myCBSEguide
- Case Study Questions Class...

Case Study Questions Class 10 Science
Table of Contents
myCBSEguide App
Download the app to get CBSE Sample Papers 2023-24, NCERT Solutions (Revised), Most Important Questions, Previous Year Question Bank, Mock Tests, and Detailed Notes.
Download Case study questions for CBSE class 10 Science in PDF format from the myCBSEguide App . We have the new pattern case study-based questions for free download. Class 10 Science case study questions
This article will guide you through:
What are case study questions?
- Sample Papers with Case Study questions
- Class 10 Science Case Study question examples
- How to get case-based questions for free?
- How to attempt the case-based questions in Science?
Questions based on case studies are some real-life examples. The questions are asked based on a given paragraph i.e. Case Study. Usually, 4-5 questions are asked on the basis of the given passage. In most cases, these are either MCQs or assertion & reason type questions. Let’s take an example to understand. There is one paragraph on how nitrogen is generated in the atmosphere. On the basis of this paragraph, the board asks a few objective-type questions. In other words, it is very similar to the unseen passages given in language papers. But the real cases may be different. So, read this article till the end to understand it thoroughly.
What is CBE?
CBSE stands for competency-based education. The case study questions are part of this CBE. The purpose of CBE is to demonstrate the learning outcomes and attain proficiency in particular competencies.
Questions on Real-life Situations
As discussed the case study questions are based on real-life situations. Especially for grade 10 science, it is very essential to have the practical knowledge to solve such questions. Here on the myCBSEguide app, we have given many such case study paragraphs that are directly related to real-life implications of the knowledge.
Sample Papers with Case Study Questions
Class 10 Science Sample Papers with case study questions are available in the myCBSEguide App . There are 4 such questions (Q.No.17 to 20) in the CBSE model question paper. If you analyze the format, you will find that the MCQs are very easy to answer. So, we suggest you, read the given paragraph carefully and then start answering the questions. In some cases, you will find that the question is not asked directly from the passage but is based on the concept that is discussed there. That’s why it is very much important to understand the background of the case study paragraph.
CBSE Case Study Sample Papers
You can download CBSE case study sample papers from the myCBSEguide App or Student Dashboard. Here is the direct link to access it.
Case Study Question Bank
As we mentioned that case study questions are coming in your exams for the last few years. You can get them in all previous year question papers issued by CBSE for class 1o Science. Here is the direct link to get them too.
Class 10 Science Case Study Question Examples
As you have already gone through the four questions provided in the CBSE model question paper , we are proving you with other examples of the case-based questions in the CBSE class 10 Science. If you wish to get similar questions, you can download the myCBSEguide App and access the Sample question papers with case study-type questions.
Case-based Question -1
Read the following and answer any four questions: Salt of a strong acid and strong base is neutral with a pH value of 7. NaCl common salt is formed by a combination of hydrochloride and sodium hydroxide solution. This is the salt that is used in food. Some salt is called rock salt bed of rack salt was formed when seas of bygone ages dried up. The common salt thus obtained is an important raw material for various materials of daily use, such as sodium hydroxide, baking soda, washing soda, and bleaching powder.
- Phosphoric acid
- Carbonic acid
- Hydrochloric acid
- Sulphuric acid
- Blue vitriol
- Washing soda
- Baking soda
- Bleaching powder
Case-based Question -2
- V 1 + V 2 + V 3
- V 1 – V 2 +V 2
- None of these
- same at every point of the circuit
- different at every point of the circuit
- can not be determined
- 20 3 Ω 203Ω
- 15 2 Ω 152Ω
Case-based Question -3
- pure strips
- impure copper
- refined copper
- none of these
- insoluble impurities
- soluble impurities
- impure metal
- bottom of cathode
- bottom of anode
How to Attempt the Case-Based Questions in Science?
Before answering this question, let’s read the text given in question number 17 of the CBSE Model Question Paper.
All living cells require energy for various activities. This energy is available by the breakdown of simple carbohydrates either using oxygen or without using oxygen.
See, there are only two sentences and CBSE is asking you 5 questions based on these two sentences. Now let’s check the first questions given there.
Energy in the case of higher plants and animals is obtained by a) Breathing b) Tissue respiration c) Organ respiration d) Digestion of food
Now let us know if you can relate the question to the paragraph directly. The two sentences are about energy and how it is obtained. But neither the question nor the options have any similar text in the paragraph.
So the conclusion is, in most cases, you will not get direct answers from the passage. You will get only an idea about the concept. If you know it, you can answer it but reading the paragraph even 100 times is not going to help you.
Test Generator
Create question paper PDF and online tests with your own name & logo in minutes.
Question Bank, Mock Tests, Exam Papers, NCERT Solutions, Sample Papers, Notes
Related Posts
- CBSE Practice Papers 2023
- Class 10 Science Sample Papers 2024
- Competency Based Learning in CBSE Schools
- Class 11 Physical Education Case Study Questions
- Class 11 Sociology Case Study Questions
- Class 12 Applied Mathematics Case Study Questions
- Class 11 Applied Mathematics Case Study Questions
- Class 11 Mathematics Case Study Questions
2 thoughts on “Case Study Questions Class 10 Science”
Where is the answer
Class 10 Science MCQ
Leave a Comment
Save my name, email, and website in this browser for the next time I comment.
Talk to our experts
1800-120-456-456
- CBSE Class 10 Science Term 2 Question Paper 2022 with Solution
- Previous Year Question Paper

CBSE Class 10 Science Term 2 Question Paper 2022|Download Free PDF with Solution
Class 10 Science is a fundamental subject in the CBSE Syllabus . This Class 10 Science Question Paper covers all the fundamental chapters that develop the conceptual foundation of the students. They study these chapters and learn the fundamental concepts well and prepare for the upcoming board exam.
To prepare for the board exam, it is ideal to practising solving the CBSE Class 10 Science Question Paper 2022. The experts of Vedantu have designed the solutions for the previous year’s Science question paper following the CBSE guidelines.

Access CBSE Class 10 Science Question Paper with Solution - Term 2
1. (a) Write the molecular formula of the following carbon compounds:
(i) Methane
(ii) Propane
(a) (i) Methane: $C{{H}_{4}}$
(ii) Propane: ${{C}_{3}}{{H}_{8}}$
(b) Carbon compounds have low melting and boiling points. Why? (2 Marks )
Ans: Carbon compounds usually have low melting points and boiling points because they are covalent in nature.
2. The electrons in the atoms of two elements X and Y are distributed in three shells having 1 and 7 electrons respectively in their outermost shells.
(a) Write the group numbers of these elements in the Modern Periodic Table.
Ans: The group number for element X is 1 and the group number for element Y is 17.
(b) Write the molecular formula of the compound formed when X and Y combine with each other.
Ans: The molecular formula of the compound formed when X and Y combine with each other is XY. We know that the 1st element is hydrogen and the 17th element is chlorine, sothe compound formed by XY is HCL.
(c) Which of the two is electropositive? (2 Marks)
Ans: Element X is an electropositive element.
3. (a) Which of the following flowers will have a higher possibility of self-pollination?
Mustard, Papaya, Watermelon, Hibiscus
Ans: a) Mustard will undergo self-pollination in the same process.
(b) List the two reproductive parts of a bisexual flower. (2 Marks)
Ans: The two reproductive parts of bisexual flower are: Stamen (male part) .It consists of anther and filament.The female part isthmus pistol .The pistol has 3 parts stigma ,style and ovary.
4. Which one of the two multicellular organisms Spirogyra and Planaria reproduces by regeneration and why? Give an example of any other organism which can also reproduce by the same process.
Ans: Some simple multicellular organisms like Planaria can be cut into pieces, and each piece can regenerate into a complete organism. Some complex multicellular organisms also undergo regeneration but not to reproduce. They can only regenerate parts like eyes and limbs.
5. (a) What is variation? List two main reasons that may lead to variation in a population. (2 Marks)
Ans: Any difference between cells, individual organisms, or groups of organisms of any species caused either by genetic differences.
The two main reasons that may lead to variation in a population are:
Variations occur as a result of recombination and mutation.
Variations enable organisms to adapt to environmental conditions.
(b) (i) In a cross between violet flowered plants and white flowered plants, state the characteristics of the plants obtained in the F2 progeny.
Ans: In a cross between plants with violet flowers and plants with white flowers the offspring of F1 generation all had violet flowers. As we know that violet flower is dominant and the white flower is recessive so in F1 generation only violet flowers can be seen but when we cross the two of springs of the F1 generation then the new of spring which is formed in F2 generation will be of both violet and white colours.
(ii) If the plants of F1 progeny are self-pollinated, then what would be observed in the plants of F2 progeny?
Ans: If plants of F1 progeny are self-pollinated then violet flowered plants and white flowered plants will have obtained in F2 progeny in ratio 3:1.
(iii) If 100 plants are produced in F2 progeny, then how many plants will show the recessive trait? ( 2 Marks)
Ans: If 100 plants are obtained in F2 progeny then 25 plants will show the recessive trait. It means that input generation out of hundred 25 plants would the of white colour.
6. (a) (i) Name and state the rule to determine the direction of force experienced by a current carrying straight conductor placed in a uniform magnetic field which is perpendicular to it.
Ans: Fleming’s Left-Hand rule gives the direction of force experienced by a current-carrying straight conductor placed in a magnetic field which is perpendicular to it. Fleming's Right-Hand rule gives the direction of current induced in a coil due to its rotation in a magnetic field.
(ii) An alpha particle while passing through a magnetic field gets projected towards north. In which direction will an electron project when it passes through the same magnetic field? (2 Marks)
Ans: Here the alpha particle projected towards the north i.e. current is towards the north (middle finger). Particles deflect towards east i.e. direction of force is towards the east (thumb). So the direction of a magnetic field will be in the upward direction (forefinger).
(b) (i) What is a solenoid?
Ans: A solenoid is a coil of wire tied across a piston that is made of iron, in the form of a corkscrew. According to the laws of electromagnetism, when an electric current passes via the wire a magnetic field is generated.
(ii) Draw the pattern of magnetic field lines of the magnetic field produced by a solenoid through which a steady current flows. (2 Marks)
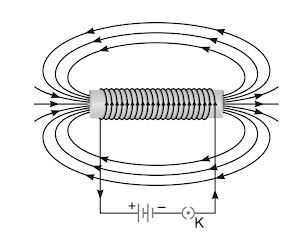
Ans: A solenoid acts as a magnet when current passes through it as it has two runs and from the positive side the electric field lines are generated and on the other side they emerge. so they form an electric field around the solenoid.
7. (a) What is ozone? How is it formed in the upper layers of the Earth's atmosphere? How does ozone affect our ecosystem? (2 Marks)
Ans: Ozone is a molecule containing three atoms of oxygen ${{O}_{3}}$ a highly poisonous gas present in the upper layer of the atmosphere.
Formation of Ozone: The UV radiations split molecular oxygen ${{O}_{2}}$ apart into free oxygen atoms O + O. These atoms then combine with molecular oxygen to form ozone. ${{O}_{2}}$ → O + O O + O 2 → ${{O}_{3}}$
Effect: Ozone layer shields the surface of the earth from damaging UV radiations of the sun.
OR
(b) (i) List two human-made ecosystems. (1 Marks)
Ans: The two human-made ecosystems are:
i) Gardens
(ii) "we do not clean the pond in the same manner as we do in an statement
aquarium. " give reason to justify this statement.
Ans: Ponds and lakes are natural habitats. They contain various decomposers that act as cleansing agents. In contrast, the aquarium is an artificial habitat. No such decomposers are present in it. Hence, it needs to be cleaned .
SECTION B
8. (a) List two advantages of adopting the atomic number of an element as the basis of classification of elements in the Modern Periodic Table.
Ans: In Modern periodic table noble gases in a separate group named as group-18. In this table transition elements are placed in separate blocks.
(b) Write the electronic configurations of the elements X (atomic number 13) and Y (atomic number 20). (3 Marks)
Ans: The electronic configurations of the elements X (atomic number 13) and Y (atomic number 20) are:
Y - 2,8,8,2
9. (a) Draw two different possible structures of a saturated hydrocarbon having four carbon atoms in its molecule. What are these two structures of the hydrocarbon having the same molecular formula called? Write the molecular formula and the common name of this compound. Also write the molecular formula of its alkyne. (3 Marks)
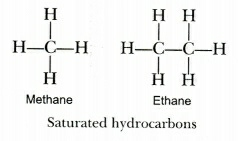
ii) They are called isomers
iii) molecular formula
a) $C{{H}_{4}}$
b) ${{C}_{2}}{{H}_{6}}$
Common name & alkyne formula
a) methane, $C{{H}_{2}}$
b) ethane, ${{C}_{2}}{{H}_{4}}$
(b) (i) Write the molecular formula of benzene and draw its structure.
Ans: Molecular formula of benzene is ${{C}_{6}}{{H}_{6}}$.
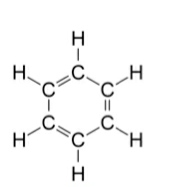
(ii) Write the number of single and double covalent bonds present in a molecule of benzene.
Ans: ${{C}_{6}}{{H}_{6}}$ has c-c single and double bonds with 9 single bonds and 3 double bonds.
(iii) Which compounds are called alkynes? (3 Marks)
Ans: Compound have one or more carbon-carbon triple bonds.
10. (a) Mention one function each of the following organs in human male
reproductive system:
(i) Testis
(ii) Scrotum
(iii) Vas deferens
(iv) Prostate gland
i) To produce sperm and to control secondary sexual character in male.Testis not only produces sperm but also produces the male androgen hormone which is essential for the development of male.
ii) lt acts as a bag to hold the testis and it maintains a cooler temperature for sperm production.(2-2.5°) lower than the body temperature.
iii) It is a conducting tube which moves sperm out of the testis. After the vas deferens, it goes into the ejaculatory duct.
iv) prostate gland provides nourishment and mobility to sperms.
(b) Name the type of germ cell which (i) is motile, and (ii) stores food. (3 Marks)
i) The male gamete is the motile germ cell that is Sperm
ii) The female gamete is the germ cell that contains the stored food that is Ovum.
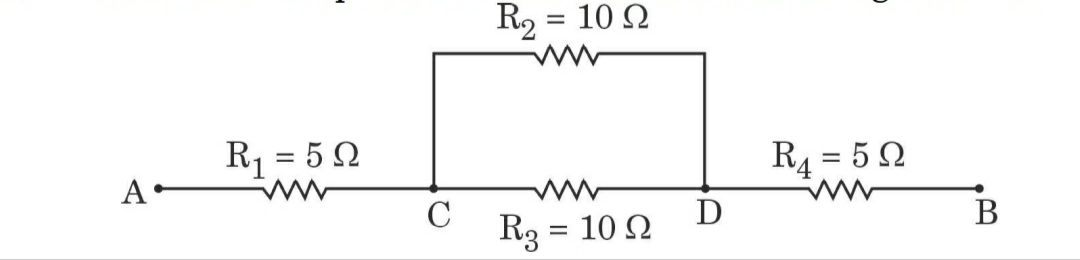
11. (a) Three resistors R 1 , R 2 and R 3 are connected in parallel and the combination is connected to a battery, an ammeter, a voltmeter and a key. Draw a suitable circuit diagram to show the arrangement of these circuit components along with the direction of current flowing.
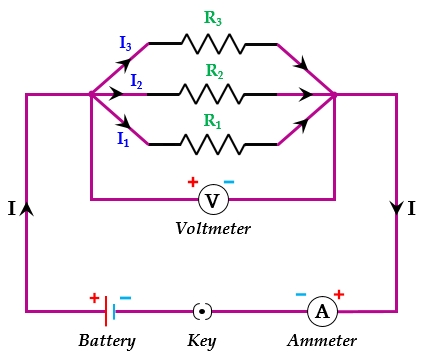
(b) Calculate the equivalent resistance of the following network:
12. (a) (i) Define Electric Power and write its SI unit. (2 Marks)
Ans: Electric power is the rate at which electrical energy is transferred by an electric circuit. The SI unit of power is the watt, one joule per second.
(ii) Two bulbs rated 100 W; 220 V and 60 W; 220 V are connected in parallel to an electric main of 220 V. Find the current drawn by the bulbs from the mains. (2 Marks)
(b) (i) state joule's law of heating. Express it mathematically when an appliance of resistance R is connected to a source of voltage V and the current I flow through the appliance for a time t.
Ans: Joule’s law of heating states that the power of heating generated by an electrical conductor is proportional to the product of its resistance and the square of the electric current passing through the conductor. It Express mathematically as: ${{I}^{2}}RT$
(ii) A 5-ohm resistor is connected across a battery of 6 volts. Calculate the energy that dissipates as heat in 10 s. (3 Marks)
Ans: Using Ohm's law
$I=\frac{V}{R}$
$I=\frac{6}{5}$
$=1.2$Ampere
Heat produced-${{I}^{2}}RT$
$\Rightarrow 1.2\times 1.2\times 5\times 10$
$=72$ joules
13. (a) Name the group of organisms which form in the first trophic level of all food chains. Why are they called so?
Ans: The group of organisms which form in the first trophic level are producers.
They called producers because they make their own food .
(b) Why are human beings most adversely affected by biomagnification?
Ans: Human beings occupy the top place of most trophic level and we know that concentration of harmful chemicals increase as we go above the trophic level. Therefore, human beings are most adversely affected by biological magnification.
(c) State one ill-effect of the absence of decomposers from a natural ecosystem. (3 Marks)
Ans: In the absence of decomposers recycling of material in the biosphere will not take place which would lead to the accumulation of dead plants and animals in the environment .
SECTION C
This section has 2 case-based questions (14 and 15). Each case is followed by 3 sub-questions (a), (b) and (c). Parts (a) and (b) are compulsory. However, an internal choice has been provided in Part (c).
14. The mechanism by which the sex of an individual is determined is called sex-determination. In human beings, sex of a newborn is genetically determined, whereas in some others it is not. There are 46 (23 pairs) chromosomes in human beings. Out of these, 44 (22 pairs) control the body characters and 2 (one pair) are known as sex chromosomes. The sex chromosomes are of two types X chromosome and Y chromosome. At the time of fertilisation, depending upon which type of male gamete fuses with the female gamete, the sex of the newborn child is decided.
(a) Why is a pair of sex chromosomes in human beings called a mismatched pair in terms of type and size? (2 Marks)
Ans: A pair sex chromosome can have two same types of chromosomes (homogamety) or two different types of chromosomes (heterogamety). Humans show male heterogamety, meaning they have two mismatched chromosomes as a pair. The other 22 pairs of chromosomes are the same for all, male and female.
(b) Out of male or female, which of them has a perfect pair of sex chromosomes? In case of a perfect pair, will the gametes produced be of the same kind or of a different kind?
Ans: Girls only. Humans have 23 pairs of chromosomes out of which 22 pairs of chromosomes are called autosomal chromosomes. In the cell nucleus of human beings, there are 46 chromosomes in total (i.e.) 23 pairs of chromosomes.
(c) (i) Name two animals whose sex is not genetically determined. Explain the process of their sex determination.
Ans: Examples of organisms in which sex is not genetically determined are crocodiles, alligators, turtles, tuatara et cetera.
In these organisms, sex is determined by environmental factors. For example, in crocodiles, if the egg is incubated at around 30 °C, it leads to the development of the female whereas if the egg is incubated at around 34 °C, it results in the development of the male.
(ii) With the help of a flowchart only, show how sex is genetically determined in human beings.
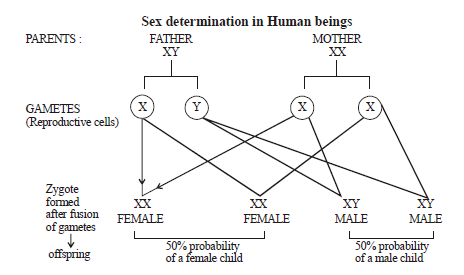
Ans: Humans have 46 chromosomes present in pairs. In male there are 22 autosomes and one pair is sex chromosome XY .In females there are 22 autosomes and one pair is sex chromosome XX. So sex of a human baby is determined by male , not by the female.
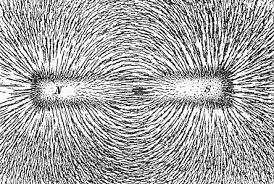
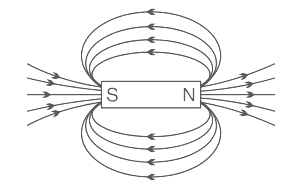
15. A student fixes a sheet of white paper on a drawing board using some adhesive materials. She places a bar magnet in the centre of it and sprinkles some iron filings uniformly around the bar magnet using a salt-sprinkler. On tapping the board gently, she observes that the iron filings have arranged themselves in a particular pattern.
(a) Draw a diagram to show this pattern of iron filings. (1 Marks)
(b) Draw the magnetic field lines of a bar magnet showing the poles of the bar magnet as well as the direction of the magnetic field lines. (1 Marks)
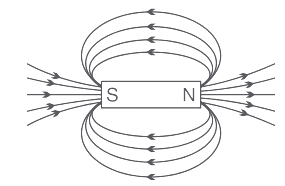
Ans: The magnetic field arise from the north pole and emerges into the South Pole of the magnet.
(c) (i) How is the direction of the magnetic field at a point determined using the field lines? Why do two magnetic field lines not cross each other? (2 Marks)
Ans: The direction of a magnetic field at a point is determined by placing a small compass needle. The N - pole of the compass indicates the direction of the magnetic field at that point.
Two magnetic field lines do not cross each other because there will be two directions of the field at the same point.
(ii) How are the magnetic field lines of a bar magnet drawn using a small compass needle? Draw one magnetic field line each on both sides of the magnet.
Ans: If you place a compass near the north pole of a magnet, the north pole of the compass needle will be repelled and point away from the magnet. Thus, the magnetic field lines point away from the north pole of a magnet and toward its south pole .
Importance of CBSE Previous Year Question Papers Class 10 Science
Class 10 Science is an important subject for engineering and medical aspirants. This subject also holds the key to a good score in the CBSE Board exam. Hence it is a crucial subject to prepare and score well.
CBSE Class 10 Science Syllabus covers the fundamental chapters based on Physics, Chemistry, and Biology. The chapters are chosen following a pattern to develop a conceptual foundation among the students. They will find it easier to understand the chapters after what they studied in the previous classes.
These chapters are included to ensure a strong base of fundamental principles is developed within the students so that they can find studying advanced chapters in the higher classes easier to comprehend.
Hence, solving the previous year’s CBSE Class 10 Science Term 2 paper is important. The questions from the important chapters are covered in this paper. Students will use this exam paper as a reference to practise answering science questions.
By referring to the solutions, they can figure out which chapter to focus more and make their preparation better.
Benefits of CBSE Class 10 Science Question Paper 2022 with Answers
The exclusive benefits of solving the CBSE Class 10 Science Term 2 2022 question paper with solution are:
Get to know your preparation level using this 2022 Term 2 paper as an evaluation tool. Check your answers and find out which chapters need more attention.
Find out how the experts have formulated the answers by following the CBSE guidelines. Follow the answering format given in the solutions to sharpen your answering skills.
Practising science questions will also make you more efficient in saving time and scoring more in the board exam.
Get to know the questions asked from the fundamental chapters of CBSE Class 10 Science. Prepare those chapters by learning the marking scheme by solving these papers.
Develop your strategy to answer the CBSE Science exam paper and learn how to use your time to score better.
Download Class 10 Science Board Question Paper 2022 with Solutions Free PDF
Perform the CBSE Class 10 Science Question Paper 2022 PDF download and complete your study material for CBSE Class 10 Science. Prepare the chapters well and practise solving this question paper. Enjoy the benefits mentioned above and make your preparation stronger for this subject.

FAQs on CBSE Class 10 Science Term 2 Question Paper 2022 with Solution
1. What is the reason for following CBSE Class 10 Science syllabus?
By following the CBSE Class 10 Science syllabus, you can easily understand the score weightage of the chapters included. In this way, you can divide your preparation time more efficiently.
2. Does solving CBSE Class 10 Science Board Exam papers make you better?
You can use the previous year’s board exam paper for Class 10 Science as an evaluation tool. You can use it to find out your preparation level and can focus on specific chapters more.
3. Why should you follow the solutions framed by Vedantu experts in the Class 10 Science 2022 Question Paper?
Following the answering format of the experts will make you better at answering fundamental science questions in the board exam. You will be able to score more.
4. Is the CBSE Class 10 Science 2022 Term 1 exam paper and solution available for free?
Yes. You can download the previous year’s question paper and solutions for free at Vedantu.
- New QB365-SLMS
- NEET Materials
- JEE Materials
- Banking first yr Materials
- TNPSC Materials
- DIPLOMA COURSE Materials
- 5th Standard Materials
- 12th Standard Materials
- 11th Standard Materials
- 10th Standard Materials
- 9th Standard Materials
- 8th Standard Materials
- 7th Standard Materials
- 6th Standard Materials
- 12th Standard CBSE Materials
- 11th Standard CBSE Materials
- 10th Standard CBSE Materials
- 9th Standard CBSE Materials
- 8th Standard CBSE Materials
- 7th Standard CBSE Materials
- 6th Standard CBSE Materials
- Tamilnadu Stateboard
- Scholarship Exams
- Scholarships

CBSE 10th Standard Science Subject Case Study Questions
By QB365 on 21 May, 2021
QB365 Provides the updated CASE Study Questions for Class 10 , and also provide the detail solution for each and every case study questions . Case study questions are latest updated question pattern from NCERT, QB365 will helps to get more marks in Exams
QB365 - Question Bank Software
10th Standard CBSE
Final Semester - June 2015
Redox reactions are those reactions in which oxidation and reduction occur Simultaneously. A redox reaction is made up of two half reactions. In the first half reaction, oxidation takes place and in second half reaction, reduction occurs. Oxidation is a process in which a substance loses electrons and in reduction, a substance gains electrons. The substance which gains electrons is reduced and acts as an oxidising agent. On the other hand, a substance which loses electrons is oxidised and acts as a reducing agent. (i) Which of the following is a redox reaction?
(ii) Identify the reaction in which H2 02 is acting as a reducing agent.
(iii) For the following reactions, identify the one in which H 2 S acts as a reducing agent.
(iv) For the following reaction, identify the correct statement. \(\mathrm{ZnO}+\mathrm{CO} \longrightarrow \mathrm{Zn}+\mathrm{CO}_{2}\)
(v) In the following reaction, which substance is reduced? \(\mathrm{PbS}+4 \mathrm{H}_{2} \mathrm{O}_{2} \longrightarrow \mathrm{PbSO}_{4}+4 \mathrm{H}_{2} \mathrm{O}\)
(ii) If the pH of a solution is 8, then its [H + ] ion is
(iii) In terms of acidic strength, which one of the following is in the correct increasing order?
(iv) Which of the following compounds does not give H + ions in aqueous solution?
(v) Four solutions labelled as P, Q, Rand Shave pH values 1, 9, 3 and 13 respectively. Which of the following statements about the given solutions is incorrect?
Baking powder produces carbon dioxide on heating, so it is used in cooking to make the batter spongy. Although, baking soda also produces CO 2 on heating, but it is not used in cooking because on heating, baking soda produces sodium carbonate along with carbon dioxide. Sodium carbonate, thus, produced, makes the taste bitter. Baking powder is the mixture of baking soda and a mild edible acid. Generally, tartaric acid is mixed with baking soda to make baking powder. When baking powder is heated, NaHCO 3 decomposes to give CO 2 which makes bread and cake fluffy. Tartaric acid helps to remove bitter taste due to formation of sodium tartrate. \(2 \mathrm{NaHCO}_{3}+ \ \ \mathrm{C}_{4} \mathrm{H}_{6} \mathrm{O}_{6} \quad \longrightarrow \quad 2 \mathrm{CO}_{2}+2 \mathrm{H}_{2} \mathrm{O}+\mathrm{Na}_{2} \mathrm{C}_{4} \mathrm{H}_{4} \mathrm{O}_{6}\) Baking soda Tartaric acid Carbon dioxide Sodium tartrate (i) On passing excess CO 2 gas in aqueous solution of sodium carbonate, the substance obtained is
(ii) When sodium hydrogen carbonate is added to acetic acid, it evolves a gas. Which of the following statements are true about the gas evolved? (I) It turns lime water milky (II) It extinguishes a burning splinter (III) It dissolves in a solution of sodium hydroxide (IV) It has a pungent odour
(iii) Select the correct statement regarding sodium hydrogen carbonate.
(iv) Acetic acid was added to a solid X kept in a test tube. A colourless and odourless gas was evolved. The gas was passed through lime water which turned milky. It was concluded that
(v) Which of the following statements are correct regarding baking soda? (I) Baking soda is sodium hydrogen carbonate (II) On heating, baking soda gives sodium carbonate (III) It is used for manufacture of soap (IV) It is an ingredient of baking powder
The chemical reactivity of an element depends upon its electronic configuration. All elements having less than eight electrons in the outermost shell show chemical reactivity. During chemical reactions, atoms of all elements tend to achieve a completely filled valence shell. Metals are electropositive in nature. They have tendency to lose one or more electrons present in the valence shell of their atoms to form cations and achieve nearest noble gas configuration. The compounds formed by the transfer of electrons from one element to other are known as ionic or electrovalent compounds. (i) The electronic configurations of three elements X, Y and Z are: X : 2 Y: 2, 8, 7 Z : 2, 8, 2 Which of the following is correct regarding these elements?
(ii) Element X reacts with element Y to form a compound Z. During the formation of compound Z, atoms of X lose one electron each whereas atoms of Y gain one electron each. Which of the following properties is not shown by compound Z?
(iii) Which of the following is correct representation of formation of magnesium chloride?
(iv) The electronic configuration of sodium ion is
(v)Which of the following represents an electropositive element?
A hydrocarbon (P) has the molecular formula C 10 H 22 .A hydrocarbon (Q) has two carbon atoms less than (P) and belong to the same homologous series. A hydrocarbon (R) has two carbon atoms more than (P) and belong to the same homologous series. (i) What is the molecular formula of (Q) ?
(ii) To which homologous series do the compound (P), (Q) and (R) belong?
(iii) What is the molecular formula of (R) ?
(iv) Identify the correct statement about compounds (P), (Q) and (R) .
(v) Compounds (P), (Q) and (R) are
The recurrence of properties of the elements after a certain regular intervals, when they are arranged in the increasing order of their atomic numbers, is called periodicity. There are a number of physical properties such as atomic size, metallic and non -metallic character, etc. which show periodic variation. In periodic table, various properties vary differently from moving left to right in a period and going down in a group. In a period, properties vary because from moving left to right in a period, number of shells remain same but valence electron increases by one number hence nuclear charge increases. In a group, on going down, number of valence shells increases while number of valence electrons remains same. (i) From top to bottom in a group of the periodic table, the electropositive character of the element
(ii) Which element has the largest size in the second period?
(iii) Which of the following elements has three valence electrons?
(iv) In the periodic table, the metallic character of elements (a) decreases from left to right and decreases down the group (b) decreases from left to right and increases down the group (c) increases from left to right and increases down the group (d) increases from left to right and decreases down the group (v) Which of the following increases along the period?
The small intestine is the longest part of the alimentary canal. It is a narrow tube of about 6 metres which lies coiled in the abdomen. The length of small intestine varies in different animals depending on the type of food they eat. (i) Humans are not able to digest cellulose whereas they are able to digest starch due to
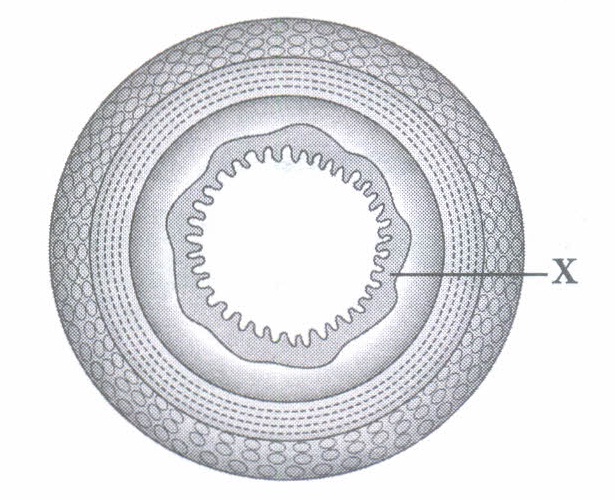
(iii) Butter cannot be digested in the stomach as lipase and bile are(a) released in small intestine
(iv) Which of the following is a correct statement? (a) Herbivores have shorter small intestine as they eat grasses (b) Carnivores have larger small intestine as they eat meat (c) Herbivores have larger small intestine as they eat grasses (d) None of these (v) Various types of movements are generated by the ______ layer of the small intestine.
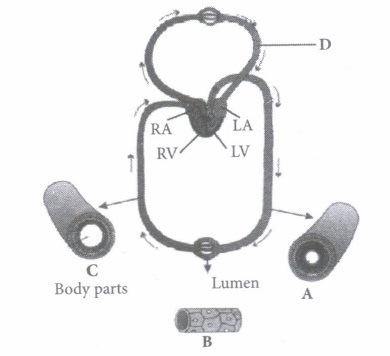
(iii) Which of the following animals shows double circulatory pathway?
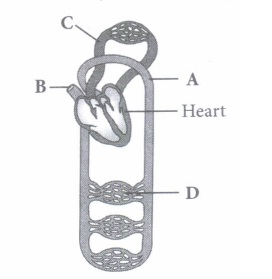
(v) Select the option which properly represents pulmonary circulation in humans. \(\text { (a) Left auricle } \frac{\text { Deoxygenated }}{\text { blood }}{\longrightarrow} \text { Lungs } \frac{\text { Oxygenated }}{\text { blood }} \text { Right ventricle }\) \(\text { (b) Left auricle } \frac{\text { Oxygenated }}{\text { blood }}{\longrightarrow} \text { Lungs } \frac{\text { Deoxygenated }}{\text { blood }}{\longrightarrow} \text { Right ventricle }\) \(\text { (c) Right ventricle } \frac{\text { Deoxygenated }}{\text { blood }}{\longrightarrow} \text { Lungs } \frac{\text { Oxygenated }}{\text { blood }} \rightarrow \text { Left auricle }\) \(\text { (d) Right ventricle } \frac{\text { Oxygenated }}{\text { blood }}>\text { Lungs } \frac{\text { Deoxygenated }}{\text { blood }} \gg \text { Left auricle }\)
Spore formation, method of asexual reproduction is used by unicellular as well as multicellular organisms.Spores are microscopic units which could be air borne or are present in soil, etc. (i) A slice of bread kept in open for sometime shows growing white cottony mass which later turns black. This happens because (a) bacterial spores present in air germinate on the surface of bread slice (b) fungal spores present in air germinate on the surface of bread slice (c) protozoan microbes start feeding on bread slice (d) none ef these. (ii) Spore formation can be seen in
(iii) Bulb like structure at top of erect hyphae where spores are produced is
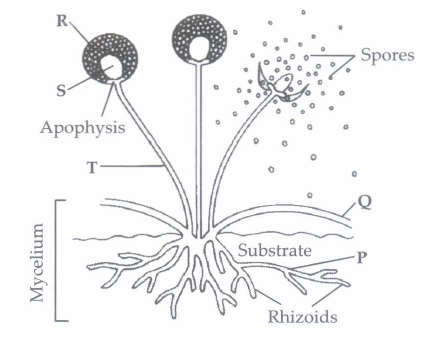
Gregor Mendel conducted hybridisation experiments on garden peas for seven years and proposed the laws of inheritance in living organisms. He investigated characters in the garden pea plant that were manifested as two opposing traits, e.g., tall or dwarf plants, yellow and green seeds, etc. (i) Among the seven pairs of contrasting traits in pea plant as studied by Mendel, the number of traits related to flower, pod and seed respectively were
(ii) The colour based contrasting traits in seven contrasting pairs, studied by Mendel in pea plant were
(iii) Refer to the given table of contrasting traits in pea plants studied by Mendel.
Which of the given traits is correctly placed? (a) (i), (ii) and (iii) only (b) (ii), (iii) and (iv) only (c) (ii) and (iii) only (d) (i), (ii), (iii) and (iv) (iv) Some of the dominant traits studied by Mendel were (a) round seed shape, green seed colour and axial flower position (b) terminal flower position, green pod colour and inflated pod shape (c) violet flower colour, green pod colour and round seed shape (d) wrinkled seed shape, yellow pod colour and axial flower position. (v) Which of the following characters was not chosen by Mendel?
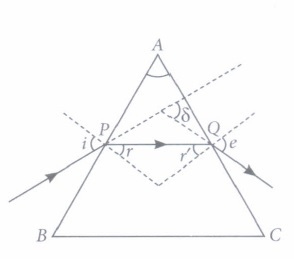
(ii) The angle between the incident ray and the emergent ray is called
(iii) When a ray is refracted through a prism, then
(iv) The angle of deviation depends on
(v) The rectangular surfaces of a prism are known as
Some harmful non-biodegradable chemicals, i.e., pesticides (e.g., DDT) and heavy metals (e.g., mercury, arsenic cadmium, etc.) enter the bodies of organism through the food chain and go on concentrating at each trophic level. This phenomenon is called bio-magnification or biological magnification. (i) Refer to the given food chain Phytoplankton \(\longrightarrow\) Zooplankton \(\longrightarrow\) Small fish \(\longrightarrow\) Large fish \(\longrightarrow\) Fish eating birds If concentration of DDT in small fish is estimated to be 0.5 ppm, then amount of DDT in zooplankton and large fish would respectively be
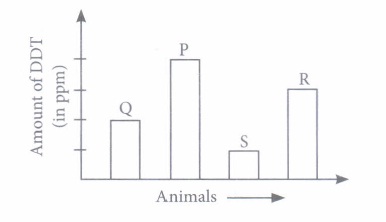
(ii) Refer to the given table.
According to the given data. The correct order in a food chain will be
(iv) Higher amount of DDT disturb calcium metabolism of birds. This results in
(v) When animals are sprayed with poisons, they may die immediately, but their bodies still contain the poison. The poison in their bodies will then be passed on to the animals which eat them. What would be the consequence of a mass poisoning of the rabbit population in a grazing food chain and why? (a) Plants would die quickly as they are eaten by rabbits (b) Grasshopper would die quickly as all the animals in the food web would be affected (c) Western rattlesnakes would quickly become poisoned as they eat rabbits (d) Hawk would become poisoned as they feed on rabbits
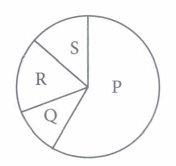
(v) Greenhouse effect is due to
Energy flow is the key function of an ecosystem. It is determined by the two basic laws of thermodynamics. Flow of energy in our ecosystem is unidirectional. Green plants capture approximately about 1% of the solar energy incident on the earth to carry out the process of photosynthesis. In an ecosystem, transfer of energy follows 10 percent law, i.e., only 10% energy is transferred from one trophic level to another and remaining 90% of energy is lost in respiration. (i) Read the given statements and select the incorrect one(s). I. At each trophic level organisms utilise energy in respiration. II. Only 10 percent of the solar radiations that fall on earth is used by green plants. III. Green plants are the ultimate source of entire energy as most of the food chain begin with them. IV. A food chain usually consist of 3-4 trophic levels.
(ii) Refer to the given flow chart. Plants \(\rightarrow\) Rat \(\rightarrow\) Snake 20 units 2 units 0.2 unit The given flow chart states that (a) flow of energy in an ecosystem is unidirectional (b) as we move along in a food chain the number of individuals at each trophic level decreases (c) only 10% of the total energy becomes available to next trophic level (d) both (a) and (c). (iii) Nearly 90% of the energy is wasted while moving from one trophic level to other. This energy is used in
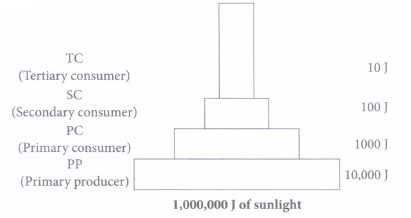
(v) Which of the following correctly states the processes involved in energy transfer between the trophic levels?
*****************************************
Cbse 10th standard science subject case study questions answer keys.
(I) (b) : H 2 is oxidised to HCI while Cl 2 is reduced to HCl. (ii) (c) \((iii) (c): 2 \mathrm{Fe} \mathrm{Cl}_{3}+\mathrm{H}_{2} \mathrm{~S} \longrightarrow 2 \mathrm{FeCl}_{2}+2 \mathrm{HCl}+\mathrm{s}\) H 2 Sitself gets oxidised to Sand reduces FeCl 3 to FeCI 2 (iv) (a ): ZnO is reduced to Zn and CO is oxidised to CO 2 (v) (b) : H 2 O 2 is reduced to water by removal of oxygen.
(i) (c): As the pH value increases from 7 to 14, it represents decrease in H+ ion concentration in the solution. (ii) (c) : pH = -log l0 [H + ] = 8 log l0 [H + ] =-8 [H + ] = 10 - 8 mol/L (iii) (a) (iv) (b): C 2 H 5 OH is not an ionic compound, it is a covalent compound and hence does not give H + ions in aqueous solution. (v) (c) : (a) Lower the pH of the solution, more acidic is the solution and higher is the [H + ] ions Thus, solution P (pH = 1) has higher [H + ] ions than solution R (pH = 3). (b) Higher the pH of the solution, more basic is the solution and higher is the [OH - ] ions Thus, solution Q (pH = 9) has lower [OH - ] ions than solution S (pH = l3). (c) Solution P (pH = 1) is acidic which turns blue litmus solution red whereas solution Q (pH = 9) is basic which turns red litmus solution blue. (d) Solution P (pH = 1) is highly acidic while solution S (pH = l3) is highly basic and solution Q (pH = 9) is weakly basic.
\({ (i) }(\mathrm{b}): \mathrm{Na}_{2} \mathrm{CO}_{3}+\mathrm{H}_{2} \mathrm{O}+\mathrm{CO}_{2} \longrightarrow 2 \mathrm{NaHCO}_{3}\) (ii) (b) : \(\mathrm{NaHCO}_{3}+\mathrm{CH}_{3} \mathrm{COOH} \longrightarrow \mathrm{CH}_{3} \mathrm{COONa}\) \(+\mathrm{CO}_{2} \uparrow+\mathrm{H}_{2} \mathrm{O}\) Carbon dioxide gas is evolved which turns limewater milky. It extinguishes a burning splinter since it is not a supporter of combustion. It dissolves in sodium hydroxide solution and it is an odourless gas. \({ (iii) }(\mathrm{c}): 2 \mathrm{NaHCO}_{3} \stackrel{\text { Heat }}{\longrightarrow} \mathrm{Na}_{2} \mathrm{CO}_{3}+\mathrm{H}_{2} \mathrm{O}+\mathrm{CO}_{2}\) NaHCO 3 is soluble in water. \({ (iv) }(\mathbf{b}): \mathrm{NaHCO}_{3}+\mathrm{CH}_{3} \mathrm{COOH} \longrightarrow\) \(\mathrm{CH}_{3} \mathrm{COONa}+\mathrm{CO}_{2}+\mathrm{H}_{2} \mathrm{O}\) (v) (c): It is not used in manufacture of soap .
(i) (d) (Ii) (b): '2' is an ionic compound \({ (iii) }(\mathrm{a}): \mathrm{Mg} \longrightarrow \mathrm{Mg}^{2+}+2 e^{-}\) \(\mathrm{Cl}+e^{-} \longrightarrow \mathrm{Cl}^{-}\) \(\mathrm{Mg}^{2+}+2 \mathrm{Cl}^{-} \longrightarrow \mathrm{MgCl}_{2}\) 2,8,2 2,8 2,8,7 2,8,8 \((\text { iv })(\mathrm{d}): \mathrm{Na} \longrightarrow \mathrm{Na}^{+}+e^{-}\) 2,8,1 2,8 (v) (c): (a) and (d) represent electronegative elements and (b) represents a noble gas.
(i) (c) : Molecular formula of (Q) is CSH1Sas it has two carbon atoms less than (P). (ii) (c): Compounds (P), (Q) and (R) are alkanes having general formula C n H 2n+2 . (iii) (a): Molecular formula of (R) is C 12 H 26 as it has two carbon atoms more than (P) (iv) (b): Compound (P), (Q) and (R) belong to same homologous series so they have different physical properties but similar chemical properties. They have same general formula C n H 2n+2 .They . differ by 2 carbon atoms and 4 hydrogen atoms. (v) (a)
(i) (a): As the size of the atom increases down the group, electropositive character increases. (ii) (c): Li is the first element of the second period. As the size decreases in the period from left to right, therefore, Li is the largest atom in the period. (iii) (c): Al (Z = 13) : 2, 8, 3 (iv) (b): Metallic character of elements decreases from left to right and increases down the group. (v) (a): As we move from left to right along a period, the number of valence electrons increases from 1 to 8.
(i) (a): In human, cellulose is indigestible as it cannot be broken into smaller molecules due to absence of cellulase enzyme. (ii) (b): Finger-like projections that come out from mucosa of intestine form villi. Cells lining the villi produce numerous microscopic projections called microvilli giving a brush border appearance which increase the surface area for absorption enormously. Villi has a good supply of capillaries and a large lymph vessel for absorption of nutrients. If the inner lining of the small intestine will be smooth, the surface for absorption will be reduced. (iii) (a) (iv) (c) (v) (b)
(i) (c): A- Artery: Carries blood from heart to different body parts. It is thick-walled and elastic. It acts as a "pressure reservoir" for maintaining the blood flow. B - Capillary : Nutrients, hormones, gases, etc. can diffuse into tissue cells through capillaries and vice versa. It is thin-walled, and only one cell layer thick resting on basement membrane. C - Vein: Brings blood from different body parts to the heart. It is thin-walled and act as low-resistance conduct for blood flow. D - Pulmonary vein: Two pulmonary veins from each lung transport the oxygenated blood to the left atrium. (ii) (d): In amphibians, the left atrium receives oxygenated blood from the gills/lungs/skin and the right atrium gets the deoxygenated blood from other body parts. However, they get mixed up in the single ventricle which pumps out mixed blood i.e., incomplete double circulation (iii) (d): Whale is a mammal and in mammals, two separate circulatory pathways are found - systemic circulation and pulmonary circulation. Oxygenated and deoxygenated bloods received by the left and right atria respectively pass on to the left and right ventricles. Thus, oxygenated and deoxygenated bloods are not mixed. This is referred to as double circulation. (iv) (a) (v) (c): Pulmonary circulation is the movement of blood between heart and lungs. During this pathway deoxygenated blood entering the right atrium, moves into the right ventricle. From here it moves through the pulmonary arch into the lungs for oxygenation. Then from lungs the oxygenated blood moves into the left atrium through pulmonary veins.
(i) (b): The tiny spores of bread mould (Rhizopus) are always present in air. On coming in contact with moist surface of bread slice they settle on it and germinate to form new fungal hyphae which first look like white cottony mass and later turns black. (ii) (a): Mucor (fungus) reproduces asexually through spore formation. (iii) (d) (iv) (c) : Bacteria produce endospore which is a dormant and tough structure that enables bacteria to remain dormant for extended periods under unfavorable conditions. (v) (d)
(i) (a) : Characters studied by Mendel are as follows:
(i) (a): The angle between the two refracting surfaces of a prism is called angle of prism. (ii) (b): The angle between the incident ray and the emergent ray is called angle of deviation. (iii) (d): As the ray of light enters from rarer medium (air) to denser medium (glass), the angle of incidence is more than angle of refraction. (iv) (c): More be the refractive index, more be the angle of deviation and it also depends on the refractive index of prism. (v) (c): The refraction of light takes place through rectangular surfaces.
(i) (c): No two magnetic field lines are found to cross each other. If two field lines crossed each other, it would mean that at the point of intersection, the compass needle would point in two directions at the same time, which is not possible. (ii) (d): The magnetic field and hence the magnetic line of force exist in all the planes all around the magnet. (iii) (d): The relative strength of the magnetic field is shown by the degree of closeness of the field lines and the direction of the magnetic field is obtained by tangent to the field lines at the point of intersect. (iv) (d): The magnetic field lines due to a bar magnet are closed continuous curves directed from N to S outside the magnet and directed from S to N inside the magnet. Hence option (d) is correct. v) (d): Inside a bar magnet, the direction of field lines is from south pole to north pole
(i) (a): Due to bio-rnagnification, the concentration of DDT will always be less in zooplanktons than large fish (ii) (c) (iii) (b) : Due to bio-rnagnification the nonbio-degradable chemicals such as DDT accumulate and go on concentrating at each trophic level. (iv) (d) : Higher amounts of DDT disturb calcium metabolism of birds resulting in thinning of egg shells and their prematllre breaking that kills the embryos. (v) (d)
( i) (b) : In the given pie chart, gases P, Q, Rand S respectively are CO 2 , CH 4 , CFCs and N 2 O. Methane is produced by incomplete combustion of biomass. (ii) (c): Methane (gas Q) is produced by incomplete biomass combustion and incomplete decomposition mostly by anaerobic methanogens. Flooded paddy fields, marshes and cattles are the major source of this gas. (iii) (c) : CO 2 is the principal greenhouse gas that helps to keep the earth warm. (iv) (d) (v) (c)
(i) (b): 1% of solar radiation is captured by plants. Sun is the ultimate source of all energy. (ii) (d) (iii) (d) (iv) (d): The given pyramid is pyramid of energy that shows the two basic laws of thermodynamics. (v) (c): Light energy from the sun is converted to chemical energy in producers via photosynthesis. This chemical energy is then transferred to primary consumer, then subsequently to secondary consumer via feeding.
Related 10th Standard CBSE Science Materials
10th standard cbse syllabus & materials, cbse 10th maths probability chapter case study question with answers, cbse 10th maths statistics chapter case study question with answers, cbse 10th maths surface areas and volumes chapter case study question with answers, cbse 10th maths areas related to circles chapter case study question with answers, cbse 10th maths circles chapter case study question with answers, cbse 10th maths some applications of trigonometry chapter case study question with answers, cbse 10th maths introduction to trigonometry chapter case study question with answers, cbse 10th maths coordinate geometry chapter case study question with answers, cbse 10th maths triangles chapter case study question with answers, cbse 10th maths arithmetic progressions chapter case study questions with answers, cbse 10th maths quadratic equations chapter case study questions with answers, cbse 10th social science the making of a global world chapter case study question with answers, cbse 10th social science nationalism in india chapter case study question with answers, cbse 10th social science the rise of nationalism in europe chapter case study question with answers, cbse 10th maths pair of linear equation in two variables chapter case study question with answers.

Class VI to XII
Tn state board / cbse, 3000+ q&a's per subject, score high marks.

10th Standard CBSE Study Materials

10th Standard CBSE Subjects

Class 10 Term 2 Case Study Questions of Science , Social Science, Mathematics PDF
- Post author: studyrate
- Post published:
- Post category: class 10th
- Post comments: 0 Comments
Class 10 Case Study Questions for Term 2 Chapter-wise PDF
Join our Telegram Channel, there you will get various e-books for CBSE 2024 Boards exams for Class 9th, 10th, 11th, and 12th.


You Might Also Like
Mcq class 10 social science history print culture and the modern world questions with answers, mcq class 10 social science geography lifelines of national economy quiz with answers, ntse daily practice problems(dpp) of all subjects (mat+sat) with solutions, leave a reply cancel reply.
Save my name, email, and website in this browser for the next time I comment.
CBSE Expert
CBSE Class 10 Science Case Study Questions Download Free PDF
If you are looking for the CBSE Class 10 Science Case Study Questions in PDF, then you are in the right place. CBSE 10th Class Case Study for the Science Subject is available here. These Case studies can help the students to solve the different types of questions that are based on the case study.

CBSE Board will be asking case study questions based on Science subjects in the upcoming board exams. Thus, it becomes an essential resource to study.
The Science Subject case study for class 10th covers a wide range of chapters from the Science. Students willing to score good marks in their board exams can use it. The questions are highly interactive and it allows students to use their thoughts and skills to solve such kinds of questions.
Case Study Questions Class 10 Science
In board exams, students will find the questions based on assertion and reasoning . Also, there will be a few questions based on case studies. In that, a paragraph will be given, and then the MCQ questions based on it will be asked.
- Case Study Questions for Chapter 1 Chemical Reactions and Equations
- Case Study Questions for Chapter 2 Acids, Bases, and Salts
- Case Study Questions for Chapter 3 Metals and Non-Metals
- Case Study Questions for Chapter 4 Carbon and Its Compounds
- Case Study Questions for Chapter 5 Periodic Classification of elements
- Case Study Questions for Chapter 6 Life Processes
- Case Study Questions for Chapter 7 Control and Coordination
- Case Study Questions for Chapter 8 How do organisms reproduce?
- Case Study Questions for Chapter 9 Heredity and Evolution
- Case Study Questions for Chapter 10 Light reflection and refraction
- Case Study Questions for Chapter 11 Human eye and colorful world
- Case Study Questions for Chapter 12 Electricity
- Case Study Questions for Chapter 13 Magnetic effects of current
- Case Study Questions for Chapter 15 Our Environment
The above Case studies for CBSE Class 10 Science will help you to score good marks in the Case Study questions that have been coming in your examinations. These CBSE Class 10 Science Case Study have been developed by experts of cbseexperts.com for benefit of Class 10 students.
Class 10 Science Assertion and Reason Questions
Case Study Type Questions in Science Class 10
Case Study Type Questions in Science Class 10 include the information or data. Students willing to solve them are required to read the passage carefully and then solve them. While solving the paragraph the ideal way is to highlight the key information or given data.
Because later it will ease them to write the final answers. Science Case study type questions consist of 4 to 5 questions that should be answered in an MCQ manner.
While reading the paragraph students will get the clue in between about the possible answer of the question. They should definitely highlight those questions. This is the best way to solve such kind of Case study Type Questions.
Leave a Comment Cancel reply
Save my name, email, and website in this browser for the next time I comment.
Download India's best Exam Preparation App Now.
Key Features
- Revision Notes
- Important Questions
- Previous Years Questions
- Case-Based Questions
- Assertion and Reason Questions
No thanks, I’m not interested!

Case Study Question for Class 10 Science (CBSE Board)
Case Study Question Class 10 Science for CBSE Board: Understudies can discover the chapter astute vital questions for course 10th Science within the table underneath. These imperative questions incorporate questions that are regularly inquired in a long time. Moreover, arrangements are to give for these questions, with extraordinary accentuation on ease-of-study. Tap on the joins underneath to begin investigating.
Case Study Question for Class 10 Science Ch. 1 to 16
Case study: 1.
1) Sahil performed an experiment to study the inheritance pattern of genes. He crossed tall pea plants (TT) with short pea plants (tt) and obtained all tall plants in F1 generation. (CBSE Sample Paper 2022)
a.) What will be set of genes present in the F1 generation? (1 Mark)
b.) Give reason why only tall plants are observed in F1 progeny. (1 Mark)
Ans. Traits like ‘T’ are called dominant traits, while those that behave like ‘t’ are called recessive traits./Alternatively accept the definition of dominant and recessive traits with examples of T and t respectively /Alternatively accept the law of Dominance with examples of T and t.
c.) When F1 plants were self – pollinated, a total of 800 plants were produced.
How many of these would be tall, medium height or short plants? Give the genotype of F 2 generation. (2 Marks)
When F1 plants were cross – pollinated with plants having tt genes, a total of 800 plants were produced. How many of these would be tall, medium height or short plants? Give the genotype of F 2 generation.
Ans. Out of 800 plants 600 plants will be tall and 200 plants will be small, 1
TT: 2Tt: 1tt (1 mark)
In the cross between Tt X tt, 400 Tall (Tt) and 400 short (tt) plants will be produced.
Case Study: 2 Question Class 10 Science
2) Ansari Sir was demonstrating an experiment in his class with the setup as shown in the figure below. (CBSE Sample Paper 2022)

case study questions class 10 science / passage based questions class 10 science cbse board / ncert class 10 science case study
A magnet is attached to a spring. The magnet can go in and out of the stationary coil.
He lifted the Magnet and released it to make it oscillate through the coil. Based on your understanding of the phenomenon, answer the following questions.
a.) What is the principle which Ansari Sir is trying to demonstrate?
Ans. Sir is trying to demonstrate the principle of Electromagnetic induction.
b.) What will be observed when the Magnet starts oscillating through the coil. Explain the reason behind this observation.
Ans. There will be induced current in the coil due to relative motion between the magnet and the coil. Changing the magnetic field around the coil generates induced current.
c.) Consider the situation where the Magnet goes in and out of the coil. State two changes which could be made to increase the deflection in the galvanometer.
Is there any difference in the observations in the galvanometer when the Magnet swings in and then out of the stationary coil? Justify your answer.
Using a stronger magnet, using a coil with more number of turns.
When the magnet moves into the coil, the ammeter shows a momentary deflection towards one side say left.
When the magnet moves out of the coil, the ammeter shows a momentary deflection now towards right.
This is due to changing magnetic field /flux associated with the coil as the magnet moves in and out.
Alternatively, the flux increases when the magnet goes in and it decreases when the magnet goes out.
- NCERT Class 10 Science Best Solution
- Exemplar Notes Solution by Expert Sir
Case Based Questions Class 10 Science Chapter-wise:
Key questions for 10th review science are outlined agreeing to the CBSE NCERT program. All address sorts are accessible within the PDF, from one-word to one-line answers, brief reply sorts to five point long reply sorts. Hence, understudies can plan for exams and indeed clarify their concepts through them. On the off chance that they refer to these questions, it’ll get ready their minds to pick up a competitive advantage. Understudies will gotten to be commonplace with question patterns and the sorts of questions that will show up on exams.
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.

- Andhra Pradesh
- Chhattisgarh
- West Bengal
- Madhya Pradesh
- Maharashtra
- Jammu & Kashmir
- NCERT Books 2022-23
- NCERT Solutions
- NCERT Notes
- NCERT Exemplar Books
- NCERT Exemplar Solution
- States UT Book
- School Kits & Lab Manual
- NCERT Books 2021-22
- NCERT Books 2020-21
- NCERT Book 2019-2020
- NCERT Book 2015-2016
- RD Sharma Solution
- TS Grewal Solution
- DK Goel Solution
- TR Jain Solution
- Selina Solution
- Frank Solution
- ML Aggarwal Solution
- Lakhmir Singh and Manjit Kaur Solution
- I.E.Irodov solutions
- ICSE - Goyal Brothers Park
- ICSE - Dorothy M. Noronhe
- Sandeep Garg Textbook Solution
- Micheal Vaz Solution
- S.S. Krotov Solution
- Evergreen Science
- KC Sinha Solution
- ICSE - ISC Jayanti Sengupta, Oxford
- ICSE Focus on History
- ICSE GeoGraphy Voyage
- ICSE Hindi Solution
- ICSE Treasure Trove Solution
- Thomas & Finney Solution
- SL Loney Solution
- SB Mathur Solution
- P Bahadur Solution
- Narendra Awasthi Solution
- MS Chauhan Solution
- LA Sena Solution
- Integral Calculus Amit Agarwal Solution
- IA Maron Solution
- Hall & Knight Solution
- Errorless Solution
- Pradeep's KL Gogia Solution
- OP Tandon Solutions
- Sample Papers
- Previous Year Question Paper
- Value Based Questions
- CBSE Syllabus
- CBSE MCQs PDF
- Assertion & Reason
- New Revision Notes
- Revision Notes
- HOTS Question
- Marks Wise Question
- Toppers Answer Sheets
- Exam Paper Aalysis
- Concept Map
- CBSE Text Book
- Additional Practice Questions
- Vocational Book
- CBSE - Concept
- KVS NCERT CBSE Worksheets
- Formula Class Wise
- Formula Chapter Wise
- JEE Crash Course
- JEE Previous Year Paper
- Important Info
- JEE Mock Test
- SRM-JEEE Mock Test
- VITEEE Mock Test
- BITSAT Mock Test
- Manipal Engineering Mock Test
- AP EAMCET Previous Year Paper
- COMEDK Previous Year Paper
- GUJCET Previous Year Paper
- KCET Previous Year Paper
- KEAM Previous Year Paper
- Manipal Previous Year Paper
- MHT CET Previous Year Paper
- WBJEE Previous Year Paper
- AMU Previous Year Paper
- TS EAMCET Previous Year Paper
- SRM-JEEE Previous Year Paper
- VITEEE Previous Year Paper
- BITSAT Previous Year Paper
- Crash Course
- Previous Year Paper
- NCERT Based Short Notes
- NCERT Based Tests
- NEET Sample Paper
- Previous Year Papers
- Quantitative Aptitude
- Numerical Aptitude Data Interpretation
- General Knowledge
- Mathematics
- Agriculture
- Accountancy
- Business Studies
- Political science
- Enviromental Studies
- Mass Media Communication
- Teaching Aptitude
- NAVODAYA VIDYALAYA
- SAINIK SCHOOL (AISSEE)
- Mechanical Engineering
- Electrical Engineering
- Electronics & Communication Engineering
- Civil Engineering
- Computer Science Engineering
- CBSE Board News
- Scholarship Olympiad
- School Admissions
- Entrance Exams
- All Board Updates
- Miscellaneous
- State Wise Books
- Engineering Exam
- STATE WISE BOOKS
- ENGINEERING EXAM
- SCHOLARSHIP OLYMPIAD
- STATE BOOKS
CBSE Class 10 Science Case Based MCQ Questions
CBSE Term 1 exam is not so far and surely you have begun the preparation for the board exam. Therefore we have dedicated this page to help you out in your preparation. This year for the first time the board has introduced the Case based Questions which will be asked in the Term 1 exam that is likely to be held in November-December.
Therefore, we have brought you the CBSE Class 10 Science Case Based MCQ on this page. The questions are given in objective types. Such types of questions are solved by reading the given scenario in the paragraph.
All these science case study questions are developed as per the new CBSE Pattern. The team of subject matter experts have crafted the given MCQs. The Case Based Questions that we are providing here are worthy to solve and practice because class 10th syllabus has been taken into consideration while preparing the problems.
CBSE Class 10 Science Case Study, Assertion & Reasoning, MCQ
The Class tenth CBSE Science Case Study, Assertion & Reasoning, MCQs are very helpful in practicing and getting a deep understanding in the topics of science. However, a comprehensible knowledge of NCERT Science Book is a must to be able to solve these types of problems.
The PDF that is available here to download contains the problems in three different variants; One is general objective types of questions that is MCQ (Multiple Choice Questions), second one is Assertion and Reasoning and the last one is Case-Based Questions.
If you Download PDF CBSE Class 10 Science Case Study from the given links, then you will be able to get the quick revision for each chapter that will help you to recall your learnings and give you information about some important Chemicals Formulas and reactions.
What are Assertion & Reasoning Questions?
Assertion & Reasoning questions are basically a type of multiple-choice question that is solved by reading the given statement and the reason. The question typically consists of one statement followed by its reason. Students' duty is to verify both statements and reason whether they are correct or not and if they are correct then it is time to look at whether the given statement truly satisfies the reason or not.
These questions should not be difficult to solve but you have to have rigorous and extensive practice. The Assertion & Reasoning questions along with the solutions are given in the CBSE Class 10 Science case study 2021-2022 PDF that is available here.
Class 10th has very basic and important chapters that are necessary to solve. A few chapters that are available in the beginning of the science books are Chemical Reactions and Equations, Acids, Bases and Salts, Metals and Non-metals, Carbon and Its Compounds, Periodic Classification of Elements, Life Processes, etc.
Since you are here to find out the CBSE Class 10 Science MCQ Based Questions, the possibilities are you need CBSE Class 10 Maths Case Study Questions too.
FAQs on CBSE Class 10th Science Case Study Questions

Case Study Questions are based on the data which are given in the form of passage. These types of questions generally consist of real life examples. Usually it contains upto 4 or 5 questions.
To prepare for Class 10 Science MCQ Be thorough with the concepts, Practice the questions regularly, Attempt online tests as much as you can. To do all these things visit the Selfstudys.com. They are providing everything for free of cost.
In class 10 Science Based Questions you will be asked to answer the questions that are explained in the standard Xth Science Syllabus. However, the problems will be related to real world examples.
To find Class 10 Science Chapter wise Assertion and Reason Questions you simply need to reach at the Selfstudys website. It provides all the study resources for free of cost. You will be able to download the assertion reason with solutions as well.
No, CBSE Class 10 Science Case Based MCQ Question is not difficult, if you pay a good attention to the given paragraph. It is important to be able to find the tiny details in the passage to answer the Case Based questions.

CBSE Class 10 Exams Finish, When Can You Expect Results? Details Here

CBSE Board Class 10 Information Technology Answer Key 2024 and Question Papers, Download PDF All SETs

CBSE Board Class 10 Computer Applications Answer Key 2024 and Question Papers, Download PDF All SETs

CBSE Class 10 Information Technology Exam 2024 : Most Important Questions Answers for Last-Minute Revision

CBSE Class 10 Computer Applications Exam 2024 : Most Important Questions Answers for Last-Minute Revision

CBSE Board Class 10 Maths Answer Key 2024 and Question Papers, Download PDF All SETs

- NCERT Solutions for Class 12 Maths
- NCERT Solutions for Class 10 Maths
- CBSE Syllabus 2023-24
- Social Media Channels
- Login Customize Your Notification Preferences

- Second click on the toggle icon

Provide prime members with unlimited access to all study materials in PDF format.
Allow prime members to attempt MCQ tests multiple times to enhance their learning and understanding.
Provide prime users with access to exclusive PDF study materials that are not available to regular users.


Maharashtra Board Class 10 Science and Technology 2 Question Paper 2024 PDF with Answer Key
i mg src="https://img.jagranjosh.com/images/2024/March/2032024/maha-ssc-10-science-tech-part-2-biology-question-paper-answer-key-2024.jpg" width="1200" height="675" />
Maharashtra 10th Science and Technology Question Paper 2024: The Maharashtra State Board of Secondary and Higher Secondary Education (MSBSHSE) Science Part I was done on March 18, 2024. The Part 1 Science and Technology exam was conducted for Physics and Chemistry. Later on March 20, 2024, the Maha SSC students appeared for the Biology exam, also called the Science and Technology Part 2 paper. The total marks, format, and number of questions were similar to paper 1.
Here, the Class 10 Biology 2024 paper is provided in PDF format along with the provisional answer key. Along with the question paper PDF and answer key, other information like exam analysis and experts' reactions are also given for better clarity. Scroll down and check out the complete article.
Maha SSC Class 10 Science and Tech Paper II 2024: Highlights
A few general details from the Maharashtra State Board Class 10 Science Part 2 exam 2024.
Maha SSC Class 10 Science and Tech Paper II 2024: Paper Design
The Maharashtra Board SSC Biology paper had four main questions that had multiple sub-parts. Each question was assigned specific marks based on the length of the answers required. All questions were compulsory to attempt to score a maximum of 40 marks.
Maha SSC Class 10 Science Part 2 Exam 2024: Student and Expert Reaction
The reaction of students and experts was not very different from the previous papers. They had similar feedback for the exam pattern and difficulty level. The exam paper was well-balanced, and the questions were distributed properly. No questions were out of the syllabus and were in easy language to understand. The paper was designed to test the basic knowledge and grasp of concepts. Overall, students felt relieved after answering the questions, and experts praised the board for setting such a good paper for students.
Maha SSC 10th Science Part 2 Question Paper 2024
Students can download the free PDF from the link below:
Maha SSC 10th Science Part 2 Answer Key 2024
To get the answers for the 2024 Science Part 2 exam conducted on March 20, 2024, for Maharashtra Board 10th students check the answer key below:
Maharashtra Board SSC Science Part 2 Biology Answer Key 2024
Keep checking for more answers....
- Maharashtra Board Class 10 Science and Technology 1 Question Paper 2024 PDF with Answer Key
- Maharashtra SSC Class 12th Board Syllabus 2023-24
- Maharashtra Board SSC, SSC 2024 Revised TimeTable

- Bihar Board
SRM University
Bseb 12th result.
- Bihar Board Result 2024
- UP Board Result 2024
- CBSE Board Result 2024
- MP Board Result 2024
- Rajasthan Board Result 2024
- Shiv Khera Special
- Education News
- Web Stories
- Current Affairs
- नए भारत का नया उत्तर प्रदेश
- School & Boards
- College Admission
- Govt Jobs Alert & Prep
- GK & Aptitude
- CBSE Class 10 Study Material
CBSE Class 10 Social Science Case Study Questions for Term 2 Exam 2022 (with Answers): Best for Last Minute Revision
Cbse class 10 social science case study questions for term 2 exam 2022 are provided here in pdf. the chapter-wise questions are curated by the subject experts. students must practice these questions for last minute revision and score good marks in exam..

CBSE Class 10 students can access from here the chapter-wise case study questions for Social Science. These questions are important for the CBSE Class 10 Social Science Term 2 Exam 2022 that will be held on 14th May (Saturday). All the questions are provided with answers for the convenience of students.
In the Social Science paper, Section D will have case based questions of 8 marks. Therefore, students must practice the important chapter-wise questions provided below for quick revision before exam and score full marks.
New* CBSE Class 10 Social Science Solved Sample Paper By Experts for Last Minute Revision (Term 2)
Check below the CBSE Class 10 Social Science Case Study Questions”
1. Read the following passage and answer the following questions
In the countryside, rich peasant communities – like the Patidars of Gujarat and the Jats of Uttar Pradesh – were active in the movement. Being producers of commercial crops, they were very hard hit by the trade depression and falling prices. As their cash income disappeared, they found it impossible to pay the government’s revenue demand. And the refusal of the government to reduce the revenue demand led to widespread resentment. These rich peasants became enthusiastic supporters of the Civil Disobedience Movement, organising their communities, and at times forcing reluctant members, to participate in the boycott programmes. For them the fight for
swaraj was a struggle against high revenues. But they were deeply disappointed when the movement was called off in 1931 without the revenue rates being revised. So when the movement was restarted in 1932, many of them refused to participate. The poorer peasantry were not just interested in the lowering of the revenue demand. Many of them were small tenants cultivating land they had rented from landlords. As the Depression continued and cash incomes dwindled, the small tenants found it difficult to pay their rent. They wanted the unpaid rent to the landlord to be remitted. They joined a variety of radical movements, often led by Socialists and Communists. Apprehensive of raising issues that might upset the rich peasants and landlords, the Congress was unwilling to support ‘no rent’ campaigns in most places. So the relationship between the poor peasants and the Congress remained uncertain.
1.a.atidars and Jats are rich Peasants of which State?
(A) Gujarat and Uttar Pradesh
(B) Gujarat and Himachal Pradesh
(C) Uttar Pradesh and Rajasthan
(D) Punjab and Haryana
1.b.What was the main demand of poor peasants?
(A) remitting of unpaid rent to land lord
(B) Reduction of land revenue
(C) Complete independence
(D) None of the above
1.c.Among the following groups which group actively participated in the Civil Disobedience Movement?
(A) Poor Peasants
(B) Muslims
(D) Rich Peasants
1.d.Which among the following groups joined in radical movements led by socialist and Communists?
(B) Industrialists
(C) Rich farmers
2. Read the following passage and answer the following questions
‘It is said of “passive resistance” that it is the weapon of the weak, but the power which is the subject of this article can be used only by the strong. This power is not passive resistance; indeed it calls for intense activity. The movement in South Africa was not passive but active ...
Satyagraha is not physical force. A satyagraha does not inflict pain on the adversary; he does not seek his destruction ... In the use of satyagraha, there is no ill-will whatever.
‘Satyagraha is pure soul-force. Truth is the very substance of the soul. That is why this force is called satyagraha. The soul is informed with knowledge. In it burns the flame of love. ... Nonviolence is the supreme dharma.
‘It is certain that India cannot rival Britain or Europe in force of arms. The British worship the war-god and they can all of them become, as they are becoming, bearers of arms. The hundreds of millions in India can never carry arms. They have made the religion of non-violence their
2.a.Whose words are given above?
(A) Jawaharlal Nehru
(B) Ambedkar
(C) C R Das
(D) Gandhiji
b.Satyagraha is a passive resistance of weak.
2.c.Satyagraha is based on
(B) Non violence
(C) Both 1 and 2
2.d.Satyagraha is based on ----------
(A) Violence
Get here latest School , CBSE and Govt Jobs notification in English and Hindi for Sarkari Naukari and Sarkari Result . Download the Jagran Josh Sarkari Naukri App . Check Board Result 2024 for Class 10 and Class 12 like CBSE Board Result , UP Board Result , Bihar Board Result , MP Board Result , Rajasthan Board Result and Other States Boards.
- BSEB Bihar Board 12th Result 2024
- BSEB बिहार बोर्ड 12th रिजल्ट 2024
- Bihar Board 12th Result 2024
- बिहार बोर्ड कक्षा 12 परिणाम 2024
- biharboardonline.bihar.gov.in परिणाम 2024
- BSEB 12th परिणाम 2024 at Jagran Josh
- बीएसईबी 12th रिजल्ट 2024
- Bihar Sakshamta Pariskha Answer Key 2024
- PNB SO Admit Card 2024
- MPNRC Result 2024
Trending Categories
- CBSE Study Material
- CBSE Class 10
Latest Education News
CGPSC State Service Prelims Result 2024 Out at psc.cg.gov.in: 3597 Qualify for mains, Here's download link
WBPSC Food SI Question Paper 2024: Direct Download Link to Shift-Wise Papers
CBSE Class 12 Accountancy Answer Key 2024 and Question Papers, Download PDF All SETs
Bihar Board Result 2024 Class 12 Link: List of Direct Official Sites to Check BSEB Inter Result Online
Bihar Sakshamta Pariksha Result 2024: Where and How to Check the Merit List
How to Check Voter Application Status?
Bihar Board 12 Vocational Result 2024: BSEB Class 12th Vocational Result Date and Time at biharboardonline.bihar.gov.in
Bihar Board 12th Result 2024 OUT Live Updates: BSEB Inter Result Announced; 5,24,939 Students Got First Division, Check Topper List
BTEUP Result 2024: यूपी पॉलिटेक्निक विषम सेमेस्टर परिणाम bteup.ac.in पर, इस लिंक से करें चेक
Bihar Board 10th Result 2024 Likely by March 30? BSEB Class 12 Results Out
Bihar Sakshamta Pariksha Result 2024: जानें कैसे चेक करें बिहार सक्षमता परीक्षा रिजल्ट
Bihar Board 12th Result Analysis 2024: 87.21% Passed, Girls Outshine Boys
Find 3 differences between the fish in the sea pictures in 14 seconds!
Calicut University Result 2024 OUT at results.uoc.ac.in: Direct Link to Download UG, PG Marksheet
BSEB Bihar Board 12th Result Analysis 2024: बिहार बोर्ड 12वीं परीक्षा में 87.21% पास, लड़कियों ने मारी बाजी
biharboardonline.bihar.gov.in BSEB Result 2024 Released: Check Bihar Board 12th Result Online with Roll Number and Roll Code
Bihar Board 12th Toppers 2024 Revealed: Tushar in Arts, Priya in Commerce, and Mrityunjay Kumar Tops in Science Stream; Check Full List Here
BSEB Bihar Board 12th Result 2024 Out Live: वेबसाइट डाउन, बिहार बोर्ड 12वीं कॉमर्स, आर्ट्स और साइंस स्ट्रीम के नतीजों का Direct Link
Bihar Board BSEB 12th Result 2024 Out LIVE: तुरंत इस डायरेक्ट लिंक से डाउनलोड करें बिहार बोर्ड इंटर का रिजल्ट, 87.21% हुए पास
BSEB 12th Result 2024: Steps To Check Bihar Board Intermediate Result Online With Roll Number And Name

IMAGES
VIDEO
COMMENTS
There will be a few questions based on case studies and passage-based as well. In that, a paragraph will be given, and then the MCQ questions based on it will be asked. Acids, Bases, and Salts Case Study Questions With Answers. Here, we have provided case-based/passage-based questions for Class 10 Science Chapter 2 Acids, Bases, and Salts
CBSE Class 10 Science paper in term 2 exam will have case study questions of 8 marks in Section C. To help students score full marks in this section, we have provided below important case study ...
Class 10 Science Sample Papers with case study questions are available in the myCBSEguide App. There are 4 such questions (Q.No.17 to 20) in the CBSE model question paper. If you analyze the format, you will find that the MCQs are very easy to answer. So, we suggest you, read the given paragraph carefully and then start answering the questions.
At Case Study Questions there will given a Paragraph. In where some Important Questions will made on that respective Case Based Study. There will various types of marks will given 1 marks, 2 marks, 3 marks, 15 marks. CBSE Case Study Questions Class 10 Science Our Environment Case Study - 1 1.) Waste management is essential in today's society.
Accurate answers of all the Case-based questions given in the PDF. Case Study class 10 Science solutions are prepared by subject experts referring to the CBSE Syllabus of class 10. Free to download in Portable Document Format (PDF) so that students can study without having access to the internet.
The Chapter wise Important case study based questions with their solved answers in CBSE Class 10 Science can be accessed from the table below: CBSE Class 10 Science Chapter 1 Chemical Reactions ...
The above Case studies for Class 10 Science will help you to boost your scores as Case Study questions have been coming in your examinations. These CBSE Class 10 Science Case Studies have been developed by experienced teachers of schools.studyrate.in for the benefit of Class 10 students. Class 10th Maths Case Study Questions
Answer: b) Photosynthesis. 1.4 Marble statues are corroded or stained when they repeatedly come into contact with polluted rain water. Identify the main reason. a) decomposition of calcium ...
This section has 2 case-based questions (14 and 15). Each case is followed by 3 sub-questions (a), (b) and (c). ... The exclusive benefits of solving the CBSE Class 10 Science Term 2 2022 question paper with solution are: ... Perform the CBSE Class 10 Science Question Paper 2022 PDF download and complete your study material for CBSE Class 10 ...
For Science subjects, there would be 5 case-based sub-parts questions, wherein a student has to attempt 4 sub-part questions. Chapters. Download. 1. Chemical Reactions & Equations. Click Here. 2. Acids, Bases & Salts. Click Here.
CBSE Case Study Questions Class 10 Science Heredity and Evolution. A scientist cross pure-bred tall (dominant) pea plant with pure-bred dwarf (recessive) pea plant he will get pea plants of F1 generation. If now self-cross the pea plant of F2 generation is done, then we obtain pea plants of F2 generation.
Physics Chapters for Case Study Questions. Light - Reflection and Refraction. The Human Eye and The Colourful World. Electricity. Magnetic Effects of Electric Current. TopperLearning provides a complete collection of case studies for CBSE Class 10 Science students. Improve your understanding of biological concepts and develop problem-solving ...
CBSE 10th Standard Science Subject Case Study Questions Answer Keys. (I) (b) : H 2 is oxidised to HCI while Cl 2 is reduced to HCl. (v) (b): H 2 O 2 is reduced to water by removal of oxygen. (i) (c): As the pH value increases from 7 to 14, it represents decrease in H+ ion concentration in the solution.
Such questions will be based on the case studies given. CBSE Class 10 Term-2 Science Important Questions Based on Important Topics. Here, we have provided the important questions based on important topics that can be asked in the Science exam. The experts curated these questions and are as per the latest paper pattern and reduced syllabus.
Class 10 Case Study Questions for Term 2 Chapter-wise PDF Download Books for Boards Join our Telegram Channel, there you will get various e-books for CBSE 2024 Boards exams for Class 9th, 10th, 11th, and 12th. Join Now We earn a commission if you make a purchase, at no additional cost to you. Class 10 […]
Download CBSE Class 10 Science Term 2 Question Paper 2022 in PDF here along with solution by experts. ... Section-C with 2 case-based questions of 4 marks each. (iv) Section C had 2 case based ...
At Case Study Questions there will given a Paragraph. In where some Important Questions will made on that respective Case Based Study. There will various types of marks will given 1 marks, 2 marks, 3 marks, 4 marks. CBSE Case Study Questions Class 10 Science Carbon and its compounds CASE STUDY : 1. Carbon has the unique ability to form bonds ...
Case Study Questions Class 10 Science. In board exams, students will find the questions based on assertion and reasoning. Also, there will be a few questions based on case studies. In that, a paragraph will be given, and then the MCQ questions based on it will be asked. Case Study Questions for Chapter 1 Chemical Reactions and Equations.
Case Study: 1. 1) Sahil performed an experiment to study the inheritance pattern of genes. He crossed tall pea plants (TT) with short pea plants (tt) and obtained all tall plants in F1 generation. (CBSE Sample Paper 2022) a.) What will be set of genes present in the F1 generation? (1 Mark) Ans. Tt.
These questions should not be difficult to solve but you have to have rigorous and extensive practice. The Assertion & Reasoning questions along with the solutions are given in the CBSE Class 10 Science case study 2021-2022 PDF that is available here. Class 10th has very basic and important chapters that are necessary to solve.
Also, Check CBSE Class 10 Science Term 2 Syllabus 2022. Section - A. 1. The table shows the electronic structures of four elements. a. Identify which element (s) will form covalent bonds with ...
Maha SSC Class 10 Science and Tech Paper II 2024: Paper Design The Maharashtra Board SSC Biology paper had four main questions that had multiple sub-parts. Each question was assigned specific ...
Global climate change is not a future problem. Changes to Earth's climate driven by increased human emissions of heat-trapping greenhouse gases are already having widespread effects on the environment: glaciers and ice sheets are shrinking, river and lake ice is breaking up earlier, plant and animal geographic ranges are shifting, and plants and trees are blooming sooner.
CBSE Class 10 Science important questions for term 2 exam 2022 are provided here. Practice questions of 2, 3 and 4 marks for effective preparation and quick revision.
CBSE Class 10 students can access from here the chapter-wise case study questions for Social Science. These questions are important for the CBSE Class 10 Social Science Term 2 Exam 2022 that will ...