- Registry Operations
- Reporting Guidelines
- COVID-19 Abstraction Guidance

COVID-19 Presentations

COVID-19 Abstraction Guidance by Margaret (Peggy) Adamo, RHIT, CTR
Access the presentation slides (PDF, 1.2 MB)

Coronavirus-Disease 19 (COVID-19) & SARS-CoV-2 Testing by Alison Van Dyke, MD, PhD
Access the presentation slides (PDF, 4.6 MB)


- Weill Cornell Medicine

Medicine Grand Rounds: COVID-19 presentations
A video of this lecture is available online to members of the Weill Cornell community. You will need to provide your CWID and password. Below are the PowerPoints that were included in the presentation.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Elsevier - PMC COVID-19 Collection

A mathematical model of Coronavirus Disease (COVID-19) containing asymptomatic and symptomatic classes ☆
Idris ahmed.
a Department of Mathematics, Faculty of Science, King Mongkut’s University of Technology Thonburi (KMUTT), 126 Pracha-Uthit Road, Bang Mod, Thung Khru, Bangkok 10140, Thailand
b KMUTTFixed Point Research Laboratory, Fixed Point Theory and Applications Research Group, Center of Excellence in Theoretical and Computational Science (TaCS-CoE), Faculty of Science, King Mongkut’s University of Technology Thonburi (KMUTT), 126 Pracha-Uthit Road, Bang Mod, Thung Khru, Bangkok 10140, Thailand
Goni Umar Modu
c Department of Statistics, Ramat Polytechnic Maiduguri, P. M. B 1070 Maiduguri, Borno State, Nigeria
Abdullahi Yusuf
d Department of Computer Engineering, Biruni University, Istanbul 34010, Turkey
e Department of Mathematics, Federal University Dutse, Jigawa 7156, Nigeria
f Department of Medical Research, China Medical University Hospital, China Medical University, Taichung 40402, Taiwan
Ibrahim Yusuf
g Department of Mathematical Sciences, Bayero University Kano, P. M. B. 3011 Kano, Nigeria
Associated Data
All data used in this analysis is provided in the Nigerian center for disease control and Word heath organization, https://ncdc.gov.ng, and https://who.int.
The research work in this paper attempts to describe the outbreak of Coronavirus Disease 2019 (COVID-19) with the help of a mathematical model using both the Ordinary Differential Equation (ODE) and Fractional Differential Equation. The spread of the disease has been on the increase across the globe for some time with no end in sight. The research used the data of COVID-19 cases in Nigeria for the numerical simulation which has been fitted to the model. We brought in the consideration of both asymptomatic and symptomatic infected individuals with the fact that an exposed individual is either sent to quarantine first or move to one of the infected classes with the possibility that susceptible individual can also move to quarantined class directly. It was found that the proposed model has two equilibrium points; the disease-free equilibrium point ( DFE ) and the endemic equilibrium point ( E 1 ) . Stability analysis of the equilibrium points shows ( E 0 ) is locally asymptotically stable whenever the basic reproduction number, R 0 < 1 and ( E 1 ) is globally asymptotically stable whenever R 0 > 1 . Sensitivity analysis of the parameters in the R 0 was conducted and the profile of each state variable was also depicted using the fitted values of the parameters showing the spread of the disease. The most sensitive parameters in the R 0 are the contact rate between susceptible individuals and the rate of transfer of individuals from exposed class to symptomatically infected class. Moreover, the basic reproduction number for the data is calculated as R 0 ≈ 1.7031 . Existence and uniqueness of solution established via the technique of fixed point theorem. Also, using the least square curve fitting method together with the fminsearch function in the MATLAB optimization toolbox, we obtain the best values for some of the unknown biological parameters involved in the proposed model. Furthermore, we solved the fractional model numerically using the Atangana-Toufik numerical scheme and presenting different forms of graphical results that can be useful in minimizing the infection.
1. Introduction
A mathematical model is a description of the workings of the real world employing mathematical symbols, equations, and formulas. Mathematical models are commonly used in many fields, such as medicine [39] , agriculture [25] , [47] , management and social sciences [41] and references cited therein. Such models can either be linear or nonlinear, stochastic or deterministic. In the health sector, mathematical models have been used to forecast disease outbreaks, avoid or cure these illnesses. Currently, there are many mathematical models used to explain disease processes and they can be found in books dedicated to mathematical models in biology and medicine, for more details, refer the reader to [10] , [18] , [26] , [35] . By the end of December 2019, the world’s deadly coronavirus pandemic, popularly known as COVID-19, erupted in the ancient town of Wuhan Hubei Province, China spreading almost through several countries in 2020 [2] . Several unexplained cases of cough, pneumonia, dyspnea, exhaustion and there were fever in Wuhan, China, for a short period. The arrival of COVID-19 resulted in the closure of business, schools, markets, traveling within and outside, intermingle, curfew, lockdown, reduction of gathering to mention but a few [3] .
Nigeria has fallen into the category of nations affected by the coronavirus pandemic. February 27, 2020, culminated in the arrival of the pioneer case of COVID-19 in Lagos state southwestern Nigeria when an Italian citizen arrived on 24 February and presented at a health facility on 26 February 2020 (see Fig. 1.1 ) [1] . Before the arrival of COVID-19 into Nigeria, the Federal government with some states in Nigeria established various medical agencies and isolation centers to handle and stop the spread of the pandemic. The Federal government through its Nigeria Center for Disease Control (NCDC) provide appropriate public health advice to Nigerians, on the symptoms, dissemination, and prevention of information with national and sub-national networks of public health staff, built capacity for contact tracing and case management and strengthened some laboratories for diagnostic activities of the pandemic.

Confirmed COVID-19 Cases-Nigeria 2020 [1] .
With the arrival of COVID-19, researchers have been using and formulating mathematical models as a technique in gaining insight into the mode of spread of the pandemic, transmission, impact of the pandemic, prevention and control of the pandemic, the influence of preventive measure on the pandemic ranging from washing hands with a disinfectant such as a hand sanitizer, 2 to 5 meters social distance and use of face mask, see the recent literature [20] , [33] , [34] , [36] , [43] , [48] , [50] , [53] , [54] , [55] . Okuonghae and Omame [38] analyzed the spread and behavior of COVID-19 in Lagos, Nigeria. Roseline et al. [37] used the linear regression method to modeled the rate of fatality of the pandemic in Nigeria. Adegboye et al. [6] dealt with early spread of COVID-19 in Nigeria. Ajisegiri et al. [9] analyzed the outbreak scenario of COVID-19 in Nigeria. Researchers in different areas have presented excellent research on preventive and curative steps to contain the pandemic and have reported better outcomes. However, a further review of the latest form of models with a justified and satisfactory evaluation is required.
In all of the research cited above, the models presented therein were based On classical derivatives with some restrictions on the order of differential equations involved. To overcome these limitations, several researchers have sought the aid of a rapidly growing field of mathematics known as fractional calculus. The differential operators used in fractional calculus are non-integer or fractional order, which have memory features and are useful in demonstrating many natural phenomena and facts having non-local dynamics behavior. The concept of fractional order has gained significant attention due to its wide-ranging advances and various applications in knowledge areas, see [13] , [14] , [21] , [22] , [32] , [40] . Recently, Atangana and Baleanu [16] proposed a new fractional derivative operator with a nonsingular and Mittag-Leffer kernel. The main advantage of the operator is that it has a nonlocal and nonsingular kernel. More recently, new advances and studies in fractional differential equations has been published from a mathematical modeling point of view, see recent papers [4] , [7] , [8] , [11] , [12] , [15] , [19] , [27] , [28] , [29] , [30] , [44] , [46] , [52] .
The paper is organized as follows: In Section 2 , we formulated the model together with the description of the parameters defined in the model. In Section 3 , we obtain the invariant region. Also, we compute the basic reproduction number and study it is disease-free equilibrium ( DFE ) , local stability, the existence of endemic equilibrium, local stability of the endemic equilibrium, and global stability of the endemic equilibrium. The existence and uniqueness of solutions were investigated via the techniques of fixed point theorems in Section 4 . In Section 5 , we present the model fitting as well as the estimation of the parameters. Besides, sensitivity analysis and numerical simulation are also highlighted. Finally, we give the conclusion of the paper in Section 6 .
2. Model formulation
In this research paper, a model of the coronavirus (COVID-19) disease using simple rates of transmission is considered. Let N ( t ) be the total population of human. This population is divided into seven classes: susceptible individuals S ( t ) , exposed individuals E ( t ) , asymptotically infected individuals I A ( t ) , symptomatic infected individuals I S ( t ) , quarantined individuals Q ( t ) , and individuals that have recovered/remove from COVID-19 R ( t ) . Based on this consideration, the total population is N ( t ) = S ( t ) + E ( t ) + Q ( t ) + I A ( t ) + I S ( t ) + R ( t ) .
The natural human natality and mortality rates are denoted by Λ and μ respectively. Susceptible individuals ( S ) gets infected from enough contact with exposed individuals ( E ) at the rate of β or just move to quarantined class at the rate of τ . The exposed individuals ( E ) may move to quarantined ( Q ) class first or get infected without symptoms (asymptomatic) ( I A ) or with symptoms (symptomatic) ( I S ) at the rates of γ , σ and η respectively. Also quarantined individuals ( Q ) may be confirmed infected through a test with symptoms ( I S ) or without symptoms ( I A ) at the rates of υ and θ respectively. The asymptomatic infected individuals ( I A ) may recover at the rate of r 1 and the symptomatic infected individuals ( I S ) at the rate of r 2 .
Each of these classes may decrease as a result of natural mortality μ , while the class of individuals infected with symptoms ( I S ) may also decrease as a result of death from the disease at the rate of δ . In the infected class of individuals without symptoms ( I A ) , the death as a result of the disease is not considered. The possibility of reinfection after recovery has not been considered in this model.
Fig. 2.2 , below depicted the schematic diagram showing the spread of COVID-19. Through the schematic diagram depicted in Fig. 2.2 , a system of nonlinear differential equations is obtained and presented below (see Table 1 ):
subject to the following initial conditions:

Transmission pattern of COVID-19.
Notations used and their meaning.
3. Qualitative analysis of the proposed model
Reproduction number is vital in the study of infection disease model [23] . This section presents the computation and presentation of basic reproduction number and invariant region for the proposed model (2.1) and study the
- • Locally asymptotically stability of its disease free equilibrium (see Theorem 3.1 ).
- • Unique endemic equilibrium point (see Theorem 3.2 ).
- • Locally asymptotically stability of its unique endemic equilibrium point (see Theorem 3.3 ).
- • Globally asymptotically stable of its endemic equilibrium (see Theorem 3.5 ).
3.1. Invariant region
Having described the human population in the model (2.1) , it is vital to show that the state parameters S ( t ) , E ( t ) , Q ( t ) , I A ( t ) , I S ( t ) , R ( t ) are nonnegative for all t ≥ 0 . Solution with positive initial data remains positive for all t ≥ 0 and are bounded. It is easy to see from systems (2.1) that dN ( t ) dt = Λ - μ N ( t ) - δ I S ( t ) and sup t → + ∞ N ( t ) ⩽ Λ μ . As such we can study the system (2.1) in the following feasible region:
(3.1) is now positive invariant in relation to (2.1) . Meaning the proposed model (2.1) is epidemiologically well posed and all solutions of the system with ( S ( t ) , E ( t ) , Q ( t ) , I A ( t ) , I S ( t ) , R ( t ) ) ∈ R + 6 remain in Ω .
3.2. Disease free equilibrium point ( DFE )
Setting the parameters E = Q = I A = I S = R = 0 , the disease free equilibrium is obtained. Therefore, the system (2.1) indicates that the DFE point is given by
The basic reproduction number denoted by R 0 is the expected value of infection rate per time unit. The infection occurs in a susceptible population, caused by an infected individual. Based on the system (2.1) , the article generates an equation that involves the classes of exposed and infected population. The disease reproduction number R 0 of the proposed model (2.1) is defined herein in the infected classes. This threshold quantity has been described in [23] , [51] . In all cases, R 0 < 1 implies that disease will decline, whereas R 0 > 1 implies that disease will persist within a community and R 0 = 1 requires further investigation. R 0 is obtained using the next generation matrix approach [23] where several authors have used it.
We implore the use of a next-generation matrix to find the basic reproduction number for the model (2.1) . Without loss of generality, it is clear from the model (2.1) , the article generates an equation that involves the classes of the exposed population, infected population without symptom, and infected population with symptom as follows:
Referring to [51] , from the equations (3.3) , the study generates matrix F and V , i.e.
The Jacobian matrix of F and V at DFE, denoted by F and V are given as follows:
Therefore, FV - 1 is the next generation matrix of the model structure (3.3) . So, as described in [23] , R 0 = ρ ( FV - 1 ) where ρ stands for spectral radius of the next-generation matrix FV - 1 . Thus,
So, ρ ( FV - 1 ) = β Λ A ( τ + μ ) = R 0 , where A = γ + μ + η + σ .
3.3. Local stability analysis of the disease free equilibrium
The disease free equilibrium DFE is locally asymptotically stable if R 0 < 1 .
The Jacobian matrix with respect to the system (2.1) is given by:
which implies
The characteristic polynomial of the Jacobian matrix at DFE is given by det ( J DFE - λ I ) = 0 , where λ is the eigenvalue and I is 6 × 6 identity matrix. Thus, the determinant of ( J DFE - λ I ) is
Simplifying and solving for λ , gives
This completes the proof. ■
3.4. Existence of endemic equilibrium point
In this subsection, we look at the existence of endemic equilibrium point. Let us denote the endemic equilibrium by E 1 = S ∗ , E ∗ , Q ∗ , I A ∗ , I S ∗ , R ∗ . For simplicity, S ( t ) = S , E ( t ) = E , Q ( t ) = Q , I A ( t ) = I A , I S ( t ) = I S and R ( t ) = R , will be used henceforth. This endemic equilibrium always satisfies:
From the first Equation of (3.6) , we obtain
Inserting (3.7) in the second Equation of (3.6) , we get
Substituting E ∗ in (3.7) , yields
Using (3.8) , (3.9) in the third Equation of (3.6) , gives
Substituting Eqs. (3.8) , (3.10) , in the fourth Equation of (3.6) , we have
Inserting Eqs. (3.8) , (3.10) , in the fifth equation of (3.6) we get
Bringing Eqs. (3.11) , (3.12) , into the sixth equation of (3.6) , yields
Thus, we conclude with the following theorem.
The system (2.1) has unique endemic equilibrium point given by
whenever R 0 > 1 and
3.5. Local stability analysis of the endemic equilibrium E 1
The endemic equilibrium E 1 is locally asymptotically stable if R 0 > 1 .
which implies,
The characteristic polynomial of the Jacobian matrix at E 1 is given by det ( J E 1 - λ I ) = 0 . where λ is the eigenvalue and I is 6 × 6 identity matrix. Thus,
Simplifying the characteristic polynomial (i.e making the characteristic equation) and solving for λ , gives
The quadratic λ 2 + ( τ + μ ) R 0 λ + ( γ + μ + η + σ ) 2 has all terms positive and thus, its roots must all be negative. Meanings λ 5 , 6 < 0 . This completes the proof. ■
3.6. Global stability analysis of the endemic equilibrium E 1
The system (2.1) has no periodic orbits.
We employ the Dulac’s criterion to achieve this. Now, let X = ( S , E , Q , I A , I S , R ) . Taking the Dulac’s function
Hence, the system (2.1) has no periodic orbit. This completes the proof. ■
Since Ω is positively invariant, it follows from Poincarè-Bendixson theorem that all solutions of the system (2.1) originate and remain in Ω for all t . We conclude with the following theorem:
The endemic equilibrium E 1 for the system (2.1) is globally asymptotically stable whenever R 0 > 1 .
4. Fractional order model in the sense of ABC-fractional operator
In the current research work, researchers have shown that mathematical models constructed with the aid of fractional operators are often more precise and reliable compared to the integer-order case due to the enhanced degree of freedom. In fractional-order models, the memory property allows more knowledge from the past to be added, which predicts and translates models more accurately. Recently, Atangana and Baleanu [16] , introduced new fractional derivatives without singularity and locality. The non-singularity and non-locality of the kernel give a better description of the memory. The aforementioned operator has found wide applications to model dynamics processes in many well-known fields of science, engineering, biology, medicine, and many other, see [31] , [45] , [5] , [17] , [17] and the references cited therein. Besides, in comparisons with classical Caputo, Caputo-Fabrizio, the ABC operator recorded the highest efficiency in terms of experimental data and numerical efficiency [42] .
Motivated by the above-mentioned application, in this section, we generalize the proposed model (2.1) to fractional order in the sense of ABC-fractional derivative and study the existence and uniqueness of solutions for the generalized model using the techniques of fixed point theorems. Now, before we generalize the model (2.1) , we recall some preliminaries definitions which are important throughout the section.
For a given function z ∈ H 1 ( 0 , T ) , T > 0 and α > 0 . The fractional operator
is called the ABC-fractional operator where N ( r ) represent the normalization function and satisfies N ( 0 ) = N ( 1 ) = 1 and E α ( · ) denotes the one-parameter Mittag–Leffler function of form:
The fractional operator
is referred to fractional integral operator associated with ABC-fractional derivative.
Therefore, the proposed nonlinear fractional model in the sense of ABC-fractional operator is of the form:
where ABC D 0 α ( · ) is the ABC-fractional derivative of order ( 0 < α ≤ 1 ) and the variables are assumed to be non-negative with appropriate initial conditions.
It is worth mentioning here that, the parameters are assumed to be non-negative and have dimension time. In this paper, the dimensionality consistency was taking into consideration when fictionalized the integer-order model as the dimension of each left-hand side equation carries time - α , ( α > 0 ) , whereas the right sides remains dimensionally time - 1 . This technique was proposed by Diethelm in [24] .
4.1. Existence and uniqueness result
This subsection presents the existence and uniqueness of solutions of the proposed model using the techniques of fixed point theory. Here, we denote E = C ( [ 0 , T ] , R ) the Banach space of all continuous real-valued function equipped with the norm defined by
Now, applying the fractional integral operator AB I 0 + α on both sides of system (4.4) , yields
The kernels in Equations (4.7) satisfies the Lipschitz condition for 0 ⩽ M i < 1 , i = 1 , 2 , … 6 , if and only if the nonlinear functions S ( t ) , E ( t ) , Q ( t ) , I A ( t ) , I S ( t ) and R ( t ) have an upper bound. Indeed, suppose S ( t ) and S ∗ ( t ) be two functions, then we get
where M 1 = ( τ α + μ α ) + β α sup t ∈ [ 0 , T ] | E ( t ) | . Thus,
Repeating the same procedure as in Eq. (4.8) above, we have
where M i ( i = 1 , 2 , … 6 ) are the corresponding Lipschitz constant for the functions F i ( · ) for i = 1 , 2 , … , 6 .
Now, Eq. (4.6) can be written in recursive form given by
Let us denote difference between successive components by Φ n i , i = 1 , 2 , … 6 . So,
taking into consideration that S n ( t ) = ∑ i = 0 n Φ i 1 ( t ) , E n ( t ) = ∑ i = 0 n Φ i 1 ( t ) , Q n ( t ) = ∑ i = 0 n Φ i 1 ( t ) , I A , n ( t ) = ∑ i = 0 n Φ i 1 ( t ) , I S , n ( t ) = ∑ i = 0 n Φ i 1 ( t ) , R n ( t ) = ∑ i = 0 n Φ i 1 ( t ) . Taking the norm on both side of Equations (4.12) and using Equations (4.9) yields
Now, we are ready to state and prove the main theorem based on the above results.
The fractional proposed model (4.4) possesses a unique solution for t ∈ [ 0 , T ] if the condition is satisfied
Since from the assumptions the functions S ( t ) , E ( t ) , Q ( t ) , I A ( t ) , I S ( t ) , R ( t ) are bounded and satisfies the Lipschitz condition. Thus, in view of Eq. (4.13) , we get
Hence, the sequences above exist and as n ⟶ ∞ , ‖ Φ n i ( t ) ‖ ⟶ 0 , i = 1 , 2 , … 6 . Also, utilizing the triangular inequality for any k , Eq. (4.15) yields
where the P i , i = 1 , 2 , … 6 , are the terms within the bracket of Equations (4.15) and the condition 1 - α N ( α ) M i + 1 N ( α ) M i Γ ( α ) T α < 1 . Thus, by uniform convergent theorem the function S n , E n , Q n , I S , n , I A , n and R n constitute a Cauchy sequence in E . So, applying the limit theory on the equation (4.11) as n → ∞ shows that the limit of these sequences is the unique solution of the proposed model (4.4) . In addition, we conclude the existence of a unique solution of the proposed model (4.4) . ■
5. Model fitting and parameter estimation
One of the essential mechanisms for evaluating the transmission dynamics of a disease is the validation of a newly developed epidemiological model. The availability of real data for the underlying ailment contributes significantly to the completion of this task and the real data gives us an insight into how to determine the best values of some of the model’s unknown biological parameters. To this end, we employ the nonlinear least-squares curve fitting method with the help of “ fminsearch ” function from the MATLAB Optimization Toolbox. This approach states that, if a theoretical model t ↦ Ξ ( t , q 1 , q 2 , … , q n ) is attained and depend on a few unknown parameters q 1 , q 2 , … , q n and a sequence of actual data points ( t 0 , y 0 ) , … , ( t j , y j ) is also at hand then the aim is to obtain values of the parameters so that the error calculated can,
attain a minimum. 12 biological parameters are associated with the proposed model. Some of these parameters have been assumed while some have been best fitted (see Fig. 5.3 ). The initial conditions for the state variables are S ( 0 ) = 0.5 , E ( 0 ) = 0.2 , Q ( 0 ) = 0.1 , I A ( 0 ) = 0.2 , I S ( 0 ) = 0.1 and R ( 0 ) = 0 (see Table 2 ).

The daily COVID-19 cumulative cases time series in Nigeria from 1 July to July 31, 2020, with the best-fitted curve from simulations of the proposed model and (b) the residuals for the best-fitted curve.
Baseline values of the parameters used in the model (2.1) .
5.1. Sensitivity analysis
In this subsection, the concept of sensitivity analysis is used to discover the robust significance of the generic parameters present in the basic reproduction number R 0 . Furthermore, both the analytical and numerical values of the R 0 parameters are derived from precise assumptions using parameter values. If and only if the dynamics follow the model (2.1) , the analytical expressions obtained can be used to shed some light on how to track the model’s onset in variant locations. The threshold value R 0 is a quantity known to be the primary way of reducing and aborting the ailment spread by reducing the number to less than unity. The sensitivity index technique is used to measure the most sensitive parameters in the model, those with the positive sign are considered to be highly and proportionally sensitive to the value of R 0 while those with the negative sign are less sensitive to decreasing R 0 and the other category is neutrally sensitive (with zero relative sensitivity). The cause of the transmission of the infringement is commonly recognized to be directly linked to the specific reproduction number R 0 . The R 0 elasticity indices is given by Eq. (3.4) :
where R 0 denotes the basic reproduction ratio and P i is as stated above. Following the described formula, we reach:
The numerical values indicating the relative significance of R 0 are given in Table 3 . Some parameters are found to be positive while some are negative. A positive relationship for the parameters implies that an increase in that parameter’s values will have a major effect on the frequency of the ailment spread. While a negative relationship means that an increase in the importance of these parameters would help to decrease the violence of the disease. The physical outlook of the numerical signs stated in Table 3 is depicted in Fig. 5.4 .
The elasticity indices for R 0 = 1.703052076 to the parameters of the model (2.1) .

Elasticity indices for significance of parameters in R 0 .
5.2. Numerical simulations
To gain insight into the behavior of the solutions, a numerical solution is needed for both the classical order and the proposed fractional-order model as it involves a nonlinear equation. For this task, we used the recent numerical scheme proposed by Toufik and Atangana in [49] . The numerical scheme for the proposed model (4.4) used in the present analysis is presented by:
To fit the model to the reality of the pandemic, we used the daily cases of the spread of the disease in Nigeria. For this study and for the current situation in the world we are only interested in infected individuals as the week’s pass. From Fig. 5.5 , Fig. 5.6 , Fig. 5.7 , we observed that both population of infected individuals I S and I A have been declining as weeks pass which may not be unconnected to existing government restrictions on movement and other contact activities. Not only that, there is a possibility that the government has put in place a public health education system that made the population take safety measures. This further confirmed the result obtained from the fact that a decrease in contact among the population plays a vital role in curtailing the spread of the disease.

Profiles for behavior of each state variable for the classical version of the model.

Profiles for behavior of each state variable for the ABC version of the fractional model.

Comparison of each state variables for classical and fractional order.
The behavior of the system further confirmed the current situation in Nigeria. Both the classical and fractional differential equations indicate that the disease is declining with a very high number of recovery. It is therefore easy to understand that in Nigeria restriction on social contact can work wonders in decreasing the number of infected individuals in addition to quarantine and testing. Meaning it should be of particular interest for all that in the fight against the pandemic is the exposure as a result of contact with infected individuals especially that there those who are asymptomatic ( I A ) . Equally important, there is also a strong agreement between the classical model and the fractional model as seen in Fig. 5.5 , Fig. 5.6 .
6. Conclusion
In this current work, we developed a simple mathematical model to investigate the transmission and control of the novel coronavirus disease (COVID-19) from human to human. Principles drawn from the literature of mathematical epidemiology have been used to model how individuals are exposed and infected with the disease and their possible recovery. The mathematical analysis was done using both the ordinary differential equation (ODE) and the fractional differential equation.
It is important for health practitioners and the world at large to understand and predict infected individuals for health concern arrangement of the citizens and to control its spread rate with restricted supply. The data used in the simulation is based on the disease spread in Nigeria. Positivity of the model is established and the basic reproduction number, R 0 is obtained for the model. It is observed that when R 0 < 1 the disease-free equilibrium is locally asymptotically stable otherwise is unstable. The behavior of the system further confirmed the current situation in Nigeria. Both the classical and fractional differential equations indicate that the disease is declining with a very high number of recovery. It is therefore easy to understand that in Nigeria restriction on social contact can work wonders in decreasing the number of infected individuals in addition to quarantine and testing. Meaning it should be of particular interest for all that in the fight against the pandemic, the exposure as a result of contact with infected individuals especially that there those who are asymptomatic ( I A ) , be curtail. Equally important, there is also a strong agreement between the classical model and the fractional model as seen in Fig. 5.5 , Fig. 5.6 . Also the endemic equilibrium E 1 exist and globally stable if R 0 > 1 . This means that the disease may persist in society. The sensitivity analysis of R 0 concerning the parameters shows that the most sensitive parameter of our model structure that represents the chance of transmission is the contact rate between susceptible persons and exposed persons. It has the most dominant sensitivity to increase the endemicity of the disease while the rate of transfer of individuals to symptomatic class decreases the endemicity of the disease. Also, using the techniques of fixed point theorems, the existence and uniqueness of solutions are presented.
Furthermore, given that the non-local (fractional order derivatives and integral) operator is better able to predict the future and better fit the experimental data compared to classical order derivatives and integrals, we have generalized the model to a fractional-order model in the sense of the Atangana-Baeanu derivative. Based on the actual data on the number of infected people in Nigeria and the best fitting techniques, we have obtained some of the values of the model’s unknown biological parameters, which successfully captured the COVID-19 pattern for the case α = 1 . Therefore, our results of the ODE form of the model present the situation in Nigeria and with this, we may conclude that authorities and health practitioners in Nigeria need to work hard to ensure that the contact between the exposed individual and susceptible individuals is minimized. This calls for strict social distance and quarantine.
7. Availability of data and materials
8. author contributions.
The authors contributed equally to this paper. All authors have read and approved the final version of the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors acknowledge the financial support provided by the Center of Excellence in Theoretical and Computational Science (TaCS-CoE), KMUTT. The first author was supported by “Petchra Pra Jom Klao Ph.D Research Scholarship from King Mongkut’s University of Technology Thonburi” (Grant No. 13/2561). Moreover, the authors express their gratitude for the positive comments received by anonymous reviewers and the editors which have improved the readability and correctness of the paper.
☆ This project was supported by the Petchra Pra Jom Klao Doctoral Scholarship for Ph.D. program of King Mongkut’s University of Technology Thonburi (KMUTT). The Center of Excellence in Theoretical and Computational Science (TaCS-CoE), KMUTT.
Disclaimer: This translation was last updated on August 2, 2022. For up-to-date content, please visit the English version of this page.
Disclaimer: The Spanish COVID-19 site is currently undergoing significant updates which may lead to a delay in translated content. We apologize for any inconvenience.
Clinical Presentation
Clinical considerations for care of children and adults with confirmed COVID-19
‹ View Table of Contents
- The clinical presentation of COVID-19 ranges from asymptomatic to critical illness.
- An infected person can transmit SARS-CoV-2, the virus that causes COVID-19, before the onset of symptoms. Symptoms can change over the course of illness and can progress in severity.
- Uncommon presentations of COVID-19 can occur, might vary by the age of the patient, and are a challenge to recognize.
- In adults, age is the strongest risk factor for severe COVID-19. The risk of severe COVID-19 increases with increasing age especially for persons over 65 years and with increasing number of certain underlying medical conditions .
Incubation Period
Data suggest that incubation periods may differ by SARS-CoV-2 variant. Meta-analyses of studies published in 2020 identified a pooled mean incubation period of 6.5 days from exposure to symptom onset. (1) A study conducted during high levels of Delta variant transmission reported an incubation period of 4.3 days, (2) and studies performed during high levels of Omicron variant transmission reported a median incubation period of 3–4 days. (3,4)
Presentation
People with COVID-19 may be asymptomatic or may commonly experience one or more of the following symptoms (not a comprehensive list) (5) :
- Fever or chills
- Shortness of breath or difficulty breathing
- Myalgia (Muscle or body aches)
- New loss of taste or smell
- Sore throat
- Congestion or runny nose
- Nausea or vomiting
The clinical presentation of COVID-19 ranges from asymptomatic to severe illness, and COVID-19 symptoms may change over the course of illness. COVID-19 symptoms can be difficult to differentiate from and can overlap with other viral respiratory illnesses such as influenza(flu) and respiratory syncytial virus (RSV) . Because symptoms may progress quickly, close follow-up is needed, especially for:
- older adults
- people with disabilities
- people with immunocompromising conditions, and
- people with medical conditions that place them at greater risk for severe illness or death.
The NIH COVID-19 Treatment Guidelines group SARS-CoV-2 infection into five categories based on severity of illness:
- Asymptomatic or pre-symptomatic infection : people who test positive for SARS-CoV-2 using a virologic test (i.e., a nucleic acid amplification test [NAAT] or an antigen test) but who have no symptoms that are consistent with COVID-19.
- Mild illness : people who may have any of the various signs and symptoms of COVID-19 but who do not have shortness of breath, dyspnea, or abnormal chest imaging.
- Moderate illness : people who have evidence of lower respiratory disease during clinical assessment or imaging and who have an oxygen saturation (SpO 2 ) ≥94% on room air at sea level.
- Severe illness : people who have oxygen saturation <94% on room air at sea level, a ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (PaO 2 /FiO 2 ) <300 mm Hg, a respiratory rate >30 breaths/min, or lung infiltrates >50%
- Critical illness : people who have respiratory failure, septic shock, or multiple organ dysfunction.
Asymptomatic and presymptomatic presentation
Studies have documented SARS-CoV-2 infection in people who never develop symptoms (asymptomatic presentation) and in people who are asymptomatic when tested but develop symptoms later (presymptomatic presentation). ( 6,7 ) It is unclear what percentage of people who initially appear asymptomatic progress to clinical disease. Multiple publications have reported cases of people with abnormalities on chest imaging that are consistent with COVID-19 very early in the course of illness, even before the onset of symptoms or a positive COVID-19 test. (9)
Radiographic Considerations and Findings
Chest radiographs of patients with severe COVID-19 may demonstrate bilateral air-space consolidation. (23) Chest computed tomography (CT) images from patients with COVID-19 may demonstrate bilateral, peripheral ground glass opacities and consolidation. (24,25) Less common CT findings can include intra- or interlobular septal thickening with ground glass opacities (hazy opacity) or focal and rounded areas of ground glass opacity surrounded by a ring or arc of denser consolidation (reverse halo sign). (24)
Multiple studies suggest that abnormalities on CT or chest radiograph may be present in people who are asymptomatic, pre-symptomatic, or before RT-PCR detection of SARS-CoV-2 RNA in nasopharyngeal specimens. (25)
Common COVID-19 symptoms
Fever, cough, shortness of breath, fatigue, headache, and myalgia are among the most commonly reported symptoms in people with COVID-19. (5) Some people with COVID-19 have gastrointestinal symptoms such as nausea, vomiting, or diarrhea, sometimes prior to having fever or lower respiratory tract signs and symptoms. (10) Loss of smell and taste can occur, although these symptoms are reported to be less common since Omicron began circulating, as compared to earlier during the COVID-19 pandemic. (11,19-21) People can experience SARS-CoV-2 infection (asymptomatic or symptomatic), even if they are up to date with their COVID-19 vaccines or were previously infected. (8)
Several studies have reported ocular symptoms associated with SARS-CoV-2 infection, including redness, tearing, dry eye or foreign body sensation, discharge or increased secretions, and eye itching or pain. (13)
A wide range of dermatologic manifestations have been associated with COVID-19; timing of skin manifestations in relation to other COVID-19 symptoms and signs is variable. (14) Some skin manifestations may be associated with increased disease severity. (15) Images of cutaneous findings in COVID-19 are available from the American Academy of Dermatology .
Uncommon COVID-19 symptoms
Less common presentations of COVID-19 can occur. Older adults may present with different symptoms than children and younger adults. Some older adults can experience SARS-CoV-2 infection accompanied by delirium, falls, reduced mobility or generalized weakness, and glycemic changes. ( 12)
Transmission
People infected with SARS-CoV-2 can transmit the virus even if they are asymptomatic or presymptomatic. ( 16) Peak transmissibility appears to occur early during the infectious period (prior to symptom onset until a few days after), but infected persons can shed infectious virus up to 10 days following infection. (22 ) Both vaccinated and unvaccinated people can transmit SARS-CoV-2. ( 17,18) Clinicians should consider encouraging all people to take the following prevention actions to limit SARS-CoV-2 transmission:
- stay up to date with COVID-19 vaccines,
- test for COVID-19 when symptomatic or exposed to someone with COVID-19, as recommended by CDC,
- wear a high-quality mask when recommended,
- avoiding contact with individuals who have suspected or confirmed COVID-19,
- improving ventilation when possible,
- and follow basic health and hand hygiene guidance .
Clinicians should also recommend that people who are infected with SARS-CoV-2, follow CDC guidelines for isolation.
Table of Contents
- › Clinical Presentation
- Clinical Progression, Management, and Treatment
- Special Clinical Considerations
- Bhaskaran K, Bacon S, Evans SJ, et al. Factors associated with deaths due to COVID-19 versus other causes: population-based cohort analysis of UK primary care data and linked national death registrations within the OpenSAFELY platform. Lancet Reg Health Eur. Jul 2021;6:100109. doi:10.1016/j.lanepe.2021.100109
- Kim L, Garg S, O'Halloran A, et al. Risk Factors for Intensive Care Unit Admission and In-hospital Mortality among Hospitalized Adults Identified through the U.S. Coronavirus Disease 2019 (COVID-19)-Associated Hospitalization Surveillance Network (COVID-NET). Clin Infect Dis. Jul 16 2020;doi:10.1093/cid/ciaa1012
- Kompaniyets L, Pennington AF, Goodman AB, et al. Underlying Medical Conditions and Severe Illness Among 540,667 Adults Hospitalized With COVID-19, March 2020-March 2021. Preventing chronic disease. Jul 1 2021;18:E66. doi:10.5888/pcd18.210123
- Ko JY, Danielson ML, Town M, et al. Risk Factors for COVID-19-associated hospitalization: COVID-19-Associated Hospitalization Surveillance Network and Behavioral Risk Factor Surveillance System. Clin Infect Dis. Sep 18 2020;doi:10.1093/cid/ciaa1419
- Wortham JM, Lee JT, Althomsons S, et al. Characteristics of Persons Who Died with COVID-19 - United States, February 12-May 18, 2020. MMWR Morb Mortal Wkly Rep. Jul 17 2020;69(28):923-929. doi:10.15585/mmwr.mm6928e1
- Yang X, Zhang J, Chen S, et al. Demographic Disparities in Clinical Outcomes of COVID-19: Data From a Statewide Cohort in South Carolina. Open Forum Infect Dis. Sep 2021;8(9):ofab428. doi:10.1093/ofid/ofab428
- Rader B.; Gertz AL, D.; Gilmer, M.; Wronski, L.; Astley, C.; Sewalk, K.; Varrelman, T.; Cohen, J.; Parikh, R.; Reese, H.; Reed, C.; Brownstein J. Use of At-Home COVID-19 Tests — United States, August 23, 2021–March 12, 2022. MMWR Morb Mortal Wkly Rep. April 1, 2022;71(13):489–494. doi:http://dx.doi.org/10.15585/mmwr.mm7113e1
- Pingali C, Meghani M, Razzaghi H, et al. COVID-19 Vaccination Coverage Among Insured Persons Aged >/=16 Years, by Race/Ethnicity and Other Selected Characteristics - Eight Integrated Health Care Organizations, United States, December 14, 2020-May 15, 2021. MMWR Morb Mortal Wkly Rep. Jul 16 2021;70(28):985-990. doi:10.15585/mmwr.mm7028a1
- Wiltz JL, Feehan AK, Molinari NM, et al. Racial and Ethnic Disparities in Receipt of Medications for Treatment of COVID-19 - United States, March 2020-August 2021. MMWR Morb Mortal Wkly Rep. Jan 21 2022;71(3):96-102. doi:10.15585/mmwr.mm7103e1
- Murthy NC, Zell E, Fast HE, et al. Disparities in First Dose COVID-19 Vaccination Coverage among Children 5-11 Years of Age, United States. Emerg Infect Dis. May 2022;28(5):986-989. doi:10.3201/eid2805.220166
- Saelee R, Zell E, Murthy BP, et al. Disparities in COVID-19 Vaccination Coverage Between Urban and Rural Counties - United States, December 14, 2020-January 31, 2022. MMWR Morb Mortal Wkly Rep. Mar 4 2022;71(9):335-340. doi:10.15585/mmwr.mm7109a2
- Burki TK. The role of antiviral treatment in the COVID-19 pandemic. Lancet Respir Med. Feb 2022;10(2):e18. doi:10.1016/S2213-2600(22)00011-X
- Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al. Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients. N Engl J Med. Feb 10 2022;386(6):509-520. doi:10.1056/NEJMoa2116044
- Sjoding MW, Dickson RP, Iwashyna TJ, Gay SE, Valley TS. Racial Bias in Pulse Oximetry Measurement. N Engl J Med. Dec 17 2020;383(25):2477-2478. doi:10.1056/NEJMc2029240
- Jordan TB, Meyers CL, Schrading WA, Donnelly JP. The utility of iPhone oximetry apps: A comparison with standard pulse oximetry measurement in the emergency department. Am J Emerg Med. May 2020;38(5):925-928. doi:10.1016/j.ajem.2019.07.020
- Iuliano AD, Brunkard JM, Boehmer TK, et al. Trends in Disease Severity and Health Care Utilization During the Early Omicron Variant Period Compared with Previous SARS-CoV-2 High Transmission Periods - United States, December 2020-January 2022. MMWR Morb Mortal Wkly Rep. Jan 28 2022;71(4):146-152. doi:10.15585/mmwr.mm7104e4
- Taylor CA, Whitaker M, Anglin O, et al. COVID-19-Associated Hospitalizations Among Adults During SARS-CoV-2 Delta and Omicron Variant Predominance, by Race/Ethnicity and Vaccination Status - COVID-NET, 14 States, July 2021-January 2022. MMWR Morb Mortal Wkly Rep. Mar 25 2022;71(12):466-473. doi:10.15585/mmwr.mm7112e2
- Johnson AG, Amin AB, Ali AR, et al. COVID-19 Incidence and Death Rates Among Unvaccinated and Fully Vaccinated Adults with and Without Booster Doses During Periods of Delta and Omicron Variant Emergence - 25 U.S. Jurisdictions, April 4-December 25, 2021. MMWR Morb Mortal Wkly Rep. Jan 28 2022;71(4):132-138. doi:10.15585/mmwr.mm7104e2
- Danza P, Koo TH, Haddix M, et al. SARS-CoV-2 Infection and Hospitalization Among Adults Aged >/=18 Years, by Vaccination Status, Before and During SARS-CoV-2 B.1.1.529 (Omicron) Variant Predominance - Los Angeles County, California, November 7, 2021-January 8, 2022. MMWR Morb Mortal Wkly Rep. Feb 4 2022;71(5):177-181. doi:10.15585/mmwr.mm7105e1
To receive email updates about COVID-19, enter your email address:
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.

Got any suggestions?
We want to hear from you! Send us a message and help improve Slidesgo
Top searches
Trending searches

solar eclipse
25 templates

8 templates

22 templates

43 templates

32 templates

49 templates
COVID-19 Explained for Middle School
Covid-19 explained for middle school presentation, premium google slides theme and powerpoint template.
The pandemic has completely changed the way we live. The youngest have had to adapt without understanding what was going on. If you want to make COVID-19 understandable for your middle school students, we recommend using this editable template from Slidesgo. With it you can explain the symptoms, prevention measures, how the transmission occurs, what treatments are available, etc. We have included tables, maps, infographics and lists that will be of great help. The design is modern, with cartoon style illustrations and a great variety of icons.
Features of this template
- 100% editable and easy to modify
- 30 different slides to impress your audience
- Contains easy-to-edit graphics such as graphs, maps, tables, timelines and mockups
- Includes 500+ icons and Flaticon’s extension for customizing your slides
- Designed to be used in Google Slides and Microsoft PowerPoint
- 16:9 widescreen format suitable for all types of screens
- Includes information about fonts, colors, and credits of the free resources used
What are the benefits of having a Premium account?
What Premium plans do you have?
What can I do to have unlimited downloads?
Don’t want to attribute Slidesgo?
Gain access to over 22100 templates & presentations with premium from 1.67€/month.
Are you already Premium? Log in
Related posts on our blog

How to Add, Duplicate, Move, Delete or Hide Slides in Google Slides

How to Change Layouts in PowerPoint

How to Change the Slide Size in Google Slides
Related presentations.

Premium template
Unlock this template and gain unlimited access

TechRepublic
Account information.
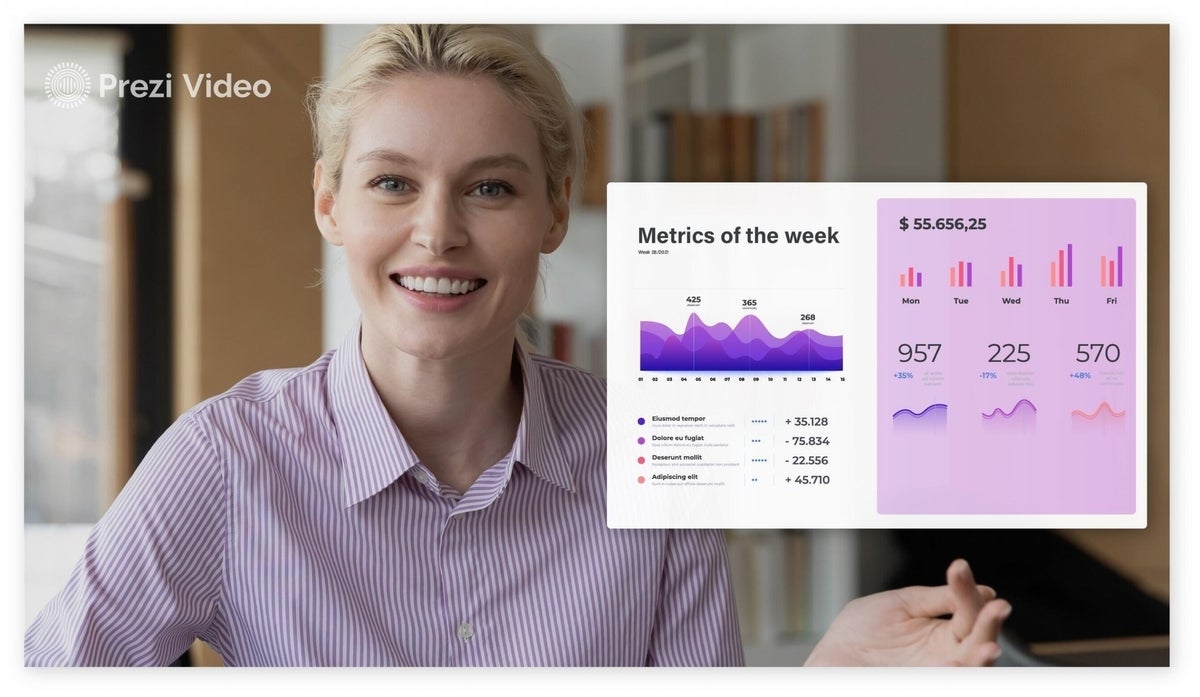
Share with Your Friends
Prezi announces Google Slides integration
Your email has been sent
Prezi users can now import presentations from Google Slides directly into Prezi Video, the company announced Thursday. This is the latest in a series of integrations with online video and collaboration tools.
This change makes it easier for people to use existing presentations built in other formats with Prezi Video.
“We’ve seen so many users come on board and use a cloud-native platform like Google Workspace,” Prezi CEO Jim Szafranski said. “They’ve been able to use Prezi Video with workarounds and now they can do it directly.”
Since the COVID-19 pandemic started in early 2020, businesses have turned to video conferencing to keep daily operations running. Most video conferencing platforms have seen a spike in use and Zoom has become a verb as well as a very profitable company. In February 2020, Zoom added more users than it did during the entire previous year . In Q1 2020, Zoom’s revenue was $122 million. In Q1 2021, that number went up to $328 million .
Prezi Video allows users to present videos, GIFs, presentations, images, and type text on-screen of a live or recorded video. In addition to Google Meet, Prezi Video works with all major video streaming platforms, including Zoom, Microsoft Teams, Webex and GoToMeeting. Prezi announced an integration with Google Workspace earlier this year.
SEE: Zoom videoconferencing: A cheat sheet (Free PDF) (TechRepublic)
“In newscaster mode, now I can very quickly have me and my content side by side without having to author a new presentation in Prezi,” he said.
Presenters see the same content that audience members see during a video session. The Prezi video app runs alongside Zoom. Prezi Video uses a virtual camera to merge the presenter’s content and camera feed and present the combined information back to the video streaming platform.
Szafranski said he has seen a new trend among business users of using Prezi Video to change how meetings are run. Business leaders record their remarks and send out the presentation before the scheduled meeting. Colleagues can watch the presentation ahead of time and then use the scheduled meeting time to discuss the content.
Prezi records presentations for board meetings ahead of time and posts the recordings in the company Slack channel.
“People like to have the flexibility around time and place by mixing live and recorded meetings,” he said. “If you give employees tools like this, they’ll find creative ways to connect with customers.”
Szafranski said he has seen much more use of the asynchronous approach in education but expects to see this tactic expand among business users.
“Remote employees feel way more included with this format and people are starting to get more comfortable with having the follow-up conversation in Slack,” he said.
He said that people also are using Prezi Video to record short snippets to promote upcoming meetings.
“We’ve seen people record the agenda slide or send a three-minute clip the day before a meeting to show that they are prepared for a meeting and have interesting content,” he said.
SEE: Prezi Video introduces live editing tool for video calls (TechRepublic)
He also has seen more people use Prezi Video to experiment with an asynchronous approach to meetings.
“If you’re doing a one-to-many lecture, you can record it and let people consume it on their own time,” he said. “When people start recording meetings, they don’t have to crowd calendars.”
Szafranski said that these recordings also are a good way to introduce new team members to a project.
Szafranski said that the company has 100 million users in education and business. Prezi Video launched in November 2019 as the company anticipated an increase in more online presentations.
“We were able to really help our users when the pandemic hit and we saw our education base start recording their lessons and sending it out to students,” Szafranski said.
According to the company, Prezi Video is used in 150,000 organizations around the world and half the school districts in the US.
Subscribe to the Innovation Insider Newsletter
Catch up on the latest tech innovations that are changing the world, including IoT, 5G, the latest about phones, security, smart cities, AI, robotics, and more. Delivered Tuesdays and Fridays
- Return to work: What the new normal will look like post-pandemic (free PDF)
- Coronavirus domain names are the latest hacker trick
- Managing accounts payable operations during COVID-19 policy
- Coronavirus: Effective strategies and tools for remote work during a pandemic
- How to track the coronavirus: Dashboard delivers real-time view of the deadly virus
- Coronavirus: More must-read coverage
Create a TechRepublic Account
Get the web's best business technology news, tutorials, reviews, trends, and analysis—in your inbox. Let's start with the basics.
* - indicates required fields
Sign in to TechRepublic
Lost your password? Request a new password
Reset Password
Please enter your email adress. You will receive an email message with instructions on how to reset your password.
Check your email for a password reset link. If you didn't receive an email don't forgot to check your spam folder, otherwise contact support .
Welcome. Tell us a little bit about you.
This will help us provide you with customized content.
Want to receive more TechRepublic news?
You're all set.
Thanks for signing up! Keep an eye out for a confirmation email from our team. To ensure any newsletters you subscribed to hit your inbox, make sure to add [email protected] to your contacts list.
Like what you're reading?
Fun PowerPoint night ideas (with a twist)
Get your team on prezi – watch this on demand video.
Anete Ezera March 28, 2024
When we think of PowerPoint presentations, we often think of slideshows. And lately, PowerPoint nights or slideshow presentations with a group of people are on the rise, and they’re being used as a form of leisure.
To create a stronger bond with family members, friends, or colleagues, or simply share exciting news and updates, PowerPoint nights have become a popular method for entertainment and bonding. In PowerPoint nights, people share funny, interesting, and personal topics that make an impression and create a fun environment for being creative with others. But if you really want to stand out and make your PowerPoint nights more fun and creative, we’ll share entertaining and witty topic ideas with an eye-catching twist – Prezi .

Fun and creative PowerPoint night ideas
Here are some fun PowerPoint night ideas that are sure to get people talking:
- Misadventures in cooking – You could share funny personal stories or widely relatable mishaps in the kitchen, from burnt dinners to bizarre recipe substitutions.
- Holiday disasters – Talk about funny stories where holidays have gone wrong, from gift exchanges to family gatherings, and how they’re memorable for all the wrong reasons.
- Everyday life hacks that shouldn’t work, but do – Present a series of unconventional life hacks that are surprisingly effective, encouraging audience members to share their own.
- The evolution of dance moves – Showcase dance trends through the decades, possibly including demonstrations or a group dance-off.
- Pets doing human things – A collection of stories, videos, or images of pets acting like humans, from cats using the toilet to dogs watching TV.
- Technological fails – Relive moments where technology let us down in hilarious ways, from autocorrect mishaps to video call blunders.
- Unexpected heroes in pop culture – Highlight secondary or overlooked characters in movies, TV shows, and books who deserve recognition for their heroism or impact.
- The art of procrastination – A humorous look into procrastination techniques, their creative outcomes, and why sometimes delaying tasks can be beneficial.
- Famous historical misunderstandings – Delve into funny or significant misunderstandings from history and their unexpected consequences.
- The world through a child’s eyes – Share funny insights, questions, or interpretations of the world from children’s perspectives.
- Vacation fails – Stories of vacations that didn’t go as planned, from getting lost in a foreign country to experiencing a series of unfortunate events.
- Bizarre world records – Explore some of the most peculiar world records ever set, inviting discussion about what records the audience might set.
- Social media mishaps – A collection of funny or embarrassing moments experienced on social media platforms, from posting on the wrong account to misunderstood hashtags.
- The journey of learning a new skill – Share the often humorous path of trying and failing to learn something new, from languages to musical instruments.
- When animals interrupt – A compilation of moments when animals hilariously interrupt important events, meetings, or broadcasts.
- Overconfident DIY Projects – Share tales of DIY projects that started with high hopes but ended in disaster, highlighting the gap between expectation and reality.
- Misadventures in Online Shopping – A humorous look at online shopping fails, from wildly inaccurate product sizes to items that look nothing like their pictures.
- The Great Outdoors Gone Wrong – Stories of camping trips, hikes, and outdoor adventures that took unexpected turns, from wild animal encounters to getting hopelessly lost.
Hopefully, these PowerPoint night ideas have been inspiring. But you could even add a personal touch to your chosen PowerPoint night themes by including a real-life scenario that you’ve experienced and encouraging the group to share their own experiences. If you’re still struggling to spark creativity, you can find lots of PowerPoint night examples on Prezi that have been created by real users.

A new and exciting way to present your PowerPoint night ideas
Once you’ve decided on a topic, this is where the fun begins – creating your presentation. While PowerPoint presentations offer a very structured format, there’s a way you can turn your slide-based presentation format into something more engaging and fun . With Prezi, you can create a more dynamic presentation that overshadows conventional slideshows.
How Prezi stands out
For people who want to put their PowerPoint night ideas into action, Prezi is a popular choice. It’s not just the way Prezi presentations look, but also the ease with which users can create them – now with Prezi AI – that makes Prezi a great platform for presentations. With many built-in tools that make the process simpler, there’s no longer a need to waste time creating traditional slideshows when Prezi can do a lot of the work for you. Let’s look at some Prezi features that would be particularly useful for bringing your PowerPoint night ideas to life.
AI presentation creator
One of Prezi’s biggest game-changers is its built-in AI assistant. Forget spending hours wrestling with layouts and agonizing over transitions. Prezi AI helps you transform your ideas, keywords, or even a rough outline into a captivating narrative . Simply jot down your key points, and Prezi AI will seamlessly arrange them into a visually stunning presentation.
This intuitive approach is perfect for creating a PowerPoint night presentation, where you might need to craft your presentation on the fly . Prezi AI handles the visual heavy-lifting, ensuring a polished presentation that keeps your audience engaged, freeing you to focus on delivering your topic and ideas with confidence.
AI text editing
Presentation nights should be light-hearted and fun, so why spend hours writing up slides when the Prezi AI text tool can speed up the process? This cool feature can recommend text for each section of your presentation, based on your chosen PowerPoint night ideas. You can prompt the AI by feeding it the information you want to talk about and it will write it up in a way that’s readable. It can also offer suggestions and edits for your text, so you don’t need to worry about spelling mistakes or whether your sentences make sense.
Layout functions
Not only can Prezi AI assist you with crafting text, but it can also help with the layout of each frame. It can condense text to bite-sized chunks and suggest a layout that’s clear for your audience, such as bullet points and lists. The AI can also offer recommendations for where each part of your slide should be placed for maximum impact and understanding, like where your text should be placed in comparison to images and visuals. This is essential when putting your PowerPoint night ideas into action, as it takes the hassle out of the design aspect and lets you focus on the content you want to talk about.
Open canvas
This is a signature feature of Prezi. The open canvas means that instead of a slide-by-slide slideshow, you can zoom in on important points and then zoom back out to the bigger picture. A good slideshow will usually tell a story, and this way of presenting your slides is perfect for storytelling, as you can walk the audience in and out of each scene as you’re telling the story. The end result will be a more dynamic, exciting slideshow than traditional methods. Presentation nights are all about humour and getting people talking, and Prezi presentations do just that.
Examples of Prezi in action
Summer plans presentation .
This presentation showcases Prezi open canvas features beautifully. As you can see, the presentation begins with a full view of the content, and then as the presentation progresses it uses the zoom feature to capture each point close up. This is much more enjoyable than traditional slideshows, and it’s a great way to put your PowerPoint night ideas into action.
International celebration of ice cream
This Prezi is a great example of how you can make a presentation that’s fun and engaging, the perfect inspiration for your PowerPoint night ideas. Again, the open canvas adds a layer of excitement that’s totally different from slideshows. The choice of lively colors and images used here would be great for a presentation night. Prezi has many visuals to choose from that you can add to your presentation, adding an extra layer of entertainment for your guests.
Powerpoint night ideas that come to life with the help of Prezi
To wrap up, Prezi stands out as a superior choice for those looking to elevate their PowerPoint nights from simple slideshows to engaging stories. Prezi’s design tools, including Prezi AI , make the creation process much easier. The standout open canvas feature allows presenters to showcase key points, making stories more dynamic. This approach makes sure your PowerPoint nights are far from dull, encouraging laughter and discussions through impactful presentations.

By choosing Prezi, you’re not just sharing slides but creating memorable moments that engage and connect your audience. So, for the perfect presentation night, all you need to do is combine good PowerPoint night topics with Prezi, and you’re good to go.
To read more about Prezi’s new AI features, read this article on mastering last-minute presentations .

Give your team the tools they need to engage
Like what you’re reading join the mailing list..
- Prezi for Teams
- Top Presentations
- The Power of mRNA
- Responsibility
News Details
Moderna advances multiple vaccine programs to late-stage clinical trials.
Announces next-generation COVID-19 vaccine candidate as fourth respiratory vaccine to successfully meet its Phase 3 endpoints
Expects two more Phase 3 readouts in 2024, including combination vaccine against flu and COVID-19, and vaccine against CMV
Announces positive clinical trial data from three new vaccines against viruses that cause significant burden (Epstein-Barr virus, Varicella-Zoster virus, norovirus) and advances programs toward Phase 3 development
Anticipates U.S. launch of vaccine against RSV following FDA approval and ACIP recommendation in 2024
Announces development and commercialization funding agreement with Blackstone Life Sciences for up to $750 million to advance flu program
"Our mRNA platform continues a remarkable track record across our broad vaccine portfolio. Today, we are excited to share that four vaccines in our pipeline have achieved successful clinical readouts across our respiratory, latent and other virus franchises," said Stéphane Bancel, Chief Executive Officer of Moderna. "With five vaccines in Phase 3, and three more moving toward Phase 3, we have built a very large and diverse portfolio addressing significant unmet medical needs. We are focused on execution to further build momentum across our pipeline and business, and to deliver for patients who are impacted by these infectious diseases."
Portfolio Overview
The vaccine portfolio seeks to address infectious diseases that cause considerable health burdens and includes 28 vaccines addressing respiratory, latent and other pathogens.
Latent and Other Vaccine Portfolio
Moderna is advancing five vaccine candidates against viruses that cause latent infections, all of which are in clinical trials. When latent, a virus is present in the body but exists in a resting state, typically without causing any noticeable symptoms. Latent viruses can reactivate and cause clinical symptoms as a person ages, during times of stress or when immunity is compromised. The capacity for latency is a defining feature of members of the Herpesviridae family, including cytomegalovirus (CMV), Epstein-Barr virus (EBV), herpes simplex virus (HSV) and Varicella-Zoster virus (VZV).
Cytomegalovirus (CMV)
CMV is the most common infectious cause of birth defects in the U.S. and is responsible for several billion dollars in annual healthcare costs. One in 200 babies in the U.S. are born with a congenital CMV infection, and of those affected, one in five will have severe, life-altering health problems. Possible short- and long-term sequelae of CMV infection include microcephaly, chorioretinitis, seizures, sensorineural hearing loss, cognitive impairment and cerebral palsy. There is currently no approved vaccine to prevent congenital CMV.
CMVictory is a pivotal Phase 3 trial evaluating mRNA-1647 against primary CMV infection in women 16 to 40 years of age. The trial is a randomized, observer-blind, placebo-controlled study designed to evaluate the efficacy, safety and immunogenicity of mRNA-1647. The trial is fully enrolled with approximately 7,300 participants from 290 clinical sites globally.
To date, 50 primary infection cases have accrued and are undergoing confirmation. The first interim analysis for the evaluation of vaccine efficacy, which will be triggered when both 81 confirmed per-protocol cases and 12 median months of safety follow-up have occurred, is expected as early as the end of 2024.
Moderna's CMV vaccine candidate mRNA-1647 has advanced to indication expansion studies in adolescents 9 to 15 years of age and adult transplant patients, both of which have begun enrollment.
Epstein-Barr virus (EBV)
EBV is a major cause of infectious mononucleosis (IM) in the U.S., accounting for more than 90% of IM cases annually. Importantly, EBV and IM are associated with a higher lifetime risk of more serious sequelae including certain cancers such as gastric carcinoma, nasopharyngeal carcinoma and multiple types of lymphoma. The lifetime risk of developing multiple sclerosis (MS) is increased by 32-fold after EBV infection. There is currently no approved vaccine to prevent EBV.
Moderna's EBV vaccine candidates are designed to tackle multiple EBV-associated conditions, including prevention of IM (mRNA-1189) and MS and post-transplant lymphoproliferative disorder, a subcategory of lymphoma in solid organ transplant patients (mRNA-1195) . The Phase 1 trial for mRNA-1189 was designed to test the safety, reactogenicity and immunogenicity of four different dose levels in participants 12 to 30 years of age in the U.S. The randomized, observer-blind, placebo-controlled study showed mRNA-1189 was immunogenic and generally well tolerated across all dose levels. The Company is advancing mRNA-1189 toward a pivotal Phase 3 trial.
The Phase 1 trial for mRNA-1195 was designed to test the safety, reactogenicity and immunogenicity of two drug products at four different dose levels in healthy EBV seropositive participants 18 to 55 years of age in the U.S. The randomized, observer-blind, placebo-controlled study is fully enrolled.
Herpes simplex virus (HSV)
Herpes simplex virus type 2 (HSV-2) infects approximately 13% of adults globally and is the primary cause of genital herpes. There are an estimated four billion people globally infected with HSV, of which 491 million cases are HSV-2. Recurrent genital herpes causes a reduction in quality of life, which antivirals (current standard of care) only partially restore. Moderna expects that if an HSV vaccine candidate could deliver similar efficacy as a suppressive antiviral treatment, compliance with recommended therapy and associated quality of life would improve. There is currently no approved vaccine to treat HSV-2.
The first in human, fully enrolled Phase 1/2 trial of mRNA-1608 is designed to test safety and immunogenicity and to establish a proof-of-concept of clinical benefit in adults 18 to 55 years of age with recurrent HSV-2 genital herpes. The randomized 1:1:1:1, observer-blind, controlled study is fully enrolled with 300 participants in the U.S.
Varicella-Zoster virus (VZV)
Herpes zoster, also known as shingles, is caused by reactivation of latent VZV, the same virus that causes chickenpox. Declining immunity in older adults decreases immunity against VZV, allowing reactivation of the virus from latently infected neurons, causing painful and itchy lesions. Herpes Zoster occurs in one out of three adults in the U.S. in their lifetime and the incidence increases at 50 years of age. There is potential to reach a growing and underserved patient population.
Moderna's VZV vaccine candidate mRNA-1468 has initial data available from a Phase 1/2 trial, which was designed to test safety and immunogenicity in healthy adults 50 years of age and older in the U.S. The randomized 1:1:1:1:1, observer-blind, active-controlled study of mRNA-1468 elicited strong antigen-specific T cell responses at one month after the second dose and was generally well tolerated. Results of the first interim analysis support the further clinical development of mRNA-1468 for the prevention of shingles. Additional results from the ongoing Phase 1/2 study will be available later this year, including persistence data. The Company is planning for a pivotal Phase 3 trial. Norovirus
Enteric viruses, including norovirus, are a leading cause of diarrheal diseases, resulting in significant morbidity and mortality worldwide, particularly among young children and older adults. Norovirus is highly contagious and a leading cause of diarrheal disease globally, associated with 18% of all acute gastroenteritis (AGE), resulting in approximately 200,000 deaths per year and substantial healthcare costs. Given the wide diversity of norovirus genotypes, a broadly effective norovirus vaccine will require a multivalent vaccine design. There is currently no approved vaccine to prevent norovirus.
The randomized, observer-blind, placebo-controlled Phase 1 trial was designed to evaluate the safety, reactogenicity and immunogenicity of trivalent (mRNA-1403) and pentavalent (mRNA-1405) norovirus vaccine candidates in 664 participants 18 to 49 years of age and 60 to 80 years of age in the U.S. An interim analysis showed that a single dose of mRNA-1403 elicited a robust immune response across all dose levels evaluated with a clinically acceptable reactogenicity and safety profile. The Company is advancing mRNA-1403 toward a pivotal Phase 3 trial.
Respiratory Vaccine Portfolio
Moderna's approach to ease the global burden of respiratory infections includes vaccine candidates against major causative pathogens, including SARS-CoV-2, respiratory syncytial virus (RSV) and influenza virus. Respiratory infections are a top cause of death in the U.S. and are particularly harmful to the young, immunocompromised, and older adults who experience more severe illness, greater incidence of hospitalization, and greater mortality than younger adults.
Moderna's respiratory pipeline includes Phase 3 trials for investigational vaccines including a next-generation COVID-19 vaccine, an RSV vaccine, a flu vaccine, and a flu and COVID-19 combination vaccine. The pipeline includes three additional flu vaccine candidates with expanded antigen coverage as well as combination vaccine programs.
Moderna continues to address the needs of the endemic COVID-19 market by focusing on public health efforts to increase vaccination coverage rates to reduce the substantial burden of COVID-19 as well as by advancing next-generation vaccines. The Company's mRNA platform can produce variant-matched vaccines on an accelerated time horizon, consistent with recent U.S. Food and Drug Administration (FDA) comments on the timing of potential strain selection for the fall booster season.
A recent announcement of positive interim results from the NEXTCove Phase 3 trial showed that mRNA-1283 elicited a higher immune response against both the Omicron BA.4/BA.5 and original virus strains of SARS-CoV-2 compared to mRNA-1273.222, Moderna's licensed COVID-19 vaccine. mRNA-1283 is designed to be refrigerator-stable and paves the way for a combination vaccine against influenza and COVID-19, mRNA-1083, enhancing the Company's overall respiratory portfolio. This is Moderna's fourth infectious disease vaccine program with Phase 3 data.
Respiratory Syncytial Virus (RSV)
RSV is the leading cause of respiratory illness in young children, and older adults are at increased risk relative to younger adults for severe outcomes. In addition to acute mortality and morbidity, RSV infection is associated with long-term sequelae such as asthma and impaired lung function in pediatric populations, and exacerbation of chronic obstructive pulmonary disease in older adults. Annually, there are approximately two million medically attended RSV infections and 58,000 to 80,000 hospitalizations in children younger than five years old in the U.S. In the U.S., each year there are up to 160,000 hospitalizations and 10,000 deaths in adults 65 years and older due to RSV. Across high-income countries in 2019, RSV caused an estimated 5.2 million cases, 470,000 hospitalizations and 33,000 in-hospital deaths in adults 60 years and older.
Moderna's RSV vaccine candidate, mRNA-1345, is in an ongoing Phase 2/3, randomized, observer-blind, placebo-controlled case-driven trial (ConquerRSV) in adults over 60 years of age. In this study, approximately 37,000 participants from 22 countries were randomized 1:1 to receive one dose of mRNA-1345 or placebo.
Based on positive data from the ConquerRSV trial, Moderna has filed for regulatory approvals for mRNA-1345 for the prevention of RSV-associated lower respiratory tract disease (RSV-LRTD) and acute respiratory disease (ARD) in adults over 60 years of age.
The trial met both its primary efficacy endpoints, with a vaccine efficacy (VE) of 83.7% (95.88% CI: 66.1%, 92.2%; p<0.0001) against RSV-LRTD as defined by two or more symptoms, and a VE of 82.4% (96.36% CI: 34.8%, 95.3%; p=0.0078) against RSV-LRTD defined by three or more symptoms. These data were published in the New England Journal of Medicine in December 2023.
A subsequent analysis from the ConquerRSV study with a longer median follow-up duration of 8.6 months (versus 3.7 months in the primary analysis), with a range of 15 days to 530 days, and including subjects from the Northern and Southern Hemispheres was recently presented at the RSVVW'24 conference . In this supplemental analysis, mRNA-1345 maintained durable efficacy, with sustained VE of 63.3% (95.88% CI: 48.7%, 73.7%) against RSV-LRTD including two or more symptoms. VE was 74.6% (95% CI: 50.7%, 86.9%) against RSV-LRTD with ≥2 symptoms, including shortness of breath and 63.0% (95% CI: 37.3%, 78.2%) against RSV-LRTD including three of more symptoms. The stringent statistical criterion of the study, a lower bound on the 95% CI of >20%, continued to be met for both endpoints.
mRNA-1345 has been granted Breakthrough Therapy designation by the FDA for the prevention of RSV-LRTD in adults over 60 years of age. The Company is awaiting regulatory approvals and the U.S. ACIP recommendation in 2024.
Indication expansion studies for mRNA-1345
mRNA-1345 has the potential to protect all vulnerable populations from RSV. Moderna has initiated multiple Phase 3 expansion studies in adults over 50 years of age to evaluate co-administration and revaccination. Additional trials (Phase 1 - Phase 3) have been initiated for high-risk adults, as well as maternal and pediatric populations. Interim data from these studies could be available as early as 2024.
Influenza (Flu)
Worldwide, influenza leads to 3-5 million severe cases of flu and 290,000-650,000 flu-related respiratory deaths annually. Two main types of influenza viruses (A and B) cause seasonal flu epidemics, and the influenza A viruses lead to most flu-related hospitalization in older adults.
The Company has several seasonal influenza vaccine candidates in clinical development. Moderna's seasonal flu vaccine, mRNA-1010 , demonstrated consistently acceptable safety and tolerability across three Phase 3 trials. In the most recent Phase 3 trial (P303), which was designed to test the immunogenicity and safety of an optimized vaccine composition, mRNA-1010 met all immunogenicity primary endpoints, demonstrating higher antibody titers compared to a currently licensed standard-dose flu vaccine. In an older adult extension study of P303, mRNA-1010 is being studied against high dose Fluzone HD ® ; the trial is fully enrolled. The Company is in ongoing discussions with regulators and intends to file in 2024.
Combination Respiratory Vaccines
Moderna's combination vaccine candidates cover respiratory viruses associated with the largest disease burden in the category. The Phase 3 combination study of the Company's investigational combination vaccine against flu and COVID-19 (mRNA-1083) for adults aged 50 years and older is fully enrolled and data are expected in 2024. mRNA-1083 was granted Fast Track designation by the FDA in May 2023.
Commercial Updates
Respiratory viruses in addition to latent and other viruses represent large unmet or underserved medical needs, and the human and economic costs from these infectious diseases highlight the need for effective vaccines. To help address this need, Moderna expects multiple vaccine product launches in the next few years, each with significant addressable markets.
The 2024 global endemic COVID-19 vaccine market alone is estimated by Moderna to be approximately $10 billion. COVID-19 continues to show a high burden of disease, and while COVID-19 hospitalizations remain high relative to RSV and flu, the risks of Long COVID are also becoming better understood. Moderna is focused on improving education and awareness to increase vaccination rates as Long COVID data suggests even traditionally low-risk groups should be vaccinated. Moderna is also working with health authorities to align the timing of COVID-19 and flu vaccine launches to help improve public health.
For RSV, Moderna estimates the peak annual market to be approximately $10 billion. The Company expects a strong RSV vaccine launch into a large market in 2024. As the only mRNA investigational vaccine with positive Phase 3 data, Moderna's RSV vaccine candidate has a strong profile with consistently strong efficacy across vulnerable and older populations, a well-established safety and tolerability profile, and ease of administration with a ready-to-use, pre-filled syringe formulation, which could relieve some of the burden that falls on pharmacies during the fall vaccination season.
An interim analysis from an ongoing time and motion study evaluating differences in preparation time between a pre-filled syringe (PFS) presentation and vaccines that require reconstitution showed that a PFS presentation could relieve some of the burden that falls on pharmacies during the fall vaccination season. Results from this study suggest that pharmacies may be capable of preparing up to four times as many doses of PFS in an hour compared to vaccines requiring reconstitution.
Moderna estimates flu vaccines represent an approximately $7 billion market in 2024. The market is expected to grow with the rise of more effective vaccines and there is an opportunity to expand the market with next-generation premium flu vaccines as well as combination respiratory vaccines, adding increased value to the health ecosystem.
CMV is expected to be a $2-5 billion annual market. With no vaccine currently on the market and a potential vaccine launch in 2026, Moderna could be the first CMV vaccine in multi-billion-dollar latent vaccine market. In addition, EBV has the potential to address and reduce the burden and cost of EBV infection in multiple populations, while VZV provides the opportunity to enter a large and growing market, which could be $5-6 billion annually. The market for norovirus vaccines is similar to that of rotavirus in pediatrics with opportunity to expand into the adult population, and represents a $3-6 billion annual market.
Moderna's vaccine portfolio targets large addressable markets, with an estimated total addressable market (TAM) of $52 billon for Moderna infectious disease vaccines, which includes a respiratory vaccines TAM of more than $27 billion and a latent and other vaccines TAM of more than $25 billion.
Manufacturing
The Company's manufacturing innovation supports expanding commercialization of a diverse pipeline through efficiency and productivity gains. Its mRNA manufacturing platform enables benefits such as quality, speed, scale and cost efficiency across a footprint that broadly includes the manufacture of plasmid, mRNA, lipid nanoparticles, as well as fill/finish and quality control capabilities.
As the Company continues to build its footprint for the future, it is developing an agile global manufacturing network to meet commercial demand and support its growing pipeline. Pre-clinical through commercial manufacturing occurs at the Moderna Technology Center in Norwood, Massachusetts, which remains central to the Company's network. New facilities being constructed in Australia, Canada and the UK are expected to come online in 2025, and drug product capacity is achieved through a flexible contract manufacturing network. Additionally, the Company has purchased and started build-out of a manufacturing site in Marlborough, Massachusetts, to enable commercial scale of its individualized neoantigen therapy program.
By continuing to pioneer new technologies, including advanced robotics, applying AI and other digital solutions, and driving network and capital efficiency, Moderna's manufacturing network is expected to also drive more predictable cost of sales.
Research and Development Investment Strategy
Today's updates provide further evidence that Moderna's mRNA technology platform is working, and with a rate of success higher than industry standard. Looking ahead, research and development will continue to be the Company's top capital allocation priority.
As Moderna looks to create value through the research and development strategy for its vaccine portfolio, it is taking three prioritization parameters into consideration: pipeline advancement, revenue diversification and risk reduction. As part of its strategy, the funding options Moderna considers are self-funding, project financing and partnerships.
Moderna recently entered into a development and commercialization funding agreement with Blackstone Life Sciences to advance the Company's flu program. As part of the agreement, Blackstone will fund up to $750 million with a return based on cumulative commercial milestones and low-single digit royalties. Moderna expects to recognize the funding as a reduction in research and development expenses and will retain full rights and control of the Company's flu program. This funding does not result in any change to Moderna's 2024 research and development framework spending of approximately $4.5 billion.
About Moderna
Moderna is a leader in the creation of the field of mRNA medicine. Through the advancement of mRNA technology, Moderna is reimagining how medicines are made and transforming how we treat and prevent disease for everyone. By working at the intersection of science, technology and health for more than a decade, the company has developed medicines at unprecedented speed and efficiency, including one of the earliest and most effective COVID-19 vaccines.
Moderna's mRNA platform has enabled the development of therapeutics and vaccines for infectious diseases, immuno-oncology, rare diseases and autoimmune diseases. With a unique culture and a global team driven by the Moderna values and mindsets to responsibly change the future of human health, Moderna strives to deliver the greatest possible impact to people through mRNA medicines. For more information about Moderna, please visit modernatx.com and connect with us on X (formerly Twitter), Facebook, Instagram, YouTube and LinkedIn.
INDICATION (U.S.)
SPIKEVAX (COVID-19 Vaccine, mRNA) is a vaccine indicated for active immunization to prevent coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in individuals 18 years of age and older.
IMPORTANT SAFETY INFORMATION
- Do not administer to individuals with a known history of severe allergic reaction (e.g., anaphylaxis) to any component of the vaccine.
- Appropriate medical treatment to manage immediate allergic reactions must be immediately available in the event an acute anaphylactic reaction occurs following administration of the vaccine.
- Postmarketing data demonstrate increased risks of myocarditis and pericarditis, particularly within 7 days following the second dose. The observed risk is higher among males under 40 years of age than among females and older males. The observed risk is highest in males 18 through 24 years of age.
- Syncope (fainting) may occur in association with administration of injectable vaccines. Procedures should be in place to avoid injury from fainting.
- Immunocompromised persons, including individuals receiving immunosuppressive therapy, may have a diminished response to the vaccine.
- The vaccine may not protect all vaccine recipients.
- Adverse reactions reported in clinical trials following administration of the vaccine include pain at the injection site, fatigue, headache, myalgia, arthralgia, chills, nausea/vomiting, axillary swelling/tenderness, fever, swelling at the injection site, and erythema at the injection site, and rash.
- The vaccination provider is responsible for mandatory reporting of certain adverse events to the Vaccine Adverse Event Reporting System (VAERS) online at https://vaers.hhs.gov/reportevent.html or by calling 1-800-822-7967.
- Please see the SPIKEVAX Full Prescribing Information . For information regarding authorized emergency uses of the Moderna COVID-19 Vaccine, please see the EUA Fact Sheet .
Spikevax ® is a registered trademark of Moderna. Fluzone ® is a registered trademark of Sanofi Pasteur. Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including statements regarding: the advancement of Moderna's programs under clinical development; the timing for anticipated approvals of vaccine candidates; the efficacy, safety and tolerability of vaccine candidates; the total addressable markets for programs under development; the efficiencies and advantages of Moderna's mRNA platform; future capital allocation and financing efforts; and anticipated spending for R&D in 2024. In some cases, forward-looking statements can be identified by terminology such as "will," "may," "should," "could," "expects," "intends," "plans," "aims," "anticipates," "believes," "estimates," "predicts," "potential," "continue," or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these words. The forward-looking statements in this press release are neither promises nor guarantees, and you should not place undue reliance on these forward-looking statements because they involve known and unknown risks, uncertainties, and other factors, many of which are beyond Moderna's control and which could cause actual results to differ materially from those expressed or implied by these forward-looking statements. These risks, uncertainties, and other factors include, among others, those risks and uncertainties described under the heading "Risk Factors" in Moderna's Annual Report on Form 10-K for the fiscal year ended December 31, 2023, filed with the U.S. Securities and Exchange Commission (SEC), and in subsequent filings made by Moderna with the SEC, which are available on the SEC's website at www.sec.gov . Except as required by law, Moderna disclaims any intention or responsibility for updating or revising any forward-looking statements contained in this presentation in the event of new information, future developments or otherwise. These forward-looking statements are based on Moderna's current expectations and speak only as of the date of this press release.
Moderna Contacts
Media: Chris Ridley Head, Global Media Relations +1 617-800-3651 [email protected]
Investors: Lavina Talukdar Senior Vice President & Head of Investor Relations +1 617-209-5834 [email protected]
SOURCE: Moderna, Inc.
Quick Links
- SEC Filings
- Investor FAQs
- Information Request Form
Investor Email Alerts
To opt-in for investor email alerts, please enter your email address in the field below and select at least one alert option. After submitting your request, you will receive an activation email to the requested email address. You must click the activation link in order to complete your subscription. You can sign up for additional alert options at any time.
At Moderna, we promise to treat your data with respect and will not share your information with any third party. You can unsubscribe to any of the investor alerts you are subscribed to by visiting the ‘unsubscribe’ section below. If you experience any issues with this process, please contact us for further assistance.
By providing your email address below, you are providing consent to Moderna to send you the requested Investor Email Alert updates.
Email Alert Sign Up Confirmation
Investor overview
Press releases
Media Center
Partnerships.
Strategic collaborators
Company culture
People behind the science
Join our team
Innovation Incubator
New venture labs
- Terms of Use
- Privacy Policy
- Contact Moderna
2024 Annual Meeting of Shareholders Webcast
The annual meeting of shareholders of The Walt Disney Company, including remarks by management regarding the Company, will be available live via webcast beginning at 1:00 p.m. ET/ 10:00 a.m. PT on April 3, 2024.
Please return here 15 minutes prior to the presentation to ensure your connection.
The presentation will be archived.

IMAGES
VIDEO
COMMENTS
COVID-19 is a new disease, caused by a novel (or new) coronavirus that has not previously been seen in humans. This new virus and disease were unknown before the outbreak began in Wuhan, China, in December 2019. In COVID-19, 'CO' stands for 'corona,' 'VI' for 'virus,' and 'D' for disease. Formerly, this disease was referred ...
AI generated presentations: simplifying the creation process; Feb. 27, 2024. Tackle the "tomorrow problem": Turn your last-minute presentation into a winning momentum; Feb. 20, 2024. Storyboard examples for presentations; Latest posts
COVID-19 is an infectious disease of the human respiratory system caused by the virus SARS-CoV-2. The disease is almost always mild and causes fever, dry cough, shortness of breath, and fatigue. Older people and other at-risk populations may develop life-threatening symptoms. There is no vaccine or treatment.
Symptoms. Fact.3: COVID-19 spread's mainly from person to person, mainly through respiratory droplets produced when an infected person coughs or sneezes. Fact.4:It may be possible that a person can get COVID-19 by touching a surface or object that has the virus on it and then touching their own mouth, nose, or possibly their eyes.
Coronavirus. Human coronaviruses were first identified in the mid-1960s. There are four main sub-groupings of coronaviruses, known as alpha, beta, gamma, and delta. The seven coronaviruses that can infect people are: Common human coronaviruses (cause common colds)
PK !aŽF | 'u [Content_Types].xml ¢ ( Ì ]oÛ6 †ï ì? º lI--ÉEœbh7`À¶ h ì-'h[¾&ÑNòïGÉ uR%-|Èò½ ¢ >'È÷% éæíC-Žv¼ª""_ZîÄ ...
COVID-19 presentation for educators - Google Slides. JavaScript isn't enabled in your browser, so this file can't be opened.
COVID-19 Presentation . Multi-purpose . Premium Google Slides theme and PowerPoint template . The coronavirus outbreak has become one of the most notorious events of the decade, if not the current century. Every bit of information helps a lot, so let us help you create useful and informative presentations about this virus with our latest template.
This is a PowerPoint presentation from the CDC's Clinician Outreach and Communication Activity (COCA) on the novel coronavirus (2019-nCoV) outbreak. It provides an overview of the epidemiology, clinical features, infection prevention and control, and guidance for clinicians. It also includes links to additional resources and updates from the CDC and the World Health Organization (WHO).
Coronavirus Presentation templates Raise awareness about one of the hottest topics of the 21st century by downloading and editing our free PPT templates and Google Slides themes about Coronavirus. Filter by. ... The COVID-19 vaccine has improved the pandemic situation. We are gradually returning to our pre-COVID-19 lifestyle.
Clinical presentation. Similar to other coronaviruses, SARS‐CoV‐2 is predominantly spread by respiratory droplets, although spread by contact with contaminated fomites also occurs, as does transmission by aerosols in certain circumstances.1 Based on the experience in China, the typical incubation period of COVID‐19 infection has been estimated to be a median of 5.1 days (95% CI, 4.5-5. ...
COVID-19 Abstraction Guidance by Margaret (Peggy) Adamo, RHIT, CTR. Access the presentation slides (PDF, 1.2 MB) View the presentation recording. Coronavirus-Disease 19 (COVID-19) & SARS-CoV-2 Testing by Alison Van Dyke, MD, PhD. Access the presentation slides (PDF, 4.6 MB) View the presentation recording
530 East 70th Street, M-522 New York, NY 10021 Phone: (212) 746-4007 Fax: (212) 746-8214
With the arrival of COVID-19, researchers have been using and formulating mathematical models as a technique in gaining insight into the mode of spread of the pandemic, transmission, impact of the pandemic, prevention and control of the pandemic, the influence of preventive measure on the pandemic ranging from washing hands with a disinfectant such as a hand sanitizer, 2 to 5 meters social ...
Here at Slidesgo we'd also like to help fight against the coronavirus spread. Creating presentations is what we excel at, so we've just designed this new template with which you can talk about COVID-19, its spread and how to prevent it. For this template, since we'd like you to reach as many people as possible, we've tried several things.
Tags. Professional Blue Illustration Medical Health Abstract Science Breakthrough Cartoon Research Coronavirus Editor's Choice Vaccine Virus. Tell your audience all about the available vaccines against COVID-19 in these blue-colored slides. The template is editable in Google Slides and PowerPoint!
What is COVID-19? 1 CORONAVIRUS COVID-19 or also know as Coronavirus, is a illnes caused by a virus called "Sever Acute Respiratory Syndrome-Related Coronavirus 2". #1 2 How does it attack? #1 How does it spread? ... Turn your last-minute presentation into a winning momentum; Feb. 20, 2024. Storyboard examples for presentations; Latest posts ...
The clinical presentation of COVID-19 ranges from asymptomatic to critical illness. An infected person can transmit SARS-CoV-2, the virus that causes COVID-19, before the onset of symptoms. Symptoms can change over the course of illness and can progress in severity. Uncommon presentations of COVID-19 can occur, might vary by the age of the ...
NOELLA M. GOZALO COVID 19 Introduction Introduction Coronavirus disease 2019 (COVID-19) is a respiratory illness that can spread from person to person. The virus that causes COVID-19 is a novel coronavirus that was first identified during an investigation into an outbreak in How
The youngest have had to adapt without understanding what was going on. If you want to make COVID-19 understandable for your middle school students, we recommend using this editable template from Slidesgo. With it you can explain the symptoms, prevention measures, how the transmission occurs, what treatments are available, etc.
Since the COVID-19 pandemic started in early 2020, businesses have turned to video conferencing to keep daily operations running. ... Prezi records presentations for board meetings ahead of time ...
This presentation showcases Prezi open canvas features beautifully. As you can see, the presentation begins with a full view of the content, and then as the presentation progresses it uses the zoom feature to capture each point close up. This is much more enjoyable than traditional slideshows, and it's a great way to put your PowerPoint night ...
Announces next-generation COVID-19 vaccine candidate as fourth respiratory vaccine to successfully meet its Phase 3 endpoints Expects two more Phase 3 readouts in 2024, including combination vaccine against flu and COVID-19, and vaccine against CMV Announces positive clinical trial data from three new vaccines against viruses that cause significant burden (Epstein-Barr virus, Varicella-Zoster ...
When Covid-19 arrived in 2020 and millions of Americans isolated in their homes, virtual PowerPoint parties became a safe, distanced way for friends to update each other on their lives and provide ...
Covid - 19. = Ausgelöste Lungenkrankheit. Fieber, Müdigkeit, trockener Husten & Schnupfen. Symptome = grippeähnlich. Ältere Menschen und Menschen mit Vorerkrankungen sind stärker. Infizierte = müssen keine Symptome zeigen. 80% erholen sich. 1 von 6 Personen -> wird schwer krank &. entwickelt Atembeschwerden.
Coronavirus (COVID-19) Pamela Andrea Castaño Sepúlveda ¿Qué es el coronavirus? 1 Los coronavirus son una amplia familia de virus que pueden causar diversas afecciones, desde el resfriado común hasta enfermedades más graves;El coronavirus fue descubierto en la decada de los 60 y se ... AI generated presentations: simplifying the creation ...
Additional factors are set forth in the Company's Annual Report on Form 10-K for the year ended October 1, 2022, including under the captions "Risk Factors," "Management's Discussion and Analysis of Financial Condition and Results of Operations," and "Business," quarterly reports on Form 10-Q, including under the captions ...