

Top 17 Clinical Research Organizations (CRO) in 2023
In clinical research and treatment development, clinical research organizations (CROs) are frequently a sponsor’s most important partner and ally.
Depending on the nature of the clinical trial, and your existing capabilities as a sponsor to run the trial, the CRO company of your choice will typically be responsible for facilitating most of the micro and macro processes that go into designing and running a successful clinical trial.
When contracting a CRO to help you with your trial, you are transferring over a large portion of responsibility into the hands of your clinical research partner. The CRO of your choice will have the responsibility to control a variety of factors and processes of a clinical trial, and depending on their expertise, team structures, service offerings, internal resources and many other capabilities.
Your ability to find and contract a top CRO company that is the right fit for your unique trial will be a determinant of whether or not you will be able to operate a high-quality clinical trial that meets your expected timelines, budget and delivers a top-notch patient experience.
At ClaraHealth (a patient-centric recruitment acceleration platform) , we have put together an extensive list of the top CRO companies in the US and around the world.
This is not a cro rankings list, but rather a compiled list of some of the top clinical research organizations around the world. We have highlighted their strengths and core service offerings to make it easier for you to find the right fit clinical research partner.
In addition, we’ve put together a list of 9 fundamental questions to ask the prospective clinical research organization , which will help you to save time and ensure a right fit in picking the CRO.
Formerly known as Quintiles and IMS Health, IQVIA is one of the largest CROs in the world, with a large range of service offerings to help advance clinical research.
The company was founded in North Carolina in 1982, and has since grown to over 88,000 employees in more than 100 countries.
Some clinical trial solutions offered by IQVIA include:
- Assistance with protocol design
- Design of phase 1 clinical trials
- Assessment and improvement of phase 2 and 3 clinical trials
- Site identification & selection
- Patient recruitment
- Access to global laboratories via their wholly owned subsidiary Q2 Solutions
Parexel is a global clinical research organization that was founded in 1982, and specializes in conducting clinical studies on behalf of its pharmaceutical partners in order to accelerate and ensure the drug approval process of up-and-coming potential treatments. It currently operates in more than 50 countries, and is run by more than 18,000 employees around the world.
The company has a wide range of service offerings, covering nearly every type of clinical trial service to assist sponsors in running successful clinical studies.
Some clinical trial solutions offered by Parexel include:
- Clinical trial design and development for early phase, phase 2 & 3, and late phase clinical trials
- Clinical data management
- Decentralized clinical trials
- Clinical supply chain management
- Medical writing
- Regulatory affairs consulting
- Pharmacovigilance
3. PRA Health Sciences
PRA Health Sciences is one of the largest contract research organizations in the world. Founded in 1976 under the name “Anti-Inflammatory Drug Study Group”, the company was renamed to PRA in 1982. PRA Health Sciences employees more than 17,000 people, and provides coverage to more than 90 countries.
In 2021, PRA Health Sciences was acquired by the Ireland-headquartered global CRO leader ICON, which is also reviewed in this list.
Some clinical trial solutions offered by PRA Health Sciences include:
- Decentralized Clinical Trials Platform
- Protocol Consultation & Study Design
- Onsite Support services
- Customized Solutions for Biotech (such as asset valuation, regulatory strategy, engagement and support, drug development strategy and funding solutions)
- Clinical Diagnostics
- Site Commercial Solutions
- PRA’s Laboratories for Drug Development
Headquartered in Ireland, ICON was founded in 1990 in Dublin by co-founders John Climax and Ronan Lambre. The company has since grown to be one of the largest CROs in the world. As of September 2020, the company employs more than 15,000 people in 94 locations and across 40 countries.
ICON offers clinical research services which include consulting, clinical development and commercialization across a wide range of therapeutic areas.
In 2021, ICON acquired PRA Health Sciences, which is another CRO and global leader in clinical research services.
Some clinical trial solutions offered by ICON:
- Commercial Positioning
- Early Phase
- Functional Services Provision
- Laboratories
- Language Services
- Medical Imaging
- Real World Intelligence
- Site & Patient Solutions
- COVID-19 Clinical Operations
5. Syneos Health
Formerly known as InVentiv Health Incorporated and INC Research, Syneos Health is a publicly listed and global contract research organization. The company is based in Morrisville, North Carolina, and specializes in assisting companies with late-stage clinical trials. Syneos Health currently employs more than 25,000 people, and has offices across 91 locations.
In early 2018, INC Research was acquired inVentiv Health, and the merged company was named Syneos Health.
Some clinical trial solutions offered by Syneos Health include:
- Decentralized Clinical Trials Solutions
- Bioanalytical Solutions
- Phase II-III/Phase IIIb-IIIV
- Medical Device Diagnostics
- Clinical Data Management
- Clinical Project Management
- Clinical Monitoring
- Drug Safety & Pharmacovigilance
- Site and Patient Access
6. Labcorp Drug Development (Formerly Covance)
Formerly known as Covance and renamed to Labcorp Drug Development in early 2021, this CRO is one of the largest contract research organizations in the world. The company claims to provide the world’s largest central laboratory network, and has been rated as one of the best places to work for LGBTQ+ equality by the Human Rights Campaign organization in 2018 to 2021. Currently, Labcorp employs over 70,000 people and is able to support clinical research efforts in almost 100 countries around the world.
Some clinical trial solutions offered by Labcorp Drug Development include:
- Preclinical Services
- Clinical Trials
- Clinical Trial Laboratory Services
- Post-Marketing Solutions
- Medical Devices
- Data & Technology
Also known as Pharmaceutical Product Development, PPD is a large global contract research organization headquartered in Wilmington, North Carolina. Started as a one-person consulting firm in 1985, PPD has grown to over 27,000 employees worldwide, and provides a wide range of clinical research services to pharmaceutical and biotech companies.
Some clinical trial solutions offered by PPD include:
- Clinical Development
- Early Development
- Peri- and Post-Approval
- PPD Biotech
- PPD Laboratories
- Product Development and Consulting
- Site and Patient Centric Solutions
8. Fisher Clinical Services
Part of Thermo Fisher Scientific, Fisher Clinical Services is a global clinical research organization with headquarters in Center Valley, Philadelphia.
The company has been in the business of clinical supply chain management for over 20 years, and is focused exclusively on working with the packaging and distribution requirements of clinical trials across the globe.
Some clinical trial solutions offered by Fisher Clinical Services include:
- Biologistics Management
- Cell & Gene Therapy
- Clinical Ancillary Management
- Clinical Label Services
- Clinical Trial Packaging & Storage
- Clinical Supply Optimization Services
- Cold Chain Management & Expertise
- Direct-to-Patient
- Distribution & Logistics
- Strategic Comparator Sourcing
- Public Health Research
Established in 1997 under the name Kiecana Clinical Research, KCR is a full-service contract research organization that provides a variety of services for clinical monitoring, safety & pharmacovigilance, clinical project management, quality assurance and regulatory affairs.
KCR operates globally, and has offices in North America, Western Europe, Central Europe and Eartern Europe. The company currently employs more than 700 staff.
Some clinical trial solutions offered by KCR include:
- Trial Execution
10. Medpace
Founded in 1992 and based in Cincinnati, Ohio, Medpace is a midsize clinical contract research organization. The company has operations in over 45 countries, and employs over 2,800 people. Medpace provides support services for Phase I-IV clinical trials for pharmaceutical and biotechnology companies, which include central laboratory services and regulatory services.
Some clinical trial solutions offered by Medpace include:
- Biostatistics and Data Sciences
- Clinical Trial Management
- Drug Safety and Pharmacovigilance
- Medical Writing
- Quality Assurance
- Regulatory Affairs
- Risk-Based Monitoring
- Medpace Laboratories
11. Clintec
Now in business for over 22 years, Clintec is a medium-sized global contract research organization for pharmaceutical, biotech and medical device industries, with large expertise in oncology and rare diseases.
The company provides the flexibility and agility of a smaller-sized CRO, while also having a wide global coverage that large CRO companies are known for. Clintec is based in more than 50 countries, and was acquired by the leading global CRO IQVIA in late 2018.
Some clinical trial solutions offered by Clintec include:
- Project Management
- Data Management
- Biostatistics
- Global Feasibilities
- Patient Recruitment & Retention
12. Worldwide Clinical Trials
Bringing over 30 years of experience to the clinical research market, Worldwide Clinical Trials is a leading medium-sized global contract research organization. Founded by physicians with a dedication and commitment to advancing medical research, Worldwide Clinical Trials was the first customer-centric CRO.
Currently the company has coverage in more than 60 countries, and has extensive experience in a wide range of therapeutic areas, including central nervous system, metabolic, cardiovascular, oncology, rare diseases and general medicine.
Some clinical trial solutions offered by Worldwide Clinical Trials include:
- Bioanalytical Lab
- Early Phase Development
- Clinical Phase IIB-II Clinical Trials
- Phase IIIB-IV Clinical Trials
- Trial Management Technologies
Named #1 CRO in the world for operational excellence at the 2021 CRO Leadership Awards, CTI Clinical Trial And Consulting Services is a medium-sized global contract research organization that has been serving pharmaceutical companies since 1999.
Based in Covington, Kentucky, CTI has offices around the world in more than 60 countries, with coverage in North America, Europe, Latin America, Middle-East, Africa, and Asia-Pacific regions.
Some clinical trial solutions offered by CTI include:
- Feasibility
- Regulatory Affairs Study Start-Up
- Medical Monitoring
- Safety & Pharmacovigilance
- Clinical Services
14. Wuxi AppTec
Founded in 2000 as WuXi PharmaTech in the city of Wuxi, China, Wuxi AppTec has grown from a single laboratory into a leading global contract research organization with more than 28,000 employees, including 23,000 scientists and more than 30 research & development and manufacturing sites around the world.
With offices in Asia, U.S, Europe and the Middle East, the company is able to provide coverage to more than 30 countries around the world.
Some clinical trial solutions offered by Wuxi AppTec include:
- Small Molecule Drug R&D and Manufacturing
- Cell Therapy and Gene Therapy
- Drug R&D and Medical Device Testing
- Clinical Services (Phase I-IV)
15. Advanced Clinical
Founded in 1994 and based out of Deerfield, Illinois, Advanced Clinical is a midsize and full-service CRO that helps sponsors with running clinical trials. The company employs more than 700 staff, and offers a wide variety of services across many therapeutic areas. Advanced Clinical has global representation in over 50 countries around the world.
Some clinical trial solutions offered by Advanced Clinical include:
- eTMF & Document Management
- Global Medical Services
- Quality & Validation
16. Pharm-Olam
Pharm-Olam is a leading midsize CRO with global headquarters located in Houston, Texas and its European headquarters in Bracknell, United Kingdom. The company employs more than 800 staff, and has 25 offices around the world, with a global coverage in more than 60 countries.
The company has therapeutic expertise in 5 areas, including Rare & Orphan Disease, Infectious Disease & Vaccine, Oncology-Hematology, Allergy and Autoimmune.
Some clinical trial solutions offered by Pharm-Olam include:
- Study Feasibility
- Site Activation
- Patient Recruitment
- Medical Affairs
- Compliance & Training
- Clinical Monitoring & Operations
17. Clinipace
Founded in 2003 and based out of Morrisville, North Carolina, Clinipace is a global midsize full-service CRO with a focus on solution customization for clinical trials. The company has a large global coverage in more than 50 countries, and has offices in North America, South America, Europe and Asia-Pacific regions.
Clinipace’s therapeutic focus areas include Oncology, Nephrology and Urology, Rare Disease, Gastroenterology and Women’s Health. The company also has complete therapeutic expertise in Infectious Disease & Vaccines, Cardiology, CNS, Immunology, and Respiratory.
Some clinical trial solutions offered by Clinipace include:
- Clinical Analytics
- Clinical Technology and Ecosystem
- Functional Service Partnership (FSP)
- Regulatory & Strategic Product Development
9 Fundamental Questions To Ask A Top CRO Company Before Signing The Contract
1. which services does the cro provide.
CROs offload a lot of operational tasks from trial sponsors, which can touch any component of clinical trial operations. From formulating an overall study strategy and implementing technologies to support the operational processes of the trial, to picking and identifying sites, and supporting patients during the trial, the range of clinical services offered by a CRO tends to be vast and inclusive of all the typical services and support you will require for running a successful clinical trial.
However, not all CROs are the same in their service offerings, or are able to offer the same depth of capability within a seemingly same clinical trial support process. For this reason it is important to understand exactly which kind of clinical services and support you are looking to receive from the prospective CRO when running your clinical trial.
While services such as clinical monitoring and clinical trial management are offered by the majority of CROs, the specific needs of each trial are unique, and for this reason it is important to first identify what will be the unique services your trial requires. Completing this internal analysis first will help you to understand the extent to which a potential CRO partner will be able to provide all of these services.
Some CROs specialize in specific clinical trial functions which the company may label as a “core services”, in which case this is a sign the company will have more expertise, experience, and will be set up in a way to maximize their capabilities in providing support for these services compared to other services that the CRO offers.
For example, a CRO may include patient recruitment as part of its “core services”, which implies that they are highly skilled in and have the necessary infrastructure to design and implement a high-quality patient recruitment strategy.
Clara Health CRO Support Services: At Clara Health our specialty services include technology-augmented digital and patient advocacy recruitment, as well as patient support via our signature patient recruitment platform, which we use to upgrade clinical trials and deliver results sponsors look for in their recruitment and retention campaigns.
At Clara, we work alongside CROs to supplement and support clinical trials with modern and personalized capabilities that CROs do not typically have the bandwidth, corporate structure or infrastructure to support.
If you would like to learn more about exactly how our platform can upgrade your unique trial, feel free to book a Free 30 Minute Consultation Session Here with one of our in-house experts.
2. What Related Experience Does The CRO Have?
It is helpful to ask the prospective CRO company if they have any relevant experience in running clinical trials that would be an asset in designing and running your study. Previous experience in a related therapeutic area or in running a trial with a similar design allows CROs to have a deeper understanding into potential opportunities and challenges, increasing the likelihood of your clinical study being successful.
For example, if a sponsor is planning to run a trial in oncology, for the purpose of site identification and selection it would be valuable to partner with a CRO vendor that has expertise in this area, as they likely already have a good understanding of which sites will lead to optimal results.
However, it is also important to consider all factors when selecting a CRO vendor and not to rely on therapeutic experience as the sole qualifier for whether or not a potential CRO is a fit for your trial. While previous experience is beneficial, some sponsors close themselves off from working with vendors that have not worked in their therapeutic area, which significantly limits options when choosing a CRO partner that is truly a good fit for their clinical study.
This can impact the end result of your clinical study, as sponsors that are not successful in choosing a CRO vendor that is the right overall fit may face difficulties if the needs of their clinical study aren’t being properly met.
Clara Health: We have worked to provide support for clinical trials across a wide range of therapeutic areas and trial designs. Our specialty is filling in the gaps that CROs traditionally did not have to think about, which include digital patient recruitment, patient advocacy recruitment, and technology-augmented patient support.
Additionally, we are constantly building our proprietary data and running tests in a variety of therapeutic areas. These research efforts allow us to have a detailed understanding of the expected level of difficulty when recruiting particular patient populations, as well as allow us to predict with accuracy which segments of the targeted population will be likely to qualify in a particular study.
3. What Are The Communication Workflows & Expectations For Performing And Delivering Contracted Services?
It is important that you clarify what the expectations for communication will be between your prospective CRO vendor and your internal teams, as you will most likely be working with the CRO of your choice for the entire duration of your clinical trial.
There are a vast variety of factors and success determinants for a clinical trial, which are continuously undergoing change as the study unfolds. For this reason, it is recommended that you work with a CRO that is proactive in their communication, so that you are kept up to date with information about important changes as your clinical trial progresses.
A vendor that is proactive rather than reactive in their communication and approach to dealing with arising issues is one of the most important qualities in CRO. Challenging situations will naturally arise, and the promptness with which they are taken care of will significantly impact your clinical trial’s degree of success. Therefore, seeking a vendor that is able to match the standard of communication that you as a sponsor would like to experience throughout the duration of your partnership is one of the most critical steps in determining which CRO is the right fit for your clinical trial.
We’ve included a few additional questions pertaining to the communication structure and reporting expectations that you can ask a prospective CRO vendor to determine the degree of fit in this particular category:
Communication Expectations:
- If we were to move forward with you, which of your team members will be our main point of contact?
- How available will you be outside of the scheduled meetings to address any of our concerns or additional requests?
- What will be the frequency at which update meetings will be conducted, and who will be present at those meetings?
- Which clinical study processes will be reported on, and what will be the workflow for how we will receive this information?
- What will be the cadence at which we will receive progress reports?
- Would we be able to access metrics electronically via an interactive dashboard, or will you send us formal reports?
Clara Health: At Clara Health, we directly interact and actively work with several key stakeholders involved in running a clinical trial, which includes sponsors, CROs, sites, and patients. This unique position allows us to have a centralized perspective which helps us to see all the moving parts of a clinical trial at the same time, which helps to identify issues and relay this vital information and insight back to the sponsor (or other appropriate stakeholders) in the shortest time possible.
The ability to access this perspective allows us to gather the most accurate, complete, and up-to-date information about how the clinical trial is unfolding, and quickly becomes very valuable to sponsors for their clinical trial.
As an example, we may receive feedback from patients about having an unsatisfactory experience with a particular study site. We are able to aggregate and analyze this information, and relay our findings back to the sponsor and the study site to improve the experience for other patients.
4. What Is The CRO’s Client Satisfaction Record?
It is a good practice to request information or metrics from the prospective CRO vendor that can point to the degree of satisfaction of their past clients. Prior to signing the contract, vendors will naturally do their best to uplift their image and future value to you during their sales conversations with you and your team. It can be tricky to get an objective understanding of what the partnership experience will actually entail, especially when there are multiple vendors fighting for your commitment.
We recommend that you ask the prospective vendor to provide success metrics regarding areas of clinical trial operations that are going to be important for your trial.
For example, you may be interested in learning about the vendor’s relationship to finances, in which case it will be useful to ask them about situations in which they went over the planned budget, and investigate into the reasons behind that. Alternatively you may be concerned about potential delays in timelines, in which case it would be helpful to learn about metrics regarding the CRO’s ability to meet timeline expectations.
You may also request to talk to the prospective CRO’s past clients, which will help you to gain insight into what the relationship was like and give you the opportunity to examine if the way in which the particular CRO manages its relationships and performs its services meets the expectations that you would have for your potential relationship and for your clinical trial.
Clara Health: At Clara Health, our relationships with our partners and with our patients are most important to us. In the unique position where we fit in the clinical trial process, we have the opportunity to directly co-create the clinical trial patient experience with a variety of stakeholders, including sponsors, sites, CROs, and patients.
Our company’s values and culture have been directed and developed to be such that the client and patient experience is at the top of priority for all of our internal teams, and we work to provide the best quality of care to all stakeholders.
We have many testimonials from every type of partner we’ve worked with which we can happily share with you.
5. How Do You Adapt When Encountering Challenges With Running A Clinical Trial?
It is inevitable that challenges and unforeseen changes will arise throughout the operational clinical trial process, and for this reason it is important to work with a CRO vendor that can provide you with evidence of their flexibility and ability to adapt to sudden changes.
The ideal CRO partner is one that is highly consultative throughout the entire process, and has an ability and the initiative to deal with challenges at their seed stage, prior to them turning into major obstacles for the success of your trial.
CROs naturally have a large reach, and there are a lot of different clinical trial mechanisms and processes that are under their control. They are able to monitor and respond to what is going on in every key link in the chain of the clinical trial operation.
It is reasonable to expect this level of oversight from a CRO, and additional questions that can help you gain insight into this include:
- What are some examples where the CRO was effective at monitoring the health of clinical trials they’ve helped operate in the past?
- How quickly does the CRO respond to challenges or opportunities for improving the clinical trial experience?
- How well does the CRO gather & process information from study sites, study teams, patients & the sponsor, and what are their typical data analysis workflows?
It is also recommended to speak to the prospective CROs past clients to help you gain insight into how well they respond and adapt to the naturally arising challenges in clinical trials.
Clara Health: While CROs do have a large reach within the clinical trial, no CRO has complete visibility into every clinical process. They are not typically set up to support full visibility, which can manifest as a potential threat to your clinical trial as it unfolds. This is especially true for parts of the clinical trial processes that CROs naturally do not specialize and often subcontract, such as clinical trial recruitment.
At Clara, we are in a unique position in relation to other key partners involved in operating the clinical trial. We are in direct and frequent contact with patients, CROs, study sites, study teams, and the sponsor, and have a very deep understanding of the patient pipeline. This allows us the unique ability to go very deep into specific parts of the recruitment chain and investigate what is working and what is not working.
In addition, Clara functions as a resource for all partners in the clinical trial. For example, we work directly with site teams to ensure that they have access to a 3rd party that they can relay their needs to and receive fast support in case there is anything they require that can improve the patient recruitment process.
6. Which Parts Of Operating The Clinical Trial Will You Be Outsourcing?
Since there are so many processes and mechanisms that go into operating a clinical trial, CROs will always outsource some parts of running and managing the study. While you can expect that the prospective CRO will subcontract some of the work, it is important to find out which exact parts the clinical study will be outsourced.
There are certain basic and key clinical processes (such as site selection) that CROs almost always help with, and if you find that these parts of your trial are going to be subcontracted to another company, it is recommended to find out why the CROs operations are set up this way and how this would impact the service you will receive.
Ultimately what matters to you as a partner and client is that the quality of service and care that you will receive will be up to standard, and meet what was promised and what you are expecting. While this trust is important after you have signed the contract, it is recommended that prior to entering into such a significant commitment that you have evidence and the conviction that the CRO of your choice is truly the right fit and will deliver the quality of service that was being discussed.
Since it is impossible to predict exactly what the quality of this relationship and services performed will actually be like in practice, it is recommended that you understand the details of what will be done for your trial and how. Investigating how the CRO outsources and subcontracts services for a clinical trial will help you to gain necessary insight that you would need to make the correct vendor selection decision.
Clara Health: At Clara, we maximize the effectiveness of the digital component across the entire digital & recruitment spectrum, which is added on top of the existing capabilities of the CROs and other vendors involved in operating your clinical trial. In addition, we offer services that augment the CROs efforts, which has the potential to significantly improve the patient experience, operations flows, recruitment and retention performance, which is so important in ensuring the success of a clinical trial.
For example, if a CRO wants to have a great site relationship, we are able to come in as a third party on behalf of the sponsor and CRO and act as a resource and additional support for sites.
In another example, If a sponsor wants to have great relationships with the patient community, Clara is able to come in on behalf of the sponsor and develop these relationships while being perceived more neutrally by the patient community.
7. Do You Have Experience Running International Trials?
If you are planning on operating an international clinical trial, it is recommended to work with a CRO that has extensive experience in this area. While many CROs will offer near-global coverage, the level of experience with specific geographic locations can significantly vary from one vendor to another.
It is important to work with a CRO that has experience running clinical trials in the specific countries and regions you are planning to conduct your research in. Being compliant with the local rules and regulations for clinical testing is a very complex process that requires existing understanding and familiarity in order to ensure logistical smoothness and to mitigate legal risks. In operating a clinical trial, there are a multitude of clinical services and processes, which can greatly vary across the many regions in which you can conduct clinical testing.
A CRO that is lacking experience in operating international trials or operating in particular regions where you plan on conducting research may not be able to meet your desired quality and agility expectations, and therefore may not be the right fit for your international clinical trial.
Clara Health: In the past, we have provided international patient recruitment and digitally-augmented trial support services for clinical trials in the EU, Canada, UK, Australia and South America.
Clara Health is fully compliant to operate international studies everywhere in the world, with the exception of Russia and China.
8. What Is Your Relationship With Patients?
Patient-centric approach to designing and operating a clinical trial is becoming more and more crucial in the clinical research space. The ability of a sponsor and their CRO partner to understand the needs and characteristics of their target patient community is a significant determinant of whether or not the study will be a success.
A sponsor that has close and authentic relationships with the patient community tends to have a deeper understanding of how to create the best clinical trial experience that will attract patients and keep their interest throughout the clinical trial.
In addition, strong relationships with patients allow sponsors and CROs to forecast recruitment and patient retention pipeline with much higher accuracy. This ability is critical for ensuring the success of the trial and mitigating the risk of low enrollment. After an understanding of the patient population is acquired, sponsors gain the necessary insight to design a clinical trial that is not only favorable to their research results, but is also practical and will result in the enrollment numbers they are looking for.
While many CROs have already recognized the importance of patient-centricity and evolved the ways in which they design and operate clinical trials, other CROs have not yet made such a pivot in their values. It is important to understand the degree of importance the prospective CRO places on creating a favorable patient experience, and what kind of infrastructure the company has to support it.
At Clara, we recommend choosing a CRO partner that is adapting to the patient-centric model which is becoming more and more important for running a successful clinical trial.
Clara Health: Since early stages of our development, we’ve had a dedicated patient advocacy team that has been integral in shaping our company’s vision and operations. We have built our entire platform and recruitment infrastructure around creating the best experience for patients. Our teams, corporate values, service offerings and company infrastructure all work in the service of the patient.
In addition, over the many years of being in business we have heavily invested in building authentic patient community relationships that span across a variety of therapeutic areas. This has given us a unique ability to receive feedback directly from patients that is genuine and authentic around marketing materials, strategy for patient recruitment, and other services that we build for specific trials.
This ability to build partnerships with the patient community in an authentic way gives us a very unique ability to engage with the patient community on behalf of a pharmaceutical company, allowing our sponsor & CRO partners the opportunity to start conversations with patients through our in-house patient advocacy team.
If you would like to learn how Clara can help you to build a strong & authentic relationship with your target patient community, get in touch with us and we’d be happy to share our capabilities and previous results with you as they relate to your current or upcoming clinical trial.
9. How Is The CRO Going To Utilize Patient Input For Developing The Trial?
In the initial stages of clinical trial design, sponsors often determine the ideal patient profiles that would help them to drive the most favorable research outcomes for their study. While it is important for the success of your trial to determine who your ideal patients are, very often these projections do not match up with what is viable in practice.
At Clara, we often encounter study protocols that are not set up realistically for successful recruitment to be possible.
Common mistakes that are made when determining trial eligibility criteria and trial design include:
- Overestimating the interest in the clinical trial from the target patient population
- A lack of patient focus in the trial design
- A lack of convenience for patients in their participation
- Complicated and/or inefficient study experience flows
- Crafting the eligibility criteria around the patient population that is most likely to lead to favorable study outcomes, without conducting sufficient research to more accurately estimate the recruitment and retention difficulty of the group for a particular study
It is natural for there to be a “push & pull” between the research ideal and the real world practicality. It is important to determine the correct balance between these two sides for your trial, as going too far in either direction will decrease the chance of your clinical study’s success.
The nature of the industry as it is right now is such that there is excess research idealization and not enough emphasis on patient centricity. This distorted orientation has resulted in many clinical trials being unsuccessful, negatively impacting sponsors, patients and the entire clinical trials industry.
The ideal CRO partner should help you make sure that your protocol design sets your study up for success. The CRO should be able to help you determine the proper balance between the research ideal and the real world practicality, and back up their findings with sufficient research and patient data that can project your trial being a success.
Clara Health: When formulating a recruitment and retention plan for our clients, we begin with conducting thorough research into the target trial patient population. This allows us to get a clear understanding of which recruitment channels will yield the best results and what kind of marketing materials will resonate with the prospective study participants.
To ensure accuracy and real-world applicability of our research, we consult and collaborate with our internal patient advocacy and patient support teams, as well as with our clients and patients representing the target trial patient profiles. We then tie our findings back with any existing proprietary data that we have in connection with the therapeutic area or the prospective target patient group.
Our unique position within the clinical recruitment chain gives us the presence and deep-rooted access needed to effectively tap into any of the three patient traffic sources: digital recruitment, offline recruitment, or patient advocacy recruitment.
Once a recruitment campaign has gone live, we constantly monitor, analyze and optimize our performance to make sure that the processes we have in place are as efficient as possible and drive the greatest results. In addition, we have the capability to layer in any traditional advertising (such as billboard ads) if requested by the study sponsor.
Therapeutic Expertise
Participate

Explore end-to-end solutions throughout development — from portfolio optimization and regulatory strategy, to Phase I-IV clinical trials, market access planning, and more.
- Portfolio management and asset valuation
- Early development and innovation
- Integrated clinical development
- Approval and access
- Value substantiation lifecycle management
HOW WE DO IT
- Operational excellence
- Delivery models
- Building patient insights into assets, profile and claims
- Portfolio Optimization
- Asset Valuation and Indication Prioritization
- Early Evidence Review
- Model-Based Drug Development
- Integrated Development Strategy and Planning
- Phase I Clinical Trials
- Proof of Concept Studies: Phase IB-IIA
- Patient Engagement Strategy and Enrollment Solutions
- Patient Inclusion
- Site Alliance Network and KOL Engagement
- Protocol Optimization
- Regulatory Strategy
- Market Access Strategy and Delivery
- Biomarker and Genomic Medicine Strategy
- Clinical Trial Supply & Logistics
- Medical Communications
- Phase IIB-IV Clinical Trials
- Real World Evidence
- Protocol-Driven, Customized Site Solution Strategy
- Regulatory Strategy, Submissions, Compliance, and Outsourcing
- Clinical Development Technology Optimization
- Global Regulatory Submissions and Outsourcing
- Compliance and Risk Management
- Real-World Evidence, Market Access Strategy and Planning
- Regulatory Compliance, Drug Safety and Pharmacovigilance
- Lifecycle Optimization
- Leveraging AI and digital in clinical development
- Biotech Clinical Trial Solutions
- Gain an advantage through FSP

Utilize our expertise across therapeutic areas, combining innovative trial designs, leading clinical and regulatory expertise, global reach, and a passion for changing patient lives.
- Neuroscience
- General medicine
- Infectious disease & vaccines
- Inflammation & immunology
Cross-Therapeutic Expertise
- Cell & gene therapies
- Rare diseases

Our experts help you stay at the forefront of the industry - and ahead of change.
New Medicines, Novel Insights
- Advancing rare disease drug development
- Accelerating development of cell and gene therapies
- Achieving patient-guided drug development
Discussions on Diversity
- Chapter 1 Bridging the Gap
- Chapter 2 Beyond the Binary
The Regulatory Navigator
- BIOSECURE Act: Implications for US-based drug developers
- Exploring ICH Q5A revision 2
- Potency assurance for CGT products: FDA's new draft guidance
Latest Report

New Medicines, Novel Insights: Achieving patient-guided drug development

Thinking about joining a clinical trial? Learn the drug development process, what it’s like to participate, how to find a trial, and answers to frequently asked questions.
INTERESTED IN PARTICIPATING?
- Healthy Volunteers
- Patient Volunteers
- Patient Advocacy Groups
HEAR FROM REAL PATIENTS
- Patient Stories
TRIAL SITES

Want to collaborate with us to offer clinical trials at your site? We would welcome the opportunity to discuss.
We are one of the largest CROs in the world, speeding life-changing medicine to market by engaging patients With Heart ™. Learn about who we are, what we do, and what we believe.
- Management team
- Global reach
Our DE&I strategy
- Compliance, Tax & Privacy
- Meet us at an event
- Jamie Macdonald
- Peyton Howell
- Michael Crowley
- Jonathan Shough
- Sheri McCoy
- Michael Bruun
- John Groetelaars
- Susan Salka
- Global Reach
- Our DE&I Strategy
Our ESG Strategy
- Compliance & Ethics
- Tax Strategy
- Privacy Policy
- Supplier Data Privacy Requirements
- Terms of Use
- French Gender Pay Data
- UK Gender Pay Data
- UK Modern Slavery Act Statement
- EU-U.S. Data Privacy Framework (EU-U.S. DPF)
- Supplier Diversity Policy
- World Vaccine Congress
- Patients as Partners U.S.
- MAPS Americas
- ASCPT 2024 Annual Meeting
- Managing Complexity in Cell and Gene Trials: Establishing an RBQM Strategy that Drives Better Performance
- EDI in clinical research: The case for adaptive strategies at every step
- 17th ISCR ANNUAL CONFERENCE 2024
- IncluDE Site Solutions Summit
- AAN Annual Meeting
- ASGCT Annual Meeting
- Dermatology Drug Development Summit EU
- World Orphan Drug Congress US
- AACR Annual Meeting
- BIO International Convention
- DIA Global Annual Meeting
- International Society for Cell & Gene Therapy
- Final countdown: How to transition your trials under EU-CTR
- SCRS: Oncology Site Solutions Summit
- Accelerating Vaccine Development Through Operational Excellence
- Alzheimer’s Disease vs Multiple Sclerosis Drug Development: Similarities, Differences & New Perspectives
What can we help you find today?
At Parexel, we speed your life-changing medicines to patients
Parexel is among the world’s largest clinical research organizations (CROs), providing the full range of Phase I to IV clinical development services to help lifesaving treatments reach patients faster. Leveraging the breadth of our clinical, regulatory, and therapeutic expertise, our team of more than 21,000 global professionals works in partnership with biopharmaceutical leaders, emerging innovators, and sites to design and deliver clinical trials with patients in mind, increasing access and participation to make clinical research a care option for anyone, anywhere.
Our depth of industry knowledge and strong track record gained over the past 40 years is moving the industry forward and advancing clinical research in healthcare’s most complex areas, while our innovation ecosystem offers quality solutions to make every phase of the clinical trial process more efficient. Our top-notch people, insight, and focus on operational excellence allow us to work every day to treat patients with dignity and continuously learn from their experiences, so every trial makes a difference.
This approach continues to earn us recognition industrywide, with Parexel being named “Best Contract Research Organization” in November 2023 by an independent panel for Citeline, “Top CRO to Work With” by investigative sites worldwide in the 2023 WCG CenterWatch Global Site Relationship Benchmark Survey and recipient of the 2023 Society for Clinical Research Sites (SCRS) Eagle Award for advancing the clinical research profession through strong site partnerships.
Read the transcript
It starts with people. They may come from anywhere. But they’re driven by a common goal: Their search for a brighter future.
They inspire us to learn from their lives across language and race, ability, ethnicity, and community.
So we design clinical trials that give them a voice, honor their sacrifice, and treat them as equals.
We are more than 21,000 professionals working with passion and perseverance to open doors. To lead change. And find new ways to work together.
We make participation easier and partnerships more productive to get to results faster and treatments sooner.
So every patient’s step forward brings them one step closer to a cure, to care, to hope.
Delivered with heart.

Leading the industry in trial inclusivity and accessibility
Within clinical research, patient diversity is critical. Inclusive studies move us closer to healthcare equity and more accurately reflect real-world populations — resulting in a far greater understanding of how the treatment will affect the people who need it. Our approach to designing more diverse, accessible trials is leading the industry forward.
Our family of brands
Parexel Academy
Your trusted partner in learning. Parexel Academy is committed to building and enhancing capabilities within the clinical research workforce so that, together, we make a positive impact on the lives of patients around the world.

Health Advances
As a trusted strategic advisor to healthcare and life science executives, Health Advances catalyzes your success with insight, innovation, and integrity.
The Medical Affairs Company
The industry’s leading provider of comprehensive outsourced medical affairs solutions.
Patients First
Empowerment and accountability.
Each of us, no matter what we do at Parexel, contributes to the development of a therapy that ultimately will benefit a patient. We take our work personally, we do it with empathy, and we're committed to making a difference.
From the smallest detail to the largest, we take quality seriously. We focus on the details while never losing sight of the big picture to drive the best possible outcome.
In our quest for innovation, we recognize and uphold the importance of all people, from our employees to our clients and the patients we all serve.
We follow our hearts, we do the right thing, and we have the courage to own the outcome.
Awards & Recognition
2024 ViE Best Contract Research Organization
Parexel was selected by a distinguished industry advisory board for its range of services in niche and core therapeutic areas, methods of performance improvement, attention to and quality of relationships with clients, reaching milestones and final outcomes, and building and maintaining existing and long-term partnerships. The annual ViE Awards, organized by Terrapin, celebrate the industry’s most outstanding achievements and showcase excellence in the global vaccine industry.
2023 Scrip Award
Parexel was named “Best Contract Research Organization” in the Full-Service Provider category at the 19th Annual Scrip Awards. The annual Scrip Awards, organized by Citeline, are designed to celebrate and recognize the very best innovations and achievements in global biopharma.
2023 HBA ACE Award
Parexel was recognized with the 2023 Healthcare Businesswomen’s Association’s (HBA) “Advancement. Commitment. Engagement (ACE) Award,” which recognizes companies for their creation and implementation of initiatives that deliver impactful outcomes designed to close the gender gap in the healthcare ecosystem. Parexel was one of two companies chosen as a result of the strong outcomes from its business initiative “Priority: Advancing Women in Leadership.”
2023 Eagle Award by Society of Clinical Research Sites
The SCRS Eagle Award recognizes the sponsor and CRO committed to outstanding leadership, professionalism, integrity, passion and dedication to advancing the clinical research profession through strong site partnerships. Recipients of the Eagle Award are selected based on votes cast from the global site community.
Ecovadis 2023
For the second year in a row, Parexel has received a “silver” rating in the 2023 EcoVadis Sustainability Rating Program. Ecovadis is the world’s largest and most trusted provider of business sustainability ratings. A silver rating is awarded to organizations with a structured and proactive sustainability approach with strong reporting on KPIs and actions.
2023 CRO Leadership Awards
Parexel has been recognized with CRO Leadership Awards for the 12 th consecutive year across all five categories – Capabilities, Compatibility, Expertise, Quality, and Reliability – for exceeding customer expectations in different customer segments. Winning CROs are chosen based on feedback from sponsor companies that they have worked on an outsourced project within the previous 18 months.
2023 WCG CenterWatch Global Site Relationship Benchmark Survey
Parexel was ranked as the “Top CRO to Work With” by investigative sites worldwide in the 2023 WCG CenterWatch Global Site Relationship Benchmark Survey for the second straight time. Among 34 CROs, Parexel received the highest average rating across all 26 performance attributes evaluated in the survey. In addition to receiving the highest average rating across all attributes, Parexel ranked highest on four out of the five attributes considered the most important to investigative sites and was selected as the CRO that investigative sites were most willing to recommend to a colleague.
2022 Catalyst Awards
Parexel was named a 2022 Catalyst Award winner by the global nonprofit organization Catalyst. Parexel was recognized for its Leveraging Gender Partnership to Advance Women in Leadership initiative that has evolved the company culture to one where women have the right resources and training to succeed.
FlexJobs’ Top 100 Companies to Watch for Remote Jobs in 2022
Parexel was recognized as a company to watch on FlexJobs' 10th annual list of the Top 100 Companies to Watch for Remote Jobs in 2023. Parexel is one of only five companies to have made the FlexJobs' list each year since its inception in 2014.
Human Rights Campaign Corporate Equality Index 2022
Parexel is proud to be featured on the Human Rights Campaign’s 2022 Corporate Equality Index, the premier survey benchmarking tool on how corporations across the US and beyond are adopting equitable workplace policies, practices and benefits for LGBTQ+ employees
Management Team
Meet our management team
Explore our environmental, social, and governance (ESG) report
- Claim $100 of Free Data

Top 15 Clinical Research Companies: Leaders in Medical Innovation
What’s on this page:

In the healthcare industry, the number of contract research organizations in the US has reached 2,823 in 2023. This marks a subtle but significant increase of 0.9% compared to the previous year.
This increase signals a vital trend: the growing complexity of finding the best clinical research companies in a crowded field. These organizations aren’t just businesses; they’re important in advancing medicine and developing drugs and therapies.
With such an important task, choosing the right company becomes essential. In this guide, we’ve looked closely at many companies along with their strengths and weaknesses and made a list of the top clinical research organizations.
By the end, you’ll know which company is the best fit for your needs.
Quick List of Top 15 Clinical Research Companies
Here is a quick overview of the best companies of clinical research:
- IQVIA: Best for data-driven insights and advanced analytics in healthcare research.
- ICON: Best for comprehensive clinical development services and therapeutic expertise.
- Parexel: Best for global biopharmaceutical services, emphasizing regulatory and clinical trial excellence.
- Syneos Health: Best for integrated biopharmaceutical solutions and clinical-commercial capabilities.
- PPD: Best for drug development services with innovative, technology-enhanced trial strategies.
- Labcorp: Best for comprehensive clinical testing and diagnostics services with global reach.
- Medpace: Best for expertise in clinical research and regulatory affairs for pharmaceutical companies.
- Charles River Laboratories: Best for preclinical research and development services, including animal testing and research models.
- PRA Health Sciences: Best for clinical trial expertise and integrated solutions for biopharmaceutical development.
- AdvanCell: Best for innovative cell and tissue-based research solutions for life science industries.
- Dynata: Best for data-driven insights and market research services for informed decision-making.
- Covance: Best for end-to-end drug development solutions, from preclinical to post-marketing.
- MedNet: Best for technology solutions and eClinical platforms for streamlined clinical trials.
- Fisher Clinical Services Inc: Best for global logistics and supply chain services for clinical trial materials.
- Worldwide Clinical Trials: Best for specialized CRO offering personalized clinical research solutions.
3 Best Clinical Research Organizations: Comparison Chart
Here’s a comparison table to highlight the key features and differences among the best companies of clinical research. This table aims to provide a quick overview of each company’s unique strengths and areas of expertise in the pharmaceutical and healthcare research sector.
3 Top Clinical Research Organization List For Advanced Medical Discoveries

Now, we’ll explore the top clinical research organizations (CROs) dedicated to advancing medical discoveries. Let’s jump into the details of these exceptional organizations.
IQVIA is a global leader in clinical research and healthcare data analytics. They play a crucial role in the medical field by providing comprehensive data, advanced analytics, and expert insights. This helps pharmaceutical and healthcare companies make smarter, more effective decisions.
Why is IQVIA among the best? Their strength lies in their vast database and advanced technology, which enable them to analyze complex healthcare data efficiently. This leads to a better understanding of diseases, more effective treatments, and faster drug development.
IQVIA’s work is essential because it speeds up the process of bringing new medicines to the market, ultimately benefiting patients worldwide. In short, IQVIA is a key catalyst in advancing global healthcare.

About IQVIA
- Founding Team: Dennis Gillings
- Founding Year: 1982
- Company Size: 86,000
Features of IQVIA
IQVIA, a prominent player in the life sciences sector, is dedicated to advancing healthcare through connected intelligence. Here are some key features of IQVIA in the world of clinical research:
Innovative Clinical Development
IQVIA is reimagining clinical development by intelligently connecting data, technology, and analytics. This approach leads to faster decision-making and reduced risk, enabling the delivery of life-changing therapies more quickly.
Efficient Payment Systems for Clinical Trials
They have simplified the process of paying sites involved in clinical trials. IQVIA offers the capability to make payments within 30 days, even in challenging locations. This significantly reduces the administrative burden of managing clinical trial payments by up to 90%.
Decentralized Trials Expertise
The company has conducted over 500 studies in more than 75 countries, covering over 30 indications using decentralized trial methodologies. This demonstrates their capability in managing complex, multinational clinical trials.
Global Reach and Impact
With a presence in various regions, including Australia, New Zealand, the Middle East, and Africa, IQVIA’s global footprint allows it to drive healthcare innovations worldwide.
AI and Technology Integration
The company is at the forefront of integrating AI and other technologies in healthcare. Their Healthcare-grade AI promises precision, speed, scale, trust, and reliability, essential for advancing health and improving patient outcomes.
- Extensive, reliable healthcare data enhances market research quality.
- Utilizes AI and machine learning for advanced healthcare insights.
- Specialized focus yields a deep understanding of healthcare dynamics.
- Broad international presence enables diverse and large-scale studies.
- Offers advanced tools for insightful healthcare data analysis.
- Advanced tools can be challenging to use without training.
- Handling sensitive health data raises privacy and security issues.
Our Review of IQVIA
IQVIA, a prominent player in the healthcare and life sciences industry, presents a mixed bag of strengths and weaknesses. On the positive side, we appreciate IQVIA’s extensive expertise in data analytics and healthcare consulting.
Their comprehensive research and analysis have undoubtedly driven valuable insights and innovations in the sector. Moreover, their global presence allows for diverse perspectives and access to critical healthcare data.
However, we must also acknowledge some shortcomings. IQVIA’s services can be prohibitively expensive for smaller organizations, limiting accessibility. Additionally, the sheer volume of data can sometimes lead to information overload, making it challenging to extract actionable insights.
ICON is a prominent company in the field of clinical research, playing a significant role in advancing medical science. They specialize in designing and conducting clinical trials for new medicines and treatments.
The work of ICON is crucial because they help determine the safety and effectiveness of these potential medical breakthroughs. They are considered one of the best in clinical research due to their high standards of accuracy, reliability, and ethical practices.
ICON’s expertise ensures that the clinical trials they manage are conducted efficiently and effectively, leading to faster approval of new treatments. This directly impacts patient care, as it allows quicker access to new, potentially life-saving medicines.
In essence, ICON’s contribution is vital in driving forward medical innovations.
- Founding Team: John Climax and Ronan Lambe
- Founding Year: 1990
- Company Size: 41,160
Features of ICON
Here are some of the key features of ICON in clinical research:
Diverse Clinical and Scientific Operations
ICON offers a wide range of clinical and scientific operations services, ensuring comprehensive support for various aspects of clinical trials. This includes everything from study design to execution and data analysis.
Decentralized Clinical Trial Solutions
They provide end-to-end services, operational models, and technology to deliver customized solutions for decentralized clinical trials. This approach is increasingly important in today’s clinical research landscape, offering flexibility and efficiency.
Specialized Therapeutic Areas
ICON has expertise across multiple therapeutic areas including cardiovascular, central nervous system, endocrine & metabolic disorders, infectious diseases, internal medicine & immunology, oncology, and more. This broad expertise allows them to handle a wide range of clinical research projects.
Innovative Solutions for Biotech
ICON provides full-service outsourcing and flexible support customized to the specific needs of biotech companies. This includes due diligence and asset valuation, which are critical for biotech firms navigating the complex landscape of drug development.
Advanced Medical Imaging Solutions
Their expert medical imaging solutions support all stages of clinical research, improving decision-making, increasing efficiency, and reducing trial costs.
- Decades of expertise ensure high-quality clinical research.
- Offers wide-reaching capabilities for multi-regional clinical studies.
- Deep understanding of global regulations enhances compliance and efficiency.
- Invests in new technologies for more efficient trial processes.
- Broad range of specialties contributes to comprehensive service offerings.
- Managing multi-regional trials can lead to logistical challenges.
- Rapid growth may strain resources and affect service quality.
Our Review of ICON
When we researched ICON, we found both commendable aspects and areas for improvement. On the positive side, we appreciate their commitment to clinical research and their global presence, which allows for diverse study options. Their experienced team and advanced technology contribute to reliable data collection and analysis.
However, there are some drawbacks to consider. We have noticed occasional delays in project timelines, which can be frustrating. Additionally, the cost of their services tends to be on the higher side, making it a potential barrier for smaller research endeavors.
Parexel is a globally recognized company in clinical research, known for its important role in developing new medical treatments. They are one of the biggest clinical research organizations. Parexel conducts clinical trials, crucial steps in testing the safety and effectiveness of new drugs.
The work of Parexel is essential because it bridges the gap between medical research and the availability of new treatments to patients. One of the reasons they stand out as one of the best in this field is their rigorous approach to research.
Their commitment to quality and their global network also enables diverse and large-scale studies, setting them apart from others in the field. These strengths allow Parexel to deliver reliable and valuable data, accelerating the process of bringing new, effective medicines to the market.
Simply put, Parexel is a key player in transforming medical research into real-world health solutions.
About Parexel
- Founding Team: Josef von Rickenbach and Anne B. Sayigh
- Company Size: 18,900
Features of Parexel
Parexel, a global biopharmaceutical services organization, offers a range of features in clinical research. Here are some key aspects of their approach:
Patient-Centric Approach
Parexel emphasizes a patient-first strategy in their clinical trials. This approach results in deeper and more relevant insights for trial design and execution. This ensures that the trials are more aligned with patient needs and experiences.
Innovative Trial Designs
Parexel employs innovative trial designs to optimize trials for maximum impact. This includes advanced modeling and simulation to predict drug effects ahead of time, which can save time, money, and resources.
Regulatory Compliance and Market Access
Parexel designs studies and endpoints with market access in mind, ensuring that they satisfy global regulations. This approach helps in getting treatments to patients safely and quickly.
Patient Advocacy and Engagement
The company includes patient advocates in their council, using their experiences to improve trial designs. This inclusion demonstrates their commitment to understanding and incorporating patient perspectives in clinical research.
Focus on Speed and Precision
Parexel aims to design neuroscience trials with speed and precision, utilizing the right experts and specializations. This focus is crucial in delivering effective treatments on time.
- Provides advanced technology and analytics for efficient data management.
- Extensive network provides global insights with regional knowledge.
- Expertise in navigating complex regulatory environments worldwide.
- Broad experience across various therapeutic areas ensures versatile solutions.
- Focuses on patient engagement for more effective trial outcomes.
- Rapid expansion can lead to challenges in resource management.
- Concentration in specific areas could pose risks in market shifts.
Our Review of Parexel
Parexel is a notable player in the field of clinical research and pharmaceutical services. We’ve thoroughly analyzed their offerings and found both strengths and areas that need improvement.
On the positive side, Parexel excels in its commitment to innovation and technology. We appreciate their continuous efforts to simplify clinical trials and drug development processes, making them more efficient.
However, we also noticed some downsides. Communication with clients could be more transparent, with clearer updates on project progress. Additionally, there’s room for improvement in terms of ensuring consistency in service quality across different projects.
Other 12 Companies of Clinical Research

In the world of clinical research, beyond the well-known names, there are 12 other companies making significant contributions. Let’s explore their vital role in advancing healthcare.
1. Syneos Health
Syneos Health helps develop medicines by managing clinical trials for new drugs. They’re essential because they ensure medicines are safe and effective. Syneos Health stands out in clinical research for its comprehensive services and global reach, making drug development smoother and faster.
About Syneos Health
- Founding Team: Colin Shannon
- Founding Year: 1980
- Company Size: 28,000
PPD is a group that tests new drugs to see if they’re good and safe. This is crucial for getting new treatments to people. They stand out for their thorough research and global reach.
- Founding Team: Fred Eshelman
- Founding Year: 1985
- Company Size: 40,000+3
Labcorp does important tests and research for health. They’re needed because they help find out if new treatments are good. They’re among the best for their big labs and fast results.
About Labcorp
- Founding Team: Matthew Benger
- Founding Year: 1978
- Company Size: 75,5000
Medpace focuses on making sure new health treatments are safe. This is key for better medicine. They’re a top choice because of their focus on quality and detail in research.
About Medpace
- Founding Team: August Troendle
- Founding Year: 1992
- Company Size: 5,400
5. Charles River Laboratories
Charles River Laboratories tests drugs and does research to help pets and people stay healthy. They’re essential for safe, new treatments. Their expertise makes them a leader in the field.
About Charles River Laboratories
- Founding Team: Henry Foster
- Founding Year: 1947
- Company Size: 21,400
6. PRA Health Science
PRA Health Science works on finding out if new medicines are safe. This helps everyone get better treatments. They’re known for their excellent research and care in studies.
About PRA Health Science
- Founding Year: 1976
- Company Size: 17,000+
7. AdvanCell
AdvanCell specializes in new treatments, checking if they’re safe and working. Their work is vital for progress in medicine. They’re recognized for their innovation in research.
About AdvanCell
- Founding Team: Andrew Adamovich
Dynata gathers data for health studies. They’re needed for understanding what works in healthcare. They’re a top name for their accurate and wide-reaching data collection.
About Dynata
- Founding Team: Mike Petrullo
- Founding Year: 1940
- Company Size: 5000-10000
Covance helps with drug tests and research to fight diseases. Their role is key for new treatments. They’re celebrated for their comprehensive services and global impact.
About Covance
- Founding Team: Fred Cummings
- Founding Year: 1981
- Company Size: 50,000
MedNet provides software for managing clinical trials. This helps in making research easier and faster. They’re among the best for their tech solutions in research.
About MedNet
- Founding Team: John “Rob” Robertson
- Founding Year: 1996
- Company Size: 51-200
11. Fisher Clinical Services Inc.
Fisher Clinical Services Inc. manages the logistics of clinical trials, ensuring that treatments are tested efficiently. Their work is crucial for the progress of medicine, and they are renowned for their reliability and global network.
About Fisher Clinical Services Inc.
- Founding Team: John Pickering
- Founding Year: 1989
12. Worldwide Clinical Trials
Worldwide Clinical Trials conducts essential research to evaluate new medical treatments. Their work is critical for advancing healthcare. They are distinguished by their global expertise and commitment to innovation in clinical research.
About Worldwide Clinical Trials
- Founding Team: Neal Cutler
- Founding Year: 1986
- Company Size: 3,147
What To Consider When Choosing the Best Clinical Research Companies?
Choosing the right clinical research company (CRC) is crucial for the success of any clinical trial. Here’s a detailed guide on what to consider:
Expertise and Specialization
Always ensure the CRC has expertise in your specific therapeutic area. Companies with experience in similar drug trials or medical devices can better navigate the complexities of your project.
Regulatory Compliance
The CRC must adhere to regulatory guidelines like FDA (US) , EMA (Europe), and others. Check their track record in meeting these standards to avoid compliance issues.
Reputation and Track Record
You should research the company’s history. Look for testimonials, case studies, and reviews from past clients. A company with a strong reputation is likely to deliver quality results.
Project Management Capabilities
Effective project management is key. Assess their ability to manage timelines, budgets, and communication. A CRC that provides transparent, regular updates is preferable.
Patient Recruitment Strategies
Patient recruitment can be challenging. Evaluate their strategies for participant recruitment and retention. Consider their demographic reach and methods for ensuring a diverse participant pool.
Data Management and Analysis
The CRC should have strong systems for data collection, management, and analysis. Ask about their use of Electronic Data Capture (EDC) systems and how they handle data security and confidentiality.
Cost and Financial Terms
Get a clear understanding of the cost structure. Consider the value for money rather than just the lowest cost. Ensure there are no hidden fees and clarify what is included in the quoted price.
How Heartbeat AI Can Help You Get the Best List of Clinical Research Organizations?
Heartbeat AI, with its advanced features, can significantly assist in identifying the best list of clinical research organizations (CROs). Here’s how its various features contribute to this process:
Data Analysis and Processing
Heartbeat AI excels in analyzing vast amounts of data. When it comes to selecting CROs, it can process and analyze information from numerous sources, including past performance records, clinical trial reports, and regulatory compliance data. This thorough analysis helps in identifying CROs with a proven track record of success and reliability.
Machine Learning Algorithms
These algorithms enable Heartbeat AI to learn from historical data and improve its recommendations over time. By understanding trends and patterns in the successful execution of clinical trials, it can better predict which CROs are likely to meet your specific needs.
Predictive Analytics
Heartbeat AI uses predictive models to forecast future trends and outcomes based on historical data. This can be invaluable in predicting the success rate of CROs in upcoming projects, thus aiding in making more informed choices.
Customization and Personalization
The AI can be customized to your specific requirements. If you’re focusing on a specific therapeutic area or clinical trial phase, Heartbeat AI can prioritize specialized CROs in these fields.
Real-time Data Updates
The healthcare and pharmaceutical landscapes are constantly changing. Heartbeat AI’s ability to integrate and analyze real-time data ensures that the recommendations are based on the most current information available.
Integration with External Databases
Heartbeat AI can integrate with various external databases and platforms. This enables it to pull in comprehensive information about CROs from diverse sources, enhancing the accuracy of its recommendations.
Claim $500 of Free Data
Summing up, we’ve explored the best clinical research companies, diving into their features, strengths, weaknesses, and more. Clinical research is vital in healthcare; it’s key for advancing medical knowledge and developing new treatments.
With this guide, you’re equipped to find the right clinical research company that meets your specific needs. Whether it’s for innovative therapies, drug development, or medical advancements, choosing the right partner is crucial. This guide serves as a valuable resource to help you make an informed decision in the complex world of clinical research.
Frequently Asked Question
What services do companies of clinical research offer.
Clinical research organizations offer a wide range of services, including protocol development, patient recruitment, data collection and analysis, regulatory compliance, and more.
What is the role of a clinical research coordinator?
A clinical research coordinator is responsible for managing various aspects of a clinical trial, including patient recruitment, data collection, and ensuring compliance with protocols.
What is informed consent in clinical research?
Informed consent is the process by which participants in a clinical trial are fully informed about the study’s purpose, risks, and benefits. They voluntarily agree to participate based on this information.
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Enter your account information
- Access 11m+ Healthcare providers
- 100% Risk Free Trial
- No credit card required
Enter your phone number

Velocity is the world's leading integrated site organization.
Sponsors and cros trust velocity to deliver high-quality clinical trial data and patient care with unprecedented efficiency., simplify everything from site selection to study close-out.
Velocity unifies operational processes to provide world-class sites, reliable enrollment, and predictably high performance for your trials.
The right sites. The right investigators. The right partner for you.
Strategically located to give you access to diverse specialty populations, Velocity's sites are supported by next-gen technologies and patient engagement capabilities. Welcome to recruitment and retention reimagined.
Scale for a purpose: Supporting research programs worldwide
From the leading pharma companies, to the most pioneering biotech startups, Velocity supports those who are exploring new frontiers in human health.
Whether you’re ready to conduct a single-site study or a complex, high-volume clinical trial, contact Velocity.

Video: MASH CARE Council Leader Kris Kowdley, MD, AGAF, FAASLD, FACP, FACG
The global prevalence of metabolic dysfunction-associated steatotic liver disease (MASLD) is rising. In light of the stark unmet needs in liver disease treatment — particularly for metabolic dysfunction-associated steatohepatitis (MASH) … Read more

How Does Site Location Impact Patient Diversity in Clinical Trials?
Velocity recently asked some key employees to come together and discuss a wide array of issues impacting diversity, equity, and inclusion (DEI) in clinical trials. Velocity’s ongoing goals are to … Read more

Video: CARE Councils | Évelyne Newton and Sylwia Kołodziej
Évelyne Newton and Sylwia Kołodziej recently made the journey overseas to join our CARE Council summit in Durham, NC. Along with dozens of our physicians and operational, commercial, and recruitment … Read more

Velocity Wins the 2024 Vaccine Industry Excellence Award for Best Clinical Trial Company
Velocity has won the 2024 Vaccine Industry Excellence (ViE) award for Best Clinical Trial Company at The World Vaccine Congress in Washington, DC. The recognition is based on performance metrics, … Read more
Quality. Continuity. Velocity.
Asia & Oceania
Middle east & africa.
- United States
- Asia Pacific
- Australia & NZ
- Southeast Asia
- Czech Republic
- Deutschland
- España
- Switzerland
- United Kingdom

EMEA Thought Leadership
Developing IQVIA’s positions on key trends in the pharma and life sciences industries, with a focus on EMEA.
- Middle East and Africa
- Research & Development
- Real World Evidence
- Commercialization
- Safety & Regulatory Compliance
- Technologies
LIFE SCIENCE SEGMENTS
- Pharmaceutical Manufacturers
- Emerging Biopharma
- Consumer Health
HEALTHCARE SEGMENTS
- Information Partner Services
- Financial Institutions
- Public Health and Government
- Patient Associations
THERAPEUTIC AREAS
- Cardiovascular
- Cell and Gene Therapy
- Central Nervous System
- GI & Hepatology
- Infectious Diseases and Vaccines
- Rare Diseases

Impacting People's Lives
"We strive to help improve outcomes and create a healthier, more sustainable world for people everywhere.

Harness the power to transform clinical development
Reimagine clinical development by intelligently connecting data, technology, and analytics to optimize your trials. The result? Faster decision making and reduced risk so you can deliver life-changing therapies faster.
Research & Development Quick Links
- Clinical Trials
- Functional Services
- Decentralized Trials
- Therapeutic Expertise
- Site and Investigators

Real World Evidence. Real Confidence. Real Results.
Generate and disseminate evidence that answers crucial clinical, regulatory and commercial questions, enabling you to drive smarter decisions and meet your stakeholder needs with confidence.
Real World Evidence Quick Links
- Real World & Health Data Sets
- Medical Affairs
- Health Data Apps & AI
- Health Data Transformation
- Study Design
- Evidence Networks
- Health Economics & Value
- Regulatory and Safety
- Natural Language Processing
- Real World Evidence Library

See markets more clearly. Opportunities more often.
Elevate commercial models with precision and speed using AI-driven analytics and technology that illuminate hidden insights in data.
Commercialization Quick Links
- COVID-19 & Commercialization
- Launch Strategy & Management
- Brand Strategy & Management
- Pricing & Market Access
- Healthcare Professional Engagement
- Patient Engagement
- Promotional Strategy

Service driven. Tech-enabled. Integrated compliance.
Orchestrate your success across the complete compliance lifecycle with best-in-class services and solutions for safety, regulatory, quality and medical information.
Safety & Regulatory Compliance Quick Links
- Safety Pharmacovigilance
- Regulatory Compliance
- Quality Compliance
- Medical Information
- Commercial Compliance

Intelligence that transforms life sciences end-to-end.
When your destination is a healthier world, making intelligent connections between data, technology, and services is your roadmap.
Technology Quick Links
- Orchestrated Clinical Trials
- Enterprise Information Management
- Performance Management & Insights
- Provider Reference Data Network
- Customer Engagement
- Safety, Regulatory, Quality Compliance
- Partner Programs
- Technology Insights
CLINICAL PRODUCTS
- Planning Suite
- Clinical Trial Payments
- Investigator Site Portal
- One Home for Sites
- Patient Engagement Suite
- Electronic Clinical Outcome Assessment (eCOA)
- Interactive Response Technology (IRT)
- Clinical Data Analytics Solutions
COMMERCIAL PRODUCTS
- Information Management
- Promotional Engagement
- Orchestrated Analytics
- Next Best Action
- Customer Engagement (OCE)
COMPLIANCE, SAFETY, REG PRODUCTS
- IQVIA Vigilance Platform
- SmartSolve eQMS
- Safety & Pharmacovigilance
REAL WORLD PRODUCTS
- Data Profiling
- Patient Profiling
- HCP Profiling
- Market Profiling

"Data in a Day
BLOGS, WHITE PAPERS & CASE STUDIES
Explore our library of insights, thought leadership, and the latest topics & trends in healthcare.
THE IQVIA INSTITUTE
An in-depth exploration of the global healthcare ecosystem with timely research, insightful analysis, and scientific expertise.

INSTITUTE REPORT
"Global Trends in R&D 2024

EMEA Insights
"IQVIA thought leadership with a focus on EMEA.

WHITE PAPER
"DCTs Deliver Big ROI

"Capturing value at scale: The $4 billion RWE imperative
FEATURED INNOVATIONS
- IQVIA Connected Intelligence™
- IQVIA Healthcare-grade AI™
- Human Data Science Cloud
- IQVIA Innovation Hub
- Patient Experience powered by Apple
Commitment to Public Health
- Code of Conduct
- Environmental Social Governance
- Executive Team
NEWS & RESOURCES
- Events & Webinars
- Industry Analyst Reports
- COVID-19 Resources
- Our Locations

INVESTOR RELATIONS
"Visit our investor relations site for more information.

Unlock your potential to drive healthcare forward
By making intelligent connections between your needs, our capabilities, and the healthcare ecosystem, we can help you be more agile, accelerate results, and improve patient outcomes.

IQVIA AI is Healthcare-grade AI
Building on a rich history of developing AI for healthcare, IQVIA AI connects the right data, technology, and expertise to address the unique needs of healthcare. It's what we call Healthcare-grade AI.

Your healthcare data deserves more than just a cloud.
The IQVIA Human Data Science Cloud is our unique capability designed to enable healthcare-grade analytics, tools, and data management solutions to deliver fit-for-purpose global data at scale.

Innovations make an impact when bold ideas meet powerful partnerships
The IQVIA Innovation Hub connects start-ups with the extensive IQVIA network of assets, resources, clients, and partners. Together, we can help lead the future of healthcare with the extensive IQVIA network of assets, resources, clients, and partners.

Proven, faster DCT solutions
IQVIA Decentralized Trials deliver purpose-built clinical services and technologies that engage the right patients wherever they are. Our hybrid and fully virtual solutions have been used more than any others.

IQVIA Patient Experience Solutions powered by Apple
Empowering patients to personalize their healthcare and connecting them to caregivers has the potential to change the care delivery paradigm. IQVIA and Apple are collaborating to bring this exciting future of personalized care directly to devices patients already have and use.
WORKING AT IQVIA
Our mission is to accelerate innovation for a healthier world. Together, we can solve customer challenges and improve patient lives.
LIFE AT IQVIA
Careers, culture and everything in between. Find out what’s going on right here, right now.

WE’RE HIRING
"Improving human health requires brave thinkers who are willing to explore new ideas and build on successes. Unleash your potential with us.
OUR MISSION
Accelerating innovation for a healthier world, powering smarter healthcare for everyone, everywhere.
IQVIA Connected Intelligence
Environmental, Social and Governance
Innovation Hub
Third Party Access Program
Creating a healthier world
We want to help make extraordinary things possible for patients. We are inspired by their stories, and it is our mission to accelerate innovations that enable better outcomes. Every day, we work alongside customers and partners to unleash potential and help improve health around the world.

In pursuit of the extraordinary
We are focused on driving healthcare forward, supported by four key principles.
We strive to make a positive impact in everything we do.
We approach every problem with curiosity and an open-mind to better support our customers’ innovations.
Collaboration
We aim to bring out the best in each other and our diverse expertise. We maximize our positive impact by working together.
We are always learning and seeking opportunities for personal, professional, and organizational growth.
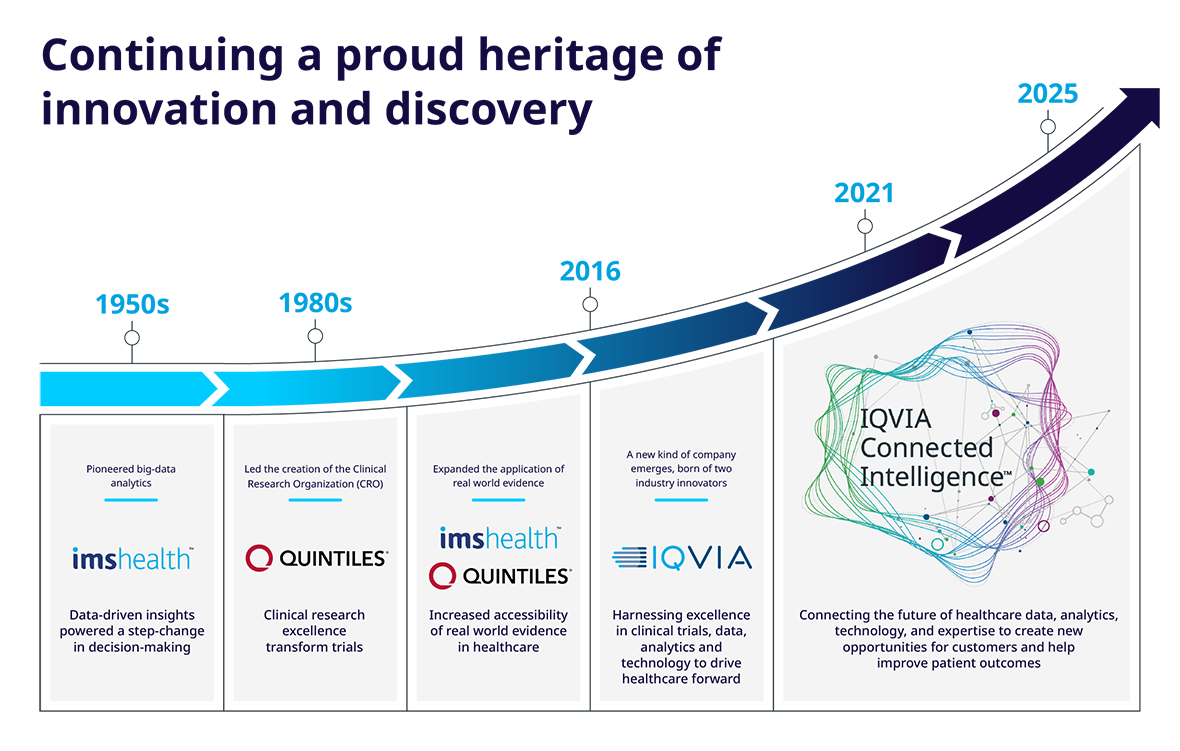

Intelligent connections to accelerate results
In today’s healthcare environment, it’s not only about how much data, information, and technology you have at your fingertips – it’s what you do with it.
IQVIA is focused on making intelligent connections for customers across the entire healthcare ecosystem to help you drive healthcare forward.
Whether that means partnering with novel technology companies to boost patient engagement, leveraging AI & machine learning to accelerate results, or using decentralized trials to reach the right patients wherever they are – we are always looking for smarter ways to move you forward.
IQVIA is a leading global provider of advanced analytics, technology solutions, and clinical research services to the life sciences industry. IQVIA creates intelligent connections across all aspects of healthcare through its analytics, transformative technology, big data resources and extensive domain expertise. IQVIA Connected Intelligence™ delivers powerful insights with speed and agility — enabling customers to accelerate the clinical development and commercialization of innovative medical treatments that improve healthcare outcomes for patients. With approximately 86,000 employees, IQVIA conducts operations in more than 100 countries.
Explore More
Code of Conduct
Featured Innovations
The IQVIA Institute
Insights Library
For this browsing session please remember my choice and don't ask again.
Discover why your CRO search is over

Your team, extended.
Partner with an expert team that seamlessly integrates with yours..

You and your work matter here.
Accessible partners. Shared commitment. Personalized for you.

Discover why it’s a world of difference at Worldwide.
Clinical research isn’t a straight path. let’s navigate it together..
At Worldwide, we believe a personalized approach is the only way to unlock the power of everyone working on a study – from operational and therapeutic experts to site partners and scientists. That means you’ll always have direct access to our experts. You’ll be able to tap into more than 30 years of therapeutic experience on a global scale. And your mission is our mission – we are dedicated to working with you to meet your goals.
White Paper
Biomarkers in Oncology Studies
The power of personalization amid the changing cro landscape.
Press Release
Worldwide Clinical Trials Expands Clinical Pharmacology Unit Pharmacy To Enhance Customer Experience; Adds Efficiencies and Decreases Timelines
Want a cro partner who gives you their full attention you’re in the right place..
Michael Murphy, MD, PhD Chief Medical and Scientific Officer
Your extended team…always within reach.
We were founded on an unwavering commitment to therapeutic excellence and personalized attention. And today, that’s more important than ever. That means you have access to our senior-level experts who will work directly with you. It means we listen to you, understand your study’s needs, and actively seek customized solutions that are tailored to your specific project – even if we need to pivot and adapt along the way.
Every study is different. Every path forward is different. And we will partner with you every step of the way.

Aman Khera, MBA, FTOPRA, FRAPS Vice President, Regulatory Science, Strategy and Innovation
Flexible? Check. Responsive and nimble? Absolutely.
We understand that no clinical trial looks the same. Your trials deserve solutions backed by decades of therapeutically relevant and adaptable expertise. That’s exactly what we bring to the table.
When you partner with Worldwide, we’ll stay by your side through the entire lifecycle of product development with nimble, solutions that are never “just off the shelf” but customized for you and your specific needs.
Let’s work together to bring clarity to complex drug development.
Schedule a consultation.
Want to see what’s possible? Connect with us!
Request a Proposal
Ready to work with a CRO partner who gives you our full attention?
Meet Us at an Event
Where in the world is Worldwide? If you’ll be at an upcoming event, let’s connect! Here’s where we’ll be:
Outsourcing Clinical Trials Southeast
Chief Medical Officer Summit
Countdown to Crunch Time: How Early and Continual Regulatory Engagement Leads to Regulatory and Commercial Success
Clinical Trials in Oncology West Coast
Send Us a Message
A member of the team is online now to help address your needs.
Explore Open Careers
Cookies on the worldwide clinical trials website.
We use cookies to improve your browsing experience and help us improve our websites. We have carefully selected third parties that use cookies to achieve purposes illustrated in the cookie policy. For more information. Read more about online privacy statement . By closing this banner or continuing to browse our website, you agree to our use of such cookies.
- Biospecimen Solutions Precision Value & Health Careers

- Clinical Trial Services Overview
Integrated Clinical Trial Services to Advance Precision Medicine
Global trials for oncology, rare diseases and other complex diseases face unique challenges. precision partners with you to meet them head-on through our extensive footprint across the globe. we succeed by pairing personalized clinical services with translational medicine excellence, making us the first fully integrated clinical research organization created expressly to help advance the promises of precision medicine., the convenience of centralized services, with people across 70+ countries, our team provides comprehensive clinical trial services that streamline and accelerate drug development at a global scale, clinical trial management.
Fully integrated biomarker-driven trial management solutions—from study start-up through full-service execution—help accelerate your path to approval.
Clinical Development Strategy
Tailored strategies consider the scientific, regulatory, and commercial factors that shape each trial, mitigating risk and advancing the development pathway.
Clinical Trial Design
Advanced trial design approaches—including basket, umbrella, and adaptive trials—deliver biomarker-driven clinical research. Deep experience in these highly complex trial designs maximizes both insights and efficiency.
Biostatistics
Seasoned biostatisticians and statistical programmers deliver insight into every trial phase, from study design to regulatory submissions, all backed by meticulous documentation and data monitoring.
Clinical Sample Management
Sample inventories from a global network of labs supply real-time processing in 55 countries; consolidated data from central labs, screening labs, and specialty labs with clinical data create actionable reports.
Global Clinical Trial Support
Sample processing labs, clinical trial sites and offices in five continents provide the clinical reach and scale to manage complex global programs.
End-to-end, biomarker-driven clinical trial solutions
Full-service, complex, global clinical research organization services designed to accelerate the path to market.
Most Recent Posts

Optimizing Investigator Databases for Oncology Trial Enrollment

Deana D. , Vincent S.
Case Study: AML Phase 1-2 Trial

Anne K. , Hasni M.
Navigating Cell and Gene Therapy Acquisitions: A Strategic Blueprint

Anshul M. , Tony K.
Clinical data science: Trial management and operations empowered by data first
Better visibility, better collaboration, better execution. targeted solutions we’ve developed based on decades of experience and being at the forefront of modern drug development – focused on the opportunities to deliver the most value for your program.
- Study design and tailored statistical analysis plans
- Clinical Data Management delivered from more than 70 team members with an average of 12 years of data management experience
- Clinical biometrics with a 100% EU regulatory agency data acceptance rate
- Create submission-ready data sets (including CDISC) using a secure 21 CFR Part 11 and GDPR compliant infrastructure
- Generate AI-derived signatures across integrated multiomic data and explore results in an intuitive, interpretable, and interactive interface
- Know precisely where every sample is with our virtual sample inventory management (vSIM)
Our clinical trial services support preclinical to phase 4 studies around the world
Case study: exceeding enrollment expectations in a rescue study: a phase 3 registrational trial in multiple myeloma.
Precision was identified by a sponsor to rescue global and US management of a phase 3 registrational trial in relapsed, refractory multiple myeloma patients. This study targeted the enrollment of 780 patients at 155 sites in 20 countries.
The sponsor’s primary motivation for pursuing the shift to Precision from the other contract research organization (CRO) was based on their recognition that clinical research associates (CRAs) with little to no multiple myeloma experience and only minimal monitoring experience had been placed on their study. As a result of inefficiencies in operations, the study’s start-up was delayed.
Discover how our clinical trial services can advance your international clinical trial
Choose a contract research organization with precision.
A contract research organization (CRO) is crucial to medical research and development to provide support that includes clinical trial management and CRO research. The right outsourced CRO services can accelerate the overall process of drug development and market placement. Whether you need a CRO for clinical trials or a CRO for pharma, Precision has a solution that can be tailored to the needs of your study.
What sets Precision apart from other contract research organizations, is the way we integrate clinical trial execution with deep scientific knowledge, laboratory expertise, and advanced data sciences. This is Precision Convergence: maximizing insights into patient biology and accelerating the pace of scientific discovery and approval.
Explore Our Other Areas of Expertise
Precision for Medicine is part of the Precision Medicine Group, an integrated team of experts that extends Precision for Medicine’s therapeutic development capabilities beyond approval and into launch strategies, marketing communication, and payer insights. As one company, the Precision Medicine Group helps pharmaceutical and life-sciences clients conquer product development and commercialization challenges in a rapidly evolving environment.
- Reach Our Healthcare List Expert +1 (786) 408 5757
- Browse Data Cards
- GET A QUOTE

The Top Players in Clinical Research: A Review of the Leading Companies
Top 10 leading clinical research companies in the usa.
The landscape of drug discovery and development is intricate, demanding significant resources at each stage of the process. As pharmaceutical companies strive to bring groundbreaking therapies to market, they increasingly rely on the expertise of Contract Research Organizations (CROs). These organizations play a vital role in supporting drug manufacturers throughout the entire drug development journey, from initial discovery to final approval. These CROs offer a comprehensive range of services, including clinical trial management, data research, and project management. Their critical role has become even more evident during the pandemic-induced challenges, where the need for rapid vaccine and drug development resulted in a surge of clinical trials.

The Impact of Covid-19 on Clinical Trials
At the outset of the pandemic, the clinical research landscape faced initial setbacks. However, the urgency to combat Covid-19 created an unprecedented rise in the number of clinical trials. The pressing demand for effective vaccines and treatments drove pharmaceutical companies and CROs to collaborate more than ever before. Looking ahead, the future of CROs appears promising, fueled by the rise of technologies that enable decentralized clinical trials. These modern approaches leverage digital tools and remote patient monitoring, reducing the need for physical site visits and expanding the reach of clinical research.
The Growth of the Global CRO Services Market
The significance of CROs is evident as the global CRO services market continues to expand exponentially. Projections indicate that this market, which was valued at US$76.6 billion , is expected to reach an impressive US$127.8 billion by 2028. This growth reflects the pivotal role CROs play in revolutionizing drug development and medical research.
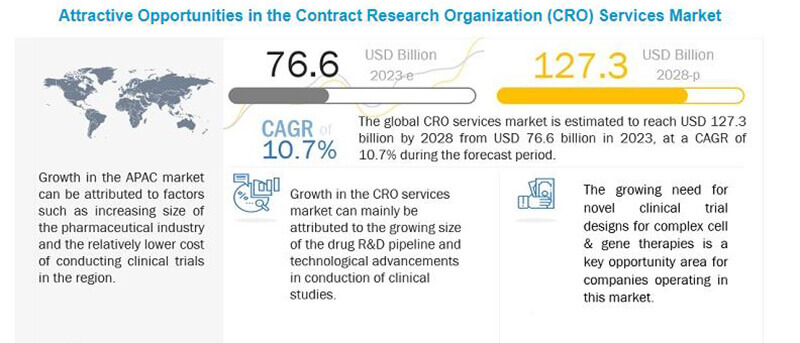
Source: Markets&Markets
Unveiling the Best 10 Clinical Research Organizations in the Field
Join us as we embark on this insightful journey, where we shine a spotlight on the ten biggest CROs shaping the pharmaceutical landscape. –
Source: Lablcorp
Labcorp, a leading provider of comprehensive drug development solutions for various industries, achieved a significant milestone in 2015 with its acquisition of Covance for an impressive $6 billion. This strategic union brought together Covance's expertise in drug development and Labcorp's unparalleled medical testing capabilities, propelling the company to become the world's foremost healthcare diagnostics company.
Over the past decade, Labcorp has continued to expand its influence through a series of strategic acquisitions. Notable additions to their portfolio include LipoScience, Inc., Bode Technology Group, Sequenom, MNG Laboratories, and Personal Genome Diagnostics (PGDx). These acquisitions have further strengthened Labcorp's position in the market, broadening its scope and enhancing its ability to provide cutting-edge solutions.
Looking forward to 2023, Labcorp unveiled its plans to spin off its Contract Research Organization (CRO) segment, creating a separate, independent publicly traded entity known as Fortrea.
Source: IQVIA
In 2016, two industry giants, Quintiles and IMS Health, joined forces and rebranded as IQVIA, establishing the largest Contract Research Organization (CRO) globally. With a presence in over 100 countries, IQVIA brings together cutting-edge advancements in data science, technology, and human science expertise, offering clients a comprehensive end-to-end clinical and commercial service. Through a series of strategic acquisitions of smaller specialist companies, IQVIA has strengthened its position and continues to lead the way in the world of CROs.
In the year 2022, IQVIA demonstrated remarkable growth and success, generating revenues of $14.494b . This impressive growth, amounting to 3.29% on a reported basis and 7.8% at constant currency , highlighted the company's continued upward trajectory. Despite uncertain market conditions, IQVIA achieved record bookings and surpassed the goals set in their Vision 22 plan, underscoring their commitment to excellence and innovation.
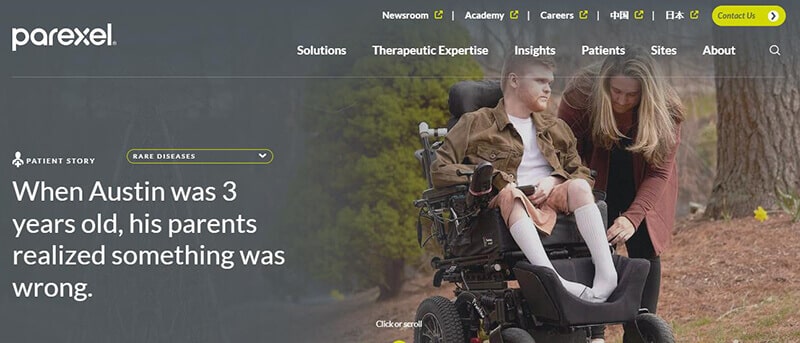
Source: Parexel
Parexel continues to hold its position as one of the largest CROs globally. Specializing in Phase I to IV clinical development services, Parexel plays a vital role in expediting and ensuring the smooth progress of the drug approval process. With a comprehensive array of service offerings, the company caters to nearly every aspect of clinical trial management, supporting sponsors in conducting successful clinical studies.
In a significant development in November 2021, Parexel was acquired by EQT Private Equity and Goldman Sachs for a remarkable $8.5 billion . Despite this transition, the commitment to putting patients first remains unwavering, ensuring that Parexel maintains its patient-centric trajectory. The newly constituted board of directors brings together a wealth of experience in the life sciences industry, further strengthening Parexel's position as a leader in the field.
4. Pharmaceutical Product Development (PPD)
Source: PPD
Pharmaceutical Product Development (PPD) stands as a leading global Contract Research Organization (CRO) with an extensive workforce of over 30,000 professionals worldwide.
The acquisition of Evidera in 2016 marked a significant milestone for PPD, solidifying their position as a leader in real-world research. Leveraging Evidera's expertise in real-world evidence, PPD has enhanced its capabilities in providing life science companies with a crucial element of the clinical development process, maintaining a competitive edge in the industry. Subsequent strategic acquisitions of Synexus (now Accelerated Enrollment Solutions) and Bioclinica, both patient recruitment and clinical research site businesses, respectively, have further augmented PPD's offerings. These collective advancements have propelled PPD's core organic growth to "high teens" in the segment, as the company achieved remarkable full-year 2022 results. With over $7.00 billion in revenue and a significant contribution of over $2.00 to adjusted earnings per share, PPD remains a dominant force in the CRO landscape, upholding their mission to facilitate groundbreaking clinical research and development.
5. Syneos Health
Source: Syneos Health
Founded in 1999, Syneos Health has its headquarters in Morrisville, North Carolina. With an impressive annual revenue of $5,213 million and a workforce of 28,000 employees, Syneos Health is a leading international Contract Research Organization (CRO) that offers a wide range of services spanning all aspects of bringing new therapies and products to the market.
While Syneos Health provides clinical development services across all stages of drug development, it has particularly specialized expertise in assisting healthcare organizations during the late stages of clinical trials. Some of them include Early phase trials, Late phase trials, Decentralized clinical trial solutions, Clinical data management, Pharmaceutical trial services, Medical device diagnostics trial services, and more.
Source: ICON
Operating across 46 locations worldwide, ICON is a top-tier Contract Research Organization (CRO) that provides a comprehensive range of services, including consulting, clinical development, and commercialization services.
In a pivotal move in 2016, ICON formed strategic partnerships with Genomics England for the UK's ambitious 100,000 Genomes Project and IBM Watson for oncology research support. These collaborations expanded ICON's service offerings and strengthened their presence in the fields of genomic science and oncology research, creating new opportunities for clinical research jobs in these cutting-edge sectors.
Additionally, the year 2022 marked a significant milestone for ICON, as it achieved impressive financial results. With full-year revenues amounting to US$7.7 billion , the company witnessed substantial growth of 41.2% compared to the previous year. On a constant currency basis, the revenue surge reached an outstanding 45%, reflecting ICON's continued success in advancing clinical research and serving as a leading partner for the life sciences industry.
7. Charles River Laboratories
Source: Charles River Lab.
Since its inception in 1947, this company has grown into an exceptional industry leader, specializing in cutting-edge cell and gene therapies. Not only does it provide vital lab services to the pharmaceutical, medical device, and biotech industries, but it also stands out with its impressive global presence. With a robust network spanning over 110+ facilities scattered robust network spanning over 110+ facilities scattered across 20+ countries, the company offers unmatched accessibility and support to clients worldwide, solidifying its reputation as a trailblazer in the field.
As of the latest data available in 2023, the company boasts an impressive annual revenue of 4.092B, a 12.73% increase year-over-year , reflecting its strong position in the market and significant contributions to the life sciences industry.
Source: CTI
CTI Clinical Trial and Consulting Services has been making waves in the industry since its establishment in 1999. With a remarkable track record of driving over 150 new drug and medical device approvals, the company has firmly established itself as a leader in the field.
Boasting a vast international footprint, CTI operates offices in more than 60 locations across the globe, ensuring its presence and support in key regions such as North America, Europe, Latin America, Middle-East, Africa, and Asia-Pacific. In a strategic move in 2021, CTI further bolstered its position by announcing the acquisition of Dynakin, a European-based strategic consulting company. This acquisition aimed to fortify CTI's standing as a comprehensive and dynamic full-service clinical research organization, ready to take on the challenges of a rapidly evolving healthcare landscape.

Source: Medpace
MEDPACE is a global organization with a workforce of over 5,000 employees spread across 40 countries. In 2022, Medpace reported an impressive full-year revenue of $1.46 billion, reflecting a substantial 27.8% increase from the previous year. In an earnings call, Jesse Geiger, the President at Medpace, highlighted the company's ability to grow its workforce by 15.8% despite facing challenges in a competitive labor environment. In 2023, Medpace places significant emphasis on employee retention and ongoing recruitment as top priorities to support its future business endeavors.
10. Fisher Clinical Services
Source: Thermo Fisher
Fisher Clinical Services is a vital division of Thermo Fisher Scientific with a rich history of over two decades in supply chain management. The company's core focus centers on efficiently handling the distribution and packaging requirements for clinical trials conducted worldwide.
With an impressive annual revenue of $20 billion and a workforce of 70,000 employees, Fisher Clinical Services is dedicated to delivering high-value products that align with the rigorous standards of pharmaceutical companies, ensuring reliability, sustainability, and optimal performance. The company plays a crucial role in supporting the success of clinical trials across the globe.
In conclusion, the best Clinical Research Companies in the USA serve as indispensable partners for pharmaceutical, biotech, and medical device industries embarking on new medicine and medical device development. With the complexities of clinical trials escalating, CROs play a pivotal role in managing and leading these trials efficiently amidst rising prices, stringent regulations, and tighter deadlines.
As the pharmaceutical landscape continues to evolve, pharmaceutical companies are increasingly turning to Clinical Research Organizations for their R&D activities to maintain competitiveness, flexibility, and profitability. With their proven track record, comprehensive services, and commitment to excellence, the best Clinical Research Companies in the USA stand as crucial allies in the quest to advance medical science and improve patient outcomes. Through fruitful partnerships with CROs, the pharmaceutical, biotech, and medical device industries continue to push the boundaries of innovation and deliver life-changing products to those in need.
Healthcare Databases
- Healthcare Email List
Doctors Email List
- Nurses Email List
- Medical Executives Email List
- Physician Email List
- Dentist Email List
- Pharmacist Email List
- Healthcare SIC Code
- Healthcare NAICS Code
- Chiropractors Email List
- Veterinarian Email List
- Radiologists Email List
- Psychiatrists Email List
- Pediatrician Email List
- Cardiologists Email List
- Neurologist Email List
- Dermatologist Email List
- Oncologists Email List
Medical Services
- Hospitals Email List
- Pharmacy Email Database
- Nursing Homes Email List
- X-Ray Laboratories Mailing List
- Pharmaceutical Email List
- Assisted Living Email List
- Dental Clinics Email List
- Clinics Email List
- Physical Fitness Facilities List
HealthcareMailing
- +1 (786) 408 5757
- [email protected]

- Privacy Policy
Top 10 largest clinical research organizations
We take a look at the 10 biggest contract research organizations in the pharma sector in 2022.

The drug discovery process is complex. The clinical stage is particularly resource-intensive, something that has led to the rise in demand for contract research organizations (CROs) that can support drug manufacturers at each stage of the process, from discovery to approval. The core activities a CRO can provide include (but are not limited to) clinical trial management, data research and project management.
Despite an initial downturn at the start of the pandemic, Covid-19 created a spike in the number of clinical trials taking place due to the need for vaccines and drugs to tackle the virus. In the coming years, we expect the rise of technologies that enable decentralized clinical trials to help expand the market share of CROs.
Currently the global CRO services market is projected to grow from US$73.38 bn to $163.48 bn by 2029. Here Pharma IQ takes a look at the 10 biggest CROs in pharma today.
Founded in 1982, IQVIA is an American multinational company formed through the merger of Quintiles, a leading provider of product development and integrated healthcare services, and IMS Health, a global information and technology services company. The latter has enabled company to have a strong focus on digital solutions and analytics. In 2017, Quintiles IMS rebranded to IQVIA.
Laboratory Corporation of America Holdings, also known as Labcorp, is an American company that operates one of the largest clinical laboratory networks in the world. In an average week Labcorp processes tests on more than 3 million patient specimens. In 2020 the company earned revenue in excess of $14 bn.
Syneos Health
Founded in 1999, Syneos Health was created following the merger of two biopharmaceutical companies: INC Research and inVentive health. Today it has offices in more than 110 countries and offers services as a CRO as well as consultancy.
Founded in 1985 as a one-person consultancy firm, Pharmaceutical Product Development (PPD) is a global contract research organization that provides drug development, lab and lifecycle management services. In 2020 the company made US$4.7bn and the following year it became part of Thermo Fisher Scientific.
Headquartered in Dublin, Icon provides strategic management and support for clinical development from the compound selection stage through to clinical trials. Its services include clinical trials management, biometric activities, investigator recruitment and outcomes research.
Parexel was founded in 1982 and acquired by private equity firm Pamplona Capital in 2017 in a deal worth $5 bn. In 2021 it was bought by EQT Private Equity and Goldman Sachs Asset Management. The company conducts clinical trials and operates in more than 50 countries. It makes around $3 bn in revenue annually.
Charles River Laboratories
Founded in 1947, today this company specializes in cell and gene therapies as well as lab services for the pharmaceutical, medical device and biotech industries. As of 2021 it operates more than 90 facilities in 20 countries and has an annual revenue of $3.54 bn.
Award-winning MedPace has offices on six continents, with headquarters in Ohio where it has a clinical research campus and a number of clinical and bioanalytical laboratories. It provides clinical trial services for Phase I-IV studies in the biotech, pharma and medical device industries. Its revenue has been growing steadily year- on-year and is currently almost $1 bn.
CTI Clinical Trial and Consulting Services
This organization was founded in 1999 to provide clinical trial services and bring new drugs to market. It operates in more than 60 countries and since its inception has contributed to the approval of more than 150 new drugs and medical devices around the world.
WuXi AppTec
Founded in 2000 in Shanghai, WuXi AppTec is the newest company in our top 10. It provides services across the entire development cycle including small molecule R&D and manufacturing, biologics R&D and manufacturing, cell and gene therapy. It currently operates in 18 locations across China, Iceland and the US.
Quick links
- How to improve the success rate of clinical trials
- Webinar: The technology enabling decentralized clinical trials
- The challenges of handling and delivering highly potent oncology drugs
Get exclusive access to member-only articles, reports, videos, interviews, webinars and other premium content from industry experts and thought leaders by signing up to Pharma IQ here .
Upcoming Events
Clinical trial supply forum.
14-16 May Brussels, Belgium

DigIT Pharma & Health 2024
September 10 - 12, 2024 Düsseldorf, Germany

Temperature Control and Logistics North America Summit
September 10 - 12, 2024 Falls Church, VA

Subscribe to our Free Newsletter
Insights from the world’s foremost thought leaders delivered to your inbox.
Latest Webinars
Pharma iq's power list 2022: in conversation with pharma's top leaders.
2022-10-18 02:00 PM - 03:00 PM BST

A post-pandemic 3D view of the patient and supply journey
2022-06-01 04:30 PM - 05:30 PM CET

Discover how targeted radiotherapy induced toxicity can be identified with imaging
2022-04-28 01:00 PM - 02:00 PM EST

RECOMMENDED

FIND CONTENT BY TYPE
- Infographics
Pharma IQ COMMUNITY
- Advertise With Us
- User Agreement
- Cookie Policy
- Become a Member Today
- Pharma IQ App
ADVERTISE WITH US
Reach Pharmaceuticals & Biotechnology professionals through cost-effective marketing opportunities to deliver your message, position yourself as a thought leader, and introduce new products, techniques and strategies to the market.
JOIN THE Pharma IQ COMMUNITY
Join Pharma & Biotech today and interact with a vibrant network of professionals, keeping up to date with the industry by accessing our wealth of articles, videos, live conferences and more.

Pharma IQ, a division of IQPC
Careers With IQPC | Contact Us | About Us | Cookie Policy
Become a Member today!
PLEASE ENTER YOUR EMAIL TO JOIN FOR FREE
Already an IQPC Community Member? Sign in Here or Forgot Password Sign up now and get FREE access to our extensive library of reports, infographics, whitepapers, webinars and online events from the world’s foremost thought leaders.
We respect your privacy, by clicking 'Subscribe' you will receive our e-newsletter, including information on Podcasts, Webinars, event discounts, online learning opportunities and agree to our User Agreement. You have the right to object. For further information on how we process and monitor your personal data click here . You can unsubscribe at any time.
Ensuring the validity of clinical outcomes assessment data
The value of rater training
Advancements in Artificial Intelligence for site selection
Using human-enabled AI to enhance decision-making and minimise risk
A multifaceted risk factor: Addressing obesity's impact across the disease spectrum
Optimising biotech funding whitepaper series.
2023 biotech sector survey
Navigating biotech's challenges and embracing a promising tomorrow.
EMA guideline on computerised systems and electronic data in clinical trials
Key considerations on the impact of the new framework of globally applicable standards.
Navigating the regulatory labyrinth of technology in decentralised clinical trials
Keeping up to date and implementing the latest guidelines.
Considerations for DTx clinical trials in CNS indications
Successful digital therapeutic clinical trials.
Featured Solutions
Digital Health Technologies
ICON acquires HumanFirst, a cloud-based technology company for life sciences supporting precision measurement in patient centred clinical research.
ICON provides full service outsourcing and flexible support for biotech specific needs such as due diligence and asset valuation.
Cardiac Safety Solutions
End-to-end cardiac safety solutions, including ECG, event monitoring, BPM, long-term Holter monitoring, ECHO and MUGA studies.
Early Clinical and Bioanalytical Solutions
Innovative early clinical solutions that will advance your drug development strategy.
Site & Patient Solutions
Transforming recruitment through patient-centric trials and real-world, real-time data.
Market Access
Expertise in mission-critical pricing, market access, and reimbursement.
Navigating the complexity of real-world healthcare data: choosing the right tools at the right time
Watch the webinar.
The future of pharmacovigilance: Exploring automation and AI in literature surveillance
Streamlining IRT in clinical trials: Simplify, optimise, succeed
Icon insights.
- 01 Apr 2024
SDoH data for efficient, patient-centred clinical trials
Insights from PHUSE US Connect 2024
- 27 Mar 2024
Diabesity: Overlapping pathophysiology informs multi-indication treatment
- 25 Mar 2024
Site Training for Clinical Trial Success
- 28 Feb 2024
Beyond awareness: Driving progress on the Rare Disease journey
- 21 Feb 2024
Rare disease trials require effective participation support strategies to succeed
Strategies for de-risking rare disease programmes during research and development
- 13 Feb 2024
Insights from the 2024 ISCR Annual Conference: India’s evolving clinical research arena
- 26 Jan 2024
Ensuring safety and compliance: The essentials of outsourcing pharmacovigilance
What’s happening in icon, how can we help.
- Site & Patient Recruitment
- Medical Device
- Real World Evidence
- Decentralised & hybrid clinical trials
- Digital Disruption
1-888-664-9690
[email protected]
- Corporate Brochure
- Case Studies
- Testimonials
- Press Release
- Vascular Surgeons Email List
- Pathologists Email List
- Gastroenterologist Email List
- Chiropractors Email List
- Anesthesiologists Email List
- Plastic Surgeons Email List
- Pediatricians Email List
- Geriatricians Email List
- General Surgeons Email List
- Diabetes Specialist Email List
- Allergist / Immunologist Email List
- Arthritis Specialists Email List
- Cardiologist Email Lists
- Dermatologist Email List
- Neurologists Email List
- Orthopedic Surgeons Email List
- Urologists Email List
- Podiatrist Email List
- Dermatopathologist Email List
- Hematologists Email List
- Pulmonologists Email List
- Nephrologists Email List
- Oncologist Email List
- Rheumatologist Email List
- Radiologist Email List
- Optometrist Email List
- Ophthalmologist Email List
- Acupuncturist Email List
- Optician Email List
- Neonatologist Email List
- Neuropathologist Email List
- Hepatologist Email List
- Traumatologist Email List
- Gynecologists Email List
- Psychiatrists Email List
- Endocrinologist Email List
- Hygienists Email List
- Primary Care Physicians Email List
- General Practitioners Email List
- Osteopathic Physicians Email List
- Emergency Physicians Email List
- Bariatric Physician Email List
- Family Medicine Specialist Email List
- Epidemiologist Email List
- Clinical Lipidologist Email List
- Registered Dieticians Email List
- Psychologists Email List
- Neurosurgeon Email List
- Nutritionists Email List
- Audiologist Email List
- Hypnotists Email List
- ENT Specialists Email List
- Sleep Medicine Specialists Email List
- Sports Medicine Physician Email List
- Physiatrists Email List
- Physician Assistants (PAs) Email List
- Athletic Trainers (ATs) Email List
- Cosmetologists Email List
- Pediatric Allergists Email List
- Naturopathic Doctors Email List
- Veterinarian Email Lists
- Phlebotomists Email List
- Pediatric Radiologists Email List
- AMA Physicians Email List
- Paramedics and EMTs Email List
- Pediatric Neurologist Email List
- Preventive Medicine Specialist Email List
- Reproductive Endocrinologist Email List
- Interventional Cardiologists Email List
- Nuclear Medicine Specialist Email List
- Infectious Disease Specialist Email List
- Radiation Oncologists Email List
- Internal Medicine Specialist Email List
- Mental Health Specialists/Professionals Email List
- Addiction Medicine Specialist Email List
- Aerospace Medicine Physicians Email List
- Hematology & Oncology Specialist Email List
- Medical Geneticist Email List
- Addiction Psychiatrist Email List
- Physical Medicine Rehabilitation Email List
- Cardiac Care Nurses Email List
- Critical Care Nurses Email List
- Emergency Nurses Email List
- Geriatric Nurses Email List
- Home Healthcare Nurses Email List
- Military Nurses Email List
- Nurse Anesthetists Email List
- Nurse Practitioners Email List
- Registered Nurses Email List and Mailing List
- Neurology Nurses Email List
- Orthopedic Nurses Email List
- Occupational Health Nurses Email List
- Pediatric Nurse Practitioners Email List
- Dermatology Nurses Email List
- Perioperative Nurses Email List
- Midwife Nurses Email List
- Family Nurse Practitioners (FNP) Email List
- Public Health Nurses (PHNs) Email List
- Endocrinology Nurses Email List
- Ambulatory Care Nurses Email List
- Women’s Health Nurse Practitioners Email List
- Adolescent Medicine Nurses Email List
- Reproductive Health Nurses Email List
- Transplant Nurses Email List
- Dialysis Nurses Email List
- Allergy / Immunology Nurses Email List
- Family Planning Nurses Email List
- ENT Nurse Practitioners Email List
- HIV/AIDS Nurses Email List
- Community Health Nurses Email List
- School Nurses Email List
- Student Nurses Email List
- Nurse Manager Email List
- Oncology Nurses Email List
- Child Psychiatric Nurses Email List
- IV Certification Nurses Email List
- Licensed Practical Nurses Email List
- Licensed Vocational Nurses Email List
- Clinical Nurse Specialist Email List
- Acute Care Nurse Practitioners Email List
- Office Based Nurses Email List
- Certified Registered Nurse Anesthetist Email List
- Certified Nursing Assistants Email List
- Psychiatric-Mental Health Nurse Practitioners Email List
- Adult Care Nurse Practitioner Email List
- Registered Pharmacist Email List
- Hospital Druggist Email List
- Druggist Email List
- Community Pharmacists Email List
- Pharmacist Clinicians (PhC) Email List
- Ambulatory Care Pharmacists Email List
- Pharmaceutical Email & Mailing List
- Prosthodontists Email List
- Periodontist Email List
- Endodontists Email List
- Pediatric Dentists Email List
- Orthodontist Email List
- Oral Pathologists Email List
- Denturist Email List
- ADA Dentists Email List
- Dental Hygienist Specialists Email List
- Oral and Maxillofacial Radiologists Email List
- Oral and Maxillofacial Surgeons Email List
- Subacute Care Centers Email List
- Cancer Centers and Services Email List
- Ambulatory Surgery Centers Email List
- Diagnostic Imaging Centers Email List
- Urgent Care Centers Email List
- Trauma Centers Email List
- Nursing Homes Email List
- Nursing Home Administrators Email List
- Hospital Email List
- Assisted Living Facilities Email List
- Medical Institutions Email List
- X-Ray Laboratories Email List
- Primary Care Centers Email List
- Dental Clinics Email List
- Blood Bank Centers Email List
- Dental Laboratories Email List
- Clinics Email List
- Physical Fitness Facilities Email List
- Med Spa Email List
- Dental Equipment / Supplies Manufacturers Email List
- Biomedical Equipment Manufacturers Email List
- Electro-Medical Equipment Manufacturers Email List
- X-Ray Apparatus and Tubes Manufacturers Email List
- Ophthalmic Goods Manufacturers Email List
- Surgical Appliances Manufacturers Email List
- Surgical / Medical Instruments Manufacturers Email List
- Neuromodulation Devices Manufacturers Email List
- Cardiovascular Devices Manufacturers Email List
- Therapeutic Devices Manufacturers Email List
- Spine Devices Manufacturers Email List
- Laboratory Equipment and Supplies Email List
- Hospital Equipment and Supplies Email List
The Top 10 Clinical Research Companies in the USA
- Dec 02, 2022
- Posted By: admin

To bring effective medicines and Innovations in healthcare industry , a Clinical Research Organization or Contract Research Organization (CRO) are a must for the Biotech, Medtech, and pharma industries. A CRO offers comprehensive support to their efforts to test, examine, refine and market the latest medicines and devices that help in improving Healthcare Industry.
In 2022, the global clinical research market stands at $63.6 billion , and by 2027, it is expected to reach around an exciting amount of 115.1 billion with a CAGR of 11.0% from 2022 to 2027.
This growth is powered by an increase in R&D activities and expenditures and a significant rise in clinical trials owing to the need for innovations in healthcare. For that reason, it is also essential to be aware of the most prominent global research organizations that offer vital support with their specialized clinical trial solutions. This post will walk you through the ten big Clinical Research Companies In USA . So, let’s begin:

- Founded: 1982
- Headquarters: Durham, North Carolina
- Annual Revenue : $13.87B
- Employee Size: 88,000
IQVIA is one of the largest Clinical Research companies in the world that works profusely to enhance healthcare by integrating the study of human science with discoveries in technology and data science to improve understanding of human health and provide better and quicker care.
It helps pharma companies, along with other medical organizations, to innovate and maximize positive outcomes. In addition to clinical research and development, IQVIA has also developed analytics and technology solutions to aid the medical sector in commercializing products and enhancing customer engagement.
Some clinical trial solutions given by IQVIA are as follows:
- Site identification & Selection
- Help with protocol design
- Design of clinical trial phase -1
- Investigation and improvements of the clinical trial in phases 2 and 3
- Accessibility to global laboratories
2. Pharmaceutical Product Development (PPD)

- Founded: 1985
- Headquarters: Wilmington, North Carolina
- Annual Revenue: $65.0M
- Employee Size: 24,000
PPD, also known as Pharmaceutical Product Development, is an extensive research organization headquartered in the United States but has a widespread global presence.
The company directs its attention to three prime areas: pharma development, lifecycle management services, and laboratory. Besides, it has clients and partners covering various sectors, including pharmaceutical companies, biotech firms, medical device manufacturers, and government or academic organizations.
Some clinical trials offered by PPD include:
- Early Development
- Consulting and Product Development
- PPD Biotech
- The site and patient-centered Solutions
- Clinical Development

- Headquarters: Waltham, Massachusetts
- Annual Revenue: $3B
- Employee Size: 18,000
Parexel is another important name in the list of Clinical Research Companies In USA that specializes on behalf of its pharmaceutical partners in carrying out clinical studies to boost and make certain that the medicine approval process takes place smoothly. It has a wide range of offerings that spreads across every type of clinical trial service to help sponsors work on creating successful trials.
Some of the clinical trial solutions which Paraxel offers:
- Management of clinical data
- Medical writing
- Regulatory affairs consulting
- Design and development of clinical trials at all phases
- Clinical supply chain management
With pride, the company operates in over 50 countries and owns over 95% of the top 200 selling biopharmaceuticals in today’s market.
4. PRA Health Services

- Founded: 1976
- Headquarters: Raleigh, North Carolina
- Annual Revenue: $64.3M
- Employee Size: 16,400
PRA Health Sciences is a contract research organization that provides coverage in more than 90 countries worldwide. It principally centers on contributing therapeutic and operational expertise through integrated applications and supplying local expertise in particular areas. Besides, it works in the early and end stages of clinical trial procedures and the domains of strategy, consultancy, technology, and bio-analytics.
Moreover, it works hard to speed up medicine development operations to introduce better and more effective medicine sooner in the market.
Let’s take a peek at the PRA Health Sciences clinical trial solutions:
- Onsite support services
- Personalized solutions for Biotech
- Clinical Diagnostics
- Site Commercial Solutions
- Protocol Consultation and Study Design
- PRA’s Laboratories for Medicine Development
5. Syneos Heath

- Founded: 1999
- Headquarters: Morrisville, North Carolina
- Annual Revenue: $5,213M
- Employee Size: 28,000
With the merger of inVentiv Health and INC Research, Syneos Health was created that combines every discipline involved in bringing new therapies or products to market, from clinical research to consulting and commercialization.
Though it provides clinical development services spanning all stages, it primarily owns a specialization in helping healthcare organizations with the late stages of clinical trials. Moreover, its commercial solutions cover communication, consulting, and medication adherence.
The clinical trial solutions it offers include:
- Early phase
- Decentralized clinical trial solutions
- Clinical data management
- Pharmaceutical
- Medical Device Diagnostics

- Founded: 1978
- Headquarters: Burlington, North Carolina
- Annual Revenue: $16.1B
- Employee Size: 70,000
Laboratory Corporation of America, or LabCorp, focuses on offering extensive clinical laboratory solutions and end-to-end medication and diagnostic development and commercialization.
The company develops special testing operations such as HIV genotyping, oncology testing, phenotyping, clinical trials, and diagnostic genetics. Moreover, its services are spread through care organizations, hospitals, government agencies, pharmaceutical companies, and physicians.
Some clinical trial solutions provided by LabCorp are:
- Preclinical Services
- Post-Marketing Solutions
- Medical Devices
- Clinical Trial Laboratory Services
- Clinical Trials
- Data & Technology
7. Charles River Lab

- Founded: 1947
- Headquarters: Wilmington, Massachusetts
- Annual Revenue: $690.4M
- Employee Size: 20,000
Charles River laboratories proudly span its capabilities throughout the medical R&D process from basic research to pre-clinical stage testing, manufacturing and commercialization within two significant services: Good Laboratory Practice (GLP) and non-GLP. It offers precise support to help its partners advance their research and efforts to introduce effective medicines in the market.
The company further serves biotechnology and pharmaceutical companies, hospitals, government agencies, and academic institutions.
Some of the clinical development solutions it offers include:
- Bioanalysis
- Clinical Kitting services
- Data management
- Quality control
- Stability testing
8. Fisher Clinical Services

- Founded: 1989
- Headquarters: Center Valley, Philadelphia
- Annual Revenue: $17B
- Employee Size: 50,000
Fisher Clinical Services is a part of Thermo Fisher Scientific that has been for over 20 years in the business of supply chain management. It focuses exclusively on working with distribution and packaging requirements of clinical trials happening around the globe.
The company is committed to providing high-value products which adhere to the standard of pharmaceutical companies and is reliable, sustainable and perform well.
Some of the clinical trial solutions offered by Fisher Clinical Services are:
- Cell & Gene Therapy
- Clinical Label Services
- Clinical Supply Optimization services
- Direct to patient
- Biologistics management
9. Medpace Holdings

- Founded: 1992
- Headquarters: Cincinnati, Ohio
- Revenue: $1B
- Employee Size: 1,001-5,000
Medpace offers support services for phases one to five of clinical trials for biotechnology, pharmaceutical companies, and medical device industries. It is a scientifically-driven organization that offers full service and helps industries make key differences with their contributions to the healthcare sector.
Some of the clinical research solutions that you receive from Medpace are:
- Medical Writing
- Quality Assurance
- Clinical Monitoring
- Clinical Trial Management
- Risk-based Monitoring
- Regulatory Affairs
- Biostatistics and Data sciences
10. Advanced Clinical

- Founded: 1994
- Headquartered: Deerfield, Illinois
- Annual Revenue: $106M
- Employee Size: 501-1,000
A mid-size and full-service Clinical Research organization that helps sponsors run clinical trials and offers various solutions across therapeutic fields. It allows clients to secure better outcomes through candid discussions, conversations, foresight, and innovative solutions.
The company works hard to improve every life touched by clinical research. As such, some clinical solutions provided by Advanced Clinical are:
- Project management
- Clinical monitoring
- eTMF and document management
- Quality & Validation
The Bottom Line
There you have it – the best Clinical Research Companies In USA to get support from while working on new medicine or medical devices.
CROs play a critical role in the pharmaceutical, biotech, and medical device industries to manage and lead their clinical trials now more than ever due to rapidly rising prices, regulations, and tighter deadlines. Also pharmaceutical companies approaching Clinical Research Organizations for their R&D activities to stay competitive, flexible, and profitable against all odds.


The Science-First CRO. ™
The scientific full service CRO and consultancy integrating strategic planning, regulatory strategy, and clinical trial execution to rapidly advance your complex or novel therapies.

READ THE PRESS RELEASE

Specialty Expertise
Our team of scientific experts is easy to work with, even for the rarest, most complex, and never done before novel therapies..
Driving Success
Advancing Novel Therapies to Success with Veristat READ SPONSOR SUCCESS STORIES

Phase I/II Trial After NH Trial

Rare Disease Gene Therapy Trial
Tackling problems big or small we can help.

What Are Your Challenges?

Be Part of the Team That Cares
At Veristat, we contribute and add value to our company’s mission - improving and saving lives. Whether you’re a scientific expert or an operations guru, explore the chance to become part of our fast-paced, agile team.


10 Best Clinical Research Organizations In The USA
With the growing population, diseases affecting mankind are also increasing. The old medications are being obsolete for certain diseases like bacterial infections, and new effective medicines are urgently needed. The clinical research institutions facilitate drug manufacture by running preclinical and clinical tests on the drugs. In addition, they also study safety, efficacy, and effectiveness of medical devices, diagnostic products, and treatment regimens intended for human health. The healthcare industry is booming with a lot of clinical research institutions in the US and all around the world.

Table of Contents
Top 10 American CROs
The United States of America has some of the top clinical research organizations in the world. Amongst them, here is the list of the 10 Best Clinical Research Organizations In The USA.

Annual Revenue: $9.6B Headquarters: Princeton, New Jersey Number of Employees: 50,000+ Total Funding: $0 Status: Public, Subsidiary of LabCorp Founded: 1996
In 2014, LabCorp acquired Covance for $6.1B. Covance, a global contract research organization under LabCorp, is the world’s leading healthcare diagnostics company, providing comprehensive clinical laboratory services and end-to-end solutions for drug and diagnostics development and commercialization. With its mission to bring new and innovative medicines to patients sooner, it has worked on more than 50 drugs available in the market today. It provides drug development services across all phases of development and multiple therapeutic areas.

Annual Revenue: $9.74B Headquarters: Durham, North Carolina Number of Employees: 55,000 Operating Income: $1.33B Status: Public, Independent Company Founded: 1982
Working to enhance healthcare by integrating health information technologies and clinical research, IQVIA explores a new path to better health outcomes via Human Data Science. It integrates the study of human science with breakthroughs in data science and technology to advance our understanding of human health, and help everyone make better, more insightful decisions.
3. Syneos Health

Annual Revenue: $1.9B Headquarters: Morrisville, North Carolina Number of Employees: 21,000 Total Funding: $0 Status: Public, Independent Company Founded: 1999
Syneos Health integrates all the disciplines involved in bringing new therapies to market, from clinical to commercial, for biopharmaceutical solutions. In January 2018, INC Research merged with inVentiv Health, the parent company of a subsidiary called Syneos, and the resulting company was named Syneos Health.
4. Parexel International

Annual Revenue: $2.4B Headquarters: Waltham, Massachusetts Number of Employees: 18,660 Total Funding: $0 Status: Public, Subsidiary of Pamplona Capital Management LLP Founded: 1982
Parexel International is involved in advancing the success of biopharmaceutical and medical device industries for the development and commercialization of new medical therapies worldwide. It is the second-largest clinical research organization in the world and has helped develop approximately 95% of the 200 top-selling biopharmaceuticals on the market today.
5. PRA Health Sciences

Annual Revenue: $2.7B Headquarters: Raleigh, North Carolina Number of Employees: 16,400 Total Funding: $305.6M Status: Public, Subsidiary of Kohlberg Kravis Roberts & Co. L. P Founded: 1976
PRA participated in the pivotal or supportive trials and/or key NDA support services that led to the approval of 85 important products currently on the market. Its aim is to improve the drug development process for getting better medicines to market sooner.
6. Pharmaceutical Product Development

Annual Revenue: $1.9B Headquarters: Wilmington, North Carolina Number of Employees: 18,583 Total Funding: $0 Status: Private, Independent Company Founded: 1985
PPD applies innovative technologies, therapeutic expertise, and a firm commitment to provide comprehensive, integrated drug development, laboratory, and lifecycle management services. Its clients and partners include pharmaceutical, biotechnology, medical device, academic, and government organizations. It helps to bend the cost and time curve of drug development and optimize value in delivering life-changing therapies to improve health.
7. Charles River Laboratories

Annual Revenue: $2.27B Headquarters: Wilmington, Massachusetts Number of Employees: 14,700 Total Funding: $224M Status: Public, Independent Company Founded: 1947
Having worked on about 85% of the drugs approved by the FDA in 2018, Charles River Laboratories operates 80+ facilities in 20 countries worldwide. It offers a wide range of products and services that span the entire drug discovery and development continuum and can be tailored to specific research conditions.
Annual Revenue: $2.2B Headquarters: Leopardstown, County Dublin Number of Employees: 13,250 Total Funding: $0 Status: Public, Independent Company Founded: 1990
Icon helps its clients to accelerate the development of drugs and devices that save lives and improve quality of life. It offers a full range of consulting, development, and commercialization services from a global network of offices in 37 countries.
9. WuXi AppTec

Annual Revenue: $674.3M Headquarters: Shanghai, Shanghai Municipality Number of Employees: 3,800 Total Funding: $2.6B Status: Public, Subsidiary of WuXi PharmaTech, Inc, Shanghai Stock Exchange Founded: 2000
WuXi AppTec Group, with 18 facilities in the USA and China combined, is a global pharmaceutical, biopharmaceutical, and medical device company. It covers the development cycle through five core operations, including small molecule R&D and manufacturing, biologics R&D and manufacturing, cell therapy and gene therapy R&D and manufacturing, medical device testing, and molecular testing and genomics.
10. Medpace Holdings

Annual Revenue: $704.6M Headquarters: Cincinnati, Ohio Number of Employees: 2,900 Total Funding: $161M Status: Public, Subsidiary of Cinven Limited Founded: 1992
Medpace provides services for Phase I-IV of drug and medical device development services, including regulatory services and central laboratory services. It combines scientific guidance, efficient clinical trial management, global regulatory leadership, and innovative technologies to deliver high-quality results.
Sharing is caring!
Related Posts

Top 10 Medical Device Companies in the World

Top 10 Jobs You Can Get in the Biotechnology Sector

Top 10 Biotechnology Universities In The World
Type above and press Enter to search. Press Esc to cancel.
Solutions to drive healthcare forward
About clinicalresearch.com.
IQVIA created ClinicalResearch.com to increase clinical research awareness, understanding, and participation.
If you are interested in taking part in a clinical trial, you can do an instant search to find clinical trials that are recruiting in your area, which requires no registration. Or, you can provide your email and location to register for updates and be notified when new clinical trials in a condition that you are interested in become available. You can also learn more about advances in medical areas of interest to you in the Communities section.
If you are a healthcare professional, you can learn more on ClinicalResearch.com about referring your patients into clinical trials, or even about how you can become a clinical trial investigator.

About IQVIA
Harness the power of human data science.
Healthcare is an industry designed to help humans. As a global community, we continuously invest and commit to advancing human health. To deliver value and real outcomes. To rise to the challenge to find the next breakthrough by making the most of increasingly limited resources.
We are inspired by the potential and propelled by the possibilities. We share the vision to drive healthcare forward. To see how we can help accelerate progress and achievements. Others are developing these medical breakthroughs. We do our part by using breakthroughs in insights, technology and human intelligence to reimagine and deliver ways to help make them a reality.
Almost everything we do in Human Data Science is powered by the IQVIA CORE.
It’s bigger than better clinical trials. Or advances in technologies and analytics. Or faster insights. It’s about exploring a new path to better health outcomes via Human Data Science. It's about harnessing the power of the IQVIA CORE™ to channel the insights, commercial and scientific depth, and executional expertise that drive maximum value for our customers.

Motivated by the industry we help, we’re committed to providing solutions that enable life sciences companies to innovate with confidence, maximize opportunities, and, ultimately, drive human health outcomes forward.
Want more information about clinical trials near you?
Register to get notifications about clinical trials in your area..
We Value Your Privacy
We use cookies to allow our site to work properly, to personalize content and ads, to provide social media features and to analyze our traffic. Data may be shared with our partners involved in the delivery and/or personalization of ads elsewhere online as explained in “ Manage Cookie Preferences ” . Please click “ I Accept ” if you agree to our use of cookies, "Manage Cookie Preferences" to manage your preferences, or “ I Reject ” if you only agree to necessary cookies.
Manage Cookie Preferences
When you visit any website, it may store or retrieve information on your browser, mostly in the form of cookies. This information might be about you, your preferences or your device and is mostly used to make the site work as you expect it to. The information does not usually directly identify you, but it can give you a more personalized web experience. Because we respect your right to privacy, you can choose not to allow some types of cookies. Click on the different category headings to find out more and change our default settings. However, blocking some types of cookies may impact your experience of the site and the services we are able to offer.
Please visit our "Cookie Policy" to find out more about our cookie policy, how to block these cookies or contact information to reach out to us if you have any questions.
Cookies strictly necessary for essential website purposes and functions Cookies strictly necessary for essential website purposes and functions ALWAYS ACTIVE These cookies are strictly necessary for the proper operation of our site. They allow us to ensure the security and efficient delivery of our site. These cookies are needed for essential functions such as administering cookie preferences. Strictly necessary cookies cannot be switched off and they don ’ t store any personally identifiable information. View Cookies
These cookies are strictly necessary for the proper operation of our site. They allow us to ensure the security and efficient delivery of our site. These cookies are needed for essential functions such as administering cookie preferences. Strictly necessary cookies cannot be switched off and they don ’ t store any personally identifiable information.
Analytics cookies and technologies Analytics cookies and technologies These cookies are used to collect information about how visitors use our site. We use the information to compile reports and to help us improve the site. The cookies collect information in an anonymous form, including the number of visitors to the site, where visitors have come to the site from and the pages they visited. View Cookies
These cookies are used to collect information about how visitors use our site. We use the information to compile reports and to help us improve the site. The cookies collect information in an anonymous form, including the number of visitors to the site, where visitors have come to the site from and the pages they visited.
Elevate Your Life Science Product Development with a Super-Powered Platform
About proxima., case studies.

Our Services
Recent news, frequently asked questions, tools &templates.

Define your future as a clinical research associate (CRA). Explore opportunities to join our global team of game changers.
Our experts outline five key approaches to ensure pediatric clinical trials are designed to meet the specific, unique needs of children.
Experts share how organizations can prepare to execute their clinical trials under the European Union’s new Clinical Trial Regulation (CTR).
Discover the transformative impact of technology and innovation in clinical trials, along with the regulatory challenges and adaptations that come with it.
PPD is a leading global contract research organization focused on delivering life-changing therapies

Clinical development
Laboratories, ppd careers, connect with us, ppd offers proven solutions, from early development to phase ii-iii to pharmacovigilance to peri- and post-approval services, to ensure your product’s success.

PPD offers a comprehensive set of lab services – from bioanalytical to vaccine sciences.
Offering proven solutions, ranging from early development to pharmacovigilance to post-approval services, to ensure your product’s success.
Clinical trial therapeutic expertise
Learn about our expertise in areas like oncology, cardiovascular, rare diseases and more.
Fuel your drug development more efficiently
Here are four key areas to consider when choosing the right FSP partner for you.
130k+ colleagues globally connected by our mission to enable our customers to make the world healthier, cleaner and safer.

Clinical Researcher
Enhancing Participation in Digital Therapeutics Clinical Trials with a Decentralized Model
Clinical Researcher April 12, 2024

Clinical Researcher—April 2024 (Volume 38, Issue 2)
RECRUITMENT & RETENTION
Anthony Brogno
Since 2021, there has been an increase among the general public in willingness to participate in clinical research studies. However, participants want the trials to be more convenient, closer to home, and more accommodating of their schedules.
Those are among the findings of the 2023 Perceptions & Insights Study from the Center for Information and Study on Clinical Research Participation (CISCRP) , which surveys clinical trial participants every two years to collect information on individual experiences during participation.
These findings are instructive for digital health companies and contract research organizations (CROs) which, by and large, are still modelling clinical trials for digital therapeutics (DTx) on those used for pharmaceutical and biotech studies. Switching to a decentralized clinical trial (DCT) model will encourage greater participation and result in more participants completing the trial. While DCTs can be used for many studies, they are particularly suited for DTx products since they are widely regarded as presenting low risk to participants, and such trials can operate under a less strict regulatory framework and be designed with a more patient-centric approach.
The pandemic proved that many more work- and health-related activities can be accomplished remotely more conveniently than we once thought possible, including accessing healthcare. People now guard their time more carefully, with staying at home often the preferred option for work, doctor appointments, shopping, entertainment, and more.
Bearing this out, the 2023 CISCRP survey of more than 4,500 individuals globally who have participated in a clinical study found that traveling to the clinic for procedures was the greatest burden for participation. Among those who quit trials before they were completed, the location of the study site was the second-most cited reason. Asked what would have encouraged them to complete their trials, 32% of respondents replied, “more virtual visits.”
Healthcare as a Commodity
None of this should come as a surprise to those who have noticed the growing trend toward the consumerization of healthcare. After decades of working around providers’ policies and schedules, patients are asserting themselves as consumers, treating healthcare like a commodity and prioritizing convenience above all. While trial participants are perhaps more magnanimous than the average patient, they, too, value their time and convenience.
Modern CROs have taken note of this and now offer hybrid or fully remote (decentralized) site capabilities for DTx sponsors to create real-word settings in which patients can carry out study activities. Much of the data collected from DTx technologies involve satisfaction measures such as emotional affect, user experience, and quality of life variables that can easily be captured without traveling to a brick-and-mortar facility. CROs are also using networks of travel nurses and study staff to perform physical assessments and collect bodily samples from participants’ homes to mitigate the need for in-clinic study visits.
Most clinical research participants today are also discovering opportunities to be in clinical trials online. CISCRP’s study reports that, of the individuals who learned about their studies online, 46% were made aware through social media channels and 26% through web advertising. DTx sponsors can ensure they are implementing a well-rounded digital marketing strategy by using a CRO that is experienced in acting as a DCT site to accelerate recruitment.
This model enables DTx sponsors to screen potential participants and allow them to participate in their trial regardless of geographic location, ultimately producing a more diverse patient population. These CROs are set up to attract the right participants through in-house clinical operations and marketing teams that screen for DTx patients who meet study inclusion/exclusion criteria and that run multi-channel recruitment campaigns.
Lastly, 86% of participants in the CISCRP survey reported they felt appreciated for their involvement in clinical studies. Even in virtual DTx trials, CROs in this space maintain excellent rapport with trial participants. Decentralized sites, although fully remote, are staffed with knowledgeable clinical research coordinators who act as the first point of contact for anything trial-related, from technical questions about the DTx technology being used to more pressing health concerns. At the end of the day, a trial is nothing without patients who are willing to participate and contribute.
Decentralized trials create an overall better experience for both sponsors and patients. Due to the nature of digital therapeutics technologies and their journey to market, there’s no reason most DTx sponsors can’t execute fully decentralized trials. When people have the autonomy to complete study activities and manage their healthcare in a real-world setting, they are happier and less likely to drop out before completion.
It’s time for DTx trial sponsors and CROs to modernize their operations and design trials to be as convenient as possible for participants.

Anthony Brogno is Director of Clinical Operations at Lindus Health .
Nashville, Tennessee
Miami, Florida
Seattle, Washington

Barriers to Clinical Trial Enrollment: Focus on Underrepresented Populations

Using Simulation to Teach Research

An Approach to a Benefit-Risk Framework
Jobs in the acrp career center.
- Clinical Research Technologist-Research | Nemours
- Research Coordinator - Research Operations | Denver Health
- Research Fellow | Nemours
- Intern-Research | Nemours
Find Info For
- Current Students
- Prospective Students
- Research and Partnerships
- Entrepreneurship and Commercialization
Quick Links
- Health and Life Sciences
- Info Security and AI
- Transformative Education
- Purdue Today
- Purdue Global
- Purdue in the News
April 11, 2024
NSF awards $275K grant to Amplified Sciences to develop new platform of ultrasensitive clinical diagnostics

Amplified Sciences, a clinical-stage life sciences diagnostic company, has received a $275,000 Small Business Innovation Research grant from the National Science Foundation. The funding will support critical development of the company’s tests for early, more accurate detection of challenging diseases, starting with pancreatic cancer. (Purdue Research Foundation photo/Vincent Walter)
State has awarded $50K matching grant through the Indiana Economic Development Corp.’s Applied Research Institute
WEST LAFAYETTE, Ind. — The National Science Foundation has awarded a $275,000 Phase 1 Small Business Innovation Research (SBIR) grant to Amplified Sciences . The clinical-stage life sciences diagnostic company is developing tests for early, more accurate detection of challenging diseases, starting with pancreatic cancer.
Amplified Sciences has received a Phase I matching grant of $50,000 from the Indiana Economic Development Corp.’s Applied Research Institute.
CEO Diana Caldwell said the funding will support critical development of Amplified Sciences’ novel ultrasensitive optical reporter platform technology.
“This, our second SBIR award, will enable further development of multiplexing capabilities for pancreatic cancer panels and beyond,” Caldwell said. “We are excited to be awarded this highly competitive NSF SBIR grant. This brings our total of nondilutive funding to over $1 million.”
Amplified Sciences also recently received investments worth $108,000 from the Flywheel Fund . In 2023 the company received a Phase 1 SBIR grant of approximately $400,000 from the National Cancer Institute and a $50,000 match from the state of Indiana to develop pancreatic cancer risk stratification tests. It was named to the Pepperdine University Graziadio Business School’s Most Fundable Companies list in 2021 and to the New York University Stern School of Business’ Endless Frontier Labs life science cohort in 2022.
The Purdue innovations
Amplified Sciences’ diagnostic tests are based on technology invented by V. Jo Davisson , professor of medicinal chemistry and molecular pharmacology in Purdue University’s College of Pharmacy and a faculty member of the Purdue Institute for Cancer Research and the Purdue Institute for Drug Discovery . Davisson serves as the company’s chief scientific officer. The company licenses Davisson’s intellectual property through the Purdue Innovates Office of Technology Commercialization .
Facts about pancreatic cancer
In its Cancer Facts & Figures 2023 report , the American Cancer Society estimated that more than 64,000 Americans were expected to be diagnosed with new cases of pancreatic cancer in 2023. It estimated that more than 50,000 would die from the disease in 2023.
Pancreatic cancer signs and symptoms, like jaundice, severe abdominal pain, weight loss and vomiting, don’t appear until advanced stages of the disease. If a diagnosis is made at an advanced stage, treatments including surgery and pharmaceuticals seldom produce a cure. The five-year relative survival rate is 12% for all patients; for those diagnosed with localized cancer, the rate is 44%.
About Amplified Sciences
Amplified Sciences is a clinical-stage life science diagnostics startup focused on detecting and preempting the risks of debilitating diseases, thus providing health care professionals the ability to treat patients earlier with better outcomes. The company’s ultrasensitive chemistry platform leverages technology licensed from Purdue University, and its headquarters is in West Lafayette, Indiana. Its lead assay has published clinical evidence in pancreatic cancer. To learn more about Amplified Sciences, visit amplifiedsciences.com .
About Purdue Innovates Office of Technology Commercialization
The Purdue Innovates Office of Technology Commercialization operates one of the most comprehensive technology transfer programs among leading research universities in the U.S. Services provided by this office support the economic development initiatives of Purdue University and benefit the university’s academic activities through commercializing, licensing and protecting Purdue intellectual property. In fiscal year 2023, the office reported 150 deals finalized with 203 technologies signed, 400 disclosures received and 218 issued U.S. patents. The office is managed by the Purdue Research Foundation, which received the 2019 Innovation & Economic Prosperity Universities Award for Place from the Association of Public and Land-grant Universities. In 2020, IPWatchdog Institute ranked Purdue third nationally in startup creation and in the top 20 for patents. The Purdue Research Foundation is a private, nonprofit foundation created to advance the mission of Purdue University. Contact [email protected] for more information.
Writer/Media contact: Steve Martin, [email protected]
Source: Diana Caldwell, [email protected]
Research Foundation News
Communication.
- OneCampus Portal
- Brightspace
- BoilerConnect
- Faculty and Staff
- Human Resources
- Colleges and Schools
Info for Staff
- Purdue Moves
- Board of Trustees
- University Senate
- Center for Healthy Living
- Information Technology
- Ethics & Compliance
- Campus Disruptions
Purdue University, 610 Purdue Mall, West Lafayette, IN 47907, (765) 494-4600
© 2015-24 Purdue University | An equal access/equal opportunity university | Copyright Complaints | Maintained by Office of Strategic Communications
Trouble with this page? Disability-related accessibility issue? Please contact News Service at [email protected] .
Státy a města
Luhanská lidová republika.

Luhanská lidová republika tvoří spolu s Doněckou lidovou republikou samozvanou konfederaci Nové Rusko (nebo také Novorusko), které bývá označované jako svaz lidových republik. Jejich vznik je důsledkem krize na Ukrajině, která začala na konci roku 2013.
Ukrajinská krize
Pozastavení podpisu asociační dohody s EU ze strany ukrajinské vlády v listopadu 2013 spustilo masové protesty , které po třech měsících přerostly v pouliční boje a otřásly celou Ukrajinou. Demonstrace konané na náměstích ukrajinských měst (tzv. euromajdan y) se později změnily v protesty proti vládě a zejména proti prezidentovi Viktoru Janukovyčovi , jehož demisi opozice požadovala.
Po " krvavém čtvrtku " 20. února 2014, během kterého zemřelo nejméně sedm desítek lidí, následovalo jednání prezidenta Janukovyče s opozicí a zahraničními diplomaty. Janukovyč oznámil, že vyhlásí předčasné prezidentské volby a že se země vrátí k ústavě z roku 2004, podle níž měla být část prezidentských pravomocí přesunuta na parlament.
Související wiki:
Vladimir Putin
Doněcká lidová republika
Nové Rusko
Den po dohodě však prezident opustil Kyjev , který zcela ovládla opozice, a odcestoval do Charkova. Později se přesunul do Ruska. Ukrajinský parlament následně schválil rezoluci, která Janukovyče vyzvala k okamžité rezignaci. Parlament po sesazení Janukovyče jmenoval do svého čela Oleksandra Turčynova , který se zároveň stal prozatimním ukrajinským prezidentem. Poslanci schválili také novou koaliční vládu, na níž se dohodli zástupci dosavadních opozičních sil. Do jejího čela se dostal Arsenij Jaceňuk , jeden z hlavních vůdců dosavadní opozice vůči režimu prezidenta Viktora Janukovyče .
Proti novému politickému uspořádání Ukrajiny se postavili představitelé autonomní republiky Krym. Poloostrov se stal hlavní baštou proruských odpůrců změny režimu . Ruský prezident Vladimir Putin požádal 1. března 2014 parlament o povolení využít ruské vojáky k "normalizaci" situace na Krymu , což se nakonec také stalo. Již 16. března 2014 proběhlo referendum o připojení Krymu k Rusku , jehož obyvatelé se vyslovili pro odtržení od Ukrajiny. Výsledek byl však vzhledem k tomu, že Ukrajinci a krymští Tataři referendum bojkotovali, zpochybňován.
Ruský prezident Vladimir Putin následně nezávislost Krymu uznal a spolu s jeho politickými představiteli a zástupci města Sevastopol podepsal smlouvu o jejich připojení k Ruské federaci. Západ ani ukrajinská vláda referendum o odtržení neuznávají.
Vznik Luhanské lidové republiky
Odtržení Krymu posílilo separatistické tendence a hlavní nepokoje se následně přenesly do převážně ruskojazyčných regionů na jihu a východě země, kde se konaly proruské a projanukovyčovské protesty - tzv. antimajdany - a vymezovaly se proti proevropskému a protijanukovyčovskému euromajdanu.
Foto: Krajina kolem Luhanska připomíná apokalypsu

V dubnu 2014 separatisté na východě Ukrajiny obsadili budovy oblastní správy. V Doněcku a Luhansku následně vyhlásili nezávislé republiky – Doněckou lidovou republiku a Luhanskou lidovou republiku. Dne 11. května 2014 se v obou "republikách" konalo referendum o tom, zda by region měl zůstat součástí Ukrajiny, nebo se stát samostatným státem. Výsledek byl v obou případech stejný – voliči se vyslovili pro samostatnost . Organizátoři tvrdí, že v Doněcku tak hlasovalo 89 procent voličů a v Luhansku 96 procent hlasujících. Samozvané republiky však žádná země neuznala. Samotná Ukrajina je označuje za teroristické organizace.
Dva týdny po referendu podepsali zástupci Luhanské lidové republiky a Doněcké lidové republiky dohodu o spojení a vytvoření státního útvaru " Nové Rusko ".
Mučili mě, protože jsem Rus a bránil jsem Ukrajinu, říká bezruký odbojář z Donbasu

Hra vabank. Kreml pořádá referenda podle krymského scénáře a sleduje několik cílů

Analytik: Rusko sílí. Boj o velké město na Donbase byl pro Ukrajince zdrcující

Nejen podpora terorismu na východě Ukrajiny, Čecha nově stíhá policie za dětské porno

Fotografie ze zákopů: Na východě Ukrajiny sílí napětí, obě strany vysílají vojáky

Šachtar pět let prchá před válkou. Stadion zničily rakety, fungoval i jako skladiště

Miny, rozstřílené domy, ale nový most. Reportáž z bojové linie v Donbasu

Právě se děje
Na andělu zemřel člověk po pádu pod soupravu metra.

ŽIVĚ Írán vyslal na Izrael drony a rakety. Útok nezůstane bez odvety, řekl Netanjahu

Řepík se vrátil po zkratu a zahodil postupovou trefu. Nebudu se schovávat, kál se

Biden si volno u moře dlouho neužil. Akutně řeší hrozící konflikt Íránu a Izraele

To jsou ty moje hospůdky, vracel se Blažek k bouřce s Nejedlým. Vystrčil vzýval mládí

Židovští osadníci na Západním břehu útočili na Palestince. Rozezlila je smrt chlapce

Třinecký Roman měl po rozhodujícím gólu blackout. Za postupovou trefu prémie neobdrží
- Podivné zločiny
- Co z tebe bude?!
- Ostatní sporty
- Den v 60 sekundách
- Historky z placu
- Politické bizáry
- Film a televize
- Klasická hudba
- Chytré Česko
- My a deziformace
- Porodnictví
Centrum.cz | Atlas.cz 1999 – 2024 © Economia, a.s. | O nás | Všechny služby | Volná místa
Inzerce | Služby firmám | Všeobecné podmínky | Cookies | Nastavení soukromí | Ochrana osobních údajů | Zpracování osobních údajů | Audiovizuální mediální služby | Nápověda
Jakékoliv užití obsahu, včetně převzetí článků, je bez souhlasu Economia, a.s. zapovězeno. Bez souhlasu Economia, a.s. je zapovězeno též rozmnožování obsahu pro účely automatizované analýzy textů nebo dat podle ustanovení § 39c autorského zákona.
Content Search
Medical staff in donetsk and luhansk oblasts to receive personal protective equipment.

UNDP and the European Union to provide 15,000 additional respirators to meet rising demand
Kramatorsk, Donetsk Oblast, 2 October 2020 – The European Union, together with the United Nations Development Programme, has donated an additional batch of 15,000 N95 valveless respirators to the Donetsk Oblast Emergency Medical Care and Disaster Medicine Centre in Kramatorsk and Clinical Hospital in Sievierodonetsk in Luhansk Oblast.
UNDP Resident Representative Dafina Gercheva said that in the face of the rapidly worsening epidemiological situation in Ukraine, it is very important to ensure there is the proper protection for health workers, who risk their own health every day while treating patients.
“We're very grateful to all medical workers in eastern Ukraine who, despite the danger and high risks of contracting COVID-19, continue to do their jobs,” Gercheva said. “Thanks to cooperation with the European Union, we're continuing to protect doctors and build the capacity of medical institutions in eastern Ukraine, so they can more effectively respond to those in need.”
Stefan Schleuning, Head of Cooperation at the EU Delegation to Ukraine, referred to joint efforts of the EU and national and international partners to withstand the COVID-19 pandemic in Ukraine. “We sincerely thank the doctors and medical staff of the Donetsk and Luhansk regions for their daily work in these extreme conditions. The European Union will continue to support economic and social recovery in eastern Ukraine and help ensure the safety of healthcare workers”, said Mr. Schleuning.
The N95 respirator can block up to 95 percent of the smallest aerosol particles and filter the air, which may contain viruses and bacteria. This respirator provides better protection than a surgical mask or homemade protective equipment, as it fits tightly over the face and can filter out both large and small air particles.
Ihor Kiiashko, Head of the Regional Centre for Emergency Care and Disaster Medicine, thanked the international partners for responding to the needs of doctors the ongoing crisis. “This new shipment of N95 respirators will further bolster our defences and strengthen the resilience of the emergency medicine system in these difficult times,” he said.
The total cost of supplying the respirators is US$46,346, and was carried out under the UN Recovery and Peacebuilding Programme, with financial support from the European Union.
The United Nations Recovery and Peacebuilding Programme (UN RPP) is being implemented by four United Nations agencies: the United Nations Development Programme (UNDP), the UN Entity for Gender Equality and the Empowerment of Women (UN Women), the United Nations Population Fund (UNFPA) and the Food and Agriculture Organization of the United Nations (FAO).
Thirteen international partners support the Programme: the European Union (EU), the European Investment Bank (EIB), the U.S. Embassy in Ukraine, and the governments of Canada, Denmark, Germany, Japan, the Netherlands, Norway, Poland, Sweden, Switzerland and the UK.
Media enquiries
Maksym Kytsiuk, Communications Associate, the UN Recovery and Peacebuilding Programme, [email protected], +380 63 576 1839
Related Content
Ukraine + 4 more
Ukraine Humanitarian Appeal - Progress Report
Ukraine + 24 more
Russia’s war on Ukraine | UCPM MEDEVAC operations - DG ECHO Daily Map | 12/04/2024
Ukraine + 1 more
UK donates £2 million lifesaving healthcare package to Ukraine
Unicef ukraine humanitarian situation report no. 37 - 29 february 2024.


IMAGES
VIDEO
COMMENTS
1. IQVIA. Formerly known as Quintiles and IMS Health, IQVIA is one of the largest CROs in the world, with a large range of service offerings to help advance clinical research. The company was founded in North Carolina in 1982, and has since grown to over 88,000 employees in more than 100 countries.
A dedicated CRO providing the and leveraging the breadth of our , our team of more than 21,000 global professionals works in partnership with to design and deliver clinical trials , to make clinical research a care option for anyone, anywhere. For over 35 years Parexel has been a trusted global CRO and biopharmaceutical services company.
Who we are. Parexel is among the world's largest clinical research organizations (CROs), providing the full range of Phase I to IV clinical development services to help lifesaving treatments reach patients faster. Leveraging the breadth of our clinical, regulatory, and therapeutic expertise, our team of more than 21,000 global professionals ...
Here is a quick overview of the best companies of clinical research: IQVIA: Best for data-driven insights and advanced analytics in healthcare research. ICON: Best for comprehensive clinical development services and therapeutic expertise. Parexel: Best for global biopharmaceutical services, emphasizing regulatory and clinical trial excellence.
Velocity Clinical Research is the world's leading organization of fully integrated research sites. The company partners with pharmaceutical and biotechnology companies to research new drugs, medical devices, and diagnostics that could improve human health and wellbeing. Velocity's unified research site solutions deliver the right patients ...
IQVIA is a leading global provider of advanced analytics, technology solutions, and clinical research services to the life sciences industry. IQVIA creates intelligent connections across all aspects of healthcare through its analytics, transformative technology, big data resources and extensive domain expertise.
At Worldwide, we believe a personalized approach is the only way to unlock the power of everyone working on a study - from operational and therapeutic experts to site partners and scientists. That means you'll always have direct access to our experts. You'll be able to tap into more than 30 years of therapeutic experience on a global scale.
Global trials for oncology, rare diseases and other complex diseases face unique challenges. Precision partners with you to meet them head-on through our extensive footprint across the globe. We succeed by pairing personalized clinical services with translational medicine excellence, making us the first fully integrated clinical research ...
Top 10 Leading Clinical Research Companies In The USA. The landscape of drug discovery and development is intricate, demanding significant resources at each stage of the process. As pharmaceutical companies strive to bring groundbreaking therapies to market, they increasingly rely on the expertise of Contract Research Organizations (CROs).
A list of the 10 biggest contract research organizations (CROs) in the pharma sector in 2022, based on revenue, services and locations. The CROs provide clinical trial management, data research and project management for drug manufacturers at each stage of the development cycle. Learn about their backgrounds, specialties and challenges.
ICON acquires HumanFirst, a cloud-based technology company for life sciences supporting precision measurement in patient centred clinical research. Biotech. ICON provides full service outsourcing and flexible support for biotech specific needs such as due diligence and asset valuation. Cardiac Safety Solutions.
So, let's begin: 1. IQVIA. IQVIA is one of the largest Clinical Research companies in the world that works profusely to enhance healthcare by integrating the study of human science with discoveries in technology and data science to improve understanding of human health and provide better and quicker care.
A distinct clinical research organization, Veristat stands alone in combining industry-leading expertise with unwavering commitment to scientific integrity. ... At Veristat, we contribute and add value to our company's mission - improving and saving lives. Whether you're a scientific expert or an operations guru, explore the chance to ...
iNGENū CRO. iNGENū is the FDA-centric Australian CRO championing disruptive, innovative biotech firms globally. Our core mission is to create access to high quality clinical research globally, for early to mid-stage biotechs by removing financial and other unnec... 💻 Website ↗ 📞 +1300 633 226 View all details.
The United States of America has some of the top clinical research organizations in the world. Amongst them, here is the list of the 10 Best Clinical Research Organizations In The USA. 1. Covance. In 2014, LabCorp acquired Covance for $6.1B. Covance, a global contract research organization under LabCorp, is the world's leading healthcare ...
It's bigger than better clinical trials. Or advances in technologies and analytics. Or faster insights. It's about exploring a new path to better health outcomes via Human Data Science. It's about harnessing the power of the IQVIA CORE™ to channel the insights, commercial and scientific depth, and executional expertise that drive maximum value for our customers.
List of clinical research organizations in the US. 1. IQVIA. IQVIA is a globally renowned medical research companies in USA that stands out for its exceptional contributions to the healthcare and pharmaceutical industries. As one of the leading providers of advanced analytics, technology solutions, and contract research services, IQVIA plays a ...
Proxima provides regulatory, quality & clinical trial life science solutions to the new class of rising stars in drugs, devices, diagnostics & digital therapeutics By clicking "Accept", you agree to the storing of cookies on your device to enhance site navigation, analyze site usage, and assist in our marketing efforts.
Discover the transformative impact of technology and innovation in clinical trials, along with the regulatory challenges and adaptations that come with it. WATCH THE WEBINAR PPD is a leading global contract research organization focused on delivering life-changing therapies
Participants want clinical trials to be more convenient, closer to home, and more accommodating of their schedules. These findings are instructive for digital health companies and contract research organizations which, by and large, are still modelling clinical trials for digital therapeutics on those used for pharmaceutical and biotech studies. Switching to a decentralized clinical trial ...
Orlando-based clinical trials facilities network Alcanza Clinical Research has purchased a Florida site of Innovation Medical Research Center, the company announced in an April 9 press release ...
Amplified Sciences, a clinical-stage life sciences diagnostic company, has received a $275,000 Small Business Innovation Research grant from the National Science Foundation. The funding will support critical development of the company's tests for early, more accurate detection of challenging diseases, starting with pancreatic cancer.
I. V. Dyachenko's 63 research works with 277 citations and 1,379 reads, including: Multicomponent Synthesis of Functionalized 2-Amino-4H-pyrans Initiated by the Knoevenagel Reaction
27. 12. 2014 23:56. Luhanská lidová republika je samozvaný státní útvar vyhlášený na části ukrajinské Luhanské oblasti. Luhanská lidová republika tvoří spolu s Doněckou lidovou republikou samozvanou konfederaci Nové Rusko (nebo také Novorusko), které bývá označované jako svaz lidových republik. Jejich vznik je ...
UNDP and the European Union to provide 15,000 additional respirators to meet rising demand. Kramatorsk, Donetsk Oblast, 2 October 2020 - The European Union, together with the United Nations ...
The actual need for social and geographical research of the region, including tourism, is determined. It is tourism that has an important social and geographical function of restoring peaceful life in the region, building a socially responsible society. Tourism should become a kind of mentor for social transformation, because when communicating with one another, people will never believe in ...