Loading metrics
Open Access
Learning Forum
Learning Forum articles are commissioned by our educational advisors. The section provides a forum for learning about an important clinical problem that is relevant to a general medical audience.
See all article types »

A 21-Year-Old Pregnant Woman with Hypertension and Proteinuria
- Andrea Luk,
* To whom correspondence should be addressed. E-mail: [email protected]
- Ching Wan Lam,
- Wing Hung Tam,
- Anthony W. I Lo,
- Enders K. W Ng,
- Alice P. S Kong,
- Wing Yee So,
- Chun Chung Chow
- Andrea Luk,
- Ronald C. W Ma,
- Ching Wan Lam,
- Wing Hung Tam,
- Anthony W. I Lo,
- Enders K. W Ng,
- Alice P. S Kong,
- Wing Yee So,

Published: February 24, 2009
- https://doi.org/10.1371/journal.pmed.1000037
- Reader Comments
Citation: Luk A, Ma RCW, Lam CW, Tam WH, Lo AWI, Ng EKW, et al. (2009) A 21-Year-Old Pregnant Woman with Hypertension and Proteinuria. PLoS Med 6(2): e1000037. https://doi.org/10.1371/journal.pmed.1000037
Copyright: © 2009 Luk et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Funding: The authors received no specific funding for this article.
Competing interests: RCWM is Section Editor of the Learning Forum. The remaining authors have declared that no competing interests exist.
Abbreviations: CT, computer tomography; I, iodine; MIBG, metaiodobenzylguanidine; MRI, magnetic resonance imaging; SDH, succinate dehydrogenase; SDHD, succinate dehydrogenase subunit D
Provenance: Commissioned; externally peer reviewed
Description of Case
A 21-year-old pregnant woman, gravida 2 para 1, presented with hypertension and proteinuria at 20 weeks of gestation. She had a history of pre-eclampsia in her first pregnancy one year ago. During that pregnancy, at 39 weeks of gestation, she developed high blood pressure, proteinuria, and deranged liver function. She eventually delivered by emergency caesarean section following failed induction of labour. Blood pressure returned to normal post-partum and she received no further medical follow-up. Family history was remarkable for her mother's diagnosis of hypertension in her fourth decade. Her father and five siblings, including a twin sister, were healthy. She did not smoke nor drink any alcohol. She was not taking any regular medications, health products, or herbs.
At 20 weeks of gestation, blood pressure was found to be elevated at 145/100 mmHg during a routine antenatal clinic visit. Aside from a mild headache, she reported no other symptoms. On physical examination, she was tachycardic with heart rate 100 beats per minute. Body mass index was 16.9 kg/m 2 and she had no cushingoid features. Heart sounds were normal, and there were no signs suggestive of congestive heart failure. Radial-femoral pulses were congruent, and there were no audible renal bruits.
Baseline laboratory investigations showed normal renal and liver function with normal serum urate concentration. Random glucose was 3.8 mmol/l. Complete blood count revealed microcytic anaemia with haemoglobin level 8.3 g/dl (normal range 11.5–14.3 g/dl) and a slightly raised platelet count of 446 × 10 9 /l (normal range 140–380 × 10 9 /l). Iron-deficient state was subsequently confirmed. Quantitation of urine protein indicated mild proteinuria with protein:creatinine ratio of 40.6 mg/mmol (normal range <30 mg/mmol in pregnancy).
What Were Our Differential Diagnoses?
An important cause of hypertension that occurs during pregnancy is pre-eclampsia. It is a condition unique to the gravid state and is characterised by the onset of raised blood pressure and proteinuria in late pregnancy, at or after 20 weeks of gestation [ 1 ]. Pre-eclampsia may be associated with hyperuricaemia, deranged liver function, and signs of neurologic irritability such as headaches, hyper-reflexia, and seizures. In our patient, hypertension developed at a relatively early stage of pregnancy than is customarily observed in pre-eclampsia. Although she had proteinuria, it should be remembered that this could also reflect underlying renal damage due to chronic untreated hypertension. Additionally, her electrocardiogram showed left ventricular hypertrophy, which was another indicator of chronicity.
While pre-eclampsia might still be a potential cause of hypertension in our case, the possibility of pre-existing hypertension needed to be considered. Box 1 shows the differential diagnoses of chronic hypertension, including essential hypertension, primary hyperaldosteronism related to Conn's adenoma or bilateral adrenal hyperplasia, Cushing's syndrome, phaeochromocytoma, renal artery stenosis, glomerulopathy, and coarctation of the aorta.
Box 1: Causes of Hypertension in Pregnancy
- Pre-eclampsia
- Essential hypertension
- Renal artery stenosis
- Glomerulopathy
- Renal parenchyma disease
- Primary hyperaldosteronism (Conn's adenoma or bilateral adrenal hyperplasia)
- Cushing's syndrome
- Phaeochromocytoma
- Coarctation of aorta
- Obstructive sleep apnoea
Renal causes of hypertension were excluded based on normal serum creatinine and a bland urinalysis. Serology for anti-nuclear antibodies was negative. Doppler ultrasonography of renal arteries showed normal flow and no evidence of stenosis. Cushing's syndrome was unlikely as she had no clinical features indicative of hypercortisolism, such as moon face, buffalo hump, violaceous striae, thin skin, proximal muscle weakness, or hyperglycaemia. Plasma potassium concentration was normal, although normokalaemia does not rule out primary hyperaldosteronism. Progesterone has anti-mineralocorticoid effects, and increased placental production of progesterone may mask hypokalaemia. Besides, measurements of renin activity and aldosterone concentration are difficult to interpret as the renin-angiotensin-aldosterone axis is typically stimulated in pregnancy. Phaeochromocytoma is a rare cause of hypertension in pregnancy that, if unrecognised, is associated with significant maternal and foetal morbidity and mortality. The diagnosis can be established by measuring levels of catecholamines (noradrenaline and adrenaline) and/or their metabolites (normetanephrine and metanephrine) in plasma or urine.
What Was the Diagnosis?
Catecholamine levels in 24-hour urine collections were found to be markedly raised. Urinary noradrenaline excretion was markedly elevated at 5,659 nmol, 8,225 nmol, and 9,601 nmol/day in repeated collections at 21 weeks of gestation (normal range 63–416 nmol/day). Urinary adrenaline excretion was normal. Pregnancy may induce mild elevation of catecholamine levels, but the marked elevation of urinary catecholamine observed was diagnostic of phaeochromocytoma. Conditions that are associated with false positive results, such as acute myocardial infarction, congestive heart failure, acute cerebrovascular event, withdrawal from alcohol, withdrawal from clonidine, and cocaine abuse, were not present in our patient.
The working diagnosis was therefore phaeochromocytoma complicating pregnancy. Magnetic resonance imaging (MRI) of neck to pelvis, without gadolinium enhancement, was performed at 24 weeks of gestation. It showed a 4.2 cm solid lesion in the mid-abdominal aorto-caval region, while both adrenals were unremarkable. There were no ectopic lesions seen in the rest of the examined areas. Based on existing investigation findings, it was concluded that she had extra-adrenal paraganglioma resulting in hypertension.
What Was the Next Step in Management?
At 22 weeks of gestation, the patient was started on phenoxybenzamine titrated to a dose of 30 mg in the morning and 10 mg in the evening. Propranolol was added several days after the commencement of phenoxybenzamine. Apart from mild postural dizziness, the medical therapy was well tolerated during the remainder of the pregnancy. In the third trimester, systolic and diastolic blood pressures were maintained to below 90 mmHg and 60 mmHg, respectively. During this period, she developed mild elevation of alkaline phosphatase ranging from 91 to 188 IU/l (reference 35–85 IU/l). However, liver transaminases were normal and the patient had no seizures. Repeated urinalysis showed resolution of proteinuria. At 38 weeks of gestation, the patient proceeded to elective caesarean section because of previous caesarean section, and a live female baby weighing 3.14 kg was delivered. The delivery was uncomplicated and blood pressure remained stable.
Following the delivery, computer tomography (CT) scan of neck, abdomen, and pelvis was performed as part of pre-operative planning to better delineate the relationship of the tumour to neighbouring structures. In addition to the previously identified extra-adrenal paraganglioma in the abdomen ( Figure 1 ), the CT revealed a 9 mm hypervascular nodule at the left carotid bifurcation, suggestive of a carotid body tumour ( Figure 2 ). The patient subsequently underwent an iodine (I) 131 metaiodobenzylguanidine (MIBG) scan, which demonstrated marked MIBG-avidity of the paraganglioma in the mid-abdomen. The reported left carotid body tumour, however, did not demonstrate any significant uptake. This could indicate either that the MIBG scan had poor sensitivity in detecting a small tumour, or that the carotid body tumour was not functional.
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pmed.1000037.g001
https://doi.org/10.1371/journal.pmed.1000037.g002
In June 2008, four months after the delivery, the patient had a laparotomy with removal of the abdominal paraganglioma. The operation was uncomplicated. There was no wide fluctuation of blood pressures intra- and postoperatively. Phenoxybenzamine and propranolol were stopped after the operation. Histology of the excised tumour was consistent with paraganglioma with cells staining positive for chromogranin ( Figures 3 and 4 ) and synaptophysin. Adrenal tissues were notably absent.
The tumour is a well-circumscribed fleshy yellowish mass with maximal dimension of 5.5 cm.
https://doi.org/10.1371/journal.pmed.1000037.g003
The tumour cells are polygonal with bland nuclei. The cells are arranged in nests and are immunoreactive to chromogranin (shown here) and synaptophysin.
https://doi.org/10.1371/journal.pmed.1000037.g004
The patient was counselled for genetic testing for hereditary phaeochromocytoma/paraganglioma. She was found to be heterozygous for c.449_453dup mutation of the succinate dehydrogenase subunit D (SDHD) gene ( Figure 5 ). This mutation is a novel frameshift mutation, and leads to SDHD deficiency (GenBank accession number: 1162563). At the latest clinic visit in August 2008, she was asymptomatic and normotensive. Measurements of catecholamine in 24-hour urine collections had normalised. Resection of the left carotid body tumour was planned for a later date. She was to be followed up indefinitely to monitor for recurrences. She was also advised to contact family members for genetic testing. Our patient gave written consent for this case to be published.
https://doi.org/10.1371/journal.pmed.1000037.g005
Phaeochromocytoma in Pregnancy
Hypertension during pregnancy is a frequently encountered obstetric complication that occurs in 6%–8% of pregnancies [ 2 ]. Phaeochromocytoma presenting for the first time in pregnancy is rare, and only several hundred cases have been reported in the English literature. In a recent review of 41 cases that presented during 1988 to 1997, maternal mortality was 4% while the rate of foetal loss was 11% [ 3 ]. Antenatal diagnosis was associated with substantial reduction in maternal mortality but had little impact on foetal mortality. Further, chronic hypertension, regardless of aetiology, increases the risk of pre-eclampsia by 10-fold [ 1 ].
Classically, patients with phaeochromocytoma present with spells of palpitation, headaches, and diaphoresis [ 4 ]. Hypertension may be sustained or sporadic, and is associated with orthostatic blood pressure drop because of hypovolaemia and impaired vasoconstricting response to posture change. During pregnancy, catecholamine surge may be triggered by pressure from the enlarging uterus and foetal movements. In the majority of cases, catecholamine-secreting tumours develop in the adrenal medulla and are termed phaeochromocytoma. Ten percent of tumours arise from extra-adrenal chromaffin tissues located in the abdomen, pelvis, or thorax to form paraganglioma that may or may not be biochemically active. The malignant potential of phaeochromocytoma or paraganglioma cannot be determined from histology and is inferred by finding tumours in areas of the body not known to contain chromaffin tissues. The risk of malignancy is higher in extra-adrenal tumours and in tumours that secrete dopamine.
Making the Correct Diagnosis
The diagnosis of phaeochromocytoma requires a combination of biochemical and anatomical confirmation. Catecholamines and their metabolites, metanephrines, can be easily measured in urine or plasma samples. Day collection of urinary fractionated metanephrine is considered the most sensitive in detecting phaeochromocytoma [ 5 ]. In contrast to sporadic release of catecholamine, secretion of metanephrine is continuous and is less subjective to momentary stress. Localisation of tumour can be accomplished by either CT or MRI of the abdomen [ 6 ]. Sensitivities are comparable, although MRI is preferable in pregnancy because of minimal radiation exposure. Once a tumour is identified, nuclear medicine imaging should be performed to determine its activity, as well as to search for extra-adrenal diseases. I 131 or I 123 MIBG scan is the imaging modality of choice. Metaiodobenzylguanidine structurally resembles noradrenaline and is concentrated in chromaffin cells of phaeochromocytoma or paraganglioma that express noradrenaline transporters. Radionucleotide imaging is contraindicated in pregnancy and should be deferred until after the delivery.
Treatment Approach
Upon confirming the diagnosis, medical therapy should be initiated promptly to block the cardiovascular effects of catecholamine release. Phenoxybenzamine is a long-acting non-selective alpha-blocker commonly used in phaeochromocytoma to control blood pressure and prevent cardiovascular complications [ 7 ]. The main side-effects of phenoxybenzamine are postural hypotension and reflex tachycardia. The latter can be circumvented by the addition of a beta-blocker. It is important to note that beta-blockers should not be used in isolation, since blockade of ß2-adrenoceptors, which have a vasodilatory effect, can cause unopposed vasoconstriction by a1-adrenoceptor stimulation and precipitate severe hypertension. There is little data on the safety of use of phenoxybenzamine in pregnancy, although its use is deemed necessary and probably life-saving in this precarious situation.
The definitive treatment of phaeochromocytoma or paraganglioma is surgical excision. The timing of surgery is critical, and the decision must take into consideration risks to the foetus, technical difficulty regarding access to the tumour in the presence of a gravid uterus, and whether the patient's symptoms can be satisfactorily controlled with medical therapy [ 8 , 9 ]. It has been suggested that surgical resection is reasonable if the diagnosis is confirmed and the tumour identified before 24 weeks of gestation. Otherwise, it may be preferable to allow the pregnancy to progress under adequate alpha- and beta-blockade until foetal maturity is reached. Unprepared delivery is associated with a high risk of phaeochromocytoma crisis, characterised by labile blood pressure, tachycardia, fever, myocardial ischaemia, congestive heart failure, and intracerebral bleeding.
Patients with phaeochromocytoma or paraganglioma should be followed up for life. The rate of recurrence is estimated to be 2%–4% at five years [ 10 ]. Assessment for recurrent disease can be accomplished by periodic blood pressure monitoring and 24-hour urine catecholamine and/or metanephrine measurements.
Genetics of Phaeochromocytoma
Approximately one quarter of patients presenting with phaeochromocytoma may carry germline mutations, even in the absence of apparent family history [ 11 ]. The common syndromes of hereditary phaeochromocytoma/paraganglioma are listed in Box 2 . These include Von Hippel-Lindau syndrome, multiple endocrine neoplasia type 2, neurofibromatosis type 1, and succinate dehydrogenase (SDH) gene mutations. Our patient has a novel frameshift mutation in the SDHD gene located at Chromosome 11q. SDH is a mitochondrial enzyme that is involved in oxidative phosphorylation. Characteristically, SDHD mutation is associated with head or neck non-functional paraganglioma, and infrequently, sympathetic paraganglioma or phaeochromocytoma [ 12 ]. Tumours associated with SDHD mutation are rarely malignant, in contrast to those arisen from mutation of the SDHB gene. Like all other syndromes of hereditary phaeochromocytoma, SDHD mutation is transmitted in an autosomal dominant fashion. However, not all carriers of the SDHD mutation develop tumours, and inheritance is further complicated by maternal imprinting in gene expression. While it may not be practical to screen for genetic alterations in all cases of phaeochromocytoma, most authorities advocate genetic screening for patients with positive family history, young age of tumour onset, co-existence with other neoplasms, bilateral phaeochromocytoma, and extra-adrenal paraganglioma. The confirmation of genetic mutation should prompt evaluation of other family members.
Box 2: Hereditary Phaeochromocytoma/Paraganglioma Syndromes
- Von Hippel-Lindau syndrome
- Multiple endocrine neoplasia type 2A and type 2B
- Neurofibromatosis type 1
- Mutation of SDHB , SDHC , SDHD
- Ataxia-telangiectasia
- Tuberous sclerosis
- Sturge-Weber syndrome
Key Learning Points
- Hypertension complicating pregnancy is a commonly encountered medical condition.
- Pre-existing chronic hypertension must be considered in patients with hypertension presenting in pregnancy, particularly if elevation of blood pressure is detected early during pregnancy or if persists post-partum.
- Secondary causes of chronic hypertension include renal artery stenosis, renal parenchyma disease, primary hyperaldosteronism, phaeochromocytoma, Cushing's syndrome, coarctation of the aorta, and obstructive sleep apnoea.
- Phaeochromocytoma presenting during pregnancy is rare but carries high rates of maternal and foetal morbidity and mortality if unrecognised.
- Successful outcomes depend on early disease identification, prompt initiation of alpha- and beta-blockers, carefully planned delivery, and timely resection of the tumour.
Phaeochromocytoma complicating pregnancy is uncommon. Nonetheless, in view of the potential for catastrophic consequences if unrecognised, a high index of suspicion and careful evaluation for secondary causes of hypertension is of utmost importance. Blood pressure should be monitored in the post-partum period and persistence of hypertension must be thoroughly investigated.
Author Contributions
All authors participated in the management of the patient or writing of the article. AL and RCWM wrote the article, with contributions from all the authors.
- View Article
- Google Scholar
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Mini Review
- Mini review series: Current topic in Hypertension
- Published: 02 June 2023
Preeclampsia up to date—What’s going on?
- Kanako Bokuda 1 &
- Atsuhiro Ichihara 1
Hypertension Research volume 46 , pages 1900–1907 ( 2023 ) Cite this article
6630 Accesses
4 Citations
5 Altmetric
Metrics details
Preeclampsia is a hypertensive disorder in pregnancy characterized by placental malperfusion and subsequent multi-organ injury. It accounts for approximately 14% of maternal deaths and 10–25% of perinatal deaths globally. In addition, preeclampsia has been attracting attentions for its association with risks for developing chronic diseases in later life for both mother and child. This mini-review discusses on latest knowledge on prediction, prevention, management, and long-term outcomes of preeclampsia and also touches on association between COVID-19 and preeclampsia.
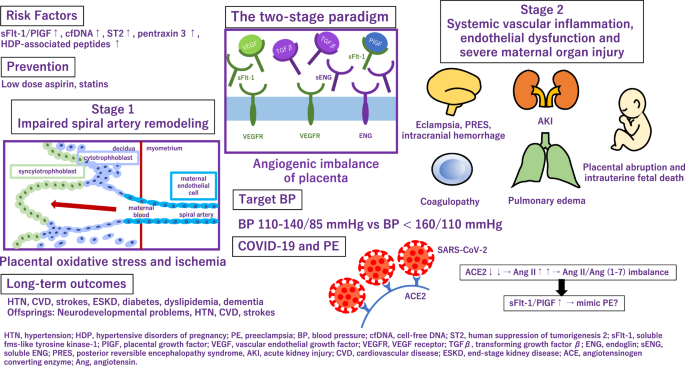
HTN hypertension, HDP hypertensive disorders of pregnancy, PE preeclampsia, BP blood pressure, cfDNA cell-free DNA, ST2 human suppression of tumorigenesis 2, sFlt-1 soluble fms-like tyrosine kinase-1, PIGF placental growth factor, VEGF vascular endothelial growth factor, VEGFR VEGF receptor, TGF β transforming growth factor β , ENG endoglin, sENG soluble ENG, PRES posterior reversible encephalopathy syndrome, AKI acute kidney injury, CVD cardiovascular disease, ESKD end-stage kidney disease, ACE angiotensinogen converting enzyme, Ang angiotensin.
Similar content being viewed by others
Unravelling the potential of angiogenic factors for the early prediction of preeclampsia
Juilee S. Deshpande, Deepali P. Sundrani, … Sadhana R. Joshi
Circulating EGFL7 distinguishes between IUGR and PE: an observational case–control study
Micol Massimiani, Silvia Salvi, … Luisa Campagnolo

Pre-eclampsia
Evdokia Dimitriadis, Daniel L. Rolnik, … Ellen Menkhorst
Introduction
Hypertensive disorders of pregnancy (HDP) affect approximately 10% of all pregnancies globally. HDP, particularly preeclampsia (PE), accounts for as high as 14% of maternal mortality and results in 10–25% of perinatal deaths. The 2019 Maternal Mortality update from the WHO report noted the major contribution of PE and eclampsia to worldwide maternal deaths. Since once it develops, termination is the only given way to ameliorate the symptoms of PE, trials and cohort studies regarding PE, give insight into the development of diagnostic and prognostic tools and preventive care.
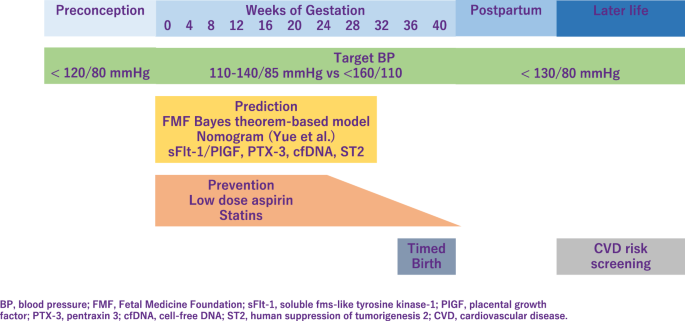
It is important to provide early screening and identify pregnant women at high risk who might benefit from prophylactic agents [ 1 ]. The Fetal Medicine Foundation proposed a Bayes theorem-based model to predict preterm PE using a combination of maternal characteristics, medical history, mean arterial pressure, uterine artery pulsatility index, and serum placental growth factor (PlGF). This model can predict ~90% of early PE cases, with delivery at <32 weeks of gestation and 75% of preterm PE cases, with delivery at <37 weeks of gestation [ 2 , 3 ]. Recently, Yue et al. have developed and validated a new nomogram for the early prediction of PE in pregnant Chinese women [ 4 ]. This nomogram included body mass index (BMI), blood pressure (BP), uterine artery ultrasound parameters, and serological indicators and can be easily utilized to facilitate the individualized prediction of PE.
Application of angiogenic and antiangiogenic biomarkers into clinical practice will help reduce the considerable burden of morbidity and mortality associated with adverse pregnancy outcomes as a consequence of PE. PE is associated with alteration of angiogenic and antiangiogenic factors such as soluble fms-like tyrosine 1 (sFlt-1), soluble endoglin (sENG) and PlGF [ 5 , 6 , 7 , 8 , 9 ] (Fig. 1 ). Quantification of the sFlt-1/PlGF ratio, has been shown to be a useful biomarker test for aiding the diagnosis and short-term prediction of PE [ 10 ]. PRediction of short-term Outcomes in preGNant wOmen with Suspected PE Study (PROGNOSIS) proposed and validated sFlt-1/PIGF ratio cutoff of 38 to predict the development of PE in women with clinical suspicion [ 11 , 12 , 13 ]. Furthermore, the sFlt-1/PlGF ratio test is likely to lessen the avoidable hospitalization of women at low risk of developing PE in the short term while identifying high-risk individuals requiring appropriate management [ 14 ].
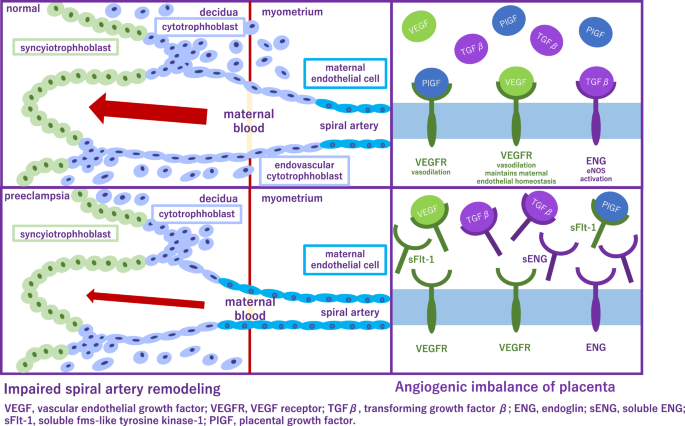
Alteration of angiogenic and antiangiogenic factors in preeclampsia
Besides sFlt-1 and PlGF, several biomarkers have been reported as candidate markers for predicting the development of PE. The first trimester pregnancy-associated plasma protein A can predict PE and superimposed PE in the third trimester [ 15 ]. Plasma cell-free DNA and human suppression of tumorigenesis served as diagnostic biomarkers for gestational hypertension (GH) and PE [ 16 ]. Pentraxin 3, an acute-phase protein which is produced and released in response to inflammatory stimuli, has been proposed as a novel biomarker predicting placental failure [ 17 ] and was also associated with PE [ 18 ]. Using an effective peptidomic analysis, Wakabayashi et al. identified seven circulating HDP-associated peptides (P-2081, P-2091, P-2127, P-2209, P-2378, P-2858, and P-3156) and proposed them as biomarkers for the diagnosis of HDP [ 19 ]. Interestingly, they have also investigated that these peptides are possible biomarkers for discriminating cardiovascular risk even in general population [ 20 ].
Low-dose aspirin is highly promising for the prevention of PE and is extensively studied. The American College of Obstetricians and Gynecologists (ACOG), International Society for the Study of Hypertension in Pregnancy (ISSHP), National Institute for Health, and Care Excellence, Japan Society for the Study of Hypertension in Pregnancy (JSSHP) recommend initiation of low-dose aspirin in women with high risk factor to reduce the risk of PE [ 21 , 22 , 23 ]. A systematic review established benefits of low-dose aspirin taken during pregnancy [ 24 ]. Aspirin use was significantly associated with lower risk of PE, perinatal mortality, preterm birth, and intrauterine growth restriction. According to the guideline proposed by the ACOG in 2018 [ 23 ], chronic hypertension (CH) is one of the risk factors for the development of PE, and aspirin is recommended for this group of patients. However, incidence of superimposed PE was not significantly different in the pre-ACOG group and post ACOG group, indicating that aspirin did not reduce the incidence of superimposed PE in patients with CH. Aspirin decreases the risk of PE, but its effectiveness in women with CH remains controversial [ 25 ]. Aspirin is preferably started before 16 weeks of gestation and continued until delivery. However, initiation of aspirin may complicate peripartum bleeding, which could be mitigated by discontinuing aspirin earlier. Recent multicenter, randomized trial showed that aspirin discontinuation at 24 to 28 weeks of gestation is noninferior to aspirin continuation for preventing preterm PE in individuals at high risk of PE and a normal sFlt-1/PlGF ratio between 24 weeks 0 days and 27 weeks 6 days of gestation.
The properties and mechanisms of action of statins make them candidates for the prevention of PE. In a meta-analysis, which included 10 studies describing 1391 women with uteroplacental insufficiency disorders: 703 treated with pravastatin and 688 not treated with statins, pravastatin prolonged pregnancy duration and improved associated obstetrical outcomes in pregnancies complicated with uteroplacental insufficiency disorders [ 26 ]. In contrast, 1120 women with singleton pregnancies at high risk of term PE were randomly assigned to receive pravastatin at a dose of 20 mg/d or placebo from 35 to 37 weeks of gestation until delivery or 41 weeks. Pravastatin in women at high risk of term PE did not reduce the incidence of delivery with PE [ 27 ].
In 2017, the American College of Cardiology/American Heart Association hypertension (ACC/AHA) treatment guidelines identified hypertension as BP ≥ 130/80 mmHg. However, the reference BP for hypertension during pregnancy as specified in international guidelines [eg. ISSHP [ 22 ], ACOG [ 28 , 29 ]], is ≥140/90 mmHg (Fig. 2 ). In Japan, guidelines of Japanese Society of Hypertension and JSSHP, both define hypertension as BP ≥ 140/90 mmHg whether the patient is pregnant or not [ 21 , 30 , 31 ]. Respecting BP control, from a preventive point of view, understanding the importance of preconception BP is of particular interest. It is relatively easy to intervene before pregnancy, and furthermore, this may have a greater impact on gestational outcome. In previous study, preconception BP and its change into early pregnancy was evaluated as risk markers for the development of HDP. Among 586 women with a pregnancy >20 weeks’ gestation, preconception BP levels were higher for preterm PE, term PE, and GH as compared with no HDP [ 32 ]. In large cohort study, which examined whether high BP in the preconception period was associated with GH and PE, when participants with normal BP were used as the reference, the adjusted ORs for GH were 1.48, 1.70, and 1.29, and for PE, the adjusted ORs were 1.55, 1.95, and 1.99 for the participants with prehypertension (SBP 120–139 mmHg or DBP 80–89 mmHg), stage 1 hypertension (SBP 140–159 mmHg or DBP 90–99 mmHg), and stage 2 hypertension (SBP ≥ 160 mmHg or DBP ≥ 100 mmHg), respectively [ 33 ]. These results support an association between hypertension and also prehypertension prior to pregnancy and an increased risk of GH and PE.
Management of preeclampsia
Target BP during pregnancy differs between guidelines. ISSHP recommends that BP ≥ 140/90 mmHg should be treated with a goal BP 110–140/85 mmHg, while ACOG recommends antihypertensive medications when BP ≥ 160/110 mmHg with goal BP below this threshold. JSSHP recommends to initiate antihypertensive medications when BP ≥ 140/90 mmHg for CH and BP ≥ 160/90 mmHg (depending on the situation, ≥140/90 mmHg) for other categories of HDP and to set target BP depending on the conditions of each case [ 21 ]. Studies reported that women in a low-risk cohort with stage 1 hypertension defined as 130–139 mmHg/80–89 mmHg, according to the ACC/AHA, are more likely to develop PE than women with normotensive in the early gestation. Based on the randomized controlled trial in China, the authors investigated whether PE was more likely to occur in stage 1 hypertensive women compared to the normotensive pregnant women between gestational age 12–20 weeks, in a high-risk cohort [ 34 ]. This subanalysis have revealed that stage 1 hypertension might be an additional risk factor for PE in high-risk pregnant women, and aspirin intervention might be useful in preventing PE. A meta-analysis established that the category of elevated BP had a risk ratio of 2.0 (95% prediction interval, 0.8–4.8), the stage 1 hypertension category had a risk ratio of 3.0 (95% prediction interval, 1.1–8.5), and the stage 2 hypertension category had a risk ratio of 7.9 (95% prediction interval, 1.8–35.1) [ 35 ]. However, none of the systolic BP measurements of <120 mmHg, <130 mmHg, or <140 mmHg were useful to rule out the development of PE. Another meta-analysis investigated that BP ≥ 120/80 mmHg, particularly ≥130/80 mmHg, at <20 weeks of gestation, is associated with increased maternal and perinatal risks and the authors proposed new BP categories in pregnancy as normal (<120/80), high normal (120–129/ < 80), and elevated (130–139/80–89) [ 36 ]. One retrospective study has shown that systolic BP < 130 mmHg within 14 weeks of gestation reduced the risk of developing early-onset superimposed PE in women with CH [ 37 ]. The benefits and safety of the treatment of mild hypertension (BP, <160/100 mm Hg) during pregnancy are still uncertain. Data are needed on whether a strategy of targeting a BP of less than 140/90 mmHg reduces the incidence of adverse pregnancy outcomes without compromising fetal growth.
Nifedipine, labetalol, and hydralazine alone or in combination are presently recommended by ACOG for the acute lowering of severe BP (≥160 mm Hg systolic and/or ≥110 mm Hg diastolic) in pregnancy [ 38 ]. A randomized controlled trial demonstrated that oral antihypertensives, methyldopa, nifedipine, and labetalol, all reduced BP in severe range to the reference range in most women [ 39 ]. As single drugs, nifedipine retard use resulted in a greater frequency of primary outcome [BP control (defined as 120–150 mm Hg SBP and 70–100 mm Hg DBP) within 6 h with no adverse outcomes.] attainment. A meta-analysis demonstrated that all commonly prescribed oral antihypertensives (labetalol, other β -blockers, methyldopa, calcium channel blockers, and mixed/multi-drug therapy) versus placebo/no therapy reduced the risk of severe hypertension by 30 to 70% in nonsevere pregnancy hypertension [ 40 ]. In addition, labetalol decreased proteinuria/PE and fetal/newborn death compared with placebo/no therapy, and proteinuria/PE compared with methyldopa and calcium channel blockers.
Currently, magnesium sulfate (MgSO4) is the primary treatment option and it is administered prophylactically to women with severe PE who are at risk of developing eclampsia. While MgSO4 is effective in preventing seizures, it is not as effective in reducing hypertension or other maternal organ injuries such as proteinuria in PE patients. Therefore, finding a therapeutic agent that improves multiple PE symptoms is urgent. In rat model, cyclosporin A (CsA) effectively attenuated PE manifestation and eclampsia-like seizure severity. In addition, CsA treatment significantly reduced the inflammatory cytokine levels and improved pregnancy outcomes following eclampsia-like seizures. The decreased inflammatory cytokines in PE are coincident with attenuated PE manifestation, suggesting that CsA treatment might decrease the PE severity through decreasing systemic inflammation [ 41 ]. Crocin, a hydrophilic carotenoid pigment, is a major compound with pharmacological activities found in Crocus sativus L. (saffron). Crocin alleviated inflammatory and oxidative stress in placental tissues, thereby protecting against GH, one of the major phenotypes of PE, and activated the Nrf-2/HO-1 pathway [ 42 ].
In women with a PE at term, immediate delivery reduces the risk of adverse maternal outcomes or progression to severe disease without affecting neonatal outcomes. However, in women with a PE diagnosed before term, benefits of delivery for the mother need to be weighed against the adverse consequences of iatrogenic preterm birth for the infant. In women with late preterm PE, the optimal time for termination is unclear because limitation of maternal disease progression needs to be balanced against infant complications. A randomized controlled study has shown that incidence of maternal death and severe hypertension was significantly lower in the planned delivery group compared with the expectant management group. However, planned delivery led to more neonatal unit admissions for the infant, principally for a listed indication of prematurity and without an excess of respiratory or other morbidity, intensity of care, or length of stay. This trade-off should be circumspectly discussed with women with late preterm PE to decide the optimal timing of delivery [ 43 ].
Long-term outcomes
Women with history of PE have increased cardiovascular disease (CVD) risk. Pregnancy has been labeled as a stress test which reveals women with cardiovascular dysfunction or poor reserve [ 44 , 45 ]. A rise of 10-year maternal CVD risk is associated with PE, while those with sustained hypertension after delivery have a two-fold increase in the risk of developing CVD in the next 10–30 years. Adverse pregnancy outcomes (APO) including PE, occur in 10 to 20% of all pregnancies and are also associated with a 1.8- to 4.0-fold risk of future CVD [ 46 , 47 ]. Women with a history of HDP are reported to have stiffer arteries and have a 2–5 times higher risk of hypertension in later life, compared to normotensive gestations [ 48 , 49 ]. Association between HDP and later hypertension was reported to be stronger in younger women and in obese women in the 30–70 age group [ 50 ]. Additionally, PE is considered a risk factor for chronic diseases such as hypothyroidism, diabetes mellitus and dyslipidemia, each of which independently increases the incidence of cardiovascular morbidity [ 45 ]. PE was included as a “risk-enhancer” in the updated 2018 cholesterol guideline [ 51 ] and in the 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease [ 52 ]. The ACOG recommends that women with APO undergo cardiovascular risk screening within 3 months postpartum [ 53 ].
Data from the Stroke Prevention in Young Women Study showed that women with a history of PE are 60% more likely to suffer from ischemic stroke after multivariable adjustment [ 54 ]. Studies from the World Health Organization also showed an increased risk of hemorrhagic stroke [ 55 ] and venous thromboembolism [ 56 ] in women with history of hypertension in pregnancy. Prior PE is also associated with an increased risk for the development of end-stage kidney disease. A meta-analysis of 2,309,946 women(among whom 103,308 women with PE) demonstrated that history of PE also increases the risk of vascular dementia [ 57 ].
The mechanisms responsible for these associations remain unclear. One possible mechanism maybe the presence of remaining angiotensin II type 1 receptor agonistic autoantibody (AT1-AA) postpartum. AT1-AAs are elevated in women with PE. AT1-AA binds to angiotensin II type 1 receptor (AT1R) and increases AT1R activity, intracellular calcium levels, and activation of intracellular mitogen activated protein kinase/extracellular signal regulated kinases (MAPK/ERK) pathways. Previous study reported that −18% of postpartum PE women have elevated circulating AT1-AAs 1 year after delivery [ 58 ]. These women with elevated AT1-AAs had increased sFlt-1, decreased free VEGF, and higher insulin resistance compared with autoantibody negative women and these correlations may suggest a mechanism by which women with PE have increased risks of severe complications later in life. JSSHP recommends to explain to women with a history of HDP that they have a higher risk of developing subsequent lifestyle diseases and cerebrovascular/cardiovascular diseases and that regular follow-up and lifestyle intervention guidance can reduce the incidence [ 21 ].
Preterm delivery is associated with long-term neurodevelopmental problems in the offspring. Zwertbroek et al. found that early delivery in women with late preterm HDP is associated with poorer neurodevelopmental outcomes in their children at 2 years of age [ 59 ]. These findings indicate an increased risk of developmental delay after early delivery compared to expectant monitoring. The infant from a PE pregnancy also appears at increased risk for CVD [ 60 ]. Infants of mothers with PE have higher BP during young adulthood and an increased risk for stroke in later life [ 61 ]. Other differences have also been shown, including increased BMI [ 62 ] and hormonal changes.
Pregnancy could potentially affect the susceptibility to and the severity of COVID-19 and pregnant women are at an increased risk of mortality and morbidity due to COVID-19. In addition, what we need to be aware of is that although pregnant women are less likely to complain of the symptoms of COVID-19, they are more than twice as likely to require critical care or mechanical ventilation than nonpregnant women [ 63 ]. Kalafat et al. have proposed that the mini-model which includes the maternal age, BMI, and pregnancy trimester can be used to estimate the risk of developing critical COVID-19 before disease onset. The addition of inflammatory markers to maternal BMI at the time of diagnosis can accurately predict critical COVID-19, PE, and the progression time from diagnosis to clinical deterioration [ 64 ].
Although few cases of intrauterine transmission of SARS-CoV-2 have been documented, it appears to be rare [ 65 ]. It is possibly related to low levels of SARS-CoV-2 viremia and the decreased coexpression of angiotensin-converting enzyme (ACE) 2 and transmembrane serine protease 2 which is needed for SARS-CoV-2 entry into cells in the placenta. However, evidence is accumulating that SARS-CoV-2 infection is associated with a number of adverse pregnancy outcomes including PE, preterm birth, and stillbirth [ 66 ]. This tendency is reported to be observed especially among pregnant women with severe COVID-19 disease, but one large, longitudinal, prospective, observational study assessing the effect of COVID-19 during pregnancy on mothers and neonates have shown that COVID-19 severity does not seem to be a factor in this association [ 67 ]. Additionally, besides the direct impact of COVID-19 on pregnancy outcomes, there is evidence that the pandemic and its effects on healthcare systems have had detrimental effects on pregnancy outcomes even among pregnant women not infected with SARS-CoV-2 [ 68 ].
Also, some severe cases of COVID-19, patients present with PE-like symptoms (Fig. 3 ). PE mimicry by COVID-19 was confirmed following the alleviation of PE symptoms without delivery of placenta [ 69 ]. In COVID-19, ACE 2 function decreases and subsequently Ang (angiotensin) II activity increases [ 70 ]. Although COVID-19 shows an increase in the sFlt-1/PlGF ratio due to pathologic Ang II/Ang (1–7) imbalance like PE [ 71 ], sFlt1/PlGF ratio did not correlate with the severity [ 72 ]. Most experts believe that SARS-Cov-2 is likely to become endemic, the continued collection of data on the effects of COVID-19 during pregnancy are needed.
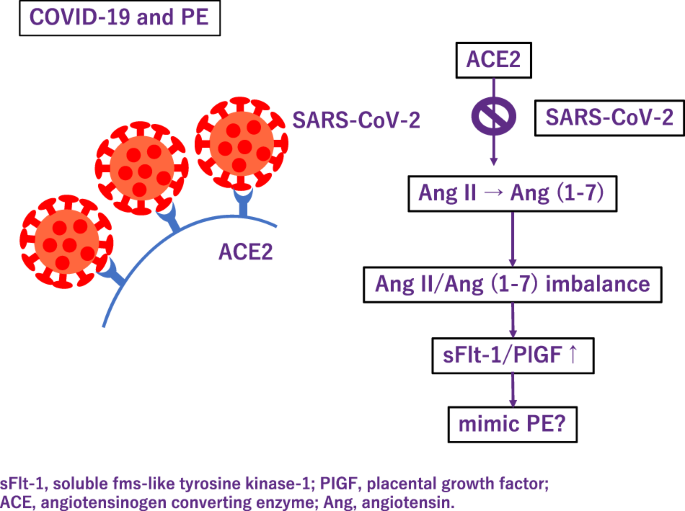
Association between COVID-19 and preeclampsia
Future perspectives
Pregnancy period is said to be a window where we can catch a glimpse of woman’s future. Though the symptoms of PE manifest typically in late pregnancy, fundamental alteration that underlies exists earlier in pregnancy or even preconceptionally and lasts throughout life. Earlier prediction, prevention and longer follow up is necessary for comprehensive management of PE. There is much to be done to decrease PE related maternal and fetal deaths and also to reduce maternal risks for chronic diseases in later life.
Rolnik DL, Wright D, Poon LC, O’Gorman N, Syngelaki A, de Paco Matallana C, et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl J Med. 2017;377:613–22.
Article CAS PubMed Google Scholar
Chaemsaithong P, Pooh RK, Zheng M, Ma R, Chaiyasit N, Tokunaka M, et al. Prospective evaluation of screening performance of first-trimester prediction models for preterm preeclampsia in an Asian population. Am J Obstet Gynecol. 2019;221:650.e1–650.e16.
Article PubMed Google Scholar
O’Gorman N, Wright D, Syngelaki A, Akolekar R, Wright A, Poon LC, et al. Competing risks model in screening for preeclampsia by maternal factors and biomarkers at 11–13 weeks gestation. Am J Obstet Gynecol. 2016;214:103.e1–103.e12.
Yue C, Gao J, Zhang C, Ni Y, Ying C. Development and validation of a nomogram for the early prediction of preeclampsia in pregnant Chinese women. Hypertens Res. 2021;44:417–25.
Duhig KE, Myers J, Seed PT, Sparkes J, Lowe J, Hunter RM, et al. Placental growth factor testing to assess women with suspected pre-eclampsia: a multicentre, pragmatic, stepped-wedge cluster-randomised controlled trial. Lancet. 2019;393:1807–18.
Article CAS PubMed PubMed Central Google Scholar
Verlohren S, Herraiz I, Lapaire O, Schlembach D, Moertl M, Zeisler H, et al. The sFlt-1/PlGF ratio in different types of hypertensive pregnancy disorders and its prognostic potential in preeclamptic patients. Am J Obstet Gynecol. 2012;206:58.e1–8.
Verlohren S, Perschel FH, Thilaganathan B, Dröge LA, Henrich W, Busjahn A, et al. Angiogenic markers and cardiovascular indices in the prediction of hypertensive disorders of pregnancy. Hypertension. 2017;69:1192–7.
Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355:992–1005.
Levine RJ, Maynard SE, Qian C, Lim K-H, England LJ, Yu KF, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–83.
Cerdeira AS, O’Sullivan J, Ohuma EO, Harrington D, Szafranski P, Black R, et al. Randomized interventional study on prediction of preeclampsia/eclampsia in women with suspected preeclampsia: INSPIRE. Hypertension. 2019;74:983–90.
Zeisler H, Llurba E, Chantraine FJ, Vatish M, Staff AC, Sennström M, et al. Soluble fms-like tyrosine kinase-1 to placental growth factor ratio: ruling out pre-eclampsia for up to 4 weeks and value of retesting. Ultrasound Obstet Gynecol J Int Soc Ultrasound Obstet Gynecol. 2019;53:367–75.
Article CAS Google Scholar
Hund M, Allegranza D, Schoedl M, Dilba P, Verhagen-Kamerbeek W, Stepan H. Multicenter prospective clinical study to evaluate the prediction of short-term outcome in pregnant women with suspected preeclampsia (PROGNOSIS): study protocol. BMC Pregnancy Childbirth. 2014;14:324.
Article PubMed PubMed Central Google Scholar
Zeisler H, Llurba E, Chantraine F, Vatish M, Staff AC, Sennström M, et al. Predictive value of the sFlt-1:PlGF ratio in women with suspected preeclampsia. N Engl J Med. 2016;374:13–22.
Ohkuchi A, Masuyama H, Yamamoto T, Kikuchi T, Taguchi N, Wolf C, et al. Economic evaluation of the sFlt-1/PlGF ratio for the short-term prediction of preeclampsia in a Japanese cohort of the PROGNOSIS Asia study. Hypertens Res J Jpn Soc Hypertens. 2021;44:822–9.
Chen Y, Wang X, Hu W, Chen Y, Ning W, Lu S, et al. A risk model that combines MAP, PlGF, and PAPP-A in the first trimester of pregnancy to predict hypertensive disorders of pregnancy. J Hum Hypertens. 2022;36:184–91.
Liu L, Li H, Wang N, Song X, Zhao K, Zhang C. Assessment of plasma cell-free DNA and ST2 as parameters in gestational hypertension and preeclampsia. Hypertens Res. 2021;44:996–1001.
Zhou P, Luo X, Qi H-B, Zong W-J, Zhang H, Liu D-D, et al. The expression of pentraxin 3 and tumor necrosis factor-alpha is increased in preeclamptic placental tissue and maternal serum. Inflamm Res. 2012;61:1005–12.
Colmenares-Mejía CC, Quintero-Lesmes DC, Bautista-Niño PK, Guio Mahecha E, Beltrán Avendaño M, Díaz Martínez LA, et al. Pentraxin-3 is a candidate biomarker on the spectrum of severity from pre-eclampsia to HELLP syndrome: GenPE study. Hypertens Res. 2020;43:884–91.
Araki Y, Yanagida M. Hypertensive disorders of pregnancy: strategy to develop clinical peptide biomarkers for more accurate evaluation of the pathophysiological status of this syndrome. Adv Clin Chem. 2020;94:1–30.
Wakabayashi I, Yanagida M, Araki Y. Associations of cardiovascular risk with circulating peptides related to hypertensive disorders of pregnancy. Hypertens Res. 2021;44:1641–51.
Takagi K, Nakamoto O, Watanabe K, Tanaka K, Matsubara K, Kawabata I, et al. A review of best practice guide 2021 for diagnosis and management of hypertensive disorders of pregnancy (HDP) - misc. - researchmap. Hypertens Res Pregnancy. 2022;10:57–73.
Article Google Scholar
Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S, et al. Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension. 2018;72:24–43.
Espinoza J, Vidaeff A, Pettker CM, Simhan H. ACOG Committee Opinion No. 743: low-dose aspirin use during pregnancy. Obstet Gynecol. 2018;132:e44–e52.
Henderson JT, Vesco KK, Senger CA, Thomas RG, Redmond N. Aspirin use to prevent preeclampsia and related morbidity and mortality: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;326:1192–206.
Magee LA, Khalil A, Kametas N, von Dadelszen P. Toward personalized management of chronic hypertension in pregnancy. Am J Obstet Gynecol. 2022;226:S1196–210.
Hirsch A, Rotem R, Ternovsky N, Hirsh Raccah B. Pravastatin and placental insufficiency associated disorders: a systematic review and meta-analysis. Front Pharm. 2022;13:1021548.
Döbert M, Varouxaki AN, Mu AC, Syngelaki A, Ciobanu A, Akolekar R, et al. Pravastatin versus placebo in pregnancies at high risk of term preeclampsia. Circulation. 2021;144:670–9.
Espinoza J, Vidaeff A, Pettker CM, Simhan H. Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obstet Gynecol. 2020;135:e237–e260.
American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin No. 203: chronic hypertension in pregnancy. Obstet Gynecol. 2019;133:e26–e50.
Metoki H, Iwama N, Hamada H, Satoh M, Murakami T, Ishikuro M, et al. Hypertensive disorders of pregnancy: definition, management, and out-of-office blood pressure measurement. Hypertens Res. 2022;45:1298–309.
Umemura S, Arima H, Arima S, Asayama K, Dohi Y, Hirooka Y, et al. The Japanese Society of Hypertension guidelines for the management of hypertension (JSH 2019). Hypertens Res. 2019;42:1235–481.
Nobles CJ, Mendola P, Mumford SL, Silver RM, Kim K, Andriessen VC, et al. Preconception blood pressure and its change into early pregnancy: early risk factors for preeclampsia and gestational. Hypertension. 2020;76:922–9.
Li N, An H, Li Z, Ye R, Zhang L, Li H, et al. Preconception blood pressure and risk of gestational hypertension and preeclampsia: a large cohort study in China. Hypertens Res. 2020;43:956–62.
Huai J, Lin L, Juan J, Chen J, Li B, Zhu Y, et al. Preventive effect of aspirin on preeclampsia in high‐risk pregnant women with stage 1 hypertension. J Clin Hypertens. 2021;23:1060–7.
Slade LJ, Mistry HD, Bone JN, Wilson M, Blackman M, Syeda N, et al. American College of Cardiology and American Heart Association blood pressure categories—a systematic review of the relationship with adverse pregnancy outcomes in the first half of pregnancy. Am J Obstet Gynecol. 2023;228:418–29.e34.
Suzuki H, Takagi K, Matsubara K, Mito A, Kawasaki K, Nanjo S, et al. Maternal and perinatal outcomes according to blood pressure levels for prehypertension: a review and meta-analysis. Hypertens Res Pregnancy. 2022;10:29–39.
Ueda A, Hasegawa M, Matsumura N, Sato H, Kosaka K, Abiko K, et al. Lower systolic blood pressure levels in early pregnancy are associated with a decreased risk of early-onset superimposed preeclampsia in women with chronic hypertension: a multicenter retrospective study. Hypertens Res. 2022;45:135–45.
Committee on Obstetric Practice. Committee Opinion No. 692: emergent therapy for acute-onset, severe hypertension during pregnancy and the postpartum period. Obstet Gynecol. 2017;129:e90–5.
Easterling T, Mundle S, Bracken H, Parvekar S, Mool S, Magee LA, et al. Oral antihypertensive regimens (nifedipine retard, labetalol, and methyldopa) for management of severe hypertension in pregnancy: an open-label, randomised controlled trial. Lancet. 2019;394:1011–21.
Bone JN, Sandhu A, Abalos ED, Khalil A, Singer J, Prasad S, et al. Oral antihypertensives for nonsevere pregnancy hypertension: systematic review, network meta- and trial sequential analyses. Hypertension. 2022;79:614–28.
Huang Q, Hu B, Han X, Yang J, Di X, Bao J, et al. Cyclosporin A ameliorates eclampsia seizure through reducing systemic inflammation in an eclampsia-like rat model. Hypertens Res. 2020;43:263–70.
Chen X, Huang J, Lv Y, Chen Y, Rao J. Crocin exhibits an antihypertensive effect in a rat model of gestational hypertension and activates the Nrf-2/HO-1 signaling pathway. Hypertens Res. 2021;44:642–50.
Chappell LC, Brocklehurst P, Green ME, Hunter R, Hardy P, Juszczak E, et al. Planned early delivery or expectant management for late preterm pre-eclampsia (PHOENIX): a randomised controlled trial. Lancet. 2019;394:1181–90.
Thilaganathan B, Kalafat E. Cardiovascular system in preeclampsia and beyond. Hypertension. 2019;73:522–31.
Vakhtangadze T, Gakhokidze N, Khutsishvili M, Mosidze S. The link between hypertension and preeclampsia/eclampsia-life-long cardiovascular risk for women. Vessel. 2019;3:19.
Google Scholar
Bellamy L, Casas J-P, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335:974.
Minissian MB, Kilpatrick S, Eastwood J-A, Robbins WA, Accortt EE, Wei J, et al. Association of spontaneous preterm delivery and future maternal cardiovascular disease. Circulation. 2018;137:865–71.
Leon LJ, McCarthy FP, Direk K, Gonzalez-Izquierdo A, Prieto-Merino D, Casas JP, et al. Preeclampsia and cardiovascular disease in a large UK pregnancy cohort of linked electronic health records. Circulation. 2019;140:1050–60.
Honigberg MC, Zekavat SM, Aragam K, Klarin D, Bhatt DL, Scott NS, et al. Long-term cardiovascular risk in women with hypertension during pregnancy. J Am Coll Cardiol. 2019;74:2743–54.
Wagata M, Kogure M, Nakaya N, Tsuchiya N, Nakamura T, Hirata T, et al. Hypertensive disorders of pregnancy, obesity, and hypertension in later life by age group: a cross-sectional analysis. Hypertens Res. 2020;43:1277–83.
Wilson PWF, Polonsky TS, Miedema MD, Khera A, Kosinski AS, Kuvin JT. Systematic review for the 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:3210–27.
Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA Guideline on the primary prevention of cardiovascular disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;74:1376–414.
American College of Obstetricians and Gynecologists’ Presidential Task Force on Pregnancy and Heart Disease and Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin No. 212: pregnancy and heart disease. Obstet Gynecol. 2019;133:e320–56.
Brown DW, Dueker N, Jamieson DJ, Cole JW, Wozniak MA, Stern BJ, et al. Preeclampsia and the risk of ischemic stroke among young women. Stroke. 2006;37:1055–9.
Poulter NR, Chang CL, Farley TMM, Meirik O, Marmot MG. Haemorrhagic stroke, overall stroke risk, and combined oral contraceptives: results of an international, multicentre, case-control study. Lancet. 1996;348:505–10.
World Health Organization Collaborative Study of Cardiovascular Disease and Steroid Hormone Contarception. Venous thromboembolic disease and combined oral contraceptives: results of international multicentre case-control study. Lancet. 1995;346:1575–82.
Samara AA, Liampas I, Dadouli K, Siokas V, Zintzaras E, Stefanidis I, et al. Preeclampsia, gestational hypertension and incident dementia: a systematic review and meta-analysis of published evidence. Pregnancy Hypertens. 2022;30:192–7.
Hubel CA, Wallukat G, Wolf M, Herse F, Rajakumar A, Roberts JM, et al. Agonistic angiotensin II type 1 receptor autoantibodies in postpartum women with a history of preeclampsia. Hypertension. 2007;49:612–7.
Zwertbroek EF, Franssen MTM, Broekhuijsen K, Langenveld J, Bremer H, Ganzevoort W, et al. Neonatal developmental and behavioral outcomes of immediate delivery versus expectant monitoring in mild hypertensive disorders of pregnancy: 2-year outcomes of the HYPITAT-II trial. Am J Obstet Gynecol. 2019;221:154.e1–154.e11.
Burton GJ, Redman CW, Roberts JM, Moffett A. Pre-eclampsia: pathophysiology and clinical implications. BMJ. 2019;366:l2381.
Davis EF, Lazdam M, Lewandowski AJ, Worton SA, Kelly B, Kenworthy Y, et al. Cardiovascular risk factors in children and young adults born to preeclamptic pregnancies: a systematic review. Pediatrics. 2012;129:e1552–61.
Davisson RL, Hoffmann DS, Butz GM, Aldape G, Schlager G, Merrill DC, et al. Discovery of a spontaneous genetic mouse model of preeclampsia. Hypertension. 2002;39:337–42.
Allotey J, Fernandez S, Bonet M, Stallings E, Yap M, Kew T, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320.
Kalafat E, Prasad S, Birol P, Tekin AB, Kunt A, Di Fabrizio C, et al. An internally validated prediction model for critical COVID-19 infection and intensive care unit admission in symptomatic pregnant women. Am J Obstet Gynecol. 2022;226:403.e1–403.e13.
Vivanti AJ, Vauloup-Fellous C, Prevot S, Zupan V, Suffee C, Do Cao J, et al. Transplacental transmission of SARS-CoV-2 infection. Nat Commun. 2020;11:3572.
Wei SQ, Bilodeau-Bertrand M, Liu S, Auger N. The impact of COVID-19 on pregnancy outcomes: a systematic review and meta-analysis. Can Med Assoc J. 2021;193:E540–E548.
Papageorghiou AT, Deruelle P, Gunier RB, Rauch S, García-May PK, Mhatre M, et al. Preeclampsia and COVID-19: results from the INTERCOVID prospective longitudinal study. Am J Obstet Gynecol. 2021;225:289.e1–289.e17.
Chmielewska B, Barratt I, Townsend R, Kalafat E, van der Meulen J, Gurol-Urganci I, et al. Effects of the COVID-19 pandemic on maternal and perinatal outcomes: a systematic review and meta-analysis. Lancet Glob Health. 2021;9:e759–e772.
Mendoza M, Garcia-Ruiz I, Maiz N, Rodo C, Garcia-Manau P, Serrano B, et al. Pre-eclampsia-like syndrome induced by severe COVID-19: a prospective observational study. BJOG Int J Obstet Gynaecol. 2020;127:1374–80.
Wu J, Deng W, Li S, Yang X. Advances in research on ACE2 as a receptor for 2019-nCoV. Cell Mol Life Sci CMLS. 2021;78:531–44.
Giardini V, Carrer A, Casati M, Contro E, Vergani P, Gambacorti-Passerini C. Increased sFLT-1/PlGF ratio in COVID-19: a novel link to angiotensin II-mediated endothelial dysfunction. Am J Hematol. 2020;95:E188–91.
Soldavini CM, Di Martino D, Sabattini E, Ornaghi S, Sterpi V, Erra R, et al. sFlt-1/PlGF ratio in hypertensive disorders of pregnancy in patients affected by COVID-19. Pregnancy Hypertens. 2022;27:103–9.
Download references
Author information
Authors and affiliations.
Department of Endocrinology and Hypertension, Tokyo Women’s Medical University, Tokyo, Japan
Kanako Bokuda & Atsuhiro Ichihara
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Kanako Bokuda .
Ethics declarations
Conflict of interest.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Bokuda, K., Ichihara, A. Preeclampsia up to date—What’s going on?. Hypertens Res 46 , 1900–1907 (2023). https://doi.org/10.1038/s41440-023-01323-w
Download citation
Received : 20 December 2022
Revised : 17 April 2023
Accepted : 02 May 2023
Published : 02 June 2023
Issue Date : August 2023
DOI : https://doi.org/10.1038/s41440-023-01323-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Soluble fms-like tyrosine kinase-1
- Placental growth factor
- Low dose aspirin
This article is cited by
Clinical features of recurrent preeclampsia: a retrospective study of 109 recurrent preeclampsia patients.
Hypertension Research (2024)
Advancements in microRNA-based electrochemical biosensors for preeclampsia detection
- Durairaj Sekar
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- Practice Test
- Fundamentals of Nursing
- Anatomy and Physiology
- Medical and Surgical Nursing
- Perioperative Nursing
- Psychiatric Mental Health Nursing
- Maternal & Child Nursing
- Community Health Nursing
- Pathophysiology
- Nursing Research
- Study Guide and Strategies
- Nursing Videos
- Work for Us!
- Privacy Policy

Pregnacy Induced Hypertension (PIH) Case Study

Pregnancy-induced hypertension (PIH) is one of the most common complications of pregnancy. This occurs during the 20 th week of gestation or late in the second trimester of pregnancy. This is a health condition wherein there is a rise in the blood pressure and disappears after the termination of pregnancy or delivery. PIH was formerly called toxaemia or the presence of toxins in the blood. This is because its occurrence was not well understood in the clinical field. Its common manifestations are hypertension, proteinuria (presence of protein in the urine), and edema. There are 2 main types of pregnancy-induced hypertension namely: pre-eclampsia and eclampsia.
- Pre-eclampsia— this is the non-convulsive form of PIH. This affects 7% of all pregnant women. Its incidence is higher in lower socio-economic groups. It may be classified either mild or severe.
- Eclampsia— this is the convulsive form of PIH. It occurs with 5% of all pre-eclampsia cases. The mortality rate among mothers is nearly 20% and fetal mortality is also high due to premature delivery.
NORMAL ANATOMY AND PHYSIOLOGY
There are a lot of bodily changes that happen during a normal pregnancy. There are external changes that are noticeable, and there are internal changes that can only be appreciated through thorough clinical examinations. Most of the changes are the body’s response to the changes in levels of hormones and the growing demands of the fetus.
The two dominant female hormones, estrogen and progesterone , change in a normal level. Along with this, a significant rise/appearance of 4 more major hormones take place; these are 1. human chorionic gonadotropin (HCG), 2. human placental lactogen, 3. prolactin, and 4. oxytocin. All these 6 hormones interact with each other simultaneously to maintain a normal pregnancy as it progresses.
The following are the major effects of these hormones in the body:
The exact cause of pregnancy-induced hypertension is unknown; however, it is highly linked to angiotensin gene T235 and the existence of other risk factors. Malnutrition and inadequate prenatal care are the greatest risk factors. The history and presence of diabetes mellitus (DM), multifetal gestation (twin pregnancies), polyhydramnios (excessive amniotic fluid), and renal diseases are also among the major contributory factors in the development of PIH. In the past, the mystery revolving around PIH postulated a lot of theories on its true origin, most of them were believed to be of toxic nature. Among these are placental infarcts, autointoxication, uremia, pyelonephritis, and maternal sensitization to total proteins.
The i ncidence of PIH among pregnant women is very high (8%), costing hundreds and thousands of lives of both mothers and fetus around the world. This commonly affects first-time pregnancies due to the presence of functioning tropoblasts (develops after the 20 th week of gestation and stays evident until after 48 hours after delivery. Age is also an important indicator in the development of PIH. Too early, as in teenage pregnancies and old primigravidas (first-time pregnancy) as in over 35 years of age put a woman higher chances of having pregnancy-induced hypertension .
PATHOPHYSIOLOGY
SIGNS AND SYMPTOMS
The signs and symptoms of the type of PIH present in a pregnant woman are based on the presentation of evident clinical manifestations. These are shown in the table below:
COMPLICATIONS
Based on the severity of the PIH present to a person or the extent of damage left/occurred, a list of possible complications can be drawn.
- Abruption placenta
- Disseminated intravascular coagulation (DIC)
- Prematurity
- Intrauterine growth retardation (IUGR)
- HELLP syndrome
- Maternal and/or fetal death
The changes of the mother and/or fetus to survive after an episode of convulsion or until delivery depends on the threshold on the effects of PIH and its complications. This can be:
- Good— if the symptoms are mild or those that are with mild pre-eclampsia and is responding well to the treatment regimen
- Poor— if there are multiple and long episodes of convulsions that are associated or lead to the development of persistent coma, hyperthermia, cyanosis, tachycardia, and liver damage.
- Congestive heart failure (CHF)
- Pulmonary edema
- Cerebral hemorrhage
- Renal failure
DIAGNOSTIC EVALUATIONS
Diagnostic evaluations are performed after episodes of convulsions or after the client has been rushed to a health care facility. These are routinely done to assess the damages and will serve as the basis for the plan of treatment.
- 24-hour urine-protein— health problem through protein determination from the involvement of the renal system.
- Serum BUN and creatinine— to evaluate renal functioning.
- Ophthalmic examination— to assess spasm, papilledema, retinal edema/detachment, and/or hemorrhages.
- Ultrasonography with stress and non- stress test— to evaluate fetal well-being after.
- Stress test —fetalheart tone (FHT) and fetal activity are electronically monitored after oxytocin induction which causes uterine contraction.
- Non-stress test —fetal heart tone (FHT) and fetal activity are electronically monitored during fetal activity (no oxytocin induction).
NURSING DIAGNOSES
- Fluid volume excess related to altered blood osmolarity and sodium/water retention.
- Altered nutrition, less than body requirements related to loss through damaged renal membrane.
- Altered tissue perfusion related to increased peripheral resistance and vasospasm in renal and cardiovascular system.
- Altered urinary elimination related to hypovolemia.
- Sensory/perceptual alterations: visual related to cerebral edema and decreased oxygenation of the brain.
- Diversional activity deficit related to decreased time for rest and sleep from stimulating environment.
- Risk for injury related to seizure episodes.
- Anxiety-related to fear of the unknown.
The overall goal of management in pregnancy-induced hypertension is directed towards the control of hypertension and the correction of developed health problems that might leadto other serious complications. Among the specially-designed treatment course for PIH are the following:
- Use of antihypertensive drugs (hydralazine-drug of choice)
- Diet-high protein, high calories
- Magnesium sulphate (MgSO4) treatment
- Diazepam and amobarbital sodium (if convulsions don’t respond to MgSO4)
- Beta-adrenergic blockers (used for acute hypertension)
- Delivery (if all treatment regimen don’t work)
NURSING MANAGEMENT
A. Assessment
- Monitor blood pressure in sitting or side-lying position.
- Monitor fetal heart tone (FHT) and fetal heart rate (FHR).
- Check for deep tendon reflexes (DTR) and clonus.
- Monitor intake and output (I&O) and proteinuria.
- Monitor daily weight and edema.
- Assess for signs of labor (possibility of abruption placenta).
- Assess for emotional status.
B. Interventions
1. Fluid balance
- Maintain patent and regulated IVF
- Strict I&O monitoring
- Monitor hematocrit level
- Vital signs monitoring every hour
- Assess breath sounds for signs of pulmonary edema
2. Tissue perfusion
- Position on left-lateral position
- Monitor fetal activity (stress and fetal activity)
3. Preventing injury
- Monitor cerebral signs and symptoms (headache, visual disturbances, and dizziness)
- Lie on left-lateral position if cerebral symptoms are present
- Secure padded side rails
- Keep oxygen suction set, tongue blade, and emergency medications (diazepam and magnesium sulphate) at all times
- Never leave an unstable patient
4. Anxiety
Discuss the health condition and planned treatment
- PIH is not lifetime
- PIH is only for the first pregnancy
- All medications and its maternal and fetal effects
Allow to ask questions and answer it truthfully
Provide emotional support to the client and family
C. Educative
- Reinforce the importance of rest and sleep
- Encourage family cooperation with the treatment course
- Discuss the laboratory procedures and alternative managements
- Include medical team, client, and significant others in the discussion
- Be realistic in discussing the possibilities of premature delivery
- No sign of pulmonary edema
- Adequate urine output
- No episode of seizure
- Stable and normal heart rate
RELATED ARTICLES MORE FROM AUTHOR
Anaphylactic shock case study -the impending doom, ectopic pregnancy case study, chronic suppurative otitis media case study, gestational diabetes mellitus case study, leave a reply cancel reply.
Save my name, email, and website in this browser for the next time I comment.
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
Enter the email address you signed up with and we'll email you a reset link.
- We're Hiring!
- Help Center

A CASE STUDY ON LIFE-THREATENING PREGNANCY-INDUCED HYPERTENSION IN PRETERM PREGNANCY AND MANAGEMENT CHALLENGES

Related Papers
Introduction: Hypertensive disorders of pregnancy are the most common causes of adverse maternal & perinatal outcomes. Such investigations in resource limited settings would help to have great design strategies in preventing maternal and perinatal morbidity and mortality. All women who presented with hypertensive disorders of pregnancy and delivered in the hospital and whose records were complete, were included in the study and divided into 5 groups namely, Gestational hypertension (GH), Mild pre-eclampsia (PE), Severe pre-eclampsia, Eclampsia and Chronic hypertension with superimposed pre-eclampsia (CHPE) based on their clinical presentation at admission. After excluding all incomplete data entries, the sample size was finalized at 200. Results: In this study, records of 2,989 women who delivered in our tertiary hospital were reviewed and of these, 256 women had hypertensive disorders of pregnancy. Fifty six of these women had either left the hospital against medical advice or their records were incomplete so their outcome could not be followed and hence were excluded from the study. Conclusion: Pre-eclampsia and Eclampsia still remains a major problem in developing countries. Pregnancy induced hypertension is one of the most extensively researched subjects in obstetrics. Still the etiology remains an enigma to us. Though the incidence of pre-eclampsia and eclampsia is on the decline, still it remains the major contributor to poor maternal and foetal outcome. The fact that pre-eclampsia, eclampsia is largely a preventable disease is established by the negligible incidence of pre-eclampsia and eclampsia with proper antenatal care and prompt treatment of pre-eclampsia. In preclampsia and eclampsia, pathology should be understood and that i-involves multiorgan dysfunction should be taken into account. The early use of antihypertensive drugs, optimum timing of delivery and strict fluid balance, anticonvulsants in cases of eclampsia will help to achieve successful outcome. Early transfer to specialist centre is important and the referral the referral centers should be well equipped to treat such critically ill patients.
IOSR Journals
Back Ground: Aim: The Aim of the study was to find out the incidence of PIH & Preeclampsia and to evaluate the risk factors, predictors of severity and obstetrical and perinatal outcome in severe preeclampsia and Eclampsia.. Place and duration Methodology: Out of total 8800 deliveries 880 were diagnosed to have pregnancy induced hypertension. Out of these 580 (66%) had gestational hypertension. 80(0.9%) cases had preeclampsia without severe features, 220(2.5%) cases had preeclampsia with severe features. The present study was conducted in 200 cases of preeclampsia with severe features. The cases were evaluated and managed as per the existing protocol in the department and Obstetrical and perinatal outcome were recorded and analyzed. Results: The incidence of pregnancy induced hypertension was 10% and preeclampsia 3.5% in our study. 50% had anemia and 30% had obesity as risk factors. Materanl mortality was seen in 12cases of severe preeclamsia, accounting to 50% of total maternal deaths in our centre. Other maternal complications were seen in 60% of cases.Most common was Eclamsia in 30% of cases followed by Abruption in 20% & DIC in 18% and 20% of cases required transfusion of blood & Blood components for thrombocytopenia and coagulation failure. 10% cases required ventilator support for dyspneoa. Perinatal mortality was seen in 16% of cases. Perinatal morality is due to premaurity, low birth weight and abruption. NICU admissions were required in 20% of cases because of severe birth Asphyxia. Conclusion: Regular antenatal checkup and regular blood pressure measurement will help in early detection of hypertensive cases. Treating anemia and educating women on significance of alarming symptoms will improve maternal and perinatal outcome. Hospitalisation, regular BP monitoring, investigations and timely delivery will improve significantly the maternal and perinatal outcome. A good maternal intensive care unit and neonatal intensive care unit will help to improve obstetrical and perinatal outcome in hypertensive disorders of pregnancy.
Hypertension in Pregnancy
Altaf shaikh
Corine Koopmans
https://www.ijhsr.org/IJHSR_Vol.11_Issue.1_Jan2021/IJHSR_Abstract.041.html
International Journal of Health Sciences and Research (IJHSR)
Background: Hypertension is one of the common medical complications of pregnancy & contributes significantly to maternal & perinatal morbidity & mortality. The World Health Organization estimates that at least one woman dies every seven minutes from complications of hypertensive disorders of pregnancy. Hence a study was undertaken to assess the impact of Pregnancy Induced Hypertension on fetal outcomes among mothers with PIH who delivered at tertiary care hospital, Dadra & Nagar Haveli. Method: It was a cross sectional study conducted at Shri Vinoba Bhave Civil Hospital, Silvassa, Dadra & Nagar Haveli from September to November 2020.The sample size of the study was 32. The data regarding demographic variables, obstetric history, clinical details & examinations, investigations & fetal outcomes was collected using Structured Interview Schedule. Result: In the present study, Gestational Hypertension was found to be 65.62%, Pre eclampsia was 28.12% and Eclampsia was found to be 6.25%. It was more prevalent among multipara mothers. The clinical representation of PIH showed that 71.87% mothers had pain in lower abdomen, 37.3% had pedal edema followed by 15.62% headache & 9.37% blurring of vision. Antihypertensive drugs (93.75%) were given to almost all the mothers whereas 9.37% were treated with anticonvulsant medicines. The most common fetal complications found were preterm births (43.75%) & LBW (37.5%). 28.12% babies required NICU admission due to various reasons whereas 6.25% neonatal deaths were reported. Conclusion: Pregnancy-related hypertensive disorders are common and adversely impact perinatal outcomes. Efforts should be made at both the community and hospital levels to increase awareness regarding hypertensive disorder of pregnancy and reduce its associated morbidity and mortality.
Clinical & Biomedical Research
Francisco Maximiliano Pancich Gallarreta
Scholar Science Journals
Background: Preeclampsia and eclampsia have been recognized as clinical entities since the times of Hippocrates. Pregnancy induced hypertension (PIH) is one of the commonest disorders associated with the increased risk of maternal and fetal complications. It is reported in the world literature that the incidence of eclampsia is on the decline, but still a menace in developing countries. Objectives: To study the maternal and foetal outcome in pregnancy induced hypertension. Material and Methods: A prospective randomized study was carried out A total of 100 pregnant women with PIH were enrolled in the study. A pre-tested interview tool was used to collect necessary information such as detailed history, clinical examination findings and investigations performed. Results were analysed using SPSS 13.0 Results: In the present study, the overall incidence of PIH was 8.96%, which includes preeclampsia in 7.26% and eclampsia in 1.70%. Preterm labour was the commonest maternal obstetrical complication observed in 18% of mild PIH and 48% of severe PIH cases. Prematurity was the commonest foetal complication seen in 17.99%, 47.62% and 52.63% of mild PIH, severe PIH and Eclampsia cases respectively. Conclusion: Pregnancy induced hypertension is a common medical disorder seen associated with pregnancy in the rural population, especially among young primigravidas, who remain unregistered during pregnancy. Maternal and fetal morbidity and mortality can be reduced by early recognition and institutional management.
American Journal of Pediatrics
Mustafa Captain
Archives of Gynecology and Obstetrics
Eray Çalışkan
Objective: The aim of the study was to determine the risk factors, prevalance, epidemiological parameters and maternal-perinatal outcome in pregnant women with hypertensive disorder. Materials and methods: A retrospective analysis was undertaken on 255 consecutive cases of hypertensive disorder in pregnancy who were managed at Kocaeli University, School of Medicine, Department of Obstetrics and Gynecology from June 1997 to November 2004. Demographic data involving age, parity, gestational week, clinical and laboratory findings were recorded from the medical files. Additionally delivery route, indications of cesarean section, fetal and maternal complications were determined. Statistical analysis was performed by SPSS programme using Kruskal Wallis nonparametric test, ANOVA (Analysis of variance) and chi-square tests. Results: Of 5,155 deliveries in our clinic during the defined period, 438 cases (8.49%) were managed as hypertensive disorder of pregnancy. Medical records of 255 cases could be avaliable. Of 255 cases, 138 patients (54.11%) were found to have severe preeclampsia while 88 cases (34.50%) were diagnosed as mild preeclampsia. Twenty-nine patients (11.37%) were suffering from chronic hypertension. Of 138 severely preeclamptic cases, 28 cases (11%) had eclamptic convulsion and another 28 patients (11%) were demonstrated to have HELLP syndrome. Intrauterine growth restriction, oligohydramnios, placental ablation were the obstetric complications in 75 (29.4%), 49 (19.2%), 19 (7.5%) cases, respectively. Additionally multiple pregnancy and gestational diabetes mellitus were noted in 5.9% (n:15) and 3.9% (n:10) of the patients. Delivery route was vaginal in 105 patients (41.2%) while 150 patients (58.8%) underwent cesarean section with the most frequent indication to be fetal distress in 69 cases (46%). Cesarean section rate seemed to be the lowest (48.3%) in chronic hypertensive women while the highest (63.8%) in severe preeclamptic patients. Maternal mortality occured in 3 cases (1.2%) and all of those cases were complicated with HELLP syndrome. Intracranial bleeding was the cause of maternal death in one case while the other two cases were lost due to acute renal failure and disseminated intravascular coagulation, respectively. Intrauterine fetal demise was recorded in 24 cases on admission. Ten fetuses died during the intrapartum period. Mean gestational age and birth weight were 28±3.5 and 1000±416 g, respectively in this group. In these ten women, five cases were diagnosed as HELLP syndrome, two were severely preeclamptic and three were eclamptic. Perinatal mortality rate was found to be 144/1,000 births Conclusion: Hypertensive disorder of pregnancy is associated with increased risk of maternal-perinatal adverse outcome. The complications of severe preeclampsia and eclampsia could be prevented by more widespread use of prenatal care, education of primary medical care personnel, prompt diagnosis of high-risk patients and timely referral to tertiary medical centers.
South African Family Practice
Nnabuike Chibuoke Ngene
RELATED PAPERS
JOURNAL OF ADVANCES IN BIOTECHNOLOGY
abdullah Qader
Hipertext. net
Pedro Pablo Sanchez
Claudia Fragelli
Tecnologia e Ambiente
Thaise Sutil
Sibgha Noureen
Discrete Dynamics in Nature and Society
Daniela Bala
Journal of Shahrekord University of Medical Sciences
Nahid Tavakoli
Cultura y Educación
Itziar Irazabal
Forensic Science International
Eligio Vacca
CENTRAL ASIAN JOURNAL OF THEORETICAL & APPLIED SCIENCES
Central Asian Studies
Lincoln Larson
Journal of Physics G: Nuclear and Particle Physics
Mohamed khaled Abdelaziz
Documents d'archéologie méridionale
Valérie Bel
MATERIALS SCIENCE-POLAND
Ewa Mijowska
2015 6th Asia Symposium on Quality Electronic Design (ASQED)
Ismat Hijazin
Journal of clinical microbiology
Journal of Hydrology
Zhengchao Tian
Estudios Latinoamericanos
gilton santos
Danuta Makowiec
Transition Metal Chemistry
Ayman El-Sawaf
Visualidad y escritura en la obra de Virginia Huneeus,
solange arroyo
Journal of Propulsion and Power
Edgar Choueiri
International Journal of bioprinting
Olga Kryukov
UoM毕业证书 曼彻斯特大学毕业证
Syaikhuna: Jurnal Pendidikan dan Pranata Islam
izza yuniati rahma
See More Documents Like This
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024

Pregnant women’s experiences with the management of hypertensive disorders of pregnancy: a qualitative study
- Amyna Helou 1 ,
- Kay Stewart 1 ,
- Kath Ryan 2 &
- Johnson George 1
BMC Health Services Research volume 21 , Article number: 1292 ( 2021 ) Cite this article
3583 Accesses
3 Citations
1 Altmetric
Metrics details
Hypertensive disorders are a leading cause of mortality and morbidity during pregnancy. Despite multiple national and international clinical guidelines and a plethora of research in the field of optimising management, there has been limited research describing the perspectives and experiences of pregnant women with the management of hypertensive disorders of pregnancy (HDP). Understanding these perceptions and experiences is imperative to the optimisation of HDP management.
A qualitative study involving face-to-face, in-depth interviews were undertaken with 27 pregnant women diagnosed with and being treated for HDP to explore their perspectives of and experiences with clinical management. Written consent was obtained individually from each participant, and the interviews ranged from 16 to 54 min. Inductive codes were generated systematically for the entire data set. Line-by-line analysis was then performed and nodes were created within NVivo, a qualitative data management software. Data collection was continued until thematic saturation was reached. Thematic analysis was employed to interpret the data.
Three major descriptive themes were discerned regarding the women’s perspectives on and experiences with the management of HDP: attitudes towards monitoring of HDP, attitudes and perceptions towards development and management of complications, and perceptions of pregnant women with chronic hypertension. Trust in the hospital system, positive attitudes towards close blood pressure monitoring as well as self-monitoring of blood pressure, and a realistic approach to emergency antenatal hospital admissions contributed to a positive attitude towards monitoring of HDP. Women with prior experiences of HDP complications, including pre-eclampsia, were more confident in their clinical management and knew what to expect. Those without prior experience were often in shock when they developed pre-eclampsia. Some women with chronic hypertension displayed limited understanding of the potential risks that they may experience during pregnancy and thus lacked comprehension of the seriousness of the condition.
Conclusions
The clinical management experiences of pregnant women with HDP were varied. Many women did not feel that they were well informed of management decisions and had a desire to be more informed and involved in decision-making. Clear, concise information about various facets of HDP management including blood pressure monitoring, prescription of the appropriate antihypertensive agent, and planning for potential early delivery are required .
Peer Review reports
Introduction
Hypertensive disorders of pregnancy (HDP) affect around 10% of pregnancies in Australia and around the world [ 1 ]. Combined, they are the second largest cause of maternal death, after haemorrhage, in the developed world [ 1 ].
In Australia, the public health system provides maternity care from pre-conception to postpartum. The main health professionals who care for the pregnant women are obstetricians, midwives, general practitioners (GP) and obstetric physicians [ 1 ]. The GP has an important role in pre-conception counselling, especially with women who have chronic diseases such as hypertension or asthma. It is also the responsibility of the GP to confirm the pregnancy and refer the woman to the relevant maternal hospital service.
Initially, the choice of model of care is given to the woman. The Midwifery Group Practice model [ 1 ] allows for one-to-one maternal care, often with the same midwife throughout the term of pregnancy, which is a suitable option for women without complications. Pregnant women with complications such as chronic hypertension or a previous pregnancy complicated by hypertensive disorders of pregnancy (HDP), however, need to be cared for by an obstetrician, who can monitor the progress of the pregnancy, blood pressure (BP), signs of pre-eclampsia, and fetal growth. The obstetric physician is usually involved in prescribing and monitoring antihypertensive medication and BP control. Pregnant women who have had pre-eclampsia previously or who have chronic hypertension are at risk of developing pre-eclampsia. Timely administration of low-dose (81-100 mg) aspirin before 16 weeks gestation has been found to reduce risk of pre-eclampsia [ 2 ].
Monitoring of BP occurs at each antenatal visit. If her BP is elevated, the woman may be referred to a day assessment unit for 4-h assessment of BP, which involves taking BP readings every half an hour for 4 h to observe the pattern of the BP and decide whether a diagnosis of HDP and/or prescription of an antihypertensive medication is warranted. In addition, test for urinary protein, full blood examination, renal function tests and fetal monitoring are performed [ 3 ]. This 4-h assessment is seen as a favourable alternative to overnight inpatient stays, both in terms of patient satisfaction and public health economics [ 3 ].
The timing of delivery in women with HDP is dependent on many maternal and fetal factors, including inability to stabilise BP, deteriorating liver and/or renal function, placental abruption, and severe fetal growth restriction [ 4 ]. Fetal morbidity and mortality are linked to the gestational age at delivery [ 4 ], so there is always a desire to prolong the pregnancy as close as possible to term (37 weeks) in the absence of an emergency. HYPITAT was a multicentre, open-label randomised controlled trial investigating induction of labour versus expectant monitoring for gestational hypertension or mild pre-eclampsia after 36 weeks’ gestation [ 5 ]. The study reported a reduction in the incidence of severe hypertension as result of induction of labour at 36 weeks gestation. No significant clinical differences were found in outcomes such as thromboembolism, eclampsia or placental abruption [ 5 ]. This study was followed by HYPITAT-II, which found that delivery should be deferred until 37 weeks as opposed to 36 weeks, unless maternal deterioration supervenes [ 6 ].
Despite multiple clinical guidelines [ 4 , 7 , 8 ] and a plethora of research in the field of optimising HDP management, there have been limited published studies describing the experiences of pregnant women with the management of HDP, as distinct from medication treatment.
A survey of women with pre-eclampsia or their partners, friends or relatives found that many had no knowledge of pre-eclampsia prior to diagnosis and once diagnosed, did not appreciate how serious or life threatening it was [ 9 ]. Women wanted access to information about pre-eclampsia and their experience contributed substantial anxiety towards future pregnancies. Partners/friends/relatives also had no prior understanding of pre-eclampsia and expressed fear for the woman and/or her baby [ 9 ]. A qualitative study of pregnant Moroccan women in the Netherlands or Morocco found that knowledge of symptoms related to hypertensive disorders of pregnancy was limited or absent [ 10 ]. The limited knowledge of hypertension-related symptoms and complications was based on their own experiences or on those of some family members or stories from their social network or internet, with little or no information on symptoms from their midwives or obstetricians [ 10 ]. The experiences, perceptions and behaviours of pregnant women with regard to the management of HDP during pregnancy remain largely unexplored. Understanding these perceptions and experiences is imperative to the optimisation of HDP management.
To explore pregnant women’s perspectives of and experiences with clinical management of HDP.
Study design
A qualitative study using in-depth interviews was conducted, with pregnant women in their second or third trimester, recruited from antenatal clinics in two large tertiary hospitals in Melbourne, Australia.
Participants were sourced via a larger mixed-methods study, which included 100 pregnant women with HDP. Eligible participants were identified by one researcher (AH), who reviewed the medical records of pregnant women attending antenatal clinics, and then approached them individually. Participants were provided with written information for the larger study and on receipt of written informed consent, a questionnaire was given for self-completion. At the end of the questionnaire, participants were asked to indicate their interest in undertaking an interview. Of the 98 women who responded to the questionnaire, 65 expressed interest in being interviewed. Combined convenience and purposive sampling was conducted among these 65 women to seek a breadth of views. All of the women who were invited accepted to participate in an interview. Informed written consent was obtained prior to each interview, which included permission to audio record the conversation and to use quotations when anonymously reporting and publishing the results.
Study sample
Face-to-face, qualitative in-depth interviews were conducted with 27 pregnant women who were diagnosed with HDP and had a prescription for an antihypertensive medication, in either the second or third trimester of pregnancy, recruited from the antenatal outpatient clinics of two large tertiary maternity hospitals in Melbourne, Australia. Together, these hospitals provide antenatal care to approximately 13,000 women annually. They were identified using hospital records and approached during subsequent clinic visits. Participation was voluntary and involved informed consent.
The study sample size was determined based on theme saturation during analysis and was not predetermined. Recruitment ceased when no new information was forthcoming from the last three interviews, with regard to replication of data relating to attitudes towards HDP monitoring, perceptions of the development and management of complications (including early delivery) and perceptions of the women who had chronic hypertension.
Data collection
Interviews were conducted face to face by a single researcher (AH) a female Pharmacist (who was a PhD candidate at the time) after receiving training in in-depth interviewing prior to the commencement of the study using an interview guide developed based on the literature [ 11 , 12 ] and was agreed upon by the authors (Table 1 ). Open-ended questions, such as “Tell me about …? ”, followed by appropriate prompts, such as “How did that make you feel?” or “Can you explain that in more detail?” were used to guide the interview and encourage the interviewee to speak freely and in-depth about their experiences and thoughts. As the interviewer had met the participants during the larger study, some rapport had been established prior to the interview. The interviewer did not disclose their healthcare background to participants to avoid requests for health advice during the interviews. Interviews on average lasted 35 min (range 16 to 54 min) and were conducted in a private room near the antenatal clinics. No repeat interviews were performed.
Socio-demographic and self-reported health information was collected from participants through the questionnaire. Health information was verified, with consent, through medical records.
All interviews were audio-recorded, transcribed verbatim and de-identified. Interviews continued until data saturation was reached, deemed to be the point after which no new information for analysis was forthcoming [ 13 ]. The transcripts were not returned to the participants for comments or correction.
Data analysis
Data analysis occurred concurrently with the interviews. Initial coding was completed by AH using the qualitative data management software QSR NVivo 10 (QSR International) [ 14 ]. Inductive codes were generated systematically for the entire data set. Line-by-line analysis was then performed and nodes were created within NVivo. To ensure reliability, a random selection of 20% of the transcripts were coded independently by another member of the research team (KS). KS and KR read all the transcripts and any differences were discussed among all three to reach consensus. The researchers were all pharmacists; KS and KR had extensive experience in conducting qualitative research. Transcripts were reread by AH and KS to ensure that coding was accurate and all relevant data were included.
Thematic analysis was employed [ 15 ]. This was done across all HDP subtypes and severities to obtain a wide range of views. AH read and reread the codes, collapsed them into potential themes, compared the developing themes with the intact transcripts and cross referenced to HDP subtypes. When a pattern was seen within a certain subtype, coding was grouped specifically for that subgroup. Codes were arranged into potential themes. Themes were reviewed, refined and prepared into a final set with KS; sub-themes were identified within this process.
Participants
Of the 98 women who responded to the questionnaire, 65 expressed interest in being interviewed. A combination of convenience and purposive sampling was conducted among these women to seek a wide breadth of views. All participants had a diagnosis of HDP and were prescribed antihypertensive medication. Interviews occurred during pregnancy except for one, which happened 1 day postpartum. All participants were aged 18 years or over and were fluent in English. Twenty-seven women were interviewed to reach data saturation. Their demographics, clinical and obstetric characteristics are shown in Table 2 . Family members were present for some interviews but none of them participated in the interview or made comments. Field notes were taken by the interviewer during the interviews. No participants dropped out of the study or refused participation.
Eight participants were primigravidae, the remainder were multigravidae, including six who had previous miscarriages. One participant had an assisted pregnancy (in vitro fertilisation). Twelve were prescribed aspirin for the prevention of pre-eclampsia. Participants ranged in age from 26 to 42 years. The annotations at the end of each quote give a description of the participant’s age, parity, gestational trimester and subtype of HDP at the time of the interview. The participants did not provide feedback on the findings.
Interview themes
Three major descriptive themes were discerned regarding the women’s experiences with the management of HDP:
attitudes towards monitoring of hypertensive disorders of pregnancy;
attitudes and perceptions towards development and management of complications; and
perceptions of pregnant women with chronic hypertension.
Theme 1: Attitudes towards monitoring of hypertensive disorders of pregnancy.
Most women had general trust in the hospital system. Some felt extra confidence knowing that they were being managed at a maternity hospital:
“Hospital is for saving lives of people...as soon as I see the hospital I know that I am in safe hands.” (#14, 40 years, 2nd pregnancy, second trimester, severe pre-eclampsia).
“I felt very comfortable here...it seems like they are well prepared for these things...I was in the section of the hospital where all women were in the same [hypertension] situation.” (#9, 30 years, 1st pregnancy, second trimester, chronic hypertension).
One woman expressed some distrust in general hospitals, which she perceived as not managing her BP well:
“My own GP at that time increased it [methyldopa] to six a day … the hospital … increased to 10 a day … but I couldn’t lift my head up … so I ended up coming to the women’s hospital to Emergency, because I felt like no one’s helping me.” (# 71, 37 years, 3rd pregnancy, third trimester, severe pre-eclampsia superimposed on chronic hypertension).
Self-monitoring of BP was often recommended to women treated with antihypertensives. For some, it gave reassurance, but for others it was a source of confusion, with different messages coming from various members of the treating team:
“I take up to eight [methyldopa tablets] a day...I take two and then I’ll see what my readings are...at home, myself...I also do it if I have any other symptoms.....” (# 71, 37 years old, 3rd pregnancy, third trimester, severe pre-eclampsia superimposed on chronic hypertension).
“One of the physicians I saw told me to do it three times a day … three times in a row and then she said take the average of the second two readings each time...Then I told the obstetrician my readings and she said the machine that I had at home is under-measuring...but...the physician, he was quite interested...he wanted to see my readings because he likes to compare his machine to home machines.” (#53, 40 years, 6th pregnancy, third trimester, secondary chronic hypertension).
Some women had milder HDP in previous pregnancies, which gave them a sense that the monitoring and management was overstated. Others with more severe cases and their prior experience brought back memories initiating action to make plans:
“With [child 1] it was really bad during pregnancy. With [child 2] it was bad just in the last couple of weeks and straight afterwards...[child 3] was bad, but not really bad enough. They just called it hypertension, pregnancy induced hypertension and they just left it at that. They didn’t make a big song and dance about it...They made a big song and dance last time [child 4] and then this time [they said] ‘You’re going for your monitoring’...come back a week later...’You’re going for your monitoring’, couple of hours later – ‘You’re being admitted’...Then a couple of hours later...’You’re starting on medicine’.” (#6, 28 years, 4th pregnancy, 1 day postpartum, gestational hypertension).
“Last Wednesday when they [found] blood pressure’s up … it just brought back memories from last time because same thing. I just went in for an appointment and I never came home...Ten days later I came home with a baby. So I think those aspects freak me out a bit because it’s like ‘Oh, it’s happening again’ … every appointment...even this appointment, we’ve got contingency plans, just in case.” (#74, 36 years, 2nd pregnancy, third trimester, chronic hypertension).
Some women wondered about why they were not told their BP readings unless they asked:
“I find it funny that when they take your blood pressure they don’t tell it to you. I always have to ask, always, no matter who it is, midwife, physician, obstetrician. They take it and they walk away. It’s my body but they don’t tell me.” (#53, 40 years, 6th pregnancy, third trimester, secondary chronic hypertension).
Close monitoring was perceived as frustrating, but also as part of the life adjustments that come with having a baby. Some women considered spending four hours in the day assessment centre for monitoring their BP better than being an inpatient and staying overnight, whilst others saw it as an annoyance:
“I am happy to come back every day as long as I don’t have to spend overnight here. I am happy to be here for 12 hours a day, but I just can’t be away from my children at night time.” (#29, 26 years, 3rd pregnancy, second trimester, secondary chronic hypertension).
“They just monitor me at that perinatal care...you just sit here four hours a day...it’s shocking...worse than taking the tablet.” (#90, 35 years, 7th pregnancy, third trimester, chronic hypertension).
“The only thing that was slightly frustrating was [that] four hours is a long time to sit around, but again, you’re having a baby so you’ve got to make a few adjustments to your life.” (#99, 34 years, 1st pregnancy, third trimester, pre-eclampsia).
Some of the women required a short inpatient stay to stabilise their BP and avert an emergency premature delivery. For many, it was an emotional experience filled with apprehension and uncertainty about the future:
“It was very emotional, very scary, and at the same time still trying to stay strong. So that when my husband and my kids came in, I was like ‘I’ve just got a little bit high blood pressure, everything’s alright’...I didn’t know that a possible side effect of having the blood pressure is that they may have to deliver the baby [early].” (#32, 42 years, 3rd pregnancy, third trimester, severe early onset pre-eclampsia).
“B.P. at first was around 160...she came back 15 minutes – 170, another 15 minutes 180, within 10 minutes 190...I got nervous … After 160 they gave me ...labetalol...but [the BP] did not go down...There were other tablets they gave to me but [the BP still] didn’t go down...All the doctors came up...surrounded with those with scrub suits, I panic...blood pressure...went to 210..they were panicked...one just looked at me and said “AAAH”. I cried...of course you feel anxious, you feel sad...worried... what’s going on with me? I cried and cried. It was just like a movie, they push my bed out from the room and sent me quickly down to the birthing suite [in case delivery was imminent].” (#14, 40 years, 2nd pregnancy, second trimester, severe pre-eclampsia).
Theme 2: Attitudes and perceptions towards development and management of complications.
For many women, the diagnosis of pre-eclampsia came as a shock. Those with prior experience knew what to expect and were hesitant to cease antihypertensive treatment even if their BP was low. One woman without prior experience self-educated about pre-eclampsia, became concerned about the symptoms and developed anxiety about developing it:
“It was a shock and it was a bit scary...I thought ‘I’ve heard of pre-eclampsia but I don’t really know what it is’...but all the staff, they explained everything quite well... [I could see] how they were being very concerned about it, so that was making me realise this isn’t just a small thing, this is obviously a serious situation.” (#32, 42 years, 3rd pregnancy, third trimester, severe early onset pre-eclampsia).
“I didn’t want to stop the medication altogether, only because I just didn’t want to go through the path of having the high blood pressure affect the baby [intra-uterine growth restriction].” (#8, 36 years, 2nd pregnancy, third trimester, chronic hypertension).
“I was reading that if you do develop pre-eclampsia... it is a risk for the baby and the mother as well. Upon reading all that information...I became a bit paranoid, swollen foot, swollen hands, they’re part of the symptoms, headaches, generally not feeling well...I became quite paranoid looking at my symptoms and [thinking] have I got this, have I not got this? But the doctors actually did say that I have got borderline pre-eclampsia, so they were waiting to see if I was going to develop it. However, they haven’t been able to reassure me that I’m not going to develop it and...that was quite scary for me.” (# 24, 35 years, 1st pregnancy, third trimester, chronic hypertension diagnosed during pregnancy).
Some women understood that low-dose aspirin was being used for prevention of pre-eclampsia, whereas others did not always perceive it as being effective for this purpose. Many women thought that aspirin helped with controlling the BP rather than for prevention of pre-eclampsia:
“I started on aspirin throughout the pregnancy … just to...prevent mild pre-eclampsia happening again.” (#64, 30 years, 3rd pregnancy, third trimester, chronic hypertension).
“Obstetrician put me on one aspirin a day which is supposed to help control blood pressure. So perhaps that’s also why my blood pressure is being well controlled.” (#21, 35 years, 1st pregnancy, second trimester, chronic hypertension).
Most women had a general understanding that the only way to stop the direct effects of pre-eclampsia was to deliver the baby for the safety of both mother and child. The level of comfort with such a decision varied depending on the gestational stage of diagnosis of pre-eclampsia:
“I was really disappointed and very worried about the effect it [pre-eclampsia] would have on the baby [at 21 weeks] and whether or not I would be able to carry the baby to a safe week. I just thought...if something had happened and I was forced, like accidentally went into labour too early or something like that, the baby’s chances of survival would be very low and I was really upset.” (#21, 35 years, 1st pregnancy, second trimester, chronic hypertension).
“All I know is that you just need to get the baby out...I mean plenty of women and plenty of babies survive it...but you need to detect it pretty quickly before it turns into the full...is it eclampsia?” (#41, 34 years, 1st pregnancy, third trimester, chronic hypertension).
Although many women understood that they would not continue to full-term, their perceptions and fears about the potential for a premature delivery were related to their week of gestation, concern about the welfare of the baby, and fear of separation after the birth:
“I know from my reading that 24 weeks, it’s still not ideal obviously, but if you had the baby at 24 weeks that the chance of survival was higher. I think it was 43% chance of survival from this...prior to that it was like 16% chance of survival...My sister-in–law, who is a midwife, had said...they consider 26 weeks more viable. So after that it was like, right (a) to get to 24, (b) get to 26.”. (#21, 35 years, 1st pregnancy, second trimester, chronic hypertension).
“I am just worried about my baby [having] to be delivered earlier because you see the consequences...you see things happen in the future...they are still very weak...no sucking reflex yet, the lungs are not fully developed, so many things not developed...she may live but maybe there are some disabilities...I am just hoping that I will reach even up to 30 weeks or 32 weeks. That would make me feel better.” (#14, 40 years, 2nd pregnancy, second trimester, severe pre-eclampsia).
“I was 28 weeks [when I developed severe pre-eclampsia]...they gave me steroid injections to increase the lung capacity of the baby...One of the doctors came from the NICU with a leaflet about possibly having a premature baby...that was very upsetting...and to think of having the baby...then me going home with the baby staying here is just a very scary thought.”(#32, 42 years, 3rd pregnancy, third trimester, severe early onset pre-eclampsia).
Intervention with the delivery process was a likely reality for many women who had a prospect of early delivery. Some women were apprehensive about the prospect of induction of labour or caesarean section but understood that it was for their benefit and that of their child. Others were hesitant to allow for intervention unless the risks were made clear:
“So a little bit scary, but in a way I want it to, because I’m starting to feel the uncomfortable risk that’s associated with pregnancy in this condition. Knowing that she’s at full term now at 37 weeks and she’s fine and healthy, I don’t want to develop pre-eclampsia if I can help it.” (# 24, 35 years, 1st pregnancy, third trimester, chronic hypertension diagnosed during pregnancy).
“I’m trying to push it off because I don’t want to do it. I like to have the baby when the baby’s ready, not when they tell me to. But if they tell me to because it’s really dangerous for me then I’ll listen to them obviously ….” (#53, 40 years, 6th pregnancy, third trimester, secondary chronic hypertension).
Concerns about lack of information sharing by health professionals led some women to feel that they were left out of the planning for potential intervention in the delivery, whilst others voiced concern about having low-dose aspirin in the context of a possible emergency caesarean section:
“I even asked her last time actually because she said...I’m happy with the baby’s growth, but the blood pressure’s going up so … she said ‘I’m formulating a plan in my mind’ but she doesn’t like to disclose it. I don’t know why. It’s about me; I don’t know why she just doesn’t tell me.” (#53, 40 years, 6th pregnancy, third trimester, secondary chronic hypertension).
“I also thought...what if I have an emergency caesarean tomorrow and I haven’t gotten off the aspirin? Is it going to cause me issues?” (#29, 26 years, 3rd pregnancy, second trimester, secondary chronic hypertension).
Theme 3: Perceptions of pregnant women with chronic hypertension.
Many women with chronic hypertension were already on antihypertensive medication not deemed safe during pregnancy when they found out they were pregnant. For some, it was changed to a safer alternative as soon as possible, whilst for others, the decision to change the medication was delayed and the patient’s assessment of potential risks was downplayed:
“I was on medication [telmisartan]...then when I had the kidney scan and I found out [that I was pregnant], my G.P. said ‘You’ve got to stop taking that medication because it’s not safe...so then she gave me another one to take.” (#2, 30 years, 1st pregnancy, third trimester, secondary chronic hypertension).
“The first time I found out I was pregnant I went to a GP...I told the GP that I’m taking atenolol, and then she told me that...atenolol is not recommended for pregnancy... so I asked....What medication do you think that I should take?’...she said she doesn’t dare to prescribe me any medicine because she knows she is going to refer me to a hospital.” (#59, 34 years, 1st pregnancy, second trimester, chronic hypertension).
Other women had their antihypertensive changed during the pre-pregnancy planning stage:
“[To be safe during pregnancy] I would just have to change my medications. The medication I was on I couldn’t be on while being pregnant. So when we decided to try for our first child, I went on the Aldomet and oxprenolol and that’s what I pretty much stayed on because we always wanted a second child.” (#1, 39 years, 2nd pregnancy, third trimester, secondary chronic hypertension).
Some women with chronic hypertension had concerns about lack of information sharing by health professionals and felt that they were not well informed of the potential risks that their hypertension may have on the pregnancy. Some mentioned that they may have ‘taken it more seriously’ if they had known about the risk of premature delivery associated with uncontrolled hypertension, whilst others had some limited awareness of pre-eclampsia:
“When they told me I had protein in my urine, I was a bit scared because I don’t know if it’s related to my BP.” (#4, 33 years, 1st pregnancy, third trimester, chronic hypertension).
One woman was very anxious about her diagnosis of severe, early-onset pre-eclampsia so she did some ‘self-research’. Unfortunately, she misinterpreted the information and caused herself extra unwarranted fear:
“I read on [US website found on Google] and found that 80% die after/during birth that have pre-eclampsia. That was really scary.” (#71, 37 years, 2nd pregnancy, third trimester, severe pre-eclampsia superimposed on chronic hypertension).
The information on the US Preeclampsia Foundation website actually states that “Nearly 80% of women who die from pre-eclampsia die post-partum” [ 16 ].
For some women with chronic hypertension, lack of knowledge of the seriousness of the condition resulted in lack of comprehension of the importance of BP monitoring and treatment:
“I think it was about 140 over 110 or something like that … which is pretty normal for me but they think it’s high … I feel alright. It’s all good.” (#90, 35 years, 7th pregnancy, third trimester, chronic hypertension).
“I really tried for weeks not to go on [the antihypertensive], but then when she said that maybe you could have a stroke, I got a bit scared, a lot scared … I got really worried because then they said … you could have problems, the baby could die. And I got really upset when she said the baby could not get enough oxygen. I just felt, oh just have whatever it is.” (#22, 37 years, 3rd pregnancy, third trimester, chronic hypertension).
Most women who had chronic hypertension were under a model of care involving both an obstetrician and a physician. One was triaged to midwife-only care, despite having a diagnosis of chronic hypertension and being prescribed an antihypertensive medication. This then caused a delay in the change of the antihypertensive to a safer alternative:
“Actually, I asked the midwife whether it [atenolol] is safe or not [at 18 weeks gestation]...and then she said that...it should be okay, but to be safe discuss with the physician. And so, because she said it should be okay, I presumed that ‘Oh that is okay’ ... but then the physician said ‘No, it’s better not to...so from now onwards you have to take this medicine [oxprenolol]’.” (#59, 34 years, 1st pregnancy, second trimester, chronic hypertension).
Many women had their first antenatal appointment at the hospital between 16 and 20 weeks gestation. Some women, especially those with chronic hypertension, had concerns about the timing of this appointment:
“It takes a long time now for women to get their first appointment through the hospital. It wasn’t like that, I think, about 10 years ago, must’ve changed by now … Now you have to wait ‘til you’re about 18, 20 weeks before you get your first actual appointment...and if you’ve got other health issues, things can go wrong, which it did with me.” (#71, 37 years, 2nd pregnancy, third trimester, severe pre-eclampsia superimposed on chronic hypertension).
Some women did not know that they had high BP before pregnancy. This may have been because they did not get regular check-ups with the GP or that their BP was not routinely checked at regular GP visits:
“I think if I had never gotten pregnant, I definitely would not have had [high BP], would not have to be on medication … because I wouldn’t be under the strain that I am. And also I wouldn’t be in with the doctor. I don’t think I would’ve gone to the doctor and said put me on medication … because I didn’t, want anything to change. But my lifestyle is changing now so I don’t have a choice.” (#18, 35 years, 2nd pregnancy, second trimester, chronic hypertension).
“It [BP] was quite normal before the pregnancy, so obviously it’s pregnancy-related according to the doctors [despite having been diagnosed at 7 weeks].” (#24, 35 years, 1st pregnancy, third trimester, chronic hypertension diagnosed during pregnancy).
Many women had developed chronic hypertension after a previous pregnancy that involved either gestational hypertension or pre-eclampsia. Some of them had routine follow-up for their hypertension postpartum and understood that it was now chronic hypertension, whilst others did not:
“Once I’d had the baby they changed my medication to the perindopril...I was then checking my BP at home...the readings were fine...when they did get too high, I’d go back to my local GP who would then once again adjust the dosage accordingly...I have been told by my local GP that generally once you’re on a blood pressure medication, you’re on it for life, whether it’s a minimal dosage or, depending on what the readings are, what they need to give...I’m happy to stay on that.” (#8, 36 years, 2nd pregnancy, third trimester, chronic hypertension).
“I got increased blood pressure at the end [of the previous pregnancy] and they put me in perinatal care, but then afterwards it was okay....I honestly just didn’t go to the doctor, and I haven’t gone to the doctor since I fell pregnant with this one.” (#90, 35 years, 7th pregnancy, third trimester, chronic hypertension).
One woman described having been prescribed an antihypertensive during her previous pregnancy and never told to stop it, so she continued with no formal review of her hypertension until the current pregnancy:
“They never told me to stop taking the tablet [labetalol] after I had him [first child] so I just kept continuing with it ... I saw the physician [during this current pregnancy] and he just said just keep taking it...he actually questioned ‘Did they ask you to stop it?...I said no one spoke to me about anything...I was here for a week after I had him [first child]...no one ever discussed it.” (#58, 38 years, 2nd pregnancy, third trimester, chronic hypertension).
Trust in the hospital system, positive attitudes towards close BP monitoring as well as self-monitoring of BP (SMBP) and a realistic approach to emergency antenatal hospital admissions contributed to a positive attitude towards monitoring of HDP. Most of the women in our study had a general trust in the healthcare system. Distrust surfaced when health services outside the women’s hospital were not seen as able to control hypertension early in the pregnancy, triggering patient-initiated referral to the women’s hospital. Trust of healthcare systems in western countries is generally declining [ 17 ]. It is, however, important to note that pregnant women with HDP are considered to be in a high-risk pregnancy and are thus more vulnerable than the general population. Therein lies dependence on the hospital system, especially in urgent situations such as needing to lower BP or planning for an early delivery, similar to the dependence reported in patients with coronary heart disease [ 18 ].
Anecdotally, it is common for healthcare professionals to mention that the BP reading is ‘good’ or ‘too high’ without telling the patient the systolic/diastolic numbers. An important factor relating to patient evaluation of care is their involvement in decision-making [ 19 ]. Most of the women in our study were not involved in decision-making, leaving some to wonder why this was so. This suggests that pregnant women with HDP would like to be better informed of their situation and be part of the decision-making process when deemed appropriate. This is consistent with the women’s views from the pilot of the CHIPS study who enjoyed being heavily involved in their BP management [ 20 ]. One way to have women more involved in their BP management is to encourage SMBP. SMBP in the general population has been shown to reduce BP [ 21 ] and improve adherence to antihypertensive medication [ 22 ]. In our study, SMBP was often recommended to women who were prescribed antihypertensive medications. This was taken up well by most, similar to the CHIPS pilot study [ 20 ]. SMBP during pregnancy has also been shown to be reassuring and not anxiety provoking [ 23 ] which was seen in our study. A recent survey of 5555 pregnant women from antenatal clinics in 16 hospitals in England, found that nearly half of the 389 hypertensive women reported SMBP, and that the majority of them (79%) shared their BP readings with their treating doctor [ 24 ]. Such partnership has been shown to improve patient adherence in the general population [ 25 ]. There is however an assumption that because these women are in a high-risk pregnancy, the healthcare professionals (HCP) tend to take over and do not acknowledge that the women are quite competent and that with the correct information can be involved in SMBP in collaboration with the HCPs. It is thus important to have a good doctor-patient relationship to reduce confusion, instil confidence in SMBP and complete the circle of care.
Those with prior experience with HDP and monitoring had varied views, often depending on the severity of disease in the previous pregnancy (ies). Good communication about how HDP can vary from one pregnancy to another, being either worse or better, may assist in reducing the cynicism of some and reassure others. Similarly, those who had prior experience with pre-eclampsia were a lot more confident in their management and knew what to expect. Those who did not have prior experience were often in shock and were at times anxious about the diagnosis, a finding similar to another study relating to the understanding of pre-eclampsia [ 26 ]. Moreover, the use of low-dose aspirin to prevent pre-eclampsia was only partially understood by women in our study. This indication should be communicated clearly to women who are at high risk of developing pre-eclampsia. The plan for the cessation of aspirin before delivery should also be communicated clearly to reduce any anxiety that may be present, especially in terms of a potential emergency delivery.
At times, antenatal inpatient admission was required to stabilise BP and closely monitor both the mother and baby. This was a particularly apprehensive time, especially for women who had not experienced HDP during a previous pregnancy. It is important to have good, clear communication with women about the need for close monitoring, affirmation concerning their status as worthy of hospital care, provision of consistent information, inclusion in decision-making and good social support [ 27 ]. A possible alternative to inpatient admission can be pregnancy day assessment monitoring. Despite limited research into this model of care, pregnant women have been found to prefer a four-hour stay rather be admitted to hospital for one or more nights, if the situation is deemed safe to do so [ 3 ]. This is consistent with our findings. Once again, clear consistent information regarding the need for this type of monitoring should be given to women who require it. Recent advancements in the integration of telemedicine into antenatal care [ 28 ] have encouraged early research into the feasibility of incorporating this for women with HDP to reduce the burden of multiple antenatal hospital visits [ 29 ]. The unpredictable course of worsening BP and the development of pre-eclampsia pose specific challenges to this monitoring and would require a holistic approach. A recent single centre study in the UK [ 29 ] developed and trialled an innovative SMBP intervention including a downloadable mobile app in for women with HDP to monitor for signs of pre-eclampsia or worsening hypertension. Although this study showed positive acceptance and compliance from the women, further research is required to meet the standard of care required for them [ 29 ].
In general, most women desire to labour spontaneously and have a natural birth [ 30 ]. When the reality that the only way to stop the direct effects of pre-eclampsia is to deliver the baby at any given gestational week is revealed to some women, it is received with disappointment. Good communication by the treating doctor about the intention to preserve the pregnancy for as close as possible to term is required. Likewise, sound communication about the need for a premature delivery should be communicated clearly. Moreover, it has been shown that pregnant women who require induction of labour or caesarean section often feel left out of the decision-making process [ 30 ]. An Australian study of women’s experiences of decision-making and attitudes in relation to induction of labour, reported a clear need for women to be provided with more information and agency when making decisions about their timing of birth, particularly when there are multiple reasonable treatment options [ 30 ]. Furthermore, emergency caesarean sections have been found to negatively contribute to several psychosocial outcomes for women, in particular post-traumatic stress [ 31 ]. There is, thus, a need for careful consideration and counselling for women after an emergency caesarean delivery. This can involve the members of the antenatal treating team but also counsellors or psychologists. Moreover, counselling of pregnant women who are at risk of emergency caesarean, either because of their HDP or otherwise, about this possibility may help to pre-empt potential trauma.
Research into the management experiences of pregnant women with chronic hypertension, as distinct from medication treatment, is scant. Our study has highlighted the need for extra attention to be given to improve management pre-conception, during the pregnancy and postpartum. A qualitative study exploring knowledge and attitudes related to pregnancy and preconception health in women with chronic medical conditions, including chronic hypertension, found that the women had limited knowledge of the specific potential complications of pregnancy [ 32 ]. Some women in our study also displayed limited understanding of the potential risks that they may endure during pregnancy and thus had a lack of comprehension of the seriousness of the condition. Counselling women pre-conception regarding potential risks during pregnancy allows them to be more aware of what to expect [ 33 ]. Moreover, an open conversation about the information that the pregnant woman may have either from prior experience or ‘self-research’ would help to improve understanding as well as avoiding confusion and unnecessary anxiety. The provision of written material so that the women can refer to it when necessary would also be appropriate. Another facet of management of chronic hypertension pre-conception is the initial diagnosis of hypertension. Some women in our study were not aware of their BP readings before pregnancy and were diagnosed with hypertension quite early in pregnancy and thus classed as having chronic hypertension. Regular checking of BP in women of reproductive age at routine GP visits may help to identify chronic hypertension earlier. This can help in the planning of a pregnancy, or ensure that the BP is under control in the case of an unexpected pregnancy. Similarly, for those who have chronic hypertension and are prescribed antihypertensives, switching the medication to one that is safer during pregnancy, either pre-conception or as early as possible, can help to reduce fetal exposure and reduce the mother’s anxiety. Both GPs and community pharmacists have a role in counselling women of reproductive age who are prescribed an antihypertensive. A simple question as to whether the women is planning a pregnancy can help initiate the necessary conversation and trigger the switch to a safer alternative in a timely manner. Various resources are available to help make the decision to change the antihypertensive, including drug information lines at maternity hospitals. Moreover, a large number of women who had entered our study with chronic hypertension had developed the condition after a previous pregnancy that was affected by gestational hypertension or pre-eclampsia. Furthermore, our study found that many women developed chronic hypertension soon after a pregnancy complicated by HDP. This is supported by a recent systematic review and meta-analysis which reported that the risk of developing hypertension after HDP is highest in the early postpartum period [ 34 ]. The authors also suggested that diagnosis and targeted interventions to improve maternal cardiovascular health may need to be commenced in the immediate postpartum period [ 33 ]. We agree with this and call for a more integrated follow up with women in the postpartum period and beyond. This may involve the GP and the community pharmacist for easy accessibility for the women.
Although most women with chronic hypertension in our study were under a model of care involving an obstetrician and a physician, one was under midwifery care despite having chronic hypertension and being prescribed an antihypertensive. Although it is recognised by Australian guidelines for the management of HDP that midwives play a role in a multidisciplinary team in relation to management of HDP [ 4 , 35 ], they are not qualified to independently prescribe medication and manage cases of pregnant women with chronic hypertension requiring treatment. Similarly, a recent scoping review found that practising midwives worldwide lack knowledge on several aspects of pre-eclampsia diagnosis and care and have called for an increase in in-service training to increase midwives’ knowledge in this area [ 36 ]. It is therefore important that all pregnant women who have chronic hypertension be under the doctor model of care to monitor both mother and baby throughout the pregnancy.
Recommendations for practice
Good communication between the HCP and the patient is important to optimise management. Clear, direct and concise information about various facets of the management of HDP should be provided for all women who experience HDP.
Women who experience any form of HDP during pregnancy should be invited to be part of the decision-making pertaining to the monitoring of BP and progression to pre-eclampsia as well as the timing and mode of delivery when appropriate.
There should be a priority for women with chronic hypertension to be seen at the hospital under an obstetrician led model of care before 18 weeks, not only for the regular monitoring of BP and fetal progress but also for the timely prescription of low-dose aspirin before 16 weeks gestation for the prevention of pre-eclampsia. Furthermore, other health professionals, including psychologists and pharmacists, can be involved in the prenatal care of these women to address potential fear and anxiety as well as the optimal use of medication.
Women who have chronic hypertension and are of reproductive age should be informed of the potential risks of pregnancy and be switched to a pregnancy safe antihypertensive in the preconception stage.
Women who experience gestational hypertension or pre-eclampsia during pregnancy should have their BP monitored postpartum by the GP or the community pharmacist to identify any risk of developing severe cardiovascular events.
Recommendations for future research
Currently, much of the monitoring of HDP requires a hospital visit. Further research into the feasibility of telehealth for the monitoring of HDP, especially in mild cases will help to include patients in the decision-making. Moreover, future research into the role of the GP and the community pharmacist in the pre-pregnancy planning stage for those with chronic hypertension and postpartum for those with gestational hypertension or pre-eclampsia is warranted.
Strengths and limitations
This qualitative study is the first to use in-depth interviews to explore pregnant women’s experiences, perceptions and behaviours with regard to the management of HDP during pregnancy. Our study included women with all forms of HDP except HELLP and eclampsia, as the interviews were done when the women were in a comfortable, non-emergency situation. Participants varied in gestation stage, subtype of HDP, severity of HDP, ethnicity and socioeconomic status allowing for a wide range of views. The interviews were conducted during pregnancy thus reducing recall bias. This is in contrast to other qualitative studies which explored aspects of HDP in retrospect [ 27 , 35 , 37 ]. Recruitment was from two major public maternity referral hospitals in Melbourne with a widespread combined catchment including metropolitan, regional and rural areas. Participants did not include those in the first trimester of pregnancy, as most were scheduled to attend the antenatal clinics after 12 weeks gestation. Views of women with chronic hypertension during the first trimester of pregnancy may vary from those in the second and third trimesters and they were not captured . Women with poor English skills were excluded from the study, therefore, caution should be taken in the extrapolation of our findings to women from non-English speaking backgrounds.
The clinical management experiences of pregnant women with HDP were varied. Many women did not feel that they were well informed of treatment and management decisions and had a desire to be more informed and more involved in decision-making. Clear, concise information about various facets of HDP management including BP monitoring, administration of low dose aspirin in women with a high risk of developing pre-eclampsia, prescription of the appropriate antihypertensive, and planning for potential early delivery are required. In addition, cardiovascular pre-pregnancy planning and postpartum follow-up should be routinely offered to women.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due to privacy surrounding participant information as stipulated in the written consent form, but are available from the corresponding author on reasonable request.
Royal Australian and New Zealand College of Obstetrians and Gynaecologists. Maternity Care in Australia. 1st ed; 2017.
Google Scholar
Roberge S, Nicolaides K, Demers S, Hyett J, Chaillet N, Bujold E. The role of aspirin dose on the prevention of preeclampsia and fetal growth restriction: systematic review and meta-analysis. Am J Obstet Gynecol. 2017;216(2):110–20 e6.
Article CAS Google Scholar
Dunlop L, Umstad M, McGrath G, Reidy K, Brennecke S. Cost-effectiveness and patient satisfaction with pregnancy day care for hypertensive disorders of pregnancy. Aust NZ J Obstet Gyn. 2003;43(3):207–12.
Article Google Scholar
Lowe SA, Bowyer L, Lust K, McMahon LP, Morton MR, North RA, et al. The SOMANZ guidelines for the Management of Hypertensive Disorders of pregnancy 2014. Aust NZ J Obstet Gyn. 2015;55(1):11–6.
Koopmans CM, Bijlenga D, Groen H, Vijgen SMC, Aarnoudse JG, Bekedam DJ, et al. Induction of labour versus expectant monitoring for gestational hypertension or mild pre-eclampsia after 36 weeks’ gestation (HYPITAT): a multicentre, open-label randomised controlled trial. Lancet. 2009;374(9694):979–88.
Broekhuijsen K, van Baaren GJ, van Pampus MG. Immediate delivery versus expectant monitoring for hypertensive disorders of pregnancy between 34 and 37 weeks of gestation (HYPITAT-II): a multicentre, open-label randomised controlled trial (vol 385, pg 2492, 2015). Lancet. 2016;387(10021):848.
Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S, et al. Hypertensive disorders of pregnancy ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension. 2018;72(1):24–43.
Magee LA, Pels A, Helewa M, Rey E, von Dadelszen P, Pr CHD. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy. Pregnancy Hypertens. 2014;4(2):105–45.
East C, Conway K, Pollock W, Frawley N, Brennecke S. Women’s experiences of preeclampsia: Australian action on preeclampsia survey of women and their confidants. J Pregnancy. 2011. Article number 375653.
Ouasmani F, Engeltjes B, Haddou Rahou B, Belayachi O, Verhoeven C. Knowledge of hypertensive disorders in pregnancy of Moroccan women in Morocco and in the Netherlands: a qualitative interview study. BMC Pregnancy Childbirth. 2018;18(1):344.
Sawicki E, Stewart K, Wong S, Leung L, Paul E, George J. Medication use for chronic health conditions by pregnant women attending an Australian maternity hospital. Aust NZ J Obstet Gynaecol. 2011;51(4):333–8.
Lim AS, Stewart K, Abramson MJ, Ryan K, George J. Asthma during pregnancy: the experiences, concerns and views of pregnant women with asthma. J Asthma. 2012;49(5):474–9.
Fusch PI, Ness LR. Are we there yet? Data saturation in qualitative research. Qual Rep. 2015;20(9):1408–16.
Microsoft Windows NVivo 10. [Available from: http://help-nv10.qsrinternational.com/desktop/concepts/about_nodes.htm .] Accessed 21 Feb 2020.
Braun VC, V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77–101.
Preeclampsia Foundation 2021 [Available from: https://www.preeclampsia.org/health-information ]. Accessed 11/02/2021.
Ward PR, Rokkas P, Cenko C, Pulvirenti M, Dean N, Carney S, et al. A qualitative study of patient (dis) trust in public and private hospitals: the importance of choice and pragmatic acceptance for trust considerations in South Australia. BMC Health Serv Res. 2015;15:297.
Meyer SB, Ward PR. Differentiating between trust and dependence of patients with coronary heart disease: furthering the sociology of trust. Health Risk Soc. 2013;15(3):279–93.
Hodnett ED. Pain and women’s satisfaction with the experience of childbirth: a systematic review. Am J Obstet Gynecol. 2002;186(5):S160–S72.
Magee LA, von Dadelszen P, Chan S, Gafni A, Gruslin A, Helewa M, et al. Women’s views of their experiences in the CHIPS (control of hypertension in pregnancy study) pilot trial. Hypertension Pregnancy. 2007;26(4):371–87.
Tucker KL, Sheppard JP, Stevens R, Bosworth HB, Bove A, Bray EP, et al. Self-monitoring of blood pressure in hypertension: a systematic review and individual patient data meta-analysis. PLoS Med. 2017;14(9):e1002389. https://doi.org/10.1371/journal.pmed.1002389 .
Ogedegbe G, Schoenthaler A. A systematic review of the effects of home blood pressure monitoring on medication adherence. J Clin Hypertens (Greenwich). 2006;8(3):174–80.
Hinton L, Tucker KL, Greenfield SM, Hodgkinson JA, Mackillop L, McCourt C, et al. Blood pressure self-monitoring in pregnancy (BuMP) feasibility study; a qualitative analysis of women’s experiences of self-monitoring. BMC Pregnancy Childbirth. 2017;17(1):427. https://doi.org/10.1186/s12884-017-1592-1 .
Tucker KL, Hodgkinson J, Wilson HM, Crawford C, Stevens R, Lay-Flurrie S, et al. Current prevalence of self-monitoring of blood pressure during pregnancy: the BUMP survey. J Hypertens. 2021;39(5):994–1001.
Sabate E, editor. Adherence to long-term therapies: evidence for action. Geneva: World Health Organization; 2003.
You WB, Wolf M, Bailey SC, Pandit AU, Waite KR, Sobel RM, et al. Factors associated with patient understanding of preeclampsia. Hypertens Pregnancy. 2012;31(3):341–9.
Barlow JHHJ. Thornton S Women’s experiences of hospitalisation with hypertension during pregnancy: feeling a fraud. J Reprod Infant Psychol. 2008;26(3):157–67.
Zairina E, Abramson MJ, McDonald CF, Li J, Dharmasiri T, Stewart K, et al. Telehealth to improve asthma control in pregnancy: a randomized controlled trial. Respirology. 2016;21(5):867–74.
Sheehan EKA, Kay L. Using a smartphone app to identify signs of pre-eclampsia and/or worsening blood pressure. Br J Midwifery. 2019;27(2):92–9.
Coates D, Goodfellow A, Sinclair L. Induction of labour: experiences of care and decision-making of women and clinicians. Women Birth. 2020;33(1):e1–e14.
Benton M, Salter A, Tape N, Wilkinson C, Turnbull D. Women’s psychosocial outcomes following an emergency caesarean section: a systematic literature review. BMC Pregnancy Childbirth. 2019;19(1):535.
Chuang CH, Velott DL, Weisman CS. Exploring knowledge and attitudes related to pregnancy and preconception health in women with chronic medical conditions. Matern Child Hlth J. 2010;14(5):713–9.
Seely EW, Ecker J. Chronic hypertension in pregnancy. Circulation. 2014;129(11):1254–61.
Giorgione V, Ridder A, Kalafat E, Khalil A, Thilaganathan B. Incidence of postpartum hypertension within 2 years of a pregnancy complicated by pre-eclampsia: a systematic review and meta-analysis. BJOG. 2021;128(3):495–503.
Roberts LM, Davis GK, Homer CS. Pregnancy with gestational hypertension or preeclampsia: a qualitative exploration of women’s experiences. Midwifery. 2017;46:17–23.
Garti I, Gray M, Tan JY, Bromley A. Midwives’ knowledge of pre-eclampsia management: a scoping review. Women Birth. 2021;34(1):87–104.
Shanmugalingam R, Mengesha Z, Notaras S, Liamputtong P, Fulcher I, Lee G, et al. Factors that influence adherence to aspirin therapy in the prevention of preeclampsia amongst high-risk pregnant women: a mixed method analysis. PLoS One. 2020;15(2):e0229622. https://doi.org/10.1371/journal.pone.0229622 .
Download references
Acknowledgements
We would like to thank the staff at both the Mercy Hospital for Women and the Royal Women’s Hospital for their help with this study. We would also like to thank all the women who participated.
This study did not receive any funding.
Author information
Authors and affiliations.
Centre for Medicine Use and Safety, Faculty of Pharmacy and Pharmaceutical Sciences, Monash University, 381 Royal Parade, Parkville, Melbourne, Victoria, Australia
Amyna Helou, Kay Stewart & Johnson George
Reading School of Pharmacy, University of Reading, Whiteknights, Reading, RG6 6AP, UK
You can also search for this author in PubMed Google Scholar
Contributions
All authors contributed to the conception and design of the study. Patient recruitment and in-depth interviews were undertaken by AH. Data analyses and interpretation were performed by AH, KS and KR. The manuscript was written by AH and critically reviewed by all authors. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Johnson George .
Ethics declarations
Ethics approval and consent to participate.
Ethical approval was obtained from Mercy Health Human Research Ethics Committee Heidelberg-Melbourne (R12/62) 08/01/2013, The Royal Women’s Hospital Research and Human Research Ethics Committee Parkville-Melbourne (R13/18) 12/07/2013 and Monash University Human Research Ethics Committee Clayton-Melbourne (CF13/117) 18/01/2013. Informed written consent was obtained prior to each interview, which included permission to audio record the conversation and use quotations when anonymously reporting and publishing the results. All methods were carried out in accordance with relevant guidelines and regulations of the ethics committees.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.

Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Helou, A., Stewart, K., Ryan, K. et al. Pregnant women’s experiences with the management of hypertensive disorders of pregnancy: a qualitative study. BMC Health Serv Res 21 , 1292 (2021). https://doi.org/10.1186/s12913-021-07320-4
Download citation
Received : 12 July 2021
Accepted : 08 November 2021
Published : 02 December 2021
DOI : https://doi.org/10.1186/s12913-021-07320-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Hypertension
- Chronic hypertension
- Pre-eclampsia
- Experiences
BMC Health Services Research
ISSN: 1472-6963
- General enquiries: [email protected]
- Open access
- Published: 02 April 2024
Post-bariatric pregnancy is associated with vitamin K1 deficiency, a case control study
- Brit Torunn Bechensteen 1 , 2 ,
- Cindhya Sithiravel 3 ,
- Ellen Marie Strøm-Roum 4 ,
- Heidi Kathrine Ruud 2 ,
- Gunnhild Kravdal 3 ,
- Jacob A. Winther 1 &
- Tone G. Valderhaug 1
BMC Pregnancy and Childbirth volume 24 , Article number: 229 ( 2024 ) Cite this article
198 Accesses
Metrics details
Maternal obesity is associated with adverse outcome for pregnancy and childbirths. While bariatric surgery may improve fertility and reduce the risk of certain pregnancy-related complications such as hypertension and gestational diabetes mellitus, there is a lack of evidence on the optimal nutritional monitoring and supplementation strategies in pregnancy following bariatric surgery. We aimed to assess the impact of bariatric surgery on micronutrients in post-bariatric pregnancy and possible differences between gastric bypass surgery and sleeve gastrectomy.
In this prospective case control study, we recruited 204 pregnant women (bariatric surgery n = 59 [gastric bypass surgery n = 26, sleeve gastrectomy n = 31, missing n = 2] and controls n = 145) from Akershus university hospital in Norway. Women with previous bariatric surgery were consecutively invited to study participation at referral to the clinic for morbid obesity and the controls were recruited from the routine ultrasound screening in gestational week 17–20. A clinical questionnaire was completed and blood samples were drawn at mean gestational week 20.4 (SD 4.5).
The women with bariatric surgery had a higher pre-pregnant BMI than controls (30.8 [SD 6.0] vs. 25.2 [5.4] kg/m2, p < 0.001). There were no differences between groups regarding maternal weight gain (bariatric surgery 13.3 kg (9.6) vs. control 14.8 kg (6.5), p = 0.228) or development of gestational diabetes ( n = 3 [5%] vs. n = 7 [5%], p = 1.000). Mean levels of vitamin K1 was lower after bariatric surgery compared with controls (0.29 [0.35] vs. 0.61 [0.65] ng/mL, p < 0.001). Multiadjusted regression analyses revealed an inverse relationship between bariatric surgery and vitamin K1 (B -0.26 ng/mL [95% CI -0.51, -0.04], p = 0.047) with a fivefold increased risk of vitamin K1 deficiency in post-bariatric pregnancies compared with controls (OR 5.69 [1.05, 30.77] p = 0.044). Compared with sleeve gastrectomy, having a previous gastric bypass surgery was associated with higher risk of vitamin K1 deficiency (OR 17.1 [1.31, 223.3], p = 0.030).
Post-bariatric pregnancy is negatively associated with vitamin K1 with a higher risk of vitamin K1 deficiency in pregnancies after gastric bypass surgery compared with after sleeve gastrectomy. Vitamin K1 deficiency in post-bariatric pregnancy have potential risk of hypocoaguble state in mother and child and should be explored in future studies.
Peer Review reports
Obesity is common in women of reproductive age, increasing the risk of several complications for mother and child [ 1 , 2 ]. Maternal metabolism in obesity may reduce the likelihood of successful pregnancy [ 3 ]. Moreover, given that weight loss before pregnancy mitigates the adverse outcomes of pregnancy related outcomes from obesity, bariatric surgery in women of reproductive age in increasing [ 4 , 5 ]. However, although bariatric surgery may reduce the risks of certain obesity related complications in pregnancy, pregnancy after bariatric surgery may carry adverse events such as malnutrition, vitamin deficiencies and inadequate weight gain as well as changes in endocrine and metabolic homeostasis [ 6 , 7 , 8 , 9 , 10 ]. Pregnancy following bariatric surgery has been associated with increased risk of preterm birth, nutritional deficiency and small for gestational age [ 7 , 8 , 11 , 12 , 13 , 14 ]. The causality of these effects are not known, but personalized nutritional counseling during post-bariatric pregnancy has been shown to improve nutrient intake of mothers and may contribute to higher weight of offspring [ 15 ].
There is a growing body of evidence suggesting that maternal nutrition and lifestyle affect fetal growth and development [ 16 , 17 ]. Micronutrients are vitamins and minerals that enable the body to produce enzymes, hormones and other substances essential for normal growth and development [ 18 ]. Micronutrient deficiencies contribute to poor growth, intellectual impairments and increased risk of morbidity and mortality [ 19 ]. Widespread global micronutrient deficiencies exist, with pregnant women and young children at highest risk [ 19 ]. Micronutrient interventions such as supplementation of folate to prevent neural tube defects zinc to reduce risk of preterm birth, and iron to reduce the risk of low birthweight are established [ 20 , 21 , 22 ]. The micronutritional deficiencies seen after bariatric surgery might be explained by poor dietary pattern in combination with gastrointestinal modification and reduced intestinal transit time [ 23 , 24 , 25 , 26 , 27 ]. Deficiencies of fatty soluble vitamins seem to be particularly prevalent in post-bariatric pregnancies, with potential risks of impaired vision, neuronal disorders, impairment of the immune system and hypocoagulability for mother and child [ 24 , 28 , 29 , 30 ].
While sleeve gastrectomy is the most common surgical procedure for the treatment of obesity worldwide, there is conflicting evidence on the optimal surgical procedure before subsequent pregnancy [ 10 , 31 ]. A large registry study showed no difference between gastric bypass and sleeve gastrectomy for preterm birth or small for gestational age [ 12 ]. Studies indicates increased risk of prematurity in pregnancy occurring less than 2 years after bariatric surgery [ 12 , 32 ]. However, other studies have not confirmed increased risks in pregnancies related to time-interval between bariatric surgery and conception [ 13 , 33 ]. As such, there is an evident knowledge gap on the impact of bariatric surgery on micronutrient status in pregnancies as well as outcomes for mother and child in order to provide optimal obstetric care in this group.
The aim of this study was to assess the impact of bariatric surgery on concentrations of micronutrients in post-bariatric pregnancies compared with non-surgical controls. Specifically, we hypothesized that fatty soluble micronutrients, including vitamin K1, was impaired after bariatric surgery. We also wanted to assess differences in maternal micronutrients concentrations following sleeve gastrectomy versus gastric bariatric surgery.
Materials and methods
Design and study population.
This observational case control study compared micronutritional status in pregnancy after bariatric surgery with non-surgical controls. Study participants were recruited from Akershus university hospital, between October 18th 2018 and December 9th 2022. Pregnant women with previous bariatric surgery were consecutively invited to study participation at referral to the clinic for morbid obesity and the controls were recruited from the routine ultrasound screening in gestational week 17–20. A total of 59 women with a previous bariatric surgery was included in the study and information on surgical procedure was available for 57 women (gastric bypass surgery n = 26 and sleeve gastrectomy n = 31). All women with post-bariatric pregnancies were closely monitored individually by a clinical doctor and a registered clinical dietitian focusing on micronutrient status and gestational weight gain. The controls received standard hospital care and dietary advice with additional advice if the blood samples revealed deficiencies.
A total of 204 women were included in this study with 92% of Caucasian ethnicity ( n = 185). We compared micronutrient status in pregnancy in women with previous bariatric surgery ( n = 59) to controls ( n = 145). Women with known intestinal conditions (i.e. known inflammatory bowel disease, uncontrolled coeliac disease) were not included in the study. The study was approved by the Regional Committee for Medical and Health Research Ethics (reference 25829). All study participants provided written informed consent before study commencement, and the study was performed in accordance with the Declaration of Helsinki [ 34 ].
Definitions
The reference intervals for micronutrients in non-pregnant women and the chosen cut-offs defining micronutrient deficiencies in pregnancy are presented in Table 1 . We defined micronutrient deficiency according to known physiological changes in blood during pregnancy combined with established reference intervals in a non-pregnant population [ 30 , 35 , 36 , 37 ]. Time interval between bariatric surgery and conception was categorized into < 18 and ≥ 18 months.
Data collection
Clinical and laboratory data were retrieved at mean gestational week 20.4 (SD 4.5) (Bariatric surgery 23.9 [6.5] vs. controls 19.0 [2.0] weeks, p < 0.001). Follow-up blood sample was available in a subgroup of 32 women with post-bariatric pregnancies at mean 30.4 (SD 5.6) gestational week. All patients completed a questionnaire on comorbidities, medications and dietary supplements. Additional information including maximum weight, time of bariatric surgery, type of bariatric surgery was retrieved during the first visit.
Blood samples and analysis
The blood samples were obtained by venipuncture and collected in Vacuette® tubes. EDTA tubes were used for analysis of hemoglobin, hemoglobin A1c and thiamine (vitamin B1). Lithium heparin gel tubes were used for analysis of zinc and selenium, and serum gel tubes for the remaining analyses. All the blood samples were non-fasting. After blood collection, all tubes were handled according to established procedures. The standard clinical chemistry parameters were analysed at the laboratory at Akershus University Hospital. Hemoglobin was analysed on Sysmex instruments (Sysmex Corporation, Kobe, Japan) and hemoglobin A1c on Tosoh instruments (Tosoh Corporation, Tokyo, Japan). Magnesium and homocysteine were analysed on Vitros 5.1 FS (Ortho Clinical Diagnostics, Raritan, NJ) until May 2021, thereafter on cobas c503 (Roche Diagnostics, Mannheim, Germany). Folate, cobalamin, ferritin and vitamin D were analysed on cobas e801 (Roche Diagnostics). Zinc and selenium were analysed using inductive coupled plasma – mass spectrometry (ICP-MS) and methylmalonic acid (MMA) with a liquid chromatography – mass spectrometry method (LC-MS/MS). Thiamine, pyridoxal 5-phosphate (vitamin B6), vitamin A and vitamin E were analysed at Oslo University Hospital, Aker and vitamin K1 was analysed at Fürst Medical Laboratorium, Oslo, all with chromatographic methods.
Statistical analysis
We estimated that the prevalence of micronutrient deficiency would be 30% in post-bariatric pregnancies and 5% in controls. To confirm a similar difference with a statistical power of more than 80% and a significance level (α) of 0.05, a total of 200 patients had to be included in the study with a 4:1 ratio of cases vs. controls (40 post-bariatric pregnancies and 160 controls). Proportions are reported as numbers with percent, continuous variables as mean ± standard deviation (SD) as appropriate. Differences between treatment groups were analysed using Pearson’s chi-square test or Fishers exact test for categorical data and Student’s t-test for continuous data. Paired sample t-test was used to assess paired observations of micronutrients in baseline and follow-up blood samples. Skewed distributed data were log-transformed to achieve normal distribution. Correlations between possible confounders and vitamin K1 variables were assessed by Spearman’s correlation (rho). Two-sided P values < 0.05 were considered statistically significant. The Bonferroni Holm correction was applied to mitigate the risk of type 1 statistical error. We used linear regression analyses to explore possible associations between bariatric surgery and vitamin K1 and logistic regression analyses to explore possible associations between bariatric surgery and vitamin K1 deficiency. Possible confounders were identified using a stepwise selection approach in which variables with p-values below 0.10 were included along with clinically significant confounders. Coefficients and odds ratio (OR) from regression analysis are presented with 95% confidence interval (CI). The analyses were performed using IBM SPSS Statistics (version 729.0.0).
We included 204 women in the study (bariatric surgery n = 59 and controls n = 145). Data on the specific type of surgical procedure were available for 57 women who had undergone bariatric surgery prior to conception (gastric bypass surgery n = 26 and sleeve gastrectomy = 31). The women in the surgical group lost on average 39.0 (16.9) kg from the time of surgery to the start of pregnancy and the time interval from bariatric surgery to pregnancy was mean 63.7 (39.2) months. Patients’ characteristics by surgical status are presented in Table 2 .
The women with bariatric surgery had a higher pre-pregnant body mass index (BMI) compared with controls (30.8 [SD 6.0] vs. 25.2 [5.4] kg/m 2 , p < 0.001). There was no difference between groups regarding age (32.1 [5.7] vs. 31.2 [4.2] years, p = 0.215), maternal weight gain (13.3 [9.6] vs. 14.8 [6.5] kg, p = 0.228), HbA1c (30.2 [7.1] vs. 31.1[3.6] mmol/mol, p = 0.234) or development of gestational diabetes (5% vs. 5%, p = 1.000). Fewer women with bariatric surgery had completed higher education and more women with bariatric surgery currently smoked compared with controls (24 [43%] vs. 103 [72%], p < 0.001 and 5 [9%] vs. 0, p = 0.001, respectively. Children of post-bariatric pregnancies had lower gestational age and lower birthweight, however neither reached statistical significance (38.5[3.1] vs. 39.3[2.1] weeks, p = 0.054 and 3363 [624] vs. 3520 [521] g, p = 0.081, respectively).
Dietary supplements and micronutrient status by surgical status are presented in Table 3 . Concentrations of ferritin, magnesium, pyridoxal 5-phosphate, vitamin A, E and K1 and selenium were significantly lower post-bariatric pregnancies compared with controls. Using micronutrients as categorical variables (deficiency yes/no) conferred a higher prevalence of micronutrient deficiencies such as iron, magnesium, pyridoxal 5-phosphate, vitamin K1 and selenium in pregnancies after bariatric surgery compared with controls and a higher prevalence of vitamin K1 deficiency after gastric bariatric surgery vs. sleeve gastrectomy (Fig. 1 ). The distribution of vitamin K1 concentrations in women with post-bariatric pregnancies and controls is presented in Fig. 2 . Paired sample t-test showed increased concentrations vitamin K1 in a subgroup of women with post-bariatric pregnancies (0.29 [0.29] ng/mL to 0.64 [0.92] ng/mL, p = 0.070).
Micronutritional deficiency in pregnancy. A : pregnancy following bariatric surgery vs. non-surgical controls. B : pregnancy after gastric bypass surgery vs. sleeve gastrectomy. * denotes statistically significance after corrections for multiple comparisons
Distribution of vitamin K1 concentrations in women with post-bariatric pregnancies and controls
The women with gastric bariatric surgery underwent surgery at a younger age and with a longer time-interval between surgery and conception compared with the women with sleeve gastrectomy (23.5 vs. 27.5 years, p = 0.002 and 85 [40] vs. 45 [28] months, p = < 0.001, respectively). One woman (4%) after gastric bariatric surgery and five women (16%) after sleeve gastrectomy, p = 0.205 became pregnant < 18 months after surgery. Both surgical groups had lost comparable weight since surgery (gastric bypass surgery 41.4 [17.1] vs. sleeve gastrectomy 37.0 [16.8] kg, p = 0.342) and they had comparable pre-pregnant BMI (gastric bypass surgery 31.9 [5.5] vs. sleeve gastrectomy 29.9 [6.4] kg/m 2 , p = 0.222). The proportion of women with vitamin K1 deficiency was higher after gastric bariatric surgery compared with sleeve gastrectomy (gastric bypass surgery 9 [38%] vs. 1 [3%], p = 0.003 and Fig. 1 ).
Univariate linear regression analysis showed that bariatric surgery was inversely associated with vitamin K1 levels (B -0.33 [95% CI -0.51, -0.15, p < 0.001]. The result remained statistically significant after multivariable adjustments (-0.26 ng/mL [-0.51, -0.04], p = 0.047) (Table 4 A). In addition, compared with sleeve gastrectomy, gastric bariatric surgery was inversely associated with vitamin K1 in univariate linear regression analysis (0.20 [0.019, 0.387], p = 0.031), but not after multivariate adjustment (Table 4 B). Using vitamin K1 as a categorical variable (deficiency yes/no), bariatric surgery was associated with a fivefold increased risk of vitamin K1 deficiency compared with controls and that gastric bariatric surgery was associated with higher adjusted risk of vitamin K1 deficiency compared with sleeve gastrectomy (Table 5 ).
In this study, we compare micronutrient concentrations in post-bariatric pregnancy with matched non-surgical controls. The study shows that the concentrations of vitamin K1, magnesium, and selenium were significantly impaired in post-bariatric pregnancies vs. controls. Moreover, our results show that bariatric surgery was consistently associated with vitamin K1 levels, both as a continuous outcome variable and as a categorical variable (vitamin K1 deficiency) in post-bariatric pregnancy compared with controls. Moreover, the associations might be driven by gastric bariatric surgery rather than sleeve gastrectomy. However, the number of pregnant women with vitamin K1 concentration below the lower reference limit was overall small and the confidence intervals were large. Thus, these results should be interpreted with caution.
Maternal nutrition and micronutrients in pregnancy after bariatric surgery
In pregnancy, there is an increased need for nutrients to support fetal and placental growth and development [ 20 ]. A detailed dietary information was not available in this study and we cannot exclude that the women with bariatric surgery had a different nutritional composition compared with controls. In a subgroup of women with post-bariatric pregnancies, an increment in vitamin K was seen. However, the changes did not reach statistical significance. Follow-up blood samples for the controls were not available. A healthy diet after bariatric surgery may differ from the general population in the composition of lean protein, fruits and vegetables and starchy carbohydrates. Nonetheless, the combination of diet, intestinal modifications and increased metabolism in pregnancy might explain the deficiencies in fatty soluble vitamins seen in this study [ 23 , 24 , 25 , 26 , 27 ]. Improved nutrient intake of mothers was seen after personalized nutritional counseling during post-bariatric pregnancy and might contribute to higher birth weight of offspring [ 15 ]. Given the complexity and heterogeneity of nutritional status in post-bariatric pregnancies, focusing on sub-groups including pre-gestational nutritional deficiencies, and type of surgery performed is of vital importance. A recent consensus report recommended specialized care in pregnancies after bariatric surgery [ 38 ]. There is however a paucity of data to support clinical practice [ 38 , 39 ]. As such, there is an imperative need to identify pregnancy and trimester specific reference intervals and clinical decision limits in order to help clinical advice on dietary supplement.
Lifelong dietary supplement is recommended after bariatric surgery, however adherence to adequate dietary supplements seems to decrease over time [ 26 , 40 , 41 ]. Our study also confers inadequate use of dietary supplements in pregnancy after bariatric surgery with 30–70% of the women not taking recommended post-bariatric surgery dietary supplements (Table 3 ). Thus, a need for increased awareness to ensure adequate microntutrional care before, during and after pregnancy is imperative.
The role of vitamin K1 in pregnancy after bariatric surgery
In line with our results, a systematic review on vitamin K1 concentrations in patients with a history of bariatric surgery reported high risk of vitamin K1 deficiency after bariatric surgery and opted for better monitoring [ 23 ]. Our results also cohere with another study of 49 pregnant women with previous bariatric surgery, showing that vitamin K1 concentrations were lower in women with a history of bariatric surgery compared with 27 controls [ 30 ]. The increased fat storage in pregnancy may lead to less bioavailability for activation of fatty soluble vitamins [ 42 ]. Furthermore, the highly fat-soluble vitamin K1 depend upon conjugated bile salts for adequate absorption. Consequently, reduced stomach acid production, reduced absorption surface and shorter interaction time between conjugated bile salts and vitamin K1 might explain the lower serum concentrations of vitamin K1 after bariatric surgery [ 43 ]. Screening for vitamin K1 deficiency is usually recommended after malabsorptive surgical procedures including biliopancreatic diversion with or without duodenal switch [ 43 ]. However, restrictive procedures may also cause vitamin deficiencies due to digestive symptoms such as vomiting and food intolerance. Interestingly, lower levels of vitamin K1 were found in the first trimester compared to a control group of women without bariatric surgery [ 30 ]. Vomiting and food intolerance may also be the main symptoms of hyperemesis gravidarum, which calls for increased vigilance of vitamin K1 insufficiency in post-bariatric pregnancies in women with symptoms of hyperemesis in pregnancy.
The impact of vitamin K1 deficiency in post-bariatric pregnancies is not clear. Low circulating levels of vitamin K1 might lead to a hypocoaguble state in mother and child [ 30 ]. Some cases of neonatal intracranial bleeding have been reported, possible due to vitamin K1 deficiency [ 44 ]. Another study reported that obesity had stronger impact on hypercoagulability than pregnancy itself [ 45 ]. Nonetheless, insufficient data exist in order to recommend interventions of vitamin K1 deficiency in post-bariatric pregnancy [ 38 ]. While optimal monitoring of vitamin K1 during pregnancy following bariatric surgery remains unclear, a major concern is raised about the consistent finding of vitamin K1 deficiency in post-bariatric pregnancy.
Bariatric surgery before pregnancy: timing and selection of procedure – dose it matter?
Few studies have assessed the impact of different surgical procedures before pregnancy. One study of 119 pregnant women found no effect of maternal weight gain on maternal and perinatal outcome after sleeve gastrectomy [ 46 ]. However, the study did not include pregnancies after gastric bariatric surgery for comparison. Another retrospective observational study showed no differences between gastric bariatric surgery and sleeve gastrectomy regarding re-interventions or obstetric outcomes [ 4 ]. Conflicting evidence exists on the possible adverse effects of sleeve gastrectomy such as dyspepsia and weight regain as compared with gastric bariatric surgery [ 47 , 48 , 49 ]. Our study adds important knowledge about the different surgical procedures, suggesting that gastric bariatric surgery holds greater risk of vitamin K1 deficiency compared with sleeve gastrectomy. The optimal surgical procedure for obesity treatment in women of reproductive age is however not clear and a person-centered approach should be advocated in future guidelines.
The timing of pregnancy after bariatric surgery is moreover under debate. Current recommendations suggest waiting at least 12 months after bariatric surgery before planning a pregnancy [ 12 , 38 , 50 ]. In our study, women with previous sleeve gastrectomy had a shorter time interval between surgery and conception than the women with gastric bariatric surgery. This might reflect that the women who underwent gastric bariatric surgery underwent surgery in an era where gastric bariatric surgery was the most common surgical procedure for weight loss [ 31 ]. Interestingly, after adjustments for the time interval since bariatric surgery, gastric bariatric surgery was not associated with vitamin K1 in the linear regression model (Table 4 B). Thus, as adherence to dietary supplements is reduced with time after bariatric surgery, we cannot rule out that patient’ adherence to dietary supplement might have influenced the differences between surgical procedure seen in the present study [ 26 , 40 , 41 ]. On the other hand, the time interval between sleeve gastrectomy and conception did not impact maternal and neonatal outcomes in a study of 15 women conceived > 18 months after surgery. The authors concluded that pregnancy after sleeve gastrectomy was overall safe and well-tolerated [ 33 ]. Furthermore, a study of 30 women who became pregnant within a mean time of 17 months after gastric bariatric surgery did not appear to confer any serious risks in pregnancy with 90% of the children were born at term with normal birthweight [ 13 ]. In our study, only six patients (11%) became pregnant earlier than 18 months after surgery and the study was not designed to assess pregnancy or birth related complications.
Future implications?
The results of this study underscore the need for increased awareness of nutritional and microntutrional status to ensure adequate obstetric care both before and during post-bariatric pregnancies. Also, this study present important information on adherence to dietary supplement that should be considered in the planning of post-bariatric pregnancies. Moreover, the results of our study rises important questions on the impact of micronutrients deficiencies on future child development.
Strengths and limitations
The strengths of this study include the prospective design with matched controls. Moreover, definitions for the chosen cut-offs for micronutrient deficiency were chosen according to pregnancy specific reference intervals if established. However, we cannot rule out that the concentrations of the micronutrients change in pregnancy. Thus, the validity of the chosen cut-offs for defining micronutrient deficiency should be assessed in future studies. This study was a small single center study and did not have the statistical power to assess pregnancy related or birth related complications. The majority of the women in this study was Caucasian and the results may not be valid in populations of other ethnicities. The observational design does not provide any causality between variables. Also, we cannot rule out if the difference in gestational week for blood sampling or non-fasting blood samples might have influenced the micronutrient analyses. Finally, use of dietary supplements was self-reported and we cannot be sure that all the study participants adhered with the recommendation.
This study shows that concentrations of the micronutrients vitamin K1, magnesium, and selenium were significantly impaired in post-bariatric pregnancies compared with controls. We found a negative association between bariatric surgery and vitamin K1 and a higher risk of vitamin K1 deficiency after gastric bariatric surgery compared with sleeve gastrectomy. Vitamin K1 deficiency in post-bariatric pregnancy have potential risk of hypocoaguble state in mother and child and should be assessed in future studies.
Data availability
The data used in the present study is not open access or publicly available. The datasets are available from the corresponding author on reasonable request.
Abbreviations
Body mass index
Confidence interval
Standard deviation
Obesity in Pregnancy. ACOG Practice Bulletin, Number 230. Obstet Gynecol. 2021;137(6):e128–44.
Article Google Scholar
Marchi J, Berg M, Dencker A, Olander EK, Begley C. Risks associated with obesity in pregnancy, for the mother and baby: a systematic review of reviews. Obes Rev. 2015;16(8):621–38.
Article CAS PubMed Google Scholar
Bou Nemer L, Shi H, Carr BR, Word RA, Bukulmez O. Effect of Body Weight on metabolic hormones and fatty acid metabolism in follicular fluid of women undergoing in Vitro fertilization: a pilot study. Reprod Sci. 2019;26(3):404–11.
Chao GF, Yang J, Peahl AF, Thumma JR, Dimick JB, Arterburn DE, Telem DA. Comparative effectiveness of sleeve gastrectomy vs Roux-en-Y gastric bypass in patients giving birth after bariatric surgery: reinterventions and obstetric outcomes. Surg Endosc. 2022;36(9):6954–68.
Article PubMed Google Scholar
Fisher SA, Stetson BT, Kominiarek MA. Pregnancy after bariatric surgery. JAMA. 2023;329(9):758–9.
Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, Navaneethan SD, Singh RP, Pothier CE, Nissen SE, et al. Bariatric surgery versus Intensive Medical Therapy for diabetes – 5-Year outcomes. N Engl J Med. 2017;376(7):641–51.
Article PubMed PubMed Central Google Scholar
Johansson K, Cnattingius S, Näslund I, Roos N, Trolle Lagerros Y, Granath F, Stephansson O, Neovius M. Outcomes of pregnancy after bariatric surgery. N Engl J Med. 2015;372(9):814–24.
Bloomberg RD, Fleishman A, Nalle JE, Herron DM, Kini S. Nutritional deficiencies following bariatric surgery: what have we learned? Obes Surg. 2005;15(2):145–54.
Yi XY, Li QF, Zhang J, Wang ZH. A meta-analysis of maternal and fetal outcomes of pregnancy after bariatric surgery. Int J Gynaecol Obstet. 2015;130(1):3–9.
Akhter Z, Rankin J, Ceulemans D, Ngongalah L, Ackroyd R, Devlieger R, Vieira R, Heslehurst N. Pregnancy after bariatric surgery and adverse perinatal outcomes: a systematic review and meta-analysis. PLoS Med. 2019;16(8):e1002866.
Stephansson O, Johansson K, Näslund I, Neovius M. Bariatric surgery and Preterm Birth. N Engl J Med. 2016;375(8):805–6.
Roos N, Neovius M, Cnattingius S, Trolle Lagerros Y, Sääf M, Granath F, Stephansson O. Perinatal outcomes after bariatric surgery: nationwide population based matched cohort study. BMJ. 2013;347:f6460.
Chagas C, Saunders C, Pereira S, Silva J, Saboya C, Ramalho A. Perinatal outcomes and the influence of maternal characteristics after Roux-en-Y gastric bypass surgery. J Womens Health (Larchmt). 2017;26(1):71–5.
Hammeken LH, Betsagoo R, Jensen AN, Sørensen AN, Overgaard C. Nutrient deficiency and obstetrical outcomes in pregnant women following Roux-en-Y gastric bypass: a retrospective Danish cohort study with a matched comparison group. Eur J Obstet Gynecol Reprod Biol. 2017;216:56–60.
Araki S, Shani Levi C, Abutbul Vered S, Solt I, Rozen GS. Pregnancy after bariatric surgery: effects of personalized nutrition counseling on pregnancy outcomes. Clin Nutr. 2022;41(2):288–97.
Barker M, Dombrowski SU, Colbourn T, Fall CHD, Kriznik NM, Lawrence WT, Norris SA, Ngaiza G, Patel D, Skordis-Worrall J, et al. Intervention strategies to improve nutrition and health behaviours before conception. Lancet. 2018;391(10132):1853–64.
Jain S, Maheshwari A, Jain SK. Maternal Nutrition and Fetal/Infant development. Clin Perinatol. 2022;49(2):313–30.
Tam E, Keats EC, Rind F, Das JK, Bhutta AZA. Micronutrient Supplementation and Fortification interventions on Health and Development outcomes among children under-five in low- and Middle-Income countries: a systematic review and Meta-analysis. Nutrients 2020, 12(2).
Bailey RL, West KP Jr., Black RE. The epidemiology of global micronutrient deficiencies. Ann Nutr Metab. 2015;66(Suppl 2):22–33.
Gernand AD, Schulze KJ, Stewart CP, West KP Jr., Christian P. Micronutrient deficiencies in pregnancy worldwide: health effects and prevention. Nat Rev Endocrinol. 2016;12(5):274–89.
Article CAS PubMed PubMed Central Google Scholar
Carducci B, Keats EC, Bhutta ZA. Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database Syst Rev. 2021;3(3):Cd000230.
PubMed Google Scholar
Kaska L, Kobiela J, Abacjew-Chmylko A, Chmylko L, Wojanowska-Pindel M, Kobiela P, Walerzak A, Makarewicz W, Proczko-Markuszewska M, Stefaniak T. Nutrition and pregnancy after bariatric surgery. ISRN Obes. 2013;2013:492060.
PubMed PubMed Central Google Scholar
Sherf-Dagan S, Goldenshluger A, Azran C, Sakran N, Sinai T, Ben-Porat T. Vitamin K-what is known regarding bariatric surgery patients: a systematic review. Surg Obes Relat Dis. 2019;15(8):1402–13.
Sherf-Dagan S, Buch A, Ben-Porat T, Sakran N, Sinai T. Vitamin E status among bariatric surgery patients: a systematic review. Surg Obes Relat Dis. 2021;17(4):816–30.
Lewis CA, de Jersey S, Hopkins G, Hickman I, Osland E. Does bariatric surgery cause vitamin A, B1, C or E Deficiency? A systematic review. Obes Surg. 2018;28(11):3640–57.
Krzizek EC, Brix JM, Stöckl A, Parzer V, Ludvik B. Prevalence of Micronutrient Deficiency after bariatric surgery. Obes Facts. 2021;14(2):197–204.
Guelinckx I, Devlieger R, Donceel P, Bel S, Pauwels S, Bogaerts A, Thijs I, Schurmans K, Deschilder P, Vansant G. Lifestyle after bariatric surgery: a multicenter, prospective cohort study in pregnant women. Obes Surg. 2012;22(9):1456–64.
Falcone V, Stopp T, Feichtinger M, Kiss H, Eppel W, Husslein PW, Prager G, Göbl CS. Pregnancy after bariatric surgery: a narrative literature review and discussion of impact on pregnancy management and outcome. BMC Pregnancy Childbirth. 2018;18(1):507.
Devlieger R, Guelinckx I, Jans G, Voets W, Vanholsbeke C, Vansant G. Micronutrient levels and supplement intake in pregnancy after bariatric surgery: a prospective cohort study. PLoS ONE. 2014;9(12):e114192.
Jans G, Guelinckx I, Voets W, Galjaard S, Van Haard PM, Vansant GM, Devlieger R. Vitamin K1 monitoring in pregnancies after bariatric surgery: a prospective cohort study. Surg Obes Relat Dis. 2014;10(5):885–90.
English WJ, DeMaria EJ, Brethauer SA, Mattar SG, Rosenthal RJ, Morton JM. American Society for Metabolic and bariatric surgery estimation of metabolic and bariatric procedures performed in the United States in 2016. Surg Obes Relat Dis. 2018;14(3):259–63.
Parent B, Martopullo I, Weiss NS, Khandelwal S, Fay EE, Rowhani-Rahbar A. Bariatric surgery in women of Childbearing Age, timing between an operation and birth, and Associated Perinatal complications. JAMA Surg. 2017;152(2):128–35.
Sancak S, Çeler Ö, Çırak E, Karip AB, Tumiçin Aydın M, Esen Bulut N, Mahir Fersahoğlu M, Altun H, Memişoğlu K. Timing of Gestation after laparoscopic sleeve gastrectomy (LSG): does it influence obstetrical and neonatal outcomes of pregnancies? Obes Surg. 2019;29(5):1498–505.
Association WM. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2000;284(23):3043–5.
Means RT. Iron Deficiency and Iron Deficiency Anemia: implications and impact in pregnancy, Fetal Development, and early childhood parameters. Nutrients 2020, 12(2).
Berger MM, Shenkin A, Schweinlin A, Amrein K, Augsburger M, Biesalski HK, Bischoff SC, Casaer MP, Gundogan K, Lepp HL, et al. ESPEN micronutrient guideline. Clin Nutr. 2022;41(6):1357–424.
Nasjonal brukerhåndbok i medisinsk biokjemi. Available from https://metodebok.no/index.php?action=book&book=biokjemi . Version 2.4. Last updated 23.06.2022. Accessed 20.06.2023.
Shawe J, Ceulemans D, Akhter Z, Neff K, Hart K, Heslehurst N, Stotl I, Agrawal S, Steegers-Theunissen R, Taheri S, et al. Pregnancy after bariatric surgery: Consensus recommendations for periconception, antenatal and postnatal care. Obes Rev. 2019;20(11):1507–22.
Makrides M, Crosby DD, Bain E, Crowther CA. Magnesium supplementation in pregnancy. Cochrane Database Syst Rev. 2014;2014(4):Cd000937.
Smelt HJM, Pouwels S, Smulders JF, Hazebroek EJ. Patient adherence to multivitamin supplementation after bariatric surgery: a narrative review. J Nutr Sci. 2020;9:e46.
Ben-Porat T, Elazary R, Goldenshluger A, Sherf Dagan S, Mintz Y, Weiss R. Nutritional deficiencies four years after laparoscopic sleeve gastrectomy-are supplements required for a lifetime? Surg Obes Relat Dis. 2017;13(7):1138–44.
Weissgerber TL, Wolfe LA. Physiological adaptation in early human pregnancy: adaptation to balance maternal-fetal demands. Appl Physiol Nutr Metab. 2006;31(1):1–11.
Pournaras DJ, le Roux CW. After bariatric surgery, what vitamins should be measured and what supplements should be given? Clin Endocrinol (Oxf). 2009;71(3):322–5.
Eerdekens A, Debeer A, Van Hoey G, De Borger C, Sachar V, Guelinckx I, Devlieger R, Hanssens M, Vanhole C. Maternal bariatric surgery: adverse outcomes in neonates. Eur J Pediatr. 2010;169(2):191–6.
Sharma S, Uprichard J, Moretti A, Boyce H, Szydlo R, Stocks G. Use of thromboelastography to assess the combined role of pregnancy and obesity on coagulation: a prospective study. Int J Obstet Anesth. 2013;22(2):113–8.
Sancak S, Altun H, Çeler Ö, Çırak E, Er C, Karip AB, Okuroğlu N, Bulut NE, Fersahoğlu MM, Sertbaş Y, et al. Impact of Gestational Weight Gain on maternal and perinatal outcomes after laparoscopic sleeve Gastrectomy. Obes Surg. 2022;32(12):4007–14.
Carabotti M, Silecchia G, Greco F, Leonetti F, Piretta L, Rengo M, Rizzello M, Osborn J, Corazziari E, Severi C. Impact of laparoscopic sleeve gastrectomy on upper gastrointestinal symptoms. Obes Surg. 2013;23(10):1551–7.
Mandeville Y, Van Looveren R, Vancoillie PJ, Verbeke X, Vandendriessche K, Vuylsteke P, Pattyn P, Smet B. Moderating the enthusiasm of Sleeve Gastrectomy: up to 50% of reflux symptoms after ten years in a Consecutive Series of one hundred laparoscopic sleeve gastrectomies. Obes Surg. 2017;27(7):1797–803.
Silveira FC, Poa-Li C, Pergamo M, Gujral A, Kolli S, Fielding GA, Ren-Fielding CJ, Schwack BF. The effect of laparoscopic sleeve gastrectomy on gastroesophageal reflux disease. Obes Surg. 2021;31(3):1139–46.
Ciangura C, Coupaye M, Deruelle P, Gascoin G, Calabrese D, Cosson E, Ducarme G, Gaborit B, Lelièvre B, Mandelbrot L, et al. Clinical practice guidelines for childbearing female candidates for bariatric surgery, pregnancy, and Post-partum Management after bariatric surgery. Obes Surg. 2019;29(11):3722–34.
Download references
Acknowledgements
We acknowledge the work of the staff at the Section for Morbid Obesity at Akershus University Hospital HF for the persistent effort of data collection.
No funding was received for conducting this study.
Author information
Authors and affiliations.
Department of Endocrinology, Akershus University Hospital HF, Lørenskog, Norway
Brit Torunn Bechensteen, Jacob A. Winther & Tone G. Valderhaug
Department of Clinical nutrition, Akershus University Hospital HF, Lørenskog, Norway
Brit Torunn Bechensteen & Heidi Kathrine Ruud
Multidisciplinary Laboratory Medicine and Medical Biochemistry, Akershus University Hospital HF, Lørenskog, Norway
Cindhya Sithiravel & Gunnhild Kravdal
Department of Gynecology, Akershus University Hospital HF, Lørenskog, Norway
Ellen Marie Strøm-Roum
You can also search for this author in PubMed Google Scholar
Contributions
TGV and EMRS designed the study. BTB, EMRS and TGV collected the data for the study. TGV analysed the data. BTB and TGV drafted the manuscript. CS ad GK were responsible for the laboratory analyses. TGV and JAW were responsible for the statistical analyses. All authors contributed to the interpretation of data, reviewed and edited the manuscript and gave their final approval of the final version to be published.
Corresponding author
Correspondence to Tone G. Valderhaug .
Ethics declarations
Ethical approval and consent to participate.
The study was approved by the Regional Committee for Medical and Health Research Ethics (reference 25829). The study was performed in accordance with the Declaration of Helsinki. All study participants provided written informed consent to participate in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Bechensteen, B.T., Sithiravel, C., Strøm-Roum, E.M. et al. Post-bariatric pregnancy is associated with vitamin K1 deficiency, a case control study. BMC Pregnancy Childbirth 24 , 229 (2024). https://doi.org/10.1186/s12884-024-06407-0
Download citation
Received : 19 October 2023
Accepted : 11 March 2024
Published : 02 April 2024
DOI : https://doi.org/10.1186/s12884-024-06407-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Morbid obesity
- Bariatric surgery
- Micronutrients
- Vitamin K1 deficiency
BMC Pregnancy and Childbirth
ISSN: 1471-2393
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Open access
- Published: 09 April 2024
Network analysis of posttraumatic stress and posttraumatic growth symptoms among women in subsequent pregnancies following pregnancy loss
- Qiaoqiao Shen 1 , 2 ,
- Qi Fu 1 &
- Chen Mao 1
BMC Psychiatry volume 24 , Article number: 266 ( 2024 ) Cite this article
Metrics details
Pregnant women who have undergone pregnancy loss often display both posttraumatic stress (PTS) and posttraumatic growth (PTG). However, the precise relationship and structure of symptomatic levels of PTS and PTG have not been well understood. This study aimed to assess the associations between PTS and PTG symptoms in women during subsequent pregnancies following a previous pregnancy loss.
A total of 406 pregnant women with a history of pregnancy loss were included in this study. The Impact of Events Scale-6 (IES-6) and the Posttraumatic Growth Inventory Short Form (PTGI-SF) were used to assess symptoms of PTS and PTG, respectively. The Graphical Gaussian Model was employed to estimate the network model. Central symptoms and bridge symptoms were identified based on “expected influence” and “bridge expected influence” indices, respectively. The stability and accuracy of the network were examined using the case-dropping procedure and nonparametric bootstrapped procedure.
The network analysis identified PTG3 (“Ability to do better things”) as the most central symptom, followed by PTS3 (“Avoidance of thoughts”) and PTG6 (“New path for life”) in the sample. Additionally, PTS3 (“Avoidance of thoughts”) and PTG9 (“Perception of greater personal strength”) were bridge symptoms linking PTS and PTG clusters. The network structure was robust in stability and accuracy tests.
Conclusions
Interventions targeting the central symptoms identified, along with key bridge symptoms, have the potential to alleviate the severity of PTS experienced by women with a history of pregnancy loss and promote their personal growth.
Peer Review reports
Pregnancy loss, also known as the unintentional demise of the fetus before reaching viability or the termination of pregnancy due to medical reasons [ 1 ], is a traumatic event that profoundly impacts a significant number of women worldwide [ 2 ]. For many women, especially those aspiring to conceive, it is likely that they will become pregnant again within 1–2 years following a pregnancy loss [ 3 ]. Throughout subsequent pregnancies, women may grapple with conflicting emotions and psychological phenomena [ 4 , 5 , 6 ]. On one hand, the previous pregnancy loss casts a shadow over them, resurfacing with the new pregnancy [ 4 , 5 ]. They may find themselves revisiting past pregnancy experiences repeatedly, questioning their efforts, and experiencing feelings of guilt or remorse for the lost child [ 4 , 5 ]. On the other hand, through the process of self-adjustment, women may gradually come to accept and confront the reality of pregnancy loss [ 6 ]. The new pregnancy can provide an opportunity for them to regain confidence in fertility and contribute to reshaping a positive mindset and emotional connection [ 7 ].
Previous research has indicated that women who experience pregnancy loss may manifest both posttraumatic stress (PTS) and posttraumatic growth (PTG) symptoms [ 8 , 9 ]. However, there is a lack of quantitative studies focusing on the concurrent presence of PTS and PTG in this specific demographic, particularly during subsequent pregnancies. Research conducted among other populations indicates that the correlation direction between PTS total scores and PTG total scores does not always remain consistent, showing positive correlations, negative correlations, and curvilinear relationships [ 10 ]. In recent times, scholars have proposed that examining the relationship between PTS and PTG solely from a holistic perspective may overlook their interaction at symptom levels [ 11 , 12 ]. As a result, some studies have attempted to utilize network analysis techniques to explore central symptoms and bridge symptoms in PTS and PTG networks, enhancing the understanding of the underlying psychopathological mechanisms associated with PTS and PTG [ 13 , 14 , 15 , 16 , 17 ].
Given the considerable heterogeneity in responses to various types of trauma among different populations [ 18 ], it is challenging to apply existing co-occurrence networks of PTS and PTG to pregnant women who have experienced pregnancy loss. Therefore, this study aimed to utilize network analysis to explore the interaction between PTS and PTG symptoms during early pregnancy in women who have undergone pregnancy loss, with the objective of establishing a theoretical foundation for future interventions by identifying crucial nodes with cascading effects within the network.
Participants
This was a multicenter, cross-sectional study conducted in Guangdong Province, China, from October 8, 2022, to July 20, 2023. Clinical staff recruited participants on-site, and upon obtaining informed consent from eligible individuals, they promptly distributed electronic versions of the survey questionnaire. The inclusion criteria for participants were as follows: (1) aged 18 years or older, (2) in the first trimester of pregnancy, (3) having a single intrauterine pregnancy, (4) having experienced miscarriage, stillbirth, or termination for medical reasons, and (5) expressing willingness to participate in this study. Individuals diagnosed with severe pregnancy complications, a history of psychological disorders, or those currently receiving psychological therapy were excluded. Ultimately, 379 pregnant women from three tertiary hospitals and 94 from a community hospital were invited to participate. A total of 406 individuals successfully completed the survey questionnaire, resulting in an effective response rate of 85.8%.
Measurement
Sociodemographic characteristics.
Participants self-reported their information, providing details on age, educational background, monthly household income, marital status, number of children, current conception method (natural conception or assisted reproductive technology), pregnancy complications, as well as the number and types of prior pregnancy losses.
PTS symptoms
The Impact of Events Scale-6 (IES-6) [ 19 ] was utilized to assess PTS symptoms related to a previous pregnancy loss. The IES-6 comprises three subscales: intrusion, avoidance, and hyperarousal, each consisting of two items. Each item is rated on a scale from 0 (not at all) to 4 (extremely), resulting in a total score ranging from 0 to 24. A total score of 10 or higher indicates the presence of posttraumatic stress disorder (PTSD) [ 19 ]. The IES-6 has been widely used among Chinese populations [ 20 ], and it demonstrated good reliability and validity in this study.
PTG symptoms
The Posttraumatic Growth Inventory Short Form (PTGI-SF) [ 21 ] was utilized to assess the manifestations of personal growth in women who had experienced pregnancy loss. The PTGI-SF comprises five dimensions: interpersonal relationships, personal strength, new possibilities, spiritual change, and appreciation of life, totaling 10 items. Each item is rated on a scale from 0 (no change) to 5 (complete change). The overall score ranges from 0 to 50, with higher scores indicating a greater level of PTG. The PTGI-SF is the most commonly used tool for measuring PTG [ 15 ], and it demonstrated acceptable reliability and validity in this study.
Statistical analysis
All analyses were conducted using R software (Version 4.2.3). Continuous variables were described as mean (standard deviation, SD), and categorical variables were presented as frequencies and percentages.
We computed polychoric correlations between all nodes to examine the edges of the network and estimated the Graphical Gaussian Model (GGM) using the graphical least absolute shrinkage and selection operator (LASSO) in combination with the Extended Bayesian Information Criterion (EBIC) model [ 22 ]. In the network model, each symptom is represented as a “node,” and the association between symptoms is defined as an “edge.” As the association between PTS and PTG symptoms can be either positive or negative, we utilized the expected influence (EI) to quantify the impact of each node in the network. Nodes with high EI values can activate other nodes within the network, making them essential components of their own network [ 11 ]. To identify bridge nodes that connect PTS and PTG, we calculated the bridge expected influence (BEI). A higher positive value of the BEI for a node indicates a greater activation capacity towards nodes in another cluster, whereas a higher negative value signifies a stronger deactivation capacity towards nodes in another cluster [ 11 , 23 ].
Additionally, we conducted verification of network accuracy and stability [ 22 ]. First, centrality stability was evaluated through a case-drop bootstrap procedure, whereby centrality indices were repeatedly computed from subsets of data with an increasing proportion of cases removed. A correlation stability (CS) coefficient value above 0.5 indicates a high level of stability for centrality indices of nodes within the network. Second, edge weight accuracy was estimated with bootstrapped 95% confidence intervals (CIs) by resampling the data 1,000 times. Finally, bootstrapped difference tests (α = 0.05) were conducted to determine if there were significant differences among edge weights and node EIs.
Characteristics of the participants
The study involved participants with an average age of 30.93 years (SD = 4.76). Out of the 406 participants, 296 (72.9%) reported experiencing a single pregnancy loss, while 110 (27.1%) reported having undergone recurrent pregnancy losses (two or more losses). Concerning the current pregnancy, the majority (89.4%) occurred through natural conception, while 43 participants utilized assisted reproductive technology. Further demographic details of the participants can be found in Table 1 . The average score for IES-6 was 6.64 (SD = 3.65), and 14.3% of participants scored above the cutoff point of 10, suggesting the presence of post-traumatic stress disorder (PTSD). The mean score for PTGI-SF was 32.29 (SD = 8.90). The mean and standard deviation for both IES-6 and PTGI-SF items can be found in Supplementary Table S1 .
Network structure
Figure 1 illustrates the network structure of the co-occurrence of PTS and PTG. The network exhibited a high density (0.72) with an average weight of 0.06. The PTS and PTG symptoms formed distinct clusters, with a few modest connections between them. Among the 86 non-zero edges connecting 16 nodes, 58 were positive edges, and 28 were negative edges. The strongest positive edge was found between PTS3 (“Avoidance of thoughts”) and PTS4 (“Avoidance of feelings”) (Edge weight value: 0.56), while the strongest negative edge was observed between PTG9 (“Perception of greater personal strength”) and PTS2 (“Intrusive rumination”) (Edge weight value: -0.25). The correlation matrix for PTS and PTG items is presented in Supplementary Table S2 .
The network structure of PTS and PTG symptoms
Nodes: Blue edges represent positive associations, and red edges indicate negative associations. Thicker edges reflect stronger associations
In terms of EI centrality, the node PTG3 (“Ability to do better things”) had the highest EI value (2.06), followed by PTS3 (“Avoidance of thoughts”) (EI value: 1.17), and PTG6 (“New path for life”) (EI value: 0.92) in the network. These findings align with the results observed in the individual network models for PTS and PTG, as depicted in Supplementary Figure S1 . Regarding BEI centrality, PTS3 (“Avoidance of thoughts”) within the PTS cluster exhibited the highest positive BEI (BEI value: 1.68), while PTG9 (“Perception of greater personal strength”) in the PTG cluster showed the highest negative BEI (BEI value: -2.21). The EI and BEI values for each node are illustrated in Fig. 2 .
Expected influence and bridge expected influence of PTS and PTG symptoms
Network stability and accuracy
Figure 3 shows the results of the case-dropping bootstrap test. In terms of network stability, the CS coefficient of centrality EI was 0.67 (95% CI: 0.59–0.75), suggesting that 67% of the nodes in the sample could be randomly dropped without significantly changing the network structure. The bootstrapped 95% CIs for most edge weights were relatively narrow, indicating an accurate network structure (see Supplementary Figure S2 ). Additionally, nonparametric bootstrapped difference tests revealed significant differences among most edge weights and node EIs (see Supplementary Figure S3 and S4 ).
Stability of expected influence indices using case-dropping bootstrap
To the best of our knowledge, this study represents the first attempt to apply network analysis to examine the co-occurring patterns of PTS and PTG in pregnant women who have experienced pregnancy loss. In the network model, the PTS symptoms and PTG symptoms displayed visually distinct clusters, along with shared positive and negative connections, consistent with findings from previous network analysis studies [ 16 , 17 ].
Our findings revealed the central role of avoidance symptoms within the network, as evidenced by their strong edge weights and high centrality. Consistent with our research, women with a history of traumatic childbirth often employ avoidance as a psychological defense mechanism, attempting to sidestep directly confronting distressing memories and emotions associated with the experience [ 24 ]. Moreover, prior experiences of pregnancy loss may cause women to feel extremely anxious about their current pregnancy or childbirth, and avoiding thoughts related to pregnancy loss could serve as a means of protecting oneself from triggering these anxious emotions and seeking psychological shelter [ 25 ]. Regarding PTG symptoms, the most influential aspect is the transformation of new possibilities, aligning with the majority of findings in current research on PTG network analysis [ 13 , 14 , 26 ]. In studies related to pregnancy loss, participants also reported experiencing personal growth by adapting to their needs, cultivating new interests, and engaging in charitable volunteer work [ 27 ]. In the realm of network theory, alterations in central symptoms can significantly impact other symptoms within the model [ 11 ]. Therefore, guiding pregnant individuals who have experienced pregnancy loss to shift their focus towards new possibilities can assist them in better adapting to their circumstances and achieving personal growth.
The observed bridge symptoms play a crucial role in comprehending the shared psychopathological structure of PTS and PTG [ 23 ]. In this study, for the sample of pregnant women, avoidance of traumatic thoughts related to prior pregnancy loss was found to activate the PTG symptom cluster. Although prior research has predominantly suggested that avoidance coping hinders adaptation to loss and inhibits PTG, some findings have indicated that short-term avoidance does not necessarily imply a negative avoidance strategy but rather signifies a flexible “adaptive avoidance” [ 28 ]. Actively redirecting attention from past painful loss experiences to the current hopeful pregnancy, refraining from dwelling on the past, and adopting a future-oriented coping approach seem to promote positive cognition and psychological transformations in pregnant women [ 6 , 27 ]. Moreover, it is intriguing to note that within the PTG cluster, perceiving greater personal strength can suppress the PTS symptom network, particularly when it exhibits a strong negative correlation with intrusive rumination. Qualitative research has demonstrated that some women, following a pregnancy loss event, discover their resilience and realize that their inner strength aids them in actively countering negative emotions and reducing intrusive thoughts [ 27 ]. Consequently, it is essential to explore methods for enhancing self-efficacy (i.e., confidence in coping with difficulties) in pregnant women with a history of pregnancy loss to alleviate their PTS symptoms.
There are some limitations that should be acknowledged. First, due to the cross-sectional study design, we could not assess the causal relationship and dynamic changes related to the association between PTS symptoms and PTG symptoms. Second, although both the IES-6 and PTGI-SF scales have good psychometric properties in Chinese populations [ 19 , 21 ], the use of simplified scales for measuring PTS and PTG symptoms may limit the comprehensiveness of symptom assessment. Third, our investigation was conducted during the COVID-19 pandemic, a period in which the post-traumatic psychological well-being of pregnant women may be influenced by the stress of contracting the virus. Fourth, this study focused on pregnant women in the early stages of pregnancy, thereby restricting the generalizability of our findings to pregnant women in different phases of gestation. Finally, while the sample size in this study is sufficient for network analysis [ 22 ], it is not adequate to support network comparison tests among different subgroups. Future research should expand the sample size to more comprehensively explore the differences in the co-occurrence networks of PTS and PTG among various samples.
In summary, this study identifies the central symptoms in the co-occurrence network of PTS and PTG as “Avoidance of thoughts,” “Ability to do better things,” and “New path for life.” The bridge nodes connecting PTS and PTG are “Avoidance of thoughts” and “Perception of greater personal strength.” This provides a theoretical foundation for targeted interventions aimed at alleviating the severity of PTS among pregnant women with a history of pregnancy loss in the future and promoting their personal growth.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- Posttraumatic stress
- Posttraumatic growth
Impact of Events Scale-6
Posttraumatic Growth Inventory-Short Form
Robinson GE. Pregnancy loss[J]. Best Pract Res Clin Obstet Gynaecol. 2014;28(1):169–78. https://doi.org/10.1016/j.bpobgyn.2013.08.012 .
Article PubMed Google Scholar
Herbert D, Young K, Pietrusinska M, et al. The mental health impact of perinatal loss: a systematic review and meta-analysis[J]. J Affect Disord. 2022;297:118–29. https://doi.org/10.1016/j.jad.2021.10.026 .
Murphy M, Savage E, O’Donoghue K, et al. Trying to conceive: an interpretive phenomenological analysis of couples’ experiences of pregnancy after stillbirth[J]. Women Birth. 2021;34(5):e475–81. https://doi.org/10.1016/j.wombi.2020.10.016 .
McCarthy FP, Moss-Morris R, Khashan AS, et al. Previous pregnancy loss has an adverse impact on distress and behaviour in subsequent pregnancy[J]. BJOG. 2015;122(13):1757–64. https://doi.org/10.1111/1471-0528.13233 .
Article CAS PubMed Google Scholar
Campbell-Jackson L, Bezance J, Horsch A. A renewed sense of purpose: mothers’ and fathers’ experience of having a child following a recent stillbirth[J]. BMC Pregnancy Childbirth. 2014;14:423. https://doi.org/10.1186/s12884-014-0423-x .
Article PubMed PubMed Central Google Scholar
de Andrade AW, DeMontigny F, Zeghiche S, et al. Experience of hope: an exploratory research with bereaved mothers following perinatal death[J]. Women Birth. 2021;34(4):e426–34. https://doi.org/10.1016/j.wombi.2020.08.011 .
Article Google Scholar
Dyer E, Bell R, Graham R, et al. Pregnancy decisions after fetal or perinatal death: systematic review of qualitative research[J]. BMJ Open. 2019;9(12):e29930. https://doi.org/10.1136/bmjopen-2019-029930 .
Farren J, Jalmbrant M, Falconieri N, et al. Posttraumatic stress, anxiety and depression following miscarriage and ectopic pregnancy: a multicenter, prospective, cohort study[J]. Am J Obstet Gynecol. 2020;222(4):361–7. https://doi.org/10.1016/j.ajog.2019.10.102 .
Freedle A, Kashubeck-West S. Core belief challenge, rumination, and posttraumatic growth in women following pregnancy loss[J]. Psychol Trauma. 2021;13(2):157–64. https://doi.org/10.1037/tra0000952 .
Shakespeare-Finch J, Lurie-Beck J. A meta-analytic clarification of the relationship between posttraumatic growth and symptoms of posttraumatic distress disorder[J]. J Anxiety Disord. 2014;28(2):223–9. https://doi.org/10.1016/j.janxdis.2013.10.005 .
McNally RJ. Network Analysis of psychopathology: controversies and Challenges[J]. Annu Rev Clin Psychol. 2021;17:31–53. https://doi.org/10.1146/annurev-clinpsy-081219-092850 .
Birkeland MS, Greene T, Spiller TR. The network approach to posttraumatic stress disorder: a systematic review[J]. Eur J Psychotraumatol. 2020;11(1):1700614. https://doi.org/10.1080/20008198.2019.1700614 .
Peters J, Bellet BW, Jones PJ, et al. Posttraumatic stress or posttraumatic growth? Using network analysis to explore the relationships between coping styles and trauma outcomes[J]. J Anxiety Disord. 2021;78:102359. https://doi.org/10.1016/j.janxdis.2021.102359 .
Ganai UJ, Sachdev S, Bhat NA, et al. Associations between posttraumatic stress symptoms and posttraumatic growth elements: a network analysis[J]. Psychol Trauma. 2022. https://doi.org/10.1037/tra0001411 .
Ma Z, Zhu Y, Tao Y, et al. Using network analysis to explore the key bridge symptoms between posttraumatic stress symptoms and posttraumatic growth among survivors 10 years after the Wenchuan earthquake in China[J]. J Psychiatr Res. 2022;150:173–9. https://doi.org/10.1016/j.jpsychires.2022.03.011 .
Kangaslampi S, Peltonen K, Hall J. Posttraumatic growth and posttraumatic stress - a network analysis among Syrian and Iraqi refugees[J]. Eur J Psychotraumatol. 2022;13(2):2117902. https://doi.org/10.1080/20008066.2022.2117902 .
Yuan G, Park CL, Birkeland SR, et al. A Network analysis of the associations between Posttraumatic stress symptoms and posttraumatic growth among disaster-exposed Chinese young Adults[J]. J Trauma Stress. 2021;34(4):786–98. https://doi.org/10.1002/jts.22673 .
Isvoranu AM, Epskamp S, Cheung MW. Network models of posttraumatic stress disorder: a meta-analysis[J]. J Abnorm Psychol. 2021;130(8):841–61. https://doi.org/10.1037/abn0000704 .
Hosey MM, Leoutsakos JS, Li X, et al. Screening for posttraumatic stress disorder in ARDS survivors: validation of the impact of event Scale-6 (IES-6)[J]. Crit Care. 2019;23(1):276. https://doi.org/10.1186/s13054-019-2553-z .
Si MY, Su XY, Jiang Y, et al. Psychological impact of COVID-19 on medical care workers in China[J]. Infect Dis Poverty. 2020;9(1):113. https://doi.org/10.1186/s40249-020-00724-0 .
Cann A, Calhoun LG, Tedeschi RG, et al. A short form of the posttraumatic growth Inventory[J]. Anxiety Stress Coping. 2010;23(2):127–37. https://doi.org/10.1080/10615800903094273 .
Epskamp S, Borsboom D, Fried EI. Estimating psychological networks and their accuracy: a tutorial paper[J]. Behav Res Methods. 2018;50(1):195–212. https://doi.org/10.3758/s13428-017-0862-1 .
Jones PJ, Ma R, McNally RJ. Bridge centrality: A Network Approach to understanding Comorbidity[J]. Multivar Behav Res. 2021;56(2):353–67. https://doi.org/10.1080/00273171.2019.1614898 .
Shorey S, Wong P. Traumatic childbirth experiences of New Parents: a Meta-Synthesis[J]. Trauma Violence Abuse. 2022;23(3):748–63. https://doi.org/10.1177/1524838020977161 .
Donegan G, Noonan M, Bradshaw C. Parents experiences of pregnancy following perinatal loss: an integrative review[J]. Midwifery. 2023;121:103673. https://doi.org/10.1016/j.midw.2023.103673 .
Graziano RC, Brown WJ, Strasshofer DR, et al. Posttraumatic stress symptoms, posttraumatic growth, and personality factors: a network analysis[J]. J Affect Disord. 2023;338:207–19. https://doi.org/10.1016/j.jad.2023.06.011 .
Alvarez-Calle M, Chaves C. Posttraumatic growth after perinatal loss: a systematic review[J]. Midwifery. 2023;121:103651. https://doi.org/10.1016/j.midw.2023.103651 .
Zhou LH, Hong JF, Qin RM, et al. Post-traumatic growth and its influencing factors among Chinese women diagnosed with gynecological cancer: a cross-sectional study[J]. Eur J Oncol Nurs. 2021;51:101903. https://doi.org/10.1016/j.ejon.2021.101903 .
Download references
Acknowledgements
The authors are grateful to all participants and medical staff involved in this study.
There was no funding for this study.
Author information
Authors and affiliations.
Department of Epidemiology, School of Public Health, Southern Medical University, 510515, Guangzhou, Guangdong, China
Qiaoqiao Shen, Qi Fu & Chen Mao
School of Nursing, Southern Medical University, Guangzhou, Guangdong, China
Qiaoqiao Shen
You can also search for this author in PubMed Google Scholar
Contributions
Q-QS and Q-F contributed to questionnaire design and data collection. Q-QS conducted the statistical analysis and drafted the manuscript. C-M reviewed the statistical results and revised the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Correspondence to Chen Mao .
Ethics declarations
Ethics approval and consent to participate.
The study received ethical approval from the Southern Medical University Ethics Committee (No. 2022-43). Participants were informed about the study’s purpose and provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Shen, Q., Fu, Q. & Mao, C. Network analysis of posttraumatic stress and posttraumatic growth symptoms among women in subsequent pregnancies following pregnancy loss. BMC Psychiatry 24 , 266 (2024). https://doi.org/10.1186/s12888-024-05702-6
Download citation
Received : 28 August 2023
Accepted : 21 March 2024
Published : 09 April 2024
DOI : https://doi.org/10.1186/s12888-024-05702-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Pregnancy loss
- Pregnant woman
- Network analysis
BMC Psychiatry
ISSN: 1471-244X
- Submission enquiries: [email protected]
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Open Respir Arch
- v.3(4); Oct-Dec 2021
- PMC10369517
Pregnancy and Pulmonary Arterial Hypertension: A Case Report
Embarazo e hipertensión arterial pulmonar: presentación de un caso.
a Pulmonology Department, Centro Hospitalar Universitário Lisboa Norte, Lisboa, Portugal
Ana Mineiro
Luísa pinto.
b Department of Obstetrics, Gynecology and Reproductive Medicine, Centro Hospitalar Universitário Lisboa Norte, Lisboa, Portugal
Filipa Lança
c Anesthesiology Department, Centro Hospitalar Universitário Lisboa Norte, Lisboa, Portugal
Rui Plácido
d Cardiology Department, Centro Hospitalar Universitário Lisboa Norte, Lisboa, Portugal
Nuno Lousada
Dear Editor:
Physiological changes during pregnancy and peri-partum period can lead to hemodynamic stress with right ventricular (RV) failure, bleeding and thrombotic complications. Although maternal survival has improved with advances in PAH therapy and multidisciplinary approach, pregnancy is still not recommended. 1 , 2 , 3 , 4
The authors present a case of successfully managed PAH pregnancy in a 28-year-old woman from Guinea Bissau, gravida 1 para 0 . In 2014, she was admitted for acute pulmonary thromboembolism. Investigation established the diagnosis of Systemic Lupus Erythematosus (SLE)/Sjögren Syndrome and Antiphospholipid Syndrome (APS). After pulmonary emboli resolved, Right Heart Catheterization (RHC) with mean Pulmonary Artery Pressure (mPAP) 66 mmHg, Pulmonary Vascular Resistance (PVR) 25 Wu and Cardiac Index (CI) 2.2 L/min/m 2 diagnosed her as PAH Group 1.4.1. Sildenafil 25 mg tid, prednisolone 7.5 mg id, hydroxycloroquine 250 id, azathioprine 100 mg id and warfarin were initiated.
In 2017, she was referred to our Pulmonary Hypertension Reference Center. She was in functional class II and in the six-minute walk test (6MWT) walked 240 m with severe dessaturation (minimum SpO 2 59%). Further evaluation was negative. The RHC showed mPAP = 42 mmHg, Right Atrial Pressure (RAP) = 2 mmHg, SvO 2 = 68%, PVR = 7.7 Wu, CI = 2.5 L/min/m 2 . Ambrisentan 10 mg id and diuretics were added, with improvement.
Pregnancy diagnosis was made at 6 weeks gestation and she refused medical interruption. Close monitoring at the PH clinic and a team of rheumatologists, obstetricians, immuno-hemotherapists and anaesthesiologists worked together to provide the optimal care.
Ambrisentan and warfarin were withheld; sildenafil and furosemide were maintained. Anticoagulation with enoxaparin 60 mg bid and folic acid supplementation were started.
At 16 weeks gestation she was admitted to the Intensive Care Unit (ICU) to initiate intravenous epoprostenol with invasive monitoring. Dosage up-titration was done during 10 days to reach 12.5 ng/kg/min. She remained clinically stable in functional class I. Fetal wellbeing was documented twice during in-hospital epoprostenol titration.
Maternal echocardiography was performed at 25 weeks gestation with no signs of RV failure.
A scheduled hospitalization for surveillance at 35 weeks and cesarean delivery at 36 weeks was decided.
At 36 weeks gestation spontaneous rupture of membranes occurred. An urgent C-section was performed under general anesthesia because of lack of security time interval for neuroaxis block since the last enoxaparin administration. No major bleeding or other complications were registered. A female newborn baby was delivered, 2380 g and Apgar score 9/10/10.
After C-section she was transferred to ICU where she remained stable on epoprostenol 12.5 ng/kg/min, sildenafil 25 mg 8/8 h, furosemide, enoxaparin and imunosuppressors; 24 h after delivery she was transferred to Intermediate Care. To allow the possibility of breastfeeding, maternal milk was collected. Hospital discharge was on the 4 th postoperative day. Follow-up was maintained weekly at the PH clinic.
She remained in functional class I. There was no dilation of the right chambers or signs of RV failure on echocardiogram performed three months after delivery. At that point, breastfeeding was stopped, macitentan reinitiated at the dose of 10 mg id and RHC was performed: PmAP = 41 mmHg, RAP = 8 mmHg, PVR = 5.19 Wu, CI = 2.64 L/min/m 2 . The cardiopulmonary exercise test showed peak VO 2 15.7 mL/kg/min (35–65% predicted) and VE/VCO 2 36–44.9 grades. Considering the low risk prognosis, epoprostenol was tapered at the rate of 1 ng/kg/min per week.
She is currently on sildenafil and ambrisentan, in functional class I, RHC mPAP = 32 mmHg, RAP = 2 mmHg, PVR = 5.27 Wu, CI = 2.64L/min/m 2 : REVEAL score = 1. Her daughter is healthy and growing normally.
To supply the growing demands of fetus and mother there is an increase in blood volume, left ventricular mass and CO during pregnancy. 2 Systemic vascular resistance and blood pressure decrease due to the increasing levels of estrogen and progesterone. 1 Red cell mass also increases but only about 25%, which may lead to physiological anemia. 2 Changes in coagulation lead to hypercoagulability and a higher risk of thromboembolic events. 5 , 6 These changes affect the RV increasing O 2 consumption and might result in RV failure PAH patients. 2
The peri-partum period is the most difficult to manage, because during labor there is volume overload with 500 mL of blood diverted to maternal circulation in each contraction. 6 , 7 On the other hand, during delivery there is significant blood loss contributing to hypotension. 7 This duality of hypotension and volume overload occurring acutely in a patient with PAH might induce RV failure.
In our patient, we also had to consider the risks of SLE per se with a higher risk of fetal loss, pre-term birth, intra-uterine growth restriction and neonatal lupus syndrome. Concerning the mother there is a risk of disease flare and pre-eclampsia. 8
A systematic review comparing overall maternal mortality in patients with PAH in the period of 1997–2007 with previously published data showed a reduction from 38% to 25%, still a very high mortality rate. 4 , 9 There are no guidelines regarding the management of pregnant women with PAH and published data refers mostly to case series. Close follow up by a multidisciplinary team including clinical and echocardiographic monitoring were put in place, according to literature recommendations. 7
Despite good control with sildenafil and ambrisentan before pregnancy, our patient presented worrisome risk factors, such as high mPAP and PVR and primiparity. 4 , 10 Considering that ambrisentan had to be interrupted due to potential teratogenic effect, epoprostenol was started after the first trimester, as in other successful cases. 11 , 12
Timing and mode of delivery is another matter of debate in PAH. Some authors recommend delivery around 34 weeks gestation because towards the term of pregnancy the physiological changes reach a peak with increased difficulty of cardiovascular system to cope. Delivery between 34 and 37 weeks gestation can be accomplished in stable patients with mild PAH without deterioration during pregnancy. 13 , 14
Recents series4 report preference for C-section over vaginal delivery as the latter is associated with increase in CO of 34% at full cervical dilation, increase in venous return during prolonged labor and deleterious hemodynamic effects associated with pushing which may induce acidosis, hypercapnia or hypoxia. 1 , 13 , 15
As in the first month after delivery is when maternal mortality is higher, 5 , 9 , 15 weekly follow-up visits and specific therapy with epoprostenol and sildenafil were maintained until reevaluation.
Management of pregnancy in PAH patients is a challenge due to the risks for the mother and fetus. The recommendation is against it and no guidelines exist in how to manage those who refuse therapeutic abortion. The strategy in our case was to guarantee PAH control with the introduction of epoprostenol and ensure the support of a well-articulated multidisciplinary team.
With the improvement of PAH therapy and more patients achieving low risk prognosis, more women will probably want to assume the risk of childbearing.

IMAGES
VIDEO
COMMENTS
Description of Case. A 21-year-old pregnant woman, gravida 2 para 1, presented with hypertension and proteinuria at 20 weeks of gestation. She had a history of pre-eclampsia in her first pregnancy one year ago. During that pregnancy, at 39 weeks of gestation, she developed high blood pressure, proteinuria, and deranged liver function.
Dr. Andrea L. Utz (Endocrinology): A 35-year-old pregnant woman (gravida 2, para 1) was admitted to this hospital at 19 weeks and 6 days of gestation because of the recent onset of hypertension ...
The prevalence of hypertension in reproductive-aged women is estimated to be 7.7%. 1 Hypertensive disorders of pregnancy, an umbrella term that includes preexisting and gestational hypertension, preeclampsia, and eclampsia, complicate up to 10% of pregnancies and represent a significant cause of maternal and perinatal morbidity and mortality. 2 ...
First, more aggressive treatment of hypertension in pregnancy prevents the development of severe hypertension, as demonstrated by both a systematic review of randomized trials 152 and CHIPS (Control of Hypertension in Pregnancy Study), in which the average BP achieved by tight control was 133/85 mm Hg. 14 Although comparison of less tight ...
A qualitative study of pregnant Moroccan women in the Netherlands or Morocco found that knowledge of symptoms related to hypertensive disorders of pregnancy was limited or absent ... Perceptions of pregnant women with chronic hypertension. ... or ensure that the BP is under control in the case of an unexpected pregnancy. Similarly, for those ...
The prevalence of pregnancy induced hypertension among women attending delivery service in this study was 33(7.9%). This might increase the morbidity and mortality of the mother and the fetus. If appropriate preventive measures are not taken for the risk of pregnant women, in long term, it might be the first cause of maternal mortality.
Hypotension in pregnant women: a population-based case-control study of pregnancy complications and birth outcomes. Hypertens Res. 2011; 34:55-61. doi: 10.1038/hr.2010.172 Crossref Medline Google Scholar; 43. Li HT, Xue M, Hellerstein S, Cai Y, Gao Y, Zhang Y, Qiao J, Blustein J, Liu JM.
Dec. 1, 2023. Photo: ThinkStock. In May 2022, the results of a long-running trial that focused on treating high blood pressure in pregnant women appeared in the New England Journal of Medicine. The multi-center Chronic Hypertension and Pregnancy (CHAP) project, which enrolled just over 2,400 women at 70 sites, including the University of ...
Introduction. Hypertension in pregnancy is a major challenge in antenatal practice due to its impact on obstetric and fetal outcomes. Hypertension plays a significant role in up to 15% of complications over the course of pregnancy and the postpartum period.[] Hypertensive disorders of pregnancy encompass preexisting (or chronic) hypertension, gestational hypertension, preeclampsia, and ...
PRediction of short-term Outcomes in preGNant wOmen with Suspected PE Study ... International Society for the Study of Hypertension in Pregnancy ... multicentre case-control study. Lancet. 1995 ...
This is because its occurrence was not well understood in the clinical field. Its common manifestations are hypertension, proteinuria (presence of protein in the urine), and edema. There are 2 main types of pregnancy-induced hypertension namely: pre-eclampsia and eclampsia. TYPES. Pre-eclampsia— this is the non-convulsive form of PIH.
ment on women with PIH. Purpose The aim of this study was to evaluate the effect of an antepartum case management program on stress, anxiety, and pregnancy outcomes in women with PIH. Methods A quasi-experimental research design was employed. A convenience sample of women diagnosed with PIH, including preeclampsia, was recruited from outpatient clinics at a medical center in southern Taiwan ...
Abstract. Background: Pregnancy-induced hypertension (PIH) is a leading cause of maternal and fetal morbidity and mortality. Although case management programs have been proposed to improve ...
However, the International Society for the Study of Hypertension in Pregnancy (ISSHP) introduced a new, ... The signs and symptoms are variable but not specific, as is the case with non-pregnant women. Hypertension is only of one of the most dominant signs. If left undiagnosed, maternal and fetal mortality is around 50%. ...
A CASE STUDY ON LIFE-THREATENING PREGNANCY-INDUCED HYPERTENSION IN PRETERM PREGNANCY AND MANAGEMENT CHALLENGES ... Material and Methods: A prospective randomized study was carried out A total of 100 pregnant women with PIH were enrolled in the study. A pre-tested interview tool was used to collect necessary information such as detailed history ...
Chronic hypertension during pregnancy increases the risk of poor pregnancy and birth outcomes. 3,4 Although consensus exists to use antihypertensive therapy to treat severe hypertension (systolic ≥160, diastolic ≥105-110 mmHg) during pregnancy, the benefits and safety for treating mild chronic hypertension during pregnancy are unclear.
A 33-year-old pregnant woman with ulcerative colitis presented at 10 weeks of gestation with fever, nausea, vomiting, abdominal pain, and headache. On hospital day 3, the systolic blood pressure ...
Risk factors for women to develop hypertension in pregnancy can be divided into obstetric, medicaland social aetiology. In obstetric aetiology, the risk factor can be further divided into maternal and fetal risk factor where: Maternal risk factors are: Nulliparity or primigravida Advanced maternal age or extreme age (<15 or >35year old) Family ...
30 Among women with severe or high risk hypertension (BP>160/110), placental abruption may affect as many as 5-10% of women. 11 One study by Sibai and colleagues looked at the maternal and fetal outcomes of 44 women with severe chronic hypertension in the first trimester of pregnancy and found that while there were no maternal deaths ...
A 45-year-old woman presented with hypertension, fatigue, and episodic confusion. After medications were administered, the blood pressure decreased but fatigue and confusion persisted. Four ...
A qualitative study exploring knowledge and attitudes related to pregnancy and preconception health in women with chronic medical conditions, including chronic hypertension, found that the women had limited knowledge of the specific potential complications of pregnancy . Some women in our study also displayed limited understanding of the ...
We included 204 women in the study (bariatric surgery n = 59 and controls n = 145).Data on the specific type of surgical procedure were available for 57 women who had undergone bariatric surgery prior to conception (gastric bypass surgery n = 26 and sleeve gastrectomy = 31).The women in the surgical group lost on average 39.0 (16.9) kg from the time of surgery to the start of pregnancy and the ...
INTRODUCTION. Pregnancy Induced Hypertension (PIH) is one of the commonest causes of both maternal 1 as well as neonatal mortality 2 and morbidity that develops as a result of pregnancy and generally regresses after delivery, affecting about 5-8 % of pregnant women, moreover 5-22% of pregnancies especially in developing countries. 3. The mortality is closely associated with the severity of ...
Pregnant women who have undergone pregnancy loss often display both posttraumatic stress (PTS) and posttraumatic growth (PTG). However, the precise relationship and structure of symptomatic levels of PTS and PTG have not been well understood. This study aimed to assess the associations between PTS and PTG symptoms in women during subsequent pregnancies following a previous pregnancy loss.
A systematic review comparing overall maternal mortality in patients with PAH in the period of 1997-2007 with previously published data showed a reduction from 38% to 25%, still a very high mortality rate.4, 9 There are no guidelines regarding the management of pregnant women with PAH and published data refers mostly to case series.