- Search by keyword
- Search by citation
Page 1 of 110

Detection of bla KPC gene among carbapenemase producing Klebsiella pneumoniae isolated from different clinical specimens at tertiary care hospital of Nepal
Klebsiella pneumoniae infections have become a major cause of hospital acquired infection worldwide with the increased rate of acquisition of resistance to antibiotics. Carbapenem resistance mainly among Gram ne...
- View Full Text
Survival of highly related ESBL- and pAmpC- producing Escherichia coli in broiler farms identified before and after cleaning and disinfection using cgMLST
Broiler chickens are frequently colonized with Extended-Spectrum Beta-Lactamase- (ESBL-) and plasmid mediated AmpC Beta-Lactamase- (pAmpC-) producing Enterobacterales, and we are confronted with the potential ...
Genomic characterization and probiotic potential assessment of an exopolysaccharide-producing strain Pediococcus pentosaceus LL-07 isolated from fermented meat
The genomic information available for Pediococcus pentosaceus is primarily derived from fermented fruits and vegetables, with less information available from fermented meat. P. pentosaceus LL-07, a strain isolate...
Gut bacterial and fungal dysbiosis in tuberculosis patients
Recent studies have more focused on gut microbial alteration in tuberculosis (TB) patients. However, no detailed study on gut fungi modification has been reported till now. So, current research explores the ch...
Production of kojic acid by Aspergillus flavus OL314748 using box-Behnken statistical design and its antibacterial and anticancer applications using molecular docking technique
Kojic acid is a wonderful fungal secondary metabolite that has several applications in the food, medical, and agriculture sectors. Many human diseases become resistant to normal antibiotics and normal treatmen...
Microbiome signatures associated with clinical stages of gastric Cancer: whole metagenome shotgun sequencing study
Gastric cancer is one of the global health concerns. A series of studies on the stomach have confirmed the role of the microbiome in shaping gastrointestinal diseases. Delineation of microbiome signatures to d...
The characteristics of microbiome in the upper respiratory tract of COVID-19 patients
Co-infection with other pathogens in coronavirus disease 2019 (COVID-19) patients exacerbates disease severity and impacts patient prognosis. Clarifying the exact pathogens co-infected with severe acute respir...
Occurrence, antibiotic resistance profiles and associated risk factors of Klebsiella pneumoniae in poultry farms in selected districts of Somalia Reginal State, Ethiopia
Klebsiella pneumoniae is an opportunistic infection that causes production losses and death in the chicken industry. A cross-sectional study was conducted on exotic chicken breeds reared at the Jigjiga poultry fa...
High prevalence of multidrug-resistant Enterobacterales carrying extended-spectrum beta-lactamase and AmpC genes isolated from neonatal sepsis in Ahvaz, Iran
In the recent years, multidrug resistant (MDR) neonatal septicemia-causing Enterobacterales has been dramatically increased due to the extended-spectrum beta-lactamases (ESBLs) and AmpC enzymes. This study aimed ...
Phenotypic and molecular characterization of β-lactamase-producing Klebsiella species among children discharged from hospital in Western Kenya
The emergence and spread of β-lactamase-producing Klebsiella spp. has been associated with a substantial healthcare burden resulting in therapeutic failures. We sought to describe the proportion of phenotypic res...
Exertional heat stroke-induced changes in gut microbiota cause cognitive impairment in mice
The incidence of exertional heat stroke (EHS) escalates during periods of elevated temperatures, potentially leading to persistent cognitive impairment postrecovery. Currently, effective prophylactic or therap...
Altered microbiome of serum exosomes in patients with acute and chronic cholecystitis
This study aimed to investigate the differences in the microbiota composition of serum exosomes from patients with acute and chronic cholecystitis.
Saliva‑microbiome‑derived signatures: expected to become a potential biomarker for pulmonary nodules (MCEPN-1)
Oral microbiota imbalance is associated with the progression of various lung diseases, including lung cancer. Pulmonary nodules (PNs) are often considered a critical stage for the early detection of lung cance...
The gut microbiota facilitate their host tolerance to extreme temperatures
Exposure to extreme cold or heat temperature is one leading cause of weather-associated mortality and morbidity in animals. Emerging studies demonstrate that the microbiota residing in guts act as an integral ...
Establishment of an in vitro model of monocyte-like THP-1 cells for trained immunity induced by bacillus Calmette-Guérin
Mycobacteria bloodstream infections are common in immunocompromised people and usually have disastrous consequences. As the primary phagocytes in the bloodstream, monocytes and neutrophils play critical roles ...
The distinct cell physiology of Bradyrhizobium at the population and cellular level
The α-Proteobacteria belonging to Bradyrhizobium genus are microorganisms of extreme slow growth. Despite their extended use as inoculants in soybean production, their physiology remains poorly characterized. In ...
Decoding the role of oxidative stress resistance and alternative carbon substrate assimilation in the mature biofilm growth mode of Candida glabrata
Biofilm formation is viewed as a vital mechanism in C. glabrata pathogenesis. Although, it plays a significant role in virulence but transcriptomic architecture and metabolic pathways governing the biofilm growth...
Occurrence, antimicrobial susceptibility, and resistance genes of Staphylococcus aureus in milk and milk products in the Arsi highlands of Ethiopia
In Ethiopia, milk production and handling practices often lack proper hygiene measures, leading to the potential contamination of milk and milk products with Staphylococcus aureus ( S. aureus ), including methicill...
Based on molecular docking and real-time PCR technology, the two-component system Bae SR was investigated on the mechanism of drug resistance in CRAB
This study aimed to explore the role of the two-component system Bae SR in the mechanism of drug resistance in carbapenem-resistant A. baumannii (CRAB) using molecular docking and real-time polymerase chain react...
Genomic characterization and related functional genes of γ- poly glutamic acid producing Bacillus subtilis
γ- poly glutamic acid (γ-PGA), a high molecular weight polymer, is synthesized by microorganisms and secreted into the extracellular space. Due to its excellent performance, γ-PGA has been widely used in vario...
In silico analysis of intestinal microbial instability and symptomatic markers in mice during the acute phase of severe burns
Severe burns may alter the stability of the intestinal flora and affect the patient’s recovery process. Understanding the characteristics of the gut microbiota in the acute phase of burns and their association...
The effect of white grub ( Maladera Verticalis ) larvae feeding on rhizosphere microbial characterization of aerobic rice ( Oryza sativa L.) in Puer City, Yunnan Province, China
Rhizosphere microorganisms are vital in plants’ growth and development and these beneficial microbes are recruited to the root-zone soil when experiencing various environmental stresses. However, the effect of...
Characterization of genes related to the efflux pump and porin in multidrug-resistant Escherichia coli strains isolated from patients with COVID-19 after secondary infection
Escherichia coli ( E. coli ) is a multidrug resistant opportunistic pathogen that can cause secondary bacterial infections in patients with COVID-19. This study aimed to determine the antimicrobial resistance profi...
Correction: Uncovering the complexity of childhood undernutrition through strain‑level analysis of the gut microbiome
The original article was published in BMC Microbiology 2024 24 :73
Optimization of fermentation conditions and medium components for chrysomycin a production by Streptomyces sp. 891-B6
Chrysomycin A (CA) is a promising antibiotic for treatment of Gram-positive bacterial infections and cancers. In order to enhance CA yield, optimization of fermentation conditions and medium components was car...
Integrative metagenomic analysis reveals distinct gut microbial signatures related to obesity
Obesity is a metabolic disorder closely associated with profound alterations in gut microbial composition. However, the dynamics of species composition and functional changes in the gut microbiome in obesity r...
Ultraviolet C inactivation of Coxiella burnetii for production of a structurally preserved whole cell vaccine antigen
Q fever, a worldwide-occurring zoonotic disease, can cause economic losses for public and veterinary health systems. Vaccines are not yet available worldwide and currently under development. In this regard, it...
Neutrophil extracellular traps formation: effect of Leishmania major promastigotes and salivary gland homogenates of Phlebotomus papatasi in human neutrophil culture
Leishmaniasis as a neglected tropical disease (NTD) is caused by the inoculation of Leishmania parasites via the bite of phlebotomine sand flies. After an infected bite, a series of innate and adaptive immune res...
Assessment of bacterial profile, antimicrobial susceptibility status, and associated factors of isolates among hospitalized patients at Dessie Comprehensive Specialized Hospital, Northeast Ethiopia
Antimicrobial resistant bacteria among hospitalized patients are becoming a major public health threat worldwide, mainly in developing countries. Infections by these multidrug resistant pathogens cause high ra...
A review of emerging health threats from zoonotic New World mammarenaviruses
Despite repeated spillover transmission and their potential to cause significant morbidity and mortality in human hosts, the New World mammarenaviruses remain largely understudied. These viruses are endemic to...
Impact of Limosilactobacillus fermentum probiotic treatment on gut microbiota composition in sahiwal calves with rotavirus diarrhea: A 16S metagenomic analysis study”
Diarrhea poses a major threat to bovine calves leading to mortality and economic losses. Among the causes of calf diarrhea, bovine rotavirus is a major etiological agent and may result in dysbiosis of gut micr...
Genetic characterizations of Cryptosporidium spp. from children with or without diarrhea in Wenzhou, China: high probability of zoonotic transmission
Cryptosporidium is a highly pathogenic parasite responsible for diarrhea in children worldwide. Here, the epidemiological status and genetic characteristics of Cryptosporidium in children with or without diarrhea...
Effect of stress urinary incontinence on vaginal microbial communities
Postpartum women often experience stress urinary incontinence (SUI) and vaginal microbial dysbiosis, which seriously affect women’s physical and mental health. Understanding the relationship between SUI and va...
Hospital distribution, seasonality, time trends and antifungal susceptibility profiles of all Aspergillus species isolated from clinical samples from 2015 to 2022 in a tertiary care hospital
Aspergillus species cause a variety of serious clinical conditions with increasing trend in antifungal resistance. The present study aimed at evaluating hospital epidemiology and antifungal susceptibility of all ...
Comparative analysis of proteomic adaptations in Enterococcus faecalis and Enterococcus faecium after long term bile acid exposure
All gastrointestinal pathogens, including Enterococcus faecalis and Enterococcus faecium , undergo adaptation processes during colonization and infection. In this study, we investigated by data-independent acquisi...
Influence of PhoPQ and PmrAB two component system alternations on colistin resistance from non- mcr colistin resistant clinical E. Coli strains
The current understanding of acquired chromosomal colistin resistance mechanisms in Enterobacterales primarily involves the disruption of the upstream PmrAB and PhoPQ two-component system (TCS) control caused by ...
Staphylococcus aureus foldase PrsA contributes to the folding and secretion of protein A
Staphylococcus aureus secretes a variety of proteins including virulence factors that cause diseases. PrsA, encoded by many Gram-positive bacteria, is a membrane-anchored lipoprotein that functions as a foldase t...
Transcriptional dynamics during Rhodococcus erythropolis infection with phage WC1
Belonging to the Actinobacteria phylum, members of the Rhodococcus genus thrive in soil, water, and even intracellularly. While most species are non-pathogenic, several cause respiratory disease in animals and, m...
A hypervirulent Acinetobacter baumannii strain has robust anti-phagocytosis ability
Acinetobacter baumannii ( A. baumannii ) is associated with both hospital-acquired infections (HAP) and community-acquired pneumonia (CAP). In this study, we present a novel CAP-associated A. baumannii (CAP-AB) str...
Restoration of gut dysbiosis through Clostridium butyricum and magnesium possibly balance blood glucose levels: an experimental study
Diabetes mellitus (DM) is a chronic metabolic disorder characterized by an elevated level of blood glucose due to the absence of insulin secretion, ineffectiveness, or lack of uptake of secreted insulin in the...
Bacillus subtilis SOM8 isolated from sesame oil meal for potential probiotic application in inhibiting human enteropathogens
While particular strains within the Bacillus species, such as Bacillus subtilis , have been commercially utilised as probiotics, it is critical to implement screening assays and evaluate the safety to identify pot...
Promiscuous, persistent and problematic: insights into current enterococcal genomics to guide therapeutic strategy
Vancomycin-resistant enterococci (VRE) are major opportunistic pathogens and the causative agents of serious diseases, such as urinary tract infections and endocarditis. VRE strains mainly include species of Ente...
Comparison of integron mediated antimicrobial resistance in clinical isolates of Escherichia coli from urinary and bacteremic sources
Antimicrobial resistance (AMR) is a global threat driven mainly by horizontal gene transfer (HGT) mechanisms through mobile genetic elements (MGEs) including integrons. The variable region (VR) of an integron ...
Structure predictions and functional insights into Amidase_3 domain containing N -acetylmuramyl-L-alanine amidases from Deinococcus indicus DR1
N -acetylmuramyl-L-alanine amidases are cell wall modifying enzymes that cleave the amide bond between the sugar residues and stem peptide in peptidoglycan. Amidases play a vital role in septal cell wall cleavag.....
Profile of non-tuberculous mycobacteria amongst tuberculosis presumptive people in Cameroon
Cameroon is a tuberculosis (TB) burden country with a 12% positivity among TB presumptive cases. Of the presumptive cases with a negative TB test, some are infected with Non-tuberculous Mycobacteria (NTM). How...
In vitro investigation of relationship between quorum-sensing system genes, biofilm forming ability, and drug resistance in clinical isolates of Pseudomonas aeruginosa
Pseudomonas aeruginosa is an opportunistic pathogen in the health-care systems and one of the primary causative agents with high mortality in hospitalized patients, particularly immunocompromised. The limitation ...
Relationship between heart failure and intestinal inflammation in infants with congenital heart disease
The association between heart failure (HF) and intestinal inflammation caused by a disturbed intestinal microbiota in infants with congenital heart disease (CHD) was investigated.
Clostridium butyricum inhibits the inflammation in children with primary nephrotic syndrome by regulating Th17/Tregs balance via gut-kidney axis
Primary nephrotic syndrome (PNS) is a common glomerular disease in children. Clostridium butyricum ( C. butyricum), a probiotic producing butyric acid, exerts effective in regulating inflammation. This study was d...
Human-derived bacterial strains mitigate colitis via modulating gut microbiota and repairing intestinal barrier function in mice
Unbalanced gut microbiota is considered as a pivotal etiological factor in colitis. Nevertheless, the precise influence of the endogenous gut microbiota composition on the therapeutic efficacy of probiotics in...
In vitro and in silico studies of enterobactin-inspired Ciprofloxacin and Fosfomycin first generation conjugates on the antibiotic resistant E. coli OQ866153
The emergence of antimicrobial resistance in bacterial pathogens is a growing concern worldwide due to its impact on the treatment of bacterial infections. The "Trojan Horse" strategy has been proposed as a po...
Important information
Editorial board
For authors
For editorial board members
For reviewers
- Manuscript editing services
Annual Journal Metrics
2022 Citation Impact 4.2 - 2-year Impact Factor 4.7 - 5-year Impact Factor 1.131 - SNIP (Source Normalized Impact per Paper) 0.937 - SJR (SCImago Journal Rank)
2023 Speed 19 days submission to first editorial decision for all manuscripts (Median) 135 days submission to accept (Median)
2023 Usage 2,970,572 downloads 1,619 Altmetric mentions
- More about our metrics
- Follow us on Twitter
BMC Microbiology
ISSN: 1471-2180
- General enquiries: [email protected]
Microbiology
Viral Genetics Confirms What On-the-Ground Activists Knew Early in the Mpox Outbreak
Molecular biology could have changed the mpox epidemic—and could stop future outbreaks
Joseph Osmundson
Cannibal Cells Inspire Cancer Treatment Improvement
Giving cells an appetite for cancer could enhance treatments
Kate Graham-Shaw

Is Raw-Milk Cheese Safe to Eat?
Recent bacterial outbreaks from consuming cheese made from unpasteurized milk, or “raw milk,” raise questions about the safety of eating these artisanal products
Riis Williams
Many Pregnancy Losses Are Caused by Errors in Cell Division
Odd cell divisions could help explain why even young, healthy couples might struggle to get pregnant
Gina Jiménez

'Microbiome of Death' Uncovered on Decomposing Corpses Could Aid Forensics
Microbes that lurk in decomposing human corpses could help forensic detectives establish a person's time of death
Christoph Schwaiger, LiveScience
Weird ‘Obelisks’ Found in Human Gut May be Virus-Like Entities
Rod-shaped fragments of RNA called “obelisks” were discovered in gut and mouth bacteria for the first time
Joanna Thompson
Semen Has Its Own Microbiome—And It Might Influence Fertility
Recent research found a species of bacteria living in semen that’s associated with infertility and has links to the vaginal microbiome
Andrew Chapman
Bacteria Make Decisions Based on Generational Memories
Bacteria choose to swarm based on what happened to their great-grandparents
Allison Parshall

Your Body Has Its Own Built-In Ozempic
Popular weight-loss and diabetes drugs, such as Ozempic and Wegovy, target metabolic pathways that gut microbes and food molecules already play a key role in regulating
Christopher Damman, The Conversation US
See Your Body’s Cells in Size and Number
The larger a cell type is, the rarer it is in the body—and vice versa—a new study shows
Clara Moskowitz, Jen Christiansen, Ni-ka Ford

Subterranean ‘Microbial Dark Matter’ Reveals a Strange Dichotomy
The genes of microbes living as deep as 1.5 kilometers below the surface reveal a split between minimalist and maximalist lifestyles
Stephanie Pappas
The Vaginal Microbiome May Affect Health More than We Thought
A recent study finds varying combinations of microbes in the vaginal microbiome may influence health outcomes such as risk of sexually transmitted disease and preterm birth
Lori Youmshajekian
Microbiology News
Top headlines, latest headlines.
- Climate Change and Transmission of Malaria?
- Giant Viruses Infect Deadly Parasite
- How Parasites Shape Complex Food Webs
- Family of Proteins and Inflammatory Diseases
- World's Chocolate Supply Threatened by Virus
- Odor-Causing Bacteria in Armpits Targeted
- Microbes May Affect Taste of Mustard Seeds
- Artificial Cells That Act Like Living Cells
- Switching Off the Light to See Better
- Pathogens Spread Unrecognized in the Body
Earlier Headlines
Monday, april 22, 2024.
- AI Tool Creates 'synthetic' Images of Cells for Enhanced Microscopy Analysis
- Protein Network Dynamics During Cell Division
Friday, April 19, 2024
- New Research Defines Specific Genomic Changes Associated With the Transmissibility of the Monkeypox Virus
- Light Show in Living Cells
- Key Protein Regulates Immune Response to Viruses in Mammal Cells
Thursday, April 18, 2024
- Why Can Zebrafish Regenerate Damaged Heart Tissue, While Other Fish Species Cannot?
- Marine Microbial Populations: Potential Sensors of the Global Change in the Ocean
- RNA's Hidden Potential: New Study Unveils Its Role in Early Life and Future Bioengineering
Wednesday, April 17, 2024
- Probiotic Feed Additive Boosts Growth, Health in Poultry in Place of Antibiotics
- New Class of Antimicrobials Discovered in Soil Bacteria
- Solving a Mini Mystery of Cell Division
- Plant Sensors Could Act as an Early Warning System for Farmers
- A Better View With New Mid-Infrared Nanoscopy
- How Soil Microbes Survive in Harsh Desert Environments
- E-Tongue Can Detect White Wine Spoilage Before Humans Can
- Copper Beads in Pig Feed Reshape Swine Gut Microbiome
- Researchers Uncover Human DNA Repair by Nuclear Metamorphosis
Tuesday, April 16, 2024
- Coral Reef Microbes Point to New Way to Assess Ecosystem Health
- Real-Time Detection of Infectious Disease Viruses by Searching for Molecular Fingerprinting
- Twisted Pollen Tubes Induce Infertility
- 'One Ring to Rule Them All': How Actin Filaments Are Assembled by Formins
Monday, April 15, 2024
- How Tardigrades Can Survive Intense Radiation
- How Blue-Green Algae Manipulate Microorganisms
- Unlocking the 'chain of Worms'
- Millions of Gamers Advance Biomedical Research
- Green-to-Red Transformation of Euglena Gracilis Using Bonito Stock and Intense Red Light
- Researchers Resolve Old Mystery of How Phages Disarm Pathogenic Bacteria
- Even the Simplest Marine Organisms Tend to Be Individualistic
- Carbon Beads Help Restore Healthy Gut Microbiome and Reduce Liver Disease Progression
Friday, April 12, 2024
- Microbial Food as a Strategy Food Production of the Future
- Innovative Antiviral Defense With New CRISPR Tool
- PFAS Exposure from High Seafood Diets May Be Underestimated
Thursday, April 11, 2024
- First Step to Untangle DNA: Supercoiled DNA Captures Gyrase Like a Lasso Ropes Cattle
- New Approach for Combating 'resting' Bacteria
- Tropical Coral-Infecting Parasites Discovered in Cold Marine Ecosystems
- Genetic Underpinnings of Environmental Stress Identified in Model Plant
Wednesday, April 10, 2024
- Size of Salty Snack Influences Eating Behavior That Determines Amount Consumed
- New Drug Prevents Flu-Related Inflammation and Lung Damage
- Cockayne Syndrome: New Insights Into Cellular DNA Repair Mechanism
- Cold-Affinity Algae Species Are Gradually Being Replaced by Warm-Affinity Ones Off the Coast of Biscay
- New Diagnostic Tool Achieves Accuracy of PCR Tests With Faster and Simpler Nanopore System
Tuesday, April 9, 2024
- A Microbial Plastic Factory for High-Quality Green Plastic
- Machine Learning Method Reveals Chromosome Locations in Individual Cell Nucleus
- Do Some Mysterious Bones Belong to Gigantic Ichthyosaurs?
- Bacteria in Cancer Unmasked
Monday, April 8, 2024
- Different Means to the Same End: How a Worm Protects Its Chromosomes
- Scientists Grow 'mini Kidneys,' Revealing New Insights Into Metabolic Defects and Potential Therapy for Polycystic Kidney Disease
- Bringing Multidrug-Resistant Pathogens to Their Knees
- How Mosquito Larva Guts Could Help Create Highly Specific Insecticides
Friday, April 5, 2024
- Can Language Models Read the Genome? This One Decoded mRNA to Make Better Vaccines
- eDNA Methods Give a Real-Time Look at Coral Reef Health
- Rapid, Simultaneous Detection of Multiple Bacteria Achieved With Handheld Sensor
Thursday, April 4, 2024
- What Four Decades of Canned Salmon Reveal About Marine Food Webs
- Heat Flows the Secret to Order in Prebiotic Molecular Kitchen
- Microbial Signature of Colorectal Cancer-Associated Mutations Identified in New Study
- New Method Reveals Hidden Activity of Life Below Ground
Wednesday, April 3, 2024
- New Tools Reveal How Genes Work and Cells Organize
- Evolution in Action? New Study Finds Possibility of Nitrogen-Fixing Organelles
- Giant Phage Holds Promise as Treatment for Lung Infections
- Discovery Could End Global Amphibian Pandemic
- Plastic-Free Vegan Leather That Dyes Itself Grown from Bacteria
- New Discovery Unravels Malaria Invasion Mechanism
- Scientists Further Our Understanding of How a Foodborne Bacterium Can Survive in Food Preparation Environments
Tuesday, April 2, 2024
- Scientists Link Certain Gut Bacteria to Lower Heart Disease Risk
- Infant Gut Microbes Have Their Own Circadian Rhythm, and Diet Has Little Impact on How the Microbiome Assembles
Monday, April 1, 2024
- New Antibiotic Class Effective Against Multidrug-Resistant Bacteria
Friday, March 29, 2024
- Connecting the Dots to Shape Growth Forces
Thursday, March 28, 2024
- When Inequality Is More Than 'skin-Deep': Social Status Leaves Traces in the Epigenome of Spotted Hyenas in Tanzania
- How the Crimean-Congo Hemorrhagic Fever Virus Enters Our Cells
- Genomic Research May Help Explain Cancer Resistance in Tasmanian Devils
- TB Vaccine May Enable Elimination of the Disease in Cattle by Reducing Its Spread
- Researchers Discover Key Gene for Toxic Alkaloid in Barley
Wednesday, March 27, 2024
- A Combination of Approved Drugs Enhances the Delivery of Anti-Bacterial Medications to Treat Tuberculosis
- Scientists Extract Genetic Secrets from 4,000-Year-Old Teeth to Illuminate the Impact of Changing Human Diets Over the Centuries
- New Enzymatic Cocktail Can Kill Tuberculosis-Causing Mycobacteria
- Old Immune Systems Revitalized in Mouse Study, Improving Vaccine Response
Tuesday, March 26, 2024
- New Testing Approach Improves Detection of Rare but Emerging Powassan Virus Spread by Deer Ticks
- Researchers Show That Introduced Tardigrade Proteins Can Slow Metabolism in Human Cells
- Silicon Spikes Take out 96% of Virus Particles
Monday, March 25, 2024
- Novel Electrochemical Sensor Detects Dangerous Bacteria
- Breakthrough Antibiotic Shows Promise Against Obstinate Mycobacterial Infections
- Humans Pass More Viruses to Other Animals Than We Catch from Them
- Researchers Discover Evolutionary 'tipping Point' In Fungi
- Maize Genes Control Little Helpers in the Soil
Friday, March 22, 2024
- Natural Recycling at the Origin of Life
- Scientists Close in on TB Blood Test Which Could Detect Millions of Silent Spreaders
- Researchers Invent Artificial Intelligence Model to Design New Superbug-Fighting Antibiotics
Thursday, March 21, 2024
- As We Age, Our Cells Are Less Likely to Express Longer Genes
- Research Finds a Direct Communication Path Between the Lungs and the Brain
- Decoding the Plant World's Complex Biochemical Communication Networks

Wednesday, March 20, 2024
- Deep Earth Electrical Grid Mystery Solved
- Bacteria Subtype Linked to Growth in Up to 50% of Human Colorectal Cancers
- Experts Warn Climate Change Will Fuel Spread of Infectious Diseases
- Fiber, Genes and the Gut Microbiome: Study Reveals Possible Triggers for Inflammatory Bowel Disease
- Craving Snacks After a Meal? It Might Be Food-Seeking Neurons, Not an Overactive Appetite
Tuesday, March 19, 2024
- A Protein Found in Human Sweat May Protect Against Lyme Disease
Monday, March 18, 2024
- Climate Change Alters the Hidden Microbial Food Web in Peatlands
- Genes Identified That Allow Bacteria to Thrive Despite Toxic Heavy Metal in Soil
- Industrial Societies Losing Healthy Gut Microbes
- New Discovery Concerning Occurrence of Antibiotic Resistance
- LATEST NEWS
- Top Science
- Top Physical/Tech
- Top Environment
- Top Society/Education
- Health & Medicine
- Mind & Brain
- Living Well
- Space & Time
- Matter & Energy
- Computers & Math
- Plants & Animals
- Agriculture & Food
- Beer and Wine
- Bird Flu Research
- Genetically Modified
- Pests and Parasites
- Cows, Sheep, Pigs
- Dolphins and Whales
- Frogs and Reptiles
- Insects (including Butterflies)
- New Species
- Spiders and Ticks
- Veterinary Medicine
- Business & Industry
- Biotechnology and Bioengineering
- CRISPR Gene Editing
- Food and Agriculture
- Endangered Animals
- Endangered Plants
- Extreme Survival
- Invasive Species
- Wild Animals
- Education & Learning
- Animal Learning and Intelligence
- Life Sciences
- Behavioral Science
- Biochemistry Research
- Biotechnology
- Cell Biology
- Developmental Biology
- Epigenetics Research
- Evolutionary Biology
- Marine Biology
- Mating and Breeding
- Molecular Biology
- Microbes and More
- Microbiology
- Zika Virus Research
- Earth & Climate
- Fossils & Ruins
- Science & Society
Strange & Offbeat
- Food in Sight? The Liver Is Ready!
- Acid Reflux Drugs and Risk of Migraine
- Do Cells Have a Hidden Communication System?
- Mice Given Mouse-Rat Brains Can Smell Again
- How Do Birds Flock? New Aerodynamics
- Cancer: Epigenetic Origin Without DNA Mutation
- Climate Change Driving Biodiversity Loss
- Why Can't Robots Outrun Animals?
- Evolution of Gliding in Marsupials
- Novel One-Dimensional Superconductor
Trending Topics

Microbiology
- Publishes experimental and theoretical articles, critical reviews, and short communications.
- The target audience is specialists at research institutions and medical workers.
- The journal welcomes manuscripts from all countries.
- Nikolai.V Pimenov

Latest issue
Volume 93, Issue 2
Proceedings of the IV Russian Microbiological Congress, 2023
Latest articles
Drug resistance of different mycobacterium tuberculosis genotypes in the omsk oblast of russia.
- A. A. Vyazovaya
- I. V. Kostyukova
- I. V. Mokrousov
Application of Flow Cytometry for Viability Assay of Mutants for Translation Termination Factors in the Yeast Saccharomyces cerevisiae
- E. P. Efremova
- O. M. Zemlyanko
- G. A. Zhouravleva

Role of Copper Ions in Resistance of Modern Polymer Composite Materials to Fungal Damage
- G. Yu. Yakovleva
- E. A. Katsyuruba
- O. N. Ilyinskaya

Destruction of Biofilms of Gram-Positive and Gram-Negative Bacteria by Serine Protease PAPC from Aspergillus ochraceus
- D. R. Baidamshina
- A. Rafea Nasr
- E. Yu. Trizna

Alkaline Phosphatase Activity and Phosphatase-Active Bacteria in Lake Baikal Water Column and Major Tributaries
- M. Yu. Suslova
- G. V. Podlesnaya
- O. I. Belykh

Journal information
- Biological Abstracts
- Chemical Abstracts Service (CAS)
- Current Contents/Life Sciences
- Google Scholar
- Japanese Science and Technology Agency (JST)
- Norwegian Register for Scientific Journals and Series
- OCLC WorldCat Discovery Service
- Science Citation Index Expanded (SCIE)
- TD Net Discovery Service
- UGC-CARE List (India)
Rights and permissions
Springer policies
© Pleiades Publishing, Ltd.
- Find a journal
- Publish with us
- Track your research
- Search Menu
- FEMS Microbiology Ecology
- FEMS Microbiology Letters
- FEMS Microbiology Reviews
- FEMS Yeast Research
- Pathogens and Disease
- FEMS Microbes
- Awards & Prizes
- Editor's Choice Articles
- Thematic Issues
- Virtual Special Issues
- Call for Papers
- Journal Policies
- Open Access Options
- Submit to the FEMS Journals
- Why Publish with the FEMS Journals
- About the Federation of European Microbiological Societies
- About the FEMS Journals
- Advertising and Corporate Services
- Conference Reports
- Editorial Boards
- Investing in Science
- Journals Career Network
- Journals on Oxford Academic
- Books on Oxford Academic

Six Key Topics in Microbiology: 2024
This collection from the FEMS journals presents the latest high-quality research in six key topic areas of microbiology that have an impact across the world. All of the FEMS journals aim to serve the microbiology community with timely and authoritative research and reviews, and by investing back into the science community .
Interested in publishing your research relevant to the six key microbiology topics?
Learn more about why the FEMS journals are the perfect home for your microbiology research.
Browse the collection categories:
Antimicrobial resistance, environmental microbiology, pathogenicity and virulence, biotechnology and synthetic biology, microbiomes, food microbiology.

FEMS and Open Access: Embracing an Open Future
As of January 2024, FEMS has flipped four of its journals to fully open access (OA), making six out of its seven journals OA. FEMS Microbiology Letters remains a subscription journal and free to publish in.
We are excited to be making high quality science freely available to anyone to read anywhere in the world and further supporting the advancement of our discipline.
View our FAQs page

Never miss the latest research from the FEMS Journals
Stay up to date on the latest microbiology research with content alerts delivered directly to your inbox. This free service from OUP allows you to create custom email alerts to make sure you never miss our on the latest research from your favorite FEMS journals.
Learn more & sign up
Latest posts on X
Affiliations.
- Copyright © 2024
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 15 April 2024
Age-specific nasal epithelial responses to SARS-CoV-2 infection
- Maximillian N. J. Woodall 1 na1 ,
- Ana-Maria Cujba 2 na1 ,
- Kaylee B. Worlock ORCID: orcid.org/0000-0002-5656-7634 3 na1 ,
- Katie-Marie Case 1 ,
- Tereza Masonou 1 ,
- Masahiro Yoshida ORCID: orcid.org/0000-0002-3521-5322 3 ,
- Krzysztof Polanski ORCID: orcid.org/0000-0002-2586-9576 2 ,
- Ni Huang 2 ,
- Rik G. H. Lindeboom ORCID: orcid.org/0000-0002-3660-504X 2 ,
- Lira Mamanova 2 ,
- Liam Bolt ORCID: orcid.org/0000-0001-7293-0774 2 ,
- Laura Richardson ORCID: orcid.org/0000-0002-8075-3816 2 ,
- Batuhan Cakir 2 ,
- Samuel Ellis 1 ,
- Machaela Palor ORCID: orcid.org/0000-0003-4276-5346 1 ,
- Thomas Burgoyne ORCID: orcid.org/0000-0002-8428-720X 4 , 5 ,
- Andreia Pinto ORCID: orcid.org/0000-0002-0840-6844 5 ,
- Dale Moulding ORCID: orcid.org/0000-0002-1431-7047 1 ,
- Timothy D. McHugh ORCID: orcid.org/0000-0003-4658-8594 6 ,
- Aarash Saleh 7 ,
- Eliz Kilich ORCID: orcid.org/0000-0003-0928-8293 3 , 8 ,
- Puja Mehta ORCID: orcid.org/0000-0001-9459-9306 3 , 8 ,
- Chris O’Callaghan 1 ,
- Jie Zhou 9 ,
- Wendy Barclay ORCID: orcid.org/0000-0002-6413-2454 9 ,
- Paolo DeCoppi ORCID: orcid.org/0000-0002-1659-0207 1 , 10 ,
- Colin R. Butler 10 , 11 ,
- Mario Cortina-Borja 1 ,
- Heloise Vinette 1 ,
- Sunando Roy 1 ,
- Judith Breuer ORCID: orcid.org/0000-0001-8246-0534 1 ,
- Rachel C. Chambers ORCID: orcid.org/0000-0003-1370-9417 3 ,
- Wendy E. Heywood 1 ,
- Kevin Mills 1 ,
- Robert E. Hynds 11 , 12 ,
- Sarah A. Teichmann ORCID: orcid.org/0000-0002-6294-6366 2 , 13 na2 ,
- Kerstin B. Meyer ORCID: orcid.org/0000-0001-5906-1498 2 na2 ,
- Marko Z. Nikolić ORCID: orcid.org/0000-0001-6304-6848 3 , 8 na2 &
- Claire M. Smith ORCID: orcid.org/0000-0002-8913-0009 1 na2
Nature Microbiology ( 2024 ) Cite this article
7075 Accesses
1 Citations
470 Altmetric
Metrics details
- Mechanisms of disease
- Molecular biology
Children infected with SARS-CoV-2 rarely progress to respiratory failure. However, the risk of mortality in infected people over 85 years of age remains high. Here we investigate differences in the cellular landscape and function of paediatric (<12 years), adult (30–50 years) and older adult (>70 years) ex vivo cultured nasal epithelial cells in response to infection with SARS-CoV-2. We show that cell tropism of SARS-CoV-2, and expression of ACE2 and TMPRSS2 in nasal epithelial cell subtypes, differ between age groups. While ciliated cells are viral replication centres across all age groups, a distinct goblet inflammatory subtype emerges in infected paediatric cultures and shows high expression of interferon-stimulated genes and incomplete viral replication. In contrast, older adult cultures infected with SARS-CoV-2 show a proportional increase in basaloid-like cells, which facilitate viral spread and are associated with altered epithelial repair pathways. We confirm age-specific induction of these cell types by integrating data from in vivo COVID-19 studies and validate that our in vitro model recapitulates early epithelial responses to SARS-CoV-2 infection.
Similar content being viewed by others

Long COVID: major findings, mechanisms and recommendations

Mechanisms of SARS-CoV-2 entry into cells

Infectious disease in an era of global change
Despite effective vaccines, age remains the single greatest risk factor for COVID-19 mortality. Children infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) rarely develop severe disease 1 , while the mortality in infected people over 85 years is currently as high as 1 in 10 (ref. 2 ). Nasal epithelial cells (NECs) are the primary target of SARS-CoV-2 (refs. 3 , 4 ), and understanding their viral response is crucial as infection of upper airway cells can progress distally 5 , 6 , leading to diffuse alveolar injury with respiratory failure and long-term complications including lung fibrosis 7 .
Initially, it was thought that higher viral entry factor expression of angiotensin-converting enzyme 2 (ACE2) and transmembrane serine protease 2 (TMPRSS2) in adults could explain increased severity, but such differences between children and adults remain uncertain 8 , 9 . Children may alternatively be protected by a pre-activated antiviral state in the upper airways 9 , 10 , but this does not fully explain the increased risk with increasing age. In addition, most in vivo studies so far were unable to identify early cellular responses, since in almost all cases the exact time of infection was unknown, symptom onset was variable and research sampling usually occurred only a few days after testing positive for SARS-CoV-2 (ref. 9 ).
Here we investigated the effects of early SARS-CoV-2 infection on human NECs from healthy children (0–11 years), adults (30–50 years) and older adults (>70 years). NEC were cultured at an air-liquid interface (ALI) and either subjected to mock infection or infected with SARS-CoV-2 for up to 3 days. This setup was used to examine epithelial-intrinsic differences in function, viral replication, gene and protein expression. We reveal age-specific epithelial responses, independent of immune cells, with a strong interferon (IFN) response in infected paediatric goblet inflammatory cells, and the appearance of older adult basaloid-like cells that sustain viral replication and are associated with fibrotic signalling pathways.
Differences in the cellular landscape of NECs with age
We first investigated the cellular composition of NECs at different ages using single-cell RNA sequencing (scRNA-seq; Fig. 1a ). We analysed a dataset of 139,598 cells and identified 24 distinct epithelial cell types or states (Fig. 1b and Extended Data Fig. 1a–c ). These included basal (KRT5 hi ), secretory (SCGB1A1 hi , MUC5AC+) and ciliated (CCDC40+) cells (markers in Extended Data Fig. 1d ). Basal cells encompassed various subpopulations, such as basal, cycling basal, hillock, basal|EMT (associated with epithelial–mesenchymal transition (EMT)) and basaloid-like cells enriched in fibrotic lungs 11 . The second domain includes secretory, goblet and squamous cells, each expressing different secretory proteins and genes related to mucosal defence. The third domain comprised ciliated cells, which were further divided into two clusters on the basis of gene expression patterns associated with cilium organization. Comparison to published nasal COVID-19 datasets 9 , 12 confirmed the accuracy of our cell annotations including ionocytes and hillock cells (Extended Data Fig. 1e,f ).
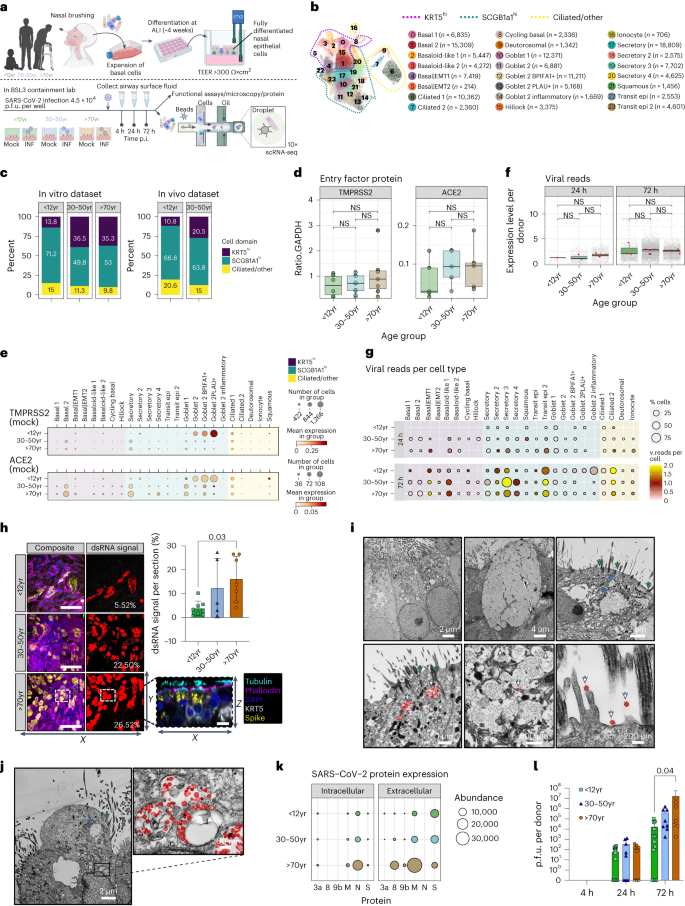
a , Schematic of method and model used to study SARS-CoV-2 infection of paediatric (P, <12 years), adult (A, 30–50 years) and older adult (O, >70 years) nasal epithelial cells. b , UMAP visualization of annotated airway epithelial cells. Cell numbers per cell type are shown in parentheses. Dotted lines indicate the three principal cell domains these fall within: KRT5 high (KRT5 hi ), SCGB1A high (SCGB1A hi ) and ciliated/other. UMAP shows the entire single-cell sequencing (scRNA-seq) dataset, including SARS-CoV-2 and mock-infected NEC cultures across all three timepoints and ages ( n = P3, A4, O4). c , Percentage of annotated airway epithelial cells with respect to age in baseline (non-infected) NEC cultures and following label transfer to an in vivo dataset of nasal brushings from age-matched donors from ref. 9 (data shown as a percentage cells in the three principal cell domains found in each age group). d , SARS-CoV-2 entry factor protein expression per culture type determined by Western blot. Comparisons of ACE2 and TMPRSS2 protein levels normalized to GAPDH were made using the Wilcoxon test. Individual values plotted for each participant, indicated by dots ( n = P9, A7, O8). e , SARS-CoV-2 entry factor gene expression by scRNA-seq. SARS-CoV-2 entry factor gene expression per cell type calculated on the basis of absolute cell numbers, with the average expression of ACE2 and TMPRSS2 indicated by colour. Dot size corresponds to the number of cells expressing ACE2 and TMPRSS2 in respective age groups in the mock condition. f , SARS-CoV-2 RNA viral reads (grey dots, per cell; red dots, per donor) as determined by viral transcript counts (encoding for the full viral genome) per nucleotide per 500 cells (grey dots) or nucleotide per 500 cells per donor (red dots) within each age group. Pairwise comparisons between donors’ age groups were performed using two-sided Wilcoxon rank-sum tests; NS, not significant. g , SARS-CoV-2 viral reads were detected within the scRNA-seq dataset (Infected condition only) at 24 (top) and 72 h (bottom) post infection, shown by cell type and age groups, with dot size and colour indicative of the percentage of cells with detectable viral reads and average reads per cell, respectively. h , Representative maximum intensity z -projections of confocal images (left) of NEC cultures immunolabelled against cilia (cyan, tubulin), dsRNA (yellow) and basal cells (KRT5, white) with DAPI (blue) and phalloidin (magenta) to indicate the nucleus and actin filaments, respectively. Scale bar, 50 μm. Representation of dsRNA signal alone for each section is indicated in red adjacent to respective maximal projections, with the value of spread given on each panel. Summarized on the bar graph to the right (mean ± s.d.), subjected to one-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test. Individual values are shown for each donor ( n = P8, A5, O6). A representative orthogonal section is given (bottom right) to indicate location of dsRNA within infected NECs. i , j , Transmission electron micrographs of epithelial cell types infected with SARS-CoV-2, with selected areas of interest shown at a higher magnitude for each; i , ciliated cells (left), goblet cell (middle), transit (right) and j , ciliated 2 cell types. Panels show components of interest within each cell type, denoted by arrows: white arrows, SARS-CoV-2; green arrows, cilia; blue arrows, secretory mucin granules; viral particles false-coloured with red to aid visualization. k , SARS-CoV-2 protein abundance in apical fluid (extracellular) and cell lysates (intracellular) from SARS-CoV-2-infected NECs for 72 h p.i. as determined by mass spectrometry. Data are shown as mean abundance of protein (dot size) and mean fold change (FC) in protein abundance per donor from mock-infected NECs (colour, age group) ( n = P5, A5, O5). l , Infectious viral titres in combined cell lysate and apical fluid of SARS-CoV-2 nasal epithelial cells from paediatric, adult and older adult donors as determined by plaque assays (mean ± s.d.). Two-way ANOVA with Tukey’s multiple comparisons test. Individual values are shown for each donor ( n = P13, A8, O8). Lines in box and whisker plots ( d , f ) indicate median, interquartile range (IQR) and minimum to maximum of the distribution.
Source data
Interestingly, we observed age-related differences in cell-type proportions in healthy control cultures, with a higher abundance of basal/progenitor subtypes in adult versus paediatric cultures (Fig. 1c and Extended Data Fig. 1g ), mirroring an in vivo nasal epithelial dataset 9 (Extended Data Fig. 1e,f ). All age groups exhibited similar apical differentiation, mucus and tubulin expression (Extended Data Fig. 1h ), and ciliary activity (Extended Data Fig. 2a,b ). There was no substantial difference in ciliary beat frequency or cellular motility with age (Extended Data Fig. 2a,c ). However, NEC cultures from older adult donors were thicker (mean ± s.d. 40 ± 18 µm) than paediatric cultures (20 ± 10 µm; P = 0.02) (Extended Data Fig. 2d ) with a distinct spiral morphology typical of NEC cultures (Extended Data Fig. 2e ), though this had no effect on the integrity of the epithelial barrier (Extended Data Fig. 2f ).
The most notable difference in paediatric cultures was an increase of goblet cell types, particularly the goblet 2 cells (Extended Data Fig. 1g ). This shift in cell state from secretory (higher in KRT5 ) to goblet (higher in BPIFA1) cells was not observed in adult and older adult cultures. Importantly, while the total protein levels of SARS-CoV-2 entry factors 13 did not vary with age (Fig. 1d ), paediatric cultures showed higher mRNA expression of TMPRSS2 and ACE2 in goblet cells (Fig. 1e ). In adult and older adult cultures, these markers were predominantly expressed in secretory and basal 2 cell types (Fig. 1e ), suggesting a shift in susceptibility to viral infection from goblet to secretory cell types with age. Other viral entry factors, BSG , CTSL , NRP1 , NRP2 and FURIN showed the same trend as ACE2 and TMPRSS2 (Extended Data Fig. 2g ).
Increased virus production in infected older adult NECs
To determine differences in viral replication between age groups, NEC cultures were infected with an early-lineage SARS-CoV-2 isolate (hCoV-19/England/2/2020; 4 × 10 4 plaque forming units (p.f.u.) per well (approximate multiplicity of infection (MOI) of 0.01 p.f.u. per cell)). Over a 5-day infection period, SARS-CoV-2 replication increased and then peaked at 72 h post infection (p.i.) (Extended Data Fig. 3a,b ); therefore, all subsequent investigations were completed before this timepoint. The total number of viral reads increased with time but did not differ between age groups (Fig. 1f ), with fewer cell types infected (showing >0 viral reads) in paediatric (3/24 cell types) versus adult and older adult cultures (7/24 and 11/24 cell types, respectively) at 24 h p.i. (Fig. 1g and Extended Data Fig. 3c–e ) and a wider range at 72 h p.i. in all age groups (Fig. 1g ). We also measured total viral spread (measured as %dsRNA+ signal coverage) by immunofluorescent analysis at 72 h p.i., which was greater in older adult (mean ± s.d. 16.1% ± 9.5) than in paediatric cultures (3.8% ± 3.1) (Fig. 1h and Supplementary Fig. 1 ). Overall, ciliated 2 and transit epi 2 cells had the highest proportion of viral reads (Fig. 1g ). Strikingly, goblet cell types appeared more infected in paediatric cultures, while adult and older adult cultures showed highest viral reads in secretory cell types (Fig. 1g and Extended Data Fig. 3c–e ). Cells expressing the highest viral reads displayed high ACE2 ( R 2 = 0.71) and TMPRSS2 ( R 2 = 0.57) expression (Extended Data Fig. 3f,g ). Transmission electron microscopy (TEM) demonstrated the presence of viral particles (red) in cells possessing both mucin-containing secretory granules and cilia (Fig. 1i,j , and Supplementary Figs. 2 and 3 ).
Key differences across the age groups were greater apical localization of the SARS-CoV-2 spike protein (Extended Data Fig. 3f ), greater abundance of intracellular and apical secreted SARS-CoV-2 proteins (Fig. 1k ) and higher levels of infectious particles in older adult than in with paediatric cultures, with a significant ( P = 0.04) >800-fold higher titre in older adult (mean ± s.d. 1.64 × 10 7 ± 3.94 × 10 7 p.f.u. per well; n = 8) than in paediatric cultures (1.71 × 10 4 ± 3.20 × 10 4 p.f.u. per well; n = 13) at 72 h p.i. (Fig. 1l and Extended Data Fig. 4i ). These findings support the conclusion that SARS-CoV-2-infected older adult NECs translate more viral protein and generate more replication-competent viruses compared with paediatric cells.
SARS-CoV-2 infection induces age-specific effects
We next profiled the phenotypic effects of infection on epithelial cells, using live cell microscopy, immunofluorescence staining, proteomics and gene expression analysis, and compared these across the age groups.
Overall, we found that compared to uninfected cultures, SARS-CoV-2-infected adult ( P < 0.05, n = 5) and older adult ( P < 0.001, n = 7) cultures had decreased culture thickness (Fig. 2a,b and Extended Data Fig. 4a ) and epithelial integrity ( P < 0.03, n = 7; Fig. 2c ), with no change in adherens junction protein expression (Extended Data Fig. 4b ). This decrease in culture thickness was accompanied by an increase in basal cell mobilization ( P < 0.03, n = 7; Fig. 2d and Extended Data Fig. 4c,d ) and epithelial escape (cell protrusion) from the pseudostratified culture in older adult cultures (Fig. 2a,e ). Some protruded cells carried viral particles (Fig. 2f ) and expressed the SARS-CoV-2 spike protein (Fig. 2g ) and others were shown to completely detach from the pseudostratified epithelium on the apical surface of the culture (Fig. 2h and Extended Data Fig. 4e ). Ultrastructural changes such as endocytosis of cilia basal bodies and sloughing of ciliated cells were observed in all age groups (Supplementary Fig. 3 ). However, there was no significant loss of ciliated cells or changes in ciliary beat frequency (Extended Data Fig. 5a–c ), or entry factor protein expression within 72 h of infection (Extended Data Fig. 5d ).
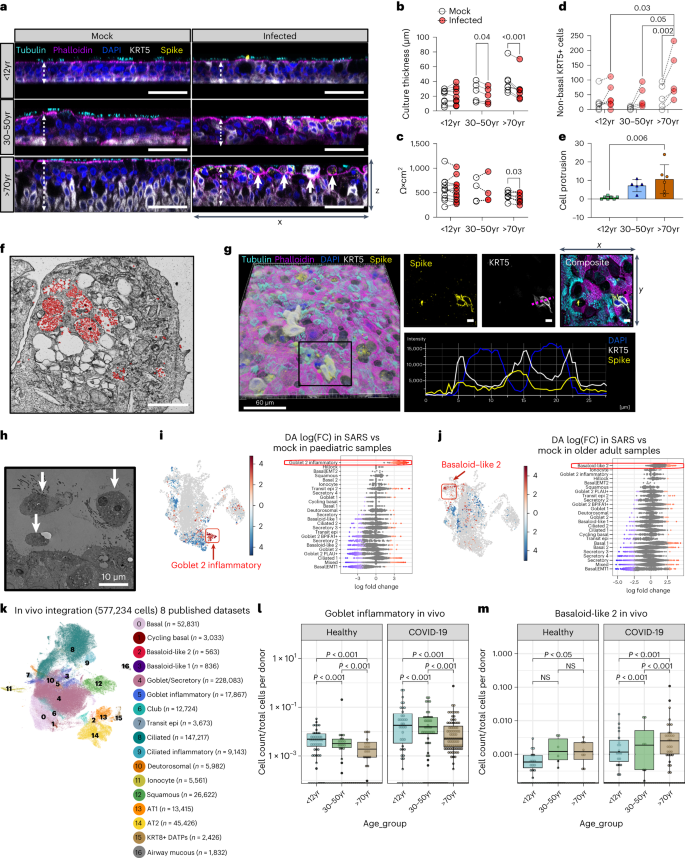
a , Representative orthogonal views of the z -stacks showing the thickness (white dashed arrow) and morphology of fixed paediatric, adult and older adult mock- or SARS-CoV-2-infected NECs at 72 h p.i. Sections were immunolabelled against cilia (cyan, tubulin), F-actin (magenta, phalloidin), DAPI (blue), SARS-CoV-2 S protein (yellow) and cytokeratin 5 (white, KRT5+). Solid white arrows indicate cells protruding from the apical surface (as quantified further in e ). Scale bar, 50 μm. b , Epithelial thickness was further measured and quantified, and subjected to a two-way ANOVA with Sidak’s multiple comparison test ( n = P9, A5, O7). c , Epithelial integrity, as measured by trans-epithelial electrical resistance (TEER) (Ω × cm 2 ) from 72 h p.i. mock- or SARS-CoV-2-infected NECs ( n = P11, A4, O7), subjected to multiple paired t -tests. d , Quantification of non-basal KRT5+ cells (for example, KRT5+ cells above and not touching the basal membrane) as a measure of basal cell mobilization, with age and infection (mock vs infection). Calculated using a cross-section of fixed NECs at 72 h p.i. ( n = P7, A5, O5), subjected to two-way ANOVA with Tukey’s multiple comparisons test. See Extended Data Fig. 4c,d for more details for analysis. e , Cell protrusion analysis, calculated by counting the number of nuclei (blue, indicated by white solid arrows in a ) above apical epithelial membrane (magenta) per section per donor. Data shown as mean ± s.d. ( n = P7, A5, O6), subjected to one-way ANOVA with Tukey’s multiple comparisons test. f , Transmission electron micrograph of protruding epithelial cell type, heavily burdened with SARS-CoV-2 virions (red) at 72 h p.i. Scale bar, 2 μm. g , Representative images of immunofluorescence staining for cells that have escaped the pseudostratified position and reside above the apical membrane, as stained in a . Of note here: SARS-CoV-2 spike (yellow) and KRT5 (white). Image 3D-rendered (left) using Imaris (Bitplane) with Blend filter; scale bar, 60 µm. Scale bar for all other images: 5 µm, rendered in ImageJ in right bottom panel, showing a histogram of distance vs fluorescence intensity for DAPI, KRT5 and SARS-CoV-2 spike staining for a single Z -slice indicated by purple dotted line. h , Transmission electron micrograph of epithelial cell shedding (white arrows) at 72 h p.i. with SARS-CoV-2. i , j , UMAP representation of the results from Milo differential abundance (DA) testing (left plot) with nodes showing cell neighbourhoods and Beeswarm plot (right plot) showing the log(FC) observed when comparing SARS-CoV-2-infected versus mock conditions in paediatric i , and older adults j , with a significant enrichment of goblet 2 inflammatory cells and basaloid-like 2 cells, respectively, observed with infection. Beeswarm plot shows the distribution of log(fold change) across annotated cell clusters when comparing SARS versus mock groups, with cell types ranked on the basis of those with the highest fold change. Grey is non-significant, red is significantly increased, blue is significantly decreased at 10% FDR. k , UMAP visualization of annotated epithelial cells from lower and upper airways of 8 in vivo integrated single-cell datasets. Cell numbers per cell type are shown in parentheses. l , m , Graph comparing the frequency of ( l ) goblet inflammatory and ( m ) basaloid-like 2 cells normalized to the total number of cells per donor. Each dot represents the ratio of the number of cells multiplied by 1,000 to the total cells contributed from one donor and are coloured on the basis of age_status group. Healthy dataset n = P49, A45, O46; COVID-19 dataset n = P41, A58, O116. Statistical analysis was performed on the normalized proportions using zero-inflated Poisson models using the gamlss package in R. Boxplots show the median and IQR, plus the minimum and maximum value distribution. Note the large frequency of donors with zero incidence.
Using Milo 14 , we tested for differential cell state abundance following infection and whether this varied with age. In paediatric cultures, the most significant change was the emergence of goblet 2 inflammatory cells, which were not present in uninfected paediatric cultures (Fig. 2i and Extended Data Fig. 5e,f ). There was a decrease in basal, secretory and goblet cell populations, while the frequency of transit epi 2 and terminally differentiated goblet cells increased (that is, goblet 2 inflammatory) (Fig. 2i and Extended Data Fig. 6e,f ).
The goblet 2 inflammatory cell type is strongly associated with type I IFN signalling, with higher levels of CXCL10 , IFIT1 and IFIT3 markers than other goblet cell subtypes (Extended Data Fig. 1d ). While goblet inflammatory cells have previously been seen in vivo 9 , it is interesting that this inflammatory phenotype is epithelial cell-intrinsic and independent of immune cells that are not present in our cultures. We later (see next section) explore the impact of this on viral replication and spread.
The biggest and consistent change in infected older adult cultures was an increase in basal ( KRT5 hi ) cell populations, indicating an older adult-specific mobilization (proliferation) of progenitor cells following SARS-CoV-2 infection (Fig. 2j , adult dataset shown in Extended Data Fig. 5f,g ) and an expansion of basaloid-like 2 cells (Fig. 2j and Extended Data Fig. 5f ). These recently identified cells are characterized by markers associated with tissue injury and fibrosis ( ITGB6 , ITGB1 , ITGAV , ITGB8 , VIM , TGFB1 ) (Extended Data Fig. 1d ). In healthy epithelial tissue, including skin and lung, integrin beta 6 ( ITGB6 ) mRNA is virtually undetectable 15 , but its expression has been reported to be considerably upregulated during wound healing 16 , tumorigenesis and fibrosis 17 . The presence of these ITGB6 + cells is a major finding as they may be involved in the exacerbation of disease in older adults.
Pseudotime trajectory analysis suggested that goblet 2 inflammatory cells (Extended Data Fig. 2h ) and basaloid-like 1 cells (Extended Data Fig. 2j ) are terminal cell states, differentiating from goblet 2 PLAU+ (Extended Data Fig. 2i ) and Basal|EMT cells (Extended Data Fig. 2k ), respectively, with ciliated 1 cells seen as a third end state (Extended Data Fig. 5h )
In vivo patient validation of induced cell states
To confirm the existence of deregulated cell states in vivo, we performed an integration of 8 scRNA-seq datasets comprising 577,243 cells, spanning upper and lower airways from paediatric (0–18 years), adult (19–50 years), and older adults (51–90 years) that are either healthy or COVID-19 patients 9 , 10 , 12 , 18 , 19 , 20 , 21 , 22 (Fig. 2k and Extended Data Fig. 6a–c ). We identified common epithelial clusters by marker genes (Extended Data Fig. 6c ). Goblet inflammatory cells were induced in response to SARS-CoV-2 across all age groups, with the highest abundances in paediatric COVID-19 and older adult COVID-19 cohorts (Fig. 2l and Extended Data Fig. 6b ). We note that in the older adult COVID-19 cohort, a single donor (mild disease, early post-symptom samples) contributed 82% of all goblet inflammatory cells (Fig. 2l ). Thus, the induction of this cluster is most robust in the paediatric cohort. In the in vivo dataset, we also identified a basaloid-like 2 cell cluster enriched across all COVID-19 patients, which were most abundant in older adult COVID-19 patients across multiple donors (Fig. 2m and Extended Data Fig. 6c ), confirming our in vitro studies. Basaloid-like 2 cells also had the highest increase in fibrosis patients (both idiopathic pulmonary fibrosis and other pulmonary fibrosis), as previously reported 11 , 23 (Extended Data Fig. 6c ).
Stronger interferon response in paediatric cultures
As described, SARS-CoV-2 infection is associated with strong interferon responses, which were particularly apparent in paediatric goblet 2 inflammatory NECs but absent in mock-infected cultures and rare in infected older age groups (proportion of total goblet 2 inflammatory cells from NEC cultures: paediatric = 1,455/1,578, adult = 90/1,578, older adult = 33/1,578) (Extended Data Fig. 1g ). These cells exhibited high levels of interferon-stimulated genes (ISGs), associated with both type I and II interferon signalling (Fig. 3a,b and Extended Data Fig. 7a,b ), and were previously shown to reduce COVID-19 severity 10 , 24 . In addition, paediatric cultures-secreted proteins also showed an association with epithelial barrier and humoral immune response pathways (Extended Data Fig. 7c–e ).
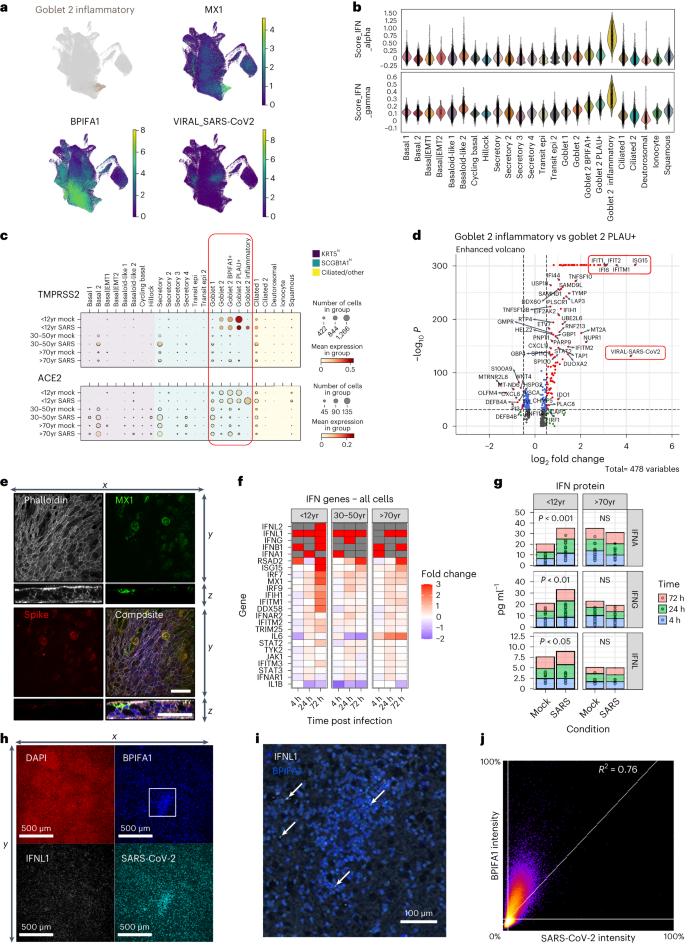
a , UMAP visualization of expression of differentially expressed genes in goblet 2 inflammatory cells. Gene expression is shown in log1p scale. b , Scores of gene ontology (GO) term gene signatures for the terms: response to type 1 interferon ( GO:0035455 or GO:0034340 ) and type 2 interferon ( GO:0034341 ) across cell types. Scores were calculated with Scanpy as the average expression of the signature genes subtracted with the average expression of randomly selected genes from bins of corresponding expression values. Each dot is a cell. c , SARS-CoV-2 entry factor gene expression per cell type calculated on the basis of absolute cell numbers with the average expression of TMPRSS2 (top) and ACE2 (bottom) indicated by colour. Dot size corresponds to infected number of cells expressing TMPRSS2 and ACE2 in respective age groups in the mock (all timepoints) and SARS-CoV-2 (all timepoints) infected condition. d , Volcano plot showing differential gene expression between goblet 2 inflammatory and their precursor goblet 2 PLAU+ cells, with a total of 478 variables. Of note were several genes associated with an interferon response (for example, IFI6 , IFITM1 , IFIT1 , IFIT2 and ISG15 ) and SARS-CoV-2 viral replication (highlighted in red) which were significantly enriched within the paediatric goblet 2 inflammatory cells. The colours indicate the genes that have adjusted P values ≤0.05 (blue), a log 2 fold-change ≥1 or ≤−1 (green), or remain unchanged (grey). The dashed horizontal line signals statistical significance threshold (adjusted P values ≤0.05). Two vertical lines show the threshold of log 2 fold-change ≥0.5 and ≤−0.5. e , Visualization of MX1 protein-expressing cells. Maximum intensity projection images of immunofluorescence staining for F-actin (white, phalloidin), MX1 (green), SARS-CoV-2 S protein (red), with DAPI (blue) in composite image. An orthogonal view of the z -stacks is given in the bottom panel. Example given is a SARS-CoV-2-infected paediatric culture at 72 h p.i. Scale bar, 50 µm. f , Fold change in the gene expression in selected IFN genes across all cell types in SARS-CoV-2-infected NECs compared to mock infections in the single-cell datasets. Shown at each timepoint and broken down by age group. Where no expression was seen in the mock infection conditions, fold change was capped at 3 (red). Grey highlights genes that were absent in both conditions. g , Level (pg ml −1 ) of interferon protein (IFNA, IFNG and IFNL) within the apical supernatant between SARS-CoV-2 and mock-infected NECs. Two-way paired t -test. * P = 0.05, ** P < 0.01. ( n = P9, O9). h , Representative immunofluorescence images of inflammatory goblet cell markers at 72 h p.i. with SARS-CoV-2. Maximum intensity projection images of immunofluorescence staining in fixed paediatric NECs. Red, DAPI; white, IFNL1; blue, BPIFA1; cyan, SARS-CoV-2 spike (S) protein. i , Higher magnification image of that shown in h with white IFNL1; blue, BPIFA1 (white arrows annotate inflammatory goblet cells). j , Co-localization plot for BPIFA1 and SARS-CoV-2 S protein.
The precursors of goblet inflammatory cells are goblet 2 PLAU+ cells (Extended Data Fig. 2i ) which expressed high levels of TMPRSS2 and ACE2 (Fig. 3c ), suggesting that the virus targeted these cells and induced the generation of goblet inflammatory cells, which again expressed high levels of entry receptors and could thus be the target for further infection. This is supported by high viral reads and high ISG expression specific to this subtype (Fig. 3d ). Coexpression of viral spike protein and the interferon-induced gene MX1 was confirmed at the protein level in our paediatric cultures (Fig. 3e ). Induction of interferon responsive genes appears to be at least partly autocrine since paediatric inflammatory cells transcribed IFNL1 , IFNL2 and IFNA1 genes (Fig. 3f and Extended Data Fig. 7c ). When comparing the ISG response across all cell types and ages, it is apparent that by 72 h p.i. paediatric cultures express more interferon genes (Fig. 3f ), a difference that was validated at the protein level (Fig. 3g ). Furthermore, immunofluorescence staining demonstrated the co-localization ( R 2 = 0.76) of IFNL1 with the goblet 2 inflammatory cell marker BPIFA1 (Fig. 3h,i ).
Goblet inflammatory cells may restrict viral replication
In paediatric cultures, despite high viral reads, the production of infectious virions is lower than in older adult cultures (Fig. 1l ). Examining the distribution of viral reads, we found that viral transcription in paediatric ciliated cells predominantly occurred towards the 3’ end, indicating active viral replication (Fig. 4a ). However, in paediatric goblet 2 inflammatory cells, viral reads were highest near the 5’ end, suggesting failed viral replication (Fig. 4a and Extended Data Fig. 8a–c ). It was concluded that this bias towards the 3’ end was not a technical artefact due to the introduction of the spike-in primer to increase the detection of viral reads, as SARS-CoV-2 reads were successfully amplified without biasing viral distribution (Extended Data Fig. 8d–f ). Moreover, using deep viral sequencing, we found that non-canonical subgenomic SARS-CoV-2 RNAs (sgRNA), particularly spike and ORF7a sgRNA, were more abundant ( P = 0.042) in paediatric and adult samples than in older adult samples (Fig. 4b,c ). These non-canonical sgRNAs can result in defective viral genomes and have been associated with increased interferon production 25 . Paediatric cultures also exhibited more low-frequency and fixed mutations in viral genomes (Fig. 4d and Extended Data Fig. 8g ), particularly before the RNA-dependent RNA polymerase (RdRp) (that is, <16 kb; Fig. 4e ). These findings suggest that there is greater pressure on the virus to mutate in younger cultures, possibly due to the production of defective viral genomes by goblet 2 inflammatory cells (Fig. 4f ). In addition, ultrastructural observations revealed fewer viral particles in paediatric goblet cells than in heavily burdened neighbouring ciliated cells (Fig. 4g and Supplementary Fig. 2 ). Our findings indicate that paediatric goblet inflammatory cells may be responsible for the discrepancy between viral reads and infectious particles.
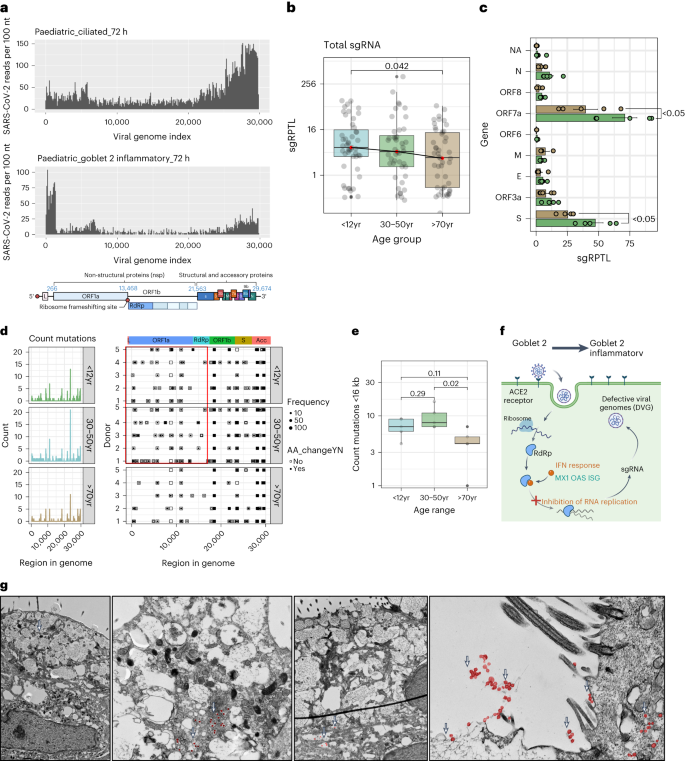
a , Coverage plot of viral reads aligned to SARS-CoV-2 genome from paediatric ciliated 2 (top) and goblet 2 inflammatory (middle) cells at 72 h p.i. Bottom panel shows the genomic organization of SARS-CoV-2 as drawn using Biorender.com . The sequencing depth was computed for each genomic position for each condition. b , Boxplot depicting the sgRPTL normalized counts for sgRNA abundances across age groups using unpaired t -test. c , The mean ± s.d. distribution of these sgRPTL counts across all genes in paediatric (green) and older adult (brown) NEC cultures, subjected to two-way ANOVA with Sidak’s multiple comparisons test ( n = P5, O5). d , Left: frequency of genomic mutations observed in different regions of the SARS-CoV-2 genome. Right: the position and whether an amino acid change was generated from that mutation. Data were generated from 72 h p.i. with SARS-CoV-2 ( n = P5, A5, O5). Bin size is 50 bases. Colour blocks indicate the start coordinates of annotated viral genes. e , Number of genomic mutations occurring <16 kb in genome, shown by age group. Data generated from n = P5, A5, O5. f , Hypothesis of SARS-CoV-2-infected goblet 2 PLAU+ cells becoming protective goblet 2 inflammatory cells through increased interferon and defective viral genome production. Drawn using Biorender.com . g , Transmission electron micrographs of goblet cells at 72 h p.i. with SARS-CoV-2 at different magnifications. Scale bar, 2 μm. Viral particles are false-coloured in red and indicated with white arrows. Lines in box and whisker plots ( b , e ) indicate median, IQR and minimum to maximum of the distribution, with individual values for each cell ( b ) or NEC culture ( e ) shown.
Infected older adult cultures express pro-fibrotic and EMT markers
As discussed above, SARS-CoV-2 infection in older adult cultures led to an increase in basaloid-like 2 cells (Fig. 5a ) associated with a pro-fibrotic state and epithelial–mesenchymal transition (EMT), including expression of ITGB6 , VIM and KRT5 (Fig. 5b and Extended Data Fig. 1d ). Typically membrane bound proteins such as ITGB6, ITGAV and TMPRSS2, produced by these cells, were more abundant in the supernatant of infected cultures from older adults (Fig. 5c and Extended Data Fig. 9a ), possibly originating from shed cells or debris. Vimentin (VIM) was upregulated in cell lysates of SARS-CoV-2-infected older adult cultures compared with mock ( n = 9; P < 0.05) (Fig. 5d and Extended Data Fig. 9b ). Immunofluorescence microscopy revealed the co-localization of ITGB6 protein with SARS-CoV-2 S protein (Fig. 5e and Extended Data Fig. 9c,d ) and the formation of vimentin cages around the virus in some infected older adult cells (Fig. 5e and Extended Data Fig. 9e–g ) 26 . Rare instances of migrating basal cell types (defined by the presence of cytokeratin bundles) burdened with viral compartments were also observed, suggesting that KRT5+, ITGB6+ and VIM+ cells are permissive to SARS-CoV-2 infection (Fig. 5f and Extended Data Fig. 9h ).
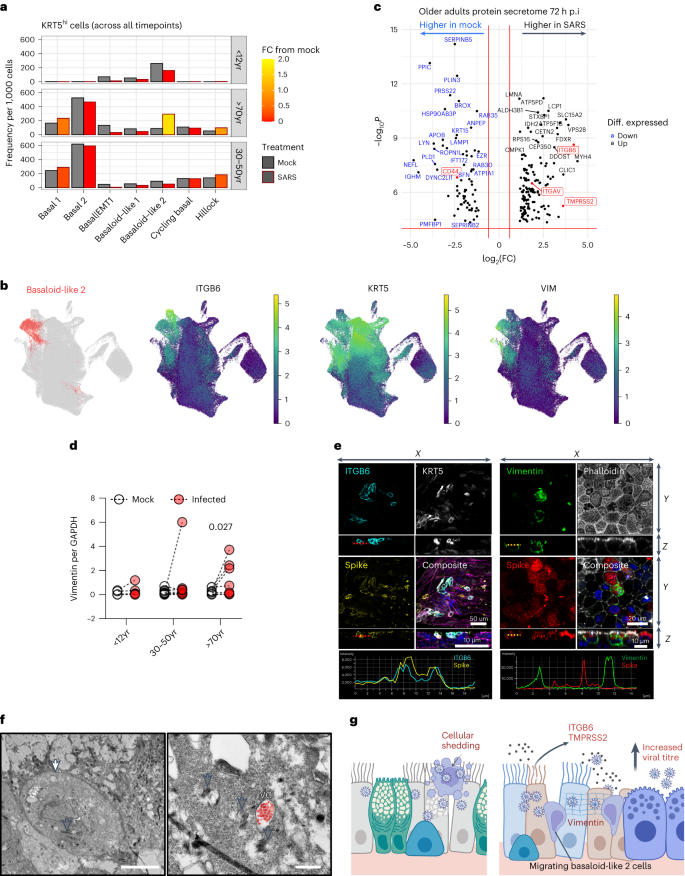
a , Frequency of KRT5 hi basal airway epithelial cells in mock (black outline) and SARS-CoV-2-infected (red outline) conditions across all timepoints (4, 24 and 72 h p.i.) with respect to age. Data shown in ratio of cell numbers per 1,000 cells per age group within scRNA-seq dataset, where the colour of the bars indicates fold change (FC) from the matched cell compartment in the mock condition. b , UMAP visualization of expression of differentially expressed genes ( ITGB6 , KRT5 and Vimentin (VIM) ) in basaloid-like 2 cells. Gene expression is shown in log1p scale. c , Volcano plot of differentially expressed proteins in the apical secretome of mock- and SARS-CoV-2-infected cultures that were unique (highly expressed) in the older adult dataset. Blue highlights those that are highly expressed in mock compared with SARS-CoV-2 infection conditions and black are enriched with infection; of note: ITGAV, ITGB6 and TMPRSS2 in red. The red horizontal line signals statistical significance threshold (adjusted P values ≤0.05). Two vertical lines show the threshold of log 2 fold-change ≥0.5 and ≤−0.5. d , Analysis of vimentin protein levels by Western blot normalized to GAPDH ( n = P5, A9, O9), subjected to multiple paired ratio t -test. e , Representative immunofluorescence images of basaloid-like 2 cell markers in older adults at 72 h p.i. with SARS-CoV-2. Maximum intensity projection images of immunofluorescence staining in fixed older adult NECs. Left: cyan, ITGB6; white, KRT5; yellow, SARS-CoV-2 spike protein; and composite with F-actin (magenta, phalloidin) and DAPI (blue). Right: green, vimentin; F-actin (grey, phalloidin); red, SARS-CoV-2 S protein; and composite with DAPI (blue). White arrows annotate the vimentin cage structure around SARS-CoV-2 S protein. f , Transmission electron micrograph of migrating basal KRT5+ epithelial cell in older adult cultures at 72 h p.i. with SARS-CoV-2 (white arrow). Cytokeratin bundles are indicated (grey arrows) and viral compartments (VC) containing viral particles false-coloured in red. Scale bars, 5 μm (left) and 0.5 μm (right). g , Hypothesis that infection of older adult cells leads to increased shedding of cells heavily burdened with viral particles, which may result in further spread of infection. Repair processes increase KRT5+ and ITGB6+ basaloid-like 2 cells, which are prioritized over the early antiviral responses from goblet 2 inflammatory cells, thereby elevating viral titre. Drawn using Biorender.com .
Although basaloid-like 2 cells showed low levels of viral transcription (Fig. 1g ), the most severely infected and damaged cells are probably shed into the secretome (as hypothesized in Fig. 5h ), leading to more ITGB6 protein (Fig. 5c ) and protruding cells (precursors of shed cells) in infected older adult cultures (Fig. 2e ).
ITGB6 expression in repair enhances viral replication
To investigate the role of basaloid-like 2 cells in SARS-CoV-2 pathogenesis, we performed gene set enrichment analysis (GSEA) and found that these cells are associated with extracellular matrix reorganization, wound response and migration processes (Fig. 6a ). Such processes may facilitate viral spread, metastasis and fibrogenic remodelling 27 , 28 , 29 . They also showed upregulation of alternative viral entry receptors CTSL , FURIN , NRP1 and NRP2 (Extended Data Fig. 10a ), suggesting their potential as targets for infection and spread.
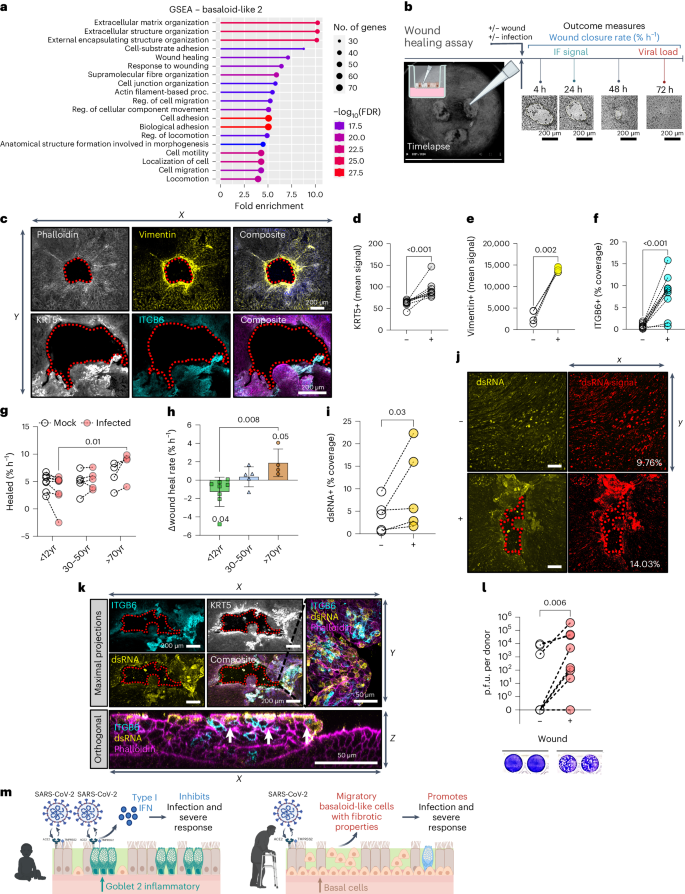
a , GSEA indicating enriched gene ontology terms for basaloid-like 2 cells obtained using ShinyGo. b , Schematic to show the different wound healing assay protocols. c , Representative immunofluorescence images of basaloid-like 2 cell markers at 24 h post-wound NECs. Top: maximum intensity projection images (left to right): F-actin (grey, phalloidin); yellow, vimentin; and composite with DAPI (blue). Bottom (left to right): white, KRT5; cyan, ITGB6; and composite with F-actin (magenta, phalloidin) and DAPI (blue). Scale bar, 200 µm. Basaloid-like 2 cell markers mean fluorescence signal around wound area. Wound area shown by dotted red outline. d – f , Analysis of maximal intensity projections of fixed NECs without (−) and with (+) wounds at 24 h post wounding. d , KRT5+ (mean) signal ( n = 9; P3, A3, O3). e , Vimentin+ (mean) signal ( n = 5; P2, A1, O2). f , ITGB6+ % coverage ( n = 10; P4, A4, O2), subjected to ratio paired t -test. Wound healing rate in NECs from different age groups with mock or SARS-CoV-2 infection. g , Percentage wound closure (healed) per hour (% h −1 ), subjected to two-way ANOVA with Sidak’s multiple comparisons test ( n = P8, A5, O4). h , The difference in wound closure per hour between mock and SARS-CoV-2-infected cells from the same donor. Mean ± s.d. ( n = P8, A5, O4), subjected to one-way ANOVA with Tukey’s multiple comparisons test. Age variable shown as shape (triangles, adults; circles, paediatric). i , dsRNA coverage for NECs irrespective of age group at 72 h p.i. Determined by percentage area covered with dsRNA signal (yellow) from maximum intensity projections of fixed NECs, subjected to ratio paired t -test ( n = 5; P2, A1, O2). j , Representative immunofluorescent images from 72 h p.i. NECs with SARS-CoV-2 without (top) and with (bottom) wounding stained for dsRNA (yellow). Percentage area covered (right) with dsRNA+ signal from maximum intensity projections of fixed NECs using threshold analysis (red) in ImageJ, with the percentage coverage given at the bottom right of each image. k , Representative immunofluorescence images of basaloid-like 2 cell markers ITGB6 (cyan), KRT5 (white), dsRNA (yellow) and F-actin (magenta, phalloidin) in SARS-CoV-2-infected NECs. Maximum intensity projection images from wounded cultures after 24 h, shown both as maximal projections (top) and as an orthogonal view (bottom). KRT5 (white) is omitted from composite images, so that overlap of ITGB6 (cyan) and dsRNA (yellow) is apparent (white). l , Infectious viral titres at 72 h p.i. in combined cell lysate and apical fluid of SARS-CoV-2 nasal epithelial cells from non-wounded (−) and wounded (+) donors that were previously shown to propagate low levels of infectious particles (<10,000 p.f.u. per donor at 72 h p.i.). Infectious viral load in combined apical and cell lysates (p.f.u. per donor) were determined by plaque assays, with representative plaque assay wells shown (bottom). Subjected to paired t -test ( n = 8; P6, A2).
To functionally observe these processes, we employed a wound healing assay (Fig. 6b ). This assay enabled us to stimulate epithelial repair pathways, resulting in the increased expression of basaloid-like 2 cell markers around the wound site including KRT5 protein (Fig. 6c,d and Extended Data Fig. 10b,c ) (mean signal ± s.d. 62.3 ± 8.40 to 94.3 ± 21.1; P < 0.001, n = 9), VIM (Fig. 6c,e and Extended Data Fig. 10d,e ) (mean signal ± s.d. 2,887 ± 1,378 to 14,088 ± 518; P < 0.002, n = 5) and ITGB6 (Fig. 6c,f and Extended Data Fig. 10f,g ) (%coverage ± s.d. 0.72 ± 0.05 to 8.13 ± 4.73; P < 0.001, n = 9). Although we found no difference in wound healing rate of uninfected cultures across ages (Extended Data Fig. 2c ), SARS-CoV-2-infected older adult cultures exhibited a faster ( P = 0.01) wound healing rate (% h −1 , mean ± s.d. 8.01 ± 2.67% n = 4) compared with infected paediatric cultures (3.67 ± 2.78%, n = 8) (Fig. 6g,h and Extended Data Fig. 10h ), indicating greater cell motility and altered repair processes in the older adult. Stimulating wound repair also correlated with an increase in SARS-CoV-2 infection, as evidenced by a higher percentage of dsRNA-positive cells (indicative of replicating virus) in wounded cultures (mean ± s.d. 4.09 ± 3.61% to 9.69 ± 9.04%; P = 0.03, n = 5) (Fig. 6i,j and Extended Data Fig. 10i ), particularly around the site of the wound (Fig. 6k and Supplementary Fig. 1 ). Finally, cultures initially generating low infectious particle counts (<10,000 p.f.u. per donor at 72 h p.i.) showed increased viral particle production upon wounding ( P = 0.006, n = 8) (Fig. 6l ). These data suggest that basaloid-like 2 cells play a role in viral spread and their involvement in the wound healing process may contribute to SARS-CoV-2 infection and replication.
In our comprehensive study of SARS-CoV-2 infection in human NECs, we identified age-associated differences in COVID-19 pathogenesis. Key findings include the induction of a strong early interferon response in paediatric epithelial cultures infected by SARS-CoV-2, leading to incomplete viral replication. In contrast, NEC cultures derived from older adults produce more infectious viruses across various epithelial cell types when compared with paediatric cultures. Moreover, infected NECs from older adults exhibit increased cell shedding, thinning and leakiness, accompanied by the migration of basaloid-like 2 cells associated with wound repair. Interestingly, we showed that previous wounding of cultures resulted in an increased expression of basaloid-like 2 cell genes, promoting viral spread and ultimately augmenting infectious viral yield. Together, these findings contribute towards a deeper understanding of the age-specific nuances of the upper airway and the effects these may have on the pathogenic mechanism underlying SARS-CoV-2 infection across different ages.
Our in vitro model using primary NECs closely resembles SARS-CoV-2 infection in the human airway, the primary site of infection 30 . It confirms age-related changes in upper airway progenitor basal cell types reported previously 10 , 31 and allows for the detection of intrinsic age-related differences in epithelial cells without confounding variations in host immunity.
Notably, we observed a significant shift in the SCGB1A1 hi cell population, transitioning from goblet cells in paediatric cultures to secretory cells with age, with the latter expressing higher levels of SARS-CoV-2 entry factors (ACE2 and TMPRSS2). In paediatric cultures, goblet 2 cell types were a primary target of infection, while in older adult cultures, infected secretory cells accounted for the highest proportion of viral reads.
Through the integration of existing in vivo COVID-19 datasets, we confirmed the existence of both basaloid-like 2 and goblet inflammatory cells identified in vitro to be induced in an age-dependent response to infection. This validates the early epithelial response to SARS-CoV-2 in our in vitro model. However, we acknowledge differences between the in vitro models and patient responses. For example, basaloid-like 2 cells are far less abundant in vivo, as has been reported 32 , which may be due to the site of sampling, cell dissociation protocols or other technical factors 33 . On the other hand, viral and cellular dynamics can be timed more precisely in vitro, while the time interval from the initial infection to sampling is largely unknown for in vivo studies as they are estimated from symptom onset.
We found that fewer paediatric cell types contained viral reads compared with adult and older adult cultures. This is consistent with previous studies indicating that infection in paediatric cells is confined to a limited number of cells due to an early interferon response that limits viral spread 34 . We suggest that this effect is attributed to goblet 2 inflammatory epithelial cells, which decrease with age. These cells have the highest viral genome burden and the strongest interferon signature of all epithelial subtypes. Interestingly, our data suggest incomplete viral replication, increased subgenomic RNA and fewer infectious viruses in paediatric cultures, indicating more defective viral genomes, presumably due to the strong interferon response. Similar observations have been made in animal challenge experiments 35 and patient studies 36 , 37 , in which discrepancies between viral RNA and infectious viral load were also reported.
SARS-CoV-2 infection in older adult NECs led to epithelial damage and early signs of repair through cell migration and basal NEC proliferation. This was not observed in younger cultures. We also detected increases in ITGAV, ITGB6 and VIM proteins, which were attributed to the emergence of basaloid-like 2 cells. ITGB6 is expressed exclusively on epithelial cells but is virtually absent or expressed at very low levels in normal healthy adult epithelium 15 . It is highly upregulated in response to injury 38 and is associated with fibrotic lung disease and epithelial cancers 17 , 39 .
Integrins also modulate cytokine expression and activate TGF-β1, implicated in fibrosis and EMT 38 , a process with distinct pathological roles in wound healing, tissue regeneration and organ fibrosis and cancer 40 . We hypothesize that age-dependent reprogramming of infected NECs contributes to COVID-19 pathogenesis by prolonging disease and enhancing viral spread.
SARS-CoV-2, influenza and other respiratory infections have previously been linked to dysregulated epithelial repair processes and disease pathogenesis 41 , 42 , 43 . We hypothesize that SARS-CoV-2 infection in older adult NECs leads to the emergence of the basaloid-like 2 cell type and drives EMT repair pathways. Elderly cultures infected with SARS-CoV-2 exhibit flattened epithelial tissue, decreased resistance and increased cell shedding, indicating EMT activation 44 . Such functional changes facilitate disease progression and potentially enhance viral spread 45 , 46 . Ultrastructural and immunofluorescence studies confirm significant SARS-CoV-2 infection in shed cells in severe COVID-19 cases 47 . Elevated vimentin levels, an EMT and basaloid cell marker, are also present in these cultures. Our immunofluorescent assays reveal unique vimentin cage-like structures known to recruit viral components for assembly and egress 26 . In addition, the virus may directly interact with ITGB6, a component of caveolae involved in viral internalization 38 , 48 , 49 . In vitro studies suggest that the SARS-CoV-2 spike protein interacts with integrins 50 , 51 , potentially serving as a viral entry route in non-ACE2-expressing cells, thereby promoting infection in older adults. These findings integrate into our proposed model, where older adult cultures are more prone to induce basaloid-like 2 cells in infection, and these cells support both viral spread and disease progression.
In summary, we have shown that SARS-CoV-2 shows age-specific tropism in nasal epithelial cells, targeting goblet cells in children and secretory cells in older adults. Paediatric cells exhibit a strong antiviral response, resulting in limited viral replication. Older adult cells undergo shedding and more epithelial damage. Altered repair pathways and an increase in basaloid-like 2 cells associated with fibrosis markers contribute to greater viral spread in older adults. These findings provide insights into age-related COVID-19 pathogenesis and demonstrate how impaired repair processes enhance SARS-CoV-2 infection in older individuals.
Participants and ethics
Participants were recruited from five large hospital sites in London, the United Kingdom: the Great Ormond Street Hospital NHS Foundation Trust, the University College London Hospitals NHS Foundation Trust, the Royal Free London NHS Foundation Trust (the Royal Free Hospital and the Barnet Hospital) and the Whittington Health NHS Trust from March 2020 to February 2021. All participants provided written informed consent. Ethics approval was given through the Living Airway Biobank, administered through the UCL Great Ormond Street Institute of Child Health (REC reference: 19/NW/0171, IRAS project ID: 261511, Northwest Liverpool East Research Ethics Committee). Exclusion criteria for the cohort included current smokers, active haematological malignancies or cancer, known immunodeficiencies, sepsis from any cause and blood transfusions within 4 weeks, known bronchial asthma, diabetes, hay fever and other known chronic respiratory diseases such as cystic fibrosis, interstitial lung disease and chronic obstructive pulmonary disease. Nasal brushings were obtained by trained clinicians from healthy paediatric (0–11 years), adult (30–50 years) and older adult (≥70 years) donors who tested negative for SARS-CoV-2 (within 24–48 h of sampling) and reported no respiratory symptoms in the preceding 7 weeks. Brushings were taken from the inferior nasal concha zone using cytological brushes (Scientific Laboratory Supplies, CYT1050). All methods were performed following the relevant guidelines and regulations. Details of the study population are shown in Supplementary Table 1 .
Differentiated human nasal epithelial cell culture
Human nasal brushings were collected fresh for this study and immediately placed in a 15 ml sterile Falcon tube containing 4 ml of transport medium (αMEM supplemented with 1× penicillin/streptomycin (Gibco, 15070), 10 ng ml −1 gentamicin (Gibco, 15710) and 250 ng ml −1 amphotericin B (ThermoFisher, 10746254)) on ice. Four matched paediatric nasal brush samples were sent directly for scRNA-seq 9 . To minimize sample variation, all samples were processed within 24 h of collection and cultured to P1 as previously described 52 . Briefly, biopsies were co-cultured with 3T3-J2 fibroblasts and Rho-associated protein kinase inhibitor (Y-27632) in epithelial cell expansion medium consisting of a 3:1 ratio DMEM:F12 (Gibco, 21765), 1× penicillin/streptomycin and 5% FBS (Gibco; 10270) supplemented with 5 μM Y-27632 (Cambridge Bioscience, Y1000), 25 ng ml −1 hydrocortisone (Sigma, H0888), 0.125 ng ml −1 EGF (Sino Biological, 10605), 5 μg ml −1 insulin (Sigma, I6634), 0.1 nM cholera toxin (Sigma, C8052), 250 ng ml −1 amphotericin B (Gibco, 10746254) and 10 μg ml −1 gentamicin (Gibco, 15710).
Basal cells were separated from the co-culture flasks by differential sensitivity to trypsin and seeded onto collagen I-coated, semi-permeable membrane supports (Transwell, 0.4 µm pore size, Corning). Cells were submerged for 24–48 h in an epithelial cell expansion medium, after which the apical medium was removed, and the basolateral medium was exchanged for epithelial cell differentiation medium to generate ‘air–liquid interface’ (ALI) conditions. PneumaCult ALI medium (STEMCELL Technologies, 05001) was used for differentiation media following manufacturer instructions. Basolateral media were exchanged in all cultures three times a week and maintained at 37 °C and 5% CO 2 . ALI cultures were maintained in PneumaCult ALI medium for 4 weeks to produce differentiated NECs for all downstream experimentation.
Wound healing assay
Mechanical injury of NEC cultures was performed by aspiration in direct contact with the apical cell layer using a P200 sterile pipette tip, creating a wound with a diameter ranging from 750 to 1,500 μm. After wounding, the apical surface of the culture was washed with 200 μl PBS to remove cellular debris. The area of the wound was tracked with the aid of time-lapse microscopy with images taken every 60 min at ×4 magnification (Promon, AIS v.4.6.0.5.). The wound area was calculated each hour using ImageJ. The initial wound area was expressed as 100% to account for variability of wound size. Wounds were considered to be closed when the calculated area fell below 2%, the effective limit of detection due to image processing. Wound closure was calculated as follows: Wound closure (%) = 100 − ((Area/Initial Area) × 100). Wound closure (%) plotted as a function of time (h) was used to calculate the rate of wound closure (% h −1 ).
Virus propagation
The SARS-CoV-2 isolate hCoV-19/England/2/2020 obtained from Public Health England (PHE) was used in this study. For virus propagation, the African green monkey kidney cell line Vero E6 (ATCC: CVCL_0574 ; a kind gift from The Francis Crick Institute, London, United Kingdom and authenticated for use in this study) was used. Vero E6 cells were maintained in DMEM supplemented with 5% FCS and 1× penicillin/streptomycin. Cell media were replenished three times a week and maintained at 37 °C and 5% CO2. Vero E6 cells were infected with an MOI of 0.01 p.f.u. per cell in serum-free DMEM supplemented with 1% NEAA, 0.3% (w/v) BSA and 1× penicillin/streptomycin. A mock condition was conducted in parallel in which an equivalent volume of PBS++ was used instead of viral inoculum. The viral and mock-inoculated cell media were collected after 48 h, centrifuged at 10,000 g for 10 min to remove cellular debris and stored at −70 °C. The viral titre was determined by plaque assay (see below).
Viral infection of NEC cultures
After 28 days, NEC cultures were rinsed with sterile PBS++ and then infected with viral inoculum suspended in PBS++ (4.5 × 10 4 p.f.u. ml −1 , ~0.1 MOI) or an equivalent volume of mock inoculum suspended in PBS++ (mock infection) for 1 h on the apical compartment at 37 °C and 5% CO 2 . The virus inocula were then removed, and the NEC cultures were washed with sterile PBS++ and incubated for up to 72 h. This timepoint was chosen as maximum viral replication was observed at days 2–3 in our pilot studies (Extended Data Fig. 3a ).
Infectious viral load quantification by plaque assay
Vero E6 cells were grown to confluence on 24-well plates and then inoculated with serial dilutions of apical supernatant and cell lysates from infected cultures for 1 h at 37 °C and 5% CO 2 . The inoculum was replaced by an overlay medium supplemented with 1.2% (w/v) cellulose and incubated for 48 h at 37 °C and 5% CO 2 . Plates were fixed with 4% (w/v) paraformaldehyde for 30 min and overlay was aspirated from individual wells. Crystal violet staining was performed for a minimum of 20 min, and then plates were washed with water. The number of visible plaques was counted.
Viral copy number quantification
Viral gene quantification was performed on apical wash supernatants from experiments. Samples were lysed in AVL buffer (Qiagen) and stored at −80 °C until further processing. Viral RNA extractions were performed using a QIAamp viral RNA kit (Qiagen) following manufacturer instructions. Extracted RNA samples (5 μl) were quantified in one-step RT–qPCR using AgPath-ID one-step RT–PCR (Applied Biosystems) with the following cycle conditions: 45 °C for 10 min, 95 °C for 15 min, (95 °C for 15 s + 58 °C for 30 s) in a total of 45 cycles.
Cellular gene quantification was performed with cultured cells collected at the end of the experiments. Cells were lysed in RLT buffer (Qiagen) and extraction was performed using an RNeasy mini kit (Qiagen) following manufacturer instructions. Total RNA was converted into cDNA with qScript cDNA supermix (Quantabio) following manufacturer instructions. RT–qPCR was performed using Taq Man Fast Advanced Master mix with the following cycle conditions: 50 °C for 2 min, 95 °C for 10 min, 95 °C for 30 s and 60 °C for 1 min in a total of 45 cycles. The expression was normalized with GAPDH and then presented as 2 −(ΔCт) in arbitrary units.
SARS-CoV-2 genomic sequencing
Viral genome read coverage.
To visualize the viral read coverage along the viral genome, we used the 10X Genomics cellranger barcoded binary alignment map (BAM) files for every sample. We filtered the BAM files to only retain reads mapping to the viral genome using the bedtools intersect tool 52 . We converted the BAM files into sequence alignment map (SAM) files to filter out cells that were removed in our single-cell data pre-processing pipeline. The sequencing depth for each base position was calculated using samtools count. To characterize read distribution along the viral genome, we counted transcripts of 10 different open reading frames (ORFs): ORF1ab, Surface glycoprotein (S), ORF3a, Envelope protein (O), Membrane glycoprotein (M), ORF6, ORF7a, ORF8, Nucleocapsid phosphoprotein (N) and ORF10.
Detection of SARS-CoV-2 subgenomic RNAs
Subgenomic RNA analysis was conducted using Periscope 53 . Briefly, Periscope distinguished sgRNA reads on the basis of the 5′ leader sequences being directly upstream from each gene’s transcription. The sgRNA counts were then normalized into a measure termed sgRPTL, by dividing the sgRNA reads by the mean depth of the gene of interest and multiplying by 1,000.
Mass spectrometry
Paired mock- and SARS-CoV-2-infected airway surface fluids from groups of 10 paediatric, adult and older adult cultures were selected for this assay. For mass spectrometry, samples were inactivated with the KeyPro UV LED decontamination system (Phoseon Technology) before removal from the Biosafety level 3 laboratory (BSL3). Proteins were precipitated using ice-cold acetone. Protein pellets were resuspended in the digestion buffer as previously described and trypsin (Promega) digested to peptides 54 . Peptides were desalted by solid phase extraction (SPE) and separated by reverse phase chromatography on a NanoAquity LC system coupled to a SYNAPT G2-Si mass spectrometer (Waters) in a UDMSE positive ion electrospray ionization mode. Raw MS data were processed using Progenesis QI analysis software (Nonlinear Dynamics). Peptide identification was performed using the UniProt human reference proteome, with one missed cleavage and 1% peptide false discovery rate (FDR). Fixed modifications were set to carbamidomethylation of cysteines and dynamic modifications of oxidation of methionine.
Western blot
Samples were resolved on 4–15% Mini-PROTEAN TGX Precast Protein Gel (Bio-rad, 4561083) with high molecular mass standards of 10–250 kDa. Proteins were transferred to a Trans-Blot Turbo Mini 0.2 µm nitrocellulose membrane in a Trans-Blot Turbo Transfer System (Bio-rad, 1704150). Membranes were blocked in Odyssey blocking buffer overnight at 4 °C. Membranes were probed with primary antibodies described in Supplementary Table 2 , with dilutions prepared in Odyssey blocking buffer. Incubation with primary antibodies was performed at room temperature (r.t.) for 1 h. These included rabbit anti-ACE2 recognizing both long and short isoforms (Abcam, ab15348, 1:2,000) and rabbit anti-ACE2 specific for the long isoform (Abcam, ab108252, 1:2,000), rat anti-alpha-tubulin (Sigma-Aldrich, MAB1864, 1:2,000) and acetylated forms (Sigma-Aldrich, T6793, 1:2,000), mouse anti-SARS-CoV-2 spike glycoprotein (Abcam, ab273433, 1:2,000), rabbit anti-GAPDH (Abcam, ab9485, 1:3,000), rabbit anti-vimentin (Abcam, ab16700, 1:500) and rabbit anti-E-cadherin (Abcam, ab40772, 1:10,000). After three 15 min washes in PBS containing 0.1% Tween 20, the membranes were incubated with the appropriate IRDye secondary antibodies: goat anti-mouse (LI-COR, 926-68070, dilution 1:18,000) and goat anti-rabbit (LI-COR, 926-32211, dilution 1:18,000), both at room temperature for 1 h. The blots were then visualized using an Odyssey CLx imager and quantified using Image Studio Lite software
Cytokine assay
Apical supernatants were collected by washing the apical surface with 200 μl of PBS. These were snap frozen at −70 °C and inactivated with the KeyPro UV LED decontamination system (Phoseon Technology) in the CL3 laboratory before handling them in a CL2 laboratory. Cytokine and chemokine levels were assessed in 25 μl of supernatants using the multiplex BD CBA bead-based immunoassay kits including: IL6: A7, 558276; IL8 (CXCL8): A9, 558277; TNFα: C4, 560112; IFNγ: E7, 558269; IP10 (CXCL10): B5, 558280; IFNα: B8, 560379; and IL10: B7, 558274. Data were acquired using the BD LSRII flow cytometer and concentrations were obtained from a standard curve (provided with the kit). Analysis was performed using the FCAP software (v.3.0, BD Biosciences).
Immunofluorescence confocal microscopy
For immunofluorescence confocal imaging, NEC cultures were fixed using 4% (v/v) paraformaldehyde for 30 min, permeabilized with 0.2% Triton X-100 (Sigma) for 15 min and blocked using 5% goat serum (Sigma) in PBS for 1 h. The cultures were then incubated overnight at 4 °C with primary antibodies described in Supplementary Table 2 , with dilutions prepared in 5% goat serum in PBS with 0.1% Triton X-100. The primary antibodies used included rabbit anti-ACE2 (Abcam, ab15348, diluted 1:200), mouse anti-MUC5AC (Sigma-Aldrich, MAB2011, diluted 1:500), rat anti-alpha-tubulin (tyrosinated) (Sigma-Aldrich, MAB1864, diluted 1:100), mouse anti-alpha-tubulin (acetylated) (Sigma-Aldrich, T6793, diluted 1:100), mouse anti-SARS-CoV-2 spike glycoprotein (Abcam, ab273433, diluted 1:500), rabbit anti-GAPDH (Abcam, ab9485, diluted 1:250), mouse anti-dsRNA (Jena Bioscience, RNT-SCI-10010500, diluted 1:100), rabbit anti-MX1 (Abcam, ab207414, diluted 1:250), rabbit anti-cytokeratin 5 conjugated with Alexa Fluor 647 (Abcam, ab193895, diluted 1:100), rabbit anti-vimentin (Abcam, ab16700, diluted 1:1,000), rabbit anti-IL28+29 (Abcam, ab191426, diluted 1:100), goat anti-BPIFA1 (Abcam, EB11482, diluted 1:100) and rat anti-integrin beta 6 (Abcam, ab97588, diluted 1:100).
Following primary antibody incubation, cultures were washed and then incubated with secondary antibodies diluted in 1.25% goat serum in PBS with 0.1% Triton X-100 at r.t. for 1 h the following day. The secondary antibodies included donkey anti-mouse Alexa Fluor 647 (Jackson ImmunoResearch, 715-605-151, 1:600), donkey anti-rat Alexa Fluor 647 (Jackson ImmunoResearch, 712-605-153, 1:600), donkey anti-mouse Alexa Fluor 594 (Jackson ImmunoResearch, 715-585-151, 1:600), donkey anti-rat Alexa Fluor 594 (Jackson ImmunoResearch, 712-585-153, 1:600), donkey anti-mouse Cy3 (Jackson ImmunoResearch, 715-165-151, 1:600), donkey anti-rabbit Cy3 (Jackson ImmunoResearch, 711-165-152, 1:600), donkey anti-rat Alexa Fluor 488 (Jackson ImmunoResearch, 712-545-153, 1:600), donkey anti-rabbit Alexa Fluor 488 (Jackson ImmunoResearch, 711-545-152, 1:600), donkey anti-mouse Alexa Fluor 488 (Jackson ImmunoResearch, 715-545-151, 1:600) and donkey anti-goat Alexa Fluor 488 (Jackson ImmunoResearch, 705-545-147, 1:600).
After the secondary antibody incubation, cultures were stained with Alexa Fluor 555 phalloidin (ThermoFisher, A34055 , 4 μg ml −1 ) for 1 h and DAPI (Sigma, 2 μg ml −1 ) for 15 min at r.t. to visualize F-actin and nuclei, respectively. Samples were washed three times with PBS containing 0.1% Tween 20 after each incubation step.
Imaging was carried out using an LSM710 Zeiss confocal microscope, and the resulting images were analysed using Fiji/ImageJ v.2.1.0/153c54 for metrics including mean intensity, cell protrusion, culture thickness, % signal coverage, wound area and pseudocolouring 55 . Intensity profiles were generated using Nikon NIS-Elements analysis module, and three-dimensional (3D) renderings of immunofluorescence images were produced with Imaris software (Bitplane, Oxford Instruments; v.9.5/9.6).
Transmission electron microscopy
Cultured NECs that were either SARS-CoV-2-infected or non-infected were fixed with 4% paraformaldehyde and 2.5% glutaraldehyde in 0.05 M sodium cacodylate buffer at pH 7.4 and placed at 4 °C for at least 24 h. The samples were incubated in 1% aqueous osmium tetroxide for 1 h at r.t. before subsequent en bloc staining in undiluted UA-Zero (Agar Scientific) for 30 min at r.t. The samples were dehydrated using increasing concentrations of ethanol (50, 70, 90, 100%), followed by propylene oxide and a mixture of propylene oxide and araldite resin (1:1). The samples were embedded in araldite and left at 60 °C for 48 h. Ultrathin sections were acquired using a Reichert Ultracut E ultramicrotome and stained using Reynold’s lead citrate for 10 min at r.t. Images were taken on a JEOL 1400Plus TEM equipped with an Advanced Microscopy Technologies (AMT) XR16 charge-coupled device (CCD) camera and using the AMT Capture Engine software.
Sample preparation for single-cell RNA sequencing
Cultured NECs were processed using an adapted cold-active protease single-cell dissociation protocol 56 , as described below, based on a previously used protocol 9 to allow for a better comparison of matched samples included in both studies. In total, NECs derived from n = 3 paediatric, 4 adult and 4 older adult donors were processed at 24, 48 and 72 h post infection (SARS-CoV-2 and mock) for scRNA-seq.
First, the transwell inserts, in which the NEC cultures were grown, were carefully transferred into a new 50 ml Falcon tube and any residual transport medium was carefully removed so as not to disturb the cell layer. Dissociation buffer (2 ml) was then added to each well, ensuring the cells were covered; 10 mg ml −1 protease from Bacillus licheniformis (Sigma-Aldrich, P5380) and 0.5 mM EDTA in HypoThermosol (STEMCELL Technologies, 07935). The cells were incubated on ice for 1 h. Every 5 min, cells were gently triturated using a sterile blunt needle, decreasing from a 21G to a 23G needle. Following dissociation, protease was inactivated by adding 400 µl of inactivation buffer (HBSS containing 2% BSA) and the cell suspension was transferred to a new 15 ml Falcon tube. The suspension was centrifuged at 400 g for 5 min at 4 °C and the supernatant was discarded. Cells were resuspended in 1 ml dithiothreitol wash (10 mM dithiothreitol in PBS) (ThermoFisher, R0861) and gently mixed until any remaining visible mucous appears to break down, or for ~2–4 min. The mixture was centrifuged at 400 g for 5 min at 4 °C and the supernatant was removed. The cells were resuspended in 1 ml of wash buffer (HBSS containing 1% BSA) and centrifuged once more under the same conditions. The single-cell suspension was then filtered through a 40 µm Flowmi cell strainer. Finally, the cells were centrifuged and resuspended in 30 µl of resuspension buffer (HBSS containing 0.05% BSA). Using trypan blue, total cell counts and viability were assessed. Cells (3,125) were then pooled together from the 4 biological replicates with corresponding conditions (for example, all mock viral treatments at 24 h) and the cell concentration was adjusted for 7,000 targeted cell recovery according to the 10x Chromium manual (between 700–1,000 cells per µl). The pools were then processed immediately for 10 × 5′ single-cell capture using the Chromium Next GEM Single Cell V(D)J reagent kit v.1.1 (Rev E Guide) or the Chromium Next GEM Single Cell 5′ V2 (Dual index) kit (Rev A guide). Each pool was run twice.
Of note, each sample was processed fresh for 5’ Next Gen single-cell RNA sequencing and thus pooled per timepoint when loading on the 10X chromium controller. Extra steps were taken where possible to balance sex, age as well as technical factors (that is, 10X chromium kit versions) within these sample pools. Furthermore, the downstream process of the sample pools, including library preparation and sequencing (see below) contained samples from the 4 h, 24 h and 72 h timepoints to mitigate additional technical effects. Timepoints can be seen to be well mixed within the single-cell dataset as visualized via a uniform manifold approximation and projection (UMAP) in Extended Data Fig. 1b .
For several samples (Supplementary Table 3 ), 1 µl viral RT oligo (either at 5 µM or 100 µM, PAGE) was spiked into the master mix (at step 1.2.b in the 10X guide, giving a final volume of 75 µl) to help with the detection of SARS-CoV-2 viral reads. The samples were then processed according to manufacturer instructions, with the viral cDNA separated from the gene expression libraries (GEX) by size selection during step 3.2. Here the supernatant was collected (159 µl) and transferred to a new PCR tube and incubated with 70 µl of SPRI beads (0.6× selection) at r.t. for 5 min. The SPRI beads were then washed according to the guide and the viral cDNA was eluted using 30 µl of EB buffer. Neither changes to the transcriptome were previously observed upon testing the addition of viral oligo 9 , nor were any significant changes observed with an increasing concentration upon comparison, outside of a small increase in the overall number of SARS-CoV-2 reads detected. The RT oligo sequence was as follows: 5′-AAGCAGTGGTATCAACGCAGAGTACTTACTCGTGTCCTGTCAACG-3′
Library generation and sequencing
The Chromium Next GEM Single Cell 5′ V2 kit (v.2.0 chemistry) was used for single-cell RNA-seq library construction. For all NEC culture samples, libraries were prepared according to manufacturer protocol (10X Genomics) using individual Chromium i7 sample indices. GEX libraries were pooled and sequenced on a NovaSeq 6000 S4 flow cell (paired-end, 150 bp reads), aiming for a minimum of 50,000 paired-end reads per cell for GEX libraries.
Single-cell RNA-seq data processing
Computational pipelines, processing and analysis.
The single-cell data were mapped to a GRCh38 ENSEMBL 93 derived reference, concatenated with 21 viral genomes (featuring SARS-CoV-2), of which the NCBI reference sequence IDs are: NC_007605.1 (EBV1), NC_009334.1 (EBV2), AF156963 (ERVWE1), AY101582 (ERVWE1), AY101583 (ERVWE1), AY101584 (ERVWE1), AY101585 (ERVWE1), AF072498 (HERV-W), AF127228 (HERV-W), AF127229 (HERV-W), AF331500 (HERV-W), NC_001664.4 (HHV-6A), NC_000898.1 (HHV-6B), NC_001806.2 (herpes simplex virus 1), NC_001798.2 (herpes simplex virus 2), NC_001498.1 (measles morbillivirus), NC_002200.1 (mumps rubulavirus), NC_001545.2 (rubella), NC_001348.1 (varicella zoster virus), NC_006273.2 (cytomegalovirus) and NC_045512.2 (SARS-CoV-2). When examining viral load per cell type, we first removed ambient RNA by SoupX 57 . The alignment, quantification and preliminary cell calling of NEC culture samples were performed using the STARsolo functionality of STAR v.2.7.3a, with the cell calling subsequently refined using the Cell Ranger v.3.0.2 version of EmptyDrops 58 . Initial doublets were called on a per-sample basis by computing Scrublet scores 59 for each cell, propagating them through an over-clustered manifold by replacing individual scores with per-cluster medians and identifying statistically significant values from the resulting distribution, replicating previous approaches 60 , 61 .
Quality control, normalization and clustering
Mixed genotype samples were demultiplexed using Souporcell 62 and reference genotypes. DNA from samples was extracted following manufacturer protocol (Qiagen, DNeasy blood and tissue kit 69504 and Qiagen Genomic DNA miniprep kit) and single nucleotide polymorphism (SNP) array-derived genotypes generated by Affymetrix UK Biobank Axiom Array kit by Cambridge Genomic Services (CGS). Cells that were identified as heterotypic doublets by Souporcell were discarded. Quality control was performed on SoupX-cleaned expression matrixes. Genes with fewer than 3 counts and cells with more than 30% mitochondrial reads were filtered out. Cells with a scrublet score >0.3 and adjusted P value < 0.8 were predicted as doublets and filtered out. Expression values were then normalized to a sum of 1 × 10 4 per cell and log-transformed with an added pseudocount of 1. Highly variable genes were selected using the scanpy.pp.highly_variable_genes() function in Scanpy. Principal component analysis (PCA) was performed and the top 30 principal components were selected as input for l. We performed graph-based batch integration with the bbknn method 63 using experimental pools and chemistry as batch covariate (encoded as ‘bbknn_batch’ in the object). Clustering was performed with the Leiden 64 algorithm on a k -nearest-neighbour graph of a PCA space derived from a log(counts per million/100 + 1) representation of highly variable genes, according to the Scanpy protocol 65 . Leiden clustering with a resolution of 1 was used to separate broad cell types (basal, goblet, secretory). For each broad cell type, clustering was then repeated, starting from highly variable gene discovery to achieve a higher resolution and a more accurate separation of refined cell types. Annotation was first performed automatically using a Celltypist 66 model built on the in vivo dataset of nasal airway brushes 9 , and then using manual inspection of each of the clusters and further manual annotation using known airway epithelial marker genes.
Developmental trajectory inference
Pseudotime inference was performed on the whole object or the basal/goblet compartment using Monocle 3 (refs. 67 , 68 ). Briefly, a cycling basal cell was chosen as a ‘root’ cell for the basal compartment, showing the highest combined expression of KRT5 , MKI67 and NUSAP1 genes. For the goblet compartment, a goblet 1 cell was chosen as a root, showing the highest combined expression of TFF3 , SERPINB3 , MUC5AC , MUC5B and AQP5 . Cells were grouped into different clusters using the group_cells() function, learning the principal graph using the learnGraph() function and ordering cells along the trajectory using the ordercells() function. A second pseudotime was inferred with Palantir (1.0.1) 69 . The cycling basal ‘root’ cell was determined as above and an unsupervised pseudotime inference was performed on a Scanpy-derived diffusion map. The five inferred endpoints were inspected and three were deemed to be very closely biologically related and replaced with a joint endpoint with the highest combined expression of OMG , PIFO and FOXJ1 . The pseudotime inference was repeated with the two remaining inferred endpoints and the marker derived one serving as the three terminal states.
Differential abundance analysis
To determine cell states that are enriched in the SARS-CoV-2 versus mock conditions for the different age groups, we used the Milo framework for differential abundance analysis using cell neighbourhoods 14 . Briefly, we computed k -nearest-neighbour graphs of cells in the whole dataset using the buildGraph() function, assigned cells to neighbourhoods using the makeNhoods() function and counted the number of cells belonging to each sample using the countCells() function. Each neighbourhood was assigned the original cluster labels using majority voting. To test for enrichment of cells in the SARS-CoV-2 condition versus the mock condition, we modelled the cell count in neighbourhoods as a negative binomial generalized linear model, using a log-linear model to model the effects of age and treatment on cell counts (logFC). We control for multiple testing using the weighted Benjamini–Hochberg correction as described in ref. 14 (spatialFDR correction). Neighbourhoods were considered enriched in SARS-CoV-2 vs mock if the spatialFDR < 0.1 and logFC > 0.
Expression signature analysis
To determine the enrichment of basaloid or interferon genes in the annotated clusters, we used the Scanpy function scanpy.tl.score_genes() to score the gene signature for each cell. The gene list for computing the basaloid score was composed of the EPCAM, CDH1, VIM, FN1, COL1A1, CDH2, TNC, VCAN, PCP4, CUX2, SPINK1, PRSS2, CPA6, CTSE, MMP7, MDK, GDF15, PTGS2, SLCO2A1, EPHB2, ITGB8, ITGAV, ITGB6, TGFB1, KCNN4, KCNQ5, KCNS3, CDKN1A, CDKN2A, CDKN2B, CCND1, CCND2, MDM2, HMGA2, PTCHD4 and OCIAD2 genes. The gene list for computing the IFN alpha score was composed of the ADAR, AXL, BST2, EIF2AK2, GAS6, GATA3, IFIT2, IFIT3, IFITM1, IFITM2, IFITM3, IFNAR1, IFNAR2, KLHL20, LAMP3, MX2, PDE12, PYHIN1, RO60, STAR and TPR genes and the gene list for computing the IFN gamma score was composed of the OAS3, OASL, OTOP1, PARP14, PARP9, PDE12, PIAS1, PML, PPARG, PRKCD, PTAFR, PTPN2, RAB20, RAB43, RAB7B, RPL13A, RPS6KB1, SHFL, SIRPA, SLC11A1, SLC26A6, SLC30A8, SNCA, SOCS1, SOCS3, SP100, STAR, STAT1, STX4, STX8, STXBP1, STXBP3, STXBP4, SUMO1, SYNCRIP, TDGF1, TLR2, TLR3, TLR4, TP53, TRIM21, TRIM22, TRIM25, TRIM26, TRIM31, TRIM34, TRIM38, TRIM5, TRIM62, TRIM68, TRIM8, TXK, UBD, VAMP3, VCAM1, VIM, VPS26B, WAS, WNT5A, XCL1, XCL2, ZYX genes, as used in ref. 9 .
Gene set enrichment analysis
Wilcoxon rank-sum test was performed to determine differentially expressed genes between clusters using the scanpy.tl.rank_genes_groups() function. Differentially expressed genes were further analysed using GSEA via ShinyGO 70 .
In vivo sub-analysis
Sex- and age-matched healthy adults and paediatric airway samples ( n = 10 total) were subsetted from our previous dataset 9 for label transfer of the in vitro cell annotation using CellTypist as described above. Selected sample IDs from the in vivo dataset are shown in Supplementary Table 4 . These were selected to match the mean age and range, and sex of the current study as the sample collection and processing were conducted in parallel between studies.
In vivo integration
We performed integration of 8 single-cell datasets comprising 614,695 cells from upper and lower airways from healthy and COVID-19 patients from paediatric (0–18 years), adult (19–50 years) and older adult (51–90 years) samples 9 , 10 , 12 , 18 , 19 , 20 , 21 , 22 . Expression values were then normalized to a sum of 1 × 10 4 per cell and log-transformed with an added pseudocount of 1. Highly variable genes were selected using the Scanpy function scanpy.pp.highly_variable_genes(). PCA was performed and the top 30 principal components were selected as input for Harmony 71 to correct for batch effects between studies and compute a batch-corrected k -nearest-neighbour graph. The clustering was performed with the Leiden 64 algorithm on a k -nearest-neighbour graph of a PCA space derived from a log(counts per million/100 + 1) representation of highly variable genes, according to the Scanpy protocol 65 . Leiden clustering with a resolution of 1 was used to separate broad cell types (basal, goblet, secretory) and subclustering was used for more accurate separation of fine-grained cell types. Annotation was first performed automatically using a Celltypist 66 model built on the in vivo dataset of nasal airway brushes 9 , and then using manual inspection of each of the clusters and further manual annotation using known epithelial marker genes.
Statistical analysis on in vivo dataset
Due to the large proportions of zero counts, we fitted zero-inflated Poisson (ZIP) models to the counts of nasal epithelial cells including the natural logarithm of the total number of cells per donor as an offset in the models for both basaloid and gobletInFam cells. This allowed us to estimate both the incidence of nasal epithelial cells () and the probability of a donor being in the zero counts class () as functions of disease and age groups. We first included interaction terms between the disease and age groups in both the incidence and the zero counts parts of the models. Generalized additive models for location, scale and shape were fitted using the packages gamlss 72 and glmmTMB 73 (to include random effects on the probability of zero class by donor), both in the R language and environment for statistical computing (v.4.2.3) 74 . Using the Bayesian Information Criterion (BIC) as a goodness-of-fit statistic, we decided to include the main effects of disease in both the linear predictors for incidence and probability of belonging to the zero class after stratifying by age group.
Statistical analysis
Statistical analysis was performed using R or GraphPad Prism 9 and details of statistical tests used are indicated. Data distribution was assumed to be normal unless stated differently, and a Kruskal–Wallis test was used to test for normality using R. The determination of sample sizes was guided by those established in previous scRNA-seq and studies using ALI cultures 9 , 75 , rather than through the application of specific statistical methods. In total, NEC cultures generated from 11 participants were used to create our in vitro single-cell dataset, including 3 paediatric (<12 years), 4 adult (30–50 years) and 4 older adult (>70 years) donors. Additional statistical power here was provided by the experimental design, sampling from multiple timepoints (4, 24 and 72 h post SARS-CoV-2 infection), with the inclusion of matched mock-infected controls for each. Samples were also carefully pooled (see Methods) to help avoid batch effects and run across multiple lanes on the 10X controller. Together, this resulted in a total of 66 NEC samples processed for single-cell sequencing, with a total of 139,598 high-quality cells sequenced. Further validation of our in vitro single-cell data and our key observation was provided using an integrated in vivo single-cell dataset (using published patient datasets) and numerous experimental validation assays. Data collection and analysis were not performed blind to the conditions of the experiments. Representative images are displayed as examples for quantified data (1 n of the total n noted in their corresponding summary graphs) unless otherwise stated in the figure legend.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
RNA-seq data are available in the European Genome-Phenome Archive (EGA) ( https://ega-archive.org/ ) under accession number EGAD00001015345 . The datasets from our study can be downloaded and explored interactively through a web portal ( https://covid19cellatlas.org , https://www.covid19cellatlas.org/ALI_COVID19/in-vitro/ , https://www.covid19cellatlas.org/ALI_COVID19/in-vivo/ ). Quality control metrics for our single-cell data are provided on the web portal page. All other data needed to evaluate the conclusions are available within the Article or its Supplementary Information . Viral sequences resulting from this study can be found on Genbank under the accession numbers PP346398 – PP346416 . Source image files for the main figures are available via Figshare at https://doi.org/10.6084/m9.figshare.25193618.v1 (ref. 76 ). Source image files for the extended figures are available via Figshare at https://doi.org/10.6084/m9.figshare.25194005.v1 (ref. 77 ). Source image files for supplementary data are available via Figshare at https://doi.org/10.6084/m9.figshare.25196396.v1 (ref. 78 ). Source data are provided with this paper.
Code availability
Custom code for the analysis performed in this study is publicly available via GitHub at https://github.com/Teichlab/ALI_COVID19 (ref. 79 ). Free data access to the single-cell objects used in this study is available at https://www.covid19cellatlas.org/ .
Lu, X. et al. SARS-CoV-2 infection in children. N. Engl. J. Med. 382 , 1663–1665 (2020).
Article PubMed Google Scholar
Yanez, N. D., Weiss, N. S., Romand, J.-A. & Treggiari, M. M. COVID-19 mortality risk for older men and women. BMC Public Health 20 , 1742 (2020).
Sungnak, W. et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 26 , 681–687 (2020).
Article CAS PubMed PubMed Central Google Scholar
Ahn, J. H. et al. Nasal ciliated cells are primary targets for SARS-CoV-2 replication in the early stage of COVID-19. J. Clin. Invest . 131 , e148517 (2021).
Chen, J. et al. Nonmuscle myosin heavy chain IIA facilitates SARS-CoV-2 infection in human pulmonary cells. Proc. Natl Acad. Sci. USA 118 , e2111011118 (2021).
Huang, J. et al. SARS-CoV-2 infection of pluripotent stem cell-derived human lung alveolar Type 2 cells elicits a rapid epithelial-intrinsic inflammatory response. Cell Stem Cell 27 , 962–973.e7 (2020).
Chu, H. et al. Comparative tropism, replication kinetics, and cell damage profiling of SARS-CoV-2 and SARS-CoV with implications for clinical manifestations, transmissibility, and laboratory studies of COVID-19: an observational study. Lancet Microbe 1 , e14–e23 (2020).
Bunyavanich, S., Do, A. & Vicencio, A. Nasal gene expression of angiotensin-converting enzyme 2 in children and adults. JAMA 323 , 2427–2429 (2020).
Yoshida, M. et al. Local and systemic responses to SARS-CoV-2 infection in children and adults. Nature 602 , 321–327 (2022).
Loske, J. et al. Pre-activated antiviral innate immunity in the upper airways controls early SARS-CoV-2 infection in children. Nat. Biotechnol. 40 , 319–324 (2022).
Article CAS PubMed Google Scholar
Habermann, A. C. et al. Single-cell RNA sequencing reveals profibrotic roles of distinct epithelial and mesenchymal lineages in pulmonary fibrosis. Sci. Adv. 6 , eaba1972 (2020).
Ziegler, C. G. K. et al. Impaired local intrinsic immunity to SARS-CoV-2 infection in severe COVID-19. Cell 184 , 4713–4733.e22 (2021).
Hoffmann, M. et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181 , 271–280.e8 (2020).
Dann, E., Henderson, N. C., Teichmann, S. A., Morgan, M. D. & Marioni, J. C. Differential abundance testing on single-cell data using k -nearest neighbor graphs. Nat. Biotechnol. 40 , 245–253 (2022).
Breuss, J. M., Gillett, N., Lu, L., Sheppard, D. & Pytela, R. Restricted distribution of integrin beta 6 mRNA in primate epithelial tissues. J. Histochem. Cytochem . 41 , 1521–1527 (1993).
Haapasalmi, K. et al. Keratinocytes in human wounds express alpha v beta 6 integrin. J. Invest. Dermatol. 106 , 42–48 (1996).
Munger, J. S. et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 96 , 319–328 (1999).
Melms, J. C. et al. A molecular single-cell lung atlas of lethal COVID-19. Nature 595 , 114–119 (2021).
Chua, R. L. et al. COVID-19 severity correlates with airway epithelium–immune cell interactions identified by single-cell analysis. Nat. Biotechnol. 38 , 970–979 (2020).
Trump, S. et al. Hypertension delays viral clearance and exacerbates airway hyperinflammation in patients with COVID-19. Nat. Biotechnol. 39 , 705–716 (2021).
Delorey, T. M. et al. COVID-19 tissue atlases reveal SARS-CoV-2 pathology and cellular targets. Nature 595 , 107–113 (2021).
Bharat, A. et al. Lung transplantation for patients with severe COVID-19. Sci. Transl. Med . 12 , eabe4282 (2020).
Adams, T. S. et al. Single-cell RNA-seq reveals ectopic and aberrant lung-resident cell populations in idiopathic pulmonary fibrosis. Sci. Adv. 6 , eaba1983 (2020).
Vono, M. et al. Robust innate responses to SARS-CoV-2 in children resolve faster than in adults without compromising adaptive immunity. Cell Rep. 37 , 109773 (2021).
Genoyer, E. & López, C. B. The impact of defective viruses on infection and immunity. Annu. Rev. Virol. 6 , 547–566 (2019).
Zhang, Y. et al. The diverse roles and dynamic rearrangement of vimentin during viral infection. J. Cell Sci. 134 , jcs250597 (2020).
Zhao, S.-H. et al. Basigin-2 is the predominant basigin isoform that promotes tumor cell migration and invasion and correlates with poor prognosis in epithelial ovarian cancer. J. Transl. Med. 11 , 92 (2013).
Stewart, C. A. et al. Lung cancer models reveal severe acute respiratory syndrome coronavirus 2-induced epithelial-to-mesenchymal transition contributes to coronavirus disease 2019 pathophysiology. J. Thorac. Oncol. 16 , 1821–1839 (2021).
Aydillo, T. et al. Shedding of viable SARS-CoV-2 after immunosuppressive therapy for cancer. N. Engl. J. Med . 383 , 2586–2588 (2020).
Woodall, M. N. J., Masonou, T., Case, K. M. & Smith, C. M. Human models for COVID-19 research. J. Physiol. 599 , 4255–4267 (2021).
Koch, C. M. et al. Age-related differences in the nasal mucosal immune response to SARS-CoV-2. Am. J. Respir. Cell Mol. Biol. 66 , 206–222 (2022).
Lang, N. J. et al. Ex vivo tissue perturbations coupled to single-cell RNA-seq reveal multilineage cell circuit dynamics in human lung fibrogenesis. Sci. Transl. Med. 15 , eadh0908 (2023).
Deprez, M. et al. A single-cell atlas of the human healthy airways. Am. J. Respir. Crit. Care Med. 202 , 1636–1645 (2020).
Beucher, G. et al. Bronchial epithelia from adults and children: SARS-CoV-2 spread via syncytia formation and type III interferon infectivity restriction. Proc. Natl Acad. Sci. USA 119 , e2202370119 (2022).
Kim, Y.-I. et al. Age-dependent pathogenic characteristics of SARS-CoV-2 infection in ferrets. Nat. Commun. 13 , 21 (2022).
Heald-Sargent, T. et al. Age-related differences in nasopharyngeal severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) levels in patients with mild to moderate coronavirus disease 2019 (COVID-19). JAMA Pediatr. 174 , 902–903 (2020).
Article PubMed PubMed Central Google Scholar
Despres, H. W. et al. Measuring infectious SARS-CoV-2 in clinical samples reveals a higher viral titer:RNA ratio for Delta and Epsilon vs. Alpha variants. Proc. Natl Acad. Sci. USA 119 , e2116518119 (2022).
Sheppard, D. Functions of pulmonary epithelial integrins: from development to disease. Physiol. Rev. 83 , 673–686 (2003).
Arun, A. S., Tepper, C. G. & Lam, K. S. Identification of integrin drug targets for 17 solid tumor types. Oncotarget 9 , 30146–30162 (2018).
Marconi, G. D. et al. Epithelial-mesenchymal transition (EMT): the type-2 EMT in wound healing, tissue regeneration and organ fibrosis. Cells. 10 , 1587 (2021).
Vareille, M., Kieninger, E., Edwards, M. R. & Regamey, N. The airway epithelium: soldier in the fight against respiratory viruses. Clin. Microbiol. Rev. 24 , 210–229 (2011).
Ruan, T. et al. H1N1 influenza virus cross-activates Gli1 to disrupt the intercellular junctions of alveolar epithelial cells. Cell Rep. 31 , 107801 (2020).
Downes, D. J. et al. Identification of LZTFL1 as a candidate effector gene at a COVID-19 risk locus. Nat. Genet . 53 , 1606–1615 (2021).
Kalluri, R. & Weinberg, R. A. The basics of epithelial-mesenchymal transition. J. Clin. Invest. 119 , 1420–1428 (2009).
Morrison, C. B. et al. SARS-CoV-2 infection of airway cells causes intense viral and cell shedding, two spreading mechanisms affected by IL-13. Proc. Natl Acad. Sci. USA 119 , e2119680119 (2022).
Taddei, M. L., Giannoni, E., Fiaschi, T. & Chiarugi, P. Anoikis: an emerging hallmark in health and diseases. J. Pathol. 226 , 380–393 (2012).
Chaudhary, S., Yadav, R. P., Kumar, S. & Yadav, S. C. Ultrastructural study confirms the formation of single and heterotypic syncytial cells in bronchoalveolar fluids of COVID-19 patients. Virol. J. 20 , 97 (2023).
Salanueva, I. J., Cerezo, A., Guadamillas, M. C. & del Pozo, M. A. Integrin regulation of caveolin function. J. Cell. Mol. Med. 11 , 969–980 (2007).
Wickham, T. J., Filardo, E. J., Cheresh, D. A. & Nemerow, G. R. Integrin alpha v beta 5 selectively promotes adenovirus mediated cell membrane permeabilization. J. Cell Biol. 127 , 257–264 (1994).
Duffney, P. F. et al. Cigarette smoke increases susceptibility to infection in lung epithelial cells by upregulating caveolin-dependent endocytosis. PLoS ONE 15 , e0232102 (2020).
Simons, P. et al. Integrin activation is an essential component of SARS-CoV-2 infection. Sci Rep. 11 , 20398 (2021).
Quinlan, A. R. & Hall, I. M. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26 , 841–842 (2010).
Parker, M. D. et al. Subgenomic RNA identification in SARS-CoV-2 genomic sequencing data. Genome Res. 31 , 645–658 (2021).
Bliss, E., Heywood, W. E., Benatti, M., Sebire, N. J. & Mills, K. An optimised method for the proteomic profiling of full thickness human skin. Biol. Proced. Online 18 , 15 (2016).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9 , 676–682 (2012).
Worlock, K. B., Yoshida, M., Meyer, K. & Nikolić, M. Z. Cell dissociation from nasal, bronchial and tracheal brushings with cold-active protease for single-cell RNA-seq v1. Protocols.io https://doi.org/10.17504/protocols.io.btpunmnw (2021).
Young, M. D. & Behjati, S. SoupX removes ambient RNA contamination from droplet-based single-cell RNA sequencing data. Gigascience. 9 , giaa151 (2020).
Lun, A. T. L. et al. EmptyDrops: distinguishing cells from empty droplets in droplet-based single-cell RNA sequencing data. Genome Biol. 20 , 63 (2019).
Wolock, S. L., Lopez, R. & Klein, A. M. Scrublet: computational identification of cell doublets in single-cell transcriptomic data. Cell Syst. 8 , 281–291.e9 (2019).
Popescu, D.-M. et al. Decoding human fetal liver haematopoiesis. Nature 574 , 365–371 (2019).
Pijuan-Sala, B. et al. A single-cell molecular map of mouse gastrulation and early organogenesis. Nature 566 , 490–495 (2019).
Heaton, H. et al. Souporcell: robust clustering of single-cell RNA-seq data by genotype without reference genotypes. Nat. Methods 17 , 615–620 (2020).
Polański, K. et al. BBKNN: fast batch alignment of single cell transcriptomes. Bioinformatics 36 , 964–965 (2020).
Traag, V. A., Waltman, L. & van Eck, N. J. From Louvain to Leiden: guaranteeing well-connected communities. Sci Rep. 9 , 5233 (2019).
Wolf, F. A., Angerer, P. & Theis, F. J. SCANPY: large-scale single-cell gene expression data analysis. Genome Biol. 19 , 15 (2018).
Domínguez Conde, C. et al. Cross-tissue immune cell analysis reveals tissue-specific features in humans. Science 376 , eabl5197 (2022).
Trapnell, C. et al. The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nat. Biotechnol. 32 , 381–386 (2014).
Qiu, X. et al. Single-cell mRNA quantification and differential analysis with Census. Nat. Methods 14 , 309–315 (2017).
Setty, M. et al. Characterization of cell fate probabilities in single-cell data with Palantir. Nat. Biotechnol. 37 , 451–460 (2019).
Ge, S. X., Jung, D. & Yao, R. ShinyGO: a graphical gene-set enrichment tool for animals and plants. Bioinformatics 36 , 2628–2629 (2020).
Korsunsky, I. et al. Fast, sensitive and accurate integration of single-cell data with Harmony. Nat. Methods 16 , 1289–1296 (2019).
Rigby, R. A. & Stasinopoulos, D. M. Generalized additive models for location, scale and shape. J. R. Stat. Soc. C Appl. Stat. 54 , 507–554 (2005).
Article Google Scholar
Brooks, M. E. et al. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 9 , 378–400 (2017).
Ripley, B. D. The R project in statistical computing. MSOR Connect. 1 , 23–25 (2001).
Smith, C. M. et al. Ciliary dyskinesia is an early feature of respiratory syncytial virus infection. Eur. Respir. J. 43 , 485–496 (2014).
Woodall, M. Main_figures_source_data_images_(Age-specific_nasal_epithelial_responses_to_SARS-CoV-2_infection). Figshare https://doi.org/10.6084/m9.figshare.25193618.v1 (2024).
Woodall, M. Extended_figures_source_data_images_(Age-specific_nasal_epithelial_responses_to_SARS-CoV-2_infection). Figshare https://doi.org/10.6084/m9.figshare.25194005.v1 (2024).
Woodall, M. Supplementary_files_source_data_images_(Age-specific_nasal_epithelial_responses_to_SARS-CoV-2_infection). Figshare https://doi.org/10.6084/m9.figshare.25196396.v1 (2024).
Teichmann Group. Analysis code for the ALI_COVID19 paper. GitHub https://github.com/Teichlab/ALI_COVID19 (2024).
Download references
Acknowledgements
This work was funded by a UKRI/BBSRC research grant (BB/V006738/1 awarded to C.M.S., M.Z.N., S.A.T., W.B., C.O., C.R.B., R.E.H.) and the NIHR Great Ormond Street Hospital Biomedical Research Centre. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. We acknowledge funding from Wellcome (WT211276/Z/18/Z and Sanger core grant WT206194 to S.A.T.). M.Z.N. and K.B.M. were funded by the Rosetrees Trust (M944) and Action Medical Research (GN2911). The project received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement 874656. M.Z.N. acknowledges funding from an MRC Clinician Scientist Fellowship (MR/W00111X/1) and the Rutherford Fund Fellowship allocated by the MRC UK Regenerative Medicine Platform 2 (MR/5005579/1). This project was made possible in part by grants 2017-174169 and 2019-202654 from the Chan Zuckerberg Foundation. C.M.S. was supported by grants from Animal Free Research UK (AFR19-20274), GOSH Children’s Charity (COVID_CSmith_017) and the Wellcome Trust (212516/Z/18/Z). Microscopy was performed at the Light Microscopy Core Facility, UCL GOS Institute of Child Health supported by the NIHR GOSH BRC award 17DD08. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. We acknowledge D. Sumanaweera (Wellcome Sanger Institute) for advice on some of the bioinformatic analysis and A. Ma (UCL GOS Institute of Child Health) for help with the analysis of NEC culture heights; Sanger IT and CellGenIT for help; Public Health England for providing the SARS-CoV-2 (hCoV-19/England/2/2020); and the Cell Services science technology platform (STP) at the Francis Crick Institute, London, UK for providing the African green monkey kidney cell line Vero E6 (ATCC: CVCL_0574 authenticated for use in this study).
Author information
These authors contributed equally: Maximillian Woodall, Ana-Maria Cujba, Kaylee B. Worlock.
These authors jointly supervised this work: Sarah A. Teichmann, Kerstin B. Meyer, Marko Z. Nikolić, Claire M. Smith.
Authors and Affiliations
Great Ormond Street UCL Institute of Child Health, London, UK
Maximillian N. J. Woodall, Katie-Marie Case, Tereza Masonou, Samuel Ellis, Machaela Palor, Dale Moulding, Chris O’Callaghan, Paolo DeCoppi, Mario Cortina-Borja, Heloise Vinette, Sunando Roy, Judith Breuer, Wendy E. Heywood, Kevin Mills & Claire M. Smith
Wellcome Sanger Institute, Cambridge, UK
Ana-Maria Cujba, Krzysztof Polanski, Ni Huang, Rik G. H. Lindeboom, Lira Mamanova, Liam Bolt, Laura Richardson, Batuhan Cakir, Sarah A. Teichmann & Kerstin B. Meyer
UCL Respiratory, Division of Medicine, University College London, London, UK
Kaylee B. Worlock, Masahiro Yoshida, Eliz Kilich, Puja Mehta, Rachel C. Chambers & Marko Z. Nikolić
UCL Institute of Ophthalmology, University College London, London, UK
Thomas Burgoyne
Royal Brompton Hospital, Guy’s and St Thomas’ NHS Foundation Trust, London, UK
Thomas Burgoyne & Andreia Pinto
UCL Centre for Clinical Microbiology, Division of Infection and Immunity, University College London, London, UK
Timothy D. McHugh
Royal Free Hospital NHS Foundation Trust, London, UK
Aarash Saleh
University College London Hospitals NHS Foundation Trust, London, UK
Eliz Kilich, Puja Mehta & Marko Z. Nikolić
Department of Infectious Disease, Imperial College London, London, UK
Jie Zhou & Wendy Barclay
Great Ormond Street Hospital NHS Foundation Trust, London, UK
Paolo DeCoppi & Colin R. Butler
Epithelial Cell Biology in ENT Research (EpiCENTR) Group, Developmental Biology and Cancer Department, Great Ormond Street UCL Institute of Child Health, University College London, London, UK
Colin R. Butler & Robert E. Hynds
UCL Cancer Institute, University College London, London, UK
Robert E. Hynds
Theory of Condensed Matter, Cavendish Laboratory/Dept Physics, University of Cambridge, Cambridge, UK
Sarah A. Teichmann
You can also search for this author in PubMed Google Scholar
Contributions
M.N.J.W., A.-M.C., K.B.W., M.Y. and K.-M.C. designed the study, conducted experiments, analysed data and reviewed the manuscript. K.B.W. and M.Y. performed 10x and isolated DNA for genotyping. K.P., N.H. and R.G.H.L. assisted with computational analysis, including providing code for viral distribution analysis and customized dotplot functions. A.S., E.K., P.M., C.O., C.R.B., P.D.C. and M.Z.N. recruited patients and collected nasal brushings and clinical metadata. L.M., L.B. and L.R. prepared sequencing libraries and conducted the sequencing. A.P. and T.B. assisted with transmission electron microscopy image analysis and review of the manuscript. M.P. assisted with cell culture. S.E. assisted with the analysis of the TEM images and the addition of pseudocolour. T.M. assisted with flow cytometric bead assays and scRNA-seq data analysis and review of the manuscript. S.R. and J.B. assisted with viral genomic data analysis and review of the manuscript. W.E.H., H.V. and K.M. assisted with mass spectrometry data analysis and review of the manuscript. D.M. assisted with microscopy data acquisition and reviewed the manuscript. C.M.S., C.O., P.D.C. and C.R.B. provided support through ethics and patient recruitment. M.C.-B. provided statistical support. S.R. and J.B. oversaw viral genomics analysis and interpretation and review of the manuscript. J.Z. and W.B. facilitated the collection of preliminary data. T.D.M. provided support in setting up and training for all BSL3 work. B.C. facilitated data upload on COVID-19 portals. M.N.J.W., A.-M.C., K.B.W., K.B.M., M.Z.N. and C.M.S. wrote the manuscript. P.D.C., C.O., W.B., S.A.T., K.B.M., C.R.B., R.E.H., M.Z.N. and C.M.S. conceived and designed the study, oversaw the funding application and reviewed the manuscript. S.A.T., R.C.C., K.B.M, R.E.H, M.Z.N. and C.M.S. oversaw data analysis and interpretation, and the write-up of the manuscript.
Corresponding authors
Correspondence to Sarah A. Teichmann , Kerstin B. Meyer , Marko Z. Nikolić or Claire M. Smith .
Ethics declarations
Competing interests.
In the past three years, S.A.T. has received remuneration for Scientific Advisory Board Membership from Sanofi, GlaxoSmithKline, Foresite Labs and Qiagen. S.A.T. is a co-founder and holds equity in Transition Bio and Ensocell. Starting 8 January 2024, S.A.T. has been a part-time employee of GlaxoSmithKline. All other authors declare no competing interests.
Peer review
Peer review information.
Nature Microbiology thanks Ivan Zanoni and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended data fig. 1 paediatric nasal epithelial cells have less basal cell subtypes compared to adult and older adult nasal epithelial cells but display comparable differentiation markers and sars-cov-2 entry factor expression..
(a) Violin plot visualization of threshold for percentage of mitochondrial reads in scRNAseq dataset. (b) Uniform manifold approximation and projection (UMAP) visualizations showing good integration of donor IDs, donor pool, treatment, age group, time after infection, 10X Chromium kit version, sex, cell cycle phase and introduced spike-in primer after batch correction (see Methods for more details). (c) Boxplot indicating comparison of cell cycle phase states (G1, G2M, S) amongst the three age groups in mock/infected/combined (All) conditions. (d) Dot plot visualization showing marker genes for annotated airway epithelial cell types, with fraction of expressing cells and average expression within each cell type indicated by dot size and colour, respectively. Broad cell domains are colour coded; KRT5 hi (purple), SCGB1A1 hi (green) and ciliated/other (yellow). Logistic regression based label transfer using Celltypist for the data sets in (e) Yoshida et al. 9 and (f) Ziegler et al. 12 , with fraction of matched cells and average probability score indicated by dot size and colour, respectively (g) Numbers of annotated airway epithelial cells in respect to age (data shown in ratio of cell numbers/per 1000 cells in age dataset). (h) Representative maximum intensity z-projections of confocal images (left and central) and transmission electron micrographs (right panels) of NEC cultures differentiated at an air–liquid interface and immunolabeled against cilia (tubulin, green) and mucin (MUC5AC, red), with DAPI (blue) and phalloidin (grey) to indicate the nucleus and actin filaments, respectively. Scale bar 10-μm applies to all images in the row.
Extended Data Fig. 2 Physiological differences in paediatric, adult and older adult nasal epithelial NEC cultures.
Physiological comparison of paediatric (P), adult (A) and older adult (O) NEC cultures as measure by; (a) ciliary beat frequency (CBF)(Hz) (n = P6, A6, O7); (b) the percentage of +ve ɑ-tubulin staining coverage per 225 μm2 section (n = P6, A4, O5); (c) motility measured by percentage scratch closure over time (n = P8, A3, O8) and (d) culture thickness (n = P9, A5, O7). For ( a-d ) data was plotted as mean ± SD, subject to one-way ANOVA with Tukey’s multiple comparison test (e) Representative light microscope images of whole well scans of NEC cultures depicting the characteristic differences in culture morphology with age (P=Paed, A=Adult, E=Older adult). (f) Comparison of epithelial integrity via trans-epithelial electrical resistance (TEER) (Ω.cm 2 ). Mean ± SD (n = P12, A8, O8) plotted across the age groups using one-way ANOVA with Tukey’s correction. (g) Alternate SARS-CoV-2 entry factor gene expression per cell type calculated based upon absolute cell numbers with the average expression of BSG, CTSL, NRP1, NRP2 and FURIN indicated by colour. Dot size corresponds to the total number of cells expressing alternate viral entry genes in respective age groups in the mock condition. ( h ) Palantir inferred probabilities of cycling basal cells differentiating into basaloid-like 2 or goblet 2 inflammatory cells. (i) Boxplot showing the distribution of pseudotime within each cluster among goblet cell subtypes. (j) Palantir inferred probabilities of cycling basal cells differentiating into basaloid-like 2 cells. (k) Boxplot showing the distribution of pseudotime within each cluster among KRT5 hi cell subtypes. Lines in box and whisker plots i, j indicates median, interquartile range, and minimum to maximum of the distribution, with individual values for each cell shown.
Extended Data Fig. 3 Elderly cells replicate more infectious viruses with a greater distribution of viral reads across epithelial subtypes.
(a) Mean viral replication shown as pfu/ml per donor over a 5-day period (n = 35: P15, A10, O10). (b) Exemplar image of plaque assay used to determine infectious viral load. (c) UMAP of airway cells with detected SARS-CoV2 mRNA in each cell type in cultures mock and SARS-CoV-2 infected for all time points and ages combined (≥1 viral UMI per donor following filtering out ambient RNA). (d) Pie charts showing the fractions of annotated airway epithelial cells containing viral reads at 4 h, 24 h and 72 h p.i. in respect to age. (e) Numbers of annotated airway epithelial cells containing viral reads at 24 h and 72 h post infection (red) in respect to age with total number of cells in each subset (grey) (data shown as cell numbers/per 1000 cells in age dataset). (f) Representative orthogonal views of z-stacks from fixed paediatric (top), adult (middle) and older adult (bottom) NECs at 72 h p.i. with SARS-CoV-2. Sections were immunolabeled against F-actin (phalloidin, grey) DAPI (blue) and SARS-CoV-2 S protein (red). The scale bar represents 10 μm. (g) Linear regression analysis of viral reads vs ACE2 and TMPRSS2 expression per cell at 72 h post infection. Data represents all age groups combined (h) Linear regression analysis of viral reads vs ACE2 and TMPRSS2 expression per cell and grouped by cell domains (i) Linear regression of infectious viral titres in combined cell lysate and apical fluid of SARS-CoV-2 nasal epithelial cells from donors of different ages as determined by plaque assays (n = 29). Subject to Pearson correlation with SE shown for line error.
Extended Data Fig. 4 Minimal cytopathology following SARS-CoV-2 infection of NECs.
(a) Orthogonal views of the z-stacks showing the thickness and morphology from fixed paediatric, adult and older adult NECs from 3 separate donors at 72 h p.i. mock or SARS-CoV-2 infected NEC cultures. Sections were immunolabeled against cilia (tubulin, cyan), F-actin (phalloidin, magenta) DAPI (blue), SARS-CoV-2 S protein (yellow) and cytokeratin 5 (KRT5+, white). Scale bar = 50 µm. (b) Representative maximal intensity projections (top panels) and orthogonal z sections (bottom panels) of NECs stained for E-Cadherin at 72 h p.i. with mock or SARS-CoV-2 (scale bar 50 μm) and expression determined via Western blots and normalised to GAPDH. Data plotted as mean ± SD, subject to one-way ANOVA with Tukey’s multiple comparison test, with individual values shown (n = P5, A5, O5). (c) Representative orthogonal views of the z-stacks showing the localisation of KRT5+ve cells (white) from example NEC cultures (summary in Fig. 2d ). KRT5+ve cells above the horizontal yellow line are classified as non-basal -layer (not in contact with basement membrane), F-actin (phalloidin, magenta) stain references apical membrane and tight-junctions. Each section is 225 µm in width. (d) Maximal projections of the z-stacks showing the localisation of KRT5+ve cells (white) from above and below the horizontal yellow line (classified as non-basal -layer) from example air–liquid interface cultures at 72 h p.i. with mock or SARS-CoV-2 (scale bar 50 μm). (e) Transmission electron micrograph of epithelial cell shedding (white arrows) at 72 h p.i. with SARS-CoV-2 (scale bar 10 μm).
Extended Data Fig. 5 Cytopathology and changes in cell types.
(a) Cilia coverage for each age group at 72 h p.i. Mock and SARS-CoV-2. (left) Representative Immunofluorescent images from 72 h p.i. NECs with mock and SARS-CoV-2 condition stained for a-tubulin (cyan) (scale bar = 50 µm). Percentage area covered (right) with αTubulin+ve signal (cyan) from maximum intensity projections of fixed NECs using threshold analysis (red) in ImageJ. Summary of cilia coverage (right) (n = P5, A4, O5). Subject to one-way ANOVA with Tukey’s multiple comparison test. For 72 h p.i. (mock and SARS-CoV-2 conditions) NECs were compared between age groups, looking at average (b) cilia beat frequency (Hz) (n = P8, A8, O8); (c) α-Tubulin protein expression (n = P11, A8, O7) and (d) SARS-CoV-2 entry factor protein expression (ACE2, TMPRSS2 and short ACE2). Protein levels were determined via Western blot and normalised to GAPDH (n = P6-8, A5-9, O5-8). Data for b-d plotted as mean ± SD, with individual values shown. (e) Total numbers of annotated airway epithelial cells in all mock infected vs all SARS-CoV-2 infected datasets in respect to age (data shown in ratio of cell numbers/per 1000 cells in age dataset) (n = P3, A4, O4). (f) Dot plots showing the log-fold change in SARS-CoV-2 versus mock at different time points for all cell types in different age groups. Calculation based upon absolute cell numbers with the average fold-change indicated by colour. The dot size corresponds to the number of that cell type per 1000 cells from each condition. (g) Uniform manifold approximation and projection (UMAP) representation of the results from Milo differential abundance testing. Nodes are neighbourhoods, coloured by their log fold change when comparing SARS-CoV-2 infected versus mock conditions in adult samples. Non-significant DA neighbourhoods at FDR 10% are coloured grey and significant DA neighbourhoods at FDR 10% are coloured with increased log fold change in red and decreased log fold change in blue. Node sizes correspond to the number of cells in a neighbourhood. The layout of nodes is determined by the position of the neighbourhood index cell in the UMAP. (h) Palantir inferred pseudotime probabilities of cycling basal cells differentiating into ciliated 1, basaloid-like 2 or goblet 2 inflammatory cells.
Extended Data Fig. 6 Basaloid-like cells were predominantly found in COVID-19 older adult patients in vivo.
(a) Dot plot visualisation showing marker genes for annotated epithelial cell types in the integrated in vivo single cell dataset (Fig. 2k ), with fraction of expressing cells and average expression within each cell type indicated by dot size and colour, respectively. Normalised goblet inflammatory cells (b) and basaloid-like 2 cells (c) per total of 5000 cells per age group in all paediatric, adult, and older adult subgroups. PF = pulmonary fibrosis, IPF = idiopathic pulmonary fibrosis. (Healthy dataset n = P49, A45, O46; COVID19 dataset n = P41, A58, O116).
Extended Data Fig. 7 Paediatric goblet 2 inflammatory cells and viral truncation in response to IFN signalling.
(a) Gene Set Enrichment Analysis (GSEA) of HVGs from goblet inflammatory cells indicating enriched gene ontology terms using ShinyGo. (b) Correlation matrix of a subset of interferon genes expressed by all paediatric or older adult cells at 72 h post SARS-CoV-2 infection as determined using the function cor_pmat() in ggcorrplot and Pearson correlation method. (c) ISG gene expression in SARS-CoV-2 infected cultures (as log-fold change compared to mock) per cell type at 4 h, 24 and 72 h p.i. for paediatric and older adult datasets. Barplot indicates the number of cells at each time point. Grey = not detected (d) Volcano plot showing differential expressed proteins of the apical secretome between mock and SARS-CoV-2 infected cultures that were unique (highly expressed) in the paediatric cohort. Blue highlights those that are highly expressed in mock compared to SARS-CoV-2 infection conditions and black enriched with infection. (e) GSEA for expression of apical secretome genes of paediatric cells at 72 h p.i. with SARS-CoV-2 obtained using ShinyGo. The data in a,b,c relates the scRNAseq dataset (n = P3, A4, O4), whilst d and e uses data generated through the analysis of the collected apical secretome (n = P5, A5, O5).
Extended Data Fig. 8 Viral truncation in response to IFN signaling.
Coverage plots of viral reads aligned to SARS-CoV-2 genome in each cell type in (a) paediatric, (b) adult (c) and older adult infected NECs grouped across all time points. Coverage plots of viral reads aligned to SARS-CoV-2 genome for all cell types, shown by age group, both (d) with and (e) without spiked-in primer grouped across all timepoints (see methods for more details of primer) and at (f) 72 h p.i. time points. Viral reads for all coverage plots are shown in 100 nucleotide (nt) bins normalised per 5,000 cells. The data in a - f relates to the scRNA-seq dataset (n = P3, A4, O4). (g) Histogram displaying frequency and position of genomic mutations in SARS-CoV-2 consensus sequences from 72 h p.i. with SARS-CoV-2 (n = P5, A5, O5). Bin size is 1000 bases (left) and 50 bases (right). Colour blocks indicate the start coordinates of annotated viral genes.
Extended Data Fig. 9 Elderly basaloid-like 2 cells drive ITGB6 production and enhance viral pathogenesis.
(a) Abundance of ITGB6 protein in the apical secretome of mock or SARS-CoV-2 infected NECs at 72 h p.i. for each age group (n = P5, A5, O5). As detected using mass spectrometry and shown in boxplot depicting the median and IQR, plus the minimum and maximum value distribution analysed using paired t test. (b) Exemplar Western blot showing vimentin (vim) in the older adult sample 72 h p.i. with mock (-) or SARS-CoV-2 (+) and 72 h p.i. Vero E6 cells as control lysate (ctl). GAPDH is the loading control, E-Cadherin is also given for reference (n = P2, A1, O1). (c) Orthogonal views of the z-stacks showing the location of ITGB6 (green) and KRT5+ve cells (white) from exemplar air–liquid interface cultures, counterstained for F-actin (phalloidin, red) and cell nucleus (DAPI, blue). (d) Representative maximum intensity projections (left) and orthogonal sections (right) of immunofluorescence z-stacks of basaloid-like 2 cell markers ITGB6 (green), KRT5 (white), spike (magenta) counterstained for F-actin (phalloidin; red) and cell nucleus (DAPI, blue) in 72 h p.i. NECs (mock top, infected bottom). (e-g) Further, example immunofluorescence images of basaloid-like 2 cell markers in 72 h p.i. with SARS-CoV-2 in older adult NEC cultures. Markers and respective counterstain colour are indicated (n = O3). (h) Transmission electron micrograph of non-basal KRT5+ve epithelial cell (white arrow) location within an NEC culture at 72 h p.i. with SARS-CoV-2. Scale is on the right of the image. For panels c-h , representative images were selected from older adult NEC cultures (n = 3) 72 hours post-infection (p.i.) with SARS-CoV for each antibody panel.
Extended Data Fig. 10 Wound repair promotes SARS-CoV-2 viral replication.
(a) Dotplot visualisation showing viral entry genes for all cell types, with fraction of expressing cells and average expression within each cell type indicated by dot size and colour, respectively. Appended bar graphs indicate absolute cell numbers per cell type. Data generated using the entire scRNAseq dataset (n = P3, A4, O4). (b) Example immunofluorescence orthogonal sections of basaloid-like 2 cell markers in unwounded or wounded older adult NECs 24 h p.i. with mock or SARS-CoV-2 infection. F-actin (Phalloidin; red), KRT5 (white), ITGB6 (cyan) and DAPI (blue). (c) Mean ± SD KRT5 fluorescence signal (RFU) around wound area in different age groups. From maximal projections of fixed NECs without (-) and with (+) wounds, 24 h post wounding (n = P3, A3, O3) (n = 6). (d) Maximum intensity projection image of a NEC culture unwounded and 24 h post wound. F-actin (Phalloidin; white), Vimentin (VIM) (yellow) and DAPI (blue). (e) Mean ± SD Vimentin fluorescence signal (RFU) around wound area in different age groups. From maximal projections of fixed NECs without (-) and with (+) wounds, 24 h post wound (n = P2, A1, O2). (f) Example immunofluorescence orthogonal sections of basaloid-like 2 cell markers in non-wounded or wounded NECs 24 h p.i. with mock or SARS-CoV-2 infection from a paediatric and older adult donor. Stained for F-actin (Phalloidin; red), ITGB6 (green), SARS-CoV-2 Spike protein (magenta), KRT5 (white) and composite with DAPI (blue). (g) Mean ± SD ITGB6 fluorescence signal (RFU) around wound area in different age groups. From maximal projections of fixed NECs without (-) and with (+) wounds, 24 h post wounding (n = P3, A3-4, O3-2). (h) Example of wound healing images taken of NECs from different age groups and with mock or SARS-CoV-2 infection. Acquired by light microscopy over 24 hours. The scale bar (bottom right) represents 1 mm. (i) Representative immunofluorescence images from 72 h p.i. NECs with SARS-CoV-2 without (-) and with (+) wounding stained for ITGB6 (cyan) and dsRNA (yellow). Percentage area covered (right) with ITGB6+ve or dsRNA+ve signal from maximum intensity projections of fixed NECs using threshold analysis (red) in ImageJ, the percentage coverage is given at the bottom right of each image. For c,e,g the average values for each NEC donor are shown and are subject to a two-way ANOVA with Sidak’s multiple comparisons test. For panels b, d, f, g, i a minimum of 5 experiments (ranging from n = 5–10) were conducted for each antibody panel, from which representative images were selected.
Supplementary information
Supplementary information.
Supplementary Figs. 1–3.
Reporting Summary
Supplementary tables.
Supplementary Tables 1–4.
Source Data Figs. 1–6
Statistical source data.
Source Data Extended Data Figs. 1–8 and 10
Source data extended data fig. 9.
Statistical source data and unprocessed Western blots.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Woodall, M.N.J., Cujba, AM., Worlock, K.B. et al. Age-specific nasal epithelial responses to SARS-CoV-2 infection. Nat Microbiol (2024). https://doi.org/10.1038/s41564-024-01658-1
Download citation
Received : 24 March 2023
Accepted : 04 March 2024
Published : 15 April 2024
DOI : https://doi.org/10.1038/s41564-024-01658-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Aged nasal epithelium is more prone to severe covid-19.
- Ivan Zanoni
Nature Immunology (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

IMAGES
VIDEO
COMMENTS
Microbiology is the study of microscopic organisms, such as bacteria, viruses, archaea, fungi and protozoa. This discipline includes fundamental research on the biochemistry, physiology, cell ...
To investigate the differences in bacterial and fungal community structure and diversity in conjunctival tissue of healthy and diabetic mice. Fengjiao Li, Shuo Yang, Ji Ma, Xiaowen Zhao, Meng Chen and Ye Wang. BMC Microbiology 2024 24 :90. Research Published on: 16 March 2024.
Linfei Shou. Tiefeng Zhou. Bin Li. Frontiers in Microbiology. doi 10.3389/fmicb.2024.1401896. The most cited microbiology journal which advances our understanding of the role microbes play in addressing global challenges such as healthcare, food security, and climate change.
Papers published in the journal cover all aspects of microbial taxonomy, phylogeny, ecology, physiology and metabolism, molecular genetics and genomics, gene regulation, viruses of prokaryotes, as well as interactions between …. View full aims & scope. Find out more about the IP. $2800. Article publishing charge.
Microbial Phytoremediation of Soil and Sediment Pollution: Mechanism, Application and Prospect. Xu Zhang. Christoph Ringli. Xinxin Li. 300 views. The most cited microbiology journal which advances our understanding of the role microbes play in addressing global challenges such as healthcare, food security, and climate change.
First published: March 11, 2024. Acetaminophen (N-acetyl-p-aminophenol; APAP) overdose-induced acute liver injury (AILI) is a huge threat to public health worldwide. Recent research clearly shows that the intestinal microbiota (IM) is a key modulator in AILI.
Microbiology January 1, 2024 See Your Body's Cells in Size and Number The larger a cell type is, the rarer it is in the body—and vice versa—a new study shows
Current Research in Microbial Sciences publishes original papers and short communications, review articles as well as graphical reviews that cover all aspects of the broad field of microbiology, the study of microorganisms, or microbes, a diverse group of unicellular organisms and subcellular genetic material, and their biological interactions with human beings and other life.
Microbiology News. Articles and images on biochemistry research, micro-organisms, cell functions and related topics, updated daily.
Microbiology is now fully Open Access (OA). Find out more about the transformation of the Society's founding journal and its OA future here.As the founding journal from the Microbiology Society, Microbiology brings together communities of scientists from all microbiological disciplines and from around the world. Originally Journal of General Microbiology, we have been publishing the latest ...
Termitidicoccus mucosus gen. nov. sp. nov. a novel Verrucomicrobiota species isolated from Reticulitermes chinensis gives insights of high adaptability of symbiotic bacteria to termite gut ecosystem. Cheng Mei, Yu Shi, Yu Wang, Zhengyong Qiu, Hong Yang. In Press, Corrected Proof, Available online 28 December 2023.
Overview. Microbiology is an international peer-reviewed journal that addresses a broad spectrum of topics in both fundamental and applied microbiology. Publishes experimental and theoretical articles, critical reviews, and short communications. The target audience is specialists at research institutions and medical workers.
Microbiology Research is an international, scientific, peer-reviewed open access journal published quarterly online by MDPI (from Volume 11 Issue 2-2020).. Open Access — free for readers, with article processing charges (APC) paid by authors or their institutions.; High Visibility: indexed within Scopus, ESCI (Web of Science), Embase, and other databases.
Six Key Topics in Microbiology: 2024. in Virtual Special Issues. This collection from the FEMS journals presents the latest high-quality research in six key topic areas of microbiology that have an impact across the world. All of the FEMS journals aim to serve the microbiology community with timely and authoritative research and reviews, and by ...
Formerly known as Zentralblatt für Mikrobiologie; ISSN: 0944-5013. Read the latest articles of Microbiological Research at ScienceDirect.com, Elsevier's leading platform of peer-reviewed scholarly literature.
About Microbiology Research Aims. Microbiology Research (ISSN 2036-7481) is an international and multidisciplinary scientific open access journal that publishes original research, review articles, editorials, perspectives, case reports, and brief reports to benefit researchers, microbiologists, physicians, veterinarians, agronomists and the like.
Age-specific differences upon SARS-CoV-2 infection are marked by emergence of goblet 2 inflammatory cells expressing antiviral interferon stimulating genes in paediatric nasal cultures, and ...
Microbiological Research is publishes research on prokaryotic and eukaryotic microorganisms such as yeasts, fungi, bacteria, archaea, and protozoa. The journal considers research on interactions between pathogenic microorganisms and their environment or hosts. The research should be original and …. View full aims & scope.