An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Cancers (Basel)


Breast Cancer—Epidemiology, Risk Factors, Classification, Prognostic Markers, and Current Treatment Strategies—An Updated Review
Sergiusz Łukasiewicz.
1 Department of Surgical Oncology, Center of Oncology of the Lublin Region St. Jana z Dukli, 20-091 Lublin, Poland; lp.lzoc@zciweisakulS (S.Ł.); [email protected] (A.S.)
Marcin Czeczelewski
2 Department of Forensic Medicine, Medical University of Lublin, 20-090 Lublin, Poland; [email protected] (M.C.); lp.teno@amrofa (A.F.)
Alicja Forma
3 Department of Human Anatomy, Medical University of Lublin, 20-090 Lublin, Poland; [email protected]
Robert Sitarz
Andrzej stanisławek.
4 Department of Oncology, Chair of Oncology and Environmental Health, Medical University of Lublin, 20-081 Lublin, Poland
Simple Summary
Breast cancer is the most common cancer among women. It is estimated that 2.3 million new cases of BC are diagnosed globally each year. Based on mRNA gene expression levels, BC can be divided into molecular subtypes that provide insights into new treatment strategies and patient stratifications that impact the management of BC patients. This review addresses the overview on the BC epidemiology, risk factors, classification with an emphasis on molecular types, prognostic biomarkers, as well as possible treatment modalities.
Breast cancer (BC) is the most frequently diagnosed cancer in women worldwide with more than 2 million new cases in 2020. Its incidence and death rates have increased over the last three decades due to the change in risk factor profiles, better cancer registration, and cancer detection. The number of risk factors of BC is significant and includes both the modifiable factors and non-modifiable factors. Currently, about 80% of patients with BC are individuals aged >50. Survival depends on both stage and molecular subtype. Invasive BCs comprise wide spectrum tumors that show a variation concerning their clinical presentation, behavior, and morphology. Based on mRNA gene expression levels, BC can be divided into molecular subtypes (Luminal A, Luminal B, HER2-enriched, and basal-like). The molecular subtypes provide insights into new treatment strategies and patient stratifications that impact the management of BC patients. The eighth edition of TNM classification outlines a new staging system for BC that, in addition to anatomical features, acknowledges biological factors. Treatment of breast cancer is complex and involves a combination of different modalities including surgery, radiotherapy, chemotherapy, hormonal therapy, or biological therapies delivered in diverse sequences.
1. Introduction
Being characterized by six major hallmarks, carcinogenesis might occur in every cell, tissue, and organ, leading to the pathological alternations that result in a vast number of cancers. The major mechanisms that enable its progression include evasion of apoptosis, limitless capacity to divide, enhanced angiogenesis, resistance to anti-growth signals and induction of own growth signals, as well as the capacity to metastasize [ 1 ]. Carcinogenesis is a multifactorial process that is primarily stimulated by both—genetic predispositions and environmental causes. The number of cancer-related deaths is disturbingly increasing every year ranking them as one of the major causes of death worldwide. Even though a significant number of cancers do not always need to result in death, they significantly lower the quality of life and require larger costs in general.
Breast cancer is currently one of the most prevalently diagnosed cancers and the 5th cause of cancer-related deaths with an estimated number of 2.3 million new cases worldwide according to the GLOBOCAN 2020 data [ 2 ]. Deaths due to breast cancer are more prevalently reported (an incidence rate approximately 88% higher) in transitioning countries (Melanesia, Western Africa, Micronesia/Polynesia, and the Caribbean) compared to the transitioned ones (Australia/New Zealand, Western Europe, Northern America, and Northern Europe). Several procedures such as preventive behaviors in general as well as screening programs are crucial regarding a possible minimization of breast cancer incidence rate and the implementation of early treatment. Currently, it is the Breast Health Global Initiative (BHGI) that is responsible for the preparation of proper guidelines and the approaches to provide the most sufficient breast cancer control worldwide [ 3 ]. In this review article, we have focused on the female breast cancer specifically since as abovementioned, it currently constitutes the most prevalent cancer amongst females.
2. Breast Cancer Epidemiology
According to the WHO, malignant neoplasms are the greatest worldwide burden for women, estimated at 107.8 million Disability-Adjusted Life Years (DALYs), of which 19.6 million DALYs are due to breast cancer. [ 4 ]. Breast cancer is the most frequently diagnosed cancer in women worldwide with 2.26 million [95% UI, 2.24–2.79 million] new cases in 2020 [ 5 ]. In the United States, breast cancer alone is expected to account for 29% of all new cancers in women [ 6 ]. The 2018 GLOBOCAN data shows that age-standardized incidence rates (ASIR) of breast cancer are strongly and positively associated with the Human Development Index (HDI) [ 7 ]. According to 2020 data, the ASIR was the highest in very high HDI countries (75.6 per 100,000) while it was more than 200% lower in medium and low HDI countries (27.8 per 100,000 and 36.1 per 100,000 respectively) [ 5 ].
Besides being the most common, breast cancer is also the leading cause of cancer death in women worldwide. Globally, breast cancer was responsible for 684,996 deaths [95% UI, 675,493–694,633] at an age-adjusted rate of 13.6/100,000 [ 5 ]. Although incidence rates were the highest in developed regions, the countries in Asia and Africa shared 63% of total deaths in 2020 [ 5 ]. Most women who develop breast cancer in a high-income country will survive; the opposite is true for women in most low-income and many middle-income countries [ 8 ].
In 2020 breast cancer mortality-to-incidence ratio (MIR) as a representative indicator of 5-year survival rates [ 9 ] was 0.30 globally [ 5 ]. Taking into consideration the clinical extent of breast cancer, in locations with developed health care (Hong-Kong, Singapore, Turkey) the 5-year survival was 89.6% for localized and 75.4% for regional cancer. In less developed countries (Costa Rica, India, Philippines, Saudi Arabia, Thailand) the survival rates were 76.3% and 47.4% for localized and regional breast cancer respectively [ 10 ].
Breast cancer incidence and death rates have increased over the last three decades. Between 1990 and 2016 breast cancer incidence has more than doubled in 60/102 countries (e.g., Afghanistan, Philippines, Brazil, Argentina), whereas deaths have doubled in 43/102 countries (e.g., Yemen, Paraguay, Libya, Saudi Arabia) [ 11 ]. Current projections indicate that by 2030 the worldwide number of new cases diagnosed reach 2.7 million annually, while the number of deaths 0.87 million [ 12 ]. In low- and medium-income countries, the breast cancer incidence is expected to increase further due to the westernization of lifestyles (e.g., delayed pregnancies, reduced breastfeeding, low age at menarche, lack of physical activity, and poor diet), better cancer registration, and cancer detection [ 13 ].
3. Risk Factors of Breast Cancer
The number of risk factors of breast cancer is significant and includes both modifiable factors and non-modifiable factors ( Table 1 ).
Modifiable and non-modifiable risk factors of breast cancer.
3.1. Non-Modifiable Factors
3.1.1. female sex.
Female sex constitutes one of the major factors associated with an increased risk of breast cancer primarily because of the enhanced hormonal stimulation. Unlike men who present insignificant estrogen levels, women have breast cells which are very vulnerable to hormones (estrogen and progesterone in particular) as well as any disruptions in their balance. Circulating estrogens and androgens are positively associated with an increased risk of breast cancer [ 14 ]. The alternations within the physiological levels of the endogenous levels of sex hormones result in a higher risk of breast cancer in the case of premenopausal and postmenopausal women; these observations were also supported by the Endogenous Hormones and Breast Cancer Collaborative Group [ 15 , 16 , 17 ].
Less than 1% of all breast cancers occur in men. However, breast cancer in men is a rare disease that’s at the time of diagnosis tends to be more advanced than in women. The average age of men at the diagnosis is about 67. The important factors increase a man’s risk of breast cancer are: older age, BRCA2/BRCA1 mutations, increased estrogen levels, Klinefelter syndrome, family history of breast cancer, and radiation exposure [ 18 ].
3.1.2. Older Age
Currently, about 80% of patients with breast cancer are individuals aged >50 while at the same time more than 40% are those more than 65 years old [ 19 , 20 , 21 ]. The risk of developing breast cancer increases as follows—the 1.5% risk at age 40, 3% at age 50, and more than 4% at age 70 [ 22 ]. Interestingly, a relationship between a particular molecular subtype of cancer and a patient’s age was observed –aggressive resistant triple-negative breast cancer subtype is most commonly diagnosed in groups under 40 age, while in patients >70, it is luminal A subtype [ 21 ]. Generally, the occurrence of cancer in older age is not only limited to breast cancer; the accumulation of a vast number of cellular alternations and exposition to potential carcinogens results in an increase of carcinogenesis with time.
3.1.3. Family History
A family history of breast cancer constitutes a major factor significantly associated with an increased risk of breast cancer. Approximately 13–19% of patients diagnosed with breast cancer report a first-degree relative affected by the same condition [ 23 ]. Besides, the risk of breast cancer significantly increases with an increasing number of first-degree relatives affected; the risk might be even higher when the affected relatives are under 50 years old [ 24 , 25 , 26 ]. The incidence rate of breast cancer is significantly higher in all of the patients with a family history despite the age. This association is driven by epigenetic changes as well as environmental factors acting as potential triggers [ 27 ]. A family history of ovarian cancer—especially those characterized by BRCA1 and BRCA2 mutations—might also induce a greater risk of breast cancer [ 28 ].
3.1.4. Genetic Mutations
Several genetic mutations were reported to be highly associated with an increased risk of breast cancer. Two major genes characterized by a high penetrance are BRCA1 (located on chromosome 17) and BRCA2 (located on chromosome 13). They are primarily linked to the increased risk of breast carcinogenesis [ 29 ]. The mutations within the above-mentioned genes are mainly inherited in an autosomal dominant manner, however, sporadic mutations are also commonly reported. Other highly penetrant breast cancer genes include TP53 , CDH1 , PTEN , and STK11 [ 30 , 31 , 32 , 33 , 34 ]. Except for the increased risk of breast cancer, carriers of such mutations are more susceptible to ovarian cancer as well. A significant number of DNA repair genes that can interact with BRCA genes including ATM , PALB2 , BRIP1 , or CHEK2 , were reported to be involved in the induction of breast carcinogenesis; those are however characterized by a lower penetrance (moderate degree) compared to BRCA1 or BRCA2 ( Table 2 ) [ 29 , 35 , 36 , 37 , 38 ]. According to quite recent Polish research, mutations within the XRCC2 gene could also be potentially associated with an increased risk of breast cancer [ 39 ].
Major genes associated with an increased risk of breast cancer occurrence.
3.1.5. Race/Ethnicity
Disparities regarding race and ethnicity remain widely observed among individuals affected by breast cancer; the mechanisms associated with this phenomenon are not yet understood. Generally, the breast cancer incidence rate remains the highest among white non-Hispanic women [ 51 , 52 ]. Contrarily, the mortality rate due to this malignancy is significantly higher among black women; this group is also characterized by the lowest survival rates [ 53 ].
3.1.6. Reproductive History
Numerous studies confirmed a strict relationship between exposure to endogenous hormones—estrogen and progesterone in particular—and excessive risk of breast cancer in females. Therefore, the occurrence of specific events such as pregnancy, breastfeeding, first menstruation, and menopause along with their duration and the concomitant hormonal imbalance, are crucial in terms of a potential induction of the carcinogenic events in the breast microenvironment. The first full-term pregnancy at an early age (especially in the early twenties) along with a subsequently increasing number of births are associated with a reduced risk of breast cancer [ 54 , 55 ]. Besides, the pregnancy itself provides protective effects against potential cancer. However, protection was observed at approximately the 34th pregnancy week and was not confirmed for the pregnancies lasting for 33 weeks or less [ 56 ]. Women with a history of preeclampsia during pregnancy or children born to a preeclamptic pregnancy are at lower risk of developing breast cancer [ 57 ]. No association between the increased breast cancer risk and abortion was stated so far [ 58 ].
The dysregulated hormone levels during preeclampsia including increased progesterone and reduced estrogen levels along with insulin, cortisol, insulin-like growth factor-1, androgens, human chorionic gonadotropin, corticotropin-releasing factor, and IGF-1 binding protein deviating from the physiological ranges, show a protective effect preventing from breast carcinogenesis. The longer duration of the breastfeeding period also reduces the risk of both the ER/PR-positive and -negative cancers [ 59 ]. Early age at menarche is another risk factor of breast cancer; it is possibly also associated with a tumor grade and lymph node involvement [ 60 ]. Besides, the earlier age of the first menstruation could result in an overall poorer prognosis. Contrarily, early menopause despite whether natural or surgical, lowers the breast cancer risk [ 61 ].
3.1.7. Density of Breast Tissue
The density of breast tissue remains inconsistent throughout the lifetime; however, several categories including low-density, high-density, and fatty breasts have been established in clinical practice. Greater density of breasts is observed in females of younger age and lower BMI, who are pregnant or during the breastfeeding period, as well as during the intake of hormonal replacement therapy [ 62 ]. Generally, the greater breast tissue density correlates with the greater breast cancer risk; this trend is observed both in premenopausal and postmenopausal females [ 63 ]. It was proposed that screening of breast tissue density could be a promising, non-invasive, and quick method enabling rational surveillance of females at increased risk of cancer [ 64 ].
3.1.8. History of Breast Cancer and Benign Breast Diseases
Personal history of breast cancer is associated with a greater risk of a renewed cancerous lesions within the breasts [ 65 ]. Besides, a history of any other non-cancerous alternations in breasts such as atypical hyperplasia, carcinoma in situ, or many other proliferative or non-proliferative lesions, also increases the risk significantly [ 66 , 67 , 68 ]. The histologic classification of benign lesions and a family history of breast cancer are two factors that are strongly associated with breast cancer risk [ 66 ].
3.1.9. Previous Radiation Therapy
The risk of secondary malignancies after radiotherapy treatment remains an individual matter that depends on the patient’s characteristics, even though it is a quite frequent phenomenon that arises much clinical concern. Cancer induced by radiation therapy is strictly associated with an individual’s age; patients who receive radiation therapy before the age of 30, are at a greater risk of breast cancer [ 69 ]. The selection of proper radiotherapy technique is crucial in terms of secondary cancer risk—for instance, tangential field IMRT (2F-IMRT) is associated with a significantly lower risk compared to multiple-field IMRT (6F-IMRT) or double partial arcs (VMAT) [ 70 ]. Besides, the family history of breast cancer in patients who receive radiotherapy additionally enhances the risk of cancer occurrence [ 71 ]. However, Bartelink et al. showed that additional radiation (16 Gy) to the tumor bed combined with standard radiotherapy might decrease the risk of local recurrence [ 72 ].
3.2. Modifiable Factors
3.2.1. chosen drugs.
Data from some research indicates that the intake of diethylstilbestrol during pregnancy might be associated with a greater risk of breast cancer in children; this, however, remains inconsistent between studies and requires further evaluation [ 73 , 74 ]. The intake of diethylstilbestrol during pregnancy is associated with an increased risk of breast cancer not only in mothers but also in the offspring [ 75 ]. This relationship is observed despite the expression of neither estrogen nor progesterone receptors and might be associated with every breast cancer histological type. The risk increases with age; women at age of ≥40 years are nearly 1.9 times more susceptible compared to women under 40. Moreover, breast cancer risk increases with greater diethylstilbestrol doses [ 76 ]. Numerous researches indicate that females who use hormonal replacement therapy (HRT) especially longer than 5 or 7 years are also at increased risk of breast cancer [ 77 , 78 ]. Several studies indicated that the intake of chosen antidepressants, mainly paroxetine, tricyclic antidepressants, and selective serotonin reuptake inhibitors might be associated with a greater risk of breast cancer [ 79 , 80 ]. Lawlor et al. showed that similar risk might be achieved due to the prolonged intake of antibiotics; Friedman et al. observed that breast risk is mostly elevated while using tetracyclines [ 81 , 82 ]. Attempts were made to investigate a potential relationship between hypertensive medications, non-steroidal anti-inflammatory drugs, as well as statins, and an elevated risk of breast cancer, however, this data remains highly inconsistent [ 83 , 84 , 85 ].
3.2.2. Physical Activity
Even though the mechanism remains yet undeciphered, regular physical activity is considered to be a protective factor of breast cancer incidence [ 86 , 87 ]. Chen et al. observed that amongst females with a family history of breast cancer, physical activity was associated with a reduced risk of cancer but limited only to the postmenopausal period [ 88 ]. However, physical activity is beneficial not only in females with a family history of breast cancer but also in those without such a history. Contrarily to the above-mentioned study, Thune et al. pointed out more pronounced effects in premenopausal females [ 89 ]. There are several hypotheses aiming to explain the protective role of physical activity in terms of breast cancer incidence; physical activity might prevent cancer by reducing the exposure to the endogenous sex hormones, altering immune system responses or insulin-like growth factor-1 levels [ 88 , 90 , 91 ].
3.2.3. Body Mass Index
According to epidemiological evidence, obesity is associated with a greater probability of breast cancer. This association is mostly intensified in obese post-menopausal females who tend to develop estrogen-receptor-positive breast cancer. Yet, independently to menopausal status, obese women achieve poorer clinical outcomes [ 92 ]. Wang et al. showed that females above 50 years old with greater Body Mass Index (BMI) are at a greater risk of cancer compared to those with low BMI [ 93 ]. Besides, the researchers observed that greater BMI is associated with more aggressive biological features of tumor including a higher percentage of lymph node metastasis and greater size. Obesity might be a reason for greater mortality rates and a higher probability of cancer relapse, especially in premenopausal women [ 94 ]. Increased body fat might enhance the inflammatory state and affects the levels of circulating hormones facilitating pro-carcinogenic events [ 95 ]. Thus, poorer clinical outcomes are primarily observed in females with BMI ≥ 25 kg/m 2 [ 96 ]. Interestingly, postmenopausal women tend to present poorer clinical outcomes despite proper BMI values but namely due to excessive fat volume [ 97 ]. Greater breast cancer risk with regards to BMI also correlates with the concomitant family history of breast cancer [ 98 ].
3.2.4. Alcohol Intake
Numerous evidences confirm that excessive alcohol consumption is a factor that might enhance the risk of malignancies within the gastrointestinal tract; however, it was proved that it is also linked to the risk of breast cancer. Namely, it is not alcohol type but rather the content of alcoholic beverages that mostly affect the risk of cancer. The explanation for this association is the increased levels of estrogens induced by the alcohol intake and thus hormonal imbalance affecting the risk of carcinogenesis within the female organs [ 99 , 100 ]. Besides, alcohol intake often results in excessive fat gain with higher BMI levels, which additionally increases the risk. Other hypotheses include direct and indirect carcinogenic effects of alcohol metabolites and alcohol-related impaired nutrient intake [ 101 ]. Alcohol consumption was observed to increase the risk of estrogen-positive breast cancers in particular [ 102 ]. Consumed before the first pregnancy, it significantly contributes to the induction of morphological alterations of breast tissue, predisposing it to further carcinogenic events [ 103 ].
3.2.5. Smoking
Carcinogens found in tobacco are transported to the breast tissue increasing the plausibility of mutations within oncogenes and suppressor genes ( p53 in particular). Thus, not only active but also passive smoking significantly contributes to the induction of pro-carcinogenic events [ 104 ]. Besides, longer smoking history, as well as smoking before the first full-term pregnancy, are additional risk factors that are additionally pronounced in females with a family history of breast cancer [ 105 , 106 , 107 , 108 ].
3.2.6. Insufficient Vitamin Supplementation
Vitamins exert anticancer properties, which might potentially benefit in the prevention of several malignancies including breast cancer, however, the mechanism is not yet fully understood. Attempts are continually made to analyze the effects of vitamin intake (vitamin C, vitamin E, B-group vitamins, folic acid, multivitamin) on the risk of breast cancer, nevertheless, the data remains inconsistent and not sufficient to compare the results and draw credible data [ 108 ]. In terms of breast cancer, most studies are currently focused on vitamin D supplementation confirming its potentially protective effects [ 109 , 110 , 111 ]. High serum 25-hydroxyvitamin D levels are associated with a lower incidence rate of breast cancer in premenopausal and postmenopausal women [ 110 , 112 ]. Intensified expression of vitamin D receptors was shown to be associated with lower mortality rates due to breast cancer [ 113 ]. Even so, further evaluation is required since data remains inconsistent in this matter [ 108 , 114 ].
3.2.7. Exposure to Artificial Light
Artificial light at night (ALAN) has been recently linked to increased breast cancer risk. The probable causation might be a disrupted melatonin rhythm and subsequent epigenetic alterations [ 115 ]. According to the studies conducted so far, increased exposure to ALAN is associated with a significantly greater risk of breast cancer compared to individuals with lowered ALAN exposure [ 116 ]. Nonetheless, data regarding the excessive usage of LED electronic devices and increased risk of breast cancer is insufficient and requires further evaluation as some results are contradictory [ 116 ].
3.2.8. Intake of Processed Food/Diet
According to the World Health Organization (WHO), highly processed meat was classified as a Group 1 carcinogen that might increase the risk of not only gastrointestinal malignancies but also breast cancer. Similar observations were made in terms of an excessive intake of saturated fats [ 117 ]. Ultra-processed food is rich in sodium, fat, and sugar which subsequently predisposes to obesity recognized as another factor of breast cancer risk [ 118 ]. It was observed that a 10% increase of ultra-processed food in the diet is associated with an 11% greater risk of breast cancer [ 118 ]. Contrarily, a diet high in vegetables, fruits, legumes, whole grains, and lean protein is associated with a lowered risk of breast cancer [ 119 ]. Generally, a diet that includes food containing high amounts of n-3 PUFA, vitamin D, fiber, folate, and phytoestrogen might be beneficial as a prevention of breast cancer [ 120 ]. Besides, lower intake of n-6 PUFA and saturated fat is recommended. Several in vitro and in vivo studies also suggest that specific compounds found in green tea might present anti-cancer effects which has also been studied regarding breast cancer [ 121 ]. Similar properties were observed in case of turmeric-derived curcuminoids as well as sulforaphane (SFN) [ 122 , 123 ].
3.2.9. Exposure to Chemical
Chronic exposure to chemicals can promote breast carcinogenesis by affecting the tumor microenvironment subsequently inducing epigenetic alterations along with the induction of pro-carcinogenic events [ 124 ]. Females chronically exposed to chemicals present significantly greater plausibility of breast cancer which is further positively associated with the duration of the exposure [ 125 ]. The number of chemicals proposed to induce breast carcinogenesis is significant; so far, dichlorodiphenyltrichloroethane (DDT) and polychlorinated biphenyl (PCB) are mostly investigated in terms of breast cancer since early exposure to those chemicals disrupts the development of mammary glands [ 126 , 127 ]. A potential relationship was also observed in the case of increased exposure to polycyclic aromatic hydrocarbons (PAH), synthetic fibers, organic solvents, oil mist, and insecticides [ 128 ].
3.2.10. Other Drugs
Other drugs that might constitute potential risk factors for breast cancer include antibiotics, antidepressants, statins, antihypertensive medications (e.g., calcium channel blockers, angiotensin II-converting enzyme inhibitors), as well as NSAIDs (including aspirin, ibuprofen) [ 129 , 130 , 131 , 132 , 133 ].
4. Breast Cancer Classification
4.1. histological classification.
Invasive breast cancers (IBC) comprise wide spectrum tumors that show a variation concerning their clinical presentation, behavior, and morphology. The World Health Organization (WHO) distinguish at least 18 different histological breast cancer types [ 134 ].
Invasive breast cancer of no special type (NST), formerly known as invasive ductal carcinoma is the most frequent subgroup (40–80%) [ 135 ]. This type is diagnosed by default as a tumor that fails to be classified into one of the histological special types [ 134 ]. About 25% of invasive breast cancers present distinctive growth patterns and cytological features, hence, they are recognized as specific subtypes (e.g., invasive lobular carcinoma, tubular, mucinous A, mucinous B, neuroendocrine) [ 136 ].
Molecular classification independently from histological subtypes, invasive breast cancer can be divided into molecular subtypes based on mRNA gene expression levels. In 2000, Perou et al. on a sample of 38 breast cancers identified 4 molecular subtypes from microarray gene expression data: Luminal, HER2-enriched, Basal-like, and Normal Breast-like [ 137 ]. Further studies allowed to divide the Luminal group into two subgroups (Luminal A and B) [ 138 , 139 ]. The normal breast-like subtype has subsequently been omitted, as it is thought to represent sample contamination by normal mammary glands. In the Cancer Genome Atlas Project (TCGA) over 300 primary tumors were thoroughly profiled (at DNA, RNA, and protein levels) and combined in biological homogenous groups of tumors. The consensus clustering confirmed the distinction of four main breast cancer intrinsic subtypes based on mRNA gene expression levels only (Luminal A, Luminal B, HER2-enriched, and basal-like) [ 140 ]. Additionally, the 5th intrinsic subtype—claudin-low breast cancer was discovered in 2007 in an integrated analysis of human and murine mammary tumors [ 141 ].
In 2009, Parker et al. developed a 50-gene signature for subtype assignment, known as PAM50, that could reliably classify particular breast cancer into the main intrinsic subtypes with 93% accuracy [ 142 ]. PAM50 is now clinically implemented worldwide using the NanoString nCounter ® , which is the basis for the Prosigna ® test. The Prosigna ® combines the PAM50 assay as well as clinical information to assess the risk of distant relapse estimation in postmenopausal women with hormone receptor-positive, node-negative, or node-positive early-stage breast cancer patients, and is a daily-used tool assessing the indication of adjuvant chemotherapy [ 143 , 144 , 145 ].
4.2. Luminal Breast Cancer
Luminal breast cancers are ER-positive tumors that comprise almost 70% of all cases of breast cancers in Western populations [ 146 ]. Most commonly Luminal-like cancers present as IBC of no special subtype, but they may infrequently differentiate into invasive lobular, tubular, invasive cribriform, mucinous, and invasive micropapillary carcinomas [ 147 , 148 ]. Two main biological processes: proliferation-related pathways and luminal-regulated pathways distinguish Luminal-like tumors into Luminal A and B subtypes with different clinical outcomes.
Luminal A tumors are characterized by presence of estrogen-receptor (ER) and/or progesterone-receptor (PR) and absence of HER2. In this subtype the ER transcription factors activate genes, the expression of which is characteristic for luminal epithelium lining the mammary ducts [ 149 , 150 ]. It also presents a low expression of genes related to cell proliferation [ 151 ]. Clinically they are low-grade, slow-growing, and tend to have the best prognosis.
In contrast to subtype A, Luminal B tumors are higher grade and has worse prognosis. They are ER positive and may be PR negative and/or HER2 positive. Additionally, it has high expression of proliferation-related genes (e.g., MKI67 and AURKA) [ 152 , 153 , 154 ]. This subtype has lower expression of genes or proteins typical for luminal epithelium such as the PR [ 150 , 155 ] and FOXA1 [ 146 , 156 ], but not the ER [ 157 ]. ER is similarly expressed in both A and B subtypes and is used to distinguish luminal from non-luminal disease.
4.3. HER2-Enriched Breast Cancer
The HER2-enriched group makes up 10–15% of breast cancers. It is characterized by the high expression of the HER2 with the absence of ER and PR. This subtype mainly expresses proliferation—related genes and proteins (e.g., ERBB2/HER2 and GRB7), rather than luminal and basal gene and protein clusters [ 154 , 156 , 157 ]. Additionally, in the HER2-enriched subtype there is evidence of mutagenesis mediated by APOBEC3B. APOBEC3B is a subclass of APOBEC cytidine deaminases, which induce cytosine mutation biases and is a source of mutation clusters [ 158 , 159 , 160 ].
HER2-enriched cancers grow faster than luminal cancers and used to have the worst prognosis of subtypes before the introduction of HER2-targeted therapies. Importantly, the HER2-enriched subtype is not synonymous with clinically HER2-positive breast cancer because many ER-positive/HER2-positive tumors qualify for the luminal B group. Moreover, about 30% of HER2-enriched tumors are classified as clinically HER2-negative based on immunohistochemistry (IHC) and/or fluorescence in situ hybridization (FISH) methods [ 161 ].
4.4. Basal-Like/Triple-Negative Breast Cancer
The Triple-Negative Breast Cancer (TNBC) is a heterogeneous collection of breast cancers characterized as ER-negative, PR-negative, and HER2-negative. They constitute about 20% of all breast cancers. TNBC is more common among women younger than 40 years of age and African-American women [ 161 ]. The majority (approximately 80%) of breast cancers arising in BRCA1 germline mutation are TNBC, while 11–16% of all TNBC harbor BRCA1 or BRCA2 germline mutations. TNBC tends to be biologically aggressive and is often associated with a worse prognosis [ 162 ]. The most common histology seen in TNBC is infiltrating ductal carcinoma, but it may also present as medullary-like cancers with a prominent lymphocytic infiltrate; metaplastic cancers, which may show squamous or spindle cell differentiation; and rare special type cancers like adenoid cystic carcinoma (AdCC) [ 163 , 164 , 165 ].
The terms basal-like and TNBC have been used interchangeably; however, not all TNBC are of the basal type. On gene expression profiling, TNBCs can be subdivided into six subtypes: basal-like (BL1 and BL2), mesenchymal (M), mesenchymal stem-like (MSL), immunomodulatory (IM), and luminal androgen receptor (LAR), as well as an unspecified group (UNS) [ 166 , 167 ]. However, the clinical relevance of the subtyping still unclear, and more research is needed to clarify its impact on TNBC treatment decisions [ 168 ].
4.5. Claudin-Low Breast Cancer
Claudin-low (CL) breast cancers are poor prognosis tumors being mostly ER-negative, PR-negative, and HER2-negative. CL tumors account for 7–14% of all invasive breast cancers [ 147 ]. No differences in survival rates were observed between claudin-low tumors and other poor-prognosis subtypes (Luminal B, HER2-enriched, and Basal-like). CL subtype is characterized by the low expression of genes involved in cell-cell adhesion, including claudins 3, 4, and 7, occludin, and E-cadherin. Besides, these tumors show high expression of epithelial-mesenchymal transition (EMT) genes and stem cell-like gene expression patterns [ 169 , 170 ]. Moreover, CL tumors have marked immune and stromal cell infiltration [ 171 ]. Due to their less differentiated state and a preventive effect of the EMT-related transcription factor, ZEB1 CL tumors are often genomically stable [ 172 , 173 ].
4.6. Surrogate Markers Classification
In clinical practice, the key question is the discrimination between patients who will or will not benefit from particular therapies. By using molecular assays, more patients can be spared adjuvant chemotherapy, but these tests are associated with significant costs. Therefore, surrogate subgroups based on pathological morphology and widely available immunohistochemical (IHC) markers are used as a tool for risk stratification and guidance of adjuvant therapy [ 174 ]. A combination of the routine pathological markers ER, PR, and HER2 is used to classify tumors into intrinsic subtypes [ 175 ]. Semiquantitative evaluation of Ki-67 and PR is helpful for further typing of the Luminal subtype [ 176 , 177 ]. Moreover, evaluation of cytokeratin 5/6 and epidermal growth factor receptor is utilized to identify the Basal-like breast cancer among the TNBC [ 178 ].
In St. Gallen’s 2013 guidelines the IHC-based surrogate subtype classification was recommended for clinical decision making [ 179 ]. However, these IHC-based markers are only a surrogate and cannot establish the intrinsic subtype of any given cancer, with discordance rates between IHC-based markers and gene-based assays as high as 30% [ 180 ].
4.7. American Joint Committee on Cancer Classification
The baseline tool to estimate the likely prognosis of patients with breast cancer is the AJCC staging system that includes grading, immunohistochemistry biomarkers, and anatomical advancement of the disease. Since its inception in 1977, the American Joint Committee on Cancer (AJCC) has published an internationally accepted staging system based on anatomic findings: tumor size (T), nodal status (N), and metastases (M). However, gene expression profiling has identified several molecular subtypes of breast cancer [ 181 ]. The eighth edition of the AJCC staging manual (2018), outlines a new prognostic staging system for breast cancer that, in addition to anatomical features, acknowledges biological factors [ 182 ]. These factors—ER, PR, HER2, grade, and multigene assays—are recommended in practice to define prognosis [ 183 , 184 ].
The most widely used histologic grading system of breast cancer is the Elston-Ellis modification [ 185 ] of Scarff-Bloom-Richardson grading system [ 186 ], also known as the Nottingham grading system. The grade of a tumor is determined by assessing morphologic features: (a) formation of tubules, (b) mitotic count, (c) variability, and the size and shape of cellular nuclei. A score between 1 (most favorable) and 3 (least favorable) is assigned for each feature. Grade 1 corresponds to combined scores between 3 and 5, grade 2 corresponds to a combined score of 6 or 7, and grade 3 corresponds to a combined score of 8 or 9.
In addition to grading and biomarkers, the commercially available multigene assays provide additional prognostic information suitable for incorporation in the AJCC 8th edition. The 21-gene assay Oncotype DX ® assessed by reverse transcription-polymerase chain reaction (RT-PCR) was the only assay sufficiently evaluated and included in the staging system. This assay is valuable in the staging of patients with hormone receptor-positive, HER2-negative, node-negative tumors that are <5 cm. Patients with results of the assay (Recurrence Score) less than 11 had excellent disease-free survival at 6.9 years of 98.6% with endocrine therapy alone [ 187 ]. Hence, adjuvant systemic chemotherapy can be safely omitted in patients with a low-risk multigene assay [ 188 ].
The AJCC staging manual includes a pathological and a clinical-stage group. The clinical prognostic stage group should be utilized in all patients on initial evaluation before any systemic therapy. Clinical staging uses the TNM anatomical information, grading, and expression of these three biomarkers. When patients undergo surgical resection of their primary tumor, the post-resection anatomic information coupled with the pretreatment biomarker findings results in the final Pathologic Prognostic Stage Group.
The recent update of breast cancer staging by the biologic markers improved the outcome prediction in comparison to prior staging based only on anatomical features of the disease. The validation studies involving the reassessment of the Surveillance, Epidemiology, and End Results (SEER) database ( n = 209,304, 2010–2014) and the University of Texas MD Anderson Cancer Center database ( n = 3327, years of treatment 2007–2013) according to 8th edition AJCC manual proved the more accurate prognostic information [ 189 , 190 ].
5. Prognostic Biomarkers
5.1. estrogen receptor.
Estrogen receptor (ER) is an important diagnostic determinant since approximately 70–75% of invasive breast carcinomas are characterized by significantly enhanced ER expression [ 191 , 192 ]. Current practice requires the measurement of ER expression on both—primary invasive tumors and recurrent lesions. This procedure is mandatory to provide the selection of those patients who will most benefit from the implementation of the endocrine therapy mainly selective estrogen receptor modulators, pure estrogen receptor downregulators, or third-generation aromatase inhibitors [ 193 ]. Even though the diagnosis of altered expression of ER is particularly relevant in terms of the proper therapy selection, ER expression might also constitute a predictive factor—patients with high ER expression usually present significantly better clinical outcomes [ 194 ]. A relationship was observed between ER expression and the family history of breast cancer which further facilitates the utility of ER expression as a diagnostic biomarker of breast cancer especially in cases of familial risk [ 195 ]. Besides, Konan et al. reported that ERα-36 expression could constitute one of the potential targets of PR-positive cancers and a prognostic marker at the same time [ 196 ].
5.2. Progesterone Receptor
PR is highly expressed (>50%) in patients with ER-positive while quite rarely in those with ER-negative breast cancer [ 197 ]. PR expression is regulated by ER therefore, physiological values of PR inform about the functional ER pathway [ 197 ]. However, both ER and PR are abundantly expressed in breast cancer cells and both are considered as diagnostic and prognostic biomarkers of breast cancer (especially ER-positive ones) [ 198 ]. Greater PR expression is positively associated with the overall survival, time to recurrence, and time to either treatment failure or progression while lowered PR levels are usually related to a more aggressive course of the disease as well as poorer recurrence and prognosis [ 199 ]. Thus, favorable management of breast cancer patients highly depends on the assessment of PR expression. Nevertheless, the predictive value of PR expression still remains controversial [ 200 ].
5.3. Human Epidermal Growth Factor Receptor 2
The expression of human epidermal growth factor receptor 2 (HER2) accounts for approximately 15–25% of breast cancers and its status is primarily relevant in the choice of proper management with breast cancer patients; HER2 overexpression is one of the earliest events during breast carcinogenesis [ 201 ]. Besides, HER2 increases the detection rate of metastatic or recurrent breast cancers from 50% to even more than 80% [ 202 ]. Serum HER2 levels are considered to be a promising real-time marker of tumor presence or recurrence [ 203 ]. HER2 amplification leads to further overactivation of the pro-oncogenic signaling pathways leading to uncontrolled growth of cancer cells which corresponds with poorer clinical outcomes in the case of HER2-positive cancers [ 204 ]. Overexpression of HER2 also correlates with a significantly shorter disease-free period [ 205 ] as well as histologic type, pathologic state of cancer, and a number of axillary nodes with metastatic cancerous cells [ 205 ].
5.4. Antigen Ki-67
The Ki-67 protein is a cellular marker of proliferation and the Ki-67 proliferation index is an excellent marker to provide information about the proliferation of cancerous cells particularly in the case of breast cancer. The proliferative activities determined by Ki-67 reflect the aggressiveness of cancer along with the response to treatment and recurrence time [ 206 ]. Thus, Ki-67 is crucial in terms of the choice of the proper treatment therapy and the potential follow-ups due to recurrence. Though, due to several limitations of the analytical validity of Ki-67 immunohistochemistry, Ki-67 expression levels should be considered benevolently in terms of definite treatment decisions. Ki-67 might be considered as a potential prognostic factor as well; according to a meta-analysis of 68 studies involving 12,155 patients, the overexpression of Ki-67 is associated with poorer clinical outcomes of patients [ 207 ]. High expression of Ki-67 also reflects poorer survival rates of breast cancer patients [ 208 ]. There are speculations whether Ki-67 could be considered as a potential predictive marker, however, such data is still limited and contradictory.
Mib1 (antibody against Ki-67) proliferation index remains a reliable diagnostic biomarker of breast cancer, similarly to Ki-67. A decrease in both Mib1 and Ki-67 expression levels is associated with a good response of breast cancer patients to preoperative treatment [ 209 ]. Mib1 levels are significantly greater in patients with concomitant p53 mutations [ 210 ]. Mib1 assessment might be especially useful in cases of biopsy specimens small in size, inappropriate for neither mitotic index nor S-phase fraction evaluation [ 211 ].
5.6. E-Cadherin
E-cadherin is a critical protein in the epithelial-mesenchymal transition (EMT); loss of its expression leads to the gradual transformation into mesenchymal phenotype which is further associated with increased risk of metastasis. The utility of E-cadherin as a breast biomarker is yet questionable, however, some research indicated that its expression is potentially associated with several breast cancer characteristics such as tumor size, TNM stage, or lymph node status [ 212 ]. Low or even total loss of E-cadherin expression might be potentially useful in the determination of histologic subtype of breast cancer [ 213 , 214 ]. E-cadherin levels do not seem to be promising in terms of patients’ survival rates assessment, however, there are some reports indicating that higher levels of E-cadherin were associated with shorter survival rates in patients with invasive breast carcinoma [ 213 , 215 ]. Lowered E-cadherin expression is positively associated with lymph node metastasis [ 216 ].
5.7. Circulating Circular RNA
Circulating circular RNAs (circRNAs) belong to the group of non-coding RNA and were quite recently shown to be crucial in terms of several hallmarks of breast carcinogenesis including apoptosis, enhanced proliferation, or increased metastatic potential [ 217 ]. One of the most comprehensively described circRNAs, mostly specific to breast cancer include circFBXW7—which was proposed as a potential diagnostic biomarker as well as therapeutic tool for patients with triple-negative breast cancer (TNBC), as well as hsa_circ_0072309 which is abundantly expressed in breast cancer patients and usually associated with poorer survival rates [ 218 ]. Has_circ_0001785 is considered to be promising as a diagnostic biomarker of breast cancer [ 219 ]. The number of circRNAs dysregulated during breast carcinogenesis is significant; their expression might be either upregulated (e.g., has_circ_103110, circDENND4C) or downregulated (e.g., has_circ_006054, circ-Foxo3) [ 220 ]. Besides, specific circRNAs have been reported in different types of breast cancer such as TNBC, HER2-positive, and ER-positive [ 221 ]. Recently it was showed that an interaction between circRNAs and micro-RNA—namely in the form of Cx43/has_circ_0077755/miR-182 post-transcriptional axis, might predict breast cancer initiation as well as further prognosis. Cx43 is transmembrane protein responsible for epithelial homeostasis that mediates junction intercellular communication and its loss dysregulates post-transcriptional axes in breast cancer initiation [ 222 ].
Loss-of-function mutations in the TP53 (P53) gene have been found in numerous cancer types including osteosarcomas, leukemia, brain tumors, adrenocortical carcinomas, and breast cancers [ 223 , 224 ]. P53 protein is essential for normal cellular homeostasis and genome maintenance by mediating cellular stress responses including cell cycle arrest, apoptosis, DNA repair, and cellular senescence [ 225 ]. The silencing mutation of the P53 gene is evident at an early stage of cancer progression. In breast cancer, the prevalence of TP53 mutations is present in approximately 80% of patients with the TNBC and 10% of patients with Luminal A disease [ 226 ].
There have been many studies showing the prognostic role of p53 loss-of-function mutation in breast cancer [ 227 , 228 ]. However, the missense mutations may alters p53 properties causing not only a loss of wild-type function, but also acquisition novel activities-gain of function [ 229 ]. The IHC status of p53 has been proposed as a specific prognostic factor in TNBC, and a feature that divides TNBC into 2 distinct subgroups: a p53-negative normal breast-like TN subgroup, and a p53-positive basal-like subgroup with worse overall survival [ 230 , 231 , 232 ]. However, there is not enough evidence to utilize p53 gene mutational status or immunohistochemically measured protein for determining standardized prognosis in patients with breast cancer [ 233 ].
5.9. MicroRNA
MicroRNAs (miRNA) are a major class of endogenous non-coding RNA molecules (19–25 nucleotides) that have regulatory roles in multiple pathways [ 234 ]. Some miRNAs are related to the development, progression, and response of the tumor to therapy [ 235 ]. Several studies have investigated abnormally expressed miRNAs as biomarkers in breast cancer tissue samples. According to meta-analysis by Adhami et al. two miRNAs (miRNA-21 and miRNA-210) were upregulated consistently and six miRNAs (miRNA-145, miRNA-139-5p, miRNA-195, miRNA-99a, miRNA-497, and miRNA-205) were downregulated consistently in at least three studies [ 236 ].
The miRNA-21 overexpression was observed in TNBC tissues and was associated with enhanced invasion and proliferation of TNBC cells as well as downregulation of the PTEN expression [ 237 ]. Similarly, the high expression of miRNA-210 is related to tumor proliferation, invasion, and poor survival rates in breast cancer patients [ 238 , 239 ].
The miRNA-145 is an anti-cancer agent having the property of inhibiting migration and proliferation of breast cancer cells via regulating the TGF-β1 expression [ 240 ]. However, the miRNA-145 is downregulated in both plasma and tumors of breast cancer patients [ 241 ]. Similarly, miRNA-139-5p and miRNA-195 have tumor suppressor activity in various cancers [ 242 , 243 ].
Nevertheless, further clinical researches focusing on these miRNAs are needed to utilize them as reproducible, disease-specific markers that have a high level of specificity and sensitivity.
5.10. Tumor-Associated Macrophages
Macrophages are known for their immunomodulatory effects and they can be divided according to their phenotypes into M1- or M2-like states [ 244 , 245 ]. M1 macrophages secrete IL-12 and tumor necrosis factor with antimicrobial and antitumor effects. M2 macrophages produce cytokines, including IL-10, IL-1 receptor antagonist type II, and IL-1 decoy receptor. Therefore, macrophages with M1-like phenotype have been linked to good disease course while M2-like phenotype has been associated with adverse outcome, potentially through immunosuppression and the promotion of angiogenesis and tumor cell proliferation and invasion [ 246 , 247 ]. In literature, tumor-associated macrophages (TAMs) are associated with M2 macrophages which promote tumor growth and metastasis.
For breast cancer, studies have shown that the density of TAMs is related to hormone receptor status, stage, histologic grade, lymph node metastasis, and vascular invasion [ 248 , 249 , 250 , 251 ]. According to meta-analysis conducted by Zhao et al. high density of TAMs was related to overall survival disease-free survival [ 252 ].
Conversely, M1 polarized macrophages are linked to favorable prognoses in various cancers [ 253 , 254 , 255 ]. In breast cancer, the high density of M1-like macrophages predicted improved survival in patients with HER2+ phenotype and may be a potential prognostic marker [ 256 ].
However, further studies are needed to clarify the influence of macrophages on breast cancer biology as well as investigate the role of their intratumoral distribution and surface marker selection.
5.11. Inflammation-Based Models
The host inflammatory and immune responses in the tumor and its microenvironment are critical components in cancer development and progression [ 257 ]. The tumor-induced systemic inflammatory response leads to alterations of peripheral blood white blood cells [ 258 ]. Therefore, the relationship between peripheral blood inflammatory cells may serve as an accessible and early method of predicting patient prognosis. Recent studies have reported the predictive role of the inflammatory cell ratios: neutrophil-to-lymphocyte ratio, the lymphocyte-to-monocyte ratio, and the platelet-to-lymphocyte ratio for prognosis in different cancers [ 258 , 259 , 260 , 261 ].
5.11.1. The Neutrophil-to-Lymphocyte Ratio (NLR)
In an extensive study on 27,031 cancer patients, Proctor et al. analyzed the prognostic value of NLR and found a significant relationship between NLR and survival in various cancers including breast cancer [ 262 ]. There are pieces of evidence of the role of lymphocytes in breast cancer immunosurveillance [ 263 , 264 ]. Opposingly neutrophils suppress the cytolytic activity of lymphocytes, leading to enhanced angiogenesis and tumor growth and progression [ 265 ].
Azab et al. first reported that NLR before chemotherapy was an independent factor for long-term mortality and related it to age and tumor size in breast cancer [ 266 ]. In a recent meta-analysis by Guo et al., performed on 17,079 individuals, the high NLR level was associated with both poor overall survival as well as disease-free survival for breast cancer patients. Moreover, it was reported that association between NLR and overall survival was stronger in TNBC patients than in HER2-positive ones [ 267 ].
5.11.2. Lymphocyte-to-Monocyte Ratio
The association of the lymphocyte-to-monocyte ratio (LMR) with patients’ prognosis has been reported for several cancers [ 268 , 269 ]. As lymphocytes have an antitumor activity by inducing cytotoxic cell death and inhibiting tumor proliferation [ 270 ], the monocytes are involved in tumorigenesis, including differentiation into TAMs [ 246 , 247 , 271 ]. In the tumor microenvironment, cytokines, and free radicals that are secreted by monocytes and macrophages are associated with angiogenesis, tumor cell invasion, and metastasis [ 271 ].
A meta-analysis investigating the prognostic effect of LMR showed that low LMR levels are associated with shorter overall survival outcomes in Asian populations, TNBC patients, and patients with non-metastatic and mixed stages [ 272 ]. Moreover, high LMR levels are associated with favorable disease-free survival of breast cancer patients under neoadjuvant chemotherapy [ 273 ].
5.11.3. Platelet-to-Lymphocyte Ratio (PLR)
A high platelet count has been associated with poor prognosis in several types of cancers [ 274 , 275 , 276 ]. Platelets contain both pro-inflammatory molecules and cytokines (P-selectin, CD40L, and interleukin (IL)-1, IL-3, and IL-6) and many anti-inflammatory cytokines. Tumor angiogenesis and growth may be stimulated by the secretion of platelet-derived growth factor, vascular endothelial growth factor, transforming growth factor-beta, and platelet factor 4 [ 277 , 278 , 279 ].
A meta-analysis study investigated the prognostic importance of PLR by analyzing 5542 breast cancer patients. High PLR level was associated with poor prognosis (overall survival and disease-free survival), yet, its prognostic value was not determined for molecular subtypes of breast cancer. Nevertheless, an association was found between PLR and clinicopathological features of the tumor, including stage, lymph node metastasis, and distant metastasis [ 280 ]. In the aforementioned meta-analysis, there was a difference in the incidence of high levels of PLR between HER2 statuses [ 280 ], while other studies found a difference between hormone ER or PR statuses [ 281 , 282 ].
6. Treatment Strategies
6.1. surgery.
There are two major types of surgical procedures enabling the removal of breast cancerous tissues and those include (1) breast-conserving surgery (BCS) and (2) mastectomy. BCS—also called partial/segmental mastectomy, lumpectomy, wide local excision, or quadrantectomy—enables the removal of the cancerous tissue with simultaneous preservation of intact breast tissue often combined with plastic surgery technics called oncoplasty. Mastectomy is a complete removal of the breast and is often associated with immediately breast reconstruction. The removal of affected lymph nodes involves sentinel lymph node biopsy (SLNB) and axillary lymph node dissection (ALND). Even though BCS seems to be highly more beneficial for patients, those who were treated with this technique often show a tendency for a further need for a complete mastectomy [ 283 ]. However, usage of BCS is mostly related to significantly better cosmetic outcomes, lowered psychological burden of a patient, as well as reduced number of postoperative complications [ 284 ]. Guidelines of the European Society for Medical Oncology (ESMO) for patients with early breast cancer make the choice of therapy dependent to tumor size, feasibility of surgery, clinical phenotype, and patient’s willingness to preserve the breast [ 285 ].
6.2. Chemotherapy
Chemotherapy is a systemic treatment of BC and might be either neoadjuvant or adjuvant. Choosing the most appropriate one is individualized according to the characteristics of the breast tumor; chemotherapy might also be used in the secondary breast cancer. Neoadjuvant chemotherapy is used for locally advanced BC, inflammatory breast cancers, for downstaging large tumors to allow BCS or in small tumors with worse prognostics molecular subtypes (HER2 or TNBC) which can help to identify prognostics and predictive factors of response and can be provided intravenously or orally. Currently, treatment includes a simultaneous application of schemes 2–3 of the following drugs—carboplatin, cyclophosphamide, 5-fluorouracil/capecitabine, taxanes (paclitaxel, docetaxel), and anthracyclines (doxorubicin, epirubicin). The choice of the proper drug is of major importance since different molecular breast cancer subtypes respond differently to preoperative chemotherapy [ 286 ]. Preoperative chemotherapy is comparably effective to postoperative chemotherapy [ 287 ].
Even though chemotherapy is considered to be effective, its usage very often leads to several side effects including hair loss, nausea/vomiting, diarrhea, mouth sores, fatigue, increased susceptibility to infections, bone marrow supression, combined with leucopenia, anaemia, easier bruising or bleeding; other less frequent side effects include cardiomyopathy, neuropathy, hand-foot syndrome, impaired mental functions. In younger women, disruptions of the menstrual cycle and fertility issues might also appear. Special form of chemotherapy is electrochemotherapy which can be used in patients with breast cancer that has spread to the skin, however, it is still quite uncommon and not available in most clinics.
6.3. Radiation Therapy
Radiotherapy is local treatment of BC, typically provided after surgery and/or chemotherapy. It is performed to ensure that all of the cancerous cells remain destroyed, minimizing the possibility of breast cancer recurrence. Further, radiation therapy is favorable in the case of metastatic or unresectable breast cancer [ 288 ]. Choice of the type of radiation therapy depends on previous type of surgery or specific clinical situation; most common techniques include breast radiotherapy (always applied after BC), chest-wall radiotherapy (usually after mastectomy), and ‘breast boost’ (a boost of high-dose radiotherapy to the place of tumor bed as a complement of breast radiotherapy after BCS). Regarding breast radiotherapy specifically, several types are distinguished including
- (1) intraoperative radiation therapy (IORT)
- (2) 3D-conformal radiotherapy (3D-CRT)
- (3) intensity-modulated radiotherapy (IMRT)
- (4) brachytherapy—which refers to internal radiation in contrast to other above-mentioned techniques.
Irritation and darkening of the skin exposed to radiation, fatigue, and lymphoedema are one of the most common side effects of radiation therapy applied in breast cancer patients. Nonetheless, radiation therapy is significantly associated with the improvement of the overall survival rates of patients and lowered risk of recurrence [ 289 ].
6.4. Endocrinal (Hormonal) Therapy
Endocrinal therapy might be used either as a neoadjuvant or adjuvant therapy in patients with Luminal–molecular subtype of BC; it is effective in cases of breast cancer recurrence or metastasis. Since the expression of ERs, a very frequent phenomenon in breast cancer patients, its blockage via hormonal therapy is commonly used as one of the potential treatment modalities. Endocrinal therapy aims to lower the estrogen levels or prevents breast cancer cells to be stimulated by estrogen. Drugs that block ERs include selective estrogen receptor modulators (SERMs) (tamoxifen, toremifene) and selective estrogen receptor degraders (SERDs) (fulvestrant) while treatments that aim to lower the estrogen levels include aromatase inhibitors (AIs) (letrozole, anastrazole, exemestane) [ 290 , 291 ]. In the case of pre-menopausal women, ovarian suppression induced by oophorectomy, luteinizing hormone-releasing hormone analogs, or several chemotherapy drugs, are also effective in lowering estrogen levels [ 292 ]. However, approximately 50% of hormonoreceptor-positive breast cancer become progressively resistant to hormonal therapy during such treatment [ 293 ]. Endocrinal therapy combined with chemotherapy is associated with the reduction of mortality rates amongst breast cancer patients [ 294 ].
6.5. Biological Therapy
Biological therapy (targeted therapy) can be provided at every stage of breast therapy– before surgery as neoadjuvant therapy or after surgery as adjuvant therapy. Biological therapy is quite common in HER2-positive breast cancer patients; major drugs include trastuzumab, pertuzumab, trastuzumab deruxtecan, lapatinib, and neratinib [ 295 , 296 , 297 , 298 , 299 ]. Further, the efficacy of angiogenesis inhibitors such as a recombinant humanized monoclonal anti-VEGF antibody (rhuMAb VEGF) or bevacizumab are continuously investigated [ 300 ].
In the case of Luminal, HER2-negative breast cancer, pre-menopausal women more often receive everolimus -TOR inhibitor with exemestane while postmenopausal women often receive CDK 4–6 inhibitor palbociclib or ribociclib simultaneously, combined with hormonal therapy [ 301 , 302 , 303 ]. Two penultimate drugs along with abemaciclib and everolimus can also be used in HER2-negative and estrogen-positive breast cancer [ 304 , 305 ]. Atezolizumab is approved in triple-negative breast cancer, while denosumab is approved in case of metastasis to the bones [ 306 , 307 , 308 ].
7. Conclusions
In this review, we aimed to summarize and update the current knowledge about breast cancer with an emphasis on its current epidemiology, risk factors, classification, prognostic biomarkers, and available treatment strategies. Since both the morbidity and mortality rates of breast cancer have significantly increased over the past decades, it is an urgent need to provide the most effective prevention taking into account that modifiable risk factors might be crucial in providing the reduction of breast cancer incidents. So far, mammography and sonography is the most common screening test enabling quite an early detection of breast cancer. The continuous search for prognostic biomarkers and targets for the potential biological therapies has significantly contributed to the improvement of management and clinical outcomes of breast cancer patients.
Author Contributions
Conceptualization, A.F., R.S. and A.S.; critical review of literature, S.Ł., M.C., A.F., J.B., R.S., A.S.; writing—original draft preparation, M.C., A.F.; writing—review and editing, S.Ł., M.C., A.F., J.B., R.S., A.S.; supervision, R.S. All authors have read and agreed to the published version of the manuscript.
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Breast Cancer - Free Essay Examples And Topic Ideas
Breast cancer is a type of cancer that develops from breast tissue. Essays on this topic could explore the causes, diagnosis, treatment, and prevention of breast cancer. Additionally, discussions might delve into the psychological and social impact of breast cancer on patients and their families, the ongoing research towards finding a cure, and the broader societal awareness and support systems available for those affected. We have collected a large number of free essay examples about Breast Cancer you can find at Papersowl. You can use our samples for inspiration to write your own essay, research paper, or just to explore a new topic for yourself.

Micro Needle Thermocouple for Detection of Breast Cancer
Hundreds and thousands of people are affected by cancer each year; it is one of the most fatal diseases and a leading cause of death and disability for humans (Iranifam 2014). There are several types of cancer than can affect different areas of the body, some being less life-threatening than others. A vast amount of patients suffer from late diagnosis or recurrence of their disease in spite of all the advances in diagnosis and treatment of breast cancer. Modern cancer […]
The Role of Histology in the Breast Cancer
Breast cancer is an uncontrolled growth of breast cell that can be benign, not dangerous, but it can also metastasize and invade different and distant tissues in our body. Breast Cancer is the most common cancer in female of any age and although the risk increases, as you get older, many different factors affect the chance of a woman to get breast cancer. I chose this specific topic because breast cancer is something that I’ve dealt with in my personal […]
Corporate Social Responsibility against Cancer
Abstract As an assistant manager at Kenta Law Firm, based in Monroe, I intend to collaborate with the Susan B. Komen Foundation a non-organization corporation that is interested in reducing issues of breast cancer among women. Kenta law firm has noted that a significant populace of Monroe’s youth especially women and young children specifically those who are homeless are suffering from breast cancer. In this CSR partnership, our law firm will collaborate with the Susan B. Komen Foundation in addressing […]
We will write an essay sample crafted to your needs.
Why is Screening for Breast Cancer Important
The impact this disease has, on not only the individual but the people around them, is powerful. Even though the tests show cancer, I am thankful that I had the annual test. It is true that stress, anxiety, and money can be saved by waiting until the age of 50 years old because of misinterpretation and overdiagnosis. However, early detection is the key to success in the battle against breast cancer. There are many different options for detection scans that […]
Breast Cancer: Casuses and Treatment
Cancer is defined as “when the body’s cells begin to divide without stopping and spread into surrounding tissues.” (“What is cancer?”, 2017), caused by mutations that lead to the cell cycle to proceed, regardless if the cell is qualified to. The mutations block the use of the G1, G2, and M checkpoints in the cell cycle. These checkpoints are important in “sensing defects that occur during essential processes, and induce a cell cycle arrest in response until the defects are […]
Breast Reconstruction after Mastectomy
Breast cancer is always personal. As a physician who counsels women at different steps during the healing process, I am acutely aware of this undeniable fact. Every decision she makes from the point at which she is diagnosed with breast cancer will require her focused engagement and a physician who is central to understanding her need for clarity of options. It is an intimate relationship where trust is a requirement and every woman faced with the many unknowns ahead will […]
Breast Cancer History Research Paper
Breast cancer is a disease in which most commonly occurs in all women no matter their size, shape, race, or ethnicity. About one in eight women will be diagnosed with breast cancer every year, a fatal disease if not discovered early. Early detection of breast cancer is key so that cancerous cells found in the breast do not spread through other parts of the body. With an increasing prevalence in breast cancer today, the evolution of technology has been improved […]
New Healthcare Inventions on Breast Cancer
Abstract Background: The Ki67 labeling index (LI) for breast carcinoma is essential for therapy. It is determined by visual assessment under a microscope which is subjective, thus has limitations due to inter-observer variability. A standardized method for evaluating Ki67 LI is necessary to reduce subjectivity and improve precision. Therefore, automated Digital Image Analysis (DIA) has been attempted as a potential method for evaluating the Ki67 index. Materials and Method: We included 48 cases of invasive breast carcinoma in this study. […]
Understanding Breast Cancer
This paper will clarify what Breast Cancer is. It will explain the symptoms, treatment options, and other useful information regarding this disease. The first thing to know about Breast Cancer is understanding what it is. According to the Cancer.org website, breast cancer begins when cells in the bosom begin to spread out of control. The tumor that is formed from these cells may be detected on an x-ray or can be felt as a lump. Malignancy can advance into neighboring […]
Breast Cancer in African American Women
Summary Despite the fact that Caucasian women in the United States have a higher incidence rate of breast cancer than any other racial group, African-Americans succumb notably worse to the disease and record the highest mortality rate. To comprehend the barriers and challenges that predispose African-American women to these disparities, this research was conducted to get a better understanding from the perspective of oncologists. With diverse ethnicity and gender representation, the participation of seven medical, surgical and radiation oncologists that […]
Essential Breast Cancer Screening Techniques and their Complements
It is with great distress that each year a large number of females suffer and die from breast cancer. Medicine practitioners and researchers have been striving to save lives from breast cancer, and how they manage to do this includes two major parts—diagnosis and treatment. What comes first on the stage of diagnosis is the detection of tumor. Thus, the development of breast imaging techniques is at the highest priority for diagnosing breast cancer, and individuals’ focus is on earlier […]
Breast Cancer Prevention and Treatment
The human body is made up of cells. When a cell dies the body automatically replaces it with a new healthy cell, but sometimes the cell is not healthy and grows out of control. These cells group together and form a lump that can be seen on an x-ray. Breast cancer is a tumor in the cells of person’s breast. It can spread throughout the breast to the person’s lymph nodes and other parts of the body. Sometimes it occurs […]
Breast Cancer Diagnosis
I. Executive Summary Breast cancer is concerning a large number of female individuals worldwide. This disease comes from abnormally developed breast tissue, which usually begins in either lobules or ducts of the breast. Generally speaking, breast cancer is divided into two types—non-invasive and invasive. The core criteria to distinguish in between these two types of breast cancers is the location of cancer cells. Cancer cells remain on their initial positions for a non-invasive breast cancer, whereas they grow, or “invade”, […]
Understanding a Breast Cancer Diagnosis
Breast cancer is often known as an aggressive cancer. It forms when cells grow uncontrollably in the tissues of the breast, leading to a tumor. Over 190,000 individuals are diagnosed yearly (Cancer Center). Breast cancer is the second leading cause of death, and the rate increases every year in women, and occasionally in men. Over 12 percent of women in the United States of America will face breast cancer in their lifetime. It is the most common cause of death […]
Breast Cancer in the Era of Precision Medicine
Introduction: Precision medicine is concerned with the diagnosis of patients according to their biological, genetic, and molecular status. As cancer is a genetic disease, its treatment comes among the first medical disciplines as an application of precision medicine. Breast cancer is a highly complex, heterogeneous, and multifactorial disease; it is also one of the most common diseases among women in the world. Usually, there are no clear symptoms, so regular screening is important for early detection. Scientists recently started using […]
Exome Sequencing to Identify Rare Mutations Associated with Breast Cancer Susceptibility
Abstract Background - Breast cancer predisposition has been known to be caused by hereditary factors. New techniques particularly exome sequencing have allowed/ helped us to identify new and novel variants that exhibit a phenotype. Method - In this review we discuss the advantages of exome sequencing and how it could help in understanding the familial breast cancer. In particular, we will discuss about the studies by Noh et al.(1), Thompson et al.(2), and Kiiski et al.(3), on how they have […]
A Novel Therapeutic Strategy for HER2 Breast Cancer by Nanoparticles Combined with Macrophages
Abstract:In recent years, the cell membrane bionic nanoparticles as a new drug delivery system is widely used in small molecule drugs, vaccines and targeted delivery of macromolecular drugs, because of its inherited the specific receptors on the cell membrane and membrane proteins can be used to implement specific targeted delivery, and the tumor showed a good treatment effect on the disease such as model, this topic with a huge bite cell membrane of the role of tumor capture, chemical modification, […]
Essays About Breast Cancer Breast Cancer is one of the most common cancers in women and is a disease by which the cells in the breast area grow out of control. Breast cancer tends to begin in the ducts or lobules of a breast and there are different types of cancer. In the US alone 1 in 8 women will develop breast cancer at some stage in their lives. In many academic fields; from science to medicine the study of breast cancer and essays about breast cancer are required as part of the curriculum. An essay on breast cancer can seem daunting due to the amount of research and several varying scientific approaches used to talk about the topic. We offer essay examples, or research paper guidance and free essay samples. These can be used to gauge how to approach the topic and are an informative look at all factors that contribute to breast cancer and prevention. We also factor breast cancer awareness into our essay samples and ensure essays for both university and college build a strong foundation to understanding the disease, but also draw criticism when necessary and a strong conclusion on whatever element of breast cancer the focus of the essay is on.
1. Tell Us Your Requirements
2. Pick your perfect writer
3. Get Your Paper and Pay
Hi! I'm Amy, your personal assistant!
Don't know where to start? Give me your paper requirements and I connect you to an academic expert.
short deadlines
100% Plagiarism-Free
Certified writers
Skip to Content
- Conquer Cancer
- ASCO Journals
- f Cancer.net on Facebook
- t Cancer.net on Twitter
- q Cancer.net on YouTube
- g Cancer.net on Google
Types of Cancer
- Navigating Cancer Care
- Coping With Cancer
- Research and Advocacy
- Survivorship
Breast Cancer: Introduction
ON THIS PAGE: You will find some basic information about this disease and the parts of the body it may affect. This is the first page of Cancer.Net’s Guide to Breast Cancer. Use the menu to see other pages. Think of that menu as a roadmap for this entire guide.
Anyone can develop breast cancer. In the United States, breast cancer is the most common cancer in women (excluding skin cancer). Male breast cancer is rare, accounting for less than 1% of all breast cancers. Learn more about male breast cancer in a separate guide on this website .
About the breast
The breast is made up of different tissues, ranging from very fatty tissue to very dense tissue. Within this tissue is a network of lobes. Each lobe is made up of small, tube-like structures called lobules that contain milk glands. Small ducts connect the glands, lobules, and lobes, carrying milk from the lobes to the nipple. The nipple is located in the middle of the areola, which is the darker area that surrounds the nipple. Blood and lymph vessels also run throughout the breast. Blood vessels nourish the cells by delivering oxygen and nutrients and also removing waste and carbon dioxide. Lymph vessels, unlike blood vessels, only carry fluid away from tissues. They connect to lymph nodes and the lymphatic system, which drains bodily waste products. Lymph nodes are the small, bean-shaped organs that are part of the body's immune system and help fight infection. Groups of lymph nodes are located in different areas throughout the body, such as in the neck, groin, and abdomen. Regional lymph nodes of the breast are those nearby the breast, such as the lymph nodes under the arm, which are called axillary lymph nodes.
About breast cancer
Cancer begins when healthy cells in the breast change and grow out of control, forming a mass or sheet of cells called a tumor. A tumor can be cancerous or noncancerous, also called benign. A cancerous tumor is malignant, meaning it can grow and spread to other parts of the body. A benign tumor means the tumor can grow but has not spread.
This guide covers both non-invasive (stage 0) as well as early-stage and locally advanced invasive breast cancer, which includes stages I, II, and III. The stage of breast cancer describes how much the cancer has grown, and if or where it has spread.
Although breast cancer most commonly spreads to nearby lymph nodes, in which case the breast cancer is still considered a local or regional disease, it can also spread further through the body through the blood vessels and/or lymph nodes to areas such as the bones, lungs, liver, and brain. This is called metastatic or stage IV breast cancer and is the most advanced stage of the disease. However, the involvement of nearby lymph nodes alone is generally not stage IV breast cancer. Learn more about metastatic breast cancer in a separate guide on this website.
If breast cancer comes back after initial treatment, it can recur locally, meaning in the same breast and/or regional lymph nodes. It can also recur elsewhere in the body, called a distant recurrence or metastatic recurrence .
Types of breast cancer
Breast cancer can be invasive or non-invasive. Invasive breast cancer is cancer that spreads into surrounding tissues and/or distant organs. Non-invasive breast cancer does not go beyond the milk ducts or lobules in the breast. About 80% of breast cancer is invasive cancer, and about 20% is non-invasive cancer. There are multiple types of breast cancers, which are classified based on how they look under a microscope.
Ductal carcinoma in situ (DCIS). This is a non-invasive cancer (stage 0) that is located only in the duct and has not spread outside the duct.
Invasive or infiltrating ductal carcinoma. This is cancer that has spread outside of the ducts. It is the most common type of invasive breast cancer.
Invasive lobular carcinoma. This is a type of breast cancer that has spread outside of the lobules.
Less common types of invasive breast cancer include:
Metaplastic
Micropapillary
Inflammatory breast cancer , which is an aggressive type of cancer that accounts for about 1% to 5% of all invasive breast cancers.
Paget’s disease is a rare type of cancer in the skin of the nipple or in the skin closely surrounding the nipple. It begins in the ducts of the nipple, then spreads to the nipple surface and the areola (dark circle of skin around the nipple). The nipple and areola often become scaly, red, itchy, and irritated. Often, Paget's disease is mistaken for eczema or an infection before the correct diagnosis is made. Although it is usually non-invasive, it can also be an invasive cancer. It is usually found with an underlying breast cancer.
Breast cancer subtypes
There are 3 main subtypes of breast cancer that are determined by doing specific tests on a sample of the tumor to determine its characteristics. These tests will help your doctor learn more about your cancer and recommend the most effective treatment plan .
Testing the tumor sample can find out if the cancer is:
Hormone receptor positive. Breast cancers expressing estrogen receptors (ER) and/or progesterone receptors (PR) are called “hormone receptor positive.” These receptors are proteins found in cells. Tumors that have estrogen receptors are called “ER positive.” Tumors that have progesterone receptors are called “PR positive.” Only 1 of these receptors needs to be positive for a cancer to be called hormone receptor positive. This type of cancer may depend on the hormones estrogen and/or progesterone to grow. Hormone receptor-positive cancers can occur at any age, but they are more common after menopause. About two-thirds of breast cancers have estrogen and/or progesterone receptors. Cancers without these receptors are called “hormone receptor negative.” Hormone receptor-positive breast cancers are commonly treated using hormone therapy (see Types of Treatment ).
HER2 positive. About 15% to 20% of breast cancers depend on the gene called human epidermal growth factor receptor 2 ( HER2 ) to grow. These cancers are called “HER2 positive” and have many copies of the HER2 gene or high levels of the HER2 protein. These proteins are also called “receptors.” The HER2 gene makes the HER2 protein, which is found on the cancer cells and is important for tumor cell growth. HER2-positive breast cancers grow more quickly. They can also be either hormone receptor positive or hormone receptor negative. HER2-positive early stage breast cancers are commonly treated using HER2-targeted therapies (see Types of Treatment ). Cancers that have no HER2 protein are called “HER2 negative." Cancers that have low levels of the HER2 protein and/or few copies of the HER2 gene are sometimes now called “HER2 low."
Triple negative. If a tumor does not express ER, PR, and HER2, the tumor is called “triple negative.” Triple-negative breast cancer makes up about 10% to 20% of invasive breast cancers. Triple-negative breast cancer seems to be more common among younger women, particularly younger Black women and Hispanic women. Triple-negative breast cancer is also more common in women with a mutation in the BRCA1 gene. Experts often recommend that people with triple-negative breast cancer be tested for BRCA gene mutations. See the Risk Factors and Prevention section for more information on these genetic mutations.
Looking for More of an Introduction?
If you would like more of an introduction, explore these related items. Please note that these links will take you to other sections on Cancer.Net:
ASCO Answers Fact Sheet: Read a 1-page fact sheet that offers an introduction to breast cancer. This free fact sheet is available as a PDF, so it is easy to print.
ASCO Answers Guide : Get this 52-page booklet that helps you better understand breast cancer and its treatment options. This free booklet is available as a PDF, so it is easy to print.
Cancer.Net Blog: Read an ASCO expert’s opinion about what newly diagnosed patients should know about breast cancer.
Cancer.Net En Español: Read about breast cancer in Spanish . Infórmase sobre cáncer de mama en español .
The next section in this guide is Statistics . It helps explain the number of people who are diagnosed with breast cancer and general survival rates. Use the menu to choose a different section to read in this guide.
Breast Cancer Guide
Cancer.Net Guide Breast Cancer
- Introduction
- Medical Illustrations
- Risk Factors and Prevention
- Symptoms and Signs
- Types of Treatment
- About Clinical Trials
- Latest Research
- Coping with Treatment
- Follow-up Care and Monitoring
- Questions to Ask the Health Care Team
- Additional Resources
View All Pages
Timely. Trusted. Compassionate.
Comprehensive information for people with cancer, families, and caregivers, from the American Society of Clinical Oncology (ASCO), the voice of the world's oncology professionals.
Find a Cancer Doctor
Coronavirus (COVID-19): Latest Updates | Visitation Policies Visitation Policies Visitation Policies Visitation Policies Visitation Policies | COVID-19 Testing | Vaccine Information Vaccine Information Vaccine Information
Health Encyclopedia
Breast cancer: introduction, what is cancer.
Cancer starts when cells in the body change (mutate) and grow out of control. Your body is made up of tiny building blocks called cells. Normal cells grow when your body needs them, and die when your body doesn't need them any longer. Cancer is made up of abnormal cells that grow even though your body doesn’t need them. In most types of cancer, the abnormal cells grow to form a lump or mass called a tumor.
Understanding the breast
The breast is made up of lobules and ducts. The lobules are the glands that can make milk. The ducts are thin tubes that carry the milk from the lobules to the nipple. The breast is also made of fat, connective tissue, lymph nodes, and blood vessels.
What is breast cancer?
Breast cancer is cancer that starts in cells in the breast. The ducts and the lobules are the two parts of the breast where cancer is most likely to start.
Breast cancer is one of the most common types of cancer in the U.S. Healthcare providers don't yet know exactly what causes it. Once breast cancer forms, cancer cells can spread to other parts of the body (metastasize), making it life-threatening. The good news is that breast cancer is often found early, when it's small and before it has spread.
There are many types of breast cancer. These are the most common types:
Ductal carcinoma. This is the most common type. It starts in the lining of the milk ducts. When breast cancer has not spread outside of the ducts, it's called ductal carcinoma in situ or intraductal carcinoma. This is the most common type of noninvasive breast cancer. Invasive ductal carcinoma is breast cancer that has spread beyond the walls of the breast ducts. It's the most common type of invasive breast cancer.
Invasive lobular carcinoma. This type starts in the milk-producing glands (lobules) and spreads outside the lobules.
Names of specific breast cancer types refer to whether they have spread or not:
Noninvasive (in situ) cancer is only in the ducts. It hasn’t spread to nearby areas. If not treated, it can grow over time into a more serious, invasive type of cancer. If you are diagnosed with noninvasive ductal carcinoma, your chances of surviving are very high if you don’t wait to treat it.
Invasive (infiltrating) cancer has the potential to spread to nearby areas. This type is much more serious than noninvasive cancer. When it starts to spread, it often invades nearby lymph nodes first. It can then spread to other parts of your body through your bloodstream and lymphatic system. Treatment for invasive cancer is often a more difficult, long-term process.
These are a few types of invasive breast cancers that you may hear about:
Inflammatory breast cancer. This is a rare form of invasive breast cancer. Often there is no lump or tumor. Instead, this cancer makes the skin of the breast look red and feel warm. The breast skin also looks thick and pitted, like an orange peel. It tends to be found in younger people and grows and spreads quickly.
Triple negative breast cancer. This is a type of breast cancer that doesn’t have estrogen receptors and progesterone receptors. It also doesn’t have an excess of the HER2 protein on the cancer cell surfaces. This type of breast cancer is most often found in younger people and in African-American people. It tends to grow and spread faster than most other types of breast cancer. Because these cancer cells don't have hormone receptors or excess HER2, medicines that target these changes don't work. The most common kind is triple-negative invasive ductal carcinoma.
Less common types of breast cancer include:
Paget disease. This is a very rare form of breast cancer that starts in the glands in the skin of the nipple. It grows slowly and occurs in only one nipple. Most people with Paget disease also have tumors in the same breast. This type causes symptoms that are like a skin infection. They include inflammation, redness, oozing, crusting, itching, and burning.
Angiosarcoma. This starts in the cells that line the blood vessels or lymph vessels. It may involve the breast tissue or the breast skin.
How breast cancer spreads
Breast cancer can spread by growing into nearby tissues in the breast. It can also spread when the cancer cells get into and travel through the blood or lymph systems. When this happens, cancer cells may be found in nearby lymph nodes, such as in the armpit. These lymph nodes are called axillary lymph nodes. They are often checked for cancer as part of the diagnosis process. If the cancer reaches these nodes, it may have spread to other parts of the body.
Breast cancer that has spread from the breast to other organs of the body is called metastatic breast cancer. When breast cancer spreads, it most often goes to the brain, bones, liver, or lungs.
A key factor in making a breast cancer diagnosis is finding out if it has spread.
Talking with your healthcare provider
If you have questions about breast cancer, talk with your healthcare provider. Your healthcare provider can help you understand more about this cancer.
Medical Reviewers:
- Jessica Gotwals RN BSN MPH
- Sabrina Felson MD
- Todd Gersten MD
- Ask a Medical Librarian Make an Appointment Physicians & Services Physicians who treat Breast Cancer
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 23 September 2019
- Breast cancer
- Nadia Harbeck 1 ,
- Frédérique Penault-Llorca 2 ,
- Javier Cortes 3 , 4 ,
- Michael Gnant 5 ,
- Nehmat Houssami 6 ,
- Philip Poortmans 7 , 8 ,
- Kathryn Ruddy 9 ,
- Janice Tsang 10 &
- Fatima Cardoso 11
Nature Reviews Disease Primers volume 5 , Article number: 66 ( 2019 ) Cite this article
88k Accesses
1455 Citations
447 Altmetric
Metrics details
- Cancer therapy
- Genetic predisposition to disease
- Radiotherapy
- Tumour biomarkers
Breast cancer is the most frequent malignancy in women worldwide and is curable in ~70–80% of patients with early-stage, non-metastatic disease. Advanced breast cancer with distant organ metastases is considered incurable with currently available therapies. On the molecular level, breast cancer is a heterogeneous disease; molecular features include activation of human epidermal growth factor receptor 2 (HER2, encoded by ERBB2 ), activation of hormone receptors (oestrogen receptor and progesterone receptor) and/or BRCA mutations. Treatment strategies differ according to molecular subtype. Management of breast cancer is multidisciplinary; it includes locoregional (surgery and radiation therapy) and systemic therapy approaches. Systemic therapies include endocrine therapy for hormone receptor-positive disease, chemotherapy, anti-HER2 therapy for HER2-positive disease, bone stabilizing agents, poly(ADP-ribose) polymerase inhibitors for BRCA mutation carriers and, quite recently, immunotherapy. Future therapeutic concepts in breast cancer aim at individualization of therapy as well as at treatment de-escalation and escalation based on tumour biology and early therapy response. Next to further treatment innovations, equal worldwide access to therapeutic advances remains the global challenge in breast cancer care for the future.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
92,52 € per year
only 92,52 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
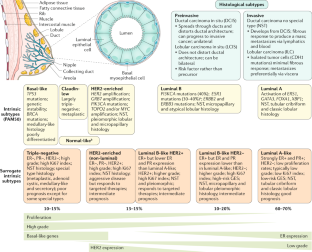
Similar content being viewed by others
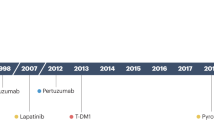
Management of patients with advanced-stage HER2-positive breast cancer: current evidence and future perspectives
Antonio Marra, Sarat Chandarlapaty & Shanu Modi

A careful reassessment of anthracycline use in curable breast cancer
Sara Alsterlind Hurvitz, Nicholas P. McAndrew, … Dennis J. Slamon
Emerging systemic therapy options beyond CDK4/6 inhibitors for hormone receptor-positive HER2-negative advanced breast cancer
Jun Ma, Jack Junjie Chan, … Yoon-Sim Yap
Perou, C. M. et al. Molecular portraits of human breast tumours. Nature 406 , 747–752 (2000).
Article CAS PubMed Google Scholar
Cardoso, F. et al. European Breast Cancer Conference manifesto on breast centres/units. Eur. J. Cancer 72 , 244–250 (2017).
Article PubMed Google Scholar
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68 , 394–424 (2018).
Bray, F. et al. Cancer Incidence in Five Continents: inclusion criteria, highlights from Volume X and the global status of cancer registration. Int. J. Cancer 137 , 2060–2071 (2015).
Mariotto, A. B., Etzioni, R., Hurlbert, M., Penberthy, L. & Mayer, M. Estimation of the number of women living with metastatic breast cancer in the United States. Cancer Epidemiol. Biomark. Prev. 26 , 809–815 (2017).
Article Google Scholar
Ren, J.-X., Gong, Y., Ling, H., Hu, X. & Shao, Z.-M. Racial/ethnic differences in the outcomes of patients with metastatic breast cancer: contributions of demographic, socioeconomic, tumor and metastatic characteristics. Breast Cancer Res. Treat. 173 , 225–237 (2019).
Torre, L. A., Siegel, R. L., Ward, E. M. & Jemal, A. Global cancer incidence and mortality rates and trends — an update. Cancer Epidemiol. Biomark. Prev. 25 , 16–27 (2016).
Ginsburg, O. et al. The global burden of women’s cancers: a grand challenge in global health. Lancet 389 , 847–860 (2017).
Allemani, C. et al. Global surveillance of cancer survival 1995–2009: analysis of individual data for 25 676 887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet 385 , 977–1010 (2015).
Winters, S., Martin, C., Murphy, D. & Shokar, N. K. Breast cancer epidemiology, prevention, and screening. Prog. Mol. Biol. Transl Sci. 151 , 1–32 (2017).
Hossain, M. S., Ferdous, S. & Karim-Kos, H. E. Breast cancer in South. Asia: a Bangladeshi perspective. Cancer Epidemiol. 38 , 465–470 (2014).
PubMed Google Scholar
Leong, S. P. L. et al. Is breast cancer the same disease in Asian and western countries? World J. Surg. 34 , 2308–2324 (2010).
Article PubMed PubMed Central Google Scholar
Bhoo Pathy, N. et al. Breast cancer in a multi-ethnic Asian setting: results from the Singapore–Malaysia hospital-based breast cancer registry. Breast 20 , S75–S80 (2011).
Raina, V. et al. Clinical features and prognostic factors of early breast cancer at a major cancer center in North India. Indian J. Cancer 42 , 40 (2005).
Agarwal, G., Pradeep, P. V., Aggarwal, V., Yip, C.-H. & Cheung, P. S. Y. Spectrum of breast cancer in Asian women. World J. Surg. 31 , 1031–1040 (2007).
Li, C. I., Malone, K. E. & Daling, J. R. Differences in breast cancer hormone receptor status and histology by race and ethnicity among women 50 years of age and older. Cancer Epidemiol. Biomark. Prev. 11 , 601–607 (2002).
Google Scholar
Wong, F. Y., Tham, W. Y., Nei, W. L., Lim, C. & Miao, H. Age exerts a continuous effect in the outcomes of Asian breast cancer patients treated with breast-conserving therapy. Cancer Commun. 38 , 39 (2018).
Kohler, B. A. et al. Annual report to the nation on the status of cancer, 1975–2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J. Natl Cancer Inst . 107 , https://doi.org/10.1093/jnci/djv048 (2015).
DeSantis, C. E. et al. Breast cancer statistics, 2015: Convergence of incidence rates between black and white women: Breast Cancer Statistics, 2015. CA Cancer J. Clin. 66 , 31–42 (2016).
DeSantis, C. E., Ma, J., Goding Sauer, A., Newman, L. A. & Jemal, A. Breast cancer statistics, 2017, racial disparity in mortality by state: Breast Cancer Statistics, 2017. CA Cancer J. Clin. 67 , 439–448 (2017).
Shiovitz, S. & Korde, L. A. Genetics of breast cancer: a topic in evolution. Ann. Oncol. 26 , 1291–1299 (2015).
CAS PubMed PubMed Central Google Scholar
Collaborative Group on Hormonal Factors in Breast Cancer. Familial breast cancer: collaborative reanalysis of individual data from 52 epidemiological studies including 58 209 women with breast cancer and 101 986 women without the disease. Lancet 358 , 1389–1399 (2001).
Brewer, H. R., Jones, M. E., Schoemaker, M. J., Ashworth, A. & Swerdlow, A. J. Family history and risk of breast cancer: an analysis accounting for family structure. Breast Cancer Res. Treat. 165 , 193–200 (2017).
Huen, M. S. Y., Sy, S. M. H. & Chen, J. BRCA1 and its toolbox for the maintenance of genome integrity. Nat. Rev. Mol. Cell Biol. 11 , 138–148 (2010).
Kuchenbaecker, K. B. et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA 317 , 2402 (2017).
Balmana, J., Diez, O., Rubio, I. T. & Cardoso, F., On behalf of the ESMO Guidelines Working Group. BRCA in breast cancer: ESMO clinical practice guidelines. Ann. Oncol. 22 , vi31–vi34 (2011).
Paluch-Shimon, S. et al. Prevention and screening in BRCA mutation carriers and other breast/ovarian hereditary cancer syndromes: ESMO Clinical Practice Guidelines for cancer prevention and screening. Ann. Oncol. 27 , v103–v110 (2016).
Daly, M. B. et al. Genetic/familial high-risk assessment: breast and ovarian, version 2.2015. J. Natl Compr. Cancer Netw. 14 , 153–162 (2016).
Forbes, C., Fayter, D., de Kock, S. & Quek, R. G. W. A systematic review of international guidelines and recommendations for the genetic screening, diagnosis, GENETIC COUNSELING and treatment of BRCA -mutated breast cancer. Cancer Manag. Res. 2019 , 2321–2337 (2019).
Robson, M. et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N. Engl. J. Med. 377 , 523–533 (2017).
Litton, J. K. et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N. Engl. J. Med. 379 , 753–763 (2018).
FDA. FDA approves olaparib germline BRCA-mutated metastatic breast cancer. Fda.gov https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-olaparib-germline-brca-mutated-metastatic-breast-cancer (2018).
FDA. FDA approves talazoparib for gBRCAm HER2-negative locally advanced or metastatic breast cancer. Fda.gov https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-talazoparib-gbrcam-her2-negative-locally-advanced-or-metastatic-breast-cancer (2018).
Pasche, B. Recent advances in breast cancer genetics. Cancer Treat. Res. 141 , 1–10 (2008).
Cobain, E. F., Milliron, K. J. & Merajver, S. D. Updates on breast cancer genetics: clinical implications of detecting syndromes of inherited increased susceptibility to breast cancer. Semin. Oncol. 43 , 528–535 (2016).
Crawford, B. et al. Multi-gene panel testing for hereditary cancer predisposition in unsolved high-risk breast and ovarian cancer patients. Breast Cancer Res. Treat. 163 , 383–390 (2017).
Article CAS PubMed PubMed Central Google Scholar
Taylor, A. et al. Consensus for genes to be included on cancer panel tests offered by UK genetics services: guidelines of the UK Cancer Genetics Group. J. Med. Genet. 55 , 372–377 (2018).
Althuis, M. D., Dozier, J. M., Anderson, W. F., Devesa, S. S. & Brinton, L. A. Global trends in breast cancer incidence and mortality 1973–1997. Int. J. Epidemiol. 34 , 405–412 (2005).
Colditz, G. A., Sellers, T. A. & Trapido, E. Epidemiology — identifying the causes and preventability of cancer? Nat. Rev. Cancer 6 , 75–83 (2006).
Britt, K., Ashworth, A. & Smalley, M. Pregnancy and the risk of breast cancer. Endocr. Relat. Cancer 14 , 907–933 (2007).
Siwko, S. K. et al. Evidence that an early pregnancy causes a persistent decrease in the number of functional mammary epithelial stem cells — implications for pregnancy-induced protection against breast cancer. Stem Cells 26 , 3205–3209 (2008).
Hilakivi-Clarke, L., de Assis, S. & Warri, A. Exposures to synthetic estrogens at different times during the life, and their effect on breast cancer risk. J. Mammary Gland. Biol. Neoplasia 18 , 25–42 (2013).
Danaei, G., Vander Hoorn, S., Lopez, A. D., Murray, C. J. & Ezzati, M. Causes of cancer in the world: comparative risk assessment of nine behavioural and environmental risk factors. Lancet 366 , 1784–1793 (2005).
Chen, W. Y., Rosner, B., Hankinson, S. E., Colditz, G. A. & Willett, W. C. Moderate alcohol consumption during adult life, drinking patterns, and breast cancer risk. JAMA 306 , 1884 (2011).
Singletary, K. W. & Gapstur, S. M. Alcohol and breast cancer: review of epidemiologic and experimental evidence and potential mechanisms. JAMA 286 , 2143 (2001).
Smith-Warner, S. A. et al. Alcohol and breast cancer in women: a pooled analysis of cohort studies. JAMA 279 , 535 (1998).
Bandera, E. V., Maskarinec, G., Romieu, I. & John, E. M. Racial and ethnic disparities in the impact of obesity on breast cancer risk and survival: a global perspective. Adv. Nutr. 6 , 803–819 (2015).
Picon-Ruiz, M., Morata-Tarifa, C., Valle-Goffin, J. J., Friedman, E. R. & Slingerland, J. M. Obesity and adverse breast cancer risk and outcome: mechanistic insights and strategies for intervention: breast cancer, inflammation, and obesity. CA Cancer J. Clin. 67 , 378–397 (2017).
Shieh, Y. et al. Body mass index, mammographic density, and breast cancer risk by estrogen receptor subtype. Breast Cancer Res. 21 , 48 (2019).
Suzuki, Y., Tsunoda, H., Kimura, T. & Yamauchi, H. BMI change and abdominal circumference are risk factors for breast cancer, even in Asian women. Breast Cancer Res. Treat. 166 , 919–925 (2017).
Del Pup, L., Codacci-Pisanelli, G. & Peccatori, F. Breast cancer risk of hormonal contraception: counselling considering new evidence. Crit. Rev. Oncol. Hematol. 137 , 123–130 (2019).
Busund, M. et al. Progestin-only and combined oral contraceptives and receptor-defined premenopausal breast cancer risk: the Norwegian Women and Cancer Study. Int. J. Cancer 142 , 2293–2302 (2018).
Mørch, L. S. et al. Contemporary hormonal contraception and the risk of breast cancer. N. Engl. J. Med. 377 , 2228–2239 (2017).
Ganz, P. A. et al. Supportive care after curative treatment for breast cancer (survivorship care): resource allocations in low- and middle-income countries. A Breast Health Global Initiative 2013 consensus statement. Breast 22 , 606–615 (2013).
Burris, J. L., Armeson, K. & Sterba, K. R. A closer look at unmet needs at the end of primary treatment for breast cancer: a longitudinal pilot study. Behav. Med. 41 , 69–76 (2015).
Coughlin, S. S., Yoo, W., Whitehead, M. S. & Smith, S. A. Advancing breast cancer survivorship among African-American women. Breast Cancer Res. Treat. 153 , 253–261 (2015).
Bodai, B. Breast cancer survivorship: a comprehensive review of long-term medical issues and lifestyle recommendations. Perm. J. 19 , 48–79 (2015).
Ho, P. J., Gernaat, S. A. M., Hartman, M. & Verkooijen, H. M. Health-related quality of life in Asian patients with breast cancer: a systematic review. BMJ Open 8 , e020512 (2018).
Miyashita, M. et al. Unmet information needs and quality of life in young breast cancer survivors in japan. Cancer Nurs. 38 , E1–E11 (2015).
Bombonati, A. & Sgroi, D. C. The molecular pathology of breast cancer progression. J. Pathol. 223 , 307–317 (2011).
Ellis, M. J. et al. Whole-genome analysis informs breast cancer response to aromatase inhibition. Nature 486 , 353–360 (2012).
Lopez-Garcia, M. A., Geyer, F. C., Lacroix-Triki, M., Marchió, C. & Reis-Filho, J. S. Breast cancer precursors revisited: molecular features and progression pathways: molecular evolution of breast cancer. Histopathology 57 , 171–192 (2010).
Nik-Zainal, S. et al. Landscape of somatic mutations in 560 breast cancer whole-genome sequences. Nature 534 , 47–54 (2016).
Yates, L. R. & Desmedt, C. Translational genomics: practical applications of the genomic revolution in breast cancer. Clin. Cancer Res. 23 , 2630–2639 (2017).
Heitzer, E., Haque, I. S., Roberts, C. E. S. & Speicher, M. R. Current and future perspectives of liquid biopsies in genomics-driven oncology. Nat. Rev. Genet. 20 , 71–88 (2019).
Ediriweera, M. K., Tennekoon, K. H. & Samarakoon, S. R. Emerging role of histone deacetylase inhibitors as anti-breast-cancer agents. Drug Discov. Today 24 , 685–702 (2019).
Munster, P. N. et al. A phase II study of the histone deacetylase inhibitor vorinostat combined with tamoxifen for the treatment of patients with hormone therapy-resistant breast cancer. Br. J. Cancer 104 , 1828–1835 (2011).
Zhou, Y., Wang, Y., Zhang, K., Zhu, J. & Ning, Z. Reverse effect of chidamide on endocrine resistance in estrogen receptor-positive breast cancer. J. Shenzhen Univ. Sci. Eng. 35 , 339 (2018).
Jiang, Z. et al. Phase III trial of chidamide, a subtype-selective histone deacetylase (HDAC) inhibitor, in combination with exemestane in patients with hormone receptor-positive advanced breast cancer [abstract]. Ann. Oncol. 29 , 283O_PR (2018).
Williams, C. & Lin, C.-Y. Oestrogen receptors in breast cancer: basic mechanisms and clinical implications. Ecancermedicalscience 7 , 370 (2013).
PubMed PubMed Central Google Scholar
Levin, E. R. & Pietras, R. J. Estrogen receptors outside the nucleus in breast cancer. Breast Cancer Res. Treat. 108 , 351–361 (2008).
Santen, R. J. Clinical review: effect of endocrine therapies on bone in breast cancer patients. J. Clin. Endocrinol. Metab. 96 , 308–319 (2011).
Ruffell, B. et al. Leukocyte composition of human breast cancer. Proc. Natl Acad. Sci. USA 109 , 2796–2801 (2012).
Solinas, C., Carbognin, L., De Silva, P., Criscitiello, C. & Lambertini, M. Tumor-infiltrating lymphocytes in breast cancer according to tumor subtype: current state of the art. Breast 35 , 142–150 (2017).
Nagarajan, D. & McArdle, S. Immune landscape of breast cancers. Biomedicines 6 , 20 (2018).
Article PubMed Central CAS Google Scholar
Savas, P. et al. Clinical relevance of host immunity in breast cancer: from TILs to the clinic. Nat. Rev. Clin. Oncol. 13 , 228–241 (2016).
Dieci, M. V. et al. Update on tumor-infiltrating lymphocytes (TILs) in breast cancer, including recommendations to assess TILs in residual disease after neoadjuvant therapy and in carcinoma in situ: a report of the International Immuno-Oncology Biomarker Working Group on Breast Cancer. Semin. Cancer Biol. 52 , 16–25 (2018).
Boudreau, A., van’t Veer, L. J. & Bissell, M. J. An ‘elite hacker’: breast tumors exploit the normal microenvironment program to instruct their progression and biological diversity. Cell Adhes. Migr. 6 , 236–248 (2012).
Smyth, M. J., Dunn, G. P. & Schreiber, R. D. Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv. Immunol. 90 , 1–50 (2006).
Schreiber, R. D., Old, L. J. & Smyth, M. J. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331 , 1565–1570 (2011).
Buonomo, O. C. et al. New insights into the metastatic behavior after breast cancer surgery, according to well-established clinicopathological variables and molecular subtypes. PLOS ONE 12 , e0184680 (2017).
Article PubMed PubMed Central CAS Google Scholar
Gobbini, E. et al. Time trends of overall survival among metastatic breast cancer patients in the real-life ESME cohort. Eur. J. Cancer 96 , 17–24 (2018).
Santé Publique France. Breast cancer [French]. Santepubliquefrance.fr https://www.santepubliquefrance.fr/maladies-et-traumatismes/cancers/cancer-du-sein (2019).
Zhang, K. et al. Clinical value of circulating ESR1 mutations for patients with metastatic breast cancer: a meta-analysis. Cancer Manag. Res. 10 , 2573–2580 (2018).
Yates, L. R. et al. Genomic evolution of breast cancer metastasis and relapse. Cancer Cell 32 , 169–184.e7 (2017).
Gingras, I., Salgado, R. & Ignatiadis, M. Liquid biopsy: will it be the ‘magic tool’ for monitoring response of solid tumors to anticancer therapies? Curr. Opin. Oncol. 27 , 560–567 (2015).
Aurilio, G. et al. A meta-analysis of oestrogen receptor, progesterone receptor and human epidermal growth factor receptor 2 discordance between primary breast cancer and metastases. Eur. J. Cancer 50 , 277–289 (2014).
Independent, U. K. Panel on breast cancer screening. the benefits and harms of breast cancer screening: an independent review. Lancet 380 , 1778–1786 (2012).
Nelson, H. D. et al. Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 U.S. Preventive Services Task Force recommendation. Ann. Intern. Med. 164 , 244–255 (2016).
Lauby-Secretan, B. et al. Breast-cancer screening — viewpoint of the IARC Working Group. N. Engl. J. Med. 372 , 2353–2358 (2015).
Houssami, N. Overdiagnosis of breast cancer in population screening: does it make breast screening worthless? Cancer Biol. Med. 14 , 1–8 (2017).
Suhrke, P. et al. Effect of mammography screening on surgical treatment for breast cancer in Norway: comparative analysis of cancer registry data. BMJ 343 , d4692–d4692 (2011).
Stang, A., Kääb-Sanyal, V., Hense, H.-W., Becker, N. & Kuss, O. Effect of mammography screening on surgical treatment for breast cancer: a nationwide analysis of hospitalization rates in Germany 2005–2009. Eur. J. Epidemiol. 28 , 689–696 (2013).
IARC Handbooks of Cancer Prevention. Breast Cancer Screening (Volume 15). Iarc.fr http://publications.iarc.fr/Book-And-Report-Series/Iarc-Handbooks-Of-Cancer-Prevention/Breast-Cancer-Screening-2016 (2016).
Nelson, H. D. et al. Harms of breast cancer screening: systematic review to update the 2009 U.S. Preventive Services Task Force recommendation. Ann. Intern. Med. 164 , 256–267 (2016).
Carter, J. L., Coletti, R. J. & Harris, R. P. Quantifying and monitoring overdiagnosis in cancer screening: a systematic review of methods. BMJ 350 , g7773 (2015).
Saslow, D. et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J. Clin. 57 , 75–89 (2007).
Phi, X.-A. et al. Magnetic resonance imaging improves breast screening sensitivity in BRCA mutation carriers age ≥ 50 years: evidence from an individual patient data meta-analysis. J. Clin. Oncol. 33 , 349–356 (2015).
Sardanelli, F. et al. Magnetic resonance imaging of the breast: recommendations from the EUSOMA working group. Eur. J. Cancer 46 , 1296–1316 (2010).
Melnikow, J. et al. Supplemental screening for breast cancer in women with dense breasts: a systematic review for the U.S. preventive services task force. Ann. Intern. Med. 164 , 268–278 (2016).
Houssami, N. & Lee, C. I. The impact of legislation mandating breast density notification — review of the evidence. Breast 42 , 102–112 (2018).
Marinovich, M. L., Hunter, K. E., Macaskill, P. & Houssami, N. Breast cancer screening using tomosynthesis or mammography: a meta-analysis of cancer detection and recall. J. Natl Cancer Inst. 110 , 942–949 (2018).
Irwig, L., Macaskill, P. & Houssami, N. Evidence relevant to the investigation of breast symptoms: the triple test. Breast 11 , 215–220 (2002).
Houssami, N., Ciatto, S., Turner, R. M., Cody, H. S. & Macaskill, P. Preoperative ultrasound-guided needle biopsy of axillary nodes in invasive breast cancer: meta-analysis of its accuracy and utility in staging the axilla. Ann. Surg. 254 , 243–251 (2011).
Morrow, M., Waters, J. & Morris, E. MRI for breast cancer screening, diagnosis, and treatment. Lancet 378 , 1804–1811 (2011).
Srigley, J. R. et al. Standardized synoptic cancer pathology reporting: a population-based approach. J. Surg. Oncol. 99 , 517–524 (2009).
World Heath Organisation. WHO Classification of Tumours of the Breast, Fourth Edition. (World Health Organization, 2012).
Elston, C. W. & Ellis, I. O. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 19 , 403–410 (1991).
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Breast Cancer. Nccn.org https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (2018).
Curigliano, G. et al. De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann. Oncol. 28 , 1700–1712 (2017).
Senkus, E. et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 24 (Suppl. 6), vi7-vi23 (2013).
Hammond, M. E. H. et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J. Clin. Oncol. 28 , 2784–2795 (2010).
Wolff, A. C. et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. J. Clin. Oncol. 36 , 2105–2122 (2018).
Dowsett, M. et al. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J. Natl Cancer Inst. 103 , 1656–1664 (2011).
Rakha, E. A. et al. The prognostic significance of lymphovascular invasion in invasive breast carcinoma. Cancer 118 , 3670–3680 (2012).
Barrio, A. V. & Morrow, M. Appropriate margin for lumpectomy excision of invasive breast cancer. Chin. Clin. Oncol. 5 , 35–35 (2016).
Chung, A. et al. Impact of consensus guidelines by the Society of Surgical Oncology and the American Society for Radiation Oncology on margins for breast-conserving surgery in stages 1 and 2 invasive breast cancer. Ann. Surg. Oncol. 22 , 422–427 (2015).
Schulman, A. M. et al. Reexcision surgery for breast cancer: an analysis of the American Society of Breast Surgeons (ASBrS) Mastery SM database following the SSO-ASTRO “no ink on tumor” guidelines. Ann. Surg. Oncol. 24 , 52–58 (2017).
Morrow, M. et al. Society of Surgical Oncology–American Society for Radiation Oncology–American Society of Clinical Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in ductal carcinoma in situ. Pract. Radiat. Oncol. 6 , 287–295 (2016).
Morrow, M. et al. Society of Surgical Oncology–American Society for Radiation Oncology–American Society of Clinical Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in ductal carcinoma in situ. J. Clin. Oncol. 34 , 4040–4046 (2016).
Moran, M. S. et al. Society of Surgical Oncology–American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. Int. J. Radiat. Oncol. Biol. Phys. 88 , 553–564 (2014).
Amin, M. B. et al. The Eighth Edition AJCC Cancer Staging Manual: continuing to build a bridge from a population-based to a more ‘personalized’ approach to cancer staging. CA Cancer J. Clin. 67 , 93–99 (2017).
Tao, L. et al. Breast cancer mortality in older and younger breast cancer patients in California. Cancer Epidemiol. Biomark. Prev. 28 , 303–310 (2018).
Salgado, R. et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann. Oncol. 26 , 259–271 (2015).
Green, A. R. et al. Nottingham Prognostic Index Plus: validation of a clinical decision making tool in breast cancer in an independent series. J. Pathol. Clin. Res. 2 , 32–40 (2016).
Candido dos Reis, F. J. et al. An updated PREDICT breast cancer prognostication and treatment benefit prediction model with independent validation. Breast Cancer Res. 19 , 58 (2017).
Phung, M. T., Tin Tin, S. & Elwood, J. M. Prognostic models for breast cancer: a systematic review. BMC Cancer 19 , 230 (2019).
Senkus, E. et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 26 (Suppl. 5), v8-v30 (2015).
Cortazar, P. et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384 , 164–172 (2014).
Cardoso, F. et al. 70-Gene signature as an aid to treatment decisions in early-stage breast cancer. N. Engl. J. Med. 375 , 717–729 (2016).
Sparano, J. A. et al. Prospective validation of a 21-gene expression assay in breast cancer. N. Engl. J. Med. 373 , 2005–2014 (2015).
Sparano, J. A. et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N. Engl. J. Med. 379 , 111–121 (2018).
Harris, L. N. et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: American Society of Clinical Oncology clinical practice guideline. J. Clin. Oncol. 34 , 1134–1150 (2016).
Krop, I. et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: American Society of Clinical Oncology clinical practice guideline focused update. J. Clin. Oncol. 35 , 2838–2847 (2017).
Nitz, U. et al. West German Study PlanB trial: adjuvant four cycles of epirubicin and cyclophosphamide plus docetaxel versus six cycles of docetaxel and cyclophosphamide in HER2-negative early breast cancer. J. Clin. Oncol. 37 , 799–808 (2019).
Sestak, I. Risk stratification in early breast cancer in premenopausal and postmenopausal women: integrating genomic assays with clinicopathological features. Curr. Opin. Oncol. 1 , 29–34 (2018).
McLaughlin, S. A. Surgical management of the breast: breast conservation therapy and mastectomy. Surg. Clin. North Am. 93 , 411–428 (2013).
Margenthaler, J. A. & Ollila, D. W. Breast conservation therapy versus mastectomy: shared decision-making strategies and overcoming decisional conflicts in your patients. Ann. Surg. Oncol. 23 , 3133–3137 (2016).
Buchholz, T. A., Mittendorf, E. A. & Hunt, K. K. Surgical considerations after neoadjuvant chemotherapy: breast conservation therapy. J. Natl Cancer Inst. Monogr. 2015 , 11–14 (2015).
Houssami, N., Macaskill, P., Luke Marinovich, M. & Morrow, M. The association of surgical margins and local recurrence in women with early-stage invasive breast cancer treated with breast-conserving therapy: a meta-analysis. Ann. Surg. Oncol. 21 , 717–730 (2014).
Morrow, M., Harris, J. R. & Schnitt, S. J. Surgical margins in lumpectomy for breast cancer — bigger is not better. N. Engl. J. Med. 367 , 79–82 (2012). This commentary and the meta-analysis by Houssami et al. (2014) settled the decade-long discussions about surgical resection margins and are, therefore, landmark contributions.
Tan, M. P., Sitoh, N. Y. & Sim, A. S. The value of intraoperative frozen section analysis for margin status in breast conservation surgery in a nontertiary institution. Int. J. Breast Cancer https://doi.org/10.1155/2014/715404 (2014).
Boughey, J. C. et al. Impact of analysis of frozen-section margin on reoperation rates in women undergoing lumpectomy for breast cancer: evaluation of the National Surgical Quality Improvement Program data. Surgery 156 , 190–197 (2014).
Haloua, M. H. et al. A systematic review of oncoplastic breast-conserving surgery: current weaknesses and future prospects. Ann. Surg. 257 , 609–620 (2013).
Benelli, L. A new periareolar mammaplasty: the ‘round block’ technique. Aesthetic Plast. Surg. 14 , 93–100 (1990).
Clough, K. B., Kaufman, G. J., Nos, C., Buccimazza, I. & Sarfati, I. M. Improving breast cancer surgery: a classification and quadrant per quadrant atlas for oncoplastic surgery. Ann. Surg. Oncol. 17 , 1375–1391 (2010).
Yao, K., Winchester, D. J., Czechura, T. & Huo, D. Contralateral prophylactic mastectomy and survival: report from the national cancer data base, 1998–2002. Breast Cancer Res. Treat. 142 , 465–476 (2013).
Vila, J., Gandini, S. & Gentilini, O. Overall survival according to type of surgery in young (≤40 years) early breast cancer patients: a systematic meta-analysis comparing breast-conserving surgery versus mastectomy. Breast 24 , 175–181 (2015).
Lucci, A. et al. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group trial Z0011. J. Clin. Oncol. 25 , 3657–3663 (2007).
Krag, D. N. et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol. 11 , 927–933 (2010). This large clinical trial confirms that there is no overall survival difference between sentinel lymph node biopsy and axillary lymph node dissection.
Veronesi, U. et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N. Engl. J. Med. 349 , 546–553 (2003).
Giuliano, A. E. et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: long-term follow-up from the American College of Surgeons Oncology Group (Alliance) ACOSOG Z0011 randomized trial. Ann. Surg. 264 , 413–420 (2016).
Balic, M., Thomssen, C., Würstlein, R., Gnant, M. & Harbeck, N. St. Gallen/Vienna 2019: a brief summary of the consensus discussion on the optimal primary breast cancer treatment. Breast Care 14 , 1–8 (2019).
Kaidar-Person, O., Meattini, I. & Poortmans, P. M. P. Between uncertainties and overtreatment. Int. J. Radiat. Oncol. 104 , 15–16 (2019).
Kuehn, T. et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 14 , 609–618 (2013).
King, T. A. & Morrow, M. Surgical issues in patients with breast cancer receiving neoadjuvant chemotherapy. Nat. Rev. Clin. Oncol. 12 , 335–343 (2015).
Giuliano, A. E. et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA 305 , 569–575 (2011).
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 378 , 1707–1716 (2011). This meta-analysis underlines that the contribution of radiation therapy should always be the standard approach for breast-conserving therapy .
Article CAS Google Scholar
EBCTCG (Early Breast Cancer Trialists’ Collaborative Group). Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 383 , 2127–2135 (2014). This meta-analysis helps us to better identify those patients who would benefit most from radiation therapy after mastectomy .
Jatoi, I., Benson, J. R. & Kunkler, I. Hypothesis: can the abscopal effect explain the impact of adjuvant radiotherapy on breast cancer mortality? NPJ Breast Cancer 4 , 8 (2018).
Bartelink, H. et al. Whole-breast irradiation with or without a boost for patients treated with breast-conserving surgery for early breast cancer: 20-year follow-up of a randomised phase 3 trial. Lancet Oncol. 16 , 47–56 (2015).
Poortmans, P. Postmastectomy radiation in breast cancer with one to three involved lymph nodes: ending the debate. Lancet 383 , 2104–2106 (2014).
Poortmans, P. M. et al. Internal mammary and medial supraclavicular irradiation in breast cancer. N. Engl. J. Med. 373 , 317–327 (2015).
Whelan, T. J. et al. Regional nodal irradiation in early-stage breast cancer. N. Engl. J. Med. 373 , 307–316 (2015).
Thorsen, L. B. J. et al. DBCG-IMN: a population-based cohort study on the effect of internal mammary node irradiation in early node-positive breast cancer. J. Clin. Oncol. 34 , 314–320 (2016).
Curigliano, G. et al. De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann. Oncol. 29 , 2153–2153 (2018).
Oliai, C. & Hurvitz, S. A. The debate over post-mastectomy radiotherapy should continue: breast cancer. Nat. Rev. Clin. Oncol. 12 , 567–568 (2015).
Recht, A. et al. Postmastectomy radiotherapy: an American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology focused guideline update. Ann. Surg. Oncol. 24 , 38–51 (2017).
Dodwell, D. et al. Abstract GS4-02: regional lymph node irradiation in early stage breast cancer: an EBCTCG meta-analysis of 13,000 women in 14 trials. in General Session Abstracts GS4-02-GS4-02 https://doi.org/10.1158/1538-7445.SABCS18-GS4-02 (American Association for Cancer Research, 2019).
Kunkler, I. H., Canney, P., van Tienhoven, G. & Russell, N. S. MRC/EORTC (BIG 2-04) SUPREMO Trial Management Group. Elucidating the role of chest wall irradiation in ‘intermediate-risk’. breast cancer: The MRC/EORTC SUPREMO trial. Clin. Oncol. R. Coll. Radiol. 20 , 31–34 (2008).
CAS PubMed Google Scholar
Poortmans, P., Aznar, M. & Bartelink, H. Quality indicators for breast cancer: revisiting historical evidence in the context of technology changes. Semin. Radiat. Oncol. 22 , 29–39 (2012).
Osman, S. O. S., Hol, S., Poortmans, P. M. & Essers, M. Volumetric modulated arc therapy and breath-hold in image-guided locoregional left-sided breast irradiation. Radiother. Oncol. 112 , 17–22 (2014).
Essers, M., Poortmans, P. M., Verschueren, K., Hol, S. & Cobben, D. C. P. Should breathing adapted radiotherapy also be applied for right-sided breast irradiation? Acta Oncol. 55 , 460–465 (2016).
Poortmans, P. M. P., Arenas, M. & Livi, L. Over-irradiation. Breast 31 , 295–302 (2017).
Blamey, R. W. et al. Radiotherapy or tamoxifen after conserving surgery for breast cancers of excellent prognosis: British Association of Surgical Oncology (BASO) II trial. Eur. J. Cancer 49 , 2294–2302 (2013).
McGuire, S. E. et al. Postmastectomy radiation improves the outcome of patients with locally advanced breast cancer who achieve a pathologic complete response to neoadjuvant chemotherapy. Int. J. Radiat. Oncol. Biol. Phys. 68 , 1004–1009 (2007).
Mamounas, E. P. et al. Predictors of locoregional recurrence after neoadjuvant chemotherapy: results from combined analysis of national surgical adjuvant breast and bowel project B-18 and B-27. J. Clin. Oncol. 30 , 3960–3966 (2012).
Krug, D. et al. Individualization of post-mastectomy radiotherapy and regional nodal irradiation based on treatment response after neoadjuvant chemotherapy for breast cancer: a systematic review. Strahlenther. Onkol. 194 , 607–618 (2018).
Amoroso, V. et al. International Expert Consensus on Primary Systemic Therapy in the Management of Early Breast Cancer: Highlights of the Fifth Symposium on Primary Systemic Therapy in the Management of Operable Breast Cancer, Cremona, Italy (2013). J. Natl Cancer Inst. Monogr. 2015 , 90–96 (2015).
Offersen, B. V. et al. ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer, version 1.1. Radiother. Oncol. 118 , 205–208 (2016).
Haviland, J. S. et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 14 , 1086–1094 (2013).
Whelan, T. J. et al. Long-term results of hypofractionated radiation therapy for breast cancer. N. Engl. J. Med. 362 , 513–520 (2010).
Wang, S.-L. et al. Hypofractionated versus conventional fractionated postmastectomy radiotherapy for patients with high-risk breast cancer: a randomised, non-inferiority, open-label, phase 3 trial. Lancet Oncol. 20 , 352–360 (2019).
Brouwers, P. J. A. M. et al. Predictors for poor cosmetic outcome in patients with early stage breast cancer treated with breast conserving therapy: results of the Young Boost trial. Radiother. Oncol. 128 , 434–441 (2018).
Polgár, C. et al. Patient selection for accelerated partial-breast irradiation (APBI) after breast-conserving surgery: recommendations of the groupe européen de curiethérapie-european society for therapeutic radiology and oncology (GEC-ESTRO) breast cancer working group based on clinical evidence (2009). Radiother. Oncol. 94 , 264–273 (2010).
Correa, C. et al. Accelerated partial breast irradiation: executive summary for the update of an ASTRO Evidence-Based. Consensus Statement. Pract. Radiat. Oncol. 7 , 73–79 (2017).
Miranda, F. A. et al. Accelerated partial breast irradiation: current status with a focus on clinical practice. Breast J. https://doi.org/10.1111/tbj.13164 (2018).
Marta, G. N. et al. Effectiveness of different accelerated partial breast irradiation techniques for the treatment of breast cancer patients: systematic review using indirect comparisons of randomized clinical trials. Rep. Pract. Oncol. Radiother. 24 , 165–174 (2019).
Veronesi, U. et al. Intraoperative radiotherapy versus external radiotherapy for early breast cancer (ELIOT): a randomised controlled equivalence trial. Lancet Oncol. 14 , 1269–1277 (2013).
Vaidya, J. S. et al. Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT-A randomised trial. Lancet 383 , 603–613 (2014).
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 378 , 771–784 (2011).
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomized trials. Lancet 379 , 432–444 (2012). This meta-analysis demonstrates the benefits of adjuvant chemotherapy in early breast cancer .
Rastogi, P. et al. Preoperative chemotherapy: updates of national surgical adjuvant breast and bowel project protocols B-18 and B-27. J. Clin. Oncol. 26 , 778–785 (2008).
Francis, P. A. et al. Tailoring adjuvant endocrine therapy for premenopausal breast cancer. N. Engl. J. Med. 379 , 122–137 (2018).
Gnant, M. et al. Zoledronic acid combined with adjuvant endocrine therapy of tamoxifen versus anastrozol plus ovarian function suppression in premenopausal early breast cancer: final analysis of the Austrian Breast and Colorectal Cancer Study Group Trial 12. Ann. Oncol. 26 , 313–320 (2015).
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet 386 , 1341–1352 (2015). This meta-analysis demonstrates the benefit of the two individual options for adjuvant endocrine therapy in postmenopausal patients with early breast cancer .
Pan, H. et al. 20-Year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N. Engl. J. Med. 377 , 1836–1846 (2017).
Gray, R. et al. Increasing the dose density of adjuvant chemotherapy by shortening intervals between courses or by sequential drug administration significantly reduces both disease recurrence and breast cancer mortality: an EBCTCG meta-analysis of 21,000 women in 16 randomised trials [abstract]. SABCS GS1-GS01 (2018).
Finn, R. S. et al. Palbociclib and letrozole in advanced breast cancer. N. Engl. J. Med. 375 , 1925–1936 (2016).
Hortobagyi, G. N. et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N. Engl. J. Med. 375 , 1738–1748 (2016).
Goetz, M. P. et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J. Clin. Oncol. 35 , 3638–3646 (2017).
Mackey, J. R. et al. Long-term outcomes after adjuvant treatment of sequential versus combination docetaxel with doxorubicin and cyclophosphamide in node-positive breast cancer: BCIRG-005 randomized trial. Ann. Oncol. 27 , 1041–1047 (2016).
Del Mastro, L. et al. Fluorouracil and dose-dense chemotherapy in adjuvant treatment of patients with early-stage breast cancer: an open-label, 2×2 factorial, randomised phase 3 trial. Lancet 385 , 1863–1872 (2015).
Article PubMed CAS Google Scholar
Blum, J. L. et al. Anthracyclines in early breast cancer: the ABC Trials-USOR 06-090, NSABP B-46-I/USOR 07132, and NSABP B-49 (NRG Oncology). J. Clin. Oncol. 35 , 2647–2655 (2017).
Gray, R. et al. Increasing the dose intensity of chemotherapy by more frequent administration or sequential scheduling: a patient-level meta-analysis of 37 298 women with early breast cancer in 26 randomised trials. Lancet 393 , 1440–1452 (2019).
Gianni, L. et al. 5-Year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 17 , 791–800 (2016).
von Minckwitz, G. et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N. Engl. J. Med. 380 , 617–628 (2018).
von Minckwitz, G. et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N. Engl. J. Med. 377 , 122–131 (2017).
Martin, M. et al. Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 18 , 1688–1700 (2017).
Tolaney, S. M. et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N. Engl. J. Med. 372 , 134–141 (2015).
Tolaney, S. M. et al. Seven-year (yr) follow-up of adjuvant paclitaxel (T) and trastuzumab (H) (APT trial) for node-negative, HER2-positive breast cancer (BC). J. Clin. Oncol. 35 , 511–511 (2017).
Earl, H. M. et al. 6 versus 12 months of adjuvant trastuzumab for HER2-positive early breast cancer (PERSEPHONE): 4-year disease-free survival results of a randomised phase 3 non-inferiority trial. Lancet 393 , 2599–2612 (2019).
Pivot, X. et al. Either 6 months versus 12 months of adjuvant trastuzumab for patients with HER2-positive early breast cancer (PHARE): a randomised phase 3 trial. Lancet Oncol. 14 , 741–748 (2013).
Joensuu, H. et al. Effect of adjuvant trastuzumab for a duration of 9 weeks vs 1 year with concomitant chemotherapy for early human epidermal growth factor receptor 2–positive breast cancer: the SOLD randomized clinical trial. JAMA Oncol. 4 , 1199 (2018).
Piccart-Gebhart, M. J. et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N. Engl. J. Med. 353 , 1659–1672 (2005).
Goldhirsch, A. et al. 2 years versus 1 year of adjuvant trastuzumab for HER2-positive breast cancer (HERA): an open-label, randomised controlled trial. Lancet 382 , 1021–1028 (2013).
Hahnen, E. et al. Germline mutation status, pathological complete response, and disease-free survival in triple-negative breast cancer: secondary analysis of the GeparSixto randomized clinical trial. JAMA Oncol. 3 , 1378–1385 (2017).
Sikov, W. M. et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J. Clin. Oncol. 33 , 13–21 (2015).
Masuda, N. et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N. Engl. J. Med. 376 , 2147–2159 (2017).
Gnant, M. et al. Adjuvant denosumab in breast cancer (ABCSG-18): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet 386 , 433–443 (2015).
Gnant, M. et al. Adjuvant denosumab in postmenopausal patients with hormone receptor-positive breast cancer (ABCSG-18): disease-free survival results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 20 , 339–351 (2019).
Coleman, R. E. et al. Adjuvant denosumab in early breast cancer: first results from the international multicenter randomized phase III placebo controlled D-CARE study [abstract]. J. Clin. Oncol. 36 (Suppl.), a501 (2018).
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet 386 , 1353–1361 (2015).
Coleman, R. E. et al. Benefits and risks of adjuvant treatment with zoledronic acid in stage II/III breast cancer. 10 years follow-up of the AZURE randomized clinical trial (BIG 01/04). J. Bone Oncol. 13 , 123–135 (2018).
Cardoso, F. et al. 4th ESO–ESMO international consensus guidelines for advanced breast cancer (ABC 4)†. Ann. Oncol. 29 , 1634–1657 (2018).
Golse, N. & Adam, R. Liver metastases from breast cancer: what role for surgery? Indications and results. Clin. Breast Cancer 17 , 256–265 (2017).
Xie, Y. et al. Surgery of the primary tumor improves survival in women with stage IV breast cancer in southwest China: a retrospective analysis. Medicine 96 , e7048 (2017).
Shien, T. & Doihara, H. Resection of the primary tumor in stage IV breast cancer. World J. Clin. Oncol. 5 , 82–85 (2014).
Badwe, R. et al. Locoregional treatment versus no treatment of the primary tumour in metastatic breast cancer: an open-label randomised controlled trial. Lancet Oncol. 16 , 1380–1388 (2015).
Soran, A., Ozbas, S., Kelsey, S. F. & Gulluoglu, B. M. Randomized trial comparing locoregional resection of primary tumor with no surgery in stage IV breast cancer at the presentation (Protocol MF07-01): a study of Turkish Federation of the National Societies for Breast Diseases. Breast J. 15 , 399–403 (2009).
Fitzal, F. et al. Impact of breast surgery in primary metastasized breast cancer: outcomes of the prospective randomized phase III ABCSG-28 POSYTIVE Trial. Ann. Surg . https://doi.org/10.1097/SLA.0000000000002771 (2018).
Barinoff, J. et al. Primary metastatic breast cancer in the era of targeted therapy — prognostic impact and the role of breast tumour surgery. Eur. J. Cancer 83 , 116–124 (2017).
Shien, T. et al. A randomized controlled trial comparing primary tumor resection plus systemic therapy with systemic therapy alone in metastatic breast cancer (JCOG1017 PRIM-BC). J. Clin. Oncol. 35 , TPS588–TPS588 (2017).
Cameron, D. Removing the primary tumour in metastatic breast cancer. Lancet Oncol. 16 , 1284–1285 (2015).
Dare, A. J. et al. Surgical Services for Cancer Care. in Cancer: Disease Control Priorities , Third Edition (Volume 3) (eds. Gelband, H., Jha, P., Sankaranarayanan, R. & Horton, S.) (The International Bank for Reconstruction and Development/The World Bank, 2015).
Phillips, C., Jeffree, R. & Khasraw, M. Management of breast cancer brain metastases: a practical review. Breast 31 , 90–98 (2017).
Thavarajah, N. et al. Continued success in providing timely palliative radiation therapy at the rapid response radiotherapy program: a review of 2008–2012. Curr. Oncol. 20 , e206–e211 (2013).
Chow, E. et al. Single versus multiple fractions of repeat radiation for painful bone metastases: a randomised, controlled, non-inferiority trial. Lancet Oncol. 15 , 164–171 (2014).
Sologuren, I., Rodríguez-Gallego, C. & Lara, P. C. Immune effects of high dose radiation treatment: implications of ionizing radiation on the development of bystander and abscopal effects. Transl Cancer Res. 3 , 18-31–31 (2014).
Morgan, S. C. & Parker, C. C. Local treatment of metastatic cancer — killing the seed or disturbing the soil? Nat. Rev. Clin. Oncol. 8 , 504–506 (2011).
Morgan, S., Caudrelier, J.-M. & Clemons, M. Radiotherapy to the primary tumor is associated with improved survival in stage IV breast cancer [abstract]. SABCS P4 , 16–06 (2012).
Bernier, J. Immuno-oncology: allying forces of radio- and immuno-therapy to enhance cancer cell killing. Crit. Rev. Oncol. Hematol. 108 , 97–108 (2016).
Fietz, T. et al. Palliative systemic therapy and overall survival of 1,395 patients with advanced breast cancer — rResults from the prospective German TMK cohort study. Breast. 34 , 122–130 (2017).
Rugo, H. S. et al. Endocrine therapy for hormone receptor-positive metastatic breast cancer: American Society of Clinical Oncology guideline. J. Clin. Oncol. 34 , 3069–3103 (2016).
Turner, N. C. et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N. Engl. J. Med. 379 , 1926–1936 (2018).
Miles, D. W. et al. First-line bevacizumab in combination with chemotherapy for HER2-negative metastatic breast cancer: pooled and subgroup analyses of data from 2447 patients. Ann. Oncol. 24 , 2773–2780 (2013).
Giordano, S. H. et al. Systemic therapy for patients with advanced human epidermal growth factor receptor 2-positive breast cancer: American Society of Clinical Oncology clinical practice guideline. J. Clin. Oncol. 32 , 2078–2099 (2014).
Partridge, A. H. et al. Chemotherapy and targeted therapy for women with human epidermal growth factor receptor 2-negative (or unknown) advanced breast cancer: American Society of Clinical Oncology clinical practice guideline. J. Clin. Oncol. 32 , 3307–3329 (2014).
Schmid, P. et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N. Engl. J. Med. 379 , 2108–2121 (2018).
Marinovich, M. L. et al. Early prediction of pathologic response to neoadjuvant therapy in breast cancer: systematic review of the accuracy of MRI. Breast 21 , 669–677 (2012).
Avril, S. et al. 18 F-FDG PET/CT for monitoring of treatment response in breast cancer. J. Nucl. Med. 57 , 34S–39SS (2016).
Marinovich, M. L. et al. Meta-analysis of magnetic resonance imaging in detecting residual breast cancer after neoadjuvant therapy. J. Natl Cancer Inst. 105 , 321–333 (2013).
Marinovich, M. L. et al. Agreement between MRI and pathologic breast tumor size after neoadjuvant chemotherapy, and comparison with alternative tests: individual patient data meta-analysis. BMC Cancer 15 , 662 (2015).
Humbert, O. et al. Role of positron emission tomography for the monitoring of response to therapy in breast cancer. Oncologist 20 , 94–104 (2015).
Pennant, M. et al. A systematic review of positron emission tomography (PET) and positron emission tomography/computed tomography (PET/CT) for the diagnosis of breast cancer recurrence. Health Technol. Assess. 14 , 1–103 (2010).
Shachar, S. S. Assessing treatment response in metastatic breast cancer. Am. J. Hematol. Oncol . 12 , (2016).
Lee, C. I. et al. Comparative effectiveness of imaging modalities to determine metastatic breast cancer treatment response. Breast 24 , 3–11 (2015).
Pagani, O. et al. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N. Engl. J. Med. 371 , 107–118 (2014).
Francis, P., Regan, M. & Fleming, G. Adjuvant ovarian suppression in premenopausal breast cancer. N. Engl. J. Med. 372 , 1672–1673 (2015).
Mao, J. J. et al. Electroacupuncture versus gabapentin for hot flashes among breast cancer survivors: a randomized placebo-controlled trial. J. Clin. Oncol. 33 , 3615–3620 (2015).
Elkins, G. et al. Randomized trial of a hypnosis intervention for treatment of hot flashes among breast cancer survivors. J. Clin. Oncol. 26 , 5022–5026 (2008).
Loprinzi, C. L. et al. Venlafaxine in management of hot flashes in survivors of breast cancer: a randomised controlled trial. Lancet 356 , 2059–2063 (2000).
Niravath, P. Aromatase inhibitor-induced arthralgia: a review. Ann. Oncol. 24 , 1443–1449 (2013).
Barton, D. L. et al. Impact of vaginal dehydroepiandosterone (DHEA) on vaginal symptoms in female cancer survivors: Trial N10C1 (Alliance). J. Clin. Oncol. 32 , 9507–9507 (2014).
Razvi, Y. et al. ASCO, NCCN, MASCC/ESMO: a comparison of antiemetic guidelines for the treatment of chemotherapy-induced nausea and vomiting in adult patients. Support. Care Cancer 27 , 87–95 (2019).
Gulati, G. et al. Prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy (PRADA): a 2×2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur. Heart J. 37 , 1671–1680 (2016).
Smith, E. M. L. et al. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. JAMA 309 , 1359–1367 (2013).
Hershman, D. L. et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J. Clin. Oncol. 32 , 1941–1967 (2014).
Hanai, A. et al. Effects of cryotherapy on objective and subjective symptoms of paclitaxel-induced neuropathy: prospective self-controlled trial. J. Natl Cancer Inst. 110 , 141–148 (2018).
Kadakia, K. C., Rozell, S. A., Butala, A. A. & Loprinzi, C. L. Supportive cryotherapy: a review from head to toe. J. Pain Symptom Manage. 47 , 1100–1115 (2014).
Hou, S., Huh, B., Kim, H. K., Kim, K.-H. & Abdi, S. Treatment of chemotherapy-induced peripheral neuropathy: systematic review and recommendations. Pain Physician 21 , 571–592 (2018).
Ahmed, R. L., Schmitz, K. H., Prizment, A. E. & Folsom, A. R. Risk factors for lymphedema in breast cancer survivors, the Iowa Women’s Health Study. Breast Cancer Res. Treat. 130 , 981–991 (2011).
Gillespie, T. C., Sayegh, H. E., Brunelle, C. L., Daniell, K. M. & Taghian, A. G. Breast cancer-related lymphedema: risk factors, precautionary measures, and treatments. Gland. Surg. 7 , 379–403 (2018).
Runowicz, C. D. et al. American Cancer Society/American Society of Clinical Oncology breast cancer survivorship care guideline. J. Clin. Oncol. 34 , 611–635 (2016).
Velikova, G. et al. Quality of life after postmastectomy radiotherapy in patients with intermediate-risk breast cancer (SUPREMO): 2-year follow-up results of a randomised controlled trial. Lancet Oncol. 19 , 1516–1529 (2018).
Hofmann, D. et al. WSG ADAPT — adjuvant dynamic marker-adjusted personalized therapy trial optimizing risk assessment and therapy response prediction in early breast cancer: study protocol for a prospective, multi-center, controlled, non-blinded, randomized, investigator initiated phase II/III trial. Trials 14 , 261 (2013).
Robertson, J. F. R., Dowsett, M. & Bliss, J. M. Peri-operative aromatase inhibitor treatment in determining or predicting long-term outcome in early breast cancer — the POETIC Trial (CRUK/07/015) [abstract]. SABCS GS1-03 (2017).
Ellis, M. J. et al. Ki67 Proliferation index as a tool for chemotherapy decisions during and after neoadjuvant aromatase inhibitor treatment of breast cancer: results from the American College of Surgeons Oncology Group Z1031 trial (Alliance). J. Clin. Oncol. 35 , 1061–1069 (2017).
Hölzel, D. et al. Improved systemic treatment for early breast cancer improves cure rates, modifies metastatic pattern and shortens post-metastatic survival: 35-year results from the munich cancer registry. J. Cancer Res. Clin. Oncol. 143 , 1701–1712 (2017).
Hölzel, D. et al. Survival of de novo stage IV breast cancer patients over three decades. J. Cancer Res. Clin. Oncol. 143 , 509–519 (2017).
Angus, L. et al. The genomic landscape of 501 metastatic breast cancer patients [abstract]. SABCS GS1-07 (2018).
Desmedt, C. et al. Unraveling lobular breast cancer progression and endocrine resistance mechanisms through genomic and immune characterization of matched primary and metastatic samples [abstract]. SABCS GS1–06 (2018).
Baselga, J. et al. Buparlisib plus fulvestrant versus placebo plus fulvestrant in postmenopausal, hormone receptor-positive, HER2-negative, advanced breast cancer (BELLE-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 18 , 904–916 (2017).
André, F. et al. Alpelisib for PIK3CA -mutated, hormone receptor-positive advanced breast cancer. N. Engl. J. Med. 380 , 1929–1940 (2019).
Baselga, J. et al. Phase III study of taselisib (GDC-0032) + fulvestrant (FULV) v FULV in patients (pts) with estrogen receptor (ER)-positive, PIK3CA-mutant (MUT), locally advanced or metastatic breast cancer (MBC): primary analysis from SANDPIPER. J. Clin. Oncol. 36 , LBA1006–LBA1006 (2018).
Kim, S.-B. et al. Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (LOTUS): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 18 , 1360–1372 (2017).
Schmid, P. et al. AZD5363 plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (PAKT): a randomised, double-blind, placebo-controlled, phase II trial. J. Clin. Oncol. 36 (15 Suppl.), 1007 (2018).
Jones, R. H. et al. Capivasertib (AZD5363) plus fulvestrant versus placebo plus fulvestrant after relapse or progression on an aromatase inhibitor in metastatic ER-positive breast cancer (FAKTION): a randomized, double-blind, placebo-controlled, phase II trial [abstract]. J. Clin. Oncol. 37 (no. 15_suppl), 1005–1005 (2019).
Yardley, D. A. et al. Randomized phase II, double-blind, placebo-controlled study of exemestane with or without entinostat in postmenopausal women with locally recurrent or metastatic estrogen receptor-positive breast cancer progressing on treatment with a nonsteroidal aromatase inhibitor. J. Clin. Oncol. 31 , 2128–2135 (2013).
Ogitani, Y. et al. DS-8201a, a novel HER2-targeting ADC with a novel DNA Topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin. Cancer Res. 22 , 5097–5108 (2016).
Tamura, K. et al. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive breast cancer previously treated with trastuzumab emtansine: a dose-expansion, phase 1 study. Lancet Oncol. 20 , 816–826 (2019).
Burris III, H. A., Giaccone, G. & Im, S. A. Updated findings of a first-in-human phase 1 study of margetuximab, an Fc-optimized chimeric monoclonal antibody, in patients with HER2-positive advanced solid tumors [abstract]. Am. Soc. Clin. Oncol. Meet. 33 (no. 15_suppl), A523 (2015).
Rugo, H. S. et al. SOPHIA primary analysis: a phase 3 (P3) study of margetuximab (M) + chemotherapy (C) versus trastuzumab (T) + C in patients (pts) with HER2+ metastatic (met) breast cancer (MBC) after prior anti-HER2 therapies (Tx) [abstract]. J. Clin. Oncol. 37 (Suppl.), Abstr 1000 (2019).
Hyman, D. M., Piha-Paul, S. & Rodon, J. Neratinib in HER2- or HER3-mutant solid tumors: SUMMIT, a global, multi-histology, open-label, phase 2 ‘basket’ study [abstract]. Am. Assoc. Cancer Res. Meet . CT001 (2017).
Saura, C. et al. Neratinib + capecitabine versus lapatinib + capecitabine in patients with HER2+ metastatic breast cancer previously treated with ≥2 HER2-directed regimens: findings from the multinational, randomized, phase III NALA trial [abstract]. J. Clin. Oncol. 37 (Suppl.), Abstract 1002 (2019).
Gucalp, A. et al. Phase II trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic breast cancer. Clin. Cancer Res. 19 , 5505–5512 (2013).
Cortes, J., Crown, J. & Awada, A. Overall survival (OS) from the phase 2 study of enzalutamide (ENZA), an androgen receptor (AR) signaling inhibitor, in AR+ advanced triple-negative breast cancer (aTNBC) [abstract]. Eur. Cancer Congr. 51 (Suppl. 3), 1802 (2015).
Gelmon, K. A. et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol. 12 , 852–861 (2011).
Nanda, R. et al. Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 Study. J. Clin. Oncol. 34 , 2460–2467 (2016).
Schmid, P., Cruz, C. & Braiteh, F. S. Atezolizumab in metastatic triple-negative breast cancer: long-term clinical outcomes and biomarker analyses [abstract]. Am. Assoc. Cancer Res. 77 , A2986 (2017).
André, F. et al. Alpelisib (ALP) + fulvestrant (FUL) for advanced breast cancer (ABC): results of the phase 3 SOLAR-1 trial [abstract]. ESMO LBA3 PR (2018).
Hyman, D. M. et al. HER kinase inhibition in patients with HER2- and HER3-mutant cancers. Nature 554 , 189–194 (2018).
Hartley, R. L., Stone, J. P. & Temple-Oberle, C. Breast cancer in transgender patients: a systematic review. Part 1: male to female. Eur. J. Surg. Oncol. 44 , 1455–1462 (2018).
Cardoso, F. et al. Characterization of male breast cancer: results of the EORTC 10085/TBCRC/BIG/NABCG International Male Breast Cancer Program. Ann. Oncol. 29 , 405–417 (2017).
PubMed Central Google Scholar
Di Oto, E. et al. X chromosome gain is related to increased androgen receptor expression in male breast cancer. Virchows Arch. 473 , 155–163 (2018).
Severson, T. M. & Zwart, W. A review of estrogen receptor/androgen receptor genomics in male breast cancer. Endocr. Relat. Cancer 24 , R27–R34 (2017).
Deb, S. et al. PIK3CA mutations are frequently observed in BRCAX but not BRCA2-associated male breast cancer. Breast Cancer Res. 15 , R69 (2013).
Gucalp, A. et al. Male breast cancer: a disease distinct from female breast cancer. Breast Cancer Res. Treat. 173 , 37–48 (2019).
Korde, L. A. et al. Multidisciplinary meeting on male breast cancer: summary and research recommendations. J. Clin. Oncol. 28 , 2114–2122 (2010).
Cardoso, F. et al. Early breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 30 , 1194–1220 (2019).
Bareche, Y. et al. Unravelling triple-negative breast cancer molecular heterogeneity using an integrative multiomic analysis. Ann. Oncol. 29 , 895–902 (2018).
Lehmann, B. D. & Pietenpol, J. A. Clinical implications of molecular heterogeneity in triple negative breast cancer. Breast 24 , S36–S40 (2015).
Lehmann, B. D. et al. Refinement of triple-negative breast cancer molecular subtypes: implications for neoadjuvant chemotherapy selection. PLOS ONE 11 , e0157368 (2016).
Burstein, M. D. et al. Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin. Cancer Res. 21 , 1688–1698 (2015).
Siu, A. L. & on behalf of the U.S. Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 164 , 279 (2016).
Klarenbach, S. et al. Recommendations on screening for breast cancer in women aged 40–74 years who are not at increased risk for breast cancer. Can. Med. Assoc. J. 190 , E1441–E1451 (2018).
Oeffinger, K. C. et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA 314 , 1599 (2015).
European Commission Initiative on Breast Cancer. Recommendations from European Breast Guidelines Europa.eu https://ecibc.jrc.ec.europa.eu/recommendations/list/Professional (2019).
Dawood, S. et al. International expert panel on inflammatory breast cancer: consensus statement for standardized diagnosis and treatment. Ann. Oncol. 22 , 515–523 (2011).
Cserni, G., Charafe-Jauffret, E. & van Diest, P. J. Inflammatory breast cancer: the pathologists’ perspective. Eur. J. Surg. Oncol. 44 , 1128–1134 (2018).
Cheang, M. C. U. et al. Defining breast cancer intrinsic subtypes by quantitative receptor expression. Oncologist 20 , 474–482 (2015).
Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 490 , 61–70 (2012). This research establishes the contemporary method of classifying breast cancer into clinically relevant molecular subtypes.
Hoadley, K. A., Andre, F., Ellis, M. J. & Perou, C. M. Breast cancer intrinsic subtypes (Poster). Nat. Rev. Clin. Oncol . https://www.nature.com/documents/nrclinonc_posters_breastcancer.pdf (2014).
Desmedt, C. et al. Genomic characterization of primary invasive lobular breast cancer. J. Clin. Oncol. 34 , 1872–1881 (2016).
Ciriello, G. et al. Comprehensive molecular portraits of invasive lobular breast cancer. Cell 163 , 506–519 (2015).
Vasudev, P. & Onuma, K. Secretory breast carcinoma: unique, triple-negative carcinoma with a favorable prognosis and characteristic molecular expression. Arch. Pathol. Lab. Med. 135 , 1606–1610 (2011).
Martelotto, L. G. et al. Genomic landscape of adenoid cystic carcinoma of the breast. J. Pathol. 237 , 179–189 (2015).
Goss, P. E. et al. Extending aromatase-inhibitor adjuvant therapy to 10 years. N. Engl. J. Med. 375 , 209–219 (2016).
Liang, M. et al. Association between CHEK2*1100delC and breast cancer: a systematic review and meta-analysis. Mol. Diagn. Ther. 22 , 397–407 (2018).
Wang, X. et al. Breast cancer risk and germline genomic profiling of women with neurofibromatosis type 1 who developed breast cancer. Genes. Chromosomes Cancer 57 , 19–27 (2018).
McCart Reed, A. E. et al. Phenotypic and molecular dissection of metaplastic breast cancer and the prognostic implications: prognostic features of metaplastic breast cancer. J. Pathol. 247 , 214–227 (2019).
Wendt, C. & Margolin, S. Identifying breast cancer susceptibility genes — a review of the genetic background in familial breast cancer. Acta Oncol. 58 , 135–146 (2019).
Couch, F. J. et al. Associations between cancer predisposition testing panel genes and breast cancer. JAMA Oncol. 3 , 1190 (2017).
Nguyen, J. et al. EORTC QLQ-BR23 and FACT-B for the assessment of quality of life in patients with breast cancer: a literature review. J. Comp. Eff. Res. 4 , 157–166 (2015).
McLachlan, S. A., Devins, G. M. & Goodwin, P. J. Factor analysis of the psychosocial items of the EORTC QLQ-C30 in metastatic breast cancer patients participating in a psychosocial intervention study. Qual. Life Res. 8 , 311–317 (1999).
Bjelic-Radisic, V. et al. An international update of the EORTC questionnaire for assessing quality of life in breast cancer patients (EORTC QLQ-BC23) — EORTC QLQ-BR45. Ann. Oncol. 29 , viii58–viii86 (2018).
Ganz, P. A., Kwan, L., Stanton, A. L., Bower, J. E. & Belin, T. R. Physical and psychosocial recovery in the year after primary treatment of breast cancer. J. Clin. Oncol. 29 , 1101–1109 (2011).
Revicki, D. A. et al. Predicting EuroQol (EQ-5D) scores from the patient-reported outcomes measurement information system (PROMIS) global items and domain item banks in a United States sample. Qual. Life Res. 18 , 783–791 (2009).
Hays, R. D., Bjorner, J. B., Revicki, D. A., Spritzer, K. L. & Cella, D. Development of physical and mental health summary scores from the patient-reported outcomes measurement information system (PROMIS) global items. Qual. Life Res. 18 , 873–880 (2009).
Bevans, M., Ross, A. & Cella, D. Patient-reported outcomes measurement information system (PROMIS): efficient, standardized tools to measure self-reported health and quality of life. Nurs. Outlook 62 , 339–345 (2014).
Download references
Acknowledgements
The authors thank N. Radosevic-Robin (Jean Perrin Comprehensive Cancer Centre, France) for her assistance in preparing Fig. 1. N. Houssami receives research support through a National Breast Cancer Foundation (NBCF, Australia) Breast Cancer Research Leadership Fellowship. K.R. acknowledges research funding from the Clinical and Translational Sciences Award (CTSA) grant number KL2 TR002379 from the National Centre for Advancing Translational Sciences, a component of the US National Institutes of Health.
Author information
Authors and affiliations.
LMU Munich, University Hospital, Department of Obstetrics and Gynecology, Breast Center and Comprehensive Cancer Center (CCLMU), Munich, Germany
Nadia Harbeck
Department of Pathology and Biopathology, Jean Perrin Comprehensive Cancer Centre, UMR INSERM 1240, University Clermont Auvergne, Clermont-Ferrand, France
Frédérique Penault-Llorca
IOB Institute of Oncology, Quironsalud Group, Madrid and Barcelona, Spain
Javier Cortes
Vall d´Hebron Institute of Oncology, Barcelona, Spain
Comprehensive Cancer Center, Medical University of Vienna, Vienna, Austria
Michael Gnant
Sydney School of Public Health, Faculty of Medicine and Health, University of Sydney, Sydney, Australia
Nehmat Houssami
Department of Radiation Oncology, Institut Curie, Paris, France
Philip Poortmans
Université PSL, Paris, France
Department of Oncology, Mayo Clinic, Rochester, MN, USA
Kathryn Ruddy
Hong Kong Breast Oncology Group, The University of Hong Kong, Hong Kong, China
Janice Tsang
Breast Unit, Champalimaud Clinical Center/Champalimaud Foundation, Lisbon, Portugal
Fatima Cardoso
You can also search for this author in PubMed Google Scholar
Contributions
Introduction (all authors); Epidemiology (J.T.); Mechanisms/pathophysiology (F.P.-L.); Diagnosis, screening and prevention (N. Houssami); Management (N. Harbeck, F.C., M.G., P.P., J.C. and N. Houssami); Quality of life (K.R.); Outlook (all authors); Overview of the Primer (N. Harbeck and F.C.).
Corresponding author
Correspondence to Nadia Harbeck .
Ethics declarations
Competing interests.
N. Harbeck reports honoraria for lectures and/or consulting from Agendia, Amgen, Astra Zeneca, Celgene, Daiichi-Sankyo, Genomic Health, Lilly, MSD, Novartis, Odonate, Pfizer, Roche, Sandoz/Hexal and Seattle Genetics. F.P.-L. declares personal financial interests in Abbvie, Agendia, Astrazeneca, BMS, Genomic Health, Janssen, Lilly, Merck Lifa, MSD, Myriad, Nanostring, Novartis, Pfizer and Roche; institutional financial interests in Astrazeneca, BMS, Genomic Health, MSD, Myriad, Nanostring and Roche; and congress invitations from Abbvie, Astrazeneca, BMS, MSD and Roche. J.C. has received honoraria from Celgene, Chugai, Eisai, Novartis, Pfizer, Roche and Samsung; has served as a consultant for Astrazeneca, Biothera, Celgene, Daichii Sankyo, Erytech Pharma, Merus, Polyphor, Roche and Seattle Genetics; has received research funding from Ariad, Astrazeneca, Baxalta GMBH, Bayer, Eisai, Guardant Health, Merch Sharp & Dohme, Pfizer, Puma and Roche; and has stocks in MedSIR. M.G. reports honoraria from Amgen, AstraZeneca, Celgene, Eli Lilly, Medison, Nanostring Technologies, Novartis and Roche; advisory fees from Accelsoir; research funding from AstraZeneca, Novartis, Pfizer and Roche; and travel expenses from Amgen, AstraZeneca, Celgene, Eli Lilly, Ipsen, Medison, Novartis and Pfizer. K.R. declares previous ownership of Merck and Pfizer stock (October 2016–February 2018). J.T. reports honoraria and consultancy or advisory roles for AstraZeneca, Astellas, De Novo, Eisai, Foundation Medicine, Nanostring, Novartis, Pfizer and Roche. F.C. declares consultancy roles for Amgen, Astellas/Medivation, AstraZeneca, Celgene, Daiichi-Sankyo, Eisai, Genentech, GE Oncology, GlaxoSmithKline, Macrogenics, Medscape, Merck-Sharp, Merus BV, Mylan, Mundipharma, Novartis, Pfizer, Pierre-Fabre, prIME Oncology, Roche, Sanofi, Seattle Genetics and Teva. The remaining authors declare no competing interests.
Additional information
Peer review information.
Nature Reviews Disease Primers thanks T. Howell, P. Neven, M. Toi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
ABC Global Alliance: https://www.abcglobalalliance.org
Adjuvant! Online: www.adjuvantonline.com
European Organization for Research and Treatment of Cancer: https://qol.eortc.org/modules/
EuroQol 5-Dimensions: https://euroqol.org/
Functional Assessment of Cancer Therapy: http://www.facit.org/FACITOrg
Patient-Reported Outcomes Measurement Information System: http://www.healthmeasures.net/explore-measurement-systems/promis
Short Form Health Survey-36: http://www.rand.org/health/surveys_tools/mos/36-item-short-form.html
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Harbeck, N., Penault-Llorca, F., Cortes, J. et al. Breast cancer. Nat Rev Dis Primers 5 , 66 (2019). https://doi.org/10.1038/s41572-019-0111-2
Download citation
Accepted : 22 July 2019
Published : 23 September 2019
DOI : https://doi.org/10.1038/s41572-019-0111-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Usp22 supports the aggressive behavior of basal-like breast cancer by stimulating cellular respiration.
- Evangelos Prokakis
- Husam Bamahmoud
- Florian Wegwitz
Cell Communication and Signaling (2024)
Targeting cholesterol impairs cell invasion of all breast cancer types
- Mauriane Maja
- Marie Verfaillie
- Donatienne Tyteca
Cancer Cell International (2024)
Identification of a Notch transcriptomic signature for breast cancer
- Eike-Benjamin Braune
- Felix Geist
- Urban Lendahl
Breast Cancer Research (2024)
Synergistic anticancer effect of Pistacia lentiscus essential oils and 5-Fluorouracil co-loaded onto biodegradable nanofibers against melanoma and breast cancer
- Obaydah Abd Alkader Alabrahim
- Hassan Mohamed El-Said Azzazy
Discover Nano (2024)
Exploring the dynamic interplay between exosomes and the immune tumor microenvironment: implications for breast cancer progression and therapeutic strategies
- Sahar Safaei
- Manouchehr Fadaee
- Tohid Kazemi
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Cancer newsletter — what matters in cancer research, free to your inbox weekly.

Breast Cancer and the Environment: A Life Course Approach (2012)
Chapter: 1 introduction.
1 Introduction
T he prospect of developing breast cancer is a source of anxiety for many women. Breast cancer remains the most common invasive cancer among women (aside from nonmelanoma skin cancers), accounting in 2011 for an estimated 230,480 new cases among women in the United States and another 2,140 new cases among men (ACS, 2011). After lung cancer, it is the second most common cause of mortality from cancer for women, with about 39,520 deaths expected in the United States in 2011. Another 450 breast cancer deaths are expected among men in 2011 (ACS, 2011). Since the mid-1970s, when the National Cancer Institute (NCI) began compiling continuous cancer statistics, the annual incidence of invasive breast cancer rose from 105 cases per 100,000 women to 142 per 100,000 women in 1999 (NCI, 2011). Since then, however, the incidence has declined. In 2008, the incidence of breast cancer was 129 cases per 100,000 women.
Further reduction of the incidence of breast cancer is a high priority, but finding ways to achieve this is a challenge. As in most types of adult cancer, breast cancer is thought to develop as a result of accumulated damage induced by both internal and external triggers resulting in initial carcinogenic events. The affected cells and tissues then progress through multiple stages, with accompanying alterations in the surrounding tissue likely playing a role in whether the damage leads to a cancer. These events contributing to subsequent cancers may occur spontaneously as a by-product of errors in normal processes, such as DNA replication, or potentially through effects of environmental exposures. The early procarcinogenic events from endogenous and exogenous processes may be sustained and
furthered by physiologic conditions such as obesity. It is likely that many such procarcinogenic events may never be entirely preventable because, although potentially modifiable, they are consequences of basic biologic processes, such as oxidative damage to DNA from endogenous metabolism, or stimulation of cell growth through normal hormonal processes. 1 Although such biological “background” mutagenesis is unavoidable, highly efficient protective pathways, such as DNA repair and immune surveillance, are effective at reducing the impacts of procarcinongenic events (Loeb and Nishimura, 2010; Bissell and Hines, 2011).
Although more needs to be learned about both the mechanisms by which breast cancers arise and the array of factors that influence risk for them, much has been established. Among the factors generally accepted as increasing women’s risk are older age, having a first child at an older age or never having a child, exposure to ionizing radiation, and use of certain forms of postmenopausal hormone therapy (HT). Inherited mutations in the BRCA1 and BRCA2 genes also markedly increase risk for breast cancer (and other cancers as well), but these mutations are rare in the general population and account for only 5 to 10 percent of cases (ACS, 2011).
Even though aging, genetics, and patterns of childbearing account for some of the risk for breast cancer, they are not promising targets for preventive measures. More helpful would be identifying modifiable risk factors. For example, the publication of findings from the Women’s Health Initiative (Writing Group for the Women’s Health Initiative Investigators, 2002) confirming earlier indications that estrogen–progestin HT was contributing to an increase in the risk of postmenopausal breast cancer was followed by a rapid reduction in use of HT and in the incidence of invasive breast cancer. As reflected in NCI data, the incidence in 2002 was 136 cases per 100,000 women, compared with 127 in 2003 (NCI, 2011). A portion of the decline in breast cancer incidence since 1999 is attributed to this reduced use of HT (e.g., Ravdin et al., 2007; Farhat et al., 2010). But there are long-standing and still unresolved concerns that aspects of diet, ambient chemicals, or other potentially modifiable environmental exposures may be contributing to high rates of breast cancer.
At present, a large but incomplete body of evidence is available on the relationship between breast cancer and the wide variety of external factors that can be said to comprise the environment. Information on interactions between genetic susceptibility and environmental factors is particularly sparse. In contrast, knowledge of the complexity of breast cancer is growing, with the characterization of multiple tumor subtypes; the possibility
_________________
1 Loeb and Nishimura (2010, p. 4270) note that each normal cell in a person’s body may be exposed to as many as 50,000 DNA-damaging events each day, and that oxygen free radicals are a major source of DNA damage.
that critical events in the origins of breast cancer can occur very early in life; the variety of pathways through which breast cancer risks may be shaped; and the potential significance of both the timing of exposures and the way combinations of factors determine the effect on risks for different types of breast cancer. This growing knowledge has stimulated a transition in breast cancer research. The new perspectives on breast cancer highlight the limitations of the current understanding of the disease, and innovative ideas are beginning to influence the design and analysis of epidemiologic studies, experimental studies in animals, and mechanistic studies of breast cancer biology, all directed toward elucidating how external factors may influence the etiology of breast cancer.
This report presents the results of a study commissioned to review the current evidence on environmental risk factors for breast cancer, consider gene–environment interactions in breast cancer, explore evidence-based actions that might reduce the risk of breast cancer, and recommend research in these areas.
STUDY CHARGE AND COMMITTEE ACTIVITIES
This study resulted from a request to the Institute of Medicine (IOM) by Susan G. Komen for the Cure and its Scientific Advisory Board. Komen for the Cure funds research on prevention, diagnosis, and treatment of breast cancer, and also provides educational information and support services for the public and health care providers. The Statement of Task for the IOM study appears in Box 1-1 .
The members of the study committee were selected to contribute expertise in epidemiology, toxicology, risk assessment, biostatistics, molecular carcinogenesis, gene–environment interactions, communication of health messages, environmental health science, exposure assessment, and health care. The committee includes a member from the patient advocacy community.
The committee met in person five times from April 2010 through February 2011 and conducted additional deliberations by conference call. During these meetings and calls, the committee reviewed and discussed the existing research literature on the topics central to its charge and developed and revised this report. At three of its meetings, the committee held public sessions during which it heard presentations by researchers, representatives of advocacy organizations, and members of the public.
The committee also commissioned work on two topics. One project was a review of data available to assess temporal changes in the potential for exposure to a selected set of chemicals and other environmental agents. The agents included in this paper have been discussed in the research literature and the popular press as possible contributors to increased risk for
BOX 1-1 Study Charge
In response to a request from Susan G. Komen for the Cure ® , the Institute of Medicine will assemble a committee to:
1. Review the evidentiary standards for identifying and measuring cancer risk factors;
2. Review and assess the strength of the science base regarding the relationship between breast cancer and the environment;
3. Consider the potential interaction between genetic and environmental risk factors;
4. Consider potential evidence-based actions that women could take to reduce their risk of breast cancer;
5. Review the methodological challenges involved in conducting research on breast cancer and the environment; and
6. Develop recommendations for future research in this area.
In addition to reviewing the published literature, the committee will seek input from stakeholders, in part by organizing and conducting a public workshop to examine issues related to the current status of evidentiary standards and the science base, research methods, and promising areas of research. The workshop will focus on the challenges involved in the design, conduct, and interpretation of research on breast cancer and the environment. The committee will generate a technical report with conclusions and recommendations, as well as a summary report for the lay public.
breast cancer. This work served as an information resource for the committee and helped to identify some data presented in Chapter 4 . The other project resulted in a paper examining temporal changes in the United States in exposure to ionizing radiation, with a particular focus on exposure from medical imaging (see Appendix F , available electronically at http://www.nap.edu/catalog.php?record_id=13263 ).
APPROACH TO THE STUDY
The committee began its work with recognition of the potentially vast scope of the study task and the need to develop a perspective and approach that could lead to a useful and timely report. The committee sought to focus its attention in areas that it considered to be the most significant and the most pertinent to the charge placed before it.
For purposes of this report, the committee interpreted “environment” broadly, to encompass all factors that are not directly inherited through
DNA. As a result, this definition includes elements that range from the cellular to the societal: the physiologic and developmental course of an individual, diet and other ingested substances, physical activity, microbial agents, physical and chemical agents encountered at home or at work, medical treatments and interventions, social factors, and cultural practices. This perspective was a foundation for the committee’s work; application of it in its broadest sense is something that the committee hopes will expand the scope of future research. For some readers, this interpretation will differ from their association of the phrase “environmental risk factors” primarily with pollutants and other products of industrial processes (Baralt and McCormick, 2010). Furthermore, throughout the report the term “breast cancer” is used to refer to disease in humans and “mammary cancer” or “mammary tumor” to refer to disease in animals.
The committee explored the available evidence concerning breast cancer risks associated with a varied but limited collection of specific substances and factors ( Chapter 3 ), and it also reviewed the many challenges that researchers have had to contend with in studying breast cancer, including those pertaining to gene–environment interactions ( Chapter 4 ). But in its examination of the relation between breast cancer and the environment, the committee chose to highlight an approach that emphasizes the biologic mechanisms through which environmental factors may be operating and the importance of the changing picture over the life course ( Chapter 5 ). This perspective played a major role in shaping the committee’s conclusions and recommendations.
A Life Course Perspective
Breast cancer is primarily (but far from exclusively) a disease of adult women who are approaching or have reached menopause. In 2009, approximately 90 percent of new cases in U.S. women were diagnosed at age 45 or older (ACS, 2009). But the breast undergoes substantial changes from the time it begins developing in the fetus through old age, especially in response to hormonal changes during puberty, pregnancy, lactation, and menopause. With the timing of these developmental events related to risk for some types of breast cancer, there has been growing interest in exploring whether the timing of a variety of environmental exposures also is important in understanding what influences breast cancer risks. In Chapter 5 , the committee has sought to link its examination of the mechanisms of carcinogenesis with a life course perspective on when and how those pathologic pathways may be particularly relevant in relation to when and how environmental exposures occur. Attention was paid to growing evidence for critical windows of susceptibility (e.g., periods with rapid cell proliferation or maturation)
when specific mechanisms that increase the likelihood of a breast cancer developing may be more likely to be activated.
Identifying Environmental Risks for Breast Cancer
Trying to determine which environmental exposures may be influencing rates of breast cancer poses substantial challenges, many of which are discussed in Chapter 4 . Cancer is a complex disease, and its “causes” are generally harder to trace than the bacteria and viruses that cause infectious diseases. People who are never exposed to the measles virus will never get measles. But the impact of removing a particular environmental exposure associated with breast cancer is less clear because many other factors can still contribute to the development of breast cancer. The role of underlying susceptibility from inherited genes appears to involve both rare variants and common ones, but it is still not well characterized. Moreover, people are exposed to a complex and changing mix of environmental agents over the course of a lifetime, so discerning the effects of an individual agent, or knowing which components of the mixture may influence the development of disease or how the mixture’s components may interact with each other or with genes, is not straightforward.
Observational epidemiologic studies are a critical tool for learning about elevated risks, but they can be difficult to do well. They typically are the basis for demonstrating correlations between risk factors and outcomes, but establishing a causal inference is much more difficult. The challenges in establishing causality in such studies include difficulties with exposure measurement and accounting for undetected or poorly measured differences that may exist between the groups designated as exposed and unexposed. Furthermore, the timing and duration of observational studies may affect whether sufficient time has elapsed to detect differences in the incidence of a cancer that may not appear until many years after an exposure. Randomized controlled trials, which assign participants to a specific exposure or a comparison condition, are easier to interpret. However, for ethical and methodological reasons, such studies are rarely possible, especially when the goal is to determine whether the exposure is associated with an adverse event.
Experimental studies in animal models and in vitro systems offer an important opportunity to study the effects of well-defined exposures and to explore mechanisms of carcinogenicity in ways that are not possible in epidemiologic studies. They can signal potential hazards to human health that cannot be identified in other ways, but their results have to be interpreted with an understanding of differences across species and the comparability of an experimental exposure to the conditions encountered in the human population.
Reviewing Evidence on Specific Risk Factors
The literature on risk factors for cancer in general and breast cancer in particular is large and varied. In the United States, the Environmental Protection Agency (EPA) and the National Toxicology Program (NTP) in the National Institute of Environmental Health Sciences have programs to review the evidence on the carcinogenicity of various substances. 2 The International Agency for Research on Cancer (IARC), which is part of the World Health Organization, is a focal point for major international collaboration in such reviews. 3 In addition, a collaborative project between the World Cancer Research Fund International and the American Institute for Cancer Research has an ongoing program to review evidence on diet, physical activity, and cancer (WCRF/AICR, 2007). 4 All of these review programs consider evidence concerning breast cancer (or mammary cancers in animal studies) when it is available, but it is not their focus. Reviews specifically concerning breast cancer have also been conducted. These reviews include one conducted by the California Breast Cancer Research Program (2007) and a review sponsored by Komen for the Cure and conducted by the Silent Spring Institute (e.g., Brody et al., 2007; Rudel et al., 2007).
Assembling a comprehensive review of evidence on the relation between a complete set of environmental factors and breast cancer was not feasible for this study. Instead, the committee chose to focus on a limited selection of various types of environmental factors and potential routes of exposure. These factors are discussed in Chapter 3 . The committee’s aim was to characterize the available evidence and identify where substantial areas of uncertainty exist.
Observations About Risk
One component of the committee’s task was to comment on actions that can be taken to reduce the risk of breast cancer. Opportunities for action are discussed in Chapter 6 , but it is important to emphasize from the outset the challenge of interpreting evidence regarding risk and risk reduction. The widely quoted estimate that women in the United States have a 1-in-8 chance of being diagnosed with breast cancer during their lifetimes
2 Information on the EPA and NTP review programs is available at http://www.epa.gov/ebtpages/pollcarcinogens.html and http://ntp.niehs.nih.gov/?objectid=72016262-BDB7-CEBA-FA60E922B18C2540 .
3 Information on IARC reviews is available at http://www.iarc.fr/ and http://monographs.iarc.fr/index.php .
4 Information on the review by the World Cancer Research Fund International and the American Institute for Cancer Research is available at http://www.wcrf.org/cancer_research/expert_report/index.php .
can be restated as approximately a 12 percent lifetime risk of developing invasive breast cancer (NCI, 2010). The risk can also be presented for shorter, more comprehensible intervals. For example, among white women who are 50 years old, 2.4 percent are likely to be diagnosed with invasive breast cancer over the next 10 years (NCI, 2010). This 10-year risk is 2.2 percent for 50-year-old black women, 2.0 percent for Asian women, and 1.7 percent for Hispanic women. For 70-year-olds, the 10-year risks are 3.9 percent for white women, 3.2 percent for black women, and 2.4 percent for both Asian and Hispanic women. Estimates for longer follow-up periods (e.g., 20 or 30 years) will only increase those risks. Within average values such as these, there are always groups of women whose particular characteristics give them a higher or lower 10-year risk.
These estimates of risk are a critical reference point for understanding the implications of findings from epidemiologic studies on factors associated with increased or decreased risk of breast cancer. These findings are typically reported in terms of relative risk, which reflects a comparison between the risk in a population exposed to a particular factor and that in a similar population that is not exposed. Thus, a relative risk of 2.0 (a doubling of risk) might mean that for women with that risk factor, the 10-year risk of breast cancer is 5 percent rather than 2.5 percent. Similarly, a relative risk of 0.5 for a protective factor means that women with that characteristic may have a 10-year risk of 1.3 percent rather than 2.5 percent. These examples are offered to illustrate the scale of the change in risk implied by typical epidemiologic findings; they are not a formal analysis.
From a public health perspective, another important piece of information is the prevalence of the risk factor in the population. Finding that an environmental factor is associated with a large relative risk may still mean that it accounts for few cases of disease if the disease or the exposure is rare in that population. Alternatively, an environmental exposure that is associated with only a small increase in risk may be contributing to a large number of cases if the exposure is very common in the population. However, if the exposure is so common that there is little variability across the population (virtually everyone is exposed), it can be extremely difficult to identify the contribution from that exposure.
Virtually all of the epidemiologic evidence regarding breast cancer risk is drawn from population-level analyses. As a result, the conclusions reached on the basis of that evidence apply to an exposed population . With current knowledge, it is not possible to apply those conclusions to predict which individuals within that population are most likely to develop breast cancer. Nevertheless, an understanding of population-based estimates of risk can help people make personal choices that may lead to better health outcomes.
TOPICS BEYOND THE SCOPE OF THE STUDY
Several topics were defined as falling beyond the scope of the study. With the focus on environmental risk factors for breast cancer, the committee chose to devote little attention to the established associations between increased risk for breast cancer and reproductive events such as younger age at menarche, older age at first birth, lack of lactation, and older age at menopause. The committee also chose not to evaluate the established associations between breast cancer risk and higher birth weight and attained stature. Although some of them might fall under the committee’s very broad definition of environmental factors, they were not the focus of its review. Background is provided on many of these other factors in Chapter 2 , and the possibility that some environmental exposures may have an indirect influence on risk for breast cancer because they may affect the timing of these reproductive events is discussed in Chapter 5 .
The committee also agreed that the nature and effectiveness of breast cancer screening, diagnosis, and treatment were generally beyond the scope of the study. It noted but did not analyze the impact of increased mammography and changes in screening practices since the 1970s on the observed incidence of breast cancer. The paper commissioned by the committee on medical sources of exposure to ionizing radiation took into account the contribution of mammography. The committee did not examine the appropriateness of screening recommendations or practices.
The committee decided as well that its charge called for a focus on risk for the initial occurrence of breast cancer and not on recurrence or factors that might be associated with the risk of recurrence. Although environmental exposures may well influence the risk of recurrence, that risk is also influenced by characteristics of tumors at the time of diagnosis and subsequent treatment and follow-up practices. Consideration of clinical practice in the treatment of women (and men) with diagnosed breast cancers is substantially different from the study’s primary focus on prevention of breast cancer through improved understanding of and response to environmental risks. Similarly, the committee concluded that its charge called for a focus on the incidence of breast cancer and not mortality. Influences on breast cancer mortality patterns include factors that affect diagnosis and treatment that are separate from the effects of environmental exposures on the incidence of the disease.
The committee did not explicitly assess environmental risk factors for male breast cancer, beyond the general assumption that some of the risk factors identified through studies in women may also be relevant to the development of breast cancer in men.
THE COMMITTEE’S REPORT
This report reviews the current evidence on the biology of breast cancer, examines the challenges of studying environmental risk factors, and presents the committee’s findings and research recommendations from its review of evidence on environmental risk factors. Specifically, Chapter 2 provides important background for evaluating factors influencing breast cancer risk with a brief review of the biology of breast cancer and trends in incidence in the United States, along with discussion of the kinds of studies used to investigate breast cancer and environmental exposures. Chapter 3 presents the committee’s review of evidence on selected environmental risk factors. Chapter 4 discusses the variety of challenges that complicate the study of environmental risk factors for breast cancer, as well as gene–environment interactions. Chapter 5 examines mechanisms of carcinogenesis and links them to a life course perspective on breast development and the potential for environmental factors to influence risk for breast cancer. In Chapter 6 , the committee examines opportunities for evidence-based action to reduce risks for breast cancer and also considers the challenges of avoiding the unintentional introduction of new risks. Chapter 7 concludes the report with the committee’s recommendations for future research efforts. Included as appendixes are agendas for the committee’s public sessions ( Appendix A ), biographical sketches of committee members ( Appendix B ), a summary of weight-of-evidence categories used by major organizations that evaluate cancer risks ( Appendix C ), a table summarizing reports of population attributable risks for breast cancer ( Appendix D ), a glossary ( Appendix E ), and the paper commissioned on exposure to ionizing radiation ( Appendix F ).
ACS (American Cancer Society). 2009. Breast cancer facts and figures 2009–2010 . Atlanta, GA: ACS. http://www.cancer.org/Research/CancerFactsFigures/BreastCancerFactsFigures/index (accessed November 17, 2010).
ACS. 2011. Breast Cancer facts and figures 2011–2012 . Atlanta, GA: ACS. http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-030975.pdf (accessed November 15, 2011).
Baralt, L. B., and S. McCormick. 2010. A review of advocate–scientist collaboration in federally funded environmental breast cancer research centers. Environ Health Perspect 118(12):1668–1675.
Bissell, M. J., and W. C. Hines. 2011. Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med 17(3):320–329.
Brody, J. G., K. B. Moysich, O. Humblet, K. R. Attfield, G. P. Beehler, and R. A. Rudel. 2007. Environmental pollutants and breast cancer: Epidemiologic studies. Cancer 109(12 Suppl):2667–2711.
California Breast Cancer Research Program. 2007. Identifying gaps in breast cancer research: Addressing disparities and the roles of the physical and social environment . http://cbcrp.org/sri/reports/identifyingGaps/index.php (accessed October 25, 2011).
Farhat, G. N., R. Walker, D. S. Buist, T. Onega, and K. Kerlikowske. 2010. Changes in invasive breast cancer and ductal carcinoma in situ rates in relation to the decline in hormone therapy use. J Clin Oncol 28(35):5140–5146.
Loeb, L. A., and S. Nishimura. 2010. Princess Takamatsu Symposium on DNA repair and human cancers. Cancer Res 70(11):4269–4273.
NCI (National Cancer Institute). 2010. SEER cancer statistics review, 1975–2007 . Edited by S. F. Altekruse, C. L. Kosary, M. Krapcho, N. Neyman, R. Aminou, W. Waldron, J. Ruhl, N. Howlader, Z. Tatalovich, H. Cho, A. Mariotto, M. P. Eisner, D. R. Lewis, K. Cronin, H. S. Chen, E. J. Feuer, D. G. Stinchcomb, and B. K. Edwards. Bethesda, MD:
NCI. http://seer.cancer.gov/csr/1975_2007/ (accessed January 6, 2011).
NCI. 2011. SEER cancer statistics review, 1975–2008. Edited by N. Howlader, A. M. Noone, M. Krapcho, N. Neyman, R. Aminou, W. Waldron, S. F. Altekruse, C. L. Kosary, J. Ruhl, Z. Tatalovich, H. Cho, A. Mariotto, M. P. Eisner, D. R. Lewis, H. S. Chen, E. J. Feuer, K. A. Cronin, and B. K. Edwards. Bethesda, MD: NCI. (Based on November 2010 SEER data submission, posted to the SEER website, 2011.) http://seer.cancer.gov/csr/1975_2008/ (accessed June 1, 2011).
Ravdin, P. M., K. A. Cronin, N. Howlader, C. D. Berg, R. T. Chlebowski, E. J. Feuer, B. K. Edwards, and D. A. Berry. 2007. The decrease in breast-cancer incidence in 2003 in the United States. N Engl J Med 356(16):1670–1674.
Rudel, R. A., K. R. Attfield, J. N. Schifano, and J. G. Brody. 2007. Chemicals causing mammary gland tumors in animals signal new directions for epidemiology, chemicals testing, and risk assessment for breast cancer prevention. Cancer 109(12 Suppl):2635–2666.
WCRF/AICR (World Cancer Research Fund/American Institute for Cancer Research). 2007. Food, nutrition, physical activity, and the prevention of cancer: A global perspective. Washington, DC: AICR.
Writing Group for the Women’s Health Initiative Investigators. 2002. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women’s Health Initiative randomized controlled trial. JAMA 288(3):321–333.
This page intentionally left blank.
Breast cancer remains the most common invasive cancer among women. The primary patients of breast cancer are adult women who are approaching or have reached menopause; 90 percent of new cases in U.S. women in 2009 were diagnosed at age 45 or older. Growing knowledge of the complexity of breast cancer stimulated a transition in breast cancer research toward elucidating how external factors may influence the etiology of breast cancer.
Breast Cancer and the Environment reviews the current evidence on a selection of environmental risk factors for breast cancer, considers gene-environment interactions in breast cancer, and explores evidence-based actions that might reduce the risk of breast cancer. The book also recommends further integrative research into the elements of the biology of breast development and carcinogenesis, including the influence of exposure to a variety of environmental factors during potential windows of susceptibility during the full life course, potential interventions to reduce risk, and better tools for assessing the carcinogenicity of environmental factors. For a limited set of risk factors, evidence suggests that action can be taken in ways that may reduce risk for breast cancer for many women: avoiding unnecessary medical radiation throughout life, avoiding the use of some forms of postmenopausal hormone therapy, avoiding smoking, limiting alcohol consumption, increasing physical activity, and minimizing weight gain.
Breast Cancer and the Environment sets a direction and a focus for future research efforts. The book will be of special interest to medical researchers, patient advocacy groups, and public health professionals.
READ FREE ONLINE
Welcome to OpenBook!
You're looking at OpenBook, NAP.edu's online reading room since 1999. Based on feedback from you, our users, we've made some improvements that make it easier than ever to read thousands of publications on our website.
Do you want to take a quick tour of the OpenBook's features?
Show this book's table of contents , where you can jump to any chapter by name.
...or use these buttons to go back to the previous chapter or skip to the next one.
Jump up to the previous page or down to the next one. Also, you can type in a page number and press Enter to go directly to that page in the book.
Switch between the Original Pages , where you can read the report as it appeared in print, and Text Pages for the web version, where you can highlight and search the text.
To search the entire text of this book, type in your search term here and press Enter .
Share a link to this book page on your preferred social network or via email.
View our suggested citation for this chapter.
Ready to take your reading offline? Click here to buy this book in print or download it as a free PDF, if available.
Get Email Updates
Do you enjoy reading reports from the Academies online for free ? Sign up for email notifications and we'll let you know about new publications in your areas of interest when they're released.
What Is Breast Cancer?
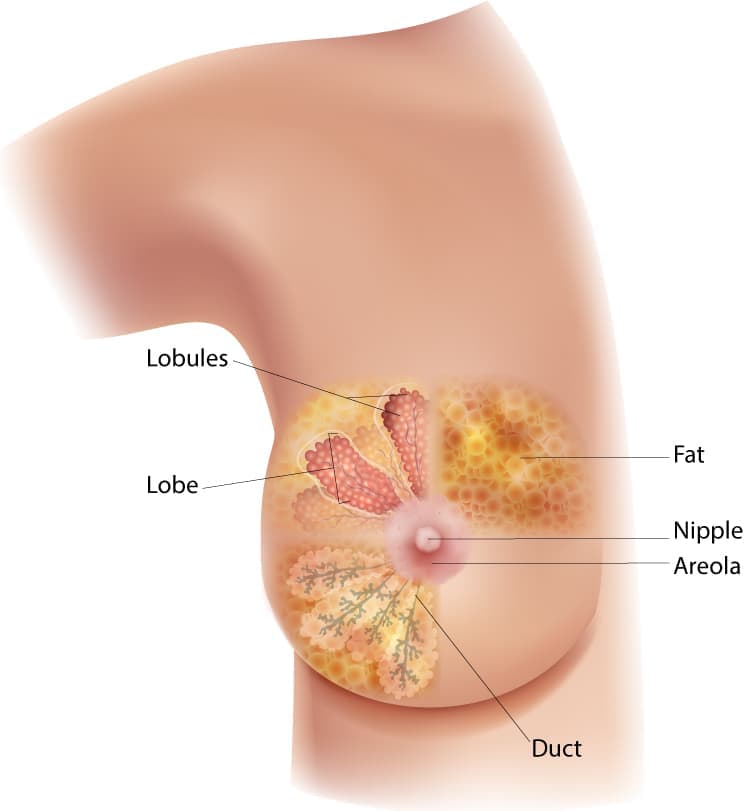
This diagram of the breast shows the location of the lobules, lobe, duct, areola, nipple, and fat.
A breast is made up of three main parts: lobules, ducts, and connective tissue. The lobules are the glands that produce milk. The ducts are tubes that carry milk to the nipple. The connective tissue (which consists of fibrous and fatty tissue) surrounds and holds everything together.
Breast cancer is a disease in which cells in the breast grow out of control. There are different kinds of breast cancer. The kind of breast cancer depends on which cells in the breast turn into cancer.
Most breast cancers begin in the ducts or lobules. Breast cancer can spread outside the breast through blood vessels and lymph vessels. When breast cancer spreads to other parts of the body, it is said to have metastasized.
Kinds of Breast Cancer
The most common kinds of breast cancer are—
- Invasive ductal carcinoma. The cancer cells begin in the ducts and then grow outside the ducts into other parts of the breast tissue. Invasive cancer cells can also spread, or metastasize, to other parts of the body.
- Invasive lobular carcinoma. Cancer cells begin in the lobules and then spread from the lobules to the breast tissues that are close by. These invasive cancer cells can also spread to other parts of the body.
There are several other less common kinds of breast cancer, such as Paget’s disease, medullary, mucinous, and inflammatory breast cancer.
Ductal carcinoma in situ (DCIS) is a breast disease that may lead to invasive breast cancer. The cancer cells are only in the lining of the ducts and have not spread to other tissues in the breast.
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
- Top Courses
- Online Degrees
- Find your New Career
- Join for Free

Introduction to Breast Cancer
Taught in English
Some content may not be translated
Financial aid available
54,159 already enrolled
Gain insight into a topic and learn the fundamentals

Instructor: Anees B. Chagpar, MD, MSc, MPH, MA, MBA, FRCS(C), FACS
Top Instructor
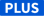
Included with Coursera Plus
(1,245 reviews)

Skills you'll gain
- Breast Cancer
Details to know

Add to your LinkedIn profile
See how employees at top companies are mastering in-demand skills

Earn a career certificate
Add this credential to your LinkedIn profile, resume, or CV
Share it on social media and in your performance review

There are 7 modules in this course
Welcome to an Introduction to Breast Cancer! In this course, we’ll learn a bit about the leading cause of cancer in women worldwide – from the basic biology of the disease, to risk factors and prevention, to treatment modalities to survivorship. We’ll talk to leading experts, explore some of the milestone studies that have pushed this field forward, and have interactive discussions on discussion boards and social media. You’ll even have an opportunity to let us know what topics you want to cover on tweetchats, so we can try to make the content fit your interests.
There is something in this course for everyone – if you’re a breast cancer survivor or the friend/family member of someone with this disease, this course will help you to better understand this disease, and give you ideas for questions you may want to ask your doctor. Maybe you’re a healthcare provider or studying to be the same, this course is a great refresher on where the state of the science is. If you’re a healthcare administrator wondering about how the interdisciplinary components of breast cancer care fit together, or an entrepreneur thinking about unmet needs in this space, or someone in public health interested in prevention, this course is also for you! Are you ready to learn a lot, and have some fun while we’re at it? If so, I hope you’ll join us! Let’s get started!!!
Welcome to the Course!
What's included.
2 videos 2 readings
2 videos • Total 3 minutes
- Welcome to the Course! • 0 minutes • Preview module
- Course tutorial • 2 minutes
2 readings • Total 20 minutes
- Resources for the Course • 10 minutes
- Disclaimer • 10 minutes
Risks and Prevention
Join me as we start to learn about what breast cancer is, the epidemiology of this disease and the risks associated with it. In these lectures, we’ll talk about genetic mutations that predispose us to developing breast cancer. As you’ll find out, this goes far beyond just BRCA!
4 videos 1 quiz
4 videos • Total 58 minutes
- An Introduction to Breast Cancer • 11 minutes • Preview module
- Genetics • 20 minutes
- Prevention • 16 minutes
- Interview with Erin Hofstatter, Co-Director of High Risk Program at Yale Cancer Center • 9 minutes
1 quiz • Total 30 minutes
- Module #1: Risks and Prevention • 30 minutes
Under the Microscope
What is cancer and how does it work? Want to learn the fundamentals of what breast cancer is? The different “types” – what is in situ vs. invasive? What is lobular vs. ductal? What is grade vs. stage? And what do molecular subtypes refer to? Well, tune in! Learn about the hallmarks of cancer – what are the processes that actually lead to this disease? Maybe this will give you some ideas about how we can stop cancers in their tracks!
2 videos 1 quiz
2 videos • Total 34 minutes
- Breast Cancer Basics • 21 minutes • Preview module
- Pathophysiology • 12 minutes
- Module #2: Under the Microscope – what is cancer and how does it work? • 30 minutes
Making the Diagnosis
Want to learn more about how to find breast cancers early, when they’re most treatable? This is the lecture for you! “Tissue is the issue” – learn how we actually do the biopsies to make the diagnosis of breast cancer. How do we stage breast cancer? Learn what tests we need to do and in whom in order to get this information!
4 videos • Total 64 minutes
- Screening • 27 minutes • Preview module
- Biopsy Techniques • 7 minutes
- Staging • 11 minutes
- Staging Updates • 17 minutes
- Module #3: Making the diagnosis – how we find and stage breast cancers • 30 minutes
All About Surgery
How do we actually remove breast cancer? Is a lumpectomy just as good as a mastectomy? Find out in this session. There are many different options for reconstructing a breast after a mastectomy – from tissue expanders and implants, to using your own tissue. In this talk, we’ll explore some of these options. Why do we take out lymph nodes, and how? What are the side effects? Learn more about this in this session! Do you have questions about lymphedema? How do you prevent it? Can/should you lift weights after a lymph node dissection? Should you wear a sleeve if you are going on a plane? What about shaving, hand surgery, and having an iv placed? We’ll answer all of these questions in this session.
5 videos 1 quiz
5 videos • Total 69 minutes
- Surgical Options • 20 minutes • Preview module
- Reconstructive Options • 7 minutes
- Interview with Alex Au, Director of Breast Reconstruction at Yale • 19 minutes
- Lymph Node Evaluation • 14 minutes
- Lymphedema • 8 minutes
- Module #4: All about Surgery • 30 minutes
Beyond the Knife
Learn all about radiation therapy – who needs it, when, what are the different types, and how do we minimize side effects. Who needs chemotherapy? What about hormonal therapy? What is targeted therapy? We’ll learn all about the drugs we use to treat breast cancer in this session.
6 videos 1 quiz
6 videos • Total 108 minutes
- Radiation Therapy • 17 minutes • Preview module
- Interview with Suzanne Evans, Breast Radiation Oncologist • 24 minutes
- Medical Oncology • 26 minutes
- Interview with Lajos Pusztai, Chief of Breast Medical Oncology at Yale • 18 minutes
- Immunotherapy • 9 minutes
- Interview with Lajos Pusztai, Chief of Breast Medical Oncology at Yale • 11 minutes
- Module #5: Beyond the Knife • 30 minutes
Not all breast cancers are the same. Let’s learn a bit more about inflammatory breast cancer, Paget’s disease, Male breast cancer, breast cancer in pregnancy and metastatic disease. Let’s talk all about clinical trials – what they are, how they are monitored, and some of the trials that have really moved the field forward. So, you or your patients have gotten through diagnosis and active treatment, and you’re now in the survivorship period. Great! But this poses a whole new set of issues as people adjust to their “new normal”. Learn about what these issues are, and a bit about survivorship care plans as well.
12 videos 1 reading 1 quiz 1 peer review
12 videos • Total 166 minutes
- Special Presentations • 20 minutes • Preview module
- Clinical Trials • 23 minutes
- Clinical Trials - TAILORx in the News • 7 minutes
- Survivorship • 4 minutes
- Interview with Tara Sanft, Director of Adult Survivorship Clinic • 19 minutes
- Diet and Exercise • 10 minutes
- Interview with Maura Harrigan, Oncology Research Registered Dietitian • 10 minutes
- Interview with Dr. Tara Sanft and Dr. Melinda Irwin, Yale Cancer Center • 14 minutes
- Office Hours 1 • 16 minutes
- Interview with Lauren Baldassarre, Director of Cardio-Oncology at Yale • 15 minutes
- Breast Cancer and COVID-19 • 21 minutes
- Conclusion • 3 minutes
1 reading • Total 10 minutes
- Send Us a Video • 10 minutes
- Module #6: Potpurri • 30 minutes
1 peer review • Total 60 minutes
- Cancer Management through Clinical Trials • 60 minutes
Instructor ratings
We asked all learners to give feedback on our instructors based on the quality of their teaching style.

For more than 300 years, Yale University has inspired the minds that inspire the world. Based in New Haven, Connecticut, Yale brings people and ideas together for positive impact around the globe. A research university that focuses on students and encourages learning as an essential way of life, Yale is a place for connection, creativity, and innovation among cultures and across disciplines.
Recommended if you're interested in Basic Science

Stanford University
Health After Cancer: Cancer Survivorship for Primary Care

Universitat de Barcelona
Manejo del enfermo semicrítico y crítico por COVID-19
Johns Hopkins University
Introduction to the Biology of Cancer

Fudan University
基于Unity引擎的游戏开发进阶
Why people choose coursera for their career.

Learner reviews
Showing 3 of 1245
1,245 reviews
Reviewed on Jan 7, 2024
An EXCELLENT course on breast cancer. Learned a lot about breast cancer, its STAGES, various treatments of breast cancer (TYPES of SURGICAL METHODS, CHEMOTHERAPY etc.) etc.
Reviewed on Jan 22, 2018
Just Perfect, it was perfect from every aspect. It had surgery, radiotherapy, medicine, genetics and clinical trials. Also, the most important the is the professor, she was amazing.
Reviewed on Sep 13, 2020
I learned so much with this course, and it will definitely help me to give people advice about breast cancer.
The spotlight of the lectures is professor Anees B. Chagpar, she is just spectacular!
New to Basic Science? Start here.

Open new doors with Coursera Plus
Unlimited access to 7,000+ world-class courses, hands-on projects, and job-ready certificate programs - all included in your subscription
Advance your career with an online degree
Earn a degree from world-class universities - 100% online
Join over 3,400 global companies that choose Coursera for Business
Upskill your employees to excel in the digital economy
Frequently asked questions
When will i have access to the lectures and assignments.
Access to lectures and assignments depends on your type of enrollment. If you take a course in audit mode, you will be able to see most course materials for free. To access graded assignments and to earn a Certificate, you will need to purchase the Certificate experience, during or after your audit. If you don't see the audit option:
The course may not offer an audit option. You can try a Free Trial instead, or apply for Financial Aid.
The course may offer 'Full Course, No Certificate' instead. This option lets you see all course materials, submit required assessments, and get a final grade. This also means that you will not be able to purchase a Certificate experience.
What will I get if I purchase the Certificate?
When you purchase a Certificate you get access to all course materials, including graded assignments. Upon completing the course, your electronic Certificate will be added to your Accomplishments page - from there, you can print your Certificate or add it to your LinkedIn profile. If you only want to read and view the course content, you can audit the course for free.
What is the refund policy?
You will be eligible for a full refund until two weeks after your payment date, or (for courses that have just launched) until two weeks after the first session of the course begins, whichever is later. You cannot receive a refund once you’ve earned a Course Certificate, even if you complete the course within the two-week refund period. See our full refund policy Opens in a new tab .
Is financial aid available?
Yes. In select learning programs, you can apply for financial aid or a scholarship if you can’t afford the enrollment fee. If fin aid or scholarship is available for your learning program selection, you’ll find a link to apply on the description page.
More questions
Home — Essay Samples — Nursing & Health — Oncology — Breast Cancer
Essays About Breast Cancer
Brief description of breast cancer.
Breast cancer is a type of cancer that forms in the cells of the breast. It is the second most common cancer in women and can also affect men. Breast cancer can be invasive or non-invasive and is often detected through screening and self-examination. Early detection and treatment are crucial for improving outcomes and survival rates.
Importance of Writing Essays on This Topic
Essays on breast cancer are significant for academic and personal exploration as they provide an opportunity to raise awareness about the disease, its risk factors, prevention, and treatment options. Writing about breast cancer also allows individuals to share personal experiences, advocate for research and support, and contribute to the ongoing dialogue surrounding this prevalent health issue.
Tips on Choosing a Good Topic
- Consider exploring the latest research and advancements in breast cancer treatment and prevention.
- Reflect on personal experiences or those of loved ones affected by breast cancer for a more personal and impactful essay.
- Investigate the societal and cultural impact of breast cancer, including awareness campaigns, advocacy, and support networks.
Essay Topics
- The Role of Genetic Testing in Breast Cancer Prevention
- The Impact of Lifestyle Choices on Breast Cancer Risk
- The Emotional and Psychological Effects of Breast Cancer Diagnosis and Treatment
- The Importance of Early Detection and Screening for Breast Cancer
- The Societal Stigma and Misconceptions Surrounding Breast Cancer
- Exploring Alternative and Complementary Therapies for Breast Cancer Patients
- The Influence of Support Networks and Advocacy Groups in Breast Cancer Awareness
- Analyzing the Economic and Social Burden of Breast Cancer on Patients and Families
- Debunking Common Myths and Misinformation about Breast Cancer
- The Role of Hormone Therapy in Breast Cancer Treatment
Concluding Thought
By writing essays on breast cancer, individuals can contribute to a better understanding of the disease, its impact, and the importance of ongoing research and support. Engaging with this topic through writing can help raise awareness, provide support, and inspire positive change within the community.
The Stages of Breast Cancer
Treatment and diagnosis of breast cancer, made-to-order essay as fast as you need it.
Each essay is customized to cater to your unique preferences
+ experts online
Breast Cancer: The Physical and Mental Effects
The ways of raising awareness about breast cancer, breast cancer: an unending battle that brought us together, the treatment of breast cancer, let us write you an essay from scratch.
- 450+ experts on 30 subjects ready to help
- Custom essay delivered in as few as 3 hours
The Benefits and Harmful Effects of Chemotherapy as a Treatment to Breast Cancer
Research on correlation of notch signaling pathway in the prognosis of breast cancer, hereditary breast and ovarian cancer, ultrasonography for the diagnosis of cancer, get a personalized essay in under 3 hours.
Expert-written essays crafted with your exact needs in mind
Mbmt Pilot Study: How This Affects The Breast Cancer Patient's Attention
Miracle in my life: my mother's battle with breast cancer, relevant topics.
- Mental Health
- Drug Addiction
- Affordable Care Act
- Teenage Pregnancy
By clicking “Check Writers’ Offers”, you agree to our terms of service and privacy policy . We’ll occasionally send you promo and account related email
No need to pay just yet!
We use cookies to personalyze your web-site experience. By continuing we’ll assume you board with our cookie policy .
- Instructions Followed To The Letter
- Deadlines Met At Every Stage
- Unique And Plagiarism Free
Breast Cancer and Its Population Burden Essay
Introduction, facts and figures, population impacts, environmental factors, social factors, potential solution to breast cancer.
The overriding objective of this paper is to provide a detailed discussion of the burden of breast cancer. The other objectives that are central to this paper are highlighted below:
- To determine which group is at a high risk of breast cancer
- To elucidate the impact of breast cancer on elderly women and those below the age of 45 years
- To highlight the possible solutions to the burden of breast cancer
- To discuss, in detail, some of the possible causes of breast cancer – environmental and social factors.
Breast cancer (BC) is regarded as the most common type of cancer globally. According to Mascara and Constantinou (2021), “about 2.3 million people are diagnosed with the disease each year” (p. 9). In the U.S., approximately 264000 and 2400 cancer cases are diagnosed each year among women and men, respectively (Mascara and Constantinou (2021, p. 6). African American women have a high mortality rate of breast cancer. The main facts about this condition are that it has a high survival rate, and women are at a higher risk than men for developing it.
While there are several types of cancer, breast cancer is regarded as the second leading cause of death among women. Women above 55 years are at a high risk of being diagnosed with breast cancer. More specifically, it is common after menopause – “longer exposure to estrogen increases a woman’s risk of breast cancer” (Madigan et al., 2020, p. 9). However, there are a few cases of this condition among women below 45 (Madigan et al., 2020, p. 9). In the U.S., for instance, about 9% of all the cases are recorded in women below 45 years
Most older women living with breast cancer are considered underdiagnosed and undertreated. This explains why this population has a low survival rate. According to Madigan et al. (2020), the majority of women who die of breast cancer are above 65 years. In addition to this, screening for this condition in the elderly population is very controversial. In fact, mammography is rarely performed in women between 65 and 70 years old (Madigan et al. (2020). Most of these women delay reporting the signs and symptoms of this condition – it is diagnosed at a more advanced stage.
The one known environmental factor that increases the risk of breast cancer is long exposure to ionizing radiation. According to Burstein et al. (2019), continued exposure to “environmental pollutants and toxic chemicals are possible risk factors for breast cancer.” However, the possibility of developing this condition depends largely on the period and type of exposure. Burstein et al.’s (2019) study focused on women exposed to polybrominated diphenyl ethers and bisphenol A. They noted that most women during the menopausal transition were at a high risk of developing breast cancer.
Social factors contribute a lot to the health and well-being of individuals. Among breast cancer patients, income and education, unemployment, social support, and neighborhood limitations are the main risks for breast cancer. Other social factors include food insecurity, poor housing, and lack of medical trust. Lack of social support, for instance, is associated with an increase in cancer-related deaths (Coughlin, 2019). This happens because most of them are socially isolated – they lack essential instrumental support. Overall, more affluent women, regardless of race, are at a higher risk of developing breast cancer.
The available solutions aim at reducing the risk of developing breast cancer. According to Montagnese et al. (2020), lifestyle changes are crucial to decreasing the risk of BC. The first possible solution requires one to maintain a healthy weight. For instance, healthy adults should strive to achieve at least 150 minutes of aerobic activity combined with up to 75 minutes of vigorous exercises (Montagnese et al., 2020). However, it is important to consult the healthcare provider regarding the available healthy strategies to help them accomplish the same.
Another possible solution to breast cancer, specifically for women below the age of 45 years, is through breastfeeding. More specifically, such women should consider breastfeeding for at least one year. This helps reduce the risk of breast cancer post-menopause. Similarly, hormone therapy in menopause should not be taken for the long term as it increases the risk of breast cancer – “whether estrogen is taken by itself or combined with progestin” (Jelly & Choudhary, 2019, p. 47). This presentation emphasizes that for those women who opt to take hormone therapy, it should be for the short-term.
As evidenced above, breast cancer is the second leading cause of death in women, especially those aged 65 years and above. Based on research, approximately 264000 and 2400 cancer cases are diagnosed each year among women and men, respectively. In addition to this, both environmental and social factors play a critical role in the development of breast cancer. For instance, ionizing radiation is one of the main environmental factors associated with this condition. Scholars recommend lifestyle changes combined with physical activity in an attempt to minimize the risk of being diagnosed with the condition.
Burstein, H. J., Curigliano, G., Loibl, S., Dubsky, P., Gnant, M., Poortmans, P., & Thurlimann, B. (2019). Estimating the benefits of therapy for early-stage breast cancer: The St. Gallen International Consensus Guidelines for the primary therapy of early breast cancer 2019 . Annals of Oncology , 30 (10), 1541-1557. Web.
Coughlin, S. S. (2019). Social determinants of breast cancer risk, stage, and survival . Breast cancer research and treatment , 177 (3), 537-548. Web.
Jelly, P., & Choudhary, S. (2019). Breastfeeding and breast cancer: A risk reduction strategy . Int J Med Paediatr Oncol , 5 (2), 47-50. Web.
Madigan, L. I., Dinh, P., & Graham, J. D. (2020). Neoadjuvant endocrine therapy in locally advanced estrogen or progesterone receptor-positive breast cancer: determining the optimal endocrine agent and treatment duration in postmenopausal women—A literature review and proposed guidelines . Breast Cancer Research , 22 (1), 1-13. Web.
Mascara, M., & Constantinou, C. (2021). Global perceptions of women on breast cancer and barriers to screening . Current Oncology Reports , 23 (7), 1-9. Web.
Montagnese, C., Porciello, G., Vitale, S., Palumbo, E., Crispo, A., Grimaldi, M., & Augustin, L. S. (2020). Quality of life in women diagnosed with breast cancer after a 12-month treatment of lifestyle modifications . Nutrients , 13 (1), 136. Web.
- Chicago (A-D)
- Chicago (N-B)
IvyPanda. (2023, December 28). Breast Cancer and Its Population Burden. https://ivypanda.com/essays/breast-cancer-and-its-population-burden/
"Breast Cancer and Its Population Burden." IvyPanda , 28 Dec. 2023, ivypanda.com/essays/breast-cancer-and-its-population-burden/.
IvyPanda . (2023) 'Breast Cancer and Its Population Burden'. 28 December.
IvyPanda . 2023. "Breast Cancer and Its Population Burden." December 28, 2023. https://ivypanda.com/essays/breast-cancer-and-its-population-burden/.
1. IvyPanda . "Breast Cancer and Its Population Burden." December 28, 2023. https://ivypanda.com/essays/breast-cancer-and-its-population-burden/.
Bibliography
IvyPanda . "Breast Cancer and Its Population Burden." December 28, 2023. https://ivypanda.com/essays/breast-cancer-and-its-population-burden/.
- Effects of Ionizing Radiation
- Issues in Menopause Analysis
- Benefits of Ionizing Radiation
- Ovulation Disorder, Menarche and Menopause
- Hillgrove High School Data Profile
- Breastfeeding and Children Immunity
- Discussion: Barriers to Breastfeeding
- Women Experiencing Menopause: A Support Group Formation
- Menopause and Associated Anatomical Changes
- Early Menopause and How to Treat Its Symptoms
- Pap Smear and Cervical Cancer: Oncology Nursing
- Colorectal Cancer Screening Methodology
- Cystic Fibrosis: A Comprehensive Overview of the Genetic Causes and Pathophysiology
- Screening Colonoscopy for Colorectal Cancer Prevention
- Prostate Cancer: Urinary Frequency and Incontinence

Introduction to Breast Cancer
Published: April 4, 2016
Description
Welcome to an Introduction to Breast Cancer! In this course, we’ll learn a bit about the leading cause of cancer in women worldwide – from the basic biology of the disease, to risk factors and prevention, to treatment modalities to survivorship. We’ll talk to leading experts, explore some of the milestone studies that have pushed this field forward, and have interactive discussions on discussion boards and social media. You’ll even have an opportunity to let us know what topics you want to cover on tweetchats, so we can try to make the content fit your interests.
There is something in this course for everyone – if you’re a breast cancer survivor or the friend/family member of someone with this disease, this course will help you to better understand this disease, and give you ideas for questions you may want to ask your doctor. Maybe you’re a healthcare provider or studying to be the same, this course is a great refresher on where the state of the science is. If you’re a healthcare administrator wondering about how the interdisciplinary components of breast cancer care fit together, or an entrepreneur thinking about unmet needs in this space, or someone in public health interested in prevention, this course is also for you!
Are you ready to learn a lot, and have some fun while we’re at it? If so, I hope you’ll join us! Let’s get started!!!
Course Takeaways
- Leading cause of cancer in women worldwide
- The basic biology of the disease
- Various risk factors and prevention, treatment and survivorship
Available on Coursera
Meet the Instructors

Anees B. Chagpar
Professor of Surgery (Oncology) MD, MBA, MPH, FACS, FRCS(C)
Also in this subject

Understanding Medical Research: Your Facebook Friend is Wrong

Evolution and Medicine

Addiction Treatment for Healthcare Providers

Anatomy of the Chest, Neck, Abdomen, and Pelvis
Introduction to Breast Cancer
Breast cancer is a malignant cell growth in the breast . If left untreated, the cancer spreads to other areas of the body. Excluding skin cancer , breast cancer is the most common type of cancer in women in the United States, accounting for one of every three cancer diagnoses.
An estimated 211,240 new invasive cases of breast cancer were expected to occur among women in the United States during 2005. About 1,690 new male cases of breast cancer were expected in 2005.
The incidence of breast cancer rises after age 40. The highest incidence (approximately 80% of invasive cases) occurs in women over age 50.
In addition to invasive breast cancer, 58,590 new cases of in situ breast cancer are expected to occur among women during 2005. Of these, approximately 88% will be classified as ductal carcinoma in situ ( DCIS ). The detection of DCIS cases is a direct result of the increased use of mammography screening . This screening method is also responsible for detection of invasive cancers at a less advanced stage than might have occurred otherwise.
An estimated 40,870 deaths (40,410 women, 460 men) were anticipated from breast cancer in 2005. Breast cancer ranks second among cancer deaths in women. According to the most recent data , mortality rates declined significantly during 1992-1998, with the largest decreases in younger women, both white and black.
Advertisement
Racial Disparities in Breast Cancer: from Detection to Treatment
- Published: 15 December 2023
- Volume 26 , pages 10–20, ( 2024 )
Cite this article
- JC Chen 1 ,
- Daniel G. Stover 2 ,
- Tarah J. Ballinger 3 ,
- Jose G. Bazan 4 ,
- Bryan P. Schneider 3 ,
- Barbara L. Andersen 5 ,
- William E. Carson 1 &
- Samilia Obeng-Gyasi ORCID: orcid.org/0000-0002-5330-7247 1 , 6
495 Accesses
13 Altmetric
Explore all metrics
Purpose of Review
Update on current racial disparities in the detection and treatment of breast cancer.
Recent Findings
Breast cancer remains the leading cause of cancer death among Black and Hispanic women. Mammography rates among Black and Hispanic women have surpassed those among White women, with studies now advocating for earlier initiation of breast cancer screening in Black women. Black, Hispanic, Asian, and American Indian and Alaskan Native women continue to experience delays in diagnosis and time to treatment. Further, racial discrepancies in receipt of guideline-concordant care, access to genetic testing and surgical reconstruction persist. Disparities in the initiation, completion, toxicity, and efficacy of chemotherapy, endocrine therapy, and targeted drug therapy remain for racially marginalized women.
Efforts to evaluate the impact of race and ethnicity across the breast cancer spectrum are increasing, but knowledge gaps remain and further research is necessary to reduce the disparity gap.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others

Survival among patients with untreated metastatic breast cancer: “What if I do nothing?”
Jennifer K. Plichta, Samantha M. Thomas, … E. Shelley Hwang

Awareness and current knowledge of breast cancer
Muhammad Akram, Mehwish Iqbal, … Asmat Ullah Khan
Breast Cancer: Epidemiology and Etiology
ZiQi Tao, Aimin Shi, … Jing Zhao
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• Giaquinto AN, Sung H, Miller KD, Kramer JL, Newman LA, Minihan A, et al. Breast Cancer Statistics, 2022. CA Cancer J Clin. 2022;72(6):524–41. https://doi.org/10.3322/caac.21754 . American Cancer Society’s most recent update on breast cancer statistics including incidence, screening behaviors, mortality, and survival.
Article PubMed Google Scholar
Giaquinto AN, Miller KD, Tossas KY, Winn RA, Jemal A, Siegel RL. Cancer statistics for African American/Black People 2022. CA Cancer J Clin. 2022;72(3):202–29. https://doi.org/10.3322/caac.21718 .
Duffy SW, Tabár L, Yen AM, Dean PB, Smith RA, Jonsson H, et al. Mammography screening reduces rates of advanced and fatal breast cancers: results in 549,091 women. Cancer. 2020;126(13):2971–9. https://doi.org/10.1002/cncr.32859 .
Ahmed AT, Welch BT, Brinjikji W, Farah WH, Henrichsen TL, Murad MH, et al. Racial disparities in screening mammography in the United States: a systematic review and meta-analysis. J Am Coll Radiol. 2017;14(2):157-65.e9. https://doi.org/10.1016/j.jacr.2016.07.034 .
Hensing WL, Poplack SP, Herman CR, Sutcliffe S, Colditz GA, Ademuyiwa FO. Racial differences in no-show rates for screening mammography. Cancer. 2021;127(11):1857–63. https://doi.org/10.1002/cncr.33435 .
Cancer Prevention & Early Detection Facts & Figures 2023-2024. Atlanta: American Cancer Society; 2023-2024.
Swami N, Nguyen T, Dee EC, Franco I, Baez YA, Lapen K, et al. Disparities in primary breast cancer stage at presentation among Hispanic subgroups. Ann Surg Oncol. 2022;29(13):7977–87. https://doi.org/10.1245/s10434-022-12302-9 .
•• Fayanju OM, Edmonds CE, Reyes SA, Arciero C, Bea VJ, Crown A, et al. The landmark series-addressing disparities in breast cancer screening: new recommendations for black women. Ann Surg Oncol. 2023;30(1):58–67. https://doi.org/10.1245/s10434-022-12535-8 . An overview of epidemiologic factors associated with breast cancer among Black women and their implications on screening, specifically reviewing evidence behind their recommendations to stratify risk and start screening earlier for Black women.
Chen T, Kharazmi E, Fallah M. Race and ethnicity-adjusted age recommendation for initiating breast cancer screening. JAMA Netw Open. 2023;6(4): e238893. https://doi.org/10.1001/jamanetworkopen.2023.8893 .
Article PubMed PubMed Central Google Scholar
La Frinere-Sandoval QNNB, Cubbin C, DiNitto DM. Racial and ethnic disparities in cervical and breast cancer screenings by nativity and length of U.S. residence. Ethn Health. 2023:1–17. https://doi.org/10.1080/13557858.2023.2174254 .
Lawson MB, Bissell MCS, Miglioretti DL, Eavey J, Chapman CH, Mandelblatt JS, et al. Multilevel Factors Associated With Time to Biopsy After Abnormal Screening Mammography Results by Race and Ethnicity. JAMA Oncol. 2022;8(8):1115–26. https://doi.org/10.1001/jamaoncol.2022.1990 .
Nguyen KH, Pasick RJ, Stewart SL, Kerlikowske K, Karliner LS. Disparities in abnormal mammogram follow-up time for Asian women compared with non-Hispanic white women and between Asian ethnic groups. Cancer. 2017;123(18):3468–75. https://doi.org/10.1002/cncr.30756 .
Article CAS PubMed Google Scholar
Ramirez AG, Pérez-Stable EJ, Talavera GA, Penedo FJ, Carrillo JE, Fernandez ME, et al. Time to definitive diagnosis of breast cancer in Latina and non-Hispanic white women: the six cities study. Springerplus. 2013;2(1):84. https://doi.org/10.1186/2193-1801-2-84 .
Dontchos BN, Achibiri J, Mercaldo SF, Wang GX, Lamb LR, Miles RC, et al. Disparities in same-day diagnostic imaging in breast cancer screening: impact of an immediate-read screening mammography program implemented during the COVID-19 pandemic. AJR Am J Roentgenol. 2022;218(2):270–8. https://doi.org/10.2214/AJR.21.26597 .
Dontchos BN, Narayan AK, Seidler M, Mercaldo SF, Miles RC, Ebert E, et al. Impact of a same-day breast biopsy program on disparities in time to biopsy. J Am Coll Radiol. 2019;16(11):1554–60. https://doi.org/10.1016/j.jacr.2019.05.011 .
Jackson DK, Li Y, Eskander MF, Tsung A, Oppong BA, Bhattacharyya O, et al. Racial disparities in low-value surgical care and time to surgery in high-volume hospitals. J Surg Oncol. 2021;123(2):676–86. https://doi.org/10.1002/jso.26320 .
Zaveri S, Nevid D, Ru M, Moshier E, Pisapati K, Reyes SA, et al. Racial disparities in time to treatment persist in the setting of a comprehensive breast center. Ann Surg Oncol. 2022;29(11):6692–703. https://doi.org/10.1245/s10434-022-11971-w .
Sukniam K, Kasbi AA, Ashary MA, Popp K, Attwood K, George A, et al. Disparities in time to treatment for breast cancer. Anticancer Res. 2022;42(12):5813–8. https://doi.org/10.21873/anticanres.16088 .
Stabellini N, Cullen J, Cao L, Shanahan J, Hamerschlak N, Waite K, et al. Racial disparities in breast cancer treatment patterns and treatment related adverse events. Sci Rep. 2023;13(1):1233. https://doi.org/10.1038/s41598-023-27578-4 .
Article ADS CAS PubMed PubMed Central Google Scholar
Corradini S, Reitz D, Pazos M, Schönecker S, Braun M, Harbeck N, et al. Mastectomy or breast-conserving therapy for early breast cancer in real-life clinical practice: outcome comparison of 7565 cases. Cancers (Basel). 2019;11(2). https://doi.org/10.3390/cancers11020160 .
Chervu N, Darbinian K, Sakowitz S, Verma A, Bakhtiyar SS, Shuch BM, et al. Disparate utilization of breast conservation therapy in the surgical management of early-stage breast cancer. Clin Breast Cancer. 2023. https://doi.org/10.1016/j.clbc.2023.04.008 .
Relation T, Obeng-Gyasi S, Bhattacharyya O, Li Y, Eskander MF, Tsung A, et al. Racial differences in response to neoadjuvant chemotherapy: impact on breast and axillary surgical management. Ann Surg Oncol. 2021;28(11):6489–97. https://doi.org/10.1245/s10434-021-09657-w .
Fwelo P, Nwosu KOS, Adekunle TE, Afolayan O, Ahaiwe O, Ojaruega AA, et al. Racial/ethnic and socioeconomic differences in breast cancer surgery performed and delayed treatment: mediating impact on mortality. Breast Cancer Res Treat. 2023;199(3):511–31. https://doi.org/10.1007/s10549-023-06941-z .
• Yu AYL, Thomas SM, DiLalla GD, Greenup RA, Hwang ES, Hyslop T, et al. Disease characteristics and mortality among Asian women with breast cancer. Cancer. 2022;128(5):1024–37. https://doi.org/10.1002/cncr.34015 . Evaluation of breast cancer characteristics and outcomes amongst Asian subgroups, highlighting the necessity to disaggregate Asian women.
Advani P, Bondy M, Thompson PA, Martínez ME, Nodora JN, Vernon SW, et al. Impact of acculturation on breast cancer treatment and survivorship care among Mexican American patients in Texas. J Cancer Surviv. 2018;12(5):659–68. https://doi.org/10.1007/s11764-018-0703-y .
Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Costantino JP, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol. 2010;11(10):927–33. https://doi.org/10.1016/S1470-2045(10)70207-2 .
Black DM, Jiang J, Kuerer HM, Buchholz TA, Smith BD. Racial disparities in adoption of axillary sentinel lymph node biopsy and lymphedema risk in women with breast cancer. JAMA Surg. 2014;149(8):788–96. https://doi.org/10.1001/jamasurg.2014.23 .
Grimmer L, Liederbach E, Velasco J, Pesce C, Wang CH, Yao K. Variation in contralateral prophylactic mastectomy rates according to racial groups in young women with breast cancer, 1998 to 2011: a report from the national cancer data base. J Am Coll Surg. 2015;221(1):187–96. https://doi.org/10.1016/j.jamcollsurg.2015.03.033 .
Scheepens JCC, Veer LV, Esserman L, Belkora J, Mukhtar RA. Contralateral prophylactic mastectomy: a narrative review of the evidence and acceptability. Breast. 2021;56:61–9. https://doi.org/10.1016/j.breast.2021.02.003 .
Kim Y, McCarthy AM, Bristol M, Armstrong K. Disparities in contralateral prophylactic mastectomy use among women with early-stage breast cancer. NPJ Breast Cancer. 2017;3:2. https://doi.org/10.1038/s41523-017-0004-z .
Verdial FC, Bartek MA, Anderson BO, Javid SH. Genetic testing and surgical treatment after breast cancer diagnosis: results from a national online cohort. J Surg Oncol. 2021;123(7):1504–12. https://doi.org/10.1002/jso.26372 .
Nikolaidis C, Duquette D, Mendelsohn-Victor KE, Anderson B, Copeland G, Milliron KJ, et al. Disparities in genetic services utilization in a random sample of young breast cancer survivors. Genet Med. 2019;21(6):1363–70. https://doi.org/10.1038/s41436-018-0349-1 .
Reid S, Cadiz S, Pal T. Disparities in genetic testing and care among black women with hereditary breast cancer. Curr Breast Cancer Rep. 2020;12(3):125–31. https://doi.org/10.1007/s12609-020-00364-1 .
Peterson JM, Pepin A, Thomas R, Biagi T, Stark E, Sparks AD, et al. Racial disparities in breast cancer hereditary risk assessment referrals. J Genet Couns. 2020;29(4):587–93. https://doi.org/10.1002/jgc4.1250 .
Centers for Medicare & Medicaid Services. Women's Health and Cancer Rights Act (WHCRA) Women's Health and Cancer Rights Act (WHCRA). 2023. CMS.gov. Retrieved November 10, 2023, from https://www.cms.gov/cciio/programs-and-initiatives/other-insurance-protections/whcra_factsheet
Sergesketter AR, Thomas SM, Lane WO, Orr JP, Shammas RL, Fayanju OM, et al. Decline in racial disparities in postmastectomy breast reconstruction: a surveillance, epidemiology, and end results analysis from 1998 to 2014. Plast Reconstr Surg. 2019;143(6):1560–70. https://doi.org/10.1097/PRS.0000000000005611 .
Article CAS PubMed PubMed Central Google Scholar
Lee C, Sunu C, Pignone M. Patient-reported outcomes of breast reconstruction after mastectomy: a systematic review. J Am Coll Surg. 2009;209(1):123–33. https://doi.org/10.1016/j.jamcollsurg.2009.02.061 .
Cordova LZ, Hunter-Smith DJ, Rozen WM. Patient reported outcome measures (PROMs) following mastectomy with breast reconstruction or without reconstruction: a systematic review. Gland Surg. 2019;8(4):441–51. https://doi.org/10.21037/gs.2019.07.02 .
Xie Y, Tang Y, Wehby GL. Federal health coverage mandates and health care utilization: the case of the women’s health and cancer rights act and use of breast reconstruction surgery. J Womens Health (Larchmt). 2015;24(8):655–62. https://doi.org/10.1089/jwh.2014.5057 .
Hamad A, Li Y, Tsung A, Oppong B, Eskander MF, Bhattacharyya O, et al. Hispanic ethnicity and breast cancer: disaggregating surgical management and mortality by race. J Racial Ethn Health Disparities. 2022;9(4):1568–76. https://doi.org/10.1007/s40615-021-01096-3 .
Johnstone T, Thawanyarat K, Rowley M, Francis S, Camacho JM, Singh D, et al. Racial Disparities in Postoperative Breast Reconstruction Outcomes: A National Analysis. J Racial Ethn Health Disparities. 2023. https://doi.org/10.1007/s40615-023-01599-1 .
Mets EJ, Chouairi FK, Gabrick KS, Avraham T, Alperovich M. Persistent disparities in breast cancer surgical outcomes among hispanic and African American patients. Eur J Surg Oncol. 2019;45(4):584–90. https://doi.org/10.1016/j.ejso.2019.01.016 .
Comprehensive Breast Reconstruction Act of 2021, H.R.469, 117th Congress, 2021.
Dehal A, Abbas A, Johna S. Racial disparities in clinical presentation, surgical treatment and in-hospital outcomes of women with breast cancer: analysis of nationwide inpatient sample database. Breast Cancer Res Treat. 2013;139(2):561–9. https://doi.org/10.1007/s10549-013-2567-1 .
Akinyemiju TF, Vin-Raviv N, Chavez-Yenter D, Zhao X, Budhwani H. Race/ethnicity and socio-economic differences in breast cancer surgery outcomes. Cancer Epidemiol. 2015;39(5):745–51. https://doi.org/10.1016/j.canep.2015.07.010 .
Oskar S, Nelson JA, Hicks MEV, Seier MSKP, Tan KS, Chu JJ, et al. The impact of race on perioperative and patient-reported outcomes following autologous breast reconstruction. Plast Reconstr Surg. 2022;149(1):15–27. https://doi.org/10.1097/PRS.0000000000008633 .
Montagna G, Zhang J, Sevilimedu V, Charyn J, Abbate K, Gomez EA, et al. Risk factors and racial and ethnic disparities in patients with breast cancer-related lymphedema. JAMA Oncol. 2022;8(8):1195–200. https://doi.org/10.1001/jamaoncol.2022.1628 .
Deldar RM, Spoer DM, Gupta NM, Towfighi PB, Boisvert MM, Wehner PM, et al. Prophylactic lymphovenous bypass at the time of axillary lymph node dissection decreases rates of lymphedema. Ann Surg Open. 2023;4(2): e278. https://doi.org/10.1097/AS9.0000000000000278 .
Jørgensen MG, Toyserkani NM, Sørensen JA. The effect of prophylactic lymphovenous anastomosis and shunts for preventing cancer-related lymphedema: a systematic review and meta-analysis. Microsurgery. 2018;38(5):576–85. https://doi.org/10.1002/micr.30180 .
Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233–41. https://doi.org/10.1056/NEJMoa022152 .
Yeboa DN, Xu X, Jones BA, Soulos P, Gross C, Yu JB. Trend in age and racial disparities in the receipt of postlumpectomy radiation therapy for stage I breast cancer: 2004–2009. Am J Clin Oncol. 2016;39(6):568–74. https://doi.org/10.1097/COC.0000000000000094 .
Parekh A, Fu W, Hu C, Shen CJ, Alcorn S, Rao AD, et al. Impact of race, ethnicity, and socioeconomic factors on receipt of radiation after breast conservation surgery: analysis of the national cancer database. Breast Cancer Res Treat. 2018;172(1):201–8. https://doi.org/10.1007/s10549-018-4881-0 .
Wakefield DV, Carnell M, Dove APH, Edmonston DY, Garner WB, Hubler A, et al. Location as destiny: identifying geospatial disparities in radiation treatment interruption by neighborhood, race, and insurance. Int J Radiat Oncol Biol Phys. 2020;107(4):815–26. https://doi.org/10.1016/j.ijrobp.2020.03.016 .
Lamm R, Woodward SG, Varshney K, Lyons W, Anne PR, George BJ, et al. A comparison of timely completion of hypofractionated and traditional adjuvant radiation therapy in early-stage breast cancer: evidence of impact on reducing racial and socioeconomic disparities. Surgery. 2022;172(1):31–40. https://doi.org/10.1016/j.surg.2022.03.019 .
Dee EC, Taunk NK, Chino FL, Deville C, McClelland S, Muralidhar V, et al. Shorter radiation regimens and treatment noncompletion among patients with breast and prostate cancer in the united states: an analysis of racial disparities in access and quality. JCO Oncol Pract. 2023;19(2):e197–212. https://doi.org/10.1200/OP.22.00383 .
• Chapman CH, Jagsi R, Griffith KA, Moran JM, Vicini F, Walker E, et al. Mediators of racial disparities in heart dose among whole breast radiotherapy patients. J Natl Cancer Inst. 2022;114(12):1646–55. https://doi.org/10.1093/jnci/djac120 . This study uses a statewide database to evaluate racial differences in receipt of breast radiotherapy, ultimately finding that Black and Asian women receive higher cardiac doses leading to greater cardiac events and deaths compared to White women.
Jagsi R, Griffith KA, Vicini F, Boike T, Burmeister J, Dominello MM, et al. Toward improving patients’ experiences of acute toxicity from breast radiotherapy: insights from the analysis of patient-reported outcomes in a large multicenter cohort. J Clin Oncol. 2020;38(34):4019–29. https://doi.org/10.1200/JCO.20.01703 .
Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351(27):2817–26. https://doi.org/10.1056/NEJMoa041588 .
Paik S, Tang G, Shak S, Kim C, Baker J, Kim W, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24(23):3726–34. https://doi.org/10.1200/JCO.2005.04.7985 .
Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med. 2018;379(2):111–21. https://doi.org/10.1056/NEJMoa1804710 .
• Kantor O, King TA, Freedman RA, Mayer EL, Chavez-MacGregor M, Korde LA, et al. Racial and ethnic disparities in locoregional recurrence among patients with hormone receptor-positive, node-negative breast cancer: a post hoc analysis of the TAILORx randomized clinical trial. JAMA Surg. 2023;158(6):583–91. https://doi.org/10.1001/jamasurg.2023.0297 . Post hoc analysis of TAILORx clinical trial noting racial discrepancies in locoregional recurrence rate, even upon adjustment for patient, tumor, and treatment factors, overall suggesting persistent differences despite similar access to care.
Hoskins KF, Danciu OC, Ko NY, Calip GS. Association of race/ethnicity and the 21-gene recurrence score with breast cancer-specific mortality among US women. JAMA Oncol. 2021;7(3):370–8. https://doi.org/10.1001/jamaoncol.2020.7320 .
Albain KS, Gray RJ, Makower DF, Faghih A, Hayes DF, Geyer CE, et al. Race, ethnicity, and clinical outcomes in hormone receptor-positive, HER2-negative, node-negative breast cancer in the randomized TAILORx trial. J Natl Cancer Inst. 2021;113(4):390–9. https://doi.org/10.1093/jnci/djaa148 .
Jasem J, Fisher CM, Amini A, Shagisultanova E, Rabinovitch R, Borges VF, et al. The 21-gene recurrence score assay for node-positive, early-stage breast cancer and impact of RxPONDER trial on chemotherapy decision-making: have clinicians already decided? J Natl Compr Canc Netw. 2017;15(4):494–503. https://doi.org/10.6004/jnccn.2017.0049 .
Abdou Y, WE Barlow, Gralow JR. Race and clinical outcomes in the RxPONDER Trial (SWOG S1007): clinical trial Rx for positive node, endocrine responsive breast cancer. 2022 San Antonio Breast Cancer Symposium. San Antonio, TX. 2022.
•• Reid S, Haddad D, Tezak A, Weidner A, Wang X, Mautz B, et al. Impact of molecular subtype and race on HR+, HER2- breast cancer survival. Breast Cancer Res Treat. 2021;189(3):845–52. https://doi.org/10.1007/s10549-021-06342-0 . This study evaluates PAM50-based genomic signatures amongst HR+, HER2- breast cancer, overall finding racial differences in subtypes that may contribute to mortality discrepancies. These findings also suggest the possible need to re-structure the current classification of breast cancer.
Killelea BK, Yang VQ, Wang SY, Hayse B, Mougalian S, Horowitz NR, et al. Racial differences in the use and outcome of neoadjuvant chemotherapy for breast cancer: results from the national cancer data base. J Clin Oncol. 2015;33(36):4267–76. https://doi.org/10.1200/JCO.2015.63.7801 .
Shubeck S, Zhao F, Howard FM, Olopade OI, Huo D. Response to treatment, racial and ethnic disparity, and survival in patients with breast cancer undergoing neoadjuvant chemotherapy in the US. JAMA Netw Open. 2023;6(3): e235834. https://doi.org/10.1001/jamanetworkopen.2023.5834 .
Terman E, Sheade J, Zhao F, Howard FM, Jaskowiak N, Tseng J, et al. The impact of race and age on response to neoadjuvant therapy and long-term outcomes in black and white women with early-stage breast cancer. Breast Cancer Res Treat. 2023;200(1):75–83. https://doi.org/10.1007/s10549-023-06943-x .
Zhao F, Miyashita M, Hattori M, Yoshimatsu T, Howard F, Kaneva K, et al. Racial disparities in pathological complete response among patients receiving neoadjuvant chemotherapy for early-stage breast cancer. JAMA Netw Open. 2023;6(3): e233329. https://doi.org/10.1001/jamanetworkopen.2023.3329 .
Knisely AT, Michaels AD, Mehaffey JH, Hassinger TE, Krebs ED, Brenin DR, et al. Race is associated with completion of neoadjuvant chemotherapy for breast cancer. Surgery. 2018;164(2):195–200. https://doi.org/10.1016/j.surg.2018.03.011 .
Vo JB, Ramin C, Lawrence WR, Barac A, Ho KL, Rhee J, et al. Racial and ethnic disparities in treatment-related heart disease mortality among US breast cancer survivors. JNCI Cancer Spectr. 2023;7(2). https://doi.org/10.1093/jncics/pkad024 .
Schneider BP, Shen F, Jiang G, O'Neill A, Radovich M, Li L, et al. Impact of genetic ancestry on outcomes in ECOG-ACRIN-E5103. JCO Precis Oncol. 2017;2017. https://doi.org/10.1200/PO.17.00059 .
Hu X, Kaplan CM, Martin MY, Walker MS, Stepanski E, Schwartzberg LS, et al. Race differences in patient-reported symptoms during chemotherapy among women with early-stage hormone receptor-positive breast cancer. Cancer Epidemiol Biomarkers Prev. 2023;32(2):167–74. https://doi.org/10.1158/1055-9965.EPI-22-0692 .
Yee MK, Sereika SM, Bender CM, Brufsky AM, Connolly MC, Rosenzweig MQ. Symptom incidence, distress, cancer-related distress, and adherence to chemotherapy among African American women with breast cancer. Cancer. 2017;123(11):2061–9. https://doi.org/10.1002/cncr.30575 .
Samuel CA, Schaal J, Robertson L, Kollie J, Baker S, Black K, et al. Racial differences in symptom management experiences during breast cancer treatment. Support Care Cancer. 2018;26(5):1425–35. https://doi.org/10.1007/s00520-017-3965-4 .
Emerson MA, Achacoso NS, Benefield HC, Troester MA, Habel LA. Initiation and adherence to adjuvant endocrine therapy among urban, insured American Indian/Alaska Native breast cancer survivors. Cancer. 2021;127(11):1847–56. https://doi.org/10.1002/cncr.33423 .
Sheppard VB, de Mendoza AH, He J, Jennings Y, Edmonds MC, Oppong BA, et al. Initiation of adjuvant endocrine therapy in black and white women with breast cancer. Clin Breast Cancer. 2018;18(5):337-46.e1. https://doi.org/10.1016/j.clbc.2017.12.002 .
Sheppard VB, Sutton AL, Hurtado-de-Mendoza A, He J, Dahman B, Edmonds MC, et al. Race and patient-reported symptoms in adherence to adjuvant endocrine therapy: a report from the women’s hormonal initiation and persistence study. Cancer Epidemiol Biomarkers Prev. 2021;30(4):699–709. https://doi.org/10.1158/1055-9965.EPI-20-0604 .
Yanez B, Gray RJ, Sparano JA, Carlos RC, Sadigh G, Garcia SF, et al. Association of modifiable risk factors with early discontinuation of adjuvant endocrine therapy: a post hoc analysis of a randomized clinical trial. JAMA Oncol. 2021;7(8):1–7. https://doi.org/10.1001/jamaoncol.2021.1693 .
Hu X, Walker MS, Stepanski E, Kaplan CM, Martin MY, Vidal GA, et al. Racial differences in patient-reported symptoms and adherence to adjuvant endocrine therapy among women with early-stage, hormone receptor-positive breast cancer. JAMA Netw Open. 2022;5(8): e2225485. https://doi.org/10.1001/jamanetworkopen.2022.25485 .
Wheeler SB, Spencer J, Pinheiro LC, Murphy CC, Earp JA, Carey L, et al. Endocrine therapy nonadherence and discontinuation in black and white women. J Natl Cancer Inst. 2019;111(5):498–508. https://doi.org/10.1093/jnci/djy136 .
Sadigh G, Gray RJ, Sparano JA, Yanez B, Garcia SF, Timsina LR, et al. Breast cancer patients’ insurance status and residence zip code correlate with early discontinuation of endocrine therapy: an analysis of the ECOG-ACRIN TAILORx trial. Cancer. 2021;127(14):2545–52. https://doi.org/10.1002/cncr.33527 .
Sadigh G, Gray RJ, Sparano JA, Yanez B, Garcia SF, Timsina LR, et al. Assessment of racial disparity in survival outcomes for early hormone receptor-positive breast cancer after adjusting for insurance status and neighborhood deprivation: a post hoc analysis of a randomized clinical trial. JAMA Oncol. 2022;8(4):579–86. https://doi.org/10.1001/jamaoncol.2021.7656 .
Hwang GS, Paranjpe R, Opsomer C, Lu K, Abajue U, Abughosh S, et al. Oral endocrine therapy agent, race/ethnicity, and time on therapy predict adherence in breast cancer patients in a large academic institution. Clin Breast Cancer. 2020;20(6):520–6. https://doi.org/10.1016/j.clbc.2020.06.004 .
Daly B, Olopade OI, Hou N, Yao K, Winchester DJ, Huo D. Evaluation of the quality of adjuvant endocrine therapy delivery for breast cancer care in the United States. JAMA Oncol. 2017;3(7):928–35. https://doi.org/10.1001/jamaoncol.2016.6380 .
Reeder-Hayes K, Peacock Hinton S, Meng K, Carey LA, Dusetzina SB. Disparities in use of human epidermal growth hormone receptor 2-targeted therapy for early-stage breast cancer. J Clin Oncol. 2016;34(17):2003–9. https://doi.org/10.1200/JCO.2015.65.8716 .
Al-Sadawi M, Hussain Y, Copeland-Halperin RS, Tobin JN, Moskowitz CS, Dang CT, et al. Racial and socioeconomic disparities in cardiotoxicity among women with her2-positive breast cancer. Am J Cardiol. 2021;147:116–21. https://doi.org/10.1016/j.amjcard.2021.02.013 .
Litvak A, Batukbhai B, Russell SD, Tsai HL, Rosner GL, Jeter SC, et al. Racial disparities in the rate of cardiotoxicity of HER2-targeted therapies among women with early breast cancer. Cancer. 2018;124(9):1904–11. https://doi.org/10.1002/cncr.31260 .
Lao C, Lawrenson R, Edwards M, Campbell I. Treatment and survival of Asian women diagnosed with breast cancer in New Zealand. Breast Cancer Res Treat. 2019;177(2):497–505. https://doi.org/10.1007/s10549-019-05310-z .
Alvarez A, Bernal AM, Anampa J. Racial disparities in overall survival after the introduction of cyclin-dependent kinase 4/6 inhibitors for patients with hormone receptor-positive, HER2-negative metastatic breast cancer. Breast Cancer Res Treat. 2023;198(1):75–88. https://doi.org/10.1007/s10549-022-06847-2 .
Schreier A, Munoz-Arcos L, Alvarez A, Sparano JA, Anampa JD. Racial disparities in neutrophil counts among patients with metastatic breast cancer during treatment with CDK4/6 inhibitors. Breast Cancer Res Treat. 2022;194(2):337–51. https://doi.org/10.1007/s10549-022-06574-8 .
Lee KWC, Lord S, Finn RS, Lim E, Martin A, Loi S, et al. The impact of ethnicity on efficacy and toxicity of cyclin D kinase 4/6 inhibitors in advanced breast cancer: a meta-analysis. Breast Cancer Res Treat. 2019;174(1):271–8. https://doi.org/10.1007/s10549-018-5054-x .
Sohn YJ, Chang CY, Miles RC. Current gaps in breast cancer screening among Asian and Asian American women in the United States. J Am Coll Radiol. 2021;18(10):1376–83. https://doi.org/10.1016/j.jacr.2021.06.002 .
Taparra K, Dee EC, Dao D, Patel R, Santos P, Chino F. Disaggregation of Asian American and Pacific Islander women with stage 0-ii breast cancer unmasks disparities in survival and surgery-to-radiation intervals: a national cancer database analysis from 2004 to 2017. JCO Oncol Pract. 2022;18(8):e1255–64. https://doi.org/10.1200/OP.22.00001 .
Cobb RJ, Thomas CS, Laster Pirtle WN, Darity WA. Self-identified race, socially assigned skin tone, and adult physiological dysregulation: assessing multiple dimensions of “race” in health disparities research. SSM Popul Health. 2016;2:595–602. https://doi.org/10.1016/j.ssmph.2016.06.007 .
Chen JC, Pawlik T, Kelly EP, Obeng-Gyasi S. Intersectionality in patients with cancer: who should care and why? Future Oncol. 2022;18(38):4137–40. https://doi.org/10.2217/fon-2022-0992 .
Download references
Author information
Authors and affiliations.
Division of Surgical Oncology, Department of Surgery, The Ohio State University Wexner Medical Center and James Cancer Hospital, Columbus, OH, USA
JC Chen, William E. Carson & Samilia Obeng-Gyasi
Department of Internal Medicine, The Ohio State University Wexner Medical Center and James Cancer Hospital, Columbus, OH, USA
Daniel G. Stover
Department of Internal Medicine, Indiana University, Indianapolis, IN, USA
Tarah J. Ballinger & Bryan P. Schneider
Department of Radiation Oncology, City of Hope, Duarte, CA, USA
Jose G. Bazan
Department of Psychology, The Ohio State University, Columbus, OH, USA
Barbara L. Andersen
The Ohio State University, N924 Doan Hall, 410 West 10th, Columbus, OH, 43210, USA
Samilia Obeng-Gyasi
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Samilia Obeng-Gyasi .
Ethics declarations
Conflict of interest.
Samilia Obeng-Gyasi and JC Chen are funded by The Ohio State University Comprehensive Cancer Center Pelotonia Grant. Samilia Obeng-Gyasi is also funded by the Paul Calabresi Career Development Award (K12 CA133250), Conquer Cancer Breast Cancer Research Foundation Advanced Clinical Research Award for Diversity and Inclusion in Breast Cancer Research, The Society of University Surgeons, and The American Cancer Society (RSG-22-106-01-CSCT). Tarah Ballinger reports personal fees from Medscape, MDEdge, and TerSera.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Chen, J., Stover, D.G., Ballinger, T.J. et al. Racial Disparities in Breast Cancer: from Detection to Treatment. Curr Oncol Rep 26 , 10–20 (2024). https://doi.org/10.1007/s11912-023-01472-8
Download citation
Accepted : 15 October 2023
Published : 15 December 2023
Issue Date : January 2024
DOI : https://doi.org/10.1007/s11912-023-01472-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Health inequities
- Breast cancer
- Find a journal
- Publish with us
- Track your research
Total Price
Margurite J. Perez

Still not convinced? Check out the best features of our service:

ORIGINAL RESEARCH article
Role and mechanism of ncapd3 in promoting malignant behaviors in gastric cancer.

- 1 Departments of Oncology Medicine, Fujian Medical University Union Hospital, Fuzhou, Fujian, China
- 2 Departments of Respiratory and Critical Care Medicine, Fujian Medical University Union Hospital, Fuzhou, Fujian, China
- 3 Department of General Surgery, Shengjing Hospital of China Medical University, Shenyang, Liaoning, China
- 4 Fujian Key Laboratory of Translational Research in Cancer and Neurodegenerative Diseases, Fuzhou, Fujian, China
Background: Gastric cancer (GC) is one of the major malignancies threatening human lives and health. Non-SMC condensin II complex subunit D3 ( NCAPD3 ) plays a crucial role in the occurrence of many diseases. However, its role in GC remains unexplored.
Materials and Methods: The Cancer Genome Atlas (TCGA) database, clinical samples, and cell lines were used to analyze NCAPD3 expression in GC. NCAPD3 was overexpressed and inhibited by lentiviral vectors and the CRISPR/Cas9 system, respectively. The biological functions of NCAPD3 were investigated in vitro and in vivo . Gene microarray, Gene set enrichment analysis (GSEA) and ingenuity pathway analysis (IPA) were performed to establish the potential mechanisms.
Results: NCAPD3 was highly expressed in GC and was associated with a poor prognosis. NCAPD3 upregulation significantly promoted the malignant biological behaviors of gastric cancer cell, while NCAPD3 inhibition exerted a opposite effect. NCAPD3 loss can directly inhibit CCND1 and ESR1 expression to downregulate the expression of downstream targets CDK6 and IRS1 and inhibit the proliferation of gastric cancer cells. Moreover, NCAPD3 loss activates IRF7 and DDIT3 to regulate apoptosis in gastric cancer cells.
Conclusion: Our study revealed that NCAPD3 silencing attenuates malignant phenotypes of GC and that it is a potential target for GC treatment.
1 Introduction
Currently, gastric cancer is one of the major malignancies threatening human lives and health ( Smyth et al., 2020 ; Sun et al., 2020 ). The global cancer statistics report (GLOBOCAN) showed that there were 1.04 million new cases of gastric cancer globally in 2018, making it the fifth most common malignancy, and 0.78 million deaths caused by this disease, making it the third leading cause of cancer death ( Bray et al., 2018 ). Gastric cancer (GC) is a multi-step process that is affected by Helicobacter pylori infection, host susceptibility, and other environmental factors. Gastric cancer is also a multifactorial process caused by the accumulation of a large number of genetic and epigenetic changes in oncogenes and tumor suppressor genes, which results in dysregulation in many signaling pathways, disruption of cell cycle, and disturbance of the equilibrium between proliferation and death ( Berger et al., 2016 ). Therefore, there are still many challenges in gastric cancer prevention and treatment, and how to improve gastric cancer diagnosis and treatment is still a global focus and hotspot. Tumor molecular biology studies have shown that tumorigenesis and tumor progression is an extremely complex biological behavior involving the participation of many genes, multifactorial interactions, and multi-stage development ( Bass et al., 2014 ). Therefore, in-depth understanding of the molecular biology mechanisms of gastric cancer occurrence and progression is vital in the search for more effective prevention and treatment measures.
Non-SMC condensin II complex subunit D3 ( NCAPD3 ) is a subunit of condensin II and the NCAPD3 gene is located in chromosome 13q25. Condensin II is a pentameric complex consisting of XCAPD3, CAP-G2, CAP-H2, SMC2, and SMC4 ( Zhang et al., 2014 ). Condensin complexes are divided into condensin I and condensin II. NCAPD3 and condensin II were first discovered in human HeLa cells and named by Ono et al. (2004) . In 2008, Maeshima et al. found that condensins play a critical role in chromosome condensation and separation during mitosis in eukaryotic cells ( Maeshima and Eltsov, 2008 ). In 2011, Abe et al. found that cyclin-dependent kinase-mediated NCAPD3 phosphorylation in the prophase of mitosis can result in chromosome condensation ( Abe et al., 2011 ). In 2015, Bakhreha et al. found that inducing a CAP-D3 T1403A mutation in the NCAPD3 ortholog in chicken DT40 cells can cause shortening of the mitotic chromosome axis, leading to disruption of cell division during the prophase ( Bakhrebah et al., 2015 ). Although little is known about the expression and role of NCAPD3 in human tumors, aberrant NCAPD3 expression and its potential effects have been observed in tumor tissues. In 2008, Lapointe pointed out that the postoperative recurrence rate is lower in prostate cancer patients with low postoperative NCAPD3 expression. Hence, NCAPD3 can be used as a prognostic predictor of prostate cancer after surgery ( Lapointe et al., 2008 ). In 2016, Dawkins et al. found that low NCAPD3 expression in pancreatic cancer is intimately associated with good prognosis ( Dawkins et al., 2016 ). However, there have been no studies on the correlation between NCAPD3 and gastric cancer.
In the present study, deep mining of gastric cancer and paracancerous tissue gene sequences in The Cancer Genome Atlas (TCGA) was performed, and RNA sequence data of gastric cancer and paracancerous tissues in the TCGA database were analyzed. The results showed that NCAPD3 is significantly upregulated in gastric cancer tissues. Then gastric cancer and normal gastric mucosal tissues were randomly selected from 67 gastric cancer patients who underwent radical subtotal gastrectomy or total gastrectomy in Fujian Medical University Union Hospital. These tissues were used for immunohistochemical staining. The results showed that NCPADC3 is highly expressed in gastric cancer tissues and is intimately associated with poor prognosis. These findings demonstrated the potential importance and clinical value of NCAPD3 in gastric cancer prevention and treatment.
Subsequently, in vitro and in vivo experiments were conducted on NCAPD3 to investigate the effects of NCAPD3 on gastric cancer cell proliferation, invasion, migration, and apoptosis through overexpression and knockout/knockdown experiments. To understand the potential molecular mechanisms of NCAPD3 knockdown on malignant cytological behavior in gastric cancer, advanced molecular biology techniques and gene chips were employed to measure the effects of NCAPD3 knockdown on gene expression and its related functional pathways, and the potential biological mechanisms of NCAPD3 were examined after obtaining the gene expression spectrum. Next, gene set enrichment analysis (GSEA) was employed to further elucidate the effects of NCAPD3 knockdown on canonical pathways, cellular components, and immune, oncogene, and transcription factor gene sets to examine the role of NCAPD3 knockdown in gastric cancer occurrence and progression at different levels ( Bustin et al., 2009 ; Liu et al., 2017 ; Zhang et al., 2021 ). In summary, this NCAPD3 research is expected to provide a new target for gastric cancer treatment and inspire new therapeutic strategies for in-depth basic research and new drug development in clinical practice for gastric cancer.
2 Materials and Methods
2.1 tcga data download.
Gastric cancer (stomach adenocarcinoma, STAD) RNAseq database and clinical information were downloaded from TCGA ( https://cancergenome.nih.gov/ ). As of 30 June 2018, there were 443 samples with useable gastric cancer data in the TCGA database, of which 416 were mRNA chip or RNAseq data samples, and 32 pairs were RNAseq v2 paired sample data with pathological information. Biological coefficient of variation (BCV) was observed for quality control, and 26 paired samples with stable data were selected. Expression spectrum analysis was performed based on this paired sample RNAseq data.
2.2 Experimental materials
AGS, SGC7901, MGC803, and BGC823 gastric cancer cell lines were purchased from Shanghai Genechem. RPMI 1640 culture medium, PBS, and fetal bovine serum were purchased from Hyclone (United States), and Opti-MEM culture medium was purchased from Gibco (United States). NCAPD3 overexpression lentivirus (LV- NCAPD3 ), blank control vector lentivirus (LV-NC1), shRNAs targeting the NCAPD3 gene (shRNA- NCAPD3 -1/2/3), single guide RNAs (sgRNAs) targeting human NCAPD3 (sgRNA- NCAPD3 -1/2/3) and their negative control (shRNA-NC and sgRNA-NC) were purchased from Shanghai Genechem. Supplementary Table S1 shows the sequences of shRNAs and sgRNAs. Knockout and Mutation Detection Kit were purchased from Shanghai Genesci Medical Technology. The TRIzol reagent was purchased from Shanghai Pufei Biotechnology. The reverse transcription kit was purchased from Promega (United States). PCR primers were synthesized by Shanghai Genechem. The MTT assay kit was purchased from Genview (United States). The AnnexinV-APC apoptosis assay kit was purchased from eBioscience (United States). Transwell chambers with matrigel were purchased from Corning (United States). NCAPD3 , TNFAIP3, FADD, IRS1, SMAD3, CD44, and MAP1LC3B antibodies were purchased from Abcam (United Kingdom). The CDK6 antibody was purchased from CST (United States). The FLAG antibody was purchased from Sigma-Aldrich (Germany). The GAPDH antibody was purchased from Santa-Cruz Biotechnology (United States). The marker (catalogue number 26619) was purchased from Thermo (United States).
2.3 Patients and tissue specimens
All clinical samples, including 67 pairs of gastric cancer and paracancerous tissues, were obtained from the tissue bank of Fujian Medical University Union Hospital. The application of archived cancer samples was approved by the Ethics Committee of Fujian Medical University Union Hospital (No. 2021WSJK042). In this study, no subjects received preoperative radiotherapy or chemotherapy. All resected specimens were stored at −80 °C for long-term storage. Written informed consents were obtained from all of patients or their guardians.
2.4 Gastric cancer cell culture
RPMI-1640/DMEM containing 10% fetal bovine serum and 1% penicillin and streptomycin solution was used for culture of the four gastric cancer cell lines (AGS, MGC803, BGC823, and SGC7901) in a 37 °C, 5% CO 2 incubator.
2.5 Cell transfection
The lentivirus plasmid containing full-length NCAPD3 was used for NCAPD3 overexpression, and the empty plasmid was used as a negative control. Additionally, shRNA plasmids targeting NCAPD3 were used for NCAPD3 knockdown, and shRNA plasmids containing non-specific scrambled shRNA sequences were used as a negative control (shNCs). Lentiviruses were used to transfect knockdown plasmids into GC cells. The culture medium was changed 6 h after lentivirus vectors were transfected into GC cells. Fetal bovine serum (FBS, 10%) was added to the new culture medium, and total RNA and total protein were extracted after 48 h of culture.
2.6 NCAPD3 gene knockout using the CRISPR/Cas9 system
The CRISPR/Cas9 system contains the LV-cas9-puro and LV-sgRNA-EGFP recombinant lentiviruses vectors. LV-cas9-puro carries a puromycin resistance gene and LV-sgRNA-EGFP contains an enhanced green fluorescent protein (EGFP) tag. The three single guide RNAs (sgRNAs) targeting human NCAPD3 were designed and synthesized by Shanghai Genechem. Sequencing was used to validate the sequences of the synthesized sgRNAs. The LV-cas9-puro and LV-sgRNA-EGFP vectors were constructed by Shanghai Genechem.
First, LV-cas9-puro lentiviruses were used to transfect AGS cells. Three days after transfection, a suitable amount of puromycin was used for 3 days of selection to obtain AGS cells with stable Cas9 expression. Following that, the three LV-sgRNA-EGFP lentiviruses were used to transfect Cas9-AGS cells. After 3 days of transfection, an inverted microscope was used to look for green fluorescent protein (GFP), and the percentage of green, fluorescent cells was calculated.
2.7 CruiserTM enzymatic cleavage experiment
The Knockout and Mutation Detection Kit was used to detect gene knockout 5 days after LV-sgRNA-EGFP lentivirus infection according to the manufacturer’s instructions. In brief, the genomic DNA extraction kit was used to extract genomic DNA for PCR amplification according to the manufacturer’s instructions. The PCR conditions used were as follows: pre-denaturation at 94 °C for 90 s, followed by 40 cycles of denaturation at 94 °C for 30 s, annealing at 60 °C for 30 s, and extension at 72 °C for 60 s, followed by final extension at 72 °C for 5 min. The PCR products were cooled to <40 °C. Then, 1 μL Cruiser™ was added to 3 μL PCR product, and the mixture was incubated at 45 °C for 20 min for enzymatic cleavage. Finally, 2% agarose gel electrophoresis was used to observe enzymatic cleavage. Supplementary Table S2 shows the PCR primer sequences.
2.8 MTT assay
Gastric cancer cells in the logarithmic growth phase were harvested and seeded at 1,500 cells/well in a 96-well plate. Triplicates were set up for every group, and the final volume of culture medium in each well was 100 µL. The cells were cultured under normal conditions. After 24, 48, 72, 96, and 120 h, 20 µL MTT (5 mg/mL) was added, and the 96-well plates were cultured normally for 4 h in an incubator. After that, the culture medium was carefully aspirated and 100 µL DMSO was added. The plates were incubated with shaking for 2–5 min before a microplate reader was used to read the optical density (OD) of each well at 490 nm.
2.9 Cell apoptosis assay
Pre-cooled PBS was used to wash the cells before trypsin was used for digestion and cells were collected. Following that, 4 °C pre-cooled D-Hanks solution was used to wash the cells, and then 1 × binding buffer was added. Cells were collected by centrifugation before 200 µL 1 × binding buffer was used to resuspend the cell pellet. Next, 10 µL AnnexinV-APC was added for staining. After incubating at room temperature in the dark for 10–15 min, a flow cytometer was used to measure changes in apoptosis rate in the various groups. This procedure was carried out in triplicate for each sample.
2.10 Transwell invasion assay
After cells had undergone trypsin digestion, serum-free culture medium was used for washing. Following that, serum-free culture medium was used to resuspend cells and enumeration was carried out. A cell suspension of 10 × 10 4 cells/200 μL was added to every Transwell chamber, and 650 μL of 30% FBS complete culture medium was added to the lower chamber, with triplicate wells for each group. The cells were incubated in a 37 °C incubator for 20 h. After 20 h of culture, 1 mL of 4% formaldehyde was added to every well, and room temperature fixation was carried out for 10 min. The cells were stained, the fixing solution was discarded, and 1× PBS was used to wash the cells once. Subsequently, 1 mL of 0.5% crystal violet solution was added to every well. At 30 min after staining, 1× PBS was used to wash the wells thrice. The plate was dried and observed. A cotton bud was used to gently remove cells that had not migrated in the Transwell chambers, and the chambers were observed under a 200 × microscope. Three random fields were selected per well for enumeration, and ImageJ was used for enumeration. Each expriment was done in triplicate.
2.11 Scratch assay
Cells in the logarithmic growth phase from the various experimental groups were digested with trypsin before complete culture medium was used to resuspend the cells (plating density was determined based on cell size to achieve >90% confluency on the following day). Next, cells were cultured in 96-well plates in a 37 °C and 5% CO 2 incubator, with quintuplicate wells per group and 100 μL/well. On the following day, a wound making tool was used to gently scratch the center of the lower part of wells in the 96-well plates. PBS was used to gently wash the plates 2–3 times, and 0.5% FBS culture medium was added. Photographs were taken, and Celigo was used to analyze the migration area at 0 h and 8 h after scratching.
2.12 Real-time fluorescence quantitative PCR
Trizol reagent was used to extract total RNA from the various groups, followed by reverse transcription to cDNA. Next, fluorescence quantitative PCR was carried out. The primer sequences for real-time PCR analysis are listed in Supplementary Table S3 . The reaction conditions were pre-denaturation at 95 °C for 30 s followed by 40 cycles of 95 °C for 5 s and 60 °C for 30 s. Triplicate wells were set up for every sample, and the 2 −ΔΔCT method was used to analyze RT-PCR data. Relative mRNA expression changes were calculated ( Bustin et al., 2009 ).
2.13 Western blotting (WB)
Cells in the logarithmic growth phase were collected and washed twice with PBS. The RIPA lysis buffer and protease inhibitor (or phosphatase inhibitor) mixture was used to extract total protein after transfection. The BCA assay kit was used for protein quantitation. Following that, SDS-PAGE was used to separate proteins (20–30 µg), and proteins were transferred to a PVDF membrane. The membrane was blocked with TBST buffer containing 5% skimmed milk at room temperature for 1 h. Next, the membrane was incubated with primary antibodies at 4 °C overnight. The membrane was washed four times with TBST followed by incubation with secondary antibody for 1.5 h. The membrane was washed four times with TBST, and the ECL reagent was used for luminescence, followed by development and imaging. Briefly, excess ECL solution was removed and membrane was put inside plastic wrap inside X-ray film cassette. Next, we expose membrane to the X-ray film in cassette in dark room for 1–2 min. We developed with the help of Carestream medical film processor using fixer and developer and then measure the band intensity using ImageJ.
2.14 Co-immunoprecipitation (Co-IP)
Co-IP was performed using the protein A/G plus-agarose immunoprecipitation kit (Santa Cruz Biotechnology, United States), according to the manufacturer’s instructions ( Liu et al., 2017 ). Briefly, the GC cells were lysed by RIPA lysis buffer, followed by total protein extraction. The concentration of proteins was detected using BCA assay kit according to previous studies ( Liu et al., 2017 ; Zhang et al., 2021 ). Then cell lysate was incubated overnight incubation at 4 °C with IP antibody, followed by incubation with 20 μL protein A/G PLUS-Agarose for 1 h to form an immune complex. The complexes were washed twice with RIPA lysis buffer and resuspended in 6× loading buffer, denatured for 10 min. The suspensions were further analyzed by Western blotting.
2.15 Immunohistochemistry
Selected gastric cancer tissue samples and their paired paracancerous tissue samples, which were fixed with 4% formaldehyde embedded in paraffin blocks, were made into 3 μm thick continuous sections. The sections were adhered to poly-L-lysine-coated glass slides and dried in a 70 °C oven for 4 h. NCAPD3 monoclonal antibody was purchased from Abcam PLC (United Kingdom). SP immunohistochemistry assay kit and DAB substrate were purchased from Fuzhou Maixin Biotech. Staining was performed according to the manufacturer’s instructions, and PBS was used instead of the primary antibody for the negative control. The positive control was provided by the company. Xylene, absolute ethanol, and PBS were analytical grade.
Immunohistochemistry staining of NCAPD3 was scored by two independent experienced pathologists. For each sample, the score of staining intensity was assigned as follows: 0, negative staining; 1, weak staining (light yellow); 2, moderate staining (yellow brown) and 3, strong staining (brown) ( Luo et al., 2020 ). And the percentage of stained cells was scored as 0 (<5% stained cells); 1 (5%–10% stained cells); 2 (11%–50% stained cells); 3 (51%–80% stained cells) and 4 (>80% stained cells). The final score was defined as staining score multiplied by proportion score ( Guo et al., 2021 ). Final scores of 0–4 and 6–12 were considered to be low and high expression, respectively ( Hou et al., 2014 ).
2.16 Animal experiments
Twenty five-week-old male nude mice were randomized into two groups: the negative control group in which untreated MGC803 gastric cancer cells were inoculated, and the NCAPD3 -knockdown group in which MGC803 gastric cancer cells that were transfected with NCAPD3 -shRNA lentivirus vector were inoculated. In each mouse, 4.0 × 10 6 cells were inoculated subcutaneously below the axilla. Tumor volume (volume = π/6×L×W×W, L: length, W: short axis, unit: mm 3 ) was calculated 30 days after inoculation for one to two times a week. On day 38, nude mice were euthanized by cervical dislocation after intraperitoneal injection of 2% pentobarbital sodium anesthesia, and the tumors were harvested and weighed. In order to minimize the bias from individual differences, the maximum and minimum mice in each group were removed from data analysis. All of the experimental protocols were approved by the Institutional Animal Care and Use Committee of Fujian Medical University (No. 2021-8CAARM125), and the animal experiments were conducted in Animal Center of Fujian Medical University.
2.17 Gene expression spectrum analysis
In order to obtain the NCAPD3 -regulated gene expression spectrum, AGS cells were transfected with sh NCAPD3 lentiviruses and control vector to construct AGS-KD and AGS-NC cells. Next, qPCR was used to validate the efficiency of RNA interference. The Trizol reagent was used to extract total RNA for an RNA quality test. The quality standards were: NanoDrop 2000 (Thermo Fisher Scientific, Waltham, MA, United States), 1.7 < A260/A280 < 2.2; Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, United States), RIN ≥7.0, 28S/18S > 0.7. The 3′ IVT Plus Kit (Affymetrix, Santa Clara, CA, United States), a reverse transcription kit, was used for labeling of RNA that passed the quality test according to the manufacturer’s instructions. The labeled RNAs were fragmented and hybridized. The Affymetrix GeneChip PrimeView human gene expression array was used for testing. The selection criteria for significantly differentially expressed genes were |Fold Change| ≥ 2.0 and FDR <0.05. The experiment was completed in Shanghai Genechem. GeneChip Scanner 3000 (Affymetrix) was used for data analysis.
In order to examine changes in overall biological processes and pathways after NCAPD3 knockdown, GSEA was carried out on expression data. First, the probe group with coefficient of variation >25% in the NCAPD3 knockdown group and control group were removed to obtain the filtered expression matrix. The filtered overall expression matrix was inputted into the GSEA. The MSigDB database was used for enrichment analysis of the background gene set, which is a combination of various gene sets, such as canonical pathway, cellular component, immunologic signatures, oncogenic signature, and transcription factor. The GSEA parameter settings were as follows: the permutation type was gene set, and “control vs. knockdown” was used for enrichment analysis. Therefore, the normalized enrichment score (NES) < 0 represents the degree of pathway enrichment of genes that were ranked in front of changes in the knockdown group. Default parameters were used for the other settings. FDR <0.05 was considered to indicate significant enrichment.
2.19 IPA analysis
The Ingenuity Pathway Analysis (IPA: Ingenuity Systems; www.ingenuity.com ; Redwood City, CA, United States) database was used for bioinformatics analysis of differentially expressed genes. Canonical pathway analysis was carried out by comparison of differentially expressed genes and pathways containing these genes, and comparison significance ( p < 0.05) was calculated to determine which pathways contained differentially expressed genes. Following that, the upstream and downstream regulatory factors of differentially expressed genes were compared. A Z-score ≥2 means that the pathway is significantly activated, whereas a Z-score ≤ −2 means that the pathway is significantly inhibited. The activation Z-score algorithm was used to analyze upstream regulatory factors to predict activation or inhibition of upstream regulatory factors. Disease and functional analysis was carried out according to IPA internal algorithm and standards. A Z-score ≥2 means that the disease or function is significantly activated, whereas a Z-score ≤ −2 means that the disease or function is significantly inhibited. Network map analysis was used to present the relationship between the disease and the differentially expressed gene. The Consistency Score is a measurement of the consistency and dense connection of causality between upstream regulatory factors and disease or function. The higher the Consistency Score, the more accurate the regulatory effect results. Therefore, IPA results were used to interpret and visualize interactions between upstream and downstream factors and global signal transduction.
2.20 Statistical methods
Excel 2016 was used for data processing, SPSS 22.0 was used for statistical analysis, GraphPad prism 6 was used for plotting of statistical graphs, and ImageJ was used for measurement of the migration area. Statistical description of quantitative data (mean ± standard deviation) was carried out. One-way ANOVA or t -test was used for comparison of inter-group differences. Qualitative data were described using ratios, and Chi-square test or Fisher’s exact probability test were used to compare differences. A difference with p < 0.05 was considered to be statistically significant.
3.1 NCAPD3 expression is increased in gastric cancer and is closely related to prognosis
Deep mining of gastric cancer and paracancerous tissue gene sequences in The Cancer Genome Atlas (TCGA) was performed, and RNA sequence data of gastric cancer and paracancerous tissues in the TCGA database were analyzed. The result showed that NCAPD3 expression level in gastric cancer tissues is significantly higher than paired paracancerous tissues, with a log2-fold change of 2.242 and FDR <0.01 ( Figure 1A ). In 26 pairs of sequencing samples, the expression level of NACPAD3 in 17 pairs of gastric cancer tissues was significantly higher than paired paracancerous tissues ( Figure 1B ).
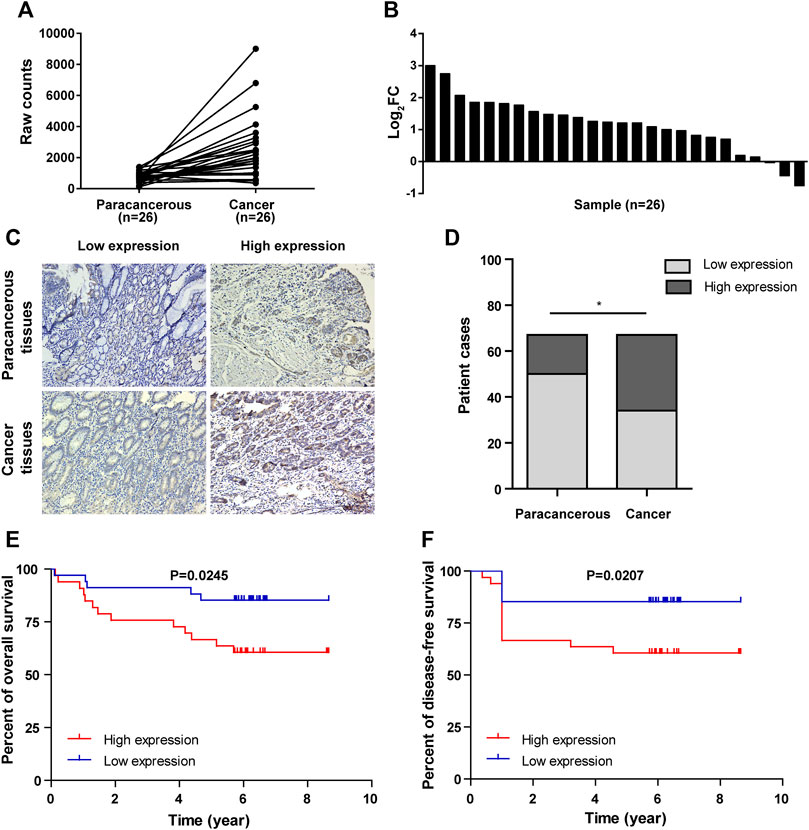
Figure 1 . NCAPD3 expression in gastric cancer tissues. (A) Line chart of differential expression of NCAPD3 in 26 pairs of sequencing samples (gastric cancer and matched paracancerous samples) from TCGA. (B) Bar chart of differential expression of NCAPD3 in 26 pairs of sequencing samples (gastric cancer and matched paracancerous samples) from TCGA. FC (fold change): ratio of expression level in cancerous samples to paracancerous samples. (C) Relative protein expression of NCAPD3 in gastric cancer tissues (n = 67) compared with paracancerous normal tissues (n = 67) with immunohistochemistry. (D) Chart of positive immunohistochemical rates and the associated statistics. A total of 49% (33/67) of gastric cancer tissues were positive for NCAPD3 expression, while 25% (17/67) of normal tissues were positive for NCAPD3 . * p < 0.05. (E) Kaplan-Meier overall survival (OS) according to NCAPD3 expression in 67 gastric cancer patients. (F) Kaplan-Meier Disease-free survival (DFS) by NCAPD3 expression in 67 gastric cancer patients.
We then detected NCAPD3 protein expression in 67 pairs of gastric cancer tissues and paracancerous tissues with immunohistochemistry. Representative IHC images posited that NCAPD3 protein was primarily expressed in the cytoplasm of gastric cancer cells ( Figure 1C ). The analysis results of Chi-square test revealed that The NCAPD3 -positive expression rate in 67 gastric cancer patients was significantly higher than in paracancerous normal gastric mucosal tissues ( p = 0.004) ( Figure 1D ). According to the relevant NCAPD3 expression in tumor tissues, 67 gastric cancer patients were classified into two groups: low-expression group (n = 34) and high-expression group (n = 33), the correlation between NCAPD3 expression and clinicopathological characteristics in gastric cancer patients were shown in Table 1 . Expression of NCAPD3 was identified to be correlated with invasion depth ( p = 0.009), lymph node metastases ( p = 0.029) and pathological TNM stage ( p < 0.001) but not with other clinicopathological characteristics in patients with gastric cancer. As for prognosis, the patients with NCAPD3 -high expression had a significantly worse overall survival (OS) and a high risk of relapse than the NCAPD3 -low patients. The overall survival rate for the NCAPD3 -low patients was 85.3 percent, as compared with 60.6 percent for the patients with high NCAPD3 expression ( p = 0.0245); the five-year disease-free survival (DFS) rate was 85.3 percent, as compared with 63.6 percent ( p = 0.0207 by the log-rank test), respectively ( Figures 1E, F ).
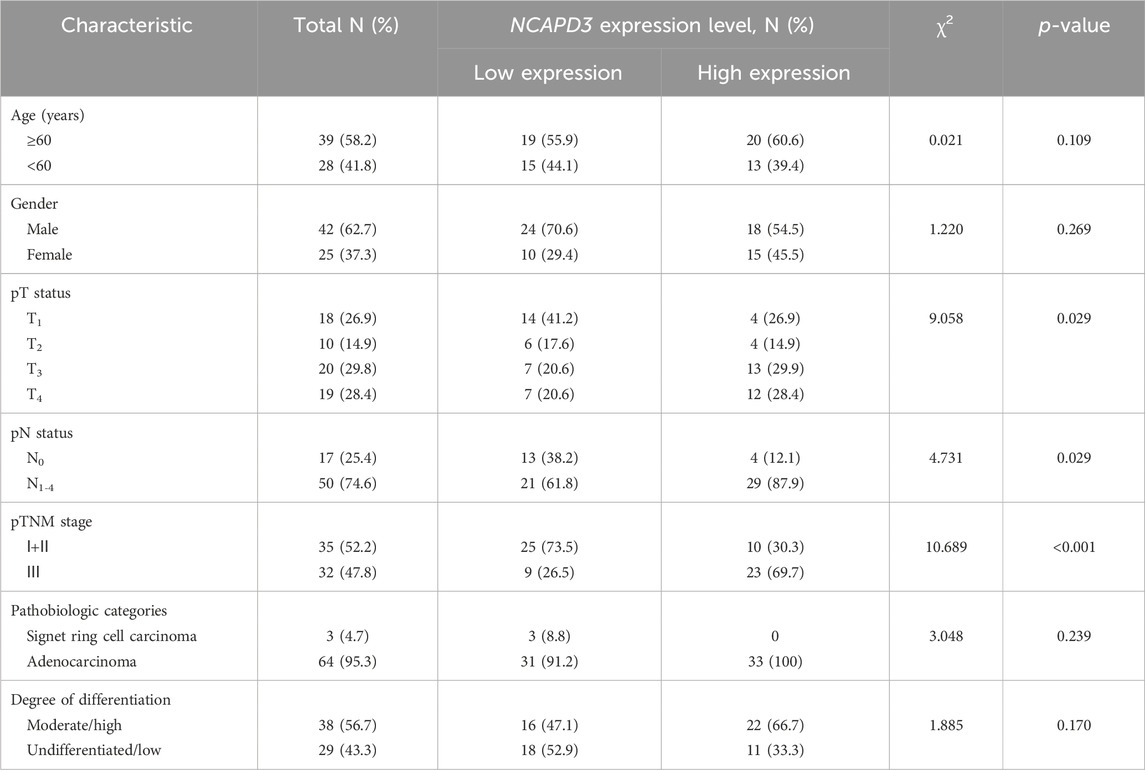
Table 1 . Association of NCAPD3 with clinicopathological characteristics from 67 gastric cancer patients.
3.2 NCAPD3 overexpression promotes the malignant phenotype of GC cells
To examine the function of NCAPD3 in gastric cancer, first, we measured NCAPD3 expression in four cancer-derived GC cell lines (AGS, BGC823, MGC803, and SGC7901) by RT-qPCR and found that the expressions of NCAPD3 mRNA in these four gastric cancer were all high-abundence ( Supplementary Material 1). Additionally, AGS cells had a lower level of NCAPD3 mRNA relative to the other cells, while MGC803 cells expressed NCAPD3 at a much higher level ( Figure 2A ). Therefore, AGS and MGC803 cells were selected for the subsequent studies.
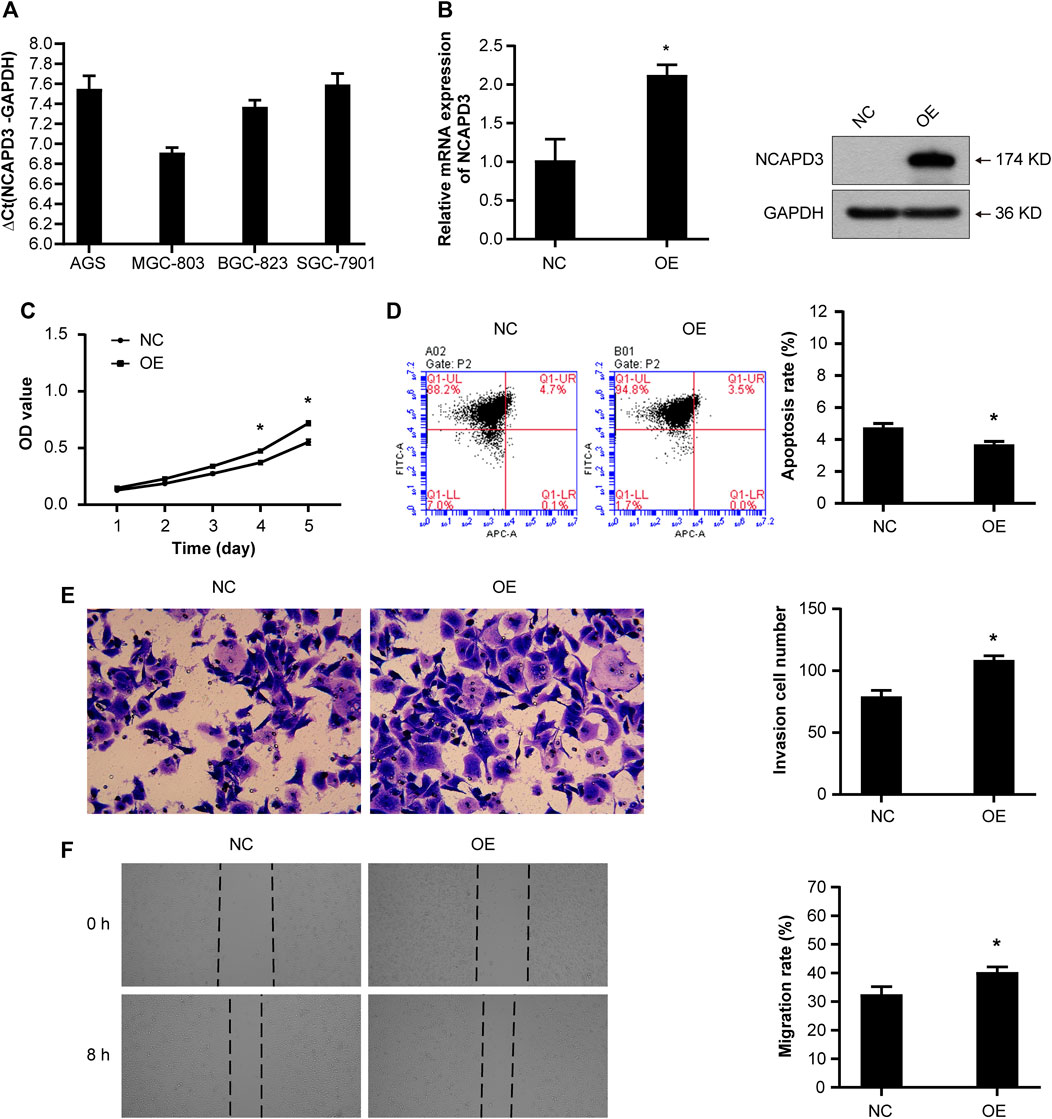
Figure 2 . NCAPD3 overexpression promotes the malignant phenotype of GC cells. (A) NCAPD3 expression in four gastric cancer cell lines (AGS, BGC823, MGC803, and SGC7901) determined by RT-qPCR. The larger the ΔCt (NCAPD3-GAPDH) , the lower the abundance of mRNA expression in cells: ΔCt≤12, high abundance; 12<ΔCt <16, medium abundance; ΔCt ≥16, low abundance. (B) mRNA and protein expression following NCAPD3 overexpression in AGS cells examined by RT-qPCR and Western blot analysis. NCAPD3 protein detected here was exogenous. (C) The proliferation of AGS cells was measured by MTT assay and presented as OD490 nm absorbance. (D) AnnexinV-APC apoptosis assay was applied to measure the effects of NCAPD3 overexpression on apoptosis in AGS cells. (E) The invasive potentials of AGS cells were determined by Transwell assays. (F) Scratch assays were performed in AGS cells to measure the effects of NCAPD3 overexpression on migration. NC: negative control. OE: NCAPD3 overexpression. * p < 0.05.
Then we established the stable AGS NCAPD3 overexpression cell line by lentiviral transduction. As shown in Figure 2B , NCAPD3 mRNA and protein expressions were significantly increased in cells transfected with NCAPD3 virus (oe NCAPD3 ) compared with negative control virus (NC). Proliferative and apoptosis abilities were evaluated using MTT assays and flow cytometry assays, respectively. Indeed, the overexpression of NCAPD3 enhanced the viability and reduced the apoptosis of GC cells ( Figures 2C, D ). The potentials of migration and invasion were also promoted by overexpression of NCAPDS in AGS cells. These effects were validated by Transwell invasion assays and scratch assays ( Figures 2E, F ).
3.3 Silencing NCAPD3 inhibits GC cell malignant biological behaviors
To further determine the oncogenic properties of NCAPD3 in GC, we used specific short hairpin RNAs (shRNAs) to transfect AGS and MGC803 cells. As demonstrated by RT-qPCR and Western blotting assays, NCAPD3 expression was successfully suppressed in both sh NCAPD3 -treated AGS and MGC 803 cells ( Figure 3A ). Then the Effects of NCAPD3 knockdown on malignant biological behaviors of gastric cancer cells were detected by MTT, flow cytometry assays, Transwell invasion assays and scratch assays. As shown in Figures 3B–E , NCAPD3 silencing significantly inhibited proliferation, enhanced apoptosis, and impaired the invasion and migration abilities of GC cells. The results were further verified by the CRISPR/Cas9 technology, which was used to construct AGS cell with stable NCAPD3 knockout ( Supplementary Figure S1 ).
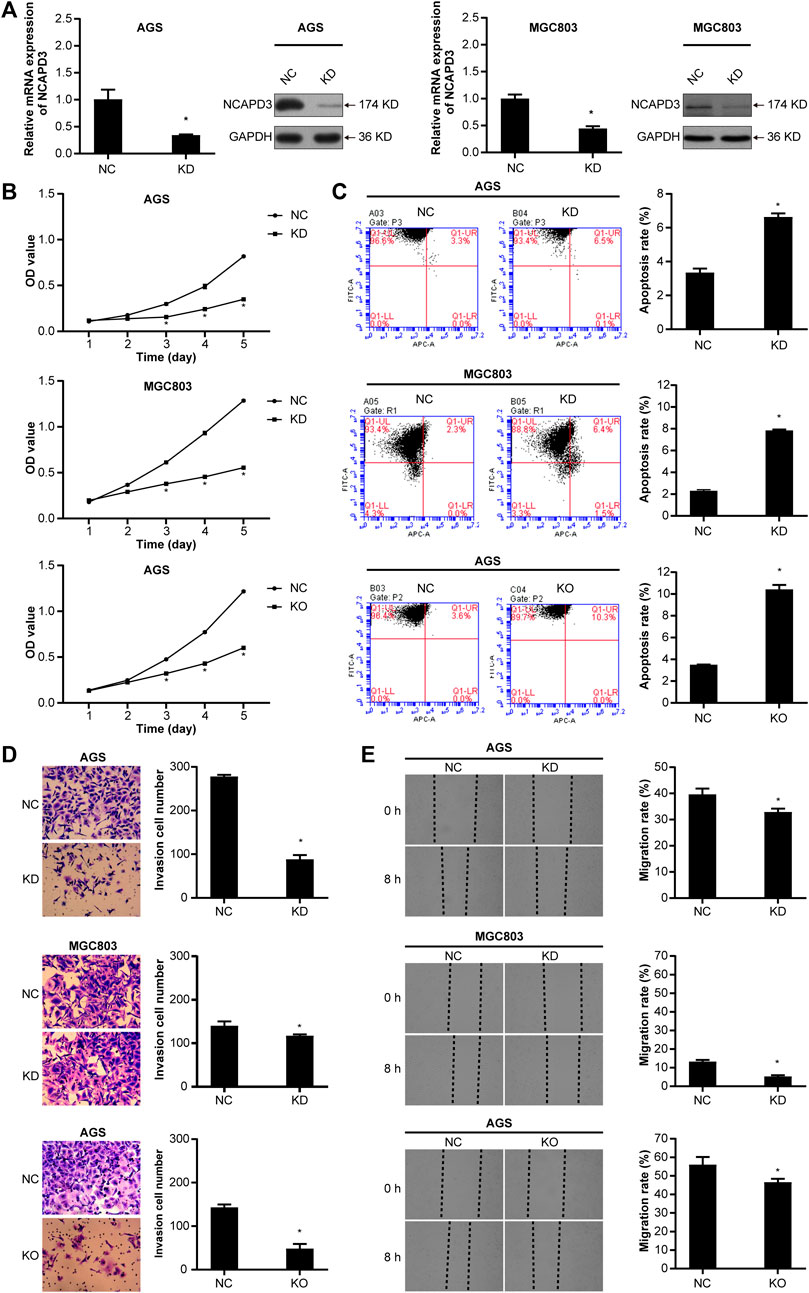
Figure 3 . Silencing NCAPD3 inhibits gastric cancer cell malignant biological. (A) RT-PCR and WB were performed to assess knockdown results. (B) MTT was used to measure the effects of NCAPD3 knockdown or knockout on gastric cancer cell proliferation. (C) AnnexinV-APC apoptosis assay was conducted to measure the effects of NCAPD3 knockdown or knockout on apoptosis in gastric cancer cells. (D) Transwell invasion assay was applied to assess the effects of NCAPD3 knockdown or knockout gastric cancer cell invasion. (E) The migratory potentials of NCAPD3 -knockdown/knockout AGS and MGC803 cells were determined by Scratch assays. NC: negative control. KD: NCAPD3 knockdown. KO: NCAPD3 knockout. * p < 0.05.
3.4 Effects of NCAPD3 knockdown on MGC803 gastric cancer subcutaneous xenografts in nude mice
MGC803 gastric cancer cells that were transfected with the empty lentivirus vector (negative control) and NCAPD3 -shRNA lentivirus vector were inoculated subcutaneously in nude mice, and tumorigenicity was observed ( Figure 4 ). The growth curve of subcutaneous tumor xenografts was plotted based on xenograft volume at different time points. Results showed that the growth speed of the NCAPD3 knockdown group was significantly lower than the negative control group ( p < 0.05).
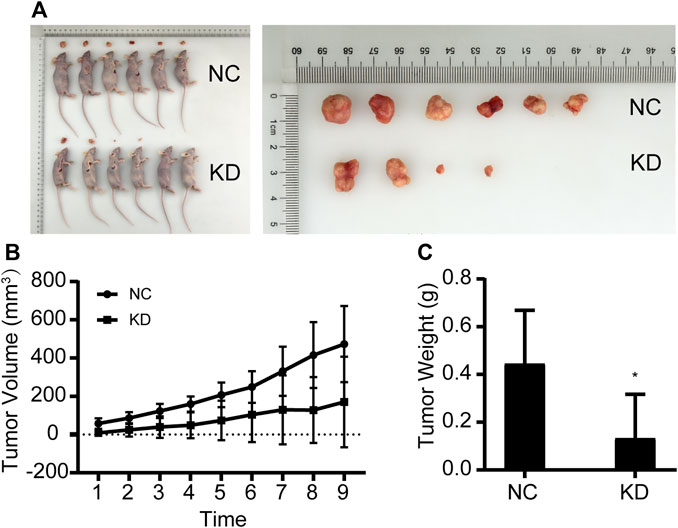
Figure 4 . Effects of NCAPD3 knockdown on BGC823 gastric cancer subcutaneous xenografts in nude mice. (A) Macroscopic appearance of representative tumor specimens at autopsy. (B) Tumor growth curves for negative control (NC) group and NCAPD3 knockdown (KD) group mice. (C) Effect of NCAPD3 knockdown on tumor weight. Data are mean ± SD of 6 animals per group. NC: negative control. KD: NCAPD3 knockdown. * p < 0.05.
Tumors were harvested and weighed after nude mice were euthanized. Results showed that the tumor weight in the NCAPD3 knockdown group was significantly lower than the negative control group ( p < 0.05). The tumor weight inhibition rate was 70.21%.
3.5 Gene expression spectrum analysis of NCAPD3 silencing
In order to study the molecular mechanisms of malignant biological behavior in gastric cancer regulated by NCAPD3 , GeneChip PrimeView human gene expression array was used to detect differentially expressed genes (DEGs) before and after NCAPD3 knockdown. The results showed that, after NCAPD3 expression was inhibited, there were significant differences in the expression levels of 1,411 genes in AGS cells, among which 562 genes were upregulated and 849 genes were downregulated after NCAPD3 silencing ( Figures 5A, B ). Hierarchical clustering was used to analyze the expression of differentially expressed genes. Results showed that there were significant changes in gene expression upregulation and downregulation ( Figure 5C ).
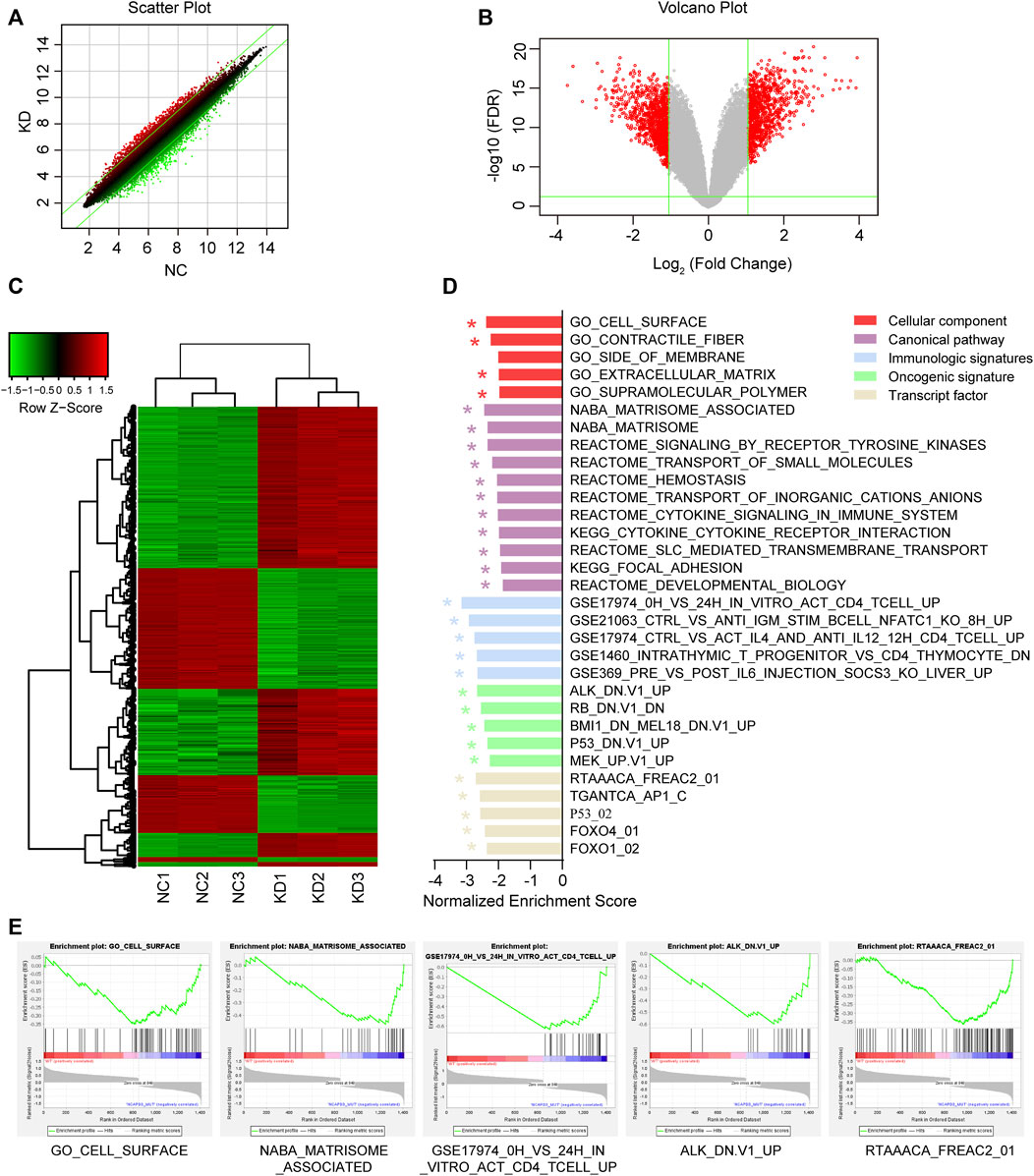
Figure 5 . Differential expression analysis and GSEA enrichment results of differentially expressed genes (DEGs) before and after NCAPD3 knockdown. (A) Scatter plot. The green line represents the reference line for differential expression, the red dots represent relatively upregulated groups in KD, and the green dots represent upregulated groups in NC. (B) Volcano plot. Red represents significant differentially expressed genes, and green represents non-significant differentially expressed genes. (C) Cluster analysis heat map. Red represents upregulated gene expression, green represents downregulated gene expression, and black represents no significant change in gene expression. (D) The GSEA enrichment results shown in a bar chart. (E) Five GESA enrichment plots of typical pathways in the canonical pathway, cellular component, immunologic signatures, oncogenic signature, and transcription factor. NC: negative control. KD: NCAPD3 knockdown. * FDR <0.05.
In order to examine changes in overall biological processes and pathways after NCAPD3 knockdown, GSEA analysis was performed on the cellular component, canonical pathway, immunologic signatures, oncogenic signature, and transcription factor ( Figures 5D, E ). Compared with the non-knockdown group, five significant enrichment results were obtained in the cellular component GSEA after NCAPD3 knockdown, among which cell surface sets were significantly enriched. A total of 11 significant signaling pathways were obtained from the canonical pathway analysis, such as signaling by receptor tyrosine kinases, cytokine signaling in the immune system, and cytokine-cytokine receptor interaction. Immunologic signatures enrichment analysis showed that 119 immune gene sets were significantly enriched in the NCAPD3 knockdown group, showing that NCAPD3 significantly activates immune-related gene sets; this result is consistent with the canonical pathway enrichment analysis results. Twenty-six significantly enriched oncogene sets were obtained from oncogenic signature enrichment analysis, and the top five significantly enriched gene sets include ALK, RB, BMI1, P53, and MEK. Thirteen significant enrichment results were obtained from transcription factor analysis. Figure 5D presents the top five significantly enriched transcription factor gene sets, such as P53, FOXO4, and FOXO1. These results showed that NCAPD3 knockdown affects many signaling pathways and biological processes. Furthermore, NCAPD3 can affect the expression of oncogenes and transcription factors to affect disease occurrence and development.
3.6 IPA analysis of differentially expressed genes relative to classical pathways, upstream regulators, disease and function, and regulatory effect
The IPA platform was used to analyze differentially expressed genes in the negative control (NC) group and NCAPD3 knockdown (KD) group. The canonical pathway analysis results showed that there were 10 signaling pathways that showed differences after NCAPD3 knockdown in the KD group. Pathways that were mainly activated were the p53 signaling, G1/S cell cycle checkpoint, and STAT3 pathways. Pathways that were inhibited mainly included the phosphatidylglycerol biosynthesis II (non-plastidic), IGF-1 signaling, and superpathway of cholesterol biosynthesis, among which the cholesterol biosynthesis pathway was significantly inhibited, with a Z-score of −2.449 ( Figure 6A ).
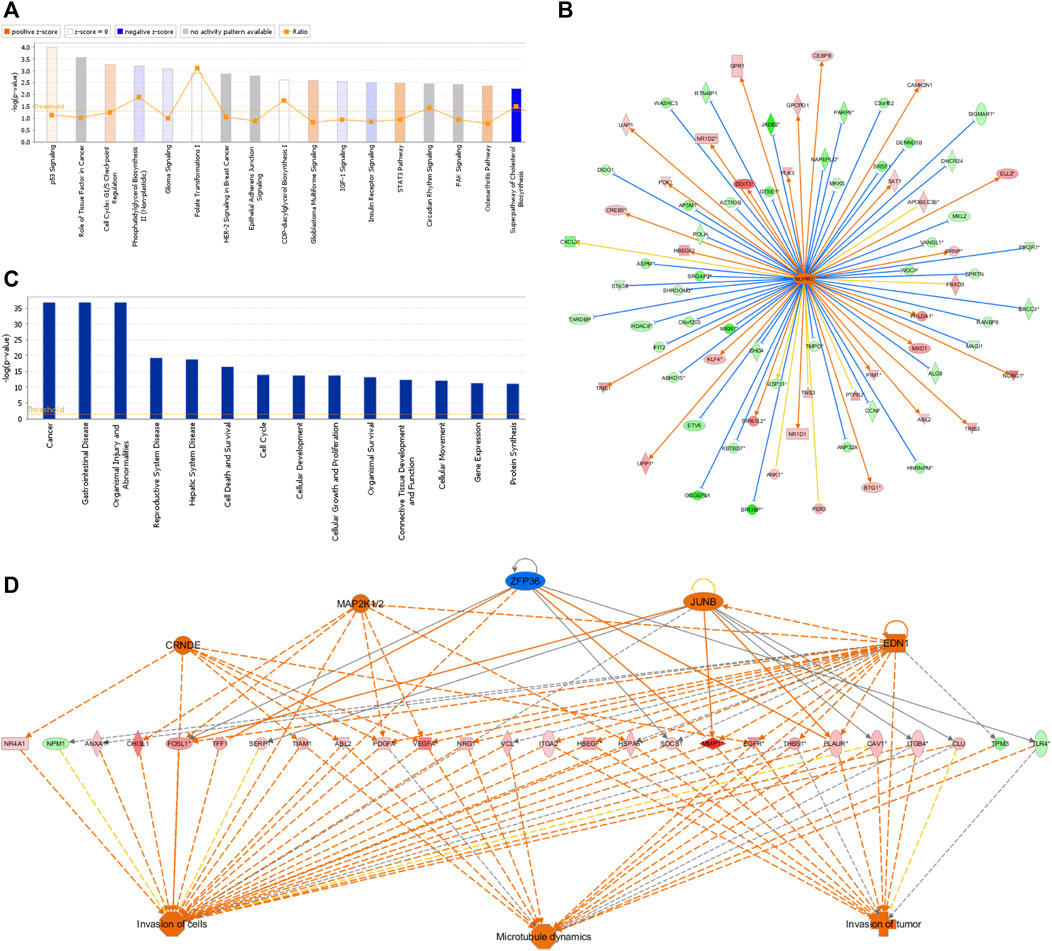
Figure 6 . IPA analysis results. (A) Canonical pathway enrichment analysis statistical chart. Orange represents pathway activation and blue represents pathway inhibition. (B) Network map of upstream regulatory factors of NUPR1. An orange line means that the upstream regulatory factor and gene were consistently activated, a blue line means that the upstream regulatory factor and gene were consistently inhibited, and a yellow line means that the expression trend of the upstream regulatory factor and gene were not consistent. (C) Disease and functional enrichment statistical graph. (D) Interactions between genes and regulatory factors and functions.
In order to determine the major upstream regulatory factors and explain differential gene expression between the two groups, upstream regulatory factor analysis was carried out. This analysis determined the number of known targets for each regulatory factor, and their direction of change was compared with that found in past papers. One hundred forty-five upstream regulators (including transcription factors, small RNAs, cytokines, kinases, and chemical molecules and drugs) were identified as activators, and 45 upstream regulators were predicted as suppressors. The prediction results for top 10 activated or inhibited upstream regulatory factors were listed ( Table 2 ), among which the transcription factor NUPR1 was predicted to be significantly activated (Z-score = 2.252). Then an interaction network between the NUPR1 upstream regulatory factor and its downstream factors was constructed ( Figure 6B ). Among these genes, ELL2 , NDRG1 , HBEGF , SAT1 , NR1D1 , PDK1 , and DDIT3 were elevated, whereas PARP9 , NAPEPLD , MKKS , PIK3R1 , MAGI1 , HDAC8 , ASPM were decreased ( Figure 6B ).
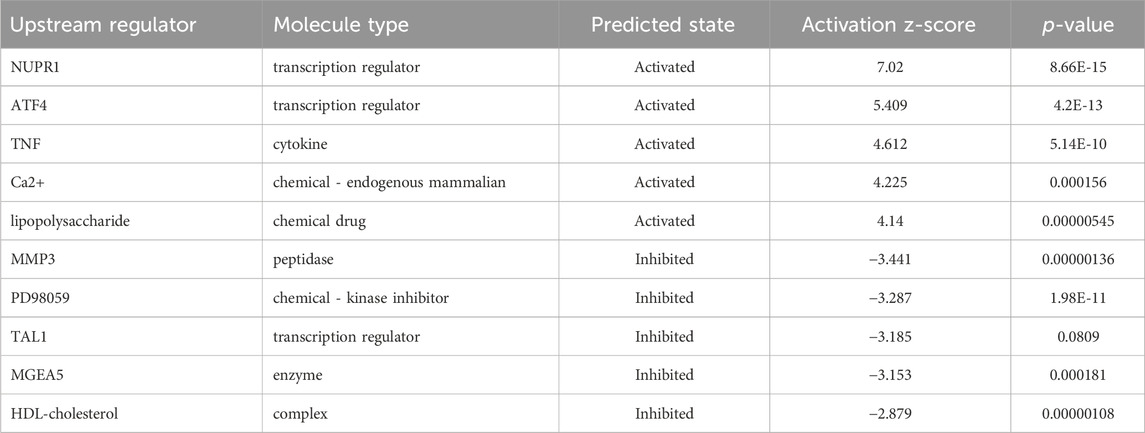
Table 2 . Predicted upstream regulators for all differentially expressed genes between NCAPD3 -knockdown and negative control AGS cells (top 10).
In the disease and functional analysis, the first analysis showed significantly enriched differentially expressed genes in disease and function, which were mainly enriched in gastrointestinal disease, cell death and survival, cell cycle, cellular development, cellular growth and proliferation, and cellular movement ( Figure 6C ). This shows that differentially expressed genes that are regulated by inhibiting NCAPD3 expression mainly participate in cell growth, development, and survival. Regulatory effect analysis was used to investigate differentially expressed genes participating in different cellular functions. Results showed that the regulatory factors CRNDE, EDN1, JUNB, and MAP2K1/2, ZFP36 mainly regulate differentially expressed genes ( VEGFA , EGFR , PLAUR ) to regulate invasion of cells, invasion of tumor, and microtubule dynamics. For example, the regulatory factor CRNDE regulates the EGFR gene to regulate invasion of cells, invasion of tumor, and microtubule dynamics. The EDI regulatory factor regulates invasion of cells, invasion of tumor, and microtubule dynamics through the PLAUR gene ( Figure 6D ).
3.7 Molecular mechanism study on inhibition of tumor cell proliferation and promotion of tumor cell apoptosis due to NCAPD3 deletion
In vivo and in vitro experiments showed that NCAPD3 loss can significantly inhibit gastric cancer cell proliferation, invasion, and migration, and promote apoptosis. In order to further study the effector molecular mechanisms, the NCAPD3 knockdown differentially expressed gene data in this study were combined with literature data, and IPA was employed to construct a molecular regulatory network map of tumor cell proliferation and apoptosis. In the molecular regulatory network map in which NCAPD3 knockdown inhibits tumor cell proliferation and promotes tumor cell apoptosis, genes related to tumor cell proliferation pathways showed overall decrease or downregulation, among which CCND1 , MYC , ESR1 were significantly decreased and their downstream genes CDK6 and IRS1 were significantly downregulated. Therefore, NCAPD3 knockdown may inhibit CCND1, MYC, and ESR1 activity to downregulate CDK6 and IRS1 expression, thereby inhibiting the proliferation of gastric cancer cells. Additionally, genes associated with tumor cell apoptosis pathways showed overall activation, among which IRF7 , DDIT3 , and HBEGF were significantly activated. Therefore, NCAPD3 knockdown may activate IRF7, DDIT3, and HBEGF expression to promote gastric cancer cell apoptosis ( Figure 7 ).
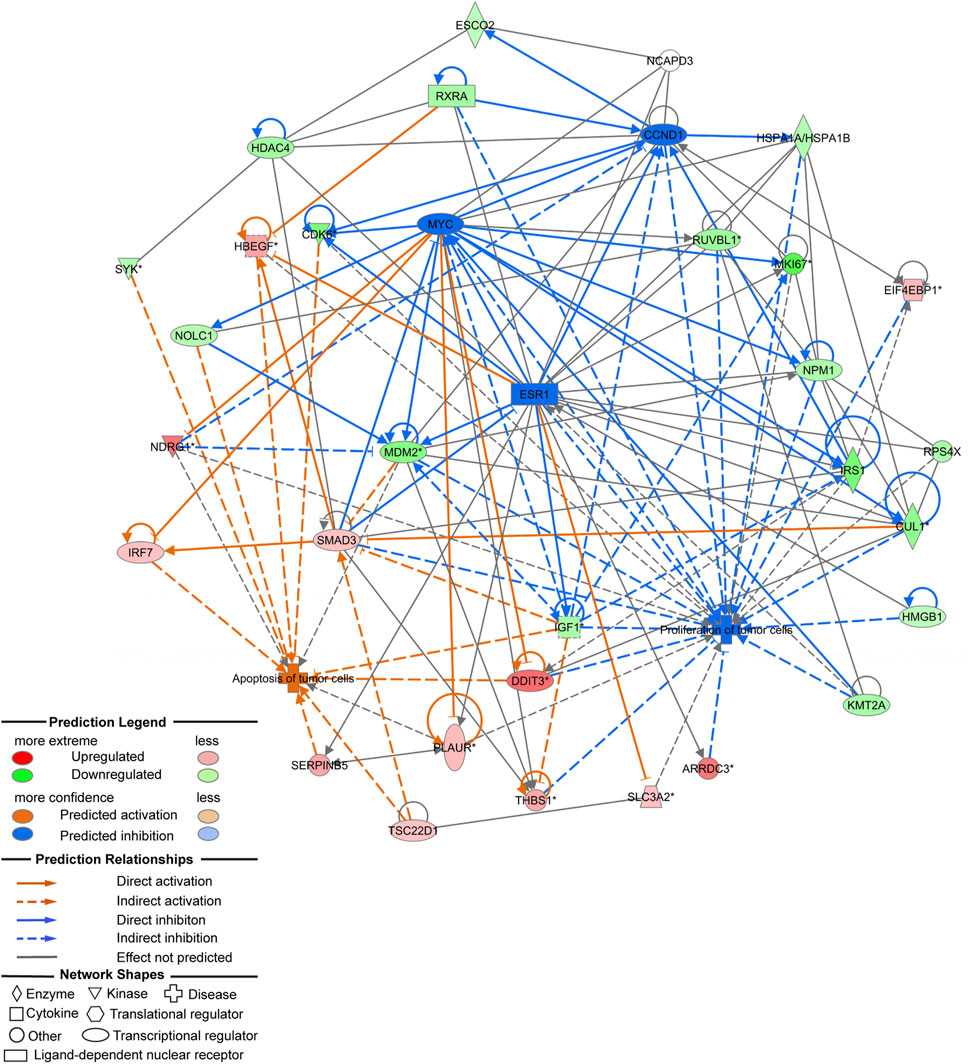
Figure 7 . Molecular regulatory network map of NCAPD3 knockdown on inhibition of tumor cell proliferation and promotion of tumor cell apoptosis.
In order to further validate whether NCAPD3 directly targets CCND1, ESR1, and MYC to regulate gastric cancer cell proliferation, Co-IP experiments were conducted to examine if NCAPD3 directly interacts with CCND1, ESR1, and MYC. The results showed that NCAPD3 NCAPD3 protein may not directly interact with MYC but may directly interact with CCND1 and ESR1 ( Figure 8A ).
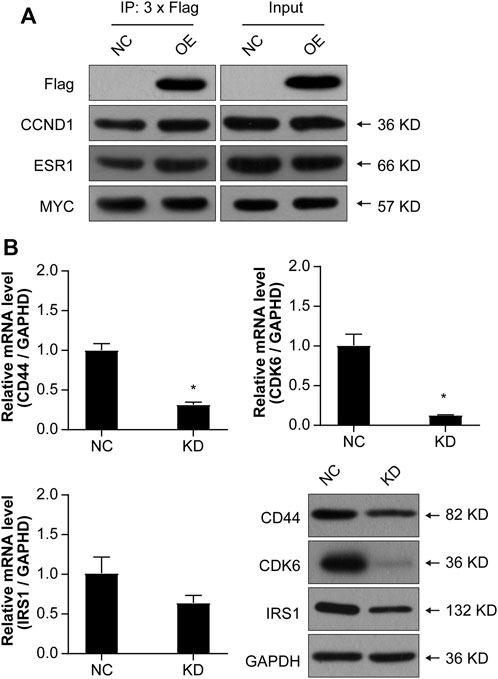
Figure 8 . Validations of the possible interactions and downstream target genes. (A) Co-IP was used to validate interactions. FLAG is a protein tag used to indicate NCAPD3 protein. (B) Validations of three target genes by qPCR and WB. NC: negative control. OE: NCAPD3 overexpression. KD: NCAPD3 knockdown. * p < 0.05.
Based on the interaction network analysis, 29 differentially expressed genes (DEGs) of interest were selected for qPCR validation. The validation results showed that the gene chip variation trends of 22 genes were consistent with PCR variation trends ( Table 3 ). From these 22 genes, three (CD44, CDK6, and IRS1) were selected for further WB validation ( Figure 8B ). The protein expression levels of CD44, CDK6, and IRS1 were downregulated by 32.30%, 84.91%, and 40.50%, respectively. Based on the above experimental and bioinformatics analysis results, the target gene NCAPD3 may upregulate CD44, CDK6, and IRS1 in AGS gastric cancer cells to carry out its effects. In summary, the NCAPD3 protein may target CCND1 or ESR1 to downregulate downstream factors such as CDK6 and IRSI to inhibit gastric cancer cell proliferation.
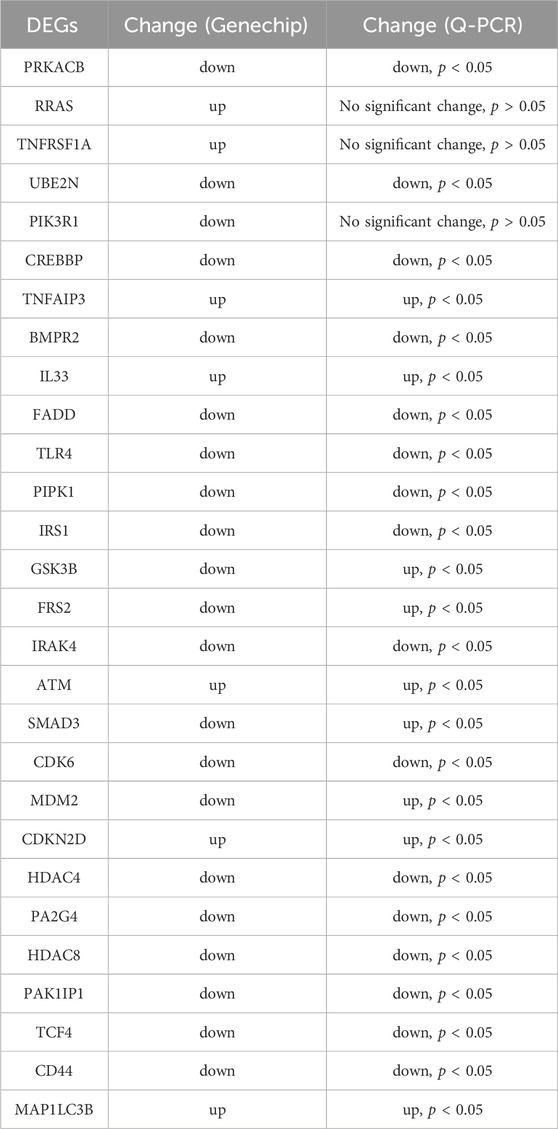
Table 3 . The qPCR validation of 29 differentially expressed genes (DEGs).
4 Discussion
Gastric cancer occurrence and progression is an extremely complex process involving oncogenes, tumor suppressor genes, cell cycle regulatory factors, and signal regulatory factors. In cancer, somatic cell changes in signaling pathways occur at different frequencies and combinations in different organs and tissues, showing complex interactions and pathway interference ( Ajani et al., 2016 ). Even though conventional gastric cancer treatments such as surgery, chemotherapy, and radiotherapy have contributed to improvements in cancer treatment, disease recurrence is common in most gastric cancer patients ( Hayakawa et al., 2015 ). The reason for this is because the pathogenesis of gastric cancer is still unclear. Therefore, in-depth studies on gastric cancer pathogenesis and discovery of new precision medicine targets are especially important.
NCAPD3 plays a crucial role in chromosome structural changes and separation during mitosis in eukaryotic cells. Previous studies found that NCAPD2/3 is intimately associated with the occurrence of many diseases ( Seipold et al., 2009 ; Zhang et al., 2014 ). Thadani et al. found that condensin II complex dynamics regulate the cell cycle ( Thadani et al., 2018 ). Yin et al. (2017) found that high expression of the NCAPD3 homologous complex, NCAPH, promotes proliferation in colon cancer cells. Kim et al. (2020) reported that knocking out the NCAPD3 homologue, NCAPH , inhibits the proliferation, colony formation, invasion, and migration of non-small cell lung cancer cells, showing that NCAPH participates in regulating non-small cell lung cancer occurrence and progression. NCAPG is a NCAPD3 homologous complex and also a subunit of the condensin complex. Studies showed that NCAPG participates in many tumors, including prostate cancer ( Goto et al., 2017 ), high-grade glioma in children ( Liang et al., 2016 ), renal cell carcinoma ( Yamada et al., 2018 ), multiple myeloma ( Cohen et al., 2014 ), and melanoma ( Ryu et al., 2007 ). Studies found that NCAPG is crucial for liver cancer occurrence and progression ( Zhang et al., 2018 ). NCAPG can activate the PI3K/AKT/FOXO4 pathway to promote liver cancer proliferation and inhibit apoptosis ( Gong et al., 2019 ). Zhang et al. found that NCAPG overexpression inhibits cardia adenocarcinoma apoptosis and promotes epithelial-mesenchymal transition ( Zhang et al., 2021 ). However, the correlation between NCAPD3 and gastric cancer occurrence and progression is still not clear. Therefore, this study aimed to evaluate the role of NCAPD3 in gastric cancer cells. The results indicated that NCAPD3 is highly expressed in gastric cancer cells and clinical tissue specimens and is intimately associated with prognosis, suggesting that high NCAPD3 expression may be the key to gastric cancer occurrence and progression. In addition, cell experiments demonstrated that NCAPD3 overexpression promotes gastric cancer cell proliferation, invasion, and migration and inhibit apoptosis. Conversely, inhibiting NCAPD3 expression attenuates gastric cancer cell proliferation, invasion, and migration and promote apoptosis. Furthermore, in vivo animal experiments showed that in vivo tumor growth is inhibited after NCAPD3 knockdown. This shows that NCAPD3 may affect cell proliferation, invasion, migration, and apoptosis to affect gastric cancer occurrence and progression. In summary, this study showed that NCAPD3 may be a crucial factor in gastric cancer occurrence and progression.
In order to examine changes in overall biological processes and related signaling pathways after NCAPD3 knockdown, GSEA was carried out. The results showed that cytokine signaling in the immune system and cytokine-cytokine receptor interaction were significantly enriched after NCAPD3 knockdown, indicating that NCAPD3 knockdown may affect immune-related signaling pathways. This finding was proven by the immunologic signature enrichment analysis, the results of which showed that 119 immune gene sets were significantly enriched in the NCAPD3 knockdown group. The tumor microenvironment is beneficial for cancer cell growth and expansion. Many types of cells participate in the tumor microenvironment, such as inflammatory cells, fibroblasts, neurons, and vascular endothelial cells. These matrix cells secrete various factors to directly activate cancer cell growth signals or remodel surrounding regions, thereby promoting tumor growth. Endothelial cells not only provide nutrients to tumors but also secrete chemokines or cytokines to interact with cancer stem cells and immune cells ( Oya et al., 2020 ). Tumor-associated immune cells have tumor suppression or tumor-promoting functions. In immune cell populations, tumor-associated macrophages, such as M1 and M2 macrophages and myeloid-derived suppressor cells, have been reported to secrete soluble factors or regulate immune responses to directly or indirectly promote gastric cancer occurrence ( Oya et al., 2020 ). Patients whose tumors show high T cell infiltration, particularly cytotoxic CD8 + T cells and memory T cells, have longer disease-free survival and overall survival, whereas patients with high neutrophil infiltration in tumors have poor prognosis ( Zeng et al., 2019 ). When one or more cells start to show uncontrollable growth, the cancer will develop. This may be the result of changes in highly regulated processes in normal cell division. These changes may be caused by germline or somatic mutations controlling normal cell proliferation, resulting in an oncogene. GSEA analysis revealed that 26 oncogene sets and 13 transcription factor gene sets were significantly enriched after NCAPD3 knockdown, such as p53 and FOXO1/4. TP53 (p53) is the most common gene mutated in human cancers and TP53 mutations are present in approximately 50% of invasive tumors. Traditionally, p53 is regarded as a gene that induces cell cycle arrest, apoptosis, or senescence or participates in DNA repair to inhibit oncogenesis and cancer progression. Although these tumor suppressor mechanisms have been confirmed in different models, recent data show that p53 can also regulate metabolism, regulate reactive oxygen species (ROS) levels, change non-coding RNA expression, and increase autophagy or ferroptosis to inhibit oncogenesis. Due to its high mutation frequency and crucial role in oncogenesis/cancer progression, p53 is a priority target in antineoplastic treatment ( Duffy et al., 2017 ). Regulation of the FOXO transcription factor mainly occurs at the post-transcriptional and post-translational levels, which are mediated by non-coding RNAs through interactions with other protein chaperones and cofactors (including phosphorylation, acetylation, methylation, and ubiquitination). FOXO regulates factors essential for cell proliferation, death, senescence, angiogenesis, migration, and metastasis and plays a role in tumorigenesis and tumor progression ( Jiramongkol and Lam, 2020 ). These findings showed that NCAPD3 affects the expression of oncogenes and transcription factors to regulate gastric cancer cell proliferation, invasion, migration, and apoptosis.
In order to further study the potential molecular mechanisms by which NCAPD3 regulates behavioral changes in gastric cancer cells, in this study, the human genome expression chip was used to detect differentially expressed genes and related signaling pathways before and after NCAPD3 knockdown. IPA canonical pathway analysis showed that the cholesterol biosynthesis pathway is significantly inhibited after NCAPD3 knockdown. There is controversy over the role of cholesterol in cancer progression and potential treatments targeting cholesterol homeostasis in oncology ( Kuzu et al., 2016 ). One study reported changes and mutations in genes in the cholesterol homeostasis pathway in cancer cells ( Murai, 2015 ). The expression of cholesterol synthesis genes is upregulated, LDL receptor-mediated cholesterol influx is increased, and cholesterol transport is decreased, which increases cellular cholesterol levels, thereby facilitating cancer cell proliferation ( Llaverias et al., 2011 ; Smith and Land, 2012 ; Krycer and Brown, 2013 ). In sarcoma, acute myeloid leukemia, and melanoma, increased activity in the cholesterol synthesis pathway is related to decreased patient survival ( Riganti and Massaia, 2013 ; Sui et al., 2015 ; Brown et al., 2016 ). However, there are still very few studies in this area, and further studies are required to comprehensively analyze the consequences of these changes and how they regulate cancer progression. This study employed IPA bioinformatics analysis and found that the cholesterol biosynthesis pathway was significantly inhibited after NCAPD3 silencing. Therefore, NCAPD3 downregulation may inhibit cholesterol synthesis, thereby affecting gastric cancer cell proliferation.
Regulatory effect analysis revealed that the biological functions of differentially expressed genes were mainly concentrated in cell invasion and tumor invasion. The expression levels of regulatory factors related to cell and tumor invasion (CRNDE, EDN1, JUNB, MAP2K1/2) were significantly activated, among which the upstream regulatory factors CRNDE and EDN1 activated the expression of the downstream target EGFR to promote cell and tumor invasion, and JUNB activated PLAUR expression to promote cell and tumor invasion. EGFR is a tyrosine kinase receptor, and binding with RGF promotes cell survival and proliferation ( Prenzel et al., 2001 ). In addition, EGFR signaling aids in vitro cell differentiation, invasion, and migration ( El-Rehim et al., 2004 ; Arteaga and Engelman, 2014 ). Dysregulated EGFR signaling has been observed in many cancers, including breast cancer, colon cancer, and lung cancer ( Nautiyal et al., 2012 ; Matalkah et al., 2016 ). EGFR can promote breast cancer invasion and migration ( Zhao et al., 2018 ). PLAUR is highly expressed in gastric cancer tissues and promotes gastric cancer invasion ( Sandoval-Bórquez et al., 2017 ). Therefore, the present study suggests that NCAPD3 -knockdown-induced differentially expressed genes (DEGs) such as CRNDE, EDN1, and JUNB can regulate cell and tumor invasion through EGFR and PLAUR.
In the molecular regulatory network map, in which NCAPD3 knockdown inhibited tumor cell proliferation and promoted tumor cell apoptosis, genes related to tumor cell proliferation pathways showed overall inhibition or downregulation, among which CCND1 , MYC , and ESR1 were significantly inhibited and their downstream genes CDK6 and IRSI were significantly downregulated. Therefore, NCAPD3 knockdown may inhibit CCND1, MYC, and ESR1 expression to downregulate CDK6 and IRSI expression, thereby inhibiting the proliferation of gastric cancer cells. Co-IP was carried out to analyze whether NCAPD3 directly targets CCND1, MYC, and ESR1. The results showed that NCAPD3 directly interacted with CCND1 and ESR1 but did not directly interact with MYC. ESR1, estrogen receptor gene, is a ligand-activated transcription factor composed of a DNA binding and a transcriptional activation domain, including the N-terminal ligand independent activation function (AF)-1 and C-terminal ligand-dependent AF-2 domains. The ligand-binding domain (LBD) and DNA binding and hinge domain in the protein core are also located at the C-terminal. Ligand-receptor binding helps co-regulate the recruitment of proteins, including co-stimulation and co-inhibitory factors to regulate many physiological processes, such as tumorigenesis and tumor progression ( Martin et al., 2017 ). ESR1 has been reported to regulate proliferation in liver cancer ( Wang et al., 2021 ), bladder cancer ( Ge et al., 2019 ), progenitor Leydig cells ( Oh et al., 2017 ), and chondrocytes ( Liu et al., 2019 ). This shows that ESR1 plays important roles in cell proliferation. CCND1 is a member of the cell cycle protein family and regulates the cell cycle by activating CDK4/CDK6 ( Marschall et al., 2016 ). Early studies showed that CCND1 and CDK6 are activated in tumor cells and their expressions are upregulated. Therefore, CCND1 and CDK6/CDK4 are potential therapeutic targets for tumors ( Malumbres and Barbacid, 2009 ; Lang et al., 2015 ). Cyclin-dependent kinase 6 (CDK6) is a member of the threonine-serine kinase subfamily that participates in controlling the cell cycle, thereby controlling cell proliferation ( Turner et al., 2019 ). Targeting CDK6 is a potential pathway for inducing cell cycle arrest and inhibiting tumor cell proliferation ( Rader et al., 2013 ). CDK6 is an important factor that regulates the cell cycle, and overexpression or activation of CDK6 will accelerate the cell cycle and promote cell proliferation, thereby resulting in transformation and promoting gastric cancer occurrence and progression ( Ilyin et al., 2003 ). Increased CDK6 protein expression can be detected in many types of cancer, and reducing CDK6 expression inhibits the growth and proliferation of tumors in vivo and in vitro ( Thammasit et al., 2015 ). Insulin receptor substrate (IRS1) is an important intracellular signaling protein and an important signaling factor for cell surface receptor activation. IRS1 regulates upstream signals and downstream effectors to regulate cell growth, metabolism, and activation ( Jellema et al., 2003 ). A study reported that IRS1 can promote tumor proliferation ( Dearth et al., 2007 ). IRS1 is a crucial regulatory factor of PI3K in malignant cells and affects tumor cell proliferation ( Houghton et al., 2010 ). A study reported that IRS1 regulates PI3K activity to inhibit gastric cancer occurrence ( Baba et al., 2007 ). Therefore, NCAPD3 knockdown may inhibit CCND1 and ESR1 expression to downregulate CDK6 and IRSI expression, thereby inhibiting the proliferation of gastric cancer cells.
In addition, molecular network regulatory maps showed that apoptosis pathway-related genes (e.g., IRF7 and DDIT3 ) in tumor cells showed overall activation after NCAPD3 inhibition. This is consistent with the result that inhibiting NCAPD3 expression promotes apoptosis in gastric cancer cells. IRF7 is an interferon regulatory factor that is intimately associated with apoptosis. Liu et al. (2017) found that IRF7 activates NF-κB/GSDMD signals in mouse adipose tissues to promote inflammasome-induced apoptosis. Zhang et al. (2017) reported that the HTLV-1 oncoprotein Tax interacts with MAVS, STING, and RIP1 to inhibit the innate interferon response, resulting in TBK1-mediated inhibition of IRF3/IRF7 phosphorylation, and also to inhibit apoptosis and autophagy in target cells. DDIT3 is also known as C/EBP homologous protein (CHOP) and is a mark of endoplasmic reticulum stress. DDIT3 can form heterodimers with other proteins in the C/EBP family. During endoplasmic reticulum stress, DDIT3 acts as a transcription factor to downregulate the expression of antiapoptotic factors BCL-2 and BCL-Xl, and it acts as a transcription activation factor to upregulate the expression of proapoptotic genes such as BIM ( Tsukano et al., 2010 ; Hu et al., 2019 ). Therefore, NCAPD3 inhibition may regulate gastric cancer cell apoptosis by activating IRF7 and DDIT3.
5 Conclusion
In summary, NCAPD3 is upregulated in gastric cancer. NCAPD3 promotes gastric cancer cell proliferation, invasion, and migration and inhibits apoptosis to accelerate gastric cancer progression. Inhibiting NCAPD3 expression can attenuate the malignant biological behaviors of gastric cancer cells. NCAPD3 loss can directly inhibit CCND1 and ESR1 expression to downregulate the expression of downstream targets CDK6 and IRS1 and inhibit the proliferation of gastric cancer cells. Moreover, NCAPD3 loss activates IRF7 and DDIT3 to regulate apoptosis in gastric cancer cells. In addition, NCAPD3 significantly affects many canonical pathways and immune and transcription factor gene sets in gastric cancer occurrence and progression. Overall, this study shows that NCAPD3 may be a potential target for gastric cancer treatment.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/ Supplementary Material . The expression data presented in the study is publicly available. This data can be found here: Gene Expression Omnibus, accession number GSE261264.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Fujian Medical University Union Hospital (No. 2021WSJK042). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. The animal study was approved by the Institutional Animal Care and Use Committee of Fujian Medical University (No. 2021-8CAARM125). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
S-YZ: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Validation, Visualization, Writing–original draft. QL: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Validation, Visualization, Writing–original draft. L-RX: Data curation, Formal Analysis, Investigation, Methodology, Software, Writing–original draft. FY: Investigation, Methodology, Software, Data curation, Formal Analysis, Writing–original draft. JZ: Conceptualization, Investigation, Project administration, Supervision, Writing–review and editing, Methodology, Software. X-QC: Conceptualization, Funding acquisition, Investigation, Project administration, Supervision, Validation, Writing–original draft, Writing–review and editing, Data curation, Resources. SY: Investigation, Writing–original draft, Conceptualization, Funding acquisition, Project administration, Supervision, Validation, Writing–review and editing, Data curation, Resources.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Joint Funds for the innovation of science and Technology, Fujian province under Grant number 2021Y9066; the Fujian Provincial Health Technology Project under Grant numbers 2020CXB016 and 2021CXA016.
Acknowledgments
The authors would like to express their gratitude to EditSprings ( https://www.editsprings.cn ) for the expert linguistic services provided.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1341039/full#supplementary-material
Abe, S., Nagasaka, K., Hirayama, Y., Kozuka-Hata, H., Oyama, M., Aoyagi, Y., et al. (2011). The initial phase of chromosome condensation requires Cdk1-mediated phosphorylation of the CAP-D3 subunit of condensin II. Genes and Dev. 25, 863–874. doi:10.1101/gad.2016411
PubMed Abstract | CrossRef Full Text | Google Scholar
Ajani, J. A., D'Amico, T. A., Almhanna, K., Bentrem, D. J., Chao, J., Das, P., et al. (2016). Gastric cancer, version 3.2016, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 14, 1286–1312. doi:10.6004/jnccn.2016.0137
CrossRef Full Text | Google Scholar
Arteaga, C. L., and Engelman, J. A. (2014). ERBB receptors: from oncogene discovery to basic science to mechanism-based cancer therapeutics. Cancer Cell 25, 282–303. doi:10.1016/j.ccr.2014.02.025
Baba, T., Endo, T., Sata, F., Honnma, H., Kitajima, Y., Hayashi, T., et al. (2007). Polycystic ovary syndrome is associated with genetic polymorphism in the insulin signaling gene IRS-1 but not ENPP1 in a Japanese population. Life Sci. 81, 850–854. doi:10.1016/j.lfs.2007.07.023
Bakhrebah, M., Zhang, T., Mann, J. R., Kalitsis, P., and Hudson, D. F. (2015). Disruption of a conserved CAP-D3 threonine alters condensin loading on mitotic chromosomes leading to chromosome hypercondensation. J. Biol. Chem. 290, 6156–6167. doi:10.1074/jbc.M114.627109
Bass, A. J., Thorsson, V., Shmulevich, I., et al. (2014). Comprehensive molecular characterization of gastric adenocarcinoma. Nature 513, 202–209. doi:10.1038/nature13480
Berger, H., Marques, M. S., Zietlow, R., Meyer, T. F., Machado, J. C., and Figueiredo, C. (2016). Gastric cancer pathogenesis. Helicobacter 21, 34–38. doi:10.1111/hel.12338
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., and Jemal, A. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA a cancer J. Clin. 68, 394–424. doi:10.3322/caac.21492
Brown, D. N., Caffa, I., Cirmena, G., Piras, D., Garuti, A., Gallo, M., et al. (2016). Squalene epoxidase is a bona fide oncogene by amplification with clinical relevance in breast cancer. Sci. Rep. 6, 19435–19513. doi:10.1038/srep19435
Bustin, S. A., Benes, V., Garson, J. A., et al. (2009). The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments . Oxford University Press .
Google Scholar
Cohen, Y., Gutwein, O., Garach-Jehoshua, O., Bar-Haim, A., and Kornberg, A. (2014). The proliferation arrest of primary tumor cells out-of-niche is associated with widespread downregulation of mitotic and transcriptional genes. Hematology 19, 286–292. doi:10.1179/1607845413Y.0000000125
Dawkins, J. B., Wang, J., Maniati, E., Heward, J. A., Koniali, L., Kocher, H. M., et al. (2016). Reduced expression of histone methyltransferases KMT2C and KMT2D correlates with improved outcome in pancreatic ductal adenocarcinoma. Cancer Res. 76, 4861–4871. doi:10.1158/0008-5472.CAN-16-0481
Dearth, R. K., Cui, X., Kim, H.-J., Hadsell, D. L., and Lee, A. V. (2007). Oncogenic transformation by the signaling adaptor proteins insulin receptor substrate (IRS)-1 and IRS-2. Cell cycle 6, 705–713. doi:10.4161/cc.6.6.4035
Duffy, M. J., Synnott, N. C., and Crown, J. (2017). Mutant p53 as a target for cancer treatment. Eur. J. Cancer 83, 258–265. doi:10.1016/j.ejca.2017.06.023
El-Rehim, A., Pinder, S., Paish, C., Bell, J. A., Rampaul, R. S., Blamey, R. W., et al. (2004). Expression and co-expression of the members of the epidermal growth factor receptor (EGFR) family in invasive breast carcinoma. Br. J. cancer 91, 1532–1542. doi:10.1038/sj.bjc.6602184
Ge, Q., Lu, M., Ju, L., Qian, K., Wang, G., Wu, C. L., et al. (2019). miR-4324-RACGAP1-STAT3-ESR1 feedback loop inhibits proliferation and metastasis of bladder cancer. Int. J. cancer 144, 3043–3055. doi:10.1002/ijc.32036
Gong, C., Ai, J., Fan, Y., Gao, J., Liu, W., Feng, Q., et al. (2019). NCAPG promotes the proliferation of hepatocellular carcinoma through PI3K/AKT signaling. OncoTargets Ther. 12, 8537–8552. doi:10.2147/OTT.S217916
Goto, Y., Kurozumi, A., Arai, T., Nohata, N., Kojima, S., Okato, A., et al. (2017). Impact of novel miR-145-3p regulatory networks on survival in patients with castration-resistant prostate cancer. Br. J. cancer 117, 409–420. doi:10.1038/bjc.2017.191
Guo, Z., Zhang, X., Zhu, H., Zhong, N., Luo, X., Zhang, Y., et al. (2021). TELO2 induced progression of colorectal cancer by binding with RICTOR through mTORC2. Oncol. Rep. 45, 523–534. doi:10.3892/or.2020.7890
Hayakawa, Y., Ariyama, H., Stancikova, J., Sakitani, K., Asfaha, S., Renz, B. W., et al. (2015). Mist1 expressing gastric stem cells maintain the normal and neoplastic gastric epithelium and are supported by a perivascular stem cell niche. Cancer Cell 28, 800–814. doi:10.1016/j.ccell.2015.10.003
Hou, T., Yang, C., Tong, C., Zhang, H., Xiao, J., and Li, J. (2014). Overexpression of ASAP1 is associated with poor prognosis in epithelial ovarian cancer. Int. J. Clin. Exp. pathology 7, 280–287.
PubMed Abstract | Google Scholar
Houghton, A. M., Rzymkiewicz, D. M., Ji, H., Gregory, A. D., Egea, E. E., Metz, H. E., et al. (2010). Neutrophil elastase–mediated degradation of IRS-1 accelerates lung tumor growth. Nat. Med. 16, 219–223. doi:10.1038/nm.2084
Hu, H., Tian, M., Ding, C., and Yu, S. (2019). The C/EBP homologous protein (CHOP) transcription factor functions in endoplasmic reticulum stress-induced apoptosis and microbial infection. Front. Immunol. 3083. doi:10.3389/fimmu.2018.03083
Ilyin, G. P., Glaise, D., Gilot, D., Baffet, G., and Guguen-Guillouzo, C. (2003). Regulation and role of p21 and p27 cyclin-dependent kinase inhibitors during hepatocyte differentiation and growth. Am. J. Physiology-Gastrointestinal Liver Physiology 285, G115–G127. doi:10.1152/ajpgi.00309.2002
Jellema, A., Zeegers, M., Feskens, E., Dagnelie, P. C., and Mensink, R. P. (2003). Gly972Arg variant in the insulin receptor substrate-1 gene and association with Type 2 diabetes: a meta-analysis of 27 studies. Diabetologia 46, 990–995. doi:10.1007/s00125-003-1126-4
Jiramongkol, Y., and Lam, E. W.-F. (2020). FOXO transcription factor family in cancer and metastasis. Cancer Metastasis Rev. 39, 681–709. doi:10.1007/s10555-020-09883-w
Kim, B., Kim, S. W., Lim, J.-Y., and Park, S.-J. (2020). NCAPH is required for proliferation, migration and invasion of non-small-cell lung cancer cells. Anticancer Res. 40, 3239–3246. doi:10.21873/anticanres.14305
Krycer, J. R., and Brown, A. J. (2013). Cholesterol accumulation in prostate cancer: a classic observation from a modern perspective. Biochimica Biophysica Acta (BBA)-Reviews Cancer 1835, 219–229. doi:10.1016/j.bbcan.2013.01.002
Kuzu, O. F., Noory, M. A., and Robertson, G. P. (2016). The role of cholesterol in cancer. Cancer Res. 76, 2063–2070. doi:10.1158/0008-5472.CAN-15-2613
Lang, E., Zelenak, C., Eberhard, M., Bissinger, R., Rotte, A., Ghashghaeinia, M., et al. (2015). Impact of cyclin-dependent kinase CDK4 inhibition on eryptosis. Cell. physiology Biochem. 37, 1178–1186. doi:10.1159/000430241
Lapointe, J., Malhotra, S., Higgins, J. P., Bair, E., Thompson, M., Salari, K., et al. (2008). hCAP-D3 expression marks a prostate cancer subtype with favorable clinical behavior and androgen signaling signature. Am. J. Surg. pathology 32, 205–209. doi:10.1097/PAS.0b013e318124a865
Liang, M.-L., Hsieh, T.-H., Ng, K.-H., Tsai, Y. N., Tsai, C. F., Chao, M. E., et al. (2016). Downregulation of miR-137 and miR-6500-3p promotes cell proliferation in pediatric high-grade gliomas. Oncotarget 7, 19723–19737. doi:10.18632/oncotarget.7736
Liu, M., Xie, W., Zheng, W., Yin, D., Luo, R., and Guo, F. (2019). Targeted binding of estradiol with ESR1 promotes proliferation of human chondrocytes in vitro by inhibiting activation of ERK signaling pathway. Nan Fang yi ke da xue xue bao= J. South. Med. Univ. 39, 134–143. doi:10.12122/j.issn.1673-4254.2019.09.02
Liu, Q., Huang, S.-Y., Yue, D.-M., Wang, J. L., Wang, Y., Li, X., et al. (2017a). Proteomic analysis of Fasciola hepatica excretory and secretory products (FhESPs) involved in interacting with host PBMCs and cytokines by shotgun LC-MS/MS. Parasitol. Res. 116, 627–635. doi:10.1007/s00436-016-5327-4
Liu, Z., Gan, L., Xu, Y., Luo, D., Ren, Q., Wu, S., et al. (2017b). Melatonin alleviates inflammasome-induced pyroptosis through inhibiting NF-κB/GSDMD signal in mice adipose tissue. J. Pineal Res. 63, e12414. doi:10.1111/jpi.12414
Llaverias, G., Danilo, C., Mercier, I., Daumer, K., Capozza, F., Williams, T. M., et al. (2011). Role of cholesterol in the development and progression of breast cancer. Am. J. pathology 178, 402–412. doi:10.1016/j.ajpath.2010.11.005
Luo, Q., Zhang, S., Zhang, D., Yuan, F., Chen, X., and Yang, S. (2020). Expression of ASAP1 and FAK in gastric cancer and its clinicopathological significance. Oncol. Lett. 20, 974–980. doi:10.3892/ol.2020.11612
Maeshima, K., and Eltsov, M. (2008). Packaging the genome: the structure of mitotic chromosomes. J. Biochem. 143, 145–153. doi:10.1093/jb/mvm214
Malumbres, M., and Barbacid, M. (2009). Cell cycle, CDKs and cancer: a changing paradigm. Nat. Rev. cancer 9, 153–166. doi:10.1038/nrc2602
Marschall, A. L., Dübel, S., and Böldicke, T. (2016). Recent advances with ER targeted intrabodies. Protein Target. Compd. 917, 77–93. doi:10.1007/978-3-319-32805-8_5
Martin, L.-A., Ribas, R., Simigdala, N., Schuster, E., Pancholi, S., Tenev, T., et al. (2017). Discovery of naturally occurring ESR1 mutations in breast cancer cell lines modelling endocrine resistance. Nat. Commun. 8, 1865–1915. doi:10.1038/s41467-017-01864-y
Matalkah, F., Martin, E., Zhao, H., and Agazie, Y. M. (2016). SHP2 acts both upstream and downstream of multiple receptor tyrosine kinases to promote basal-like and triple-negative breast cancer. Breast Cancer Res. 18, 2–14. doi:10.1186/s13058-015-0659-z
Murai, T. (2015). Cholesterol lowering: role in cancer prevention and treatment. Biol. Chem. 396, 1–11. doi:10.1515/hsz-2014-0194
Nautiyal, J., Du, J., Yu, Y., Kanwar, S. S., Levi, E., and Majumdar, A. P. N. (2012). EGFR regulation of colon cancer stem-like cells during aging and in response to the colonic carcinogen dimethylhydrazine. Am. J. Physiology-Gastrointestinal Liver Physiology 302, G655–G663. doi:10.1152/ajpgi.00323.2011
Oh, Y. S., Koh, I. K., Choi, B., and Gye, M. C. (2017). ESR1 inhibits hCG-induced steroidogenesis and proliferation of progenitor Leydig cells in mice. Sci. Rep. 7, 43459–43513. doi:10.1038/srep43459
Ono, T., Fang, Y., Spector, D. L., and Hirano, T. (2004). Spatial and temporal regulation of Condensins I and II in mitotic chromosome assembly in human cells. Mol. Biol. Cell 15, 3296–3308. doi:10.1091/mbc.e04-03-0242
Oya, Y., Hayakawa, Y., and Koike, K. (2020). Tumor microenvironment in gastric cancers. Cancer Sci. 111, 2696–2707. doi:10.1111/cas.14521
Prenzel, N., Fischer, O., Streit, S., Hart, S., and Ullrich, A. (2001). The epidermal growth factor receptor family as a central element for cellular signal transduction and diversification. Endocrine-related cancer 8, 11–31. doi:10.1677/erc.0.0080011
Rader, J., Russell, M. R., Hart, L. S., Nakazawa, M. S., Belcastro, L. T., Martinez, D., et al. (2013). Dual CDK4/CDK6 inhibition induces cell-cycle arrest and senescence in neuroblastoma. Clin. cancer Res. 19, 6173–6182. doi:10.1158/1078-0432.CCR-13-1675
Riganti, C., and Massaia, M. (2013). Inhibition of the mevalonate pathway to override chemoresistance and promote the immunogenic demise of cancer cells: killing two birds with one stone. Oncoimmunology 2, e25770. doi:10.4161/onci.25770
Ryu, B., Kim, D. S., DeLuca, A. M., and Alani, R. M. (2007). Comprehensive expression profiling of tumor cell lines identifies molecular signatures of melanoma progression. PloS one 2, e594. doi:10.1371/journal.pone.0000594
Sandoval-Bórquez, A., Polakovicova, I., Carrasco-Véliz, N., Lobos-González, L., Riquelme, I., Carrasco-Avino, G., et al. (2017). MicroRNA-335-5p is a potential suppressor of metastasis and invasion in gastric cancer. Clin. epigenetics 9, 114–116. doi:10.1186/s13148-017-0413-8
Seipold, S., Priller, F. C., Goldsmith, P., Harris, W. A., Baier, H., and Abdelilah-Seyfried, S. (2009). Non-SMC condensin I complex proteins control chromosome segregation and survival of proliferating cells in the zebrafish neural retina. BMC Dev. Biol. 9, 40–14. doi:10.1186/1471-213X-9-40
Smith, B., and Land, H. (2012). Anticancer activity of the cholesterol exporter ABCA1 gene. Cell Rep. 2, 580–590. doi:10.1016/j.celrep.2012.08.011
Smyth, E. C., Nilsson, M., Grabsch, H. I., van Grieken, N. C., and Lordick, F. (2020). Gastric cancer. Lancet 396, 635–648. doi:10.1016/S0140-6736(20)31288-5
Sui, Z., Zhou, J., Cheng, Z., and Lu, P. (2015). Squalene epoxidase (SQLE) promotes the growth and migration of the hepatocellular carcinoma cells. Tumor Biol. 36, 6173–6179. doi:10.1007/s13277-015-3301-x
Sun, D., Li, H., Cao, M., He, S., Lei, L., Peng, J., et al. (2020). Cancer burden in China: trends, risk factors and prevention. Cancer Biol. Med. 17, 879–895. doi:10.20892/j.issn.2095-3941.2020.0387
Thadani, R., Kamenz, J., Heeger, S., Muñoz, S., and Uhlmann, F. (2018). Cell-cycle regulation of dynamic chromosome association of the condensin complex. Cell Rep. 23, 2308–2317. doi:10.1016/j.celrep.2018.04.082
Thammasit, P., Sangboonruang, S., Suwanpairoj, S., Khamaikawin, W., Intasai, N., Kasinrerk, W., et al. (2015). Intracellular acidosis promotes mitochondrial apoptosis pathway: role of EMMPRIN down-regulation via specific single-chain Fv intrabody. J. Cancer 6, 276–286. doi:10.7150/jca.10879
Tsukano, H., Gotoh, T., Endo, M., Miyata, K., Tazume, H., Kadomatsu, T., et al. (2010). The endoplasmic reticulum stress-C/EBP homologous protein pathway-mediated apoptosis in macrophages contributes to the instability of atherosclerotic plaques. Arteriosclerosis, thrombosis, Vasc. Biol. 30, 1925–1932. doi:10.1161/ATVBAHA.110.206094
Turner, N. C., Liu, Y., Zhu, Z., Loi, S., Colleoni, M., Loibl, S., et al. (2019). Cyclin E1 expression and palbociclib efficacy in previously treated hormone receptor–positive metastatic breast cancer. J. Clin. Oncol. 37, 1169–1178. doi:10.1200/JCO.18.00925
Wang, L., Cui, M., Cheng, D., Qu, F., Yu, J., Wei, Y., et al. (2021). miR-9-5p facilitates hepatocellular carcinoma cell proliferation, migration and invasion by targeting ESR1. Mol. Cell. Biochem. 476, 575–583. doi:10.1007/s11010-020-03927-z
Yamada, Y., Arai, T., Kojima, S., Sugawara, S., Kato, M., Okato, A., et al. (2018). Regulation of antitumor miR-144-5p targets oncogenes: direct regulation of syndecan-3 and its clinical significance. Cancer Sci. 109, 2919–2936. doi:10.1111/cas.13722
Yin, L., Jiang, L.-P., Shen, Q.-S., Xiong, Q. X., Zhuo, X., Zhang, L. L., et al. (2017). NCAPH plays important roles in human colon cancer. Cell death Dis. 8, e2680. doi:10.1038/cddis.2017.88
Zeng, D., Li, M., Zhou, R., Zhang, J., Sun, H., Shi, M., et al. (2019). Tumor microenvironment characterization in gastric cancer identifies prognostic and immunotherapeutically relevant gene signatures. Cancer Immunol. Res. 7, 737–750. doi:10.1158/2326-6066.CIR-18-0436
Zhang, L., Huang, Y., Ling, J., Zhuo, W., Yu, Z., Shao, M., et al. (2018). Screening and function analysis of hub genes and pathways in hepatocellular carcinoma via bioinformatics approaches. Cancer Biomarkers 22, 511–521. doi:10.3233/CBM-171160
Zhang, L.-L., Wei, J.-Y., Wang, L., Huang, S. L., and Chen, J. L. (2017). Human T-cell lymphotropic virus type 1 and its oncogenesis. Acta Pharmacol. Sin. 38, 1093–1103. doi:10.1038/aps.2017.17
Zhang, P., Liu, L., Huang, J., Shao, L., Wang, H., Xiong, N., et al. (2014). Non-SMC condensin I complex, subunit D2 gene polymorphisms are associated with Parkinson’s disease: a Han Chinese study. Genome 57, 253–257. doi:10.1139/gen-2014-0032
Zhang, S., Luo, Q., Feng, R., Yang, F., Xu, Q., Chen, X., et al. (2021a). ADP ribosylation factor guanylate kinase 1 promotes the malignant phenotype of gastric cancer by regulating focal adhesion kinase activation. Life Sci. 273, 119264. doi:10.1016/j.lfs.2021.119264
Zhang, X., Zhu, M., Wang, H., Song, Z., Zhan, D., Cao, W., et al. (2021b). Overexpression of NCAPG inhibits cardia adenocarcinoma apoptosis and promotes epithelial-mesenchymal transition through the Wnt/β-catenin signaling pathway. Gene 766, 145163. doi:10.1016/j.gene.2020.145163
Zhao, Y., Ma, J., Fan, Y., Wang, Z., Tian, R., Ji, W., et al. (2018). TGF-β transactivates EGFR and facilitates breast cancer migration and invasion through canonical Smad3 and ERK/Sp1 signaling pathways. Mol. Oncol. 12, 305–321. doi:10.1002/1878-0261.12162
Keywords: gastric cancer, NCAPD3 , proliferation, apoptosis, molecular mechanism
Citation: Zhang S-Y, Luo Q, Xiao L-R, Yang F, Zhu J, Chen X-Q and Yang S (2024) Role and mechanism of NCAPD3 in promoting malignant behaviors in gastric cancer. Front. Pharmacol. 15:1341039. doi: 10.3389/fphar.2024.1341039
Received: 19 November 2023; Accepted: 30 March 2024; Published: 22 April 2024.
Reviewed by:
Copyright © 2024 Zhang, Luo, Xiao, Yang, Zhu, Chen and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sheng Yang, [email protected] ; Xiang-Qi Chen, [email protected] ; Jian Zhu, [email protected]
‡ ORCID: Su-Yun Zhang, orcid.org/0000-0001-8813-6751 ; Qiong Luo, orcid.org/0000-0001-8216-4524 ; Li-Rong Xiao, orcid.org/0009-0004-0783-3635 ; Fan Yang, orcid.org/0000-0002-9634-7003 ; Jian Zhu, orcid.org/0000-0003-3596-4339 ; Xiang-Qi Chen, orcid.org/0000-0003-3367-6261 ; Sheng Yang, orcid.org/0000-0003-4226-2159
† These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.

IMAGES
VIDEO
COMMENTS
Introduction. Being characterized by six major hallmarks, carcinogenesis might occur in every cell, tissue, and organ, leading to the pathological alternations that result in a vast number of cancers. ... Breast cancer is currently one of the most prevalently diagnosed cancers and the 5th cause of cancer-related deaths with an estimated number ...
17 essay samples found. Breast cancer is a type of cancer that develops from breast tissue. Essays on this topic could explore the causes, diagnosis, treatment, and prevention of breast cancer. Additionally, discussions might delve into the psychological and social impact of breast cancer on patients and their families, the ongoing research ...
ASCO Answers Fact Sheet: Read a 1-page fact sheet that offers an introduction to breast cancer. This free fact sheet is available as a PDF, so it is easy to print. ASCO Answers Guide: Get this 52-page booklet that helps you better understand breast cancer and its treatment options. This free booklet is available as a PDF, so it is easy to print.
INTRODUCTION BREAST cancer is currently the most common cancer affecting women worldwide [1]. In European women, it is the leading cause of cancer death, causing one in six of all deaths from cancers [2].In the U.S., a woman has a 12.15% (about one in eight) risk of developing breast cancer during her lifetime [3].
Breast cancer is a disease in which abnormal breast cells grow out of control and form tumours. If left unchecked, the tumours can spread throughout the body and become fatal. Breast cancer cells begin inside the milk ducts and/or the milk-producing lobules of the breast. The earliest form (in situ) is not life-threatening and can be detected ...
Breast cancer is cancer that starts in cells in the breast. The ducts and the lobules are the two parts of the breast where cancer is most likely to start. Breast cancer is one of the most common types of cancer in the U.S. Healthcare providers don't yet know exactly what causes it. Once breast cancer forms, cancer cells can spread to other ...
The lifetime risk for breast cancer in men is 1 in 833 compared with 1 in 10 for a woman. Of affected men, 20% have a first-degree family history of cancer; 4-14% of cases in males are ...
The term "breast cancer" refers to a malignant tumor that has developed from cells in the breast. The types of cells that most commonly give rise to breast cancers are the milk-secreting cells and duct cells, which drain milk from the lobules to the nipple. However, a small proportion of breast cancers develop from fatty or fibrous tissue.
The breast is an organ that sits on top of the upper ribs and chest muscles. There is a left and right breast and each one has mainly glands, ducts, and fatty tissue. In women, the breast makes and delivers milk to feed newborns and infants. The amount of fatty tissue in the breast determines the size of each breast.
The article by Esteva and Hortobagyi discusses breast cancer from the aspect of increased survival rates, the novel treatments that have necessitated this and the promise in even more enhanced management of breast cancer. Effects of Hypoxia, Surrounding Fibroblasts, and p16 Expression on Breast Cancer.
1 Introduction. T he prospect of developing breast cancer is a source of anxiety for many women. Breast cancer remains the most common invasive cancer among women (aside from nonmelanoma skin cancers), accounting in 2011 for an estimated 230,480 new cases among women in the United States and another 2,140 new cases among men (ACS, 2011).
Ductal carcinoma in situ (DCIS) is a breast disease that may lead to invasive breast cancer. The cancer cells are only in the lining of the ducts and have not spread to other tissues in the breast. Last Reviewed: July 25, 2023. Source: Division of Cancer Prevention and Control, Centers for Disease Control and Prevention.
There are 7 modules in this course. Welcome to an Introduction to Breast Cancer! In this course, we'll learn a bit about the leading cause of cancer in women worldwide - from the basic biology of the disease, to risk factors and prevention, to treatment modalities to survivorship. We'll talk to leading experts, explore some of the ...
By writing essays on breast cancer, individuals can contribute to a better understanding of the disease, its impact, and the importance of ongoing research and support. ... Breast Cancer Speech Outline Introduction Brief overview of breast cancer awareness and its goals Breast Cancer Advocacy and Awareness Role of breast cancer advocates in ...
Breast cancer is a life-threatening cancer and a leading cause of death among women. Breast cancer cases are increasing constantly due to the risk factors including age, menopause, obesity, use of hormone replacement therapy, family history, along with the environment and lifestyle factors. The increased awareness and newer diagnosis techniques ...
Facts and Figures. Breast cancer (BC) is regarded as the most common type of cancer globally. According to Mascara and Constantinou (2021), "about 2.3 million people are diagnosed with the disease each year" (p. 9). In the U.S., approximately 264000 and 2400 cancer cases are diagnosed each year among women and men, respectively (Mascara and ...
Welcome to an Introduction to Breast Cancer! In this course, we'll learn a bit about the leading cause of cancer in women worldwide - from the basic biology of the disease, to risk factors and prevention, to treatment modalities to survivorship. We'll talk to leading experts, explore some of the milestone studies that have pushed this field forward, and have interactive discussions on ...
Introduction to Breast Cancer. Breast cancer is a malignant cell growth in the breast.If left untreated, the cancer spreads to other areas of the body. Excluding skin cancer, breast cancer is the most common type of cancer in women in the United States, accounting for one of every three cancer diagnoses.. An estimated 211,240 new invasive cases of breast cancer were expected to occur among ...
Purpose of Review Update on current racial disparities in the detection and treatment of breast cancer. Recent Findings Breast cancer remains the leading cause of cancer death among Black and Hispanic women. Mammography rates among Black and Hispanic women have surpassed those among White women, with studies now advocating for earlier initiation of breast cancer screening in Black women. Black ...
Introduction. Breast cancer is known as a hormone dependent malignant tumour. Currently, oestrogen receptor (ER) status is widely applied to provide prognostic information and guide treatment strategies [Citation 1].However, the underlying molecular mechanisms of ER in relation to breast cancer prognosis has not been fully disentangled [Citation 2, Citation 3].
The Big Five personality traits—neuroticism, extroversion, openness to experience, agreeableness, and conscientiousness—represent continuous, individual features that affect a number of vital health aspects, including morbidity, self-reported health status, or lifestyle. The aim of this study was to analyze the relationship between the eating behaviors and engagement in physical activity ...
Introduction To Breast Cancer Essay, Application Letter For Job Word, Should I Start My Essay With Hello, Network Security Breach Case Study, Sheets For Writing First Grade, Nrel Research Papers, Pay For Reflective Essay On Civil War 100% Success rate
1 Introduction. Currently, gastric cancer is one of the major malignancies threatening human lives and health (Smyth et al., 2020; Sun et al., 2020).The global cancer statistics report (GLOBOCAN) showed that there were 1.04 million new cases of gastric cancer globally in 2018, making it the fifth most common malignancy, and 0.78 million deaths caused by this disease, making it the third ...