- ASH Foundation
- Log in or create an account
- Publications
- Diversity Equity and Inclusion
- Global Initiatives
- Resources for Hematology Fellows
- American Society of Hematology
- Hematopoiesis Case Studies

Case Study: New Therapies for Acute Myeloid Leukemia
- Agenda for Nematology Research
- Precision Medicine
- Genome Editing and Gene Therapy
- Immunologic Treatment
- Research Support and Funding
A 76-year-old woman presents to the emergency department following two weeks of progressive dyspnea and fatigue, and a new rash. Her medical history is significant for stage 2 chronic kidney disease, coronary artery disease, and diabetes.
Physical examination results are within normal limits, except for skin pallor and a petechial rash on the lower extremities bilaterally. She has an Eastern Cooperative Oncology Group (ECOG) performance status score of 1. Complete blood count with differential is significant for a white blood cell count of 18 × 10 9 /L with 40 percent circulating blasts, hemoglobin 6.7 g/dL, and platelet count of 20 × 10 9 /L. A bone marrow biopsy reveals a hypercellular marrow with 22 percent blasts, consistent with a diagnosis of acute myeloid leukemia (AML). Flow cytometry demonstrates CD33 negativity, and classic cytogenetic analysis revealed a normal karyotype. Molecular markers are pending for FLT3, IDH1, IDH2, and NPM1.
A pre-treatment echocardiogram is performed and is notable for mild global systolic dysfunction and a left-ventricular ejection fraction of 45 percent.
Which of the following is the most appropriate therapy?
- Gilternitinib
- Azacitidine and venetoclax
- Liposomal daunorubicin and cytarabine
- Gemtuzumab ozogamicin
Explanation
The best treatment option for this patient is azacitidine and venetoclax. Recently, the U.S. Food and Drug Administration (FDA) approved the BCL-2 inhibitor venetoclax in combination with a hypomethylating agent for patients with newly diagnosed AML who are 75 years or older, or those with comorbidities that preclude the use of intensive induction chemotherapy. 1 Approval was based on preliminary data published in February 2018 from a phase Ib study of 57 patients to evaluate the safety and efficacy of either azacitidine or decitabine in combination with venetoclax. 2
Eligibility criteria included previously untreated patients aged 65 years and older with AML who were ineligible for standard induction therapy, ECOG performance status of 0 to 2, and intermediate-risk or poor-risk cytogenetics. During dose escalation, oral venetoclax was administered daily in combination with either decitabine (days 1-5) or azacitidine (days 1-7). Results from this study population showed a complete remission (CR) or CR with incomplete marrow recovery (CRi) in 61 percent of patients. 2 A follow-up of the same clinical trial was recently published in January 2019 evaluating 145 patients. 3 This study demonstrated a CR + CRi rate at all doses of 67 percent, with notable responses in those with poor-risk cytogenetics and those who were at least 75 years old. The median duration of CR + CRi was 11.3 months, with a median overall survival of 17.5 months. 3
While this patient has newly diagnosed AML, her age and comorbidities, including CKD, borderline heart function, and diabetes, likely preclude her from being able to tolerate a standard induction chemotherapy regimen. 4 Gilteritinib (answer A) is an oral kinase inhibitor that was recently approved for treatment of relapsed or refractory AML, with a FLT3 mutation based on interim analysis of 138 patients in the ADMIRAL trial, showing CR or CRh in 21 percent of patients. 5 Answer D is incorrect because the liposomal form of daunorubicin and cytarabine is approved for indications of newly diagnosed therapy-related AML (t-AML) or AML with myelodysplasia-related changes, 6 which is not the case with this patient. Additionally, the left ventricular dysfunction is a relative contraindication to the danunorubicin. Ivosidenib is an IDH-1 inhibitor approved for patients with relapsed or refractory AML with a mutation in the IDH-1 gene. 7 While gemtuzumab ozogamicin (GO), an anti-CD33 monoclonal antibody, is well tolerated in older patients with newly diagnosed or relapsed AML, its approval is for treatment of CD33+ disease. 8 It would be an inappropriate choice for this patient because her flow cytometry demonstrated CD33 negativity. It is also not used as a single agent for initial induction therapy.
In summary, for older patients with newly diagnosed AML, a hypomethylating agent in combination with venetoclax should be considered when comorbidities preclude the use of standard induction chemotherapy.
Case study submitted by Nicole Held, DO, and Talha Badar, MD, of Medical College of Wisconsin, Milwaukee, WI.
Resources
- U.S. Food and Drug Administration FDA approves venetoclax in combination for AML in adults. . 2018.
- DiNardo CD, Pratz KW, Letai A, et al Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid luekaemia: a non-randomised, open-label, phase 1b study . Lancet Oncol. 2018 19:216-228.
- DiNardo CD, Pratz K, Pullarkat V, et al Venetoclax combined with decitabine or azacitidine in treatment-naïve, elderly patients with acute myeloid leukemia . Blood. 2019 133:7-17.
- Kantarjian H, O’brien S, Cortes J, et al Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: predictive prognostic models for outcome . Cancer. 2006 106:1090-1098.
- U.S. Food and Drug Administration FDA approves gilteritinib for relapsed or refractory acute myeloid leukemia (AML) with a FLT3 mutation . 2018.
- Vyxeos (daunorubicin and cytarabine) package insert . Jazz Pharmaceuticals. 2017.
- U.S. Food and Drug Administration FDA approves first targeted treatment for patients with relapsed or refractory acute myeloid leukemia who have a certain genetic mutation . 2018.
- Sievers EL, Larson RA, Stadtmauer EA, et al Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukemia in first relapse . J Clin Oncol. 2001 19:3244-3254.
American Society of Hematology. (1). Case Study: New Therapies for Acute Myeloid Leukemia. Retrieved from https://www.hematology.org/education/trainees/fellows/case-studies/new-therapies-for-acute-myeloid-leukemia .
American Society of Hematology. "Case Study: New Therapies for Acute Myeloid Leukemia." Hematology.org. https://www.hematology.org/education/trainees/fellows/case-studies/new-therapies-for-acute-myeloid-leukemia (label-accessed March 28, 2024).
"American Society of Hematology." Case Study: New Therapies for Acute Myeloid Leukemia, 28 Mar. 2024 , https://www.hematology.org/education/trainees/fellows/case-studies/new-therapies-for-acute-myeloid-leukemia .
Citation Manager Formats
Introduction
Volume–outcome relationship in aml, are there distinct patient subsets that derive particular benefit from treatment at higher-volume or academic centers, transition to outpatient delivery of intensive aml-directed therapy and supportive care, will treatment at a higher-volume/academic center remain important with new lower-intensity and/or oral drugs, conclusion and future perspective, acknowledgments, practice patterns and outcomes for adults with acute myeloid leukemia receiving care in community vs academic settings.
- Split-Screen
- Request Permissions
- Cite Icon Cite
- Search Site
- Open the PDF for in another window
Anna B. Halpern , Roland B. Walter; Practice patterns and outcomes for adults with acute myeloid leukemia receiving care in community vs academic settings. Hematology Am Soc Hematol Educ Program 2020; 2020 (1): 129–134. doi: https://doi.org/10.1182/hematology.2020000097
Download citation file:
- Ris (Zotero)
- Reference Manager
Visual Abstract

Consistent with observations in other disease settings, retrospective studies have indicated that treatment outcomes for adults with acute myeloid leukemia (AML) are better in higher- vs lower-volume hospitals and academic vs nonacademic centers, with greatest benefits noted in acute promyelocytic leukemia. Younger age, more frequent receipt of chemotherapy and hematopoietic cell transplantation, and differences in comorbidities and socioeconomic factors may partially account for these differences. With new therapeutic options including oral small molecule inhibitors and parenteral drugs suitable for outpatient administration, there is increasing interest from patients and physicians in treating AML in the community setting and avoiding referral to academic centers. This may be particularly true for older adults, for whom treatment rates in the community have historically been low, and for those with comorbidities, because treatment benefits are estimated to be low, and thus travel to academic centers is perceived as especially burdensome. How the volume-outcome relationship is affected by the shift of the treatment landscape in AML over the last few years is unknown. Additionally, improvements in supportive care (transfusion support, broad-spectrum oral antimicrobials), resulting in gradually decreasing early death rates over time, and the growing focus on the impact of AML therapy on quality of life and treatment cost concerns further fuel the larger trend toward an increasing proportion of care delivered in the outpatient setting. Here, we examine whether the current shift of administering chemotherapy and supportive care to the outpatient setting can be translated to the community setting without compromising patient outcomes.
Understand the impact of center type on outcomes in patients with AML
Recognize characteristics of academic centers that may account for differences in outcomes compared with community settings
Consider how emerging diagnostic and monitoring techniques, together with the availability of new drugs, will affect care delivery to AML patients in the future
Until recently, treatment options for acute myeloid leukemia (AML) were relatively limited, and decision making followed an algorithm in place for almost 50 years. 1,2 For medically fit patients, cure was assumed possible with intensive chemotherapy and possibly allogeneic hematopoietic cell transplantation (HCT). Because of transfusion needs and risks of disease/treatment-related complications, patients receiving intensive therapies typically remained in the hospital, either at academic centers or nonacademic facilities, until resolution of cytopenias. In contrast, if the patient was judged medically unfit, cure was considered rare, prompting either nonintensive chemotherapy, most commonly low-dose cytarabine or single-agent azacitidine or decitabine, or an approach purely focused on supportive care.
Since 2017, the US Food and Drug Administration has approved 8 new drugs for AML in the United States. 3 With these, treatment options have substantially increased, and the line dividing intensive and less-intensive therapies has become less clear. With oral small-molecule inhibitors and parenteral drugs suitable for outpatient administration included, and helped by improvements in supportive care with resulting declines in early death rates, interest is mounting from patients and physicians in treating AML in the community. This may be especially true for older adults and/or those with comorbid illnesses, because expectations for treatment benefits may be low; hence, travel to and treatment at academic centers be perceived as particularly burdensome. Treatment rates for such individuals in the community have historically been low. This trend is further fueled by an increasing interest in the impact of AML therapy on quality of life (QOL) and growing concerns over costs. Here, we review the evidence for and against the need to treat AML at academic centers and examine whether the current shift of transitioning therapy and supportive care to the outpatient setting can be translated to the community without compromising patient outcomes.
For many medical conditions and surgical procedures, both in oncologic and nononcologic settings, numerous studies and meta-analyses have shown a strong correlation between increasing patient volumes and better outcomes. 4,5 A study reported several years ago 6 suggested AML is no exception to this. A more recent analysis of patterns of care and clinical outcomes with conventional induction chemotherapy (IC) across diverse practice settings supports this conclusion by showing AML patients treated in high-IC–volume hospitals were less likely to die or be discharged to hospice than those treated at low-IC–volume hospitals. 7
Rather than examining patient volume in the strict sense, however, most studies (all retrospective) have compared academic with community centers, with individual studies differing in how to define academic centers (patient volume vs designation as comprehensive cancer center by the National Cancer Institute or academic center by the Commission on Cancer), complicating comparisons and data interpretation. As one example, data from >60 000 AML patients treated in the United States between 2003 and 2011 suggested lower early death risks and better 1- and 5-year overall survival at academic centers. 8 The benefit was greatest for patients with acute promyelocytic leukemia (APL), consistent with previous observations. 9,10 However, although many studies have suggested better outcomes at higher-volume or academic institutions, findings have not been entirely uniform. In the European Organization for Research and Treatment/Gruppo Italiano Malattie Ematologiche dell’ Adulto AML 8A study, for instance, which included patients treated at both transplant centers and referring centers (analogous to community centers), early death rates were higher and initial remission rates lower at referring centers, but remission rates after the second induction course and 6-year overall survival were similar. 11
There are many reasons why AML patients may do better with nontransplant therapies at academic (or higher-volume) centers ( Table 1 ). For one, patients treated at academic centers, especially when older, are more likely to receive chemotherapy than their counterparts in the community. 12,13 Furthermore, physicians, other medical providers, and supportive and ancillary staff (eg, physical therapists and nutritionists) at academic centers may have more experience managing disease/treatment-related complications of AML and have greater access to multidisciplinary teams and subspecialists dedicated to managing challenging complications The observation of lower early mortality when treated at academic centers 8 supports the notion of better supportive care playing a pivotal role in survival differences, with one study showing half the risk of early death at National Cancer Institute–designated cancer centers compared with private hospitals with lower rates of renal failure, respiratory failure, and cardiac arrest 14 . Similarly, another study found patients treated at higher-volume hospitals were more likely to undergo bone marrow assessment and receive prophylactic antimicrobials than those treated at lower-volume hospitals. 7 Whether better access to clinical trials at academic centers translates into better outcomes (trial effect) remains controversial 13,15-17 ; restrictive eligibility criteria for trial participation may bias such analyses. 18
Potential reasons for improved outcomes at academic centers
ATRA, all trans retinoic acid; DIC, disseminated intravascular coagulation.
Perhaps unsurprisingly, the center effect in AML extends to survival outcomes with allogeneic stem cell transplantation (HCT), which are closely linked to overall AML outcomes. 19 Whether such benefits extend to other post-HCT composite endpoints such as graft-versus-host disease–free, relapse-free survival is, although plausible, currently unknown. Underlying reasons may include that patients treated at academic centers more likely undergo allografting, possibly because of earlier HLA typing and donor identification, easier care coordination facilitating the transplant workup and reducing the time to transplant, and access to a broader range of transplant protocols. Moreover, expansion of eligibility for allogeneic HCT (eg, to include larger numbers of older adults) combined with the fact that older patients are more likely to receive antileukemia therapy may contribute to improved overall outcomes at academic centers.
Attempts to decipher whether outcomes are better at higher-volume/academic centers are complicated by the increasing heterogeneity within academic settings as private oncology practices are bought by academic health systems, because this academic affiliation does not necessarily come along with the expertise or supportive care capabilities that long-standing academic centers provide. There are also likely unaccounted-for differences in the characteristics of patients treated at different sites. These confounders could be addressed by multivariate analyses accounting for prognostic covariates and site of treatment, but such models have limited prognostic ability, indicating many important prognostic factors remain unknown. Only randomization between treatment at academic or community centers can account for such latent variables.
Certain subsets of leukemia patients have unique needs and challenges and may particularly benefit from the treatment environment offered at high-volume centers. As mentioned above, this is particularly true for patients with APL when treatment is initiated at academic centers. 9 This may be partially reflected in the substantially lower early death rates observed in the context of clinical trials (which are largely conducted at academic centers) compared with those seen in the general APL population, 20 although selection bias may play a role as well. Earlier diagnosis, faster availability and initiation of ATRA, 21 and improved diagnosis and management of complications (eg, disseminated intravascular coagulation, differentiation syndrome) may be contributing factors. Another distinct subset of patients particularly benefiting from higher-volume/academic centers are adolescents and young adults (AYA). As one example, one study evaluating outcomes of AML patients 18 to 39 years of age reported improved survival specifically in those with good-risk cytogenetics and those with APL when treated at academic centers, with the latter having half the risk of early death compared with patients treated at community centers. 10 Similarly, available evidence suggests that AYA with acute lymphoblastic leukemia (ALL) likewise benefit from treatment at academic sites, even after controlling for sociodemographic features. 22 Conceivably, this observation is closely linked to better outcomes of AYA with ALL following pediatric-inspired treatment regimens, which, because of their complexity, are more likely to be given at academic sites. 23 Increased enrollment on clinical trials of AYA with ALL at academic sites may also be contributing. Although data are lacking, it is likely that the same features that contribute to better outcomes in AYA with ALL and AML in general (better access to diagnostic testing and complex supportive care) may also apply to older adults with ALL given the complexity of the treatment regimens, many of which are now administered in the outpatient setting.
Several studies have evaluated whether improved outcomes seen at academic centers extend to older adults, for whom travel to these centers might be more burdensome and overall treatment outcomes worse. Prospective and registry data suggest that such patients do benefit from both intensive and less-intensive chemotherapy compared with no therapy. 24-26 However, intensive induction strategies are very rarely used in the community setting. In fact, most older patients with AML do not receive any type of AML-directed therapy, as indicated by data from a large retrospective Surveillance, Epidemiology, and End Results Medicare study showing only 40% of adults >65 years of age received anti-AML therapy within 3 months of diagnosis. 27 Results were similar in an analysis of US community oncology practice data, 12 with another Surveillance, Epidemiology, and End Results study showing >50% of AML patients >65 years of age received no anti-AML therapy even 9 years after azanucleosides (eg, azacitidine and decitabine) became available. Patients living in large metropolitan areas (with easier access to academic centers) were more likely to receive treatment, as were patients with a previous diagnosis of a solid or hematologic malignancy despite reduced performance status, possibly because of already having established specialist care. 28 Finally, for those who do receive azanucleoside therapy, published dose schedules are often not adhered to in the community (partially related to limited weekend infusion hours), and only a minority of patients surveyed in a recent population-based study in the United States received the recommended ≥4 cycles of therapy, potentially limiting the efficacy of these agents. 29
Historically, intensive therapy for AML has been delivered in the hospital in both academic and community settings because of the need for frequent transfusions and the likely occurrence of treatment/disease-related complications. However more recently, with improvements in supportive care such as introduction of broad-spectrum oral antifungals, ready availability of high-quality blood products, approval of new antileukemia drugs, and increasing focus on QOL 30 and treatment-associated costs, there has been a shift in care patterns with increasing efforts to administer chemotherapy and supportive care in the outpatient setting.
With availability of new drugs such as CPX-351 that have limited immediate toxicities and relatively convenient dosing schedules, there are now intensive treatment options that can be administered in the outpatient clinic even to older patients, both in the community and academic settings. Difficulties in recovering inpatient costs provide an additional incentive, prompting many centers to shift to outpatient administration of CPX-351, 31 with pilot data suggesting patients may remain outpatient after therapy, 32 although some centers currently routinely admit patients to the hospital for monitoring once CPX-351 is administered. Likewise, although treatment with venetoclax in combination with either low-dose cytarabine or an azanucleoside can be given in the outpatient setting, validated guidelines on how best to administer such therapies are currently missing, and many institutions admit patients routinely at the beginning for close monitoring of potential treatment-related complications (tumor lysis syndrome). Nonetheless, even conventional intensive induction and postremission therapy can be safely delivered in the outpatient setting in many patients. 33,34 However, given the complexities surrounding drug administration schedules and management of postchemotherapy care, a multidisciplinary team including social workers, nurses, pharmacists, and providers with expertise in the care of AML (largely available in academic centers only) is critical for successfully implementing this approach.
Despite this interest in administering newer therapeutics in the outpatient setting, most patients are still hospitalized for prolonged periods of time after intensive induction chemotherapy because of disease/treatment-related cytopenias, transfusion needs, and management of related complications. 35 This may, however, not be necessary for many patients. Based on multiple small studies suggesting feasibility of outpatient management after conventional induction chemotherapy, 36 we conducted 2 prospective clinical trials at our institution evaluating an early hospital discharge (EHD) strategy within 3 days after completion of intensive induction chemotherapy. 37,38 These studies supported the notion that early transition to outpatient care is feasible, safe, and associated with reduced care costs, which has since become standard of care at our institution, logistics permitting. We recently evaluated our experience with this approach in the 4-year period since we completed our trials, with the application of the EHD strategy to a much broader patient base than was captured in the prospective trials, with confirmation of safety and reduced medical resource use. 39 Of note, we found no significant differences in care needs for patients undergoing initial induction treatment and those receiving postremission therapy. 40 This suggests that an EHD care strategy after induction therapy may be practically (and safely) implemented at many institutions that already have the infrastructure available enabling them to manage patients in the outpatient setting after standard postremission chemotherapy. At our institution, infrastructure available to support outpatient management of AML patients during the time of prolonged pancytopenia includes 24-hour phone access for patients to a provider familiar with outpatient management of AML, an infusion center with extended daily hours (including weekends and holidays) for transfusion needs, and the ability to rapidly evaluate and initiate treatment of neutropenic fever in the outpatient clinic before hospital transfer. These features (summarized in Table 2 ) are more likely present at academic than community cancer centers.
Infrastructure required to support delivery of supportive care after intensive chemotherapy in the outpatient setting
CVC, central venous catheter; SOP, standard operating policy.
Unlike induction therapy, follow-up care after postremission chemotherapy has already shifted to the outpatient setting at both academic and community centers, 35 with multiple studies demonstrating feasibility, safety, and cost-effectiveness. For some patients, receiving outpatient care at the center where the chemotherapy was administered may pose logistic challenges. Here, a shared care model may address this barrier by allowing patients to receive their supportive care after postremission therapy at community centers closer to their homes. 41 Finally, the COVID-19 pandemic has forced rapid improvements in technology supporting telehealth, along with reimbursement for this service, potentially allowing academic sites to oversee some aspects of patient care with less travel for patients. How the rapid increase in access to telehealth will play out for the care of leukemia patients over the next few years remains unknown.
What about other major drivers for the shift from inpatient to outpatient care: QOL and health care costs? Studies in patients with hematologic malignancies undergoing autologous or allogeneic HCT have shown hospitalization is associated with reductions in QOL and increased depression. 42 The same has been found in AML. 30 Although there are no data comparing QOL of AML patients treated at academic vs community centers, more time spent in the outpatient setting (and closer to home) for both treatment and follow-up care may lead to gains in overall QOL. The same might apply to care costs, which remain dominated by inpatient charges. 43 The shift to more outpatient care and potentially more community-based care that newer medications facilitate may ultimately offset at least part of their high costs.
Many of the new drugs in AML are molecularly targeted agents that are given orally and can be used at various stages along the AML treatment path. Whether there is a measurable benefit when such agents are given at a higher-volume/academic center vs the community setting is unclear. Undoubtedly, the availability of effective oral drugs (eg, venetoclax and oral decitabine/azacitidine in the AML pipeline) used alone or along with parenteral lower-intensity therapeutics (eg, azanucleosides) will increase the proportion of patients treated at community centers, including older and less-fit individuals. Thus far, the risk/benefits of delivering lower-intensity therapies at academic vs community centers are unknown (an important research agenda item for the near future), especially because these therapies can still lead to prolonged cytopenias and toxicities; thus, the issue of which centers provide better supportive care will likely remain relevant. In what way a center’s access to molecular testing influences treatment outcomes is also unknown. For some of the newer agents (eg, inhibitors of mutated IDH1/IDH2/FLT3), anti-AML efficacy is primarily seen in patients carrying the corresponding mutations in their leukemia cells. Appropriate use of these therapeutics therefore requires access to timely molecular testing. One could therefore argue better testing availability at academic institutions may ultimately translate into better outcomes for patients getting care at such centers. However, although some data suggest molecular testing is more widely available at academic centers, 44 there is no clear evidence yet linking this availability to improved outcomes. It also remains uncertain whether targeted therapy in general will yield longer survival than nonspecific clinical trials. 45 Finally, many of these new drugs are costly to patients (particularly the oral drugs that come with high copays) that many, especially older individuals, cannot afford. With their resources, academic centers may be better positioned to help them find financial support from foundations and pharmaceutical companies to obtain these therapies.
Increasing access to new drugs has shifted the care of AML patients from academic to community settings. Thus far, this change in care pattern remains unsupported by data, especially for older and less fit patients who historically have not received antileukemia therapy in the community. Some of the potential advantages that academic centers provide (access to more rapid molecular testing, a broader range of clinical trials and allogeneic HCT, greater disease expertise, and availability of multidisciplinary teams for supportive care) have to be weighed against potential advantages of community settings (eg, less disrupted life, better family support, and QOL). With the rapidly changing treatment landscape in AML, the pros and cons of academic vs community setting treatment will need to be revisited constantly, ideally via randomized trial, as challenging as this would be. In the absence of strong data arguing for/against a particular care scenario, a shared academic-community care approach may currently best serve the interests of many patients.
The authors thank Elihu H. Estey, Charles A. Schiffer, and Wendy Stock for critical reading of the manuscript.
Competing Interests
Conflict-of interest disclosure: The authors declare no competing financial interests.
Author notes
Off-label drug use: None disclosed.
Correspondence Anna B. Halpern, 825 Eastlake Ave E, Box CE3-300, Seattle, WA 98109-1024; e-mail: [email protected] .
- Previous Article
- Next Article
Email alerts
Affiliations, american society of hematology.
- 2021 L Street NW, Suite 900
- Washington, DC 20036
- TEL +1 202-776-0544
- FAX +1 202-776-0545
ASH Publications
- Blood Advances
- Hematology, ASH Education Program
- ASH Clinical News
- The Hematologist
- Publications
- Privacy Policy
- Cookie Policy
- Terms of Use
This Feature Is Available To Subscribers Only
Sign In or Create an Account
- Sign In to save searches and organize your favorite content.
- Not registered? Sign up
Recently viewed (0)
- Save Search
- Subscriptions
- Join E-mail List
Patient Case Studies and Panel Discussion: Leukemia – Rare and Emerging Subtypes
- Get Citation Alerts
- Download PDF to Print
Rare and emerging subtypes of leukemia can be incredibly challenging to diagnose and even more challenging to treat. At the NCCN 2019 Annual Congress: Hematologic Malignancies, a panel of experts, moderated by Andrew D. Zelenetz, MD, PhD, were presented with particularly challenging cases in these malignancies and asked to discuss best approaches to treatment.
- Patient Case Study 1
In the first case study, a 77-year-old woman presented with multiple nodular lesions and plaques on her face, chest, and back. She had a history of type 2 diabetes, stage 3 hypertension, hyperlipidemia, coronary heart disease, cerebral infarction, glaucoma, lens extracapsular extraction and posterior chamber intraocular lens implantation, Sjögren syndrome, rheumatoid arthritis, and left axillary vein and brachial vein thrombosis.
She had previously received a conventional therapy of Chinese medicine, but her condition did not improve. Her clinicians performed a bone marrow biopsy and an aspiration biopsy of a nodule on the right side of her face, and immunostaining results revealed the following immunophenotype: CD4+, CD123+, CD43+, CD56+, with Ki-67 level of 30% to 40%.
The patient was diagnosed with blastic plasmacytoid dendritic cell neoplasm, which is a rare blood cancer in the myeloid malignancies family. Andrew D. Zelenetz, MD, PhD, Memorial Sloan Kettering Cancer Center, noted that this disease used to be classified as a variant of acute lymphoblastic leukemia (ALL) and has a distinctive immunophenotype and clinical appearance, characterized by purple skin lesions.
He said a helpful tool for remembering the immunophenotype of this disease is to think “123456”: CD123, CD4, and CD56. Conversely, Nitin Jain, MD, The University of Texas MD Anderson Cancer Center, noted that although this rule of thumb can be helpful, it is important to keep in mind that approximately 10% of patients with this malignancy are actually CD56-negative.
Daniel A. Pollyea, MD, MS, University of Colorado Cancer Center, emphasized the unique phenotypic expression pattern in this malignancy, and the risk of cytopenias due to bone marrow involvement. “Certainly there are patients with bone marrow involvement who don't have cytopenias and have predominant expression of these skin manifestations,” he said. “But I think the CD123 is really the key, because this is a very, very difficult diagnosis to make, and that can be the linchpin.” He added that CD123 expression status is important to know not only for diagnostic purposes but also from a therapeutic perspective. However, many clinical pathologists do not possess the capabilities to test for CD123, so if a diagnosis of blastic plasmacytoid dendritic cell neoplasm is even being entertained, a discussion with a pathologist regarding testing for CD123 is critical.
The nodule on the right side of the patient’s face was surgically excised, and she was treated with gemcitabine, nedaplatin (a second-generation platinum drug used in China that is not approved by the FDA; it is similar to carboplatin and cisplatin), and bleomycin. The patient experienced an initial response to therapy but subsequently developed additional nodular lesions on her arm.
According to Dr. Pollyea, regardless of what transpired with this particular patient, surgical resection of skin lesions did not have a role in this case. “Typically, if the disease is going to respond, the skin lesions are very, very sensitive,” he said. “So there are issues with wound healing if you perform a large resection.”
The panel then discussed tagraxofusp-erzs, a recently approved drug for the treatment of this disorder that has been shown to be highly effective. 1 Dr. Pollyea noted that the mechanism of action of this drug is “quite brilliant.”
“You're taking one of nature's most potent toxins and delivering it directly to a cell population of critical importance in this disease, and potentially the precursor or primitive population of the disease,” he said.
A trial of tagraxofusp treatment in patients with blastic plasmacytoid dendritic cell neoplasms led to durable responses and high complete response rates, particularly in the first-line setting (72%). 1 In relapsed/refractory disease, it was less effective, but “still very effective,” according to Dr. Zelenetz, with a complete response rate of 38%. However, significant toxicity was seen, with capillary leak syndrome a fatal toxicity.
Jae Park, MD, Memorial Sloan Kettering Cancer Center, noted that because of the limited clinical experience with this agent, it is critical to administer the drug in an inpatient setting whenever possible and to closely monitor any patient-related physical changes, including weight fluctuations, kidney function, and respiratory status.
William G. Wierda, MD, PhD, The University of Texas MD Anderson Cancer Center, agreed, adding that he actually treated patients with this compound on a clinical trial before its approval. “During the trial, we were closely monitoring daily weight, albumin, and [liver function], and making daily adjustments in dosing based on what was happening with patients clinically,” he said. “So it's important to be very familiar with the prescribing information.”
Given this particular patient’s age, history, and comorbidities, stem cell transplantation was not an option. However, according to Dr. Park, allotransplant should be considered in these cases whenever possible, and earlier rather than later. “Even with a good response, it becomes difficult to continue this regimen,” he said. “And after [patients] relapse, there are very few treatment options available.”
- Patient Case Study 2
A 28-year-old woman presented with fatigue and lymphadenopathy. Her initial WBC count was 11.1 k/uL with 40% blasts, and she showed hypercellular bone marrow. Her immunophenotype included the following: 88.0% CD45+/–, CD34+, CD19+, CD10+ (variable), CD20– (∼4% of cells stain), sCD22+, CD13–, CD33–, CD38+, CD56–, CD2+/–, CD3–, CD4–, CD8–, CD7–, CD5–, CD117, HLA-DR+, sIg light chain–, cCD79a+, cCD22+, MPO–, cIgM+, and TdT+. After noting the complexity of the patient’s immunophenotype, Dr. Pollyea emphasized the importance of working with a skilled hematopathologist in cases such as this.
The patient was diagnosed with B-cell ALL and treated with the CALGB 10403 regimen. 2 At day 30, bone marrow biopsy showed residual disease with 16% blasts by flow. As her next course of treatment, the patient received blinatumomab for one cycle.
Dr. Jain agreed that this was a reasonable next step, but added that an additional cycle of chemotherapy would also have been feasible. Although the patient was high-risk, he would not yet say treatment had failed after only one treatment cycle.
“I think on the adult side we have to take our cues from the pediatricians who have been so incredibly successful with this disease,” said Dr. Pollyea. “And CALGB 10403 is a regimen that attempts to apply the pediatric regimens to an adolescent/young adult population.” 2
He added that pediatricians tend to stick to protocol, and the protocol for this particular regimen allows for a more extended induction period. “So at this point you should have a lot of concerns about this patient, but I think the protocol allows you to continue.”
About 4 weeks after starting blinatumomab, the patient experienced complete remission confirmed by bone marrow biopsy. She also received 6 cycles of intrathecal chemotherapy throughout the course of her treatment and showed no evidence of central nervous system involvement.
A month later, she presented with enlarged lymph nodes in her groin and neck, and bone marrow biopsy confirmed 63% blasts with an ALL phenotype. A same-day inguinal lymph node biopsy was consistent with lymphoblastic leukemia involvement.
Although the patient experienced a complete remission initially, Dr. Park noted that minimal residual disease (MRD) status was never confirmed. This factor is critical in assessing a patient’s depth of remission, and MRD-positive patients should receive additional therapy sooner rather than later to get to MRD-negative status, he said.
Dr. Jain said that additional diagnostic testing in the form of RNA sequencing would be appropriate in this case, but noted a caveat of the limited availability of this type of testing. The patient underwent next-generation sequencing (NGS), which revealed the following: DIAPH1-PDGFRB fusion; CDKN2A/B - p14 ARF loss exon 1 and CDKN2b loss; PIK3R1 splice site 1746-2A>6; and TP53 N288fs*60.
According to Dr. Park, interpreting NGS data can be difficult, and misinterpretation can lead to the wrong choice of treatment. This again underlines the importance of consulting with a skilled pathologist or other experienced ALL expert to assist in interpreting mutation profiles.
The patient was determined to have Ph-like ALL (a newly recognized entity of Ph-negative ALL with a poor prognosis) and was enrolled in the KTE-CA19 CAR-T (axicabtagene ciloleucel [axi-cel]) trial ( ClinicalTrials.gov identifier: NCT02614066). She received cytoreductive chemotherapy with hyperCVAD part A before apheresis for CAR-T generation, and experienced favorable cytoreduction (she received fludarabine/cyclophosphamide for lymphodepletion). She then received a post–CAR-T infusion and showed no response; her blast count increased from 0.42 to 80.35 within a week.
“This is just a tough case,” said Dr. Park, noting the unusually refractory nature of the disease. “Initial response rates to CAR-T cell therapy are approximately 80%, so she’s already in the very unlucky 20% of cases,” he said.
Dr. Jain described 2 subtypes of Ph-like ALL: approximately half are CRLF2 -rearranged, 3 and these patients should ideally be referred to a clinical trial. The other half are nonrearranged, 3 and these patients should be referred for RNA sequencing to determine fusion genes.
No response was seen to further treatment, and the patient chose to continue care in hospice.
According to Dr. Zelenetz, incorporation of comprehensive genetic analysis and fluorescence in situ hybridization testing is important to identify high-risk patients (such as those with Ph-like phenotype) and plan for allogeneic hematopoietic stem cell transplantation (alloHSCT) or referral to clinical trials as early as possible.
MRD assessment by flow and/or NGS is critical to assess depth of response, modification of therapy, and candidacy for early alloHSCT. Dr. Park noted that both gene sequencing tests are validated, so patient preference should take priority.
Incorporation of tyrosine kinase inhibitors (TKIs) in Ph-like ALL is being investigated in clinical trials, and patients with this disease should be referred earlier rather than later, added Dr. Zelenetz. “But the nuance to that is understanding how to integrate TKIs into this entity, which is going to be dependent on understanding the mechanisms involved in the disease,” he said. “It won’t be just one TKI [that everyone receives]; it's much more complicated than that, unfortunately.”
Dr. Jain added that although Ph-like ALL has been established as high risk in the setting of chemotherapy, its classification remains to be determined in the new era of targeted therapies. “Some emerging data suggest that blinatumomab, inotuzumab, and CAR-T-cell therapy may overcome the negative prognostication of Ph-like ALL,” he said. “So those are some data we’ll hopefully see at the ASH Annual Meeting.”
Jarrod Holmes, MD, Annadel Medical Group, also participated in the panel discussion.
Pemmaraju N , Lane AA , Sweet KL , et al. . Tagraxofusp in blastic plasmacytoid dendritic-cell neoplasm . N Engl J Med 2019 ; 380 : 1628 – 1637 .
- Search Google Scholar
- Export Citation
Stock W , Luger SW , Advani AS , et al. . A pediatric regimen for older adolescents and young adults with acute lymphoblastic leukemia: results of CALGB 10403 . Blood 2016 ; 133 : 1548 – 1559 .
Jain N , Roberts KG , Jabbour E , et al. . Ph-like acute lymphoblastic leukemia: a high-risk subtype in adults . Blood 2017 ; 129 : 572 – 581 .
Disclosures: Dr. Zelenetz has disclosed that he receives research support from Genentech/Roche, Gilead, MEI, and BeiGene; he has been a consultant for Celegene/JUNO, Genentech/Roche, Gilead, BeiGene, Pharmacyclics, Jansen, Amgen, Astra‐Zeneca, Novartis, and MEI Pharma; and he is on the Scientific Advisory Board of the Lymphoma Research Foundation and Adaptive Biotechnologies. Dr. Jain has disclosed that he is a consultant for AbbVie, Inc., AstraZeneca Pharmaceuticals LP, Genentech, Inc., Janssen Pharmaceutica Products, LP, Adaptive Biotechnologies, Precision Biosciences, Verastem, and Pharmacyclics; receives grant/research support from AbbVie, Inc., AstraZeneca Pharmaceuticals LP, Bristol-Myers Squibb Company, Genentech, Inc., Incyte Corporation, Adaptive Biotechnologies, ADC Therapeutics, Cellectis, Precision Biosciences, Servier, Verastem, Pfizer, Inc., and Pharmacyclics; is a scientific advisor for AbbVie, Inc., AstraZeneca Pharmaceuticals LP, Genentech, Inc., Janssen Pharmaceutica Products, LP, Adaptive Biotechnologies, Precision Biosciences, Verastem, and Pharmacyclics; and has received honoraria from AbbVie, Inc., AstraZeneca Pharmaceuticals LP, Genentech, Inc., Janssen Pharmaceutica Products, LP, Adaptive Biotechnologies, Precision Biosciences, Verastem, and Pharmacyclics. Dr. Park has disclosed that he receives grant/research support from Amgen Inc., Genentech, Inc., Incyte Corporation, Juno Therapeutics, Inc., Kite Pharma, Novartis Pharmaceuticals Corporation, and Servier; and is a scientific advisor for from Amgen Inc., AstraZeneca Pharmaceuticals LP, GlaxoSmithKline, Incyte Corporation, Kite Pharma, Novartis Pharmaceuticals Corporation, Allogene Therapeutics, Autolus Therapeutics plc, and Takeda Pharmaceuticals North America, Inc. Dr. Pollyea has disclosed that he is a scientific advisor for AbbVie, Inc., Agios, Inc., Celgene Corporation, Daiichi-Sankyo Co., Forty Seven, Inc., Janssen Pharmaceutica Products, LP, Pfizer Inc., and Takeda Pharmaceuticals North America, Inc. Dr. Wierda has disclosed that he is a consultant for Genzyme Corporation and receives grant/research support from AbbVie, Inc., Acerta Pharma, Genentech, Inc., Gilead Sciences, Inc., Janssen Pharmaceutica Products, LP, Juno Therapeutics, Inc., Karyopharm Therapeutics, Kite Pharma, Cyclacel Pharmaceuticals, Inc., GlaxoSmithKline/Novartis Pharmaceuticals Corporation, Loxo Oncology, Inc., miRagen Therapeutics, Inc., Oncternal Therapeutics, Inc., Xencor, Inc., Pharmacyclics, and Sunesis Pharmaceuticals, Inc. Dr. Holmes has disclosed that he has no financial interests, arrangements, affiliations, or commercial interests with the manufacturers of any products discussed in this article or their competitors.
Article Sections
Article information.
- Get Permissions
- Similar articles in PubMed
Google Scholar
Related articles.
- Advertising
- Terms of Use
- Privacy Policy
- Permissions

© 2019-2024 National Comprehensive Cancer Network
Powered by:
- [66.249.64.20|185.80.151.9]
- 185.80.151.9
Character limit 500 /500
All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know AML .
The AML Hub uses cookies on this website. They help us give you the best online experience. By continuing to use our website without changing your cookie settings, you agree to our use of cookies in accordance with our updated Cookie Policy
Introducing

Now you can personalise your AML Hub experience!
Bookmark content to read later
Select your specific areas of interest
View content recommended for you
The AML Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the AML Hub cannot guarantee the accuracy of translated content. The AML Hub and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help .
Lymphoma Hub
Multiple Myeloma Hub
“How I Treat”: Case studies in relapsed/refractory acute myeloid leukemia
By dylan barrett, jan 29, 2024, learning objective: after reading this article, learners will be able to discuss the clinical management of patients with relapsed/refractory acute myeloid leukemia..
Bookmark this article

The “How I Treat” series, featured in Blood , highlights expert perspectives on the diagnosis and treatment of patients using sample patient cases, and the AML Hub has previously covered topics in these series on the use of new therapeutics and updated classification systems . Although there have been some major advancements in the treatment of patients with acute myeloid leukemia (AML) in recent years, relapsed/refractory (R/R) AML is still associated with poor outcomes. 1
Here, we summarize key points from a recent “How I Treat” article by Thor et al., 1 discussing the treatment of patients with R/R AML, in three patient cases.
Treatment of R/R AML 1
The 2022 European LeukemiaNet (ELN) recommendations include response criteria for R/R AML, incorporating both hematologic measures and the assessment of measurable residual disease (MRD). Molecular reevaluation should be carried out when diagnosing a patient with R/R AML as this may influence treatment decisions ( Figure 1 ). Due to the poor outcomes associated with R/R AML, patients should be enrolled in clinical trials where possible ( Figure 1 ). Allogeneic hematopoietic stem cell transplantation (allo-HSCT) rescues around one-third of R/R patients, and a donor search in all transplantable patients should be carried out if not already done at the initial diagnosis.
Figure 1. header...
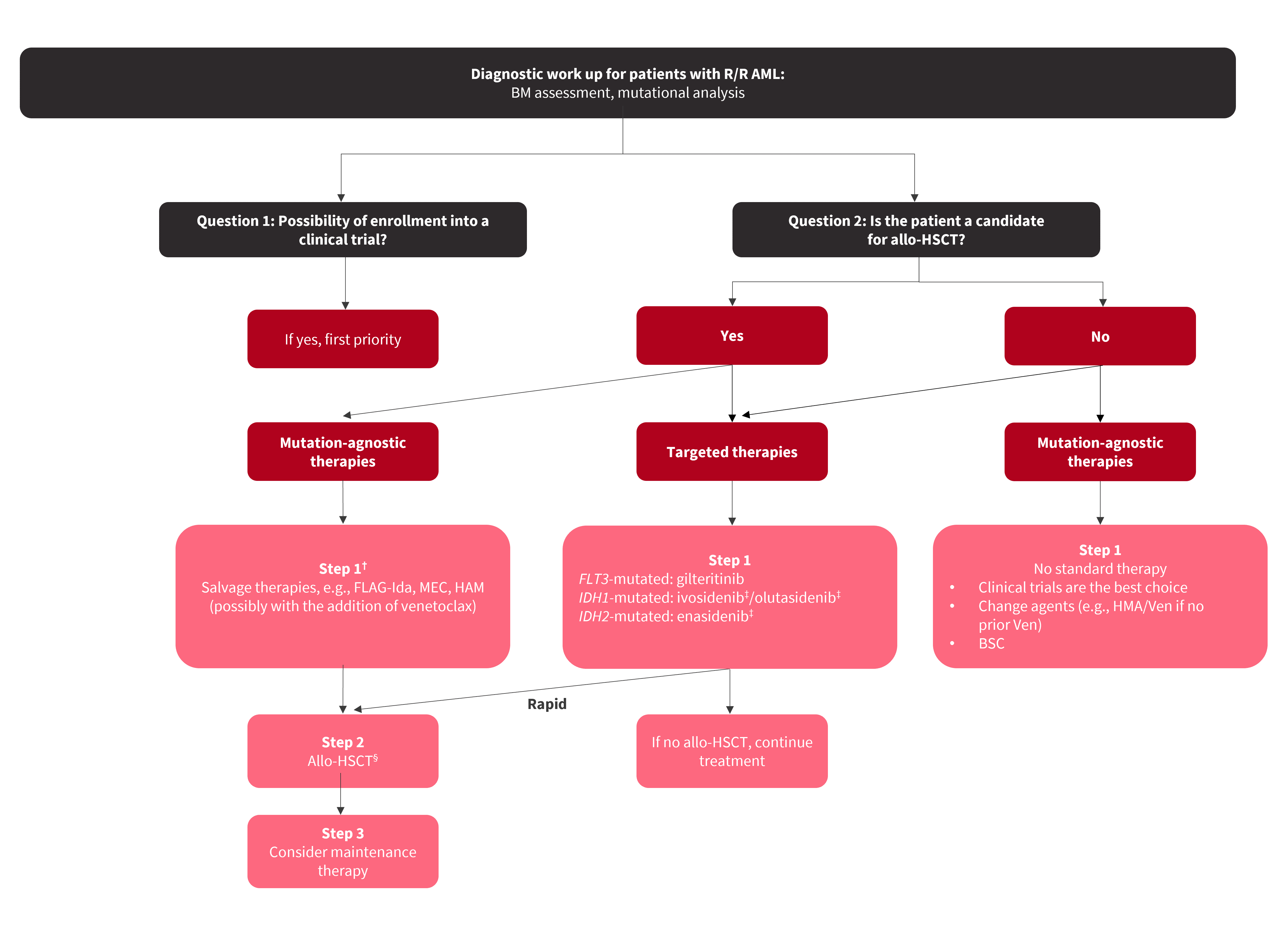
Allo-HSCT, allogeneic hematopoietic stem cell transplantation; AML, acute myeloid leukemia; BM, bone marrow; BSC, best supportive care; FLAG-Ida, fludarabine, cytarabine, granulocyte colony-stimulating factor, and idarubicin; HAM, high-dose cytarabine, mitoxantrone; HMA, hypomethylating agent; MEC, mitoxantrone, etoposide, cytarabine; R/R, relapsed/refractory; Ven, venetoclax. *Adapted from Thor, et al. 1 † Some patients may go directly to allo-HSCT or receive lower-intensity regimens. ‡ Not approved by the European Medicines Agency for patients with R/R AML.
Case 1. 52-year-old female
Figure 2. Case study 1 presentation*
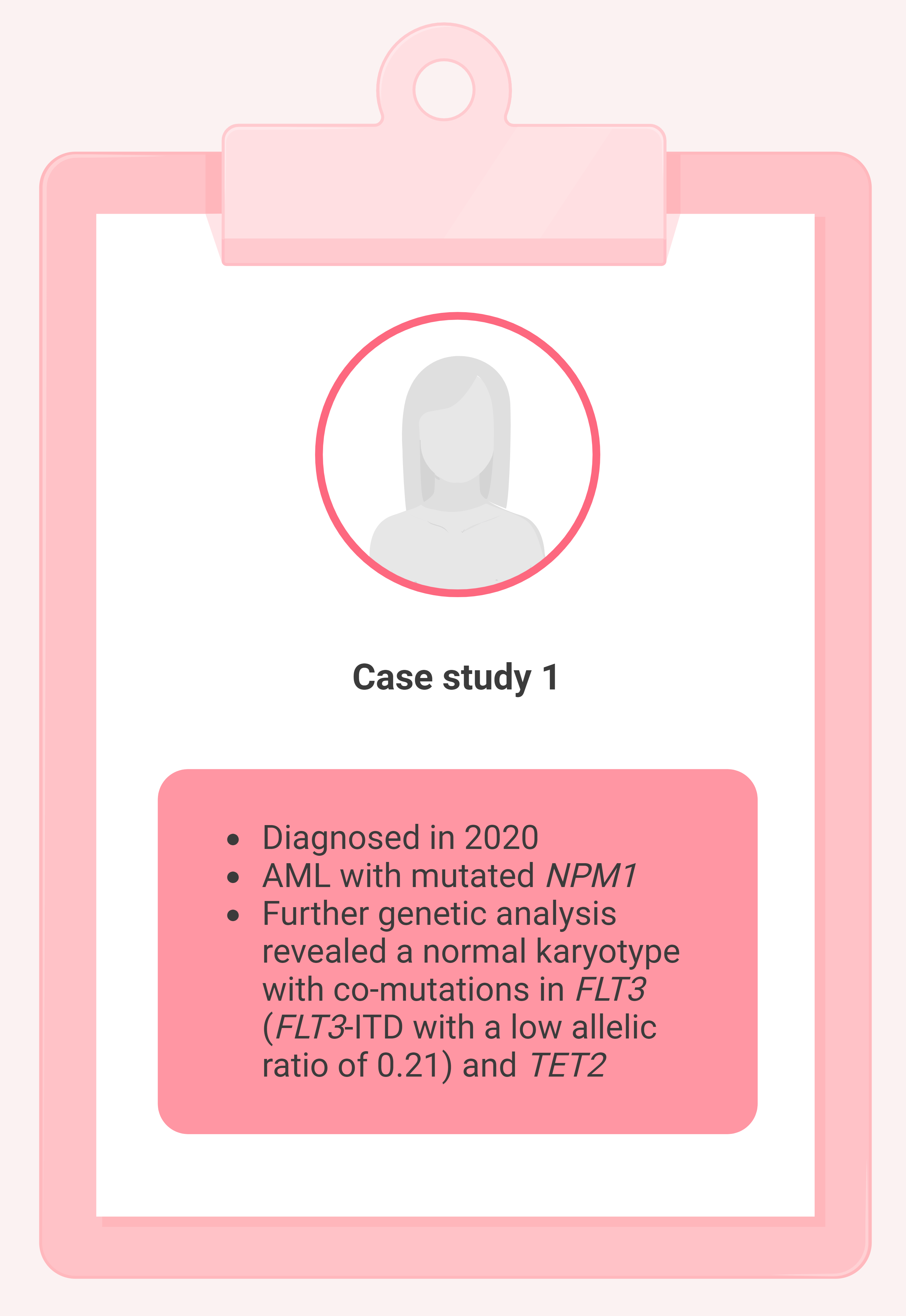
AML, acute myeloid leukemia; FLT3 , FMS‐like tyrosine kinase 3; ITD, internal tandem duplication; NPM1 , nucleophosmin; TET2 , tet methylcytosine dioxygenase 2. *Adapted from Thor, et al. 1 Created with BioRender.com.
- Classified as favorable risk based on the 2017 ELN classification
- Received 7 + 3 plus midostaurin and achieved a complete remission (CR)
- Subsequently received 3 cycles of intermediate-dose cytarabine with midostaurin consolidation therapy
- NPM1 MRD was assessed using real-time quantitative polymerase chain reaction (RT-qPCR); MRD negativity was achieved
- 1-year post-consolidation, became MRD-positive with >400 mutated NPM1 copies/ ABL × 10 4 in the bone marrow and >600 mutated NPM1 copies/ ABL × 10 4 in the peripheral blood
Authors question
What does MRD relapse mean, and how can we clinically react to prevent morphological relapse?
- Based on the ELN recommendations, RT-qPCR is used for MRD assessment in patients with NPM1 -mutated or core-binding factor AML, and multi-parameter flow cytometry is used for other AML subtypes
- NPM1 is a robust MRD marker and can be detected at very low levels using RT-qPCR
- TET2 is a marker of clonal hematopoiesis and, similarly to the other DTA mutations ( DNMT3A and ASXL1 ), should not be used as a marker of MRD
- FLT3 -internal tandem duplication (ITD) is another clinically relevant MRD marker
- While there is no approved therapy or treatment standard for MRD relapse, donors should be identified for all transplant-eligible patients, and the authors recommend allo-HSCT in eligible patients with mutant transcript levels >200 NPM1 mutations/ ABL × 10 4 and with confirmatory analysis, or enrollment in a clinical trial
Further treatment
- Morphological relapse occurred 4 weeks after MRD detection when genetic analysis confirmed an NPM1 , FLT3 -ITD (with an increase in the allelic ratio to 2.7), TET2 , and a novel NRAS mutation
- Received gilteritinib monotherapy at 120 mg/day
- Achieved CR with incomplete count recovery and underwent allo-HSCT with a matched unrelated donor
- Posttransplant maintenance therapy included sorafenib ; currently in CR 180 days posttransplant
How do first-line therapy and mutational profile influence treatment options at relapse?
- Repeated molecular analysis is necessary to identify alterations of leukemic clones. In this case, the FLT3 -ITD allelic ratio increased from 0.21 to 2.7; however, the FLT3 -ITD clone is often lost at relapse following treatment with midostaurin, highlighting the need for molecular testing at relapse
- Although this patient was not initially a candidate for allo-HSCT due to their favorable risk, they received gilteritinib (an FLT3 inhibitor approved by the U.S Food and Drug Administration [FDA] and the European Medicines Agency [EMA] for the treatment of R/R FLT3 -mutated AML) as a bridge to allo-HSCT
- While the primary endpoint of relapse-free survival was not met, a benefit was observed in patients who were pre or posttransplant MRD-positive, suggesting that maintenance with a tyrosine kinase inhibitor could be tailored according to pre and posttransplant MRD status
- Novel agents such as the B-cell lymphoma 2 inhibitor venetoclax are being investigated in combination with salvage chemotherapy to improve responses, and the authors also highlighted the potential of fludarabine, cytarabine, granulocyte colony-stimulating factor, and idarubicin (FLAG-Ida) plus venetoclax
- Other future potential treatment options may include first-line venetoclax plus intensive chemotherapy, menin inhibitors such as revumenib and ziftomenib , and the E-selectin antagonist uproleselan
- The authors recommend that allo-HSCT is the highest priority for all transplantable patients with R/R AML
Case 2. 75-year-old male
Figure 3. Case study 2 presentation*

AML, acute myeloid leukemia; ASXL1, additional sex comb-like 1; IDH2, isocitrate dehydrogenase 2; NF1, neurofibromatosis 1. *Adapted from Thor, et al. 1 Created with BioRender.com.
- Venetoclax and azacitidine combination therapy led to CR with incomplete count recovery following Cycle 1
- Received an additional 12 cycles of venetoclax and azacitidine, followed by a 10-week treatment holiday, requested by the patient
- On treatment reinitiation, 35% blasts were observed on blood smear
- Mutation analysis then revealed IDH2 , ASXL1 , NF1, and TP53 mutations
What is the outlook for R/R AML patients after treatment with azacitidine/venetoclax, and what are their treatment options?
- Based on the VIALE-A trial, hypomethylating agents (HMAs) plus venetoclax is the standard of care for patients who are unfit for intensive chemotherapy, although this combination is not curative in most patients
- While patients with NPM1 mutations who are MRD-negative have favorable outcomes, most other patients become R/R with a poor prognosis
- If patients become transplant-eligible during treatment with HMAs plus venetoclax, allo-HSCT should be considered before relapse occurs
- For those who are R/R following HMAs plus venetoclax and ineligible for allo-HSCT, switching to other chemotherapy agents is not beneficial, and clinical trials should be considered alongside a discussion about the limitations of approved agents; best supportive care and palliative care should also be considered
- Targeted therapies, such as ivosidenib ( IDH1 ), olutasidenib ( IDH1 ), enasidenib ( IDH2 ), gemtuzumab ozogamicin (CD33 + ), and gilteritinib ( FLT 3) are an option for patients with the relevant targetable mutation, although further studies are warranted to clarify their use in those who are R/R to HMAs plus venetoclax and there is also a need for novel treatments to improve the currently poor outcomes
Case 3. 65-year-old male
Figure 4. Case study 3 presentation*
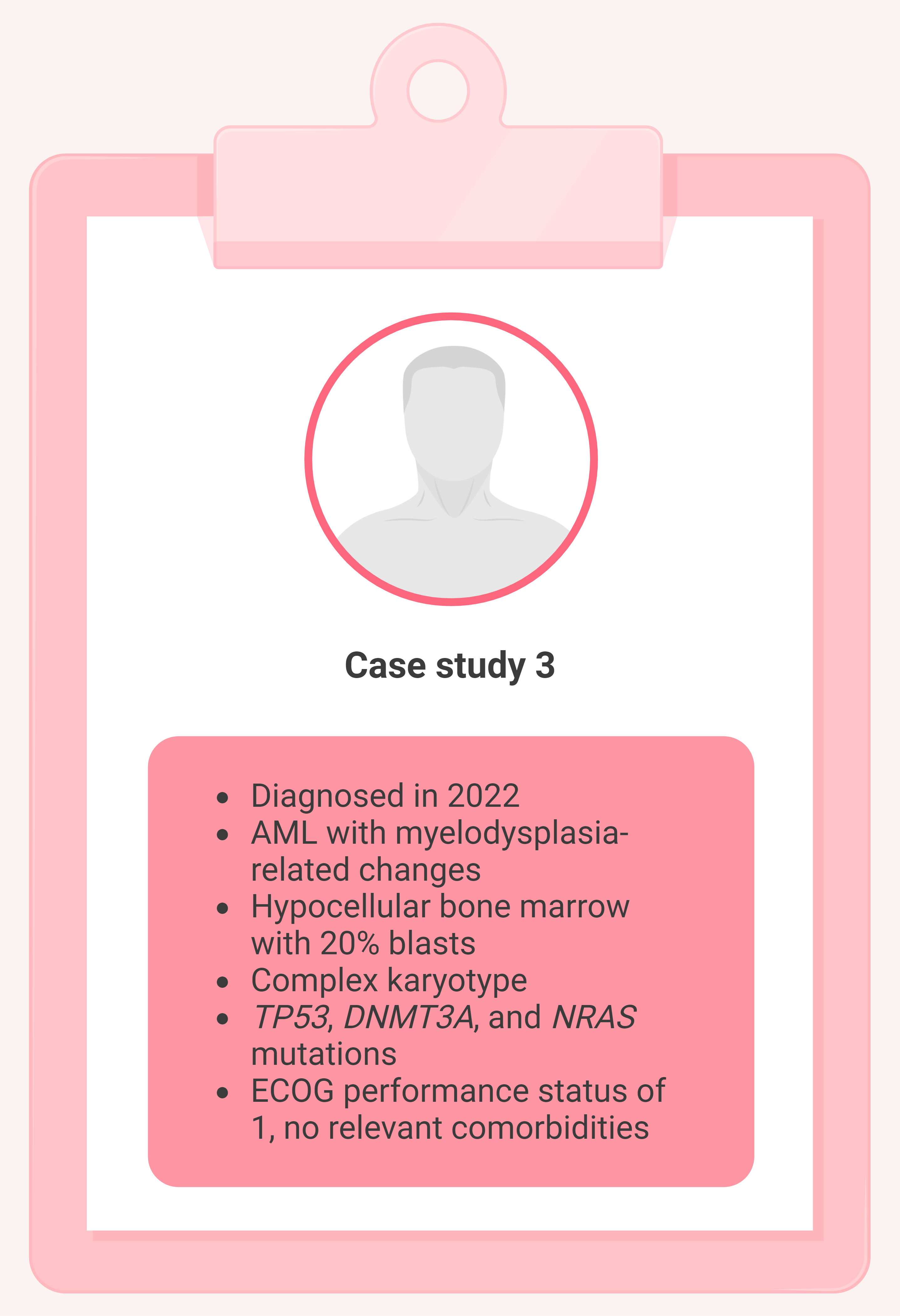
AML, acute myeloid leukemia; DNMT3A , DNA methyltransferase 3 alpha; ECOG, Eastern Cooperative Oncology Group; NRAS , neuroblastoma RAS viral oncogene homolog; TP53 , tumor protein p53.
*Adapted from Thor, et al. 1 Created with BioRender.com.
Treatment
- Received one cycle of CPX-351, and achieved CR
- Underwent allo-HSCT from a matched unrelated donor, but relapsed 5 months later
How could we prevent relapse after allo-HSCT in this patient, and what can we do if relapse occurs?
- While allo-HSCT is the most effective consolidation therapy, relapse still occurs in 45–55% of patients with adverse risk AML, and the rate is even higher for patients with TP53 mutations
- The 2-year overall survival rate in patients who relapse after allo-HSCT is 14–25%, with particularly poor outcomes in those who relapse in the first 6 months
- Donor-lymphocyte infusions can be combined with other therapies, but the potential development of graft-versus-host disease must be assessed continually
- For a small number of patients, a second allo-HSCT may be possible
The landscape of treatment for patients with R/R AML is evolving. Triplet combinations of azacitidine and venetoclax plus targeted therapy or novel agents are currently being investigated, with promising initial results. Novel immunotherapies, including chimeric antigen receptor T cells, bispecific T-cell engaging antibodies, or dual-affinity retargeting antibodies are currently under evaluation. However, substantial effort is required to improve outcomes for this patient population.
References ( 1 )
- Thol FR, Döhner H, Ganser A. How I treat refractory and relapsed acute myeloid leukemia. Blood . 2023. Online ahead of print. DOI: 1182/blood.2023022481
More about...
Your opinion matters
0 vote s - 6 days left ...
Related articles.
AML World Awareness Day 2020 | Clinical trials involving novel agents in AML
On April 21, 2020, communities will unite on Acute Myeloid Leukemia (AML) World Awareness Day (WAD) to raise awareness of AML. Both scientific and patient communities will...
Apr 20, 2020
On April 21, 2020, communities will unite on Acute Myeloid Leukemia (AML) World Awareness Day (WAD) to raise awareness of...
Prof. Agnieszka Wierzbowska | ASH 2017 | Take home messages for Poland from ASH 2017
59th ASH Annual Meeting and Exposition, 9 - 12 Dec 2017, Atlanta, GA Professor Agnieszka Wierzbowska Copernicus Memorial Hospital, Lodz, PL
Dec 21, 2017
59th ASH Annual Meeting and Exposition, 9 - 12 Dec 2017, Atlanta, GA Professor Agnieszka Wierzbowska Copernicus...
Subscribe to get the best content related to AML delivered to your inbox

Outcomes After Stem Cell Transplant in Elderly Patients With Acute Myeloid Leukemia Have Improved Since 2000
© 2024 Smart + Strong . All Rights Reserved. Terms of use and Your privacy . Smart + Strong ® is a registered trademark of CDM Publishing, LLC.
Historically, many patients over age 65 have been considered too infirm to receive intensive chemotherapy or stem cell transplants.
March 25, 2024 • By American Association for Cancer Research
Among patients over 65 who received an allogeneic hematopoietic stem cell transplant (allo-HCT) for the treatment of acute myeloid leukemia (AML) between 2000 and 2021, leukemia-free and overall survival improved significantly over time, according to results from a study published in Clinical Cancer Research , a journal of the American Association for Cancer Research (AACR).
AML is typically treated with targeted therapies or intensive chemotherapy, but if those treatments fail, allo-HCT—in which blood stem cells are taken from a matched donor and transplanted into a patient—is often required. Historically, however, many patients over age 65 have been considered too infirm to receive intensive chemotherapy or allo-HCT.
Medical advances over the past 20 years—such as improved supportive care, newer generation anti-infectious agents, and high-resolution human leukocyte antigen (HLA) typing—have made allo-HCT a safer and more popular option for older patients with AML, which has a median age at diagnosis of 68, explained Ali Bazarbachi, MD, PhD , senior author of the study and a professor at the American University of Beirut in Lebanon.
“Over time, significant progress in allo-HCT has decreased mortality and allowed for the delivery of allo-HCT to older patients,” Bazarbachi said. “However, little information is available about the global impact of these changes and the predictive factors for post-transplant outcomes, and available data on outcomes from retrospective and prospective studies are mixed.”
Bazarbachi and colleagues sought to examine how the outcomes of elderly patients after allo-HCT have changed over time, using data from a large transplant registry.
“We hoped these large-scale, real-world data could serve as a benchmark for future studies in this setting,” Bazarbachi said. “Our study represents one of the largest analyses to date assessing trends over time and predictive factors for outcomes in elderly AML patients after allo-HCT.”
Bazarbachi and colleagues analyzed a data set from the European Society for Blood and Marrow Transplantation, a working group of more than 600 transplant centers that report transplantation and follow-up data to a central registry. The data set consisted of 7,215 patients who received their first allo-HCT for AML at age 65 or older, between 2000 and 2021. At the time of their transplants, 64% of the patients were in their first complete AML remission, 14% were in their second complete AML remission, and 22% had active disease.
The researchers assessed outcomes in the three years immediately following allo-HCT. They compared outcomes between patients treated from 2000 to 2009 (728 patients), 2010 to 2014 (1,775 patients), and 2015 to 2021 (4,712 patients). Relapse incidence decreased significantly from 37% to 31% to 30% across the three time periods; similarly, non-relapse mortality (death from anything other than AML) was 31% from 2000 to 2014 and decreased to 27% from 2015 to 2021.
Both leukemia-free survival and overall survival steadily increased over time. Leukemia-free survival climbed from 32% to 38% to 44% across the three time periods, while overall survival climbed from 37% to 42% to 49%.
The researchers also studied the incidence of graft-versus-host-disease (GvHD), a potentially serious condition in which donor cells recognize the patient’s body as foreign and attack healthy tissues. The incidence of chronic GvHD decreased from 35% between 2000 and 2014 to 31% between 2015 and 2021, and GvHD- and relapse-free survival rose from 22% to 29% to 34%.
Improvements in all outcomes except non-relapse mortality were observed regardless of whether patients were in their first complete response, second complete response, or had active disease at the time of transplant. For patients with active disease, however, these differences were only significant in the most recent time period (2015 to 2021). Decreases in non-relapse mortality were only observed for patients experiencing their second complete response.
Bazarbachi emphasized that the steady improvement in outcomes after allo-HCT indicates the importance of offering the option to more patients over 65.
“In tandem with the marked increase in elderly patients receiving allo-HCT, we observed an impressive improvement over time in leukemia-free and overall survival,” he said. “These data indicate that allo-HCT should no longer be optional but should be mandatory for elderly patients.”
Limitations of this study include a lack of information about minimal residual disease for most patients, especially those treated before 2015. Additionally, no information was available about the maintenance therapies patients received after allo-HCT.
Funding for this study was provided by the European Society for Blood and Marrow Transplantation. Bazarbachi declares no conflicts of interest.
This news release was published by the American Association for Cancer Research on March 22, 2024.
Read More About:
- #acute myeloid leukemia
- #cancer treatment
- #GVHD (graft-versus-host disease)
- #stem cell transplant
RELATED articles

Colorado Chef Experiences Significant Tumor Shrinkage with Immunotherapy Clinical Trial [VIDEO]

Sensory Nerves Appear to Drive Head and Neck Cancer Growth

FDA Approves First CAR-T Therapy for Chronic Lymphocytic Leukemia

Can a New Drug Candidate Cure Pancreatic Cancer?
Stay logged in.
You have been inactive for 60 minutes and will be logged out in . Any updates not saved will be lost.
You Have Been Logged Out
Click here to log back in.
Cancer Health uses cookies to provide necessary website functionality, improve your experience, analyze our traffic and personalize ads. Our Privacy Policy
Cancer Health uses cookies to provide necessary website functionality, improve your experience, analyze our traffic and personalize ads. By remaining on our website, you indicate your consent to our Privacy Policy and our Cookie Usage .
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 26 March 2024
ACUTE MYELOID LEUKEMIA
Proteomics for optimizing therapy in acute myeloid leukemia: venetoclax plus hypomethylating agents versus conventional chemotherapy
- Eduardo Sabino de Camargo Magalhães ORCID: orcid.org/0000-0002-7726-4827 1 ,
- Stefan Edward Hubner 2 ,
- Brandon Douglas Brown 3 ,
- Yihua Qiu 4 &
- Steven Mitchell Kornblau ORCID: orcid.org/0000-0002-5990-9548 4
Leukemia ( 2024 ) Cite this article
Metrics details
- Acute myeloid leukaemia
- Chemotherapy
- Combination drug therapy
- Translational research
The use of Hypomethylating agents combined with Venetoclax (VH) for the treatment of Acute Myeloid Leukemia (AML) has greatly improved outcomes in recent years. However not all patients benefit from the VH regimen and a way to rationally select between VH and Conventional Chemotherapy (CC) for individual AML patients is needed. Here, we developed a proteomic-based triaging strategy using Reverse-phase Protein Arrays (RPPA) to optimize therapy selection. We evaluated the expression of 411 proteins in 810 newly diagnosed adult AML patients, identifying 109 prognostic proteins, that divided into five patient expression profiles, which are useful for optimizing therapy selection. Furthermore, using machine learning algorithms, we determined a set of 14 proteins, among those 109, that were able to accurately recommend therapy, making it feasible for clinical application. Next, we identified a group of patients who did not benefit from either VH or CC and proposed target-based approaches to improve outcomes. Finally, we calculated that the clinical use of our proteomic strategy would have led to a change in therapy for 30% of patients, resulting in a 43% improvement in OS, resulting in around 2600 more cures from AML per year in the United States.
Similar content being viewed by others

Targetable lesions and proteomes predict therapy sensitivity through disease evolution in pediatric acute lymphoblastic leukemia
Amanda C. Lorentzian, Jenna Rever, … Philipp F. Lange
Proteogenomics refines the molecular classification of chronic lymphocytic leukemia
Sophie A. Herbst, Mattias Vesterlund, … Sascha Dietrich

RPPA-based proteomics recognizes distinct epigenetic signatures in chronic lymphocytic leukemia with clinical consequences
Anneke D. van Dijk, Ti’ara L. Griffen, … Steven M. Kornblau
Introduction
Acute Myeloid Leukemia (AML) is characterized by the uncontrolled clonal expansion of hematopoietic precursors. Although the majority of patients achieve remission, most ultimately relapse. Despite recent innovation in therapy [ 1 ], AML remains a fatal diagnosis for the majority, especially the elderly population [ 2 , 3 ]. The identification of recurrent chromosomal abnormalities and common somatic mutations has improved the understanding of leukemogenesis, leading to revision in both diagnostic and prognostic categorization of AML [ 4 , 5 , 6 , 7 ]. However, most of these mutations lack therapies that can directly target them [ 8 ].
Since the 1970s, anthracycline combined with cytosine arabinoside (AraC), hereafter referred to as conventional chemotherapy (CC), has been the standard of care in AML induction therapy [ 9 ]. Despite being the backbone of AML treatment, it has been challenged with more target-based therapies [ 10 , 11 ]. Increasing evidence has demonstrated that some patients with newly diagnosed AML benefit from the combination of venetoclax (VEN) and hypomethylating agents (HMA), such as Azacytidine or Decitabine, hereafter referred to as VH [ 12 , 13 ]. Moreover, achieving long-term remission is still challenging in AML [ 14 ], and the VH combination has proven advantageous for use in patients with relapse [ 15 ]. However, it has been reported that specific groups of patients may not benefit from VH [ 16 ]. Moreover, despite the improved molecular classification of AML and the resulting improvement in prognostication for outcome, these schemas do not predict which of the available regimens individual patients will respond best to, especially older patients [ 17 , 18 ]. Most patients are selected for CC or VH treatments based on clinical characteristics such as age, performance status, or occasionally cytogenetics and/or individual mutations, rather than on characteristics of the underlying pathophysiology of the leukemic blasts that cause differential responses to different therapeutic options [ 19 ]. Therefore, incorrect therapy triaging reduces the effectiveness and cure fraction achieved.
The ability to recognize which patients are more likely to respond to one regimen versus another is crucial for maximizing outcomes with existing therapies. Previous studies from our group using reverse-phase protein array (RPPA)-based proteomics have demonstrated that leukemia (AML, ALL, CML, and CLL) is characterized by a limited number of recurrent proteomic signatures, which are prognostic for outcome [ 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 ]. RPPA is a high-throughput microarray that can quantitatively measure the levels of hundreds of proteins in more than 1000 samples in a single array, using very little biological material [ 29 , 30 ]. We investigated whether this technique could be leveraged to identify proteomic signatures associated with a superior response to CC vs. VH therapies in AML.
In the present study, we identified specific protein profiles associated with an improved response to CC or VH therapy using machine learning algorithms to develop a Protein Classifier based on the expression of a limited set of proteins that could be utilized clinically to recommend either VH, CC, or neither. Revised triaging based on these calculated predictions was estimated to increase the 5-year cure rate by 43%. Furthermore, we identified potentially targetable signaling hubs for a group of patients who did not benefit from either VH or CC.
Materials and methods
Study design, ethics statement, and patient population.
The use of AML samples in the present study was approved by the MD Anderson Cancer Center (MDACC) Investigational Review Board (IRB), according to previously approved protocols (LAB01-473, Lab05-0654). Informed consent was obtained for sample use in compliance with the Declaration of Helsinki. PB and BM samples were collected from 810 adult patients (>17 years old) with newly diagnosed AML admitted to the MDACC between April 2012 and June 2020. Patients were included in the analysis if they received VH combination therapy ( N = 85) or Conventional Chemotherapy (CC) ( N = 369), predominantly anthracycline and cytosine arabinoside. Patients who were not treated at the MDACC ( N = 115), or did not receive VH nor CC ( N = 241) were excluded.
Sample collection and processing
Immediately after harvesting, the samples were cooled to 4° C and processed within two hours. Fresh samples were layered on a Ficoll gradient, washed with PBS, and then counted. When T and B cells represented more than 5% of the post-Ficoll cells, CD3 and CD19 positive cells were removed by Magnetic Activated Cell Sorting (MACS) using the Miltenyi AutoMACS Magnetic Cell Sorter. Sample concentrations were normalized to 1 × 10 4 cells/mL, and whole-cell lysates were prepared as previously described [ 31 ].
Reverse-phase protein arrays (RPPA)
RPPA was performed in the MDACC RPPA Core Facility as described previously [ 20 , 21 , 23 , 31 , 32 ]. Briefly, whole-cell lysates were subjected to five serial 2× dilutions (1:1, 1:2, 1:4, 1:8, and 1:16) and printed onto nitrocellulose-coated glass slides. To determine protein expression levels, slides were probed with 411 validated primary antibodies (322 total and 89 post-translational modified (PTM)), together with secondary antibodies conjugated to an infrared molecule. The primary antibodies used were validated, as previously described [ 33 ]. Stained slides were quantitated with Microvigene (Version 3.4, Vigene Tech), and expression was normalized to normal bone marrow (NBM)-derived CD34+ cells. More specifically, the mean expression of NBM was normalized to zero and the values of each AML sample are expressed in Log2-fold-change (LFC) values compared to NBM. The antibodies used are listed in Supplementary Table S1 .
Computational analysis
Data analysis was performed using R v4.3.2 (“Eye Holes”) and Python3. To identify the proteins that significantly affected patient prognosis, the expression level of a single protein was split into quantiles: median split, tertiles, quartiles, quintiles, and sextiles, resulting in the formation of five groups. Overall survival (OS) was compared between quantiles in each case. This was repeated for each of the 411 proteins, resulting in the generation of a p -value table (Supplementary Table S2 ). Prognostic proteins were defined using two significance cutoffs: p < 0.05 and p < 0.01. Next, patients underwent unbiased hierarchical clustering according to their protein expression using the progeny clustering algorithm [ 34 ]. The protein set that showed clusters with clearly distinct protein expression profiles and most significant cluster separation in Kaplan–Meier (KM) plots for OS and complete remission duration (CRD), was chosen for further analysis and named protein selector set (PS). Three protein selector sets (PS1, PS2, and PS3) were developed to cover different population subsets. In order to create a stricter contrast between VH and CC for outcome analyses, patients who received HMA + VEN and AraC were removed from the VH group after the generation of PS1, leaving a total of 79. Similarly, the CC population was filtered for AraC-treated patients only, reducing the number of patients in this group to 340. The list of selected proteins for the PS1, PS2, and PS3, along with their respective p -values generated from the initial assessment can be found in Supplementary Table S3 . Protein networks were made with Cytoscape v3.10.1 (ref. [ 35 ]), the StringApp [ 36 ], and the R package Rcy3(ref. [ 37 ]). Pathway enrichment analysis was performed using the Enrichr webtool. To assess the significancy of each biological process, a combination of adjusted p -values and odds-ratio, entitled ‘combined score’ was used. Ontologies were filtered using an adjusted p -value cutoff <0.01, and the combination of lowest adjusted p -value and highest odds-ratio (i.e., highest combined score) were considered the most significant. Further details of the methodology can be found elsewhere [ 38 , 39 , 40 ].
For Machine learning analysis, datasets were separated into developmental (dev) and test sets using an 80/20 split. Dev sets were further separated into training and validation sets using a 75/25 split. Model weights were initialized using replicable random states. Random forest machine learning algorithms were used in Python3 from the sklearn.ensemble package (scikit-learn) with specific importation of the RandomForestClassifier function. Hyperparameter tuning involved the application of two individually assembled Python functions: holdout_grid_search and random_forest_grid_search. Grid search was performed to optimize hyper-parameters, including the number of trees in the random forest and their maximum depth. 150 hyperparameter search-spaces were evaluated based on the unique n_estimators, max_depth, and min_samples_leaf hyperparameter combinations. Shapley Additive Explanations (SHAP) values were calculated to explain the model predictions by quantifying the additive importance of each feature. SHAP functions were imported from the shap library. For each of the 3 protein classifier models, all available proteins served as inputs into the aforementioned random forest algorithm, and the output was a SHAP-based hierarchy of the most predictive proteins. Few proteins (defined as 6 or less proteins) were tested from the top 6 proteins in each model to train the final version of each random forest model. The combination of proteins that generated the highest C-index for each model were isolated and reported. C-index calculation was used to evaluate model accuracy, using the formula: ((#concordant pairs + 0.5*#ties)/(#permissible pairs)).
Statistical analysis
LogRank tests with p -values adjusted by the Benjamini–Hochberg (BH) method were used to compare outcomes. Pearson’s correlation coefficient was used to measure the linear the correlation between proteins. Fisher’s exact test, Wilcoxon or Kruskal–Wallis tests were used to compare measured variables. Univariate (UV) and multivariate (MV) models were build using Cox proportional-hazards (CoxPH). Wilcoxon tests adjusted by the False Discovery Rate (FDR), with the cutoff p < 0.05, and mean Log2-fold change values, with a threshold of 0.5, were used for differential expression analysis. Statistical significance was defined as a p -value < 0.05, and significance symbols were determined as **** p < 0.0001, *** p < 0.001, ** p < 0.01, * p < 0.05, and ns not significant.
Protein selector sets (PS) identify patient groups with distinct clinical outcomes
We developed an algorithm to identify the most therapeutically discriminating proteins and generated Protein Selector Sets (see “Materials and Methods” section). The first one, entitled PS1, was comprised of 55 proteins, which identified three clusters (C1, C2, and C3) with unique expression signatures. Protein levels across the clusters are shown in Fig. 1A . Although the protein signature of each cluster was the same in both patients with VH and CC, their overall survival (OS) varied greatly between treatments. As shown in Fig. 1B , patients in C1 (red) treated with VH (solid line) had diametrically different and superior responses compared to those treated with CC (dashed line), with a Median OS (MS) of 68.5 months (mo.) in the VH group versus (vs.) MS of 19.4 mo. in the CC population. The opposite was true for C3 (yellow), where CC patients had a MS of 16.8 mo. and the VH population displayed a very poor MS of 8.7 mo. However, PS1 did not identify an optimal therapy for patients in cluster C2 (light blue). Therefore, to identify the preferred therapy for PS1-C2 patients ( N = 182), we generated PS2, using the same strategy described previously. As shown in Fig. 1C , PS2 separated the population into two clusters with distinct expression profiles. In Fig. 1E , cluster PS2-C1 (blue color) treated with CC (dashed line) had a markedly better OS (>120 mo.), compared to C1-VH (solid blue), which has a MS of 12.7 mo. The same was true for cluster PS2-C2 (purple color), where CC (dashed line) had a MS 12.2 mo., and VH (solid line) had a MS of 6.4 mo. Moreover, as shown in Fig. 1B , the best PS1-C3 curve (dashed yellow, CC-treated) has an OS comparable to the worst PS1-C1 group (dashed red, CC-treated). Therefore, we generated a PS3 for PS1-C3 patients ( N = 146) in an attempt to identify a group with better OS. Within PS3, two clusters with contrasting protein expression levels were defined, and separated by treatment (Fig. 1D ). As shown in Fig. 1F , patients in cluster PS3-C1 (green color) had a very good prognosis when treated with CC (dashed line), with MS > 120 mo., and a very poor outcome when treated with VH (solid line), having a MS of 10.4 mo. In contrast, OS of patients in PS3-C2 (orange color) were similarly poor for both therapies.
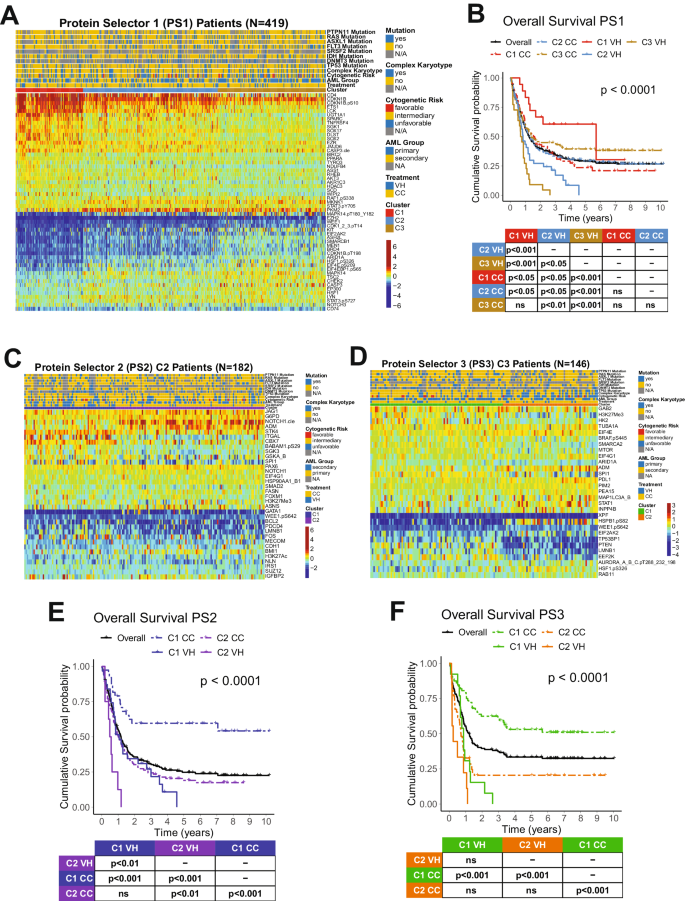
A Heatmap depicting the protein expression of PS1 patients ( N = 419). B Kaplan–Meier plots of Overall Survival PS1 patients ( N = 419) separated by cluster and treatment modality (VH = solid line, CC = dashed line; PS1-C1 = red, PS1-C2 = light blue, PS1-C3 = yellow). C Heatmap depicting the protein expression of PS2 patients ( N = 182) and D PS3 patients ( N = 146). E Kaplan–Meier plots of Overall Survival from PS2 patients ( N = 182) and F PS3 patients ( N = 146) separated by cluster and treatment modality (VH = solid line, CC = dashed line; PS2-C1 = blue, PS2-C2 = purple, PS3-C1 = orange, PS3-C2 = green). Annotations above the heatmaps, starting closest to the heatmap, show the clusters, VH vs. CC treatment modality (second from bottom), and then other annotations for several previously recognized prognostic features including AML group, cytogenetic risk, and presence of complex karyotype and mutations. Colors for the annotations have the value shown in the legends along the right side. Protein expression ranging from above normal (red) to normal (yellow-green-aqua) to below normal (dark blue) as shown in the color legend.
The combination of the PS sets led to the generation of five clusters separated by the expression levels of 109 proteins as shown in Fig. 2A . C1 derived from PS1, C2 and C3 from PS2 (former PS2-C1 and PS2-C2), and C4 and C5 from PS3 (former PS3-C1 and PS3-C2). In Fig. 2B , the OS was better for C1 patients (red) treated with VH (solid) compared to CC (dashed) (MS = 68.5 mo. vs. 19.4 mo.). In contrast, both C2-CC (dashed blue) and C4-CC (dashed green) displayed MS > 120 mo., outperforming both C2-VH (solid blue), with a MS of 12.7 mo., and C4-VH (solid green), which has a MS of 10.4 mo. Moreover, although C3-CC (purple dashed) do better than C3-VH (purple solid) (MS of 12.2 mo. vs. 6.4 mo.), their OS are worse than the C2-CC and C4-CC populations. Finally, our PS system could not determine which treatment patients in cluster C5 (orange) should receive. Considering their poor outcomes in both VH (MS = 2.9 mo.) and CC (MS = 8.6 mo), it seems that this population might benefit from another treatment regimen (e.g., target-based therapies). Analysis of CRD for all PS sets showed a similar outcome pattern (Supplementary Fig. S1 ). Comparison of VH vs. CC for each cluster separately is shown in Supplementary Fig. S2 .
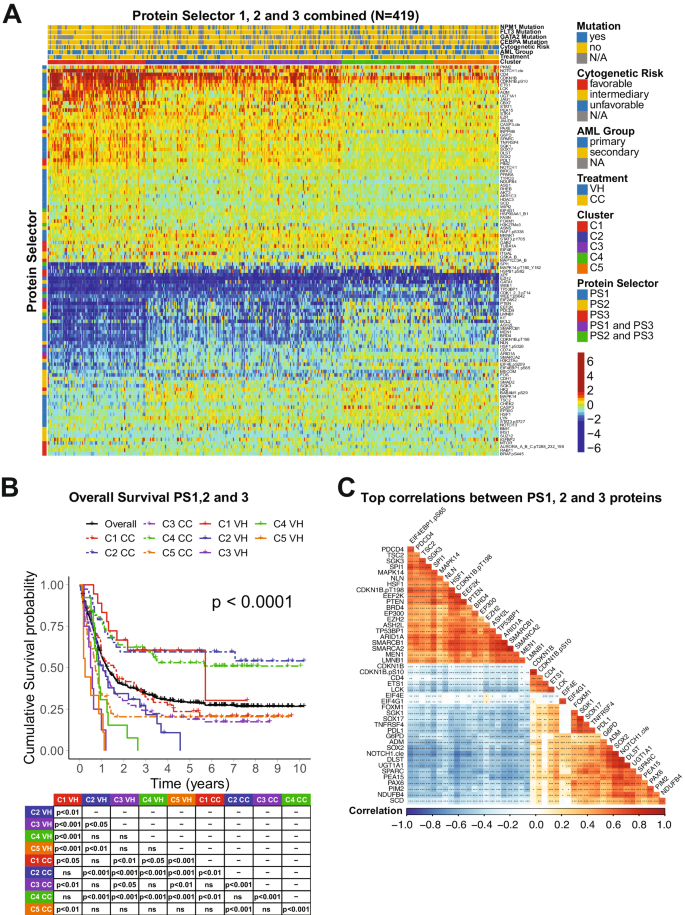
A Heatmap depicting the protein expression of all patients ( N = 419). Annotations above the heatmap, starting closest to the heatmap, show the cluster membership and treatment modality, and then other previously recognized prognostic features (AML group, cytogenetic risk, and presence of complex karyotype and mutations). Legends are as described in Fig. 1. B Kaplan–Meier plots of Overall Survival and C Top correlations between all the PS proteins ( N = 45). Squares represent the correlation between each protein are colored according to the degree of the linear correlation, which varies between (−1, 1) and follows a ‘blue’ (−1), ‘white’ (0), and ‘red’ (1) gradient, as shown in the color legend. Significant correlations are highlighted according to the following: *** p < 0.001, ** p < 0.01, * p < 0.05, and blank = not significant.
To better assess the biological meaning of the PS analyses, we evaluated the correlation of the expression levels of the 109 prognostic proteins between each other. In Fig. 2C , the top most correlated proteins, defined as having a correlation coefficient > 0.60, are shown. Among the biological processes related to those proteins, the most common were ribosomal and transcriptional activity (10 proteins), histone modifiers (8 proteins), cell cycle and DNA damage response (7 proteins), cell metabolism (6 proteins). For an expanded view of these protein relationships, the complete correlation plot, together with protein networks of the PS proteins divided by functional group are shown in Supplementary Fig. S3 . The correlation coefficients for all proteins, along with p -values of each comparison are shown in Supplementary Table S4 . The stratification of all 109 proteins by biological process with their respective Protein Selector Set is shown in Supplementary Table S5 .
Clusters associations with demographic, clinical, and molecular features
We examined how the clusters differed considering demographic (age, gender, race), clinical (AML group and laboratory parameters), and molecular features (cytogenetics and mutation profiles), as shown in Table 1 . There were significant differences in age distribution, as well as the frequency of many clinical variables (primary vs. secondary AML, white blood cell count, percentage of blasts and platelets number), cytogenetics (by risk group, simple vs. complex karyotype, or for specific events, such as −5/5q-, −7/7q- and inv16), and for several individual mutations (ASXL1, CEBPA, DNMT3A, EZH2, FLT3 [individually for ITD and D835, and in combination], NPM1, and TP53). An expanded table with all variables assessed is shown in Supplementary Table S6 .
Since many of these features with unbalanced distributions among the clusters are known to be prognostic, we wondered whether the cluster prognostic impact was just a reflection of these imbalances or if the clusters were independently predictive. Here, we generated KM plots to verify whether cluster membership is prognostic for OS and CRD when the population is filtered for specific variables (e.g., males only, secondary AML only, etc.). KM plots with p -values are shown in Supplementary Figs. S4 and S5 . The prognostic impact of the five clusters was sustained for almost all the variables, including gender, all three age groups, all races, both primary and secondary AML, and major cytogenetic groupings (whether divided into three prognostic groups or for complex karyotypes). Since most individual cytogenetic and mutation events occur at a low frequency when the five clusters are subdivided by treatment modality (ten groups in total), the small sample sizes often preclude reaching statistical thresholds. However, similar trends (C1, C2, and C4, better than C3 and C5) were maintained for the majority, with exceptions noted for FLT3, IDH1, IDH2, JAK2, MLL, PTPN11, and TP53 mutations.
Next, we measured the prognostic value of the clusters and other variables using univariate (UV) and multivariate (MV) Cox proportional-hazards models (CoxPH) for both OS and CRD. In both analyses, clusters were condensed into three groups to avoid a large number of levels in a single variable, which might negatively influence the CoxPH models. Therefore, clusters with good prognosis (C1-VH, C2-CC, and C4-CC) were joined and renamed Group1; the ones with intermediate OS and CRD (C1-CC, C2-VH, C3-CC) were compacted into Group2; and finally, the remaining clusters, with poor prognosis, (C3-VH, C4-VH, C5-VH and C5-CC) were merged into Group3. As demonstrated in Table 2 , all cluster groups were predictive of survival and remission in both the UV and MV models, reinforcing their prognostic value. Moreover, a few demographic (age, white race, and Asian race), clinical (secondary AML, blasts, Hbg, and serum B2M), cytogenetic (complex karyotype, −5/5q-, −7/7q-, t(8;21), Inv16, and Del12), and mutational (ASLX1, CEBPA, FLT3 [individually for ITD and D835, and in combination], IDH2, JAK2, MLL, NPM1, PTPN11, and TP53 mutations) features were also prognostic in the UV model for OS. However, only clusters, secondary AML, complex karyotype, Inv16, and IDH2 and PTPN11 mutations remained significant in the MV analysis. Regarding CRD, in the UV analysis clusters remained highly significant along with other characteristics (age, black race, AML group, complex karyotype, −5/5q-, Inv16, and FLT3, RUNX1, and TP53 mutations), with only clusters, black race, and complex karyotype, which remained significant in the MV model. Taken together, these findings corroborate the independent prognostic value of the PS protein signatures. An expanded table containing all variables evaluates in the UV model for both OS and CRD is shown in Supplementary Table S7 .
Development of a protein classifier (PC) for treatment recommendation
Although the PS system can efficiently separate patients who should receive VH from those who would do better with CC, it is not feasible to measure more than 100 different proteins in the clinical setting. The number of proteins required to be assessed is excessive and poses a major cost-benefit challenge for the application of the method. Instead, the identification of a few proteins that can be measured using a Clinical Laboratory Improvement Amendments (CLIA)-certified test to accurately assign an individual patient to a specific protein expression profile is practical. Therefore, we designed a classification algorithm using the random forest machine learning technique entitled Protein Classifier (PC). The system can identify the most predictive proteins for treatment recommendation, based on previously developed cluster memberships and protein expression data. In other words, we recommended VH treatment for patients belonging to cluster C1 ( N = 91); CC therapy for patients in clusters C2, C3, and C4 ( N = 267); and neither VH nor CC for the C5 patient population ( N = 61). The system was developed with the goal of defining clusters using three different models sequentially:(1) Define C1 patients ( N = 91); (2) Distinguish C2 and C4 groups ( N = 154) from the C3 and C5 populations ( N = 174); and (3) Separate C3 ( N = 113) from C5 ( N = 61) patients. In Fig. 3A , the top predictive proteins are visualized together with their respective SHAP values. The first step of the PC system identified the six most predictive proteins for C1: SPI1, ASH2L, EIF4EBP1.pS65, EZH2, NFE2L2 and SOX2 (C-index: 0.951). Thus, according to our previous OS and CRD analyses, patients with this protein signature should receive VH therapy. In the second step of the PC system, TGM2, NOTCH1.cle, DUSP4, and RAD51 were the best proteins to differentiate C2 + C4 from C3 + C5 (C-index: 0.903). Of note, distinguishing C3 from C2 and C4 is necessary, because although both patient groups should receive CC, the OS and CRD for C3 is much lower, so this patient group may benefit from additional therapy (e.g., CC and stem cell transplant in first remission), whereas C2 and C4 seem to do well with CC alone. Finally, SMAD2.pS245_250_255, MAPK14.pT180_Y182, EIF4E.pS209, and NDUFB4 were identified as the best proteins to segregate C3 and C5, defining the last step of our system (C-index:0.923). The expression of all proteins in the PC system by cluster is shown in Fig. 3B . Importantly, the C-index, a measure of individual patient discriminatory power, of all models in our PC system is above 0.90, demonstrating that it robustly predicts optimal therapy choice (a C-index higher than 0.7 is considered predictive, while a measure of 1 would indicate perfection). Moreover, by considering all three models working together, we predicted that 87.3% of patients would receive the correct therapy, and only a small fraction of 5.5% would be misassigned. The proportion of patients in the C5 group who could be assigned to either CC or VH, instead of being defined as ‘undetermined’, was 7.1%. Overall sensitivity, specificity, and accuracy were 84.2%, 79.6%, and 82.8%, respectively. The predictive calculations for the PC model are presented in Supplementary Table S8 . Therefore, the development of a kit that determines the expression of the aforementioned 14 proteins would be useful and financially feasible for triaging patients and guiding the recommendation for VH or CC.
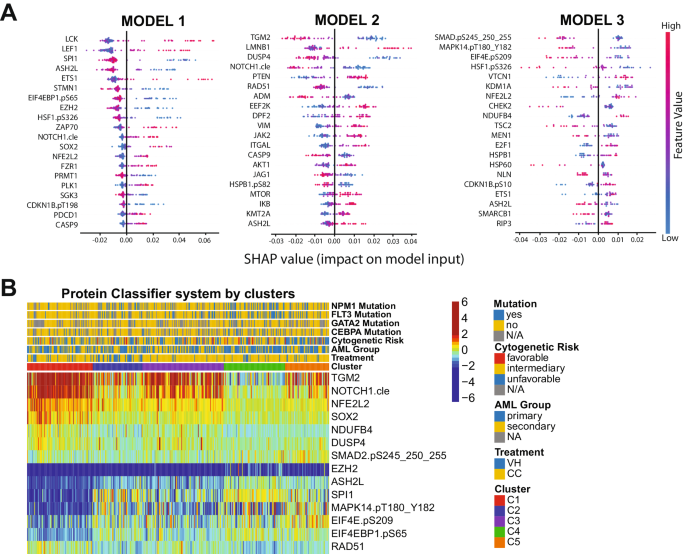
A Top predictive proteins for each of the three models developed (y-axis), for all test-set patients, according to each protein’s calculated SHAP value (x-axis). Color legend indicates the value of a given prognostic protein’s expression relative to other expression values of that protein among test-set patients. (pink = high predictive value; blue = low predictive value). B Heatmap showing the expression levels of the proteins selected for the protein classifier by cluster and treatment modality. Annotations and legends are as described in Figs. 1 – 3 .
Patients with the worst outcomes have a unique and targetable protein signature
Since our PS system was unable to recommend either VH or CC for cluster C5 patients, we decided to determine the most associated signaling pathways within this population. We identified 24 proteins among the 411 in our database which in combination form a unique expression profile in C5 patients, compared to all the other clusters. In Fig. 4A , the Log2-fold-change (LFC) values of each each cluster against all the others is shown for each differentially expressed (DE) protein of cluster C5. Proteins from ZAP70 until VIM have lower LFC values and, thus, were considered down-regulated in C5, whereas the proteins from HSPB1.pS82 to RB1.pS807_811 were classified as up-regulated since their LFC values are higher in C5 compared to the others. A table with FDR-adjusted p -values and LFC values comparing each cluster against all the others is shown in Supplementary Table S9 . To better visualize connections of the C5 DE proteins with each other, we generated a protein network, annotating the mean expression values of each one compared to normal bone marrow (node fill color), and whether the protein is up- or down-regulated (node border). Importantly, although a few proteins are up-regulated compared to the other clusters, their mean expression is below the levels of normal bone marrow (e.g., CHEK1, BIRC5, CCNB1). A table with all the DE proteins and their directionality (up- or down-regulated), stratified by cluster is in Supplementary Table S10 . Volcano plots highlighting the directionality of DE proteins for every cluster are shown in Supplementary Fig. S6 .
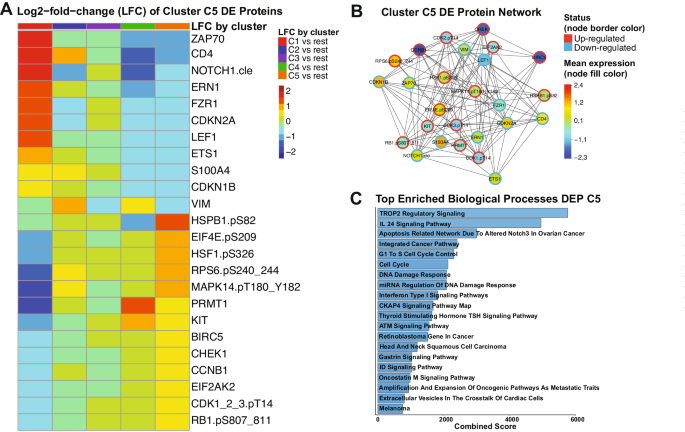
A Heatmap demonstrating the mean Log2-fold change (LFC) values of every assessed comparison, regarding the 24 proteins that are differentially expressed in cluster C5 patients. Heatmap annotation refer to the comparison as established by the legend on the right. The mean LFC ranges according to the values and color legend on the right B Protein network of DE proteins of Cluster C5. Network nodes are colored according to the mean expression value, ranging from above normal (red) to normal (yellow-green-aqua) to below normal (dark blue) as shown in the color legend (right). Node borders are colored according to the Differential Expression status of protein (up-regulated or down-regulated), following the colors shown in the legend (right). C Top twenty enriched biological processes related to the differentially expressed proteins of cluster C5. Y-axis shows the name of each ontology and x-axis shows the combined scores of each process. Bargraphs are colored according to a blue gradient, where darker blue corresponds to lower values and lighter blue to higher values.
To gain insights about the biological meaning of our data, we performed pathway enrichment analysis of the 24 DE proteins. As shown in Fig. 4C , processes with the highest combined scores (i.e., lowest p -value and highest odds-ratio) were most significantly correlated to these proteins. Most of those were related to cell cycle regulation and the DNA damage response (DDR), but specific pathways were also enriched (e.g., TROP2, IL-24, and CKAP4 signaling). The complete table with all the processes and their combined scores, along with adjusted p -values and odds ratios can be found in Supplementary Table S11 . Altogether, even though we were unable to recommend a specific treatment for C5 patients, our DE analysis revealed potential druggable signaling pathways that could be useful for developing target-based therapies.
Proteomic profiling studies, developed previously by our group using the RPPA methodology, identified proteomic signatures that create a novel proteomic-based categorization system that was prognostic in leukemia [ 20 , 21 , 22 , 23 , 24 ]. In this study, we applied a similar proteomic-based strategy to a large cohort of AML patients and identified unique and recurrent protein signatures that could be useful for recommending either HMA + VEN or Conventional Chemotherapy treatments. We identified five protein signatures: one (22% of cases) that should optimally receive VH, three (63%) in which CC is superior, and the last one (15% of cases) for which neither VH nor CC was preferable (especially after removing favorable cytogenetics patients that are known to do well with CC). However, for this group, the PS system and differential expression (DE) analysis identified major signaling hubs connected to the protein profile of those patients, providing insights for possible target-based therapies in the remaining 15% (61/419) of patients. A therapy triaging system, optimized by the evaluation of protein expression, would have reassigned 30% of cases (125/419), with great impact on the five-year survival and remission rates. Considering the adequate treatment for cluster C1 as VH and the best treatment for clusters C2, C3, and C4 as CC, if the patients were triaged by the PS system, the overall five-year survival rate would be predicted to increase from 30% (126 patients) to 43% (181 patients), a 43% increase in survival. The proportion in remission at the five-year timepoint jumps from 52 to 63%, an increase of 21%. Considering the US annual incidence of 20,000 newly diagnosed AML cases, our proteomic triaging system using proteomics-optimized therapy selection could result in 2600 more cures using existing therapies (full calculations are in Supplementary Table S12 ). Of note, centralized proteomic assessment as part of a clinical trial or for routine testing is feasible, since protein levels, including phosphorylation, have been shown by us to remain stable for up to 72 h if the samples are refrigerated, even if they are shipped across long distances [ 41 ].
Furthermore, most demographic, clinical, and molecular characteristics were not exclusively associated with a single protein signature, although some showed biased distribution among the five clusters. However, cluster membership by treatment was an independent prognostic factor for OS, and to a lesser extent, for CRD, in both univariate and univariate models. Therefore, proteomic analysis provides new prognostic information regarding responses that are not available for known prognostic factors. Since most of the assessed molecular and cytogenetic features were equally common in all protein signatures, it seems that several distinct associations of independent molecular events may lead to a similar proteomic signature, and a similar corresponding pathophysiology, which is being captured by our PS system.
Interestingly, the PS system was also able to identify recurrent biological processes relevant to patient prognosis. Since the three selector sets (PS1, PS2 and PS3) were sequentially derived from patient subsets of a larger population, it is not surprising that most proteins ( N = 100) were unique to a single selector set, while only three (ARID1A, EIF2AK2 and HSF1.pS326) were common to PS1 and PS2, and just six showed overlap between PS2 and PS3 (H3K27Me3, WEE1.pS642, EIF4G1, SP1, ADM and LMNB1). However, while the proteins in each selector set tended to be unique, the cellular functions involved were recurrent in all of them. Among the 15 functionally related groups of proteins defined by us, 10 showed substantial convergence between the PS sets: histone modifiers, cell cycle and DDR, ribosomal and transcriptional activity, cell metabolism, proliferative pathways, cell adhesion and cytoskeleton regulation, apoptosis, signaling regulation, heatshock proteins, and cell differentiation (see Supplementary Table S4 ). Importantly, considering the distinct expression pattern of all five protein signatures, it seems that each cluster has its own biases regarding those functional groups. This suggests that these biological processes are not only are related to prognosis but also might represent a therapeutic opportunity worth exploring to improve patient response.
Finally, our PS system identified a particular patient population for whom neither VH nor CC was recommended as the main therapy. By exploring the protein expression profiles of those patients, we identified a small number of differentially expressed proteins that were up- or down-regulated in comparison to the other clusters. We also correlated those proteins with ontologies related to cell cycle and DDR and other more specific pathways. Furthermore, two proteins caught our attention: RPS6.pS240_244, which is up-regulated in C5 and has higher expression levels compared to normal bone marrow (NBM), and FZR1, which is down-regulated has low expression compared to NBM. RPS6 composes part of the 40 S unit of the ribosome and is a downstream target of several proliferative pathways, such as PI3K/AKT/mTORC1 and MAPK/ERK axis, both of which converge to activate S6K, responsible for the phosphorylation of RPS6 at S240/S244(refs. [ 42 , 43 ]). Phospho-RSP6 increases translation of specific mRNAs, ultimately inducing cell growth, and its overexpression has been observed in many cancer types, including AML [ 43 , 44 , 45 ]. In contrast, loss of FZR1, a cell cycle and DDR regulator, increases the sensitivity to genotoxic agents in B-cell acute leukemia and also contributes to the selection therapy-resistant subclones [ 46 ]. Interestingly, phosphorylation of FZR1 by ERK facilitates melanomagenesis, and loss of FZR1 cooperates with AKT to transform primary melanocytes [ 47 ]. Therefore, high RPS6.pS240_244 and low FZR1 might actually be directly correlated to PI3K/AKT/mTORC1 and/or MAPK/ERK activation in C5 patients, and inhibition of those pathways with FDA-approved drugs (e.g., sirolimus, capivasertib, sorafenib) could potentially improve outcomes.
In summary, we developed a proteomic-based triaging system to recommend either VH or CC for patients with AML. We predict that by applying our proteomic approach both overall survival and complete remission duration of AML patients will experience a significant increase, resulting in 2 600 more cures per year in the USA using existing therapies. Moreover, we identified potential therapeutic targets to improve the therapy of patients who would not be predicted to benefit from either VH or CC treatment regimens.
Data availability
Patient datasets and code scripts are freely available at https://github.com/escmagalhaes/23-LEU-1445 and will be transferred to http://www.leukemiaatlas.org upon publication.
Kantarjian H, Kadia T, DiNardo C, Daver N, Borthakur G, Jabbour E, et al. Acute myeloid leukemia: current progress and future directions. Blood Cancer J. 2021;11:41.
Article PubMed PubMed Central Google Scholar
Yilmaz M, Wang F, Loghavi S, Bueso-Ramos C, Gumbs C, Little L, et al. Late relapse in acute myeloid leukemia (AML): clonal evolution or therapy-related leukemia? Blood Cancer J. 2019;9:7.
Almeida AM, Ramos F. Acute myeloid leukemia in the older adults. Leuk Res Rep. 2016;6:1–7.
PubMed PubMed Central Google Scholar
Padmakumar D, Chandraprabha VR, Gopinath P, Vimala Devi ART, Anitha GRJ, Sreelatha MM, et al. A concise review on the molecular genetics of acute myeloid leukemia. Leuk Res. 2021;111:106727.
Article CAS PubMed Google Scholar
Welch JS, Ley TJ, Link DC, Miller CA, Larson DE, Koboldt DC, et al. The origin and evolution of mutations in acute myeloid leukemia. Cell. 2012;150:264–78.
Article CAS PubMed PubMed Central Google Scholar
Pourrajab F, Zare-Khormizi MR, Hashemi AS, Hekmatimoghaddam S. Genetic characterization and risk stratification of acute myeloid leukemia. Cancer Manag Res. 2020;12:2231–53.
Di Nardo CD, Cortes JE. Mutations in AML: prognostic and therapeutic implications. Hematol Am Soc Hematol Educ Program. 2016;2016:348–55.
Article Google Scholar
Yu J, Jiang PYZ, Sun H, Zhang X, Jiang Z, Li Y, et al. Advances in targeted therapy for acute myeloid leukemia. Biomark Res. 2020;8:17.
Yates JW, Wallace HJ, Ellison RR, Holland JF. Cytosine arabinoside (NSC-63878) and daunorubicin (NSC-83142) therapy in acute nonlymphocytic leukemia. Cancer Chemother Rep. 1973;57:485–8.
CAS PubMed Google Scholar
Tamamyan G, Kadia T, Ravandi F, Borthakur G, Cortes J, Jabbour E, et al. Frontline treatment of acute myeloid leukemia in adults. Crit Rev Oncol Hematol. 2017;110:20–34.
Article PubMed Google Scholar
Tang K, Schuh AC, Yee KW. 3+7 Combined chemotherapy for acute myeloid leukemia: is it time to say goodbye? Curr Oncol Rep. 2021;23:120.
Mustafa Ali MK, Corley EM, Alharthy H, Kline KAF, Law JY, Lee ST, et al. Outcomes of newly diagnosed acute myeloid leukemia patients treated with hypomethylating agents with or without venetoclax: a propensity score-adjusted cohort study. Front Oncol. 2022;12:858202.
Pollyea DA, Bixby D, Perl A, Bhatt VR, Altman JK, Appelbaum FR, et al. NCCN guidelines insights: acute myeloid leukemia, version 2.2021. J Natl Compr Cancer Netw. 2021;19:16–27.
de Lima M, Roboz GJ, Platzbecker U, Craddock C, Ossenkoppele G. AML and the art of remission maintenance. Blood Rev. 2021;49:100829.
Tenold ME, Moskoff BN, Benjamin DJ, Hoeg RT, Rosenberg AS, Abedi M, et al. Outcomes of adults with relapsed/refractory acute myeloid leukemia treated with venetoclax plus hypomethylating agents at a comprehensive cancer center. Front Oncol. 2021;11:649209.
Jonathan BK, Blanding D, Rangel CA, Pasyar S, Hill EG, Davis J, et al. Outcomes in AML patients receiving HMA + venetoclax combination with prior HMA exposure. JCO. 2021;39:e19011–e19011.
Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–47.
Pogosova-Agadjanyan EL, Moseley A, Othus M, Appelbaum FR, Chauncey TR, Chen IML, et al. AML risk stratification models utilizing ELN-2017 guidelines and additional prognostic factors: a SWOG report. Biomark Res. 2020;8:29.
Aldoss I, Pullarkat V, Stein AS. Venetoclax-containing regimens in acute myeloid leukemia. Ther Adv Hematol. 2021;12:2040620720986646.
van Dijk AD, Hoff FW, Qiu YH, Chandra J, Jabbour E, de Bont ESJM, et al. Loss of H3K27 methylation identifies poor outcomes in adult-onset acute leukemia. Clin Epigenetics. 2021;13:21.
Hoff FW, Hu CW, Qiu Y, Ligeralde A, Yoo SY, Mahmud H, et al. Recognition of recurrent protein expression patterns in pediatric acute myeloid leukemia suggests new therapeutic targets. Mol Cancer Res. 2018;16:1275–86.
van Dijk AD, Griffen TL, Qiu YH, Hoff FW, Toro E, Ruiz K, et al. RPPA-based proteomics recognizes distinct epigenetic signatures in chronic lymphocytic leukemia with clinical consequences. Leukemia. 2021;36:712–22.
Griffen TL, Hoff FW, Qiu Y, Lillard JW, Ferrajoli A, Thompson P, et al. Proteomic profiling based classification of CLL provides prognostication for modern therapy and identifies novel therapeutic targets. Blood Cancer J. 2022;12:43.
van Dijk AD, Hu CW, de Bont ESJM, Qiu YH, Hoff FW, Yoo SY, et al. Histone modification patterns using RPPA-based profiling predict outcome in acute myeloid leukemia patients. Proteomics. 2018;18:e1700379.
Quintás-Cardama A, Qiu YH, Post SM, Zhang Y, Creighton CJ, Cortes J, et al. Reverse phase protein array profiling reveals distinct proteomic signatures associated with chronic myeloid leukemia progression and with chronic phase in the CD34-positive compartment. Cancer. 2012;118:5283–92.
Hoff FW, Hu CW, Qiu Y, Ligeralde A, Yoo SY, Scheurer ME, et al. Recurrent patterns of protein expression signatures in pediatric acute lymphoblastic leukemia: recognition and therapeutic guidance. Mol Cancer Res. 2018;16:1263–74.
Hoff FW, Van Dijk AD, Qiu Y, Hu CW, Ries RE, Ligeralde A, et al. Clinical relevance of proteomic profiling in de novo pediatric acute myeloid leukemia: a Children’s Oncology Group study. Haematologica. 2022;107:2329–43.
Hu CW, Qiu Y, Ligeralde A, Raybon AY, Yoo SY, Coombes KR, et al. A quantitative analysis of heterogeneities and hallmarks in acute myelogenous leukemia. Nat Biomed Eng. 2019;3:889–901.
Coarfa C, Grimm SL, Rajapakshe K, Perera D, Lu HY, Wang X, et al. Reverse-phase protein array: technology, application, data processing, and integration. J Biomol Tech. 2021;32:15–29.
Lu Y, Ling S, Hegde AM, Byers LA, Coombes K, Mills GB, et al. Using reverse-phase protein arrays (RPPAs) as pharmacodynamic assays for functional proteomics, biomarker discovery, and drug development in cancer. Semin Oncol. 2016;43:476–83.
Kornblau SM, Womble M, Yi HQ, Jackson CE, Chen W, Konopleva M, et al. Simultaneous activation of multiple signal transduction pathways confers poor prognosis in acute myelogenous leukemia. Blood. 2006;108:2358–65.
Kornblau SM, Coombes KR. Use of reverse phase protein microarrays to study protein expression in leukemia: technical and methodological lessons learned. Methods Mol Biol. 2011;785:141–55.
Tibes R, Qiu YH, Lu Y, Hennessy B, Andreeff M, Mills GB, et al. Reverse phase protein array: validation of a novel proteomic technology and utility for analysis of primary leukemia specimens and hematopoietic stem cells. Mol Cancer Ther. 2006;5:2512–21.
Hu CW, Kornblau SM, Slater JH, Qutub AA. Progeny clustering: a method to identify biological phenotypes. Sci Rep. 2015;5:12894.
Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–504.
Doncheva NT, Morris JH, Gorodkin J, Jensen LJ. Cytoscape StringApp: network analysis and visualization of proteomics data. J Proteome Res. 2019;18:623–32.
Gustavsen JA, Pai S, Isserlin R, Demchak B, Pico AR. RCy3: network biology using Cytoscape from within R. F1000Res. 2019;8:1774.
Xie Z, Bailey A, Kuleshov MV, Clarke DJB, Evangelista JE, Jenkins SL, et al. Gene set knowledge discovery with enrichr. Curr Protoc. 2021;1:e90.
Kuleshov MV, Jones MR, Rouillard AD, Fernandez NF, Duan Q, Wang Z, et al. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016;44:W90–7.
Chen EY, Tan CM, Kou Y, Duan Q, Wang Z, Meirelles GV, et al. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinform. 2013;14:128.
Horton TM, Hoff FW, van Dijk A, Jenkins GN, Morrison D, Bhatla T, et al. The effects of sample handling on proteomics assessed by reverse phase protein arrays (RPPA): functional proteomic profiling in leukemia. J Proteom. 2021;233:104046.
Article CAS Google Scholar
Meyuhas O. Ribosomal protein S6 phosphorylation: four decades of research. Int Rev Cell Mol Biol. 2015;320:41–73.
Yi YW, You KS, Park JS, Lee SG, Seong YS. Ribosomal protein S6: a potential therapeutic target against cancer? Int J Mol Sci. 2022;23:48.
Grundy M, Jones T, Elmi L, Hall M, Graham A, Russell N, et al. Early changes in rpS6 phosphorylation and BH3 profiling predict response to chemotherapy in AML cells. PLoS ONE. 2018;13:e0196805.
Pallis M, Harvey T, Russell N. Phenotypically dormant and immature leukaemia cells display increased ribosomal protein S6 phosphorylation. PLoS ONE. 2016;11:e0151480.
Ishizawa J, Sugihara E, Kuninaka S, Mogushi K, Kojima K, Benton CB, et al. FZR1 loss increases sensitivity to DNA damage and consequently promotes murine and human B-cell acute leukemia. Blood. 2017;129:1958–68.
Wan L, Chen M, Cao J, Dai X, Yin Q, Zhang J, et al. The APC/C E3 ligase complex activator FZR1 restricts BRAF oncogenic function. Cancer Discov. 2017;7:424–41.
Download references

Author information
Authors and affiliations.
Department of Ageing Biology/ERIBA, University of Groningen, University Medical Center Groningen, Groningen, 9713 AV, the Netherlands
Eduardo Sabino de Camargo Magalhães
John Sealy School of Medicine, The University of Texas Medical Branch at Galveston, Galveston, TX, 77555, USA
Stefan Edward Hubner
Division of Pediatrics, The University of Texas MD Anderson Cancer Center, Houston, TX, 77030-4009, USA
Brandon Douglas Brown
Department of Leukemia, The University of Texas MD Anderson Cancer Center, Houston, TX, 77030-4009, USA
Yihua Qiu & Steven Mitchell Kornblau
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization was done by ESCM and SMK. Methodology was performed by ESCM, SEH, BDB, YQ and SMK. Investigation and visualization were done by ESCM and SEH. Writing was done by ESCM (original draft, review and editing) and SMK (original draft, review and editing). SMK was responsible for funding acquisition, project administration, and supervision.
Corresponding author
Correspondence to Steven Mitchell Kornblau .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary files, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
de Camargo Magalhães, E.S., Hubner, S.E., Brown, B.D. et al. Proteomics for optimizing therapy in acute myeloid leukemia: venetoclax plus hypomethylating agents versus conventional chemotherapy. Leukemia (2024). https://doi.org/10.1038/s41375-024-02208-8
Download citation
Received : 18 November 2023
Revised : 22 February 2024
Accepted : 26 February 2024
Published : 26 March 2024
DOI : https://doi.org/10.1038/s41375-024-02208-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
SYSTEMATIC REVIEW article
Venetoclax combined chemotherapy versus chemotherapy alone for acute myeloid leukemia: a systematic review and meta-analysis.

- 1 Department of Hematology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 2 Department of Cardiology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
Objective: To compare the efficacy and safety of venetoclax (VEN) in combination with chemotherapy (chemo) versus chemo alone in the treatment of acute myeloid leukemia (AML).
Method: To compare the efficacy and/or safety of VEN+chemo versus chemotherapy alone for AML, PubMed, Embase, Web of Science, and the Cochrane Library were used to searching up to June 2023. Comparisons included complete remission (CR), CR with incomplete hematologic recovery (CRi), morphologic leukemia-free state (MLFS), overall response rate (ORR), and adverse events (AEs).
Result: A total of 9 articles were included, including 3124 patients. The baseline characteristics between two patient groups were similar. The combined analysis showed that compared with the group receiving chemo alone, the VEN+chemo group exhibited higher rates of CR, CRi, MLFS and ORR. Additionally, the VEN+chemo group had longer event-free survival (EFS) and overall survival (OS) durations. The incidence rates of AEs and serious AEs (SAEs) were similar between the two groups, but the early 30-day mortality rate was lower in the VEN+chemo group than in the chemo alone group.
Conclusion: The VEN+chemo therapy demonstrates significant efficacy and safety profile in AML patients. However, more prospective studies are needed in the future to provide more accurate and robust evidence for treatment selection in patients.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42023439288 , identifier CRD42023439288.
Introduction
Acute myeloid leukemia (AML) manifests as a remarkably heterogeneous hematological malignancy, marked by impediments in myeloid differentiation and aberrant proliferation of immature myeloid progenitor cells ( 1 ). With a median age of 68 years at diagnosis, AML emerges as the most prevalent form of acute leukemia in adults, and its incidence rises with age ( 2 , 3 ).The current standard intensive induction therapy for newly diagnosed acute myeloid leukemia (ND-AML) is a 7 + 3 regimen comprising cytarabine in combination with anthracyclines, followed by consolidation therapy upon achieving remission. Elderly patients and individuals with substantial capabilities comorbidities are generally deemed inappropriate candidates for intensive chemotherapy (chemo). This frequently leads to a reduced response rate when subjected to low-intensity chemo protocols, such as those involving hypomethylating agents (HMA) and low-dose cytarabine ( 4 ). Moreover, the absence of standardized treatment protocols leads to a long-term survival rate of less than 20% and a bleak prognosis for relapsed or refractory AML (R/R-AML) ( 5 ). The majority of AML patients have limited opportunities for effective treatment options. Consequently, there is a pressing need for research and the development of more potent treatment strategies to improve patient prognosis.
Venetoclax (VEN) is a selective small molecule inhibitor of B cell lymphoma 2 (BCL-2), effectively interrupting BCL-2’s inhibitory effects on pro-apoptotic proteins BAX and BIM. It demonstrates anti-tumor activity against a range of hematologic malignancies by increasing the permeability of the mitochondrial outer membrane, facilitating the release of cytochrome C, and thereby inducing apoptosis ( 6 ). This study suggests that, compared to chemo alone, VEN+chemo can improve the prognosis of AML patients ( 7 ).Nevertheless, research also indicates that patients undergoing VEN+chemo have lower rates of complete remission (CR) and shorter overall survival (OS) compared to those in the chemo-alone group ( 8 ). Concurrently, there is controversy surrounding the question of whether VEN+chemo leads to an increased occurrence of adverse events (AEs) and/or serious AEs (SAEs) in patients ( 9 , 10 ). Presently, a deficiency exists in accessible meta-analyses for comparing outcomes between the two groups. Consequently, we conducted a thorough systematic literature review and meta-analysis to evaluate the effectiveness and safety of VEN+chemo in comparison to chemo alone in AML patients.
This study was conducted according to the Preferred Reporting Items for Systematic Evaluation and Meta-Analysis (PRISMA statement) and registered in the PROSPERO International Registry of Prospective Systematic Reviews (registration number: CRD42023439288).
Literature search
Until June 2023, we conducted an extensive literature search utilizing multiple databases (PubMed, Embase, Web of Science, and the Cochrane Library) to compare the efficacy and safety of VEN+chemo to chemo alone for AML patients. The search terms used were “venetoclax,” “chemotherapy,” and “Acute Myeloid Leukemia”. The comprehensive search strategy is outlined in Supplementary Data Sheet 1 . Additionally, we manually reviewed the reference lists of all eligible studies. Two investigators (HZ and RG) independently retrieved and evaluated the selected studies, resolving any discrepancies in the literature search through collaborative consensus.
Inclusion and exclusion criteria
The inclusion criteria were as follows (1): the study design encompassed cohort or case-control, and randomized controlled trial (RCT) (2);adult patients with AML were involved in this study (3); study comparing the combination of VEN with chemo to chemo alone; and (4) the study reported outcome metrics, such as efficacy and AEs.
Exclusion criteria were as follows (1): reviews, meta-analyses, letters, editorial comments, case reports, conference abstracts, pediatric articles, unpublished articles, animal studies, non-English language articles (2); duplicate publications.
Data extraction
The two investigators (JZ and JF) independently extracted the data, which included (1) basic information of the included studies, such as authors, year of publication, type of study, sample size, intervention, etc. (2); basic characteristics of the study subjects, such as median age, gender, etc.; and (3) outcome metrics, such as CR, CR with incomplete hematologic recovery (CRi), morphologic leukemia-free state (MLFS), and AEs. In case of disagreement, a third investigator (TX) was involved in the discussion to resolve it.
Quality assessment
The quality of the included cohort studies was assessed according to the Newcastle-Ottawa Scale (NOS), a frequently employed tool for assessing the quality of observational studies. The NOS examines the potential bias stemming from the selection of study participants, misclassification, and confounding in association measurements. Studies scoring 7-9 points are generally regarded as high quality, whereas those scoring 3 or lower are deemed low quality. Furthermore, the quality evaluation of randomized controlled trials (RCTs) was carried out through the Cochrane Risk of Bias Assessment tool ( 11 ). Which is widely used for evaluating the quality of RCTs and primarily examines bias risks in several domains including random sequence generation, allocation concealment, blinding, completeness of outcome reporting, management of incomplete outcome data, and other possible biases. The utilization of this tool enables reviewers to develop a thorough comprehension of bias in RCT studies, facilitating an effective assessment of research quality and result reliability. The quality and level of evidence of the eligible studies were assessed independently by two researchers (JZ and JF), and any disagreements were resolved through discussion (TX).
Statistical analysis
Statistical analyses were performed using RevMan 5.3 software. Heterogeneity of the studies was assessed by χ2 and I^2 (heterogeneity was considered significant when χ2 P < 0.05 or I^2>50%), and if the heterogeneity was significant, a random-effects model was used, otherwise a fixed-effects model was used. Odds ratio (OR) were used to compare categorical variables and hazard ratio (HR) were used to compare survival variables and 95% confidence intervals (CI) were reported. Publication bias was assessed by funnel plot and Egger regression test. In addition, we performed subgroup analyses and one-way sensitivity analyses for outcomes with significant heterogeneity. For the other tests, P < 0.05 was considered statistically significant.
Literature search and characteristics
Initially, a preliminary search was performed on a total of 2,606 articles relevant to the study (794 articles from PubMed, 1,081 from Embase, 611 from Web of Science, and 120 from the Cochrane Library). Following the removal of duplicate papers, a comprehensive screening of titles and abstracts for1,588 papers was conducted. Eventually, a total of 9 articles were selected, consisting of data from 3,124 patients ( 5 , 8 – 10 , 12 – 16 ). Of these, seven were cohort studies and two were RCTs. The flow chart of the selection process was shown in Figure 1 . Table 1 exhibits the baseline characteristics of included studies. The cytogenetic/molecular/ELN risk information listed in Supplementary Table S1 .
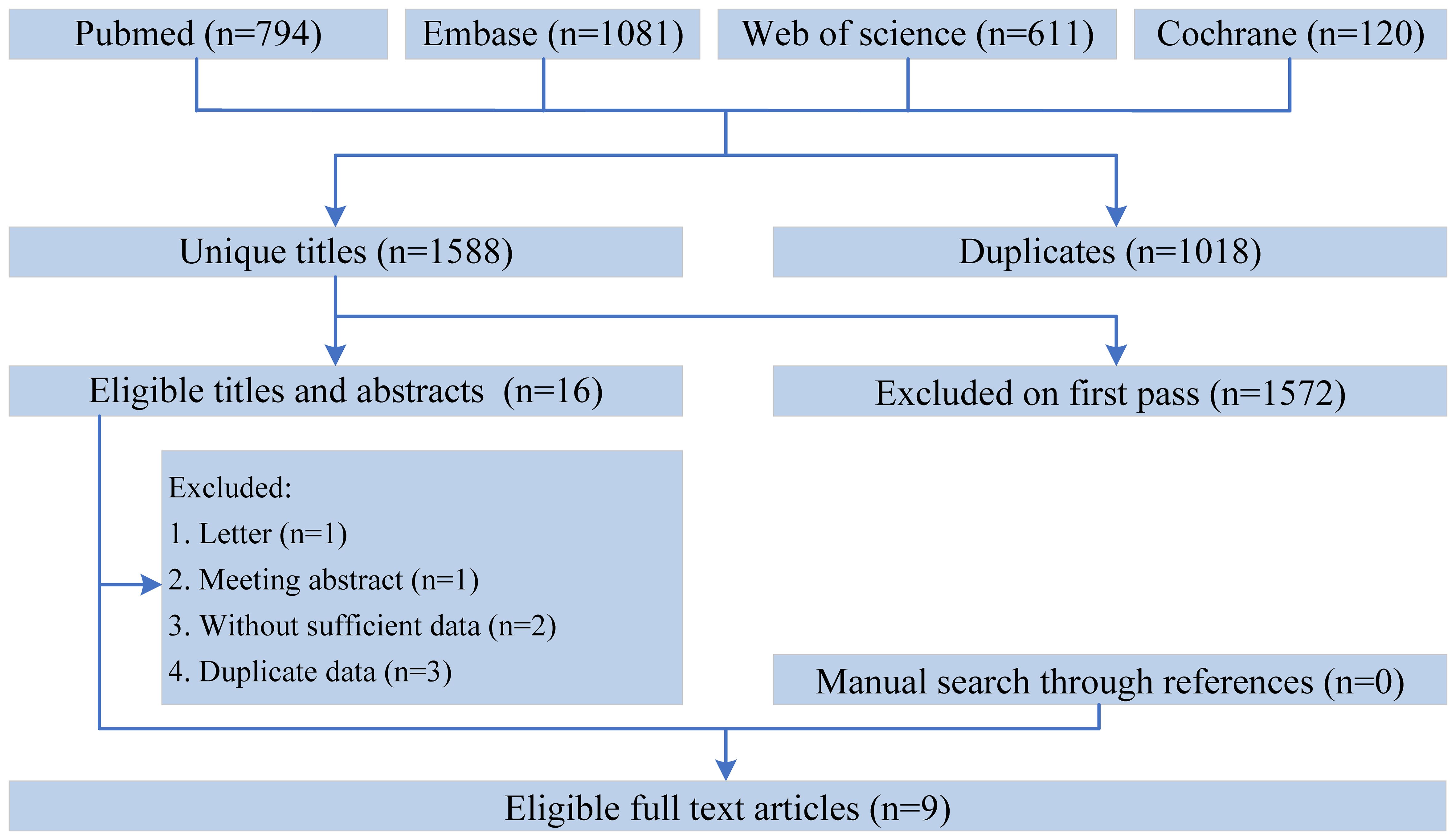
Figure 1 The flow chart of the selection process.
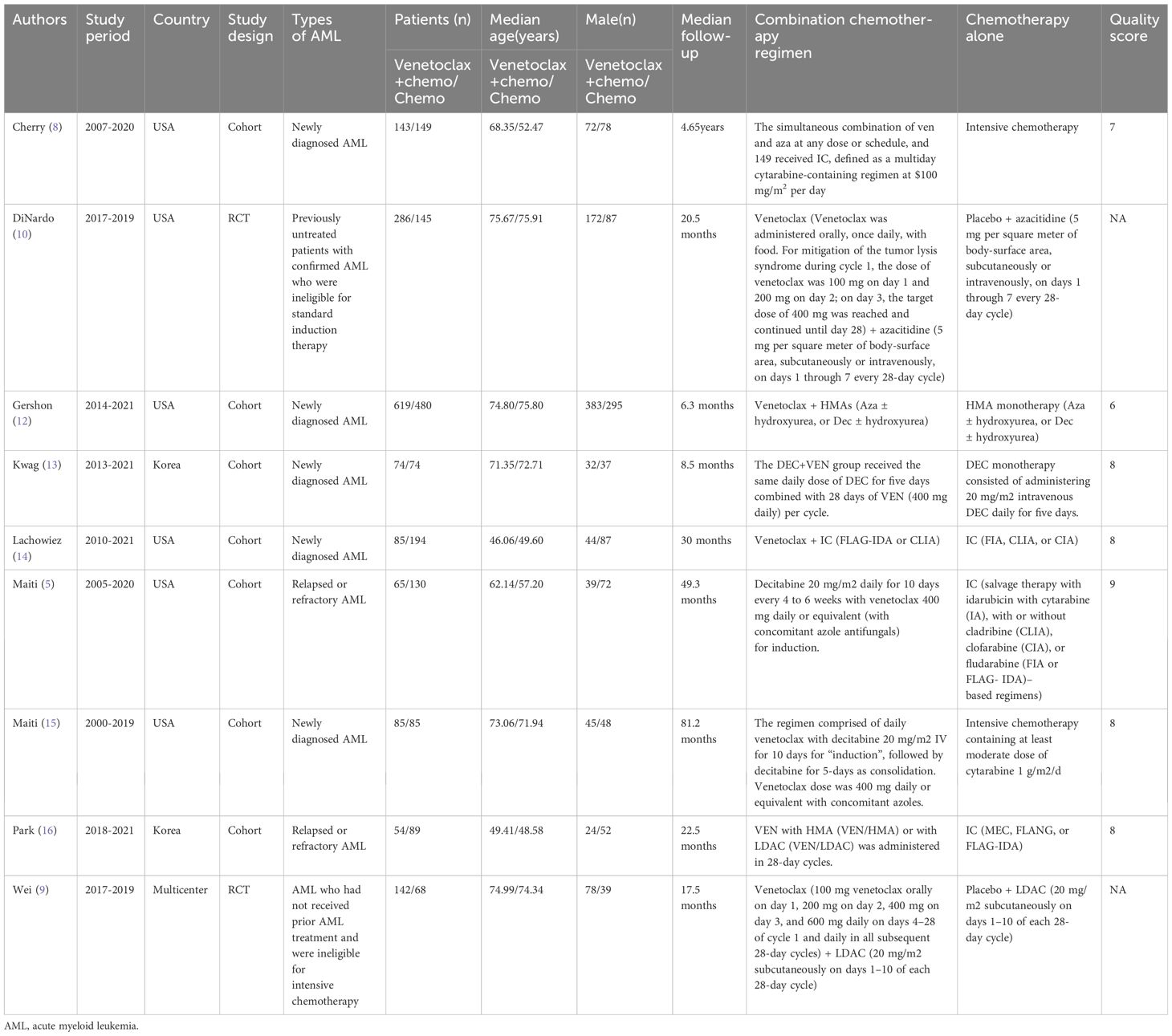
Table 1 Baseline characteristics of include studies and methodological assessment.
Quality assessment and risk of bias
Of the 7 cohort studies, 6 were high quality studies with a score of 7-9 ( Table 1 ). Details of the quality ratings of all eligible cohort studies are provided in Supplementary Table S2 . The quality ratings of the 2 RCT studies were shown in Supplementary Figure 1 .
Treatment response
1. CR: Seven studies reported the CR of patients, and the results revealed that the CR was higher in the VEN+chemo group compared to the chemo alone group (48.3% vs 44.6%). The combined effect was statistically significant (OR=1.74, 95%CI: 1.12-2.69), and significant heterogeneity (I^2 = 65%, P =0.009) depicted in Figure 2A . Without obvious publication bias exhibited in Funnel plot ( Figure 3A ), but the Egger’s test with ( P =0.017);
2. CRi: The meta-analysis results of CRi in patients from six studies indicated that CRi was higher in the VEN+chemo group compared to the chemo alone group (25.1% vs 11.4%). The combined effect was statistically significant (OR=2.88, 95%CI: 1.99-4.18). The study results showed heterogeneity (I^2 = 35%, P =0.17), as shown in Figure 2B . Publication bias was observed in the funnel plot ( Figure 3B ), but not in the Egger’s test ( P =0.193);
3. MLFS: Five studies used MLFS to assess treatment efficacy, and there was no heterogeneity among the study results (I^2 = 0%, P =0.49). The study results indicated that the MLFS was higher in the VEN+chemo group compared to the chemo alone group (6.9% vs 2.0%, OR=3.49, 95%CI: 1.80-6.74) ( Figure 2C ). The funnel plot ( Figure 3C ) and the Egger’s test did not reveal any significant publication bias ( P =0.203);
4. Overall response rates (ORR): The five included studies used ORR as the measure of therapeutic effect. The results showed that the ORR was higher in the VEN+chemo group compared to the chemo alone group (75.1% vs 57.1%), and the combined effect was statistically significant (OR=3.05, 95%CI: 1.58-5.86). There was significant heterogeneity in the study results (I^2 = 77%, P =0.002) ( Figure 2D ), the funnel plot ( Figure 3D ), and Egger’s test( P =0.355) had no obvious publication bias.
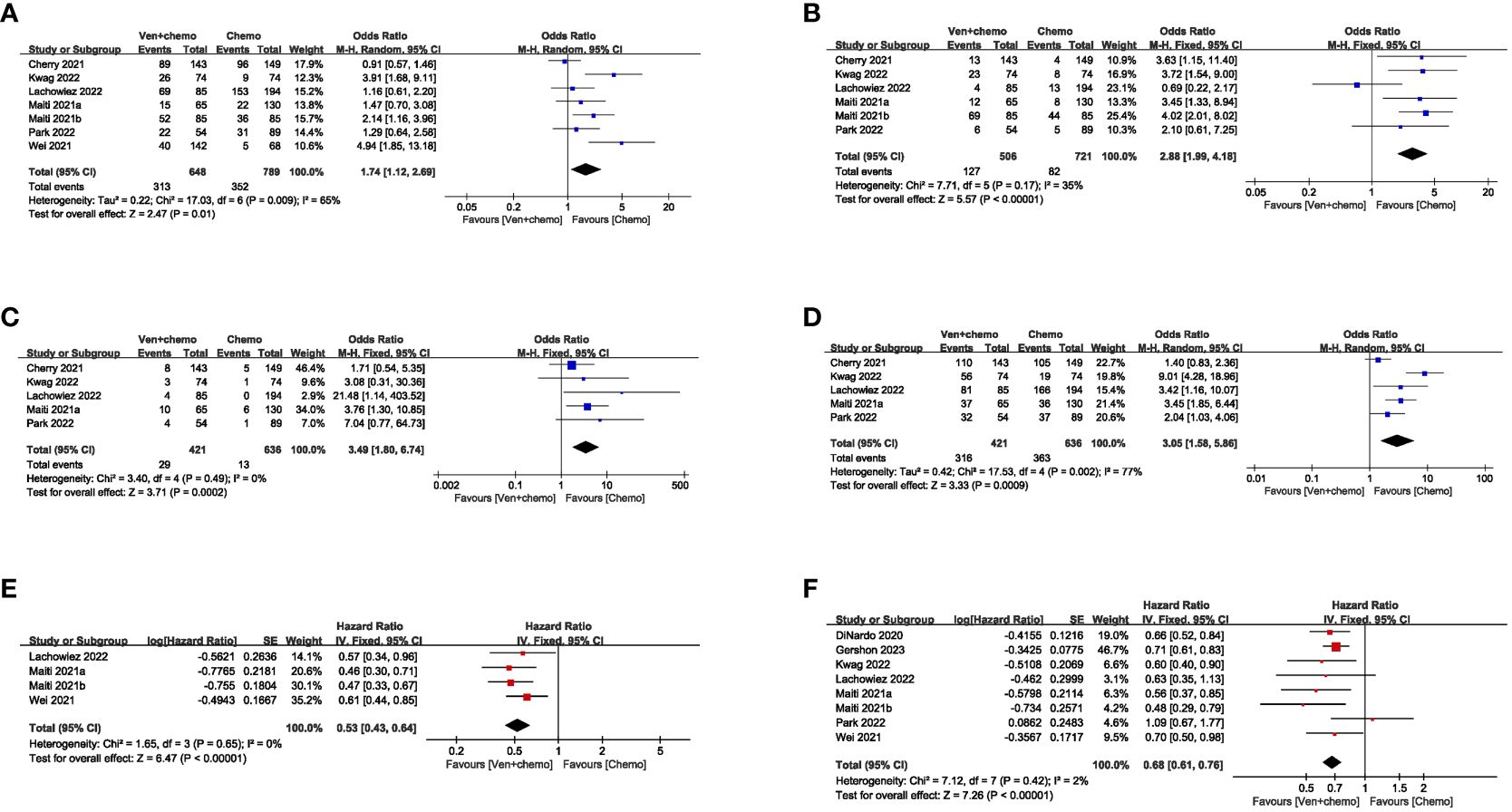
Figure 2 Assessment of heterogeneity in outcome measures.
Figure 3 Funnel plot of outcome measures in meta-analysis.
Event-free survival and OS
(1) EFS: Seven studies reported the EFS of the patients. The results showed that the VEN+chemo group had a longer EFS compared to the chemo alone group, and the combined effect was statistically significant (HR=0.53, 95%CI: 0.43-0.64). There was no significant heterogeneity in the study results (I^2 = 0%, P =0.65), as depicted in Figure 2E . The funnel plot ( Figure 3E ), and Egger’s test ( P =0.781) found no obvious publication bias;
(2) OS: Eight studies used OS as the evaluation measure. The results showed that the VEN+chemo group had a longer OS compared to the chemo alone group, and the combined effect was statistically significant (HR=0.68, 95%CI: 0.61-0.76). There was no significant heterogeneity in the study results (I^2 = 2%, P =0.42), as depicted in Figure 2F . The funnel plot ( Figure 3F ), and Egger’s test ( P =0.551) exhibited no significant publication bias.
We found that almost all patients experienced at least one AE (99%). The most prevalent AEs observed in both study groups included neutropenia, thrombocytopenia, nausea, and infection. Although the SAEs incidence in VEN+chemo group was higher than chemo alone group, but the difference was not statistically significant( P >0.05) in AEs and SAEs. Early 30-day mortality, of VEN+chemo group was superior to the chemo alone group (OR=0.23, 95%CI=0.12-0.48, P <0.0001).
Subgroup analysis and sensitivity analysis
We performed subgroup analyses of efficacy measures (CR, CRi, MLFS, overall response, OS, EFS) according to different study design, combining scheme, region, and types of AML. The results indicate that the VEN+AZA, VEN+IC, Asia, America, and R/R-AML subgroups were unsatisfactory in some of the efficacy indices, while the other subgroups showed no significant changes (detailed analysis in Table 2 ). Specifically, the VEN+AZA group exhibited inconsistency with the overall results in CR, MLFS, and overall response; the VEN+IC group showed inconsistency in CR, CRi, and OS; the Asia and America groups were inconsistent with the overall results in CR; and the R/R-AML group showed inconsistency in CR and OS.
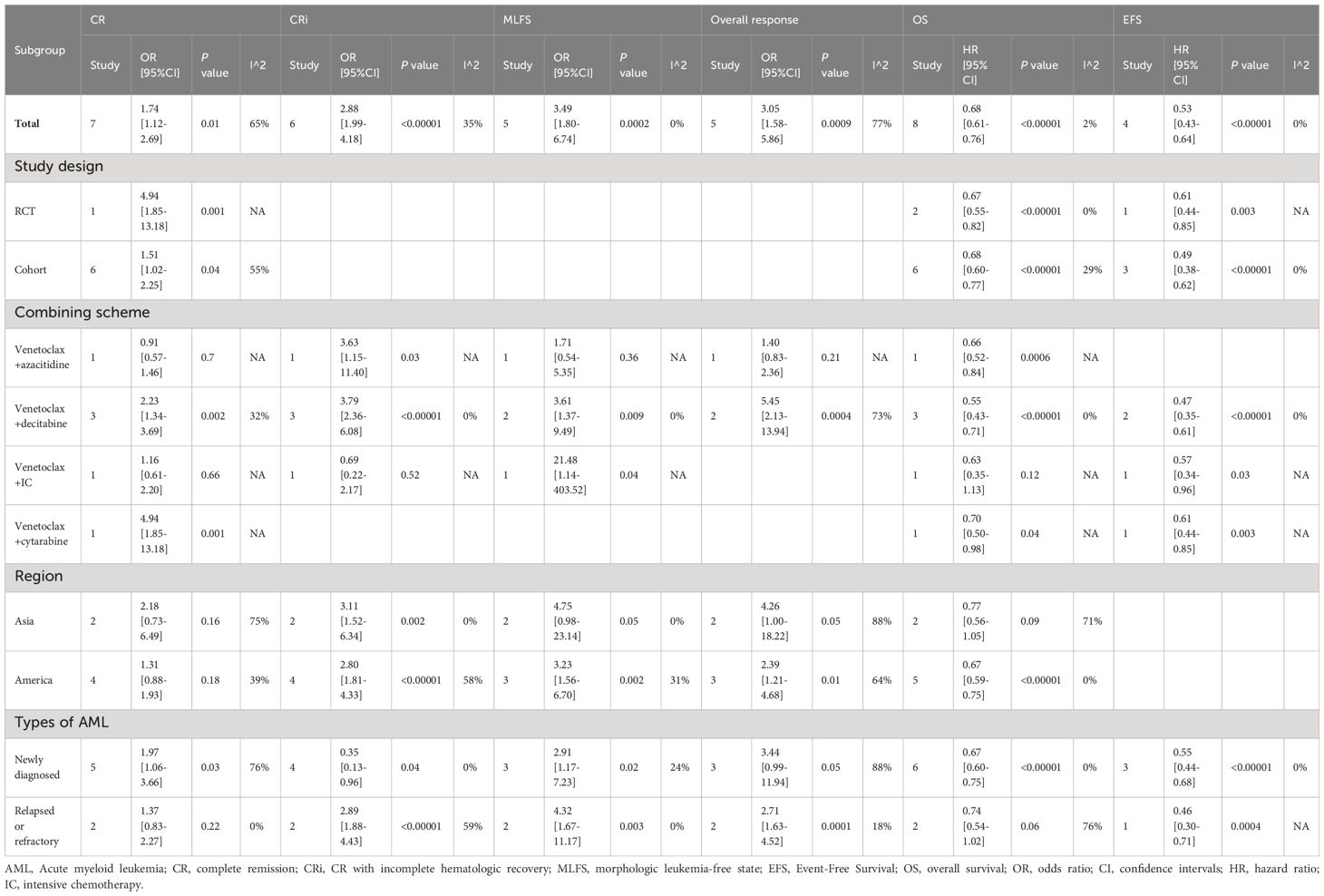
Table 2 Subgroup analysis.
In addition, we performed a one-way sensitivity analysis on CR and ORR, evaluating the influence of each study on the stability and heterogeneity of CR and ORR using the method of one-by-one exclusion. The results showed that the statistical differences in CR ( Figure 4A ) and ORR ( Figure 4B ) remained unchanged after excluding any individual literature, and significant heterogeneity still existed after excluding any individual literature.
Figure 4 Sensitivity analysis of complete response (CR) and overall response rate (ORR).
VEN has significant anti-tumor activity against various hematologic malignancies, including AML. However, the efficacy and safety of VEN combined with chemo in AML patients are still controversial, more high-quality research still needed. To our knowledge, this is the first systematic review and meta-analysis comparing the efficacy of VEN combined with chemo versus chemo alone in AML patients. In this study, we conducted a meta-analysis of 3124 patients from 9 publications to resolve this clinical controversy. The results of this study showed that the VEN combined with chemotherapy group had significantly better treatment response rates and survival time than the chemo alone group. The CR, CRi, and ORR rates in the VEN combined with chemo group were 48.3%, 25.1%, and 75.1%, respectively. These findings are similar to the meta-analysis results of previous studies on VEN combined therapy for AML, further validating the effectiveness of combination therapy ( 17 ).
VEN-based combination regimens are currently approved for the treatment of ND-AML patients who are elderly or unsuitable for IC, but there is still a lack of studies in R/R-AML patients ( 18 ). The subgroup analysis results in this study showed that R/R-AML patients who received combination therapy had higher CR/CRi/ORR rates, which may be associated with the targeting and synergistic effects of VEN. AML cells, particularly leukemia stem cells, are dependent on BCL-2 for their survival. VEN’s inhibitory action has the capacity to stimulate intrinsic apoptosis pathways, resulting in the prompt induction of apoptosis in AML cells and the elimination of dormant leukemia stem cells. VEN possesses the ability to activate T cells directly, both in vivo and in vitro , thereby enhancing their cytotoxicity against AML. By inhibiting the formation of respiratory chain super complexes, VEN ultimately boosts the effector function of T cells by enhancing the generation of reactive oxygen species ( 19 ). VEN used alone may lead to drug resistance, highlighting the importance of combining it with other chemo drugs. The mechanism of VEN resistance is not yet clear, but it may be related to the RAS/MAPK/MCL-1 pathway, leading to the upregulation of anti-apoptotic BCL-2 family proteins (such as BCL-XL and MCL-1), which effectively enhance the survival of leukemia cells ( 20 ). The concurrent use of VEN with chemo can synergistically induce cell apoptosis, collaboratively trigger mitochondrial apoptosis in AML cells, lower MCL-1 levels, thereby overcoming resistance in AML, and heightening anti-tumor efficacy ( 19 ).
The study conducted by Lee et al. ( 19 ), as reported in BLOOD, elucidated that VEN exerts a direct enhancement on the anti-leukemic effector function of T cells. Conversely, azacitidine induces a type I interferon response by activating the STING-cGAS pathway, thereby eliciting a virus-like infection response in leukemia cells. The increased susceptibility of AML cells to T cell-mediated cytotoxicity is observed in this study. Notably, the treatment response rate in the group with ND-AML surpassed that in the group with R/R-AML. This could be attributed to T cell dysfunction following chemo in the group with R/R-AML and could additionally be associated with the heightened probability of R/R-AML patients harboring adverse prognostic chromosomal karyotypes and gene mutations (e.g., TP53, SF3B1, EZH2) that make them less responsive to VEN ( 21 , 22 ). In this study, the efficacy of the VEN+AZA and VEN+IC subgroups was suboptimal. This may be attributed to the higher incidence of neutropenia in patients receiving combination therapy in the VEN+AZA group, leading to treatment interruption for hematologic recovery. Moreover, within the VEN+IC cohort, a greater percentage of patients receiving IC exhibited FLT3-ITD mutations. Consequently, patients receiving IC treatment also received FLT3 inhibitor therapy. However, heterogeneity exists in our study, and may related with the differences in types of AML and treatment protocols. Specifically, the response to VEN in ND-AML and R/R-AML patients varies. For instance, R/R-AML patients receiving combination therapy demonstrate improved treatment efficacy compared to those undergoing chemo alone. Additionally, the treatment response rate in the ND-AML group was higher than that in the R/R-AML group.
Different molecular features can significantly influence the efficacy of VEN. It has been reported that patients with mutations in NPM1, TET2, IDH1/2, ASXL1 and DDX41 have a higher response rate to VEN ( 23 – 30 ). In which, DDX41 is a DEAD-box type helicase that participates in various cellular processes including RNA metabolism and splicing ( 31 ). DDX41 mutations affect small nucleolar RNA maturation, impair ribosomal rRNA modification, hinder cellular protein synthesis, leading to cell cycle arrest and promoting apoptosis of mutated blood cells ( 32 ). In addition, splice factor (SF) mutations such as SRSF2, U2AF1, SF3B1, and ZRSR2 are commonly found in elderly AML patients and portend a poor prognosis ( 33 ). Lachowiez et al. ( 34 ) revealed that the outcome of patients with SF mutations treated with VEN+ hypomethylating agents was comparable to that of the wild-type patients. The improved prognosis of patients with DDX41 and SF mutations following VEN-based therapy treatment may be attributed to the potential influence of these mutations on the expression of BCL-2 family genes, thereby impacting the response to VEN-based therapy ( 35 ). It is worth noting that Stahl et al ( 23 ) found the mutation status of DNMT3A and the treatment history of HMA can predict the treatment response of patients with R/R-AML to VEN+HMA. For R/R-AML patients without DNMT3A mutations, regardless of previous HMA treatment, their survival rates after VEN+HMA therapy are similar. For R/R-AML patients with DNMT3A mutations who have not received prior HMA treatment, the response rate to VEN+HMA is higher, and their survival period is longer. Conversely, for R/R-AML patients with DNMT3A mutations who have a history of HMA treatment, the response rate to VEN+HMA is lower, and their survival period is shorter. Although it is not possible to conduct a quantitative analysis of median OS due to the different follow-up periods in each study, our research still indicates that VEN+chemo can prolong EFS and OS in AML patients. At the same time, the use of VEN-based combination therapy may improve the poor prognosis associated with certain genetic mutations. This discovery provides new possibilities for personalized treatment of AML patients.
The safety analysis results indicate that there were no significant differences in AEs and SAEs between the two groups of patients. Compared to chemo alone, VEN+chemo does not increase the incidence of AEs and/or SAEs in patients, and the early 30-day mortality rate was lower than the chemo alone group. Similar to the previous meta-analysis results, almost all patients experienced at least one AE during the study. The most common AEs in patients treated with the combination of VEN and chemotherapy were neutropenia, thrombocytopenia, nausea, and infection ( 17 , 36 ). However, it is important to note that the safety assessment results are based on limited data. Therefore, in clinical practice, it is still necessary to consider individual differences in patients in order to better evaluate potential risks and benefits.
This study provides the first systematic comparison of the efficacy and safety between VEN-based combination therapy and chemo alone in AML patients. In order to ensure the reliability of the results, we employed a comprehensive search strategy, clearly defined selection criteria, conducted rigorous quality assessments, and reported according to the PRISMA statement. The study confirms the superiority of VEN-based combination therapy over chemo alone in AML patients. However, our study has the following limitations: First, this meta-analysis included seven cohort studies and two RCTs, lacking prospective studies, which may impact the reliability of the results. Therefore, more RCTs and prospective clinical studies are needed to confirm our findings. Second, the results in this meta-analysis exhibit high heterogeneity. Subgroup analysis and sensitivity analysis were performed to evaluate the sources of heterogeneity, but it is difficult to determine all the factors contributing to heterogeneity. Considering the potential confounders, the results of this meta-analysis should be interpreted with caution. Third, due to small sample sizes in some subgroup analyses, it was challenging to quantitatively synthesize the data, and larger sample size needed for further analysis. Fourth, influenced by the limitations of the original study, we were unable to assess safety outcomes such as cycle length and hospitalization rates in patients. Consequently, it is essential to conduct further research to thoroughly investigate these aspects in the future. Ultimately, the existing literature remains limited despite conducting comprehensive searches across multiple databases. It is important to acknowledge the potential presence of publication bias, as this may compromise the statistical power and reliability of the study results. More studies needed for update our meta-analysis in the further.
VEN-based combination therapy demonstrates significant efficacy and a favorable safety profile in patients with AML, potentially providing a more appropriate treatment option. Nevertheless, due to the limited available literature and the presence of heterogeneity and potential publication bias, it is imperative to undertake further prospective studies in the future. These studies are essential for providing more accurate and convincing evidence to guide therapeutic decisions in patients.
Data availability statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding authors.
Author contributions
JZ: Writing – original draft. JF: Writing – original draft. TX: Writing – original draft. HZ: Writing – review & editing. RL: Writing – review & editing. YZ: Writing – review & editing. YL: Writing – review & editing. XX: Writing – review & editing. DW: Writing – review & editing. ZJ: Writing – review & editing. FH: Writing – review & editing. RG: Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Funding for this study was provided by Natural Science Foundation of Henan Province (182300410301), Medical Science and Technology Research Project of Henan Province (SBGJ202102147, SBGJ202003036, 2018020118), Science and Technology Plan of Henan Province (182102310160), and Project of Higher Education of Henan Province (18A320050).
Acknowledgments
We would like to acknowledge the authors of the original studies included in this meta-analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1361988/full#supplementary-material
1. Walker CJ, Kohlschmidt J, Eisfeld A-K, Mrózek K, Liyanarachchi S, Song C, et al. Genetic characterization and prognostic relevance of acquired uniparental disomies in cytogenetically normal acute myeloid leukemia. Clin Cancer Res . (2019) 25:6524–31. doi: 10.1158/1078-0432.CCR-19-0725
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Neuendorff NR, Loh KP, Mims AS, Christofyllakis K, Soo W-K, Bölükbasi B, et al. Anthracycline-related cardiotoxicity in older patients with acute myeloid leukemia: A young siog review paper. Blood Adv . (2020) 4:762–75. doi: 10.1182/bloodadvances.2019000955
3. DiNardo CD, Erba HP, Freeman SD, Wei AH. Acute myeloid leukaemia. Lancet . (2023) 401:2073–86. doi: 10.1016/S0140-6736(23)00108-3
4. Wei AH, Montesinos P, Ivanov V, DiNardo CD, Novak J, Laribi K, et al. Venetoclax plus ldac for newly diagnosed aml ineligible for intensive chemotherapy: A phase 3 randomized placebo-controlled trial. Blood . (2020) 135:2137–45. doi: 10.1182/blood.2020004856
5. Maiti A, DiNardo CD, Qiao W, Kadia TM, Jabbour EJ, Rausch CR, et al. Ten-day decitabine with venetoclax versus intensive chemotherapy in relapsed or refractory acute myeloid leukemia: A propensity score-matched analysis. Cancer . (2021) 127:4213–20. doi: 10.1002/cncr.33814
6. Griffioen MS, de Leeuw DC, Janssen JJWM, Smit L. Targeting acute myeloid leukemia with venetoclax; biomarkers for sensitivity and rationale for venetoclax-based combination therapies. Cancers (Basel) . (2022) 14(14):3456. doi: 10.3390/cancers14143456
7. Wang H, Mao L, Yang M, Qian P, Lu H, Tong H, et al. Venetoclax plus 3 + 7 daunorubicin and cytarabine chemotherapy as first-line treatment for adults with acute myeloid leukaemia: A multicentre, single-arm, phase 2 trial. Lancet Haematol . (2022) 9:e415–e24. doi: 10.1016/S2352-3026(22)00106-5
8. Cherry EM, Abbott D, Amaya M, McMahon C, Schwartz M, Rosser J, et al. Venetoclax and azacitidine compared with induction chemotherapy for newly diagnosed patients with acute myeloid leukemia. Blood Adv . (2021) 5:5565–73. doi: 10.1182/bloodadvances.2021005538
9. Wei AH, Panayiotidis P, Montesinos P, Laribi K, Ivanov V, Kim I, et al. 6-month follow-up of viale-C demonstrates improved and durable efficacy in patients with untreated aml ineligible for intensive chemotherapy (141/150). Blood Cancer J . (2021) 11:163. doi: 10.1038/s41408-021-00555-8
10. DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med . (2020) 383:617–29. doi: 10.1056/NEJMoa2012971
11. Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ . (2011) 343:d5928. doi: 10.1136/bmj.d5928
12. Gershon A, Ma E, Xu T, Montez M, Naqvi K, Ku G, et al. Early real-world first-line treatment with venetoclax plus hmas versus hma monotherapy among patients with aml in a predominately us community setting. Clin Lymphoma Myeloma Leuk . (2023) 23:e222–e31. doi: 10.1016/j.clml.2023.02.002
13. Kwag D, Cho B-S, Bang S-Y, Lee JH, Min G-J, Park S-S, et al. Venetoclax with decitabine versus decitabine monotherapy in elderly acute myeloid leukemia: A propensity score-matched analysis. Blood Cancer J . (2022) 12:169. doi: 10.1038/s41408-022-00770-x
14. Lachowiez CA, Reville PK, Kantarjian H, Jabbour E, Borthakur G, Daver N, et al. Venetoclax combined with induction chemotherapy in patients with newly diagnosed acute myeloid leukaemia: A post-hoc, propensity score-matched, cohort study. Lancet Haematol . (2022) 9:e350–e60. doi: 10.1016/S2352-3026(22)00076-X
15. Maiti A, Qiao W, Sasaki K, Ravandi F, Kadia TM, Jabbour EJ, et al. Venetoclax with decitabine vs intensive chemotherapy in acute myeloid leukemia: A propensity score matched analysis stratified by risk of treatment-related mortality. Am J Hematol . (2021) 96:282–91. doi: 10.1002/ajh.26061
16. Park S, Kwag D, Kim TY, Lee JH, Lee JY, Min GJ, et al. A retrospective comparison of salvage intensive chemotherapy versus venetoclax-combined regimen in patients with relapsed/refractory acute myeloid leukemia (Aml). Ther Adv Hematol . (2022) 13:20406207221081637. doi: 10.1177/20406207221081637
17. Shimony S, Rozental A, Bewersdorf JP, Goldberg AD, Stein EM, Grimshaw AA, et al. Investigational venetoclax combination therapy in acute myeloid leukemia - a systematic review and meta-analysis. Haematologica . (2022) 107:2955–60. doi: 10.3324/haematol.2022.281453
18. DiNardo CD, Pratz KW, Letai A, Jonas BA, Wei AH, Thirman M, et al. Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid leukaemia: A non-randomised, open-label, phase 1b study. Lancet Oncol . (2018) 19:216–28. doi: 10.1016/S1470-2045(18)30010-X
19. Lee JB, Khan DH, Hurren R, Xu M, Na Y, Kang H, et al. Venetoclax enhances T cell-mediated antileukemic activity by increasing ros production. Blood . (2021) 138:234–45. doi: 10.1182/blood.2020009081
20. Thol F, Ganser A. Treatment of relapsed acute myeloid leukemia. Curr Treat Options Oncol . (2020) 21:66. doi: 10.1007/s11864-020-00765-5
21. Pei S, Pollyea DA, Gustafson A, Stevens BM, Minhajuddin M, Fu R, et al. Monocytic subclones confer resistance to venetoclax-based therapy in patients with acute myeloid leukemia. Cancer Discovery . (2020) 10:536–51. doi: 10.1158/2159-8290.CD-19-0710
22. Tsai CH, Hou HA, Tang JL, Liu CY, Lin CC, Chou WC, et al. Genetic alterations and their clinical implications in older patients with acute myeloid leukemia. Leukemia . (2016) 30:1485–92. doi: 10.1038/leu.2016.65
23. Stahl M, Menghrajani K, Derkach A, Chan A, Xiao W, Glass J, et al. Clinical and molecular predictors of response and survival following venetoclax therapy in relapsed/refractory aml. Blood Adv . (2021) 5:1552–64. doi: 10.1182/bloodadvances.2020003734
24. DiNardo CD, Rausch CR, Benton C, Kadia T, Jain N, Pemmaraju N, et al. Clinical experience with the bcl2-inhibitor venetoclax in combination therapy for relapsed and refractory acute myeloid leukemia and related myeloid Malignancies. Am J Hematol . (2018) 93:401–7. doi: 10.1002/ajh.25000
25. Konopleva M, Pollyea DA, Potluri J, Chyla B, Hogdal L, Busman T, et al. Efficacy and biological correlates of response in a phase ii study of venetoclax monotherapy in patients with acute myelogenous leukemia. Cancer Discovery . (2016) 6:1106–17. doi: 10.1158/2159-8290.CD-16-0313
26. Huemer F, Melchardt T, Jansko B, Wahida A, Jilg S, Jost PJ, et al. Durable remissions with venetoclax monotherapy in secondary aml refractory to hypomethylating agents and high expression of bcl-2 and/or bim. Eur J Haematol . (2019) 102:437–41. doi: 10.1111/ejh.13218
27. Ram R, Amit O, Zuckerman T, Gurion R, Raanani P, Bar-On Y, et al. Venetoclax in patients with acute myeloid leukemia refractory to hypomethylating agents-a multicenter historical prospective study. Ann Hematol . (2019) 98:1927–32. doi: 10.1007/s00277-019-03719-6
28. Aldoss I, Yang D, Pillai R, Sanchez JF, Mei M, Aribi A, et al. Association of leukemia genetics with response to venetoclax and hypomethylating agents in relapsed/refractory acute myeloid leukemia. Am J Hematol . (2019) 94:E253–E5. doi: 10.1002/ajh.25567
29. Gangat N, Karrar O, Iftikhar M, McCullough K, Johnson IM, Abdelmagid M, et al. Venetoclax and hypomethylating agent combination therapy in newly diagnosed acute myeloid leukemia: genotype signatures for response and survival among 301 consecutive patients. Am J Hematol . (2024) 99:193–202. doi: 10.1002/ajh.27138
30. Alkhateeb HB, Nanaa A, Viswanatha D, Foran JM, Badar T, Sproat L, et al. Genetic features and clinical outcomes of patients with isolated and comutated ddx41-mutated myeloid neoplasms. Blood Adv . (2022) 6:528–32. doi: 10.1182/bloodadvances.2021005738
31. Nanaa A, He R, Foran JM, Badar T, Gangat N, Pardanani A, et al. Venetoclax plus hypomethylating agents in ddx41-mutated acute myeloid leukaemia and myelodysplastic syndrome: mayo clinic series on 12 patients. Br J Haematol . (2024) 204:171–6. doi: 10.1111/bjh.19105
32. Chlon TM, Stepanchick E, Hershberger CE, Daniels NJ, Hueneman KM, Kuenzi Davis A, et al. Germline ddx41 mutations cause ineffective hematopoiesis and myelodysplasia. Cell Stem Cell . (2021) 28(11):1966–81. doi: 10.1016/j.stem.2021.08.004
33. Senapati J, Urrutia S, Loghavi S, Short NJ, Issa GC, Maiti A, et al. Venetoclax abrogates the prognostic impact of splicing factor gene mutations in newly diagnosed acute myeloid leukemia. Blood . (2023) 142:1647–57. doi: 10.1182/blood.2023020649
34. Lachowiez CA, Loghavi S, Furudate K, Montalban-Bravo G, Maiti A, Kadia T, et al. Impact of splicing mutations in acute myeloid leukemia treated with hypomethylating agents combined with venetoclax. Blood Adv . (2021) 5:2173–83. doi: 10.1182/bloodadvances.2020004173
35. Crews LA, Balaian L, Delos Santos NP, Leu HS, Court AC, Lazzari E, et al. Rna splicing modulation selectively impairs leukemia stem cell maintenance in secondary human aml. Cell Stem Cell . (2016) 19:599–612. doi: 10.1016/j.stem.2016.08.003
36. Du Y, Li C, Yan J. The efficacy and safety of venetoclax and azacytidine combination treatment in patients with acute myeloid leukemia and myelodysplastic syndrome: systematic review and meta-analysis. Hematology . (2023) 28:2198098. doi: 10.1080/16078454.2023.2198098
Keywords: meta-analysis, acute myeloid leukemia, venetoclax, chemotherapy, efficacy, adverse events
Citation: Zhu J, Fan J, Xie T, Zhao H, Lu R, Zhang Y, Li Y, Xie X, Wan D, Jiang Z, He F and Guo R (2024) Venetoclax combined chemotherapy versus chemotherapy alone for acute myeloid leukemia: a systematic review and meta-analysis. Front. Oncol. 14:1361988. doi: 10.3389/fonc.2024.1361988
Received: 27 December 2023; Accepted: 14 March 2024; Published: 26 March 2024.
Reviewed by:
Copyright © 2024 Zhu, Fan, Xie, Zhao, Lu, Zhang, Li, Xie, Wan, Jiang, He and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rong Guo, [email protected] ; Fei He, [email protected]
† These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
- Biomarker-Driven Lung Cancer
- HER2-Positive Breast Cancer
- Chronic Lymphocytic Leukemia
- Small Cell Lung Cancer
- Renal Cell Carcinoma

- CONFERENCES
- PUBLICATIONS
Revumenib Earns FDA Priority Review in R/R KMT2Ar Acute Leukemia

The FDA granted priority review to the NDA for revumenib in adult and pediatric R/R KMT2A -rearranged acute leukemia. 1
Revumenib is a first-in-class Menin inhibitor. The NDA filing for the agent is currently being reviewed under the FDA's real-time oncology review program, which allows for a more efficient review and engagement between the sponsor and the FDA throughout the submission process.
A PDUFA date for the agent has been set for September 26, 2024.
"The receipt of priority review for the revumenib NDA filing is a significant milestone as we transition to a leading commercial-stage oncology company with the planned launches of 2 first- and best-in class drugs in 2024," said Michael A. Metzger, chief executive officer of Syndax Pharmaceuticals, in a press release. "With 2 regulatory filings now under FDA priority review, our team is focused on commercial preparations to enable Syndax's continued success as we enter this next stage of growth."

a close-up of red blood cells flowing through a vein, displaying the characteristic sickle shape Generative AI: © catalin - stock.adobe.com
Data From the AUGMENT-101 Trial
Findings from the AUGMENT-101 trial of revumenib in adult and pediatric patients with KMT2A -rearranged AML and ALL support this approval. At the protocol-defined interim analysis, the trial met its primary end point by showing a complete remission (CR) or a CR with partial hematologic recovery (CRh) rate of 23% (95% CI, 12.7%-35.8%; P =.0036).2 This was seen among the 57 patients who were in the pooled KMT2Ar acute leukemia population and were efficacy-evaluable.
In both the overall population and among patients with AML, a durable CR/CRh response was seen with a 6.4-month (95% CI, 3.4-not reached) median duration as of the data cutoff date of July 24, 2023. At this time, 46% of patients remained in response.
Ten of the 13 patients who achieved CR/CRh had minimal residual disease (MRD) status assessed, and 70% of patients were MRD-negative. In the overall cohort of 57 patients, 36 achieved an overall response, 39% (14/36) of whom underwent hematopoietic stem cell transplant. Additionally, 50% of these patients (7/14) restarted revumenib as post transplant maintenance at the time of the data cutoff.
Further findings from phase 2 AUGMENT-101 trial in this patient population were presented at the 2023 American Society of Hematology Annual Meeting. Data from the phase 1 portion of the AUGMENT-101 trial in acute leukemia were also published in Nature . 1
Previously, in December 2022, the FDA granted a breakthrough therapy designation to revumenib for the treatment of adult and pediatric patients with R/R acute leukemia who harbor a KMT2A rearrangement.
REFERENCES:
1. syndax announces fda priority review of nda for revumenib for the treatment of relapsed/refractory kmt2ar acute leukemia. news release. syndax pharmaceuticals. march 26, 2024. accessed march 26, 2024. https://tinyurl.com/27x4vena, 2. aldoss i, issa gc, thirman m, et al. revumenib monotherapy in patients with relapsed/refractory acute leukemia topline efficacy and safety results from the pivotal augment-101 phase 2 study. blood. 2023;142(supple 2): lba-5. doi:10.1182/blood-2023-192042.

FDA Clears IND of BAT8006 in Platinum-Resistant Ovarian Cancers
A phase 2 trial plans to evaluate BAT8006 for the treatment of patients with platinum-resistant epithelial ovarian, fallopian tube, or primary peritoneal cancer.

FDA Grants Full Approval to Mirvetuximab for Platinum-Resistant Ovarian Cancer
Mirvetuximab soravatansine is now FDA-approved for the treatment of patients with folate receptor alpha-positive, platinum-resistant ovarian cancer

FDA Approves Safety Label Changes for Fluorouracil Injection
The FDA strengthened safety warnings for fluorouracil due to dihydropyrimidine dehydrogenase deficiency that can cause severe adverse effects.

Breaking Ground: FDA Approves Ponatinib and Chemotherapy for Ph+ ALL
In an interview with Targeted Oncology, Elias Jabbour, MD, discussed the approval of ponatinib and what it means for patients with Philadelphia chromosome-positive acute lymphoblastic leukemia.

FDA Fast Tracks PT886 in CLDN18.2+ Pancreatic Cancer
A fast track designation from the FDA has been given to PT886 for the treatment of patients with metastatic claudin 18.2-positive pancreatic adenocarcinoma.
2 Commerce Drive Cranbury, NJ 08512
609-716-7777

- Get 7 Days Free
MEI Pharma Reports Update from Clinical Study Evaluating Oral CDK9 Inhibitor Voruciclib in Combination with Venetoclax in Patients with Relapsed and Refractory Acute Myeloid Leukemia
– Enrollment Initiated in 12-Patient Expansion Cohort Evaluating Voruciclib Plus Venetoclax in Ongoing Phase 1 Study –
– Anti-leukemic Activity Across Multiple Heavily Pretreated Patients Demonstrated Along with Anticipated Decreases in Mcl-1 –
– No Evidence of Overlapping Toxicity, and No Dose Limiting Toxicities Observed to Date in Dose Escalation Cohorts –
MEI Pharma, Inc. (Nasdaq: MEIP), a clinical-stage pharmaceutical company evaluating novel drug candidates to address known resistance mechanisms to standard-of-care cancer therapies, today reported initiation of enrollment in a 12-patient expansion cohort in the ongoing Phase 1 study evaluating voruciclib, an investigational selective oral cyclin-dependent kinase 9 (“CDK9”) inhibitor, in combination with venetoclax (Venclexta ® ), a B-cell lymphoma 2 (“BCL2”) inhibitor, in relapsed and refractory (“R/R”) acute myeloid leukemia (“AML”) patients. The Safety Review Committee recommended initiating the expansion cohort after observing anti-leukemic activity in multiple heavily pretreated patients in the dose escalation cohorts, including responses, anticipated decreases in myeloid leukemia cell differentiation protein (“Mcl-1”) in available patient samples, no overlapping toxicity or dose limiting toxicities, and favorable safety results to date.
“MCL-1 overexpression has been associated with a poor prognosis and development of resistance to BCL-2 inhibition by venetoclax in patients with AML and CLL. Voruciclib is a potent, oral CDK9 inhibitor that indirectly also suppresses MCL-1. We are participating in the ongoing multicenter phase 1 study, where preliminary results are demonstrating good treatment tolerance and safety to date,” said Yesid Alvarado-Valero, M.D., Associate Professor, Department of Leukemia, University of Texas MD Anderson Cancer Center and study chair of the combination therapy stage of the Phase 1 study. “When Voruciclib is used in combination with venetoclax, the combination appears to have no added toxicity, in addition there is evidence of synergistic, early clinical activity, with disease responses, in a group of heavily pretreated acute myeloid leukemia patients.”
“Increasingly, venetoclax is being used as a standard treatment in patients with AML, but resistance to salvage therapy after venetoclax use is common and yields limited benefit upon relapse; only about 10% of patients respond to salvage therapy after venetoclax failure, representing a significant need for patients with AML,” said Richard Ghalie, M.D., chief medical officer of MEI Pharma. “We see the voruciclib data to date demonstrating anti-leukemic activity as promising, particularly alongside the consistent reductions of Mcl-1 that provide evidence we are eliciting the anticipated biological response in patients, and we are excited to share additional updates as appropriate in the second half of 2024.”
Dr. Ghalie continued: “As we enroll the expansion cohort evaluating the potential of voruciclib in combination with venetoclax among a larger group of patients, I would like to thank and recognize the continued engagement of our investigators, and the participation of the patients enrolling in this study.”
Phase 1 Study Details
The Phase 1 study is a multiple stage, open-label, 3+3 dose escalation and expansion study evaluating voruciclib, an oral CDK9 inhibitor, as a monotherapy and in combination with venetoclax, a BCL2 inhibitor. The first stage of the study evaluated the dose and schedule of voruciclib as a single-agent in patients with AML or B-cell malignances after failure of standard therapies. This stage is complete.
The second stage of the study, evaluating voruciclib in combination with standard dose venetoclax in patients with R/R AML, has completed enrollment in the dose escalation cohorts evaluating seven voruciclib dose levels from 50 mg every other day to 300 mg daily for two weeks in a four-week cycle. The study is currently enrolling a 12-patient expansion cohort evaluating voruciclib administered at 300 mg daily for two weeks in a four-week cycle in combination with venetoclax. Considering the tolerability results for the combination to date, another arm of the study will evaluate escalating doses of voruciclib administered over three weeks in a four-week cycle in combination with venetoclax to increase dose intensity and potentially optimize patient response.
A total of 29 patients with R/R AML, median age 67 years (range 34-89), enrolled in the dose escalation stage of the study evaluating voruciclib in combination with venetoclax. These patients were generally heavily pretreated; the median number of prior therapies was 3 (range 1-7), and 15 (52%) patients had ≥3 prior lines. Almost all patients (28/29) were treated with venetoclax in an earlier line of therapy. Additionally, 21 (72%) patients were noted as being in an adverse 2017 ELN Risk Category due to adverse cytogenetics and molecular mutations.
The primary objectives of the study are to determine the safety and biologic effective dose of voruciclib monotherapy or voruciclib in combination with venetoclax. Secondary objectives of the study include assessing the preliminary efficacy, pharmacokinetics, pharmacodynamics, and biomarkers of voruciclib monotherapy or voruciclib in combination with venetoclax.
Voruciclib Plus Venetoclax Combination: Initial Safety and Tolerability Data
Voruciclib at doses up to 300 mg administered on 14 consecutive days in a 28-day cycle in combination with standard dose venetoclax was well tolerated with no dose limiting toxicities observed. The maximum tolerated dose of voruciclib administered on this schedule with venetoclax has not been established. There were no discontinuations due to drug-related adverse events. No evidence of overlapping toxicity has been observed to date. The most common (≥5% of patients) grade 3 adverse events were myelosuppression associated with AML. Only 1 patient was observed as having a non-hematologic grade 3 drug-related adverse event (diarrhea).
Voruciclib Plus Venetoclax Combination: Initial Efficacy Data
In the 20 patients administered voruciclib at a dose of 100 mg or more, three patients achieved a response, including two patients that achieved a complete response with incomplete hematologic recovery (CRi) and one patient that achieved a morphologic leukemia-free state (MLFS), in each case having received venetoclax in an earlier line of treatment. Responses lasted 7 months in one patient, 5 months and ongoing in the second patient, and the third patient was referred to stem cell transplant. Further, an additional 14 patients had stable disease which lasted more than 90 days in 5 patients.
In the patients administered voruciclib at a dose of 100 mg or more, initial results from correlative biomarker assay studies of available samples from patients treated with the combination demonstrate the anticipated decrease of Mcl-1. Further, the available assays from the dose escalation cohorts demonstrated dose proportional decreases in Mcl-1. Reductions in Mcl-1 are consistent with the known mechanism of action of CDK9, which regulates Mcl-1.
About Voruciclib
Voruciclib is an investigational orally administered cyclin-dependent kinase 9 (“CDK9”) inhibitor with potential to treat both hematological malignancies and solid tumors. It is in clinical development for acute myeloid leukemia and B-cell malignancies. Applications in solid tumors are also being considered.
The CDK family of proteins are important cell cycle regulators responsible for the control of cell proliferation, differentiation, apoptosis, and DNA repair. CDK9, one of several members of the CDK family of proteins, functions as a gene transcription controller and is also involved in regulating protein degradation. Specifically, CDK9 is a promising target to treat a range of cancers because of its role in controlling two other proteins often dysregulated in cancerous cells: myeloid leukemia cell differentiation protein (“Mcl-1”) and the MYC proto-oncogene protein ("MYC").
Mcl-1 is a member of the family of anti-apoptotic proteins which, when elevated, may prevent the cell from undergoing cell death. Inhibition of CDK9 blocks the production of Mcl-1, which is an established resistance mechanism to the B-cell lymphoma 2 ("BCL2") inhibitor venetoclax (marketed as Venclexta ® ).
MYC regulates cell proliferation and growth. Upregulation of MYC is implicated in many human cancers and is frequently associated with poor prognosis and unfavorable patient survival. CDK9, in addition to being a transcription factor for MYC, also decreases phosphorylation of MYC protein that is implicated in stabilizing MYC in KRAS mutant cancers. Targeting MYC directly has historically been difficult, but CDK9 is a promising approach to target this oncogene.
About MEI Pharma
MEI Pharma, Inc. (Nasdaq: MEIP) is a clinical-stage pharmaceutical company committed to developing novel and differentiated cancer therapies. We build our pipeline by acquiring promising cancer agents and creating value in programs through development, strategic partnerships, out-licensing and commercialization, as appropriate. Our approach to oncology drug development is to evaluate our drug candidates in combinations with standard-of-care therapies to overcome known resistance mechanisms and address clear medical needs to provide improved patient benefit. The drug candidate pipeline includes voruciclib, an oral cyclin-dependent kinase 9 ("CDK9") inhibitor, and ME-344, an intravenous small molecule mitochondrial inhibitor targeting the oxidative phosphorylation pathway. For more information, please visit www.meipharma.com . Follow us on X (formerly Twitter) @MEI_Pharma and on LinkedIn.
Forward-Looking Statements
Certain information contained in this press release that are not historical in nature are “forward-looking statements” within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995 including, without limitation, statements regarding: the potential, safety, efficacy, and regulatory and clinical progress of our product candidates, including the anticipated timing for initiation of clinical trials and release of clinical trial data and our expectations surrounding potential regulatory submissions, approvals and timing thereof, our business strategy and plans; the sufficiency of our cash, cash equivalents and short-term investments to fund our operations; and our ability to fund future capital returns. You should be aware that our actual results could differ materially from those contained in the forward-looking statements, which are based on management’s current expectations and are subject to a number of risks and uncertainties, including, but not limited to our failure to successfully commercialize our product candidates; the availability or appropriateness of utilizing the FDA’s accelerated approval pathway for our product candidates; final data from our pre-clinical studies and completed clinical trials may differ materially from reported interim data from ongoing studies and trials; costs and delays in the development and/or FDA approval, or the failure to obtain such approval, of our product candidates; uncertainties or differences in interpretation in clinical trial results; uncertainty regarding the impact of rising inflation and the increase in interest rates as a result; potential economic downturn; geopolitical conflicts; activist investors; our inability to maintain or enter into, and the risks resulting from, our dependence upon collaboration or contractual arrangements necessary for the development, manufacture, commercialization, marketing, sales and distribution of any products; competitive factors; our inability to protect our patents or proprietary rights and obtain necessary rights to third party patents and intellectual property to operate our business; our inability to operate our business without infringing the patents and proprietary rights of others; general economic conditions; the failure of any products to gain market acceptance; our inability to obtain any additional required financing; technological changes; government regulation; changes in industry practice; and one-time events. We do not intend to update any of these factors or to publicly announce the results of any revisions to these forward-looking statements. Under U.S. law, a new drug cannot be marketed until it has been investigated in clinical studies and approved by the FDA as being safe and effective for the intended use.
David A. Walsey MEI Pharma Tel: 858-369-7104 [email protected]
View source version on businesswire.com: https://www.businesswire.com/news/home/20240326048347/en/
Market Updates
Q2 stock market outlook: contrarian plays increasingly attractive, what the next bitcoin halving means for etf investors, 5 undervalued stocks to buy after they’ve been dumped, morningstar market insights: central banks, inflation, and patient investing, where to invest in bonds now, what’s happening in the markets this week, reddit stock surges in ipo, why big bank stocks are no longer cheap, stock picks, investors can find more than gold in the mining industry, an ultracheap stock to buy in a rallying sector, celsius stock has soared 206%. is it a buy or a sell, 10 undervalued wide-moat stocks, investment opportunities in us renewable energy, we just upgraded these 6 companies. is it time to buy the stocks, the best international stock index funds, boeing: management shakeup may foster more change—no simple task, sponsor center.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts

Bone marrow evaluation for diagnosis and monitoring of acute myeloid leukemia
Mary-elizabeth percival.
a Department of Medicine, University of Washington, Seattle, WA, USA
b Clinical Research Division, Fred Hutchinson Cancer Research Center, Seattle, WA, USA
Catherine Lai
c Myeloid Malignancies Section, Hematology Branch, National Heart, Lung and Blood Institute, National Institutes of Health, Bethesda, MD, USA
Elihu Estey
Christopher s. hourigan.
The diagnosis of acute myeloid leukemia (AML) can be made based on peripheral blood or bone marrow blasts. In this review, we will discuss the role of bone marrow evaluation and peripheral blood monitoring in the diagnosis, management, and follow up of AML patients. For patients with circulating blasts, it is reasonable to perform the necessary studies needed for diagnosis and risk stratification, including multiparametric flow cytometry, cytogenetics, and molecular analysis, on a peripheral blood specimen. The day 14 marrow is used to document hypocellularity in response to induction chemotherapy, but it is unclear if that assessmentis necessary as it often does not affect immediate management. Currently, response assessments performed at count recovery for evaluation of remission and measurable residual disease rely on bone marrow sampling. For monitoring of relapse, peripheral blood evaluation may be adequate, but the sensitivity of bone marrow testing is in some cases superior. While bone marrow evaluation can certainly be avoided in particular situations, this cumbersome and uncomfortable procedure currently remains the de facto standard for response assessment.
1. Use of marrow in initial diagnosis
1.1 bone marrow evaluation.
When the diagnosis of acute myeloid leukemia (AML) is suspected, the treating physician typically recommends a bone marrow evaluation for further morphologic assessment. Indeed, the practice of morphologic assessment of the bone marrow is recommended in the initial diagnostic work-up for suspected AML by the European LeukemiaNet (ELN), the National Comprehensive Cancer Network (NCCN) Guidelines, and the World Health Organization (WHO) 2016 guidelines. 1 - 3 Typically, both bone marrow aspiration and bone marrow trephine biopsy are performed. Some centers perform aspiration alone when possible, with biopsy only in cases of a “dry tap” or diagnostic uncertainty (e.g., distinguishing whether peripheral pancytopenia is related to AML or myelodysplastic syndrome); a biopsy is always indicated if there are no circulating blasts in the peripheral blood and AML is suspected. Analyses typically performed on the bone marrow sample include flow cytometry, metaphase cytogenetics, fluorescence in situ hybridization (FISH), and molecular analyses, which often utilize polymerase chain reaction-based or next-generation sequencing technology to examine specific markers ( NPM1 , CEBPA , FLT3 ) or panels of markers with demonstrated importance in myeloid malignancy. 4 , 5 This combined information collected at the time of AML diagnosis is used in prognostication for patients overall, 6 , 7 as well as in recommendations for individual patients regarding whether to proceed to investigational induction chemotherapy and/or allogeneic hematopoietic cell transplant (HCT). 8
1.2 Can AML be diagnosed and characterized without bone marrow evaluation?
The diagnosis of AML requires the presence of ≥20% blasts in the peripheral blood or bone marrow; in certain cases, the presence of recurrent cytogenetic abnormalities, such as the characteristic translocation (8;21) define AML even at a lower blast count. 3 Frequently the diagnosis of AML can be made without resorting to invasive bone marrow sampling. For patients with high peripheral leukemic blast counts, many of the requisite tests can be performed on peripheral blood. The immunophenotype obtained by flow cytometry has been found to be the same in peripheral blood and bone marrow blasts, though the antibody panel used therein was relatively limited in scope and complexity in this relatively older study 9 . A small case series confirmed this finding by comparing peripheral blood and bone marrow in patients with acute leukemias, which demonstrated no differences in morphology or immunophenotyping if the peripheral blood blasts were 30% or more. 11 Differences have also been found in the cell cycle phase of blasts in these two compartments, though the clinical significance of this finding is yet to be determined. 10 . In contrast, the particular leukemia-associated immunophenotype (LAIP) for a particular patient may be variable and meaningful, particularly for later monitoring of residual disease. 12
In the small case series mentioned previously, the karyotype was insufficient for analysis in 17% of the AML peripheral blood samples (5 out of 29 patients), but in none of the bone marrow samples. 11 However, no account was made by the authors of the total blood blast count (i.e., white blood cell count multiplied by percentage of blasts), only the blast percentage in peripheral blood. Since the comparison of karotype between the blood and bone marrow was performed only in patients with a high percentage of peripheral blasts, bone marrow aspiration for karyotype should be performed if few or no circulating blasts are present. Similar findings were demonstrated by the study of Hussein et al, in which patients with high numbers of circulating blasts (at least 0.1 × 10 9 cells/L) were likely to have successful peripheral blood karyotype (90% success rate or higher). 13 In AML patients specifically, peripheral blood karyotyping produced successful metaphases in 32 out of 42 patients (76%). 13 Conventional karyotypeing with chromosome banding is the current standard in the diagnosis and work-up of a patient with AML, and several AML entities are defined by in the WHO classification by their recurrent karyotypic abnormalities. 1 , 3 It is possible that next generation sequencing approaches will make conventional chromosomal banding redundant in the future. 14
FISH testing, typically performed as part of an AML or myelodysplastic syndrome-specific panel, is less clearly correlated between blood and bone marrow. In a review of 48 cases of AML with paired peripheral blood and bone marrow samples, abnormal peripheral blood FISH results were found in 69% of patients with abnormal bone marrow FISH results (18 of 26), but also in 23% of cases with normal bone marrow FISH results (5 of 22). 15 There is uncertainty whether patients with abnormal cytogenetics as assessed by FISH but not standard 20-metaphase karyotype have a prognosis more befitting the FISH results or the standard results; pathologist consultation may help the treating physician in cases of discordance. Molecular analysis has similarly been compared between peripheral blood and bone marrow samples, and peripheral blood has high sensitivity and specificity for detection of FLT3 -ITD and NPM1 mutations when the blast count is >2000 cells per microliter. 16 , 17 More recently, the protein expression pattern (so-called proteome) has been shown to be closely correlated between peripheral blood and bone marrow blasts, though this finding is not clinically relevant at the current time. 18
Overall, if peripheral blood blasts are high at the time of AML diagnosis (>2000 cells per microliter), we posit that bone marrow examination is an unnecessary adjunct to peripheral blood sampling, which is able to provide morphologic, immunophenotypic, and molecular data; however, discrepancies still remain between the consistency of FISH results in peripheral blood and bone marrow.
1.3 Is morphologic assessment at diagnosis necessary?
Morphologic assessment of the bone marrow for the evaluation of acute leukemias was initially standardized through pathologic review by the French-American-British (FAB) cooperative group in 1976, and revised a decade later. 19 , 20 While the small study mentioned previously showed good correlation between morphology in the peripheral blood and bone marrow, 11 the question remains whether morphology is necessary at all for the diagnosis of AML. In fact, at times, the bone marrow may be inaspirable, making morphology moot. In limited subtypes, including acute megakaryoblastic leukemia, acute panmyelosis with myelofibrosis, and acute myeloid leukemia with myelodysplasia-related changes, some have argued that immunohistochemistry performed on a bone marrow biopsy is a crucial adjunct to peripheral blood analysis in order to make the final diagnosis. 21 It should be noted that one way for the diagnosis of AML with myelodysplasia-related changes to be made in the 2016 WHO classification is with multilineage dysplasia (defined as >50% of cells with dysplasia in at least two cell lines), which can only be assessed on bone marrow sampling. 3 It has recently been suggested that the presence of a mutation in any of SRSF2, SF3B1, U2AF1, ZRSR2, ASXL1, EZH2, BCOR, or STAG2 in AML is greater than 95% specific for the diagnosis of secondary AML 22 suggesting the possibility that future revisions of the WHO classification will continue the trend to move away from morphological descriptors as surrogates of underlying etiology.
However, shortly after the FAB classification was released, flow cytometry was developed and rapidly incorporated into clinical diagnosis, allowing for precise surface marker characterization of acute leukemias at diagnosis and beyond. 9 , 23 Furthermore, it is increasingly becoming evident that morphological and even immunophenotypic assessments cannot accurately reflect the diverse genetic etiology of this class of diseases, 24 , 25 including even clonal heterogeneity within a single patient. 26 - 31 Increasingly sophisticated molecular tools are able to better refine an AML diagnosis based on genetic abnormality, 4 , 14 , 32 with the important caveats that 1) somatic mutations are not typically in themselves disease defining and can be seen in healthy older adults 33 - 35 or patients in prolonged remissions after chemotherapy 36 and that 2) such genomic profiling pre-treatment is currently suboptimal in predicting resistance to induction therapy. 37
2. Use of marrow in response assessment
2.1 utility of the day 14 marrow.
Soon after initial diagnosis with AML, fit patients are treated with intensive induction chemotherapy. The NCCN guidelines for AML recommend performing bone marrow evaluation 7-10 days after completion of induction chemotherapy, which is around day 14 if traditional 7+3 chemotherapy (combining continuous low-dose cytarabine with three days of an anthracycline) is used. 2 If persistent or residual disease is identified (generally >5-10% blasts, though the background marrow cellularity may also be important), the recommendation is to administer more chemotherapy. A study of clinical flow cytometry examined a novel method to calculate the degree of cytoreduction with induction chemotherapy; 38 when combined with knowledge that time to complete remission (CR) is an important prognostic factor, 39 , 40 it seems intuitive that a day 14 marrow would provide important clinical information. Indeed, Liso et al. examined the prognostic value of a day 14 marrow in 198 de novo AML patients in an attempt to derive a predictive tool based on blast percentage. 41 Similarly, a German study evaluated outcomes in 449 patients enrolled in the German AML Cooperative Group 1992 trial, and found that day 16 blasts as a continuous variable were significantly related to rates of CR and persistent disease, as well as to overall and relapse-free survival. 42 It should be noted that patients in this study and similar studies from European groups received a double induction regardless of early marrow status and achievement of CR after first induction course. 42 - 44 Additionally, there is wide variation when assessing blast clearance in the aforementioned and other studies, having a nadir bone marrow blast cut-off ranging from ‘too few to count’ to <22%. Patients who had blast cut-offs below these ranges had higher rates of CR and improved outcomes. However, intra-observer variability remains an important factor when evaluating hypoplastic marrows. 45 More recent studies have questioned the utility of the day 14 marrow, as the marrow results are not always correlated with level of disease and may not reliably predict achievement of CR.
In a retrospective analysis of 194 untreated AML patients, Hussein et al. found that day 14 marrow was highly sensitive in predicting CR (90% sensitivity), but did not predict overall survival. 46 In fact, some patients with a high BM blast percentage at day 14 were still able to achieve CR at day 21 47 or day 28 without re-induction chemotherapy, a finding also seen in other retrospective analyses using morphology and even flow cytometry. 48 - 50 The method of assessment of residual disease at day 14 or later by flow cytometry may also be important, evaluating a leukemia-associated immunophenotype (so-called LAIP) or a “different-fron-normal” phenotype. 12 Further, the treatment algorithm for patients with evidence of disease on a day 14 marrow is not standardized even by practitioners at a single institution, 51 as summarized in a recent review. 52
2.2 Marrow sampling after induction
It is difficult to debate the necessity of an end of treatment bone marrow performed at count recovery after the first cycle of induction chemotherapy. Remission status is formally assessed around day 28-35, as the peripheral blood counts recover from induction chemotherapy. The definition of CR requires peripheral blood count recovery, generally defined as neutrophils > 1000/microliter, platelets > 100,000/microliter, and independence from red blood cell transfusion, along with a concomitant decrease in marrow blasts to <5%. Such criteria were first proposed in 1956 53 and were updated in 2003 54 and 2010, 1 and are expected to be updated again within the next year in the 2017 ELN guidelines. 55 However, despite patients being in CR by morphology, patients often have measurable residual disease (MRD) detectable by more sensitive flow or polymerase chain reaction (PCR)-based assays. Patients with MRD at the end of treatment or prior to transplant have similar outcomes as patients who have bone marrow blasts > 5%. 56 - 59 Prospective randomized studies need to be peformed evaluating patients in CR but with MRD to determine if further treatment improves outcomes (see section 3.2 Measurable Residual Disease below).
2.3 Use of marrow in patients receiving less intensive therapy
Increasingly, less intensive therapies are being used in the management of patients with AML who are considered not to be candidates for induction chemotherapy; these are typically “less fit” newly-diagnosed patients or relapsed/refractory patients who have not responded to conventional chemotherapy. While the ELN response guidelines do not specify required intensity of treatment, remission status is generally determined after one or two cycles of induction chemotherapy. Less intensive therapies, including hypomethylating agents such as azacitidine and decitabine, may take months in order to achieve CR. 60 - 63 The frequency at which bone marrow evaluation should be performed is not clear; the AZA-AML-001 study, in which patients were randomized to azacitidine or a conventional care regimen, specified that peripheral blood and bone marrow aspirate/biopsy would be collected every second cycle beginning at cycle 3, but the authors did not comment on time to best response. 62 A retrospective analysis of patients treated with three cycles of decitabine at MD Anderson suggested patients were more likely to achieve CR if they had a significant (p < 0.05) reduction in the ratio of number of blasts to number of non-blasts in the bone marrow (Estey, unpublished data).
Targeted therapies, whether used alone or combined with intensive chemotherapy, are growing in importance, as investigators seek to exploit the molecular heterogeneity of the disease. 64 FLT3 inhibitors have been under investigation for over a decade, but the multikinase inhibitor midostaurin is the first to show an overall survival benefit when studied in a randomized controlled fashion in combination with 7+3 chemotherapy in newly-diagnosed FLT3 -mutated patients. 65 While midostaurin has primarily been studied in combination with other drugs, the IDH inhibitors used in patients with IDH1 and IDH2 mutations have been used as single agents to date. 66 Anecdotally, these drugs have led to a differentiation syndrome with high numbers of blasts seen both in the circulation and in the marrow after multiple weeks of therapy, a finding which seems to correspond to clinical response. 67 Such phenomena are provocative, and require a reassessment of the standard timing of blood and marrow response assessments for patients receiving these novel targeted drugs. Simultaneously, some might argue that bone marrow assessment may be advantageous in these patients to avoid prolonged, expensive treatment without demonstrable clinical benefit.
3. Peripheral Blood Monitoring
3.1 prognostic value of clearing peripheral blood blasts.
One non-invasive marker to consider using in place of an invasive day 14 marrow would be kinetics of peripheral blood blast clearance in applicable patients. Clearance of leukemic blasts in the periphery has been correlated with day 14 marrow when analyzed by prospective daily flow cytometry in a small group of 30 patients; in 17 of 19 patients who had a decrease in peripheral blasts of > 2 logs by day 6 of therapy with induction chemotherapy, CR was achieved. 68 , 69 In another study, time to blast clearance monitored by manual differentials in 162 AML patients receiving induction chemotherapy showed that early blast clearance (prior to day 6 of treatment) was able to predict for early marrow blast clearance, CR, relapse free survival, and overall survival. 70 Similarly, a retrospective analysis by Elliott et al stratified relapse-free survival in 73 patients with de novo AML who ultimately achieved CR; time of peripheral blast clearance (at or before day 3, on days 4 or 5, and on day 6 or later) was highly significant, with early clearance associated with a relapse rate of 12.5% and late clearance with a much higher relapse rate of 78%. 71 These findings were confirmed by an analysis examining peripheral blood blast clearance by more sensitive multiparametric flow cytometry in 130 AML patients. 72 Mathematical modeling of the peripheral blood blast clearance has been performed by at least two groups to evaluate kinetics, and rapid blast clearance is strong and independent predictor of CR. 72 , 73 Lacombe et al. evaluated the slope of blast cell decrease in each individual patient over the first four days of treatment, and the slope was strongly correlated with the achievement of CR and risk of relapse. 72 Vainstein V et al. examined peripheral blood blast dynamics by modeling an exponential decay curve for 106 patients, and using this methodology calculated an area under the ROC curve of 0.79. 73
A major limitation of examining peripheral blood blasts is that not all patients have circulating blasts at diagnosis. Changes in treatment course in response to rate of peripheral blood blast clearance have not been studied in a prospective fashion. An association between mutation clearance (as measured by next-generation sequencing of bone marrow) and clinical outcome has been reported in a retrospective cohort. 74 It is possible that kinetics of changes measured with high sensitivity tools, such as used for MRD, on peripheral blood samples during induction may provide early information regarding clinical response; a clinical trial is currently underway at the NIH to test this hypothesis ( {"type":"clinical-trial","attrs":{"text":"NCT02527447","term_id":"NCT02527447"}} NCT02527447 ).
3.2 Measurable Residual Disease (MRD)
As discussed above, CR requires peripheral blood count recovery in addition to morphologic remission in the marrow with blasts <5%. Subtypes of CR include CR with incomplete platelet recovery (CRp) and CR with incomplete neutrophil recovery (CRi), and these subtypes have a significantly worse overall prognosis in terms of both response to chemotherapy and survival. 55 , 75 - 77
It is possible to further risk stratify patients in a CR by using high sensitivity techniques to detect biomarkers associated with increased relapse risk. These can include flow cytometry, PCR for gene expression, PCR for abnormal gene sequence, and increasingly next generation sequencing. 12 , 78 - 82 The 2017 ELN guidelines for the diagnosis and treatment of AML that are currently under development will move toward such MRD-based response criteria. 55 That is, the most stringent definition of CR will require no evidence of MRD, as detected by multiparametric flow cytometry (MFC) 83 or molecular techniques where appropriate for individual patients in the bone marrow. This change is made in response to the fact that post-induction factors, particularly MRD status, have a very strong correlation with outcomes after either further chemotherapy or after allogeneic HCT. MRD provides prognostic information independent of type of response to induction chemotherapy, which can be important in future treatment planning for younger and older patients. 77 , 84 - 86 Additionally, presence of MRD is a critical factor in determining outcomes for AML patients following allogeneic HCT, to such a degree that patients with MRD, but morphologic remission, behave similarly poorly to those with active disease at the time of allogeneic HCT. 58 , 87 - 90 These observations have led investigators to suggest that “minimal” in the traditional definition of MRD should be replaced with “measurable,” since any detectable evidence of disease leads to a worse prognosis. The sensitivity of any particular MRD technique used is likely of lesser importance than issues of amount, type, and frequency of sampling; clonal heterogeneity and antigen drift; technical reproducibility; and interpretation and integration of such measurements into clinical care. 79 , 91 The sensitivities of multiple targets assessed by MFC or PCR, which range from 1:100 to 1:200,000, have been comprehensively summarized by Hokland et al. 82
Persistence of cytogenetic abnormalities for those patients in remission after therapy is known to be associated with worse outcomes, 92 , 93 though this technique is not sensitive for the presence of residual disease. A number of more sensitive tools exist to detect MRD, 81 many of which may be used on peripheral blood, lessening the need for invasive bone marrow sampling. No clear superiority of one MRD technology over another in AML has been proven, with typically at least one hundred fold improvement in sensitivity compared with morphology alone, however flow cytometry methods may suffer from greater variability between centers than molecular approaches. 81 PCR-based monitoring of disease in the peripheral blood has been used successfully to monitor for patients with favorable risk AML for translocation(15;17), inversion(16) and translocation(8;21) AML 94 - 97 and more recently somatic mutations such as in NPM1 . 98 The ELN performed extensive testing on expression based MRD using WT1. 99 Despite being expressed in approximately 90% cases of AML, it was overexpressed to a level useful for MRD monitoring in only around 50% of cases. This limitation may be mitigated, in part, by using a multiple gene approach as studied in patients receiving allogeneic HCT 59 , 100 and autologous HCT. 101 Though sampling of peripheral blood every three months is typically used for monitoring, different molecular aberrations may require more frequent testing or even bone marrow sampling, due to distinct differences in the doubling time of abnormal clones. 102 , 103
MRD assessment using flow cytometric techniques has traditionally been done on marrow samples, but there is increasing evidence that peripheral blood can be used to monitor for MRD. A recent study examined the cumulative incidence of relapse and 3-year overall survival for patients with MRD detected by immunophenotyping of the peripheral blood, and found that both differences were significant. Specifically, the cumulative incidence of relapse at 1 year for patients with peripheral blood MRD positivity was 89% vs. 29% (p<0.001). 104 Caveats include that the study included only 114 AML patients with paired bone marrow and peripheral blood samples, primarily at a single center.
Importantly, however, though each of these methods has shown that detection of disease is associated with a worse prognosis, early intervention for MRD-positive patients has not been studied in a systematic manner to demonstrate improvement in outcome when MRD is detected. There is provocative evidence in childhood AML that a risk-stratified approach based on genetic classification and MRD may improve outcomes. 105
In the future, there may also be a role for whole-genome or whole-exome sequencing to follow patients for MRD. In an analysis of comprehensive sequencing data for 50 patients with paired samples from diagnosis and remission, the 24 patients who had persistent leukemia-associated mutations had significantly worse survival. 74 Though the cost of large-scale sequencing has decreased considerably in recent years, concerns still remain about the interpretation and utility of the large amount of data generated for each individual AML patient. Additionally, the sensitivity and specificity of MRD on outcomes, however the MRD is detected, are such that it can be difficult to counsel individual patients about treatment planning; indeed, though a patient with MRD after induction chemotherapy may be more likely to relapse after allogeneic HCT than one without MRD, that same patient may be more likely to benefit from a graft-versus-leukemia effect than from more cytotoxic chemotherapy.
The optimal frequency of monitoring for the development of MRD is unknown at this time, and likely depends on the specific type of mutations that are identified, as discussed above, because of differences in both test sensitivity and leukemic clone doubling time. 98 , 102 , 103 , 106 Whether early intervention will be beneficial for relapsed disease is also unknown; for example, an older study suggested that routine bone marrow examination was not beneficial during first CR, 107 and it remains to be proven that early detection of MRD leads to improved survival outcomes. Given the current limitations of the technology, our practice is not to make clinical decisions on the basis of a single MRD result; our viewpoints regarding necessary times for bone marrow evaluation are summarized in Table 1 .
The authors' viewpoint on necessity of bone marrow evaluation at standard times during the course of AML diagnosis therapy.
Abbreviations: AML (acute myeloid leukemia); MRD (measureable residual disease).
4. Novel approaches to track disease burden
Bone marrow biopsies at diagnosis and count recovery time-points are currently still the “gold standard.” With the improvement of peripheral blood monitoring techniques, in combination with better imaging modalities, it may be possible to create a new standard for evaluating response to treatment. There are limited studies exploring imaging as a prognostic and predictive indicator of response and survival, likely related to cost and time needed to complete these studies. While provocative, these radiologic studies are not yet ready for incorporation into routine clinical practice.
4.1 FDG PET
Positron emission tomography (PET) is a functional imaging technique used to evaluate metabolic processes. In fludeoxyglucose (FDG) PET, a biologically active analogue of glucose is used as a tracer and is very sensitive at measuring glucose uptake as a function of metabolic activity. However, FDG PET is not specific for distinguishing inflammation secondary to tumor versus infection in the majority of cases. Interestingly, FDG PET has shown efficacy in visualizing extramedullary disease (EMD). In a small study of 10 patients, FDG PET was able to detect known EMD in 90% of patients and additional EMD in 60% with an SUV max range 2.1-8.1. 108 Cribe et al. evaluated 26 patients with newly diagnosed AML in which FDG PET found 65% of patients to have EMD compared to 31% by clinical exam. There was a high degree of concordance with bone marrow response and FDG PET response at the end of treatment, with 4 of 6 patients achieving a PR on FDG PET but CR on bone marrow biopsy experiencing an early relapse. 109 The utility of FDG PET is unknown, but given the sensitivity of the imaging, FDG PET may be useful as an adjunct at diagnosis for patients with EMD AML to determine extent of disease, and at the end of treatment to document response. 110
4.2 18 F-FLT PET
18 F-FLT PET may be more suitable for the evaluation of AML patients given that 3′-deoxy-3′- 18 F-fluorothymidine (FLT) is a thymidine analog that is resistant to in vivo degradation and accumulates in proliferating tissues, including rapidly dividing hematopoietic stem cells in the bone marrow. 111 The first demonstration of FLT PET in AML patients showed higher rates of biodistribution in the bone marrow, spleen and EMD compared to normal healthy controls. 112 In a pilot study of eight patients, 18 F-FLT PET was used as an early assessment of treatment response. Eight newly diagnosed AML patients were treated with induction chemotherapy and completed 18 F-FLT PET during therapy (range from 2-6 days from start of treatment). Patients with a CR showed SUV uptake < 2 while patients with resistant disease (RD) displayed SUV > 2. SUV mean and SUV max were also significantly lower in patients with CR compared to RD and normal controls had SUVs similar to patients in CR. 113 While the numbers in this study are too small to generalize to larger populations, it addresses an interesting question of using imaging as an early assessment tool of response. For patients who are not responding, it may be worth changing therapy early to avoid unnecessary toxicity from an unsuccessful regimen. Taken in combination with peripheral blood monitoring, there is potential to predict response minimizing the need for an invasive testing. Currently, ECOG-ACRIN Cancer Research Group is conducting a phase 2 study of FLT PET/CT at the time of the nadir bone marrow (days 10-17) in newly diagnosed AML patients being treated with standard induction chemotherapy ( {"type":"clinical-trial","attrs":{"text":"NCT02392429","term_id":"NCT02392429"}} NCT02392429 ).
4.3 DCE-MRI
Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) provides global and functional imaging of bone marrow angiogenesis as compared to traditional MRI which uses radio waves in a magnetic field to identify anatomy. In the studies conducted, DCE-MRI has been examined as a predictor for overall survival using a calculated peak enhancement ratio (Peak) and quantification of vascularity (Amp). In 78 de novo AML patients, those with a low Peak and Amp at diagnosis had an improved disease free survival and overall survival compared to patients with a high Peak and Amp. 114 Another study by the same group looked at DCE-MRI at day 0 and day 7 of chemotherapy and found that patients with a decrease in Peak values (decrease in angiogenesis compared to baseline) had a higher chance of achieving CR and longer disease free survival compared to patients that had an increase in Peak values (increase in angiogenesis compared to baseline). 115 Similar to the results seen with FLT PET, imaging modalities have the potential to strengthen our current testing methods for response assessment.
Future imaging studies have the potential to answer some important outstanding questions before imaging technology can be incorporated into standard assessments for AML monitoring: 1) Which imaging technique most accurately reflects the total burden of disease? 2) When is the ideal time, during or after treatment, for imaging to take place? 3) Can imaging accurately predict which patients will go into a complete remission and measure depth of response? and 4) What is the role of imaging in surveillance?
5. Future directions
Bone marrow evaluation, therefore, remains an important adjunct to peripheral blood analysis in patients with AML, and perhaps always will since some patients do not have circulating peripheral blood blasts. However, we feel that some “standard” tests such as the day 14 marrow are of questionable importance in the management of AML patients and should not be incorporated into routine clinical practice. The recently published WHO guidelines for the diagnosis of AML still espouse morphology as the most important characteristic for the diagnosis and management of myeloid neoplasms, 3 though more modern techniques may threaten the hegemony of morphology, as the biology underlying this diverse set of malignancies is better elucidated. While it is likely that highly sensitive tools will be increasingly used on peripheral blood for response assessment and to monitor for clinical relapse, at present, sampling of the bone marrow compartment remains an important component of initial AML diagnosis and at the end of induction treatment in the majority of patients. Figure 1 summarizes the current recommendations for bone marrow evaluation, as well as areas for possible future modifications to the algorithm as methods for diagnosis and monitoring of AML are refined.

The possible future landscape for diagnosis and monitoring of acute myeloid leukemia at various time points during treatment and subsequent surveillance. The current schema follows the guidelines of the National Comprehensive Cancer Network and others. In the future, we posit that advances in flow cytometry and sequencing (and possibly imaging) may circumvent our current reliance on morphology and cytogenetics. Though the current sensitivity of bone marrow testing is generally 10-fold higher than in the peripheral blood, many tests may be done on peripheral blood only in the future. Timing of surveillance monitoring for measurable residual disease on the peripheral blood will likely depend on the abnormalities being followed for a particular patient.
In an aging population experiencing an increased incidence in AML (de novo, progression from MDS, and treatment related), it is imperative that we consider the patient's ability, willingness, and pain threshold in continuing to do bone marrows. Table 2 summarizes the pros and cons of peripheral blood versus bone marrow sampling for diagnosis and monitoring of AML. Notably, clinical trials often include patients with the best performance status, and the findings generated by such patients may not hold true for the general population. 116 , 117 Work on alternatives to bone marrow examination in AML will continue, primarily using sensitive assays on the peripheral blood and newer imaging technologies, but for now bone marrow evaluation remains an important diagnostic tool in the care of AML patients.
Pros and cons of sampling from the peripheral blood and bone marrow in AML.
Abbreviations: AML (acute myeloid leukemia); MRD (measurable residual disease)
6. Practice points
- Morphologic diagnosis of AML, immunophenotyping, and karyotype can all be performed on a peripheral blood sample if the absolute blast count is > 2,000/μl.
- The day 14 marrow is of questionable clinical utility, since it is predictive of CR rates, but not of OS.
- Marrow sampling at count recovery after induction chemotherapy is critical for response assessment; patients with a morphologic CR but evidence of MRD have worse outcomes
7. Research agenda
- The 2017 ELN guidelines for AML will include a response category of so-called “stringent” CR (i.e., CR without MRD); meanwhile, new techniques are being developed for monitoring MRD, including next-generation sequencing and array-based approaches
- The recommended frequency of marrow sampling for less intensive therapies, such as with targeted inhibitors, is unclear
- Novel imaging technology, using both PET and MRI, may have a role in future monitoring of AML patients, though many questions still remain about the predictive ability, utility, and cost
Acknowledgments
Funding : This work was supported in part by the Intramural Research Program of the National Heart, Lung, and Blood Institute of the National Institutes of Health.
Conflict of interest : C.S.H. receives research funding from SELLAS Life Sciences Group AG.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.

IMAGES
VIDEO
COMMENTS
A phase 3 study of gemtuzumab ozogamicin during induction and postconsolidation therapy in younger patients with acute myeloid leukemia. Blood. 2013; 121:4854-4860. Blood. 2013; 121:4854-4860. Citations
Relapsed acute myeloid leukemia. Discussion of Management. Dr. Andrew M. Brunner: This 49-year-old man had a subtype of AML that had arisen de novo, with a normal male karyotype and mutations in ...
Case Study: New Therapies for Acute Myeloid Leukemia. A 76-year-old woman presents to the emergency department following two weeks of progressive dyspnea and fatigue, and a new rash. Her medical history is significant for stage 2 chronic kidney disease, coronary artery disease, and diabetes. Physical examination results are within normal limits ...
Discussion. The incidence of acute leukemia is approximately 2.3 per 100000 people per year.AML M-7 is a rare subtype of leukemia and represents 1.2% of cases of adult leukemia, compared to 3-10% of childhood leukemia ( 10 ).It is classified under M-7 in the French-American-British classification ( 11 ). The patient was a 26 month years old ...
1 Introduction. Acute myeloid leukemia (AML) is a malignantly clonal disorder characterized by blockage of differentiation in the myeloid lineage and an accumulation of its immature progenitors in bone marrow, leading to hematopoietic failure. In China, it was predicted that there were about 75,300 newly diagnosed leukemia cases in 2015; meanwhile, it was estimated that about 53,400 Chinese ...
Presentation of Case. Dr. Richard A. Newcomb: A 49-year-old man was evaluated at this hospital because of relapsed acute myeloid leukemia (AML). Fourteen months before the current presentation ...
Acute myeloid leukemia developed in a woman approximately 5.5 years after she had received LentiGlobin for sickle cell disease as part of the initial cohort (Group A) of the HGB-206 study. An ...
Patients with primary cancer receiving chemotherapy and/or radiotherapy may develop therapy-related acute leukemia (t-AL). Therapy-related acute myeloid leukemia (t-AML) accounts for the majority of these cases and is frequently associated with a variety of cytogenetic and molecular abnormalities. The aim of the present study was to explore the ...
Acute myeloid leukemia is a hematologic disorder resulting from chromosomal translocations and rearrangements resulting in the uncontrolled proliferation of myeloid blast cells and impaired production ... Case Study Continued. The hematology-oncology team was notified for continued management of Thomas' acute leukemia, and the medical ...
Leukemia (2024) Progress in the understanding of the biology and therapy of acute myeloid leukemia (AML) is occurring rapidly. Since 2017, nine agents have been approved for various indications in ...
The patient then evolved to therapy-related acute myeloid leukemia in fall 2019 after the bone marrow blast count passed 20%. Therapy-related acute myeloid leukemia is thought to make up 5%-20% of all myelodysplastic syndrome/acute myeloid leukemia (AML) cases according to the National Comprehensive Cancer Network Acute Leukemia guidelines.
Until recently, treatment options for acute myeloid leukemia (AML) were relatively limited, and decision making followed an algorithm in place for almost 50 years. 1,2 For medically fit patients, cure was assumed possible with intensive chemotherapy and possibly allogeneic hematopoietic cell transplantation (HCT). Because of transfusion needs and risks of disease/treatment-related ...
Rare and emerging subtypes of leukemia can be incredibly challenging to diagnose and even more challenging to treat. At the NCCN 2019 Annual Congress: Hematologic Malignancies, a panel of experts, moderated by Andrew D. Zelenetz, MD, PhD, were presented with particularly challenging cases in these malignancies and asked to discuss best approaches to treatment.
The "How I Treat" series, featured in Blood, highlights expert perspectives on the diagnosis and treatment of patients using sample patient cases, and the AML Hub has previously covered topics in these series on the use of new therapeutics and updated classification systems.Although there have been some major advancements in the treatment of patients with acute myeloid leukemia (AML) in ...
Progress in acute myeloid leukaemia treatment is occurring at an unprecedented pace. The past decade has witnessed an increasingly improved scientific understanding of the underlying biology of acute myeloid leukaemia, leading to enhanced prognostication tools and refined risk assessments, and most especially incorporating measurable residual disease (MRD) into longitudinal risk assessments.
December 23, 2019. Video. Naval G. Daver, MD: This is the case of a 64-year-old patient with newly diagnosed acute myeloid leukemia. The patient has significant comorbidities, including an elevated BMI [body mass index], signifying a significant obesity, as well as underlying pneumonia and prior history of high blood pressure and diabetes.
Among patients over 65 who received an allogeneic hematopoietic stem cell transplant (allo-HCT) for the treatment of acute myeloid leukemia (AML) between 2000 and 2021, leukemia-free and overall survival improved significantly over time, according to results from a study published in Clinical Cancer Research, a journal of the American Association for Cancer Research (AACR).
Acute myeloid leukemia (AML) is a type of blood cancer that specifically targets myeloid stem cells and its differentiation process. ... Myeloblasts are immature WBC and, in the case of AML, ... Monalizumab also generated promising therapeutic effects in pre-clinical research studies in AML models by promoting NK cell-mediated killing of ...
Abstract. Acute myeloid leukaemia (AML) is a cancer of the myeloid line of blood cells, characterized by the rapid growth of abnormal cells that build up in the bone marrow and blood and interfere ...
Acute myeloid leukemia (AML) with T lymphoblastic lymphoma (T-LBL) is a hematologic tumor of two origins, myeloid and lymphoblastic, and is relatively rare in the same patient. ... (allo-HSCT) may be the only option to cure this disease. Considering that the present study was a case report without a control group, our conclusions would be more ...
Study design, ethics statement, and patient population. The use of AML samples in the present study was approved by the MD Anderson Cancer Center (MDACC) Investigational Review Board (IRB ...
Acute myeloid leukemia (AML) ... The inclusion criteria were as follows (1): the study design encompassed cohort or case-control, and randomized controlled trial (RCT) (2);adult patients with AML were involved in this study (3); study comparing the combination of VEN with chemo to chemo alone; and (4) the study reported outcome metrics, such as ...
Introduction. Acute promyelocytic leukemia with t (15;17) is a cancerous disease of the white blood cells in the bone marrow and peripheral blood. It is a subtype of the acute myelogenous leukemia (AML), and is commonly seen in adults. An adolescent male was admitted by referral to a large hospital with symptoms of thrombocytopenia and ...
Acute myeloid leukemia (AML) is the most common leukemia among the adult population and accounts for about 80% of all cases. It is characterized by clonal expansion of immature "blast cells" in the peripheral blood and bone marrow resulting in ineffective erythropoiesis and bone marrow failure. With recent advancements in the management guidelines, the cure rates have increased up to 15% ...
Pseudo-Chediak-Higashi granules are large cytoplasmic inclusions commonly encountered in myeloblasts or other myeloid precursors in acute myeloid leukemia and myelodysplastic syndromes. ... pseudo-Chediak-Higashi granules are rarely found in acute lymphoblastic leukemia (ALL). We present the case of an 8-year-old boy who was diagnosed with ALL ...
The FDA granted priority review to the NDA for revumenib in adult and pediatric R/R KMT2A-rearranged acute leukemia. 1. Revumenib is a first-in-class Menin inhibitor. The NDA filing for the agent is currently being reviewed under the FDA's real-time oncology review program, which allows for a more efficient review and engagement between the sponsor and the FDA throughout the submission process.
MEI Pharma, Inc. (Nasdaq: MEIP), a clinical-stage pharmaceutical company evaluating novel drug candidates to address known resistance mechanisms to standard-of-care cancer therapies, today ...
The diagnosis of acute myeloid leukemia (AML) can be made based on peripheral blood or bone marrow blasts. In this review, we will discuss the role of bone marrow evaluation and peripheral blood monitoring in the diagnosis, management, and follow up of AML patients. For patients with circulating blasts, it is reasonable to perform the necessary ...