Case Report: β-thalassemia major on the East African coast
Alexander W. Macharia Roles: Conceptualization, Data Curation, Formal Analysis, Investigation, Methodology, Visualization, Writing – Original Draft Preparation, Writing – Review & Editing George Mochamah Roles: Conceptualization, Formal Analysis, Investigation, Writing – Review & Editing Johnstone Makale Roles: Data Curation, Formal Analysis, Methodology, Project Administration Thad Howard Roles: Data Curation, Formal Analysis, Investigation, Methodology Neema Mturi Roles: Investigation, Methodology, Resources Peter Olupot-Olupot Roles: Validation, Writing – Review & Editing Anna Färnert Roles: Validation, Writing – Review & Editing Russell E. Ware Roles: Methodology, Visualization, Writing – Review & Editing Thomas N. Williams Roles: Funding Acquisition, Methodology, Supervision, Visualization, Writing – Review & Editing
β-thalassemia major, rs33941849, East Africa, HbA2, sequencing

Introduction
β-thalassemia is rare in most of Africa, with the exception of North Africa where the prevalence, causal pathogenic variants and disease outcomes have all been well described previously ( Hamamy & Al-Allawi, 2013 ). We recently reported elevated levels of HbA 2, suggestive of β-thalassemia, in a small proportion of children participating in a cohort study conducted in Kilifi county on the coast of Kenya ( Macharia et al ., 2019 ). We subsequently sequenced samples from the same children and found that 0.6% were carriers of one of four different β-thalassemia pathogenic variants: the β 0 -thalassemia variants CD22 (GAA➝TAA) (rs33959855), initiation codon (ATG➝ACG) (rs33941849) and IVS1-3ʹ end del 25bp (rs193922563) and the β + -thalassemia variant IVS-I-110 (G➝A) (rs35004220). Whereas the mutations observed in North Africa resemble those found in Middle Eastern countries, those identified in Kilifi were a mixture of mutations reported from Asia and the Middle East. To the best of our knowledge, no cases of β-thalassemia major – a condition in which both HBB genes are affected by a β 0 -thalassemia mutation to result in the complete loss of normal β 0 -globin production - have yet been reported from the East Africa region. Here, we describe what is, to the best of our knowledge, the first case of β 0 -thalassemia major to be recognised from within this region.
Written informed consent was provided by the parents of the study participant. Ethical approval for the study was granted by the Kenya Medical Research Institute Ethical Review Committee in Nairobi, Kenya (Number: SCC3891).
Patient report
The child, a two-and-a-half-year-old female, presented to Kilifi County Hospital in Kenya, with a one-week history of left sided abdominal swelling. No previous hospital admissions were reported. Clinical history suggested delayed developmental milestones; specifically, she was unable to walk without support. The child was the fourth born of five siblings, all of whom were alive and well as were both of her parents. Both her parents were of Mijikenda ethnolinguistic ancestry and no recent genetic admixture was apparent from the clinical history. On physical examination, the child was pale but had no signs of clinical jaundice. Her vital signs were essentially normal with the exception of a fever measured at 38.8 ° C per axilla. Fronto-maxillary skull bossing was apparent. Her abdomen was distended, soft and non-tender, massive splenomegaly being detected at 8cm below the costal margin. She was severely malnourished with a weight of 8.8 kg, a height of 78.5 cm, a height for age z-score (HAZ) of -3.79, a weight for age z-score (WAZ) of -3.20 and a weight for height z-score (WHZ) of -1.23. Further examination was essentially normal. The timeline of events is given in Table 1 .
Table 1. Timeline of events.
A full hemogram revealed marked anemia (Hb 6.6 g/dL), a low mean corpuscular volume (MCV) of 64 fL, a low mean corpuscular hemoglobin (MCH) of 19.4 pg, and a raised total white blood cell (WBC) count of 49.6 × 10 9 /µl which were predominantly lymphocytes. Her platelet count was normal at 321 × 10 6 /L and her creatinine mildly elevated at 32 μmol/l. Blood cultures and tests for malaria were negative. A peripheral blood film revealed nucleated red blood cells (RBCs), microcytes, dacrocytes, acanthocytes, giant platelets and a marked lymphocytosis ( Table 2 ).
Table 2. Complete blood count and peripheral blood film from the child with β-thalassemia.
Abbreviations: WBC, white blood cells; RBC, red blood cells; Hb, hemoglobin; HCT, hematocrit; MCV, mean cell volume; MCH, mean cell hemoglobin; PBF, peripheral blood film. ¢Age=2.5 years, ‡Age=3.5 years.
The child was admitted to the general pediatric ward with a working diagnosis of iron deficiency anemia, potentially complicated by bacterial sepsis, and with a differential diagnosis of sickle cell anemia. She was treated empirically with iron and folic acid supplementation for her anemia and with intravenous penicillin and gentamicin to cover sepsis. She was also prescribed malaria prophylaxis with proguanil pending analysis for sickle cell anemia by high-performance liquid chromatography (HPLC). Her fever subsided within two days of admission, at which point she was discharged home on oral amoxicillin, with follow-up planned for the following week.
The results of her HPLC analysis, received after discharge from hospital, revealed the absence of normal adult hemoglobin (HbA), normal levels of HbA 2 at 2.5% and elevated levels of fetal hemoglobin (HbF) (>80% of total Hb) that eluted in adjacent peaks A1b (16%) and LA1C/cHb1 (76.5%) ( Figure 1 ). The complete absence of HbA suggested a diagnosis of β 0 -thalassemia major. We therefore sequenced her HBB gene region as described in detail previously ( Clark & Thein, 2004 ), which revealed that the child was homozygous for the initiation codon (ATG➝ACG) mutation (rs33941849).
Figure 1. HPLC chromatograms from study participants with normal hemoglobin A individual (HbAA), homozygous hemoglobin S (HbSS) and homozygous β-thalassemia patient at first admission (age 2.5 years) and at second admission (age 3.5 years).
Initially lost to follow-up, the child re-presented at the age of three years 11 months with a one-week history of a cough and fever. On examination at that time, her spleen remained grossly enlarged at 10 cm, and she remained malnourished with a HAZ of -4.98, a WAZ of -4.01 and a WHZ of -0.99. Although hemodynamically stable, she was profoundly anemic (Hb 2.2 g/dL) and was therefore transfused and treated with folic acid supplementation and nutritional support. Repeat HPLC analysis revealed the continued absence of HbA together with elevated levels of HbF (>80%) and HbA 2 (at 5%) ( Figure 1 ). PCR for the α -3.7 deletional form of α-thalassemia was negative.
To the best of our knowledge, this is the first case of homozygous β-thalassemia to be reported from the East Africa region. The mutation responsible disrupts the transfer RNA binding site to result in a β 0 form of the disease. It appears to be rare in other populations: only 45 carriers have been reported in the literature to date, 20 of which were from our recently reported study ( Macharia et al ., 2020 ). Other reports of carriers have come from a wide range of countries including Switzerland ( Beris et al ., 1993 ), Belgium ( Wildmann et al ., 1993 ), Russia ( Molchanova et al ., 1998 ), India ( Gorakshakar et al ., 2018 ; Gupta et al ., 2002 ) and the former Yugoslavia ( Jankovic et al ., 1990 ). The only homozygous case described to date was a male child of Pakistani origin who presented at 10 months of age with a palpable liver and spleen at 7 cm and 3 cm below costal margin, respectively. His Hb was 9.2 g/dl, MCV of 73 fl and MCH of 33 pg. He was also found to be homozygous for the α -3.7 -thalassemia deletion and to have a Bantu β-globin gene cluster haplotype. He was managed with regular blood transfusions ( Khan et al ., 2000 ).
On comparing the current and previously described cases, all had anemia, a low MCV and massive splenomegaly. In our current patient, we also observed elevated levels of HbF and varying levels of HbA 2 at the two points of testing, an observation which is common in β-thalassemia major ( Steinberg & Rodgers, 2015 ). Options for the treatment of this condition in our context are limited. Throughout much of the world, first line management includes the provision of regular, leuco-depleted blood transfusions together with extended antigen typing of transfused blood to reduce the risk of alloimmunization. Iron-chelation is also used to mitigate the risk of iron overload ( Steinberg et al ., 2009 ) while more recently, allogeneic hematopoietic cell transplantation (HCT) is also being used as a potentially curative therapy. However, all these strategies are beyond the capacity of our local health-care system. Nevertheless, there is growing evidence to support the use of hydroxyurea, an HbF inducer, in the treatment of transfusion and non-transfusion dependent β-thalassemia ( Algiraigri et al ., 2017a ; Algiraigri et al ., 2017b ). We will investigate this strategy together with surgical splenectomy if the child returns for further follow-up in the hope that these will reduce the frequency at which transfusions will be required.
Conclusions
We have previously estimated the birth prevalence of β-thalassemia major in our local community at approximately 1 in 100,000 ( Macharia et al ., 2020 ). Nevertheless, low awareness of this condition among clinicians and the low availability of diagnostic facilities within the region mean that historically, individuals with β-thalassemia major have probably been misdiagnosed with other conditions such as sickle cell anemia or iron deficiency anemia as was the case with this child. As such, we hope that our case study will raise awareness about the existence and clinical importance of β-thalassemia major as a public health problem within the East Africa region and lead to the development of locally appropriate diagnostic and treatment guidelines.
Data availability
Underlying data.
All data underlying the results are available as part of the article and no additional source data are required.
Written informed consent for publication of their clinical details was obtained from the parents of the patient.
- Algiraigri AH, Wright NAM, Paolucci EO, et al. : Hydroxyurea for lifelong transfusion-dependent β-thalassemia: A meta-analysis. Pediatr Hematol Oncol. 2017a; 34 (8): 435–448. PubMed Abstract | Publisher Full Text
- Algiraigri AH, Wright NAM, Paolucci EO, et al. : Hydroxyurea for nontransfusion-dependent β-thalassemia: A systematic review and meta-analysis. Hematol Oncol Stem Cell Ther. 2017b; 10 (3): 116–125. PubMed Abstract | Publisher Full Text
- Beris P, Darbellay R, Speiser D, et al. : De novo initiation codon mutation (ATG-->ACG) of the beta-globin gene causing beta-thalassemia in a Swiss family. Am J Hematol. 1993; 42 (3): 248–253. PubMed Abstract | Publisher Full Text
- Clark BE, Thein SL: Molecular diagnosis of haemoglobin disorders. Clin Lab Haematol. 2004; 26 (3): 159–176. PubMed Abstract | Publisher Full Text
- Gorakshakar AC, Breganza PV, Colaco SP, et al. : Rare β- and δ-Globin Gene Mutations in the Pathare Prabhus: Original Inhabitants of Mumbai, India. Hemoglobin. 2018; 42 (5–6): 297–301. PubMed Abstract | Publisher Full Text
- Gupta A, Hattori Y, Agarwal S: Initiation codon mutation in an Asian Indian family. Am J Hematol. 2002; 71 (2): 134–136. PubMed Abstract | Publisher Full Text
- Hamamy HA, Al-Allawi NA: Epidemiological profile of common haemoglobinopathies in Arab countries. J Community Genet. 2013; 4 (2): 147–167. PubMed Abstract | Publisher Full Text | Free Full Text
- Jankovic L, Efremov GD, Josifovska O, et al. : An initiation codon mutation as a cause of a beta-thalassemia. Hemoglobin. 1990; 14 (2): 169–176. PubMed Abstract | Publisher Full Text
- Khan SN, Riazuddin S, Galanello R: Identification of three rare beta-thalassemia mutations in the Pakistani population. Hemoglobin. 2000; 24 (1): 15–22. PubMed Abstract | Publisher Full Text
- Macharia AW, Mochamah G, Uyoga S, et al. : β-Thalassemia pathogenic variants in a cohort of children from the East African coast. Mol Genet Genomic Med. 2020; 8 (7): e1294. PubMed Abstract | Publisher Full Text | Free Full Text
- Macharia AW, Uyoga S, Ndila C, et al. : The population dynamics of hemoglobins A, A 2 , F and S in the context of the hemoglobinopathies HbS and α-thalassemia in Kenyan infants. Haematologica. 2019; 104 (5): e184–e186. PubMed Abstract | Publisher Full Text | Free Full Text
- Molchanova TP, Postnikov Yu V, Gu LH, et al. : Historical note: the beta-thalassemia allele in the noble Russian family Lermontov is identified as the ATG-->ACG change in the initiation codon. Hemoglobin. 1998; 22 (3): 283–286. PubMed Abstract | Publisher Full Text
- Steinberg MH, Forget BG, Higgs DR, et al. : Disorders of Hemoglobin: Genetics Pathophysiology, and Clinical Management. 2nd edn. Cambridge University Press, Cambridge. 2009. Reference Source
- Steinberg MH, Rodgers GP: HbA 2 : biology, clinical relevance and a possible target for ameliorating sickle cell disease. Br J Haematol. 2015; 170 (6): 781–787. PubMed Abstract | Publisher Full Text
- Wildmann C, Larondelle Y, Vaerman JL, et al. : An initiation codon mutation as a cause of beta-thalassemia in a Belgian family. Hemoglobin. 1993; 17 (1): 19–30. PubMed Abstract | Publisher Full Text
Comments on this article Comments (0)
Open peer review.
Is the background of the case’s history and progression described in sufficient detail?
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Is the case presented with sufficient detail to be useful for other practitioners?
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Hemoglobinopathies and thalassemia
- Respond or Comment
- COMMENT ON THIS REPORT
Reviewer Expertise: disorders of hemoglobin
Reviewer Status
Alongside their report, reviewers assign a status to the article:
Reviewer Reports
- Martin H. Steinberg , Boston Medical Center, Boston, USA
- Paloma Ropero , Hospital Clínico San Carlos, Madrid, Spain
Comments on this article
All Comments (0)
Competing Interests Policy
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
- Within the past 4 years, you have held joint grants, published or collaborated with any of the authors of the selected paper.
- You have a close personal relationship (e.g. parent, spouse, sibling, or domestic partner) with any of the authors.
- You are a close professional associate of any of the authors (e.g. scientific mentor, recent student).
- You work at the same institute as any of the authors.
- You hope/expect to benefit (e.g. favour or employment) as a result of your submission.
- You are an Editor for the journal in which the article is published.
- You expect to receive, or in the past 4 years have received, any of the following from any commercial organisation that may gain financially from your submission: a salary, fees, funding, reimbursements.
- You expect to receive, or in the past 4 years have received, shared grant support or other funding with any of the authors.
- You hold, or are currently applying for, any patents or significant stocks/shares relating to the subject matter of the paper you are commenting on.
Stay Updated
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with Wellcome Open Research
Already registered? Sign in
Not now, thanks
Are you a Wellcome-funded researcher?
If you are a previous or current Wellcome grant holder, sign up for information about developments, publishing and publications from Wellcome Open Research.
We'll keep you updated on any major new updates to Wellcome Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here .
If you still need help with your Google account password, please click here .
You registered with F1000 via Facebook, so we cannot reset your password.
If you still need help with your Facebook account password, please click here .
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Extramedullary hematopoiesis in β-thalassemia major patient: a case report and review of the literature
- Case Report
- Published: 15 July 2022
- Volume 15 , pages 185–190, ( 2022 )
Cite this article

- Bijan Keikhaei 1 ,
- Daryush Purrahman 1 ,
- Batool Marashi 1 ,
- Meisam Moezi 2 &
- Mohammad Reza Mahmoudian-Sani 1
814 Accesses
1 Altmetric
Explore all metrics
Extramedullary hematopoiesis (EMH), as a compensatory phenomenon, refers to the blood cell formation outside of the bone marrow that occurs once the cells in the circulatory system fail to meet individuals’ needs. EMH is rare in moderate to severe beta thalassemia because most symptomatic patients are effectively managed with transfusion. However, patients that fail to receive transfusions like β-thalassemia intermedia (β-TI) as indicated are at increased risk for developing EMH. This paper describes the case of a 15-year-old female adolescent with β-thalassemia major (β-TM), suffering from a rare form of EMH affecting the sinus cavities, characterized by headache, sinusitis, and nasal obstruction, as confirmed by physical-pathological examinations and computerized tomography (CT) scan findings. The EMH in this patient could be significantly attributed to the lack of regular blood transfusions in recent years. It was concluded that β-TM along with the occurrence of EMH in the sinus cavities had led to a complex case, carrying a heavy burden of the disease for the patient.
Similar content being viewed by others
Newborn Screening for Severe T and B Cell Lymphopenia Using TREC/KREC Detection: A Large-Scale Pilot Study of 202,908 Newborns

The 5th Edition of the World Health Organization (WHO) Classification of Hematolymphoid Tumors: What’s New in Pediatrics?

Thrombocytosis in children and adolescents—classification, diagnostic approach, and clinical management
Avoid common mistakes on your manuscript.
Introduction
Hematopoiesis is the process of blood cell formation that begins in the yolk sac in the embryonal period, followed by production in the liver, and ultimately occurring almost exclusively in the bone marrow past the age of five. In spite of this, some conditions, such as inflammation, anemia (e.g., due to thalassemia), myelofibrosis, and certain pathological factors may induce hematopoiesis outside of the bone marrow. This condition, called extramedullary hematopoiesis (EMH), was first illustrated by Guizetti in 1912 [ 1 , 2 ]. EMH represents a nonneoplastic compensatory mechanism that arises once the bone marrow fails to produce enough blood cells [ 3 ]. While EMH can have effect in different parts of the body, it is much more common in certain organs, like the liver and spleen [ 4 ]. EMH also tends to be latent and asymptomatic but problematic for some patients. For example, there are several case reports of severe neurological symptoms due to EMH in the spinal cord [ 5 , 6 ]. There is even one case report of EMH in the brain of a patient with β-T, causing ossicular fixation and bilateral conductive hearing loss [ 7 ]. In one patient with β-TI, thoracic EMH had thus led to shortness of breath and dry cough [ 8 ]. Furthermore, EMH occurring due to the formation of tumefactive masses may make it more difficult to perform a differential diagnosis among pathologies and tumors with similar conditions, which highlights the importance of paying much attention to EMH. β-TM is further a serious disease that requires the affected patients to undergo regular blood transfusions; the risk of developing EMH in these patients is very low. Because the body’s deficiency of need is compensated by blood transfusions. This paper describes the case of a patient with β-TM who has developed EMH in her sinus cavities (viz. maxillary, ethmoid, and sphenoid) most likely because of not undergoing regular blood transfusions in recent years.
Case presentation
The patient here was a 15-year-old Iranian female adolescent suffering from β-TM (IVSII-I(G-A)/IVSII(G-A)- β 0 /β 0 ) admitted for facial swelling and splenomegaly to Shafa Hospital, affiliated to Jundishapur University of Medical Sciences, Ahvaz, Iran.
Despite needing regular blood transfusions, the patient had not undergone any blood transfusions in recent years. Moreover, examinations revealed pallor and jaundice, bilateral maxillary swelling, and thalassemic facies (Fig. 1 ). Of note, the patient was 145 cm tall and weighed 35 kg, which was not proportional to her age.
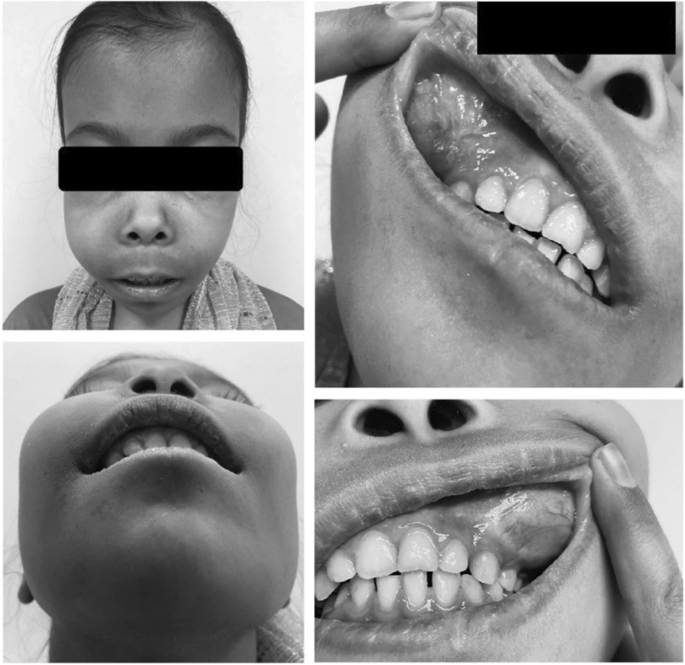
Bilateral maxillary swelling, swollen gums, and thalassemic facies
The ultrasound results also showed splenomegaly of size 58 × 129 mm and hepatomegaly of size 132 mm. As well, the brain magnetic resonance imaging (MRI) revealed the asymmetric swelling of the sinuses, putting more pressure on the nasal septum and causing a deviated septum.
The maxillary sinuses of both sides had been dilated because of hyperplasia and had a reduced wall thickness (Fig. 2 ). Hyperplasia was even more intense in the right maxillary sinus, leading to the closure of the right airway. Although this condition was also present in the left-side maxillary sinus, it was mild. The dilatation of the right-side maxillary sinus had further put pressure on the nasal septum, causing nasal deviation. Moreover, the calvaria had an irregular texture with the signs of thickening and a spongy appearance in the frontal and posterolateral sections. Accordingly, it was concluded that the progression of this condition could bring about head deformity.
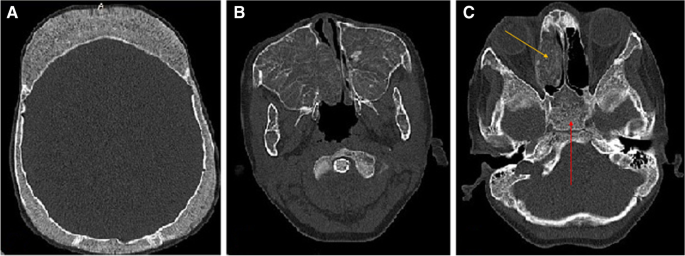
CT scan showed dilation, thinning, and opacification in the right ethmoid sinus (yellow arrow). The sphenoid sinus was also fully opacified, with a spongy appearance (red arrow)
The laboratory findings were as follows:
Blood urea nitrogen (BUN), 15 mmol/L; creatinine (CR), 1.0 mg/dL; bilirubin (BR, total), 4.1 µmol/L; BR (direct), 0.4 mg/dL; aspartate transaminase (AST), 40 U/L; alanine aminotransferase (ALT), 38 IU/L; alkaline phosphatase (ALP), 535 IU/L; phosphor (P), 5.4 mg/dL; ferritin (FER), 311.1 ng/mL; 25-OH-vitamin D, 17.4 ng/mL; white blood count (WBC), 8.500 × 10 9 /L; red blood count (RBC), 3.81 × 10 12 /L; hemoglobin (Hb), 9.2 g/dL; mean corpuscular volume (MCV) 72.4 fl; mean corpuscular hemoglobin (MCH), 24.1 pg; mean corpuscular hemoglobin concentration (MCHC), 33.3 g/dL; red cell distribution width-coefficient of distribution (RDW-CV), 28.9%; RDW-standard deviation (RDW-SD), 63.8 fL; and platelet (PLT) 318 × 10 9 /L. In addition, the electrophoresis outcomes revealed fetal hemoglobin (HbF), 98.1%, and hemoglobin A2 (HbA2), 1.9% (Fig. 3 ).
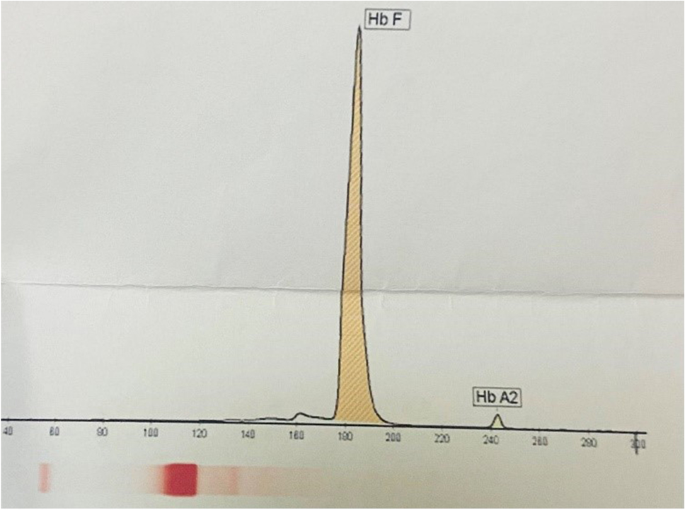
The graph for the patient’s electrophoresis results
For the final diagnosis, a biopsy was taken from the patient’s sinuses that it showed the presence of trilineage hematopoiesis that confirmed the diagnosis of EMH (Fig. 4 ). The patient was also prescribed folic acid 5 mg/day, hydroxyurea 500 mg/day, and zinc-plus, and then received 10 sessions of 10 Gray (GY) radiotherapy. After 9 months, the size of the mass has slightly decreased, and the patient undergoes surgery in the next stage.
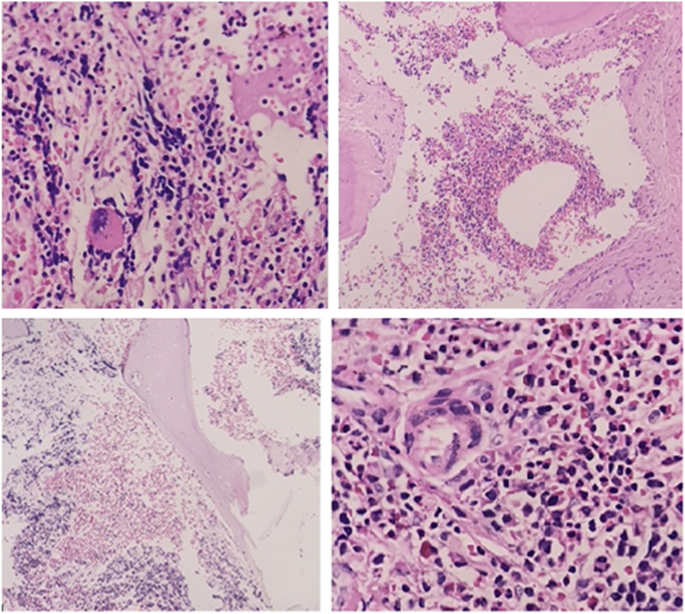
The specimen received in the fixative formalin, consisting of multiple tiny fragments of the bone and the soft tissue totally M: 1 × 1 × 0.5 cm. The sections showed sinonasal mucosa and connective tissues with mild inflammation, admixed with the fragments of the bony trabeculae with areas of trilineage hematopoiesis, including erythroid, myeloid, and megakaryocytic, which confirmed the diagnosis of EMH
Discussion and conclusion
A small amount of hematopoietic stem cells (HSCs) circulates in the peripheral blood and the spleen in a steady state. Furthermore, in patients with TM, who stop undergoing blood transfusions, the bone marrow ramps up to overcome chronic anemia, which leads to osteoporosis and bone deformities [ 3 ]. This causes erythroid precursors to separate from the bone marrow and move to other organs. The liver and spleen are also two organs with the highest likelihood of EMH because of their filtration capability, which causes the entrapment and homing of erythroid precursors in these tissues, ultimately inducing hepatosplenomegaly [ 3 ]. However, this does not mean that EMH cannot take place in other organs, and indeed this condition can occur, as was the case for the patient here, because of the formation of erythroid mass and the start of hematopoiesis in a tertiary site (e.g., the sinus cavity) in response to the inability to meet circulatory demands.
Recently emerged molecular evidence has further shed some light on the molecular background of EMH. Some studies have pointed to several genetic factors involved in the EMH progression, including Ras homolog enriched in brain 1 ( Rheb1 ) loss, ASXL transcriptional regulator 2 ( ASXL2 ) deficiency, zinc finger E-box binding homeobox 2 ( Zeb2 ) inactivation, and SET domain containing 1B, histone lysine methyltransferase ( Setd1b ) deletion [ 9 ]. These molecules appear to be part of the network that regulates hematopoiesis. For example, Setd1b has a regulatory effect on key lineage specification components, such as CCAAT enhancer-binding protein alpha ( Cebpa ), GATA-binding protein 1 ( Gata1 ), and Kruppel-like factor 1 ( klf1 ), and results in the loss of expression in myeloid-biased mice EMH in the spleen [ 10 ].
In one study, a correlation had been accordingly found between the T-cell leukemia homeobox 1 ( Tlx1 ) expression in mesenchymal progenitor-like cells in the spleen along with the recruitment and mobilization of circulating hematopoietic stem/progenitor cells (HSPCs) in this organ, which seemed to be involved in EMH in the spleen [ 9 ]. In fact, it appears that niche-forming cells, such as mesenchymal stem/progenitor cells (MSPCs) located in places other than the bone marrow play a significant role in the recruitment and cell fate processes as well as the triggering of EMH in the organ [ 1 ].
It has been also reported that nitrogen-containing bisphosphonate (NBP) medications, such as alendronate, neridronate, and ibandronate, often used to treat osteoporosis in thalassemia patients can augment the risk of EMH by inducing histidine decarboxylase (HDC) production [ 11 , 12 , 13 ].
Histidine decarboxylase (HDC) is a primary and unique enzyme that catalyzes the synthesis of histamine through the decarboxylation of the amino acid L-histidine in mouse and human.
HDC is expressed in the hematopoietic progenitor cell. Comparison of morphological analysis findings in the liver of HDC-knockout mice with wild-type showed more significant hematopoietic colonies and megakaryocytes in the former. It seems that high levels of HDC in the culture medium cause detachment of hematopoietic stem cells and their disappearance [ 14 ]. On the other hand, HDC as wells also involved in maintaining a suitable environment for hematopoiesis by inducing the expression of interleukin 3 ( IL-3 ), IL-17 , granulocyte colony-stimulating factor ( G-CSF ), erythropoietin (EPO), and C-X-C motif chemokine ligand 12 ( CXCL12 ) [ 14 , 15 ]. As well, EPO is one of the main factors affecting the onset of EMH, and anemic patients show increased EPO expression due to lower oxygen delivery to the tissues in the liver and the kidneys regardless of the NBP consumption. Moreover, EPO plays a direct role in the release of HSPCs from the bone marrow and the rise of the HSPC level in circulation [ 16 ]. EPO similarly activates the erythropoietin receptor/Janus kinase 2 ( EPOR/JAK2 ) pathway, whose signaling leads to the excessive proliferation of erythroid cells and increases the number of these cells in the secondary hematopoietic organ [ 9 , 17 ].
While the common practice for the final diagnosis of EMH is a biopsy, other noninvasive methods such as CT scan, MRI, and chest roentgenograms can be effective [ 5 , 8 ]. One of the major challenges to deal with EMH is the difficulty of early detection and diagnosis. In a study comparing the levels of different factors in non-transfusion-dependent thalassemia (NTDT) patients, EMH-positive cases had thus shown significantly higher serum FER, growth differentiation factor-15 ( GDF-15 ), and EPO levels than EMH-negative ones [ 18 ]. This study had also reported that GDF15 , EPO , GDF15/EPO , and GDF15/ soluble transferrin receptors ( sTfR ) could be exploited for the early risk prediction of EMH in the NTDT patients [ 18 ]. In another study, sTfR could act as a prognostic factor for EMH in β-TI patients [ 19 ]. In view of this, these factors can be used as prognostic markers to help identify patients with a high risk of EMH in order to prevent an elevation in the burden of the disease.
In general, there are still no clear guidelines for treating EMH, and physicians often consider radiotherapy (10–30 Gy), surgical interventions, hydroxyurea therapy, hyper-transfusion, or a combination of these methods as treatment options [ 20 ]. However, some methods have certain drawbacks that make them less preferable. For example, while blood stem cells are highly sensitive to radiotherapy, this treatment can cause secondary bone marrow capacity reduction. Similarly, surgical interventions can be associated with the risk of excessive bleeding [ 21 ]. Furthermore, there is a significant risk of recurrence (19–37%) in patients undergoing surgery or radiotherapy for EMH [ 5 , 8 ]. Thus, it seems that a more appropriate method for dealing with EMH is to utilize a combination therapy comprised of—for example—hydroxyurea therapy or EPO treatment with radiotherapy ( 22 ). In any case, there is a dire need for a gold standard for the treatment of EMH in order to reduce the burden of this condition. It might be also helpful to develop a diagnostic method or a score system for identifying patients with a high risk of EMH.
Data availability
The data and materials were taken from medical records and archives.
Oda A, Tezuka T, Ueno Y, Hosoda S, Amemiya Y, Notsu C et al (2018) Niche-induced extramedullary hematopoiesis in the spleen is regulated by the transcription factor Tlx1. Sci Rep 8(1):1–16
Article Google Scholar
Kim CH (2010) Homeostatic and pathogenic extramedullary hematopoiesis. Journal of blood medicine 1:13
Malla S, Razik A, Das C, Naranje P, Kandasamy D, Kumar R (2020) Marrow outside marrow: imaging of extramedullary haematopoiesis. Clin Radiol 75(8):565–578
Article CAS Google Scholar
Tabesh H, Shekarchizadeh A, Mahzouni P, Mokhtari M, Abrishamkar S, Abbasi FS (2011) An intracranial extramedullary hematopoiesis in a 34-year-old man with beta thalassemia: a case report. J Med Case Reports 5(1):1–4
Yathiraj PH, Singh A, Vidyasagar S, Varma M, Mamidipudi V (2016) Excellent and durable response to radiotherapy in a rare case of spinal cord compression due to extra-medullary hematopoiesis in β-thalassemia intermedia: case report and clinicoradiological correlation. Annals of palliative medicine 6(2):195–199
Keikhaei B, Zandian K, Rahim F (2008) Existence of cord compression in extramedullary hematopoiesis due to beta thalassemia intermedia. Hematology 13(3):183–186
Lanigan A, Fordham MT (2017) Temporal bone extramedullary hematopoiesis as a causeof pediatric bilateral conductive hearing loss: case report and review of the literature. Int J Pediatr Otorhinolaryngol 97:135–138
Abdulla MA, Yassin MA, Abdelrazek M, Mudawi D, Ibrahim F, Soliman DS et al (2018) A persistent cough as atypical clinical presentation of intrathoracic extramedullary hematopoiesis (EMH) in a female with thalassemia intermedia. Acta Bio Medica: Atenei Parmensis 89(Suppl 2):41
Google Scholar
Yang X, Chen D, Long H, Zhu B (2020) The mechanisms of pathological extramedullary hematopoiesis in diseases. Cell Mol Life Sci 77(14):2723–2738
Schmidt K, Zhang Q, Tasdogan A, Petzold A, Dahl A, Arneth BM et al (2018) The H3K4 methyltransferase Setd1b is essential for hematopoietic stem and progenitor cell homeostasis in mice. Elife 7:e27157
Otsuka H, Endo Y, Ohtsu H, Inoue S, Noguchi S, Nakamura M et al (2021) Histidine decarboxylase deficiency inhibits NBP-induced extramedullary hematopoiesis by modifying bone marrow and spleen microenvironments. Int J Hematol 113(3):348–361
Kowsaryan M, Zafari M (2016) Which pamidronate protocol is the best for treating osteoporosis in beta-thalassemia major? Ann Hematol 95(3):383–386
Forni GL, Perrotta S, Giusti A, Quarta G, Pitrolo L, Cappellini MD et al (2012) Neridronate improves bone mineral density and reduces back pain in β-thalassaemia patients with osteoporosis: results from a phase 2, randomized, parallel-arm, open-label study. Br J Haematol 158(2):274–282
Otsuka H, Endo Y, Ohtsu H, Inoue S, Kuraoka M, Koh M et al (2021) Changes in histidine decarboxylase expression influence extramedullary hematopoiesis in postnatal mice. Anat Rec 304(5):1136–1150
Horváth Z, Pállinger É, Horváth G, Jelinek I, Veszely G, Fűrész J et al (2010) Extramedullary hematopoiesis is dysregulated in histamine-free histidine decarboxylase knockout (HDC−/−) mice. Inflamm Res 59(6):429–436
Ricchi P, Meloni A, Grigoratos C, Toia P, Fina P, Pistoia L et al (2019) Prevalence of extramedullary hematopoiesis, renal cysts, splenic and hepatic lesions, and vertebral hemangiomas among thalassemic patients: a retrospective study from the Myocardial Iron Overload in Thalassemia (MIOT) network. Ann Hematol 98(6):1333–1339
Kessinger A, Bishop M, Jackson J, O’Kane-Murphy B, Vose J, Bierman P et al (1995) Erythropoietin for mobilization of circulating progenitor cells in patients with previously treated relapsed malignancies. Exp Hematol 23(7):609–612
CAS PubMed Google Scholar
Huang Y, Liu R, Wei X, Liu J, Pan L, Yang G, et al. Erythropoiesis and iron homeostasis in non-transfusion-dependent thalassemia patients with extramedullary hematopoiesis. BioMed research international. 2019;2019
Ricchi P, Ammirabile M, Costantini S, Di Matola T, Verna R, Diano A et al (2012) A useful relationship between the presence of extramedullary erythropoeisis and the level of the soluble form of the transferrin receptor in a large cohort of adult patients with thalassemia intermedia: a prospective study. Ann Hematol 91(6):905–909
Hisamud-Din N, Mustafah NM, Fauzi AA, Hashim NM (2017) Incomplete paraplegia caused by extramedullary hematopoiesis in a patient with thalassemia intermedia. Spinal Cord Series and Cases 3(1):1–3
Musallam KM, Taher AT, Rachmilewitz EA (2012) β-thalassemia intermedia: a clinical perspective. Cold Spring Harb Perspect Med 2(7):a013482
Goerner M, Gerull S, Schaefer E, Just M, Sure M, Hirnle P (2008) Painful spinal cord compression as a complication of extramedullary hematopoiesis associated with β-thalassemia intermedia. Strahlenther Onkol 184(4):224–226
Download references
Acknowledgements
The authors thank their colleagues in Shafa Hospital for their collaboration.
Author information
Authors and affiliations.
Thalassemia & Hemoglobinopathy Research Center, Health Research Institute, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
Bijan Keikhaei, Daryush Purrahman, Batool Marashi & Mohammad Reza Mahmoudian-Sani
Department of Emergency Medicine, Faculty of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
Meisam Moezi
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Mohammad Reza Mahmoudian-Sani .
Ethics declarations
Ethics approval.
Written informed consent was obtained from the patient and her parents for the publication of this case report as well as any accompanying images. A copy of the written consent is available for review by the editor-in-chief of this journal.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Keikhaei, B., Purrahman, D., Marashi, B. et al. Extramedullary hematopoiesis in β-thalassemia major patient: a case report and review of the literature. J Hematopathol 15 , 185–190 (2022). https://doi.org/10.1007/s12308-022-00506-7
Download citation
Received : 14 March 2022
Accepted : 11 July 2022
Published : 15 July 2022
Issue Date : September 2022
DOI : https://doi.org/10.1007/s12308-022-00506-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Extramedullary hematopoiesis
- β-Thalassemia major
- Sinus cavity
Advertisement
- Find a journal
- Publish with us
- Track your research
CLINICAL CASE 1: USE OF LUSPATERCEPT
Clinical case 2: autologous genetic therapies, clinical case 3: targeting abnormal iron metabolism, clinical case 4: fetal hemoglobin induction and other mechanisms, conflict-of-interest disclosure, off-label drug use, β-thalassemia: evolving treatment options beyond transfusion and iron chelation.
- Split-Screen
- Request Permissions
- Cite Icon Cite
- Search Site
- Open the PDF for in another window
Arielle L. Langer , Erica B. Esrick; β-Thalassemia: evolving treatment options beyond transfusion and iron chelation. Hematology Am Soc Hematol Educ Program 2021; 2021 (1): 600–606. doi: https://doi.org/10.1182/hematology.2021000313
Download citation file:
- Ris (Zotero)
- Reference Manager
Visual Abstract

After years of reliance on transfusion alone to address anemia and suppress ineffective erythropoiesis in β-thalassemia, many new therapies are now in development. Luspatercept, a transforming growth factor–β inhibitor, has demonstrated efficacy in reducing ineffective erythropoiesis, improving anemia, and possibly reducing iron loading. However, many patients do not respond to luspatercept, so additional therapeutics are needed. Several medications in development aim to induce hemoglobin F (HbF): sirolimus, benserazide, and IMR-687 (a phosphodiesterase 9 inhibitor). Another group of agents seeks to ameliorate ineffective erythropoiesis and improve anemia by targeting abnormal iron metabolism in thalassemia: apotransferrin, VIT-2763 (a ferroportin inhibitor), PTG-300 (a hepcidin mimetic), and an erythroferrone antibody in early development. Mitapivat, a pyruvate kinase activator, represents a unique mechanism to mitigate ineffective erythropoiesis. Genetically modified autologous hematopoietic stem cell transplantation offers the potential for lifelong transfusion independence. Through a gene addition approach, lentiviral vectors have been used to introduce a β-globin gene into autologous hematopoietic stem cells. One such product, betibeglogene autotemcel (beti-cel), has reached phase 3 trials with promising results. In addition, 2 gene editing techniques (CRISPR-Cas9 and zinc-finger nucleases) are under investigation as a means to silence BCL11A to induce HbF with agents designated CTX001 and ST-400, respectively. Results from the many clinical trials for these agents will yield results in the next few years, which may end the era of relying on transfusion alone as the mainstay of thalassemia therapy.
Understand the strengths and limitations of luspatercept for the treatment of TDT and NTDT
Understand the potential benefits and toxicity of genetically modified autologous HSCT for TDT
Describe therapies under development for the treatment of TDT and NTDT
After many years without novel disease-modifying therapeutics, numerous agents are now in development for β-thalassemia. We review therapies that have been recently approved or are in development for transfusion-dependent thalassemia (TDT) and non-transfusion-dependent thalassemia (NTDT) β-thalassemia using 4 patient cases ( Table 1 ).
Current limitations of thalassemia care
A 54-year-old woman with congestive heart failure, paroxysmal atrial fibrillation on warfarin, and pulmonary hypertension due to β-thalassemia intermedia has a hemoglobin ranging from 7.5 to 8.5 g/dL without packed red blood cell (RBC) transfusion. Transfusion to maintain hemoglobin nadir of approximately 9.5 g/dL is indicated because of end-organ damage. However, she has been unable to tolerate the transfusion volumes, as attempts at transfusion frequently trigger a heart failure exacerbation and acute worsening of her pulmonary pressures. She is started on luspatercept, which leads to a rise in hemoglobin to 9.2 to 10.1 g/dL with drops below this range only in the setting of acute illness.
Luspatercept is a recombinant fusion protein that blocks transforming growth factor–β (TGF-β) superfamily ligands and promotes late-stage erythroid maturation, resulting in effective reduction in transfusion requirements in some patients with TDT. 1 In a randomized, placebo-controlled trial, 21.4% of patients met the primary end point of at least a 33% reduction in transfusion volume during weeks 13 through 24 compared with 4.5% receiving placebo ( P < .001). This equated to a least mean square difference in transfusion burden of 1.35 fewer RBC transfusions in weeks 13 through 24 in the TDT population. Follow-up data show transfusion reduction continued at 48 weeks and up to 4.8 years into treatment. 2 , 3 Common side effects included headache, myalgia, and bone pain. Although rare, 8 of 223 (3.6%) patients in the luspatercept arm had thromboembolism compared with 1 of 109 patients in the placebo arm ( Table 2 ). 1
Luspatercept considerations 1-6
In the NTDT population, data from a phase 1/2 nonrandomized trial showed an increase in hemoglobin of at least 1.5 g/dL in 58% of patients receiving higher dose levels. 4 A randomized, placebo-controlled trial for luspatercept in patients with NTDT has finished accrual, and preliminary results showed hemoglobin of at least 1.5 g/dL in 52.1% in the luspatercept arm compared with 0% in the placebo arm and no thromboembolic events in either arm (NCT03342404). 5
Case 1 demonstrates several strengths and limitations of luspatercept. This medication is most likely to be useful in patients for whom a moderate increase in hemoglobin or a moderate reduction in transfusion volume would be impactful. This may be the case because of difficulty with volume status, as was the case in this patient; for patients with alloimmunization for whom obtaining RBC units is more difficult; or for patients whose quality of life would improve by spending less time at transfusion appointments. The risk of thromboembolism should be considered when initiating this medication; although low in clinical trials, the incidence of thrombosis may compound other risk factors, such as prior splenectomy, and this may affect the risk-benefit ratio of initiating luspatercept. In case 1, the patient was already on lifelong anticoagulation because of atrial fibrillation, so this risk was mitigated. Finally, published data primarily address the TDT population; forthcoming results will help clarify how best to tailor the use of luspatercept for patients with NTDT.
Further follow-up will be required to determine if luspatercept may improve iron overload, although preliminary results are promising. 6 Early findings that ferritin and liver iron concentration (LIC) improved in both those who met the primary end point and those who did not suggest that reduced iron loading from the gut is likely playing a role, rather than just reduced transfusion burden. If this bears out, it would represent a significant additional benefit, particularly for individuals in poor iron balance. Although luspatercept represents an exciting new therapeutic option and is the first novel approved therapy in many years, caution is merited in setting expectations, as most patients will not be able to forgo transfusion, and increasing the interval between transfusions is often not practically feasible or appealing since visits every 3 weeks are required for this subcutaneous medication. As such, a great unmet need remains.
A 17-year-old boy with TDT on a stable transfusion regimen and in good iron balance wishes to consider curative intent therapy. He is considering his goals for adulthood, including attending college that is not near a large medical center, and is interested in avoiding frequent medical and transfusion appointments. He has no available donor for allogeneic stem cell transplantation. He opts to enroll in a clinical trial of genetically modified autologous hematopoietic stem cell transplantation (HSCT) using a lentivirus vector.
Patients may desire a curative intent therapy given the long-term impact on morbidity, mortality, and quality of life associated with chronic transfusion therapy. Until recently, the only curative option was allogeneic HSCT. Allogeneic HSCT has provided a cure to many patients with TDT, with the best outcomes reported for matched sibling donors and recipients who underwent transplantation in teenage years. 7 However, the risk of acute and chronic complications and the lack of available donors have limited its use.
Ex vivo modification of CD34+ hematopoietic stem cells using different techniques has opened the possibility of autologous HSCT as a curative therapy, thereby eliminating the need for a donor as well as the risks specific to allogeneic HSCT, such as graft-versus-host disease. In this context, genetically modified autologous HSCT is an area of active research with significant advancements in the past few years.
One ex vivo gene therapy, betibeglogene autotemcel (beti-cel; formerly LentiGlobin), uses a lentiviral vector to add a modified β-globin gene into CD34+ cells for infusion by autologous HSCT after myeloablative conditioning. In phase 1/2 trials, beti-cel treatment led to transfusion independence (TI) in 12 of 13 non-β 0 /β 0 genotype and 3 of 9 β 0 /β 0 genotype patients with TDT, or 68% overall. 8 The remaining patients had a reduction in transfusion volume. Toxicity was primarily related to busulfan used for HSCT conditioning. The original beti-cel trial established the potential for genetically modified autologous HSCT, although most patients with the β 0 /β 0 genotype did not achieve TI. Preliminary data from 2 phase 3 trials of beti-cel for β 0 /β 0 (NCT03207009) and non-β 0 /β 0 (NCT02906202) patients have demonstrated TI at >12 months of follow-up in 88% of 34 evaluable patients, including 24 of 27 non-β 0 /β 0 and 6 of 7 β 0 /β 0 patients, with no additional toxicities noted. 9 Preliminary reports of long-term follow up of 44 patients with >2 years of follow-up after beti-cel treatment (23-76 months) showed a durable hemoglobin response, reduction in iron burden, and favorable safety profile. 10 Initial evaluation of 27 pediatric patients treated with beti-cel, 16 of whom were under 12 years old, showed a comparable TI rate of 84.6% ( Table 3 ). 11
Gene therapy trials for β-thalassemia
Another phase 1/2 trial uses a lentiviral vector (the GLOBE vector) to add a β-globin gene to autologous hematopoietic stem cells, with intrabone administration of the transduced cells. Three of 4 evaluable pediatric patients achieved TI, whereas 3 adult patients had a reduced transfusion burden without achieving TI. 12 At present, a subsequent trial is not registered.
In contrast to the gene addition approach, another strategy for autologous genetic therapies for β-hemoglobinopathies is to decrease BCL11A expression in order to induce expression of γ-globin and hemoglobin F (HbF). The first trial targeting BCL11A uses a lentiviral vector, BCH-BB694, to introduce short hairpin RNA to decrease BCL11A expression. 13 Six patients with sickle cell disease who received BCH-BB694 demonstrated safety and feasibility and had substantial induction of HbF. This vector has not been evaluated in patients with TDT. Trials are currently open for patients with thalassemia, in whom a gene editing technique, either CRISPR-Cas9 or zinc-finger nuclease (ZFN), is employed to disrupt an erythroid enhancer of BCL11A. CTX001 is a CRISPR-Cas9–modified autologous HSCT product being investigated in TDT as well as sickle cell disease (NCT03655678). Preliminary results from the first 10 patients treated with CTX001 for TDT showed substantial HbF induction, broad distribution of HbF, and achievement of TI in all patients. 14 , 15 Toxicity was related to busulfan conditioning. A phase 1/2 study of ST-400, an autologous HSCT product in which the BCL11A enhancer is disrupted by a ZFN, is currently under way (NCT03432364), but only very preliminary data on the first 2 patients have been reported. 16
Case 2 demonstrates the clinical demand for curative intent therapy and its potential impact on quality of life as well as health. Although genetically modified autologous HSCT may offer a more feasible and less toxic option for curative intent therapy compared with allogeneic HSCT, limitations are likely to remain. HSCT of any kind is a costly therapy and not likely to be available in low-resource settings. In addition to monetary costs, undergoing autologous HSCT requires a significant time commitment and interruption of other life events. The potential for reduced fertility or infertility due to exposure to myeloablative alkylator conditioning is an important consideration for many patients. Finally, 2 cases of acute myeloid leukemia (AML) in patients with sickle cell disease treated with beti-cel have raised concerns. 17 , 18 Evaluation suggests that neither case was caused by insertional mutagenesis. To date, no patients with thalassemia have developed myelodysplastic syndrome or AML after genetically modified autologous HSCT, but additional data will be necessary before final conclusions may be drawn about what factors influence malignancy risk.
A 34-year-old man with NTDT is concerned about ongoing iron overload. His hemoglobin averages 8.4 g/dL, and he receives transfusion a few times per year. His LIC is estimated at 8 mg per gram of dry weight on magnetic resonance imaging. In addition to intensifying his iron chelation, he inquires whether there are any therapies that may raise his hemoglobin or reduce his iron absorption. He is open to participation in clinical trials.
Currently, there are no established therapies to address this patient's anemia. However, several medications in development may improve anemia in patients with NTDT that also target iron metabolism. Apotransferrin has been shown to upregulate hepcidin and downregulate transferrin receptor 1. 19 , 20 This leads to decreased iron absorption from the gut, 19 as well as potentially less cardiac iron loading. 21 The correction of pathologic iron metabolism led to more effective erythropoiesis in mouse models. 20 These preclinical findings have spurred a phase 2 trial of intravenous apotransferrin every 2 weeks in patients with β-thalassemia intermedia that seeks to raise hemoglobin and reduce transfusion requirements (NCT03993613).
Similarly, a ferroportin inhibitor (VIT-2763) has begun a phase 2 trial (NCT04364269) after demonstrating improvement in anemia and dysregulated iron metabolism in a mouse model of thalassemia. 22 Like the apotransferrin trial, the VIT-2753 is focused on correction of anemia but includes secondary outcomes measures of iron overloading. Notably, VIT-2753 is administered orally. In considering treatment for patients with NTDT, this is particularly impactful for quality of life, as many of these patients do not otherwise have recurring infusion center appointments ( Table 4 ).
Novel medications for β-thalassemia
EMA, European Medicines Agency; FDA, Food and Drug Administration; JAK, Janus-associated kinase; MDS, myelodysplastic syndrome; mTOR, mechanistic target of rapamycin.
Hepcidin itself has also been a target for drug development, because increasing hepcidin has been shown to both reduce iron absorption and ameliorate ineffective erythropoiesis. 23 A hepcidin mimetic, PTG-300, has completed accrual for a phase 2 study, including both NTDT and TDT arms (NCT03802201). A recent mouse study of an erythroferrone antibody showing improved anemia as well as iron homeostasis provides another potential therapeutic target in the iron regulation pathway. 24
Case 3 emphasizes the large unmet need of therapies for NTDT and the appeal of a therapy that may also address nontransfusional hemosiderosis in these patients. These trials not only highlight the role of altered iron metabolism in ineffective erythropoiesis but also provide several promising therapeutic targets for ameliorating anemia.
A 21-year-old woman with TDT and in good iron balance recently tried luspatercept with only a minimal reduction in transfusion burden. She inquires if there are other therapies that might reduce her transfusion requirement. She is not interested in allogeneic or genetically modified autologous HSCT, as she is worried about the upfront risk and disruption in her life, because she is doing quite well overall on her current transfusion and chelation regimen.
Several agents currently in clinical trials could provide good options for this patient. Sirolimus, the mechanistic target of rapamycin inhibitor, is being investigated as a method of increasing HbF expression. Sirolimus appears to upregulate the expression of HbF in erythroid cell cultures derived from patients with β-thalassemia, as well as sickle cell patients, and may increase clearance of α-globin in RBC precursors. 25-28 The latter finding may have the potential to reduce ineffective erythropoiesis separate from HbF induction. In this context, 2 ongoing phase 2 trials of sirolimus in patients with TDT (NCT03877809, NCT04247750) will track markers of ineffective erythropoiesis in addition to the primary end point of HbF induction.
Another pathway for HbF induction may be phosphodiesterase 9 inhibition with IMR-687. 29 , 30 A phase 2 trial of IMR-687 for both patients with TDT and NTDT is currently under way (NCT04411082). Benserazide, which is currently used in combination with levodopa for the treatment of Parkinson disease, showed promising induction of HbF, although the study was not of thalassemia models, 31 but it did not induce HbF in patients on this medication for Parkinson disease. 32 A phase 1 trial of benserazide in patients with NTDT is ongoing (NCT04432623).
Mitapivat (formerly AG-348), an allosteric activator RBC-specific pyruvate kinase, represents a distinct and novel mechanism. RBC-specific pyruvate kinase activation increases adenosine triphosphate synthesis, as well as reduces the production of reactive oxygen species and concentration of 2,3-diphosphoglycerate. 33 Preliminary results of a phase 2 trial of mitapivat in α and β NTDT showed a response, defined as an increase in hemoglobin of at least 1 g/dL in 16 of 20 patients, including all 5 patients with α- thalassemia. 34 Phase 3 studies of mitapivat in TDT (NCT04770779) and NTDT (NCT04770753) patients are under way. 35 Like the phase 2 trial, these trials include patients with α-thalassemia, a patient population that is underserved but outside the scope of this review.
Case 4 is a reminder that even in well-managed patients, there is significant room for improvement in care. All 4 patients presented highlight the nuanced needs of patients with thalassemia and the importance of tailoring therapeutic choices to the individual as new treatments emerge. Although the medications and interventions reviewed here are grounded in strong preclinical research on thalassemia, the results of these trials are needed to understand which agents will be clinically impactful and which agents will not be fruitful for patient care. If early studies are borne out, it is likely that both patients with TDT and NTDT will have several options beyond transfusion and iron chelation in the coming years.
Arielle L. Langer: no competing financial interests to declare.
Erica B. Esrick: steering committee (consulting) for bluebird bio and research funding to institution from Celgene (site for luspatercept trial).
Arielle L. Langer: nothing to disclose. The majority of the drugs discussed are not yet approved.
Erica B. Esrick: nothing to disclose. The majority of the drugs discussed are not yet approved.
- Previous Article
- Next Article
Email alerts
Affiliations, american society of hematology.
- 2021 L Street NW, Suite 900
- Washington, DC 20036
- TEL +1 202-776-0544
- FAX +1 202-776-0545
ASH Publications
- Blood Advances
- Blood Neoplasia
- Blood Vessels, Thrombosis & Hemostasis
- Hematology, ASH Education Program
- ASH Clinical News
- The Hematologist
- Publications
- Privacy Policy
- Cookie Policy
- Terms of Use
This Feature Is Available To Subscribers Only
Sign In or Create an Account
- Case Report
- Open access
- Published: 09 March 2023
Myocardial infarction in patients with severe beta thalassaemia: a case series
- Anuja Premawardhena 1 ,
- Shamila De Silva 1 ,
- Megha Rajapaksha 2 ,
- Vishaka Ratnamalala 3 ,
- Jemimah Nallarajah 4 &
- Gamini Galappatthy 4
International Journal of Emergency Medicine volume 16 , Article number: 16 ( 2023 ) Cite this article
1684 Accesses
1 Citations
Metrics details
Cardiac disease remains a dominant if not the most important cause of morbidity and mortality in patients with thalassaemia, particularly in those with thalassaemia major. Myocardial infarction and coronary artery disease however are rarely reported.

Case presentations
Three older patients with three distinct thalassaemia syndromes presented with acute coronary syndrome. Two were heavily transfused whilst the other was a minimally transfused patient. Both heavily transfused patients had ST-elevation myocardial infarctions (STEMI) while the minimally transfused patient had unstable angina. Coronary angiogram (CA) was normal in two patients. One patient who developed a STEMI had a 50% plaque. All three were managed as standard ACS, although the aetiology appeared non-atherogenic.
Conclusions
The exact etiology of the presentation, remains a mystery and therefore the rational use of thrombolytic therapy, carrying out angiogram in the primary setting, using and continuing antiplatelet and high dose statins all remains unclear in this sub group of patients.
Cardiac disease due to iron overload is the commonest cause of death in patients with thalassaemia major, accounting for almost 70% of deaths in some series [ 1 , 2 ]. Cardiac iron overload related deaths may be less common in patients with non-transfusion dependent thalassaemia (NTDT), including in patients with haemoglobin E-beta thalassaemia where malignancies and infections seem to be the dominant causes for mortality [ 3 , 4 , 5 ]. Pulmonary hypertension that develops due to a multitude of reasons is also recognized as an important contributor to cardiac related mortality, particularly in older splenectomised patients with thalassaemia intermedia [ 6 ].
Myocardial infarction has only rarely been reported in patients with thalassaemia. There is an observation that those with beta thalassaemia trait may be protected against developing coronary artery disease (CAD), and it is well known that patients with thalassaemia tend to have hypocholesterolemia with low LDL level [ 7 , 8 ]. However, there is also a suggestion that patients with thalassaemia, especially those with the intermediate form, have a higher chance of developing premature atherosclerosis and vascular abnormalities resembling pseudo- xanthoma elasticum [ 9 , 10 ]. Many studies have demonstrated a prothrombotic state in patients with thalassaemia [ 11 , 12 , 13 ].
The first documented myocardial infarction in a person with thalassaemia was in a patient with thalassaemia major in 2004. The patient was thrombolysed with streptokinase for a ST-elevation myocardial infarction (STEMI) but there were no plaques on a coronary angiogram carried out a month later. The authors suggested that thromboembolism or vasospasm may have been causative [ 14 ]. A patient with thalassaemia intermedia reported with a STEMI in 2008 however had evidence of two coronary plaques, one of which caused a 90% obstruction in the left anterior descending (LAD) artery. He underwent primary percutaneous intervention (PCI). [ 15 ].
We were unable to find any further reports of acute coronary syndrome (ACS) in persons with thalassemia syndromes in the literature. Currently, there is no clear idea on pathogenesis or the best strategy for management of such persons in the emergency care setting.
It is in this backdrop that we encountered three coronary events over a period of four months in adult patients with thalassaemia receiving treatment at a single center in Sri Lanka.
A 34-year-old woman with haemoglobin E-beta thalassaemia (IVS1-5(G-C)/Hb E; α- 4.2 /αα) presented to a hospital in Southern Sri Lanka with epigastric pain and severe central chest pain lasting for more than 18 hours. She complained of radiating pain to the neckleft arm pain and difficulty in breathing. She was diagnosed to have an anterior STEMI based on ECGs and was treated with aspirin 300 mg, clopidogrel 300 mg, atorvastatin 40 mg and enoxaparin 40 mg sub-cutaneously on admission. She had not been thrombolysed due to the late presentation. High sensitivity cardiac troponin was 136.6 ng/dL on admission and rose to 1036 ng/dL within six hours. She had an uneventful recovery after a 6-day hospital stay. Results of further investigations are in Table 1 .
Echocardiogram at discharge showed an ejection fraction of 60%. Coronary angiogram done a week later showed a normal main coronary, a 50% occlusion of the proximal LAD, and mild disease in the mid vessel. The right coronary artery was normal.
The patient was diagnosed with thalassemia at seven years of age, when she presented with a haemoglobin of 6.2 g/dL. She had been managed at three different transfusion centers across the country during different stages of her life, but throughout she had been transfused either monthly or once in 2–3 months. Mean pre-transfusion haemoglobin was 7.8 g/dL.
Splenectomy was performed at the age of 22 years. Mean platelet count post-splenectomy was 550,000/mm 3 . The highest recorded serum ferritin value was 8500 ng/ml in 2016 and the most recent was 1130 ng/ml in May 2022. Cardiac and liver T2 * MRI had been done once in 2017 which read 9.2 ms and 10.7 ms respectively. She was on hormone replacement therapy for hypogonadism, thyroxine for hypothyroidism, and calcium and vitamin D3 replacement for hypoparathyroidism. She was also on treatment for diabetes mellitus for the past five years with premixed insulin and had variable glycaemic control. Her BMI was 21.8 kg/m 2 . At the time of admission, she was on dual chelators with oral deferasirox 30 mg/kg/day and desferrioxamine 30 mg/kg/day subcutaneously, a regime she was following for the previous six months. Her pre-event echocardiogram was normal with an ejection fraction of 60% and no evidence of pulmonary hypertension.
She was vaccinated against COVID-19 with two doses of the Sinopharm vaccine, with the second dose given two months before the acute coronary event. She developed a mild COVID-19 infection in October 2021 which was managed at home without any complications.
A 44-year-old woman with beta thalassaemia intermedia diagnosed at 24 years (genotype IVS1-5(G-C))/ β;ααα/ααα] presented to the emergency department with severe bilateral submammary pain lasting for more than one hour, associated sweating, an episode of fainting and vomiting. ECG on admission showed T inversion in LII, LIII, aVf, V3, and V4. Subsequent ECGs showed dynamic changes spreading to V5. Troponin titers on admission and six hours later remained un-elevated. She was treated for unstable angina with four doses of enoxaparin subcutaneously. She received one unit of blood transfusion while in hospital. Investigations showed elevated liver enzymes which gradually reduced over the hospital stay of three days. Results of further investigations are shown in Table 1 .
The patient had a mild form of beta thalassaemia intermedia and attended clinic only occasionally. Examination did not show a clinically palpable spleen. She had been transfused only 10 units of blood during her lifetime, with the last transfusion in 2011. Almost all the blood transfusions had been given during her pregnancy. Her mean haemoglobin level was 7.5 g/dL and mean platelet count was 250–300,000/μL. She had no previous history of chest pain, shortness of breath or symptoms suggestive of angina. Serum ferritin level was 644 ng/dL in 2019 at her last clinic visit. She had no major complications of thalassemia of note and had never been on iron chelators.
She had been given two doses of the Sinopharm COVID-19 vaccine, the second dose in July 2021. She has a mild upper respiratory infection in February 2022 but did not test for COVID-19.
A 21-year-old male patient with beta thalassaemia major presented to the emergency department with severe retrosternal chest pain and shortness of breath of three hours duration. He had associated symptoms of sweating and palpitations. ECG on admission showed striking ST elevations in the inferior and lateral leads. Troponin titer was highly positive at 236 ng/dL. He was thrombolysed with tenecteplase in the emergency treatment unit, followed by subcutaneous enoxaparin. The chest pain reduced but ECG changes did not show resolution and he was transferred for urgent per-cutaneous intervention (PCI). At the coronary care unit, a coronary angiogram showed normal coronary vasculature. Echocardiogram showed global hypokinesia with an ejection fraction < 30%. The patient developed repeated episodes of ventricular tachycardia refractory to treatment and succumbed during one episode.
The patient was on regular transfusions from the time of diagnosis at 6 months of age. Mean pre-transfusion haemoglobin was 9.5 g/dl during the past year but he was not always well transfused. He had a BMI of 17.4 kg/m 2 . He had undergone splenectomy at the age of 9 years and the mean platelet count was 505,000/μL over the past four years. He had hypogonadism and puberty was induced at the age of 13 years. He had no other endocrine abnormalities. The last serum ferritin available was 5080 ng/ml and there was a rising trend. T2* cardiac MRI done in November 2021 was 5/ms confirming severe myocardial iron load. He was on dual chelators (deferasirox and desferrioxamine) but was poorly compliant with chelator use. Pre-event echocardiogram done six months ago was normal with an ejection fraction > 60% and no evidence of pulmonary hypertension.
He had two Sinopharm vaccines against COVID -19 in July and August of 2021, and there was no record of infection with COVID 19 at any point.
We present case histories of three older patients with three distinct thalassemia syndromes followed up at the same thalassaemia unit, who presented with acute coronary syndrome during a four-month period. Acute coronary syndrome is rare in patients with thalassemia, with literature discussing only a few cases. The coronary arterial pathology in the three patients in our series did not show significant atheromatous disease. The female with unstable angina and the 21-year-old man with STEMI who succumbed during the episode had normal coronary arteries. The 34-year-old woman who developed an anterior STEMI had only a 50%-occluding plaque in the left anterior descending (LAD) coronary artery. In a previous report of a STEMI in a patient with thalassemia major from Israel the authors were not able to identify any plaques on coronary angiography [ 14 ].
In our series the two patients who developed STEMI were heavily transfused although their genetic make-up was different, with one having beta thalassaemia major and the other having haemoglobin E-beta thalassaemia. The latter individual had a plaque albeit of moderate significance. It is likely that the pathogenesis of the cardiac events was due to thromboembolism. There is sufficient literature to support a pro-coagulant state in thalassaemia syndromes. Both patients had undergone splenectomy at a young age and had moderately elevated platelet counts. DRVVT and KCT were done to exclude acquired thrombophilic conditions but more extensive testing including testing for Factor V Leiden and protein C, protein S and anti-thrombin level were not done due to cost limitations. In the two previous case reports in the literature no significant association with inherited thrombophilc factors had been reported [ 14 , 15 ].
Based on the 4th Universal Definition of Myocardial Infarction (MI), in a patient if a demonstrable obstruction > 50% is absent in the coronary angiogram but other criteria fulfill a diagnosis of MI the term ‘myocardial infarction with non-obstructive coronary arteries’ (MINOCA) is used, an example of type 2 MI. In the two patients with definite evidence of myocardial infarction, in the absence of significant atheromatous disease a type 2 myocardial infarction could be postulated. Likewise, the aetiology of unstable angina in patient 2 may also be related to a similar pathology. However, the source of the thrombus or embolus if any could not be identified. Coronary arterial vasospasm has been postulated as a probable mechanism for causation of the myocardial infarction in one of the previous cases but no explanations as to why vasospasm should occur in patients in thalassemia could be found in the literature.
We were intrigued by the occurrence of three coronary events over a short period of time in a single unit and wondered if COVID-19 infection could have played a role in the pathogenesis. All three patients had received at least two vaccines for COVID-19 but had contracted mild infections subsequently. There was no evidence of acute COVID infection in any one of them at the time of the events.
Just as much as the aetiology remains unclear how should a similar event be managed in the future? The rational use of thrombolytic and anticoagulants in an acute event remains unclear as is the use of high doses of lipid lowering treatment in the follow-up of these patients. Its perhaps too early to discourage early angiography in thalassaemic patients with suspected ACS because the aetiology remains uncertain.
Availability of data and materials
Will be made available on request from the authors, including ECHO cardiograms.
Abbreviations
Acute coronary syndrome
Body mass index
Coronary angiogram
Coronary artery disease
Dilute Russel viper venom test
Electro cardiogram
Kaolin clotting time
Left anterior descending artery
Low density lipoproteins
Myocardial infarction
Magnetic resonance imaging
Non- transfusion dependent thalassaemia
Percutaneous coronary intervention
ST elevation myocardial infarction
Modell B, Khan M, Darlison M, et al. Improved survival of thalassaemia major in the UK and relation to T2* cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2008;10:42. https://doi.org/10.1186/1532-429X-10-42 .
Article PubMed PubMed Central Google Scholar
Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596–646.
PubMed PubMed Central Google Scholar
Musallam KM, Vitrano A, Meloni A, Pollina SA, Karimi M, El-Beshlawy A, Hajipour M, Di Marco V, Ansari SH, Filosa A, Ricchi P, Ceci A, Daar S, Vlachaki E, Singer ST, Naserullah ZA, Pepe A, Scondotto S, Dardanoni G, Bonifazi F, Sankaran VG, Vichinsky E, Taher AT, Maggio A. Survival and causes of death in 2,033 patients with non-transfusion-dependent β-thalassemia. Haematologica. 2021;106(9):2489–92. https://doi.org/10.3324/haematol.2021.278684.PMID:33882642;PMCID:PMC8409024 .
Vitrano A, Calvaruso G, Lai E, Colletta G, Quota A, Gerardi C, Concetta Rigoli L, Pitrolo L, Cuccia L, Gagliardotto F, Filosa A, Caruso V, Argento C, Campisi S, Rizzo M, Prossomariti L, Fidone C, Fustaneo M, Di Maggio R, Maggio A. The era of comparable life expectancy between thalassaemia major and intermedia: Is it time to revisit the major-intermedia dichotomy? Br J Haematol. 2017;176(1):124–30. https://doi.org/10.1111/bjh.14381 . (Epub 2016 Oct 17 PMID: 27748513).
Article CAS PubMed Google Scholar
Premawardhena AP, Ediriweera DS, Sabouhanian A, Allen A, Rees D, de Silva S, et al. Survival and complications in patients with haemoglobin E thalassaemia in Sri Lanka: a prospective, longitudinal cohort study. Lancet Glob Health. 2022;10(1):e134–41. https://doi.org/10.1016/S2214-109X(21)00446-0 . Epub 2021 Nov 26.
S, Lamabadusuriya SP, Weatherall DJ, Olivieri NF. Survival and complications in patients with haemoglobin E thalassaemia in Sri Lanka: a prospective, longitudinal cohort study. Lancet Glob Health. 2022 Jan;10(1):e134-e141. doi: https://doi.org/10.1016/S2214-109X(21)00446-0 . Epub 2021 Nov 26. PMID: 34843671; PMCID: PMC8672061.
Wood JC. Pulmonary hypertension in thalassemia: a call to action. Blood. 2022;139(13):1937–8. https://doi.org/10.1182/blood.2021015340.PMID:35357479;PMCID:PMC8972095 .
Article CAS PubMed PubMed Central Google Scholar
Gallerani M, Scapoli C, Cicognani I, Ricci A, Martinelli L, Cappato R, Manfredini R, Dall’Ara G, Faggioli M, Pareschi PL. Thalassaemia trait and myocardial infarction: low infarction incidence in male subjects confirmed. J Intern Med. 1991;230(2):109–11. https://doi.org/10.1111/j.1365-2796.1991.tb00416.x . (PMID: 1865160).
Haghpanah S, Davani M, Samadi B, Ashrafi A, Karimi M. Serum lipid profiles in patients with beta-thalassemia major and intermedia in southern Iran. J Res Med Sci. 2010;15(3):150–4 PMID: 21526074; PMCID: PMC3082801.
CAS PubMed PubMed Central Google Scholar
Hahalis G, Kalogeropoulos A, Terzis G, Tselepis AD, Kourakli A, Mylona P, Grapsas N, Alexopoulos D. Premature Atherosclerosis in Non-Transfusion-Dependent β-Thalassemia Intermedia. Cardiology. 2011. https://doi.org/10.1159/000327997,118,3,(159-163) .
Article PubMed Google Scholar
Farmakis D, Moyssakis I, Perakis A, Rombos Y, Deftereos S, Giakoumis A, Polymeropoulos E, Aessopos A. Unstable angina associated with coronary arterial calcification in a thalassemia intermedia patient with a pseudoxanthoma elasticum-like syndrome. Eur J Haematol. 2003;70(1):64–6 (PMID: 12631261).
Sirachainan N. Thalassemia and the hypercoagulable state. Thromb Res. 2013;132(6):637–41. https://doi.org/10.1016/j.thromres.2013.09.029 . (Epub 2013 Sep 27 PMID: 24125597).
Eldor A, Rachmilewitz EA. The hypercoagulable state in thalassemia. Blood. 2002;99(1):36–43. https://doi.org/10.1182/blood.v99.1.36 . (PMID: 11756150).
Fridlender ZG, Rund D. Myocardial infarction in a patient with beta-thalassemia major: first report. Am J Hematol. 2004;75(1):52–5. https://doi.org/10.1002/ajh.10454 . (PMID: 14695633).
El Rassi FA, Ismaeel HA, Koussa SC, Taher AT. Myocardial Infarction in a 28-Year-Old Thalassemia Intermedia Patient. Clin Appl Thromb Hemost. 2008. https://doi.org/10.1177/1076029608315166,15,4,(467-469) .
Download references
Self-funded.
Author information
Authors and affiliations.
Department of Medicine: Faculty of Medicine, University of Kelaniya, Kelaniya, Sri Lanka
Anuja Premawardhena & Shamila De Silva
Adolescent & Adult Thalassaemia Unit, North Colombo (Teaching) Hospital, Ragama, Sri Lanka
Megha Rajapaksha
Department of Haematology, National Hospital of Sri Lanka, Colombo, Sri Lanka
Vishaka Ratnamalala
Institute of Cardiology, National Hospital of Sri Lanka, Colombo, Sri Lanka
Jemimah Nallarajah & Gamini Galappatthy
You can also search for this author in PubMed Google Scholar
Contributions
AP is the principle investigator, and the clinician in charge of the thalassaemia unit, MR is the Nursing officer in charge of the thalassaemia Unit, JN and GG are the cardiologists who carried out work on patient 2 and 3 and VR is the Consultant Hematologist who carried out the thrombophilia work. S De S and AP weres involved in writing the primary manuscript and all authors participated in the subsequent editing of it. The author(s) read and approved the final manuscript.
Corresponding author
Correspondence to Anuja Premawardhena .
Ethics declarations
Ethics approval and consent to participate.
The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. No Ethical permission was deemed necessary as the patients are anonymized. The Ethics Review committee of University of Kelaniya exempts case reports from seeking ERC approval. Written consent is available from patient 1 and 2 and from the family members of patient 3.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Premawardhena, A., De Silva, S., Rajapaksha, M. et al. Myocardial infarction in patients with severe beta thalassaemia: a case series. Int J Emerg Med 16 , 16 (2023). https://doi.org/10.1186/s12245-023-00495-z
Download citation
Received : 10 December 2022
Accepted : 02 March 2023
Published : 09 March 2023
DOI : https://doi.org/10.1186/s12245-023-00495-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
International Journal of Emergency Medicine
ISSN: 1865-1380
- General enquiries: [email protected]
International Open Access, Peer-reviewed Platform
We provide a global open access platform for journals to promote the publication of high quality research in multiple fields of medicine and technology by promoting unrestricted access to our journal., doi (digital object identifier)- we provide doi to all published papers to facilitate higher citation and classification of articles. peertechz publisher id: 10.17352, quick links.
PTZ: We're glad you're here. Please click "create a new query" if you are a new visitor to our website and need further information from us.
If you are already a member of our network and need to keep track of any developments regarding a question you have already submitted, click "take me to my Query."
Peertechz Publications e-Library (Count-6627)
Search the metadata of 11155 journals, articles, special issues, proceedings, books, editors, editor-in-chief, executive editors and guest editors, reviewers database & more.

- Google Reviews 11
Member Information
Peer techz Publications Google Reviews
Each step from submission to publication was handled efficiently. The review comments were accurate which increased the value of our revised manuscript. I will certainly publish more works with Peertechz. More
cantrella caralynn
It was found that each step in the submission and publishing process was handled efficiently. The reviewers were clearly experts in the field and our revised manuscript was dealt with value. More
Samantha Wyatt
The entire review and production process for the article has been handled with remarkable professionalism and efficiency. As the editor, I can appreciate the enormous organizational effort and discipline shown. More

MUSHTAQ CHALKOO
Tomorrow is a search of today and if we research,we add an another day,that is how the time pulls on and on.Science keeps on changing its facies and thus paradigm and makes our living interesting and awesome.The knowledge of science needs to be dissipated to the progeny for better living ahead and the way we do that is through scientific journals published by different publication houses.Being associated with peertechz publications has been a wonderful experience.i have enjoyed the true spirit of scientific spice serving the organisation as an editor.It is author friendly,and makes the publication process for you very easy taking care not to jeopardise the standards of true science.I would suggest the research scholars and young scientists to associate with this publication house ,submit your reseach to brighten your career ahead in science.let us keep up the spirit of spreading the true science to the world ahead. More

Dr Shivaji Jadhav
Wonderful ..process and indeed very good way in terms of communication and i recommend for publishing with this publishers...Great support. More
Search in Journals, Articles and more
- Reference Manager
- Simple TEXT file
People also looked at
Case report article, case report: clinical and hematological characteristics of ε γδβ thalassemia in an italian patient.

- 1 Department of Pediatric Hematology/Oncology and Hematopoietic Stem Cell Transplantation (HSCT), Meyer Children's University Hospital, Florence, Italy
- 2 Department of Health Science, University of Florence, Florence, Italy
- 3 Human Genetics Laboratory, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Giannina Gaslini, Genoa, Italy
Introduction: ε γδβ thalassemia is a rare form of β-thalassemia mostly described in children originating from Northern Europe. Only anecdotic cases from the Mediterranean area are reported. The diagnosis is challenging, considering the rarity of the disease and its heterogeneous clinical presentation. Most patients have neonatal microcytic anemia, sometimes requiring in utero and/or neonatal transfusions, and typically improving with age.
Case Description: We report on an Italian newborn presenting with severe neonatal anemia that required red blood cell transfusion. After the first months of life, hemoglobin levels improved with residual very low mean corpuscular volume. β and α thalassemia, IRIDA syndrome, and sideroblastic anemia were excluded. Finally, a diagnosis of ε γδβ thalassemia was made after microarray analysis of single nucleotide polymorphisms revealed a 26 kb single copy loss of chromosome 11p15.4, including the HBD, HBBP1, HBG1, and HBB genes.
Conclusions: Despite its rarity, the diagnosis of ε γδβ thalassemia should be considered in newborns with severe neonatal anemia requiring in utero and/or neonatal transfusions, but also in older infants with microcytic anemia, after excluding more prevalent red blood cell disorders.
Introduction
The ε γδβ thalassemia is an extremely rare heterozygous form of β-thalassemia, with around 40 reported cases in 2019 ( 1 ). In most cases, patients originated from ethnic backgrounds where β-thalassemia was not prevalent ( Table 1 ). Despite the extreme heterogeneity of the molecular bases of β-thalassemia in Italy, the first ε γδβ thalassemia deletion has been only identified in 2016 ( 23 ).
Table 1 . Origin and presentation of previously described patients with ε γδβ thalassemia.
ε γδβ thalassemias are caused by long deletions in the β-globin cluster and exist only in heterozygous form. Except for one case ( 8 , 27 ), the reported deletions are almost exclusively unique and in most cases de novo , explaining the phenotypic heterogeneity of the disease. Indeed, multiple clinical phenotypes of ε γδβ thalassemia have been reported, ranging from normal blood cell count to severe anemia requiring in utero and/or neonatal transfusions ( Table 1 ) ( 20 ). The underlying reasons for such a spectrum of clinical characteristics are unknown, but the type and length of the deletion are not responsible, as contrasting phenotypes have been reported in heterozygotes with identical deletions within the same family ( 8 ). At the molecular level ε γδβ thalassemias fall into two distinct categories: in group I all, or a greater part of the β-globin cluster, are removed, including the β-globin gene, whereas in group II extensive upstream regions are removed, leaving the β-globin gene itself intact although its expression is silenced because of inactivation of the upstream β-locus control region ( 23 ). Furthermore, co-existent α-globin gene triplication has been suggested to exacerbate the phenotype of ε γδβ thalassemia increasing the imbalance between the α and non-α globin chain ratio during fetal life ( 16 ).
Most patients with ε γδβ -thalassemia had neonatal erythroblastosis, reticulocytosis, hypochromia, and microcytosis ( Table 1 ), that later improved with age. Anemia usually remitted spontaneously during the first months of life, and the adult phenotype is similar to that of the β-thalassemia trait, but with more severe microcytosis ( 13 ).
Herein, we describe the clinical phenotype of a novel Italian ε γδβ deletion, the second patient from Italy described in the literature and the third from the Mediterranean Area, presenting with severe microcytic anemia in the neonatal period.
Case Description
A male, full-term infant of Tuscanian origin was born by induced vaginal delivery due to meconium-stained amniotic fluid. He presented with clinical and laboratory signs of sepsis (increased white blood cell count, C-reactive protein, and indirect bilirubin) and received wide spectrum antibiotics. Laboratory evaluations revealed microcytic anemia (hemoglobin, Hb, 10.8 g/dL, mean corpuscular volume, MCV, 65.4 fL). The clinical condition rapidly improved and hemoglobin rose to 12 g/dL, with persistent microcytemia. At the first follow-up visit at 1 month of age, hemoglobin had dropped to 6.4 g/dL, with a MCV of 53.8 fL, a mean cell hemoglobin concentration of 18.3 g/dL, a hematocrit of 19.9%, and an increased reticulocyte count (0.2 × 10 6 /L) ( Figure 1 ). No other signs of hemolysis were detected (normal bilirubin and lactate dehydrogenase levels). The peripheral blood smear revealed microcytic hypochromic erythrocytes with anisopoikilocytosis ( Figure 2 ). Hemoglobin electrophoresis showed a normal pattern with an unusually high proportion of HbA (HbF 47%, HbA2 0.8%, HbA 52.2%), no abnormal hemoglobin variants, nor evidence of β-thalassemia; abdominal ultrasound showed splenomegaly. The patient received a red blood cell transfusion and supplementation of iron and folic acid, which proved ineffective. Therefore, bone marrow aspiration was performed to exclude the presence of ring sideroblasts with Prussian blue staining ( Figure 2 ); normal plasmatic hepcidin values ruled out an Iron-Refractory Iron Deficiency Anemia (IRIDA) syndrome.
Figure 1 . Timeline graph showing the chronological evolution of the values of hemoglobin and mean corpuscular volume from birth to last follow-up. Hemoglobin electrophorese studies and transfusions are also reported. Hb, hemoglobin; EPh, electrophoresis; MCV, mean corpuscular volume.
Figure 2 . Peripheral blood smear (A) performed in the neonatal period, showing hypochromic erythrocytes with anisopoikilocytosis; isolated target cells, ovalocytes, ellissocytes, and dacrocytes are also visible (600x magnification, MGG). Bone marrow aspirate (B) performed at 2 months of age showing mild dyserythropoiesis (1000x magnification, MGG); no ring sideroblasts were found in the smear [ (C) 1000x magnification, Pearls coloration].
At 6 months of age, the blood cell count of the patient was consistent with a thalassemia trait (hemoglobin 9 g/dL, red blood cell 6.19 × 10 12 /L, MCV 52 fL, mean cell hemoglobin, MCH, 6.3 pg). The hemoglobin electrophoresis showed HbA2 value of 3.3%, and α gene deletions were excluded using Multiplex Ligation Probe Amplification (MLPA). Conversely, MLPA showed a heterozygous deletion in the short arm of chromosome 11 ( Figure 3 ). This was confirmed by microarray analysis of single nucleotide polymorphisms that revealed a 26 kb single-copy loss of a genomic region localized at 11p15.4. The lost genetic material included the HBD, HBBP1, HBG1 - and partially HBB - genes, a finding consistent with ε γδβ thalassemia. The family history was negative for similarly affected individuals and targeted parental testing via quantitative polymerase chain reaction confirmed the presence of a de novo deletion.
Figure 3 . Multiplex Ligation Probe Amplification (MLPA) showing a deletion of the OR51V1-1, HBB, HBD, HBBP1, HBG1 and HBG2 genes (red dots) on the short arm of chromosome 11. All deletion were detected in the heterozygous form. The first deleted probe was the 486 on the OR51V1-1 gene (hg18 loc.11–005,177842), while the last was the 373, after the end of the HBG2 gene (hg18 loc.11–005,233895).
At last follow-up (5 years of age), the patient had a hemoglobin of 11.2 g/dL, a MCV of 52.9 fL, and a MCH of 17 pg; hemoglobin electrophoresis revealed 0.1% of HbF and 3.3% of HbA2 ( Figure 1 ). The patient was in good clinical condition, with normal growth (72 nd centile of height and 80 th centile of weight, WHO curves) and cognitive development. No splenomegaly was found at the abdominal ultrasound, nor signs of iron overload/deficiency. Therefore, no specific follow-up plan nor specific interventions in case of minor ailments were deemed necessary, as for β-trait carriers.
Anemia during the neonatal period represents a challenge for the pediatrician, mainly for the multiplicity of conditions that are responsible for the condition during the first weeks of life. The etiology of neonatal anemia usually falls into three major categories: blood loss, decreased production, and increased destruction of erythrocytes ( 28 ). The differential diagnosis for hemolytic anemia in the newborn period includes alloimmunity, erythrocyte membrane defects, enzyme deficiencies, and hemoglobinopathies. The most frequent hemoglobinopathy associated with critically ill infants and hemolytic anemia is α thalassemia with deletion of three α globin genes ( 28 , 29 ).
ε γδβ thalassemia usually presents as severe neonatal hemolytic anemia that requires in utero and/or neonatal transfusions but this condition is rarely considered among the causes of neonatal anemia and therefore misdiagnosed, as in our case. A reduced MCV without abnormalities on hemoglobin electrophoresis in a newborn is not always detected in ε γδβ thalassemia ( Table 1 ), but when it is found, it can orient toward the diagnosis. Despite the high incidence of thalassemias in Italy, the significant microcytosis in our patient was initially deemed secondary to iron deficiency, as the intercurrent sepsis misdirected high indirect bilirubin values as a sign of hemolysis.
Although uncommon during the neonatal period, microcytosis can occur secondary to iron deficiency following feto-maternal hemorrhage. However, in most cases, it is associated with thalassemia, also depending on the α thalassemia allele frequency, which varies in different populations ( 30 ). After the neonatal period, the hematologic phenotype of microcytosis associated with normal hemoglobin electrophoresis, which is typical of ε γδβ thalassemia, can be associated to or confused with α thalassemia, but also, in presence of normal ferritin levels, with IRIDA. Unlike previously suggested, the severe phenotype of our patient was not justified by the presence of α triplication, which was excluded by MLPA analysis.
There is no established explanation for the phenotypic heterogeneity of the disease, but it is not dependent on the type and length of deletion ( 8 ). Although at the molecular level ε γδβ thalassemias fall into two distinct categories, the associated phenotypes of the two groups are similar. Therefore, the variable severity is likely to be influenced by other genetic and environmental factors.
The remission of anemia after the first months of life is a consequence of the increasing production of β-globin that reduces the imbalance between α/non-α globin chain synthesis. The residual adult phenotype is similar to that of the β-thalassemia trait but with normal, rather than increased, levels of hemoglobin A2 due to the loss of one δ locus, while the fetal hemoglobin is normal or minimally increased ( 13 ). The normal HbA2 levels make the hematologic phenotype also similar to that of carriers of α-thalassemia ( 23 ). However, data collected by Rooks et al. suggest that adult heterozygotes for ε γδβ -thalassemias tend to have more severe microcytosis and hypochromia even than β 0 -thalassemia carriers ( 13 ).
In conclusion, this case remarks the importance of considering the ε γδβ thalassemia in the differential diagnosis of hypochromic microcytic hemolytic anemias in the newborn period. In the post-natal period, microcytosis with normal ferritin values and without abnormalities on hemoglobin electrophoresis should also raise the suspicion for ε γδβ thalassemia.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin. Written informed consent was obtained from the minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author Contributions
IF and FP wrote the manuscript and MV and CF critically reviewed it. IF, EC, and TC followed the patient. MM performed the genetic analysis. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Repnikova E, Roberts J, Mc Dermott S, Farooqi MS, Iqbal NT, Silvey M, et al. Clinical and molecular characterization of novel deletions causing epsilon gamma delta beta thalassemia: report of two cases. Pathol Res Pract. (2019) 215:152578. doi: 10.1016/j.prp.2019.152578
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Kan YW, Forget BG, Nathan DG. Gamma-beta thalassemia: a cause of hemolytic disease of the newborn. N Engl J Med. (1972) 286:129–34. doi: 10.1056/NEJM197201202860304
3. Oort M, Heerspink W, Roos D, Flavell RA, Bernini LF. Hemolytic disease of the newborn and chronic hypochromic microcytic anemia in one family: gamma-delta-beta thalassemia. Tijdschr Kindergeneeskd. (1981) 49:199–207.
PubMed Abstract | Google Scholar
4. Fearon ER, Kazazian HH, Waber PG, Lee JI, Antonarakis SE, Orkin SH, et al. The entire beta-globin gene cluster is deleted in a form of gamma delta beta-thalassemia. Blood. (1983) 61:1269–74. doi: 10.1182/blood.V61.6.1269.1269
5. Curtin P, Pirastu M, Kan YW, Gobert-Jones JA, Stephens AD, Lehmann H, et al. distant gene deletion affects beta-globin gene function in an atypical gamma delta beta-thalassemia. J Clin Invest. (1985) 76:1554–8. doi: 10.1172/JCI112136
6. Diaz-Chico JC, Huang HJ, Juricić D, Efremov GD, Wadsworth LD, Huisman TH. Two new large deletions resulting in epsilon gamma delta beta-thalassemia. Acta Haematol. (1988) 80:79–84. doi: 10.1159/000205607
7. Driscoll MC, Dobkin CS, Alter BP. Gamma delta beta-thalassemia due to a de novo mutation deleting the 5' beta-globin gene activation-region hypersensitive sites. Proc Natl Acad Sci U S A. (1989) 86:7470–4. doi: 10.1073/pnas.86.19.7470
8. Trent RJ, Williams BG, Kearney A, Wilkinson T, Harris PC. Molecular and hematologic characterization of Scottish-Irish type (epsilon gamma delta beta)zero thalassemia. Blood. (1990) 76:2132–8. doi: 10.1182/blood.V76.10.2132.2132
9. Fortina P, Delgrosso K, Werner E, Haines K, Rappaport E, Schwartz E, et al. A greater than 200 kb deletion removing the entire beta-like globin gene cluster in a family of Irish descent. Hemoglobin. (1991) 15:23–41. doi: 10.3109/03630269109072482
10. Abels J, Michiels JJ, Giordano PC, Bernini LF, Baysal E, Smetanina NS, et al. A de novo deletion causing epsilon gamma delta beta-thalassemia in a Dutch patient. Acta Haematol. (1996) 96:108–9. doi: 10.1159/000203726
11. Game L, Bergounioux J, Close JP, Marzouka BE, Thein SL. A novel deletion causing (epsilon gamma delta beta) degrees thalassaemia in a Chilean family. Br J Haematol. (2003) 123:154–9. doi: 10.1046/j.1365-2141.2003.04564.x
12. Harteveld CL, Osborne CS, Peters M, van der Werf S, Plug R, Fraser P, et al. Novel 112 kb (epsilonGgammaAgamma) deltabeta-thalassaemia deletion in a Dutch family. Br J Haematol. (2003) 122:855–8. doi: 10.1046/j.1365-2141.2003.04505.x
13. Rooks H, Bergounioux J, Game L, Close JP, Osborne C, Best S, et al. Heterogeneity of the epsilon gamma delta beta-thalassaemias: characterization of three novel English deletions. Br J Haematol. (2005) 128:722–9. doi: 10.1111/j.1365-2141.2005.05368.x
14. Furuya C, Yamashiro Y, Hattori Y, Hino M, Nishioka H, Shimizu Y, et al. A novel epsilon gamma delta beta thalassemia of 1. 4 Mb deletion found in a Japanese patient. Am J Hematol. (2008) 83:84–6. doi: 10.1002/ajh.21040
15. Brantberg A, Eik-Nes SH, Roberts N, Fisher C, Wood WG. Severe intrauterine anemia: a new form of epsilongammagammadeltabeta thalassemia presenting in utero in a Norwegian family. Haematologica. (2009) 94:1157–9. doi: 10.3324/haematol.2009.007534
16. Rose C, Rossignol J, Lambilliotte A, Depret S, Le Metayer N, Pissard S, et al. novel (epsilongammadeltabeta)(o)-thalassemia deletion associated with an alpha globin gene triplication leading to a severe transfusion dependent fetal thalassemic syndrome. Haematologica. (2009) 94:593–4. doi: 10.3324/haematol.2008.002675
17. Verhovsek M, Shah NR, Wilcox I, Koenig SC, Barros T, Thornburg CD, et al. Severe fetal and neonatal hemolytic anemia due to a 198 kb deletion removing the complete β-globin gene cluster. Pediatr Blood Cancer. (2012) 59:941–4. doi: 10.1002/pbc.24094
18. Rooks H, Clark B, Best S, Rushton P, Oakley M, Thein OS, et al. A novel 506kb deletion causing ε γδβ thalassemia. Blood Cells Mol Dis. (2012) 49:121–7. doi: 10.1016/j.bcmd.2012.05.010
19. Von Kanel T, Röthlisberger B, Schanz U, Dutly F, Huber AR, Saller E, et al. Swiss (ε γδβ )°-thalassemia patient with a novel 3-Mb deletion associated with mild mental impairment. Am J Hematol. (2013) 88:158–9. doi: 10.1002/ajh.23364
20. Shalev H, Landau D, Pissard S, Krasnov T, Kapelushnik J, Gilad O, et al. A novel epsilon gamma delta beta thalassemia presenting with pregnancy complications and severe neonatal anemia. Eur J Haematol. (2013) 90:127–33. doi: 10.1111/ejh.12047
21. Zebisch A, Schulz E, Grosso M, Lombardo B, Acierno G, Sill H, et al. Identification of a novel variant of epsilon-gamma-delta-beta thalassemia highlights limitations of next generation sequencing. Am J Hematol. (2015) 90:E52–4. doi: 10.1002/ajh.23913
22. Shooter C, Rooks H, Thein SL. Barnaby Clark. Next generation sequencing identifies a novel rearrangement in the HBB cluster permitting to-the-base characterization. Hum Mutat. (2015) 36:142–50. doi: 10.1002/humu.22707
23. Cardiero G, Prezioso R, Dembech S, Del Vecchio Blanco F, Scarano C, Lacerra G. Identification and molecular characterization of a novel 163 kb deletion: The Italian (ϵ γδβ )(0)-thalassemia. Hematology. (2016) 21:317–24. doi: 10.1080/10245332.2015.1133007
24. Goel R, Snow J, Pri-Paz SM, Cushing M, Vasovic LV. Intrauterine transfusions for severe fetal anemia and hydrops due to de novo ε γδβ -thalassemia. Transfusion. (2017) 57:876. doi: 10.1111/trf.13926
25. Hui ASY, Au PKC, Ting YH, Kan ASY, Cheng YKY, Leung AWK, et al. First Report of a Novel Deletion Due to ε γδβ -Thalassemia in a Chinese Family. Hemoglobin. (2017) 41:175–9. doi: 10.1080/03630269.2017.1366918
26. Muñoz Tormo-Figueres Á, Sanchis Calvo A, Guibert Zafra B. Epsilon gamma delta beta thalassemia: A rare cause of fetal and neonatal anemia. Med Clin (Barc). (2018) 150:368–9. doi: 10.1016/j.medcli.2017.10.011
27. Pirastu M, Kan YW, Lin CC, Baine RM, Holbrook CT. Hemolytic disease of the newborn caused by a new deletion of the entire beta-globin cluster. J Clin Invest. (1983) 72:602–9. doi: 10.1172/JCI111008
28. Allali S, Brousse V, Sacri AS, Chalumeau M, de Montalembert M. Anemia in children: prevalence, causes, diagnostic work-up, and long-term consequences. Expert Rev Hematol. (2017) 10:1023–8. doi: 10.1080/17474086.2017.1354696
29. Cappellini MD, Russo R, Andolfo I, Iolascon A. Inherited microcytic anemias. Hematology Am Soc Hematol Educ Program. (2020) 2020:465–70. doi: 10.1182/hematology.2020000158
30. Piel FB, Weatherall DJ. The α-thalassemias. N Engl J Med. (2014) 371:1908–16. doi: 10.1056/NEJMra1404415
Keywords: thalassemia, ε γδβ , children, newborn, anemia
Citation: Fotzi I, Pegoraro F, Chiocca E, Casini T, Mogni M, Veltroni M and Favre C (2022) Case Report: Clinical and Hematological Characteristics of ε γδβ Thalassemia in an Italian Patient. Front. Pediatr. 10:839775. doi: 10.3389/fped.2022.839775
Received: 20 December 2021; Accepted: 07 February 2022; Published: 17 March 2022.
Reviewed by:
Copyright © 2022 Fotzi, Pegoraro, Chiocca, Casini, Mogni, Veltroni and Favre. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Claudio Favre, claudio.favre@meyer.it
† These authors share first authorship
[Beta-thalassemia major: a clinical case]
Affiliation.
- 1 Facultatea de Medicină, U.M.F., Iaşi.
- PMID: 1305327
Publication types
- Case Reports
- Diagnosis, Differential
- Heterozygote
- beta-Thalassemia / blood
- beta-Thalassemia / diagnosis*
- beta-Thalassemia / genetics
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Thalassemia.
Hamza Bajwa ; Hajira Basit .
Affiliations
Last Update: August 8, 2023 .
- Continuing Education Activity
Thalassemia is a heterogeneous group of blood disorders affecting the hemoglobin genes and resulting in ineffective erythropoiesis. The decreased production of hemoglobin results in anemia in early age and frequent blood transfusions are required to keep up the hemoglobin levels. This activity outlines the evaluation and treatment of thalassemia and highlights the role of an interprofessional team in managing the patients with this condition.
- Summarize the etiology of thalassemias.
- Review of different laboratory and bedside evaluation techniques in the management of thalassemia patients.
- Outline the treatment and management options available for thalassemia.
- Identify interprofessional team strategies for improving care coordination and communication to improve outcomes in thalassemia.
- Introduction
Thalassemias are a heterogeneous grouping of genetic disorders that result from a decreased synthesis of alpha or beta chains of hemoglobin (Hb). Hemoglobin serves as the oxygen-carrying component of the red blood cells. It consists of two proteins, an alpha, and a beta. If the body does not manufacture enough of one or the other of these two proteins, the red blood cells do not form correctly and cannot carry sufficient oxygen; this causes anemia that begins in early childhood and lasts throughout life. Thalassemia is an inherited disease, meaning that at least one of the parents must be a carrier for the disease. It is caused by either a genetic mutation or a deletion of certain key gene fragments.
Alpha thalassemia is caused by alpha-globin gene deletion which results in reduced or absent production of alpha-globin chains. Alpha globin gene has 4 alleles and disease severity ranges from mild to severe depending on the number of deletions of the alleles. Four allele deletion is the most severe form in which no alpha globins are produced and the excess gamma chains (present during the fetal period) form tetramers. It is incompatible with life and results in hydrops fetalis. One allele deletion is the mildest form and is mostly clinically silent.
Beta thalassemia results from point mutations in the beta-globin gene. It is divided into three categories based on the zygosity of the beta-gene mutation. A heterozygous mutation (beta-plus thalassemia) results in beta-thalassemia minor in which beta chains are underproduced. It is mild and usually asymptomatic. Beta thalassemia major is caused by a homozygous mutation (beta-zero thalassemia) of the beta-globin gene, resulting in the total absence of beta chains. It manifests clinically as jaundice, growth retardation, hepatosplenomegaly, endocrine abnormalities, and severe anemia requiring life-long blood transfusions. The condition in between these two types is called beta-thalassemia intermedia with mild to moderate clinical symptoms.
- One mutated gene: Mild signs and symptoms. The condition is called thalassemia minor.
- Two mutated genes: Signs and symptoms will be moderate to severe. This condition is called thalassemia major, or Cooley anemia. Babies born with two mutated beta hemoglobin genes are usually healthy at birth but disease starts to manifest after 6 months of life when fetal hemoglobin (Hb-gamma) disappears and is replaced by adult Hb.
The excess unpaired alpha-globin chains in beta-thalassemia aggregate and form precipitates that damage red cell membranes and result in intravascular hemolysis. This premature death of erythroid precursor cells leads to ineffective erythropoiesis and later results in extramedullary expansion of hematopoiesis.
Coinheritance of alpha thalassemia: Beta-thalassemia patients with coinheritance of alpha thalassemia have a milder clinical course due to a less severe alpha-beta chain imbalance.
Coexistence of sickle cell trait: The presence of sickle cell trait with beta-thalassemia is a major hemoglobinopathy and results in manifestations of sickle cell disease. Unlike sickle cell trait in which major Hb is HbA, in the co-existence state the major Hb is HbS which constitutes more than 60% of Hb depending on the nature of the disease (beta-zero or beta-plus0.)
Hemoglobin (HbE) is also a common Hb variant found in Southeast Asia population. It has a correlation with a beta-thalassemia phenotype, as people with thalassemia in this territory are commonly found to have HbE.
Two new terminologies being used more often in clinical settings are transfusion requiring and non-transfusion requiring thalassemias and all the basic classification falls into these two types depending on the requirement of frequent blood transfusions or not. [1] [2] [3]
Thalassemia is autosomal recessive, which means both the parents must be affected with or carriers for the disease to transfer it to the next generation. It is caused by mutations or deletions of the Hb genes, resulting in underproduction or absence of alpha or beta chains. There are over 200 mutations identified as the culprits for causing thalassemias. Alpha thalassemia is caused by deletions of alpha-globin genes, and beta thalassemias are caused by a point mutation in splice site and promoter regions of the beta-globin gene on chromosome 11. [4]
- Epidemiology
Alpha thalassemia is prevalent in Asian and African populations while beta-thalassemia is more prevalent in the Mediterranean population, although it is relatively common in Southeast Asia and Africa too. Prevalence in these regions may be as high as 10%. The true numbers of thalassemia affected patients in the United States are unknown, as there is no effective screening method in place. [4]
- History and Physical
Thalassemia presentation varies widely depending on the type and severity. A complete history and physical examination can give several clues that are sometimes not obvious to the patient themselves. The following findings can be noted:
Skin can show pallor due to anemia and jaundice due to hyperbilirubinemia resulting from intravascular hemolysis. Patients usually report fatigue due to anemia as the first presenting symptom. Extremities examination can show ulcerations. Chronic iron deposition due to multiple transfusions can result in bronze skin.
Musculoskeletal
Extramedullary expansion of hematopoiesis results in deformed facial and other skeletal bones and an appearance known as chipmunk face.
Iron deposition in cardiac myocytes due to chronic transfusions can disrupt the cardiac rhythm, and the result is various arrhythmias. Due to chronic anemia, overt heart failure can also result.
Chronic hyperbilirubinemia can lead to precipitation of bilirubin gall stones and manifest as typical colicky pain of cholelithiasis. Hepatosplenomegaly can result from chronic iron deposition and also from extramedullary hematopoiesis in these organs. Splenic infarcts or autophagy result from chronic hemolysis due to poorly regulated hematopoiesis.
Hepatic involvement is a common finding in thalassemias, particularly due to the chronic need for transfusions. Chronic liver failure or cirrhosis can result from chronic iron deposition or transfusion-related viral hepatitis.
Slow Growth Rates
Anemia can inhibit a child's growth rate, and thalassemia can cause a delay in puberty. Particular attention should focus on the child's growth and development according to age.
Endocrinopathies
Iron overload can lead to its deposition in various organ systems of the body and resultant decreased functioning of the respective systems. The deposition of iron in the pancreas can lead to diabetes mellitus; in the thyroid or parathyroid glands can lead to hypothyroidism and hypoparathyroidism, respectively. The deposition in joints leads to chronic arthropathies. In the brain, iron prefers to accumulate in the substantia nigra and manifests as early-onset Parkinson's disease and various other physiatry problems. These symptoms fall in the vast kingdom of hemochromatosis. [5]
Several laboratory tests have been developed to screen and diagnose thalassemia:
Complete blood count (CBC): CBC is often the first investigation in a suspected case of thalassemia. A CBC showing low hemoglobin and low MCV is the first indication of thalassemia, after ruling out iron deficiency as the cause of anemia. The calculation of the Mentzer index (mean corpuscular volume divided by red cell count) is useful. A Mentzer lower than 13 suggests that the patient has thalassemia, and an index of more than 13 suggests that the patient has anemia due to iron deficiency. [6]
Peripheral blood smear: A blood smear (also called peripheral smear and manual differential) is next, to assess additional red cell properties. Thalassemia can present with the following findings on the peripheral blood smear:
- Microcytic cells (low MCV)
- Hypochromic cells
- Variation in size and shape (anisocytosis and poikilocytosis)
- Increased percentage of reticulocytes
- Target cells
- Heinz bodies
Iron studies (serum iron, ferritin, unsaturated iron-binding capacity (UIBC), total iron-binding capacity (TIBC), and percent saturation of transferrin) are also done to rule out iron deficiency anemia as the underlying cause.
Erythrocyte porphyrin levels may be checked to distinguish an unclear beta-thalassemia minor diagnosis from iron deficiency or lead poisoning. Individuals with beta-thalassemia will have normal porphyrin levels, but those with the latter conditions will have elevated porphyrin levels.
Hemoglobin electrophoresis: Hemoglobinopathy (Hb) evaluation assesses the type and relative amounts of hemoglobin present in red blood cells. Hemoglobin A (HbA), composed of both alpha and beta-globin chains, is the type of hemoglobin that typically makes up 95% to 98% of hemoglobin for adults. Hemoglobin A2 (HbA2) is normally 2% to 3% of hemoglobin, while hemoglobin F usually makes up less than 2% of hemoglobin in adults.
Beta thalassemia disturbs the balance of beta and alpha hemoglobin chain formation. Patients with the beta-thalassemia major usually have larger percentages of HbF and HbA2 and absent or very low HbA. Those with beta-thalassemia minor usually have a mild elevation of HbA2 and mild decrease of HbA. HbH is a less common form of hemoglobin that may be seen in some cases of alpha thalassemia. HbS is the hemoglobin prevalent in people with sickle cell disease.
Hemoglobinopathy (Hb) assessment is used for prenatal screening when parents are at high risk for hemoglobin abnormalities and state-mandated newborn hemoglobin screening.
DNA analysis: These tests serve to help confirm mutations in the alpha and beta globin-producing genes. DNA testing is not a routine procedure but can be used to help diagnose thalassemia and to determine carrier status if needed.
Since having relatives carrying mutations for thalassemia increases a person's risk of carrying the same mutant gene, family studies may be necessary to assess carrier status and the types of mutations present in other family members.
Genetic testing of amniotic fluid is useful in those rare instances where a fetus has an increased risk for thalassemia. This is particularly important if both parents likely carry a mutation because that increases the risk that their child may inherit a combination of abnormal genes, causing a more severe form of thalassemia. Prenatal diagnosis with chorionic villi sampling at 8 to 10 weeks or by amniocentesis at 14 to 20 weeks’ gestation can be carried out in high-risk families. [7] [6]
Multisystem evaluation: Evaluation of all related systems should be done on a regular basis due to their frequent involvement in the disease progression. Biliary tract and gall bladder imaging, abdominal ultrasonography, cardiac MRI, serum hormone measurements are a few examples that can be done or repeated depending on the clinical suspicion and case description.
- Treatment / Management
Thalassemia treatment depends on the type and severity of the disease.
Mild thalassemia (Hb: 6 to 10g/dl):
Signs and symptoms are generally mild with thalassemia minor and little if any, treatment is needed. Occasionally, patients may need a blood transfusion, particularly after surgery, following childbirth, or to help manage thalassemia complications.
Moderate to severe thalassemia (Hb less than 5 to 6g/dl):
- Frequent blood transfusions: More severe forms of thalassemia often require regular blood transfusions, possibly every few weeks. The goal is to maintain Hb at around 9 to 10 mg/dl to give the patients a sense of well being and also to keep a check on erythropoiesis and suppress extramedullary hematopoiesis. To limit transfusion-related complications, washed, packed red blood cells (RBCs) at approximately 8 to 15 mL cells per kilogram (kg) of body weight over 1 to 2 hours are recommended.
- Chelation therapy: Due to chronic transfusions, iron starts to get deposited in various organs of the body. Iron chelators (deferasirox, deferoxamine, deferiprone) are given concomitantly to remove extra iron from the body.
- Stem cell transplant: Stem cell transplant, (bone marrow transplant), is a potential option in selected cases, such as children born with severe thalassemia. It can eliminate the need for lifelong blood transfusions. [8] However, this procedure has its own complications, and the clinician must weigh these against the benefits. Risks include including graft vs. host disease, chronic immunosuppressive therapy, graft failure, and transplantation-related mortality. [9]
- Gene therapy: It is the latest advancement in severe thalassemia management. It involves harvesting the autologous hematopoietic stem cells (HSCs) from the patient and genetically modifying them with vectors expressing the normal genes. These are then reinfused to the patients after they have undergone the required conditioning to destroy the existing HSCs. The genetically modified HSCs produce normal hemoglobin chains, and normal erythropoiesis ensues.
- Genome editing techniques: Another recent approach is editing genomic libraries, such as zinc-finger nucleases, transcription activator-like effectors, and cluster regulated interspaced short palindromic repeats (CRISPR) with Cas9 nuclease system. These techniques target specific mutation sites and replace them with the normal sequence. The limitation of this technique is to produce a large number of corrected genes sufficient to cure the disease. [10]
- Splenectomy: Patients with thalassemia major often undergo splenectomy to limit the number of required transfusions. Splenectomy is the usual recommendation when the annual transfusion requirement increases to or more than 200 to 220 mL RBCs/kg/year with a hematocrit value of 70%. Splenectomy not only limits the number of required transfusions but also controls the spread of extramedullary hematopoiesis. Postsplenectomy immunizations are necessary to prevent bacterial infections, including Pneumococcus , Meningococcus , and Haemophilus influenzae . Postsplenectomy sepsis is possible in children, so this procedure is deferred until 6 to 7 years of age, and then penicillin is given for prophylaxis until they reach a certain age.
- Cholecystectomy : Patients can develop cholelithiasis due to increased Hb breakdown and bilirubin deposition in the gallbladder. If it becomes symptomatic, patients should undergo cholecystectomy at the same time when they are undergoing splenectomy.
Diet and exercise:
Reports exist that drinking tea aids in reducing iron absorption from the intestinal tract. So, in thalassemia patients tea might be a healthy drink to use routinely. Vitamin C helps in iron excretion from the gut, especially when used with deferoxamine. But using vitamin C in large quantities and without concomitant deferoxamine use, there is a higher risk for fatal arrhythmias. So, the recommendation is to use low quantities of vitamin C along with iron chelators (deferoxamine). [10]
- Differential Diagnosis
- Iron deficiency anemia: This is ruled out by iron studies and Mentzer index.
- Anemia of chronic disease and renal failure: Elevated markers of inflammation (CRP, ESR) point in this direction.
- Sideroblastic anemias: These are ruled out by iron studies and peripheral blood smear.
- Lead poisoning: This is ruled out by measuring serum protoporphyrin level.
Thalassemia minor is usually asymptomatic and has a good prognosis. It normally does not increase morbidity or mortality.
Thalassemia major is a severe disease, and the long-term prognosis depends on the treatment adherence to transfusion and iron chelation therapies. [11]
- Complications
Thalassemia major can produce the following complications [12] [13] :
- Jaundice and gall stones due to hyperbilirubinemia
- Cortical thinning and distortion of bones due to extramedullary hematopoiesis
- High output cardiac failure due to severe anemia, cardiomyopathies, and arrhythmias - cardiac involvement is the major cause of mortality in thalassemia patients
- Hepatosplenomegaly due to extramedullary hematopoiesis and excess iron deposition due to repeated blood transfusions
- Excess iron can lead to findings of primary hemochromatosis such as endocrine abnormalities, joint problems, skin discoloration, etc.
- Neurological complications such as peripheral neuropathies
- Slow growth rate and delayed puberty
- Increased risk of parvovirus B19 infection
- Deterrence and Patient Education
Patients should be educated to keep a check on their disease by following an appropriate treatment plan and adopting healthy living habits.
- Avoid excess iron. Unless the doctor recommends otherwise, patients should avoid multivitamins or other supplements that contain iron.
- Eat a healthy diet. Eating a balanced diet that contains plenty of nutritious foods can help the patient feel better and boost energy. Doctors sometimes also recommend taking a folic acid supplement to help make new red blood cells.
- Avoid infections. Patients should try maximally to protect themselves from infections, especially following a splenectomy. An annual flu shot, meningitis, pneumococcal, and hepatitis B vaccines are recommended to prevent infections.
Patients should also receive education about the hereditary nature of the disease. If both parents have thalassemia minor, there is a 1/4th chance that they will have a child with thalassemia major. If one parent has beta-thalassemia minor and the other parent has some form of beta-globin gene defect, i.e., sickle cell defect, they should also be counseled about the possibility of disease transfer to their children. Patients with thalassemias should understand that their disease is not due to iron deficiency and that iron supplements will not cure the anemia; in fact, it will lead to more iron buildup if they are already receiving blood transfusions. [14]
- Enhancing Healthcare Team Outcomes
Thalassemia has negative repercussions for many organs, and without a cure, it has high morbidity. The disorder is best managed by an interprofessional team that includes a thalassemia care team, cardiologist, hepatologist, endocrinologist, and psychologist. Also, family care, nursing support, and social support are an integral part of the management. A lead consultant should be in charge of the patient care, and a nurse specialist, along with other specialists in the respective fields, should be involved to cover all the aspects of the disease. Patient education is crucial, and social worker involvement, including a geneticist, is essential. In some parts of the world, preventive strategies include prenatal screening, restrictions on issuing marriage licenses to two people with the same disease. The screening of children and pregnant women who visit clinicians is an effective strategy to limit the disease morbidity. The social worker should ensure that the caregiver/patient has adequate support and financial resources so that they can continue with treatment. Nurses should educate patients on the importance of treatment compliance to avoid serious complications, as well as monitoring treatment progress. Pharmacists may soon play a greater role as there are new drug products to assist in gene therapy on the horizon that can eliminate the need for ongoing transfusions.
Active collaboration and discussion between interprofessional team members help in the better understanding of the progression or control of the disease.[Level 5]
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Electrophoresis Patterns in Beta-Thalassemia Modified from: Wilson et al. Chapter 11 Evaluation of Anemia, Leukopenia, and Thrombocytopenia. In Jaffe et al: Hematopathology. 2nd ed. Elsevier Health Sciences. Pg197, 2011.
Peripheral blood with features of beta-thalassemia minor. Microcytosis and frequent target cells are characteristic. Contributed by David T Lynch, MD
Peripheral blood picture of beta thalassemia major patient showing hypochromic, microcytic red blood cells along with target cells. Contributed by Hamza Bajwa, MD
Disclosure: Hamza Bajwa declares no relevant financial relationships with ineligible companies.
Disclosure: Hajira Basit declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Bajwa H, Basit H. Thalassemia. [Updated 2023 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- Review Alpha-Thalassemia. [GeneReviews(®). 1993] Review Alpha-Thalassemia. Tamary H, Dgany O. GeneReviews(®). 1993
- Review Beta-Thalassemia. [GeneReviews(®). 1993] Review Beta-Thalassemia. Langer AL. GeneReviews(®). 1993
- Review Sickle Cell Disease. [GeneReviews(®). 1993] Review Sickle Cell Disease. Bender MA, Carlberg K. GeneReviews(®). 1993
- Review Beta-thalassemia. [Orphanet J Rare Dis. 2010] Review Beta-thalassemia. Galanello R, Origa R. Orphanet J Rare Dis. 2010 May 21; 5:11. Epub 2010 May 21.
- Review Laboratory investigation of hemoglobinopathies and thalassemias: review and update. [Clin Chem. 2000] Review Laboratory investigation of hemoglobinopathies and thalassemias: review and update. Clarke GM, Higgins TN. Clin Chem. 2000 Aug; 46(8 Pt 2):1284-90.
Recent Activity
- Thalassemia - StatPearls Thalassemia - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers

IMAGES
VIDEO
COMMENTS
INTRODUCTION. Thalassemia is inherited autosomal recessive disorders characterized by reduced rate of hemoglobin synthesis due to a defect in alpha or beta globin chain synthesis. 1 It has high cases in Mediterranean, Middle east, Central Asia, Indian Subcontinent and Far East. 2 Maldives has a beta thalassemia prevalence rate of 1618 %. 3 Major reason for high cases in these regions is due to ...
Thalassemia and sickle cell disease are some of the most common single-gene inherited hemoglobin disorders worldwide. Unlike sickle cell disease, which is a qualitative globin chain defect, thalassemia results from quantitative defects (beta+ and beta0) in one or more globin chains of hemoglobin and causes hypochromic microcytic anemia. Dr. Cooley was the first to report beta-thalassemia in ...
Citation: Ambarkova V, Krmzova T, Nonkulovski Z (2021) Thalassemia-Beta major-Case report. Arch Hematol Case Rep Rev 6 (1): 021-025. Beta thalassemia ( thalassemia) is a group of inherited blood ...
Patients who are homozygous or compound heterozygous for β-thalassemia mutations can have β-thalassemia major or intermedia. 16 Patients with β-thalassemia major generally present early in life ...
Background: β-thalassemia is rare in sub-Saharan Africa and to our knowledge there has been no case of homozygous β-thalassemia major reported from this region. In a recent cohort study, we identified four β-thalassemia mutations among 83 heterozygous carriers in Kilifi, Kenya. One of the mutations identified was a rare β-globin gene initiation codon mutation (ATG ACG) (rs33941849).
Extramedullary hematopoiesis (EMH), as a compensatory phenomenon, refers to the blood cell formation outside of the bone marrow that occurs once the cells in the circulatory system fail to meet individuals' needs. EMH is rare in moderate to severe beta thalassemia because most symptomatic patients are effectively managed with transfusion. However, patients that fail to receive transfusions ...
The beyond study: results of a phase 2, double-blind, randomized, placebo-controlled multicenter study of luspatercept in adult patients with non-tranfusion dependent beta-thalassemia Paper presented at the 26th Congress of the European Hematology Association (EHA)
Cardiac disease due to iron overload is the commonest cause of death in patients with thalassaemia major, accounting for almost 70% of deaths in some series [1, 2].Cardiac iron overload related deaths may be less common in patients with non-transfusion dependent thalassaemia (NTDT), including in patients with haemoglobin E-beta thalassaemia where malignancies and infections seem to be the ...
Background: Iron chelation, blood transfusions, and complication management are typical hospital requirements for children with beta-thalassemia major. This affects their health-related quality of life (HRQoL). The purpose of this study was to evaluate how the Supportive and Coping strategies, Ongoing Assessment, Prevention of Complications, and Empowerment (SCOPE) Program impacted the HRQoL ...
In a study conducted by Hatab, et al. involving 54 patients with Thalassemia major aged 5-18 years, clinical and radiological features were analyzed. In addition, poor oral hygiene was registered in 60% and gingivitis in 43%. More than half had a frontal anomaly, saddle nose and maximal protrusion.
Introductionεγδβ thalassemia is a rare form of β-thalassemia mostly described in children originating from Northern Europe. Only anecdotic cases from the Mediterranean area are reported. The diagnosis is challenging, considering the rarity of the disease and its heterogeneous clinical presentation. Most patients have neonatal microcytic anemia, sometimes requiring in utero and/or neonatal ...
What causes beta thalassemia? Beta thalassemia is caused by damaged or missing genes. Two specific genes are involved. There are several types of this disorder: Beta thalassemia major (Cooley's anemia). There are two damaged genes. This is the most severe form of this disorder. People with this condition will need frequent blood transfusions.
Abstract. BACKGROUND HbE is a variant haemoglobin with a mutation in beta-globin gene, which is the most common Hb variant in South-East Asia. Here, we present a case report of 17 yrs. old boy ...
In the United Kingdom, total healthcare expenditure attributable to managing β‐thalassemia major over 50 years was estimated to be USD 720 201 at 2013‐2014 prices. 78 A study of 331 patients with β‐thalassemia in Greece found that the mean annual cost per patient, including all treatment strategies, was EUR 32 064 for the period 2009 to ...
Background/Objectives: in β-thalassemia, important clinical complications are caused by the presence of free α-globin chains in the erythroid cells of β-thalassemia patients. These free α-globin chains are present in excess as a result of the lack of β-globin chains to bind with; they tend to aggregate and precipitate, causing deleterious effects and overall cytotoxicity, maturation ...
Case Study on Thalassemia in Children Yohana Sheikha, Ravi Christianb and Nimisha Shrivastavac aIntern, BSc MRIT , School of Allied health Sciences, Department Of Radiodiagnosis, Jawaharlal Nehru Medical College, Datta Meghe Institute of Medical Sciences, Wardha; Email:[email protected]; Ph no:-9284850862,9561256158.
[Beta-thalassemia major: a clinical case] [Beta-thalassemia major: a clinical case] Rev Med Chir Soc Med Nat Iasi. 1992;96 Suppl:33-6. [Article in Romanian] Author L I Zamfir 1 Affiliation 1 Facultatea de Medicină, U.M.F., Iaşi. PMID: 1305327 No abstract available ...
Beta thalassemia major is caused by a homozygous mutation (beta-zero thalassemia) of the beta-globin gene, resulting in the total absence of beta chains. ... (CBC): CBC is often the first investigation in a suspected case of thalassemia. A CBC showing low hemoglobin and low MCV is the first indication of thalassemia, after ruling out iron ...