [Requirements artifacts] Second IEEE International Symposium on Requirements Engineering (RE‘95), IEEE Computer Society.
Gregor S (2006) The nature of theory in information systems. MIS Quarterly 30 (3), 611–642.
Gregor S and Jones D (2007) The anatomy of a design theory. Journal of the Association for Information Systems (JAIS) 8 (5), Article 19.
Hevner A, March S, Park J and Ram S (2004) Design science in information systems research. MIS Quarterly 28 (1), 75–105.
Iivari J (1986) Dimensions of information systems design: a framework for a long-range research program. Information Systems Frontiers 11 (2), 185–197.
Jou J and Shanteau J (1996) An information processing view of framing effects: the role of causal schemas in decision making. Memory and Cognition 24 (1), 1–15.
Kuechler W and Vaishnavi V (2006) So, talk to me: the effect of explicit goals on the comprehension of business process narratives. MIS Quarterly 30 (4), 961–996.
Kuechler W and Vaishnavi V (2008) The emergence of design science research in information systems in North America. Journal of Design Research 7 (1), 1–16.
Kuechler W, Vaishnavi V and Petter S (2005) The aggregate general design cycle as a perspective on the evolution of computing communities of interest. Computing Letters 1 (3), 123–128.
Lethbridge T, Singer J and Forward A (2003) How software engineers use documentation: the state of the practice. IEEE Software 20 (6), 35–39.
Lewalter D (2003) Cognitive strategies for learning from static and dynamic visuals. Learning and Instruction 13, 177–189.
Maiden N, Manning S, Jones S and Greenwood J (2005) Generating requirements from systems models using patterns: a case study. Requirements Engineering 10, 276–288.
March S and Smith G (1995) Design and natural science research on information technology. Decision Support Systems 15, 251–266.
Markus L and Lee A (2000) Foreward: special issue on intensive research. MIS Quarterly 24 (3), 473–474.
Mayer R and Jackson J (2005) The case for coherence in scientific explanations: quantitative details can hurt qualitative understanding. Journal of Experimental Psychology: Applied 11 (1), 13–18.
Merton R (1968) Social Theory and Social Structure. Free Press, New York, NY.
Mylopoulos J, Chung L and Nixon B (1992) Representing and using nonfunctional requirements: a process-oriented approach. IEEE Transactions on Software Engineering 18 (6), 483–497.
Nelson K, Nadkarni S, Narayanan V and Ghods M (2000) Understanding software operations support expertise: a revealed causal mapping approach. MIS Quarterly 24 (3), 475–507.
Nissen HW, Jeusfeld M, Jarke M, Zemanek G and Huber H (1996) Managing multiple requirements perspectives with metamodels. IEEE Software 13 (2), 37–48.
Nunamaker J, Chen M and Purdin T (1991) Systems development in information systems research. Journal of Management Information Systems 7 (3), 89–106.
Orlikowski W and Iacono C (2001) Desperately seeking the “IT” in IT research – a call to theorizing the IT artifact. Information Systems Research 12 (2), 121–134.
Parsons J and Cole L (2005) What do the pictures mean? Guuidelines for experimental evaluation of representation fidelity in diagrammatical conceptual modeling techniques. Data & Knowledge Engineering 55, 327–342.
Purao S (2002) Truth or dare: design research in information technology. GSU CIS Department Working Paper, 2002.
Seufert T, Janen I and Bruken R (2007) The impact of intrinsic cognitive load on the effectiveness of graphical help for coherence formation. Computers in Human Behavior 23, 1055–1071.
Simon A (1996) The Sciences of the Artificial, 3rd edn, MIT Press, Cambridge, MA.
Stefansen C and Borch S (2008) Using soft constraints to guide users in flexible business process management systems. International Journal of Business Process Integration and Management 3 (1), 26–35.
Takeda H, Veerkamp P, Tomiyama T and Yoshikawam H (1990) Modeling design processes. AI Magazine (Winter), 37–48.
Tversky A and Kahneman D (1981) The framing of decisions and the psychology of choice. Science 211, 453–458.
Vaishnavi V and Kuechler W (2004) Design research in information systems. 20 January 2004, last updated 29 June 2007. [WWW document] http://www.isworld.org/Researchdesign/drisISworld.htm .
Vaishnavi V and Kuechler W (2007) Design Science Research Methods and Patterns: Innovating Information and Communication Technology. Auerbach, New York.
Book Google Scholar
Vans A and Von Mayrhauser A (1999) Program understanding behavior during corrective maintenance of large scale software. International Journal of Human–Computer Studies 51, 31–70.
Venable J (2006a) The role of theory and theorizing in design science research. In Proceedings DESRIST 2006 (C HATTERJEE S and H EVNER A, Eds), Claremont, CA, http://ncl.cgu.edu/designconference/index.htm .
Venable J (2006b) A framework for design science research activities. In Proceedings of the 2006 Information Resource Management Association Conference (K HOSROW -P OUR M, Ed), Washington, DC, 24–26 May 2006, IRMA, Hershey, PA.
Walls J, Widmeyer G and El Sawy O (1992) Building an information system design theory for vigilant EIS. Information Systems Research 3 (1), 36–59.
Walls J, Widmeyer G and El Sawy O (2004) Assessing information system design theory in perspective: how useful was our 1992 initial rendition. Journal of Information Technology Theory and Application 6 (2), 43–58.
Wand Y and Weber R (2002) Information systems and conceptual modeling – a research agenda. Information Systems Research 13 (4).
Yu E (1995) Models for supporting the redesign of organizational work. In The Proceedings of the Conference on Organizational Computing Systems (C OMSTOCK N and E LLIS C, Eds), pp. 225–236, ACM, New York.
Yu E and Mylopoulos J (1994) Understanding “Why” in software process modeling. 16th International Conference on Software Engineering, Sorrento, Italy.
Zukier H (1986) The paradigmatic and narrative modes in goal-guided inference. In Handbook of Motivation and Cognition: Foundations of Social Behavior (S ORRENTINO RM and H IGGINS ET, Eds), Guilford, New York.
Zukier H (1990) Aspects of narrative thinking. In The Legacy of Solomon Asch: Essays in Cognition and Social Psychology (R OCK I, Ed), pp 195–209, Lawrence Earlbaum and Associates, Hillsdale, NJ.
Zukier H and Pepitone A (1984) Social roles and strategies in prediction: some determinants of the use of base-rate information. Journal of Personality and Social Psychology 47 (2), 349–360.
Download references
Acknowledgements
We are greatly indebted to the anonymous reviewers whose suggestions have greatly strengthened the paper. We especially appreciate the suggestion that what we originally referred to as refinement of kernel theories might in fact be the development of mid-range theory. A paper by the reviewer on this topic may well precede this paper to publication.
Author information
Authors and affiliations.
Accounting and Information Systems, University of Nevada, Reno, NV, U.S.A.
Bill Kuechler
Computer Information Systems, Georgia State University, Atlanta, Georgia, U.S.A.
Vijay Vaishnavi
You can also search for this author in PubMed Google Scholar
A process change scenario illustrating ‘soft context information’ (a true story)
Note that this scenario describes the revision of a significant organizational process that involves both information technology and nonautomated process actions. The overall process is sometimes referred to as a ‘composite system’ ( Fickas & Helm, 1992 ). The mission-critical ‘soft context’ information for this particular process revision is shown in italics in the scenario description below.
A medium sized U.S. university made an administrative decision to transition from paper-based student course evaluations to a web-based system. One of the university IT department's senior analysts gathered requirements for the system and was placed in charge of the project. The analyst was told the primary driver for the new system was the high cost of processing the paper forms. The analyst was also cautioned during interviews with several administrators that the system needed to generate very near the number of evaluations per course that the current system produced or the results would not be accepted. Not uncommonly this soft context information was never translated into a composite system requirement. A web-based system was developed that, when used, generated exactly the information required by the faculty and administration at a fraction of the cost per response. Unfortunately, the students saw no reason to take on the additional work of entering information into the system at a very busy time in the semester, and the system did not generate enough results to be usable. Several ‘obvious’ paths to greater use, such as requiring the students to enter evaluation information before grades would be issued for them, are politically unpalatable at the university. After several semesters of unsuccessful attempts to exhort students to greater system use, the university is on the verge of abandoning the system.
See Figures B1 , B2 and B3 .
AND/OR graphs used to represent system quality (taken from Cysneiros et al., 2001 ) http://www.palgrave-journals.com .
i * graphs used to represent system context for an air traffic control system (a very small portion of the total graph, taken from Maiden et al., 2005 ) http://www.palgrave-journals.com .
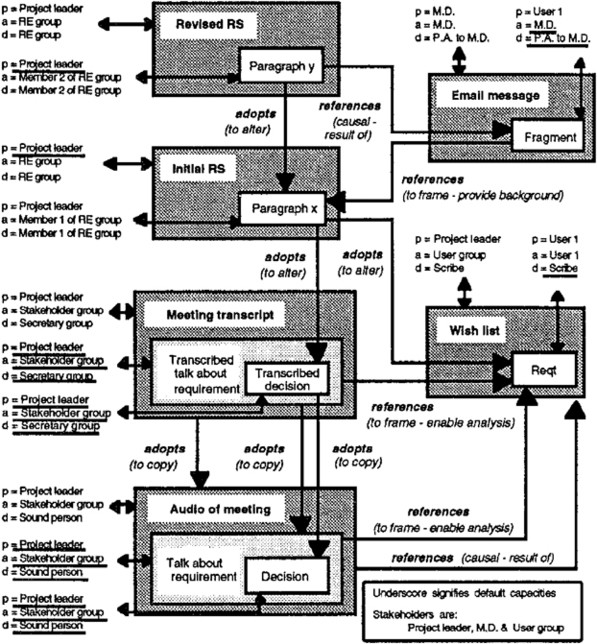
Connectivity structures (taken from Gotel & Finkelstein, 1995 ) http://www.palgrave-journals.com .
Sample process graph ‘slices’ and associated text description and micro-rationale as used in our evaluation prototype
http://www.palgrave-journals.com
With reference to the diagram above, the prototype works as follows for the treatment session:
In the actual prototype, the screen is wide enough to display a 50 character wide text section on the left of the screen and the full diagram on the right of the screen. Initially, instructions are displayed on the left and only slice 0 – the swim lane names and the graphic heading – is visible. The subject must click on the text to view the next information segment. Information segments alternate between narrative – descriptive text and micro-rationales – and the next sequential graphic slice. Text segments are displayed in sequential positions down the text display portion of the screen. Each piece of information, whether text or graphic, fades from view in 9 s. The subject must click on the information to make it reappear for 9 s. The only exception to this is the initial display of the graphic associated with a given text segment. That is, on clicking a text segment, the associated graphic is displayed and both are visible. However, after clicking on the associated graphic slice, both the graphic and its associated text disappear, and the next text segment appears. The prototype records the time and object for every mouse click. During final data analysis the click traces will augment coded transcriptions of the concurrent verbal protocols that were recorded as the subjects proceeded through the process display.
Rights and permissions
Reprints and permissions
About this article
Kuechler, B., Vaishnavi, V. On theory development in design science research: anatomy of a research project. Eur J Inf Syst 17 , 489–504 (2008). https://doi.org/10.1057/ejis.2008.40
Download citation
Received : 29 April 2008
Accepted : 08 September 2008
Published : 18 November 2008
Issue Date : 01 October 2008
DOI : https://doi.org/10.1057/ejis.2008.40
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- design science
- theory building
- mid-range theory
- kernel theory
- research methods
- design theory
- Find a journal
- Publish with us
- Track your research
National Institutes of Health ( NIH )
National Eye Institute ( NEI ) National Human Genome Research Institute ( NHGRI ) National Institute on Aging ( NIA ) National Institute on Alcohol Abuse and Alcoholism ( NIAAA ) National Institute of Allergy and Infectious Diseases ( NIAID ) National Institute of Arthritis and Musculoskeletal and Skin Diseases ( NIAMS ) National Institute on Drug Abuse ( NIDA ) National Institute on Deafness and Other Communication Disorders ( NIDCD ) National Institute of Dental and Craniofacial Research ( NIDCR ) National Institute of Environmental Health Sciences ( NIEHS ) National Institute on Minority Health and Health Disparities ( NIMHD ) National Institute of Nursing Research ( NINR ) National Library of Medicine ( NLM ) National Center for Complementary and Integrative Health ( NCCIH ) Note: Not all NIH Institutes and Centers (ICs) participate in Parent Announcements. Applicants should carefully note which ICs participate in this announcement and view their respective areas of research interest at the R21 IC-Specific Scientific Interests and Contact website. ICs that do not participate in this announcement will not consider applications for funding.
R21 Exploratory/Developmental Research Grant
Reissue of PA-16-161 for due dates on or after January 25, 2018
- March 5, 2020 - Notice of Special Interest (NOSI): Harnessing computational tools for sophisticated analyses of Substance Use Disorder-related behaviors. See Notice NOT-DA-20-017 .
- November 26, 2018 - NIH & AHRQ Announce Upcoming Updates to Application Instructions and Review Criteria for Research Grant Applications. See Notice NOT-OD-18-228 .
- November 5, 2018 - This PA has been reissued as PA-19-053 .
- May 18, 2018 - Notice of Information: NIMH Council Workgroup on Genomics' Recommendations for Basic and Clinical Research. See Notice NOT-MH-18-035 .
PA-18-344 - Parent R21 Clinical Trial Required Check Components of Participating Organizations and Related Notices for restrictions.
See Section III. 3. Additional Information on Eligibility .
93.273, 93.866, 93.855, 93.846, 93.213, 93.279, 93.173, 93.121, 93.113, 93.867, 93.172, 93.879, 93.307, 93.361
The NIH Exploratory/Developmental Grant supports exploratory and developmental research projects by providing support for the early and conceptual stages of these projects. These studies may involve considerable risk but may lead to a breakthrough in a particular area, or to the development of novel techniques, agents, methodologies, models, or applications that could have a major impact on a field of biomedical, behavioral, or clinical research.
December 6, 2017
January 16, 2018
Not Applicable
Standard dates apply by 5:00 PM local time of applicant organization. All types of non-AIDS applications allowed for this funding opportunity announcement are due on these dates.
The first standard application due date for this FOA is February 16, 2018.
Applicants are encouraged to apply early to allow adequate time to make any corrections to errors found in the application during the submission process by the due date.
Standard AIDS dates apply by 5:00 PM local time of applicant organization. All types of AIDS and AIDS-related applications allowed for this funding opportunity announcement are due on these dates.
The first AIDS application due date for this FOA is May 7, 2018. Applicants are encouraged to apply early to allow adequate time to make any corrections to errors found in the application during the submission process by the due date.
Standard dates apply
Standard dates apply or Month(s) Year(s)
New Date January 8, 2019 per issuance of PA-19-053 . (Original Expiration Date: January 8, 2021)
It is critical that applicants follow the Research (R) Instructions in the SF424 (R&R) Application Guide , except where instructed to do otherwise (in this FOA or in a Notice from the NIH Guide for Grants and Contracts ). Conformance to all requirements (both in the Application Guide and the FOA) is required and strictly enforced. Applicants must read and follow all application instructions in the Application Guide as well as any program-specific instructions noted in Section IV . When the program-specific instructions deviate from those in the Application Guide, follow the program-specific instructions. Applications that do not comply with these instructions may be delayed or not accepted for review.
Part 1. Overview Information Part 2. Full Text of the Announcement
Section I. Funding Opportunity Description Section II. Award Information Section III. Eligibility Information Section IV. Application and Submission Information Section V. Application Review Information Section VI. Award Administration Information Section VII. Agency Contacts Section VIII. Other Information
The evolution and vitality of the biomedical, behavioral, and clinical sciences require a constant infusion of new ideas, techniques, and points of view. These may differ substantially from current thinking or practice and may not yet be supported by substantial preliminary data. Through the NIH Exploratory/Developmental Research Grant Program, the NIH seeks to foster the introduction of novel scientific ideas, model systems, tools, agents, targets, and technologies that have the potential to substantially advance biomedical, behavioral, and clinical research.
This program is intended to encourage new exploratory and developmental research projects. For example, such projects could assess the feasibility of a novel area of investigation or a new experimental system that has the potential to enhance health-related research. Another example could include the unique and innovative use of an existing methodology to explore a new scientific area. These studies may involve considerable risk but may lead to a breakthrough in a particular area, or to the development of novel techniques, agents, methodologies, models, or applications that could have a major impact on a field of biomedical, behavioral, or clinical research.
Applications for Exploratory/Developmental Research Grant awards should include projects distinct from those supported through the traditional R01 activity code. For example, long-term projects, or projects designed to increase knowledge in a well-established area, are not appropriate for this FOA. Applications submitted to this FOA should be exploratory and novel. These studies should break new ground or extend previous discoveries toward new directions or applications. Projects of limited cost or scope that use widely accepted approaches and methods within well-established fields are better suited for the NIH Small Research Grant Program .
This Funding Opportunity Announcement does not accept applications proposing clinical trial(s)
Applications are assigned to participating Institutes and Centers (ICs) based on receipt and referral guidelines and many applications are assigned to multiple participating ICs with related research interests. Applicants are encouraged to identify a participating IC that supports their area of research via the R21 IC-Specific Scientific Interests and Contact website and contact Scientific/Research staff from relevant ICs to inquire about their interest in supporting the proposed research project.
See Section VIII. Other Information for award authorities and regulations.
Grant: A support mechanism providing money, property, or both to an eligible entity to carry out an approved project or activity.
New Resubmission Revision
The OER Glossary and the SF424 (R&R) Application Guide provide details on these application types.
Not Allowed: Only accepting applications that do not propose clinical trials
Need help determining whether you are doing a clinical trial?
The number of awards is contingent upon NIH appropriations and the submission of a sufficient number of meritorious applications.
The combined budget for direct costs for the two-year project period may not exceed $275,000. No more than $200,000 may be requested in any single year.
The total project period may not exceed 2 years.
NIH grants policies as described in the NIH Grants Policy Statement will apply to the applications submitted and awards made from this FOA.
Higher Education Institutions
- Public/State Controlled Institutions of Higher Education
- Private Institutions of Higher Education
The following types of Higher Education Institutions are always encouraged to apply for NIH support as Public or Private Institutions of Higher Education:
- Hispanic-serving Institutions
- Historically Black Colleges and Universities (HBCUs)
- Tribally Controlled Colleges and Universities (TCCUs)
- Alaska Native and Native Hawaiian Serving Institutions
- Asian American Native American Pacific Islander Serving Institutions (AANAPISIs)
Nonprofits Other Than Institutions of Higher Education
- Nonprofits with 501(c)(3) IRS Status (Other than Institutions of Higher Education)
- Nonprofits without 501(c)(3) IRS Status (Other than Institutions of Higher Education)
For-Profit Organizations
- Small Businesses
- For-Profit Organizations (Other than Small Businesses)
Governments
- State Governments
- County Governments
- City or Township Governments
- Special District Governments
- Indian/Native American Tribal Governments (Federally Recognized)
- Indian/Native American Tribal Governments (Other than Federally Recognized)
- Eligible Agencies of the Federal Government
- U.S. Territory or Possession
- Independent School Districts
- Public Housing Authorities/Indian Housing Authorities
- Native American Tribal Organizations (other than Federally recognized tribal governments)
- Faith-based or Community-based Organizations
- Regional Organizations
- Non-domestic (non-U.S.) Entities (Foreign Institutions)
Non-domestic (non-U.S.) Entities (Foreign Institutions) are eligible to apply. Non-domestic (non-U.S.) components of U.S. Organizations are eligible to apply. Foreign components, as defined in the NIH Grants Policy Statement , are allowed.
Applicant Organizations
Applicant organizations must complete and maintain the following registrations as described in the SF 424 (R&R) Application Guide to be eligible to apply for or receive an award. All registrations must be completed prior to the application being submitted. Registration can take 6 weeks or more, so applicants should begin the registration process as soon as possible. The NIH Policy on Late Submission of Grant Applications states that failure to complete registrations in advance of a due date is not a valid reason for a late submission.
- Dun and Bradstreet Universal Numbering System (DUNS) - All registrations require that applicants be issued a DUNS number. After obtaining a DUNS number, applicants can begin both SAM and eRA Commons registrations. The same DUNS number must be used for all registrations, as well as on the grant application.
- System for Award Management (SAM) (formerly CCR) Applicants must complete and maintain an active registration, which requires renewal at least annually . The renewal process may require as much time as the initial registration. SAM registration includes the assignment of a Commercial and Government Entity (CAGE) Code for domestic organizations which have not already been assigned a CAGE Code.
- NATO Commercial and Government Entity (NCAGE) Code Foreign organizations must obtain an NCAGE code (in lieu of a CAGE code) in order to register in SAM.
- eRA Commons - Applicants must have an active DUNS number and SAM registration in order to complete the eRA Commons registration. Organizations can register with the eRA Commons as they are working through their SAM or Grants.gov registration. eRA Commons requires organizations to identify at least one Signing Official (SO) and at least one Program Director/Principal Investigator (PD/PI) account in order to submit an application.
- Grants.gov Applicants must have an active DUNS number and SAM registration in order to complete the Grants.gov registration.
Program Directors/Principal Investigators (PD(s)/PI(s))
All PD(s)/PI(s) must have an eRA Commons account. PD(s)/PI(s) should work with their organizational officials to either create a new account or to affiliate their existing account with the applicant organization in eRA Commons. If the PD/PI is also the organizational Signing Official, they must have two distinct eRA Commons accounts, one for each role. Obtaining an eRA Commons account can take up to 2 weeks.
Any individual(s) with the skills, knowledge, and resources necessary to carry out the proposed research as the Program Director(s)/Principal Investigator(s) (PD(s)/PI(s)) is invited to work with his/her organization to develop an application for support. Individuals from underrepresented racial and ethnic groups as well as individuals with disabilities are always encouraged to apply for NIH support.
For institutions/organizations proposing multiple PDs/PIs, visit the Multiple Program Director/Principal Investigator Policy and submission details in the Senior/Key Person Profile (Expanded) Component of the SF424 (R&R) Application Guide.
This FOA does not require cost sharing as defined in the NIH Grants Policy Statement .
Applicant organizations may submit more than one application, provided that each application is scientifically distinct.
The NIH will not accept duplicate or highly overlapping applications under review at the same time. This means that the NIH will not accept:
- A new (A0) application that is submitted before issuance of the summary statement from the review of an overlapping new (A0) or resubmission (A1) application.
- A resubmission (A1) application that is submitted before issuance of the summary statement from the review of the previous new (A0) application.
- An application that has substantial overlap with another application pending appeal of initial peer review (see NOT-OD-11-101 ).
Buttons to access the online ASSIST system or to download application forms are available in Part 1 of this FOA. See your administrative office for instructions if you plan to use an institutional system-to-system solution.
It is critical that applicants follow the Research (R) Instructions in the SF424 (R&R) Application Guide , except where instructed in this funding opportunity announcement to do otherwise. Conformance to the requirements in the Application Guide is required and strictly enforced. Applications that are out of compliance with these instructions may be delayed or not accepted for review.
For information on Application Submission and Receipt, visit Frequently Asked Questions Application Guide, Electronic Submission of Grant Applications .
All page limitations described in the SF424 Application Guide and the Table of Page Limits must be followed.
The following section supplements the instructions found in the SF424 (R&R) Application Guide and should be used for preparing an application to this FOA.
All instructions in the SF424 (R&R) Application Guide must be followed.
All instructions in the SF424 (R&R) Application Guide must be followed, with the following additional instructions:
Research Strategy: Since the goal of this program is to support exploratory and developmental research projects, extensive background material and preliminary data are not required. Appropriate justification for the proposed work can be provided through literature citations, data from other sources, or, when available, from investigator-generated data.
Resource Sharing Plan : Individuals are required to comply with the instructions for the Resource Sharing Plans as provided in the SF424 (R&R) Application Guide.
Only limited Appendix materials are allowed. Follow all instructions for the Appendix as described in the SF424 (R&R) Application Guide.
When involving NIH-defined human subjects research, clinical research, and/or clinical trials (and when applicable, clinical trials research experience) follow all instructions for the PHS Human Subjects and Clinical Trials Information form in the SF424 (R&R) Application Guide, with the following additional instructions:
If you answered Yes to the question Are Human Subjects Involved? on the R&R Other Project Information form, you must include at least one human subjects study record using the Study Record: PHS Human Subjects and Clinical Trials Information form or Delayed Onset Study record.
Study Record: PHS Human Subjects and Clinical Trials Information
Delayed Onset Study
Foreign (non-U.S.) institutions must follow policies described in the NIH Grants Policy Statement , and procedures for foreign institutions.
See Part 1. Section III.1 for information regarding the requirement for obtaining a unique entity identifier and for completing and maintaining active registrations in System for Award Management (SAM), NATO Commercial and Government Entity (NCAGE) Code (if applicable), eRA Commons, and Grants.gov
Part I. Overview Information contains information about Key Dates and times. Applicants are encouraged to submit applications before the due date to ensure they have time to make any application corrections that might be necessary for successful submission. When a submission date falls on a weekend or Federal holiday , the application deadline is automatically extended to the next business day.
Organizations must submit applications to Grants.gov (the online portal to find and apply for grants across all Federal agencies). Applicants must then complete the submission process by tracking the status of the application in the eRA Commons , NIH’s electronic system for grants administration. NIH and Grants.gov systems check the application against many of the application instructions upon submission. Errors must be corrected and a changed/corrected application must be submitted to Grants.gov on or before the application due date and time. If a Changed/Corrected application is submitted after the deadline, the application will be considered late. Applications that miss the due date and time are subjected to the NIH Policy on Late Application Submission.
Applicants are responsible for viewing their application before the due date in the eRA Commons to ensure accurate and successful submission.
Information on the submission process and a definition of on-time submission are provided in the SF424 (R&R) Application Guide.
This initiative is not subject to intergovernmental review.
All NIH awards are subject to the terms and conditions, cost principles, and other considerations described in the NIH Grants Policy Statement .
Pre-award costs are allowable only as described in the NIH Grants Policy Statement .
Applications must be submitted electronically following the instructions described in the SF424 (R&R) Application Guide. Paper applications will not be accepted.
Applicants must complete all required registrations before the application due date. Section III. Eligibility Information contains information about registration.
For assistance with your electronic application or for more information on the electronic submission process, visit Applying Electronically . If you encounter a system issue beyond your control that threatens your ability to complete the submission process on-time, you must follow the Guidelines for Applicants Experiencing System Issues . For assistance with application submission, contact the Application Submission Contacts in Section VII .
Important reminders:
All PD(s)/PI(s) must include their eRA Commons ID in the Credential field of the Senior/Key Person Profile Component of the SF424(R&R) Application Package . Failure to register in the Commons and to include a valid PD/PI Commons ID in the credential field will prevent the successful submission of an electronic application to NIH. See Section III of this FOA for information on registration requirements.
The applicant organization must ensure that the DUNS number it provides on the application is the same number used in the organization’s profile in the eRA Commons and for the System for Award Management. Additional information may be found in the SF424 (R&R) Application Guide.
See more tips for avoiding common errors.
Upon receipt, applications will be evaluated for completeness and compliance with application instructions by the Center for Scientific Review, NIH. Applications that are incomplete or non-compliant will not be reviewed.
Applicants are required to follow the instructions for post-submission materials, as described in the policy . Any instructions provided here are in addition to the instructions in the policy.
Only the review criteria described below will be considered in the review process. As part of the NIH mission , all applications submitted to the NIH in support of biomedical and behavioral research are evaluated for scientific and technical merit through the NIH peer review system.
For this particular announcement, note the following:
The R21 exploratory/developmental grant supports investigation of novel scientific ideas or new model systems, tools, or technologies that have the potential for significant impact on biomedical or biobehavioral research. An R21 grant application need not have extensive background material or preliminary information. Accordingly, reviewers will emphasize the conceptual framework, the level of innovation, and the potential to significantly advance our knowledge or understanding. Appropriate justification for the proposed work can be provided through literature citations, data from other sources, or, when available, from investigator-generated data. Preliminary data are not required for R21 applications; however, they may be included if available.
Reviewers will provide an overall impact score to reflect their assessment of the likelihood for the project to exert a sustained, powerful influence on the research field(s) involved, in consideration of the following review criteria and additional review criteria (as applicable for the project proposed).
Reviewers will consider each of the review criteria below in the determination of scientific merit, and give a separate score for each. An application does not need to be strong in all categories to be judged likely to have major scientific impact. For example, a project that by its nature is not innovative may be essential to advance a field.
Does the project address an important problem or a critical barrier to progress in the field? Is there a strong scientific premise for the project? If the aims of the project are achieved, how will scientific knowledge, technical capability, and/or clinical practice be improved? How will successful completion of the aims change the concepts, methods, technologies, treatments, services, or preventative interventions that drive this field?
Are the PD(s)/PI(s), collaborators, and other researchers well suited to the project? If Early Stage Investigators or those in the early stages of independent careers, do they have appropriate experience and training? If established, have they demonstrated an ongoing record of accomplishments that have advanced their field(s)? If the project is collaborative or multi-PD/PI, do the investigators have complementary and integrated expertise; are their leadership approach, governance and organizational structure appropriate for the project?
Does the application challenge and seek to shift current research or clinical practice paradigms by utilizing novel theoretical concepts, approaches or methodologies, instrumentation, or interventions? Are the concepts, approaches or methodologies, instrumentation, or interventions novel to one field of research or novel in a broad sense? Is a refinement, improvement, or new application of theoretical concepts, approaches or methodologies, instrumentation, or interventions proposed?
Are the overall strategy, methodology, and analyses well-reasoned and appropriate to accomplish the specific aims of the project? Have the investigators presented strategies to ensure a robust and unbiased approach, as appropriate for the work proposed? Are potential problems, alternative strategies, and benchmarks for success presented? If the project is in the early stages of development, will the strategy establish feasibility and will particularly risky aspects be managed? Have the investigators presented adequate plans to address relevant biological variables, such as sex, for studies in vertebrate animals or human subjects?
If the project involves human subjects and/or NIH-defined clinical research, are the plans to address 1) the protection of human subjects from research risks, and 2) inclusion (or exclusion) of individuals on the basis of sex/gender, race, and ethnicity, as well as the inclusion or exclusion of children, justified in terms of the scientific goals and research strategy proposed?
Will the scientific environment in which the work will be done contribute to the probability of success? Are the institutional support, equipment and other physical resources available to the investigators adequate for the project proposed? Will the project benefit from unique features of the scientific environment, subject populations, or collaborative arrangements?
As applicable for the project proposed, reviewers will evaluate the following additional items while determining scientific and technical merit, and in providing an overall impact score, but will not give separate scores for these items.
For research that involves human subjects but does not involve one of the six categories of research that are exempt under 45 CFR Part 46, the committee will evaluate the justification for involvement of human subjects and the proposed protections from research risk relating to their participation according to the following five review criteria: 1) risk to subjects, 2) adequacy of protection against risks, 3) potential benefits to the subjects and others, 4) importance of the knowledge to be gained, and 5) data and safety monitoring for clinical trials.
For research that involves human subjects and meets the criteria for one or more of the six categories of research that are exempt under 45 CFR Part 46, the committee will evaluate: 1) the justification for the exemption, 2) human subjects involvement and characteristics, and 3) sources of materials. For additional information on review of the Human Subjects section, please refer to the Guidelines for the Review of Human Subjects .
When the proposed project involves human subjects and/or NIH-defined clinical research, the committee will evaluate the proposed plans for the inclusion (or exclusion) of individuals on the basis of sex/gender, race, and ethnicity, as well as the inclusion (or exclusion) of children to determine if it is justified in terms of the scientific goals and research strategy proposed. For additional information on review of the Inclusion section, please refer to the Guidelines for the Review of Inclusion in Clinical Research .
The committee will evaluate the involvement of live vertebrate animals as part of the scientific assessment according to the following criteria: (1) description of proposed procedures involving animals, including species, strains, ages, sex, and total number to be used; (2) justifications for the use of animals versus alternative models and for the appropriateness of the species proposed; (3) interventions to minimize discomfort, distress, pain and injury; and (4) justification for euthanasia method if NOT consistent with the AVMA Guidelines for the Euthanasia of Animals. Reviewers will assess the use of chimpanzees as they would any other application proposing the use of vertebrate animals. For additional information on review of the Vertebrate Animals section, please refer to the Worksheet for Review of the Vertebrate Animal Section .
Reviewers will assess whether materials or procedures proposed are potentially hazardous to research personnel and/or the environment, and if needed, determine whether adequate protection is proposed.
For Resubmissions, the committee will evaluate the application as now presented, taking into consideration the responses to comments from the previous scientific review group and changes made to the project.
For Revisions, the committee will consider the appropriateness of the proposed expansion of the scope of the project. If the Revision application relates to a specific line of investigation presented in the original application that was not recommended for approval by the committee, then the committee will consider whether the responses to comments from the previous scientific review group are adequate and whether substantial changes are clearly evident.
As applicable for the project proposed, reviewers will consider each of the following items, but will not give scores for these items, and should not consider them in providing an overall impact score.
Reviewers will assess whether the project presents special opportunities for furthering research programs through the use of unusual talent, resources, populations, or environmental conditions that exist in other countries and either are not readily available in the United States or augment existing U.S. resources.
Reviewers will assess the information provided in this section of the application, including 1) the Select Agent(s) to be used in the proposed research, 2) the registration status of all entities where Select Agent(s) will be used, 3) the procedures that will be used to monitor possession use and transfer of Select Agent(s), and 4) plans for appropriate biosafety, biocontainment, and security of the Select Agent(s).
Reviewers will comment on whether the following Resource Sharing Plans, or the rationale for not sharing the following types of resources, are reasonable: (1) Data Sharing Plan ; (2) Sharing Model Organisms ; and (3) Genomic Data Sharing Plan (GDS) .
For projects involving key biological and/or chemical resources, reviewers will comment on the brief plans proposed for identifying and ensuring the validity of those resources.
Reviewers will consider whether the budget and the requested period of support are fully justified and reasonable in relation to the proposed research.
Applications will be evaluated for scientific and technical merit by (an) appropriate Scientific Review Group(s) in accordance with NIH peer review policy and procedures , using the stated review criteria . Assignment to a Scientific Review Group will be shown in the eRA Commons.
As part of the scientific peer review, all applications:
- May undergo a selection process in which only those applications deemed to have the highest scientific and technical merit (generally the top half of applications under review) will be discussed and assigned an overall impact score.
- Will receive a written critique.
Applications will be assigned on the basis of established PHS referral guidelines to the appropriate NIH Institute or Center. Applications will compete for available funds with all other recommended applications. Following initial peer review, recommended applications will receive a second level of review by the appropriate national Advisory Council or Board. The following will be considered in making funding decisions:
- Scientific and technical merit of the proposed project as determined by scientific peer review.
- Availability of funds.
- Relevance of the proposed project to program priorities.
After the peer review of the application is completed, the PD/PI will be able to access his or her Summary Statement (written critique) via the eRA Commons . Refer to Part 1 for dates for peer review, advisory council review, and earliest start date.
Information regarding the disposition of applications is available in the NIH Grants Policy Statement .
If the application is under consideration for funding, NIH will request "just-in-time" information from the applicant as described in the NIH Grants Policy Statement .
A formal notification in the form of a Notice of Award (NoA) will be provided to the applicant organization for successful applications. The NoA signed by the grants management officer is the authorizing document and will be sent via email to the grantee’s business official.
Awardees must comply with any funding restrictions described in Section IV.5. Funding Restrictions . Selection of an application for award is not an authorization to begin performance. Any costs incurred before receipt of the NoA are at the recipient's risk. These costs may be reimbursed only to the extent considered allowable pre-award costs.
Any application awarded in response to this FOA will be subject to terms and conditions found on the Award Conditions and Information for NIH Grants website. This includes any recent legislation and policy applicable to awards that is highlighted on this website.
All NIH grant and cooperative agreement awards include the NIH Grants Policy Statement as part of the NoA. For these terms of award, see the NIH Grants Policy Statement Part II: Terms and Conditions of NIH Grant Awards, Subpart A: General and Part II: Terms and Conditions of NIH Grant Awards, Subpart B: Terms and Conditions for Specific Types of Grants, Grantees, and Activities . More information is provided at Award Conditions and Information for NIH Grants .
Recipients of federal financial assistance (FFA) from HHS must administer their programs in compliance with federal civil rights law. This means that recipients of HHS funds must ensure equal access to their programs without regard to a person’s race, color, national origin, disability, age and, in some circumstances, sex and religion. This includes ensuring your programs are accessible to persons with limited English proficiency. HHS recognizes that research projects are often limited in scope for many reasons that are nondiscriminatory, such as the principal investigator’s scientific interest, funding limitations, recruitment requirements, and other considerations. Thus, criteria in research protocols that target or exclude certain populations are warranted where nondiscriminatory justifications establish that such criteria are appropriate with respect to the health or safety of the subjects, the scientific study design, or the purpose of the research.
For additional guidance regarding how the provisions apply to NIH grant programs, please contact the Scientific/Research Contact that is identified in Section VII under Agency Contacts of this FOA. HHS provides general guidance to recipients of FFA on meeting their legal obligation to take reasonable steps to provide meaningful access to their programs by persons with limited English proficiency. Please see https://www.hhs.gov/civil-rights/for-individuals/special-topics/limited-english-proficiency/index.html. The HHS Office for Civil Rights also provides guidance on complying with civil rights laws enforced by HHS. Please see http://www.hhs.gov/ocr/civilrights/understanding/section1557/index.html ; and https://www.hhs.gov/civil-rights/for-providers/laws-regulations-guidance/index.html . Recipients of FFA also have specific legal obligations for serving qualified individuals with disabilities. Please see http://www.hhs.gov/ocr/civilrights/understanding/disability/index.html . Please contact the HHS Office for Civil Rights for more information about obligations and prohibitions under federal civil rights laws at https://www.hhs.gov/ocr/about-us/contact-us/index.html or call 1-800-368-1019 or TDD 1-800-537-7697. Also note it is an HHS Departmental goal to ensure access to quality, culturally competent care, including long-term services and supports, for vulnerable populations. For further guidance on providing culturally and linguistically appropriate services, recipients should review the National Standards for Culturally and Linguistically Appropriate Services in Health and Health Care at http://minorityhealth.hhs.gov/omh/browse.aspx?lvl=2&lvlid=53 .
In accordance with the statutory provisions contained in Section 872 of the Duncan Hunter National Defense Authorization Act of Fiscal Year 2009 (Public Law 110-417), NIH awards will be subject to the Federal Awardee Performance and Integrity Information System (FAPIIS) requirements. FAPIIS requires Federal award making officials to review and consider information about an applicant in the designated integrity and performance system (currently FAPIIS) prior to making an award. An applicant, at its option, may review information in the designated integrity and performance systems accessible through FAPIIS and comment on any information about itself that a Federal agency previously entered and is currently in FAPIIS. The Federal awarding agency will consider any comments by the applicant, in addition to other information in FAPIIS, in making a judgement about the applicant’s integrity, business ethics, and record of performance under Federal awards when completing the review of risk posed by applicants as described in 45 CFR Part 75.205 Federal awarding agency review of risk posed by applicants. This provision will apply to all NIH grants and cooperative agreements except fellowships.
Cooperative Agreement Terms and Conditions of Award
When multiple years are involved, awardees will be required to submit the Research Performance Progress Report (RPPR) annually and financial statements as required in the NIH Grants Policy Statement.
A final RPPR, invention statement, and the expenditure data portion of the Federal Financial Report are required for closeout of an award, as described in the NIH Grants Policy Statement .
The Federal Funding Accountability and Transparency Act of 2006 (Transparency Act), includes a requirement for awardees of Federal grants to report information about first-tier subawards and executive compensation under Federal assistance awards issued in FY2011 or later. All awardees of applicable NIH grants and cooperative agreements are required to report to the Federal Subaward Reporting System (FSRS) available at www.fsrs.gov on all subawards over $25,000. See the NIH Grants Policy Statement for additional information on this reporting requirement.
In accordance with the regulatory requirements provided at 45 CFR 75.113 and Appendix XII to 45 CFR Part 75, recipients that have currently active Federal grants, cooperative agreements, and procurement contracts from all Federal awarding agencies with a cumulative total value greater than $10,000,000 for any period of time during the period of performance of a Federal award, must report and maintain the currency of information reported in the System for Award Management (SAM) about civil, criminal, and administrative proceedings in connection with the award or performance of a Federal award that reached final disposition within the most recent five-year period. The recipient must also make semiannual disclosures regarding such proceedings. Proceedings information will be made publicly available in the designated integrity and performance system (currently FAPIIS). This is a statutory requirement under section 872 of Public Law 110-417, as amended (41 U.S.C. 2313). As required by section 3010 of Public Law 111-212, all information posted in the designated integrity and performance system on or after April 15, 2011, except past performance reviews required for Federal procurement contracts, will be publicly available. Full reporting requirements and procedures are found in Appendix XII to 45 CFR Part 75 Award Term and Conditions for Recipient Integrity and Performance Matters.
We encourage inquiries concerning this funding opportunity and welcome the opportunity to answer questions from potential applicants.
eRA Service Desk (Questions regarding ASSIST, eRA Commons registration, submitting and tracking an application, documenting system problems that threaten submission by the due date, post submission issues) Finding Help Online: http://grants.nih.gov/support/ (preferred method of contact) Telephone: 301-402-7469 or 866-504-9552 (Toll Free)
Grants.gov Customer Support (Questions regarding Grants.gov registration and submission, downloading forms and application packages) Contact Center Telephone: 800-518-4726 Email: [email protected]
GrantsInfo (Questions regarding application instructions and process, finding NIH grant resources) Email: [email protected] (preferred method of contact) Telephone: 301-945-7573
Participating NIH Institutes and Centers are listed in Components of Participating Organizations in Part 1. Overview . Scientific/Research Contact information is listed on the R21 IC-Specific Scientific Interests and Contact website.
Examine your eRA Commons account for review assignment and contact information (information appears two weeks after the submission due date).
Participating NIH Institutes and Centers are listed in Components of Participating Organizations in Part 1. Overview . Financial/Grants Management Contact information is listed on the R21 IC-Specific Scientific Interests and Contact website.
Recently issued trans-NIH policy notices may affect your application submission. A full list of policy notices published by NIH is provided in the NIH Guide for Grants and Contracts . All awards are subject to the terms and conditions, cost principles, and other considerations described in the NIH Grants Policy Statement .
Awards are made under the authorization of Sections 301 and 405 of the Public Health Service Act as amended (42 USC 241 and 284) and under Federal Regulations 42 CFR Part 52 and 45 CFR Part 75.

Note: For help accessing PDF, RTF, MS Word, Excel, PowerPoint, Audio or Video files, see Help Downloading Files .

COMMENTS
Project Number: COR 19-489: Title: Pain/Opioid CORE: Principal Investigator: Alicia Heapy . Location: West Haven, CT: Research Service: Health Services R&D
The HIST 489 Research Project—a 10,000-word essay—gives students the chance to pursue their own research topic with guidance and support from a faculty member as supervisor. Students gain experience with high-level historical research, managing an independent project, and producing an extended piece of written work. Supervisors help
research project. Organising material, sharpening the argument, checking and making consistent citations in footnotes and bibliography and polishing expression is all part of the stages of producing a completed piece of research. HIST 489 Research Essays should be around 10,000 words, approximately 40 pages of double-spaced typing, excluding
This project represents the first step toward producing a new tool which can be used by growers, processors, researchers, and testing laboratories to detect and quantify Cc quickly and cost-effectively. ... Home > Funded Research Projects > Development of an infrared-functionalized microbalance sensor for Cyclospora cayetanensis detection and ...
Kaela Cantrell SCI-489 11 September 2022 Milestone One: Data For the final research project, I've had to identify various resources to compile data pertinent to my statement problem and research hypothesis. It is important to show if there is a correlatio ... SCI 489 Module 3 Project Log.docx. 3-1 Project Log: Research Status Report Geosciences ...
Research questions give your project a clear focus. They should be specific and feasible, but complex enough to merit a detailed answer. 2607. How to Write a Literature Review | Guide, Examples, & Templates A literature review is a survey of scholarly knowledge on a topic. Our guide with examples, video, and templates can help you write yours.
statement of (potential) research questions, a brief methodology, and at least a few appropriate scholarly sources. Supervisors should not be expected to provide funding (e.g. for travel), but may do so at their discretion. Students may undertake a research project for GEOG 489 as part of a lab or research group. In
Research Essay: Due 2pm Friday 11 October. 100% of final grade. This piece of assessment relates to all learning objectives The largest component of MDIA 489 is the individual research project you develop and complete as a 10,000 word research essay in consultation with your supervisor. This is a theoretically informed piece of
The ENCODE Project Consortium Nature 489, 57-74 (2012). International Human Genome Sequencing Consortium Nature 431 , 931-945 (2004). The ENCODE Project Consortium Nature 447 , 799-816 (2007).
NIH Research Project Grant (Parent R01 Clinical Trial Not Allowed) R01 Research Project Grant. Reissue of PA-16-160 for due dates on or after January 25, 2018. March 5, 2020 - Notice of Special Interest (NOSI): Harnessing computational tools for sophisticated analyses of Substance Use Disorder-related behaviors.
Volume 17, pages 489-504, (2008) Cite this article; Download PDF. European Journal of Information Systems ... The in-progress research project described in this paper is an example of design science research that can yield not only a prescriptive design theory for a class of artifacts, but can also refine and extend the kernel theory that ...
Faculty sponsors are assigned research course sections in BIO 486, BIO 487, or BIO 489 based on their main area of interest. Once you have identified your area of interest, investigate faculty that are conducting research in that area. ... you'll discuss and design a research project with your sponsor. Will I Get BIO Major Credit? You can ...
CSSE 489 - Senior Research Project III. Credit Hours: 4C; Term Available: - Graduate Studies Eligible: No; Prerequisites: CSSE 488; Corequisites: None Individual or group research on an unsolved technical problem. The problem is expected to be at an advanced level and have an appropriate client. A prototype system, a technical report, and a ...
HIST 489 Research Project Student Guide 2021 History Programme School of History, Philosophy, Political Science and International Relations Te Kura Aro Whakamuri, Rapunga Whakaaro, Matai Tōrangapū me te Ao. 2.
Students will also have an opportunity to present their project and findings to the department through an oral presentation. Prerequisite(s): Admission to the Honours program in Biochemistry, Microbiology and Immunology. Note: Permission of the department is required. Students with credit for BIOC 489.6 or MCIM 491.6 may not take this course ...
Undergraduate Research Projects 489. displacement of the wall of an intracranial saccular aneurysm that was filled. with blood and surrounded by cerebral spinal fluid [12]. 4.4. Developing ...
NCHRP REPORT 489 Research Sponsored by the American Association of State Highway and Transportation Officials in Cooperation with the Federal Highway Administration ... Research projects to fulfill these needs are defined by the Board, and qualified research agencies are selected from those that have
Stigmas have been widely used to describe organizations and individuals that have negative reputations or that have engaged in illegitimate practices. Extensive research has been done on the effects of positive reputation and personal reputation. However, there is still much to learn about the effects and consequences of organizational stigmas. The purpose of this paper is to provide a ...
Research Project Status: Completed End Date: August 31,2013 UTC Grant Cycle: OTREC 2012 UTC Funding: $138,633 TRB RIP: 25809 ... Project Brief: Hiding Private Locations by Anonymizing Data (PROJECT_BRIEF) OTREC-RR-489 Wider Dissemination of Household Travel Survey Data Using Geographical Perturbation Methods ...
The DSRIS project we describe in this paper uses and refines kernel theory as it aims to create a design theory for a new class of artifacts. Refinement of theory in DSRIS is somewhat unusual and a brief overview of the positions set out for the use of theory (of any type) within DSRIS will situate our approach.
A longitudinal study was conducted of transformational leadership and the performance of project groups in three industrial research and development organizations. As hypothesized, transformational leadership predicted higher project quality and budget/schedule performance ratings at time I and one-year later at time 2. A moderator effect was hypothesized and found for type of research and ...
PA-18-489 . Companion Funding Opportunity. PA-18-344 - Parent R21 Clinical Trial Required ... HHS recognizes that research projects are often limited in scope for many reasons that are nondiscriminatory, such as the principal investigator's scientific interest, funding limitations, recruitment requirements, and other considerations. ...
487. British Studies: Research. 3 hrs. Prerequisite: Consent of instructor. Provides the opportunity for in-depth research projects. 489. Library Practicum. 1-4 hrs. Arr. Supervised work in a li brary to provide the student with operational library experience. This is writing intensive capstone course. 491. Library Instruction. 3 hrs.