
The Asthma and Allergy Foundation of America (AAFA), a not-for-profit organization founded in 1953, is the leading patient organization for people with asthma and allergies, and the oldest asthma and allergy patient group in the world.
- Board of Directors
- Medical Scientific Council
- Senior Leadership
- Sources of Financial Support
- AAFA Alaska Chapter
- AAFA Michigan Chapter
- AAFA New England Chapter
- AAFA St. Louis Chapter
- Support Groups

More than 27 million people in the United States have asthma. The best way to manage asthma is to avoid triggers, take medications to prevent symptoms, and prepare to treat asthma episodes if they occur.
- Asthma Facts
- Air Pollution
- Allergens and Allergic Asthma
- Emotions, Stress, and Depression
- Other Health Conditions
- Tobacco Smoke
- Respiratory Infections
- Asthma Symptoms
- Asthma-Like Conditions
- Lung Function Tests
- Physical Exam
- Asthma Action Plan
- Asthma Medicines
- Biologics for Asthma
- Oral Corticosteroids
- Vaccine Recommendations
- Asthma, Allergies & the ADA
- Asthma During Pregnancy
- Asthma in Infants
- Asthma in Children
- Asthma in Adults
- Asthma in Older Adults
- Managing Asthma and Allergies at School
- Traveling with Asthma & Allergies
- Work-Related Asthma

Allergies are one of the most common chronic diseases. An allergy occurs when the body’s immune system sees a substance as harmful and overreacts to it. The substances that cause allergic reactions are allergens .
- Drug Allergies
- Food Allergies
- Insect Allergies
- Latex Allergy
- Mold Allergy
- Pet Allergy
- Pollen Allergy
- Allergy Facts
- Anaphylaxis (Severe Allergic Reaction)
- Eye Allergies (Allergic Conjunctivitis)
- Nasal Allergies (Rhinitis)
- Skin Allergies
- Allergy Diagnosis
- Allergy Treatments
- Control Indoor Allergens
- Healthier Home
- The Allergic March

AAFA offers a variety of educational programs, resources and tools for patients, caregivers, and health professionals. AAFA launches educational awareness campaigns throughout the year. We teach the general public about asthma and allergic diseases.
- ACT for Asthma & Allergy
- No Appetite for Bullying
- Type 2 Inflammation
- Health Equity
- AAFA’s Certification Program
- Kids with Food Allergies
- ASTHMA Care for Adults
- Asthma Management Education
- Continuing Medical Education (CME) Programs
- Managing Food Allergies
- Patient and Caregiver Webinars
- Spanish Resources
- Understanding Research Basics


Research & Reports
Research is an important part of our pursuit of better health. Through research, we gain better understanding of illnesses and diseases, new medicines, ways to improve quality of life and cures. The Asthma and Allergy Foundation of America (AAFA) conducts and promotes research for asthma and allergic diseases.
- Asthma Capitals
- Allergy Capitals
- Asthma Disparities in America
- AFFORD Asthma Study
- Anaphylaxis in America
- Atopic Dermatitis in America
- Food Allergy Anaphylaxis in Infants and Toddlers
- Life with Eosinophilic Esophagitis (EoE)
- Life with Nasal Polyps
- My Life With Asthma
- My Life With Food Allergy
- Climate Change & Health Report
- Patient Focused Drug Development
- Access to Pseudoephedrine
- Clinical Trials
- Patient Engagement in Asthma Research
- For Researchers
- Research Publications

AAFA works to support public policies that will benefit people with asthma and allergies. Advocacy and public policy work are important for protecting the health and safety of those with asthma and allergies. We advocate for federal and state legislation as well as regulatory actions that will help you.
- Become an Advocate
- AAFA’s Positions & Statements
- State Honor Roll 2019
- Health Insurance Programs
- Drug Assistance Programs
- Accessing Your Medical Records
- Cost of Asthma on Society
- Patient and Family Engagement
- Asthma in Schools
- Access to Health Care
- Albuterol in Schools
- Epinephrine in Schools and Public Places
- National Asthma Control Program
- Food Allergies in Child Care Settings
- Food Allergen Labeling
- Health Disparities
- Healthy Settings

Get Involved
There are several ways you can support AAFA in its mission to provide education and support to patients and families living with asthma and allergies. You can make a donation, fundraise for AAFA, take action in May for Asthma and Allergy Awareness Month, and join a community to get the help and support you need.
- Coming Events
- Get Support
- Planned Giving
- Fundraise for AAFA
- Take Action
- Social Media Tools
- Eczema Awareness Month

AAFA can connect you to all of the information and resources you need to help you learn more about asthma and allergic diseases.
- AAFA’s e-Newsletters
- Press Releases
- FreshAAIR Magazine – Previous Issues
- Join the Community
Types of Allergies
An allergy occurs when your body’s immune system sees a certain substance as harmful. It reacts by causing an allergic reaction. Substances that cause allergic reactions are allergens.
There are many types of allergies. Some allergies are seasonal and others are year-round. Some allergies may be life-long. It is important to work with your health care provider to create a plan to manage your allergy. Avoiding your allergens is the best way to prevent an allergic reaction.

True allergies to drugs (medicines) occur in only a small number of people. Most drug reactions are not allergic, but are side effects of the properties of the medicine. A diagnosis of the cause of the drug reaction is usually based only upon the patient’s history and symptoms. Sometimes skin testing for drug allergy is also done.

Non-stinging insects can also cause allergic reactions. The most common are cockroaches and the insect-like dust mite. Allergies to these two insects may be the most common cause of year-round allergy and asthma.

Medical Review: October 2015
Support Our Work
Over the past 15 years, there have been moderate advances in U.S. public policy, health care and research, but racial gaps in asthma outcomes have not changed. Minority groups continue to bear disproportionate hardship in managing asthma.
Donate Today >

Related Content
Healthier Home Checklist: How to Improve Your Home’s Asthma and Allergy Hot Spots
Ask the Allergist: Can I Develop Tolerance to Antihistamines?

2024 Allergy Capitals Report Ranks the Most Challenging Cities to Live in with Seasonal Allergies
View more blog articles >
Search AAFA and the AAFA Community
- Search the site GO Please fill out this field.
- Newsletters
- Health Conditions A-Z
What Are Allergies?
Lindsay Curtis is a freelance health & medical writer in South Florida. Prior to becoming a freelancer, she worked as a communications professional for health nonprofits and the University of Toronto’s Faculty of Medicine and Faculty of Nursing.
:max_bytes(150000):strip_icc():format(webp)/ScreenShot2022-10-03at4.00.52PM-LindsayCurtis-31f4c08afe2a4bfab4f4c1336af79e3c.png)
Risk Factors
Comorbid conditions.
- Living With Allergies
An allergy is an overactive immune response to a substance (called an allergen) that is usually harmless, such as pollen or dust. Exposure to an allergy causes the body’s cells to release chemicals, such as histamines, that can cause inflammation and swelling that affects the skin, respiratory system, and digestive tract. This results in symptoms such as sneezing, itching, congestion, and rashes. In some people, allergic reactions can be life-threatening ( anaphylaxis ).
There are many types of allergies, including seasonal (pollen), food, drug, pet, mold, insect, and latex allergies. Healthcare providers such as allergists and immunologists (doctors who specialize in allergic conditions) diagnose allergies through physical examination, a review of symptoms and medical history, and allergy testing. Treatment options include medications, immunotherapy, and lifestyle changes.
Allergies are very common, affecting more than 50 million people in the United States alone. Worldwide, allergies affect up to 20% of the global population.
Types of Allergy
There are several different types of allergies, each with specific triggers and symptoms. Understanding the different types of allergies is important for proper diagnosis and treatment.
- Drug allergy: Drug (medicine) allergies occur when your body reacts to a specific medication.
- Food allergy: Food allergies occur when your body views a specific food ( e.g., peanuts ) as harmful and causes an allergic reaction. Symptoms can include itching, hives, and difficulty breathing.
- Pollen allergy: Also known as hay fever or allergic rhinitis, pollen allergy occurs when your body reacts to pollen from grasses, trees, and weeds.
- Latex allergy: A latex allergy occurs when your body reacts to natural rubber latex, which is found in many products such as bandages and balloons.
- Insect allergy: Insect allergies occur when your body reacts to the venom of stinging insects, such as bees and wasps .
- Mold allergy: Mold (fungus) allergies occur when spores from the mold enter your airways, and your body reacts to the spores, which are found in damp or humid environments.
- Pet allergy: Pet allergies occur when your body reacts to proteins in an animal's skin cells, urine, or saliva.
- Other indoor allergies: Cockroach and dust mites are other common indoor allergens.
Allergy Symptoms
Symptoms of allergies vary widely and depend on the type of allergy. Allergic reactions usually affect the area of the body that comes into contact with the allergen. For example, seasonal allergies (hay fever) occur when pollen is breathed in, so respiratory tract symptoms such as sneezing and a runny nose usually occur.
Skin Allergy Symptoms
Allergic skin reactions can occur when the skin comes into contact with an allergen (contact dermatitis). Common environmental allergens include pollen, mold, animal dander, and dust.
Common skin allergy symptoms include:
- Cracked skin
- Redness
- Scaly, flaking skin
Nasal Allergy Symptoms
Nasal allergies, also known as rhinitis or hay fever, happen when the nasal passages become inflamed after exposure to allergens. Allergic rhinitis can last for weeks or months, depending on the trigger. Common nasal allergy symptoms include:
- Itchy nose and eyes
- Congestion and sinus pressure
- Postnasal drip (mucus in the throat)
Eye Allergy Symptoms
Eye allergies ( allergic conjunctivitis ) occur when the eyes come into contact with an allergen, causing the eyes to become irritated and inflamed. Pollen, dust mites, mold spores, and pet dander can trigger eye allergies. Common eye allergy symptoms include:
- Red, itchy eyes
- Burning or gritty sensation in the eyes
- Watery eyes
- Swollen eyelids
Gastrointestinal Allergy Symptoms
Gastrointestinal allergies refer to allergic reactions that affect the digestive system. Food allergies are the most common cause of gastrointestinal allergies and can be triggered by various foods such as peanuts, tree nuts, dairy, eggs, soy, and wheat. Common gastrointestinal allergy symptoms include:
- Abdominal pain
Anaphylaxis
Anaphylaxis is a severe and potentially life-threatening allergic reaction that can occur quickly and without warning. It can affect multiple systems in the body, including the skin, respiratory, cardiovascular, and digestive systems. Symptoms of anaphylaxis can include:
- Hives or skin rash
- Swelling of the face or tongue
- Difficulty breathing
- Chest tightness
- Rapid or weak pulse
- Nausea or vomiting
- Uterine cramps
- A sense of dread or that something bad is going to happen
If You Have a Severe Allergic Reaction
If you are experiencing anaphylaxis, get help right away by calling 911. Anaphylaxis needs to be treated promptly with a shot of epinephrine—a hormone that’s also called adrenaline. This treatment is highly effective at slowing or stopping an allergic reaction and can be life-saving.
Even if you use epinephrine, you still need to seek immediate medical care.
What Causes Allergies?
Allergies occur when the body's immune system mistakenly views a normally harmless substance, such as pollen or food, as a threat and releases chemicals, including histamines, leukotrienes, and cytokines, to combat it. These chemicals can trigger the onset of allergy symptoms.
The exact cause of allergies is not fully understood, but genetic and environmental factors are believed to play a role. People with a family history of allergies are more likely to develop allergies. Exposure to allergens when the immune system is weakened, such as during pregnancy or after an illness, may play a role in the development of allergies.
Certain risk factors can raise the risk of developing allergies. Common allergy risk factors include:
- Family history of allergies
- Having asthma or eczema
- Living in an urban area
- Age (allergies are more likely to develop during childhood/adolescence)
How Is Allergy Diagnosed?
If you have symptoms of allergies but aren’t sure what is causing them, visit your healthcare provider. They may refer you to an allergist.
To diagnose allergies, your allergist will first ask about your symptoms, including how often you experience them and how severe they are. They will also ask about your home and work environments to identify potential allergens you are exposed to and ask about your health history and whether you have family members with allergies.
Your healthcare provider will also perform a physical examination of your eyes, ears, nose, and lungs. If you have respiratory symptoms of allergy (e.g., cough, runny nose), they may perform a lung function test to determine how well you exhale air from your lungs.
Your allergist may recommend allergy testing to determine what allergens you are allergic to provide an accurate diagnosis. Common allergy diagnostic tests include:
- Skin prick test: This involves exposing the skin to a small amount of an allergen. If you are allergic to a specific allergen, the exposed skin will be swollen, red, and itchy within 15 minutes.
- Blood test: A blood test called specific IgE (sIgE) involves taking a sample of your blood. This test measures the levels of allergen-specific antibodies to confirm an allergy.
- Intradermal test: Similar to the skin prick test, an allergen is injected into the top layer of the skin. This test may be performed if the skin prick test is negative but your allergist suspects you have an allergy.
- Patch test: Commonly used to determine what allergen causes contact dermatitis, this test involves applying small patches containing an allergen onto your skin, covering it with a bandage, and examining it for an allergic reaction after 48-96 hours. A rash will develop if you are allergic to that allergen.
- Challenge test: A physician-supervised challenge test involves taking or inhaling a small amount of a suspected allergen in the doctor’s office (usually food or medication) to look for signs of an allergic reaction.
In some cases, your healthcare provider may also perform additional tests, such as an X-ray of your chest and sinuses, to rule out other conditions or better evaluate the extent of your symptoms.
There is no cure for allergies. The goal of allergy treatment is to reduce the severity of symptoms and prevent future allergic reactions. The type of treatment your healthcare provider recommends will depend on the type of allergy you have and your symptoms.
Allergen Avoidance
Avoiding contact with known allergens is crucial in managing allergies. Though avoiding certain allergens is difficult, taking steps to reduce your exposure can help reduce allergic reactions. Depending on your allergy, this may involve avoiding certain foods, avoiding outdoor activities or wearing a mask during peak pollen season , installing air filters in your home, and using protective clothing to avoid insect bites.
Medications
Over-the-counter and prescription medications can help control allergy symptoms. Common allergy medications include:
- Antihistamines: These oral drugs block histamine production after exposure to an allergic to relieve symptoms such as itching, redness, hives, runny nose, and sneezing.
- Decongestants: These oral drugs arrow the blood vessels in the nasal passages to reduce inflammation and relieve nasal congestion.
- Corticosteroids: These decrease inflammation and swelling to help reduce allergy symptoms. Corticosteroids may be applied topically, taken orally, or used as a nasal spray.
- Mast cell stabilizers: These oral or inhaled medications revent the release of histamine and other chemicals to reduce allergy symptoms.
- Leukotriene modifiers: These oral drugs modify the immune system's response to allergens to prevent symptoms.
- Epinephrine: Administered as an injection (Epi-Pen), epinephrine is used in the case of severe, life-threatening allergic reactions (anaphylaxis).
Emergency Treatment for Anaphylaxis: Epinephrine
If you have a history of an allergy, talk to your healthcare provider about getting epinephrine auto-injectors. A common brand is EpiPen. Experts recommend carrying two epinephrine auto-injectors injections in case of an emergency. Epinephrine works by rapidly:
- Increasing low blood pressure
- Improving breathing
- Decreasing swelling
If your healthcare provider has not told you otherwise, plan to go to the emergency room after using an epinephrine auto-injector that is prescribed to you. This is to ensure you receive any additional medication or care that you may need if symptoms return or worsen.
Immunotherapy
Immunotherapy involves gradually exposing small amounts of known allergens to build immunity over time. Allergen-specific immunotherapy may be recommended for people with persistent and severe allergies that are not effectively managed with other treatments or when multiple allergens are causing symptoms.
Immunotherapy is administered in two different ways: subcutaneous immunotherapy (SCIT) and sublingual immunotherapy (SLIT).
Subcutaneous Immunotherapy (SCIT)
Known as allergy shots, SCIT is injections given 1-2 times per week for about six months until a maintenance dose is reached. After that, the maintenance dose is usually continued at least once a month for three to five years. Each injection contains a small amount of the allergen(s) when the therapy begins. Over time, the dose gradually increases to reduce sensitivity to the allergen and shots are given less frequently. People usually notice improvement by the time they reach maintenance doses.
Sublingual Immunotherapy (SLIT)
With SLIT, small doses of an allergen are given under the tongue to make the immune system less sensitive to the allergen and reduce symptoms. It may take up to 14 weeks of SLIT to notice a difference in symptoms.
How to Prevent Allergy Attacks
Preventing an allergic reaction isn’t always possible, but preventive measures can help reduce the frequency and severity of allergic reactions. Here are some ways to avoid allergy attacks:
- Identify and avoid allergens: Understanding what triggers your allergies is the first step in avoiding them. Allergy testing and tracking your symptoms will help you identify which allergens to avoid and when to take extra precautions (e.g., during pollen season).
- Reduce indoor allergens: Keep windows closed, use allergy-proof bedding, install air filters, and vacuum regularly to reduce allergens in the home.
- Take allergy medications: Follow your healthcare provider’s treatment recommendations and take your medications as prescribed to help control symptoms.
Your healthcare provider can work with you to determine the best approach for preventing allergy attacks and managing symptoms when they do occur.
Allergies are often associated with other medical conditions, which can occur together (comorbidity). Some of the most common comorbid conditions associated with allergies include:
- Asthma : Asthma is a chronic lung disease that causes the airways to be inflamed and narrow, making breathing difficult.
- Eczema (atopic dermatitis): Eczema is a skin condition characterized by itchy, red, and dry skin.
- Sinusitis : Sinusitis is an inflammation of the sinuses that can cause headaches, facial pain, and nasal congestion.
- Gastrointestinal disorders: People with allergies are more likely to develop gastrointestinal conditions like eosinophilic esophagitis (EoE).
- Mental health conditions: Living with allergies can impact your mental health, and research shows that people with allergic conditions have an increased risk of depression and anxiety .
Living With Allergies
Living with allergies isn’t easy. Experiencing symptoms and taking extra steps to avoid allergens can take a toll on your physical and mental health. The good news is proper management of allergies can help reduce the impact allergies have on your daily activities and improve your quality of life. Learning about your allergy, avoiding allergens, and following your treatment plan can help you live well.
Merck Manual: Consumer Version. Overview of allergic reactions .
Asthma and Allergy Foundation of America. Types of allergies .
Allergy & Asthma Network. Allergy statistics in the US .
Dierick BJH, van der Molen T, Flokstra-de Blok BMJ, et al. Burden and socioeconomics of asthma, allergic rhinitis, atopic dermatitis and food allergy . Expert Rev Pharmacoecon Outcomes Res . 2020;20(5):437-453. doi:10.1080/14737167.2020.1819793
Allergy and Asthma Foundation of America. Drug allergy .
Allergy and Asthma Foundation of America. Food allergy .
Allergy and Asthma Foundation of America. Pollen allergy .
Allergy and Asthma Foundation of America. Latex allergy .
Allergy and Asthma Foundation of America. Insect allergy .
Allergy and Asthma Foundation of America. Mold allergy .
Allergy and Asthma Foundation of America. Pet allergy .
InformedHealth.org: Institute for Quality and Efficiency in Health Care. Allergies: Overview .
Allergy and Asthma Foundation of America. Skin allergies .
Allergy and Asthma Foundation of America. Nasal allergies (rhinitis) .
Asthma and Allergy Foundation of America. Eye allergy (allergic conjunctivitis) .
Food Allergy Canada. Reaction signs and symptoms .
Asthma and Allergy Foundation of America. Severe allergic reaction: Anaphylaxis .
Thangam EB, Jemima EA, Singh H, et al. The role of histamine and histamine receptors in mast cell-mediated allergy and inflammation: The hunt for new therapeutic targets . Front Immunol. 2018;9:1873. doi:10.3389/fimmu.2018.01873
American College of Allergy, Asthma & Immunology. Who gets allergies?
Portelli MA, Hodge E, Sayers I. Genetic risk factors for the development of allergic disease identified by genome‐wide association . Clin Exp Allergy . 2015;45(1):21-31. doi:10.1111/cea.12327
American Academy of Dermatology Association. Can eczema increase risk of asthma, hay fever and food allergy?
Tizek L, Redlinger E, Ring J, Eyerich K, Biedermann T, Zink A. Urban vs rural - Prevalence of self-reported allergies in various occupational and regional settings . World Allergy Organ J. 2022;15(1):100625. doi:10.1016/j.waojou.2022.100625
Nemours Kids Health. Season allergies (hay fever) .
Allergy & Asthma Network. Allergy diagnosis and testing .
Allergy and Asthma Foundation of America. How do doctors diagnose allergies?
Allergy and Asthma Foundation of America. Allergy prevention .
Allergy & Asthma Network. How are allergies treated?
Bekić S, Martinek V, Talapko J, Majnarić L, Vasilj Mihaljević M, Škrlec I. Atopic dermatitis and comorbidity . Healthcare (Basel). 2020;8(2):70. doi:10.3390/healthcare8020070
Related Articles
- Patient Care & Health Information
- Diseases & Conditions
To evaluate whether you have an allergy, your health care provider will likely:
- Ask detailed questions about signs and symptoms
- Perform a physical exam
- Have you keep a detailed diary of symptoms and possible triggers
If you have a food allergy, your provider will likely:
- Ask you to keep a detailed diary of the foods you eat
- Ask if you've stopped eating the suspected food during the allergy evaluation
Your provider might also recommend one or both of the following tests. However, be aware that these allergy tests can be falsely positive or falsely negative.
- Skin test. Your skin will be pricked with small amounts of the proteins found in common allergens. If you're allergic, you'll likely develop a raised bump (hive) at the test location on your skin.
- Blood test. Specific IgE (sIgE) blood testing, commonly called radioallergosorbent test (RAST) or ImmunoCAP testing, measures the amount of allergy-causing antibodies in your bloodstream, known as immunoglobulin E (IgE) antibodies. A blood sample is sent to a medical laboratory, where it can be tested for evidence of sensitivity to possible allergens.
If your provider suspects your problems are caused by something other than an allergy, other tests might help identify — or rule out — other medical problems.
More Information
- Allergy skin tests
Allergy treatments include:
- Allergen avoidance. Your provider will help you take steps to identify and avoid your allergy triggers. This is generally the most important step in preventing allergic reactions and reducing symptoms.
- Medications. Depending on your allergy, medications can help reduce your immune system reaction and ease symptoms. Your provider might suggest nonprescription or prescription medication in the form of pills or liquid, nasal sprays, or eyedrops.
Immunotherapy. For severe allergies or allergies not completely relieved by other treatment, your provider might recommend allergen immunotherapy. This treatment involves a series of injections of purified allergen extracts, usually given over a period of a few years.
Another form of immunotherapy is a tablet that's placed under the tongue (sublingual) until it dissolves. Sublingual drugs are used to treat some pollen allergies.
- Emergency epinephrine. If you have a severe allergy, you might need to carry an emergency epinephrine shot at all times. Given for severe allergic reactions, an epinephrine shot (Auvi-Q, EpiPen, others) can reduce symptoms until you get emergency treatment.
- Allergy medications: Know your options
Clinical trials
Explore Mayo Clinic studies testing new treatments, interventions and tests as a means to prevent, detect, treat or manage this condition.
Lifestyle and home remedies
Some allergy symptoms improve with home treatment.
- Sinus congestion and hay fever symptoms. These often improve with saline nasal irrigation — rinsing out the sinuses with a salt and water solution. You can use a neti pot or a specially designed squeeze bottle to flush out thickened mucus and irritants from your nose. However, improper use of a neti pot or other device can lead to infection.
- Household airborne allergy symptoms. Reduce your exposure to dust mites or pet dander by frequently washing bedding and stuffed toys in hot water, maintaining low humidity, regularly using a vacuum with a fine filter such as a high-efficiency particulate air (HEPA) filter and replacing carpeting with hard flooring.
- Mold allergy symptoms. Reduce moisture in damp areas, such as your bath and kitchen, by using ventilation fans and dehumidifiers. Fix leaks inside and outside your home.
Alternative medicine
Clinical practice guidelines suggest that some people with allergic rhinitis may benefit from acupuncture.
Preparing for your appointment
For symptoms that could be caused by an allergy, see your primary health care provider. You might be referred to a provider who specializes in treating allergies (allergist).
What you can do
Ask if you should stop taking allergy medications before your appointment, and for how long. For example, antihistamines can affect the results of an allergy skin test.
Make a list of:
- Your symptoms, including any that seem unrelated to allergies, and when they began
- Your family's history of allergies and asthma, including specific types of allergies, if you know them
- All medications, vitamins and other supplements you take, including doses
- Questions to ask during your appointment
Some basic questions to ask include:
- What is the most likely cause of my signs and symptoms?
- Are there other possible causes?
- Will I need allergy tests?
- Should I see an allergy specialist?
- What treatment do you recommend?
- I have these other health conditions. How can I best manage them together?
- What emergency symptoms should my friends and family be aware of?
Don't hesitate to ask other questions.
What to expect from your doctor
Your provider is likely to ask you questions, including:
- Have you recently had a cold or other respiratory infection?
- Are your symptoms worse at certain times of the day?
- Does anything seem to improve or worsen your symptoms?
- Are your symptoms worse in certain areas of your house or at work?
- Do you have pets, and do they go into bedrooms?
- Is there dampness or water damage in your home or workplace?
- Do you smoke, or are you exposed to secondhand smoke or other pollutants?
- What treatments have you tried so far? Have they helped?
- Allergy overview. Asthma and Allergy Foundation of America. https://www.aafa.org/allergies.aspx. Accessed July 28, 2020.
- Allergic reactions. American Academy of Allergy, Asthma and Immunology. https://www.aaaai.org/conditions-and-treatments/library/allergy-library/allergic-reactions. Accessed July 28, 2020.
- Seidman MD, et al. Clinical practice guideline: Allergic rhinitis. Otolaryngology — Head and Neck Surgery. 2015;152:S1.
- Allergies. American College of Allergy, Asthma & Immunology. https://acaai.org/allergies. Accessed July 28, 2020.
- Allergies and hay fever. American Academy of Otolaryngology — Head and Neck Surgery. https://www.entnet.org/node/1347. Accessed July 28, 2020.
- Anaphylaxis. American Academy of Allergy, Asthma and Immunology. https://www.aaaai.org/conditions-and-treatments/allergies/anaphylaxis. Accessed July 28, 2020.
- Types of allergies. Asthma and Allergy Foundation of America. https://www.aafa.org/types-of-allergies/. Accessed July 28, 2020.
- Who has allergies? American Academy of Allergy, Asthma and Immunology. https://acaai.org/allergies/who-has-allergies. Accessed July 28, 2020.
- What causes asthma? American Lung Association. https://www.lung.org/lung-health-diseases/lung-disease-lookup/asthma/asthma-symptoms-causes-risk-factors/what-causes-asthma. Accessed July 28, 2020.
- Is rinsing your sinuses with neti pots safe? U.S. Food and Drug Administration. https://www.fda.gov/consumers/consumer-updates/rinsing-your-sinuses-neti-pots-safe. Accessed July 28, 2020.
- Allergy-proof your home
- Nasal Cleaning
Associated Procedures
News from mayo clinic.
- Expert advice on breathing issues due to wildfire smoke June 08, 2023, 04:31 p.m. CDT
- How does red tide impact beachgoers? March 17, 2023, 03:30 p.m. CDT
- Mayo Clinic Q and A: Is it allergies or a sinus infection? March 14, 2023, 01:30 p.m. CDT
Products & Services
- A Book: Mayo Clinic Book of Home Remedies
- Assortment of Health Products from Mayo Clinic Store
- Symptoms & causes
- Diagnosis & treatment
- Doctors & departments
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
Make twice the impact
Your gift can go twice as far to advance cancer research and care!
Frontiers for Young Minds

- Download PDF
Allergy: Concepts and Treatments

Allergy is a syndrome characterized by an undesirable bodily reaction against a harmless substance. Allergies are very common, and the prevalence of allergic diseases has continued to grow over the last 50 years. It is not completely clear why some people develop allergic reactions and others do not. In this article, we explore how an allergic reaction occurs, as well as discuss the causes of allergy and its treatments.
Importance to Children and Teens
Allergy is a worldwide problem, and there is a high prevalence of allergic syndromes like allergic asthma and food allergies among children. It is important to understand what triggers and how to prevent/treat an allergic reaction. Not only that but highlight the usage of immunotherapy for allergic disorders and how they can improve the quality of life of non-drugs responders.
What Is an Allergy?
We all have a recognition and defense system in our bodies, which is called the immune system. The immune system is made up of cells, substances called antibodies, and many other components. When we are vaccinated or when we get infected by a microorganism that can cause disease, our immune system aims to develop cells and antibodies that will protect us against that specific microorganism. Antibodies are molecules produced by cells called B cells, and antibodies have the ability to bind to the invading microorganism, to prevent or combat an infection. However, in an allergy, the immune system reacts in a different way.
An allergy occurs when, for some reason, the immune system creates a response against a harmless substance, like pollen or a certain kind of food. Allergies are very common, and it is estimated that around one in three people has some type of allergy. Allergy can generate a reaction in any part of the body. For example, a food allergy can cause problems in the intestines, with symptoms, such as abdominal pain and diarrhea. Allergy can show up in the lungs in the form of allergic asthma, causing difficulty breathing and overproduction of mucus.
How Does an Allergic Response Happen?
When the body of an allergic person encounters an allergen (the substance that causes the allergic reaction), the immune system produces cells and antibodies against that harmless substance. Common allergens are shown in Figure 1 . Some of the cells produced in an allergic response are called type 2 T helper cells (Th2), and these Th2 cells help other cells, called B cells, to produce a certain type of antibody called immunoglobulin E (IgE) . The IgE then binds to other immune cells, making those cells very sensitive to the allergen. When the person comes into contact with that same allergen again, the immune cells coated with IgE release chemicals that cause inflammation and swelling, and those chemicals are the cause of the itching and sneezing symptoms that often occur in the body’s attempt to remove the allergen. The Th2 cells can also go to the site where the allergen entered the body and promote inflammation in that site. For example, the eyes of an allergic person can become swollen, red, and itchy in response to an allergen in the air, like pollen.
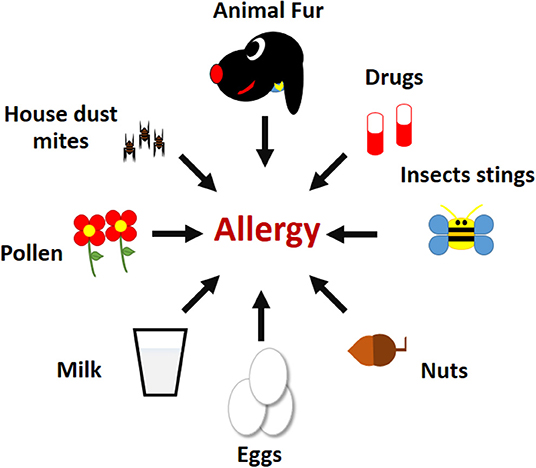
- Figure 1 - Examples of common allergens.
- Mites present in house dust, fur from domestic animals, compounds in drugs, venom and other substances presents in insect stings, nuts, eggs, milk, and pollen from flowers.
Why Do Only Some People Have Allergies?
Although we know a lot about how allergic reactions occur, the exact reason why some people develop allergies while others do not is still a mystery. We know that there is a genetic predisposition, meaning that if your mother or father have allergies, you are more likely to also have allergies. Factors in the environment also play a role in the likelihood of developing allergies [ 1 – 3 ], such as:
- Where you live (if it is rural or urban);
- Lifestyle factors (whether you exercise, for example);
- Dietary habits (the food you eat); and
- Medicines (antibiotics for example).
How Are Allergies Diagnosed?
To be sure that a person is allergic to something, a medical doctor can perform some simple tests. The most popular ones are called the skin-prick test and the IgE test.
In the skin-prick test, the doctor or nurse puts tiny amounts of different allergens just under your skin. If you are allergic, you will develop a red bump where the allergen was placed. The doctor can tell how allergic you are by the size of the red bump. In the IgE test, the doctor will do some blood tests to see whether you have a high amount of IgE in your bloodstream, or IgE specific to some common allergens.
How Are Allergies Treated?
If you have a positive allergy test, and you know which allergen(s) you are allergic to, the best treatment is to avoid contact with those allergens. However, it is easier to avoid contact with some allergens, like peanuts or milk, than it is to avoid airborne allergens, like dust or pollen. Can you imagine not being exposed to any pollen during spring? Since it is very hard to avoid some allergens completely, particularly the airborne ones, medicines have been developed to reduce inflammation and stop the allergic reaction from occurring.
If an allergic person is exposed to a very large amount of allergen, or if the person has a very severe allergy, that person may develop a serious, body-wide reaction that can be life-threatening. This reaction is called anaphylactic shock . Anaphylactic shock can occur very quickly after exposure to the allergen. The person’s blood pressure drops, he or she can have extreme difficulty breathing, and death can even result. Therefore, if you see someone having an anaphylactic shock, quickly call for help, because this is a true medical emergency.
Is There a Cure for Allergy?
The medicines developed to treat allergies are safe and helpful for relieving allergy symptoms, but they do not cure allergies [ 4 ]. For more than 100 years, scientists have been working on developing a cure for allergic disorders [ 5 ]. This is especially important because a part of the allergic population does not respond well to regular allergy medicines.
Currently, the only treatment with the potential for cure is called immunotherapy. Immunotherapy is a treatment that regulates a person’s immune system so that the person responds differently to an allergen. Immunotherapy is used for other diseases in addition to allergy, including as a treatment for certain types of cancer.
Immunotherapy for allergies involves giving increasing doses of the allergen to the allergic person, over time. There are two different allergy immunotherapies: subcutaneous, when the allergen is put under the skin of the patient (also called allergy shots) and sublingual, when the allergen is put under the tongue of the patient (also called allergy tablets). Both types of immunotherapy have strong and weak points, but they are both effective, to some extent, in reducing allergic reactions.
In general, allergy immunotherapy aims to teach the immune system to act differently, producing another kind of T cells, called T regulatory cells (Treg), and other types of antibodies, the blocking immunoglobulin G (IgG) ( Figure 2 ). Treg cells encounter the Th2 cells and prevent their activation. IgG antibodies encounter the allergen before the allergen meets the IgE antibodies on immune cells, and this can help prevent the allergic reaction.
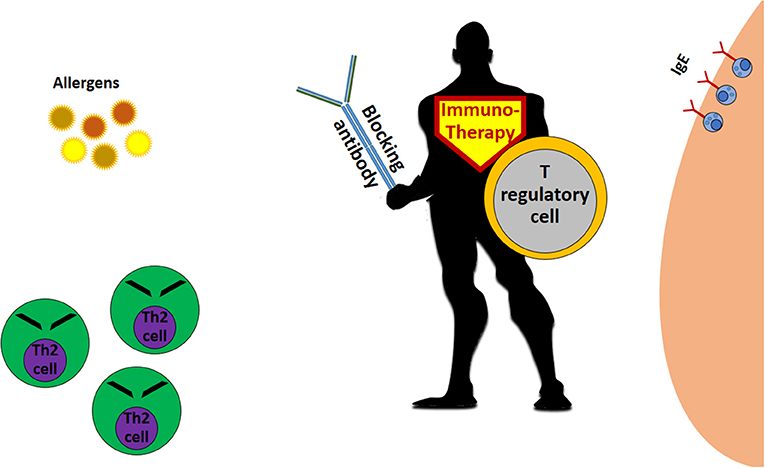
- Figure 2 - The allergy immunotherapy “superhero.”
- Immunotherapy for allergy works mainly through the creation of T regulatory cells, which inhibit allergy-causing Th2 cells and stimulate the production of blocking antibodies that prevent the allergen from coming in contact with the “allergy antibody,” IgE.
In conclusion, allergies are a serious health problem. Although there are medicines that relieve allergy symptoms, the only cure for allergy is the use of immunotherapy, which changes the patient’s response to the allergen. Immunotherapy is usually prescribed for patients that do not respond to the usual medicines. Scientists all over the world are still working on understanding allergies so that they can develop better treatments and possibly even a cure for allergies.
Immunotherapy : ↑ Is a type of treatment that aims to modify how your immune system reacts to something, in the case of allergy immunotherapy it aims to modify the reaction to the allergen.
Allergen : ↑ Is normally a harmless substance, which in the case of an allergic person can trigger a response for the immune system and results in an allergic reaction.
Antibody : ↑ A Y-shaped protein produced B cells. Antibodies bind to microorganisms that infect the body, to neutralize them, and also to allergens, which creates an allergic response.
IgE : ↑ A type of antibody produced by an allergic person. IgE binds to certain cells of the immune system and causes an immediate allergic reaction if the person comes in contact with the allergen again.
Anaphylactic Shock : ↑ It is a severe, potentially life-threatening, allergic reaction. Some common symptoms include swelling of throat or tongue, difficulty breathing, vomiting, dizziness, and drop blood pressure.
IgG : ↑ A type of antibody that can be produced after allergy immunotherapy. IgG can prevent the immediate allergic reaction by binding to the allergen and blocking it, before the allergen binds to the IgE.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
[1] ↑ Alberca-Custódio, R. W., Greiffo, F. R., MacKenzie, B., Oliveira-Junior, M. C., Andrade-Sousa, A. S., Graudenz, G. S., et al. 2016. Aerobic exercise reduces asthma phenotype by modulation of the leukotriene pathway. Front. Immunol. 7:237. doi: 10.3389/fimmu.2016.00237
[2] ↑ Schröder, P. C., Li, J., Wong, G. W., and Schaub, B. 2015. The rural-urban enigma of allergy: what can we learn from studies around the world? Pediatr. Allergy Immunol. 26:95–102. doi: 10.1111/pai.12341
[3] ↑ Yilmaz, B., Carvalho, J. C., and Marialva, M. 2019. The intestinal universe—full of gut heroes who need sidekicks. Front. Young Minds 7:111. doi: 10.3389/frym.2019.00111
[4] ↑ Larsen, J. N., Broge, L., and Jacobi, H. 2016. Allergy immunotherapy: the future of allergy treatment. Drug Discov. Today 21:26–37. doi: 10.1016/j.drudis.2015.07.010
[5] ↑ Ring, J., and Gutermuth, J. 2011. 100 years of hyposensitization: history of allergen-specific immunotherapy (ASIT). Allergy 66:713–24. doi: 10.1111/j.1398-9995.2010.02541.x
- Type 2 Diabetes
- Heart Disease
- Digestive Health
- Multiple Sclerosis
- COVID-19 Vaccines
- Occupational Therapy
- Healthy Aging
- Health Insurance
- Public Health
- Patient Rights
- Caregivers & Loved Ones
- End of Life Concerns
- Health News
- Thyroid Test Analyzer
- Doctor Discussion Guides
- Hemoglobin A1c Test Analyzer
- Lipid Test Analyzer
- Complete Blood Count (CBC) Analyzer
- What to Buy
- Editorial Process
- Meet Our Medical Expert Board
How Do You Know If You Have Allergies?
What are allergies, types of allergies.
- Ways To Recognize an Allergy
Management and Prevention
When to see a doctor, frequently asked questions.
It can sometimes be difficult to tell the difference between allergies and a cold , or other illnesses that cause similar symptoms. This is especially true if your allergy symptoms are mild. Though symptoms can vary, if you have itchy, watery eyes , and a runny nose, it's likely that you have allergies. In this article, we'll discuss allergy symptoms and their diagnosis and treatment.
Verywell / Jiaqi Zhou
Allergies are the ways your body responds to substances it sees as harmful. However, these substances are often harmless. For example, some people may sneeze and have watery eyes when they come in contact with pollen . The pollen, which causes your body to react in that way, is an allergen.
During allergies, your immune system produces antibodies called Immunoglobulin E (IgE) that tag a harmless allergen as harmful. When you come across an allergen, your immune system then inflames different parts of your body like your skin, sinuses, and airway.
Your immune system keeps an eye out for an allergen so that it can release antibodies when it detects it again. These antibodies release chemicals like histamine, which cause allergic reactions.
Common allergens include:
- Grass and tree pollen
- Pet dander, tiny flakes of skin or hair
- Food , such as peanuts and tree nuts, wheat, soy, fish, shellfish, and dairy
- Insect bites and stings
- Medications, including ibuprofen and certain antibiotics like penicillin
- Household chemicals like those used in detergents and hair dyes
Note that you’ll be more likely to have an allergy if:
- Your family has a history of asthma or allergies
- You are a child, and your siblings or parents have allergies or asthma
- You have asthma or an allergic condition
Allergies are caused by an overreaction of your immune system to allergens. Common allergens include food, grass and tree pollen, dust mites, mold, medications, and insect bites.
Signs and Symptoms of Allergies
Allergic reactions depend on the allergen involved and can affect different parts of your body. Allergy symptoms can be mild to severe. Severe allergies can trigger a dangerous reaction known as anaphylaxis . Common symptoms of allergies include:
- Runny or stuffy nose
- Watery eyes
- Wheezing or coughing
- Worsening asthma or eczema symptoms
There are many types of allergies. They can be caused by different allergens and have different types of symptoms.
Hay fever , also known as allergic rhinitis, is a type of allergy triggered by pollen from trees, weeds, and grasses. Each spring, summer , and fall, trees, weeds, and grasses release tiny pollen grains into the air. Some of the pollen ends up in your nose and throat. Hay fever affects 40 million to 60 million Americans.
Symptoms of allergic rhinitis can include:
- Sneezing, often with a runny or clogged nose
- Coughing and postnasal drip
- Itchy eyes, nose and throat
- Red, watery eyes
- Dark circles under the eyes
Atopic Dermatitis
Atopic dermatitis is a type of eczema, and causes your skin to become red and itchy. The American Academy of Dermatology estimates that one in 10 people have this condition.
Atopic dermatitis is due to a reaction in the skin. The reaction leads to ongoing itching, swelling, and redness. People with atopic dermatitis may be more sensitive because their skin lacks specific proteins that maintain the skin's barrier to water.
Atopic dermatitis can be caused by allergies. In some children, food allergies and dust mite allergies play a role in the development of atopic dermatitis.
The following can make atopic dermatitis symptoms worse:
- Allergies to pollen, mold, dust mites, or animals
- Cold and dry air in the winter
- Colds or the flu
- Contact with irritants and chemicals
- Contact with rough materials, such as wool
- Emotional stress
- Drying out of the skin from taking frequent baths or showers and from swimming
- Getting too hot or too cold, as well as sudden changes in temperature
- Perfumes or dyes added to skin lotions or soaps
Drug Allergies
A drug allergy occurs when your body’s immune system becomes sensitized to a substance in a medication, perceives it as a foreign invader, and releases chemicals to defend against it. Common triggers of drug allergies:
- Penicillin and related antibiotics
- Antibiotics containing sulfonamides (sulfa drugs)
- Anticonvulsants
- Aspirin, ibuprofen, and other nonsteroidal anti-inflammatory drugs (NSAIDs)
- Chemotherapy drugs
An allergic reaction to drugs can affect any part of your body. Common symptoms include:
Anaphylaxis, a potentially life-threatening reaction, can also occur.
Food Allergies
A food allergy is an abnormal response to a food triggered by your body's immune system. In adults, the foods that most often trigger allergic reactions include fish, shellfish, peanuts, and tree nuts, such as walnuts. Problem foods for children can include eggs, milk , peanuts, tree nuts, soy, and wheat.
A food allergy can cause:
- Itching or swelling in your mouth
- Vomiting, diarrhea, or abdominal cramps and pain
- Tightening of the throat and trouble breathing
- Drop in blood pressure
When you have food allergies, you must be prepared to treat an accidental exposure. Wear a medical alert bracelet or necklace and carry an auto-injector device containing epinephrine.
Insect Sting Allergies
Insect sting allergies occur when your immune system overreacts to the venom in insect stings. Stinging insects include:
- Yellow jackets
- Honeybees and bumblebees
- Paper wasps
Insect sting allergies can cause the following symptoms:
- Swelling at the sting site
- Difficulty breathing
- Anaphylaxis
Ways to Recognize an Allergy
Even though it may not be easy to tell if you have an allergy, there are a few ways that you can differentiate an allergy from other conditions.
Make a Checklist of Symptoms
It may be difficult for you to differentiate between an allergy and a cold because both come with similar symptoms. It that is the case, it would be best to write down the symptoms you are experiencing.
If you have a fever, green mucus, and body aches , then you most likely have a cold. However, if you have sneezing, watery eyes, clear mucus, and itchy eyes, ears, nose, or throat, you most likely have an allergy.
Note What Time Allergy Symptoms Occur
Noting the time span and exact time you have these allergic reactions can help you discover the cause. A cold generally lasts between five to seven days. If allergy symptoms last for more than two weeks or for months at a time, you may have a seasonal allergy.
If your symptoms worsen during the spring or fall, when pollen counts are higher, then you are more likely to have a seasonal allergy. If you have allergic reactions around the clock, you should check to see if there are allergens in your environment like dust mites.
Rule Out Other Conditions
Some disorders are often misdiagnosed as food allergies. Also, food intolerance is often confused with allergies. Food intolerance is your body's response to what you eat. For example, people who are lactose-intolerant react to milk products. As a result, they experience abdominal pain due to gas. Although the symptoms may be similar to those of a food allergy, they should not be confused.
It’s best to see an allergist or immunologist if your allergic reaction lasts more than two weeks or more and recurs often.
Skin tests are commonly used to identify the allergens that are causing your allergy symptoms. Your allergist will prick your skin with the extract of an allergen and then check for a reaction.
A blood test can also be performed. It checks the amount of antibodies your immune system produces. A higher count shows that you may be allergic to the allergen. You should note, however, that this test is not as sensitive as a skin test.
Even though staying away from the allergen seems like the best way to treat an allergy, it isn’t the most efficient in emergencies. Some common treatment methods include the following:
- Medications : Antihistamines or steroids are commonly used to treat allergies like allergic rhinitis and allergic conjunctivitis (inflammation of the whites of the eyes). These drugs come in tablets, injections, and nasal sprays. Your doctor may also recommend steroid creams.
- Allergen-specific immunotherapy ( desensitization ) : Also known as desensitization, this method exposes you to little bits of the allergen at regular intervals. These can be given as drops under the tongue or by injection. It takes three to five years to complete the therapy. This treatment method is used in treating pollen, dust, or insect sting allergies.
Preventing allergic reactions is easier than treating them. General preventive strategies include:
- Avoiding allergy triggers : Despite whether you are undergoing treatment, it helps if you avoid the allergens that cause your reaction. For example, people allergic to pollen should stay indoors when the pollen count is high. Those allergic to dust or pets should keep their environment clean and stay away from pet dander. Look for a vacuum with a high-efficiency particulate air (HEPA) filter.
- Wearing a medical alert device/mobile medical alert app : If you've experienced severe allergies, it is important you wear a medical alert device or have a mobile app that will let close friends and families know when you run into that kind of trouble.
- Noting the symptoms and triggers: A good way to avoid allergies is by finding out the cause. Write down what you do or eat and if there are any symptoms associated with your actions. This may help you and your allergist discover your allergens.
Avoiding allergy triggers is a common way to manage and prevent allergies, but that is not always enough. Over-the-counter (OTC) medications and immunotherapy can treat allergies. Make a note of your triggers so you and your doctor can determine ways to keep your allergy under control. Also, wear a medical bracelet in case you can't communicate when you experience a severe allergic reaction.
If OTC allergy drugs don’t stop the allergic reaction, see your doctor immediately. Also, if you notice an allergic reaction after starting a new drug, reach the doctor that recommended it immediately.
In severe cases, like anaphylaxis, seek emergency medical assistance. If you have epinephrine on you, self-administer the medication as soon as you notice symptoms of an allergic reaction.
You should still visit the emergency room after the injection. If you have had an anaphylactic reaction before, schedule an appointment to visit your doctor.
Allergies are your body’s way of fighting off substances that it sees as harmful even when they are harmless. Your body's immune system reacts when triggers to the allergens invade your body.
Some common causes of these reactions are pollen, pet dander, dust mites, chemicals, or even insect bites.
Ways to manage allergies are by taking over-the-counter medications or prescription medications as recommended by your healthcare professional. It is also important to avoid potential triggers that might cause reactions. In serious cases, dial 911 or visit the nearest emergency ward to receive adequate medical attention.
A Word From Verywell
Allergies are common but not deadly as long as they are kept under control. Educating yourself and taking the right precautions can help you live through these episodes. However, don’t forget to always inform your doctor if you notice symptoms that are not normal in your body.
Allergies and colds share symptoms like sneezing and stuffy or runny nose, headache, and fatigue. However, what they don’t share is a fever. You will not get a fever if you’re having an allergic reaction.
Also, you don’t experience itchy ears with the common cold the way you would with allergies. Note, too, that it is rare to experience muscle aches or sore throats when having allergies.
Both allergies and sinus infections come with a stuffy nose. Nevertheless, they have their differences. A sinus infection arises from an allergy. With sinusitis, you may have thick mucus, postnasal drip, cough, sore throat, and fatigue. Whereas with allergies, you mainly experience a runny nose, sneezing, watery eyes, and wheezing.
The time it takes for an allergic reaction to stop depends on the type of reaction (allergic rhinitis, rash, anaphylaxis) and whether exposure to the allergy trigger (allergen) is continuing.
You are likely to have allergy symptoms with seasonal allergies as long as you are exposed to the allergy trigger (such as pollen), which can be two or more months each year. You may have ongoing exposure when you have allergies to mold, dust mites, or pet dander.
You may continue to have an allergy for the rest of your life once you develop it. However, some people find that over the years they no longer have symptoms when exposed to an allergen.
Information NC for B, Pike USNL of M 8600 R, MD B, Usa 20894. Allergies: Overview. Institute for Quality and Efficiency in Health Care (IQWiG); 2020
National Health Service. Allergies overview .
Identifying causes of food allergy & assessing strategies for prevention | NIH: National Institute of Allergy and Infectious Diseases.
Asthma and Allergy Foundation of America. Types of allergies .
MedlinePlus. Hay fever .
American Academy of Dermatology Association. Eczema types: Atopic dermatitis overview .
MedlinePlus. Atopic dermatitis .
American College of Allergy, Asthma & Immunology. Drug allergy .
MedlinePlus. Food allergy .
National Institute of Allergy and Infectious Diseases. Characterizing food allergy & addressing related disorders .
American Academy of Allergy, Asthma & Immunology. Allergy testing .
Asthma and Allergy Foundation of America. Rhinitis (nasal allergies) .
Cleveland Clinic. Can you outgrow hay fever or other allergies?
Allergies - symptoms . nhs.uk.
Information NC for B, Pike USNL of M 8600 R, MD B, Usa 20894. Allergies: Overview. Institute for Quality and Efficiency in Health Care (IQWiG).
Nadolpho. Hay fever . ACAAI Patient.
By Margaret Etudo Etudo is a medical writer pursuing her Bachelor of Pharmacy.
Allergies: A Scientific Explanation
Some say a sneeze and a stuffy nose come from allergies. However, a rash coming from touching or eating something can also be called an allergy. What are allergies then? What causes them? What happens inside the body during an allergic reaction?
Figure of an antibody and how they match with certain antigens.
WHO IS THE BAD GUY?
First, allergies are always the result of an external stimulant that ranges from airborne particles like pollen or animal hair, to ingested matter like cinnamon or certain medicines. The immune system considers these outside particles called allergens foreign to the body and will react to them accordingly. When the immune system first encounters an allergen, it releases antibodies, a group of Y-shaped proteins (1). The antibodies are also shaped to attach to specific types of allergens. This can be imagined like a lock and key. Each type of antibody (key) attaches to a specific allergen (lock). During an allergic response, the antibodies will attach to the incoming allergen. Then, this conglomerate will travel to the mast cells located in all body tissues. Most mast cells are found in surface tissues such as the skin, nose, and mucus membranes in the nose. Their main job is to secrete certain chemicals to flush out this allergen. These chemicals and the effects they have on the body result in the allergic reaction (2, 5, 6, 7). One of the chemicals secreted by the mast cell is histamine. Histamine constricts the airways causing difficulty in breathing. It also expands the vessels leading to fluid leakage. Another chemical released is leukotrienes. This causes the excessive secretion of mucus and thus a runny nose (2, 6).
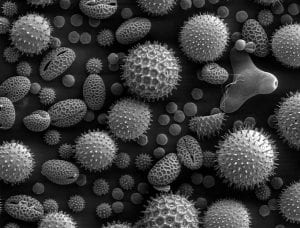
A collection of plant pollen, a common allergen, taken with an electron microscope magnified 500x.
The most common allergy most people think of is allergic rhinitis, also known as hay fever. This allergy is seasonal and often triggered by air particles like pollen, molds, dust mites, cleaning products, or pet hairs (2, 6). As mentioned above, the pollen and dust are the allergens that cause the body’s immune system to release antibodies. The release of these chemicals causes the constriction of airways, overproduction of mucus, and inflammation in the sinuses, which usually manifests as sneezing and a stuffy nose. Preventing hay fever can often be done by adding more omega-3 fats (fish or krill oil) into the diet, which reduces inflammation (5). Rubbing eucalyptus oil on the nose and temples is also a very soothing treatment because the strong smell of the eucalyptus clears the airways and thins out the mucus and stuffiness.
WHAT DID I TOUCH?
Another form of allergic reaction is contact dermatitis (6, 7, 10). This occurs when the body comes into contact with an object or chemical that the body reacts to as an allergen. These allergens can be any everyday object such as leather or nickel or ingredients in the soap or detergent or even the fabric of one’s clothes. For people who aren’t allergic to the allergen, nothing may happen when the allergen touches their skin. However, it is possible for a person to develop an allergic reaction to certain allergens over time. One such allergen is the formaldehyde. This compound is an adhesive used to hold together leather, or attach rubber to rubber or rubber to metal. It can be found in simple items like shoes, belts, or watch straps. When the immune system recognizes the allergen, it sends the antibodies to the mast cells on the skin and release large amounts of histamine. The histamine will cause the area of the skin touching the allergen to become inflamed, itchy, and display hives. At times, coming into contact with the allergen will cause the antibodies to travel to different areas on the body not touching the allergen. For example, it’s possible for a person who is allergic to formaldehyde to wear a leather watch on the wrist and develop a rash not only on that wrist, but also on the neck area. However, a skin patch test can determine the allergen. In this test, a doctor will stick multiple patches onto the patient’s back (11). Each patch is soaked with a liquid solution of a type of allergen. The patches stay on the patient’s back for five days. The doctor then looks at the patient’s back on the third and fifth day and determines which patch causes its respective skin area to react (11). Occasionally, an additional week is required to test more allergens. To treat contact dermatitis, it is best to wash the area with water, milk, or a saline solution to reduce the itchiness and inflammation. The area must be cleaned of any oil or residue from the allergen. Afterwards, a topical cream is usually added to prevent further irritation (10).
WHAT DID I EAT?
Another form of allergy is food allergy, which occurs when a person ingests a substance that they are allergic to. Normally the allergen is a protein that the body mistakenly identifies as a foreign substance due to a mutation in one of a few specific genes. This mutation causes the body to produce large amounts of the antibody that will react to that specific food. Since this is a genetic mutation, this allergy is often hereditary (12). Depending on where the immune system sends the antibody to interact with the mast cells, ingesting the allergen can have variable effects (12). One allergen is amoxicillin. This medicine may be a helpful antibiotic some people use to fight infections, but to others, it can be a detrimental allergen. To a person allergic to amoxicillin, ingesting it can cause them to break out into rashes all over his body and suffer from bloody diarrhea. The antibodies causes the mast cells in the skin and intestines to release histamine. The histamine makes the blood vessels more permeable, increases blood flow, and causes the blood to be excreted. Diagnosing food allergies can be a bit difficult. If the allergic reaction hasn’t proved to be dangerous, the doctor may prescribe a food challenge to test the allergen food. The patient would eat the types of food he’s had within the past few days and sees whether or not his body reacts. Skin tests can also be used, where an extract of a food is be injected just beneath the skin. If there is redness or swelling around the area of injection, this means the body’s antibodies are reacting to the food. For a safer test, a blood test may be administered. These tests will measure the levels of food-specific antibodies within the patient’s blood. Large amounts of a food-specific antibody could point to the possible allergen but it may not be correct (12).

Example of patch testing for contact dermatitis.
A more severe allergic reaction is anaphylaxis. This occurs when someone is heavily allergic to an allergen (food, medications, air particles, insects, or contact with a substance). Exposure to this allergen can cause a cascade of symptoms in various parts of the body. Some of these symptoms include a feeling of warmth, redness and itching in the mouth, light headed-ness, shortness of breath, throat tightness, anxiety, pain, cramps, vomiting, diarrhea, or even a drop in blood pressure. Without an immediate injection of epinephrine, anaphylaxis can be fatal (5).
Although there are many ways to treat the symptoms of an allergic reaction as they appear, the best way to avoid suffering from a reaction is to avoid the allergen. As mentioned before, allergies occur as the body reacts to a foreign stimulant. Therefore, to prevent and stop the reaction, avoid the allergen. In the case of leather belts or nickel belt buckles, alternative products are available such as rope belts. For medications such as amoxicillin, alternative medications that prove to be just as potent can also be taken. In general, to avoid having a severe allergic reaction, it’s best to avoid the allergen causing the problem all together.
1) Britannica, T. E. (2014, March 12). Antibody . Retrieved from Encyclopedia Britannica: http://www.britannica.com/EBchecked/topic/27783/antibody
2) Busse, P. (2011, June 29). How Allergic Reactions Work . Retrieved from Penn State Hershey Medical Center: http://pennstatehershey.adam.com/content.aspx?productId=28&pid=28&gid=000036
3) Colored Version of Antibody . 2007. Retrieved February 18, 2015, from http://commons.wikimedia.org/wiki/File:Antibody.svg
4) Dartmouth College Electron Microscope Facility. Pollen from a variety of common plants. 2004. Retrieved February 18, 2015 from http://commons.wikimedia.org/wiki/File:Misc_pollen.jpg
5) Henochowicz, S. (2014, May 10). Allergic Reactions . Retrieved from Medline Plus: http://www.nlm.nih.gov/medlineplus/ency/article/000005.htm
6) Immunology, A. A. (2014). Allergic Reactions . Retrieved from American Academy of Allergy Asthma & Immunology: http://www.aaaai.org/conditions-and-treatments/library/at-a-glance/allergic-reactions.aspx
7) Immunology, A. A. (2014). Skin Allergy . Retrieved from American Academy of Allergy Asthma & Immunology: http://www.aaaai.org/conditions-and-treatments/allergies/skin-allergy.aspx
8) Jan Polák. 2011. Patch Test . Retrieved February 18, 2015 from http://en.wikipedia.org/wiki/Patch_test
9) Mercola, J. (2013, April 18). How and Why Do Allergies Develop? Retrieved from Mercola.com: http://articles.mercola.com/sites/articles/archive/2013/04/18/allergy-season.aspx
10) Moskowitz, R. (2013, October 18). Contact Dermatitis . Retrieved from Medline Plus: http://www.nlm.nih.gov/medlineplus/ency/article/000869.htm
11) Seidu, L. (2014, September 24). Skin Testing for Allergies . Retrieved from WebMD: http://www.webmd.com/allergies/guide/skin-test?page=2
12) Stöppler, M. (2014, March 24). Food Allergy . Retrieved from Medicine.net: http://www.medicinenet.com/food_allergy/page2.htm
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
- Bovine Brain Damage: New Findings in the Field of Wildlife Neuroscience
- The Heredity of Mental Disorders
- Levels of Empathy in Apes and Humans
- Stochastic Volatility Models and its Effect on the Asset Market
- Corporate Psychopathy: Does Empathy Cripple Leaders?
- Dementia Villages – Experimenting with Universal Design Treatment
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Clin Transl Allergy

Research needs in allergy: an EAACI position paper, in collaboration with EFA
Nikolaos g papadopoulos.
1 Allergy Department, 2nd Pediatric Clinic, University of Athens, Athens, Greece
Ioana Agache
2 Transylvania University, Brasov, Romania
Sevim Bavbek
3 Ankara University, Ankara, Turkey
Beatrice M Bilo
4 University Hospital Ospedali Riuniti, Ancona, Italy
Fulvio Braido
5 Allergy & Respiratory Diseases Clinic – DIMI, University of Genoa, IRCCS AOU San Martino-IST, Genoa, Italy
Victoria Cardona
6 Hospital Vall d'Hebron, Barcelona, Spain
Adnan Custovic
7 University of Manchester, Manchester, United Kingdom
Jan deMonchy
8 University Hospital Groningen, Groningen, The Netherlands
Pascal Demoly
9 University Hospital of Montpellier, Montpellier, France
Philippe Eigenmann
10 Children's Hospital, Geneva, Switzerland
Jacques Gayraud
11 Polyclinique de l’Ormeau, Tarbes, France
Clive Grattan
12 Norfolk & Norwich University Hospital, Norwich, United Kingdom
Enrico Heffler
13 Dipartimento di Scienze Mediche, University of Torino, Torino, Italy
Peter W Hellings
14 University Hospitals Leuven, Leuven, Belgium
Marek Jutel
15 Wroclaw Medical University, Wroclaw, Poland
Edward Knol
16 University Medical Center Utrecht, Utrecht, The Netherlands
Jan Lötvall
17 Krefting Research Centre, Gothenburg, Sweden
Antonella Muraro
18 Department of Pediatrics, University of Padua, Padova, Italy
Lars K Poulsen
19 Copenhagen University Hospital at Gentofte, Gentofte, Denmark
Graham Roberts
20 University of Southampton Faculty of Medicine, Southampton, United Kingdom
Peter Schmid-Grendelmeier
21 University of Zurich, Zurich, Switzerland
Chrysanthi Skevaki
Massimo triggiani.
22 University of Salerno, Fisciano, Salerno, Italy
Ronald vanRee
23 Academic Medical Center, Amsterdam, The Netherlands
Thomas Werfel
24 Department of Dermatology and Allergy, Hannover Medical School, Hannover, Germany
Breda Flood
25 European Federation of Allergy and Airways Diseases Patients‘ Associations (EFA), Brussels, Belgium
Susanna Palkonen
Roberta savli, pia allegri.
26 Uveitis Center, Ophthamology epmn, Rapallo Hospital, Genova, Italy
Isabella Annesi-Maesano
27 Epidemiology of Allergic and Respiratory Disease, UMR-S 707 INSERM and UPMC Paris Sorbonnes, Paris, France
Francesco Annunziato
28 University of Florence, Florence, Italy
Dario Antolin-Amerigo
29 Hospital Universitario Príncipe de Asturias, Madrid, Spain
Christian Apfelbacher
30 University of Regensburg, Germany / Brighton and Sussex Medical School, Brighton, United Kingdom
Miguel Blanca
31 Research Laboratory, Carlos Hava Hospital, Malaga, Spain
Ewa Bogacka
32 Department of Internal Diseases, Geriatrics and Allergology, University of Medicine, Wroclaw, Poland
Patrizia Bonadonna
33 Allergy Unit, Azienda Ospedaliera Universitaria Integrata, Padova, Italy
Matteo Bonini
34 Department of Internal Medicine, Lung Function Unit, "Sapienza", University of Rome, Rome, Italy
Onur Boyman
Knut brockow.
35 Department of Dermatology, Technische Universität München, Munich, Germany
Peter Burney
36 Imperial College London, London, United Kingdom
Jeroen Buters
37 CK-CARE, ZAUM – Center of Allergy & Environment, Helmholtz Zentrum München/Technische Universität, Munich, Munich, Germany
Indre Butiene
38 Vilnius University, Vilnius, Lithuania
Moises Calderon
39 Royal Brompton Hospital, London, United Kingdom
Lars Olaf Cardell
40 Karolinska University Hospital, Stockholm, Sweden
Jean-Christoph Caubet
41 University Hospitals of Geneva, Medical School of the University of Geneva, Department of Child and Adolescent Medicine, Geneva, Switzerland
Sevcan Celenk
42 Uludag University, Bursa, Turkey
Ewa Cichocka-Jarosz
43 Chair and Department of Pediatrics, Jagiellonian University Medical College, Krakow, Poland
Cemal Cingi
44 Department of Otorhinolaryngology, Head and Neck Surgery, Eskisehir Osmangazi University, Eskisehir, Turkey
Mariana Couto
45 Centro Hospitalar São João EPE, Porto, Portugal
Nicolette deJong
46 ErasmusMC, Rotterdam, The Netherlands
Stefano Del Giacco
47 Department of Medical Sciences “M. Aresu”, University of Cagliari, Cagliari, Italy
Nikolaos Douladiris
Filippo fassio.
48 Careggi Hospital, Florence, Italy
Jean-Luc Fauquert
49 Pédiatre A, CHRU Clermont Ferrand, Clermont Ferrand, France
Javier Fernandez
50 UMH University, Alicante, Spain
Montserrat Fernandez Rivas
51 Hospital Clínico San Carlos, Madrid, Spain
Marta Ferrer
52 Universidad de Navarra, Pamplona, Spain
Carsten Flohr
53 St Thomas' Hospital & King's College London, London, UK
James Gardner
54 Royal Free Hospital, London, United Kingdom
Jon Genuneit
55 Institute of Epidemiology and Medical Biometry, Ulm University, Ulm, Germany
Philippe Gevaert
56 University Hospital Ghent, Ghent, Belgium
Anna Groblewska
57 Polish Mother’s Memorial Hospital - Research Institute, Department of Opthalmology, Lodz, Poland
Eckard Hamelmann
58 Klinik für Kinder und Jugendmedizin, St. Josef Hospital Ruhr University, Bochum, Germany
Hans Jürgen Hoffmann
59 Aarhus University Hospital, Aarhus, Denmark
Karin Hoffmann-Sommergruber
60 Medical University Vienna, Wien, Austria
Lilit Hovhannisyan
61 Institute of Molecular Biology, Yerevan, Armenia
Valérie Hox
Frode l jahnsen.
62 Oslo University Hospital, Rikshospitalet, Oslo, Norway
Ömer Kalayci
63 Pediatric Allergy and Asthma Unit, Ihsan Dogramaci Children’s Hospital, Hacettepe Univirsity School of Medicine, Ankara, Turkey
Ayse Füsun Kalpaklioglu
64 Allergie- & Asthma-Zentrum Berlin Westend, Berlin, Germany
Jörg Kleine-Tebbe
George konstantinou.
65 Department of Allergy and Clinical Immunology, 424 General Military Training Hospital, Thessaloniki, Greece
Marcin Kurowski
66 Department of Immunology, Rheumatology and Allergy, Medical University of Lodz, Lodz, Poland
Susanne Lau
67 Charité C. Virchow University Children`s Hospital, Berlin, Germany
Roger Lauener
68 Hochgebirgsklinik, Davos-Wolfgang, Davos, Switzerland
Antti Lauerma
69 Skin and Allergy Hospital, Helsinki, Finland
Kirsty Logan
70 King's College London, London, United Kingdom
Antoine Magnan
71 L'Institut du Thorax, Nantes, France
Joanna Makowska
72 Department of Allergy and Clinical Immunology, Lodz, Poland
Heidi Makrinioti
Paraskevi mangina, felicia manole.
73 Faculty of Medicine, ENT Department, University of Oradea, Oradea, Romania
Adriano Mari
74 Center for Molecular Allergology, IDI-IRCCS, Rome, Italy
Angel Mazon
75 Unit of Pediatric allergy and Pneumology, Children’s Hospital La Fe, Valencia, Spain
Clare Mills
76 Manchester Interdisciplinary Biocentre, Manchester, United Kingdom
ErvinÇ Mingomataj
77 Department of Allergology and Clinical Immunology, Mother Theresa School of Medicine, Tirana, Albania
Bodo Niggemann
78 German Red Cross Hospital Westend, Berlin, Germany
Gunnar Nilsson
79 Centre for Allergy Research at Karolinska Institutet, Stockholm, Sweden
Markus Ollert
80 Technical University of Munich, Munich, Germany
Liam O'Mahony
81 Swiss Institute of Allergy and Asthma Research (SIAF), University of Zurich, Christine Kühne-Center for Allergy Research and Education (CK-CARE), Davos, Switzerland
Serena O'Neil
Gianni pala.
82 Allergy and Immunology Unit, Fondazione ‘Salvatore Maugeri’, Pavia, Italy
Alberto Papi
83 University of Ferrara at St. Anna Hospital, Ferrara, Italy
Gianni Passalacqua
84 Internal Medicine Pad Maragliano, Genoa, Italy
Michael Perkin
Oliver pfaar.
85 Center for Rhinology and Allergology Wiesbaden, University Hospital Mannheim, Mannheim, Germany

Constantinos Pitsios
86 Dietetics and Nutritional Science Dept, Harokopio University, Athens, Greece
Santiago Quirce
87 Hospital La Paz Institute for Health Research, Madrid, Spain
Ulrike Raap
Monika raulf-heimsoth.
88 Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Allergology/Immunology; Ruhr-University, Bochum, Germany
Claudio Rhyner
Paula robson-ansley.
89 School of Life Sciences, Northumbria University, Newcastle upon Tyne, United Kingdom
Rodrigo Rodrigues Alves
90 Hospital Divino Espirito Santo de Ponta Delgada, Ponta Delgada, Portugal
Zeljka Roje
91 ENT Department, University Hospital Split, Split, Croatia
Carmen Rondon
92 Hospital Civil, Malaga, Spain
Odilija Rudzeviciene
93 Nr. 101 - Odilija Rudzeviciene, Vilnius University, Vilnius, Lithuania
Franziska Ruëff
94 Ludwig-Maximilians-Universität, Munich, Germany
Maia Rukhadze
95 Center of Allergy & Immunology, Tbilisi, Georgia
Gabriele Rumi
96 Complesso Integrato Columbus, Rome, Italy
Cansin Sackesen
97 Department of Pediatric Allergy, Hacettepe University, Ankara, Turkey
Alexandra F Santos
98 Department of Pediatric Allergy, King's College London, MRC & Asthma UK Centre for the Allergic Mechanisms of Asthma, London, United Kingdom
99 Immunoallergology Department, Coimbra University Hospital, Coimbra, Portugal
Annalisa Santucci
100 Rimini Infermi Hospital, Rimini, Italy
Christian Scharf
101 Greifswald University Medical School, Greifswald, Germany
Carsten Schmidt-Weber
102 Klinikum rechts der Isar der TU München, ZAUM, Munich, Germany
Benno Schnyder
103 Clinic for Rheumatology and Clinical Immunology/Allergology, University Hospital of Bern, Switzerland, Bern, Switzerland
Jürgen Schwarze
104 University of Edinburgh, Edinburgh, United Kingdom
Gianenrico Senna
105 Azienda Ospedaliero-Universitaria, Verona, Italy
Svetlana Sergejeva
106 Institute of Technology, Tartu University, Tartu, Estonia
107 Catholic University of Leuven, Leuven, Belgium
Andrea Siracusa
108 Occupational Medicine, Terni Hospital, University of Perugia, Perugia, Italy
Isabel Skypala
Milena sokolowska.
109 National Institutes of Health, Bethesda, MD, 20892, USA
Francois Spertini
110 Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland
Radoslaw Spiewak
111 Department of Experimental Dermatology and Cosmetology, Jagiellonian University, Krakow, Poland
Aline Sprikkelman
112 Emma Children's Hospital Academic Medical Center, Amsterdam, The Netherlands
Gunter Sturm
113 Department of Dermatology, Medical University of Graz, Graz, Austria
Ines Swoboda
Ingrid terreehorst.
114 Department of ENT and Pediatrics, Academic Medical Center, Amsterdam, the Netherlands
Elina Toskala
115 Dept. of Otolaryngology-Head and Neck Surgery, Temple University, Philadelphia, USA
Claudia Traidl-Hoffmann
Carina venter.
116 The David Hide Asthma and Allergy Research Centre, Isle of Wight, England, United Kingdom
Berber Vlieg-Boerstra
Paul whitacker.
117 St James’s Hospital, England, United Kingdom
Margitta Worm
118 Charité - Universitaetsmedizin Dpt. of Dermatology, Berlin, Germany
Paraskevi Xepapadaki
Cezmi a akdis.
In less than half a century, allergy, originally perceived as a rare disease, has become a major public health threat, today affecting the lives of more than 60 million people in Europe, and probably close to one billion worldwide, thereby heavily impacting the budgets of public health systems. More disturbingly, its prevalence and impact are on the rise, a development that has been associated with environmental and lifestyle changes accompanying the continuous process of urbanization and globalization. Therefore, there is an urgent need to prioritize and concert research efforts in the field of allergy, in order to achieve sustainable results on prevention, diagnosis and treatment of this most prevalent chronic disease of the 21 st century.
The European Academy of Allergy and Clinical Immunology (EAACI) is the leading professional organization in the field of allergy, promoting excellence in clinical care, education, training and basic and translational research, all with the ultimate goal of improving the health of allergic patients. The European Federation of Allergy and Airways Diseases Patients’ Associations (EFA) is a non-profit network of allergy, asthma and Chronic Obstructive Pulmonary Disorder (COPD) patients’ organizations. In support of their missions, the present EAACI Position Paper, in collaboration with EFA, highlights the most important research needs in the field of allergy to serve as key recommendations for future research funding at the national and European levels.
Although allergies may involve almost every organ of the body and an array of diverse external factors act as triggers, there are several common themes that need to be prioritized in research efforts. As in many other chronic diseases, effective prevention, curative treatment and accurate, rapid diagnosis represent major unmet needs. Detailed phenotyping/endotyping stands out as widely required in order to arrange or re-categorize clinical syndromes into more coherent, uniform and treatment-responsive groups. Research efforts to unveil the basic pathophysiologic pathways and mechanisms, thus leading to the comprehension and resolution of the pathophysiologic complexity of allergies will allow for the design of novel patient-oriented diagnostic and treatment protocols. Several allergic diseases require well-controlled epidemiological description and surveillance, using disease registries, pharmacoeconomic evaluation, as well as large biobanks. Additionally, there is a need for extensive studies to bring promising new biotechnological innovations, such as biological agents, vaccines of modified allergen molecules and engineered components for allergy diagnosis, closer to clinical practice. Finally, particular attention should be paid to the difficult-to-manage, precarious and costly severe disease forms and/or exacerbations. Nonetheless, currently arising treatments, mainly in the fields of immunotherapy and biologicals, hold great promise for targeted and causal management of allergic conditions. Active involvement of all stakeholders, including Patient Organizations and policy makers are necessary to achieve the aims emphasized herein.
Allergies represent the most frequent chronic diseases in Europe today, affecting, with the most conservative estimates, the daily lives of more than 60 million people. While at the beginning of the 20 th century, allergies were viewed as rare diseases, the last few decades have seen a dramatic increase in disease burden. The industrial and technological revolution has led to environmental changes, including climate variation, pollution and microbial sterilization, but also to an urban, sedentary life style, affecting on one hand the intensity, type and diversity of external exposures, while on the other hand altering the normal immune/inflammatory responses.
Allergies involve almost every organ of the body in variable combinations with a broad spectrum of possible symptoms, and thus their manifestations cover a wide range of phenotypes. Studies in Europe have shown that up to 30% of the population suffers from allergic rhinoconjunctivitis, while up to 20% suffer from asthma and 15% from allergic skin conditions [ 1 , 2 ]. These numbers match those reported for other parts of the world, such as the USA and Australia. Food allergies, are becoming more frequent and severe; occupational allergies, drug allergies and allergies to insect stings (occasionally fatal), further aggravate the burden of the allergy epidemic. In contrast to the popular belief that allergies are mild conditions, a considerable and increasing proportion of patients (15%-20%) have severe, debilitating disease and are under constant fear of death from a possible asthma attack or anaphylactic shock [ 3 ]. Within the EU, there are nevertheless wide geographical variations in the incidence of allergies with a south to north and east to west gradient [ 4 , 5 ]. An alarming observation is that most allergic conditions start in childhood and peak during highly productive years of individuals, with allergic rhinitis affecting up to 45% of 20–40 year old Europeans. The numbers may even be an underestimation, as many patients do not report their symptoms or are not properly diagnosed. Indeed, it is estimated that approximately 45% of patients have never received a diagnosis [ 6 ]. Notwithstanding evidence suggesting a plateau in some areas, the European Academy of Allergy and Clinical Immunology (EAACI) warns that in less than 15 years more than half of the European population will suffer from some type of allergy!
Major knowledge gaps in allergy
• Causes of allergy, as well as reasons for recent increase in prevalence are unknown
• The natural history, including mechanisms of spontaneous resolution, are unknown
• There is marginal understanding of interactions among microbes, immune system and allergic disorders
• Therapeutic targets with potential for a complete cure are scarce
Independent of incidence, age group or nationality, it is important to realize that allergic diseases have a detrimental impact on the quality of life of patients and their families, affecting their personal development, career plans and lifestyle choices. Allergies may affect sleep and mood, school or work competence, and social interaction [ 7 ]. For example 43% of patients with allergic rhinoconjunctivitis have sleep disturbances and 39% have difficulty in falling asleep [ 6 ]. The possibility of failing an examination increases by 40%-70% in school-age children with rhinitis [ 6 ]. Allergic individuals have a higher risk of developing depression [ 7 ]. The impact of allergies on quality of life can be as high or even higher than that of diseases commonly perceived as being more ‘serious’ [ 6 , 8 ].
At the society level, the rising prevalence of allergic diseases poses a multifaceted, major socioeconomic burden on national and European budgets. The increased use of health services, hospitalization and pharmaceutical costs, in addition to the billions of days of lost productivity through absenteeism or presenteeism (people going to work but being unable to perform), reveal a worrying prospect for public health when it comes to allergies [ 9 ]. It is estimated that the annual cost of asthma in Europe is over €18 billion [ 8 ]; allergic rhinitis may cost several times more (up to €100 billion, according to unpublished data from the Global Allergy and Asthma Network of Excellence, GA 2 LEN, investigators). Skin allergy care costs may be as high as that of asthma [ 10 , 11 ]. Given the nature of current lifestyles, the ageing population and continuing environmental changes, these numbers are likely to increase, unless a concerted effort is devised to understand the causes and mechanisms of allergy and design effective strategies for prevention and/or treatment.
Special attention should be paid to childhood allergies as these usually demonstrate a persistent and varying course: many children first develop eczema, followed by asthma and allergic rhinitis, the so called “allergic march”. Therefore, early diagnosis and adequate control of allergy is crucial especially for children. The Polish Presidency of the Council of the EU underlined this problem in its conclusions on “Prevention, early diagnosis and treatment of chronic respiratory diseases in children” (unanimously adopted by the EU Ministers of Health in December 2011) [ 12 ].
EAACI is the leading professional organization in the field; its members are nearly 8000 physicians, researchers and academicians, as well as all of the European National Allergy Societies. EAACI is dedicated to improving the health of people affected by allergic diseases by promoting adequate patient care, advancing basic and clinical research and encouraging education and training. The European Federation of Allergy and Airways Diseases Patients’ Associations (EFA) is a non-profit network of allergy, asthma and chronic obstructive pulmonary disorder (COPD) patients’ organizations, representing 35 national associations in 22 countries and over 400,000 patients. EFA is dedicated to making Europe a place where people with allergies, asthma and COPD have the right to the best quality of care, a safe environment, the right to live uncompromised lives and be actively involved in all decisions influencing their health. More information about the organizations and their programs can be found at http://www.eaaci.org and http://www.efanet.org .
Following their missions, EAACI and EFA, advocate for feasible, sustainable and patient-centered strategies for allergy research. There is currently a great need for a sustained investment in allergy research. Key mechanisms need to be further understood. Several important findings at the basic immunological level are close to being translated into bedside treatments. New preventative approaches require large-scale studies to be confirmed so that they can be used for public health purposes.
This article, written by Section and Interest Group Board members of EAACI and co-authored by EFA, intends to create a milestone, describing the current research needs in the wide spectrum of allergic diseases, and provide key recommendations to provide input for consultations on current and future research programs at the national and European levels. By prioritizing various projects of basic, translational and clinical allergy research, and efficient networking, such programs may not only yield ground-breaking results, but also crucially inform patient and public health strategies, reducing health-related costs and improving the quality of life of millions in Europe and around the world, while providing opportunities for a strong European Research Area and an innovative knowledge-based European industry. They can also inform novel National Plans following the model of the Finnish Asthma and Allergy Programs [ 13 ] , implementing wide stakeholder participation.
Objectives and methodology
The aim of this article is to briefly describe the current understanding of the whole spectrum of allergic diseases and conditions, and to identify the immediate knowledge gaps amenable to research at the national and European levels. Input was obtained by all EAACI Sections and Interest Groups; the Chair, Secretary and Board Members of each group prepared a paragraph related to their specific domain. EFA was also consulted and provided input to the text. These were subsequently integrated and reviewed by the EAACI Executive Committee members, who provided by consensus a concluding part and summary of key points.
Understanding the allergy epidemic
Epidemiology, research needs in the epidemiology of allergy.
• Europe-wide epidemiological studies on occupational allergy, drug allergy, venom hypersensitivity, exposure to various environmental agents
• Interactions between various risk factors of allergy development to explain prevalence differences
• Link between sensitization and clinical allergy development
• Incidence, prevalence studies of various disease phenotypes and endotypes
• Development of pan-European registries
Epidemiology is the study of health and disease in populations. Several landmark studies have defined the global burden of allergic diseases, and have demonstrated that the problem is growing. Major demographic, socio-economic and environmental developments such as urbanization, globalization, upcoming economies (BRIC countries) and climate change will most likely contribute to further increases. The International Study of Asthma and Allergies in Childhood (ISAAC) reports that well over 20% of children in European countries suffer from an allergic disease at some point during their childhood [ 14 ]. This study also assessed allergy prevalence sequentially and observed a rise in prevalence especially in areas where allergic diseases were previously less common [ 2 ]. Large-scale population-based data from the European Community Respiratory Health Survey (ECRHS) [ 15 ] and the Global Allergy and Asthma Network of Excellence (GA2LEN) [ 16 ] clearly show that allergic diseases are significant health problems in adults too. A similar initiative for food allergy (EuroPrevall) [ 17 ], has been recently completed, but population-based information is currently lacking for occupational allergy, drug and insect venom hypersensitivity, as well as daily exposures to environmental agents such as pollutants and cosmetics. These studies have revealed significant differences in allergy prevalence even amongst those of the same ethnic background, advocating that, notwithstanding the importance of genetic factors, environmental factors are likely to be responsible for the observed prevalence gradients and time trends. Socio-economic background, family size, urban dwelling, farm exposure, infection history, diet, obesity, use of certain drugs, tobacco smoke exposure and indoor and outdoor air pollution are among the factors associated with atopic disease.
In spite of these clear indications, we are currently missing studies that aim at explaining the prevalence differences and trends between populations taking all known risk factors into account as well as the interactions between risk factors (including gene-environment interactions). Rapid developments in the field of information technologies allowing systems approaches now offer the opportunity to study these complex issues in a comprehensive way.
When it comes to allergies, it is vital to maintain an open mind about the nature of causal influences and be prepared to follow up clues or signals emerging even in, at first sight, unlikely places: for instance, the protective effect of Alpine farms has been an important stimulus to aetiological enquiry [ 18 ]. In addition, the link between allergic sensitization and clinical allergy remains poorly understood and new molecular methods may shed further light on this fundamental issue.
One limitation of large population-based studies has been the use of questionnaires in the diagnosis of asthma or allergic rhinitis and that more objective markers, such as spirometry and bronchial hyper responsiveness, do not capture the episodic nature of the disease and often do not correspond to the clinical phenotype. An advantage in atopic dermatitis is that the skin is readily accessible to examination. The strong association between clinical atopic dermatitis and filaggrin skin barrier gene mutation carriage has opened up new avenues to explore gene-environment interactions, not only in the context of atopic dermatitis, but also allergic respiratory disease and food allergy [ 19 ].
Another important area of epidemiological research is the application of epidemiological methods to explore the quality and safety of medical care provided for patients with allergic diseases, for instance through establishing registries with a main focus on the efficacy and safety of treatments, such as immunotherapy, systemic immunosuppressive drugs and biologicals. Registries should also capture trends in disease prevalence and severity and differences between age and/or social groups. Data on emerging allergens, including work-related exposure, could also be monitored.
Overall, we need to find better ways of identifying the phenotypes of allergic disease, using standardized methodology between studies to facilitate direct comparability. This will then allow us to revisit known and hitherto unexplored risk and protective factors, preferably in longitudinal population-based cohorts that take into account environmental, genetic and immunological factors (biomarkers) as well as the fluctuant nature of allergic diseases and disease severity. Novel approaches to genetic and gene-environmental interaction studies will be helpful to identify the ‘missing heritability’.
Immunology, molecular and cellular mechanisms
Major gaps in understanding the allergic immune response.
• Immunological basis of allergy epidemic
• Innate immune response to molecules that are coexposed with allergens
• Role of novel subsets of T cells, B cells and innate lymphoid cells in allergy development
• Epithelial barrier function and its role in allergy development and chronicity
• Mechanisms of development of immune tolerance to allergens and novel ways to induce this
• Understanding epigenetic regulation of the allergic inflammation
• Development of novel biologicals to treat allergy
• Identification of novel biomarkers for endotyping patients for the prediction of treatment response and prognosis
• Development of immunological registries and Europe-wide disease-specific biobanks
In diseases involving the immune system such as allergy, autoimmunity, transplantation rejection, cancer and infections, antigens are either the direct or indirect cause of the disease and can be targeted for treatment [ 20 ]. The investigation of what makes a protein an allergen has been a prerequisite of understanding allergic disease to develop strategies for immune intervention. Allergens are almost always proteins, but not all proteins are allergens. A protein with allergenic activity should display two properties a) induction of IgE response, which involves the sensitization phase including T cell, B cell and dendritic cell cooperation, and b) induction of a clinical response to the same or similar protein on subsequent exposures, which involves immediate and late phase responses [ 21 ]. Many allergens contain potent stimulatory properties for the epithelium, such as the protease activity of house dust mite. Besides proteases and oxidases, extracts of pollen contain low molecular weight molecules such as pollen-associated lipid mediators or adenosine exhibiting a potential to stimulate and modulate immune cells [ 22 ]. Therefore, when exposed to e.g. pollen, it is more than just allergenic proteins that we inhale. More knowledge on the immune stimulatory properties (adjuvant-like activity) of allergens, in addition to their antigenic potential will be of great importance and might help in modifying allergens in the application of allergen specific immunotherapy.
An increasing body of evidence indicates that immune responses in allergy involve a comprehensive network of cellular and molecular interactions (Figure (Figure1). 1 ). Novel insights from implicated environmental influences on the development of allergy have increased interest in innate immune responses preceding and directing the adaptive immune response. In parallel, we have now identified a role of the local tissue immune response, not only in determining the implication of a specific tissue in allergy (i.e. atopic asthma vs. atopic dermatitis) but also the role of structural cells such as epithelial cells in the innate immune responses to environmental triggers, including allergens.

Mechanisms of allergic inflammation in asthma. Epithelial cell activation by allergens, viruses, bacteria and pollutants takes place and their proinflammatory cytokines and chemokines induce inflammation and contribute to Th2 response with TNF-α, IL-13, TSLP, IL-31, IL-33. Th2 response involves multiple cytokines such as IL-4, IL-5, IL-9, IL-13, IL-25, IL-33, eosinophilia, and local and systemic IgE production. A series of chemokines are produced and migration of inflammatory cells to the allergic tissues takes place. In addition, other effector T cell subsets, such as Th9, Th17 and Th22 cells play inflammatory roles. Cross-linking of IgE receptor FcεRI on the surface of mast cells and basophils and their degranulation, induces a type 1 allergic response. The activation of smooth muscle cells, myofibroblasts, angiogenesis and subepithelial fibrosis, lead to remodeling. Bronchial hyperreactivity takes place, with enhanced susceptibility to bronchoconstriction. Innate lymphoid cells may contribute to many aspects of allergic inflammation by the help of multiple cytokines. Epithelial apoptosis and shedding are essential in the mechanisms of eczema and asthma [ 23 , 24 ]. Survival and reactivation of migrating inflammatory cells and their interaction with resident tissue cells and other inflammatory cells augment allergic inflammation.
As a direct result of epidemiological findings of reduced incidence of allergy in farm environments, and many others on the so-called hygiene hypothesis, there has been interest in the development of early and innate immune responses. Pattern recognition receptors such as Toll like receptors (TLR) are important in these reactions, essentially deciding over the pro- or anti-inflammatory nature of subsequent adaptive immune responses. It is becoming clear that these receptors do not only play a role on innate immune cells directing their response to e.g. lipopolysaccharide, double stranded RNA or bacterial DNA, but also on tissue cells such as epithelial cells or keratinocytes [ 25 ]. Very recently, a novel category of innate lymphoid cells with still a wide variety of names, such as nuocytes, lymphoid tissue–inducer cells (LTi cells) or innate lymphoid cells (ILCs) have been described. The demonstration of IL-13 and IL-5 production by so-called type-2 ILCs in allergic inflamed tissue is a strong hint that these cells can have an important role in the Th2-type skewing in allergic tissues, even in the absence of direct involvement of antigens [ 26 ]. The epithelial cells at environmental interfaces (skin, respiratory tract and gut mucosae) are also important for allergy development, possibly through reduced barrier function and/or the inherent properties of epithelial cells to induce Th2 responses [ 27 ]. There is increasing evidence that the composition of the microbiota of the gut (and possibly of the airways or even skin) play an important role in deciding over development towards allergy or protection against it [ 28 ]. The epigenetic regulation of allergic inflammation rendering certain genes or gene areas more accessible to translation should be extensively studied and is expected to provide better links to the influence of the environmental changes. Also, multigenerational epigenetic memory can help us to explain the inherited influence of the environmental exposure [ 29 ].
In addition to the almost classical definition of T helper cells in Th1 and Th2, many more T cell subsets have recently been described, including regulatory T cells, Th17, Th9 and Th22 cells [ 30 - 33 ]. Moreover, for all T cell subsets it is evident that their role can differ in different tissue types. Accordingly, there is increasing evidence that differentiated Th cell populations can alter the range of cytokines they produce, indicating a certain degree of “cell plasticity”. Understanding the molecular basis of the process through which cells modify their cytokine-producing potential is likely to provide interesting insights that may allow the development of strategies to alter Th cell function in immune-mediated pathologies, including allergic diseases. Amongst T-cell subsets, it is becoming clearer that CD8 T cells, classically called cytotoxic T cells, contain potent immunomodulatory subsets, which may be potent in the release of several cytokines, including IL-13. In addition, the immune regulatory and immune suppressive roles of various T and B cell subsets, such as T regulatory cells and B regulatory cells still remain to be elucidated.
In many inflammatory diseases, application of biologicals is an important option in treating severe forms of the disease. It is surprising that there is only limited potential of the known biologicals developed specifically for allergy [ 34 ]. Anti-IgE has major IgE decreasing effects, but is only indicated in a small subset of allergic asthmatics [ 35 ] at present although licensing studies for chronic urticaria are in an advanced stage. Blocking eosinophil differentiation and activation by anti-IL-5 is effective in vitro, but results from clinical studies [ 36 ], suggest the need for identifying responsive populations. The outcome of the application of these agents has indicated that our knowledge of the immunological mechanisms responsible for allergic inflammatory diseases remains incomplete. Although improved forms of anti-IgE or blocking of IL-5 effects may provide better drug efficacy, it is also important to design new biologicals and find novel application of established ones. Combined application of allergen-specific immunotherapy (SIT) with anti-IgE treatment, might improve safety and efficacy of immunotherapy.
SIT is the only treatment that alters the immunological basis of the disease. The immune responses to allergen observed during SIT are being described at increasing level of detail, but there is a continuous debate around mechanisms of induction and maintenance of tolerance. It is absolutely essential to further elucidate the immune mechanism of SIT. Only in this way new leads can be found to transform SIT from a therapy requiring “chronic administration” for years in a row into an effective therapeutic vaccination based on a small number of shots, and perhaps even a preventive vaccination [ 34 ].
Evidence is gathering that the complex interplay between the innate and adaptive immune response both of the myeloid and lymphoid lineages, in combination with the immune response of the tissue ultimately determines the development and expression of allergic diseases. A further important issue comprises adjuvant factors from the allergen carriers. We face an interesting and challenging era that will implicate all novel immunological insights in understanding better the immune-pathophysiological mechanisms in allergic diseases. This will lead to improved insight in the disease itself and also in new treatment options that may even result in allergy prevention, which is a major concern not only for parents thinking about their children, but for society as a whole.
Diagnostics
Next steps in allergy diagnosis.
• Improvement of molecular diagnostics methods
• Diagnosis of biomarkers for endotyping
• Easy and standardized tests for cellular diagnosis
• Development of point of care assays and devices
• Development of tests for endotyping, follow up of exacerbations and treatment response
The challenges in allergy diagnosis lie in developing methods that are objective, rapid, reproducible, cost-effective, sensitive and specific for the disease. They need to be able to identify exposures and contribute to decision making for treatment, preferably also in primary care. In addition, tests monitoring the response to treatment and allowing the comparison of preclinical and clinical effects across large cohorts and in several countries are needed. As recombinant molecular therapies are becoming available, there is a need to tailor treatment to disease burden, phenotype and/or endotype more effectively, which has always been a key request by patient groups; in this respect improved allergy diagnosis is a prerequisite. Molecular diagnostic methods enable a much more detailed analysis of the allergen molecules and their association with clinical presentations. Molecular approaches have great potential to significantly improve the prognostic value of diagnostic tests, but large-scale clinical studies are needed for their validation. Both allergen molecules and recombinant antibodies are made available to enlarge the repertoire of diagnostic tests [ 37 ]. The first allergen molecule-based multiplex tests have reached the market [ 38 ], significantly increasing diagnostic and epidemiological options [ 39 ].
Improvement and standardization of cellular tests, such as the basophil activation test, [ 40 ] will enable laboratories to more closely monitor disease status and advance allergy diagnosis and monitoring from the mere assay of IgE affinity, concentration and avidity to an integrated evaluation of the burden of allergic disease on the individual. Such an approach is not feasible at the moment. A similar methodology to the one used to produce standardized T cell tests, should be adopted. The involvement of reference centers and standardized comparisons of efficacy can lead to standardized methods of analysis. In combination with molecular tools, this will enable improved-resolution diagnosis with a higher prognostic value, which in turn will result into more precise diagnosis and superior monitoring of allergic disease. Recent advances in multicolor flow-cytometry are expected to contribute to cellular diagnosis and disease phenotyping/endotyping. In addition, detection of recently discovered microRNAs and/or exosomes in body fluids may open new ways for non-invasive diagnosis and monitoring of patients. Development of a standardized mast cell line similar to resident tissue mast cells would revolutionize the understanding and diagnosis of mast cell-mediated immediate hypersensitivity reactions.
In the diagnosis of drug allergy, cellular tests can be more useful both for diagnosis of type I allergy [ 41 ] and for diagnosis of the more severe type IV allergy [ 42 ]. As treatment options expand and life expectancy increases, people will require more frequent medical treatment and ex vivo diagnostic approaches will become more important.
Ex vivo tests for type I allergy will play a role in both the diagnosis of occupational allergy and food allergy. Moreover, they are an important element in diagnosis of paediatric allergy, since they constitute an alternative to allergen exposure tests currently considered as the gold standard in diagnosis of allergy.
In the near future, individualized –omics analyses will provide a wealth of detailed information.
A new integrated approach, combining data of genomics (genome-wide SNP association studies) and metabolomics with disease-specific proteome and transcriptome data from biopsies and body fluids (blood, saliva, nasal lavage) will allow a comprehensive characterization of complex diseases for the first time. Furthermore, diagnostic tools to characterize the environment such as microbiome, pollen counts, mite counts, allergen quantification, mould counts, smoke, and other pollutants need to be developed and standardized across Europe.
In addition to diagnostic approaches based on large laboratory-intensive methods, individualized point of care testing should be developed as this can/will remove a burden from the health sector with patients eventually capable of partly monitoring their own allergic disease and optimizing their treatment in order to rely more on a predefined plan and electronic support (ICT and e-health) and less on frequent visits to the allergy clinic.
Patient-reported outcomes
Patient-reported outcomes (PROs) refers to all health-related reports coming from the patient, without involvement or interpretation by physician or others [ 43 ] (i.e. Health Related Quality of Life (HRQoL), symptoms, illness perception, satisfaction, well-being, perceived disease control).
PROs recently gained great attention in clinical research and by regulatory bodies due to their importance in the overall treatment efficacy assessment [ 44 - 46 ].
The role of patient’s perspectives is now underlined by the GRADE system [ 47 ], which also includes patients’ preferences and values as cornerstones in the process of formulating recommendations towards diagnostic and therapeutic interventions, thereby contributing to the translation of scientific research into real life.
A critical aspect in the management of allergic diseases is their impact on subjective experience. Available data show that, from the patients’ perspective, allergy is more than just an annoying disease: when compared to healthy subjects, patients with asthma, rhinitis, chronic urticaria, atopic dermatitis and food allergy reported markedly reduced HRQoL [ 48 ]. The burden of the disease, besides functional and practical problems, includes some emotional aspects: the presence of a chronic condition, the need to take medication and change habits and lifestyle may cause anxiety, tension and irritability and an unsatisfactory social life.
Among PROs, HRQoL and patient-reported symptoms have been extensively evaluated in asthma and rhinitis [ 49 , 50 ], and more recently, also in chronic urticaria and food allergy [ 51 ].
In contrast to the advanced stage of research in respiratory and skin allergy, little is known about HRQoL in allergic conditions such as drug hypersensitivity, occupational allergy and insect venom allergy.
Although some evidence about subjective view of allergic diseases and their treatment has been achieved, further unmet needs and unexplored areas should be underlined. First, there is a need to develop clinical trials in which PROs are the primary or co-primary outcome. Another point is the necessity to assess patients’ viewpoint with a rigorous methodological procedure (use of validated tools, correct administration of the questionnaires and report of complete results).
Furthermore, there is a need to reach a global picture of patient’s perspective about allergy and its treatment by exploring the following uncovered areas: other PROs (satisfaction, preferences, well-being, illness perception) besides HRQoL; burden of comorbidity on PROs; specific population (children, adolescents, elderly, parents of allergic children); relation of PROs with other clinical measures of health impact; relationships among different PROs and between PROs and psychological variables.
Overall, there is a need for a more correct and extensive assessment of PROs, both in clinical trials and in routine practice, to capture information unavailable from other sources, which is crucial for predicting health outcomes, for establishing health policy and for the optimal management of allergic diseases.
Age-specific tools for patient information, education and peer support, major objectives of patient organizations, should be further developed.
Chronic Respiratory Allergies
In the last decade, it has been clearly demonstrated that allergies of the respiratory tract (asthma and rhinitis) very frequently coexist in the same patient, have similar epidemiology, share mechanisms and interact in terms of treatment and risk for persistence. Therefore, asthma and rhinitis are currently considered as part of a common syndrome, for which different terms, including ‘chronic respiratory allergy’ have been proposed. Additionally, the most common forms of allergic conjunctivitis occur together with rhinitis, in which case, allergic rhinoconjunctivitis is the prevailing term.
EFA’s 4-year Respiratory Allergies Project ‘Raise Awareness, Relieve the Burden’ aims at increasing the awareness of allergy as a serious chronic disease, to receive earlier concrete diagnosis with proper management, to avoid the increase of severity and to relieve the burden that it may impose on the lives of affected people. EFA published the Book on Respiratory Allergies – ‘Raise Awareness, Relieve the Burden’ in 15 languages and launched a “Call to Action” at the European Parliament (EP) to call upon policy makers to act in this crucial topic [ 6 ].
Asthma is a major global health problem contributing greatly to socio-economic burden: more than 200 million people of all ages suffer from asthma worldwide [ 52 ], and 250,000 people die prematurely every year due to asthma. Although the majority of patients with asthma can potentially be sufficiently controlled using currently available treatments, present management practices have considerable limitations in a relatively small, but important subgroup of patients with severe asthma, who suffer from ongoing symptoms, frequent exacerbations and reduced quality of life, despite receiving the best available treatment. So, although this patient group comprises about ~10% of the overall asthmatic population, it accounts for more than 75% of the costs attributable to asthma. Already in 2003, the total cost of asthma in Europe was calculated at 17.7 billion EUR per year, and productivity lost to patients’ poor control of their asthma was estimated at 9.8 billion EUR per annum [ 8 ]. The necessity to improve the management of asthma has frequently been underlined [ 53 ].
Major unmet needs in chronic respiratory allergies
• Mechanisms and management of severe cases
• Characterization of phenotypes and identification of novel biomarkers for endotypes
• Devise a consensus definition for severe asthma and exacerbations
• Identification of factors increasing the risk of asthma exacerbations and preventing them
• Vaccine development against viruses that trigger exacerbations and severity
• Patient-tailored treatment
• Development of biologicals
• Establish European registry and biobank for respiratory allergies in meticulously phenotyped patients
An additional significant unresolved problem in the management of asthma is the failure to prevent and/or promptly treat asthma exacerbations, which are associated with significant morbidity, risk of death and high treatment cost [ 54 ] and whose prevention in children may reduce the risk of subsequent adulthood asthma [ 55 ] (Figure (Figure2). 2 ). Identifying the factors increasing the risk of asthma exacerbations and designing novel treatments that reduce the risk are key priorities. Virus infections have a major role in asthma exacerbations and preventive vaccines or passive immunizations are awaited to prevent virus infection-induced exacerbations. In addition, to allow for a rational approach to the management of asthma, we must consider the emerging evidence for pathophysiological heterogeneity of the disease, particularly in its more severe forms. The conceptual framework of asthma endo/phenotypes developed by the EAACI [ 56 , 57 ] can offer the basis for an improved perception of the pathophysiological mechanisms involved in severe asthma and asthma exacerbations and enable target identification and development of novel treatments. Prediction of the outcomes and personalization of treatment through innovative approaches and new technologies can contribute to an effective intervention in terms of prevention and reduction of healthcare costs, with improved quality of life for asthma patients [ 58 ]. So, asthma research should aim to implement a consensus definition of severe asthma and asthma exacerbations that could usefully guide treatment and match this with diagnostic tools for “scoring” the asthma patient in terms of disease severity and future risk for the use in primary care. The recognition of asthma as a heterogeneous disease is a prerequisite for planning studies aimed at differentiating between different endo/phenotypes and the sequential creation of robust clinically-relevant and endo/phenotype-specific biomarkers and therapies targeting specific pathophysiology, including longitudinal outcomes. In this effort, we must also identify relevant environmental factors and the influence of current and prior medication. The accomplishment of the above aims also necessitates the establishment of large Research Tissue Banks (including DNA, serum, lavage fluids and other biological samples) in meticulously clinically phenotyped patients and the execution of clinical studies to improve the evidence-base, since most randomized, controlled trials to date have been carried out in highly selected patients; we should now aim for effectiveness, rather than efficacy studies.

Pathophysiology of asthma at steady state and during an exacerbation. The airways of asthmatic individuals are characterized by pathological changes, including thickened basement membrane, collagen deposition and hypertrophic smooth muscle, collectively called ‘airway remodeling’. Inflammation is triggered by a variety of factors, including allergens and respiratory viruses. These factors also induce hyperreactive responses in the asthmatic airways, associated with mucus and cell debris released into the lumen, oedema and bronchoconstriction, leading to airway obstruction and related acute exacerbations. Although pathophysiological changes related to asthma are generally reversible, recovery may be partial.
Allergic rhinitis affects up to one third of the adult population, hence having a major socio-economic impact. In spite of the high prevalence of the disease and the availability of effective and safe treatments [ 59 ], a significant number of patients remain deprived of an accurate diagnosis and specific anti-allergic treatment [ 60 ], while another group of patients remain symptomatic in spite of adequate medical therapy [ 61 ].
There is a need for the better characterization of the different phenotypes of allergic rhinitis, which should be matched with improved understanding of both the environmental and occupational factors determining severity of disease and the endogenous and exogenous factors that contribute to the progression from rhinitis to asthma in childhood and adolescence. In this respect, we must also define control of the disease, through improving insights into the mechanisms underlying uncontrolled allergic rhinitis. The similarities and differences between rhinitis and asthma should be further characterized [ 62 ]. Diagnosis-wise, there is a need for objective evaluation tools of disease severity and impact [ 63 ]. Treatment-wise, we need to both define patient-related reasons for undertreatment, reasons for underdiagnosis and non-compliance of patients to treatment and therefore design patient-oriented treatment strategies for optimal efficacy of treatment and compliance to therapy [ 64 ]. One aspect, which also requires attention, is the role of complimentary/alternative medicine in allergic rhinitis, an approach that is widely used and is a common question from patients.
Ocular allergies
Ocular allergies have been studied to a lesser extent, compared to other allergies. The evaluation of prevalence and severity range of ocular allergies in Europe requires additional effort. The more serious forms, such as allergic keratoconjunctivitis (AKC), one of the most serious complications of atopic dermatitis, and vernal keratoconjunctivitis (VKC), which may have serious ocular complications such as keratoconus, cataract and retinal detachment, are of particular interest. Pharmacological and immunological research is needed to evaluate new possibilities in modifying the ocular immune response in these diseases. Although evidence on ocular immunopathology is available, no comprehensive solution exists.
The roles of allergens and cross-reactivity, as well as criteria for consequent immunotherapy, are well established in seasonal allergic rhinoconjunctivitis, but far from clear in perennial conjunctivitis, AKC and VKC. Phenotyping and genotyping of these diseases is needed. This will allow us to effectively choose treatments (pharmacological and/or allergen immunotherapy).
Furthermore, evidence-based recommendations for use of available treatment options in ocular allergy are needed.
Dermatological allergies
The skin plays a major role in allergy by acting as the first frontier of the body to allergen contact and sensitization. An intact epidermal barrier protects the immune system from exposure to exogenous allergens, whilst an impairment of the skin barrier, either mechanically or functionally, allows allergen penetration into the sub-epidermal layer and promotes sensitization. The skin as route of sensitization is no longer seen as necessarily being limited to allergy of the skin, but is more and more considered as a route of sensitization for allergy involving other organs.
The atopic march starts in newborn skin. The role of skin integrity and skin microbiota in childhood as interventional targets to prevent atopic sensitizations should be studied in large-scale studies with well-defined populations [ 65 ]. The role of allergen avoidance versus early allergen ingestion in promoting tolerance versus sensitization to food allergens and subsequent atopic symptoms must be evaluated [ 66 ]. The possibilities of tolerance induction to prevent symptoms caused by food allergens for both children and adults should be studied in more depth, with new monitoring methodologies. There is an opportunity to study the effect of newer biological drugs on prevention of the atopic march.
Major unmet needs in skin allergy
• Identification of molecular, genetic and environmental determinants of the atopic march
• Targeting skin barrier function for novel treatments and prevention
• Role of skin microbiota and colonizing pathogens
• Better characterization and identification of novel biomarkers for endotypes
• Monitor new contact sensitizers and promote patch testing
• Develop and evaluate new drug classes including biologicals
• Epidemiological monitoring of urticaria and hereditary angioedema
Atopic dermatitis is one of the most common chronic inflammatory skin diseases with an estimated prevalence of 20% in children and 5% in adults [ 67 , 68 ]. The direct and indirect costs of atopic dermatitis are significant and comparable to other common chronic diseases with large annual economic burdens. However, compared to psoriasis, the most closely analogous complex inflammatory skin disease, treatment options are limited. There is a need for new classes of drugs, such as biologicals for treating it, including those targeting the immune system, skin barrier, and skin microbiota. Developing methods for improving the skin barrier function should be an important focus of future research [ 69 ]. It is increasingly clear from advances in molecular genetics that a close interaction and reciprocal regulation of epithelial function by immunological events underlie allergic disease, and epithelial barrier function is becoming more and more important in other forms of allergic diseases, such as asthma and rhinitis [ 70 ]. A bench to bedside approach is necessary to identify the key elements of these interactions systematically.
Allergic contact dermatitis is one of the most common work-related diseases and is of appreciable public health importance because of its significant economic impact. As knowledge on its diagnostics through patch testing is waning, promoting patch test education is a paramount issue. New contact sensitizers continue to appear [ 71 ] and older ones become important in new situations, emphasizing the need to monitor their appearance in Europe and worldwide and integrate new knowledge to relevant regulations at all levels. Contact allergy in children continues to be underdiagnosed and should be studied, tested and publicized more often. The potential role of food chemicals as a possible cause of dermatitis and food allergy requires study and, conversely, exposure to shared haptens in cosmetics and food could potentially be a cause of food allergies [ 72 ]. Increasing exposure to palladium, with its high sensitizing potential, in electronic appliances, dental alloys, jewellery and car exhaust catalytic converters, presents a real risk of it becoming the ‘metal allergy of the 21 st century’ [ 73 ]. The epidemiology and dynamics of palladium sensitization call for an in depth study of palladium allergy from the scientific viewpoint and further regulation of its use.
Urticaria in all its presentations has an estimated lifetime prevalence of around 20% [ 74 ]. However, reliable epidemiologic data are scarce, and most of the published literature deals with adults. Thus, there is a need for epidemiological research into urticaria, as well as good diagnostic markers defining the subgroups within urticaria patients. In addition, treatment with new classes of drugs, including novel antihistamines, mast cell stabilizing drugs and biologicals, such as omalizumab [ 75 ], should be evaluated thoroughly for their clinical effectiveness and understanding the biology of mast cell disease. Development of specific mediator antagonists for hereditary angioedema and cryopyrin associated periodic fever syndrome will help to define the phenotype of specific subsets of disease.
Environmental determinants of allergy
Aerobiology and pollution.
Airborne allergens are a major trigger of allergic diseases, especially of the respiratory tract. Monitoring of biological airborne particles (pollen and moulds) is currently done by private networks. All over Europe, these national networks are underfunded and on the verge of collapse. In addition, European Union (EU)-citizens and scientists have limited access to pollen and mould data due to diffuse property rights, as most stations are funded with private money.
Therefore, there is an urgent need to secure, harmonize and open the existing networks with a European-scale pollen, allergen and air pollution/air quality forecasting network, also capable of predicting future developments in migration of allergenic plants (like Ambrosia artemisiifolia ), and the environmental change in allergen exposure due to climate change. Thus, we will be able to sufficiently serve allergic sufferers and policy makers. The existing networks could be incorporated in the Global Monitoring for Environment and Security (GMES) Network for air quality monitoring and forecasting, in combination with the modeling communities.
Major unmet needs in aerobiology
• Harmonization of existing networks for monitoring biological airbourne particles
• Monitoring of the ‘exposome’ in conjunction with epidemiology in sensitization and symptom monitoring
• Identification of the socioeconomic burden of different biological airbourne particles to define strategies to fight pollution
• Identification of the role of climate change in allergic diseases
• Epigenetics studies to link the environmental factors with inherited inflammatory thresholds
The causes of allergic diseases are multifaceted. Thus, multiple exposure monitoring (‘exposome’) in conjunction with symptom and sensitization monitoring may reveal novel associations and interactions. Assessment of the ‘exposome’ (biological and chemical, indoor and outdoor) EU-wide, in concert with ongoing epidemiological studies/registries of allergic symptoms and sensitizations, will produce important synergies [ 76 ]. The climate change and its impact on development of allergies, microbiota, virus epidemics, parasites, moulds and food resources should be specifically focused on [ 77 ].
Pollen, moulds, parasites, viruses and bacteria are the carriers of allergens and/or innate immune system danger signals. These biological properties (not the pollen or the mould but the allergen and the danger signal) in ambient air are currently only partly monitored and should be an addition to current research. Cross border comparison, also with non-EU countries will enlarge the biological variance and thus facilitate the finding of allergy modulating factors [ 78 , 79 ].
Infections and allergy
Major research gaps on the role of infections in allergy.
• Differential immune response to various microorganisms
• Helminth infections as a paradigm to prevent/treat allergy
• Evolution and dynamics of the microbiome
• Viral-bacterial interactions
• Development of vaccination and other targeted interventions against human rhinoviruses
• Determine mechanisms underlying deficient antiviral immune responses in asthma
Both infections and commensal micro-organisms have a crucial role in the development and severity of allergic disorders and are involved in their resolution or chronicity. To harness their properties for prevention and treatment of allergies, we need to better understand which micro-organisms affect allergies naturally and how they interact with immune and inflammatory responses in allergy. This requires considerable epidemiological, clinical and mechanistic research both in human and animal models.
Helminth infections are associated with reduced skin prick test responsiveness to environmental allergens, especially if the parasite burden is high and occurs in early life. The evidence with regard to allergic disease is more heterogeneous, but some studies show a negative relationship between allergy and certain parasite infections, such as hookworm and schistosomiasis [ 80 ]. However, clinical trials treating people with established allergy with a single parasite dose have not shown beneficial effects so far. Future research will need to include trials of multi-dose administration resulting in higher worm burden and stronger immunomodulation. Another approach could be to develop allergy therapies and prophylaxis based on parasite-derived products; such products should be assessed for efficacy and safety, especially in young children, who are likely targets for primary prevention of allergy [ 81 ].
There is also emerging evidence that exposure to both commensal and pathogenic bacteria may influence the development of allergy [ 82 ]. We need to understand in detail how the microbiomes of gut, skin, and airways evolve from early life to senescence, which factors (e.g. birth mode, breastfeeding, diet, puberty, pregnancy, infections) influence their composition and how this relates to the development and severity of allergies. Such studies will also contribute to investigations aiming at the development of preventive and therapeutic applications of pre-, pro- and symbiotics.
During the last decades, it has become evident that viral infections, particularly those caused by human rhinoviruses (RV) are the most frequent triggers of acute exacerbations of asthma; in some cases viral agents have been detected in more than 90% of such events [ 83 , 84 ]. RVs are also responsible for the majority of mild rhinitis, i.e. common colds, therefore contributing further to symptoms in respiratory allergic patients. Viral bronchiolitis in infants is associated with an increased asthma risk. Once again RVs appear to be the agents most strongly associated, with a remarkable 40-fold increase of risk of wheezing at age 6 after an early symptomatic RV infection [ 85 ]. Whether early life episodes of respiratory viral infections also predispose to the induction of sensitization is less well-established, but has potentially great impact on strategies for allergy prevention. Given the lack of effective treatment or prevention of virus-induced asthma symptoms, future research must focus on developing diagnostic, therapeutic and prophylactic modalities for virus induced respiratory allergy. Our incomplete understanding of the role that respiratory viruses play in immune and inflammatory responses of the respiratory tract, their regulation and resolution, requires research in both animal models and in well-defined patient populations [ 86 ]. This needs to include studies to determine mechanisms underlying deficient antiviral immune responses reported in asthma [ 87 ] and define the roles of interactions between different viruses, including the recently identified rhinovirus-C [ 88 ], viral-bacterial synergistic effects, which accentuate airway inflammation [ 89 ], repeated infections, and interactions between viruses and other factors including allergens and pollutants, in establishing chronic respiratory allergies. Prospective cohort studies involving comprehensive microbiological detection and identification of other potential triggers of asthma development and exacerbations may be required to provide accurate information on natural microbial exposures and the relative impact of individual respiratory pathogens.
Insect stings
Hymenoptera, which represent one of the largest orders of insects, are responsible for the majority of anaphylactic reactions in adults, of which some are fatal. In Europe, wasp or bee stings are the most common elicitors of Hymenoptera venom allergy (HVA) having a great impact on quality of life in adults, children and their parents [ 90 ]. Population-based studies should establish the regional frequency of HVA in different European countries [ 91 ]. Identification of the genetic background, and of the pathophysiological mechanisms involved in venom allergic reactions also require further research [ 92 ]. Healthcare providers and the public alike will benefit from proper diagnosis and long-term treatment of this potentially life-threatening allergy. To improve the diagnosis of HVA, certain techniques such as molecular diagnosis and peptide microarray immunoassay need further evaluation. A better knowledge on eliciting allergens might not only help to make a correct diagnosis but might also help to optimize venom extracts, or even replace them with recombinant allergens, for treatment. Parallel studies should aim at improving the clinical interpretation of well-established diagnostic procedures such as the intradermal tests, baseline serum tryptase concentration and sting challenge test [ 93 ].
Unlike food and medication allergy, which are managed primarily by allergen avoidance, the prospective management of HVA relies on venom immunotherapy (VIT), where treatment is mediated through administration of gradually increasing doses of the venom-allergen.
The efficacy of VIT in adults has progressed considerably, reaching a protective level of up to 98%. Still, there are many open questions regarding patient selection for VIT and duration of treatment. The long-term prognosis of untreated in comparison to treated HVA in children and adults needs further evaluation; only few data are available on risk factors of severe reactions in children with HVA [ 94 ]. Various treatment protocols mainly differ from each other with respect to the degree of tolerance of VIT, and it should be examined whether specific patients particularly profit from specific types of treatment protocols [ 95 ].
Food, diet & nutrition
Food allergies affect a considerable proportion of children and adults in Europe and are the leading cause of severe anaphylaxis [ 96 ]. Furthermore, there is increased recognition of disorders associated with food allergy, such as eosinophilic oesophagitis.
Our current knowledge about the molecular mechanisms and factors for the allergic sensitization and elicitation phase is limited. Therefore, in depth characterization studies of both allergens and their co-factors are needed to understand how immune tolerance towards dietary antigens is disrupted and allergic sensitization initiated. An integrative approach is needed, that addresses the role of exposure (timing and dose) and host factors, including investigations on the role of genetic predisposition, the extent and composition of the intestinal microbiome and the involvement of the innate defense system [ 19 , 97 ].
In particular the interplay between the innate immune system and food should be studied in more detail. While we know that in spite of a close botanical relationship, peanut seems to be a strong sensitizer and soy a much weaker sensitizer, we still do not know whether the reason for this discrepancy is differences on the allergen level (including different digestibility), on the level of innate immune response stimulation, on the level of the different food matrices, on eating habits (oral exposure) or even on the level of non-oral exposures such as transdermal or inhalation. Thus both clinical studies of allergen exposure and uptake and in vitro models of the human intestine by which these features can be studied should be given high priority.
Unmet needs in food allergy
• Understand molecular mechanisms of tolerance and its disruption in food allergy
• Improve diagnosis with individual allergen components, food matrix interactions and threshold identification
• Establish food allergy phenotypes
• Establish novel immunotherapeutic approaches
• Evaluate the socioeconomic cost of food allergies
• Standardize nutritional interventions
For improved diagnosis of food allergy, complete panels of well characterized individual allergens and knowledge on their food matrix interactions regarding allergenicity, are still lacking. Identification of thresholds for each individual food is also needed. In component resolved diagnosis, investigation of allergen specific IgE recognition with clinical implications and the identification of marker allergens is needed at least for the most important food allergen sources. These well-characterized diagnostic tools will contribute to patient tailored dietary recommendations and to design avoidance strategies, accordingly; such approaches have been initiated within the context of the FP6-funded project EuroPrevall. On the other hand in depth knowledge of allergens and certified reference material available for the food industry will help them to fine tune their allergenic risk management. Furthermore, individual food allergens including their hypoallergenic variants will contribute to novel immunotherapeutic applications for food allergy.
Epidemiological and clinical studies suggest that there are different phenotypes in food allergy and that the onset (early, late), natural course (short term, persistent), and clinical presentation (mild, severe) differ. Multicentre studies involving a comprehensive clinical, immunological and (epi)genetic evaluation are needed to establish the different phenotypes in food allergy. This will further guide the management and specific treatment of food allergic patients.
Specific immunotherapy in food allergy, especially oral but also sublingual, has provided promising results. With the availability of hypoallergens, also subcutaneous immunotherapy has regained interest [ 98 ]. Studies ranging from basic science to clinical application are urgently needed regarding indications, underlying immunological mechanisms, safety, efficacy and cost-effectiveness of this treatment [ 98 ].
Recently, methods were developed to assess and record the reduced quality of life of the food allergic patient. However, the implementation of these findings in improved dietary recommendations, training of the patient or production of safer foods is still lacking and calls for a multidisciplinary approach to address the relevant issue. In parallel, the socioeconomic cost of food allergy is largely unknown. Capitalizing on a specific instrument built in the EuroPrevall project [ 99 ], the economic burden of food allergy can be studied across Europe, and the impact on costs of diagnostic and therapeutic interventions evaluated.
Despite the importance of diet and nutrition in immune development, only a small number of studies have evaluated their role on the prevention and natural course of food allergy and allergic diseases in general. There is therefore a need for descriptive studies on preventive nutritional aspects. These would include nutritional analysis around conception, during pregnancy, but also in early life and evaluation of atopic disease outcomes longitudinally. Nutritional analysis in lactating women as related to the composition of breast milk may also provide important insights. The role of specific nutrient levels, e.g. vitamin D, should be part of an extensive nutritional analysis [ 100 ].
The influence of the nutritional composition of avoidance diets on both nutritional status, but also disease severity, of children and adults with food allergy should be further evaluated [ 101 ].
Unlike other diagnostic tools, the removal of a suspected food from the diet has not been properly standardized nor validated. Furthermore, there is an important diagnostic value of a standardized allergy-focused diet history. Based on expert consensus opinion, both the recent NIH US guidelines and the UK NICE guidelines [ 102 ] reported that diet history is the cornerstone of food allergy diagnosis. Research efforts should gather evidence to substantiate this opinion, including the role of the dietitian in both diagnosis and individual dietary counseling.
Food labeling remains an important issue and EFA is actively involved in the elaboration and follow-up of the new EU regulation on food information to consumers.
Drug allergies
Drug hypersensitivity reactions (DHRs) affect more than 7% of the general population and represent an important public health problem [ 103 ]. They are unpredictable, cause morbidity and mortality, compromise optimal medical care and are a major cause of post-marketing drug withdrawal [ 104 ]. While urticaria and maculopapular exanthemas are the most frequent, other drug-induced reactions, although less frequent may have a high fatality rate, including systemic anaphylaxis and toxic epidermal necrolysis (TEN).
DHRs represent a growing health problem and their world-wide prevalence is expected to increase over the next decades. A number of reasons will contribute to this phenomenon and will amplify its impact on the general population. First, new pharmacological molecules are continuously going to the market and more and more patients will be exposed to these agents. In particular, biological drugs, such as monoclonal antibodies, have profound interactions with the immune system and may cause severe hypersensitivity reactions. Second, there is an increasing use of over-the-counter medications that may potentially raise the risk of adverse reactions to drugs because of lack of medical supervision. Finally, the concomitant administration of multiple drugs (polychemotherapy), particularly in the elderly, is expected to increase the rate and severity of reactions to drugs: It is worth to mention, for example, that aspirin (ASA) and other drugs widely used for cardiovascular diseases (ACE inhibitors, beta-blockers) may exacerbate or aggravate allergic and anaphylactic reactions.
The complexity in DHRs is high; despite the common occurrence of DHRs, the mechanisms, diagnostic/therapeutic opportunities and differential diagnoses differ between drugs and manifestations and experience towards specific reactions is lacking. Every centre alone, experiences a limited and mostly biased spectrum of the disease and physicians frequently do not attempt to clarify a suspected reaction at all [ 105 ]. With diversity and diagnostic challenges, both individual patient phenotyping and epidemiological studies are affected. Additionally, most therapeutic recommendations, including new approaches such as desensitization are predominantly based on case reports or small case series. As we do not know the natural course of disease, it is not clear whether lifelong avoidance is really necessary. Taking into account that DHR research has not been supported for a long time by either the pharmaceutical industry or national programs [ 106 ], there is a clear need for training, standardized criteria, and large, multicentre studies and data collection to provide answers to the above questions. National and international registries should also be implemented or potentiated to provide useful information on the real dimension of the problem and to identify reliable parameters to assess the individual risk of DHRs.
New diagnostic tools have to be developed, as the available ones are poorly validated and may lead to misclassification (underreporting or overdiagnosis). The development of new procedures, such as standardization of skin tests, test concentrations, advancement and improvement of diagnostic test agents and tests, such as basophil activation test (BAT), Elispot, lymphocyte transformation test (LTT), is indispensable to confirm a DHR and identify the culprit drug to be avoided in the future. Existing diagnostic tests must be critically validated and standard operation procedures should be tailored to specific drugs, specific manifestations and age groups (children versus adults). Drug re-exposure by standardized challenges are most needed procedures in order to limit medication withdrawal based on an unclear diagnosis. Nevertheless, they are often non-standardized and lack good correlation with currently used diagnostic in-vitro tests.
Research agenda for drug hypersensitivity
• Training (multiple drugs, complex manifestations and mechanisms)
• Standardization of test procedures (still not achieved)
• Development of new diagnostic tools
• Reliable epidemiological data with proven hypersensitivity
• Clinical multicenter studies on diagnosis and therapy with well-phenotyped patients
• Mechanistic studies (pharmacological interaction with immune receptors (PI) concept, genetic pretesting, risk factors)
The availability of tissue and serum samples from immune-mediated drug hypersensitivity reaction (IDHR) patients is a prerequisite for basic research in the mechanism of DHRs, which may be allergic or non-allergic, with immunological or pharmacological recognition and with the allergenic determinants mostly unknown. In this effort, the study of genetic variants, especially of the major histocompatibility complex (MHC) molecules, should be included, in order to enable personalized recommendations for the risk to develop a DHR. To generate preclinical testing methods to assess the risk of potential DHRs in new drugs, research should also encompass the characterization of drug-specific (chemical structure, metabolites, exposure), intrinsic (genetics) and extrinsic risk factors (viral infections, other danger signals), complemented by preclinical prediction models.
On the socioeconomic level, as in other allergy manifestations, studies should define the impact of DHRs on the quality of life of patients and their cost on the health system.
Activity-related allergies
Allergy and asthma in sports.
A moderate physical activity favors health and well-being whereas a sedentary lifestyle and obesity are associated with a higher prevalence of allergic diseases in both children and adults [ 107 ]. Interestingly, however, an intense physical training, as a stress event, may induce several changes in immune parameters and response to environmental agents, which essentially result in an increased susceptibility to infections and in a preferential Th2 (allergic) phenotype [ 108 ].
Major research gaps on the role of allergy and asthma in sports
• Describe mechanisms of exercise-induced bronchoconstriction and identify biomarkers
• Understand the immune derangement after strenuous exercise
• Therapeutic trials in athletes
• Standardization of specific diagnostic tests
Physical exercise may also play a direct role on target organs triggering symptoms such as bronchial obstruction and anaphylaxis. Indeed, allergic diseases, asthma and exercise-induced bronchoconstriction (EIB) are present with high and increasing prevalence in athletes, significantly affecting their health status and performance and therefore representing a major concern in Sports Medicine [ 109 ]. The necessity therefore in Sports Allergy research is to describe the mechanisms of EIB, so as to clarify the clinical relevance and natural course of this phenotype and also identify markers of epithelial stress and inflammation. In this line of research, it would also be feasible to identify putative danger signals of immune derangement linked to the transient immune deficiency, which forms the basis for recurrent infections in elite athletes and is caused by strenuous and continuous training loads. Research efforts recruiting the –omics approaches should allow understanding of the impact of acute and chronic exercise as well as any correlation of gender on the immune functions.
With regard to clinical pharmacology in Sports Allergy, this is often extrapolated from data obtained in the general population, creating a need for randomized controlled clinical trials solely in athletes. Allergy and asthma in athletes is underdiagnosed [ 110 ] but this would be remedied if athletes were to be routinely evaluated through a standardized protocol for sensitization and immune function; such a policy would also allow improved management of their condition and permit the accurate study and screening of the effects on physical performance of anti-allergic and anti-asthmatic drugs (with special reference to beta-2 agonists). There is a need for guidelines for the diagnosis and management of allergic diseases and these should be widely diffused and applied by Sports physicians, in compliance with the World Anti-Doping Agency (WADA) rules, in the adequate management of these clinical pictures [ 111 ]. Some special attention should be paid to the usefulness of different methods of non-specific bronchial provocations in diagnosis of athletes’ asthma. Since metacholine provocation does not prove useful in a substantial percentage of cases, more comparative research involving other stimuli is needed in order to create feasible and unified algorithms to be used in professional athletes.
Occupational allergy
Major research gaps in occupational allergy.
• Changes of occupational allergy with time
• Public health impact of occupational allergy
• Monitor allergen exposure at workplaces
Occupational allergy and particularly occupational asthma can be caused by more than 300 agents and about 15% of adult-onset asthma can be attributed to workspace exposures [ 112 , 113 ]. Exposure-specific studies on occupational asthma have focused on substances of high and low molecular weight- e.g. flour, enzymes, isocyanates and latex [ 114 ]. While in developing countries the workforces probably have more extensive occupational exposures than in high-income countries, lower figures of the occurrence of occupational asthma have been reported, probably indicating a problem of underdetection and methodology. In addition, protective mechanisms described for respiratory allergies (hygiene hypothesis) may also play a role for occupational allergies. Still, the public health impact and burden to society related to occupational or work-related asthma have been scarcely investigated. Assessment of excess burden of disease due to specific occupational exposure is a useful measure, when there is valid information on population exposure and attributable fractions. Changes in the working conditions and implementation of new substances lead to the introduction of new allergens in some workplaces and the onset of new, so far unknown cases of occupational allergy. However, there is only little available information on the changes in the pattern of occupational allergy over time. An objective evaluation of the time trends in the incidence and causes of immunological occupational asthma in the EU, using workers' compensation data or registry-based data would be of great importance [ 115 - 118 ]. Prevention of occupational asthma related to a work-sensitizing agent would ideally be achieved by avoidance of exposure causing immunological sensitization and subsequent asthma. Especially apprenticeship is a period of increased risk of developing work-related respiratory allergic diseases and therefore is a need for appropriate professional advice to young adults aiming to reduce unsuitable job choices and prevent impairment from their careers [ 119 ]. Different forms and different steps of prevention exist, like primary prevention (the ideal form of prevention), where workers do not become sensitized to agents that can cause asthma. Since primary prevention is not always successful, secondary prevention by medical surveillance has some evidence to support benefit for those working with common occupational sensitizers. On the other hand it is necessary to optimize workers’ education and also the diagnosis and medical management to minimize further impairment of the airways [ 120 - 124 ]. There is a need to increase the implementation of airborne allergen quantification at workplaces to be used as scientific background e.g. for the discussion of health-based occupational exposure limits for high molecular weight sensitizers.
Allergic diseases in children
Childhood is a key period as many allergies start early in life. It is also characterized by a large number of research needs, mainly due to the lack of reliable evidence to guide practice that has resulted from the challenges associated with undertaking paediatric research. We are still uncertain how to prevent children from developing allergic diseases. For example, important advances in our knowledge of genetic associations with allergic disease, have not clarified the underlying pathological pathways, probably because we have yet to understand their interactions with environmental exposures. We also lack knowledge on epigenetic mechanisms, now thought to be important in allergy. A relatively recent hypothesis in the prevention field is that high dose oral exposure to food allergens in early infancy may promote the development of immunological tolerance [ 125 ]. We must await the results of large, interventional studies to assess the validity of this hypothesis and it will be important to explore whether it also holds for aeroallergens, opening up possibilities for preventive vaccination. Additionally, with our improved understanding of the infant immune system, new therapeutic approaches addressing early immune stimulation and tolerance induction using bacterial compounds need further exploration [ 126 ].
Important gaps exist in the diagnostic tools that are available for young patients with possible allergy. In practice, it is not uncommon to find children with symptoms highly suggestive of clinical allergy, but negative allergy skin or blood tests. There is a need to investigate the potential importance of novel allergens and whether we can detect the local presence of relevant specific IgE in relevant end organs in addition to the skin or serum. Furthermore, our current allergy skin or blood tests fail to distinguish between clinical allergy and irrelevant sensitization or to identify patients at high risk of severe allergy mediated reactions [ 127 ]. We need a new improved approach to diagnostic testing to reduce the need for potentially dangerous provocations challenges (e.g. food challenges).
Research needs in paediatric allergy
• More studies in children of different ages are needed, both on the development of allergy and to evaluate differential responses to treatment
• Large, primary prevention intervention programs to confirm current hypotheses and reverse the epidemic
• Improved, non-invasive diagnostic tools
• Prevention and/or treatment of asthma exacerbations
• Evaluate the long-term effects of immunotherapy in children
• Focus on adolescence
Asthma is an important problem in childhood affecting many children and resulting in significant morbidity [ 128 ]. It has been suggested that vitamin D deficiency may play an important role in the development of asthma as well as being associated with severe asthma [ 129 ]. Like many findings in paediatric allergy, these are based on observational data that are very susceptible to confounding. There is a research need for multi-centre randomized controlled trials to assess the role of vitamin D in the development of asthma and/or severe asthma. Another unmet need in paediatric asthma is the study of exacerbations, many of which are associated with viral respiratory tract infections [ 83 ]; we also do not currently have any therapies capable of preventing or treating such exacerbations. A possible role of early respiratory virus exposure in facilitating sensitization to allergens requires attention as well. A further unexplained association exists between food allergy and severe exacerbations of asthma [ 130 ], which is important particularly as asthma is also associated with severe allergic reactions to foods. Severe asthma in childhood, and adult life, is poorly understood. In an attempt to understand this area, a novel unbiased “omics” systems biology approach is currently being used to explore the underlying pathophysiological mechanisms [ 131 ]. A similar approach is also being used in other allergic diseases and both studies can be expected to highlight many novel pathways that have the potential to deliver new therapeutic approaches for allergic disease.
The majority of our therapies for allergic disease only suppress the disease process while they are taken. The exception is immunotherapy, which would appear to have the potential to alter the natural history of allergic disease in childhood, for example preventing the development of asthma in children with hay fever [ 132 ]. Again though, as the quality of the paediatric evidence in this area is not optimal there is a need for well designed, double-blind, placebo-controlled studies to assess the long-term effects of immunotherapy in childhood. While such studies are ethically challenging, it is important to characterize the long-term effects, as this would substantially alter the perceived cost-effectiveness and trade-off between efficacy and safety of immunotherapy in childhood.
Adolescents and young adults are at higher than expected risk of morbidity and mortality from both asthma and food allergy. While this is poorly understood, it may result from the disengagement of adolescent patients from their medical needs as they are challenged by the usual process of adolescence [ 133 ]. It is also not assisted by the lack of continuity between paediatric and adult medical care in many countries. There is a need to develop a better, adolescent-focused approach to this at risk group to tackle this unmet need [ 134 ] and to develop better transition strategies to ease the progression from paediatric to adult services.
Causal treatment of allergy: allergen specific immunotherapy
Allergen specific immunotherapy is the only currently available medical intervention that has the potential to affect the natural course of the disease [ 135 ]. Accumulating evidence have convincingly shown that in addition to alleviating symptoms, allergen specific immunotherapy can improve quality of life, reduce long-term costs and burden of allergies, and has the potential to change the course of the disease. Several appropriately designed and powered clinical trials have proven its good safety profile and effectiveness in allergic rhinitis, asthma and venom allergy [ 59 , 136 , 137 ].
However, there are still important questions to be answered. The unmet needs of this causal treatment should be evaluated in an integrated multinational academic, research, industry and regulatory agencies effort in Europe. Research should focus on optimizing clinical and immunological efficacy, safety and compliance, possibly through improved time-schedules. Novel approaches such as the use of adjuvants, modified allergens and molecular allergen components should be widely evaluated [ 138 ].
Major knowledge gaps in allergen-specific immunotherapy
• Mechanisms of induction and long term maintenance of allergen tolerance
• Molecular mechanisms of how T-reg cells and B-reg cells are generated in vivo and how to affect their life span
• Role of resident tissue cells in immune tolerance
• Molecular mechanisms of spontaneous healing, remissions and exacerbations of allergic disease
• Local tissue events during SLIT and epicutaneous SIT
• Better adjuvants that specifically induce immune tolerance
• Early biomarkers and predictors to decide to start, stop and success
• Phase III clinical trial primary outcomes equivalent to real-life exposure
• Efficient short term and long lasting treatment modalities
Outcome measures for both clinical trials and routine clinical practice should be further standardized and validated. To date, the required primary outcome is real-life exposure, which by its nature is very poorly standardized. Alternatives are urgently needed, not only because costs of failed trials due to lack of pollen exposure are a great financial burden to the sponsor, but perhaps even more importantly it is not ethical to subject patients to double-blind, placebo-controlled (DBPC) trials in which exposure is at best poorly controlled. Another issue is the dominance of poly-sensitization in clinical trials conducted for one allergy. Clear indications and guidelines, especially in regard to polysensitized patients are therefore needed. Multi-allergen immunotherapy needs more supporting data to be validated. The use of surrogate antigen challenges in the eye and nose and their relationship to clinical efficacy of SIT should be evaluated for both, seasonal and perennial allergens. The use of pollen chambers in dose–response studies has to be adequately studied. Surrogate allergen challenges should also be evaluated as an alternative primary outcome for phase III clinical trials. New modalities of allergen-applications such as the epicutaneous and the direct injection of allergens in the lymph node are promising and need to be further evaluated. The long-term efficacy and safety of these modalities needs to be confirmed.
In children, there is a need to identify an optimal dose, dosing frequency and duration. Most importantly however, long-term efficacy, including the preventive capacity for asthma and new sensitisations, and the safety profile of allergen specific immunotherapy should be further addressed in the paediatric population. This will facilitate the recognition and approval of allergen specific immunotherapy in children by regulatory national and international agencies. The demonstration of long-term efficacy is required for the current mandatory paediatric investigation plan (PIP) that must accompany applications for marketing authorization submitted to the European Medicine Agency (EMA). In addition, a role of SIT for secondary prevention in already sensitized children has been evoked, but requires further evaluation. Studies exploring this hypothesis are definitely needed. Furthermore, there is a need to perform economic assessments in high-quality prospective and long-term clinical studies comparing immunotherapy with pharmacotherapy in real-life practice.
Understanding the mechanisms of immunotherapy will ultimately lead to advanced, curative treatments of allergic diseases, therefore research focusing on the generation, life span and different roles of T-regulatory cells is needed [ 34 , 139 ]. Furthermore, long-term immune tolerance and local tissue events should be studied in detail, in parallel to the understanding of spontaneous healing, remission and exacerbations of allergic disease. Specific issues in relation to immune tolerance include the molecular mechanisms of how T regulatory cells are generated in vivo, their life span induced by allergen immunotherapy, whether they have any deleterious roles, tolerance to tumour antigens and chronic infectious agents and the role of resident tissue cells [ 140 ]. Increased knowledge on tolerance induction may also lead to strategies for preventive vaccination [ 141 ].
Biomarkers able to predict clinical response, treatment outcome and/or monitor progression of the treatment are also key to enhance clinical decisions. Any differences in the mechanisms of high-dose and low-dose allergen specific immunotherapy need also to be assessed.
Conclusions
The advances in allergy diagnosis, management and basic science to date have been substantial. There remain, nevertheless, many unmet needs as a consequence of the modern epidemic of allergy. Extensive research is needed to counteract the consequences for both individual patients and public health. Despite the variety of external factors that may trigger allergic reactions, as well as the pathophysiological complexity of these chronic diseases, common themes in relation to research needs arise: phenotyping/endotyping seems to be widely required in order to arrange or re-arrange clinical syndromes in more coherent and treatment-responsive groups. Using different –omics approaches in combination with systems biology and systems medicine appear promising in this direction. Using these approaches, patient-tailored management, including gender specific focusing, may become realistic. The need for epidemiological description or surveillance, including exposome monitoring, remains in many fields, and real-life, patient-centered research supported by registries is required. Basic mechanisms are still incompletely understood, therefore, any diagnostic, classification or treatment effort should be supported by attempts to better understand pathophysiology. Allergen components promise to be the next generation of diagnostic and therapeutic tools; large-scale studies are required to bring them to the clinics. Finally, severe disease, co-morbidities and/or exacerbations over a chronic course, are usually the most difficult to manage, more costly and dangerous and therefore require particular attention. Nevertheless, currently arising treatments, particularly in the fields of immunotherapy and biologicals, hold great promise for targeted and causal management of allergic diseases.
Major research themes in allergy
• Mechanisms
• Prevention
• Epidemiological surveillance - Registries
• Biomarkers for prediction, diagnosis, classification, treatment response
• Phenotypes / Endotypes
• Severe Disease/Exacerbations
• Novel treatments (biologicals, vaccines, new drugs)
Abbreviations
AD: Atopic dermatitis; AKC: Atopic keratoconjunctivitis; AR: Allergic rhinitis; DBPC: Double-blind, placebo-controlled; DHR: Drug hypersensitivity reaction; EAACI: European Academy of Allergy and Clinical Immunology; ECHRS: European community respiratory health survey; EIB: Exercise-induced bronchoconstriction; EU: European Union; GA 2 LEN: Global Allergy and Asthma European Network; HVA: Hymenoptera venom allergy; IDHR: Immune-mediated drug hypersensitivity reaction; ILCs: Innate lymphoid cells; ISAAC: International study of asthma and allergies in childhood; LTi: Lymphoid tissue–inducer; PAC: Perennial allergic conjunctivitis; VIT: Venom immunotherapy; VKC: Vernal keratoconjunctivitis; WADA: World anti-doping agency.
Competing interests
All authors are members of EAACI or EFA Boards and declare no other competing interests in respect to this publication.
- World Health Organization FSn307. http://www.who.int/mediacentre/factsheets/fs307/en/index.html .
- Asher MI, Montefort S, Bjorksten B, Lai CK, Strachan DP, Weiland SK, Williams H. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006; 368 (9537):733–743. doi: 10.1016/S0140-6736(06)69283-0. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- European Federation of Allergy and Airway diseases, Patients Associations, (EFA) Fighting for breath. http://wwwefanetorg/activities/documents/Fighting_For_Breath1pdf .
- Jarvis D, Burney P. ABC of allergies. The epidemiology of allergic disease. BMJ. 1998; 316 (7131):607–610. doi: 10.1136/bmj.316.7131.607. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- European Community Respiratory Health Survey. Variations in the prevalence of respiratory symptoms, selfreported asthma attacks and the use of asthma medication in the European Community respiratory health survey (ECRHS) Eur Respir J. 1996; 9 :687–695. [ PubMed ] [ Google Scholar ]
- E V. EFA Book on Respiratory Allergies – Raise Awareness, Relieve the Burden. 2011. http://wwwefanetorg/documents/EFABookonRespiratoryAllergiesFINALpdf .
- Wertz DA, Pollack M, Rodgers K, Bohn RL, Sacco P, Sullivan SD. Impact of asthma control on sleep, attendance at work, normal activities, and disease burden. Ann Allergy Asthma Immunol. 2010; 105 (2):118–123. doi: 10.1016/j.anai.2010.05.009. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- European Respiratory Society, [ERS] European Lung Book. The First Comprehensive Survey on Respiratory Health in Europe.
- Foundation EL. Economic Impact of Lung Diseases. 2011.
- Verboom P, Hakkaart-Van L, Sturkenboom M, De Zeeuw R, Menke H, Rutten F. The cost of atopic dermatitis in the Netherlands: an international comparison. Br J Dermatol. 2002; 147 (4):716–724. doi: 10.1046/j.1365-2133.2002.04964.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mancini AJ, Kaulback K, Chamlin SL. The socioeconomic impact of atopic dermatitis in the United States: a systematic review. Pediatr Dermatol. 2008; 25 (1):1–6. doi: 10.1111/j.1525-1470.2007.00572.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Council of the European Union. Council conclusions on prevention, early diagnosis and treatment of chronic respiratory diseases in children. http://wwwconsiliumeuropaeu/uedocs/cms_Data/docs/pressdata/en/lsa/126522pdf .
- Kauppi P, Linna M, Martikainen J, Makela MJ, Haahtela T. Follow-up of the Finnish Asthma Programme 2000–2010: reduction of hospital burden needs risk group rethinking. Thorax. 2012. [Epub ahead of print] PMID 22504963. [ PubMed ]
- Beasley RKU, von Mutius E, Pearce N. International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. Lancet. 1998; 351 :1225–1232. doi: 10.1016/S0140-6736(97)07302-9. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sunyer J, Anto JM, Tobias A, Burney P. Generational increase of self-reported first attack of asthma in fifteen industrialized countries. European Community Respiratory Health Study (ECRHS) Eur Respir J. 1999; 14 (4):885–891. doi: 10.1034/j.1399-3003.1999.14d26.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bousquet J, Kauffmann F, Demoly P, Leynaert B, Bousquet PJ, Demenais F, Lenzen G, Burney PG, Zuberbier T, Van Cauwenberge P. [GA2LEN (Global Allergy and Asthma European Network)] Rev Mal Respir. 2009; 26 (6):577–586. doi: 10.1016/S0761-8425(09)74689-3. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Burney P, Summers C, Chinn S, Hooper R, van Ree R, Lidholm J. Prevalence and distribution of sensitization to foods in the European Community Respiratory Health Survey: a EuroPrevall analysis. Allergy. 2010; 65 (9):1182–1188. [ PubMed ] [ Google Scholar ]
- von Mutius E, Vercelli D. Farm living: effects on childhood asthma and allergy. Nat Rev Immunol. 2010; 10 (12):861–868. doi: 10.1038/nri2871. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Brown SJ, Asai Y, Cordell HJ, Campbell LE, Zhao Y, Liao H, Northstone K, Henderson J, Alizadehfar R, Ben-Shoshan M. et al. Loss-of-function variants in the filaggrin gene are a significant risk factor for peanut allergy. J Allergy Clin Immunol. 2011; 127 (3):661–667. doi: 10.1016/j.jaci.2011.01.031. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Akdis CA, Akdis M, Trautmann A, Blaser K. Immune regulation in atopic dermatitis. Curr Opin Immunol. 2000; 12 :641–646. doi: 10.1016/S0952-7915(00)00156-4. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Larche M, Akdis CA, Valenta R. Immunological mechanisms of allergen-specific immunotherapy. Nat Rev Immunol. 2006; 6 (10):761–771. doi: 10.1038/nri1934. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gilles S, Fekete A, Zhang X, Beck I, Blume C, Ring J, Schmidt-Weber C, Behrendt H, Schmitt-Kopplin P, Traidl-Hoffmann C. Pollen metabolome analysis reveals adenosine as a major regulator of dendritic cell-primed T(H) cell responses. J Allergy Clin Immunol. 2011; 127 (2):454–461. doi: 10.1016/j.jaci.2010.12.1082. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Basinski TM, Holzmann D, Eiwegger T, Zimmermann M, Klunker S, Meyer N, Schmid-Grendelmeier P, Jutel M, Akdis CA. Dual nature of T cell-epithelium interaction in chronic rhinosinusitis. J Allergy Clin Immunol. 2009; 124 (1):74–80. doi: 10.1016/j.jaci.2009.04.019. e71-78. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Akdis CA. Allergy and hypersensitivity: mechanisms of allergic disease. Curr Opin Immunol. 2006; 18 (6):718–726. doi: 10.1016/j.coi.2006.09.016. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat Med. 2009; 15 (4):410–416. doi: 10.1038/nm.1946. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mjosberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, Fokkens WJ, Cupedo T, Spits H. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011; 12 (11):1055–1062. doi: 10.1038/ni.2104. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Strid J, Sobolev O, Zafirova B, Polic B, Hayday A. The intraepithelial T cell response to NKG2D-ligands links lymphoid stress surveillance to atopy. Science. 2011; 334 (6060):1293–1297. doi: 10.1126/science.1211250. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature. 2011; 474 (7351):327–336. doi: 10.1038/nature10213. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ashe A, Sapetschnig A, Weick EM, Mitchell J, Bagijn MP, Cording AC, Doebley AL, Goldstein LD, Lehrbach NJ, Le Pen J. et al. PiRNAs Can Trigger a Multigenerational Epigenetic Memory in the Germline of C. elegans. Cell. 2012; 150 (1):88–99. doi: 10.1016/j.cell.2012.06.018. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Eyerich S, Eyerich K, Pennino D, Carbone T, Nasorri F, Pallotta S, Cianfarani F, Odorisio T, Traidl-Hoffmann C, Behrendt H. et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest. 2009; 119 (12):3573–3585. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Burgler S, Ouaked N, Bassin C, Basinski TM, Mantel PY, Siegmund K, Meyer N, Akdis CA, Schmidt-Weber CB. Differentiation and functional analysis of human T(H)17 cells. J Allergy Clin Immunol. 2009; 123 (3):588–595. doi: 10.1016/j.jaci.2008.12.017. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Stassen M, Schmitt E, Bopp T. From interleukin-9 to T helper 9 cells. Ann N Y Acad Sci. 2012; 1247 :56–68. doi: 10.1111/j.1749-6632.2011.06351.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wisniewski JA, Borish L. Novel cytokines and cytokine-producing T cells in allergic disorders. Allergy Asthma Proc. 2011; 32 (2):83–94. doi: 10.2500/aap.2011.32.3428. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Akdis CA. Therapies for allergic inflammation: refining strategies to induce tolerance. Nat Med. 2012; 18 (5):736–749. doi: 10.1038/nm.2754. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pelaia G, Renda T, Romeo P, Busceti MT, Maselli R. Omalizumab in the treatment of severe asthma: efficacy and current problems. Ther Adv Respir Dis. 2008; 2 (6):409–421. doi: 10.1177/1753465808100431. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wenzel SE. Eosinophils in asthma--closing the loop or opening the door? N Engl J Med. 2009; 360 [ PubMed ] [ Google Scholar ]
- Eberlein B, Krischan L, Darsow U, Ollert M, Ring J. Double positivity to bee and wasp venom: Improved diagnostic procedure by recombinant allergen-based IgE testing and basophil activation test including data about cross-reactive carbohydrate determinants. J Allergy Clin Immunol. 2012; 14 :14. [ PubMed ] [ Google Scholar ]
- Harwanegg C, Hiller R. Protein microarrays in diagnosing IgE-mediated diseases: spotting allergy at the molecular level. Expert Rev Mol Diagn. 2004; 4 (4):539–548. doi: 10.1586/14737159.4.4.539. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Scala E, Alessandri C, Bernardi ML, Ferrara R, Palazzo P, Pomponi D, Quaratino D, Rasi C, Zaffiro A, Zennaro D. et al. Cross-sectional survey on immunoglobulin E reactivity in 23,077 subjects using an allergenic molecule-based microarray detection system. Clin Exp Allergy. 2010; 40 (6):911–921. doi: 10.1111/j.1365-2222.2010.03470.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hoffmann HJ, Bogebjerg M, Nielsen LP, Dahl R. Lysis with Saponin improves detection of the response through CD203c and CD63 in the basophil activation test after crosslinking of the high affinity IgE receptor FcepsilonRI. Clin Mol Allergy. 2005; 3 :10. doi: 10.1186/1476-7961-3-10. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ebo DG, Bridts CH, Hagendorens MM, Mertens CH, De Clerck LS, Stevens WJ. Flow-assisted diagnostic management of anaphylaxis from rocuronium bromide. Allergy. 2006; 61 (8):935–939. doi: 10.1111/j.1398-9995.2006.01094.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- El-Ghaiesh S, Monshi MM, Whitaker P, Jenkins R, Meng X, Farrell J, Elsheikh A, Peckham D, French N, Pirmohamed M. et al. Characterization of the antigen specificity of T-cell clones from piperacillin-hypersensitive patients with cystic fibrosis. J Pharmacol Exp Ther. 2012; 341 (3):597–610. doi: 10.1124/jpet.111.190900. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Patrick DL, Burke LB, Powers JH, Scott JA, Rock EP, Dawisha S, O'Neill R, Kennedy DL. Patient-reported outcomes to support medical product labeling claims: FDA perspective. Value Health. 2007; 10 (Suppl 2):S125–137. [ PubMed ] [ Google Scholar ]
- Baiardini I, Bousquet PJ, Brzoza Z, Canonica GW, Compalati E, Fiocchi A, Fokkens W, van Wijk RG, La Grutta S, Lombardi C. et al. Recommendations for assessing patient-reported outcomes and health-related quality of life in clinical trials on allergy: a GA(2)LEN taskforce position paper. Allergy. 2010; 65 (3):290–295. doi: 10.1111/j.1398-9995.2009.02263.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- European Medicines Agency, Committee for medicinal products for human use, (CHMP) Reflection paper on the regulatory guidance for the use of health-related quality of life (HROL) measures in the evaluation of medicinal products. European Medicines Agency website. 2005. (cited 2007 April) 2005.
- US Department of Health and Human Services FDA Center for Drug Evaluation and Research. Guidance for Industry: patient reported outcome measures: use in medical product development to support labelling claims: draft guidance. Health Qual Life Outcomes. 2006; 4 :79. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schunemann HJ. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008; 336 :924–926. doi: 10.1136/bmj.39489.470347.AD. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Leynaert B, Soussan D. Monitoring the quality-of-life in allergic disorders. Curr Opin Allergy Clin Immunol. 2003; 3 (3):177–183. doi: 10.1097/00130832-200306000-00005. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Baiardini I, Braido F, Brandi S, Tarantini F, Bonini S, Bousquet PJ, Zuberbier T, Demoly P, Canonica GW. The impact of GINA suggested drugs for the treatment of asthma on Health-Related Quality of Life: a GA(2)LEN review. Allergy. 2008; 63 (8):1015–1030. doi: 10.1111/j.1398-9995.2008.01823.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Baiardini I, Braido F, Tarantini F, Porcu A, Bonini S, Bousquet PJ, Zuberbier T, Demoly P, Canonica GW. ARIA-suggested drugs for allergic rhinitis: what impact on quality of life? A GA2LEN review. Allergy. 2008; 63 (6):660–669. doi: 10.1111/j.1398-9995.2008.01649.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- van derVelde JL, Flokstra-de Blok BM, Vlieg-Boerstra BJ, Oude Elberink JN, Schouten JP, Dunngalvin A, Hourihane JO, Duiverman EJ, Dubois AE. Test-retest reliability of the Food Allergy Quality of Life Questionnaires (FAQLQ) for children, adolescents and adults. Qual Life Res: Inst J Qual Life Aspects Treatment, Care Rehabil. 2009; 18 (2):245–251. doi: 10.1007/s11136-008-9434-2. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- World Health Organization. Fact Sheet n°307. 2011. http://www.who.int/mediacentre/factsheets/fs307/en/index.html .
- Holgate S, Bisgaard H, Bjermer L, Haahtela T, Haughney J, Horne R, McIvor A, Palkonen S, Price DB, Thomas M. et al. The Brussels Declaration: the need for change in asthma management. Eur Respir J. 2008; 32 (6):1433–1442. doi: 10.1183/09031936.00053108. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lane S, Molina J, Plusa T. An international observational prospective study to determine the cost of asthma exacerbations (COAX) Respir Med. 2006; 100 (3):434–450. doi: 10.1016/j.rmed.2005.06.012. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sears MR, Greene JM, Willan AR, Wiecek EM, Taylor DR, Flannery EM, Cowan JO, Herbison GP, Silva PA, Poulton R. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N Engl J Med. 2003; 349 (15):1414–1422. doi: 10.1056/NEJMoa022363. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lotvall J, Akdis CA, Bacharier LB, Bjermer L, Casale TB, Custovic A, Lemanske RF Jr, Wardlaw AJ, Wenzel SE, Greenberger PA. Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. J Allergy Clin Immunol. 2011; 127 (2):355–360. doi: 10.1016/j.jaci.2010.11.037. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Agache I, Akdis C, Jutel M, Virchow JC. Untangling asthma phenotypes and endotypes. Allergy. 2012; 67 (7):835–846. doi: 10.1111/j.1398-9995.2012.02832.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Auffray C, Charron D, Hood L. Predictive, preventive, personalized and participatory medicine: back to the future. Genome Med. 2010; 2 (8):57. doi: 10.1186/gm178. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Greiner AN, Hellings PW, Rotiroti G, Scadding GK. Allergic rhinitis. Lancet. 2011; 378 (9809):2112–2122. doi: 10.1016/S0140-6736(11)60130-X. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Maurer M, Zuberbier T. Undertreatment of rhinitis symptoms in Europe: findings from a cross-sectional questionnaire survey. Allergy. 2007; 62 (9):1057–1063. doi: 10.1111/j.1398-9995.2007.01367.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bousquet J, Bachert C, Canonica GW, Mullol J, Van Cauwenberge P, Jensen CB, Fokkens WJ, Ring J, Keith P, Gopalan G. et al. Efficacy of desloratadine in persistent allergic rhinitis - a GA(2)LEN study. Int Arch Allergy Immunol. 2010; 153 (4):395–402. doi: 10.1159/000316351. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Watelet JB, Van Zele T, Gjomarkaj M, Canonica GW, Dahlen SE, Fokkens W, Lund VJ, Scadding GK, Mullol J, Papadopoulos N. et al. Tissue remodelling in upper airways: where is the link with lower airway remodelling? Allergy. 2006; 61 (11):1249–1258. doi: 10.1111/j.1398-9995.2006.01226.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Scadding G, Hellings P, Alobid I, Bachert C, Fokkens W, van Wijk RG, Gevaert P, Guilemany J, Kalogjera L, Lund V. et al. Diagnostic tools in Rhinology EAACI position paper. Clin Transl Allergy. 2011; 1 (1):2. doi: 10.1186/2045-7022-1-2. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hellings PW, Dobbels F, Denhaerynck K, Piessens M, Ceuppens JL, De Geest S. Explorative study on patients' perceived knowledge level, expectations, preferences and fear for side effects for treatment for allergic rhinitis. Clin Transl Allergy. 2012; 2 (1):9. doi: 10.1186/2045-7022-2-9. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kong HH OJ, Deming C, Conlan S, Grice EA, Beatson MA, Nomicos E, Polley EC, Komarow HD, Murray PR, Turner ML, Segre JA, NISC Comparative Sequence Program. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012. Epub ahead of print. [ PMC free article ] [ PubMed ]
- Fox ATSP, du Toit G, Syed H, Lack G. Household peanut consumption as a risk factor for the development of peanut allergy. J Allergy Clin Immunol. 2009; 123 (2):417–423. doi: 10.1016/j.jaci.2008.12.014. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Schultz Larsen F, Diepgen T, Svensson A. The occurrence of atopic dermatitis in north Europe: an international questionnaire study. J Am Acad Dermatol. 1996; 34 (5 Pt 1):760–764. [ PubMed ] [ Google Scholar ]
- Williams H, Robertson C, Stewart A, Ait-Khaled N, Anabwani G, Anderson R, Asher I, Beasley R, Bjorksten B, Burr M. et al. Worldwide variations in the prevalence of symptoms of atopic eczema in the international study of asthma and allergies in childhood. J Allergy Clin Immunol. 1999; 103 (1 Pt 1):125–138. [ PubMed ] [ Google Scholar ]
- Kawasaki HNK, Kubo A, Hata T, Shimizu A, Mizuno H, Yamada T, Amagai M. Altered stratum corneum barrier and enhanced percutaneous immune responses in filaggrin-null mice. J Allergy Clin Immunol. 2012. [ PubMed ]
- Soyka MB, Wawrzyniak P, Eiwegger T, Holzmann D, Treis A, Wanke K, Kast JI, Akdis CA. Defective epithelial barrier in chronic rhinosinusitis: The regulation of tight junctions by IFN-gamma and IL-4. J Allergy Clin Immunol. 2012. [ PubMed ]
- Rantanen T. The cause of the Chinese sofa/chair dermatitis epidemic is likely to be contact allergy to dimethylfumarate, a novel potent contact sensitizer. Br J Dermatol. 2008; 159 (1):218–221. doi: 10.1111/j.1365-2133.2008.08622.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Spiewak R. Food-provoked eczema: a hypothesis on the possible role of systemic contact allergy to haptens present in both cosmetics and foods. Estetol Med Kosmetol. 2011; 1 (1):35–40. [ Google Scholar ]
- Faurschou AMT, Johansen JD, Thyssen JP. Metal allergen of the 21st Century - a review on exposure, epidemiology and clinical manifestations of palladium allergy95. Contact Dermatitis. 2011; 644 :185–195. [ PubMed ] [ Google Scholar ]
- Hellgren L. The prevalence of urticaria in the total population. Acta Allergol. 1972; 27 (3):236–240. [ PubMed ] [ Google Scholar ]
- Saini SRK, Hsieh HJ, Wong DA, Conner E, Kaplan A, Spector S, Maurer M. A randomized, placebo-controlled, dose-ranging study of single-dose omalizumab in patients with H1-antihistamine-refractory chronic idiopathic urticaria. J Allergy Clin Immunol. 2011; 128 (3):567–573. doi: 10.1016/j.jaci.2011.06.010. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Peden D, Reed CE. Environmental and occupational allergies. J Allergy Clin Immunol. 2010; 125 (2 Suppl 2):S150–160. [ PubMed ] [ Google Scholar ]
- Cecchi L, D’Amato G, Ayres JG, Galan C, Forastiere F, Forsberg B, Gerritsen J, Nunes C, Behrendt H, Akdis C. et al. Projections of the effects of climate change on allergic asthma: the contribution of aerobiology. Allergy. 2010; 65 (9):1073–1081. [ PubMed ] [ Google Scholar ]
- Buters JT, Kasche A, Weichenmeier I, Schober W, Klaus S, Traidl-Hoffmann C, Menzel A, Huss-Marp J, Kramer U, Behrendt H. Year-to-year variation in release of Bet v 1 allergen from birch pollen: evidence for geographical differences between West and South Germany. Int Arch Allergy Immunol. 2008; 145 (2):122–130. doi: 10.1159/000108137. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Laatikainen T, von Hertzen L, Koskinen JP, Makela MJ, Jousilahti P, Kosunen TU, Vlasoff T, Ahlstrom M, Vartiainen E, Haahtela T. Allergy gap between Finnish and Russian Karelia on increase. Allergy. 2011; 66 (7):886–892. doi: 10.1111/j.1398-9995.2010.02533.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Flohr C, Quinnell RJ, Britton J. Do helminth parasites protect against atopy and allergic disease? Clin Exp Allergy. 2009; 39 (1):20–32. doi: 10.1111/j.1365-2222.2008.03134.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Erb KJ. Can helminths or helminth-derived products be used in humans to prevent or treat allergic diseases? Trends Immunol. 2009; 30 (2):75–82. doi: 10.1016/j.it.2008.11.005. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bisgaard H, Li N, Bonnelykke K, Chawes BL, Skov T, Paludan-Muller G, Stokholm J, Smith B, Krogfelt KA. Reduced diversity of the intestinal microbiota during infancy is associated with increased risk of allergic disease at school age. J Allergy Clin Immunol. 2011; 128 (3):646–652. doi: 10.1016/j.jaci.2011.04.060. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Papadopoulos NG, Christodoulou I, Rohde G, Agache I, Almqvist C, Bruno A, Bonini S, Bont L, Bossios A, Bousquet J. et al. Viruses and bacteria in acute asthma exacerbations–a GA(2) LEN-DARE systematic review. Allergy. 2011; 66 (4):458–468. doi: 10.1111/j.1398-9995.2010.02505.x. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Skevaki CL, Psarras S, Volonaki E, Pratsinis H, Spyridaki IS, Gaga M, Georgiou V, Vittorakis S, Telcian AG, Maggina P. et al. Rhinovirus-induced basic fibroblast growth factor release mediates airway remodeling features. Clinical and translational allergy. 2012; 2 (1):14. doi: 10.1186/2045-7022-2-14. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jackson DJ, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Pappas TE, Printz MC, Lee WM, Shult PA, Reisdorf E. et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008; 178 (7):667–672. doi: 10.1164/rccm.200802-309OC. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Papadopoulos NG, Xepapadaki P, Mallia P, Brusselle G, Watelet JB, Xatzipsalti M, Foteinos G, Van Drunen CM, Fokkens WJ, D’Ambrosio C. et al. Mechanisms of virus-induced asthma exacerbations: State-of-the-art. A GA2LEN and InterAirways document. Allergy: European J Allergy Clin Immunol . 2007; 62 (5):457–470. doi: 10.1111/j.1398-9995.2007.01341.x. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Contoli M, Message SD, Laza-Stanca V, Edwards MR, Wark PA, Bartlett NW, Kebadze T, Mallia P, Stanciu LA, Parker HL. et al. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med. 2006; 12 (9):1023–1026. doi: 10.1038/nm1462. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Miller EK, Edwards KM, Weinberg GA, Iwane MK, Griffin MR, Hall CB, Zhu Y, Szilagyi PG, Morin LL, Heil LH. et al. A novel group of rhinoviruses is associated with asthma hospitalizations. J Allergy Clin Immunol. 2009; 123 (1):98–104. doi: 10.1016/j.jaci.2008.10.007. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sun K, Metzger DW. Inhibition of pulmonary antibacterial defense by interferon-gamma during recovery from influenza infection. Nat Med. 2008; 14 (5):558–564. doi: 10.1038/nm1765. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Oude Elberink J. Venom immunotherapy (VIT): clinical efficacy and improvement in quality of life. Drugs Today. 2008; 44 :43–45. [ PubMed ] [ Google Scholar ]
- Bilo BM, Bonifazi F. Advances in hymenoptera venom immunotherapy. Curr Opin Allergy Clin Immunol. 2007; 7 (6):567–573. doi: 10.1097/ACI.0b013e3282f1eca5. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Niedoszytko M, Bruinenberg M, de Monchy J, Weersma RK, Wijmenga C, Jassem E, Elberink JN. Changes in gene expression caused by insect venom immunotherapy responsible for the long-term protection of insect venom-allergic patients. Ann Allergy Asthma Immunol. 2011; 106 (6):502–510. doi: 10.1016/j.anai.2011.01.007. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Borer-Reinhold M, Haeberli G, Bitzenhofer M, Jandus P, Hausmann O, Fricker M, Helbling A, Muller U. An increase in serum tryptase even below 11.4 ng/mL may indicate a mast cell-mediated hypersensitivity reaction: a prospective study in Hymenoptera venom allergic patients. Clin Exp Allergy. 2011; 41 (12):1777–1783. doi: 10.1111/j.1365-2222.2011.03848.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rueff F, Przybilla B, Bilo MB, Muller U, Scheipl F, Aberer W, Birnbaum J, Bodzenta-Lukaszyk A, Bonifazi F, Bucher C. et al. Predictors of side effects during the buildup phase of venom immunotherapy for Hymenoptera venom allergy: the importance of baseline serum tryptase. J Allergy Clin Immunol. 2010; 126 (1):105–111. doi: 10.1016/j.jaci.2010.04.025. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bilo MB, Cinti B, Brianzoni MF, Braschi MC, Bonifazi M, Antonicelli L. Honeybee venom immunotherapy: a comparative study using purified and nonpurified aqueous extracts in patients with normal Basal serum tryptase concentrations. J Allergy. 2012; 869243 :12. Epub 2012 Jan 12. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Scurlock AM, Vickery BP, Hourihane JO, Burks AW. Pediatric food allergy and mucosal tolerance. Mucosal Immunol. 2010; 3 (4):345–354. doi: 10.1038/mi.2010.21. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fernandez-Rivas M, Bolhaar S, Gonzalez-Mancebo E, Asero R, van Leeuwen A, Bohle B, Ma Y, Ebner C, Rigby N, Sancho AI. et al. Apple allergy across Europe: how allergen sensitization profiles determine the clinical expression of allergies to plant foods. J Allergy Clin Immunol. 2006; 118 (2):481–488. doi: 10.1016/j.jaci.2006.05.012. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zuidmeer-Jongejan L, Fernandez-Rivas M, Poulsen LK, Neubauer A, Asturias J, Blom L, Boye J, Bindslev-Jensen C, Clausen M, Ferrara R. et al. FAST: Towards safe and effective subcutaneous immunotherapy of persistent life-threatening food allergies. Clinical and translational allergy. 2012; 2 (1):5. doi: 10.1186/2045-7022-2-5. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mills EN, Mackie AR, Burney P, Beyer K, Frewer L, Madsen C, Botjes E, Crevel RW, van Ree R. The prevalence, cost and basis of food allergy across Europe. Allergy. 2007; 62 (7):717–722. doi: 10.1111/j.1398-9995.2007.01425.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Vassallo MF, Camargo CA Jr. Potential mechanisms for the hypothesized link between sunshine, vitamin D, and food allergy in children. J Allergy Clin Immunol. 2010; 126 (2):217–222. doi: 10.1016/j.jaci.2010.06.011. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Noimark L, Cox HE. Nutritional problems related to food allergy in childhood. Pediatr Allergy Immunol. 2008; 19 (2):188–195. doi: 10.1111/j.1399-3038.2007.00700.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Excellence NIfHaC. NICE Clinical Guideline 116. Food Allergy in Children and Young People. Diagnosis and assessment of food allergy in children and young people in primary care and community settings. 2011. http://guidance.nice.org.uk/CG116 . [ PMC free article ] [ PubMed ]
- Gomes ECM, Praca F, Gomes L, Marino E, Demoly P. Self-reported drug allergy in a general adult Portuguese population. Clin Exp Allergy. 2004; 34 :1597–1601. doi: 10.1111/j.1365-2222.2004.02070.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Demoly PPW, Pirmohamed M, Romano A. Important questions in Allergy: 1–drug allergy/hypersensitivity. Allergy Asthma Clin Immunol. 2008; 63 (5):616–619. [ PubMed ] [ Google Scholar ]
- Brockow KRA, Blanca M, Ring J, Pichler W, Demoly P. General considerations for skin test procedures in the diagnosis of drug hyper-sensitivity. Allergy. 2002; 57 :45–51. [ PubMed ] [ Google Scholar ]
- Adkinson NF ED Jr, Gruchalla R, Haggerty H, Kawabata T, Sandler JD, Updyke L, Shear NH, Wierda D. Health and environmental sciences institute task force: Task force report: future research needs for the prevention and management of immune-mediated drug hypersensitivity reactions. J Allergy Clin Immunol. 2002; 1098 (3):S461–467. [ PubMed ] [ Google Scholar ]
- Gleeson M. Immune function in sport and exercise. J Appl Physiol. 2007; 103 (2):693–699. doi: 10.1152/japplphysiol.00008.2007. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lakier Smith L. Overtraining, excessive exercise, and altered immunity: is this a T helper-1 versus T helper-2 lymphocyte response? Sports Med. 2003; 33 (5):347–364. doi: 10.2165/00007256-200333050-00002. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fitch KD. An overview of asthma and airway hyper-responsiveness in Olympic athletes. Br J Sports Med. 2012; 46 (6):413–416. doi: 10.1136/bjsports-2011-090814. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bonini M, Lapucci G, Petrelli G, Todaro A, Pamich T, Rasi G, Bonini S. Predictive value of allergy and pulmonary function tests for the diagnosis of asthma in elite athletes. Allergy. 2007; 62 (10):1166–1170. doi: 10.1111/j.1398-9995.2007.01503.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Schwartz LB, Delgado L, Craig T, Bonini S, Carlsen KH, Casale TB, Del Giacco S, Drobnic F, van Wijk RG, Ferrer M. et al. Exercise-induced hypersensitivity syndromes in recreational and competitive athletes: a PRACTALL consensus report (what the general practitioner should know about sports and allergy) Allergy. 2008; 63 (8):953–961. doi: 10.1111/j.1398-9995.2008.01802.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jaakkola MS, Jaakkola JJ. Assessment of public health impact of work-related asthma. BMC Med Res Methodol. 2012; 12 :22. doi: 10.1186/1471-2288-12-22. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Henneberger PK, Mirabelli MC, Kogevinas M, Anto JM, Plana E, Dahlman-Hoglund A, Jarvis DL, Kromhout H, Lillienberg L, Norback D. et al. The occupational contribution to severe exacerbation of asthma. Eur Respir J. 2010; 36 (4):743–750. doi: 10.1183/09031936.00135109. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kogevinas M, Zock JP, Jarvis D, Kromhout H, Lillienberg L, Plana E, Radon K, Toren K, Alliksoo A, Benke G. et al. Exposure to substances in the workplace and new-onset asthma: an international prospective population-based study (ECRHS-II) Lancet. 2007; 370 (9584):336–341. doi: 10.1016/S0140-6736(07)61164-7. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Vandenplas O, Lantin AC, D’Alpaos V, Larbanois A, Hoet P, Vandeweerdt M, Thimpont J, Speybroeck N. Time trends in occupational asthma in Belgium. Respir Med. 2011; 105 (9):1364–1372. doi: 10.1016/j.rmed.2011.05.002. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Paris C, Ngatchou-Wandji J, Luc A, McNamee R, Bensefa-Colas L, Larabi L, Telle-Lamberton M, Herin F, Bergeret A, Bonneterre V. et al. Work-related asthma in France: recent trends for the period 2001–2009. Occup Environ Med. 2012; 69 (6):391–397. doi: 10.1136/oemed-2011-100487. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Moscato G, Pala G, Barnig C, De Blay F, Del Giacco SR, Folletti I, Heffler E, Maestrelli P, Pauli G, Perfetti L. et al. EAACI consensus statement for investigation of work-related asthma in non-specialized centres. Allergy. 2012; 67 (4):491–501. doi: 10.1111/j.1398-9995.2011.02784.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Moscato G, Vandenplas O, Van Wijk RG, Malo JL, Perfetti L, Quirce S, Walusiak J, Castano R, Pala G, Gautrin D. et al. EAACI position paper on occupational rhinitis. Respir Res. 2009; 3 (10):16. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Moscato G, Pala G, Boillat MA, Folletti I, Gerth van Wijk R, Olgiati-Des Gouttes D, Perfetti L, Quirce S, Siracusa A, Walusiak-Skorupa J. et al. EAACI position paper: prevention of work-related respiratory allergies among pre-apprentices or apprentices and young workers. Allergy. 2011; 66 (9):1164–1173. doi: 10.1111/j.1398-9995.2011.02615.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cowl CT. Occupational asthma: review of assessment, treatment, and compensation. Chest. 2011; 139 (3):674–681. doi: 10.1378/chest.10-0079. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tarlo SM, Liss GM. Prevention of occupational asthma. Curr Allergy Asthma Rep. 2010; 10 (4):278–286. doi: 10.1007/s11882-010-0118-y. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Baur X, Sigsgaard T. The new guidelines for management of work-related asthma. Eur Respir J. 2012; 39 (3):518–519. doi: 10.1183/09031936.00096211. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- de Groene GJ, Pal TM, Beach J, Tarlo SM, Spreeuwers D, Frings-Dresen MH, Mattioli S, Verbeek JH. Workplace interventions for treatment of occupational asthma. Cochrane Database Syst Rev. 2011; 11 (5) [ PubMed ] [ Google Scholar ]
- Vandenplas O, Dressel H, Wilken D, Jamart J, Heederik D, Maestrelli P, Sigsgaard T, Henneberger P, Baur X. Management of occupational asthma: cessation or reduction of exposure? A systematic review of available evidence. Eur Respir J. 2011; 38 (4):804–811. doi: 10.1183/09031936.00177510. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lack G. Epidemiologic risks for food allergy. J Allergy Clin Immunol. 2008; 121 (6):1331–1336. doi: 10.1016/j.jaci.2008.04.032. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Martinez FD. New insights into the natural history of asthma: primary prevention on the horizon. J Allergy Clin Immunol. 2011; 128 (5):939–945. doi: 10.1016/j.jaci.2011.09.020. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- DunnGalvin A, Daly D, Cullinane C, Stenke E, Keeton D, Erlewyn-Lajeunesse M, Roberts GC, Lucas J, Hourihane JO. Highly accurate prediction of food challenge outcome using routinely available clinical data. J Allergy Clin Immunol. 2011; 127 (3):633–639. doi: 10.1016/j.jaci.2010.12.004. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Papadopoulos NG, Arakawa H, Carlsen KH, Custovic A, Gern J, Lemanske R, Le Souef P, Makela M, Roberts G, Wong G. et al. International consensus on (ICON) pediatric asthma. Allergy. 2012; 67 (8):976–997. doi: 10.1111/j.1398-9995.2012.02865.x. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bozzetto S, Carraro S, Giordano G, Boner A, Baraldi E. Asthma, allergy and respiratory infections: the vitamin D hypothesis. Allergy. 2012; 67 (1):10–17. doi: 10.1111/j.1398-9995.2011.02711.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Roberts G, Patel N, Levi-Schaffer F, Habibi P, Lack G. Food allergy as a risk factor for life-threatening asthma in childhood: a case-controlled study. J Allergy Clin Immunol. 2003; 112 (1):168–174. doi: 10.1067/mai.2003.1569. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Auffray C, Adcock IM, Chung KF, Djukanovic R, Pison C, Sterk PJ. An integrative systems biology approach to understanding pulmonary diseases. Chest. 2010; 137 (6):1410–1416. doi: 10.1378/chest.09-1850. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Niggemann B, Jacobsen L, Dreborg S, Ferdousi HA, Halken S, Host A, Koivikko A, Koller D, Norberg LA, Urbanek R. et al. Five-year follow-up on the PAT study: specific immunotherapy and long-term prevention of asthma in children. Allergy. 2006; 61 (7):855–859. doi: 10.1111/j.1398-9995.2006.01068.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Edgecombe K, Latter S, Peters S, Roberts G. Health experiences of adolescents with uncontrolled severe asthma. Arch Dis Child. 2010; 95 (12):985–991. doi: 10.1136/adc.2009.171579. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Monks H, Gowland MH, MacKenzie H, Erlewyn-Lajeunesse M, King R, Lucas JS, Roberts G. How do teenagers manage their food allergies? Clin Exp Allergy. 2010; 40 (10):1533–1540. doi: 10.1111/j.1365-2222.2010.03586.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- EAACI. A European Declaration of Immunotherapy. Designing the future of allergen specific immunotherapy. Clinical and translational allergy. 2012; 2 :20. doi: 10.1186/2045-7022-2-20. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bilo BM, Bonifazi F. Hymenoptera venom immunotherapy. Immunotherapy. 2011; 3 (2):229–246. doi: 10.2217/imt.10.88. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Warrington R. Immunotherapy in asthma. Immunotherapy. 2010; 2 (5):711–725. doi: 10.2217/imt.10.47. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Calderon M, Cardona V, Demoly P. One hundred years of allergen immunotherapy European Academy of Allergy and Clinical Immunology celebration: review of unanswered questions. Allergy. 2012; 67 (4):462–476. doi: 10.1111/j.1398-9995.2012.02785.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jutel M, Akdis CA. Immunological mechanisms of allergen-specific immunotherapy. Allergy. 2011; 66 (6):725–732. doi: 10.1111/j.1398-9995.2011.02589.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fujita H, Soyka MB, Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy. Clin Transl Allergy. 2012; 2 (1):2. doi: 10.1186/2045-7022-2-2. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Palomares O, Rückert B, Jartti T, Kücüksezer UC, Puhakka T, Gomez E, Fahrner HB, Speiser A, Jung A, Kwok WW. et al. Induction and maintenance of allergen-specific FOXP3+ Treg cells in human tonsils as potential first-line organs of oral tolerance. J Allergy Clin Immunol. 2012; 129 :510–520. doi: 10.1016/j.jaci.2011.09.031. [ PubMed ] [ CrossRef ] [ Google Scholar ]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Open access
- Published: 24 March 2023
Pathogenesis of allergic diseases and implications for therapeutic interventions
- Ji Wang 1 na1 ,
- Yumei Zhou 1 na1 ,
- Honglei Zhang 1 ,
- Linhan Hu 1 ,
- Juntong Liu 1 ,
- Lei Wang 2 ,
- Tianyi Wang 1 ,
- Haiyun Zhang 1 ,
- Linpeng Cong 1 &
- Qi Wang 1
Signal Transduction and Targeted Therapy volume 8 , Article number: 138 ( 2023 ) Cite this article
17k Accesses
28 Citations
11 Altmetric
Metrics details
- Immunological disorders
Allergic diseases such as allergic rhinitis (AR), allergic asthma (AAS), atopic dermatitis (AD), food allergy (FA), and eczema are systemic diseases caused by an impaired immune system. Accompanied by high recurrence rates, the steadily rising incidence rates of these diseases are attracting increasing attention. The pathogenesis of allergic diseases is complex and involves many factors, including maternal-fetal environment, living environment, genetics, epigenetics, and the body’s immune status. The pathogenesis of allergic diseases exhibits a marked heterogeneity, with phenotype and endotype defining visible features and associated molecular mechanisms, respectively. With the rapid development of immunology, molecular biology, and biotechnology, many new biological drugs have been designed for the treatment of allergic diseases, including anti-immunoglobulin E (IgE), anti-interleukin (IL)-5, and anti-thymic stromal lymphopoietin (TSLP)/IL-4, to control symptoms. For doctors and scientists, it is becoming more and more important to understand the influencing factors, pathogenesis, and treatment progress of allergic diseases. This review aimed to assess the epidemiology, pathogenesis, and therapeutic interventions of allergic diseases, including AR, AAS, AD, and FA. We hope to help doctors and scientists understand allergic diseases systematically.
Similar content being viewed by others
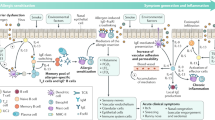
Allergic rhinitis
Allergic asthma is a risk factor for human cardiovascular diseases
The translational revolution in atopic dermatitis: the paradigm shift from pathogenesis to treatment
Introduction.
Allergic diseases are systemic disorders caused by an impaired immune system. Different allergic diseases, including AR, AAS, AD, FA and eczema, are caused by complex interactions between genetic and environmental factors. Allergic diseases are listed by the World Health Organization (WHO) as one of the top three disorders to be prevented and controlled in the 21st century. An allergic disease, whilst a systemic disease, can also manifest as different local maladies, all of which may lead to anaphylactic shock in severe cases. The incidence of allergic diseases is high, bringing much suffering to patients. It is estimated that nearly 500 million and 300 million individuals worldwide have AR and AAS, respectively, 1 with an increasing number of cases. For AAS, mortality rates in women and men are 90 and 170 per million individuals, respectively. About 96% of asthma deaths occur in low-and-middle-income countries. 2 It is currently estimated that FA affects 1–10% of the total population. 3 The global prevalence rate of AD is 8%, 4 , 5 with a lifetime prevalence reaching 20%. 6 In 2019, there were 171.17 million patients worldwide with AD. 7
Due to the different sites of allergic diseases, the clinical and pathological manifestations also differ. In AR, after stimulation by allergens, including airborne dust mites associated with fecal particles, cockroach remains, pet dander, molds and pollens, inflammatory cells such as mast cells, CD4 + T cells, B cells, macrophages and eosinophils infiltrate the lining of the nasal cavity, with infiltration into the nasal mucosa. T helper 2 (Th2) cells promote the release of immunoglobulin and cytokines, including interleukin (IL)-3, IL-4, IL-5, and IL-13; meanwhile, IgE is also produced by plasma cells. There is, however, still some uncertainty around the source of IgE production. Follicular helper T (Tfh) cells are a subpopulation of CD4 + T-effector cells, and in recent years it has been discovered that the key cells regulating IgE production are not Th2 cells, but Tfh cells. Allergens cross-link IgE that interact with mast cells, which further induces the release of multiple mediators (including histamine and leukotrienes), promotes arteriole dilation and vascular permeability, and causes pruritus, runny nose, mucus secretion, and pulmonary smooth muscle contraction. 8 Over the next 4–8 hours, the released mediators and cytokines induce subsequent cellular inflammatory reactions (late inflammatory response), leading to the recurrence of symptoms, often nasal congestion, which generally persist. 8 , 9 The immunopathological profiles of AR and AAS are very similar in terms of eosinophil, mast cell and Th2 cell infiltration. Although structural changes in airway remodeling are well characterized in AAS, they may also occur in AR. There are also pathophysiological differences between AR and AAS. In the AAS disease, mucosal pathological alterations comprise epithelial hyperplasia, goblet cell metaplasia and increased mucus generation. In the submucosal layer, smooth muscle hypertrophy, collagen accumulation and large mucus glands prevail, leading to airway narrowing and enhanced mucus generation during an asthma attack, 10 with symptoms such as difficulty breathing, wheezing, chest pain, and coughing. 11 The pathogenesis of AD is mainly reflected by a complex interplay between epidermal barrier dysfunction, abnormal skin microbiota and dysregulated type 2 T cell immunity. 12 , 13 The above pathogenesis induces a series of pathological manifestations such as filamentous aggregation; weak skin barrier due to protein shortage promoting inflammatory reactions and T cell infiltration; S. aureus colonization or infection disrupting the skin barrier and inducing an inflammatory response as well as the development of epidermal edema (“spongy sclerosis”); local Th2 immune reactions further reducing the barrier function, promoting dysregulation that favors Staphylococcus species, especially S. aureus , which triggers pruritus. FA is an IgE-dependent type I hypersensitivity to a specific food allergen. Its pathological process is divided into two stages: in the allergic sensitization stage, the initial exposure to the allergen results in tolerance breakdown, with subsequent generation of specific IgE, vasoactive substances and allergic response mediators such as histamine and platelet activating factor. 14 During the provocation phase, degranulation of effector cells, such as mast cells, induces allergic inflammation, and serotonin or 5-hydroxytryptamine is released in large amounts, resulting in acute gastrointestinal symptoms, including diarrhea. Besides mast cells, an allergen also reacts with sensitized basophils in the circulation, triggering a life-threatening systemic reactions featuring multiple-organ and system involvements, hypotension and shock. 15 After repeated exposure to food allergens, persistent allergic inflammatory reactions occur and tissue mast cells increase, resulting in persistent gastrointestinal reactions. 16
The pathogenesis of allergic diseases is complex, involving many factors such as genetics, epigenetics, environmental factors, microecology and the body’s immune function. Their recurrence rate is high, which brings great pain to and imposes a severe financial burden on patients. Therefore, this manuscript comprehensively analyzes allergic diseases, from a brief introduction of their history to their mechanism and treatment, hoping to provide not only a systematic understanding of such diseases, but also a reference for clinical doctors and scientists.
A brief history of allergic diseases
Since ancient times, people have always been drawn to allergic diseases, studying their pathogenesis and developing related treatments. The earliest recorded allergic reaction in human history was the death of the Egyptian pharaoh Menes after being bitten by a bumblebee around 2641 BCE. 17 Theories on the actual causes and diagnosis of allergic diseases were further developed in the 19 th century, precisely in 1819, when the British physician John Bostock, at the Royal Society of Reported Medicine, attributed summer eye and nose discomfort to hay, naming the condition “hay fever”. 18 Later in 1868, Eosinophilia was first observed by Henry Hyde Salter, in the sputum of a patient with an allergic disease. 19
The pathogenesis and treatment of allergic diseases have made rapid progress in the 20 th century. The word “allergy” was coined by Clemens von Pirquet in 1906, 20 , 21 which is considered to be the beginning of modern allergy science. In 1911, Leonard Noon was the first to be successful in the treatment of pollen-associated AR with low-dose flower infusion, setting a precedent for immunotherapy. 22 Edward Calvin Kendall discovered the adrenocortical hormone and determined its structure and physiological effects in 1935, earning the Nobel Prize in Physiology or Medicine in 1950. 23 Daniel Bovet synthesized antihistamines in 1937 and earned the Nobel Prize in Physiology or Medicine in 1957, which brought hope in the treatment of allergic diseases and has been in clinical use to this day. 24 , 25 In 1953, James F. Riley was the first to discover that histamine in the human body mainly comes from mast cell granules. 26 Up to this point in history, basic treatment methods for allergic diseases had been established, but no breakthrough had been made in mechanistic research. In terms of pathogenesis, the Ishzaka couple discovered in 1966 that the reactive hormone in the serum of patients with allergic diseases was IgE, 27 providing a new experimental tool and concept for serological research. In 1989, the epidemiologist Strachan proposed the “hygiene hypothesis” on the basis that “compared with an only child, children in large families have a lower risk of developing pollen allergy and eczema”. 28 The hygiene hypothesis has laid a solid foundation for studying the pathogenesis of allergic diseases from the perspectives of modern immunology, microecology, and antibiotic application. Current guidelines suggest a combined application of allergen avoidance, pharmacotherapy, and/or allergen-specific immunotherapy (AIT) 29 , 30 (Fig. 1 ).
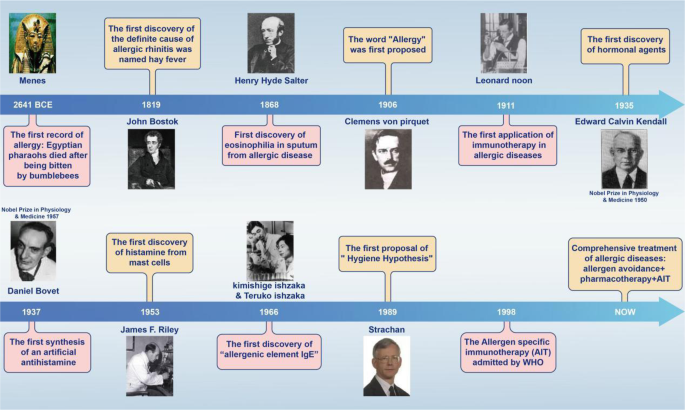
Timeline of major findings related to allergic diseases. Allergic reactions in Western countries were first recorded in 2641 BCE, when the Egyptian Pharaoh Menes died after being bitten by a bumblebee. In 1911, Leonard Noon published an article in The Lancet , reporting a clinical paper treating pollinosis by subcutaneous injection of grass pollen extract, which marked the beginning of modern immunotherapy. In 1966, the Japanese scientist Ishizaka and his wife discovered IgE, which led to a leap in the understanding of immediate allergy. Following their discovery, IgE became a new indicator for the diagnosis of allergy
Genetics and epigenetics
Gene-environment interactions in allergy diseases.
Allergic disease is a complex disorder, whose etiology and development may involve genetic and environmental factors. Although the innate and adaptive immune systems are critical in regulating the adaptation to the external microenvironment, 31 allergic diseases are considered a major cause of immune dysfunction caused by the interactions of multiple genes and the external environment in cells. 32 , 33
The research boom in genetics and epigenetics has substantially promoted research progress for allergic diseases. The application of genome-wide association studies (GWASs), single nucleotide polymorphism (SNP) analysis, and epigenome-wide association studies (EWASs) has laid a solid foundation for exploring the genetics of allergic diseases. Epigenetic studies mainly focus on DNA methylation, post-translational histone modifications, and non-coding RNAs. Epigenetics can explain the occurrence and development of allergic diseases in the external environment from various aspects, elucidate the mechanism of immune response plasticity in allergic disorders, and even provide diagnostic biomarkers and therapeutic targets for allergic disorders 34 , 35 (Fig. 2 ).
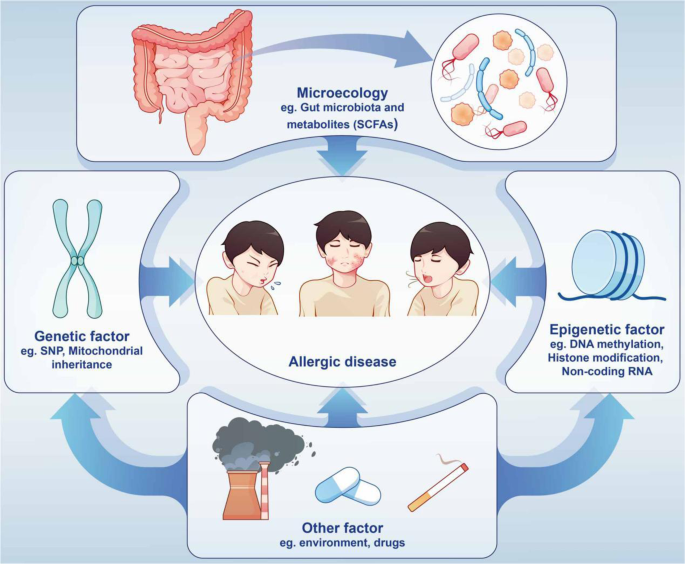
Allergic diseases are caused by a variety of factors. External factors include changes in gut microbiota and metabolites, drugs, and air pollution. Internal influencing factors include genetic and epigenetic changes
SNPs and related GWASs in allergic diseases
When the Human Genome Project was completed in 2001, 36 scientists were surprised to find that most genome sequence variations involve SNPs. SNP diversity can be found throughout different regions of the genome, 37 including introns, exons, promoters, enhancers and intergenic regions, with SNPs considered as the basis of DNA sequence variation. 38
Hereditary studies
Both genetics and the environment are critical to the etiology and development of allergic diseases, and it is difficult to distinguish their independent roles in allergic diseases when they are combined. Twins’ studies can be used to separate genetics from environmental factors, providing clues to the genetic component of allergic diseases. In fact, research into the genetics of allergic disease also began in twins. Twin studies revealed that FA had high heritability, and GWAS and candidate gene studies indicated marked associations of the human leukocyte antigen(HLA)-DR and HLA-DQ region with genetic variants in multiple genes, including Filaggrin (FLG), the HLA locus, and Forkhead Box Protein P3 (FOXP3). 39 Peanut allergy in 82% of identical twins far exceeds the 20% concordance rate observed in dizygotic twins, 40 , 41 further supported by the fact that children whose parents or siblings have peanut allergy are 7 times more likely to develop the disease compared with children without family risk factors. In general, heritability estimates for FA are as high as 81%, 42 while the heritability of AR is estimated at approximately 91%. 42 Twin studies reveal that about 25% of phenotypic variation in asthma severity can be explained by genetic factors; for example, RAD50- IL13 on chromosome 5q and the ORMDL sphingolipid biosynthesis regulator 3 (ORMDL3)- gasdermin B (GSDMB) locus on chromosome 17q21 were found to be associated with asthma severity. 43 Besides, the concordance rate for AD in identical twins is about 80%, which is remarkably elevated compared with the 20% found in fraternal twins. 44
SNP and GWAS analyses in allergic diseases
To date, SNPs in no less than 34 loci and 46 genes are considered to have AD risk in different populations around the world. 45 Loss-of-function mutations in FLG, which encodes filaggrin (a skin barrier protein), are considered the most important genetic risk factor for AD, although variants affecting skin and systemic immune reactions also play critical roles. 46 In an AAS disease study, after analysis by GWAS, Sarnowski et al. recently detected five genetic variants related to age at onset in 5,462 asthma cases, at or around recombinant Cylindromatosis (CYLD) on 16q12 (rs1861760), IL1RL1 on 2q12 (rs10208293), HLA-DQA1 on 6p21 (rs9272346), IL33 on 9p24 (rs928413) and GSDMA on 17q12 (rs9901146), with the last four also showing associations with susceptibility to allergic diseases. Recombinant Mucin 5 Subtype AC (MUC5AC) is considered an essential factor in the natural barrier function of the airway and has a potential association with moderate to severe asthma. 47 Studies have shown that SNP changes of ORMDL3 and the TSLP promoter gene are involved in AAS; 48 , 49 polymorphisms in the alpha chain coding region of IL-4 receptor are also associated with AAS. 50 Susceptibility-related GWAS data showed that asthma-related IL-33 genes are all associated with asthma and AR. 51 , 52 Changes in IL-4 gene single nucleotide polymorphisms can increase the risk of AR; 52 individuals with the Vitamin D (1,25- dihydroxyvitamin D3) receptor (VDR) rs2228570 CC and vitamin D-binding protein (VDBP) rs7041 GG genotypes have a high risk of asthma progression. 53 In FA analysis, peanut allergy is clearly associated with specific locus changes in the HLA-DR and HLA-DQ genes. 54 Molecular genetic analysis of the GG, GA, and AA genotypes of the IL-13 R130Q gene polymorphism revealed markedly elevated incidence rates of the GA and AA genotypes in comparison with healthy control individuals. 55 Besides, the serpin B serpin (SERPINB) and cytokine gene clusters increase the risk of any FA, as well as the C11orf30/LRRC32 locus. 56
In the study of AR, the largest GWAS revealed 20 novel loci associated with AR risk, 57 including IL7R at 5p13.2 and SH2B adaptor protein 3 (SH2B3) on chromosome 12q24.12, which separately participate in V(D)J recombination of T cell and B cell receptors, 58 blood eosinophil count 59 and T cell activation pathways. 60 Furthermore, we noted that AR risk loci have important effects on innate and adaptive immune responses. Loci near C-X-C chemokine receptor type 5 (CXCR5) on 11q23 and Fc Fragment of IgE Receptor Ig (FCER1G) on 1q23.3 separately encode chemokine receptor in B cells and follicular T cells 61 and the γ chain of IgE receptor. 62 Broad-complex,tramtrack and bric-a-brac and cap’n’collar homology 2 (BACH2) on 6q15 is critical to the induction immunomodulatory function of memory B and T cells, 63 , 64 Leukocyte tyrosine kinase (LTK) and TYRO3 protein tyrosine kinase (TYRO3) modulate Th2-type immune responses; RAR-related orphan receptor A (RORA) regulates the development and inflammatory response of Th2 innate lymphocytes, and tumor necrosis factor (ligand) superfamily, member 11 (TNFSF11) is involved in dendritic cell activation of T cells. The Viral protein R binding protein (VPRBP) gene controls T cell proliferation and contributes to V(D)J recombination in B cells. 65 , 66 , 67 , 68 Surprisingly, the frequencies of the tumor necrosis factor-α (TNF-α) and MRPL4 genes are starkly elevated in AR cases in Han individuals 69 (Table 1 ).
Mitochondrial inheritance in allergic disease
Maternal inheritance is considered the most critical player in allergic disease occurrence. Most offspring mitochondria are inherited from the mother, and mitochondrial inheritance is tightly associated with asthma occurrence. 70 , 71 , 72 , 73 Mitochondrial DNA (mtDNA) variants are significantly associated with allergic disorders, including AD and asthma. A report demonstrated that in 69 mtDNA variants, the rs28357671 locus of the MT-ND6 gene was significantly associated with mitochondrial function genes in allergic diseases, including: NLR Family Member X1 (NLRX1), oculocutaneous albinism II (OCA2) and coiled-coil-helix-coiled-coil-helix domain containing 3 (CHCHD3). 74 Interestingly, the genetic cause of asthma in females may be associated with a dysfunction of mitochondrial MT-ND2 and MT-RNR2 genes, while in males, mutations in the mitochondrial cytochrome-b (CYB) gene leads to changes in reactive oxygen species (ROS) and asthma development. 75
Mutations affecting mitochondrial tRNA genome sequences have been observed in the placenta of asthmatic mothers and are associated with AAS; 76 for example, a rare mutation in the A3243G-tRNA Leu (UUR) MELAS gene, which is thought to be associated with asthma, was found in asthmatic patients. Maternally inherited mitochondrial diseases have been reported. 77 Fukuda’s team found that 9 of the 13 differentially expressed genes in allergic patients were mitochondria-related genes, including those producing cytochrome oxidases II and III, and NADH dehydrogenase. 76 , G of the cytochrome b gene is a novel genetic marker of predisposition to bronchial asthma. Ter. Arkh 81, 5–31 (2009)." href="/articles/s41392-023-01344-4#ref-CR78" id="ref-link-section-d110983919e2199">78 , 79 In addition, polymorphisms in the ADAM metallopeptidase domain 33 (ADAM33) and cytochrome b genes located on chromosome 20 have been associated with asthma susceptibility, both of which are closely associated with mitochondrial oxidative function. 80 Mitochondrial haploidy and elevated serum IgE amounts are associated in Europeans, 73 which may involve diverse mutations in genes that encode mitochondrial tRNAs. 76 The ATP synthase mitochondrial F1 complex assembly factor 1 gene was implicated in asthma in Caucasian children. 81 Some AAS is closely related to mtDNA deficiency, and alterations in more than 25 genes (ORMDL3, 2PBP2, GSDMB, PDE4D, VEGF, Wnt, MMP-12, PRKCA, JAG1, ANKRD5, TGF-β1, IL-12β, IL-10, IL-13, IL-17, IL-25, and β2-adrenergic receptors) are associated with abnormal changes in the immune system in AAS. 80 , 82 , 83 , 84
Epigenetics and Epigenome-wide Association Study (EWAS)-related analysis of allergic diseases
Epigenetics are heritable features that affect gene expression without altering the DNA sequence. 85 DNA methylation is an effective factor to distinguish allergic patients from healthy people. 44 DNA methylation is reflected by a methyl group added to cytosine at position 5 by DNA methyltransferases to form 5-methylcytosine, 86 where a cytosine nucleotide is called a CpG, followed by a guanine nucleotide. 87 CpG islands typically contain more than 200 bases, of which more than 60%-80% are guanines and cytosines (G + C). 88 Methylation of CpG islands at transcription start sites (TSSs) of genes leads to gene activation or repression, and is generally thought to repress gene transcription. 89
A cross-sectional study found that DNA methylation of allergy-related genes in the whole blood of allergic children may be a common parameter affecting asthma, rhinitis, and eczema; a total of 21 differential CpG loci were screened, 10 of which were in the pulmonary epithelium. The sites of replication, related to acyl-CoA thioesterase 7 (ACOT7), Lectin, Mannose Binding 2 (LMAN2) and Claudin 23 (CLDN23) genes, were all derived from eosinophils. 90 Thus, changes in eosinophil levels are reflected by changes in methylation, unveiling a possible mechanism for phenotypic alterations in immune response-associated features.
In addition, methylation plays an important role in AD pathogenesis. The TSLP gene promoter is hypomethylated in the damaged skin of AD patients. 91 Methylation is not specific to DNA, but is closely related to disease. Demethylation of histone H3 residues in the FOXP3 gene promoter region and hypermethylation of histone H3 residues in the RORC gene promote the differentiation of Th0 cells towards a regulatory T (Treg) phenotype. Conversely, these events cause Treg deficiency, one of the hallmarks of AD pathogenesis. 34 , 92 , 93 , 94 , 95 Furthermore, CpG hypermethylation in IL-4 is negatively correlated with serum total IgE levels, explaining the role of Th2 immunity in AD. 96 Enhanced hypermethylation of S100 calcium binding protein A5 (S100A5) was found in the epidermal part of lesions in AD cases in comparison with healthy individuals. 97 Hypomethylation was observed in Recombinant Keratin 6 A (KRT6A) in keratinocytes, and methylation of cg07548383 in FLG also elevates AD risk. 98
DNA methylation is also critical for AAS pathogenesis, occurrence, and development. An EWAS detected a total of 40,892 CpG sites methylated in important genes C-C motif chemokine ligand 26 (CCL26, a chemokine) and mucin 5 AC (MUC5AC, a mucin with airway defense function) among AAS patients compared with control cases. 99 Chromosome 17q12-q21 hypermethylation contributes to asthma pathogenesis, with regulatory effects on all five protein-coding genes of this region, including IKAROS family zinc finger 3 (Aiolos) (IKZF3), zona pellucida-binding protein 2 (ZPBP2), ORMDL3, gasdermin A (GSDMA) and GSDMB. 100 The STAT5A gene is hypermethylated in the 17q21.2 region and has been linked to increased Th1 responses and reduced infiltration of eosinophils in the airway epithelium. 101 In childhood asthma, cg23602092 gene methylation status was linked to asthma symptoms, 102 and hypomethylation of arachidonate 15-lipoxygenase (ALOX15) gene 17p13.2 and periostin, osteoblast specific factor (POSTN) gene 13q13.3 in nasal epithelial cells is associated with increased Th2 function. 103 Methylation sites in multiple white blood cell (WBC) genes show significant associations with total IgE amounts, with the two most significant genes (ACOT7 and ZFPM1) associated with asthma. 104 In adults, WNT2 gene hypermethylation in the 7q31.2 region in blood specimens is involved in neutrophilic asthma, 105 and ORMDL3 hypermethylation in endobronchial airway epithelial cells contributes to asthma. 106 Following hypermethylation at CpG sites, FOXP3 (12q15) and interferon-γ (IFN-γ) (Xp11.23) lead to altered T cell function and repressed Treg and T effector cell-related genes in blood. 107 In adolescents, interleukin-5 receptor alpha (IL-5RA) (3p26.2) hypomethylation in blood was linked to asthma. 108
In the AR disease, DNA methylation levels are tightly associated with CD4 + T cell amounts. DNA hypermethylation may downregulate IFN-γ in AR cases, 109 while DNA hypomethylation increases the mRNA amounts of IL-13 and IgE. 110 Alterations in hypermethylation at CpG sites in the melatonin receptor 1 A gene may be caused by paternal genetic variations in AR. 111
DNA methylation might also contribute to FA pathogenesis. Reports have shown differences in DNA methylation in some mitogen-activated protein kinase (MAPK) signaling genes, e.g., human leukocyte antigen (HLA)-DQB1 and the Treg-specific demethylation region (TSDR) of FOXP3. Differential genetic DNA methylation might also contribute to FA diagnosis. 39 In a pilot study of cow’s milk protein (CMA, milk allergy), hypermethylation was found in the DEXH (Asp-Glu-X-His) box polypeptide 58 (Dhx58), zinc finger protein 81 (ZNF281) and HtrA serine peptidase 2 (HTRA2) regions. 112 Maternal peanut allergy also induces epigenetic changes in the IL-4 promoter in the offspring, which is associated with Th2 immune response (production of IL-4 and IgE). 113 In a study of identical monozygotic (MZ) twins, the distance between peanut allergy and nonallergy in methylation profiles containing 12 DNAm signatures was reduced compared with randomly paired individuals without genetic relationships, indicating peanut allergy-associated DNAm signatures might be linked to genetic factors. 114
Histone modifications
The DNA is packaged into an organized chromatin structure formed by a core histone protein consisting of H2A, H2B, H3 and H4. 115 Post-translational histone modifications mainly comprise acetylation, methylation, phosphorylation, ubiquitination, SUMOylation, and adenosine diphosphate (ADP) ribosylation of core histone tails, which reflect the epigenetic inheritance of many diseases, including AAS. 115 Histone acetyltransferase (HAT)-mediated histone acetylation often loosens chromatin structure, facilitating access to transcription factors that induce gene expression. Conversely, histone deacetylation by histone deacetylases (HDACs) also leads to gene silencing. Higher levels of histone acetylation are generally associated with increased gene transcriptional activity and expression. Whether histone methylation is transcriptionally permissive or repressive depends largely on the number of methyl groups added and the position of the target amino acid residue in the histone tail. 34 , 116 , 117 , 118
In adult asthmatic patients, lysine 18 acetylation of histone 3 (H3K18) and lysine 9 trimethylation of histone 3 (H3K9me3) are elevated in epithelial cells, and acetylation of H3K18 increases ΔNp63 (a p63 splice variant), epidermal growth factor receptor (EGFR) and signal transducer and activator of transcription 6 (STAT6) mRNA amounts. 119 An imbalance of HAT and HDAC underlies impaired gene expression and is a determinant of asthma. 120 HATs and HDACs have opposite functions, as the acetylation function of HATs promotes gene expression, while the deacetylation function of HDACs is responsible for gene silencing. In children with asthma, H3 acetylation of the FOXP3 gene contributes to Treg differentiation, and H3 histone acetylation critically affects the IL-13 gene promoter. 121 Stefanowicz and collaborators assessed gene-specific alveolar epithelial histone acetylation and methylation statuses in asthma and healthy control cases, and found increased levels of H3K18ac and H3K9me3 in asthmatic patients. 119 Acetylation of C-C Motif Chemokine 8 (CCL8), a neutrophil activator found in macrophages, as well as H3K18, results in elevated secreted amounts of this activator in airway smooth muscle. 122 In asthma, the levels of CCR4 and CCL5 are high. CCR4 controls Th2 cell infiltration, while CCL5 is a leukocyte chemokine, and a single nucleotide polymorphism of CCR4 and CCL5 dimethylation (H3K4me2) is associated with Th2 differentiation. 123 Resistance to steroid therapy in AAS has emerged, mainly due to IL-17A-induced steroid resistance resulting from decreased HDAC2 activity. 123 Increased enrichment of transcriptionally active H3ac and H4ac histone markers found in AAS cases are associated with IL-13 upregulation in CD4 + T cells. 121
In AR, increased HDAC activity may be involved in the pathogenetic mechanism by elevating pro-inflammatory cytokine amounts and reducing anti-inflammatory cytokine levels. Early responses are characterized by increased IL-4 expression, 124 H3K9 acetylation, and H3K4 trimethylation at the IL4 locus. 125 A study showed HDAC1 upregulation in nasal epithelial cells from AR patients, 126 and IL-4 increased HDAC1 expression, leading to nasal epithelial barrier dysfunction. HDAC1 inhibition promotes the master regulators of T cell function, including IL-10 and CCL8, and prevents excessive activation of immune cells. 127 Histone acetylation is also critical for AD pathogenesis. In AD pathogenesis, the demethylation, acetylation, and methylation of the H3 residue in the FOXP3 promoter gene region, along with the hypermethylation of the RORC gene and the methylation of the H3 residue, promote the regulation of Th0 cells. The differentiation of Tregs, 34 , 92 , 94 , 95 thereby reduce the levels of histone acetylation at Th1 and regulatory sites. 128
Non-coding RNAs in allergic diseases
Long noncoding RNAs (lncRNAs) are defined as transcripts longer than 200 nucleotides in length; functional RNAs that are not translated include micro-RNAs (miRNAs), small interfering RNAs (siRNAs), lncRNAs and Pivi-interacting RNAs (piRNAs). They are essential signaling and regulatory tools that affect transcriptional processes and may also alter gene expression post-transcriptionally, with critical roles in the development of allergic diseases. We take microRNAs as an example to explain their important roles in allergic diseases.
miRNA-21, high expression of miRNA155, and low levels of Let-7a were detected in peripheral blood specimens from asthmatic children. These markers can be used for the diagnosis and prognosis of childhood asthma; 129 up-regulation of miR-126 in peripheral circulation is related to immune imbalance and is considered a biomarker for asthma diagnosis. 130
MicroRNAs have critical functions in the development of allergic diseases. MiR-19b reduced airway remodeling and inflammation as well as oxidative stress by downregulating TSLP to inhibit Stat3 signaling in mice with experimental asthma. 131 MMP-16 and ATG7 via miR-192-5p molecules reduce airway inflammation and remodeling. 132 MiR-221 can control the enhanced airway smooth muscle cell proliferation in severe asthma cases. 133 The circular RNA (circHIPK3) contributes to smooth muscle cell proliferation and airway remodeling in asthma patients via miR-326/ stromal interaction molecule 1 (STIM1) signaling. 134 MiR-130a-3p and miR-142-5p mediate lung macrophage polarization and are associated with airway remodeling. 135 MiR-155 and miR-221 are closely associated with the regulation of Th2 responses and airway smooth muscle hyperproliferation in asthmatic patients. 133 , 136 Vascular endothelial growth factor A (VEGF-A) amounts are elevated in sputum and serum samples from asthmatics, and has-miR-15a is associated with VEGF-A downregulation in CD4 + T cells. 137 MiR-21 downregulates IL-3, IL-5 and IL-12, and inhibits IFN-γ and IL-12 production by dendritic cells, and reduces IFN-γ biosynthesis in CD4 + T cells. 138 MiR-21 overexpression was also associated with the differentiation of Th2 cells in vitro. In granulocyte-infiltrating asthma, miR-221-3p in epithelial cells and sputum was inversely associated with airway eosinophilia. 139 Downregulated miR-28-5p and miR-146a/b activate blood CD8 + T cells in severe asthma. 140 MiR-223-3p, miR-142-3p, and miR-629-3p are involved in severe neutrophilic cellular asthma. 141 MiR-126 induces Th2-type eosinophilic asthma, 142 and miR-23-27-24 regulates T cell function and differentiation; meanwhile, miR-24 and miR-27 suppress Th2 cell differentiation, leading to IL-4 cytokines. 143 Recently published reports revealed miR-200a is involved in asthma pathogenesis via phosphatidylinositol 3 kinase (PI3K)/ RAC-alpha serine/threonine-protein kinase (AKT) signaling. 144 , 145 , 146 In childhood asthma, the miR-29c/B7-H3 axis controls the differentiation of Th2/Th17 cells, and the above microRNA studies might point to novel research directions for developing treatments for AAS. 147
In AR, aberrantly expressed circulating lnc-NEAT1 and miR-125a were associated with Th2 cell percentage and symptoms in pediatric AR. 148 In AD, Liew et al. found decreased expression of miR-335 in AD lesions compared with healthy control skin. 149 Nuclear factor kappa-B (NF-κB) (p65) is a critical modulator of inflammatory immune response, and miR-124 is associated with inflammatory response and may constitute a new effector and regulator of NF-κB. 150 , 151 In diseased skin, miR-124 is downregulated in AD patients. 152 In macrophages, miR-155 targets IL-13Rα1. 153 In dendritic cells (DCs), miR-221 knockdown or miR-155 overexpression promotes apoptosis, while miR-155 overexpression in mDCs enhances the production of IL-12p70. 154 The study of microRNA would bring new hope in the treatment and understanding of allergic diseases.
Cell signaling pathways play critical roles in allergic diseases
Allergic diseases are immune disorders caused by an imbalance of the immunity, in which immune cells play an important role. Signaling pathways are important in intercellular signaling. This review provides a systematic review of the signaling pathways involved in allergic diseases from the nucleus to the cell membrane, in the hope of laying a solid foundation for the study of allergic diseases.
Notch signaling pathway
In the 1910s, the Notch gene was detected in Drosophila melanogaster with notched wings. 155 , 156 Notch signaling is highly conserved. In mammals, NOTCH has four paralogs, including NOTCH1-4, with redundancy and distinct roles. 157 Human Notch1-4 genes map to chromosomes 9, 1, 19 and 6, respectively. The NOTCH receptor undergoes three cleavages and is transferred to the nuclear compartment to regulate target genes transcriptionally. Notch signaling is divided into the canonical and non-canonical pathways with complex functions, but the pathway is now well known. 158 It is mainly involved in diverse molecular events across species, including tissue functional damage and repair; abnormal Notch pathway might lead to different pathological processes.
In AAS, eosinophilic asthma is dominated by Th2-type immune responses, and Notch signaling upregulates the key transcription factor Gata3. 159 , 160 NOTCH4 is known to be critical for asthma development (Fig. 3 ). Repeated allergen exposure induces Tregs that produce high amounts of Notch4, which activates downstream Wnt and Hippo pathways, thereby promoting the transformation of iTregs into Th2 and Th17 cells, and exacerbating AAS. 160 , 161
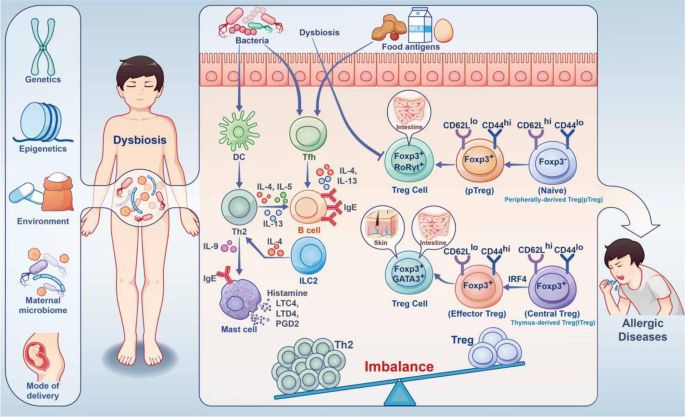
Immune imbalance caused by dysbiosis under the combined effect of gene environment in IgE-related FA. During childhood, the human microbiota is influenced by a combination of the maternal microbiome, mode of delivery, genetics, epigenetics, environment, etc. Dysbiosis resulting from aberrant damage to the gut microbiota early in life impairs Treg differentiation. This results in imbalance of Treg and Th2 cells. Food allergens and the microbiota promote T follicular helper (Tfh) responses to induce B cells, which produce large amounts of IgE through IL-4, IL-13 cytokines, causing allergic reactions
In other allergic diseases, such as AR, FA, and AD, the exact underpinning mechanism remains unclear, Notch signaling also plays important roles. In a study of AR, the serum amounts of Notch1 and Jagged1 (Jag1) in AR patients were significantly increased, which was confirmed in mouse experiments. In this study, Notch signaling could downregulate Foxp3 expression and inhibit Treg differentiation, thereby promoting AR occurrence and development. 162 In a FA study, blocking the Notch signaling pathway could suppress Th2 polarization, increase Th1 cell differentiation and promote Th1/Th2 balance in a mouse model, thereby preliminarily verifying that blocking the Notch signaling pathway inhibits ovalbumin (OVA)-induced FA. 163 In addition, Tfh cell production and function are dependent on Notch signaling, Notch receptors 1 and 2 are required for Tfh cell production, and Notch signaling enhances the production of type 2 cytokines in Tfh cells. 164 Administration of a Notch signaling inhibitor inhibits IgE-mediated proliferation of intestinal mucosal mast cells (MMCs) in mice with food hypersensitivity, thereby attenuating allergic diarrhea and anaphylaxis. 165 In AD, the epidermis of AD patients exhibits a marked deficiency in Notch receptors, leading to the upregulation of alarm TSLP, which triggers Th2-associated responses as well as TSLP and IL-31-related pruritus. 166 , 167 Keratinocyte-produced TSLP and granulocyte-macrophage colony stimulating factor (GM-CSF) induce macrophage and DC activation, drive Th2 polarization, and promote eosinophil and mast cell infiltration, thereby enhancing the immune response. 168 , 169
JAK/STAT signaling pathway
Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling represents a relatively simple membrane-nucleus pathway that mainly upregulates diverse key modulators involved in cancer and inflammatory processes. The well-conserved JAK/STAT pathway consists of ligand-receptor complexes, JAK and STAT. JAK consists of four cytoplasmic tyrosine kinases, i.e., JAK1, JAK2, JAK3 and TYK2. STAT proteins comprise STAT1, STAT2, STAT3, STAT4, STAT5a, STAT5b and STAT6. Each cytokine requires interaction with specific receptors on its target cells to activate this pathway, and these receptors contain associated intracellular domains composed of JAK family members. 170 JAKs are inactive before exposure to cytokines, which induce JAK activation through phosphorylation by binding to their receptors. 171 , 172 , 173 Activated JAKs phosphorylate the receptors on specific tyrosine moieties in the intracellular tail. 174 STATs at receptor sites are also phosphorylated by JAKs. 175 , 176 STATs are phosphorylated and translocated into the nuclear compartment to transcriptionally upregulate specific genes, 177 often leading to cell proliferation or differentiation.
JAK/STAT signaling is highly involved in the differentiation of Th cell subsets; Th1 cell differentiation is controlled by the IFN-γ/STAT1 and IL-12/STAT4 signaling pathways. 178 , 179 Meanwhile, Th2 cell differentiation is modulated by IL-2/STAT5 and IL-4/STAT6 signaling. 180 , 181 The differentiation of Th17 cells mainly requires the involvement of STAT3/STAT4 signaling induced by IL-6 or IL-23. 182 In allergic diseases, the JAK/STAT pathway is critical for cell proliferation and differentiation. In both rat and human bronchial smooth muscle cells, IL-13 induces JAK1-STAT6 signaling, which regulates Ras Homolog Family Member A (RhoA) activation that promotes smooth muscle contraction. 183 , 184 IL-4 and IL-13 induce STAT6 in target cells with JAK involvement, and gene-targeted knockout mouse assays revealed STAT6 contributes to IgE synthesis, bronchial hyperresponsiveness and airway remodeling upon allergen sensitization. 185 , 186 Further type 2 asthma-related cytokines, including IL-5 and TSLP, signal through JAK-dependent pathways. The JAK signaling pathway is critical for the differentiation of naive precursors into CD4 + Th2 cells, and the key cytokines involved are IL-2 and IL-4, which bind to cytokine receptors coupled to JAK1 and JAK3, respectively, then induce STAT5 and STAT6. 187
In AAS, cytokine receptors, e.g., IL-4, IL-5, IL-13, IL-31, and TSLP, promote JAK/STAT signaling activation. 188 , 189 In AD, Th2 immune enhancement induced by JAK/STAT signaling downstream of multiple cytokines, including IL-4, IL-5 and IL-13, is considered an essential pathogenic pathway. 190 It was demonstrated that the JAK-STAT pathway regulates inflammatory processes and induces changes in the natural skin barrier, increasing TEWL (transepidermal water loss) by upregulating IFN-γ, IL-31, IL-23, and IL-22. 190 STAT3 is one of the factors responsible for IL-23 expression induced by IL-6 from DCs, which is critical for Th17 lymphocyte differentiation and cellular memory, leading to a disruption of epithelial barrier integration. 191
NF-κB/MAPK signaling pathway
By 2022, NF-κB has been known for 36 years. NF-κB (nuclear factor) is a protein factor with gene transcriptional regulation, which is present in almost all nucleated cells. When cells are stimulated by inflammatory mediators, NF-κB protein is activated in the cytoplasm and enters the nucleus to regulate the expression of various inflammatory factors, playing an important role in allergic diseases.
In AAS, the NF-κB/MAPK pathway controls inflammatory and immune responses by regulating TNF-α and IL-6. 192 , 193 , 194 A 2019 study demonstrated that after nuclear translocation of phosphorylated P65, inhibited NF-κB/MAPK signaling may modulate IgE and IL-4 production. 195 Enhanced NF-κB nuclear binding or production was also found in inflammatory cells collected from induced sputum in asthmatic patients. 196 Besides, experiments have shown enhanced NF-κB activation in airway tissues and inflammatory cells challenged by allergens such as ovalbumin (OVA) and house dust mite (HDM) extract. 197 , 198 , 199 In addition, the studies related to increased Tfh cells in allergic diseases, NF-κB deficiency led to a decrease in CXCR5 (Tfh cells expressing chemokine receptors) in mice and a consequent decrease in the number of Tfh cells. 200 All these studies suggest that NF-κB signaling is essential in the cellular immune response of allergic diseases, especially AAS. The following focuses on the functions of NF-κB in immune cells in allergic diseases (Fig. 4 ).
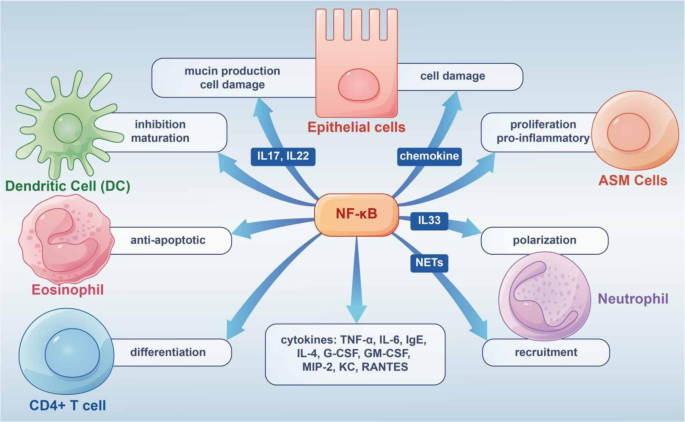
Graphical summary of NF-κB pathway’s role in allergic diseases. The NF-κB pathway is highly involved in the occurrence and development of allergic diseases by acting on different cells and releasing inflammatory factors
Epithelial Cells
Epithelial cells play a key role in airway diseases and constitute a critical interface between the body and the environment. 201 The epithelium coordinates responses to diverse invasive injuries via the production of multiple immunomodulators and inflammatory factors controlled by NF-κB. The latter mediators mostly comprise chemoattractants that induce inflammatory cell infiltration affecting epithelial function; among these, TSLP synthesized by bronchial epithelial cells is essential in Th2 responses that trigger allergic airway inflammation. 201 The neutrophil-associated proteins S100A8 and S100A9 induce mucin production in airway epithelial cells through toll-like receptor 4 (TLR4)-related NF-κB pathway activation during AAS attacks. 202
In isolated mouse airway epithelial cells, NF-kB activation upregulates IL-6, granulocyte colony-stimulating factor (G-CSF), GM-CSF, macrophage inflammatory Protein 2 (MIP-2), keratinocyte-derived chemokine (KC), and RANTES. NF-κB activation in mouse epithelial cells also leads to the recruitment of neutrophils for innate immune response. 203 In the OVA-induced model of allergic diseases, NF-κB activation leads to airway inflammation, goblet cell hyperplasia, and induced expression inflammatory cytokines, including IL-15, IL-10, and IL-9. 204
Airway Smooth Muscle (ASM) Cells, Neutrophils and Eosinophils
In AAS, acute contraction of ASM is the main factor that causes bronchospasm. ASM cells contribute to persistent histological alterations in the airway wall, ASM and inflammatory cells are important players in inflammation, 205 in which thrombin and IL-1α stimulate NF-κB signaling in ASM cells. 206 IL-8 over-secretion by ASM cells may increase NF-κB’s binding to the IL-8 promoter. 207
AAS includes eosinophilic, neutrophilic, oligogranulocytic and mixed granulocytic types, based on the type of inflammatory infiltrating cells. Of these, neutrophil infiltration is an important cause of AAS exacerbation and resistance to hormone therapy. 208 Th17 lymphocytes are critical for neutrophilic asthma, and are the major producers of IL-17A, IL-17F and IL-22, whose amounts are elevated in the airways of severe steroid-refractory asthma cases. 209 NF-κB signaling is associated with IL-17 and/or IL-22-related production of epithelial mucin and ASM cell proliferation. 210 , 211 , 212 IL-33 promotes neutrophil polarization via c-Jun N-terminal kinase and NF-κB-related pathways. NETs induce CXCL1, CXCL2, and CXCL8 expression in airway cells through TLR4/NF-κB signaling, thereby recruiting neutrophils to inflammatory sites. 213 In neutrophilic asthma (NA) mice, NETs trigger the expression of chemokines by airway and alveolar epithelial cells that promote the recruitment of more neutrophils through the TLR4/NF-κB pathway, leading to epithelial cell damage. 214
Airway inflammation in eosinophilic allergic asthma features infiltrated and activated eosinophils, and co-culture of epithelial cells with mast cells or eosinophils induce NF-κB-dependent cytokine production by airway epithelial cells. 215 , 216 NF-κB signaling is critical in the survival of eosinophils, exerting an anti-apoptotic effect through autocrine TNF-α. 217 NF-κB suppressors on the other hand, including MG-132, reduce eosinophil amounts and alleviate allergic inflammation. 218
Dendritic cells and Lymphocytes
DCs interconnect innate and adaptive immune systems, with crucial roles in promoting immune defense and maintaining immune tolerance. Previous reports have established NF-κB signaling involvement in DC development, with NF-κB suppression preventing DC maturation associated with the upregulation of MHC and co-stimulatory molecules. 219
In eosinophilic allergic asthma, naive T cells differentiate and mature into Th2 cells, which biosynthesize IL-4, IL-5 and IL-13 with NF-κB involvement, and stimulate B lymphocytes to produce immunoglobulin E (IgE). 220 During CD4 + T cell differentiation, IL-6 and TGF-β are highly involved in Th17 cell differentiation. 221 NF-κB signaling regulates antigen-presenting cell function and controls CD4 + T cell differentiation into Th effector cells. 222 , 223 However, the present study is still inconclusive.
Hippo signaling and allergic disease
Hippo signaling was first discovered in Drosophila and is a highly conserved pathway. 224 It mainly comprises the cascade kinase cascade transcription molecule mammalian STE20-like kinase 1/2 (MST1/2), WW domain of Sav family containing protein 1 (SAV1), and MOB kinase activator 1 (MOB1), with large tumor suppressor 1/2 (LATS1/2) upstream and Yes-associated protein (YAP)/effector molecules with PDZ- binding motif (TAZ) downstream. When Hippo signaling is not activated, unphosphorylated YAP undergoes nuclear translocation and interacts with TEAD, thereby triggering the transcription of target genes. After Hippo signaling activation, TAOK induces MST1/2 phosphorylation, and phosphorylated MST1/2 interacts with SAV1 for MST1/2-SAV1 complex formation. With activated MOB1, the latter complex phosphorylates LATS1/2. In turn, LATS1/2 phosphorylation triggers YAP activation, causing YAP capture by 4-3-3 proteins in the cytosol or degradation by SCFβ-TRCP E3 ubiquitin ligase-mediated ubiquitin-proteasome signaling. 225
In AAS, Hippo signaling mainly induces cell differentiation. The Notch4 protein mediates immune tolerance and leads to Treg dysfunction, thereby promoting allergic airway inflammation. 226 After alveolar macrophage engulfment of allergens and particulate pollutants, Jag1 is highly expressed on alveolar macrophages, thereby activating Notch on CD4 + T cells and promoting inflammation associated with Th2 and Th17 effector T (Teff) cells; 227 , 228 at the same time, alveolar macrophages secrete a large amount of IL-6, which promote the expression of Notch4 on induced regulatory T (iTreg) cells, thereby activating Hippo signaling, which further exacerbates Th17 cell-induced inflammation (Fig. 5 ). 161 In other allergic diseases, including AR, AD and FA, no associations with Hippo signaling have been reported.
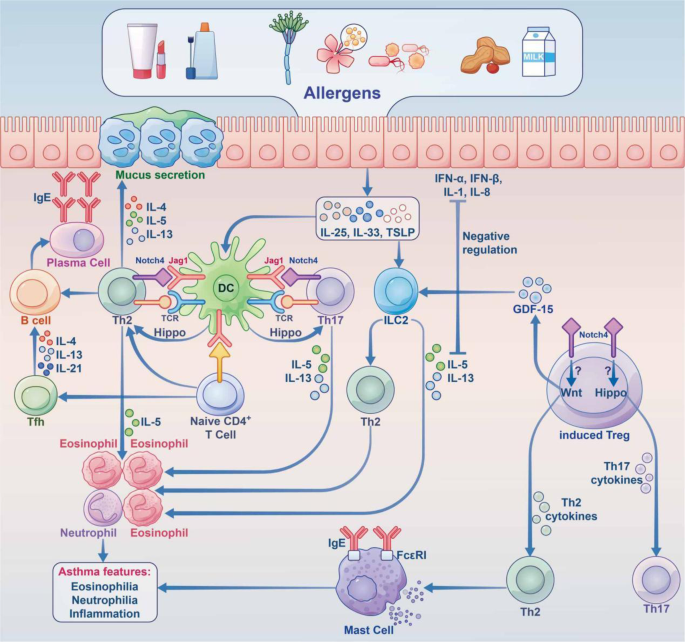
The roles of the hippo and Notch pathways in AAS. Under stimulation by allergens, epithelial cells synthesize large amounts of proinflammatory cytokines (IL-25, IL-33, TSLP, etc.), thereby acting on innate lymphocytes (ILC2 cells) and DCs. Jag1 on DCs interacts with Notch receptors on T cells for Notch pathway induction. Notch transforms induced Tregs into Th2 and Th17 cells. Naive CD4 + cells affect Tfh cell class switch recombination by secreting IL-5, thus acting on B cells to induce plasma cells, which produce IgE. At the same time, Th2 cells secrete IL-4 and others to activate B cells to synthesize IgE, which interacts with IgE receptors on mast cells. In case the allergen invades the body again, it directly cross-links with IgE on the cell surface and releases a variety of active mediators, which trigger the clinical symptoms of asthma. Th17 cells are activated through the Hippo pathway; Th2 cells are activated through the Wnt pathway, and GDF-15 molecules are stimulated to act on ILC2 cells to enhance the expression of IL-13, although this remains controversial. Notch converts induced Tregs into Th2 and Th17 cells via hippo pathway-dependent mechanisms. IL interleukin, TSLP thymic stromal lymphopoietin, ILC2 group 2 innate lymphoid cell, DC dendritic cell, Jag1 jagged1
The newly discovered Hippo pathway plays a critical role in the immune function of the body, with complex crosstalk with other signaling pathways, and is regulated by other signaling pathways (such as Notch, Wnt signaling pathway, etc.). As the study of Hippo signaling pathway continues to deepen, its important function in allergic diseases will be gradually discovered.
TOLL-like receptor (TLR) signaling pathway
The increasing prevalence of allergic diseases is not only related to changes in the modern living environment (such as pollution, low-endotoxin living environments, smoking, etc.), which may induce the disorder of immune system, 229 , 230 but also closely related to the loss of microbial biodiversity.
TLRs represents an important group of transmembrane protein receptors that are critical for proper activation of innate immunity, are highly conserved, and comprise binding domains containing arginine-rich repeats. As molecules involved in the first line of defense, TLRs are induced by pathogen-associated molecular patterns (PAMPs), which are found in diverse pathogenic organisms and absent from the host. There are eleven known human TLRs (TLR1 to TLR11), all with functions except for TLR11. 231 , 232 TLRs are located on and within immune and non-immune cells, respectively, and are critical for initiating adaptive immune responses, including in alveolar macrophages, mast cells, epithelial cells, neutrophils, natural killer cells, and antigen presenting cells (APCs). Different TLRs recognize various groups of molecules in diverse pathogens. For example, multiple diacyl peptides can be recognized by TLR1 and TLR6, while liposomes are recognized by TLR1/2. 233 TLR5 cooperates with TLR4 to recognize bacterial flagellin. 234 Studies have shown that gut microbiota regulates the activity of Th1 and Th2, thereby affecting the formation of immune tolerance in the body. 235 To maintain immune homeostasis, the activation of innate immune cells requires TLR signaling molecules, and innate immunity should be activated correctly to stimulate the body’s immune response. 231
The strength of the TLR signaling pathway determines the possibility of allergic diseases. Accumulation of TLR4 signaling on DC was detected in house dust extract (HDE) hypersensitivity. 236 , 237 Bacteria belonging to the healthy lung microbiome elicit baseline TLR response, whereas those involved in asthma show stronger TLR response. Mice administered asthma-associated proteobacteria show elevated amounts of neutrophils and cytokines in comparison with animals administered commensal Provetella . 238
The strength of TLR signaling does determine the occurrence of allergic reactions or its absence. Strong TLR signals are protective against allergic airway disease, while low airway amounts of TLR ligands cause airway sensitization and Th2-type immunity. 239 , 240 , 241 Studies have shown that low-level flagellin (TLR5 ligand) can enhance OVA-associated hypersensitivity in mice, whereas a high flagellin dose protects the animals from hypersensitivity by producing CD25 + Treg-dependent regulatory DCs and T cells. 242 Differential responses to TLR activation have been considered a shift from Th2-type immunity to Th1-type immunity with increasing stimulation intensity.
In AAS, alveolar macrophages are immune cells with critical roles in the clearance of immune antigens. Excessive inflammatory response of these cells, however, might induce tissue damage. 243 Alveolar macrophages are important in developing tolerance to inhaled allergens by inhibiting T cell proliferation and APC function. 244 Asthmatic patients have reduced monocyte and macrophage amounts in comparison with healthy control individuals. 245 TLR2, 4, 5, 6, 7, 8 and 9 are expressed on macrophages. TLRs have a critical function in AAS; among the TLRs expressed by human lung cells, TLR1-5, 7 and 8 have the highest expression. 246 TLR2-6 and TLR9 are highly expressed on human airway epithelial cells. 247 , 248 Mouse alveolar macrophages highly express TLR2, 4 and 9, 249 and mouse macrophages produce TLR1-7 and 9 mRNAs. 250 , 251
Lipopeptides in Gram-positive bacterial organisms and mycoplasmas show diacylation, while lipopeptides in Gram-negative bacterial and mycobacterial species show triacylation. When TLR2 polymerizes with TLR1 or TLR6, the lipopeptide is recognized by TLR2. The TLR2/TLR6 heterodimer recognizes diacylated lipopeptides such as S-FSL1 (TLR2/6), R-FSL1 (TLR2/6/CD36) and MALP-2 (macrophage-activating lipopeptide-2). Cell wall constituents in Gram-positive bacteria, including lipoteichoic acid, also bind to the TLR2/TLR6 heterodimer. 252 Phagocytic cells, airway epithelial cells, smooth muscle cells, glia, mouse bone marrow-derived mast cells, and B cells all can express the TLR2 receptor. 253 In OVA-sensitized animal model, TLR2 receptor pathway induction also results in enhanced pause and increased bronchioalveolar lavage fluid (BALF) eosinophil amounts. 254 , 255 Elevated TLR2 ligand amounts can also elevate serum IgE concentration. Though TLR6 is heterodimerized with TLR2, and the heterodimer plays an important role in AAS, TLR6 is decreased in PBMC compared with healthy controls, and is also overexpressed in severe asthma cases compared with mild asthmatic patients. 256 TLR4 uses the adaptor protein TRIF via myeloid differentiation primary response gene 88 (MyD88)-dependent and MyD88-independent pathway, respectively, enhancing IRF-3 induction and IFN-β production. 257 In bronchial asthma cases, it was shown that TLR4 activation of macrophages produces cytokines that affects immune balance and thus affects the Th1/Th2 balance. 258 After TLR5 recognizes flagellin, it induces NF-κB signaling through MyD88 and TNF receptor associated factor 6 (TRAF6), producing cytokines to trigger an inflammatory response. 245 Recently, Nawijn et al. showed that intranasal TLR2 induction by aerosolized allergens promotes allergen-specific Treg proliferation to suppress asthma in a mouse model. 259 Numerous studies have shown that TLR2 stimulation by parenteral or mucosal treatment with synthetic agonists prevents APCs from triggering Th2-polarizing responses, reducing IgE antibodies and immunogenicity in a mouse model of asthma. 260 , 261 , 262 , 263
In FA, Treg activation is an important pathway by which gut DCs and macrophages induce immune tolerance, disrupting the normal immune homeostasis of the intestine. 264 All microbial pattern recognition receptors (PRRs) may be involved in food tolerance and allergen presentation. TLR2 is highly expressed by intestinal epithelial cells (IECs) and DCs, and most commensal bacteria in the intestine are gram-positive organisms, meaning they have a high ability to induce TLR2. 265 , 266 In AR, researchers were surprised to find that TLR4 inhibits allergic response in OVA-induced AR in a mouse model. 267 TLR2, 3 and 4 were highly expressed in nasal mucosa specimens from AR cases in a study of 27 healthy control individuals and 42 cases of seasonal allergic rhinitis. 268 All these studies have once again confirmed that TLR is critical for the etiology and progression of allergic disorders, which is worthy of further exploration.
Wnt/β-catenin signaling
In 1982, the Wnt gene was described as integrase-1 in murine breast cancer cells and the wingless gene in Drosophila. 269 Wnt signaling plays core roles in the maintenance of progenitor cells and stem cells, the differentiation of T cells, and the regulation of cellular immunity. Among the Wnt-mediated signaling pathways, the most classical Wnt/β-catenin pathway contributes to maintaining human tissue homeostasis. Wnt, which mediates extracellular signals, is a secretory glycoprotein, with 19 human Wnt proteins reported as of now. This pathway relies on β-catenin, which is activated by binding extracellular Wnt ligands to membrane receptors through autocrine and paracrine processes, inhibiting the degradation of β-catenin so that it can be stably accumulated in the cytoplasm and transferred to the nuclear compartment, where it can work together with T cell factor/lymphoenhancer binding factor to stimulate the transcription of target genes. 270 , 271
Wnt/β-catenin contributes to airway remodeling in asthma by upregulating the tenascin C/platelet-derived growth factor receptor (PDGFR) or activating p38 MAPK and its target genes c-Myc and cyclin D1 to induce proliferation in airway smooth muscle cells. 272 , 273 A study by Trischler et al. revealed that Wnt10b, known as the classical Wnt ligand, is highly produced by T cells in AAS, and its absence increases the activation of cultured T cells and enhances immune response in animal models. 274 In addition, after mesenchymal stem cell-derived exosomes and vitamin D inhibit Wnt/β-catenin signaling, airway remodeling is reduced, thereby inhibiting chronic allergic inflammation in the airway. 275 , 276 Upregulated Notch4 in blood Tregs from asthma patients differentiates Tregs into Th2 and Th17 T cells through a Wnt and Hippo pathway-dependent mechanism, and Wnt induction upregulates growth and differentiation factor 15 (GDF15) in Tregs, and this feedforward mechanism exacerbates inflammation. 226 However, Wnt-1/β-catenin pathway induction promotes allergic airway diseases. Overexpression of Wnt1 reduces DC migration to draining lymph nodes and induces an appropriate T cell tolerance response without causing T cell proliferation. 277
Related literature reported that inactivation of Wnt/β-catenin signaling could reduce nasal mucosa damage and eosinophil infiltration, decrease the infiltration of nasal mast cells and enhance red blood cell immune adhesion, thereby reducing the progression of AR. 278
There are few reports on this pathway in FA and AR. Research in allergic dermatitis shows that Notch deficiency is the basis for inhibiting epidermal differentiation and skin barrier defects, thus enhancing Wnt pathway, which is very important for the proliferation of epidermis cells. 279
PI3K/AKT signaling
PI3K in the lipid kinase family interacts with the PH domain of the AKT protein (also known as PKB), inducing its conformational change and AKT phosphorylation. Activated AKT is transferred from the cytosol to the plasma membrane, and subsequently induces its downstream effectors, including mammalian target of rapamycin (mTOR). 280
PI3K inhibitors have been considered to have great potential in the treatment of inflammation. Current evidence shows that PI3K participates in the pathogenesis of asthma through two main mechanisms. First, PI3K increases the permeability of mouse blood vessels to enhance antigen-induced airway inflammation and high reactivity. 281 , 282 , 283 In the OVA-induced asthma model, inhibition of PI3K110δ subtype (PI3K-δ) reduces the activation of HIF-1α in airway epithelial cells, as well as antigen-induced airway inflammatory reactions and hyperresponsiveness, by regulating vascular leakage mediated by VEGF. 284 Secondly, the PI3K pathway induces airway smooth muscle cell proliferation, promotes airway smooth muscle thickening and luminal stenosis, thereby participating in airway remodeling. 285 With respect to immune cells, PI3K regulates the differentiation of Th cells and eosinophils in asthma, 286 , 287 and affects the occurrence and development of inflammation in asthma. In an animal model of AAS, it was found that PI3K and AKT activities in lung tissue are increased, as well as the expression of mTOR. After treatment with a PI3K inhibitor, some pathological manifestations of asthma (such as increased amounts of activated chemokines in eosinophils, bronchoalveolar lavage fluid IL-5 and IL-13, lung tissue eosinophilia, increased mucus secretion in the respiratory tract, airway hyperresponsiveness, etc.) are obviously inhibited, and PI3K/AKT signaling highly regulates asthma pathogenesis. 288
In AR, drug development research found that the anti-allergic drug α-TCP (alpha-tocopherol) and the androgen receptor antagonist bicalutamide both play anti-inflammatory and alleviating roles in AR in animal models by inhibiting the PI3K/AKT/mTOR pathway in mast cells. 289 , 290 A mouse model with AR shows that increased leptin enhances the expression of type II innate lymphoid cell (ILC2) transcription factor and type II cytokines through the PI3K/AKT pathway. 291 For mice lacking CCR3 gene in the bone marrow, the activity of PI3K/AKT signaling was also significantly reduced, and nasal eosinophil infiltration was inhibited; in addition, serum Th2 cytokines were reduced, and the symptoms of AR in mice were alleviated. 292 It was found in cell experiments that ST2/PI3K/mTOR-mediated autophagy is inhibited by IL-33 secreted by nasal epithelial cells, thereby promoting mast cell degranulation in allergic asthma. 293
In the skin, dysregulated PI3K/AKT pathway might result in serious pathologies featuring unchecked cell proliferation and inflammatory response. 294 In addition, PI3K/AKT signaling also modulates mast cell degranulation via miRNAs in allergic skin diseases. High-expression miR-126 induces IgE-mediated mast cell degranulation related to PI3K/AKT signaling by increasing Ca 2+ influx. 295
Serum chitinase 3-like 1 (CHI3L1) amounts are increased in individuals with allergic disorders and promote Th2-related immunity and the polarization of M2 macrophages through PI3K/AKT signaling in FA. 296
mTOR signaling pathway
mTOR represents a serine/threonine protein kinase that belongs to the PI3K-associated protein kinase (PIKK) family. 297 While associated with the above paths, mTOR signaling may serve as an upstream response to other signaling pathways. mTOR has catalytic subunits in two different complexes, including mTOR complex 1 (mTORC1) and mTORC2, 298 which have different susceptibilities to rapamycin, substrates, and functions. 299 Studies in allergic diseases have shown that mTOR is a key molecule for sensing the immune microenvironment and determining the function and differentiation of immune cells, 300 because it regulates a variety of immune cells and limits pro-inflammatory mediators. 301 , 302 For example, increasing evidence supports that mTOR is an important regulator of Tfh cell differentiation. The balance of Tfh and Th1 cell differentiation in vivo is regulated by IL-2 signaling through PI3K, AKT and mTOR, and both mTORC1 and mTORC2 essentially promote Tfh cell differentiation and germinal centers (GC) formation, which cannot be ignored in allergic diseases. 303 , 304
In AAS, the progenitor cells of granulocytes originate from the bone marrow and move to blood vessels and lungs when inflammation occurs. Among them, eosinophils regulate Th2 immune response, which is related to the severity of the disease, 305 while ablation of mTOR leads to Gata-1 overexpression and increases eosinophil differentiation. 306 The angiogenic factor fibroblast growth factor-binding protein 1 (FGFBP1) is highly expressed in asthma models with airway remodeling features, because activating mTORC1 and signal transducer and activator of transcription 3 (STAT3) signaling pathways enhances FGFBP1 expression and secretion, thus inducing angiogenesis. 307
The target protein mTOR of rapamycin is involved in the growth of keratinocytes. Studies have found IL-13 activates the mTOR signaling pathway and downregulates miR-143, followed by the downregulation of epidermal barrier related proteins. Therefore, rapamycin could treat allergic dermatitis by inhibiting mTOR. 308
FcƐRI signaling pathway
Fc receptors play major roles in adaptive immunity by interacting with immunoglobulins, among which FcεRI represents a high-affinity IgE receptor found on mast cells, basophils, eosinophils and APCs. 309 When IgE binds to FcεRI to trigger immunity, FcεRI aggregation induces a variety of signaling pathways to regulate the secretion of allergy-associated mediators, including histamines and leukotrienes, and induces the transcription of Th2 cytokines and tumor necrosis factor (TNF) genes, 310 which leads to potentially life-threatening allergic diseases. The tetrameric form of FcεRI is present in mast cells and basophils, while the trimeric form is found in other immune cells; FcεRIα, FcεRIβ and FcεRIγ (αβγ2) are encoded by the FcεR1A, FcεR1B (MS4A2) and FcεR1G genes, respectively. 311 , 312
The pro-inflammatory effects mediated by FcεRI in different allergic disorders have the following similar mechanisms. Signal transduction in mast cells is induced by the phosphorylation of immune receptor tyrosine activation motif (ITAM) of FcεRIβ and FcεRIγ subunits by Src-protein tyrosine kinase. This results in the recruitment of tyrosine kinase Syk, which mediates the activation of some adaptor molecules (SLP76, LAT, etc.), leading to calcium mobilization. 313 Therefore, dephosphorylation of tyrosine kinase activating signals downstream of the IgE-FcεRI complex may prevent allergic diseases, 314 and TLR-mediated release of cytokines from mast cells depends on the expansion effect of FcεRI, which is more important in the late reactions associated with inflammation. 315 There is a synergistic effect between TLR and FcεRI-mediated activation in basophils, which promotes Th2 cell differentiation and induces degranulation and cytokine release. 316 , 317 Platelets depend on the interaction between allergens and allergen-specific IgE and FcεRI, and are directly involved in allergic asthma. 318 In addition, FcεRI signaling can also activate the PI3K signaling pathway. 314
In allergic diseases, sensory neurons exposed to allergens produce action potentials; the Ca 2+ flux mediated by immune complexes increases, the action potentials discharge and neuropeptides are released, thereby causing pain or itching. The IgE receptor FcɛRI highly contributes to the development and remodeling of airway inflammation in allergic asthma. 319 Studies in animals with experimental allergic asthma demonstrated that when the vagus nerve, which dominates the airway, senses the invasion of allergens, pain receptor neurons overexpress the immunoglobulin receptor FcɛRI and release Substance P, which drives the polarization of Th2 cells, thus triggering allergic inflammation. 320
IgE-mediated FA is very common, and FcɛRI is also upregulated in the abdominal vagus nerve of mice with experimental food allergy, which promotes the skewed Th2 polarization in the intestine. 321 Functional FcɛRI also exists in intestinal neurons, and stimulation of IgE antigen activates intermuscular neurons. 322
NOD-like receptors signaling pathway
Nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) are located in the cytoplasm, and 23 species have been found in the human body to date. 323 , 324 This pattern recognition receptor recognizes microbial compounds such as PAMPs and Damage-Associated Molecular Patterns (DAMPs) and cooperate with TLR and related pathways to trigger antibacterial immune response. The NOD-like receptor consists of the following three domains: (i) the nucleotide sequence located in the center has a domain that binds to NACHT, which drives the activation of downstream inflammatory caspase and NF-κB; (ii) the effector domain located at the N-terminal end mediates the interaction with adaptor proteins and downstream effectors and transmitting receptor excitability information; (iii) the C-terminal region is composed of leucine-rich repeats (LRRs), which constitute the microbial pattern recognition domain. 325 , 326 NLRs recognize many pathogen-related model molecules, including microorganisms and toxins secreted by microorganisms 327 which could be used for immune surveillance and host defense. The NLR signaling pathway mainly has the following functions: signal transduction, inflammasome formation, gene transcription stimulation and autophagy. 328 , 329
After an NLR recognizes bacteria-related ligands, γ-D-glutamyl-meso- diaminopimelic acid (iE-DAP) and muramyl dipeptides (MDP), it promotes the expression of the adhesion molecule ICAM-1 on eosinophils and bronchial epithelial cells, and thus drive the cell adhesion, chemotaxis and migration of leukocytes. NLRs also induce eosinophils and bronchial epithelial cells to produce pro-inflammatory molecules such as IL-1β, IL-6, CXCL8, CCL2, CCL3, CCL4, and CCL5, thereby causing lung inflammation. 330
In animal models, treatment of allergic asthma mice with NOD1 ligand induces subcutaneous fibrosis and significantly increases serum amounts of total IgE, eosinophils and the chemokine CCL5 as well as bronchoalveolar lavage fluid amounts of the Th2 cytokine IL-13. 331 However, intranasal NOD2 ligand induces the expression of TSLP, IL-25 and OX40L in the lung, 332 and these three molecules have been reported to promote asthma-related inflammation, 333 , 334 , 335 blunt the production of antigen-specific CD4 + Foxp3 + adaptive Tregs, and simultaneously drive CD4 T cells to produce IL-4, change the Treg/Th2 balance, block tolerance, and promote the susceptibility of airway inflammation dominated by eosinophils. 332
The nucleotide-binding oligomeric domain-like receptor family Pyrin domain 3 (NLRP3) inflammasome contains NLRP3, ASC and Caspase-1, which are important constituents of the innate immune system, with critical roles in allergic disorders. 331 The NLRP3-retinoid X receptor (RXR) axis drives airway epithelial cell apoptosis as well as the production of inflammatory cytokines in the lungs of asthmatic mice. 336 Further study found that NLRP3 in bone marrow cells promotes the development and progression of AAS in an inflammasome-dependent manner, and RRx-001 (an inhibitor of NLRP3) could significantly decrease inflammatory cell infiltration and mucus secretion in the airway. 337 PMs can cause acute exacerbation of allergic airway inflammation, activate TLR2/NF-κB/NLRP3 signaling and aggravate allergic airway inflammation. 338
It was demonstrated that NOD1 and NLRP3 in AR patients are downregulated during the pollen season. 339 Activation of the NLRP3/gasdermin D/IL-1β signaling pathway mediates macrophage pyroptosis and releases inflammatory mediators to local tissues, which is involved in nasal mucosa inflammation of AR. 340
The microbiota plays an essential role in the occurrence and development of allergic diseases
In 1989, Strachan observed that children with more siblings in the family were less likely to develop hay fever or eczema; 28 children are frequently exposed to allergens, hence the original “hygiene hypothesis” was proposed. This hypothesis is a good explanation for the phenomenon that AAS is significantly more prevalent in developed countries in comparison with underdeveloped countries. From an immunological point of view, it could be understood that the development of immune tolerance to allergens depends on the amount and degree of stimulation by microbial colonization and immune stimulating environmental signals transmitted in early life. 341
With the rapid development of urbanization and industrialization, excessive use of hygiene products and antibiotics, coupled with changes in diet such as fast food, etc., would decrease microbial diversity in early life, 342 resulting in impaired immune protection and the destruction of normal microorganisms. 343 There is increasing evidence that the microbiota is critical for the occurrence and development of allergic disorders. 344 The human microbiota mainly colonizes the gastrointestinal tract (GIT), with microorganisms also present in other body parts such as the oral cavity, nasal cavity, skin, and respiratory and reproductive tracts. 345 Symbiotic microorganisms in the GIT and other organs mediate the innate and adaptive immune systems through the gut-lung and gut-skin axes. Reports have shown that many environmental factors influence the colonization, composition and metabolic activities of microbial communities in early life, thereby affecting the immune function of the body and leading to the occurrence of allergic diseases. 346 , 347 , 348 , 349
Early-life activities and dysbiosis is tightly associated with allergic diseases
It is well established that microbial colonization starts at birth, and that microbiota composition is affected by related factors such as the prenatal and postnatal environment, which are also critical for the body’s immune function. Multiple factors, including mode of delivery, 350 , 351 , 352 feeding choice, 353 and use of antibiotics or not, 354 , 355 can alter the composition of the gut microbiota and modulate infant tolerance to different allergens.
The use of antibiotics during pregnancy and the early postpartum period can affect the gut microbiota in normal infants and increase the risk of developing allergic diseases. 356 , 357 Mothers exposed to antibiotics during childbirth had significantly lower microbial diversity compared with infants born to antibiotic-free mothers. The microbiota of antibiotic-exposed infants shows reduced amounts of Bacteroidetes and Bifidobacterium , alongside increased Proteus amounts. Studies have shown antibiotic utilization during pregnancy and childbirth is associated with elevated risk of AD and asthma. 358 , 359 , 360 A study of 14,572 children, 10,220 of whom were administered antibiotics in the initial 2 years of life, revealed that early exposure to antibiotics had tight associations with childhood asthma, AR and AD. 361 Studies in germ-free laboratory animals further demonstrated an interdependent association of gut microbiota with immune system development. 362 , 363 , 364 , 365
Dysbiosis is tightly associated with allergic disease occurrence, and in some way affects the balance of immune cells in allergic diseases. Most AAS begins in childhood, and HDM, cockroach remains, pet dander, fungi and pollen are the main allergens. 366 A study found lower abundances of Lachnospira , Veillonella , Faecalibacterium , and Rothia in the gut of infants are associated with higher asthma risk, and inoculating these bacteria in germ-free (GF) mice could alleviate airway inflammation and prevent the development of asthma. 367 Furthermore, decreased abundance of Bifidobacterium was found in adult asthmatic patients. 368 Haemophilus , Moraxella and Neisseria spp . were also observed in the airway microbial composition of asthmatic patients, and Proteus was also found in mild cases not receiving inhaled corticosteroids as well as in severe asthma cases. Actinobacteria and Klebsiella species were markedly enriched in severe asthma cases in comparison with healthy controls or mild-to-moderate asthma cases. 369 In AD patients, Staphylococcus aureus colonization is an important exacerbating factor in AD pathogenesis, and gut dysbiosis is also considered an important factor in AD pathogenesis. A metagenomic analysis data showed that S. aureus constituted approximately 90% of AD skin, leading to dramatically decreased skin microbial diversity. 370 In animal models, alpha-hemolysin and extracellular vesicles produced by S. aureus lead to skin barrier dysfunction and promote atopic skin inflammation. 371 , 372 , 373 Enterotoxins secreted by staphylococci promote allergic skin inflammation by triggering substantial T cell activation, and staphylococcal delta-toxins also cause allergic skin diseases via mast cell activation. 374 Clinical cohort trials have shown an early reduction in gut microbiota diversity is strongly related to elevated AD risk, 375 and the presence of gut microbiota subspecies such as Clostridium Perfringens , Clostridium difficile and Faecalibacterium prausnitzii is closely associated with reduced capability of producing short-chain fatty acids (SCFAs), while L. paracasei abundance reduces the susceptibility to AD. 376 , 377 , 378 AR is a respiratory disease that occurs in the upper respiratory tract, which includes the nose and oropharynx. Firmicutes and Actinobacteria represent key microbiota constituents in the nasal cavity of humans, while Proteobacteria , Firmicutes , and Bacteroidetes represent key phyla in the oropharynx. 379 , 380 , 381 Microbial diversity in seasonal AR (hay fever) did not decrease, but was instead elevated during allergy season. 382 Staphylococcus aureus is a microorganism involved in perennial (non-seasonal) AR. 383 The etiology of the elevated incidence of FA remains undefined and may be related to the mode of delivery (natural vs. surgery) or the changes in the microbiome in early life due to antibiotic use even in low amounts. 384 , 385 , 386
The microbiota contributes to the innate and adaptive immune systems in allergic diseases
Human mucosal tissues, such as intestinal mucosa, nasal mucosa, and other surfaces, have regular exposures to complex microbial populations comprising commensal and pathogenic organisms. The host utilizes many molecular mechanisms to mediate mucosal innate immunity for microbial homeostasis. PRRs, including TLRs and NLRs, play major roles in recognizing pathogens and inducing innate immune responses.
Different gut microbiota have different activation pathways, and some gut bacteria, e.g., Escherichia coli , Salmonella typhimurium , Klebsiella pneumoniae , and Proteus vulgaris , activate HEK-293 cells through the TLR2 and TLR4 pathways. 387 Flagellate bacteria, including Salmonella , Listeria , Pseudomonas , and Escherichia coli , contain flagellin, which acts by binding to TLR5. 388 TLR9 functions by recognizing unmethylated CpG motifs, especially GTCGTT motifs, in the DNA of gut bacteria, including Proteu s, Bacteroides and Actinobacteria , as well as Lactobacillus plantarum , etc. 389 TLR signaling stimulates the maturation of innate immune cells, to properly activate APCs and initiate a moderate immune response. 231 Both exposure to allergic environments and the mother’s allergic status are all factors affecting an infant’s susceptibility to allergic diseases. 390 Microorganisms are present in the placenta, amniotic fluid and meconium, which are in contact with the fetus in early fetal stage; therefore, the fetus needs to develop immune tolerance during the mother-fetal period to prevent infection. 391 Natural killers, DCs and macrophages in the endometrium and trophoblasts are already induced during fetal development. 392 , 393
In allergic disorders, the microbiome exhibits a crucial function in establishing adaptive and innate immune protection. For instance, children with reduced IgG responses to specific microbial antigens than healthy counterparts are prone to allergic diseases, including asthma, AD and FD. 344 , 394 , 395 In studies of babies, higher AD risk had associations with reduced levels of Proteobacteria and elevated innate inflammatory response induced by TLR-4, and Ruminococcus decrease was associated with elevated TLR2-dependent innate inflammatory response. 396 , 397 , 398 FA in early stage is also closely associated with reduced gut microbial abundance. 344
Epithelial cells
Epithelial cells on the nasal and bronchial mucosal surfaces are critical for maintaining the healthy state of respiratory mucosa. Continuous and coordinated ciliary movement enables the removal of foreign invading substances such as pollutants or allergens from surfaces. Epithelial cells also produce different cytokines and chemokines to activate inflammatory cells; meanwhile, hyperactivation of epithelial cells may trigger the onset of several disorders, including asthma and AR. 399
Epithelial cells recognize PAMPs through innate PRRs, e.g., TLRs and NLRs, and such interactions affect the proliferation of epithelial cells. Microbiota-derived metabolites, including SCFAs, also have effects on epithelial cells. SCFAs, for example, stimulate inflammatory pathways by interacting with GPRs on epithelial cells in the intestine. 400 P-cresol sulfate (PCS) is a microbial-derived product produced in the gut. PCS selectively reduces CCL20 production by airway epithelial cells due to uncoupling of epidermal growth factor receptor (EGFR) and Toll-like receptor 4 (TLR4) signaling, a pathway that acts distally on airway epithelial cells to reduce allergic airway responses. 401
DCs, as APCs, are critical for immune responses in contact with commensal microbiota. The presence of SCFA butyrate, an end product of microbial fermentation, stimulates human monocyte-derived dendritic cell (moDC) maturation, increasing IL-10 amounts while decreasing IL-6 and IL-12 levels. 402 Meanwhile, mice treated with SCFA propionate could produce new myeloid DC precursors with strong phagocytic capability but poor capability of promoting Th2 responses in the lung.
In humans, TLR9 is highly produced by macrophages and plasmacytoid DCs. 403 The CpG motif is prevalent in bacteria, and the CpG motif also recognizes plasmacytoid dendritic cells (pDC) expressing TLR9 that produce pro-inflammatory cytokines, induce Th1-like immune activation patterns and activate their migration, while CpG-A oligodeoxynucleotides (ODN) induces extremely high levels of IFN-α production and CpG-B ODN induces activation of murine bone marrow-derived DCs to secrete IL-12 and IL-6. 404 , 405
In reports assessing AAS disease models in mice and rhesus macaques, macrophage responses to TLR9 activation mainly induce Th1 type immune response, including upregulated TNFα, IL6, IL-12, IL-18, IFN-α and IFN-γ, 406 resulting in the immune imbalance of Th1/2 cells. 407
Macrophages
The microbiome and associated metabolites, including SCFAs, affect the function of macrophages resident in tissues. In the gut, the SCFA butyrate promotes the anti-inflammatory response of macrophages and induces Treg differentiation by activating its receptor GPR109a, which is essential in inducing tolerance to food. Besides, butyrate exerts anti-inflammatory effects on macrophages by inhibiting IL-6, IL-12 and NO production. 408 In the lungs of antibiotic-treated mice, macrophages are polarized towards an M2 hypersensitivity phenotype by prostaglandin E2 (PGE 2) produced by commensal fungi. 409
Lipopolysaccharide (LPS) represents a soluble cell wall constituents in common Gram-negative bacterial organisms. 410 LPS can interact with complex host systems, including cellular and humoral components of the immune system, to induce the production of multiple immunomodulatory cytokines. 411 It has been shown that LPS regulates lung inflammation in asthmatic mice via the TLR4 pathway in alveolar macrophages and that different doses of LPS exposure determine the type of inflammatory response. 412 Improvement of AD by modulation of Th1/Th2 immune system homeostasis with LPS-activated macrophages derived from pantoid epimer (IP-PA1). 413
Mast cells (MCs)
Mast cells (MCs) represent major effector cells in allergic diseases. Increasing evidence suggests that the microbiota can modulate MC function, influence MC activation through direct interactions or secreted metabolites. 414 In human MCs, co-culture with Lactobacillus rhamnosus downregulates high-affinity IgE and histamine H4 receptors, while upregulating IL-8, IL-10, CCL2 and TNF-α. 414 In murine experiments, Lactobacillus paracasei inhibits IgE-mediated MC activation via TLR2. However, the inhibitory effect of Lactobacillus casei is mainly through direct cell contact and not dependent on TLR or NOD1/2. 414
Eosinophils
Elevated numbers of eosinophils in AD, AR and eosinophilic allergic asthma are typical symptoms for allergic diseases. 415 , 416 , 417 , 418 Eosinophils act as major effector cells driving innate immunity in allergy and other inflammatory diseases, with an important role in clearing microbes resident in tissues. 419 Meanwhile, eosinophil function is also regulated by pathogenic microorganisms; for example, Clostridium difficile stimulates the release of eosinophil-derived neurotoxins by eosinophils. 420 However, when eosinophils ingest the probiotic strain Bifidobacterium , neurotoxin release is significantly reduced. 420 Very interestingly, probiotics such as Lactobacillus fermentum and Lactobacillus rhamnosus , alleviate allergic inflammation involving reduced eosinophil infiltration in multiple mouse models of asthma and AD, although probiotic strains have not been shown to have a direct effect on eosinophils. 421 , 422 NLRP12 induces allergic skin inflammation by promoting peripheral DC retention as well as neutrophil migration. 423
TLR2 was identified as the primary receptor against S. aureus , 244 and intracellular NLRs also modulate microbial pathogen recognition. Intracellular NLRs, including NOD1, NOD2, NLRP1, NLRP3, NLRP4, and the interferon-inducible protein AIM2 produce a series of inflammatory molecules to resist the invasion of microorganisms. 424 NOD2 is considered a major player in innate immune response to S. aureus in the skin. 425 Studies have found that NOD2 expression on basophils in the peripheral blood of AD cases is markedly reduced compared with that of healthy people, 426 and basophils are the main effector cells involved in Th2 polarization in allergic inflammation. Acinetobacter in the skin prevents allergic inflammation and is critical for the regulation of Th1/Th2 cell balance and anti-inflammatory responses. 427
Innate Lymphoid Cells (ILCs)
ILCs, a major group of innate immune cells, are present in all parts of the respiratory tract; ILC2 cells are predominantly found in mice, while ILC3 cells are predominantly found in the human respiratory tract. 428 , 429 Microbial signaling affects the maturation of ILC tissue-specific functions. Clostridia has been shown to stimulate ILC3 to produce IL-22, which helps to strengthen epithelial barrier and reduce intestinal permeability to dietary protein. 430 ILC3 cells tolerize T-cell response and prevent IL-22 production, leading to a loss of gut bacteria. 409 Furthermore, when gut macrophages release IL-1β upon microbial insult, ILC3 cells release GM-CSF and induce immune tolerance. 409 TNF-β produced by ILC3 cells is essential for maintaining gut microbiota homeostasis, and IL-25 is produced by epithelial tuft cells in a microbiota-dependent manner. 409 The microbiota and its metabolites induce different types of ILCs and regulate their capability of preventing allergic reactions.
Tregs are important immune regulatory cells, which suppress allergic diseases and have critical functions in controlling the immune response. Bifidobacterium longum 35624, Clostridium fragilis , and Bacteroides fragilis all induce Tregs in the intestine, whereas other bacteria do not induce Tregs. 431 , 432 Activation of PRRs on DCs is a critical mechanism by which gut microbial organisms mediate Treg differentiation. 433 Recently, it was found that infants with reduced levels of Bifidobacterium and Faecalibacterium have elevated relative risk of asthma, characterized by elevated amounts of IL-4 + Th2 cells and reduced Treg levels, 434 attenuating the adaptive immune response. In IgE-induced FA studies, dysbiosis affected the differentiation of Tregs, resulting in an imbalance of Treg and Th2 cells and leading to allergic diseases (Fig. 3 ). 435 , 436
At present, the treatment of allergic diseases mainly includes five aspects: management and therapeutic education for patients and allergen avoidance, traditional pharmacotherapy, allergen immunotherapy, biologics administration and other therapies.
Management and therapeutic education for patients and allergen avoidance
Daily management of patients with allergic diseases and educational intervention plays an essential role in managing difficult-to-treat allergic cases. It is frequent and may lead to treatment failure due to poor adherence to the prescribed treatment. 437 Ensuring patient education and confidence in prescribed drug is urgently required, to achieve disease control. For example, it is necessary to inform patients regarding the correct use of intranasal spray and other pharmaceutical preparations. 438 What’s more, psychosomatic aspects can also contribute to complementing topical and systemic therapies. 439 Furthermore, it is also a critical issue in allergic disease management to combine the limited resources and increased digitalization. For example, mobile applications can monitor symptoms, drug use, and quality of life timely with higher efficiency, e.g., the Allergy Diary. 440 , 441
Avoiding allergens or minimizing exposure to allergens is the first step of treatment. However, since the pathogenic allergens of different patients are often different, mainly including food allergens, aeroallergens, contact allergies, and allergens in the environment that are frequently unknown, the strategy of avoiding allergens on the basis of an allergy diagnosis is often challenging. 438 Therefore, teaching patients how to avoid contacting with allergens may help achieve the best treatment effect. For example, the current standard of FA care is to avoid allergens, because there is no Food and Drug Administration (FDA) approved treatment for FA at present. 442 As for AR or AD, due to their multifactorial etiologies, elimination of food or environmental allergens represents an adjuvant treatment to pharmacotherapy; thus, complete remission may not be expected after mere allergen elimination. 443 Other allergen avoidance measures such as environmental intervention and use of protective devices are available to reduce exposure to causative substances for maximum effectiveness, which apply to all forms of asthma aggravated by the working environment. 444 It is noteworthy that determining the individual’s relevant triggers for allergic diseases may be more difficult than diagnosing the disease itself. 439 To sum up, allergic diseases normally need drugs for treatment.
Traditional pharmacotherapy
Traditional pharmacotherapy is currently an efficient and rapid treatment method, which helps improve the symptoms of most patients and enhance their quality of life. At present, there are many kinds of drug treatments for allergic diseases, with good therapeutic effects (Table 2 ).
H1-antihistamines
H1-antihistamines, which serve as neutral receptor antagonists or inverse agonists of the histamine H1 receptor, can block the action of histamine. 445 According to brain H1 receptor occupancy (H1RO, an indicator of antihistamines), H1‐antihistamines can be divided into the non-sedating, less-sedating and sedating groups, whose diverse chemical structures, pharmacokinetic features and potential for drug-drug and drug-food interactions make them different. First-generation H1-antihistamines have not been well studied, and because of their adverse effects, particularly sedation, they should be avoided and not recommended for use. 438 New second-generation H1-antihistamines have great efficacy and safety profiles, as well as good tolerance. 446 The development of second-generation H1-antihistamines occurred in the 1980s, revolutionizing allergy therapy due to no or only minimal sedative potential, including the less-sedating oral H1-antihistamines and non-sedating H1-antihistamines. 447 However, because of cardiotoxic side effects, two early second-generation H1-antihistamines, i.e., astemizole and terfenadine, have now been withdrawn from the market. 447 , 448 Third-generation oral antihistamines have improved efficacy, safety, and pharmacological and pharmacokinetic features, representing suitable candidates for the treatment of seasonal or perennial allergies, which may improve the allergic symptoms of patients. 449
Corticosteroids
Corticosteroids were considered the most effective therapeutic approach for atopic disorders in the past, and could control virtually all cases of allergic diseases at high dose. 450 The main role of corticosteroids is to inhibit a variety of inflammatory molecules such as cytokines, pro-inflammatory proteins, adhesion molecules and inflammatory receptors, which explain their high efficacy in complex inflammatory diseases. Corticosteroids are divided into intranasal corticosteroids (INCS), topical corticosteroids (TCS) and systemic corticosteroids (SCS).
INCS are a well-established first-line therapeutic option for adults and children with persistent or moderate-to-severe symptoms, decrease inflammation associated with AR and alleviate nasal and ocular symptoms. 438 , 451 Their efficiency is more obvious than that of oral or intranasal antihistamines and antileukotrienes. In addition, INCS are comparable to the combination of antihistamines and antileukotrienes. 452 Mechanistically, INCS exert local anti-inflammatory effects on nasal mucosal cells. New-generation inhaled corticosteroids for asthma show enhanced anti-inflammatory effects with minimal adverse effects. Undoubtedly, inhaled corticosteroids (ICS) have revolutionized asthma therapy, and are currently considered the first-line therapeutic option for all chronic asthma cases.
Topical corticosteroids (TCS) induce fewer systemic side effects, especially the recently developed topical steroids that have short half-lives, and are the first-line anti-inflammatory approach in AD. 439 , 453 Studies have shown that topical corticosteroids used as adjunctive therapy alongside dupilumab may provide additional benefits. 454 Systemic corticosteroids (SCS), one of the groups of drugs available for systemic anti-inflammatory therapy, are required for AD not sufficiently controllable with adequate topical therapies and UV light therapy. Although SCS have rapid effects, their use should be limited to 1-2 weeks because of significant risk of severe long-term side effects, as observed with methylprednisolone. 439
Combination of H1-antihistamines and nasal corticosteroids
Conceptually, it is meaningful to use both nasal corticosteroids and local nasal antihistamines at the same time, because such treatment can block the main cause of direct response and reduce inflammation through significantly different mechanisms. 455 A study addressed the possibility that a combination of azelastine and fluticasone (both as nasal spray) can confer significant clinical benefits in individuals with seasonal allergic rhinitis (SAR) in comparison with any drug alone. 456 It has been shown that the nasal symptoms of SAR are more significantly relieved by the fixed-dose combination (FDC), containing an intranasal antihistamine (azelastine hydrochloride [HCl]) and a corticosteroid (fluticasone propionate) compared with placebo or monotherapy, and could also alleviate the nasal symptoms of perennial allergic rhinitis (PAR) more substantially than fluticasone monotherapy. 457
Leukotriene receptor antagonists
Leukotrienes (LTS) are indispensable for the pathogenetic mechanisms of allergic inflammation, so inhibiting LTS represents an effective and feasible strategy in the treatment of allergic diseases, including AAS, AR, and AD. Leukotriene receptor antagonist (LTRA) is an additional treatment option for asthmatics and AR cases. In addition, some leukotriene receptor antagonists are currently used clinically for exercise-induced bronchoconstriction. 458 Leukotriene is an important lipid mediator in asthma-related research. 459 Leukotriene receptor antagonists improve small airway function and reduce airway inflammation in the treatment of AAS. 460 In Europe, LTRAs have been approved by European Medicine Agency(EMA) only for the treatment of asthma and AR. 438 Studies have shown that early intervention with a 4-week anti-leukotriene course is also beneficial for some pollen allergies. 461 LTRAs are not known to cause significant congenital malformations or adverse perinatal outcomes in pregnancy safety studies. Some reports point out that LTRAs may be considered in second-line treatment of pregnant women if better treatments fail. 462
Other drugs
Other drugs can also help treat allergic diseases. Mast cells can regulate angiogenesis, tissue inflammation and repair. The cells play an important role in innate and adaptive immune response, immune tolerance, and host defense. Therefore, mast cells are essential for allergic reactions. Mast cell stabilizer such as sodium cromoglicate is targeted at mast cells, which can inhibit the degranulation of allergic mediators, thus preventing allergic diseases. The side effects of sodium cromoglicate are less, and it has been clinically approved for the treatment of asthma and AR. However, the therapeutic time window of mast cell stabilizer is relatively narrow. The patient needs to be given the drug immediately before the allergen stimulation, so that the drug can play a stable and effective role. 463 , 464 , 465
Some drugs are used for symptomatic treatment of allergic diseases. For example, most chromones can be administered as monotherapy for local symptoms. They are relatively safe drugs, but with low efficacy. 438 Theophylline, a Phosphodiesterase 4 (PDE4) inhibitor, is another drug that promotes apoptosis by reducing the anti-apoptotic protein Bcl-2, which inhibits neutrophils and eosinophils in vitro. It was also noted that theophylline inhibits reactive oxygen species accumulation by neutrophils and reduces neutrophil chemotaxis. 466 At present, there are four PDE4 inhibitors approved for treating human diseases, including AD and bronchial asthma. 467 However, considering the associated systemic adverse effects, topical administration of inhaled PDE4 inhibitors might represent a promising alternative instead of systemic utilization, although these drugs are not recommended to use before or during pregnancy. 462 , 468
Topical calcineurin inhibitors (TCIs) have substantial anti-inflammatory effects, inhibiting the biosynthesis of proinflammatory cytokines by T cells and mast cells, as well as antipruritic effects that are attributed to specific effects on skin Transient receptor potential vanilloid 1 (TRPV1) neurons. 439 TCIs are especially useful in individuals requiring long-term treatment, and two of them have been approved for topical AD therapy; nevertheless, reports assessing the application of topical calcineurin inhibitors during pregnancy are limited. 469 It is important to note that other immunosuppressants, including azathioprine and cyclosporine, do not induce congenital malformations; besides, cyclosporine is considered as first-line drug for long-term treatment of diseases. 462 , 470
Bronchodilators, which are significant for preventing and relieving bronchoconstriction, including long‐acting muscarinic antagonist (LAMA) and long‐acting beta‐agonist (LABA) addition to ICS, sometimes have adverse effects, including tremors, palpitations and tachycardia. 466 , 471 , 472
In general, H1-antihistamines are usually safe and widely used for the treatment of various allergic diseases, but some patients experience adverse effects, such as cardiotoxicity, central depression and anticholinergic effects. In addition, there are individual differences in the efficacy of antihistamines in clinical practice. 473 INCS are effective in combating nasal and ocular symptoms and improving quality of life, and are safe for short-term use, but long-term safety data are lacking. Although generally well tolerated, adverse events can be observed that may lead to serious ocular complications. 474 As for TCS and SCS, long-term continuous use of corticosteroids may result in local and systemic toxicity risks. LTRAs are well tolerated, non-hormonal anti-inflammatory drugs. LTRAs are primarily employed for long-term control treatment of patients with mild asthma and comorbid AR. It has also been shown that LTRAs are not associated with the risk of major congenital malformations and can be safely used during pregnancy to treat asthma. 475 Nevertheless, the anti-inflammatory effect of LTRAs is not as strong as that of corticosteroids, so they are often used in combination with inhaled corticosteroids to enhance their efficacy in clinical practice. However, Some experiments have shown that LTRAs and oral H1-antihistamines have comparable effects in AR. 438 In short, based on the side effects of these traditional drugs, other therapies are emerging as well, such as allergen immunotherapy (AIT) and biologics, etc.
AIT constitutes the sole disease-modifying therapy for patients with IgE-mediated inhalation allergic diseases. 476 Compared with traditional pharmacotherapy, the advantage of AIT is that it is a medical intervention that can limit the natural process of the disease. 477 The purpose of this therapy is to reduce the symptoms of allergic diseases by inducing tolerance to allergens. 438 AIT is based on the administration of allergens that cause a given disease. Through long-term repeated exposure to specified doses of allergens, it can change the immune response and induce protective immunity, so that patients may tolerate future allergen exposure. 478 , 479 In addition, this therapy reduces the long-term treatment cost for patients and the economic burden of allergy. From the perspective of public health, AIT plays a crucial role in allergy management. 477
Application of AIT in allergic diseases
In allergic diseases, immune dysfunction is the key pathogenic factor, so the concept of inducing immune tolerance has gradually become one of the goals set for preventing and treating allergic diseases. 477 In the routine use of AIT, it is recommended to take a 3-year course of treatment to achieve long-term efficacy, and long-term clinical benefits are achievable after stopping treatment. 476 , 480 In adolescents and adults, AIT can be used to treat moderate-to-severe rhinitis and moderate asthma. In children, AIT can prevent rhinitis cases from further developing asthma symptoms. 481 Researchers analyzed AR progress and changes in asthma symptoms after HDM allergen immunotherapy. The results showed that treatment of AR patients with HDM allergy drugs could indeed reduce the overall incidence rate of asthma, improve asthma symptoms and slow down the progression of asthma. 482 , 483 Many experiments have revealed that AIT markedly reduces the symptoms in patients, changes the disease process, and improves the quality of life in allergic individuals.
Therapeutic principles of AIT in allergic diseases
The tolerance induced by AIT is related to changes in allergen-specific memory T and B cells as well as allergen-specific IgE and IgG antibody amounts. In addition, AIT has some impact on the activation threshold of mast cells, basophils and dendritic cells. 438 Some cells utilize interleukin-10 (IL-10), IL-35, transforming growth factor-β (TGF-β), IL-10R, TGF-βR, cytotoxic T lymphocyte-associated antigen 4 (CTLA4), programmed cell death protein 1 (PD1) and other inhibitors to directly or indirectly alleviate the anaphylactic environment. 484 Studies have shown that AIT regulates the follicular helper T (TFH) cell-to-follicular regulatory T (TFR) cell balance in allergic cases. The decrease and functional defect of TFR cells are associated with AR. 485 Studies have also shown that AIT reduces the expression of CD23 on switched memory B cells, which has a positive correlation with the clinical efficacy of AIT in AR. 480
Biomarkers in AIT can predict or monitor immune responses to determine efficacy as early as possible
In AIT, biomarkers can reflect some clinical or laboratory characteristics of the immune process, which is essential for monitoring the health status of patients at all times. However, there is a lack of validated genetic or blood markers that could help predict or monitor the efficacy of AIT at the individual patient level. 481 In recent years, researchers have been screening for biomarkers to detect the success of AIT. Many technologies are expected to be used to examine these biomarkers, including genomics, transcriptomics, immunology, lipidomics, metabolomics, microbiology, epigenetics and proteomics. 478 According to the mechanism of anaphylaxis and the development and application of the above technologies, relatively reliable candidate biomarkers for immune detection have been reported, including immunoglobulins (allergen-specific IgD, IgE and IgG4) and some cytokines or chemokines (IL-4, IL-13, IL-9, IL-25, IL-33 and TSLP). In addition, candidate biomarkers can also include specific genes or immune-related cells such as cytokine and immunoglobin transcripts, myeloid cells, innate lymphoid cells (ILC), T cells and B cells. 478
The drug delivery route affects the quality of AIT
In AIT, different drug delivery routes show different side effects and final efficacy. Conventional AIT includes subcutaneous immunotherapy (SCIT) and sublingual immunotherapy (SLIT). 476 , 486 Traditional SCIT has certain shortcomings that cannot be ignored. It requires frequent medical treatment and multiple injections, and in some rare cases, life-threatening allergic reactions and adverse events may occur. 487 The typical local reaction in SCIT is redness and swelling at the injection site, and more serious systemic reactions include sneezing, nasal congestion and urticaria. Some serious symptoms often occur within 30 minutes, so individuals are usually allowed to leave the hospital 30 minutes after completing the injection. 481 , 488 SLIT has become a particularly attractive alternative to AIT because of its rather easy administration. It usually involves allergen drops or tablets, which can be administered at home. 489 Some new routes of AIT administration are also being developed in order to improve the safety and convenience of patients and maintain or even obtain better curative effects. 476 , 477 , 490 Oral immunotherapy (OIT) is another SCIT option. OIT seems to have no effects on most respiratory allergens because they are easily digested in the gastrointestinal tract. Therefore, OIT is mainly used for anti-digestive food allergens, including milk, eggs, peanuts and some wheat. 479 Studies have shown that OIT for IgE-mediated FA desensitizes patients who are at risk or have experienced severe allergies to peanuts, eggs and milk. 477 Intralymphatic immunotherapy (ILIT) is also another better choice for SCIT. This method involves intralymphatic administration, because lymph nodes contain a large number of immune cells, so their direct contact with allergens would be faster than in SCIT and produce stronger protective IgG antibodies and immune regulation at the same time. 479 , 491 In addition, studies have proposed epicutaneous immunotherapy (EPIT), which aims to cause fewer systemic side effects when administered through the epidermis. 479
In conclusion, the current goal of improving AIT is to shorten the treatment time, improve its efficiency in order to promote the absorption and presentation of allergens, reduce side effects to improve safety, improve patient compliance, and ultimately significantly increase the utilization of this treatment method. 492
Application of biologics in allergic diseases
New studies on allergic diseases are underway, especially the development and application of new biologics. 441 So far, many biologics targeting Th2/1/17 inflammatory biomarkers are available, many of which are clinically applied. 493 Identifying new and reliable biomarkers and clarifying novel molecular mechanisms that persist in specific reactions have become important research directions. 438 We summarized some existing biologics according to differences in the mechanisms of action and target sites, providing a certain reference for follow-up studies of biologics (Table 3 , Fig. 6 ).
Application of biologics in allergic diseases. Multiple cell interactions trigger allergic reactions. Therefore, treatment with biologics aims to target the cytokines produced by various cells, with a potential impact on the interaction between cells. Some biologics exert their effects by targeting IgE, IL-5, IL-4, IL-13, IL-31, IL-9, IL-33, and TSLP, among others
Targeting IgE
Targeting IgE molecules is one of the most important methods for nipping allergic reactions in the body with lasting effects. Anti-IgE antibody treatment markedly reduces the serum amounts of free IgE molecules in allergic individuals, thus exerting a certain effect. 494 Recombinant humanized anti-IgE antibodies, such as omalizumab, have been approved by the FDA for severe asthma, substantially improving clinical symptoms in patients with poor disease control. 441 , 495
Targeting IL-5
Eosinophils are critical in asthma pathogenesis, and airway eosinophil inflammation is related to the severity of asthma. Biologics, including mepolizumab, reslizumab and benralizumab, have been developed to target IL-5 or IL-5Rα and then affect the survival and differentiation of eosinophils. Studies have shown that blocking the IL-5 receptor could indeed alleviate severe eosinophilic asthma and severe uncontrolled asthma. 496 , 497 , 498
Targeting IL-4/IL-13
IL-4 and IL-13 represent key driving factors in type 2 immune diseases. For example, dupilumab was approved by the FDA and the EMA (EU) for adults with moderate-to-severe AD. Dupilumab is a fully humanized antibody that targets IL-4Rα subunits, thus inhibiting the IL-4/IL-13 axis. These two cytokines induce IgE production in B cells, goblet cell metaplasia, mucus production, basement membrane thickening and fibrosis. A recent experiment showed that blocking the IL-4/IL-13 pathway alleviates glucocorticoid-dependent severe asthma, moderate-to-severe uncontrolled asthma and AD. Biologics also significantly alleviate AR symptoms, especially nasal symptoms. 441 , 496 , 497 , 499 Many biologics have been developed to target IL-4 and IL-13, inhibiting the dimerization of IL-13Rα1 and IL-4Rα or directly targeting the IL-4Rα subunits to play a role. 499 , 500 , 501 , 502 More and more biologics that block IL-4 and IL-13 activities have been shown to alleviate nasal symptoms in patients with uncontrolled asthma and AR. 503
Targeting IL-31
Monoclonal antibodies, including nemolizumab, a humanized monoclonal antibody targeting IL-31 receptor A (IL-31RA), relieve itching symptoms and IL-31 signal transduction in the pathogenesis of AD. In mice, IL-31 and its receptor IL-31RA are involved in AD-induced pruritus. In another animal experiment, the monkey scratch model, it was found that the scratch induced by IL-31 is significantly alleviated after a single injection of nemolizumab. 501 , 504
Targeted signaling pathways
Some biologics target signaling pathways, including baricitinib, tofacitinib, upadacitinib and ruxolitinib, which target JAK/STAT signaling. Targeting JAK/STAT signaling is critical for T cell activation, and could also block the downstream pathways of many important cytokine receptors. T cells are essential for atopic inflammation. The JAK protein in cells activates STAT protein dimerization and transfer to the nucleus, thereby increasing the gene expression of inflammatory mediators. Therefore, JAK/STAT pathway suppression represents an effective and feasible therapeutic approach in AD. 500 , 501 , 505 Asthma is often accompanied by inflammation, and many pro-inflammatory chemokines, cytokines, adhesion molecules, airway mucins, growth and angiogenesis factors are upregulated through Rel/Nuclear Factor-κB (NF-κB) transcription factor family. 218 , 506 So asthma and NF-κB mediated signal transduction is inextricably linked, and a series of NF-κB signaling intermediate inhibitors have been produced, e.g., DNA oligonucleotides and DNA-peptide molecules acting as NF-κB bait sequences; in addition, small molecule inhibitors and some proteasome inhibitors affect NF-κB signal transduction. 218 For example, small molecule inhibitors such as TPCA-1 and AS602868 inhibit IκB kinase (IKK)-β to block NF-κB signaling. In addition, proteasome inhibitors can block NF-κB signal transduction by targeting the 20 S proteasome, ultimately regulating eosinophil function. 218 , 507 Some biologics regulate the signal cascade by inhibiting PI3K or any downstream target, because PI3K-mediated signals pass through IKKα phosphorylation, and protein kinase B (PKB, AKT) phosphorylation directly activates IKK, which then enters NF-κB signal cascade reaction pathway. For example, idelalisib, alpelisib, copanlisib and duvelisib are PI3K inhibitors. 508 The mechanistic target of rapamycin (mTOR) pathway is also considered a signaling pathway regulating innate and adaptive immune cells. It was found that rapamycin targeting mTOR inhibits eosinophil differentiation and reduces allergic airway inflammation in mouse models. 505 , 509 Notch signaling is also associated with the pathogenesis of allergic airway inflammation. Notch is necessary to maintain Th1 and Th2 programs. As a biological agent, stapled α-helical peptide derived from mastermind-like 1 (SAHM1) targets Notch to effectively reduce the inflammatory symptoms of mice with experimental allergic airway inflammation and accelerate recovery. In addition to being related to T cells, Notch also is critical for the differentiation of lung organs and alveoli. For example, in a mouse asthma model, intranasal administration of γ-secretase inhibitors (GSIs) could block the Notch signaling pathway and reduce allergic pulmonary edema. 510 Avasimibe (Ava), as a specifically targets acetyl-CoA acetyltransferase 1 (ACAT1) inhibitor, has been proven to alleviate the disruption of the airway epithelial barrier by inhibiting the Wnt/β-catenin signaling pathway. It has good safety and may be a promising drug for the clinical treatment of AAS. 511
Other biologics
Currently, the use of new therapeutic targets is also being explored. For example, Toll-like receptors are involved in the activation of innate immunity in the respiratory mucosa. Some agonists can induce cytokines, including TNF-α, IL-6 and IFN-α; such biologics are well tolerated and may not cause systemic immune activation. 512 , 513 In the study of AAS, due to its complex pathogenesis, certain new biological targets have attracted attention from researchers, e.g., epithelial cell-derived cytokines (IL-1, IL-33, IL-25 and TSLP). 496 , 503 , 514 , 515 In addition, IL-9 is also associated with allergic diseases. In the study of asthma, mouse models overexpressing IL-9 show enhanced eosinophilic airway inflammation, IgE production and mast cell proliferation. Therefore, biologics targeting IL-9 have also been designed. 516 There are also antibodies that target the costimulatory molecule OX40 (CD134), which is critical for T cell expansion. 500
Chemoattractant receptor-homologous molecule expressed on TH2 cells (CRTH2), also known as prostaglandin D2 receptor 2, is a 7-transmembrane G protein-coupled receptor expressed by Th2 cells, eosinophils and basophils. The CRTH2 receptors are involved in inducing the migration and activation of Th2 lymphocytes, eosinophils and basophils. At the same time, it is also involved in the up regulation of adhesion molecules and the release of proinflammatory Th2 cytokines such as IL-4, IL-5 and IL-13. Therefore, it plays a certain role in the course of allergic diseases. It has been proved that the number of CRTH2-positive cells is related to the severity of asthma. At present, some CRTH2 antagonists, such as AMG853, OC000459 and BI671800, have been developed to treat asthma, AD and AR. These CRTH2 inhibitors reduce the Th2-mediated inflammatory response by blocking the activation of mast cells, basophils and eosinophils. 189 , 517 , 518
Nitric oxide (NO) is an important signal molecule in many physiological processes, such as maintenance of vascular tone and endothelial barrier function, immune defense and apoptosis. NO can also regulate cell function through post-translational modification of proteins. NO can react with glutathione to form S-nitrosoglutathione (GSNO), which effectively transduces NO signal. GSNO concentration can be regulated by GSNO-reductase (GSNOR), which provides the “brake” for signal transduction. There is evidence that GSNOR polymorphisms increase the expression of GSNOR and are associated with an increased risk of asthma. Inhibition of GSNOR can lead to the preservation of endogenous GSNO and limit eosinophilic inflammation, mucus production and airway hyper reactivity (AHR). N6022 is the first small molecule inhibitor of GSNOR in the treatment of asthma. Some studies have shown that in the mouse asthma model, N6022 significantly reduces airway hyperreactivity and shows a strong anti-inflammatory effect. 519 , 520 , 521
Biologics-targeted therapies target the endotypes of allergic diseases based on pathogenesis, reducing the occurrence of adverse events and improving the efficiency of treatment, and are considered promising therapeutic approaches. However, many biologics have not been developed far enough and have not been better evaluated. The response to treatment varies greatly from individual to individual. Therefore, the dose and duration of treatment need to be better defined and the details need to be further optimized. In addition, the relatively expensive price of biologics also limits their application to some extent. 522 , 523 , 524
Other therapies
Different treatment options have varying degrees of side effects; thus, a growing number of alternatives therapies have also been developed. In addition to the aforementioned treatment methods, other therapies also offer different therapeutic effects in the treatment of allergic diseases, including antibacterial and antimycotic therapies, phototherapy, early introduction therapy, circadian regulation therapy and so on.
The microbiota and allergic diseases are closely related. For example, Staphylococcus aureus is the main cause of AD, and Malassezia furfur is also associated with skin immune response and barrier function. Therefore, antibacterial and antimycotic therapies have also become an option for treating allergic diseases. 439 Phototherapy has immunosuppressive and immunomodulatory effects, which can inhibit the effects of anaphylaxis and histamine release triggered by mast cell antigens. Therefore, rhinophototherapy is considered a promising non-invasive alternative treatment option for perennial or seasonal AR cases, especially low-level laser therapy (LLLT). 525 , 526 However, it has been demonstrated that photochemotherapy has certain carcinogenicity. In addition, in AD, oral psoralen plus ultraviolet A (PUVA) as a type of phototherapy also has many side effects. Therefore, PUVA for AD treatment has been abandoned to a large extent, and it is recommended to apply combination therapy. 439 In patients with FA, early consumption is more beneficial than delayed administration. A trial showed that early introduction of eggs combined with appropriate eczema treatment is practical, and may effectively reduce the odds of egg allergy in high-risk infants. 527 One of the important research fields to further examine new methods to prevent or treat modern allergic diseases is to understand the relationship between circadian biology and allergy; correspondingly, it is suggested to develop a lifestyle in which the endogenous biological clock is consistent with the environmental cycle, or undergo appropriate therapeutic interventions to prevent or treat allergic disease in the future. 528
Bousquet, J., Van Cauwenberge, P., Khaltaev, N., Aria Workshop, G. & World Health, O. Allergic rhinitis and its impact on asthma. J. Allergy Clin. Immunol. 108 , S147–S334 (2001).
Article CAS PubMed Google Scholar
Meghji, J., Mortimer, K., Jayasooriya, S. & Marks, G. B. Lung health in LMICs: tackling challenges ahead – Authors’ reply. Lancet 398 , 490 (2021).
Article PubMed Google Scholar
Kattan, J. D. & Wang, J. Allergen component testing for food allergy: ready for prime time? Curr. Allergy Asthma Rep. 13 , 58–63 (2013).
Article CAS PubMed PubMed Central Google Scholar
Tsoi, L. C. et al. Progression of acute-to-chronic atopic dermatitis is associated with quantitative rather than qualitative changes in cytokine responses. J. Allergy Clin. Immunol. 145 , 1406–1415 (2020).
Lloyd-Lavery, A. et al. What’s new in atopic eczema? An analysis of systematic reviews published in 2016. Part 2: epidemiology, aetiology and risk factors. Clin. Exp. Dermatol 44 , 370–375 (2019).
Weidinger, S., Beck, L. A., Bieber, T., Kabashima, K. & Irvine, A. D. Atopic dermatitis. Nat. Rev. Dis. Prim. 4 , 1 (2018).
Dong, W. L. et al. The prevalence and year lived with disability of atopic dermatitis in China: Findings from the global burden of disease study 2019. World Allergy Organ J. 14 , 100604 (2021).
Article PubMed PubMed Central Google Scholar
Small, P. et al. In J. Otolaryngol . S5-S27 (2007).
Dykewicz, M. S. & Hamilos, D. L. Rhinitis and sinusitis. J. Allergy Clin. Immunol. 125 , S103–S115 (2010).
Fahy, J. V. Type 2 inflammation in asthma–present in most, absent in many. Nat. Rev. Immunol. 15 , 57–65 (2015).
Mims, J. W. Asthma: definitions and pathophysiology. Int Forum Allergy Rhinol. 5 (Suppl 1), S2–S6 (2015).
Feld, M. et al. The pruritus- and TH2-associated cytokine IL-31 promotes growth of sensory nerves. J. Allergy Clin. Immunol. 138 , 500–508 e524 (2016).
Wilson, S. R. et al. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell 155 , 285–295 (2013).
Chinthrajah, R. S. et al. Diagnosis of food allergy. Pediatr. Clin. North Am. 62 , 1393–1408 (2015).
Deschildre, A. & Lejeune, S. How to cope with food allergy symptoms? Curr. Opin. Allergy Clin. Immunol. 18 , 234–242 (2018).
Chinthrajah, R. S., Hernandez, J. D., Boyd, S. D., Galli, S. J. & Nadeau, K. C. Molecular and cellular mechanisms of food allergy and food tolerance. J. Allergy Clin. Immunol. 137 , 984–997 (2016).
Krombach, J. W., Kampe, S., Keller, C. A. & Wright, P. M. Pharaoh Menes’ death after an anaphylactic reaction–the end of a myth. Allergy 59 , 1234–1235 (2004).
Ramachandran, M. & Aronson, J. K. John Bostock’s first description of hayfever. J. R. Soc. Med. 104 , 237–240 (2011).
Bjermer, L. History and future perspectives of treating asthma as a systemic and small airways disease. Respir. Med. 95 , 703–719 (2001).
Huber, B. [100 years of allergy: Clemens von Pirquet - his idea of allergy and its immanent concept of disease]. Wien. Klin. Wochenschr. 118 , 573–579 (2006).
German, A. I. [Munchener Medizinische Wochenschrift/24 July 1906: Allergy by Clemens v. Pirquet, Vienna]. MMW Munch. Med Wochenschr. 120 , 474 (1978).
Google Scholar
Noon, L. Prophylactic inoculation against hay fever. Int Arch. Allergy Appl Immunol. 4 , 285–288 (1953).
de Herder, W. W. Heroes in endocrinology: Nobel Prizes. Endocr. Connect 3 , R94–R104 (2014).
WB, S. The uses and abuses of antihistamine drugs. Bull. N. Y Acad. Med. 27 , 309–324 (1951).
Knowles, S. & Shear, N. H. Antihistamines. Clin. Dermatol. 7 , 48–59 (1989).
Riley, J. F. Editorial: heparin, histamine and mast cells. Blood 9 , 1123–1126 (1954).
Ishizaka, K. & I, T. Biological function of gamma E antibodies and mechanisms of reaginic hypersensitivity. Clin. Exp. Immunol. 6 , 25–42 (1970).
CAS PubMed PubMed Central Google Scholar
DP, S. Hay fever, hygiene, and household size. BMJ 299 , 1259–1260 (1989).
Article Google Scholar
Larsen, J. N., Broge, L. & Jacobi, H. Allergy immunotherapy: the future of allergy treatment. Drug Disco. Today 21 , 26–37 (2016).
Article CAS Google Scholar
Bousquet J, L. R. & Malling, H. J. Allergen immunotherapy: therapeutic vaccines for allergic diseases. A WHO position paper. J. Allergy Clin. Immunol. 102 , 558–562 (1998).
Agache, I. & Akdis, C. A. Precision medicine and phenotypes, endotypes, genotypes, regiotypes, and theratypes of allergic diseases. J. Clin. Investig. 130 (2019).
Palmer, L. J., C. W. In Analysis of multifactorial disease 22 (2000).
Agache, I. et al. Emerging concepts and challenges in implementing the exposome paradigm in allergic diseases and asthma: a Practall document. Allergy (2019).
Potaczek, D. P. et al. Epigenetics and allergy: from basic mechanisms to clinical applications. Epigenomics 9 , 539–571 (2017).
Tost, J. O. & France clinical reviews in allergy and immunology a translational perspective on epigenetics in allergic diseases. J. Allergy Clin. Immunol. 142 , 715–726 (2018).
Sachidanandam, R., Weissman, D., Schmidt, S. C., Kakol, J. M. & Willey, D. L. A map of human genome sequence variation containing 1.42 million single nucleotide polymorphisms. Nature 409 , 928–933 (2001).
Schork, N. J., Fallin, D. & Lanchbury, J. S. Single nucleotide polymorphisms and the future of genetic epidemiology. Clin. Genet. 58 , 250–264 (2010).
MarkSonkn Single nucleotide polymorphisms: from the evolutionary past. Nature (2001).
Demirdag, Y. & Bahna, S. The role of genetics in food allergy. Expert Rev. Clin. Immunol. 18 , 401–411 (2022).
Sicherer, S. H. et al. Genetics of peanut allergy: a twin study. J. Allergy Clin. Immunol. 106 , 53–56 (2000).
Liu, X., Zhang, S., Tsai, H. J., Hong, X. & Wang, X. Genetic and environmental contributions to allergen sensitization in a Chinese twin study. Clin. Exp. Allergy 39 , 991–998 (2010).
Ober, C. & Yao, T. C. The genetics of asthma and allergic disease: a 21st century perspective. Immunol. Rev. 242 , 10–30 (2011).
Raby, B. A. Asthma severity, nature or nurture: genetic determinants. Curr. Opin. Pediatr. 31 , 1 (2019).
Choi, B. Y., Han, M., Kwak, J. W. & Kim, T. H. Genetics and Epigenetics in Allergic Rhinitis. (2021).
Yazd, N. K. K., Patel, R. R., Dellavalle, R. P. & Dunnick, C. A. Genetic risk factors for development of atopic dermatitis: a systematic review. Curr. Dermatol. Rep. 6 , 297–308 (2017).
Brown, S. J., Elias, M. S. & Bradley, M. Genetics in atopic dermatitis: historical perspective and future prospects. Acta Derm. Venereol. 100 , adv00163 (2020).
Schoettler, N. & Ober, C. Genetic architecture of moderate-to-severe asthma mirrors that of mild asthma. J. Allergy Clin. Immunol. 144 , 1521–1523 (2019).
Agache, I., Akdis, C., Jutel, M. & Virchow, J. C. Untangling asthma phenotypes and endotypes. Allergy 67 , 835–846 (2012).
Andiappan, A. K., Yang, Y. S., Lee, B., Suri, B. K. & Chew, F. T. Functional variants of 17q12-21 are associated with allergic asthma but not allergic rhinitis. J. allergy Clin. Immunol. 137 , 758–766.e753 (2015).
Beghé, B., Barton, S., Rorke, S., Peng, Q. & Holloway, J. W. Polymorphisms in the interleukin-4 and interleukin-4 receptor α chain genes confer susceptibility to asthma and atopy in a Caucasian population. Clin. Exp. Allergy 33 , 1111–1117 (2003).
Bønnelykke, K. et al. A genome-wide association study identifies CDHR3 as a susceptibility locus for early childhood asthma with severe exacerbations. Nat. Genet. 46 , 51–55 (2014).
Jiang, F. & Yan, A. IL-4 rs2243250 polymorphism associated with susceptibility to allergic rhinitis: a meta-analysis. Biosci. Rep. 41 , BSR20210522 (2021).
Zhang, W. & Xu, Y. Association between vitamin D receptor gene polymorphism rs2228570 and allergic rhinitis. Pharmacogenomics Pers. Med. ume 13 , 327–335 (2020).
Bonnelykke, K. et al. Meta-analysis of genome-wide association studies identifies ten loci influencing allergic sensitization. Nat. Genet. 45 , 902–906 (2013).
Ashley, S. E. et al. Genetic variation at the Th2 immune gene IL13 is associated with IgE‐mediated paediatric food allergy. Clin. Exp. Allergy (2017).
Marenholz, I. et al. Genome-wide association study identifies the SERPINB gene cluster as a susceptibility locus for food allergy. Nat. Commun. 8 , 1056 (2017).
Waage, J. et al. Genome-wide association and HLA fine-mapping studies identify risk loci and genetic pathways underlying allergic rhinitis. Nat. Genet. 50 , 1072–1080 (2018).
Consortium, T. E. G. A. Multi-ancestry genome-wide association study of 21,000 cases and 95,000 controls identifies new risk loci for atopic dermatitis. Nat. Genet. advance online publication (2015).
Gudbjartsson, D. F. et al. Sequence variants affecting eosinophil numbers associate with asthma and myocardial infarction. Nat. Genet. 41 , 342–347 (2009).
Mori, T. et al. Lnk/Sh2b3 controls the production and function of dendritic cells and regulates the induction of IFN-gamma-producing T cells. J. Immunol. 193 , 1728–1736 (2014).
León, B. et al. Regulation of T(H)2 development by CXCR5+ dendritic cells and lymphotoxin-expressing B cells. Nat. Immunol. 13 , 681–690 (2013).
Lawrence, T. The nuclear factor NF-κB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 1 , a001651 (2009).
Shinnakasu, R. et al. Regulated selection of germinal-center cells into the memory B cell compartment. Nat. Immunol. (2016).
Roychoudhuri, R. et al. BACH2 regulates CD8(+) T cell differentiation by controlling access of AP-1 factors to enhancers. Nat. Immunol .
Rothlin, C. TAM receptors and pleiotropic inhibitors of the innate immune response. Cell 131 (2007).
Kassmeier, M. D. et al. VprBP binds full-length RAG1 and is required for B-cell development and V(D)J recombination fidelity. EMBO J. 31 , 945–958 (2014).
Hamblet, C. E., Makowski, S. L., Tritapoe, J. M. & Pomerantz, J. L. NK cell maturation and cytotoxicity are controlled by the intramembrane aspartyl protease SPPL3. J. Immunol. 196 , 2614 (2016).
Anderson, D. M., Maraskovsky, E., Billingsley, W. L., Dougall, W. C. & Galibert, L. A. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature 390 , 175–179 (1997).
Xin, W., Yuan, Z., Zheng, F. & Luo, Z. The association between polymorphisms in the MRPL4 and TNF-α genes and susceptibility to allergic rhinitis. Plos One 8 , e57981 (2013).
Oliveti, J. F., Kercsmar, C. M. & Susan, R. Pre- and perinatal risk factors for asthma in inner city African-American Children. Am. J. Epidemiol. , 570.
Litonjua, A. A., Carey, V. J. & Burge, H. A. Parental history and the risk for childhood asthma. Does mother confer more risk than father?. Am. J. Respir. Crit. Care Med. 158 , 176–181 (1998).
Maternal history, sensitization to allergens, and current wheezing, rhinitis, and eczema among children in Costa Rica. Pediat. Pulmonol. 33 (2002).
Raby, B. A. et al. A common mitochondrial haplogroup is associated with elevated total serum IgE levels. J. Allergy Clin. Immunol. 120 , 351–358 (2007).
Jang, H., Kim, M., Hong, J. Y., Cho, H. J. & Kim, K. W. Mitochondrial and nuclear mitochondrial variants in allergic diseases. Allergy Asthma Immunol. Res. 12 , 877 (2020).
Flaquer, A., Heinzmann, A., Rospleszcz, S., Mailaparambil, B. & Grychtol, R. Association study of mitochondrial genetic polymorphisms in asthmatic children. Mitochondrion 14 (2014).
Zifa, E. et al. Mitochondrial genetic background plays a role in increasing risk to asthma. Mol. Biol. Rep. 39 , 4697–4708 (2012).
Qian, L., Mehrabi, N. E., Athari, S. M. & Athari, S. S. Mitochondria signaling pathways in allergic asthma. J. Investig. Med 70 , 863–882 (2022).
Polonikov, A. V. et al. Polymorphism -930A > G of the cytochrome b gene is a novel genetic marker of predisposition to bronchial asthma. Ter. Arkh 81 , 5–31 (2009).
Fukuda, T., Mochida, S., Fukushima, Y. & Makino, S. Detection of allergen-induced genes in peripheral blood mononuclear cells of patients with allergic asthma using subtractive hybridization. J. Allergy Clin. Immunol. 96 , 1076–1082 (1995).
Xu, Y. D. et al. The early asthmatic response is associated with glycolysis, calcium binding and mitochondria activity as revealed by proteomic analysis in rats. Respir. Res. 11 , 107 (2010).
Schauberger, E. M. et al. Identification of ATPAF1 as a novel candidate gene for asthma in children. J. Allergy Clin. Immunol. 128 , 753–760.e711 (2011).
Raby, B. A. et al. Importin-13 genetic variation is associated with improved airway responsiveness in childhood asthma. Respir. Res. 10 , 67 (2009).
Silverman, E. K. et al. Family-based association analysis of beta2-adrenergic receptor polymorphisms in the childhood asthma management program. J. Allergy Clin. Immunol. 112 , 870–876 (2003).
Reddy, P. H. Mitochondrial dysfunction and oxidative stress in asthma: implications for mitochondria-targeted antioxidant therapeutics. Pharmaceuticals 4 , 429 (2011).
Devries, A. & Vercelli, D. Epigenetic mechanisms in asthma. Ann. Am. Thorac. Soc. 13 (Suppl 1), S48 (2016).
Moen, E. L. et al. New themes in the biological functions of 5‐methylcytosine and 5‐hydroxymethylcytosine. Immunol. Rev. 263 (2015).
Neidhart, M. DNA methylation and complex human disease (Oxford: Academic Press, 2016).
Zemach, A., McDaniel, I. E., Silva, P. & Zilberman, D. Genome-Wide Evolutionary Analysis of Eukaryotic DNA Methylation. Science 328 , 916–919 (2010).
Jones, P. A. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat. Rev. Genet. 13 , 484–492 (2012).
Xu, C. J., Gruzieva, O., Qi, C., Esplugues, A. & Koppelman, G. H. Shared DNA methylation signatures in childhood allergy: the MeDALL study. J. Allergy Clin. Immunol. (2020).
Luo, Y., Zhou, B., Zhao, M., Tang, J. & Lu, Q. Promoter demethylation contributes to TSLP overexpression in skin lesions of patients with atopic dermatitis. Clin. Exp. Dermatol. 39 , 48–53 (2013).
Harb, H. & Renz, H. Update on epigenetics in allergic disease. J. Allergy Clin. Immunol. 135 , 15–24 (2015).
Botchkarev, V. A., Gdula, M. R., Mardaryev, A. N., Sharov, A. A. & Fessing, M. Y. Epigenetic regulation of gene expression in keratinocytes. J. Investig. Dermatol. 132 , 2505–2521 (2012).
Nedoszytko, B. et al. The role of regulatory T cells and genes involved in their differentiation in pathogenesis of selected inflammatory and neoplastic skin diseases. Part III: polymorphisms of genes involved in Tregs’ activation and function. Postepy Dermatol. Allergol. 34 , 517–525 (2017).
Kehrmann, J. et al. Impact of 5-aza-2’-deoxycytidine and epigallocatechin-3-gallate for induction of human regulatory T cells. Immunology 142 (2014).
Boorgula, M. P. et al. Replicated methylation changes associated with eczema herpeticum and allergic response. Clin. Epigenetics 11 , 122 (2019).
Schmidt, A. D. & Strong, C. D. G. Current understanding of epigenetics in atopic dermatitis. Exp. Dermatol. 30 (2021).
Ziyab, A. H., Karmaus, W., Holloway, J. W., Zhang, H. & Arshad, S. H. DNA methylation of the filaggrin gene adds to the risk of eczema associated with loss-of-function variants. J. Eur. Acad. Dermatol. Venereol. 27 , e420–e423 (2013).
Nicodemus-Johnson, J. et al. DNA methylation in lung cells is associated with asthma endotypes and genetic risk. Jci Insight 1 (2016).
Moussette, S., Tuwaijri, A. A., Kohan-Ghadr, H. R., Elzein, S. & Naumova, A. K. Role of DNA methylation in expression control of the IKZF3-GSDMA region in human epithelial cells. Plos ONE 12 , e0172707 (2017).
Stefanowicz, D. et al. DNA methylation profiles of airway epithelial cells and PBMCs from healthy, atopic and asthmatic children. PLoS ONE 7 (2012).
Hong et al. Nasal DNA methylation is associated with childhood asthma.
Yang, I. V. et al. The nasal methylome and childhood atopic asthma. J. Allergy Clin. Immunol. (2017).
Ivana et al. DNA methylation and childhood asthma in the inner city. J. Allergy Clin. Immunol. (2015).
Gunawardhana, L. P. et al. Characteristic DNA methylation profiles in peripheral blood monocytes are associated with inflammatory phenotypes of asthma. Epigenetics 9 , 1302–1316 (2014).
Nicodemus-Johnson, J. et al. Genome-wide methylation study identifies an il-13-induced epigenetic signature in asthmatic airways. Am. J. Respir. Crit. Care Med. 193 , 376–385 (2016).
Scott, R. R. et al. Asthma discordance in twins is linked to epigenetic modifications of T cells. PLoS ONE 7 , e48796 (2012).
Arathimos, R. et al. Epigenome-wide association study of asthma and wheeze in childhood and adolescence. Clin. Epigenetics 9 , 112 (2017).
Bayrak Degirmenci, P. et al. Allergic Rhinitis and Its Relationship with IL-10, IL-17, TGF-β, IFN-γ, IL 22, and IL-35. Dis. Markers 2018 , 9131432 (2018).
Li, J. Y. et al. Association between DNA hypomethylation at IL13 gene and allergic rhinitis in house dust mite‐sensitized subjects. Clin. Exp. Allergy 46 (2016).
Sarnowski, C. et al. DNA methylation within melatonin receptor 1 A (MTNR1A) mediates paternally transmitted genetic variant effect on asthma plus rhinitis. J. Allergy Clin. Immunol. , 748-753 (2016).
Petrus, N. C. M. et al. Cow’s milk allergy in Dutch children: an epigenetic pilot survey. Clin. Transl. Allergy 6 (2016).
Song, Y. et al. Maternal allergy increases susceptibility to offspring allergy in association with TH2-biased epigenetic alterations in a mouse model of peanut allergy. J. Allergy Clin. Immunol. (2014).
Zhou, X., Han, X., Lyu, S. C., Bunning, B. J. & Nadeau, K. C. Targeted DNA methylation profiling reveals epigenetic signatures in peanut allergy. JCI insight 6 , 143058.
Salam, M. T. Asthma epigenetics. Adv. Exp. Med. Biol. 795 , 183 (2014).
Bilal, A. A. et al. Histone modifications and their role in epigenetics of atopy and allergic diseases. Allergy Asthma Clin. Immunol. 14 , 39 (2018).
Angiolilli et al. The acetyl code in rheumatoid arthritis and other rheumatic diseases.
Kaniskan, H. Ü., Martini, M. L. & Jin, J. Inhibitors of Protein Methyltransferases and Demethylases. Chem. Rev. (2018).
Stefanowicz, D. et al. Elevated H3K18 acetylation in airway epithelial cells of asthmatic subjects. Respir. Res. 16 , 95 (2015).
Kabesch, M. & Adcock, I. M. Epigenetics in asthma and COPD. Biochimie 94 , 2231–2241 (2012).
Harb, H. et al. Childhood allergic asthma is associated with increased IL-13 and FOXP3 histone acetylation. J. Allergy Clin. Immunol. (2015).
Clifford, R. L., Patel, J. K., John, A. E., Tatler, A. L. & Knox, A. J. CXCL8 Histone H3 acetylation is dysfunctional in airway smooth muscle in asthma: regulation by BET. AJP Lung Cell. Mol. Physiol. 308 , ajplung.00021.02015 (2015).
Brook, P. O., Perry, M. M., Adcock, I. M. & Durham, A. L. Epigenome-modifying tools in asthma. Epigenomics 7 , 1017–1032 (2015).
Fields, P. E., Kim, S. T. & Flavell, R. A. Cutting edge: changes in histone acetylation at the IL-4 and IFN-γ loci accompany Th1/Th2 differentiation. J. Immunol. 169 , 647–650 (2002).
Wang, Y., Lv, L., Zang, H., Gao, Z. & Zhou, X. Regulation of Trek1 expression in nasal mucosa with allergic rhinitis by specific immunotherapy. Cell Biochem. Funct. 33 , 23–28 (2015).
Cho, J. S., Kang, J. H., Han, I. H., Um, J. Y. & Lee, H. M. Antiallergic effects of trichostatin A in a murine model of allergic rhinitis. Clin. Exp. Otorhinolaryngol. 8 , 243–249 (2015).
Zhu, H., Shan, L., Schiller, P.W., Mai, A. & Peng, T. Histone deacetylase-3 activation promotes tumor necrosis factor-α (TNF-α) expression in cardiomyocytes during Lipopolysaccharide stimulation. J. Biol. Chem. (2010).
Alashkar Alhamwe, B. et al. Decreased histone acetylation levels at Th1 and regulatory loci after induction of food allergy. Nutrients 12 (2020).
Karam, R. A. & Elrahman, D. A. Differential expression of miR-155 and Let-7a in the plasma of childhood asthma: potential biomarkers for diagnosis and severity. Clin. Biochem. (2019).
Tian, M. et al. Changes in circulating microRNA-126 levels are associated with immune imbalance in children with acute asthma. Int J. Immunopathol. Pharm. 32 , 2058738418779243 (2018).
Ye, L., Yan, M., Jian, W. & Jin, M. L. Effects of microRNA-19b on airway remodeling, airway inflammation and degree of oxidative stress by targeting TSLP through the Stat3 signaling pathway in a mouse model of asthma. Oncotarget 8 , 47533–47546 (2017).
Lou, L., Tian, M., Chang, J., Li, F. & Zhang, G. MiRNA-192-5p attenuates airway remodeling and autophagy in asthma by targeting MMP-16 and ATG7. Biomed. Pharmacother. 122 , 109692 (2019).
Perry, M. M., Baker, J. E., Gibeon, D. S., Adcock, I. M. & Chung, K. F. Airway smooth muscle hyperproliferation is regulated by microRNA-221 in severe asthma. Am. J. Respir. Cell. Mol. Biol. , 7–17 (2014).
Lin, J., Feng, X. & Zhang, J. Circular RNA circHIPK3 modulates the proliferation of airway smooth muscle cells by miR-326/STIM1 axis. Life Sci. 255 , 117835 (2020).
Shi, J. et al. miR‐142‐5p and miR‐130a‐3p regulate pulmonary macrophage polarization and asthma airway remodeling. Immunol. Cell Biol. 98 (2020).
Malmh, Ll, C. et al. MicroRNA-155 is essential for TH2-mediated allergen-induced eosinophilic inflammation in the lung. J. Allergy Clin. Immunol. 133 , 1429–1438.e1427 (2014).
Nakano, T. et al. Lower levels of hsa-mir-15a, which decreases VEGFA, in the CD4+ T cells of pediatric patients with asthma. J. Allergy Clin. Immunol. 132 , 1224–1227.e1212 (2013).
Lu, T. X. et al. MicroRNA-21 limits in vivo immune response-mediated activation of the IL-12/IFN-gamma pathway, Th1 polarization, and the severity of delayed-type hypersensitivity. J. Immunol. 187 , 3362–3373 (2011).
Kan, Z. et al. Decreased epithelial and sputum miR-221-3p associates with airway eosinophilic inflammation and CXCL17 expression in asthma. AJP Lung Cell. Mol. Physiol. 315 , ajplung.00567.02017- (2018).
Tsitsiou, E. et al. Transcriptome analysis shows activation of circulating CD8+ T cells in patients with severe asthma. J. Allergy Clin. Immunol. 129 , 95–103 (2012).
Tm, A. et al. Asthma inflammatory phenotypes show differential microRNA expression in sputum - ScienceDirect. J. Allergy Clin. Immunol. 137 , 1433–1446 (2016).
Collison, A., Herbert, C., Siegle, J. S., Mattes, J. & Kumar, R. K. Altered expression of microRNA in the airway wall in chronic asthma: miR-126 as a potential therapeutic target. BMC Pulm. Med. 11 , 29 (2011).
Cho, S. et al. miR-23 ∼ 27 ∼ 24 clusters control effector T cell differentiation and function. J. Exp. Med. (2016).
Ghosh, J. A. & Ulaganathan Resveratrol attenuates experimental allergic asthma in mice by restoring inositol polyphosphate 4 phosphatase (INPP4A). Int. Immunopharmacol. (2012).
Xia, H., Li, Y. & Lv, X. MicroRNA-107 inhibits tumor growth and metastasis by targeting the BDNF-mediated PI3K/AKT pathway in human non-small lung cancer. Int J. Oncol. 49 , 1325–1333 (2016).
Lin, Y. J. et al. Tumor hypoxia regulates Forkhead Box C1 to promote lung cancer progression. Theranostics 7 , 1177–1191 (2017).
Zhang, X. et al. The role of miR-29c/B7-H3 axis in children with allergic asthma. J. Transl. Med. 16 , 218 (2018).
Wu, X. et al. Aberrant expressions of circulating lncRNA NEAT1 and microRNA-125a are linked with Th2 cells and symptom severity in pediatric allergic rhinitis. J. Clin. Lab Anal. 36 , e24235 (2022).
Wen, C. L., Sundaram, G. M., Shan, Q., Guang, L. G. & Sampath, P. Belinostat resolves skin barrier defects in atopic dermatitis by targeting the dysregulated miR-335:SOX6 axis. J. Allergy Clin. Immunol. 146 (2020).
O’Connell, R. M., Rao, D. S. & Baltimore, D. microRNA Regulation of Inflammatory Responses. Annu. Rev. Immunol. 30 , 295–312 (2011).
Boldin, M. P. & Baltimore, D. MicroRNAs, new effectors and regulators of NF-κB. Immunol. Rev. 246 , 205–220 (2012).
Yang, Z. et al. MicroRNA-124 alleviates chronic skin inflammation in atopic eczema via suppressing innate immune responses in keratinocytes. Cell. Immunol. , S0008874917301235 (2017).
Martinez-Nunez, R. T., Louafi, F. & Sanchez-Elsner, T. The Interleukin 13 (IL-13) pathway in human macrophages is modulated by microRNA-155 via direct targeting of interleukin 13 receptor α1 (IL13Rα1). J. Biol. Chem. 286 , 1786 (2011).
C, L. et al. miR-221 and miR-155 regulate human dendritic cell development, apoptosis, and IL-12 production through targeting of p27kip1, KPC1, and SOCS-1. blood (2011).
Metz, C. W. & Bridges, C. B. Incompatibility of Mutant Races in Drosophila. Proc. Natl Acad. Sci. USA 3 , 673–678 (1917).
Mohr, O. L. Character changes caused by mutation of an entire region of a chromosome in Drosophila. Genetics 4 , 275–282 (1919).
Kopan, R. & Ilagan, M. X. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell 137 , 216–233 (2009).
Zhou, B. et al. Notch signaling pathway: architecture, disease, and therapeutics. Signal Transduct. Target Ther. 7 , 95 (2022).
Tindemans, I. et al. Increased surface expression of NOTCH on memory T cells in peripheral blood from patients with asthma. J. Allergy Clin. Immunol. 143 , 769–771.e763 (2019).
Tindemans, I. et al. Notch signaling in T cells is essential for allergic airway inflammation, but expression of the Notch ligands Jagged 1 and Jagged 2 on dendritic cells is dispensable. J. Allergy Clin. Immunol. 140 , 1079–1089 (2017).
Hammad, H. & Lambrecht, B. N. Wnt and Hippo pathways in regulatory T cells: a NOTCH above in asthma. Nat. Immunol. 21 , 1313–1314 (2020).
Jiao, W. E. et al. Notch signaling promotes development of allergic Rhinitis by suppressing Foxp3 expression and Treg cell differentiation. Int Arch. Allergy Immunol. 178 , 33–44 (2019).
Jiang, S., Han, S., Chen, J., Li, X. & Che, H. Inhibition effect of blunting Notch signaling on food allergy through improving T(H)1/T(H)2 balance in mice. Ann. Allergy Asthma Immunol. 118 , 94–102 (2017).
Dell’Aringa, M. & Reinhardt, R. L. Notch signaling represents an important checkpoint between follicular T-helper and canonical T-helper 2 cell fate. Mucosal Immunol. 11 , 1079–1091 (2018).
Honjo, A. et al. Pharmacologic inhibition of Notch signaling suppresses food antigen-induced mucosal mast cell hyperplasia. J. Allergy Clin. Immunol. 139 , 987–996.e910 (2017).
Ziegler, S. F. & Artis, D. Sensing the outside world: TSLP regulates barrier immunity. Nat. Immunol. 11 , 289–293 (2010).
Niebuhr, M., Mamerow, D., Heratizadeh, A., Satzger, I. & Werfel, T. Staphylococcal alpha-toxin induces a higher T cell proliferation and interleukin-31 in atopic dermatitis. Int Arch. Allergy Immunol. 156 , 412–415 (2011).
Liu, Y. J. Thymic stromal lymphopoietin: master switch for allergic inflammation. J. Exp. Med. 203 , 269–273 (2006).
Yoo, J. et al. Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. J. Exp. Med. 202 , 541–549 (2005).
Stark, G. R. & Darnell, J. E. Jr. The JAK-STAT pathway at twenty. Immunity 36 , 503–514 (2012).
Velazquez, L., Fellous, M., Stark, G. R. & Pellegrini, S. A protein tyrosine kinase in the interferon alpha/beta signaling pathway. Cell 70 , 313–322 (1992).
Wilks, A. F. et al. Two novel protein-tyrosine kinases, each with a second phosphotransferase-related catalytic domain, define a new class of protein kinase. Mol. Cell Biol. 11 , 2057–2065 (1991).
Kawamura, M. et al. Molecular cloning of L-JAK, a Janus family protein-tyrosine kinase expressed in natural killer cells and activated leukocytes. Proc. Natl Acad. Sci. USA 91 , 6374–6378 (1994).
Argetsinger, L. S. et al. Identification of JAK2 as a growth hormone receptor-associated tyrosine kinase. Cell 74 , 237–244 (1993).
Schindler, C., Shuai, K., Prezioso, V. R. & Darnell, J. E. Jr. Interferon-dependent tyrosine phosphorylation of a latent cytoplasmic transcription factor. Science 257 , 809–813 (1992).
Shuai, K., Schindler, C., Prezioso, V. R. & Darnell, J. E. Jr. Activation of transcription by IFN-gamma: tyrosine phosphorylation of a 91-kD DNA binding protein. Science 258 , 1808–1812 (1992).
Schindler, C. & Darnell, J. E. Jr. Transcriptional responses to polypeptide ligands: the JAK-STAT pathway. Annu Rev. Biochem. 64 , 621–651 (1995).
Lighvani, A. A. et al. T-bet is rapidly induced by interferon-gamma in lymphoid and myeloid cells. Proc. Natl Acad. Sci. USA 98 , 15137–15142 (2001).
Afkarian, M. et al. T-bet is a STAT1-induced regulator of IL-12R expression in naïve CD4+ T cells. Nat. Immunol. 3 , 549–557 (2002).
Zhu, J., Guo, L., Watson, C. J., Hu-Li, J. & Paul, W. E. Stat6 is necessary and sufficient for IL-4’s role in Th2 differentiation and cell expansion. J. Immunol. 166 , 7276–7281 (2001).
Zhu, J., Cote-Sierra, J., Guo, L. & Paul, W. E. Stat5 activation plays a critical role in Th2 differentiation. Immunity 19 , 739–748 (2003).
Zhou, L. et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat. Immunol. 8 , 967–974 (2007).
Chiba, Y., Goto, K., Matsusue, K., Kimura, S. & Misawa, M. Identification and characterization of rat RhoA gene promoter. J. Pharm. Sci. 112 , 467–472 (2010).
Chiba, Y., Goto, K. & Misawa, M. Interleukin-13-induced activation of signal transducer and activator of transcription 6 is mediated by an activation of Janus kinase 1 in cultured human bronchial smooth muscle cells. Pharm. Rep. 64 , 454–458 (2012).
Kuperman, D. A. et al. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat. Med 8 , 885–889 (2002).
Kuperman, D. A. et al. Dissecting asthma using focused transgenic modeling and functional genomics. J. Allergy Clin. Immunol. 116 , 305–311 (2005).
Ruterbusch, M., Pruner, K. B., Shehata, L. & Pepper, M. In vivo CD4(+) T cell differentiation and function: revisiting the Th1/Th2 paradigm. Annu Rev. Immunol. 38 , 705–725 (2020).
Hsieh, Y. Y. et al. JAK-1 rs2780895 C-related genotype and allele but not JAK-1 rs10789166, rs4916008, rs2780885, rs17127114, and rs3806277 are associated with higher susceptibility to asthma. Genet Test. Mol. Biomark. 15 , 841–847 (2011).
Howell, M. D., Fitzsimons, C. & Smith, P. A. JAK/STAT inhibitors and other small molecule cytokine antagonists for the treatment of allergic disease. Ann. Allergy Asthma Immunol. 120 , 367–375 (2018).
Leung, D. Y. & Guttman-Yassky, E. Deciphering the complexities of atopic dermatitis: shifting paradigms in treatment approaches. J. Allergy Clin. Immunol. 134 , 769–779 (2014).
Bao, L., Zhang, H. & Chan, L. S. The involvement of the JAK-STAT signaling pathway in chronic inflammatory skin disease atopic dermatitis. Jakstat 2 , e24137 (2013).
PubMed PubMed Central Google Scholar
Paplińska, M. et al. Expression of eotaxins in the material from nasal brushing in asthma, allergic rhinitis and COPD patients. Cytokine 60 , 393–399 (2012).
Shieh, Y. H. et al. Zerumbone enhances the Th1 response and ameliorates ovalbumin-induced Th2 responses and airway inflammation in mice. Int Immunopharmacol. 24 , 383–391 (2015).
Gao, G. M. The progress of eotaxin and the association with ashtma. Int. J. Immunol. (2006).
Yuan, F. et al. JAX2, an ethanol extract of Hyssopus cuspidatus Boriss, can prevent bronchial asthma by inhibiting MAPK/NF-κB inflammatory signaling. Phytomedicine 57 , 305–314 (2019).
Hart, L. A., Krishnan, V. L., Adcock, I. M., Barnes, P. J. & Chung, K. F. Activation and localization of transcription factor, nuclear factor-kappaB, in asthma. Am. J. Respir. Crit. Care Med. 158 , 1585–1592 (1998).
Tully, J. E. et al. Epithelial NF-κB orchestrates house dust mite-induced airway inflammation, hyperresponsiveness, and fibrotic remodeling. J. Immunol. 191 , 5811–5821 (2013).
Poynter, M. E. et al. NF-kappa B activation in airways modulates allergic inflammation but not hyperresponsiveness. J. Immunol. 173 , 7003–7009 (2004).
Poynter, M. E., Irvin, C. G. & Janssen-Heininger, Y. M. Rapid activation of nuclear factor-kappaB in airway epithelium in a murine model of allergic airway inflammation. Am. J. Pathol. 160 , 1325–1334 (2002).
Oh, H. & Ghosh, S. NF-κB: roles and regulation in different CD4(+) T-cell subsets. Immunol. Rev. 252 , 41–51 (2013).
Gras, D., Chanez, P., Vachier, I., Petit, A. & Bourdin, A. Bronchial epithelium as a target for innovative treatments in asthma. Pharm. Ther. 140 , 290–305 (2013).
Kang, J. H., Hwang, S. M. & Chung, I. Y. S100A8, S100A9 and S100A12 activate airway epithelial cells to produce MUC5AC via extracellular signal-regulated kinase and nuclear factor-κB pathways. Immunology 144 , 79–90 (2015).
Cheng, D. S. et al. Airway epithelium controls lung inflammation and injury through the NF-kappa B pathway. J. Immunol. 178 , 6504–6513 (2007).
Sheller, J. R. et al. Nuclear factor kappa B induction in airway epithelium increases lung inflammation in allergen-challenged mice. Exp. Lung Res. 35 , 883–895 (2009).
Koziol-White, C. J. & Panettieri, R. A. Jr. Airway smooth muscle and immunomodulation in acute exacerbations of airway disease. Immunol. Rev. 242 , 178–185 (2011).
Tran, T. et al. Stimulus-dependent glucocorticoid-resistance of GM-CSF production in human cultured airway smooth muscle. Br. J. Pharm. 145 , 123–131 (2005).
John, A. E., Zhu, Y. M., Brightling, C. E., Pang, L. & Knox, A. J. Human airway smooth muscle cells from asthmatic individuals have CXCL8 hypersecretion due to increased NF-kappa B p65, C/EBP beta, and RNA polymerase II binding to the CXCL8 promoter. J. Immunol. 183 , 4682–4692 (2009).
Radermecker, C., Louis, R., Bureau, F. & Marichal, T. Role of neutrophils in allergic asthma. Curr. Opin. Immunol. 54 , 28–34 (2018).
Chien, J. W. et al. Increased IL-17A secreting CD4+ T cells, serum IL-17 levels and exhaled nitric oxide are correlated with childhood asthma severity. Clin. Exp. Allergy 43 , 1018–1026 (2013).
Fujisawa, T. et al. NF-κB mediates IL-1β- and IL-17A-induced MUC5B expression in airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 45 , 246–252 (2011).
Dragon, S., Hirst, S. J., Lee, T. H. & Gounni, A. S. IL-17A mediates a selective gene expression profile in asthmatic human airway smooth muscle cells. Am. J. Respir. Cell Mol. Biol. 50 , 1053–1063 (2014).
Chang, Y. et al. Th17-associated cytokines promote human airway smooth muscle cell proliferation. FASEB J. 26 , 5152–5160 (2012).
Wan, R. et al. Neutrophil extracellular traps amplify neutrophil recruitment and inflammation in neutrophilic asthma by stimulating the airway epithelial cells to activate the TLR4/ NF-κB pathway and secrete chemokines. Aging 12 , 16820–16836 (2020).
Wenzel, S. E. et al. Bronchoscopic evaluation of severe asthma. Persistent inflammation associated with high dose glucocorticoids. Am. J. Respir. Crit. Care Med 156 , 737–743 (1997).
Wong, C. K. et al. Induction of adhesion molecules upon the interaction between eosinophils and bronchial epithelial cells: involvement of p38 MAPK and NF-kappaB. Int. Immunopharmacol. 6 , 1859–1871 (2006).
Wang, C. B. et al. Induction of IL-6 in co-culture of bronchial epithelial cells and eosinophils is regulated by p38 MAPK and NF-kappaB. Allergy 60 , 1378–1385 (2005).
Kankaanranta, H. et al. Tumour necrosis factor-α regulates human eosinophil apoptosis via ligation of TNF-receptor 1 and balance between NF-κB and AP-1. PLoS ONE 9 , e90298 (2014).
Edwards, M. R. et al. Targeting the NF-kappaB pathway in asthma and chronic obstructive pulmonary disease. Pharm. Ther. 121 , 1–13 (2009).
Rescigno, M., Martino, M., Sutherland, C. L., Gold, M. R. & Ricciardi-Castagnoli, P. Dendritic cell survival and maturation are regulated by different signaling pathways. J. Exp. Med. 188 , 2175–2180 (1998).
Pelaia, G. et al. Cellular mechanisms underlying eosinophilic and neutrophilic airway inflammation in asthma. Mediators Inflamm. 2015 , 879783 (2015).
Ahern, P. P., Izcue, A., Maloy, K. J. & Powrie, F. The interleukin-23 axis in intestinal inflammation. Immunol. Rev. 226 , 147–159 (2008).
Ouaaz, F., Arron, J., Zheng, Y., Choi, Y. & Beg, A. A. Dendritic cell development and survival require distinct NF-kappaB subunits. Immunity 16 , 257–270 (2002).
O’Keeffe, M. et al. Distinct roles for the NF-kappaB1 and c-Rel transcription factors in the differentiation and survival of plasmacytoid and conventional dendritic cells activated by TLR-9 signals. Blood 106 , 3457–3464 (2005).
Nicolay, B. N., Bayarmagnai, B., Islam, A. B., Lopez-Bigas, N. & Frolov, M. V. Cooperation between dE2F1 and Yki/Sd defines a distinct transcriptional program necessary to bypass cell cycle exit. Genes Dev. 25 , 323–335 (2011).
Tang, W. et al. Hippo signaling pathway and respiratory diseases. Cell Death Disco. 8 , 213 (2022).
Harb, H. et al. A regulatory T cell Notch4-GDF15 axis licenses tissue inflammation in asthma. Nat. Immunol. 21 , 1359–1370 (2020).
Kumar, P. et al. Soluble OX40L and JAG1 Induce Selective Proliferation of Functional Regulatory T-Cells Independent of canonical TCR signaling. Sci. Rep. 7 , 39751 (2017).
Fermino, M. L. et al. Galectin-3 negatively regulates the frequency and function of CD4(+) CD25(+) Foxp3(+) regulatory T cells and influences the course of Leishmania major infection. Eur. J. Immunol. 43 , 1806–1817 (2013).
Begin, P. & Nadeau, K. C. Epigenetic regulation of asthma and allergic disease. Allergy Asthma Clin. Immunol. 10 , 27 (2014).
Palmer, L. J. et al. Independent inheritance of serum immunoglobulin E concentrations and airway responsiveness. Am. J. Respir. Crit. Care Med. 161 , 1836–1843 (2000).
Gangloff, S. C. & Guenounou, M. Toll-like receptors and immune response in allergic disease. Clin. Rev. Allergy Immunol. 26 , 115–125 (2004).
Takeda, K. & Akira, S. Roles of Toll-like receptors in innate immune responses. Genes Cells 6 , 733–742 (2001).
Sioud, M. Induction of inflammatory cytokines and interferon responses by double-stranded and single-stranded siRNAs is sequence-dependent and requires endosomal localization. J. Mol. Biol. 348 , 1079–1090 (2005).
Mizel, S. B., Honko, A. N., Moors, M. A., Smith, P. S. & West, A. P. Induction of macrophage nitric oxide production by Gram-negative flagellin involves signaling via heteromeric Toll-like receptor 5/Toll-like receptor 4 complexes. J. Immunol. 170 , 6217–6223 (2003).
Riiser, A. The human microbiome, asthma, and allergy. Allergy Asthma Clin. Immunol. 11 , 35 (2015).
McAlees, J. W. et al. Distinct Tlr4-expressing cell compartments control neutrophilic and eosinophilic airway inflammation. Mucosal Immunol. 8 , 863–873 (2015).
Thomas, S. Y. et al. MyD88-dependent dendritic and epithelial cell crosstalk orchestrates immune responses to allergens. Mucosal Immunol. 11 , 796–810 (2018).
Larsen, J. M. et al. Chronic obstructive pulmonary disease and asthma-associated Proteobacteria, but not commensal Prevotella spp., promote Toll-like receptor 2-independent lung inflammation and pathology. Immunology 144 , 333–342 (2015).
Karvonen, A. M. et al. Quantity and diversity of environmental microbial exposure and development of asthma: a birth cohort study. Allergy 69 , 1092–1101 (2014).
Remot, A. et al. Bacteria isolated from lung modulate asthma susceptibility in mice. ISME J. 11 , 1061–1074 (2017).
Eisenbarth, S. C. et al. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J. Exp. Med. 196 , 1645–1651 (2002).
Shim, J. U. et al. Flagellin suppresses experimental asthma by generating regulatory dendritic cells and T cells. J. Allergy Clin. Immunol. 137 , 426–435 (2016).
Holt, P. G. et al. Downregulation of the antigen presenting cell function(s) of pulmonary dendritic cells in vivo by resident alveolar macrophages. J. Exp. Med. 177 , 397–407 (1993).
Strickland, D. H., Thepen, T., Kees, U. R., Kraal, G. & Holt, P. G. Regulation of T-cell function in lung tissue by pulmonary alveolar macrophages. Immunology 80 , 266–272 (1993).
Lun, S. W., Wong, C. K., Ko, F. W., Hui, D. S. & Lam, C. W. Expression and functional analysis of toll-like receptors of peripheral blood cells in asthmatic patients: implication for immunopathological mechanism in asthma. J. Clin. Immunol. 29 , 330–342 (2009).
Zarember, K. A. & Godowski, P. J. Tissue expression of human Toll-like receptors and differential regulation of Toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J. Immunol. 168 , 554–561 (2002).
Sha, Q., Truong-Tran, A. Q., Plitt, J. R., Beck, L. A. & Schleimer, R. P. Activation of airway epithelial cells by toll-like receptor agonists. Am. J. Respir. Cell Mol. Biol. 31 , 358–364 (2004).
Armstrong, L. et al. Expression of functional toll-like receptor-2 and -4 on alveolar epithelial cells. Am. J. Respir. Cell Mol. Biol. 31 , 241–245 (2004).
Fernandez, S., Jose, P., Avdiushko, M. G., Kaplan, A. M. & Cohen, D. A. Inhibition of IL-10 receptor function in alveolar macrophages by Toll-like receptor agonists. J. Immunol. 172 , 2613–2620 (2004).
Applequist, S. E., Wallin, R. P. & Ljunggren, H. G. Variable expression of Toll-like receptor in murine innate and adaptive immune cell lines. Int Immunol. 14 , 1065–1074 (2002).
Kanehisa, M. et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 36 , D480–D484 (2008).
Long, E. M., Millen, B., Kubes, P. & Robbins, S. M. Lipoteichoic acid induces unique inflammatory responses when compared to other toll-like receptor 2 ligands. PLoS ONE 4 , e5601 (2009).
Fuchs, B. & Braun, A. Modulation of asthma and allergy by addressing toll-like receptor 2. J. Occup. Med. Toxicol. 3 (Suppl. 1), S5 (2008).
Redecke, V. et al. Cutting edge: activation of Toll-like receptor 2 induces a Th2 immune response and promotes experimental asthma. J. Immunol. 172 , 2739–2743 (2004).
Akdis, C. A. et al. Inhibition of T helper 2-type responses, IgE production and eosinophilia by synthetic lipopeptides. Eur. J. Immunol. 33 , 2717–2726 (2003).
Chun, E. et al. Toll-like receptor expression on peripheral blood mononuclear cells in asthmatics; implications for asthma management. J. Clin. Immunol. 30 , 459–464 (2010).
Yamamoto, M. et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science 301 , 640–643 (2003).
Siwiec, J. et al. [Evaluation of Th1/Th2 lymphocyte balance and lipopolysaccharide receptor expression in asthma patients]. Pneumonol. Alergol. Pol. 77 , 123–130 (2009).
PubMed Google Scholar
Nawijn, M. C. et al. TLR-2 activation induces regulatory T cells and long-term suppression of asthma manifestations in mice. PLoS ONE 8 , e55307 (2013).
Zhou, C., Kang, X. D. & Chen, Z. A synthetic Toll-like receptor 2 ligand decreases allergic immune responses in a mouse rhinitis model sensitized to mite allergen. J. Zhejiang Univ. Sci. B 9 , 279–285 (2008).
Patel, M. et al. TLR2 agonist ameliorates established allergic airway inflammation by promoting Th1 response and not via regulatory T cells. J. Immunol. 174 , 7558–7563 (2005).
Taylor, R. C., Richmond, P. & Upham, J. W. Toll-like receptor 2 ligands inhibit TH2 responses to mite allergen. J. Allergy Clin. Immunol. 117 , 1148–1154 (2006).
Fuchs, B. et al. A Toll-like receptor 2/6 agonist reduces allergic airway inflammation in chronic respiratory sensitisation to Timothy grass pollen antigens. Int Arch. Allergy Immunol. 152 , 131–139 (2010).
Kim, K. S. et al. Dietary antigens limit mucosal immunity by inducing regulatory T cells in the small intestine. Science 351 , 858–863 (2016).
Eckburg, P. B. et al. Diversity of the human intestinal microbial flora. Science 308 , 1635–1638 (2005).
Qin, J. et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464 , 59–65 (2010).
Xu, H., Shu, H., Zhu, J. & Song, J. Inhibition of TLR4 inhibits allergic responses in murine allergic rhinitis by regulating the NF-kappaB pathway. Exp. Ther. Med. 18 , 761–768 (2019).
Fransson, M. et al. Up-regulation of Toll-like receptors 2, 3 and 4 in allergic rhinitis. Respir. Res. 6 , 100 (2005).
Nusse, R. & Varmus, H. E. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell 31 , 99–109 (1982).
Liu, J. et al. Wnt/beta-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target Ther. 7 , 3 (2022).
Matsuda-Hirose, H. et al. Selective inhibition of beta-Catenin/Co-Activator cyclic AMP response element-binding protein-dependent signaling prevents the emergence of hapten-induced atopic dermatitis-like dermatitis. Ann. Dermatol. 31 , 631–639 (2019).
Kwak, H. J. et al. The Wnt/beta-catenin signaling pathway regulates the development of airway remodeling in patients with asthma. Exp. Mol. Med. 47 , e198 (2015).
Jia, X. X. et al. Wnt/beta-catenin signaling pathway regulates asthma airway remodeling by influencing the expression of c-Myc and cyclin D1 via the p38 MAPK-dependent pathway. Exp. Ther. Med. 18 , 3431–3438 (2019).
Trischler, J. et al. Immune modulation of the T cell response in asthma through Wnt10b. Am. J. Respir. Cell Mol. Biol. 54 , 584–593 (2016).
Song, J., Zhu, X. M. & Wei, Q. Y. MSCs reduce airway remodeling in the lungs of asthmatic rats through the Wnt/β-catenin signaling pathway. Eur. Rev. Med Pharm. Sci. 24 , 11199–11211 (2020).
CAS Google Scholar
Huang, Y., Wang, L., Jia, X. X., Lin, X. X. & Zhang, W. X. Vitamin D alleviates airway remodeling in asthma by down-regulating the activity of Wnt/beta-catenin signaling pathway. Int Immunopharmacol. 68 , 88–94 (2019).
Reuter, S. et al. The Wnt/beta-catenin pathway attenuates experimental allergic airway disease. J. Immunol. 193 , 485–495 (2014).
Li, J., Xue, K., Zheng, Y., Wang, Y. & Xu, C. RORA overexpression alleviates nasal mucosal injury and enhances red blood cell immune adhesion function in a mouse model of allergic rhinitis via inactivation of the Wnt/beta-catenin signaling pathway. Int Arch. Allergy Immunol. 180 , 79–90 (2019).
Leung, D. Y. M., Berdyshev, E. & Goleva, E. Cutaneous barrier dysfunction in allergic diseases. J. Allergy Clin. Immunol. 145 , 1485–1497 (2020).
Tasioudi, K. E. et al. Immunohistochemical and molecular analysis of PI3K/AKT/mTOR pathway in esophageal carcinoma. APMIS 123 , 639–647 (2015).
Choi, Y. H., Jin, G. Y., Li, L. C. & Yan, G. H. Inhibition of protein kinase C delta attenuates allergic airway inflammation through suppression of PI3K/Akt/mTOR/HIF-1 alpha/VEGF pathway. PLoS ONE 8 , e81773 (2013).
Zou, W., Ding, F., Niu, C., Fu, Z. & Liu, S. Brg1 aggravates airway inflammation in asthma via inhibition of the PI3K/Akt/mTOR pathway. Biochem. Biophys. Res. Commun. 503 , 3212–3218 (2018).
Lee, K. S. et al. Phosphoinositide 3-kinase-delta inhibitor reduces vascular permeability in a murine model of asthma. J. Allergy Clin. Immunol. 118 , 403–409 (2006).
Kim, S. R. et al. HIF-1alpha inhibition ameliorates an allergic airway disease via VEGF suppression in bronchial epithelium. Eur. J. Immunol. 40 , 2858–2869 (2010).
Burgess, J. K. et al. Dual ERK and phosphatidylinositol 3-kinase pathways control airway smooth muscle proliferation: differences in asthma. J. Cell Physiol. 216 , 673–679 (2008).
Huang, L. et al. OX40L induces helper T cell differentiation during cell immunity of asthma through PI3K/AKT and P38 MAPK signaling pathway. J. Transl. Med. 16 , 74 (2018).
Stark, A. K., Sriskantharajah, S., Hessel, E. M. & Okkenhaug, K. PI3K inhibitors in inflammation, autoimmunity and cancer. Curr. Opin. Pharm. 23 , 82–91 (2015).
Zhao, Y. et al. PI3K-AKT-mTOR signaling pathway: the intersection of allergic asthma and cataract. Pharmazie 74 , 598–600 (2019).
CAS PubMed Google Scholar
Wu, G. et al. Anti-allergic function of α-Tocopherol is mediated by suppression of PI3K-PKB activity in mast cells in mouse model of allergic rhinitis. Allergol. Immunopathol. 48 , 395–400 (2020).
Zhang, Y. et al. Bicalutamide, an androgen receptor antagonist, effectively alleviate allergic rhinitis via suppression of PI3K-PKB activity. Eur. Arch. Otorhinolaryngol. 280 , 703–711 (2023).
Zeng, Q., Luo, X., Tang, Y., Liu, W. & Luo, R. Leptin regulated ILC2 Cell through the PI3K/AKT pathway in allergic rhinitis. Mediators Inflamm. 2020 , 4176082 (2020).
Yuan, J. et al. Gene knockdown of CCR3 reduces eosinophilic inflammation and the Th2 immune response by inhibiting the PI3K/AKT pathway in allergic rhinitis mice. Sci. Rep. 12 , 5411 (2022).
Nian, J. B. et al. Epithelial cells expressed IL-33 to promote degranulation of mast cells through inhibition on ST2/PI3K/mTOR-mediated autophagy in allergic rhinitis. Cell Cycle 19 , 1132–1142 (2020).
Karagianni, F., Pavlidis, A., Malakou, L. S., Piperi, C. & Papadavid, E. Predominant role of mTOR signaling in skin diseases with therapeutic potential. Int. J. Mol. Sci. 23 (2022).
Bao, Y. et al. MicroRNA-126 accelerates IgE-mediated mast cell degranulation associated with the PI3K/Akt signaling pathway by promoting Ca(2+) influx. Exp. Ther. Med 16 , 2763–2769 (2018).
Kim, E. G. et al. Chitinase 3-like 1 contributes to food allergy via M2 macrophage polarization. Allergy Asthma Immunol. Res. 12 , 1012–1028 (2020).
Keith, C. T. & Schreiber, S. L. PIK-related kinases: DNA repair, recombination, and cell cycle checkpoints. Science 270 , 50–51 (1995).
Zou, Z., Tao, T., Li, H. & Zhu, X. mTOR signaling pathway and mTOR inhibitors in cancer: progress and challenges. Cell Biosci. 10 , 31 (2020).
Liu, G. Y. & Sabatini, D. M. mTOR at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell Biol. 21 , 183–203 (2020).
Delgoffe, G. M. & Powell, J. D. mTOR: taking cues from the immune microenvironment. Immunology 127 , 459–465 (2009).
Powell, J. D., Pollizzi, K. N., Heikamp, E. B. & Horton, M. R. Regulation of immune responses by mTOR. Annu Rev. Immunol. 30 , 39–68 (2012).
Zou, H. et al. MTOR-mediated autophagy is involved in the protective effect of ketamine on allergic airway inflammation. J. Immunol. Res 2019 , 5879714 (2019).
Preite, S., Huang, B., Cannons, J. L., McGavern, D. B. & Schwartzberg, P. L. PI3K orchestrates T follicular helper cell differentiation in a context dependent manner: implications for autoimmunity. Front Immunol. 9 , 3079 (2018).
Wang, P. et al. The Regulatory Effects of mTOR Complexes in the Differentiation and Function of CD4(+) T Cell Subsets. J. Immunol. Res. 2020 , 3406032 (2020).
Jacobsen, E. A. et al. Allergic pulmonary inflammation in mice is dependent on eosinophil-induced recruitment of effector T cells. J. Exp. Med. 205 , 699–710 (2008).
Zhu, C. et al. mTOR complexes differentially orchestrates eosinophil development in allergy. Sci. Rep. 8 , 6883 (2018).
Chen, X. et al. Poly-L-arginine promotes asthma angiogenesis through induction of FGFBP1 in airway epithelial cells via activation of the mTORC1-STAT3 pathway. Cell Death Dis. 12 , 761 (2021).
Jia, Q. N. & Zeng, Y. P. Rapamycin blocks the IL-13-induced deficiency of epidermal barrier related proteins via upregulation of miR-143 in HaCaT Keratinocytes. Int. J. Med. Sci. 17 , 2087–2094 (2020).
Méndez-Enríquez, E. & Hallgren, J. Mast cells and their progenitors in allergic asthma. Front Immunol. 10 , 821 (2019).
Turner, H. & Kinet, J. P. Signalling through the high-affinity IgE receptor Fc epsilonRI. Nature 402 , B24–B30 (1999).
Amo, G. et al. FCERI and histamine metabolism gene variability in selective responders to NSAIDS. Front Pharm. 7 , 353 (2016).
Kanagaratham, C., El Ansari, Y. S., Lewis, O. L. & Oettgen, H. C. IgE and IgG antibodies as regulators of mast cell and basophil functions in food allergy. Front Immunol. 11 , 603050 (2020).
Shin, J. S. & Greer, A. M. The role of FcεRI expressed in dendritic cells and monocytes. Cell Mol. Life Sci. 72 , 2349–2360 (2015).
Suber, J. & Iweala, O. I. Strategies for mast cell inhibition in food allergy. Yale J. Biol. Med 93 , 719–731 (2020).
Suurmond, J., Dorjée, A. L., Knol, E. F., Huizinga, T. W. & Toes, R. E. Differential TLR-induced cytokine production by human mast cells is amplified by FcɛRI triggering. Clin. Exp. Allergy 45 , 788–796 (2015).
Suurmond, J. et al. Activation of human basophils by combined toll-like receptor- and FcεRI-triggering can promote Th2 skewing of naive T helper cells. Eur. J. Immunol. 44 , 386–396 (2014).
Pellefigues, C. IgE Autoreactivity in atopic dermatitis: paving the road for autoimmune diseases? Antibodies 9 (2020).
Pitchford, S. C. et al. Allergen induces the migration of platelets to lung tissue in allergic asthma. Am. J. Respir. Crit. Care Med. 177 , 604–612 (2008).
Guo, W. et al. IgE aggravates the senescence of smooth muscle cells in abdominal aortic aneurysm by upregulating LincRNA-p21. Aging Dis. 10 , 699–710 (2019).
Crosson, T. et al. FcεR1-expressing nociceptors trigger allergic airway inflammation. J. Allergy Clin. Immunol. 147 , 2330–2342 (2021).
Liang, H. et al. Vagal activities are involved in antigen-specific immune inflammation in the intestine. J. Gastroenterol. Hepatol. 26 , 1065–1071 (2011).
Yashiro, T. et al. Pathophysiological roles of neuro-immune interactions between enteric neurons and mucosal mast cells in the gut of food allergy mice. Cells 10 (2021).
Chandler, C. E., Harberts, E. M. & Ernst, R. K. Pathogen sensing: toll-like receptors and NODs (Innate Immunity). Ency. Microbiol. , 443–456 (2019).
Wicherska-Pawłowska, K., Wróbel, T. & Rybka, J. Toll-Like Receptors (TLRs), NOD-like receptors (NLRs), and RIG-I-like receptors (RLRs) in innate immunity. TLRs, NLRs, and RLRs ligands as immunotherapeutic agents for hematopoietic diseases. Int. J. Mol. Sci. 22 (2021).
Motta, V., Soares, F., Sun, T. & Philpott, D. J. NOD-like receptors: versatile cytosolic sentinels. Physiol. Rev. 95 , 149–178 (2015).
Kim, Y. K., Shin, J. S. & Nahm, M. H. NOD-like receptors in infection, immunity, and diseases. Yonsei Med J. 57 , 5–14 (2016).
Gurung, P. & Kanneganti, T. D. Immune responses against protozoan parasites: a focus on the emerging role of Nod-like receptors. Cell Mol. Life Sci. 73 , 3035–3051 (2016).
Kufer, T. A. & Sansonetti, P. J. NLR functions beyond pathogen recognition. Nat. Immunol. 12 , 121–128 (2011).
Travassos, L. H., Carneiro, L. A., Girardin, S. & Philpott, D. J. Nod proteins link bacterial sensing and autophagy. Autophagy 6 , 409–411 (2010).
Tsang, M. S., Hou, T., Chan, B. C. & Wong, C. K. Immunological roles of NLR in allergic diseases and its underlying mechanisms. Int. J. Mol. Sci . 22 (2021).
Wong, C. K. et al. NOD-like receptors mediated activation of eosinophils interacting with bronchial epithelial cells: a link between innate immunity and allergic asthma. Cell Mol. Immunol. 10 , 317–329 (2013).
Duan, W. et al. Innate signals from Nod2 block respiratory tolerance and program T(H)2-driven allergic inflammation. J. Allergy Clin. Immunol. 126 , 1284–1293.e1210 (2010).
Zhou, B. et al. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat. Immunol. 6 , 1047–1053 (2005).
Tamachi, T. et al. IL-25 enhances allergic airway inflammation by amplifying a TH2 cell-dependent pathway in mice. J. Allergy Clin. Immunol. 118 , 606–614 (2006).
Jember, A. G., Zuberi, R., Liu, F. T. & Croft, M. Development of allergic inflammation in a murine model of asthma is dependent on the costimulatory receptor OX40. J. Exp. Med. 193 , 387–392 (2001).
Lv, J. et al. Heme oxygenase-1 protects airway epithelium against apoptosis by targeting the proinflammatory NLRP3-RXR axis in asthma. J. Biol. Chem. 293 , 18454–18465 (2018).
Ma, M., Li, G., Qi, M., Jiang, W. & Zhou, R. Inhibition of the inflammasome activity of NLRP3 attenuates HDM-induced allergic asthma. Front Immunol. 12 , 718779 (2021).
Dai, M. Y. et al. Particulate matters induce acute exacerbation of allergic airway inflammation via the TLR2/NF-κB/NLRP3 signaling pathway. Toxicol. Lett. 321 , 146–154 (2020).
Bogefors, J. et al. Nod1, Nod2 and Nalp3 receptors, new potential targets in treatment of allergic rhinitis? Allergy 65 , 1222–1226 (2010).
Zhou, H. et al. Activation of NLRP3 inflammasome contributes to the inflammatory response to allergic rhinitis via macrophage pyroptosis. Int Immunopharmacol. 110 , 109012 (2022).
Haspeslagh, E., Heyndrickx, I., Hammad, H. & Lambrecht, B. N. The hygiene hypothesis: immunological mechanisms of airway tolerance. Curr. Opin. Immunol. 54 , 102–108 (2018).
Sbihi, H. et al. Thinking bigger: how early-life environmental exposures shape the gut microbiome and influence the development of asthma and allergic disease. Allergy 74 , 2103–2115 (2019).
Coyte, K. Z., Rao, C., Rakoff-Nahoum, S. & Foster, K. R. Ecological rules for the assembly of microbiome communities. PLoS Biol. 19 , e3001116 (2021).
Peroni, D. G., Nuzzi, G., Trambusti, I., Di Cicco, M. E. & Comberiati, P. Microbiome composition and its impact on the development of allergic diseases. Front Immunol. 11 , 700 (2020).
Dekaboruah, E., Suryavanshi, M. V., Chettri, D. & Verma, A. K. Human microbiome: an academic update on human body site specific surveillance and its possible role. Arch. Microbiol. 202 , 2147–2167 (2020).
Sugahara H, O. T., Hashikura, N., Abe, F. & Xiao, J. Z. Differences in folate production by bifidobacteria of different origins. Biosci. Microbiota Food Health 34 , 87–93 (2015).
Rowland, I. et al. Gut microbiota functions: metabolism of nutrients and other food components. Eur. J. Nutr. 57 , 1–24 (2018).
Gomez de Agüero, M. et al. The maternal microbiota drives early postnatal innate immune development. Science 351 , 1296–1302 (2016).
Francino, M. P. Early development of the gut microbiota and immune health. Pathogens 3 , 769–790 (2014).
Stokholm, J. et al. Cesarean section changes neonatal gut colonization. J. Allergy Clin. Immunol. 138 , 881–889 e882 (2016).
Shaterian, N., Abdi, F., Ghavidel, N. & Alidost, F. Role of cesarean section in the development of neonatal gut microbiota: a systematic review. Open Med. 16 , 624–639 (2021).
Kim, G. et al. Delayed establishment of gut microbiota in infants delivered by Cesarean section. Front. Microbiol. 11 , 2099 (2020).
Ma, J. et al. Comparison of gut microbiota in exclusively breast-fed and formula-fed babies: a study of 91 term infants. Sci. Rep. 10 , 15792 (2020).
Zhong, H. et al. Impact of probiotics supplement on the gut microbiota in neonates with antibiotic exposure: an open-label single-center randomized parallel controlled study. World J. Pediatr. 17 , 385–393 (2021).
Yousuf, E. I. et al. Persistence of suspected probiotic organisms in preterm infant gut microbiota weeks after probiotic supplementation in the NICU. Front. Microbiol. 11 , 574137 (2020).
Dierikx, T. H. et al. The influence of prenatal and intrapartum antibiotics on intestinal microbiota colonisation in infants: a systematic review. J. Infect. 81 , 190–204 (2020).
Milliken, S., Allen, R. M. & Lamont, R. F. The role of antimicrobial treatment during pregnancy on the neonatal gut microbiome and the development of atopy, asthma, allergy and obesity in childhood. Expert Opin. Drug Saf. 18 , 173–185 (2019).
Panduru, M. et al. Antibiotics administration during last trimester of pregnancy is associated with atopic dermatitis - a cross-sectional study. Sciendo (2020).
Stensballe, L. G., Simonsen, J., Jensen, S. M., Bonnelykke, K. & Bisgaard, H. Use of antibiotics during pregnancy increases the risk of asthma in early childhood. J. Pediatr. 162 , 832–838 e833 (2013).
Geng, M. et al. Prenatal low-dose antibiotic exposure and children allergic diseases at 4 years of age: a prospective birth cohort study. Ecotoxicol. Environ. Saf. 225 , 112736 (2021).
Aversa, Z. et al. Association of Infant antibiotic exposure with childhood health outcomes. Mayo Clin. Proc. 96 , 66–77 (2021).
Moreau MC, D. R., Guy-Grand, D. & Muller, M. C. Increase in the population of duodenal immunoglobulin A plasmocytes in axenic mice associated with different living or dead bacterial strains of intestinal origin. Infect. Immun. 21 , 532–539 (1978).
Fulde, M. & H, M. Maturation of the enteric mucosal innate immune system during the postnatal period.pdf. Immunol. Rev. 260 , 21–34 (2014).
Wesemann, D. R. et al. Microbial colonization influences early B-lineage development in the gut lamina propria. Nature 501 , 112–115 (2013).
Sansonetti, P. J. To be or not to be a pathogen: that is the mucosally relevant question. Mucosal Immunol. 4 , 8–14 (2011).
Holgate, S. T. Innate and adaptive immune responses in asthma. Nat. Med. 18 , 673–683 (2012).
Arrieta, M. C. et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci. Transl. Med. 7 , 307ra152 (2015).
Hevia, A. et al. Allergic patients with long-term asthma display low levels of Bifidobacterium adolescentis. PLoS ONE 11 , e0147809 (2016).
Huang, Y. J. et al. The airway microbiome in patients with severe asthma: associations with disease features and severity. J. Allergy Clin. Immunol. 136 , 874–884 (2015).
Kong, H. H. et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 22 , 850–859 (2012).
Yao, Y. et al. Identification of staphylococcal protein A in infected atopic dermatitis lesions. J. Invest. Dermatol. 130 , 2502–2504 (2010).
Niebuhr, M., Scharonow, H., Gathmann, M., Mamerow, D. & Werfel, T. Staphylococcal exotoxins are strong inducers of IL-22: a potential role in atopic dermatitis. J. Allergy Clin. Immunol. 126 , 1176–1183 e1174 (2010).
Hong, S. W. et al. Extracellular vesicles derived from Staphylococcus aureus induce atopic dermatitis-like skin inflammation. Allergy 66 , 351–359 (2011).
Ando, T. et al. Mast cells are required for full expression of allergen/SEB-induced skin inflammation. J. Invest. Dermatol. 133 , 2695–2705 (2013).
Abrahamsson, T. R. et al. Low diversity of the gut microbiota in infants with atopic eczema. J. Allergy Clin. Immunol. 129 , 434–440 (2012). 440 e431-432.
Marrs, T. & Flohr, C. The role of skin and gut microbiota in the development of atopic eczema. Br. J. Dermatol. 175 (Suppl. 2), 13–18 (2016).
Penders, J. et al. Establishment of the intestinal microbiota and its role for atopic dermatitis in early childhood. J. Allergy Clin. Immunol. 132 , 601–607 e608 (2013).
Song, H., Yoo, Y., Hwang, J., Na, Y. C. & Kim, H. S. Faecalibacterium prausnitzii subspecies-level dysbiosis in the human gut microbiome underlying atopic dermatitis. J. Allergy Clin. Immunol. 137 , 852–860 (2016).
Bassis CM, T. A., Young, V. B. & Pynnonen, M. A. The nasal cavity microbiota of healthy adults. Microbiome 2 , 27 (2014).
Liu CM, P. L. et al. Staphylococcus aureus and the ecology of the nasal microbiome. Sci. Adv. 1 , e1400216 (2015).
Lemon, K. P. et al. Comparative analyses of the bacterial microbiota of the human nostril and oropharynx. mBio 1 , e00129–00110 (2010).
Choi, C. H. et al. Seasonal allergic rhinitis affects sinonasal microbiota. Am. J. Rhinol. Allergy 28 , 281–286 (2014).
Shiomori, T., Yoshida, S., Miyamoto, H. & Makishima, K. Relationship of nasal carriage of Staphylococcus aureus to pathogenesis of perennial allergic rhinitis. J. Allergy Clin. Immunol. 105 , 449–454 (2000).
Bager, P., Wohlfahrt, J. & Westergaard, T. Caesarean delivery and risk of atopy and allergic disease: meta-analyses. Clin. Exp. Allergy 38 , 634–642 (2008).
Hirsch, A. G. et al. Early-life antibiotic use and subsequent diagnosis of food allergy and allergic diseases. Clin. Exp. Allergy 47 , 236–244 (2017).
Sanchez-Valverde, F. et al. The impact of caesarean delivery and type of feeding on cow’s milk allergy in infants and subsequent development of allergic march in childhood. Allergy 64 , 884–889 (2009).
Erridge, C., Duncan, S. H., Bereswill, S. & Heimesaat, M. M. The induction of colitis and ileitis in mice is associated with marked increases in intestinal concentrations of stimulants of TLRs 2, 4, and 5. PLoS ONE 5 , e9125 (2010).
Frank, D. N. et al. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl Acad. Sci. USA 104 , 13780–13785 (2007).
Kant, R., de Vos, W. M., Palva, A. & Satokari, R. Immunostimulatory CpG motifs in the genomes of gut bacteria and their role in human health and disease. J. Med. Microbiol. 63 , 293–308 (2014).
Aagaard, K. et al. The placenta harbors a unique microbiome. Sci. Transl. Med. 6 , 237ra265 (2014).
Chu, D. M. et al. The early infant gut microbiome varies in association with a maternal high-fat diet. Genome Med. 8 , 77 (2016).
Goldenberg, R. L., Hauth, J. C. & Andrews, W. W. Intrauterine infection and preterm delivery. N. Engl. J. Med. 342 , 1500–1507 (2000).
Koga, K. & Mor, G. Toll-like receptors at the maternal-fetal interface in normal pregnancy and pregnancy disorders. Am. J. Reprod. Immunol. 63 , 587–600 (2010).
Christmann, B. S. et al. Human seroreactivity to gut microbiota antigens. J. Allergy Clin. Immunol. 136 , 1378–1386 e1371-1375 (2015).
Tanaka, M. & Nakayama, J. Development of the gut microbiota in infancy and its impact on health in later life. Allergol. Int. 66 , 515–522 (2017).
West, C. E. et al. Gut microbiome and innate immune response patterns in IgE-associated eczema. Clin. Exp. Allergy 45 , 1419–1429 (2015).
Yiu, J. H., Dorweiler, B. & Woo, C. W. Interaction between gut microbiota and toll-like receptor: from immunity to metabolism. J. Mol. Med. 95 , 13–20 (2017).
Sun, L., Liu, W. & Zhang, L. J. The role of toll-like receptors in skin host defense, psoriasis, and atopic dermatitis. J. Immunol. Res 2019 , 1824624 (2019).
Lambrecht, B. N. & Hammad, H. The airway epithelium in asthma. Nat. Med. 18 , 684–692 (2012).
Lin, L. & Zhang, J. Role of intestinal microbiota and metabolites on gut homeostasis and human diseases. BMC Immunol. 18 , 2 (2017).
Wypych, T. P. et al. Microbial metabolism of l-tyrosine protects against allergic airway inflammation. Nat. Immunol. 22 , 279–286 (2021).
Nastasi, C. et al. The effect of short-chain fatty acids on human monocyte-derived dendritic cells. Sci. Rep. 5 , 16148 (2015).
Müller, T., Hamm, S. & Bauer, S. TLR9-mediated recognition of DNA. Handb. Exp. Pharmacol. 183 , 51–70 (2008).
Hartmann, G., Weiner, G. J. & Krieg, A. M. CpG DNA: a potent signal for growth, activation, and maturation of human dendritic cells. Proc. Natl Acad. Sci. USA 96 , 9305–9310 (1999).
Krug, A. et al. Identification of CpG oligonucleotide sequences with high induction of IFN-alpha/beta in plasmacytoid dendritic cells. Eur. J. Immunol. 31 , 2154–2163 (2001).
3.0.CO;2-U" data-track-action="article reference" href="https://doi.org/10.1002%2F1521-4141%28200107%2931%3A7%3C2154%3A%3AAID-IMMU2154%3E3.0.CO%3B2-U" aria-label="Article reference 405" data-doi="10.1002/1521-4141(200107)31:7 3.0.CO;2-U">Article CAS PubMed Google Scholar
Krieg, A. M. CpG motifs in bacterial DNA and their immune effects. Annu Rev. Immunol. 20 , 709–760 (2002).
Choudhury, B. K. et al. In vivo role of p38 mitogen-activated protein kinase in mediating the anti-inflammatory effects of CpG oligodeoxynucleotide in murine asthma. J. Immunol. 169 , 5955–5961 (2002).
Chang, P. V., Hao, L., Offermanns, S. & Medzhitov, R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl Acad. Sci. USA 111 , 2247–2252 (2014).
Thaiss, C. A., Zmora, N., Levy, M. & Elinav, E. The microbiome and innate immunity. Nature 535 , 65–74 (2016).
Bortolatto, J. et al. Toll-like receptor 4 agonists adsorbed to aluminium hydroxide adjuvant attenuate ovalbumin-specific allergic airway disease: role of MyD88 adaptor molecule and interleukin-12/interferon-gamma axis. Clin. Exp. Allergy 38 , 1668–1679 (2008).
O’Hara, A. M., Moran, A. P., Würzner, R. & Orren, A. Complement-mediated lipopolysaccharide release and outer membrane damage in Escherichia coli J5: requirement for C9. Immunology 102 , 365–372 (2001).
Dong, L., Li, H., Wang, S. & Li, Y. Different doses of lipopolysaccharides regulate the lung inflammation of asthmatic mice via TLR4 pathway in alveolar macrophages. J. Asthma 46 , 229–233 (2009).
Yoshida, A., Kohchi, C., Inagawa, H., Nishizawa, T. & Soma, G. Improvement of allergic dermatitis via regulation of the Th1/Th2 immune system balance by macrophages activated with lipopolysaccharide derived from Pantoea agglomerans (IP-PA1). Anticancer Res. 29 , 4867–4870 (2009).
Siebenhaar, F., Redegeld, F. A., Bischoff, S. C., Gibbs, B. F. & Maurer, M. Mast cells as drivers of disease and therapeutic targets. Trends Immunol. 39 , 151–162 (2018).
Simon, D., Braathen, L. R. & Simon, H.-U. Eosinophils and atopic dermatitis. Allergy 59 , 561–570 (2004).
Fitzhugh, D. J. & Lockey, R. F. Allergen immunotherapy: a history of the first 100 years. Curr. Opin. Allergy Clin. Immunol. 11 , 554–559 (2011).
Price, D. B. et al. Blood eosinophil count and prospective annual asthma disease burden: a UK cohort study. Lancet Respir. Med. 3 , 849–858 (2015).
Woodruff, P. G. et al. Relationship between airway inflammation, hyperresponsiveness, and obstruction in asthma. J. Allergy Clin. Immunol. 108 , 753–758 (2001).
Rosenberg, H. F., Masterson, J. C. & Furuta, G. T. Eosinophils, probiotics, and the microbiome. J. Leukoc. Biol. 100 , 881–888 (2016).
Hosoki, K. et al. Differential activation of Eosinophils by ‘Probiotic’ Bifidobacterium bifidum and ‘Pathogenic’ Clostridium difficile. Int. Arch. Allergy Immunol. 152 (Suppl. 1), 83–89 (2010).
Choi, C. Y. et al. Anti-inflammatory potential of a heat-killed Lactobacillus strain isolated from Kimchi on house dust mite-induced atopic dermatitis in NC/Nga mice. J. Appl. Microbiol. 123 , 535–543 (2017).
Wang, X. et al. Oral administration of Lactobacillus paracasei L9 attenuates PM2.5-induced enhancement of airway hyperresponsiveness and allergic airway response in murine model of asthma. PLoS ONE 12 , e0171721 (2017).
Arthur, J. C. et al. Cutting edge: NLRP12 controls dendritic and myeloid cell migration to affect contact hypersensitivity. J. Immunol. 185 , 4515–4519 (2010).
Michels, K. R., Lukacs, N. W. & Fonseca, W. TLR activation and allergic disease: early life microbiome and treatment. Curr. Allergy Asthma Rep. 18 , 61 (2018).
Hruz, P. et al. NOD2 contributes to cutaneous defense against Staphylococcus aureus through alpha-toxin-dependent innate immune activation. Proc. Natl Acad. Sci. USA 106 , 12873–12878 (2009).
Wong, C. K., Chu, I. M., Hon, K. L., Tsang, M. S. & Lam, C. W. Aberrant expression of bacterial pattern recognition receptor NOD2 of basophils and microbicidal peptides in atopic dermatitis. Molecules 21 , 471 (2016).
Fyhrquist, N. et al. Acinetobacter species in the skin microbiota protect against allergic sensitization and inflammation. J. Allergy Clin. Immunol. 134 , 1301–1309 e1311 (2014).
Montaldo, E. et al. Human innate lymphoid cells. Immunol. Lett. 179 , 2–8 (2016).
Bal, S. M. et al. IL-1beta, IL-4 and IL-12 control the fate of group 2 innate lymphoid cells in human airway inflammation in the lungs. Nat. Immunol. 17 , 636–645 (2016).
Stefka, A. T. et al. Commensal bacteria protect against food allergen sensitization. Proc. Natl Acad. Sci. USA 111 , 13145–13150 (2014).
Konieczna, P. et al. Immunomodulation by Bifidobacterium infantis 35624 in the murine lamina propria requires retinoic acid-dependent and independent mechanisms. PLoS ONE 8 , e62617 (2013).
Lyons, A. et al. Bacterial strain-specific induction of Foxp3+ T regulatory cells is protective in murine allergy models. Clin. Exp. Allergy 40 , 811–819 (2010).
Konieczna, P. et al. Bifidobacterium infantis 35624 administration induces Foxp3 T regulatory cells in human peripheral blood: potential role for myeloid and plasmacytoid dendritic cells. Gut 61 , 354–366 (2012).
Fujimura, K. E. et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat. Med. 22 , 1187–1191 (2016).
Koizumi, S. I. & Ishikawa, H. Transcriptional regulation of differentiation and functions of effector T regulatory cells. Cells 8 (2019).
Rachid, R., Stephen-Victor, E. & Chatila, T. A. The microbial origins of food allergy. J. Allergy Clin. Immunol. 147 , 808–813 (2021).
Eicher, L. et al. A systematic review of factors influencing treatment adherence in chronic inflammatory skin disease - strategies for optimizing treatment outcome. J. Eur. Acad. Dermatol Venereol. 33 , 2253–2263 (2019).
Bousquet, J. et al. Allergic rhinitis. Nat. Rev. Dis. Prim. 6 , 95 (2020).
Wollenberg, A. et al. ETFAD/EADV Eczema task force 2020 position paper on diagnosis and treatment of atopic dermatitis in adults and children. J. Eur. Acad. Dermatol. Venereol. 34 , 2717–2744 (2020).
Bousquet, J. et al. Treatment of allergic rhinitis using mobile technology with real-world data: The MASK observational pilot study. Allergy 73 , 1763–1774 (2018).
Meng, Y., Wang, C. & Zhang, L. Recent developments and highlights in allergic rhinitis. Allergy 74 , 2320–2328 (2019).
Tordesillas, L., Berin, M. C. & Sampson, H. A. Immunology of food allergy. Immunity 47 , 32–50 (2017).
Katoh, N. et al. Japanese guidelines for atopic dermatitis 2020. Allergol. Int 69 , 356–369 (2020).
Nakamura, Y. et al. Japanese guidelines for adult asthma 2020. Allergol. Int 69 , 519–548 (2020).
Kawauchi, H., Yanai, K., Wang, D. Y., Itahashi, K. & Okubo, K. Antihistamines for allergic rhinitis treatment from the viewpoint of nonsedative properties. Int. J. Mol. Sci. 20 (2019).
Brozek, J. L. et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines: 2010 revision. J. Allergy Clin. Immunol. 126 , 466–476 (2010).
Wang, X. Y., Lim-Jurado, M., Prepageran, N., Tantilipikorn, P. & Wang de, Y. Treatment of allergic rhinitis and urticaria: a review of the newest antihistamine drug bilastine. Ther. Clin. Risk Manag. 12 , 585–597 (2016).
Estelle, F. & Simons, R. M.D. Advances in H 1 -Antihistamines. N. Engl. J. Med. 351 , 2203–2217 (2004).
Casale, T. B. & Oppenheimer, J. J. Next generation antihistamines: therapeutic rationale, accomplishments and advances. Expert Opin. Investig. Drugs 11 , 807–817 (2002).
Barnes, P. J. Therapeutic strategies for allergic diseases. Nature 402 (1999).
Wu, E. L. et al. Epistaxis risk associated with intranasal corticosteroid sprays: a systematic review and meta-analysis. Otolaryngol. Head. Neck Surg. 161 , 18–27 (2019).
Rodrigo, G. J. & Neffen, H. Efficacy of fluticasone furoate nasal spray vs. placebo for the treatment of ocular and nasal symptoms of allergic rhinitis: a systematic review. Clin. Exp. Allergy 41 , 160–170 (2011).
Bieber, T. Atopic dermatitis: an expanding therapeutic pipeline for a complex disease. Nat. Rev. Drug Disco. 21 , 21–40 (2022).
Blauvelt, A. et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet 389 , 2287–2303 (2017).
Kaliner, M. A. A novel and effective approach to treating rhinitis with nasal antihistamines. Ann. Allergy Asthma Immunol. 99 , 383–391 (2007).
Hampel, F. C. et al. Double-blind, placebo-controlled study of azelastine and fluticasone in a single nasal spray delivery device. Ann. Allergy Asthma Immunol. 105 , 168–173 (2010).
Segall, N., Prenner, B., Lumry, W., Caracta, C. F. & Tantry, S. K. Long-term safety and efficacy of olopatadine-mometasone combination nasal spray in patients with perennial allergic rhinitis. Allergy Asthma Proc. 40 , 301–310 (2019).
Shim, J. S., Kim, M. H., Kim, M. H., Cho, Y. J. & Chun, E. M. Risk of neuropsychiatric diseases according to the use of a leukotriene receptor antagonist in middle-aged and older adults with asthma: a nationwide population-based study using health claims data in Korea. J. Allergy Clin. Immunol. Pr. 9 , 4290–4297 (2021).
Hamri, S. et al. Convenient approach for the synthesis of ONO-LB-457, a potent leukotriene B4 receptor antagonist. Tetrahedron 77 (2021).
Kim, J. H. et al. Airway mechanics after withdrawal of a leukotriene receptor antagonist in children with mild persistent asthma: Double-blind, randomized, cross-over study. Pediatr. Pulmonol. 55 , 3279–3286 (2020).
Osada, T. & Okano, M. Japanese cedar and cypress pollinosis updated: new allergens, cross-reactivity, and treatment. Allergol. Int 70 , 281–290 (2021).
Pfaller, B., Bendien, S., Ditisheim, A. & Eiwegger, T. Management of allergic diseases in pregnancy. Allergy 77 , 798–811 (2022).
Lin, P. et al. A phosphatase-mimetic nano-stabilizer of mast cells for long-term prevention of allergic disease. Adv. Sci. 8 , 2004115 (2021).
Xu, H., Bin, N. R. & Sugita, S. Diverse exocytic pathways for mast cell mediators. Biochem Soc. Trans. 46 , 235–247 (2018).
Jackson, C. W., Pratt, C. M., Rupprecht, C. P., Pattanaik, D. & Krishnaswamy, G. Mastocytosis and mast cell activation disorders: clearing the air. Int. J. Mol. Sci. 22 (2021).
Sze, E., Bhalla, A. & Nair, P. Mechanisms and therapeutic strategies for non-T2 asthma. Allergy 75 , 311–325 (2020).
Peng, T., Qi, B., He, J., Ke, H. & Shi, J. Advances in the development of phosphodiesterase-4 inhibitors. J. Med. Chem. 63 , 10594–10617 (2020).
Janosova, V. et al. Phosphodiesterase 4 inhibitors in allergic rhinitis/rhinosinusitis. Front. Pharm. 11 , 1135 (2020).
Vestergaard, C. et al. European task force on atopic dermatitis position paper: treatment of parental atopic dermatitis during preconception, pregnancy and lactation period. J. Eur. Acad. Dermatol. Venereol. 33 , 1644–1659 (2019).
Drucker, A. M. et al. Systemic immunomodulatory treatments for patients with atopic dermatitis: a systematic review and network meta-analysis. JAMA Dermatol. 156 , 659–667 (2020).
Arakawa, H. et al. Japanese guidelines for childhood asthma 2020. Allergol. Int. 69 , 314–330 (2020).
Barnes, P. J. New therapies for asthma: is there any progress? Trends Pharm. Sci. 31 , 335–343 (2010).
Li, L., Liu, R., Peng, C., Chen, X. & Li, J. Pharmacogenomics for the efficacy and side effects of antihistamines. Exp. Dermatol. 31 , 993–1004 (2022).
Rollema, C. et al. Pharmacology, particle deposition and drug administration techniques of intranasal corticosteroids for treating allergic rhinitis. Clin. Exp. Allergy 52 , 1247–1263 (2022).
Hatakeyama, S. et al. The safety of pranlukast and montelukast during the first trimester of pregnancy: a prospective, two-centered cohort study in Japan. Congenit. Anom. 62 , 161–168 (2022).
Pfaar, O., Lou, H., Zhang, Y., Klimek, L. & Zhang, L. Recent developments and highlights in allergen immunotherapy. Allergy 73 , 2274–2289 (2018).
Hoffmann, H. J. et al. Novel approaches and perspectives in allergen immunotherapy. Allergy 72 , 1022–1034 (2017).
van Zelm, M. C., McKenzie, C. I., Varese, N., Rolland, J. M. & O’Hehir, R. E. Recent developments and highlights in immune monitoring of allergen immunotherapy. Allergy 74 , 2342–2354 (2019).
Dorofeeva, Y. et al. Past, present, and future of allergen immunotherapy vaccines. Allergy 76 , 131–149 (2021).
Yao, Y. et al. CD23 expression on switched memory B cells bridges T-B cell interaction in allergic rhinitis. Allergy 75 , 2599–2612 (2020).
Bousquet, J. et al. 2019 ARIA Care pathways for allergen immunotherapy. Allergy 74 , 2087–2102 (2019).
Cevhertas, L. et al. Advances and recent developments in asthma in 2020. Allergy 75 , 3124–3146 (2020).
Jutel, M., Bruggenjurgen, B., Richter, H. & Vogelberg, C. Real-world evidence of subcutaneous allergoid immunotherapy in house dust mite-induced allergic rhinitis and asthma. Allergy 75 , 2050–2058 (2020).
Pfaar, O. et al. Perspectives in allergen immunotherapy: 2019 and beyond. Allergy 74 Suppl 108 , 3–25 (2019).
Yao, Y. et al. Allergen immunotherapy improves defective follicular regulatory T cells in patients with allergic rhinitis. J. Allergy Clin. Immunol. 144 , 118–128 (2019).
Muraro, A. et al. EAACI guidelines on allergen immunotherapy: executive statement. Allergy 73 , 739–743 (2018).
Malling, H.-J. Minimising the risks of allergen-specific injection immunotherapy. Drug Saf. 23 , 323–332 (2000).
Cox, L., Larenas-Linnemann, D., Lockey, R. F. & Passalacqua, G. Speaking the same language: the World Allergy Organization subcutaneous immunotherapy systemic reaction grading system. J. Allergy Clin. Immunol. 125 , 569–574, 574 e561–574 e567 (2010).
Bousquet, J. et al. Highlights and recent developments in airway diseases in EAACI journals (2018). Allergy 74 , 2329–2341 (2019).
Pfaar, O. et al. Perspectives in allergen immunotherapy: 2017 and beyond. Allergy 73 Suppl 104 , 5–23 (2018).
Senti, G. et al. Intralymphatic allergen administration renders specific immunotherapy faster and safer: a randomized controlled trial. Proc. Natl Acad. Sci. USA 105 , 17908–17912 (2008).
Komlosi, Z. I. et al. Highlights of novel vaccination strategies in allergen immunotherapy. Immunol. Allergy Clin. North Am. 40 , 15–24 (2020).
Fokkens, W. J. et al. EUFOREA consensus on biologics for CRSwNP with or without asthma. Allergy 74 , 2312–2319 (2019).
Kraft, S. & Novak, N. Fc receptors as determinants of allergic reactions. Trends Immunol. 27 , 88–95 (2006).
Tsabouri, S., Tseretopoulou, X., Priftis, K. & Ntzani, E. E. Omalizumab for the treatment of inadequately controlled allergic rhinitis: a systematic review and meta-analysis of randomized clinical trials. J. Allergy Clin. Immunol. Pr. 2 , 332–340.e331 (2014).
Hammad, H. & Lambrecht, B. N. The basic immunology of asthma. Cell 184 , 1469–1485 (2021).
Breiteneder, H. et al. Future research trends in understanding the mechanisms underlying allergic diseases for improved patient care. Allergy 74 , 2293–2311 (2019).
Eschenbacher, W., Straesser, M., Knoeddler, A., Li, R. C. & Borish, L. Biologics for the treatment of allergic rhinitis, chronic rhinosinusitis, and nasal polyposis. Immunol. Allergy Clin. North Am. 40 , 539–547 (2020).
Weinstein, S. F. et al. Efficacy and safety of dupilumab in perennial allergic rhinitis and comorbid asthma. J. Allergy Clin. Immunol. 142 , 171–177.e171 (2018).
Newsom, M., Bashyam, A. M., Balogh, E. A., Feldman, S. R. & Strowd, L. C. New and emerging systemic treatments for atopic dermatitis. Drugs 80 , 1041–1052 (2020).
Misery, L., Huet, F., Gouin, O., Stander, S. & Deleuran, M. Current pharmaceutical developments in atopic dermatitis. Curr. Opin. Pharm. 46 , 7–13 (2019).
Meurs, H., Zaagsma, J., Maarsingh, H. & van Duin, M. Recent Patents in Allergy/Immunology: use of arginase inhibitors in the treatment of asthma and allergic rhinitis. Allergy 74 , 1206–1208 (2019).
Breiteneder, H. et al. Biomarkers for diagnosis and prediction of therapy responses in allergic diseases and asthma. Allergy 75 , 3039–3068 (2020).
Oyama, S. et al. Cynomolgus monkey model of interleukin-31-induced scratching depicts blockade of human interleukin-31 receptor A by a humanized monoclonal antibody. Exp. Dermatol 27 , 14–21 (2018).
Kruse, R. L. & Vanijcharoenkarn, K. Drug repurposing to treat asthma and allergic disorders: progress and prospects. Allergy 73 , 313–322 (2018).
Schuliga, M. NF-kappaB signaling in chronic inflammatory airway disease. Biomolecules 5 , 1266–1283 (2015).
Sherman, D. J. & Li, J. Proteasome inhibitors: harnessing proteostasis to combat disease. Molecules 25 (2020).
Ramadass, V., Vaiyapuri, T. & Tergaonkar, V. Small molecule NF-kappaB pathway inhibitors in clinic. Int. J. Mol. Sci. 21 (2020).
Hua, W. et al. Rapamycin inhibition of eosinophil differentiation attenuates allergic airway inflammation in mice. Respirology 20 , 1055–1065 (2015).
KleinJan, A. et al. The Notch pathway inhibitor stapled alpha-helical peptide derived from mastermind-like 1 (SAHM1) abrogates the hallmarks of allergic asthma. J. Allergy Clin. Immunol. 142 , 76–85.e78 (2018).
Zhou, Z. et al. Avasimibe alleviates disruption of the airway epithelial barrier by suppressing the Wnt/beta-Catenin signaling pathway. Front Pharm. 13 , 795934 (2022).
Jha, A. et al. Increased nasal mucosal interferon and CCL13 response to a TLR7/8 agonist in asthma and allergic rhinitis. J. Allergy Clin. Immunol. 147 , 694–703.e612 (2021).
Siddall, H. et al. Intranasal GSK2245035, a Toll-like receptor 7 agonist, does not attenuate the allergen-induced asthmatic response in a randomized, double-blind, placebo-controlled experimental medicine study. PLoS ONE 15 , e0240964 (2020).
Menzies-Gow, A. et al. Tezepelumab in adults and adolescents with severe, uncontrolled asthma. N. Engl. J. Med 384 , 1800–1809 (2021).
Chinthrajah, S. et al. Phase 2a randomized, placebo-controlled study of anti-IL-33 in peanut allergy. JCI Insight 4 (2019).
Angkasekwinai, P. & Dong, C. IL-9-producing T cells: potential players in allergy and cancer. Nat. Rev. Immunol. 21 , 37–48 (2021).
Kupczyk, M. & Kuna, P. Targeting the PGD2/CRTH2/DP1 signaling pathway in asthma and allergic disease: current status and future perspectives. Drugs 77 , 1281–1294 (2017).
Corren, J. New targeted therapies for uncontrolled asthma. J. Allergy Clin. Immunol. Pr. 7 , 1394–1403 (2019).
Colagiovanni, D. B. et al. A nonclinical safety and pharmacokinetic evaluation of N6022: a first-in-class S-nitrosoglutathione reductase inhibitor for the treatment of asthma. Regul. Toxicol. Pharm. 62 , 115–124 (2012).
Sun, X. et al. Discovery of s-nitrosoglutathione reductase inhibitors: potential agents for the treatment of asthma and other inflammatory diseases. ACS Med. Chem. Lett. 2 , 402–406 (2011).
Que, L. G. et al. Effect of the S-nitrosoglutathione reductase inhibitor N6022 on bronchial hyperreactivity in asthma. Immun. Inflamm. Dis. 6 , 322–331 (2018).
Zhang, Y., Lan, F. & Zhang, L. Update on pathomechanisms and treatments in allergic rhinitis. Allergy 77 , 3309–3319 (2022).
Licari, A. et al. Biologics in children with allergic diseases. Curr. Pediatr. Rev. 16 , 140–147 (2020).
Cox, L. Biologics and allergy immunotherapy in the treatment of allergic diseases. Immunol. Allergy Clin. North Am. 40 , 687–700 (2020).
Jung, H. J., Chung, Y. J., Choi, Y .S., Chung, P. S. & Mo, J. H. Clinical efficacy and safety of low-level laser therapy in patients with perennial allergic rhinitis: a randomized, double-blind, Placebo-controlled trial. J. Clin. Med . 10 (2021).
Costa, T. M. R. et al. Rhinophototherapy, an alternative treatment of allergic rhinitis: Systematic review and meta-analysis. Braz. J. Otorhinolaryngol. 87 , 742–752 (2021).
Natsume, O. et al. Two-step egg introduction for prevention of egg allergy in high-risk infants with eczema (PETIT): a randomised, double-blind, placebo-controlled trial. Lancet 389 , 276–286 (2017).
Nakao, A. Circadian regulation of the biology of allergic disease: clock disruption can promote allergy. Front. Immunol. 11 , 1237 (2020).
Hong, X. et al. Genome-wide association study identifies peanut allergy-specific loci and evidence of epigenetic mediation in US children. Nat. Commun. 6 , 6304 (2015).
Andiappan, A. K. et al. Genome-wide association study for atopy and allergic rhinitis in a Singapore Chinese population. PLoS ONE 6 , e19719 (2011).
Ramasamy, A. et al. A genome-wide meta-analysis of genetic variants associated with allergic rhinitis and grass sensitization and their interaction with birth order. J. Allergy Clin. Immunol. 128 , 996–1005 (2011).
Shirkani, A. et al. The Role of interleukin-4 and 13 gene polymorphisms in allergic rhinitis: a case control study. Rep. Biochem. Mol. Biol. 8 , 111–118 (2019).
Amo, G. et al. A Nonsynonymous FCER1B SNP is associated with risk of developing allergic rhinitis and with IgE levels. Sci. Rep. 6 , 19724 (2016).
Zhao, C. N. et al. The association of GSDMB and ORMDL3 gene polymorphisms with asthma: a meta-analysis. Allergy Asthma Immunol. Res. 7 , 175–185 (2015).
Nieto-Fontarigo, J. J. et al. The CD14 (-159 C/T) SNP is associated with sCD14 levels and allergic asthma, but not with CD14 expression on monocytes. Sci. Rep. 8 , 4147 (2018).
Nowakowska, J. et al. Nitric oxide synthase 2 promoter polymorphism is a risk factor for allergic asthma in children. Med. (Kaunas.) 57 , 1341 (2021).
Beigh, A. H. et al. Influence of single gene variants of FOXP3 on allergic asthma predisposition. Gene 763 , 145073 (2020).
Stevens, M. L. et al. Disease-associated KIF3A variants alter gene methylation and expression impacting skin barrier and atopic dermatitis risk. Nat. Commun. 11 , 4092 (2020).
Li, Y. et al. Association of UBASH3A gene polymorphism and atopic dermatitis in the Chinese Han population. Genes Immun. 18 , 158–162 (2017).
Dytiatkovskyi, V., Drevytska, T., Lapikova-Bryhinska, T., Dosenko, V. & Abaturov, O. Genotype associations with the different phenotypes of atopic dermatitis in children. Acta Med. 64 , 96–100 (2021).
Hirota, T. et al. Genome-wide association study identifies eight new susceptibility loci for atopic dermatitis in the Japanese population. Nat. Genet 44 , 1222–1226 (2012).
Ramadass, V., Vaiyapuri, T. & Tergaonkar, V. Small molecule NF-κB pathway inhibitors in clinic. Int. J. Mol. Sci. 21 (2020).
Download references
Acknowledgements
We thank Professor Qianfei Wang (Beijing Institute of genomics, Chinese Academy of Sciences) and Professor Qianben Wang (Department of Pathology, Duke University School of Medicine, Durham, USA) for critical comments and advice on the manuscript. This work was supported by the General project of National Natural Science Foundation of China (No. 82174243, 81973715 and 82204948), National Key R&D Program of China (2020YFC2003100), Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (No: ZYYCXTD-C-202001), General project of Beijing Natural Science Foundation (No.7202110).
Author information
These authors contributed equally: Ji Wang, Yumei Zhou
Authors and Affiliations
National Institute of TCM constitution and Preventive Medicine, School of Chinese Medicine, Beijing University of Chinese Medicine, Beijing, 100029, P.R. China
Ji Wang, Yumei Zhou, Honglei Zhang, Linhan Hu, Juntong Liu, Tianyi Wang, Haiyun Zhang, Linpeng Cong & Qi Wang
National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 1000210, China
You can also search for this author in PubMed Google Scholar
Contributions
J.W., Y.Z., L.H., H.Z., J.L., L.W., H.Z. and T.W. wrote the manuscript and created the figures and table. J.W., Y.Z. and Q.W. provided the conceptual idea. L.C. edited the manuscript. All authors have read and approved the article.
Corresponding author
Correspondence to Qi Wang .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Wang, J., Zhou, Y., Zhang, H. et al. Pathogenesis of allergic diseases and implications for therapeutic interventions. Sig Transduct Target Ther 8 , 138 (2023). https://doi.org/10.1038/s41392-023-01344-4
Download citation
Received : 02 September 2022
Revised : 20 January 2023
Accepted : 03 February 2023
Published : 24 March 2023
DOI : https://doi.org/10.1038/s41392-023-01344-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Association between interleukin-6-174g/c gene polymorphism and asthma severity: exploring the role of total serum ige, blood eosinophils, and feno as markers of type 2 inflammation.
- Mona Al-Ahmad
- Mohammad Z. Haider
Allergy, Asthma & Clinical Immunology (2024)
Nanozyme-Engineered Hydrogels for Anti-Inflammation and Skin Regeneration
- Amal George Kurian
- Rajendra K. Singh
- Hae-Won Kim
Nano-Micro Letters (2024)
B-cell-derived IL-10 promotes allergic sensitization in asthma regulated by Bcl-3
- Guojun Qian
- Wenxia Jiang
- Xiaoren Zhang
Cellular & Molecular Immunology (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
60 Allergy Essay Topic Ideas & Examples
🏆 best allergy topic ideas & essay examples, 👍 good research topics about allergy, 📌 most interesting allergy topics to write about.
- Allergic Rhinitis: The Case Study The objective of this paper is to discuss the case of a 35-year-old woman with a history of nasal congestion. Management and treatment of illnesses are often intended to alleviate the symptoms of a disease, […]
- Diabetes and Allergies: A Statistical Check The current dataset allowed us to test the OR for the relationship between family history of diabetes and the presence of diabetes in a particular patient: all variables were dichotomous and discrete and could take […] We will write a custom essay specifically for you by our professional experts 808 writers online Learn More
- Allergic Rhinitis: Dianostic and Treatment Allergic rhinitis is an intermittent or persistent inflammation of the mucous membrane of the upper respiratory tract, characterized by nasal congestion, discharge, itching, sneezing, and a combination of several symptoms is possible.
- Use of Scientific Method in Asthma and Allergic Reactions Study As in the case of asthma and allergic reactions investigations, descriptive studies can be used to describe the nature of the relationship between asthma and asthma attack, therefore explaining the cause and effect.
- Amoxicillin Allergies in Patients In a study, pediatric patients with histories of allergic reactions to amoxicillin were tested with the antibiotic. In a recent study, allergists observed that patients, especially children, with amoxicillin-associated allergic reactions became non-allergic when rechallenged […]
- Characteristics of Allergic Contact Dermatitis It is known that “allergic contact dermatitis is caused by a delayed-type hypersensitivity response to contact allergens. Patients must then be provided with practical behavioral modifications to help decrease the inflammatory response of this disease.
- Allergic Diseases and the Hygiene Hypothesis In 2009, a large cohort study set to investigate the effect of antibiotic exposure in early age and development of asthma found a positive association between antibiotic use in early years of life and development […]
- Peanut Allergy: Teaching Plan for Child With Anaphylaxis Reaction In this case, the 11-year old boy has swellings on the face, tongue and the lips, which show that he is likely to be diagnosed with the reaction.
- Allergic Rhinitis Case Donna While referring to Donna’s history, it is possible to state that the possible disease causing the observed symptoms is allergic rhinitis.
- Biological Basis of Asthma and Allergic Disease The immunological response in asthmatic people fails in the regulation of the production of the Th2 cells and the anti-inflammatory cells.
- Diagnostics and Immunotherapy in Allergic Reactions: Food Allergies In review articles, it is generally stated that oral tolerance can be induced by a variety of factors and immune system cells, the most important of which are the regulatory T cells.
- Review of Hygiene Hypothesis for Allergies According to Gibbs et al, the concept that non-exposure to infections in early life leads to the development of Atopic disease has come to be referred to as hygiene hypothesis.
- Dry Eye Syndrome and Allergic Conjunctivitis As it can be seen, AC can be related to various diseases in children, including severe and progressing conditions; it can also be complicated by DES, which is frequently hard to diagnose due to a […]
- Food Allergies Management When a person who is allergic to tomatoes decides to visit a Mexican restaurant and orders chili con carne, it is necessary to have a special plan of action to guarantee the customer’s safety.
- Allergic Patient Experiences and Disease Awareness The following section of results includes information presented by the interview and involves her experience regarding the course of the disease, its occurrence and treatment, and the limitations it set on her life.
- Recent and Promising Food Allergy Treatments Generally, the effect of the two allergies is the same, and the treatment may also be similar. In the United States, the amount of money allocated to study food allergies and how to control their […]
- Feeding Baby: How to Avoid Food Allergies Food allergies have become a menace in contemporary society, but the unfortunate thing is that there is no known underlying cause for this upsurge of the food allergies.
- Allergic Rhinitis: A Critical Discussion This view is reinforced by Liu et al, who argue that the production of high levels of allergen-specific IgE in certain individuals adversely interacts with inflammatory cells found in the respiratory and upper airways, particularly […]
- Tregs in Allergies and Autoimmune Diseases In the investigation of the role of Tregs in the autoimmunity, depletion of the Tregs resulted in increased sensitivity to LPS.
- Survey of Food Allergies in the UAE The purpose of this research paper is to create a survey about food allergies in the UAE with the aim of establishing the seriousness of the situation within the region.
- Hematopoietic Stem and Progenitor Cells in Inflammation and Allergy
- Desensitization Therapy for Allergy: Theory and Practice
- Asthma and Common Allergy Symptoms
- Convincing Outcomes From Sensitizing the Wistar Strain Rats Used as Peanut Allergy Model
- American Academy of Asthma, Allergy and Immunology Analysis
- Asthma and Food Allergy in Children: Is There a Connection or Interaction?
- The Ironic Allergy Treatment
- Host- and Microbe-Dependent Dietary Lipid Metabolism in the Control of Allergy, Inflammation, and Immunity
- Food Allergy and Intolerance Products Market to Grab the Largest Market Share by 2018
- The Peanut Allergy Problem
- Getting The Upper Hand On Asthma Allergy
- Microbiology: Allergy and Common Skin Reactions
- Allergy and Emergency Response Essay
- Food Allergy and Food Intolerance Identification and Treatment
- Allergy Medications Which Ones Are the Best
- Parsing the Peanut Panic: The Social Life of a Contested Food Allergy
- Penicillin Allergy and Its Effects on the Cost of Medical
- Lipophilic Allergens, Different Modes of Allergen-Lipid Interaction and Their Impact on Asthma and Allergy
- The Rule With Any Allergy Restriction
- Ige Mediated Peanut Allergy Development Diagnosis and Treatment
- Drug Allergy Profile From a National Drug Allergy Registry
- Preventive Allergen-Specific Vaccination Against Allergy: Mission Possible?
- Sphingolipids and Epoxidized Lipid Metabolites in the Control of Gut Immunosurveillance and Allergy
- Severe Asthma and Allergy: A Pediatric Perspective
- Nutrition and Avoidance Diets in Children With Food Allergy
- Skin Care Products May Cause Your Skin Allergy
- Penicillin Allergy De-labeling Results in Significant Changes in Outpatient Antibiotic Prescribing Patterns
- Phenotype Profiling and Allergy in Otitis-Prone Children
- Oral Immunotherapy and Basophil and Mast Cell Reactivity in Food Allergy
- Helpful Household Tips For Allergy Sufferers
- Adolescents With Food Allergy Health and Social Care
- Intestinal Epithelial Cells Regulate Gut Eotaxin Responses and Severity of Allergy
- Insights Into the Etiology, Prevention, and Treatment of Food Allergy
- Maternal Schistosomiasis: Immunomodulatory Effects With Lasting Impact on Allergy and Vaccine Responses
- Hymenoptera Venom Allergy: How Does Venom Immunotherapy Prevent Anaphylaxis From Bee and Wasp Stings?
- What Are the Symptoms of a Food Allergy
- Recruiting The Most Suitable Laboratory Animals For Food Allergy Modeling
- Shaping the Gut Microbiota by Breastfeeding: The Gateway to Allergy Prevention?
- Ragweed Allergy Home Remedies
- Chicago (A-D)
- Chicago (N-B)
IvyPanda. (2023, September 26). 60 Allergy Essay Topic Ideas & Examples. https://ivypanda.com/essays/topic/allergy-essay-topics/
"60 Allergy Essay Topic Ideas & Examples." IvyPanda , 26 Sept. 2023, ivypanda.com/essays/topic/allergy-essay-topics/.
IvyPanda . (2023) '60 Allergy Essay Topic Ideas & Examples'. 26 September.
IvyPanda . 2023. "60 Allergy Essay Topic Ideas & Examples." September 26, 2023. https://ivypanda.com/essays/topic/allergy-essay-topics/.
1. IvyPanda . "60 Allergy Essay Topic Ideas & Examples." September 26, 2023. https://ivypanda.com/essays/topic/allergy-essay-topics/.
Bibliography
IvyPanda . "60 Allergy Essay Topic Ideas & Examples." September 26, 2023. https://ivypanda.com/essays/topic/allergy-essay-topics/.
- Asthma Paper Topics
- Microbiology Questions
- Food Essay Ideas
- Epigenetics Essay Titles
- Genetics Research Ideas
- Intensive Care Research Topics
- Dermatology Topics
- Health Promotion Research Topics
- Disease Questions
- Evidence-Based Practice Titles
- Hygiene Essay Topics
- Malnutrition Titles
- Nursing Care Plan Paper Topics
- Metabolism Research Topics
- Arthritis Titles
University of Utah Hospital
General questions.
- Billing & Insurance

- Health Care Home
Uncover Your Triggers With Allergy Testing: 5 Common Questions Answered
Allergies can affect everyone differently, including the symptoms you have and the treatments that work. Mili Shum, MD , an allergist at University of Utah Health, helps patients suffering with asthma and allergy symptoms every day, often through the process of allergy testing.
“Allergy testing is a great way to safely identify allergens that are making you uncomfortable or even endangering your life,” Shum says. “If you don’t know what’s causing your allergy or asthma symptoms, an allergy test can help us pinpoint the cause and develop a plan to help you manage those symptoms moving forward.”
If you’re not sure about allergy testing, here are some commonly asked questions that may help guide you in deciding whether or not to seek out an allergist’s help .
1. When should I consider allergy testing?
You may want to consider allergy testing if you are experiencing allergy or asthma symptoms that are unexplained, or that you cannot control with antihistamines or over-the-counter allergy medications. Allergy testing can identify allergens that will help you and your allergist come up with an effective treatment plan.
2. What are the types of allergy tests?
The type of allergy testing depends on the type of reaction that the patient is experiencing. These testing methods include:
- Skin Prick: Skin prick testing is the most common test used to evaluate environmental, food, drug, and venom allergies. Small amounts of allergens are placed just barely into the surface of the skin through the use of small needles, which are then monitored for an immediate reaction. This test may be done on the upper back or forearm. Many common allergens can be tested at once.
- Intradermal Skin: Intradermal skin testing involves a small amount of allergen being carefully injected just barely into the skin of your arm. It is then monitored for up to 15 minutes for any immediate reactions. This may be recommended by your allergist for further investigation following a skin prick test.
- Patch: Patch testing is performed for contact dermatitis or reactions to products and some drugs. It can detect delayed reactions, which may take several days to develop. Allergens are applied to patches that are placed on top of your skin and are worn for 48 hours. When taken off, irritated skin at the site of the patch indicates a possible allergy.
3. How long does allergy testing take?
The length of time depends on the type of allergen, the number of allergens, and the method of testing. For skin prick and intradermal skin testing, results are monitored and recorded after 15 minutes, while patch testing takes at least 48 hours to develop results. Your allergist will also need to review the results and possibly schedule you for a follow-up appointment.
4. Does allergy testing hurt?
Allergy testing does not hurt, but it can cause some minor irritation.
5. Does allergy testing cause anaphylaxis?
For the vast majority of people, the amount of allergen applied to your skin is too small to cause a severe allergic reaction. Patients who undergo the skin prick or intradermal skin testing are monitored during the entirety of the test.
If you or your child are experiencing allergy symptoms, consider allergy testing. It is a safe and effective way to help your allergist diagnose an allergy and can be the first step in helping you get on a treatment plan that will improve your symptoms and quality of life.

ENG 101: English Composition I - Tangvik
Selecting sources for research papers.
- Find Articles
- Find Streaming Videos

You can now chat with us by text message!
Text (857) 877 - 2255 to chat with a librarian ., your carrier's text rates may apply., spring 2024 library hours, monday - thursday: 8:30am - 9:00pm, friday: 8:30am - 4:30pm, saturday: 8:30am - 4:00pm, sun. closed.
You can find the services we offer at a distance via Zoom, Teams, and other internet-based methods on our remote services page.
Evening class instruction is available by request. To see if the RCC Library is closed on school holidays and semester breaks, please consult the Library Calendar.
857-701-1380
or via text at 857-877-2255
1234 Columbus Avenue Building 3, Room 211 Boston, MA 02120

Types of Sources
Information comes from many places. We may get information from books, the news, or social media.
When using information for an academic paper, some sources may be more helpful than others. The library provides access to the following kinds of sources:
- Scholarly articles (peer reviewed research from academic journals, usually written by professors or graduate students)
- News and magazine articles
- Ebooks
- Reference books, such as encyclopedias and dictionaries
- Streaming videos
Evaluating Sources
When you locate a source that you may want to use in your research, you should first make sure that the source is reliable, up to date, and relevant to your research topic. No matter what your source type (scholarly article, book, encyclopedia, etc.), ask yourself the following questions:
- When was this source published? Is the information outdated, or is it still up to date? (Currency)
- Who created this source, and what are their qualifications? Are they an expert on the topic? (Authority)
- Is the information true? Can you fact-check it? (Accuracy)
- Why was this information published? To inform you? To sell you something? To convince you? (Purpose)
- Does the source help you answer your research question? Is it a relevant source? (Relevance)
Asking these questions will help you choose the best sources for your paper.
- << Previous: Welcome
- Next: Find Books >>
- Last Updated: Apr 27, 2024 10:13 AM
- URL: https://libguides.rcc.mass.edu/ENG101Ken
- Share full article
Advertisement
Supported by
Guest Essay
What Sentencing Could Look Like if Trump Is Found Guilty

By Norman L. Eisen
Mr. Eisen is the author of “Trying Trump: A Guide to His First Election Interference Criminal Trial.”
For all the attention to and debate over the unfolding trial of Donald Trump in Manhattan, there has been surprisingly little of it paid to a key element: its possible outcome and, specifically, the prospect that a former and potentially future president could be sentenced to prison time.
The case — brought by Alvin Bragg, the Manhattan district attorney, against Mr. Trump — represents the first time in our nation’s history that a former president is a defendant in a criminal trial. As such, it has generated lots of debate about the case’s legal strength and integrity, as well as its potential impact on Mr. Trump’s efforts to win back the White House.
A review of thousands of cases in New York that charged the same felony suggests something striking: If Mr. Trump is found guilty, incarceration is an actual possibility. It’s not certain, of course, but it is plausible.
Jury selection has begun, and it’s not too soon to talk about what the possibility of a sentence, including a prison sentence, would look like for Mr. Trump, for the election and for the country — including what would happen if he is re-elected.
The case focuses on alleged interference in the 2016 election, which consisted of a hush-money payment Michael Cohen, the former president’s fixer at the time, made in 2016 to a porn star, Stormy Daniels, who said she had an affair with Mr. Trump. Mr. Bragg is arguing that the cover-up cheated voters of the chance to fully assess Mr. Trump’s candidacy.
This may be the first criminal trial of a former president in American history, but if convicted, Mr. Trump’s fate is likely to be determined by the same core factors that guide the sentencing of every criminal defendant in New York State Court.
Comparable cases. The first factor is the base line against which judges measure all sentences: how other defendants have been treated for similar offenses. My research encompassed almost 10,000 cases of felony falsifying business records that have been prosecuted across the state of New York since 2015. Over a similar period, the Manhattan D.A. has charged over 400 of these cases . In roughly the first year of Mr. Bragg’s tenure, his team alone filed 166 felony counts for falsifying business records against 34 people or companies.
Contrary to claims that there will be no sentence of incarceration for falsifying business records, when a felony conviction involves serious misconduct, defendants can be sentenced to some prison time. My analysis of the most recent data indicates that approximately one in 10 cases in which the most serious charge at arraignment is falsifying business records in the first degree and in which the court ultimately imposes a sentence, results in a term of imprisonment.
To be clear, these cases generally differ from Mr. Trump’s case in one important respect: They typically involve additional charges besides just falsifying records. That clearly complicates what we might expect if Mr. Trump is convicted.
Nevertheless, there are many previous cases involving falsifying business records along with other charges where the conduct was less serious than is alleged against Mr. Trump and prison time was imposed. For instance, Richard Luthmann was accused of attempting to deceive voters — in his case, impersonating New York political figures on social media in an attempt to influence campaigns. He pleaded guilty to three counts of falsifying business records in the first degree (as well as to other charges). He received a sentence of incarceration on the felony falsification counts (although the sentence was not solely attributable to the plea).
A defendant in another case was accused of stealing in excess of $50,000 from her employer and, like in this case, falsifying one or more invoices as part of the scheme. She was indicted on a single grand larceny charge and ultimately pleaded guilty to one felony count of business record falsification for a false invoice of just under $10,000. She received 364 days in prison.
To be sure, for a typical first-time offender charged only with run-of-the-mill business record falsification, a prison sentence would be unlikely. On the other hand, Mr. Trump is being prosecuted for 34 counts of conduct that might have changed the course of American history.
Seriousness of the crime. Mr. Bragg alleges that Mr. Trump concealed critical information from voters (paying hush money to suppress an extramarital relationship) that could have harmed his campaign, particularly if it came to light after the revelation of another scandal — the “Access Hollywood” tape . If proved, that could be seen not just as unfortunate personal judgment but also, as Justice Juan Merchan has described it, an attempt “to unlawfully influence the 2016 presidential election.”
History and character. To date, Mr. Trump has been unrepentant about the events alleged in this case. There is every reason to believe that will not change even if he is convicted, and lack of remorse is a negative at sentencing. Justice Merchan’s evaluation of Mr. Trump’s history and character may also be informed by the other judgments against him, including Justice Arthur Engoron’s ruling that Mr. Trump engaged in repeated and persistent business fraud, a jury finding that he sexually abused and defamed E. Jean Carroll and a related defamation verdict by a second jury.
Justice Merchan may also weigh the fact that Mr. Trump has been repeatedly held in contempt , warned , fined and gagged by state and federal judges. That includes for statements he made that exposed witnesses, individuals in the judicial system and their families to danger. More recently, Mr. Trump made personal attacks on Justice Merchan’s daughter, resulting in an extension of the gag order in the case. He now stands accused of violating it again by commenting on witnesses.
What this all suggests is that a term of imprisonment for Mr. Trump, while far from certain for a former president, is not off the table. If he receives a sentence of incarceration, perhaps the likeliest term is six months, although he could face up to four years, particularly if Mr. Trump chooses to testify, as he said he intends to do , and the judge believes he lied on the stand . Probation is also available, as are more flexible approaches like a sentence of spending every weekend in jail for a year.
We will probably know what the judge will do within 30 to 60 days of the end of the trial, which could run into mid-June. If there is a conviction, that would mean a late summer or early fall sentencing.
Justice Merchan would have to wrestle in the middle of an election year with the potential impact of sentencing a former president and current candidate.
If Mr. Trump is sentenced to a period of incarceration, the reaction of the American public will probably be as polarized as our divided electorate itself. Yet as some polls suggest — with the caveat that we should always be cautious of polls early in the race posing hypothetical questions — many key swing state voters said they would not vote for a felon.
If Mr. Trump is convicted and then loses the presidential election, he will probably be granted bail, pending an appeal, which will take about a year. That means if any appeals are unsuccessful, he will most likely have to serve any sentence starting sometime next year. He will be sequestered with his Secret Service protection; if it is less than a year, probably in Rikers Island. His protective detail will probably be his main company, since Mr. Trump will surely be isolated from other inmates for his safety.
If Mr. Trump wins the presidential election, he can’t pardon himself because it is a state case. He will be likely to order the Justice Department to challenge his sentence, and department opinions have concluded that a sitting president could not be imprisoned, since that would prevent the president from fulfilling the constitutional duties of the office. The courts have never had to address the question, but they could well agree with the Justice Department.
So if Mr. Trump is convicted and sentenced to a period of incarceration, its ultimate significance is probably this: When the American people go to the polls in November, they will be voting on whether Mr. Trump should be held accountable for his original election interference.
What questions do you have about Trump’s Manhattan criminal trial so far?
Please submit them below. Our trial experts will respond to a selection of readers in a future piece.
Norman L. Eisen investigated the 2016 voter deception allegations as counsel for the first impeachment and trial of Donald Trump and is the author of “Trying Trump: A Guide to His First Election Interference Criminal Trial.”
The Times is committed to publishing a diversity of letters to the editor. We’d like to hear what you think about this or any of our articles. Here are some tips . And here’s our email: [email protected] .
Follow the New York Times Opinion section on Facebook , Instagram , TikTok , WhatsApp , X and Threads .
購読者会員サイト Club Alphaへ
- 英文記事のバックナンバー
Essay 2024.5.3
A new type of woman 新しいタイプの女性たち

Level What’s your image of a typical Japanese woman? Delicate? Small and slim? Black hair? Fair skin? The advertising images I see are pretty similar. I’ve got the black hair and fair skin, but I’ve neve...
この記事は会員限定です。 全文見るには、会員登録・ログインが必要です
会員限定の記事・バックナンバーが 毎月5本無料 で読むことができます。
定期購読している方、 メルマガ会員の方はこちら
Recommended for you

現在ログインしていません
- Webサイトご利用案内
- 購読・購入方法のご案内

- Video Lectures
- Listening & Speaking
- Describe This!
- 留学生Lillianの教えて 日本のコト!
- Error Corrector
- Fujisanデジタル版について

The Japan Times Alpha 公式 @japantimes_alpha
Alpha 編集長 @st_takahashi
The Japan Times Alpha 公式
The Japan Times Alpha 公式 @thejapantimesalpha
Alpha Online ログイン
初回ログインの手順は こちら をご確認ください。
ID・PW・Passコードをお持ちの方
パスワードをお忘れですか?
Alpha Passコード
※Fujisan以外でご購読の方は初回にメルマガ会員登録を行い、ログイン時にID・パスワード・Passコードの入力が必要です。
紙面2面の下部に記載され、毎月更新されます。
メルマガ会員へのご登録 ※新聞を購読していなくても登録できます
メルマガ会員になると、毎週の見どころを紹介するメルマガが届くほか、Alpha Onlineの英文記事を月5本までお読みいただけます。
※Fujisanマガジンサービスの提携サイトにてご登録となります
定期購読申込み 無料試読申込み

IMAGES
VIDEO
COMMENTS
Some of the common types of triggers of allergies include the following: Food: Common allergy triggers include peanuts, nuts, fish, soy, wheat, shellfish, milk, and eggs. Medications: Reaction to certain drugs, most commonly penicillin or penicillin-based antibiotics. Latex. An allergy that can be triggered by both contact with the skin or ...
Allergies are common, affecting more than 50 million Americans. Types of allergens include those that are airborne or those that arise from contact with your skin, from food, and from medications. Common allergy symptoms include runny nose, congestion, cough, and sneezing, among others. Less commonly, an allergen can produce a severe allergic ...
An allergic reaction causes inflammation and irritation. However, the specific symptoms will depend on the type of allergen. For example, allergic reactions may occur in the gut, skin, sinuses ...
There are different types of allergic reactions to foods. There are differences between IgE-mediated allergies, non-IgE mediated allergies and food intolerances. Bees, wasps, hornets, yellow jackets and fire ants are the most common stinging insects that cause an allergic reaction. Non-stinging insects can also cause allergic reactions.
Allergies arise if the body's immune system overreacts to foreign substances (allergens) that are usually harmless in most people, such as pollen or certain foods. In some cases the symptoms are quite mild, but they can also be a real nuisance and have a considerable impact in everyday life. There are various treatment options for allergies.
Symptoms. Allergy symptoms, which depend on the substance involved, can affect your airways, sinuses and nasal passages, skin, and digestive system. Allergic reactions can range from mild to severe. In some severe cases, allergies can trigger a life-threatening reaction known as anaphylaxis. Hay fever, also called allergic rhinitis, can cause:
Eye allergies ( allergic conjunctivitis) occur when the eyes come into contact with an allergen, causing the eyes to become irritated and inflamed. Pollen, dust mites, mold spores, and pet dander ...
Your symptoms, including any that seem unrelated to allergies, and when they began; Your family's history of allergies and asthma, including specific types of allergies, if you know them; All medications, vitamins and other supplements you take, including doses; Questions to ask during your appointment; Some basic questions to ask include:
Type of Allergy Symptoms Prevalence Affected Organ Causes Reference; Allergic rhinatisis: Sneezing, itchy, watery, and red eyes, stuffy or runny nose, swelling around the eyes. ... Many research papers on allergen bioinformatics and immunoinformatics have been published by various groups of researchers [198,199].
An allergy occurs when, for some reason, the immune system creates a response against a harmless substance, like pollen or a certain kind of food. Allergies are very common, and it is estimated that around one in three people has some type of allergy. Allergy can generate a reaction in any part of the body.
Sneezing, difficulty breathing, cramps, and vomiting-all are allergy symptoms. Learn the types of allergies, specific allergy symptoms, and emergency warning signs.
Allergic reactions may happen only when you are exposed to a substance, such as with a food, latex, or pet dander allergy. Sometimes, they can occur seasonally, such as with allergies to pollen. Or, an allergy to something like mold or dust mites can be year-round depending on where a person lives. 1. The most common types of allergies are ...
shortness of breath. wheezing. runny nose and eyes. pain over the sinuses (at the bridge of the nose, near the eyes, over cheeks and at the forehead) coughing. skin rashes (nettle rashes or hives ...
Allergies and colds share symptoms like sneezing and stuffy or runny nose, headache, and fatigue. However, what they don't share is a fever. You will not get a fever if you're having an allergic reaction. Also, you don't experience itchy ears with the common cold the way you would with allergies.
The antibodies are also shaped to attach to specific types of allergens. This can be imagined like a lock and key. Each type of antibody (key) attaches to a specific allergen (lock). During an allergic response, the antibodies will attach to the incoming allergen. Then, this conglomerate will travel to the mast cells located in all body tissues.
An allergy occurs when the body's immune system becomes hyperreactive to a substance that could be harmless in itself, called an allergen. The external substances that provoke allergies are ...
Ex vivo tests for type I allergy will play a role in both the diagnosis of occupational allergy and food allergy. Moreover, they are an important element in diagnosis of paediatric allergy, since they constitute an alternative to allergen exposure tests currently considered as the gold standard in diagnosis of allergy.
Allergic diseases such as allergic rhinitis (AR), allergic asthma (AAS), atopic dermatitis (AD), food allergy (FA), and eczema are systemic diseases caused by an impaired immune system.
Allergic Rhinitis: Dianostic and Treatment. Allergic rhinitis is an intermittent or persistent inflammation of the mucous membrane of the upper respiratory tract, characterized by nasal congestion, discharge, itching, sneezing, and a combination of several symptoms is possible. Use of Scientific Method in Asthma and Allergic Reactions Study.
The type of allergy testing depends on the type of reaction that the patient is experiencing. These testing methods include: Skin Prick: Skin prick testing is the most common test used to evaluate environmental, food, drug, and venom allergies. Small amounts of allergens are placed just barely into the surface of the skin through the use of small needles, which are then monitored for an ...
Allergies is an international, peer-reviewed, open access journal on allergy and immunology published quarterly online by MDPI.. Open Access — free for readers, with article processing charges (APC) paid by authors or their institutions.; Rapid Publication: manuscripts are peer-reviewed and a first decision is provided to authors approximately 28.1 days after submission; acceptance to ...
Selecting Sources for Research Papers. Types of Sources. Information comes from many places. We may get information from books, the news, or social media. When using information for an academic paper, some sources may be more helpful than others. The library provides access to the following kinds of sources:
Prison time is a possibility. It's uncertain, of course, but plausible. Nevertheless, there are many previous cases involving falsifying business records along with other charges where the ...
Essay 2024.5.3. A new type of woman ... Instead, all women should go for whatever body type gives them the most confidence.(Samantha Loong)