- Department of Health and Human Services
- National Institutes of Health


Nursing at the NIH Clinical Center
Clinical research nurses are an essential component of the intramural clinical research program. The career path for Clinical Research Nurses includes a core component of advancement in research skills and supports graduate and post-graduate training. Research training for nurses, like its medical counterpart, is integrated into specialty practice training and supports progression through increasingly complex levels of clinical practice and research involvement. Nurses with advanced academic training and clinical practice experience serve in different capacities. Clinical Nurse Specialists support the development of specialty practice and improvement of patient outcomes in all our practice areas. Advanced practice nurses, including nurse consultants and nurse educators work in a variety of inpatient and ambulatory care settings. They collaborate with research teams to provide continuity of care for patients enrolled in clinical trials.
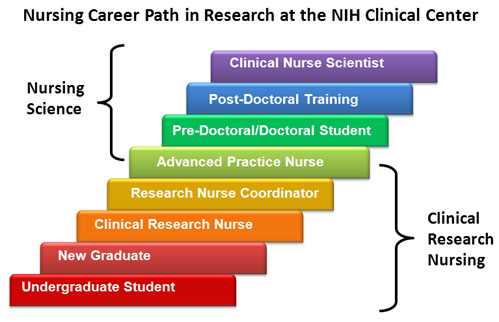
For more information on these roles visit Nursing Role Descriptions.
NOTE: PDF documents require the free Adobe Reader .
This page last updated on 04/08/2022
You are now leaving the NIH Clinical Center website.
This external link is provided for your convenience to offer additional information. The NIH Clinical Center is not responsible for the availability, content or accuracy of this external site.
The NIH Clinical Center does not endorse, authorize or guarantee the sponsors, information, products or services described or offered at this external site. You will be subject to the destination site’s privacy policy if you follow this link.
More information about the NIH Clinical Center Privacy and Disclaimer policy is available at https://www.cc.nih.gov/disclaimers.html
Resume Builder
- Resume Experts
- Search Jobs
- Search for Talent
- Employer Branding
- Outplacement
Research Nurse Job Description
Research nurse duties & responsibilities.
To write an effective research nurse job description, begin by listing detailed duties, responsibilities and expectations. We have included research nurse job description templates that you can modify and use.
Sample responsibilities for this position include:
Research Nurse Qualifications
Qualifications for a job description may include education, certification, and experience.
Licensing or Certifications for Research Nurse
List any licenses or certifications required by the position: BLS, CPR, HIPAA, CITI, IRB, CCRC
Education for Research Nurse
Typically a job would require a certain level of education.
Employers hiring for the research nurse job most commonly would prefer for their future employee to have a relevant degree such as Bachelor's and Associate Degree in Nursing, Education, Associates, School of Nursing, Communication, Graduate, Patient Care, Computer, Management, Medical
Skills for Research Nurse
Desired skills for research nurse include:
Desired experience for research nurse includes:
Research Nurse Examples
- Microsoft Word (.docx) .DOCX
- PDF Document (.pdf) .PDF
- Image File (.png) .PNG
- Assesses research subject
- Support the safety of study participants, and maintain communication with their families/caregivers and clinicians
- Provide clinical and administrative nursing support for research clinical trials based on scope of practice
- Administer medications and treatments per study protocol for out study participant research participants
- Identify, recruit, and enroll study participants
- Participate and host monitor visits
- Assist sponsor investigator research with Investigational New Drug/Investigational Device Exemption applications
- Incorporates and Clinical Research Services mission, vision, values and code of Business Conduct into planning patient care and clinical trials activities
- Provides clinical trial support in the Hospital and clinic setting to ensure patients are offered appropriate clinical trials following Good Clinical Practice standards
- Assesses and documents the patient's compliance and response to protocol treatment
- Clinical or Research experience in Pediatrics preferred
- Attention to detail a must ability to accurately prioritize workflow
- Screens patients/patient records to identify potential trial candidates and notify the treating physician and Clinical Trials support staff
- Provides Clinical Trial support staff with the documentation needed to determine eligibility and maintain compliance
- Enters applicable orders related to research specific tests and procedures
- Provides conduit between physicians, allied health professionals, nursing staff and clinical trial patients regarding trial related issues to maintain a safe environment for the patient while maintaining compliance
- Provides potential and registered clinical trial patients with contact information to ensure that patients and families have an avenue to direct questions about the clinical trial that they were offered
- Provides a consistent and accessible resource for physicians and clinical staff regarding clinical trials
- Completes mandatory education and training that includes review of age-specific needs and other competencies required
- Participates in the development of general goals
- Review (and adhere to) guidelines for each project
- Obtain HIPAA, IRB CITI course training certifications, and all other required training certifications
- Review (and adhere to) guidelines and protocols for each project
- Oversee and administer medications and treatments per study protocol for out study participant research participants
- May coordinate multi-site studies
- May collect, process, and ship lab specimens
- Three years of nursing experience required with related research experience in designated area of work preferred
- Computer and Internet experience required ‐ familiarity and comfort with MS Office products is essential for success in this position (Word and Excel‐ required
- Current chemotherapy/biotherapy certification preferred
- Manage essential regulatory documents and handle confidential information (verbal and written) with tact and diplomacy, adhering to all HIPAA guidelines
- Graduate of an accredited schools of nursing
- To screen and recruit new participants
- Ensure adherence to protocol, IRB, GCP and HIPAA (if applicable)
- Ascertain that study staff are working within IRB, GCP, and HIPAA (if applicable) guidelines
- To provide information, education and support to children, carers and families regarding the Project
- To ensure blood samples are collected for the study as required by the protocol
- The patient/family (and significant others) fully understand the nature of the Project
- The patient/family is aware that entry into the Project is voluntary and they can withdraw at any point without prejudice
- The participants are aware of any extra procedures required by the Project
- The consent/assent form is completed accurately and filed as required
- Ensure that the dignity, health, safety, and welfare of participants are given the highest priority at all times
- Be current with appropriate emergency certifications (ILS) and company emergency policy and procedures
- Utilize skills, knowledge, and clinical judgment in order to provide a high standard of care for participants in clinical trials
- Maintains an understanding of current regulatory requirements
- Typically 2-3 related experience
- Identify study locations (boxing and MMA gyms, athletic centers, ) to recruit subjects and meet with location management to arrange for study subject involvement
- Coordinate with study locations and Transcranial Doppler Ultrasound (TCD) technologist for optimal subject enrollment and execution of study evaluations
- Carry out study tests as outlined in protocol to include arterial blood pressure, reaction time, heart rate variability, Military Acute Concussion Evaluation (MACE), electrocardiogram, oxygen saturation, and autonomic testing using standard ICU monitor and non-invasive measurement attachments
- Maintain updated study documents in accordance with Good Clinical Practice guidelines including preparation of project reports and study amendments
- Collect data using clinical skills as required to include drawing blood, administering medications and operating critical care and data acquisition monitoring equipment
- Maintain and submit records for regular Institutional Review Boards and grant reviews coordinating activities for protocol compliance and implementation
- Utilising nursing assessment skills to observe participant general well-being and potential adverse events
- Oversee and manage day-to-day activities of the Phase II/III research nurses and research assistants
- Offer suggestions for improvement in processes and overall quality and monitors outcome of such if deficiencies are identified
- Assist with the process of training new research nurses
- Problem-solve clinical situations along with research nurses as they arise
- Assess compliance of the research nurses
- Monitor patient enrollment at sites through weekly and monthly reporting
- The minimum personnel background investigation will be a National Agency Check with Inquiries (NACI)
- Shall provide all information required for background checks to meet installation access requirements to be accomplished by installation Provost Marshal Office, Director of Emergency Services or Security Office corresponding to the location of performance
- Identify opportunities and develop action plans as necessary to increase patient accrual
- Assist in development and tracking of quarterly goals for each research nurse and clinic
- Meet with physicians and administrators as needed to assess performance of clinic
- Evaluate staffing needs and provides optimal coverage of research offices
- Communicate to the research nurses the overall performance standards and expectations day to day
- Make frequent visits to the sites
- Interview Research Nurse candidates and provides input to upper management
- Work with other team members in Clinical Operations department to ensure communication and responsibility are effective among the two groups
- Represent the company vision at the clinics
- Approve planned Paid Time Off (PTO) and submits benefit time usage to HR per payroll schedule
- Responsible for individual site goals, financial performance, quality assurance, oversight and contracted patient enrollment
- Assist Directors to ensure continuing education and training to investigators and coordinators
- Meet with supervisor weekly and submit brief clinic summaries
- Submit annual performance evaluations to supervisor for review and conduct the evaluations
- Administer medications and treatments per study protocol for research participants
- The research nurse coordinates and implements the research studies according to the study protocol
- The research nurse works with physicians, nurses and patients in the selection of and participation by appropriate patients
- The research nurse obtains all necessary approvals and informed consent forms
- The research nurse documents informed consent process and all components of the study procedures
- Using the nursing process, the research nurse delivers professional nursing care, including administration of the device during study procedures
Related Job Descriptions
Create a Resume in Minutes with Professional Resume Templates
I am an Employer
I am a candidate.
National Cancer Institute - Cancer.gov
Research Nurse - Clinical trials, Patient care
Job description.
We are looking for research nurses to join our clinical program to help us manage the care of patients participating in clinical trials. Duties include, but are not limited to, ensuring adherence to ethical practice in the conduct of clinical trials, research protocol compliance and good clinical practice, ensuring patient comprehension of informed consent, management of care for patients participating in clinical trials, and maintenance of essential documentation and collection of data.
We are the NCI’s internal, federally funded cancer center where world leading physician-scientists working on the cutting edge of medicine developing clinical trials of new sometimes “first-in-human” drugs and new treatment approaches. Closely knit clinical support teams are providing excellent quality of care of our patients and carrying out our clinical protocols with professionalism and compassion at the world’s largest dedicated research hospital on the NIH campus in Bethesda, Maryland. Join our unique and rewarding clinical team.
Qualifications and Job Details
Required and preferred skills.
Must have a degree from an accredited nursing program and a minimum of one (1) year oncology nursing and/or related clinical research experience. The ideal candidate will have experience in an oncology research setting, be highly organized, pleasant and energetic.
About the NCI Center for Cancer Research
The Center for Cancer Research (CCR) is home to nearly 250 basic and clinical research groups located on two campuses just outside of Washington, D.C. CCR is part of the National Cancer Institute (NCI) and makes up the largest component of the research effort at the National Institutes of Health (NIH). Centrally supported by long-term funding and a culture of complete intellectual freedom, CCR scientists are able to pursue the most important and challenging problems in cancer research. We collaborate with academic and commercial partners and advocacy groups across the world in efforts to prevent, diagnose and treat cancer and HIV/AIDS. The CCR research portfolio covers the full spectrum of biological and biomedical research. Our work ranges from basic to translational and clinical, and our clinical trials are conducted in the NIH Clinical Center, the world’s largest hospital dedicated to clinical research that offers a robust infrastructure to support CCR’s patients on an estimated 250 open studies. The success of CCR is grounded in an exceptionally strong discovery research program that provides the foundation for the seamless translation of insights from bench to bedside. Read more about CCR , the benefits of working at CCR and hear from our staff on their CCR experiences.
Bethesda is one of the most highly educated communities in the United States and has a nationally renowned school system. The city is a thriving suburban center close to Washington, D.C., and home to many restaurants, retailers and a flourishing arts and entertainment district.
- 3 References
- Cover Letter
To apply, please send your CV and a cover letter to Corrine Keen at [email protected]

Clinical Research Training For Nurses: A Guide to Becoming a Clinical Research Nurse
Clinical research training for nurses, guide to becoming a clinical research nurse, what is clinical nursing research.
Nurses are known for providing direct care for patients. However, nurses may take up roles that are completely new to them within the world of clinical research. These roles include clinical research coordinator , educator and manager. They can also take up less traditional role like regulatory specialist, study monitor and IRB (institutional board review) admin.
Regulatory specialist: their activities relate mainly with preparing regulatory documents and communicating with regulatory bodies. Nurses can work as a regulatory affairs specialist, a regulatory operation coordinator, or a regulatory coordinator. They can work within government agencies, pharmaceutical companies, academic medical centers.
Study monitor: they monitor clinical research practices and make sure that it complies with necessary research protocols and regulations. They tend work at government agencies, biotechnology companies, pharmaceutical companies, contract research organizations, device manufacturers etc.
Institutional Review Board (IRB) administrator: they are the professionals in charge of overseeing, administrating, implementing and managing IRB activities, like policies and procedures that relates to protecting human welfare. They can work at all IRBs: local, commercial or central IRB.
Nurses that have developed interest in the field of clinical research can join professional organizations. This provides them with the opportunity to network and continue their education through mediums like conferences, webinars, discussion groups, publications and online resources. These avenues serve as part of their clinical research training .
Certification is often a parameter used to measure professional expertise. This is based on criterion that reflects skill, knowledge, educational preparation, ability, and competence that are developed from experience in that area of specialization. Nurses that developed an interest in clinical research and have taken a clinical research training program have an opportunity to be certified through the:
Society for Clinical Research Professionals, Inc. (Certified Clinical Research Professionals)
Association for Clinical Research Professionals (Certified Clinical Research Associate or Certified Clinical Research Coordinator)
This field of clinical research gives nurses a chance, an opportunity to advance themselves professionally in a field that might not have been explored by them before. The benefits of having a registered nurse cover letter are insurmountable. This also provides a career path that can show family members the benefits of working in the medical field.
Nurses that have gone through the clinical research for nurses , otherwise called research nurse can carry out research on the various aspects of the human health, such as illness, pharmaceutical and health care methods and treatment plans. The main aim of this research is to improve the quality of health care service delivery. Helping patients and their family in a healthcare facility also brings a level of joy that is hard to find in many other career paths.
Roles of Research Nurses
They are responsible for designing and implementing research studies.
They observe procedures for treatment, collect and analyze data.
They report their research results to appropriate quarters.
They write articles and report their research findings in nursing or medical professional publications and journals.
They help in recruiting participants for studies and are involved in providing direct care for the participants.
Clinical research nurse salary can make use of their communication skills as well as their critical thinking skills gotten from their knowledge and experience in healthcare to further their career in this exciting way.
Know that future CRNs can speak to our 24/7 chat and phone advisors to request information on partial scholarships and payment plans for nurses.
2. Clinical Research Nurse Salary
The average pay for a Clinical Research Nurse is $31.28 per hour.
MD Anderson Cancer Center Clinical Research Nurse salaries - $71,503/yr
Northwestern University Clinical Research Nurse salaries - $75,005/yr
NIH Clinical Research Nurse salaries - $77,331/yr
CLINICAL RESEARCH NURSE JOB Description
A clinical research nurse conducts scientific research on different aspects of human health like illnesses, pharmaceuticals, treatment plans and healthcare methods. Their major goal is to improve the quality of healthcare services that are administered to the patients.
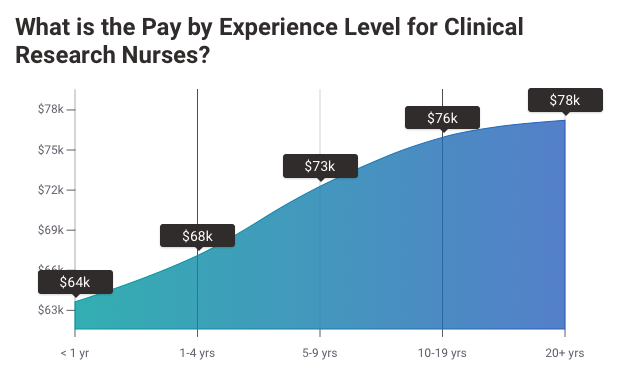
Source: Payscale
3. How do I get Clinical Research Nurse Experience?
Experience don’t just jump on you, you have to get it by practice. CCRPS affords you an opportunity to acquire knowledge in clinical research, and not just knowledge but experience as well. Registering for the appropriate course will boost your knowledge base and as well you get experience of clinical research first hand.
As a clinical research nurse, you will be at the forefront of new medical discoveries, and help develop breakthrough cures and medical treatments. The work that you do during your career can help some patients live longer or better quality of life. You may be responsible for studying diseases and disorders, as well as developing new treatment plans. You will also help test new treatments and medications that could possibly change the way a disease or disorder is perceived.
The field of clinical research can be very rewarding and fulfilling. A good research nurse is dedicated to their work and ready to take on everything that the profession throws their way. If you’re looking to pursue a research nursing career, you should have an excellent understanding of the research process as well as the specialty area that you’re studying.
Excellent communication skills are also a must. You must be able to effectively communicate with scientists, physicians, researchers, patients, and corporate executives.
4. What Does a Clinical Research Nurse Do?
The duties of a research nurse will typically depend on their employer and role. Some research nurses may be responsible for studying diseases, while others may help create and improve new medications and other treatments.
clinical research nursing scope and Standards of Practice
Clinical research nurses can take up clinical research jobs in institutions like research organizations, pharmaceutical companies, universities, research laboratories, government agencies and teaching hospitals.
The work that a research nurse does is quite exhaustive and it includes;
They use their knowledge of the basics of clinical research in designing and implementation of research studies.
Observation of the procedures for patient treatment, collection and analyzing of data.
They report their research findings to the relevant authorities. They may also have to present their results at health conferences and publish them in journals.
They write grant applications in order to secure funds to carry out the research.
They render assistance in the process of recruiting study subjects.
They provide direct treatment for research participants.
Research nurses that study diseases and illnesses will often perform a great deal of research, both by studying previous findings and observing patients. They may be required to examine medical journals, for instance, as well as observe, study, and care for patients suffering from a particular disease.
They make decisions based on the observations made as to which patients are the best candidates for certain clinical trials. During clinical trials , the research nurse will administer medications or perform other treatment procedures, During this process, research nurses must closely monitor each patient’s progress. This includes documenting side effects, drug interactions, and the overall efficiency of the medication.
Aside from caring for patients, documenting and recording information during clinical trials are the most important responsibility that a research nurse has. The information and data gathered during the research must be compiled into reports and handed over to senior clinical researchers or specialists.

5. How Do I Become a Research Nurse?
Don’t expect to become a research nurse overnight. It's a lot of work and you are expected to undergo years of training and experience.
The clinical research nurse job is a competitive one and certificates are not just handed out to anybody. The conditions to be eligible to take the certificate exam is that you must be an experienced registered nurse and your experience must include having thousands of hours of experience in the area of clinical research.
How to Become a Registered Nurse (RN) in 2020 that contains everything a person pursuing a nursing job should know - responsibilities, education, salaries and more.
The first step toward becoming a research nurse is to obtain a proper education. You can start with a bachelor’s degree in nursing, although many employers prefer that their research nurses have master’s degrees or even doctoral degrees in their chosen specialty. During your schooling, classes in research and statistics are a must and are courses in your chosen area of expertise.
According to clinical research job websites , many research nurses have a MSN degree and some have a PhD in nursing. Many of them attain these degrees of education in order to give them an edge on getting clinical research positions . While studying, courses in statistics and research are mandatory.
There are two main certifications that clinical research nurses can get from the Association of Clinical Research Professionals (ACRP). You can get certification to become a certified clinical research associate or you can choose to become a certified clinical research coordinator.
Take courses from CCRPS and learn more on how to become a clinical research nurse.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
6. Clinical Research Nurse Requirements and Certifications & Nursing Cover Letter
A bachelor's degree in nursing does meet licensure requirements for graduates to become registered nurses (RNs), which qualifies individuals for the specialized certification. Bridge programs, such as an RN-to-Bachelor of Science in Nursing (BSN), require previous nursing education for admission. Nursing students complete traditional classroom courses, laboratory experiences, and a clinical practicum in a medical setting, which includes a hospital, assisted living facility, and long term care center.
For specific education in clinical research , trained RNs enroll in graduate certificate and degree programs. There students are introduced to case studies, ethical research practices, and financial matters affecting the design, implementation, and funding of clinical research trials. In a master's program, studies in research ethics point students towards ethical research practices, including a discussion on human rights, misconduct, and conflicts of interest. Graduate programs will also include quantitative research and a capstone project.
All RN-to-BSN programs will require an RN license to enroll. Master's and graduate certificates will need a bachelor's degree with sufficient prerequisite coursework in the field. In addition, they will need letters of recommendation or reference, a personal statement, and GRE scores.
Becoming a nurse researcher which is a highly specialized career requires an advanced degree and training in informatics and research methodology and tools. The initial step for these individuals, or for any aspiring advanced practice nurse, is to earn a Bachelor of Science in Nursing degree and pass the NCLEX-RN exam. Once a nurse has completed their degree and attained an RN license, the next step is to complete a Master's of Science (MSN) in Nursing program with a focus on research and writing. MSN courses prepare nurses for a career in research and usually include coursework in statistics, research for evidence-based practice, design and coordination of clinical trials , and advanced research methodology.
A TYPICAL JOB POSTING FOR A RESEARCH NURSE POSITION WOULD LIKELY INCLUDE THE FOLLOWING QUALIFICATIONS, AMONG OTHERS SPECIFIC TO THE TYPE OF EMPLOYER AND LOCATION:
MSN degree and valid RN license.
Experience conducting clinical research, including enrolling patients in research studies, Implementing research protocol and presenting findings.
Excellent attention to detail required in collecting and analyzing data.
Strong written and verbal communication skills for interacting with patients and reporting research findings.
For a person to practice nursing legally, acquiring of nursing credentials and certifications is very important. For instance, some nurses who achieve a master's degree (MSN) leave the patient care aspect of nursing, and practice in a more managerial role.
CRA JOB OPPORTUNITIES
If you choose to become a Clinical Research Associate (CRA), you will have a key role in the success of clinical trials. Most CRAs have a nursing background, like yours. You will be the primary contact and support for trial sites, ensuring that the study is conducted according to the protocol, ICH-GCP, regulatory requirements and standard operating procedures (SOPs).
The Clinical Research Associates also offers you the unique opportunity to have an exciting career in the research of drug and medical device development while making a difference in the lives of those around them.
Take courses from CCRPS and learn more on how to become a clinical research professional.
Speak to our 24/7 chat and phone advisors to request information on partial scholarships and payment plans for nurses.

About CCRPS
How much is a clinical research coordinator’s salary.
Internet Explorer is no longer supported by Microsoft. To browse the NIHR site please use a modern, secure browser like Google Chrome, Mozilla Firefox, or Microsoft Edge.

The Role of the Clinical Research Nurse

Published: 17 May 2019
Version: 1.0 - June 2019
Clinical Research Nurses: In their own words
We spoke to nurses about their experience of working in this exciting space and the variety of roles our clinical research nurses undertake. All speak of having started their research careers with an uninformed view of what a research role could bring them.
All speak of their surprise at the autonomy of the role, the skills they have developed and the variety of work they undertake. All speak of working in great teams, the career opportunities that have opened for them and the importance of their relationships with the clinical research nursing community.
All speak of the challenges they have faced and overcome in research. And all speak of their passion for research. Most importantly they all speak of their crucial role in delivering high quality patient care.
Here are their stories, in their own words.
Building Local Research
Anne Suttling, Senior Research Nurse, Portsmouth Hospitals Trust
After qualifying as a nurse, I commenced a rotation programme for 18 months. I worked in surgery, medicine, Accident and Emergency, critical care and also coronary care – where I gained a permanent post. That’s where my love is in coronary care and cardiology.
After seven years, I needed a change but still wanted to remain in cardiology. That’s when the opportunity to set up a research study came up. On the first day I was faced with an empty six – bedded bay, on an empty ward and told this was the available space to set up the clinic. The study was a success and on the back of this, the PI got funding for a full – time research nurse, to run interventional studies.
I remember the first complex commercial portfolio study I set up. Before I recruited my first patient I did not sleep the night before. I took home the packaging for all the bloods and biomarkers and had it all out in my living room … there was so much to get my head around. It did go ok – we became one of the top UK recruiters for the study. Because research in the department was working well, more PIs wanted to come on board. They could see research wasn’t such a demanding workload for them because research nurses were organising what they had to do and carrying out the study management.
I currently manage 17 staff in eight specialities. This brings its own challenges. A ward manager has one speciality on their ward and can see what is happening. In this role, you can’t be in renal, gastro and surgery if problems arise. So it’s slightly more difficult to manage. But I am learning so much about other specialities. I enjoy the patient contact and the patients really enjoy being in research. Patients have a hotline to consultants and any problems or issues they call the research nurse.
Our role is so diverse – it is not just recruiting patients.
I go to monthly meetings with the industry manager. If a company want 8 sites in the UK, they will send out expression of interest forms. If they get 20 back, they will do site selection visits. We also get selected for commercial studies off the back of our success in recruiting to other studies … you start to build up a name for yourself. We also get selected for commercial studies off the back of our success in recruiting to other studies. You start to build up a name for yourself.
A clinical research nurse has a certain amount of autonomy. You have to be able to manage your own time, prioritise and pay attention to detail. Data queries can drive you insane, but that is what research is about. It is all recruit, recruit, but, what is the point if the data is not correct? Part of being a research nurse is having the determination to meet targets. Follow ups don’t necessarily count (as part of the target). You are under pressure to recruit but you still have to follow-up patients … that is what I find difficult. You are perceived to be successful if your recruitment figures are high. Follow up and maintaining consent throughout the trial is just as important – this is when the majority of data is collected.
Our role is so diverse – it is not just recruiting patients. There are follow-ups, collecting data for the CRFs, maintaining site files, knowing about the agencies, regulatory bodies, protocols, consent and giving presentations to inform colleagues about what we do in research.
Cardiology were nominated in 3 or 4 categories at the Portsmouth Hospitals Research Conference and won a “Research Merit Award”. This was in recognition of how we built up cardiology research over the last three years. In cardiology we are getting more PIs on board because they can see we are organised. The PIs are understanding that they don’t have to do all the work. They have a team of experienced research nurses to co-ordinate their trials.
Change Research Cultures
Alison Mortimer, Lead Nurse, NIHR Clinical Research Facility, Sheffield
I fell into research. I bumped into an old colleague on the stairs, she was working in research and had a job going. My first reaction was negative, as research was like a swear word and I hated anything to do with research in my training but I went away, did some reading and decided to apply. I was shocked when I actually got it. I had no idea what I was walking into.
I absolutely loved it. It was so fast-paced, the workload was immense but the patient benefit was amazing. I could see positive outcomes, but it wasn’t only that. I was working with the same patients for a year or longer and built up rapport with them, and they spoke to you about everything that was going on with them. I loved it.
For me research has the best mix of autonomy and teamwork. You manage your own caseload but good communication across the team is essential. I also love the element of surprise … on one occasion I came in to find an email saying we needed to pull all of the patients on one of our trials off the drug immediately. I love that fast-paced excitement, it makes you grateful for the rare moments you do get to sit down at your desk and answer a data query.
My main passion is thinking about how we can unite clinical and research nursing.
I moved into the Comprehensive Local Research Network (CLRN) in 2009. To me it felt like a completely new way of thinking. You were working across such a wide area and with acute and primary care organisations that weren’t at all geared up for research. We had to be flexible so we could be responsive to the different needs of the Trusts. We also had to be sensitive to the internal politics, we were perceived as outsiders. It took a lot of thought and time to ensure we didn’t mess that up. But it was definitely rewarding.
In my role I have responsibilities for the Clinical Research Facility, research nurses within the trust and the CLRN. After that first conversation on the stairs I would have laughed if you had said I would be in this position now. I think research is still a dirty word amongst nurses. The most common reaction is ‘why would you want to do that’. It’s mainly a misunderstanding of the role, research used to be an easy role that people took when they are coming up to retirement. Once that perception exists it is hard to change. My main passion is thinking about how we can unite clinical and research nursing.
One of my main struggles is getting buy in from matrons on the ward. A lot hate research because they feel keeping posts open while nurse go on research secondments depletes their staff. For ward matrons they have targets and certain expectations to make their ward high quality and forward thinking. They don’t realise that we are feeding into that. I think some of the tensions arise because clinical nurses don’t realise that patient welfare and good patient outcomes are as central to our work. We need to stop speaking our own research language, go back to our roots and speak the same language.
After the Francis Report, nurses developed the 6 Cs to guide nursing – Care, Communication, Compassion, Courage, Competency and Commitment. If you think about what a research nurse does these are as essential to us as they are to clinical nurses. We should use this as a common language to unite us.
Supporting Surgical Trials
Joyce Katebe Clinical Trials Nurse, Surgery/Gastroenterology
I am a Surgical Clinical Trials Nurse and I trained and qualified in Zambia. My research started during post basic nursing training, during my BSc Nursing. Research was not part of the pre-registration nursing diploma/certificate then, but it was a requirement for the completion of the BSc Nursing.
After this, I became interested in research and was encouraged as I worked with my lecturers. I helped with data collection which I found very interesting and thought it was something I would like to do more of in the future. I was fortunate to be involved in research for the World Health Organization (WHO) on family planning in Zambia, and this inspired me to want to do more and helped me to develop my own questions about improving nursing practices to improve patient care.
While working in a local teaching hospital, I helped come up with a proposal about teenage pregnancy and the provision of ante-natal clinics for them. It was noted that most of them were first seen in labour when they were admitted to give birth. This prompted me to ask about what services were available for these mothers. I thought I would use this project to help set up ante-natal clinics for teenagers.
In research I find that there are many opportunities to learn.
Having moved to the UK and having spent time working in the NHS, I applied for a position at the Oxford University Hospitals. It was my first research nurse role and I worked with an enthusiastic professor who was very keen to involve nurses in his research. Very exciting. There were always new studies and each of the studies had different research questions to answer.
In my role, I was required to attend research meetings as well as having regular meetings with the principal investigators. I really got a buzz from these meetings as I felt really involved in trying to improve health of patients for the future. I lived in Oxford during the week and went home to Bristol at the weekends. Family life was difficult because my family stayed behind in Bristol and could not relocate as the children felt settled in their schools in Bath and were not keen to move to Oxford.
Many people when they hear the word research think having a career in research is beyond them, but in research I find that there are many opportunities to learn different things. I applied for my current position in Bath to help set up a research unit in the department of General Surgery and Gastroenterology. I was the first research nurse recruited to work purely for the two units.
Initially, it was a challenge because I had to find my way around the system. With the help of the surgeons and colleagues from oncology clinical trials unit, I had to look for office space, desk and all the equipment needed. I had to ensure that everyone joining the unit had Good Clinical Practice training and I went around the wards meeting the different specialists and nurses to discuss the research we did in the unit and this was repeated as required.
It is important that I develop good working relations with non-research nurses because most of my patients are in their care. It also allows them opportunity to understand the research we are doing. I meet patients in pre-op assessment unit, wards and in outpatients. The majority of them are keen to participate in research, the phrase I hear a lot from patients is "I am doing this because I want to give something back to the NHS and community at large” and some say "If no one did this years back, we would not have the treatment we have today."
To hear these words from patients is very encouraging. Many people when they hear the word research think having a career in research is beyond them, but in research I find that there are many opportunities to learn different things as well as witnessing how research is improving lives.
Developing Nurses
Lisa Berry, Senior Research Nurse, NIHR Wellcome Trust Clinical Research Facility, Southampton
The desire to be a research nurse came from a passionate belief that healthcare needs to be evidence based. It combines all the things that I enjoy; law, ethics, clinical care and working in complete partnership with research participants. At times, healthcare can be paternalistic. Patients come to us unwell and we do things to make them better.
Whereas in research the balance of power shifts considerably, we cannot achieve medical advances without help from patients (research participants). We work with them to assess the efficacy and safety of novel therapies and there is no guarantee that participating in a research study will be of benefit to the participant. In research, the safety and wellbeing of our participants is at the centre of everything we do and the research nurse is crucial to supporting them through the whole process of taking part in research.
Research nurses bring a study to life.
There are a specific set of skills that a research nurse needs. All the skills you learn on the ward are transferable and it is essential to have a good clinical grounding. You also need to pay attention to detail, understand the principles and importance of informed consent and be extremely organised. You need to understand not only the science behind the protocol but what participation will entail for the patients/healthy volunteers taking part.
Our nurses need to have the confidence to act as an advocate for the participant and must remain clinically relevant. We specialise in experimental medicine and provide care to healthy volunteers and patients with a wide range of disease and conditions. It is possible that a participant could become very unwell during a trial and therefore it is essential that research nurses remain sufficiently engaged with their clinical training to act appropriately and quickly.
Part of my role is to ensure that researchers are allocated appropriate levels of support and that the studies are set-up in a timely, safe and efficient manner and that we deliver an excellent standard of clinical and research care. Research nurses bring a study to life; they make a huge contribution to advancing healthcare and are a valuable asset for any research team. I was a Health Care Assistant before qualifying as a nurse. Although I am passionate about research nursing, this is not enough to build a career. I would not have progressed as quickly to the role of Senior Research Sister without support, mentoring and developmental opportunities.
Since I started in research in 2006 I have seen more career opportunities. More training has become available and there is a greater understanding of what clinical research nursing is. Even I didn’t really know what research nursing was when I started. We try to encourage our nurses to consider all their development options. We facilitate academic development as needed and also strive to provide career opportunities. A few of our band 6 and 7 nurses have been very fortunate in obtaining MRes funding. The NIHR funds the course fees, salary and also backfill for their position. The NIHR fund one person to do a Masters degree in research, but really they are funding the development of two people because someone else can then act-up into a more senior role and is also developed.
Our aim is to ensure that research is fully embedded within healthcare at this hospital. All research nurses now wear a dark grey uniform. This has given us a very visible identity and it is exciting to see how integrated into and dispersed around the hospital we are. Suddenly people become very much aware of the research presence in every division.
Informed Consent
Arshiya Pereira, Research Nurse, Renal Transplant Department, Central Manchester University Hospitals NHS Foundation Trust
I was trained in India to become a nurse. My first placement was in renal dialysis. I was interested in learning more about renal because of its vast subject area; renal medicine, renal transplant, research, transplant clinic and dialysis.
The main aim was to get more knowledge and experience working in a specialised unit. After moving to the UK I worked on the renal ward and dialysis unit at Sunderland Royal Hospital. I moved to Manchester as I wanted to gain more knowledge and experience of transplant. Initially working in the renal transplant clinic conducting follow up I became aware of research and I was curious about the research studies my patients had been recruited to. When a vacancy in Renal Transplant Research was advertised I applied.
I was a bit apprehensive in taking the role initially as I had heard many people say you lose your clinical skills and you do not get to take care of the patients as you would on the ward. I realise that those assumptions are inaccurate. I get to spend more time with the patients and I have discussions about the research. What we do in research today may change the way we practice medicine in the future.
Every day is different in renal research.
We work with two different types of donors, live donor transplant and cadaveric donors. With live donor transplant we know when they are coming to us. With cadaveric donors we don’t know when we are going to get the kidney. So I have to organise myself on the day itself. Recruitment always takes priority. The first thing I do each day is check if there are any transplant operations and if there are, I see if the patients are eligible for my study, and recruit them if they are happy to take part.
At times I have found it difficult to get the Principal Investigator (PI) to consent the patients because they were either in surgery or clinic. I began to wonder whether it would be possible for me to conduct informed consent? At the same time, the Trust was undertaking a scoping exercise to assess the need for clinical staff who were not doctors to take informed consent and developed policies and procedures to support us to take on this role. This is a wonderful opportunity for clinical staff who were not doctors to extend their role. Initially the role was delegated by the Principal Investigator who had to justify the need for a clinical research nurse to take the informed consent for a specific study.
The main aim was to get more knowledge and experience working in a specialised unit. A half day training programme was developed to gain more in depth knowledge of informed consent and group activities to explore the issues and processes involved. My competency in obtaining informed consent was assessed by the PI. I passed and felt really proud of myself.
To take consent I screen the patients’ eligibility and send information sheets two weeks prior to clinic visit, so they have time to read the information and speak to family. I also consult with the respective surgeon to see whether they are happy for their patient to be approached for the particular study. When the patient comes to clinic I discuss the study and if they are happy to take part, I make sure they are fully aware of what the study involves. In total, I have taken 20 informed consents so far, which has enabled the team to recruit to time and target.
I have now been working in research for over five years. I feel that due to the skills and expertise gained in particular informed consent my leadership qualities have improved significantly. I ensure the patient feels valued, they are followed closely from their pre-transplant appointment to their aftercare and they always remember me for the care I provide for them.
Ruth Hulbert, Lead Nurse, Kent and Medway Comprehensive Local Research Network
I came into the NIHR from the pharmaceutical industry, working with GSK and then Pfizer, I was used to an environment where money was no object and it wasn’t necessary to get people on board with the idea of research. The need to influence the right people in order to get research done was completely new to me.
Clinical research nursing is definitely not for the fainthearted. Most people get into nursing for the patient contact. You still have that but you also get other experiences like handling data, project managing and making direct approaches to very senior managers and consultants. You have to be proactive which can be difficult. The patients don’t come to you, you have to go out and find them.
When I began in the Cancer Research Network my personal worry was about approaching patients to join a study. It is an unusual position for a nurse, you are asking them to help you. The first patient I recruited was a lung cancer patient for an observational trial. He was very receptive which gave me the confidence going forward.
Clinical research nursing is definitely not for the fainthearted.
Clinical Research Nursing comes with a lot of autonomy, you don’t get that freedom in other areas of nursing. Nurses are in a much better position now in clinical research as there is a much clearer career structure. Most nurses come into our CLRN as a Band 5 with some nursing experience. Our goal is to develop them, and within a year to 18 months, most become Band 6s.
Training is passion of mine. I think there is a lot of satisfaction to be gained in passing on your knowledge and skills to people who are new and inexperienced. It is great when you see people growing and becoming a more confident and competent version of themselves. I am one of the Network’s Good Clinical Practice facilitators. At the last facilitators meeting it was announced that we had now trained 30,000 people across the network, to be even a small part of that it great.
Clinical research nursing is definitely not for the fainthearted. I was twice involved in developing new networks in Kent and Medway; the Cancer Research Network and then the CLRN. There was very little research activity at the time but awareness of research is definitely starting to change. A major culture shift but there are still areas within our CLRN where there is no research activity. In the early days there was a mixture of lack of knowledge and lack of interest in research, but most of all the clinical staff didn’t realise we were there as a resource for them to handle the more time consuming aspects of starting up a trial. That has changed.
My hope is that within my lifetime research will be embedded into the NHS in Kent to such an extent that the public can go to their doctor and ask what clinical trials are available for them and their doctor will know. Wherever my career takes me from here, I know that I want to stay within research I have developed a passion for it.
Research Management
Debbie Beirne, Nurse Consultant, Cancer Research UK, Leeds
I loved research from the start. I loved the autonomy, responsibility, the degree of change, the degree of learning. When new nurses start with me I tell them that they will probably feel like a fish out of water for six months. I explain it is a very dynamic and interesting environment, not suitable for anyone who likes things to stay the same.
Adapting to change is probably the most important thing. With research we don’t want things to stay still, we want them to move forward and nurses have to be able to move with that. A big misconception is that research nurses float around with a clipboard, drink tea and work very standard hours. None of that is true. I don’t think that there is the appreciation that we are actually delivering care, not just writing protocols for others.
Research nurses can now have a role that is much broader.
I have several parts to my role – my day-to-day operational role, a translational development role, a role within my trust as a research expert for other departments, and my Cancer Research UK role in engaging with the public at events. I work with some of our clinician scientists to deliver their protocols. I help them look at what they are currently doing in the labs and how that could translate into patient care. As a result, I have some co-investigator roles on a few grants.
I have seen huge changes in almost every aspect of research since 1999, except for the fundamental of how we care for the patient. Research Governance has changed, the way we structure and deliver clinical research has changed, the way we inform people has changed. Clinical trials are much more complex than they were ten years ago, and so the role of a research nurse is much more complex too. It's a very dynamic and interesting environment, not suitable for anyone who likes things to stay the same.
Obviously medical science wants to engage with the public and keep them aware of advances but when a newspaper runs a 'magic bullet' headline it impacts the work I do. I frequently get calls from patients who don’t realise that the headline doesn’t relate to their situation or refers to something in a lab which could take us 18 months to translate. I think we have a duty to give people hope but make sure it is a realistic hope.
Research allows you a degree of personal and professional development in a more flexible framework than traditional nursing. There are lots of different avenues; Network managers and lead nurses, Trust and R&D lead nurses and new roles are always coming up. As recently as five years ago if you wanted to move beyond a Band 7 you had to leave nursing, now I am a Band 8b and still a nurse.
We need to move away from the idea that as a research nurse you are just picking up the trial and delivering it. Research nurses can now have a role that is much broader. You can be involved in writing the protocol, be a patient voice with scientists, change the research culture within the wider trust.
Patient and Public Involvement
Maggie Peat, Lead Research Nurse, Harrogate and District Foundation Trust and Patient and Public Involvement Lead North and East Yorkshire and Lincolnshire Comprehensive Local Research Network
I was working as a nurse giving chemotherapy. It was just at the start of the cancer research networks. I didn’t really have much idea about what research networks might do, it just sounded like a really interesting job. When I started there was a lot of feeling your way, there wasn’t a lot of guidance around. There is a lot more now. We mentor people.
We recognised fairly early on that most student nurses didn’t really know anything about research. I wanted to show them that it wasn’t just about systematic reviews and all the really dull stuff but about actually recruiting patients into studies and the really exciting stuff of being at the sharp end of research. Student nurses absolutely loved it.
Often patients will take part in research because it's for the greater good.
Some of the Patient and Public Involvement work has been about raising awareness because patients and the public have all sorts of good ideas that we don’t think of, like putting information up on screens in patient waiting areas. Everybody is doing that now but none of us had thought of that because we didn’t wait in the waiting areas. Accessibility to information is really important. The people who need properly accessible information the most, are the people who are least likely to ask because they don’t want to look stupid or think that they are going to be judged.
It’s a simple thing that after taking consent to say to the person "right I want to be really sure that you understand what you are taking on. So can you say to me, what you might say to your wife when you get home?" It is simple but nurses are not taught how to do that. It is important that we have tools to measure understanding.
The power imbalance between a nurse and a patient is less than between a consultant and patient. It makes it easier for a patient to say no to taking part in a study. It is important, that people can say no to a trial. Patients understand the incremental process of research. One of the things they say is "all that I have benefited from has come from someone else doing a study." Often patients will take part in research because it is for the greater good or sometimes it is a positive thing to come out of something bad that has happened to them.
I wanted to show them that it wasn’t just about systematic reviews and all the really dull stuff. I think people are sometimes terrified of signing up for Patient and Public Involvement, thinking that they may have to do more than they want to do. So all our stuff is about saying to people, you can be involved as much as you want to be, you can do the occasional information sheet, you can look at a questionnaire and comment on it or you can come and be part of a steering group. People and patients can be involved in research as much or as little as they like.
NIHR has made it easier for consultants to take a study on, partly because of the nursing infrastructure. Nurses and support staff can work with consultants and we are here to stay. If you are interested in research nursing just do it, it suits most people. We have not had anyone work here that doesn’t love doing research.
Informing the Public
Karen Doyle, Senior Nurse, Cancer Research UK
When I started, research nursing was on the fringe of nursing. At the time we were told "you look after the doctors that is your role". That old fashioned view of nursing was still there.
I started in clinical research nursing because I wanted to be using all of the knowledge that I had gathered in my career. There were nurse specialist posts but I wanted something more intriguing, more complex. I didn’t want to do nurse management because it would take me away from patients. I always wanted to be patient centred. Research nursing offered all of that. I loved the fact that research nurses were involved in the science.
There were a lot of cancer patients with horrible side effects from the treatments available at the time. I wanted to be part of something that was not just accepting what we had because there was room for improvement. I wanted to be with the team that was making things better for these patients. Initially we were given early phase work such as toxicity and safety of the drug or treatment. So lots of additional testing. I loved the intensity and you got to know patients really well. I loved that in-depth interaction.
We are getting out into the communities with the right messages.
Medical teams sometimes want to get their patients into trials for compassionate, for misguided reasons. Sometimes you will get medical teams saying "But we have no other treatment to give them." You have to be strong. A clinical trial is not a treatment option and I think people forget that. We have to make sure that it is the right thing for the patient. That is what we are there for. When deciding if a trial is the right thing for a patient it is not only the science that matters. Sometimes it is the simple questions that matter for patients. Can you take tablets? Will you be able to cope with travelling to the trial? Those questions are missed if you haven’t got a nurse. We are the practical voice that makes the trial work.
I have developed my research nursing role to include informing members of the public about research. I love talking in the community because that is where the information is needed. We are getting out into the communities with the right messages – myth busting about clinical trials. Public understanding is better than it was but it has a long way to go. I have discovered that in addition to being face to face with the patient making the difference, I can also become the person (as a trainer) who will influence the nurses who are face-to-face. I can benefit many more patients through training than I could with nursing alone. I get a lot of reward from influencing other nurses. It is not management to me, management was taking you away from patients.
Research nurse leaders should be proud. We have taken the role of nurses in research from setting out someone’s lunch to a dynamic career. We have got national research nurse networks, we have got training and we have got the support of the NIHR. The change really is dramatic. I don’t know of any other type of nursing where it has improved as much and got more respect over time. You are really working as a specialist team member. That is the way it should be.
Claire Merritt, Lead Research Nurse Manager, Dementias & Neurodegenerative Diseases Research Network, Oxford
I caught the research bug in my first research post about 10 years ago. A consultant colleague had a grant to do a pilot study and asked me if I would be interested in working as a research nurse on their research study.
I was the only research nurse and was responsible for recruitment and study delivery and very quickly learnt about how challenging it was identifying patients and accessing patients for research. Rightly, there are people who want to protect patients, but it’s about persuading them that research is a good idea. Encouraging them to introduce the idea to a patient can be hard. People think there is a large cohort of people out there for trials. In reality, the numbers are much lower than you expect them to be.
What we have done for the last seven years within DeNDRoN is about trying to facilitate a culture change within the Trusts and make research part of everyday thinking. We’ve introduced what we call link workers into teams. Each of the community mental health teams has an honorary research worker linked to it. This has been successful as they develop an understanding of what that teams needs and wants and how they work.
Working with patients is what nurses do best.
As well as building up a team it’s about building up an infrastructure to support research to happen. During the time I have been working with DeNDRoN the portfolio of research studies within Trusts has grown, as has the complexity of studies we are able to support. The key message is that research becomes embedded in patient pathways so it becomes everyone’s business and not just ours.
Our job is about helping to facilitate research to happen. Partly, that’s just continuously giving that message that research is an important activity. We’ve had a degree of success, but I think we still have a way to go to persuade all clinicians that research is their business. Some clinicians have bought into this concept well, some still argue they do not have the time to do research and some say they find research is something scary. However, research is about empowering patients and their caregivers and the general population to help.
You get a very interesting range of people who are keen to take part in research. Some people want to be able to access something that may make a difference to their relatives or themselves. Others have altruistic motives and want to help, because it will help others rather than help themselves. On the other hand some people don’t really understand what we mean by research, or feel it’s too scary or risky or too much of a burden. So education is important. We must always remember we work with people in crisis and sometimes it is not the right time for them to consider research.
Working with patients is what nurses do best. So for most research nurses the contact with patients bit deals with itself. There is though a lot to learn and it’s all the other stuff around research you have to work hard at. As a lead nurse most of my job is about providing clinical leadership to staff, making sure that things happen and supporting workforce development. Key to what I do is making sure we have the right people with the right training in order for us to do research that can go on to make a difference to people in the future. Everything we do is about forward thinking, you always have to be thinking about the future.
Toward the end of a long career in mental health nursing I am really pleased to have found myself following a career in research. I am quite passionate about working in research. You have to believe in research to make this job a success.
Patient Care
Kathryn Kennedy, Trainee Advanced Clinical Research Nurse Practitioner, Manchester Clinical Research Facility
When I worked as a clinical nurse we did not have that much interaction with the research nurses who came onto the ward, even though most of the children were on trial drugs with a protocol I don’t think I really understood what that meant. Because I was nosy and interested I got to know more about the research side of things.
When I started in clinical research nursing the studies were lower intensity to what we have now, generally well children in an out-patient setting. At first it felt like a little bit of step back on the clinical side but that gave me the opportunity to really develop my research knowledge.
The team is now dealing in phase 1, phase 2 trials now in children who have no other treatment options, sometimes quite unwell, so we are in the heart of clinical nursing on a daily basis. The role of the research nurse is critical to keeping families motivated to stay on the trial … we spend a lot of time ensuring that their journey through the trial is a positive experience.
Our expertise is essential in ensuring that the study runs smoothly.
I think the perception of losing the clinical part of the nursing role perhaps puts some people off. Friends have expressed that they would not consider research because they see it as a very academic, very administrative based role. That could not be further from the truth. We don’t cut corners with informed consent. Parents need to understand at a level where they are able to put their emotions to one side and make a decision based on the knowledge we have given them.
Some of our children come from Europe and can be re located in this country for up to 6 months with their family. The research nurse is very much responsible for that relocation, as the liaison with hospital services or sponsors, ensuring bank accounts are in place, other children are getting educated. A lot of biotech companies are new companies. It might only be their third or fourth clinical trial.
Our expertise is essential in ensuring that the study runs smoothly. It is not reasonable to involve parents in that process if these things have not been thought through. We are invited earlier now to go to (sponsor and management) meetings because they are recognising our expertise. We are the ones who understand how to get things right from the very beginning.
I am a trainee advanced practitioner due to qualify in September. It is really exciting to be the first. This is a brand new role to research; able to recruit children to clinical trials, deal with physical examinations, prescribe and see patients without the support of a medic. As an advanced practitioner we can provide support for PIs hopefully this will ease some of that pressure so that trials that we wouldn’t perhaps have been able to do, because there wasn’t the medical support, get done.
I wasn’t sure that research nursing was somewhere I would stay forever. I wanted the research knowledge and experience but knew that my heart would be in clinical nursing. This new opportunity coming along has allowed me to be even more clinical in research nursing.
My new role has generated interest from other nurses that did not know this kind of role would be possible in research. You get to make a difference in a very different way. I still get to look after sick children which is what I always liked about nursing. But now there is a deeper level to this.
Supporting Primary Care
Julia Rooney, NIHR Primary Care Research Nurse, Kent, Surrey and Sussex Clinical Research Network
I used to manage the cardiac care unit at Brighton for many years. I also had a period in the Middle East working in a heart centre. When I came back I worked for the heart network in Sussex where I also worked within primary care which was great experience as it is very different from secondary care.
I am now a research nurse working in primary care, coordinating and running studies in practices where there isn’t capacity to carry out research. I started in this post six months ago and I have found it to be a very fulfilling job for many reasons. It is so rewarding what you get back from patients who want to be involved in research for the greater good
In nursing you cannot move forward without research and we are an evidenced based profession, for example we wouldn’t have made the advances we have in the treatment of heart attack patients without research. The autonomy you get within this role and the one-to-one patient contact you have means the whole process is extremely worthwhile. I cannot recommend the role highly enough. I was looking for a something that would get me more contact with patients and a new challenge … it allows me to utilise my clinical background and experience within research and means I can make a difference that way.
As a nurse you always are an advocate for patients. You make sure they are the priority in the research.
I was apprehensive recruiting my first patient despite years of experience in nursing. I am a bit of a perfectionist and wanted to get it right, taking the informed consent, making sure the patients understood what they were entering into. Once you start it becomes very natural. In my previous position I was moving further away from patients and then this opportunity came up. Everyone who asks about my job I say 'I love it! There isn’t anything I don’t like about it'. People are probably getting a bit bored of me talking about it now.
I underestimated the job in the beginning. I knew it was something I wanted to do but I underestimated how much I would love it and how much of a difference I could make to patients’ lives. This will change lives for future generations. You actually get time with a patient. In that hour or so you can hear other concerns they have and you can talk to them and advise them. You get the opportunity to discuss issues that may be of concern to the patient.
My working days vary so much, for example for one study I arrive in clinic, order a courier to collect the bloods, get the clinic room ready and all of the paperwork. Then you consent the patient and run the study. I might have to then go to another clinic in another practice to complete paperwork or run a shorter clinic. I genuinely don’t have two days the same. If you have the clinical background just do clinical research. Until you are doing the job you cannot be sure how fantastic it is. The studies I am involved in will change lives. You cannot put a price on that.
Susan Read, NIHR Primary Care Research Nurse, West Midlands Local Clinical Research Network
Five years ago the Midlands Research Practices Consortium (MidRec) secured funding to recruit some half-time research nurse posts based in local GP research active practices. I’ve always been interested in research and was working in a very busy GP surgery at the time, seeing patients every 5-10 minutes, with no quality time available to spend with my patients. I thought this job would give me the opportunity to spend more satisfactory time with patients while becoming involved in gathering accurate information to provide evidence based medicine.
I was the first nurse appointed and now part of a successful team of six nurses, based in our own individual surgeries, overseen by a Lead Research Nurse. Before we started running research studies in our nominated surgeries our Lead Nurse manager ensured that we underwent a programme of mandatory training so we had an understanding of what was required to safely be involved in research.
We have this supportive network of research nurses which makes for a powerful effective team.
Gradually the studies came in and I remember being asked by Professor McManus how many studies we were running and I said we were currently running about 20 studies. He was surprised. I think that opened the GPs’ eyes and they realised the opportunity they had with the support of the nurses.
Many studies are observational where you are looking and extracting data. We also look at feasibility of studies, so we are contacted by a study manager and asked to find out the number of patients we have. So we can go back to the study team and say these are the numbers for this head of population which we think you will be able to access at these surgeries.
The medical knowledge nurses have comes in useful when running feasibility studies. We can search effectively for eligible patients. We significantly help the GPs. I remember my first patient recruited to a study. I was absolutely thrilled because patients were willing to participate in the study where we were doing near patient testing and baseline health measurements with immediate feedback of results which we then had time to discuss. If necessary the patients could be referred back to their GP for any concerns that were highlighted.
The patient contact, the communication pathway that opened up in the last five years with the team has given me job satisfaction. We really feel included more so now than ever before. What pleases me the most is that although we are autonomous within our own environment there are five other host nurses. We have this supportive network of research nurses which makes a powerful effective team. We have opened up effective pathways between the university, study teams and other professionals. If they need help or an answer to a query we can normally provide the information quickly and efficiently because we have close contact with the GPs.
If someone is considering a career in research I would say come and join me for a day and see things first hand. Many of the host nurses have come from a Practice nurse background and have a wide range of knowledge because you can in any given day look after babies, give travel jabs, look after women’s health to caring for a patients with a chronic condition. Over the years our training has covered an enormous remit.
You learn a lot from problems, you have to be pragmatic and always look for solutions. When they set up this scheme, we were a pilot study. The success of the Pilot enabled funding to continue and currently the NIHR fund us through their networks even after MidRec itself came to an end. Patients deserve the opportunity to be involved in research.
Building Social Media Networks
Nathaniel Mills, Research Nurse Manager, Clinical Research Network: Yorkshire and Humber
My research career started in 2007. I was working in a large teaching hospital, part-time research and part-time primary care. When I started there was some negativity around working in research from colleagues who thought it was not real nursing. They thought it wasn’t direct patient care. But for me it was something I felt I could do to make a difference.
The whole notion of improving health and wellbeing through research appeals to me. I joined the NIHR Clinical Research Facility in Sheffield when it was a relatively new facility and I was in one of the first cohorts of research nurses. As a novice to clinical research you think ‘what’s it all about?’ but with experience and as time goes on, you physically see a patient’s symptoms improving. That gave me a great deal of job satisfaction.
In some cases the patient you see at the beginning of a trial is different to the same patient at the end of the trial. This is not just because they have had an trial intervention but because you, the nurse and the research team have given them the support and care that comes with trial participation.
I am passionate about twitter because it pulls together these groups of people who have common themes and needs.
When the NIHR Coordinating Centre began to consider social media as a tool to support research staff and utilise established tools, I became involved in the work of the NIHR Clinical Research Network Nurses Strategy Board. I met up with Fiona O’Neill, a few colleagues and Teresa Chinn from ‘@WeNurses’ to start up a clinical research nursing network on twitter. Teresa was inspiring in the sense that she’s a nurse working on her own and used social media to connect with other nurses. She now has a community of over 10,000 active and innovative followers.
So we developed a social media strategy (#crnnurse) with the aim of connecting the clinical research nursing community - especially reaching out to those nurses working in silos, something which is common in clinical research. We advertised this through the Clinical Research Network newsletter, and widely on Twitter we have regular ‘tweet chat’ debates and anyone who has anything to say about clinical research can participate - this has led to an active and vibrant community on social media.
Sometimes I get the odd negative responses such as 'I don’t want to do this in my own time' or 'what’s the point?' and that’s the beauty of it, you don’t have to, you can participate as much or as little as you like, the conversation is always going on.
Since its launch we have achieved a lot. We have a community of nurses and international nurses from the USA, Australia, South America and North Africa. We promote good practice and social media brings the learning to the community ... it’s free, cheap and easy. People who link in to our network can find out which Trusts are running trials. Problems can be shared rather than dealing with them by yourself, because it is highly likely someone will have already encountered the problem. So I would encourage nurses to get out there and start networking through social media, it can help make the life of clinical research nurses much easier.
I think the future is whatever you want it to be in terms of social media. If we do it right we can respond to what the nursing community wants. I am passionate about twitter because it pulls together these groups of people who have common themes and needs. Of course we have to consider what we say on a public forum, but we are all professional nurses and we are accountable for our actions. Follow me @natwm10 or @resnurse.

Home » Job Descriptions » Supv, Clinical Research Nurse
*This is an Exempt position. Employees in this position are paid a salary on a monthly basis and are not eligible to receive overtime pay.
The above statements are intended to describe the work being performed by people assigned to this job. they are not intended to be an exhaustive list of all responsibilities, duties and skills required of the personnel so classified., equal employment opportunity / affirmative action employer:, emory university is dedicated to providing equal opportunities to all individuals regardless of race, color, religion, ethnic or national origin, gender, age, disability, sexual orientation, gender identity, gender expression, veteran's status, or any other factor that is a prohibited consideration under applicable law..
- Current Employees
- Duke & Durham
- Human Resources
- Connect With Us
- External Applicants
- Current Duke Employees
- Duke Health Careers
CLINICAL RESEARCH NURSE COORDINATOR
Durham, NC, US, 27710
School of Medicine
Established in 1930, Duke University School of Medicine is the youngest of the nation's top medical schools. Ranked sixth among medical schools in the nation, the School takes pride in being an inclusive community of outstanding learners, investigators, clinicians, and staff where interdisciplinary collaboration is embraced and great ideas accelerate translation of fundamental scientific discoveries to improve human health locally and around the globe. Composed of more than 2,600 faculty physicians and researchers, nearly 2,000 students, and more than 6,200 staff, the Duke University School of Medicine along with the Duke University School of Nursing, and Duke University Health System comprise Duke Health, a world-class academic medical center. The Health System encompasses Duke University Hospital, Duke Regional Hospital, Duke Raleigh Hospital, Duke Health Integrated Practice, Duke Primary Care, Duke Home Care and Hospice, Duke Health and Wellness, and multiple affiliations.
Occ Summary
Type of Research: Duke Neurosurgery performs a variety of research in both adults and pediatric patients. Trials involving research for Parkinson's Disease, Alzheimer's Disease, other movement disorders, rare genetic disorders, bleeding and/or tumors of the brain, epilepsy, and chronic pain are just some of what our department loves to invest our time and effort towards. The focus for this role will primarily be injuries to the brain through a collaboration with other departments for treatment options of acute events. We are involved in device trials as well as drug trials. Studies may be complex with randomization, blinding, crossovers, etc. We have a diverse research group that love working together as a team, are very supportive of one another, and have our own core team values. Special skills: The Duke Department of Neurosurgery is looking for motivated and talented individuals with a desire to participate in a fast-paced academic, medical, and scientific research environment. The Duke Neurosurgery research team is led by a productive group of clinically active neurosurgeons, neuro-oncologists, surgeon scientists, and dedicated PhD researchers. This role will include on-call pay for after hours and weekend/holidays due to the acute nature of the brain event.
Description of Clinical Responsibilities: Clinical responsibilities: • Ambulatory Medication Administration • Adult Medication • Pediatric Medication • Draw blood from arterial lines Operations: Recognizes when typical agreements (MTAs, CDAs, DUAs, DTAs, etc.) are necessary and alerts appropriate parties. Prepares FDA regulatory submissions in collaboration with ORAQ, including development, submission, and maintenance of relevant documentation. Addresses FDA review and/or potential hold issues in collaboration with the Principal Investigator (PI). May train or oversee others. Knowledgeable in regulatory and institutional policies and processes; applies appropriately in study documentation, protocol submissions, and SOPs. May train others in these policies and processes. Maintains study level documentation for international studies. May develop resources and tools for management of international studies, and/or coordinate with other entities or offices. Is responsible for all aspects of managing and documenting Investigational Product (IP); including arrival, storage, tracking, and provision to research participants. Serves as the primary liaison with sponsors, IDS, and other parties as necessary. Follows protocol schema for randomization and blinding/unblinding. May train others. Prepares for and provides support for study monitoring and audit visits, including support for the reviewer. Addresses and corrects findings. May train others. Maintains participant level documentation for all studies, including those that are complex in nature (e.g., procedural and interventional studies) and/or require access to the Duke EHR. May train or oversee others. Employ strategies to maintain retention rates. Evaluate processes to identify problems with retention. May train or oversee others. Employs and may develop strategies to maintain recruitment rates and evaluate processes to identify problems. Escalates issues. May train or oversee others. Screens participants for complex studies (e.g., procedural and interventional studies). May train or oversee others. Develops or helps develop SOPs. May train or oversee others. Collects, prepares, processes, ships, and maintains the inventory of human research specimens, primarily those requiring complex procedures. May train or oversee others. Maintains study level documentation for all studies, including those that are complex in nature (e.g., procedural and interventional studies). May train or oversee others. Conducts activities for study visits in compliance with the protocol. May train staff. Contributes to the effective facilitation of team meetings to achieve predetermined objectives. May lead multidisciplinary meetings with various stakeholders. Ethics: Identifies all AEs, and determines whether or not they are reportable. Collaborates with the PI to determine AE attributes, including relatedness to study. May train or oversee others. Conducts and documents consent for participants for all types of studies, including those that are complex in nature and/or require any orders in Maestro Care. May train or oversee others. Develops consent plans and documents for participants in a variety of studies. May train or oversee others. Develops and submits documentation and information for IRB review. Communicates with the IRB staff and reviewers and handles issues appropriately. May train or oversee others. Prepares and submits documents needed for regulatory and safety reporting to sponsors and other agencies. May train or oversee others. Data: Under direct supervision from Biostatistician and PI may perform basic analyses on structured data. Enters and collects data. Develops data entry or collection SOPs or tools. May provide oversight or training to study team members collecting or entering data. Assists with quality control and data cleaning as directed. Independently responds to queries created by a CRO or the data manager. Ensures accuracy and completeness of data for all studies, including those that are complex in nature. Recognizes data quality trends and escalates as appropriate. May develop tools for, and train others in, data quality assurance procedures. Recognizes and reports security of physical and electronic data vulnerabilities. May develop or review data lifecycle and management plans for multiple study protocols. Follows predetermined SOPs to assist in documenting and maintaining documentation to facilitated data sharing. Identifies when various data standards should be used in creating eCRFs and EDCs and integrates as according to best practices. Maps a protocol's data flow plan including data capture, storage, transfer, management, quality, and preparation for analysis (may include data from EDCs, EHR, mobile apps, etc.). May train or oversee others. Innovatively uses technology to enhance a research process. May train others. Prepares tables, data visualizations, and lay summaries to communicate study results to participants. Develops reports on study progress for the PI and other study team members and collaborators. Creates clear visualizations to help communicate key information to stakeholders. Under supervision, executes testing process after the completion of a build, or following any project changes or system upgrades and may conduct some testing and documentation for Part 11 projects. Science: Assists with or contributes to the development of funding proposals. Independently conducts literature searches and reviews. Demonstrates and applies a basic understanding of open science practices and the FAIR data principals. Using scientific proposals from the PI, develops elements of research protocols. Demonstrates a basic understanding of the elements of research study designs. Contributes to the development of scientific publications or presentations. Serves as an author on poster presentations or publications. Study and Site Management: Prepares for, coordinates, and actively participates in site visits. Communicates effectively with sponsors and/or CROs. Uses clinical research management system and its reports to manage research participants' activities, calendars, tracking/marking financial milestones, and all aspects of study visits. Uses required EMR functionalities to manage participants and study visits. May train others. Uses clinical research management system and its reports to manage all protocol activities, including minimum footprint, SIP counsel, and all aspects of maintaining current protocol information. May train others. Collects appropriate information to determine whether the study team's participation in a specific trial is feasible. May make recommendations. For studies with supplies or equipment, ensures that there are ample supplies and that equipment is in good working order. May forecast effort needs. May train or oversee others. Ensure that studies are conducted in compliance with institutional requirements and other policies. Follow, and may develop or implement, operational plans (e.g. protocol-specific systems and documents including process flows). May train or oversee others. Prepares studies for closeout and document storage. May train or oversee others. Leadership: Proactively seeks opportunities to add relevant skills and certifications to own portfolio. Keeps current with research updates by attending key external offerings (i.e. Research Wednesday, RPN, events outside of Duke, etc.) and applies the learned material to the job. May disseminate information to others. Serves on committees and workgroups internal to Duke or externally in therapeutic area of research. Navigates processes and people involved in Duke clinical research, demonstrates the organizational awareness, and has the interpersonal skills necessary to get work done efficiently. Demonstrates resilience and is adaptive to change. Uses advanced subject matter expertise in the therapeutic area or clinical research to solve problems. Communicates effectively with others, regardless of reporting relationship, to accomplish shared work objectives.
Minimum Qualifications
Work requires graduation from an accredited BSN or Associates Degree in Nursing or Nursing Diploma program. All registered nurses without a Bachelors degree in Nursing (or higher) will be required to enroll in an appropriate BSN program within two years of their start date and to complete the program within five years of their start date. Must have current or compact RN licensure in the state of North Carolina. BLS required. Maintain compliance with required hospital and unit specific training competencies as well as an active RN status with the North Carolina Board of Nursing (NCBON).
Twelve months of appropriate clinical nursing experience is required.
Duke is an Affirmative Action/Equal Opportunity Employer committed to providing employment opportunity without regard to an individual's age, color, disability, gender, gender expression, gender identity, genetic information, national origin, race, religion, sex, sexual orientation, or veteran status.
Duke aspires to create a community built on collaboration, innovation, creativity, and belonging. Our collective success depends on the robust exchange of ideas—an exchange that is best when the rich diversity of our perspectives, backgrounds, and experiences flourishes. To achieve this exchange, it is essential that all members of the community feel secure and welcome, that the contributions of all individuals are respected, and that all voices are heard. All members of our community have a responsibility to uphold these values.
Essential Physical Job Functions: Certain jobs at Duke University and Duke University Health System may include essentialjob functions that require specific physical and/or mental abilities. Additional information and provision for requests for reasonable accommodation will be provided by each hiring department.
Nearest Major Market: Durham Nearest Secondary Market: Raleigh
Duke is an Affirmative Action / Equal Opportunity Employer committed to providing employment opportunity without regard to an individual’s age, color, disability, gender, gender expression, gender identity, genetic information, national origin, race, religion, sex, sexual orientation, or veteran status. Read more about Duke’s commitment to affirmative action and nondiscrimination at hr.duke.edu/eeo.

IMAGES
VIDEO
COMMENTS
Clinical Research Nurse - Job Description The Clinical Research Nurse, under the guidance and supervision of the Principal Investigator (PI), ensures the integrity and quality of clinical trials are maintained and conducted in accordance w/ federal, state,
The Nurse Scientist is a nurse with advanced preparation (PhD in nursing or related field) in research principles and methodology, who also has expert content knowledge in a specific clinical area. The primary focus of the role is to (1) provide leadership in the development, coordination and management of clinical research studies; (2) provide ...
Research Nurse Job Description. Conduct health screenings and tests to ensure that patients are clear of any disease or infection that might affect the study; ... Clinical research nurses report greater job satisfaction per the research by Fawcett and McCulloch in 2014. This specialized career offers more predictable schedules and an ...
Responsibilities for clinical research nurse. Adhering to trial protocols and legislation. Dose investigational product via- oral, topical, inhalation, IM, SC, intranasal, and IV route. Perform standard nursing care like vital signs, ECG and holter monitoring in a controlled and timed environment. Collecting trial data.
Registered Nurse (RN)- Clinical Research. Capital Cardiology Associates 3.6. Albany, NY 12211. $37 - $42 an hour. Full-time. Easily apply. Registered Nurse will be responsible for supporting clinical research as a coordinator for multiple research studies. And clinical trials within the practice. Posted.
They collect blood samples, administer vaccines, check lab work, and use critical thinking to assess a patient's health and review adverse events or treatment toxicities. Throughout, they ensure patients meet protocol goals. Data, data and more data: Collecting clinical trial results. Another significant role of a research nurse is to collect ...
Glassdoor.com states an annual median salary of $95,396 for Research Nurses and Payscale reports that Clinical Research Nurses earn an average annual salary of $75,217 or $36.86/hr.. Research Nurse Salary by Years of Experience. Research Nurses can earn a higher annual salary with increased years of experience. Less than 1 year of experience earn an average salary of $68,000
This domain description was validated in 2008 with the assistance of a consensus panel representing CC and IC nurses as well as nurses in clinical research from across the country. We now have eight 2009 CRN2010Teams charged with taking the domain to the next level of application, including competency assessment, course development and a ...
Year 4: Get licensed by taking the NCLEX-RN exam for registered nurses. (Optional) Years 5-7: Obtain an MSN degree. This program typically takes up to three years to complete. (Optional) Years 5-9: Obtain a Doctor of Philosophy in Nursing (Ph.D.) degree, which can take three to five years to complete. 2+ years of work experience: Whether ...
Careers. Clinical research nurses are an essential component of the intramural clinical research program. The career path for Clinical Research Nurses includes a core component of advancement in research skills and supports graduate and post-graduate training. Research training for nurses, like its medical counterpart, is integrated into ...
Responsibilities for research nurse. Assesses research subject. Support the safety of study participants, and maintain communication with their families/caregivers and clinicians. Provide clinical and administrative nursing support for research clinical trials based on scope of practice. Administer medications and treatments per study protocol ...
JOB DESCRIPTION: Provides clinical and administrative nursing support for research trials, studies, and projects. Recruits and screens potential participants. Performs patient/research subject evaluations, administers medications and research instruments and collects data. May assist with surgical procedures. Provides patient education related ...
JOB DESCRIPTION: Functions as an expert research nurse or coordinates a multi-center trial. Provides direction in auditing and monitoring activities. Collaborates with multidisciplinary researchers to establish research programs that integrate new advances in clinical trials management. Develops and conducts patient and family education.
Job Description. We are looking for research nurses to join our clinical program to help us manage the care of patients participating in clinical trials. Duties include, but are not limited to, ensuring adherence to ethical practice in the conduct of clinical trials, research protocol compliance and good clinical practice, ensuring patient ...
The work of clinical research nurses can have a wide-ranging impact. Through their involvement, new pharmaceutical interventions can be developed with a clear sense of how those drugs impact patient safety and well-being. Clinical research nurses also assist in the formation of new preventative measures and clinical procedures. Steps to ...
Maintains a working knowledge of the principles that guide clinical research nursing (participant and study team member safety; participant self-determination; research informed consent; fidelity to the research protocol; regulatory compliance; and research ethics and guiding principles). ... Review the employee's job description and identify ...
CLINICAL RESEARCH NURSE JOB Description. A clinical research nurse conducts scientific research on different aspects of human health like illnesses, pharmaceuticals, treatment plans and healthcare methods. Their major goal is to improve the quality of healthcare services that are administered to the patients.
Annually: $62,500. *This is an Exempt position. Employees in this position are paid a salary on a monthly basis and are not eligible to receive overtime pay. JOB DESCRIPTION: Coordinates implements and evaluates clinical research trials, studies and projects. Provides direction in the development of research protocols.
Nathaniel Mills, Research Nurse Manager, Clinical Research Network: Yorkshire and Humber. My research career started in 2007. I was working in a large teaching hospital, part-time research and part-time primary care. When I started there was some negativity around working in research from colleagues who thought it was not real nursing.
Clinical Research Nurse Coordinator. University of North Carolina at Chapel Hill. Chapel Hill, NC 27514. $70,000 - $85,000 a year. Full-time. Monday to Friday + 1. The Clinical Research Nurse Coordinator is responsible for independently providing specialized nursing services for various clinical research protocols. Posted.
Clinical and Professional Responsibilities. Working autonomously to assist in the management of a caseload of clinical trial patients, whilst working as part of a multi-disciplinary team. Maintain effective communication with patients, carers and professionals to ensure high quality service delivery. Manage and oversee a portfolio of research ...
Annually: $74,400. *This is an Exempt position. Employees in this position are paid a salary on a monthly basis and are not eligible to receive overtime pay. JOB DESCRIPTION: Supervises, trains and evaluates a minimum of two Research Nurses in the performance of clinical and administrative responsibilities related to one or more research trials ...
Nurse Practitioner/Clinical Instructor of Medicine (Practitioner) USC. Los Angeles, CA. $161,562 - $226,192 a year. Assists with medical research projects, following medical research protocols. The nurse practitioner/physician assistant works in collaboration with other….
The Duke Neurosurgery research team is led by a productive group of clinically active neurosurgeons, neuro-oncologists, surgeon scientists, and dedicated PhD researchers. This role will include on-call pay for after hours and weekend/holidays due to the acute nature of the brain event. Description of Clinical Responsibilities: Clinical ...