
Get work done right, and right-on-time with our industry leading BPM platform.

Project Approval Process Made Easy: 5 Steps & Best Practices
The project approval process is often rushed.
It’s understandable that you want to quickly and efficiently evaluate and launch new projects to stay competitive.
But a well-defined project approval process is critical to achieving these goals.
This article will guide you through the steps, benefits, and considerations you should consider before implementing this process .
What is the project approval process?
The project approval process incorporates a structured set of steps that a project must go through before it can begin.
It is essentially a roadmap that ensures that the right projects are undertaken at the right time and with the right resources.
A well-defined project approval process can also help you reduce risks , make better decisions, and increase accountability. That’s why it’s important to get it right to avoid problems later on down the line.
Let me show you how.
Project approval process steps
Because the process involves obtaining approval from various stakeholders, it can be tricky to manage it well.
That’s why I’ve broken down the project approval process into several stages that you may find useful to follow:
- Project Initiation: The first stage is about defining the project’s scope, objectives, and requirements. Potential stakeholders are identified, and their needs and expectations are assessed.
- Planning : Next, you’ll develop a detailed project plan, which will include timelines, budgets, and resource requirements. This is also the right time to create a risk management proposal and identify potential roadblocks .
- Execution : Once you’re happy with all the above, you can begin to implement the project plan.
- Monitoring and Control : As with any process, you should make sure that the project’s progress is tracked. This will help you to identify any potential issues and take action fast.
- Closure : Finally, you may move on to reviewing the project’s results and outcomes, assessing lessons learned, and documenting the project’s success.
Remember that throughout the whole process, approvals must be obtained before proceeding to the next stage.
The Benefits of a Project Approval Process
Having a well-thought-through project approval process in place comes with a number of benefits:
Benefit 1: Risk management
It helps reduce risk by ensuring that projects are aligned with business objectives and that resources are allocated efficiently.
This is particularly important in industries such as healthcare or finance, where regulatory compliance and risk management are critical.
Benefit 2: Better decision-making
A project approval process can improve decision-making by providing a structured framework for evaluating projects.
What this means is that you can ensure the projects are assessed objectively and based on pre-defined criteria.
As a result, you’ll be able to make informed decisions about which projects to pursue and which to reject.
Benefit 3: Improved accountability
A project approval process can increase accountability by defining roles and responsibilities and establishing clear metrics for success.
In turn, this guaranteed that everyone involved in the project understands their role and the project’s objectives.
A project approval process makes it easy for the project to be delivered on time, within budget, and to the required quality standards.
Benefit 4: Enhanced communication
A project approval process boosts communication by ensuring that stakeholders are involved in the project from the outset.
The result? Everyone involved in the project is aware of its status and progress, which facilitates transparency and improves trust.
Project approval process examples
Now, I’d like to show you a couple of examples to help me better illustrate how the project approval process may look in action.
Example of a good approval process
I’ve devised this rough approvals plan to demonstrate how you would go about your approval process.
So let’s say you are running a software development company and have a new feature you want to release to your customers:
1. Development
The development team creates and tests the new feature thoroughly in a staging environment.
2. Quality Assurance (QA)
Then, the QA team reviews the new feature to ensure it meets the company’s quality standards and works as expected.
3. Product Management
The product management team reviews the new feature to confirm it aligns with the overall product strategy and customer needs.
The legal team reviews the new feature to make sure it complies with legal or regulatory requirements.
5. Executive Approval
The executive team then reviews and approves the new feature for release to customers.
The new feature is released to customers.
By having a clear approval process in place, the company can ensure that they are releasing high-quality features that meet the needs of their customers while minimizing the risk of any legal or regulatory issues.
This approval process example is more or less the starting point we’d like to see when releasing a new feature.
Example of a bad approval process
Now, let’s move on to what you don’t want to be doing.
Let’s say you work for a marketing agency, and your approval process for creating new marketing campaigns is as follows:
- Creatives develop a new marketing campaign idea and present it to the account manager.
- The account manager approves the campaign idea and sends it to the client for review.
- The client reviews the campaign and provides feedback.
- The creatives make changes based on the client’s feedback.
- The revised campaign is sent back to the account manager for final approval.
- The account manager approves the final version of the campaign, and it is launched.
This approval process has a few issues:
Lack of cross-functional collaboration
There is no involvement from other teams, such as legal, compliance, or even the client’s marketing team.
This can lead to potential legal or compliance issues and a lack of alignment with the client’s strategy.
Too many revisions
The approval process includes multiple rounds of revisions, which can delay the campaign launch and increase costs.
Best practices
Clearly define the project scope and objectives.
Before seeking approval for a project, you should define what the project intends to achieve and the scope of work needed.
This will help ensure that stakeholders clearly understand what the project is for.
Identify and involve all stakeholders
Determinate all stakeholders impacted by the project and involve them in the approval process.
When I say “stakeholders”, I mean, for example:
- team members
Involving relevant people in the approval process will ensure the project aligns with their needs and requirements.
Develop a detailed project plan
A detailed project plan that outlines all project tasks, timelines, and dependencies is essential for getting approval for the project.
The project plan should be comprehensive, outlining all aspects, from initial scoping to final delivery.
Establish a clear approval process
Try to establish as transparent and streamlined approval process as possible.
This could include:
- Who has the authority to approve the project at each stage
- What documentation is required
- How feedback will be incorporated
Communicate effectively
Effective communication is crucial throughout the project approval process.
Stakeholders should be kept informed of progress, milestones, and any changes to the project plan or scope.
Monitor progress
Once the project is approved, it’s you can monitor progress to ensure that the project is staying on track and meeting its objectives.
Provide regular progress reports to stakeholders, and address any issues promptly.
While you could deal with all the approvals yourself, there is a better and faster way to do this – software.
With Process Street, you’ll create custom workflows that align with your specific project approval criteria so all projects are properly vetted and meet the necessary standards before proceeding.
One of the key benefits of using Process Street is the ability to automate the approval process, such as:
- Sending notifications to stakeholders when projects are ready for review
- Tracking project progress in real-time
- Gathering and storing all necessary documentation
- Collaborating with team members, regardless of their location.
Here is a free process management process checklist that you can start to use right now. This template provides the basic framework to keep every one of your projects on track and on target.
So why not take a look at Process Street’s website to see how we can help your project approval process?
Project approval process is vital
There’s no doubt that a clear and effective project approval process is essential for success.
By having a project approval process in place, you’ll:
- minimize risk
- increase accountability
- take your decision-making to the next level!
Yes, implementing a project approval process may come with challenges and pitfalls, but these can be overcomed by careful planning.
And don’t be afraid of utilizing relevant tools and software! These can come significantly speed up the whole process and streamline your operations, too.
So involve your stakeholders in the development process, communicate clearly and frequently, and establish clear metrics for success.
If you do, the project approval process will be implemented, and your business will achieve its goals.
Related posts
- /business-process-management/">Business Process Management
- /process-management/">Process Management
- /bpm-software/">BPM Software
- /business-process-management-template/">Business Process Management Template
- /process-management-software/">Process Management Software
- /process-management-tools/">Process Management Tools
- /bpm-tools/">BPM Tools
- /process-checklist/">Process Checklist
- /definition-of-process-management/">Definition of Process Management
- /business-operations-management-software/">Business Operations Management Software
Take control of your workflows today
Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, generate accurate citations for free.
- Knowledge Base
- Starting the research process
A Beginner's Guide to Starting the Research Process
When you have to write a thesis or dissertation , it can be hard to know where to begin, but there are some clear steps you can follow.
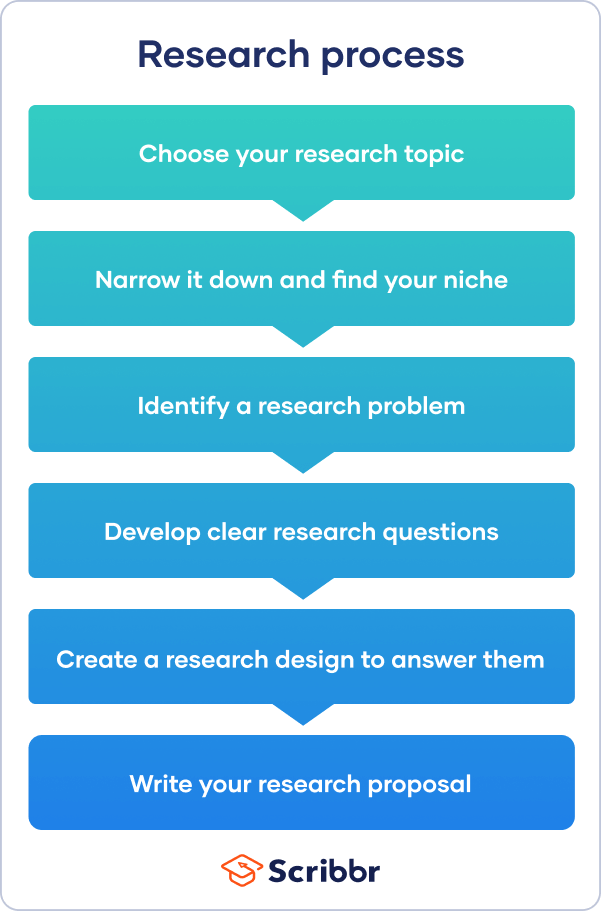
The research process often begins with a very broad idea for a topic you’d like to know more about. You do some preliminary research to identify a problem . After refining your research questions , you can lay out the foundations of your research design , leading to a proposal that outlines your ideas and plans.
This article takes you through the first steps of the research process, helping you narrow down your ideas and build up a strong foundation for your research project.
Table of contents
Step 1: choose your topic, step 2: identify a problem, step 3: formulate research questions, step 4: create a research design, step 5: write a research proposal, other interesting articles.
First you have to come up with some ideas. Your thesis or dissertation topic can start out very broad. Think about the general area or field you’re interested in—maybe you already have specific research interests based on classes you’ve taken, or maybe you had to consider your topic when applying to graduate school and writing a statement of purpose .
Even if you already have a good sense of your topic, you’ll need to read widely to build background knowledge and begin narrowing down your ideas. Conduct an initial literature review to begin gathering relevant sources. As you read, take notes and try to identify problems, questions, debates, contradictions and gaps. Your aim is to narrow down from a broad area of interest to a specific niche.
Make sure to consider the practicalities: the requirements of your programme, the amount of time you have to complete the research, and how difficult it will be to access sources and data on the topic. Before moving onto the next stage, it’s a good idea to discuss the topic with your thesis supervisor.
>>Read more about narrowing down a research topic
Receive feedback on language, structure, and formatting
Professional editors proofread and edit your paper by focusing on:
- Academic style
- Vague sentences
- Style consistency
See an example

So you’ve settled on a topic and found a niche—but what exactly will your research investigate, and why does it matter? To give your project focus and purpose, you have to define a research problem .
The problem might be a practical issue—for example, a process or practice that isn’t working well, an area of concern in an organization’s performance, or a difficulty faced by a specific group of people in society.
Alternatively, you might choose to investigate a theoretical problem—for example, an underexplored phenomenon or relationship, a contradiction between different models or theories, or an unresolved debate among scholars.
To put the problem in context and set your objectives, you can write a problem statement . This describes who the problem affects, why research is needed, and how your research project will contribute to solving it.
>>Read more about defining a research problem
Next, based on the problem statement, you need to write one or more research questions . These target exactly what you want to find out. They might focus on describing, comparing, evaluating, or explaining the research problem.
A strong research question should be specific enough that you can answer it thoroughly using appropriate qualitative or quantitative research methods. It should also be complex enough to require in-depth investigation, analysis, and argument. Questions that can be answered with “yes/no” or with easily available facts are not complex enough for a thesis or dissertation.
In some types of research, at this stage you might also have to develop a conceptual framework and testable hypotheses .
>>See research question examples
The research design is a practical framework for answering your research questions. It involves making decisions about the type of data you need, the methods you’ll use to collect and analyze it, and the location and timescale of your research.
There are often many possible paths you can take to answering your questions. The decisions you make will partly be based on your priorities. For example, do you want to determine causes and effects, draw generalizable conclusions, or understand the details of a specific context?
You need to decide whether you will use primary or secondary data and qualitative or quantitative methods . You also need to determine the specific tools, procedures, and materials you’ll use to collect and analyze your data, as well as your criteria for selecting participants or sources.
>>Read more about creating a research design
Prevent plagiarism. Run a free check.
Finally, after completing these steps, you are ready to complete a research proposal . The proposal outlines the context, relevance, purpose, and plan of your research.
As well as outlining the background, problem statement, and research questions, the proposal should also include a literature review that shows how your project will fit into existing work on the topic. The research design section describes your approach and explains exactly what you will do.
You might have to get the proposal approved by your supervisor before you get started, and it will guide the process of writing your thesis or dissertation.
>>Read more about writing a research proposal
If you want to know more about the research process , methodology , research bias , or statistics , make sure to check out some of our other articles with explanations and examples.
Methodology
- Sampling methods
- Simple random sampling
- Stratified sampling
- Cluster sampling
- Likert scales
- Reproducibility
Statistics
- Null hypothesis
- Statistical power
- Probability distribution
- Effect size
- Poisson distribution
Research bias
- Optimism bias
- Cognitive bias
- Implicit bias
- Hawthorne effect
- Anchoring bias
- Explicit bias
Is this article helpful?
Other students also liked.
- Writing Strong Research Questions | Criteria & Examples
What Is a Research Design | Types, Guide & Examples
- How to Write a Research Proposal | Examples & Templates
More interesting articles
- 10 Research Question Examples to Guide Your Research Project
- How to Choose a Dissertation Topic | 8 Steps to Follow
- How to Define a Research Problem | Ideas & Examples
- How to Write a Problem Statement | Guide & Examples
- Relevance of Your Dissertation Topic | Criteria & Tips
- Research Objectives | Definition & Examples
- What Is a Fishbone Diagram? | Templates & Examples
- What Is Root Cause Analysis? | Definition & Examples
"I thought AI Proofreading was useless but.."
I've been using Scribbr for years now and I know it's a service that won't disappoint. It does a good job spotting mistakes”

Business Research Methodology pp 109–121 Cite as
Research Proposal
- Sergey K. Aityan 2
- First Online: 01 January 2022
3420 Accesses
Part of the book series: Classroom Companion: Business ((CCB))
The proposal submission and approval are the final activities in the preparation for research. The goal of the research proposal is to present the prospective research proposal for review and approval. The research proposal is a structured document that describes what you plan to do in the research project and how you plan to do it. The proposal should include all the necessary information that allow the approval organization to make decision on the prospective research. As the proposal is approved, you get the green light to conduct the research.
This is a preview of subscription content, log in via an institution .
Buying options
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Durable hardcover edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Purchases are for personal use only
Sergey K. Aityan (2020). Practical Guide to PC and Microsoft Office 365, Amazon KDP, 180p
Author information
Authors and affiliations.
Lincoln University - California, Oakland, CA, USA
Sergey K. Aityan
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Sergey K. Aityan .
Rights and permissions
Reprints and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter.
Aityan, S.K. (2022). Research Proposal. In: Business Research Methodology. Classroom Companion: Business. Springer, Cham. https://doi.org/10.1007/978-3-030-76857-7_7
Download citation
DOI : https://doi.org/10.1007/978-3-030-76857-7_7
Published : 01 January 2022
Publisher Name : Springer, Cham
Print ISBN : 978-3-030-76856-0
Online ISBN : 978-3-030-76857-7
eBook Packages : Business and Management Business and Management (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Indian J Anaesth
- v.60(9); 2016 Sep
How to write a research proposal?
Department of Anaesthesiology, Bangalore Medical College and Research Institute, Bengaluru, Karnataka, India
Devika Rani Duggappa
Writing the proposal of a research work in the present era is a challenging task due to the constantly evolving trends in the qualitative research design and the need to incorporate medical advances into the methodology. The proposal is a detailed plan or ‘blueprint’ for the intended study, and once it is completed, the research project should flow smoothly. Even today, many of the proposals at post-graduate evaluation committees and application proposals for funding are substandard. A search was conducted with keywords such as research proposal, writing proposal and qualitative using search engines, namely, PubMed and Google Scholar, and an attempt has been made to provide broad guidelines for writing a scientifically appropriate research proposal.
INTRODUCTION
A clean, well-thought-out proposal forms the backbone for the research itself and hence becomes the most important step in the process of conduct of research.[ 1 ] The objective of preparing a research proposal would be to obtain approvals from various committees including ethics committee [details under ‘Research methodology II’ section [ Table 1 ] in this issue of IJA) and to request for grants. However, there are very few universally accepted guidelines for preparation of a good quality research proposal. A search was performed with keywords such as research proposal, funding, qualitative and writing proposals using search engines, namely, PubMed, Google Scholar and Scopus.
Five ‘C’s while writing a literature review

BASIC REQUIREMENTS OF A RESEARCH PROPOSAL
A proposal needs to show how your work fits into what is already known about the topic and what new paradigm will it add to the literature, while specifying the question that the research will answer, establishing its significance, and the implications of the answer.[ 2 ] The proposal must be capable of convincing the evaluation committee about the credibility, achievability, practicality and reproducibility (repeatability) of the research design.[ 3 ] Four categories of audience with different expectations may be present in the evaluation committees, namely academic colleagues, policy-makers, practitioners and lay audiences who evaluate the research proposal. Tips for preparation of a good research proposal include; ‘be practical, be persuasive, make broader links, aim for crystal clarity and plan before you write’. A researcher must be balanced, with a realistic understanding of what can be achieved. Being persuasive implies that researcher must be able to convince other researchers, research funding agencies, educational institutions and supervisors that the research is worth getting approval. The aim of the researcher should be clearly stated in simple language that describes the research in a way that non-specialists can comprehend, without use of jargons. The proposal must not only demonstrate that it is based on an intelligent understanding of the existing literature but also show that the writer has thought about the time needed to conduct each stage of the research.[ 4 , 5 ]
CONTENTS OF A RESEARCH PROPOSAL
The contents or formats of a research proposal vary depending on the requirements of evaluation committee and are generally provided by the evaluation committee or the institution.
In general, a cover page should contain the (i) title of the proposal, (ii) name and affiliation of the researcher (principal investigator) and co-investigators, (iii) institutional affiliation (degree of the investigator and the name of institution where the study will be performed), details of contact such as phone numbers, E-mail id's and lines for signatures of investigators.
The main contents of the proposal may be presented under the following headings: (i) introduction, (ii) review of literature, (iii) aims and objectives, (iv) research design and methods, (v) ethical considerations, (vi) budget, (vii) appendices and (viii) citations.[ 4 ]
Introduction
It is also sometimes termed as ‘need for study’ or ‘abstract’. Introduction is an initial pitch of an idea; it sets the scene and puts the research in context.[ 6 ] The introduction should be designed to create interest in the reader about the topic and proposal. It should convey to the reader, what you want to do, what necessitates the study and your passion for the topic.[ 7 ] Some questions that can be used to assess the significance of the study are: (i) Who has an interest in the domain of inquiry? (ii) What do we already know about the topic? (iii) What has not been answered adequately in previous research and practice? (iv) How will this research add to knowledge, practice and policy in this area? Some of the evaluation committees, expect the last two questions, elaborated under a separate heading of ‘background and significance’.[ 8 ] Introduction should also contain the hypothesis behind the research design. If hypothesis cannot be constructed, the line of inquiry to be used in the research must be indicated.
Review of literature
It refers to all sources of scientific evidence pertaining to the topic in interest. In the present era of digitalisation and easy accessibility, there is an enormous amount of relevant data available, making it a challenge for the researcher to include all of it in his/her review.[ 9 ] It is crucial to structure this section intelligently so that the reader can grasp the argument related to your study in relation to that of other researchers, while still demonstrating to your readers that your work is original and innovative. It is preferable to summarise each article in a paragraph, highlighting the details pertinent to the topic of interest. The progression of review can move from the more general to the more focused studies, or a historical progression can be used to develop the story, without making it exhaustive.[ 1 ] Literature should include supporting data, disagreements and controversies. Five ‘C's may be kept in mind while writing a literature review[ 10 ] [ Table 1 ].
Aims and objectives
The research purpose (or goal or aim) gives a broad indication of what the researcher wishes to achieve in the research. The hypothesis to be tested can be the aim of the study. The objectives related to parameters or tools used to achieve the aim are generally categorised as primary and secondary objectives.
Research design and method
The objective here is to convince the reader that the overall research design and methods of analysis will correctly address the research problem and to impress upon the reader that the methodology/sources chosen are appropriate for the specific topic. It should be unmistakably tied to the specific aims of your study.
In this section, the methods and sources used to conduct the research must be discussed, including specific references to sites, databases, key texts or authors that will be indispensable to the project. There should be specific mention about the methodological approaches to be undertaken to gather information, about the techniques to be used to analyse it and about the tests of external validity to which researcher is committed.[ 10 , 11 ]
The components of this section include the following:[ 4 ]
Population and sample
Population refers to all the elements (individuals, objects or substances) that meet certain criteria for inclusion in a given universe,[ 12 ] and sample refers to subset of population which meets the inclusion criteria for enrolment into the study. The inclusion and exclusion criteria should be clearly defined. The details pertaining to sample size are discussed in the article “Sample size calculation: Basic priniciples” published in this issue of IJA.
Data collection
The researcher is expected to give a detailed account of the methodology adopted for collection of data, which include the time frame required for the research. The methodology should be tested for its validity and ensure that, in pursuit of achieving the results, the participant's life is not jeopardised. The author should anticipate and acknowledge any potential barrier and pitfall in carrying out the research design and explain plans to address them, thereby avoiding lacunae due to incomplete data collection. If the researcher is planning to acquire data through interviews or questionnaires, copy of the questions used for the same should be attached as an annexure with the proposal.
Rigor (soundness of the research)
This addresses the strength of the research with respect to its neutrality, consistency and applicability. Rigor must be reflected throughout the proposal.
It refers to the robustness of a research method against bias. The author should convey the measures taken to avoid bias, viz. blinding and randomisation, in an elaborate way, thus ensuring that the result obtained from the adopted method is purely as chance and not influenced by other confounding variables.
Consistency
Consistency considers whether the findings will be consistent if the inquiry was replicated with the same participants and in a similar context. This can be achieved by adopting standard and universally accepted methods and scales.
Applicability
Applicability refers to the degree to which the findings can be applied to different contexts and groups.[ 13 ]
Data analysis
This section deals with the reduction and reconstruction of data and its analysis including sample size calculation. The researcher is expected to explain the steps adopted for coding and sorting the data obtained. Various tests to be used to analyse the data for its robustness, significance should be clearly stated. Author should also mention the names of statistician and suitable software which will be used in due course of data analysis and their contribution to data analysis and sample calculation.[ 9 ]
Ethical considerations
Medical research introduces special moral and ethical problems that are not usually encountered by other researchers during data collection, and hence, the researcher should take special care in ensuring that ethical standards are met. Ethical considerations refer to the protection of the participants' rights (right to self-determination, right to privacy, right to autonomy and confidentiality, right to fair treatment and right to protection from discomfort and harm), obtaining informed consent and the institutional review process (ethical approval). The researcher needs to provide adequate information on each of these aspects.
Informed consent needs to be obtained from the participants (details discussed in further chapters), as well as the research site and the relevant authorities.
When the researcher prepares a research budget, he/she should predict and cost all aspects of the research and then add an additional allowance for unpredictable disasters, delays and rising costs. All items in the budget should be justified.
Appendices are documents that support the proposal and application. The appendices will be specific for each proposal but documents that are usually required include informed consent form, supporting documents, questionnaires, measurement tools and patient information of the study in layman's language.
As with any scholarly research paper, you must cite the sources you used in composing your proposal. Although the words ‘references and bibliography’ are different, they are used interchangeably. It refers to all references cited in the research proposal.
Successful, qualitative research proposals should communicate the researcher's knowledge of the field and method and convey the emergent nature of the qualitative design. The proposal should follow a discernible logic from the introduction to presentation of the appendices.
Financial support and sponsorship
Conflicts of interest.
There are no conflicts of interest.
Chapter 2: Approval of Research Projects
Share this:.
- Click to email this to a friend
- Click to print
What Needs Approval
PIs planning to conduct research using recombinant or synthetic nucleic acid molecules and/or hazardous biological materials that pose a potential risk to the health of humans, animals, or plants, either directly through infection or indirectly through damage to the environment, must submit an IBC Application entitled “Biological Use Authorization” (BUA) to the IBC Program for review and approval by the IBC. Researchers using any of the following must complete and submit a “Biological Use Authorization” (a.k.a. IBC protocol) for review and approval:
- Recombinant and synthetic nucleic acid molecules;
- Hazardous biological agents (e.g., virus, bacteria, fungi, parasite, prion, rickettsia etc.);
- Other potentially infectious materials (e.g., human or non-human primate blood, serum, plasma, body fluids, unfixed tissue, organ and cells);
- Inactivated Biological Samples derived from BSL3 or higher agents;
- Select agents and biological Toxins;
- Transgenic animals or plants;
- Human gene transfer;
- Sheep and any tissues derived from them (these tissues can transmit Coxiella burnetii, the causative agent of Q-fever); and
- Field studies with wild animals and vectors and their tissues potentially infected or would be experimentally infected with BSL2 or higher agents.
For work with potentially infectious agents on human subjects or experimental animals, IBC review is necessary in addition to review by the appropriate IRB or IACUC.
PIs whose research will involve the above mentioned category agents or infectious materials are required to complete a BUA and obtain IBC approval prior to receiving such materials or commencing such research. PIs are also required, where applicable, to maintain a medical surveillance program for laboratory employees ( see Appendix P ). Relevant federal, state, and local governmental regulations include the following (see Chapter 3 for more details):
- NIH Guidelines for Research Involving Recombinant or synthetic nucleic acid molecules (NIH Guidelines);
- Recombinant DNA Technology: Use Regulations
- Biological Laboratory Regulation with accompanying Guidelines;
- Disease Surveillance and Reporting Regulation with accompanying Guidelines;
- OSHA Bloodborne Pathogens Standard;
- OSHA Tuberculosis Standard;
- OSHA Laboratory Standards;
- Minimum Requirements for the Management of Medical or Biological Waste
- Reportable Diseases, Surveillance, and Isolation and Quarantine Requirements;
- Biosafety in Microbiological and Biomedical Laboratories (BMBL) 6th edition;
- The Centers for Disease Control and Prevention (CDC) and United States Department of Agriculture (USDA) Select Agent regulations; and
- International, federal, and state transport regulations.
Note: A PI performing this type of work without IBC approval is non-compliant with current NIH and local regulations and the IBC will take appropriate corrective actions and may impose sanctions.
Types of IBC Protocols
New applications.
A new BUA must be submitted and reviewed by the IBC program for any research mentioned previously. PIs seeking IBC approval for the first time also need to submit a curriculum vitae (CV) or two-page NIH biosketch with their application. Applications need to be submitted via the Research Information Management System (RIMS) .
The PI may designate others to assist in completing the application, however, only the PI may submit the application. The BU IBC requires that all PIs be members of the faculty. Projects from post-docs or other non-faculty members require a faculty PI sponsor.
All individuals listed on a new BUA must complete the following training and ROHP medical health clearance requirements before engaging in any approved protocol related activities:
- Laboratory Safety Training
- Health Questionnaire (must be filled out, submitted, reviewed, and cleared by ROHP); and
- Laboratory Specific Training.
All Principal Investigators (PIs) listed on a new BUA, and all individuals listed on a new BUA involving recombinant or synthetic nucleic acids, must complete the following training before engaging in any approved protocol related activities:
- IBC Policy /Recombinant DNA Training and Quiz.
Personnel will need a RIMS Training Profile in order to complete these requirements. Other training will be required based on the nature of project including, but not limited to: BSL3 annual training; shipping biologicals training; select agent training; and agent specific training for Francisella tularensis, Mycobacterium tuberculosis, and Yersinia pestis.
All applications must be received by the meeting date deadline to allow time for review by the committee members and a Biological Use Authorization Site Assessment by EHS. Meeting date deadlines can be found on the IBC Program website. Each application will be assigned to a primary and secondary reviewer. Reviewer comments will be discussed at a convened IBC meeting.
A renewal of the original BUA approval must the completed in RIMS at the third year after initial approval of a protocol. Email notice of 3-Year Renewal will be sent to the PIs. Detailed instructions on completing the 3-Year Renewal in RIMS is found in the IBC website. The PI is asked to list all proposed modifications from the protocol as initially approved (or since the last renewal notice), including: changes in laboratory location or equipment, changes in laboratory staff working on the project, any project titles to be added, and any agent of experimental changes.
If there are significant modifications to the protocol, especially those that affect the biosafety containment level (e.g., new study on organisms, a new host-vector-donor system, or any other modifications that may affect the containment level), the IBC Program will send the protocol for committee review and approval. When a project is renewed, the PI and all staff listed on the protocol must complete the “Laboratory Safety Annual Refresher Training” and the annual ROHP clearance update (contact ROHP at [email protected] or at (617) 358-7647), and any other training requirements applicable to the project.
Amendments must be submitted to the IBC Program for changes within an approved project. All changes should be detailed in RIMS and submitted to the IBC Program and the IBC must review and approve the amendment prior to implementing the changes. Detailed instructions on completing the amendment in RIMS are found in the IBC Amendment Instructions document. Title additions approval may be applied to several different granting agencies, but all grant titles must be registered with the IBC Program. Approval of lab space additions applies only to work performed in registered lab space.
For non-PI personnel changes, individuals must complete the “Initial Laboratory Safety Training” and “ROHP Clearance” requirements. Individuals on protocols involving recombinant or synthetic nucleic acids, must also complete the IBC Policy /Recombinant DNA Training and Quiz. Additions of titles, lab space, and personnel are reviewed administratively reviews and approvals, and do not to go the full IBC.
Some amendments may require full committee review and when extensive, may require the PI to submit a completely new application. A change in PI also requires submission of an amendment where CV (or NIH biosketch) of the new PI must be attached to the amendment, and this must be reviewed by the IBC Chair and the BSO.
The IBC Program will notify the PI when the Amendment has been processed and approved. IBC approval is required before any changes in the work can commence.
Protocol Review Outcomes
The IBC has the authority to decide to approve, conditionally approve, defer or withhold approval of the proposed research activity. These actions will be taken by a vote of a majority of the members present. At the conclusion of discussion during its scheduled meeting, the IBC determines the outcome of its review, which will be one of the following:
A protocol that receives full approval requires no (additional) changes or clarifications to comply with IBC policies. Work may commence immediately upon full approval of a protocol. Approval is valid for the study as described in the protocol form for a period of three years from the original approval date. PIs must complete a renewal form tri-annually (annually in the first year for BSL 3 and BSL4) after the first and second year following initial approval and must submit a new application to continue the work beyond the approved three-year time period.
Conditionally Approved
“Conditionally Approved” is a determination that the IBC makes when minor changes or clarifications are required to bring the protocol into compliance with IBC policies. The investigator must respond in writing to the IBC’s notice of conditional approval.
“Deferred” is used when numerous and/or major changes or clarifications are required to bring the protocol into compliance with IBC policies or there is insufficient time at the IBC meeting to conduct a thorough review.
Withhold Approval
The IBC may withhold approval of a protocol if it fails to meet one or more criteria used by the IBC for approval of research.
Use of Materials that Require Clarification for the IBC Approval
Biohazardous and potentially infectious materials.
Biohazardous agents and potentially infectious materials present a risk or potential risk to the health of humans or animals, either directly through infection or indirectly through damage to the environment. Infectious agents have the ability to replicate and give rise to potentially large populations in nature when small numbers are released from a controlled situation.
PIs should follow the instructions in the BUA carefully to ensure that all appropriate sections of the application are completed. It is important that the PI clarifies the intended use of the agents, how risks associated with its handling are mitigated, and the management of the wastes generated from such work. If a PI intends to use biological agents that are not listed in this section, he or she should contact the IBC Program or BSO for advice regarding proper completion of the BUA.
For the list of biological agents with the potential to cause Laboratory Acquired Infection (LAI) see the Appendix G . The ROHP has developed additional “Agent Information Sheets” that are available on the IBC website. Where applicable, agent specific vaccines will be offered at no charge to all persons who are approved by the IBC to work with or could potentially get exposed to these materials.
Recombinant or synthetic nucleic acid Materials
When a BUA protocol involves the use of recombinant or synthetic nucleic materials, most of those work require IBC approval before initiation of work. The NIH Guidelines for Recombinant and Synthetic Nucleic Acids provide specific guidelines on the facility containment and specific regulatory requirements depending on the exact detail of the proposed work. A PI must follow the defined containment directive. There may be experiments not covered by the guidelines that would require review and approval by outside agencies before initiation. If the experimental protocol is not covered by the NIH’s guidelines, contact the BSO at (617) 358-7840 at BUMC and (617) 353-4094 at CRC to determine further review requirements.
Human Gene Transfer
All protocols involving human gene transfer must be submitted to both the IBC and the IRB for review. However a public review and discussion by the NIH Recombinant DNA Advisory Committee (RAC) is no longer required as it has been decided that existing FDA guidelines and requirements for the review of gene therapy protocol is sufficient. The NIH Office of Science Policy will also not accept annual reports, safety reports, amendments or other documentation for any previously registered human gene transfer protocols under the NIH Guidelines. The roles and responsibilities of local IBCs will continue as described in the NIH Guidelines 2019 Section III-C. These studies remain subject to FDA and other clinical trial regulations, and only after FDA, IBC, and other relevant approvals are in place can these protocols proceed.
However, the deliberate transfer of recombinant or synthetic nucleic acids into one human research participant, conducted under a Food and Drug Administration (FDA) regulated individual patient expanded access Investigational New Drug (IND) or protocol, including for emergency use, is not research subject to the NIH Guidelines and thus does not need to be submitted to an IBC for review and approval.
For more details about IBC approval of human gene therapy protocols, contact the IBC Office at (617) 358-7910 or [email protected]. For information about IRB submissions, contact the BUMC IRB at (617) 358-5372 or the CRC IRB at (617) 358-6115.
Use of Animals
All PIs planning to use recombinant or synthetic nucleic acid molecules and/or biohazardous materials in their laboratory or in research animals must receive IBC approval prior to commencing the work. The use of animals in research also requires compliance with the “Animal Welfare Act,” administered by the USDA’s Animal and Plant Health Inspection Service (APHIS); the “Public Health Service Policy on Humane Care and Use of Laboratory Animals,” administered by NIH’s Office of Laboratory Animal Welfare (OLAW); and all applicable state, local or University regulations covering the care and use of animals. All protocols involving the use of live animals must be reviewed and approved by the IACUC before their implementation.

Transgenic Animals
PIs who create transgenic animals, either in the PI’s lab or through the BU Transgenic Core facility, as well as PIs who use transgenic animals at ABSL2, or at ABSL1 if not considered exempt under the NIH Guidelines (Section III-E, Appendix C-VIII), must complete an IBC application and submit it to the IBC for approval prior to initiation of experimentation. In addition, the IACUC must approve the protocol. The IACUC can be reached at (617) 358-5586 (BUMC) or (617) 358-3867 (CRC).
Tissue Culture/Cell Lines
All tissue and cell cultures derived from humans are considered potentially infectious under the OSHA Bloodborne Pathogens Standard, Title 29 Code of Federal Regulations (CFR) Part 1910.1030 and must be handled in a laboratory using BSL2 principles, practices and facilities (the concept of “universal precautions”). Nonhuman primate tissues and cell cultures are also considered potentially infectious and must be handled in the BSL2 laboratory. Persons who are exposed to these materials in the laboratory are considered to have potential exposure to bloodborne pathogens, such as human immunodeficiency virus (HIV) and hepatitis B virus (HBV), and must be included in the Bloodborne Pathogens program. These persons must be offered the HBV vaccination and complete annual bloodborne pathogens training.
When cell cultures are known to contain any Risk Group 3 and higher organism, the cell line will be classified at the same higher containment level. Consequently, they must be handled using BSL3 and higher containment criteria.
Select and Biological Toxins
Use of biological toxins including the select agent toxins generally needs approval by the IBC ( see Appendix F “Guidelines for Work with Toxins of Biological Origin” for more details). Please check with IBC staff directly if you have questions for a particular biological toxin. Use of select agent toxins such as Abrin, Bacillus cereus Biovar anthracis, Botulinum neurotoxins, Botulinum neurotoxin producing species of Clostridium, Conotoxins (Short, paralytic alpha conotoxins containing the following amino acid sequence X1CCX2PACGX3X4X5X6CX7) Diacetoxyscirpenol (DAS), Ricin, Saxitoxin, Staphylococcal Enterotoxins (Subtypes A, B, C, D, and E), T-2 toxin, Tetrodotoxin requires IBC approval.
Testing and Certification of Containment Equipment Required
Handling infectious or potentially infectious materials could potentially generate infectious aerosols. Such work must be performed in the Biological Safety Cabinet (BSC) and wearing appropriate PPE. All BSCs are required to be tested and certified when they are first installed. They are also required to be tested and recertified annually. BU works with an outside vendor that is experienced and certified to test and repair this equipment. Laboratories must ensure that their BSCs certifications are current before initiation of protocols. EHS will verify their status during BUA follow up of protocols under review by the IBC.
Completion of Laboratory Safety Trainings
The PI and all laboratory personnel that are listed on the protocol must complete their required lab safety trainings. The trainings are available on BioRAFT and can be accessed online. These trainings must be completed prior to initiation of lab work and they need to be refreshed annually. EHS will verify that the PI and personnel that are listed on the protocol have completed the required training during BUA follow up of protocols under review by the IBC.
What does the IBC Review?
When reviewing a research protocol the IBC is responsible for assessing:
- Recombinant or synthetic nucleic acid molecule research for compliance with the NIH rDNA Guidelines;
- Use of hazardous biological materials in the laboratory;
- Use of hazardous biological materials on animals;
- Potential risk to the lab personnel, environment and public health;
- Appropriate Risk Category and BSL/ABSL Containment levels per NIH Guidelines and CDC BMBL 6th edition;
- Adequacy of facilities, safety equipment. PPE, procedures, practices, training, transport and shipping of specimen samples, and experience and expertise of personnel;
- Adverse event report.
Risk Group Definition
The investigator must make an initial risk assessment based on the Risk Group (RG) of an agent. Agents are classified into four RGs according to their relative pathogenicity for healthy adult humans by the following criteria:
As a general rule, a biosafety level should be used that matches the highest risk group classification of the agents involved (see chapter 4). More information can be found in Appendix B of NIH Guidelines. For information about the risk group of a specific pathogen, please check the resource below:
- Pathogen Safety Data Sheets and Risk Assessment (from Public Health Agency of Canada)
- Risk Group Classification for Infectious Agents (from American Biological Safety Association)
- Classification Of Human Etiologic Agents On The Basis Of Hazard (Appendix B of NIH Guidelines
Biohazardous Pathogen Examples
Tuberculosis.
Since 1985, the incidence of tuberculosis in the United States has been increasing steadily, reversing a 30-year downward trend. Recently, drug-resistant strains of Mycobacterium tuberculosis have become a serious concern. Outbreaks of tuberculosis, including drug-resistant strains, have occurred in health-care environments.
In 2005 the CDC published Guidelines for Preventing the Transmission of Mycobacterium tuberculosis in Health-care Settings. MMWR 2005; 54 (No. rr-17, 1-141). (Errata published September 25, 2006.) The guidelines contain specific information on ventilation requirements, respiratory protection, medical surveillance, and training for those personnel who are considered at-risk for exposure to tuberculosis. For more information, contact the BSO.
PIs intending to work with Mycobacterium tuberculosis in the laboratory must obtain full approval from the IBC via the IBC application process before beginning work. Propagation and manipulation of Mycobacterium tuberculosis cultures must be performed at BSL3. PI’s and all other persons approved to work with Mycobacterium tuberculosis are required to undergo semi-annual tuberculosis screenings.
Vaccinia Virus
PIs wishing to use vaccinia virus must obtain full approval from the IBC via the application process. BSL2 practices and procedures must be followed. All employees who directly handle cultures or animals contaminated or infected with vaccinia virus, recombinant or defective vaccinia viruses, or other orthopox viruses that infect humans shall be offered the smallpox vaccine. PI’s and all other persons approved to work with the vaccinia virus shall be offered the vaccinia virus vaccine at no charge. Individuals that decline the vaccinia virus vaccine must sign a declination form which will be kept on file with ROHP.
Francisella tularensis Bacteria
Tularemia, or rabbit fever, is a bacterial disease associated with both animals and humans. According to the CDC, “Tularemia is a potentially serious illness that occurs naturally in the United States. It is caused by the bacterium Francisella tularensis found in animals (especially rodents, rabbits, and hares).” PI’s and all other persons approved to work with F. tularensis shall be offered the tularemia vaccine at no charge. Individuals that decline the tularemia vaccine must sign a declination form that will be kept on file with ROHP.
The use of tularemia-causing bacteria is strictly regulated by the IBC and must be conducted in designated BSL3 facilities.
Herpes B virus
Cercopithecine herpesvirus 1 (Herpesvirus simiae or B-virus) is an endemic infection in Old World primates of the genus Macaca, which is the species most commonly used in biomedical research. It is assumed that all existing animals in the United States are carriers. Although herpes B virus is relatively benign in the macaque monkey, it can cause rapidly ascending encephalomyelitis in humans with a fatality rate of approximately 80%. The most common routes of transmission of the virus are through bites, scratches, splashes, or cuts.
All research involving non-human primates must be approved by both the IACUC and the IBC. Additional occupational health screening and PPE requirements are described in this manual.
For a detailed description of a specific pathogen that has the potential to cause a laboratory acquired infection (LAI), please check on ROHP website for “Agent Information Sheets” .
Information For...

- SHSU Library
- Research Guides
- Scholarly Communication
Undergraduate's Guide to Creating & Communicating Research
- Getting Approval for Research
- What Do I Search For?
- Where Do I Search?
- Can I Get Help Searching?
- Now What? A Lit Review How-To
- Asking a Research Question
- Data Analysis and Visualization
- Research Posters
- Conference Presentations
- How to Write About Research
- Where Could I Publish a Paper?
- SHSU Campus Resources
- Upcoming Training & Events

IRB Application Walk-Through for Students
- Introduction
- Create a New Study or Re-Open a Draft
- Complete Application Information Page and Sections 1-2
- Complete Section 3
- Additional Sections
- Finalizing a Submission
- Modifications
What is the Institutional Review Board (IRB)?
IRB is a committee of faculty who review proposals for human subjects research to ensure that studies are safe, ethical, and in compliance with relevant laws. At SHSU, the IRB is a division of the Office of Research and Sponsored Programs (ORSP).
What research must be approved by the IRB?
Most research involving living humans cannot begin until the IRB approves it. Depending on how high-risk or low-risk the research is, more or less detail may be required, and IRB review may be more in-depth or more expedited.
If our research only involves compiling existing data, or studying inanimate objects (like literature), then IRB approval is not required.
If our research involves living animals, then we will instead need approval from Institutional Animal Care and Use Committee (IACUC).
What steps should I complete before I request IRB review?
Before you request IRB review of a research study, you must complete CITI training with scores of 80% or greater in each module. If multiple people will collaborate on a research project, every one of them must complete CITI training. The ORSP Compliance website provides a link to the training and instructions for registering on the CITI site.
You should discuss your project with your faculty advisor before you begin your IRB application. You may also want to consult them along the way if you are not sure of the best way to answer a certain question. Be aware that IRB applications take time to review—this may be faster or slower depending on the complexity of the project and the risks involved, as well as how many other applications are waiting for review—so start your application as early as you can. ORSP provides some additional information about student-led and class projects and things to take into account concerning application timing.
You should have some general notes about your planned project before you begin, but it’s ok if you aren’t ready to complete every part of the application in one sitting. You can begin an IRB application, save it, and come back to it at a future time to add or edit information.
How do I submit my research for IRB review?
IRB review processes are done online in a special software program, Cayuse Human Ethics. To access Cayuse Human Ethics:
- Start at the SHSU IRB homepage .
- Click the blue “Submit IRB” button at the top of the page.

The tabbed pages of information below will offer detailed guidance through a student IRB application.
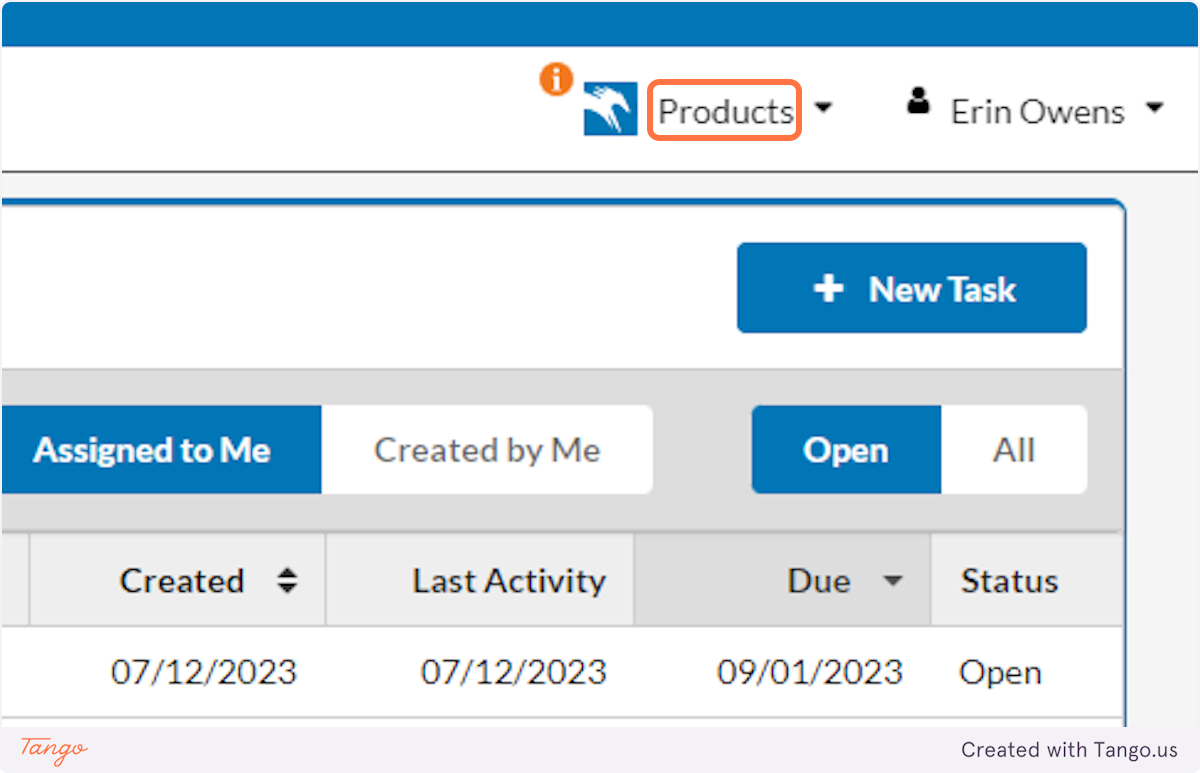
Start by logging in to SHSU's Cayuse Research Suite
1. click on products.
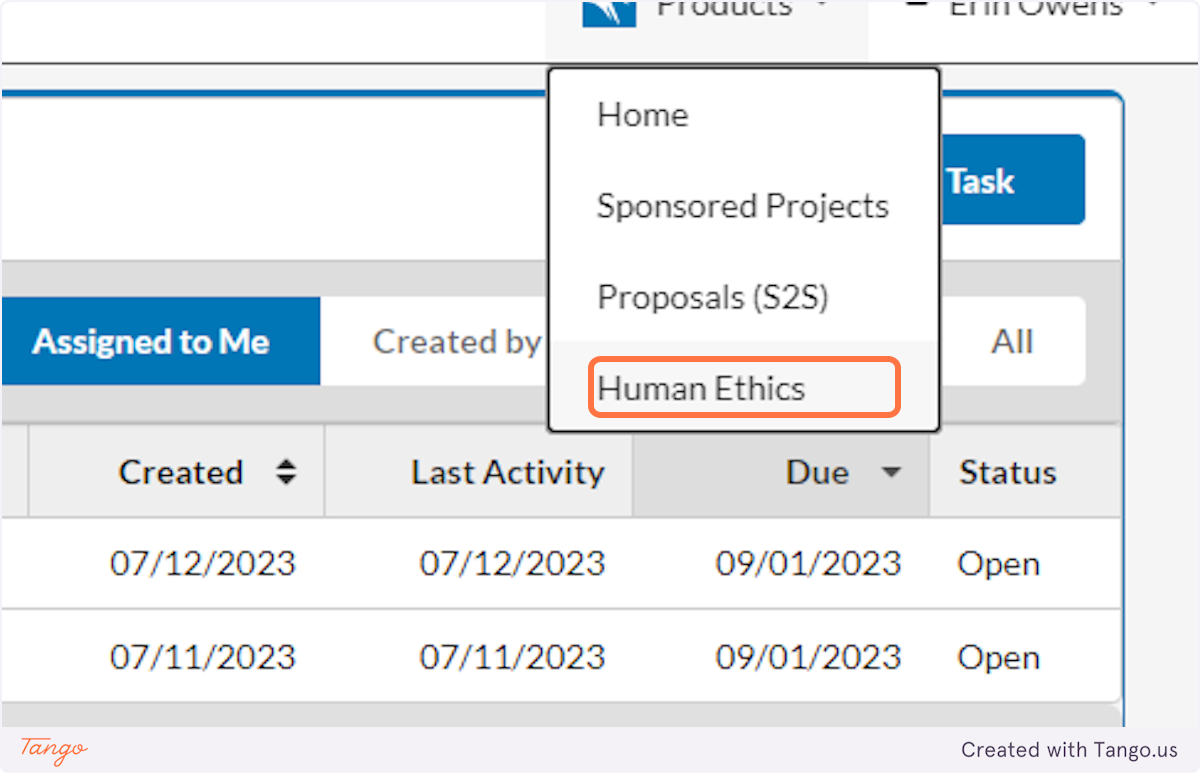
2. Click on Human Ethics
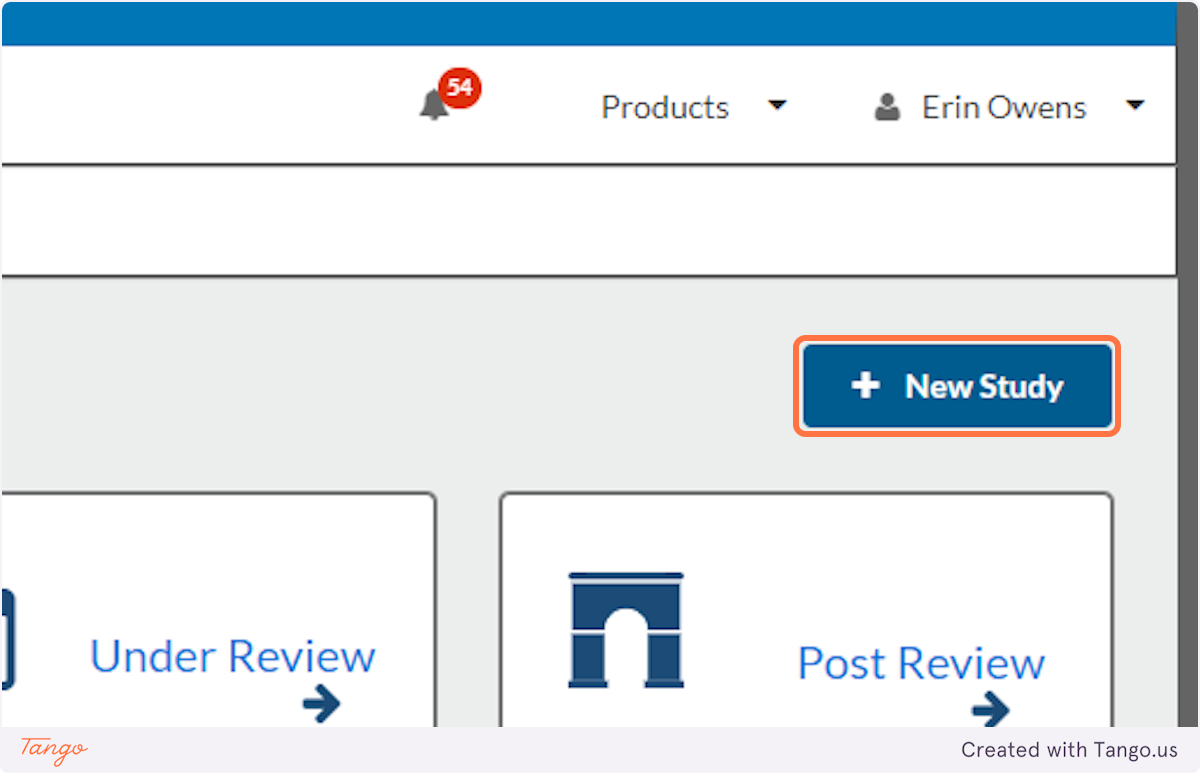
3. Click on the New Study button
4. Enter a clear and concise title describing your project
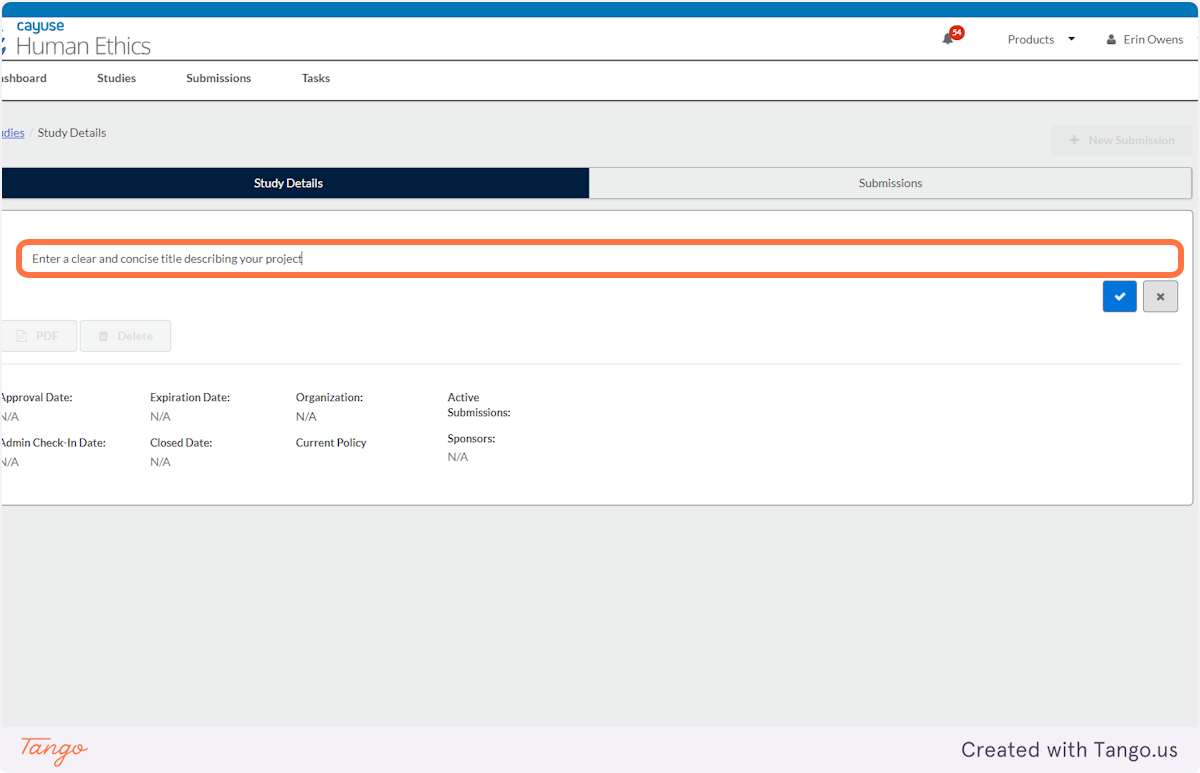
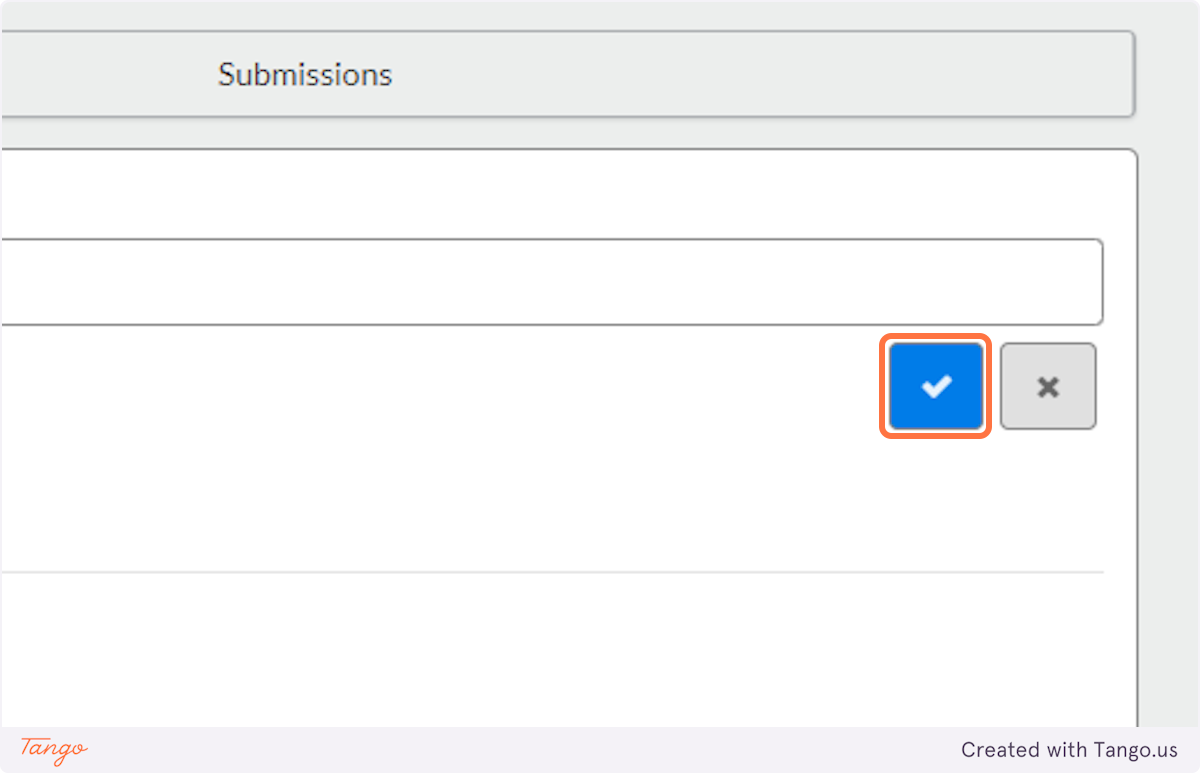
5. Click on the blue checkmark to confirm
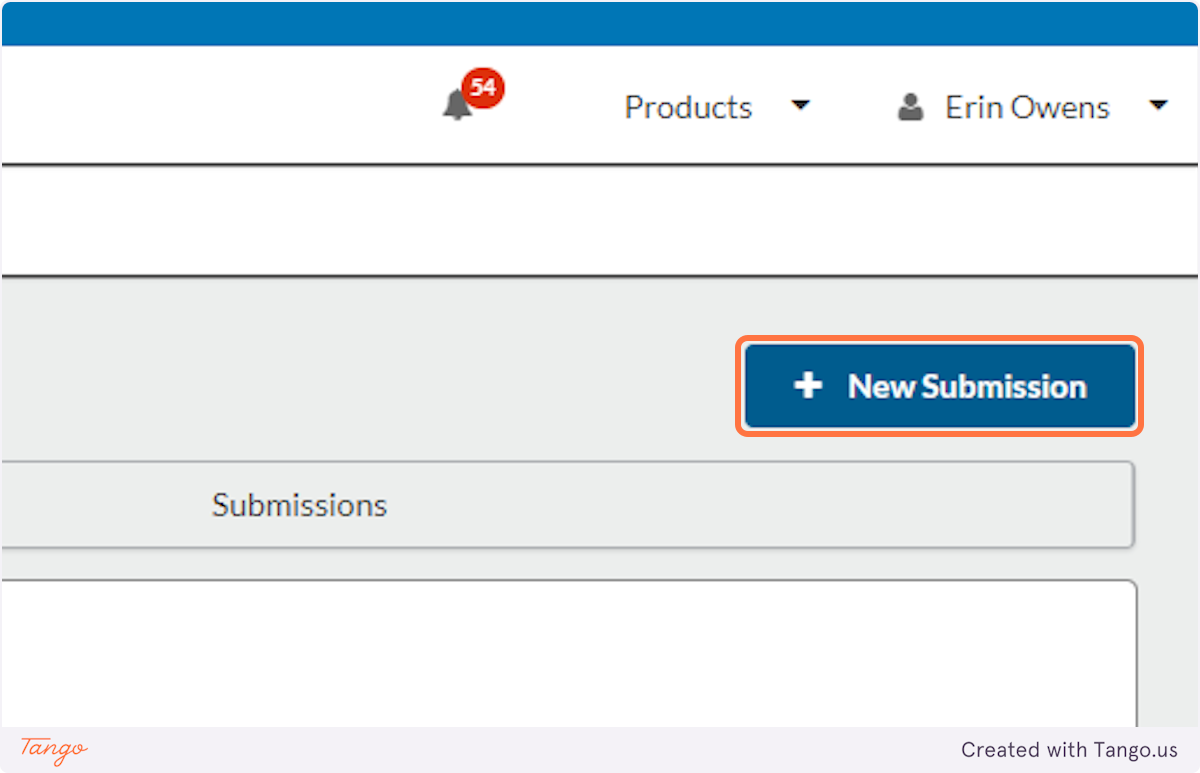
6. Click on the "New Submission" button
7. Click on Initial
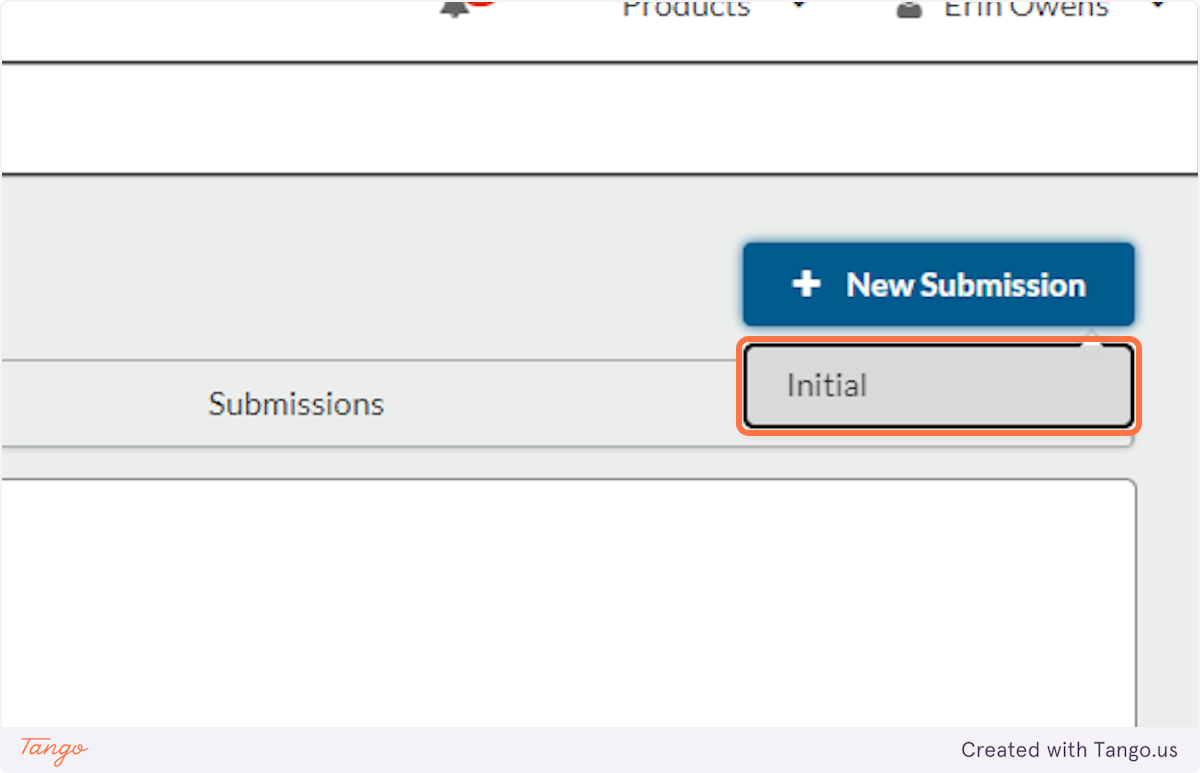
8. Click on Edit this submission
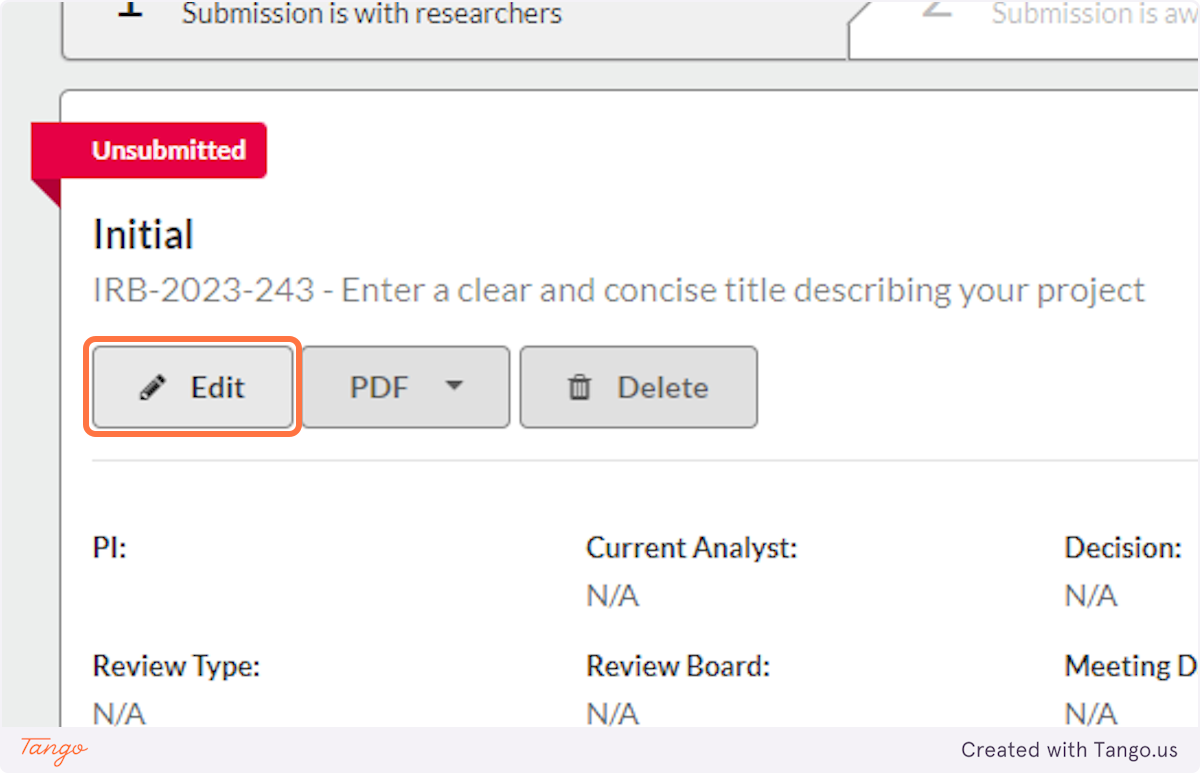
9. Here is where you will begin entering details about your study (see the walk-through on the next tab for details). You can always save a draft and come back to work on it another time.
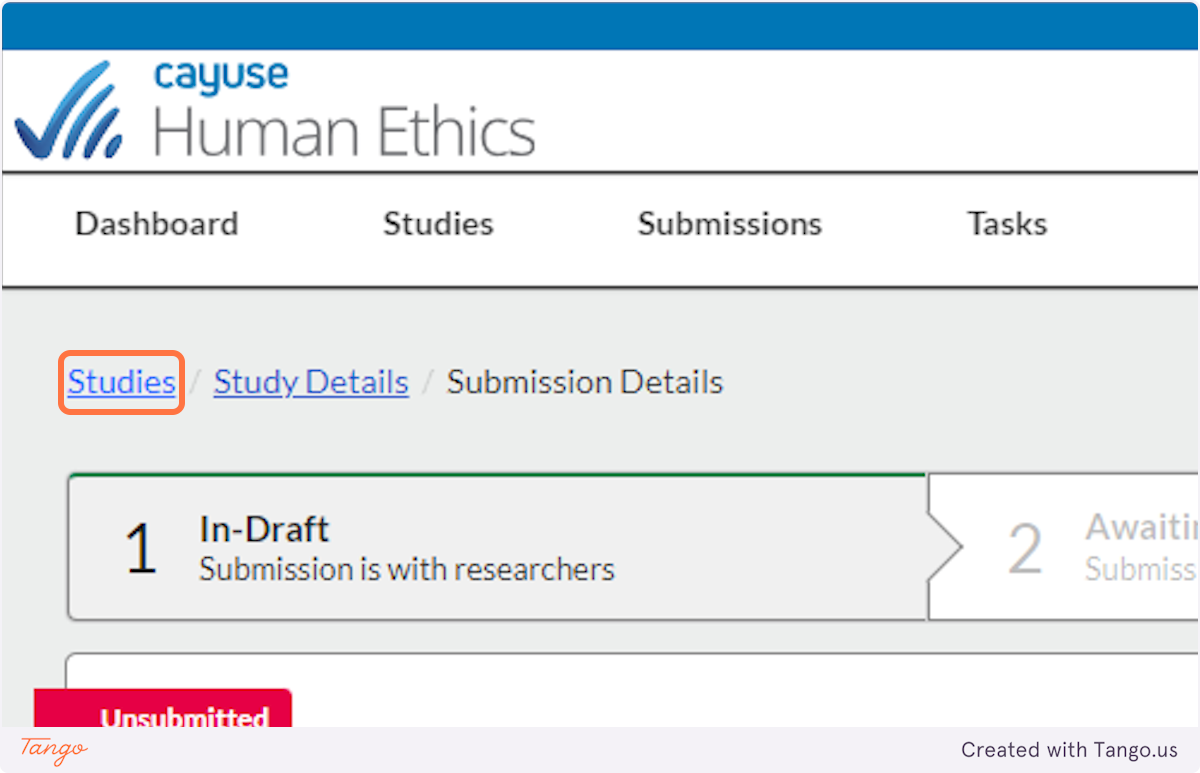
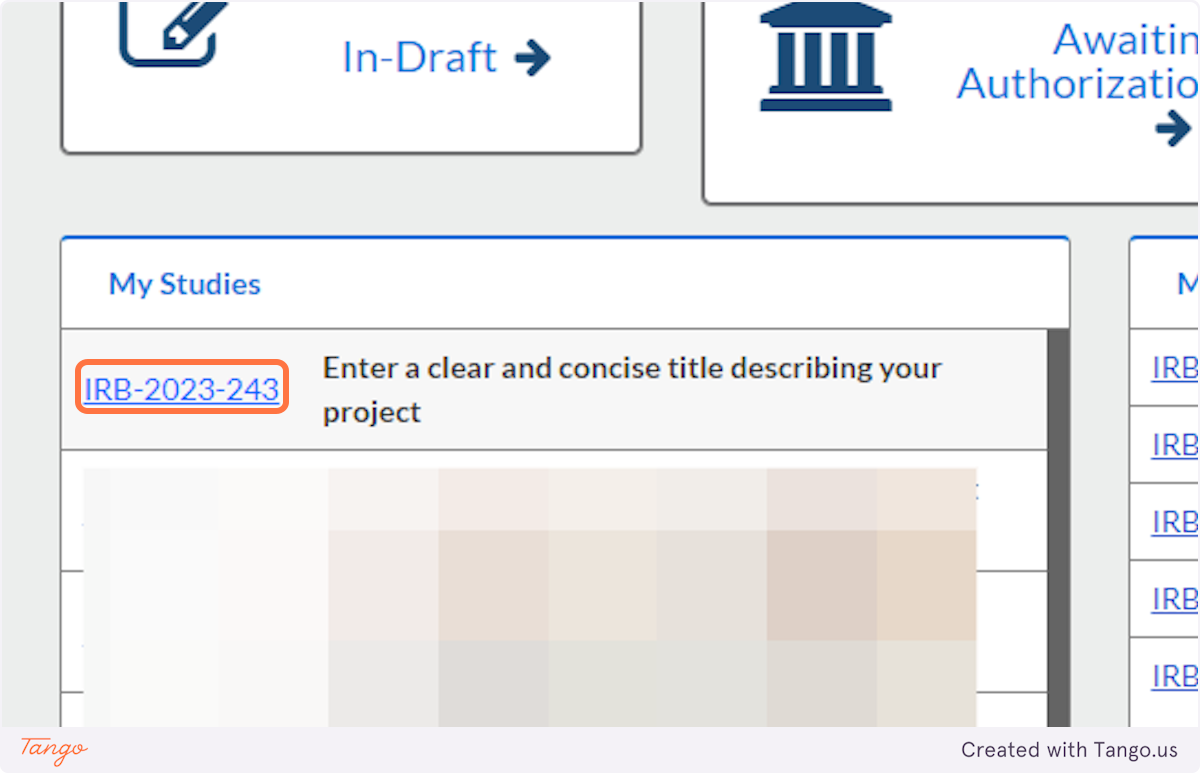
10. When you revisit Cayuse Human Ethics in the future, you will see a list of "My Studies" where you can click on the title to resume editing your draft study.
11. After you choose the study title, you will have to click on the Submissions tab...
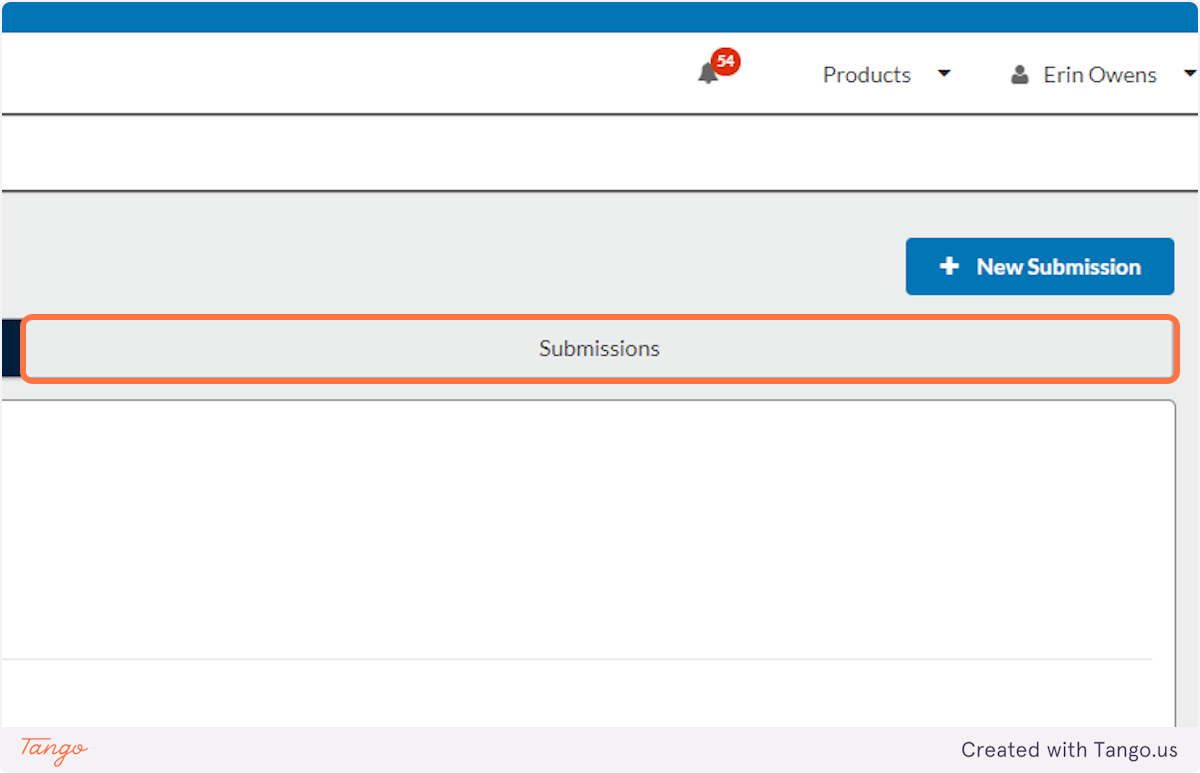
12. ...and click to open the "Initial" submission that you had begun drafting.
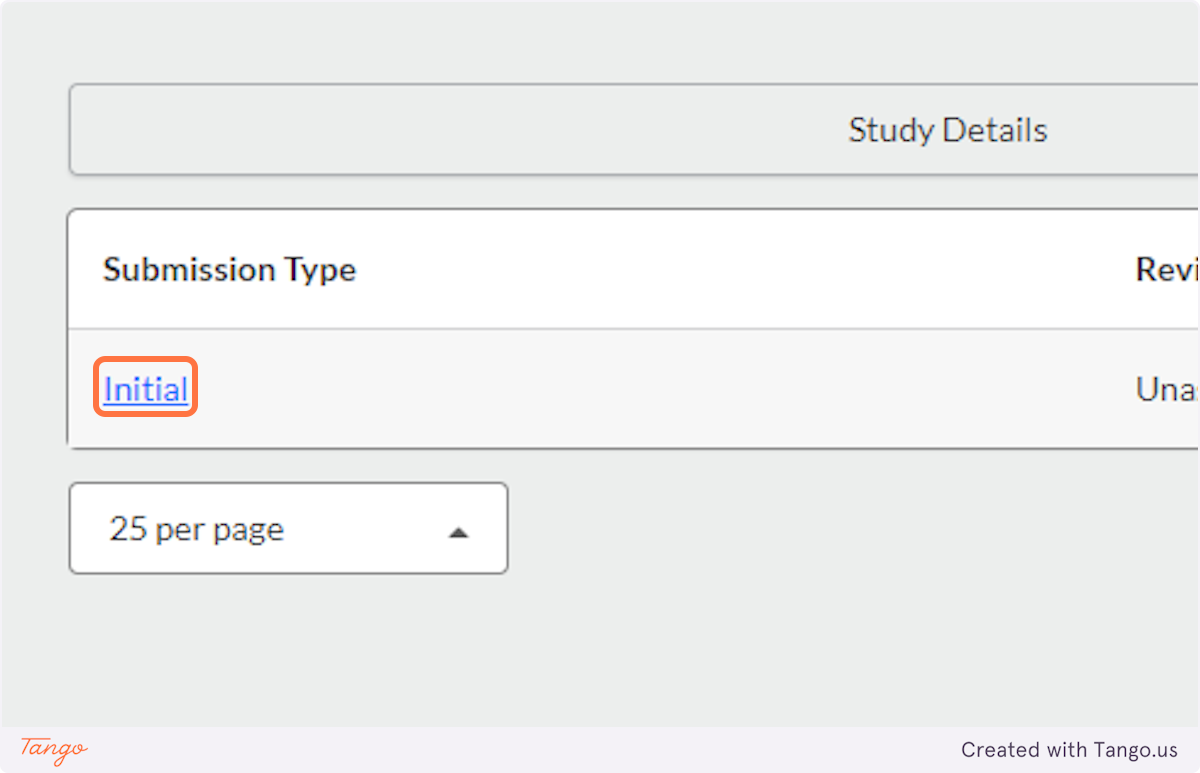
13. Click on Edit this submission to resume entering details about your study
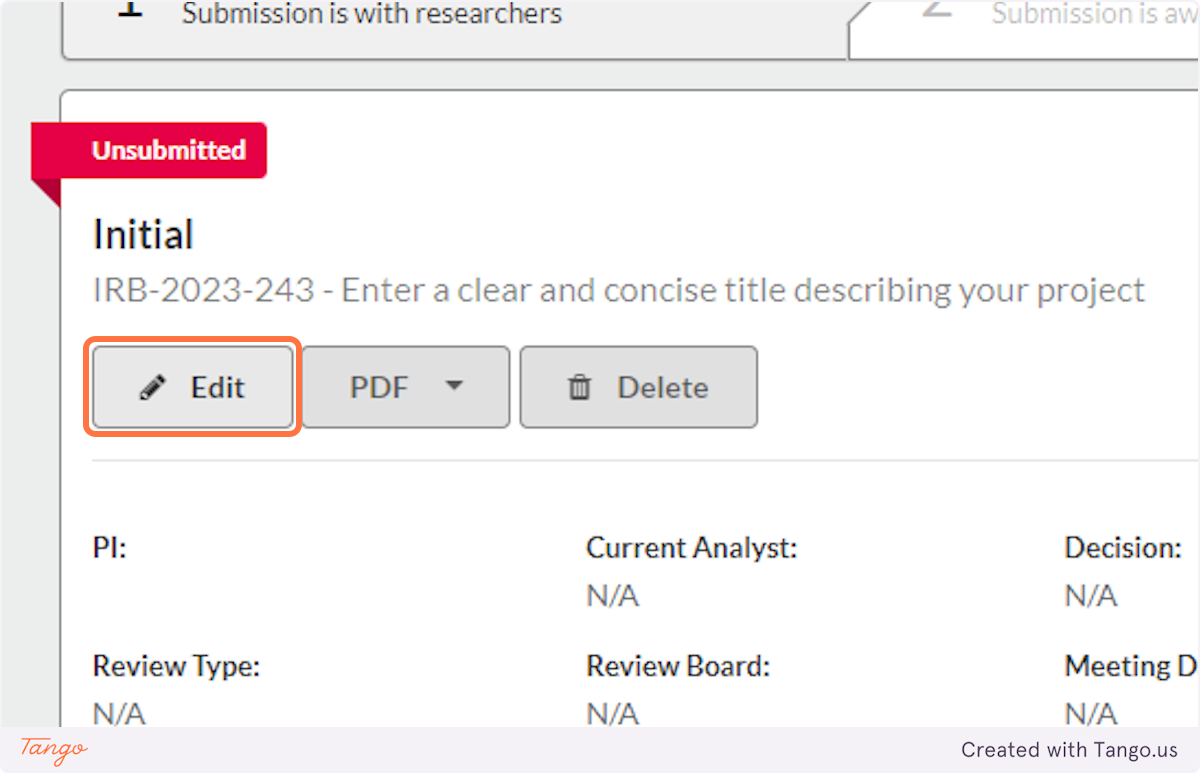
Creating a New Study or Accessing a Draft Study
Begin by opening the Initial Submission for your project in Cayuse Human Ethics
1. read all details on the application information page carefully, including (but not limited to) the fact that all project personnel must have completed the appropriate citi human ethics training..
If you (or any collaborators) have not completed CITI, you will need to do so BEFORE you complete this submission.
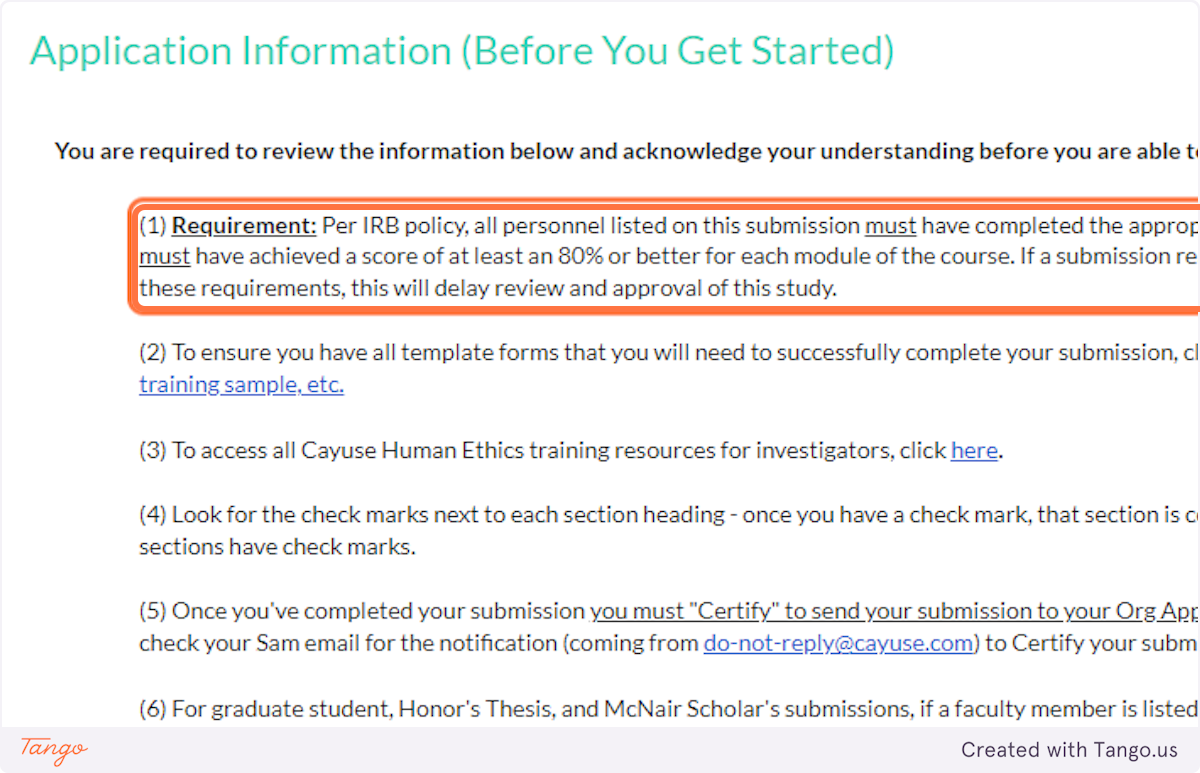
2. The application details explain to look for a white checkmark beside each application section in the blue menu.
If a section does not yet have a checkmark, you have an incomplete item somewhere in that section.
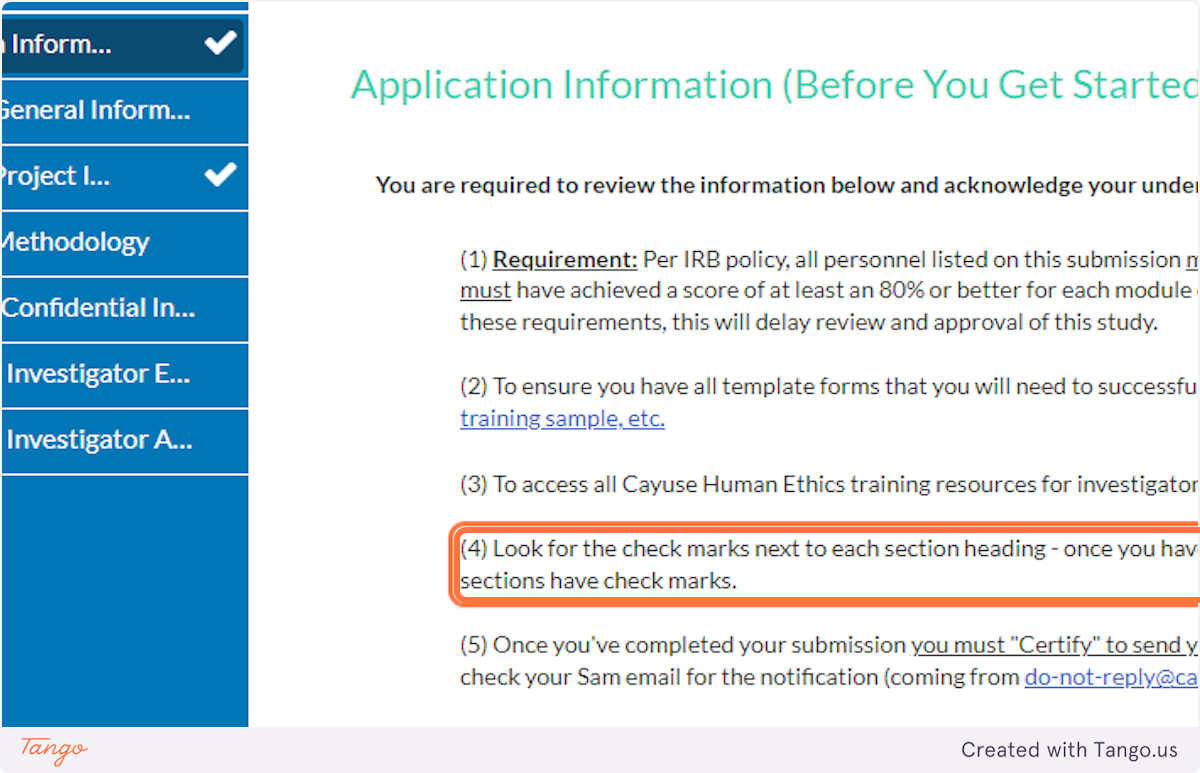
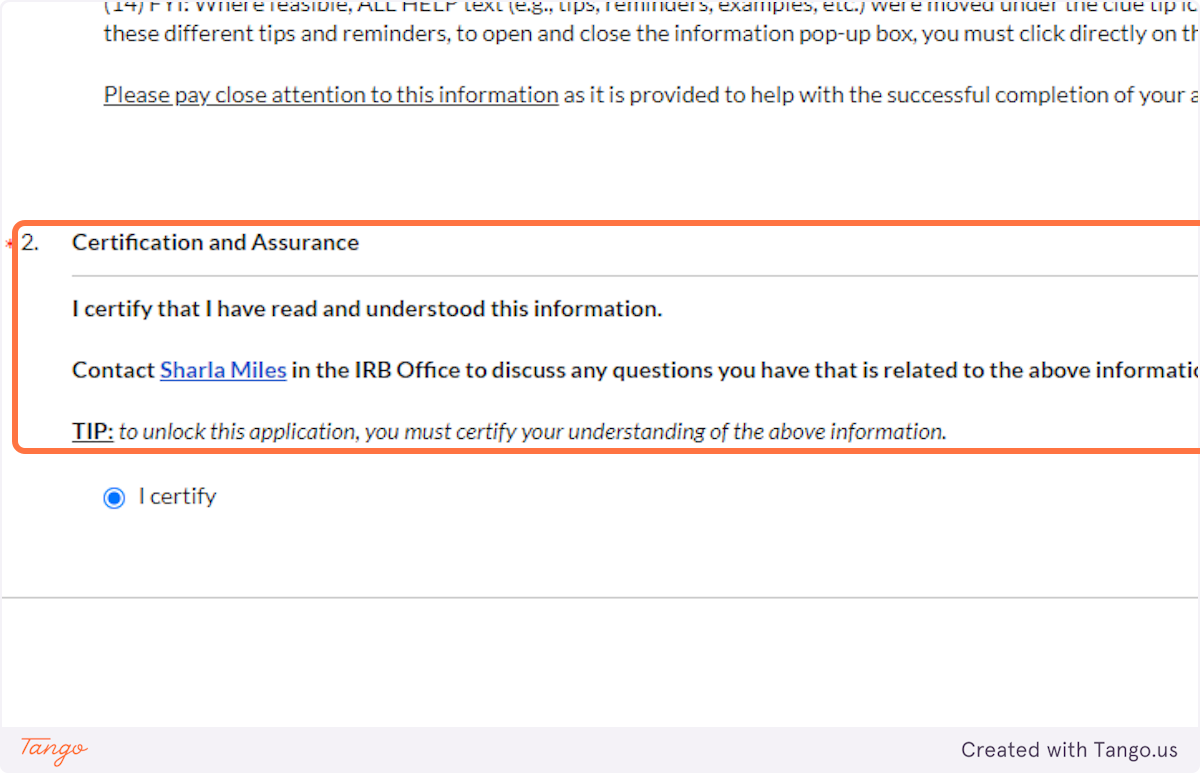
3. After you have read all details CAREFULLY, click the button to certify your understanding.
4. Click on the arrow at the lower right to go to Section 1.
5. The first field asks for the Primary Contact and likely already lists you as the creator of the submission. Verify that this is correct for this project -- that you want all IRB communication to come to you.
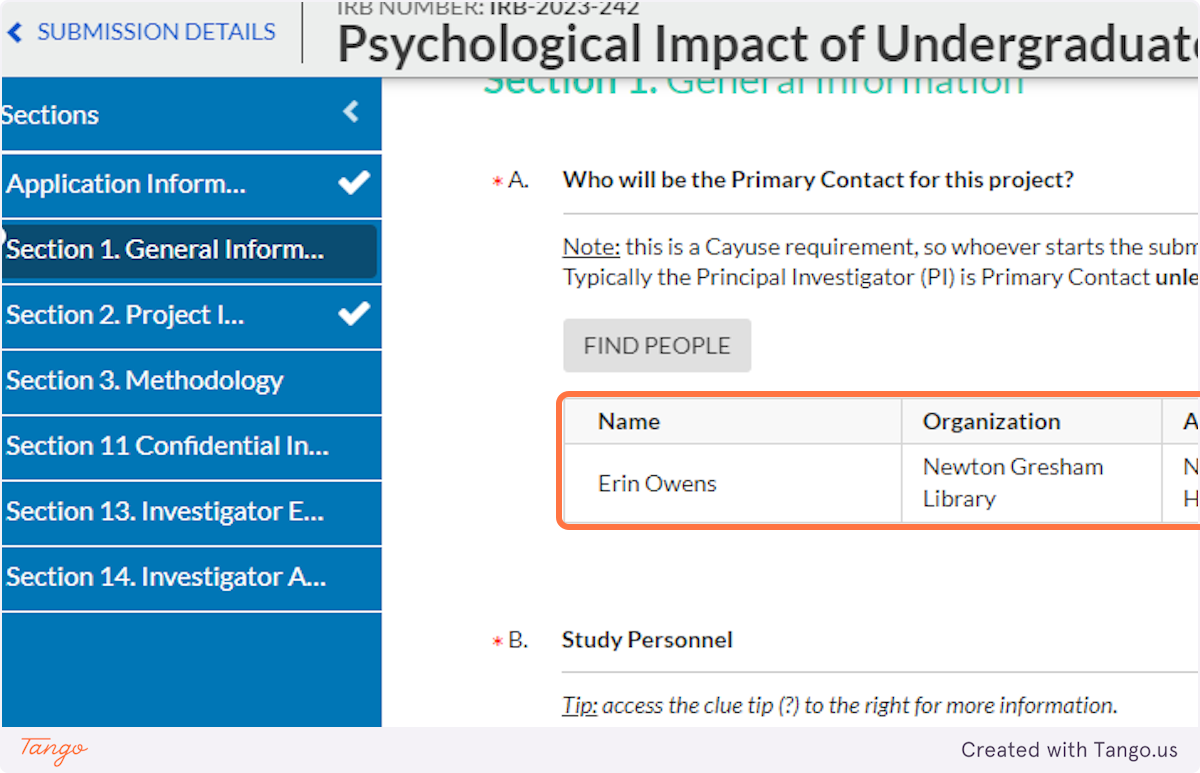
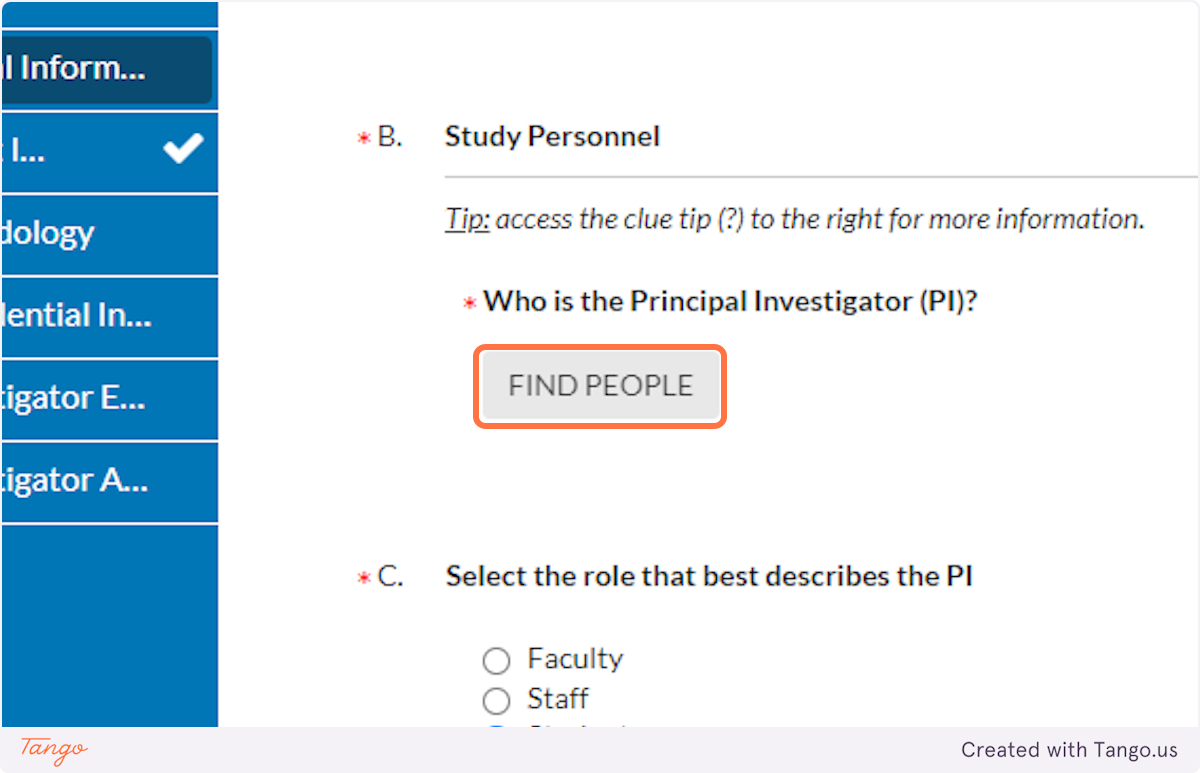
6. The next question asks who the actual Primary Investigator (PI, or head researcher) will be. Click the "Find People" button to select the appropriate person.
This might be you! Or it might be your faculty advisor, or even another student collaborator. Think carefully about your situation and " who is ultimately responsible for the conduct and oversight." If you are unsure who to enter, ask your faculty advisor.
7. Enter a name (yours or another) to search for.
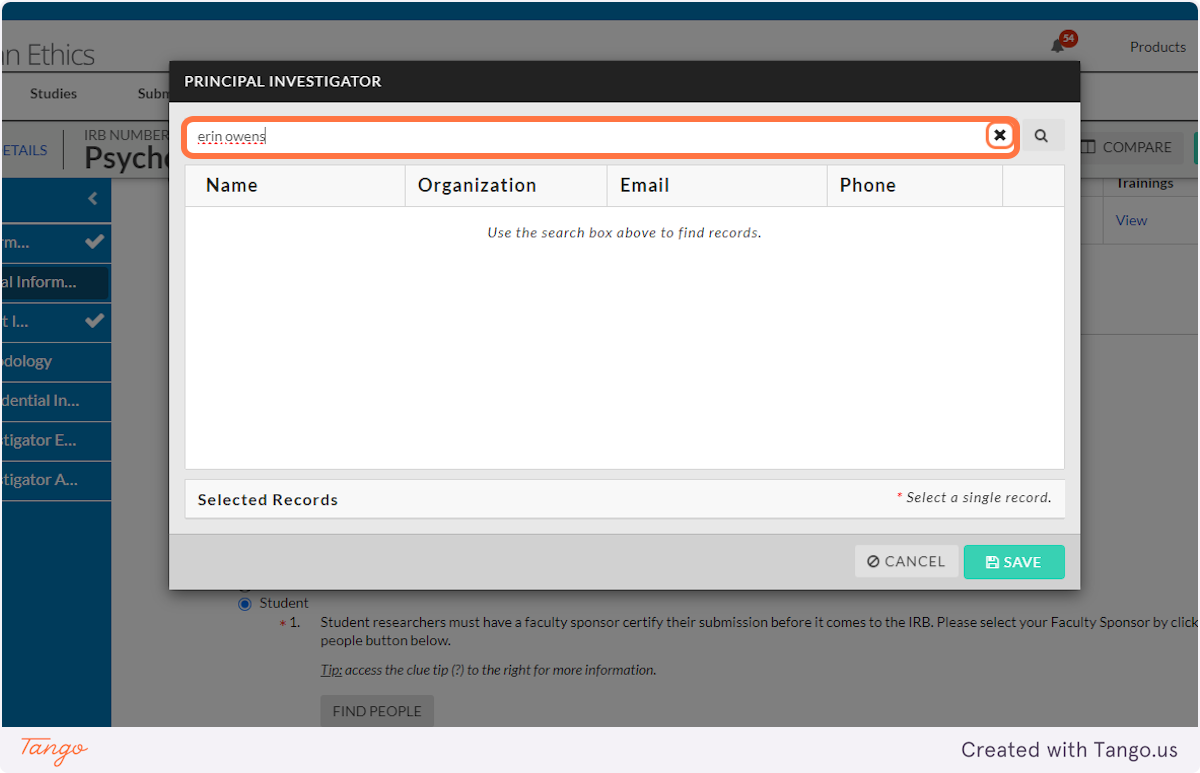
8. Press Enter or click the magnifying glass to search.
9. Click to select the correct name from those listed.
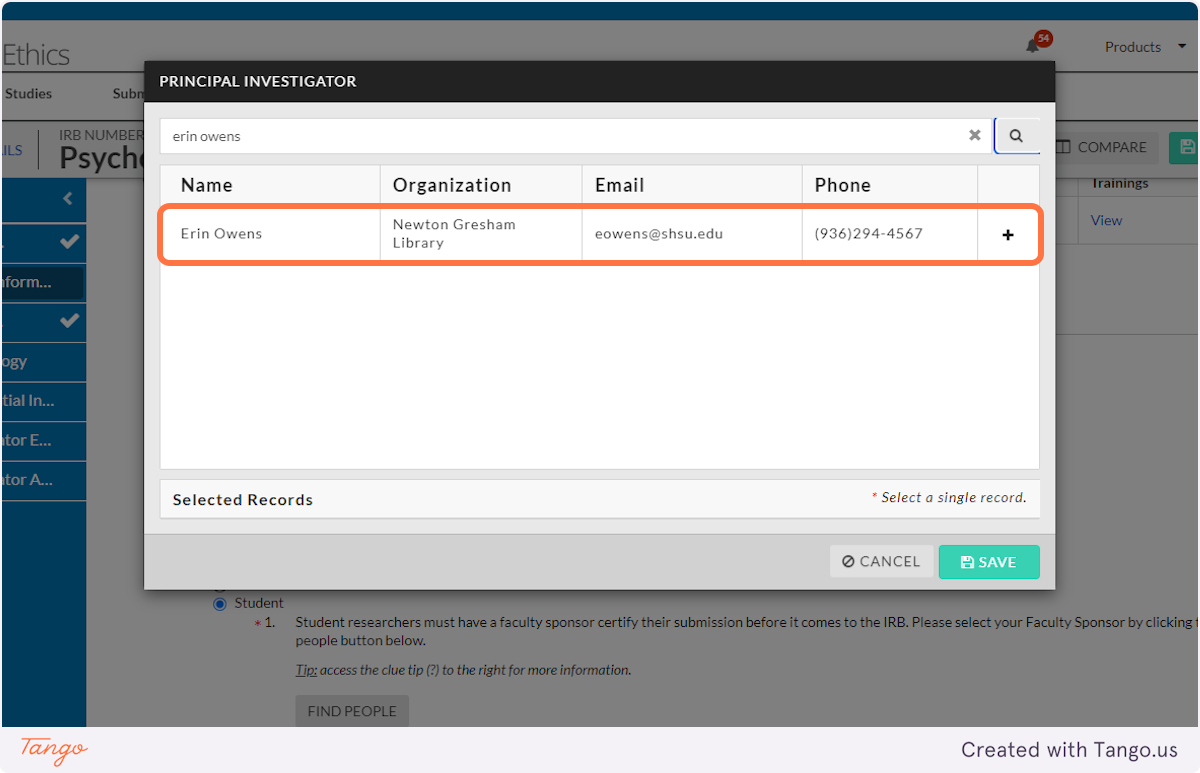
10. Click on SAVE
11. Based on who you selected above as the PI, click the correct button to describe that PI's role at SHSU. If you are the PI, you will choose Student.
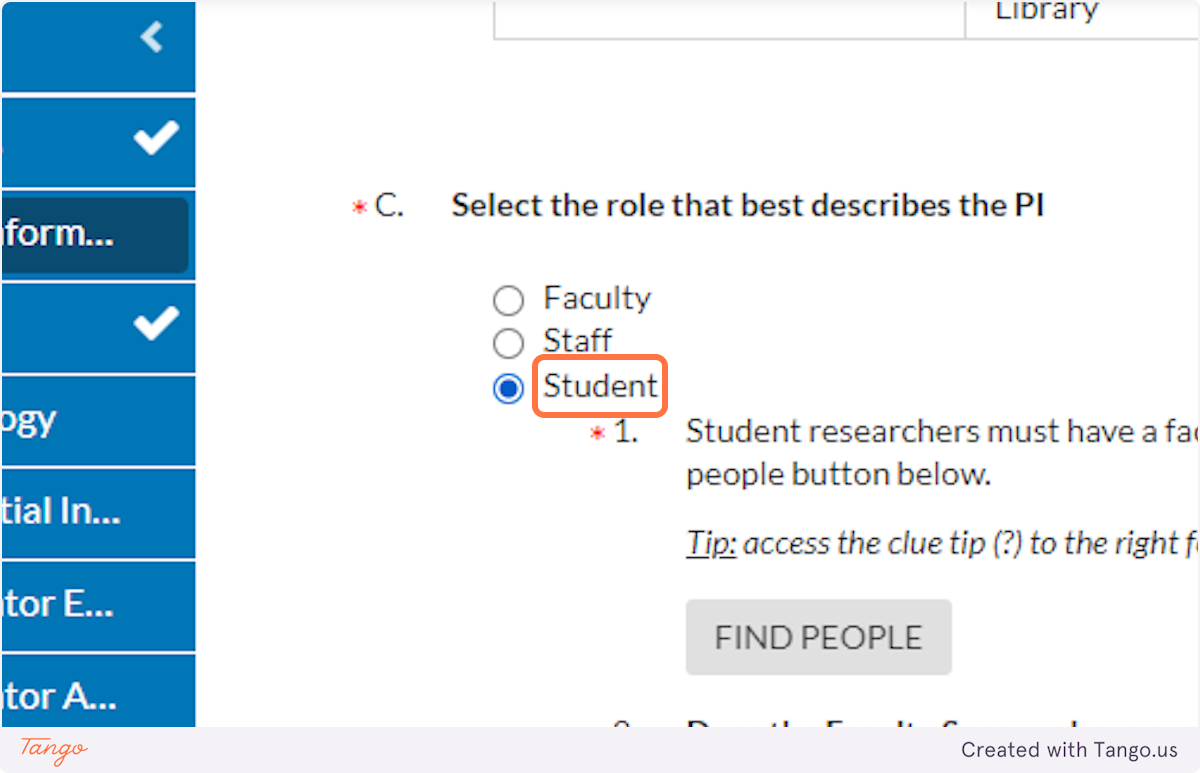
12. A note will inform you that student PIs must have a faculty sponsor, who must be identified in this IRB submission.
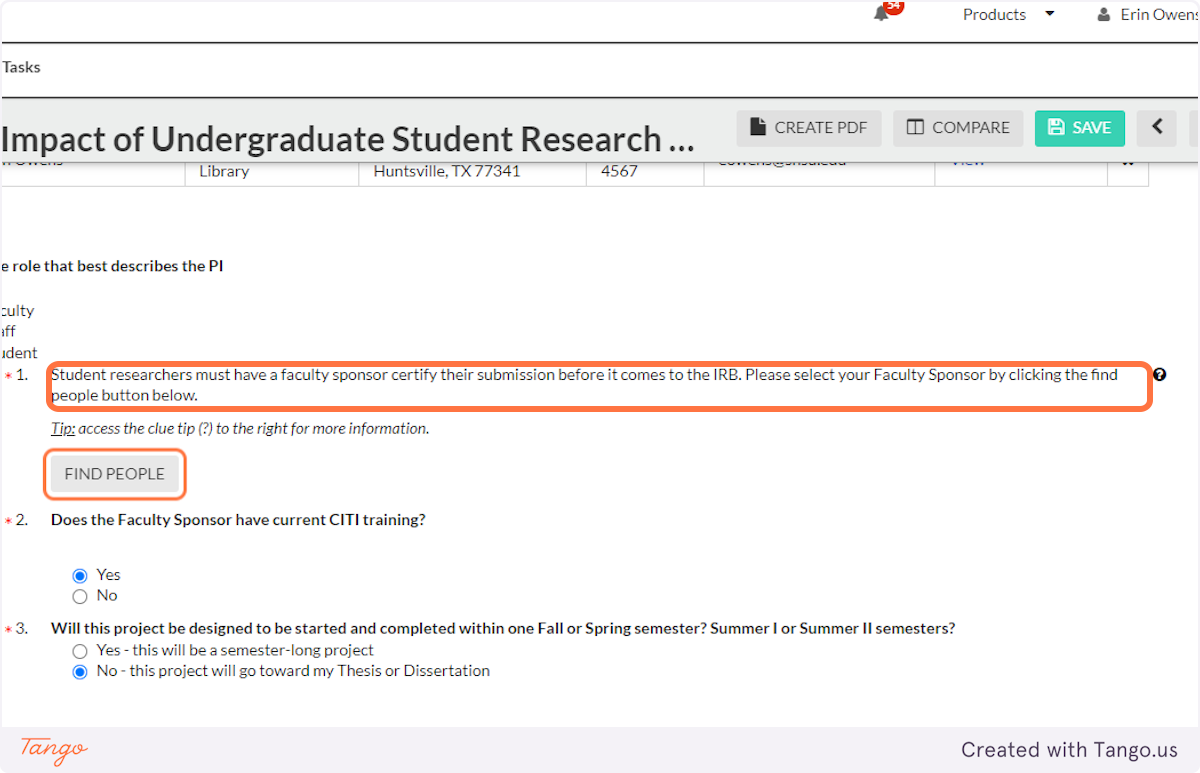
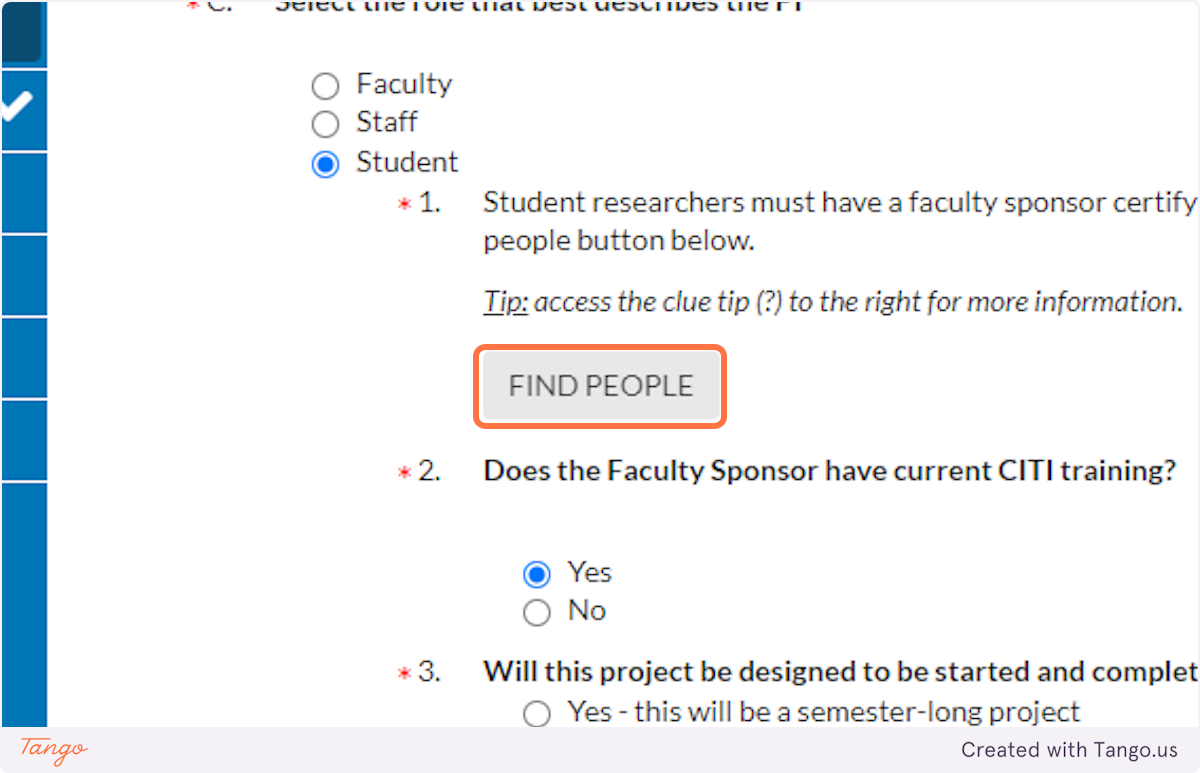
13. Click on FIND PEOPLE…
14. Enter a name to search for.
15. Press Enter or click the magnifying glass to search.
16. Click to select the correct name from those listed.
17. Click on SAVE
18. Indicate whether your faculty sponsor has up-to-date CITI training on record with SHSU's Office of Research. If you aren't sure, ask your sponsor!
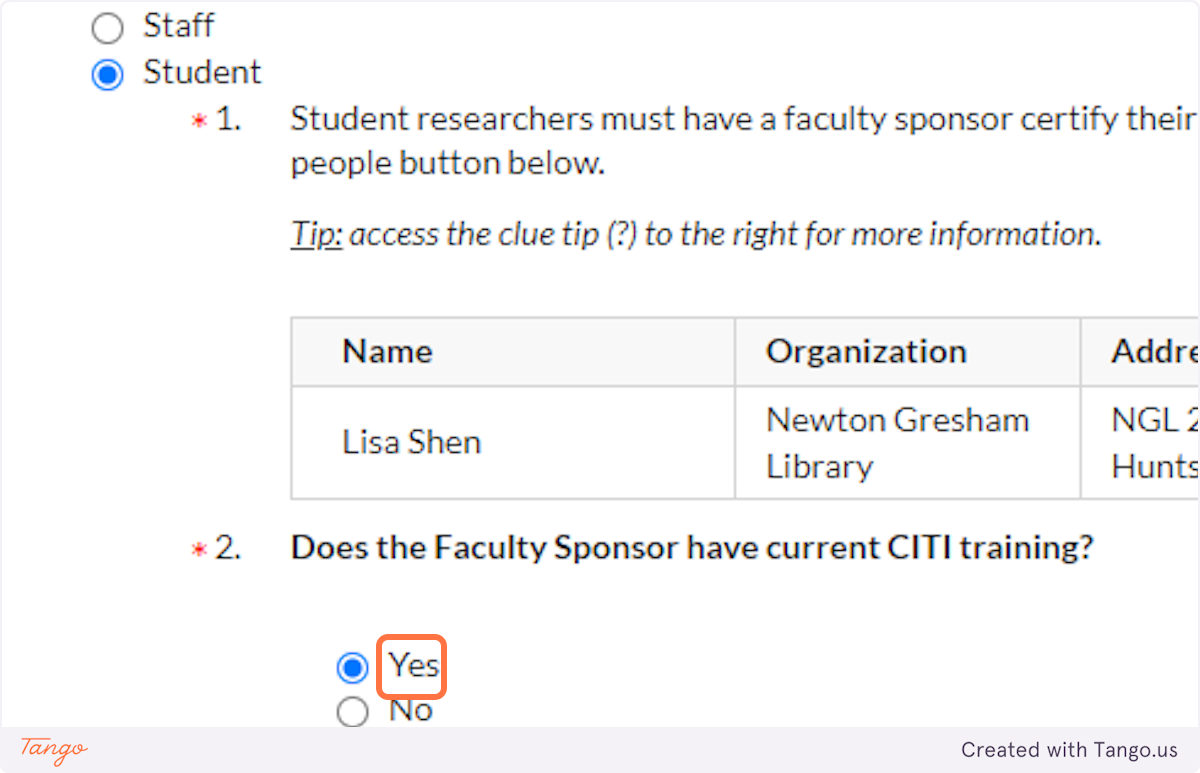
19. Select whether this is a class project that will only last for a semester...
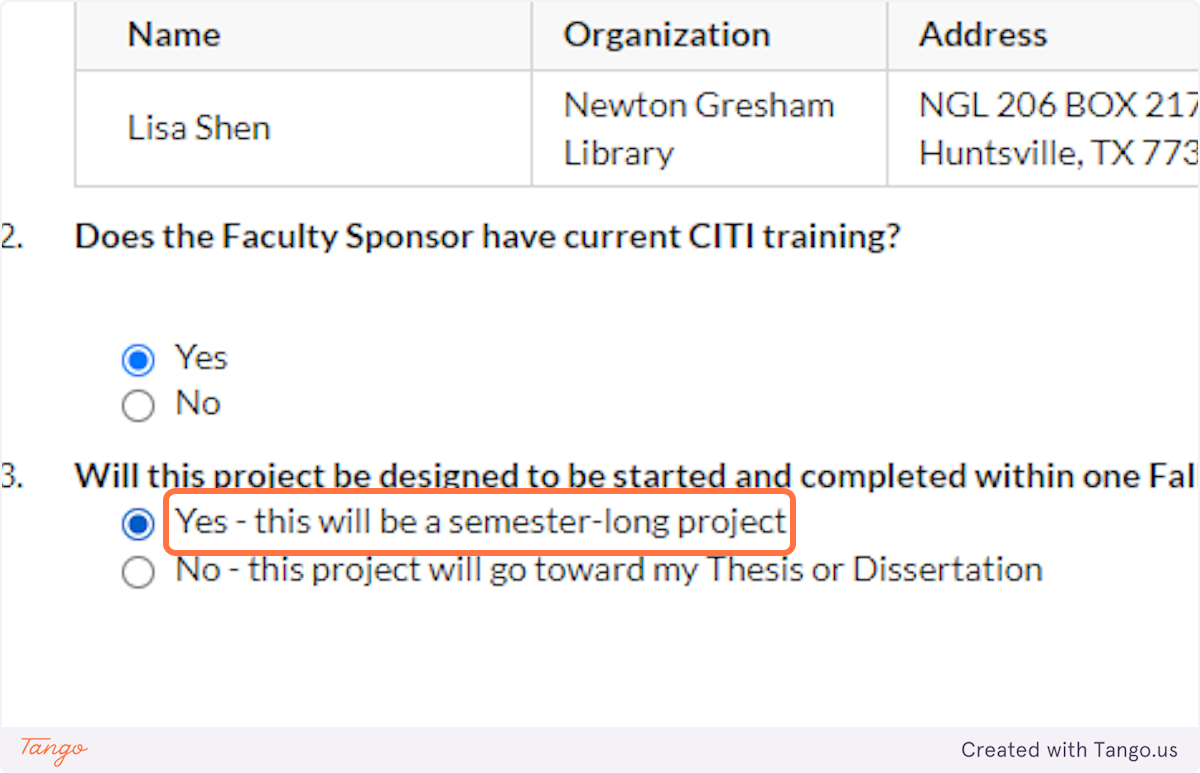
20. ...Or whether this project will be longer-term, towards your thesis or dissertation.
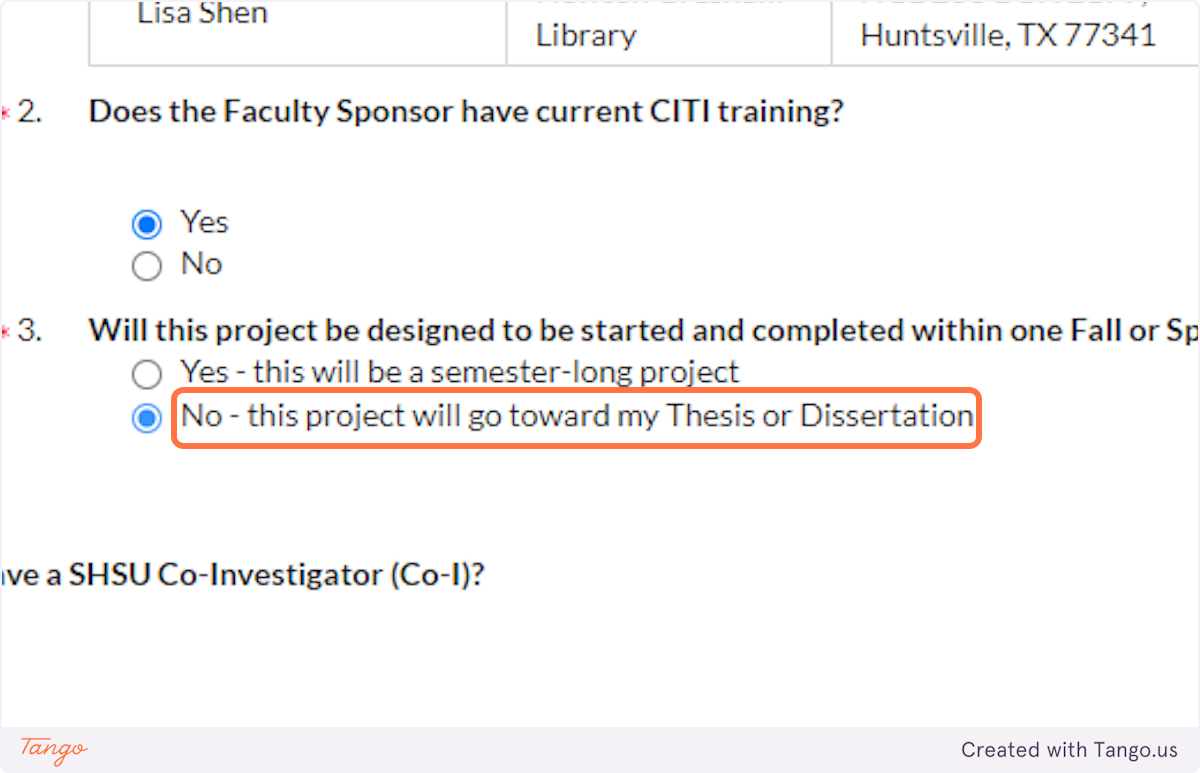
21. If you have other collaborators INSIDE of SHSU, such as other student researchers, click Yes and identify them here.
Anyone who was already identified in Section 1 does NOT need to be added again here.
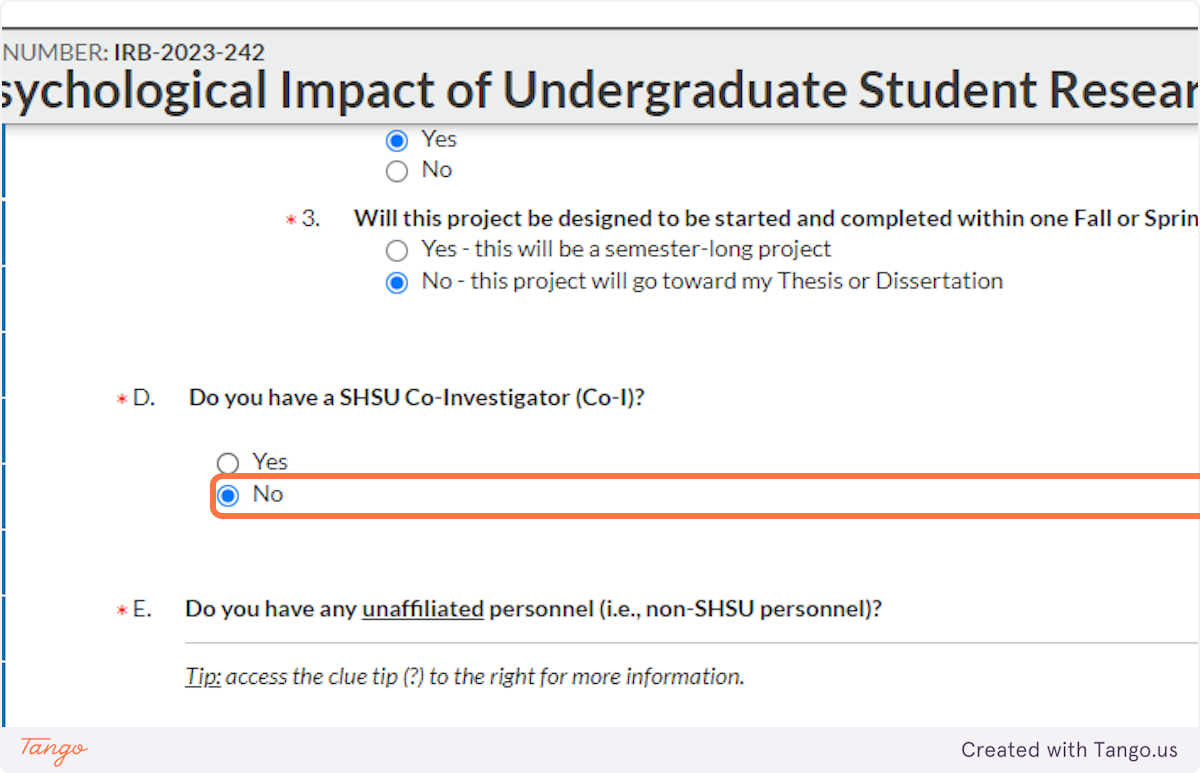
22. If you are collaborating with any other researchers OUTSIDE of SHSU, click Yes and identify them here. This will not be common for most student projects.
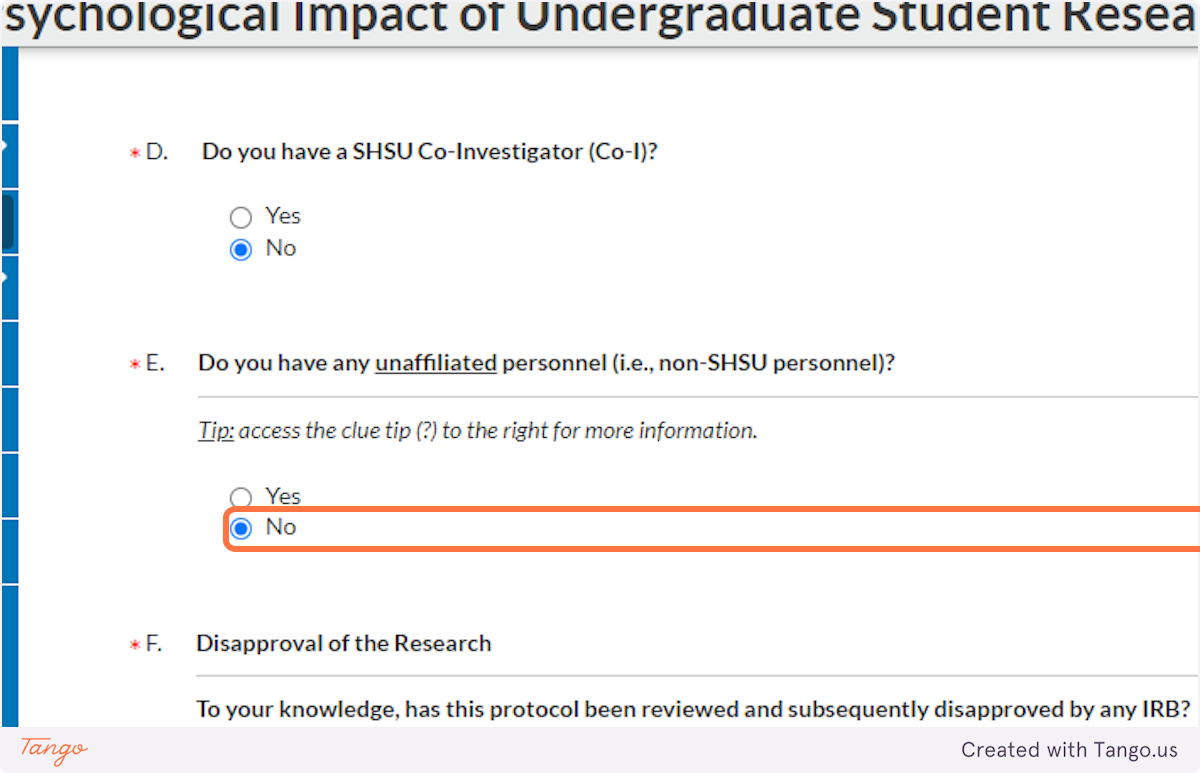
23. If this is the first time this project has been proposed to any IRB (at or outside of SHSU), click No. If this project has been proposed before AND BEEN REJECTED, select Yes.
For past disapprovals, you will need to provide details and documentation.
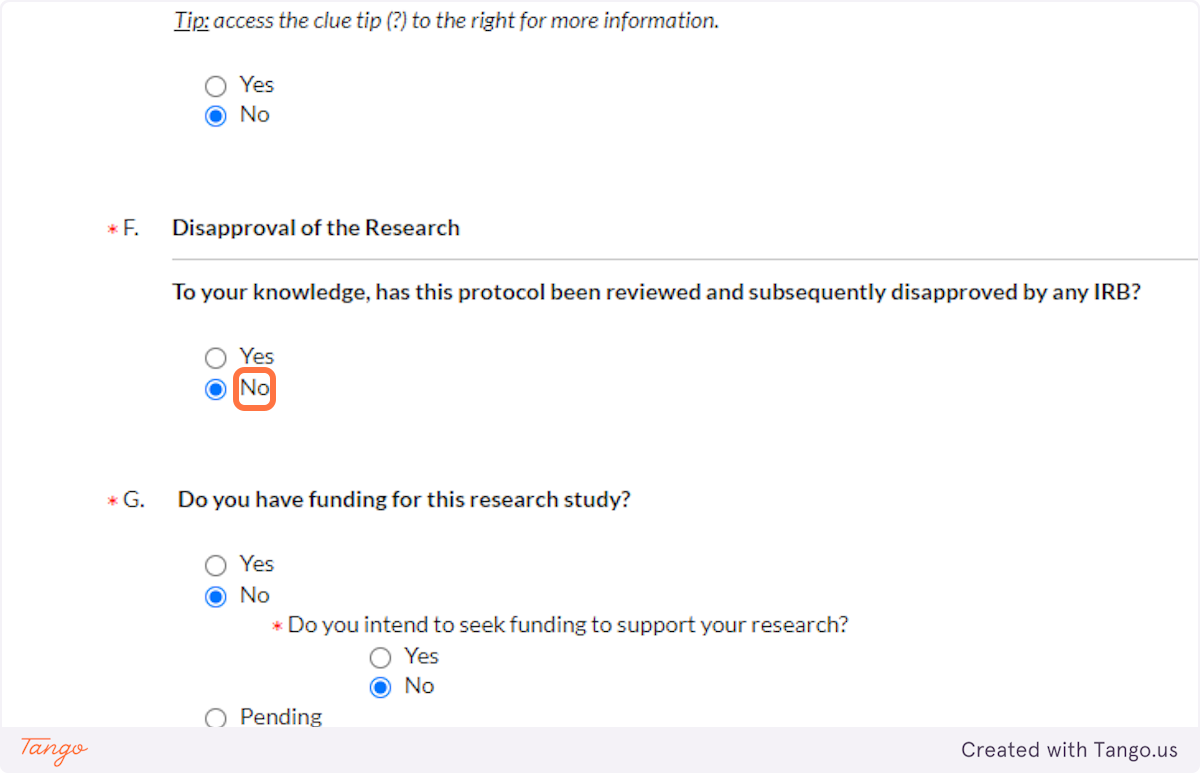
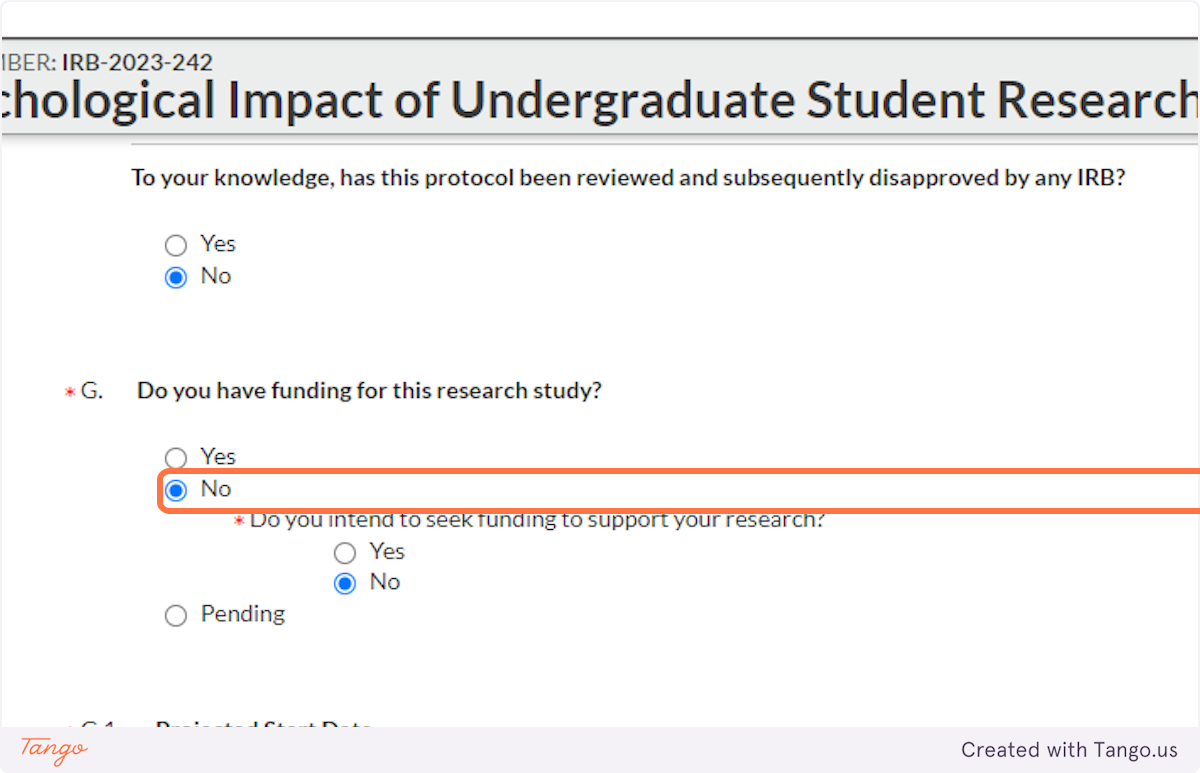
24. If you have secured grant funding, or intend to apply for funding, indicate that here. This DOES include grants from within SHSU, such as a EURECA or ORSP grant.
Note that if a funding application is pending but not yet decided, you will mark Pending, but then you will also eventually need to send the IRB an update when that funding application is either received or rejected.
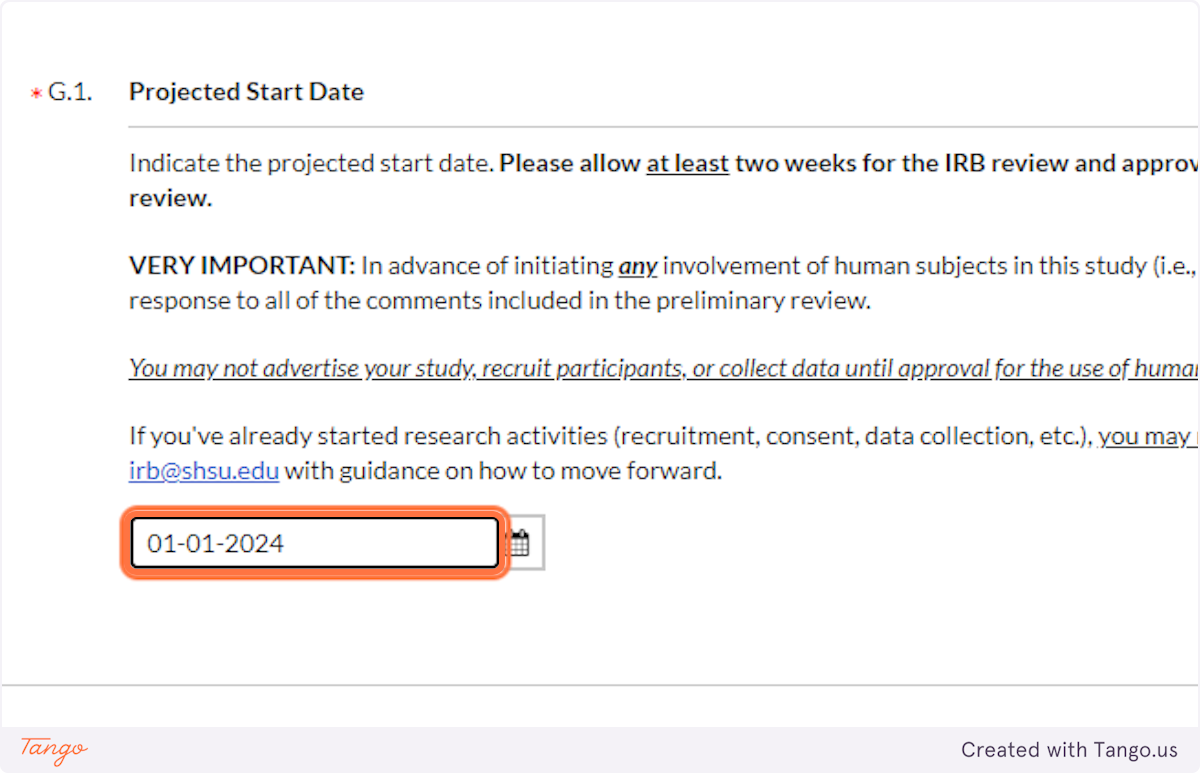
25. Enter the date AT LEAST two weeks in the future (or further) when you expect to start collecting data for this project, if it is approved.
26. Click on the arrow at the lower right to go to Section 2.
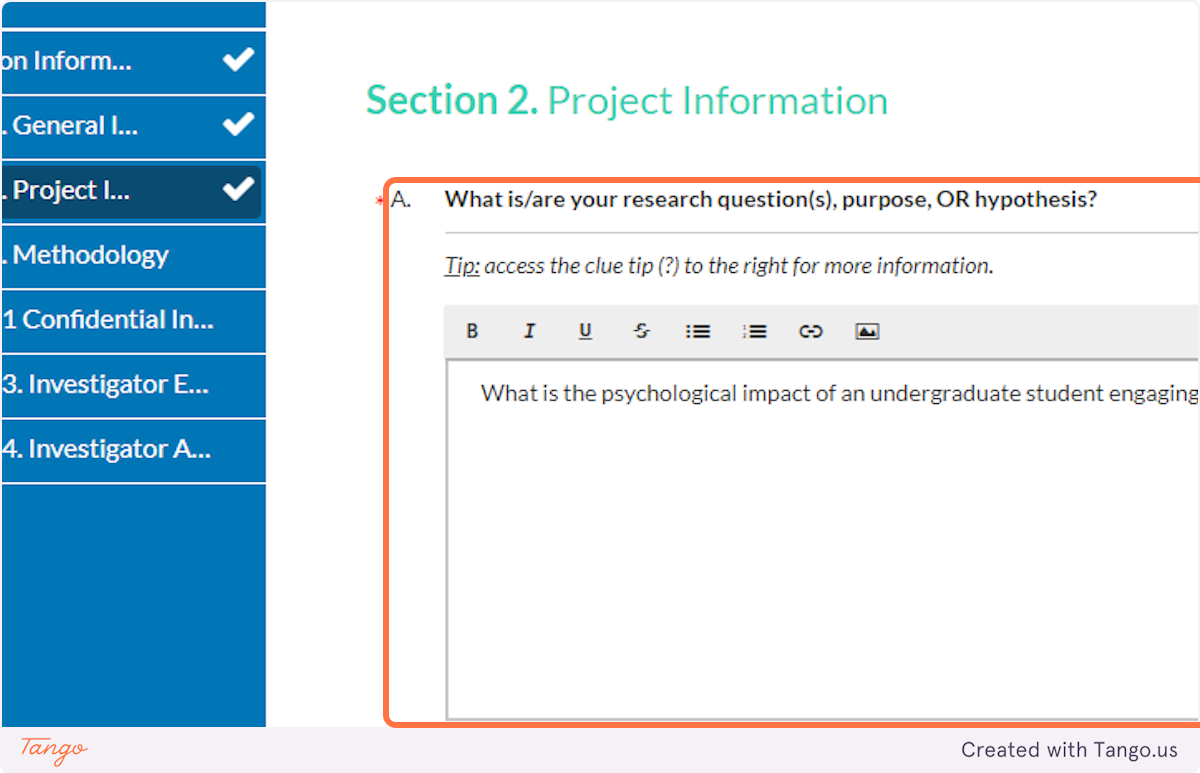
27. In this field, enter your research question(s), hypothesis, or a clear, concise statement of the research purpose. If you are completing a thesis or dissertation, the purpose/questions here should match those in your thesis or dissertation.
Note: Do NOT include explanatory background of the problem or literature in this field. Just state clearly and concisely the overall question or phenomenon you are studying and what you hope to learn about it.
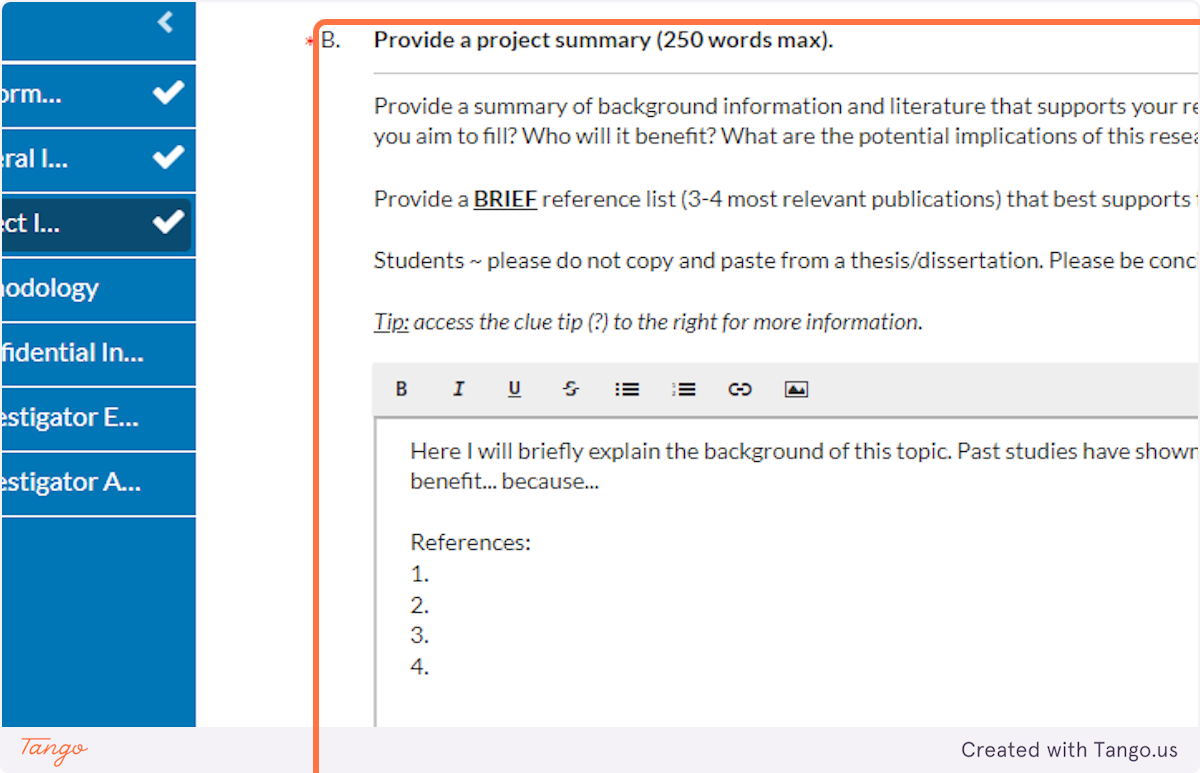
28. Your project summary in this field will SUMMARIZE what would be the introduction/background and literature review in your complete paper. This is where you should briefly explain the context and importance of your research question for someone who is not yet familiar with it.
Do not cut and paste full sections from your thesis or dissertation; this should be a very short summary. Cite only the 3-4 most crucial publications to support what you will be doing and why.
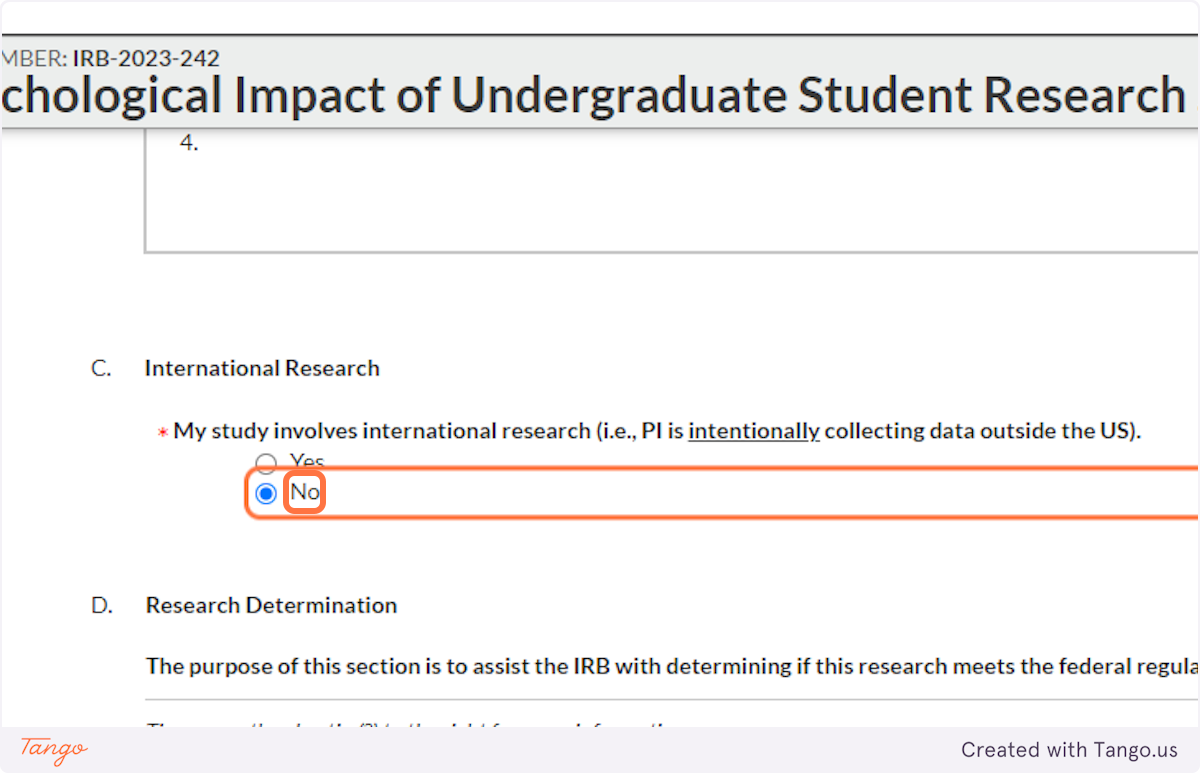
29. Are you deliberately seeking to collect data from outside the U.S.? If so, additional regulations may apply.
If the answer to this question is Yes, be prepared to provide additional details about what country or countries your data will come from and whether you have identified their equivalent of an IRB for approval of your research.
30. If this research will be applied to support a thesis, dissertation, presentation, publication, or similar, select YES for this question.
If your project will not be applied in ANY way that contributes to generalizable knowledge, then it may not qualify as "research" as defined by federal regulations, and you may not need IRB approval. You are encouraged to call the SHSU IRB office and ask for clarification or guidance.
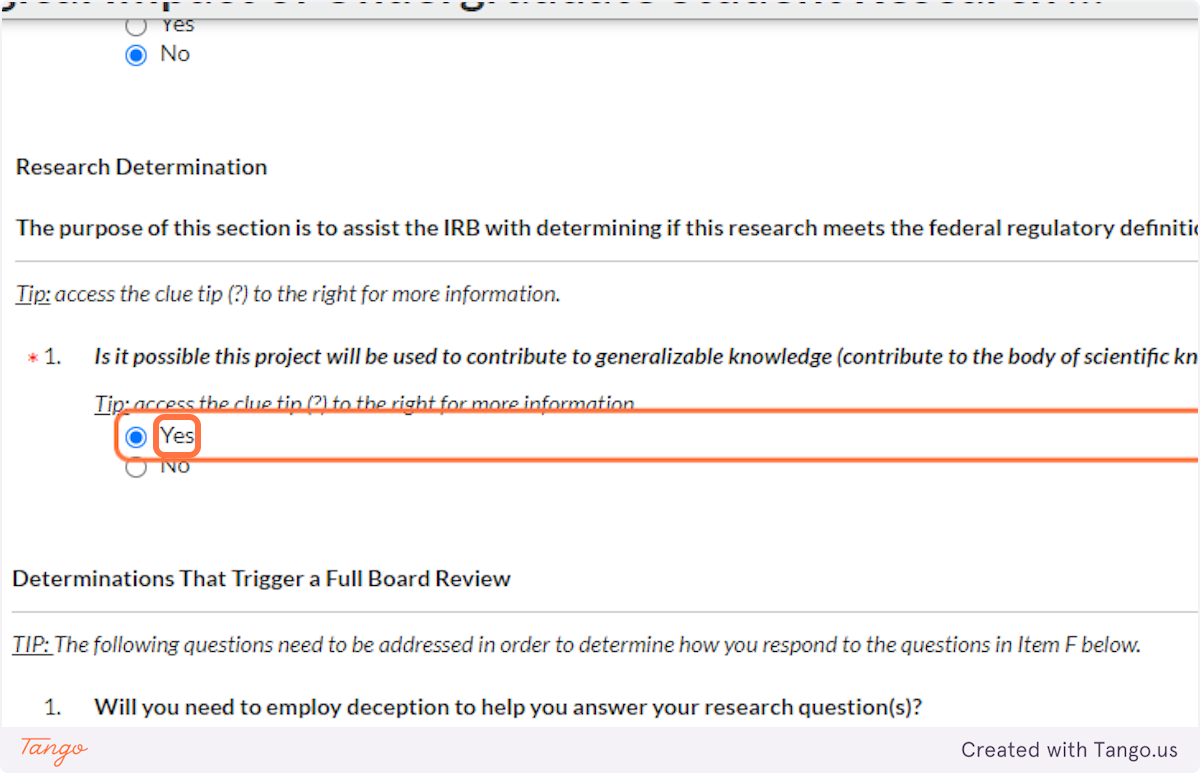
31. In most disciplines, student projects are unlikely to involve deception -- not being truthful with your research participants -- but if that applies to your study, you must indicate it here. This will trigger a more rigorous review of your proposal.
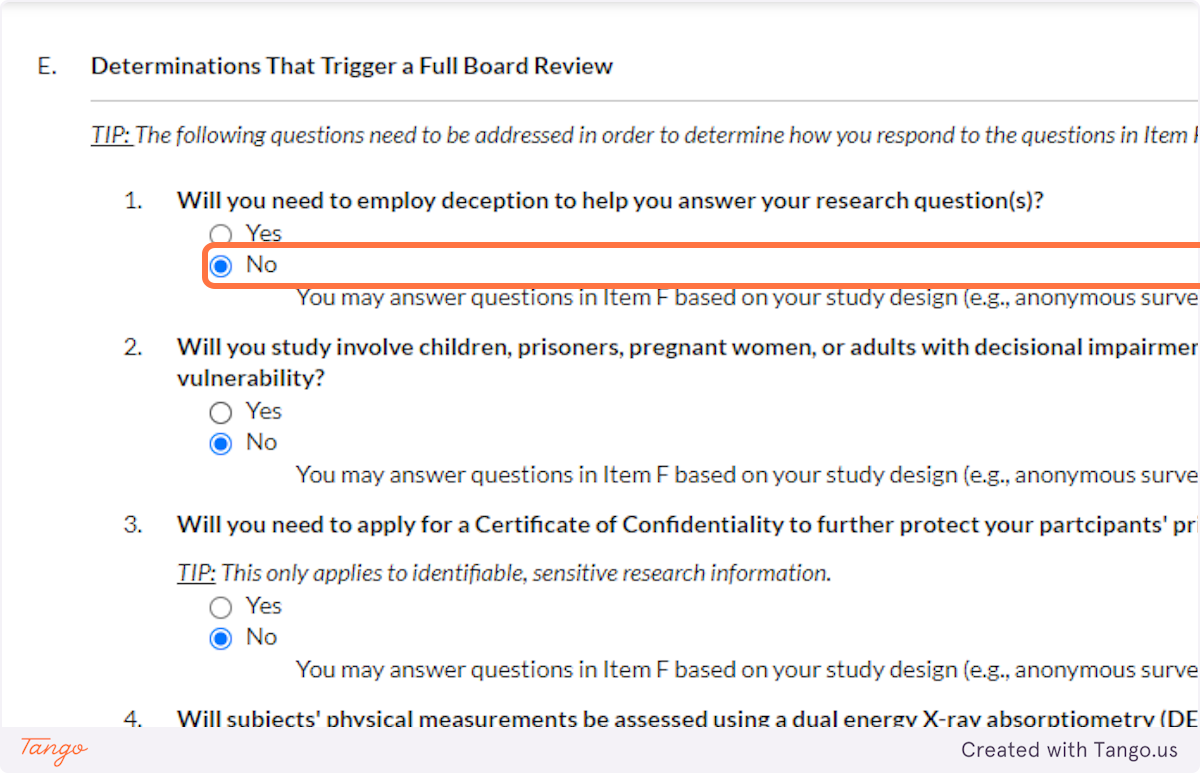
32. Indicate whether you will be working with vulnerable populations such as those listed. Again, answering Yes to this question will trigger a more rigorous review to ensure participant safety.
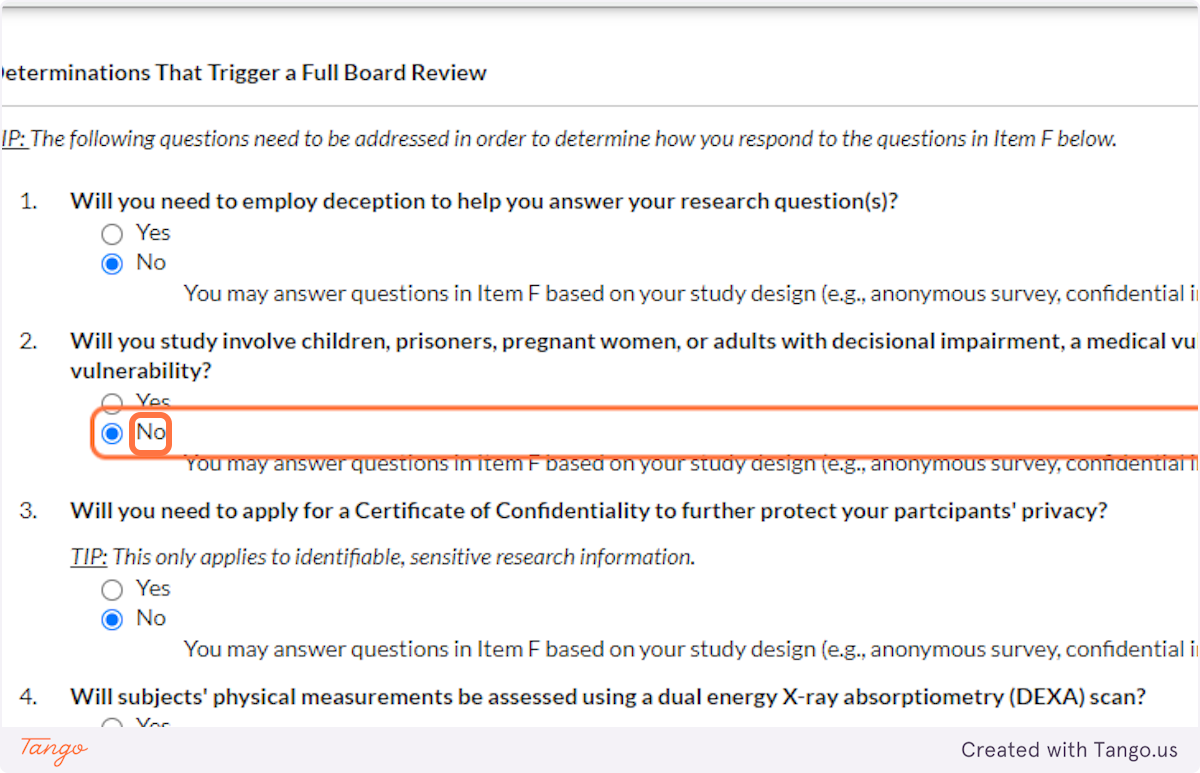
33. A Certificate of Confidentiality will most often NOT apply to student research projects. If this applies to you, it is likely something that you and your faculty advisor have already discussed.
If you are uncertain, speak with your advisor or the SHSU IRB for clarification. Answering yes here will trigger a more rigorous review of your proposal.
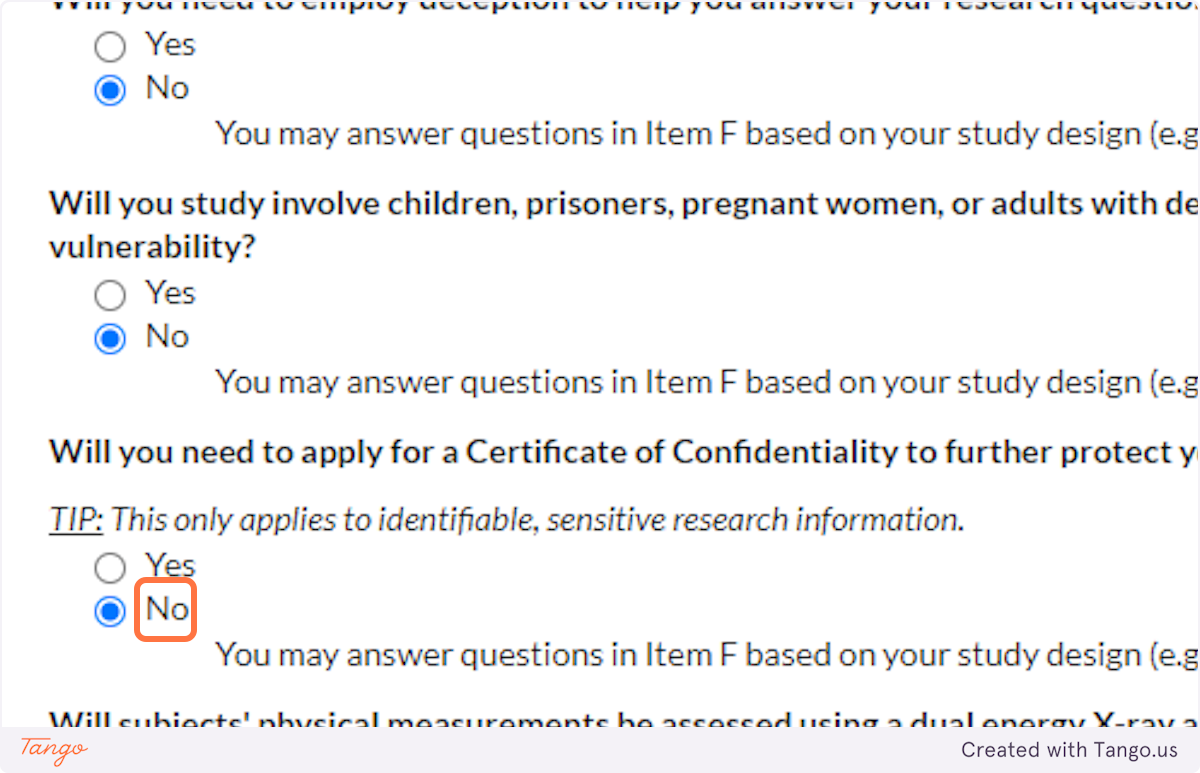
34. Unless your methods involve a DEXA scan, select NO for this question. Answering yes here will trigger a more rigorous review of your proposal.
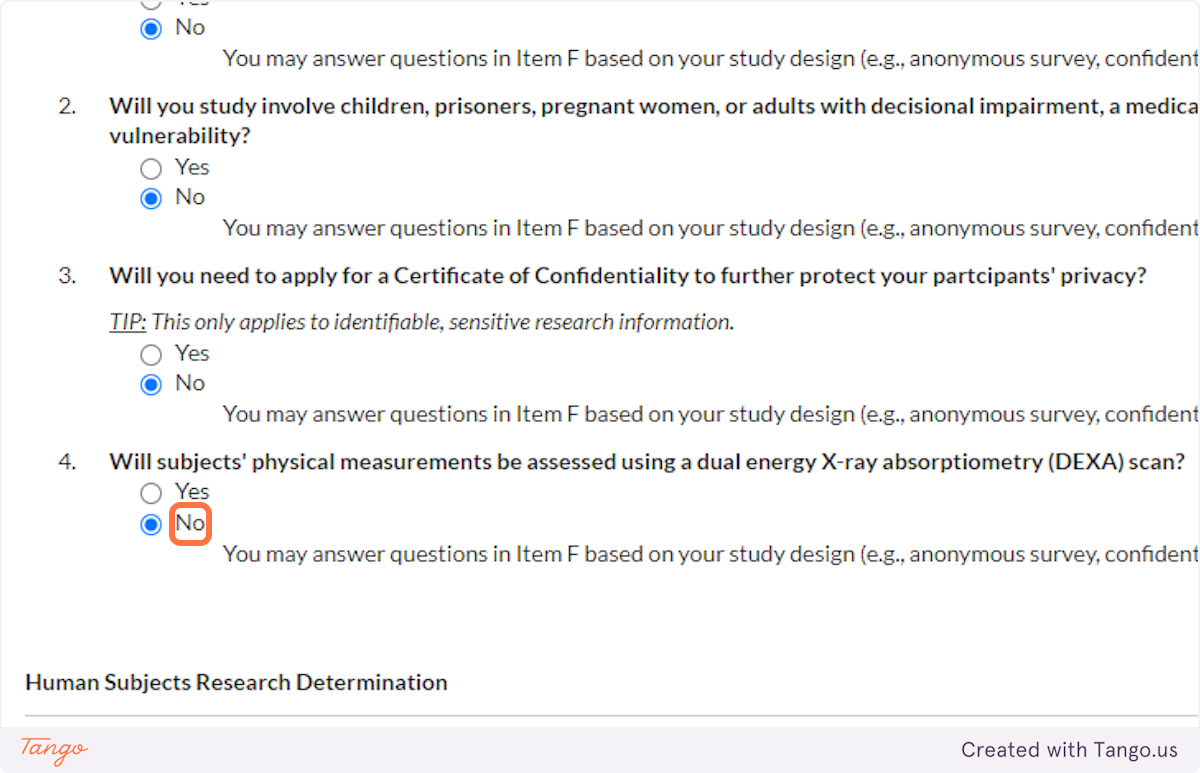
35. If you will be interacting with your human participants in some way -- whether through face to face conversation, email, online survey, syringe, blood pressure cuff, etc. -- you will answer NO to this question.
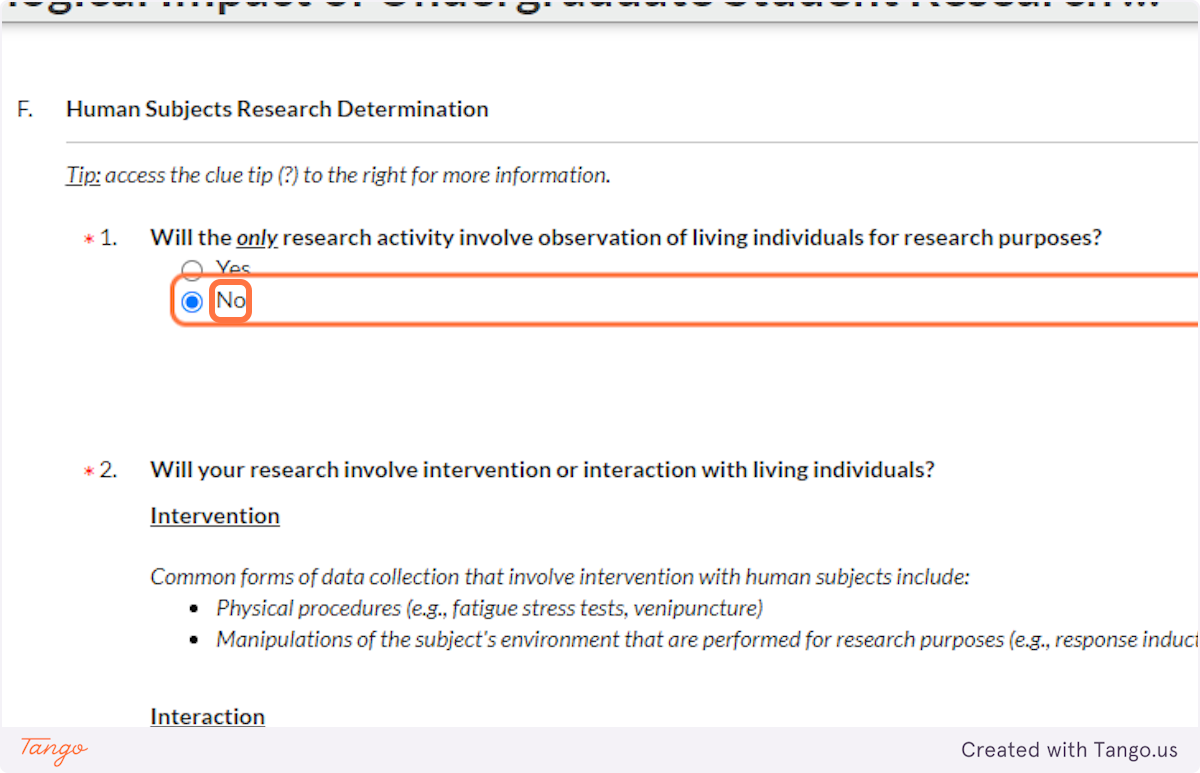
You should also answer NO if you will be observing participants, with or without interaction, in a PRIVATE place. Note that a "public school" is a "private place" for the purposes of this question.
If you will be sitting in a PUBLIC place like a park and watching people without interaction, you may need to choose YES here. You will likely not need to enter any more information about your project or receive IRB approval, if you are not actually interacting with human participants.
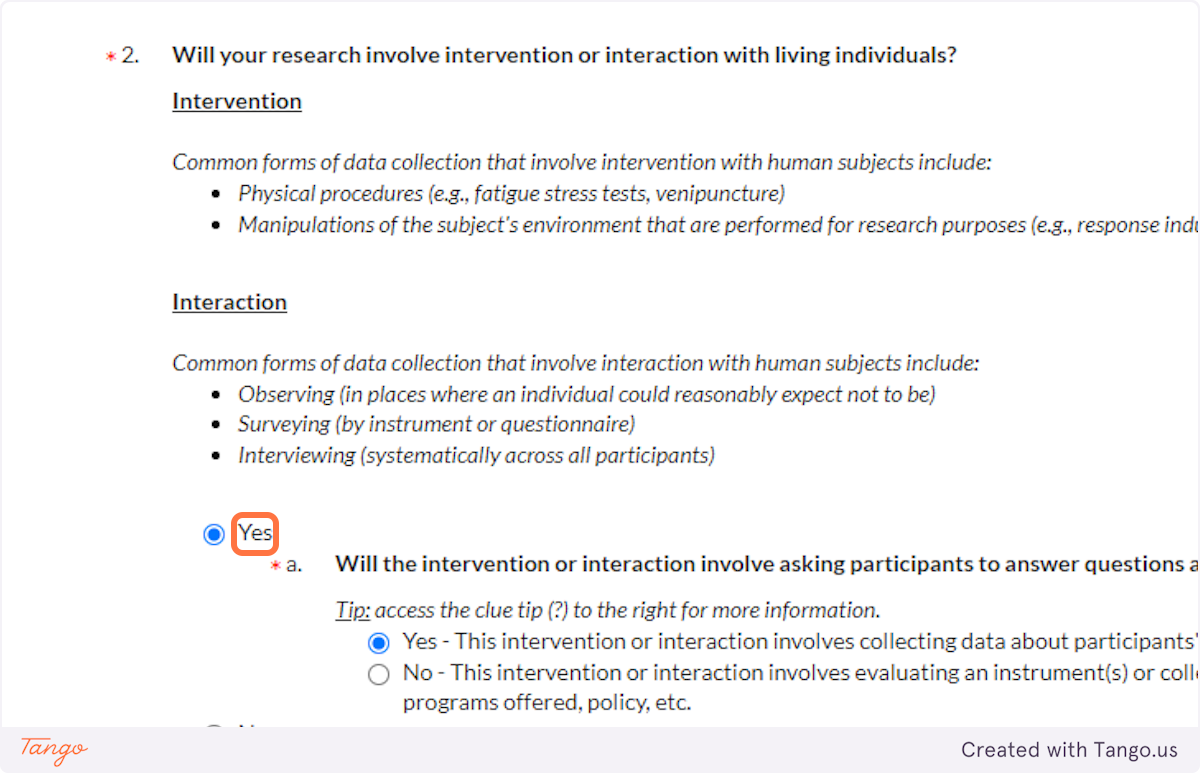
36. Most research projects that require IRB approval involve interacting with human participants in some way -- whether through face to face conversation, email, online survey, syringe, blood pressure cuff, etc. If that's your case, choose YES here.
There are some exceptions to this, for example if you are conducting secondary research with identifiable private information or biospecimens whose collection was unrelated to this project. If your research falls under one of these exceptions, and you are truly NOT interacting with human participants, then choose NO here. You will then answer accordingly to the question 4 regarding secondary research, and you will need to complete an additional application section detailing the secondary data source and the original purpose for its collection.
37. If your research only involves analyzing a public dataset in which participants are unidentifiable, you will not require IRB approval; select Yes here. Otherwise, select NO to continue pursuing IRB approval.
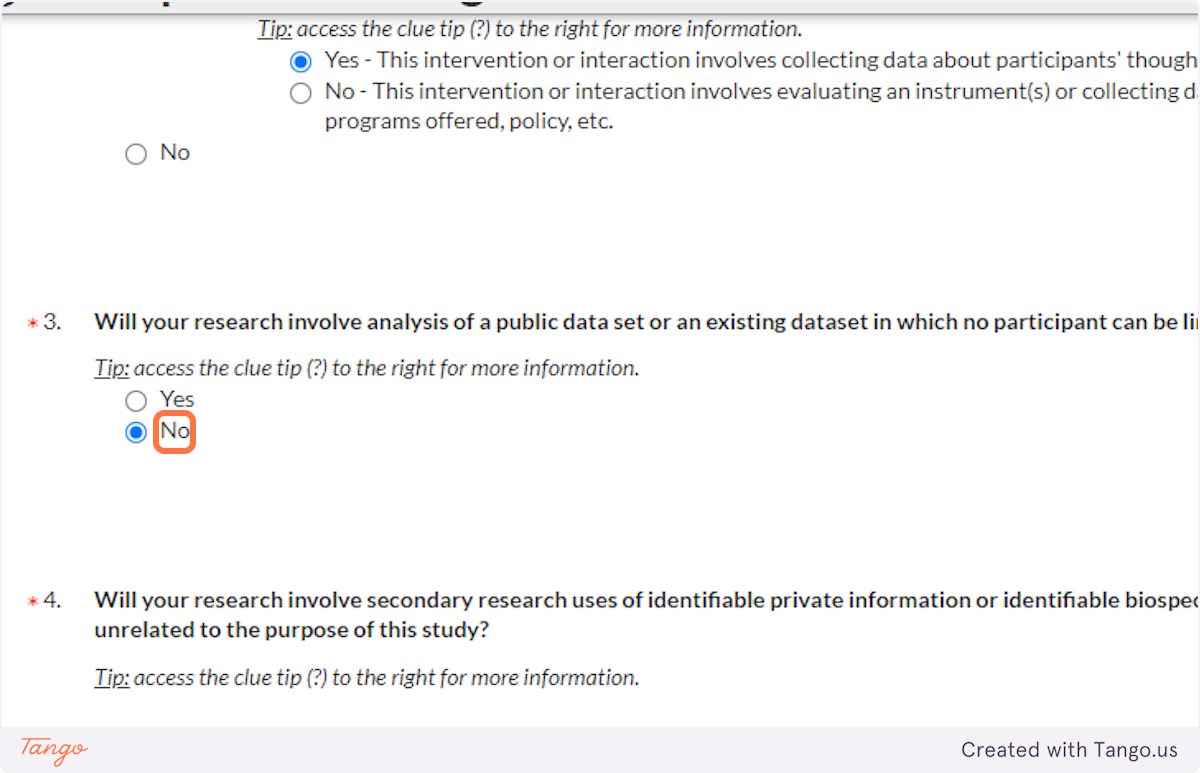
38. As discussed above, if you are doing secondary research with identifiable information or specimens, select Yes here. Otherwise, select NO.
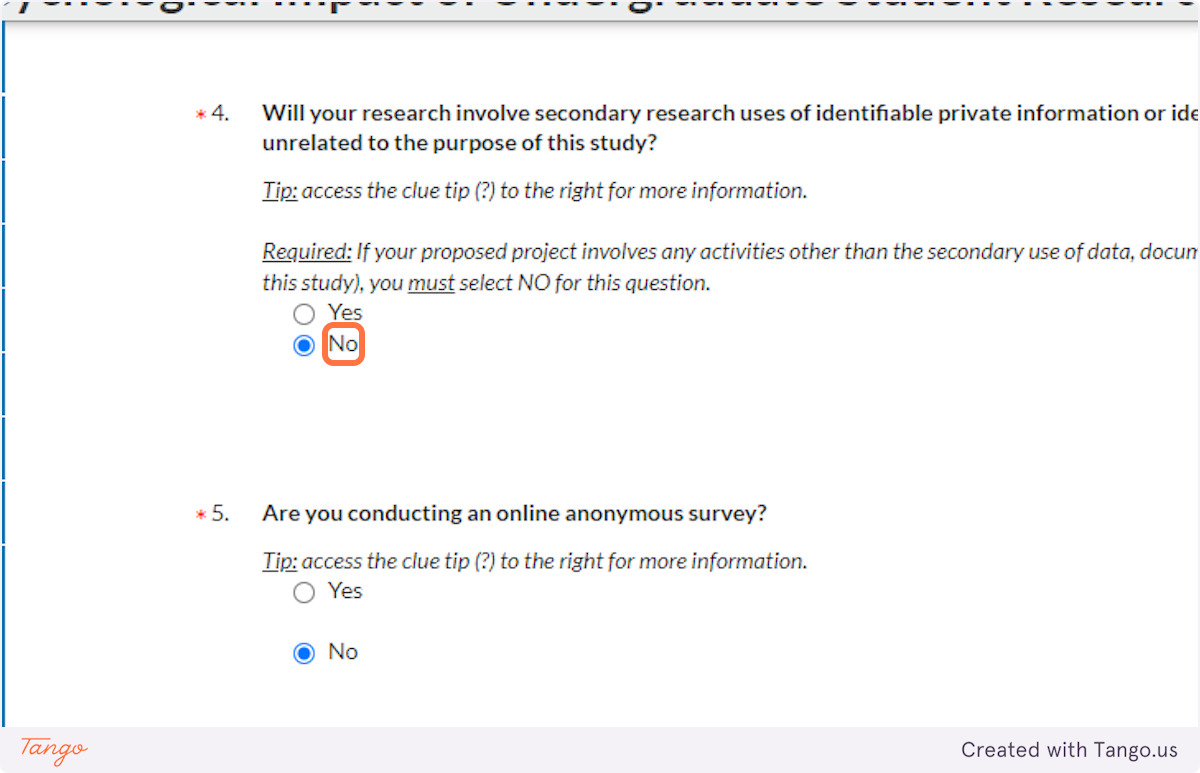
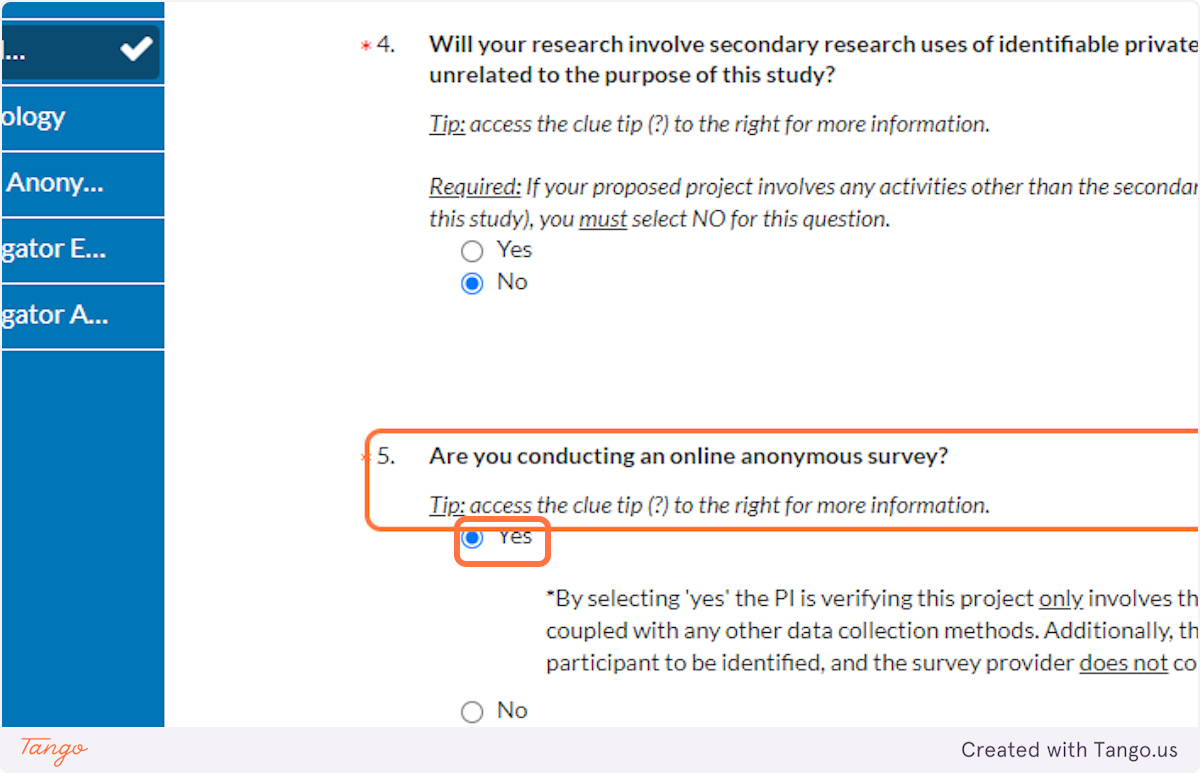
39. If you are conducting ONLY an anonymous online survey, choose Yes here. This will trigger creation of a new section in the application that will ask you more detailed questions to ensure anonymity of the survey.
If you are NOT conducting an online survey, or it is not online and anonymous, select No instead.
Note: For logical consistency, if you select Yes here, you also should have selected Yes above for "interaction or intervention."
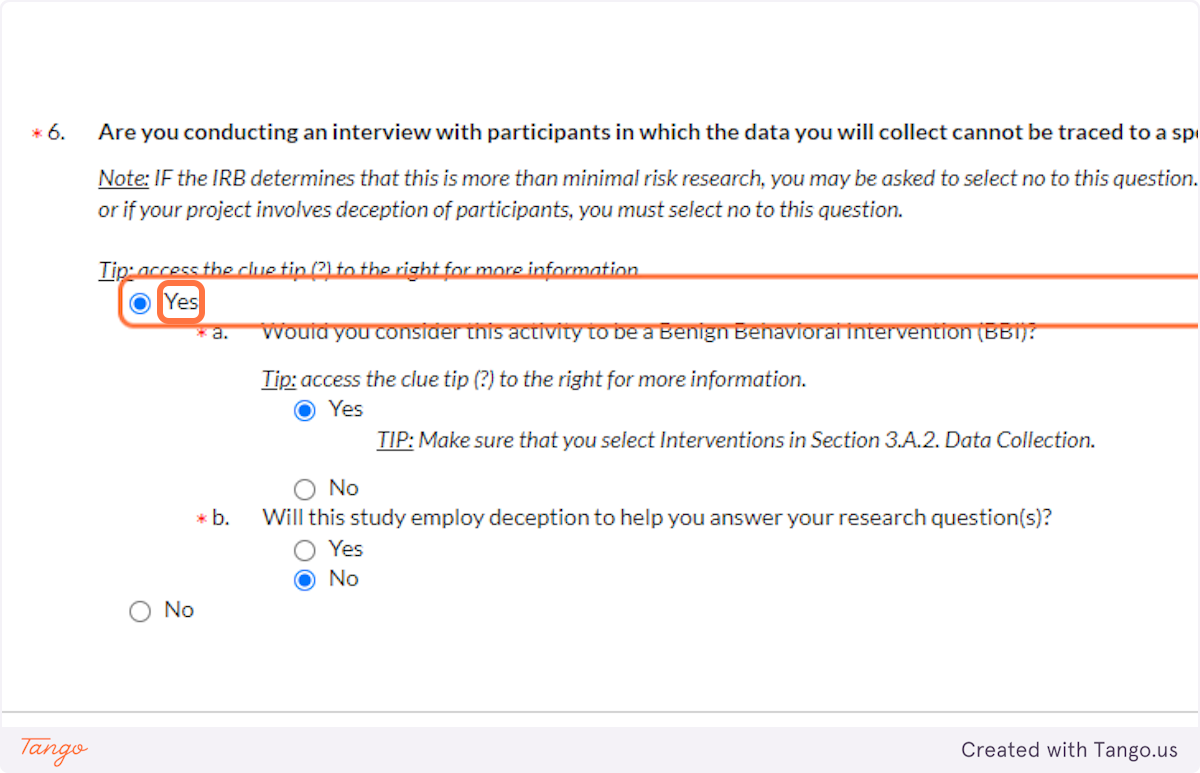
40. If you are not conducting interviews at all, your answer to question 6 will be NO. If you are: Ask yourself whether the data could be traced to a participant, and answer Yes or No accordingly.
If you answer Yes:
You will be asked further whether or not you consider this activity "benign" (brief, painless, not invasive or embarrassing). If you are unsure, definitely click on the question mark icon to read more details about benign interventions. Check with your faculty advisor if you find you still need clarification.
You will also be asked again whether your study involves deception, or being untruthful with participants.
41. Click the arrow at the lower right to on go to Section 3.
Completing the Application Information Page and Application Sections 1 and 2
- << Previous: Asking a Research Question
- Next: Data & Research Methods >>
- Last Updated: Mar 29, 2024 12:37 PM
- URL: https://shsulibraryguides.org/undergradresearch
Newton Gresham Library | (936) 294-1614 | (866) NGL-INFO | Ask a Question | Share a Suggestion Sam Houston State University | Huntsville, Texas 77341 | (936) 294-1111 | (866) BEARKAT © Copyright Sam Houston State University | All rights reserved. | A Member of The Texas State University System
Search code, repositories, users, issues, pull requests...
Provide feedback.
We read every piece of feedback, and take your input very seriously.
Saved searches
Use saved searches to filter your results more quickly.
To see all available qualifiers, see our documentation .
- Notifications

IMAGES
VIDEO
COMMENTS
Before starting any coding, you will need to research and define your portfolio project over the course of 3 weeks. In the first week, the Project Proposal will be approved, followed by the second week where the MVP will be defined, and finally presented as a collection of tasks on a Trello board. This first part is focused on creating a ...
Research & Project approval alx build your own project have fun
Pro Manager is a website to help busy individuals to stay afloat daily despite of their busy schedules, I noticed that in the new world we are today, people barely have time to catch up with ...
The ReadME Project. GitHub community articles Repositories. Topics Trending Collections Pricing; Search or jump to... Search code, repositories, users, issues, pull requests... Search Clear. Search syntax tips Provide feedback We read every piece of feedback, and take your input very seriously. ...
Research & Project approval (Part 1) \n. Portfolio project Presentation \n Concepts \n. For this project, students are expected to look at these concepts: \n \n; Maze project \n; Portfolio Project Overview \n \n \n What's this \"Research & project approval\"? \n
Luckily, there's an easy seven-step method you can follow to establish the smoothest project approval process possible. #1: Specify your project approval requirements ... As you can see, when using a proofing tool as part of your approval workflow, things get easier. You're more likely to reach milestones faster, stay on top of due dates ...
Research proposal examples. Writing a research proposal can be quite challenging, but a good starting point could be to look at some examples. We've included a few for you below. Example research proposal #1: "A Conceptual Framework for Scheduling Constraint Management".
Project Initiation: The first stage is about defining the project's scope, objectives, and requirements. Potential stakeholders are identified, and their needs and expectations are assessed. Planning: Next, you'll develop a detailed project plan, which will include timelines, budgets, and resource requirements.
Recognize the important, value-added role that your research advisor and committee members have in approving your research proposal. Understand that it is not unusual for a proposed research project to receive tentative approval since some parts may be too difficult based on a candidate's current skill level, due to the availability of necessary data, or because the proposed project cannot ...
Step 1: Choose your topic. First you have to come up with some ideas. Your thesis or dissertation topic can start out very broad. Think about the general area or field you're interested in—maybe you already have specific research interests based on classes you've taken, or maybe you had to consider your topic when applying to graduate school and writing a statement of purpose.
The goal of the research proposal is to present the prospective research proposal for review and approval. The research proposal is a structured document that describes what you plan to do in the research project and how you plan to do it. The proposal should include all the necessary information that allow the approval organization to make ...
Introduction this is ALX Research & Project approval (Part 1) Team Fitsum Belay => working alone Technologies Laravel — Backend Vuejs — Frontend Challenge As ALX student we need community ...
Research Project Part 1: Target: TGT FINC 330 - Business Finance 09/22/ Background and Industry. Target has been growing worldwide ever since the 1900s. What first started as George D. Dayton becoming a partner in Goodfellow's Dry Goods Company, flourished into the birth of Target in 1962 (Target History, N.). This department store offers ...
Part 1: Research Project Proposal The research project provides students the opportunity to demonstrate that they can design and implement an empirical investigation. All projects must have the prior approval of the Graduate Studies Committee (or First Year Project Committee for Systems Neuroscience students) based on its review of a project ...
INTRODUCTION. A clean, well-thought-out proposal forms the backbone for the research itself and hence becomes the most important step in the process of conduct of research.[] The objective of preparing a research proposal would be to obtain approvals from various committees including ethics committee [details under 'Research methodology II' section [Table 1] in this issue of IJA) and to ...
There may be experiments not covered by the guidelines that would require review and approval by outside agencies before initiation. If the experimental protocol is not covered by the NIH's guidelines, contact the BSO at (617) 358-7840 at BUMC and (617) 353-4094 at CRC to determine further review requirements.
Before starting any coding, you will need to research and define your portfolio project over the course of 3 weeks. In the first week, the Project Proposal will be approved, followed by the second week where the MVP will be defined, and finally presented as a collection of tasks on a Trello board. This first part is focused on creating a ...
This Research Project Approval Form is to be completed and approved before research begins. The completed form should be sent to the Office of Institutional Research at least one month before the planned start date of the project. CCRRB will review the study, discuss changes/implications with the author, and make final project recommendations ...
Explanation of my final portfolio project More of the explanation of my project can be found in my GitHub portfolio www.github.com/Pascalchinedu
936-294-4567 [email protected] NGL 223D ORCID: 0000-0001-9520-9314 I'm Creative Commons Certified! I can advise on CC licenses to reuse, remix, and publish open resources.
jobootwori/Research-Project-approval-Part-1-This commit does not belong to any branch on this repository, and may belong to a fork outside of the repository.
This is the alternative 'Maze project' for the 'Research & Project approval (Part 1)' project in the ALX Software Engineering program. Maze project. Background Context. The goal of this project is to create a game in 3D using raycasting ! Tasks. Walls ! In this first part, you'll have to:
Find and fix vulnerabilities Codespaces. Instant dev environments