Ohio State nav bar
The Ohio State University
- BuckeyeLink
- Find People
- Search Ohio State

Patient Case Presentation
Vital Signs and Measurements
- BP: 135/85 mm Hg
- HR: 99 bpm
- RR: 21 b / min
- Temperature: 36.1 ℃
- Pulse oxygenation: 99%
- Height: 167 cm
- Weight: 58.2 kg
Past medical history
- Gastritis with Helicobacter pylori (H. pylori) infection, diagnosed 5 years ago. Resolved with pharmacotherapy, frequent recurrence.
- Heart attack 3 months ago, has started taking aspirin since then.
- Osteoarthritis, diagnosed 3 years ago. Long-term use of the non-steroidal anti-inflammatory drug (NSAID) since diagnosis.
- Acute pancreatitis 5 years ago, resolved with pharmacotherapy, no recurrence.
- Chronic obstructive pulmonary disease, diagnosed 11 years ago.
- Diabetes Mellitus and hypertension diagnosed 6 years ago.
- No surgical history.
Pertinent family history
- Mother died from gastric cancer at age 67 years old.
- Father alive, has smoked for 50 years and now has a small-cell carcinoma. Live with the patient since she divorced her husband.
- Brother alive and well at age 56 years, had a history of duodenal ulcers. Heavy smoker.
- Ex-husband at age 62, alive and has COPD.
- One son alive and well at age 29.
- One daughter at age 35, alive and has recurrent Gastritis.
Pertinent social history
- Has worked full-time as a TV program producer for 30 years.
- Divorced with her husband 20 years prior, raised 2 children by herself.
- Hobbies include drinking, eating spicy food, watching dramas and talkshow.
- Has smoked for 30 years, even after the diagnosis of COPD, still cannot quit smoking.
Peptic Ulcer
Case Presentation
Harold, a fifty-eight year old grocery store manager, had recently been waking up in the middle of the night with abdominal pain. This was happening several nights a week. He was also experiencing occasional discomfort in the middle of the afternoon. Harold decided to schedule an appointment with his physician.
The doctor listened as Harold described his symptoms and then asked Harold some questions. He noted that Harold's appetite had suffered as a result of the pain he was experiencing and as a result of the fear that what he was eating may be responsible for the pain. Otherwise, Harold seemed fine.
The doctor referred Harold to a physician that specialized in internal medicine and had Harold make an appointment for a procedure called an endoscopy. The endoscopy was performed at a hospital later that week. During the procedure, a long, thin tube was inserted into Harold's mouth and directed into his digestive tract. The end of the tube was equipped with a light source and a small camera which allowed the doctor to observe the interior of Harold's stomach. The endoscope was also equipped with a small claw-like structure that the doctor could use in order to obtain a small tissue sample from the lining of Harold's stomach, if required.
The endoscopy revealed that Harold had a peptic ulcer. Analysis of a tissue sample taken from the site showed that Harold also had an infection that was caused by Helicobacter pylori bacteria. The doctor who performed the endoscopy gave Harold prescriptions for two different antibiotics and a medication that would decrease the secretion of stomach acid. The doctor also instructed Harold to schedule an appointment for another endoscopy procedure in 6 months.
Case Background
A peptic ulcer is a sore that occurs in the lining of a part of the gastrointestinal tract that is exposed to pepsin and acid secretions. Most peptic ulcers occur in the lining of the stomach or duodenum. 90% of all duodenal ulcers and 80% of all gastric ulcers are caused by H. pylori infection. Most of the remaining peptic ulcers are caused by long-term usage of certain anti-inflammatory medications like aspirin.
There is still some question as to how H. pylori is spread. However, H. pylori has been identified in the saliva of infected individuals and may be spread via this fluid. H. pylori bacteria have the ability to survive the acid environment in the stomach because they produce enzymes that neutralize stomach acids. They also have the ability to move through the mucous membrane lining the stomach or duodenum and take up residence in the underlying connective tissue. The damage to the mucous membrane that results from a H. pylori infection allows pepsin and hydrochloric acid to further damage the wall of the stomach or duodenum. The sore that results is the peptic ulcer.
Describe the functions of the following components of gastric juice.
Peptic Ulcer Disease Case Study (60 min)
Watch More! Unlock the full videos with a FREE trial
Included In This Lesson
Access More! View the full outline and transcript with a FREE trial
Mrs. Baker is a 54 year old female who presented to the ED complaining of nausea and severe epigastric pain x 3 days. She reports a history of osteoarthritis and reports taking ibuprofen 400 mg 3-4 times a day regularly for the last few months since her “arthritis has gotten really bad”.
What initial nursing assessments should be performed?
- Put the patient on a monitor to assess EKG. A 12-lead EKG should be done to rule out cardiac involvement, request order for cardiac enzymes from provider
- Auscultate heart and lung sounds
- Full abdominal assessment – inspect, auscultate, palpate and percuss. Assess for tenderness over specific areas, feel for masses, and look for guarding.
- Get more detailed history questions – vomiting? Bloody stools? Has this happened before?
Patient demonstrates guarding when palpating epigastric region, no tenderness to palpation over RLQ, LLQ, or LUQ. Some tenderness over RUQ. Bowel sounds are hyperactive, lungs are clear to auscultation, S1 and S2 heard clearly with no murmurs. As you finish your assessment, Mrs. Baker reports she is going to be sick and vomits approximately 300 mL of coffee-ground emesis.
Explain the significance of coffee-ground emesis.
- Coffee-ground emesis is vomit that looks like it has coffee-grounds in it.
- These black specs are actually hemolyzed blood cells/clots.
- Coffee-ground emesis is indicative of a slow source of bleeding within the stomach or refluxing from the duodenum.
You notify the provider of the coffee-ground emesis, administer Ondansetron 4 mg IV per provider orders, and assist Mrs. Baker with oral care.
What further diagnostic testing do you expect to be performed for this patient?
- Complete Blood Count
- Occult blood testing of stool and emesis
- Patient may need an EGD (esophagogastroduodenoscopy) to check for bleeding ulcers
Mrs. Baker is now weak and drowsy. Her fecal occult test is positive and her CBC shows a Hemoglobin of 10 g/dL and a Hematocrit of 31%. Per provider orders, you insert an NG tube to evaluate stomach contents and decompress the stomach. You connect the NG tube to intermittent low wall suction.
What is likely going on with Mrs. Baker physiologically?
- Mrs. Bakers chronic heavy use of NSAID’s may have caused ulcers to form in the lining of her stomach and/or duodenum
- It is possible that these ulcers are now bleeding
What is the benefit to decompressing the stomach via NG tube?
- Decompressing the stomach removes the majority of stomach acid, thereby decreasing the irritation on the stomach lining
- The hope is to prevent further irritation to any bleeding ulcers
The UAP notifies you that Mrs. Baker’s blood pressure has dropped to 96/60. You enter the room and see that the suction canister is over halfway full of bright red blood.
What is your priority assessment at this time?
- Assess Mrs. Baker – LOC, heart and lung sounds, confirm the accuracy of vital signs
- Protect airway – suction if needed, minimize risk for aspiration
What may be happening to Mrs. Baker?
- She may have an ulcer that is bleeding more actively than before. With that amount of blood, it could possibly be an arterial bleed.
Mrs. Baker is pale, diaphoretic, and drowsy. Her heart rate is up to 122. You notify the provider who orders to transfuse 2 units of PRBC’s and calls the Gastroenterology team for a STAT EGD. Within 30 minutes the patient is taken to the GI lab for an EGD, where they find two slow-bleeding gastric ulcers, which they cauterize, and 1 arterial bleed which they repair as well. Mrs. Baker returns to the unit post-procedure for observation.
What are nursing priorities for Mrs. Baker after this procedure?
- Keep NPO until gag reflex returns
- Assess and monitor output from NG tube
- Monitor vital signs closely
- Monitor LOC as she awakens from sedatives used during the procedure
- Ensure the full 2 units of PRBC’s were administered. If not, continue transfusion.
View the FULL Outline
When you start a FREE trial you gain access to the full outline as well as:
- SIMCLEX (NCLEX Simulator)
- 6,500+ Practice NCLEX Questions
- 2,000+ HD Videos
- 300+ Nursing Cheatsheets
“Would suggest to all nursing students . . . Guaranteed to ease the stress!”
Nursing Case Studies

This nursing case study course is designed to help nursing students build critical thinking. Each case study was written by experienced nurses with first hand knowledge of the “real-world” disease process. To help you increase your nursing clinical judgement (critical thinking), each unfolding nursing case study includes answers laid out by Blooms Taxonomy to help you see that you are progressing to clinical analysis.We encourage you to read the case study and really through the “critical thinking checks” as this is where the real learning occurs. If you get tripped up by a specific question, no worries, just dig into an associated lesson on the topic and reinforce your understanding. In the end, that is what nursing case studies are all about – growing in your clinical judgement.
Nursing Case Studies Introduction
Cardiac nursing case studies.
- 6 Questions
- 7 Questions
- 5 Questions
- 4 Questions
GI/GU Nursing Case Studies
- 2 Questions
- 8 Questions
Obstetrics Nursing Case Studies
Respiratory nursing case studies.
- 10 Questions
Pediatrics Nursing Case Studies
- 3 Questions
- 12 Questions
Neuro Nursing Case Studies
Mental health nursing case studies.
- 9 Questions
Metabolic/Endocrine Nursing Case Studies
Other nursing case studies.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- BMJ Case Rep

Case Report
Atypical presentation of perforated peptic ulcer disease in a 12-year-old boy, simon mbarushimana.
1 Cardiothoracic Surgery, Belfast, UK
Gareth Morris-Stiff
2 Department of General Surgery, Western Trust, Derry, UK
George Thomas
3 Department of General Surgery, Western Trust, Enniskillen, UK
A 12-year-old boy was referred to the surgical unit with 4 h history of severe lower abdominal pain and bilious vomiting. No other symptoms were reported and there was no significant medical or family history. Examination revealed tenderness in the lower abdomen, in particular the left iliac fossa. His white cell count was elevated at 19.6×10 9 /L, with a predominant neutrophilia of 15.8×10 9 /L and a C reactive protein of <0.3 mg/L. An abdominal X-ray revealed intraperitoneal gas and a chest X-ray identified free air under both hemidiaphragms. Subsequent diagnostic laparoscopy identified a perforated duodenal ulcer that was repaired by means of an omental patch. The case illustrates that although uncommon, alternate diagnoses must be borne in mind in children presenting with lower abdominal pain and diagnostic laparoscopy is a useful tool in children with visceral perforation as it avoids treatment delays and exposure to excess radiation.
In a recent multicentre European study, the prevalence of peptic ulceration was 8.1% in children presenting with abdominal pain, the majority of patients being males in the second decade of life. 1 Helicobacter pylori infection and non-steroidal anti-inflammatory drug ingestion are the main aetiological risk factors in the paediatric age. 2 The classic presentation of patients with peptic ulcers is one of epigastric pain, often associated with vomiting.
Perforated peptic ulcer disease in children is rare, seen in only 5% of cases, and is usually associated with a preceding history of typical pain, and presentation with generalised peritonitis. In the largest study in the literature, 52 cases of perforated duodenal ulcer disease were reported over a 20-year period. 3 All patients in this series reported a history of abdominal pain and 94.2% had signs of peritonitis at presentation.
As with all acute abdominal emergencies, rapid diagnosis and prompt treatment are the keys to a successful outcome, this being of particular importance in cases of visceral perforation. Faced with radiological evidence of perforation but an uncertain origin, options include cross-sectional imaging or immediate surgery. Diagnostic laparoscopy, as selected, excludes the radiation exposure of abdominal CT as well as its associated time delay. It also allows direct visualisation of the whole peritoneal cavity, thorough evacuation of food material and gastric secretions as well as providing direct visualisation of the perforation and facilitating repair.
Case presentation
A 12-year-old boy presented to the emergency surgical intake via the out of hours general practitioner service with very severe lower abdominal pain that woke him from sleep. The pain was constant in nature, scoring 10 out of 10 in severity, but did not radiate and no exacerbating factors were reported. The pain was associated with vomiting but no alteration in bowel habit. There was no medical or family history of note. He had no urinary or respiratory symptoms, took no medications and lived with four siblings who were all well.
On examination, he appeared flushed, with tenderness in the lower abdomen and peritonism that was markedly worse over the left iliac fossa. He was tachycardic with a heart rate of 140 bpm, blood pressure of 110/89 mm Hg, a temperature of 36.6°C and a respiratory rate of 20 bpm. Peripheral intravenous access was established and a standard blood profile sent for evaluation. The child was maintained nil per mouth and provided with adequate analgesia and antiemetics. Abdominal and chest radiographs were also requested.
Blood work revealed an elevated WCC at 19.6×10 9 /L (neutrophilia of 15.8 × 10 9 /L) but a normal CRP of <0.3 mg/L. The abdominal X-ray revealed intraperitoneal air and free air was seen under both hemidiaphragms in the chest radiograph ( figures 1 and and2). 2 ). A diagnosis of perforated viscus was established, and given the location of the pain in the lower abdomen, the perforation was believed to originate from the appendix or a Meckel's diverticulum.

Abdominal X-ray demonstrating free intraperitoneal air as arrowed.

Erect chest X-ray showing bilateral subdiaphragmatic air (arrow).
The patient was consented for diagnostic laparoscopy and to proceed appropriately dependent on the diagnosis. Laparoscopy revealed a large volume of turbid fluid tracking to the pelvis and a 0.5 cm perforation in the anterior wall of the first part of the duodenum was observed. The perforation was repaired with an omental patch and the peritoneal cavity thoroughly washed with warm saline.
Outcome and follow-up
His postoperative recovery was unremarkable and he was discharged 6 days later on empirically prescribed H. pylori eradication therapy. Prior to discharge a serum gastrin level was sent, and returned as being normal. At follow-up, he was symptom free and was prescribed a maintenance dose of 20 mg omeprazole. He was also referred to a paediatric gastroenterologist for on-going care.
The current case is unusual in that the location of pain was atypical, there being no preceding upper abdominal pain, and the clinical signs were limited to the lower abdomen, specifically the left iliac fossa. The existing literature would suggest that the majority of children with perforated peptic ulcers report severe abdominal pain with evidence of generalised peritonitis. 1 3
Right iliac fossa pain as a presentation of a perforated peptic ulcer has been documented. 4 Indeed, the eponym Valentino's syndrome has been applied to this presentation and relates to the famous actor Rudolph Valentino who underwent an appendicectomy for suspected appendicitis but then developed multiorgan failure and died. At autopsy, a perforated peptic ulcer was identified as the cause of his initial presentation.
The likely mechanism accounting for lower abdominal pain rather than epigastric pain, as confirmed by laparoscopy, is that gastric contents descend under gravity along the paracolic gutters. However, it is uncertain why in this case the fluid preferentially gathered in the left iliac fossa. A detailed review of the published English language literature by means of a comprehensive electronic search of MEDLINE and manual review of the bibliographies of relevant papers failed to identify a previously documented similar presentation of perforated peptic ulcer disease.
In the largest study to date, the mean age for paediatric perforated peptic ulcer disease was 14.2 years, with 90% being adolescents. 3 The majority of children (>80%) are males, with most reporting a predisposing risk factor such as abdominal pain of greater than 3 months duration; underlying medical illness; family history of peptic ulcer disease; active smoker and alcohol use. 3
In the case reported herein, the preoperative diagnosis was of perforated viscus but the origin was unclear. Faced with this clinical scenario, there are two available options namely to try and define the defect preoperatively with further imaging or to proceed to surgical exploration. In a study of 85 patients with visceral perforation, CT scan was able to accurately identify the point of perforation in 86% of cases, 5 and while there are no series specifically looking at diagnostic laparoscopy in the evaluation of visceral perforation, a series of 1320 patients undergoing evaluation for abdominal pain showed a diagnosis was established in 90% of cases. 6 Furthermore, laparoscopy changed the preoperative diagnosis in 30% of cases, and allowed for immediate laparoscopic operation in 83% with the remaining 7% converted to an open operation.
In the current paediatric case, with a lesser range of differential diagnoses available for the perforation, rather than requesting a CT scan, a decision was made to progress immediately to laparoscopy. This decision omitted the radiation exposure and reduced the interval from admission to definitive management. Reducing the time interval delay from presentation to surgery with paediatric perforated peptic ulcers, as with all surgical conditions, is associated with a reduction in morbidity and mortality. 3 In adults with left iliac fossa pain and intraperitoneal air present, perforated diverticular disease becomes an important consideration and CT may be of value in determining the need/urgency of surgery and so taking into account each case independently is important.
It is clear from the literature that perforated peptic ulcer disease is frequently not considered in the differential diagnosis of a child with peritonism leading to delays in management. 3 7 8 It is also clear from a large Danish registry report that delays in diagnosing and treating perforated ulcers is associated with poorer outcome, with each hour leading to a 2.4% decreased probability of survival. 9
The published series illustrate that there is no consensus as to the investigation of children with abdominal pain, with significant intercentre variation. In the current case, the abdominal and chest radiographs confirmed free intraperitoneal gas, and so rather than investigating using radiological means, a laparoscopy was performed to allow diagnosis and management within a reduced time frame.
After managing the acute presentation of peptic ulceration in the paediatric patient, it is important to treat, if present, with appropriate eradication therapy. 3 Indeed, evidence from a systematic review and meta-analysis of this approach has suggested empirical treatment with H. pylori eradication therapy is superior to antisecretory treatment alone. 10 Other risk factors such as hypersecretory states should also be sought and treated. All children should be referred for endoscopic evaluation to ensure the ulcer has healed. 11

Learning points
- Peptic ulcer disease is not uncommon in the paediatric population accounting for 8.1% of patients investigated for abdominal pain; however, ulcer perforation is rare.
- Suspect perforated peptic ulcer in adolescents who present with acute abdominal pain and peritoneal signs, in particular if upper abdominal pain has been reported over the preceding months.
- Once visceral perforation is diagnosed in a child, diagnostic laparoscopy with a view to definitive surgery would appear to be the appropriate option to expedite treatment and reduce delays.
Acknowledgments
The authors would like to thank Mr Alan Miller and Mr Seamus Dolan, Consultant Surgeons, South West Acute Hospital, Enniskillen, Northern Ireland.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
- Research article
- Open access
- Published: 10 February 2022
The global, regional and national burden of peptic ulcer disease from 1990 to 2019: a population-based study
- Xin Xie 1 , 2 ,
- Kaijie Ren 1 ,
- Zhangjian Zhou 3 ,
- Chengxue Dang 1 , 4 &
- Hao Zhang ORCID: orcid.org/0000-0001-8986-3854 1 , 4
BMC Gastroenterology volume 22 , Article number: 58 ( 2022 ) Cite this article
27k Accesses
53 Citations
Metrics details
Peptic ulcer disease (PUD) is a common digestive disorder, of which the prevalence decreased in the past few decades. However, the decreasing tendency has plateaued in recent years due to changes in risk factors associated with the etiology of PUD, such as non-steroidal anti-inflammatory drug use. In this study, we investigated the epidemiological and the sociodemographic characteristics of PUD in 204 countries and territories from 1990 to 2019 based on data from the Global Burden of Disease, Injuries and Risk Factors (GBD) Study.
Demographic characteristics and annual prevalence, incidence, mortality, disability-adjusted life years (DALYs) and age-standardized death rate (ASR) data associated with PUD were obtained and analyzed. According to the sociodemographic index (SDI), the numbers of patients, ASRs, estimated annual percentage changes and geographical distributions were assessed with a generalized linear model and presented in world maps. All evaluations of numbers and rates were calculated per 100,000 population with 95% uncertainty intervals (UIs).
In 2019, the global prevalence of PUD was approximately 8.09 [95% UI 6.79–9.58] million, representing a 25.82% increase from 1990. The age-standardized prevalence rate was 99.40 (83.86–117.55) per 100,000 population in 2019, representing a decrease of 143.37 (120.54–170.25) per 100,000 population from 1990. The age-standardized DALY rate in 2019 was decreased by 60.64% [74.40 (68.96–81.95) per 100,000 population] compared to that in 1990. In both sexes, the numbers and ASRs of the prevalence, incidence, deaths and DALYs were higher in males than in females over 29 years. Regionally, South Asia had the highest age-standardized prevalence rate [156.62 (130.58–187.05) per 100,000 population] in 2019. A low age-standardized death rate was found in the high-income super-region. Among nations, Kiribati had the highest age-standardized prevalence rate [330.32 (286.98–379.81) per 100,000 population]. Regarding socioeconomic status, positive associations between the age-standardized prevalence, incidence, death rate, DALYs and SDI were observed globally in 2019.
Conclusions
Morbidity and mortality due to PUD decreased significantly from 1990 to 2019, while a gradual upward inclination has been observed in recent 15 years, which might be associated with changes in risk factors for PUD. Attention and efforts by healthcare administrators and society are needed for PUD prevention and control.
Peer Review reports
Peptic ulcer disease (PUD), a common disorder of the digestive system, is defined as digestive tract injury that results in a mucosal break greater than 3–5 mm, with a visible depth reaching the submucosa [ 1 , 2 ]. Mainly occurring in the stomach and proximal duodenum, PUD accounts for an estimated lifetime prevalence of 5–10% and an annual incidence of 0.1–0.3% in the general population in Western countries [ 2 , 3 ]. Due to nonspecific symptoms, PUD assessment and treatment requires clinical caution due to severe complications such as bleeding, perforation, penetration into adjacent organs and gastrointestinal obstruction, all of which could require acute endoscopic or surgical treatment [ 1 , 4 , 5 ].
Similar to several digestive disorders, the prevalence of PUD initially increased and then subsequently decreased. Jennings et al . analyzed PUD epidemiological data spanning 150 years and found that the incidence of and mortality due to PUD increased markedly during the nineteenth century and then decreased steadily due to improvements in environmental hygiene and medical therapeutic strategies [ 6 ]. During the first 50 years of the twentieth century in the United States, PUD affected approximately 10% of the adult population [ 7 ]. Several studies which were conducted in the past 20–30 years indicated a sharp decreasing tendency in the PUD prevalence, PUD-related hospital admissions and PUD-associated mortality due to new anti-PUD therapies application, such as Helicobacter pylori ( H. pylori ) eradication and proton-pump inhibitors (PPIs) using [ 8 , 9 , 10 , 11 ]. However, the widespread use of nonsteroidal anti-inflammatory drugs (NSAIDs), histamine 2 receptor antagonists, and selective serotonin reuptake inhibitors, as well as increased physiological stress, have been reported as risk factors and have changed the landscape of PUD in recent years [ 12 , 13 ]. The details of the epidemiological changes caused by these relatively new risk factors are still controversial.
In this study, we analyzed PUD burdens in 204 countries or territories from 1990 to 2019 based on data from the Global Burden of Disease, Injuries and Risk Factors (GBD) Study, which is updated in 2020 and contains epidemiological and socioeconomic data of 354 diseases globally, allowing evaluations of the burdens, distributions and trends of PUD in different regions. Our study aims to investigate the current landscape and changes in the epidemiological characteristics of PUD to support healthcare-associated policy makers in developing improved PUD prevention strategies.
Data acquisition
The GBD study provides comprehensive epidemiological estimates of the prevalence of, incidence of, disability-adjusted life years (DALYs) due to, and mortality associated with diseases and injuries across specific groups of countries and territories by sex, age and year. Annual (inclusive dates: Jan 1st, 1990 to Dec 31st, 2019) prevalence rates, incidence rates, DALYs and deaths and the corresponding age-standardized rates (ASRs) were extracted from the Global Health Data Exchange (GHDx) database ( http://ghdx.healthdata.org/ ). These data were socioecologically classified into seven GBD super-regions, 21 GBD regions and 204 countries/territories by sociodemographic index (SDI) quintiles, which is a composite indicator of lag-distributed income per capita, ranging from 0 (less developed) to 1 (most developed). SDI value reflects the degree of social development and correlates with total fertility, per capita income, and average years of education [ 14 ]. All countries and territories were classified into five quintiles based on the SDI ( http://ghdx.healthdata.org/record/ihme-data/gbd-2019-sociodemographic-index-sdi-1950-2019 ). Besides, super-regions and regions in GBD database are groups of countries rather than geological concepts for analysis ( http://www.healthdata.org/sites/default/files/files/Data_viz/GBD_2017_Tools_Overview.pdf ).
Statistical analyses
ASRs and numbers were analyzed to compare PUD prevalence and mortality trends among different cohorts. DALYs refer to the years lived with disability and years of life lost [ 15 ]. Estimated annual percentage changes (EAPCs) indicate ASR trends during a defined period. The specific EAPC was calculated using a generalized linear model (GLM) considering a Gaussian distribution for the ASR. Under the assumption of linearity on the log scale, which is equivalent to a constant change assumption, the EAPC was calculated. An EAPC estimation greater than zero indicates an increasing ASR trend, while an estimation less than zero indicates a decreasing ASR trend. If the 95% confidence interval (CI) of the EAPC crossed zero, the change in ASR was not obvious over time.
World maps and graphs were generated to display the distribution and change trends in global, regional, and national disease burdens attributable to PUD. Uncertainty was incorporated by sampling 1,000 draws combining uncertainty from a number of sources, including input data, corrections of measurement errors and estimates of residual nonsampling errors. The 2.5th and 97.5th centiles of the ordered draws were defined as uncertainty intervals (UIs). All calculations and figures were performed and made in EXCEL 2019 (Microsoft Corporation) and R software (version 4.0.0) with the “Rcan”, “ggplot2” and other packages.
Global burden and demographic profiles of PUD
Our study indicated that there were approximately 8.09 million (95% UI 6.79 to 9.58 million) prevalent cases of PUD in 2019, which represented an increase of 25.82% from 1990 [6.43 million (95% UI 5.41 to 7.63 million)]. Moreover, the age-standardized prevalence rate in 2019 was 99.40 per 100,000 (95% UI 83.86 to 117.55 per 100,000) population, which represented a decrease from 1990 [143.37 per 100,000 (95% UI 120.54 to 170.25 per 100,000) population](Additional file 1 : Table S1). Between 1990 and 2019, the number of incident cases of PUD increased from 2.82 million (95% UI 2.36 to 3.30 million) to more than 3.59 million (95% UI 3.03 to 4.22), representing an increase of 27.3% in the global incident cases of PUD. However, the global age-standardized incidence rate of PUD showed a decreasing trend, at 63.84 (95% UI 54.09 to 75.54) per 100,000 population in 1990 and 44.26 (95% UI 37.32 to 51.87) per 100,000 population in 2019 (Additional file 1 : Table S2). At the global level, nearly 6.03 (95% UI 5.59 to 6.64) million DALYs were attributable to PUD, with an age-standardized rate of 74.40 (95% UI 68.96 to 81.95) DALYs per 100,000 population in 2019. The age-standardized rate of DALYs decreased by 60.64% from 1990. Similar trends were also found in PUD-related deaths (Additional file 1 : Tables S3, S4).
Both the number of prevalent cases and age-standardized prevalence rate were higher in males than in females in all years from 1990 to 2019. However, the difference between the two groups decreased, mainly because the number of prevalent cases and age-standardized prevalence rate in males decreased faster than those in females. Overall, in 2019, 3.92 (95% UI 3.29 to 4.64) million prevalent cases occurred in females, whereas 4.17 (95% UI 3.49 to 4.97) million prevalent cases occurred in males. The proportion of prevalent cases between males and females was 1:0.94. The age-standardized prevalence rate was 94.23 (95% UI 79.10 to 111.93) per 100,000 population in females and 104.98 (95% UI 88.26 to 124.10) per 100,000 population in males in 2019 (Fig. 1 a).
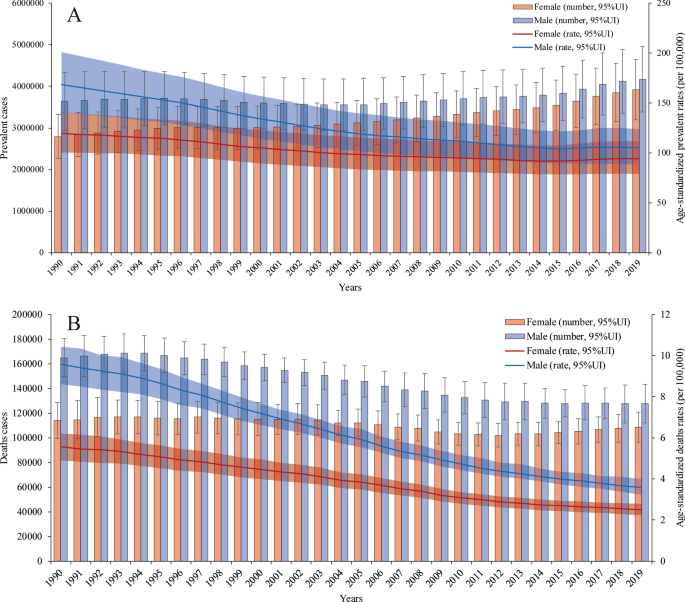
Prevalence rates and deaths with age-standardized rate changes in all years from 1990 to 2019. a The numbers of prevalent cases and age-standardized prevalence rates in males and females. b The numbers of deaths and age-standardized death rates in males and females
From Jan.1st, 1990 to Dec 31st, 2019, the number of PUD-related deaths has shown a gradual, fluctuating decreasing trend in females and a relatively significant decreasing trend in males. Moreover, the age-standardized death rates in both groups showed downward trends. Among males, there were 127,522.08 (95% UI 115,260.65 to 143,079.71) PUD-related deaths and 3.57 (95% UI 3.23 to 4.00) per 100,000 population PUD-related age-standardized deaths in 2019, whereas there were 164,933.87 (95% UI 146,881.12 to 180,422.89) deaths and 9.58 (95% UI 8.62 to 10.43) age-standardized deaths in 1990. Among females, there were 108,617.41 (95% UI 96,020.68 to 120,954.17) PUD-related deaths and 2.50 (95% UI 2.21 to 2.79) per 100,000 population PUD-related age-standardized deaths in 2019, whereas there were 114,044.63 (95% UI 99,995.18 to 128,749.67) death and 5.56 (95% UI 4.91 to 6.22) age-standardized deaths in 1990. The number of PUD-related deaths was lowest in 2012 [102,041.21 (95% UI 92,732.31 to 111,554.31)]. This may be related to a variety of factors, such as the age distributions of the different sexes and the proportions of aging populations around the world (Fig. 1 b). The patterns of incidence (Additional file 1 : Figure S1) and DALYs (Additional file 1 : Figure S2) by sex and year were relatively similar to those of prevalence and death, respectively.
The global prevalence of PUD was higher in females than in males on both ends of the age spectrum (more than 70 and less than 24 years old). The PUD prevalence rates peaked in 65- to 69-year-old females [330,974.81 (95% UI 223,943.66 to 485,784.86)] and 55- to 59-years-old males [391,973.56 (95% UI 259,447.97 to 569,117.47)] in 2019. In addition, the age-standardized prevalence rates increased with age, peaking at 80–84 years in both males [393.04 (95% UI 275.22 to 537.73)] and females [385.16 (95% UI 270.52 to 525.41)] and then decreased until patients reached the oldest age group in 2019. Age-standardized prevalence rates were also higher in females than in males at both ends of the age spectrum (more than 85 and less than 24 years old) (Fig. 2 a). However, the age-standardized incidence rates were higher in males than in females and increased with age, peaking in the more than 95-year-old group in both males and females in 2019 (Fig. 2 b). For the age-standardized death rates (Additional file 1 : Figure S3), there was a sharp increase in those aged more than 70 years, and the trend in males increased more than that in females; there was a similar trend in DALYs (Additional file 1 : Figure S4). PUD-related deaths peaked in 80- to 84-year old female patients; at this point, the number of deaths in female patients exceeded that in male patients, which peaked in 75- to 79-year-old males.
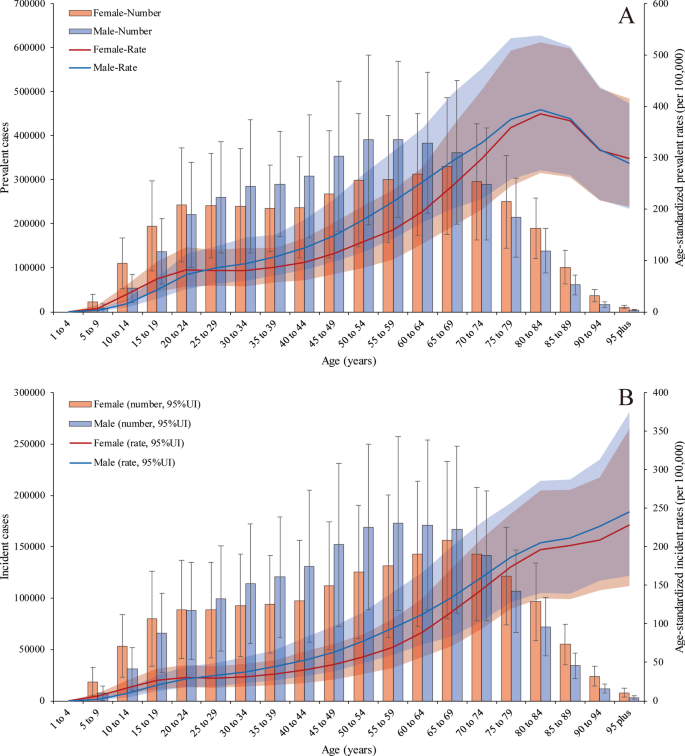
Prevalent and incident cases with age-standardized rate changes in 2019. a The numbers of prevalent cases and age-standardized prevalence rates in males and females. b The numbers of incident cases and age-standardized death rates in males and females
Regional burden of PUD
For 30 years, the age-standardized prevalence rate in South Asia [156.62 (95% UI 130.58 to 187.05) in 2019] was highest among all the GBD super-regions. However, it showed a sharp decreasing trend. The super-regions with lowest age-standardized prevalence rates were Latin America and the Caribbean [41.77 (95% UI 35.53 to 49.29) in 2019] (Additional file 1 : Figure S5). Moreover, the age-standardized incidence rate trends of the 7 super-regions were similar to the prevalence rate trends from 1990 to 2019 (Additional file 1 : Figure S6). The high-income super-region had the lowest age-standardized death rate from 1990 to 2019 [1.08 (95% UI 0.96 to 1.19) in 2019]. South Asia presented the largest decreasing trend in the age-standardized death rate during the study period. The percent change from 1990 to 2019 was 66.92% (95% UI 59.25% to 73.22%). Central Europe, Eastern Europe, and Central Asia experienced a fluctuating but gradually decreasing trend (Additional file 1 : Figure S7, S8). Among the 21 GBD regions, the prevalence (Additional file 1 : Figure S9) and incidence cases (Additional file 1 : Figure S10) were highest in South Asia [2.52 (95% UI 2.09 to 3.01) million for prevalence in 2019] and East Asia [1.49 (95% UI 1.22 to 1.82) million for prevalence in 2019], with increasing trends. Although the numbers of PUD-related DALYs (Additional file 1 : Figure S11) and deaths (Additional file 1 : Figure S12) in South Asia and East Asia accounted for the largest proportions, the number of DALYs showed a decreasing trend. The number of deaths in East Asia showed a slight but fluctuating decrease.
In 2019, the age-standardized prevalence rates in males were higher than those in females in 16 GBD regions, with the exception of Western sub-Saharan Africa, South Asia, North Africa and the Middle East, High-income North America and Central sub-Saharan Africa. The region with the greatest difference in prevalence between males and females was High-income Asia Pacific (male:female, 2.35:1), followed by Central Asia (male:female, 2.13:1)(Additional file 1 : Figure S13). The age-standardized incidence rate showed almost the same trend (Additional file 1 : Figure S14). The age-standardized DALY rate was slightly higher in females than in males in only South Asia; in the rest of the GBD regions, males had a higher DALY rate than females. The region with the greatest difference in DALYs between males and females was Eastern Europe (male:female, 2.97:1), followed by Central Asia (male:female, 2.46:1)(Additional file 1 : Figure S15). The trend of death was similar to that of DALYs in 2019 (Additional file 1 : Figure S16). From 1990 to 2019, the age-standardized prevalence rate in males in all GBD regions decreased to varying degrees, but among females, four regions showed variable increases: Eastern Europe, Southern sub-Saharan Africa, Western sub-Saharan Africa and Central sub-Saharan Africa. The age-standardized death rates decreased among all 21 GBD regions, except in females in Eastern Europe and Central Asia. After estimating the EAPCs in the age-standardized prevalence and DALY rates, only females in Eastern Europe showed a consistent increasing trend (Additional file 1 : Figure S17, S18, S19, S20).
National burden of PUD
The age-standardized prevalence rate estimated for PUD in 2019 ranged from 15.19 to 330.32 per 100,000 population. Kiribati [330.32 (95% UI 286.98 to 379.81)], Vanuatu [247.62 (95% UI 214.30 to 284.91)] and Greenland [209.77 (95% UI 182.50 to 239.31)] had the highest age-standardized prevalence rates in 2019. Israel [15.19 (95% UI 11.83 to 18.85)], Costa Rica [17.28 (95% UI 14.59 to 20.33)] and Panama [19.95 (95% UI 16.25 to 23.98)] had the lowest rates (Fig. 3 a). The EAPCs in the age-standardized prevalence rates from 1990 to 2019 differed substantially between countries and territories. Turkey [1.39 (95% CI 1.01 to 1.77)], Norway [1.27 (95% CI 0.86 to 1.69)] and Ghana [0.84 (95% CI 0.43 to 1.26)] showed the largest increases, and Bangladesh [-6.80 (95% CI − 7.07 to − 6.53)], Brazil [-4.76 (95% CI − 5.16 to − 4.35)] and Bhutan [-4.24 (95% CI − 4.55 to − 3.94)] showed the largest decreases (Fig. 3 b). The countries and territories with the highest age-standardized PUD prevalence rates in 2019 also had the highest age-standardized incidence rates (Additional file 1 : Figure S21). The country with the highest EAPC in incidence rate was Norway [1.08 (95% CI 0.45 to 1.71)] (Additional file 1 : Figure S22).
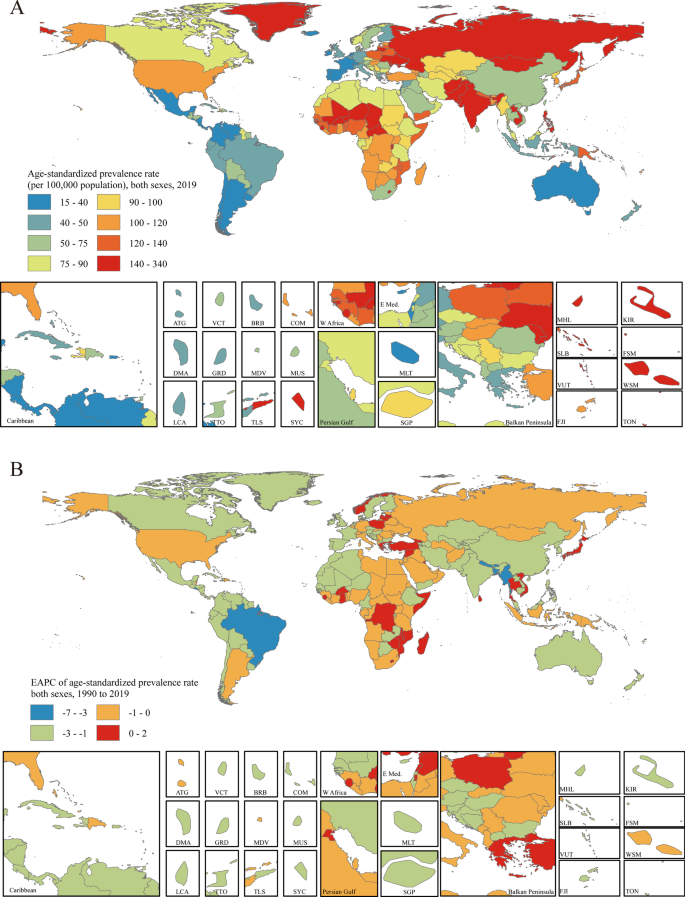
Distribution of age-standardized prevalence rates and EAPCs in age-standardized prevalence rates of PUD globally. a The age-standardized prevalence rate (per 100,000 population) in both sexes globally in 2019. b The EAPC in the age-standardized prevalence rate in both sexes globally from 1990 to 2019. Maps in Fig. 3 were designed and plotted by ArcGIS (version 9.0). ATG Antigua and Barbuda, BRB Barbados, COM Comoros, DMA Dominica, FJI Fiji, FSM Federated States of Micronesia, GRD Grenada, KIR Kiribati, LCA Saint Lucia, MDV Maldives, MHL Marshall Islands, MLT Malta, MUS Mauritius, SGP Singapore, SLB Solomon Islands, SYC Seychelles, TLS Timor-Leste, TON Tonga, TTO Trinidad and Tobago, VCT Saint Vincent and the Grenadines, VUT Vanuatu, WSM Samoa
Age-standardized PUD-associated death rates in 2019 varied from 0.46 to 22.48 per 100,000 population. Cambodia [22.48 (95% UI 17.42 to 28.98)], Kiribati [21.78 (95% UI 16.38 to 27.96)] and Laos [19.24 (95% UI 14.04 to 26.14)] had the highest age-standardized death rates in 2019. Sri Lanka [0.46 (95% UI 0.32 to 0.64)], Italy [0.58 (95% UI 0.50 to 0.64)] and Israel [0.60 (95% UI 0.48 to 0.74)] had the lowest rates (Additional file 1 : Figure S23a). Only eight of the 204 countries and territories showed potential increasing EAPCs in age-standardized death rates (red regions in Additional file 1 : Figure S23b). Among them, only one country, Lesotho [1.19 (95% CI 0.04 to 2.36)], showed a definite increase, with all 95% CIs greater than zero. Bangladesh [-10.08 (95% CI − 11.83 to − 8.29)], the Republic of Korea [-7.34 (95% CI − 9.61 to − 5.02)] and Spain [-7.24 (95% CI − 10.46 to − 3.91)] had the largest decreases in age-standardized death rates from 1990 to 2010 (Additional file 1 : Figure S23b).
Socioeconomic profiles of PUD
A lower SDI was associated with higher age-standardized prevalence rates, incidence rates, DALYs and deaths associated with PUD, with values that were higher than the global rate in the two highest SDI quintiles and lower than the global rate in the three lowest SDI quintiles. The age-standardized prevalence rates in the high-SDI and low-SDI quintiles were 80.98 (95% UI 68.19 to 95.81) and 145.35 (95% UI 123.83 to 169.90) per 100,000 population in 2019, respectively (Additional file 1 : Figure S24). The high-SDI quintile [1.18 (95% UI 1.04 to 1.29)] was associated with the lowest age-standardized death rate in 2019, while the low-SDI quintile [6.15 (95% UI 5.31 to 7.04)] had the second-highest rate, which was just slightly lower than that of the low-middle-SDI quintile (Additional file 1 : Figure S25). In contrast, the largest decreases in age-standardized prevalence, incidence, DALY and death rates from 1990 to 2019 occurred in the low-SDI and low-middle-SDI quintiles. For the 21 GBD regions, positive associations were found between the age-standardized prevalence (Fig. 4 a), incidence (Additional file 1 : Figure S26), DALY (Additional file 1 : Figure S27) and death (Fig. 4 b) rates and SDI between 1990 and 2019. However, in the Saharan African regions, the downward trend of the prevalence rate associated with increasing SDI was not obvious. In the Southern sub-Saharan African region, the death rate showed an inverted U-shaped pattern; the death rate and SDI were positively correlated when the SDI was < 0.59 and negatively correlated when the SDI > 0.59. Positive associations between age-standardized prevalence, incidence, DALY and death rates and SDI for 204 countries and territories in 2019 were observed. The age-standardized prevalence rate was higher than the expected level in some countries such as Kiribati, Vanuatu and Greenland (Fig. 5 ). The estimated age-standardized DALY rate decreased when the SDI improved. Some countries and territories also had DALY rates that were significantly higher than expected, such as Kiribati, Cambodia and Laos (Additional file 1 : Figure S28).
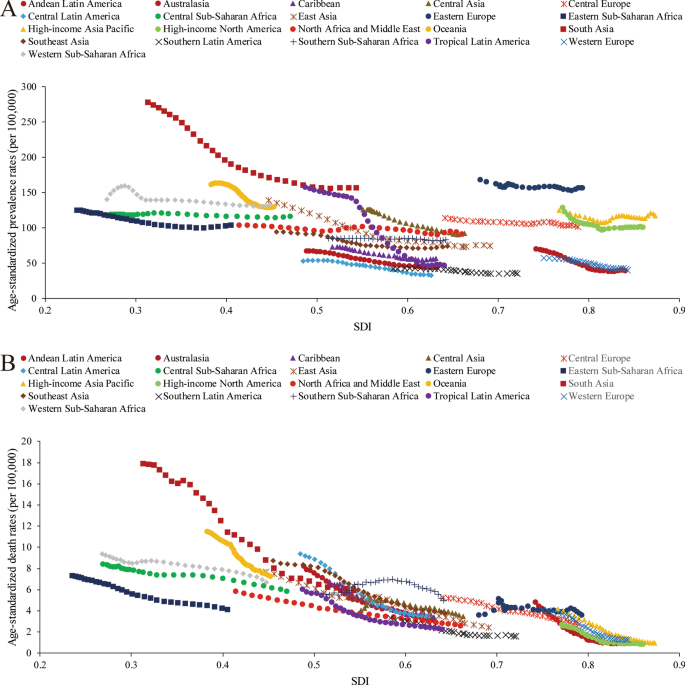
Trends of age-standardized prevalence and death rates (per 100,000 population) in 21 GBD regions by SDI from 1990 to 2019. a Trends of age-standardized prevalence rates; b trends of age-standardized death rates by SDI
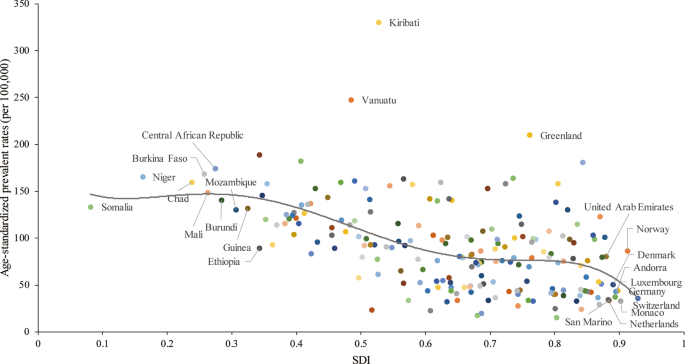
Age-standardized prevalence rates (per 100,000 population) of PUD in 204 countries globally by SDI in 2019. The gray line represents the expected age-standardized prevalence rate based on the SDI in 2019
PUD is usually defined as a greater than 3- to 5-mm rupture of the gastric or duodenal mucosa, which is caused by an imbalance in factors that protect the gastric and duodenal mucosa and factors that can cause damage. In this study, we analyzed the prevalence trends of PUD at the global, regional and national levels from 1990 to 2019, along with PPIs medication use over the span of thirty years. The prevalence of PUD in 2019 was approximately 8.09 million worldwide, and this study exhibited continues increasing tendency in the number of prevalent. Similar to the results of other research reports [ 16 , 17 ], the incidence of PUD showed a slight increase from 2006 to 2019, but the ASR showed a decreasing trend. However, in recent years, this downward trend has plateaued, which may be related to the fact that the main ulcer etiology has shifted in many countries from H. pylori infection to non-steroidal anti-inflammatory drug (NSAIDs) use [ 1 ]. For PUD, which is a chronic disease, it is often necessary to take appropriate drugs for a long period of time, and its recurrent characteristics and potentially serious complications have a significant impact on the social economy and medical and health costs.
PUD may be attributed to many etiologies, such as H. pylori infection, NSAIDs use, gastric bypass surgery, smoking, selective serotonin reuptake inhibitor use, stress, lifestyle habits and genetic characteristics, which have been identified as the main risk factors [ 1 , 2 ]. During the study period, especially in the first 20 years, the incidence of and mortality due to PUD showed significant decreasing trends, which were closely related to PPIs use and the widespread administration of anti- H. pylori treatment, which started in the late 1980s. In the last 10 years of the study period, the incidence of and mortality due to PUD showed relatively stable trends that did not decline with the further promotion of anti- H. pylori treatment. However, there was an increase in the use of NSAIDs, especially aspirin and other drugs, and these drugs often lead to serious complications in patients with PUD. In previous studies, especially in Australia, a country with an inexplicable history of H. pylori , H. pylori infection was associated with 70% to 90% of PUD cases [ 18 , 19 ]. Although these values are lower in some other studies, H. pylori infection is still a key factor in the pathogenesis of PUD [ 20 ]. Despite anti-inflammatory effect, NSAIDs are always used in antipyrexia and analgesic therapy, which makes NSAIDs as most commonly prescribed medicine [ 21 ]. Targeting cyclooxygenases enzymes (COXs), NSAIDs are divided into non-selective NSAIDs and selective COX-2 inhibitors, such as aspirin and celecoxib repectively [ 22 ]. However, NSAIDs could cause gastrointestinal adverse effects including ulcers, bleeding or perforation [ 21 , 23 ]. Drugs such as aspirin and other NSAIDs account for approximately 10% of PUD cases. NSAIDs have a stronger correlation with duodenal ulcers than with gastric ulcers. In recent decades, the use of these drugs has increased dramatically [ 24 , 25 ]. They account for approximately 5% to 10% of all prescription drugs each year and have shown an increasing trend [ 26 ]. In general practice, the prevalence of NSAIDs use in patients over 65 years old is as high as 96% [ 27 ]. A study from Norway indicated that approximately 7.3% of elderly patients over 60 years old took at least one NSAIDs prescription within one year period [ 28 ]. Differ from aspirin, selective COX-2 inhibitors have a weaker association with PUD than nonselective NSAIDs, which suppress COX-1 activity to inhibit gastric mucosa repair [ 29 , 30 ].
For sever obesity patients, bariatric surgery, such as Roux-en-Y gastric bypass (RYGBP) surgery and duodenal switch (DS) surgery, could be a proper therapy to reduce weight and comorbidities [ 31 ]. Due to well-established procedure and nearly 70 years of surgical experience, RYGBP is considered as gold standard for bariatric surgery [ 32 ]. Although overweight and related metabolic symptoms would be reduced after gastric bypass surgery, several complications still might influence the recovery of operated patients, such as marginal ulcer (MU). MU developed at or distal to gastroenteral anastomosis and occurs in approximately 5% of obese patients undergoing gastric bypass surgery [ 31 , 33 ]. In patients with upper gastrointestinal symptoms after gastric bypass surgery, the incidence can reach 27% to 36%, indicating gastric bypass surgery history might contribute to the development of PUD [ 1 , 34 ].
In the vast majority of GBD regions, the incidence of and mortality due to PUD showed significant downward trends with increasing SDI; this is closely related to the awareness of H. pylori treatment and the appropriate management of other chronic diseases. However, in some areas, this relationship is questionable, especially in the southern sub-Saharan African region. The cause of this phenomenon is still controversial and may be related to the epidemiological characteristics and risk factors for PUD in such countries and territories. Although the incidence of H. pylori infection had decreased as SDIs increased in these areas, in some countries, the infection rate remains very high. NSAIDs use rates in these areas were significantly lower than those in developed countries [ 35 , 36 ]. Studies have also shown that the rates of severe PUD-related complications, such as perforation of the digestive tract, are high in these countries and territories and appear to be associated with Khat intake [ 37 ]. When stratified by SDI, the incidence of PUD showed decreasing trends in different groups over time. The decrease was more obvious in low- and low-middle-SDI regions than in high-SDI regions, and the incidence of PUD tended to plateau in high-SDI regions; however, it was still far lower than those in low-SDI regions. PUD-related deaths decreased in all groups. In 2019, in different countries and regions, the prevalence of PUD generally decreased with increasing SDI, but in some Pacific islands, such as Kiribati and Vanuatu, the prevalence of PUD remained abnormally high. This may be related to the high H. pylori infection rate among and the ethnic characteristics of Pacific islanders. Some studies found that Maori and Pacific Island adults and children living in South Auckland had a high rate of infection with H. pylori compared to Europeans from the same area [ 38 ]. Data showed that household crowding involving children in New Zealand contributed to 44% of H. pylori infections in Pacific Islanders, 36% in Maori people and 14% in Europeans [ 39 , 40 ].
The age-standardized incidence rate showed an increasing annual trend with increasing age. This is different from the incidence rate of H. pylori . A multicenter cross-sectional study showed that the positive rate of H. pylori serum antibody increased linearly from the 30- to 39-year-old age group to the 60- to 69-year-old group, while the infection rates of H. pylori in people aged 20 to 29 and over 70 years old were low. The change in the H. pylori infection rate showed a trend of initially increasing and then decreasing with age [ 41 ]. The consistent increase in the age-standardized incidence rate may be associated with an increased incidence of drug-related PUD in older people who are more likely to suffer from other chronic diseases and require other medications, such as NSAIDs. Studies have shown that the use of NSAIDs peaks at approximately 50 years old, and after 70 years old, the use of NSAIDs, especially aspirin, decreases, which is related to the increased incidence of NSAID-related adverse reactions in the elderly population [ 42 , 43 ]. Estrogen can prevent ulcers by inhibiting the synthesis and release of gastrin and reducing the secretion of gastric acid. The decrease in estrogen levels in elderly females may be a protective factor for PUD [ 44 ] and may also cause a slight decrease in the growth trend of the age-standardized incidence rate in elderly women over 80 years old.
Regarding sex, at the end of the twentieth century, the incidence of and mortality due to PUD showed significant decreases, and the trends in males were more obvious than those in females. Over time, the decreasing trends of the age-standardized incidence and mortality rates became less steep, but the rates in males remained higher than those in females. PUD-related deaths and DALYs were significantly lower in females than in males. However, further analysis of PUD-related factors revealed that the incidence in females was slightly higher than that in males aged less than 20 years. In 2019, among the different GBD regions, the incidence rates of PUD in the Oceania, High-income Asia Pacific, Eastern Europe, East Asia and Central Asia regions were significantly higher in males than in females, while in other regions, there was no significant difference in the incidence between the sexes. In some regions, such as Western sub-Saharan Africa, the rate in females was significantly higher than that in males. However, the PUD-related death rate was higher in males than in females, except in Central sub-Saharan Africa. In West African countries, such as Ghana and Nigeria, the incidence of PUD in females accounted for approximately 54–57% [ 35 ]. Research results have shown that there is a sex difference in the influence of acetic acid-induced gastric ulcer formation in rats. After the administration of certain interventions, the natural defensive mechanism in the gastric mucosa was adversely disturbed in male rats but activated in female rats [ 45 ]. This phenomenon is similar to previous clinical and the current research results, although the specific mechanism is not completely clear.
There were increasing trends in the EAPCs in the age-standardized prevalence and DALY rates among only female patients in Eastern Europe (marginally increasing incidence and death rate trends) from 1990 to 2019, mainly due to the increase in the number of females in Russia and the Ukraine. In these two countries, especially in Siberia, the H. pylori infection rates were significantly higher than those in other regions. However, there were no differences in H. pylori infection rates between the sexes and among ethnicities [ 46 , 47 ]. Therefore, the consistently increased risk of PUD among females in these areas may be related to factors such as drugs or bypass surgery [ 48 , 49 ]. These reasons require further study to produce high-level evidence-based medical evidence.
The roles of anti- H. pylori therapy and acid suppression therapy in the treatment and prevention of PUD are clear. Identifying high-risk populations and preventing drug-related complications are particularly important at this stage. Anti- H. pylori treatment can reduce the incidence of PUD and reduce the risk of gastric cancer in high-risk groups. Similar to acid suppression therapy, it will not increase the economic burden of disease treatment. However, there is still some controversy regarding whether to administer anti- H. pylori treatment in all positive patients considering the extensive administration of antibiotics, as it may promote the production of other resistant bacteria, leading to the occurrence of Clostridium difficile -associated diarrhea, and increase the potential risk of cardiovascular disease [ 50 , 51 ]. Acid suppressant drugs, mainly PPIs, seem to be the most effective class of gastroprotectants for the management of PUD [ 52 ]. Especially in recent years, the risk factors for PUD have gradually changed from H. pylori infection to drug-related PUD, so treatment with PPIs has gradually increased in importance. Especially in patients with gastrointestinal bleeding, PPIs can significantly reduce the incidence of adverse outcomes. However, PPIs also have certain medication risks, such as an increased risk of fracture and the development of other infections. These risks still lack strong evidence, and the benefits of PPIs greatly outweigh their associated risks. The current controversy mainly concerns the durations and doses of PPIs and whether oral PPIs have the same effect as intravenous medication in maintenance treatment [ 53 ].
The study has some limitations. First, critical information about disease burden in some countries does not exist or was unavailable, making it difficult to illustrate and understand health trends. Second, compared with other chronic noncommunicable diseases with high mortality rates, the mortality rate associated with PUD is relatively low, but PUD has the characteristic of recurrence. The high prevalence rate also results in a high disease burden. However, compared with that of malignant tumors, diabetes, and cardiovascular and cerebrovascular diseases, evidence indicating that PUD should be considered a high-priority disease is lacking [ 54 ].
In conclusion, this study focused on the epidemiological characteristics of PUD in different countries and territories, different age groups and different sexes. Overall, the risk of morbidity and mortality due to PUD decreased significantly, but with the passage of time for H. pylori eradication, the downward trend gradually weakened, which might be related to the gradual shift in the main risk factors for PUD from H. pylori infection to wide use of NSAIDs. Therefore, additional medical and health-related attention is needed to control the incidence of PUD and the occurrence of adverse events.
Availability of data and materials
The datasets generated and/or analyzed during the current study are available in the Global Burden of Disease, Injuries and Risk Factors Study ( http://ghdx.healthdata.org/ ).
Abbreviations
- Peptic ulcer disease
Proton-pump inhibitor
Nonsteroidal anti-inflammatory drugs
Global Burden of Disease, Injuries and Risk Factors Study
Disability-adjusted life years
Age-standardized rate
Global Health Data Exchange database
Sociodemographic index
Estimated annual percentage change
Generalized linear model
Confidence interval
Uncertainty interval
Sverdén E, Agréus L, Dunn J, Lagergren J. Peptic ulcer disease. BMJ (Clinical research ed). 2019;367:l5495.
Lanas A, Chan F. Peptic ulcer disease. Lancet (London, England). 2017;390(10094):613–24.
Article Google Scholar
Rosenstock S, Jørgensen T. Prevalence and incidence of peptic ulcer disease in a Danish County—a prospective cohort study. Gut. 1995;36(6):819–24.
Article CAS PubMed PubMed Central Google Scholar
Gralnek I, Dumonceau J, Kuipers E, Lanas A, Sanders D, Kurien M, et al. Diagnosis and management of nonvariceal upper gastrointestinal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015;47(10):a1-46.
Article PubMed Google Scholar
Lau J, Sung J, Hill C, Henderson C, Howden C, Metz D. Systematic review of the epidemiology of complicated peptic ulcer disease: incidence, recurrence, risk factors and mortality. Digestion. 2011;84(2):102–13.
Jennings D. Perforated peptic ulcer: changes in age-incidence and sex-distribution in the last 150 years. The Lancet. 1940;235(6080):444–7.
Sonnenberg A, Everhart JE. The prevalence of self-reported peptic ulcer in the United States. Am J Public Health. 1996;86(2):200–5.
Sonnenberg A. Review article: historic changes of Helicobacter pylori-associated diseases. Aliment Pharmacol Ther. 2013;38(4):329–42.
Article CAS PubMed Google Scholar
Lanas A, García-Rodríguez L, Polo-Tomás M, Ponce M, Quintero E, Perez-Aisa M, et al. The changing face of hospitalisation due to gastrointestinal bleeding and perforation. Aliment Pharmacol Ther. 2011;33(5):585–91.
Goh K-L., Liew W-C, et al. Time trends in upper gastrointestinal diseases and Helicobacter pylori infection in a multiracial Asian population—a 20-year experience over three time periods. Aliment Pharmacol Therap. 2016.
Malmi H, Kautiainen H, Virta L, Färkkilä N, Koskenpato J, Färkkilä M. Incidence and complications of peptic ulcer disease requiring hospitalisation have markedly decreased in Finland. Aliment Pharmacol Ther. 2014;39(5):496–506.
Dall M, Schaffalitzky de Muckadell O, Lassen A, Hallas J. There is an association between selective serotonin reuptake inhibitor use and uncomplicated peptic ulcers: a population-based case-control study. Aliment Pharmacol Therap 2010;32:1383–91.
Krag M, Perner A, Wetterslev J, Wise M, Borthwick M, Bendel S, et al. Prevalence and outcome of gastrointestinal bleeding and use of acid suppressants in acutely ill adult intensive care patients. Intensive Care Med. 2015;41(5):833–45.
Collaborators GCoD. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet (London, England). 2018;392(10159):1736–88.
Collaborators GMaCoD. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet (London, England). 2015;385(9963):117–71.
Sung J, Kuipers E, El-Serag H. Systematic review: the global incidence and prevalence of peptic ulcer disease. Aliment Pharmacol Ther. 2009;29(9):938–46.
Agréus L, Hellström P, Talley N, Wallner B, Forsberg A, Vieth M, et al. Helicobacter pyloriTowards a healthy stomach? Prevalence has dramatically decreased over 23 years in adults in a Swedish community. United Eur Gastroenterol J. 2016;4(5):686–96.
Eslick G, Tilden D, Arora N, Torres M, Clancy R. Clinical and economic impact of “triple therapy” for Helicobacter pylori eradication on peptic ulcer disease in Australia. Helicobacter. 2020;25(6):e12751.
Ford A, Gurusamy K, Delaney B, Forman D, Moayyedi P. Eradication therapy for peptic ulcer disease in Helicobacter pylori-positive people. Cochrane Database Syst Rev. 2016;4:CD003840.
PubMed Google Scholar
Ford A, Forman D, Hunt R, Yuan Y, Moayyedi P. Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: systematic review and meta-analysis of randomised controlled trials. BMJ (Clinical research ed). 2014;348:g3174.
Abdulla A, Adams N, Bone M, Elliott AM, Gaffin J, Jones D, et al. Guidance on the management of pain in older people. Age Ageing. 2013;42(Suppl 1):i1-57.
Rainsford KD. Anti-inflammatory drugs in the 21st century. Subcell Biochem. 2007;42:3–27.
Sabzwari SR, Qidwai W, Bhanji S. Polypharmacy in elderly: a cautious trail to tread. J Pak Med Assoc. 2013;63(5):624–7.
Zhou Y, Boudreau DM, Freedman AN. Trends in the use of aspirin and nonsteroidal anti-inflammatory drugs in the general U.S. population. Pharmacoepidemiology and drug safety. 2014;23(1):43–50.
Fosbøl EL, Gislason GH, Jacobsen S, Abildstrom SZ, Hansen ML, Schramm TK, et al. The pattern of use of non-steroidal anti-inflammatory drugs (NSAIDs) from 1997 to 2005: a nationwide study on 4.6 million people. Pharmacoepidemiology and drug safety. 2008;17(8):822–33.
Wongrakpanich S, Wongrakpanich A, Melhado K, Rangaswami J. A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging Dis. 2018;9(1):143–50.
Article PubMed PubMed Central Google Scholar
Pilotto A, Franceschi M, Leandro G, Di Mario F. NSAID and aspirin use by the elderly in general practice: effect on gastrointestinal symptoms and therapies. Drugs Aging. 2003;20(9):701–10.
Vandraas K, Spigset O, Mahic M, Slørdal L. Non-steroidal anti-inflammatory drugs: use and co-treatment with potentially interacting medications in the elderly. Eur J Clin Pharmacol. 2010;66(8):823–9.
Silverstein F, Faich G, Goldstein J, Simon L, Pincus T, Whelton A, et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: A randomized controlled trial. Celecoxib Long-term Arthritis Safety Study JAMA. 2000;284(10):1247–55.
CAS PubMed Google Scholar
Harirforoosh S, Asghar W, Jamali F. Adverse effects of nonsteroidal antiinflammatory drugs: an update of gastrointestinal, cardiovascular and renal complications. J Pharm Pharmaceut Sci. 2013;16(5):821–47.
Google Scholar
Bekhali Z, Sundbom M. Low risk for marginal ulcers in duodenal switch and gastric bypass in a well-defined cohort of 472 patients. Obes Surg. 2020;30(11):4422–7.
Angrisani L, Santonicola A, Iovino P, Vitiello A, Zundel N, Buchwald H, et al. Bariatric surgery and endoluminal procedures: IFSO worldwide survey 2014. Obes Surg. 2017;27(9):2279–89.
Coblijn U, Goucham A, Lagarde S, Kuiken S, van Wagensveld B. Development of ulcer disease after Roux-en-Y gastric bypass, incidence, risk factors, and patient presentation: a systematic review. Obes Surg. 2014;24(2):299–309.
El-Hayek K, Timratana P, Shimizu H, Chand B. Marginal ulcer after Roux-en-Y gastric bypass: What have we really learned? Surg Endosc. 2012;26(10):2789–96.
Archampong T, Asmah R, Richards C, Martin V, Bayliss C, Botão E, et al. Gastro-duodenal disease in Africa: literature review and clinical data from Accra. Ghana World J Gastroenterol. 2019;25(26):3344–58.
Smith S, Fowora M, Pellicano R. Helicobacter pyloriInfections with and challenges encountered in Africa. World J Gastroenterol. 2019;25(25):3183–95.
Bupicha J, Gebresellassie H, Alemayehu A. Pattern and outcome of perforated peptic ulcer disease patient in four teaching hospitals in Addis Ababa, Ethiopia: a prospective cohort multicenter study. BMC Surg. 2020;20(1):135.
Fraser A, Scragg R, Metcalf P, McCullough S, Yeates N. Prevalence of Helicobacter pylori infection in different ethnic groups in New Zealand children and adults. Aust N Z J Med. 1996;26(5):646–51.
Mitchell H, Katelaris P. Epidemiology, clinical impacts and current clinical management of Helicobacter pylori infection. Med J Aust. 2016;204(10):376–80.
McDonald A, Sarfati D, Baker M, Blakely T. Trends in Helicobacter pylori infection among Māori, Pacific, and European Birth cohorts in New Zealand. Helicobacter. 2015;20(2):139–45.
Lim S, Kim N, Kwon J, Kim S, Baik G, Lee J, et al. Trends in the seroprevalence of Helicobacter pylori infection and its putative eradication rate over 18 years in Korea: A cross-sectional nationwide multicenter study. PLoS ONE. 2018;13(10):e0204762.
Sarganas G, Buttery A, Zhuang W, Wolf I, Grams D, Rosario A, et al. Prevalence, trends, patterns and associations of analgesic use in Germany. BMC Pharmacol Toxicol. 2015;16:28.
Gómez-Acebo I, Dierssen-Sotos T, de Pedro M, Pérez-Gómez B, Castaño-Vinyals G, Fernández-Villa T, et al. Epidemiology of non-steroidal anti-inflammatory drugs consumption in Spain The MCC-Spain study. BMC Public Health. 2018;18(1):1134.
Gadzhanova S, Ilomäki J, Roughead E. COX-2 inhibitor and non-selective NSAID use in those at increased risk of NSAID-related adverse events: a retrospective database study. Drugs Aging. 2013;30(1):23–30.
Liu E, Wong B, Cho C. Influence of gender difference and gastritis on gastric ulcer formation in rats. J Gastroenterol Hepatol. 2001;16(7):740–7.
Matysiak-Budnik T, Mégraud F. Helicobacter pylori in eastern European countries: what is the current status? Gut. 1994;35(12):1683–6.
Tkachenko M, Zhannat N, Erman L, Blashenkova E, Isachenko S, Isachenko O, et al. Dramatic changes in the prevalence of Helicobacter pylori infection during childhood: a 10-year follow-up study in Russia. J Pediatr Gastroenterol Nutr. 2007;45(4):428–32.
Wysocki A, Budzyński P, Kulawik J, Drożdż W. Changes in the localization of perforated peptic ulcer and its relation to gender and age of the patients throughout the last 45 years. World J Surg. 2011;35(4):811–6.
Thorsen K, Søreide J, Kvaløy J, Glomsaker T, Søreide K. Epidemiology of perforated peptic ulcer: age- and gender-adjusted analysis of incidence and mortality. World J Gastroenterol. 2013;19(3):347–54.
Skole K, Mahpour N. There Are Several Reasons to Not Treat H. pylori. Am J Gastroenterol. 2020;115(8):1301.
Desai T, Edhi A, Hakim S. Eradicating H. pylori. Am J Gastroenterol. 2019;114(12):1827–8.
Scally B, Emberson J, Spata E, Reith C, Davies K, Halls H, et al. Effects of gastroprotectant drugs for the prevention and treatment of peptic ulcer disease and its complications: a meta-analysis of randomised trials. Lancet Gastroenterol Hepatol. 2018;3(4):231–41.
Kuipers E. PPIs for prevention and treatment of peptic ulcer. Lancet Gastroenterol Hepatol. 2018;3(4):214–5.
Voigt K, King N. Out of alignment? Limitations of the Global Burden of Disease in assessing the allocation of global health aid. Public Health Ethics. 2017;10(3):244–56.
Download references
Acknowledgements
We thank the Global Burden of Disease, Injuries and Risk Factors (GBD) Study which provided the free data for this study.
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Author information
Authors and affiliations.
Department of Surgical Oncology, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, 710061, Shaanxi, China
Xin Xie, Kaijie Ren, Chengxue Dang & Hao Zhang
Department of Nuclear Medicine, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, 710061, Shaanxi, China
Department of Oncology, The Second Affiliated Hospital of Xi’an Jiaotong University, Xi’an, 710004, Shaanxi, China
Zhangjian Zhou
Clinical Medicine and Cancer Research Center of Shaanxi Province, Xi’an, 710061, Shaanxi, China
Chengxue Dang & Hao Zhang
You can also search for this author in PubMed Google Scholar
Contributions
XX, KJR and HZ prepared the manuscript. XX, ZJZ and HZ analyzed and managed the data of this study. XX and HZ reviewed the final manuscript. CXD and HZ designed and supervised this study. All authors reviewed the data and agreed the publication of this manuscript.
Corresponding author
Correspondence to Hao Zhang .
Ethics declarations
Ethics approval and consent to participate.
All data involved in this study were available from the public and open access database GBD, no patient contact was made. Therefore, ethics approval, informed consent and access permission of database for this study was not required.
Consent for publication
Not applicable.
Competing interests
All authors declare that they have no conflicts of interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file.
1 : Table S1 . PUD prevalence in 1990 and 2019 for both sexes and estimated annual percentage change in age-standardized rates by location; Table S2 . PUD incidence in 1990 and 2019 for both sexes and estimated annual percentage change in age-standardized rates by location; Table S3 . DALYs of PUD in 1990 and 2019 for both sexes and estimated annual percentage change in age-standardized rates by location; Table S4 . PUD death in 1990 and 2019 for both sexes and estimated annual percentage change in age-standardized rates by location; Figure S1 . Incident cases with age-standardized incidence rate (per 100,000 population) changes in all years from 1990 to 2019; Figure S2 . DALYs with age-standardized rate (per 100,000 population) changes in all years from 1990 to 2019; Figure S3 . Deaths with age-standardized death rate (per 100,000 population) changes by age in 2019; Figure S4 . DALYs with age-standardized DALY rate (per 100,000 population) changes by age in 2019; Figure S5 . Age-standardized prevalent rate changes in PUD in seven super-regions in all years from 1990 to 2019; Figure S6 . Trends of age-standardized incidence rates (per 100,000 population) in seven super-regions in all years from 1990 to 2019; Figure S7 . Trends of age-standardized death rates (per 100,000 population) in seven super-regions in all years from 1990 to 2019; Figure S8 . Trends of age-standardized DALY rates (per 100,000 population) in seven super-regions in all years from 1990 to 2019; Figure S9 . Trends of prevalent cases of PUD in 21 GBD regions in all years from 1990 to 2019; Figure S10 . Trends of incident cases of PUD in 21 GBD regions in all years from 1990 to 2019; Figure S11 . Trends of DALYs due to PUD in 21 GBD regions in all years from 1990 to 2019; Figure S12 . Trends of PUD-related deaths in 21 GBD regions in all years from 1990 to 2019; Figure S13 . Age-standardized prevalence rates (per 100,000 population) of PUD in males and females in 21 GBD regions in 2019; Figure S14 . Age-standardized incident rates (per 100,000 population) of PUD in males and females in 21 GBD regions in 2019; Figure S15 . Age-standardized DALY rates (per 100,000 population) due to PUD in males and females in 21 GBD regions in 2019; Figure S16 . Age-standardized death rates (per 100,000 population) due to PUD in males and females in 21 GBD regions in 2019; Figure S17 . Estimated annual percentage changes in age-standardized prevalent rates in different regions between 1990 and 2019; Figure S18 . Estimated annual percentages of age-standardized incident rates (per 100,000 population) in 21 GBD regions between 1999 and 2019; Figure S19 . Estimated annual percentages of age-standardized DALY rates (per 100,000 population) in 21 GBD regions between 1999 and 2019; Figure S20 . Estimated annual percentages of age-standardized death rates (per 100,000 population) in 21 GBD regions between 1999 and 2019; Figure S21 . Distributions of age-standardized incidence rates (per 100,000 population) of PUD in different regions in 2019; Figure S22 . Distributions of age-standardized incidence rates (per 100,000 population) of PUD in different regions from 1999 to 2019; Figure S23 . Distributions of age-standardized death rates and EAPCs in age-standardized prevalence rates of PUD globally; Figure S24 . Trends of age-standardized prevalence rates (per 100,000 population) in different SDI regions from 1990 to 2019; Figure S25 . Age-standardized death rates (per 100,000 population) from 1990 to 2019 in different SDI regions; Figure S26 . Trends of age-standardized incidence rates (per 100,000 population) of PUD in 21 GBD regions by SDI; Figure S27 . Trends of age-standardized DALY rates (per 100,000 population) of PUD in 21 GBD regions by SDI; Figure S28 . Age-standardized DALY rates (per 100,000 population) due to PUD globally in 204 countries and territories by SDI in 2019.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Xie, X., Ren, K., Zhou, Z. et al. The global, regional and national burden of peptic ulcer disease from 1990 to 2019: a population-based study. BMC Gastroenterol 22 , 58 (2022). https://doi.org/10.1186/s12876-022-02130-2
Download citation
Received : 16 April 2021
Accepted : 31 January 2022
Published : 10 February 2022
DOI : https://doi.org/10.1186/s12876-022-02130-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Disease burden
- Sociodemographic characteristics
- Global Burden of Disease
- Injuries and Risk Factors Study
BMC Gastroenterology
ISSN: 1471-230X
- Submission enquiries: [email protected]
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
Patient Case Presentation. A 61-year-old American woman was referred to a Gastroenterology Clinic from primary care provider due to consistent discomfort and significant weight loss. She looked for a PCP’s advice as she had a tarry stool in the early morning which she had never experienced before. She presented with a 2-month history of ...
In 2009, the Japanese Society of Gastroenterology (JSGE) developed evidence-based clinical practice guidelines for peptic ulcer disease. The guidelines were revised in 2015 and again in 2020. Of the 90 clinical questions (CQs) included in the previous guidelines, those with a clear conclusion were considered background questions (BQs) and those ...
Case Background. A peptic ulcer is a sore that occurs in the lining of a part of the gastrointestinal tract that is exposed to pepsin and acid secretions. Most peptic ulcers occur in the lining of the stomach or duodenum. 90% of all duodenal ulcers and 80% of all gastric ulcers are caused by H. pylori infection.
2,000+ HD Videos. 300+ Nursing Cheatsheets. Start Free Trial. “Would suggest to all nursing students . . . Guaranteed to ease the stress!”. ~Jordan. Peptic Ulcer Disease Case Study (60 min) is mentioned in these lessons. Check out this case study on peptic ulcer disease & learn everything you will need to about it. View the lesson today!
Figure 3. An esophagogastroduodenoscopy revealed a double pylorus. A clean-based ulcer is also visible. The patient was diagnosed with double pylorus, an uncommon complication of peptic ulcer disease. The patient was started on a proton pump inhibitor (PPI) and discharged in stable condition.
Background. In a recent multicentre European study, the prevalence of peptic ulceration was 8.1% in children presenting with abdominal pain, the majority of patients being males in the second decade of life. 1 Helicobacter pylori infection and non-steroidal anti-inflammatory drug ingestion are the main aetiological risk factors in the paediatric age. 2 The classic presentation of patients with ...
Paimela, H, Oksala, NK, Kivilaakso, E. Surgery for peptic ulcer today: a study on the incidence, methods and mortality in surgery for peptic ulcer in Finland between 1987 and 1999. Dig Surg 2004 ...
Background Peptic ulcer disease (PUD) is a common digestive disorder, of which the prevalence decreased in the past few decades. However, the decreasing tendency has plateaued in recent years due to changes in risk factors associated with the etiology of PUD, such as non-steroidal anti-inflammatory drug use. In this study, we investigated the epidemiological and the sociodemographic ...