- Privacy Policy

Home » Validity – Types, Examples and Guide

Validity – Types, Examples and Guide
Table of Contents

Definition:
Validity refers to the extent to which a concept, measure, or study accurately represents the intended meaning or reality it is intended to capture. It is a fundamental concept in research and assessment that assesses the soundness and appropriateness of the conclusions, inferences, or interpretations made based on the data or evidence collected.
Research Validity
Research validity refers to the degree to which a study accurately measures or reflects what it claims to measure. In other words, research validity concerns whether the conclusions drawn from a study are based on accurate, reliable and relevant data.
Validity is a concept used in logic and research methodology to assess the strength of an argument or the quality of a research study. It refers to the extent to which a conclusion or result is supported by evidence and reasoning.
How to Ensure Validity in Research
Ensuring validity in research involves several steps and considerations throughout the research process. Here are some key strategies to help maintain research validity:
Clearly Define Research Objectives and Questions
Start by clearly defining your research objectives and formulating specific research questions. This helps focus your study and ensures that you are addressing relevant and meaningful research topics.
Use appropriate research design
Select a research design that aligns with your research objectives and questions. Different types of studies, such as experimental, observational, qualitative, or quantitative, have specific strengths and limitations. Choose the design that best suits your research goals.
Use reliable and valid measurement instruments
If you are measuring variables or constructs, ensure that the measurement instruments you use are reliable and valid. This involves using established and well-tested tools or developing your own instruments through rigorous validation processes.
Ensure a representative sample
When selecting participants or subjects for your study, aim for a sample that is representative of the population you want to generalize to. Consider factors such as age, gender, socioeconomic status, and other relevant demographics to ensure your findings can be generalized appropriately.
Address potential confounding factors
Identify potential confounding variables or biases that could impact your results. Implement strategies such as randomization, matching, or statistical control to minimize the influence of confounding factors and increase internal validity.
Minimize measurement and response biases
Be aware of measurement biases and response biases that can occur during data collection. Use standardized protocols, clear instructions, and trained data collectors to minimize these biases. Employ techniques like blinding or double-blinding in experimental studies to reduce bias.
Conduct appropriate statistical analyses
Ensure that the statistical analyses you employ are appropriate for your research design and data type. Select statistical tests that are relevant to your research questions and use robust analytical techniques to draw accurate conclusions from your data.
Consider external validity
While it may not always be possible to achieve high external validity, be mindful of the generalizability of your findings. Clearly describe your sample and study context to help readers understand the scope and limitations of your research.
Peer review and replication
Submit your research for peer review by experts in your field. Peer review helps identify potential flaws, biases, or methodological issues that can impact validity. Additionally, encourage replication studies by other researchers to validate your findings and enhance the overall reliability of the research.
Transparent reporting
Clearly and transparently report your research methods, procedures, data collection, and analysis techniques. Provide sufficient details for others to evaluate the validity of your study and replicate your work if needed.
Types of Validity
There are several types of validity that researchers consider when designing and evaluating studies. Here are some common types of validity:
Internal Validity
Internal validity relates to the degree to which a study accurately identifies causal relationships between variables. It addresses whether the observed effects can be attributed to the manipulated independent variable rather than confounding factors. Threats to internal validity include selection bias, history effects, maturation of participants, and instrumentation issues.
External Validity
External validity concerns the generalizability of research findings to the broader population or real-world settings. It assesses the extent to which the results can be applied to other individuals, contexts, or timeframes. Factors that can limit external validity include sample characteristics, research settings, and the specific conditions under which the study was conducted.
Construct Validity
Construct validity examines whether a study adequately measures the intended theoretical constructs or concepts. It focuses on the alignment between the operational definitions used in the study and the underlying theoretical constructs. Construct validity can be threatened by issues such as poor measurement tools, inadequate operational definitions, or a lack of clarity in the conceptual framework.
Content Validity
Content validity refers to the degree to which a measurement instrument or test adequately covers the entire range of the construct being measured. It assesses whether the items or questions included in the measurement tool represent the full scope of the construct. Content validity is often evaluated through expert judgment, reviewing the relevance and representativeness of the items.
Criterion Validity
Criterion validity determines the extent to which a measure or test is related to an external criterion or standard. It assesses whether the results obtained from a measurement instrument align with other established measures or outcomes. Criterion validity can be divided into two subtypes: concurrent validity, which examines the relationship between the measure and the criterion at the same time, and predictive validity, which investigates the measure’s ability to predict future outcomes.
Face Validity
Face validity refers to the degree to which a measurement or test appears, on the surface, to measure what it intends to measure. It is a subjective assessment based on whether the items seem relevant and appropriate to the construct being measured. Face validity is often used as an initial evaluation before conducting more rigorous validity assessments.
Importance of Validity
Validity is crucial in research for several reasons:
- Accurate Measurement: Validity ensures that the measurements or observations in a study accurately represent the intended constructs or variables. Without validity, researchers cannot be confident that their results truly reflect the phenomena they are studying. Validity allows researchers to draw accurate conclusions and make meaningful inferences based on their findings.
- Credibility and Trustworthiness: Validity enhances the credibility and trustworthiness of research. When a study demonstrates high validity, it indicates that the researchers have taken appropriate measures to ensure the accuracy and integrity of their work. This strengthens the confidence of other researchers, peers, and the wider scientific community in the study’s results and conclusions.
- Generalizability: Validity helps determine the extent to which research findings can be generalized beyond the specific sample and context of the study. By addressing external validity, researchers can assess whether their results can be applied to other populations, settings, or situations. This information is valuable for making informed decisions, implementing interventions, or developing policies based on research findings.
- Sound Decision-Making: Validity supports informed decision-making in various fields, such as medicine, psychology, education, and social sciences. When validity is established, policymakers, practitioners, and professionals can rely on research findings to guide their actions and interventions. Validity ensures that decisions are based on accurate and trustworthy information, which can lead to better outcomes and more effective practices.
- Avoiding Errors and Bias: Validity helps researchers identify and mitigate potential errors and biases in their studies. By addressing internal validity, researchers can minimize confounding factors and alternative explanations, ensuring that the observed effects are genuinely attributable to the manipulated variables. Validity assessments also highlight measurement errors or shortcomings, enabling researchers to improve their measurement tools and procedures.
- Progress of Scientific Knowledge: Validity is essential for the advancement of scientific knowledge. Valid research contributes to the accumulation of reliable and valid evidence, which forms the foundation for building theories, developing models, and refining existing knowledge. Validity allows researchers to build upon previous findings, replicate studies, and establish a cumulative body of knowledge in various disciplines. Without validity, the scientific community would struggle to make meaningful progress and establish a solid understanding of the phenomena under investigation.
- Ethical Considerations: Validity is closely linked to ethical considerations in research. Conducting valid research ensures that participants’ time, effort, and data are not wasted on flawed or invalid studies. It upholds the principle of respect for participants’ autonomy and promotes responsible research practices. Validity is also important when making claims or drawing conclusions that may have real-world implications, as misleading or invalid findings can have adverse effects on individuals, organizations, or society as a whole.
Examples of Validity
Here are some examples of validity in different contexts:
- Example 1: All men are mortal. John is a man. Therefore, John is mortal. This argument is logically valid because the conclusion follows logically from the premises.
- Example 2: If it is raining, then the ground is wet. The ground is wet. Therefore, it is raining. This argument is not logically valid because there could be other reasons for the ground being wet, such as watering the plants.
- Example 1: In a study examining the relationship between caffeine consumption and alertness, the researchers use established measures of both variables, ensuring that they are accurately capturing the concepts they intend to measure. This demonstrates construct validity.
- Example 2: A researcher develops a new questionnaire to measure anxiety levels. They administer the questionnaire to a group of participants and find that it correlates highly with other established anxiety measures. This indicates good construct validity for the new questionnaire.
- Example 1: A study on the effects of a particular teaching method is conducted in a controlled laboratory setting. The findings of the study may lack external validity because the conditions in the lab may not accurately reflect real-world classroom settings.
- Example 2: A research study on the effects of a new medication includes participants from diverse backgrounds and age groups, increasing the external validity of the findings to a broader population.
- Example 1: In an experiment, a researcher manipulates the independent variable (e.g., a new drug) and controls for other variables to ensure that any observed effects on the dependent variable (e.g., symptom reduction) are indeed due to the manipulation. This establishes internal validity.
- Example 2: A researcher conducts a study examining the relationship between exercise and mood by administering questionnaires to participants. However, the study lacks internal validity because it does not control for other potential factors that could influence mood, such as diet or stress levels.
- Example 1: A teacher develops a new test to assess students’ knowledge of a particular subject. The items on the test appear to be relevant to the topic at hand and align with what one would expect to find on such a test. This suggests face validity, as the test appears to measure what it intends to measure.
- Example 2: A company develops a new customer satisfaction survey. The questions included in the survey seem to address key aspects of the customer experience and capture the relevant information. This indicates face validity, as the survey seems appropriate for assessing customer satisfaction.
- Example 1: A team of experts reviews a comprehensive curriculum for a high school biology course. They evaluate the curriculum to ensure that it covers all the essential topics and concepts necessary for students to gain a thorough understanding of biology. This demonstrates content validity, as the curriculum is representative of the domain it intends to cover.
- Example 2: A researcher develops a questionnaire to assess career satisfaction. The questions in the questionnaire encompass various dimensions of job satisfaction, such as salary, work-life balance, and career growth. This indicates content validity, as the questionnaire adequately represents the different aspects of career satisfaction.
- Example 1: A company wants to evaluate the effectiveness of a new employee selection test. They administer the test to a group of job applicants and later assess the job performance of those who were hired. If there is a strong correlation between the test scores and subsequent job performance, it suggests criterion validity, indicating that the test is predictive of job success.
- Example 2: A researcher wants to determine if a new medical diagnostic tool accurately identifies a specific disease. They compare the results of the diagnostic tool with the gold standard diagnostic method and find a high level of agreement. This demonstrates criterion validity, indicating that the new tool is valid in accurately diagnosing the disease.
Where to Write About Validity in A Thesis
In a thesis, discussions related to validity are typically included in the methodology and results sections. Here are some specific places where you can address validity within your thesis:
Research Design and Methodology
In the methodology section, provide a clear and detailed description of the measures, instruments, or data collection methods used in your study. Discuss the steps taken to establish or assess the validity of these measures. Explain the rationale behind the selection of specific validity types relevant to your study, such as content validity, criterion validity, or construct validity. Discuss any modifications or adaptations made to existing measures and their potential impact on validity.
Measurement Procedures
In the methodology section, elaborate on the procedures implemented to ensure the validity of measurements. Describe how potential biases or confounding factors were addressed, controlled, or accounted for to enhance internal validity. Provide details on how you ensured that the measurement process accurately captures the intended constructs or variables of interest.
Data Collection
In the methodology section, discuss the steps taken to collect data and ensure data validity. Explain any measures implemented to minimize errors or biases during data collection, such as training of data collectors, standardized protocols, or quality control procedures. Address any potential limitations or threats to validity related to the data collection process.
Data Analysis and Results
In the results section, present the analysis and findings related to validity. Report any statistical tests, correlations, or other measures used to assess validity. Provide interpretations and explanations of the results obtained. Discuss the implications of the validity findings for the overall reliability and credibility of your study.
Limitations and Future Directions
In the discussion or conclusion section, reflect on the limitations of your study, including limitations related to validity. Acknowledge any potential threats or weaknesses to validity that you encountered during your research. Discuss how these limitations may have influenced the interpretation of your findings and suggest avenues for future research that could address these validity concerns.
Applications of Validity
Validity is applicable in various areas and contexts where research and measurement play a role. Here are some common applications of validity:
Psychological and Behavioral Research
Validity is crucial in psychology and behavioral research to ensure that measurement instruments accurately capture constructs such as personality traits, intelligence, attitudes, emotions, or psychological disorders. Validity assessments help researchers determine if their measures are truly measuring the intended psychological constructs and if the results can be generalized to broader populations or real-world settings.
Educational Assessment
Validity is essential in educational assessment to determine if tests, exams, or assessments accurately measure students’ knowledge, skills, or abilities. It ensures that the assessment aligns with the educational objectives and provides reliable information about student performance. Validity assessments help identify if the assessment is valid for all students, regardless of their demographic characteristics, language proficiency, or cultural background.
Program Evaluation
Validity plays a crucial role in program evaluation, where researchers assess the effectiveness and impact of interventions, policies, or programs. By establishing validity, evaluators can determine if the observed outcomes are genuinely attributable to the program being evaluated rather than extraneous factors. Validity assessments also help ensure that the evaluation findings are applicable to different populations, contexts, or timeframes.
Medical and Health Research
Validity is essential in medical and health research to ensure the accuracy and reliability of diagnostic tools, measurement instruments, and clinical assessments. Validity assessments help determine if a measurement accurately identifies the presence or absence of a medical condition, measures the effectiveness of a treatment, or predicts patient outcomes. Validity is crucial for establishing evidence-based medicine and informing medical decision-making.
Social Science Research
Validity is relevant in various social science disciplines, including sociology, anthropology, economics, and political science. Researchers use validity to ensure that their measures and methods accurately capture social phenomena, such as social attitudes, behaviors, social structures, or economic indicators. Validity assessments support the reliability and credibility of social science research findings.
Market Research and Surveys
Validity is important in market research and survey studies to ensure that the survey questions effectively measure consumer preferences, buying behaviors, or attitudes towards products or services. Validity assessments help researchers determine if the survey instrument is accurately capturing the desired information and if the results can be generalized to the target population.
Limitations of Validity
Here are some limitations of validity:
- Construct Validity: Limitations of construct validity include the potential for measurement error, inadequate operational definitions of constructs, or the failure to capture all aspects of a complex construct.
- Internal Validity: Limitations of internal validity may arise from confounding variables, selection bias, or the presence of extraneous factors that could influence the study outcomes, making it difficult to attribute causality accurately.
- External Validity: Limitations of external validity can occur when the study sample does not represent the broader population, when the research setting differs significantly from real-world conditions, or when the study lacks ecological validity, i.e., the findings do not reflect real-world complexities.
- Measurement Validity: Limitations of measurement validity can arise from measurement error, inadequately designed or flawed measurement scales, or limitations inherent in self-report measures, such as social desirability bias or recall bias.
- Statistical Conclusion Validity: Limitations in statistical conclusion validity can occur due to sampling errors, inadequate sample sizes, or improper statistical analysis techniques, leading to incorrect conclusions or generalizations.
- Temporal Validity: Limitations of temporal validity arise when the study results become outdated due to changes in the studied phenomena, interventions, or contextual factors.
- Researcher Bias: Researcher bias can affect the validity of a study. Biases can emerge through the researcher’s subjective interpretation, influence of personal beliefs, or preconceived notions, leading to unintentional distortion of findings or failure to consider alternative explanations.
- Ethical Validity: Limitations can arise if the study design or methods involve ethical concerns, such as the use of deceptive practices, inadequate informed consent, or potential harm to participants.
Also see Reliability Vs Validity
About the author
Muhammad Hassan
Researcher, Academic Writer, Web developer
You may also like

Alternate Forms Reliability – Methods, Examples...

Construct Validity – Types, Threats and Examples

Internal Validity – Threats, Examples and Guide

Reliability Vs Validity

Internal Consistency Reliability – Methods...

Split-Half Reliability – Methods, Examples and...
Validity In Psychology Research: Types & Examples
Saul Mcleod, PhD
Editor-in-Chief for Simply Psychology
BSc (Hons) Psychology, MRes, PhD, University of Manchester
Saul Mcleod, PhD., is a qualified psychology teacher with over 18 years of experience in further and higher education. He has been published in peer-reviewed journals, including the Journal of Clinical Psychology.
Learn about our Editorial Process
Olivia Guy-Evans, MSc
Associate Editor for Simply Psychology
BSc (Hons) Psychology, MSc Psychology of Education
Olivia Guy-Evans is a writer and associate editor for Simply Psychology. She has previously worked in healthcare and educational sectors.
In psychology research, validity refers to the extent to which a test or measurement tool accurately measures what it’s intended to measure. It ensures that the research findings are genuine and not due to extraneous factors.
Validity can be categorized into different types based on internal and external validity .
The concept of validity was formulated by Kelly (1927, p. 14), who stated that a test is valid if it measures what it claims to measure. For example, a test of intelligence should measure intelligence and not something else (such as memory).
Internal and External Validity In Research
Internal validity refers to whether the effects observed in a study are due to the manipulation of the independent variable and not some other confounding factor.
In other words, there is a causal relationship between the independent and dependent variables .
Internal validity can be improved by controlling extraneous variables, using standardized instructions, counterbalancing, and eliminating demand characteristics and investigator effects.
External validity refers to the extent to which the results of a study can be generalized to other settings (ecological validity), other people (population validity), and over time (historical validity).
External validity can be improved by setting experiments more naturally and using random sampling to select participants.
Types of Validity In Psychology
Two main categories of validity are used to assess the validity of the test (i.e., questionnaire, interview, IQ test, etc.): Content and criterion.
- Content validity refers to the extent to which a test or measurement represents all aspects of the intended content domain. It assesses whether the test items adequately cover the topic or concept.
- Criterion validity assesses the performance of a test based on its correlation with a known external criterion or outcome. It can be further divided into concurrent (measured at the same time) and predictive (measuring future performance) validity.
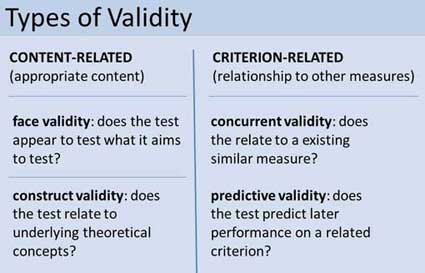
Face Validity
Face validity is simply whether the test appears (at face value) to measure what it claims to. This is the least sophisticated measure of content-related validity, and is a superficial and subjective assessment based on appearance.
Tests wherein the purpose is clear, even to naïve respondents, are said to have high face validity. Accordingly, tests wherein the purpose is unclear have low face validity (Nevo, 1985).
A direct measurement of face validity is obtained by asking people to rate the validity of a test as it appears to them. This rater could use a Likert scale to assess face validity.
For example:
- The test is extremely suitable for a given purpose
- The test is very suitable for that purpose;
- The test is adequate
- The test is inadequate
- The test is irrelevant and, therefore, unsuitable
It is important to select suitable people to rate a test (e.g., questionnaire, interview, IQ test, etc.). For example, individuals who actually take the test would be well placed to judge its face validity.
Also, people who work with the test could offer their opinion (e.g., employers, university administrators, employers). Finally, the researcher could use members of the general public with an interest in the test (e.g., parents of testees, politicians, teachers, etc.).
The face validity of a test can be considered a robust construct only if a reasonable level of agreement exists among raters.
It should be noted that the term face validity should be avoided when the rating is done by an “expert,” as content validity is more appropriate.
Having face validity does not mean that a test really measures what the researcher intends to measure, but only in the judgment of raters that it appears to do so. Consequently, it is a crude and basic measure of validity.
A test item such as “ I have recently thought of killing myself ” has obvious face validity as an item measuring suicidal cognitions and may be useful when measuring symptoms of depression.
However, the implication of items on tests with clear face validity is that they are more vulnerable to social desirability bias. Individuals may manipulate their responses to deny or hide problems or exaggerate behaviors to present a positive image of themselves.
It is possible for a test item to lack face validity but still have general validity and measure what it claims to measure. This is good because it reduces demand characteristics and makes it harder for respondents to manipulate their answers.
For example, the test item “ I believe in the second coming of Christ ” would lack face validity as a measure of depression (as the purpose of the item is unclear).
This item appeared on the first version of The Minnesota Multiphasic Personality Inventory (MMPI) and loaded on the depression scale.
Because most of the original normative sample of the MMPI were good Christians, only a depressed Christian would think Christ is not coming back. Thus, for this particular religious sample, the item does have general validity but not face validity.
Construct Validity
Construct validity assesses how well a test or measure represents and captures an abstract theoretical concept, known as a construct. It indicates the degree to which the test accurately reflects the construct it intends to measure, often evaluated through relationships with other variables and measures theoretically connected to the construct.
Construct validity was invented by Cronbach and Meehl (1955). This type of content-related validity refers to the extent to which a test captures a specific theoretical construct or trait, and it overlaps with some of the other aspects of validity
Construct validity does not concern the simple, factual question of whether a test measures an attribute.
Instead, it is about the complex question of whether test score interpretations are consistent with a nomological network involving theoretical and observational terms (Cronbach & Meehl, 1955).
To test for construct validity, it must be demonstrated that the phenomenon being measured actually exists. So, the construct validity of a test for intelligence, for example, depends on a model or theory of intelligence .
Construct validity entails demonstrating the power of such a construct to explain a network of research findings and to predict further relationships.
The more evidence a researcher can demonstrate for a test’s construct validity, the better. However, there is no single method of determining the construct validity of a test.
Instead, different methods and approaches are combined to present the overall construct validity of a test. For example, factor analysis and correlational methods can be used.
Convergent validity
Convergent validity is a subtype of construct validity. It assesses the degree to which two measures that theoretically should be related are related.
It demonstrates that measures of similar constructs are highly correlated. It helps confirm that a test accurately measures the intended construct by showing its alignment with other tests designed to measure the same or similar constructs.
For example, suppose there are two different scales used to measure self-esteem:
Scale A and Scale B. If both scales effectively measure self-esteem, then individuals who score high on Scale A should also score high on Scale B, and those who score low on Scale A should score similarly low on Scale B.
If the scores from these two scales show a strong positive correlation, then this provides evidence for convergent validity because it indicates that both scales seem to measure the same underlying construct of self-esteem.
Concurrent Validity (i.e., occurring at the same time)
Concurrent validity evaluates how well a test’s results correlate with the results of a previously established and accepted measure, when both are administered at the same time.
It helps in determining whether a new measure is a good reflection of an established one without waiting to observe outcomes in the future.
If the new test is validated by comparison with a currently existing criterion, we have concurrent validity.
Very often, a new IQ or personality test might be compared with an older but similar test known to have good validity already.
Predictive Validity
Predictive validity assesses how well a test predicts a criterion that will occur in the future. It measures the test’s ability to foresee the performance of an individual on a related criterion measured at a later point in time. It gauges the test’s effectiveness in predicting subsequent real-world outcomes or results.
For example, a prediction may be made on the basis of a new intelligence test that high scorers at age 12 will be more likely to obtain university degrees several years later. If the prediction is born out, then the test has predictive validity.
Cronbach, L. J., and Meehl, P. E. (1955) Construct validity in psychological tests. Psychological Bulletin , 52, 281-302.
Hathaway, S. R., & McKinley, J. C. (1943). Manual for the Minnesota Multiphasic Personality Inventory . New York: Psychological Corporation.
Kelley, T. L. (1927). Interpretation of educational measurements. New York : Macmillan.
Nevo, B. (1985). Face validity revisited . Journal of Educational Measurement , 22(4), 287-293.
Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, automatically generate references for free.
- Knowledge Base
- Methodology
- The 4 Types of Validity | Types, Definitions & Examples
The 4 Types of Validity | Types, Definitions & Examples
Published on 3 May 2022 by Fiona Middleton . Revised on 10 October 2022.
In quantitative research , you have to consider the reliability and validity of your methods and measurements.
Validity tells you how accurately a method measures something. If a method measures what it claims to measure, and the results closely correspond to real-world values, then it can be considered valid. There are four main types of validity:
- Construct validity : Does the test measure the concept that it’s intended to measure?
- Content validity : Is the test fully representative of what it aims to measure?
- Face validity : Does the content of the test appear to be suitable to its aims?
- Criterion validity : Do the results accurately measure the concrete outcome they are designed to measure?
Note that this article deals with types of test validity, which determine the accuracy of the actual components of a measure. If you are doing experimental research, you also need to consider internal and external validity , which deal with the experimental design and the generalisability of results.
Table of contents
Construct validity, content validity, face validity, criterion validity.
Construct validity evaluates whether a measurement tool really represents the thing we are interested in measuring. It’s central to establishing the overall validity of a method.
What is a construct?
A construct refers to a concept or characteristic that can’t be directly observed but can be measured by observing other indicators that are associated with it.
Constructs can be characteristics of individuals, such as intelligence, obesity, job satisfaction, or depression; they can also be broader concepts applied to organisations or social groups, such as gender equality, corporate social responsibility, or freedom of speech.
What is construct validity?
Construct validity is about ensuring that the method of measurement matches the construct you want to measure. If you develop a questionnaire to diagnose depression, you need to know: does the questionnaire really measure the construct of depression? Or is it actually measuring the respondent’s mood, self-esteem, or some other construct?
To achieve construct validity, you have to ensure that your indicators and measurements are carefully developed based on relevant existing knowledge. The questionnaire must include only relevant questions that measure known indicators of depression.
The other types of validity described below can all be considered as forms of evidence for construct validity.
Prevent plagiarism, run a free check.
Content validity assesses whether a test is representative of all aspects of the construct.
To produce valid results, the content of a test, survey, or measurement method must cover all relevant parts of the subject it aims to measure. If some aspects are missing from the measurement (or if irrelevant aspects are included), the validity is threatened.
Face validity considers how suitable the content of a test seems to be on the surface. It’s similar to content validity, but face validity is a more informal and subjective assessment.
As face validity is a subjective measure, it’s often considered the weakest form of validity. However, it can be useful in the initial stages of developing a method.
Criterion validity evaluates how well a test can predict a concrete outcome, or how well the results of your test approximate the results of another test.
What is a criterion variable?
A criterion variable is an established and effective measurement that is widely considered valid, sometimes referred to as a ‘gold standard’ measurement. Criterion variables can be very difficult to find.
What is criterion validity?
To evaluate criterion validity, you calculate the correlation between the results of your measurement and the results of the criterion measurement. If there is a high correlation, this gives a good indication that your test is measuring what it intends to measure.
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the ‘Cite this Scribbr article’ button to automatically add the citation to our free Reference Generator.
Middleton, F. (2022, October 10). The 4 Types of Validity | Types, Definitions & Examples. Scribbr. Retrieved 22 April 2024, from https://www.scribbr.co.uk/research-methods/validity-types/
Is this article helpful?
Fiona Middleton
Other students also liked, qualitative vs quantitative research | examples & methods, a quick guide to experimental design | 5 steps & examples, what is qualitative research | methods & examples.
Validity in research: a guide to measuring the right things
Last updated
27 February 2023
Reviewed by
Cathy Heath
Validity is necessary for all types of studies ranging from market validation of a business or product idea to the effectiveness of medical trials and procedures. So, how can you determine whether your research is valid? This guide can help you understand what validity is, the types of validity in research, and the factors that affect research validity.
Make research less tedious
Dovetail streamlines research to help you uncover and share actionable insights
- What is validity?
In the most basic sense, validity is the quality of being based on truth or reason. Valid research strives to eliminate the effects of unrelated information and the circumstances under which evidence is collected.
Validity in research is the ability to conduct an accurate study with the right tools and conditions to yield acceptable and reliable data that can be reproduced. Researchers rely on carefully calibrated tools for precise measurements. However, collecting accurate information can be more of a challenge.
Studies must be conducted in environments that don't sway the results to achieve and maintain validity. They can be compromised by asking the wrong questions or relying on limited data.
Why is validity important in research?
Research is used to improve life for humans. Every product and discovery, from innovative medical breakthroughs to advanced new products, depends on accurate research to be dependable. Without it, the results couldn't be trusted, and products would likely fail. Businesses would lose money, and patients couldn't rely on medical treatments.
While wasting money on a lousy product is a concern, lack of validity paints a much grimmer picture in the medical field or producing automobiles and airplanes, for example. Whether you're launching an exciting new product or conducting scientific research, validity can determine success and failure.
- What is reliability?
Reliability is the ability of a method to yield consistency. If the same result can be consistently achieved by using the same method to measure something, the measurement method is said to be reliable. For example, a thermometer that shows the same temperatures each time in a controlled environment is reliable.
While high reliability is a part of measuring validity, it's only part of the puzzle. If the reliable thermometer hasn't been properly calibrated and reliably measures temperatures two degrees too high, it doesn't provide a valid (accurate) measure of temperature.
Similarly, if a researcher uses a thermometer to measure weight, the results won't be accurate because it's the wrong tool for the job.
- How are reliability and validity assessed?
While measuring reliability is a part of measuring validity, there are distinct ways to assess both measurements for accuracy.
How is reliability measured?
These measures of consistency and stability help assess reliability, including:
Consistency and stability of the same measure when repeated multiple times and conditions
Consistency and stability of the measure across different test subjects
Consistency and stability of results from different parts of a test designed to measure the same thing
How is validity measured?
Since validity refers to how accurately a method measures what it is intended to measure, it can be difficult to assess the accuracy. Validity can be estimated by comparing research results to other relevant data or theories.
The adherence of a measure to existing knowledge of how the concept is measured
The ability to cover all aspects of the concept being measured
The relation of the result in comparison with other valid measures of the same concept
- What are the types of validity in a research design?
Research validity is broadly gathered into two groups: internal and external. Yet, this grouping doesn't clearly define the different types of validity. Research validity can be divided into seven distinct groups.
Face validity : A test that appears valid simply because of the appropriateness or relativity of the testing method, included information, or tools used.
Content validity : The determination that the measure used in research covers the full domain of the content.
Construct validity : The assessment of the suitability of the measurement tool to measure the activity being studied.
Internal validity : The assessment of how your research environment affects measurement results. This is where other factors can’t explain the extent of an observed cause-and-effect response.
External validity : The extent to which the study will be accurate beyond the sample and the level to which it can be generalized in other settings, populations, and measures.
Statistical conclusion validity: The determination of whether a relationship exists between procedures and outcomes (appropriate sampling and measuring procedures along with appropriate statistical tests).
Criterion-related validity : A measurement of the quality of your testing methods against a criterion measure (like a “gold standard” test) that is measured at the same time.
- Examples of validity
Like different types of research and the various ways to measure validity, examples of validity can vary widely. These include:
A questionnaire may be considered valid because each question addresses specific and relevant aspects of the study subject.
In a brand assessment study, researchers can use comparison testing to verify the results of an initial study. For example, the results from a focus group response about brand perception are considered more valid when the results match that of a questionnaire answered by current and potential customers.
A test to measure a class of students' understanding of the English language contains reading, writing, listening, and speaking components to cover the full scope of how language is used.
- Factors that affect research validity
Certain factors can affect research validity in both positive and negative ways. By understanding the factors that improve validity and those that threaten it, you can enhance the validity of your study. These include:
Random selection of participants vs. the selection of participants that are representative of your study criteria
Blinding with interventions the participants are unaware of (like the use of placebos)
Manipulating the experiment by inserting a variable that will change the results
Randomly assigning participants to treatment and control groups to avoid bias
Following specific procedures during the study to avoid unintended effects
Conducting a study in the field instead of a laboratory for more accurate results
Replicating the study with different factors or settings to compare results
Using statistical methods to adjust for inconclusive data
What are the common validity threats in research, and how can their effects be minimized or nullified?
Research validity can be difficult to achieve because of internal and external threats that produce inaccurate results. These factors can jeopardize validity.
History: Events that occur between an early and later measurement
Maturation: The passage of time in a study can include data on actions that would have naturally occurred outside of the settings of the study
Repeated testing: The outcome of repeated tests can change the outcome of followed tests
Selection of subjects: Unconscious bias which can result in the selection of uniform comparison groups
Statistical regression: Choosing subjects based on extremes doesn't yield an accurate outcome for the majority of individuals
Attrition: When the sample group is diminished significantly during the course of the study
Maturation: When subjects mature during the study, and natural maturation is awarded to the effects of the study
While some validity threats can be minimized or wholly nullified, removing all threats from a study is impossible. For example, random selection can remove unconscious bias and statistical regression.
Researchers can even hope to avoid attrition by using smaller study groups. Yet, smaller study groups could potentially affect the research in other ways. The best practice for researchers to prevent validity threats is through careful environmental planning and t reliable data-gathering methods.
- How to ensure validity in your research
Researchers should be mindful of the importance of validity in the early planning stages of any study to avoid inaccurate results. Researchers must take the time to consider tools and methods as well as how the testing environment matches closely with the natural environment in which results will be used.
The following steps can be used to ensure validity in research:
Choose appropriate methods of measurement
Use appropriate sampling to choose test subjects
Create an accurate testing environment
How do you maintain validity in research?
Accurate research is usually conducted over a period of time with different test subjects. To maintain validity across an entire study, you must take specific steps to ensure that gathered data has the same levels of accuracy.
Consistency is crucial for maintaining validity in research. When researchers apply methods consistently and standardize the circumstances under which data is collected, validity can be maintained across the entire study.
Is there a need for validation of the research instrument before its implementation?
An essential part of validity is choosing the right research instrument or method for accurate results. Consider the thermometer that is reliable but still produces inaccurate results. You're unlikely to achieve research validity without activities like calibration, content, and construct validity.
- Understanding research validity for more accurate results
Without validity, research can't provide the accuracy necessary to deliver a useful study. By getting a clear understanding of validity in research, you can take steps to improve your research skills and achieve more accurate results.
Get started today
Go from raw data to valuable insights with a flexible research platform
Editor’s picks
Last updated: 21 December 2023
Last updated: 16 December 2023
Last updated: 6 October 2023
Last updated: 25 November 2023
Last updated: 12 May 2023
Last updated: 15 February 2024
Last updated: 11 March 2024
Last updated: 12 December 2023
Last updated: 18 May 2023
Last updated: 6 March 2024
Last updated: 10 April 2023
Last updated: 20 December 2023
Latest articles
Related topics, log in or sign up.
Get started for free

What is the Significance of Validity in Research?

Introduction
- What is validity in simple terms?
Internal validity vs. external validity in research
Uncovering different types of research validity, factors that improve research validity.
In qualitative research , validity refers to an evaluation metric for the trustworthiness of study findings. Within the expansive landscape of research methodologies , the qualitative approach, with its rich, narrative-driven investigations, demands unique criteria for ensuring validity.
Unlike its quantitative counterpart, which often leans on numerical robustness and statistical veracity, the essence of validity in qualitative research delves deep into the realms of credibility, dependability, and the richness of the data .
The importance of validity in qualitative research cannot be overstated. Establishing validity refers to ensuring that the research findings genuinely reflect the phenomena they are intended to represent. It reinforces the researcher's responsibility to present an authentic representation of study participants' experiences and insights.
This article will examine validity in qualitative research, exploring its characteristics, techniques to bolster it, and the challenges that researchers might face in establishing validity.

At its core, validity in research speaks to the degree to which a study accurately reflects or assesses the specific concept that the researcher is attempting to measure or understand. It's about ensuring that the study investigates what it purports to investigate. While this seems like a straightforward idea, the way validity is approached can vary greatly between qualitative and quantitative research .
Quantitative research often hinges on numerical, measurable data. In this paradigm, validity might refer to whether a specific tool or method measures the correct variable, without interference from other variables. It's about numbers, scales, and objective measurements. For instance, if one is studying personalities by administering surveys, a valid instrument could be a survey that has been rigorously developed and tested to verify that the survey questions are referring to personality characteristics and not other similar concepts, such as moods, opinions, or social norms.
Conversely, qualitative research is more concerned with understanding human behavior and the reasons that govern such behavior. It's less about measuring in the strictest sense and more about interpreting the phenomenon that is being studied. The questions become: "Are these interpretations true representations of the human experience being studied?" and "Do they authentically convey participants' perspectives and contexts?"
Differentiating between qualitative and quantitative validity is crucial because the research methods to ensure validity differ between these research paradigms. In quantitative realms, validity might involve test-retest reliability or examining the internal consistency of a test.
In the qualitative sphere, however, the focus shifts to ensuring that the researcher's interpretations align with the actual experiences and perspectives of their subjects.
This distinction is fundamental because it impacts how researchers engage in research design , gather data , and draw conclusions . Ensuring validity in qualitative research is like weaving a tapestry: every strand of data must be carefully interwoven with the interpretive threads of the researcher, creating a cohesive and faithful representation of the studied experience.
While often terms associated more closely with quantitative research, internal and external validity can still be relevant concepts to understand within the context of qualitative inquiries. Grasping these notions can help qualitative researchers better navigate the challenges of ensuring their findings are both credible and applicable in wider contexts.
Internal validity
Internal validity refers to the authenticity and truthfulness of the findings within the study itself. In qualitative research , this might involve asking: Do the conclusions drawn genuinely reflect the perspectives and experiences of the study's participants?
Internal validity revolves around the depth of understanding, ensuring that the researcher's interpretations are grounded in participants' realities. Techniques like member checking , where participants review and verify the researcher's interpretations , can bolster internal validity.
External validity
External validity refers to the extent to which the findings of a study can be generalized or applied to other settings or groups. For qualitative researchers, the emphasis isn't on statistical generalizability, as often seen in quantitative studies. Instead, it's about transferability.
It becomes a matter of determining how and where the insights gathered might be relevant in other contexts. This doesn't mean that every qualitative study's findings will apply universally, but qualitative researchers should provide enough detail (through rich, thick descriptions) to allow readers or other researchers to determine the potential for transfer to other contexts.
Try out a free trial of ATLAS.ti today
See how you can turn your data into critical research findings with our intuitive interface.
Looking deeper into the realm of validity, it's crucial to recognize and understand its various types. Each type offers distinct criteria and methods of evaluation, ensuring that research remains robust and genuine. Here's an exploration of some of these types.
Construct validity
Construct validity is a cornerstone in research methodology . It pertains to ensuring that the tools or methods used in a research study genuinely capture the intended theoretical constructs.
In qualitative research , the challenge lies in the abstract nature of many constructs. For example, if one were to investigate "emotional intelligence" or "social cohesion," the definitions might vary, making them hard to pin down.
To bolster construct validity, it is important to clearly and transparently define the concepts being studied. In addition, researchers may triangulate data from multiple sources , ensuring that different viewpoints converge towards a shared understanding of the construct. Furthermore, they might delve into iterative rounds of data collection, refining their methods with each cycle to better align with the conceptual essence of their focus.
Content validity
Content validity's emphasis is on the breadth and depth of the content being assessed. In other words, content validity refers to capturing all relevant facets of the phenomenon being studied. Within qualitative paradigms, ensuring comprehensive representation is paramount. If, for instance, a researcher is using interview protocols to understand community perceptions of a local policy, it's crucial that the questions encompass all relevant aspects of that policy. This could range from its implementation and impact to public awareness and opinion variations across demographic groups.
Enhancing content validity can involve expert reviews where subject matter experts evaluate tools or methods for comprehensiveness. Another strategy might involve pilot studies , where preliminary data collection reveals gaps or overlooked aspects that can be addressed in the main study.
Ecological validity
Ecological validity refers to the genuine reflection of real-world situations in research findings. For qualitative researchers, this means their observations , interpretations , and conclusions should resonate with the participants and context being studied.
If a study explores classroom dynamics, for example, studying students and teachers in a controlled research setting would have lower ecological validity than studying real classroom settings. Ecological validity is important to consider because it helps ensure the research is relevant to the people being studied. Individuals might behave entirely different in a controlled environment as opposed to their everyday natural settings.
Ecological validity tends to be stronger in qualitative research compared to quantitative research , because qualitative researchers are typically immersed in their study context and explore participants' subjective perceptions and experiences. Quantitative research, in contrast, can sometimes be more artificial if behavior is being observed in a lab or participants have to choose from predetermined options to answer survey questions.
Qualitative researchers can further bolster ecological validity through immersive fieldwork, where researchers spend extended periods in the studied environment. This immersion helps them capture the nuances and intricacies that might be missed in brief or superficial engagements.
Face validity
Face validity, while seemingly straightforward, holds significant weight in the preliminary stages of research. It serves as a litmus test, gauging the apparent appropriateness and relevance of a tool or method. If a researcher is developing a new interview guide to gauge employee satisfaction, for instance, a quick assessment from colleagues or a focus group can reveal if the questions intuitively seem fit for the purpose.
While face validity is more subjective and lacks the depth of other validity types, it's a crucial initial step, ensuring that the research starts on the right foot.
Criterion validity
Criterion validity evaluates how well the results obtained from one method correlate with those from another, more established method. In many research scenarios, establishing high criterion validity involves using statistical methods to measure validity. For instance, a researcher might utilize the appropriate statistical tests to determine the strength and direction of the linear relationship between two sets of data.
If a new measurement tool or method is being introduced, its validity might be established by statistically correlating its outcomes with those of a gold standard or previously validated tool. Correlational statistics can estimate the strength of the relationship between the new instrument and the previously established instrument, and regression analyses can also be useful to predict outcomes based on established criteria.
While these methods are traditionally aligned with quantitative research, qualitative researchers, particularly those using mixed methods , may also find value in these statistical approaches, especially when wanting to quantify certain aspects of their data for comparative purposes. More broadly, qualitative researchers could compare their operationalizations and findings to other similar qualitative studies to assess that they are indeed examining what they intend to study.
In the realm of qualitative research , the role of the researcher is not just that of an observer but often as an active participant in the meaning-making process. This unique positioning means the researcher's perspectives and interactions can significantly influence the data collected and its interpretation . Here's a deep dive into the researcher's pivotal role in upholding validity.
Reflexivity
A key concept in qualitative research, reflexivity requires researchers to continually reflect on their worldviews, beliefs, and potential influence on the data. By maintaining a reflexive journal or engaging in regular introspection, researchers can identify and address their own biases , ensuring a more genuine interpretation of participant narratives.
Building rapport
The depth and authenticity of information shared by participants often hinge on the rapport and trust established with the researcher. By cultivating genuine, non-judgmental, and empathetic relationships with participants, researchers can enhance the validity of the data collected.
Positionality
Every researcher brings to the study their own background, including their culture, education, socioeconomic status, and more. Recognizing how this positionality might influence interpretations and interactions is crucial. By acknowledging and transparently sharing their positionality, researchers can offer context to their findings and interpretations.
Active listening
The ability to listen without imposing one's own judgments or interpretations is vital. Active listening ensures that researchers capture the participants' experiences and emotions without distortion, enhancing the validity of the findings.
Transparency in methods
To ensure validity, researchers should be transparent about every step of their process. From how participants were selected to how data was analyzed , a clear documentation offers others a chance to understand and evaluate the research's authenticity and rigor .
Member checking
Once data is collected and interpreted, revisiting participants to confirm the researcher's interpretations can be invaluable. This process, known as member checking , ensures that the researcher's understanding aligns with the participants' intended meanings, bolstering validity.
Embracing ambiguity
Qualitative data can be complex and sometimes contradictory. Instead of trying to fit data into preconceived notions or frameworks, researchers must embrace ambiguity, acknowledging areas of uncertainty or multiple interpretations.

Make the most of your research study with ATLAS.ti
From study design to data analysis, let ATLAS.ti guide you through the research process. Download a free trial today.

Validity & Reliability In Research
A Plain-Language Explanation (With Examples)
By: Derek Jansen (MBA) | Expert Reviewer: Kerryn Warren (PhD) | September 2023
Validity and reliability are two related but distinctly different concepts within research. Understanding what they are and how to achieve them is critically important to any research project. In this post, we’ll unpack these two concepts as simply as possible.
This post is based on our popular online course, Research Methodology Bootcamp . In the course, we unpack the basics of methodology using straightfoward language and loads of examples. If you’re new to academic research, you definitely want to use this link to get 50% off the course (limited-time offer).
Overview: Validity & Reliability
- The big picture
- Validity 101
- Reliability 101
- Key takeaways
First, The Basics…
First, let’s start with a big-picture view and then we can zoom in to the finer details.
Validity and reliability are two incredibly important concepts in research, especially within the social sciences. Both validity and reliability have to do with the measurement of variables and/or constructs – for example, job satisfaction, intelligence, productivity, etc. When undertaking research, you’ll often want to measure these types of constructs and variables and, at the simplest level, validity and reliability are about ensuring the quality and accuracy of those measurements .
As you can probably imagine, if your measurements aren’t accurate or there are quality issues at play when you’re collecting your data, your entire study will be at risk. Therefore, validity and reliability are very important concepts to understand (and to get right). So, let’s unpack each of them.

What Is Validity?
In simple terms, validity (also called “construct validity”) is all about whether a research instrument accurately measures what it’s supposed to measure .
For example, let’s say you have a set of Likert scales that are supposed to quantify someone’s level of overall job satisfaction. If this set of scales focused purely on only one dimension of job satisfaction, say pay satisfaction, this would not be a valid measurement, as it only captures one aspect of the multidimensional construct. In other words, pay satisfaction alone is only one contributing factor toward overall job satisfaction, and therefore it’s not a valid way to measure someone’s job satisfaction.
Oftentimes in quantitative studies, the way in which the researcher or survey designer interprets a question or statement can differ from how the study participants interpret it . Given that respondents don’t have the opportunity to ask clarifying questions when taking a survey, it’s easy for these sorts of misunderstandings to crop up. Naturally, if the respondents are interpreting the question in the wrong way, the data they provide will be pretty useless . Therefore, ensuring that a study’s measurement instruments are valid – in other words, that they are measuring what they intend to measure – is incredibly important.
There are various types of validity and we’re not going to go down that rabbit hole in this post, but it’s worth quickly highlighting the importance of making sure that your research instrument is tightly aligned with the theoretical construct you’re trying to measure . In other words, you need to pay careful attention to how the key theories within your study define the thing you’re trying to measure – and then make sure that your survey presents it in the same way.
For example, sticking with the “job satisfaction” construct we looked at earlier, you’d need to clearly define what you mean by job satisfaction within your study (and this definition would of course need to be underpinned by the relevant theory). You’d then need to make sure that your chosen definition is reflected in the types of questions or scales you’re using in your survey . Simply put, you need to make sure that your survey respondents are perceiving your key constructs in the same way you are. Or, even if they’re not, that your measurement instrument is capturing the necessary information that reflects your definition of the construct at hand.
If all of this talk about constructs sounds a bit fluffy, be sure to check out Research Methodology Bootcamp , which will provide you with a rock-solid foundational understanding of all things methodology-related. Remember, you can take advantage of our 60% discount offer using this link.
Need a helping hand?
What Is Reliability?
As with validity, reliability is an attribute of a measurement instrument – for example, a survey, a weight scale or even a blood pressure monitor. But while validity is concerned with whether the instrument is measuring the “thing” it’s supposed to be measuring, reliability is concerned with consistency and stability . In other words, reliability reflects the degree to which a measurement instrument produces consistent results when applied repeatedly to the same phenomenon , under the same conditions .
As you can probably imagine, a measurement instrument that achieves a high level of consistency is naturally more dependable (or reliable) than one that doesn’t – in other words, it can be trusted to provide consistent measurements . And that, of course, is what you want when undertaking empirical research. If you think about it within a more domestic context, just imagine if you found that your bathroom scale gave you a different number every time you hopped on and off of it – you wouldn’t feel too confident in its ability to measure the variable that is your body weight 🙂
It’s worth mentioning that reliability also extends to the person using the measurement instrument . For example, if two researchers use the same instrument (let’s say a measuring tape) and they get different measurements, there’s likely an issue in terms of how one (or both) of them are using the measuring tape. So, when you think about reliability, consider both the instrument and the researcher as part of the equation.
As with validity, there are various types of reliability and various tests that can be used to assess the reliability of an instrument. A popular one that you’ll likely come across for survey instruments is Cronbach’s alpha , which is a statistical measure that quantifies the degree to which items within an instrument (for example, a set of Likert scales) measure the same underlying construct . In other words, Cronbach’s alpha indicates how closely related the items are and whether they consistently capture the same concept .

Recap: Key Takeaways
Alright, let’s quickly recap to cement your understanding of validity and reliability:
- Validity is concerned with whether an instrument (e.g., a set of Likert scales) is measuring what it’s supposed to measure
- Reliability is concerned with whether that measurement is consistent and stable when measuring the same phenomenon under the same conditions.
In short, validity and reliability are both essential to ensuring that your data collection efforts deliver high-quality, accurate data that help you answer your research questions . So, be sure to always pay careful attention to the validity and reliability of your measurement instruments when collecting and analysing data. As the adage goes, “rubbish in, rubbish out” – make sure that your data inputs are rock-solid.

Psst… there’s more!
This post is an extract from our bestselling short course, Methodology Bootcamp . If you want to work smart, you don't want to miss this .
You Might Also Like:

THE MATERIAL IS WONDERFUL AND BENEFICIAL TO ALL STUDENTS.
THE MATERIAL IS WONDERFUL AND BENEFICIAL TO ALL STUDENTS AND I HAVE GREATLY BENEFITED FROM THE CONTENT.
Submit a Comment Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
- Print Friendly

Validity of Research and Measurements
Chris nickson.
- Nov 3, 2020
In general terms, validity is “the quality of being true or correct”, it refers to the strength of results and how accurately they reflect the real world. Thus ‘validity’ can have quite different meanings depending on the context!
- Reliability is distinct from validity, in that it refers to the consistency or repeatability of results
- internal validity
- external validity
- Validity applies to an outcome or measurement, not the instrument used to obtain it and is based on ‘validity evidence’
INTERNAL VALIDITY
- The extent to which the design and conduct of the trial eliminate the possibility of bias, such that observed effects can be attributed to the independent variable
- refers to the accuracy of a trial
- a study that lacks internal validity should not applied to any clinical setting
- power calculation
- details of study context and intervention
- avoid loss of follow up
- standardised treatment conditions
- control groups
- objectivity from blinding and data handling
- Clinical research can be internally valid despite poor external validity
EXTERNAL VALIDITY
- The extent to which the results of a trial provide a correct basis for generalizations to other circumstances
- Also called “generalizability or “applicability”
- Studies can only be applied to clinical settings the same, or similar, to those used in the study
- population validity – how well the study sample can be extrapolated to the population as a whole (based on randomized sampling)
- ecological validity – the extent to which the study environment influences results (can the study be replicated in other contexts?)
- internal/ construct validity – verified relationships between dependent and independent variables
- Research findings cannot have external validity without being internally valid
FACTORS THAT AFFECT EXTERNAL VALIDITY OF CLINICAL RESEARCH (Rothwell, 2006)
Setting of the trial
- healthcare system
- recruitment from primary, secondary or tertiary care
- selection of participating centers
- selection of participating clinicians
Selection of patients
- methods of pre-randomisation diagnosis and investigation
- eligibility criteria
- exclusion criteria
- placebo run-in period
- treatment run-in period
- “enrichment” strategies
- ratio of randomised patients to eligible non-randomised patients in participating centers
- proportion of patients who decline randomisation
Characteristics of randomised patients
- baseline clinical characteristics
- racial group
- uniformity of underlying pathology
- stage in the natural history of disease
- severity of disease
- comorbidity
- absolute risk of a poor outcome in the control group
Differences between trial protocol and routine practice
- trial intervention
- timing of treatment
- appropriateness/ relevance of control intervention
- adequacy of nontrial treatment – both intended and actual
- prohibition of certain non-trial treatments
- Therapeutic or diagnostic advances since trial was performed
Outcome measures and follow up
- clinical relevance of surrogate outcomes
- clinical relevance, validity, and reproducibility of complex scales
- effect of intervention on most relevant components of composite outcomes
- identification of who measured outcome
- use of patient outcomes
- frequency of follow up
- adequacy of length of follow-up
Adverse effects of treatment
- completeness of reporting of relevant adverse effects
- rate of discontinuation of treatment
- selection of trial centers on the basis of skill or experience
- exclusion of patients at risk of complications
- exclusion of patients who experienced adverse events during a run in period
- intensity of trial safety procedures
MEASUREMENT VALIDITY (Downing & Yudkowsky, 2009)
Validity refers to the evidence presented to support or to refute the meaning or interpretation assigned to assessment data or results. It relates to whether a test, tool, instrument or device actually measures what it intends to measure.
Traditionally validity was viewed as a trinatarian concept based on:
- degree to which the the test measures what it is meant to be measuring
- e.g. the ideal depression score would include different variants of depression and be able to distinguish depression from stress and anxiety
- Concurrent validity – compares measurements with an outcome at the same time (e.g. a concurrent “gold standard” test result)
- Predictive validity – compares measurements with an outcome at the same time (e.g. do high exam marks predict subsequent incomes?)
- the degree to which the content of an instrument is an adequate reflection of all the components of the construct
- e.g. a schizophrenia score would need to include both positive and negative symptoms
According to current validity theory in psychometrics, validity is a unitary concept and thus construct validity is the only form of validity. For instance in health professions education, validity evidence for assessments comes from (:
- relationship between test content and the construct of interest
- theory; hypothesis about content
- independent assessment of match between content sampled and domain of interest
- solid, scientific, quantitative evidence
- analysis of individual responses to stimuli
- debriefing of examinees
- process studies aimed at understanding what is measured and the soundness of intended score interpretations
- quality assurance and quality control of assessment data
- data internal to assessments such as: reliability or reproducibility of scores; inter-item correlations; statistical characteristics of items; statistical analysis of item option function; factor studies of dimensionality; Differential Item Functioning (DIF) studies
- a. Convergent and discriminant evidence: relationships between similar and different measures
- b. Test-criterion evidence: relationships between test and criterion measure(s)
- c. Validity generalization: can the validity evidence be generalized? Evidence that the validity studies may generalize to other settings.
- intended and unintended consequences of test use
- differential consequences of test use
- impact of assessment on students, instructors, schools, society
- impact of assessments on curriculum; cost/benefit analysis with respect to tradeoff between instructional time and assessment time.
- Note that strictly speaking we cannot comment on the validity of a test, tool, instrument, or device, only on the measurement that is obtained. This is because the the same test used in a different context (different operator, different subjects, different circumstances, at a different time) may not be valid. In other words, validity evidence applies to the data generated by an instrument, not the instrument itself.
- Validity can be equated with accuracy, and reliability with precision
- Face validity is a term commonly used as an indicator of validity – it is essential worthless! It means at ‘face value’, in other words, the degree to which the measure subjectively looks like what it is intended to measure.
- The higher the stakes of measurement (e.g. test result), the higher the need for validity evidence.
- You can never have too much validity evidence, but the minimum required varies with purpose (e.g. high stakes fellowship exam versus one of many progress tests)
References and Links
Journal articles and Textbooks
- Downing SM, Yudkowsky R. (2009) Assessment in health professions education, Routledge, New York.
- Rothwell PM. Factors that can affect the external validity of randomised controlled trials. PLoS Clin Trials. 2006 May;1(1):e9. [ pubmed ] [ article ]
- Shankar-Hari M, Bertolini G, Brunkhorst FM, et al. Judging quality of current septic shock definitions and criteria. Critical care. 19(1):445. 2015. [ pubmed ] [ article ]

Critical Care
Chris is an Intensivist and ECMO specialist at the Alfred ICU in Melbourne. He is also a Clinical Adjunct Associate Professor at Monash University . He is a co-founder of the Australia and New Zealand Clinician Educator Network (ANZCEN) and is the Lead for the ANZCEN Clinician Educator Incubator programme. He is on the Board of Directors for the Intensive Care Foundation and is a First Part Examiner for the College of Intensive Care Medicine . He is an internationally recognised Clinician Educator with a passion for helping clinicians learn and for improving the clinical performance of individuals and collectives.
After finishing his medical degree at the University of Auckland, he continued post-graduate training in New Zealand as well as Australia’s Northern Territory, Perth and Melbourne. He has completed fellowship training in both intensive care medicine and emergency medicine, as well as post-graduate training in biochemistry, clinical toxicology, clinical epidemiology, and health professional education.
He is actively involved in in using translational simulation to improve patient care and the design of processes and systems at Alfred Health. He coordinates the Alfred ICU’s education and simulation programmes and runs the unit’s education website, INTENSIVE . He created the ‘Critically Ill Airway’ course and teaches on numerous courses around the world. He is one of the founders of the FOAM movement (Free Open-Access Medical education) and is co-creator of litfl.com , the RAGE podcast , the Resuscitology course, and the SMACC conference.
His one great achievement is being the father of three amazing children.
On Twitter, he is @precordialthump .
| INTENSIVE | RAGE | Resuscitology | SMACC
Leave a Reply Cancel reply
This site uses Akismet to reduce spam. Learn how your comment data is processed .
Privacy Overview
- Bipolar Disorder
- Therapy Center
- When To See a Therapist
- Types of Therapy
- Best Online Therapy
- Best Couples Therapy
- Best Family Therapy
- Managing Stress
- Sleep and Dreaming
- Understanding Emotions
- Self-Improvement
- Healthy Relationships
- Student Resources
- Personality Types
- Guided Meditations
- Verywell Mind Insights
- 2023 Verywell Mind 25
- Mental Health in the Classroom
- Editorial Process
- Meet Our Review Board
- Crisis Support
Internal Validity vs. External Validity in Research
Both help determine how meaningful the results of the study are
Arlin Cuncic, MA, is the author of The Anxiety Workbook and founder of the website About Social Anxiety. She has a Master's degree in clinical psychology.
:max_bytes(150000):strip_icc():format(webp)/ArlinCuncic_1000-21af8749d2144aa0b0491c29319591c4.jpg)
Rachel Goldman, PhD FTOS, is a licensed psychologist, clinical assistant professor, speaker, wellness expert specializing in eating behaviors, stress management, and health behavior change.
:max_bytes(150000):strip_icc():format(webp)/Rachel-Goldman-1000-a42451caacb6423abecbe6b74e628042.jpg)
Verywell / Bailey Mariner
- Internal Validity
- External Validity
Internal validity is a measure of how well a study is conducted (its structure) and how accurately its results reflect the studied group.
External validity relates to how applicable the findings are in the real world. These two concepts help researchers gauge if the results of a research study are trustworthy and meaningful.
Conclusions are warranted
Controls extraneous variables
Eliminates alternative explanations
Focus on accuracy and strong research methods
Findings can be generalized
Outcomes apply to practical situations
Results apply to the world at large
Results can be translated into another context
What Is Internal Validity in Research?
Internal validity is the extent to which a research study establishes a trustworthy cause-and-effect relationship. This type of validity depends largely on the study's procedures and how rigorously it is performed.
Internal validity is important because once established, it makes it possible to eliminate alternative explanations for a finding. If you implement a smoking cessation program, for instance, internal validity ensures that any improvement in the subjects is due to the treatment administered and not something else.
Internal validity is not a "yes or no" concept. Instead, we consider how confident we can be with study findings based on whether the research avoids traps that may make those findings questionable. The less chance there is for "confounding," the higher the internal validity and the more confident we can be.
Confounding refers to uncontrollable variables that come into play and can confuse the outcome of a study, making us unsure of whether we can trust that we have identified the cause-and-effect relationship.
In short, you can only be confident that a study is internally valid if you can rule out alternative explanations for the findings. Three criteria are required to assume cause and effect in a research study:
- The cause preceded the effect in terms of time.
- The cause and effect vary together.
- There are no other likely explanations for the relationship observed.
Factors That Improve Internal Validity
To ensure the internal validity of a study, you want to consider aspects of the research design that will increase the likelihood that you can reject alternative hypotheses. Many factors can improve internal validity in research, including:
- Blinding : Participants—and sometimes researchers—are unaware of what intervention they are receiving (such as using a placebo on some subjects in a medication study) to avoid having this knowledge bias their perceptions and behaviors, thus impacting the study's outcome
- Experimental manipulation : Manipulating an independent variable in a study (for instance, giving smokers a cessation program) instead of just observing an association without conducting any intervention (examining the relationship between exercise and smoking behavior)
- Random selection : Choosing participants at random or in a manner in which they are representative of the population that you wish to study
- Randomization or random assignment : Randomly assigning participants to treatment and control groups, ensuring that there is no systematic bias between the research groups
- Strict study protocol : Following specific procedures during the study so as not to introduce any unintended effects; for example, doing things differently with one group of study participants than you do with another group
Internal Validity Threats
Just as there are many ways to ensure internal validity, there is also a list of potential threats that should be considered when planning a study.
- Attrition : Participants dropping out or leaving a study, which means that the results are based on a biased sample of only the people who did not choose to leave (and possibly who all have something in common, such as higher motivation)
- Confounding : A situation in which changes in an outcome variable can be thought to have resulted from some type of outside variable not measured or manipulated in the study
- Diffusion : This refers to the results of one group transferring to another through the groups interacting and talking with or observing one another; this can also lead to another issue called resentful demoralization, in which a control group tries less hard because they feel resentful over the group that they are in
- Experimenter bias : An experimenter behaving in a different way with different groups in a study, which can impact the results (and is eliminated through blinding)
- Historical events : May influence the outcome of studies that occur over a period of time, such as a change in the political leader or a natural disaster that occurs, influencing how study participants feel and act
- Instrumentation : This involves "priming" participants in a study in certain ways with the measures used, causing them to react in a way that is different than they would have otherwise reacted
- Maturation : The impact of time as a variable in a study; for example, if a study takes place over a period of time in which it is possible that participants naturally change in some way (i.e., they grew older or became tired), it may be impossible to rule out whether effects seen in the study were simply due to the impact of time
- Statistical regression : The natural effect of participants at extreme ends of a measure falling in a certain direction due to the passage of time rather than being a direct effect of an intervention
- Testing : Repeatedly testing participants using the same measures influences outcomes; for example, if you give someone the same test three times, it is likely that they will do better as they learn the test or become used to the testing process, causing them to answer differently
What Is External Validity in Research?
External validity refers to how well the outcome of a research study can be expected to apply to other settings. This is important because, if external validity is established, it means that the findings can be generalizable to similar individuals or populations.
External validity affirmatively answers the question: Do the findings apply to similar people, settings, situations, and time periods?
Population validity and ecological validity are two types of external validity. Population validity refers to whether you can generalize the research outcomes to other populations or groups. Ecological validity refers to whether a study's findings can be generalized to additional situations or settings.
Another term called transferability refers to whether results transfer to situations with similar characteristics. Transferability relates to external validity and refers to a qualitative research design.
Factors That Improve External Validity
If you want to improve the external validity of your study, there are many ways to achieve this goal. Factors that can enhance external validity include:
- Field experiments : Conducting a study outside the laboratory, in a natural setting
- Inclusion and exclusion criteria : Setting criteria as to who can be involved in the research, ensuring that the population being studied is clearly defined
- Psychological realism : Making sure participants experience the events of the study as being real by telling them a "cover story," or a different story about the aim of the study so they don't behave differently than they would in real life based on knowing what to expect or knowing the study's goal
- Replication : Conducting the study again with different samples or in different settings to see if you get the same results; when many studies have been conducted on the same topic, a meta-analysis can also be used to determine if the effect of an independent variable can be replicated, therefore making it more reliable
- Reprocessing or calibration : Using statistical methods to adjust for external validity issues, such as reweighting groups if a study had uneven groups for a particular characteristic (such as age)
External Validity Threats
External validity is threatened when a study does not take into account the interaction of variables in the real world. Threats to external validity include:
- Pre- and post-test effects : When the pre- or post-test is in some way related to the effect seen in the study, such that the cause-and-effect relationship disappears without these added tests
- Sample features : When some feature of the sample used was responsible for the effect (or partially responsible), leading to limited generalizability of the findings
- Selection bias : Also considered a threat to internal validity, selection bias describes differences between groups in a study that may relate to the independent variable—like motivation or willingness to take part in the study, or specific demographics of individuals being more likely to take part in an online survey
- Situational factors : Factors such as the time of day of the study, its location, noise, researcher characteristics, and the number of measures used may affect the generalizability of findings
While rigorous research methods can ensure internal validity, external validity may be limited by these methods.

Internal Validity vs. External Validity
Internal validity and external validity are two research concepts that share a few similarities while also having several differences.
Similarities
One of the similarities between internal validity and external validity is that both factors should be considered when designing a study. This is because both have implications in terms of whether the results of a study have meaning.
Both internal validity and external validity are not "either/or" concepts. Therefore, you always need to decide to what degree a study performs in terms of each type of validity.
Each of these concepts is also typically reported in research articles published in scholarly journals . This is so that other researchers can evaluate the study and make decisions about whether the results are useful and valid.
Differences
The essential difference between internal validity and external validity is that internal validity refers to the structure of a study (and its variables) while external validity refers to the universality of the results. But there are further differences between the two as well.
For instance, internal validity focuses on showing a difference that is due to the independent variable alone. Conversely, external validity results can be translated to the world at large.
Internal validity and external validity aren't mutually exclusive. You can have a study with good internal validity but be overall irrelevant to the real world. You could also conduct a field study that is highly relevant to the real world but doesn't have trustworthy results in terms of knowing what variables caused the outcomes.
Examples of Validity
Perhaps the best way to understand internal validity and external validity is with examples.
Internal Validity Example
An example of a study with good internal validity would be if a researcher hypothesizes that using a particular mindfulness app will reduce negative mood. To test this hypothesis, the researcher randomly assigns a sample of participants to one of two groups: those who will use the app over a defined period and those who engage in a control task.
The researcher ensures that there is no systematic bias in how participants are assigned to the groups. They do this by blinding the research assistants so they don't know which groups the subjects are in during the experiment.
A strict study protocol is also used to outline the procedures of the study. Potential confounding variables are measured along with mood , such as the participants' socioeconomic status, gender, age, and other factors. If participants drop out of the study, their characteristics are examined to make sure there is no systematic bias in terms of who stays in.
External Validity Example
An example of a study with good external validity would be if, in the above example, the participants used the mindfulness app at home rather than in the laboratory. This shows that results appear in a real-world setting.
To further ensure external validity, the researcher clearly defines the population of interest and chooses a representative sample . They might also replicate the study's results using different technological devices.
A Word From Verywell
Setting up an experiment so that it has both sound internal validity and external validity involves being mindful from the start about factors that can influence each aspect of your research.
It's best to spend extra time designing a structurally sound study that has far-reaching implications rather than to quickly rush through the design phase only to discover problems later on. Only when both internal validity and external validity are high can strong conclusions be made about your results.
San Jose State University. Internal and external validity .
Michael RS. Threats to internal & external validity: Y520 strategies for educational inquiry .
Pahus L, Burgel PR, Roche N, Paillasseur JL, Chanez P. Randomized controlled trials of pharmacological treatments to prevent COPD exacerbations: applicability to real-life patients . BMC Pulm Med . 2019;19(1):127. doi:10.1186/s12890-019-0882-y
By Arlin Cuncic, MA Arlin Cuncic, MA, is the author of The Anxiety Workbook and founder of the website About Social Anxiety. She has a Master's degree in clinical psychology.
9 Types of Validity in Research

Validity refers to whether or not a test or an experiment is actually doing what it is intended to do.
Validity sits upon a spectrum. For example:
- Low Validity: Most people now know that the standard IQ test does not actually measure intelligence or predict success in life.
- High Validity: By contrast, a standard pregnancy test is about 99% accurate , meaning it has very high validity and is therefore a very reliable test.
There are many ways to determine validity. Most of them are defined below.
Types of Validity
1. face validity.
Face validity refers to whether a scale “appears” to measure what it is supposed to measure. That is, do the questions seem to be logically related to the construct under study.
For example, a personality scale that measures emotional intelligence should have questions about self-awareness and empathy. It should not have questions about math or chemistry.
One common way to assess face validity is to ask a panel of experts to examine the scale and rate it’s appropriateness as a tool for measuring the construct. If the experts agree that the scale measures what it has been designed to measure, then the scale is said to have face validity.
If a scale, or a test, doesn’t have face validity, then people taking it won’t be serious.
Conbach explains it in the following way:
“When a patient loses faith in the medicine his doctor prescribes, it loses much of its power to improve his health. He may skip doses, and in the end may decide doctors cannot help him and let treatment lapse all together. For similar reasons, when selecting a test one must consider how worthwhile it will appear to the participant who takes it and other laymen who will see the results” (Cronbach, 1970, p. 182).
2. Content Validity
Content validity refers to whether a test or scale is measuring all of the components of a given construct. For example, if there are five dimensions of emotional intelligence (EQ), then a scale that measures EQ should contain questions regarding each dimension.
Similar to face validity, content validity can be assessed by asking subject matter experts (SMEs) to examine the test. If experts agree that the test includes items that assess every domain of the construct, then the test has content validity.
For example, the math portion of the SAT contains questions that require skills in many types of math: arithmetic, algebra, geometry, calculus, and many others. Since there are questions that assess each type of math, then the test has content validity.
The developer of the test could ask SMEs to rate the test’s construct validity. If the SMEs all give the test high ratings, then it has construct validity.
3. Construct Validity
Construct validity is the extent to which a measurement tool is truly assessing what it has been designed to assess.
There are two main methods of assessing construct validity: convergent and discriminant validity.
Convergent validity involves taking two tests that are supposed to measure the same construct and administering them to a sample of participants. The higher the correlation between the two tests, the stronger the construct validity.
With divergent validity, two tests that measure completely different constructs are administered to the same sample of participants. Since the tests are measuring different constructs, there should be a very low correlation between the two.
4. Internal Validity
Internal validity refers to whether or not the results of an experiment are due to the manipulation of the independent, or treatment, variables. For example, a researcher wants to examine how temperature affects willingness to help, so they have research participants wait in a room.
There are different rooms, one has the temperature set at normal, one at moderately warm, and the other at very warm.
During the next phase of the study, participants are asked to donate to a local charity before taking part in the rest of the study. The results showed that as the temperature of the room increased, donations decreased.
On the surface, it seems as though the study has internal validity: room temperature affected donations. However, even though the experiment involved three different rooms set at different temperatures, each room was a different size. The smallest room was the warmest and the normal temperature room was the largest.
Now, we don’t know if the donations were affected by room temperature or room size. So, the study has questionable internal validity.
Another way internal validity is assessed is through inter-rater reliability measures, which helps bolster both the validity and reliability of the study.
5. External Validity
External validity refers to whether the results of a study generalize to the real world or other situations. A lot of psychological studies take place in a university lab. Therefore, the setting is not very realistic.
This creates a big problem regarding external validity. Can we say that what happens in a lab would be the same thing that would happen in the real world?
For example, a study on mindfulness involves the researcher randomly assigning different research participants to use one of three mindfulness apps on their phones at home every night for 3 weeks. At the end of three weeks, their level of stress is measured with some high-tech EEG equipment.
This study has external validity because the participants used real apps and they were at home when using those apps. The apps and the home setting are realistic, so the study has external validity.
See More: Examples of External Validity
6. Concurrent Validity
Concurrent validity is a method of assessing validity that involves comparing a new test with an already existing test, or an already established criterion.
For example, a newly developed math test for the SAT will need to be validated before giving it to thousands of students. So, the new version of the test is administered to a sample of college math majors along with the old version of the test.
Scores on the two tests are compared by calculating a correlation between the two. The higher the correlation, the stronger the concurrent validity of the new test.
7. Predictive Validity
Predictive validity refers to whether scores on one test are associated with performance on a given criterion. That is, can a person’s score on the test predict their performance on the criterion?
For example, an IT company needs to hire dozens of programmers for an upcoming project. But conducting interviews with hundreds of applicants is time-consuming and not very accurate at identifying skilled coders.
So, the company develops a test that contains programming problems similar to the demands of the new project. The company assesses predictive validity of the test by having their current programmers take the test and then compare their scores with their yearly performance evaluations.
The results indicate that programmers with high marks in their evaluations also did very well on the test. Therefore, the test has predictive validity.
Now, when new applicants’ take the test, the company can predict how well they will do at the job in the future. People that do well on the predictor variable test will most likely do well at the job.
8. Statistical Conclusion Validity
Statistical conclusion validity refers to whether the conclusions drawn by the authors of a study are supported by the statistical procedures.
For example, did the study apply the correct statistical analyses, were adequate sampling procedures implemented, did the study use measurement tools that are valid and reliable?
If the answers to those questions are all “yes,” then the study has statistical conclusion validity. However, if the some or all of the answers are “no,” then the conclusions of the study are called into question.
Using the wrong statistical analyses or basing the conclusions on very small sample sizes, make the results questionable. If the results are based on faulty procedures, then the conclusions cannot be accepted as valid.
9. Criterion Validity
Criterion validity is sometimes called predictive validity. It refers to how well scores on one measurement device are associated with scores on a given performance domain (the criterion).
For example, how well do SAT scores predict college GPA? Or, to what extent are measures of consumer confidence related to the economy?
An example of low criterion validity is how poorly athletic performance at the NFL’s combine actually predicts performance on the field on gameday. There are dozens of tests that the athletes go through, but about 99% of them have no association with how well they do in games.
However, nutrition and exercise are highly related to longevity (the criterion). Those constructs have criterion validity because hundreds of studies have identified that nutrition and exercise are directly linked to living a longer and healthier life.
There are so many types of validity because the measurement precision of abstract concepts is hard to discern. There can also be confusion and disagreement among experts on the definition of constructs and how they should be measured.
For these reasons, social scientists have spent considerable time developing a variety of methods to assess the validity of their measurement tools. Sometimes this reveals ways to improve techniques, and sometimes it reveals the fallacy of trying to predict the future based on faulty assessment procedures.
Cook, T.D. and Campbell, D.T. (1979) Quasi-Experimentation: Design and Analysis Issues for Field Settings. Houghton Mifflin, Boston.
Cohen, R. J., & Swerdlik, M. E. (2005). Psychological testing and assessment: An introduction to tests and measurement (6th ed.). New York: McGraw-Hill.
Cronbach, L. J. (1970). Essentials of Psychological Testing . New York: Harper & Row.
Cronbach, L. J., and Meehl, P. E. (1955) Construct validity in psychological tests. Psychological Bulletin , 52 , 281-302.
Simms, L. (2007). Classical and Modern Methods of Psychological Scale Construction. Social and Personality Psychology Compass, 2 (1), 414 – 433. https://doi.org/10.1111/j.1751-9004.2007.00044.x

Dave Cornell (PhD)
Dr. Cornell has worked in education for more than 20 years. His work has involved designing teacher certification for Trinity College in London and in-service training for state governments in the United States. He has trained kindergarten teachers in 8 countries and helped businessmen and women open baby centers and kindergartens in 3 countries.
- Dave Cornell (PhD) https://helpfulprofessor.com/author/dave-cornell-phd/ 25 Positive Punishment Examples
- Dave Cornell (PhD) https://helpfulprofessor.com/author/dave-cornell-phd/ 25 Dissociation Examples (Psychology)
- Dave Cornell (PhD) https://helpfulprofessor.com/author/dave-cornell-phd/ 15 Zone of Proximal Development Examples
- Dave Cornell (PhD) https://helpfulprofessor.com/author/dave-cornell-phd/ Perception Checking: 15 Examples and Definition

Chris Drew (PhD)
This article was peer-reviewed and edited by Chris Drew (PhD). The review process on Helpful Professor involves having a PhD level expert fact check, edit, and contribute to articles. Reviewers ensure all content reflects expert academic consensus and is backed up with reference to academic studies. Dr. Drew has published over 20 academic articles in scholarly journals. He is the former editor of the Journal of Learning Development in Higher Education and holds a PhD in Education from ACU.
- Chris Drew (PhD) #molongui-disabled-link 25 Positive Punishment Examples
- Chris Drew (PhD) #molongui-disabled-link 25 Dissociation Examples (Psychology)
- Chris Drew (PhD) #molongui-disabled-link 15 Zone of Proximal Development Examples
- Chris Drew (PhD) #molongui-disabled-link Perception Checking: 15 Examples and Definition
Leave a Comment Cancel Reply
Your email address will not be published. Required fields are marked *

Research validity in surveys relates to the extent at which the survey measures right elements that need to be measured. In simple terms, validity refers to how well an instrument as measures what it is intended to measure.
Reliability alone is not enough, measures need to be reliable, as well as, valid. For example, if a weight measuring scale is wrong by 4kg (it deducts 4 kg of the actual weight), it can be specified as reliable, because the scale displays the same weight every time we measure a specific item. However, the scale is not valid because it does not display the actual weight of the item.
Research validity can be divided into two groups: internal and external. It can be specified that “internal validity refers to how the research findings match reality, while external validity refers to the extend to which the research findings can be replicated to other environments” (Pelissier, 2008, p.12).
Moreover, validity can also be divided into five types:
1. Face Validity is the most basic type of validity and it is associated with a highest level of subjectivity because it is not based on any scientific approach. In other words, in this case a test may be specified as valid by a researcher because it may seem as valid, without an in-depth scientific justification.
Example: questionnaire design for a study that analyses the issues of employee performance can be assessed as valid because each individual question may seem to be addressing specific and relevant aspects of employee performance.
2. Construct Validity relates to assessment of suitability of measurement tool to measure the phenomenon being studied. Application of construct validity can be effectively facilitated with the involvement of panel of ‘experts’ closely familiar with the measure and the phenomenon.
Example: with the application of construct validity the levels of leadership competency in any given organisation can be effectively assessed by devising questionnaire to be answered by operational level employees and asking questions about the levels of their motivation to do their duties in a daily basis.
3. Criterion-Related Validity involves comparison of tests results with the outcome. This specific type of validity correlates results of assessment with another criterion of assessment.
Example: nature of customer perception of brand image of a specific company can be assessed via organising a focus group. The same issue can also be assessed through devising questionnaire to be answered by current and potential customers of the brand. The higher the level of correlation between focus group and questionnaire findings, the high the level of criterion-related validity.
4. Formative Validity refers to assessment of effectiveness of the measure in terms of providing information that can be used to improve specific aspects of the phenomenon.
Example: when developing initiatives to increase the levels of effectiveness of organisational culture if the measure is able to identify specific weaknesses of organisational culture such as employee-manager communication barriers, then the level of formative validity of the measure can be assessed as adequate.
5. Sampling Validity (similar to content validity) ensures that the area of coverage of the measure within the research area is vast. No measure is able to cover all items and elements within the phenomenon, therefore, important items and elements are selected using a specific pattern of sampling method depending on aims and objectives of the study.
Example: when assessing a leadership style exercised in a specific organisation, assessment of decision-making style would not suffice, and other issues related to leadership style such as organisational culture, personality of leaders, the nature of the industry etc. need to be taken into account as well.
My e-book, The Ultimate Guide to Writing a Dissertation in Business Studies: a step by step assistance offers practical assistance to complete a dissertation with minimum or no stress. The e-book covers all stages of writing a dissertation starting from the selection to the research area to submitting the completed version of the work within the deadline. John Dudovskiy

- How it works
Reliability and Validity – Definitions, Types & Examples
Published by Alvin Nicolas at August 16th, 2021 , Revised On October 26, 2023
A researcher must test the collected data before making any conclusion. Every research design needs to be concerned with reliability and validity to measure the quality of the research.
What is Reliability?
Reliability refers to the consistency of the measurement. Reliability shows how trustworthy is the score of the test. If the collected data shows the same results after being tested using various methods and sample groups, the information is reliable. If your method has reliability, the results will be valid.
Example: If you weigh yourself on a weighing scale throughout the day, you’ll get the same results. These are considered reliable results obtained through repeated measures.
Example: If a teacher conducts the same math test of students and repeats it next week with the same questions. If she gets the same score, then the reliability of the test is high.
What is the Validity?
Validity refers to the accuracy of the measurement. Validity shows how a specific test is suitable for a particular situation. If the results are accurate according to the researcher’s situation, explanation, and prediction, then the research is valid.
If the method of measuring is accurate, then it’ll produce accurate results. If a method is reliable, then it’s valid. In contrast, if a method is not reliable, it’s not valid.
Example: Your weighing scale shows different results each time you weigh yourself within a day even after handling it carefully, and weighing before and after meals. Your weighing machine might be malfunctioning. It means your method had low reliability. Hence you are getting inaccurate or inconsistent results that are not valid.
Example: Suppose a questionnaire is distributed among a group of people to check the quality of a skincare product and repeated the same questionnaire with many groups. If you get the same response from various participants, it means the validity of the questionnaire and product is high as it has high reliability.
Most of the time, validity is difficult to measure even though the process of measurement is reliable. It isn’t easy to interpret the real situation.
Example: If the weighing scale shows the same result, let’s say 70 kg each time, even if your actual weight is 55 kg, then it means the weighing scale is malfunctioning. However, it was showing consistent results, but it cannot be considered as reliable. It means the method has low reliability.
Internal Vs. External Validity
One of the key features of randomised designs is that they have significantly high internal and external validity.
Internal validity is the ability to draw a causal link between your treatment and the dependent variable of interest. It means the observed changes should be due to the experiment conducted, and any external factor should not influence the variables .
Example: age, level, height, and grade.
External validity is the ability to identify and generalise your study outcomes to the population at large. The relationship between the study’s situation and the situations outside the study is considered external validity.
Also, read about Inductive vs Deductive reasoning in this article.
Looking for reliable dissertation support?
We hear you.
- Whether you want a full dissertation written or need help forming a dissertation proposal, we can help you with both.
- Get different dissertation services at ResearchProspect and score amazing grades!
Threats to Interval Validity
Threats of external validity, how to assess reliability and validity.
Reliability can be measured by comparing the consistency of the procedure and its results. There are various methods to measure validity and reliability. Reliability can be measured through various statistical methods depending on the types of validity, as explained below:
Types of Reliability
Types of validity.
As we discussed above, the reliability of the measurement alone cannot determine its validity. Validity is difficult to be measured even if the method is reliable. The following type of tests is conducted for measuring validity.
Does your Research Methodology Have the Following?
- Great Research/Sources
- Perfect Language
- Accurate Sources
If not, we can help. Our panel of experts makes sure to keep the 3 pillars of Research Methodology strong.

How to Increase Reliability?
- Use an appropriate questionnaire to measure the competency level.
- Ensure a consistent environment for participants
- Make the participants familiar with the criteria of assessment.
- Train the participants appropriately.
- Analyse the research items regularly to avoid poor performance.
How to Increase Validity?
Ensuring Validity is also not an easy job. A proper functioning method to ensure validity is given below:
- The reactivity should be minimised at the first concern.
- The Hawthorne effect should be reduced.
- The respondents should be motivated.
- The intervals between the pre-test and post-test should not be lengthy.
- Dropout rates should be avoided.
- The inter-rater reliability should be ensured.
- Control and experimental groups should be matched with each other.
How to Implement Reliability and Validity in your Thesis?
According to the experts, it is helpful if to implement the concept of reliability and Validity. Especially, in the thesis and the dissertation, these concepts are adopted much. The method for implementation given below:
Frequently Asked Questions
What is reliability and validity in research.
Reliability in research refers to the consistency and stability of measurements or findings. Validity relates to the accuracy and truthfulness of results, measuring what the study intends to. Both are crucial for trustworthy and credible research outcomes.
What is validity?
Validity in research refers to the extent to which a study accurately measures what it intends to measure. It ensures that the results are truly representative of the phenomena under investigation. Without validity, research findings may be irrelevant, misleading, or incorrect, limiting their applicability and credibility.
What is reliability?
Reliability in research refers to the consistency and stability of measurements over time. If a study is reliable, repeating the experiment or test under the same conditions should produce similar results. Without reliability, findings become unpredictable and lack dependability, potentially undermining the study’s credibility and generalisability.
What is reliability in psychology?
In psychology, reliability refers to the consistency of a measurement tool or test. A reliable psychological assessment produces stable and consistent results across different times, situations, or raters. It ensures that an instrument’s scores are not due to random error, making the findings dependable and reproducible in similar conditions.
What is test retest reliability?
Test-retest reliability assesses the consistency of measurements taken by a test over time. It involves administering the same test to the same participants at two different points in time and comparing the results. A high correlation between the scores indicates that the test produces stable and consistent results over time.
How to improve reliability of an experiment?
- Standardise procedures and instructions.
- Use consistent and precise measurement tools.
- Train observers or raters to reduce subjective judgments.
- Increase sample size to reduce random errors.
- Conduct pilot studies to refine methods.
- Repeat measurements or use multiple methods.
- Address potential sources of variability.
What is the difference between reliability and validity?
Reliability refers to the consistency and repeatability of measurements, ensuring results are stable over time. Validity indicates how well an instrument measures what it’s intended to measure, ensuring accuracy and relevance. While a test can be reliable without being valid, a valid test must inherently be reliable. Both are essential for credible research.
Are interviews reliable and valid?
Interviews can be both reliable and valid, but they are susceptible to biases. The reliability and validity depend on the design, structure, and execution of the interview. Structured interviews with standardised questions improve reliability. Validity is enhanced when questions accurately capture the intended construct and when interviewer biases are minimised.
Are IQ tests valid and reliable?
IQ tests are generally considered reliable, producing consistent scores over time. Their validity, however, is a subject of debate. While they effectively measure certain cognitive skills, whether they capture the entirety of “intelligence” or predict success in all life areas is contested. Cultural bias and over-reliance on tests are also concerns.
Are questionnaires reliable and valid?
Questionnaires can be both reliable and valid if well-designed. Reliability is achieved when they produce consistent results over time or across similar populations. Validity is ensured when questions accurately measure the intended construct. However, factors like poorly phrased questions, respondent bias, and lack of standardisation can compromise their reliability and validity.
You May Also Like
Textual analysis is the method of analysing and understanding the text. We need to look carefully at the text to identify the writer’s context and message.
Action research for my dissertation?, A brief overview of action research as a responsive, action-oriented, participative and reflective research technique.
What are the different types of research you can use in your dissertation? Here are some guidelines to help you choose a research strategy that would make your research more credible.
USEFUL LINKS
LEARNING RESOURCES

COMPANY DETAILS

- How It Works
- Skip to main content
- Skip to primary sidebar
- Skip to footer
- QuestionPro

- Solutions Industries Gaming Automotive Sports and events Education Government Travel & Hospitality Financial Services Healthcare Cannabis Technology Use Case NPS+ Communities Audience Contactless surveys Mobile LivePolls Member Experience GDPR Positive People Science 360 Feedback Surveys
- Resources Blog eBooks Survey Templates Case Studies Training Help center
Home Market Research
Reliability vs. Validity in Research: Types & Examples

When it comes to research, getting things right is crucial. That’s where the concepts of “Reliability vs Validity in Research” come in.
Imagine it like a balancing act – making sure your measurements are consistent and accurate at the same time. This is where test-retest reliability, having different researchers check things, and keeping things consistent within your research plays a big role.
As we dive into this topic, we’ll uncover the differences between reliability and validity, see how they work together, and learn how to use them effectively.
Understanding Reliability vs. Validity in Research
When it comes to collecting data and conducting research, two crucial concepts stand out: reliability and validity.
These pillars uphold the integrity of research findings, ensuring that the data collected and the conclusions drawn are both meaningful and trustworthy. Let’s dive into the heart of the concepts, reliability, and validity, to comprehend their significance in the realm of research truly.
What is reliability?
Reliability refers to the consistency and dependability of the data collection process. It’s like having a steady hand that produces the same result each time it reaches for a task.
In the research context, reliability is all about ensuring that if you were to repeat the same study using the same reliable measurement technique, you’d end up with the same results. It’s like having multiple researchers independently conduct the same experiment and getting outcomes that align perfectly.
Imagine you’re using a thermometer to measure the temperature of the water. You have a reliable measurement if you dip the thermometer into the water multiple times and get the same reading each time. This tells you that your method and measurement technique consistently produce the same results, whether it’s you or another researcher performing the measurement.
What is validity?
On the other hand, validity refers to the accuracy and meaningfulness of your data. It’s like ensuring that the puzzle pieces you’re putting together actually form the intended picture. When you have validity, you know that your method and measurement technique are consistent and capable of producing results aligned with reality.
Think of it this way; Imagine you’re conducting a test that claims to measure a specific trait, like problem-solving ability. If the test consistently produces results that accurately reflect participants’ problem-solving skills, then the test has high validity. In this case, the test produces accurate results that truly correspond to the trait it aims to measure.
In essence, while reliability assures you that your data collection process is like a well-oiled machine producing the same results, validity steps in to ensure that these results are not only consistent but also relevantly accurate.
Together, these concepts provide researchers with the tools to conduct research that stands on a solid foundation of dependable methods and meaningful insights.
Types of Reliability
Let’s explore the various types of reliability that researchers consider to ensure their work stands on solid ground.
High test-retest reliability
Test-retest reliability involves assessing the consistency of measurements over time. It’s like taking the same measurement or test twice – once and then again after a certain period. If the results align closely, it indicates that the measurement is reliable over time. Think of it as capturing the essence of stability.
Inter-rater reliability
When multiple researchers or observers are part of the equation, interrater reliability comes into play. This type of reliability assesses the level of agreement between different observers when evaluating the same phenomenon. It’s like ensuring that different pairs of eyes perceive things in a similar way.
Internal reliability
Internal consistency dives into the harmony among different items within a measurement tool aiming to assess the same concept. This often comes into play in surveys or questionnaires, where participants respond to various items related to a single construct. If the responses to these items consistently reflect the same underlying concept, the measurement is said to have high internal consistency.
Types of validity
Let’s explore the various types of validity that researchers consider to ensure their work stands on solid ground.
Content validity
It delves into whether a measurement truly captures all dimensions of the concept it intends to measure. It’s about making sure your measurement tool covers all relevant aspects comprehensively.
Imagine designing a test to assess students’ understanding of a history chapter. It exhibits high content validity if the test includes questions about key events, dates, and causes. However, if it focuses solely on dates and omits causation, its content validity might be questionable.
Construct validity
It assesses how well a measurement aligns with established theories and concepts. It’s like ensuring that your measurement is a true representation of the abstract construct you’re trying to capture.
Criterion validity
Criterion validity examines how well your measurement corresponds to other established measurements of the same concept. It’s about making sure your measurement accurately predicts or correlates with external criteria.
Differences between reliability and validity in research
Let’s delve into the differences between reliability and validity in research.
While both reliability and validity contribute to trustworthy research, they address distinct aspects. Reliability ensures consistent results, while validity ensures accurate and relevant results that reflect the true nature of the measured concept.
Example of Reliability and Validity in Research
In this section, we’ll explore instances that highlight the differences between reliability and validity and how they play a crucial role in ensuring the credibility of research findings.
Example of reliability
Imagine you are studying the reliability of a smartphone’s battery life measurement. To collect data, you fully charge the phone and measure the battery life three times in the same controlled environment—same apps running, same brightness level, and same usage patterns.
If the measurements consistently show a similar battery life duration each time you repeat the test, it indicates that your measurement method is reliable. The consistent results under the same conditions assure you that the battery life measurement can be trusted to provide dependable information about the phone’s performance.
Example of validity
Researchers collect data from a group of participants in a study aiming to assess the validity of a newly developed stress questionnaire. To ensure validity, they compare the scores obtained from the stress questionnaire with the participants’ actual stress levels measured using physiological indicators such as heart rate variability and cortisol levels.
If participants’ scores correlate strongly with their physiological stress levels, the questionnaire is valid. This means the questionnaire accurately measures participants’ stress levels, and its results correspond to real variations in their physiological responses to stress.
Validity assessed through the correlation between questionnaire scores and physiological measures ensures that the questionnaire is effectively measuring what it claims to measure participants’ stress levels.
In the world of research, differentiating between reliability and validity is crucial. Reliability ensures consistent results, while validity confirms accurate measurements. Using tools like QuestionPro enhances data collection for both reliability and validity. For instance, measuring self-esteem over time showcases reliability, and aligning questions with theories demonstrates validity.
QuestionPro empowers researchers to achieve reliable and valid results through its robust features, facilitating credible research outcomes. Contact QuestionPro to create a free account or learn more!
LEARN MORE FREE TRIAL
MORE LIKE THIS

NPS Survey Platform: Types, Tips, 11 Best Platforms & Tools
Apr 26, 2024
User Journey vs User Flow: Differences and Similarities
Best 7 Gap Analysis Tools to Empower Your Business
Apr 25, 2024

12 Best Employee Survey Tools for Organizational Excellence
Other categories.
- Academic Research
- Artificial Intelligence
- Assessments
- Brand Awareness
- Case Studies
- Communities
- Consumer Insights
- Customer effort score
- Customer Engagement
- Customer Experience
- Customer Loyalty
- Customer Research
- Customer Satisfaction
- Employee Benefits
- Employee Engagement
- Employee Retention
- Friday Five
- General Data Protection Regulation
- Insights Hub
- Life@QuestionPro
- Market Research
- Mobile diaries
- Mobile Surveys
- New Features
- Online Communities
- Question Types
- Questionnaire
- QuestionPro Products
- Release Notes
- Research Tools and Apps
- Revenue at Risk
- Survey Templates
- Training Tips
- Uncategorized
- Video Learning Series
- What’s Coming Up
- Workforce Intelligence
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Bras Pneumol
- v.44(3); May-Jun 2018
Internal and external validity: can you apply research study results to your patients?
Cecilia maria patino.
1 . Methods in Epidemiologic, Clinical, and Operations Research-MECOR-program, American Thoracic Society/Asociación Latinoamericana del Tórax, Montevideo, Uruguay.
2 . Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA, USA.
Juliana Carvalho Ferreira
3 . Divisão de Pneumologia, Instituto do Coração, Hospital das Clínicas, Faculdade de Medicina, Universidade de São Paulo, São Paulo (SP) Brasil.
CLINICAL SCENARIO
In a multicenter study in France, investigators conducted a randomized controlled trial to test the effect of prone vs. supine positioning ventilation on mortality among patients with early, severe ARDS. They showed that prolonged prone-positioning ventilation decreased 28-day mortality [hazard ratio (HR) = 0.39; 95% CI: 0.25-0.63]. 1
STUDY VALIDITY
The validity of a research study refers to how well the results among the study participants represent true findings among similar individuals outside the study. This concept of validity applies to all types of clinical studies, including those about prevalence, associations, interventions, and diagnosis. The validity of a research study includes two domains: internal and external validity.
Internal validity is defined as the extent to which the observed results represent the truth in the population we are studying and, thus, are not due to methodological errors. In our example, if the authors can support that the study has internal validity, they can conclude that prone positioning reduces mortality among patients with severe ARDS. The internal validity of a study can be threatened by many factors, including errors in measurement or in the selection of participants in the study, and researchers should think about and avoid these errors.
Once the internal validity of the study is established, the researcher can proceed to make a judgment regarding its external validity by asking whether the study results apply to similar patients in a different setting or not ( Figure 1 ). In the example, we would want to evaluate if the results of the clinical trial apply to ARDS patients in other ICUs. If the patients have early, severe ARDS, probably yes, but the study results may not apply to patients with mild ARDS . External validity refers to the extent to which the results of a study are generalizable to patients in our daily practice, especially for the population that the sample is thought to represent.

Lack of internal validity implies that the results of the study deviate from the truth, and, therefore, we cannot draw any conclusions; hence, if the results of a trial are not internally valid, external validity is irrelevant. 2 Lack of external validity implies that the results of the trial may not apply to patients who differ from the study population and, consequently, could lead to low adoption of the treatment tested in the trial by other clinicians.
INCREASING VALIDITY OF RESEARCH STUDIES
To increase internal validity, investigators should ensure careful study planning and adequate quality control and implementation strategies-including adequate recruitment strategies, data collection, data analysis, and sample size. External validity can be increased by using broad inclusion criteria that result in a study population that more closely resembles real-life patients, and, in the case of clinical trials, by choosing interventions that are feasible to apply. 2
- Open access
- Published: 19 April 2024
A first look at the reliability, validity and responsiveness of L-PF-35 dyspnea domain scores in fibrotic hypersensitivity pneumonitis
- Jeffrey J. Swigris ORCID: orcid.org/0000-0002-2643-8110 1 ,
- Kerri Aronson 2 &
- Evans R. Fernández Pérez 1
BMC Pulmonary Medicine volume 24 , Article number: 188 ( 2024 ) Cite this article
162 Accesses
Metrics details
Dyspnea impairs quality of life (QOL) in patients with fibrotic hypersensitivity pneumonitis (FHP). The Living with Pulmonary Fibrosis questionnaire (L-PF) assesses symptoms, their impacts and PF-related QOL in patients with any form of PF. Its scores have not undergone validation analyses in an FHP cohort.
We used data from the Pirfenidone in FHP trial to examine reliability, validity and responsiveness of the L-PF-35 Dyspnea domain score (Dyspnea) and to estimate its meaningful within-patient change (MWPC) threshold for worsening. Lack of suitable anchors precluded conducting analyses for other L-PF-35 scores.
At baseline, Dyspnea’s internal consistency (Cronbach’s coefficient alpha) was 0.85; there were significant correlations with all four anchors (University of California San Diego Shortness of Breath Questionnaire scores r = 0.81, St. George’s Activity domain score r = 0.82, percent predicted forced vital capacity r = 0.37, and percent predicted diffusing capacity of the lung for carbon monoxide r = 0.37). Dyspnea was significantly different between anchor subgroups (e.g., lowest percent predicted forced vital capacity (FVC%) vs. highest, 33.5 ± 18.5 vs. 11.1 ± 9.8, p = 0.01). There were significant correlations between changes in Dyspnea and changes in anchor scores at all trial time points. Longitudinal models further confirmed responsiveness. The MWPC threshold estimate for worsening was 6.6 points (range 5–8).
The L-PF-35 Dyspnea domain appears to possess acceptable psychometric properties for assessing dyspnea in patients with FHP. Because instrument validation is never accomplished with one study, additional research is needed to build on the foundation these analyses provide.
Trial registration
The data for the analyses presented in this manuscript were generated in a trial registered on ClinicalTrials.gov; the identifier was NCT02958917.
Peer Review reports
Introduction
Fibrotic hypersensitivity pneumonitis (FHP) is a form of fibrosing interstitial lung disease (fILD) that, like other fILDs is incurable, induces burdensome symptoms, confers the risk of shortened survival [ 1 , 2 ], and robs patients of their quality of life (QOL) [ 3 , 4 ]. Although in FHP there has not been as much research into the patient experience as with idiopathic pulmonary fibrosis (IPF), available data reveal that FHP-induced dyspnea, fatigue and cough affect how patients feel and function in their daily lives [ 3 , 4 ].
Given the potential for FHP to progress and respond poorly to immunosuppression and antigen avoidance (if one can be identified), Fernández Pérez and colleagues conducted a placebo-controlled trial of the antifibrotic, pirfenidone, in patients with FHP [ 5 ]. In that trial (Pirfenidone in FHP), among other patient-reported outcome measures (PROMs), the Living with Pulmonary Fibrosis (L-PF) questionnaire was used to examine the effects of pirfenidone on FHP-related QOL, symptoms and their impacts.
Here, we present findings from a hypothesis-based analysis of the reliability, validity and responsiveness of the Dyspnea domain from the 35-item L-PF (or L-PF-35; these 35 items are the same 35 that compose the Living with Idiopathic Pulmonary Fibrosis questionnaire (L-IPF) [ 6 ]).
The design and primary results for the single-center, double-blinded Pirfenidone in FHP trial (ClinicalTrials.gov identifier NCT02958917) from which the data for our analyses were generated have been published [ 5 ]. Briefly, 40 subjects with FHP were randomized 2:1 to receive pirfenidone or a matching placebo for 52 weeks. Study visits occurred at baseline, 13, 26, 39 and 52 weeks. At each visit, subjects completed three patient-reported outcome measures (PROMs) and performed spirometry to capture forced vital capacity (FVC). Diffusing capacity (DLCO) was assessed at baseline, 26 and 52 weeks only. This analysis was performed under an approved research protocol by the National Jewish Health central Institutional Review Board (HS# 3034).
PROMs used in the Pirfenidone in FHP trial
The l-pf-35 (living with pulmonary fibrosis 35-item questionnaire).
The L-PF-35 is designed to assess PF-related QOL, symptoms and their impacts. L-PF-35 is equivalent to the Living with Idiopathic Pulmonary Fibrosis Questionnaire (L-IPF) but with the word “idiopathic” removed from the title and a single item from the Impacts Module. L-IPF began as a 44-item questionnaire, but in a previously published validation study that included 125 patients with IPF, psychometric analyses supported reducing numbers from 44 to 35 items [ 6 ]. The intent of the developer of the L-PF is to have a single, 35-item questionnaire for all forms of PF (IPF and non-IPF, including FHP). Thus, although the 44-item version (again, with the word “idiopathic” removed) was administered in the Pirfenidone in FHP trial, our analyses here were conducted on the Dyspnea domain from the 35-item version resulting from the IPF analysis. From here on, we refer to this instrument as the L-PF-35.
Percentage-of-total-possible points is used to generate the Dyspnea domain, Cough domain, Energy/Fatigue domain, and Impacts module from the L-PF-35. The Symptoms module score is derived as the average of the Dyspnea, Cough and Energy/Fatigue domain scores. The total score is the average of the Symptoms and Impacts module scores. The Symptoms module contains 15 items (Dyspnea domain 7 items, Cough domain 5 items, Energy/Fatigue domain 3 items), each with a 24-hour recall period. The Impacts module contains 20 items, each with a 7-day recall period. The range for each of the six scores is 0-100, and higher scores connote greater impairment.
The SGRQ (St. George’s Respiratory Questionnaire)
The SGRQ is a 50-item questionnaire that yields four scores (total, Symptoms, Activity, Impacts). For the version used in the trial, the recall period for some items is three months and for others, it is “these days”. The range for each score is 0-100, and higher scores indicate worse respiratory health status [ 7 , 8 ].
The UCSD (University of California San Diego Shortness of Breath Questionnaire)
The UCSD is a 24-item questionnaire that assesses dyspnea severity while performing each of 21 activities, and it includes another 3 items that ask about limitations induced by shortness of breath [ 9 ]. Each item is scored on a 0–5 scale. There is no stated recall period. Scores range from 0 to 120, and higher scores indicate greater dyspnea severity.
Statistical analyses
Baseline data were tabulated and summarized using counts, percentages and measures of central tendency. We formulated hypotheses (included in the Supplementary material) for the L-PF-35 Dyspnea domain and conducted analyses in accordance with COSMIN recommendations for studies on the measurement properties of PROMs [ 10 , 11 ]. We used SGRQ Activity domain change scores, UCSD change scores, percent predicted FVC (FVC%) change, and percent predicted DLCO (DLCO%) change as anchors. Analyses included the following: (1) internal consistency and test-retest reliability, (2) convergent and known-groups analyses to assess content validity, (3) responsiveness, and (4) an estimation of the meaningful within-patient change (MWPC) threshold for worsening.
For applicable analyses, we defined worsening for the anchors in the following way: 1) ≥ 5 point increase for SGRQ Activity domain [ 12 , 13 ]; 2) ≥ 5 point increase in UCSD score [ 14 ]; 3) > 2% drop in FVC% (e.g., 70% to less than 68%) [ 15 ]; and 4) ≥ 5% drop in DLCO% (e.g., 70–65% or lower). Analyses were conducted in SAS, version 9.4 (SAS Institute Inc.; Cary, NC).
Internal consistency
We used Cronbach’s raw coefficient alpha as the measure of internal consistency (IC). Values > 0.7 are considered acceptable.
Test-retest reliability
We used a two-way mixed effects model for absolute agreement to generate the intraclass correlation coefficient (ICC (2,1)) as a measure of test-retest reliability of L-PF-35 Dyspnea domain scores (from baseline to week 26) among subjects considered stable according to change (also from baseline to week 26) scores for the various anchors. Values > 0.7 are considered acceptable.
Convergent and known-groups validity
Convergent validity was examined using pairwise Spearman correlations between L-PF-35 Dyspnea domain scores and anchors at baseline. We used analysis of variance with secondary, p-value corrected (Tukey) pairwise comparisons to look for statistically significant differences in L-PF-35 Dyspnea domain scores between most and least severe anchor subgroup strata (with anchors di- or trichotomized based on clinically relevant cut-points; e.g., FVC: ≤55, 55 < FVC < 70, or ≥ 70).
Responsiveness
We used pairwise correlation, longitudinal models and empirical cumulative distribution function (eCDF) plots to assess the responsiveness of L-PF-35 Dyspnea domain scores among subjects whose dyspnea changed as defined by the applicable anchor. In the correlational analyses, for 13-, 26-, 39- and 52-week timepoints, we examined pairwise Spearman correlations between L-PF-35 Dyspnea domain change scores and anchor change. In the modeling analyses, for each anchor, we built a repeated-measures, longitudinal model with L-PF-35 Dyspnea domain change score (from baseline to each subsequent time point) as the outcome variable and anchor change (from baseline to each subsequent time point) as the lone predictor variable. Visit (week 13, 26, 39, 52) was included in each model as a class variable, and an unstructured covariance structure was used (i.e., type = un in SAS). For the eCDF, we graphed the cumulative distribution of L-PF-35 Dyspnea domain change scores from baseline to week 26 for each of two dichotomized anchor change strata (worse vs. not at week 26 as defined above).
Meaningful within patient change (MWPC) threshold
We used predictive modeling (anchor as the outcome and L-PF-35 Dyspnea domain as the lone predictor) and adjustment for the correlation between L-PF-35 Dyspnea domain score change and anchor score change [ 16 ] to generate MWPC threshold estimates for worsening at 26 weeks. We used the method of Trigg and Griffiths [ 17 ] to generate a correlation-weighted point estimate.
Baseline characteristics and PROM scores from the trial population are presented in Table 1 . Most subjects were of non-Hispanic white ethnicity/race and supplemental oxygen users, with moderate pulmonary physiological impairment.
Internal consistency and test-retest reliability
IC for the L-PF-35 Dyspnea domain was at least 0.85 at all time points. Test-retest reliability (TRR) coefficients for L-PF-35 Dyspnea were 0.81 or greater for each anchor. Table S1 contains IC and TRR values.
Pairwise correlations at baseline are presented in Table 2 . Correlations between L-PF-35 Dyspnea domain scores and UCSD or SGRQ Activity scores are very strong, statistically significant and in the expected directions. Correlations between L-PF-35 Dyspnea and FVC% or DLCO% are low-moderately strong, statistically significant and in the expected directions.
Table 3 shows results for known-groups validity analyses. For each of the four anchors, compared to the least impaired anchor subgroup, L-PF-35 Dyspnea scores were significantly worse (i.e., higher and of large effect; e.g., worse by > 1 standard deviation) for the more impaired anchor subgroup.
Across study timepoints, 12 of 14 correlations between L-PF-35 Dyspnea domain score change and anchor change values were statistically significant and at least moderately strong (Table S2).
Longitudinal modeling showed significant ( p < 0.0001 for all) associations between L-PF-35 Dyspnea domain score change and anchor change values over the course of the trial (Fig. 1 ). Table S3 shows results for all longitudinal models.
eCDF plots of L-PF-35 Dyspnea domain 26-week change scores are displayed in Fig. 2 . They show separation between subgroups that worsened vs. not at 26 weeks according to each of the four anchors. Table 4 provides values of L-PF-35 Dyspnea domain 26-week change scores for the cohort using percentile cut-points.
MWPC threshold
Predictive modeling yielded estimates for MWPC for worsening in L-PF-35 Dyspnea domain scores of 6.3, 4.8, 8.0 and 6.9 for the four anchors: UCSD, SGRQ Activity, FVC%, and DLCO% respectively. The corresponding point-biserial correlations between L-PF-35 Dyspnea domain score change and the dichotomized UCSD, SGRQ Activity, FVC%, and DLCO% anchors (worse vs. not) were the following: 0.30, 0.49, 0.47, and 0.65. Thus, the weighted MWPC threshold estimate for worsening of L-PF-35 Dyspnea domain scores was 6.6 points (range 5–8).
In this study, we conducted analyses whose results offer a first glance at the psychometric properties of the L-PF-35 Dyspnea domain and support its reliability, validity and the responsiveness of its score as a measure of dyspnea in patients with FHP. Measurement experts and regulatory bodies have compiled criteria that, when met, deem clinical outcome assessments (COAs)– like PROMs– fit for the purpose of measuring outcomes in a target population [ 10 , 18 ]. The internal structure of the PROM must be sound, with sufficiently strong correlations among grouped items (internal consistency); PROM scores from respondents who are stable on the construct being measured should be similarly stable (test-retest reliability); PROM scores should differ between subgroups of respondents known– or hypothesized– to differ on the construct being measured (known-groups validity); and PROM scores should change for respondents who change on the underlying construct (responsiveness).
Because there are no gold standards for any of the constructs assessed by L-PF-35 scores (including dyspnea), anchors are employed as surrogates for gold standards, and hypotheses are formulated around the surrogates while incorporating the fit-for-purpose criteria outlined above. Anchors, themselves, must be suitable and ideally have undergone validity assessments of their own. Reassuringly, in their studies of patients with PF, other investigators have employed the anchors we used in our analyses [ 19 ]. Additionally, self-report anchors (like the UCSD and SGRQ Activity domain) generally surpass expert-endorsed suitability criteria [ 20 ], and the FVC and DLCO are universally accepted metrics of PF severity.
As hypothesized, the L-PF-35 Dyspnea domain surpassed the acceptability criteria (0.7) for internal consistency and test-retest reliability. Likewise, L-PF-35 Dyspnea domain scores distinguished respondents hypothesized to have the greatest dyspnea severity (e.g., those with the highest (worst) UCSD scores, highest (worst) SGRQ Activity scores, lowest FVC% or lowest DLCO%) from those with the least dyspnea severity. L-PF-35 Dyspnea domain change scores correlated with anchor change scores, and longitudinal modeling and eCDF plots further supported the L-PF-35 Dyspnea domain score as responsive to changes in dyspnea severity over time.
When the recall period for a PROM is 24 h, variability can be accommodated by averaging scores over a given time frame (e.g., a week). That was not done in the Pirfenidone in FHP trial. However, reassuringly, despite the difference in recall periods (L-PF-35 Dyspnea domain 24 h, UCSD no timeframe, SGRQ Activity domain three months), correlations between anchor change scores were generally moderately strong, statistically significant and always in the hypothesized directions. These results, and previously published data showing a < 1 point day-to-day variability in scores from the L-IPF Dyspnea domain scores over a 14 day period in 125 patients with IPF [ 6 ], provide indirect evidence that a single administration of L-PF-35 at each data collection timepoint/visit will likely suffice. And administration on consecutive days with averaging of scores is unlikely to yield significant differences from single administration.
In a previously published study, using different methodology than us, the MWPC threshold for deterioration in the L-PF-44 Dyspnea domain was estimated at 6–7 points in the INBUILD trial population (which included patients with all forms of PF, including FHP, who had progressed within 24 months of enrollment) [ 21 ]. The population in the Pirfenidone in FHP trial was similar to the INBUILD population; in both trials, subjects had to have fibrosis and meet the same progression criteria. In our MWPC analysis, we employed predictive modeling, which is argued to yield the most precise MWPC estimates [ 16 ]. We did not include distribution-based estimates, because they fail to capture patients’ perspectives, ignore the concept of “minimal”, and arguably, should not be included at all in MWPC estimates [ 22 , 23 ]. We used a weighting approach that appropriately incorporated the correlation between the L-PF-35 Dyspnea domain score change and anchor change. Doing so yields a less biased estimate than taking the mean or median of all estimates [ 17 ]. Regardless, it is reassuring that our point estimate perfectly aligns with the estimate generated from the INBUILD data.
Limitations
A lack of suitable anchors were available to conduct analyses for the other L-PF-35 scores, so those must be left for future studies (e.g., there were no cough or fatigue questionnaires included in the trial; SGRQ “total” and L-PF-35 “total” are similar in name but not necessarily in the constructs they capture. The same is true for the L-PF-35 Symptoms module and the SGRQ Symptoms domain). Moving forward, investigators would greatly help advance the science of measurement in the ILD field by including patient global impression (PGI) items for all the constructs being evaluated (e.g., here, these could have included PGI Dyspnea Severity, PGI Cough Severity/Frequency, PGI Fatigue Severity, PGI pulmonary fibrosis-related QOL or PGI general QOL). Additional limitations in our study include the low number of subjects (of predominantly the same ethnic/racial background) and the single-center design of the trial that generated the data, both of which potentially limit generalizing results to the broader FHP population. Because “validation” is not a threshold phenomenon and can not be achieved in a single study, our results should be viewed as only a first– but important– step in the process of confirming L-PF-35 Dyspnea domain scores as fit-for-purpose in this population. Additional research, including validation work, concept elicitation, and cognitive debriefing studies in patients with FHP and other non-IPF populations, is encouraged.
Conclusions
L-PF-35 Dyspnea domain scores appear to possess acceptable reliability, validity and responsiveness for assessing dyspnea severity in patients with FHP. Additional studies are needed to further support its validity and to assess the psychometric properties of the other five L-PF-35 scores for assessing their respective constructs. For now, it is reasonable to use 5–8 points as the estimated range for the MWPC threshold for worsening for the L-PF-35 Dyspnea domain in patients with FHP.
Results for mixed-effects longitudinal models showing the relationship between baseline-to-weeks 13/26/39/52 changes in L-PF-35 Dyspnea domain scores and baseline-to-weeks 13/26/39/52 changes in anchor values (Panel A: UCSD anchor, Panel B: SGRQ Activity Domain anchor, Panel C: FVC% anchor, Panel D: DLCO% anchor). Footnote: UCSD = University of California San Diego Shortness of Breath Questionnaire; SGRQ = St. George’s Respiratory Questionnaire; FVC% = percentage of the predicted forced vital capacity; DLCO% = percentage of the predicted diffusing capacity of the lung for carbon monoxide; L-PF = 35-item Living with Pulmonary Fibrosis Questionnaire
CDF (Cumulative Distribution Function) plots showing baseline-to-week 26 changes in L-PF-35 Dyspnea domain scores for subgroups defined by anchor change, worse or not from baseline to week 26 (Panel A: UCSD anchor, Panel B: SGRQ Activity Domain anchor, Panel C: FVC% anchor, Panel D: DLCO% anchor) values. Footnote: Red = worsened according to anchor; Blue = not worsened (stable/improved) according to anchor; UCSD = University of California San Diego Shortness of Breath Questionnaire; SGRQ = St. George’s Respiratory Questionnaire; FVC% = percentage of the predicted forced vital capacity; DLCO% = percentage of the predicted diffusing capacity of the lung for carbon monoxide; L-PF = 35-item Living with Pulmonary Fibrosis Questionnaire. Definitions for anchors worsened: 1) ≥ 5 point increase for SGRQ Activity domain; 2) ≥ 5 point increase in UCSD score; 3) > 2% drop in FVC% (e.g., 70% to less than 68%); and 4) ≥ 5% drop in DLCO% (e.g., 70–65% or lower)
Data availability
Data are not publicly available. Parties interested in accessing the data used in this study are encouraged to contact Dr. Fernandez Perez ([email protected]).
Fernandez Perez ER, Swigris JJ, Forssen AV, Tourin O, Solomon JJ, Huie TJ, Olson AL, Brown KK. Identifying an inciting antigen is associated with improved survival in patients with chronic hypersensitivity pneumonitis. Chest. 2013;144:1644–51.
Article PubMed PubMed Central Google Scholar
Hanak V, Golbin JM, Ryu JH. Causes and presenting features in 85 consecutive patients with hypersensitivity pneumonitis. Mayo Clin Proc. 2007;82:812–6.
Article PubMed Google Scholar
Aronson KI, Hayward BJ, Robbins L, Kaner RJ, Martinez FJ, Safford MM. It’s difficult, it’s life changing what happens to you’ patient perspective on life with chronic hypersensitivity pneumonitis: a qualitative study. BMJ Open Resp Res. 2019;6:e000522.
Article PubMed Central Google Scholar
Lubin M, Chen H, Elicker B, Jones KD, Collard HR, Lee JS. A Comparison of Health-Related Quality of Life in Idiopathic Pulmonary Fibrosis and Chronic Hypersensitivity Pneumonitis. Chest. 2014.
Fernandez Perez ER, Crooks JL, Lynch DA, Humphries SM, Koelsch TL, Swigris JJ, Solomon JJ, Mohning MP, Groshong SD, Fier K. Pirfenidone in fibrotic hypersensitivity pneumonitis: a double-blind, randomised clinical trial of efficacy and safety. Thorax. 2023.
Swigris JJ, Andrae DA, Churney T, Johnson N, Scholand MB, White ES, Matsui A, Raimundo K, Evans CJ. Development and initial validation analyses of the living with idiopathic pulmonary fibrosis questionnaire. Am J Respir Crit Care Med. 2020;202:1689–97.
Jones PW, Quirk FH, Baveystock CM. The St George’s Respiratory Questionnaire. Respiratory medicine 1991; 85 Suppl B: 25–31; discussion 33– 27.
Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George’s respiratory questionnaire. Am Rev Respir Dis. 1992;145:1321–7.
Article CAS PubMed Google Scholar
Eakin EG, Resnikoff PM, Prewitt LM, Ries AL, Kaplan RM. Validation of a new dyspnea measure: the UCSD Shortness of Breath Questionnaire. University of California, San Diego. Chest. 1998; 113: 619–624.
Mokkink LB, Terwee CB, Patrick DL, Alonso J, Stratford PW, Knol DL, Bouter LM, de Vet HC. The COSMIN checklist for assessing the methodological quality of studies on measurement properties of health status measurement instruments: an international Delphi study. Qual life Research: Int J Qual life Aspects Treat care Rehabilitation. 2010;19:539–49.
Article Google Scholar
Terwee CB, Prinsen CAC, Chiarotto A, Westerman MJ, Patrick DL, Alonso J, Bouter LM, de Vet HCW, Mokkink LB. COSMIN methodology for evaluating the content validity of patient-reported outcome measures: a Delphi study. Qual life Research: Int J Qual life Aspects Treat care Rehabilitation. 2018;27:1159–70.
Article CAS Google Scholar
Swigris JJ, Brown KK, Behr J, du Bois RM, King TE, Raghu G, Wamboldt FS. The SF-36 and SGRQ: validity and first look at minimum important differences in IPF. Respir Med. 2010;104:296–304.
Swigris JJ, Wilson H, Esser D, Conoscenti CS, Stansen W, Kline Leidy N, Brown KK. Psychometric properties of the St George’s respiratory questionnaire in patients with idiopathic pulmonary fibrosis: insights from the INPULSIS trials. BMJ open Respiratory Res. 2018;5:e000278.
Chen T, Tsai APY, Hur SA, Wong AW, Sadatsafavi M, Fisher JH, Johannson KA, Assayag D, Morisset J, Shapera S, Khalil N, Fell CD, Manganas H, Cox G, To T, Gershon AS, Hambly N, Halayko AJ, Wilcox PG, Kolb M, Ryerson CJ. Validation and minimum important difference of the UCSD Shortness of Breath Questionnaire in fibrotic interstitial lung disease. Respir Res. 2021;22:202.
Article CAS PubMed PubMed Central Google Scholar
du Bois RM, Weycker D, Albera C, Bradford WZ, Costabel U, Kartashov A, King TE, Lancaster L, Noble PW, Sahn SA, Thomeer M, Valeyre D, Wells AU. Forced Vital Capacity in Patients with Idiopathic Pulmonary Fibrosis: Test Properties and Minimal Clinically Important Difference. American journal of respiratory and critical care medicine. 2011.
Terluin B, Eekhout I, Terwee C, De Vet H. Minimal important change (MIC) based on a predictive modeling approach was more precise than MIC based on ROC analysis. J Clin Epidemiol 2015; 68.
Trigg A, Griffiths P. Triangulation of multiple meaningful change thresholds for patient-reported outcome scores. Qual life Research: Int J Qual life Aspects Treat care Rehabilitation. 2021;30:2755–64.
US Department of Health and Human Services and the Food and Drug Administration (CDER). Guidance for Industry, Food and Drug Administration Staff, and other stakeholders: patient-focused Drug Development. Incorporating Clinical Outcome Assessments Into Endpoints for Regulatory Decision-Making Silver Spring, MD; 2023.
Swigris JJ, Esser D, Conoscenti CS, Brown KK. The psychometric properties of the St George’s respiratory questionnaire (SGRQ) in patients with idiopathic pulmonary fibrosis: a literature review. Health Qual Life Outcomes. 2014;12:124.
Devji T, Carrasco-Labra A, Qasim A, Phillips M, Johnston BC, Devasenapathy N, Zeraatkar D, Bhatt M, Jin X, Brignardello-Petersen R, Urquhart O, Foroutan F, Schandelmaier S, Pardo-Hernandez H, Vernooij RW, Huang H, Rizwan Y, Siemieniuk R, Lytvyn L, Patrick DL, Ebrahim S, Furukawa T, Nesrallah G, Schunemann HJ, Bhandari M, Thabane L, Guyatt GH. Evaluating the credibility of anchor based estimates of minimal important differences for patient reported outcomes: instrument development and reliability study. BMJ. 2020;369:m1714.
Swigris JJ, Bushnell DM, Rohr K, Mueller H, Baldwin M, Inoue Y. Responsiveness and meaningful change thresholds of the living with pulmonary fibrosis (L-PF) questionnaire Dyspnoea and Cough scores in patients with progressive fibrosing interstitial lung diseases. BMJ open Respiratory Res 2022; 9.
Swigris J, Foster B, Johnson N. Determining and reporting minimal important change for patient-reported outcome instruments in pulmonary medicine. Eur Respir J 2022; 60.
Terwee CB, Peipert JD, Chapman R, Lai JS, Terluin B, Cella D, Griffith P, Mokkink LB. Minimal important change (MIC): a conceptual clarification and systematic review of MIC estimates of PROMIS measures. Qual life Research: Int J Qual life Aspects Treat care Rehabilitation. 2021;30:2729–54.
Download references
Acknowledgements
Authors’ information (This is optional): N/A.
There was no funding for this study. Genentech/Roche was the sponsor of the Pirfenidone in Chronic HP trial.
Author information
Authors and affiliations.
Center for Interstitial Lung Disease, National Jewish Health, 1400 Jackson Street, G07, 80206, Denver, CO, USA
Jeffrey J. Swigris & Evans R. Fernández Pérez
Division of Pulmonary and Critical Care Medicine, Weill Cornell College of Medicine, New York, NY, USA
Kerri Aronson
You can also search for this author in PubMed Google Scholar
Contributions
Study conceptualization: JJS, KA, ERFP. Data acquisition: ERFP. Data analysis: JJS. Interpretation of results: JJS, KA, ERFP. Manuscript preparation and approval of submitted version: JJS, KA, ERFP.
Corresponding author
Correspondence to Jeffrey J. Swigris .
Ethics declarations
Ethics approval and consent to participate.
This analysis was performed under an approved research protocol by the National Jewish Health central Institutional Review Board (HS# 3034). All methods were carried out in accordance with relevant guidelines and regulations. Informed consent was obtained from all subjects enrolled in the trial.
Consent for publication
Not applicable.
Competing interests
JJS is the developer of L-PF-44, L-PF-35 and other questionnaires designed to assess outcomes in patients with various forms of interstitial lung disease. KA and ERFP report no conflict related to this study.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Take-home message: Our analyses begin to build the foundation supporting scores from the 35-item Living with Pulmonary Fibrosis Dyspnea domain as possessing psychometric characteristics that make it a suitable measure of dyspnea severity in patients with fibrotic hypersensitivity pneumonitis. The estimate for the meaningful within patient threshold for deterioration in this patient population is 6.6 points with a range of 5–8.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Swigris, J.J., Aronson, K. & R. Fernández Pérez, E. A first look at the reliability, validity and responsiveness of L-PF-35 dyspnea domain scores in fibrotic hypersensitivity pneumonitis. BMC Pulm Med 24 , 188 (2024). https://doi.org/10.1186/s12890-024-02991-1
Download citation
Received : 25 July 2023
Accepted : 02 April 2024
Published : 19 April 2024
DOI : https://doi.org/10.1186/s12890-024-02991-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Hypersensitivity pneumonitis
- Patient-reported outcome
BMC Pulmonary Medicine
ISSN: 1471-2466
- Submission enquiries: [email protected]
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
The 4 Types of Validity in Research | Definitions & Examples. Published on September 6, 2019 by Fiona Middleton.Revised on June 22, 2023. Validity tells you how accurately a method measures something. If a method measures what it claims to measure, and the results closely correspond to real-world values, then it can be considered valid.
Research validity refers to the degree to which a study accurately measures or reflects what it claims to measure. In other words, research validity concerns whether the conclusions drawn from a study are based on accurate, reliable and relevant data. ... Here are some examples of validity in different contexts: Logical Validity: Example 1: All ...
In psychology research, validity refers to the extent to which a test or measurement tool accurately measures what it's intended to measure. It ensures that the research findings are genuine and not due to extraneous factors. Validity can be categorized into different types, including construct validity (measuring the intended abstract trait), internal validity (ensuring causal conclusions ...
Face validity. Face validity considers how suitable the content of a test seems to be on the surface. It's similar to content validity, but face validity is a more informal and subjective assessment. Example: Face validity. You create a survey to measure the regularity of people's dietary habits. You review the survey items, which ask ...
Validity in research is the ability to conduct an accurate study with the right tools and conditions to yield acceptable and reliable data that can be reproduced. Researchers rely on carefully calibrated tools for precise measurements. However, collecting accurate information can be more of a challenge.
In this vein, there are many different types of validity and ways of thinking about it. Let's take a look at several of the more common types. Each kind is a line of evidence that can help support or refute a test's overall validity. In this post, learn about face, content, criterion, discriminant, concurrent, predictive, and construct ...
Validity is an important concept in establishing qualitative research rigor. At its core, validity in research speaks to the degree to which a study accurately reflects or assesses the specific concept that the researcher is attempting to measure or understand. It's about ensuring that the study investigates what it purports to investigate.
For this reason, we are going to look at various validity types that have been formulated as a part of legitimate research methodology. Here are the 7 key types of validity in research: Face validity. Content validity. Construct validity. Internal validity. External validity. Statistical conclusion validity.
Validity and reliability are two related but distinctly different concepts within research. Understanding what they are and how to achieve them is critically important to any research project. In this post, we'll unpack these two concepts as simply as possible. This post is based on our popular online course, Research Methodology Bootcamp. In ...
Validity can be demonstrated by showing a clear relationship between the test and what it is meant to measure. This can be done by showing that a study has one (or more) of the four types of validity: content validity, criterion-related validity, construct validity, and/or face validity. Understanding Methods for Research in Psychology.
The concept of validity is also applied to research studies and their findings. Internal validity examines whether the study design, conduct, and analysis answer the research questions without bias. External validity examines whether the study findings can be generalized to other contexts. Ecological validity examines, specifically, whether the ...
OVERVIEW. In general terms, validity is "the quality of being true or correct", it refers to the strength of results and how accurately they reflect the real world. Thus 'validity' can have quite different meanings depending on the context! Reliability is distinct from validity, in that it refers to the consistency or repeatability of ...
Differences. The essential difference between internal validity and external validity is that internal validity refers to the structure of a study (and its variables) while external validity refers to the universality of the results. But there are further differences between the two as well. For instance, internal validity focuses on showing a ...
In assessing validity of qualitative research, the challenge can start from the ontology and epistemology of the issue being studied, ... Set off in different pathways, qualitative research regarding the individual's wellbeing will be concluded with varying validity. Choice of methodology must enable detection of findings/phenomena in the ...
Types of Validity. 1. Face Validity. Face validity refers to whether a scale "appears" to measure what it is supposed to measure. That is, do the questions seem to be logically related to the construct under study. For example, a personality scale that measures emotional intelligence should have questions about self-awareness and empathy.
Creswell and Millers' work advances the debate on validity in qualitative research in several ways. It elegantly unites different worldviews or paradigms within qualitative research with key perspectives by which the validity of qualitative research can be assessed: that of the researcher, the respondent, and the external reader.
Research validity in surveys relates to the extent at which the survey measures right elements that need to be measured. In simple terms, validity refers to how well an instrument as measures what it is intended to measure. Reliability alone is not enough, measures need to be reliable, as well as, valid. For example, if a weight measuring scale ...
Reliability refers to the consistency of the measurement. Reliability shows how trustworthy is the score of the test. If the collected data shows the same results after being tested using various methods and sample groups, the information is reliable. If your method has reliability, the results will be valid. Example: If you weigh yourself on a ...
That's where the concepts of "Reliability vs Validity in Research" come in. Imagine it like a balancing act - making sure your measurements are consistent and accurate at the same time. This is where test-retest reliability, having different researchers check things, and keeping things consistent within your research plays a big role.
The validity of a research study includes two domains: internal and external validity. Internal validity is defined as the extent to which the observed results represent the truth in the population we are studying and, thus, are not due to methodological errors. In our example, if the authors can support that the study has internal validity ...
Validity in research is composed two parts, internal validity and external validity. Internal validity is the extent in which a study is legitimate based on the way the sample group was
Research outcomes in mental health disciplines are usually assessed using rating instruments that were developed as English language versions. However, in countries such as India, English is not the native language, and patients at even a single research center may speak in different regional tongues.
Dyspnea impairs quality of life (QOL) in patients with fibrotic hypersensitivity pneumonitis (FHP). The Living with Pulmonary Fibrosis questionnaire (L-PF) assesses symptoms, their impacts and PF-related QOL in patients with any form of PF. Its scores have not undergone validation analyses in an FHP cohort. We used data from the Pirfenidone in FHP trial to examine reliability, validity and ...
This research presents four studies that developed and validated the Organizational Climate Perception Scale for Public Service (OCPS-PS). The first qualitative study consulted the literature and conducted a focus group to develop the initial version of the scale. The second study involved expert evaluation and pre-testing, aiming at the semantic and face validation of the items. This study ...