Lung Cancer Trial of Osimertinib Draws Praise—and Some Criticism
July 5, 2023 , by Carmen Phillips
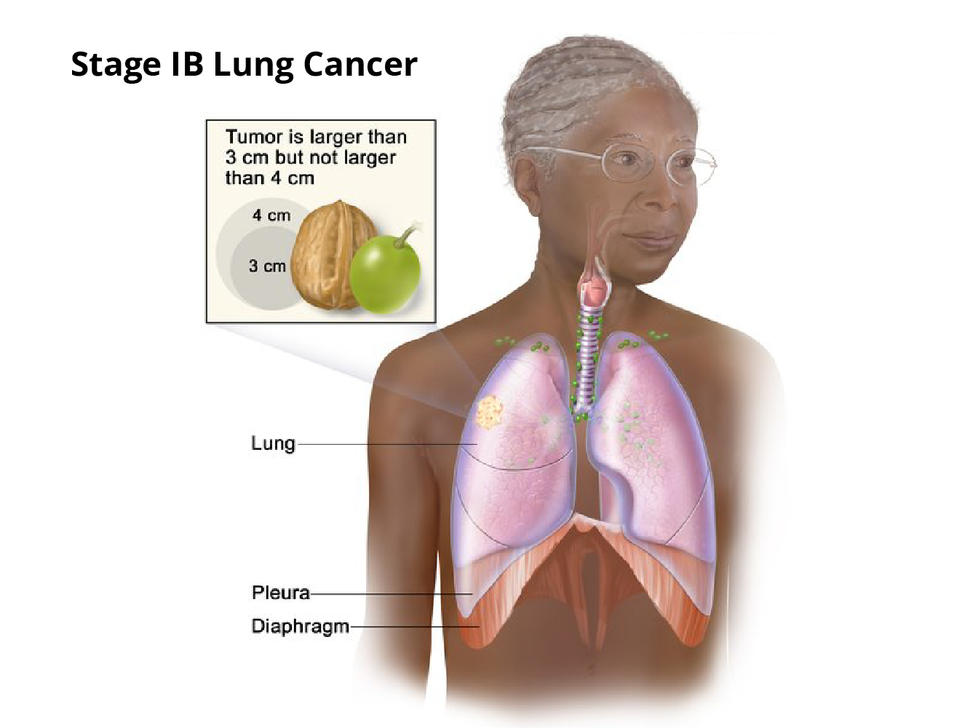
Results from the ADAURA trial are expected to have an impact on the treatment of early-stage lung cancer, including people with stage IB disease.
Highly anticipated results from a large study of people with early-stage lung cancer are generating praise, and some controversy, among oncologists who specialize in treating people with this cancer.
The clinical trial , called ADAURA, tested giving the drug osimertinib (Tagrisso) after surgery in people with early-stage non-small cell lung cancer (NSCLC). All trial participants’ tumors had specific mutations in a gene called EGFR . Osimertinib, which is taken daily as a pill, specifically targets and kills lung cancer cells with these genetic mutations.
In the nearly 700-patient study, people who were randomly assigned to receive osimertinib after surgery to remove their tumor(s)—known as adjuvant therapy — lived substantially longer overall than people assigned to receive a placebo after surgery . At 5 years after starting adjuvant treatment, 88% of people treated with osimertinib were still alive, compared with 78% of those treated with a placebo.
The findings “herald a new era of targeted therapy in early-stage [lung cancer],” said the trial’s lead investigator, Roy Herbst, M.D., of the Yale Cancer Center, who presented the data on June 4 at the 2023 annual meeting of the American Society of Clinical Oncology (ASCO). The results were published the same day in the New England Journal of Medicine .
Many other lung cancer experts agreed. In a commentary on the ADAURA results delivered at the ASCO meeting, Ben Solomon, M.B.B.S., Ph.D., of the Peter MacCallum Cancer Center in Australia, called the findings “groundbreaking.”
But other oncologists who specialize in treating lung cancer or conducting cancer clinical trials were less enthusiastic. Their primary criticism focused on the treatments received by participants in the placebo group, or control arm, whose cancer came back: Only about 40% received osimertinib.
In the United States and much of Europe, osimertinib is the standard therapy in people with early-stage lung cancer that relapses.
So the extent to which adjuvant osimertinib increased how long participants in ADAURA lived is unclear “because so many patients [in the control arm] didn’t get the standard care ,” said Bishal Gyawali, M.D., Ph.D., an oncologist at Queen’s University in Ontario, Canada.
“This is a very good drug,” Dr. Gyawali continued. But in people with early-stage lung cancer whose tumors have EGFR mutations, he said, it’s still unclear from the trial’s results if it’s “appropriate for everyone to receive [osimertinib] immediately after surgery versus receiving the same drug at the time of relapse.”

EGFR inhibitors: From advanced- to early-stage lung cancer
Drugs that target lung cancers with EGFR mutations have been among the most successful of the targeted cancer therapy era that began more than 2 decades ago.
Osimertinib is often referred to as a third-generation EGFR inhibitor , because it was designed to address some of the shortcomings of earlier EGFR inhibitors. Key among the properties of osimertinib is an improved ability to get into the brain and, thus, shrink tumors that have spread there from the lungs—a common occurrence in NSCLC.
Osimertinib is already the standard therapy for people with metastatic NSCLC whose tumors have EGFR mutations and in those initially diagnosed with early-stage disease but whose cancer comes back, explained Charu Aggarwal, M.D., who specializes in treating lung cancer at the University of Pennsylvania Abramson Cancer Center.
Lung-Sparing Surgery Effective for Some with Lung Cancer
A clinical trial showed sublobar surgery is an option for certain people instead of lobectomy.
And even with the dramatic improvements in the treatment of metastatic lung cancer, once the cancer has spread, it’s often fatal. Less than half of people initially diagnosed with stage IIIA NSCLC, in fact, will still be alive 5 years later.
So the ADAURA trial—which was funded by AstraZeneca, osimertinib’s manufacturer—was launched in late 2014 to see if adding osimertinib as an adjuvant therapy for early-stage NSCLC with EGFR mutations might improve outcomes.
Specifically, the ADAURA research team wanted to know if it could prevent, or at least substantially delay, the disease from returning, and increase how long people live overall.
Improved overall survival with osimertinib
The initial results from ADAURA, published in October 2020, offered signs of possible survival improvements with adjuvant osimertinib. This treatment, they showed, dramatically improved how long people lived without any evidence of their cancer returning .
Not long after, the Food and Drug Administration (FDA) approved osimertinib as an adjuvant therapy for people with early-stage NSCLC ( stages IA to IIIB) with certain EGFR mutations. That led at least some US oncologists to start routinely using osimertinib as an adjuvant therapy for these patients, Dr. Aggarwal said—at least in hospitals and cancer centers where mutation testing in tumors is a standard practice.
Although the reasons vary, she continued, failure to routinely perform testing for EGFR mutations “has been an issue.”
But some oncologists argued that, before any wholesale changes in patient care, it was important to see if the drug also helped people live longer. This is particularly important, they argued, because osimertinib can have persistent side effects and can cost nearly half a million dollars a year.
Both are important considerations, because in ADAURA, people assigned to receive osimertinib were to take it for 3 years.
Improved overall survival with adjuvant osimertinib
In total, 682 people participated in the study. Participants were allowed to receive chemotherapy after surgery (but before starting adjuvant osimertinib or placebo), and about 60% did. Treatment with adjuvant chemotherapy was most frequent in people with stage II and IIIA disease (71% and 80%, respectively).
The large improvement in survival, Dr. Solomon noted, was seen regardless of the extent of participants’ cancer. When the analysis was limited to people with stage II to IIIA tumors, for example, the 5-year survival rate was 85% in the osimertinib group and 73% in the placebo group.
FDA Immunotherapy Approval Marks a First for Lung Cancer
Atezolizumab is approved to treat some people with non-small cell lung cancer after surgery.
Osimertinib’s side effects in the trial were consistent with what is typically seen with this drug, with diarrhea and rash among the most common. In the osimertinib group, 13% of people stopped taking the drug entirely because of side effects, and another 27% stopped taking it for periods but then resumed treatment.
Dr. Solomon cautioned that 3 years is a long time to be on a therapy, and that side effects, even if they seem minor, shouldn’t be discounted.
“It’s important for clinicians not to underestimate the impact of continued [low-grade] toxicities on patients,” he said. Such side effects can include diarrhea, dry skin and rash, frequent coughing, and fingernail and toenail infections, among others.
But was the ADAURA placebo group an adequate comparison?
With the trial’s details published, some oncologists began to point out what they viewed as problems with the treatments given to those in the control arm—specifically, the limited number who received osimertinib once their cancer relapsed.
On Twitter, H. Jack West, M.D., a lung cancer expert at City of Hope Comprehensive Cancer Center in Los Angeles, argued that “ADAURA is unfortunately a trial of 100% access to [osimertinib] vs <50% access to [osimertinib].” Instead, he wrote, the control arm should have been osimertinib “for all [patients] at relapse, as needed.”
At the ASCO meeting, Dr. Herbst noted that in the first few years of the trial, osimertinib was not yet approved for treating metastatic NSCLC in the United States and some other countries. So early on, at least, the drug wasn’t available to patients in the control arm whose cancer returned, he said.
Later, after the initial trial results showed the improvement in disease-free survival , control arm participants could be offered the drug if their cancer relapsed after surgery.
Even with that change, however, many patients in the control arm whose cancer relapsed ended up getting earlier-generation EGFR inhibitors. And 15% received no additional treatment at all.
Importance of testing for EGFR mutations
Despite the concerns expressed about the control arm, there seems to be little disagreement that oncologists should discuss adjuvant osimertinib therapy with their patients with early-stage NSCLC that has EGFR mutations.
Dr. West wrote that he believed the overall survival improvement with adjuvant osimertinib “would hold up even if issues around [ADAURA’s] conduct were not present.”
Dr. Gyawali agreed with Dr. West, while also highlighting a larger concern. “That does not mean we should not criticize the trial’s flaws,” he said. Voicing legitimate criticisms is important, he continued, so similar problems “don’t happen in future trials.”
For her part, Dr. Aggarwal said there was now little doubt about what should be happening in everyday clinical practice. “This confirms our practice to test for EGFR mutations and recommend adjuvant targeted therapy with osimertinib” for those whose tumors have the genetic changes, she said.
Osimertinib isn’t an option, however, if patients aren’t tested for EGFR mutations. And as another study presented at the ASCO meeting showed, although testing rates appear to be increasing, they are far from 100% .
Ensuring that all patients with early-stage NSCLC are tested for EGFR mutations is essential, Dr. Herbst said. “The only way we’re going to find these mutations is if we look for them.”
Other studies are ongoing to help better guide the treatment of early-stage lung cancer and how best to use osimertinib, he explained.
One clinical trial, called NeoADAURA , for example, is looking at the impact on patient outcomes of using osimertinib before surgery. And some researchers have already shown the potential of analyzing bits of tumor DNA floating in the blood to determine the need for adjuvant therapy or to monitor if the cancer has come back .
“We need to be able to personalize therapy,” Dr. Aggarwal said. These and other studies are pushing in that direction, she added. “We don’t have the right tools yet. But I think in the near future we’ll get there.”
Featured Posts
February 22, 2024, by Carmen Phillips
January 23, 2024, by Elia Ben-Ari
January 12, 2024, by Shana Spindler
- Biology of Cancer
- Cancer Risk
- Childhood Cancer
- Clinical Trial Results
- Disparities
- FDA Approvals
- Global Health
- Leadership & Expert Views
- Screening & Early Detection
- Survivorship & Supportive Care
- February (6)
- January (6)
- December (7)
- November (6)
- October (7)
- September (7)
- February (7)
- November (7)
- October (5)
- September (6)
- November (4)
- September (9)
- February (5)
- October (8)
- January (7)
- December (6)
- September (8)
- February (9)
- December (9)
- November (9)
- October (9)
- September (11)
- February (11)
- January (10)
- Skip to main content
- Keyboard shortcuts for audio player
- Your Health
- Treatments & Tests
- Health Inc.
- Public Health
After 40 years of smoking, she survived lung cancer thanks to new treatments

Yuki Noguchi

Denise Lee on her last day of chemo. In addition to chemo and surgery, she was treated with immunotherapy. She's currently in remission. Denise Lee hide caption
Denise Lee on her last day of chemo. In addition to chemo and surgery, she was treated with immunotherapy. She's currently in remission.
Denise Lee grew up in Detroit in the mid-1970s and went to an all-girls Catholic high school. She smoked her first cigarette at age 14 at school, where cigarettes were a popular way of trying to lose weight.
Instead, her nicotine addiction lasted four decades until she quit in her mid-50s.
"At some point it got up as high as 2.5 packs a day," Lee, 62, recalls.
Yet she didn't think about lung cancer risk — until she saw a billboard urging former smokers to get screened. Lee, a retired lawyer living in Fremont, Calif., used to drive past it on her way to work.
"The thing that caught my attention was the fact that it was an African American female on the front," she recalls.

Shots - Health News
The american cancer society says more people should get screened for lung cancer.
She eventually got the low-dose CT scan recommended for current and former smokers. When doctors found an early, but dangerous, tumor, Lee cried and panicked. Her mother had cared for her father, who'd died of prostate cancer. "My biggest concern was telling my mom," she says.
But that was six years ago, and Lee is cancer free today. Surgery removed the 2-inch tumor in her lung, then new treatments also boosted her immune system, fighting off any recurrence.
Lung cancer remains the most lethal form of the disease, killing about 135,000 Americans a year – more than breast, prostate and colon cancer combined – which is why many people still think of a diagnosis as synonymous with a death sentence. But with new treatments and technology, the survival rates from lung cancer are dramatically improving, allowing some patients with relatively late-stage cancers to live for years longer.
"If you're gonna have lung cancer, now is a good time," Lee says of the advances that saved her.

Denise Lee has been cancer-free for six years. She says she's grateful she got screened and caught her lung cancer early enough that treatment has been effective. Denise Lee hide caption
Denise Lee has been cancer-free for six years. She says she's grateful she got screened and caught her lung cancer early enough that treatment has been effective.
The key breakthrough, says Robert Winn, a lung cancer specialist at Virginia Commonwealth University, is the ability to better pinpoint the mutations of a patient's particular form of cancer. In the past, treatments were blunt tools that caused lots of collateral damage to healthy parts of the body while treating cancer.
"We've gone from that to molecular characterization of your lung cancer, and it has been a game changer," Winn says. "This is where science and innovation has an impact."
One of those game-changing treatments is called targeted therapy . Scientists identify genetic biomarkers in the mutated cancer cells to target and then deliver drugs that attack those targets, shrinking tumors.

CRISPR gene-editing may boost cancer immunotherapy, new study finds
Another is immunotherapy, usually taken as a pill, which stimulates the body's own defense system to identify foreign cells, then uses the immune system's own power to fight the cancer as if it were a virus.
As scientists identify new cancer genes, they're creating an ever-broader array of these drugs.
Combined, these treatments have helped increase national survival rates by 22% in the past five years – a rapid improvement over a relatively short time, despite the fact that screening rates are very slow to increase. Winn says as these treatments get cheaper and readily available, the benefits are even reaching rural and Black populations with historic challenges accessing health care.
The most remarkable thing about the drugs is their ability to, in some cases, reverse late-stage cancers. Chi-Fu Jeffrey Yang, a thoracic surgeon at Massachusetts General Hospital and faculty at Harvard Medical School, recalls seeing scans where large dark shadows of tumor would disappear: "It was remarkable to see the lung cancer completely melting away."
To Yang, such progress feels personal. He lost his beloved grandfather to the disease when Yang was in college. If he were diagnosed today, he might still be alive.
"Helping to take care of him was a big reason why I wanted to be a doctor," Yang says.
But the work of combating lung cancer is far from over; further progress in lung cancer survival hinges largely on getting more people screened.
Low-dose CT scans are recommended annually for those over 50 who smoked the equivalent of a pack a day for 20 years. But nationally, only 4.5% of those eligible get those scans , compared to rates of more than 75% for mammograms.
Andrea McKee, a radiation oncologist and spokesperson for the American Lung Association, says part of the problem is that lung cancer is associated with the stigma of smoking. Patients often blame themselves for the disease, saying: "'I know I did this to myself. And so I don't I don't think I deserve to get screened.'"
McKee says that's a challenge unique to lung cancer. "And it just boggles my mind when I hear that, because, of course, nobody deserves to die of lung cancer."
Denise Lee acknowledges that fear. "I was afraid of what they would find," she admits. But she urges friends and family to get yearly scans, anyway.
"I'm just so grateful that my diagnosis was early because then I had options," she says. "I could have surgery, I could have chemotherapy, I could be a part of a clinical trial."
And all of that saved her life.
- lung cancer screening
- immunotherapy
- lung cancer
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Front Pharmacol
Immunotherapy and targeted therapy for lung cancer: Current status and future perspectives
Bilal zulfiqar.
1 Griffith Institute for Drug Discovery, Griffith University, Brisbane, QLD, Australia
Asim Farooq
2 Department of Clinical Research, Shaukat Khanum Memorial Cancer Hospital and Research Centre, Lahore, Pakistan
Shahzina Kanwal
3 Institute of Molecular Physiology at Shenzhen Bay Laboratory, Shenzhen, China
Kashif Asghar
4 Department of Basic Sciences Research, Shaukat Khanum Memorial Cancer Hospital and Research Centre, Lahore, Pakistan
Owais Bhat , Virginia Commonwealth University, United States
Namratha Sheshadri , Rutgers University, United States
Lung cancer has the highest incidence of morbidity and mortality throughout the globe. A large number of patients are diagnosed with lung cancer at the later stages of the disease. This eliminates surgery as an option and places complete dependence on radiotherapy or chemotherapy, and/or a combination of both, to halt disease progression by targeting the tumor cells. Unfortunately, these therapies have rarely proved to be effective, and this necessitates the search for alternative preventive approaches to reduce the mortality rate of lung cancer. One of the effective therapies against lung cancer comprises targeting the tumor microenvironment. Like any other cancer cells, lung cancer cells tend to use multiple pathways to maintain their survival and suppress different immune responses from the host’s body. This review comprehensively covers the role and the mechanisms that involve the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) in lung adenocarcinoma and methods of treating it by altering the tumor microenvironment. It focuses on the insight and understanding of the lung cancer tumor microenvironment and chemokines, cytokines, and activating molecules that take part in angiogenesis and metastasis. The review paper accounts for the novel and current immunotherapy and targeted therapy available for lung cancer in clinical trials and in the research phases in depth. Special attention is being paid to mark out single or multiple genes that are required for malignancy and survival while developing targeted therapies for lung cancer treatment.
- • The tumor microenvironment is intricate and complex and involves a wide variety of chemokines and cytokines.
- • Disease progression is promoted in the tumor environment, resulting in inflammatory responses via the activation of NF-κB.
- • With the development of new targeted therapies, molecular-based therapies have extended their spectrum beyond EGFR, VEGFR, and HER2/neu receptors to the receptor tyrosine kinases (RTKs).
- • It is also imperative to evaluate optimal combinatorial approaches, optimal drug sequencing, and redefining and streamlining clinical trials.
Introduction
Lung cancer has the highest mortality rate compared to all other cancers ( Mao et al., 2016 ; Siegel et al., 2016 ). In 2012, 1.8 million cases were reported worldwide for lung cancer, which constituted 13% of all cancers reported globally ( Torre et al., 2012 ). In the United States alone, 243,820 new cases of lung cancer were reported, which claimed 162,510 lives ( Siegel et al., 2016 ). The male-to-female ratio is 2:1 and is diagnosed mostly in men aged 60 and above ( Mao et al., 2016 ). Its occurrence is the highest in the regions of Eastern, Central, and Southern Europe for both genders and among women in eastern Asia and North America ( Stuber et al., 2008 ). The major cause of its occurrence is considered to be environmental factors, such as the presence of radon, lead, and other toxic pollutants in the air ( Pope et al., 2002 ). It is also noted that with the prevalence of smoking, particularly in developing countries, the number of cases being reported for lung cancer is proportionally increasing. The mortality rate of lung cancer was recorded to be over 75% with a ratio of 2.2:1 for men to women among people of age 60 years and more ( Siegel et al., 2016 ).
Based on the morphological forms, lung cancer is divided into two main categories, non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). The non-small cell lung cancer (NSCLC) is further divided into adenocarcinoma, squamous cell carcinoma, and large cell carcinoma ( Cheng et al., 2016 ). Adenocarcinoma is more prevalent than squamous cell carcinoma in most of the countries around the globe ( de Groot and Munden, 2012 ). It has been observed that a fivefold more number of cases are reported in women as compared to men in Japan, China, and Saudi Arabia ( de Groot and Munden, 2012 ). The reason for the rise in adenocarcinoma cases is linked with cigarette components, use of electronic cigarette (e-cigarette), and environmental factors ( Lortet-Tieulent et al., 2014 ). Before 1979, squamous cell carcinoma was regarded as more prevalent than other forms of cancer and is still a more common type of lung cancer in India, Russia, and the Netherlands ( Cheng et al., 2016 ).
Tumor microenvironment in lung cancer
Lung adenocarcinoma is a complex disease with a wide array of oncogenes involved along with the cytokines and chemokines, all of which play a significant role in tumor growth and angiogenesis ( Grunewald et al., 2006 ; Li et al., 2011 ) ( Figure 1 ). The study of cell and molecular biology of lung cancer has emanated from the circuit pathways comprising different key factors that play critical roles in the development of a full-fledged lung cancer. Among these factors, several factors have also been studied for their role at the genetic and epigenetic level and, thus, are considered important for carcinogenesis and metastasis. A variety of compounds/drugs have been developed to specifically target farnesyltransferase, epidermal growth factor receptor (EGFR), and vascular endothelial growth factor receptor (VEGFR). These compounds/drugs have shown encouraging results in clinical trials ( Gore et al., 2000 ; Morabito, 2016 ).

Tumor microenvironment. The characteristics of lung tumors are often determined by fibroblasts, endothelial cells, and myeloid cells existing in the tumor microenvironment. Extracellular matrix (ECM) constituting keratin, fibronectin, and collagen functions to provide structural support to tumor cells. Angiogenesis occurs due to the presence of platelet-derived growth factor (PDGF) and vascular endothelial growth factor (VEGF) at the tumor site. The CXC-chemokine ligand (CXCL) family members bind to the neutrophil receptor CXCR2 to help the tumor cells recruit neutrophils.
Signal transduction pathways that are responsible for cell proliferation and survival include mitogen-activated protein kinases (MAPKs) ( Vicent et al., 2004 ), a serine/threonine kinase AKT ( Brognard et al., 2001 ), and NF-κB ( Jones et al., 2000 ), which are hijacked or altered to facilitate these functions and maintain tumorous growth.
NF-κB is the key mediator of the tumor microenvironment and is constitutively active in different tumor cells. The key signaling pathway, involved in a wide array of functions, is activated in the case of lung adenocarcinoma both in murine models and humans ( Karin and Greten, 2005 ; Karin, 2006 ; Meylan et al., 2009 ; Basseres et al., 2010 ). The T-cell infiltration in the tumors is associated with immunosurveillance and tumor immunoediting, thus increasing the patient quality of life and survival rate. NF-κB has been a potent factor involved in protumor responses by boosting and recruiting the immunosuppressive cells, which include the regulatory T cells (Tregs) and myeloid dendritic cells (mDCs). These cells activate and release chemokines and cytokines along with the growth factors such as VEGF that initiate tumor growth and angiogenesis. Mutations in NF-κB enhance angiogenesis and metastasis by ultimately inducing mutations. Type 1 interferons including IFN alpha and beta and interferon gamma have pivotal roles in cancer immunosurveillance and priming of T cells in tumors. The effector functions of interferon gamma play a significant role in cancer immunoediting and natural killer cell activation. T-cell priming also activates the complement system and mediates the antitumor responses. A crosstalk at the molecular level between the interferon and the NF-κB pathway plays a significant role in the tumor microenvironment ( Muthuswamy et al., 2012 ; Hopewell et al., 2013 ).
The antitumor responses of the NF-κB trigger signaling cascade result in T-cell recruitment at the tumor site, leading to tumor regression and activation of chemokines and cytokines possessing the C–C motif and CCL2, respectively ( Xia et al., 2014 ). NF-κB mediates both protumor and antitumor responses along with interferon activation and T-cell activation ( Figure 2 ) ( Zhang et al., 2021 ). The roles of different mediators, which include Toll-like receptors, lymphotoxin beta (LTB), intercellular adhesion molecule 1 (ICAM1), interferon beta, chemokines, and cytokines, are linked to the NF-κB activation and promotion of tumor regression, leading to better disease prognosis ( Liu et al., 2017 ). Under the action of the aforementioned mediators and immunomodulatory genes, NF-κB regulation in inflammatory and immune responses opens up new avenues of research and a better prognosis of lung adenocarcinoma, which can be solved by immunotherapy. The Pathways and inhibitors for NF-κB activation have been shown in Figure 3 .

Role of NF-κB cancer immunosurveillance. Proinflammatory cytokines and oncogenes activate NF-κB resulting in the expression of proinflammatory mediators such as chemokine CXCL 1-3, interleukin-8 (IL-8), and (C-X-C motif) ligand. The recruitment of myeloid-derived suppressor cells (MDSCs) inhibits the antitumor response. Inflammation, angiogenesis, and metastasis are stimulated via multiple chemokines. On the other hand, interferon (IFN)-γ produced by T cells or natural killer (NK) cells stimulates the secretion of CXCL9–11, which, in turn, inflicts antiangiogenic and antimetastatic effects.

Pathways and inhibitors for NF-κB activation. These signaling cascades are modulated within the non-canonical and canonical pathways using the receptor, adapter protein, IKKα, proteasome, NF-κB-inducing kinase (NIK), nuclear translocation, REL-associated protein A (RELA), REL-associated protein B (RELB), and NF-κB essential modulator (NEMO) inhibitors.
T-cell proliferation is regulated by the cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and programmed death cell receptor 1 (PD-1). They are associated with NF-κB. The tumor microenvironment is complex and consists of intricate crosstalk of different signaling factors, chemokines, cytokines, and genes that need investigation and focus to target the soil rather than the seed of the tumor cells ( Muthuswamy et al., 2012 ).
Disease progression is promoted in the tumor environment, resulting in inflammatory responses via the activation of NF-κB ( Akca et al., 2011 ). It has recently been found that in tumor cells, T cell-mediated immune response is also regulated by the activation of NF-κB, hence actively participating in cancer immunosurveillance ( Zhu et al., 2016 ).
Domains of the nuclear factor kappa-light-chain-enhancer of activated B cells
Five family members of NF-κB have been identified in mammalian cells. These are RelA (REL-associated protein A) (p65), RelB (REL-associated protein B), c-Rel, NF-κB1 (p50/p105), and NF-κB2 (p52/p100). All of these contain an N-terminal domain called RHD (Rel homology domain) that makes them a member of this family and is used in forming a homo/heterodimer that can bind to the DNA ( Hayden and Ghosh, 2004 ) . p65, RelB, and c-Rel also contain a domain called the trans-activator domain (TAD), through which they bind with p50 or p52 members, resulting in their activation in a trans manner. p50 and p52 lack TAD on their C-terminals. Also, the p50 and p52 homodimers are transcription repressors and, in this configuration, develop a threshold for NF-κB activation ( Ghosh et al., 1998 ).
However, in a normal physiological condition, NF-κB dimers are present in cells but are withheld within the cytoplasm by their inhibitors that mask their NLS (nuclear localization sequence) domain. These inhibitors are considered to be specific for each member of the family that includes IκBα, IκBβ, IκBγ, IκBϵ, and BCL-3, and they keep a tight check on the activation of NF-κB pathways ( Lin et al., 2010 ).
Pathways for the nuclear factor kappa-light-chain-enhancer of activated B cells
NF-κB is a multifunctional transcription factor that can be activated via various extracellular signals generated due to genotoxic or endoreticulum stress, including growth factors, cytokines, carcinogens, intracellular stimuli, and tumor promoters ( Tak and Firestein, 2001 ).
A canonical pathway can be activated by proinflammatory growth factors, microbial infections, and cytokines including TNFα. TNFα on binding with TNFR1 (TNFα receptor 1) causes its transmerization leading to the recruitment of several proteins that phosphorylate and activate IKK (IκB kinase complex) ( Lin et al., 2010 ) . The IKK complex consists of three subunits involved in its catalytic reactions: IKKα/IKK1, IKKβ/IKK2, and an essential regulatory subunit, IKKγ/nuclear factor-κB essential modulator (NEMO) ( Karin, 1999 ). In the canonical pathway, IKKβ plays an important role as it gets phosphorylated on its serine residues 32 and 36 and results in its ubiquitination and degradation, thereby freeing NF-κB p50, p65, and c-Rel ( Karin, 1999 ). The NLS domains present on these NF-κB molecules are now exposed and modified to allow binding to the DNA or to transcriptional factors such as CBP (cAMP response element-binding protein) ( Chen and Greene, 2004 ).
Also, in the case of DNA damage by radiation and genotoxic agents, the IKKB-NF-κB cascade can be elicited. In this scenario, the pathway is activated by the activation of ATM (ataxia telangiectasia-mutated kinase) that phosphorylates the IKKγ domain bound to a complex called PIDDsome ( Tinel and Tschopp, 2004 ). This complex consists of a receptor-interacting protein (RIP1), p53-induced death domain, and NF-κB essential modulator (NEMO). When NEMO is phosphorylated, it detaches itself from the complex and moves into the cytoplasm, resulting in the transactivation of IKKβ, and this serves as the initiation of the canonical pathway ( Lee et al., 2012 ).
Apart from the aforementioned pathway, cells have a non-canonical pathway involving non-death receptor members of the TNF receptor family ( Muppidi et al., 2004 ). These include the cluster of differentiation 40 (CD40), lymphotoxin beta, and B-cell activating factors ( Muppidi et al., 2004 ). These receptors are activated by their specific ligands, resulting in the stabilization and auto-activation of NIK (NF-κB-inducing kinase), which further phosphorylates the IKKα member of the NF-κB family ( Kratz et al., 2016 ). IKKα, in response to its activation, undergoes a conformational change and cleaves its p100 to produce a functional NF-κB heterodimer containing the newly cleaved p52 and RelB, which is then translocated to the nucleus to act as a functional transcription factor ( Kratz et al., 2016 ).
Canonical and non-canonical pathways are regulated by c-IAP (inhibitor of apoptosis) proteins. These proteins suppress the non-canonical pathway by causing ubiquitination of NIK under normal conditions ( Kocab and Duckett, 2016 ). However, K-Ras can bind to and activate NF-κB through TBK1 (TANK-binding kinase 1) in the non-canonical pathway and contribute to oncogenic K-Ras-mediated lung carcinogenesis. NF-κB can also be activated by the components contained in tobacco; among these, nicotine and methylnitrosamino-1-(3-pyridyl)-1-butanone (NNK) are seen in a panel of NSCLC cell lines ( Kim et al., 2016 ). As in smokers’ lungs, NF-κB is constantly activated, and it is possible that it allows cancer cell proliferation and escape from apoptosis in the very early stage of lung cancer development ( Chen, 2005 ).
Chemokines and cytokines in the tumor microenvironment
The tumor microenvironment is intricate and involves a wide variety of chemokines and cytokines. In this section, we will discuss these chemokines and cytokines.
Vascular endothelial growth factor
Vascular endothelial growth factor (VEGF) is the most significant regulator of angiogenesis and is the requisite for the growth and viability of tumors in the microenvironment ( Koch et al., 2011 ). VEGF chemokine induces the expression of the C-X-C motif Chemokine ligand 12 (CXCL12) are chemokines formed by the activation of myofibroblasts and tumor macrophages. CXCL12 chemokines have a high expression of the epidermal growth factor and promotes the formation of new vessels in the tumor cells, consequently increasing the chances of metastasis ( Koch et al., 2011 ). A strong interaction exists between the cell surface receptors and C-X-C chemokine receptor type 4 (CXCR4) in lung cancer ( Takahashi, 2011 ).
Role of chemokine receptors in non-small cell lung cancer
The CXCR4 chemokine plays a very significant role in non-small cell lung cancer (NSCLC) metastasis and is an important component of the tumor microenvironment ( Wu et al., 2010 ). The high levels of CXCR4 chemokine are investigated using immunohistochemistry. CXCR4 is involved in the role of pleural spaces with its levels correlated with the expression of the CXCL12 chemokine, which is present in the advanced stages of the disease. The CXCL12 chemokine has a high expression on stromal cells, neoplastic cells, and vascular and endothelial cells in the patients suffering from lung adenocarcinoma study of cancer patients at stages I and II ( Chen, 2005 ; Grunewald et al., 2006 ; Li et al., 2011 ). CXCL12 expression in NSCLC cells ( in vitro ) indicates the correlation between CXCL12 and CXCR4 chemokines, which induces the extracellular signal-regulated kinase (ERK) pathways and growth-forming factor activation. They are the keynote chemokines associated with tumor growth along with the accessory cells such as the T regulatory cells. These chemokines act in the paracrine and autocrine fashion and attract other growth-promoting and inflammatory cytokines, which mediate the process of angiogenesis and tumor growth ( Chen, 2005 ; Grunewald et al., 2006 ; Li et al., 2011 ).
In the later part of this review, we turn our focus to the current and available immunotherapies, anticancer drugs, and vaccines that are available for lung adenocarcinoma.
Molecular-based targeted therapies for lung cancer
Extensive research is being carried out to pinpoint the key players playing pivotal roles in malignancy and/or cell survival while exploiting this knowledge to develop targeted therapies for lung cancer treatment. As described in Table 1 , several new drugs have been developed, which target these specific factors, and their clinical trials have revealed positive and encouraging results ( Dunn et al., 2006 ).
Novel and current immunotherapies available for lung cancer in clinical trials and in the research phases.
Epidermal growth factor receptor inhibitors
Epidermal growth factor receptor (EGFR) pathways are mostly observed to be dysregulated in human cancers, attracting researchers for targeted anticancer therapy ( Sharma et al., 2007 ). The noted EGFR family includes the following members, EGFR (epidermal growth factor receptor 1, also known as HER1 or ERBB1), HER2 (ERBB2/NEU or EGFR2), HER3 (ERBB3 or EGFR3), and HER4 (ERBB4 or EGFR4). The EGFR family consists of receptor tyrosine kinase (TK), a transmembrane receptor involved in cellular growth and proliferation ( Sharma et al., 2007 ). Upon binding of the ligand, the EGFR intracellular domain dimerizes and activates the TK domain and its autophosphorylation, which runs an intracellular cascade that leads to the inhibition of apoptosis, while the increase in cellular proliferation, angiogenesis, and invasion ultimately leads to tumor generation and metastasis ( Shigematsu and Gazdar, 2006 ). Of note, mostly EGFR (ERBB1) along with ligands is found overexpressed in NSCLC tumors. It is possible that the members of the EGFR family of receptors can heterodimerize with each other, so in order to identify the pharmacological therapeutic target, it is important to have a robust grip of knowledge about the ERB receptors expressed in tumor cells ( Dunn et al., 2006 ).
Erlotinib and gefitinib are molecular TKIs of EGFR, of which only the former is presently approved for NSCLC treatment in the United States ( Miller et al., 2012 ). Significant improvement was observed in phase III clinical trials of erlotinib along with a placebo given to the patient previously treated antecedently with an advanced NSCLC ( Miller et al., 2012 ). For this study practice, 731 subjects who had previously received one to two chemotherapies were recruited in a ratio pattern of 2:1 in order to administer erlotinib/placebo. The response rate observed was 8.9% and <1% in the erlotinib and placebo categories, respectively.
Gefitinib also responded positively in phase II trials, but its adequate survival rate was not observed in phase III trials. Some researchers theorized that it was because erlotinib was administered at MTD (maximum tolerated dose), while gefitinib was below its MTD ( Cataldo et al., 2011 ). Moreover, the acceptability criteria for both were also different in gefitinib trials, and the patients recruited made progress within 90 days of the previous chemotherapy. Gefitinib is currently provided to a patient who benefits from it or who is involved in clinical trials ( Cataldo et al., 2011 ; Fuertes et al., 2013 ).
Gefitinib and erlotinib have both been studied in different groups of patients along with cytotoxic chemotherapy, but no overall positive response has been observed ( Cataldo et al., 2011 ). However, it is proposed in some retrospective analytical studies that patients who never smoked may derive benefits from this combination. However, tumor mutation in EGFR and its amplification status are strongly associated with EGFR TKI therapy’s positive response. All of these trials have also revealed that a patient deprived of these features can also respond positively ( Sun et al., 2007 ).
Kirsten rat sarcoma virus gene mutations and inhibitors
KRAS is a proto-oncogene product that plays a role in the cellular proliferation mechanism. Among the mutations observed in the RAS family, 90% are found in KRAS proteins in smoker NSCLC patients with rare survival ( Pao et al., 2005 ). Normally, EGFR and KRAS mutations are not associated, but KRAS mutations have been observed to develop as a result of resistance to the EGFR therapy at the primary level ( Riely et al., 2009 ). Currently, many agents targeting Kirsten rat sarcoma virus gene (KRAS) pathways at their different steps have been developed and are in clinical trials. Among these, farnesyltransferase inhibitors (FTIs) have been studied; in particular, tipifarnib and lonafarnib are orally available TKIs that are being analyzed in combination with cytotoxic chemotherapy ( Kim et al., 2005 ).
B-Raf proto-oncogene (BRAF) is also found to be an important downregulating agent for the RAS pathway and is considered a balanced therapeutic target ( Brose et al., 2002 ). Sorafenib is an orally available dual-action multikinase inhibitor drug that acts as an antiangiogenic agent and functions as a BRAF inhibitor. Additionally, it inhibits VEGFR and PDGFR ( Wilhelm et al., 2008 ; Scagliotti et al., 2010 ). Early trials of this drug revealed adequate tolerance as a cytostatic agent and with prolonged disease stabilization. Phase II trials for sorafenib are in progress in previously treated NSCLC patients ( Scagliotti et al., 2010 ). MEK inhibitors have recently been developed, which downregulate the RAS/RAF pathway reaction. The preclinical and initial clinical trials have revealed their covenant antitumor activity in NSCLC patients, while phase II studies are in progress ( Brose et al., 2002 ; Wilhelm et al., 2008 ; Fuertes et al., 2013 ).
Histone deacetylase inhibitors
Histone deacetylase (HDAC) inhibitors have been observed to arrest cellular differentiation, growth, and apoptosis in tumors acquired in cell culture in melanoma, leukemia, prostate, breast, ovarian, and lung cancers ( Marks et al., 2004 ). Many HDAC inhibitors have been observed in arresting tumor proliferation in cancerous animal models. The inhibitors include depsipeptide MS-27-275, oxamflatin, and suberanilohydroxamic acid (SAHA) ( Kumar et al., 2015 ). It has been observed that SAHA inhibits tumor growth in methylnitrosourea-induced mammary carcinoma ( Zhu et al., 2013 ). SAHA and its second hybrid polar hydroxamic acid-based HDAC inhibitor have been approved for clinical trials ( Sun et al., 2007 ).
Angiogenesis inhibitors
High expression of vascular endothelial growth factor receptors (including all family receptors VEGF-A, -B, -C, -D, and -E) is observed in NSCLC patients and is strongly related to tumor progression and poor prognosis ( Smith et al., 2010 ). Several molecular therapeutic agents designed to target these receptors are in clinical and preclinical trials ( Batchelor et al., 2010 ). The monoclonal antibodies against these receptors are extensively studied ( Perren et al., 2011 ).
A monoclonal antibody named bevacizumab, possessing the equal potential to bind with all VEGF isoforms, gained success in clinical trials ( Perren et al., 2011 ). Recently, different studies have revealed that the addition of carboplatin and paclitaxel to bevacizumab showed encouraging survival benefits in first-line treatment of advanced nonsquamous NSCLC patients ( Dahlberg et al., 2010 ). In combination with other therapies, bevacizumab is still in trials for lung cancer treatment.
VEGFR TKIs are molecular inhibitors designed to target the ATP pocket of TK in the intracellular domain of VEGFR that leads to the blockage of its cellular cascade ( Choueiri et al., 2010 ). Zactima is an orally available molecular inhibitor that is capable of binding to VEGFR2 to a greater extent as compared to EGFR ( Robert, 2010 ). The recent use of zactima in combination with docetaxel in phase II trials on patients with advanced NSCLC has revealed an improved and progression-free survival rate as compared to only docetaxel therapy and has been approved for phase III trials recently ( Robert, 2010 ). AZD2171, along with carboplatin and paclitaxel, showed an efficient antitumor activity as second-line therapy and is well-tolerated in advanced NSCLC patients ( Ramalingam et al., 2010 ). The phase II/III trials of this combination therapy are also in progress ( Dunn et al., 2006 ).
New targets and perspectives
With the development of new targeted therapies, molecular- based therapies have extended their spectrum beyond EGFR, VEGFR, and HER2/ neu receptors to the receptor tyrosine kinases (RTKs). The most important RTK is the platelet-derived growth factor (PDGF), which is an attractive target for oncology field researchers. Its expression has been observed in fibroblasts, smooth muscles, the brain, testes, and kidneys ( Clark, 2013 ). The overexpression of PDGF and PDGFR has also been observed in a large proportion of glioblastoma tumors. It establishes an autocrine stimulatory loop that is thought to be important in tumor establishment and proliferation ( Zarghooni et al., 2010 ). The same loop is diagnosed in various cancers like meningioma, neuroendocrine cancer, ovarian, pancreatic, gastrointestinal, prostate, and lung cancers. As far as the inhibitors of PDGF/PDGFR are concerned, CDP680 (cell tech) is under phase I trials ( Raica and Cimpean, 2010 ), whereas clinical trials for SU101 are stopped at phase III due to their acute pharmacokinetic variability ( Raica and Cimpean, 2010 ). In addition to the RTK-targeted therapy, many other kinases in the cytoplasm are thought to play a major role in cell cycle regulation, gene expression, cell death, and metabolism. These kinases are considered an important joint for these pathways and could be important molecular targets for anticancer therapy ( Marks et al., 2001 ; Heist and Christiani, 2009 ). One of the very first anti-CTLA-4 blocking antibodies ipilimumab (IgG1) was tested and approved for melanoma cancer patients ( Phan et al., 2003 ). Tremelimumab (IgG2) also belongs to the same pharmacological class, and both these monoclonal antibodies are undergoing clinical trials for NSCLC patients. T-lymphocyte-associated protein 4 (CTLA-4) (CD152) belongs to the B7/CD28 family that inhibits T-cell functions ( Chan et al., 2014 ). It is regarded as an immune checkpoint receptor as it diminishes signaling through CD28, which induces immunosuppression ( Rudd et al., 2009 ). CTLA-4 is expressed on tumor cells, exhausted conventional T cells, and infiltrating Tregs ( Huang et al., 2016 ). Apart from its involvement in immunosuppression, its role in disease progression is still unknown.
Indoleamine 2,3-dioxygenase
Indoleamine 2,3-dioxygenase (IDO) is an immunosuppressive enzyme that mediates the catabolism of tryptophan. IDO is produced both in tumor cells and antigen-presenting cells ( Platten et al., 2012 ).
IDO induces immune tolerance in the tumor microenvironment through the depletion of tryptophan, and its toxic catabolites subsequently inhibit T-cell proliferation and T-cell immune response ( Hwu et al., 2000 ). Furthermore, IDO has the ability to inhibit T-cell immunity by inducing the differentiation and maturation of Tregs ( Nakamura et al., 2007 ). IFN-γ is the most potent inducer of IDO ( Basu et al., 2006 ). NF-κB transcription factors are crucial for the expression of proinflammatory cytokines in DCs ( Ouaaz et al., 2002 ) and have been implicated in IDO induction ( Du et al., 2000 ). It has been recognized that IDO emerging from tumors has the capacity to inhibit antitumor immunity and promote metastasis ( Uyttenhove et al., 2003 ; Sakurai et al., 2005 ). Smith et al. (2012) observed that IDO is involved in the development of lung cancer metastasis in a mouse model. Chung et al. (2014) identified that the IDO activity contributes to interferon-γ-induced apoptosis in NSCLC. Karanikas et al demonstrated that IDO is not only contributing to tumor immune escape but may also mediate the immune conditioning of the peri-tumoral lung area ( Karanikas et al., 2007 ). A comprehensive study published by Creelan et al explicated that IDO may partake in the resistance of NSCLC to therapy, and further studies will be necessary to investigate the antineoplastic effects of IDO inhibitors, such as 1-methyl-D-tryptophan (D-1MT) ( Creelan et al., 2013 ). Yang et al. (2013) established that IDO inhibitors reduced the number of regulatory T cells and presented therapeutic activities against Lewis lung cancer in a mouse model. Astigiano et al. (2005) suggested that IDO has the potential to be used as a prognostic marker in NSCLC. Another conclusive study published by Schafer et al. (2016) pointed out that IDO inhibitors, as an adjuvant therapy, can promote antitumor immunity against lung cancer. Further studies will be required to investigate the immunosuppressive role of IDO in lung cancer, in order to facilitate the development of efficient anticancer immunotherapy.
Non-small cell lung cancer stem cells
The aggressiveness of non-small cell lung cancer and resistance to different drugs depicted its heterogeneity and increased the plausibility of stem cell presence. The gross root hindrance for taking control of cancerous cells is to stop uncontrolled proliferation, which is the hallmark of undifferentiated/stem cells. Moreover, cancer stem cells have the ability to hideout in the dormant/quiescent phase of growth, which can also be contributed by stem cells, and this capability acts as one of the devils causing intrinsic and acquired drug resistance. Several studies demonstrated the plasticity of different cancer cells including NSCLC ( Gupta et al., 2009 ; Leung et al., 2010 ; Akunuru et al., 2012 ; Sterlacci et al., 2014 ). A number of studies observed a correlation between metastatic invasion and stemness of NSCLC, reviewed by Gottschling et al. (2012 ). An epithelial-to-mesenchymal transition (EMT), which is considered another hallmark of cancer cells, has also been found to be associated with stem cell presence. NSCLC possessing stem cells showed low sensitivity to different cancer drugs ( Perona et al., 2011 ). Moreover, ionization surviving cancers exhibit the mesenchymal phenotype with a higher expression of stem cell markers, for example, CD44 and CD24 ( Gomez-Casal et al., 2013 ; Sterlacci et al., 2014 ). The aforementioned studies are indicating the significance of stem cell studies in prognosis and in stem cell therapeutics. Therefore, we need to focus on the exploitation of stem cells in NSCLC as these hidden culprits need to be targeted for effective therapy.
Development of immunotherapy for non-small cell lung cancer
The future of immunotherapy lies with the perpetual research in tumor immunology ( Mikulski et al., 1979 ). In 1991, 16 patients with metastatic NSCLC were treated with IL-2 in combination with TNF- α. The results of phase-I clinical trials showed that low doses of TNF-α and IL-2 mediate tumor regression in advanced-stage NSCLC patients ( Yang et al., 1991 ). In 1992, Jansen et al. (1992) concluded that a combination of IL-2 and IFN-α was ineffective for the treatment of NSCLC patients. A study published in 1993 stated that the administration of recombinant IL-2 therapy resulted in increased circulating immune cells with a potential antitumor activity ( Scudeletti et al., 1993 ). In 1995, Ratto et al. (1995) showed that adoptive immunotherapy might be given to patients with stage-III NSCLC.
In 2001, Palmer et al. (2001) conducted a phase-I clinical trial on the BLP25 liposomal vaccine and concluded that the vaccine generated an immune response in lung cancer patients. In 2005, Ishikawa et al. (2005) conducted a phase-I clinical trial of α-galactosylceramide (KRN7000)-pulsed dendritic cells and concluded that it was well tolerated and could be administrated safely in patients with advanced diseases. In 2006, telomerase peptide vaccination was shown to induce immunogenic responses in patients with NSCLC, and further clinical studies of these peptides were warranted ( Brunsvig et al., 2006 ). In 2008, Wu et al. (2008) concluded that the combination of chemotherapy with cytokine-induced killer cells could ameliorate patients’ cellular response and help patients in recovery. In a different study, Li et al. (2009) stated that dendritic cell-activated cytokine-induced killer cells enhanced the outcomes of chemotherapy in NSCLC patients. A group of researchers conducted a study of adoptive immunotherapy in patients with NSCLC and suggested that T-cell immunotherapy might be safe and feasible for patients with recurrent NSCLC ( Nakajima et al., 2010 ).
In 2011, Jensen et al. (2011) proposed that radioimmunotherapy with cetuximab was particularly efficacious in elderly patients with various comorbidities. In 2012, Pan et al. (2012) carried out a study on the monoclonal antibody NJ001 and concluded that it selectively reacted to NSCLC and exhibited an antitumor activity. Wang et al. (2014) indicated that haploidentical cytokine-induced killer cells were effective in prolonging the survival of NSCLC patients. In 2015, Pujol et al. (2015) showed that MAGE-A3 induced a specific immune response in resected and unresected NSCLC patients. In 2016, Rodriguez et al. (2016) conducted a study on CIMAvax-EGF (an epidermal growth factor vaccine) and showed its efficacy in the control of EGF-dependent NSCLC tumors. A pilot study was carried out to analyze the efficacy of an autologous tumor-derived autophagosome vaccine (DRibbles), and it was reported that the vaccine given in combination with GM-CSF was capable of inducing an immune response against tumor cells ( Sanborn et al., 2017 ). Zhao et al. (2018) showed that blocking PD-1 in combination with retronectin-activated cytokine-induced killer cells was valuable in NSCLC patients with advanced diseases. In 2019, Koopman et al. (2019) demonstrated that enapotamab vedotin (an AXL-specific antibody–drug conjugate) shows promising therapeutic potential in NSCLC. Recently, Martin et al. (2020) have showed that nivolumab is a promising antibody for NSCLC patients. Another study conducted by Arrieta et al. (2020) concluded that pembrolizumab in combination with docetaxel improved the overall response rate and progression-free survival in patients with advanced diseases.
It has been established up until now that the tumor microenvironment plays a major role in tumor formation, survival, and in immune evasion in lung cancer. NF-κB plays a dual function of either tumor clearance or tumor survival depending upon the environment. In the presence of interferons or generally a more TH-1 environment, it performs an antitumor activity and helps in immune clearance of the tumors, but in a more Th-2 cytokine-mediated environment, NF-κB plays the opposite role and helps in tumor survival and progression. The recent advances in immunotherapy and targeted therapy have offered a glimmer of hope in lung cancer treatment. It is also imperative to evaluate optimal combinatorial approaches, optimal drug sequencing, and redefining and streamlining clinical trials.
Author contributions
BZ reviewed the literature, wrote the manuscript, and made the figures; AF reviewed the literature and wrote the manuscript; SK reviewed the literature and wrote the manuscript; and KA reviewed the literature, and wrote and approved the final version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
- Akca H., Demiray A., Tokgun O., Yokota J. (2011). Invasiveness and anchorage independent growth ability augmented by PTEN inactivation through the PI3K/AKT/NFkB pathway in lung cancer cells . Lung Cancer 73 ( 3 ), 302–309. 10.1016/j.lungcan.2011.01.012 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Akunuru S., James Zhai Q., Zheng Y. (2012). Non-small cell lung cancer stem/progenitor cells are enriched in multiple distinct phenotypic subpopulations and exhibit plasticity . Cell Death Dis. 3 , e352. 10.1038/cddis.2012.93 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Arrieta O., Barrón F., Ramírez-Tirado L. A., Zatarain-Barrón Z. L., Cardona A. F., Díaz-García D., et al. (2020). Efficacy and safety of pembrolizumab plus docetaxel vs docetaxel alone in patients with previously treated advanced non–small cell lung cancer: The PROLUNG phase 2 randomized clinical trial . JAMA Oncol. 6 ( 6 ), 856–864. 10.1001/jamaoncol.2020.0409 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Astigiano S., Morandi B., Costa R., Mastracci L., D'Agostino A., Ratto G. B., et al. (2005). Eosinophil granulocytes account for indoleamine 2, 3-dioxygenase-mediated immune escape in human non-small cell lung cancer . Neoplasia 7 ( 4 ), 390–396. 10.1593/neo.04658 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Basseres D. S., Ebbs A., Levantini E., Baldwin A. S. (2010). Requirement of the NF-kappaB subunit p65/RelA for K-Ras-induced lung tumorigenesis . Cancer Res. 70 ( 9 ), 3537–3546. 10.1158/0008-5472.can-09-4290 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Basu G. D., Tinder T. L., Bradley J. M., Tu T., Hattrup C. L., Pockaj B. A., et al. (2006). Cyclooxygenase-2 inhibitor enhances the efficacy of a breast cancer vaccine: Role of Ido . J. Immunol. 177 ( 4 ), 2391–2402. 10.4049/jimmunol.177.4.2391 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Batchelor T. T., Duda D. G., di Tomaso E., Ancukiewicz M., Plotkin S. R., Gerstner E., et al. (2010). Phase II study of cediranib, an oral pan–vascular endothelial growth factor receptor tyrosine kinase inhibitor, in patients with recurrent glioblastoma . J. Clin. Oncol. 26 , 3988. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Brognard J., Clark A. S., Ni Y., Dennis P. A. (2001). Akt/protein kinase B is constitutively active in non-small cell lung cancer cells and promotes cellular survival and resistance to chemotherapy and radiation . Cancer Res. 61 ( 10 ), 3986–3997. [ PubMed ] [ Google Scholar ]
- Brose M. S., Volpe P., Feldman M., Kumar M., Rishi I., Gerrero R., et al. (2002). BRAF and RAS mutations in human lung cancer and melanoma . Cancer Res. 62 ( 23 ), 6997–7000. [ PubMed ] [ Google Scholar ]
- Brunsvig P. F., Aamdal S., Gjertsen M. K., Kvalheim G., Markowski-Grimsrud C. J., Sve I., et al. (2006). Telomerase peptide vaccination: A phase I/II study in patients with non-small cell lung cancer . Cancer Immunol. Immunother. 55 ( 12 ), 1553–1564. 10.1007/s00262-006-0145-7 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cataldo V. D., Gibbons D. L., Pérez-Soler R., Quintás-Cardama A. (2011). Treatment of non–small-cell lung cancer with erlotinib or gefitinib . N. Engl. J. Med. 364 ( 10 ), 947–955. 10.1056/NEJMct0807960 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chan D. V., Gibson H. M., Aufiero B. M., Wilson A. J., Hafner M. S., Mi Q-S., et al. (2014). Differential CTLA-4 expression in human CD4+ versus CD8+ T cells is associated with increased NFAT1 and inhibition of CD4+ proliferation . Genes Immun. 15 ( 1 ), 25–32. 10.1038/gene.2013.57 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chen L-F., Greene W. C. (2004). Shaping the nuclear action of NF-kappaB . Nat. Rev. Mol. Cell Biol. 5 ( 5 ), 392–401. 10.1038/nrm1368 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chen Z. J. (2005). Ubiquitin signalling in the NF-kappaB pathway . Nat. Cell Biol. 7 ( 8 ), 758–765. 10.1038/ncb0805-758 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cheng T-Y. D., Cramb S. M., Baade P. D., Youlden D. R., Nwogu C., Reid M. E. (2016). The international epidemiology of lung cancer: Latest trends, disparities, and tumor characteristics . J. Thorac. Oncol. 11 ( 10 ), 1653–1671. 10.1016/j.jtho.2016.05.021 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Choueiri T. K., Schutz F. A., Je Y., Rosenberg J. E., Bellmunt J. (2010). Risk of arterial thromboembolic events with sunitinib and sorafenib: A systematic review and meta-analysis of clinical trials . J. Clin. Oncol. 28 ( 13 ), 2280–2285. 10.1200/JCO.2009.27.2757 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chung T. W., Tan K. T., Chan H. L., Lai M. D., Yen M. C., Li Y. R., et al. (2014). Induction of indoleamine 2, 3-dioxygenase (Ido) enzymatic activity contributes to interferon-gamma induced apoptosis and death receptor 5 expression in human non-small cell lung cancer cells . Asian pac. J. Cancer Prev. 15 ( 18 ), 7995–8001. 10.7314/apjcp.2014.15.18.7995 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ciardiello F., Caputo R., Bianco R., Damiano V., Pomatico G., De Placido S., et al. (2000). Antitumor effect and potentiation of cytotoxic drugs activity in human cancer cells by ZD-1839 (Iressa), an epidermal growth factor receptor-selective tyrosine kinase inhibitor . Clin. Cancer Res. 6 ( 5 ), 2053–2063. [ PubMed ] [ Google Scholar ]
- Clark R. (2013). The molecular and cellular biology of wound repair . Germany: Springer Science & Business Media. [ Google Scholar ]
- Commander H., Whiteside G., Perry C. (2011). Vandetanib: First global approval . Drugs 71 ( 10 ), 1355–1365. 10.2165/11595310-000000000-00000 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cordier P-Y., Nau A., Ciccolini J., Oliver M., Mercier C., Lacarelle B., et al. (2011). 5-FU-induced neurotoxicity in cancer patients with profound DPD deficiency syndrome: A report of two cases . Cancer Chemother. Pharmacol. 68 ( 3 ), 823–826. 10.1007/s00280-011-1666-0 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Creelan B. C., Antonia S., Bepler G., Garrett T. J., Simon G. R., Soliman H. H. (2013). Indoleamine 2, 3-dioxygenase activity and clinical outcome following induction chemotherapy and concurrent chemoradiation in Stage III non-small cell lung cancer . Oncoimmunology 2 ( 3 ), e23428. 10.4161/onci.23428 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dahlberg S. E., Sandler A. B., Brahmer J. R., Schiller J. H., Johnson D. H. (2010). Clinical course of advanced non–small-cell lung cancer patients experiencing hypertension during treatment with bevacizumab in combination with carboplatin and paclitaxel on ECOG 4599 . J. Clin. Oncol. 28 ( 6 ), 949–954. 10.1200/JCO.2009.25.4482 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- de Groot P., Munden R. F. (2012). Lung cancer epidemiology, risk factors, and prevention . Radiol. Clin. North Am. 50 ( 5 ), 863–876. 10.1016/j.rcl.2012.06.006 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Du M. X., Sotero-Esteva W. D., Taylor M. W. (2000). Analysis of transcription factors regulating induction of indoleamine 2, 3-dioxygenase by IFN-gamma . J. Interferon Cytokine Res. 20 ( 2 ), 133–142. 10.1089/107999000312531 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dunn G. P., Koebel C. M., Schreiber R. D. (2006). Interferons, immunity and cancer immunoediting . Nat. Rev. Immunol. 6 ( 11 ), 836–848. 10.1038/nri1961 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Eisen T., Ahmad T., Flaherty K., Gore M., Kaye S., Marais R., et al. (2006). Sorafenib in advanced melanoma: A phase II randomised discontinuation trial analysis . Br. J. Cancer 95 ( 5 ), 581–586. 10.1038/sj.bjc.6603291 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Forde P. M., Rudin C. M. (2012). Crizotinib in the treatment of non-small-cell lung cancer . Expert Opin. Pharmacother. 13 ( 8 ), 1195–1201. 10.1517/14656566.2012.688029 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fuertes M. B., Woo S. R., Burnett B., Fu Y. X., Gajewski T. F. (2013). Type I interferon response and innate immune sensing of cancer . Trends Immunol. 34 ( 2 ), 67–73. 10.1016/j.it.2012.10.004 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ghosh S., May M. J., Kopp E. B. (1998). NF-κB and rel proteins: Evolutionarily conserved mediators of immune responses . Annu. Rev. Immunol. 16 ( 1 ), 225–260. 10.1146/annurev.immunol.16.1.225 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gomez-Casal R., Bhattacharya C., Ganesh N., Bailey L., Basse P., Gibson M., et al. (2013). Non-small cell lung cancer cells survived ionizing radiation treatment display cancer stem cell and epithelial-mesenchymal transition phenotypes . Mol. Cancer 12 ( 1 ), 94. 10.1186/1476-4598-12-94 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gore J. M., Brophy C. J., Greenstone M. (2000). How well do we care for patients with end stage chronic obstructive pulmonary disease (copd)? A comparison of palliative care and quality of life in copd and lung cancer . Thorax 55 ( 12 ), 1000–1006. 10.1136/thorax.55.12.1000 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gottschling S., Schnabel P. A., Herth F. J., Herpel E. (2012). Are we missing the target? Cancer stem cells and drug resistance in non-small cell lung cancer . Cancer Genomics Proteomics 9 ( 5 ), 275–286. [ PubMed ] [ Google Scholar ]
- Gridelli C., Maione P., Del Gaizo F., Colantuoni G., Guerriero C., Ferrara C., et al. (2007). Sorafenib and sunitinib in the treatment of advanced non-small cell lung cancer . Oncologist 12 ( 2 ), 191–200. 10.1634/theoncologist.12-2-191 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Grunewald M., Avraham I., Dor Y., Bachar-Lustig E., Itin A., Jung S., et al. (2006). VEGF-Induced adult neovascularization: Recruitment, retention, and role of accessory cells . Cell 124 ( 1 ), 175–189. 10.1016/j.cell.2005.10.036 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gupta P. B., Chaffer C. L., Weinberg R. A. (2009). Cancer stem cells: Mirage or reality? Nat. Med. 15 ( 9 ), 1010–1012. 10.1038/nm0909-1010 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Han K., Peyret T., Marchand M., Quartino A., Gosselin N. H., Girish S., et al. (2016). Population pharmacokinetics of bevacizumab in cancer patients with external validation . Cancer Chemother. Pharmacol. 78 ( 2 ), 341–351. 10.1007/s00280-016-3079-6 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hayden M. S., Ghosh S. (2004). Signaling to NF-kappaB . Genes Dev. 18 ( 18 ), 2195–2224. 10.1101/gad.1228704 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Heist R. S., Christiani D. (2009). EGFR-Targeted therapies in lung cancer: Predictors of response and toxicity . Pharmacogenomics 10 ( 1 ), 59–68. 10.2217/14622416.10.1.59 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hopewell E. L., Zhao W., Fulp W. J., Bronk C. C., Lopez A. S., Massengill M., et al. (2013). Lung tumor NF-κB signaling promotes T cell-mediated immune surveillance . J. Clin. Invest. 123 ( 6 ), 2509–2522. 10.1172/jci67250 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Huang P-Y., Guo S-S., Zhang Y., Lu J-B., Chen Q-Y., Tang L-Q., et al. (2016). Tumor CTLA-4 overexpression predicts poor survival in patients with nasopharyngeal carcinoma . Oncotarget 7 ( 11 ), 13060–13068. 10.18632/oncotarget.7421 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hwu P., Du M. X., Lapointe R., Do M., Taylor M. W., Young H. A. (2000). Indoleamine 2, 3-dioxygenase production by human dendritic cells results in the inhibition of T cell proliferation . J. Immunol. 164 ( 7 ), 3596–3599. 10.4049/jimmunol.164.7.3596 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ishikawa A., Motohashi S., Ishikawa E., Fuchida H., Higashino K., Otsuji M., et al. (2005). A phase I study of α-galactosylceramide (KRN7000)–pulsed dendritic cells in patients with advanced and recurrent non–small cell lung cancer . Clin. Cancer Res. 11 ( 5 ), 1910–1917. 10.1158/1078-0432.CCR-04-1453 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jansen R. L., Slingerland R., Goey S. H., Franks C. R., Bolhuis R. L., Stoter G. (1992). Interleukin-2 and interferon-α in the treatment of patients with advanced non-small-cell lung cancer . J. Immunother. 12 ( 1 ), 70–73. 10.1097/00002371-199207000-00009 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jensen A. D., Münter M. W., Bischoff H. G., Haselmann R., Haberkorn U., Huber P. E., et al. (2011). Combined treatment of nonsmall cell lung cancer NSCLC stage III with intensity‐modulated RT radiotherapy and cetuximab: The NEAR trial . Cancer 117 ( 13 ), 2986–2994. 10.1002/cncr.25888 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jones D. R., Broad R. M., Madrid L. V., Baldwin A. S., Jr., Mayo M. W. (2000). Inhibition of NF-kappaB sensitizes non-small cell lung cancer cells to chemotherapy-induced apoptosis . Ann. Thorac. Surg. 70 ( 3 ), 930–936. 10.1016/s0003-4975(00)01635-0 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Karanikas V., Zamanakou M., Kerenidi T., Dahabreh J., Hevas A., Nakou M., et al. (2007). Indoleamine 2, 3-dioxygenase (Ido) expression in lung cancer . Cancer Biol. Ther. 6 ( 8 ), 1258–1262. 10.4161/cbt.6.8.4446 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Karin M., Greten F. R. (2005). NF-kappaB: Linking inflammation and immunity to cancer development and progression . Nat. Rev. Immunol. 5 ( 10 ), 749–759. 10.1038/nri1703 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Karin M. (1999). How NF-kappaB is activated: The role of the IkappaB kinase (IKK) complex . Oncogene 18 ( 49 ), 6867–6874. 10.1038/sj.onc.1203219 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Karin M. (2006). Nuclear factor-kappaB in cancer development and progression . Nature 441 ( 7092 ), 431–436. 10.1038/nature04870 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kim E. S., Kies M. S., Fossella F. V., Glisson B. S., Zaknoen S., Statkevich P., et al. (2005). Phase II study of the farnesyltransferase inhibitor lonafarnib with paclitaxel in patients with taxane‐refractory/resistant nonsmall cell lung carcinoma . Cancer 104 ( 3 ), 561–569. 10.1002/cncr.21188 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kim S., Yoon T. M., Lee D. H., Lee J. K., Park Y. L., Chung I. J., et al. (2016). Livin enhances tumorigenesis by regulating the mitogen-activated protein kinase signaling pathway in human hypopharyngeal squamous cell carcinoma . Mol. Med. Rep. 14 ( 1 ), 515–520. 10.3892/mmr.2016.5242 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kim T., Murren J. (2000). Erlotinib OSI/Roche/Genentech . Curr. Opin. Investig. Drugs 3 ( 9 ), 1385–1395. [ PubMed ] [ Google Scholar ]
- Kim Y. B., Lee K-H., Sugita K., Yoshida M., Horinouchi S. (1999). Oxamflatin is a novel antitumor compound that inhibits mammalian histone deacetylase . Oncogene 18 ( 15 ), 2461–2470. 10.1038/sj.onc.1202564 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kocab A. J., Duckett C. S. (2016). Inhibitor of apoptosis proteins as intracellular signaling intermediates . FEBS J. 283 ( 2 ), 221–231. 10.1111/febs.13554 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Koch S., Tugues S., Li X., Gualandi L., Claesson-Welsh L. (2011). Signal transduction by vascular endothelial growth factor receptors . Biochem. J. 437 ( 2 ), 169–183. 10.1042/BJ20110301 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Koopman L. A., Terp M. G., Zom G. G., Janmaat M. L., Jacobsen K., Gresnigt-Van den Heuvel E., et al. (2019). Enapotamab vedotin, an AXL-specific antibody-drug conjugate, shows preclinical antitumor activity in non-small cell lung cancer . JCI insight 4 ( 21 ), 128199. 10.1172/jci.insight.128199 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kratz A., Zang C., Eucker J., Liu H. (2016). Abstract 943: Retinoid sensitizes Tumor Necrosis Factor (TNF) -related apoptosis inducing ligand -induced cytotoxic effect in breast cancer by upregulating cell surface receptor Death Receptor-4 expression . Cancer Res. 76 , 943. 10.1158/1538-7445.am2016-943 [ CrossRef ] [ Google Scholar ]
- Kumar R., Sharma P. K., Qadri F., Saraswat P. (2015). Histone deacetylase inhibitor as a novel anticancer agent: A review . Glob. J. Pharmacol. 9 ( 2 ), 179–189. [ Google Scholar ]
- Lee H-Y., Moon H., Chun K-H., Chang Y-S., Hassan K., Ji L., et al. (2004). Effects of insulin-like growth factor binding protein-3 and farnesyltransferase inhibitor SCH66336 on Akt expression and apoptosis in non–small-cell lung cancer cells . J. Natl. Cancer Inst. 96 ( 20 ), 1536–1548. 10.1093/jnci/djh286 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lee S. H., Toth Z., Wong L. Y., Brulois K., Nguyen J., Lee J. Y., et al. (2012). Novel phosphorylations of IKKγ/NEMO . mBio 3 ( 6 ), e00411–e00412. 10.1128/mBio.00411-12 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Leung E. L., Fiscus R. R., Tung J. W., Tin V. P., Cheng L. C., Sihoe A. D., et al. (2010). Non-small cell lung cancer cells expressing CD44 are enriched for stem cell-like properties . PLoS One 5 ( 11 ), e14062. 10.1371/journal.pone.0014062 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Li C., Fang R., Sun Y., Han X., Li F., Gao B., et al. (2011). Spectrum of oncogenic driver mutations in lung adenocarcinomas from East Asian never smokers . PLoS One 6 ( 11 ), e28204. 10.1371/journal.pone.0028204 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Li H., Wang C., Yu J., Cao S., Wei F., Zhang W., et al. (2009). Dendritic cell-activated cytokine-induced killer cells enhance the anti-tumor effect of chemotherapy on non-small cell lung cancer in patients after surgery . Cytotherapy 11 ( 8 ), 1076–1083. 10.3109/14653240903121252 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lin Y., Bai L., Chen W., Xu S. (2010). The NF-kappaB activation pathways, emerging molecular targets for cancer prevention and therapy . Expert Opin. Ther. Targets 14 ( 1 ), 45–55. 10.1517/14728220903431069 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Liu T., Zhang L., Joo D., Sun S-C. (2017). NF-κB signaling in inflammation . Signal Transduct. Target. Ther. 2 ( 1 ), 17023–17029. 10.1038/sigtrans.2017.23 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lortet-Tieulent J., Soerjomataram I., Ferlay J., Rutherford M., Weiderpass E., Bray F. (2014). International trends in lung cancer incidence by histological subtype: Adenocarcinoma stabilizing in men but still increasing in women . Lung cancer 84 ( 1 ), 13–22. 10.1016/j.lungcan.2014.01.009 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mao Y., Yang D., He J., Krasna M. J. (2016). Epidemiology of lung cancer . Surg. Oncol. Clin. N. Am. 25 ( 3 ), 439–445. 10.1016/j.soc.2016.02.001 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Marks P. A., Richon V. M., Miller T., Kelly W. K. (2004). Histone deacetylase inhibitors . Adv. Cancer Res. 91 , 137–168. 10.1016/S0065-230X(04)91004-4 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Marks P. A., Rifkind R. A., Richon V. M., Breslow R. (2001). Inhibitors of histone deacetylase are potentially effective anticancer agents . Clin. Cancer Res. 7 ( 4 ), 759–760. [ PubMed ] [ Google Scholar ]
- Martin C., Lupinacci L., Perazzo F., Bas C., Carranza O., Puparelli C., et al. (2020). Efficacy and safety of nivolumab in previously treated patients with non-small cell lung cancer: Real world experience in Argentina . Clin. Lung Cancer 21 , e380–e387. 10.1016/j.cllc.2020.02.014 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- McDermott J., Pembrolizumab J. A. (1998). Pembrolizumab: PD-1 inhibition as a therapeutic strategy in cancer . Drugs Today 51 ( 1 ), 7–20. 10.1358/dot.2015.51.1.2250387 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Meylan E., Dooley A. L., Feldser D. M., Shen L., Turk E., Ouyang C., et al. (2009). Requirement for NF-kappaB signalling in a mouse model of lung adenocarcinoma . Nature 462 ( 7269 ), 104–107. 10.1038/nature08462 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mikulski S. M., McGuire W. P., Louie A. C., Chirigos M. A., Muggia F. M. (1979). Immunotherapy of lung cancer I. Review of clinical trials in non-small cell histologic types . Cancer Treat. Rev. 6 ( 3 ), 177–190. 10.1016/s0305-7372(79)80069-9 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Miller V. A., Hirsh V., Cadranel J., Chen Y-M., Park K., Kim S-W., et al. (2012). Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): A phase 2b/3 randomised trial . Lancet. Oncol. 13 ( 5 ), 528–538. 10.1016/S1470-2045(12)70087-6 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Morabito A. (2016). The evolution of the treatment of advanced NSCLC . Recenti Prog. Med. 107 ( 10 ), 510–514. 10.1701/2454.25700 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Muppidi J. R., Tschopp J., Siegel R. M. (2004). Life and death decisions: Secondary complexes and lipid rafts in TNF receptor family signal transduction . Immunity 21 ( 4 ), 461–465. 10.1016/j.immuni.2004.10.001 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Muthuswamy R., Berk E., Junecko B. F., Zeh H. J., Zureikat A. H., Normolle D., et al. (2012). NF-κB hyperactivation in tumor tissues allows tumor-selective reprogramming of the chemokine microenvironment to enhance the recruitment of cytolytic T effector cells . Cancer Res. 72 ( 15 ), 3735–3743. 10.1158/0008-5472.can-11-4136 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nakajima J., Murakawa T., Fukami T., Goto S., Kaneko T., Yoshida Y., et al. (2010). A phase I study of adoptive immunotherapy for recurrent non-small-cell lung cancer patients with autologous gammadelta T cells . Eur. J. Cardiothorac. Surg. 37 ( 5 ), 1191–1197. 10.1016/j.ejcts.2009.11.051 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nakamura T., Shima T., Saeki A., Hidaka T., Nakashima A., Takikawa O., et al. (2007). Expression of indoleamine 2, 3-dioxygenase and the recruitment of Foxp3-expressing regulatory T cells in the development and progression of uterine cervical cancer . Cancer Sci. 98 ( 6 ), 874–881. Epu7. 10.1111/j.1349-7006.2007.00470.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ouaaz F., Arron J., Zheng Y., Choi Y., Beg A. A. (2002). Dendritic cell development and survival require distinct NF-kappaB subunits . Immunity 16 ( 2 ), 257–270. 10.1016/s1074-7613(02)00272-8 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Palmer M., Parker J., Modi S., Butts C., Smylie M., Meikle A., et al. (2001). Phase I study of the BLP25 (MUC1 Peptide) liposomal vaccine for active specific immunotherapy in stage IIIB/IV non–small-cell lung cancer . Clin. Lung Cancer 3 ( 1 ), 49–57. 10.3816/clc.2001.n.018 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pan S., Wang F., Huang P., Xu T., Zhang L., Xu J., et al. (2012). The study on newly developed McAb NJ001 specific to non-small cell lung cancer and its biological characteristics . PloS one 7 ( 3 ), e33009. 10.1371/journal.pone.0033009 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pao W., Wang T. Y., Riely G. J., Miller V. A., Pan Q., Ladanyi M., et al. (2005). KRAS mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinib . PLoS Med. 2 ( 1 ), e17. 10.1371/journal.pmed.0020017 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Perona R., Lopez-Ayllon B. D., de Castro Carpeno J., Belda-Iniesta C. (2011). A role for cancer stem cells in drug resistance and metastasis in non-small-cell lung cancer . Clin. Transl. Oncol. 13 ( 5 ), 289–293. 10.1007/s12094-011-0656-3 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Perren T. J., Swart A. M., Pfisterer J., Ledermann J. A., Pujade-Lauraine E., Kristensen G., et al. (2011). A phase 3 trial of bevacizumab in ovarian cancer . N. Engl. J. Med. 365 ( 26 ), 2484–2496. 10.1056/NEJMoa1103799 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Phan G. Q., Yang J. C., Sherry R. M., Hwu P., Topalian S. L., Schwartzentruber D. J., et al. (2003). Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma . Proc. Natl. Acad. Sci. U. S. A. 100 ( 14 ), 8372–8377. 10.1073/pnas.1533209100 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Platten M., Wick W., Van den Eynde B. J. (2012). Tryptophan catabolism in cancer: Beyond Ido and tryptophan depletion . Cancer Res. 72 ( 21 ), 5435–5440. 10.1158/0008-5472.can-12-0569 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pope C. A., 3rd, Burnett R. T., Thun M. J., Calle E. E., Krewski D., Ito K., et al. (2002). Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution . JAMA 287 ( 9 ), 1132–1141. 10.1001/jama.287.9.1132 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pujol J. L., Vansteenkiste J. F., De Pas T. M., Atanackovic D., Reck M., Thomeer M., et al. (2015). Safety and immunogenicity of MAGE-A3 cancer immunotherapeutic with or without adjuvant chemotherapy in patients with resected stage IB to III MAGE-A3-positive non-small-cell lung cancer . J. Thorac. Oncol. 10 ( 10 ), 1458–1467. 10.1097/jto.0000000000000653 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Raica M., Cimpean A. M. (2010). Platelet-derived growth factor (PDGF)/PDGF receptors (PDGFR) axis as target for antitumor and antiangiogenic therapy . Pharmaceuticals 3 ( 3 ), 572–599. 10.3390/ph3030572 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ramalingam S. S., Belani C. P., Mack P. C., Vokes E. E., Longmate J., Govindan R., et al. (2010). Phase II study of Cediranib (AZD 2171), an inhibitor of the vascular endothelial growth factor receptor, for second-line therapy of small cell lung cancer (National Cancer Institute# 7097) . J. Thorac. Oncol. 5 ( 8 ), 1279–1284. 10.1097/JTO.0b013e3181e2fcb0 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ratto G. B., Melioli G., Zino P., Mereu C., Mirabelli S., Fantino G., et al. (1995). Immunotherapy with the use of tumor-infiltrating lymphocytes and interleukin-2 as adjuvant treatment in stage III non-small-cell lung cancer: A pilot study . J. Thorac. Cardiovasc. Surg. 109 ( 6 ), 1212–1217. 10.1016/S0022-5223(95)70205-9 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Riely G. J., Marks J., Pao W. (2009). KRAS mutations in non–small cell lung cancer . Proc. Am. Thorac. Soc. 6 ( 2 ), 201–205. 10.1513/pats.200809-107LC [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Robert C. (2010). Vandetanib (Zactima®). Cutaneous side effects induced by targeted anticancer therapies: A new dermatology . France: Privat Toulouse. [ Google Scholar ]
- Rodriguez P. C., Popa X., Martínez O., Mendoza S., Santiesteban E., Crespo T., et al. (2016). A phase III clinical trial of the epidermal growth factor vaccine CIMAvax-EGF as switch maintenance therapy in advanced non–small cell lung cancer patients . Clin. Cancer Res. 22 ( 15 ), 3782–3790. 10.1158/1078-0432.CCR-15-0855 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rudd C. E., Taylor A., Schneider H. (2009). CD28 and CTLA‐4 coreceptor expression and signal transduction . Immunol. Rev. 229 ( 1 ), 12–26. 10.1111/j.1600-065X.2009.00770.x [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sakurai K., Amano S., Enomoto K., Kashio M., Saito Y., Sakamoto A., et al. (2005). Study of indoleamine 2, 3-dioxygenase expression in patients with breast cancer . Gan Kagaku Ryoho. 32 ( 11 ), 1546–1549. [ PubMed ] [ Google Scholar ]
- Sanborn R. E., Ross H. J., Aung S., Acheson A., Moudgil T., Puri S., et al. (2017). A pilot study of an autologous tumor-derived autophagosome vaccine with docetaxel in patients with stage IV non-small cell lung cancer . J. Immunother. Cancer 5 ( 1 ), 103. 10.1186/s40425-017-0306-6 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Scagliotti G., Novello S., Von Pawel J., Reck M., Pereira J. R., Thomas M., et al. (2010). Phase III study of carboplatin and paclitaxel alone or with sorafenib in advanced non–small-cell lung cancer . J. Clin. Oncol. 28 ( 11 ), 1835–1842. 10.1200/JCO.2009.26.1321 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Schafer C. C., Wang Y., Hough K. P., Sawant A., Grant S. C., Thannickal V. J., et al. (2016). Indoleamine 2, 3-dioxygenase regulates anti-tumor immunity in lung cancer by metabolic reprogramming of immune cells in the tumor microenvironment . Oncotarget 7 , 75407–75424. 0. 10.18632/oncotarget.12249 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Scudeletti M., Filaci G., Imro M. A., Motta G., Di Gaetano M., Pierri I., et al. (1993). Immunotherapy with intralesional and systemic interleukin-2 of patients with non-small-cell lung cancer . Cancer Immunol. Immunother. 37 ( 2 ), 119–124. 10.1007/BF01517044 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sharma S. V., Bell D. W., Settleman J., Haber D. A. (2007). Epidermal growth factor receptor mutations in lung cancer . Nat. Rev. Cancer 7 ( 3 ), 169–181. 10.1038/nrc2088 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Shigematsu H., Gazdar A. F. (2006). Somatic mutations of epidermal growth factor receptor signaling pathway in lung cancers . Int. J. Cancer 118 ( 2 ), 257–262. 10.1002/ijc.21496 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Siegel R. L., Miller K. D., Jemal A. (2016). Cancer statistics . Ca. Cancer J. Clin. 66 ( 1 ), 7–30. 10.3322/caac.21332 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Smith C., Chang M. Y., Parker K. H., Beury D. W., DuHadaway J. B., Flick H. E., et al. (2012). Ido is a nodal pathogenic driver of lung cancer and metastasis development . Cancer Discov. 2 ( 8 ), 722–735. 10.1158/2159-8290.cd-12-0014 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Smith N. R., Baker D., James N. H., Ratcliffe K., Jenkins M., Ashton S. E., et al. (2010). Vascular endothelial growth factor receptors VEGFR-2 and VEGFR-3 are localized primarily to the vasculature in human primary solid cancers . Clin. Cancer Res. 16 ( 14 ), 3548–3561. 10.1158/1078-0432.CCR-09-2797 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sterlacci W., Savic S., Fiegl M., Obermann E., Tzankov A. (2014). Putative stem cell markers in non-small-cell lung cancer: A clinicopathologic characterization . J. Thorac. Oncol. 9 ( 1 ), 41–49. 10.1097/jto.0000000000000021 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Stuber J., Galea S., Link B. G. (2008). Smoking and the emergence of a stigmatized social status . Soc. Sci. Med. 67 ( 3 ), 420–430. 10.1016/j.socscimed.2008.03.010 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sun S., Schiller J. H., Spinola M., Minna J. D. (2007). New molecularly targeted therapies for lung cancer . J. Clin. Invest. 117 ( 10 ), 2740–2750. 10.1172/jci31809 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Suwa T., Ueda M., Jinno H., Ozawa S., Kitagawa Y., Ando N., et al. (1999). Epidermal growth factor receptor-dependent cytotoxic effect of anti-EGFR antibody-ribonuclease conjugate on human cancer cells . Anticancer Res. 19 ( 5 B ), 4161–4165. [ PubMed ] [ Google Scholar ]
- Tak P. P., Firestein G. S. (2001). NF-kappaB: A key role in inflammatory diseases . J. Clin. Invest. 107 ( 1 ), 7–11. 10.1172/JCI11830 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Takahashi S. (2011). Vascular endothelial growth factor (VEGF), VEGF receptors and their inhibitors for antiangiogenic tumor therapy . Biol. Pharm. Bull. 34 ( 12 ), 1785–1788. 10.1248/bpb.34.1785 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Taneja S. S. (2012). Re: Safety and activity of anti-PD-L1 antibody in patients with advanced cancer . J. Urol. 188 ( 6 ), 2148–2149. 10.1016/j.juro.2012.08.169 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tinel A., Tschopp J. (2004). The PIDDosome, a protein complex implicated in activation of caspase-2 in response to genotoxic stress . Sci. (New York, NY) 304 ( 5672 ), 843–846. 10.1126/science.1095432 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Torre L. A., Bray F., Siegel R. L., Ferlay J., Lortet‐Tieulent J., Jemal A. (2012). Global cancer statistics . Ca. Cancer J. Clin. 65 ( 2 ), 87–108. 10.3322/caac.21262 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Uyttenhove C., Pilotte L., Theate I., Stroobant V., Colau D., Parmentier N., et al. (2003). Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2, 3-dioxygenase . Nat. Med. 9 ( 10 ), 1269–1274. 10.1038/nm934 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Venkatasubbarao K., Choudary A., Freeman J. W. (2005). Farnesyl transferase inhibitor (R115777)–induced inhibition of STAT3 (Tyr705) phosphorylation in human pancreatic cancer cell lines require extracellular signal-regulated kinases . Cancer Res. 65 ( 7 ), 2861–2871. 10.1158/0008-5472.CAN-04-2396 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Vicent S., Garayoa M., Lopez-Picazo J. M., Lozano M. D., Toledo G., Thunnissen F. B., et al. (2004). Mitogen-activated protein kinase phosphatase-1 is overexpressed in non-small cell lung cancer and is an independent predictor of outcome in patients . Clin. Cancer Res. 10 ( 11 ), 3639–3649. 10.1158/1078-0432.ccr-03-0771 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wang S., Zhang H., Liu C., Jiao X., Liu D., Du W., et al. (2014). Human leukocyte antigen-haploidentical donor-derived cytokine-induced killer cells are safe and prolong the survival of patients with advanced non-small cell lung cancer . Oncol. Lett. 8 ( 6 ), 2727–2733. 10.3892/ol.2014.2558 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wedge S. R., Kendrew J., Hennequin L. F., Valentine P. J., Barry S. T., Brave S. R., et al. (2005). AZD2171: A highly potent, orally bioavailable, vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor for the treatment of cancer . Cancer Res. 65 ( 10 ), 4389–4400. 10.1158/0008-5472.CAN-04-4409 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wilhelm S. M., Adnane L., Newell P., Villanueva A., Llovet J. M., Lynch M. (2008). Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling . Mol. Cancer Ther. 7 ( 10 ), 3129–3140. 10.1158/1535-7163.MCT-08-0013 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wind S., Schnell D., Ebner T., Freiwald M., Stopfer P. (2017). Clinical pharmacokinetics and pharmacodynamics of afatinib . Clin. Pharmacokinet. 56 ( 3 ), 235–250. 10.1007/s40262-016-0440-1 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wu B., Chien E. Y., Mol C. D., Fenalti G., Liu W., Katritch V., et al. (2010). Structures of the CXCR4 chemokine GPCR with small-molecule and cyclic peptide antagonists . Science 330 ( 6007 ), 1066–1071. 10.1126/science.1194396 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wu C., Jiang J., Shi L., Xu N. (2008). Prospective study of chemotherapy in combination with cytokine-induced killer cells in patients suffering from advanced non-small cell lung cancer . Anticancer Res. 28 ( 6B ), 3997–4002. [ PubMed ] [ Google Scholar ]
- Xia Y., Shen S., Verma I. M. (2014). NF-κB, an active player in human cancers . Cancer Immunol. Res. 2 ( 9 ), 823–830. 10.1158/2326-6066.cir-14-0112 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Xu W., Parmigiani R., Marks P. (2007). Histone deacetylase inhibitors: Molecular mechanisms of action . Oncogene 26 ( 37 ), 5541–5552. 10.1038/sj.onc.1210620 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Yang S., Li X., Hu F., Li Y., Yang Y., Yan J., et al. (2013). Discovery of tryptanthrin derivatives as potent inhibitors of indoleamine 2, 3-dioxygenase with therapeutic activity in Lewis lung cancer (LLC) tumor-bearing mice . J. Med. Chem. 56 ( 21 ), 8321–8331. 10.1021/jm401195n [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Yang S. C., Grimm E. A., Parkinson D. R., Carinhas J., Fry K. D., Mendiguren-Rodriguez A., et al. (1991). Clinical and immunomodulatory effects of combination immunotherapy with low-dose interleukin 2 and tumor necrosis factor α in patients with advanced non-small cell lung cancer: a phase I trial . Cancer Res. 51 ( 14 ), 3669–3676. [ PubMed ] [ Google Scholar ]
- Zarghooni M., Bartels U., Lee E., Buczkowicz P., Morrison A., Huang A., et al. (2010). Whole-genome profiling of pediatric diffuse intrinsic pontine gliomas highlights platelet-derived growth factor receptor α and poly (ADP-ribose) polymerase as potential therapeutic targets . J. Clin. Oncol. 28 ( 8 ), 1337–1344. 10.1200/JCO.2009.25.5463 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zhang T., Ma C., Zhang Z., Zhang H., Hu H. (2021). NF-κB signaling in inflammation and cancer . MedComm 2 ( 4 ), 618–653. 10.1002/mco2.104 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zhao L., Han L., Zhang Y., Li T., Yang Y., Li W., et al. (2018). Combination of PD-1 blockade and RetroNectin®-activated cytokine-induced killer in preheavily treated non-small-cell lung cancer: A retrospective study . Immunotherapy 10 ( 15 ), 1315–1323. 10.2217/imt-2018-0125 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zhu J., Luo J., Li Y., Jia M., Wang Y., Huang Y., et al. (2016). HMGB1 induces human non-small cell lung cancer cell motility by activating integrin αvβ3/FAK through TLR4/NF-κB signaling pathway . Biochem. Biophys. Res. Commun. 480 ( 4 ), 522–527. 10.1016/j.bbrc.2016.10.052 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zhu Z., Jiang W., McGinley J. N., Thompson H. J. (2013). Defining the role of histone deacetylases in the inhibition of mammary carcinogenesis by dietary energy restriction (DER): Effects of suberoylanilide hydroxamic acid (SAHA) and DER in a rat model . Cancer Prev. Res. 6 ( 4 ), 290–298. 10.1158/1940-6207.CAPR-12-0449-T [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]

LCFA believes
makes living with lung cancer possible
New discoveries in lung cancer research, such as LCFA-funded research, result in new treatments for lung cancer patients.
Because of research, the survival rate for lung cancer rate has doubled.
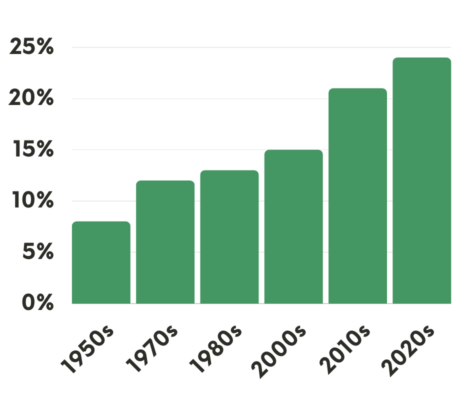
Improvement of the 5-year Survival Rate for Lung Cancer
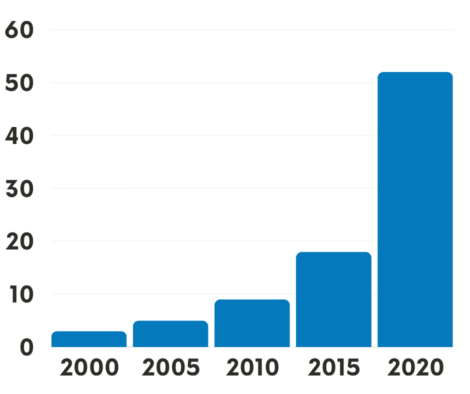
# of FDA-approved Treatments for Lung Cancer over the past 20 years
Learn about these 5 Exciting Areas of Lung Cancer Research
Treatments that target specific biomarkers that are present in lung cancer tumors continue to have great success, especially in treating patients with:
It is recommended that all patients get comprehensive biomarker testing when they are first diagnosed. A biomarker is a feature of the cancer that can help determine a personalized treatment. It is advisable for patients to discuss with their doctors how and when to schedule a complete panel of biomarker testing. This is preferably before any treatment is started. Comprehensive biomarker testing, specified in the NCCN guidelines , will provide the most complete assessment. This assessment can help to determine the most effective treatment plan.
Immunotherapy is another very effective treatment option that uses the body’s own immune response to fight the cancer without damaging healthy cells. In the past few years, lung cancer research has yielded new medications that can be used to stop cancer cells from being able to turn off the immune system. This allows it to do its work in battling the cancer.
In addition, various forms of liquid biopsy are being studied that can identify molecular abnormalities of lung cancer without the need for invasive procedures. These specialized blood tests make use of readily accessible specimens like blood, saliva, or even exhaled breath.
Lung cancer research has rapidly advanced robotics used in surgeries, which allow for quicker recovery for lung cancer patients who have tumors resected. Board certified thoracic surgeons are able to remove the cancerous tissue with minimally invasive techniques, which allow for far smaller incision sites and more rapid recovery for patients.
Stereotactic radiation is a type of external radiation therapy that uses special equipment to position the patient and precisely deliver radiation to a tumor. Advances in techniques using stereotactic radiation allow for effective treatment of brain metastases.

We are in about the most exciting age in lung cancer. It’s an age of rapid fire discovery. Every six months there is a new drug, just about. Dr. Osarogiagbon, Lung Cancer Foundation of America’s Scientific Advisory Board

And so what we find is that, we can give these drugs that turn off this alteration specifically in the cancer cells. And sometimes almost like magic, the cancer melts away and the patients often have no side effects at all. It’s really a dramatic efficacy. Dr. David Carbone, The Ohio State University

Lung cancer research…I know I wouldn’t be here without it. It’s like someone flipped a switch. I went from going downhill fast, to climbing back up. Frank McKenna, after started on a new oral medication that had just been approved for his particular biomarker.
LCFA Funds Impactful Young Investigator Research Grants
“Based on my own personal experience, the LCFA Young Investigator award was the very first grant that I was ever awarded. I’ve been able to leverage that now into several million dollars worth of funding.”
~Dr. Kellie Smith, assistant professor of oncology at Johns Hopkins Medicine
Meet the Researchers
Jonathan villena-vargas, md.
Thoracic Surgeon
Characterizing T cell PD-1 response in the tumor-draining lymph nodes of early-stage NSCLC patients

Jarushka Naidoo, MRCPI
Clinical/Translational Investigator
Identifying novel biomarkers of response and toxicity to immunotherapy

Triparna Sen, PhD
Cell and molecular biologist
Novel therapies for small cell lung cancer (SCLC)

New discoveries in lung cancer are happening at a rapid pace.
Join our community to get the latest new on lung cancer research.
Follow the Latest Research News
Lung cancer research is making significant strides. Find what’s new in lung cancer research for diagnosis, treatment, and prevention.
Colorless, odorless gas likely linked to alarming rise in non-smoking lung cancer
Consumer survey reveals most americans are unaware of dangerous, preventable risk factor.
Although lung cancer is traditionally thought of as a "smoker's disease," a surprising 15-20% of newly diagnosed lung cancers occur in people who have never smoked, many of whom are in their 40s or 50s.
Doctors say this concerning rise in non-smoking lung cancer cases is likely linked to long-term, high exposures of radon gas. This colorless, odorless gas is emitted from the breakdown of radioactive material naturally occurring underground that then seeps through building foundations. The gas can linger and accumulate in people's homes and lungs silently unless they know to test for it.
Although the U.S. Environmental Protection Agency (EPA) recommends regular radon testing and corrective measures to lower exposure levels in homes, a new consumer survey conducted on behalf of The Ohio State University Comprehensive Cancer Center -- Arthur G. James Cancer Hospital and Richard J. Solove Research Institute (OSUCCC -- James) showed that a stunning 75% of Americans have not had their homes tested for radon, and over half (55%) are not concerned about radon exposure in their homes, community or schools.
"Anyone with lungs can develop lung cancer, and as a community we should be aware and concerned about radon exposure because it's thought to be one of the leading causes of lung cancer in never-smokers -- and there is something we can do reduce our risk," said David Carbone, MD, PhD, a thoracic medical oncologist and director of the OSUCCC -- James Thoracic Oncology Center. "There are relatively simple tests to measure radon in the home and actions to reduce radon exposure."
This includes installing outside the home a radon remediation system that sucks air from the basement, where radon gas typically lingers. Increasing air flow by opening windows and using fans/venting in your home, and sealing cracks in the floors, walls and foundation is also important.
Lung cancer rising in young non-smokers
The No. 1 risk factor for lung cancer is long-term cigarette smoking; however, rates of lung cancer among non-smokers continue to rise. The symptoms of the disease are the same regardless of whether the person has smoked: generally not feeling well or feeling tired all the time, frequent cough, chest pain, wheezing, shortness of breath or coughing up blood. These symptoms happen with other illnesses too, but Carbone notes anyone -- regardless of age -- who has a lingering symptom that doesn't resolve despite initial treatment should insist on having it checked out.
Lung cancer screening is currently available only to people at the highest risk for the disease -- that means people aged 50 to 80 who also have a 20 pack-year history (one pack of cigarettes per day for 20 years, are current smokers or someone who has quit within the past 15 years.
If detected in its earliest stages, the cure rate for lung cancer can be 90-95%. The bulk of cases, however, are not detected until the disease has spread throughout the lung or to other parts of the body, when treatments aren't as effective. It is important that anyone deemed at risk for lung cancer get timely screening, and that people who might be at increased risk due to secondhand smoke, radon or occupational exposures (like firefighting) talk to their doctors about testing.
"Your health and the health of your family are the most important things you have. Really push to get your concerns addressed if your symptoms aren't resolving, even if you don't fit the typical 'picture' of lung cancer. It could truly save your life," said Carbone.
Requiring radon testing in homes, schools and workplaces
Carbone noted that having high levels of radon exposure at school or work is just as much a health hazard as having high-level exposure in your basement.
He says he strongly supports potential legislation to require radon testing at schools, at places of business and during home sales to help reduce community risk. The effects of radon on your lungs is cumulative and can be delayed by decades.
"So your children playing in your basement or going to school today, exposed to unknown levels of radon, could be at risk for developing lung cancer 10, 20, 30 years from now," Carbone said. "And because the gas is totally colorless and odorless, you would have no idea you were being exposed unless you knew the importance of proactively testing."
Survey methodology and results
This survey was conducted by SSRS on its Opinion Panel Omnibus platform. The SSRS Opinion Panel Omnibus is a national, twice-per-month, probability-based survey. Data collection was conducted from February 2- February 4, 2024, among a sample of 1,006 respondents. The survey was conducted via web (n=976) and telephone (n=30) and administered in English. The margin of error for total respondents is +/- 3.5 percentage points at the 95% confidence level. All SSRS Opinion Panel Omnibus data are weighted to represent the target population of U.S. adults ages 18 or older.
- Lung Cancer
- Lung Disease
- Diseases and Conditions
- Mesothelioma
- Breast Cancer
- Colon Cancer
- Lung cancer
- Colorectal cancer
- Ovarian cancer
- Breast cancer
- Stomach cancer
Story Source:
Materials provided by Ohio State University Wexner Medical Center . Note: Content may be edited for style and length.
Cite This Page :
Explore More
- Quantum Effects in Electron Waves
- Star Trek's Holodeck Recreated Using ChatGPT
- Cloud Engineering to Mitigate Global Warming
- Detecting Delayed Concussion Recovery
- Genes for Strong Muscles: Healthy Long Life
- Brightest Gamma-Ray Burst
- Stellar Winds of Three Sun-Like Stars Detected
- Fences Causing Genetic Problems for Mammals
- Ozone Removes Mating Barriers Between Fly ...
- Parkinson's: New Theory On Origins and Spread
Trending Topics
Strange & offbeat.
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
April 13, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
reputable news agency

Adjuvant alectinib improves disease-free survival in lung cancer
by Elana Gotkine
Adjuvant alectinib improves disease-free survival compared with platinum-based chemotherapy among patients with resected ALK-positive non-small cell lung cancer (NSCLC), according to a study published in the April 11 issue of the New England Journal of Medicine .
Yi-Long Wu, M.D., from the Guangdong Lung Cancer Institute in Guangzhou, China, and colleagues conducted a global, phase 3, open-label, randomized trial involving patients with completely resected, ALK-positive NSCLC of stage IB, II, or IIIA. Participants were randomly assigned to receive oral alectinib (600 mg twice daily) for 24 months or intravenous platinum-based chemotherapy in four 21-day cycles (130 and 127 patients, respectively).
The researchers found that the percentage of patients alive and disease-free was 93.8 and 63.0 percent in the alectinib and chemotherapy groups, respectively, at two years, among patients with stage II or IIIA disease (hazard ratio for disease recurrence or death, 0.24), and 93.6 and 63.7 percent, respectively, in the intention-to-treat population (hazard ratio, 0.24). A clinically meaningful benefit with respect to central nervous system disease-free survival was seen in association with alectinib versus chemotherapy (hazard ratio, 0.22). The overall survival data were immature. There were no unexpected safety findings.
"The disease-free survival benefit was seen consistently across prespecified subgroups, including those defined according to disease stage, race, sex, and smoking status," the authors write.
The study was funded by F. Hoffmann-La Roche, the manufacturer of alectinib.
Antonio Passaro et al, Adjuvant Alectinib in ALK -Rearranged NSCLC — Here and Now, New England Journal of Medicine (2024). DOI: 10.1056/NEJMe2402015
Copyright © 2024 HealthDay . All rights reserved.
Explore further
Feedback to editors

Carbon beads help restore healthy gut microbiome and reduce liver disease progression, researchers find
5 hours ago

Untangling dreams and our waking lives: Latest findings in cognitive neuroscience
11 hours ago

Researchers demonstrate miniature brain stimulator in humans
Apr 13, 2024
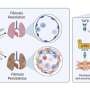
Study reveals potential to reverse lung fibrosis using the body's own healing technique
Apr 12, 2024
Researchers discover cell 'crosstalk' that triggers cancer cachexia

Study improves understanding of effects of household air pollution during pregnancy

Wearable sensors for Parkinson's can improve with machine learning, data from healthy adults

New insights on B cells: Researchers explore building better antibodies and curbing autoimmune diseases

Grieving pet owners comforted by 'supernatural' interactions

Study shows AI improves accuracy of skin cancer diagnoses
Related stories.
AACR: Cadonilimab plus chemo beneficial for gastric adenocarcinoma
Apr 8, 2024
Alectinib: ALEX and ALUR trials show CNS benefit in NSCLC
Sep 6, 2017

Overall survival interim analysis of a Phase III study of atezolizumab vs best supportive care in resected NSCLC
Aug 8, 2022
Researchers report on ctDNA-based detection of residual disease prognostic for resected CRC
Jan 29, 2024

IMpower010 shows improved disease-free survival for resected stage II to III non-small cell lung cancer patients
Sep 9, 2021
Lenvatinib + pembrolizumab treats advanced endometrial cancer
Jan 31, 2022
Recommended for you
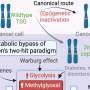
Scientists uncover a missing link between poor diet and higher cancer risk

Genomic deletions explain why some types of melanoma resist targeted therapies

Metformin may help the immune system better identify breast cancer cells
Softer tumors fuel more aggressive spread of triple-negative breast cancer, research shows
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
- See us on facebook
- See us on twitter
- See us on youtube
- See us on linkedin
- See us on instagram
Stanford Medicine study flags unexpected cells in lung as suspected source of severe COVID
A previously overlooked type of immune cell allows SARS-CoV-2 to proliferate, Stanford Medicine scientists have found. The discovery has important implications for preventing severe COVID-19.
April 10, 2024 - By Bruce Goldman
In an uninfected interstitial macrophage, the nucleus (purple) and outer cell membrane (blue) are intact. In an infected interstitial macrophage, the nucleus is shattered, copious newly made viral components (red) clump together, and the cell broadcasts inflammatory and scar-tissue-inducing chemical signals (yellow). Emily Moskal
The lung-cell type that’s most susceptible to infection by SARS-CoV-2, the virus that causes COVID-19, is not the one previously assumed to be most vulnerable. What’s more, the virus enters this susceptible cell via an unexpected route. The medical consequences may be significant.
Stanford Medicine investigators have implicated a type of immune cell known as an interstitial macrophage in the critical transition from a merely bothersome COVID-19 case to a potentially deadly one. Interstitial macrophages are situated deep in the lungs, ordinarily protecting that precious organby, among other things, engorging viruses, bacteria, fungi and dust particles that make their way down our airways. But it’s these very cells, the researchers have shown in a study published online April 10 in the Journal of Experimental Medicine , that of all known types of cells composing lung tissue are most susceptible to infection by SARS-CoV-2.
SARS-CoV-2-infected interstitial macrophages, the scientists have learned, morph into virus producersand squirt out inflammatory and scar-tissue-inducing chemical signals, potentially paving the road to pneumonia and damaging the lungs to the point where the virus, along with those potent secreted substances, can break out of the lungs and wreak havoc throughout the body.
The surprising findings point to new approaches in preventing a SARS-CoV-2 infection from becoming a life-threatening disease. Indeed, they may explain why monoclonal antibodies meant to combat severe COVID didn’t work well, if at all — and when they did work, it was only when they were administered early in the course of infection, when the virus was infecting cells in the upper airways leading to the lungs but hadn’t yet ensconced itself in lung tissue.
The virus surprises
“We’ve overturned a number of false assumptions about how the virus actually replicates in the human lung,” said Catherine Blish , MD, PhD, a professor of infectious diseases and of microbiology and immunology and the George E. and Lucy Becker Professor in Medicine and associate dean for basic and translational research.
Blish is the co-senior author of the study, along with Mark Krasnow , MD, PhD, the Paul and Mildred Berg Professor of biochemistry and the Executive Director of the Vera Moulton Wall Center for pulmonary vascular disease.
“The critical step, we think, is when the virus infects interstitial macrophages, triggering a massive inflammatory reaction that can flood the lungs and spread infection and inflammation to other organs,” Krasnow said. Blocking that step, he said, could prove to be a major therapeutic advance. But there’s a plot twist: The virus has an unusual way of getting inside these cells — a route drug developers have not yet learned how to block effectively — necessitating a new focus on that alternative mechanism, he added.

Catherine Blish
In a paper published in Nature in early 2020, Krasnow and his colleagues including then-graduate student Kyle Travaglini, PhD — who is also one of the new study’s co-lead authors along with MD-PhD student Timothy Wu — described a technique they’d worked out for isolating fresh human lungs; dissociating the cells from one another; and characterizing them, one by one, on the basis of which genes within each cell were active and how much so. Using that technique, the Krasnow lab and collaborators were able to discern more than 50 distinct cell types, assembling an atlas of healthy lung cells.
“We’d just compiled this atlas when the COVID-19 pandemic hit,” Krasnow said. Soon afterward, he learned that Blish and Arjun Rustagi , MD, PhD, instructor of infectious diseases and another lead co-author of the study, were building an ultra-safe facility where they could safely grow SARS-CoV-2 and infect cells with it.
A collaboration ensued. Krasnow and Blish and their associates obtained fresh healthy lung tissue excised from seven surgical patients and five deceased lung donors whose lungs were virus-free but for one reason or another not used in transplants. After infecting the lung tissue with SARS-CoV-2 and waiting one to three days for the infection to spread, they separated and typed the cells to generate an infected-lung-cell atlas, analogous to the one Krasnow’s team had created with healthy lung cells. They saw most of the cell types that Krasnow’s team had identified in healthy lung tissue.
Now the scientists could compare pristine versus SARS-CoV-2-infected lungs cells of the same cell type and see how they differed: They wanted to know which cells the virus infected, how easily SARS-CoV-2 replicated in infected cells, and which genes the infected cells cranked up or dialed down compared with their healthy counterparts’ activity levels. They were able to do this for each of the dozens of different cell types they’d identified in both healthy and infected lungs.
“It was a straightforward experiment, and the questions we were asking were obvious,” Krasnow said. “It was the answers we weren’t prepared for.”
It’s been assumed that the cells in the lungs that are most vulnerable to SARS-CoV-2 infection are those known as alveolar type 2 cells. That’s because the surfaces of these cells, along with those of numerous other cell types in the heart, gut and other organs, sport many copies of a molecule known as ACE2. SARS-CoV-2 has been shown to be able to grab onto ACE2 and manipulate it in a way that allows the virus to maneuver its way into cells.
Alveolar type 2 cells are somewhat vulnerable to SARS-CoV-2, the scientists found. But the cell types that were by far the most frequently infected turned out to be two varieties of a cell type called a macrophage.
Virus factories
The word “macrophage” comes from two Greek terms meaning, roughly, “big eater.” This name is not unearned. The air we inhale carries not only oxygen but, unfortunately, tiny airborne dirt particles, fungal spores, bacteria and viruses. A macrophage earns its keep by, among other things, gobbling up these foreign bodies.

Mark Krasnow
The airways leading to our lungs culminate in myriad alveoli, minuscule one-cell-thick air sacs, whichare abutted by abundant capillaries. This interface, called the interstitium, is where oxygen in the air we breathe enters the bloodstream and is then distributed to the rest of the body by the circulatory system.
The two kinds of SARS-CoV-2-susceptible lung-associated macrophages are positioned in two different places. So-called alveolar macrophages hang out in the air spaces within the alveoli. Once infected, these cells smolder, producing and dribbling out some viral progeny at a casual pace but more or less keeping a stiff upper lip and maintaining their normal function. This behavior may allow them to feed SARS-CoV-2’s progression by incubating and generating a steady supply of new viral particles that escape by stealth and penetrate the layer of cells enclosing the alveoli.
Interstitial macrophages, the other cell type revealed to be easily and profoundly infected by SARS-CoV-2, patrol the far side of the alveoli, where the rubber of oxygen meets the road of red blood cells. If an invading viral particle or other microbe manages to evade alveolar macrophages’ vigilance, infect and punch through the layer of cells enclosing the alveoli, jeopardizing not only the lungs but the rest of the body, interstitial macrophages are ready to jump in and protect the neighborhood.
At least, usually. But when an interstitial macrophage meets SARS-CoV-2, it’s a different story. Rather than get eaten by the omnivorous immune cell, the virus infects it.
And an infected interstitial macrophage doesn’t just smolder; it catches on fire. All hell breaks loose as the virus literally seizes the controls and takes over, hijacking a cell’s protein- and nucleic-acid-making machinery. In the course of producing massive numbers of copies of itself, SARS-CoV-2 destroys the boundaries separating the cell nucleus from the rest of the cell like a spatula shattering and scattering the yolk of a raw egg. The viral progeny exit the spent macrophage and move on to infect other cells.
But that’s not all. In contrast to alveolar macrophages, infected interstitial macrophages pump out substances that signal other immune cells elsewhere in the body to head for the lungs. In a patient, Krasnow suggested, this would trigger an inflammatory influx of such cells. As the lungs fill with cells and fluid that comes with them, oxygen exchange becomes impossible. The barrier maintaining alveolar integrity grows progressively damaged. Leakage of infected fluids from damaged alveoli propels viral progeny into the bloodstream, blasting the infection and inflammation to distant organs.
Yet other substances released by SARS-CoV-2-infected interstitial macrophages stimulate the production of fibrous material in connective tissue, resulting in scarring of the lungs. In a living patient, the replacement of oxygen-permeable cells with scar tissue would further render the lungs incapable of executing oxygen exchange.
“We can’t say that a lung cell sitting in a dish is going to get COVID,” Blish said. “But we suspect this may be the point where, in an actual patient, the infection transitions from manageable to severe.”
Another point of entry
Compounding this unexpected finding is the discovery that SARS-CoV-2 uses a different route to infect interstitial macrophages than the one it uses to infect the other types.
Unlike alveolar type 2 cells and alveolar macrophages, to which the virus gains access by clinging to ACE2 on their surfaces, SARS-CoV-2 breaks into interstitial macrophages using a different receptor these cells display. In the study, blocking SARS-CoV-2’s binding to ACE2 protected the former cells but failed to dent the latter cells’ susceptibility to SARS-CoV-2 infection.
“SARS-CoV-2 was not using ACE2 to get into interstitial macrophages,” Krasnow said. “It enters via another receptor called CD209.”
That would seem to explain why monoclonal antibodies developed specifically to block SARS-CoV-2/ACE2 interaction failed to mitigate or prevent severe COVID-19 cases.
It’s time to find a whole new set of drugs that can impede SARS-CoV-2/CD209 binding. Now, Krasnow said.
The study was funded by the National Institutes of Health (grants K08AI163369, T32AI007502 and T32DK007217), the Bill & Melinda Gates Foundation, Chan Zuckerberg Biohub, the Burroughs Wellcome Fund, Stanford Chem-H, the Stanford Innovative Medicine Accelerator, and the Howard Hughes Medical Institute.

About Stanford Medicine
Stanford Medicine is an integrated academic health system comprising the Stanford School of Medicine and adult and pediatric health care delivery systems. Together, they harness the full potential of biomedicine through collaborative research, education and clinical care for patients. For more information, please visit med.stanford.edu .
Artificial intelligence
Exploring ways AI is applied to health care
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- 18 November 2020
Research round-up: lung cancer
- Bianca Nogrady
You can also search for this author in PubMed Google Scholar
Genes predict effect of immunotherapy
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
185,98 € per year
only 3,65 € per issue
Rent or buy this article
Prices vary by article type
Prices may be subject to local taxes which are calculated during checkout
Nature 587 , S8-S9 (2020)
doi: https://doi.org/10.1038/d41586-020-03153-z
This article is part of Nature Outlook: Lung cancer , an editorially independent supplement produced with the financial support of third parties. About this content .
Related Articles
- Therapeutics
- Medical research
Biological age surges in survivors of childhood cancer
Research Highlight 11 APR 24
How to supercharge cancer-fighting cells: give them stem-cell skills
News 10 APR 24
FOXO1 is a master regulator of memory programming in CAR T cells
Article 10 APR 24

The rise of eco-anxiety: scientists wake up to the mental-health toll of climate change
News Feature 10 APR 24

Metabolic rewiring promotes anti-inflammatory effects of glucocorticoids
Use fines from EU social-media act to fund research on adolescent mental health
Correspondence 09 APR 24
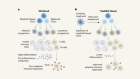
Anti-ageing antibodies revive the immune system
News & Views 27 MAR 24

How AI is being used to accelerate clinical trials
Nature Index 13 MAR 24

Yaws could soon be eradicated — 70 years behind schedule
Outlook 11 JAN 24
Junior Group Leader Position at IMBA - Institute of Molecular Biotechnology
The Institute of Molecular Biotechnology (IMBA) is one of Europe’s leading institutes for basic research in the life sciences. IMBA is located on t...
Austria (AT)
IMBA - Institute of Molecular Biotechnology
Research Group Head, BeiGene Institute
A cross-disciplinary research organization where cutting-edge science and technology drive the discovery of impactful Insights
Pudong New Area, Shanghai
BeiGene Institute
Open Rank Faculty, Center for Public Health Genomics
Center for Public Health Genomics & UVA Comprehensive Cancer Center seek 2 tenure-track faculty members in Cancer Precision Medicine/Precision Health.
Charlottesville, Virginia
Center for Public Health Genomics at the University of Virginia
Husbandry Technician I
Memphis, Tennessee
St. Jude Children's Research Hospital (St. Jude)
Lead Researcher – Department of Bone Marrow Transplantation & Cellular Therapy
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Skip to Content
- Conquer Cancer
- ASCO Journals
- f Cancer.net on Facebook
- t Cancer.net on Twitter
- q Cancer.net on YouTube
- g Cancer.net on Google
Types of Cancer
- Navigating Cancer Care
- Coping With Cancer
- Research and Advocacy
- Survivorship
Lung Cancer - Non-Small Cell: Latest Research
ON THIS PAGE: You will read about the scientific research being done to learn more about this type of cancer and how to treat it. Use the menu to see other pages.
Doctors are working to learn more about non-small cell lung cancer (NSCLC), ways to prevent it, how to best treat it, and how to provide the best care to people diagnosed with this disease. The following areas of research may include new options for patients through clinical trials. Always talk with your doctor about the best diagnostic and treatment options for you.
Personalized drug therapy . Researchers are looking at features of lung tumors that can predict whether a specific drug, such as chemotherapy or targeted therapy, may be effective. To collect this information, patients are increasingly being asked to have additional analyses of the tumor samples taken when the disease is first diagnosed. In many patients for whom chemotherapy is recommended, the amount of tumor tissue removed during the biopsy to diagnose their cancer is not enough for these additional tests. These patients may be asked to have another biopsy to help plan treatment and, if part of a clinical trial, to help researchers find better ways to treat lung cancer. Learn more about personalized therapy .
Targeted therapy. Researchers are looking at gene and protein changes that could be new targets for treatment. These include changes called NRG fusion. Additional research is also being done to study drugs that can help patients after an initial targeted therapy stops working.
Immunotherapy. Promising results in immunotherapy for NSCLC and the recent approval of multiple types of immunotherapy are leading to more research on using these types of drugs to help the immune system control NSCLC growth.
Better techniques for surgery and radiation therapy. Doctors are finding ways to improve the effectiveness of surgery and radiation therapy while reducing the side effects of these procedures. For example, a current study is comparing the removal of early-stage NSCLC by lobectomy to removal by wedge resection or segmentectomy. This is to preserve nearby lung tissue. Stereotactic radiation therapy is also being studied for NSCLC. This technique is used to focus radiation therapy more directly on the cancer and avoids more of the healthy tissue. Advances in all types of treatment will improve doctors’ ability to combine medication, radiation therapy, and surgery for the treatment of all stages of NSCLC.
Liquid biopsies: Free-floating cancer DNA from blood tests can be used to find molecular changes in your cancer. These are typically used at initial diagnosis and at the time when certain targeted therapies are no longer working (at the time of acquired resistance to a treatment). Research is ongoing to evaluate new ways to use liquid biopsies to assess response to treatments or detect remaining cancer DNA after surgery.
Improved screening. NSCLC is more successfully treated in its early stages, which has raised interest in screening people for lung cancer before it causes signs and symptoms. Researchers are studying improved screening techniques, including genetic testing and blood tests to learn which people have a higher risk of lung cancer.
Stopping tobacco use. Even with the best methods for the early detection and treatment of lung cancer, the best way to save lives from lung cancer is through programs to encourage people to never begin smoking and, if they have, quit cigarette smoking . For most people, lung cancer is a highly preventable disease. Even for people diagnosed with lung cancer, stopping smoking lengthens their lives, lowers side effects, and lessens their chance of getting a second lung cancer. Quitting smoking is hard at any time, and even more so during cancer treatment. The health care team can help make it easier to quit smoking with nicotine replacement and other techniques. Read about one recent study that confirmed stopping smoking helps people with lung cancer. Research continues into new ways to help people stop smoking.
Palliative care/supportive care . Clinical trials are underway to find better ways of reducing symptoms and side effects of current lung cancer treatments to improve comfort and quality of life for patients.
Looking for More About the Latest Research?
If you would like more information about the latest areas of research in NSCLC, explore these related items that take you outside of this guide:
To find clinical trials specific to your diagnosis, talk with your doctor or search online clinical trial databases .
Visit the Cancer.Net Blog to review lung cancer news and information, including a study from a recent scientific meeting about inherited genetic mutations that are known to increase lung cancer risk.
Listen to a podcast from an ASCO expert about recent clinical trials that studied molecular testing in early-stage lung cancer.
Visit the website of Conquer Cancer, the ASCO Foundation to find out how to help support cancer research. Please note that this link takes you to a different ASCO website.
The next section in this guide is Coping with Treatment . It offers some guidance in how to cope with the physical, emotional, social, and financial changes that cancer and its treatment can bring. Use the menu to choose a different section to read in this guide.
Lung Cancer - Non-Small Cell Guide
Cancer.Net Guide Lung Cancer - Non-Small Cell
- Introduction
- Medical Illustrations
- Risk Factors and Prevention
- Symptoms and Signs
- Types of Treatment
- About Clinical Trials
- Latest Research
- Coping with Treatment
- Follow-Up Care
- Questions to Ask the Health Care Team
- Additional Resources
View All Pages
Timely. Trusted. Compassionate.
Comprehensive information for people with cancer, families, and caregivers, from the American Society of Clinical Oncology (ASCO), the voice of the world's oncology professionals.
Find a Cancer Doctor

Some breast cancer patients could be at risk of another type of cancer, study reveals
Women with breast cancer who have received chemotherapy are at an increased risk of developing lung cancer, a new study suggests.
Epic Research, a health data group based in Delaware, found that women in this category have a 57% higher lung cancer risk than those who received radiation.
In comparison to patients who received endocrine therapy, those who have undergone chemo have a 171% increase in lung cancer risk, the study found.
BREAST CANCER DRUG COULD HAVE POTENTIALLY SERIOUS SIDE EFFECT, NEW RESEARCH REVEALS
In a statement sent to Fox News Digital, the Epic Research team said the key takeaway from their research is that primary lung cancer is more than twice as prevalent in women who were previously diagnosed with breast cancer — compared to those who did not have it.
"Furthermore, women who had breast cancer and received chemotherapy have the greatest risk of subsequent primary lung cancer," the researchers wrote.
READ ON THE FOX NEWS APP
"This suggests that patients diagnosed with breast cancer are at an increased risk of developing second primary lung cancer, especially if their treatment included chemotherapy."
BREAST CANCER BREAKTHROUGH: AI PREDICTS A THIRD OF CASES PRIOR TO DIAGNOSIS IN MAMMOGRAPHY STUDY
The research group studied more than two million women ages 50 to 84 who received a screening mammogram between 2010 and 2023.
Patients with an elevated breast cancer risk due to a previous breast or lung cancer diagnosis, those who had been screened within the past three months and those who started mammogram screenings prior to age 50 were excluded from the study.
"This could potentially limit the generalizability of our findings," the researchers said.
The team encouraged patients with a history of breast cancer — especially those who have had chemotherapy — to monitor for the development of primary lung cancer.
"It is important to remember that while our study found a correlation between breast cancer, its treatments and subsequent primary lung cancer, this does not mean that every woman who has had breast cancer will develop lung cancer," the researchers said.
CLICK HERE TO SIGN UP FOR OUR HEALTH NEWSLETTER
Fox News medical contributor Dr. Marc Siegel, clinical professor of medicine at NYU Langone Medical Center, told Fox News Digital that one cancer can cause a " higher genetic risk " for others.
"We don't know the exact etiology, but one cancer puts you in a higher genetic risk pool for other cancers, either because of cancer genes that increase the risk of both, or because of a tendency for mutations that is increased in this pool," he said.
"It could also be because of environmental factors or carcinogens, including diet, or the result of toxicities from the treatment for breast cancer," Siegel added.
Jack Manley, M.D., head of new markets and growth at Viz.ai, a San Francisco-based AI-powered disease detection platform, shared with Fox News Digital that Epic Research’s findings and methodology speak to "the power of incorporating multi-modal data in predictive algorithms."
Said Manley as well, "Companies with capabilities to incorporate both structured and unstructured EHR (electronic health record) data with conventional imaging will have a higher predictive performance than those that don't."
He was not involved in the study.
"Currently, a large majority of patients with pulmonary nodules (a possible indicator of early lung cancer) are missed on conventional imaging, while less than half of these detected patients receive subsequent guideline-recommended follow up," he said.
Artificial intelligence tools are "well-positioned" to address these challenges, Manley noted — but EHR integration is "key to finding those patients at the highest risk."
For more Health articles, visit www.foxnews.com/health .
Original article source: Some breast cancer patients could be at risk of another type of cancer, study reveals


IMAGES
VIDEO
COMMENTS
This page highlights some of the latest research in non-small cell lung cancer (NSCLC), the most common form of lung cancer, including clinical advances that may soon translate into improved care, NCI-supported programs that are fueling progress, and research findings from recent studies.
The FDA approved sotorasib for treating lung cancers with this specific KRAS mutation. At the American Association for Cancer Research annual meeting in April 2022, researchers presented longer-term follow-up data for the clinical trial that led to sotorasib's approval. Notably, nearly one-third of patients continued to survive after two ...
Lung cancer is one of the most frequently diagnosed cancers and the leading cause of cancer-related deaths worldwide with an estimated 2 million new cases and 1·76 million deaths per year. Substantial improvements in our understanding of disease biology, application of predictive biomarkers, and refinements in treatment have led to remarkable progress in the past two decades and transformed ...
Lung cancer arises in tissues of the lung, usually in the cells lining air passages. The two main types are small-cell lung cancer and non-small-cell lung cancer, according to the shape of cells ...
Lung cancer is a very aggressive and highly prevalent disease worldwide, with an estimated 2.2 million new cases and 1.8 million deaths in 2020.
Improved overall survival with adjuvant osimertinib. In total, 682 people participated in the study. Participants were allowed to receive chemotherapy after surgery (but before starting adjuvant osimertinib or placebo), and about 60% did. Treatment with adjuvant chemotherapy was most frequent in people with stage II and IIIA disease (71% and 80 ...
Read the latest Research articles in Lung cancer from Nature Reviews Clinical Oncology. ... Lung cancer is a disease typically associated with tobacco smoking; however, lung cancer in individuals ...
But with new treatments and technology, the survival rates from lung cancer are dramatically improving, allowing some patients with relatively late-stage cancers to live for years longer.
The year 2021 was marked by new approaches to adjuvant therapy and the availability of agents to target new subsets of nonsmall cell lung cancer. Funding information Nisha A. Mohindra is supported by grant AHRQ-K12HS02638 from the Agency for Healthcare Research and Quality, US Department of Health and Human Services.
Screening lowers the risk of death from lung cancer. Ongoing studies are looking at new ways to improve early detection of lung cancer: Ways to use molecular markers from your body fluid (i.e., sputum or blood) for lung cancer screening. Ways to use new forms of bronchoscopies for lung cancer screening, such as autofluorescence bronchoscopy.
The more can do at the beginning of treatment to essentially cure the cancer is where our research lies and where these exciting new changes come into play." Screening for Early Stage Lung Cancer The identification of early stage lung cancer has increased significantly in the past five years because of low-dose CT screening for smokers.
Update: On May 28, 2021, the US Food and Drug Administration granted accelerated approval to sotorasib (Lumakras TM) for the treatment of advanced non-small cell lung cancer driven by the KRAS-G12C mutation in patients who have already received at least one other treatment.The approval was based on the clinical trials co-led by Memorial Sloan Kettering medical oncologist Bob Li.
C.M. LovlyN Engl J Med 2023;389:560-561. Lung cancer remains the leading cause of cancer-related deaths worldwide. 1 However, the surge of new therapies and the use of these therapies in earlier ...
Information about lung cancer symptoms and treatments. Explore the latest medical research on cancers including experimental treatments.
Read the latest Research articles in Lung cancer from Nature Medicine. ... In the first stage of the BR.36 adaptive trial in patients with non-small cell lung cancer receiving anti-PD1 ...
Molecular-based targeted therapies for lung cancer. Extensive research is being carried out to pinpoint the key players playing pivotal roles in malignancy and/or cell survival while exploiting this knowledge to develop targeted therapies for lung cancer treatment. ... The international epidemiology of lung cancer: Latest trends, disparities ...
Learn about these 5 Exciting Areas of Lung Cancer Research. Biomarkers. Immunotherapy. Liquid Biopsy. Robotics. Stereotactic Radiation. We are in about the most exciting age in lung cancer. It's an age of rapid fire discovery. Every six months there is a new drug, just about.
Lung cancer is a significant public health concern, causing a considerable number of deaths globally. GLOBOCAN 2020 estimates of cancer incidence and mortality produced by the International Agency for Research on Cancer (IARC) show as lung cancer remains the leading cause of cancer death, with an estimated 1.8 million deaths (18%) in 2020.
Although lung cancer is traditionally thought of as a 'smoker's disease,' a surprising 15-20% of newly diagnosed lung cancers occur in people who have never smoked, many of whom are in their 40s ...
For resectable non-small-cell lung cancer (NSCLC), data from the phase III CheckMate 77T study, ... Get what matters in cancer research, free to your inbox weekly.
More information: Yi-Long Wu et al, Alectinib in Resected ALK -Positive Non-Small-Cell Lung Cancer, New England Journal of Medicine (2024). DOI: 10.1056/NEJMoa2310532
The PD-1 pathway may be very important in the immune system's ability to control cancer growth. Blocking this pathway with PD-1 and PD-L1 antibodies has stopped or slowed the growth of lung cancer for some patients. Research into new immunotherapies and new ways to use these treatments is ongoing. Learn more about immunotherapy and lung cancer.
In a paper published in Nature in early 2020, Krasnow and his colleagues including then-graduate student Kyle Travaglini, PhD — who is also one of the new study's co-lead authors along with MD-PhD student Timothy Wu — described a technique they'd worked out for isolating fresh human lungs; dissociating the cells from one another; and characterizing them, one by one, on the basis of ...
Among the screen-detected lung cancers, only 9.4% were at stage IV, whereas in the non-screened group, 45.7% of lung cancers diagnosed were at stage IV. The authors reported that adherence to ...
Research is ongoing to evaluate new ways to use liquid biopsies to assess response to treatments or detect remaining cancer DNA after surgery. Improved screening. NSCLC is more successfully treated in its early stages, which has raised interest in screening people for lung cancer before it causes signs and symptoms.
Women with breast cancer who have received chemotherapy are at an increased risk of developing lung cancer, a new study suggests. Epic Research, a health data group based in Delaware, found that ...