CBSE NCERT Solutions
NCERT and CBSE Solutions for free

Class 9 Science Assignments
We have provided below free printable Class 9 Science Assignments for Download in PDF. The Assignments have been designed based on the latest NCERT Book for Class 9 Science . These Assignments for Grade 9 Science cover all important topics which can come in your standard 9 tests and examinations. Free printable Assignments for CBSE Class 9 Science , school and class assignments, and practice test papers have been designed by our highly experienced class 9 faculty. You can free download CBSE NCERT printable Assignments for Science Class 9 with solutions and answers. All Assignments and test sheets have been prepared by expert teachers as per the latest Syllabus in Science Class 9. Students can click on the links below and download all Pdf Assignments for Science class 9 for free. All latest Kendriya Vidyalaya Class 9 Science Assignments with Answers and test papers are given below.
Science Class 9 Assignments Pdf Download
We have provided below the biggest collection of free CBSE NCERT KVS Assignments for Class 9 Science . Students and teachers can download and save all free Science assignments in Pdf for grade 9th. Our expert faculty have covered Class 9 important questions and answers for Science as per the latest syllabus for the current academic year. All test papers and question banks for Class 9 Science and CBSE Assignments for Science Class 9 will be really helpful for standard 9th students to prepare for the class tests and school examinations. Class 9th students can easily free download in Pdf all printable practice worksheets given below.
Topicwise Assignments for Class 9 Science Download in Pdf

Advantages of Class 9 Science Assignments
- As we have the best and largest collection of Science assignments for Grade 9, you will be able to easily get full list of solved important questions which can come in your examinations.
- Students will be able to go through all important and critical topics given in your CBSE Science textbooks for Class 9 .
- All Science assignments for Class 9 have been designed with answers. Students should solve them yourself and then compare with the solutions provided by us.
- Class 9 Students studying in per CBSE, NCERT and KVS schools will be able to free download all Science chapter wise worksheets and assignments for free in Pdf
- Class 9 Science question bank will help to improve subject understanding which will help to get better rank in exams
Frequently Asked Questions by Class 9 Science students
At https://www.cbsencertsolutions.com, we have provided the biggest database of free assignments for Science Class 9 which you can download in Pdf
We provide here Standard 9 Science chapter-wise assignments which can be easily downloaded in Pdf format for free.
You can click on the links above and get assignments for Science in Grade 9, all topic-wise question banks with solutions have been provided here. You can click on the links to download in Pdf.
We have provided here topic-wise Science Grade 9 question banks, revision notes and questions for all difficult topics, and other study material.
We have provided the best collection of question bank and practice tests for Class 9 for all subjects. You can download them all and use them offline without the internet.
Related Posts

Class 9 Physics Assignments

Class 9 Mathematics Graphical Distribution of Data Assignments

Class 9 Social Science Economics Assignments
WorkSheets Buddy
Download Math, Science, English and Many More WorkSheets
CBSE Worksheets for Class 9 Science
CBSE Worksheets for Class 9 Science: One of the best teaching strategies employed in most classrooms today is Worksheets. CBSE Class 9 Science Worksheet for students has been used by teachers & students to develop logical, lingual, analytical, and problem-solving capabilities. So in order to help you with that, we at WorksheetsBuddy have come up with Kendriya Vidyalaya Class 9 Science Worksheets for the students of Class 9. All our CBSE NCERT Class 9 Science practice worksheets are designed for helping students to understand various topics, practice skills and improve their subject knowledge which in turn helps students to improve their academic performance. These chapter wise test papers for Class 9 Science will be useful to test your conceptual understanding.
Board: Central Board of Secondary Education(www.cbse.nic.in) Subject: Class 9 Science Number of Worksheets: 30
CBSE Class 9 Science Worksheets PDF
All the CBSE Worksheets for Class 9 Science provided in this page are provided for free which can be downloaded by students, teachers as well as by parents. We have covered all the Class 9 Science important questions and answers in the worksheets which are included in CBSE NCERT Syllabus. Just click on the following link and download the CBSE Class 9 Science Worksheet. CBSE Worksheets for Class 9 Science can also use like assignments for Class 9 Science students.
- CBSE Worksheets for Class 9 Science All Chapters Assignments
- CBSE Worksheets for Class 9 Science Diversity in Living Organisms Animals Assignment
- CBSE Worksheets for Class 9 Science Diversity in Living Organisms Plants Assignment
- CBSE Worksheets for Class 9 Science Experiments Assignment
- CBSE Worksheets for Class 9 Science Force and Laws of Motion Assignment
- CBSE Worksheets for Class 9 Science Fundamental Unit of Life Assignment
- CBSE Worksheets for Class 9 Science Gravitation Assignment
- CBSE Worksheets for Class 9 Science Improvement in Food Resources Assignment 1
- CBSE Worksheets for Class 9 Science Improvement in Food Resources Assignment 2
- CBSE Worksheets for Class 9 Science Law of Conservation of Mass Assignment
- CBSE Worksheets for Class 9 Science Matter around Us Assignment
- CBSE Worksheets for Class 9 Science Melting and Boiling Point Assignment
- CBSE Worksheets for Class 9 Science Physical and Chemical Changes Assignment
- CBSE Worksheets for Class 9 Science Preparation of Mixture and Compound Assignment
- CBSE Worksheets for Class 9 Science Seperation of Components of Mixture Assignment
- CBSE Worksheets for Class 9 Science Sound Assignment
- CBSE Worksheets for Class 9 Science Structure of Atom Assignment
- CBSE Worksheets for Class 9 Science Tissues Animals Assignment
- CBSE Worksheets for Class 9 Science Tissues Plants Assignment
- CBSE Worksheets for Class 9 Science Why do we fall Ill Assignment
- CBSE Worksheets for Class 9 Science Assignment 1
- CBSE Worksheets for Class 9 Science Assignment 2
- CBSE Worksheets for Class 9 Science Assignment 3
- CBSE Worksheets for Class 9 Science Assignment 4
- CBSE Worksheets for Class 9 Science Assignment 5
- CBSE Worksheets for Class 9 Science Assignment 6
- CBSE Worksheets for Class 9 Science Assignment 7
- CBSE Worksheets for Class 9 Science Assignment 8
- CBSE Worksheets for Class 9 Science Assignment 9
- CBSE Worksheets for Class 9 Science Assignment 10
Advantages of CBSE Class 9 Science Worksheets
- By practising NCERT CBSE Class 9 Science Worksheet , students can improve their problem solving skills.
- Helps to develop the subject knowledge in a simple, fun and interactive way.
- No need for tuition or attend extra classes if students practise on worksheets daily.
- Working on CBSE worksheets are time-saving.
- Helps students to promote hands-on learning.
- One of the helpful resources used in classroom revision.
- CBSE Class 9 Science Workbook Helps to improve subject-knowledge.
- CBSE Class 9 Science Worksheets encourages classroom activities.
Worksheets of CBSE Class 9 Science are devised by experts of WorksheetsBuddy experts who have great experience and expertise in teaching Maths. So practising these worksheets will promote students problem-solving skills and subject knowledge in an interactive method. Students can also download CBSE Class 9 Science Chapter wise question bank pdf and access it anytime, anywhere for free. Browse further to download free CBSE Class 9 Science Worksheets PDF .
Now that you are provided all the necessary information regarding CBSE Class 9 Science Worksheet and we hope this detailed article is helpful. So Students who are preparing for the exams must need to have great solving skills. And in order to have these skills, one must practice enough of Class 9 Science revision worksheets . And more importantly, students should need to follow through the worksheets after completing their syllabus. Working on CBSE Class 9 Science Worksheets will be a great help to secure good marks in the examination. So start working on Class 9 Science Worksheets to secure good score.
CBSE Worksheets For Class 9
Share this:.
- Click to share on Twitter (Opens in new window)
- Click to share on Facebook (Opens in new window)
Leave a Comment Cancel reply
Notify me of follow-up comments by email.
Notify me of new posts by email.
NCERT Books and Solutions for all classes
Assignments Class 9 Science Pdf Download
Students can refer to Assignments for Class 9 Science available for download in Pdf. We have given below links to subject-wise free printable Assignments for Science Class 9 which you can download easily. All assignments have a collection of questions and answers designed for all topics given in your latest NCERT Books for Class 9 Science for the current academic session. All Assignments for Science Grade 9 have been designed by expert faculty members and have been designed based on the type of questions asked in standard 9 class tests and exams. All Free printable Assignments for NCERT CBSE Class 9, practice worksheets, and question banks have been designed to help you understand all concepts properly. Practicing questions given in CBSE NCERT printable assignments for Class 9 with solutions and answers will help you to further improve your understanding. Our faculty have used the latest syllabus for Class 9. You can click on the links below to download all Pdf assignments for class 9 for free. You can get the best collection of Kendriya Vidyalaya Class 9 Science assignments and questions workbooks below.
Class 9 Science Assignments Pdf Download
CBSE NCERT KVS Assignments for Science Class 9 have been provided below covering all chapters given in your CBSE NCERT books. We have provided below a good collection of assignments in Pdf for Science standard 9th covering Class 9 questions and answers for Science. These practice test papers and workbooks with question banks for Class 9 Science Pdf Download and free CBSE Assignments for Class 9 are really beneficial for you and will support in preparing for class tests and exams. Standard 9th students can download in Pdf by clicking on the links below.
Subjectwise Assignments for Class 9 Science

Benefits of Solving Class 9 Science Assignments
- The best collection of Grade 9 assignments for Science have been provided below which will help you in getting better marks in class tests and exams.
- The solved question for Class 9 Science will help you to gain more confidence to attempt all types of problems in exams
- Latest NCERT Books for Class 9 Science have been referred to for designing these assignments
- We have provided step by step solutions for all questions in the Class 9 assignments so that you can understand the solutions in detail.
- We have provided single click download links to all chapterwise worksheets and assignments in Pdf.
- Class 9 practice question banks will support to enhance subject knowledge and therefore help to get better marks in exam
FAQs by Science Students in Class 9
At https://www.ncertbooksolutions.com is the best website that has the biggest collection of free printable assignments for Class 9 Science.
We provide here Standard 9 subject-wise assignments which can be easily downloaded in Pdf format for free. Our teachers have provided these Grade 9 Science test sheets for English given in your books.
You can click on the links above and get assignments for Science in Grade 9, all chapters and topic-wise question banks with solutions have been provided here. You can click on the links to download in Pdf.
We have provided here subject-wise Grade 9 Science question banks, revision notes and questions for all difficult topics, and other study material. You can download it all without any charge by clicking on the links provided above.
We have provided the best quality question bank for Class 9 for Science available for Pdf Download. You can download them all and use them offline without the internet.
Related Posts:

Related Posts

Assignments Class 9 Mathematics Herons Formula Pdf Download

Assignments Class 9 Biology Pdf Download

Assignments Class 9 Urdu Pdf Download
Talk to our experts
1800-120-456-456
- NCERT Solutions for Class 9 Science Chapter 1 - Matters In Our Surroundings
- NCERT Solutions

NCERT Solutions for Class 9 Science Chapter 1 - Matter in Our Surroundings
The NCERT Answer for Class 9 Science Chapter 1 was written with the sole purpose of assisting students in obtaining more marks and improving their grades in mind. The answer for class 9 Science chapter 1 addresses students' academic expectations and needs. The themes in Chapter 1 on Matter in Our Surroundings are covered in accordance with the CBSE syllabus . The solution's strategy incorporates a step-by-step method that assists in improved knowledge of the issues.
NCERT Solution of class 9 Science chapter 1 has been created, keeping in mind the primary goal of helping students to secure more marks and improve grades. The academic requirements and needs of students have been addressed in the solution for class 9 Science chapter 1. The topics in chapter 1 related to Matter in Our Surrounding are discussed according to the CBSE syllabus. The approach in the solution involves a step-by-step process which aids in better comprehension of the topics.
CBSE class 9 Science chapter 1 solutions explain all the foundational concepts in detail for students to understand better. The solutions are made available to everybody over the Vedantu app. These free PDFs can be downloaded easily from Vedantu’s official portal.
You can also download NCERT Solutions for Class 9 Maths and NCERT Solution for Class 9 Science to help you to revise complete syllabus and score more marks in your examinations.

Access NCERT solutions for Class 9 Science Chapter – 1 Matter in our Surroundings
Intext exercise -1.
1. Which of the following are the matter?
Chair, air, love, smell, hate, almonds, thought, cold, cold drink, smell of perfume.
Ans: Matter is anything that occupies space and has some mass. There are three states of matter called Solid, Liquid and Gas. On the basis of these three states, we can define that which of this is a matter:
Chair and almond are said to be in a solid state of matter as these have fixed shape., cold drink is in liquid state as it has the tendency to flow., air and the smell of perfume have gaseous particles which are free to move so this will also be considered as a gaseous state of matter..
While Love, hate, cold, smell and thought are not having any mass or neither do they occupy space these are just emotions or sensations felt by human beings so they are not considered as matter.
2. Give reasons for the following observation:
The smell of hot sizzling food reaches you several meters away, but to get the smell from cold food you have to go close.
Ans: The smell of hot sizzling food prepared by our mom reaches to us in our room from kitchen but if the food gets cold after some time, we did not feel any smell of that food this phenomenon can be defined on the basis of rate of diffusion which gets increases when the temperature get increases as high temperature increases the kinetic energy of food particles to get diffused in air. The temperature of hot food particles is high as compared to old one so its molecules get easily diffused in the air as compared to cold ones.
3: a diver is able to cut through water in a swimming pool. which property of matter does this observation show, ans: this can be explained on the properties of matter to attract the particles towards themselves and this will also decide their shape and rigidity. the force of attraction is highest in case of solid as compared to liquid and gas this defines that particles of solid are tightly bound to each other. while in case of liquid particles they have less forces of attraction which defines that there is space between the particles and due to this reason that we can cut them easily. that is why we can say that due to less forces of attraction between water molecules a diver is able to cut through water in a swimming pool., 4: what are the characteristics of particles of matter, ans: matter is anything that occupies space and has some mass. there are three states of matter called solid, liquid and gas., the main characteristics of matter can be described as follows:, particles of matter have space between them and the order of spacing is highest in gas after that liquid and solid have very less space between their particles., particles of matter are continuously moving in all the three states of matter., particles of matter attract each other with strong forces which help them to bind with each other. in solid particles attraction is very high whereas in liquid it is low and in gases it is quite low. , intext exercise -2.
1: The mass per unit volume of a substance is called density (density = mass/volume). Arrange the following in order of increasing density − air, exhaust from chimney, honey, water, chalk, cotton, and iron.
Ans: Density is depending on mass and volume hence higher the mass higher will be the density and out of these heavier particles have higher mass as compare to lighter one so the order of increasing density of given substances can be written as follows:
Air < Exhaust from chimney < Cotton < Water < Honey < Chalk < Iron.
Tabulate the differences in the characteristics of states of matter.
Matter is anything that occupies space and has some mass. There are three states of matter called Solid, Liquid and Gas.
Comment upon the following: rigidity, compressibility, fluidity, filling a gas container, shape, kinetic energy, and density.
Ans:
Rigidity: it is defined as the tendency of matter to resist a change in shape..
Compressibility: The ability of matter to reduce in volume when any type of external force is applied on it.
Fluidity: Tendency of particles to flow this property can be seen in case of liquid and gases which can also be known as fluids.
Filling a Gas Container: Gases neither have definite shape nor have definite volume. Gases take the shape of that container in which it gets filled. Hence by filling a gas container, it means the attainment of shape of the container by the gas.
Shape: Shape corresponds to fixed volume and boundary. Only solids have a fixed shape.
Kinetic Energy: Particles which produce energy possessed due to its continuous motion.
Density: It is defined as the mass per unit volume.
3: Give Reasons:
A Gas Fills Completely the Vessel in Which it is Kept.
The gas particles have a tendency to move freely in all directions as they have very less force of attraction between their particles. Like water, gas can also take the shape of the container in which it is kept. Therefore, we can say that gas completely fills the vessel in which it is kept.
A Gas Exerts Pressure on the Walls of the Container.
The gas particles move freely due to its lesser forces of attraction between the particles. Therefore, these gaseous particles continuously collide with each other and with the walls of the container with a greater force. Pressure is known as the force produced by the gas particles per unit area. By this we can say that gas exerts pressure on the walls of the container.
A wooden table should be called a solid.
A wooden table is very rigid in nature which means that it has a definite shape and its shape cannot be changed easily and has definite volume too. The shape is fixed due to strong intermolecular forces hence it attains all the properties of solid therefore, it is considered as a solid.
We can easily move our hands in air, but to do the same through a solid block of wood, we need a karate expert.
Air particles have very less forces of attraction between their particles so they have large space in between them. But wood has very little space between the particles due to its high force of attraction therefore, wood is considered to be of rigid nature. Due to this reason, we can easily move our hands in air but the same will not happen through a solid block of wood. For this we need a karate expert.
4: Liquids generally have lower density as compared to solids. But you must have observed that ice floats on water. Find out why.
Ans: as we know that density is defined as the mass per unit volume. this corresponds with the increase of the volume of any substance density will decrease as they are inversely proportional to each other. .
Ice is solid in nature therefore it contains strong intermolecular forces which tightly bound them and they contain lesser volume but on the other hand liquid has tendency to move freely due to weak intermolecular forces and contain large volume.
From this we can say that water has larger volume and lesser density so it has a tendency to float on water.
Intext Exercise -3
1: convert the following temperature to celsius scale:.
Celsius and Kelvin are two main scales to measure the temperature. By subtracting the 273K from the given value we can get the value in degrees Celsius. The formula corresponds to degree Celsius can be shown as:
\[X{}^\circ C=(X-273)\]
\[300K=(300-273){}^\circ C=27{}^\circ C\]
573 K
$573K=(573-273){}^\circ C=300{}^\circ C$
2: What is the physical state of water at:
$\mathbf{\text{25}{{\text{0}}^{\text{o}}}\text{C}}$

Ans: Physical state corresponds to the state of matter whether it exists in solid, liquid or gas.
$\text{25}{{\text{0}}^{\text{o}}}\text{C}$– As we know that water starts boiling at 100°C and above this temperature water exists in gaseous state. So, this defines that water at 250°C exists in a gaseous state.
$\mathbf{{{100}^{o}}C}$
${{100}^{o}}C$ – It is the starting point where water starts boiling so at this temperature water exists in both liquid and gaseous states.
3: For any substance, why does the temperature remain constant during the change of state?
Ans: this can be explained as the whole heat which we are providing to the substance to increase its temperature is used to break the intermolecular forces of attraction between them. this heat will also correspond to the latent heat i.e., the heat which gets absorbed or released during change of state. hence all the energy gets used so temperature remains constant. , 4: suggest a method to liquefy atmospheric gases., ans: atmospheric gases can be defined as the gases present in the atmosphere. it can be liquified i.e., converted into liquid by applying suitable conditions of applying pressure and by reducing their temperature., intext exercise -4, 1: why does a desert cooler cool better on a hot dry day, ans: this can be explained by the process called evaporation, this is the process in which the liquid particles absorb energy from the surroundings and cause cooling. the rate of evaporation generally depends on the amount of water vapour present in the air. if the amount of water vapor present in air is more than the rate of evaporation is more or vice-versa. on a hot dry day, the amount of water vapor present in air is quite low so this will evaporate easily and make its surroundings cooler. thus, from this we can say that a desert cooler cools better on a hot dry day as compared to rainy one., 2: how does water kept in an earthen pot (matka) become cool during summers, ans: an earthen pot or matka is generally made up of sand particles in which many tiny pores exist and this helps the water inside the pot to evaporate and surroundings makes the water cool. this is the reason why people kept the water in an earthen pot during summers., 3: why does our palm feel cold when we put some acetone or petrol or perfume on it, ans: acetone, petrol or perfume are considered as organic compounds which are volatile in nature whereas volatile substances are those which easily get vaporized and go through the process of evaporation. we know that during the process of evaporation particles of these organic liquids absorb energy from the surroundings or the surface of the palm and make the surroundings or surface of the palm somewhat cool. this is the reason why our palm feels cold when we put some acetone, petrol or perfume on it. , 4: why are we able to sip hot tea or milk faster from a saucer than a cup, ans: this can also be explained on the basis of rate of evaporation as we know that evaporation produces a cooling effect and evaporation depends on the surface area, larger the surface area higher the evaporation. as in saucer the area is larger as compared to cup so evaporation will be high in case of greater surface area. thus, we can say that liquid cools faster in a saucer than in a cup and due to this reason, we are able to sip hot tea or milk faster from a saucer than a cup., 5: what type of clothes should we wear in the summer, ans: in summer we usually sweat so we have to wear cotton or light-colored clothes because cotton or light-colored clothes can absorb more sweat from our body and transfers the sweat which is in the form of liquid to the atmosphere and makes the evaporation process faster. evaporation process causes a cooling effect which makes our body cool in cotton clothes as compared to synthetic or woolen ones. , ncert exercise, 2: convert the following temperature to kelvin scale:.
$\mathbf{25{}^\circ C}$
Celsius and Kelvin are two main scales to measure the temperature. By adding the 273K from the given value we can get the value in degrees Celsius. The formula corresponds to degree Celsius can be shown as:
\[0{}^\circ C=273K\]
\[{{27}^{o}}C=(27+273)K=300K\]
$\mathbf{373{}^\circ C}$
${{373}^{o}}C=(373+273)K=646K$
3: Give reason for the following observations.
Naphthalene balls disappear with time without leaving any solid.
This phenomenon can be explained on the basis of sublimation which defines that solid is directly converted into gaseous form without turning it into liquid. naphthalene is one that substances which undergo through the process of sublimation easily at room temperature. that is why we can say that naphthalene balls disappear after some time without leaving any solid., we can get the smell of perfume sitting several meters away., gaseous particles have very less internuclear forces due to which its molecules are very free to move and it possesses high kinetic energy. due to this reason particles of perfumes diffuse into the atmosphere and its molecules will mix in the environment which enables us to smell the perfume from several meters away., 4: arrange the following substances in increasing order of forces of attraction between particles − water, sugar, oxygen., ans: forces of attraction is the attracting power of molecules which keep them together and intermolecular forces are very strong in case of solid as compared to liquid or gas. , here sugar is said to be solid and contains higher forces of attraction., water is liquid comparatively containing lesser forces of attraction but higher as compared to gases., oxygen is a gas which contains very less attraction between forces., thus, the increasing order of forces of attraction between the particles of water, sugar and oxygen is.
Oxygen < Water < Sugar
5: What is the physical state of water at:
$\mathbf{{{25}^{o}}C}$
Ans: Physical state corresponds to the state of matter whether it exists in solid, liquid or gas.
$25{}^\circ C$– As we know that water melts at 0°C and above this temperature water exists in liquid state. So, this defines that water at 25°C exists in a liquid state
$\mathbf{{{0}^{o}}C}$
Ans: $0{}^\circ C$– It is the temperature at which water starts melting i.e., water gets converted into liquid from ice so at this temperature water exists as both solid and liquid state.
Ans: $100{}^\circ C$– It is the starting point where water starts boiling so at this temperature water exists in both liquid and gaseous states.
6: Give two reasons to justify:
water at room temperature is a liquid.
We find water is in liquid state at room temperature this can be justified as follows:
Water does not have any fixed shape; it can take the shape of the container in which it is kept and water has definite volume.
It has a tendency to flow.
Have weak intermolecular forces between their particles.
These all describe the property of liquid so we can say that water is liquid at room temperature.
An Iron Almirah is a Solid at Room Temperature.
Iron almirah kept in our room is said to be solid due to following reasons:
It has a fixed shape and definite volume.
It contains strong intermolecular forces between their particles.
Rigid in nature, difficult to compress.
These all describe the property of solid so we can say that almirah is solid at room temperature.
7: Why is ice at 273 K more effective in cooling than water at the same temperature?
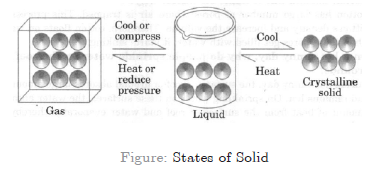
Ans: here condition given that both ice and water are at same temperature i.e. 273 k. but ice at 273 k has less energy as compared to water this can be explained on the basis of latent heat of fusion which is possessed by water as an additional energy but ice does not have such type of energy. therefore, we can say that at 273 k ice is more effective in cooling as compared to water., 8: what produces more severe burns, boiling water or steam, ans: steam and water both are said to be at the same temperature i.e., 373 k. but steam contains more energy as compared to boiling water. this can be explained on the basis of latent heat of fusion which is possessed by water as an additional energy. therefore, steam produces more severe burns than boiling water., 9: name a, b, c, d, e and f in the following diagram showing change in its state..
A is the process of converting solid into liquid is called Melting.
B is the process which converts liquid into gaseous state, this is called vaporisation.
C in which gases get converted into liquid this is called condensation.
D is the process which converts liquid into solid. It is called solidification.
E and F are the processes which convert solid into gas or vice versa is known as sublimation.
You can opt for Chapter 1 - Matter in Our Surroundings NCERT Solutions for Class 9 Science PDF for Upcoming Exams and also You can Find the Solutions of All the Maths Chapters below.
NCERT Solutions for Class 9 Science
Chapter 1 - Matter in Our Surroundings
Chapter 2 - Is Matter Around us Pure
Chapter 3 - Atoms and Molecules
Chapter 4 - Structure of Atom
Chapter 5 - The Fundamental Unit of Life
Chapter 6 - Tissues
Chapter 7 - Diversity in Living Organisms
Chapter 8 - Motion
Chapter 9 - Force and Laws of Motion
Chapter 10 - Gravitation
Chapter 11 - Work and Energy
Chapter 12 - Sound
Chapter 13 - Why do We Fall ill
Chapter 14 - Natural Resources
Chapter 15 - Improvement in Food Resources
Important Concepts Covered in Chapter 1 - Matter in Our Surroundings of Class 9 Science NCERT Solutions
The first chapter delves into the meaning of the matter, its definition, and the various physical states of matter. The matter is defined as everything that takes up space and has mass. For example, water and sugar, sand and sugar, hydrogen and oxygen, and so forth. The matter is made up of minuscule, microscopic particles. Because matter particles have space between them, they are attracted to one another.
Matter in Our Surrounding is the first chapter of Class 9 Science and it covers the following concepts.
Class 9 Science Chapter 1 Matter in Our Surroundings
Even though the topic of Matter in Our Surroundings can be very wide in scope, NCERT solution of Class 9 Science Chapter 1 strictly adheres to the CBSE syllabus. Here are the topics covered –
Matter in Our Surroundings
The introductory chapter elucidates the concept of matter, its definition and the and the physical states in which it exists in nature.
Physical State of Matter
The three physical states of matter are discussed – solid, liquid and gas. The point is elaborated with instances, one of which exemplifies the property of matter when a diver cuts through water.
Characteristics of Particles of Matter
In this chapter, students get to know various characteristics of particles of matter. It discusses that particles of matter – (1) have intermolecular spaces, (2) are in continuous motion and (3) experiences force between one another.
States of Matter
Chapter 4 deals with the features of states of matter – compressibility, rigidity, fluidity, kinetic energy, shape and density.
Can Matter Change its State?
The occurrence of change in state of matter is discussed through the example of ice converting into water and eventually into gaseous form.
Evaporation
The concept of evaporation is exemplified through the question as to why a desert cooler functions better on a hot, dry day as opposed to other weather conditions.
Why is it Beneficial for Students to Study from NCERT solutions class 9 Science Matter in Our Surroundings?
Teachers and mentors have always stressed on the importance of self-study in order for a student to succeed. The material that is referred to in the course of self-study also holds a critical value. Here are some of the benefits of studying NCERT solution of Class 9 Science Chapter 1 that would provide students with that much-needed competitive edge –
The solutions are framed by domain experts possessing much experience. The language of the material has been purposely kept lucid; however, the content remains comprehensive. It enables students to gain in-depth knowledge on the topic
If there is any concern about the relevance and accuracy of the Class 9 Science Ch 1 NCERT solutions, it can be put to rest. The solution strictly adheres to the CBSE curriculum, without any scope for deviation
It is ensured that the basic concepts are suitably explained so that students remain clear on the topic
Ample problem exercises are provided so that students get sufficient practice
The NCERT Solutions for Matter in Our Surroundings (Chapter 8) for Class 9 are given in this article.
Conclusion
This solution contains all of the chapter's questions so that students may prepare for their examinations properly. We have also provided NCERT activities to help pupils grasp the kind or pattern of questions in the CBSE Class 9 test.
While your devotion and constant practice will put you on the right track, NCERT answers will only pave the road. Therefore, get the answer right now and get started on your preparation!

FAQs on NCERT Solutions for Class 9 Science Chapter 1 - Matters In Our Surroundings
1. What are the different characteristics of state of matter explained in NCERT solutions class 9 Science chapter 1?
The different characteristics as elaborated in CBSE Class 9 Science Chapter 1 solutions are based on six parameters - (1) Shape, (2) Volume, (3) Rigidity or Fluidity, (4) Intermolecular force, (5) Intermolecular space, and (6) Compressibility.
A few of the properties listed in Matter in Our Surroundings Class 9 NCERT answers suggest that solids have a set form, volume, and are rigid, whereas liquids have no fixed shape or volume and are less rigid than solids. Gas, on the other hand, has no definite shape or volume and is not hard.
2. Which are the chapters included in Class 9 Science as per the NCERT Science Book?
There are altogether fifteen chapters included in CBSE class 9 Science – (1) Matter in Our Surroundings, (2) Is Matter Around Us Pure, (3) Atoms and Molecules, (4) Structure of Atom, (5) The Fundamental Unit of Life, (6) Tissues, (7) Diversity in Living Organisms, (8) Motion, (9) Force and Laws of Motion, (10) Gravitation, (11) Work and Energy, (12) Sound, (13) Why do We Fall Ill, (14) Natural Resources, (15) Improvement in Food Resources.
3. Can NCERT Solutions be an effective way to prepare Science Class 9 Chapter 1?
Yes, NCERT Solutions will help the studnets to prepare for chapter 1 of Class 9 pretty well. While there can be a multitude of ways to prepare Ch 1 Class 9 Science, it is only when such preparation is done in a systematic manner, can an optimal output be gained. It is advised that instead of starting with a topic in Class 9 Chapter 1 solution of one’s preference, proceed according to the flow of the chapter.
4. How many questions are present in each exercise of NCERT Solutions for Class 9 Science Chapter 1?
There are five exercises in the NCERT Solutions for Class 9 Science Chapter 1. In Exercise 1, there are four questions. In Exercise 2, there are four questions. Exercise 3 also consists of four questions. In Exercise 4, there are four questions. Lastly, the NCERT exercise has nine questions. All of these questions are provided along with their solutions in the NCERT Solutions for Class 9 Science Chapter 1.
5. Are the NCERT Solutions for Class 9 Science Chapter 1 sufficient for the exam preparation?
Yes. To do well in the test, students must study and prepare through self-study in addition to what is given in school. Students must use the best study resources provided on Vedantu for self-study. Students should refer to NCERT Answers for Class 9 Science Chapter 1 and others when studying for the Class 9 Science test. They are developed by qualified teachers and are simple to grasp.
6. What is the matter in our surroundings?
Matter in our surroundings is the first topic covered in Chapter 1 of Class 9 Science. In this chapter, students learn what matter is and which entities can not be considered as matter. The five basic elements that constitute matters in our surroundings are fire, earth, sky, water and air. The building blocks of matter are very small particles that can only be seen under a microscope.
7. Write in short answer format what is matter according to the syllabus of Class 9.
Matter is defined as everything that fills space and has mass. Matter may be perceived by humans through their senses. Water, earth, fire, sky, and air are components that make up matter. It is made up of microscopic particles that can only be seen under a microscope. These are the fundamental building components of matter. Light, heat, electricity, and magnetism are not considered matter since they do not occupy space or have mass.
8. How to download the NCERT Solutions Class 9 Science Chapter 1?
Students can use NCERT Solutions for Class 9 Science Chapter 1 to download the NCERT Solutions Class 9 Science Chapter 1 from Vedantu (vedantu.com). These solutions contain the answers to all the questions from Chapter 1 of the Class 9 Science NCERT textbook in a concise and structured manner. These are available free of cost on Vedantu (vedantu.com). Students can download these using the Vedantu app as well. By going through the solutions, students can learn how to write their answers in the exam. Apart from the solutions, students can also find other study material on Vedantu, including important questions, revision notes and previous year question papers.
NCERT Solutions for Class 9

CBSE Worksheets for Class 9 Science
CBSE Worksheets for Class 9 Science: One of the best teaching strategies employed in most classrooms today is Worksheets. CBSE Class 9 Science Worksheet for students has been used by teachers & students to develop logical, lingual, analytical, and problem-solving capabilities. So in order to help you with that, we at CoolGyan have come up with Kendriya Vidyalaya Class 9 Science Worksheets for the students of Class 9. All our CBSE NCERT Class 9 Science practice worksheets are designed for helping students to understand various topics, practice skills and improve their subject knowledge which in turn helps students to improve their academic performance. These chapter wise test papers for Class 9 Science will be useful to test your conceptual understanding. Board: Central Board of Secondary Education(www.cbse.nic.in) Subject: Class 9 Science Number of Worksheets: 30
CBSE Class 9 Science Worksheets PDF
All the CBSE Worksheets for Class 9 Science provided in this page are provided for free which can be downloaded by students, teachers as well as by parents. We have covered all the Class 9 Science important questions and answers in the worksheets which are included in CBSE NCERT Syllabus. Just click on the following link and download the CBSE Class 9 Science Worksheet. CBSE Worksheets for Class 9 Science can also use like assignments for Class 9 Science students.
- CBSE Worksheets for Class 9 Science All Chapters Assignments
- CBSE Worksheets for Class 9 Science Diversity in Living Organisms Animals Assignment
- CBSE Worksheets for Class 9 Science Diversity in Living Organisms Plants Assignment
- CBSE Worksheets for Class 9 Science Experiments Assignment
- CBSE Worksheets for Class 9 Science Force and Laws of Motion Assignment
- CBSE Worksheets for Class 9 Science Fundamental Unit of Life Assignment
- CBSE Worksheets for Class 9 Science Gravitation Assignment
- CBSE Worksheets for Class 9 Science Improvement in Food Resources Assignment 1
- CBSE Worksheets for Class 9 Science Improvement in Food Resources Assignment 2
- CBSE Worksheets for Class 9 Science Law of Conservation of Mass Assignment
- CBSE Worksheets for Class 9 Science Matter around Us Assignment
- CBSE Worksheets for Class 9 Science Melting and Boiling Point Assignment
- CBSE Worksheets for Class 9 Science Physical and Chemical Changes Assignment
- CBSE Worksheets for Class 9 Science Preparation of Mixture and Compound Assignment
- CBSE Worksheets for Class 9 Science Seperation of Components of Mixture Assignment
- CBSE Worksheets for Class 9 Science Sound Assignment
- CBSE Worksheets for Class 9 Science Structure of Atom Assignment
- CBSE Worksheets for Class 9 Science Tissues Animals Assignment
- CBSE Worksheets for Class 9 Science Tissues Plants Assignment
- CBSE Worksheets for Class 9 Science Why do we fall Ill Assignment
- CBSE Worksheets for Class 9 Science Assignment 1
- CBSE Worksheets for Class 9 Science Assignment 2
- CBSE Worksheets for Class 9 Science Assignment 3
- CBSE Worksheets for Class 9 Science Assignment 4
- CBSE Worksheets for Class 9 Science Assignment 5
- CBSE Worksheets for Class 9 Science Assignment 6
- CBSE Worksheets for Class 9 Science Assignment 7
- CBSE Worksheets for Class 9 Science Assignment 8
- CBSE Worksheets for Class 9 Science Assignment 9
- CBSE Worksheets for Class 9 Science Assignment 10
Advantages of CBSE Class 9 Science Worksheets
- By practising NCERT CBSE Class 9 Science Worksheet , students can improve their problem solving skills.
- Helps to develop the subject knowledge in a simple, fun and interactive way.
- No need for tuition or attend extra classes if students practise on worksheets daily.
- Working on CBSE worksheets are time-saving.
- Helps students to promote hands-on learning.
- One of the helpful resources used in classroom revision.
- CBSE Class 9 Science Workbook Helps to improve subject-knowledge.
- CBSE Class 9 Science Worksheets encourages classroom activities.
Worksheets of CBSE Class 9 Science are devised by experts of CoolGyan experts who have great experience and expertise in teaching Maths. So practising these worksheets will promote students problem-solving skills and subject knowledge in an interactive method. Students can also download CBSE Class 9 Science Chapter wise question bank pdf and access it anytime, anywhere for free. Browse further to download free CBSE Class 9 Science Worksheets PDF . Now that you are provided all the necessary information regarding CBSE Class 9 Science Worksheet and we hope this detailed article is helpful. So Students who are preparing for the exams must need to have great solving skills. And in order to have these skills, one must practice enough of Class 9 Science revision worksheets . And more importantly, students should need to follow through the worksheets after completing their syllabus. Working on CBSE Class 9 Science Worksheets will be a great help to secure good marks in the examination. So start working on Class 9 Science Worksheets to secure good score.
CBSE Worksheets For Class 9
If you're seeing this message, it means we're having trouble loading external resources on our website.
If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
To log in and use all the features of Khan Academy, please enable JavaScript in your browser.
Class 9 Physics (India)
Class 9 chemistry (india), class 9 biology (india).
- NCERT Solutions
- NCERT Class 9
- NCERT 9 Science
- Chapter 1: Matter In Our Surroundings
NCERT Solutions for Class 9 Science Chapter 1 Matter in Our Surroundings
Ncert solutions class 9 science chapter 1 – free pdf download.
NCERT Solutions for Class 9 Science (Chemistry) Chapter 1 Matter in Our Surroundings is an important study material from the standpoint of your CBSE Class 9 Science examination. Detailed NCERT Solutions for Class 9 Chemistry provided here will help you understand the fundamental concepts taught in the chapter.
Download Exclusively Curated Chapter Notes for Class 9 Science Chapter – 1 Matter in Our Surroundings
Download most important questions for class 9 science chapter – 1 matter in our surroundings.
Matter is an important concept in Science, and it forms the basis for topics that are taught in later classes. Learn more by referring to NCERT Solutions for Class 9 Science (Chemistry) Chapter 1 – Matter in Our Surroundings. Solutions are prepared with the help of dedicated teachers with a thorough conceptual understanding and years of experience. The content is well-structured so that it becomes easier for students to learn and understand. Also, content in the NCERT Solutions has been updated as per the latest NCERT syllabus.
We also ensure that the NCERT Solutions provided are designed to meet multiple criteria that an evaluator typically looks for. This ensures that the answers are highly relevant and useful for higher classes.
- Chapter 2 Is Matter Around Us Pure?
- Chapter 3 Atoms and Molecules
- Chapter 4 Structure of the Atom
- Chapter 5 The Fundamental Unit of Life
- Chapter 6 Tissues
- Chapter 7 Diversity in Living Organisms
- Chapter 8 Motion
- Chapter 9 Force and Laws of Motion
- Chapter 10 Gravitation
- Chapter 11 Work and Energy
- Chapter 12 Sound
- Chapter 13 Why Do We Fall Ill?
- Chapter 14 Natural Resources
- Chapter 15 Improvement in Food Resources
NCERT Solutions for Class 9 Science Chapter 1 – Matter in Our Surroundings
carouselExampleControls111

Previous Next
Access Answers to NCERT Class 9 Science (Chemistry) Chapter 1 – Matter in Our Surroundings (All in-text and Exercise Questions solved)
Exercise-1.1-1.2 page: 3.
1. Which of the following are matter?
Chair, air, love, smell, hate, almonds, thought, cold, lemon water, the smell of perfume .
The following substances are matter:
Lemon water
The smell of perfume (Smell is considered as a matter due to the presence of some volatile substances in air that occupy space & have mass.)
2. Give reasons for the following observation:
The smell of hot sizzling food reaches you several meters away, but to get the smell from cold food, you have to go close.
Particles in the air, if fueled with higher temperatures, acquire high kinetic energy, which aids them to move fast over a stretch. Hence, the smell of hot sizzling food reaches a person even at a distance of several meters.
3. A diver is able to cut through water in a swimming pool. Which property of matter does this observation show?
The diver is able to easily cut through the water in the swimming pool because of the weak forces of attraction between water molecules. It is this property of water that attributes to easy diving.
4. What are the characteristics of the particles of matter?
The characteristics of particles of matter are as follows:
(a) Presence of intermolecular spaces between particles
(b) Particles are in constant motion
(c) They attract each other
(d) All matter is composed of very small particles which can exist independently.
Exercise-1.3 Page: 6
1. The mass per unit volume of a substance is called density. (density=mass/volume). Arrange the following in the order of increasing density – air, exhaust from the chimneys, honey, water, chalk, cotton and iron.
The following substances are arranged in increasing density:
Exhaust from chimney
2. Answer the following.
a) Tabulate the differences in the characteristics of matter.
b) Comment upon the following: rigidity, compressibility, fluidity, filling a gas container, shape, kinetic energy and density.
(a) The difference in the characteristics of the three states of matter.
(b) (i) Rigidity: It is the property of matter to continue to remain in its shape when treated
with an external force.
(ii) Compressibility: It is the attribute of the particles to contract their intermolecular space when
exposed to an external force, thereby escalating its density.
(iii) Fluidity: It is the ability of a substance to flow or move about freely.
(iv) Filling the gas container: The particles in a container take their shape as they randomly vibrate in all possible directions.
(v) Shape: It is the definite structure of an object within an external boundary
(vi) Kinetic energy: Motion allows particles to possess energy which is referred to as kinetic energy. The increasing order of kinetic energy possessed by various states of matter are:
Solids < Liquids < Gases
Mathematically, it can be expressed as K.E = 1/2 mv 2 , where ‘m’ is the mass and ‘v’ is the velocity of the particle.
(vii) Density: It is the mass of a unit volume of a substance. It is expressed as:
d = M/V, where ‘d’ is the density, ‘M’ is the mass and ‘V’ is the volume of the substance
3. Give reasons
a) A gas fills completely the vessel in which it is kept.
b) A gas exerts pressure on the walls of the container.
c) A wooden table should be called a solid.
d) We can easily move our hand in the air, but to do the same through a solid block of wood, we need a karate expert.
(a) There is a low force of attraction between gas particles. The particles in the filled vessel are free to move about.
(b) Gaseous particles have the weakest attraction force. They are always moving in a haphazard manner. When a gas particle collides with the container’s walls, it exerts force and, thus pressure on the wall.
(c) There is a distinct contour and volume to the hardwood table. The wood particles are tightly packed. They do not conform to the container’s shape. As a result, the solid features of a hardwood table are satisfied.
(d) The boundaries between air particles are quite loose. They are a long way apart and have a lot of space between them. As a result, we may move our hands freely in the air. The particles in a solid block, on the other hand, are bound together by a strong force of attraction. As a result, there is either some or no space between them. As a result, we will require a karate expert.
4. Liquids generally have a lower density than solids. But you must have observed that ice floats on water. Find out why.
In general, the volume of a liquid is more than the volume of a solid because liquid particles are freer to move, resulting in more volume. Ice, on the other hand, has a maximum density of water at 4 degrees Celsius. Ice is lighter than water and has a lower density. As a result, it floats on water.
Exercise – 1.4 Page: 9
1. Convert the following temperature to Celsius scale:
a. 300K b. 573K
a. 0°C=273K
300K= (300-273)°C = 27°C
b. 573K= (573-273)°C = 300°C
2. What is the physical state of water at:
a. 250 °C b. 100 °C ?
(a) At 250°C – Gaseous state since it is beyond its boiling point.
(b) At 100°C – It is at the transition state as the water is at its boiling point. Hence it would be present in both liquid and gaseous states.
3. For any substance, why does the temperature remain constant during the change of state?
It is due to the latent heat as the heat supplied to increase the temperature of the substance is used up to transform the state of matter of the substance; hence, the temperature stays constant.
4. Suggest a method to liquify atmospheric gases.
It can be achieved by either increasing the pressure or decreasing the temperature, which ultimately leads to the reduction of spaces between molecules.
Exercise – 1.5 Page:10
1. Why d oes a desert cooler cool better on a hot dry day?
It is because the temperature is high and less humid on a hot dry day, enabling better evaporation. High levels of this evaporation provide better cooling effects.
2. How does the water kept in an earthen pot (matka) become cool during summer?
An earthen pot is porous in nature. These tiny pores facilitate the penetration of water and hence their evaporation from the pot surface. The process of evaporation requires energy which is contributed by water in the pot as a result of which water turns cooler.
3.Why does our palm feel cold when we put on some acetone or petrol, or perfume on it?
Acetone, petrol, and perfume are volatile substances that evaporate when they come in contact with air. Evaporation is facilitated as it uses energy from the palm, hence leaving a cooling effect on our palms.
4. Why are we able to sip hot tea or milk faster from a saucer rather than a cup?
A saucer has a larger surface area than a cup, promoting quicker evaporation. Hence, the tea or milk in a saucer cools down faster.
5. What type of clothes should we wear in summer?
In summer, it is preferred to wear light-coloured cotton clothes because light colour reflects heat and cotton materials have pores that absorb sweat, facilitating evaporation, and hence causing a cooling effect on the skin.
Chapter Exercise – Page: 12
1. Convert the following temperature to Celsius scale.
(a) 293K (b) 470K
(a) 293K= (293 – 273)°C = 20°C
(b) 470K= (470 – 273)°C = 197°C
2.Convert the following temperatures to the Kelvin scale.
(a) 25 °C (b) 373 °C
(a) 25°C = (25+273)K = 298K
(b) 373°C = (373+273)K = 646K
3. Give reason for the following observations:
(a) Naphthalene balls disappear with time without leaving any solid.
(b) We can get the smell of perfume while sitting several metres away.
(a) At room temperature, naphthalene balls undergo sublimation wherein they directly get converted from a solid to a gaseous state without having to undergo the intermediate state, i.e., the liquid state.
(b) Molecules of air move at a higher speed and have large intermolecular spaces. Perfumes comprise substances that are volatile, which scatter quickly in air, becoming less concentrated over a distance. Hence, we are able to smell perfume sitting several metres away.
4. Arrange the following in increasing order of forces of attraction between the particles – water, sugar, oxygen.
Oxygen (gas) < water (liquid) < sugar (solid)
5. What is the physical state of water at –
(a) 25 ° C (b) 0 ° C (c) 100 ° C?
(a) At 25°C, the water will be in liquid form (normal room temperature)
(b) At 0°C, the water is at its freezing point, hence both solid and liquid phases are observed.
(c) At 100°C, the water is at its boiling point, hence both liquid and gaseous states of water (water vapour) are observed.
6. Give two reasons to justify –
(a) Water at room temperature is a liquid.
(b) An iron almirah is a solid at room temperature.
(a) Water persists as a liquid at room temperature since its melting point is lower than room temperature and its boiling point (100 o C) is higher.
(i). A fixed volume is occupied by a fixed mass of water.
(ii). At room temperature, water does not have a fixed shape and flows to fit the container’s shape.
As a result, water is a liquid at room temperature.
(b) Because its melting and boiling points are above room temperature, an iron almirah is a solid at room temperature. In the same way,
(i) An iron almirah is rigid and has a predetermined shape.
(ii) Metals have a relatively high density.
As a result, at room temperature, iron almirah is a solid.
7. Why is ice at 273K more effective in cooling than water at the same temperature?
At 273 K, ice will absorb heat energy or latent heat from the medium to overcome fusion and transform into water. As a result, ice has a greater cooling impact than water at the same temperature since water does not absorb the excess heat from the medium.
8. What produces more severe burns, boiling water or steam?
Steam produces severe burns. It is because it is an exothermic reaction that releases a high amount of heat which it had consumed during vaporization.
9. Name A, B, C, D, E and F in the following diagram showing a change in its state.

Interconversion of three states of matter: Using temperature or pressure, any state of matter can be turned into another.
(A) Solid to Liquid → Melting (or) fusion (or) liquefaction
(B) Liquid to Gas → Evaporation (or) vaporization
(C) Gas to liquid → Condensation
(D) Liquid to Solid → Solidification
(E) Solid to Gas → Sublimation
(F) Gas to Solid → solidification
Chapter 1 – Matter in Our Surroundings is a part of Unit 1: Matter – Its Nature and Behavior. According to the past trends and previous years’ question papers, this particular unit carries 23 marks out of 100. Therefore, it is important to ensure that this chapter is studied thoroughly.
The topics and subtopics from NCERT Solutions Class 9 Science Chapter 1 Matter in Our Surroundings are given below:
1. Physical Nature of Matter
- Matter Is Made Up of Particles
- How Small Are These Particles of Matter?
2. Characteristics of Particles of Matter
- Particles of Matter Have Space between Them
- Particles of Matter Are Continuously Moving
- Particles of Matter Attract Each Other
3. States of Matter
- The Solid State
- The Liquid State
- The Gaseous State
4. Can Matter Change Its State?
- Effect of Change of Temperature
- Effect of Change of Pressure
5. Evaporation
- Factors Affecting Evaporation
- How Does Evaporation Cause Cooling?
Students can utilise the NCERT Solutions for Class 9 Science Chemistry Chapter 1 for any quick references to comprehend complex topics.
Matter is one of the fundamental constituents that make up everything in the universe – from minute sand particles on Earth to the enigmatic black holes at the centre of many galaxies. Matter has a role to play in everything we see around us, interacting to form new materials, some familiar and others exotic.
Explore how matter works and discover its molecular components. Also, learn how the term matter was coined and its significance in various fields of science. Find more important NCERT Solutions For Class 9 Science to aid your studies.
Exercises with Question count covered in NCERT Solutions for Class 9 Chapter 1: Matter in Our Surroundings
Exercise 1.1 & 1.2, Page number 3 – Solutions of 4 Questions Exercise 1.3, Page number 6 – Solutions of 4 Questions Exercise 1.4, Page number 9 – Solutions of 4 Questions Exercise 1.5, Page number 10 – Solutions of 5 Questions Chapter Exercise, Page number 12 – Solutions of 9 Questions
Key Features of NCERT Solutions for Class 9 Science Chapter 1 – Matter in Our Surroundings
- Content elaborated in detail, ensuring all concepts are explained
- Solutions have been written in an easy-to-understand language
- Crafted by qualified teachers and industry experts
- Includes questions from the latest prescribed syllabus
- Comprehensive analysis of previous year exam questions
- Explore additional learning tools such as sample papers and previous year question papers
Disclaimer:
Dropped Topics – Box item titled ‘Plasma and Bose–Einstein Condensate’.
Frequently Asked Questions on NCERT Solutions for Class 9 Science Chapter 1
Explain the different characteristics of the state of matter covered in chapter 1 of ncert solutions for class 9 science., how many questions are present in each exercise of ncert solutions for class 9 science chapter 1, is the ncert solutions for class 9 science chapter 1 sufficient for the exam preparation, leave a comment cancel reply.
Your Mobile number and Email id will not be published. Required fields are marked *
Request OTP on Voice Call
Post My Comment
I really like byjus and answer are hundred percent good 👌👌👌👌👌👌 best experience ever
Thanks for this science book chapter 1 qus. And ans. Thank you very much 👏👏👏
My experience with byju’s is 2 good. It’s amazing
It’s very helpful Thnx byjus.
It’s very helpful Thnx byjus.
Thanks byjus for ch -1 questions answers of science
- Share Share
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.

Trial Class
AssignmentsBag.com
Matter in Our Surroundings Chapter 1 Class 9 Science Assignments
Please refer to Matter in Our Surroundings Chapter 1 Class 9 Science Assignments below. We have provided important questions and answers for Matter in Our Surroundings which is an important chapter in Class 9 Science. Students should go through the notes and also learn the solved assignment with exam solved questions provided below. All examination and class tests questions are as per the latest syllabus and books issued by CBSE, NCERT, and KVS. We have also provided Class 9 Science Assignments for all chapters on our website.
Chapter 1 Matter in Our Surroundings Class 9 Science Assignments
Question. Predict the physical state of melting point of a substance is below the room temperature.
Question. We can get the smell of perfume sitting several metres away, why?
This is because perfumes diffuse very fast and can reach to people sitting several metres away.
Question. The boiling point of alcohol is 78°C. What is this temperature on Kelvin scale?
K = °C + 273 = 78 + 273 = 351 K
Question. We can easily move our hand in the air but to do the same through a solid block of wood. We need a karate expert. Why?
In air, the inter-particle attractive forces are negligible and hence, it is easy to separate the particles in air and we can easily move our hand in air. The interparticle forces are very strong in solids. So, it is not easy to separate the particles and it is not easy to move our hand through a solid block of wood.
Question. Give two examples of diffusion.
Milk drops dissolved in water and perfume sprayed in a room.
Question. Express the boiling point of water in Celsius as well as Kelvin scale.
100°C and 373 K.
Question. Name the state of water at 100 degree Celsius, zero degree Celsius and 4 degree Celsius.
The state of water at 100 degree Celsius is gas, at 0 degree Celsius it is solid and at 4 degree Celsius it is liquid.
Question. Name two processes from which it may be concluded that the particles of a gas move continuously.
Compressibility and Brownian movement.
Question. Is it possible to turn a liquid into vapour without heating?
Yes, by the process of evaporation as evaporization of water occur below the boiling point under atmospheric pressure.
Question. What is diffusion?
The intermingling of molecules of one substance with that of the other is called diffusion.
Question. What happens to the rate of diffusion if the temperature is increased?
With increased temperature, the rate of diffusion also increases as the particles gain energy and vibrate more.
Question. What is dry ice?
Solid carbon dioxide obtained by cooling and applying pressure on carbon dioxide gas. It does not melt so it is called dry ice.
Question. What is humidity?
The air holds water vapour, this air with water is called humid air and the amount of water vapour present in the air is called humidity.
Question. What is normal atmospheric pressure?
The atmospheric pressure at sea level is 1 atmosphere and taken as the normal atmospheric pressure.
Question. Write the SI unit of temperature?
Question. Give the temperature at which water exists in two different phases/states.
(i) At 0°C water can be in solid or in liquid state. (ii) At 100°C water can be in liquid or in gaseous state.
Question. What are fluids?
The states of matter that can flow due to less intermolecular force of attraction are liquids and gases and are called fluids.
Question. Define matter.
Anything that occupies space and has mass and is felt by senses is called matter.
Question. Why are light and sound not considered as matter?
Light and sound are not considered as matter because they have no mass and do not occupy space.
Question. What happens if you put copper sulphate crystals in water?
Copper sulphate crystals mixed between the spaces of molecules of water and disappear.
Question. Give state of a matter if this substance has neither a fixed shape nor a fixed volume.
Question. What do you mean by vapour?
A substance that is found in gaseous state only at room temperature is called vapour.
Question. Is it true to say that fluorescent tube contains plasma? Explain Answer. It is right to say that fluorescent tube contains plasma. As fluorescent tube has helium or some other rare gas.The particles of the gas get ionized in the presence of high voltage applied. These charged particles are called plasma which glows.
Question. A karate expert can easily move his hand through a solid block of wood but we cannot. Why? Answer. In a solid block of wood, the inter-particle forces are very strong and hence, it is not easy to separate the particles. Therefore, it is not easy to move our hand through a solid block of wood, only a karate expert can do it as he has expertise in this.
Question. What is latent heat of fusion? Answer. The heat required to change 1 kg of a solid substance into liquid state at the melting point of the substance. For example : Amount of heat required to melt ice at 0°C into water, at 0°C will be known as the latent heat of fusion of ice.
Question. What is compressibility? How it is negligible in solids? Answer. Compressibility is the ability of a substance to be reduced to its volume under pressure. Solids are incompressible as their particles are held together. So, we can tell that compressibility is negligible in solids.
Question. Two cubes of ice are pressed hard between two palms and after releasing the pressure, the cubes join together. Why? Answer. Pressure is directly proportional to temperature when we apply pressure, temperature increases then the ice in contact melts and it turns into water. When pressure is removed, the temperature decreases again and melted ice again freezes. Hence, cubes join together.
Question. What is the reason that “Ice has lower density than water”? Answer. The mass per unit volume of a substance is called density (density = mass/volume). The density of substance decreases as the volume of a substance increases. Space between particles increases when water changes into ice. These spaces are larger as compared to the spaces present between the particles of water. Thus, the volume of ice become greater as compared to the water. Hence, the density of ice become lower than that of water. And, a substance with lower density than water can float on water. Thus, ice floats on water.
Question. Why does the temperature remain constant during the change of state, for any substance? Answer. On increasing the temperature of solids, the kinetic energy of the particles increases which is used up in changing the state as it overcome the forces of attraction between the particles, therefore, the temperature remains constant during the change of state.
Question. Why cannot you smell its perfume at a short distance when incense stick is not lighted? Answer. The particles of the perfume (matter) do not have sufficient energy to drift through the air. Thus, we cannot smell it at a few steps away from incense stick.
Question. How can you show that evaporation causes cooling? Answer. When we put some acetone on our hand, after some time we will feel coolness on our hand because the acetone absorbs kinetic energy from our hand and evaporates and evaporation causes cooling.
Question. What do you mean by latent heat of vaporization? Answer. The latent heat of vaporization of a liquid is the quantity of heat in joules required to convert 1 kilogram of the liquid to vapour or gas at its boiling point, without any change in temperature.
Question. Explain, why solids have fixed shape but liquids and gases do not have fixed shape? Answer. Solids have fixed shape due to strong intermolecular force of attraction between them. The liquids and gases have molecules with less intermolecular force of attraction, and hence they can flow and take shape of the container.
Question. Liquids and gases can be compressed but it is difficult to compress solids. Why? Answer. Liquids and gases have intermolecular space; on applying pressure externally on them the molecules can come closer thereby minimizing the space between them. But in case of solids, there is no intermolecular space to do so.
Question. Is it not proper to regard the gaseous state of ammonia as vapours? Explain. Answer. The gaseous state of a substance can be regarded as vapours only in case it is a liquid at room temperature. Since ammonia is a gas at room temperature, its gaseous state cannot be regarded as vapours. Naphthalene is volatile solid and has a tendency to sublime. So, it changes into vapours completely, thus disappear into the air and no solid is left.
Question. Cotton is solid but it floats on water. Why? Answer. Cotton has large number of pores where air is trapped.This process reduces cotton’s density and increase the volume. Therefore, cotton floats on water. But, when these pores get filled with water, it starts sinking.
Question. Why do we see water droplets on the outer surface of a glass containing ice-cold water? Answer. If we take some ice-cold water in a glass, after some time we will see small droplets of water deposited on the outer walls of the glass. Because water vapour present in air come into the contact of cold wall of glass, lose energy and converted into liquid state which can be seen in the form of small droplets.
Question. Pressure and temperature determine the state of a substance. Explain this in detail. Answer. Any matter, i.e. solid, liquid or gas when experiences an increase in temperature then they change their state. Example :
When we take ice cubes in a beaker or heat them slowly, the temperature increases and ice melts to form liquid. We heat this liquid further it will become steam. On lowering down the temperature of any matter,show change in their state. Example :
We take the steam that is coming out of boiling water and allow it to cool down, it condenses to form water and on further cooling of this water we get ice. On applying pressure and reducing temperature we can liquefy gases or change them into solid. Example : We take carbon dioxide gas, reduce its temperature and apply lot of pressure on it so that it changes into solid carbon dioxide, called dry ice, which is used as refrigerant for cooling. If pressure on it is decreased it directly changes into gas. In LPG cylinders, the petroleum gas is cooled and with lot of pressure changes it into liquid state. While using this LPG, we release the pressure exerted on it and hence, it comes out in the form of gas.
Question. Give difference between Evaporation and Boiling. Answer. Evaporation Boiling 1. It takes place at any It takes place at definite temperature called boiling point of liquid. place. 2. Temperature of liquid Temperature of liquid does not change during boiling. decreases during evaporation. 3. Evaporation is a Boiling is the bulk phenomenon; it takes place in the whole mass of the liquid. surface phenomenon; it takes place only at the surface of the liquid. 4. Evaporation is a slow Boiling is a rapid and violent process. and silent process. Question. Explain the inter-conversion of three states in terms of force of attraction and kinetic energy of the molecules. Answer. The force working between the particles of a matter is called intermolecular force. Intermolecular forces are strong in solids and the particles are close to each other and thus make the substance rigid. In liquids,intermolecular force is less than solids and more than gases. So, they cannot have rigid shape and kinetic energy of the molecules is not enough to hold gas in open container.
Related Posts
Diversity in living organisms chapter 7 class 9 science assignments.

Assignments For Class 9 Mathematics Coordinate Geometry

Assignments For Class 9 Mathematics Graphical Distribution of Data

IMAGES
VIDEO
COMMENTS
All Assignments and test sheets have been prepared by expert teachers as per the latest Syllabus in Science Class 9. Students can click on the links below and download all Pdf Assignments for Science class 9 for free. All latest Kendriya Vidyalaya Class 9 Science Assignments with Answers and test papers are given below.
CBSE Class 9 Science Worksheet for students has been used by teachers & students to develop logical, lingual, analytical, and problem-solving capabilities. So in order to help you with that, we at WorksheetsBuddy have come up with Kendriya Vidyalaya Class 9 Science Worksheets for the students of Class 9. All our CBSE NCERT Class 9 Science ...
NCERT Solutions for Class 9 Science help students to clear any doubts instantly and efficiently. These NCERT Solutions guide students to learn the important concepts which are included in the CBSE Class 9 Science syllabus. Students are required to solve the exercise questions included in the textbook to create a proper understanding of the topics.
All Assignments for Science Grade 9 have been designed by expert faculty members and have been designed based on the type of questions asked in standard 9 class tests and exams. All Free printable Assignments for NCERT CBSE Class 9, practice worksheets, and question banks have been designed to help you understand all concepts properly.
NCERT Solutions For class 9 science: Download class 9 science NCERT Solutions Chapters wise FREE PDF solved by master teachers updated for the year 2024-25. Courses. Courses for Kids. Free study material. ... The solutions will even help students to do their assignments and score well. Given below are all the chapters that students must learn ...
Class 9 Science NCERT Solutions is given here. Students can click on the links of the particular chapter for which they are finding the solutions. Chapter 1 Matter in Our Surroundings. Chapter 2 Is Matter Around Us Pure. Chapter 3 Atoms and Molecules. Chapter 4 Structure of the Atom. Chapter 5 The Fundamental Unit of Life.
The NCERT Answer for Class 9 Science Chapter 1 was written with the sole purpose of assisting students in obtaining more marks and improving their grades in mind. The answer for class 9 Science chapter 1 addresses students' academic expectations and needs. The themes in Chapter 1 on Matter in Our Surroundings are covered in accordance with the ...
CBSE Worksheets for Class 9 Science Assignment 9; CBSE Worksheets for Class 9 Science Assignment 10; Advantages of CBSE Class 9 Science Worksheets. By practising NCERT CBSE Class 9 Science Worksheet, students can improve their problem solving skills. Helps to develop the subject knowledge in a simple, fun and interactive way.
Science. Class 9. Science. Class 9. Course summary; Class 9 Physics (India) ... The fundamental unit of life: Class 9 Biology (India) Tissues: Class 9 Biology (India) Diversity in living organisms: Class 9 Biology (India) Our mission is to provide a free, world-class education to anyone, anywhere.
NCERT Solutions For Class 9 Science Chapter 1 - Extra Questions Solved. I. Multiple Choice Questions in Class 9 Science Chapter 1 Matter in Our Surroundings. Choose the correct option: 1. Evaporation of a liquid occurs at. (a) boiling point. (b) a fixed temperature. (c) temperature lower than boiling point.
Assignments for Class 9 Science have been developed for Standard 9 students based on the latest syllabus and textbooks applicable in CBSE, NCERT and KVS schools. Parents and students can download the full collection of class assignments for class 9 Science from our website as we have provided all topic wise assignments free in PDF format which ...
2. Write the steps you would use for making tea. Use the words solution, solvent, solute, dissolve, soluble, insoluble, filtrate, and residue. Solution: (a) Into a vessel, add a cup of milk, which is the solvent, and supply it with heat. (b) Add tea powder or tea leaves to the boiling milk, which acts as a solute.
So this page contains notes of most of the class 9 chapters and we also have assignments of most of the chapters that you can practice. We also have a free class 9 E-book or pdf download page where you can download lots of files. So, to learn any chapter you have to follow these steps. First read and understand the notes.
NCERT Solutions Class 9 Science Chapter 1 - Free PDF Download. NCERT Solutions for Class 9 Science (Chemistry) Chapter 1 Matter in Our Surroundings is an important study material from the standpoint of your CBSE Class 9 Science examination. Detailed NCERT Solutions for Class 9 Chemistry provided here will help you understand the fundamental concepts taught in the chapter.
NCERT Solutions for Class 9 Science. August 19, 2021 by Prasanna. NCERT Syllabus for Class 9 involves concepts from biology, physics, and chemistry. Candidates must have a detailed knowledge of the NCERT Solutions based on the syllabus to secure great grade points in the final exams. NCERT Science syllabus for Class 9 is designed to provide a ...
In liquids,intermolecular force is less than solids and more than gases. So, they cannot have rigid shape and kinetic energy of the molecules is not enough to hold gas in open container. Please refer to Matter in Our Surroundings Chapter 1 Class 9 Science Assignments below. We have provided important questions and answers for Matter in Our.