- Introduction
- Conclusions
- Article Information
Data were obtained from the 2006-2019 National Surveys on Drug Use and Health. Each estimate was adjusted for sex, race and ethnicity, educational attainment, family income, employment status, health insurance status, marital status, metropolitan statistical area status, major depressive episode, and substance use disorder (includes alcohol use disorder, cannabis use disorder, cocaine use disorder, and heroin use disorder). Error bars represent SEs.
Data were obtained from the 2006-2019 National Surveys on Drug Use and Health. Each estimate was adjusted for sex, race and ethnicity, educational attainment, family income, employment status, health insurance status, marital status, metropolitan statistical area status, and survey year. Error bars represent SEs. MDE indicates major depressive episode; and SUD, substance use disorder.
Data Sharing Statement

See More About
Sign up for emails based on your interests, select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Get the latest research based on your areas of interest.
Others also liked.
- Download PDF
- X Facebook More LinkedIn
Han B , Einstein EB , Compton WM. Patterns and Characteristics of Nicotine Dependence Among Adults With Cigarette Use in the US, 2006-2019. JAMA Netw Open. 2023;6(6):e2319602. doi:10.1001/jamanetworkopen.2023.19602
Manage citations:
© 2024
- Permissions
Patterns and Characteristics of Nicotine Dependence Among Adults With Cigarette Use in the US, 2006-2019
- 1 National Institute on Drug Abuse, National Institutes of Health, Bethesda, Maryland
Question Does the national prevalence of nicotine dependence vary by year, age, psychiatric comorbidities (substance use disorder and/or depression), and sociodemographic characteristics?
Findings In this cross-sectional study of 152 354 US community-dwelling adults with past-month cigarette use, the adjusted prevalence of nicotine dependence decreased from 59.52% in 2006 to 56.00% in 2019 among adults with past-month cigarette smoking and among each age group, except for ages 18 to 25 years. Adults 50 years and older (especially those with substance use disorder and/or depression) had the highest prevalence of nicotine dependence.
Meaning These findings suggest that evidence-based tobacco cessation strategies tailored to age and psychiatric comorbidities are needed.
Importance Nicotine dependence increases the risk of persistent smoking, which is the leading preventable cause of morbidity and death. However, evidence regarding the associations of nicotine dependence with age, psychiatric conditions, and sociodemographic characteristics is limited.
Objective To assess whether and how nicotine dependence among US adults with cigarette use varies by year, age, psychiatric comorbidities, and sociodemographic characteristics.
Design, Setting, and Participants This exploratory serial cross-sectional study used data from 152 354 US community-dwelling individuals 18 years or older who participated in the 2006-2019 National Surveys on Drug Use and Health. Data analyses were conducted from January 15 to February 15, 2023.
Exposure Past-month cigarette use. Past-year major depressive episode (MDE) and/or substance use disorder (SUD) based on criteria from the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition), Text Revision.
Main Outcomes and Measures Past-month nicotine dependence based on criteria from the Nicotine Dependence Syndrome Scale or the Fagerström Test of Nicotine Dependence.
Results Among 152 354 adults with past-month cigarette use (54.1% male; 40.2% aged 18-34 years; 29.0% aged 35-49 years; 69.8% non-Hispanic White), the adjusted prevalence of nicotine dependence decreased from 59.52% (95% CI, 57.93%-61.10%) in 2006 to 56.00% (95% CI, 54.38%-57.60%) in 2019 (average annual percentage change [AAPC], −0.4%; 95% CI, −0.5% to −0.4%; P < .001) and among each examined age group, except for stability among those aged 18 to 25 years (AAPC, −0.5%; 95% CI, −1.4% to 0.4%; P = .27). Compared with those 50 years and older with past-month cigarette smoking, the adjusted prevalence of nicotine dependence among those aged 18 to 49 years was 32% lower for those aged 18 to 25 years (adjusted risk ratio [ARR], 0.68; 95% CI, 0.66-0.70), 18% lower for those aged 26 to 34 years (ARR, 0.82; 95% CI, 0.80-0.84), and 6% lower for those aged 35 to 49 years (ARR, 0.94; 95% CI, 0.92-0.96). The adjusted prevalence of nicotine dependence varied by age, MDE and/or SUD status, and sociodemographic characteristics. For example, by 2019, prevalence was 41.27% (95% CI, 39.21%-43.37%) among those aged 18 to 25 years and 64.43% (95% CI, 60.98%-67.74%) among those 50 years and older. Differences in nicotine dependence prevalence between those with co-occurring MDE and SUD and those without both conditions were more than 2 times larger for those 50 years and older vs those aged 18 to 49 years (eg, ages ≥50 years vs 18-25 years: 18.69 percentage point difference [83.32% vs 64.63%] vs 7.67 percentage point difference [48.88% vs 41.21%]; P < .001).
Conclusions and Relevance In this cross-sectional study, there were significant reductions in nicotine dependence prevalence from 2006 to 2019 among US adults with cigarette use and all examined subgroups 26 years and older. Adults 50 years and older (especially those with MDE and/or SUD) had the highest nicotine dependence prevalence compared with other age groups, highlighting the importance of assisting with smoking cessation efforts and addressing nicotine dependence for this older population. Evidence-based tobacco cessation strategies tailored to age and comorbidities are needed.
Nicotine dependence increases the risk of smoking persistence and is the leading preventable cause of morbidity and death. 1 The prevalence of cigarette smoking has declined in the US over the past decades among adults with and without psychiatric conditions (eg, major depressive episode [MDE] and substance use disorder [SUD]). 2 , 3 A nicotine hardening hypothesis has emerged. This hypothesis proposes that declines in nicotine use resulting from population-level tobacco control measures leave a higher proportion of people with intractable nicotine dependence and consumption over time. 4
However, findings on the hardening hypothesis from existing literature 4 - 6 are inconsistent. Based on nationally representative data collected from 2001 to 2002 and 2012 to 2013, Grant et al 4 reported increases in nicotine dependence prevalence among US adults with nicotine use, supporting the hardening hypothesis. Methodological differences between the 2001 to 2002 and 2012 to 2013 data collections may limit comparability for the 2 time periods. Another study 5 based on representative data from 18 European countries provided empirical evidence against this theory. One study 6 reviewed 26 population-based studies and did not find that conversion from current to former smoking, number of quit attempts, or success on a given quit attempt decreased over time, suggesting hardening may not be occurring in noninstitutionalized civilian populations with cigarette use.
Moreover, evidence regarding whether and how nicotine dependence varies by age is inconsistent. Studies have reported that a significant decrease in nicotinic activation in the hippocampus (a brain region involved in addiction) occurs around age 40 years, 7 , 8 and reduced nicotinic activation is associated with reduced addictive potential of nicotinic agonists. 9 Another study 10 based on nationally representative data from 1991 to 1993 reported that US adults 50 years and older who smoke cigarettes had the highest consumption yet experienced the lowest prevalence of nicotine dependence. One study 11 suggested that this older age group may be more responsive to nonpharmacological cessation programs because of their reduced nicotine dependence. However, the study from 18 European countries 5 found that high nicotine dependence was associated with older age. Another recent study 12 based on nationally representative data from China found that the prevalence of nicotine dependence among adults with cigarette use increased with age for men but peaked at age 40 years for women. In addition, although nicotine dependence is associated with lower socioeconomic status 1 and psychiatric disorders, 13 little is known about whether and how associations of sociodemographic characteristics and psychiatric conditions with nicotine dependence vary by age.
Given continuing declines in smoking and because the hardening hypothesis could play a role in US tobacco cessation strategies, this study assessed patterns in nicotine dependence among US adults with cigarette use based on nationally representative data from 2006 through 2019. Among adults with cigarette use, patterns and characteristics of nicotine dependence were examined to understand whether and how associations of sociodemographic characteristics and psychiatric conditions with nicotine dependence varied by age.
Data were from 152 354 US community-dwelling individuals 18 years and older who participated in the 2006-2019 National Surveys on Drug Use and Health (NSDUH); the NSDUH provides nationally representative data on cigarette smoking, nicotine dependence, MDE, and SUD among US noninstitutionalized civilian adult populations. 14 , 15 The NSDUH data collection protocol was approved by the institutional review board of Research Triangle Institute International. Each participant provided verbal informed consent. 14 , 15 This cross-sectional study used publicly available deidentified data from the NSDUH and was exempt from review and the requirement for informed consent per the institutional review board of the National Institutes of Health. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology ( STROBE ) reporting guideline.
For the NSDUH, data were collected by interviewers through personal visits to households and noninstitutional group facilities. Audio computer-assisted self-administered interviewing was used, providing respondents with a private and confidential way to record answers. From 2006 to 2019, the annual mean weighted screening response rate was 82.9%, and the annual mean weighted interview response rate was 71.4%. Data collected for this cross-sectional study were analyzed from January 15 to February 15, 2023.
Among NSDUH respondents who reported past-month cigarette smoking, past-month nicotine dependence was defined as meeting criteria from either the Nicotine Dependence Syndrome Scale or the Fagerström Test of Nicotine Dependence. 13 Using diagnostic criteria specified in the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition), Text Revision ( DSM-IV-TR ), 2 , 14 - 17 the NSDUH estimated the prevalence of past year–specific SUD, and respondents were classified as having past-year SUD if they had any of 4 specific SUDs (eg, alcohol use disorder, cannabis use disorder, cocaine use disorder, and heroin use disorder). 2 The NSDUH also assessed past-year MDE using DSM-IV-TR diagnostic criteria. 2 , 14 - 17 These measures of SUDs and MDE have been found to have good validity and reliability. 18 , 19 In addition, the NSDUH asked respondents about sociodemographic characteristics (eg, age, sex, race and ethnicity [including Hispanic; non-Hispanic Black; non-Hispanic White; and other non-Hispance race, including American Indian or Alaska Native, Asian, Native Hawaiian or other Pacific Islander, and more than 1 race]), educational attainment, employment status, family income, marital status, health insurance status, and metropolitan statistical area [MSA] status). Self-reported race and ethnicity classification was included because tobacco use has previously been documented to vary according to these socially determined factors. 1 Additional details about NSDUH methods and survey questionnaires have been published previously. 14 - 16
Bivariable and multivariable logistic regression analyses were conducted to understand factors associated with past-month nicotine dependence among adults with past-month cigarette smoking. Model-adjusted prevalence (predicted marginal proportion) and differences in model-adjusted prevalence (differences in predicted marginal proportions) were estimated. Multicollinearity and potential interaction effects were examined. Although multicollinearity was not found in the final pooled model, significant interaction effects were identified between age and selected covariates. Thus, stratified multivariable logistic regression models by age were conducted.
All of these analyses used SUDAAN software, release 11.0.3 (RTI International), 20 to account for the complex sample design and sample weights used in the NSDUH. The Joinpoint regression program, version 4.8.0.1 (National Cancer Institute), 21 was used to test for significant changes in nonlinear patterns in the adjusted prevalence of nicotine dependence using permutation tests and to estimate average annual percentage changes (AAPCs) from 2006 to 2019, which are considered valid even if the Joinpoint models suggest changes in patterns during a given study period. 22 For each analysis, 2-tailed P < .05 was considered statistically significant.
Among 152 354 community-dwelling adults with past-month cigarette use, 54.1% were male, 45.9% were female, 18.8% were ages 18 to 25 years, 21.4% were ages 26 to 34 years, 29.0% were ages 35 to 49 years, and 30.8% were 50 years or older. With regard to race and ethnicity, 12.2% were Hispanic, 12.3% were non-Hispanic Black, 69.8% were non-Hispanic White, and 5.6% were of other non-Hispanic race (including American Indian or Alaska Native, Asian, Native Hawaiian or other Pacific Islander, and more than 1 race). Overall, the adjusted prevalence of nicotine dependence decreased from 59.52% (95% CI, 57.93%-61.10%) in 2006 to 56.00% (95% CI, 54.38%-57.60%) in 2019 (AAPC, −0.4%; 95% CI,−0.5% to −0.4%; P < .001), representing a percentage point difference of 3.52 and a percentage change of 5.91% ( Table 1 ). Prevalence was lower from 2012 to 2019 (eg, 2012: 56.83% [95% CI, 55.30%-58.34%]; 2015: 56.80% [95% CI, 55.46%-58.13%]; 2018: 55.64% [95% CI, 54.39%-56.87%]) than in 2006. Moreover, higher prevalence of nicotine dependence was associated with older age. Compared with those 50 years and older with past-month cigarette smoking, the adjusted prevalence of nicotine dependence was 32% lower among those aged 18 to 25 years (adjusted risk ratio [ARR], 0.68; 95% CI, 0.66-0.70), 18% lower among those aged 26 to 34 years (ARR, 0.82; 95% CI, 0.80-0.84), and 6% lower among those aged 35 to 49 years (ARR, 0.94; 95% CI, 0.92-0.96) ( Table 2 ).
Nicotine dependence was also positively associated with male sex, non-Hispanic White race and ethnicity, non–full-time employment status, low family income, low educational attainment, nonprivate health insurance status, divorced or separated status, residence in a non-MSA, and the presence of MDE and/or SUD ( Table 1 and Table 2 ). The pooled model identified 8 interaction effects (race and ethnicity and age, employment status and age, family income and age, educational attainment and age, health insurance status and age, marital status and age, MSA and age, and psychiatric condition and age), suggesting that associations of these characteristics with nicotine dependence varied by age. Thus, age-stratified multivariable logistic regression analysis was conducted.
Among adults with past-month cigarette smoking from 2006 to 2019, the adjusted prevalence of nicotine dependence remained statistically unchanged among those aged 18 to 25 years (AAPC, −0.5%; 95% CI, −1.4% to 0.4%; P = .27) ( Figure 1 ; Table 1 and Table 2 ) but decreased among those aged 26 to 49 years (ages 26-34 years: AAPC, −0.5% [95% CI, −0.9% to −0.2%]; P = .008; ages 35-49 years: AAPC, −0.8% [95% CI, −1.0% to −0.5]; P < .001) and those 50 years and older (AAPC, −0.3%; 95% CI, −0.5% to 0.0%; P = .04). By 2019, adjusted nicotine dependence prevalence was 41.27% (95% CI, 39.21%-43.37%) among those aged 18 to 25 years, 50.64% (95% CI, 47.99%-53.30%) among those aged 26 to 34 years, 60.71% (95% CI, 58.27%-63.09%) among those aged 34 to 49 years, and 64.43% (95% CI, 60.98%-67.74%) among those 50 years and older.
Nicotine dependence prevalence was 4% higher in men aged 26 to 34 years (ARR, 1.04; 95% CI, 1.01-1.07) and 3% higher in men aged 35 to 49 years (ARR, 1.03; 95% CI, 1.00-1.05) compared with women ( Table 2 ). Prevalence was lower among minority racial and ethnic adults than non-Hispanic White adults across examined age groups; difference in prevalence ranged from 9% lower for non-Hispanic individuals of other race who were 50 years and older (ARR, 0.91; 95% CI, 0.85-0.97) to 52% lower for Hispanic individuals aged 18 to 25 years (ARR, 0.48; 95% CI, 0.46-0.51). However, compared with those 50 years and older, differences in nicotine dependence prevalence between non-Hispanic White vs non-Hispanic Black groups were smaller for those aged 18 to 25 years (difference, 7.55 percentage points [48.29% vs 40.74%] vs 14.13 percentage points [70.50% vs 56.37%]; P < .001) and those aged 26 to 34 years (difference, 7.23 percentage points [57.62% vs 50.39%] vs 14.13 percentage points [70.50% vs 56.37%]; P < .001) ( Table 1 ). Compared with those 50 years and older, differences in prevalence between non-Hispanic White and Hispanic groups were larger for those aged 26 to 34 years (difference, 29.14 percentage points [57.62% vs 28.48%] vs 23.81 percentage points [70.50% vs 46.69%]; P = .004) and aged 35 to 49 years (difference, 30.03 percentage points [67.40% vs 37.37%] vs 23.81 percentage points [70.50% vs 46.69%]; P = .002).
Compared with adults with a family income of $75 000 or higher, nicotine dependence prevalence was higher among adults with a family income of less than $50 000 across all age groups, ranging from 6% higher for those aged 18 to 25 years with income of less than $20 000 (ARR, 1.06; 95% CI, 1.02-1.09) to 16% higher for those aged 35 to 49 years with income of less than $20 000 (ARR, 1.16; 95% CI, 1.10-1.21) ( Table 2 ). In addition, prevalence was higher among those aged 18 to 49 years with a family income between $50 000 and $74 999 vs $75 000 or higher, ranging from 4% higher for those aged 26 to 34 years (ARR, 1.04; 95% CI, 1.00-1.09) to 8% higher for those aged 35 to 49 years (ARR, 1.08; 95% CI, 1.04-1.12). Differences in nicotine dependence prevalence between adults with family incomes of less than $20 000 vs $75 000 or higher were greater among those aged 35 to 49 years than those aged 18 to 25 years (difference, 8.80% percentage points [65.10% vs 56.30%] vs 2.36% percentage points [42.91% vs 40.55%]; P < .001) ( Table 1 ).
Nicotine dependence prevalence did not differ by employment, health insurance, or marital status among those 50 years and older with cigarette use. However, prevalence was 18% lower among those aged 18 to 25 years with part-time employment than their counterparts with full-time employment (ARR, 0.82; 95% CI, 0.79-0.84) ( Table 2 ). Among adults aged 18 to 49 years, prevalence was higher among those who were unemployed vs those who had full-time employment, ranging from 5% higher for those aged 35 to 49 years (ARR, 1.05; 95% CI, 1.00-1.09) to 17% higher for those aged 26 to 34 years (ARR, 1.17; 95% CI, 1.11-1.23).
Among adults aged 18 to 49 years, nicotine dependence prevalence was higher for those with Medicaid vs private insurance only, ranging from 13% higher among those aged 35 to 49 years (ARR, 1.13; 95% CI, 1.09-1.17) to 45% higher among those aged 18 to 25 years (ARR, 1.45; 95% CI, 1.40-1.49) ( Table 2 ). In addition, prevalence was higher among those aged 18 to 49 years with no insurance vs private insurance only, ranging from 8% higher for those aged 35 to 49 years (ARR, 1.08; 95% CI, 1.04-1.11) to 34% higher for those aged 18 to 25 years (ARR, 1.34; 95% CI, 1.30-1.37). Among adults aged 18 to 49 years, prevalence was higher for those who were divorced or separated vs married, ranging from 6% higher among those aged 35 to 49 years (ARR, 1.06; 95% CI, 1.03-1.09) to 14% higher among those aged 26 to 34 years (ARR, 1.14; 95% CI, 1.10-1.19). Among adults aged 26 to 49 years, prevalence was 4% higher for those who were never married vs married (ages 26-34 years: ARR, 1.04 [95% CI, 1.01-1.07]; ages 35-49 years: ARR, 1.04 [95% CI, 1.01-1.08]). Among adults aged 18 to 25 years, prevalence was 9% lower for those who were never married vs married (ARR, 0.91; 95% CI, 0.88-0.94).
Across age groups, nicotine dependence prevalence was higher among those without a college degree vs those with a college degree, ranging from 20% higher for those 50 years and older with some college education (ARR, 1.20; 95% CI, 1.12-1.27) to 98% higher for those aged 26 to 34 years (ARR, 1.98; 95% CI, 1.86-2.13) and 136% higher for those aged 18 to 25 years with less than a high school diploma (ARR, 2.36; 95% CI, 2.20-2.53) ( Table 2 ). Compared with adults 50 years and older, differences in nicotine dependence prevalence between those without a high school diploma vs those with a college degree were higher among those aged 18 to 25 years (difference, 31.58 percentage points [54.79% vs 23.21%] vs 19.40 percentage points [73.68% vs 54.28%]; P < .001) and those aged 26 to 34 years (difference, 31.55 percentage points [63.71% vs 32.16%] vs 19.40 percentage points; P < .001) ( Table 1 ).
Across age groups, nicotine dependence prevalence was lower among those residing in large MSAs than those in non-MSAs, ranging from 3% lower for those 50 years and older (ARR, 0.97; 95% CI, 0.94-0.99) to 11% lower for those aged 26 to 34 years (ARR, 0.89; 95% CI, 0.86-0.92) ( Table 2 ). Compared with adults 50 years and older, differences in nicotine dependence prevalence between those residing in non-MSAs vs large MSAs were greater among those aged 26 to 34 years (difference, 6.11 percentage points [55.17% vs 49.06%] vs 2.29 percentage points [68.00% vs 65.71%]; P = .01) and those aged 35 to 49 years (difference, 5.42 percentage points [65.12% vs 59.70%] vs 2.29 percentage points [68.00% vs 65.71%]; P = .03) ( Table 1 ). Prevalence was lower among adults aged 26 to 49 years residing in small MSAs vs non-MSAs, ranging from 4% lower for those aged 26 to 34 years (ARR, 0.96; 95% CI, 0.93-0.99) to 5% lower for those aged 35 to 49 years (ARR, 0.95; 95% CI, 0.92-0.98) ( Table 2 ).
Overall, nicotine dependence was higher among adults with cigarette use who also had SUD and/or MDE than among those without these comorbid conditions, but the pattern of associations varied according to age group ( Table 1 and Table 2 ; Figure 2 ). For example, the differences in nicotine dependence prevalence between those with co-occurring SUD and MDE and those with neither condition were more than 2 times larger for adults 50 years and older vs those aged 18 to 49 years (eg, ages ≥50 years vs 18-25 years: 18.69 [83.32 vs 64.63] percentage point difference vs 7.67 [48.88 vs 41.21] percentage point difference; P < .001; ages ≥50 years vs 35-49 years: 18.69 vs 8.28 percentage point difference [57.76% vs 49.48%]; P < .001) ( Table 1 ). In all age groups of adults with cigarette use who had neither SUD nor MDE, nicotine dependence prevalence was lower than among adults with cigarette use who had SUD but not MDE, ranging from 6% lower for those aged 35 to 49 years (ARR, 0.94; 95% CI, 0.91-0.98) to 15% lower for those 50 years and older (ARR, 0.85; 95% CI, 0.81-0.88) ( Table 2 ). However, also compared with those with SUD but without MDE, nicotine dependence prevalence was higher only among those aged 18 to 25 years (ARR, 1.09; 95% CI, 1.04-1.14), those aged 26 to 34 years (ARR, 1.07; 95% CI, 1.01-1.14), and those 50 years and older (ARR, 1.09; 95% CI, 1.02-1.17) with co-occurring SUD and MDE. Prevalence was also higher among those aged 26 to 34 years (ARR, 1.10; 95% CI, 1.04-1.16) and those aged 35 to 49 years (ARR, 1.11; 95% CI, 1.06-1.16) with MDE but without SUD compared with those with SUD but without MDE.
Consistent with the findings based on representative data from 18 European countries, 5 this cross-sectional study found that the adjusted prevalence of nicotine dependence declined from 2006 to 2019 among the US general adult population who smoked cigarettes and among each examined age group, except for stability among those aged 18 to 25 years. Another study 23 based on nationally representative data from the US reported significant increases in quit attempts and significant decreases in the average number of cigarettes smoked, even among those with psychological distress, from 1997 to 2015. These European 5 and US 23 results are consistent with those of a previous qualitative review, 6 which found that conversion from current to former smoking, number of quit attempts, and success on a given quit attempt increased rather than declined with time. 6 Overall, direct evidence provided by our study and indirect evidence from other studies do not support the hardening hypothesis for the general adult population with cigarette use.
We found that nicotine dependence prevalence decreased from 59.52% in 2006 to 56.00% in 2019 (difference, 3.52 percentage points; percentage change, 5.91%) overall among adults who smoked cigarettes. These findings of declines in nicotine dependence among US adults with cigarette use are consistent with the benefits and safety of pharmacotherapy (eg, varenicline) and the benefits of nicotine replacement therapy and counseling that have been reported, even for people with high nicotine dependence. 1 , 24 Furthermore, our results suggest that decreases in nicotine dependence prevalence started in 2012, a timeline that contrasts that of a recent study 3 suggesting that decline in cigarette use may be associated with Affordable Care Act–related increases in health insurance coverage that began in 2014.
Although the recent Surgeon General report on smoking cessation has emphasized that “it is never too late to quit smoking,” 1 there has been a lack of attention specific to those 50 years and older. Contrary to earlier findings that adults 50 years and older who smoked had lower nicotine dependence prevalence compared with their younger counterparts, 7 , 8 , 10 , 11 our results revealed that among US adults with cigarette use, those 50 years and older had the highest nicotine dependence prevalence compared with all younger groups. Moreover, nicotine dependence prevalence was positively associated with older age for both men and women. We did not find an interaction effect between age and sex on nicotine dependence. We did find that the pattern of nicotine dependence, which is associated with psychiatric comorbidity, 13 varied according to age. For example, nicotine dependence prevalence was even higher among those 50 years and older with neither MDE nor SUD than those aged 18 to 34 years with co-occurring MDE and SUD.
High nicotine dependence is associated with increased difficulties in quitting smoking, low quality of life, low work productivity, high health care costs, and high morbidity, disability, and mortality, especially in those 50 years and older, among whom age-related common chronic conditions are often exacerbated by smoking. 1 , 25 - 28 Smoking can reduce the benefits of medications prescribed for conditions common in later life. 1 In contrast, smoking cessation in this older population has been found to reduce the increased risk of death and improve recovery from acute and chronic illness. 1 One systematic review 28 based on 29 randomized clinical trials reported that for adults 50 years and older, consistent with current clinical practice guidelines, multimodal interventions produced the highest abstinence rates, and pharmacotherapy and behavioral interventions were 2 complementary modalities that improved smoking cessation synergistically. Yet, most older adults who smoked did not try to quit smoking in the past year. 29 Our results revealed that nicotine dependence prevalence decreased by an AAPC of only 0.3% among those 50 years and older from 2006 to 2019. We also found that differences in nicotine dependence prevalence between those with co-occurring MDE and SUD and those without both conditions were more than 2 times larger for those 50 years and older (18.69 percentage points) than those aged 18 to 49 years (ranging from 7.67 percentage points for ages 18-25 years to 8.28 percentage points for ages 35-49 years).
We also found that nicotine dependence varied by both age and presence of MDE and SUD, with significantly higher nicotine dependence for those with co-occurring MDE and SUD in most age groups and for those with MDE but without SUD in the age groups of 26 to 34 years and 35 to 49 years. High prevalence of depression among adults with cigarette use may be associated with reductions in dopamine receptors (directly or through mechanisms involving the habenula). 30 Thus, adults with depression or SUD should be prioritized for tobacco control interventions, especially those aged 26 to 49 years with depression.
Notably, primary care clinicians and mental health care professionals can play important roles in encouraging and assisting with smoking cessation efforts among these populations, who may visit them regularly for medical or behavioral health conditions other than nicotine dependence. The recent Surgeon General report 1 on smoking cessation has covered adults, young adults, and youths, but evidence-based strategies are needed to improve smoking cessation efforts for those 50 years and older and especially those with psychiatric conditions.
This study provided detailed results on how associations of sociodemographic characteristics with nicotine dependence vary by age, highlighting the need to implement evidence-based age-specific tobacco cessation strategies. For example, although previous research 1 found that low educational attainment was associated with nicotine dependence, our study revealed that differences in nicotine dependence prevalence between those without a high school diploma and those with a college degree were significantly higher among those aged 18 to 34 years than those 50 years and older. Because low educational attainment is associated with lower cessation success, 31 our results suggest that further research is needed to help understand whether education-related differences in cessation success are markedly higher for young adults than older adults and how to implement evidence-based age-specific nicotine cessation strategies accordingly.
This study has several limitations. First, the cross-sectional nature of NSDUH data precludes the establishment of causal relationships. Second, this study may underestimate the prevalence of nicotine dependence because the NSDUH excluded people experiencing homelessness who were not living in shelters and people who were institutionalized (eg, jail and prison populations), who often have higher nicotine dependence than the general population. Third, this study cannot examine e-cigarette use because the 2006 to 2019 NSDUH did not assess it. Fourth, the NSDUH is a self-report survey and is subject to recall and social desirability bias. Fifth, age is a categorical variable in the NSDUH public use files; thus, this study cannot examine the association of cohort with patterns in nicotine dependence among adults with cigarette use. Future research is needed to examine the associations of age, cohort, and period with nicotine dependence. Sixth, success of cessation treatments, increases in cigarette prices through taxation, and implementation of smoking-free air laws help reduce cigarette consumption and increase cessation rates over time. 1 , 24 , 32 Future research is needed to fully understand how these factors impact patterns in nicotine dependence among US adults with cigarette use. Seventh, it is important to understand patterns in nicotine dependence overall and by age among US adults with cigarette use during the COVID-19 pandemic. However, because the NSDUH data collection modes changed during this period, it is inappropriate to examine any changes between the COVID-19 era (eg, 2020-2022) and the 2006 to 2019 period based on NSDUH data. More research is needed to continue monitoring the patterns.
This cross-sectional study found that nicotine dependence prevalence declined slightly from 2006 to 2019 among the general adult population with cigarette use and all subgroups 26 years and older. For adults overall with cigarette use, nicotine dependence prevalence had an AAPC decrease of 0.4% from 2006 to 2019. This study also found that both men and women 50 years and older (especially those with MDE and/or SUD) had the highest nicotine dependence prevalence compared with other age groups, highlighting the importance of assisting smoking cessation efforts and addressing nicotine dependence for this older population. Moreover, those aged 18 to 49 years with MDE or SUD also had higher nicotine dependence prevalence than those in the same age group without corresponding psychiatric comorbidities. These results suggest the need to implement evidence-based tobacco cessation strategies that are specific to age and psychiatric comorbidities.
Accepted for Publication: May 7, 2023.
Published: June 23, 2023. doi:10.1001/jamanetworkopen.2023.19602
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2023 Han B et al. JAMA Network Open .
Corresponding Author: Wilson M. Compton, MD, MPE, National Institute on Drug Abuse, National Institutes of Health, 301 N Stonestreet Ave, 3WFN Room 09D18, MSC 6025, Bethesda, MD 20892-6025 ( [email protected] ).
Author Contributions: Dr Han had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: All authors.
Acquisition, analysis, or interpretation of data: Han, Compton.
Drafting of the manuscript: Han, Compton.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Han.
Administrative, technical, or material support: Einstein.
Supervision: Compton.
Conflict of Interest Disclosures: Dr Compton reported owning stock in 3M, General Electric Company, and Pfizer outside the submitted work. No other disclosures were reported.
Funding/Support: This study was supported by funding from the National Institute on Drug Abuse of the National Institutes of Health in that all authors are full-time employees of the National Institute on Drug Abuse.
Role of the Funder/Sponsor: The sponsor supported the authors, who were responsible for preparation, review, and approval of the manuscript and the decision to submit the manuscript for publication. The sponsor had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The sponsor reviewed and approved the manuscript.
Disclaimer: The findings and conclusions of this study are those of the authors and do not necessarily reflect the views of the National Institute on Drug Abuse of the National Institutes of Health and the US Department of Health and Human Services.
Data Sharing Statement: See the Supplement .
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 21 January 2021
The effects of tobacco control policies on global smoking prevalence
- Luisa S. Flor ORCID: orcid.org/0000-0002-6888-512X 1 ,
- Marissa B. Reitsma 1 ,
- Vinay Gupta 1 ,
- Marie Ng ORCID: orcid.org/0000-0001-8243-4096 2 &
- Emmanuela Gakidou ORCID: orcid.org/0000-0002-8992-591X 1
Nature Medicine volume 27 , pages 239–243 ( 2021 ) Cite this article
31k Accesses
105 Citations
366 Altmetric
Metrics details
- Risk factors
Substantial global effort has been devoted to curtailing the tobacco epidemic over the past two decades, especially after the adoption of the Framework Convention on Tobacco Control 1 by the World Health Organization in 2003. In 2015, in recognition of the burden resulting from tobacco use, strengthened tobacco control was included as a global development target in the 2030 Agenda for Sustainable Development 2 . Here we show that comprehensive tobacco control policies—including smoking bans, health warnings, advertising bans and tobacco taxes—are effective in reducing smoking prevalence; amplified positive effects are seen when these policies are implemented simultaneously within a given country. We find that if all 155 countries included in our counterfactual analysis had adopted smoking bans, health warnings and advertising bans at the strictest level and raised cigarette prices to at least 7.73 international dollars in 2009, there would have been about 100 million fewer smokers in the world in 2017. These findings highlight the urgent need for countries to move toward an accelerated implementation of a set of strong tobacco control practices, thus curbing the burden of smoking-attributable diseases and deaths.
Similar content being viewed by others

Reductions in smoking due to ratification of the Framework Convention for Tobacco Control in 171 countries
Guillermo Paraje, Mauricio Flores Muñoz, … Prabhat Jha
Accelerating tobacco control at the national level with the Smoke-free Generation movement in the Netherlands
Marc C. Willemsen & Jasper V. Been
A dynamic modelling analysis of the impact of tobacco control programs on population-level nicotine dependence
Adam Skinner, Jo-An Occhipinti & Nathaniel D. Osgood
Decades after its ill effects on human health were first documented, tobacco smoking remains one of the major global drivers of premature death and disability. In 2017, smoking was responsible for 7.1 (95% uncertainty interval (UI), 6.8–7.4) million deaths worldwide and 7.3% (95% UI, 6.8%–7.8%) of total disability-adjusted life years 3 . In addition to the health impacts, economic harms resulting from lost productivity and increased healthcare expenditures are also well-documented negative effects of tobacco use 4 , 5 . These consequences highlight the importance of strengthening tobacco control, a critical and timely step as countries work toward the 2030 Sustainable Development Goals 2 .
In 2003, the World Health Organization (WHO) led the development of the Framework Convention on Tobacco Control (FCTC), the first global health treaty intended to bolster tobacco use curtailment efforts among signatory member states 1 . Later, in 2008, to assist the implementation of tobacco control policies by countries, the WHO introduced the MPOWER package, an acronym representing six evidence-based control measures (Table 1 ) (ref. 6 ). While accelerated adoption of some of these demand reduction policies was observed among FCTC parties in the past decade 7 , many challenges remain to further decrease population-level tobacco use. Given the differing stages of the tobacco epidemic and tobacco control across countries, consolidating the evidence base on the effectiveness of policies in reducing smoking is necessary as countries plan on how to do better. In this study, we evaluated the association between varying levels of tobacco control measures and age- and sex-specific smoking prevalence using data from 175 countries and highlighted missed opportunities to decrease smoking rates by predicting the global smoking prevalence under alternative unrealized policy scenarios.
Despite the enhanced global commitment to control tobacco use, the pace of progress in reducing smoking prevalence has been heterogeneous across geographies, development status, sex and age 8 ; in 2017, there were still 1.1 billion smokers across the 195 countries and territories assessed by the Global Burden of Diseases, Injuries, and Risk Factors Study. Global smoking prevalence in 2017 among men and women aged 15 and older, 15–29 years, 30–49 years and 50 years and older are shown in Extended Data Figs. 1 , 2 , 3 and 4 , respectively. We found that, between 2009 and 2017, current smoking prevalence declined by 7.7% for men (36.3% (95% UI, 35.9–36.6%) to 33.5% (95% UI, 32.9–34.1%)) and by 15.2% for women globally (7.9% (95% UI, 7.8–8.1%) to 6.7% (95% UI, 6.5–6.9%)). The highest relative decreases were observed among men and women aged 15–29 years, at 10% and 20%, respectively. Conversely, prevalence decreased less intensively for those aged over 50, at 2% for men and 9.5% for women. While some countries have shown an important reduction in smoking prevalence between 2009 and 2017, such as Brazil, suggesting sustained progress in tobacco control, a handful of countries and territories have shown considerable increases in smoking rates among men (for example, Albania) and women (for example, Portugal) over this time period.
In an effort to counteract the harmful lifelong consequences of smoking, countries have, overall, implemented stronger demand reduction measures after the FCTC ratification. To assess national-level legislation quality, the WHO attributes a score to each of the MPOWER measures that ranges from 1 to 4 for the monitoring component (M) and 1–5 for the other components. A score of 1 represents no known data, while scores 2–5 characterize the overall strength of each measure, from the lowest level of achievement (weakest policy) to the highest level of achievement (strongest policy) 6 . Between 2008 and 2016, although very little progress was made in treatment provision (O) 7 , 9 , the share of the total population covered by best practice (score = 5) P, W and E measures increased (Fig. 1 ). Notably, however, a massive portion of the global population is still not covered by comprehensive laws. As an example, less than 15% of the global population is protected by strongly regulated tobacco advertising (E) and the number of people (2.1 billion) living in countries where none or very limited smoke-free policies (P) are in place (score = 2) is still nearly twice as high as the population (1.1 billion) living in locations with national bans on smoking in all public places (score = 5).
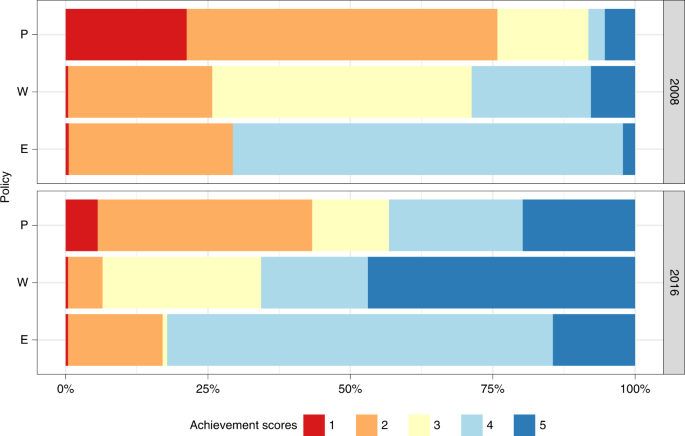
To assess national-level legislation quality, the WHO attributes a score to each MPOWER component that ranges from 1 to 5 for smoke-free (P), health warning (W) and advertising (E) policies. A score of 1 represents no known data or no recent data, while scores 2–5 characterize the overall strength of each policy, from 2 representing the lowest level of achievement (weakest policy), to 5 representing the highest level of achievement (strongest policy).
Source data
In terms of fiscal policies (R), the population-weighted average price, adjusted for inflation, of a pack of cigarettes across 175 countries with available data increased from I$3.10 (where I$ represents international dollars) in 2008 to I$5.38 in 2016. However, from an economic perspective, for prices to affect purchasing decisions, they need to be evaluated relative to income. The relative income price (RIP) of cigarettes is a measure of affordability that reflects, in this study, what proportion of the country-specific per capita gross domestic product (GDP) is needed to purchase half a pack of cigarettes a day for a year. Over time, cigarettes have become less affordable (RIP 2016 > RIP 2008) in about 75% of the analyzed countries, with relatively more affordable cigarettes concentrated across high-income countries.
Our adjusted analysis indicates that greater levels of achievement on key measures across the P, W and E policy categories and higher RIP values were significantly associated with reduced smoking prevalence from 2009 to 2017 (Table 2 ). Among men aged 15 and older, each 1-unit increment in achievement scores for smoking bans (P) was independently associated with a 1.1% (95% UI, −1.7 to −0.5, P < 0.0001) decrease in smoking prevalence. Similarly, an increase of 1 point in W and E scores was associated with a decrease in prevalence of 2.1% (95% UI, −2.7 to −1.6, P < 0.0001) and 1.9% (95% UI, −2.6 to −1.1, P < 0.0001), respectively. Furthermore, a 10 percentage point increase in RIP was associated with a 9% (95% UI, −12.6 to −5.0, P < 0.0001) decrease in overall smoking prevalence. Results were similar for men from other age ranges.
Among women, the magnitude of effect of different policy indicators varied across age groups. For those aged over 15, each 1-point increment in W and E scores was independently associated with an average reduction in prevalence of 3.6% (95% UI, −4.5 to −2.9, P < 0.0001) and 1.9% (95% UI, −2.9 to −1.8, P = 0.002), respectively, and these findings were similar across age groups. Smoking ban (P) scores were not associated with reduced prevalence among women aged 15–29 years or over 50 years. However, a 1-unit increase in P scores was associated with a 1.3% (95% UI, −2.3 to −0.2, P = 0.016) decline in prevalence among women aged 30–49 years. Lastly, while a 10 percentage point increase in RIP lowered women smoking prevalence by 6% overall (95% UI, −10.0 to −2.0, P = 0.014), this finding was not statistically significant when examining reductions in prevalence among those aged 50 and older (Table 2 ).
If tobacco control had remained at the level it was in 2008 for all 155 countries (with non-missing policy indicators for both 2008 and 2016; Methods ) included in the counterfactual analysis, we estimate that smoking prevalence would have been even higher than the observed 2017 rates, with 23 million more male smokers and 8 million more female smokers (age ≥ 15) worldwide (Table 3 ). Out of the counterfactual scenarios explored, the greatest progress in reducing smoking prevalence would have been observed if a combination of higher prices—resulting in reduced affordability levels—and strictest P, W and E laws had been implemented by all countries, leading to lower smoking rates among men and women from all age groups and approximately 100 million fewer smokers across all countries (Table 3 ). Under this policy scenario, the greatest relative decrease in prevalence would have been seen among those aged 15–29 for both sexes, resulting in 26.6 and 6.5 million fewer young male and female smokers worldwide in 2017, respectively.
Our findings reaffirm that a wide spectrum of tobacco demand reduction policies has been effective in reducing smoking prevalence globally; however, it also indicates that even though much progress has been achieved, there is considerable room for improvement and efforts need to be strengthened and accelerated to achieve additional gains in global health. A growing body of research points to the effectiveness of tobacco control measures 10 , 11 , 12 ; however, this study covers the largest number of countries and years so far and reveals that the observed impact has varied by type of control policy and across sexes and age groups. In high-income countries, stronger tobacco control efforts are also associated with higher cessation ratios (that is, the ratio of former smokers divided by the number of ever-smokers (current and former smokers)) and decreases in cigarette consumption 13 , 14 .
Specifically, our results suggest that men are, in general, more responsive to tobacco control interventions compared to women. Notably, with prevalence rates for women being considerably low in many locations, variations over time are more difficult to detect; thus, attributing causes to changes in outcome can be challenging. Yet, there is already evidence that certain elements of tobacco control policies that play a role in reducing overall smoking can have limited impact among girls and women, particularly those of low socioeconomic status 15 . Possible explanations include the different value judgments attached to smoking among women with respect to maintaining social relationships, improving body image and hastening weight control 16 .
Tax and price increases are recognized as the most impactful tobacco control policy among the suite of options under the MPOWER framework 10 , 14 , 17 , particularly among adolescents and young adults 18 . Previous work has also demonstrated that women are less sensitive than men to cigarette tax increases in the USA 19 . Irrespective of these demographic differences, effective tax policy is underutilized and only six countries—Argentina, Chile, Cuba, Egypt, Palau and San Marino—had adopted cigarette taxes that corresponded to the WHO-prescribed level of 70% of the price of a full pack by 2017 (ref. 20 ). Cigarettes also remain highly affordable in many countries, particularly among high-income nations, an indication that affordability-based prescriptions to countries, instead of isolated taxes and prices reforms, are possibly more useful as a tobacco control target. In addition, banning sales of single cigarettes, restricting legal cross-border shopping and fighting illicit trade are required so that countries can fully experience the positive effect of strengthened fiscal policies.
Smoke-free policies, which restrict the opportunities to smoke and decrease the social acceptability of smoking 17 , also affect population groups differently. In general, women are less likely to smoke in public places, whereas men might be more frequently influenced by smoking bans in bars, restaurants, clubs and workplaces across the globe due to higher workforce participation rates 16 . In addition to leading to reduced overall smoking rates, as indicated in this study, implementing complete smoking bans (that is, all public places completely smoke-free) at a faster pace can also play an important role in minimizing the burden of smoking-attributable diseases and deaths among nonsmokers. In 2017 alone, 2.18% (95% UI, 1.8–2.7%) of all deaths were attributable to secondhand smoke globally, with the majority of the burden concentrated among women and children 21 .
Warning individuals about the harms of tobacco use increases knowledge about the health risks of smoking and promotes changes in smoking-related behaviors, while full advertising and promotion bans—implemented by less than 20% of countries in 2017 (ref. 20 )—are associated with decreased tobacco consumption and smoking initiation rates, particularly among youth 17 , 22 , 23 . Large and rotating pictorial graphic warnings are the most effective in attracting smokers’ attention but are lacking in countries with high numbers of smokers, such as China and the USA 20 . Adding best practice health warnings to unbranded packages seems to be an effective way of informing about the negative effects of smoking while also eliminating the tobacco industry’s marketing efforts of using cigarette packages to make these products more appealing, especially for women and young people who are now the prime targets of tobacco companies 24 , 25 .
While it is clear that strong implementation and enforcement are crucial to accelerating progress in reducing smoking and its burden globally, our heterogeneous results by type of policy and demographics highlight the challenges of a one-size-fits-all approach in terms of tobacco control. The differences identified illustrate the need to consider the stages 26 of the smoking epidemics among men and women and the state of tobacco control in each country to identify the most pressing needs and evaluate the way ahead. Smoking patterns are also influenced by economic, cultural and political determinants; thus, future efforts in assessing the effectiveness of tobacco control policies under these different circumstances are of value. As tobacco control measures have been more widely implemented, tobacco industry forces have expanded and threaten to delay or reverse global progress 27 . Therefore, closing loopholes through accelerated universal adoption of the comprehensive set of interventions included in MPOWER, guaranteeing that no one is left unprotected, is an urgent requirement as efforts toward achieving the Sustainable Development Goals by 2030 are intensified.
This was an ecological time series analysis that aimed to estimate the effect of four key demand reduction measures on smoking rates across 175 countries. Country-year-specific achievement scores for P, W and E measures and an affordability metric measured by RIP—to capture the impact of fiscal policy (R)—were included as predictors in the model. Although the WHO also calls for monitoring (M) and tobacco cessation (O) interventions, these were not evaluated. Monitoring tobacco use is not considered a demand reduction measure, while very little progress has been made in treatment provision over the last decade 7 , 9 . Further information on research design is available in the Life Sciences Reporting Summary linked to this paper.
Smoking outcome data
The dependent variable is represented by country-specific, age-standardized estimates of current tobacco smoking prevalence, defined as individuals who currently use any smoked tobacco product on a daily or occasional basis. Complete time series estimates of smoking prevalence from 2009 to 2017 for men and women aged 15–29, 30–49, 50 years and older and 15 years and older, were taken from the Global Burden of Disease (GBD) 2017 study.
The GBD is a scientific effort to quantify the comparative magnitude of health loss due to diseases, injuries and risk factors by age, sex and geography for specific points in time. While full details on the estimation process for smoking prevalence have been published elsewhere, we briefly describe the main analytical steps in this article 3 . First, 2,870 nationally representative surveys meeting the inclusion criteria were systematically identified and extracted. Since case definitions vary between surveys, for example, some surveys only ask about daily smoking as opposed to current smoking that includes both daily and occasional smokers, the extracted data were adjusted to the reference case definition using a linear regression fit on surveys reporting multiple case definitions. Next, for surveys with only tabulated data available, nonstandard age groups and data reported as both sexes combined were split using observed age and sex patterns. These preprocessing steps ensured that all data used in the modeling were comparable. Finally, spatiotemporal Gaussian process regression, a three-step modeling process used extensively in the GBD to estimate risk factor exposure, was used to estimate a complete time series for every country, age and sex. In the first step, estimates of tobacco consumption from supply-side data are incorporated to guide general levels and trends in prevalence estimates. In the second step, patterns observed in locations, age groups and years with smoking prevalence data are synthesized to improve the first-step estimates. This step is particularly important for countries and time periods with limited or no available prevalence data. The third step incorporates and quantifies uncertainty from sampling error, non-sampling error and the preprocessing data adjustments. For this analysis, the final age-specific estimates were age-standardized using the standard population based on GBD population estimates. Age standardization, while less important for the narrower age groups, ensured that the estimated effects of policies were not due to differences in population structure, either within or between countries.
Using GBD-modeled data is a strength of the study since nearly 3,000 surveys inform estimates and countries are not required to have complete survey coverage between 2009 and 2017 to be included in the analysis. Yet, it is important to note that these estimates have limitations. For example, in countries where a prevalence survey was not conducted after the enactment of a policy, modeled estimates may not reflect changes in prevalence resulting from that policy. Nonetheless, the prevalence estimates from the GBD used in this study are similar to those presented in the latest WHO report 28 , indicating the validity and consistency of said estimates.
MPOWER data
Summary indicators of country-specific achievements for each MPOWER measure are released by the WHO every two years and date back to 2007. Data from different iterations of the WHO Report on the Global Tobacco Epidemic (2008 6 , 2009 29 , 2011 30 , 2013 31 , 2015 32 and 2017 20 ) were downloaded from the WHO Tobacco Free Initiative website ( https://www.who.int/tobacco/about/en/ ). To assess the quality of national-level legislation, the WHO attributes a score to each MPOWER component that ranges from 1 to 4 for the monitoring (M) dimension and 1–5 for the other dimensions. A score of 1 represents no known data or no recent data, while scores 2–5 characterize the overall strength of each policy, from the lowest level of achievement (weakest policy) to the highest (strongest policy).
Specifically, smoke-free legislation (P) is assessed to determine whether smoke-free laws provide for a complete indoor smoke-free environment at all times in each of the respective places: healthcare facilities; educational facilities other than universities; universities; government facilities; indoor offices and workplaces not considered in any other category; restaurants or facilities that serve mostly food; cafes, pubs and bars or facilities that serve mostly beverages; and public transport. Achievement scores are then based on the number of places where indoor smoking is completely prohibited. Regarding health warning policies (W), the size of the warnings on both the front and back of the cigarette pack are averaged to calculate the percentage of the total pack surface area covered by the warning. This information is combined with seven best practice warning characteristics to construct policy scores for the W dimension. Finally, countries achievements in banning tobacco advertising, promotion and sponsorship (E) are assessed based on whether bans cover the following types of direct and indirect advertising: (1) direct: national television and radio; local magazines and newspapers; billboards and outdoor advertising; and point of sale (indoors); (2) indirect: free distribution of tobacco products in the mail or through other means; promotional discounts; nontobacco products identified with tobacco brand names; brand names of nontobacco products used or tobacco products; appearance of tobacco brands or products in television and/or films; and sponsorship.
P, W and E achievement scores, ranging from 2 to 5, were included as predictors into the model. The goal was to not only capture the effect of adopting policies at its highest levels but also assess the reduction in prevalence that could be achieved if countries moved into the expected direction in terms of implementing stronger measures over time. Additionally, having P, W and E scores separately, and not combined into a composite score, enabled us to capture the independent effect of different types of policies.
Although compliance is a critical factor in understanding policy effectiveness, the achievement scores incorporated in our main analysis reflect the adoption of legislation rather than degree of enforcement, representing a limitation of these indicators.
Prices in I$ for a 20-cigarette pack of the most sold brand in each of the 175 countries were also sourced from the WHO Tobacco Free Initiative website for all available years (2008, 2010, 2012, 2014 and 2016). I$ standardize prices across countries and also adjust for inflation across time. This information was used to construct an affordability metric that captures the impact of cigarette prices on smoking prevalence, considering the income level of each country.
More specifically, the RIP, calculated as the percentage of per capita GDP required to purchase one half pack of cigarettes a day over the course of a year, was computed for each available country and year. Per capita GDP estimates were drawn from the Institute for Health Metrics and Evaluation; the estimation process is detailed elsewhere 33 .
Given that the price data used in the analysis refer to the most sold brand of cigarettes only, it does not reflect the full range of prices of different types of tobacco products available in each location. This might particularly affect our power in detecting a strong effect in countries where other forms of tobacco are more popular.
Statistical analysis
Sex- and age-specific logit-transformed prevalence estimates from 2009 to 2017 were matched to one-year lagged achievement scores and RIP values using country and year identifiers 34 . The final sample consisted of 175 countries and was constrained to locations and years with non-missing indicators. A multiple linear mixed effects model fitted by restricted maximum likelihood was used to assess the independent effect of P, W and E scores and RIP values on the rates of current smoking. Specifically, a country random intercept and a country random slope on RIP were included to account for geographical heterogeneity and within-country correlation. The regression model takes the following general form:
where y c,t is the prevalence of current smoking in each country ( c ) and year ( t ), β 0 is the intercept for the model and β p , β w , β e and β r are the fixed effects for each of the policy predictors. \(\mathrm{P}_{c,\,t - 1},\,\mathrm{W}_{c,\,t - 1},\,\mathrm{E}_{c,\,t - 1}\) are the P, W and E scores and R c , t −1 is the RIP value for country c in year t − 1. Finally, α c is the random intercept for country ( c ), while δ c represent the random slope for the country ( c ) to which the RIP value (R t − 1 ) belongs. Variance inflation factor values were calculated for all the predictor parameters to check for multicollinearity; the values found were low (<2) 35 . Bivariate models were also run and are shown in Extended Data Fig. 5 . The one-year lag introduced into the model may have led to an underestimation of effect sizes, particularly as many MPOWER policies require a greater period of time to be implemented effectively. However, due to the limited time range of our data (spanning eight years in total), introducing a longer lag period would have resulted in the loss of additional data points, thus further limiting our statistical power in detecting relevant associations between policies and smoking prevalence.
In addition to a joint model for smokers from both sexes, separate regressions were fitted for men and women and the four age groups (15–29, 30–49, ≥50 and ≥15 years old). To assess the validity of the mixed effects analyses, likelihood ratio tests comparing the models with random effects to the null models with only fixed effects were performed. Linear mixed models were fitted by maximum likelihood and t -tests used Satterthwaite approximations to degrees of freedom. P values were considered statistically significant if <0.05. All analyses were executed with RStudio v.1.1.383 using the lmer function in the R package lme4 v.1.1-21 (ref. 36 ).
A series of additional models to examine the impact of tobacco control policies were developed as part of this study. In each model, cigarette affordability (RIP) and a different set of policy metrics was used to capture the implementation, quality and compliance of tobacco control legislation. In models 1 and 2, we replaced the achievements scores by the proportion of P, W and E measures adopted by each country out of all possible measures reported by the WHO. In model 3, we used P and E (direct and indirect measures separately) compliance scores provided by the WHO to represent actual legislation implementation. Finally, an interaction term for compliance and achievement to capture the combined effect of legislation quality and performance was added to model 4. Results for men and women by age group for each of the additional models are presented in the Supplemental Information (Supplementary Tables 1–4 ).
The main model described in this study was chosen because it includes a larger number of country-year observations ( n = 823) when compared to models including compliance scores and because it is more directly interpretable.
Counterfactual analysis
To further explore and quantify the impact of tobacco control policies on current smoking prevalence, we simulated what smoking prevalence across all countries would have been achieved in 2017 under 4 alternative policy scenarios: (1) if achievement scores and RIP remained at the level they were at in 2008; (2) if all countries had implemented each of P, W and E component at the highest level (score = 5); (3) if the price of a cigarette pack was I$7.73 or higher, a price that represents the 90th percentile of observed prices across all countries and years; and (4) if countries had implemented the P, W and E components at the highest level and higher cigarette prices. To keep our results consistent across scenarios, we restricted our analysis to 155 countries with non-missing policy-related indicators for both 2008 and 2016.
Random effects were used in model fitting but not in this prediction. Simulated prevalence rates were calculated by multiplying the estimated marginal effect of each policy by the alternative values proposed in each of the counterfactual scenarios for each country-year. The global population-weighted average was computed for status quo and counterfactual scenarios using population data sourced from the Institute for Health Metrics and Evaluation. Using the predicted prevalence rates and population data, the additional reduction in the number of current smokers in 2017 was also computed. Since models were ran using age-standardized prevalence, the number of smokers was proportionally redistributed across age groups using the sex-specific numbers from the age group 15 and older as an envelope.
The UIs for predicted estimates were based on a computation of the results of each of the 1,000 draws (unbiased random samples) taken from the uncertainty distribution of each of the estimated coefficients; the lower bound of the 95% UI for the final quantity of interest is the 2.5 percentile of the distribution and the upper bound is the 97.5 percentile of the distribution.
Reporting Summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The dataset generated and analyzed during the current study is publicly available at http://ghdx.healthdata.org/record/ihme-data/global-tobacco-control-and-smoking-prevalence-scenarios-2017 ( https://doi.org/10.6069/QAZ7-6505 ). The dataset contains all data necessary to interpret, replicate and build on the methods or findings reported in the article. Tobacco control policy data that support the findings of this study are released every two years as part of the WHO’s Global Report on Tobacco Control; these data are also directly accessible at https://www.who.int/tobacco/global_report/en/ . Source data are provided with this paper.
Code availability
All code used for these analyses is available at http://ghdx.healthdata.org/record/ihme-data/global-tobacco-control-and-smoking-prevalence-scenarios-2017 and https://github.com/ihmeuw/team/tree/effects_tobacco_policies .
World Health Organization. WHO Framework Convention on Tobacco Control https://www.who.int/fctc/text_download/en/ (2003).
United Nations. Transforming Our World: the 2030 Agenda for Sustainable Development https://sustainabledevelopment.un.org/post2015/transformingourworld/publication (2015).
Stanaway, J. D. et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392 , 1923–1994 (2018).
Article Google Scholar
Jha, P. & Peto, R. Global effects of smoking, of quitting, and of taxing tobacco. N. Engl. J. Med. 370 , 60–68 (2014).
Article CAS Google Scholar
Ekpu, V. U. & Brown, A. K. The economic impact of smoking and of reducing smoking prevalence: review of evidence. Tob. Use Insights 8 , 1–35 (2015).
World Health Organization. WHO Report on the Global Tobacco Epidemic, 2008: the MPOWER Package https://www.who.int/tobacco/mpower/2008/en/ (2008).
Chung-Hall, J., Craig, L., Gravely, S., Sansone, N. & Fong, G. T. Impact of the WHO FCTC over the first decade: a global evidence review prepared for the Impact Assessment Expert Group. Tob. Control 28 , s119–s128 (2019).
Reitsma, M. B. et al. Smoking prevalence and attributable disease burden in 195 countries and territories, 1990–2015: a systematic analysis from the Global Burden of Disease Study 2015. Lancet 389 , 1885–1906 (2017).
Nilan, K., Raw, M., McKeever, T. M., Murray, R. L. & McNeill, A. Progress in implementation of WHO FCTC Article 14 and its guidelines: a survey of tobacco dependence treatment provision in 142 countries. Addiction 112 , 2023–2031 (2017).
Dubray, J., Schwartz, R., Chaiton, M., O’Connor, S. & Cohen, J. E. The effect of MPOWER on smoking prevalence. Tob. Control 24 , 540–542 (2015).
Anderson, C. L., Becher, H. & Winkler, V. Tobacco control progress in low and middle income countries in comparison to high income countries. Int. J. Environ. Res. Public Health 13 , 1039 (2016).
Gravely, S. et al. Implementation of key demand-reduction measures of the WHO Framework Convention on Tobacco Control and change in smoking prevalence in 126 countries: an association study. Lancet Public Health 2 , e166–e174 (2017).
Ngo, A., Cheng, K.-W., Chaloupka, F. J. & Shang, C. The effect of MPOWER scores on cigarette smoking prevalence and consumption. Prev. Med. 105S , S10–S14 (2017).
Feliu, A. et al. Impact of tobacco control policies on smoking prevalence and quit ratios in 27 European Union countries from 2006 to 2014. Tob. Control 28 , 101–109 (2019).
Google Scholar
Greaves, L. Gender, equity and tobacco control. Health Sociol. Rev. 16 , 115–129 (2007).
Amos, A., Greaves, L., Nichter, M. & Bloch, M. Women and tobacco: a call for including gender in tobacco control research, policy and practice. Tob. Control 21 , 236–243 (2012).
Hoffman, S. J. & Tan, C. Overview of systematic reviews on the health-related effects of government tobacco control policies. BMC Public Health 15 , 744 (2015).
Chaloupka, F. J., Straif, K. & Leon, M. E. Effectiveness of tax and price policies in tobacco control. Tob. Control 20 , 235–238 (2011).
Rice, N., Godfrey, C., Slack, R., Sowden, A. & Worthy, G. A Systematic Review of the Effects of Price on the Smoking Behaviour of Young People (Centre for Reviews and Dissemination, 2009); https://www.crd.york.ac.uk/crdweb/ShowRecord.asp?LinkFrom=OAI&ID=12013060057&LinkFrom=OAI&ID=12013060057
World Health Organizaion. WHO Report on the Global Tobacco Epidemic 2017: Monitoring Tobacco Use and Prevention Policies https://www.who.int/tobacco/global_report/2017/en/ (2017).
Institute for Health Metrics and Evaluation. GBD Compare https://vizhub.healthdata.org/gbd-compare/ (2017).
Saffer, H. & Chaloupka, F. The effect of tobacco advertising bans on tobacco consumption. J. Health Econ. 19 , 1117–1137 (2000).
Noar, S. M. et al. The impact of strengthening cigarette pack warnings: systematic review of longitudinal observational studies. Soc. Sci. Med. 164 , 118–129 (2016).
Moodie, C., Brose, L. S., Lee, H. S., Power, E. & Bauld, L. How did smokers respond to standardised cigarette packaging with new, larger health warnings in the United Kingdom during the transition period? A cross-sectional online survey. Addict. Res. Theory 28 , 53–61 (2020).
Wakefield, M. et al. Australian adult smokers’ responses to plain packaging with larger graphic health warnings 1 year after implementation: results from a national cross-sectional tracking survey. Tob. Control 24 , ii17–ii25 (2015).
Thun, M., Peto, R., Boreham, J. & Lopez, A. D. Stages of the cigarette epidemic on entering its second century. Tob. Control 21 , 96–101 (2012).
Bialous, S. A. Impact of implementation of the WHO FCTC on the tobacco industry’s behaviour. Tob. Control 28 , s94–s96 (2019).
World Health Organization. Global Report on Trends in Prevalence of Tobacco Smoking 2000–2025 http://www.who.int/tobacco/publications/surveillance/trends-tobacco-smoking-second-edition/en/ (2018).
World Health Organization. WHO Report on the Global Tobacco Epidemic 2009: Implementing Smoke-Free Environments https://www.who.int/tobacco/mpower/2009/en/ (2009).
World Health Organization. WHO Report on the Global Tobacco Epidemic 2011: Warning About the Dangers of Tobacco https://www.who.int/tobacco/global_report/2011/en/ (2011).
World Health Organization. WHO Report on the Global Tobacco Epidemic 2013: Enforcing Bans on Tobacco Advertising, Promotion and Sponsorship https://www.who.int/tobacco/global_report/2013/en/ (2013).
World Health Organization. WHO Report on the Global Tobacco Epidemic 2015: Raising Taxes on Tobacco https://www.who.int/tobacco/global_report/2015/en/ (2015).
James, S. L., Gubbins, P., Murray, C. J. & Gakidou, E. Developing a comprehensive time series of GDP per capita for 210 countries from 1950 to 2015. Popul. Health Metr. 10 , 12 (2012).
Institute for Health Metrics and Evaluation. Global Tobacco Control and Smoking Prevalence Scenarios 2017 (dataset) (Global Health Data Exchange, 2020).
Zuur, A. F., Ieno, E. N. & Elphick, C. S. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 1 , 3–14 (2010).
Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. https://doi.org/10.18637/jss.v067.i01 (2015).
Download references
Acknowledgements
The study was funded by Bloomberg Philanthropies (grant 47386, Initiative to Reduce Tobacco Use). We thank the support of the Tobacco Metrics Team Advisory Group, which provided valuable comments and suggestions over several iterations of this manuscript. We also thank the Tobacco Free Initiative team at the WHO and the Campaign for Tobacco-Free Kids for making the tobacco control legislation data available and providing clarifications when necessary. We thank A. Tapp, E. Mullany and J. Whisnant for assisting in the management and execution of this study. We thank the team who worked in a previous iteration of this project, especially A. Reynolds, C. Margono, E. Dansereau, K. Bolt, M. Subart and X. Dai. Lastly, we thank all GBD 2017 Tobacco collaborators for their valuable work in providing feedback to our smoking prevalence estimates throughout the GBD 2017 cycle.
Author information
Authors and affiliations.
Institute for Health Metrics and Evaluation, Department of Health Metrics Sciences, University of Washington, Seattle, WA, USA
Luisa S. Flor, Marissa B. Reitsma, Vinay Gupta & Emmanuela Gakidou
IBM Watson Health, San Jose, CA, USA
You can also search for this author in PubMed Google Scholar
Contributions
L.S.F., M.N. and E.G. conceptualized the study and designed the analytical framework. M.B.R. and V.G. provided input on data, results and interpretation. L.S.F. and E.G. wrote the first draft of the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Correspondence to Emmanuela Gakidou .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Peer review information Jennifer Sargent was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended data fig. 1 prevalence of current smoking for men (a) and women (b) aged 15 years and older (age-standardized) in 2017..
Age-standardized smoking prevalence (%) estimates from the 2017 Global Burden of Disease Study for men (a) and women (b) aged 15 years and older for 195 countries. Smoking is defined as current use of any type of smoked tobacco product. Details on the estimation process can be found in the Methods section and elsewhere 3 .
Extended Data Fig. 2 Prevalence of current smoking for men (a) and women (b) aged 15 to 29 years old (age-standardized) in 2017.
Age-standardized smoking prevalence (%) estimates from the 2017 Global Burden of Disease Study for men (a) and women (b) aged 15–29 years old for 195 countries. Smoking is defined as current use of any type of smoked tobacco product. Details on the estimation process can be found in the Methods section and elsewhere 3 .
Extended Data Fig. 3 Prevalence of current smoking for men (a) and women (b) aged 30 to 49 years old (age-standardized) in 2017.
Age-standardized smoking prevalence (%) estimates from the 2017 Global Burden of Disease Study for men (a) and women (b) aged 30–49 years old for 195 countries. Smoking is defined as current use of any type of smoked tobacco product. Details on the estimation process can be found in the Methods section and elsewhere 3 .
Extended Data Fig. 4 Prevalence of current smoking for men (a) and women (b) aged 50 years and older (age-standardized) in 2017.
Age-standardized smoking prevalence (%) estimates from the 2017 Global Burden of Disease Study for men (a) and women (b) aged 50 years and older for 195 countries. Smoking is defined as current use of any type of smoked tobacco product. Details on the estimation process can be found in the Methods section and elsewhere 3 .
Extended Data Fig. 5 Percentage changes in current smoking prevalence based on fixed effect coefficients from bivariate mixed effect linear regression models, by policy component, sex and age group.
Bivariate models examined the unadjusted association between smoke-free (P), health warnings (W), and advertising (E) achievement scores, and cigarette’s affordability (RIP) and current smoking prevalence, from 2009 to 2017, across 175 countries (n = 823 country-years). Linear mixed models were fit by maximum likelihood and t-tests used Satterthwaite approximations to degrees of freedom. P values were considered statistically significant if lower than 0.05.
Supplementary information
Supplementary information.
Supplementary Tables 1–4: additional models results.
Source Data Fig. 1
Input data for Fig. 1 replication.
Source Data Extended Data Fig. 1
Input data for Extended Data 1 replication.
Source Data Extended Data Fig. 2
Input data for Extended Data 2 replication.
Source Data Extended Data Fig. 3
Input data for Extended Data 3 replication.
Source Data Extended Data Fig. 4
Input data for Extended Data 4 replication.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Flor, L.S., Reitsma, M.B., Gupta, V. et al. The effects of tobacco control policies on global smoking prevalence. Nat Med 27 , 239–243 (2021). https://doi.org/10.1038/s41591-020-01210-8
Download citation
Received : 28 May 2020
Accepted : 10 December 2020
Published : 21 January 2021
Issue Date : February 2021
DOI : https://doi.org/10.1038/s41591-020-01210-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Marketing claims, promotional strategies, and product information on malaysian e-cigarette retailer websites-a content analysis.
- Sameeha Misriya Shroff
- Chandrashekhar T Sreeramareddy
Substance Abuse Treatment, Prevention, and Policy (2024)
Challenges in legitimizing further measures against smoking in jurisdictions with robust infrastructure for tobacco control: how far can the authorities allow themselves to go?
- Karl Erik Lund
- Gunnar Saebo
Harm Reduction Journal (2024)
Health effects associated with exposure to secondhand smoke: a Burden of Proof study
- Luisa S. Flor
- Jason A. Anderson
- Emmanuela Gakidou
Nature Medicine (2024)
Impacts of heavy smoking on non-coding RNA expression for patients with esophageal carcinoma
- Jianming Wang
BMC Medical Genomics (2023)
Shifting the gaze on implementation: examining the association between the implementation of tobacco control laws and prevalence of tobacco using data from a nationally representative survey
- Pragati B. Hebbar
- Upendra Bhojani
- Gera E. Nagelhout
BMC Public Health (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Search Menu
- Supplements
- Advance articles
- Editor's Choice
- Special Issues
- Author Guidelines
- Submission Site
- Why Publish With Us?
- Open Access
- About Nicotine & Tobacco Research
- About Society for Nicotine & Tobacco Research
- Editorial Board
- Advertising and Corporate Services
- Journals Career Network
- Self-Archiving Policy
- Dispatch Dates
- Journals on Oxford Academic
- Books on Oxford Academic

Editor-in-Chief
Marcus Munafo, PhD
The International Impacts of Alcohol Use Disorder and Nicotine and Tobacco
Explore a collaborative collection showcasing crucial research into the global impacts of alcohol use disorder and nicotine and tobacco use.
Latest articles
Latest posts on x, editor's choice, the biological impact of menthol on tobacco dependence.
Understanding menthol’s biological functions as it pertains to nicotine dependence will be helpful in crafting novel pharmacotherapies that might better serve menthol smokers.
Use of Mentholated Cigarettes and Likelihood of Smoking Cessation in the United States: A Meta-Analysis
This study adds a quantitative summary of the association between menthol cigarette use and smoking cessation in the United States.
2018 Langley Award for Basic Research on Nicotine and Tobacco: Bringing Precision Medicine to Smoking Cessation
This review describes the journey from genetic discovery to the potential implementation of genetic knowledge for the treatment of tobacco use disorder.
Tobacco Cost of Illness Studies: A Systematic Review
The review systematically documents the post-2007 literature on tobacco cost-of-illness estimations and details conditions and costs included.

Committee on Publication Ethics (COPE)
This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE)
publicationethics.org

Ready to format your manuscript?
If you’re ready to prepare your manuscript for submission, be sure to read our Instructions for Authors to make the process as simple as possible.
You can read our latest guidelines here .


Diversity and Equity in Nicotine & Tobacco Research
We are committed to reducing health inequities associated with commercial tobacco products and, in particular, where discriminatory practices have contributed to higher smoking rates among some ethnic and racial groups. As a part of these efforts, we have curated a collection of articles that foreground diversity, equity, and inclusion in their research.
Read the collection here .

About the Journal
Nicotine & Tobacco Research aims to provide a forum for empirical findings, critical reviews, and conceptual papers on the many aspects of nicotine and tobacco, including research from the biobehavioral, neurobiological, molecular biologic, epidemiological, prevention, and treatment arenas.
Find out more here.
Related Titles
- About Nicotine & Tobacco Research
- Recommend to your Library
Affiliations
- Online ISSN 1469-994X
- Copyright © 2024 Society for Research on Nicotine and Tobacco
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Change Password
Your password must have 6 characters or more:.
- a lower case character,
- an upper case character,
- a special character
Password Changed Successfully
Your password has been changed
Create your account
Forget yout password.
Enter your email address below and we will send you the reset instructions
If the address matches an existing account you will receive an email with instructions to reset your password
Forgot your Username?
Enter your email address below and we will send you your username
If the address matches an existing account you will receive an email with instructions to retrieve your username

- April 01, 2024 | VOL. 181, NO. 4 CURRENT ISSUE pp.255-346
- March 01, 2024 | VOL. 181, NO. 3 pp.171-254
- February 01, 2024 | VOL. 181, NO. 2 pp.83-170
- January 01, 2024 | VOL. 181, NO. 1 pp.1-82
The American Psychiatric Association (APA) has updated its Privacy Policy and Terms of Use , including with new information specifically addressed to individuals in the European Economic Area. As described in the Privacy Policy and Terms of Use, this website utilizes cookies, including for the purpose of offering an optimal online experience and services tailored to your preferences.
Please read the entire Privacy Policy and Terms of Use. By closing this message, browsing this website, continuing the navigation, or otherwise continuing to use the APA's websites, you confirm that you understand and accept the terms of the Privacy Policy and Terms of Use, including the utilization of cookies.
Substance Use Disorders and Addiction: Mechanisms, Trends, and Treatment Implications
- Ned H. Kalin , M.D.
Search for more papers by this author
The numbers for substance use disorders are large, and we need to pay attention to them. Data from the 2018 National Survey on Drug Use and Health ( 1 ) suggest that, over the preceding year, 20.3 million people age 12 or older had substance use disorders, and 14.8 million of these cases were attributed to alcohol. When considering other substances, the report estimated that 4.4 million individuals had a marijuana use disorder and that 2 million people suffered from an opiate use disorder. It is well known that stress is associated with an increase in the use of alcohol and other substances, and this is particularly relevant today in relation to the chronic uncertainty and distress associated with the COVID-19 pandemic along with the traumatic effects of racism and social injustice. In part related to stress, substance use disorders are highly comorbid with other psychiatric illnesses: 9.2 million adults were estimated to have a 1-year prevalence of both a mental illness and at least one substance use disorder. Although they may not necessarily meet criteria for a substance use disorder, it is well known that psychiatric patients have increased usage of alcohol, cigarettes, and other illicit substances. As an example, the survey estimated that over the preceding month, 37.2% of individuals with serious mental illnesses were cigarette smokers, compared with 16.3% of individuals without mental illnesses. Substance use frequently accompanies suicide and suicide attempts, and substance use disorders are associated with a long-term increased risk of suicide.
Addiction is the key process that underlies substance use disorders, and research using animal models and humans has revealed important insights into the neural circuits and molecules that mediate addiction. More specifically, research has shed light onto mechanisms underlying the critical components of addiction and relapse: reinforcement and reward, tolerance, withdrawal, negative affect, craving, and stress sensitization. In addition, clinical research has been instrumental in developing an evidence base for the use of pharmacological agents in the treatment of substance use disorders, which, in combination with psychosocial approaches, can provide effective treatments. However, despite the existence of therapeutic tools, relapse is common, and substance use disorders remain grossly undertreated. For example, whether at an inpatient hospital treatment facility or at a drug or alcohol rehabilitation program, it was estimated that only 11% of individuals needing treatment for substance use received appropriate care in 2018. Additionally, it is worth emphasizing that current practice frequently does not effectively integrate dual diagnosis treatment approaches, which is important because psychiatric and substance use disorders are highly comorbid. The barriers to receiving treatment are numerous and directly interact with existing health care inequities. It is imperative that as a field we overcome the obstacles to treatment, including the lack of resources at the individual level, a dearth of trained providers and appropriate treatment facilities, racial biases, and the marked stigmatization that is focused on individuals with addictions.
This issue of the Journal is focused on understanding factors contributing to substance use disorders and their comorbidity with psychiatric disorders, the effects of prenatal alcohol use on preadolescents, and brain mechanisms that are associated with addiction and relapse. An important theme that emerges from this issue is the necessity for understanding maladaptive substance use and its treatment in relation to health care inequities. This highlights the imperative to focus resources and treatment efforts on underprivileged and marginalized populations. The centerpiece of this issue is an overview on addiction written by Dr. George Koob, the director of the National Institute on Alcohol Abuse and Alcoholism (NIAAA), and coauthors Drs. Patricia Powell (NIAAA deputy director) and Aaron White ( 2 ). This outstanding article will serve as a foundational knowledge base for those interested in understanding the complex factors that mediate drug addiction. Of particular interest to the practice of psychiatry is the emphasis on the negative affect state “hyperkatifeia” as a major driver of addictive behavior and relapse. This places the dysphoria and psychological distress that are associated with prolonged withdrawal at the heart of treatment and underscores the importance of treating not only maladaptive drug-related behaviors but also the prolonged dysphoria and negative affect associated with addiction. It also speaks to why it is crucial to concurrently treat psychiatric comorbidities that commonly accompany substance use disorders.
Insights Into Mechanisms Related to Cocaine Addiction Using a Novel Imaging Method for Dopamine Neurons
Cassidy et al. ( 3 ) introduce a relatively new imaging technique that allows for an estimation of dopamine integrity and function in the substantia nigra, the site of origin of dopamine neurons that project to the striatum. Capitalizing on the high levels of neuromelanin that are found in substantia nigra dopamine neurons and the interaction between neuromelanin and intracellular iron, this MRI technique, termed neuromelanin-sensitive MRI (NM-MRI), shows promise in studying the involvement of substantia nigra dopamine neurons in neurodegenerative diseases and psychiatric illnesses. The authors used this technique to assess dopamine function in active cocaine users with the aim of exploring the hypothesis that cocaine use disorder is associated with blunted presynaptic striatal dopamine function that would be reflected in decreased “integrity” of the substantia nigra dopamine system. Surprisingly, NM-MRI revealed evidence for increased dopamine in the substantia nigra of individuals using cocaine. The authors suggest that this finding, in conjunction with prior work suggesting a blunted dopamine response, points to the possibility that cocaine use is associated with an altered intracellular distribution of dopamine. Specifically, the idea is that dopamine is shifted from being concentrated in releasable, functional vesicles at the synapse to a nonreleasable cytosolic pool. In addition to providing an intriguing alternative hypothesis underlying the cocaine-related alterations observed in substantia nigra dopamine function, this article highlights an innovative imaging method that can be used in further investigations involving the role of substantia nigra dopamine systems in neuropsychiatric disorders. Dr. Charles Bradberry, chief of the Preclinical Pharmacology Section at the National Institute on Drug Abuse, contributes an editorial that further explains the use of NM-MRI and discusses the theoretical implications of these unexpected findings in relation to cocaine use ( 4 ).
Treatment Implications of Understanding Brain Function During Early Abstinence in Patients With Alcohol Use Disorder
Developing a better understanding of the neural processes that are associated with substance use disorders is critical for conceptualizing improved treatment approaches. Blaine et al. ( 5 ) present neuroimaging data collected during early abstinence in patients with alcohol use disorder and link these data to relapses occurring during treatment. Of note, the findings from this study dovetail with the neural circuit schema Koob et al. provide in this issue’s overview on addiction ( 2 ). The first study in the Blaine et al. article uses 44 patients and 43 control subjects to demonstrate that patients with alcohol use disorder have a blunted neural response to the presentation of stress- and alcohol-related cues. This blunting was observed mainly in the ventromedial prefrontal cortex, a key prefrontal regulatory region, as well as in subcortical regions associated with reward processing, specifically the ventral striatum. Importantly, this finding was replicated in a second study in which 69 patients were studied in relation to their length of abstinence prior to treatment and treatment outcomes. The results demonstrated that individuals with the shortest abstinence times had greater alterations in neural responses to stress and alcohol cues. The authors also found that an individual’s length of abstinence prior to treatment, independent of the number of days of abstinence, was a predictor of relapse and that the magnitude of an individual’s neural alterations predicted the amount of heavy drinking occurring early in treatment. Although relapse is an all too common outcome in patients with substance use disorders, this study highlights an approach that has the potential to refine and develop new treatments that are based on addiction- and abstinence-related brain changes. In her thoughtful editorial, Dr. Edith Sullivan from Stanford University comments on the details of the study, the value of studying patients during early abstinence, and the implications of these findings for new treatment development ( 6 ).
Relatively Low Amounts of Alcohol Intake During Pregnancy Are Associated With Subtle Neurodevelopmental Effects in Preadolescent Offspring
Excessive substance use not only affects the user and their immediate family but also has transgenerational effects that can be mediated in utero. Lees et al. ( 7 ) present data suggesting that even the consumption of relatively low amounts of alcohol by expectant mothers can affect brain development, cognition, and emotion in their offspring. The researchers used data from the Adolescent Brain Cognitive Development Study, a large national community-based study, which allowed them to assess brain structure and function as well as behavioral, cognitive, and psychological outcomes in 9,719 preadolescents. The mothers of 2,518 of the subjects in this study reported some alcohol use during pregnancy, albeit at relatively low levels (0 to 80 drinks throughout pregnancy). Interestingly, and opposite of that expected in relation to data from individuals with fetal alcohol spectrum disorders, increases in brain volume and surface area were found in offspring of mothers who consumed the relatively low amounts of alcohol. Notably, any prenatal alcohol exposure was associated with small but significant increases in psychological problems that included increases in separation anxiety disorder and oppositional defiant disorder. Additionally, a dose-response effect was found for internalizing psychopathology, somatic complaints, and attentional deficits. While subtle, these findings point to neurodevelopmental alterations that may be mediated by even small amounts of prenatal alcohol consumption. Drs. Clare McCormack and Catherine Monk from Columbia University contribute an editorial that provides an in-depth assessment of these findings in relation to other studies, including those assessing severe deficits in individuals with fetal alcohol syndrome ( 8 ). McCormack and Monk emphasize that the behavioral and psychological effects reported in the Lees et al. article would not be clinically meaningful. However, it is feasible that the influences of these low amounts of alcohol could interact with other predisposing factors that might lead to more substantial negative outcomes.
Increased Comorbidity Between Substance Use and Psychiatric Disorders in Sexual Identity Minorities
There is no question that victims of societal marginalization experience disproportionate adversity and stress. Evans-Polce et al. ( 9 ) focus on this concern in relation to individuals who identify as sexual minorities by comparing their incidence of comorbid substance use and psychiatric disorders with that of individuals who identify as heterosexual. By using 2012−2013 data from 36,309 participants in the National Epidemiologic Study on Alcohol and Related Conditions–III, the authors examine the incidence of comorbid alcohol and tobacco use disorders with anxiety, mood disorders, and posttraumatic stress disorder (PTSD). The findings demonstrate increased incidences of substance use and psychiatric disorders in individuals who identified as bisexual or as gay or lesbian compared with those who identified as heterosexual. For example, a fourfold increase in the prevalence of PTSD was found in bisexual individuals compared with heterosexual individuals. In addition, the authors found an increased prevalence of substance use and psychiatric comorbidities in individuals who identified as bisexual and as gay or lesbian compared with individuals who identified as heterosexual. This was most prominent in women who identified as bisexual. For example, of the bisexual women who had an alcohol use disorder, 60.5% also had a psychiatric comorbidity, compared with 44.6% of heterosexual women. Additionally, the amount of reported sexual orientation discrimination and number of lifetime stressful events were associated with a greater likelihood of having comorbid substance use and psychiatric disorders. These findings are important but not surprising, as sexual minority individuals have a history of increased early-life trauma and throughout their lives may experience the painful and unwarranted consequences of bias and denigration. Nonetheless, these findings underscore the strong negative societal impacts experienced by minority groups and should sensitize providers to the additional needs of these individuals.
Trends in Nicotine Use and Dependence From 2001–2002 to 2012–2013
Although considerable efforts over earlier years have curbed the use of tobacco and nicotine, the use of these substances continues to be a significant public health problem. As noted above, individuals with psychiatric disorders are particularly vulnerable. Grant et al. ( 10 ) use data from the National Epidemiologic Survey on Alcohol and Related Conditions collected from a very large cohort to characterize trends in nicotine use and dependence over time. Results from their analysis support the so-called hardening hypothesis, which posits that although intervention-related reductions in nicotine use may have occurred over time, the impact of these interventions is less potent in individuals with more severe addictive behavior (i.e., nicotine dependence). When adjusted for sociodemographic factors, the results demonstrated a small but significant increase in nicotine use from 2001–2002 to 2012–2013. However, a much greater increase in nicotine dependence (46.1% to 52%) was observed over this time frame in individuals who had used nicotine during the preceding 12 months. The increases in nicotine use and dependence were associated with factors related to socioeconomic status, such as lower income and lower educational attainment. The authors interpret these findings as evidence for the hardening hypothesis, suggesting that despite the impression that nicotine use has plateaued, there is a growing number of highly dependent nicotine users who would benefit from nicotine dependence intervention programs. Dr. Kathleen Brady, from the Medical University of South Carolina, provides an editorial ( 11 ) that reviews the consequences of tobacco use and the history of the public measures that were initially taken to combat its use. Importantly, her editorial emphasizes the need to address health care inequity issues that affect individuals of lower socioeconomic status by devoting resources to develop and deploy effective smoking cessation interventions for at-risk and underresourced populations.
Conclusions
Maladaptive substance use and substance use disorders are highly prevalent and are among the most significant public health problems. Substance use is commonly comorbid with psychiatric disorders, and treatment efforts need to concurrently address both. The papers in this issue highlight new findings that are directly relevant to understanding, treating, and developing policies to better serve those afflicted with addictions. While treatments exist, the need for more effective treatments is clear, especially those focused on decreasing relapse rates. The negative affective state, hyperkatifeia, that accompanies longer-term abstinence is an important treatment target that should be emphasized in current practice as well as in new treatment development. In addition to developing a better understanding of the neurobiology of addictions and abstinence, it is necessary to ensure that there is equitable access to currently available treatments and treatment programs. Additional resources must be allocated to this cause. This depends on the recognition that health care inequities and societal barriers are major contributors to the continued high prevalence of substance use disorders, the individual suffering they inflict, and the huge toll that they incur at a societal level.
Disclosures of Editors’ financial relationships appear in the April 2020 issue of the Journal .
1 US Department of Health and Human Services: Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality: National Survey on Drug Use and Health 2018. Rockville, Md, SAMHSA, 2019 ( https://www.samhsa.gov/data/nsduh/reports-detailed-tables-2018-NSDUH ) Google Scholar
2 Koob GF, Powell P, White A : Addiction as a coping response: hyperkatifeia, deaths of despair, and COVID-19 . Am J Psychiatry 2020 ; 177:1031–1037 Link , Google Scholar
3 Cassidy CM, Carpenter KM, Konova AB, et al. : Evidence for dopamine abnormalities in the substantia nigra in cocaine addiction revealed by neuromelanin-sensitive MRI . Am J Psychiatry 2020 ; 177:1038–1047 Link , Google Scholar
4 Bradberry CW : Neuromelanin MRI: dark substance shines a light on dopamine dysfunction and cocaine use (editorial). Am J Psychiatry 2020 ; 177:1019–1021 Abstract , Google Scholar
5 Blaine SK, Wemm S, Fogelman N, et al. : Association of prefrontal-striatal functional pathology with alcohol abstinence days at treatment initiation and heavy drinking after treatment initiation . Am J Psychiatry 2020 ; 177:1048–1059 Abstract , Google Scholar
6 Sullivan EV : Why timing matters in alcohol use disorder recovery (editorial). Am J Psychiatry 2020 ; 177:1022–1024 Abstract , Google Scholar
7 Lees B, Mewton L, Jacobus J, et al. : Association of prenatal alcohol exposure with psychological, behavioral, and neurodevelopmental outcomes in children from the Adolescent Brain Cognitive Development Study . Am J Psychiatry 2020 ; 177:1060–1072 Link , Google Scholar
8 McCormack C, Monk C : Considering prenatal alcohol exposure in a developmental origins of health and disease framework (editorial). Am J Psychiatry 2020 ; 177:1025–1028 Abstract , Google Scholar
9 Evans-Polce RJ, Kcomt L, Veliz PT, et al. : Alcohol, tobacco, and comorbid psychiatric disorders and associations with sexual identity and stress-related correlates . Am J Psychiatry 2020 ; 177:1073–1081 Abstract , Google Scholar
10 Grant BF, Shmulewitz D, Compton WM : Nicotine use and DSM-IV nicotine dependence in the United States, 2001–2002 and 2012–2013 . Am J Psychiatry 2020 ; 177:1082–1090 Link , Google Scholar
11 Brady KT : Social determinants of health and smoking cessation: a challenge (editorial). Am J Psychiatry 2020 ; 177:1029–1030 Abstract , Google Scholar
- Cited by None

- Substance-Related and Addictive Disorders
- Addiction Psychiatry
- Transgender (LGBT) Issues
Vaping: The new wave of nicotine addiction
Affiliations.
- 1 Cleveland Clinic Lerner College of Medicine of Case Western Reserve University, Cleveland, OH.
- 2 Head, Center for Adolescent Medicine, Department of General Pediatrics, Cleveland Clinic; Professor, Cleveland Clinic Lerner College of Medicine of Case Western Reserve University, Cleveland, OH [email protected].
- PMID: 31821136
- DOI: 10.3949/ccjm.86a.19118
Vaping devices, introduced to the US market in 2007 as aids for smoking cessation, have become popular among youth and young adults because of their enticing flavors and perceived lack of negative health effects. However, evidence is emerging that vaping may introduce high levels of dangerous chemicals into the body and cause severe lung injury and death. This article reviews the history and prevalence of vaping and available research on its health effects and efficacy in smoking cessation, and proposes recommendations for clinicians and legislators to reduce harms associated with vaping.
Copyright © 2019 Cleveland Clinic.
Publication types
- Adolescent Behavior
- Behavior, Addictive* / etiology
- Behavior, Addictive* / prevention & control
- Behavior, Addictive* / psychology
- Electronic Nicotine Delivery Systems*
- United States
- Vaping* / adverse effects
- Vaping* / epidemiology
- Vaping* / prevention & control
- Vaping* / psychology
- Study protocol
- Open access
- Published: 18 November 2021
Study protocol: evaluation of the addictive potential of e-cigarettes (EVAPE): neurobiological, sociological, and epidemiological perspectives
- Sabine Vollstädt-Klein ORCID: orcid.org/0000-0002-6210-672X 1 , 2 na1 ,
- Nadja Grundinger 1 na1 ,
- Tatiana Görig 3 ,
- Daria Szafran 3 ,
- Astrid Althaus 4 ,
- Ute Mons 4 na1 &
- Sven Schneider 3 na1
BMC Psychology volume 9 , Article number: 181 ( 2021 ) Cite this article
2643 Accesses
3 Citations
Metrics details
Tobacco use is the largest preventable cause of diseases and deaths; reducing tobacco intake is, therefore, an urgent public health goal. In recent years, e-cigarettes have been marketed as a 'healthier' alternative to tobacco smoking, whilst product features have evolved tremendously in the meantime. A lively scientific debate has developed regarding the potential benefits and risks of e-cigarettes although, surprisingly, there are few studies investigating the addictive potential of nicotine-containing e-cigarettes. The present work comprises three work packages investigating the addictive potential of e-cigarettes from different perspectives: (1) the neurobiological addictive potential of e-cigarettes; (2) the experience and perception of dependence symptoms among users of e-cigarettes in a social context; and (3) the epidemiological perspective regarding factors influencing the potential for dependence.
Work package I: the neurobiological study will investigate the key elements of addiction in e-cigarettes compared to tobacco cigarettes using neurobiological and neuropsychological correlates associated with craving, incentive motivation, cue reactivity and attentional bias. Work package II: the sociological study part examines self-reports on the experience and perception of dependence symptoms in a social context, using focus group interviews and the analysis of posts in online discussion forums on e-cigarettes. Work package III: the epidemiological study part focuses on tolerance development and the role of psychosocial and product factors by analyzing longitudinal data from the International Tobacco Control Policy Evaluation Project (ITC).
The present study offers a chosen mix of three methodological approaches, thereby comprehensively examining core symptoms of positive and negative reinforcement in addiction. Whether e-cigarettes are as reinforcing and addictive as combustible tobacco cigarettes is an important public health issue with implications for prevention and treatment programs.
Trial registration: Work package I: Registered at clinicaltrials.gov/ct2/show/NCT04772014. Work package II: Registered at OSF Registries: https://osf.io/dxgya (2021, January 14).
Tobacco use causes more than 8 million deaths worldwide annually, making it the biggest preventable cause of disease [ 1 ]. Hence, reducing tobacco consumption and the associated health burden is an important goal. In 2006, e-cigarettes entered the market as alternatives to smoking tobacco cigarettes and the product characteristic have evolved tremendously since then. E-cigarettes can make nicotine available to users without exposing them to the harmful toxicants of tobacco smoke. Nevertheless, researchers have expressed different views on potential benefits and risks associated with e-cigarettes. Proponents see e-cigarettes as an innovative step in tobacco harm reduction, as switching from tobacco to e-cigarettes significantly reduces users’ exposure to the main toxicants of tobacco smoke [ 2 , 3 , 4 , 5 , 6 ] and may help smokers quit tobacco use [ 7 ]. Opponents on the other hand point to the lack of long-term data regarding potential health risks [ 8 , 9 ], and fear that the marketing of e-cigarettes as lifestyle products leads to nicotine dependence and might be a gateway or a catalyst to tobacco use, especially among young non-smokers [ 10 , 11 , 12 , 13 , 14 ]. Although this 'gateway hypothesis' is highly controversial [ 15 ], research indicates that e-cigarettes—as compared to other nicotine replacement therapies (NRTs)—are mostly not limited to short-term use [ 16 , 17 ]. Ex-smokers, who switch to e-cigarettes, often maintain their nicotine levels. While most e-cigarettes allow a gradual reduction of nicotine levels, longitudinal research findings indicates that a reduction in the concentration of nicotine may be accompanied by a higher consumption of liquid [ 18 ]. Such data suggests a maintenance of nicotine addiction that is initially acquired by tobacco use. It is however surprising that there are only few studies to date that examine the addictive potential of e-cigarettes containing nicotine.
Addictive behavior is determined by positive and negative reinforcement mechanisms. The rewarding potential of a substance includes its euphoric effect and the latency period until the effect occurs. The faster the drug enters the brain, the greater the euphoric effect [ 19 , 20 ]. This positive reinforcement effect leads to an initial repetition of drug use. Neuroadaptive changes occur, which lead to a dysregulation of the neurochemical circuits and thus to withdrawal symptoms when the drug is discontinued [ 21 ]. The shorter the elimination half-life of a substance, the more severe the withdrawal symptoms [ 20 ]. To avoid withdrawal symptoms, the substance is consumed repeatedly and frequently, leading to increasing tolerance (e.g., higher dosage to achieve the same effect) [ 19 ]. Accordingly, in addition to positive reinforcement in the early stages of the addiction process, this negative reinforcement mechanisms are increasingly recruited as a source of motivation [ 21 ].
Tobacco dependence is primarily produced by the pharmacological effects of nicotine [ 22 ]. Cigarette smoke releases significant amounts of nicotine into the bloodstream, where it quickly reaches the brain and triggers the release of dopamine by stimulating nicotinic acetylcholine receptors [ 23 ]. Other characteristics and additives of tobacco smoke further enhance the addictive potential [ 24 , 25 , 26 , 27 ]. This causes the rapid positive reinforcement, making smoking of tobacco the most addictive form of nicotine administration [ 22 , 28 ]. Nicotine uptake is significantly slower and lower with NRTs, which can explain the absence of the addiction-inducing 'kick' [ 29 ]. In most studies, e-cigarettes also showed lower nicotine absorption than tobacco cigarettes [ 30 ]. If they deliver nicotine less effectively, e-cigarettes might, thus, have less addictive properties. Nevertheless, depending on the device, liquid, and user behavior, it is possible to achieve equal or even higher plasma nicotine levels [ 30 , 31 ].
However, nicotine is a necessary but not a sufficient component in the development of dependence [ 32 ]. Non-pharmacological motives for smoking include psychological, behavioral, sensorimotor and social manipulative factors [ 33 , 34 ]. Thus, cigarette smoke is known to have very characteristic sensory effects on the respiratory tract that are perceived as pleasant and reduce the urge to smoke even more effectively than the direct pharmacological effect of nicotine [ 32 , 35 , 36 , 37 ]. The combination of pleasant stimuli associated with smoking behavior and the drug itself act synergistically. Multisensory experiences of smoking (visual, tactile, auditory, olfactory, gustatory) quickly acquire the quality of a conditioned cue stimulus that can trigger the urge to smoke [ 38 ]. Therefore, craving can be induced by the substance itself, substance-associated stimuli or by emotional states such as stress [ 39 , 40 ]. This is not necessarily associated with physical discomfort, but also includes preoccupation with thoughts of the drug. Expectations of positive outcomes from smoking (e.g., social interaction, stress coping, stop craving) as well as expectations of the negative consequences of quitting (e.g., physical withdrawal symptoms, weight gain) play a crucial role in addiction. Thus, it has been shown that 79% of interviewed ex-smokers are afraid of relapsing if they would stop using their e-cigarette [ 41 ]. Therefore, psychological dependence is also characterized by repeated drug use, but this is based less on tolerance development or physical withdrawal symptoms and more on classical and operant conditioning processes and craving [ 19 ]. With repeated consumption, the initial hedonic effects finally diminish, while consumption increasingly becomes habitual and eventually compulsive [ 42 ]. Cigarette smoking is such a compulsive behavioral pattern: rigid, automatic, and habitual actions that require little mental elaboration and are triggered by internal or external stimuli. E-cigarettes are the only tobacco-free nicotine delivery devices that closely resemble the smoking ritual of cigarette smoking: Hand-to-mouth movement, tactile action of puffing, inhalation and exhalation, the sensory stimulus in the airways, vapor production and social aspects such as smoking breaks. Therefore, e-cigarettes might be expected to produce the same psychological, behavioral, and social effects that can promote or maintain nicotine dependence.
Epidemiological data suggest that e-cigarettes may lead to dependence symptoms, such as craving, or e-cigarette use within 30 min of waking. In these studies, the severity of dependence, however, was significantly lower with e-cigarettes compared to tobacco cigarettes [ 43 , 44 , 45 ]. This is consistent with self-reporting by users, many of whom state that they are less dependent upon their e-cigarette than they were previously upon tobacco cigarettes [ 17 , 46 , 47 ]. Some experimental studies show that e-cigarettes, compared to other ‘high- and low-abuse liability’—nicotine products, have some risk of abuse that appears to be higher than for NRTs but lower compared to tobacco cigarettes [ 48 , 49 , 50 ]. Nevertheless, e-cigarette users show greater discounting for liquid compared to money, which was associated with more unsuccessful attempts to quit vaping [ 51 ].
Research to date shows that e-cigarettes have the potential for abuse liability and to maintain an existing nicotine dependence and lead to dependence symptoms. Whether e-cigarettes have a similar addictive potential as tobacco cigarettes has not yet been sufficiently clarified, especially since many studies were still conducted on old devices from earlier generations. Newer, more powerful devices can deliver nicotine more efficiently. It is still unclear what role dependence symptoms such as craving, tolerance and withdrawal symptoms play in e-cigarette use and how they develop.
This research hence comprises three work packages with the aim of investigating the addictive potential of e-cigarettes from three different perspectives, combining neurobiological, sociological, and epidemiological research methods and levels of observation. In particular, craving is examined as a correlate for reward potential and tolerance development as a correlate for punishment potential. By combining these complementary methodological approaches, the overall project aims to cover all relevant sub-constructs of the addictive potential of e-cigarettes.
Work package I: neurobiological study part
Registered at clinicaltrials.gov/ct2/show/NCT04772014.
In this work package (WP), the focus is on the investigation of positive reinforcement mechanisms of e-cigarettes utilizing neurobiological and neuropsychological methods. One of the most discussed theories in this context is the incentive sensitization theory by Berridge and Robinson: Accordingly, mesolimbic sensitization occurs with repeated drug use, leading to a realignment of the reward and motivation system, resulting in the attribution of incentive salience of drug-associated stimuli, making them attractive and 'wanted' [ 52 , 53 , 54 ]. Thus, the substance and its stimuli are attributed a high reward value, which can be measured in terms of effort, time, money, or other goods one is willing to spend to acquire it. Some experimental studies on tobacco smokers [ 50 , 55 ] and experienced dual users [ 56 ] show that tobacco cigarettes have a higher reward value than e-cigarettes. However, most participants smoked more frequently and for a much longer period of time, which is why the reward value for tobacco cigarettes could be more established. Thus, the reward value of cigarettes itself was found to differ between dependent and occasional smokers. Occasional smokers exert more physical effort to obtain money and showed increased reactivity of the mesocorticolimbic system (including ventral striatum) to stimuli that predicted a money reward compared with a cigarette reward. Dependent smokers, in contrast, exerted similar physical effort and showed equivalent anticipatory activity for both reward types [ 57 ]. Measuring brain activity in a heterogeneous e-cigarette consumer group during reward announcement and acquisition for tobacco cigarettes and e-cigarettes could provide additional information about their reward value.
In addition, numerous meta-analyses in tobacco smokers show that smoking-related cues elicit significantly greater craving in smokers than neutral cue stimuli. This is associated with distinctive neural activation patterns in, e.g., the striatum, amygdala, orbitofrontal cortex, anterior cingulate cortex, medial prefrontal cortex and insula [ 58 , 59 , 60 , 61 ]. There are few studies on cue reactivity with e-cigarettes and they deliver conflicting results. In a study with merely seven participants, Nichols and colleagues failed to detect e-cigarette cue-related activity in brain areas associated with cue reactivity; but in regions related to episodic memory retrieval and motor control [ 62 ]. Another study by Wall and colleagues examined 10 subjects using e-cigarettes during functional magnetic resonance imaging (fMRI) to visualize brain activity associated with active vaping. Activation clusters were seen in cortical regions, (e.g., the insula, amygdala, and the anterior cingulate gyrus) as well as in sub-cortical regions (e.g., in the thalamus and putamen). Relative deactivations associated with vaping were detected in parts of the ventral striatum [ 63 ]. One possible explanation may be the transition from goal‐directed to habitual behavior in e-cigarette use that has been associated with a dysfunction of fronto-striatal circuits and a shift from ventral to dorsal striatal responses [ 64 ].
Furthermore, cognitive processes play an essential role in reactivity to drug cues. Through mesolimbic sensitization drug stimuli automatically and involuntary become the focus of attention. Attentional bias has been consistently found in various substance use disorders [ 65 ] and also in smokers [ 66 , 67 , 68 , 69 ]. Studies have shown that smokers have an initial orientation to smoking-related cues [ 70 ] and maintain their gaze on smoking-related images longer than on control images [ 67 , 71 ]. In fact, current tobacco smokers also have a longer dwell time on e-cigarette cues compared to neutral cues, which was associated with greater baseline craving [ 72 ]. However, to our best knowledge, there is no study investigating attentional bias for tobacco cigarette and e-cigarette stimuli in e-cigarette users.
In sum, the neuropsychological and neurobiological mechanisms of cigarette dependence have been relatively well studied. The extent to which this can be applied to e-cigarettes has not yet been adequately elucidated. Therefore, the neurobiological study will investigate the key elements of addiction in e-cigarettes compared to tobacco cigarettes using neurobiological and neuropsychological correlates associated with craving, incentive motivation, cue reactivity and attentional bias. We hypothesize that (1) participants who mainly use e-cigarettes work harder for e-cigarettes and show increased activation in the ventral striatum in the anticipation phase for e-cigarettes compared to tobacco cigarettes; (2) e-cigarette users show increased cue reactivity (compared to nicotine naïve users) in response to e-cigarette stimuli compared to neutral stimuli. Moreover, dual users will show activations in the same neural networks for tobacco cigarette and e-cigarette stimuli; (3) e-cigarette users (compared to nicotine naïve and dual users) show an increased attentional bias towards e-cigarette cues, which correlates positively with e-cigarette use. Dual users' attentional bias towards smoking cues, on the other hand, correlates positively with tobacco cigarette use.
Study sample
We intend to include 70 e-cigarette users (daily e-cigarette use, additional smoking of tobacco cigarettes is not an exclusion criterion) and 30 nicotine naïves (lifetime consumption of less than 20 e-cigarettes or tobacco cigarettes) aged 18–65 years. Exclusion criteria for both groups are contraindications for an MRI examination, severe internal, neurological, and psychiatric comorbidities, pharmacotherapy with psychoactive substances within the past 14 days, current substance abuse (THC, amphetamine, opiates, benzodiazepines, barbiturates, and cocaine) and axis I disorders according to ICD-10 and DSM-5 (except tobacco use disorder and specific phobias).
Power calculation
Sample size was estimated with an assumed effect size of ρ = 0.3 using G*Power software tool version 3 [ 73 ]. In this case, n = 64 smokers would be sufficient to detect a correlative relationship at p < 0.05 with a power of 80%. Since dropouts due to artifacts or lack of compliance are to be expected, a total of 70 subjects will be examined. The number of cases in the healthy control group (n = 30) is also sufficient to detect group differences between smokers and nicotine naïve subjects (effect size d = 0.6; 80% power, p < 0.05).
Study design
Inclusion and exclusion criteria are checked in advance in a telephone interview. The subjects are comprehensively informed about the objectives and the procedure of the planned examinations. On the examination day, smoking status is checked by measuring carbon monoxide levels in exhaled air and by taking a saliva sample to determine cotinine levels. Sociodemographic data are collected. Drug urine screening is performed, as well as a pregnancy test for women. This is followed by diagnostic interviews and the recording of smoking and vaping behavior, the severity of dependence symptoms, craving, and expected consequences for the use of tobacco cigarettes and e-cigarettes, as well as withdrawal symptoms using standardized questionnaires. Psychiatric and neurological, as well as somatic pre-existing conditions are recorded, as is the subject's current medication. In a neuropsychological assessment, subjects complete an Implicit Association Task, Delay Discounting Task, and Iowa Gambling Task. The fMRI examination is performed with a 3 T whole-body tomograph (Siemens Healthineers, Erlangen, Germany) including resting-state, MPRAGE and three different tasks: (1) To measure the reward value of e-cigarettes, we use an instrumental motivation task (adapted from [ 57 ]). Thereby, we examine brain activity to reward-predicting stimuli (cigarette, liquid, and money) and the subsequent instrumental response to obtain the reward. Physical effort (pressing a button) is thus used as a measure of motivation. (2) In a cue reactivity paradigm (adapted from [ 74 ]) participants’ physiological and neural responses, as well as self-reported craving, are examined while viewing images of e-cigarettes and tobacco cigarettes compared to neutral pictures. (3) Attentional bias for e-cigarette and tobacco cigarette cues and its neural correlates are tested using a visual dot-probe task (adapted from [ 69 ]). One problem is that the reaction time-based index only provides a 'snapshot' of attention, which can be overcome by directly measuring participants' eye movements during the task. To improve reliability, this task is, therefore, combined with eye tracking (see also [ 75 , 76 ]). For a comprehensive list of questionnaires and tasks used, please see Table 1 . For a graphical representation of the paradigms used during fMRI, please see Fig. 1 .
Experimental tasks used during fMRI. Note. ( 1 ) Instrumental motivation task (adapted from [ 57 ]); ( 2 ) Cue-reactivity paradigm (adapted from [ 74 ]); ( 3 ) visual dot-probe task (adapted from [ 69 ])
Work package II: sociological study part
Registered at OSF Registries: https://osf.io/dxgya .
In this part of the study program, the question is whether the reward potential (e.g., craving) and the punishment potential (e.g., tolerance development) are actually subjectively perceived by e-cigarette users. Therefore, the aim of the sociological study part (WP II) is to investigate whether aspects of addiction defined in the current DSM-5 are also reported by the users themselves. To this end, two different qualitative approaches will be combined.
Focus group interviews
One approach within the sociological study part (WP II) is to conduct four focus group interviews, each with 9–10 e-cigarette users. The planned focus group interviews aim to capture potential experiences of craving and tolerance development. An open-ended guide will be developed for conducting the focus group interviews. Inclusion criteria for participants will be: (1) age ≥ 18 years, (2) sufficient understanding of the German language, and (3) daily e-cigarette use. Dual use of tobacco and e-cigarettes will be set as a specific exclusion criterion for participation in this study part to exclude dependence symptoms resulting from the tobacco cigarette use. The composition of the focus groups will be as heterogeneous as possible [ 91 ] in order to generate a wide variety of opinions and to discuss as many different experiences as possible.
Prior to the focus group interview, each participant will be personally informed about the objectives of the study and data protection procedures. A written consent will be obtained from each participant. After completion of each focus group (max. 2 h), all participants will receive information on the current state of research on health risks and dependence potential of e-cigarettes. In addition, participants will receive an expense allowance of 50€ and the opportunity to receive information about the study results at a later date. All focus group discussions will be audiotaped, transcribed verbatim, and analyzed using qualitative content analysis [ 92 ].
Online forums
In addition to the focus group interviews, an analysis of posts in online discussion forums on e-cigarettes will be conducted. Online forums represent anonymous places of exchange for users [ 93 ] and an opportunity to share ideas about problematic or taboo topics without fear of stigmatization [ 94 ]. We hypothesize that the internet, and in particular anonymous online forums, are among the few places where e-cigarette users report possible dependence symptoms without feeling shame. Therefore, they provide a venue to gain health- and dependence-related experiences without the social-desirability bias [ 95 ]. Examining posts in online forums complements focus group interviews in an innovative and useful way, as shame-related experiences may be reported more detailed than in focus group interviews.
A three-step procedure will be used to collect the data for this study part. In the first step, the relevant online forums will be identified via Google search using different combinations with various spellings of the German words “e-cigarette” and “(online) forum”. Following inclusion criteria will be applied to select relevant online forums: (1) e-cigarettes as the main topic of the forum, (2) the forum is in German language; (3) the forum is publicly accessible (i.e., no registration is required to read the users contributions, (4) the forum was active over the previous 4 weeks, (5) at least 500,000 posts, (6) at least 5000 forum members, (7) search function within the forum, (8) no affiliation with tobacco industry, (9) public disclaimer in terms and conditions. In the second step, the identified forums will be searched for previously defined keywords describing dependence criteria derived from current DSM-5 covering the reward potential (e.g., craving) and the punishment potential (e.g., tolerance development) as accurate as possible. In the third step, the identified user contributions will be analyzed using qualitative content analysis in regard to the reported dependence symptoms.
As suggested in a previous discussion about ethics of using of online data [ 96 ], formal ethical clearance is not necessary for analyses of such kind of posts in online discussion forums. We will use data that is publicly accessible at the time of data collection, so that forum members can be assumed to be aware of the public availability of their posts. Nicknames of users will not be included in data analyses, and no further information on individuals is available in the forums. The team members will not participate actively in any discussions in the forums.
Work package III: epidemiological study part
The aim of WP III is to quantify dependence symptoms, in particular the development of tolerance, in e-cigarette users and to investigate associations with user and product factors within the framework of a secondary data analysis of a representative large-scale longitudinal study of tobacco and e-cigarette use. In particular, we will investigate how dependence symptoms develop over time. For this purpose, transitions to e-cigarette use or from tobacco cigarette use will be investigated. Associations of such transitions with individual factors (e.g., age, gender, socioeconomic factors) and attitudes and perceptions (e.g., perceived dependence and harm potential of tobacco and e-cigarettes, perceived societal norms regarding tobacco and e-cigarette use) will be studied. In addition, because most e-cigarette users were previously long-time smokers of conventional tobacco cigarettes or continued to smoke tobacco cigarettes, the comparison of the perceived addictive potential of both products is of interest.
WP III mainly includes a secondary data analysis of already collected and available longitudinal data from the International Tobacco Control Policy Evaluation Project (ITC)—a multinational consortium comprising longitudinal surveys on representative samples of smokers using largely standardized survey instruments and methods [ 97 ]. Conceptually, the survey instruments and models of the ITC cigarette project are based on psychosocial behavioral theories [ 98 ]. For the planned analyses, two survey waves are used, which were collected in six European countries (Germany, Greece, Poland, Romania, Spain, Hungary) within the EU-funded project EUREST-PLUS [ 99 ]. The baseline survey took place from June to September 2016 and included approximately 1000 smokers per country (total: N = 6011). Participants were recruited using multistage cluster sampling, geographically stratified by Nomenclature of Territorial Units for Statistics-Region (NUTS) region. A random walk procedure was used to randomly select addresses of households from 100 clusters in each country. Households were eligible if at least one smoker (> 100 cigarettes smoked in lifetime and at least monthly cigarette consumption) lived there. A maximum of one male and one female smoker from each selected household were randomly selected for a computer-assisted interview [ 100 ]. The second wave of the survey took place between February and May 2018, during which participants from the first wave of the survey were interviewed a second time. Overall, 54% of participants from the first wave participated a second time. For participants who refused to participate a second time or could not be reached (so-called panel mortality), replacement participants were recruited analogous to the initial sample selection to enable cross-sectional analyses in addition to longitudinal analyses [ 101 ]. This database is supplemented by two further surveys of the ITC cigarette Europe project (the Netherlands: approx. N = 2000 smokers, and England: approx. N = 4300 smokers, former smokers, and vapers), which were not collected within the scope of the EUREST-PLUS project, but which have good comparability given the use of the ITC cigarette sampling design and data collection methods across all involved countries [ 101 ]. All study participants provided informed consent and all study procedures and material were approved by the ethics research committee at the University of Waterloo (Ontario, Canada), and local ethics committees in all countries.
Of relevance for this work package are dependence symptoms, which were recorded with identical question wording at both survey time points and separately for tobacco cigarettes and e-cigarettes, depending on which products are being used. Pertinent measures include, in particular, self-reports (e.g., time of first use of cigarettes or e-cigarettes after getting up in the morning, failed attempts at abstinence, need for daily functioning) and self-assessments (e.g., of the degree of dependence). Furthermore, measures of perceived addictiveness of products are available. Tolerance development can be mapped longitudinally via detailed recording of dose for both tobacco cigarettes and e-cigarettes.
Measures of interest and associations with individual and product factors will be studied cross-sectionally using regression models. To examine the course of dependence symptoms over time based on longitudinal data, generalized linear models are the method of choice to account for intra-individual correlation. In order to investigate to what extent trajectories or transitions are influenced by individual and product characteristics, these are introduced into the models as influencing factors (i.e., modeled as interaction terms with the time factor).
The addictive potential of tobacco cigarettes is undisputed. E-cigarettes are very similar to tobacco cigarettes in their nicotine delivery and smoking behavior: Hand-to-mouth movement, tactile action of puffing, inhalation and exhalation, sensory stimulation in the airways, nicotine uptake via the pulmonary route, vapor production and social aspects such as smoking breaks. Therefore, e-cigarettes could produce the same pharmacological, psychological, behavioral, and social effects that can promote or maintain nicotine dependence. However, there are few studies on the addictive potential of e-cigarettes containing nicotine—with conflicting results. With the present project, we aim to close this gap by investigating the addictive potential of e-cigarettes from three perspectives, combining different research methods and levels of observation:
The neurobiological study part focuses on the positive reinforcement mechanisms of e-cigarettes using neurological and neuropsychological research methods. On the neurobiological level, the reward value of e-cigarettes and craving will be investigated in an experimental approach through presentation of conditioned stimuli and measurement of motivational and attentional processes. The aim is to test the assumption that chronic use of e-cigarettes leads to similar conditioning processes and motivational aspects as with traditional tobacco cigarettes.
The sociological study part uses a qualitative approach to investigate the extent to which e-cigarette users actually experience and report craving and tolerance development. This involves a qualitative description of the typical experience and perception of dependence symptoms in a social context. The self-reports of users cover psychological, physiological, and behavioral aspects of dependence disorders.
The epidemiological study part examines the factors influencing the potential for dependence and the development of dependence symptoms in a longitudinal study. The focus is on the development of tolerance and the role played by psychosocial and product factors with regard to transitions into and out of e-cigarette use. Using readily available quantitative longitudinal data, the development of tolerance among e-cigarette users will be quantified and associations with user and product factors examined.
During the conduct of this project, we will have to deal with certain limitations. Most e-cigarette users are former or current smokers. Here, the dependence symptoms or the dependent behavior could reflect the transfer of nicotine dependence from the previous use of combustible tobacco. Thus, it cannot be clarified whether the use of e-cigarettes alone actually leads to the development of addiction. Ideally, an evaluation of the addictive potential would be done with a group of individuals using nicotine-containing e-cigarettes but who have never used tobacco products. However, the prevalence of exclusive e-cigarette users who have never smoked cigarettes in their life is very low. Furthermore, previous or concurrent cigarette use also plays a role in the examination of reward effects such as craving and incentive motivation. Thus, most users have a longer smoking history and may have a greater sensitivity to tobacco cigarette and smoking stimuli. Therefore, we collect extensive smoking variables (age of smoking initiation, duration of smoking, exposure to tobacco cigarettes measured by pack years, severity of dependence on tobacco cigarette) to statistically account for these potential confounders. From a methodological point of view, the heterogeneous product group of e-cigarettes and different liquids and nicotine concentrations must also be taken into account. This can make it difficult to analyze and compare the data collected. Therefore, extensive information about e-cigarette consumption is collected (e-cigarette device, coil model, nicotine concentration, flavor etc.). Nevertheless, e-cigarettes differ not only in their characteristics, nicotine delivery and consumption patterns, but also in their design, which makes it difficult to select suitable stimuli. This is problematic given that personalized, familiar stimuli can best trigger craving and attention biases [ 75 ].
The strength of the present study is the chosen mix of three methodological approaches, whereby core symptoms of positive and negative reinforcement in addiction are investigated comprehensively. The reward effects of e-cigarette and craving can be experimentally validated at the individual level, while longitudinal designs are the method of choice for measuring tolerance development as a correlate of the punishment potential. The simultaneous individual occurrence of both phenomena can furthermore be investigated through qualitative analysis of self-reports.
Whether e-cigarettes are as reinforcing and addictive as combustible tobacco cigarettes is an important public health question with implications for prevention and treatment programs. In particular, the development of tolerance towards e-cigarettes is relevant from a public health perspective, as health risks are usually higher with increasing consumption. In contrast, a systematic assessment of the reward value of e-cigarettes, especially in comparison to tobacco cigarettes, plays an important role for therapy offers. The results will provide important insights into the motivational properties of e-cigarettes and could expand our understanding of whether and to what extent e-cigarettes can be used in smoking cessation treatments.
Availability of data and materials
Not applicable.
Abbreviations
Functional magnetic resonance imaging
International tobacco control policy evaluation project
Nicotine replacement therapy
Work package
World Health Organization. WHO report on the global tobacco epidemic 2019: offer help to quit tobacco use. Geneva: World Health Organization; 2019.
Google Scholar
Goniewicz ML, Knysak J, Gawron M, Kosmider L, Sobczak A, Kurek J, Prokopowicz A, Jablonska-Czapla M, Rosik-Dulewska C, Havel C, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014;23(2):133–9.
PubMed Google Scholar
Hajek P, Etter JF, Benowitz N, Eissenberg T, McRobbie H. Electronic cigarettes: review of use, content, safety, effects on smokers and potential for harm and benefit. Addiction (Abingdon, England). 2014;109(11):1801–10.
Levy DT, Borland R, Lindblom EN, Goniewicz ML, Meza R, Holford TR, Yuan Z, Luo Y, O’Connor RJ, Niaura R, et al. Potential deaths averted in USA by replacing cigarettes with e-cigarettes. Tob Control Int J. 2018;27(1):18–25.
McNeill A, Brose LS, Calder R, Hitchman SC, Hajek P, McRobbie H. E-cigarettes: the need for clear communication on relative risks. Lancet. 2015;386(10000):1237.
National Academies of Sciences, Engineering, and Medicine. Public health consequences of e-cigarettes. Washington: The National Academies Press; 2018.
Hartmann-Boyce J, McRobbie H, Lindson N, Bullen C, Begh R, Theodoulou A, Notley C, Rigotti NA, Turner T, Butler AR, et al. Electronic cigarettes for smoking cessation. Cochrane Database Syst Rev. 2021;4(4):Cd010216.
Hiemstra PS, Bals R. Basic science of electronic cigarettes: assessment in cell culture and in vivo models. Respir Res. 2016;17(1):127.
PubMed PubMed Central Google Scholar
Pisinger C, Døssing M. A systematic review of health effects of electronic cigarettes. Prev Med. 2014;69:248–60.
Barrington-Trimis JL, Urman R, Berhane K, Unger JB, Cruz TB, Pentz MA, Samet JM, Leventhal AM, McConnell R. E-cigarettes and future cigarette use. Pediatrics. 2016;138(1):e20160379.
Loukas A, Marti CN, Cooper M, Pasch KE, Perry CL. Exclusive e-cigarette use predicts cigarette initiation among college students. Addict Behav. 2018;76:343–7.
Morgenstern M, Nies A, Goecke M, Hanewinkel R. E-cigarettes and the use of conventional cigarettes. Deutsch Ärzteblatt Int. 2018;115(14):243–8.
Pierce JP, Chen R, Leas EC, White MM, Kealey S, Stone MD, Benmarhnia T, Trinidad DR, Strong DR, Messer K. Use of E-cigarettes and other tobacco products and progression to daily cigarette smoking. Pediatrics. 2021;147(2):e2020025122.
Schneider S, Diehl K. Vaping as a catalyst for smoking? An initial model on the initiation of electronic cigarette use and the transition to tobacco smoking among adolescents. Nicotine Tob Res Off J Soc Res Nicotine Tob. 2016;18(5):647–53.
Chan GCK, Stjepanović D, Lim C, Sun T, Shanmuga Anandan A, Connor JP, Gartner C, Hall WD, Leung J. Gateway or common liability? A systematic review and meta-analysis of studies of adolescent e-cigarette use and future smoking initiation. Addiction (Abingdon, England). 2021;116(4):743–56.
Hajek P, Phillips-Waller A, Przulj D, Pesola F, Myers Smith K, Bisal N, Li J, Parrott S, Sasieni P, Dawkins L, et al. A randomized trial of e-cigarettes versus nicotine-replacement therapy. N Engl J Med. 2019;380(7):629–37.
Foulds J, Veldheer S, Yingst J, Hrabovsky S, Wilson SJ, Nichols TT, Eissenberg T. Development of a questionnaire for assessing dependence on electronic cigarettes among a large sample of ex-smoking E-cigarette users. Nicotine Tob Res Off J Soc Res Nicotine Tob. 2015;17(2):186–92.
Etter JF. A longitudinal study of cotinine in long-term daily users of e-cigarettes. Drug Alcohol Depend. 2016;160:218–21.
Nutt D, King LA, Saulsbury W, Blakemore C. Development of a rational scale to assess the harm of drugs of potential misuse. Lancet. 2007;369(9566):1047–53.
Salerian A. Addictive potency of substances. Pharm Pharmacol Int J. 2015. https://doi.org/10.15406/ppij.2015.02.00030 .
Article Google Scholar
Koob GF. Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology. 2009;56(Suppl 1):18–31.
Benowitz NL, Hukkanen J, Jacob P 3rd. Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol. 2009;192:29–60.
Hukkanen J, Jacob P, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57(1):79–115.
Fowler JS, Logan J, Wang GJ, Volkow ND. Monoamine oxidase and cigarette smoking. Neurotoxicology. 2003;24(1):75–82.
Guillem K, Vouillac C, Azar MR, Parsons LH, Koob GF, Cador M, Stinus L. Monoamine oxidase inhibition dramatically increases the motivation to self-administer nicotine in rats. J Neurosci Off J Soc Neurosci. 2005;25(38):8593–600.
Lewis A, Miller JH, Lea RA. Monoamine oxidase and tobacco dependence. Neurotoxicology. 2007;28:182–95.
Stevenson T, Proctor RN. The secret and soul of Marlboro: Phillip Morris and the origins, spread, and denial of nicotine freebasing. Am J Public Health. 2008;98(7):1184–94.
Henningfield JE, Keenan RM. Nicotine delivery kinetics and abuse liability. J Consult Clin Psychol. 1993;61(5):743–50.
West R, Hajek P, Foulds J, Nilsson F, May S, Meadows A. A comparison of the abuse liability and dependence potential of nicotine patch, gum, spray and inhaler. Psychopharmacology. 2000;149(3):198–202.
Jacobson K, Martinez J, Larroque S, Jones IW, Paschke T. Nicotine pharmacokinetics of electronic cigarettes: a pooled data analysis from the literature. Toxicol Rep. 2021;8:84–95.
Breland A, Soule E, Lopez A, Ramôa C, El-Hellani A, Eissenberg T. Electronic cigarettes: What are they and what do they do? Ann N Y Acad Sci. 2017;1394(1):5–30.
Rose JE. Nicotine and nonnicotine factors in cigarette addiction. Psychopharmacology. 2006;184(3):274–85.
Buchhalter AR, Acosta MC, Evans SE, Breland AB, Eissenberg T. Tobacco abstinence symptom suppression: the role played by the smoking-related stimuli that are delivered by denicotinized cigarettes. Addiction (Abingdon, England). 2005;100(4):550–9.
Tate JC, Pomerleau CS, Pomerleau OF. Pharmacological and non-pharmacological smoking motives: a replication and extension. Addiction. 1994;89(3):321–30.
Barrett SP. The effects of nicotine, denicotinized tobacco, and nicotine-containing tobacco on cigarette craving, withdrawal, and self-administration in male and female smokers. Behav Pharmacol. 2010;21(2):144–52.
Naqvi NH, Bechara A. The airway sensory impact of nicotine contributes to the conditioned reinforcing effects of individual puffs from cigarettes. Pharmacol Biochem Behav. 2005;81(4):821–9.
Rose JE, Behm FM, Westman EC, Johnson M. Dissociating nicotine and nonnicotine components of cigarette smoking. Pharmacol Biochem Behav. 2000;67(1):71–81.
Niaura R, Shadel WG, Abrams DB, Monti PM, Rohsenow DJ, Sirota A. Individual differences in cue reactivity among smokers trying to quit: effects of gender and cue type. Addict Behav. 1998;23(2):209–24.
Shaham Y, Shalev U, Lu L, de Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168(1):3–20.
Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217–38.
Etter JF, Bullen C. Electronic cigarette: users profile, utilization, satisfaction and perceived efficacy. Addiction (Abingdon, England). 2011;106(11):2017–28.
Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3(8):760–73.
Liu G, Wasserman E, Kong L, Foulds J. A comparison of nicotine dependence among exclusive e-cigarette and cigarette users in the PATH study. Prev Med. 2017;104:86–91.
Rostron BL, Schroeder MJ, Ambrose BK. Dependence symptoms and cessation intentions among US adult daily cigarette, cigar, and e-cigarette users, 2012–2013. BMC Public Health. 2016;16(1):814.
Strong DR, Pearson J, Ehlke S, Kirchner T, Abrams D, Taylor K, Compton WM, Conway KP, Lambert E, Green VR, et al. indicators of dependence for different types of tobacco product users: descriptive findings from wave 1 (2013–2014) of the population assessment of tobacco and health (PATH) study. Drug Alcohol Depend. 2017;178:257–66.
Etter JF, Eissenberg T. Dependence levels in users of electronic cigarettes, nicotine gums and tobacco cigarettes. Drug Alcohol Depend. 2015;147:68–75.
Farsalinos KE, Romagna G, Tsiapras D, Kyrzopoulos S, Voudris V. Evaluating nicotine levels selection and patterns of electronic cigarette use in a group of “vapers” who had achieved complete substitution of smoking. Subst Abuse. 2013;7:139–46.
Steinberg MB, Zimmermann MH, Delnevo CD, Lewis MJ, Shukla P, Coups EJ, Foulds J. E-cigarette versus nicotine inhaler: comparing the perceptions and experiences of inhaled nicotine devices. J Gen Int Med. 2014;29(11):1444–50.
Stiles MF, Campbell LR, Graff DW, Jones BA, Fant RV, Henningfield JE. Pharmacodynamic and pharmacokinetic assessment of electronic cigarettes, combustible cigarettes, and nicotine gum: implications for abuse liability. Psychopharmacology. 2017;234(17):2643–55.
Vansickel AR, Weaver MF, Eissenberg T. Clinical laboratory assessment of the abuse liability of an electronic cigarette. Addiction. 2012;107(8):1493–500.
Pericot-Valverde I, Yoon JH, Gaalema DE. Single- and cross-commodity delay discounting of money and e-cigarette liquid in experienced e-cigarette users. Drug Alcohol Depend. 2020;206:107740.
Berridge KC, Robinson TE. Liking, wanting, and the incentive-sensitization theory of addiction. Am Psychol. 2016;71(8):670–9.
Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8(11):1481–9.
Robinson TE, Berridge KC. Review. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond Ser B Biol Sci. 2008;363(1507):3137–46.
McPherson S, Howell D, Lewis J, Barbosa-Leiker C, Bertotti Metoyer P, Roll J. Self-reported smoking effects and comparative value between cigarettes and high dose e-cigarettes in nicotine-dependent cigarette smokers. Behav Pharmacol. 2016;27(2 and 3—Special Issue):301–7.
Dowd AN, Tiffany ST. Comparison of tobacco and electronic cigarette reward value measured during a cue-reactivity task: an extension of the choice behavior under cued conditions procedure. Nicotine Tob Res Off J Soc Res Nicotine Tob. 2019;21(10):1394–400.
Bühler M, Vollstädt-Klein S, Kobiella A, Budde H, Reed LJ, Braus DF, Büchel C, Smolka MN. Nicotine dependence is characterized by disordered reward processing in a network driving motivation. Biol Psychiatry. 2010;67(8):745–52.
Chase HW, Eickhoff SB, Laird AR, Hogarth L. The neural basis of drug stimulus processing and craving: an activation likelihood estimation meta-analysis. Biol Psychiatry. 2011;70(8):785–93.
Engelmann JM, Versace F, Robinson JD, Minnix JA, Lam CY, Cui Y, Brown VL, Cinciripini PM. Neural substrates of smoking cue reactivity: a meta-analysis of fMRI studies. Neuroimage. 2012;60(1):252–62.
Kühn S, Gallinat J. Common biology of craving across legal and illegal drugs—a quantitative meta-analysis of cue-reactivity brain response. Eur J Neurosci. 2011;33(7):1318–26.
Lin X, Deng J, Shi L, Wang Q, Li P, Li H, Liu J, Que J, Chang S, Bao Y, et al. Neural substrates of smoking and reward cue reactivity in smokers: a meta-analysis of fMRI studies. Transl Psychiatry. 2020;10(1):97.
Nichols TT, Foulds J, Yingst JM, Veldheer S, Hrabovsky S, Richie J, Eissenberg T, Wilson SJ. Cue-reactivity in experienced electronic cigarette users: novel stimulus videos and a pilot fMRI study. Brain Res Bull. 2016;123:23–32.
Wall MB, Mentink A, Lyons G, Kowalczyk OS, Demetriou L, Newbould RD. Investigating the neural correlates of smoking: feasibility and results of combining electronic cigarettes with fMRI. Sci Rep. 2017;7(1):11352.
Everitt BJ. Neural and psychological mechanisms underlying compulsive drug seeking habits and drug memories–indications for novel treatments of addiction. Eur J Neurosci. 2014;40(1):2163–82.
Field M, Cox WM. Attentional bias in addictive behaviors: a review of its development, causes, and consequences. Drug Alcohol Depend. 2008;97(1–2):1–20.
Kwak SM, Na DL, Kim G, Kim GS, Lee JH. Use of eye movement to measure smokers’ attentional bias to smoking-related cues. Cyberpsychol Behav Impact Internet Multimed Virtual Real Behav Soc. 2007;10(2):299–304.
Mogg K, Bradley BP, Field M, De Houwer J. Eye movements to smoking-related pictures in smokers: relationship between attentional biases and implicit and explicit measures of stimulus valence. Addiction. 2003;98(6):825–36.
Mogg K, Field M, Bradley BP. Attentional and approach biases for smoking cues in smokers: an investigation of competing theoretical views of addiction. Psychopharmacology. 2005;180(2):333–41.
Vollstädt-Klein S, Loeber S, Winter S, Leménager T, von der Goltz C, Dinter C, Koopmann A, Wied C, Winterer G, Kiefer F. Attention shift towards smoking cues relates to severity of dependence, smoking behavior and breath carbon monoxide. Eur Addict Res. 2011;17(4):217–24.
Bradley B, Field M, Mogg K, De Houwer J. Attentional and evaluative biases for smoking cues in nicotine dependence: component processes of biases in visual orienting. Behav Pharmacol. 2004;15(1):29–36.
Bradley BP, Mogg K, Wright T, Field M. Attentional bias in drug dependence: vigilance for cigarette-related cues in smokers. Psychol Addict Behav. 2003;17(1):66–72.
Lochbuehler K, Wileyto EP, Tang KZ, Mercincavage M, Cappella JN, Strasser AA. Do current and former cigarette smokers have an attentional bias for e-cigarette cues? J Psychopharmacol (Oxf Engl). 2018;32(3):316–23.
Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–91.
Vollstädt-Klein S, Loeber S, Richter A, Kirsch M, Bach P, von der Goltz C, Hermann D, Mann K, Kiefer F. Validating incentive salience with functional magnetic resonance imaging: association between mesolimbic cue reactivity and attentional bias in alcohol-dependent patients. Addict Biol. 2012;17(4):807–16.
Christiansen P, Mansfield R, Duckworth J, Field M, Jones A. Internal reliability of the alcohol-related visual probe task is increased by utilising personalised stimuli and eye-tracking. Drug Alcohol Depend. 2015;155:170–4.
Schmukle SC. Unreliability of the dot probe task. Eur J Personal. 2005;19(7):595–605.
Beesdo-Baum K, Zaudig M, Wittchen H-U, editors. SCID-5-CV: strukturiertes klinisches Interview für DSM-5-Störungen—Klinische Version: deutsche Bearbeitung des structured clinical interview for DSM-5 disorders—clinician version von Michael B. First, Janet B.W. Williams, Rhonda S. Karg, Robert L. Spitzer, 1, Auflage edn. Göttingen: Hogrefe; 2019.
Scheurich A, Müller MJ, Anghelescu I, Lörch B, Dreher M, Hautzinger M, Szegedi A. Reliability and validity of the form 90 interview. Eur Addict Res. 2005;11(1):50–6.
Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom K-O. The Fagerström test for nicotine dependence: a revision of the Fagerstrom tolerance questionnaire. Br J Addict. 1991;86:9S.
Vollstädt-Klein S, Leménager T, Jorde A, Kiefer F, Nakovics H. Development and validation of the craving automated scale for alcohol. Alcohol Clin Exp Res. 2015;39(2):333–42.
Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3:7–16.
Dowd AN, Motschman CA, Tiffany ST. Development and validation of the questionnaire of vaping craving. Nicotine Tob Res. 2019;21:63–70.
Rash C, Copeland A. The brief smoking consequences questionnaire-adult (BSCQ-A): development of a short form of the SCQ-A. Nicotine Tob Res. 2008;10:1633–43.
Hughes J. Effects of abstinence from tobacco: valid symptoms and time course. Nicotine Tob Res. 2007;9:315–27.
Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–96.
Crawford JR, Henry JD. The positive and negative affect schedule (PANAS): construct validity, measurement properties and normative data in a large non-clinical sample. Br J Clin Psychol. 2004;43:21S.
Preuss UW, Rujescu D, Giegling I, Watzke S, Koller G, Zetzsche T, Meisenzahl EM, Soyka M, Möller HJ. Psychometrische evaluation der deutschsprachigen version der Barratt-Impulsiveness-Skala psychometric evalutation of the German version of the Barratt Impulsiveness Scale. Nervenarzt. 2008;79:305–19.
Greenwald AG, McGhee DE, Schwartz JLK. Measuring individual differences in implicit cognition: the implicit association test. J Personal Soc Psychol. 1998;74:1464–80.
Kirby KN, Maraković NN. Delay-discounting probabilistic rewards: rates decrease as amounts increase. Psychon Bull Rev. 1996;3(1):100–4.
Bechara A, Tranel D, Damasio H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain (London, England: 1878). 2000;123:2189–202.
Schulz M, Mack B, Renn O, editors. Fokusgruppen in der empirischen Sozialwissenschaft: Von der Konzeption bis zur Auswertung. Wiesbaden: VS Verl. für Sozialwiss; 2012.
Mayring P: Qualitative Inhaltsanalyse : Grundlagen und Techniken, 11., aktualisierte und überarb. Aufl. edn. Weinheim [u.a.]: Beltz; 2010.
Colloseus C. Grenzen erfahren—von Grenzen erzählen. Die Hebamme. 2016;29:33–8.
Karlheim C. Depressions-online-foren: Präventiver Nutzen oder ein Risiko für Betroffene und Angehörige? In: Hahn S, Harald S, Abderhalden C, Needham I, Schulz M, Hegedüs A, Schoppmann S, editors. Gesundheitsförderung und Gesundheitskompetenz": eine Herausforderung für die psychiatrische Pflege in Praxis, Management, Ausbildung Forschung: Vorträge, Workshops und Posterpräsentationen 9 Dreiländerkongress Pflege in der Psychiatrie in Wien. Bern: Abt. Forschung/Entwicklung Pflege und Pädagogik, Universitäre Psychiatrische Dienste Bern; 2012. p. 166–7.
Kahr MK, Padgett S, Shope CD, Griffin EN, Xie SS, Gonzalez PJ, Levison J, Mastrobattista J, Abramovici AR, Northrup TF, et al. A qualitative assessment of the perceived risks of electronic cigarette and hookah use in pregnancy. BMC Public Health. 2015;15:1273.
Roberts LD. Ethical issues in conducting qualitative research in online communities. Qual Res Psychol. 2015;12:314–25.
Thompson ME, Fong GT, Hammond D, Boudreau C, Driezen P, Hyland A, Borland R, Cummings KM, Hastings GB, Siahpush M, et al. Methods of the international tobacco control (ITC) four country survey. Tob Control. 2006;15(Suppl 3):iii12-18.
Fong GT, Cummings KM, Borland R, Hastings G, Hyland A, Giovino GA, Hammond D, Thompson ME. The conceptual framework of the international tobacco control (ITC) policy evaluation project. Tob Control. 2006;15(Suppl 3):iii3-11.
Vardavas CI, Bécuwe N, Demjén T, Fernández E, McNeill A, Mons U, Tountas Y, Trofor AC, Tsatsakis A, Rohde G, et al. Study protocol of European regulatory science on tobacco (EUREST-PLUS): policy implementation to reduce lung disease. Tob Induc Dis. 2018;16:A2.
Fong GT, Thompson ME, Boudreau C, Bécuwe N, Driezen P, Agar TK, Quah ACK, Zatoński WA, Przewoźniak K, Mons U, et al. The conceptual model and methods of wave 1 (2016) of the EUREST-PLUS ITC 6 European countries survey. Tob Induc Dis. 2018;16:A3.
Thompson ME, Driezen P, Boudreau C, Bécuwe N, Agar TK, Quah ACK, Zatoński W, Przewoźniak K, Mons U, Demjén T, et al. Methods of the international tobacco control (ITC) EUREST-PLUS ITC Europe surveys. Eur J Public Health. 2020;30(Suppl_3):4–9.
Download references
Acknowledgements
The authors would like to thank Falk Kiefer for providing the infrastructure and support to conduct the study and Ronald Fischer for technical support during the study set-up.
Open Access funding enabled and organized by Projekt DEAL. The project described in this study protocol is funded with a grant from the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation), Project-ID 437718741. It is partly supported by another grant from the DFG, Project-ID 402170461 (recipient SVK, Heinz et al., Addict Biol. 2019). UM is supported by the Marga and Walter Boll-Foundation. The sources of funding have no role in study design, data collection, data analysis, data interpretation, or writing of the manuscript.
Author information
Sabine Vollstädt-Klein, Nadja Grundinger, Ute Mons and Sven Schneider have contributed equally to this work
Authors and Affiliations
Department of Addictive Behavior and Addiction Medicine, Central Institute of Mental Health, Medical Faculty Mannheim, University of Heidelberg, PO Box 12 21 20, 68072, Mannheim, Germany
Sabine Vollstädt-Klein & Nadja Grundinger
Mannheim Center for Translational Neurosciences (MCTN), Medical Faculty of Mannheim, University of Heidelberg, Mannheim, Germany
Sabine Vollstädt-Klein
Mannheim Institute of Public Health, Social and Preventive Medicine (MIPH), Medical Faculty of Mannheim, University of Heidelberg, Mannheim, Germany
Tatiana Görig, Daria Szafran & Sven Schneider
Heart Center, Faculty of Medicine, University Hospital Cologne, University of Cologne, Cologne, Germany
Astrid Althaus & Ute Mons
You can also search for this author in PubMed Google Scholar
Contributions
SV, UM, and SS were responsible for the study design and procured study funding. SV, NG, UM, and SS drafted the manuscript with extensive input from TG, AA, DS. All authors made substantial contributions to the subsequent draft of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Sabine Vollstädt-Klein .
Ethics declarations
Ethics approval and consent to participate.
WP I was approved by the Ethics Committee II of the Medical Faculty Mannheim at the University of Heidelberg, Germany (Ethics approval number: 2018-595 N with amendment from 2021–01-13) and conforms to the requirements of the World Medical Association’s Declaration of Helsinki. All procedures of WP II were approved by the Ethics Committee II of the Medical Faculty Mannheim at the University of Heidelberg (Ethics approval number: 2017-567 N-MA with amendment from 2019–07-15). Study procedures and material used by WP III was approved by the ethics research committee at the University of Waterloo (Ontario, Canada), and local ethics committees in all countries where surveys took place. For WP I and II, written informed consent is obtained from all participants. For WP III, informed consent was obtained from other research institutions where the surveys took place.
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Vollstädt-Klein, S., Grundinger, N., Görig, T. et al. Study protocol: evaluation of the addictive potential of e-cigarettes (EVAPE): neurobiological, sociological, and epidemiological perspectives. BMC Psychol 9 , 181 (2021). https://doi.org/10.1186/s40359-021-00682-8
Download citation
Received : 29 September 2021
Accepted : 05 November 2021
Published : 18 November 2021
DOI : https://doi.org/10.1186/s40359-021-00682-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Electronic cigarettes
- Tobacco use disorder
- Focus groups
- Longitudinal data
BMC Psychology
ISSN: 2050-7283
- General enquiries: [email protected]
- Featured Paper of the Month
- Reviews to Read
- Hot off the Press
- Technology Development Initiative Paper of the Month
- Seminar Series
- Addiction Grand Rounds
- About NIDA IRP
- Directions and Map
- Careers at NIDA IRP
- Emergency Contacts
- Office of the Scientific Director
- Office of the Clinical Director
- Office of Education and Career Development
- Administrative Management Branch
- Molecular Targets and Medications Discovery Branch
- Cellular and Neurocomputational Systems Branch
- Molecular Neuropsychiatry Research Branch
- Neuroimaging Research Branch
- Behavioral Neuroscience Research Branch
- Integrative Neuroscience Research Branch
- Translational Addiction Medicine Branch
- Core Facilities
- Women Scientist Advisors
- Community Outreach Group
- OECD Awards
- Summer Internship Program
- Postbaccalaureate Program
- Graduate Partnership Program
- Postdoctoral Program
- Diversity, Equity, Inclusion and Access Initiatives (DEIA)
- NIDA Speakers Bureau
- Clinical Electives Program
- Study Volunteers
- Skip to primary navigation
- Skip to main content
- Skip to primary sidebar
National Institute on Drug Abuse - Intramural Research Program
GPR55 is expressed in glutamate neurons and functionally modulates drug taking and seeking in rats and mice

Study Authors Yi He and Hui Shen
Hot Off the Press – April 4, 2024
Published in Translational Psychiatry by Yi He , Hui Shen and Zheng-Xiong Xi , et al. from the NIDA IRP Addiction Biology Unit and Magnetic Resonance Imaging and Spectroscopy Section .
G protein-coupled receptor 55 (GPR55) is a putative cannabinoid receptor, which has been considered as the “CB3” receptor, as multiple cannabinoids such as Δ9-THC, CP55,940, HU-210, anandamide, and 2-AG, have high binding affinities to this receptor. However, its functional role in cannabis action and substance abuse has not been explored. Through a comprehensive array of neuroimaging techniques, including RNAscope ISH, IHC, and fluorescent ligand binding assays, we found that GPR55 is highly expressed in cortical and subcortical glutamate neurons, but not in midbrain DA neurons, in mice. Based on this cellular distribution in the brain, we then conducted a series of neurochemical, physiological, and behavioral assays. We found that GPR55 activation augments glutamate release in the nucleus accumbens without affecting extracellular dopamine levels. Systemic administration of O-1602, a potent GPR55 agonist, neither altered Δ9-THC-induced triad effects (analgesia, hypothermia, and catalepsy) nor altered optical brain-stimulation reward in DAT-cre mice. Unexpectedly, systemic administration of O-1602 dose-dependently inhibited cocaine-enhanced brain-stimulation reward and intravenous cocaine and nicotine self-administration under fixed-ratio and/or progressive-ratio reinforcement schedules in rats, wild-type mice, but not in GPR55-KO mice. These groundbreaking findings revealed a pivotal role of glutamatergic GPR55 mechanisms in the reward processes associated with cocaine and nicotine addiction, and therefore, GPR55 deserves further studies as a promising therapeutic target for treating substance use disorders.
Publication Information
He, Yi; Shen, Hui; Bi, Guo-Hua; Zhang, Hai-Ying; Soler-Cedeño, Omar; Alton, Hannah; Yang, Yihong; Xi, Zheng-Xiong
GPR55 is expressed in glutamate neurons and functionally modulates drug taking and seeking in rats and mice Journal Article
In: Transl Psychiatry, vol. 14, no. 1, pp. 101, 2024 , ISSN: 2158-3188 .
Abstract | Links
- https://pubmed.ncbi.nlm.nih.gov/38374108/
- doi:10.1038/s41398-024-02820-3

- National Institute on Drug Abuse
- NIH Intramural Research Program
- National Institutes of Health
- Health and Human Services
- Treatment Information
- Accessibility
- HHS Vulnerability Disclosure
- Freedom of Information Act
- Document Viewing Tools
- Offsite Links
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Int J Angiol
- v.16(3); Autumn 2007

Cigarette smoke and adverse health effects: An overview of research trends and future needs
Sibu p saha.
1 Gill Heart Institute, University of Kentucky, Lexington, Kentucky
Deepak K Bhalla
2 Department of Pharmaceutical Sciences, Wayne State University, Detroit, Michigan
Thomas F Whayne, Jr
3 Graduate Center for Toxicology, University of Kentucky, Lexington, Kentucky, USA
A large volume of data has accumulated on the issues of tobacco and health worldwide. The relationship between tobacco use and health stems initially from clinical observations about lung cancer, the first disease definitively linked to tobacco use. Almost 35 years ago, the Office of the Surgeon General of the United States Health Service reviewed over 7000 research papers on the topic of smoking and health, and publicly recognized the role of smoking in various diseases, including lung cancer. Since then, numerous studies have been published that substantiate the strong association of tobacco use with a variety of adverse human health effects, most prominently with cancer and cardiovascular diseases. Cigarette smoking is regarded as a major risk factor in the development of lung cancer, which is the main cause of cancer deaths in men and women in the United States and the world. Major advances have been made by applying modern genetic technologies to examine the relationship between exposure to tobacco smoke and the development of diseases in human populations. The present review summarizes the major research areas of the past decade, important advances, future research needs and federal funding trends.
A repository for the collection, analysis, validation and dissemination of all smoking and health-related data was established by the World Health Organization. The data received from various member countries were compiled into a book entitled Tobacco or Health: A Global Status Report, 1997 ( 1 ). This report showed smoking prevalence and other tobacco use-related data from various countries and presented an analysis. It is estimated that there are approximately 1.1 billion smokers worldwide, of which 900 million are men and 200 million are women. The sex ratio of men to women is 2:1 for developed nations and 7:1 for developing nations. Smoking prevalence in men and women averages 42% and 24%, respectively, for developed countries, and 48% and 7%, respectively, for less developed countries. In comparison, approximately 47 million people smoke cigarettes in the United States ( 2 ), and smoking prevalence in the United States is estimated at 28% and 23% for men and women, respectively. The Surgeon General’s report in 2004 concluded that in the United States, cigarette smoking has caused 12 million deaths since 1964, at a cost to the nation of approximately US$157.7 billion each year ( 3 ). There has been a significant decline in the consumption of cigarettes in the United States since 1964. The production of cigarettes continues at a steady pace mainly to meet export demands, which continue to rise due to increasing tobacco use in the rest of the world, especially in far eastern and southeastern Asia. On the basis of consumption and disease incidence trends, it is predicted that there will be an epidemic of tobacco-related diseases in various countries of the world in the next 20 to 30 years.
EPIDEMIOLOGY OF TOBACCO-RELATED DISEASE
As part of the Global Burden of Disease Study carried out by the Harvard University School of Public Health in 1997 ( 4 ), it was projected that mortality and morbidity from tobacco use will increase by almost threefold worldwide in 20 to 25 years. Similar predictions have been made by the Oxford University Center headed by Sir Richard Doll, who was one of the first researchers to link cigarette smoking with lung cancer in the 1950s ( 5 , 6 ). Cancer, cardiovascular diseases and chronic obstructive pulmonary disease continue to be the main health problems associated with cigarette smoking. An extensive database has accumulated, which has consistently documented a relationship between smoking and these specific diseases. The strength of the association is further demonstrated by measuring the RR and the presence of a dose-response relationship (ie, direct relationship between the intensity of exposure to cigarette smoke and the risk of disease). According to a 2004 Centers for Disease Control and Prevention report ( 3 ), approximately 2600 people die of cardiovascular disease in the United States every day, which translates into one death every 33 s. Furthermore, the likelihood of dying from heart disease increases fourfold as a result of smoking. The cost of heart disease and stroke in terms of health care expenses and lost productivity was estimated at US$351 billion in the United States alone in 2003.
An analysis by European health experts ( 7 ) determined that in developed countries as a whole, tobacco is responsible for 24% of all male deaths and 7% of all female deaths; these figures rise to over 40% in men in some countries of central and eastern Europe and to 17% in women in the United States. The average decreased life span of smokers is approximately eight years. Among United Kingdom doctors followed for 40 years, overall death rates in middle age were approximately three times higher among physicians who smoked cigarettes than in nonsmokers. In those United Kingdom physicians who stopped smoking, even in middle age, a substantial improvement in life expectancy was noticed. These same experts found that worldwide, smoking kills three million people each year and this figure is increasing. They predict that in most countries, the worst is yet to come, because by the time the young smokers of today reach middle or old age, there will be approximately 10 million deaths per year from tobacco use. Approximately 500 million individuals alive today can expect to be killed by tobacco and 250 million of these deaths will occur in the middle age group. Tobacco is already the biggest cause of adult death in developed countries. Over the next few decades tobacco is expected to become the biggest cause of adult death in the world. For men in developed countries, the full effects of smoking can already be seen. Tobacco causes one-third of all male deaths in the middle age group (plus one-fifth in the old age group) and is the cause of approximately one-half of all male cancer deaths in the middle age group (plus one-third in the old age group). Of those who start smoking in their teenage years and continue smoking, approximately one-half will be killed by tobacco. One-half of these deaths will be in middle-aged individuals (35 to 69 years of age) and each will lose an average of 20 to 25 years of nonsmoker life expectancy. In contrast, the total mortality is decreasing rapidly and cancer mortality is decreasing slowly in nonsmokers in many countries. Throughout Europe in the 1990s, tobacco smoking caused three-quarters of a million deaths in the middle age group. In the Member States of the European Union in the 1990s, there were over one-quarter of a million deaths in the middle age group directly caused by tobacco smoking, which included 219,700 deaths in men and 31,900 in women. There were many more deaths caused by tobacco at older ages. In countries of central and eastern Europe, including the former Union of Soviet Socialist Republics, there were 441,200 deaths in middle-aged men and 42,100 deaths in women. Several epidemiological studies examining the factors responsible for the interindividual differences in the susceptibility to tobacco-related cancers and cardiovascular diseases are being performed in the United States, Europe and Japan. Although still not common practice, many of the newer studies are employing molecular genetic assays in conjunction with epidemiology to identify genotypes susceptible to disease development and select suitable biomarkers of tobacco smoke exposure.
The frequency of investigations in the area of cigarette smoke composition and chemistry decreased during the last decade. Nonetheless, there are ample data to suggest that cigarette smoke is a highly complex mixture that contains approximately 4800 different compounds ( 8 ). Approximately 100 of these compounds are known carcinogens, cocarcinogens and/or mutagens. The complex mixture also contains gases such as ozone, formaldehyde, ammonia, carbon monoxide, toluene and benzene, and about 10 10 particles of different sizes in each mL of mainstream smoke. In addition, a number of other toxic, mutagenic, tumour promoter and/or cocarcinogenic substances have been identified in both mainstream and sidestream cigarette smoke over the years. Many chemical and biological assays of smoke condensates have also documented the presence of potent inhibitors of carcinogenesis in smoke. Such a complex chemical composition of smoke has made it difficult to determine the active constituent(s) responsible for the tobacco-related health risks of smoking and has led to studies of individual constituents of smoke such as polycyclic aromatic hydrocarbons (PAH), nitrosamines and nicotine. Thus, over the years, various individual groups of smoke constituents have been the focus of research at different times. For example, studies of PAH were in vogue during the 1970s and 1980s, followed by nitrosamines in the 1990s. Tobacco alkaloids have long been studied because of their pharmacological activity and have attracted increased attention because of their suspected role in addiction, smoking behaviour and cessation. However, it is also being realized now that the health effects of this complex mixture are likely to result from a combined effect of these chemicals through multiple mechanisms rather than as result of the effects of a single smoke constituent. The mixture contains compounds belonging to almost every class of chemicals that are toxic and protective, agonist and antagonist, carcinogenic and anticarcinogenic, and exists in the gaseous as well as the particulate phase. Extensive studies on the chemical constituents of tobacco smoke and their relationship to disease were published by Hoffmann and Hoffmann of the American Health Foundation ( 8 ). Newer studies have largely focused on the comparative chemistry of mainstream and sidestream smoke. Interest in the free radical chemistry of smoke has resurfaced due to the realization that smoke-induced oxidative injury may play an important role in the etiology of a variety of tobacco-related diseases. Pioneering studies on the free radical chemistry of tobacco smoke, performed in the laboratory of William Pryor at the Louisiana State University ( 9 ), identified short- and long-lived radicals in mainstream and sidestream cigarette smoke, and implicated them in various smoking-associated disease etiologies.
TOBACCO-RELATED CARDIOVASCULAR DISEASE
Cardiovascular diseases, and atherosclerosis in particular, are the leading causes of death in industrial societies. The predominant underlying cause of coronary artery disease (CAD) is atherogenesis, which also causes atherosclerotic aortic and peripheral vascular diseases. Cigarette smoking, independently and synergistically with other risk factors such as hypertension and hypercholesterolemia, contributes to the development and promotion of the atherosclerotic process. Various studies have shown that the risk of developing CAD increases with the number of cigarettes smoked per day, total number of smoking years and the age of initiation, thus indicating a dose-related response. In contrast, cessation of smoking is reported to reduce mortality and morbidity from atherosclerotic vascular disease.
The mechanisms through which smoking influences the development and progression of atherosclerosis are poorly understood at present, but recent studies point to an adverse effect of smoking on endothelial and smooth muscle cell functions as well as thrombotic disturbances produced by tobacco smoke ( 10 , 11 ). With the use of modern ultrasonographic techniques, three independent studies performed in the United States, Europe and Australia have demonstrated that both active and passive smokers exhibit impaired endothelium-dependent vasoregulation ( 12 – 14 ). Some degree of recovery of endothelial function in ex-passive smokers who have stayed away from smoke-contaminated environments further supported a secondary role of smoke in endothelial dysfunction ( 15 ).
Evidence has been presented that tobacco-related impairment of endothelial function may be related to its adverse effects on endothelial nitric oxide (NO) synthase ( 16 , 17 ). An association between a genetic polymorphism of the endothelial NO synthase gene and the predisposition of smokers to CAD was reported ( 18 , 19 ). Additionally, studies report that smoke interferes with L-arginine and NO metabolism, resulting in reduced NO formation ( 20 ). Upregulation of the expression of endothelial cell adhesion molecules (CAMs) such as vascular CAM-1 and intercellular CAM-1 by smoke condensates, and stimulation of leukocyte and endothelium attachment by exposure to cigarette smoke was demonstrated ( 21 ). Cigarette smoke extract has been shown to induce expression of CAMs ( 22 ). However, the expression of a specific adhesion molecule is determined in vivo and the relationship between various events is poorly understood.
Exposure to tobacco smoke is known to increase oxidative stress in the body by various mechanisms, including depletion of plasma antioxidants such as vitamin C. At least two studies have been performed to determine the role of oxidative stress in increasing leukocyte-endothelial interactions that precede the development of atherosclerosis in smokers. One study showed that a high intake of vitamin C by smokers significantly reduced the adhesiveness of their monocytes to endothelial cells ( 23 ). However, in a second study, sera from young smokers was collected before and after a single oral supplementation with vitamin C and L-arginine (a substrate for NO production). The sera were tested for promotion of the adherence of human monocytes to human umbilical vein endothelial cell monolayers. It was shown that while oral L-arginine caused reduction in such leukocyte adherence, no reduction was seen with vitamin C supplementation ( 24 ). This suggested that the NO levels may be important in smoking-induced leukocyte-endothelial interactions, at least during the early stages. Neither NO nor any other markers of oxidative stress were measured in either of these studies.
The levels of 8-hydroxydeoxyguanosine, an oxidized DNA product, and F2-isoprostane, an oxidative arachidonic acid product, were found to be elevated in passive smokers ( 25 , 26 ). Oxidation of low-density lipoprotein (LDL), which is a gold standard risk factor of the atherosclerotic process, was also found to be elevated in smokers, as determined by the presence of increased levels of autoantibodies against oxidized LDL. It was further demonstrated that dietary supplementation with a lipid-soluble antioxidant, α-tocopherol, significantly reduced plasma levels of oxidized LDL autoantibodies ( 27 ). Similarly, intake of a mixture of antioxidants was found to increase the resistance of smoker LDL to oxidative modification ( 28 ) and reduce the plasma levels of 8-hydroxydeoxyguanosine in passive smokers ( 25 ). These studies have thus identified newer, more specific markers of oxidative stress that can be used as biomarkers of oxidant injury and used for the development of dietary and/or pharmacological interventions against disease development.
Relatively few studies related to cardiovascular effects of cigarette smoke have been performed in rodent models. Such animal studies are, however, needed to delineate the role of different mechanisms in promoting atherosclerotic disease and for developing appropriate interventions.
TOBACCO-RELATED CANCERS
Tobacco carcinogenesis has remained a focus of research during the past 10 years, and various epidemiological and experimental studies have not only confirmed the major role of tobacco smoke exposure in lung and bladder cancers, but have also reported on its association with cancers of various other sites, such as the oral cavity, esophagus, colon, pancreas, breast, larynx and kidney. It is also associated with leukemia, especially acute myeloid leukemia.
In addition to the highly recognized role of cigarette smoking in lung cancer, it has been implicated in many other chronic diseases, including chronic bronchitis and pulmonary emphysema. In the United States, the reduction in smoking has resulted in a decline in death due to lung cancer in men since the mid 1980s. However, the incidence of lung cancer in women has surpassed that of breast cancer and continues to rise; it will likely be the focus of future studies ( 29 , 30 ). Both active and passive smoking are implicated in this increase, and several studies of smoking behaviour and disease incidence in women suggest greater susceptibility of women to tobacco carcinogens ( 31 ). It is believed that 80% to 90% of all respiratory cancers are related to active smoking.
Because of the antiestrogenic protective effects of smoking, the role of smoking in breast cancer is controversial. However, recent studies suggest that both active and passive smoking may have a role in the occurrence of breast cancer. One example is a study that found an OR of 4.5 for breast cancer among women who were exposed to passive smoke before 12 years of age and an OR of 7.5 for active smokers. Women who were first exposed to passive smoke after 12 years of age had a lower, although still elevated, OR ( 32 ).
In both men and women, cancers of the head and neck are also on the rise, and this has been attributed to increased use of smokeless tobacco products. Also, a synergistic interaction between cigarette smoking and radon exposure was confirmed in a large study that showed that lung cancer incidence due to an interaction between smoking and radon exposure exceeded incidence accounted for by additive effects and, therefore, indicated multiplicative effects ( 33 ).
Comparative toxicity studies have shown that in comparison with standard cigarettes, the new experimental cigarettes that heat tobacco have a relatively low toxicity ( 34 ). In comparing lung cancer risk in smokers of different types of cigarettes, Lee ( 35 ) determined in 2001 that the risk was 36% lower in individuals smoking filtered cigarettes than in those smoking unfiltered cigarettes, and the risk was 23% lower for smokers of low-tar cigarettes than smokers of high-tar cigarettes. The risk increased by 42% in hand-rolled cigarette smokers and by 75% in smokers using black tobacco.
One interesting observation relates to the nature of lung cancer, which has changed over the years with respect to the location and the types of lung tumours observed in smokers. In the past, the primary tumours observed among smokers were the centrally located squamous cell carcinomas of the airways. Now, the predominant lung tumours in smokers are peripheral adenocarcinomas and other non-small-cell lung cancers. This shift in tumour types has been attributed to changes in the composition of cigarettes and its effect on the smoking patterns of tobacco users over the past 30 years ( 8 , 36 ). Significant reductions in cigarette tar and nicotine and increased levels of nitrates in cigarettes have markedly altered the manner in which cigarettes are smoked. The number and volume of puffs taken by smokers have increased from a single 35 mL puff/min with 1950s cigarettes to two to four 50 mL puffs/min of low-tar or low-nicotine cigarettes; the depth of inhalation has also increased. These changes in smoking patterns have promoted greater deposition of smoke constituents into the peripheral lungs, where adenocarcinomas develop.
Major advances are being made in the area of molecular epidemiology of tobacco-related cancers in human populations. Many recent epidemiological studies have focused on the differential susceptibility to tobacco-related cancers; they have employed polymerase chain reaction-based molecular assays that permit genotypic analysis of small human samples and supplement the information generated by enzymatic and immunological assays. These assays are increasingly being used in human and experimental studies to examine various gene-gene and gene-environment interactions. One area that has received considerable attention in recent years is the role of polymorphic enzymes in the development of diseases. It is now well recognized that genetic polymorphism strongly influences cancer susceptibility and incidence. The frequencies of mutated alleles of proto-oncogenes, tumour suppressor genes and xenobiotic bio-transformation genes vary significantly among different populations and impact substantially on their susceptibility to cancer. Nearly every enzyme in the carcinogen metabolism pathways has been found to exist in multiple forms, many of which vary in binding affinity and/or turnover efficiency. Some are even entirely absent in individuals, thereby influencing their susceptibility to disease development.
The chemical complexity of tobacco smoke and the metabolic activation requirements for many of its carcinogenic constituents have drawn particular attention to genetic polymorphisms of biotransformation enzymes that metabolize tobacco smoke carcinogens. Thus, genes for various activating enzymes such as cytochrome P450 (CYP) proteins, and deactivating enzymes such as glutathione S-transferase (GST), N-acetyl transferase (NAT) and uridine diphosphate-glucose transferase have been the main target of many recent studies in the context of tobacco carcinogenesis. Also, pre-existing inherited mutations and/or mutation susceptibility of tumour suppressor genes such as p53 , which are known to play a major role in determining cancer susceptibility, have been a subject of investigations in tobacco-related carcinogenesis ( 37 , 38 ).
Several human studies have suggested a strong interplay of various polymorphic CYP1A1, CYP1A2, CYP2E1, NAT1, NAT2, GSTM1 and GSTT1 enzymes in modulating the formation of DNA adducts, induction of mutations and chromosomal damage, and/or the incidence of cancers of various sites in different populations ( 39 – 47 ).
The CYP1A1 gene has been extensively studied in Japanese populations. Two polymorphic variants that interact with smoking to modify lung cancer risk have been identified ( 47 , 48 ). Thus, a homozygous minor allele combined with smoking was found to increase lung cancer risk. Studies of the same gene in Western populations have, however, yielded negative or conflicting results ( 49 ), although an interaction of CYP1A1 variants with the GST null genotype has been reported to significantly increase lung cancer risks in non-Japanese populations ( 50 , 51 ).
NATs are polymorphic conjugation enzymes (produced by the NAT1 and NAT2 genes) involved in the detoxification of aromatic amines by N-acetylation. Depending on the presence or absence of a particular variant, individuals can be categorized as slow or fast acetylators, which in turn can influence the incidence of bladder cancer. It was shown that slow acetylator NAT2 is an important modifier of the amount of aromatic amine-DNA adduct formation even at a low dose of tobacco smoke exposure ( 52 ). Slow acetylator NAT2 genotype was also a significant risk factor for bladder cancer in moderate and heavy smokers, but had no effect in nonsmokers ( 53 ).
GSTs are another group of metabolic detoxification enzymes that have attracted a great deal of interest in recent years because of their association with risks for different types of cancers. Based on their sequences, these enzymes are divided into five classes. Three of these classes – GSTM1, GSTT1 and GSTPi – are important in the context of tobacco-related cancers. Extensive studies on the relationship of these genes to cancer risks have shown that most populations studied have very high frequencies (20% to 50%) of homozygous GSTM1 and GSTT1 deletion carriers. GSTM1 and GSTT1 may be involved in the etiology of cancer at more than one site. Furthermore, the risk to individuals who carry homozygous deletions is generally small but increases significantly on interaction with cigarette smoking ( 54 ). Among all metabolic cancer susceptibility genes, the association of GSTM1 deficiency with cancer risk is the most consistent and unidirectional. Various experimental and epidemiological observations support the role of this gene in tobacco-related cancers. For example, it has been observed that the excretion of urinary mutagens and the number of lung tissue DNA adducts in GSTM1-deficient smokers is significantly greater than those carrying the wild-type allele ( 55 – 57 ). Various epidemiological studies also support the premise that deficiency of this enzyme predisposes for lung and bladder cancers ( 58 ). Furthermore, low activity alleles of GSTPi have been often found in association with different types of human cancers ( 59 , 60 ).
In addition to anomalies of biotransformation enzyme genes, inactivation of tumour suppressor genes such as p53 , and activation of the proto-oncogene K-ras are also involved in tobacco-related cancers. Various mutated forms of tumour suppressor gene p53 have been commonly detected in lung tumours and it has been found that these mutations are predominantly located in exons 5 to 8. The nature of point mutations in this gene has been extensively investigated and studies show that the most common mutant allele of the p53 gene possesses a G:C to A:C transversion ( 61 ), which is associated with tobacco use ( 62 , 63 ).
The above studies show that several genetically controlled polymorphic enzymes and enzyme systems are linked to tobacco carcinogen activation and deactivation. Some of these genes have been identified and characterized, but others remain undiscovered. Not only the independent effects of single gene polymorphisms, but an interplay of multiple gene interactions appear to be involved. The complexity of epidemiological studies, which have many uncontrollable variables, makes it difficult to study such interactions and their control in human studies. Additionally, many of the enzymes involved in tobacco carcinogen metabolism are also induced by other environmental factors such as alcohol use, dietary constituents, pesticide and xenobiotic exposure, hormonal status, etc, further complicating the interpretation of data. The interaction of many of these genes with each other and the effect of environmental factors are just beginning to be examined. Experimental studies in specifically constructed transgenic and knock-out animals will be important for a systematic evaluation of the contribution of specific cancer genes and/or cancer susceptibility genes to the tobacco carcinogenic process, and to help identify the mechanisms through which environmental agents, such as cigarette smoke, influence these processes.
SECONDHAND SMOKE
The adverse effects of cigarette smoke on human health are widely recognized. It is the main etiological agent in chronic obstructive pulmonary disease and lung cancer, and is a known human carcinogen. While the risks to human health from active smoking are accepted, evidence supporting the risk of involuntary exposure to environmental tobacco smoke (ETS) has accumulated in recent years. It is the main source of toxicant exposure by inhalation in nonsmokers. Despite recent regulations, smoking in public enterprises is not uncommon. However, despite an occasional report on the effect of secondhand smoke in nonsmokers, little attention was given to this aspect of smoking until about 1970. ETS is now regarded as a risk factor for development of lung cancer, cardiovascular disease and altered lung functions in passive smokers ( 64 ). In general, children exposed to ETS show deterioration of lung function, more days of restricted activity, more pulmonary infections, more days in bed, more absences from school and more hospitalization than children living in nonsmoking homes ( 65 ).
Passive smoking is also implicated in increasing atherosclerosis in individuals 15 to 65 years of age. Children exposed to ETS are at higher risk of developing cardiovascular disorders. Quantitative risk estimates were obtained by measuring the intimal-medial thickness of the carotid artery in a large longitudinal atherosclerosis risk study of 10,914 individuals. Increases of 50%, 25% and 20% were shown over nonsmokers in current, ex-and passive smokers, respectively, thus suggesting a role of all types of tobacco smoke exposure in the progression of atherosclerosis ( 66 ). A recent meta-analysis ( 67 ) of 18 epidemiological studies (10 cohort and eight case-control) further showed an increased RR of CAD in ETS-exposed individuals. These investigators also identified a significant dose-response relationship between the intensity of smoke exposure and risk of CAD in passive smokers. Cardiovascular health risks of smoke-exposed women are of particular concern. Although the exposure to ETS is a current topic of debate in tobacco-related cancers and other lung diseases, the limited research at the basic experimental level provides a strong argument for launching experimental studies to support human data and explore disease mechanisms.
Follow-up of news stories, and local and state ordinances, leads to the conclusion that more communities and states are restricting exposure to secondhand smoke.
NATIONAL INSTITUTES OF HEALTH RESEARCH FUNDING FOR STUDIES OF HEALTH EFFECTS OF CIGARETTE SMOKE
To determine the extent of federal support for experimental studies in the area of health effects of cigarette smoke, the National Institutes of Health (NIH) database of all R01 research grant awards was searched for titles and abstracts containing the words ‘cigarette smoke’ from 1985 to 1998. The results are summarized below. A total of 127 hits were obtained and a careful review of the abstracts provided the following distribution:
- Grants involving experimental animal studies = 12 (9.4%)
- Grants involving experimental animal studies in which whole tobacco smoke was used = 3 (2.3%)
- Grants involving experimental animal studies using smoke components (nicotine, PAH, cadmium and quinones) = 8 (6.2%)
- One grant involved aging
A similar search of the NIH database from 1999 to 2006 revealed 907 grants in all award categories. The grant distribution by category was as follows:
- Total number of R01s = 383
- Grants involving experimental animal studies = 77 (20.1%)
- Grants involving experimental animal studies in which whole tobacco smoke was used = 29 (7.6%)
- Grants involving experimental animal studies using smoke components (nicotine, PAH, cadmium and quinones) = 29 (7.6%)
All the remaining grants generally supported behavioural and epidemiological studies in humans or other systems. Although the number of grants supporting animal studies increased between 1999 and 2006 compared with 1985 to 1998, a significant portion of NIH funding still went to research projects in the area of tobacco use and smoking behaviour, tobacco use among youth and interventions, nicotine addiction and neurobiology of nicotine (areas not covered in this review), presumably in agreement with the NIH’s recent goal of finding effective smoking cessation programs to reduce tobacco usage in the general population. Thus, it is clear that the need for basic experimental research in the field of smoking-associated diseases and the mechanisms through which tobacco smoke causes various diseases remain as important as they ever were. The escalation of health care costs makes it even more necessary to find ways to protect the health of smokers and smoke-exposed individuals with any dietary or therapeutic interventions that hold promise.
DIRECTIONS FOR FUTURE RESEARCH
The most benefit is likely to result from detailed epidemiological studies complemented by specific molecular genotyping of various populations. Ideally, studies of this type will re-evaluate the prevalence of smoking and tobacco use and determine the exact nature of tobacco-related disease incidence, the role of contributory factors such as dietary habits, exposure to other substances and the genetic composition of subpopulations most at risk. Various biochemical and molecular assays will need to be applied to screen nonsmoker and smoker populations for a variety of health risks. Analysis of the results from such studies will help identify the main interacting factors for various health risks and define relationships among various epidemiological parameters. It would appear necessary to assemble teams of multidisciplinary investigators to perform these coordinated human studies in the field and in the laboratory. By nature, such studies are expensive and will involve commitment of resources, time and substantial amounts of funds to obtain meaningful results. Given the limited resources and competing priorities for research funding, it is not easy to undertake such human studies. Hence, the experimental studies in animal models using inhalation exposure to whole smoke, and not individual constituents of smoke, is probably the next best approach for smoking and health programs.
The human epidemiological studies described in the present review have identified a number of genes that appear to have a distinct role in various tobacco-related diseases, and cancers in particular. Inability to control all the different variables in human studies has made it difficult to clearly define the contribution of various suspect genes in tobacco carcinogenesis. With the recent commercial availability of a variety of transgenic and knock-out animals for research, it would be most desirable, as a first step, to use these animals to establish experimental models of various tobacco-related diseases which can then be used for determining the contribution of different genes to disease processes and for elucidation of the mechanism(s) of disease development. Furthermore, these animal models can be used to identify various agents possessing protective and therapeutic potential.
Research efforts in the area of smoking and health would benefit by focusing on studies of the in vivo effects of inhaled whole cigarette smoke in animal models of known specific genetic composition. Selection of the genetic composition would also require a thorough consideration of the information available from human molecular epidemiological studies. As indicated earlier, there are a number of genes that clearly influence the development of smoke-related diseases. In this context, many relevant transgenic and knock-out animals that can be effectively used for the study of tobacco-related diseases are now becoming available.
Tobacco abuse is a major public health problem and includes secondhand smoke exposure. Continued efforts to control and eliminate this abuse are a medical necessity.

IMAGES
VIDEO
COMMENTS
We review current advances in research on nicotine addiction treatment and recovery, with a focus on conventional combustible cigarette use. Our review covers evidence-based methods to treat smoking in adults and policy approaches to prevent nicotine product initiation in youth. ... Tobacco rolled in paper for smoking: A typical cigarette ...
1. Introduction. Oral diseases appear to be a global problem that should be addressed as a matter of global health concern. Oral health issues include various behavioral and social features such as habits, oral health knowledge, practices, availability, modifiable risk factors, and accessibility to oral health treatments [].Health is considered as a significant factor in making life valuable [].
Nicotine dependence increases the risk of smoking persistence and is the leading preventable cause of morbidity and death. 1 The prevalence of cigarette smoking has declined in the US over the past decades among adults with and without psychiatric conditions (eg, major depressive episode [MDE] and substance use disorder [SUD]). 2,3 A nicotine ...
We identified three outcomes with a 4-star association with smoking: COPD (72% increase in risk based on the BPRF, 0.54 ROS), lower respiratory tract infection (54%, 0.43) and pancreatic cancer ...
Abstract. Tobacco smoking is a major determinant of preventable morbidity and mortality worldwide. More than a billion people smoke, and without major increases in cessation, at least half will ...
There is little research on perceptions of nicotine addiction, and more is needed, particularly considering the increasing use of nontobacco nicotine products and the potential for long-term nicotine maintenance. 19 Researchers should be deliberate in their choice of terms used in surveys and interviews to examine understandings of addiction to ...
Despite growing knowledge of the adverse effects of cigarette smoking on general health, smoking is one of the most widely prevalent addictions around the world. Globally, about 1.1 billion smokers and over 8 million people die each year because of cigarette smoking. Smoking acts as a source for a variety of oral and systemic diseases. Various periodontal issues such as increased pocket depth ...
We review current advances in research on nicotine addiction treatment and recovery, with a focus on conventional combustible cigarette use. Our review covers evidence-based methods to treat smoking in adults and policy approaches to prevent nicotine product initiation in youth. In closing, we discuss emerging areas of evidence and consider new ...
Further information on research design is available in the Life Sciences Reporting Summary linked to this paper. Smoking outcome data ... Addiction 112 , 2023-2031 (2017 ... gender in tobacco ...
Keywords: smoking; tobacco; addiction Introduction ... This paper provides a broad overview of smoking in terms of: the health effects, benefits of stopping, prevalence and patterns of use, psychological, pharmacological ... has been the subject of by far the largest volume of research and is the most harmful
tobacco smoking remains one of the main preventable causes of ill-health and. premature death worldwide. This paper reviews the extent and nature of harms. caused by smoking, the bene fits of ...
Nicotine & Tobacco Research aims to provide a forum for empirical findings, critical reviews, and conceptual papers on the many aspects of nicotine and tobacco, including research from the biobehavioral, neurobiological, molecular biologic, epidemiological, prevention, and treatment arenas. Find out more here.
Trends in Nicotine Use and Dependence From 2001-2002 to 2012-2013. Although considerable efforts over earlier years have curbed the use of tobacco and nicotine, the use of these substances continues to be a significant public health problem. As noted above, individuals with psychiatric disorders are particularly vulnerable.
Cutting-edge neuroimaging technologies have identified brain changes associated with nicotine dependence and smoking. Using functional magnetic resonance imaging (fMRI), scientists can visualize smokers' brains as they respond to cigarette-associated cues that can trigger craving and relapse. 231 Such research may lead to a biomarker for ...
The Tobacco Addiction Cycle. The first cigarette of the day has a substantial pharmacologic effect, primarily arousal, but at the same time, tolerance to nicotine begins to develop. ... Supported by grants from the Flight Attendants Medical Research Institute and the National Institute on Drug Abuse (U.S. Public Health Service grants DA02277 ...
In 2014, the Nation marked the 50th anniversary of the first Surgeon General's Report on Smoking and Health. In 1964, more than 40 percent of the adult population smoked. Once the link between smoking and its medical consequences—including cancers and heart and lung diseases—became a part of the public consciousness, education efforts and public policy changes were enacted to reduce the ...
We review current advances in research on nicotine addiction treatment and recovery, with a focus on conventional combustible cigarette use. Our review covers evidence-based methods to treat smoking in adults and policy approaches to prevent nicotine product initiation in youth. ... Tobacco rolled in paper for smoking: A typical cigarette ...
Abstract. Vaping devices, introduced to the US market in 2007 as aids for smoking cessation, have become popular among youth and young adults because of their enticing flavors and perceived lack of negative health effects. However, evidence is emerging that vaping may introduce high levels of dangerous chemicals into the body and cause severe ...
Tobacco use is the largest preventable cause of diseases and deaths; reducing tobacco intake is, therefore, an urgent public health goal. In recent years, e-cigarettes have been marketed as a 'healthier' alternative to tobacco smoking, whilst product features have evolved tremendously in the meantime. A lively scientific debate has developed regarding the potential benefits and risks of e ...
Nicotine addiction remains the most common form of chemical dependence in the United States ().Consequently, despite considerable public health investment, tobacco use is still the leading cause of preventable illness and death in the United States ().Exploring Databases engages students in examining how environmental and genetic factors contribute to smoking addiction by using the Smoking ...
The research investigated determinants of current tobacco use, encompassing any tobacco use in any form, smoked and smokeless tobacco (SLT). ... Nicotine addiction. N Engl J Med, 2010; 362(24): 2295-2303. Crossref. ... et al. White paper on smokeless tobacco & women's health in India. Indian J Med Res, 2020; 151(6): 513. Crossref. Google ...
The habitual use of tobacco and tobacco products continues to be a significant contributor to health problems worldwide. The increasing affordability of tobacco and nicotine products has been a contributing factor as well as marketing strategies that increasingly use social media to reach consumers globally. Smoking and chewing tobacco remain the main source of nicotine exposure in humans.
Hot Off the Press - April 4, 2024. Published in Translational Psychiatry by Yi He, Hui Shen and Zheng-Xiong Xi, et al. from the NIDA IRP Addiction Biology Unit and Magnetic Resonance Imaging and Spectroscopy Section.. Summary. G protein-coupled receptor 55 (GPR55) is a putative cannabinoid receptor, which has been considered as the "CB3" receptor, as multiple cannabinoids such as Δ9-THC ...
Almost 35 years ago, the Office of the Surgeon General of the United States Health Service reviewed over 7000 research papers on the topic of smoking and health, and publicly recognized the role of smoking in various diseases, including lung cancer. ... nicotine addiction and neurobiology of nicotine (areas not covered in this review ...
The data suggests a significant trend towards increasing addiction to electronic devices among students, with 7.1% using their gadgets for 1 hour a day, 26% for 2 hours, the majority (48%) for 4 hours, and 18.9% for 6 hours daily. The rising prevalence of screen addiction necessitates immediate action.