McCombs School of Business
- Español ( Spanish )
Videos Concepts Unwrapped View All 36 short illustrated videos explain behavioral ethics concepts and basic ethics principles. Concepts Unwrapped: Sports Edition View All 10 short videos introduce athletes to behavioral ethics concepts. Ethics Defined (Glossary) View All 58 animated videos - 1 to 2 minutes each - define key ethics terms and concepts. Ethics in Focus View All One-of-a-kind videos highlight the ethical aspects of current and historical subjects. Giving Voice To Values View All Eight short videos present the 7 principles of values-driven leadership from Gentile's Giving Voice to Values. In It To Win View All A documentary and six short videos reveal the behavioral ethics biases in super-lobbyist Jack Abramoff's story. Scandals Illustrated View All 30 videos - one minute each - introduce newsworthy scandals with ethical insights and case studies. Video Series
Case Studies UT Star Icon

Case Studies
More than 70 cases pair ethics concepts with real world situations. From journalism, performing arts, and scientific research to sports, law, and business, these case studies explore current and historic ethical dilemmas, their motivating biases, and their consequences. Each case includes discussion questions, related videos, and a bibliography.

A Million Little Pieces
James Frey’s popular memoir stirred controversy and media attention after it was revealed to contain numerous exaggerations and fabrications.

Abramoff: Lobbying Congress
Super-lobbyist Abramoff was caught in a scheme to lobby against his own clients. Was a corrupt individual or a corrupt system – or both – to blame?

Apple Suppliers & Labor Practices
Is tech company Apple, Inc. ethically obligated to oversee the questionable working conditions of other companies further down their supply chain?

Approaching the Presidency: Roosevelt & Taft
Some presidents view their responsibilities in strictly legal terms, others according to duty. Roosevelt and Taft took two extreme approaches.

Appropriating “Hope”
Fairey’s portrait of Barack Obama raised debate over the extent to which an artist can use and modify another’s artistic work, yet still call it one’s own.

Arctic Offshore Drilling
Competing groups frame the debate over oil drilling off Alaska’s coast in varying ways depending on their environmental and economic interests.

Banning Burkas: Freedom or Discrimination?
The French law banning women from wearing burkas in public sparked debate about discrimination and freedom of religion.

Birthing Vaccine Skepticism
Wakefield published an article riddled with inaccuracies and conflicts of interest that created significant vaccine hesitancy regarding the MMR vaccine.

Blurred Lines of Copyright
Marvin Gaye’s Estate won a lawsuit against Robin Thicke and Pharrell Williams for the hit song “Blurred Lines,” which had a similar feel to one of his songs.

Bullfighting: Art or Not?
Bullfighting has been a prominent cultural and artistic event for centuries, but in recent decades it has faced increasing criticism for animal rights’ abuse.

Buying Green: Consumer Behavior
Do purchasing green products, such as organic foods and electric cars, give consumers the moral license to indulge in unethical behavior?

Cadavers in Car Safety Research
Engineers at Heidelberg University insist that the use of human cadavers in car safety research is ethical because their research can save lives.

Cardinals’ Computer Hacking
St. Louis Cardinals scouting director Chris Correa hacked into the Houston Astros’ webmail system, leading to legal repercussions and a lifetime ban from MLB.

Cheating: Atlanta’s School Scandal
Teachers and administrators at Parks Middle School adjust struggling students’ test scores in an effort to save their school from closure.

Cheating: Sign-Stealing in MLB
The Houston Astros’ sign-stealing scheme rocked the baseball world, leading to a game-changing MLB investigation and fallout.

Cheating: UNC’s Academic Fraud
UNC’s academic fraud scandal uncovered an 18-year scheme of unchecked coursework and fraudulent classes that enabled student-athletes to play sports.
Cheney v. U.S. District Court
A controversial case focuses on Justice Scalia’s personal friendship with Vice President Cheney and the possible conflict of interest it poses to the case.

Christina Fallin: “Appropriate Culturation?”
After Fallin posted a picture of herself wearing a Plain’s headdress on social media, uproar emerged over cultural appropriation and Fallin’s intentions.

Climate Change & the Paris Deal
While climate change poses many abstract problems, the actions (or inactions) of today’s populations will have tangible effects on future generations.

Cover-Up on Campus
While the Baylor University football team was winning on the field, university officials failed to take action when allegations of sexual assault by student athletes emerged.

Covering Female Athletes
Sports Illustrated stirs controversy when their cover photo of an Olympic skier seems to focus more on her physical appearance than her athletic abilities.

Covering Yourself? Journalists and the Bowl Championship
Can news outlets covering the Bowl Championship Series fairly report sports news if their own polls were used to create the news?

Cyber Harassment
After a student defames a middle school teacher on social media, the teacher confronts the student in class and posts a video of the confrontation online.

Defending Freedom of Tweets?
Running back Rashard Mendenhall receives backlash from fans after criticizing the celebration of the assassination of Osama Bin Laden in a tweet.

Dennis Kozlowski: Living Large
Dennis Kozlowski was an effective leader for Tyco in his first few years as CEO, but eventually faced criminal charges over his use of company assets.

Digital Downloads
File-sharing program Napster sparked debate over the legal and ethical dimensions of downloading unauthorized copies of copyrighted music.

Dr. V’s Magical Putter
Journalist Caleb Hannan outed Dr. V as a trans woman, sparking debate over the ethics of Hannan’s reporting, as well its role in Dr. V’s suicide.

East Germany’s Doping Machine
From 1968 to the late 1980s, East Germany (GDR) doped some 9,000 athletes to gain success in international athletic competitions despite being aware of the unfortunate side effects.
Ebola & American Intervention
Did the dispatch of U.S. military units to Liberia to aid in humanitarian relief during the Ebola epidemic help or hinder the process?

Edward Snowden: Traitor or Hero?
Was Edward Snowden’s release of confidential government documents ethically justifiable?

Ethical Pitfalls in Action
Why do good people do bad things? Behavioral ethics is the science of moral decision-making, which explores why and how people make the ethical (and unethical) decisions that they do.
Ethical Use of Home DNA Testing
The rising popularity of at-home DNA testing kits raises questions about privacy and consumer rights.

Flying the Confederate Flag
A heated debate ensues over whether or not the Confederate flag should be removed from the South Carolina State House grounds.

Freedom of Speech on Campus
In the wake of racially motivated offenses, student protests sparked debate over the roles of free speech, deliberation, and tolerance on campus.

Freedom vs. Duty in Clinical Social Work
What should social workers do when their personal values come in conflict with the clients they are meant to serve?

Full Disclosure: Manipulating Donors
When an intern witnesses a donor making a large gift to a non-profit organization under misleading circumstances, she struggles with what to do.

Gaming the System: The VA Scandal
The Veterans Administration’s incentives were meant to spur more efficient and productive healthcare, but not all administrators complied as intended.

German Police Battalion 101
During the Holocaust, ordinary Germans became willing killers even though they could have opted out from murdering their Jewish neighbors.
Head Injuries & American Football
Many studies have linked traumatic brain injuries and related conditions to American football, creating controversy around the safety of the sport.
Head Injuries & the NFL
American football is a rough and dangerous game and its impact on the players’ brain health has sparked a hotly contested debate.
Healthcare Obligations: Personal vs. Institutional
A medical doctor must make a difficult decision when informing patients of the effectiveness of flu shots while upholding institutional recommendations.

High Stakes Testing
In the wake of the No Child Left Behind Act, parents, teachers, and school administrators take different positions on how to assess student achievement.

In-FUR-mercials: Advertising & Adoption
When the Lied Animal Shelter faces a spike in animal intake, an advertising agency uses its moral imagination to increase pet adoptions.

Krogh & the Watergate Scandal
Egil Krogh was a young lawyer working for the Nixon Administration whose ethics faded from view when asked to play a part in the Watergate break-in.

Limbaugh on Drug Addiction
Radio talk show host Rush Limbaugh argued that drug abuse was a choice, not a disease. He later became addicted to painkillers.

U.S. Olympic swimmer Ryan Lochte’s “over-exaggeration” of an incident at the 2016 Rio Olympics led to very real consequences.

Meet Me at Starbucks
Two black men were arrested after an employee called the police on them, prompting Starbucks to implement “racial-bias” training across all its stores.

Myanmar Amber
Buying amber could potentially fund an ethnic civil war, but refraining allows collectors to acquire important specimens that could be used for research.

Negotiating Bankruptcy
Bankruptcy lawyer Gellene successfully represented a mining company during a major reorganization, but failed to disclose potential conflicts of interest.

Pao & Gender Bias
Ellen Pao stirred debate in the venture capital and tech industries when she filed a lawsuit against her employer on grounds of gender discrimination.

Pardoning Nixon
One month after Richard Nixon resigned from the presidency, Gerald Ford made the controversial decision to issue Nixon a full pardon.

Patient Autonomy & Informed Consent
Nursing staff and family members struggle with informed consent when taking care of a patient who has been deemed legally incompetent.
Prenatal Diagnosis & Parental Choice
Debate has emerged over the ethics of prenatal diagnosis and reproductive freedom in instances where testing has revealed genetic abnormalities.

Reporting on Robin Williams
After Robin Williams took his own life, news media covered the story in great detail, leading many to argue that such reporting violated the family’s privacy.

Responding to Child Migration
An influx of children migrants posed logistical and ethical dilemmas for U.S. authorities while intensifying ongoing debate about immigration.
Retracting Research: The Case of Chandok v. Klessig
A researcher makes the difficult decision to retract a published, peer-reviewed article after the original research results cannot be reproduced.
Sacking Social Media in College Sports
In the wake of questionable social media use by college athletes, the head coach at University of South Carolina bans his players from using Twitter.

Selling Enron
Following the deregulation of electricity markets in California, private energy company Enron profited greatly, but at a dire cost.

Snyder v. Phelps
Freedom of speech was put on trial in a case involving the Westboro Baptist Church and their protesting at the funeral of U.S. Marine Matthew Snyder.

Something Fishy at the Paralympics
Rampant cheating has plagued the Paralympics over the years, compromising the credibility and sportsmanship of Paralympian athletes.

Sports Blogs: The Wild West of Sports Journalism?
Deadspin pays an anonymous source for information related to NFL star Brett Favre, sparking debate over the ethics of “checkbook journalism.”

Stangl & the Holocaust
Franz Stangl was the most effective Nazi administrator in Poland, killing nearly one million Jews at Treblinka, but he claimed he was simply following orders.

Teaching Blackface: A Lesson on Stereotypes
A teacher was put on leave for showing a blackface video during a lesson on racial segregation, sparking discussion over how to teach about stereotypes.

The Astros’ Sign-Stealing Scandal
The Houston Astros rode a wave of success, culminating in a World Series win, but it all came crashing down when their sign-stealing scheme was revealed.

The Central Park Five
Despite the indisputable and overwhelming evidence of the innocence of the Central Park Five, some involved in the case refuse to believe it.

The CIA Leak
Legal and political fallout follows from the leak of classified information that led to the identification of CIA agent Valerie Plame.

The Collapse of Barings Bank
When faced with growing losses, investment banker Nick Leeson took big risks in an attempt to get out from under the losses. He lost.

The Costco Model
How can companies promote positive treatment of employees and benefit from leading with the best practices? Costco offers a model.

The FBI & Apple Security vs. Privacy
How can tech companies and government organizations strike a balance between maintaining national security and protecting user privacy?

The Miss Saigon Controversy
When a white actor was cast for the half-French, half-Vietnamese character in the Broadway production of Miss Saigon , debate ensued.

The Sandusky Scandal
Following the conviction of assistant coach Jerry Sandusky for sexual abuse, debate continues on how much university officials and head coach Joe Paterno knew of the crimes.

The Varsity Blues Scandal
A college admissions prep advisor told wealthy parents that while there were front doors into universities and back doors, he had created a side door that was worth exploring.

Providing radiation therapy to cancer patients, Therac-25 had malfunctions that resulted in 6 deaths. Who is accountable when technology causes harm?

Welfare Reform
The Welfare Reform Act changed how welfare operated, intensifying debate over the government’s role in supporting the poor through direct aid.

Wells Fargo and Moral Emotions
In a settlement with regulators, Wells Fargo Bank admitted that it had created as many as two million accounts for customers without their permission.
Stay Informed
Support our work.

Site Search
- How to Search
- Advisory Group
- Editorial Board
- OEC Fellows
- History and Funding
- Using OEC Materials
- Collections
- Research Ethics Resources
- Ethics Projects
- Communities of Practice
- Get Involved
- Submit Content
- Open Access Membership
- Become a Partner
Discussion Tools: Case Studies
Instructional tools that promote active, participatory learning are widely recognized as the most effective way to engage trainees, convey knowledge, develop skills, and change attitudes.
What is Research Ethics
Why Teach Research Ethics
Animal Subjects
Biosecurity
Collaboration
Conflicts of Interest
Data Management
Human Subjects
Peer Review
Publication
Research Misconduct
Social Responsibility
Stem Cell Research
Whistleblowing
Descriptions of educational settings , including in the classroom, and in research contexts.
Case Studies
Other Discussion Tools
Information about the history and authors of the Resources for Research Ethics Collection
Case studies are a tool for discussing scientific integrity. Although one of the most frequently used tools for encouraging discussion, cases are only one of many possible tools. Many of the principles discussed below for discussing case studies can be generalized to other approaches to encouraging discussion about research ethics. Cases are designed to confront readers with specific real-life problems that do not lend themselves to easy answers. Case discussion demands critical and analytical skills and, when implemented in small groups, also fosters collaboration (Pimple, 2002). By providing a focus for discussion, cases help trainees to define or refine their own standards, to appreciate alternative approaches to identifying and resolving ethical problems, and to develop skills for analyzing and dealing with hard problems on their own. The effective use of case studies is comprised of many factors, including:
- appropriate selection of case(s) (topic, relevance, length, complexity)
- method of case presentation (verbal, printed, before or during discussion)
- format for case discussion (Email or Internet-based, small group, large group)
- leadership of case discussion (choice of discussion leader, roles and responsibilities for discussion leader)
- outcomes for case discussion (answers to specific questions, answers to general questions, written or verbal summaries)
It should be noted that ethical decision-making is a process rather than a specific correct answer. In this sense, unethical behavior is defined by a failure to engage in the process of ethical decision-making. It is always unacceptable to have made no reasonable attempt to define a consistent and defensible basis for conduct.
Leading Case Discussions
For the sake of time and clarity of purpose, it is essential that one individual have responsibility for leading the group discussion. As a minimum, this responsibility should include:
- Reading the case aloud.
- Defining, and re-defining as needed, the questions to be answered.
- Encouraging discussion that is "on topic".
- Discouraging discussion that is "off topic".
- Keeping the pace of discussion appropriate to the time available.
- Eliciting contributions from all members of the discussion group.
- Summarizing both majority and minority opinions at the end of the discussion.
How should cases be analyzed?
Many of the skills necessary to analyze case studies can become tools for responding to real world problems. Cases, like the real world, contain uncertainties and ambiguities. Readers are encouraged to identify key issues, make assumptions as needed, and articulate options for resolution. In addition to the specific questions accompanying each case, readers might consider the following questions:
- Who are the affected parties (individuals, institutions, a field, society) in this situation?
- What interest(s) (material, financial, ethical, other) does each party have in the situation? Which interests are in conflict?
- Were the actions taken by each of the affected parties acceptable (ethical, legal, moral, or common sense)? If not, are there circumstances under which those actions would have been acceptable? Who should impose what sanction(s)?
- What other courses of action are open to each of the affected parties? What is the likely outcome of each course of action?
- For each party involved, what course of action would you take, and why?
- What actions could have been taken to avoid the conflict?
If consensus is not possible, then written or oral summaries should reflect majority and minority opinions.
Is there a right answer?
ACCEPTABLE SOLUTIONS: Most problems will have several acceptable solutions or answers, but it will not always be the case that a perfect solution can be found. At times, even the best solution will still have some unsatisfactory consequences. UNACCEPTABLE SOLUTIONS: While more than one acceptable solution may be possible, not all solutions are acceptable. For example, obvious violations of specific rules and regulations or of generally accepted standards of conduct would typically be unacceptable. However, it is also plausible that blind adherence to accepted rules or standards would sometimes be an unacceptable course of action.
- Bebeau MJ with Pimple KD, Muskavitch KMT, Borden SL, Smith DH (1995): Moral Reasoning in Scientific Research: Cases for Teaching and Assessment . Indiana University.
- Elliott D, Stern JE (1997): Research Ethics - A Reader. University Press of New England, Hanover, NH.
- OEC Resources: Cases
- Ellison, Karin and Karin Wellner. (2013) Research, Ethics, and Society Cases: Discussion Guide , Online Ethics Center.
- The Case Method , Center for Innovation in Teaching & Learning, University of Illinois at Urbana-Champaign
- Herreid CF: National Center for Case Study Teaching in Science, State University of New York at Buffalo. This comprehensive site offers methodology, a case study collection, case study teachers, workshops, and links to additional resources. https://web.archive.org/web/20071006070923/http://ublib.buffalo.edu/libraries/projects/cases/case.html
- Korenman SG, Shipp AC (1994): Teaching the Responsible Conduct of Research through a Case Study Approach: A Handbook for Instructors. Association of American Medical Colleges, Washington, DC.
- Macrina FL (2005): Scientific Integrity: An Introductory Text with Cases. 3rd edition, American Society for Microbiology Press, Washington, DC.
- National Academy of Sciences (2009): On Being a Scientist: Responsible Conduct in Research . 3rd Edition. Publication from the Committee on Science, Engineering, and Public Policy, National Academy of Sciences, National Academy of Engineering, and Institute of Medicine. National Academy Press, Washington DC.
- Penslar RL, ed. (1995): Research Ethics: Cases and Materials. Indiana University Press, Bloomington, IN.
- Pimple, KD (2002): Using Case Studies in Teaching Research Ethics
- Pimple KD (2002): Using Small Group Assignments in Teaching Research Ethics
- Schrag B, ed. (1996-2007): Graduate Research Ethics: Cases and Commentaries , Volumes 1-7, Association for Practical and Professional Ethics, Bloomington, Indiana.
The Resources for Research Ethics Education site was originally developed and maintained by Dr. Michael Kalichman, Director of the Research Ethics Program at the University of California San Diego. The site was transferred to the Online Ethics Center in 2021 with the permission of the author.
Related Resources
Submit Content to the OEC Donate

This material is based upon work supported by the National Science Foundation under Award No. 2055332. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Publication ethics: Role and responsibility of authors
- POSTGRADUATE CORNER: RESEARCH TECHNIQUES
- Published: 22 January 2021
- Volume 40 , pages 65–71, ( 2021 )
Cite this article
- Shubha Singhal 1 &
- Bhupinder Singh Kalra ORCID: orcid.org/0000-0003-4217-5530 1
36k Accesses
24 Citations
23 Altmetric
Explore all metrics
Publication of scientific paper is critical for modern science evolution, and professional advancement. However, it comes with many responsibilities. An author must be aware of good publication practices. While refraining from scientific misconduct or research frauds, authors should adhere to Good Publication Practices (GPP). Publications which draw conclusions from manipulated or fabricated data could prove detrimental to society and health care research. Good science can blossom only when research is conducted and documented with complete honesty and ethics. Unfortunately, publish or perish attitude has led to unethical practices in scientific research and publications. There is need to identify, acknowledge, and generate awareness among junior researchers or postgraduate students to curb scientific misconduct and adopt GPP. This article discusses various unethical publication practices in research. Also, the role and responsibilities of authors have been discussed with the purpose of maintaining the credibility and objectivity of publication.
Similar content being viewed by others

Publication Ethics
Kirtisudha Mishra & Aashima Dabas

Scientific Electronic Library Online/SciELO: Good Practices Guide for the Enhancement of Ethics in Scientific Publication (Version 09/2018)

Peer Review in Scholarly Journal Publishing
Avoid common mistakes on your manuscript.
Introduction
Need to publish.
A scientific paper is an organized description of hypothesis, data, and conclusions, intended to instruct the readers. Research conducted has to be published or documented; otherwise, it is considered not done. Publication of paper is critical for the evolution of modern science, in which the work of one scientist builds upon that of others [ 1 ]. The roots of scholarly, scientific publishing can be traced to 1665, when Henry Oldenburg of the British Royal Society established the journal Philosophical Transactions of the Royal Society . The aim of the journal was to create a public record of original contribution to knowledge and also to encourage scientists to “speak” directly to others [ 2 ]. Documentation of research work followed by publication helps in the dissemination of observations and findings. This flow of knowledge guides and contributes towards research coalition. Established and budding researchers do get benefited by published literature and consolidates their research.
Publication of research in peer-reviewed journal not only validates the research and boosts confidence of the authors but also gives national and international recognition to an author, department, university, and institution [ 3 ]. Unfortunately, in some establishments, the most compelling reason for publication is to fulfill specific job requirements by employers. It may include promotion to an academic position and improving prospects of success in research grant application. The importance of publication in the career is further emphasized by the adage “Publish or perish,” i.e. publish your research or lose your identity.
Ethics-related organizations and their role
A good research involves many coordinated steps. It starts from hypothesis, selection of appropriate study design, study execution, data collection, analysis, and finally publication. Not only the conduct of the study requires ethics to be adhered to but also the process of publication comes under the purview of ethics. Any publication that reports the results and draws the conclusion from the data which have been manipulated is considered research fraud or scientific misconduct [ 4 ]. Recently, Lancet retracted a study entitled “Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis” because the veracity of the data underlying this observational study could not be assured by the study authors [ 5 ].
There are organizations which give recommendations and develop guidelines to assist authors, editors, and reviewers. The purpose is to create and disseminate accurate, clear, reproducible, unbiased research papers. The organizations involved with publication ethics are
International Committee of Medical Journals Editors (ICMJE).
World Association of Medical Editors (WAME)
Committee on Publication Ethics (COPE)
The ICMJE was established in 1978, in Vancouver, British Columbia, Canada, by a group of medical journal editors. ICMJE developed recommendations which are primarily for authors who want to submit their work in ICMJE member journals. These recommendations discuss the role and responsibilities of the authors, contributors, reviewers, and editors. Steps of manuscript preparation, submission, and editorial issues related to publication in medical journals are also discussed and drafted. The uniform requirements for manuscript submitted to biomedical journals, which most of the journals are following were drafted by ICMJE [ 6 ].
The WAME is a nonprofit voluntary association, which was established in 1995 by a group of members of the ICMJE. The goal was to improve editorial standards, promote professionalism in medical editing, and encourage research on the principals and practice of medical editing. The role of WAME is to facilitate worldwide cooperation and communication among editors of peer-reviewed medical journal. Membership in WAME is free and all decision-making editors of peer-reviewed journals are eligible to join. WAME has more than 1830 members representing more than 1000 journals from 92 countries [ 7 ].
The COPE also helps in ethical publication. COPE was founded in 1997 by a small number of UK medical editors as a self-help group to discuss troubling ethical cases in the publication process. It provides paid membership and currently has more than 7000 members in various disciplines from all parts of the world. The purpose of COPE is to find the practical ways to deal with the misconduct cases and to develop codes of conduct for good publication practice. It also generates the funding for the research based on the issues related to publication misconduct [ 8 ].
Process of publication
The scientific publication is a team effort. Transforming the research findings and observations into a published article is an art as well as science, which involves multiple steps. The very first step is the preparation of the manuscript as per the journal’s requirement. The language in which the manuscript has been drafted is important. It should be checked by an expert or native language speaker and the senior authors. Clear and concise language helps editors and reviewers to concentrate on the content. For up-to-date information, recent references should be cited. Final manuscript must be shared with all the authors and it should have approval of all the authors. Copyright transfer form should be signed by all the authors before submitting to the journal. Signing the copyright form brings responsibility.
Submitted manuscripts are first screened by the editors for its suitability, content, novelty, and what it adds to existing knowledge. The subject of research work should be synchronized with the target journal. It should comply with journal’s manuscript drafting guidelines. After the editorial screening, if some technical issues or non-adherence to manuscript guidelines are observed, it is sent back to the author for technical modifications. The peer review process gets initiated after technical modifications are acceptable. It may take a couple of weeks/months.
In light of reviewer’s recommendations, the editor sends the decision letter to the author mentioning the status of the manuscript, i.e. accepted, rejected, or requires revision. In case of revision, author(s) reply in detail to all comments of reviewers and submit to the journal again within stipulated time. After deliberation on replies and revised manuscript submitted, the editor decides for suitability of publication or if it needs to be sent out for review again. These steps get repeated until the manuscript is accepted or rejected. Once it gets accepted, it goes under proof read stage and finally gets published. The author is never in direct communication with the reviewer. He communicates with the Editorial board only. The reviewer should declare conflicts of interest (COI), if any, before reviewing the manuscript. Manuscripts are usually mailed to reviewers without information of the authors and their affiliations; hence, reviewers are blinded.
What is publishable or not publishable?
Writing for publication is an important yet challenging form of knowledge dissemination. Journals like to publish articles that present an exhaustive meaningful research. It should contribute towards the knowledge building and awareness of readers. At the very minimum, a publishable article needs to be original. It should be conducted and drafted with robust methodology and significant findings, well organized, well written, and concise yet clear. It should be drafted with clear explanation of how the article addresses the existing knowledge gap. Conclusion drawn should be relevant to the audience or readers with a comprehensive list of up-to-date references. Papers that are poorly organized, cluttered with unnecessary information, and consist of routine extension of previous reports or fragmentary reports of research results are not accepted for publication. Violation of ethical or legal norms, including plagiarism, duplicates publication lead to immediate rejection of the paper [ 9 ].
- Scientific misconduct
Scientific misconduct is the violation of the standard codes of scholarly conduct and ethical behavior in the publication of scientific research [ 10 ]. Misconduct in the scientific publication process by the authors is detrimental for integrity of the whole system and is considered unethical. Falsification or fabrication of data is the gravest form of scientific misconduct wherein authors either manipulate skewed data to look favorable or generate data where no data exists. Different forms of scientific misconduct are plagiarism or misappropriation of the ideas of others, improprieties of authorship, simultaneous publications, duplicate publications, salami slicing, and non-declaration of COI. Conducting research without informed consent or ethics approval and not maintaining data confidentiality is a form of scientific misconduct. Editors or publication houses do take disciplinary action as per COPE recommendations against scientific misconduct. Authors are blacklisted or banned to submit articles in the respective journal in the future [ 11 ].
Criteria of authorship
Academic life revolves around publications. The publication adds to the credibility of the research and brings fame and recognition. An author is an individual who fulfills enlisted criteria collectively: (1) substantial contributions to conception and design; (2) acquisition of data, or analysis and interpretation of data; (2) drafting the article or revising it critically for important intellectual content; and (3) final approval of the version to be published. Individuals who have provided technical services/translating text/identifying patients for study/supplying material/providing funds/applied statistics/medical writers are not eligible for authorship. However, all those contributors who do not meet the criteria for authorship should be listed in the acknowledgement section [ 12 , 13 ]. Because of the important role of publication in clinical practice and academic setting, the authorship of articles must be honest, reliable, trustworthy, and transparent.
Types of authors
Since authorship is sought after, many unethical practices are also prevalent. Ghost, guest, or gift authors are the examples of such practices. A ghost author is a person who has made a substantial contribution to the research or writing of a manuscript but is not listed as an author. A ghost author might be a direct employee or hired contract employee of pharmaceutical company and hence, listing him as an author amounts to COI [ 14 ]. It is dishonest to omit an author who has made significant contributions. In contrast to ghost author, guest or gift/honorary author is someone who is named as an author, but who did not contribute in a meaningful way to the design, research, analysis, or writing of a paper. Often guest or gift authors are well known and well respected in the field of research. The inclusion of their name in the author list might increase chances of acceptance for publication.
However, sometimes senior investigators may also give honorary authorship to their colleagues for encouraging collaborations and maintaining good working relations or as repayment of favors. Whatever the cause, the gift or guest authorship is an unacceptable practice in publication. The presence of well-known author on the board as a guest author can influence the opinion of clinicians, academicians, and politicians about a particular drug or device. Secondly, due to gift authorship, the person is perceived as being more skilled than his colleague who has not published [ 12 , 13 ]. In multicenter trials, since investigators from different sites have contributed, they qualify for the authorship and all those who qualify for authorship should be listed [ 15 ]. One should always remember that authorship brings responsibility and authors have to be accountable to the data and results which are published.

Authorship issues/disputes
Authorship issues or disputes account for 2% to 11% of all disagreement in the scientific community. The authorship disputes could range from order of authorship, inclusion or exclusion of authors, number of authors etc. Request for addition of authors after submission or even after publication is quite common. In contrast, there are examples where a co-author denies becoming a part of a manuscript, once any scientific misconduct including plagiarism is detected [ 16 ].
The order of authorship should be mutually decided before taking up the study. It has to be a joint decision of all co-authors. In multicenter trials, research group includes large number of researchers. Hence, the corresponding author specifies and registers the group name and clearly identifies the group members who can take credit and responsibility for the work as an author.
ICMJE and other organizations issued the guidelines regarding group authorship and stated that in case of group authorship the byline of the article identifies who is directly responsible for the manuscript, and MEDLINE lists as authors. If the byline includes a group name, MEDLINE will list the names of individual group members who are authors or who are collaborators [ 17 ]. Despite these guidelines, authorship battles for inappropriate attribution of credit are witnessed in this area also.
Usually, the dispute is for the “First author” place because most of the articles are cited by the name of the first author. Conventionally, the extent of involvement decides the order of authorship; for example, the person who has done the majority of the groundwork would be considered eligible for being the first author (junior researcher) and the person who planned and conceived the study would be the last author (supervisor). There is no general consensus in order of authorship, and there are different schools of thoughts [ 16 ]. During submission of revised manuscript, order of authorship should not be altered without any justification. Approval from all authors is warranted in case of revision of order of authorship. It affects the credibility of manuscript too.
How to resolve authorship issues
The best way to prevent disputes in authorship is to generate awareness among research groups about authorship criteria and to develop Standard Operating Procedure (SOP) for the conduct and publication of research. COPE guidelines are to be referred in case of authorship or conflicts [ 18 ]. The next best option to prevent disputes is to have open discussion among all the authors involved in multidisciplinary research prior to initiating research, i.e. at the time of protocol drafting. Defining the role and responsibility of each author further reduces the chances of disputes within the research team. Editors do ask for individual contributions of authors in designing manuscript. The journal can blacklist guest or ghost authors [ 12 ].
Plagiarism: do’s and don’ts
The word plagiarism was first used in the English language in the year 1601 by the dramatist Ben Jonson to describe someone who was guilty of theft. Plagiarism is derived from the Latin word “plagiare” which means to “kidnap.” A plagiarist is the person who commits plagiarism [ 19 ]. By definition, plagiarism is the use of previously published work by another author in one’s own manuscript without consent, credit, or acknowledgement. It is the most common form of scientific misconduct [ 4 ]. Plagiarism can be intentional or unintentional. Unintentional plagiarism is usually seen in articles written by students or junior researchers. Lack of awareness and ignorance lead to unintentional plagiarism. Intentional plagiarism happens when an author deliberately copies documented or published work and presents it as his/her own. Both types of plagiarism are unethical and illegal, which can ruin the career and reputation of the writer [ 19 ].
Plagiarism of idea occurs when a plagiarist copies or steals the idea or thought of someone else and presents it as his/her own. Such type of plagiarism is difficult to detect; however, once detected, it is considered serious offense. The example of plagiarism of idea is presenting or documenting an idea of someone else which is being discussed or presented in any conference or seminar without citing proper sources. Plagiarism of text or direct plagiarism, i.e. word to word writing, is when a researcher takes large section of an article from another source and pastes it in his/her own research without providing proper citation. One of the hybrid varieties of plagiarism is Mosaic plagiarism where the author steals the idea, opinion, words, and phrases from different sources and merges words without acknowledging the original author.
Self-plagiarism is the practice of an author using portions of their previous writings on the same topic in their subsequent publications, without specifically citing it formally in quotes. There is no consensus as to whether this is a form of scientific misconduct, or how many of one’s own words one can use before it is truly “plagiarism.” To be on the safer side, authors should cite source or give reference of their previous publications. There are examples in which plagiarism engulfed the entire career of authors and writers and it became the reason of article retraction or rejection [ 20 ].
Culture of publish or perish is one of the important causes of plagiarism. The researcher needs to publish a large number of papers in limited time period to get more opportunities in career and research. In addition, lack of knowledge, laziness, and fear of failure and desire of getting recognition also lead to plagiarism. Many softwares, which can detect plagiarism are available online. It is the responsibility of the author to run their manuscript through software before submitting it to the journal [ 19 , 21 ].
The very first step to prevent plagiarism is the awareness about plagiarism, the consequences, and how to avoid plagiarism. Authors can avoid plagiarism by acknowledging the original source of the idea or word and enclosing them within quotation marks. In case of paraphrasing, where the writer writes the text in his own word, authors must properly cite the original source. Authors must always obtain permission for use of published illustration. Authors should avoid writing multiple separate articles if he can present a large, complex study in a cohesive manner in a single article [ 21 ].
- Conflict of interest
Conflict of interest is an attribute which is invisible to the reader or editor, but which may affect or influence his or her judgment or objectivity. Academicians/physicians and researchers often work in collaboration with pharmaceutical and biotechnology companies to develop a product for the well-being of society. However, there are examples where financial and non-financial ties of researches or physicians with the company have compromised the integrity of research [ 22 ].
Conflict of interest describes the situations where the impartiality of the research may be compromised because the researcher stands to profit in some way from the conclusions they draw [ 23 ]. Examples of potential conflicts of interests that are directly or indirectly related to the research may include research grants from funding agencies, honorarium for speaking at symposium, financial support for educational programs, employment, and multiple affiliations. In addition, non-financial benefits including recognition, career advancement, advocacy for a strongly held position, and support for friends and colleagues can also affect the research work and result biases in the research. These biases, when hidden, can affect clinical decision-making by making interventions appear safer or more effective than they really are [ 24 ].
Disclosure of COI is the basic requirement to prevent attribution-related bias in the research. The ICMJE has produced a common form to disclose any COI and that has to be individually signed by each co-author. It has to be uploaded along with the manuscript files. The intent of the disclosure form is not to prevent authors with a potential COI from publication. It is merely intended that any potential conflict should be declared so that the readers may form their own judgment about the findings and observations. It is for the readers to determine whether the authors outside interest may reflect a possible bias in either the exposition of the conclusions presented [ 25 ]. Authors are supposed to declare COI in the manuscript text too which is meant for readers.
- Duplicate publication
Duplicate publication or redundant publication is a publication of a paper that substantially overlaps with one which is already published, without clear, visible reference to the previous publication [ 26 ]. As per copyright law and publication ethics, whatever is available in the journal for reading would be original unless there is a clear statement that the author and editor are intentionally republishing an article. Hence, duplication of publication is the breach in the copyright law and against the ethical conduct. In addition, duplication of publication causes waste of limited resources and also leads to inappropriate weighting of the result of a single study. It was observed that duplicate publications of Ondansetron led to overestimation of its efficacy by 23% in one of the meta-analyses [ 26 , 27 ].
The COPE classifies duplicate publication into major and minor offenses. The major offense is the one where duplicate publication is based on the same data set and findings which are already published. It is also considered if there is evidence that the author tried to hide duplication by changing the title or order of authorship or by not referring previous publication [ 28 ]. Minor or salami slicing is considered segmental publication or part publication of results or reanalysis derived from a single study. Authors do it to increase the number of publications and citations. It is considered unethical and it is taken in a bad taste because for a reader it may cause distortion in the conclusions drawn. Publication of the results of a single study in parts in different journals might lead to over-judgement. Wrong conclusions may be drawn from a study if it is done on a fixed number of subjects but the data are being presented in fragments in different journals.
When an author needs to submit a report that has been already published or closely related to another paper that has been submitted elsewhere, the letter of submission should clearly say so. The authors should declare and provide copies of the related submission to help the editor decide how to handle the submission. Authors who attempt to duplicate publication without such notification can face prompt rejection of the submitted manuscript. If the editor was not aware of the violations and the article has already been published, then the article might warrant retraction with or without the author’s explanation or approval.
Duplicate publication does not prevent the author to disseminate important public health information in case of public health emergency. In fact, ICMJE encourages editors to give priority to authors who have made crucial data publicly available without delay [ 26 ]. Duplicate publications are justified if it is about combined editorials, clinical guidelines, and translation of archives.
Predatory publishing
Predatory publishing is the publication of an article in the journal that lacks the usual feature of editorial oversight, transparent policies, and operating procedure of legitimate peer review journals. Predatory journals exploit the authors by charging the publication fee and deceiving them by providing the false claim about the journal’s impact factor, indexing, and peer review [ 29 ].
Predatory publishing is harmful for both the author and the community. Predatory publishing may tarnish the image of the author. Articles published in predatory journals are usually not appreciated by the subject expert. It can misinform the readers and propagate wrong science because of poor quality control. Sometimes genuine information also gets missed because most of the predatory journals are not indexed in the database, so papers are not easily traceable [ 30 ].
Predatory publishing can be avoided by educating researchers, supervisors, and administrators about fake journals. Authors should also learn how to identify trustworthy journals. If the journal website mentions of indexing, then it is important to cross check the inclusion of the journal in the mentioned databases. For an open-access journal, the inclusion in Directory of Open Access Journals (DOAJ) can be checked at the DOAJ website. The journal’s claim of the Journal Citation Report (JCR) impact factor can be verified by its International Standard Serial Number (ISSN) number in the JCR Master list. Another approach to check trustworthy journals is to self-asses the journal through websites like https://thinkchecksubmit.org/ [ 30 ].
Responsibility of author
Authorship is not just a list of names. It is the matter of pride that has to be deserved, earned, and declared [ 15 ]. To maintain the integrity and credibility of medical research and to nourish the trust of public in scientific endeavors, all authors must follow the rules of good scientific publication practice and should stick to the following responsibilities (Table 1 ):
Do not fabricate or manipulate the data
Avoid plagiarism and give proper acknowledgment to other works
Decide the order of authorship prior to writing the paper to avoid future conflicts
Declare whether research work has been published or presented before
Declare COI
Avoid ghost/gift/guest authorship
Do not submit the manuscript to more than one journal for simultaneous consideration
Take approval from the Institutional Ethics Committee before conducting research
Last but not the least, take direct responsibility for appropriate portions of the content.
Awareness of good publication practices should be generated among novice authors to prevent unethical practices in publication of scientific research. Each institute or department should resort to COPE or ICMJE recommendations for publications and draft their own SOP for authors who are actively involved in research. Unethical practices on the part of the authors or scientific misconduct should be discouraged and addressed by appropriate training and guidance.
Whitesides GM. Whitesides’ group: writing a paper. Adv Mater. 2004;16:1375–7.
Article CAS Google Scholar
National Research Council. Sharing publication related data and materials: responsibilities of authorship in the life sciences. Washington, DC: National Academies Press; 2003. https://doi.org/10.17226/10613 .
Book Google Scholar
Asnake M. The importance of scientific publication in the development of public health. Cienc Saude Coletiva. 2015;20:1973.
Article Google Scholar
Sengupta S, Honavar SG. Publication ethics. Indian J Ophthalmol. 2017;65:429–2.
Mehra M, Desai S, Ruschitzka F, Patel A. Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet. 2020;22:S0140–6.
Google Scholar
ICMJE. Recommendations for the conduct, reporting, editing and publication of scholarly work in medical journals. 2019. http://www.icmje.org/icmje-recommendations.pdf . Accessed 22 Aug 2020.
WAME. About Us: What is WAME? 2016. http://wame.org/about . Accessed 22 Aug 2020.
Wager E. The Committee on Publication Ethics (COPE): objectives and achievements 1997–2012. Presse Med. 2012;41:861–6.
Pickett ST, Donnell MJ. The art and science of writing a publishable article. J Urban Ecol. 2017;3:1–6.
Nylenna M, Anderson D, Dahlquist G, et al. Handling of scientific dishonesty in the Nordic countries. National Committees on Scientific Dishonesty in the Nordic countries. Lancet. 1999;354:57–1.
Fitzpatrick JJ. Scientific misconduct. Appl Nurs Res. 2018;41:87.
Schofferman J. Ghost and guest authors: you can’t always trust who you read. Pain Med. 2015;16:416–20.
Zaki. Gift authorship – a cause for concern. Lung India. 2011;28:232–3.
Gøtzsche PC, Hróbjartsson A, Johansen HK, et al. Ghost authorship in industry-initiated randomized trials. PLoS Med. 2007;4:e19.
Bavdekar SB. Authorship issue. Lung India. 2012;29:76–80.
Desai C. Authorship issues. Indian J Pharm. 2012;44:433–4.
ICMJE. Defining the role of authors and contributors. 2020. http://www.icmje.org/recommendations/browse/roles-and-responsibilities/defining-the-role-of-authors-and-contributors.html . Accessed 22 Aug 2020.
Albert T, Wager E, on behalf of COPE Council. How to handle authorship disputes: a guide for new research. Version1. 2003. https://publicationethics.org/files/2003pdf12_0.pdf . Accessed 22 Aug 2020.
Mohammed RAA, Shaaban OM, Mahran DG, et al. Plagiarism in medical scientific research. J Taibah Univ Med Sci. 2015;10:6–11.
White C. Suspected research fraud: difficulties of getting at the truth. BMJ. 2005;331:281.
Das N, Panjabi M. Plagiarism: why is it such a big issue for medical writers? Perspect Clin Res. 2011;2:67–1.
Lo B, Field MJ. Institute of medicine. Conflict of interest in medical research, education, and practice. Washington DC: The National Academy Press; 2009. https://www.nap.edu/download/12598 . Accessed 23 Aug 2020.
Dunn AG, Coilia E, Mandi KD, Bourgeois F. Conflict of interest disclosure in biomedical research: a review of current practices biases, and the role of public registries in improving transparency. Res Integr Peer Rev. 2016;1:1.
Romain PL. Conflicts of interest in research: looking out for number one means keeping the primary interest front and center. Curr Rev Musculoskelet Med. 2015;8:122–7.
ICMJE. Conflicts of Interest. 2020. http://www.icmje.org/conflicts-of-interest/ . Accessed 22 Aug 2020.
ICMJE.Overlappingpublications. 2020. http://www.icmje.org/recommendations/browse/publishing-and-editorial-issues/overlapping-publications.html . Accessed 22 Aug 2020.
Villar R. What is this duplicate publication thing? J Hip Preserv Surg. 2015;2:203–5.
Elston DM. Duplicate publication. J Am Acad Dermatol. 2019;81:339.
COPE Council. COPE Discussion document: predatory publishing. 2020. https://publicationethics.org/files/cope_dd_a4_pred_publishing_nov19_screenaw.pdf . Accessed 22 Aug 2020.
Vasudevan S, Mehta A. Predatory publishing: what a researcher should know about. J Curr Oncol. 2020;3:4–7.
Download references
Author information
Authors and affiliations.
Department of Pharmacology, Maulana Azad Medical College, New Delhi, 110 002, India
Shubha Singhal & Bhupinder Singh Kalra
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Bhupinder Singh Kalra .
Ethics declarations
SS, and BSK declare that they have no conflict of interest.
The authors are solely responsible for the data and the contents of the paper. In no way, the Honorary Editor-in-Chief, Editorial Board Members, the Indian Society of Gastroenterology or the printer/publishers are responsible for the results/findings and content of this article.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Singhal, S., Kalra, B.S. Publication ethics: Role and responsibility of authors. Indian J Gastroenterol 40 , 65–71 (2021). https://doi.org/10.1007/s12664-020-01129-5
Download citation
Received : 28 October 2020
Accepted : 23 November 2020
Published : 22 January 2021
Issue Date : February 2021
DOI : https://doi.org/10.1007/s12664-020-01129-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Biomedical ethics
- Editorial policies
- Journal article
- Peer review
- Retracted publication
- Find a journal
- Publish with us
- Track your research
CONDUCTING RESEARCH
Manuscript preparation, ready to submit, research promotion, solutions for every need, top subject areas.
Advanced Editing
Premium Editing
Scientific Editing
Digital Editing
Journal Finder
AI Literature Search
Illustration Creation Tool
Academic Writing Tool
Silver Pack
Platinum Pack
Custom Pack
Case Studies on Ethics
Case 1: suspected data fabrication and image duplication.
While reviewing the author’s manuscript, our publication expert made the following observations: 1. The study documented a novel surgical procedure that was complicated and had high animal mortality. Such research is usually performed on a large number of animals (to account for animal death during the procedure). Moreover, at the end, the experimental groups have unequal numbers. However, in this specific study, the animal numbers were uniform in all groups. This seemed highly improbable and was an issue that the authors needed to account for. 2. Two figures appeared to have been taken from the same slide. Both images had the same cellular distribution, which was unlikely because the paper mentioned both slides as having been stained with two different antibodies.
Our publication expert communicated these concerns to the author and intimated him of the possible problems if the paper was found to be violating ethical standards of publication. When the author expressed his desire to continue with his submission to the target journal, our expert requested him to offer a clear explanation for the data’s credibility to the journal.
Data fabrication and image manipulation are issues that cannot be spotted through the use of any software. They are best identified by a trained eye in the field. At Editage, we try our best to get subject-specialist expert editors to work on a paper so that our clients’ papers are free from ethical concerns.
Case 2: Suspected duplicate publication
A major concern with the manuscript was that the study appeared to be exactly the same as a previous study by the same authors published in a Chinese journal. The original Chinese study had not been cited or discussed in the manuscript. Our expert concluded that the similarities/differences between the two studies needed to be highlighted transparently.
Our expert informed the authors that they needed to disclose the existence of the previous paper, which would imply that their manuscript was a duplicate paper (and thus a potentially redundant publication). We recommended that this disclosure be made in the cover letter to the English-language target journal. The authors seemed to believe that using parts from their own previously published paper was perfectly acceptable and were very much surprised by our information. Our expert shared links to a number of Editage resources on publication ethics to help them understand the issue better.
Lack of awareness about the do’s and don’ts of ethical publication practices is a serious contributor to the incidence of apparent misconduct, especially among early-career researchers who are non-native-English speakers and unfamiliar with industry ethical guidelines. In such cases, Editage uses the opportunity to share the essential know-how on various missteps that a young researcher might make in the academic publication process.
Case 3: Suspected lack of informed consent
The study proposed a new wound-healing technique and involved needle puncture on patients from two medical centers. The paper mentioned that an institutional ethical review board had given ethical approval for the study. However, the processes of puncturing, healing, and cleaning vascular access were performed without informed consent.
On being informed of the need for patient consent, the authors asked if they could correct the situation by obtaining informed consent retrospectively. Our expert explained to the authors that journals would have an issue with human experimentation conducted without active consent of the subjects and that this requirement ought to be fulfilled before experimentation.
Any study on patients, patient groups, and/or volunteer participants requires informed consent, which must be documented in the paper before submission to a journal. This is a non-negotiable requirement for experiments/studies that involve human subjects. At Editage, we try and make authors aware of all the ethical requirements of scientific research so that their work is not rendered ineligible for publication because of an ethical lapse.
Trust our experts to improve your chances of publication in high-impact journals
*Disclaimer: All third-party trademarks (including logos and icons) of journals / publishers, etc. referred to on this website remain the property of their respective owners. Use of third-party trademarks does not indicate any affiliation, sponsorship with or endorsement by them. Any references to third-party trademarks is to identify the corresponding services and shall be considered fair use under The Trademarks Law. We are not implying that purchasing this service will ensure publication in any journal.
ENGLISH EDITING SERVICES
- Advanced editing
- Premium editing
- Scientific editing
- Proofreading services
- Book editing
- Thesis editing
- Abstract editing
- Case report editing
- Compare editing plans
- Manuscript editing
- Copy editing services
- Post editing
- Post editing support
OTHER EDITING SERVICES
- English language editing
- English language check
- Academic editing
- Dissertation editing
- Essay editing
- Personal statement editing
- Report editing
- ESL academic editing
- Research paper editing
- Journal article editing
- Substantive editing
- Medical editing
- Medical editing and writing
- Digital Editing Services
TRANSLATION SERVICES
- Chinese to english translation
- Brazilian portuguese to english
- Japanese to english translation
- Korean to english translation
- Spanish to english translation
- Turkish to english translation
PUBLICATION SUPPORT SERVICES
- Gold publication support pack
- Silver publication support pack
- Platinum publication support pack
- Custom pack
- Compare PSS plans
OTHER SERVICES
- Research promotion
- Graphical Abstract
- Journal selection
- Journal submission
- Artwork preparation
- Plagiarism checker
- Literature search
- Pre-submission peer review
- Resubmission support
- Rapid technical review
- Statistical review
- Experimental design
- Manuscript resubmission
- Alternate text writing
- Medical writing
- Conference presentation packs
- Call: +1 (833) 979-0061 [email protected] Request a call
SOCIAL MEDIA
Regional websites.
- 英文校正 英文润色 영문교정 Revisão Inglês Editage USA Editage Indonesia
Quick Links:
Cactus Communications. All Rights Reserved
Case Study: Research Ethics
Answer (scenario 1).
Unfortunately it is not. BMC Editorial Policy clearly states: Research involving human subjects, human material, or human data, must have been approved by an appropriate ethics committee. A statement detailing this, including the name of the ethics committee and the reference number where appropriate, must appear in all manuscripts reporting such research. If a study has been granted an exemption from requiring ethics approval, this should also be detailed in the manuscript (including the name of the ethics committee that granted the exemption).
Answer (Scenario 2)
Unfortunately it is not. BMC Editorial Policy dictates : Research involving human subjects, human material, or human data, must have been approved by a local Ethics Committee based in the same country from where the participants have been recruited . Therefore if the study has taken place at multiple locations (such as Ethiopia and Nigeria), we require ethical approval from a committee at all locations.
If you are unsure whether or not your study requires ethical approval, check with the local Ethics Committee. Do not rely on online tools.
Equity in academic publishing
1. Where you publish matters!
2. What if English is not my first language?
3. Research and publication ethics to consider
- Follow us on Twitter
Annual Journal Metrics
2022 Citation Impact 4.5 - 2-year Impact Factor 4.7 - 5-year Impact Factor 1.661 - SNIP (Source Normalized Impact per Paper) 1.307 - SJR (SCImago Journal Rank)
2023 Speed 32 days submission to first editorial decision for all manuscripts (Median) 173 days submission to accept (Median)
2023 Usage 24,332,405 downloads 24,308 Altmetric mentions
- More about our metrics
BMC Public Health
ISSN: 1471-2458
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Publication Ethics and Editorial Policies
Publication ethics and research integrity help to ensure the accuracy and trustworthiness of an article. They cover a broad range of subjects including authorship, ethical approval for the study, conflicts of interest, availability of data, peer review amongst other topics.
Karger Publishers is a member of the Committee on Publication Ethics (COPE). Karger journals aim to adhere to the COPE Code of Conduct and Best Practice Guidelines . Below is guidance for authors when preparing their manuscript and details about our Editorial policies relating to research integrity and publication ethics.
Manuscripts may be rejected if the editors believe that the research has not been carried out within an appropriate ethical framework, and concerns raised after publication may lead to a correction, retraction, or expression of concern in line with COPE guidelines .
Studies Involving Human Subjects, Identifiable Materials or Data
Manuscripts presenting research based on human subjects, materials or data must contain a Statement of Ethics outlining the steps taken to ensure compliance with internationally-accepted standards for ethical research practice and reporting.
Studies must have been performed with the approval of an appropriate ethics committee and with appropriate participants’ informed consent in compliance with the Helsinki Declaration . In the manuscript, authors should specify the name of the ethics committee or other relevant authority who approved the study protocol and provide the reference number where appropriate. If ethics approval was not required, or if the study has been granted an exemption from requiring ethics approval, this should also be detailed in the manuscript (including the name of the ethics committee that made that decision). Note if authors are submitting to a journal with a double blind peer review policy, the Ethics Statement should be anonymized where appropriate.
For all research involving human subjects, written informed consent to participate in the study should be obtained from participants (or their parent/legal guardian where appropriate) and a statement detailing this should appear in the manuscript. For studies involving vulnerable participants or participants at risk of potential coercion, detailed information regarding the steps taken to ensure informed consent must be provided. If consent was not obtained, please specify why this was not obtained and whether this was approved by the ethics committee.
Identifying Participant Information
In line with the ICMJE recommendations on the protection of research participants, authors must avoid providing identifying information unless strictly necessary for the submission and participants’ identifiable attributes must be anonymized in the manuscript and its supplementary files, if any. If identifying information is necessary, authors must confirm that the individual has provided written consent for the use of that information in a publication and clearly state this in the statement of ethics.
Research Using Cell Lines
The source of all cell lines used in a study must be detailed in the methods section of the manuscript. An appropriate statement of ethics must be provided for studies using or creating de novo cell lines. Research involving human embryonic stem cells, embryonic germ cells or induced pluripotent stem cells should comply with the ISSCR 'Guidelines for the Conduct of Human Embryonic Stem Cell Research' or an equivalent set of guidelines or applicable regulations.
Additional Requirements for Case Reports
Manuscripts reporting a case report must include a statement detailing that written informed consent for publication was obtained and from whom (e.g. “Written informed consent was obtained from the patient for publication of this case report and any accompanying images.”). If the patient has died, consent for publication must be obtained from their next of kin. If the patient described in the case report is a minor or vulnerable, then consent for publication must be obtained from the parent/legal guardian. The completed consent form must be made available to the Editor if requested and will be treated confidentially. If consent to publish any directly or indirectly identifiable data or images has not been obtained, we will not consider the manuscript for publication.
Additional Requirements for Clinical Trials
In accordance with the ICMJE recommendations , all clinical trials should be registered in a publicly available registry approved by the WHO or ICMJE and the clinical trial number must be clearly stated in the manuscript. Manuscripts reporting clinical trials must adhere to the relevant reporting guidelines for their study design, such as CONSORT for randomized controlled trials, TREND for non-randomized trials, or other relevant reporting guidelines as detailed on the Equator network website .
Karger follows the WHO definition of clinical trials "A clinical trial is any research study that prospectively assigns human participants or groups of humans to one or more health-related interventions to evaluate the effects on health outcomes [...] Interventions include but are not restricted to drugs, cells and other biological products, surgical procedures, radiologic procedures, devices, behavioural treatments, process-of-care changes, preventive care, etc. This definition includes Phase I to Phase IV trials.”
Studies Involving Animals
Manuscripts presenting research involving animals must contain a Statement of Ethics outlining the steps taken to ensure compliance with internationally-accepted standards for research practice and reporting.
Experimental research on vertebrates or any regulated invertebrates must have been approved by the authors' Institutional Animal Care and Use Committee (IACUC) or equivalent ethics committee and must follow internationally recognized guidelines such as the ARRIVE guidelines. In the manuscript, authors should specify the name of the ethics committee or other relevant authority who approved the study protocol and provide the reference number where appropriate. Please note if authors are submitting to a journal with a double blind peer review policy, the Ethics Statement should be anonymized where appropriate.
If ethics approval was not required, or if the study has been granted an exemption from requiring ethics approval, this should also be detailed in the manuscript, including the name of the ethics committee that made that decision. Additional information on welfare and the steps taken to minimise suffering is expected for studies reporting the death of a regulated animal as a likely outcome or planned endpoint. Other types of studies including field studies and non-interventional research on animals must comply with local or international guidelines, and where appropriate must have been approved by an appropriate ethics committee.
Conflict of Interest
Karger endorses the ICMJE recommendations on the ‘Disclosure of Financial and Non-Financial Relationships and Activities, and Conflicts of Interest’. This states that “The potential for conflict of interest and bias exists when professional judgment concerning a primary interest (such as patients' welfare or the validity of research) may be influenced by a secondary interest (such as financial gain). Perceptions of conflict of interest are as important as actual conflicts of interest.”
What to Declare
When submitting a manuscript, authors must declare any activity or relationship that could be perceived as a conflict of interest in the Conflict of Interest statement. All forms of support and financial involvement (e.g. employment, consultancies, honoraria, stock ownership and options, expert testimony, grants or patents received or pending, royalties) that occurred within the previous three years should be declared, regardless of their potential relevance to the paper. The role of the funder(s) in study design; collection, analysis, and interpretation of data; writing of the report; any restrictions regarding the submission of the report for publication should be declared. If the funder(s) had no role in any of the above, this should be clearly stated in the manuscript’s Funding Sources statement. Nonfinancial relationships (personal, political, or professional) that could be perceived as potentially influencing the writing of the manuscript should also be declared.
Note if authors are submitting to a journal with a double blind peer review policy, the Conflict of Interest statement should be anonymized where appropriate.
Editors and reviewers must declare any relationship or activity that could be perceived as potentially interfering with a full and objective evaluation of a manuscript. For more detail on what should be declared by Editors and Reviewers see our Peer Review page .
Funding Sources
Authors must give full details about the funding of any research relevant to their study, including sponsor names and explanations of the roles of these sources in the study design, execution and analysis, and manuscript conception, planning, writing and decision to publish. If the sponsor or funder had no role in any of the above, please state this in the funding statement. Please ensure to include any support that could be perceived as a potential conflict of interest in the Conflict of Interest Statement, in line with the below policy. It is strongly advised to write out the funding body in full and add the grant number in brackets. Multiple grant numbers should be separated by commas and spaces.
Authorship and Contributorship
When submitting a manuscript, the names and affiliations of all individuals who are authors of the manuscript must be provided. The affiliation listed for an author in a manuscript must be the affiliation where the author was based when the work described in the manuscript was carried out. When preparing the author list, authors can refer to the ICMJE which provides four criteria for authorship and recommends that all individuals who meet all of these criteria be listed as an author As outlined by the ICMJE, these authorship criteria are intended to reserve the status of authorship for those who deserve credit and can take responsibility for the work but are not intended be to used to disqualify an individual from authorship. Contributors to the study who do not fulfil the criteria for authorship but whose contribution should be recognized should be credited in the Acknowledgement section.
Every manuscript must contain an Author Contribution Statement. In this, the contributions of each person named as an author must be detailed. This statement must contain the full name of each author as it appears in the author list. Note if authors are submitting to a journal with a double blind peer review policy, the Author Contributions statement should be anonymized.
The Contributor Roles Taxonomy (CRediT) is a useful structure to refer to when preparing the Author Contribution Statement. Follow the link to find out more about the 14 contributor roles and their definitions according to CRediT . Example Author Contribution Statement using CRediT roles from Rivera et al., Glomerular Dis 2022. doi: 10.1159/000526868.
“Frederick B. Rivera, MD, and Pia Gabrielle I. Alfonso, MD: conceptualization, data curation, formal analysis, investigation, methodology, validation, writing - original draft, and writing - review and editing; Marie Francesca Mapua Ansay, MD: conceptualization, validation, and writing - review and editing; Jem Marie Golbin, MD: data curation, formal analysis, investigation, methodology, validation, writing - original draft, and writing - review and editing; Gerard Francis E. Mangubat, MD; Rajiv Hans Solita Menghrajani, MD; Siena Placino; Marianne Taliño, MD; Deogracias de Luna, MD; Nicolo Cabrera, MD; Carlo Nemesio Trinidad, MD; and Amir Kazory, MD: conceptualization, data curation, methodology, validation, and writing - review and editing”
Group Authorship
For group authorship, the ICMJE recommends that:
"When a large multi-author group has conducted the work, the group ideally should decide who will be an author before the work is started and confirm who is an author before submitting the manuscript for publication. All members of the group named as authors should meet all four criteria for authorship, including approval of the final manuscript, and they should be able to take public responsibility for the work and should have full confidence in the accuracy and integrity of the work of other group authors."
When submitting a manuscript authored by a group , the submitting author should specify the group name in the manuscript file and enter the information of the group members who can take credit and responsibility for the work as authors in the submission system. The non-author group members should be listed elsewhere in the manuscript, typically in the Acknowledgements or as supplementary material. For display of the collaborators' names in the Medline citation, the first name, last name and affiliations of the non-author members of this group should be provided in a datasheet file using the template below, named “List of Collaborators”, and uploaded as supplementary material when submitting the manuscript.
List of Collaborators Template (DOCX, 19 KB)
Data Availability
All journal’s data sharing policy strongly encourages authors to make all datasets on which the conclusions of the paper rely available to editors, reviewers and readers without unnecessary restriction wherever possible. Authors are required to provide a Data Availability Statement in their manuscript that details whether data are available and where they can be found. In cases where research data are not publicly available on legal or ethical grounds, this should be clearly stated in the Data Availability Statement along with any conditions for accessing the data. The decision to publish will not be affected by whether or not authors share their research data.
Examples of Data Availability Statements
- The data that support the findings of this study are openly available in [repository name e.g “figshare”] at http://doi.org/[doi], reference number [reference number]
- Publicly available datasets were used in this study. These can be found in [repository name e.g “figshare”] at http://doi.org/[doi], reference number [reference number]
- All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
- All data generated or analyzed during this study are included in this article and its supplementary material files. Further enquiries can be directed to the corresponding author.
- The data that support the findings of this study are not publicly available due to [REASON WHY DATA ARE NOT PUBLIC e.g. their containing information that could compromise the privacy of research participants] but are available from [e.g. the corresponding author [author initials] OR Data sharing committee [PROVIDE CONTACT DETAILS including email address] upon reasonable request]
- The data in this study was obtained from [third party source] where [RESTRICTIONS/LICENCE] may apply. Datasets may be requested from [source contact information]
Note if authors are submitting to a journal with a double blind peer review policy, the data availability statement should be anonymized where appropriate.
Definition of Research Data
This policy applies to the research data that would be required to verify the results of the research reported in articles. Research data includes data produced by the authors (“primary data”) and data from other sources that are analysed by authors in their study (“secondary data”). Research data includes any recorded factual materials that are used to produce the results in digital and non-digital forms. This includes, but is not limited to, tabular data, code, images, audio, documents, video, maps, raw and/or processed data.
Policy Exceptions
This policy does not require public sharing of quantitative or qualitative data that could identify a research participant unless participants have consented to data release. The policy also does not require public sharing of other sensitive data, such as the locations of endangered species. Alternatives to public sharing of sensitive or personal data include:
- Depositing research data in controlled access repositories
- Anonymizing or deidentifying data before public sharing
- Only sharing metadata about the research data
- Stating the procedures for accessing your research data in the article and managing data access requests from other researchers
Embargoes on data sharing are permitted but should be clearly stated in the data availability statement, including the reason for embargo, date of the end of the embargo period and how and where the data can be accessed following the end of the embargo period. Please note that all datasets on which the conclusions of the paper rely must be made available to editors and reviewers if requested to facilitate the review process.
Data Repositories
The preferred mechanism for sharing research data is via public data repositories. Authors are encouraged to select a data repository with data curation procedures, that issues a persistent identifier, preferably a Digital Object Identifier (DOI), and has established a robust preservation plan to ensure the data is preserved in perpetuity. Additionally, we highly encourage researchers to consider the FAIR Data Principles when depositing data. Authors are encouraged to deposit their research data in a repository that has been widely adopted within their research community, suitable repositories per each area and data type can be searched using the FAIRsharing database tool or via Repository Finder .
If no such database is available authors may use a general data repository. Examples of general data repositories include:
Figshare Dryad Zenodo Open Science Framework
If authors are submitting an article to a journal with a double blind peer review policy, they should deposit their data in a repository that allows them to temporarily preserve anonymity such as Figshare (“private sharing link”) or Dryad (“private for peer review”).
Data and Software Citation
The journal encourages authors to cite any publicly available research data or software used in their reference list. References to datasets (data citations) and software code must include a persistent identifier (such as a DOI). Citations of datasets, when they appear in the reference list, should include the minimum information recommended by DataCite (e.g. author(s), title, publisher (repository name), DOI) and follow journal style. References to software, when they appear in the reference list should include the minimum information recommended by the FORCE11 Software Citation Implementation Working Group (e.g. creator(s), software title, publication venue (repository name), date the software was published, identifier (DOI, if available)) and follow journal style.
Generative Artificial Intelligence (AI)
If a Large Language Model (LLM), or other generative AI-based tool (e.g. chatbots or image creators), has been used as part of this study or manuscript, the use must be clearly declared in the manuscript Methods or Acknowledgements section, if the article type does not include a Methods section. Generative AI tools should not be listed as an author of the work, in line with our Authorship and Contributorship policy. Any software used must be cited in the References, in line with our software citation policy. Authors are responsible for guaranteeing the accuracy and originality of the content of their manuscript. The manuscript must include detail on how the accuracy of any generative AI-based output was verified. Authors are encouraged to include the original input prompts and outputs from the tools used as supplementary material. Failure to comply with the above will be considered a violation of our Editorial Policies and may result in the rejection of a manuscript or post-publication notice, in line with our policy on Misconduct.
Data Licensing
The journal encourages research data to be made available under open licences that permit reuse freely. The journal does not enforce particular licenses for research data, where research data are deposited in third party repositories. The publisher of the journal does not claim copyright for research data.
Reproducibility
Availability of materials.
The Methods section of the article must contain sufficient information to allow a reader to replicate the study. Karger encourages authors to use protocols.io as an open access repository for their detailed methodology. For protocols registered in protocols.io cite the record in your methods section and include the full record and DOI in the references. Karger supports the inclusion of Research Resource Identifiers in the methods section, for further information see the Resource Identification Portal. Supplier and catalogue numbers should be included for any chemicals, reagents, antibodies (etc.) used in the study.
Inclusion of Representative Images
Where authors include a representative image of an experimental group or outcome it is expected that no image enhancements or adjustments are applied to that image. Where necessary for clarity of interpretation, for example, image cropping or brightness adjustment, this must be applied equally to the whole image, be detailed in the Methods section of the article and the original images must be uploaded as supplementary material.
Images in submitted material may be subjected to investigation using image integrity software. Any concerns raised over inappropriate image modification will be investigated in accordance with COPE guidelines .
Blot and gel images
If blot or gel images are included in the Figures of the submission, the original, uncropped and unedited images must be uploaded as supplementary material. The marker should be visible in the original images, including the size of each marker band. If there are off-target or non-specific bands in the original image, the size of the intended product should be noted on the image and specified in the manuscript text.
Histology and Cytology images
All brightfield, fluorescent or other histology or cytology images must include a scale bar in each image. Relevant image capture information, such as aperture size and exposure time, should be consistent between comparator images and any necessary deviations between comparators must be described in the text.
Karger recommends following The SAMPL Guidelines when reporting statistical analyses. The sample size must be reported for each study in the methods section tables and Figure legends. Where statistical testing for the significance of an effect is carried out, a dedicated section for statistical methodology must be included in the Methods. This section should provide sufficient information that would allow, with access to the full data set, reproduction of the article's results. The choice of statistical tests and any post-hoc tests must be justified in this section. The threshold for significance, alpha, should be defined here as well as how multiple comparisons are adjusted for, where applicable. When reporting the results of statistical tests it is not sufficient to only report the p-value or that the p-value was <0.05. For example, if reporting the result of a Student’s t-test, it is necessary to report the degrees of freedom, t-statistic as well as the exact p-value.
Materials Design Analysis Reporting Framework
Karger Publishers endorses the Materials Design Analysis Reporting (MDAR) Framework for minimum reporting standards in the life sciences and encourages authors to consider all aspects of the MDAR Framework relevant to their study when submitting a manuscript. Authors are encouraged to submit a completed MDAR Checklist with their manuscripts.
Preprints may be shared without restriction prior to publication in any Karger journal. The availability of a preprint should be declared in the Acknowledgements section of the manuscript and included in the reference list. For example, “A preprint version of this article is available on medRxiv [reference number]”. To cite the document, include the Digital Object Identifier and the document type (e.g., preprint, protocol) in the citation. Also list any information about the document version (e.g., most recent date modified), and if relevant, the date the document was cited and follow the journal’s style. Example: Bar DZ, Atkatsh K, Tavarez U, Erdos MR, Gruenbaum Y, Collins FS. Biotinylationby antibody recognition-A novel method for proximity labeling. BioRxiv069187v1 [Preprint]. doi: https://doi.org/10.1101/069187. For further information please visit Open Science
Open Science
For further information please visit Open Science .
Karger takes seriously all allegations of potential misconduct and will follow relevant COPE Guidelines . Concerns regarding a published article should be raised to the Research Integrity and Publication Ethics team at publication.ethics[at]karger.com. All efforts will be made to resolve concerns raised about a published article without undue delay and an Erratum or Retraction will be issued, where necessary. An Expression of Concern may be published to inform readers of ongoing matters in line with COPE guidance . In cases of suspected research or publication misconduct, it may be necessary for the Editor or Publisher to contact and share submission details with third parties including authors’ institutions and ethics committees in line with COPE Guidelines. Advice may also be sought directly from COPE.
Plagiarism, whether intentional or not, is not tolerated in Karger’s journals. Plagiarism includes, but is not limited to, copying or reusing text, ideas, images or data from other sources without clear attribution, and goes against the principle of academic publishing. Karger may subject any manuscripts to text overlap detection software (Crossref Similarity Check, powered by iThenticate) and if the software raises any concerns, there will be a follow-up investigation in line with COPE guidelines . At any stage of peer review, publication, or post-publication, if plagiarism is detected the manuscript may be rejected, corrected or retracted, as appropriate, and we reserve the right to inform the authors' institutions about any plagiarism detected. We expect that our editors and reviewers will inform the journal about any concerns related to plagiarism.
Errata and Retractions
Karger is committed to maintaining the accuracy and integrity of the scientific record. Retractions will be issued where required in accordance with COPE guidelines . Errors in an article that affect the interpretation or conclusion of the article, such as figures or results, or the article metadata, such as the author list, will be corrected through the publication of an Erratum. Please note that the corrections of other errors introduced by authors and missed during the final manuscript proofing stage may be declined. Authors should contact us to report errors in their articles. Please state the journal name, volume, issue and page numbers, the DOI number if the article has not yet been printed, as well as the article title and the nature of the error.
In the case of a publisher error that does not impact the article content, for example, an error in the pagination, the Version of Record will be republished accompanied by a Publisher’s Note to indicate the date and nature of the error that was rectified. A separate Erratum will not be published.
Karger Publishers remains neutral in cases of jurisdictional claims made or implied in published texts, maps or authors’ institutional affiliations.
INFORMATION
- Contact & Support
- Information & Downloads
- Rights & Permissions
- Terms & Conditions
- Catalogue & Pricing
- Policies & Information
- People & Organization
- Stay Up-to-Date
- Regional Offices
- Community Voice
SERVICES FOR
- Researchers
- Healthcare Professionals
- Patients & Supporters
- Health Sciences Industry
- Medical Societies
- Agents & Booksellers
Karger International
- S. Karger AG
- P.O Box, CH-4009 Basel (Switzerland)
- Allschwilerstrasse 10, CH-4055 Basel
- Tel: +41 61 306 11 11
- Fax: +41 61 306 12 34
- Email: [email protected]
- Experience Blog
- Privacy Policy
- Terms of Use
This Feature Is Available To Subscribers Only
Sign In or Create an Account
- Open access
- Published: 03 August 2023
Raising concerns on questionable ethics approvals – a case study of 456 trials from the Institut Hospitalo-Universitaire Méditerranée Infection
- Fabrice Frank ORCID: orcid.org/0000-0002-6524-4570 1 ,
- Nans Florens ORCID: orcid.org/0000-0002-0960-6055 2 ,
- Gideon Meyerowitz-katz ORCID: orcid.org/0000-0002-1156-9345 3 ,
- Jérôme Barriere ORCID: orcid.org/0000-0003-1113-8948 4 ,
- Éric Billy ORCID: orcid.org/0000-0002-8859-0089 5 ,
- Véronique Saada ORCID: orcid.org/0000-0001-8021-9050 6 ,
- Alexander Samuel ORCID: orcid.org/0000-0003-4547-5280 7 ,
- Jacques Robert ORCID: orcid.org/0000-0003-4380-1476 8 &
- Lonni Besançon ORCID: orcid.org/0000-0002-7207-1276 9
Research Integrity and Peer Review volume 8 , Article number: 9 ( 2023 ) Cite this article
15k Accesses
1 Citations
1536 Altmetric
Metrics details
The practice of clinical research is strictly regulated by law. During submission and review processes, compliance of such research with the laws enforced in the country where it was conducted is not always correctly filled in by the authors or verified by the editors. Here, we report a case of a single institution for which one may find hundreds of publications with seemingly relevant ethical concerns, along with 10 months of follow-up through contacts with the editors of these articles. We thus argue for a stricter control of ethical authorization by scientific editors and we call on publishers to cooperate to this end.
We present an investigation of the ethics and legal aspects of 456 studies published by the IHU-MI (Institut Hospitalo-Universitaire Méditerranée Infection) in Marseille, France.
We identified a wide range of issues with the stated research authorization and ethics of the published studies with respect to the Institutional Review Board and the approval presented. Among the studies investigated, 248 were conducted with the same ethics approval number, even though the subjects, samples, and countries of investigation were different. Thirty-nine (39) did not even contain a reference to the ethics approval number while they present research on human beings. We thus contacted the journals that published these articles and provide their responses to our concerns. It should be noted that, since our investigation and reporting to journals, PLOS has issued expressions of concerns for several publications we analyze here.
This case presents an investigation of the veracity of ethical approval, and more than 10 months of follow-up by independent researchers. We call for stricter control and cooperation in handling of these cases, including editorial requirement to upload ethical approval documents, guidelines from COPE to address such ethical concerns, and transparent editorial policies and timelines to answer such concerns. All supplementary materials are available.
Peer Review reports
There are over 27 million scientific articles listed on the National Health Institute Platform PubMed. Previous investigations have shown that about 2% of scientists admitted to have fabricated, falsified or modified data or results at least once [ 1 ]. The business of scientific publication has surged during the last decade, including a staggering growth in the number of articles submitted and finally accepted for publication [ 2 ]. The peer review process is crucial for assessing the quality of hypotheses, methods, reliability of the data, and identifying any obvious ethical shortcomings. The Covid-19 pandemic was a stress test for the academic publishing system and unveiled several failures in processes evaluating quality of scientific publications [ 3 , 4 , 5 , 6 , 7 ]. Neglected or non-existent review procedures [ 3 , 6 ], editorial conflicts of interests combined with expeditive peer review [ 3 ], inconsistent publications with, e.g., missing data [ 8 ], failure to retract or delayed retractions [ 6 , 7 ], and irregularities in legal permissions are among the most common concerns seen in biomedical publications relating to the COVID-19 pandemic. The latter is particularly critical as it can directly put study participants at risk.
Regulation of clinical research became gradually an issue after the crimes perpetrated by the Nazis during World War II [ 9 ]. Later, these issues were reinforced by notoriously unethical studies conducted in the following decades, such as the Tuskegee Syphilis Study [ 10 ]. In the 1950 and 1960 s, the thalidomide adverse effects scandal reinforced this tendency by defining vulnerable groups such as, pregnant women [ 11 ]. More recently, ethics has evolved with new concerns for the protection of healthy volunteers following the TGN1412 disaster [ 12 ].
To prevent these ethical quandaries, countries have different governance system for ensuring appropriate moral conduct in clinical research. This commonly includes both legal requirements for conducting research, as well as guidelines for the oversight into and approval of ethical research. In France, this is done through the legislative framework. In this context, the French legislation was updated in 2016 with the Jardé Law on good practices in clinical research [ 13 ]. It should be noted that the legislative framework encountered in France is not the same as in other countries which may have other governance instead. French regulation requires that any experimentation on human beings must be approved by an independent ethics committee and depending on the complexity of the protocol, additional authorizations are required, especially regarding the collection of body fluids such as stool, vaginal secretions or urine.
In this paper, we present an investigation into papers published by the Institut Hospitalo-Universitaire Méditerranée Infection (IHU-MI ), a large clinical and research center in the city of Marseilles in the south of France. The IHU-MI employs over 700 people, covering a range of research topics related to infectious disease including basic biomedical research, epidemiological work, clinical trials,
This center has been the subject of research controversy since the advent of the COVID-19 pandemic, largely due to the actions of Professor Didier Raoult, former head of the institute. The original notoriety of the IHU-MI came from the now-infamous promotion of hydroxychloroquine, an anti-malarial medication, in combination with azithromycin for the treatment of COVID-19 [ 14 ]. This regimen was promoted as the most effective treatment for COVID-19 based on the results of a small, poorly-controlled observational study that has been described as having “major methodological shortcomings which make it nearly if not completely uninformative” and “fully irresponsible” in an independent review commissioned by the parent publishing company Elsevier [ 15 , 16 , 17 , 18 ]. This study also elicited negative peer-review comments on PubPeer, an independent review site that collates commentary on scientific studies, and from the French authorities [ 19 ]. The hydroxychloroquine/azithromycin regimen has remained popular in some minds despite increasingly robust evidence that it is ineffective in the treatment of COVID-19 and furthermore may increase the risk of death [ 18 ], demonstrating once more the danger of problematic and potentially unethical research [ 20 ]. Of note, IHU-MI has had previous retractions for alleged data fabrication, but thus far not due to ethical concerns [ 20 ].
Concerns on ethical approvals from IHU-MI have been raised outside scientific journals [ 21 ]. In August 2021, Elisabeth Bik pointed out issues about ethical approvals including research on vulnerable populations like homeless study participants [ 22 ]. These initial reports prompted us to further investigate potential concerns about ethics in the published literature from the institute. We have found and report below about highly worrying data in IHU-MI publications.
For post-publication critiques, the Committee on Publication Ethics (COPE) recommends referring to the journal or publisher policy [ 23 ], depending on the availability of an editorial policy for the reported issue. In case editorial policy does not take post-publication critiques into account, this policy should be amended. We can thus hope that the critiques formulated here might be helpful for journals to improve their peer reviewing policies. This is the whole meaning of our approach.
Investigation
Due to a great deal of national interest following publicization of poor research practices at IHU-MI, the French government investigated the unit and then launched legal actions in early September 2022. This followed a damning report from IGAS (Inspection Générale des Affaires Sociales) on the ethics and conduct of research taking place at IHU-MI during the period of investigation [ 24 ]. The seriousness of the accusations reported, combined with previous reports of questionable conduct and publication ban [ 20 ], made us question whether current academic editorial processes could have caught such concerns regarding the legal framework implemented at IHU-MI when conducting clinical trials. This paper provides the results of a detailed review covering the work of researchers at IHU-MI, analyzing published ethical statements.
After noticing that some Institutional Review Board (IRB) identification numbers were identical in several publications from the IHU-MI, we started by screening studies on IRB numbers on “Google Scholar”. We found several repetitions, and we finally noticed that one of the IRB approval numbers (09–022) appeared in hundreds of publications while the publications’ topics and the patients involved in studies were significantly different. We then used “Google Scholar” to identify all occurrences of this approval number.
We then decided to further investigate the bibliography of this institute by screening PubPeer reports and analyzing them. We did not contact directly IHU-MI since their answer to the PubPeer posts showed they were already aware of the concerns reported here. Furthermore, cyber and legal harassment made any direct contact with this institute more difficult than it should have been [ 21 , 25 , 26 ]. We only recently contacted Prof. Didier Raoult, former head of IHU-MI, since he was listed as editor-in-chief of the journals where some of the articles we report here were published but received no answer to this day. This list has also been reported to French Health Authority, namely the “Agence Nationale de Sécurité du Médicament” (ANSM), in charge of the evaluation of the legality of clinical studies, in July 2022. These publications were only evaluated regarding ethics criteria and our findings have no significance regarding the validity of their content.
We attempted to conduct a relatively systematic review of all recent biomedical research published by key authors at the IHU-MI, using “Google Scholar” profiles and PubPeer. This involved searching the bibliographies of senior scholars (i.e., Professors) at the institute, however given the sheer number of studies, we limited the search parameters to those published in the last 25 years. Screening on PubPeer was conducted by searching author names, and reviewing the resulting comments on papers. The results from PubPeer were then used to complete the data obtained from analysis of “Google Scholar” results. Frequently-repeating IRB-approval numbers were entered into “Google Scholar” to list of all their occurrences.
Request for the official IRB approval
We qualitatively determined whether IRB approval was likely to have been granted for different studies. This was done by discussion among the authors, and we present the tabulated findings in the results of the number of separate published works that use the same IRB approval number.
Contact of editors
While papers have been retracted in the past for issues concerning ethical approval [ 27 ], most of the Retraction Watch database entries seem to contain only Expressions of Concern (EOCs) or retractions relating to “ethical violations by author” or “lack of IRB/IACUC approval.” As such, it is not surprising that COPE did not provide guidelines on reporting and investigating IRB approval duplications such as what we have found. Indeed, current COPE guidelines on issues on ethics approval only focus on handling concerns at the time of submission of a manuscript [ 28 ] and not after publication or for cases of potentially fraudulent duplication of IRB approval numbers. As a result, we have further analysed the dataset to append the journal Editor-in-Chief’s name and email after screening the journals websites for information. In some cases, such as discontinued journals, the last known editor-in-chief was contacted. When no contact form or mail was available on the journal’s page, we contacted the editor-in-chief directly through academic mail found on the university affiliation’s website.
We thus contacted all editors of journals that published papers for which our analysis could raise concerns. The number of editors we contacted may seem very low compared to the number of publications we report, but many of them have been published in the same journal: for example “New Microbes and New Infections” published 135 of the studies we report. The low number of answers (despite reminder emails) led us to contact some publishers including Elsevier, for “New Microbes and New Infections”, without any satisfactory answer to this day. It should be noted that some of the authors of the papers we investigated are in many cases on the editorial team of the journals which have published the papers. Such concerns had already been highlighted with respect to this institute and some of their COVID-19 papers which had been peer-reviewed under 24 h in journals in which the authors presented with editorial conflicts of interest [ 3 ]. Related to our concerns, the journal “New Microbes and New Infections” is famous for being closely related to the team whose work we investigated [ 29 ] and had many members of the IHU-MI on the editorial board [ 30 ]. As such, editorial contact to report on the issues we have identified was indeed difficult.
At the time of writing, we have not approached the STM integrity hub. We have only contacted the ethics officer at Elsevier, since this publisher counts for most of the articles we report; from some answers of the editor-in-chief, we have no doubt that they did not intend to take any action.
After cleaning, we noticed that the IRB approval number 09–022 had been used 248 times over 12 years (between 2009 and 2021). Reusing approvals is allowed if results are from samples originally approved by the committee and in compliance with local laws related to clinical research. However, we found that those 248 publications covered a large variety of samples (stool, vaginal secretions, urine, samples taken during surgical procedures), a wide array of populations (adults, children, healthy volunteers, obese patients, etc.) and countries (France, Senegal, Niger, Gabon, Saudi Arabia, etc.) as depicted in Fig. 1 (see the Additional file 2 “Table S1 – Studies_with_09–022_IRB.csv”). Among the 248 studies identified, we have found at least one that was conducted after the Jardé Law was implemented, as well as many more published after 2016 with no dates of patient enrollment identifiable.
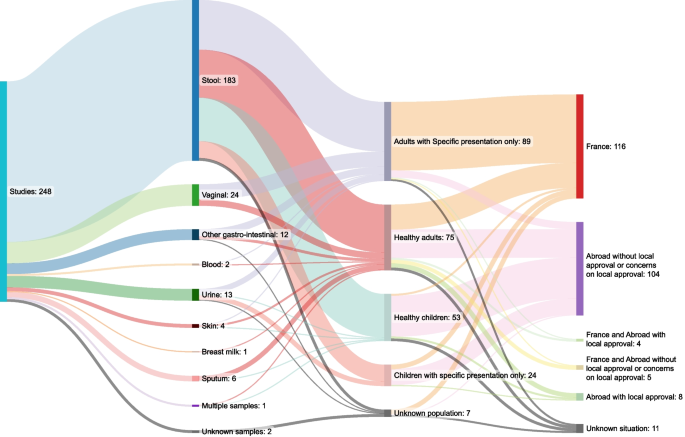
Various subjects, samples, and countries for the 248 studies with the IRB number 09–022
Further investigation in the bibliography from IHU-MI showed a total of 456 studies that could have ethical and legal concerns of the same type: multiple and different studies with the same IRB, absence of legal authorization, recruitment starting before authorization was obtained, etc. (see Additional file 2 Table S2 “Table S2-Clinical_Research_Papers_With_Ethical_Concerns.csv”).
In biomedical research, researchers, authors, sponsors, editors and publishers all have ethical obligations with regard to the publication and dissemination of the results of research. Most, if not all, scientific publishing companies have subscribed to the declaration of Helsinki [ 31 , 32 , 33 , 34 ], which is also recommended by COPE [ 35 ].
We could not access the original file with the IRB number 09–022, even after requesting this document from French authorities. However, we obtained a copy of the outline of the document (see Additional file 3 ). Based on our analysis, this form does not allow such a wide variety of samples, clinical conditions, and geographical origin to be documented. We could not find any reasonable explanation for such a multiplicity of identical occurrences in the literature. The original document should mention all those samples, conditions, and countries. If amendments have been made, they have not been explicitly mentioned in the articles from IHU-MI cited herein.
Contact of the editors
An overview of the journals that have published most of these articles is available in Fig. 2 .
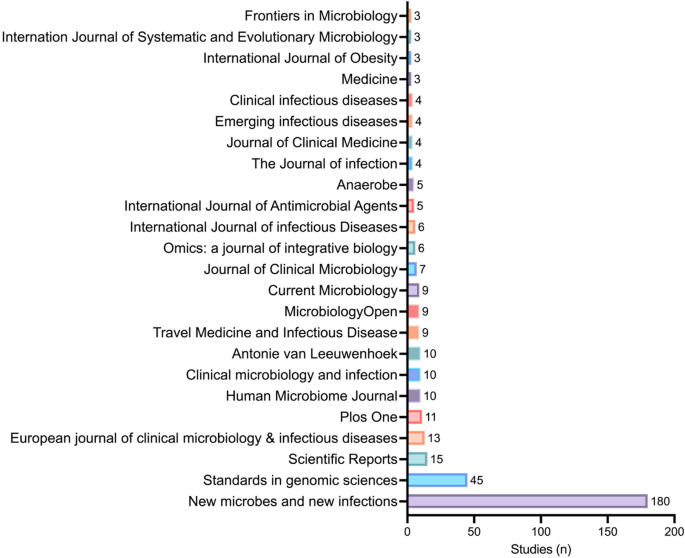
Journals involved in the 456 studies with legal authorization concerns
Out of the 85 journals that we contacted, 19 editorial teams have responded to our email while 66 have not replied to us yet. A complete list of the journals, dates at which they have been contacted, as well as date and summary of the response is available in Additional file 2 (“Table S3 – Editors contact.csv”).
In summary, among the studies we have investigated, 248 were conducted with the same ethics approval number, even though the subjects, samples, and countries of investigation were different. Thirty-nine (39) of the manuscripts we considered did not even contain a reference to the ethics approval number although they contained research on human beings. We have contacted the 85 journals that published these articles and only 19 of them have replied to our queries so far. However, a publisher, PLOS [ 36 ], has issued expressions of concerns for several publications we have analyzed over ethical concerns.
Taking French legal framework into account, we made a rapid analysis and this showed these studies could include different categories of research involving human beings (RIPH) i.e. 6 RIPH 1, 67 RIPH 2 and 202 RIPH 3 for which no legally-required authorization has been reported by the authors.
Since we have reached out to editors, PLOS Biology has issued a wide expression of concern for 49 articles published by this institute, including 12 for which we have reported concerns on ethics approval. Out of these 12 articles, ten use the same ethics approval number, namely 09–022. Given that we have not raised concerns about the remaining 37 articles, there appears to be some concern at the editorial level of the work done by IHU-MI more broadly than this investigation identified. Thus, our figures should serve as a baseline estimate of the total number of papers which may have issues that are associated with IHU-MI. To our best knowledge, no other public editorial decision has been made from other publishing venues yet, although we hope that the concerns from PLOS Biology will help either obtaining clarifications from the authors on such concerns, or drive other journals to publicly respond too.
Editorial practices in verifying ethics and lawfulness of clinical research are still very heterogeneous and all journal are not members of COPE. We wish to initiate a conversation to improve the ethical controls at the editorial level across published academic research, and for changes to facilitate post-hoc investigations in future work, despite not suggesting any mean of enforcing these controls.
While some publishers already require the upload of ethics approval, this is not a largely adopted requirement. We thus argue that the practice should become more widely and rigorously adopted, or that, at the very least, ethics approval numbers are provided as metadata along a submission such that post-hoc analysis could be done in a more systematically fashion through mining of submission’s metadata. This metadata could be made available through PubMed along with a basic description of the study and its targeted population, intervention(s), and country of study.
We argue that submission processes should be amended to require the potentially confidential upload of ethical documents linked to clinical research, and that editorial procedures should pay attention to the international (and potentially local) ethical framework for research by including, for instance, basic verification steps. This responsibility should absolutely not be placed on reviewers whose primary mission is to ensure the scientific robustness of the research as well as its relevance for publication. Indeed, much of this process could be easily automated by publishing companies such as Elsevier to avoid precisely the issues identified in this review. Placing the weight on an editorial responsibility would also facilitate further verification. Indeed, as we have ourselves experienced, independent researchers investigating the adequacy of ethical documents are not likely to obtain an answer from IRBs or ethical committees, while editors and publishers would have an easier and more legally anchored claim to request those documents. In conclusion, there is an urgent need for publishers to require clinical research approvals. This could be done by requesting validation from the sponsoring organization or from the authority that issued the IRB number. There is also a critical need for COPE to provide clear guidelines on how to report (for researchers) and how to handle (for editors and journals) issues with ethics approval in published manuscript. While we are aware that further editorial verifications could create additional and potentially difficult to navigate publication steps, we however believe that the recommendations we put forward here could easily be put in place without an increased bureaucratic cost. Indeed, our most drastic recommendation would be to normalize the upload of IRB documents for which IRB and publishers could easily implement regulations based on existing policies from journals which already implement such requirements.
We finally argue that editorial responses within a strict timeline should be put in place, such that journals and editorial teams have a responsibility to respond to ethical queries from researchers within a reasonable time [ 7 ] as well as disclose reasonable concerns that have been publicly raised on articles, and this even before reaching out to authors.
Since we cannot ask every editor to know every framework of every country for every type of clinical research, this would of course not solve all the problems, but we think that this might be a step forward to a better respect of ethics and thus of patient rights. While additional editorial constraints are unlikely to eliminate fraud or questionable practices altogether, they can help limit them, raise awareness about them, and facilitate their detection. We believe that the new editorial policies on ethics and IRB approval that we suggest would advance scientific processes in the same way that requiring the publication of clinical trials registration has facilitated research on the spin of medical research [ 37 , 38 ], the prevalence of outcome switching, and other questionable reporting practices and likely help reduce these practices and adopt new standards to produce more robust and ethical research.
We have presented a case study of 456 trials from the Institut Hospitalo-Universitaire Méditerranée Infection. Our investigation has revealed serious concerns on the ethics approvals of these trials ranging from the re-use of the same ethics approval number 248 times on trials with significantly different subjects, samples, and countries of investigation, to potential lack of local ethics approval for studies conducted abroad. To the best of our knowledge, our investigation is the first to reveal concerns over the potentially inappropriate reuse of ethics approval numbers on such a massive scale. While our concerns have been acted on by one publisher (PLoS), most publishers are either still investigating the issue or have not yet responded to us. This investigation thus highlights the needs for guidelines and processes for readers, reviewers, and editorial teams, to report and respond to ethics approval misuse and concerns.
Availability of data and materials
The datasets supporting this article are available.
Abbreviations
Institut Hospitalo-Universitaire.
IHU Méditerannée Infection.
Institutional Review Board.
Hydroxychloroquine.
Agence Nationale de Sécurité du Médicament.
Recherche impliquant la personne humaine i.e. research involving human subjects.
Fanelli D. How many scientists fabricate and falsify Research? A systematic review and Meta-analysis of Survey Data. PLoS ONE. 2009;4:e5738. https://doi.org/10.1371/journal.pone.0005738 .
Article Google Scholar
Bornmann L, Haunschild R, Mutz R. Growth rates of modern science: a latent piecewise growth curve approach to model publication numbers from established and new literature databases. Humanit Soc Sci Commun. 2021;8. https://doi.org/10.1057/s41599-021-00903-w .
Besançon L, Peiffer-Smadja N, Segalas C, et al. Open science saves lives: lessons from the COVID-19 pandemic. BMC Med Res Methodol. 2021;21. https://doi.org/10.1186/s12874-021-01304-y .
Fraser N, Brierley L, Dey G, et al. The evolving role of preprints in the dissemination of COVID-19 research and their impact on the science communication landscape. PLoS Biol. 2021;19:e3000959. https://doi.org/10.1371/journal.pbio.3000959 .
Lawrence JM, Meyerowitz-Katz G, Heathers JAJ, et al. The lesson of ivermectin: meta-analyses based on summary data alone are inherently unreliable. Nat Med. 2021;27:1853–4. https://doi.org/10.1038/s41591-021-01535-y .
Barrière J, Frank F, Besançon L, et al. Scientific Integrity requires Publishing Rebuttals and Retracting Problematic Papers. Stem Cell Rev and Rep. 2022. https://doi.org/10.1007/s12015-022-10465-2 .
Besançon L, Bik E, Heathers J, et al. Correction of scientific literature: too little, too late! PLoS Biol. 2022;20:e3001572. https://doi.org/10.1371/journal.pbio.3001572 .
Mehra MR, Ruschitzka F, Patel AN. Retraction-hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet. 2020;395:1820. https://doi.org/10.1016/S0140-6736(20)31324-6 .
Kim WO. Institutional review board (IRB) and ethical issues in clinical research. Korean J Anesthesiol. 2012;62:3–12. https://doi.org/10.4097/kjae.2012.62.1.3 .
Corbie-Smith G. The continuing legacy of the Tuskegee syphilis study: considerations for clinical investigation. Am J Med Sci. 1999;317:5–8. https://doi.org/10.1016/S0002-9629(15)40464-1 .
Waggoner MR, Lyerly AD. Clinical trials in pregnancy and the “shadows of thalidomide”: revisiting the legacy of Frances Kelsey. Contemp Clin Trials. 2022;119:106806. https://doi.org/10.1016/j.cct.2022.106806 .
Attarwala H. TGN1412: from discovery to disaster. J Young Pharm. 2010;2:332–6. https://doi.org/10.4103/0975-1483.66810 .
French legal approach to clinical research. Anaesthesia Critical Care & Pain Medicine.2018;37:607–14. https://doi.org/10.1016/j.accpm.2018.10.013.
Gautret P, Lagier J-C, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56:105949. https://doi.org/10.1016/j.ijantimicag.2020.105949 .
Rosendaal FR. Review of: “Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial Gautret et al. Int J Antimicrob Agents. 2020;56:106063. https://doi.org/10.1016/j.ijantimicag.2020.106063 .
Oransky I. French hydroxychloroquine study has “major methodological shortcomings” and is “fully irresponsible,” says review, but is not being retracted. Retraction Watch 2020. https://retractionwatch.com/2020/07/19/french-hydroxychloroquine-study-has-major-methodological-shortcomings-and-is-fully-irresponsible-says-review-but-is-not-being-retracted/ . Accessed 19 Jan 2022.
Machiels JD, Bleeker-Rovers CP, ter Heine R, et al. Reply to Gautret et al: hydroxychloroquine sulfate and azithromycin for COVID-19: what is the evidence and what are the risks? Int J Antimicrob Agents. 2020;56:106056. https://doi.org/10.1016/j.ijantimicag.2020.106056 .
Axfors C, Schmitt AM, Janiaud P, et al. Mortality outcomes with hydroxychloroquine and chloroquine in COVID-19 from an international collaborative meta-analysis of randomized trials. Nat Commun. 2021;12. https://doi.org/10.1038/s41467-021-22446-z .
Contrôle de l’IHU Méditerranée infection - IGAS. - Inspection générale des affaires sociales. https://www.igas.gouv.fr/spip.php?article861 . Accessed 19 Jan 2022.
6 Mary C. Didier Raoult profile. Sound and fury in the microbiology lab. Science 2012;335:1033–5. https://doi.org/10.1126/science.335.6072.1033 .
Schneider L. Didier Raoult fraud: “Je ne regrette rien.” For Better Science 2021. https://forbetterscience.com/2021/03/23/didier-raoult-fraud-je-ne-regrette-rien/ . Accessed 21 Feb 2023.
Concerns about Marseille’s IHU-MI/AMU papers – part 2. Science Integrity Digest 2021. https://scienceintegritydigest.com/2021/08/31/concerns-about-marseilles-IHU-MI-amu-papers-part-2/ . Accessed Feb 21 2023.
Handling of post-publication critiques. Committee on Publication Ethics. ; 2021. https://doi.org/10.24318/o1VgCAih . Accessed 19 Feb 2022.
IHU de Didier Raoult. Une information judiciaire ouverte à Marseille suite au rapport de l’ANSM. Ouest-france.fr. https://www.ouest-france.fr/sante/ihu-de-didier-raoult-une-information-judiciaire-ouverte-a-marseille-suite-au-rapport-de-l-ansm-7d300800-2ddd-11ed-82ab-ca288831284e#:~:text=de%20Didier%20Raoult.-,Une%20information%20judiciaire%20ouverte%20%C3%A0%20Marseille%20suite%20au%20rapport%20de,%C3%A9tait%20dirig%C3%A9%20par%20Didier%20Raoult . Accessed 15 Feb 2023.
Besançon L, Samuel A, Sana T, Rebeaud ME, Guihur A, Robinson-Rechavi M, Berre NL, Mulot M, Meyerowitz-Katz G, Hervé, Maisonneuve, Brian A, Nosek. (2021). “Open Letter: Scientists Stand up to Protect Academic Whistleblowers and Post-publication Peer Review.” OSF Preprints. May 18. doi: https://doi.org/10.31219/osf.io/2awsv .
Else H. Scientific image sleuth faces legal action for criticizing research papers. Nature. 2021;594:17–8. https://doi.org/10.1038/d41586-021-01430-z .
Marcus A. 250th COVID-19 retraction is for faked ethics approval. Retraction Watch. 2022. https://retractionwatch.com/2022/07/21/250th-covid-19-retraction-is-for-faked-ethics-approval/ (Accessed 28 Feb 2023).
What to do if you suspect an ethical problem. Committee on Publication Ethics. 2006. https://publicationethics.org/sites/default/files/ethical-problem-in-submitted-manuscript-cope-flowchart.pdf . Accessed 28 Feb 2023.
Locher C, Moher D, Cristea IA, et al. Publication by association: how the COVID-19 pandemic has shown relationships between authors and editorial board members in the field of infectious diseases. BMJ Evid Based Med. 2022;27:133–6. https://doi.org/10.1136/bmjebm-2021-111670 .
Editorial Board - New Microbes and New Infections - Journal – Elsevier. Archived 04. August 2021. https://web.archive.org/web/20210804233115/https://www.journals.elsevier.com/new-microbes-and-new-infections/editorial-board .
Mandatory author declaration. https://www.elsevier.com/__data/promis_misc/JBMTauthor_declaration.pdf . Accessed 19 Jan 2022.
Research Ethics | Nature Portfolio. https://www.nature.com/nature-portfolio/editorial-policies/ethics-and-biosecurity . Accessed 19 Jan 2022.
Best Practice Guidelines on Research Integrity and Publishing Ethics. https://authorservices.wiley.com/ethics-guidelines/index.html . Accessed 19 Jan 2022.
Research involving human. participants, their data or biological material. https://www.springer.com/gp/editorial-policies/research-involving-human-and-or-animal-participants . Accessed 19 Jan 2022.
Code of conduct and best practice guidelines for, journal editors. https://publicationethics.org/files/Code_of_conduct_for_journal_editors_Mar11.pdf . Accessed 19 Jan 2022.
PLOS flags nearly 50 papers by controversial French COVID researcher for ethics concerns. https://retractionwatch.com/2022/12/13/plos-flags-nearly-50-papers-by-controversial-french-covid-researcher-for-ethics-concerns/ . Accessed 19 Jan 2022.
Austin J, Smith C, Natarajan K, et al. Evaluation of spin within abstracts in obesity randomized clinical trials: a cross-sectional review: spin in obesity clinical trials. Clin Obes. 2019;9:e12292. https://doi.org/10.1111/cob.12292 .
Jellison S, Roberts W, Bowers A, et al. Evaluation of spin in abstracts of papers in psychiatry and psychology journals. BMJ Evid Based Med. 2019;25:178–81. https://doi.org/10.1136/bmjebm-2019-111176 .
Download references
The authors received no specific funding for this work.
Author information
Authors and affiliations.
Independent researcher, Essaouira, Morocco
Fabrice Frank
Department of Nephrology, Hôpitaux Universitaires de Strasbourg, Université de Strasbourg, Strasbourg, France
Nans Florens
School of Health and Society, University of Wollongong, Wollongong, Australia
Gideon Meyerowitz-katz
Medical Oncology Department, Polyclinique Saint-Jean, Cagnes-sur-Mer, France
Jérôme Barriere
Independent researcher, Strasbourg, France
Biopathology department, Gustave Roussy Anti-Cancer Center, Villejuif, France
Véronique Saada
Independent researcher, Nice, France
Alexander Samuel
Université de Bordeaux, INSERM Unité 1312, Bordeaux, France
Jacques Robert
Media and Information Technology, Linköping University, Norrköping, Sweden
Lonni Besançon
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization: FF, NF, LB. Methodology: FF, NF, LB. Investigation: FF. Writing – original draft: FF, NF, EB. Writing – review & editing: FF, NF, GMK, JB, EB, VS, AS, LB, JR. The author(s) read and approved the final manuscript.
Corresponding author
Correspondence to Lonni Besançon .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
No competing interest. EB is a full-time Novartis employee. Views are his own.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: figure 1..
Various subjects, samples, and countries for the 248 studieswith the IRB number 09-022. Figure 2. Journals involved in the 444 studies with legal authorization concerns.
Additional file 2: Table S1.
Studies_with_09–022_IRB. Table S2. Clinical_Research_Papers_With_Ethical_Concerns. Table S3. Editors contact.
Additional file 3:
Outline of IRB approval.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Frank, F., Florens, N., Meyerowitz-katz, G. et al. Raising concerns on questionable ethics approvals – a case study of 456 trials from the Institut Hospitalo-Universitaire Méditerranée Infection. Res Integr Peer Rev 8 , 9 (2023). https://doi.org/10.1186/s41073-023-00134-4
Download citation
Received : 13 February 2023
Accepted : 22 May 2023
Published : 03 August 2023
DOI : https://doi.org/10.1186/s41073-023-00134-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Clinical research
- Scientific publications
- Scientific publishing
- IRB (Institutional Review Board)
Research Integrity and Peer Review
ISSN: 2058-8615
- Submission enquiries: [email protected]
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts

Plagiarism, Cheating and Research Integrity: Case Studies from a Masters Program in Peru
Andres m. carnero.
1 School of Public Health and Administration, Universidad Peruana Cayetano Heredia, Lima, Peru
Percy Mayta-Tristan
2 School of Medicine, Universidad Peruana de Ciencias Aplicadas, Lima, Peru
Kelika A. Konda
3 David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA
Edward Mezones-Holguin
Antonio bernabe-ortiz.
4 CRONICAS, Center of Excellence in Chronic Diseases, Universidad Peruana Cayetano Heredia, Lima, Peru
German F. Alvarado
Carlos canelo-aybar, jorge l. maguiña.
5 Department of Parasitology, U.S. Naval Medical Research Unit No. 6 (NAMRU-6), Lima, Peru
Eddy R. Segura
Antonio m. quispe.
6 Department of International Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA
Edward S. Smith
7 School of Medicine, Universidad San Martin de Porres, Lima, Peru
Angela M. Bayer
Andres g. lescano.
Plagiarism is a serious, yet widespread type of research misconduct, and is often neglected in developing countries. Despite its far-reaching implications, plagiarism is poorly acknowledged and discussed in the academic setting, and insufficient evidence exists in Latin America and developing countries to inform the development of preventive strategies. In this context, we present a longitudinal case study of seven instances of plagiarism and cheating arising in four consecutive classes (2011–2014) of an Epidemiology Masters program in Lima, Peru, and describes the implementation and outcomes of a multifaceted, “zero-tolerance” policy aimed at introducing research integrity. Two cases involved cheating in graded assignments, and five cases correspond to plagiarism in the thesis protocol. Cases revealed poor awareness of high tolerance to plagiarism, poor academic performance, and widespread writing deficiencies, compensated with patchwriting and copy-pasting. Depending on the events’ severity, penalties included course failure (6/7) and separation from the program (3/7). Students at fault did not engage in further plagiarism. Between 2011 and 2013, the Masters program sequentially introduced a preventive policy consisting of: (i) intensified research integrity and scientific writing education, (ii) a stepwise, cumulative writing process; (iii) honor codes; (iv) active search for plagiarism in all academic products; and (v) a “zero-tolerance” policy in response to documented cases. No cases were detected in 2014. In conclusion, plagiarism seems to be widespread in resource-limited settings and a greater response with educational and zero-tolerance components is needed to prevent it.
Science aims at expanding knowledge through systematic generation and testing of hypotheses, which can then be used for the benefit of humanity. To achieve this goal, science is guided by several values, including objectivity, honesty and unselfishness ( Allchin 1999 ; Committee on Science Engineering and Public Policy et al. 2009 ). Disregard to these values can result in research misconduct ( Steneck 2006 ; Committee on Science Engineering and Public Policy et al. 2009 ), which distorts the scientific record, wastes resources, and undermines the trust of society in science ( Steneck 2006 ). Plagiarism, the misappropriation of other’s intellectual contribution, is a serious form of research misconduct, and probably one of the most frequently reported type of research misconduct ( Smith 2000 ). Despite the challenges in ascertaining the true frequency of plagiarism, recent estimates (around 2 %) suggest that it is much more common than previously thought ( Pupovac and Fanelli 2015 ). However, this high frequency compared to other forms of research misconduct may partially result from enhanced detection by electronic methods.
Plagiarism can occur at any point in the career of a researcher, but it is more frequently reported in the early stages ( Martinson et al. 2005 ), and relatively few studies have explored its origins during undergraduate and early post-graduate research ( Swazey et al. 1993 ; Wadja-Johnston et al. 2001 ; Krstic 2015 ). Early training stages may constitute a critical period to prevent plagiarism, when students begin to actively engage in research. If uncorrected, plagiarism and cheating may continue throughout the researcher’s career, and can potentially lead to other misconduct ( Lovett-Hopper et al. 2007 ; Park 2003 ). During training, plagiarism can become part of a broader set of dishonest behaviors aimed at obtaining undeserved academic advantage (such as copying in an exam, taking credit for another’s work, and prohibited collaboration between students), which are collectively termed “cheating” ( Park 2003 ).
Plagiarism is a global problem, yet evidence of its occurrence comes almost exclusively from developed countries ( Ana et al. 2013 ). Studies exploring plagiarism in developing countries are critically needed ( Ana et al. 2013 ), given that cultural and economic factors may affect the perception of and engagement in plagiarism ( Davis 2003 ; Martin 2012 ). In developing countries, several unique factors may enable plagiarism such as: (i) lack of training in the Responsible Conduct of Research (RCR) ( Rodriguez and Lolas 2011 ; Davis 2003 ; Vasconcelos et al. 2009 ; Cameron et al. 2012 ); (ii) poor development of writing skills ( Heitman and Litewka 2011 ; Vasconcelos et al. 2009 ; Cameron et al. 2012 ); (iii) tolerance to misconduct during education and professional activities ( Heitman and Litewka 2011 ; Vasconcelos et al. 2009 ); (iv) lack of institutional policies and oversight of academic centers and journals ( Rodriguez and Lolas 2011 ; Heitman and Litewka 2011 ; Vasconcelos et al. 2009 ); (v) differing perceptions of intellectual property and misconduct ( Heitman and Litewka 2011 ; Davis 2003 ; Cameron et al. 2012 ); (vi) the pervasive effect of corruption ( Heitman and Litewka 2011 ); and (vii) cultural differences in values ( Rodriguez and Lolas 2011 ; Heitman and Litewka 2011 ; Davis 2003 ; Vasconcelos et al. 2009 ; Cameron et al. 2012 ). Discussing plagiarism in Latin America is an important issue, given the dramatic growth of research activities in the region in the last two decades ( Van Noorden 2014 ; Catanzaro et al. 2014 ). In particular, sporadic reports have highlighted the occurrence of plagiarism in research conducted in Latin America ( Vasconcelos et al. 2009 ; Alfaro-Tolosa et al. 2013 ), and the reaction of scientific journals ( Alfaro-Tolosa et al. 2013 ; Almeida et al. 2015 ). In addition, Latin American countries share many cultural features, arising from their common colonial history, that may affect how plagiarism and cheating are perceived ( Martin 2012 ; Salter and Guffey 2001 ), including collectivism, high uncertainty avoidance, high power distance, high indulgence, and a short-term orientation ( Hofstede 2011 ). Finally, the fact that Latin American countries share a common language (mainly Spanish, but also Portuguese, which are closely related) and culture may facilitate the development of effective control strategies with the potential to reach >10 % of the world’s population. Despite its importance, plagiarism has not been systematically studied in Latin America ( Vasconcelos et al. 2009 ; Alfaro-Tolosa et al. 2013 ), and little evidence exists on its frequency, determinants, and consequences in the Latin American setting. In particular, there is a lack of evidence about the implementation of effective, affordable, and context-specific interventions targeted at preventing plagiarism and promoting research and academic integrity among research students in Latin America ( Vasconcelos et al. 2009 ).
In this article, we present a case study of seven instances of plagiarism and cheating detected between 2011 and 2014 in our Masters program in Epidemiologic Research in Lima, Peru, that receives students from a broad range of countries in Latin America. We also describe the implementation and outcomes of a feasible, low-cost, “zero tolerance” policy tailored to promote research integrity among postgraduate research students in Latin America.
The Program
The Masters in Epidemiologic Research of Universidad Peruana Cayetano Heredia is a postgraduate program offered annually since 2007 in Peru. The program aims at training epidemiologists capable of designing and executing high-quality research and publishing in top-tier peer-reviewed international journals. It was created jointly by Universidad Peruana Cayetano Heredia (UPCH), the leading university in biomedical research in Peru ( SCImago Research Group 2015 ), and the U.S. Naval Medical Research Unit No. 6 (NAMRU-6), and was created with funding from the Fogarty International Center (grant 2D43 TW007393). The courses are structured in four 10-week terms, and an overall coursework of 10 months. It is coordinated and taught almost entirely by young scientists with international graduate training, many of them doctorates from U.S. and European universities. The core coursework includes three series of courses taught in each of the four terms of the program, progressively advancing into more complex topics: Biostatistics, Epidemiology, and Research Methods. Additional compulsory courses address complementary research topics: Outbreak investigation (Term 1), Epidemiologic surveillance (Term 2), Validation of instruments (Term 2), Health situation analysis (Term 3), Qualitative research (Term 3), Program evaluation (Term 4), and Writing research proposals (Term 4). Topics on career-advancement are discussed as part of the Research Methods I-IV courses. Since 2013, the program is offered by the UPCH School of Public Health and Administration, whereas past editions were offered by the School of Medicine (2007–2009) and the School of Postgraduate Studies (2010–2012). Academic and research misconduct are critically important issues, and lectures addressing RCR, research ethics, and scientific writing have been part of the program since its inception. Students also complete the CITI research ethics course early in the program ( Braunschweiger and Goodman 2007 ; Litewka et al. 2008 ). Contents on research integrity have evolved in time, expanding the discussion of plagiarism, responsible authorship, and adequate referencing as needed ( Table 1 ). Each class has 20–30 students, usually junior researchers with a biomedical background from local research groups, governmental agencies and clinical/medical centers. Since 2011, the program has received an increasing number of international students from countries in South and Central America and the Caribbean.
Summary of RCR and scientific writing contents in the Masters in Epidemiologic Research Program curriculum (2011–2013), Lima, Peru
CITI Collaborative institutional training initiative ( www.citiprogram.org ), IRB institutional review board
Case Studies
We present here all seven cases of plagiarism and cheating discovered between the fourth (2011) and seventh (2014) classes of the Masters program, although other cases probably remained undetected because of limited surveillance, particularly before 2011. The information presented is based on the experiences of faculty directly handling the cases. All conversations with the students at fault took place in private settings, and class discussions about the events preserved their anonymity. All cases are described as male here to further support anonymity. Figure 1 summarizes key information of the cases and the response measures implemented by our program.

Timeline of cases of academic misconduct in the Masters in Epidemiologic Research Program (2011–2013), Lima, Peru
Cheating Case 1: Epidemiology I Course, April 2011
During the first term Epidemiology course, students were asked to complete a brief individual take-home assignment consisting of short-answer questions, and e-mail their responses to the teaching assistant (TA). Explicit instructions regarding the individual nature of the assignment were given and no discussion was allowed between students. One hour before the deadline, the TA received an e-mail with a student’s homework attached, which had been shared with the rest of the class:
Hi guys! Continuing with the love chain!!!! Hahahaha. I’m sending Epi’s exercise 2, for those of you that are on a tight schedule … please let me know if you find anything wrong!:) ….
The student’s behavior violated the standards of conduct by sharing individual work and requesting review of an individual assignment by other students. The event was immediately communicated to the course and program coordinators, and was discussed with the class 3 h later, preserving the anonymity of the student involved in the case. During the discussion, the class tried to minimize the importance of the event, and faculty required substantial effort to explain that the incident constituted severe academic misconduct and would not be tolerated. Coordinators evaluated potential sanctions to both the student who shared the assignment and the whole class, given that no student reported the incident. Finally, the coordinators decided jointly to fail the student on the assignment, and initiate disciplinary probation for the rest of the academic year. Penalty to the class was waived, given the short time students had to report the event (3 h). Additional sessions to discuss plagiarism and research integrity were added to the curricula. Given that the incident occurred early in the academic year, no information exists regarding the student’s academic performance prior to the incident. The student completed all the required coursework that year under close supervision and intensive counseling, maintaining a low academic performance (ranked 18 of 22), without any evidence of further misconduct.
Plagiarism Case 1: Research Methods I Course, May 2011
A student’s final assignment (first draft of the thesis proposal) exhibited highly heterogeneous writing, with clear and well-written sections interspersed with less-developed sections and poorly presented arguments. In addition, some of the cited material was unrelated to the sources quoted, and the text included uncommon terminology (e.g. general practitioners were referred as “generalist physicians”). The coordinator searched the suspiciously-written sections in Google ® , as described by Rojas-Revoredo et al. (2007) . Several paragraphs were found to be unacknowledged verbatim fragments of published articles. The next day, the course and program coordinator met at the student’s workplace to discuss the incident with the student in private. After initial denial, the student finally accepted committing plagiarism, and was failed in the course and separated from the program for the rest of the year. The university authorities were informed and a misconduct report was filed in the student’s permanent academic record. In addition, the student was warned that consideration of future readmission was conditional on preparing an RCR guide for future students. The incident was discussed with the class at the beginning of the second term and substantial knowledge gaps and ambivalence towards plagiarism were noted. Plagiarism was thoroughly discussed, and five writing workshops were added to each term, at the class’ request. The student contacted the program coordinator in 2012 and was readmitted to the program after completing the required material. Prior to the event, the student had poor performance (ranked 29 of 30). After readmission, the student completed all the required courses under close monitoring and intensive counselling, exhibiting average performance (ranked 16 of 30), and without evidence of any further incidents. After this incident, a paragraph describing plagiarism and its potential sanctions (including course failure) was added to the syllabi of all courses.
Plagiarism Cases 2 and 3: Research Methods II Course, July 2011
The final assignment (final draft of the thesis proposal) of two separate students presented evidence highly suggestive of plagiarism. One case exhibited partial use of quotation marks, while the other presented evidence of self-plagiarism. After searching for the suspicious fragments in the web, plagiarism and self-plagiarism were confirmed. Upon confrontation, both students initially denied the events, but eventually one accepted the misconduct, while the student who committed self-plagiarism did not accept having engaged in any misbehavior. Both students were failed in the course, separated from the program for the rest of the year, and the event was notified to the School of Postgraduate Studies. Two weeks later, the student who did not admit fault contacted the university authorities to start legal action. The authorities from the School of Postgraduate Studies discussed the case in depth with the program coordinator and endorsed program’s decision. Finally, the student desisted from taking legal action, and contacted the program in early 2013 to inquire about readmission, but did not complete the re-admission process. Both students had low academic performance in the program (ranked 26 out of 27). The other student was readmitted in 2012 but exhibited poor performance (ranked 26 of 26), and has not completed all the required coursework yet. The event was discussed with the class, and some students argued that throughout their education they repeatedly witnessed and resorted to similar behavior without any indication that it constituted a dishonest practice. One student even mentioned that a mentor in medical residency once said: “all has been written already, (publishing) only requires putting the pieces together”, which seemed to be an invitation to plagiarize. Starting the following year, all students were required to sign an integrity agreement accepting to avoid plagiarism, disclose any misconduct cases witnessed (whistle-blowing) and acknowledge that failure to do so would make them accomplices. The document also specified the potential sanctions for such behaviors. Finally, content on RCR, responsible authorship, plagiarism and adequate referencing was thoroughly enhanced in the first term Research Methods course.
Cheating Case 2: Biostatistics I Course, April 2012
On April 2012, during an individual quiz, two students turned in identical solutions, even with the same variable names and Stata ® code. The next day, the TA and course coordinator interviewed both students, one of which admitted having requested repeatedly the exam to the other student, whom eventually shared the answers. One day later, the program coordinator received an e-mail from the student apologizing for the misconduct, accepting all the responsibility for the incident, relieving the other student from any liability, and resigning from the program. The e-mail was promptly answered with the indication that resignation from the program was not possible, as the student was going to be expelled from the program. The School of Postgraduate Studies was then notified about this event, and the student was expelled from the program. After extensive discussion among the coordinators, the student that shared the exam was failed in the exam with a grade of zero, and was allowed to continue in the program at the end of the term. As the program had just started, no evidence is available on the academic performance of the two students prior to this event. The student that shared the exam eventually failed the Research Methods IV course, nearly failing the program due to low academic performance (ranked 19 of 20). No evidence exists of involvement in further events. An 8-week Research Integrity course was added to the first term’s curriculum the following year, addressing extensively research integrity, RCR, plagiarism and appropriate referencing, among other topics.
Plagiarism Case 4: Research Methods III Course, October 2013
The introduction section of a thesis proposal contained passages highly suggestive of plagiarism. After searching for these sections in Google ® , literal plagiarism from research articles and the web was confirmed. Upon questioning by the course and program coordinator, the student admitted committing plagiarism, albeit without realizing that it constituted misconduct. The student was failed in the course, and the incident was discussed anonymously with the rest of the class, reiterating the severity of plagiarism and how to avoid it. Also, students were warned that any further plagiarism cases would be expelled from the program. Until the event occurred, the student had very low academic performance (ranked 26 of 26). The student completed the rest of the program’s coursework under close monitoring and intensive counseling, with low performance (ranked 25 of 25), and was not involved in other misconduct incidents.
Plagiarism Case 5: Research Methods III Course, October 2013
One week after the class discussion of the previous case of plagiarism, the final assignment of a student (full thesis proposal) had several sections strongly suggestive of literal plagiarism. A Google ® search evidenced that these paragraphs were identical to the content of several websites, including Wikipedia ® . The program and course coordinator discussed the incident with the student, and after a long explanation of the definition of plagiarism, the student recognized having plagiarized inadvertently. Given the thorough discussion of plagiarism in the Research Integrity course, writing workshops, and the previous plagiarism case a week before, the student was failed in the course and separated from the program for the rest of the year. The event was reported to the university, and a misconduct report was filed in the student’s permanent academic record. When given the opportunity to address the class, the student described the case, accepted all responsibility for having plagiarized, and warned the class about the severity and importance of preventing plagiarism. The class recognized the severity of the event, but unanimously asked for a more lenient sanction, arguing that the student may have missed prior warnings. Despite accepting misconduct, the student argued the sanction was too harsh and presented a notarized letter requesting a formal decision. The student’s work supervisors contacted the program coordinator in coordination with the student, inquiring about the incident and the program’s response, and full details were provided. The university confirmed the sanction imposed by the program and the student recently contacted the program to try to finish the coursework. Prior to the event, the student had a low performance (ranked 24 of 26).
Most of the cases of plagiarism and cheating detected involved students with a record of suboptimal academic performance in the program. Indeed, 20 % of students in the lowest quartile of their class were involved in plagiarism and cheating compared to only 2 % of students in higher grade quartiles (risk ratio = 12.2; 95 % confidence interval: 2.5–60.2, Fisher’s exact p value = 0.008). Also, none of the four cases described above who actually completed their coursework later had successfully defended their dissertations. No cases were detected in the 2014 class, which suggests a very strong impact of the policy implemented, despite the fact that the reduction in the incidence of plagiarism and cheating is only marginally significant (Fisher’s exact p value = 0.187).
Discussion In three consecutive annual classes of our Epidemiology Masters in Peru, we detected five cases of plagiarism and two cases of cheating, including literal plagiarism, self-plagiarism, inappropriate sharing of work, and appropriation of other students’ work. We believe that these are not isolated events, but rather the manifestation of a widespread and frequent misconduct that has probably gone undetected beyond our program. This is consistent with the high rates of cheating and plagiarism reported worldwide among high school and undergraduate students ( McCabe 2005 ; McCabe et al. 2001 ), including students of medical and allied health sciences ( Rennie and Crosby 2001 ; Taradi et al. 2010 ). It is likely that plagiarism and cheating may originate in high school and undergraduate education, and continue to graduate education. Thus, the widespread occurrence of plagiarism at all levels of education suggests that prevention, detection and response to plagiarism should hold a much higher priority in academic institutions in contexts like Peru and Latin America.
Students committing plagiarism and cheating shared several predisposing characteristics, including poor awareness of research integrity and plagiarism, widespread deficiencies in writing and referencing skills, poor academic performance, and a high tolerance to plagiarism. However, a significant portion of the rest of the class also shared a limited awareness of research integrity and tolerance to plagiarism, and many students had difficulty in grasping research integrity concepts. This is consistent with previous reports evidencing insufficient knowledge of RCR and plagiarism in graduate students in the U.S., particularly among international graduates ( Heitman et al. 2007 ; Ryan et al. 2009 ). These knowledge gaps may be particularly severe in Latin America, where shortcomings in higher education neglect the discussion of plagiarism and academic and research integrity. In addition, lack of development of analytic and writing skills may lead some students to use plagiarism as a maladaptive, compensatory writing strategy. The situation is further complicated by a widespread tolerance to plagiarism throughout the education system in Latin America ( Vasconcelos et al. 2009 ; Heitman and Litewka 2011 ). In Peru, for example, the National Assembly of Rectors reduced the sanction of two undergraduate law students guilty of literal plagiarism from a semester suspension to a simple reprimand, arguing that “copying without indicating the source is a natural behavior in students” ( Tantaleán Odar 2014 ), and that “teaching consists fundamentally in a constant repetition of external ideas, often omitting the sources for brevity” ( Tantaleán Odar 2014 ). Furthermore, several authors have reported that a large proportion of undergraduate research and approved theses contain plagiarism ( Saldana-Gastulo et al. 2010 ; Huamani et al. 2008 ). The synergic effect of limited awareness of plagiarism, RCR, and scientific writing, and the widespread tolerance to plagiarism highlights the need to couple intensive anti-plagiarism education with stronger response policies.
Any attempt to expunge plagiarism is unlikely to succeed without institutional commitment with scientific integrity ( Whitley and Keith-Spiegel 2001 ; Park 2004 ). Institutions should have a transparent, comprehensive and uniformly applied policy that is embedded in a context of promotion of academic integrity. UPCH has an established institutional policy against academic misconduct, which is supplemented by the regulations of each school ( Universidad Peruana Cayetano Heredia 2009 ). However, such a framework focuses almost exclusively on punitive aspects, neglecting preventive and detection strategies. Additionally, regulations have not been widely disseminated and/or discussed across the university’s academic programs, and their application seems inconsistent across programs. Nevertheless, our findings are probably not an isolated case, as lack of comprehensive policies against and widespread tolerance to plagiarism appear to be nearly universal in educational institutions in countries such as Peru. Thus, the institutions’ commitment and proactivity to address plagiarism is critical for the implementation of any effective and sustainable intervention against cases of plagiarism in the future. As a program, we are disappointed to see our students falling due to misconduct, but are not embarrassed to admit we had these issues. We believe many other programs face the same challenges and should come forward to admit it openly and therefore create greater awareness and response.
In this complex scenario, we adopted a “zero tolerance” policy against plagiarism ( Titus et al. 2008 ), in which we actively searched for potential research misconduct and all suspected cases are reported, investigated and sanctioned as dictated by the severity of the case. Although there is no current consensus worldwide on the best way to respond to plagiarism findings, we believe that a zero tolerance approach is the most acceptable alternative, as it results in a clear, strong message that plagiarism and other forms of research misconduct are wrong and can never be justified. In low-resource settings, resource constraints and dependence on external funding may discourage investigating apparently “mild” cases to avoid the associated costs and potential damages in reputation. However, the long term adverse consequences of tolerating plagiarism and therefore graduating student with poor RCR knowledge, outweigh any of these short term apparent benefits. None of the students who committed/attempted plagiarism were known to engage in further events during the program and no additional misconduct events have been detected in the 2014 class.
Our “zero tolerance” policy was actively complemented by intensive education on research integrity and scientific writing. Also, policies were reinforced through discussion sessions, written statements describing the policy in all course syllabi, and a modified honor code in the form of a signed agreement to maintain research and academic integrity and report any observed cases. Honor codes constitute a simple, low-cost strategy that has been shown to prevent academic misconduct ( McCabe et al. 2001 ). However, our experience collaborating with several Latin American educational institutions, has led us to believe that honor codes are not frequently used in Latin America. Furthermore, we feel that although many Latin American educational institutions may have codes of conduct, these are probably not discussed with students, faculty and researchers. We feel that signing a short but very clear and explicit honor code may be a more effective alternative for preventing misconduct by directly engaging students and all the academic and scientific community.
Education in the RCR is a critical pillar for maintaining research integrity and preventing plagiarism ( Steneck and Bulger 2007 ; Kalichman 2007 ), and comprised the medullar aspect of our policy. Seminars on plagiarism and scientific writing were upgraded into an obligatory course on research integrity. Short online research integrity courses were used as additional activities, including both the required CITI basic RCR course for biomedical researchers ( Braunschweiger and Goodman 2007 ; Litewka et al. 2008 ), as well as the optional, free, online RCR course recently created by UPCH and NAMRU-6 ( http://www.cri.andeanquipu.org/index.php/es/ ). The definition, forms, implications and case studies of plagiarism were thoroughly discussed, and practical advice was given on preventing plagiarism ( Roig 2009 ; Fischer and Zigmond 2011 ). Frequent maladaptive forms of writing, such as “patchwriting”, in which original and borrowed text are intermixed ( Cameron et al. 2012 ), and “copy/paste” were thoroughly discussed, emphasizing their intimate relation to plagiarism. Students were advised to express ideas taken from external sources in their own words, always linking each idea to its original source, and never to copy and paste. Other educative interventions implemented included: (i) breaking down extensive written assignments into multiple, smaller assignments, to allow the incremental development of writing skills ( Fischer and Zigmond 2011 ); (ii) provision of templates, so that students have a clear idea of what is expected for each assignment ( Fischer and Zigmond 2011 ); (iii) review of progress in an increased number of writing workshops, to provide detailed and timely guidance, allow early detection and correction of maladaptive writing strategies ( Fischer and Zigmond 2011 ); and, (iv) requirement of more student-advisor meetings, in order to increase the oversight of the students’ work, and promote mentoring, an important strategy for maintaining research integrity ( Anderson et al. 2007 ).
As a complement to educative interventions, we now screen academic products for plagiarism ( Barret et al. 2003 ; McKeever 2006 ) using widely-available search engines (e.g. Google ® ) ( McKeever 2006 ). Searching actively for plagiarism allowed close monitoring the policy’s efficacy, and early identification and guidance of students with inadequate referencing skills ( Barret et al. 2003 ; McKeever 2006 ). This measure closely parallels the routine screening of submissions that has been increasingly implemented by scientific journals ( Butler 2010 ). In Peru, NAMRU-6 requires that the final version of all articles reporting research conducted at the institution is checked for plagiarism before being submitted using iThenticate ® (Andres G. Lescano personal communication, April 2015). In our program, plagiarism is evaluated on a case-by-case basis, after investigation and discussion among all coordinators and the faculty involved in the case. Penalties were also defined individually, following the program and university’s policy, and were complemented with rehabilitative measures ( Whitley and Keith-Spiegel 2001 ), such as intensive counseling by an experienced faculty and remedial educative activities.
The case study approach we adopted does not allow a formal evaluation of the efficacy of our program’s policy against plagiarism and cheating, but it may expand the extant literature in Latin America. Our experience delivered several important learning points. First, plagiarism seems to be widespread, likely involving all stages of the educative system. Second, it is possible to implement a “zero tolerance” plagiarism prevention policy with a strong educational component in postgraduate research programs. We implemented a promising, feasible, low-cost policy tailored for postgraduate research students in Latin America, with the aim to offer educators and researchers practical alternatives to prevent and address plagiarism that they could continue to evaluate in their practice. Third, key features associated with plagiarism in Latin America that should be considered when discussing plagiarism in the classroom include the unawareness of plagiarism and its implications, the pervasiveness of poorly-developed writing skills, and the extensive use of “patchwriting” and “copy/paste”. Fourth, students with low academic performance may be at higher risk of committing plagiarism, and implement personalized tutoring and close surveillance to prevent them from plagiarizing. Given that our experience pertains a taught Masters program that receives students from several Latin American countries, we believe that our findings are applicable to postgraduate research students in Latin America. However, we emphasize that our findings may also be useful for educators and postgraduate research programs in other resource-limited, non-English speaking settings after critical assessment and a context-sensitive adaptation. Finally, it is urgent that educative institutions at all levels recognize the frequent occurrence of academic and research misconduct and integrity as an active, institutional duty. Furthermore, as the methods for engaging in dishonesty have expanded in the Internet era, preventive approaches coupled with zero tolerance for plagiarism and cheating will have a major role for controlling academic and research misconduct, even in low resource settings ( Grieger 2007 ).
Plagiarism and cheating appear to be a frequent problem in research training programs in resource-limited settings, such as Peru. These instances of misconduct should be addressed at institutional and programmatic levels through policies that prioritize preventive strategies, instead of purely punitive actions. Educational activities and mentoring should be complemented with strict, active detection and zero tolerance to misconduct.
Acknowledgments
This study was funded by the training Grant 2D43 TW007393-06 awarded to AGL by the Fogarty International Center of the U.S. National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Copyright Statement One author of this manuscript is an employee of the U.S. Government. This work was prepared as part of his duties. Title 17 U.S.C. § 105 provides that ‘Copyright protection under this title is not available for any work of the United States Government.’ Title 17 U.S.C. § 101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person’s official duties. The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, a worldwide license to the Publishers and its licensees in perpetuity, in all forms, formats and media (whether known now or created in the future), to (i) publish, reproduce, distribute, display and store the Contribution, (ii) translate the Contribution into other languages, create adaptations, reprints, include within collections and create summaries, extracts and/or, abstracts of the Contribution, (iii) create any other derivative work(s) based on the Contribution, (iv) to exploit all subsidiary rights in the Contribution, (v) the inclusion of electronic links from the Contribution to third party material wherever it may be located; and, (vi) license any third party to do any or all of the above.
Compliance with Ethical Standards
Conflict of interest All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: all authors had financial support from the NIH Fogarty International Center for the submitted work; all authors had paid teaching positions at Universidad Peruana Cayetano Heredia in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
Disclaimer The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the U.S. Government.
- Alfaro-Tolosa P, Mayta-Tristan P, Rodriguez-Morales AJ. Publication misconduct and plagiarism retractions: A Latin American perspective. Current Medical Research and Opinion. 2013; 29 (2):1–2. [ PubMed ] [ Google Scholar ]
- Allchin D. Values in science: An educational perspective. Science & Education. 1999; 8 :1–12. [ Google Scholar ]
- Almeida RM, de Albuquerque Rocha K, Catelani F, Fontes-Pereira AJ, Vasconcelos SM. Plagiarism allegations account for most retractions in major latin American/Caribbean databases. Science and Engineering Ethics. 2015 [ PubMed ] [ Google Scholar ]
- Ana J, Koehlmoos T, Smith R, Yan LL. Research misconduct in low- and middle-income countries. PLoS Medicine. 2013; 10 (3):e1001315. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Anderson MS, Horn AS, Risbey KR, Ronning EA, De Vries R, Martinson BC. What do mentoring and training in the responsible conduct of research have to do with scientists’ misbehavior? Findings from a national survey of NIH-funded scientists. Academic Medicine. 2007; 82 (9):853–860. [ PubMed ] [ Google Scholar ]
- Barret R, Malcolm J, Lyon C. Are we ready for large scale use of plagiarism detection tools?; 4th Annual LTSN-ICS Conference, 2003; 2003. [Accessed Jan 2012]. http://www.ics.heacademy.ac.uk/Events/conf2003/Ruth%20Barrett.pdf . [ Google Scholar ]
- Braunschweiger P, Goodman KW. The CITI program: An international online resource for education in human subjects protection and the responsible conduct of research. Academic Medicine. 2007; 82 (9):861–864. [ PubMed ] [ Google Scholar ]
- Butler D. Journals step up plagiarism policing. Nature. 2010; 466 (7303):167. [ PubMed ] [ Google Scholar ]
- Cameron C, Zhao H, McHugh MK. Publication ethics and the emerging scientific workforce: Understanding “Plagiarism” in a global context. Academic Medicine. 2012; 87 (1):51–54. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Catanzaro M, Miranda G, Palmer L, Bajak A. South American science: Big players. Nature. 2014; 510 (7504):204–206. [ PubMed ] [ Google Scholar ]
- Committee on Science Engineering and Public Policy, National Academy of Sciences, National Academy of Engineering, & Institute of Medicine. On being a scientist: A guide to responsible conduct in research. 3rd. Washington, DC: The National Academies Press; 2009. [ PubMed ] [ Google Scholar ]
- Davis MS. The role of culture in research misconduct. Accountability in Research. 2003; 10 (3):189–201. [ PubMed ] [ Google Scholar ]
- Fischer BA, Zigmond MJ. Educational approaches for discouraging plagiarism. Urologic Oncology. 2011; 29 (1):100–103. [ PubMed ] [ Google Scholar ]
- Grieger MC. Ghostwriters and commerce of scientific papers on the internet: science at risk. Revista Da Associacao Medica Brasileira. 2007; 53 (3):247–251. [ PubMed ] [ Google Scholar ]
- Heitman E, Litewka S. International perspectives on plagiarism and considerations for teaching international trainees. Urologic Oncology. 2011; 29 (1):104–108. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Heitman E, Olsen CH, Anestidou L, Bulger RE. New graduate students’ baseline knowledge of the responsible conduct of research. Academic Medicine. 2007; 82 (9):838–845. [ PubMed ] [ Google Scholar ]
- Hofstede G. Dimensionalizing cultures: The Hofstede model in contex. Online Readings in Psychology and Culture. 2011; 2 (1):26. [ Google Scholar ]
- Huamani C, Dulanto-Pizzorni A, Rojas-Revoredo V. ‘Copy and paste’ in undergraduate research: Abusing Internet. Anales de la Facultad de Medicina. 2008; 69 (2):117–119. [ Google Scholar ]
- Kalichman MW. Responding to challenges in educating for the responsible conduct of research. Academic Medicine. 2007; 82 (9):870–875. [ PubMed ] [ Google Scholar ]
- Krstic SB. Research integrity practices from the perspective of early-career researchers. Science and Engineering Ethics. 2015; 21 (5):1181–1196. [ PubMed ] [ Google Scholar ]
- Litewka S, Goodman K, Braunschweiger P. The ClTI program: An alternative for research ethics education in Latin America. Acta Bioethica. 2008; 14 (1):54–60. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lovett-Hopper G, Komarraju M, Weston R, Dollinger SJ. Is plagiarism a forerunner of other deviance? Imagined futures of academically dishonest students. Ethics and Behavior. 2007; 17 (3):323–336. [ Google Scholar ]
- Martin DE. Culture and unethical conduct: Understanding the impact of individualism and collectivism on actual plagiarism. Management Learning. 2012; 43 (3):261–273. [ Google Scholar ]
- Martinson BC, Anderson MS, de Vries R. Scientists behaving badly. Nature. 2005; 435 :737–738. [ PubMed ] [ Google Scholar ]
- McCabe DL. Cheating among college and university students: A North American perspective. International Journal for Educational Integrity. 2005; 1 (1):1–11. [ Google Scholar ]
- McCabe DL, Treviño LK, Butterfield KD. Cheating in academic institutions: A decade of research. Ethics and Behavior. 2001; 11 (3):219–222. [ Google Scholar ]
- McKeever L. Online plagiarism detection services-saviour or scourge? Assessment and Evaluation in Higher Education. 2006; 31 (2):155–165. [ Google Scholar ]
- Park C. In other (People’s) words: Plagiarism by university students—literature and lessons. Assessment and Evaluation in Higher Education. 2003; 28 (5):471–488. [ Google Scholar ]
- Park C. Rebels without a clause: Towards an institutional framework for dealing with plagiarism by students. Journal of Further and Higher Education. 2004; 28 (3):291–306. [ Google Scholar ]
- Pupovac V, Fanelli D. Scientists admitting to plagiarism: A meta-analysis of surveys. Science and Engineering Ethics. 2015; 21 (5):1331–1352. [ PubMed ] [ Google Scholar ]
- Rennie SC, Crosby JR. Are “tomorrow’s doctors” honest? Questionnaire study exploring medical students’s attitudes and reported behaviour on academic misconduct. BMJ (Clinical Research Ed.) 2001; 322 :274–275. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rodriguez E, Lolas F. The topic of research integrity in Latin America. Bioethikos. 2011; 5 (4):362–368. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Roig M. Avoiding plagiarism, self-plagiarism, and other questionable writing practices: A guide to ethical writing. [Accessed 12 Sept, 2011]; 2009 http://ori.hhs.gov/education/products/plagiarism/ [ Google Scholar ]
- Rojas-Revoredo V, Huamani C, Mayta-Tristan P. Plagiarism in undergraduate publications: Experiences and recommendations. Revista Medica de Chile. 2007; 135 :1087–1088. [ PubMed ] [ Google Scholar ]
- Ryan G, Bonanno H, Krass I, Scouller K, Smith L. Undergraduate and postgraduate pharmacy students’ perceptions of plagiarism and academic honesty. American Journal of Pharmaceutical Education. 2009; 73 (6):105. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Saldana-Gastulo JJ, Quezada-Osoria CC, Pena-Oscuvilca A, Mayta-Tristan P. High frequency of plagiarism in medical thesis from a Peruvian public university. Revista Peruana de Medicina Experimental y Salud Publica. 2010; 27 (1):63–67. [ PubMed ] [ Google Scholar ]
- Salter SB, Guffey DM. Truth, consequences and culture: A comparative examination of cheating and attitudes about cheating among U.S. and U.K. students. Journal of Business Ethics. 2001; 31 :37–50. [ Google Scholar ]
- SCImago Research Group. SCImago Institution Rankings. [Accessed 2015 Mar 19]; Ranking Iberoamericano SIR 2015. 2015 http://www.scimagoir.com/pdf/iber_new/SIR%20Iber%202015%20HE.pdf . [ Google Scholar ]
- Smith R. What is research misconduct. Journal of the Royal College of Physicians of Edinburgh. 2000; 30 :4–8. [ Google Scholar ]
- Steneck NH. Fostering integrity in research: Definitions, current knowledge, and future directions. Science and Engineering Ethics. 2006; 12 :53–74. [ PubMed ] [ Google Scholar ]
- Steneck NH, Bulger RE. The history, purpose, and future of instruction in the responsible conduct of research. Academic Medicine. 2007; 82 (9):830–834. [ PubMed ] [ Google Scholar ]
- Swazey J, Anderson M, Louis K. Ethical problems in academic research. American Scientist. 1993; 81 :542–553. [ Google Scholar ]
- Tantaleán Odar RM. ¡¿Ya podemos plagiar?! Crítica a las resoluciones No 191-2009 y 288-2009-CODACUN. Derecho y Cambio Social, Año XI. 2014;(36):23. [ Google Scholar ]
- Taradi SK, Taradi M, Knezevic T, Dogas Z. Students come to medical school prepared to cheat: A multi-campus investigation. Journal of Medical Ethics. 2010; 36 (1):666–670. [ PubMed ] [ Google Scholar ]
- Titus SL, Wells JA, Rhoades LJ. Repairing research integrity. Nature. 2008; 453 :980–982. [ PubMed ] [ Google Scholar ]
- Universidad Peruana Cayetano Heredia. Reglamento Disciplinario. 2009 http://www.upch.edu.pe/portal/images/stories/files/REGLAMENTO_DISCIPLINARIO.pdf . [ Google Scholar ]
- Van Noorden R. The impact gap: South America by the numbers. Nature. 2014; 510 (7504):202–203. [ PubMed ] [ Google Scholar ]
- Vasconcelos S, Leta J, Costa L, Pinto A, Sorenson ME. Discussing plagiarism in Latin American science. EMBO Reports. 2009; 10 (7):677–682. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wadja-Johnston VA, Handal PJ, Brawer PA, Fabricatore AN. Academic dishonesty at the graduate level. Ethics and Behavior. 2001; 11 (3):287–305. [ Google Scholar ]
- Whitley BE, Jr, Keith-Spiegel P. Academic integrity as an institutional issue. Ethics and Behavior. 2001; 11 (3):325–342. [ Google Scholar ]

IMAGES
VIDEO
COMMENTS
More than 70 cases pair ethics concepts with real world situations. From journalism, performing arts, and scientific research to sports, law, and business, these case studies explore current and historic ethical dilemmas, their motivating biases, and their consequences. Each case includes discussion questions, related videos, and a bibliography.
Introduction. Ethical misconduct is not a new issue in the history of science and literature. As a classical example, in the 11 th century al-Hajvery al-Ghaznawi the author of Revelation of the Veiled (famous treatise on Sufis) claimed that two of his previous works have been the subjects of plagiarism. He asked the readers not to use his words without citing him as the author.
According to Resnik (2011), many people think of ethics as a set of rules distinguishing right from wrong, but actually the term "ethics" refers to norms of conduct or of action and in disciplines of study. Research ethics or norms promote the "knowledge, truth, and avoidance of error" (p. 1) and protect against "fabricating ...
Case. COPE Members bring specific (anonymised) publication ethics issues to the COPE Forum for discussion and advice. The advice from the COPE Forum meetings is specific to the particular case under consideration and may not necessarily be applicable to similar cases either past or future. The advice is given by the Forum participants (COPE ...
Information about the history and authors of the Resources for Research Ethics Collection. Case Studies. Case studies are a tool for discussing scientific integrity. Although one of the most frequently used tools for encouraging discussion, cases are only one of many possible tools. ... Publication from the Committee on Science, Engineering ...
We have noticed a lot of variety in the way that ethical approval for Case Reports are published in different journals. For example, some state that the study was determined not to require Ethics Committee (EC) or Institutional Review Board (IRB) review especially if it was a retrospective review. Others state that all procedures were carried ...
Core practices are the policies and practices journals and publishers need, to reach the highest standards in publication ethics. We include cases with advice, guidance for day-to-day practice, education modules and events on topical issues, to support journals and publishers fulfil their policies.
Not only the conduct of the study requires ethics to be adhered to but also the process of publication comes under the purview of ethics. ... In case of paraphrasing, where the writer writes the text in his own word, authors must properly cite the original source. ... The Committee on Publication Ethics (COPE): objectives and achievements 1997 ...
Ethical issues concerning publication of this case study. Owing to the recent amalgamation of health services, the health service's regional boundaries and senior management have changed since the time of the original research (1999). In addition, a number of nurse practitioner models have been implemented in NSW.
Case Study: Publication Ethics Scenario 2 Back to top- A paper is submitted to a journal with the corresponding author listing three authors- When revisions are submitted, the corresponding author leaves one author off- The Editor pauses the peer review process and asks the corresponding author why one of the authors has been removed
Case. The study proposed a new wound-healing technique and involved needle puncture on patients from two medical centers. The paper mentioned that an institutional ethical review board had given ethical approval for the study. However, the processes of puncturing, healing, and cleaning vascular access were performed without informed consent.
Publication ethics. Academic research involves many coordinated steps and processes - appropriate study design, study execution, data collection, data analysis, and finally publication. While going through these steps and culminating in a publication can be an exhilarating experience, one should be aware of ethical code of conduct that binds ...
Case Study: Publication Ethics. Answer (Scenario 1) 1. Methods (original article): This study was a Randomised Controlled Trial (RCT) conducted in four teaching hospitals in London. Patients were recruited between January 2007 and December 2008. Patients were eligible for inclusion in the study if they were over the age of 18 and had undergone ...
Answer (Scenario 1) Unfortunately it is not. BMC Editorial Policy clearly states: Research involving human subjects, human material, or human data, must have been approved by an appropriate ethics committee. A statement detailing this, including the name of the ethics committee and the reference number where appropriate, must appear in all ...
Jonathan S. Towner, PhD, Luke Nyakarahuka, PhD, MPH, BVM, and Patrick Atimnedi, BVM. Marburg virus is carried by the Egyptian rousette bat, a common cave-dwelling fruit bat endemic to sub-Saharan Africa, where populations can exceed 50 000. AMA J Ethics. 2024;26 (2):E109-115. doi: 10.1001/amajethics.2024.109. Case and Commentary.
A case study is one of the most commonly used methodologies of social research. This article attempts to look into the various dimensions of a case study research strategy, the different epistemological strands which determine the particular case study type and approach adopted in the field, discusses the factors which can enhance the effectiveness of a case study research, and the debate ...
Editorial board participation. An overview of editorial board appointment and engagement, roles and responsibilities, and key details to consider during recruitment such as participation on multiple editorial boards. COPE Guidelines are formal COPE policy and are intended to advise editors and publishers on expected publication ethics practice
Publication of scientific paper is critical for modern science evolution, and professional advancement. However, it comes with many responsibilities. An author must be aware of good publication practices. While refraining from scientific misconduct or research frauds, authors should adhere to Good Publication Practices (GPP).
Some Resources for Case Studies in Research Ethics. (initially compiled by Karen Muskavitch in 2005; checked, updated and added to by Julie Hollowell in 2009; checked and updated 2010 by Hannah Harp in 2010 and in 2011 and 2012 by Julie Hollowell) There has been an explosion in case studies accessible on the Internet for use in teaching ethics ...
Case Study 2: Issue: What are your responsibilities for accurately conveying qualifications and competencies? Relevant ISMPP Code of Ethics Sections: Ethical Principles I. 3, 4, 5. Scenario: A medical communications agency has been invited to take on a publications program in a therapeutic area in which they have no expertise. However, this is a fantastic opportunity for the agency to expand ...
Publication ethics and research integrity help to ensure the accuracy and trustworthiness of an article. They cover a broad range of subjects including authorship, ethical approval for the study, conflicts of interest, availability of data, peer review amongst other topics. Karger Publishers is a member of the Committee on Publication Ethics ...
The practice of clinical research is strictly regulated by law. During submission and review processes, compliance of such research with the laws enforced in the country where it was conducted is not always correctly filled in by the authors or verified by the editors. Here, we report a case of a single institution for which one may find hundreds of publications with seemingly relevant ethical ...
Publication ethics (2 h) Open in a separate window. ... The case study approach we adopted does not allow a formal evaluation of the efficacy of our program's policy against plagiarism and cheating, but it may expand the extant literature in Latin America. Our experience delivered several important learning points.