Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, generate accurate citations for free.
- Knowledge Base

Hypothesis Testing | A Step-by-Step Guide with Easy Examples
Published on November 8, 2019 by Rebecca Bevans . Revised on June 22, 2023.
Hypothesis testing is a formal procedure for investigating our ideas about the world using statistics . It is most often used by scientists to test specific predictions, called hypotheses, that arise from theories.
There are 5 main steps in hypothesis testing:
- State your research hypothesis as a null hypothesis and alternate hypothesis (H o ) and (H a or H 1 ).
- Collect data in a way designed to test the hypothesis.
- Perform an appropriate statistical test .
- Decide whether to reject or fail to reject your null hypothesis.
- Present the findings in your results and discussion section.
Though the specific details might vary, the procedure you will use when testing a hypothesis will always follow some version of these steps.
Table of contents
Step 1: state your null and alternate hypothesis, step 2: collect data, step 3: perform a statistical test, step 4: decide whether to reject or fail to reject your null hypothesis, step 5: present your findings, other interesting articles, frequently asked questions about hypothesis testing.
After developing your initial research hypothesis (the prediction that you want to investigate), it is important to restate it as a null (H o ) and alternate (H a ) hypothesis so that you can test it mathematically.
The alternate hypothesis is usually your initial hypothesis that predicts a relationship between variables. The null hypothesis is a prediction of no relationship between the variables you are interested in.
- H 0 : Men are, on average, not taller than women. H a : Men are, on average, taller than women.
Here's why students love Scribbr's proofreading services
Discover proofreading & editing
For a statistical test to be valid , it is important to perform sampling and collect data in a way that is designed to test your hypothesis. If your data are not representative, then you cannot make statistical inferences about the population you are interested in.
There are a variety of statistical tests available, but they are all based on the comparison of within-group variance (how spread out the data is within a category) versus between-group variance (how different the categories are from one another).
If the between-group variance is large enough that there is little or no overlap between groups, then your statistical test will reflect that by showing a low p -value . This means it is unlikely that the differences between these groups came about by chance.
Alternatively, if there is high within-group variance and low between-group variance, then your statistical test will reflect that with a high p -value. This means it is likely that any difference you measure between groups is due to chance.
Your choice of statistical test will be based on the type of variables and the level of measurement of your collected data .
- an estimate of the difference in average height between the two groups.
- a p -value showing how likely you are to see this difference if the null hypothesis of no difference is true.
Based on the outcome of your statistical test, you will have to decide whether to reject or fail to reject your null hypothesis.
In most cases you will use the p -value generated by your statistical test to guide your decision. And in most cases, your predetermined level of significance for rejecting the null hypothesis will be 0.05 – that is, when there is a less than 5% chance that you would see these results if the null hypothesis were true.
In some cases, researchers choose a more conservative level of significance, such as 0.01 (1%). This minimizes the risk of incorrectly rejecting the null hypothesis ( Type I error ).
Prevent plagiarism. Run a free check.
The results of hypothesis testing will be presented in the results and discussion sections of your research paper , dissertation or thesis .
In the results section you should give a brief summary of the data and a summary of the results of your statistical test (for example, the estimated difference between group means and associated p -value). In the discussion , you can discuss whether your initial hypothesis was supported by your results or not.
In the formal language of hypothesis testing, we talk about rejecting or failing to reject the null hypothesis. You will probably be asked to do this in your statistics assignments.
However, when presenting research results in academic papers we rarely talk this way. Instead, we go back to our alternate hypothesis (in this case, the hypothesis that men are on average taller than women) and state whether the result of our test did or did not support the alternate hypothesis.
If your null hypothesis was rejected, this result is interpreted as “supported the alternate hypothesis.”
These are superficial differences; you can see that they mean the same thing.
You might notice that we don’t say that we reject or fail to reject the alternate hypothesis . This is because hypothesis testing is not designed to prove or disprove anything. It is only designed to test whether a pattern we measure could have arisen spuriously, or by chance.
If we reject the null hypothesis based on our research (i.e., we find that it is unlikely that the pattern arose by chance), then we can say our test lends support to our hypothesis . But if the pattern does not pass our decision rule, meaning that it could have arisen by chance, then we say the test is inconsistent with our hypothesis .
If you want to know more about statistics , methodology , or research bias , make sure to check out some of our other articles with explanations and examples.
- Normal distribution
- Descriptive statistics
- Measures of central tendency
- Correlation coefficient
Methodology
- Cluster sampling
- Stratified sampling
- Types of interviews
- Cohort study
- Thematic analysis
Research bias
- Implicit bias
- Cognitive bias
- Survivorship bias
- Availability heuristic
- Nonresponse bias
- Regression to the mean
Hypothesis testing is a formal procedure for investigating our ideas about the world using statistics. It is used by scientists to test specific predictions, called hypotheses , by calculating how likely it is that a pattern or relationship between variables could have arisen by chance.
A hypothesis states your predictions about what your research will find. It is a tentative answer to your research question that has not yet been tested. For some research projects, you might have to write several hypotheses that address different aspects of your research question.
A hypothesis is not just a guess — it should be based on existing theories and knowledge. It also has to be testable, which means you can support or refute it through scientific research methods (such as experiments, observations and statistical analysis of data).
Null and alternative hypotheses are used in statistical hypothesis testing . The null hypothesis of a test always predicts no effect or no relationship between variables, while the alternative hypothesis states your research prediction of an effect or relationship.
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the “Cite this Scribbr article” button to automatically add the citation to our free Citation Generator.
Bevans, R. (2023, June 22). Hypothesis Testing | A Step-by-Step Guide with Easy Examples. Scribbr. Retrieved March 22, 2024, from https://www.scribbr.com/statistics/hypothesis-testing/
Is this article helpful?
Rebecca Bevans
Other students also liked, choosing the right statistical test | types & examples, understanding p values | definition and examples.
Hypothesis and hypothesis testing in the clinical trial
Affiliation.
- 1 Department of Psychiatry and Mental Health and Neuroscience Clinical Research Center, University of North Carolina School of Medicine, Chapel Hill 27599-7160, USA.
- PMID: 11379832
The hypothesis provides the justification for the clinical trial. It is antecedent to the trial and establishes the trial's direction. Hypothesis testing is the most widely employed method of determining whether the outcome of clinical trials is positive or negative. Too often, however, neither the hypothesis nor the statistical information necessary to evaluate outcomes, such as p values and alpha levels, is stated explicitly in reports of clinical trials. This article examines 5 recent studies comparing atypical antipsychotics with special attention to how they approach the hypothesis and hypothesis testing. Alternative approaches are also discussed.
- Antipsychotic Agents / therapeutic use*
- Benzodiazepines
- Clinical Trials as Topic / methods*
- Clinical Trials as Topic / standards
- Clinical Trials as Topic / statistics & numerical data
- Dibenzothiazepines / therapeutic use
- Periodicals as Topic / standards
- Pirenzepine / analogs & derivatives*
- Pirenzepine / therapeutic use
- Psychotic Disorders / drug therapy
- Publishing / standards
- Quetiapine Fumarate
- Reproducibility of Results
- Research Design* / standards
- Risperidone / therapeutic use
- Schizophrenia / drug therapy*
- Terminology as Topic
- Antipsychotic Agents
- Dibenzothiazepines
- Pirenzepine
- Risperidone

- Physician Physician Board Reviews Physician Associate Board Reviews CME Lifetime CME Free CME
- Student USMLE Step 1 USMLE Step 2 USMLE Step 3 COMLEX Level 1 COMLEX Level 2 COMLEX Level 3 96 Medical School Exams Student Resource Center NCLEX - RN NCLEX - LPN/LVN/PN 24 Nursing Exams
- Nurse Practitioner APRN/NP Board Reviews CNS Certification Reviews CE - Nurse Practitioner FREE CE
- Nurse RN Certification Reviews CE - Nurse FREE CE
- Pharmacist Pharmacy Board Exam Prep CE - Pharmacist
- Allied Allied Health Exam Prep Dentist Exams CE - Social Worker CE - Dentist
- Point of Care
- Free CME/CE
Hypothesis Testing, P Values, Confidence Intervals, and Significance
Definition/introduction.
Medical providers often rely on evidence-based medicine to guide decision-making in practice. Often a research hypothesis is tested with results provided, typically with p values, confidence intervals, or both. Additionally, statistical or research significance is estimated or determined by the investigators. Unfortunately, healthcare providers may have different comfort levels in interpreting these findings, which may affect the adequate application of the data.
Issues of Concern
Register for free and read the full article, learn more about a subscription to statpearls point-of-care.
Without a foundational understanding of hypothesis testing, p values, confidence intervals, and the difference between statistical and clinical significance, it may affect healthcare providers' ability to make clinical decisions without relying purely on the research investigators deemed level of significance. Therefore, an overview of these concepts is provided to allow medical professionals to use their expertise to determine if results are reported sufficiently and if the study outcomes are clinically appropriate to be applied in healthcare practice.
Hypothesis Testing
Investigators conducting studies need research questions and hypotheses to guide analyses. Starting with broad research questions (RQs), investigators then identify a gap in current clinical practice or research. Any research problem or statement is grounded in a better understanding of relationships between two or more variables. For this article, we will use the following research question example:
Research Question: Is Drug 23 an effective treatment for Disease A?
Research questions do not directly imply specific guesses or predictions; we must formulate research hypotheses. A hypothesis is a predetermined declaration regarding the research question in which the investigator(s) makes a precise, educated guess about a study outcome. This is sometimes called the alternative hypothesis and ultimately allows the researcher to take a stance based on experience or insight from medical literature. An example of a hypothesis is below.
Research Hypothesis: Drug 23 will significantly reduce symptoms associated with Disease A compared to Drug 22.
The null hypothesis states that there is no statistical difference between groups based on the stated research hypothesis.
Researchers should be aware of journal recommendations when considering how to report p values, and manuscripts should remain internally consistent.
Regarding p values, as the number of individuals enrolled in a study (the sample size) increases, the likelihood of finding a statistically significant effect increases. With very large sample sizes, the p-value can be very low significant differences in the reduction of symptoms for Disease A between Drug 23 and Drug 22. The null hypothesis is deemed true until a study presents significant data to support rejecting the null hypothesis. Based on the results, the investigators will either reject the null hypothesis (if they found significant differences or associations) or fail to reject the null hypothesis (they could not provide proof that there were significant differences or associations).
To test a hypothesis, researchers obtain data on a representative sample to determine whether to reject or fail to reject a null hypothesis. In most research studies, it is not feasible to obtain data for an entire population. Using a sampling procedure allows for statistical inference, though this involves a certain possibility of error. [1] When determining whether to reject or fail to reject the null hypothesis, mistakes can be made: Type I and Type II errors. Though it is impossible to ensure that these errors have not occurred, researchers should limit the possibilities of these faults. [2]
Significance
Significance is a term to describe the substantive importance of medical research. Statistical significance is the likelihood of results due to chance. [3] Healthcare providers should always delineate statistical significance from clinical significance, a common error when reviewing biomedical research. [4] When conceptualizing findings reported as either significant or not significant, healthcare providers should not simply accept researchers' results or conclusions without considering the clinical significance. Healthcare professionals should consider the clinical importance of findings and understand both p values and confidence intervals so they do not have to rely on the researchers to determine the level of significance. [5] One criterion often used to determine statistical significance is the utilization of p values.
P values are used in research to determine whether the sample estimate is significantly different from a hypothesized value. The p-value is the probability that the observed effect within the study would have occurred by chance if, in reality, there was no true effect. Conventionally, data yielding a p<0.05 or p<0.01 is considered statistically significant. While some have debated that the 0.05 level should be lowered, it is still universally practiced. [6] Hypothesis testing allows us to determine the size of the effect.
An example of findings reported with p values are below:
Statement: Drug 23 reduced patients' symptoms compared to Drug 22. Patients who received Drug 23 (n=100) were 2.1 times less likely than patients who received Drug 22 (n = 100) to experience symptoms of Disease A, p<0.05.
Statement:Individuals who were prescribed Drug 23 experienced fewer symptoms (M = 1.3, SD = 0.7) compared to individuals who were prescribed Drug 22 (M = 5.3, SD = 1.9). This finding was statistically significant, p= 0.02.
For either statement, if the threshold had been set at 0.05, the null hypothesis (that there was no relationship) should be rejected, and we should conclude significant differences. Noticeably, as can be seen in the two statements above, some researchers will report findings with < or > and others will provide an exact p-value (0.000001) but never zero [6] . When examining research, readers should understand how p values are reported. The best practice is to report all p values for all variables within a study design, rather than only providing p values for variables with significant findings. [7] The inclusion of all p values provides evidence for study validity and limits suspicion for selective reporting/data mining.
While researchers have historically used p values, experts who find p values problematic encourage the use of confidence intervals. [8] . P-values alone do not allow us to understand the size or the extent of the differences or associations. [3] In March 2016, the American Statistical Association (ASA) released a statement on p values, noting that scientific decision-making and conclusions should not be based on a fixed p-value threshold (e.g., 0.05). They recommend focusing on the significance of results in the context of study design, quality of measurements, and validity of data. Ultimately, the ASA statement noted that in isolation, a p-value does not provide strong evidence. [9]
When conceptualizing clinical work, healthcare professionals should consider p values with a concurrent appraisal study design validity. For example, a p-value from a double-blinded randomized clinical trial (designed to minimize bias) should be weighted higher than one from a retrospective observational study [7] . The p-value debate has smoldered since the 1950s [10] , and replacement with confidence intervals has been suggested since the 1980s. [11]
Confidence Intervals
A confidence interval provides a range of values within given confidence (e.g., 95%), including the accurate value of the statistical constraint within a targeted population. [12] Most research uses a 95% CI, but investigators can set any level (e.g., 90% CI, 99% CI). [13] A CI provides a range with the lower bound and upper bound limits of a difference or association that would be plausible for a population. [14] Therefore, a CI of 95% indicates that if a study were to be carried out 100 times, the range would contain the true value in 95, [15] confidence intervals provide more evidence regarding the precision of an estimate compared to p-values. [6]
In consideration of the similar research example provided above, one could make the following statement with 95% CI:
Statement: Individuals who were prescribed Drug 23 had no symptoms after three days, which was significantly faster than those prescribed Drug 22; there was a mean difference between the two groups of days to the recovery of 4.2 days (95% CI: 1.9 – 7.8).
It is important to note that the width of the CI is affected by the standard error and the sample size; reducing a study sample number will result in less precision of the CI (increase the width). [14] A larger width indicates a smaller sample size or a larger variability. [16] A researcher would want to increase the precision of the CI. For example, a 95% CI of 1.43 – 1.47 is much more precise than the one provided in the example above. In research and clinical practice, CIs provide valuable information on whether the interval includes or excludes any clinically significant values. [14]
Null values are sometimes used for differences with CI (zero for differential comparisons and 1 for ratios). However, CIs provide more information than that. [15] Consider this example: A hospital implements a new protocol that reduced wait time for patients in the emergency department by an average of 25 minutes (95% CI: -2.5 – 41 minutes). Because the range crosses zero, implementing this protocol in different populations could result in longer wait times; however, the range is much higher on the positive side. Thus, while the p-value used to detect statistical significance for this may result in "not significant" findings, individuals should examine this range, consider the study design, and weigh whether or not it is still worth piloting in their workplace.
Similarly to p-values, 95% CIs cannot control for researchers' errors (e.g., study bias or improper data analysis). [14] In consideration of whether to report p-values or CIs, researchers should examine journal preferences. When in doubt, reporting both may be beneficial. [13] An example is below:
Reporting both: Individuals who were prescribed Drug 23 had no symptoms after three days, which was significantly faster than those prescribed Drug 22, p = 0.009. There was a mean difference between the two groups of days to the recovery of 4.2 days (95% CI: 1.9 – 7.8).
Clinical Significance
Recall that clinical significance and statistical significance are two different concepts. Healthcare providers should remember that a study with statistically significant differences and large sample size may be of no interest to clinicians, whereas a study with smaller sample size and statistically non-significant results could impact clinical practice. [14] Additionally, as previously mentioned, a non-significant finding may reflect the study design itself rather than relationships between variables.
Healthcare providers using evidence-based medicine to inform practice should use clinical judgment to determine the practical importance of studies through careful evaluation of the design, sample size, power, likelihood of type I and type II errors, data analysis, and reporting of statistical findings (p values, 95% CI or both). [4] Interestingly, some experts have called for "statistically significant" or "not significant" to be excluded from work as statistical significance never has and will never be equivalent to clinical significance. [17]
The decision on what is clinically significant can be challenging, depending on the providers' experience and especially the severity of the disease. Providers should use their knowledge and experiences to determine the meaningfulness of study results and make inferences based not only on significant or insignificant results by researchers but through their understanding of study limitations and practical implications.
Nursing, Allied Health, and Interprofessional Team Interventions
All physicians, nurses, pharmacists, and other healthcare professionals should strive to understand the concepts in this chapter. These individuals should maintain the ability to review and incorporate new literature for evidence-based and safe care.
Jones M, Gebski V, Onslow M, Packman A. Statistical power in stuttering research: a tutorial. Journal of speech, language, and hearing research : JSLHR. 2002 Apr:45(2):243-55 [PubMed PMID: 12003508]
Sedgwick P. Pitfalls of statistical hypothesis testing: type I and type II errors. BMJ (Clinical research ed.). 2014 Jul 3:349():g4287. doi: 10.1136/bmj.g4287. Epub 2014 Jul 3 [PubMed PMID: 24994622]
Fethney J. Statistical and clinical significance, and how to use confidence intervals to help interpret both. Australian critical care : official journal of the Confederation of Australian Critical Care Nurses. 2010 May:23(2):93-7. doi: 10.1016/j.aucc.2010.03.001. Epub 2010 Mar 29 [PubMed PMID: 20347326]
Hayat MJ. Understanding statistical significance. Nursing research. 2010 May-Jun:59(3):219-23. doi: 10.1097/NNR.0b013e3181dbb2cc. Epub [PubMed PMID: 20445438]
Ferrill MJ, Brown DA, Kyle JA. Clinical versus statistical significance: interpreting P values and confidence intervals related to measures of association to guide decision making. Journal of pharmacy practice. 2010 Aug:23(4):344-51. doi: 10.1177/0897190009358774. Epub 2010 Apr 13 [PubMed PMID: 21507834]
Infanger D, Schmidt-Trucksäss A. P value functions: An underused method to present research results and to promote quantitative reasoning. Statistics in medicine. 2019 Sep 20:38(21):4189-4197. doi: 10.1002/sim.8293. Epub 2019 Jul 3 [PubMed PMID: 31270842]
Dorey F. Statistics in brief: Interpretation and use of p values: all p values are not equal. Clinical orthopaedics and related research. 2011 Nov:469(11):3259-61. doi: 10.1007/s11999-011-2053-1. Epub [PubMed PMID: 21918804]
Liu XS. Implications of statistical power for confidence intervals. The British journal of mathematical and statistical psychology. 2012 Nov:65(3):427-37. doi: 10.1111/j.2044-8317.2011.02035.x. Epub 2011 Oct 25 [PubMed PMID: 22026811]
Tijssen JG, Kolm P. Demystifying the New Statistical Recommendations: The Use and Reporting of p Values. Journal of the American College of Cardiology. 2016 Jul 12:68(2):231-3. doi: 10.1016/j.jacc.2016.05.026. Epub [PubMed PMID: 27386779]
Spanos A. Recurring controversies about P values and confidence intervals revisited. Ecology. 2014 Mar:95(3):645-51 [PubMed PMID: 24804448]
Freire APCF, Elkins MR, Ramos EMC, Moseley AM. Use of 95% confidence intervals in the reporting of between-group differences in randomized controlled trials: analysis of a representative sample of 200 physical therapy trials. Brazilian journal of physical therapy. 2019 Jul-Aug:23(4):302-310. doi: 10.1016/j.bjpt.2018.10.004. Epub 2018 Oct 16 [PubMed PMID: 30366845]
Dorey FJ. In brief: statistics in brief: Confidence intervals: what is the real result in the target population? Clinical orthopaedics and related research. 2010 Nov:468(11):3137-8. doi: 10.1007/s11999-010-1407-4. Epub [PubMed PMID: 20532716]
Porcher R. Reporting results of orthopaedic research: confidence intervals and p values. Clinical orthopaedics and related research. 2009 Oct:467(10):2736-7. doi: 10.1007/s11999-009-0952-1. Epub 2009 Jun 30 [PubMed PMID: 19565303]
Gardner MJ, Altman DG. Confidence intervals rather than P values: estimation rather than hypothesis testing. British medical journal (Clinical research ed.). 1986 Mar 15:292(6522):746-50 [PubMed PMID: 3082422]
Cooper RJ, Wears RL, Schriger DL. Reporting research results: recommendations for improving communication. Annals of emergency medicine. 2003 Apr:41(4):561-4 [PubMed PMID: 12658257]
Doll H, Carney S. Statistical approaches to uncertainty: P values and confidence intervals unpacked. Equine veterinary journal. 2007 May:39(3):275-6 [PubMed PMID: 17520981]
Colquhoun D. The reproducibility of research and the misinterpretation of p-values. Royal Society open science. 2017 Dec:4(12):171085. doi: 10.1098/rsos.171085. Epub 2017 Dec 6 [PubMed PMID: 29308247]
Use the mouse wheel to zoom in and out, click and drag to pan the image

Quick Guide to Biostatistics in Clinical Research: Hypothesis Testing
In this article series, we will be looking at some of the important concepts of biostatistics in clinical trials and clinical research. Statistics is frequently used to analyze quantitative research data. Clinical trials and clinical research both often rely on statistics. Clinical trials proceed through many phases . Contract Research Organizations (CRO) can be hired to conduct a clinical trial. Clinical trials are an important step in deciding if a treatment can be safely and effectively used in medical practice. Once the clinical trial phases are completed, biostatistics is used to analyze the results.
Research generally proceeds in an orderly fashion as shown below.

Once you have identified the research question you need to answer, it is time to frame a good hypothesis. The hypothesis is the starting point for biostatistics and is usually based on a theory. Experiments are then designed to test the hypothesis. What is a hypothesis ? A research hypothesis is a statement describing a relationship between two or more variables that can be tested. A good hypothesis will be clear, avoid moral judgments, specific, objective, and relevant to the research question. Above all, a hypothesis must be testable.
A simple hypothesis would contain one predictor and one outcome variable. For instance, if your hypothesis was, “Chocolate consumption is linked to type II diabetes” the predictor would be whether or not a person eats chocolate and the outcome would be developing type II diabetes. A good hypothesis would also be specific. This means that it should be clear which subjects and research methodology will be used to test the hypothesis. An example of a specific hypothesis would be, “Adults who consume more than 20 grams of milk chocolate per day, as measured by a questionnaire over the course of 12 months, are more likely to develop type II diabetes than adults who consume less than 10 grams of milk chocolate per day.”
Null and Alternative Hypothesis
In statistics, the null hypothesis (H 0 ) states that there is no relationship between the predictor and the outcome variable in the population being studied. For instance, “There is no relationship between a family history of depression and the probability that a person will attempt suicide.” The alternative hypothesis (H 1 ) states that there is a relationship between the predictor (a history of depression) and the outcome (attempted suicide). It is impossible to prove a statement by making several observations but it is possible to disprove a statement with a single observation. If you always saw red tulips, it is not proof that no other colors exist. However, seeing a single tulip that was not red would immediately prove that the statement, “All tulips are red” is false. This is why statistics tests the null hypothesis. It is also why the alternative hypothesis cannot be tested directly.
The alternative hypothesis proposed in medical research may be one-tailed or two-tailed. A one-tailed alternative hypothesis would predict the direction of the effect. Clinical studies may have an alternative hypothesis that patients taking the study drug will have a lower cholesterol level than those taking a placebo. This is an example of a one-tailed hypothesis. A two-tailed alternative hypothesis would only state that there is an association without specifying a direction. An example would be, “Patients who take the study drug will have a significantly different cholesterol level than those patients taking a placebo”. The alternative hypothesis does not state if that level will be higher or lower in those taking the placebo.
The P-Value Approach to Test Hypothesis
Once the hypothesis has been designed, statistical tests help you to decide if you should accept or reject the null hypothesis. Statistical tests determine the p-value associated with the research data. The p-value is the probability that one could have obtained the result by chance; assuming the null hypothesis (H 0 ) was true. You must reject the null hypothesis if the p-value of the data falls below the predetermined level of statistical significance. Usually, the level of statistical significance is set at 0.05. If the p- value is less than 0.05, then you would reject the null hypothesis stating that there is no relationship between the predictor and the outcome in the sample population.
However, if the p-value is greater than the predetermined level of significance, then there is no statistically significant association between the predictor and the outcome variable. This does not mean that there is no association between the predictor and the outcome in the population. It only means that the difference between the relationship observed and the relationship that could have occurred by random chance is small.
For example, null hypothesis (H 0 ): The patients who take the study drug after a heart attack did not have a better chance of not having a second heart attack over the next 24 months.
Data suggests that those who did not take the study drug were twice as likely to have a second heart attack with a p-value of 0.08. This p-value would indicate that there was an 8% chance that you would see a similar result (people on the placebo being twice as likely to have a second heart attack) in the general population because of random chance.
The hypothesis is not a trivial part of the clinical research process. It is a key element in a good biostatistics plan regardless of the clinical trial phase. There are many other concepts that are important for analyzing data from clinical trials. In our next article in the series, we will examine hypothesis testing for one or many populations, as well as error types.
Thank you for this very informative article. You describe all the things very well. I am doing a fellowship in Clinical research training. This information really helps me a lot in my research studies. I have been connected with your site since a long time for such updates. Thank you once again
Rate this article Cancel Reply
Your email address will not be published.

Enago Academy's Most Popular Articles

- AI in Academia
- Infographic
- Manuscripts & Grants
- Reporting Research
- Trending Now
Can AI Tools Prepare a Research Manuscript From Scratch? — A comprehensive guide
As technology continues to advance, the question of whether artificial intelligence (AI) tools can prepare…

Abstract Vs. Introduction — Do you know the difference?
Ross wants to publish his research. Feeling positive about his research outcomes, he begins to…

- Old Webinars
- Webinar Mobile App
Demystifying Research Methodology With Field Experts
Choosing research methodology Research design and methodology Evidence-based research approach How RAxter can assist researchers

- Manuscript Preparation
- Publishing Research
How to Choose Best Research Methodology for Your Study
Successful research conduction requires proper planning and execution. While there are multiple reasons and aspects…

Top 5 Key Differences Between Methods and Methodology
While burning the midnight oil during literature review, most researchers do not realize that the…
How to Draft the Acknowledgment Section of a Manuscript
Discussion Vs. Conclusion: Know the Difference Before Drafting Manuscripts

Sign-up to read more
Subscribe for free to get unrestricted access to all our resources on research writing and academic publishing including:
- 2000+ blog articles
- 50+ Webinars
- 10+ Expert podcasts
- 50+ Infographics
- 10+ Checklists
- Research Guides
We hate spam too. We promise to protect your privacy and never spam you.
I am looking for Editing/ Proofreading services for my manuscript Tentative date of next journal submission:

What should universities' stance be on AI tools in research and academic writing?

Introduction to Basics of Pharmacology and Toxicology pp 865–875 Cite as
Hypothesis Testing
- Lakshmi Balasundaram 4
- First Online: 16 November 2022
889 Accesses
- The original version of this chapter was revised. Revised figure updated this chapter. A correction to this chapter can be found at https://doi.org/10.1007/978-981-19-5343-9_64
Hypothesis testing forms an important domain of inferential statistics, it also forms a stepping stone to evidence-based medicine (EBM). A narrow research question leads to the formulation of a research hypothesis which is put into test, in order to draw a statistical inference. A researcher believes in the alternative hypothesis which states that there is a treatment difference between the two groups, in contrast to null hypothesis which believes in no difference. The hypothesis is tested on a sample population after setting the significance level. The researcher uses the right statistical procedure to arrive at a statistical decision which helps is rejecting the null hypothesis or otherwise. A p -value with confidence interval helps in interpreting the results of the hypothesis testing better. In case where multiple hypotheses need to be tested simultaneously, the alpha error rate must be controlled, which can be done by various methods. A newer method of hypothesis testing is by using Bayesian methods which uses Bayes factor in assessing the plausibility of the hypotheses.
- Null hypothesis
- Alternative hypothesis
- Confidence interval
This is a preview of subscription content, log in via an institution .
Buying options
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Purchases are for personal use only
Change history
24 january 2023.
The original version of this chapter was inadvertently published with an error in Fig. 60.1, which has been updated with the correct figure as follows
Bibliography
Biau DJ, Jolles BM, Porcher R. P value and the theory of hypothesis testing: an explanation for new researchers. Clin Orthop Relat Res. 2010 Mar;468(3):885–92.
Article Google Scholar
Faulkenberry TJ. A simple method for teaching Bayesian hypothesis testing in the brain and behavioral sciences. J Undergrad Neurosci Educ. 2018 Jun 15;16(2):A126–30.
Google Scholar
Guyatt G, Jaeschke R, Heddle N, Cook D, Shannon H, Walter S. Basic statistics for clinicians: 1 Hypothesis testing. CMAJ. 1995;152(1):27–32.
CAS Google Scholar
Hazra A, Gogtay N. Biostatistics series module 2: overview of hypothesis testing. Indian J Dermatol. 2016;61(2):137–45.
Sacha V, Panagiotakos DB. Insights in hypothesis testing and making decisions in biomedical research. Open Cardiovasc Med J. 2016 Sep 30;10:196–200.
Shreffler J, Huecker MR. Hypothesis testing, P Values, confidence intervals, and significance. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020. [cited 2021 Feb 1]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK557421/ .
Download references
Author information
Authors and affiliations.
Department of Pharmacology, Jawaharlal Institute of Postgraduate Medical Education & Research (JIPMER), Puducherry, India
Lakshmi Balasundaram
You can also search for this author in PubMed Google Scholar
Editor information
Editors and affiliations.
Department of Pharmacology, Thanjavur Medical College, Thanjavur, Tamil Nadu, India
Mageshwaran Lakshmanan
Department of Pharmacology, Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Puducherry, Pondicherry, India
Deepak Gopal Shewade
Department of Pharmacology, All India Institute of Medical Sciences (AIIMS) Bibinagar, Hyderabad, Telangana, India
Gerard Marshall Raj
Rights and permissions
Reprints and permissions
Copyright information
© 2022 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter.
Balasundaram, L. (2022). Hypothesis Testing. In: Lakshmanan, M., Shewade, D.G., Raj, G.M. (eds) Introduction to Basics of Pharmacology and Toxicology. Springer, Singapore. https://doi.org/10.1007/978-981-19-5343-9_60
Download citation
DOI : https://doi.org/10.1007/978-981-19-5343-9_60
Published : 16 November 2022
Publisher Name : Springer, Singapore
Print ISBN : 978-981-19-5342-2
Online ISBN : 978-981-19-5343-9
eBook Packages : Medicine Medicine (R0)

Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
- Research article
- Open access
- Published: 19 May 2010
The null hypothesis significance test in health sciences research (1995-2006): statistical analysis and interpretation
- Luis Carlos Silva-Ayçaguer 1 ,
- Patricio Suárez-Gil 2 &
- Ana Fernández-Somoano 3
BMC Medical Research Methodology volume 10 , Article number: 44 ( 2010 ) Cite this article
38k Accesses
23 Citations
18 Altmetric
Metrics details
The null hypothesis significance test (NHST) is the most frequently used statistical method, although its inferential validity has been widely criticized since its introduction. In 1988, the International Committee of Medical Journal Editors (ICMJE) warned against sole reliance on NHST to substantiate study conclusions and suggested supplementary use of confidence intervals (CI). Our objective was to evaluate the extent and quality in the use of NHST and CI, both in English and Spanish language biomedical publications between 1995 and 2006, taking into account the International Committee of Medical Journal Editors recommendations, with particular focus on the accuracy of the interpretation of statistical significance and the validity of conclusions.
Original articles published in three English and three Spanish biomedical journals in three fields (General Medicine, Clinical Specialties and Epidemiology - Public Health) were considered for this study. Papers published in 1995-1996, 2000-2001, and 2005-2006 were selected through a systematic sampling method. After excluding the purely descriptive and theoretical articles, analytic studies were evaluated for their use of NHST with P-values and/or CI for interpretation of statistical "significance" and "relevance" in study conclusions.
Among 1,043 original papers, 874 were selected for detailed review. The exclusive use of P-values was less frequent in English language publications as well as in Public Health journals; overall such use decreased from 41% in 1995-1996 to 21% in 2005-2006. While the use of CI increased over time, the "significance fallacy" (to equate statistical and substantive significance) appeared very often, mainly in journals devoted to clinical specialties (81%). In papers originally written in English and Spanish, 15% and 10%, respectively, mentioned statistical significance in their conclusions.
Conclusions
Overall, results of our review show some improvements in statistical management of statistical results, but further efforts by scholars and journal editors are clearly required to move the communication toward ICMJE advices, especially in the clinical setting, which seems to be imperative among publications in Spanish.
Peer Review reports
The null hypothesis statistical testing (NHST) has been the most widely used statistical approach in health research over the past 80 years. Its origins dates back to 1279 [ 1 ] although it was in the second decade of the twentieth century when the statistician Ronald Fisher formally introduced the concept of "null hypothesis" H 0 - which, generally speaking, establishes that certain parameters do not differ from each other. He was the inventor of the "P-value" through which it could be assessed [ 2 ]. Fisher's P-value is defined as a conditional probability calculated using the results of a study. Specifically, the P-value is the probability of obtaining a result at least as extreme as the one that was actually observed, assuming that the null hypothesis is true. The Fisherian significance testing theory considered the p-value as an index to measure the strength of evidence against the null hypothesis in a single experiment. The father of NHST never endorsed, however, the inflexible application of the ultimately subjective threshold levels almost universally adopted later on (although the introduction of the 0.05 has his paternity also).
A few years later, Jerzy Neyman and Egon Pearson considered the Fisherian approach inefficient, and in 1928 they published an article [ 3 ] that would provide the theoretical basis of what they called hypothesis statistical testing . The Neyman-Pearson approach is based on the notion that one out of two choices has to be taken: accept the null hypothesis taking the information as a reference based on the information provided, or reject it in favor of an alternative one. Thus, one can incur one of two types of errors: a Type I error, if the null hypothesis is rejected when it is actually true, and a Type II error, if the null hypothesis is accepted when it is actually false. They established a rule to optimize the decision process, using the p-value introduced by Fisher, by setting the maximum frequency of errors that would be admissible.
The null hypothesis statistical testing, as applied today, is a hybrid coming from the amalgamation of the two methods [ 4 ]. As a matter of fact, some 15 years later, both procedures were combined to give rise to the nowadays widespread use of an inferential tool that would satisfy none of the statisticians involved in the original controversy. The present method essentially goes as follows: given a null hypothesis, an estimate of the parameter (or parameters) is obtained and used to create statistics whose distribution, under H 0 , is known. With these data the P-value is computed. Finally, the null hypothesis is rejected when the obtained P-value is smaller than a certain comparative threshold (usually 0.05) and it is not rejected if P is larger than the threshold.
The first reservations about the validity of the method began to appear around 1940, when some statisticians censured the logical roots and practical convenience of Fisher's P-value [ 5 ]. Significance tests and P-values have repeatedly drawn the attention and criticism of many authors over the past 70 years, who have kept questioning its epistemological legitimacy as well as its practical value. What remains in spite of these criticisms is the lasting legacy of researchers' unwillingness to eradicate or reform these methods.
Although there are very comprehensive works on the topic [ 6 ], we list below some of the criticisms most universally accepted by specialists.
The P-values are used as a tool to make decisions in favor of or against a hypothesis. What really may be relevant, however, is to get an effect size estimate (often the difference between two values) rather than rendering dichotomous true/false verdicts [ 7 – 11 ].
The P-value is a conditional probability of the data, provided that some assumptions are met, but what really interests the investigator is the inverse probability: what degree of validity can be attributed to each of several competing hypotheses, once that certain data have been observed [ 12 ].
The two elements that affect the results, namely the sample size and the magnitude of the effect, are inextricably linked in the value of p and we can always get a lower P-value by increasing the sample size. Thus, the conclusions depend on a factor completely unrelated to the reality studied (i.e. the available resources, which in turn determine the sample size) [ 13 , 14 ].
Those who defend the NHST often assert the objective nature of that test, but the process is actually far from being so. NHST does not ensure objectivity. This is reflected in the fact that we generally operate with thresholds that are ultimately no more than conventions, such as 0.01 or 0.05. What is more, for many years their use has unequivocally demonstrated the inherent subjectivity that goes with the concept of P, regardless of how it will be used later [ 15 – 17 ].
In practice, the NHST is limited to a binary response sorting hypotheses into "true" and "false" or declaring "rejection" or "no rejection", without demanding a reasonable interpretation of the results, as has been noted time and again for decades. This binary orthodoxy validates categorical thinking, which results in a very simplistic view of scientific activity that induces researchers not to test theories about the magnitude of effect sizes [ 18 – 20 ].
Despite the weakness and shortcomings of the NHST, they are frequently taught as if they were the key inferential statistical method or the most appropriate, or even the sole unquestioned one. The statistical textbooks, with only some exceptions, do not even mention the NHST controversy. Instead, the myth is spread that NHST is the "natural" final action of scientific inference and the only procedure for testing hypotheses. However, relevant specialists and important regulators of the scientific world advocate avoiding them.
Taking especially into account that NHST does not offer the most important information (i.e. the magnitude of an effect of interest, and the precision of the estimate of the magnitude of that effect), many experts recommend the reporting of point estimates of effect sizes with confidence intervals as the appropriate representation of the inherent uncertainty linked to empirical studies [ 21 – 25 ]. Since 1988, the International Committee of Medical Journal Editors (ICMJE, known as the Vancouver Group ) incorporates the following recommendation to authors of manuscripts submitted to medical journals: "When possible, quantify findings and present them with appropriate indicators of measurement error or uncertainty (such as confidence intervals). Avoid relying solely on statistical hypothesis testing, such as P-values, which fail to convey important information about effect size" [ 26 ].
As will be shown, the use of confidence intervals (CI), occasionally accompanied by P-values, is recommended as a more appropriate method for reporting results. Some authors have noted several shortcomings of CI long ago [ 27 ]. In spite of the fact that calculating CI could be complicated indeed, and that their interpretation is far from simple [ 28 , 29 ], authors are urged to use them because they provide much more information than the NHST and do not merit most of its criticisms of NHST [ 30 ]. While some have proposed different options (for instance, likelihood-based information theoretic methods [ 31 ], and the Bayesian inferential paradigm [ 32 ]), confidence interval estimation of effect sizes is clearly the most widespread alternative approach.
Although twenty years have passed since the ICMJE began to disseminate such recommendations, systematically ignored by the vast majority of textbooks and hardly incorporated in medical publications [ 33 ], it is interesting to examine the extent to which the NHST is used in articles published in medical journals during recent years, in order to identify what is still lacking in the process of eradicating the widespread ceremonial use that is made of statistics in health research [ 34 ]. Furthermore, it is enlightening in this context to examine whether these patterns differ between English- and Spanish-speaking worlds and, if so, to see if the changes in paradigms are occurring more slowly in Spanish-language publications. In such a case we would offer various suggestions.
In addition to assessing the adherence to the above cited statistical recommendation proposed by ICMJE relative to the use of P-values, we consider it of particular interest to estimate the extent to which the significance fallacy is present, an inertial deficiency that consists of attributing -- explicitly or not -- qualitative importance or practical relevance to the found differences simply because statistical significance was obtained.
Many authors produce misleading statements such as "a significant effect was (or was not) found" when it should be said that "a statistically significant difference was (or was not) found". A detrimental consequence of this equivalence is that some authors believe that finding out whether there is "statistical significance" or not is the aim, so that this term is then mentioned in the conclusions [ 35 ]. This means virtually nothing, except that it indicates that the author is letting a computer do the thinking. Since the real research questions are never statistical ones, the answers cannot be statistical either. Accordingly, the conversion of the dichotomous outcome produced by a NHST into a conclusion is another manifestation of the mentioned fallacy.
The general objective of the present study is to evaluate the extent and quality of use of NHST and CI, both in English- and in Spanish-language biomedical publications, between 1995 and 2006 taking into account the International Committee of Medical Journal Editors recommendations, with particular focus on accuracy regarding interpretation of statistical significance and the validity of conclusions.
We reviewed the original articles from six journals, three in English and three in Spanish, over three disjoint periods sufficiently separated from each other (1995-1996, 2000-2001, 2005-2006) as to properly describe the evolution in prevalence of the target features along the selected periods.
The selection of journals was intended to get representation for each of the following three thematic areas: clinical specialties ( Obstetrics & Gynecology and Revista Española de Cardiología) ; Public Health and Epidemiology ( International Journal of Epidemiology and Atención Primaria) and the area of general and internal medicine ( British Medical Journal and Medicina Clínica ). Five of the selected journals formally endorsed ICMJE guidelines; the remaining one ( Revista Española de Cardiología ) suggests observing ICMJE demands in relation with specific issues. We attempted to capture journal diversity in the sample by selecting general and specialty journals with different degrees of influence, resulting from their impact factors in 2007, which oscillated between 1.337 (MC) and 9.723 (BMJ). No special reasons guided us to choose these specific journals, but we opted for journals with rather large paid circulations. For instance, the Spanish Cardiology Journal is the one with the largest impact factor among the fourteen Spanish Journals devoted to clinical specialties that have impact factor and Obstetrics & Gynecology has an outstanding impact factor among the huge number of journals available for selection.
It was decided to take around 60 papers for each biennium and journal, which means a total of around 1,000 papers. As recently suggested [ 36 , 37 ], this number was not established using a conventional method, but by means of a purposive and pragmatic approach in choosing the maximum sample size that was feasible.
Systematic sampling in phases [ 38 ] was used in applying a sampling fraction equal to 60/N, where N is the number of articles, in each of the 18 subgroups defined by crossing the six journals and the three time periods. Table 1 lists the population size and the sample size for each subgroup. While the sample within each subgroup was selected with equal probability, estimates based on other subsets of articles (defined across time periods, areas, or languages) are based on samples with various selection probabilities. Proper weights were used to take into account the stratified nature of the sampling in these cases.
Forty-nine of the 1,092 selected papers were eliminated because, although the section of the article in which they were assigned could suggest they were originals, detailed scrutiny revealed that in some cases they were not. The sample, therefore, consisted of 1,043 papers. Each of them was classified into one of three categories: (1) purely descriptive papers, those designed to review or characterize the state of affairs as it exists at present, (2) analytical papers, or (3) articles that address theoretical, methodological or conceptual issues. An article was regarded as analytical if it seeks to explain the reasons behind a particular occurrence by discovering causal relationships or, even if self-classified as descriptive, it was carried out to assess cause-effect associations among variables. We classify as theoretical or methodological those articles that do not handle empirical data as such, and focus instead on proposing or assessing research methods. We identified 169 papers as purely descriptive or theoretical, which were therefore excluded from the sample. Figure 1 presents a flow chart showing the process for determining eligibility for inclusion in the sample.
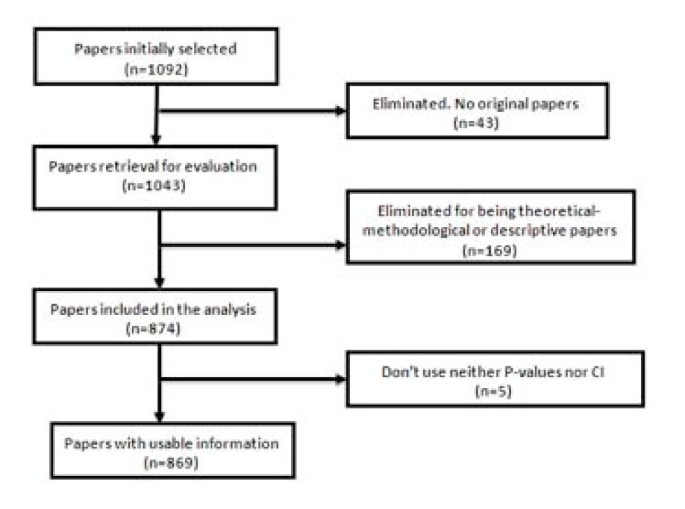
Flow chart of the selection process for eligible papers .
To estimate the adherence to ICMJE recommendations, we considered whether the papers used P-values, confidence intervals, and both simultaneously. By "the use of P-values" we mean that the article contains at least one P-value, explicitly mentioned in the text or at the bottom of a table, or that it reports that an effect was considered as statistically significant . It was deemed that an article uses CI if it explicitly contained at least one confidence interval, but not when it only provides information that could allow its computation (usually by presenting both the estimate and the standard error). Probability intervals provided in Bayesian analysis were classified as confidence intervals (although conceptually they are not the same) since what is really of interest here is whether or not the authors quantify the findings and present them with appropriate indicators of the margin of error or uncertainty.
In addition we determined whether the "Results" section of each article attributed the status of "significant" to an effect on the sole basis of the outcome of a NHST (i.e., without clarifying that it is strictly statistical significance). Similarly, we examined whether the term "significant" (applied to a test) was mistakenly used as synonymous with substantive , relevant or important . The use of the term "significant effect" when it is only appropriate as a reference to a "statistically significant difference," can be considered a direct expression of the significance fallacy [ 39 ] and, as such, constitutes one way to detect the problem in a specific paper.
We also assessed whether the "Conclusions," which sometimes appear as a separate section in the paper or otherwise in the last paragraphs of the "Discussion" section mentioned statistical significance and, if so, whether any of such mentions were no more than an allusion to results.
To perform these analyses we considered both the abstract and the body of the article. To assess the handling of the significance issue, however, only the body of the manuscript was taken into account.
The information was collected by four trained observers. Every paper was assigned to two reviewers. Disagreements were discussed and, if no agreement was reached, a third reviewer was consulted to break the tie and so moderate the effect of subjectivity in the assessment.
In order to assess the reliability of the criteria used for the evaluation of articles and to effect a convergence of criteria among the reviewers, a pilot study of 20 papers from each of three journals ( Clinical Medicine , Primary Care , and International Journal of Epidemiology) was performed. The results of this pilot study were satisfactory. Our results are reported using percentages together with their corresponding confidence intervals. For sampling errors estimations, used to obtain confidence intervals, we weighted the data using the inverse of the probability of selection of each paper, and we took into account the complex nature of the sample design. These analyses were carried out with EPIDAT [ 40 ], a specialized computer program that is readily available.
A total of 1,043 articles were reviewed, of which 874 (84%) were found to be analytic, while the remainders were purely descriptive or of a theoretical and methodological nature. Five of them did not employ either P-values or CI. Consequently, the analysis was made using the remaining 869 articles.
Use of NHST and confidence intervals
The percentage of articles that use only P-values, without even mentioning confidence intervals, to report their results has declined steadily throughout the period analyzed (Table 2 ). The percentage decreased from approximately 41% in 1995-1996 to 21% in 2005-2006. However, it does not differ notably among journals of different languages, as shown by the estimates and confidence intervals of the respective percentages. Concerning thematic areas, it is highly surprising that most of the clinical articles ignore the recommendations of ICMJE, while for general and internal medicine papers such a problem is only present in one in five papers, and in the area of Public Health and Epidemiology it occurs only in one out of six. The use of CI alone (without P-values) has increased slightly across the studied periods (from 9% to 13%), but it is five times more prevalent in Public Health and Epidemiology journals than in Clinical ones, where it reached a scanty 3%.
Ambivalent handling of the significance
While the percentage of articles referring implicitly or explicitly to significance in an ambiguous or incorrect way - that is, incurring the significance fallacy -- seems to decline steadily, the prevalence of this problem exceeds 69%, even in the most recent period. This percentage was almost the same for articles written in Spanish and in English, but it was notably higher in the Clinical journals (81%) compared to the other journals, where the problem occurs in approximately 7 out of 10 papers (Table 3 ). The kappa coefficient for measuring agreement between observers concerning the presence of the "significance fallacy" was 0.78 (CI95%: 0.62 to 0.93), which is considered acceptable in the scale of Landis and Koch [ 41 ].
Reference to numerical results or statistical significance in Conclusions
The percentage of papers mentioning a numerical finding as a conclusion is similar in the three periods analyzed (Table 4 ). Concerning languages, this percentage is nearly twice as large for Spanish journals as for those published in English (approximately 21% versus 12%). And, again, the highest percentage (16%) corresponded to clinical journals.
A similar pattern is observed, although with less pronounced differences, in references to the outcome of the NHST (significant or not) in the conclusions (Table 5 ). The percentage of articles that introduce the term in the "Conclusions" does not appreciably differ between articles written in Spanish and in English. Again, the area where this insufficiency is more often present (more than 15% of articles) is the Clinical area.
There are some previous studies addressing the degree to which researchers have moved beyond the ritualistic use of NHST to assess their hypotheses. This has been examined for areas such as biology [ 42 ], organizational research [ 43 ], or psychology [ 44 – 47 ]. However, to our knowledge, no recent research has explored the pattern of use P-values and CI in medical literature and, in any case, no efforts have been made to study this problem in a way that takes into account different languages and specialties.
At first glance it is puzzling that, after decades of questioning and technical warnings, and after twenty years since the inception of ICMJE recommendation to avoid NHST, they continue being applied ritualistically and mindlessly as the dominant doctrine. Not long ago, when researchers did not observe statistically significant effects, they were unlikely to write them up and to report "negative" findings, since they knew there was a high probability that the paper would be rejected. This has changed a bit: editors are more prone to judge all findings as potentially eloquent. This is probably the frequent denunciations of the tendency for those papers presenting a significant positive result to receive more favorable publication decisions than equally well-conducted ones that report a negative or null result, the so-called publication bias [ 48 – 50 ]. This new openness is consistent with the fact that if the substantive question addressed is really relevant, the answer (whether positive or negative) will also be relevant.
Consequently, even though it was not an aim of our study, we found many examples in which statistical significance was not obtained. However, many of those negative results were reported with a comment of this type: " The results did not show a significant difference between groups; however, with a larger sample size, this difference would have probably proved to be significant ". The problem with this statement is that it is true; more specifically, it will always be true and it is, therefore, sterile. It is not fortuitous that one never encounters the opposite, and equally tautological, statement: " A significant difference between groups has been detected; however, perhaps with a smaller sample size, this difference would have proved to be not significant" . Such a double standard is itself an unequivocal sign of the ritual application of NHST.
Although the declining rates of NHST usage show that, gradually, ICMJE and similar recommendations are having a positive impact, most of the articles in the clinical setting still considered NHST as the final arbiter of the research process. Moreover, it appears that the improvement in the situation is mostly formal, and the percentage of articles that fall into the significance fallacy is huge.
The contradiction between what has been conceptually recommended and the common practice is sensibly less acute in the area of Epidemiology and Public Health, but the same pattern was evident everywhere in the mechanical way of applying significance tests. Nevertheless, the clinical journals remain the most unmoved by the recommendations.
The ICMJE recommendations are not cosmetic statements but substantial ones, and the vigorous exhortations made by outstanding authorities [ 51 ] are not mere intellectual exercises due to ingenious and inopportune methodologists, but rather they are very serious epistemological warnings.
In some cases, the role of CI is not as clearly suitable (e.g. when estimating multiple regression coefficients or because effect sizes are not available for some research designs [ 43 , 52 ]), but when it comes to estimating, for example, an odds ratio or a rates difference, the advantage of using CI instead of P values is very clear, since in such cases it is obvious that the goal is to assess what has been called the "effect size."
The inherent resistance to change old paradigms and practices that have been entrenched for decades is always high. Old habits die hard. The estimates and trends outlined are entirely consistent with Alvan Feinstein's warning 25 years ago: "Because the history of medical research also shows a long tradition of maintaining loyalty to established doctrines long after the doctrines had been discredited, or shown to be valueless, we cannot expect a sudden change in this medical policy merely because it has been denounced by leading connoisseurs of statistics [ 53 ]".
It is possible, however, that the nature of the problem has an external explanation: it is likely that some editors prefer to "avoid troubles" with the authors and vice versa, thus resorting to the most conventional procedures. Many junior researchers believe that it is wise to avoid long back-and-forth discussions with reviewers and editors. In general, researchers who want to appear in print and survive in a publish-or-perish environment are motivated by force, fear, and expedience in their use of NHST [ 54 ]. Furthermore, it is relatively natural that simple researchers use NHST when they take into account that some theoretical objectors have used this statistical analysis in empirical studies, published after the appearance of their own critiques [ 55 ].
For example, Journal of the American Medical Association published a bibliometric study [ 56 ] discussing the impact of statisticians' co-authorship of medical papers on publication decisions by two major high-impact journals: British Medical Journal and Annals of Internal Medicine . The data analysis is characterized by methodological orthodoxy. The authors just use chi-square tests without any reference to CI, although the NHST had been repeatedly criticized over the years by two of the authors:
Douglas Altman, an early promoter of confidence intervals as an alternative [ 57 ], and Steve Goodman, a critic of NHST from a Bayesian perspective [ 58 ]. Individual authors, however, cannot be blamed for broader institutional problems and systemic forces opposed to change.
The present effort is certainly partial in at least two ways: it is limited to only six specific journals and to three biennia. It would be therefore highly desirable to improve it by studying the problem in a more detailed way (especially by reviewing more journals with different profiles), and continuing the review of prevailing patterns and trends.
Curran-Everett D: Explorations in statistics: hypothesis tests and P values. Adv Physiol Educ. 2009, 33: 81-86. 10.1152/advan.90218.2008.
Article PubMed Google Scholar
Fisher RA: Statistical Methods for Research Workers. 1925, Edinburgh: Oliver & Boyd
Google Scholar
Neyman J, Pearson E: On the use and interpretation of certain test criteria for purposes of statistical inference. Biometrika. 1928, 20: 175-240.
Silva LC: Los laberintos de la investigación biomédica. En defensa de la racionalidad para la ciencia del siglo XXI. 2009, Madrid: Díaz de Santos
Berkson J: Test of significance considered as evidence. J Am Stat Assoc. 1942, 37: 325-335. 10.2307/2279000.
Article Google Scholar
Nickerson RS: Null hypothesis significance testing: A review of an old and continuing controversy. Psychol Methods. 2000, 5: 241-301. 10.1037/1082-989X.5.2.241.
Article CAS PubMed Google Scholar
Rozeboom WW: The fallacy of the null hypothesissignificance test. Psychol Bull. 1960, 57: 418-428. 10.1037/h0042040.
Callahan JL, Reio TG: Making subjective judgments in quantitative studies: The importance of using effect sizes and confidenceintervals. HRD Quarterly. 2006, 17: 159-173.
Nakagawa S, Cuthill IC: Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev. 2007, 82: 591-605. 10.1111/j.1469-185X.2007.00027.x.
Breaugh JA: Effect size estimation: factors to consider and mistakes to avoid. J Manage. 2003, 29: 79-97. 10.1177/014920630302900106.
Thompson B: What future quantitative social science research could look like: confidence intervals for effect sizes. Educ Res. 2002, 31: 25-32.
Matthews RA: Significance levels for the assessment of anomalous phenomena. Journal of Scientific Exploration. 1999, 13: 1-7.
Savage IR: Nonparametric statistics. J Am Stat Assoc. 1957, 52: 332-333.
Silva LC, Benavides A, Almenara J: El péndulo bayesiano: Crónica de una polémica estadística. Llull. 2002, 25: 109-128.
Goodman SN, Royall R: Evidence and scientific research. Am J Public Health. 1988, 78: 1568-1574. 10.2105/AJPH.78.12.1568.
Article CAS PubMed PubMed Central Google Scholar
Berger JO, Berry DA: Statistical analysis and the illusion of objectivity. Am Sci. 1988, 76: 159-165.
Hurlbert SH, Lombardi CM: Final collapse of the Neyman-Pearson decision theoretic framework and rise of the neoFisherian. Ann Zool Fenn. 2009, 46: 311-349.
Fidler F, Thomason N, Cumming G, Finch S, Leeman J: Editors can lead researchers to confidence intervals but they can't make them think: Statistical reform lessons from Medicine. Psychol Sci. 2004, 15: 119-126. 10.1111/j.0963-7214.2004.01502008.x.
Balluerka N, Vergara AI, Arnau J: Calculating the main alternatives to null-hypothesis-significance testing in between-subject experimental designs. Psicothema. 2009, 21: 141-151.
Cumming G, Fidler F: Confidence intervals: Better answers to better questions. J Psychol. 2009, 217: 15-26.
Jones LV, Tukey JW: A sensible formulation of the significance test. Psychol Methods. 2000, 5: 411-414. 10.1037/1082-989X.5.4.411.
Dixon P: The p-value fallacy and how to avoid it. Can J Exp Psychol. 2003, 57: 189-202.
Nakagawa S, Cuthill IC: Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev Camb Philos Soc. 2007, 82: 591-605. 10.1111/j.1469-185X.2007.00027.x.
Brandstaetter E: Confidence intervals as an alternative to significance testing. MPR-Online. 2001, 4: 33-46.
Masson ME, Loftus GR: Using confidence intervals for graphically based data interpretation. Can J Exp Psychol. 2003, 57: 203-220.
International Committee of Medical Journal Editors: Uniform requirements for manuscripts submitted to biomedical journals. Update October 2008. Accessed July 11, 2009, [ http://www.icmje.org ]
Feinstein AR: P-Values and Confidence Intervals: two sides of the same unsatisfactory coin. J Clin Epidemiol. 1998, 51: 355-360. 10.1016/S0895-4356(97)00295-3.
Haller H, Kraus S: Misinterpretations of significance: A problem students share with their teachers?. MRP-Online. 2002, 7: 1-20.
Gigerenzer G, Krauss S, Vitouch O: The null ritual: What you always wanted to know about significance testing but were afraid to ask. The Handbook of Methodology for the Social Sciences. Edited by: Kaplan D. 2004, Thousand Oaks, CA: Sage Publications, Chapter 21: 391-408.
Curran-Everett D, Taylor S, Kafadar K: Fundamental concepts in statistics: elucidation and illustration. J Appl Physiol. 1998, 85: 775-786.
CAS PubMed Google Scholar
Royall RM: Statistical evidence: a likelihood paradigm. 1997, Boca Raton: Chapman & Hall/CRC
Goodman SN: Of P values and Bayes: A modest proposal. Epidemiology. 2001, 12: 295-297. 10.1097/00001648-200105000-00006.
Sarria M, Silva LC: Tests of statistical significance in three biomedical journals: a critical review. Rev Panam Salud Publica. 2004, 15: 300-306.
Silva LC: Una ceremonia estadística para identificar factores de riesgo. Salud Colectiva. 2005, 1: 322-329.
Goodman SN: Toward Evidence-Based Medical Statistics 1: The p Value Fallacy. Ann Intern Med. 1999, 130: 995-1004.
Schulz KF, Grimes DA: Sample size calculations in randomised clinical trials: mandatory and mystical. Lancet. 2005, 365: 1348-1353. 10.1016/S0140-6736(05)61034-3.
Bacchetti P: Current sample size conventions: Flaws, harms, and alternatives. BMC Med. 2010, 8: 17-10.1186/1741-7015-8-17.
Article PubMed PubMed Central Google Scholar
Silva LC: Diseño razonado de muestras para la investigación sanitaria. 2000, Madrid: Díaz de Santos
Barnett ML, Mathisen A: Tyranny of the p-value: The conflict between statistical significance and common sense. J Dent Res. 1997, 76: 534-536. 10.1177/00220345970760010201.
Santiago MI, Hervada X, Naveira G, Silva LC, Fariñas H, Vázquez E, Bacallao J, Mújica OJ: [The Epidat program: uses and perspectives] [letter]. Pan Am J Public Health. 2010, 27: 80-82. Spanish.
Landis JR, Koch GG: The measurement of observer agreement for categorical data. Biometrics. 1977, 33: 159-74. 10.2307/2529310.
Fidler F, Burgman MA, Cumming G, Buttrose R, Thomason N: Impact of criticism of null-hypothesis significance testing on statistical reporting practices in conservation biology. Conserv Biol. 2005, 20: 1539-1544. 10.1111/j.1523-1739.2006.00525.x.
Kline RB: Beyond significance testing: Reforming data analysis methods in behavioral research. 2004, Washington, DC: American Psychological Association
Book Google Scholar
Curran-Everett D, Benos DJ: Guidelines for reporting statistics in journals published by the American Physiological Society: the sequel. Adv Physiol Educ. 2007, 31: 295-298. 10.1152/advan.00022.2007.
Hubbard R, Parsa AR, Luthy MR: The spread of statistical significance testing: The case of the Journal of Applied Psychology. Theor Psychol. 1997, 7: 545-554. 10.1177/0959354397074006.
Vacha-Haase T, Nilsson JE, Reetz DR, Lance TS, Thompson B: Reporting practices and APA editorial policies regarding statistical significance and effect size. Theor Psychol. 2000, 10: 413-425. 10.1177/0959354300103006.
Krueger J: Null hypothesis significance testing: On the survival of a flawed method. Am Psychol. 2001, 56: 16-26. 10.1037/0003-066X.56.1.16.
Rising K, Bacchetti P, Bero L: Reporting Bias in Drug Trials Submitted to the Food and Drug Administration: Review of Publication and Presentation. PLoS Med. 2008, 5: e217-10.1371/journal.pmed.0050217. doi:10.1371/journal.pmed.0050217
Sridharan L, Greenland L: Editorial policies and publication bias the importance of negative studies. Arch Intern Med. 2009, 169: 1022-1023. 10.1001/archinternmed.2009.100.
Falagas ME, Alexiou VG: The top-ten in journal impact factor manipulation. Arch Immunol Ther Exp (Warsz). 2008, 56: 223-226. 10.1007/s00005-008-0024-5.
Rothman K: Writing for Epidemiology. Epidemiology. 1998, 9: 98-104. 10.1097/00001648-199805000-00019.
Fidler F: The fifth edition of the APA publication manual: Why its statistics recommendations are so controversial. Educ Psychol Meas. 2002, 62: 749-770. 10.1177/001316402236876.
Feinstein AR: Clinical epidemiology: The architecture of clinical research. 1985, Philadelphia: W.B. Saunders Company
Orlitzky M: Institutionalized dualism: statistical significance testing as myth and ceremony. Accessed Feb 8, 2010, [ http://ssrn.com/abstract=1415926 ]
Greenwald AG, González R, Harris RJ, Guthrie D: Effect sizes and p-value. What should be reported and what should be replicated?. Psychophysiology. 1996, 33: 175-183. 10.1111/j.1469-8986.1996.tb02121.x.
Altman DG, Goodman SN, Schroter S: How statistical expertise is used in medical research. J Am Med Assoc. 2002, 287: 2817-2820. 10.1001/jama.287.21.2817.
Gardner MJ, Altman DJ: Statistics with confidence. Confidence intervals and statistical guidelines. 1992, London: BMJ
Goodman SN: P Values, Hypothesis Tests and Likelihood: implications for epidemiology of a neglected historical debate. Am J Epidemiol. 1993, 137: 485-496.
Pre-publication history
The pre-publication history for this paper can be accessed here: http://www.biomedcentral.com/1471-2288/10/44/prepub
Download references
Acknowledgements
The authors would like to thank Tania Iglesias-Cabo and Vanesa Alvarez-González for their help with the collection of empirical data and their participation in an earlier version of the paper. The manuscript has benefited greatly from thoughtful, constructive feedback by Carlos Campillo-Artero, Tom Piazza and Ann Séror.
Author information
Authors and affiliations.
Centro Nacional de Investigación de Ciencias Médicas, La Habana, Cuba
Luis Carlos Silva-Ayçaguer
Unidad de Investigación. Hospital de Cabueñes, Servicio de Salud del Principado de Asturias (SESPA), Gijón, Spain
Patricio Suárez-Gil
CIBER Epidemiología y Salud Pública (CIBERESP), Spain and Departamento de Medicina, Unidad de Epidemiología Molecular del Instituto Universitario de Oncología, Universidad de Oviedo, Spain
Ana Fernández-Somoano
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Patricio Suárez-Gil .
Additional information
Competing interests.
The authors declare that they have no competing interests.
Authors' contributions
LCSA designed the study, wrote the paper and supervised the whole process; PSG coordinated the data extraction and carried out statistical analysis, as well as participated in the editing process; AFS extracted the data and participated in the first stage of statistical analysis; all authors contributed to and revised the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Authors’ original file for figure 1
Rights and permissions.
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Reprints and permissions
About this article
Cite this article.
Silva-Ayçaguer, L.C., Suárez-Gil, P. & Fernández-Somoano, A. The null hypothesis significance test in health sciences research (1995-2006): statistical analysis and interpretation. BMC Med Res Methodol 10 , 44 (2010). https://doi.org/10.1186/1471-2288-10-44
Download citation
Received : 29 December 2009
Accepted : 19 May 2010
Published : 19 May 2010
DOI : https://doi.org/10.1186/1471-2288-10-44
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Clinical Specialty
- Significance Fallacy
- Null Hypothesis Statistical Testing
- Medical Journal Editor
- Clinical Journal
BMC Medical Research Methodology
ISSN: 1471-2288
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Ind Psychiatry J
- v.18(2); Jul-Dec 2009
Hypothesis testing, type I and type II errors
Amitav banerjee.
Department of Community Medicine, D. Y. Patil Medical College, Pune, India
U. B. Chitnis
S. l. jadhav, j. s. bhawalkar, s. chaudhury.
1 Department of Psychiatry, RINPAS, Kanke, Ranchi, India
Hypothesis testing is an important activity of empirical research and evidence-based medicine. A well worked up hypothesis is half the answer to the research question. For this, both knowledge of the subject derived from extensive review of the literature and working knowledge of basic statistical concepts are desirable. The present paper discusses the methods of working up a good hypothesis and statistical concepts of hypothesis testing.
Karl Popper is probably the most influential philosopher of science in the 20 th century (Wulff et al ., 1986). Many scientists, even those who do not usually read books on philosophy, are acquainted with the basic principles of his views on science. The popularity of Popper’s philosophy is due partly to the fact that it has been well explained in simple terms by, among others, the Nobel Prize winner Peter Medawar (Medawar, 1969). Popper makes the very important point that empirical scientists (those who stress on observations only as the starting point of research) put the cart in front of the horse when they claim that science proceeds from observation to theory, since there is no such thing as a pure observation which does not depend on theory. Popper states, “… the belief that we can start with pure observation alone, without anything in the nature of a theory, is absurd: As may be illustrated by the story of the man who dedicated his life to natural science, wrote down everything he could observe, and bequeathed his ‘priceless’ collection of observations to the Royal Society to be used as inductive (empirical) evidence.
STARTING POINT OF RESEARCH: HYPOTHESIS OR OBSERVATION?
The first step in the scientific process is not observation but the generation of a hypothesis which may then be tested critically by observations and experiments. Popper also makes the important claim that the goal of the scientist’s efforts is not the verification but the falsification of the initial hypothesis. It is logically impossible to verify the truth of a general law by repeated observations, but, at least in principle, it is possible to falsify such a law by a single observation. Repeated observations of white swans did not prove that all swans are white, but the observation of a single black swan sufficed to falsify that general statement (Popper, 1976).
CHARACTERISTICS OF A GOOD HYPOTHESIS
A good hypothesis must be based on a good research question. It should be simple, specific and stated in advance (Hulley et al ., 2001).
Hypothesis should be simple
A simple hypothesis contains one predictor and one outcome variable, e.g. positive family history of schizophrenia increases the risk of developing the condition in first-degree relatives. Here the single predictor variable is positive family history of schizophrenia and the outcome variable is schizophrenia. A complex hypothesis contains more than one predictor variable or more than one outcome variable, e.g., a positive family history and stressful life events are associated with an increased incidence of Alzheimer’s disease. Here there are 2 predictor variables, i.e., positive family history and stressful life events, while one outcome variable, i.e., Alzheimer’s disease. Complex hypothesis like this cannot be easily tested with a single statistical test and should always be separated into 2 or more simple hypotheses.
Hypothesis should be specific
A specific hypothesis leaves no ambiguity about the subjects and variables, or about how the test of statistical significance will be applied. It uses concise operational definitions that summarize the nature and source of the subjects and the approach to measuring variables (History of medication with tranquilizers, as measured by review of medical store records and physicians’ prescriptions in the past year, is more common in patients who attempted suicides than in controls hospitalized for other conditions). This is a long-winded sentence, but it explicitly states the nature of predictor and outcome variables, how they will be measured and the research hypothesis. Often these details may be included in the study proposal and may not be stated in the research hypothesis. However, they should be clear in the mind of the investigator while conceptualizing the study.
Hypothesis should be stated in advance
The hypothesis must be stated in writing during the proposal state. This will help to keep the research effort focused on the primary objective and create a stronger basis for interpreting the study’s results as compared to a hypothesis that emerges as a result of inspecting the data. The habit of post hoc hypothesis testing (common among researchers) is nothing but using third-degree methods on the data (data dredging), to yield at least something significant. This leads to overrating the occasional chance associations in the study.
TYPES OF HYPOTHESES
For the purpose of testing statistical significance, hypotheses are classified by the way they describe the expected difference between the study groups.
Null and alternative hypotheses
The null hypothesis states that there is no association between the predictor and outcome variables in the population (There is no difference between tranquilizer habits of patients with attempted suicides and those of age- and sex- matched “control” patients hospitalized for other diagnoses). The null hypothesis is the formal basis for testing statistical significance. By starting with the proposition that there is no association, statistical tests can estimate the probability that an observed association could be due to chance.
The proposition that there is an association — that patients with attempted suicides will report different tranquilizer habits from those of the controls — is called the alternative hypothesis. The alternative hypothesis cannot be tested directly; it is accepted by exclusion if the test of statistical significance rejects the null hypothesis.
One- and two-tailed alternative hypotheses
A one-tailed (or one-sided) hypothesis specifies the direction of the association between the predictor and outcome variables. The prediction that patients of attempted suicides will have a higher rate of use of tranquilizers than control patients is a one-tailed hypothesis. A two-tailed hypothesis states only that an association exists; it does not specify the direction. The prediction that patients with attempted suicides will have a different rate of tranquilizer use — either higher or lower than control patients — is a two-tailed hypothesis. (The word tails refers to the tail ends of the statistical distribution such as the familiar bell-shaped normal curve that is used to test a hypothesis. One tail represents a positive effect or association; the other, a negative effect.) A one-tailed hypothesis has the statistical advantage of permitting a smaller sample size as compared to that permissible by a two-tailed hypothesis. Unfortunately, one-tailed hypotheses are not always appropriate; in fact, some investigators believe that they should never be used. However, they are appropriate when only one direction for the association is important or biologically meaningful. An example is the one-sided hypothesis that a drug has a greater frequency of side effects than a placebo; the possibility that the drug has fewer side effects than the placebo is not worth testing. Whatever strategy is used, it should be stated in advance; otherwise, it would lack statistical rigor. Data dredging after it has been collected and post hoc deciding to change over to one-tailed hypothesis testing to reduce the sample size and P value are indicative of lack of scientific integrity.
STATISTICAL PRINCIPLES OF HYPOTHESIS TESTING
A hypothesis (for example, Tamiflu [oseltamivir], drug of choice in H1N1 influenza, is associated with an increased incidence of acute psychotic manifestations) is either true or false in the real world. Because the investigator cannot study all people who are at risk, he must test the hypothesis in a sample of that target population. No matter how many data a researcher collects, he can never absolutely prove (or disprove) his hypothesis. There will always be a need to draw inferences about phenomena in the population from events observed in the sample (Hulley et al ., 2001). In some ways, the investigator’s problem is similar to that faced by a judge judging a defendant [ Table 1 ]. The absolute truth whether the defendant committed the crime cannot be determined. Instead, the judge begins by presuming innocence — the defendant did not commit the crime. The judge must decide whether there is sufficient evidence to reject the presumed innocence of the defendant; the standard is known as beyond a reasonable doubt. A judge can err, however, by convicting a defendant who is innocent, or by failing to convict one who is actually guilty. In similar fashion, the investigator starts by presuming the null hypothesis, or no association between the predictor and outcome variables in the population. Based on the data collected in his sample, the investigator uses statistical tests to determine whether there is sufficient evidence to reject the null hypothesis in favor of the alternative hypothesis that there is an association in the population. The standard for these tests is shown as the level of statistical significance.
The analogy between judge’s decisions and statistical tests
TYPE I (ALSO KNOWN AS ‘α’) AND TYPE II (ALSO KNOWN AS ‘β’)ERRORS
Just like a judge’s conclusion, an investigator’s conclusion may be wrong. Sometimes, by chance alone, a sample is not representative of the population. Thus the results in the sample do not reflect reality in the population, and the random error leads to an erroneous inference. A type I error (false-positive) occurs if an investigator rejects a null hypothesis that is actually true in the population; a type II error (false-negative) occurs if the investigator fails to reject a null hypothesis that is actually false in the population. Although type I and type II errors can never be avoided entirely, the investigator can reduce their likelihood by increasing the sample size (the larger the sample, the lesser is the likelihood that it will differ substantially from the population).
False-positive and false-negative results can also occur because of bias (observer, instrument, recall, etc.). (Errors due to bias, however, are not referred to as type I and type II errors.) Such errors are troublesome, since they may be difficult to detect and cannot usually be quantified.
EFFECT SIZE
The likelihood that a study will be able to detect an association between a predictor variable and an outcome variable depends, of course, on the actual magnitude of that association in the target population. If it is large (such as 90% increase in the incidence of psychosis in people who are on Tamiflu), it will be easy to detect in the sample. Conversely, if the size of the association is small (such as 2% increase in psychosis), it will be difficult to detect in the sample. Unfortunately, the investigator often does not know the actual magnitude of the association — one of the purposes of the study is to estimate it. Instead, the investigator must choose the size of the association that he would like to be able to detect in the sample. This quantity is known as the effect size. Selecting an appropriate effect size is the most difficult aspect of sample size planning. Sometimes, the investigator can use data from other studies or pilot tests to make an informed guess about a reasonable effect size. When there are no data with which to estimate it, he can choose the smallest effect size that would be clinically meaningful, for example, a 10% increase in the incidence of psychosis. Of course, from the public health point of view, even a 1% increase in psychosis incidence would be important. Thus the choice of the effect size is always somewhat arbitrary, and considerations of feasibility are often paramount. When the number of available subjects is limited, the investigator may have to work backward to determine whether the effect size that his study will be able to detect with that number of subjects is reasonable.
α,β,AND POWER
After a study is completed, the investigator uses statistical tests to try to reject the null hypothesis in favor of its alternative (much in the same way that a prosecuting attorney tries to convince a judge to reject innocence in favor of guilt). Depending on whether the null hypothesis is true or false in the target population, and assuming that the study is free of bias, 4 situations are possible, as shown in Table 2 below. In 2 of these, the findings in the sample and reality in the population are concordant, and the investigator’s inference will be correct. In the other 2 situations, either a type I (α) or a type II (β) error has been made, and the inference will be incorrect.
Truth in the population versus the results in the study sample: The four possibilities
The investigator establishes the maximum chance of making type I and type II errors in advance of the study. The probability of committing a type I error (rejecting the null hypothesis when it is actually true) is called α (alpha) the other name for this is the level of statistical significance.
If a study of Tamiflu and psychosis is designed with α = 0.05, for example, then the investigator has set 5% as the maximum chance of incorrectly rejecting the null hypothesis (and erroneously inferring that use of Tamiflu and psychosis incidence are associated in the population). This is the level of reasonable doubt that the investigator is willing to accept when he uses statistical tests to analyze the data after the study is completed.
The probability of making a type II error (failing to reject the null hypothesis when it is actually false) is called β (beta). The quantity (1 - β) is called power, the probability of observing an effect in the sample (if one), of a specified effect size or greater exists in the population.
If β is set at 0.10, then the investigator has decided that he is willing to accept a 10% chance of missing an association of a given effect size between Tamiflu and psychosis. This represents a power of 0.90, i.e., a 90% chance of finding an association of that size. For example, suppose that there really would be a 30% increase in psychosis incidence if the entire population took Tamiflu. Then 90 times out of 100, the investigator would observe an effect of that size or larger in his study. This does not mean, however, that the investigator will be absolutely unable to detect a smaller effect; just that he will have less than 90% likelihood of doing so.
Ideally alpha and beta errors would be set at zero, eliminating the possibility of false-positive and false-negative results. In practice they are made as small as possible. Reducing them, however, usually requires increasing the sample size. Sample size planning aims at choosing a sufficient number of subjects to keep alpha and beta at acceptably low levels without making the study unnecessarily expensive or difficult.
Many studies s et al pha at 0.05 and beta at 0.20 (a power of 0.80). These are somewhat arbitrary values, and others are sometimes used; the conventional range for alpha is between 0.01 and 0.10; and for beta, between 0.05 and 0.20. In general the investigator should choose a low value of alpha when the research question makes it particularly important to avoid a type I (false-positive) error, and he should choose a low value of beta when it is especially important to avoid a type II error.
The null hypothesis acts like a punching bag: It is assumed to be true in order to shadowbox it into false with a statistical test. When the data are analyzed, such tests determine the P value, the probability of obtaining the study results by chance if the null hypothesis is true. The null hypothesis is rejected in favor of the alternative hypothesis if the P value is less than alpha, the predetermined level of statistical significance (Daniel, 2000). “Nonsignificant” results — those with P value greater than alpha — do not imply that there is no association in the population; they only mean that the association observed in the sample is small compared with what could have occurred by chance alone. For example, an investigator might find that men with family history of mental illness were twice as likely to develop schizophrenia as those with no family history, but with a P value of 0.09. This means that even if family history and schizophrenia were not associated in the population, there was a 9% chance of finding such an association due to random error in the sample. If the investigator had set the significance level at 0.05, he would have to conclude that the association in the sample was “not statistically significant.” It might be tempting for the investigator to change his mind about the level of statistical significance ex post facto and report the results “showed statistical significance at P < 10”. A better choice would be to report that the “results, although suggestive of an association, did not achieve statistical significance ( P = .09)”. This solution acknowledges that statistical significance is not an “all or none” situation.
Hypothesis testing is the sheet anchor of empirical research and in the rapidly emerging practice of evidence-based medicine. However, empirical research and, ipso facto, hypothesis testing have their limits. The empirical approach to research cannot eliminate uncertainty completely. At the best, it can quantify uncertainty. This uncertainty can be of 2 types: Type I error (falsely rejecting a null hypothesis) and type II error (falsely accepting a null hypothesis). The acceptable magnitudes of type I and type II errors are set in advance and are important for sample size calculations. Another important point to remember is that we cannot ‘prove’ or ‘disprove’ anything by hypothesis testing and statistical tests. We can only knock down or reject the null hypothesis and by default accept the alternative hypothesis. If we fail to reject the null hypothesis, we accept it by default.
Source of Support: Nil
Conflict of Interest: None declared.
- Daniel W. W. In: Biostatistics. 7th ed. New York: John Wiley and Sons, Inc; 2002. Hypothesis testing; pp. 204–294. [ Google Scholar ]
- Hulley S. B, Cummings S. R, Browner W. S, Grady D, Hearst N, Newman T. B. 2nd ed. Philadelphia: Lippincott Williams and Wilkins; 2001. Getting ready to estimate sample size: Hypothesis and underlying principles In: Designing Clinical Research-An epidemiologic approach; pp. 51–63. [ Google Scholar ]
- Medawar P. B. Philadelphia: American Philosophical Society; 1969. Induction and intuition in scientific thought. [ Google Scholar ]
- Popper K. Unended Quest. An Intellectual Autobiography. Fontana Collins; p. 42. [ Google Scholar ]
- Wulff H. R, Pedersen S. A, Rosenberg R. Oxford: Blackwell Scientific Publicatons; Empirism and Realism: A philosophical problem. In: Philosophy of Medicine. [ Google Scholar ]

IMAGES
VIDEO
COMMENTS
Medical providers often rely on evidence-based medicine to guide decision-making in practice. Often a research hypothesis is tested with results provided, typically with p values, confidence intervals, or both. Additionally, statistical or research significance is estimated or determined by the investigators. Unfortunately, healthcare providers may have different comfort levels in interpreting ...
The investigator formulates a specific hypothesis, evaluates data from the sample, and uses these data to decide whether they support the specific hypothesis. The first step in testing hypotheses is the transformation of the research question into a null hypothesis, H 0, and an alternative hypothesis, H A. 6 The null and alternative hypotheses ...
The present paper attempts to put the P value in proper perspective by explaining different types of probabilities, their role in clinical decision making, medical research and hypothesis testing. Keywords: Hypothesis testing, P value, Probability. The clinician who wishes to remain abreast with the results of medical research needs to develop ...
A hypothesis test is a procedure used in statistics to assess whether a particular viewpoint is likely to be true. They follow a strict protocol, and they generate a 'p-value', on the basis of which a decision is made about the truth of the hypothesis under investigation.All of the routine statistical 'tests' used in research—t-tests, χ 2 tests, Mann-Whitney tests, etc.—are all ...
Step 5: Present your findings. The results of hypothesis testing will be presented in the results and discussion sections of your research paper, dissertation or thesis.. In the results section you should give a brief summary of the data and a summary of the results of your statistical test (for example, the estimated difference between group means and associated p-value).
Abstract. The role of hypothesis testing, and especially of p-values, in evaluating the results of scientific experiments has been under debate for a long time.At least since the influential article by Ioannidis (Citation 2005) awareness is growing in the scientific community that the results of many research experiments are difficult or impossible to replicate.
Hypothesis testing is usually performed using a test statistic, which summarizes the sample information. Under a certain set of assumptions, a test statistic follows an exact or approximate distribution under H 0 that reflects the randomness associated with the sample. The P value, the probability of obtaining a statistic at least as extreme as the test statistic in the direction of H A if H 0 ...
Hypothesis and hypothesis testing in the clinical trial. 2001:62 Suppl 9:5-8; discussion 9-10. Department of Psychiatry and Mental Health and Neuroscience Clinical Research Center, University of North Carolina School of Medicine, Chapel Hill 27599-7160, USA. The hypothesis provides the justification for the clinical trial.
p>"Hypothesis testing" is an integral and most important component of research methodology, in all researches, whether in medical sciences, social sciences or any such allied field. It is a ...
In hypothesis testing, the research question of interest is simplified into one of two possible hypothesis types: the null hypothesis, denoted H 0, or the research hypothesis (sometimes called the alternative hypothesis), denoted H 1.In most cases, it is easier to reject a statement that is false (null hypothesis); therefore, we generally assume the opposite of our research question of interest.
Often a research hypothesis is tested with results provided, typically with p values, confidence intervals, or both. Additionally, statistical or research significance is estimated or determined by the investigators. ... Confidence intervals rather than P values: estimation rather than hypothesis testing. British medical journal (Clinical ...
In 1973, the Medical Subject Heading (MeSH) of the U.S. National Library of Medicine introduced "Research Design" as a structured keyword that referred to the importance of collecting data and properly testing hypotheses, and indirectly linked the term to ethics, methods and standards, among many other subheadings.
The alternative hypothesis proposed in medical research may be one-tailed or two-tailed. A one-tailed alternative hypothesis would predict the direction of the effect. Clinical studies may have an alternative hypothesis that patients taking the study drug will have a lower cholesterol level than those taking a placebo.
Abstract and Figures. Research is an integral part of the medical curriculum. Postgraduate students are exposed to the concept of literature search and research methodologies in the form of their ...
Abstract. Statistical hypothesis testing is one of the key steps in modern medical research. Initially, scientists formulate a research hypothesis based on which the statistical hypothesis is then ...
6.1 Example of Hypothesis Testing. We shall work out an example using one-sample Z test to do hypothesis testing.. A medical officer claims that in a particular village, women of reproductive age group have higher haemoglobin levels, a random sample of 100 women showed a mean haemoglobin level of 16.5 g/dL.
The p-value is a practical tool gauging the "strength of evidence" against the null hypothesis. It informs investigators that a p-value of 0.001, for example, is stronger than 0.05. However, p-values produced in significance testing are not the probabilities of type I errors as commonly misconceived.
There is a close relationship between CIs of effect size estimates and hypothesis testing. When the 95% CI of an effect size does not contain the null hypothesis value that indicates "no effect" (eg, an odds ratio of exactly 1), this corresponds to a "statistically significant" result with a .05 alpha level in a hypothesis test.
Like other application fields in medical sciences as well , the analytitical frame work in respect of testing of hypothesis comprises of 1. formulating research hypothesis 2. framing corresponding ...
Abstract. Generating a testable working hypothesis is the first step towards conducting original research. Such research may prove or disprove the proposed hypothesis. Case reports, case series, online surveys and other observational studies, clinical trials, and narrative reviews help to generate hypotheses.
Major Problems Using the p Values as Result of a Hypothesis Test. Many investigators, in various research fields refer to Neyman & Pearson hypothesis tests and their associated p values. Indeed, the p value is a widely used tool for inference in studies. However, despite the numerous books, papers and other scientific literature published on this topic, there still seems to be serious misuses ...
The null hypothesis significance test (NHST) is the most frequently used statistical method, although its inferential validity has been widely criticized since its introduction. In 1988, the International Committee of Medical Journal Editors (ICMJE) warned against sole reliance on NHST to substantiate study conclusions and suggested supplementary use of confidence intervals (CI).
Hypothesis testing is an important activity of empirical research and evidence-based medicine. A well worked up hypothesis is half the answer to the research question. ... as measured by review of medical store records and physicians' prescriptions in the past year, is more common in patients who attempted suicides than in controls ...