- CBSE Class 11 Chemistry Chapter 2 – Structure of Atom Revision Class 11 Notes

Structure of Atom Class 11 Revision Notes
Structure of Atom Class 11 Notes – This topic talks about atoms. In addition, it also talks about electron, neutron, and protons. Furthermore, the who and how these particles are discovered by the scientists. Moreover, various principles, functions, and structures are also discussed in the chapter. Most noteworthy, the chapter talks about sub-atomic particles. Also, shell configuration and how elements complete their shells. Besides, the chapter also discusses atom valency.
Download Toppr app for Android and iOS or signup for free.
Sub-topics covered under Structure of Atom
- Introduction: Structure of Atom – This topic explains the atom and discovery of electron, neutron, and proton.
- Atomic Number – Also, the topic talks about the atomic number and how to find them.
- Bohr’s Model of Atom – This topic overview Bohr’s model of an atom.
- Charged Particles in Matter – Furthermore, the topic discusses charged particles and the discovery of sub-atomic particles.
- Isobars – Moreover, this topic highlights isobars and differences between isobars and isotopes.
- Isotopes – The topic defines isotopes in detail.
- Mass Number – Also, this topic describes the mass numbers, its uses, and properties.
- Neutrons – The topic explains the functions, properties, and discovery of the neutron.
- Rutherford’s Model of an Atom – In addition, this topic overviews Rutherford’s model of an atom.
- Thomson’s Model of an Atom – The topic defines Thomson’s model of an atom.
- Valency – This topic highlights what is valency of an atom.
- How are Electrons Distributed in Different Orbits (Shells)? – Furthermore, the topic describes the electron distribution.
- Sub-Atomic Particles – This topic talks about sub-atomic and important properties.
- Atomic Models – Moreover, the topic describes various atomic models.
- Shapes of Atomic Orbitals – This topic explains the shape of atomic orbitals.
- Energies of Orbitals – The topic talks about the energies of orbitals.
- Quantum Numbers – This topic overviews the quantum numbers.
- Development Leading to Bohr’s Model of Atom – The topic highlights the latest development in Bohr’s model.
- Emission and Absorption Spectra – This topic discusses the emission and absorption of spectra.
- Towards Quantum Mechanical Model of Atom – The topic describes the quantum mechanical model of an atom.
You can download CBSE Class 11 Chemistry Structure of Atom Revision Notes by clicking on the download button below

Customize your course in 30 seconds
Which class are you in.

CBSE Class 12 Chemistry Revision Notes
- CBSE Class 12 Chemistry Chapter 16 – Chemistry in Everyday Life Class 12 Notes
- CBSE Class 12 Chemistry Chapter 15 – Polymers Class 12 Notes
- CBSE Class 12 Chemistry Chapter 14 – Biomolecules Class 12 Notes
- CBSE Class 12 Chemistry Chapter 13 – Amines Class 12 Notes
- CBSE Class 12 Chemistry Chapter 12 – Aldehydes, Ketones and Carboxylic Acids Class 12 Notes
- CBSE Class 12 Chemistry Chapter 11 – Alcohols, Phenols and Ethers Class 12 Notes
- CBSE Class 12 Chemistry Chapter 10 | Haloalkanes and Haloarenes Notes
- CBSE Class 12 Chemistry Chapter 9 – Coordination Compounds Class 12 Notes
- CBSE Class 12 Chemistry Chapter 8 – The d and f Block Elements Class 12 Notes
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Download the App

Chemistry Worksheet Class 11 on Chapter 2 Structure of Atom with Answers - Set 4
The atomic number (Z) is the number of protons in the nucleus; the atomic number defines the element. N represents the number of neutrons in the nucleus. The nucleus’ mass number (A) equals Z + N. The mass number of the nucleus is usually slightly different from its mass in atomic mass units (amu).
Atoms of the same element with different numbers of neutrons are known as isotopes of that element. Hydrogen, for example, has three (3) isotopes: hydrogen-1 (hydrogen), deuterium-2 (deuterium), and hydrogen-3 (deuterium-3) (tritium).
There are currently 118 known elements, which are typically represented on an elemental periodic table. Elements numbered 1 through 98 have all been discovered in nature, whereas elements numbered 99 through 118 have only ever been created artificially.
Download PDF of Class 11 Chemistry Chapter 2 Structure of Atom – Set 4

CBSE Class 11 Chemistry Worksheet Chapter 2 Structure of Atom – Set 4
Q1. The orbital nearest to the nucleus is:
Q2. The electronic configuration of Cu 2+ ion is:
a.) [Ar] 3d 8 4s 1
b.) [Ar] 3d 9 4s 0
c.) [Ar] 3d 7 4s 2
d.) [Ar] 3d 8 4s 0
Q3. What is the maximum number of emission lines obtained when the excited electrons of a hydrogen atom in n = 5 drop to the ground state?
Q4. The maximum number of electrons in a subshell is given by the expression:
Q5. In Bohr’s theory, the radius r of the orbit is proportional to ____.
Q6. Symbols 79 35 Br and 79 Br can be written, whereas symbols 35 79 Br and 35 Br are not acceptable. Answer briefly.
Q7. List the properties of anode rays.
Q8. What were the observations of Rutherford’s scattering experiment?
Q9. Calculate the wave number of radiations having a frequency of 4 × 10 11 kHz.
Q10. What is the experimental evidence in support of the idea that electronic energies in an atom are quantised?
Q11. How many protons, electrons, and neutrons are there in the following nuclei?
ii.) 25 12 Mg
iii.) 80 35 Br
Q12. Calculate the kinetic energy of the ejected electron when ultraviolet radiation of frequency 1.6 × 10 15 s –1 strikes the surface of potassium metal.
[Threshold frequency of potassium metal is 5 × 10 14 s –1 .]
Q13. An atom of an element contains 29 electrons and 35 neutrons. Deduce:
i.) The number of protons
ii.) The electronic configuration of the element
Q14. A hydrogen atom has only one electron, so mutual repulsion between electrons is absent. However, in multielectron atoms, mutual repulsion between the electrons is significant. How does this affect the energy of an electron in the orbitals of the same principal quantum number in multielectron atoms?
Q15. The wavelength of the first spectral line in the Balmer series is 6561 Å. Calculate the wavelength of the second spectral line in the Balmer series.
Q16. Threshold frequency, v 0, is the minimum frequency that a photon must possess to eject an electron from a metal. It is different for different metals. When a photon of frequency 1.0 × 10 15 s –1 was allowed to hit a metal surface, an electron having 1.988 × 10 –19 J of kinetic energy was emitted. Calculate the threshold frequency of this metal. Show that an electron will not be emitted if a photon with a wavelength equal to 600 nm hits the metal surface.
Q17. According to Bohr’s theory, the electronic energy of a hydrogen atom in the nth Bohr orbit is given by

Calculate the longest wavelength of light that will be needed to remove an electron from the third orbit of the He + ion.
Q18. Differentiate between electromagnetic waves and matter waves.
Q19. If the uncertainty in the position of a moving electron is equal to its de-Broglie wavelength, show that its velocity is completely uncertain.
Q20. Calculate the energy emitted when electrons of 1.0 g atom of hydrogen undergo transition giving the spectral line of largest energy in the visible region of its atomic spectrum. (R H = 1.1 × 10 7 m –1 , c = 3 × 10 8 m s –1 , h = 6.62 × 10 –34 J s)
Download PDF to access answers of the Chemistry Worksheet for Class 11 Chemistry Chapter 2 Structure of Atom Set – 4.
Download PDF
- Class 11 Chemistry Chapter 2 Structure of Atom MCQs
- Important Questions for Class 11 Chemistry Chapter 2 Structure of Atom
- Chemistry Concept Questions and Answers
- CBSE Class 11 Chemistry Practical
- Aufbau Principle
- Subatomic Particles
- Rutherford Atomic Model
- Planck’s Quantum Theory: Quantisation of Energy
- Bohr’s Model of an Atom
- Share Share
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.

AssignmentsBag.com
Assignments Class 11 Chemistry Structure of Atom
Please refer to Assignments Class 11 Chemistry Structure of Atom Chapter 2 with solved questions and answers. We have provided Class 11 Chemistry Assignments for all chapters on our website. These problems and solutions for Chapter 2 Structure of Atom Class 11 Chemistry have been prepared as per the latest syllabus and books issued for the current academic year. Learn these solved important questions to get more marks in your class tests and examinations.
Structure of Atom Assignments Class 11 Chemistry
Question. A hydrogen atom in its ground state absorbs 10.2 eV of energy. The orbital angular momentum is increased by (Given Planck constant h = 6.6 × 10 -34 Jsec) (a) 1.05 × 10 -34 Jsec (b) 3.16 × 10 -34 Jsec (c) 2.11 × 10 -34 Jsec (d) 4.22 × 10 -34 Jsec
Question. In chromium atom, in ground state, the number of occupied orbitals is (a) 14 (b) 15 (c) 7 (d) 12
Question. The value of Plancks constant is 6.63 × 10 -34 Js. The velocity of light is 3.0 × 10 8 ms -1 . Which value is closest to the wavelength in nanometres of a quantum of light with frequency of 8 × 10 15 s -1 (a) 3 × 10 7 (b) 2 × 10 -25 (c) 5 × 10 -18 (d) 4 × 10 1
Question. Maximum number of electrons in a subshell with l = 3 and n = 4 is (a) 10 (b) 12 (c) 14 (d) 16
Question. The magnetic quantum number specifies (a) Size of orbitals (b) Shape of orbitals (c) Orientation of orbitals (d) Nuclear Stability
Question. In a multi – electron atom, which of the following orbitals described by the three quantum numbers will have the same energy in the absence of magnetic acid and electric fields? (a) n = 1, l = 0, m = 0 (b) n = 2, l = 0, m = 0 (c) n = 2, l = 1, m = 1 (d) n = 3, l = 2, m = 1 (e) n = 3, l = 2, m = 0 (a) (a) and (b) (b) (b) and (c) (c) (c) and (d) (d) (d) and (e)
Question. For principal quantum number n = 4, the total number of orbitals having l = 3 is (a) 3 (b) 7 (c) 5 (d) 9
Question. In Bohr series of lines of hydrogen spectrum, the third line from the red end corresponds to which one of the following inner-orbit jumps of the electron for Bohr orbits in an atom of hydrogen? (a) 3 → 2 (b) 5 → 2 (c) 4 → 1 (d) 2 → 5
Question. The electronic transitions from n = 2 to n = 1 will produce shortest wavelength in (where n = principal quantum state) (a) Li 2+ (b) He + (c) H (d) H +
Question. The ionization enthalpy of hydrogen atom is 1.312 × 10 6 J mol -1 . The energy required to excite the electron in the atom from n = 1 to n = 2 is (a) 8.51 × 10 5 Jmol -1 (b) 6.56 × 10 5 Jmol -1 (c) 7.56 × 10 5 Jmol -1 (d) 9.84 × 10 5 Jmol -1 /sup>
Related Posts
Assignments class 11 mathematics principle of mathematical induction.

Assignments For Class 11 Mathematics Limits And Derivatives

Assignments Class 11 Chemistry The s-Block Elements

- Book Solutions
- State Boards
Case Study Questions Class 11 Chemistry Structure of Atom
Case study questions class 11 chemistry chapter 2 structure of atom.
CBSE Class 11 Case Study Questions Chemistry Structure of Atom. Important Case Study Questions for Class 11 Board Exam Students. Here we have arranged some Important Case Base Questions for students who are searching for Paragraph Based Questions Structure of Atom.
At Case Study Questions there will given a Paragraph. In where some Important Questions will made on that respective Case Based Study. There will various types of marks will given 1 marks, 2 marks, 3 marks, 4 marks.
CBSE Case Study Questions Class 11 Chemistry Structure of Atom
The atomic theory of matter was first proposed on afirm scientific basis by JohnDalton, a British schoolteacher in 1808. His theory, called Dalton’s atomictheory, regarded the atom as the ultimate particle ofmatter Dalton’s atomic theory was able to explainthe law of conservation of mass, law of constantcomposition and law of multiple proportion verysuccessfully. However, it failed to explain the results ofmany experiments.In mid 1850s many scientists mainlyFaraday began to study electrical dischargein partially evacuated tubes, known ascathode ray discharge tubes.Electrical discharge carried out in the modifiedcathode ray tube led to the discovery of canalrays carrying positively charged particles. Thecharacteristics of these positively chargedparticles are listed below.
1) Unlike cathode rays, mass of positivelycharged particles depends upon thenature of gas present in the cathode raytube. These are simply the positivelycharged gaseous ions.
2)The charge to mass ratio of the particlesdepends on the gas from which theseoriginate.
3) Some of the positively charged particlescarry a multiple of the fundamental unitof electrical charge.
4) The behaviour of these particles in themagnetic or electrical field is opposite tothat observed for electron or cathoderays.
The smallest and lightest positive ion wasobtained from hydrogen and was called
proton. This positively charged particle wascharacterised in 1919. Later, a need was feltfor the presence of electrically neutral particleas one of the constituent of atom. Theseparticles were discovered by Chadwick (1932)by bombarding a thin sheet of beryllium byα-particles. When electrically neutral particleshaving a mass slightly greater than that ofprotons were emitted. He named theseparticles as neutrons.J. J. Thomson, in 1898, proposed that an atom possesses a spherical shape (radiusapproximately 10–10 m) in which the positivecharge is uniformly distributed. The electronsare embedded into it in such a manner as togive the most stable electrostatic arrangementMany different names are given tothis model, for example, plum pudding, raisinpudding or watermelon. This model can be visualised as a pudding or watermelon ofpositive charge with plums or seeds (electrons)embedded into it. An important feature of thismodel is that the mass of the atom is assumed to be uniformly distributed over theatom.Rutherford and his students (Hans Geiger andErnest Marsden) bombarded very thin gold foilwith α–particles. Rutherford’s famous α–particle scattering experiment.The observations of Scattering experiment are as follows-:
(i) most of the α–particles passed throughthe gold foil undeflected.
(ii) a small fraction of the α–particles wasdeflected by small angles.
(iii) a very few α–particles (∼1 in 20,000)bounced back, that is, were deflected bynearly 180°.
On the basis of observations andconclusions from this experiment, Rutherford proposed the nuclearmodel of atom. According to this model:
(i) The positive charge and most of the massof the atom was densely concentrated inextremely small region. This very smallportion of the atom was called nucleusby Rutherford.
(ii) The nucleus is surrounded by electronsthat move around the nucleus with a veryhigh speed in circular paths called orbits.Thus, Rutherford’s model of atomresembles the solar system in which thenucleus plays the role of sun and theelectrons that of revolving planets.
(iii) Electrons and the nucleus are held together by electrostatic forces of attraction.
1) The atomic theory of matter was first proposed on afirm scientific basis by
(a) John Dalton
(b) Ernest Rutherford
(c) J.Thomson
(d) Henry Moseley
Ans – a) John Dalton
2) The cathode rays start from … and move towards the ….
(a) Anode , Cathode
(b) Centre , Anode
(c) Cathod , Anode
(d) Cathod , Centre
Ans – c) Cathod , Anode
3) negativelycharged particles in atoms , called …
(a) Protons
(b) electrons
(c) Neutron
(d) Positron
Ans – b) electrons
4) The smallest and lightest positive ion wasobtained from …. and was called proton .
(b) Nitrogen
(d) Hydrogen
Ans- d) Hydrogen
5) Electrically neutral particles having a mass slightly greater than that of protons, these particles termed as ….
Ans – c) Neutron
6) J.J. Thomson’s atomic model is also named as
(a) plum pudding
(b) raisin pudding
(c) watermelon
(d) All the above
Ans- d) All the above
[B] Short Answers
1) Explain Thomson’s Atomic Model
Ans – J. J. Thomson, in 1898, proposed that an atompossesses a spherical shape (radius approximately 10–10 m) in which the positivecharge is uniformly distributed. The electronsare embedded into it in such a manner as togive the most stable electrostatic arrangement.Many different names are given tothis model, for example, plum pudding, raisinpudding or watermelon. This model can be visualised as a pudding or watermelon ofpositive charge with plums or seeds (electrons)embedded into it. An important feature of thismodel is that the mass of the atom is assumed to be uniformly distributed over the atom.
2) What are the observations of Rutherfords scattering experiment ?
Ans – The observations of Scattering experiment are as follows-:
[C] Long Answers
1) What are the Characteristics of positively charged particles carrying by canel rays ?
Ans – Thecharacteristics of these positively chargedparticles are listed below.
1) Unlike cathode rays, mass of positively charged particles depends upon the nature of gas present in the cathode ray tube. These are simply the positively charged gaseous ions.
2) The charge to mass ratio of the particlesdepends on the gas from which theseoriginate.
2) Write the postulates of Rutherford Atomic Model .
Ans – On the basis of observations andconclusions from this experiment, Rutherford proposed the nuclearmodel of atom. According to this model:
The presence of positive charge on thenucleus is due to the protons in the nucleus.As established earlier, the charge on the proton is equal but opposite to that of electron.Atomic number (Z) = number of protons inthe nucleus of an atom = number of electrons in a nuetral atom. protons and neutrons present in thenucleus are collectively known as nucleons.The total number of nucleons is termed asmass number (A) of the atom.
mass number (A) = number of protons (Z) + number of neutrons (n).
Isobars are the atoms with same massnumber but different atomic number forexample, 6 14 C and 7 14 N. On the other hand, atomswith identical atomic number but differentatomic mass number are known as Isotopes.For example,considering of hydrogen atom again, 99.985%of hydrogen atoms contain only one proton.This isotope is called protium ( 1 1 H). Rest of thepercentage of hydrogen atom contains two otherisotopes, the one containing 1 proton and 1neutron is called deuterium ( 2 1 D, 0.015%)and the other one possessing 1 proton and 2neutrons is called tritium ( 1 3 T )..the studiesof interactions of radiations with matter haveprovided immense information regarding thestructure of atoms and molecules. Neils Bohrutilised these results to improve upon themodel proposed by Rutherford. Twodevelopments played a major role in theformulation of Bohr’s model of atom. Thesewere:
(i) Dual character of the electromagneticradiation which means that radiations possess both wave like and particle likeproperties, and
(ii) Experimental results regarding atomicspectra.
James Maxwell (1870) was the first to givea comprehensive explanation about theinteraction between the charged bodies andthe behaviour of electrical and magnetic fieldson macroscopic level. He suggested that whenelectrically charged particle moves underaccelaration, alternating electrical and magnetic fields are produced and transmitted.These fields are transmitted in the forms ofwaves called electromagnetic waves orelectromagnetic radiation.radiations are characterised by theproperties, namely, frequency (ν ) and wavelength (λ).The SI unit for frequency (ν) is hertz(Hz, s –1 ), after Heinrich Hertz. It is defined asthe number of waves that pass a given pointin one second.Wavelength should have the units of lengthand as you know that the SI units of length ismeter (m). Since electromagnetic radiationconsists of different kinds of waves of muchsmaller wavelengths, smaller units are used.In vaccum all types of electromagneticradiations, regardless of wavelength, travel atthe same speed, i.e., 3.0 × 10 8 m s –1 (2.997925× 10 8 ms –1 , to be precise). This is called speedof light and is given the symbol ‘c‘. Thefrequency (ν ), wavelength (λ) and velocity of light(c) are related by the following equation .
The other commonly used quantityspecially in spectroscopy, is the wavenumber.It is defined as the number of wavelengthsper unit length. Its units are reciprocal ofwavelength unit, i.e., m –1 . However commonlyused unit is cm –1
1) The presence of positive charge on the nucleus is due to the …. in the nucleus .
(b) Neutrons
(c) Electron
(d) Nucleons
Ans- a) Protons
2) Atomic Number is denoted by ..
3) Atomic Mass number is denoted by ..
4) … are the atoms with same mass number but different atomic number.
(a) Isotopes
(b) Allotropes
(c) Isobars
(d) None of above
Ans- c) Isobars
5) Atoms with identical atomic number but different atomic mass number are known as ..
Ans- a) Isotopes
1) What are the developments that played major role in theformulation of Bohr’smodel of atom ?
Ans – Two developments played a major role in the formulation of Bohr’s model of atom. These were:
(i) Dual character of the electromagnetic radiation which means that radiations possess both wave like and particle like properties, and
(ii) Experimental results regarding atomic spectra.
2) Explain the term isotope with suitable example?
Ans- Atomswith identical atomic number but differentatomic mass number are known as Isotopes.For example,considering of hydrogen atom again, 99.985%of hydrogen atoms contain only one proton.This isotope is called protium ( 1 1 H). Rest of thepercentage of hydrogen atom contains two otherisotopes, the one containing 1 proton and 1neutron is called deuterium ( 2 1 D, 0.015%)and the other one possessing 1 proton and 2neutrons is called tritium ( 1 3 T ).

[C] Long Answer
1) Who give a comprehensive explanation about the interaction between the charged bodies and the Behaviour of electrical and magnetic fields? Explain in brief.
Ans- James Maxwell (1870) was the first to give a comprehensive explanation about the interaction between the charged bodies and the behaviour of electrical and magnetic fields on macroscopic level. He suggested that when electrically charged particle moves under accelaration, alternating electrical and magnetic fields are produced and transmitted. These fields are transmitted in the forms of waves called electromagnetic waves or electromagnetic radiation.
2) State and Explain characteristic properties of Radiation .
Ans- Radiations are characterised by the properties, namely, frequency (ν ) and wavelength (λ).The SI unit for frequency (ν) is hertz (Hz, s–1), after Heinrich Hertz. It is defined as the number of waves that pass a given point in one second. Wavelength should have the units of length and as you know that the SI units of length is meter (m). Since electromagnetic radiation consists of different kinds of waves of much smaller wavelengths, smaller units are used. In vaccum all types of electromagnetic radiations, regardless of wavelength, travel at the same speed, i.e., 3.0 × 108 m s–1 (2.997925 × 108 ms–1, to be precise). This is called speed of light and is given the symbol ‘c‘. The frequency (ν ), wavelength (λ) and velocity of light © are related by the following equation .
The other commonly used quantity specially in spectroscopy, is the wavenumber. It is defined as the number of wavelengths per unit length. Its units are reciprocal of wavelength unit, i.e., m–1.
The first concreteexplanation for the phenomenon of the blackbody radiation was given byMax Planck in 1900.An ideal body, which emits and absorbs radiations of allfrequencies uniformly, is called a black bodyand the radiation emitted by such a body is called black body radiation. Max Planck arrived at a satisfactory relationshipbymaking an assumption that absorption andemmission of radiation arises from oscillatori.e., atoms in the wall of black body.He suggested that atoms andmolecules could emit or absorb energy onlyin discrete quantities and not in a continuousmanner. He gave the name quantum to thesmallest quantity of energy that can be emitted or absorbed in the form of electromagnetic radiation. The energy (E ) of aquantum of radiation is proportionalto its frequency (ν ) and is expressed byequation .
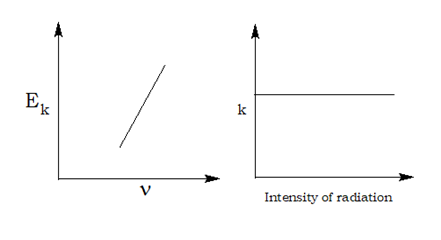
The proportionality constant, ‘h’ is knownas Planck’s constant and has the value6.626×10 –34 Js.In 1887, H. Hertz performed a very interestingexperiment in which electrons (or electriccurrent) were ejected when certain metals (forexample potassium, rubidium, caesium etc.)were exposed to a beam of light. The phenomenon is calledPhotoelectric effect. The results observed inthis experiment were:
(i) The electrons are ejected from the metalsurface as soon as the beam of light strikesthe surface, i.e., there is no time lagbetween the striking of light beam and theejection of electrons from the metal surface.
(ii) The number of electrons ejected is proportional to the intensity or brightness
(iii) For each metal, there is a characteristicminimum frequency,ν0(also known asthreshold frequency) below which photoelectric effect is not observed. At afrequency ν >ν 0 , the ejected electrons comeout with certain kinetic energy. The kineticenergies of these electrons increase withthe increase of frequency of the light used.
The particle nature of light posed a dilemmafor scientists. Theonly way to resolve the dilemma was to acceptthe idea that light possesses both particle andwave-like properties, i.e., light has dualbehaviour. Depending on the experiment, wefind that light behaves either as a wave or as astream of particles. Whenever radiationinteracts with matter, it displays particle likeproperties in contrast to the wavelike properties (interference and diffraction), whichit exhibits when it propagates. This conceptwas totally alien to the way the scientiststhought about matter and radiation and it tookthem a long time to become convincedof itsvalidity.
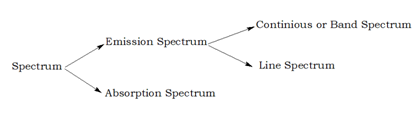
The study of emission or absorption spectra is referred to as spectroscopy.The emission spectra of atoms inthe gas phase, on the other hand, do not showa continuous spread of wavelength from redto violet, rather they emit light only at specificwavelengths with dark spaces between them.Such spectra are called line spectra or atomicspectra.The Swedishspectroscopist, Johannes Rydberg, noted that
all series of lines in the hydrogen spectrumcould be described by the following expression :
The value 109,677 cm –1 is called theRydberg constant for hydrogen. The first fiveseries of lines that correspond to n 1 = 1, 2, 3,4, 5 are known as Lyman, Balmer, Paschen,Bracket and Pfund series, respectively.Neils Bohr (1913) was the first to explainquantitatively the general features of thestructure of hydrogen atom and its spectrum.He used Planck’s concept of quantisation ofenergy. Though the theory is not the modernquantum mechanics, it can still be used to rationalize many points in the atomic structureand spectra. Bohr’s model for hydrogen atomis based on the following postulates:
i) The electron in the hydrogen atom canmove around the nucleus in a circular pathof fixed radius and energy. These paths arecalled orbits, stationary states or allowedenergy states. These orbits are arrangedconcentrically around the nucleus.
ii) The energy of an electron in the orbit doesnot change with time. However, theelectron will move from a lower stationarystate to a higher stationary state whenrequired amount of energy is absorbedby the electron or energy is emitted when electron moves from higher stationarystate to lower stationary state. The energychange does not takeplace in a continuous manner.
iii) The frequency of radiation absorbed oremitted when transition occurs between two stationary states that differ in energyby ∆E, is given by:
Where E1 and E2 are the energies of the lower and higher allowed energy statesrespectively. This expression is commonly known as Bohr’s frequency rule.
iv) The angular momentum of an electron isquantised. In a given stationary state itcan be expressed as in equation

1)The first concrete explanation for the phenomenon of the black body radiation was given by ….in 1900 .
(a) Max Planck
(b) De Broglie
(c) Albert Einstein,
(d) Niels Bohr
Ans- a) Max Planck
2) Which of the following equation is Planck’s equation ?
(a) E= mc 2
(c) E= hc 2
(d) E= vc 2 .
Ans- b)E = hυ
3) What is nature of light ?
(b) Particle
(c) Wave and Particle
Ans- c) Wave and Particle
4) The value …. is called theRydberg constant for hydrogen.
(a) 109,674 cm –1
(b) 109,675 cm –1
(c) 109,676cm –1
(d) 109,677 cm –1
Ans – d) 109,677 cm –1
5) … was the first to explain quantitatively the general features of the structure of hydrogen atom and its spectrum.
Ans- d) Niels Bohr
1) What is line spectra?
Ans- The emission spectra of atoms inthe gas phase, do not show a continuous spread of wavelength from redto violet, rather they emit light only at specificwavelengths with dark spaces between them.Such spectra are called line spectra or atomicspectra.
2) Define – Black body and black body radiation .
Ans- An ideal body, which emits and absorbs radiations of allfrequencies uniformly, is called a black bodyand the radiation emitted by such a body is called black body radiation.
3) Explain Dual nature of light .
Light possesses both particle andwave-like properties, i.e., light has dualbehaviour. Depending on the experiment, wefind that light behaves either as a wave or as astream of particles. Whenever radiationinteracts with matter, it displays particle likeproperties in contrast to the wavelike properties (interference and diffraction), whichit exhibits when it propagates.
1) What are the experimental results of photoelectric effects ?
Ans- The results observed in experiment were:
(ii) The number of electrons ejected is proportional to the intensity or brightness of light.
(iii) For each metal, there is a characteristic minimum frequency,ν0(also known asthreshold frequency) below which photoelectric effect is not observed. At a frequency ν >ν 0 , the ejected electrons comeout with certain kinetic energy. The kinetic energies of these electrons increase withthe increase of frequency of the light used.
2) Write the postulates of Bohr’s model of hydrogen atom.
Ans- Bohr’s model for hydrogen atomis based on the following postulates:
i)The electron in the hydrogen atom can move around the nucleus in a circular path of fixed radius and energy. These paths are called orbits, stationary states or allowed energy states. These orbits are arranged concentrically around the nucleus.
ii) The energy of an electron in the orbit does not change with time. However, the electron will move from a lower stationary state to a higher stationary state when required amount of energy is absorbed by the electron or energy is emitted when electron moves from higher stationary state to lower stationary state. The energy change does not take place in a continuous manner.
iii) The frequency of radiation absorbed oremitted when transition occurs between two stationary states that differ in energy by ∆E, is given by:
Where E1 and E2 are the energies of thelower and higher allowed energy statesrespectively. This expression is commonlyknown as Bohr’s frequency rule.
The French physicist, de Broglie, in 1924proposed that matter, like radiation, shouldalso exhibit dual behaviour i.e., both particleand wavelike properties. This means that justas the photon has momentum as well aswavelength, electrons should also havemomentum as well as wavelength, de Broglie,from this analogy, gave the following relationbetween wavelength (λ) and momentum (p) ofa material particle
where m is the mass of the particle, v itsvelocity and p its momentum.
Werner Heisenberg a German physicist in1927, stated uncertainty principle which is theconsequence of dual behaviour of matter andradiation. It states that it is impossible todetermine simultaneously, the exact position and exact momentum (or velocity)of an electron.Mathematically, it can be given as inequation

where ∆x is the uncertainty in position and ∆p x (or ∆v x ) is the uncertainty in momentum (orvelocity) of the particle.
One of the important implications of theHeisenberg Uncertainty Principle is that itrules out existence of definite paths ortrajectories of electrons and other similarparticles. The effect of Heisenberg Uncertainty Principle is significant only for motion of microscopic objects and is negligible for that of macroscopic objects. It, therefore, means that theprecise statements of the position andmomentum of electrons have to bereplaced by the statements of probability,that the electron has at a given positionand momentum. This is what happens inthe quantum mechanical model of atom. In Bohr model, anelectron is regarded as a charged particlemovingin well defined circular orbits aboutthe nucleus. The wave character of the electronis not considered in Bohr model. Further, anorbit is a clearly defined path and this pathcan completely be defined only if both theposition and the velocity of the electron areknown exactly at the same time. This is notpossible according to the Heisenberguncertainty principle. Bohr model of thehydrogen atom, therefore, not only ignoresdual behaviour of matter but also contradictsHeisenberg uncertainty principle. The structure of the atom was needed which could account for wave-particle duality of matter and be consistent with Heisenberg uncertainty Principle. This came with the advent of Quantum mechanics. This is mainly becauseof the fact thatclassical mechanics ignores theconcept of dual behaviour of matter especiallyfor sub-atomic particles and the uncertaintyprinciple. The branch of science that takes intoaccount this dual behaviour of matter is calledquantum mechanics.Quantum mechanics is a theoreticalscience that deals with the study of the motionsof the microscopic objects that have bothobservable wave like and particle likeproperties.When Schrödinger equation is solved forhydrogen atom, the solution gives the possibleenergy levels the electron can occupy and thecorresponding wave function(s) (ψ) of theelectron associated with each energy level. A large number of orbitals are possible in anatom. Qualitatively these orbitals can bedistinguished by their size, shape andorientation. An orbital of smaller size meansthere is more chance of finding the electron nearthe nucleus. Similarly shape and orientationmean that there is more probability of findingthe electron along certain directions thanalong others. Atomic orbitals are preciselydistinguished by what are known as quantumnumbers. Each orbital is designated by threequantum numbers labelled as n, l and m 1.
The principal quantum number ‘n’ isa positive integer with value of n = 1,2,3…….The principal quantum number determines thesize and to large extent the energy of theorbital. Azimuthal quantum number. ‘l’ is alsoknown as orbital angular momentum orsubsidiary quantum number. It defines thethree-dimensional shape of the orbital.. For agiven value of n, l can have n values rangingfrom 0 to n – 1, that is, for a given value of n,the possible value of l are : l = 0, 1, 2, ……….(n–1)
Magnetic orbital quantum number. ‘mlgives information about the spatialorientation of the orbital with respect tostandard set of co-ordinate axis. For anysub-shell (defined by ‘l’ value) 2l+1 valuesof ml are possible and these values are givenbuy :ml = – l, – (l –1), – (l–2)… 0,1… (l –2), (l–1)..
In 1925, George Uhlenbeck and SamuelGoudsmit proposed the presence of the fourthquantum number known as the electronspin quantum number (ms). electron has, besides charge and mass,intrinsic spin angular quantum number. Spinangular momentum of the electron — a vectorquantity, can have two orientations relative tothe chosen axis. These two orientations aredistinguished by the spin quantum numbersms which can take the values of +½ or –½.These are called the two spin states of theelectron and are normally represented by twoarrows, ↑ (spin up) and ↓ (spin down).the four quantum numbersprovide the following information :
i) n defines the shell, determines the size ofthe orbital and also to a large extent theenergy of the orbital.
ii) There are n subshells in the n the shell. Lidentifies the subshell and determines the shape of the orbital (see section 2.6.2).There are (2l+1) orbitals of each type in asubshell, that is, one s orbital (l = 0), threep orbitals (l = 1) and five d orbitals (l = 2)per subshell. To some extent l alsodetermines the energy of the orbital in amulti-electron atom.
iii) ml designates the orientation of the orbital.For a given value of l, mlhas (2l+1) values,the same as the number of orbitals persubshell. It means that the number oforbitals is equal to the number of ways inwhich they are oriented.
iv) ms refers to orientation of the spin of the electron.
1) Uncertainty principle was given by ..
(a) Werner Heisenberg
(b) George Uhlenbeck
(c) Samuel Goudsmit
(d) De Broglie
Ans- a)Werner Heisenberg
2) Quantum mechanics is a theoretical science that deals with the study of the motions of the ….. objects.
(a) Macroscopic
(b) Microscopic
(c) Laparoscopic
Ans- b) Microscopic
3) The principal quantum number …
4) …is also known as orbital angular momentum or subsidiary quantum number.
(a) principal quantum number
(b) electron spin quantum number
(c) Magnetic orbital quantum number.
(d) Azimuthal quantum number
Ans- d) Azimuthal quantum number
5) George Uhlenbeck and Samuel Goudsmit proposed the presence of the fourth quantum number known as the …
Ans- b) electron spin quantum number.
1) State Heisenberg uncertainty principle
Ans- It states that it is impossible todetermine simultaneously, the exact position and exact momentum (or velocity)of an electron.Mathematically, it can be given as inequation
2) Define -Azimuthal quantum number.
Ans- Azimuthal quantum number. ‘ l ’ is alsoknown as orbital angular momentum orsubsidiary quantum number. It defines thethree-dimensional shape of the orbital.. For agiven value of n, l can have n values rangingfrom 0 to n – 1, that is, for a given value of n,the possible value of l are : l = 0, 1, 2, ……….(n–1)
3) What is Magnetic orbital quantum number ?
Ans- Magnetic orbital quantum number. ‘m l ‘gives information about the spatial orientation of the orbital with respect to standard set of co-ordinate axis. For anysub-shell (defined by ‘l’ value) 2l+1 values of ml are possible and these values are given buy :m l = – l, – (l –1), – (l–2)… 0,1… (l –2), (l–1)..
1) Explain in brief – Electron spin quantum number.
Ans- In 1925, George Uhlenbeck and SamuelGoudsmit proposed the presence of the fourthquantum number known as the electronspin quantum number (ms). electron has, besides charge and mass,intrinsic spin angular quantum number. Spinangular momentum of the electron — a vectorquantity, can have two orientations relative tothe chosen axis. These two orientations aredistinguished by the spin quantum numbersms which can take the values of +½ or –½.These are called the two spin states of theelectron and are normally represented by twoarrows, ↑ (spin up) and ↓ (spin down).
2) What information do the Quantum numbers provide?
Ans- The four quantum numbersprovide the following information :
ii) There are n subshells in the n the shell. Lidentifies the subshell and determines theshape of the orbital (see section 2.6.2).There are (2l+1) orbitals of each type in asubshell, that is, one s orbital (l = 0), threep orbitals (l = 1) and five d orbitals (l = 2)per subshell. To some extent l alsodetermines the energy of the orbital in amulti-electron atom.
iii) ml designates the orientation of the orbital. For a given value of l, mlhas (2l+1) values, the same as the number of orbitals persub shell. It means that the number of orbitals is equal to the number of ways in which they are oriented.
The orbital wave function or ψ for an electronin an atom has no physical meaning. It issimply a mathematical function of thecoordinates of the electron. However, fordifferent orbitals the plots of correspondingwave functions as a function of r (the distancefrom the nucleus) are different. According to the German physicist, MaxBorn, the square of the wave function(i.e.,ψ 2 ) at a point gives the probability densityof the electron at that point. Boundary surface diagrams of constantprobability density for different orbitals give afairly good representation of the shapes of theorbitals. In this representation, a boundarysurface or contour surface is drawn in spacefor an orbital on which the value of probabilitydensity |ψ|2 is constant. In principle manysuch boundary surfaces may be possible.However, for a given orbital, only thatboundary surface diagram of constantprobability density* is taken to be goodrepresentation of the shape of the orbital whichencloses a region or volume in which theprobability of finding the electron is very high,say, 90%.
In hydrogen atom, electron has the same energy when it is in the2s orbital as when it is present in 2p orbital.The orbitals having the same energy are calleddegenerate. The 1s orbital in a hydrogenatom, as said earlier, corresponds to the moststable condition and is called the ground stateand an electron residing in this orbital is moststrongly held by the nucleus.
An electron inthe 2s, 2p or higher orbitals in a hydrogen atomis in excited state.The filling of electrons into the orbitals ofdifferent atoms takes place according to theaufbau principle which is based on the Pauli’sexclusion principle, the Hund’s rule ofmaximum multiplicity and the relativeenergies of the orbitals. Theaufbausprinciple states : In the ground state of theatoms, the orbitals are filled in order oftheir increasing energies. In other words,electrons first occupy the lowest energy orbitalavailable to them and enter into higher energyorbitals only after the lower energy orbitals arefilled.The number of electrons to be filled in variousorbitals is restricted by the exclusion principle,given by the Austrian scientist Wolfgang Pauli(1926). According to this principle : No twoelectrons in an atom can have the sameset of four quantum numbers. Pauliexclusion principle can also be stated as : “Onlytwo electrons may exist in the same orbitaland these electrons must have oppositespin.” This means that the two electrons canhave the same value of three quantum numbersn, l and m l , but must have the opposite spinquantum number.Hund’s Rule of Maximum Multiplicity rule deals with the filling of electrons into the orbitals belonging to the same subshell. It states : pairing ofelectrons in the orbitals belonging to thesame subshell (p, d or f) does not take placeuntil each orbital belonging to thatsubshell has got one electron each i.e., itis singly occupied.
The distribution of electrons into orbitals of anatom is called its electronic configuration.If one keeps in mind the basic rules whichgovern the filling of different atomic orbitals,the electronic configurations of different atomscan be written very easily.The electronic configuration of differentatoms can be represented in two ways. Forexample :
(i) s a p b d c …… notation
(ii) Orbital diagram
1) …at a point gives the probability density of the electron at that point.
Ans- d) ψ 2
2) Only …. electrons may exist in the same orbital and these electrons must have opposite spin .
3) …deals with the filling of electrons into the orbitals belonging to the same subshell.
(a) Hund’s Rule of Maximum Multiplicity rule
(b) Pauli’s exclusion principle
(c) Aufbau principle
(d) Werner Heisenberg
Ans- a) Hund’s Rule of Maximum Multiplicity rule
4) electrons first occupy the …. energy orbital available to them and enter into … energy orbitals
(a) Lowest, Higher
(b) Higher , Lowest
(c) Middle , Higher
(d) Higher, Middle
Ans- a) Lowest, Higher.
1) Explain the following terms 1) Degenerate 2)Ground state
Ans- In hydrogen atom, electron has the same energy when it is in the 2s orbital as when it is present in 2p orbital. The orbitals having the same energy are called degenerate. The 1s orbital in a hydrogen atom, as said earlier, corresponds to the most stable condition and is called the ground state and an electron residing in this orbital is most strongly held by the nucleus.
2) State Hund’s Rule of Maximum Multiplicity.
Ans – Hund’s Rule of Maximum Multiplicity rule deals with the filling of electrons into the orbitals belonging to the same subshell. It states : pairing ofelectrons in the orbitals belonging to thesame subshell (p, d or f) does not take placeuntil each orbital belonging to thatsubshell has got one electron each i.e., itis singly occupied.
3) State and explain aufbau principle
Ans- Aufbausprinciple states : In the ground state of theatoms, the orbitals are filled in order oftheir increasing energies. In other words,electrons first occupy the lowest energy orbitalavailable to them and enter into higher energyorbitals only after the lower energy orbitals arefilled.
1) State and explain pauli Exclusion Principle.
Ans – The number of electrons to be filled in various orbitals is restricted by the exclusion principle, given by the Austrian scientist Wolfgang Pauli(1926). According to this principle : No two electrons in an atom can have the same set of four quantum numbers. Pauli exclusion principle can also be stated as : “Only two electrons may exist in the same orbital and these electrons must have opposites pin.” This means that the two electrons can have the same value of three quantum numbersn, l and m l , but must have the opposite spin quantum number.
2) Write Electronic Configuration of the following Elements-
boron (B, 1s22s22p1), carbon (C, 1s22s22p2), nitrogen(N, 1s22s22p3), oxygen (O,1s22s22p4), fluorine(F, 1s22s22p5) and neon (Ne, 1s22s22p6)
Ans- The orbital picture of these elements can be represented as follows :

Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
We have a strong team of experienced Teachers who are here to solve all your exam preparation doubts
Delhi public school (dps) chandmari, patna admission 2024 – 2025 details, factors promoting growth of nationalism foundation of the indian national congress class 10 icse chapter 2 complete notes, premalok mission school bairiya, patna admission 2024 – 2025 details, duff and dutt class 10 2024 chapter 10 miscellaneous practice solution.
Sign in to your account
Username or Email Address
Remember Me

Gurukul of Excellence
Classes for Physics, Chemistry and Mathematics by IITians
Join our Telegram Channel for Free PDF Download
Chemistry MCQs for Class 11 Chapter 2 Structure of Atom
- Last modified on: 3 years ago
- Reading Time: 26 Minutes
Q.1. Number of protons, neutrons and electrons in the element 89 X 231 is (a) 89, 89, 242 (b) 89, 142, 89 (c) 89, 71, 89 (d) 89, 231, 89
Q.2. Atoms with same mass number but different atomic numbers are called (a) isotopes (b) isobars (c) isochores (d) None of these
Q.3. The increasing order (lowest first) for the values of e/m (charge/mass) for (a) e, p, n, α (b) n, p, e, α (c) n, p, α, e (d) n, α, p, eAnswer
Q.4. The energy of second Bohr orbit of the hydrogen atom is -328 kJ mol-1; hence the energy of fourth Bohr orbit would be: (a) -41 kJ mol -1 (b) -82 kJ mol -1 (c) -164 kJ mol -1 (d) -1312 kJ mol -1
Q.5. The ionisation potential of a hydrogen atom is –13.6 eV. What will be the energy of the atom corresponding to n = 2. (a) – 3.4 eV (b) – 6.8 eV (c) – 1.7 eV (d) –2.7 eV
Q.6. The ionization enthalpy of hydrogen atom is 1.312 × 10 6 J mol -1 . The energy required to excite the electron in the atom from n = 1 to n = 2 is (a) 8.51 × 10 5 J mol -1 (b) 6.56 × 10 5 J mol -1 (c) 7.56 × 10 5 J mol -1 (d) 9.84 × 10 5 J mol -1
Answer: (d) 9.84 × 10 5 J mol -1 Energy required when an electron makes transition from n = 1 to n = 2 E 2 =−(1.312 × 10 6 × (1)²)/(2²) = −3.28 × 10 5 J mol -1 E 1 = −1.312 × 10 6 J mol -1 ΔE = E 2 − E 1 =−3.28 × 10 5 −(−13.2 × 10 6 ) ΔE = 9.84×10 5 J mol -1
Q.7. In which of the following Bohr’s stationary state, the electron will be at maximum distance from the nucleus ? (a) IInd (b) Ist (c) Vth (d) IIIrd
Q.8. For a given principal level n = 4, the energy of its subshells is in the order (a) s < p < d < f (b) s > p > d > f (c) s < p < f < d (d) f < p < d < s
Q.9. A gas absorbs a photon of 355 nm and emits at two wavelengths. If one of the emissions is at 680 nm, the other is at: (a) 518 nm (b) 1035 nm (c) 325 nm (d) 743 nm
Answer: (d) 743 nm Explanation: From Law of Conservation of energy, energy of absorbed photon must be equal to combined energy of two emitted photons. ET = E 1 + E 2 ….. (1) Where E 1 is Energy of first emitted photon emitted and E 2 is Energy of second emitted photon. Energy E and wavelength λ of a photon are related by the equation E= (hc)/ (λ)….. (2) Where Plancks constant = h, c is velocity of light. Substituting the values from (2) in (1) we get (hc/λ T ) = (hc)/ (λ 1 ) + (hc)/ (λ 2 ) Or (1λ 𝑇 ) = (1λ1) + (1λ2) …… (3) Substituting given values in (3) we get (1355) = (1680) + (1λ2) Or (1)(λ 𝑇 ) = (1355) − (1680) ⇒ (1λ2) = (680 − 355)/ (355 × 680) ⇒ λ 2 = 742.77nm
Q.10. Which of the following statements do not form a part of Bohr’s model of hydrogen atom ? (a) Energy of the electrons in the orbits are quantized (b) The electron in the orbit nearest the nucleus has the lowest energy (c) Electrons revolve in different orbits around the nucleus (d) The position and velocity of the electrons in the orbit cannot be determined simultaneously.
Q.11. Which of the following statements in relation to the hydrogen atom is correct? (a) 3s orbital is lower in energy than 3p orbital (b) 3p orbital is lower in energy than 3d orbital (c) 3s and 3p orbitals are of lower energy than 3d orbital (d) 3s, 3p and 3d orbitals all have the same energyAnswer
Q.12. The magnetic quantum number specifies (a) Size of orbitals (b) Shape of orbitals (c) Orientation of orbitals (d) Nuclear Stability
Q.13. If electron, hydrogen, helium and neon nuclei are all moving with the velocity of light, then the wavelength associated with these particles are in the order (a) Electron > hydrogen > helium > neon (b) Electron > helium > hydrogen > neon (c) Electron < hydrogen < helium < neon (d) Neon < hydrogen < helium < electron
Q.14. The electronic configuration of silver atom in ground state is (a) [Kr]3d 10 4s 1 (b) [Xe]4f 14 5d 10 6s 1 (c) [Kr]4d 10 5s 1 (d) [Kr]4d 9 5s 2 Answer
Q.15. Which of the following element has least number of electrons in its M-shell? (a) K (b) Mn (c) Ni (d) ScAnswer
Q.16. Which one of the following sets of ions represents a collection of isoelectronic species? (Atomic nos.: F = 9, Cl = 17, Na = 11, Mg = 12, Al = 13, K = 19, Ca = 20, Sc = 21) (a) K + , Ca 2+ , Sc 3+ , Cl – (b) Na + , Ca 2+ , Sc 3+ , F – (c) K + , Cl – , Mg 2+ , Sc 3+ (d) Na + , Mg 2+ , Al 3+ , Cl – Answer
Q.17. The total number of electrons that can be accommodated in all orbitals having principal quantum number 2 and azimuthal quantum number 1 is (a) 2 (b) 4 (c) 6 (d) 8
Q.18. In the ground state, an element has 13 electrons in its M-shell. The element is_____. (a) Copper (b) Chromium (c) Nickel (d) Iron
Q.19. The electrons of the same orbitals can be distinguished by (a) Principal quantum number (b) Azimuthal quantum number (c) Spin quantum number (d) Magnetic quantum number
Q.20. What will be the sum of all possible values of l and m for n = 5 ? (a) 12 (b) 13 (c) 4 (d) 9
Q.21. Consider the ground state of Cr atom (Z = 24). The numbers of electrons with the azimuthal quantum numbers, l = 1 and 2 are, respectively: (a) 12 and 4 (b) 12 and 5 (c) 16 and 4 (d) 16 and 5
Q.22. A body of mass 10 mg is moving with a velocity of 100 ms -1 . The wavelength of de-Broglie wave associated with it would be (Note: h = 6.63 × 10 -34 Js) (a) 6.63 × 10 -37 m (b) 6.63 × 10 -31 m (c) 6.63 × 10 -34 m (d) 6.63 × 10 -35 m
Q.23. The orbitals are called degenerate when (a) they have the same wave functions (b) they have the same wave functions but different energies (c) they have different wave functions but same energy (d) they have the same energy
Q.24. The ionization enthalpy of hydrogen atom is 1.312 × 10 6 J mol -1 . The energy required to excite the electron in the atom from n = 1 to n = 2 is (a) 8.51 × 10 5 J mol -1 (b) 6.56 × 10 5 J mol -1 (c) 7.56 × 10 5 J mol -1 (d) 9.84 × 10 5 J mol -1 Answer
Q.25. In Hydrogen atom, energy of first excited state is – 3.4 eV. Then find out KE of same orbit of Hydrogen atom (a) 3.4 eV (b) 6.8 eV (c) -13.6 eV (d) +13.6 eV
Q.26. Which of the following sets of quantum numbers represents the highest energy of an atom? (a) n = 3, l = 0, m = 0, s = + 12 (b) n = 3, l = 1, m = 1, s = + 12 (c) n = 3, l = 2, m = 1, s = + 12 (d) n = 4, l = 0, m = 0, s = + 12
Q.27. In the Bohr’s model of the hydrogen atom, the ratio of the kinetic energy to the total energy of the electron in a quantum state n is: (a) 1 (b) 2 (c) -1 (d) -2
Q.28. The number of spherical nodes in 3p orbitals are (a) one (b) three (c) two (d) None of these
Q.29. Which of the following statements does not form a part of Bohr’s model of hydrogen atom? (a) Energy of the electrons in the orbit is quantised. (b) The electron in the orbit nearest the nucleus has the lowest energy (c) Electrons revolve in different orbits around the nucleus (d) The position and velocity of the electrons in the orbit cannot be determined simultaneously
Q.30. Which of the following statements in relation to the hydrogen atom is correct? (a) 3s orbital is lower in energy than 3p orbital (b) 3p orbital is lower in energy than 3d orbital (c) 3s and 3p orbitals are of lower energy than 3d orbital (d) 3s, 3p and 3d orbitals all have the same energy.
Q.31. A sub-shell with n = 6 , l = 2 can accommodate a maximum of (a) 12 electrons (b) 36 electrons (c) 10 electrons (d) 72 electrons
Quiz on Structure of Atom
General Instructions:
(1) Total number of question is 5.
(2) Each question has 4 choices, out of which ONLY ONE CHOICE is correct.

In order to equip the students with the latest methodology of testing and evaluation in competitive exam segment in India Physics Gurukul has introduced successrouter.com for Online Testing and Assessment. Try our free tests for JEE and NEET Today!
Download CBSE Books
Exam Special Series:
- Sample Question Paper for CBSE Class 10 Science (for 2024)
- Sample Question Paper for CBSE Class 10 Maths (for 2024)
- CBSE Most Repeated Questions for Class 10 Science Board Exams
- CBSE Important Diagram Based Questions Class 10 Physics Board Exams
- CBSE Important Numericals Class 10 Physics Board Exams
- CBSE Practical Based Questions for Class 10 Science Board Exams
- CBSE Important “Differentiate Between” Based Questions Class 10 Social Science
- Sample Question Papers for CBSE Class 12 Physics (for 2024)
- Sample Question Papers for CBSE Class 12 Chemistry (for 2024)
- Sample Question Papers for CBSE Class 12 Maths (for 2024)
- Sample Question Papers for CBSE Class 12 Biology (for 2024)
- CBSE Important Diagrams & Graphs Asked in Board Exams Class 12 Physics
- Master Organic Conversions CBSE Class 12 Chemistry Board Exams
- CBSE Important Numericals Class 12 Physics Board Exams
- CBSE Important Definitions Class 12 Physics Board Exams
- CBSE Important Laws & Principles Class 12 Physics Board Exams
- 10 Years CBSE Class 12 Chemistry Previous Year-Wise Solved Papers (2023-2024)
- 10 Years CBSE Class 12 Physics Previous Year-Wise Solved Papers (2023-2024)
- 10 Years CBSE Class 12 Maths Previous Year-Wise Solved Papers (2023-2024)
- 10 Years CBSE Class 12 Biology Previous Year-Wise Solved Papers (2023-2024)
- ICSE Important Numericals Class 10 Physics BOARD Exams (215 Numericals)
- ICSE Important Figure Based Questions Class 10 Physics BOARD Exams (230 Questions)
- ICSE Mole Concept and Stoichiometry Numericals Class 10 Chemistry (65 Numericals)
- ICSE Reasoning Based Questions Class 10 Chemistry BOARD Exams (150 Qs)
- ICSE Important Functions and Locations Based Questions Class 10 Biology
- ICSE Reasoning Based Questions Class 10 Biology BOARD Exams (100 Qs)
✨ Join our Online JEE Test Series for 499/- Only (Web + App) for 1 Year
✨ Join our Online NEET Test Series for 499/- Only for 1 Year
2 thoughts on “ Chemistry MCQs for Class 11 Chapter 2 Structure of Atom ”
Liked it finally got some best
Leave a Reply Cancel reply
Join our Online Test Series for CBSE, ICSE, JEE, NEET and Other Exams

Editable Study Materials for Your Institute - CBSE, ICSE, State Boards (Maharashtra & Karnataka), JEE, NEET, FOUNDATION, OLYMPIADS, PPTs
Discover more from Gurukul of Excellence
Subscribe now to keep reading and get access to the full archive.
Type your email…
Continue reading
What happens when ammonium dichromate is heated?
How nitrogen is prepared in the laboratory, what happens when calcium phosphide is treated with water, what happens when magnesium nitride is treated with water.

ALL ABOUT CHEMISTRY
Explore the Mystery of Chemistry
- Disclaimer New
- Privacy Policy
- Terms and Conditions
- Quora Space

What happens when phosphorous acid is heated?

What happen when nitrous acid is heated?

FAQ|Structure of Atom

FAQ | Solutions| CBSE|ISC|JEE|NEET

Chemical Kinetics |JEE Main|JEE Advanced

Solutions|JEE main |JEE Advanced|MCQ question and answers
Structure of atom|class-11.

Describe the discharge tube experiment
The discharge tube filled with a gas is connected to a vacuum pump in order to reduce the pressure. The electrodes are connected to a source of high voltage of 10000 volts.
- Under normal pressure (1 atm), nothing is observed.
- At 10 -2 atm, the gas is found to emit light and the colour of the light depends upon the nature of the gas.
- At 10 -4 atm, the emission of light ceases but the wall of the discharge tube opposite to the cathode starts glowing with a faint greenish light called fluorescence. This is due to the bombardment on the wall by cathode rays.
Mention some properties of cathode rays
- It travels in a straight line, can caste shadow, consists of negatively charged particles, and can exert mechanical pressure.
- It can produce a heating effect, can ionize the gas through which they pass, can produce X-rays when striking on the surface of hard metals like tungsten, copper, molybdenum, etc.
- It is deflected by electric and magnetic field and can pass through thin foils of metals like Al.
- It affects the photographic plate. This is called fogging.
- The e/m ratio of the particles constituting the cathode rays does not depend on the nature of the gas or the material of the cathode.

Mention some properties of anode rays
- It travels in a straight line and can caste shadow.
- It can exert mechanical pressure and is deflected by electric and magnetic field.
- The anode rays are formed by the expulsion of electrons from the atoms of the gas in the space between anode and cathode.
- The e/m ratio of the particles constituting the anode rays depends on the nature of the gas.
Mention the observations of Rutherford’s Experiment
- Most of the a- particles passed through the foil without any deflection from their path.
- A few of them were deflected at some angles.
- Very few (1 in 10000) turned back on their original path.
Mention the conclusions of Rutherford’s experiment
- Most part of the atom is empty.
- The entire mass of the atom is concentrated in a small area that carries positive charge.
- Electrons revolve around the nucleus at a large distance from it.
Different types of atomic species
Atoms of the same elements with different mass numbers but the same atomic number are called isotopes. Ex: 1 H 1 , 1 H 2 , 1 H 3 .
Atoms of different elements which possess same mass number but a different atomic number. Ex: 18 Ar 40 and 20 Ca 40 , 1 H 3 and 2 He 3 , 7 N 14 and 8 O 17 .
Atoms of different elements which possess different atomic and mass number but same number of neutrons. Ex: 6 C 13 and 7 N 14 , 4 Be 9 and 5 B 10 .
Isodiaphers:
Nuclides which have different atomic and mass number but same difference between neutron and proton numbers. Ex: 90 Th 234 and 92 U 238
Mirror nuclei:
Nuclei having the same mass number but with the proton and neutron number interchanged. Ex : 1 H 3 and 2 He 3 , 3 Li 7 and 4 Be 7 .
Isoelectronic species:
Species having the same number of electrons. Ex: N 3- , O 2- , F – , Ne.
Electromagnetic Radiation
The invisible radiation caused by the combined effect of electric and magnetic field.
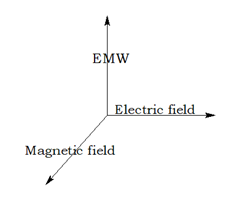
Photoelectric effect:
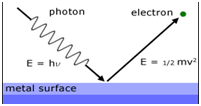
The phenomenon of ejection of electrons from the metal surface when light of suitable frequency strikes the metal. The electrons ejected are called photo-electrons.
hν= hν 0 + ½ mv 2
hν = Energy of the incident ray
hν 0 = Threshold energy= work function = Ionisation potential
ν 0 = Threshold frequency
½ mv 2 = kinetic energy of the emitted electrons
Planck’s Quantum Theory:
- Radiant energy is not emitted or absorbed continuously; rather it is absorbed or emitted by a body discontinuously in the form of small packets of energy known as quanta. For light one quantum of energy is called photon.
- The amount of energy associated with a quantum of radiation is directly proportional to its frequency. E α ν or E = hν
- A body can emit or absorb energy only in terms of integral multiples of quantum.E = nhν
Different types of spectrum
Mention the defects of Rutherford’s Model:
- According to Classical electromagnetic theory of Maxwell, whenever a charged body revolves it gives out radiations and loses energy. Thus electron which carries negative charge, and revolves around the nucleus should continuously emit radiations and loses energy. As a result, the electron follows a spiral path and collides with the nucleus.
- When gases under pressure are subjected to electric discharge, line spectrum is produced. This cannot be explained by Rutherford.
Hydrogen spectra:

Mention the main postulates of Bohr’s Theory
- The electrons move around the nucleus in certain specifically permitted circular orbits called energy levels or energy states. An electron in a particular energy level is associated with a definite amount of energy. While moving in a particular level, it neither absorbs nor loses energy.
- Only those energy levels are permitted in which the angular momentum of an electron is an integral multiple of h/2Π(The angular momentum are quantized)
- The electron absorbs energy when it jumps from lower to higher energy levels and emits energy when it moves from higher to lower levels. The energy gained or lost is equal to the difference between the energies of the two transitions.
Mention the significance of negative sign of energy:
- The energy of the electron in the atom is less than the energy of a free electron and thus the atom is a stable entity.
- Electron is attracted by the nucleus.
- To remove an electron, energy must be supplied from outside.
Mention the merits and demerits of Bohr’s Theory
Difference between rutherford’s and bohr’s concept:, de- broglie equation:.
De- Broglie suggested that electrons shows dual nature of particles (mass) and wave ( wave length).
From Planck’s Equation, E = hc/λ
From Einstein equation, E=mc 2
Where λ is the wave length
h is Planck’s Constant= 6.626 x 10 -27 erg sec
m and v are the mass and velocity respectively
Heisenberg’s Uncertainty Principle:
It states that it is impossible to measure simultaneously the position and exact velocity of a sub-atomic particle.
∆x.∆v≥ h/ 4Πm
∆x= Uncertainty in position
∆v = Uncertainty in velocity
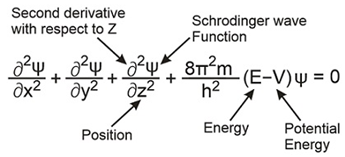
Schrodinger’s Wave Equation:
Quantum numbers:
Set of four numbers which can depict the shape, location, energy, orientation etc of the electrons.
Principal Quantum Number(n):
- It represents the number of shells
- It can have values like 1,2,3,4….
- Determines the energy of the shell
- Provides the maximum number of electrons a shell can hold(2n 2 )
Azimuthal Quantum Number(l):
- The line spectrum when highly resolved shows that each such line is made up of finer lines called the fine spectrum. These fine lines are due to the elliptical orbits.
- The angular momentum of a moving electron in an elliptical path is
- The minimum value of l is 0 and the maximum is n-1. Spectroscopic terms-(s- Sharp,p- Principal,d-Diffuse,f- fundamental)
- The penetrating power and screening effect of an elliptical orbital is: s> p>d>f
- The order of energy: s< p< d <f
Magnetic Quantum Number(m):
- The splitting of fine lines under the magnetic field is called the Zeeman effect.
- Under the influence of the external magnetic field, the electrons present in the subshells orient themselves in certain regions of space called orbitals.
- The value of m is –L to +L including zero.
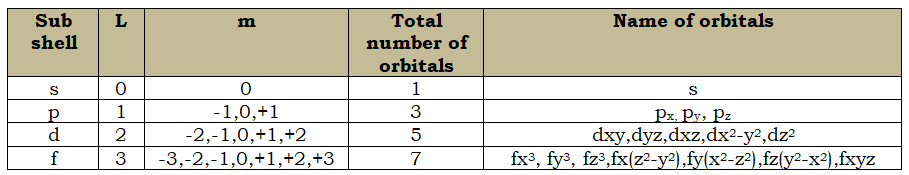
- The negative values of the magnetic quantum numbers signify that these orbitals are inclined in the direction opposite to the magnetic field and positive value indicates that these orbitals are inclined in the direction of the magnetic field.
The three dimensional region or space around the nucleus of an atom where there is maximum probability of finding an electron having certain energy.
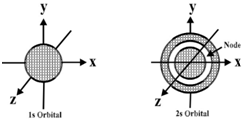
- 1s, 2s and 3s orbitals are all spherical shaped.
- Size and energy of the orbitals -1s<2s<3s
- Nodes are the region where the probability of getting the electrons is zero.
- p-orbitals are dumble shaped.
- Each p-orbitals possess two lobes, where the probability of getting the electron is max.
- All the 3 orbitals possess the same energy and thus are degenerate.
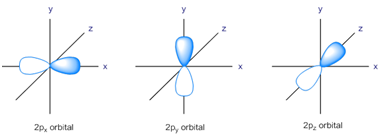
Total no of nodes= Radial + angular nodes = n-1
d-Orbitals:
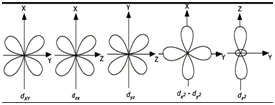
- d-orbitals are double dumble shaped with four lobes.
- dz 2 has two lobes. The annular portion orbital is called doughnut or belly band.
- All the 5 orbitals are degenerate.
Spin Quantum Number(s):
- It expresses the two opposite types of spinning motions of each electron.
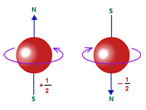
- Its values are +1/2 and -1/2
- It signifies the mode of electron spin- clockwise or anticlockwise.
- It is the only quantum no which is not obtained from Schrodinger’s wave equation.
Some important graphs:
- Variation of radial part of wave function with distance from the nucleus.
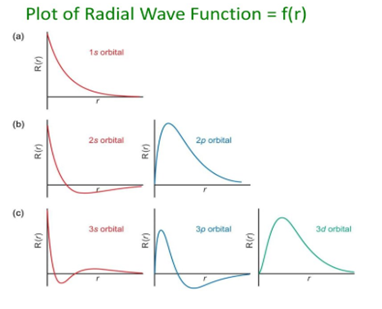
- Variation of the square of radial wave function(radial probability density) with distance from the nucleus:
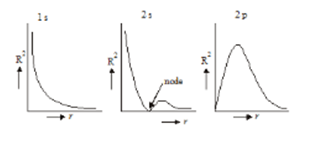
- Variation of radial probability distribution function with distance from the nucleus:
Pauli’s Exclusion Principle:
In an atom , no two electrons can have four identical quantum numbers.
Rules for filling up of orbitals:
1.aufbau principle:.
The orbitals are filled up with electrons in the increasing order of their energy.
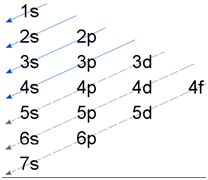
2. (n+l) rule:
The electron comes in the vacant sub-shell with lowest value of (n+l). If the value of (n+l) is same, then electron enters the sub shell having lowest value of n.
Lanthanides and actinides are exception to the (n+l) rule.
3. Hund’s Multiplicity Rule:
- Electrons continue to enter different orbitals as long as possible. Electron pairing in any orbital cannot take place until each orbital of the same sub-shell contains 1 electron.
- Two or more electrons each occupying different orbitals must have parallel spins. (Same direction of spin ↑ or ↓).
- Half filled and full filled subshells possess maximum stability. i.e., s 1 , s 2 , p 3 ,p 6 ,d 5 , d 10, f 7 , f 14 are stable configurations.
Why half-filled and full filled subshells possess maximum stability?
Symmetrical distribution of electrons:.
Subshells with half filled or completely filled electrons are found to have a more symmetrical distribution of electrons. Consequently, they have less energy and more stability.
Exchange energy:
The electrons occupying degenerate orbitals can exchange their positions with other electrons with the same spin. In this process, exchange energy is released. Greater the exchange energy, more stable the configuration is.
Elements with exceptional E.C-Cr(24), Cu(29), Nb(41), Mo(42),Ru(44), Rh(45), Pd(46), Ag(47), La(57), Ce(58), Gd(64), Pt(78), Au(79)
Magnetic nature of atoms and ions:
- Atoms with at least one unpaired electron are paramagnetic in nature( attracted by magnet).
- Atoms with no unpaired electron(s) are diamagnetic in nature(repelled by magnet).
- More the number of unpaired electrons, more the magnetic moment.
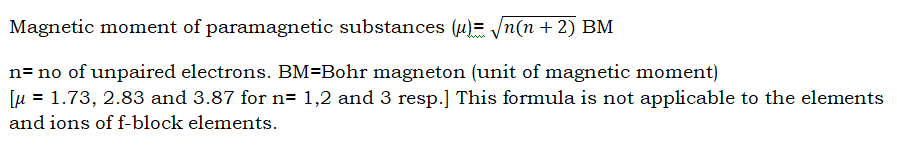
Related Posts

Mole Concept|JEE Main|JEE Advanced|MCQ question and answers

IMAGES
VIDEO
COMMENTS
Q2. Show the distribution of electrons in an oxygen atom (atomic number 8) using an orbital diagram. Answer. The distribution of electrons in an oxygen atom using an orbital diagram is-. From the above diagram, it can be concluded that oxygen has two unpaired electrons. Q3.
NCERT Solutions for Class 11 Chemistry Chapter 2 Structure of Atom. " Structure of Atom " is the second chapter in the NCERT Class 11 Chemistry textbook. The topics covered under this chapter include subatomic particles, Thomson's atomic model, Rutherford's atomic model, Bohr's model and the quantum mechanical model of the atom.
2.1.3 Charge on the Electron. R.A. Millikan (1868-1953) devised a method known as oil drop experiment (1906-14), to determine the charge on the electrons. He found that the charge on the electron to be. 1.6 × 10-19 C. The present accepted value of electrical charge is - 1.6022 × 10-19 C.
Practice. Atoms are the foundation of chemistry. They are the basis for everything in the Universe. In this second unit of class 11 chemistry, we will learn the discovery of subatomic particles- electrons, neutrons, & protons, atomic models of J.J Thomson's, Rutherford's, Bohr's. We will learn the dual nature of light and electron, quantum ...
The Important Questions For Class 11 Chemistry Chapter 2 are provided to the students so that they can have some help when it comes to the preparation for their examinations in the best way. The Class 11 Chemistry Chapter 2 important questions deals with the questions in the chapter Structure of Atom and hence is a really good addition to the syllabus for the students of class 11.
Free NCERT Solutions for Class 11 Chemistry Chapter 2 Structure of Atom solved by expert teachers from latest edition books and as per NCERT (CBSE) guidelines.Class 11 Chemistry Structure of Atom NCERT Solutions and Extra Questions with Solutions to help you to revise complete Syllabus and Score More marks.
Structure of Atom Class 11 Notes - This topic talks about atoms. In addition, it also talks about electron, neutron, and protons. Furthermore, the who and how these particles are discovered by the scientists. Moreover, various principles, functions, and structures are also discussed in the chapter. Most noteworthy, the chapter talks about sub ...
These Revision Notes for Class 11 Chemistry Chapter 2 Structure of Atom are available in a PDF format and can be downloaded for free. Structure of atoms is the important chapter for class 11. It has covered the base for class 12 chemistry and many competitive exams. We covered several fundamental chemistry concepts in the previous chapter, but ...
Structure of Atom Class 11 Notes Chemistry Chapter 2 • Discovery of Electron—Discharge Tube Experiment In 1879, William Crooks studied the conduction of electricity through gases at low pressure. He performed the experiment in a discharge tube which is a cylindrical hard glass tube about 60 cm in length. It is sealed at both the […]
Chemistry Worksheet Class 11 on Chapter 2 Structure of Atom with Answers - Set 4. The atomic number (Z) is the number of protons in the nucleus; the atomic number defines the element. N represents the number of neutrons in the nucleus. The nucleus' mass number (A) equals Z + N. The mass number of the nucleus is usually slightly different from ...
Learn. Quantum Wavefunction. The quantum mechanical model of the atom. Quantum numbers. Quantum numbers for the first four shells. Shells, subshells, and orbitals. The periodic table, electron shells, and orbitals.
The ionization enthalpy of hydrogen atom is 1.312 × 106 J mol-1. The energy required to excite the electron in the atom from n = 1 to n = 2 is. (a) 8.51 × 10 5 Jmol -1. (b) 6.56 × 10 5 Jmol -1. (c) 7.56 × 10 5 Jmol -1. (d) 9.84 × 10 5 Jmol -1 /sup>. Answer. Please refer to Assignments Class 11 Chemistry Structure of Atom Chapter 2 with ...
CBSE Case Study Questions Class 11 Chemistry Structure of Atom Case - I. The atomic theory of matter was first proposed on afirm scientific basis by JohnDalton, a British schoolteacher in 1808. His theory, called Dalton's atomictheory, regarded the atom as the ultimate particle ofmatter Dalton's atomic theory was able to explainthe law of ...
Q.1. Number of protons, neutrons and electrons in the element 89X231 is(a) 89, 89, 242 (b) 89, 142, 89(c) 89, 71, 89 (d) 89, 231, 89 Answer (b) Number of p = number of e- = 89 and neutrons 231 - 89 = 142. Q.2. Atoms with same mass number but different atomic numbers are … Continue reading Chemistry MCQs for Class 11 Chapter 2 Structure of Atom
An, atomic orbital, on the other hand, is a quantum mechanical concept and refers to the one, electron wave function ψ in an atom. It is characterized by three quantum numbers (n, l and, ml) and its value depends upon the coordinates of the electron. ψ has, by itself, no physical, 2, 2, meaning.
1) Space around the nucleus where the probability of finding the electron is maximum. 2)Two orbits in an atom cannot have the same energy. 2) Two orbitals can have the same energy. 3) Have two dimensional representation. 3) Have three dimensional representation. 4) Circular or elliptical in shape.