- Quick Links

Tools & Resources
- Events Calendar
- Strauss Health Sciences Library
- Department A-Z Directory
- Campus Directory
- Faculty & Staff Resources
- Supporter & Alumni Resources
- Student Resources
- Mental Health Resources
- University Policies
CU Campuses
Cu anschutz medical campus.
- CU Colorado Springs
- School of Dental Medicine
- Graduate School
School of Medicine
- College of Nursing
- Skaggs School of Pharmacy and Pharmaceutical Sciences
- Colorado School of Public Health
University of Colorado Alzheimer's and Cognition Center
Thinking of participating in a research study here are some frequently asked questions:, what does a typical research visit day look like.
Every research study is different, so we recommend that you talk to the study coordinator for the study you are interested in for more details. However, the majority of our research visits follow a similar pattern. If you participate in an initial study visit, the first thing you will do is go through an informed consent form with the study coordinator. Depending on the study, this may be done virtually or in person. The informed consent form will describe many things about the study, including but not limited to the purpose of the study, the risks and benefits associated with the study, who will have access to the information collected during the research study, and who to contact if you have more questions. If you are in a clinical drug trial, this discussion may also include a medical doctor on the study to discuss the drug that is being studied.
After reviewing the informed consent form, it will be up to you to decide if you would like to sign. This process is completely voluntary. If you choose to sign and participate in the study, the visit will begin. Most of our studies include brief cognitive screenings, conversations with you and sometimes a study partner about your thinking and memory, and a review of your medical history and medications to determine eligibility, although not all studies will require the same things. Some other things you may encounter in your study visit are an extended cognitive testing period, health questionnaires asking questions about things such as your thinking, mood, sleeping patterns, etc., a neurological and physical exam with the study doctor, blood work, and vital signs such as blood pressure, weight, and temperature.
A research study visit can take anywhere from 1 hour to 6-7 hours, depending on the procedures involved. Please discuss the expected time with the study coordinator. Sometimes, visits can be broken up into multiple days or breaks can be scheduled in for the longer visits. Depending on the study, some procedures may be able to be completed virtually.
What is cognitive testing and how long does it take?
Cognitive testing is conducted to provide an objective indicator of how people are functioning in different areas such as memory, language, attention, planning and organization, and processing speed. These tests can detect very subtle changes in cognition, even in healthy adults with no memory complaints, over time. Because of this, cognitive tests are designed to be challenging. If a healthy person gets 100% correct on a test, the test is not as useful as it could be. Therefore, cognitive tests are designed to show a person’s strengths and weaknesses, which differ among people at all levels of cognitive functioning.
The length of cognitive testing varies per study. On average, a full cognitive battery for research can take about 2-4 hours depending on the testing protocol for the study. Please check with the study coordinator for the length of the cognitive testing portion of the study.
What is the difference between an MRI I get through a research study, and an MRI I get through my primary care provider or neurologist?
Magnetic Resonance Imaging (MRI) is often used in a clinical setting to help a doctor diagnose a disease or to evaluate the extent of an injury. In our research, MRI scans help us track structural changes in the brain over time and allow us to see whether those changes coincide with other symptoms. From the participant’s perspective, the experience of having a research MRI scan is very similar to having an MRI scan as part of a normal clinic visit.
As a result, we often get asked by a participant if they can share a copy of their research MRI scan with their primary care physician, so they can look at it and see whether they may have a disease, such as Alzheimer’s. However, research MRI scans are actually very different from clinical MRI scans and the two are not interchangeable. When you go to a doctor with a complaint or an injury and they put in an order for you to have an MRI scan, the images obtained from that scan are standardized by a radiologist, who is a doctor trained to interpret medical images and make a diagnosis based on specific symptoms.
For a research MRI scan, the type of images that we obtain are chosen specifically based on the research question that the research team is trying to answer, and they are not designed to provide a diagnosis for the participant. MRI scans that are performed for research purposes are usually not formally interpreted by a radiologist. In the rare case that a clinically-relevant abnormality is noticed on a research MRI scan, the participant is notified. They are then encouraged to receive a doctor-ordered clinical MRI scan to assess the abnormality and to determine whether any further treatment is necessary.
In research, the MRI scan itself is used to help answer the questions we are studying. However, the results from the research MRI scan may not start to be meaningful until the end of the study, when all of the data are grouped together and analyzed, and even then, what those results mean for any individual participant may still be unclear. Research MRI scans are very important for understanding how brain structure relates to cognitive symptoms, and hopefully research in this area will contribute to our ability to use MRI data to help diagnose and inform treatment plans for patients with neurodegenerative diseases, such as Alzheimer’s and Parkinson’s.
The study I am interested in says results cannot be provided - why would that be?
Many of the tests we are conducting are done so to find out more information or test ideas, and are not yet proven clinically. Because of this, we don't actually know what they mean for an individual - that is why we are doing the research. Therefore, the results would not actually mean anything to you or your doctor were we to provide them. Also, many of the procedures we do are done through a research lens, meaning that we are not doing the same tests or looking for the same results as your doctor might.
The study I am interested in has an optional or required lumbar puncture/spinal tap. What is that?
A lumbar puncture involves placing a needle between the bones of the lower back, below the end of the spinal cord, to draw the fluid known as cerebrospinal fluid or CSF. This fluid bathes the brain and contains the proteins of interest and other molecules that are markers of brain health and disease.
Adopted from the University of Wisconsin’s Alzheimer’s Center
To learn more about lumbar punctures and cerebrospinal fluid, visit the Colorado Aging Brain Lab website
What is cerebrospinal fluid (CSF) and why is it important for research?
Cerebrospinal fluid (CSF) is a fluid made in the brain that helps protect the brain and the spinal cord. Importantly, it contains numerous proteins that are important for brain health. It can also contain inflammatory markers and other aging related proteins that are associated with disease states, including early markers that are potential risks for Alzheimer’s disease. The CSF provides an important and unique window into brain health that helps us better understanding factors that contribute to both healthy aging and Alzheimer’s disease.
CSF is a special gift to aging research, as it allows us to examine brain proteins during a person’s life. To help detail why this is such an important part of research, please see the following videos, which were developed at the Knight Alzheimer’s Disease Center in St. Louis
- Donating Cerebrospinal Fluid - A Profound Gift
- Cerebrospinal Fluid: A Window into the Brain
- The Procedure
To learn more about lumbar punctures and cerebrospinal fluid, visit the Colorado Aging Brain Lab website
Scheduled for an upcoming research visit? Here are some frequently asked questions:
What should i bring with me the day of a research visit.
Please bring the following items to a research visit:
- List of current medications, that includes the name of the medication, dosage, dosage schedule, and when you began taking the medication
- Water and snacks - most studies will provide refreshments but if there is something specific you would like to bring, please do so
- Book or something else to do in case there is down time.
- If you are participating in a study and have cognitive impairment to the point where someone else has been designated to make medical decisions for you, please bring Medical Durability/Power of Attorney paperwork with you
- Light sweater - our visits often take place in medical facilities, which can be cold
If your study requires that a study partner attend the appointment with you, we highly recommend the study partner also bring water and snacks as well as a book or something to do. Many times the study partner will have down time while the person enrolled in the study is doing one of the research tasks.
Where do I go for my research appointment?
We have multiple locations on campus where research visits can be held, so please confirm with your study coordinator where your visit will be taking place. The most commonly used locations are:
Building 400
12469 East 17th Pl, Aurora, CO 80045
Parking: Cheyenne Wells Parking lot
Your study coordinator will meet you when you park, and either provide you with a code or a parking tag for your visit. Please do not pay for parking. If provided a code, please have your license plate number ready as it will be needed to pay for the parking.
Clinical Trial Research Center (CTRC) in the Anschutz Health Sciences Building
1890 N Revere Ct, 6th floor, Aurora, CO 80045
The Clinic is in the NE corner of the 6th floor, accessible by the elevators in the NW end of the first floor. Front Desk Number: 303-724-1225
Parking: AHSB Parking lot (12795 E 19th Ave Aurora, CO 80045)
Prior to arrival you will have received a code from your study team that will grant you access to the parking lot for your visit. Please note the code changes frequently, so please make sure you have the current code from the study coordinator. More information and directions to the CTRC can be found here .
Fitzsimmons Building (Building 500)
12401 E 17th Ave, Room #3201, Aurora, CO 80045
Parking: Ignacio Lot ( Google Map )
The study coordinator will meet you in the parking lot and input a code to ensure you are not charged for parking. Please have your license plate number ready as they will need it to pay for the parking.
If the Ignacio lot is full, please park in the Georgetown lot.
I have an MRI visit today - what do I wear?
For our MRI visits, we require participants to wear scrubs that will be provided by us for you to wear. However, please make sure that any underclothes do not have any metal or metallic threads on them. For women, this may mean considering the type of bra that you wear, as the clasps are often composed of metal, even if they look plastic. For men, this may mean making sure you do not wear any underwear that has metal buttons on them. We will also ask you to remove your jewelry and eyeglasses when it is time for the scan.
My study visit will include a lumbar puncture - do I need to do anything special for that visit?
If you take a daily baby aspirin (81 mg), you will need to stop taking your aspirin for 7 days prior to lumbar puncture visit. We also recommend that you eat a hearty meal and drink lots of water the day before and the day of your appointment. Being hydrated is the best way to prevent headaches, a potential side effect of lumbar punctures.
On the day of your lumbar puncture, you should wear loose clothing or lounge wear (such as leggings or sweatpants), so that the provider doing the procedure can easily adjust clothing as needed.
My appointment has a blood draw - how do I prepare?
We ask that you please eat a good meal before the visit (unless the study coordinator specifically directs you to fast) and drink a lot of water both the day before the visit and the day of the visit. We also suggest wearing a shirt that is either short sleeved (and bring a jacket) or a long sleeved shirt with loose sleeves that can be rolled up comfortably above the elbow.
My visit today has a PET scan - do I have to wear anything specific?
Neurology (som).
CU Anschutz
Research Complex II
12700 East 19th Avenue
Aurora, CO 80045
303-724-4328
- School of Medicine Home
- Find a Doctor
- Find a Researcher
- Departments
- School Profiles
- Affiliate/Partner Hospitals
- CU Medicine
- Map and Parking
- Health Science Library
- Student Life
- Colorado Springs Branch
Documentation of Study Visit Interventions/Observations
The purpose of source documentation is to reconstruct the research study as it happened. It should allow for data to be confirmed, validated, and support the fundamental principle of protecting the participant’s rights, safety and well-being.
These templates are intended to assist in documenting study procedures, interventions, observations, etc.
Below are several examples that can be customized to meet your specific study needs/requirements.
Best Practice Considerations
The research record should contain documentation of each participant’s study involvement from the time they sign consent through to whatever point they complete participation.
- Anything not completed should include record of why and be signed and dated by the person completing the note.
- Attaching a note to file or documenting in a running note missing items, dates, incomplete questions, missing assessments, etc., is another way to document why a record is incomplete, but the note to file entry must also be signed and dated.
- With electronic study documentation, consider creating a data field to capture any general notes about why study interventions were not completed or were partially complete
Hardcopy source documents/data collections forms should contain at the very least the HRPO#, participant ID, and study visit date on each loose page.
Research team members should document each task they perform.
Screening/Enrollment
- Demographics and Contact Form (DOC)
- Including Health History (DOC)
- Without Health History (DOC)
- Guide to Using the Screening & Enrollment Log (DOC)
- Screening & Enrollment Log Template (XLS)
- Subject Contact Information
Study Visits/Phone Visit Documentation
- Urine Pregnancy Test Record
- Vital Signs CRF Example 1
- Vital Signs CRF Example 2
- Vital Signs CRF Example 3
- Multiple Visits
- Single Visit
- End of Study/Early Termination Visit Source Document
- First Dose Documentation
- Follow-up Phone Call
- Telephone Communication Record
- Inclusion-Exclusion Checklist
- Inclusion-Exclusion Checklist with Interview Section
- Medical History
- Phone Screen Checklist
- Physical Exam Form Example 1
- Physical Exam Form Example 2
- Study Visit Source Document
- Study Specific Source Document Template
- Protocol Deviation Form
Memos/Notes to Files
- Action Form
- Note to File Example 1
- Note to File Example 2
- Note to File Example 3
- Running Note
- Correspondence (Research Team/Participant)
- COVID-19 Note to File
Adverse/Serious Adverse Event Documentation
- Adverse Event Example Template
- Adverse Event Tracking Log
Medication Logs
- Concurrent Medications CRF – Extended Version
- Concurrent Medications Sheet Example 1
- Concurrent Medications Sheet Example 2
- Daily Temperature Log
- Deviation Log
- Device Dispensing Log
- Drug Dispensing Log
- PI Delegation Log
- Receipt Log
- Specimen Collection Log
- Specimen Shipping Log
- Study Device Accountability Log
- Study Drug Accountability Log
- Study Personnel Log
- Study Team Contact Log
- Telephone Call Log
- Screening and Enrollment Log Guide and Template
- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
U.S. Food and Drug Administration
- Search
- Menu
- For Patients
- Clinical Trials: What Patients Need to Know
Informed Consent for Clinical Trials

On this page you will find information on:
What is Informed Consent
Before enrolling in a clinical trial, the following information must be given to each potential research subject
When Appropriate, one or more of the following elements of information must also be provided in the informed consent document
A potential research subject must have an opportunity to
Informed consent may not include language that
To many, the term informed consent is mistakenly viewed as the same as getting a research participant's signature on the consent form. FDA believes that obtaining a research participant's verbal or written informed consent is only part of the process. Informed consent involves providing a potential participant with:
adequate information to allow for an informed decision about participation in the clinical investigation.
facilitating the potential participant's understanding of the information.
an appropriate amount of time to ask questions and to discuss with family and friends the research protocol and whether you should participate.
obtaining the potential participant's voluntary agreement to participate.
continuing to provide information as the clinical investigation progresses or as the subject or situation requires.
To be effective, the process must provide sufficient opportunity for the participant to consider whether to participate. ( 21 CFR 50.20 .) FDA considers this to include allowing sufficient time for participants to consider the information and providing time and opportunity for the participant to ask questions and have those questions answered. The investigator (or other study staff who are conducting the informed consent interview) and the participant should exchange information and discuss the contents of the informed consent document. This process must occur under circumstances that minimize the possibility of coercion or undue influence. (21 CFR 50.20.)
What is Informed Consent?
As new medical products are being developed, no one knows for sure how well they will work, or what risks they will find. Clinical trials are used to answer questions such as:
Are new medical products safe enough to outweigh the risks related to the underlying condition?,
How should the product be used? (for example, the best dose, frequency, or any special precautions necessary to avoid problems),
How effective is the medical product at relieving symptoms, treating or curing a condition.
The main purpose of clinical trials is to “study” new medical products in people. It is important for people who are considering participation in a clinical trial to understand their role, as a “subject of research” and not as a patient.
While research subjects may get personal treatment benefit from participating in a clinical trial, they must understand that they:
may not benefit from the clinical trial,
may be exposed to unknown risks,
are entering into a study that may be very different from the standard medical practices that they currently know
To make an informed decision about whether to participate or not in a clinical trial, people need to be informed about:
what will be done to them,
how the protocol (plan of research) works,
what risks or discomforts they may experience,
participation being a voluntary decision on their part.
This information is provided to potential participants through the informed consent process. Informed consent means that the purpose of the research is explained to them, including what their role would be and how the trial will work.
A central part of the informed consent process is the informed consent document. The Food and Drug Administration (FDA) does not dictate the specific language required for the informed consent document, but does require certain basic elements of consent be included.
Before enrolling in a clinical trial, the following information must be given to each potential research subject:
A statement explaining that the study involves research.
An explanation of the purposes of the research.
The expected length of time for participation.
A description of all the procedures that will be completed during enrollment on the clinical trial.
Information about all experimental procedures the will be completed during the clinical trial.
A description of any predictable risks.
Any possible discomforts (e.g., injections, frequency of blood test etc.) that could occur as a result of the research.
Any possible benefits that may be expected from the research.
Information about any alternative procedures or treatment (if any) that might benefit the research subject.
A statement describing:
the confidentiality of information collected during the clinical trial,
how records that identify the subject will be kept
the possibility that the FDA may inspect the records.
For research involving more than minimal risk information including
an explanation as to whether any compensation or medical treatments are available if injury occurs,
what they consist of, or
where more information may be found.
questions about the research,
research subjects' rights,
injury related to the clinical trial.
Research subject participation is voluntary,
Research subjects have the right to refuse treatment and will not losing any benefits for which they are entitled,
Research subjects may choose to stop participation in the clinical trial at any time without losing benefits for which they are entitled.
Contact information will be provided for answers to :
A statement that:
When Appropriate, one or more of the following elements of information must also be provided in the informed consent document:
A statement that the research treatment or procedure may involve unexpected risks (to the subject, unborn baby, if the subject is or may become pregnant).
Any reasons why the research subject participation may be ended by the clinical trial investigator (e.g., failing to follow the requirements of the trial or changes in lab values that fall outside of the clinical trial limits).
Added costs to the research subject that may result from participating in the trial.
The consequence of leaving a trial before it is completed (e.g. if the research and procedures require a slow and organized end of participation).
A statement that important findings discovered during the clinical trial will be provided to the research subject.
The approximate number of research subjects that will be enrolled in the study.
A potential research subject must have an opportunity to:
read the consent document,
ask questions about anything they do not understand.
Usually, if one is considering participating in a clinical trial, he or she may take the consent document home to discuss with family, friend or advocate.
An investigator should only get consent from a potential research subject if:
enough time was given to the research subject to consider whether or not to participate
the investigator has not persuaded or influenced the potential research subject.
The information must be in language that is understandable to the research subject.
Informed consent may not include language that:
the research subject is made to ignore or appear to ignore any of the research subject's legal rights,
releases or appears to release the investigator, the sponsor, the institution, or its agents from their liability for negligence.
Participating in clinical trials is voluntary. You have the right not to participate, or to end your participation in the clinical trial at any time. Read the informed consent document carefully. Ask questions about any information you don’t understand or find confusing.
Draft Guidance: Informed Consent Information Sheet Guidance for IRBs, Clinical Investigators, and Sponsors
- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español
You are here
Nih clinical research trials and you, finding a clinical trial, around the nation and worldwide.

NIH conducts clinical research trials for many diseases and conditions, including cancer , Alzheimer’s disease , allergy and infectious diseases , and neurological disorders . To search for other diseases and conditions, you can visit ClinicalTrials.gov.
ClinicalTrials.gov [ How to Use Search ] This is a searchable registry and results database of federally and privately supported clinical trials conducted in the United States and around the world. ClinicalTrials.gov gives you information about a trial's purpose, who may participate, locations, and phone numbers for more details. This information should be used in conjunction with advice from health care professionals.
Listing a study does not mean it has been evaluated by the U.S. Federal Government. Read the disclaimer on ClinicalTrials.gov for details.
Before participating in a study, talk to your health care provider and learn about the risks and potential benefits.
At the NIH Clinical Center in Bethesda, Maryland

Search NIH Clinical Research Studies The NIH maintains an online database of clinical research studies taking place at its Clinical Center, which is located on the NIH campus in Bethesda, Maryland. Studies are conducted by most of the institutes and centers across the NIH. The Clinical Center hosts a wide range of studies from rare diseases to chronic health conditions, as well as studies for healthy volunteers. Visitors can search by diagnosis, sign, symptom or other key words.
Join a National Registry of Research Volunteers

ResearchMatch This is an NIH-funded initiative to connect 1) people who are trying to find research studies, and 2) researchers seeking people to participate in their studies. It is a free, secure registry to make it easier for the public to volunteer and to become involved in clinical research studies that contribute to improved health in the future.
This page last reviewed on November 6, 2018
Connect with Us
- More Social Media from NIH
Administration of Study Treatments and Participant Follow-Up
- Living reference work entry
- First Online: 24 February 2021
- Cite this living reference work entry

- Jennifer J. Gassman 3
141 Accesses
After clinical trial participants have consented, provided baseline data, and been randomized, each participant begins study treatment and follow-up. This chapter covers administering a participant’s randomly assigned treatment regimen and collecting the participant’s trial data through the end of their time in the study, along with tracking and reporting data on timeliness and quality of treatment administration and of follow-up visit attendance and trial data collection. Treatment administration can include providing study medications or, in a lifestyle intervention trial, teaching the participant to follow a diet, exercise, or smoking cessation intervention. Trial data collection includes, for example, questionnaires completed via smartphone, laboratory sample collection details and results of lab analyses, imaging data, treatment adherence data, measurements taken at clinic visits, and adverse event data. Monitoring participant follow-up and capturing reasons why patients discontinue treatment or end follow-up early can aid in interpretation of a trial’s results. Treatment administration, treatment adherence, and participant follow-up metrics should be captured in real time, with the Data Coordinating Center (DCC) providing continuous performance feedback to study leadership and to the participating sites themselves. These aspects of trial conduct are described in the context of a multicenter trial in which two or more clinical sites enroll participants and study data are managed in a centrally administered database. Timeliness and accuracy of study treatment administration is key to the success of a trial. Participants providing required data according to the protocol-defined schedule allow a trial to attain its goals.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
Institutional subscriptions
Ahmed I, Ahmad NS, Ali S, George A, Saleem-Danish H, Uppal E, Soo J, Mobasheri MH, King D, Cox B, Darzi A (2018) Medication adherence apps: review and content analysis. JMIR Mhealth Uhealth 6(3):e62. https://doi.org/10.2196/mhealth.6432
Article Google Scholar
Booker C, Harding S, Benzeval M (2011) A systematic review of the effect of retention methods in population-based cohort studies. BMC Public Health 11:249. https://doi.org/10.1186/1471-2458-11-249
Brueton VC, Tierney JF, Stenning S, Meredith S, Harding S, Nazareth I, Rait G (2014) Strategies to improve retention in randomised trials: a Cochrane systematic review and meta-analysis. BMJ Open 4(2):e003821. https://doi.org/10.1136/bmjopen-2013-003821
Byar DP, Simon RM, Friedewald WT, Schlesselman JJ, DeMets DL, Ellenberg JH, Gail MH, Ware JH (1976) Randomized clinical trials – perspectives on some recent ideas. N Engl J Med 295:74–80. https://doi.org/10.1056/NEJM197607082950204
Dayer L, Heldenbrand S, Anderson P, Gubbins PO, Martin BC (2013) Smartphone medication adherence apps: potential benefits to patients and providers. J Am Pharm Assoc (JAPhA) 53(2):172–181. https://doi.org/10.1111/j.1547-5069.2002.00047.x
Dunbar-Jacob J, Rohay JM (2016) Predictors of medication adherence: fact or artifact. J Behav Med 39(6):957–968. https://doi.org/10.1007/s10865-016-9752-8
Farmer KC (1999) Methods for measuring and monitoring medication regimen adherence in clinical trials and clinical practice. Clin Ther 21(6):1074–1090. https://doi.org/10.1016/S0149-2918(99)80026-5
Ferris M, Norwood V, Radeva M, Gassman JJ, Al-Uzri A, Askenazi D, Matoo T, Pinsk M, Sharma A, Smoyer W, Stults J, Vyas S, Weiss R, Gipson D, Kaskel F, Friedman A, Moxey-Mims M, Trachtman H (2013) Patient recruitment into a multicenter randomized clinical trial for kidney disease: report of the focal segmental glomerulosclerosis clinical trial (FSGS CT). Clin Transl Sci 6(1):13–20. https://doi.org/10.1111/cts.12003
Fewtrell MS, Kennedy K, Singhal A, Martin RM, Ness A, Hadders-Algra M, Koletzko B, Lucas A (2008) How much loss to follow-up is acceptable in long-term randomised trials and prospective studies? Arch Dis Child 93(6):458–461. https://doi.org/10.1136/adc.2007.127316
FHN Trial Group, Chertow GM, Levin NW, Beck GJ, Depner TA, Eggers PW, Gassman JJ, Gorodetskaya I, Greene T, James S, Larive B, Lindsay RM, Mehta RL, Miller B, Ornt DB, Rajagopalan S, Rastogi A, Rocco MV, Schiller B, Sergeyeva O, Schulman G, Ting GO, Unruh ML, Star RA, Kliger AS (2010) In-center hemodialysis six times per week versus three times per week. N Engl J Med 363:2287–2300. https://doi.org/10.1056/NEJMoa1001593
Garber M, Nau D, Erickson S, Aikens J, Lawrence J (2004) The concordance of self-report with other measures of medication adherence: a summary of the literature. Med Care 42(7):649–652. https://doi.org/10.1097/01.mlr.0000129496.05898.02
Gassman J, Agodoa L, Bakris G, Beck G, Douglas J, Greene T, Jamerson K, Kutner M, Lewis J, Randall OS, Wang S, Wright JT, the AASK Study Group (2003) Design and statistical aspects of the African American Study of Kidney Disease and Hypertension (AASK). J Am Soc Nephrol 14:S154–S165. https://doi.org/10.1097/01.ASN.0000070080.21680.CB
Gipson DS, Trachtman H, Kaskel FJ, Greene TH, Radeva MK, Gassman JJ, Moxey-Mims MM, Hogg RJ, Watkins SL, Fine RN, Hogan SL, Middleton JP, Vehaskari VM, Flynn PA, Powell LM, Vento SM, McMahan JL, Siegel N, D’Agati VD, Friedman AL (2011) Clinical trial of focal segmental glomerulosclerosis (FSGS) in children and young adults. Kidney Int 80(8):868–878. https://doi.org/10.1038/ki.2011.195
Isakova T, Ix JH, Sprague SM, Raphael KL, Fried L, Gassman JJ, Raj D, Cheung AK, Kusek JW, Flessner MF, Wolf M, Block GA (2015) Rationale and approaches to phosphate and fibroblast growth factor 23 reduction in CKD. J Am Soc Nephrol 26(10):2328–2339. https://doi.org/10.1681/ASN.2015020117
Ix JH, Isakova T, Larive B, Raphael KL, Raj D, Cheung AK, Sprague SM, Fried L, Gassman JJ, Middleton J, Flessner MF, Wolf M, Block GA, Wolf M (2019) Effects of nicotinamide and lanthanum carbonate on serum phosphate and fibroblast growth factor-23 in chronic kidney disease: The COMBINE trial. J Am Soc Nephrol 30(6):1096–1108. https://doi.org/10.1681/ASN.2018101058
Kravitz RL, Hays RD, Sherbourne CD (1993) Recall of recommendations and adherence to advice among patients with chronic Medical conditions. Arch Intern Med 153(16):1869–1878. https://doi.org/10.1001/archinte.1993.00410160029002
Meinert CL (2012) Clinical trials: design, conduct and analysis, 2nd edn. Oxford University Press, New York
Google Scholar
Miskulin DC, Gassman J, Schrader R, Gul A, Jhamb M, Ploth DW, Negrea L, Kwong RY, Levey AS, Singh AK, Harford A, Paine S, Kendrick C, Rahman M, Zager P (2018) BP in dialysis: results of a pilot study. J Am Soc Nephrol 29(1):307–316. https://doi.org/10.1681/ASN.2017020135
Morawski K, Ghazinouri R, Krumme A et al (2018) Association of a smartphone application with medication adherence and blood pressure control: the MedISAFE-BP randomized clinical trial. JAMA Intern Med 178(6):802–809. https://doi.org/10.1001/jamainternmed.2018.0447
Osterberg L, Blashchke T (2005) Adherence to medication August 4, 2005. N Engl J Med 353:487–497. https://doi.org/10.1056/NEJMra050100
Piantadosi S (2017) Clinical trials a methodologic perspective. Wiley series in probability and statistics, 3rd edn. Wiley, New York
MATH Google Scholar
Raphael KL, Isakova T, Ix JH, Raj DS, Wolf M, Fried LF, Gassman JJ, Kendrick C, Larive B, Flessner MF, Mendley SR, Hostetter TH, Block GA, Li P, Middleton JP, Sprague SM, Wesson DE, Cheung AK (2020). A Randomized Trial Comparing the Safety, Adherence, and Pharmacodynamics Profiles of Two Doses of Sodium Bicarbonate in CKD: the BASE Pilot Trial. J Am Soc Nephrol 31(1):161–174. https://doi.org/10.1681/ASN.2019030287
Rocco MV, Lockridge RS, Beck GJ, Eggers PW, Gassman JJ, Greene T, Larive B, Chan CT, Chertow GM, Copland M, Hoy C, Lindsay RM, Levin NW, Ornt DB, Pierratos A, Pipkin M, Rajagopalan S, Stokes JB, Unruh ML, Star RA, Kliger AS, the FHN Trial Group (2011) The effects of nocturnal home hemodialysis: the frequent hemodialysis network nocturnal trial. Kidney Int 80:1080–1091. https://doi.org/10.1038/ki.2011.213
Sackett DL, Richardson WS, Rosenberg W (1997) Evidence-based medicine: how to practice and teach EBM. Churchill Livingstone, New York
Santo K, Richtering SS, Chalmers J, Thiagalingam A, Chow CK, Redfern J (2016) Mobile phone apps to improve medication adherence: a systematic stepwise process to identify high-quality apps. JMIR Mhealth Uhealth 4(4):e132. https://doi.org/10.2196/mhealth.6742
Schwed A, Fallab C-L, Burnier M, Waeber B, Kappenberger L, Burnand B, Darioli R (1999) Electronic monitoring of adherence to lipid- lowering therapy in clinical practice. J Clin Pharmacol 39(4):402–409. https://doi.org/10.1177/00912709922007976
Stewart M (1987) The validity of an interview to assess a patient’s drug taking. Am J Prev Med 3:95–100
Trachtman H, Vento S, Gipson D, Wickman L, Gassman J, Joy M, Savin V, Somers M, Pinsk M, Greene T (2011) Novel therapies for resistant focal segmental glomerulosclerosis (FONT) phase II clinical trial: study design. BMC Nephrol 12:8. https://doi.org/10.1186/1471-2369-12-8
Weinberg JM, Appel LJ, Bakris G, Gassman JJ, Greene T, Kendrick CA, Wang X, Lash J, Lewis JA, Pogue V, Thornley-Brown D, Phillips RA, African American Study of Hypertension and Kidney Disease Collaborative Research Group (2009) Risk of hyperkalemia in nondiabetic patients with chronic kidney disease receiving antihypertensive therapy. Arch Intern Med 169(17):1587–1594. https://doi.org/10.1001/archinternmed.2009.284
Zulig LL, Phil Mendys HB (2017) Bosworth, Medication adherence: A practical measurement selection guide using case studies. Patient Educ Couns 100(7):1410–1414. https://doi.org/10.1016/j.pec.2017.02.001 . ISSN 0738-3991
Download references
Author information
Authors and affiliations.
Department of Quantitative Health Sciences, Cleveland Clinic, Cleveland, OH, USA
Jennifer J. Gassman
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Jennifer J. Gassman .
Editor information
Editors and affiliations.
Samuel Oschin Comprehensive Cancer Insti, WEST HOLLYWOOD, CA, USA
Steven Piantadosi
Bloomberg School of Public Health, Johns Hopkins Center for Clinical Trials Bloomberg School of Public Health, Baltimore, MD, USA
Curtis L. Meinert
Section Editor information
Frontier Science (Scotland) Ltd, Grampian View, Kincraig, UK
Eleanor McFadden
Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA
The Johns Hopkins Center for Clinical Trials and Evidence Synthesis, Johns Hopkins University, Baltimore, MD, USA
Rights and permissions
Reprints and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this entry
Cite this entry.
Gassman, J.J. (2021). Administration of Study Treatments and Participant Follow-Up. In: Piantadosi, S., Meinert, C.L. (eds) Principles and Practice of Clinical Trials. Springer, Cham. https://doi.org/10.1007/978-3-319-52677-5_39-1
Download citation
DOI : https://doi.org/10.1007/978-3-319-52677-5_39-1
Received : 15 October 2020
Accepted : 04 November 2020
Published : 24 February 2021
Publisher Name : Springer, Cham
Print ISBN : 978-3-319-52677-5
Online ISBN : 978-3-319-52677-5
eBook Packages : Springer Reference Mathematics Reference Module Computer Science and Engineering
- Publish with us
Policies and ethics
- Find a journal
- Track your research

Transforming the understanding and treatment of mental illnesses.
Información en español
Celebrating 75 Years! Learn More >>
- Opportunities & Announcements
- Funding Strategy for Grants
- Grant Writing & Approval Process
- Managing Grants
- Clinical Research
- Small Business Research
NIMH Clinical Research Toolbox
The NIMH Clinical Research Toolbox serves as an information repository for NIMH staff and the clinical research community, particularly those receiving NIMH funding. The Toolbox contains resources such as NIH and NIMH policy and guidance documents, templates, sample forms, links to additional resources, and other materials to assist clinical investigators in the development and conduct of high-quality clinical research studies.
Use of these templates and forms is optional; the resources can be used as-is or customized to serve study team needs. In cases where institutions provide research teams with institution-specific templates and forms for clinical research documentation, NIMH expects researchers to follow their institutional policies for document use. Nevertheless, the materials on this page can be consulted to assure that study teams are meeting NIMH expectations.
Protocol Templates
Protocol associated documents, regulatory documents and associated case report forms, clinical research education, support, and training (crest) program overview.
- Human Subject Risk
Data and Safety Monitoring for Clinical Trials
Reportable events, recruitment, suicide prevention research, good clinical practice training, data sharing, educational presentations, clinical research start up.
NIMH encourages investigators to consider using one of the protocol templates below when developing a clinical research protocol. In cases where an institutional review board (IRB) has a recommended or required protocol template, reviewing the documents included below is still suggested as there may be sections that a study team may opt to include in an effort to develop a comprehensive research protocol.
NIH has developed a Clinical e-Protocol Writing Tool to support the collaborative writing and review of protocols for behavioral and social sciences research involving humans, and of phase 2 and 3 clinical trial protocols that require a Food and Drug Administration (FDA) Investigational New Drug (IND) or Investigational Device Exemption (IDE) Application.
NIH-FDA Phase 2 and 3 IND/IDE Clinical Trial Protocol Template
This clinical trial protocol template is a suggested format for Phase 2 and 3 clinical trials funded by NIH that are being conducted under a FDA IND or IDE Application.
Investigators for such trials are encouraged to use this template when developing protocols for NIH-funded clinical trial(s). This template may also be useful to others developing phase 2 and 3 IND/IDE clinical trials.
NIH Behavioral and Social Clinical Trials Template
This clinical trial protocol template is a suggested format for behavioral or psychosocial clinical trials funded by NIH. Investigators for such studies are encouraged to use this template when developing protocols for NIH-funded clinical trial(s). This template may also be useful to others developing behavioral of psychosocial research studies.
Back to Table of Contents
NIMH Clinical Manual of Procedures (MOP) Template [Word]
This template provides a recommended structure for developing consistent instructions on study procedure implementation and data collection across participant and clinical site activities. It details the study’s organization, operations, procedures, data management, and quality control.
NIMH Clinical Monitoring Plan Template [Word]
This template provides a recommended structure for a plan to conduct internal or independent review of Good Clinical Practices (GCP), human subject safety, and data integrity throughout the lifecycle of a study.
Informed Consent Materials
Often study teams will be provided with informed consent form templates and guidance on requirements for the informed consent process by their institutions. Below is additional guidance and materials to support a thorough informed consent process.
Sample NDA Informed Consent Language
The NIMH Data Archive (NDA) receives de-identified human subjects data collected from hundreds of research projects across many scientific domains, and makes these data available to enable collaborative science. This NDA sample informed consent language for data sharing can be adapted when using one of the NDA platforms.
Regulatory Document Checklists by Study Type The following checklists are intended to help the investigator community identify a set of core documents to be organized within a single study specific folder, either electronically, hard copy, or a mixture of both formats. NIMH encourages study teams to verify what additional documents, or alternative formats of the documents in the checklists, their institution and IRB require.
NIMH Regulatory Document Checklist for non-Clinical Trial Human Subjects Research [Word]
Study teams can use this checklist to compile essential documents for the conduct of a NIMH-funded study that does not meet the NIH definition of a clinical trial and is research on human subjects.
NIMH Regulatory Document Checklist for Clinical Trials without Investigational Product [Word]
Study teams can use this checklist to compile essential documents for the conduct of a NIMH-funded NIH defined clinical trial that does not involve an investigational drug or device.
NIMH Regulatory Document Checklist for Human Subjects Research Clinical Trials with Investigational Product not under a FDA IND/IDE [Word]
Study teams can use this checklist to compile essential documents for the conduct of a NIMH-funded NIH defined clinical trial with an investigational drug or device that is not under a FDA IND or IDE.
NIMH Regulatory Document Checklist for a Study under a FDA IND or IDE [Word]
Study teams can use this checklist to compile essential documents for the conduct of a NIMH-funded NIH defined clinical trial or non-clinical trial with an investigational drug or device under a FDA IND or IDE.
Necessary Documents for Reportable Events
NIMH Reportable Events Log Template [Word]
This document provides a log template for documenting reportable events. The types of events that require reporting may vary by institution, IRB, sponsor, state, and other factors.
NIMH Study-Wide Protocol Deviation Log Template [Word]
This document provides a log template for tracking all protocol deviations/violations across a study.
NIMH Subject-Specific Protocol Deviation Log Template [Word]
This document provides a log template for tracking subject-specific protocol deviations/violations. If captured electronically, subject-specific deviation logs can be exported into a study-wide deviation log.
NIMH Study-Wide Adverse Events (AE) Log Template [Word]
This document provides a log template for tracking all adverse events (AEs), including serious adverse events (SAEs), across a study.
NIMH Subject-Specific Adverse Event (AE) Log Template [Word]
This document provides a log template for tracking adverse events (AEs), including serious adverse events (SAEs), for each subject. If captured electronically, subject-specific AE logs can be exported into an electronic study-wide AE log.
Necessary Documents for Studies with Pharmacy/Investigational Product
FDA Form 1572 Statement of Investigator
This FDA form should be signed by the investigator prior to study initiation to provide certain information to the sponsor, and assure that he/she will comply with FDA regulations related to the conduct of a clinical investigation of an investigational drug or biologic.
NIMH Investigational Product (IP) Management Standard Operating Procedure (SOP) Template [Word]
This document provides a sample standard operating procedures (SOP) template to document how investigational product (IP) will be received, stored, monitored, labeled, dispensed, and destroyed.
NIMH Investigational Product Storage Temperature Log Template [Word]
This document provides a log template for recording the daily temperatures for investigational product (IP).
NIMH Master Investigational Product Dispensing and Accountability Log Template [Word]
This document provides a log template for capturing all investigational product (IP) dispensed to and returned by participants for the duration of the study.
NIMH Subject-Specific Investigational Product Dispensation and Accountability Log Template [Word]
This document provides a log template for capturing all investigational product (IP) dispensed to an individual participant and returned by that participant. This log is typically placed in each subject’s study binder (study blind is maintained, if applicable).
Screening and Enrollment Logs and Materials
NIMH Participant Pre-Screening Log Template [Word]
This document provides a log template for all potential participants who have completed initial screening procedures (i.e. phone screens or internet screening surveys; typically, prior to signing written informed consent). This log should capture the number of participants eligible for an official screening visit, as well as the number ineligible with the reasons for ineligibility listed.
NIMH Participant Enrollment Log Template [Word]
This document provides a log template for chronologically documenting the participants who have been enrolled in the study.
NIMH Inclusion/Exclusion Checklist Template [Word]
This document provides a sample checklist to customize according to protocol-specific eligibility criteria. A qualified and appropriately-delegated study team member should sign and date to confirm eligibility once all criteria have been assessed. If criteria are assessed on different visit dates, this checklist should be reformatted to reflect which criteria are assessed on which visit dates, and who is responsible for assessing them.
NIMH Documentation of Informed Consent Template [Word]
This document provides a sample form template for documenting the informed consent process.
Additional Participant Tracking Logs and Materials
NIMH Concomitant Medication Log Template [Word]
This document provides a log template for recording each participant’s medications throughout the study. This log is typically reviewed at all subject study visits and is located in each participant’s study binder.
NIMH Research Sample Inventory/Tracking Log [Word]
This document provides a log template for tracking the collection and storage of research samples.
Staff Training and Administrative Tracking Logs and Materials
NIMH Good Clinical Practice (GCP) Training Log Template [Word]
This document provides a log template for documenting completion of Good Clinical Practice (GCP) training requirements. Note: all NIH-funded investigators and staff who are involved in the conduct, oversight, or management of clinical trials should be trained in Good Clinical Practice (GCP), consistent with principles of the International Conference on Harmonisation (ICH) E6 (R2). Individual institutions may require GCP training regardless of funding source or clinical trial status.
NIMH Study Training Log Template [Word]
This document provides a log template for documenting staff trainings for study-specific procedures (i.e., trainings for diagnostic interview administration, study protocol adherence, phlebotomy, outcomes measures, OSHA Bloodborne Pathogens, etc.).
NIMH Delegation of Authority Log Template [Word]
This document can be used to record all study staff members’ significant study-related duties, as delegated by the Principal Investigator (PI). Most studies opt to use a log format, such as the Delegation of Authority log, because it captures study staff on one page and includes space to document the addition or removal of specific study tasks for individual staff members.
NIMH Monitoring Visit Log Template [Word]
This document is typically completed by the clinical site monitor to document dates and purpose of clinical site monitoring visits.
NIMH Note to File (NTF) Template [Word]
This document provides a sample template for generating notes-to-file, which are written to acknowledge a discrepancy or problem with the study’s conduct, or for other administrative purposes (such as to document where study materials are stored).
On-Site Monitoring
Even though it is the NIMH’s expectation that grantees will provide adequate oversight of their clinical research, NIMH Program Officials may require additional levels of on-site monitoring conducted by NIMH staff. Clinical monitoring helps ensure the rights and well-being of human subjects are protected; the reported clinical research study data are accurate, complete, and verifiable; and the conduct of the study is in compliance with the study protocol, Good Clinical Practice (GCP), and the regulations of applicable agencies.
The NIMH Clinical Research Education, Support, and Training (CREST) Program provides ongoing educational and technical support from NIMH staff for clinical research project grants selected for consultation and/or site visit(s). The CREST Program aims to ensure that the reported clinical research study data are accurate, complete, and verifiable, the conduct of the study is in compliance with the study protocol, Good Clinical Practice (GCP) and the regulations of applicable agencies, and the rights and well-being of human subjects are protected, in accordance with 45 CFR 46 (Protection of Human Subjects) and, as applicable, 21 CFR part 50 (Protection of Human Subjects).
To promote clinical research that is compliant with GCP and human subject regulations, the CREST Program includes phone conversations, email consultation, and/or site visit(s) from NIMH staff, as needed, to assess and provide written feedback and recommendations on planned or ongoing clinical research protocols. Documents relating to the conduct of the clinical research, such as current IRB approved protocols, informed consent documents, source documents, and drug accountability records, as applicable, may be reviewed for compliance with applicable Federal regulations, and institutional and IRB policies.
Research project grants selected for inclusion in the CREST Program might include clinical research studies with “significantly-greater-than-minimal risk” to subjects (e.g., an intervention or invasive procedure with high potential for serious adverse events; see NIMH Risk-Based Monitoring Guidance ); a study intervention under a FDA Investigational New Drug or Investigational Device Exemption; or other studies identified by NIMH staff that may benefit from inclusion in CREST. CREST is separate and distinct from “for cause” audits of clinical research. Research grants may be included in CREST at any time during the study lifecycle, although projects are generally identified and selected for the program at the initiation of the grant.
NIMH Clinical Research Education Support and Training (CREST) Program Overview
This page provides a description of the NIMH CREST Program’s purpose, process for inclusion, and operating procedures.
Site Visits
NIMH Clinical Research Education, Support, and Training Program (CREST): Comprehensive Visit Report Template [Word]
This template provides a recommended structure for a CREST site visit report, as well as a sample matrix of regulatory criteria that CREST monitors look at while at site initiation visits (SIVs), interim monitoring visits (IMVs) and close out visits (COVs). It is to be used as a starting point for preparing for a CREST site visit or for writing a site visit report.
NIMH CREST Site Initiation Visit (SIV) Sample Agenda [Word]
This document provides a sample site initiation visit agenda to be customized by the Principal Investigator (PI) and site monitor prior to the visit.
Human Subjects Research
This section provides resources, including policy and guidance documents related to the conduct of human subject research. The resources included below represent those frequently of interest to NIMH investigators, specifically: overviews of human subject research, data and safety monitoring, human subject risk, reportable events, and recruitment. There are numerous other NIH webpages devoted to human subjects research; see Research Involving Human Subjects , NIH Human Subjects Policies and Guidance , and New Human Subjects and Clinical Trial Information Form .
Human Subject Regulations Decision Charts
The Office for Human Research Protections (OHRP) has developed graphic aids to help guide investigators in deciding if an activity is research involving human subjects that must be reviewed by an IRB under the requirements of the U.S. Department of Health and Human Services (HHS) regulations ( 45 CFR 46 ).
Human Subjects in Research: Things to Consider
This NIMH webpage presents items which investigators should pay particular attention to when proposing to use human subjects in NIMH-funded studies.
Human Subjects Risk
NIMH Guidance on Risk-Based Monitoring
This NIMH guidance aims to clarify risk level definitions and the NIMH’s monitoring expectations to mitigate these risks. This guidance will assist study teams in determining the level of data and safety monitoring that should be established for a study based on the probability and magnitude of anticipated harm and discomfort.
The policies, guidance and documentation in this section outline NIMH expectations for data and safety monitoring of clinical trials . For human subject research that does not meet criteria for NIH clinical trial designation, investigators still have an option of including a data and safety monitoring plan (DSMP; i.e., in studies that may have significant risk to participants). The initial links below apply to all NIMH-funded clinical trials, while the second section provides documentation for clinical trials under the oversight of a NIMH-constituted data and safety monitoring board (DSMB).
All Clinical Trials
NIMH Policy Governing the Monitoring of Clinical Trials
This NIMH policy outlines NIH and NIMH expectations for data and safety monitoring of clinical trials. This policy also assures that the NIMH is notified by NIMH-funded researchers in a timely manner of all directives emanating from monitoring activities.
Guidance for Developing a Data and Safety Monitoring Plan for Clinical Trials Sponsored by NIMH
This guidance was created to aid investigators developing a data and safety monitoring plan (DSMP) to ensure the safety of research participants and to protect the validity and integrity of study data in clinical trials supported by NIMH. This guidance applies to data and safety monitoring for all NIMH-supported clinical trials (including grants, cooperative agreements, and contracts).
NIMH Policy Governing Independent Safety Monitors and Independent Data and Safety Monitoring Boards
This policy establishes expectations for the monitoring of NIMH-supported clinical trials by Independent Safety Monitors (ISMs) and/or independent data and safety monitoring boards (DSMBs) to assure the safety of research participants, regulatory compliance, and data integrity.
Trials Reviewed by a NIMH-Constituted DSMB
The materials below are for studies designated for review by a NIMH-constituted DSMB. Study teams developing materials for a study-constituted independent DSMB may benefit from reviewing the data report template and the protocol amendment memo.
NIMH Clinical Trials Operations Branch Liaison Orientation Letter [Word]
This letter provides an orientation to working with the NIMH Clinical Trials Operations Branch which supports study teams reporting to the NIMH DSMB.
NIMH DSMB Reporting Guide Full Report Template [PDF]
This template provides a recommended structure for data reports used for DSMB review and oversight. The report template includes standard data tables. Study teams are encouraged to utilize this template as a starting point, and use, remove, and/or modify the existing tables as appropriate for the study under review.
NIMH DSMB Amendment Memo Template [Word]
This template may be used when submitting a study protocol or consent document amendment to the NIMH DSMB.
NIMH Reportable Events Policy
This policy outlines the expectations of NIMH-funded researchers relating to the submission of reportable events (i.e., Adverse Events (AEs); Serious Adverse Events (SAEs); Unanticipated Problems Involving Risks to Subjects or Others ; protocol violations; non-compliance (serious or continuing); suspensions or terminations by monitoring entities (i.e., Institutional Review Board (IRB), Independent Safety Monitor (ISM)); and suspensions or terminations by regulatory agencies (i.e., Office for Human Research Protections (OHRP) or the Food and Drug Administration (FDA)).
( For associated documentation, see: Guidance on Regulatory Documents and Associated Case Report Forms )
NIMH Policy for the Recruitment of Participants in Clinical Research
This policy is intended to support effective and efficient recruitment of participants into all NIMH extramural-funded clinical research studies proposing to enroll 150 or more subjects per study, and all clinical trials, regardless of size.
NIMH Recruitment of Participants in Clinical Research Policy
This policy outlines NIMH expectations regarding the establishment of recruitment plans and milestones for overall study enrollment, and as appropriate, recruitment plans for females and males, members of racial and ethnic minority groups, and children, as well as recruitment reporting.
Frequently Asked Questions (FAQ) about Recruitment Milestone Reporting (RMR)
This NIMH FAQ document provides responses to several of the most common questions surrounding RMR.
Points to Consider about Recruitment and Retention While Preparing a Clinical Research Study
These “points to consider” are meant to serve as a resource as investigators plan a clinical research study and a NIMH grant application. It also outlines common barriers that can impact clinical recruitment and retention.
Additional Resources and Trainings
Conducting Research with Participants at Elevated Risk for Suicide: Considerations for Researchers
This web document is intended to support the development of NIMH research grant applications in suicide research, including those related to clinical course, risk and detection, and interventions and implementation, as well as to support research conduct that is safe, ethical and feasible.
Based on the NIH Good Clinical Practice (GCP) policy , all NIH-funded clinical investigators and clinical trial staff who are involved in the design, conduct, oversight, or management of clinical trials are requirement to be trained in GCP. Below are links to some GCP courses that meet NIH GCP training expectations.
Good Clinical Practice for Social and Behavioral Research – E-Learning Course
The NIH Office of Behavioral and Social Sciences Research (OBSSR) offers a self-paced Good Clinical Practice (GCP) training course with nine video modules. Learners complete knowledge checks and exercises throughout the course.
National Institute of Allergies and Infectious Diseases (NIAID) GCP Learning Center
NIAID has created a self-paced Good Clinical Practice (GCP) training course that includes four modules. These modules educate the learner on the history of human subject research, the regulatory framework, planning human subject research, and conducting human subject research.
National Drug Abuse Treatment (NDAT) Clinical Trials Network
This NDAT course includes 12 modules based on International Council for Harmonisation (ICH) Good Clinical Practice (GCP) and the Code of Federal Regulations (CFR) for clinical research studies in the U.S. The course is self-paced and takes approximately six hours to complete.
The following notices and links present NIMH expectations and tools for data sharing.
Data Sharing Expectations for NIMH-Funded Clinical Trials
This notice establishes NIMH’s data sharing expectations, including the request to include a detailed data sharing plan as part of grant applications.
Data Harmonization
This notice encourages investigators in the mental health research community to utilize data collection protocols using a common set of tools and resources to facilitate sharing, comparing, and integration of data from multiple sources.
NIMH Data Archive
The NIMH Data Archive is an informatics platform for the sharing of de-identified human subject data from all clinical research funded by the NIMH.
Educational Materials
The following educational materials are provided to support the training of NIMH-funded clinical research investigators and staff.
Good Clinical Practices (GCP) for NIMH-Sponsored Studies [PowerPoint]
This training presentation defines Good Clinical Practice (GCP) and describes its application in NIMH-funded research. Topics include: investigator responsibilities, training and qualifications, resources and staffing, delegation of responsibilities, informed consent, documentation and storage of data, assessment and reporting, protocol adherence, drug accountability, adverse events/unanticipated problems and noncompliance. Note that this presentation does not replace the Good Clinical Practice (GCP) training required for NIH funded investigators.
Good Documentation Practices for NIMH-Sponsored Studies [PowerPoint]
This training presentation provides an overview of good documentation practices to follow throughout the duration of NIMH-funded research. The presentation defines and gives examples of good documentation practices.
Introduction to Site-Level Quality Management for NIMH-Sponsored Studies [PowerPoint]
This training presentation provides an overview of the process of establishing and ensuring the quality of processes, data, and documentation associated with clinical research activities. Quality Management (QM) is defined in relationship to site-level documentation, processes, and activities. Tools that are available to support site-level QM are also described.
NIMH Clinical Monitoring and Clinical Research Education, Support, and Training Program (CREST) Overview [PowerPoint]
This training presentation provides an overview of Clinical Monitoring, types of site monitoring visits and what takes place during these visits as well as an overview of follow-up activities. The presentation specifically describes the NIMH Clinical Research Education Support and Training (CREST) Program, its goals, study portfolio selection process, and standard procedures.
Additional NIMH Links and Contacts:
- Office of Clinical Research
- Clinical Trials Operations Branch (CTOB)
- NIMH Clinical Research Policies, Guidance, and Resources
- Human Research Protection Branch (HRPB)

Not a member?
Find out what The Global Health Network can do for you. Register now.
Member Sites A network of members around the world. Join now.
- 1000 Challenge
- ODIN Wastewater Surveillance Project
- Global Health Research Management
- UK Overseas Territories Public Health Network
- Global Malaria Research
- Coronavirus
- Sub-Saharan Congenital Anomalies Network
- Global Health Data Science
- AI for Global Health Research
- MRC Clinical Trials Unit at UCL
- Virtual Biorepository
- Epidemic Preparedness Innovations
- Rapid Support Team
- The Global Health Network Africa
- The Global Health Network Asia
- The Global Health Network LAC
- Global Health Bioethics
- Global Pandemic Planning
- EPIDEMIC ETHICS
- Global Vector Hub
- Global Health Economics
- LactaHub – Breastfeeding Knowledge
- Global Birth Defects
- Antimicrobial Resistance (AMR)
- Human Infection Studies
- EDCTP Knowledge Hub
- CHAIN Network
- Brain Infections Global
- Research Capacity Network
- Global Research Nurses
- ZIKAlliance
- TDR Fellows
- Global Health Coordinators
- Global Health Laboratories
- Global Health Methodology Research
- Global Health Social Science
- Global Health Trials
- Zika Infection
- Global Musculoskeletal
- Global Pharmacovigilance
- Global Pregnancy CoLab
- INTERGROWTH-21ˢᵗ
- East African Consortium for Clinical Research
- Women in Global Health Research
- Global Pathogen Variants
Research Tools Resources designed to help you.
- Site Finder
- Process Map
- Global Health Training Centre
- Resources Gateway
- Global Health Research Process Map
- About This Site
Downloadable Templates and Tools for Clinical Research
Welcome to global health trials' tools and templates library. please note that this page has been updated for 2015 following a quality check and review of the templates, and many new ones have been added. please click on the orange text to download each template., the templates below have been shared by other groups, and are free to use and adapt for your researchstudies. please ensure that you read and adapt them carefully for your own setting, and that you reference global health trials and the global health network when you use them. to share your own templates and sops, or comment on these, please email [email protected]. we look forward to hearing from you.
These templates and tools are ordered by category, so please scroll down to find what you need.
To share your own templates and SOPs, or comment on these, please email [email protected]. We look forward to hearing from you!
- Webinar on community engagement in clinical research involving pregnant women
- Free Webinar: Science, technology and innovation for upskilling knowledge-based economies in Africa
- Open Public Consultation on “Strengthened cooperation against vaccine preventable diseases”
Trial Operations Trial Management Ethics and Informed Consent Resources Trial Design Data Management and Statistics
training
This is Degena Bahrey Tadesse from Tigray, Ethiopia. I am new for this web I am assistant professor in Adult Health Nursing Could you share me the sample/templet research proposal for Global Research Nurses Pump-priming Grants 2023: Research Project Award
I have learned lot..Thanks..
i was wondering why there is no SOP on laboratory procedures ?
Hi, Can you provide me the SOP for electronic signatures in Clinical trial
Do you have an "SOP for Telephonic site selection visit". Kindly Share on my registered mail ID
Thank you for sharing the resources. It is very kind of you.
Hi These tolls are very useful! Thank you
Do you have a task and responsability matrix template for clinical trial managment ? Best
I am very much happy to find myself here as a clinician
Dear Getrude
We have a free 14-module course on research ethics on our training centre; you'll receive a certificate if you complete all the modules and quizzes. You can take it in your own time. Just visit 'Training centre' in the tabs above, then 'short courses'.
Kind regards The Editorial Team
need modules on free online gcp course on research ethics
Estimados: me parece excelente el aporte que han hecho dado que aporta. por un lado a mejorar la transparencia del trabajo como a facilitar el seguimiento y supervisión de los mismos. Muchas gracias por ello
We also have an up to date list of global health events available here: https://globalhealthtrials.tghn.org/community/training-events/
Dear Nazish
Thank you, I am glad you found the seminars and the training courses useful. We list many training events (all relevant to Global Health, and as many of them as possible are either free or subsidised) on the 'community' web pages above. Keep an eye on those for events and activities which you can get involved with. Also, if you post an 'introduction' on the introduction group stating where you are from and your research interests, we can keep you updated of relevant local events.
Thanks so much. These are very helpful seminars. Please let me know any other websites/links that provide free or inexpensive lectures on clinical Research. Appreciate your help.
Hi Nazish, and welcome to the Network. The items here are downloadable templates for you to use; it sounds like you may be seeking lectures and eLearning courses? If so - no problem! You can find free seminars with sound and slides here: https://globalhealthtrainingcentre.tghn.org/webinars/ , and you can find free, certified eLearning courses here: https://globalhealthtrials.tghn.org/elearning . Certificates are awarded for the eLearning courses for those scoring over 80% in the quiz at the end of each course. If you need anything else, do ask! Kind regards The Editorial Team
Hi, I am new to this website and also to the Clinical Research Industry for that matter I only am able to see the PDF of these courses, just wanted to know are these audio lectures and also happen to have audio clips that go with the pdf?
This site is impeccable and very useful for my job!!!!
Thank you for your kind comments.
Fantastic resources
I am delighted you found this website. I earlier introduced it to you because of your prolific interest in health care information and resource sharing....
Please Sign in (or Register ) to view further.
Useful Resources
Related articles.
- PRISMA for Abstracts: Reporting Systematic Reviews in Journal and Conference Abstracts BY Jai K Das
- 5 ways statistics can fool you—Tips for practicing clinicians BY Jai K Das
- How to prepare for a job interview and predict the questions you’ll be asked BY The Editorial Team
- Preparing for and Executing a Randomised Controlled Trial of Podoconiosis Treatment in Northern Ethiopia BY Henok Negussie, Thomas Addissie, Adamu Addissie, Gail Davey
- Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control BY WHO/ TDR
Most popular tags
- Archive (303)
- archive (104)
- data sharing (70)
- sharing (63)
- training (49)
- malaria (30)
- ACT consortium (25)
- informed consent (7)
- data management (6)
- trial management (6)
- careers (5)
- guidelines (5)
- monitoring (5)
- workshop (5)
- administration (4)
- clinical research (4)
Study record managers: refer to the Data Element Definitions if submitting registration or results information.
Search for terms
- Department of Health and Human Services
- National Institutes of Health

COVID-19 Research Studies
More information, about clinical center, clinical trials and you, participate in a study, referring a patient, about clinical research.
Research participants are partners in discovery at the NIH Clinical Center, the largest research hospital in America. Clinical research is medical research involving people The Clinical Center provides hope through pioneering clinical research to improve human health. We rapidly translate scientific observations and laboratory discoveries into new ways to diagnose, treat and prevent disease. More than 500,000 people from around the world have participated in clinical research since the hospital opened in 1953. We do not charge patients for participation and treatment in clinical studies at NIH. In certain emergency circumstances, you may qualify for help with travel and other expenses Read more , to see if clinical studies are for you.
Medical Information Disclaimer
Emailed inquires/requests.
Email sent to the National Institutes of Health Clinical Center may be forwarded to appropriate NIH or outside experts for response. We do not collect your name and e-mail address for any purpose other than to respond to your query. Nevertheless, email is not necessarily secure against interception. This statement applies to NIH Clinical Center Studies website. For additional inquiries regarding studies at the National Institutes of Health, please call the Office of Patient Recruitment at 1-800-411-1222

Find NIH Clinical Center Trials
The National Institutes of Health (NIH) Clinical Center Search the Studies site is a registry of publicly supported clinical studies conducted mostly in Bethesda, MD.

Clinical Trial Templates to Start Your Clinical Research
By Kate Eby | May 13, 2019
- Share on Facebook
- Share on LinkedIn
Link copied
In this article, you will find everything you need to start your clinical research trials, with easy-to-understand guidance and terminology, 26 adaptable templates, and project plans in Microsoft Word, Excel, Project, and SharePoint formats.
Included on this page, you'll find details on what a research protocol is, project management for clinical trials , research compliance templates , and post-clinical study research documentation and templates
What Is the Research Protocol?
All clinical research starts with the research protocol , a document that details all aspects of the trial: its background, rationale, objectives, design, methodology, statistical analysis plan, and organization. With the protocol, you can make sure you protect the participants and collect the data. Using protocol templates, you can start thinking through what you need to meet compliance standards with the Food and Drug Administration (FDA) and clinical study best practices.

Download Research Protocol Template - Word
The full research protocol includes the following sections and topics:
- Title Pages: These pages provide general information about the protocol, including name, number, version number and date, trial phase, investigational product name, investigational new drug (IND) number, sponsor (or principal investigator in academia), funding organization, medical monitor, and coordinating center. The pages include the principal investigator’s signature (or sponsor), as well as site-specific information, such as the agreement, and protocol details. They also detail the study team and site, particularly in the case of multiple teams and sites.
- Objectives: List the study’s primary and secondary objectives.
- Background Information: Describe the problem under study and priority. Include the medical and scientific rationale that justifies researching the problem. Include data from other studies relevant to this proposed research. Include the name and description of the proposed intervention, including the dosage, route of administration, period, and frequency of intervention.
- Study Design: Describe the methodology and how it will answer the study question. This should include the type of study, primary and secondary outcome(s), population, sample size, study location, period of enrollment and follow-up, intervention and route of administration, randomization (as necessary), and any other relevant protocol information.
- Selection and Exclusion of Subjects: Provide statements describing how the participants must meet all the inclusion and exclusion criteria, and list the criteria. Clearly define the study population. For example, list the demographic criteria, required laboratory data, any prior therapies allowed or disallowed, ability to understand and meet all study requirements, if contraception is necessary, exclusion criteria such as specific health status, use of excluded drugs, cancer status, and chemical dependency status.
- Study Enrollment Procedures: Describe the methods and procedures for identifying and enrolling subjects, how they are documented, how consent is obtained, and any randomization procedures.
- Study Intervention, Duration, and Route of Administration: This section should describe each intervention and duration, as well as how each is administered. List expected adverse effects and dose escalation, if applicable. Discuss how the intervention is acquired, stored, and disposed of, as well as documentation for intervention accountability. In addition, note the medications restricted, allowed, and required, along with the extent to which these medications are tracked and documented.
- Study Procedures: This section includes a study evaluation schedule (presented as a chart) and explanations of the required assessments, what each period is, and any special considerations or instructions necessary. These should match what is available in the column headers of the chart above, and they should include information on the screening or baseline assessments, randomization, blinding, follow-up visits, and final assessments.
- Safety Assessment: List any expected adverse events, and how these could be managed. Mention any toxicities seen in earlier IND studies here. Also, include safety measures as identified in laboratory findings, methods and timing for safety parameters based on the risk profile, definitions for adverse events (AE) and serious adverse events (SAE) and laboratory values used to identify their possibility, timeframes for reporting and collecting information on AEs and SAEs, the reporting system, how you will follow up on AEs, and the specific guidelines for independent monitoring.
- Intervention Discontinuation: List criteria for intervention discontinuation and how you could meet them. Also list possible reasons for discontinuation, any modifications to the schedule should it be discontinued, duration of follow-up, any temporary discontinuation criteria, or any evaluations should participants be temporarily or permanently discontinued from the study.
- Statistical and Analytical Considerations: Include primary and secondary statistical hypotheses, why you chose the study design, the primary and secondary outcome measures, and the validity and reliability of these measures. Also discuss sample size and randomization, treatment assignment procedures, how you define the population, any interim analyses, primary and secondary outcome analyses, the statistical methods you use to consider any necessary intervention effect between groups, and if necessary, the expected positive within group correlations among different study arms.
- Data Collection: Detail how you will gather the data, the required forms, how to keep these forms confidential, and what source data to expect. Note site responsibility for data collection and management, and (if necessary) the responsibilities of the coordinating center.
- Quality Assurance: Describe training for study staff, whether there is a control committee and their required practices, any quality control metrics, how you will identify and document protocol deviations, how you will assure protocol compliance, and the schedule for reviews. If you have a manual of procedures (MOP), reference it here.
- Participants Rights: Include references to the Institutional Review Board (IRB) requirements, informed consent documents, procedures for participant confidentiality, and study discontinuation requirements.
- Committees: List any committees associated with the study, along with their roles.
- Publication: Outline the requirements and procedures for publication.
- References: List any citations referenced in this protocol.
- Supplements/Appendices: Include any additional documentation.
To track every aspect of the proposed research for each participant, create a case report form (CRF) that you can use in both paper and electronic formats. With CRFs, you can collect and analyze data for analysis, and then generate a conclusion for your study. For more information on the distinct phases of clinical trials, see “ Understanding the Phases of Clinical Trials .”
Concept Protocol Template
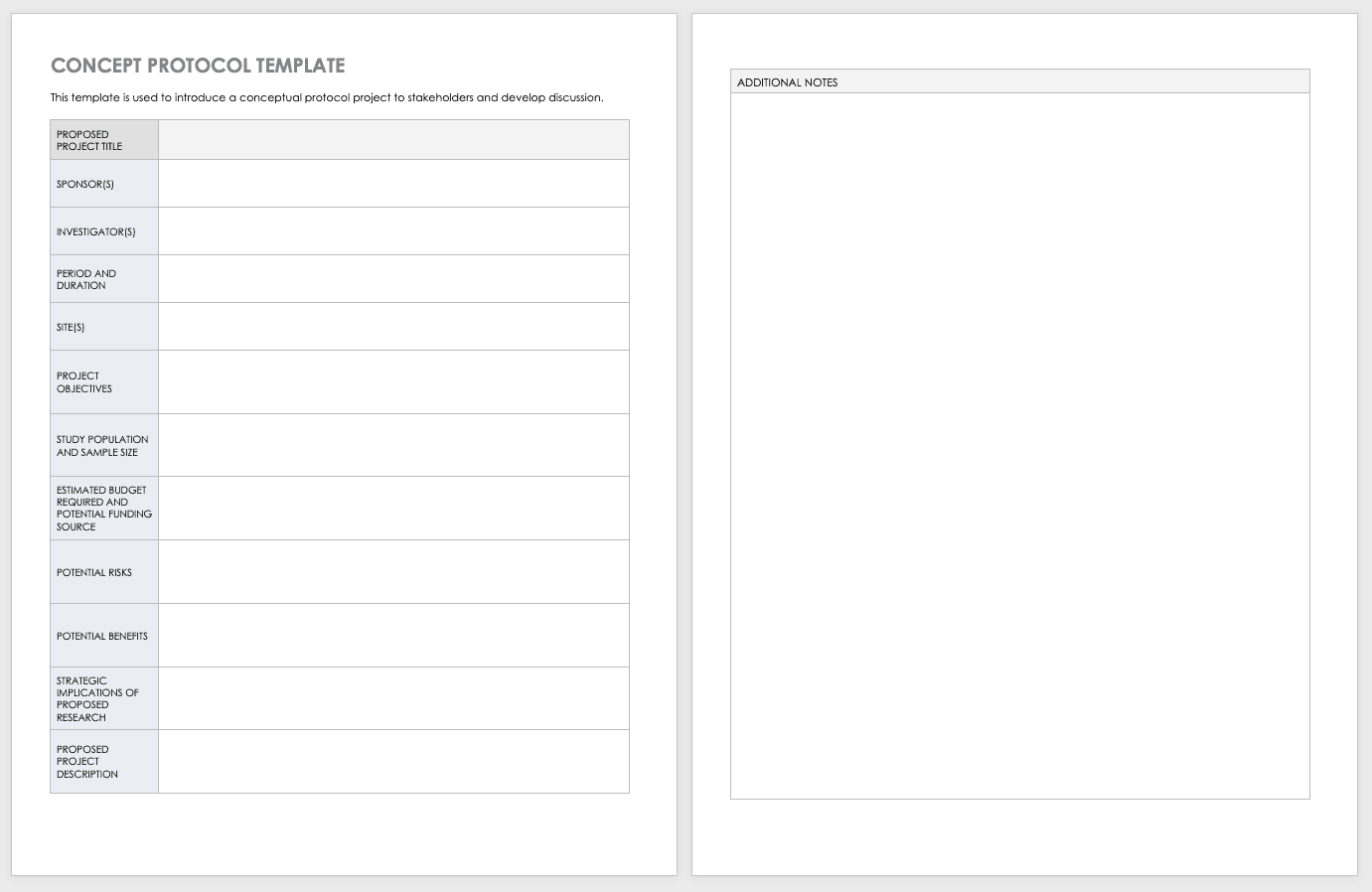
Before you start your full protocol, consider putting together a concept protocol. A concept protocol helps you introduce an abstract project to stakeholders and encourage discussion around the proposed project.
Download Concept Protocol Template for Clinical Research
Phase 1 Clinical Trial Protocol Template

For nonclinical research or clinical trials that are Phase 0 or Phase 1, use this free template. Phase 1 or nonclinical trials do not require the same amount of detail as a full study protocol.
Download Phase 1 Clinical Trial Protocol Template - Word
Research Compliance Templates
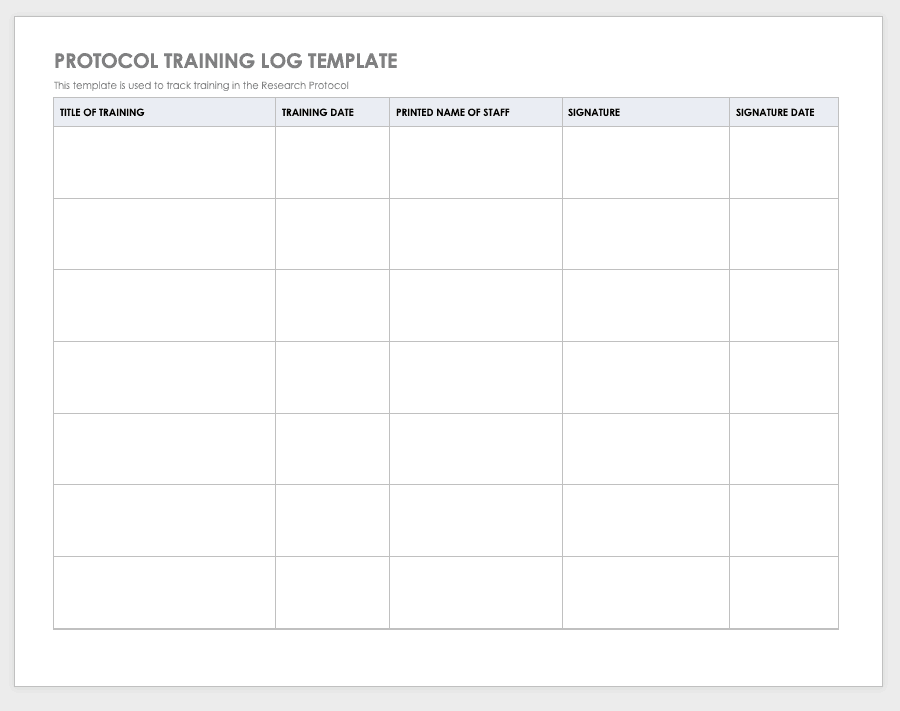
By training staff members on the research protocol, you’ll help them meet compliance standards and understand the purpose and details of the study. Use a training log to record all training that the site study staff completes, signing the log entry for verification.
Download Protocol Training Log Template
Excel | Word | PDF | Smartsheet
Protocol Deviation Template
Protocol deviations are inadvertent or unplanned changes or noncompliance with the research protocol. These events do not increase risk or decrease benefit, nor do they impinge on participants’ safety or rights. They do not compromise study data, but you should capture the deviation for reference.
Download Protocol Deviation Log Template
Excel | Word | PDF
Delegation of Authority Log Template
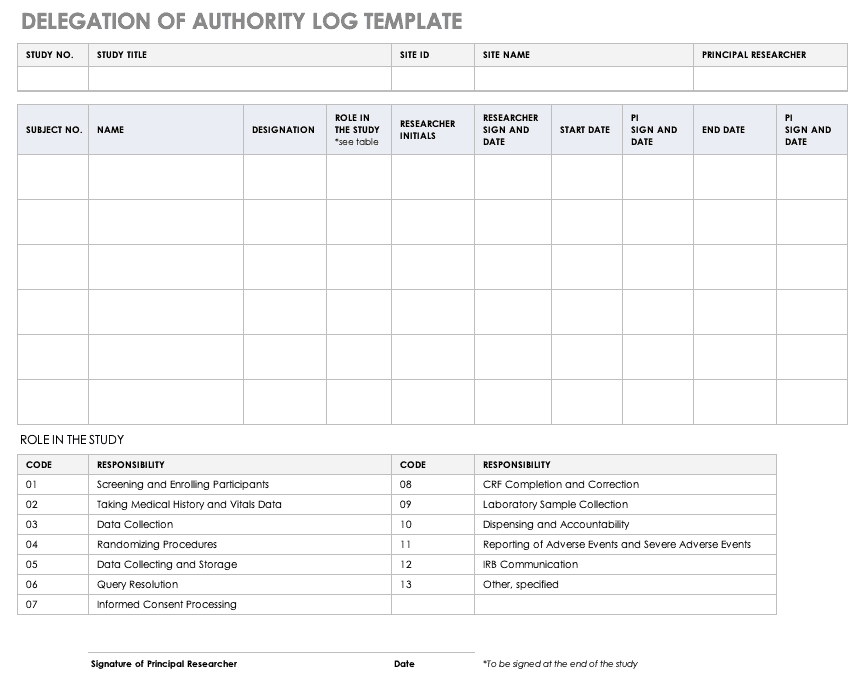
Once you’ve trained your staff and figured out their roles and responsibilities, the principal investigator must delegate authority. The delegation of authority log should be filled out and signed prior to the study’s start.
Download Delegation of Authority Log Template
Site Selection Visit Form Template
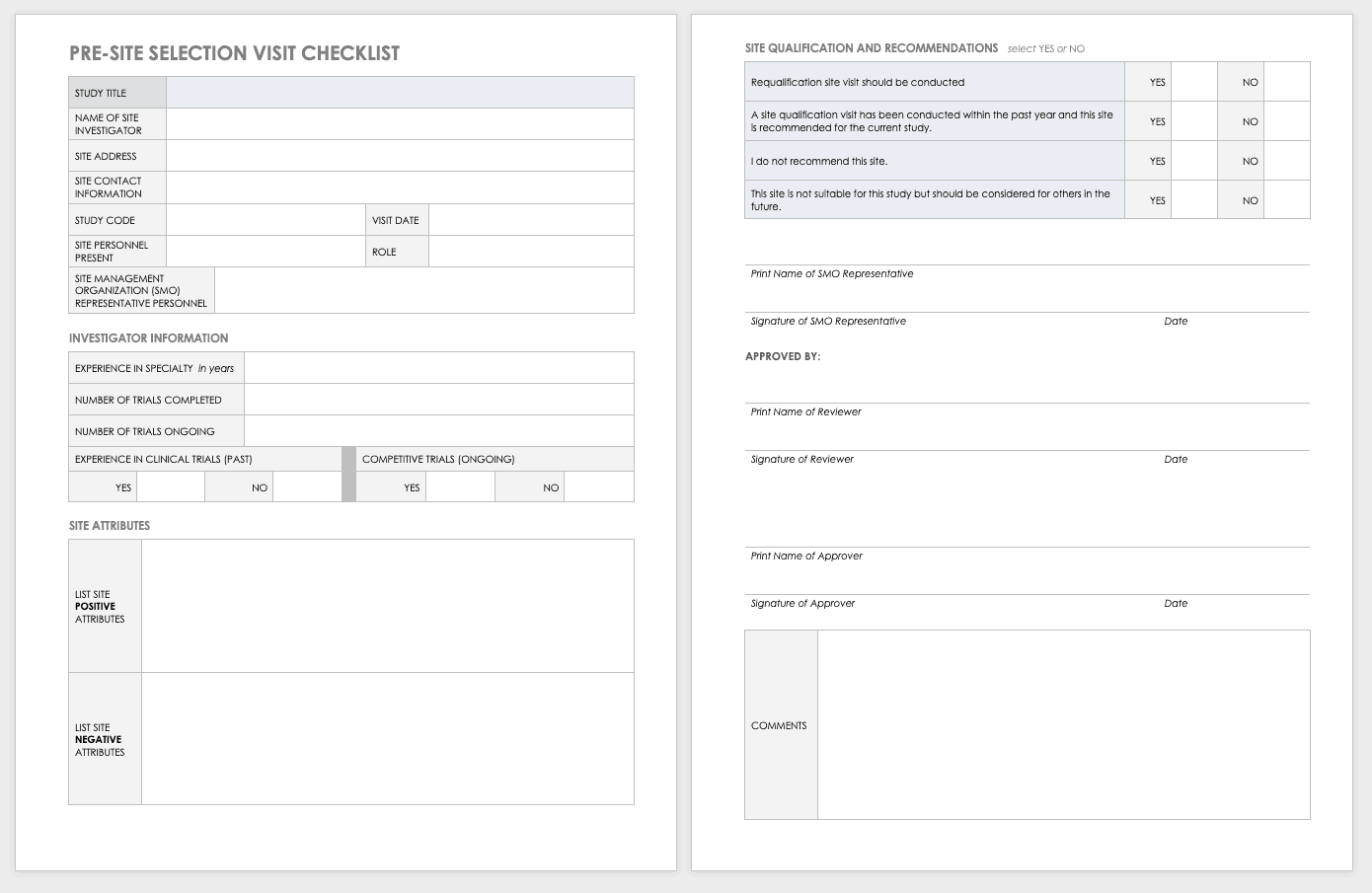
The sponsor must perform a site visit to determine its suitability as part of a multisite study. This means taking a tour to determine whether the site has the capabilities to meet the sponsor’s goals.
Download Site Selection Visit Form Template
Word | PDF | Smartsheet
Study Site Initiation Checklist
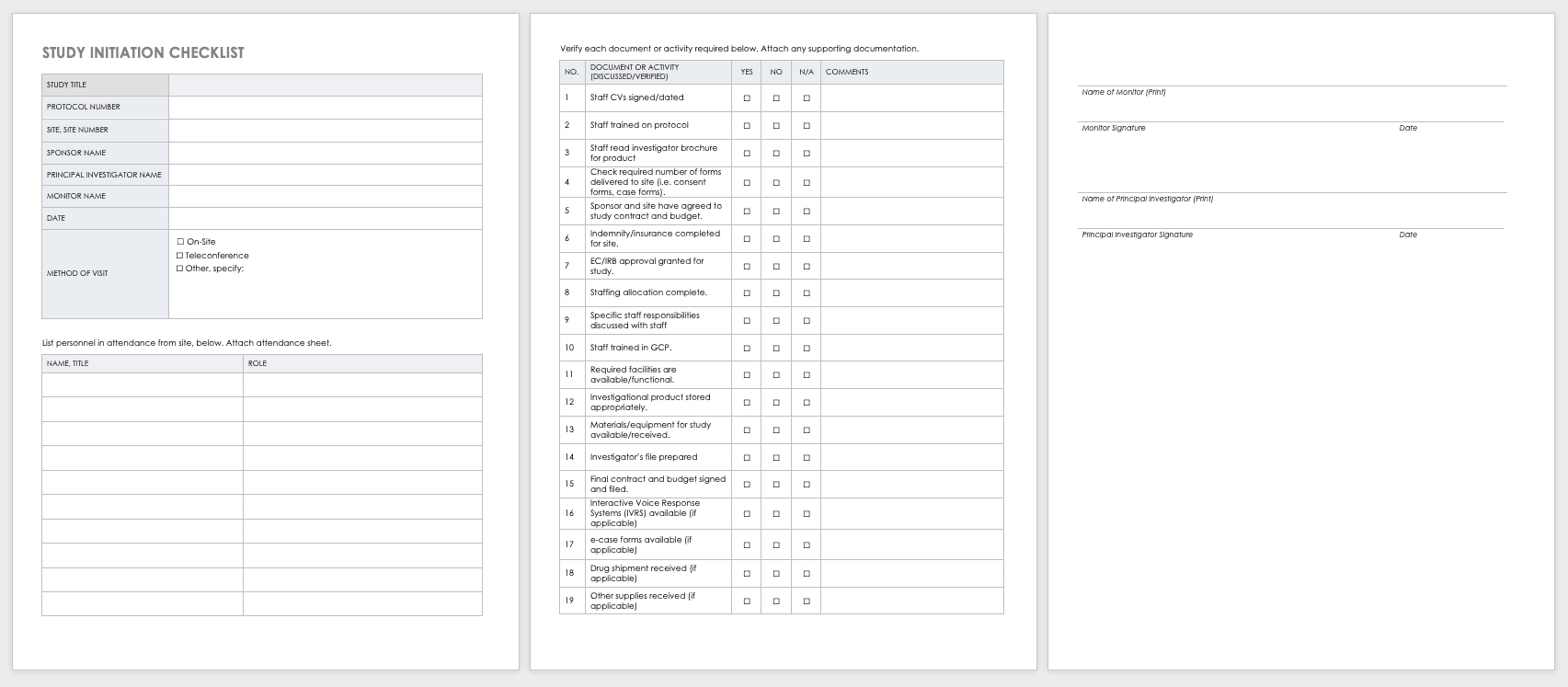
Teams must also perform an inspection to determine if a site has the appropriate staff, training, equipment, and supplies to be part of a multisite trial.
Download Study Site Initiation Checklist
Project Management for Clinical Trials, Practices, Templates, and Documents
Clinical trials are big projects. If the organization is not used to planning and wants to conduct clinical research, it must hire a project manager and work with senior leadership to introduce planning into the organization.
Together, they should develop the main goals and define their limits and the terms of success. They should set out a strategy for which tasks and sets of tasks to perform and in what manner. Test any planning tools or software before the trials start. When possible, use templates to ensure consistency and best practices.
Once the trial starts, evaluate your systems with standardized metrics. The project manager can track study deviations and apply corrective actions. Use the lessons learned from past and current projects to help guide future projects. Employing consistent tools gives you the opportunity to draw from a reservoir of data.
Clinical research can cost billions of dollars and years of time, resources, and effort. As
such, project management best practices and methodologies are critical to the success of a clinical trial, according to experts .
Many software systems are available to manage clinical trials. When very specialized, these are referred to as clinical trial management systems (CTMSs). However, other platforms can also manage clinical trials and may already be embedded with your information technology. Regardless of the platform you use, you should have full project management functionality, such as planning and reporting modules, as well as the ability to track participant contact information, deadlines, and milestones.
You may want to consider the following project management documents for your clinical research.
Project Management Plan (PMP) for Clinical Trials
A PMP delineates and acts as an agreed-upon document of scope, responsibilities, and guidance. You can use it throughout the project to help stay on track. Every clinical trial has difficult milestones, but a good project management plan can help you sidestep some of the regular issues.
You have many PMP software platforms to choose from, but regardless of your ultimate decision, your PMP must focus on protocol adherence, subject care, and service quality, along with how to achieve each standard. Here are the sections you should include in your PMP for a clinical trial:
- Project Objectives: This is an outline of the research objectives for the study, your quantifying standards, and your goals.
- Background and Strategic Context: By documenting background and context, you establish a foundation for decisions and discussion to follow.
- Study Governance: The governance covers the roles and responsibilities in the project, encouraging open communication, sharing, and accountability.
- Stakeholder Management Plan: This plan details how the staff and investigators will collaborate and effectively communication with stakeholders. This could include (as per the roles and responsibilities) regular emails, newsletters, consultation, oversight, training, and documentation.
- Scope: This document delineates assumptions, constraints, and deliverables (and their expected dates).
- Project Risk Assessment: This document helps you prepare for risks and decide on the risk profile.
Clinical Research Project Activity List
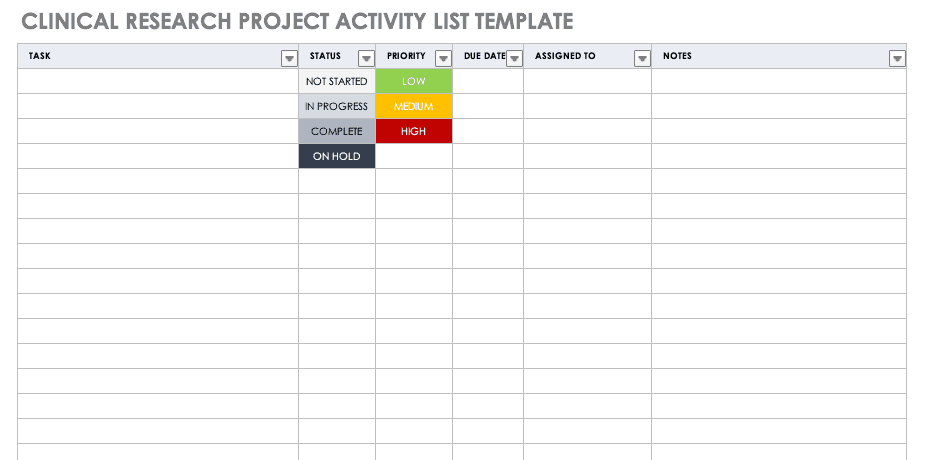
A project activity list is an itemized documentation of all the activities scheduled as part of the project. This list should be very detailed, including the status and priority of the task, when it is due, and to whom it is assigned.
Download Clinical Research Project Activity List Template
Excel | Smartsheet
Clinical Trial Timeline Template
A timeline enables you and your staff to track each major portion or milestone of your clinical trial. Your timeline should include these steps:
- Choose Research Questions and Study Design: Research always begins with questions. Your research question will determine how you design your study.
- Choose Outcomes: The outcomes for any trial are dependent on many factors, including scope, health conditions under study, target population, type of intervention. One resource to help develop outcomes is Core Outcome Measures in Effectiveness Trials (COMET) . This database details core outcome sets for comparison in clinical trials.
- Prospectively Register the Trial: Whether you are working through the FDA, World Health Organization (WHO), or another national agency, study transparency is critical. Prospective registration of trials is recommended. One resource for registration is the ISRCTN registry .
- Obtain Ethics Approval: Any trial involving human participants must go through an ethics review to safeguard the subjects’ rights, safety, well-being, and dignity. There are many options for institutional review, including through a university or a private or governmental organization. Without this step, research cannot commence.
- Prospectively Publish Protocol and Analysis Plan: Before a clinical trial, you must complete some pilot research. When you publish the research leading up to a clinical trial, along with the protocol and analysis for the trial itself, you increase transparency and accountability of the research.
- Planning for the Trial and Data Management: Many clinical research professionals recommend including patients in the planning phase of clinical trials, at least as stakeholders to review the plan. By completing the plan early and allowing potential participants to review it, you help improve recruitment and retention during the trial.
- Recruitment and Retention: Recruitment is getting the right people to take part in your trial, and retention is about keeping their interest and trust. A source of unending frustration for researchers, recruitment and retention can make or break a trial.
- Identify and Manage Trial Sites and Staff: This process is not as straightforward as it is often thought to be. Study coordinators must use feasibility checklists to choose sites and figure out how to get bring on staff who have the bandwidth to recruit for the study.
- Data Collection: The methods for collecting data are critical to any study. Advance planning and structure help you stay organized, comprehensive, and transparent so that your study can have a seamless analysis and solid conclusions.
- Data analysis: Flaws in analysis can generate poor, biased, or erroneous outcomes. In advance, researchers should consider patient blinding, randomization procedures, and sequence generation.
- Findings dissemination: Some researchers recommend threading all research on a trial topic. One resource for this is CrossRef , a database that links similar research. Regardless, the point of research is to capitalize on scientific progress and move it along. By having a plan to disseminate your results, you ensure that others capitalize on your research and move the knowledge forward.
Use this free template to develop your own clinical trial timeline. Add your own steps, milestones, and dates for a comprehensive, expansive view.

Download Clinical Trial Timeline Template
For a different perspective, add your project details to this free template so you can view your timeline visually.
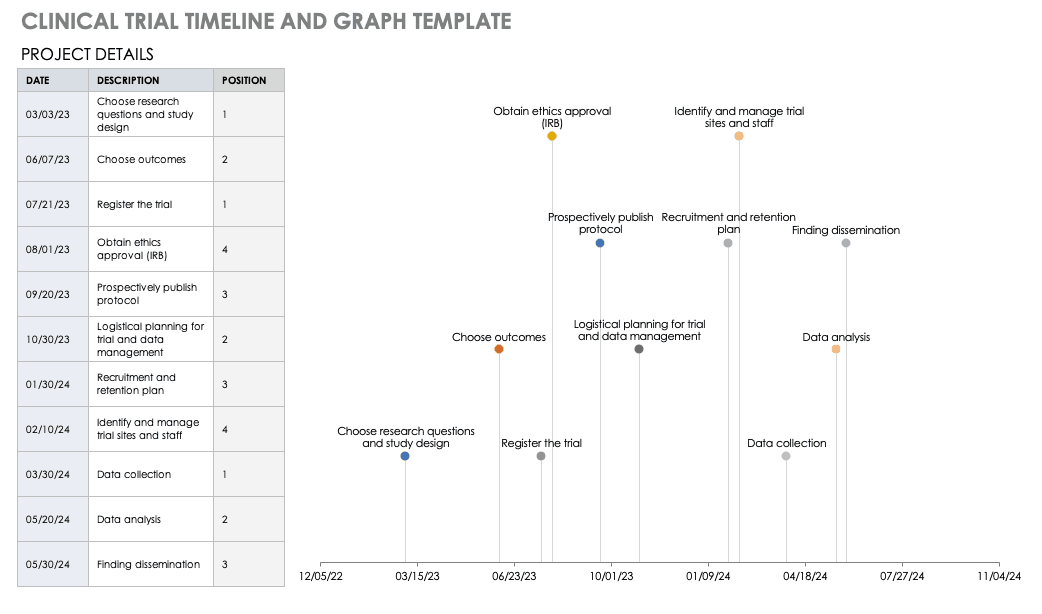
Download Trial Timeline and Graph Template
Microsoft Project Management for Clinical Trials
First released in 1985, Project is a well-respected Microsoft product for project management. Microsoft Project was not traditionally available as a part of Office Suites, a package of programs for professionals and professional organizations. However, Microsoft recently included it as a part of the Windows 2016 suite.
Microsoft Project Management has the following features:
- Built-in templates
- Project portfolio management
- IT management
- Presentations
- Out-of-the-box reports
- Multiple timelines
- Real-time reporting
- Dependency management
- Priority assignment
- Lean management
- Gantt charts/project mapping
- Calendar views
- Setting baselines/KPIs
- Project budgeting
- Issue tracking
- Task creation
- Resource management
- Cloud access
Microsoft Project has built-in templates that you can apply to clinical trial management.
Microsoft SharePoint for Clinical Trials
SharePoint is a collaboration platform that is integrated with Microsoft Office. SharePoint manages and stores documents , and it enables multiple users to access the documents via their own site or a standardized Microsoft site. A subscription to Microsoft Office 365’s SharePoint does not require a server, but customization options are limited; the flexible authentication and authorization systems are built in.
SharePoint Server, available in Standard or Enterprise versions, can be developed as either
virtual or hosted services in a business’s IT department. SharePoint Server enables the organization to control the SharePoint features available to staff, and you can scale it to meet different numbers of users.
Windows SharePoint Services 3.0 is a Microsoft-hosted version that comes with Microsoft Office. Microsoft provides a template in SharePoint for Clinical Trials: Clinical Trial Initiation and Management application template for Windows SharePoint Services 3.0 . You can download and add this template to your SharePoint Services, which enables you to create the following:
- Clinical Trial Protocols: This includes the objectives, study design, project plan, subject selection, and budget.
- Protocol Documents: This includes additional documents relative to your study.
- Calendar: Track milestones in the project.
- Threaded Document Discussions: Team members can start and track discussions within documents.
- Task Creation and Assignment: You can create and assign tasks to users, who receive email notifications.
- Archiving: You can move documents or groups of documents to archive status, keeping them but not making them visible.
The clinical trial template has site lists of libraries for clinical trial protocols, protocol documents, announcements, calendars, issues, tasks, and document discussions. These can be further customized with different versions of SharePoint. To download this template, you will need access to SharePoint Server 3.0.
Clinical Research Budget Plan Template
In many instances, you set the clinical trial budget after much negotiation with a sponsor. Other times, you need to build a budget before the sponsor is even on board, as a way to convince them of the project’s feasibility. The key cost drivers for any clinical research project are the following:
- Patient Grants: These include the costs for screening failures, baseline patient measurements, and procedural costs.
- Site Costs: This covers any expenses associated with the site, such as start-up fees, IRB fees, storage fees, and site management costs.
- Non-Patient Costs: This includes consultation fees, monitoring board fees, and any medical device costs.
- Labor Costs: You must account for all the staff required for the project and their full-time equivalency (FTE).
- Site Management: These costs include pre-study visits, initiation fees, monitoring, and close-out fees.
- Miscellaneous: These include investigator meetings, any technology needs, and ad hoc travel.
- Unexpected Costs: These are costs resulting from protocol amendments, value added tax (VAT), delays, and inflation.
Before you start putting together your research budget, you must gather the following:
- Schedule of assessments from the protocol
- Standard institutional fees from your institution, if applicable
- Evaluation and procedural costs
- Staff allocation and their hourly rates
- Indirect cost rate
- Subject compensation costs
- Data storage fee estimate
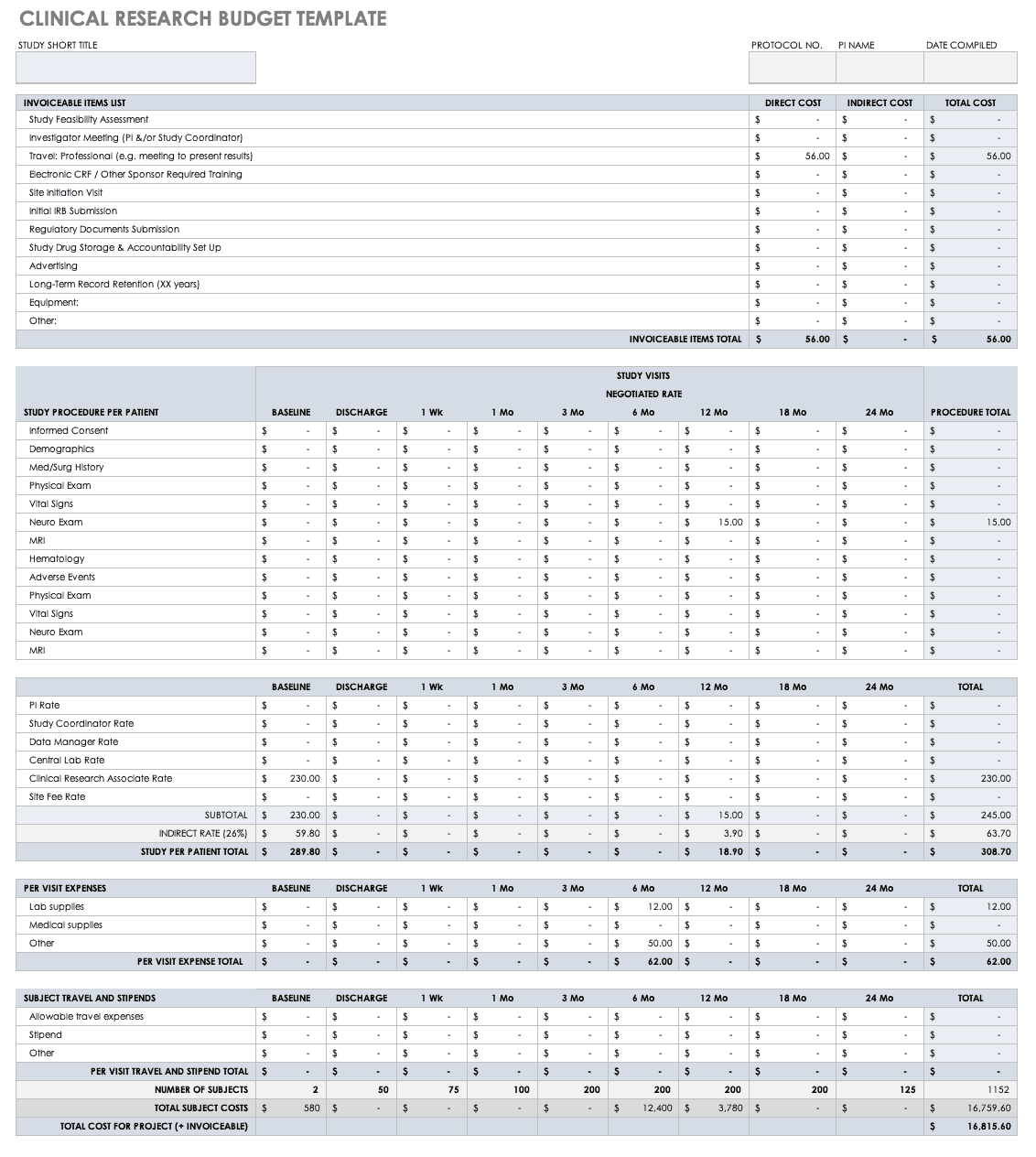
Put together your own clinical trial budget with this free clinical research budget template.
Download Clinical Research Budget Template - Excel
Clinical Research Tracking Log Templates
Clinical research requires scrupulous planning, a well-developed team, regulatory adherence, and above all, excellent documentation. It is therefore critical for clinical trial project managers to have a completed scope of work and to develop all the forms and templates before the trial begins. Some of these documents are for planning, and some, like those included below, are for operational purposes.
Regulatory Binder Checklist

Strong clinical practice thrives with a regulatory binder checklist. This checklist keeps track of all paper versions of essential regulatory study documents. Each document should also include any electronic locations. This document should be regularly updated, customized for unique studies, and stored in reverse chronological order.
Download Regulatory Binder Checklist
Clinical Study Document Tracking Log
It is important to not only track all paperwork related to a clinical trial, but also be able to locate it easily between various staff and sites. A clinical trial document tracking log can help you keep a written trail of the documents and when they were submitted and approved. You should also keep copies of the documents with the log. Use this free template to develop your own clinical study document tracking log. You can also adapt the log for specific correspondence, such as documents relating to FDA or IRB submissions, but it should not be mixed with regulatory documentation.
Download Clinical Study Document Tracking Log
Data and Safety Monitoring Plan (DSMP) Template
Before you can undertake a study, you must develop a DSMP for how to keep participants safe and how to secure data and ensure accuracy. The DSMP has several sections:
- The study purpose
- An adherence statement
- Any protocol amendments
- Multisite agreements
- A plan for subject privacy
- Confidentiality during adverse event reporting
- Expected risks
- Adverse events, unanticipated problems, and serious adverse events: how they are defined, their relation to the study, expectations, severity grading, and reporting procedures in single-site and multisite trials, and whether they are IND or non-IND studies
- Events of special interest
- Pregnancy reporting
- Rules to halt the study for participants
- Quality control and quality assurance
- Subject accrual and compliance
- Sample size justification
- Stoppage rules
- Monitoring committee designation
- Safety review plan
- Study report plan for independent monitors
- Plan to submit reports from onsite monitoring and audits
- Data handling and record keeping
- Informed consent
- Reporting changes in study status

Create your own data and safety monitoring plan using this free template. It lays out each section so you can specify them for your research. The principal investigator should sign and date this document once it is complete so that it may be filed.
Download Data and Safety Monitoring Plan Template - Word
Research Communication Plan Template
A communication plan should describe how you will converse with internal and external stakeholders during your project. Your communication plan should include a brief overview of your project and a breakdown of the messages you need to get out. You should adapt the messages for different audiences and define who will deliver these messages. The messages should include the following:
- The purpose and benefits of the research
- The known effectiveness of the intervention, or (if the intervention is under study) the disclosure that the effectiveness is unknown
- How participants will be protected
- The risks and benefits of participating

Develop your own communication plan using this free clinical trial communication plan template. This template also includes a section for situation analysis and risk analysis that asks for inputs on strengths, weaknesses, opportunities, and threats.
Download Clinical Trial Communication Plan Template - Word
Participant Management in Clinical Trials Using Templates
A few main documents help ensure that your participants are tracked and well-cared for before and during your research study.
Enrollment Log for Clinical Trials Template
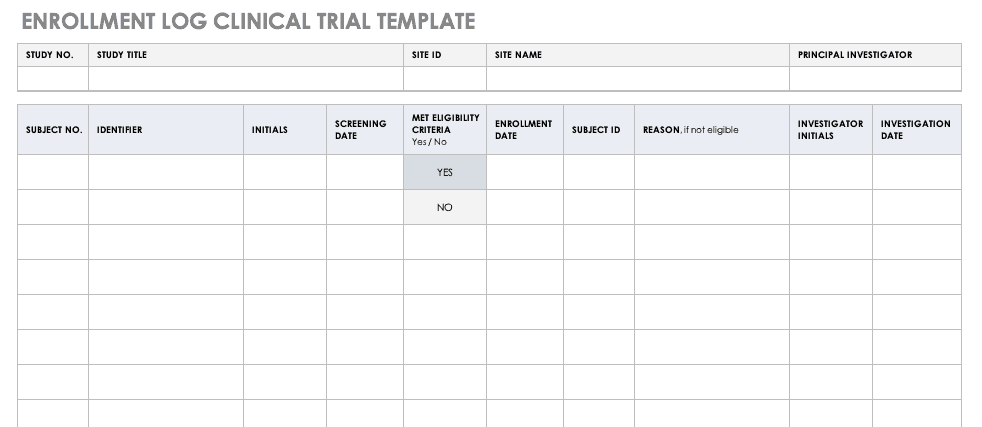
This log keeps track of everyone that has been enrolled for participation in your study. This does not mean that they have met the eligibility requirements or have been otherwise screened, but it is a record that they have signed up to be admitted.
Download Enrollment Log for Clinical Trials Template
Informed Consent Form Templates
Informed consent is the central tenet of ethical research with human subjects. The consent process typically involves a researcher delineating what is involved in the study, its risks and benefits, what a participant’s duties entail, and answering any questions they have. Before you perform any research, make sure the informed consent document is signed and the participant receives a copy, unless the informed consent document has been waived by an institutional review board (IRB). Federal regulations 45 CFR 46.116 govern what you must provide in the informed consent process in the United States.
To prepare informed consent documentation, researchers must do the following:
- Use plain, easily understandable language no higher than an 8th-grade reading level.
- Tailor documents to the potential population.
- Avoid technical jargon.
- Use the second or third person (you/he/she) to present study details.
- Include a statement of agreement.
- Ensure that the consent document is consistent with information in the IRB application.
These templates assist the principal investigator in the design of their informed consent forms (ICFs). You can adapt them to accommodate the details of any study and include both the information sheet and the consent form. Modify each section with the appropriate description described in italics. Use the general template for any type of research.
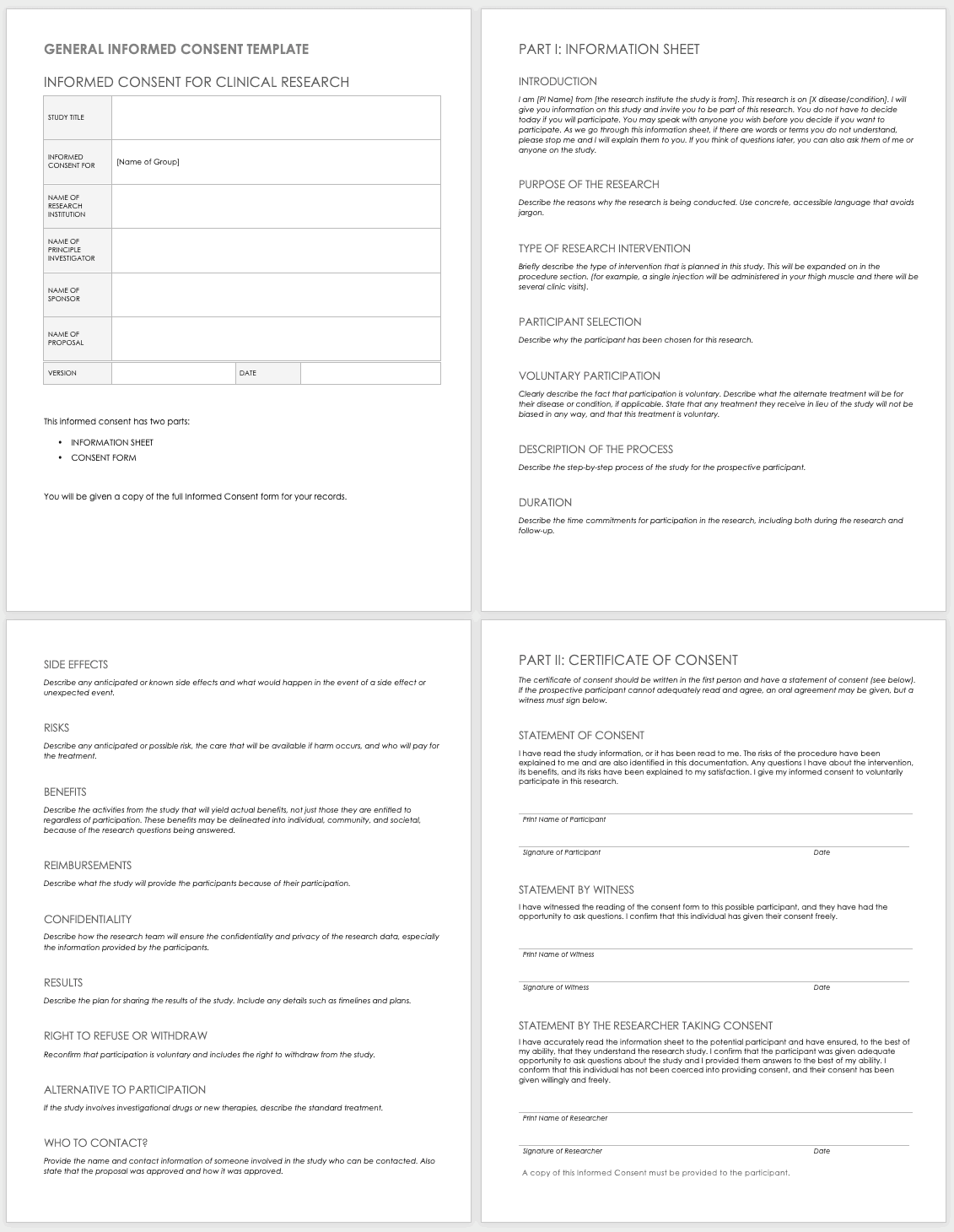
Download General Informed Consent Template - Word
Use the clinical trial template for medical research.

Download Informed Consent for Clinical Trials Template - Word
Eligibility Criteria (Inclusion/Exclusion) Checklist
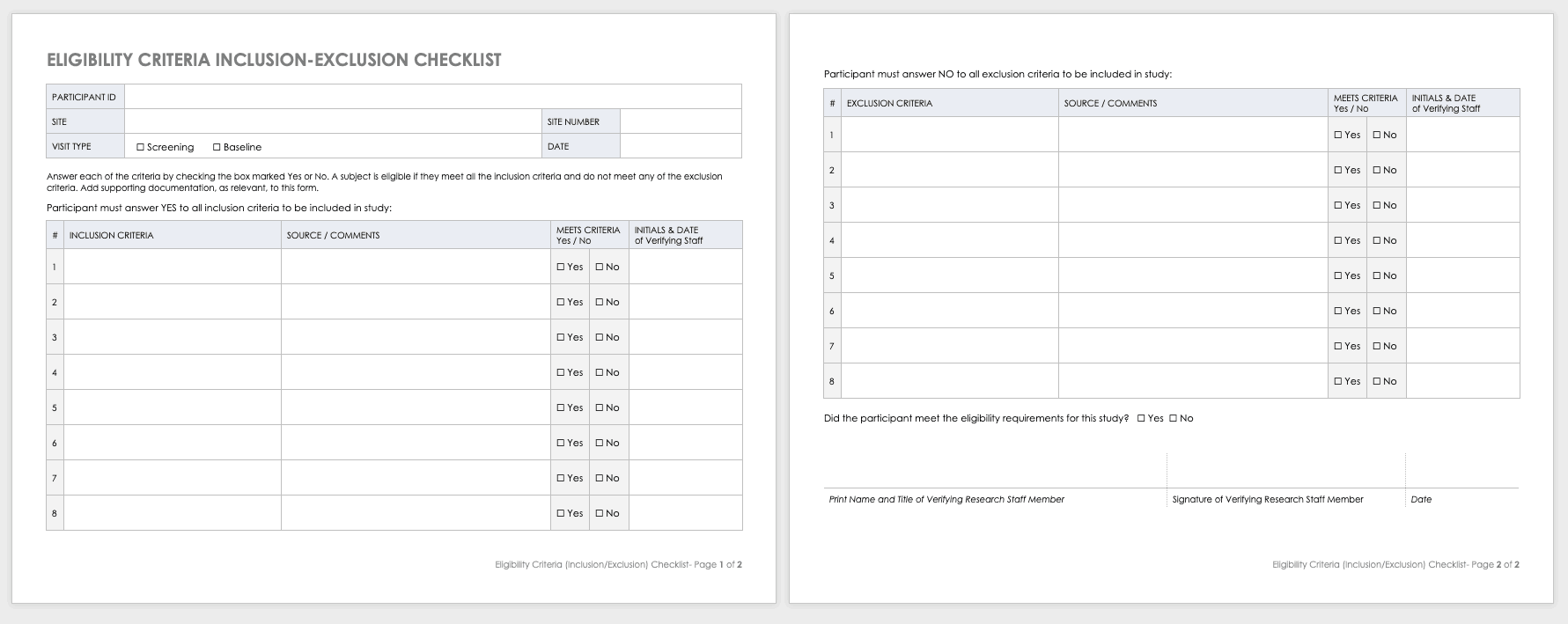
Eligibility criteria are an essential part of clinical trials. They define the population under investigation.
Inclusion criteria are the standards that participants must meet to enroll in the study. For example, in a study on a new diabetes medication, you would likely want participants who have already been diagnosed with diabetes.
Exclusion criteria specify the characteristics that disqualify participants from taking part in the research. For example, in the diabetes study above, the proposed diabetes drug may target a specific age demographic. One exclusion criterion could be a participant whose age falls outside of the range.
Download Eligibility Checklist Inclusion-Exclusion Template
Concomitant Medication Log Template
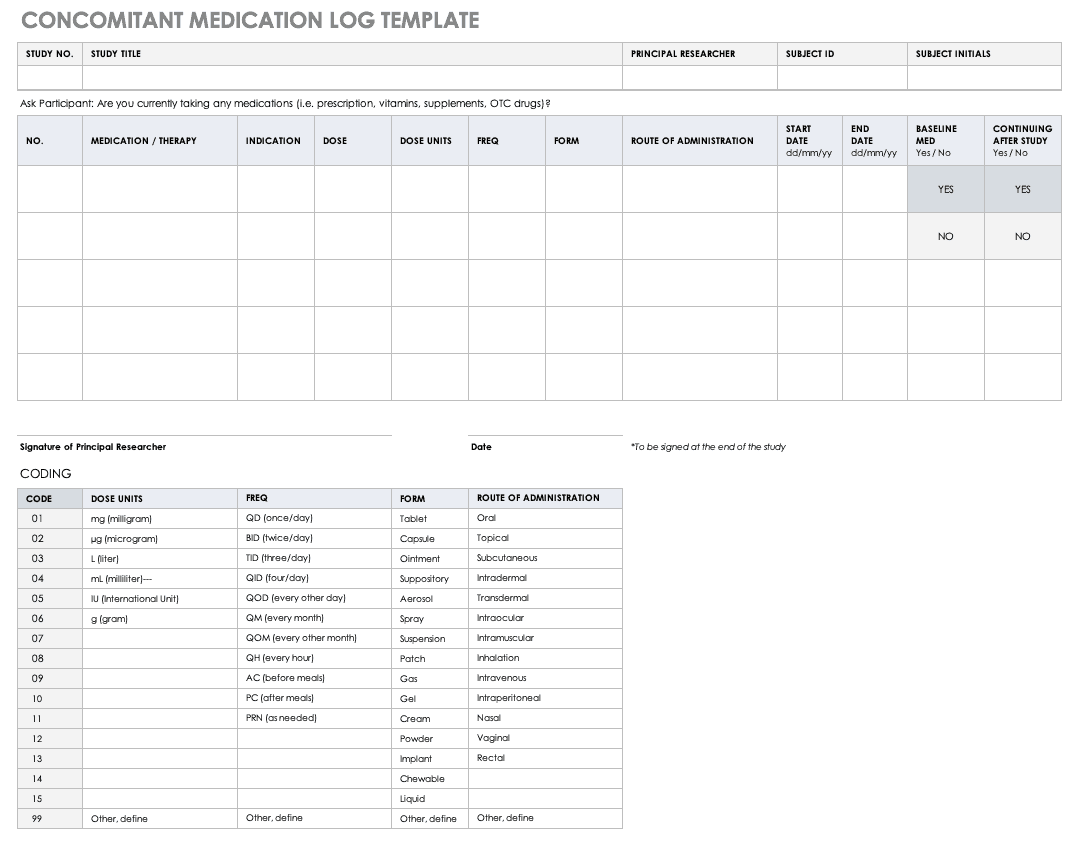
Properly documenting any medications that participants are taking is imperative to understanding the reactions occurring in their bodies, as well as what could spur adverse and severe adverse events during the study. Fill out a concomitant medication log for every participant and account for everything participants take, even seemingly innocuous items like multivitamins.
Download Concomitant Medication Log Template
Excel | Word | PDF
Adverse Event Form
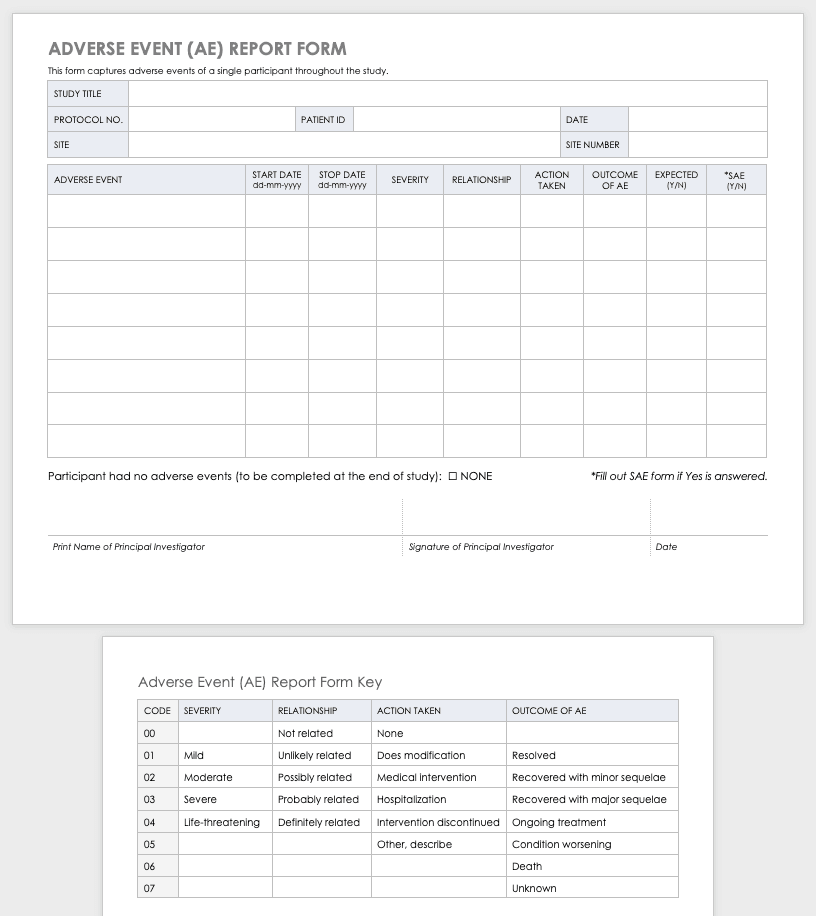
Clinical research can result in complications for the participants and trigger an adverse or severe adverse event. An adverse or severe adverse event is when participants in a clinical trial have negative medical symptoms that can be shown in laboratory or physical testing. Each participant in a clinical trial should have an adverse event log that tracks any adverse events through the duration of the study.
Download Adverse Event Form Template
Severe Adverse Event Form

A severe adverse event (SAE) is a special case of an adverse event in which the outcomes are acute. Examples of SAEs include death, life-threatening complications, or anything leading to immediate hospitalization, physical disability, or congenital abnormalities. Log SAEs in the AE form, but fill out an additional SAE form.
Download Severe Adverse Event Form Template
Word | PDF | Smartsheet
Post-Clinical Study Research Documentation and Templates
After you complete or terminate a clinical trial, you should prepare several additional documents. Here are some examples of this documentation:
- Investigational Product Accountability Log: You generally provide an accountability log to the authorities that tracks drug products to show product disposition and accountability per participant. It also helps you track the drug product stock and any imbalance at the end of the study.
- Investigational Product Destruction: Due to regulations governing the proper disposition of investigational products in clinical research, you must properly dispose of products left at the end of a study (as evidenced by the product accountability log). This form describes and ensures that you have properly handled any leftover products.
- Close-out Checklist/Report: A study close-out checklist and report helps ensure that you complete all closing procedures, archive the paperwork, and resolve electronic data.
Clinical Study Summary Report Template

Assemble the summary report at the end of a study to get results into the sponsor’s or public’s hands while you complete the full report. A summary report is typically about 2-3 page-long document that encompasses the highlights from the trial.
Download Study Summary Report Template - Word
Clinical Study Report (Full) Template

The full clinical study report (CSR) encompasses all aspects and details of the research you’ve conducted. It is not a sales or marketing tool; instead, it is a scientific report details the methodology and shows scientific rigor.
Download Clinical Study Report Template - Word
Public Links and Resources for Clinical Trials
The following are publicly available resources, tools, and links for clinical trial practitioners and principal investigators:
- PROMIS : Patient-Reported Outcomes Measurement Information System (PROMIS) software gives clinicians health status patient measures that are physical, mental, and social patient-reported metrics. Funded by the National Institutes of Health (NIH), PROMIS can be used in clinical trials as measures of conditions and disease and as a comparison to the general population. The measures in PROMIS are free to administer on paper, by computer (computer adaptive tests), or with an app. The computer adaptive tests may be conducted on REDCap , Assessment Center , or Epic .
- REDCap: REDCap (Research Electronic Data Capture) is an electronic data capture system that works on browsers to develop research databases. It was developed at Vanderbilt University to support clinical research data collection and is a free resource to nonprofit organizations. It is limited to organizations joining the REDCap consortium and is not open-source or available for commercial use.
- Good Clinical Practice (GCP) Training: GCP is an international quality standard designed for use by staff involved in clinical trials. The guidelines for this are from the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). These regulate the ethical guidelines, documentation, record keeping, training, facilities, technology, and inspections. The purpose of these guidelines is to keep clinical trials scientifically rigorous and to delineate the roles and responsibilities of research staff. The National Institutes of Health administers training for GCP.
- Quality Management Study-wide Review Tool: Developed by the NIH, this review tool is for PIs and study teams to manage their quality reviews, and may be customized for unique studies.
- Quality Management Subject Review Tool: Also developed by the NIH, this review tool provides study teams the structure for review of participant data, and may be customized for the unique study. This should be developed in concert with the DSMP.
- AccrualNet: AccrualNet is sponsored by the National Cancer Institute (NCI), and offers advice and training to staff on how to recruit study participants.
- Regulatory Education for Industry (REdI): The FDA offers a Clinical Investigator Training Course for researchers conducting investigational new drug (IND) or device exemption (IDE) studies.
- ResearchMatch: Available to volunteers and researchers affiliated with the NIH Clinical and Translational Science Award (CTSA) program, this site helps match prospective participants with specific studies.
- Grant Policies and Guidance: The NIH and National Center for Complementary and Integrative Health (NCCIH) offer links to many resources that are policy- and grant-specific to the NIH and NCCIH, updated regularly.
- Protocol Amendments: The NIH and NCCIH offer regularly updated guidance for NIH policy and protocol changes.
- Clinical Terms of Award for Human Subjects Research: The NIH and NCCIH offer guidance for clinical trial grant awardees for compliance.
- NIH Single IRB (sIRB) Policy for Multisite Research: The NIH offers a FAQ page for multisite research that includes policy, contract and application information, responsibilities, exceptions, and costs.
- Dictionary of Cancer Terms: The National Cancer Institute (NCI) offers a dictionary of cancer terms for researchers and laypersons. You can add this dictionary to your website as a widget.
- Informed Consent FAQs: The U.S. Department of Health and Human Services (HHS) and the Office for Human Research Protections (OHRP) offer a FAQ page about informed consent for researchers and lay persons.\Informed Consent Language (ICL) Database: The National Comprehensive Cancer Network (NCCN) offers a database to help write informed consents. This database is specific to medical conditions and different risk language.
Improve Clinical Trial Research with Smartsheet for Healthcare
Empower your people to go above and beyond with a flexible platform designed to match the needs of your team — and adapt as those needs change.
The Smartsheet platform makes it easy to plan, capture, manage, and report on work from anywhere, helping your team be more effective and get more done. Report on key metrics and get real-time visibility into work as it happens with roll-up reports, dashboards, and automated workflows built to keep your team connected and informed.
When teams have clarity into the work getting done, there’s no telling how much more they can accomplish in the same amount of time. Try Smartsheet for free, today.
Discover why over 90% of Fortune 100 companies trust Smartsheet to get work done.
- Introduction
- Conclusions
- Article Information
Emergency department visit rates are per 1000 person-years in children younger than 2 years are shown. Symbols are the crude annual (April 1 to March 31) rates from April 1, 2004, to March 31, 2022, among groups. Trends were quantified using the Joinpoint regression program, version 5.0.5 (National Cancer Institute). Annual percent change (APC) was estimated for years 2004-2005 to 2019-2020. There was no evidence of a significant joinpoint for sex, residence location, or material resources quintile (Q), indicating that the observed increases in emergency department visit rates were linear.
a The average APC was significantly different from zero at P < .001.
b The average APC was significantly different from zero at P < .01.
Hospitalization rates are per 1000 person-years in children younger than 2 years are shown. Symbols are the crude annual (April 1 to March 31) rates from April 1, 2004, to March 31, 2022, among groups. Trends were quantified using the Joinpoint regression program, version 5.0.5 (National Cancer Institute). Annual percent change (APC) was estimated for years 2004-2005 to 2019-2020. There was no evidence of a significant joinpoint for sex and residence location. For material resources, in quintile (Q) 4, there was an initial decrease in hospitalization rate from 2004 to 2008 (trend 1: APC, −5.72; 95% CI, −14.10 to 3.49; P = .19) followed by an increase from 2008 to 2019 (trend 2: APC, 1.72; 95% CI, −0.28 to 3.76; P = .09). In Q5, there was an initial decrease in hospitalization rate from 2004 to 2012 (trend 1: APC, −3.05; 95% CI, −6.51 to 0.53; P = .09) followed by an increase from 2012 to 2019 (trend 2: APC, 3.81; 95% CI, −0.70 to 8.53; P = .09). However, these trends for Q4 and Q5 were not statistically significant. The average annual percent change (AAPC) for each of the Q1 to Q5 subgroups was not significantly different from zero at the α = .05 level. The AAPC difference between Q2 to Q5 and referent Q1 were not significantly different from zero at the α = .05 level.
eMethods. Supplemental Methods
eTable 1. Trends in Bronchiolitis Emergency Department Visit Rates Among Equity Stratifiers: Female vs. Male, 2004-2005 to 2021-2022
eTable 2. Trends in Bronchiolitis Emergency Department Visit Rates Among Equity Stratifiers: Rural vs. Urban, 2004-2005 to 2021-2022
eTable 3. Trends in Bronchiolitis Emergency Department Visit Rates Among Equity Stratifiers: Material Resources Quintile, 2004-2005 to 2021-2022
eTable 4. Trends in Bronchiolitis Hospitalization Rates Among Equity Stratifiers: Female vs. Male, 2004-2005 to 2021-2022
eTable 5. Trends in Bronchiolitis Hospitalization Rates Among Equity Stratifiers: Rural vs. Urban, 2004-2005 to 2021-2022
eTable 6. Trends in Bronchiolitis Hospitalization Rates Among Equity Stratifiers: Material Resources Quintile, 2004-2005 to 2021-2022
eAppendix. Canadian Paediatric Inpatient Research Network Members
Data Sharing Statement
See More About
Sign up for emails based on your interests, select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Get the latest research based on your areas of interest.
Others also liked.
- Download PDF
- X Facebook More LinkedIn
Mahant S , Borkhoff CM , Parkin PC, et al. Sociodemographic Factors and Trends in Bronchiolitis-Related Emergency Department Visit and Hospitalization Rates. JAMA Netw Open. 2024;7(4):e248976. doi:10.1001/jamanetworkopen.2024.8976
Manage citations:
© 2024
- Permissions
Sociodemographic Factors and Trends in Bronchiolitis-Related Emergency Department Visit and Hospitalization Rates
- 1 Child Health Evaluative Sciences, The Hospital for Sick Children Research Institute, Toronto, Ontario, Canada
- 2 Division of Pediatric Medicine, Department of Pediatrics, University of Toronto, Toronto, Ontario, Canada
- 3 Institute of Health Policy, Management, and Evaluation, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada
- 4 Division of Pediatric Medicine and the Pediatric Outcomes Research Team, The Hospital for Sick Children, Toronto, Ontario, Canada
- 5 ICES, Ottawa, Ontario, Canada
- 6 The Ottawa Hospital Research Institute, Ottawa, Ontario, Canada
Question Have inequalities based on sex, residence location, and material resources in bronchiolitis emergency department visits and hospitalizations improved over time?
Findings In a population-based cohort study of 2 921 573 children from 2004 to 2022 in Ontario, Canada, bronchiolitis emergency department and hospitalization rates were highest for boys, those with rural residence, and those with lowest material resources. There were no between-group differences in the average annual percentage change in bronchiolitis emergency department visit and hospitalization rates for sex (female vs male), residence (rural vs urban), and material resources (greatest vs least).
Meaning These findings indicate that inequalities in bronchiolitis emergency department visit and hospitalization rates did not improve over time.
Importance Bronchiolitis is the most common and most cumulatively expensive condition in pediatric hospital care. Few population-based studies have examined health inequalities in bronchiolitis outcomes over time.
Objective To examine trends in bronchiolitis-related emergency department (ED) visit and hospitalization rates by sociodemographic factors in a universally funded health care system.
Design, Setting, and Participants This repeated cross-sectional cohort study was performed from April 1, 2004, to March 31, 2022, using population-based health administrative data from children younger than 2 years in Ontario, Canada.
Main Outcome and Measures Bronchiolitis ED visit and hospitalization rates per 1000 person-years reported for the equity stratifiers of sex, residence location (rural vs urban), and material resources quintile. Trends in annual rates by equity stratifiers were analyzed using joinpoint regression and estimating the average annual percentage change (AAPC) with 95% CI and the absolute difference in AAPC with 95% CI from April 1, 2004, to March 31, 2020.
Results Of 2 921 573 children included in the study, 1 422 088 (48.7%) were female and 2 619 139 (89.6%) lived in an urban location. Emergency department visit and hospitalization rates were highest for boys, those with rural residence, and those with least material resources. There were no significant between-group absolute differences in the AAPC in ED visits per 1000 person-years by sex (female vs male; 0.22; 95% CI, −0.92 to 1.35; P = .71), residence (rural vs urban; −0.31; 95% CI −1.70 to 1.09; P = .67), or material resources (quintile 5 vs 1; −1.17; 95% CI, −2.57 to 0.22; P = .10). Similarly, there were no significant between-group absolute differences in the AAPC in hospitalizations per 1000 person-years by sex (female vs male; 0.53; 95% CI, −1.11 to 2.17; P = .53), residence (rural vs urban; −0.62; 95% CI, −2.63 to 1.40; P = .55), or material resources (quintile 5 vs 1; −0.93; 95% CI −3.80 to 1.93; P = .52).
Conclusions and Relevance In this population-based cohort study of children in a universally funded health care system, inequalities in bronchiolitis ED visit and hospitalization rates did not improve over time.
Quantifying and assessing health inequalities in a population is important as health care systems seek to reduce health inequities. 1 - 3 The World Health Organization defines health inequalities as “differences in health status or the distribution of health determinants between different population groups.” 4 Health inequalities are assessed by comparing differences in health outcomes among population groups. Health inequalities may be due to biological variations, personal preferences, or an individual’s environment and conditions outside their control. When the differences in health outcomes are avoidable or deemed unfair and unjust, they are termed health inequities. 5 Equity stratifiers, also called dimensions of inequality, are characteristics chosen to investigate perceived inequalities. The PROGRESS (place of residence, race/ethnicity/culture/language, occupation, gender/sex, religion, education, socioeconomic status, and social capital) framework summarizes the equity stratifiers most frequently used when grouping individuals into population subgroups to measure health inequalities 6
Bronchiolitis is a common cause of childhood emergency department (ED) visits and hospitalizations and is among the costliest conditions in pediatric hospital care. Health inequalities in bronchiolitis may occur for several reasons. 7 , 8 Material hardship, rural residence, and male sex have been associated with greater health care use and poorer outcomes. 6 , 9 - 14 Although several studies have examined health inequalities in bronchiolitis outcomes, 15 - 19 few studies have examined bronchiolitis ED visit and hospitalization inequalities over time using population-based data. 20 Such research is important to understand trends in inequality and whether the inequality gap is narrowing or widening. Therefore, the objective of this study was to conduct a population-based cohort study within a universally funded health care system in Ontario, Canada, to examine temporal trends in bronchiolitis-related ED visit and hospitalization rates from 2004 to 2022. Specifically, we examined trends in bronchiolitis outcomes by equity stratifiers: sex, residence location, and material resources.
We conducted a population-based cohort study using a repeated cross-sectional study design to examine trends in bronchiolitis-related ED and hospitalization rates by sociodemographic factors in Ontario, Canada. In a similar approach to previous studies, 13 , 14 the data sources were provincial health administrative databases housed at ICES (formerly known as the Institute for Clinical Evaluative Sciences), an independent, nonprofit research institute whose legal status under Ontario’s health information privacy law allows it to collect and analyze health data for health system evaluation without patient consent. Data were collected from children younger than 2 years from April 1, 2004, to March 31, 2022. Annual (April 1 to March 31) rates of bronchiolitis ED visits and hospitalizations were determined for the entire study period. The trend analysis, which determined the average annual percentage change (AAPC) in rates and differences in AAPC for the equity stratifiers, focused on April 1, 2004, to March 31, 2020. We did not include the period from April 1, 2020, to March 31, 2022 (the last 2 annual time points), in the trend analysis given the unprecedented reduction in bronchiolitis incidence during the early COVID-19 pandemic period. Research ethics board approval was obtained from The Hospital for Sick Children. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology ( STROBE ) reporting guideline. 21
All children younger than 2 years who were residents of Ontario and enrolled in the provincially funded Ontario Health Insurance Plan (OHIP) were included. Data from all children’s and community hospitals in the province were included. Bronchiolitis encounters were identified using International Statistical Classification of Diseases and Related Problems, Tenth Revision, Canada ( ICD-10-CA ) discharge diagnosis codes for bronchiolitis, including both respiratory syncytial virus (RSV) and non-RSV causes, and whether bronchiolitis was the primary or secondary discharge diagnosis (eMethods in Supplement 1 ). Viral pneumonia diagnosis codes (eg, RSV, influenza, and adenovirus pneumonia) were also included because viral pneumonia presents similarly and is managed similarly to bronchiolitis in children younger than 2 years.
Health administrative databases, 22 linked using unique encoded identifiers derived from the OHIP number of every resident in Ontario with public health insurance, were used (eMethods in Supplement 1 contains information on the databases). The Registered Persons Database includes demographic information on all Ontarians registered for public health insurance and was used to obtain age, sex, and residence postal code. The National Ambulatory Care Reporting System database was used to obtain ED visit data, the Canadian Institute for Health Information Discharge Abstract Database was used for hospitalization data, and the MOMBABY database was used to capture birth records for inpatient births (the database captures more than 97% of all births in Ontario). 23
The study population was described by the following characteristics: sex, gestational age, and comorbidity status. Comorbidity status was determined by the presence of a comorbidity ICD-10-CA discharge diagnosis code on a hospital encounter from birth to 2 years. Additionally, procedural codes were used for children with a complex chronic condition (eg, gastrostomy), following the Canadian Institute for Health Information methods. 24 Comorbidities were not mutually exclusive and included complex chronic condition, 24 , 25 chronic lung disease or bronchopulmonary dysplasia arising from the perinatal period, 18 congenital heart disease, 26 neurologic impairment, 27 immunodeficiency, and trisomy 21. 26
Health inequalities were determined for the following equity stratifiers: sex (male or female), geography (rural or urban residence), and material resources quintile. Race and ethnicity data are not available through Ontario health administrative databases. The primary residence location was assessed using the Rurality Index of Ontario, which defines neighborhoods into urban (score of <40) or rural (score of ≥40). 28 The Rurality Index of Ontario score is calculated using community population size, density, and travel time to the nearest basic and advanced health care referral centers. We used the 2008 version of the rurality index, which is the most recent version available and currently used by the Ontario government. Material resources is 1 of 4 dimensions of the Ontario Marginalization Index that is used as a comprehensive area-based measure of socioeconomic status and poverty at the neighborhood level. 29 It is based on level of education (proportion of population 25 years or older without a certificate, diploma, or degree), family structure (proportion of lone-parent families), housing quality (proportion of homes needing a major repair), employment (proportion of population 15 years or older who are unemployed), and income (proportion of population below the low-income cutoff and proportion receiving government transfer payments). Material resources quintiles were created at the dissemination area level and assigned to individual records, with quintile 1 representing the most resourced (ie, least deprived) and quintile 5 representing the least resourced (ie, most deprived). The material resources index is available for 2001, 2006, 2011, 2016, and 2021 for tracking changes over time.
Bronchiolitis outcomes included the population-based bronchiolitis ED visit and hospitalization rate. Outcomes were estimated annually (April 1 to March 31) and for the entire study period (April 1, 2004, to March 31, 2022). The population-based ED visit rate was estimated per 1000 person-years with 95% CIs. The numerator was the total number of bronchiolitis ED visits for all children younger than 2 years, and the denominator was the number of person-years contributed by all children younger than 2 years in that period. The hospitalization rate was estimated per 1000 person-years with 95% CIs. The numerator was the total number of bronchiolitis hospitalizations for all children younger than 2 years, and the denominator was the number of person-years contributed by all children younger than 2 years in that period. Crude rates were estimated because the focus was on understanding the health system and population burden of bronchiolitis hospital use.
We used joinpoint regression analysis to examine temporal trends in bronchiolitis ED visit rates and hospitalization rates by sex, residence location, and material resources quintile from April 1, 2004, to March 31, 2020. 30 The joinpoint regression software calculates the number and temporal location of points representing a statistically significant change in trend (ie, a joinpoint). 31 We used Joinpoint, version 5.0.2 (National Cancer Institute) to run the joinpoint regression models, starting with a model with 0 joinpoints (a linear relationship) and testing whether 1 or more joinpoints should be added to the final model. We selected the grid search method for fitting the model and the permutation test (n = 4499 permutations) for determining the optimal number of joinpoints. We selected log transformation to calculate annual average percent change (AAPC), the average rate of change in ED rate (or hospitalization rate) per year during the study period. The AAPC was tested against the null hypothesis that the percent change was zero (no increase or decrease over time). A pairwise comparison between trends (with the null hypothesis that the 2 lines are parallel) was performed to examine whether the rate of change (AAPC) differed between groups. A 95% CI around the absolute difference in the AAPC between groups (eg, male vs female) that includes 0 indicates no increase or decrease in the inequality gap over time between groups. Statistical significance was defined as P < .05; all statistical tests were 2-sided.
The study cohort included 2 921 573 children, of whom 1 499 485 (51.3%) were male and 1 422 088 (48.7%) were female, 545 378 (18.7%) were preterm and/or had a comorbidity, 105 189 (3.6%) had a chronic complex condition, and 2 619 139 (89.6%) lived in an urban location ( Table 1 ). Bronchiolitis ED visits occurred at 214 hospitals (185 [86.4%] community and 29 [13.6%] pediatric or teaching) and hospitalizations at 151 hospitals (134 [88.7%] community and 17 (11.2%) pediatric or teaching).
During the study period, there were 141 045 bronchiolitis ED visits for an overall population-based ED visit rate of 27.8 (95% CI, 27.6-27.9) per 1000 person-years. eTables 1-3 in Supplement 1 provide the annual ED visit rates by equity stratifier and rate ratios with 95% CIs. The ED visit rates were lower for girls than boys, greater for children living in a rural location than an urban location, and greater for children with the least material resources. The ED visit rates increased from years 2004-2005 to 2019-2020. The 2020-2021 rates saw a marked reduction associated with the pandemic; in 2021-2022 rates showed an increase but not to prepandemic rates. There was no evidence of a significant joinpoint for sex, residence location, or material deprivation quintile, indicating that the observed increases in ED visit rates were linear over time to 2019-2020 ( Figure 1 ).
The ED visit rates increased over time for both boys (AAPC, 2.38 [95% CI, 1.48-3.30] visits per 1000 person-years; P < .001) and girls (AAPC, 2.60 [95% CI, 1.76-3.45]) visits per 1000 person-years; P < .001), with no significant between-group difference in the rate of change (0.22 [95% CI, −0.92 to 1.35] visits per 1000 person years; P = .71) ( Figure 1 and Table 2 ).
The ED visit rates increased over time for both children with an urban (AAPC, 2.53 [95% CI 1.72-3.34] visits per 1000 person-years; P < .001) and rural (AAPC, 2.22 [95% CI, 0.94-3.52]) visits per 1000 person-years; P = .002) residence, with no significant between-group difference in the rate of change (−0.31 [95% CI, −1.70 to 1.09] visits per 1000 person-years; P = .67) ( Figure 1 and Table 2 ).
The ED visit rates increased for all material resources quintiles, with the greatest increase for the group with greatest material resources (quintile 1) (AAPC, 3.07 [95% CI, 1.83-4.32] visits per 1000 person- years; P < .001) and lowest for the group with lowest material resources (quintile 5) (1.89 [95% CI, 1.00-2.79] visits per 1000 person-years; P < .001) ( Figure 1 and Table 2 ). However, there were no statistically significant between-group differences in the rate of change for each quintile compared with the group with the greatest material resources (eg, quintile 5 vs 1: −1.17; 95% CI, −2.57 to 0.22; P = .10) ( Table 2 ).
There were 58 215 bronchiolitis hospitalizations during the study period for an overall hospitalization rate of 11.4 (95% CI, 11.4-11.6) hospitalizations per 1000 person-years. As displayed in Figure 2 , there was no evidence of a significant joinpoint for sex and residence location, indicating that the observed stable hospitalization rates were linear over time to 2019-2020. There was 1 joinpoint for material resources quintiles 4 and 5, which was not statistically significant. Similar to ED rates, hospitalization rates in 2020-2021 saw a marked reduction associated with the pandemic, and then the 2021-2022 rates showed an increase, but not to prepandemic rates.
Hospitalization rates were lower for girls compared with boys (eTables 4-6 in Supplement 1 ). Hospitalization rates were stable over time for both boys (AAPC, 0.22 [95% CI, −0.94 to 1.40] visits per 1000 person-years) and girls (AAPC, 0.75 [95% CI, −0.60 to 2.12] visits per 1000 person-years), with no significant between-group difference in the rate of change (0.53 [95% CI, −1.11 to 2.17] visits per 1000 person-years; P = .53) ( Figure 2 and Table 3 ).
Hospitalization rates were greater for children living in a rural location compared with an urban location (eTable 5 in Supplement 1 ). Hospitalization rates were stable over time for both children with an urban (AAPC, 0.52 [95% CI, −0.65 to 1.70] visits per 1000 person-years) and rural (AAPC, −0.10 [95% CI, −1.95 to 1.79] visits per 1000 person-years) residence, with no significant between-group difference in the rate of change (−0.62 [95% CI, −2.63 to 1.40] visits per 1000 person-years; P = .55) ( Figure 2 and Table 2 ).
During the study period, the hospitalization rate was lowest for the group with the greatest material resources (quintile 1) (eTable 6 in Supplement 1 ). The hospitalization rate was stable for all material resources quintiles, and there were no statistically significant between-group differences in the rate of change for each quintile compared with the group with greatest material resources (eg, quintile 5 vs 1: −0.93; 95% CI −3.80 to 1.93; P = .52) ( Figure 2 and Table 2 ).
This population-based cohort study examined trends in bronchiolitis-related ED visit and hospitalization rates by sociodemographic factors in a universally funded health care system from 2004 to 2022. Crude rates were highest for boys, rural residents, and those with the least material resources. We found no significant differences in the AAPC in the ED visit and hospitalization rates between groups based on sex, residence, and material resources. These data indicate that health inequalities among children with bronchiolitis in Ontario (based on an examination of sex, location of residence, and material resources as potential equity stratifiers) did not improve over time. Although health inequalities also did not get worse over time, the existence of inequalities suggests that we need to reallocate effort and resources to those groups experiencing poorer outcomes to improve children’s health.
Few previous studies 15 , 20 have examined trends in bronchiolitis inequalities to examine whether the inequality gap is narrowing or widening. Fujiogi et al 15 examined bronchiolitis hospitalizations in the US from 2000 to 2016 in a serial cross-sectional analysis using the Kids’ Inpatient Database. In contrast to our data source, the Kids’ Inpatient Database does not contain data on the denominator of at-risk children. Thus, Fujiogi et al 15 could not determine population-based hospitalization rates by equity stratifiers to examine temporal trends. Chung et al 20 reported increasing hospitalization rates in Scotland from 2001 to 2016, with a hospitalization rate of 45 per 1000 children in the most deprived group vs 23 per 1000 in the least deprived group. In contrast to our findings, Chung et al 20 found a greater increase in the hospitalization rate in the most deprived compared with the least deprived group during 5 years, comparing 2011 with 2015. Other studies 16 , 19 , 20 , 32 - 35 quantify inequalities at one time point rather than examining trends in inequalities. These studies found higher rates of bronchiolitis ED visits or hospitalization based on race, Indigenous status, material deprivation, health care insurance provider, and maternal age in the US, Canada, New Zealand, and the UK.
Health system or bronchiolitis-specific interventions implemented during the study period might have affected bronchiolitis ED or admission rates. Between 2001 and 2006, the Ontario government introduced new primary care models characterized by group-based care, physician remuneration by salary (vs fee for service), pay for performance, and a requirement for after-hours services. 36 These primary care interventions are aimed at increasing primary care access and reducing ED visits and hospitalizations. However, we observed an increase in bronchiolitis-related ED visits over time.
For this 18-year study, we observed persistent health inequalities in bronchiolitis ED visit and hospitalization rates. Several underlying factors may contribute to the sustained inequality. Disparities in bronchiolitis outcomes based on sex have been reported in previous studies. 11 , 13 , 15 , 37 Both biological (ie, smaller airways in males and hormonal and immunologic difference) and possibly social factors have been suggested as explanations for higher rates of ED visits, hospitalizations, and severity of illness in males. 37 - 39 Residents of rural areas often face chronic health care infrastructure limitations from primary to tertiary care, leading to inadequate health care access and/or delayed medical care. 40 Socioeconomic disparities, including material deprivation, remain a pervasive issue, creating barriers to access and timely use of health care services. 41 , 42 Emerging preventive interventions hold promise in addressing inequalities in sex, residence location, material resources, and other equity stratifiers (eg, race and/or ethnicity). Vaccination for RSV and new RSV monoclonal antibodies (eg, nirsevimab) could prevent a substantial proportion of RSV-related bronchiolitis and, if implemented equitably, may narrow the health inequalities gap. 43 - 46 Evaluation of inequalities after implementation is important because it is possible that these interventions could also increase disparities in bronchiolitis outcomes if access to them is not equitable.
Several limitations to this study are important to consider. Data on individuals’ race, ethnicity, or indigeneity were unavailable in the data source; thus, we could not describe trends in health inequalities based on these important strata. Children with comorbidities are at risk for severe bronchiolitis and may not have been equally distributed across the groups examined. Rurality was measured using the most recent version of the rurality index of Ontario from 2008. It is possible that some jurisdictions moved from rural to urban since 2008. Such misclassification could bias the results toward finding less inequality. Our study focused on ED visits and hospitalization outcomes. Further studies examining inequalities in other important outcomes, such as length of stay and intensive care unit admission, are needed. Lastly, the findings of this study may not be generalizable to jurisdictions without universal health care coverage.
This population-based cohort study in a universally funded health care system found that inequalities in bronchiolitis ED and hospitalization rates from 2004 to 2022 did not improve. Future research should evaluate whether emerging preventive interventions, such as RSV vaccination and newer monoclonal antibodies, narrow the bronchiolitis inequalities gap.
Accepted for Publication: February 28, 2024.
Published: April 29, 2024. doi:10.1001/jamanetworkopen.2024.8976
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2024 Mahant S et al. JAMA Network Open .
Corresponding Author: Sanjay Mahant, MD, MSc, Department of Pediatrics and Institute for Health Policy, Management, and Evaluation, University of Toronto, The Hospital for Sick Children Research Institute, Peter Gilgan Centre for Research and Learning, 686 Bay St, Toronto, ON M5G 0A4, Canada ( [email protected] ).
Author Contributions: Drs Mahant and Borkhoff had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Drs Mahant and Borkhoff made equal contributions to the manuscript as first authors.
Concept and design: Mahant, Borkhoff, Parkin, Imsirovic, Gill.
Acquisition, analysis, or interpretation of data: Mahant, Borkhoff, Parkin, Imsirovic, Tuna, Macarthur, To.
Drafting of the manuscript: Mahant, Borkhoff, Macarthur, Gill.
Critical review of the manuscript for important intellectual content: All authors.
Statistical analysis: Borkhoff, Imsirovic, Tuna, Gill.
Administrative, technical, or material support: Mahant, Tuna, To.
Supervision: Mahant, Tuna.
Conflict of Interest Disclosures: Dr Parkin reported receiving grants from The Hospital for Sick Children Foundation and Canadian Institutes of Health Research and nonfinancial support from Mead Johnson outside the submitted work. Dr Tuna reported receiving grants from The Ottawa Hospital Research Institute during the conduct of the study. Dr Gill reported receiving grants from Physicians’ Services Incorporated Foundation, Canadian Institutes of Health Research, grants from The Hospital for Sick Children, serving as a member of the Canadian Institutes of Health Research’s Institute of Human Development Child and Youth Health advisory board, and serving on the EBMLive Steering Committee outside the submitted work. No other disclosures were reported.
Funding/Support: This study was supported by the Ontario Child Health Support Unit and by ICES, which is funded by an annual grant from the Ontario Ministry of Health and the Ministry of Long-Term Care. This manuscript used data adapted from the Statistics Canada Postal Code Conversion File, which is based on data licensed from Canada Post Corporation, and/or data adapted from the Ontario Ministry of Health Postal Code Conversion File, which contains data copied under license from Canada Post Corporation and Statistics Canada.
Role of the Funder/Sponsor: The Ontario Child Health Support Unit provided methodologic support for the study; otherwise, the funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Group Information: The members of the Canadian Paediatric Inpatient Research Network are listed in the eAppendix in Supplement 1 .
Disclaimer: Parts of this material are based on data and information compiled and provided by the Canadian Institute for Health Information and the Ontario Ministry of Health. The analyses, conclusions, opinions, and statements expressed herein are those of the authors and do not necessarily reflect those of the funding or data sources; no endorsement is intended or should be inferred.
Data Sharing Statement: See Supplement 2 .
Additional Contributions: Shan Dhaliwal, BSc, ICES, Ottawa, Ontario, Canada, made substantial contributions to the data analysis and did not receive payment for this work.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
Women are less likely to die when treated by female doctors, study suggests
Hospitalized women are less likely to die or be readmitted to the hospital if they are treated by female doctors, a study published Monday in the Annals of Internal Medicine found.
In the study of people ages 65 and older, 8.15% of women treated by female physicians died within 30 days, compared with 8.38% of women treated by male physicians.
Although the difference between the two groups seems small, the researchers say erasing the gap could save 5,000 women’s lives each year.
The study included nearly 800,000 male and female patients hospitalized from 2016 through 2019. All patients were covered by Medicare. For male hospitalized patients, the gender of the doctor didn’t appear to have an effect on risk of death or hospital readmission.
The data alone doesn’t explain why women fare better when treated by other women. But other studies suggest that women are less likely to experience “miscommunication, misunderstanding and bias” when treated by female doctors, said lead study author Dr. Atsushi Miyawaki, a senior assistant professor of health services research at the University of Tokyo Graduate School of Medicine.
The new research is part of a growing field of study examining why women and minorities tend to receive worse medical care than men and white patients. For example, women and minority patients are up to 30% more likely to be misdiagnosed than white men.
“Our pain and our symptoms are often dismissed,” said Dr. Megan Ranney, dean of the Yale School of Public Health. “It may be that women physicians are more aware of that and are more empathetic.”
Research shows that women are less likely than men to receive intensive care but more likely to report having negative experiences with health care, having their concerns dismissed, and having their heart or pain symptoms ignored, the authors wrote in the new study. Male physicians are also more likely than female doctors to underestimate women’s risk of stroke .
Part of the problem, Miyawaki said, is that medical students get “limited training in women’s health issues.”
Dr. Ronald Wyatt, who is Black, said his 27-year-old daughter recently had trouble getting an accurate diagnosis for her shortness of breath. An emergency room physician told her the problem was caused by asthma. It took two more trips to the emergency room for his daughter to learn that she actually had a blood clot in her lungs, a potentially life-threatening situation.
“There is a tendency for doctors to harbor sexist stereotypes about women, regardless of age, such as the notion that women’s symptoms are more emotional or their pain is less severe or more psychological in origin,” said Wyatt, former chief science and chief medical officer at the Society to Improve Diagnosis in Medicine, a nonprofit research and advocacy group.
Women seem to experience fewer of these problems when treated by other women.
For example, a study published JAMA Surgery in 2021 found that women patients developed fewer complications if their surgeon was female. Another JAMA Surgery study published in 2023 found all patients had fewer complications and shorter hospital stays if they were operated on by female surgeons, who worked more slowly than their male counterparts.
Women primary care doctors also tend to spend more time with their patients , Ranney said. Although that extra attention is great for patients, it also means that women see fewer patients per day and earn less, on average, than male doctors.
Dr. Ashish Jha, dean of the Brown University School of Public Health, said several studies suggest that female doctors follow medical evidence and guidelines , and that their patients have better outcomes.
“There’s lots of variation between women and men physicians,” said Jha, who was not involved in the new study. Women “tend to be better at communication, listening to patients, speaking openly. Patients report that communication is better. You put these things together, and you can understand why there are small but important differences.”
The authors of the study said it’s also possible that women are more forthcoming about sensitive issues with female physicians, allowing them to make more informed diagnoses.
That doesn’t mean that women should switch doctors, said Dr. Preeti Malani, a professor of medicine at the University of Michigan. For an individual patient, the differences in mortality and readmission rates seen in the new study are tiny.
“It would be a mistake to suggest that people need to find physicians of the same gender or race as themselves,” Jha said. “The bigger issue is that we need to understand why these differences exist.”
Malani said she’s curious about what women doctors are doing to prevent patients from needing to be readmitted soon after discharge. “How much care and thought is going into that discharge plan?” Malani asked. “Is that where women are succeeding? What can we learn about cultural humility and asking the right questions?”
Others aren’t convinced that the new study proves a physician’s gender makes a big difference.
Few hospitalized patients are treated by a single doctor, said Dr. Hardeep Singh, a professor at Baylor College of Medicine in Houston and a patient safety researcher at the Michael E. DeBakey VA Medical Center.
Hospital patients are treated by teams of physicians, especially if they need specialist care, in addition to nurses and other professionals, Singh said.
“How often do you see the same doc every day in the hospital?” Singh asked. “The point is that it’s not a one-man or one-woman show. Outcomes are unlikely to depend on one individual, but rather on a clinical team and the local context of care. … One name may appear on your bill, but the care is team-based.”
However, Singh said his research on misdiagnoses shows that doctors in general need to do a better job listening to patients.
Jha said he’d like the health system to learn what women doctors are doing right when they treat other women, then teach all physicians to practice that way.
“We should train everyone to be better at generating trust and being worthy of trust,” Jha said.
Wyatt said the country needs to take several steps to better care for women patients, including “de-biasing training” to teach doctors to overcome stereotypes. The health care system also needs to increase the number of women physicians in leadership, recruit more female doctors and do a better job at retaining them. All physicians also need more understanding of how adverse childhood experiences affect patient health, particularly for women, he said.
“More than once I’ve had white female patients tell me they came to be because I listened and they trusted me,” Wyatt said.
Liz Szabo is an independent health and science journalist. Her work has won multiple national awards. One of her investigations led to a new state law in Virginia.

UCL Queen Square Institute of Neurology
- Equality, Diversity & Inclusion
- Translation & Enterprise
- Building your career
- Sustainability

Study finds half of people living with Dravet Syndrome experience feeding problems
30 April 2024
We are pleased to share the results of our research partnership with Dravet Syndrome UK, exploring an aspect of living with Dravet Syndrome which is rarely talked about but has a huge impact on family life.

A study to explore the impact of feeding difficulties in children and adults living with Dravet Syndrome, found that over half (52%) reported that they had experienced some form of feeding difficulty and some experienced many different problems with feeding. The findings also showed that around 1 in 5 people with Dravet Syndrome may require a feeding tube (called a gastrostomy) at some point during their life to help address the feeding issues.
The research conducted with Dravet Syndrome UK revealed that 88% of carers of someone with Dravet Syndrome had a high level of concern and fear about having a feeding tube fitted and that there was a lack of information available. However, once the gastrostomy was inserted, the opinions of caregivers completely changed with 88% happy that their child had the gastrostomy, and more than 90% agreed that they had a better-quality life, better nutrition, got their medication on time and that caregivers found that it was easy to use.
Lisa Clayton, Clinical Research Fellow, Department of Clinical and Experimental Epilepsy, UCL Queen Square Institute of Neurology and Neurology Registrar, who led this research said: “We found that feeding problems were common in people living with Dravet Syndrome and can emerge at any age. Severe problems with eating can be particularly worrying for caregivers as it can affect both nutrition, hydration and giving medicines to the person they care for. It is vital that health professionals have early discussions with caregivers about feeding issues to avoid unnecessary delays in intervention if needed.”
Galia Wilson, Chair of Trustees, Dravet Syndrome UK, said: This important research highlights one of the many challenges faced by children and adults living with Dravet Syndrome and their families on a daily basis. Our strategy is to create a better understanding of the realities of caring for people with Dravet Syndrome, and to empower families to access the support they need by providing an evidence base through studies like this.
Underlying causes and risk factors for feeding issues in people living with Dravet Syndrome are currently unknown and are likely to be complex. More research is needed to explore the causes and find ways to try and address them.
Taliah's story
Edwina Williams, mother of Taliah who lives with Dravet Syndrome, contributed to this research as her daughter had feeding problems for a number of years and then had a gastrostomy fitted. She said: “As a parent you always want to do the best for your child, but sometimes it is difficult to figure out what the best thing is. As time went on, Taliah’s feeding issues worsened, and I was watching my daughter wither away in front of my eyes. This is when I contacted her medical team to arrange for her to have a feeding tube fitted as I knew that we needed help. It has been a long journey, but I can honestly say that the feeding tube has been the absolute best thing for Taliah, and our family. She is no longer dehydrated, she no longer sleeps for most of the day as her body has the correct nutrients, her hair is glossy, and she is a chatterbox now too!”

About the research
The anonymous, cross-sectional survey regarding feeding difficulties and gastrostomy was developed by adult neurologists working with individuals with Dravet Syndrome, alongside representatives, and caregivers, from Dravet Syndrome UK (DSUK). The survey was shared through email and private Facebook group to DSUK-registered families, and open for responses between 7th and 28th January 2022. Survey questions were multiple choice, including Likert scale responses, with opportunities for open-ended responses. 124 families took part in the survey.
Clayton et al. Feeding Difficulties and Gastrostomy in Dravet Syndrome : A UK-Wide Survey and 2-Center Experience . Neurology: Clinical Practice 2024;14:e200288. doi:10.1212/CPJ.0000000000200288
- Dravet Syndrome UK (DSUK)
- Dr Lisa Clayton's academic profile
- Dr Lisa Clayton explains the results of her study looking at feeding issues in Dravet Syndrome
YouTube Widget Placeholder https://www.youtube.com/watch?v=41mPbNP4uHw
Study in Wageningen! The university is part of Wageningen University & Research and is the only university in the Netherlands to focus specifically on the theme ‘healthy food and living environment’. We do so by working closely together with governments and the business community.

Wageningen University & Research offers bachelor's programmes in the domain of healthy food, agriculture and living environment.

Learn more about the study programmes Wageningen University & Research offers in the fields of life, social and environmental sciences.

Study orientation
We offer you an overview of all upcoming events here below!

Education for Professionals
With innovative programmes, from MBAs to online courses, our professors translate their cutting edge research into actionable knowledge and skills.

Summer School
Enjoy summer and pick up the latest scientific insights, either at the Wageningen Campus or online.

On this page you can find information on how to support your son or daughter in choosing the right study programme and how you can help them in the first year of studying.

IMAGES
VIDEO
COMMENTS
Access Subject Schedule by Enrollment. Prior to each study visit, the research team must be prepared for all known and unknown tasks that may need to be completed per protocol. If applicable, physician orders need to be completed and authorized for lab draws, study medication and additional testing; research lab kits should be prepared and ...
Study Participant Visit Checklist. This template helps track a research participant's study visit to ensure that protocol-designated procedures for each visit are completed. Access this template. Study Participant Telephone Call Log. This template can be used to document study-specific conversations with or about a study participant.
The visit flow provides an overview of the activities that take place at each study visit, and may be customized for each study site. Study Drug/Investigational Product Tracker (MS Excel, 12K) - Used to track study drug/investigational product disposition and accountability by the clinical research site. For multi-site studies under an ...
Regular site monitor visits can be broken down into four types: pre-study visits, initiation visits, periodic monitoring visits, and close-out visits. Study sites may also be monitored or audited by the FDA, Clinical Research Organizations (CROs), IRBs and sponsors. For more information on site audits by outside entities please visit the training documents on Quality Management: Audits.
A research study visit can take anywhere from 1 hour to 6-7 hours, depending on the procedures involved. Please discuss the expected time with the study coordinator. Sometimes, visits can be broken up into multiple days or breaks can be scheduled in for the longer visits. Depending on the study, some procedures may be able to be completed ...
Purpose The purpose of source documentation is to reconstruct the research study as it happened. It should allow for data to be confirmed, validated, and support the fundamental principle of protecting the participant's rights, safety and well-being. These templates are intended to assist in documenting study procedures, interventions, observations, etc. Below are several examples that […]
This visit will confirm that you meet the study requirements. It includes a more detailed review of your medical history and a physical exam. ... Taking part in a research study is different from regular medical care. When in a study, you'll primarily interact with the study team—the study doctor, nurses, and potentially others who work ...
OFFICE OF RESEARCH COMPLIANCE 11100 Euclid Avenue, Lakeside 1400 Cleveland, Ohio 44106 Phone: (216) 286-2283 Version: December 2020 Site Initiation/Study Start-Up Visit Tip Sheet A Site Initiation Visit (SIV) or Study Start-Up is an organized meeting to discuss the new protocol before
FDA believes that obtaining a research participant's verbal or written informed consent is only part of the process. Informed consent involves providing a potential participant with: adequate ...
Issue 98 March 2016 39. Hints and Tips: Advice for an academic study vis it. Philip I.N. Ulrich. Studying for a PhD requires learning new skills and building a body of research for a thesis. One ...
NIH conducts clinical research trials for many diseases and conditions, including cancer, Alzheimer's disease, allergy and infectious diseases, and neurological disorders. To search for other diseases and conditions, you can visit ClinicalTrials.gov. This is a searchable registry and results database of federally and privately supported ...
For each trial participant, the last on-treatment-study visit marks the participant's closeout, and remaining medications should be collected at the participating site. ... steering committees balance their hopes for pragmatism with special research interests, and a study protocol may be full of visit requirements including, questionnaires ...
The CREST Program aims to ensure that the reported clinical research study data are accurate, complete, and verifiable, the conduct of the study is in compliance with the study protocol, Good Clinical Practice (GCP) and the regulations of applicable agencies, and the rights and well-being of human subjects are protected, in accordance with 45 ...
Industry sponsors typically conduct an in-person or remote initiation visit with the entire study team prior to study start. In many cases the study coordinator is responsible for working with the sponsor or Clinical Research Organization (CRO) to set up this visit.
SOME OF THE TOOLS AND TEMPLATES ON THIS SITE MAY NOT BE THE MOST RECENT VERSION. CONTACT THE EQUIP OFFICE AT 617-355-5308 IF YOU HAVE QUESTIONS ABOUT WHETHER A NEWER VERSION IS AVAILABLE. The study tools and templates provided are intended to help research teams: Document required information. Organize study documents.
The Study Close-out Visit is a visit and process arranged by the sponsor of the research study to ensure that all necessary aspects of the study closure have been addressed, to include organization and completion of documentation and reporting. The study close-out visit occurs once participants are no longer receiving investigational treatment ...
prepare for the scheduled monitoring visit by ensuring that all clinical research study -related documents are current, organized, complete, and accurate prior to the monitoring visit. They will also ensure that all requested source documents and clinical research study -related documents are available to the monitor during the monitoring visit.
initial monitoring visit. If you were recently hired for a CRA position in a new pharmaceutical company, you would need to do the next steps prior to scheduling the first monitoring visit: - Familiarize with the company's general SOPs and Sponsor's study-specific SOPs (if applicable) relating to the clinical study initiation, conduct, and oversight;
On Study Visit Checklist - Sample. 1. (Note: If this CRF is used as a source document, it must be signed and dated by study personnel.) Visit ChecklistVersion 3.0. On Study Visit Checklist - Sample. STUDY NAME. Site Name: Pt_ID: Visit Date:
Recruiting study participants SOP. Subject screening log template. Follow up visits SOP. Subject visit log vaccine trial. Pre and post admission study team meetings SOP. Subject visit log any trial. Blood Sampling SOP. Subject withdrawal and termination log. Reimbursement of Study Subjects SOP. Pre-screening eligibility check template. Transfer ...
8600 Rockville Pike, Bethesda, MD 20894. ClinicalTrials.gov. An official website of the U.S. Department of Health and Human Services, National Institutes of Health, National Library of Medicine, and National Center for Biotechnology Information.
More than 500,000 people from around the world have participated in clinical research since the hospital opened in 1953. We do not charge patients for participation and treatment in clinical studies at NIH. In certain emergency circumstances, you may qualify for help with travel and other expenses Read more, to see if clinical studies are for you.
Clinical Subject Management. Last revised: 10/16/2018. Master Subject Log. Most clinical trials involve multiple study visits over an extended period of time. While it is very important to maintain subject privacy and confidentiality, a study coordinator's job also involves making sure that subjects come for their follow up visits on schedule.
How to Start a Clinical Research Study "My PI wants to conduct a clinical research study. Where do I begin?" ... Industry sponsors typically conduct an in-person or remote initiation visit with the entire study team prior to study start. Read more > Mock Run Through of a Patient Visit. More information about types of binders.
All clinical research starts with the research protocol, a document that details all aspects of the trial: its background, rationale, objectives, design, methodology, statistical analysis plan, and organization.With the protocol, you can make sure you protect the participants and collect the data. Using protocol templates, you can start thinking through what you need to meet compliance ...
13. You can be referring to two things, a visit to another institution (usually to give an invited talk) or you could mean a visiting student/researcher role: Someone who visits a different institution for some prolonged period of time, usually to do research. You may even be able to register for classes. It gives you the opportunity to work ...
During the study period, there were 141 045 bronchiolitis ED visits for an overall population-based ED visit rate of 27.8 (95% CI, 27.6-27.9) per 1000 person-years. eTables 1-3 in Supplement 1 provide the annual ED visit rates by equity stratifier and rate ratios with 95% CIs. The ED visit rates were lower for girls than boys, greater for ...
Although the difference between the two groups seems small, the researchers say erasing the gap could save 5,000 women's lives each year. The study included nearly 800,000 male and female ...
More research is needed to explore the causes and find ways to try and address them. Taliah's story Edwina Williams, mother of Taliah who lives with Dravet Syndrome, contributed to this research as her daughter had feeding problems for a number of years and then had a gastrostomy fitted.
Study in Wageningen! The university is part of Wageningen University & Research and is the only university in the Netherlands to focus specifically on the theme 'healthy food and living environment'. We do so by working closely together with governments and the business community.